
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 54
AMMONIA
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154194 6
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1986
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR AMMONIA
1. SUMMARY
1.1. Properties and analytical methods
1.2. Sources in the environment
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.5.1. Uptake and absorption
1.5.2. Distribution
1.5.3. Metabolic transformation
1.5.4. Excretion and turnover
1.5.5. Plant metabolism of ammonia
1.6. Effects on aquatic organisms
1.7. Effects on experimental animals and in vitro test systems
1.7.1. Single exposures
1.7.2. Short-term exposures
1.7.3. Skin and eye irritation; sensitization
1.7.4. Long-term exposure
1.7.5. Reproduction, embryotoxicity, and teratogenicity
1.7.6. Mutagenicity
1.7.7. Carcinogenicity
1.7.8. Mechanisms of toxicity
1.8. Effects on man
1.8.1. Organoleptic effects
1.8.2. Clinical, controlled human studies and accidental
exposure
1.8.3. Endogenous ammonia
1.9. Evaluation of the health risks for man and effects on the
environment
1.10. Conclusions
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and chemical properties of ammonia and ammonium
compounds
2.1.1. Gaseous and anhydrous liquid ammonia
2.1.2. Aqueous solutions
2.1.3. Chemical reactions
2.1.4. Ammonium compounds
2.2. Sampling and analytical methods
2.2.1. Air and water samples
2.2.2. Soil samples
2.2.3. Blood and tissue samples
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production and use
3.2. Sources releasing ammonia into the air
3.3. Sources discharging ammonia into water
3.3.1. Point sources of ammonia
3.3.2. Non-point sources of ammonia
3.3.3. Comparison between point and non-point sources
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Uptake and transformation in atmosphere
4.2. Transport to the earth's surface
4.2.1. Wet and dry deposition
4.2.2. Contribution to acid rain
4.3. Transformation in surface water
4.4. Uptake and transformation in soils
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Atmospheric levels
5.1.2. Levels in water
5.1.3. Levels in soil
5.1.4. Food
5.1.5. Other products
5.2. General population exposure
5.2.1. Inhalation
5.2.2. Ingestion from water and food
5.2.3. Dermal exposure
5.3. Occupational exposure
5.4. Exposure of farm animals
5.4.1. Oral exposure
5.4.2. Inhalation exposure
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Microorganisms
6.2. Plants
6.2.1. Terrestrial plants
6.2.2. Aquatic plants
6.2.3. Fresh-water plants
6.2.4. Salt-water plants
6.3. Aquatic invertebrates
6.3.1. Fresh-water invertebrates: acute toxicity
6.3.2. Fresh-water invertebrates: chronic toxicity
6.3.3. Salt-water invertebrates: acute and chronic toxicity
6.4. Fish
6.4.1. Ammonia metabolism in fish
6.4.1.1 Ammonia production and utilization
6.4.1.2 Ammonia excretion
6.4.2. Fish: acute toxicity
6.4.2.1 Salt-water fish
6.4.3. Factors affecting acute toxicity
6.4.3.1 pH
6.4.3.2 Temperature
6.4.3.3 Salinity
6.4.3.4 Dissolved oxygen
6.4.3.5 Carbon dioxide
6.4.3.6 Prior acclimatization to ammonia
6.4.4. Fish: chronic toxicity
6.5. Wild and domesticated animals
6.5.1. Wildlife
6.5.2. Domesticated animals
6.5.2.1 Oral exposure
6.5.2.2 Inhalation exposure
7. KINETICS AND METABOLISM
7.1. Absorption
7.1.1. Respiratory tract
7.1.2. Gastrointestinal tract
7.1.3. Skin and eye
7.2. Distribution
7.2.1. Human studies
7.2.2. Animal studies
7.3. Metabolic transformation
7.4. Reaction with body components
7.5. Elimination and excretion
7.5.1. Expired air
7.5.2. Urine and faeces
7.6. Retention and turnover
7.7. Uptake and metabolism in plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Inhalation exposure
8.1.2. Oral exposure
8.1.2.1 Effects of metabolic acidosis induced by
ammonium chloride
8.1.2.2 Organ effects following oral
administration
8.1.2.3 Influence of diet on the effects of
ammonia
8.1.3. Dermal exposure
8.1.4. Effects due to parenteral routes of exposure
8.1.4.1 Lethality
8.1.4.2 Central nervous system effects
8.1.4.3 Effects on the heart
8.2. Short-term exposures
8.2.1. Inhalation exposure
8.2.2. Oral exposure
8.2.2.1 Histopathological effects
8.2.2.2 Effects of ammonium as a dietary nitrogen
supplement
8.2.3. Dermal exposure
8.3. Skin and eye irritation; sensitization
8.4. Long-term exposures
8.4.1. Inhalation exposure
8.4.2. Oral exposure
8.5. Reproduction, embryotoxicity, and teratogenicity
8.6. Mutagenicity
8.7. Carcinogenicity
8.8. Factors modifying effects
8.8.1. Synergistic effects
8.8.2. Antagonistic effects
8.9. Mechanisms of toxicity
9. EFFECTS ON MAN
9.1. Organoleptic aspects
9.1.1. Taste
9.1.2. Odour
9.2. Clinical and controlled human studies
9.2.1. Inhalation exposure
9.2.2. Oral exposure
9.2.2.1 Effects of acute oral exposure
9.2.3. Endogenous hyperammonaemia
9.2.3.1 Inborn errors of metabolism
9.2.3.2 Hepatic features
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Atmospheric exposure and effects
10.1.1. General population exposure
10.1.2. Occupational exposure
10.2. Exposure through food and water
10.3. Ocular and dermal exposure
10.4. Accidental exposure
10.5. Evaluation of risks for the environment
10.5.1. The aquatic environment
10.5.2. The terrestrial environment
10.6. Conclusions
10.6.1. General population
10.6.2. Sub-populations at special risk
10.6.3. Occupational exposure
10.6.4. Farm animals
10.6.5. Environment
11. RECOMMENDATIONS
11.1. Research needs
REFERENCES
ANNEX I
REFERENCES TO ANNEX I
ANNEX II
WHO TASK GROUP ON AMMONIA
Members
Professor E.A. Bababunmi, Department of Biochemistry, University of
Ibadan, Ibadan, Nigeria (Chairman)
Dr J.R. Jackson, Albright and Wilson, Ltd., Occupational Health and
Hygiene Service, Warley, United Kingdom (Rapporteur)
Professor I. Kundiev, Research Institute of Labour, Hygiene, and
Occupational Diseases, Saksaganskogo, Kiev, USSR
Dr M. Piscator, Department of Environmental Hygiene, Karolinska
Institute, Stockholm, Sweden
Professor D. Randall, Department of Zoology, University of British
Columbia, Vancouver, British Columbia, Canada
Dr V.R. Rao, Department of Toxicology, Haffkine Institute, Parel,
Bombay, India
Dr J.A.A.R. Schuurkes, Laboratory of Aquatic Ecology, Faculty of
Natural Sciences, Catholic University, Nijmegen, The Netherlands
Dr J.R. Stara, Office of Research and Development, US Environmental
Protection Agency, Cincinnati, Ohio, USA
Dr H. Suzuki, Department of Hygiene, Fukushima Medical College,
Fukushima, Japan
Dr R.V. Thurston, Fisheries Bioassay Laboratory, Montana State
University, Bozeman, Montana, USA
Dr E. Weisenberg, Institute for the Standardization and Control of
Pharmaceuticals, Ministry of Health, Jerusalem, Israel
(Vice-Chairman)
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mrs I.-M. Linquist, Occupational Safety and Health Branch,
International Labour Office, Geneva, Switzerland
Dr C. Xintaras, Office of Occupational Health, World Health
Organization, Geneva, Switzerlanda
-------------------------------------------------------------------
a Attended half day only.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort is made to present information in the criteria
documents as accurately as possible. In the interest of all users
of the environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A data profile and information on the various limits set by
countries can be obtained from the International Register of
Potentially Toxic Chemicals, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 988400 - 985850).
Concentrations in this document are expressed in the terms used
in original references.
1 mmol is equivalent to 14 mg ammonia-nitrogen/litre
17 mg NH3/litre
18 mg NH4+/litre
1 mg ammonia-nitrogen is equivalent to 1.21 mg NH3
1.29 mg NH4+
In air, 1 mg/m3 is equal to about 1.42 ppm, depending on the
temperature and pressure.
ENVIRONMENTAL HEALTH CRITERIA FOR AMMONIA
Following the recommendations of the United Nations Conference
on the Human Environment held in Stockholm in 1972, and in response
to a number of resolutions of the World Health Assembly and a
recommendation of the Governing Council of the United Nations
Environment Programme, a programme on the integrated assessment of
the health effects of environmental pollution was initiated in
1973. The programme, known as the WHO Environmental Health
Criteria Programme, has been implemented with the support of the
Environment Fund of the United Nations Environment Programme. In
1980, the Environmental Health Criteria Programme was incorporated
into the International Programme on Chemical Safety (IPCS), a joint
venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The Programme is responsible for the publication of
a series of criteria documents.
A WHO Task Group on Environmental Health Criteria for Ammonia
was held in Geneva on 8-13 July, 1985. Dr E.M. Smith opened the
meeting on behalf of the Director-General. The Task Group reviewed
and revised the draft criteria document and made an evaluation of
the health risks of exposure to ammonia.
The original draft of this document was prepared by THE UNITED
STATES ENVIRONMENTAL PROTECTION AGENCY ENVIRONMENTAL CRITERIA AND
ASSESSMENT OFFICE under the direction of DR J.F. STARA. Additional
contributions were made by DR J.R. JACKSON, PROFESSOR D. RANDALL,
and DR R.V. THURSTON.
The efforts of these contributors and of all who helped in the
preparation and finalization of the document are gratefully
acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY
1.1. Properties and Analytical Methods
Ammonia (NH3) is a colourless acrid-smelling gas at ambient
temperature and pressure. It can be stored and transported as a
liquid at a pressure of 10 atm at 25 °C.
Ammonia dissolves readily in water where it forms, and is in
equilibrium with, ammonium ions (NH4+). The sum of ammonia and
ammonium concentrations is termed "total ammonia" and, because of
the slightly different relative molecular masses, may be expressed
as "total ammonia-nitrogen (NH3-N)". In most waters, NH4+
predominates, but increases in pH or temperature or decreases in
ionic strength may materially increase levels of non-ionized
ammonia.
Ammonia will adsorb on various solids. At concentrations of
between 16 and 27% by volume, it can form explosive mixtures with
air. Catalytic oxygenation is an important reaction in the
manufacture of nitric acid. Ammonia dissolves in dilute acids to
form ionized ammonium salts, which are similar in solubility to
alkali metal salts, and can be crystallized. Some of these salts
are found in nature. Heating solutions or crystals of the salts
yields gaseous ammonia. Ammonia forms chloramines in water
containing hypochlorous acid.
There are difficulties in sampling media for the determination
of ammonia, and in preventing contamination and losses before
analysis. A variety of analytical techniques are available; many
have interactions. For measurements, the flourescent
derivatization technique has advantages.
1.2. Sources in the Environment
Ammonia is present in the environment as a result of natural
processes and industrial activity, including certain types of
intensive farming. Atmospheric ammonia is volatilized from the
earth's surface in quantities of about 108 tonnes/year, mostly from
natural biological activity. Industrial activity may cause local
and regional elevations in emission and atmospheric concentrations.
Surface waters receive ammonia from point sources, such as effluent
from sewage treatment and industrial plants, in quantities
estimated in the USA to be about half a million tonnes annually.
Much more significant quantities arise from non-point sources, such
as atmospheric deposition, the breakdown of vegetation and animal
wastes, applied artificial fertilizers and urban runoff, and these
are significant, even in industrial areas.
1.3. Environmental Transport, Distribution, and Transformation
Ammonia in the environment is a part of the nitrogen cycle. It
volatilizes into the atmosphere where it may undergo a variety of
reactions. Photolytic reactions destroy some of the ammonia and
reactions with sulfur dioxide or ozone produce aerosols, most
importantly of ammonium sulfate or nitrate, which return to the
earth's surface as wet or dry deposition. In surface waters,
ammonium may undergo microbiological nitrification, which yields
hydrogen and utilizes oxygen so that, in certain systems,
acidification and oxygen depletion may result. In one study, one-
third of the acidifying effect of precipitation was attributed to
ammonium deposition. Ammonia may be assimilated by aquatic plants
as a nitrogen source or transferred to sediments or volatilized.
In soil, major sources of ammonia are the aerobic degradation of
organic matter and the application and atmospheric deposition of
synthetic fertilizers. The ammonium cation is adsorbed on
positively charged clay particles and is relatively immobile. Most
ammonium undergoes nitrification; the nitrate ion is mobile and is
removed by leaching, plant root uptake, or denitrification.
1.4. Environmental Levels and Human Exposure
Atmospheric concentrations vary according to underlying land
usage. Urban concentrations are typically in the range of 5 -
25 µg/m3 and rural concentrations, 2 - 6 µg/m3. Areas with
intensive manure production or use may produce concentrations of
100 - 200 µg/m3. Particulate ammonium concentrations above oceans,
remote from land, have been found to be 10 - 115 ng/m3. In most
situations, atmospheric particulate ammonium concentrations are
comparable to gaseous ammonia concentrations.
Surface waters contain concentrations of total ammonia that
vary both regionally and seasonally. In the USA, most surface
waters contain less than 0.18 ng/litre, though those near large
metropolitan areas may contain 0.5 ng/litre, as total ammonia. In
hydrologically isolated acidified small lakes, concentrations may
reach 3 mg NH4+-N/litre, and values near intensive farms of 12 mg
NH4+-N/litre have been recorded. Ground water usually contains low
concentrations of ammonia, because of ammonium adsorption and/or
nitrification; this, and the conversion of ammonia to chloramines
on chlorination, results in low levels of ammonia in most treated
drinking-water.
Ammonia in soil is largely fixed; that in solution is in
dynamic equilibrium with nitrate and is not directly available to
plants. Ammonia occurs in unprocessed foods, but ammonium salts
are added to processed foods. Acceptable Daily Intakes (ADIs),
where specified, relate to the anion. Cigarette smoking and
certain medicines may contribute to intake, in some cases, but the
intake from all sources is small in comparison with endogenous
intestinal ammonia production.
Occupational exposure to low levels of ammonia is common, but,
in certain occupations, work-place concentrations may exceed
100 mg/m3. At such levels, the daily ammonia intake is small in
relation to endogenous production, but it is significant, since
inhaled ammonia enters the systemic circulation.
1.5. Kinetics and Metabolism
1.5.1. Uptake and absorption
At low concentrations, inhaled ammonia dissolves in the mucous
fluid lining the upper respiratory tract and little reaches the
lower airways. Initial retention is about 80% in both the dog and
man, but, in man, it falls to less than 30% in less than 27 min.
In rats, increases in blood-ammonia were measured following short-
term exposure to ammonia at 220 mg/m3 but not at 23 mg/m3. The
increases were less marked with longer exposure. Calculated blood-
ammonia increases with exposure to air containing 18 mg/m3 are
about 10% of fasting levels.
Ammonia is formed in the human intestinal tract by the
biological degradation of nitrogenous matter, including secreted
urea, in quantities of about 4 g/day. Nearly all of this is
absorbed (mainly passively) and is metabolized in the liver on
first passage, so that only small amounts reach the systemic
circulation.
1.5.2. Distribution
Ammonia is normally present in all tissues constituting a
metabolic pool. Its distribution is pH dependent, since NH3
diffuses more easily than NH4+. Oral administration of ammonium
chloride to healthy male and female volunteers at 9 mg/kg body
weight produced transient increases in blood-ammonia in about half
of the subjects. Patients with cirrhosis showed a greater and more
prolonged increase over a higher baseline. This confirms
substantial first pass metabolism in the liver.
Administration of 15N-labelled ammonium compounds to
experimental animals indicated that the initial distribution of 15N
depended on the route of administration and that, after parenteral
administration, more was distributed to organs other than the
liver.
1.5.3. Metabolic transformation
Ammonia is taken up by glutamic acid in many tissues, and this
will take part in a variety of transamination and other reactions,
the nitrogen being incorporated in non-essential amino acids. In
the liver, ammonia is used in the synthesis of protein by the
Krebs-Henseleit cycle.
1.5.4. Excretion and turnover
The principal means of ammonia excretion varies between phyla.
Mammals excrete urea and secrete ammonium in the kidney tubules as
a means of hydrogen ion excretion. Faecal and respiratory
excretion are insignificant. Exhaled air may contain volatilized
ammonia from the microfloral degradation of salivary urea. In man,
on a 70 g protein/day diet, 70% of administered ammonium 15N is
lost in a week; on a 20 g protein/day diet, 35% is lost.
1.5.5. Plant metabolism of ammonia
Ammonia is toxic in plants and cannot be excreted. It is
detoxified by combination with carbon skeletons, and so excess
ammonia may strain carbohydrate metabolism. Some plants have
special means of handling ammonia, enabling them to tolerate it or
use it preferentially.
1.6. Effects on Aquatic Organisms
Concentrations of ammonia that are toxic for aquatic animals
are generally expressed as non-ionized ammonia (NH3), because, in
the environment, NH3 and not the ammonium ion (NH4+) has been
demonstrated to be the principal toxic form of ammonia.
Concentrations of ammonia, acutely toxic for fish, can cause
loss of equilibrium, hyperexcitability, increased breathing,
cardiac output, and oxygen uptake, and, in extreme cases,
convulsions, coma, and death. At lower concentrations, ammonia
produces many effects in fish including a reduction in egg hatching
success, a reduction in growth rate and morphological development,
and pathological changes in the tissue of the gills, liver, and
kidney.
Several factors have been shown to modify acute ammonia
toxicity in fresh water. Some factors alter the concentration
of NH3 in the water by affecting the aqueous ammonia equilibrium,
while other factors affect the toxicity of NH3 itself, either
ameliorating or exacerbating its effects. Factors that have been
shown to affect ammonia toxicity include dissolved oxygen
concentration, temperature, pH, previous acclimatization to
ammonia, fluctuating or intermittent exposures, carbon dioxide
concentration, salinity, and the presence of other toxic
substances. The best studied of these is pH; the acute toxicity
of NH3 has been shown to increase as pH decreases. Data on
temperature effects on acute NH3 toxicity are limited and
variable, but there are indications that NH3 toxicity is greater
at low (< 10 °C) temperatures.
Data concerning concentrations of NH3 that are toxic for fresh-
water phytoplankton and vascular plants, although limited, indicate
that fresh-water plant species are appreciably more tolerant to NH3
than invertebrates or fish.
(a) Fresh-water organisms
Mean 48- and 96-h LC50 values reported for fresh-water
invertebrates and fish ranged from 1.10 to 22.8 mg NH3/litre for
invertebrate species, and from 0.56 to 2.48 mg/litre for fish
species. Mean 96-h LC50 values ranged from 0.56 to 2.37 mg
NH3/litre for salmonid fish and from 0.76 to 2.48 mg/litre for non-
salmonids. In terms of LC50, Percidae and Salmonidae are
considered to be the most sensitive families and walleye and
rainbow trout are the most sensitive species within these families.
For fresh-water organisms, the families most sensitive in terms
of chronic toxicity are Salmonidae and Catostomidae, pink salmon
and white sucker being the most sensitive species within these
families. Limited chronic toxicity data for invertebrates, mostly
cladocerans and one insect species, indicate that they are
generally more tolerant than fish, although the fingernail clam
appears to be as sensitive as salmonids.
(b) Salt-water organisms
Available acute and chronic ammonia toxicity data for salt-
water organisms are very limited. Mean LC50 values for marine
invertebrate species range from 0.94 to 18.3 mg NH3/litre and, for
marine fish species, from 0.32 to 1.31 mg/litre. The prawn,
Macrobrachium rosenbergii, appears to be the most sensitive
invertebrate species tested, and the red drum, the most sensitive
fish species.
1.7. Effects on Experimental Animals and In Vitro Test Systems
1.7.1. Single exposures
There have been many estimates of inhalational toxicity in
which the theoretical relationship between concentration, duration
of exposure, and lethality has been observed. Typical results are
LC50 values in rats ranging from 31 612 mg/m3 for a 10-min exposure
to 11 620 mg/m3 for a 60-min exposure. The corresponding value for
a 2-h exposure was 7600 mg/m3. Exposed mice exhibited avoidance
behaviour at concentrations above 350 mg/m3, and ciliary activity
was arrested above this level in in vitro studies on rabbit
tracheal epithelium. Other effects of exposure include bradypnoea
and bradycardia, changes in various serum-enzyme levels, and
histological changes in the lung. At high concentrations,
convulsions occurred.
There have been a number of studies on the oral toxicity of
various ammonium salts, some of which have been complicated by the
acidity or alkalinity of the preparations used. Median lethal
doses for ammonium sulfamate or sulfate were in the range 3 -
4.5 g/kg body weight in both rats and mice. Ammonium chloride
causes substantial acidosis and has been reported to produce
pulmonary oedema by a different mechanism by gavage, but not by
intraperitoneal injection. There is also evidence that ammonium
ions exert a direct effect on the appetite by their effect on
prepyriform cortical areas. Ammonium chloride, even after
administration for periods of a few days, produces hypertrophy of
the kidney, but the extent to which this results from acidosis, a
solute load, or a direct effect of the ammonium ion is not clear.
Diet and the clinical condition of the liver are important
modulators of ammonia toxicity, and it has been shown that the
administration of ornithine, aspartic acid, or adenosine
triphosphate (ATP) exerts a protective effect against ammonia
toxicity.
No information is available regarding systemic toxicity from
single dermal exposures to ammonia or ammonium compounds.
Symptoms after intravenous injection of ammonium salts are
characterized by immediate hyperventilation and clonic convulsions,
followed by either fatal tonic extensor convulsion or the onset of
coma, in which tonic convulsions and death can occur at any time.
After 30 - 45 min, surviving animals recover rapidly and
completely. After injection, neurological symptoms commenced when
the blood-ammonia concentration doubled above basal values. Brain-
ammonia levels did not increase until blood levels reached 20 times
basal values; at this stage, brain levels suddenly increased to
about 100 mg ammonia-nitrogen/kg wet weight. However, immediate
increases in brain-ammonia after intravenous injection have also
been observed, and it has been suggested that there is no critical
blood-ammonia concentration for diffusion of ammonia through the
blood-brain barrier. Some workers have demonstrated the induction
of ventricular fibrillation of the heart following injections of
ammonium salts.
1.7.2. Short-term exposures
Ninety-day inhalation exposures of rats to 127 mg/m3 and
262 mg/m3 did not produce any, or only minimal, changes. Continuous
exposure to 455 mg/m3 was fatal for 50 out of 51 rats by the 69th
day of exposure. Similar results were obtained in guinea-pigs.
The principal pathological findings were eye irritation, corneal
opacities, and diffuse lung inflammation. Similar results have
been published by a number of authors. Concentration-dependent
increases in susceptibility to infection during ammonia exposure
have been reported. Blood-ammonia levels increased with
inhalational exposure to increasing concentrations of ammonia above
70 mg/m3, for periods of 1 - 7 days.
Studies on the effects of ingestion of ammonium chloride
(10 g/litre drinking-water - about 1 g/kg body weight per day)
and ammonium sulfamate (5 g/kg body weight per day for 6 days
per week) did not show any significant toxic effects. Cyclical
administration of various ammonium salts, at moderate doses, for 3
weeks out of 4 affected the reproductive system of virgin female
rabbits. Ammonium salts have been given as a dietary supplement to
animals on diets deficient in non-essential amino acids, with
resultant increases in weight gain. Ammonium salts can prevent and
reduce the weight loss associated with 10% and 20% reduction of the
crude protein content of the diet of pigs.
There is no information regarding the systemic effects of
short-term dermal exposure.
1.7.3. Skin and eye irritation; sensitization
There is little information on animals to complement the
extensive human experience. In rabbits, ammonia has been shown to
penetrate the cornea rapidly and to cause corneal burns. Ammonium
persulfate is a recognized skin sensitizer in man. No data on
sensitization potential in animal models are available.
1.7.4. Long-term exposure
Inhalation exposure studies did not extend beyond 130 days. A
130-day study demonstrated congestion of parenchymatous organs at
18 weeks, but not at 12 weeks, in guinea-pigs exposed to about
119 mg/m3 for 6 h/day, 5 days/week. Long-term studies have not
been carried out according to modern protocols, and observed
effects have mainly been related to changes in acid-base balance.
1.7.5. Reproduction, embryotoxicity, and teratogenicity
There have not been any formal studies based on modern
protocols, but studies have been undertaken to investigate the
effects of ammonia in hen-houses on the egg-laying performance of
intensively reared poultry. No systematic conclusions could be
drawn.
1.7.6. Mutagenicity
Ammonium sulfate has been reported non-mutagenic in Salmonella
and Saccharomyces test systems, but mutagenic in E. coli at toxic
levels and may affect mutagenic responses to other agents. Various
workers have described effects on Drosophila, which were minimal
or achieved only at toxic levels. There is no evidence that ammonia
is mutagenic in mammals.
1.7.7. Carcinogenicity
There is no evidence that ammonia is carcinogenic, though it
can produce inflammatory lesions of the colon and cellular
proliferation, which could increase susceptibility to malignant
change. There was no evidence that ammonia was responsible for the
increased incidence of tumours with increased dietary protein
intake. Ammonia did not either cause tumours or increase the
spontaneous incidence of tumours in life-time studies on mice.
1.7.8. Mechanisms of toxicity
Although there are a number of hypotheses, there is no
established mechanism for the toxicity of ammonia or ammonium
salts.
1.8. Effects on Man
1.8.1. Organoleptic effects
Ammonia can be tasted in water at levels above about 35
mg/litre. Odour thresholds have been variously reported according
to the definition used and technique of measurement. Most people
can identify ammonia in air at about 35 mg/m3 and can detect it at
about one-tenth of this level.
1.8.2. Clinical, controlled human studies and accidental exposure
Exposure to ammonia in air at a concentration of 280 mg/m3
produced throat irritation; 1200 mg/m3 produced cough; 1700 mg/m3
was life-threatening, and more than 3500 mg/m3 caused a high
mortality. Respiratory symptoms were usually reversible, but
chronic bronchitis has been reported to develop. Volunteers
exposed by oro-nasal mask experienced irritation and increased
minute volumes. Retention of inspired ammonia decreased
progressively to about 24% after about 19 min of exposure. The
blood chemistry remained normal. Respiratory indices were
insignificantly altered at concentrations up to 98 mg/m3 (which was
tolerable). Other studies have demonstrated a high incidence of
symptoms at this level. Irritation occurred at 35 mg/m3, which was
neither discomforting nor painful. Industrial exposure at 88 mg/m3
was described as "definitely irritating".
Ingestion of ammonia solutions has produced caustic burns of
the upper gastrointestinal tract. Ingestion of ammonium chloride
produces metabolic acidosis and diuresis and is administered for
these effects.
1.8.3. Endogenous ammonia
Ammonia plays a key role in nitrogen metabolism, and its level
in the body may be increased as a result, either of in-born errors
of metabolism, or, as a result of impaired liver function. The
role of hyperammonaemia in causing the encephalopathy associated
with the latter is not completely clear, but there is sufficient
evidence to indicate a significant contribution.
1.9. Evaluation of the Health Risks for Man and Effects on the
Environment
Atmospheric exposure of the general population is toxicologically
insignificant. Occupational exposure can give rise to symptoms,
particularly in occupations exposed to decaying organic matter.
Accidental exposure to ammonia in any of its forms produces
irritant or caustic effects.
Exposure to ammonia in the water supply and food is
insignificant in comparison with the nitrogen intake through the
diet which becomes available as metabolic ammonia.
The most significant effects of ammonia are in the aquatic and
terrestrial environments where, as a result of urbanization,
industry, and farming and as a result of deposition to sensitive
environments, significant toxic effects of ammonia may arise.
1.10. Conclusions
Ammonia does not present a direct threat to man except as a
result of accidental exposure, particularly in industry. Farm
animals may be adversely affected when reared intensively in closed
conditions. Localized effects of point-source emissions of ammonia
and of deposition in sensitive environments is a cause of concern.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties of Ammonia and Ammonium Compounds
2.1.1. Gaseous and anhydrous liquid ammonia
Ammonia (NH3) is a colourless gas at atmospheric pressure,
which is lighter than air and possesses a strong penetrating odour.
Some of the relevant physical properties of ammonia are summarized
in Table 1.
The vapour pressure of ammonia gas over pure ammonia liquid can
be calculated using the equation (NRC, 1979):
log10P = 9.95028 - 0.003863T - 1473.17/T,
where P = partial pressure in mm Hg, and T = temperature at K.
Ammonia may be liquefied under pressure at about 10 atm and is
stored and transported in this state.
2.1.2. Aqueous solutions
Ammonia dissolves readily in water where it ionizes to form the
ammonium ion.
\
NH3 + H2O ========= NH4+ + OH-
\
The solubility of ammonia in water is influenced by the
atmospheric pressure, temperature, and by dissolved or suspended
materials. Solubility values at moderate concentrations and
temperatures can be obtained from the graphic (Sherwood, 1925) and
tabular (Perry et al., 1963) compilations, and from empirical
formulae (Jones, 1973).
The total ammonia content of water is the sum of non-ionized
(NH3) and ionized (NH4+) species. Ammonia is readily soluble in
aqueous systems (Table 1) and, at the pH of most biological
systems, exists predominantly in the ionized form. At low
concentrations, the molarity of total dissolved ammonia is given by
(Drewes & Hales, 1980):
[NH3] + [NH4+] = H[NH3(gas)] + KbH[NH3(gas)],
where [NH3(gas)] is the molar concentration of gas-phase ammonia,
Kb is the dissociation constant given by:
[NH4+] [OH-]
Kb = ------------ = 1.774 x 10-5 (at 25°C)
[NH3]
and H is a Henry's law constant given by (NRC, 1979):
log10H = 1477.8/T - 1.6937
Table 1. Physical properties of ammoniaa
------------------------------------------------------------------
Properties Values
------------------------------------------------------------------
Boiling point at one atm -33.42 °C
Melting point -77.74 °C
Density (liquid) at -33.35 °C and 1 atm 0.6818 gm/cm2
Density (gas) 0.7714 g/litre
Viscosity at -33 °C 0.254 centipoise
Viscosity at 20 °C 9.821 x 109 poise
Refractive index at 25 °C 1.325
Dielectric constant at 25 °C 16.9
Surface tension at 11 °C 23.38 dyn/cm
Specific conductance at -38 °C 1.97 x 10-7 cm-1
Thermal conductivity at 12 °C 5.51 x 10-5 gcal/cm
Vapour pressure at 25 °C 10 atm
Critical temperature 132.45 °C
Critical pressure 112.3 atm
Critical density 0.2362 g/cm3
Solubility in water, 101 kPa
at 0 °C 895 g/litre
20 °C 529 g/litre
40 °C 316 g/litre
60 °C 168 g/litre
------------------------------------------------------------------
a From: Jones (1973) and Windholz et al., ed. (1976).
The pKa for the ammonia/ammonium equilibrium can be calculated at
all temperatures, T(K), between 0 and 50 °C (273 < T < 323) by
the equation (Emerson et al., 1975):
Ka = [NH3] [H+]/[NH4+],
pKa = 0.09018 + 2729.92/T
Theoretically, the fraction (f) of total ammonia that is non-
ionized depends on both water temperature and pH, according to the
preceding and the following equations (Emerson et al., 1975):
(pKa-pH)
f = 1/[10 + 1]
Thus, in water at 0 °C and a pH of 6, less than 0.01% of the total
ammonia present is in the non-ionized form, whereas, at 30 °C and a
pH of 10, 89% of total ammonia is non-ionized.
The above relationship holds in most fresh waters. However,
the concentration of non-ionized ammonia will be lower at the
higher ionic strengths of very hard fresh waters or saline waters.
Using the appropriate activity coefficients, in sea water of ionic
strength = 0.7, the above relationship can be restated as follows
(API, 1981):
(pKa-pH + 0.221)
f = 1/[10 + 1]
At 25 °C, the pKa can be calculated to be 9.24, from the
equation of Emerson et al. (1975). Therefore, at pH 8, and at a
temperature of 25 °C, the above equation shows that 3.31% of the
total ammonia in sea water exists in the non-ionized form. The
corresponding value in fresh water can be calculated to be 5.38%.
Thus, at this pH and temperature, sea water with an ionic strength
of 0.7 would contain 62% as much non-ionized ammonia as fresh
water.
2.1.3. Chemical reactions
Gaseous ammonia is readily adsorbed on certain solids. The
adsorption characteristics of ammonia on metal surfaces are
important in its synthesis and other catalytic reactions (Cribb,
1964). Because of the adsorption of ammonia on charcoal, acid-
impregnated charcoal masks are used for protection against ammonia
gas.
Ammonia can form explosive mixtures with air at atmospheric
temperature and pressure, if present in concentrations of 16 - 27%
by volume. The products of combustion are mainly nitrogen and
water, but small traces of ammonium nitrate (NH4NO3) and nitrogen
dioxide (NO2) are also formed.
Another important reaction involving the oxidation of ammonia
is its catalytic oxidation to nitric oxide (NO) and nitrous oxide
(N2O) (Miles, 1961; Matasa & Matasa, 1968). This reaction is an
important step in the manufacture of nitric acid.
Under normal atmospheric conditions, ammonia does not undergo
any primary photochemical reactions at wavelengths greater than
290 nm.
When exposed to radicals or other photochemically excited
species, ammonia undergoes secondary decomposition:
NH3 + -OH -> -NH2 + H2O
NH3 + O -> -NH2 + -OH
Some of these reactions may be important in the balance of
atmospheric nitrogen.
Ammonia also undergoes decomposition to nitrogen and hydrogen,
when exposed to an electric discharge (Jones, 1973). It reacts
with sulfur dioxide gas to form ammonium sulfate in the atmosphere
(Kushnir et al., 1970).
Aqueous ammonia can take part in substitution reactions with
organic halide, sulfonate, hydroxyl, and nitro compounds, and, in
the presence of metallic catalysts, it is used to produce amino
acids from keto acids. Ammonia reacts with hypochlorous acid
(HOCl) to form monochloramine, dichloramine, or nitrogen
trichloride (Morris, 1967; Lietzke, 1978). The formation of these
N-chloramines depends on the pH, the relative concentrations of
hypochlorous acid and NH3, the reaction time, and the temperature.
When pH values are greater than 8, and when the molar ratio of HOCl
to NH3 is 1:1 or less, the monochloramine predominates. At higher
Cl2:NH3 ratios or, at lower pH values, dichloramine and
trichloramine are formed. These, and various organic chloramines,
are produced during the chlorination of water containing NH3 or
organic amines. The presence of these chloramines may contribute
to the taste and odour of drinking-water, and to various
associated health problems (Morris, 1978).
2.1.4. Ammonium compounds
Ammonium compounds comprise a large number of salts, many of
which are of industrial importance; ammonium chloride, ammonium
nitrate, and ammonium sulfate are produced on a large scale. With
the exception of metal complexes, the ammonium salts are very
similar in solubility to the salts of the alkali metals, but differ
in that they are completely volatilized on heating or ashing.
Ammonium salts undergo slight hydrolysis in aqueous solution.
Most dissociate at elevated temperatures to give ammonia and the
protonated anion. The physical and chemical properties of ammonium
compounds of environmental importance are discussed below, and some
of their physical properties are summarized in Table 2.
Ammonium chloride [NH4Cl] occurs naturally in volcanic
crevices as a sublimation product. When it sublimes, the
vapour is completely dissociated into hydrogen chloride and
ammonia. Like other ammonium salts of strong acids, the
chloride hydrolyses in aqueous solution to lower the pH of the
solution. The solid tends to lose ammonia during storage. Aqueous
solutions of ammonium chloride have a notable tendency to attack
ferrous metal and other metals and alloys, particularly copper,
bronze, and brass. Ammonium chloride can be oxidized to nitrosyl
chloride and chlorine by strong oxidizing agents, such as nitric
acid.
Ammonium nitrate [NH4NO3] does not occur in nature. It is
soluble in water and liquid ammonia and slightly soluble in
absolute ethyl alcohol, methanol, and acetone. Although ammonium
salts of strong acids generally tend to lose ammonia during
storage, ammonium nitrate can be considered a very stable salt. It
undergoes decomposition at elevated temperatures or under extreme
shock, as in commercial explosives. Ammonium nitrate acts as an
oxidizing agent in many reactions, and, in aqueous solution, it is
reduced by various metals. Solutions of ammonium nitrate attack
metals, particularly copper and its alloys.
Ammonium sulfate [(NH4)2SO4] is found naturally in volcanic
craters. It is soluble in water and insoluble in alcohol and
acetone. The melting point of ammonium sulfate is 230 °C. On
heating in an open system, the compound begins to decompose at
100 °C, yielding ammonium bisulfate (NH4HSO4) that has a melting
point of 146.9 °C.
Ammonium acetate [CH3COONH4] is a deliquescent material that is
highly soluble in cold water and in alcohol. Solubility does not
increase greatly with increasing temperature, at least up to 25 °C.
In aqueous solution at atmospheric pressures, ammonium acetate
readily loses ammonia, especially in alkaline conditions.
Ammonium carbonate [(NH4)2CO3] and ammonium bi-carbonate
[NH4HCO3] have long been known because of their occurrence in
association with animal wastes. Ammonium bicarbonate is the more
readily formed and the more stable. It decomposes below its
melting point (35 °C), dissociating into ammonia, carbon dioxide,
and water. Ammonium bicarbonate reacts with, and dissolves,
calcium sulfate scale. Ammonium carbonate decomposes on exposure
to air with the loss of ammonia and carbon dioxide, becoming white
and powdery and converting into ammonium bicarbonate. Ammonium
carbonate volatilizes at about 60 °C. It dissolves slowly in water
at 20 °C, but decomposes in hot water.
2.2. Sampling and Analytical Methods
2.2.1. Air and water samples
Measurement of ammonia levels in air is difficult. Atmospheric
levels are low, and samples can be contaminated by emissions from
man; thus, the analyst should remain remote from the sampling
device. In addition, air samples are bubbled through acid media to
form an aqueous solution of ammonia, predominately in its ionic
form. The extraction of ammonia is variable and both gaseous
ammonia and that contained in aerosols will be extracted. In some
instances filters are used to remove aerosols from the gas stream
so that only ammonia gas is sampled. There are, however, some
problems with aerosol filters as they may interact with gaseous
ammonia when the aerosol is collected on the filter (NRC 1979).
Table 2. Physical properties of some ammonium compoundsa
-----------------------------------------------------------------------------------------------------------------
Property Ammonium Ammonium Ammonium Ammonium Ammonium Ammonium
chloride nitrate sulfate acetate carbonate bicarbonate
-----------------------------------------------------------------------------------------------------------------
Synonyms ammonium ammonium ammonium ammonium ammonium ammonium bicarbonate;
chloride; nitrate sulfate; acetate carbonate; ammonium hydrogen
sal ammoniac mascagnite monohydrate carbonate
Colour colourless colourless colourless white colourless colourless
Physical state cubic rhombic rhombic crystals, cubic rhombic or monoclinic
(25 °C, 1 atm) crystals crystals crystals hygroscopic crystals crystals
Formula NH4Cl NH4NO3 (NH4)2SO4 CH3COONH4 (NH4)2CO3 x H2O NH4HCO3
Relative 53.49 80.04 132.14 77.08 114.10 79.06
molecular mass
Melting point 340 sublimes 169.6 230 114 58 35
(°C) decomposes decomposes decomposes
Boiling point 520 > 210 decomposes sublimes
(°C) decomposes
Density 1.527 (20 °C) 1.725 (25 °C) 1.769 (50 °C) 1.17 (20 °C) 1.58 (20 °C)
Refractive 1.642 1.533 1.423, 1.555
index, nb20
Solubility in 370 (20 °C) 1920 (20 °C) 754 (20 °C) 1480 (4 °C) 1000 (15 °C) 217 (20 °C)
water
(g/litre)
-----------------------------------------------------------------------------------------------------------------
a From: Dean (1979) and Weast (1979).
Air samples collected by liquid impinger yield aqueous
solutions. Fabric filters used for collecting aerosols may be
extracted with water for analysis. Generally, air and water
samples are analysed using similar techniques, which are summarized
in Table 3.
Various methods for preventing interference can be used, but
distillation at pH 9.5 is often carried out. Care must be taken
with water samples to prevent oxidation, volatilization, or
microbiological assimilation of ammonia. Thus, samples should be
acidified and refrigerated in sealed containers (and may be treated
with reagents) and analysed within 24 h (APHA, 1976; NRC, 1979; US
EPA, 1979b; ASTM, 1980; API, 1981; Analytical Quality Control
(Harmonised Monitoring) Committee, 1982).
2.2.2. Soil samples
Soil samples are usually collected by the grab method. To
inhibit microbial activity during transport and storage, reagents
(e.g., mercury (II) chloride) can be added to the soil (NRC, 1979).
Rapid drying at 55 °C, then sealing the samples in air-tight
containers is a more satisfactory method of preservation for
ammonium determination (NRC, 1979), but even this may not prevent
erroneous results, and samples should be analysed soon after sample
collection (NRC, 1979). Analytical methods for the determination
of ammonia and ammonium in soils have been reviewed by NRC (1979).
2.2.3. Blood and tissue samples
The various techniques used for the determination of ammonia in
blood and tissues ultimately incorporate the ammonia detection
methods described in Table 3, but with various conditions, such as
distillation, aeration, and diffusion to minimize interference
(NRC, 1979). Because of the higher protein concentration in
tissues, determination of ammonia is subject to greater glutamine-
caused error than in body fluids (NRC, 1979).
Table 3. Ammonia detection methods
----------------------------------------------------------------------------------------------------------------------
Medium Particular Method Principle Interferants Sensitivity Reference
application
----------------------------------------------------------------------------------------------------------------------
Air silo air NH3 alkalimetric air is drawn through other acidic or 70 - 700 Elkins (1959);
sulfuric acid until alkaline mg/m3 Leithe (1971)
bromophenol indicator contaminants
changes colour; volume
of air is inversely
proportional to
ammonia concentration
Water high titrimetric NH3 in water is distilled 1 - 25 API (1981)
concentrations off into distilled water mg/litre
which is titrated with
acid to a methyl red/
methylene blue end-point
Air Nesslerization NH3/NH4 in dilute amines, cyanate, 14 - 95 Leithe (1971);
sulfuric or boric acid alcohols, aldehyde, mg/m3 air NIOSH (1977)
is reacted with alkaline ketones, colour,
Water mercuric and potassium turbidity, residual 1 - 25 Stern (1968);
iodide solution (Hg I2 x chlorine mg/litre API (1981)
KI); absorbence at 440 nm water
is compared with a
standard curve; distil-
lation can preceed
analysis
Air low indophenol NH3 in solution is monoalkyl amines, 7 - 7000 Leithe (1971)
concentrations reaction reacted with hypochlorite formaldehyde µg/m3
and phenol (slow-warm
reagents)
Water turbidity, colour 10 - 2000 API (1981)
salt (sea water) µg/litre
Air measurement ammonia measurement of ionization mercury, volatile 14 - 2100 Sloan & Morie
of tobacco electrode potential of NH3 --> amines µg/m3 (1974)
smoke (potentiometric) NH+4
----------------------------------------------------------------------------------------------------------------------
Table 3. (contd.)
----------------------------------------------------------------------------------------------------------------------
Medium Particular Method Principle Interferants Sensitivity Reference
application
----------------------------------------------------------------------------------------------------------------------
Water 0.05 - 1400 API (1981)
mg/litre
Air continuous chemiluminescent air is passed through 3.5 - 3500 Spicer (1977)
measurement high- and low-temperature µg/m3
catalytic converters,
which respectively
measure NOx + NH3 and
NOx; NH3 is obtained by
subtraction
Air tobacco gas chromato- gas chromatography with 7 - 70 Sloan & Morie
smoke graphy thermal conductivity mg/m3 (1974)
detector
Air continuous UV spectro- NH3(gas) exhibits several 0.7 - 7 Leithe (1971)
measurement photometry strong absorption bonds mg/m3
between 190 and 230 nm;
absorption in 10 cm
quartz cells at 204.3 nm
has been used (molecular
extinction coefficient =
2790)
Air continuous Fluorescent 1-phthaldehyde 0.07 µg/m3 Abbas & Tanner
measurement derivatization derivatization upwards (1981)
high technique
sensitivity
----------------------------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Ammonia is present in the environment as a result of natural
processes and through the industrial activities of man. It is
generally accepted that, of the ammonia present in the atmosphere,
99% is produced by natural biological processes. Ammonia is
continually released throughout the biosphere by the breakdown or
decomposition of organic waste matter. Thus, any natural or
industrial process that concentrates and makes nitrogen-containing
organic matter available for decomposition represents a potential
source of high local concentrations of ammonia in water, air, and
soil. Industrially-produced ammonia, from non-biological nitrogen,
also represents an environmental source, by release through
agricultural fertilization and industrial emissions. Coal
gasification or liquefaction may provide a major local source of
ammonia. The natural occurrence of ammonia compounds is indicated
in section 2.1.4.
3.1. Production and Use
Ammonia is one of the most widely-used industrial chemicals.
It is ranked fourth in production volume in the USA after sulfuric
acid, lime, and oxygen (Chemical and Engineering News, 1980).
Total production of ammonia-nitrogen in the USA increased from
5.8 x 106 tonnes in 1964 to 11.5 x 106 tonnes in 1974 (Keyes,
1975), and had further increased to 17.6 x 106 tonnes by 1979
(Chemical and Engineering News, 1980). The demand in the USA for
the production of ammonia is projected to reach 25 x 106 tonnes by
1990 (Mai, 1977).
Ammonia is mainly produced industrially by the Haber-Bosch
process in which nitrogen and hydrogen are combined under high
pressure in the presence of a catalyst (Harding, 1959; Matasa &
Matasa, 1968). Prior to the Haber-Bosch process, ammonia was
produced by the hydrolysis of cyanamides or cyanides. A smaller
scale method for ammonia production is regeneration from ammonium
salts by heating with a base. Alkaline earth metal oxides and
hydroxides have been used with the naturally-occurring ammonium
chloride.
Most of the ammonia produced in the USA is consumed as
fertilizers (80%), fibres and plastics (10%), and explosives (5%)
(Chemical and Engineering News, 1980). It is also used in the
production of animal feed (1.5%), pulp and paper (0.6%), and rubber
(0.5%) (Keyes, 1975) and in a variety of other chemical production
processes. Ammonia and ammonium compounds are used as cleaning
fluids, scale-removing agents, and in food as leavening agents,
stabilizers, and for flavouring purposes. A survey by the US Food
and Drug Administration (US FDA) indicated that about 6000 tonnes
of ammonium compounds were used in food in 1970 (FASEB, 1974)
comprising ammonium bicarbonate, 317 tonnes; ammonium carbonate,
24 tonnes; ammonium hydroxide, 535 tonnes; monobasic ammonium
phosphate, 52 tonnes; dibasic ammonium phosphate, 434 tonnes; and
ammonium sulfate, 1468 tonnes. Information for ammonium chloride
was not available. The use of ammonium compounds in food nearly
doubled during the period 1960 - 70.
3.2. Sources Releasing Ammonia into the Air
Ammonia is released into the atmosphere by agricultural, waste-
disposal, and industrial activities. Ammonia global release has
been estimated at 113 - 244 x 106 tonnes ammonia-nitrogen/year
(Söderlund & Svensson, 1976). In the USA, industrial emissions
from ammonia and fertilizer production (anhydrous ammonia, aqueous
ammonia, ammonium nitrate, ammonium phosphates, urea), from
petroleum refineries, coke ovens, and sodium carbonate manufacture,
and loss of anhydrous ammonia during distribution, handling, and
application have been estimated to be approximately 328 x 103
tonnes, annually (NRC, 1979; US EPA, 1981). This figure does not
include volatilization of ammonia after soil applications of
nitrogen fertilizer, which may amount to 5 - 10% of the ammonia and
urea fertilizer applied. These losses were estimated to comprise
another 285 x 103 tonnes, annually (US EPA, 1981).
Combustion processes release ammonia as a by-product in amounts
that are dependent on the substance being burned and the conditions
of combustion. Assuming that 2% of the municipal wastes generated
in the USA are incinerated, about 0.8 x 103 tonnes of ammonia would
be emitted annually from this source. On the other hand, fossil
fuel combustion in the USA is estimated to release 783 x 103
tonnes/year (US EPA, 1981).
On the basis of the number of cattle in the USA and an average
excretion of 31 kg urea per animal per year, it has been estimated
that 3400 x 103 tonnes/year of ammonia are produced by cattle in
the USA (API, 1981). Similar calculations made in the Netherlands
on the basis of the manure production of cattle, pigs, and poultry
give a figure of 114 x 103 tonnes/year (Buysman, 1984). Estimates
of atmospheric emissions from the Netherlands and the USA are shown
in Table 4.
It must be emphasized that substantial uncertainties are
associated with these estimates, which are given for rough
comparison only. Ammonia from sources that cannot be quantified
includes that which volatilizes from livestock wastes or polluted
water, and emissions from the combustion of wood. These sources
must be considered in perspective with natural sources, especially
the microbial fixation of nitrogen and the mineralization of
nitrogenous organic matter. Emissions from these natural sources
far outweigh those from man-made sources, on a global scale;
however, man-made sources can result in locally elevated
atmospheric concentrations.
Table 4. Estimated atmospheric emissions of
ammonia in the USA and the Netherlands
--------------------------------------------------
Source Annual emission
(103 tonnes NH3)
----------------------
USAa the Netherlandsb
--------------------------------------------------
animal manure 3400 114 (1)
fertilizer volatilization 285 6.1 - 10.6
industrial activities 1111 7.6
other sources 0.8 0.5
--------------------------------------------------
a From: US EPA (1981).
b From: Buysman (1984).
The very high contribution to ammonia emission from animal
manure production in the Netherlands is remarkable. More than 80%
of the annually emitted ammonia results from the production of
manure on intensive livestock farms and its use as an agricultural
fertilizer. In all areas with intensive livestock farming, ammonia
emission from animal manure production contributes 90 - 99% to the
total NH3 emission. The number of poultry and pigs used in
livestock farms increased 3 - 5 times between 1950 and 1980, and it
can be expected that the ammonia emission from animal manure
production has increased similarly. In only a few areas does most
of the emitted ammonia result from industrial activities, such as
the production of coke and fertilizers, and the combustion of
fossil fuel.
In Denmark, Belgium, and some parts of the Federal Republic of
Germany and France, animal manure production contributes
significantly to atmospheric emissions of NH3 (Buysman et al.,
1985).
3.3. Sources Discharging Ammonia into Water
Ammonia is released into the aquatic environment from a variety
of man-made point source discharges and from natural and man-made
non-point sources.
3.3.1. Point sources of ammonia
Major man-made point sources discharging ammonia into surface
waters include sewage treatment plants, and plants producing
fertilizers, steel, petroleum, leather, inorganic chemicals,
non-ferrous metals, and ferroalloys, and meat processing plants.
Amounts of ammonia discharged annually by these industries in the
USA were estimated to be nearly 5.6 x 105 tonnes (API, 1981)
(Table 5). These estimates show that the industries examined
contribute < 5% of the total ammonia discharged into surface
waters while publicly owned sewage treatment plants (POTWs)
contribute > 95% of the total. It is important to note that the
POTW figure is based on an estimated actual discharge, while
several of the industrial figures are based on Best Practicable
Control Technology (BPT) guidelines and other industrial data.
An estimate of ammonia discharge by sewage treatment plants was
based on an average ammonia concentration of 15 mg/litre in
secondary treatment waste waters (Metcalf & Eddy, Inc., 1972) and a
total discharge of 104 billion litres per day. However, some
sewage treatment plants discharge waste waters containing much
higher ammonia concentrations. Using data from Mearns (1981), the
US EPA (1981) estimated that the mean effluent concentration of
ammonia from 5 major POTWs in southern California was 107 mg NH3-
N/litre (130 mg NH3/litre).
The iron and steel industries release ammonia, as a by-product
of the conversion of coal to coke, and during blast furnace
operations. The source estimate in Table 5 is based on proposed
BPT effluent control limits and steel production data.
The estimated ammonia contribution from the fertilizer industry
was based on 1978 production figures for ammonia, ammonium nitrate,
urea solutions, and urea solids, and on BPT guideline limits. This
contribution may be underestimated because relatively few of these
producers meet BPT limits and because production is increasing (US
EPA, 1981).
The estimated contribution of ammonia for all other industry
groups in Table 5, except the meat processing and leather
industries, was based on production figures and BPT guideline
limits. The effluents of the meat processing and leather
industries were reported to contain about 40 and 100 mg NH3/litre,
respectively (API, 1981).
3.3.2. Non-point sources of ammonia
Non-point sources of ammonia for surface waters are not as easy
to quantify as point sources. Non-point sources include releases
not discharged by a discrete conveyance. They are variable,
discontinuous, diffuse, and differ according to specific land use.
They may be the result of runoff from urban, agricultural,
silvicultural, or mined lands. Urban runoff may sometimes be
considered a point source, as it is frequently collected and
discharged from drainage systems. Several hydrological models are
available to predict runoff and estimate pollutant loading, but
there is still difficulty with this subject. Major non-point
sources of ammonia for surface waters include fertilizer runoff,
animal feedlots, animal wastes spread on the soil, urban runoff,
and precipitation.
Table 5. Estimates of aquatic emissions of ammoniaa from
point sources in the USA
----------------------------------------------------------
Point source Estimated contribution
(tonnes NH3-N/year)
----------------------------------------------------------
Sewage treatment plants (POTWs) 535 922.3b
Steel industry 12 951.0c
Fertilizer industry 5955.9c
Petroleum industry 2826.1c or 2767.2b
Meat processing industry 1099.3b
Leather industry 687.1b
Inorganic chemicals industry 99.8c
Non-ferrous metals manufacturing 0.9c
Ferroalloy manufacturing industry 0.3c
Total 559 542.7 tonnes/year
----------------------------------------------------------
a Adapted from: API (1981).
b Estimated contribution based on reported or estimated
actual discharge concentrations.
c Estimated contribution based on production data and BPT
guidelines, not actual discharges.
The ammonia content of urban runoff is variable, depending, in
part, on specific land use. In a study of urban runoff, the amount
of ammonia present varied with the seasons of the year. Ammonia
concentrations ranged from 0.18 mg N/litre in the autumn to 1.4 mg
N/litre in the early spring (Kluesener & Lee, 1974). In another
study, Struzewski (1971) reported that ammonia-nitrogen in urban
storm water ranged from 0.1 to 2.5 mg/litre.
The ammonia content of rural runoff originates from natural
and man-made sources, including wastes from wildlife and livestock,
decaying vegetation, fertilizer applications, material originally
present in the soil, and precipitation. Estimating total rural
runoff quantities and ammonia concentrations is extremely complex
and no overall estimates are available. Loehr (1974) reported
that the ammonium-nitrogen concentrations in the drainage from 4
forested watersheds ranged from 0.03 to 0.08 mg/litre. The
ammonium-nitrogen concentrations were 8 - 14% of the nitrate-
nitrogen concentrations.
Precipitation is also a significant non-point source of
ammonia. Concentrations may vary locally, reflecting local
atmospheric sources. The average concentration in rainfall at one
rural location on Long Island, New York (0.18 mg NH3-N/litre) was
less than half those at 2 other Long Island sites closer to the New
York urban area (0.43 and 0.459 mg NH3-N/litre) (Frizzola & Baier,
1975). Among collection sites throughout Wisconsin, ammonia levels
in urban rain samples differed little from those in rural samples
not taken near barnyards (range, 0 - 3 mg NH3-N/litre); however,
values for locations near barnyards were 4 - 5 times higher (range
0 - 3 mg NH3-N/litre) indicating contamination from locally-
generated atmospheric ammonia (Hoeft et al., 1972).
Similar tendencies have been observed in the Netherlands,
though the absolute data are much higher. In agricultural areas
with dense livestock farming, ammonia levels ranging from 2.9 mg
NH4+-N/litre near slurry manured croplands to 5.4 mg NH4+-N/litre
at a distance of 100 m from a poultry farm have been found. In
relatively unaffected areas along the northern coast, the average
value was 1.2 mg NH4+-N/litre, because of the relatively high
background levels of atmospheric ammonia. The average
concentration in wet deposition was 2.4 mg NH4+-N/litre (Schuurkes,
in press).
Watershed studies from pristine forests (Fisher et al., 1968),
rural wood and pasture lands (Taylor et al., 1971), and heavily
fertilized crop lands (Schuman & Burwell, 1974) have all shown that
rainfall nitrogen, including ammonia-nitrogen, accounts for a
substantial proportion (50 - 100%) of nitrogen in surface runoff.
3.3.3. Comparison between point and non-point sources
Little information is available for the accurate comparison of
point and non-point sources of ammonia for surface waters. In one
study, Wilkin & Flemal (1980) examined 3 Illinois river basins to
determine the relative sources of various pollution loadings (by
mass balance accounting) and the possible extent of water quality
improvement by controlling various types of sources. The three
river basins, showed differences in point sources, patterns of land
use (which influences non-point sources), and ammonia-nitrogen
concentrations. The east DuPage basin (42% industrial and urban)
contained 4.73 mg NH3-N/litre, the upper Sangamon basin (19% urban
and industrial), 2.51 NH3-N/litre, and the west DuPage basin (2%
urban and industrial), 0.22 mg NH3-N/litre. The fraction of
ammonia-nitrogen load from undefined (non-point) sources in the
heavily-industrialized east branch DuPage was only 0.54 compared
with 0.84 in the rural upper Sangamon. The authors concluded that
much of the pollution loading appeared to be related to undefined
sources and that further restrictions on point-source contributions
might not result in improved water quality.
These data indicate that, although point sources contribute a
large fraction of ammonia loading to surface waters, the
contribution of undefined non-point sources is also significant.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Ammonia in the environment is a part of the total biotic and
abiotic nitrogen balance as represented by the nitrogen cycle. The
processes of the nitrogen cycle consist of nitrogen fixation,
assimilation, ammonification, nitrification, and denitrification.
Nitrogen fixation and ammonification are microbially-mediated
processes that produce ammonium ions from nitrogen gas and organic
nitrogen. Assimilation is the uptake and incorporation of
inorganic nitrogen into organic molecules by microbes and plants.
Nitrification is the microbial oxidation of the ammonium ion to
nitrite (NO2-) and nitrate (NO3-). Denitrification converts
nitrate to nitrogen gas or nitrous oxide.
4.1. Uptake and Transformation in Atmosphere
Ammonia enters the atmosphere as a result of both natural and
artificial processes on the Earth's surface; there is no known
photochemical reaction by which ammonia could be produced in the
atmosphere (NRC, 1979). Atmospheric ammonia undergoes four main
types of reaction, namely aqueous-phase reactions, thermal
reactions, photochemical reactions, and heterogeneous reactions.
In the aqueous-phase reactions, oxidation of aqueous sulfur
dioxide in the presence of ammonia results in the formation of
atmospheric ammonium sulfate aerosols. This process is favoured by
high humidity, high ammonia concentrations, and low temperatures
(NRC, 1979).
Thermal reactions involving anhydrous ammonia and sulfur
dioxide may, via heteromolecular nucleation, also result in the
formation of ammonium sulfate aerosols. Thermal reactions of
ammonia with ozone result in the formation of ammonium nitrate, but
the importance of this mechanism in the production of atmospheric
ammonium nitrate aerosols is not known (NRC, 1979).
Photolytic degradation and reaction with photolytically
produced hydroxyl radicals (-OH) in the troposphere are major
pathways for the removal of atmospheric ammonia. While there is
limited information on the relative importance of these different
reactions, it has been suggested that one-half of the atmospheric
ammonia may be destroyed by the reaction with hydroxyl radicals,
with the balance being destroyed by reaction with soot particles or
by deposition (wet and dry) as particulate ammonium (NRC, 1979).
In addition to the formation of ammonium sulfate and nitrate,
various ammonium surface complexes may be formed by the
heterogeneous reaction of atmospheric ammonia with nitric oxide-
soot surfaces in the atmosphere (NRC, 1979). While these
heterogeneous reactions are significant in combustion reactions,
their importance in the atmosphere at much lower concentrations of
both ammonia and soot particles, is not known (NRC, 1979).
Comparison of the findings of Robinson & Robbins (1971) and
Söderlund & Svensson (1976) on global nitrogen balances for ammonia
reveals differences and it is difficult to evaluate which is the
more accurate.
4.2. Transport to the Earth's Surface
Most of the ammonia entering the atmosphere will be transported
back to the earth by both wet and dry deposition. Wet deposition
includes rainfall, snow, hail, fog, and dew, while dry deposition
mainly concerns gaseous ammonia. In the Netherlands, a comparison
has been made between the total emission and total deposition of
ammonia- and ammonium-nitrogen. Almost 95% of the emitted ammonia
(119 x 103 tonnes/year) is deposited back on the surface (van
Aalst, 1984). In this way, ammonia contributes 60 - 90% of the
nitrogen loading of water and soil, with nitrogen oxides making up
the other part.
4.2.1. Wet and dry deposition
A part of the ammonia in the atmosphere is removed by washout
and rainout. Ammonium sulfate aerosols are produced by aqueous-
phase reactions. Thus, wet deposition of ammonia can be estimated
by measuring ammonium concentrations in precipitation. Annual
average concentrations in wet deposition at locations in 21
European countries vary from 0.12 to 1.74 mg NH4+-N/litre (Fuhrer,
1985). In the Netherlands, the mean annual concentration for the
period 1978 - 82 was 2.4 mg NH4+-N/litre, corresponding to a wet
deposition of 12.2 kg/ha per year. The wet deposition in Norway
ranges from 1.3 kg/ha per year in the centre of the country to
8.6 kg/ha per year in the south (calculated from Overrein et al.,
1980). In the United Kingdom, it varies between 3.2 and 6.0 kg/ha
per year (calculated from Warren Spring Laboratory, 1982).
A comparison has been made of the amounts of dry and wet
deposition of ammonia and ammonium per area in the Netherlands.
The data are summarized in Table 6.
Wet deposition plays only a minor part (1/3) in the total
deposition of NH3 and NH4+. On average, 28.4 kg NH3 + NH4+-N is
deposited per ha per year. However, in rural areas with dense
livestock farming, values may reach up to 50 - 100 kg per year.
Table 6. Dry and wet deposition of NH3 + NH+4
per ha per year in the Netherlandsa
------------------------------------------------
mol/ha per year kg/ha per year
------------------------------------------------
dry deposition 1150 16.2
wet deposition 790 12.2
Total 1940 28.4
------------------------------------------------
a From: Van Aalst (1984).
4.2.2. Contribution to acid rain
Although ammonia is a base and thus increases the pH of rain
water, it contributes to the acidifying action of deposition. In
particular, the conversion of ammonium to nitrate appears to be
important in the acidification of soil and water in carbonate-poor
environments (Roelofs, in press; Schuurkes, in press). This
potentially-acidifying action has been implicated in the acid rain
problem in the Netherlands. On average, NH3 contributes about 32%
of the total deposition of potentially-acidifying substances (Table
7).
Table 7. Average deposition of acid
and acidifying substances (acid eq.,/ha
per year) in the Netherlandsa
---------------------------------------
SO2 NOx NH3
---------------------------------------
Total deposition 2750 1310 1940
(46%) (22%) (32%)
---------------------------------------
a From: The Netherlands Ministry of
Housing, Physical Planning, and
Environment (1984).
4.3. Transformation in Surface Water
Nitrification is important in preventing the persistence or
accumulation of high ammonia levels in waters receiving sewage
effluent or runoff. The overall reaction is:
NH4+ + SO2 ---> 2H+ + NO3- + H2O
It occurs in two steps, involving primarily two bacterial genera,
and forming nitrite as an intermediate.
Nitrosomonas
NH4+ ------------> NO2-
Nitrobacter
NO2- -----------> NO3-
The process depends on many factors, including the amount of
dissolved oxygen, temperature, pH, the microbial population, and
the nitrogen forms present. Nitrification is an oxygen-consuming
process, requiring 2 moles of O2 per mole of NH4+ consumed and
yielding hydrogen ion (NRC, 1979). Nitrification may thus lead to
a depletion of dissolved oxygen and acidification, which may, in
turn, inhibit microbiological nitrification (Knowles et al., 1965;
Schuurkes et al., 1985).
Anthonisen et al. (1976) reported that, at high levels of
total ammonia and a high pH, the resulting concentrations of free
ammonia were toxic to both nitrifying forms, but especially to
nitrobacters, occasionally leading to the accumulation of nitrite.
Other authors (Kholdebarin & Oertli, 1977) have reported that high
pH alone, in the absence of ammonia, can inhibit nitrite oxidation.
At high nitrite levels, formation of free nitrous acid caused
inhibition of nitrosomonad bacteria, resulting in the persistence
of both ammonia and nitrite. However, inhibitory conditions and
persistence of reduced forms are usually transient (Anthonisen et
al., 1976), and reports of high nitrite levels are rare (Ecological
Analysts, Inc., 1981).
Other mechanisms also act to remove ammonia from natural
waters. Ammonia is assimilated by aquatic algae and macrophytes
for use as a nitrogen source. Ammonia in water may be transferred
to sediments by adsorption on particulates, or to the atmosphere by
volatilization at the air-water interface. Both processes have
been described as having measurable effects on ammonia levels in
water; however, the relative significance of each will vary
according to specific environmental conditions (API, 1981).
4.4. Uptake and Transformation in Soils
Ammonia levels in soils are a function of the balance between
natural and man-made activities. As a result of aerobic
degradation processes, ammonia is the first inorganic nitrogenous
compound to be released from organic matter together with amines,
which are rapidly converted to ammonia (Powers et al., 1977).
Other important sources of ammonia in soil are fertilizers
(primarily anhydrous ammonia, ammonium nitrate, and urea, which is
rapidly converted to ammonia), wet and dry deposition, and animal
wastes.
The ammonium cation is relatively immobile in soils, because
it is adsorbed on the negatively-charged clay colloids present
in all soils (Wallingford, 1977). Ammonia may be lost from
soils by volatilization, especially after the application of
ammonia fertilizers (Walsh, 1977), sewage, or manures, and by
uptake of ammonium ions into root systems. However, the most
likely fate of ammonium ions in soils is conversion to nitrate by
nitrification. Nitrate is, in turn, lost from soils by: leaching,
which occurs readily, since it is repulsed by the clay particles;
denitrification, which occurs rapidly within a few days or weeks in
warm, moist soils; and by uptake by the plant root system.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Atmospheric levels
Ammonia is present in the atmosphere in very low
concentrations, which vary with underlying land use. In most
situations, urban atmospheres contain more than non-urban, but
certain rural areas, for example, those characterized by intensive
animal husbandry or use of organic manure, have atmospheric ammonia
levels that exceed urban values. Atmospheric ammonia levels also
show a seasonal variation, the highest levels being attained during
the winter and the lowest during the summer months. In urban
areas, the ammonia levels may increase substantially during
pollution episodes. However, they do not show any circadian
patterns.
Urban and non-urban atmospheric levels of ammonia at some
locations around the world are shown in Tables 8 and 9. It can
be seen that ammonia levels of 4 - 5 µg/m3 and 20 µg/m3 are
typical of non-urban and urban sites, respectively. Levels of
particulate NH4+ ions in the atmosphere above the main oceans
(Atlantic, Pacific, Indian, and Antartic) have been studied; in the
southern hemisphere, remote from terrestrial sources, the NH4+
concentrations were found to be between 10 and 115 ng/m3. The
authors concluded that the oceans are a source of ammonia for the
atmosphere (Servant & Delaporte, 1983).
Atmospheric levels of particulate ammonium at some non-urban
and urban locations around the world are shown in Table 10. It can
be seen that concentrations of 1 µg/m3 and 4 - 5 µg/m3 are typical
for non-urban and urban sites, respectively.
5.1.2. Levels in water
The concentration of ammonia in surface waters varies
regionally and seasonally. Wolaver (1972) studied US Geological
Survey data for total ammonia and reported average concentrations
of < 0.18 mg/litre in most surface waters, and around 0.5 mg/litre
in waters near large metropolitan areas. Analysis of data from the
Water Quality Control Information (STORET) System for the years
1972 - 77 (US EPA, 1979a) showed that, although total ammonia-
nitrogen concentrations in surface waters in the USA tended to be
slightly lower during summer months than during winter months, the
percentage of areas in which non-ionized ammonia concentrations
occasionally exceeded 0.02 mg/litre increased from 11% during
winter to 23% during summer; these percentages were higher when
waters had elevated pH values.
Table 8. Urban and industrial atmospheric levels of ammonia in a
few global locationsa
--------------------------------------------------------------------
Location Year Concentration Reference
(µg/m3)
--------------------------------------------------------------------
Germany, Federal
Republic of
Frankfurt-am-Main pre-1963 8 - 20 Georgii (1963)
Italy
Cagliari - 37 - 280 Spinazzola et
(highest conc. al. (1966)
in the vicinity
of port)
Japan
Tokyo - up to 210 (down- TMRI (1971)
wind from two major
pharmaceutical
plants)
Tokyo 1969 4.8 - 25.8 Okita &
Kanamori (1971)
Tsuruga - up to 6.8 FEPCC (1972)
Netherlands
Bilthoven 1983 5 Van Aalst
(1984)
Delft 1979-81 4.4 Van Aalst
(1984)
USA
Seattle, Washington 1975 0.8 - 77.0 Farber &
Rossano (1975)
St. Louis, Missouri 1972-73 up to 17.5 Breeding et al.
(1976)
Five urban sites - 3 - 60 Hidy (1974)
in California (average 20.0)
Chino-Corona area, 1975 up to 315 Pitts &
California (vicinity of Grosjean (1976)
dairy farm)
USSR
Environment of 1967 190 Saifutdinov
metallurgical plant (1966)
West Berlin - up to 97 Hantzsch &
(average 17.6) Lahmann (1970)
--------------------------------------------------------------------
a Adapted from: NRC (1979). Industrial activities include intensive
farming activities.
Table 9. Non-urban atmospheric levels of ammonia in a few global
locationsa
--------------------------------------------------------------------------
Location Year Concentration Reference
(µg/m3)
--------------------------------------------------------------------------
Harwell, England 1969 up to 5.1 Healy et al. (1970)
(typical level
0.85 - 1.7)
Maritime stations pre-1963 2 - 5 Georgii (1963)
(North Sea, Italian
coast, and Hawaii)
Rural and mountain pre-1963 5 - 8 Georgii (1963)
locations in Switzerland
and the Federal Republic
of Germany
Non-urban locations - 4 - 5 Robinson & Robbins
(1968); McKay (1969)
Rural sites in USA 1971 1.4 - 4.2 Breeding et al. (1973)
Boulder, Colorado, USA 1975 2.0 - 3.1 Axelrod & Greenberg
(1976)
American tropic 1967-68 3.5 - 21.7 Lodge et al. (1974)
(average 10.5)
Non-urban sites in 1972 4.6 - 9.7 Hidy (1974)
California, USA
--------------------------------------------------------------------------
a Adapted from: NRC (1979).
In the Netherlands, enhanced ammonium levels are also present
in waters that are not influenced by surface run-off. In
particular, in hydrologically-isolated acidified small lakes,
concentrations may reach up to 3 mg NH4+-N/litre. In rural areas
with high atmospheric ammonia levels, the loading of these small
lakes with airborne ammonia substances appears to be responsible.
The highest measured value near intensive pig and poultry farms was
12 mg NH4+-N/litre (Leuven & Schuurkes, 1984).
There are few data on the concentrations of ammonia in
drinking-water. This is possibly because of the conversion of most
of the available ammonia to N-chloramines (mono-, di-, and tri-
chloramines) during the chlorination of drinking-water (Morris,
1978), which reduces ammonia concentrations to levels below
analytical detectability. The presence of these N-chloramines may
contribute to the taste, odour, and also the potential health
problems of drinking-water.
Table 10. Urban and non-urban atmospheric levels of particulate
ammonium in some global locationsa
-------------------------------------------------------------------
Location Year Concentration Reference
(µg/m3)
-------------------------------------------------------------------
Non-urban:
England
Harwell - 3 - 4 Healy (1974)
(troposphere) 1971-73 1.3 Reiter et al. (1976)
Germany, Federal
Republic of
Bavaria - 1.0 Georgii & Muller
(lower troposphere) (1974)
USA
28 non-urban sites 1968 0 - 1.2 US EPA (1972)
Point Arguello, - 0.36 Hidy (1974)
California
Goldstone, - 0.76 Hidy (1974)
California
Urban:
Belgium
Ghent 1972 1.3 - 33.0 Demuynck et al.
(severe (1976)
pollution
episode)
Japan
Nagoya 1973-74 2.7 - 4.2 Kadowaki (1976)
Netherlands
Delft 1979-81 4.6 Van Aalst (1984)
Terschelling 1982 2.7 Van Aalst (1984
Houtakker 1983 19 Van Aalst (1984)
Sweden
Rao - 2.2 - 7.2 Brosset et al. (1975)
(aerosol
originating
from England)
United Kingdom
Tees River Valley 1967 up to 33.0 Eggelton (1969)
(severe
pollution
episode)
-------------------------------------------------------------------
Table 10. (contd.)
-------------------------------------------------------------------
Location Year Concentration Reference
(µg/m3)
-------------------------------------------------------------------
USA
Urban areas 1968 0 - 15.1 US EPA (1972)
Five cities 1970-72 0 - 21 Lee & Goranson (1976)
Tuscon, Arizona 1973-74 0 - 6.5 Keesee et al. (1975)
Los Angeles, 1969-70 2.8 - 3.4 Gordon & Bryan (1973)
California
15 urban sites in - average 5.3 Hidy (1974)
California
Riverside, 1975 up to 30.1 Grosjean et al.
California (average 7.6) (1976)
-------------------------------------------------------------------
a Adapted from: NRC (1979).
Ground water is frequently used as drinking-water, without
prior chlorination. Ammonia levels in ground water are usually low
because the adsorption of the ammonium ion on clay minerals, or its
bacterial oxidation to nitrate, limit its mobility in soil (Feth,
1966; Liebhardt et al., 1979). However, nitrogen fertilizers,
livestock wastes, or septic tanks may contribute significant
amounts of ammonia to shallow ground waters, especially those
underlying poorly-drained soils (Gilliam et al., 1974; Rajagopal,
1978). In domestic tap water from Michigan wells averaging 20 m in
depth, mean levels of ammonia-nitrogen were between 0.04 and 0.18
mg/litre; the highest reported single value was 0.57 mg/litre from
a well 12.5 m in depth (Rajagopal, 1978).
In wells drilled for research purposes and not supplying
drinking-water, levels of ammonia-nitrogen in shallow (3 m) wells
beneath wood and crop land usually averaged less than 2 mg/litre
(Gilliam et al., 1974). Levels in shallow (3 - 6 m) ground water
beneath plots spread with poultry manure varied typically between 1
and 15 mg NH3-N/litre (Liebhardt et al., 1979); those in ground
water beneath 29 feedlots averaged 4.5 mg NH3-N/litre and ranged up
to 38 mg/litre (Stewart et al., 1967). Levels in hot springs and
other ground waters have been reported to reach > 1000 mg NH3-
N/litre (Feth, 1966).
The ammonia levels present in the runoff of receiving surface
waters have been measured in various studies. Kluesner & Lee
(1974) found that levels ranged from approximately 0.23 mg
ammonia/litre in the autumn to 1.8 mg ammonia/litre in the early
spring in the urban runoff of Madison, Wisconsin. Struzewski
(1971) reported that ammonia levels in urban storm water ranged
from 0.1 to 3.2 mg/litre.
Only limited data are available on nitrogen pools in the ocean.
Söderlund & Svensson (1976) used values of 5 µg NH3-N/litre for
deep areas and 50 µg NH3-N/litre for near-shore areas and estimated
an ammonia inventory of approximately 9 x 103 µg/litre in coastal
upwelling systems.
Interstitial water in sediments rich in organic matter contain
higher concentrations of ammonia. Sholkovitz (1973) reported
values of 1.4 - 23.8 µg ammonia/litre in the interstitial waters
of the Santa Barbara Basin. The interstitial water of the Long
Island Sound, 2 km off shore, contained concentrations ranging
from 11.2 to 42 µg ammonia/litre (Gold-haber & Kaplan, 1974).
5.1.3. Levels in soil
The quantity of ammonia bound to clay in soil has not been
estimated. The ammonia present in soil is in dynamic equilibrium
with nitrate and other substrates of the nitrogen cycle and is
difficult to measure as its concentration is in constant flux (NRC,
1979).
5.1.4. Food
There is very little ammonia in unprocessed food and in
drinking-water derived from deep ground-water or chlorinated
sources. Various salts of ammonia are added to foods (Annex I).
5.1.5. Other products
Ammonium chloride is a common ingredient in expectorant
cough mixtures and is a component of tobacco smoke (about
40 µg/cigarette) (Sloan & Morie, 1974).
5.2. General Population Exposure
Exposure via inhalation and ingestion must be compared to the
endogenous production of ammonia in the intestinal tract, which is
of the order of several grams per day (section 7.1.2). The
relative importance of the different sources is indicated in Table
11.
5.2.1. Inhalation
Assuming ammonia and ammonium concentrations in non-urban and
urban air are 2 and 6 µg/m3 and 24 and 25 µg/m3, respectively, and
that the amount of air breathed per day by an individual is 20 m3,
the intake of total ammonia through inhalation can be calculated to
be 0.1 - 0.5 mg/day; the amounts exhaled are considerably higher.
The average amount of ammonia inhaled from the smoking of one
cigarette is approximately 42 µg (Sloan & Morie, 1974). Assuming
an individual smokes 20 cigarettes per day, the inhalation of
ammonia through cigarette smoking would be 0.8 mg/day.
5.2.2. Ingestion from water and food
Most drinking-water in the USA is chlorinated, which
effectively eliminates ammonia. However, assuming the direct
consumption of 2 litres per day of untreated surface water, at an
average total ammonia concentration of 0.18 mg/litre (Wolaver,
1972), the average human uptake from this source would be 0.36 mg
per day.
Table 11. Intake of ammonia from
different sources
------------------------------------
Source mg/day
------------------------------------
Endogenous 4000
Exogenous
Ingestion (food and drink) ~18
Inhalation < 1
Cigarette smoking (20/day) < 1
------------------------------------
Although ammonia is a negligible natural constitutent of food,
it is formed in the intestine by deamination of the amino groups of
food proteins. In addition, ammonium compounds are added in small
amounts (< 0.01 - 20 g/kg) to various foods as stabilizers,
leavening agents, flavourings, and for other purposes (FASEB,
1974). Information concerning the usual concentrations of ammonium
salt additives in foods and the estimated total quantities of these
compounds used for this purpose in the USA in 1970 has been used to
estimate the average daily intake of 6 ammonium salt additives
(FASEB, 1974). The estimates for ammonium bicarbonate, carbonate,
hydroxide, monobasic phosphate, dibasic phosphate, and sulfate were
42, 0.3, 7, < 0.1, 6, and 20 mg, respectively. No estimate was
available for ammonium chloride. On this basis, the average daily
ammonia intake from these compounds has been calculated to be
18 mg.
5.2.3. Dermal exposure
Very few data are available concerning levels of dermal
exposure to ammonia or ammonium compounds. Dermal exposure of
human beings mainly occurs through the use of household cleaning
products, accidental spillage, or under occupational conditions.
5.3. Occupational Exposure
Exposure to ammonia or ammonium compounds can occur in certain
occupations involving their production, transportation, and use in
agricultural and farm settings, during fertilizer application, or
as a result of animal waste decomposition.
It is estimated that about half a million workers in the USA,
in a wide variety of occupations, have potential exposure to
ammonia (NIOSH, 1974).
Ammonia is generated as a by-product in a wide variety of
industrial activities, and workplace atmospheric concentrations
are given in Table 12. Municipal waste incineration and gas-fired
industrial incinerators generate concentrations of 20 and 0.4
mg/m3, respectively (NRC, 1979) and shipboard and quayside levels
for natural gas tankers may be about 30 mg/m3 (Avot et al., 1977).
Levels in intensive livestock-rearing buildings are frequently
reported to be up to 30 mg/m3 (Poliak, 1981) or more (Anderson et
al., 1964b; Taiganides & White, 1969; Marschang & Petre, 1971).
Ammonia levels in dairy farms and cattle-fattening facilities in
Romania have been reported to range from 0.7 mg/m3 to 140 mg/m3
(Marchang & Crainiceanu, 1971; Marschang & Petre, 1971).
Maximum daily intake from work-place concentrations such as
these would normally be less than 300 mg/day and this may be
compared with endogenous production (Table 11).
Occupational exposure limits for some countries in the world
are shown in Table 13.
5.4. Exposure of Farm Animals
Farm animals are exposed to ammonia through feed containing
urea or various ammonium salts and to atmospheric ammonia due to
bacterial decomposition and volatilization of ammonia from animal
wastes.
5.4.1. Oral exposure
(a) Non-protein nitrogen additives
Urea and various ammonium salts have been used for several
years as non-protein nitrogen sources in ruminant nutrition. It is
used much more widely for this purpose than the ammonium compounds.
Urea is hydrolysed to ammonia and carbon dioxide by the ruminal
bacteria and, therefore, represents a source of ammonia exposure.
The ammonia released is used by the ruminal microorganisms to
synthesize microbial protein, which is then digested in the small
intestine of the ruminant and used as a source of dietary amino
acids.
(b) Refeeding of livestock wastes
Results of studies on the refeeding of livestock wastes
(Bhattacharya & Taylor, 1975; Arndt et al., 1979; Smith & Wheeler,
1979) have indicated that manure could be of nutritive value,
salvaging some nutrients ordinarily lost (Yeck et al., 1975). The
non-protein nitrogen (e.g., urea, uric acid) present in livestock
wastes is available to ruminants because of microbial conversion in
the rumen. Wastes are of limited value for monogastrics such as
swine.
Table 12. Ammonia levels in some industrial processesa
--------------------------------------------------------
Operation Level (mg/m3)
--------------------------------------------------------
Machinery manufacturing (cleaning) 10.5
Diazo-reproducing machine 5.6
Mildew-proofing 87.5
Electroplating 38.5
Galvanizing, ammonium chloride flux 7 - 61.6
Blueprint machine 7 - 31.5
Printing machine 0.7 - 31.5
Etching 25.2
Refrigeration equipment 6.3 - 25.9
Cementing insoles 5.6 - 19.6
Chemical mixing 42 - 308
Fabric impregnating ND
--------------------------------------------------------
a From: NIOSH (1974).
ND = Not detectable.
5.4.2. Inhalation exposure
(a) Ruminants
Marschang & Crainiceanu (1971) measured the ammonia
concentrations in air (sampled at nose level of animals) in calf
stables at 4 dairy farms in Romania. The ammonia levels ranged
from 0.7 to 140 mg/m3 (1 - 200 ppm). Most of the observed values
greatly exceeded the permissible upper limit of 18.2 mg/m3 (26
ppm). In a second study, Marschang & Petre (1971) measured the
ammonia concentrations in the air of 3 cattle-fattening facilities
in Romania in which the animals were being fed in total
confinement; the capacities of the 3 operations were 3000, 3000,
and 4900 animals. The ammonia concentration ranged from 2 to
1400 mg/m3 (3 to 200 ppm). In general, the ammonia content was
below the admissible upper limit during the summer months but
exceeded it during the winter months, when extremely high
concentrations were observed. These high concentrations were
primarily due to the blocking of the ventilation system, in order
to maintain necessary stall temperatures. The highest value
(1400 mg/m3) was measured when the cleaning mechanism of the
manure canals malfunctioned.
Table 13. Occupational exposure limits (mg/m3)a
-------------------------------------------------
Country Ammonia Ammonium
Ammonia chloride sulfamate
A B A B A B
-------------------------------------------------
Australia 18 10 10
Belgium 18 10 10
Czechoslovakia 40 80
Finland 18
German Democratic 20 10
Republic
Germany, Federal 35 10 15
Republic of
Hungary 20
Italy 20 10
Japan 18
Netherlands 18 10 10
Poland 20
Romania 20 30 5 10 10 15
Sweden 18 36
Switzerland 18 6 10
USA (NIOSH/OSHA) 35 15
(ACGIH) 18 27 10 20 10 20
USSR 20 10
Yugoslavia 35 15
Council of Europe 18 15
-------------------------------------------------
a From: ILO (1970).
Column A represents average values.
Column B is higher quoted limits, which may
variously be ceiling values, short-term exposure
limits, etc.
Note: Occupational exposure levels and limits are derived in
different ways, possibly using different data, and expressed
and applied in accordance with national practices. These
aspects should be taken into account when making comparisons.
(b) Swine
The increased use of confined housing for swine has caused
concern about the purity of the air within the buildings and its
effects on swine growth. Bacterial decomposition of excreta
collected and stored beneath slotted floors in enclosed buildings
produces a number of gases, including ammonia, carbon dioxide,
hydrogen sulfide, and methane (Curtis, 1972). Miner & Hazen (1969)
reported a range of ammonia concentrations of 4.2 - 24.5 mg/m3
(6 - 35 ppm) determined 30 cm above the floor level in a swine-
rearing facility. Levels in solid-floor confinement units were
normally found to be < 35 mg/m3 (< 50 ppm), but they could be
higher during cold months, when ventilation was at a minimum,
particularly when the floor was heated (Taiganides & White, 1969).
The normal ammonia concentration in the air above slotted floors
was reported to be ~7 mg/m3 (~10 ppm), but this was increased by a
factor of 5 - 10 by stirring the stored manure.
(c) Poultry
Poultry are usually exposed to ammonia, together with hydrogen
sulfide, carbon dioxide, and methane, in the air of poultry houses.
These compounds result from bacterial action on poultry wastes
(Ringer, 1971). In cold climates, proper ventilation rates cannot
be maintained in many poultry houses, and gas production in the
manure may build up to toxic levels. Ammonia has been found at
concentrations exceeding 35 mg/m3 (50 ppm) in modern poultry
houses, and at up to 140 mg/m3 (200 ppm) in poorly-ventilated
poultry houses (Anderson et al., 1964b; Valentine, 1964). The
toxic effects in poultry can be prevented through proper management
practices (Lillie, 1970).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Microorganisms
Many microorganisms are able to use ammonia as a nitrogen
source for cellular nutrition. Nitrifying organisms derive energy
from the oxidation of ammonia to nitrate. High levels of ammonia
and high pH, which may occur, for example, in waste waters or
fertilized fields, may inhibit nitrification and cause persistance
or accumulation of ammonia and/or nitrite. Improper maintenance of
conditions in waste treatment processes may result in ammonia
overloading and inhibition of the nitrification process, with
consequent ammonia and/or nitrite pollution of receiving surface
waters. Other soil microorganisms may also be inhibited; fungi
reportedly are more sensitive than bacteria. However, these
inhibitory effects are temporary. Aqueous and gaseous ammonia have
been used to control microbial growth in stored fruits, hay, and
grains. Ammonia treatment has proved more effective against fungal
than against bacterial spoilage of food.
The bacterial species Escherichia coli and Bacillus subtilis
were found to be sensitive to ammonium chloride (NH4Cl) (Deal et
al. 1975); exposure to 1100 mg NH3/litre killed 90% of an E. coli
population in 78 min. B. subtilis, an aerobic, spore-forming
bacterium, was destroyed in less than 2 h in a solution of 620 mg
NH3/litre. Anthonisen et al. (1976) and Neufeld et al. (1980)
studied NH3 inhibition of the bacterium Nitrosomonas (which
converts ammonium to nitrite) and the bacterium Nitrobacter (which
converts nitrite to nitrate). The nitrification process was
inhibited by NH3 at a concentration of 10 mg/litre (Neufeld et al.,
1980). Concentrations that inhibited Nitrosomonas (10 - 150
mg/litre) were greater than those that inhibited Nitrobacter
(0.1 - 1.0 mg/litre), and NH3, not NH4+, was reported to be the
inhibiting chemical species (Anthonisen et al. 1976).
Acclimatization of the nitrifying bacteria to NH3, temperature,
and the number of active nitrifying organisms are factors that may
affect the inhibitory concentrations of NH3 in a nitrification
system.
Langowska & Moskal (1974) investigated the inhibitory effects
of NH3 on bacteria during 24-h exposure periods. Ammonifying and
denitrifying bacteria were most resistant to NH3; proteolytic and
nitrifying bacteria were the most sensitive. Concentrations of up
to 170 mg NH3/litre did not adversely affect denitrifying and
ammonifying bacteria; a concentration of 220 mg/litre caused a
reduction in metabolic processes. Proteolytic bacteria were
unaffected at concentrations of 0.8 mg NH3/litre, but were affected
at 13 - 25 mg/litre.
Jones & Hood (1980) conducted studies on 2 species of
Nitrosomonas isolated from 2 wetland environments, one estuarine
and the other fresh water. At 30 °C and pH 8.0, the estuarine
isolate showed peak ammonium oxidation activity at 18 mg NH3/litre;
activity gradually declined to 30% of the peak at 80 mg NH3/litre.
However, the fresh-water isolate was not inhibited by ammonia
concentrations of up to 80 mg NH3/litre.
Application of anhydrous ammonia to soil may strongly affect
soil microorganisms; however, the effect has been attributed more
to alterations in pH than to ammonia toxicity per se. Henis & Chet
(1967) found that ammonia reduced sclerotial germinability of the
fungus Sclerotium rolfsii only when the soil pH rose to 9.8 or
higher. According to Müller & Gruhn (1969), fungi were more
sensitive than bacteria to ammonia application, the fungi
disappearing at pH values above 8. At pH 8.38, bacterial numbers
initially decreased but then increased above control levels, 7 days
after application, with an increased number of protein-decomposing
and nitrifying forms.
Ammonia has been used to control microbial growth in food and
cattle feed. The growth of mould (Penicillium digitatum) on fresh
fruit was inhibited by 70 - 300 mg ammonia/m3 in the air, either
applied directly or in the form of ammonia-releasing compounds
(Eckert et al., 1963; Eckert, 1967). Anhydrous ammonia has also
been used to prevent spoilage in high-moisture hay (Lechtenberg et
al., 1977; Wilkinson et al., 1978; Butterworth, 1979) and cattle
feed derived from corn leaves and stalks (Johanning et al., 1978).
Ammonium hydroxide and ammonium isobutyrate were shown to control
the growth of mould in stored corn (Bothast et al., 1973, 1975a,b;
Rempel, 1975; Peplinski et al., 1978). Periodic addition of
ammonia to stored corn, to a total of 30.4 g/kg, prevented the
growth of mould, but was not fully effective as a bactericide
(Peplinski et al., 1978).
6.2. Plants
Ammonia may affect vegetation directly by acting on plant
structure and function, and, indirectly, via its influence on soil
condition after being deposited. The effects of NH3 are shown in
Fig. 1.
6.2.1. Terrestrial plants
It is well recognized that nitrogen plays an important role in
both plant metabolism and growth. The principal nitrogen sources
are ammonium and nitrate ions. Ammonia is used primarily by the
root system, but uptake of non-ionized ammonia or ammonium salts by
the leaves also occurs. Ammonia is a nitrogen source for the
synthesis of proteins. This use of ammonia in the synthesis of
organic molecules can be regarded as a process for storing a
valuable nutrient, but is also an important detoxifying mechanism.
As ammonia is toxic, its uptake in large quantities can place a
severe strain on the carbohydrate metabolism. Since carbohydrates
are used in the synthesis of amino acids and amides, carbohydrate
availability is an important factor in ammonia metabolism.
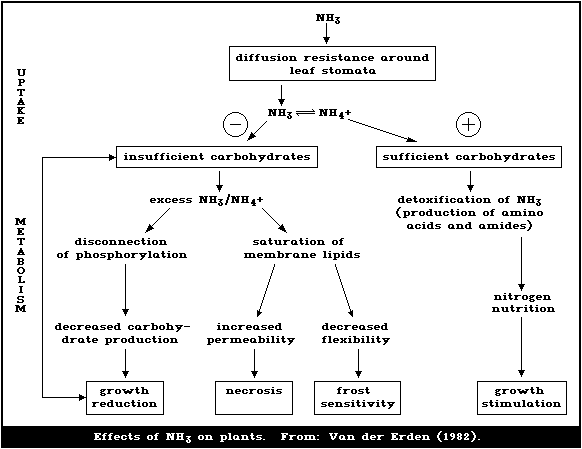 Manifestations of ammonia toxicity can be traced to several
metabolic disturbances. Both photosynthetic and respiratory
pathways are affected adversely by ammonia. There is a direct
relationship between ammonia concentration and respiratory
metabolism, including oxygen uptake, glycolysis, and the
tricarboxylic acid cycle (Matsumoto et al., 1976). Ammonium ions
may restrict photosynthesis through the uncoupling of noncyclic
photophosphorylation in isolated chloroplasts (Gibbs & Calo, 1959;
Losada & Arnon, 1963), though the mechanism of action is not known
(Losada et al., 1973). It is well documented that uncoupling leads
to an increase in reducing power in the cell. There is evidence
that 1 - 3 mmol ammonium inhibits respiration in plants (Wedding &
Vines, 1959; Vines & Wedding, 1960). It is suspected that similar
or identical inhibition occurs in ammonium toxicity in animals.
The site of toxicity is thought to be in the electron-transport
system; specifically, at the oxidation of NADH to NAD. This may
have a mechanism similar to the interference by ammonium ion with
electron transfer in the photosynthetic reaction.
High atmospheric levels may have direct toxic effects. Plant
growth is inhibited by a decrease in the carbohydrate production as
a consequence of inhibition of photosynthesis (Losada & Arnon,
1963). Ammonium increases the permeability of the cell membrane
causing plasmolysis and necrosis. Furthermore, the flexibility of
cells may be decreased, which results in an increased sensitivity
to frost. Excess ammonia can be detoxified as long as amino acids
are converted in the presence of carbohydrates (Fig. 1) (Van der
Eerden, 1982).
Most data on the toxic exposure of plants to ammonia have been
derived from controlled fumigation studies. Such experiments have
provided the following relative susceptibilities to ammonia:
leaves > stems, fungi, and bacteria < seeds, sclerotia, and
animals (McCallan & Setterstrom, 1940).
An exposure of 175 mg/m3 (250 ppm) for 4 min produced 50%
foliar necrosis in tomatoes, whereas the same foliar injury was
only produced in buckwheat and tobacco with exposure to 700 mg/m3
(1000 ppm) for 5 and 8 min, respectively (Thornton & Setterstrom,
1940). Zimmerman (1949) reported that fumigation with ammonia at
28 mg/m3 (40 ppm) for 60 min injured tomato, sunflower, and coleus
completely, and a concentration of 2.1 mg/m3 (3 ppm) severely
injured mustard (Benedict & Breen, 1955). Other plant parts are
more resistant to ammonia injury than the foliage. Thornton &
Setterstrom (1940) found 50% injury in tomato stems after exposure
to 700 mg/m3 (1000 ppm) for 60 min. Barton (1940) observed that
moist spring rye seeds were killed in a 4-h exposure to 700 mg
ammonia/m3 (100 ppm), whereas moist radish seeds were still viable
after 16 h. Exposure to a concentration of 175 mg/m3 (250 ppm) for
16 h reduced germination of rye seeds by half, but had no effect on
radish seeds.
Foliar injury is the most common toxic effect of anhydrous
ammonia on vegetation (Linzon, 1971). In broad-leaved woody plants
exposed to high concentrations of ammonia, the injury begins as
large, dark green water-soaked areas on leaves, which darken into
black or brownish-gray bifacial necrotic lesions, widely
distributed over the leaf surface. In lightly injured leaves,
these symptoms occur mainly on the upper surface. Conifer foliage
injured by ammonia exposure darkens to shades of gray-brown,
purple, or black. The entire needle is usually affected.
Abscission of severely damaged leaves is often seen in broad-leaved
and conifer species. On trees or shrubs with crowded leaves,
injury may be confined to particular sections of the leaf.
Occasionally, the foliage of woody species turns a variety of
colours, mimicking autumn colours. Symptoms of injury in
herbaceous plants are more variable, ranging from irregular,
bleached, bifacial, necrotic lesions to dark upper-surface
discolouration (Treshow, 1970). Grasses and cereal grains develop
tan to reddish-brown marginal or interveinal necrosis, and broad-
leaved weeds show red-brown to dark-brown upper surface
discolouration on terminal or marginal portions of the leaf
(Benedict & Breen, 1955). Coleus leaves lose their brilliant
colour after exposure to ammonia and appear green (Zimmerman,
1949). Injury to flowers by ammonia is rarely seen in the field,
but the development of small necrotic spots on azalea flowers has
been reported following ammonia exposure (Treshow, 1970).
Although parts of plants, other than the foliage, are less
susceptible to the injurious effects of ammonia (McCallan &
Setterstrom, 1940), injury to apples, peaches, and other fruits and
vegetables, accidentally exposed during cold storage, has been
reported (Ramsey, 1953). Apparently, ammonia entered the fruit and
turned red-pigmented tissues black, or brown, and yellow tissues,
dark-brown. Immediately on exposure to ammonia, the outer skins of
red onions became greenish-black, and the skin of yellow and brown
onions became dark brown. These colour changes were usually
permanent. Fumigation of fruit with ammonia also caused overall
darkening of the skin. Peaches and apples showed such symptoms at
140 mg/m3 (200 ppm) and 210 mg/m3 (300 ppm), respectively (Brennan
et al., 1962). These symptoms of injury were similar to those seen
on fruits injured by the accidental release of ammonia.
In studies in the Netherlands, several vegetables and trees
showed leaf damage by necrosis, growth reduction, and increased
frost senstivity at concentrations of 75 µg/m3 (annual average),
600 µg/m3 (during 24 h), and 10 000 µg/m3 (during 1 h) (Van der
Eerden, 1982). The sensitivity of pine trees to exposure to
ammonia differed between species (den Boer & Bastiaens, 1984). A
survey is given in Table 14. Airborne ammonium sulfate deposits
damaged the needles of pine trees. Owing to the enhanced uptake of
ammonium, the excretion of potassium, magnesium, and calcium
increases, often resulting in potassium and/or magnesium
deficiency, which may lead to premature shedding of needles
(Roelofs et al., 1985).
Effects of ammonia on the root system have generally been
studied by exposing the roots to different aqueous ammonium
solutions.
Table 14. Relative sensitivity of some pine trees to NH3a
-----------------------------------------------------------------
High sensitivity Moderate sensitivity Low sensitivity
-----------------------------------------------------------------
Picea abies Picea omorika Pinus sylvestris
Picea sitchensis Pinus nigra var. Pinus nigra nigra
Taxus maritima Tsuga canadensis
Cupressus leylandii Taxus baccata Taxus media
Pseutsuga menziesii Pinus mugo var. mughus
-----------------------------------------------------------------
a From: den Boer & Bastiaens (1984).
Exposure of tomato seedlings to ammonium solutions as the sole
nitrogen source showed reduced growth. The root system was
sparsely branched and discoloured and the stem was easily bruised.
There was considerable wilting of leaves, which developed marginal
necrosis (Pierpont & Minotti, 1977). Earlier, Maynard & Barker
(1969), using cucumber (C. sativus), bean (P. vulgaris), and pea
(Pisum sativum L.) in sand culture, demonstrated that ammonium
toxicity was generally characterized by an immediate reduction in
growth rate, wilting, marginal necrosis, interveinal chlorosis of
terminal leaves and, finally, death of the entire plant. However,
these symptoms did not occur in the ammonium medium with added
calcium carbonate (CaCO3) (Maynard & Barker, 1969; Pierpont &
Minotti, 1977). Several studies have shown that an increase in the
ammonium concentration is more deleterious to root than to shoot
growth (Bennett et al., 1964; Haynes & Goh, 1977). Warncke &
Barber (1973) observed that the ratio of root dry weight to shoot
dry weight decreased significantly with increasing concentrations
of nitrogen, but was not affected by the ammonium-to-nitrate ratio.
The roots appeared darker and less branched. The authors
attributed the observed decrease in root production to greater
acidity around the roots of ammonium-fed plants (Klemm, 1967;
Warncke & Barber, 1973).
In the Netherlands, field observations and experiments have
shown effects of ammonia and ammonium on pine forests, and
heathland vegetation (Heil & Diemont, 1983; Van Breemen & Jordens,
1983; den Boer & Bastiaens, 1984; Roelofs, in press; Schuurkes, in
press). In the United Kingdom, ombrotrophic mires (upland bogs
deriving water only from rain) are affected by eutrophication (Lee,
1985). Forest ecosystems in the Federal Republic of Germany may
also suffer from ammonia exposure (Hunger, 1978; Ulrich, 1983).
Although only little information is available for other countries,
it is expected that similar effects may occur in Belgium, Denmark,
and the northwestern part of France, where high ammonia emissions
occur as a consequence of the production of animal manure (Buysman
et al., 1985).
The condition of forests may deteriorate as a consequence of
direct exposure to ammonia. Pine trees in areas near intensive
livestock farms are particularly affected (Hunger, 1978; Janssen,
1982). In the Netherlands, there is a clear correlation between
ammonia emission and forest condition (Van Aalst, 1984). The
damage observed is the result of both direct and indirect effects,
and it is often difficult to distinguish between the two.
Field observations and experiments have shown deleterious
effects on pine trees, namely:
- decreased vitality (growth reduction and necrosis);
- higher susceptibility to fungal diseases and attack
by insects; and
- increased sensitivity to meteorological stress
factors, e.g., hard frost.
Most of these phenomena can be explained in terms of disturbed
nutrient budgets in trees. Enhanced ammonium sulfate uptake
results in the excretion of essential nutrients, such as potassium
and magnesium, by the needles, and the uptake of these ions by the
root system is inhibited as a result of ammonium accumulation and
cation leaching in soils (den Boer & Bastiaens, 1984; Roelofs, in
press). Increased ammonium uptake ultimately leads to enhanced
nitrogen levels in the leaves of beech (Nihlgard, 1970) and pine
tress (Roelofs et al., 1985).
More attention is being paid to the role of ammonium in the
forest dieback in Europe (Nihlgard, 1985).
Heather communities on poorly-buffered, slightly-acidic, and
nutrient-poor heathland soils are also disturbed as a result of the
deposition of airborne ammonia compounds, the number of plant
species declining in acidified and nitrogen-enriched heathland
soils. A succession from heather-dominated to grass-dominated
heathlands has been observed (Heil & Diemont, 1983; Roelofs, in
press). The same effects can be expected in other western European
countries where similar plant communities are present.
6.2.2. Aquatic plants
In the aquatic environment, nitrogen plays an important role in
determining the composition of phytoplankton and vascular plant
communities; in some cases, it can act as a limiting factor in
primary production. Ammonia is important in nitrogen metabolism,
because it functions as a nitrogen source in the synthesis of amino
acids.
Most species can use either ammonium or nitrate as the sole
nitrogen source, though, when both forms are available, ammonium is
used first. Uptake takes place through both roots and leaves. The
relative importance of ammonium as a nutrient depends on the
absolute concentration and the ratio of ammonium to nitrate.
Assimilation of ammonium is less expensive in terms of energy than
nitrate, because the first metabolic step in which nitrate is
reduced is not needed. Although ammonia is an important nutrient,
it appears to be toxic at higher concentrations. Its uptake in
large quantities may put a severe strain on the carbohydrate
metabolism of the species, because carbon skeletons are used for
detoxification (Bidwell, 1974). Increasing ammonia levels within
the cell inhibit the utilization of nitrate. Ammonia solutions
seem to be more toxic at high than at low pH, indicating that
toxicity is probably due primarily to NH3 rather than to NH4+.
Surface waters that are poorly buffered, nutrient poor, and
hydrologically dependent on rainfall and/or snow melt are most
sensitive to ammonia. Deposition of ammonia and other nitrogen
compounds may contribute significantly to the nitrogen enrichment
of susceptible waters. Ombrotrophic mire plant communities can be
altered by this atmospheric pollutant (Lee, 1985). In these
Sphagnum-dominated wetlands, seed plants become more dominant. In
small, poorly-buffered and nutrient-poor clear water lakes, both
water composition and macrophyte composition are altered.
Atmospheric ammonia deposition plays a major role in the
acidification and nitrogen enrichment of surface waters in the
Netherlands (Schuurkes, in press). Typical plant species belonging
to the Littorellion alliance disappear from acidified waters.
These species may be supressed by the luxuriant growth of Sphagnum
species and Juncus bulbosus. Ammonium enrichment enables the last
two species to form extremely high biomasses (Roelofs et al., 1984;
Schuurkes, in press; Schuurkes et al., 1985). It should be noted
that these changes in the plant community also influence the
structure of the animal population.
6.2.3. Fresh-water plants
Experimental data concerning the toxicity of ammonia for fresh-
water phytoplankton are limited. Przytocka-Jusiak (1976) reported
the effects of ammonia on the growth of Chlorella vulgaris. A 50%
inhibition was seen in 5 days of exposure to a concentration of
2.4 mg NH3/litre; complete growth inhibition occurred in 5 days
at 5.5 mg/litre. The NH3 concentration resulting in 50% survival
of C. vulgaris after 5 days was found to be 9.8 mg/litre. A C.
vulgaris strain in which the tolerance to elevated ammonia
concentrations was enhanced by prolonged incubation of the alga in
ammonium carbonate solutions was isolated by Przytocka-Jusiak et
al. (1977). C. vulgaris was reported to grow well in solutions
containing 4.4 mg NH3/litre, but growth was inhibited at 7.4
mg/litre (Matusiak, 1976). Tolerance to elevated concentrations of
NH3 seemed to show a slight increase, when other forms of nitrogen
were available to the alga, rather than when ammonia was the only
form of nitrogen in the medium. Bretthauer (1978) found that a
concentration (assuming pH 6.5 and 30 °C) of 0.6 mg NH3/litre
killed Ochromonas sociabilis, and that, at 0.3 mg/litre,
development of the population was reduced. Concentrations of 0.06
to 0.15 mg NH3/litre had an insignificant effect on growth, and
concentrations of 0.015 to 0.03 mg/litre enhanced growth.
Ammonia at concentrations exceeding 2.5 mg NH3/litre inhibited
photosynthesis and growth in the algal species Scenedesmus obliquus
and inhibited photosynthesis in the algae Chlorella pyrenoidosa,
Anacystis nidulans, and Plectonema boryanum (Abelovich & Azov,
1976). Mosier (1978) reported that NH3 concentrations causing a
50% reduction in oxygen production by the green alga Chlorella
ellipsoidea and blue-green alga Anabaena subcylindrica were
16.0 x 10-8 and 251.0 x 10-8 µg NH3-N/cell, respectively.
The rate of photosynthesis in the blue-green alga P. boryanum
was stimulated by NH4+, but inhibited by NH3 (Solomonson 1969); the
magnitude of these effects was dependent on the sodium-potassium
composition of the suspension media. Inhibition of photosynthesis
by NH3 was associated with a conversion of inorganic polyphosphate,
stored in the cells, to orthophosphate.
Champ et al. (1973) treated a small natural water pond with
ammonia to achieve a mean concentration of 25.6 mg NH3/litre. A
diverse population of dinoflagellates, diatoms, desmids, and blue-
green algae was present before ammonia treatment. Twenty-four
hours after treatment, the mean number of phytoplankton cells/litre
was reduced by 84%. By the end of 2 weeks (3.6 mg NH3/litre), the
original concentration of cells had been reduced by 95%.
Some research has been carried out to investigate the possible
use of ammonia as an aquatic herbicide. Champ et al. (1973)
reported virtually complete eradication of rooted aquatic
vegetation (water shield, Brasenia schreberi, and American lotus,
Nelumbo sp.). The NH3 concentration was 25.6 mg/litre 24 h after
ammonia addition, and 3.6 mg/litre, 2 weeks later. The use of high
concentrations of ammonia to eradicate aquatic vegetation has been
described by Ramachandran & Ramaprabhu (1976) and Ramachandran et
al. (1975).
In experiments with Potamogeton lucens, Litav & Lehrer (1978)
observed that ammonia caused appreciable injury to detached
branches. Ammonia inhibition of the growth of Eurasian
watermilfoil (Myriophyllum spicatum) affected both the length and
weight of roots and shoots (Stanley, 1974).
Grube (1973) found that Sium erectum was slightly injured at
15 mg NH3-N/litre, and completely eradicated at 35 mg/litre after a
10-week exposure. Callitriche sp. showed slight injury at 5 mg
NH3-N/litre, and the lethal dose was between 10 and 15 mg/litre.
Injury was estimated from the amount of black colouring and the
death of leaves. Roelofs et al. (1984) reported that exposure of
the isoebid Littorella uniflora to 50 µmol NH4+/litre for 10 weeks
resulted in excretion of an equivalent amount of potassium, which
may lead to discolouration and starvation.
Changes in the vegetation in 2 rivers subject to increased
pollution from agricultural fertilizers, urban sewage, and
industrial wastes, were studied by Litav & Agami (1976), who
attributed the changes in the composition of the plant species
primarily to a combination of ammonia and detergents. Agami et al.
(1976) transplanted 7 species of "clean water" macrophytes to
various sections of a river, and found that ammonia affected only
Nymphaea caerulea.
6.2.4. Salt-water plants
A concentration of 0.24 mg NH3/litre retarded the growth of
most of 10 species of benthic diatoms cultured for 10 days by
Admiraal (1977). Pinter & Provasoli (1963) found that Coccolithus
huxleyi was the most sensitive, and Pavlova gyrans and Hymenomonas
sp. the most tolerant to ammonium sulfate with intermediate tolerance
exhibited by Syracosphaera sp. and Ochrosphaera neapolitana.
Shilo & Shilo (1953, 1955) reported that the euryhaline alga
Prymnesium parvum was effectively controlled by applications of
ammonium sulfate, which exerted a lytic effect that decreased with
increasing pH, indicating that NH3 and not NH4+ is responsible for
the lytic activity of ammonium sulfate on P. parvum. It was
reported by Byerrum & Benson (1975) that added ammonium ion at
concentrations found to stimulate the photosynthetic rate also
caused the alga Amphidinium carterae to release up to 60% of fixed
14CO2 to the medium.
Natarajan (1970) found that the concentrations of fertilizer
plant effluent that were toxic for natural phytoplankton
(predominantly diatoms) ranged between 1.1 and 11 mg NH3/litre.
Thomas et al. (1980) concluded that increased ammonium
concentrations found near sewage outlets would not be inhibiting to
phytoplankton in the vicinity. Provasoli & McLaughlin (1963)
reported that ammonium sulfate was toxic for some marine
dinoflagellates, but only at concentrations far exceeding those in
sea water.
6.3. Aquatic Invertebrates
The toxicity of ammonia has been less extensively studied in
invertebrates than in fish. Most of the available invertebrate
data consists of studies on arthropods, primarily crustaceans and
insects. Many of these studies are laboratory tests in which test
animals were exposed to known concentrations of a toxic agent for
specified periods of time. Results may be expressed as a median
lethal concentration (LC50) for a given time period (e.g., 48-h
LC50) or, occasionally, as an effective concentration (EC), that
is, the concentration at which the test animal is completely
immobilized, though it might still be respiring (e.g., 48-h EC).
Another method of reporting test results is as the estimated time
required to kill 50% of a test population (LT50) at a given
concentration of toxin (e.g., LT50 = 250 min).
6.3.1. Fresh-water invertebrates: acute toxicity
The acute toxicity of ammonia for Daphnia magna has been
studied by Parkhurst et al. (1979, 1981) and Reinbold & Pescitelli
(1982a) with reported 48-h LC50s values of 2.08 and 4.94 mg
NH3/litre. DeGraeve et al. (1980) reported a similar 48-h LC50
value for Daphnia pulicaria of 1.16 mg NH3/litre. A threshold
toxicity value for D. magna of 2.4 - 3.6 mg NH3/litre lake water
was reported by Anderson (1948). Threshold concentration was taken
as the highest concentration that would just fail to immobilize the
test animals, under conditions of prolonged exposure (Anderson,
1948). A minimum lethal concentration of 0.55 mg NH3/litre was
reported for D. magna by Malacea (1966), and a 24-h LC50 value of
1.50 mg NH3/litre was reported by Györe & Oláh (1980) for Moina
rectirostris.
Buikema et al. (1974) reported an EC50 for NH3 toxicity in the
rotifer, Philodina acuticornis, to be 2.9 - 9.1 mg NH3/litre
(calculated using reported pH values of 7.4 - 7.9). Tests of
ammonia toxicity for the flatworm, Dendrocoelum lacteum (Procotyla
fluviatilis), and a tubificid worm (Tubifex tubifex) gave LC50
values of 1.4 and 2.7 mg NH3/litre, respectively (Stammer, 1953).
Thurston et al. (1984a) conducted 25 flow-through toxicity
tests with 3 mayfly, 2 stonefly, 1 caddisfly, and 1 isopod species;
all tests were conducted with water of similar chemical
composition. The 96-h LC50 values ranged from 1.8 to 5.9 mg
NH3/litre. The results also indicated that a 96-h test is not long
enough to determine the acutely lethal effects of ammonia on the
species tested, because an asymptotic LC50 was not always obtained
within 96 h. Percentage survival data were reported for some
mayfly, stonefly, and caddisfly tests in which LC50 values were not
obtained; there was 60 - 100% survival at test concentrations
ranging from 1.5 to 7.5 mg NH3/litre. Gall (1980) tested NH4Cl
with Ephemerella sp. (near excrucians). Organisms were exposed
to ammonia for 24 h, followed by 72 h in ammonia-free water;
mortality observations were made at the end of the overall 96-h
period. An EC50 value of 4.7 mg NH3/litre was obtained. Hazel et
al. (1979) reported a LC50 value of 8.0 mg NH3/litre for the beetle
Stenelmis sexlineata.
No deaths occurred in ammonia toxicity tests conducted on scud,
Gammarus lacustris, or D. magna, using dilution water from a
river, after a 96-h exposure to 0.08 mg NH3/litre. In a second
test, using river water buffered with sodium bicarbonate, 13%
mortality occurred with scud at the several concentrations tested,
including the highest and lowest of 0.77 and 0.12 mg NH3/litre,
respectively; 7 and 13% mortality occurred with D. magna at the
same concentrations (Miller et al., 1981).
Five fresh-water mussel species, Amblema p. plicata, Anodonta
imbecillis, Corbicula manilensis, Cyrtonaias tampicoensis, and
Toxolasma texasensis, were exposed for 165 h to a concentration of
0.32 mg NH3/litre; T. texasensis was most tolerant to ammonia, and
A. p. plicata was most sensitive (Horne & McIntosh, 1979). During
the tests, the more tolerant species generally had their shells
tightly shut, whereas the least tolerant species continued
siphoning or had their mantles exposed. In 2 studies, acute
exposure of the fresh-water crayfish, Orconectes nais, to ammonium
chloride gave LC50 values of 3.15 and 3.82 mg NH3/litre,
respectively (Evans, 1979; Hazel et al., 1979).
6.3.2. Fresh-water invertebrates: chronic toxicity
Few studies have been conducted on the long-term exposure of
fresh-water invertebrates to ammonia. In a long-term test
conducted by Reinbold & Pescitelli (1982a), reproduction and growth
of D. magna were affected at a concentration of 1.6 mg NH3/litre.
Two tests lasting 42 days were conducted by Anderson et al.
(1978) on the effects of ammonium chloride on the fingernail clam,
Musculium transversum. Significant mortality (67 and 72%) occurred
in both tests at a concentration of 0.7 mg NH3/litre. In one of
the studies, significant reduction in growth was observed after 14
days of exposure to 0.41 mg NH3/litre. Sparks & Sandusky (1981)
reported that fingernail clams exposed to 0.23 and 0.63 mg
NH3/litre showed 36 and 23% mortality, respectively, in 4 weeks;
after 6 weeks, there was 47% mortality at 0.073 mg NH3/litre, and
83% mortality at 0.23 and 0.63 mg NH3/litre. After 6 weeks there
was no growth in any test chamber (concentrations of 0.036 mg
NH3/litre and higher), other than in the controls.
Two partial tests, lasting 24 and 30 days, respectively, were
conducted by Thurston et al. (1984a) on the stonefly Pteronarcella
badia. Adult stonefly emergence was delayed with increasing
ammonia concentration, and little or no emergence occurred at
concentrations exceeding 3.4 mg NH3/litre. There was no significant
relationship between the food consumption rates of nymphs and
concentrations up to 6.9 mg NH3/litre. LC50 values for 24- and
30-day exposures were 1.45 and 4.57 mg NH3/litre, respectively.
The effects of ammonia on the ciliary beating rate of clam
gills were investigated by Anderson et al. (1978). Concentrations
of 0.036 - 0.11 mg NH3/litre caused a reduction in the ciliary
beating rate of fingernail clams; the effects ranged from a 50%
reduction in beating rate to complete inhibition. Adult clams (> 5
mm) were more sensitive than juveniles (< -5 mm); adults were also
slightly more sensitive than the unionid mussel, Elliptio
complanata, and the Asiatic clam, C. manilensis. Shaw (1960)
investigated the effects of ammonium chloride on sodium influx in
the fresh-water crayfish, Astacus pallipes. Ammonia produced an
inhibition of sodium influx; a concentration of 18 mg NH4+/litre
reduced the influx to about 20% of its normal value, and influx
reduction was related to increasing ammonia concentration. This
effect was attributed to NH4+ ions and not to any toxic effect
exerted on the transporting cells by non-ionized ammonia. NH4+ did
not affect chloride influx nor the rate of sodium loss.
Ammonia was added to a stream at a 24-h average concentration
of 1.4 mg NH3/litre, and a 24-h drift net sampling was conducted
(Liechti & Huggins, 1980). No change in diel drift pattern was
observed, but there was an increase in the magnitude of drift, a
shift in the kinds of organisms present, and changes in benthic
standing crop estimates; this ammonia concentration was non-lethal.
6.3.3. Salt-water invertebrates: acute and chronic toxicity
Data on the acute toxicity of ammonia for salt-water
invertebrate species are very limited. A 96-h LC50 value of 1.5 mg
NH3/litre has been reported for the copepod, Nitocra spinipes
(Linden et al., 1979). Lethal effects of ammonium chloride on the
quahog clam, Mercenaria mercenaria, and eastern oyster,
Crassostrea virginica, were studied by Epifano & Srna (1975).
There were no observed differences in susceptibility between
juveniles and adults of the 2 species. Armstrong et al. (1978)
conducted acute toxicity tests (6 days) on ammonium chloride using
prawn larvae, Macrobrachium rosenbergii. LC50 values were highly
pH-dependent. The acute toxicity of ammonium chloride for penaeid
shrimp was reported as a 48-h composite LC50 value of 1.6 mg
NH3/litre for 7 species pooled, including the resident species
Penaeus setiferus (Wickins, 1976). The acute toxicity of ammonium
chloride for the caridean prawn, M. rosenbergii, was reported
(Wickins, 1976) as LT50 values of 1700 - 560 min at concentrations
of 1.74 - 3.41 mg NH3/litre. From the data of Hall et al. (1978),
48-h LC50 values of 0.34 - 0.53 were estimated for grass shrimp,
Palaemonetes pugio. Catedral et al. (1977a,b) investigated the
effects of ammonium chloride on the survival and growth of Penaeus
monodon; larvae had a lower tolerance to ammonia compared with
post-larvae. Brown (1974) reported a time to 50% mortality of
106 min for the nemertine worm, Cerebratulus fuscus, at a
concentration of 2.3 mg NH3/litre.
Effects of ammonium chloride solutions on the American lobster,
Homarus americanus, were studied by Delistraty et al. (1977).
Their studies were performed on fourth-stage larvae, which was
considered to be the most sensitive life stage. A 96-h LC50 value
of 2.2 mg NH3/litre and an incipient LC50 value of 1.7 mg NH3/litre
were reported. A "safe" concentration of 0.17 mg NH3/litre was
tentatively recommended.
The sublethal toxicity of ammonium chloride for the quahog clam
and eastern oyster was studied by Epifano & Srna (1975) who
measured the effect of a 20-h exposure to ammonia on the rate of
removal of the alga, Isochrysis galbana, from suspension (clearing
rate) by the clams and oysters. Concentrations of 0.06 - 0.2 mg
NH3/litre affected removal; no differences were observed between
juveniles and adults. The effect of ammonia on the ciliary beating
rate of the mussel, Mytilus edulis, was studied by Anderson et al.
(1978). Concentrations of 0.097 - 0.12 mg NH3/litre resulted in a
reduction in the ciliary beating rate ranging from 50% to complete
inhibition.
Exposure of unfertilized sea urchin, Lytechinus pictus, eggs to
ammonium chloride resulted in stimulation of the initial rate of
protein synthesis, an event that normally follows fertilization
(Winkler & Grainger, 1978). Exposure of unfertilized eggs of
Strongylocentrotus purpurpatus, L. pictus, and Strongylocentrotus
drobachiensis to ammonium chloride (Johnson et al., 1976; Paul et
al., 1976) caused "fertilization acid" to be released more rapidly
and in greater amounts than after insemination. Activation of
unfertilized L. pictus eggs by ammonium chloride exposure was
also evidenced by an increase in intracellular pH (Steinhardt &
Mazia, 1973; Shen & Steinhardt, 1978). Ammonia treatment has also
been reported to activate phosphorylation of thymidine and
synthesis of histones in unfertilized eggs of the sea urchin,
S. purpuratus, (Nishioka, 1976). Premature chromosome
condensation was induced by ammonia treatment of eggs of L. pictus
and S. purpuratus (Epel et al., 1974; Wilt & Mazia, 1974; Krystal
& Poccia, 1979). Treatment of S. purpuratus and S. drobachiensis
fertilized eggs with ammonia resulted in an absence of the normal
uptake of calcium following insemination. However, calcium uptake
was not inhibited when ammonia treatment preceded insemination
(Paul & Johnston, 1978).
The polychetous annelid, Nereis succinea, the channelled whelk,
Busycon canaliculatum, and the brackish-water clam, Rangia
cuneata, were exposed to concentrations of 0.85, 0.37, and 0.27 mg
NH3/litre and the ammonia excretion measured (Mangum et al., 1978).
Excretion was inhibited by non-lethal concentrations of ammonia,
and the authors concluded that ammonia crosses the excretory
epithelium in the ionized form, and that the process is linked to
the activity of the Na++K+ ATPases. When blue crabs, Callinectes
sapidus, were moved from water of 28 parts per thousand salinity
to water of 5 parts per thousand, a doubling of the ammonia
excretion rate occurred; addition of excess ammonium chloride to
the low-salinity water inhibited ammonia excretion and decreased
net acid output (Mangum et al., 1976). The effect of gaseous NH3
on haemoglobin from the blood of the common marine bloodworm,
Glycera dibrachiata, was examined by Sousa et al. (1977) in an
attempt to determine whether there was competition between NH3 and
oxygen in binding to haemoglobin; such an NH3/O2 relationship was
not found.
6.4. Fish
Ammonia is highly toxic for fish, and, because of its
occurrence at high concentrations in some water systems, it can
present a major pollution problem. It enters aquatic environments
from several sources, including sewage effluent, deposition of
human wastes without treatment, industrial discharges, and runoff
from animal culture and agricultural operations. It is also a
metabolic waste product of fish and, therefore, can be a problem in
facilities involved with intensive fish culture.
Elevated ammonium ion (NH4+) concentrations within the bodies
of fish, as with other vertebrates, cause convulsions and death.
The concentration of non-ionized ammonia (NH3) in the environment
of the fish is important, because ammonia is transferred between
the water and fish largely in this form. Thus, while NH3 is the
more toxic chemical species in the water, within the fish, toxicity
is related to the NH4 concentration.
Research by Chipman (1934), Wuhrman et al. (1947), Wuhrman &
Woker (1948), and Tabata (1962) implicated NH3 as the ammonia
species in water that is mainly toxic for fish, and reported that
NH4+ was non-toxic or considerably less toxic. More recent
research by Robinson-Wilson & Seim (1975), Armstrong et al. (1978),
and Thurston et al. (1981c) has demonstrated that the role of water
pH in the toxicity of ammonia for fish is more than the regulation
of the NH3/NH4+ equilibrium. NH3 is considerably more toxic in
water when pH values are lower than 7 - 9, and there is some
evidence that the toxicity of NH3 is also increased above this
range. Temperature and dissolved oxygen and the ionic composition
of the background water all play a role in the toxicity of NH3 for
some fish.
In a comprehensive analysis of the data, it was concluded that
a number of fish species that are phylogenetically similar are also
similar in their sensitivity to the toxicity of ammonia, and that
many of the factors that affect the toxicity of ammonia similarly
affect all the species that have been studied (US EPA, 1985).
It was formerly considered that fish of the family Salmonidae
were among the most sensitive to the effects of many pollutants,
whereas other fish species, which have evolved in warm-water, low
oxygen, or more turbid aquatic environments, may be less sensitive
to many naturally-occuring pollutants, such as ammonia. However,
other fish species, including some that are frequently referred to
as "warm water" fishes, are of comparable sensitivity (US EPA,
1985). In the "resident-species" approach for establishing water
quality criteria for a toxic agent in a given water body, the
tolerance of selected fish and invertebrate species, naturally
resident in the water body, is used. It must be borne in mind
that, if these selected species are less sensitive than other
species in different water bodies, the standards may be
inappropriate for other water bodies.
Because of variation among different background test water
conditions in different laboratories, as well as differences in the
genetic pools of the same species, results of a single test on a
given species may not be as meaningful as composite results from
several tests conducted at different laboratories.
Much of the evidence on ammonia toxicity is empirical, so, for
a more complete understanding of the biological actions of this
chemical, the results of toxicity tests must be integrated with a
knowledge of ammonia metabolism in fish.
6.4.1. Ammonia metabolism in fish
6.4.1.1. Ammonia production and utilization
The major pathway for the production of ammonia in fish, as in
other vertebrates, is through the transamination of various amino
acids (Forster & Goldstein, 1969; Watts & Watts, 1974). The
primary site for ammonia production is probably the liver (Pequin &
Serfaty, 1963), but the necessary enzymes have also been located in
the kidneys, gills, and skeletal muscle tissue (Goldstein &
Forster, 1961; McBean et al., 1966; Walton & Cowey, 1977). Ammonia
is also produced by the deamination of adenylates in fish muscle
(Driedzic & Hochachka, 1976). The quantitative importance of
muscle ammoniogenesis in total ammonia excretion depends on the
level of activity of the animal, and increases with increasing
workload (Suyama et al., 1960; Fraser et al., 1966; Driedzic &
Hochachka, 1976).
Ammonia toxicity can be ameliorated by the formation of less
toxic compounds, namely glutamine and urea. Levi et al. (1974)
recorded high levels of glutamine in the brain of goldfish,
Carrasius auratus, and found that brain-glutamine levels increased
with ambient ammonia concentrations. Webb & Brown (1976) found
high glutamine synthetase (EC 6.3.1.2) activity in the brains of
teleosts and elasmobranchs, and this may be important in protecting
the brain from sudden surges in ammonia concentration. Walton &
Cowey (1977) were able to detect glutaminase activity in the gills
of rainbow trout, but were unable to measure any in vivo
utilization of glutamine by the gills.
Ammonia can be converted, through carbamyl phosphate, to urea
either via purines (uricolysis) or via the ornithine cycle. The
enzymes required for uricolysis have been found in most fish
studied (Forster & Goldstein, 1969; Watts & Watts, 1974), but
Florkin & Duchateau (1943) were unable to detect any activity of
uricolytic enzymes in the cyclostome, Lampetra. The ratio of urea
production via the ornithine cycle to production via uricolysis is
about 100 to 1 in elasmobranchs and dipnoi, whereas in teleosts
most of the urea is formed via uricolysis (Gregory, 1977).
6.4.1.2. Ammonia excretion
The gills are the major site of ammonia excretion in fish, but
smaller quantities of ammonia may also be eliminated by the kidneys
(Edwards & Condorelli, 1928; Grollman, 1929; Fromm, 1963; Maetz,
1972) and skin (Morii et al., 1978). Although the majority of
branchial ammonia excretion represents clearance from the blood,
gill metabolism may contribute between 20% (Payan & Matty, 1975)
and 5 - 8% (Cameron & Heisler, 1983) of the net ammonia excretion.
The excretion of ammonia by fish is variable, depending on the
state of the animal, the environmental conditions, and the species.
Ammonia excretion tripled in sockeye salmon, Oncorhynchus nerka,
following daily feeding (Brett & Zala, 1975) but remained low and
unchanging during starvation (Brett & Zala, 1975; Guerin-Ancey,
1976a). In fresh-water fish, ammonia excretion increases in
response to exercise (Sukumaran & Kutty, 1977; Holeton et al.,
1983), long-term acid exposure (McDonald & Wood, 1981; Ultsch et
al., 1981), hypercapnia (Claiborne & Heisler, 1984), and NH4Cl
infusion (Hillaby & Randall, 1979). In contrast, increased levels
of environmental ammonia (Guerin-Ancey, 1976b) and short-term
exposure to acid or alkaline water (Wright & Wood, 1985) cause a
decrease in ammonia excretion. It is not known if these changes in
excretion reflect change in the rate of ammonia production or in
the ammonia content of the body. The ammonia content of fish is
likely to be the equivalent of the ammonia excreted in about 2 h,
most of the ammonia being in the tissues with a lower pH, such as
muscle. Blood levels are around 0.2 to 0.3 mmol, but muscle at a
lower pH may contain levels of up to 1 mmol; thus, a 1-kg fish may
contain about 0.5 to 0.7 mmol of ammonia and have an excretion rate
of about 0.3 mmol/h. There is increased ammonia production in
muscle during exercise (Driedzic & Hochachka, 1976). Ammonia
excretion by the spiny dogfish, Squalus acanthias, in sea water is
unaffected by temperature change, exercise, hyperoxia, hypercapnia,
or the infusion of either HCl or NaHCO3 or anything that induces
acid-base stress (Heisler, 1984). This is surprising, because many
of these changes affect pH and therefore would be expected to alter
the ammonia content of body compartments and consequently ammonia
excretion.
There is an elevation in blood-ammonia during starvation (Morii
et al., 1978; Hillaby & Randall, 1979), even though ammonia
excretion does not change (Brett & Zala, 1975). Blood-ammonia
concentrations also rise with increases in both temperature
(Fauconneau & Luquet, 1979) and ammonia concentrations in the water
(Fromm & Gillette, 1968; Thurston et al., 1984b). Exposure of fish
to either air (Gordon, 1970) or increased ammonia levels in water
(Fromm, 1970; Guerin-Ancey, 1976b), raises blood-ammonia levels and
reduces ammonia excretion; this is associated with a rise in urea
production in many, but not all fish. Unlike the authors of the
above studies, Buckley et al. (1979) did not find any change in
blood-total ammonia when coho salmon, Oncorhynchus kisutch, were
exposed to elevated ammonia levels in the environment. However, a
significant rise in plasma-sodium, indicating some coupling between
sodium uptake and ammonia excretion, was observed.
The study of ammonia movement is complicated by the fact that,
with present analytical techniques, it is impossible to distinguish
between the transfer of a molecule of NH3 plus a H+ ion from the
transfer of an NH4+ ion. Thus, only indirect evidence can be
obtained regarding the relative gas and ion movements across the
gill epithelium.
Three possible mechanisms of ammonia excretion have received
the most attention: passive NH3 flux, ionic exchange of NH4+ for
Na+, and passive NH4+ flux. There seems to be little doubt that a
significant pathway for branchial ammonia excretion is by the
passive diffusion of NH3 down its partial pressure gradient.
Changes in the NH3 partial pressure gradient are positively
correlated with changes in net ammonia excretion in the channel
catfish, Ictalurus punctatus, (Kormanik & Cameron, 1981), and
rainbow trout (Cameron & Heisler, 1983; Wright & Wood, 1985).
Ammonia entry into the fish has also been shown to be dependent on
the NH3 gradient (Wuhrmann et al., 1947; Wuhrmann & Woker, 1948;
Fromm & Gillette, 1968).
The excretion of NH4+ is strongly coupled with the movement
of other ions. Many studies have attempted to link the
transepithelial exchange of Na+ uptake to NH4+ efflux. Although
there is considerable indirect evidence for the presence of a
coupled ionic exchange mechanism under certain conditions (Maetz &
Garcéa-Romeu, 1964; Maetz, 1973; Payan & Maetz, 1973; Evans, 1977,
1980; Payan, 1978; Girard & Payan, 1980; Wright & Wood, 1985), the
ubiquity and stoichiometry of this exchange remain controversial.
While Na+ influx can be monitored with isotopes, it is difficult to
determine NH4+ efflux. Investigators have attempted to quantify
the relationship between Na+ uptake and NH4+ excretion by
manipulating Na+ levels in the environmental water, by
pharmaceutical inhibition of the Na+ influx mechanism, or by
loading the fish with ammonia.
In goldfish (Maetz, 1973), and in irrigated rainbow trout gills
(Kirschner et al., 1973), Na+ influx was best correlated with the
sum of H+ and NH4+ ion efflux. The possibility of a Na+ uptake
carrier coupled to either NH4+ or H+ appears likely in other fish
as well (Kerstetter et al., 1970; Payan & Maetz, 1973; Evans,
1977). In perfused heads of trout, Na+ uptake was tightly coupled
with NH4+ efflux (Payan et al., 1975; Payan, 1978). Wright & Wood
(1985) demonstrated that, in intact trout, the rate of ion exchange
was influenced by external water pH, increasing from no exchange at
pH 4 to maximal rates at pH 8. The relationship between Na+ and
NH4+ was one-to-one at a pH of less than 8. However, Payan et al.
(1975) and Wright & Wood (1985) found that the majority of the
ammonia was eliminated by gaseous diffusion, and only when NH3 was
subtracted from total ammonia efflux was the NH4+/Na+ exchange
evident. In common carp, Cyprinus carpio (de Vooys, 1968), and
little skate, Raja erinacea (Evans et al., 1979), ammonia
excretion was unaffected by a reduction in environmental Na+
levels. Cameron & Heisler (1983) found that, under resting
conditions, diffusive movement of NH3 could account for ammonia
excretion in trout, but when the ammonia gradient was reversed and
directed inwards, an NH4+/Na+ exchange could counter-balance the
diffusive uptake of NH3 from the water. If this hypothesis is
correct, then it would explain the unchanging blood-ammonia levels
and increased Na+ levels in coho salmon exposed to elevated
concentrations of ammonia in the water (Buckley et al., 1979).
The Na+/NH4+ (H+) exchange is probably located on the
epithelial apical membrane. Either acid conditions or amiloride in
the water inhibits Na+ influx across the gills and both these
conditions result in a reduction in ammonia excretion (Wright &
Wood, 1985).
The ammonium ion can displace potassium in many membrane
processes in, for example, the giant axon of squid, Loligo pealei
(Binstock & Lecar, 1969), and this is the probable reason that
elevated ammonia causes convulsions in so many vertebrates. In
various aquatic animals, it is possible that NH4+ can substitute
for potassium in oubain-sensitive sodium/potassium exchange (Payan
et al., 1975; Towle & Taylor, 1976; Towle et al., 1976; Mallery,
1979; Girard & Payan, 1980). NH4+ ions will substitute for K+ ions
across the epithelial basolateral border (Richards & Fromm, 1970;
Shuttleworth & Freeman, 1974; Karnaky et al., 1976), but the
importance to net ammonia transfer in fresh-water fish is unknown.
The passive movement of NH4+ down its electrochemical gradient
may also contribute to net ammonia excretion (Claiborne et al.,
1982; Goldstein et al., 1982). Lipid membranes are relatively
impermeable to cations (Jacobs, 1940) and, because respiratory
epithelial cells of fresh-water fishes are joined by tight
junctions (Girard & Payan, 1980), it appears unlikely that NH4+
diffusion is of quantitative importance (Kormanik & Cameron, 1981).
Indeed, Wright & Wood (1985) found a negative correlation between
ammonia excretion and the NH4+ concentration gradient in rainbow
trout exposed to 5 different water pH regimes. Although it appears
that NH4+ diffusion may be of minor importance, simultaneous
measurements of the electrical and chemical gradient have not been
made and are necessary before conclusions can be drawn.
6.4.2. Fish: acute toxicity
The acute toxicity of ammonia for rainbow trout has been
studied by many investigators, with reported 96-h LC50 values
ranging from 0.16 to 1.1 mg NH3/litre. Thurston & Russo (1983)
conducted 71 toxicity tests on rainbow trout ranging in size from
sac fry (< 0.1 g) to 4-year-old adults (2.6 kg), in water of
uniform chemical composition. LC50 values ranged from 0.16 to
1.1 mg NH3/litre for 96-h exposures. Fish susceptibility to NH3
decreased with increasing weight over the range 0.06 - 2.0 g, but
gradually increased above that weight range. LC50 values for 12-
and 35-day exposures did not differ greatly from 96-h values. No
statistically-significant differences in results were observed when
different ammonium salts [NH4Cl, NH4HCO3, (NH4)2HPO4, (NH4)2SO4]
were used. Grindley (1946) also reported that there were no
appreciable differences in toxicity between toxic solutions of
NH4Cl and (NH4)2SO4 in rainbow trout tests. However, Calamari et
al. (1977, 1981) reported that embryos and fingerlings were less
sensitive than the other life stages studied. LC50 values (96-h)
ranging from 0.16 to 1.02 mg NH3/litre for rainbow trout exposed to
ammonia were reported by Calamari et al. (1977, 1981), Broderius &
Smith (1979), Holt & Malcolm (1979), DeGraeve et al. (1980), and
Reinbold & Pescitelli (1982b).
Although acute toxicity studies with salmonids have mainly been
conducted on rainbow trout, some data are available for a few other
salmonid species. Thurston et al. (1978) investigated the
toxicity of ammonia for cutthroat trout, Salmo clarki, and
reported 96-h LC50 values of 0.52 - 0.80 mg NH3/litre. Thurston &
Russo (1981) reported a 96-h LC50 value of 0.76 mg NH3/litre for
golden trout, Salmo aguabonita. Brown trout, Salmo trutta, were
exposed to 0.15 mg NH3/litre for 18 h, resulting in a 36%
mortality; when returned to ammonia-free water, the test fish
recovered after 24 h (Taylor, 1973). No mortality occurred during
the 96-h exposure at 0.090 mg NH3/litre, although fish would not
feed. Exposure to 0.8 mg NH3/litre was not acutely toxic for brown
trout according to Woker & Wuhrmann (1950). However, Thurston &
Meyn (1984) reported 96-h LC50 values of 0.60 - 0.70 mg NH3/litre,
and Miller et al. (1981) reported a 96-h LC50 value of 0.47 mg
NH3/litre for brown trout using test dilution river water. Brook
trout, Salvelinus fontinalis, showed distress within 1.75 h at a
concentration of 3.25 mg NH3/litre and within 2.5 h at 5.5 mg/litre
(Phillips, 1950). Thurston & Meyn (1984) reported 96-h LC50 values
of 0.96 - 1.05 mg NH3/litre for brook trout, 0.40 - 0.48 mg
NH3/litre for chinook salmon, Oncorhynchus tshawytscha, and 0.14 -
0.47 mg NH3/litre for mountain whitefish, Prosopium williamsoni.
Toxicity tests with (NH4)2SO4 on pink salmon, Oncorhynchus
gorbuscha, at different early stages of development (Rice & Baily,
1980) showed that late alevins near swim-up stage were the most
sensitive (96-h LC50 = 0.083 mg NH3/litre), and eyed embryos were
the most tolerant, surviving 96 h at > 1.5 mg NH3/litre. Buckley
(1978) reported a 96-h LC50 value of 0.55 mg NH3/litre for
fingerling coho salmon, Oncorhynchus kisutch, and Herbert &
Shurben (1965) reported a 24-h LC50 value of 0.28 mg NH3/litre for
Atlantic salmon, Salmo salar.
There are acute toxicity data for ammonia in a variety of non-
salmonid fish species. Thurston et al. (1983) studied the toxicity
of ammonia for fathead minnows, Pimephales promelas, ranging from
0.1 to 2.3 g in weight and found that 96-h LC50 values in 29 tests
ranged from 0.75 to 3.4 mg NH3/litre; toxicity was not dependent on
the test fish size or source. LC50 values ranging from 0.73 to
2.35 mg NH3/litre for fathead minnows were also reported by Sparks
(1975), DeGraeve et al. (1980), Reinbold & Pescitelli (1982b),
Swigert & Spacie (1983). LC50 values for white sucker, Catostomus
commersoni, exposed to ammonium chloride solutions for 96 h
(Reinbold & Pescitelli 1982c) were 1.40 and 1.35 mg/litre NH3,
though a somewhat lower 96-h LC50 of 0.79 mg NH3/litre was
determined by Swigbert & Spacie (1983). Thurston & Meyn (1984)
reported 96-h LC50 values of 0.67 - 0.82 mg NH3/litre for the
mountain sucker, Caststomus platyrhynchus.
Reported LC50 values for 96-h exposures of bluegill, Lepomis
macrochirus, ranged from 0.26 to 4.60 mg NH3/litre (Emery & Welch,
1969; Lubinski et al., 1974; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982b; Swigert & Spacie, 1983). LC50 values (96-h) of
0.7 - 1.8 mg NH3/litre for smallmouth bass, Micropterus dolomieui,
and 1.0 - 1.7 mg NH3/litre for largemouth bass, Micropterus
salmoides, were reported by Broderius et al. (in press) and
Roseboom & Richey (1977), respectively. Sparks (1975) reported
48-h LC50 values for bluegill of 2.30 mg NH3/litre, and for channel
catfish of 2.92 mg NH3/litre. For goldfish, Carassius auratus,
Dowden & Bennett (1965) reported a 24-h LC50 of 7.2 mg NH3/litre,
and Chipman (1934) reported lethal threshold values of 0.97 -
3.8 mg NH3/litre. Turnbull et al. (1954) reported a 48-h LC50 for
bluegill to be within the range 0.024 - 0.093 mg NH3/litre; during
the exposure, they observed that the fish exhibited a lack of
ability to avoid objects.
Reported 96-h LC50 values for channel catfish, Ictalurus
punctatus, ranged from 1.5 to 4.2 mg NH3/litre (Colt &
Tchobanoglous, 1976; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982d; Swigert & Spacie, 1983). Vaughn & Simco (1977)
reported a 48-h LC50 for channel catfish of 1.24 - 1.96 mg
NH3/litre, and Knepp & Arkin (1973) reported 1-week LC50 values of
0.97 - 2.0 mg NH3/litre. The results of studies on bluegill,
channel catfish, and largemouth bass (Roseboom & Richey, 1977)
showed that bluegill susceptibility was dependent on fish weight,
fish weighing 0.07 g being slightly more sensitive than those
weighing either 0.22 or 0.65 g; size had little effect on the
susceptibility of channel catfish or bass.
Hazel et al. (1979) reported 96-h LC50 values of 0.90 and
1.07 mg NH3/litre for the orangethroat darter, Etheostoma
spectabile, and red shiner, Notropis lutrensis. Largemouth bass,
channel catfish, and bluegill were also exposed for 96 h to a
concentration of 0.21 mg NH3/litre resulting in zero mortality for
bluegill and channel catfish and one death (6%) among the
largemouth bass tested. A 96-h LC50 value for walleye,
Stizostedion vitreum, of 0.85 mg/litre NH3 was reported by
Reinbold & Pescitelli (1982a).
LC50 values ranging from 2.4 to 3.2 mg NH3/litre for
(NH4)2CO3, NH4Cl, NH4C2H3O2, and NH4OH, in 96-h exposures of
mosquitofish, Gambusia affinis, in waters with suspended solids
ranging from < 25 to 1400 mg/litre were reported by Wallen et al.
(1957). Susceptibility of mosquito fish to ammonia was studied by
Hemens (1966) who reported a 17-h LC50 of 1.3 mg NH3/litre; he also
observed that male fish were more susceptible than females. Rubin
& Elmaraghy (1976, 1977) tested guppy, Poecilia reticulata, fry
and reported 96-h LC50 values averaging 1.50 mg NH3/litre; mature
guppy males were more tolerant, with 100% survival for 96 h at
concentrations of 0.17 - 1.58 mg NH3/litre. LC50 values (96-h) of
0.15 and 0.20 mg NH3/litre at pH 6.0, and of 0.52 and 2.13 mg
NH3/litre at pH 8.0, were reported by Stevenson (1977) for white
perch, Morone americana. LC50 values (96-h) of 1.20 and 1.62 mg
NH3/litre for spotfin shiner, Notropis spilopterus, were reported
by Rosage et al. (1979), and of 1.20 mg NH3/litre for golden
shiner, Notemigonus crysoleucas, by Baird et al. (1979). Swigert
& Spacie (1983) reported 96-h LC50 values of 0.72 mg NH3/litre for
golden shiner, 1.35 mg NH3/litre for spotfin shiner, 1.25 mg
NH3/litre for steelcolour shiner, Notropis whipplei, and 1.72 mg
NH3/litre for stoneroller, Campostoma anomalum.
Jude (1973), Reinbold & Pescitelli (1982a), and McCormick et
al. (1984) reported 96-h LC50 values ranging from 0.6 to 2.1 mg
NH3/litre for green sunfish, Lepomis cyanellus. In studies on the
pumpkinseed sunfish, Lepomis gibbosus, by Jude (1973) and Thurston
(1981), 96-h LC50 values ranged from 0.14 to 0.86 mg NH3/litre.
Mottled sculpin, Cottus bairdi, were tested by Thurston & Russo
(1981), yielding a 96-h LC50 value of 1.39 mg NH3/litre. An
asymptotic (6-day) LC50 of 0.44 mg NH3/litre was determined for
rudd, Scardinius erythropthalmus (Ball, 1967).
Rao et al. (1975) reported a 96-h LC50 value for the common
carp, Cyprinus carpio, of 1.1 mg NH3/litre. Carp exposed to
0.24 mg NH3/litre exhibited no adverse effects in 18 h (Vámos
1963). However, exposure to 0.67 mg NH3/litre caused gasping and
equilibrium disturbance within 18 min, frenetic swimming activity
at 25 min, then sinking to the tank bottom after 60 min; after
75 min, the fish were placed in ammonia-free water and all revived.
Kempinska (1968) reported a lethal concentration of 7.5 mg
NH3/litre for carp. Studies on the acute exposure of bitterling,
Rhodeus sericeus, and carp to ammonium sulfate revealed minimum
lethal concentrations of 0.76 mg NH3/litre for bitterling and
1.4 mg NH3/litre for carp (Malacea, 1966). Nehring (1963) reported
survival times for carp at concentrations of 9.7 and 2.1 mg
NH3/litre of 2.4 and 6.0 h, respectively. The survival time for
tench, Tinca tinca, was reported to be 20 - 24 h at 2.5 mg
NH3/litre by Danecker (1964). In a 24-h exposure of creek chub,
Semotilus atromaculatus, to ammonium hydroxide solution, the
"critical range" below which all test fish lived and above which
all died was reported to be 0.26 - 1.2 mg NH3/litre (Gillette et
al., 1952).
In static exposures lasting 9 - 24 h, with a gradual increase
in NH3 content, mortalities occurred in oscar, Astronutus
ocellatus, at 0.50 mg NH3/litre (4%) to 1.8 mg/litre (100%)
(Magalhaes Bastos, 1954). Tests on oscar of two different sizes
showed no difference in susceptibility, in relation to size. A
72-h LC50 value of 2.85 mg NH3/litre was reported by Redner &
Stickney (1979) for blue tilapia, Tilapia aurea.
6.4.2.1. Saltwater fish
Very few acute toxicity data are available for salt-water fish
species. Holland et al. (1960) reported the critical level for
chinook salmon, Oncorhynchus tshawytscha, to be between 0.04 and
0.11 mg NH3/litre and for coho salmon to be 0.134 mg NH3/litre. A
static test with coho salmon provided a 48-h LC50 value of 0.50 mg
NH3/litre (Katz & Pierro, 1967). Atlantic salmon smolts and
yearling rainbow trout exposed for 24 h in 50 and 75% saltwater
solutions exhibited similar sensitivities to ammonia (United
Kingdom Ministry of Technology, 1963). Holt & Arnold (1983)
reported a 96-h LC50 value of 0.47 mg NH3/litre for red drum,
Sciaenops ocellatus. LC50 values (96-h) of 1.2 - 2.4 mg NH3/litre
were reported by Venkataramiak et al. (1981) for striped mullet,
Mugil caphalus, and 0.69 mg NH3/litre for planehead filefish,
Monacanthus hispidus.
6.4.3. Factors affecting acute toxicity
A number of factors can affect the toxicity of ammonia for
aquatic organisms. These include the effects of pH, temperature,
dissolved oxygen concentration, previous acclimatization to
ammonia, fluctuating or intermittent exposures, carbon dioxide
concentration, salinity, and the presence of other toxicants.
Almost all studies of factors affecting ammonia toxicity have been
carried out using only acute exposures.
6.4.3.1. pH
The toxicity for fish of aqueous solutions of ammonia and
ammonium compounds has been attributed to the non-ionized
(undissociated) ammonia present in the solution. The earliest
reported thorough study of the pH dependence of ammonia toxicity
was that of Chipman (1934), who concluded from studies on goldfish,
amphipods, and cladocerans that ammonia toxicity was a function of
pH and therefore of the concentration of undissociated ammonia in
the solution. Downing & Merkens (1955) tested rainbow trout at
different concentrations of ammonia at both pH 7 and pH 8. The
results were consistent when ammonia concentration was expressed as
NH3. Tabata (1962) conducted 24-h tests on the toxicity of ammonia
for Daphnia (species not specified) and guppy at different pH
values and calculated the relative toxicity of NH3/NH4+ to be 48
for Daphnia and 190 for guppy (i.e., NH3 is 190 times more toxic
than NH4+).
More recently, Robinson-Wilson & Seim (1975) studied the
toxicity of ammonium chloride for juvenile coho salmon in flow-
through bioassays within the pH range 7.0 - 8.5; the reported
96-h LC50 for NH3 was approximately 60% less at pH 7.0 than at pH
8.5. The toxicity of ammonium chloride for larvae of prawn,
Macrobrachium rosenbergii, was studied by Armstrong et al. (1978)
in 6-day tests within the pH range 6.8 - 8.3 with test solutions
being renewed every 24 h. The 96-h LC50 for NH3 at pH 6.83 was
approximately 70% less than that at pH 8.34. It was concluded that
the toxicity of ammonia was not due solely to the NH3 molecule and
that in solutions of different pH, but equal NH3 concentrations,
survival was greatly reduced as NH4+ levels increased. Tomasso et
al. (1980) studied the toxicity of ammonia (NH3) for channel
catfish at pH values of 7, 8, and 9 and reported that 24-h LC50
values were significantly higher at pH 8 than at pH 7 or pH 9.
Thurston et al. (1981c) tested the toxicity of ammonia for
rainbow trout and fathead minnows in 96-h flow-through tests at
different pH levels within the range 6.5 - 9.0. Results showed
that the toxicity of ammonia, in terms of NH3, increased at lower
pH values, and could also increase at higher pH values. It was
concluded that NH4+ exerts some measure of toxicity, and/or that
increased H+ concentration increases the toxicity of NH3. Acute
(96-h) exposures of green sunfish and smallmouth bass at 4
different pH levels over the range 6.5 - 8.7 showed that, for both
species, NH3 toxicity increased markedly with a decrease in pH,
with LC50 values at the lowest pH tested (6.6 for sunfish, 6.5 for
bass) being 3.6 (sunfish) and 2.6 (bass) times smaller than those
at the highest pH (8.7) tested (McCormick et al., 1984; Broderius
et al., in press).
It is concluded that NH3 is more toxic for fish at lower pH
values than within the pH range 7 - 9; the toxicity of NH3 may
increase again above this range.
6.4.3.2. Temperature
Information in the literature on the effects of temperature on
ammonia toxicity is varied. The concentration of NH3 increases
with increasing temperature. Several researchers have reported an
effect of temperature on the toxicity of the non-ionized ammonia
species, independent of the effect of temperature on the aqueous
ammonia equilibrium.
McCay & Vars (1931) reported that it took three times as long
for the brown bullhead, Ictalurus nebulosus, to succumb to the
toxicity of ammonia in water at 10 - 13 °C than at 26 °C. The pH
of the tested water was not reported but with the probable range
tested (pH 7 - 8), the percent NH3 at the higher test temperature
would have been approximately three times that at the mean lower
temperature. The toxicity of ammonium chloride for goldfish,
bluntnose minnow, Pimephales notatus, and the straw-coloured
minnow or river shiner, Notropis blennius, was reported (Powers,
1920) to be greater at high temperatures than at low, but no
consideration was given to the increase in the relative
concentration of NH3 as the temperature increased. Herbert (1962)
suggested that the effects of temperature on the susceptibility of
rainbow trout to NH3 toxicity was only slightly, if at all,
affected by temperature change. In studies on striped bass, Morone
saxtilis, and stickleback, Gasterosteus aculeatus, Hazel et al.
(1971) found a slight difference in toxicity between 15° and 23 °C
in fresh water, with both fish species being slightly more
resistant at the lower temperature.
However, there are other studies in which the toxicity of NH3
decreased with increasing temperature over the ranges studied. The
toxicity of NH3 for rainbow trout has been reported to be much
higher at 5 °C than at 18 °C (United Kingdom Ministry of
Technology, 1968). Brown (1968) reported that the 48-h LC50 for
rainbow trout increased with increase in temperature over the range
3 °C - 18 °C; the reported increase in tolerance between ~12 °C -
~18 °C was considerably less than that between ~3 °C - ~12 °C. A
relationship between temperature and 96-h LC50 was reported for
rainbow trout over the temperature range 12 °C - 19 °C with ammonia
toxicity decreasing with increasing temperature (Thurston & Russo,
1983).
Thurston et al. (1983) reported that the acute toxicity of NH3
for fathead minnows decreased with a rise in temperature over the
range 12 °C - 22 °C. Bluegill and fathead minnow were tested at
low and high temperatures of 4.0 °C - 4.6 °C and 23.9 °C - 25.2 °C,
respectively, and rainbow trout were tested at 3 °C and 14 °C
(Reinbold & Pescitelli, 1982b). All three species were more
sensitive to NH3 at the low temperatures, with toxicity being 1.5 -
5 times higher in the colder water. Bluegill appeared to be the
most sensitive of the three species to the effects of low
temperature on ammonia toxicity. Colt & Tchobanoglous (1976)
reported that the toxicity of NH3 for channel catfish decreased
with increasing temperature over the range 22 °C - 30 °C. LC50
values for bluegill, channel catfish, and largemouth bass at 28 °C
- 30 °C were approximately twice those at 22 °C (Roseboom & Richey,
1977). An effluent containing ammonia as the principal toxic
component showed a marked decrease in toxicity for channel catfish
over the temperature range 4.6 °C - 21.3 °C (Cary, 1976).
Lloyd & Orr (1969) investigated the effects of temperature
(range 10 - 20 °C) on urine flow rates in rainbow trout exposed to
0.30 mg NH3/litre, and did not find any apparent temperature effect
on the total diuretic response of the fish, though the relative
increase in urine production was less at higher temperatures. From
a study of the behavioural response of bluegill to gradients of
ammonium chloride, it was hypothesized that low temperatures
increased the sensitivity of the bluegill and interfered with the
ability, either to detect ammonia after a certain period of
exposure, or, to compensate behaviourally for physiological stress
caused by ammonia gradients (Lubinski, 1979; Lubinski et al.,
1980).
The European Inland Fisheries Advisory Commission (1970)
has stated that, at temperatures below 5 °C, the toxic effects of
non-ionized ammonia may be greater than at above 5 °C, though the
basis for this is not clearly documented. The evidence that
temperature, independent of its role in the aqueous ammonia
equilibrium, affects the toxicity of NH3 for fish argues for
further consideration of the temperature/ammonia toxicity
relationship.
6.4.3.3. Salinity
Herbert & Shurben (1965) reported that the resistance of
yearling rainbow trout to ammonium chloride increased with
increasing salinity up to levels of 30 - 40% sea water; above this
level, resistance appeared to decrease. Fingerling coho salmon
were tested at salinity levels of 20 - 30 parts per thousand (57 -
86% salt water), and it was found that the toxicity of an ammonia-
ammonium waste increased as salinity increased (Katz & Pierro,
1967). These findings are in agreement, at the levels tested, with
those of Herbert & Shurben (1965). Atlantic salmon were exposed to
ammonium chloride solutions for 24 h under both fresh-water and 30%
salt-water conditions; LC50 values were 0.15 and 0.3 mg NH3/litre,
respectively, in the 2 different waters (Alabaster et al., 1979).
Harader & Allen (1983) reported that the resistance to ammonia of
chinook salmon parr increased by about 500%, as salinity increased
to almost 30% sea water, but declined as salinity increased beyond
that.
There is a slight decrease in the NH3 fraction of total ammonia
as ionic strength increases in dilute saline solutions, but the
relative changes in NH3 toxicity, as salinity increases, are more
directly attributable to changes in the rate of exchange of NH3 and
NH4+ across the fish gill membranes.
6.4.3.4. Dissolved oxygen
A decrease in dissolved oxygen concentration in the water can
increase ammonia toxicity. There is a reduction in fish blood
oxygen-carrying capacity following ammonia exposure (Brockway,
1950; Danecker, 1964; Reichenback-Klinke, 1967; Körting, 1969a,b;
Waluga & Flis, 1971). Hypoxia would further exacerbate problems of
oxygen delivery and could lead to the early demise of the fish.
Vámos & Tasnádi (1967) observed deaths of carp in ponds at
ammonia concentrations lower than would normally be lethal, and
attributed this to periodic low concentrations of oxygen. On the
basis of research in warm-water (20 °C - 22 °C) fish ponds, Selesi
& Vámos (1976) projected a "lethal line", relating acute ammonia
toxicity and dissolved oxygen, below which carp died. The line ran
between 0.2 mg NH3/litre at 5 mg dissolved oxygen/litre and 1.2 mg
NH3/litre at 10 mg dissolved oxygen/litre. Thurston et al. (1983)
compared the acute toxicity of ammonia for fathead minnows at
reduced and normal dissolved oxygen concentrations; seven 96-h
tests were conducted within the range 2.6 - 4.9 mg dissolved
oxygen/ litre, and 3 between 8.7 and 8.9 mg/litre. There was a
slight positive trend between 96-h LC50 values and dissolved
oxygen, though it was not shown to be statistically significant.
Atlantic salmon smolts were tested in both fresh water and 30% salt
water at 9.6 - 9.5 and 3.5 - 3.1 mg dissolved oxygen/litre. The
reported 24-h LC50 values at the higher oxygen concentrations were
about twice those at the lower (Alabaster et al., 1979).
Several studies have been reported on rainbow trout. Allan
(1955) reported that below 0.12 mg NH3/litre and at about 30%
oxygen saturation, the median survival time was greater than 24 h,
but at the same concentration with oxygen saturation below 30%, the
median survival time was less than 24 h. In studies by Downing &
Merkens (1955), fingerling rainbow trout were tested at 3 different
concentrations of NH3 at 5 different levels of dissolved oxygen.
In tests lasting up to 17 h, decreasing the oxygen level from 8.5
to 1.5 mg/litre shortened the period of survival at all ammonia
concentrations, and a decrease in survival time produced by a given
decrease in oxygen was greatest at the lowest concentration of NH3.
Merkens & Downing (1957), in tests lasting up to 13 days, also
reported that the effects of low concentrations of dissolved oxygen
on the survival of rainbow trout were more pronounced at low
concentrations of NH3. Ammonia (NH3) was found to be up to 2.5
times more toxic when the dissolved oxygen concentration was
reduced from 100 to about 40% saturation (Lloyd, 1961). It was
reported by Danecker (1964) that the toxicity of ammonia increased
rapidly when the oxygen concentration decreased below two-thirds of
the saturation value. Thurston et al. (1981b) conducted 15, 96-h
acute toxicity tests on rainbow trout over the dissolved oxygen
range 2.6 - 8.6 mg/litre. A positive linear correlation between
96-h LC50 and dissolved oxygen was reported over the entire range
tested.
When rainbow trout were treated in a channel receiving sewage
discharge containing 0.05 - 0.06 mg NH3/litre, it was found that,
at 25 - 35% dissolved oxygen saturation, more than 50% of the fish
died within 24 h, compared with 50% mortality of test fish in the
laboratory, at 15% dissolved oxygen saturation (Herbert, 1956).
The difference was attributed to unfavourable water conditions
below the sewage outflow, including ammonia, which increased the
sensitivity of the fish to the lack of oxygen.
6.4.3.5. Carbon dioxide
An increase in carbon dioxide (CO2) concentrations up to
30 mg/litre decreased total ammonia toxicity (Alabaster & Herbert,
1954; Allan et al., 1958). Carbon dioxide causes a decrease in pH,
thereby decreasing the proportion of non-ionized ammonia in
solution. However, Lloyd & Herbert (1960) found that, though total
ammonia toxicity was reduced at elevated CO2 levels, the inverse
was true when considering non-ionized ammonia alone; more NH3 was
required in low CO2, high pH water to exert the toxic effect seen
in fish in high CO2, low pH water. The explanation presented by
Lloyd & Herbert (1960) for the decreased toxicity of NH3 in low CO2
water was that CO2 excretion across the gills would reduce the pH
and, therefore, the NH3 concentration, in water flowing over the
gills. A basic flaw in this hypothesis has been discussed by
Broderius et al. (1977). Carbon dioxide will only form protons
very slowly in water at the tested temperature. The uncatalysed
CO2 hydration reaction has a half-time of seconds or even min
(e.g., at pH 8: 25 seconds at 25 °C; 300 seconds at 0 °C) (Kern
1960), and water does not remain in the opercular cavity for more
than a few seconds, and at the surface of a gill lamella for about
0.5 - 1 second (Randall, 1970; Cameron, 1979). Thus, the
liberation of CO2 across the gills will have little, if any, effect
on water pH or NH3 levels and the NH3 gradient across the gills
between water and blood.
6.4.3.6. Prior acclimatization to ammonia
The question of whether fishes can acquire an increased
tolerance to ammonia by acclimatization to low ammonia
concentrations is an important one. If fish were able to develop
such tolerance, they might be able to survive what would otherwise
be lethal ammonia concentrations.
Observations by McCay & Vars (1931) indicated that brown
bullheads subjected to several successive exposures to ammonia,
alternating with recovery in fresh water, did not acquire
tolerance. However, a number of research workers have reported
that previous exposure of fish to low concentrations of ammonia
increases their resistance to lethal concentrations. Vámos (1963)
reported that carp exposed to 0.67 or 0.52 mg NH3/litre for 75 min,
then transferred to fresh water for 12 h, followed by a solution
containing 0.7 mg NH3/litre, exhibited symptoms of ammonia toxicity
in 60 - 85 min, whereas control fish, exposed initially to
0.7 mg/litre NH3, developed symptoms within 20 min. Blue tilapia
acclimatized for 35 days to 0.52 - 0.64 mg NH3/litre subsequently
survived 48 h at 4.1 mg/litre, compared with the 48-h value for
unacclimatized fish of 2.9 mg/litre (Redner & Stickney, 1979).
Malacea (1968) studied the effects on bitterling of acclimatization
to ammonium sulfate solutions. A group of 10 fish was held in an
acclimatization solution of 0.26 mg NH3/litre for 94 h, after which
the fish were exposed to a 5.1 mg NH3/litre solution for 240 min.
A control group of 10 bitterling received identical treatment,
except that the acclimatization aquarium did not contain added
(NH4)2SO4. The ratio of the mean survival times of "adapted" to
"unadapted" fish was 1:13, indicating a slightly higher ammonia
tolerance for the adapted fish.
Schulze-Wiehenbrauck (1976) subjected 2 groups of rainbow trout
(mean weights 56 g and 110 g) that had been held for at least 3
weeks at sublethal ammonia concentrations, to lethal ammonia
concentrations. In the study on the 110-g fish, the
acclimatization concentrations were 0.007, 0.131, and 0.167 mg
NH3/litre. The fish were then subjected for 8.5 h to
concentrations of 0.45, 0.42 and 0.47 mg NH3/litre, respectively.
Fish from the 2 higher sublethal concentrations showed 100%
survival after 8.5 h in the 0.42 and 0.47 mg NH3/litre solutions,
whereas fish from the 0.007 mg NH3/litre concentration showed only
50% survival in 0.45 mg NH3/litre. In the study on the 56-g fish,
the acclimatization concentrations were 0.004 mg NH3/litre and
0.159 mg NH3/litre; these fish were placed for 10.25 h in NH3
concentrations of 0.515 and 0.523 mg/litre, respectively. There
was 100% survival in the acclimatized fish, and 85% survival in the
fish acclimatized to 0.004 mg/litre. The results of these studies
showed an increase in resistance of trout to high ammonia levels
after prior exposure to sublethal ammonia levels.
Alabaster et al. (1979) determined 24-h LC50 values of NH3 for
Atlantic salmon smolts under reduced dissolved oxygen test
conditions. Fish acclimatized to ammonia before oxygen reduction
had LC50 values 38% and 79% higher than fish without prior ammonia
acclimatization.
In studies by Brown et al. (1969), rainbow trout were tested by
moving back and forth between tanks in which the ammonia
concentrations were 0.5 and 2.5 times a previously determined 48-h
LC50 value. If fish were transferred on an hourly basis, the
median period of survival for the fluctuating exposure was reported
to be the same as that for constant exposure (> 700 min). When
the fish were transferred at 2-h intervals, the median survival
time for the fluctuating exposure was reported to be less (370
min), indicating that the toxic effects from exposure to the
fluctuating concentrations of ammonia were greater than those from
exposure to the constant concentration. Thurston et al. (1981a)
conducted acute toxicity tests in which rainbow trout and cutthroat
trout were exposed to short-term cyclical fluctuations of ammonia.
Companion tests were conducted in which test fish were subjected to
ammonia at constant concentrations. The LC50 values for both
average and peak concentrations of ammonia for the fluctuating
concentration tests were compared with the LC50 values for the
constant concentration tests. Comparison of total exposures showed
that fish were more tolerant to constant, than to fluctuating
concentrations of ammonia. Fish subjected to fluctuating
concentrations of ammonia at levels below those acutely toxic were
better able to withstand subsequent exposure to high fluctuating
concentrations than unacclimatized fish. There is reasonable
evidence that fish with a history of prior exposure to sublethal
concentrations of ammonia are better able to withstand an acutely
lethal concentration for a period of hours and possibly days.
Limited data on fluctuating exposures indicate that fish are more
susceptible to fluctuating than to constant exposure with the same
average NH3 concentrations.
6.4.4. Fish: chronic toxicity
"Full-chronic" tests cover the entire life cycle of the test
animal, beginning at a given stage of development of one generation
(frequently as the fertilized egg) and continuing through to this
same stage in the next generation. Common end-points for measuring
toxicity are survival, growth, and reproductive success, though
recent research on ammonia toxicity for fish has demonstrated the
desirability of also conducting histological examinations.
"Partial-chronic" tests on fish most frequently cover a period
of 30 days or longer, from the egg incubation stage to the free-
swimming stage; for many toxins it has been demonstrated that
these stages are the most sensitive. However, in the case of
ammonia it has been demonstrated that older, mature rainbow trout,
Salmo gairdneri, are potentially as susceptible to the effects of
ammonia as newly-hatched larvae.
Long-term ammonia exposure of fishes, including complete life-
cycle tests on rainbow trout and fathead minnows with several end-
points, including effects on spawning and egg incubation, growth,
survival and tissues, have been studied. The effects of prolonged
exposure to ammonia (up to 61 days) on the early life stages of of
pink salmon were studied by Rice & Bailey (1980). Three series of
exposures were carried out, beginning at selected times after
hatching. These were for 21 days prior to completion of yolk
absorption, for 40 days up to 21 days before yolk absorption, and
for 61 days up to yolk absorption. All test fish were sampled for
size when the controls had completed yolk absorption. Test
concentrations ranged from zero up to 0.004 mg NH3/litre. For fry
at the highest concentration of 0.004 mg NH3/litre, significant
decreases in weight were observed in all 3 exposed groups. At a
concentration of 0.0024 mg NH3/litre, the groups of fry exposed for
40 and 61 days were significantly smaller, whereas a concentration
of 0.0012 mg/litre had no significant effect on growth. Effects
were consistently more marked for the 61-day-exposed fish.
In a 3-generation, 5-year laboratory study, rainbow trout
exposed for 5 months to concentrations of ammonia ranging from 0.01
to 0.07 mg NH3/litre, spawned of their own volition. There was no
correlation between ammonia concentration and numbers of egg lots
spawned, total numbers of eggs produced, or numbers of eggs
subsequently hatched. Parental fish were exposed for 11 months,
the first filial generation (F1) for 4 years, and the second filial
generation (F2) for 5 months. Pathological lesions were observed in
both parental and F1 fish, when ammonia concentrations reached and
exceeded 0.04 mg NH3/litre. Measurements of blood-ammonia
concentrations in 4-year-old F1 fish showed an increase when test
water concentrations reached or exceeded 0.04 mg NH3/litre. The F1
fish exposed for 52 months from day of hatching showed no
relationship between growth and concentration at 10, 15, 21, and 52
months (Thurston et al., 1984b).
Burkhalter & Kaya (1977) tested ammonia at concentrations
ranging from 0.06 to 0.45 mg/litre on fertilized eggs and the
resultant sac fry of rainbow trout. Eggs were incubated at 12 °C
for 25 days in one test and at 10 °C for 33 days in another; fry
were maintained for 42 days. No concentration response was seen in
egg mortality or incubation time in either test. Retardation in
early growth and development occurred at 0.06 mg NH3/litre, the
lowest concentration tested. Fish exposed to 0.12 mg NH3/litre
required 1 week longer than the controls to achieve a free-swimming
state; fish at 0.34 and 0.45 mg NH3/litre did not achieve a free-
swimming state during a 42-day test period. A 21-day LC50 value of
0.30 mg NH3/litre was obtained. For sac fry exposed for 42 days
after hatching, hypertrophy of secondary gill lamellae epithelium
occurred at 0.23 mg NH3/litre, and karyolysis and karyorrhexis in
the secondary gill lamellae were observed after 28 days at 0.34 mg
NH3/litre and higher.
Calamari et al. (1977, 1981) exposed rainbow trout to
ammonium chloride solutions for 71 days, beginning 1 day after
fertilization and ending when fry had been feeding for 30 days.
A 72-day LC50 of 0.056 mg NH3/litre was calculated; 23% mortality
occurred at a concentration of 0.025 mg/litre. Examination of 986
rainbow trout embryos at the hatching stage after exposure to
concentrations of 0.010 - 0.193 mg NH3/litre for 24 days showed
an increase in gross malformations with increasing ammonia
concentration. The deformities observed were various degrees
of curvature from the median body axis, and various kinds of
malformations in the head region with a number of cases of double
heads. At the highest concentration tested, 0.193 mg NH3/litre,
60% of the observed fish were malformed. Microscopic examination,
at hatching, of 128 larvae from the same exposure showed
abnormalities of the epidermis and pronephros, which were
correlated with ammonia concentrations. The epidermis was
thickened with an irregular arrangement of the various layers of
cells and an increase in the number and dimensions of mucous cells.
The pronephros showed widespread vacuolization of the tubule cells,
together with a thickening of the wall. Increasing abnormalities
were observed after exposure to concentrations exceeding 0.025 mg
NH3/litre for the epidermis and 0.063 mg/litre for the pronephros.
Four 4-week-old rainbow trout fry were exposed for 30 days to
concentrations of ammonia (reported graphically) ranging from
~0.06 to 0.31 mg NH3/litre. Growth rate at ~0.06 mg NH3/litre was
comparable with that of controls, but, above ~0.10 mg NH3/litre,
growth rate decreased, in correlation with increased NH3
concentration. Survival at 0.32 mg NH3/litre was 70% of that of
the controls (Broderius & Smith, 1979). Schulze-Wiehenbrauck
(1976) tested juvenile rainbow trout of different sizes, for
periods of time ranging from 2 to 7 weeks, and at ammonia
concentrations ranging from 0.012 to 0.17 mg/litre. He concluded
that a concentration of 0.05 mg NH3/litre caused a slight decrease
in growth during the first 14-day interval in non-acclimatized
fish, but that the decrease was completely compensated for in the
next growth interval. Exposure to 0.13 mg NH3/litre (apparently
for 3 or 4 weeks) did not affect growth, food consumption, or food
conversion.
Young rainbow trout were reared in 3 concentrations of ammonia
(averaging 0.006, 0.012, and 0.017 mg/litre) for a period of 1
year. At 4 months, there was no significant difference in fish
growth at the 3 concentrations. At 11 months, there was a
difference with the fish at 0.012 and 0.017 mg NH3/litre, which
weighed 9% and 38% less, respectively, than the fish at 0.006 mg
NH3/litre. Microscopic examination of tissues from fish exposed to
the highest concentration, examined at 6, 9, and 12 months, showed
severe pathological changes in gill and liver tissues. Gills
showed extensive proliferation of the epithelium, which resulted in
severe fusion of gill lamellae preventing normal respiration.
Livers showed reduced glycogen storage and scattered areas of dead
cells; these became more extensive with increase in exposure time
(Smith, 1972; Smith & Piper, 1975).
Rainbow trout were exposed for 3 months to concentrations of
0.069, 0.14, and 0.28 mg NH3/litre. The cumulative mortality of a
control group (0.005 mg NH3/litre) was ~2%; cumulative mortality at
0.069 and 0.14 mg/litre was ~5%, and that at 0.28 mg/litre was ~15%
(United Kingdom Ministry of Technology, 1968). Reichenbach-Klinke
(1967) performed a series of 1-week tests on 240 fish of 9 species
(including rainbow trout, goldfish, northern pike, Esox lucius,
carp, and tench) at concentrations of 0.1 - 0.4 mg NH3/litre.
Swelling of, and diminution of the number of, red blood cells,
inflammation, and hyperplasia were observed. Irreversible blood
damage occurred in rainbow trout fry at concentrations above
0.27 mg NH3/litre. Low NH3 concentrations also inhibited the
growth of young trout and lessened their resistance to disease.
In rainbow trout exposed to 0.30 to 0.36 mg NH3/litre, 81%
mortality occurred over the 36-day duration of the test, with most
deaths occurring between days 14 and 21. Microscopic examination
of the gills revealed some thickening of the lamellar epithelium
and an increased mucous production. The most characteristic
feature was a large proportion of swollen, rounded secondary
lamellae in which the pillar system was broken down and the
epithelium enclosed a disorganized mass of pillar cells and
erythrocytes. Gill hyperplasia was not a characteristic
observation (Smart, 1976).
In rainbow trout exposed to < 0.0005 or 0.005 mg NH3/litre for
8 weeks, examination of the gill lamellae of fish from the lower
concentration showed them to be long and slender with no
significant pathology. Fish exposed to 0.005 mg NH3/litre had
shorter and thicker gill lamellae with bulbous ends, and some
consolidation of lamellae was noticed. Many filaments showed a
definite hyperplasia of the epithelial layer, evidenced by an
increase in the number of cell nuclei (Fromm, 1970).
Thurston et al. (1978) studied the toxicity of ammonia for
cutthroat trout fry in tests that lasted up to 36 days. Results of
duplicate tests on 1-g fish showed 29- and 36-day LC50 values of
0.56 mg NH3/litre. Duplicate tests on 3-g fish provided 29-day
LC50 values of 0.37 and 0.34 mg NH3/litre, slightly less than those
of the 1-g fish. The heart, gastrointestinal tract, and thymus of
cutthroat trout fry exposed to 0.34 mg NH3/litre for 29 days were
comparable with those of control fish, but the gills and kidneys
showed degenerative changes. The gills showed hypertrophy of
epithelium, some necrosis of epithelial cells, and separation of
epithelium due to oedema. The kidneys had mild hydropic
degeneration and accumulation of hyaline droplets in the renal
tubular epithelium. Reduced vacuolation was observed in livers.
Samylin (1969) studied the effects of ammonium carbonate on the
early stages of development of Atlantic salmon. The first set of
studies, at 13 °C, lasted 53 days and was conducted within the
range 0.001 to > 6.6 mg NH3/litre beginning with the "formed
embryo" stage. Accelerated hatching was observed with increasing
(NH4)2CO3 concentrations, but concentrations of > 0.16 mg
NH3/litre were lethal for emerging larvae within 12 - 36 h.
Because (NH4)2CO3 was used as the toxin, the pH in the test aquaria
increased from 6.7 to 7.6 with increasing NH3 concentration. Growth
inhibition was observed at 0.07 mg NH3/litre. Tissue changes were
observed in eyes, brains, fins, and blood of Atlantic salmon
embryos and larvae exposed to concentrations ranging from 0.16
to > 6.6 mg NH3/litre, with more marked changes at higher ammonia
concentrations. The effects observed included erosion of membranes
of the eyes and shedding of the crystalline lens, dilatation of
blood vessels in the liver and brain, accumulation of blood in the
occipital region and in the intestines. Reaction to light and
mechanical stimulation gradually disappeared with increased ammonia
concentration, and the heart rate slowed. Morphological
differences in development between experimental and control larvae
were observed from the tenth day of exposure, including a lag in
yolk resorption, decrease in growth of the skin fold, and
contraction of skin pigment cells causing the skin colour to become
paler than it was after hatching. At concentrations up to 0.07 mg
NH3/litre, no significant morphological differences were observed.
A second series of studies, at 16.5 °C, was carried out in
the 0.001 - 0.32 mg NH3/litre concentration range, beginning
with larval salmon (Samylin 1969). Concentrations of > 0.21
mg/litre were lethal and caused weight loss in fry; 0.001 -
0.09 mg NH3/litre caused a decrease in weight gain, though there
were no differences in feeding activity, behaviour, or development
at these concentrations compared with controls. Dissolved oxygen
concentrations in this second series of studies dropped as low as
3.5 mg/litre.
Burrows (1964) tested fingerling chinook salmon for 6 weeks in
outdoor water channels into which ammonium hydroxide was
introduced. Two studies were conducted, one at 6.1 °C and the
other at 13.9 °C, both at pH 7.8. The fish were then maintained in
fresh water for an additional 3 weeks. A recalculation of the
reported non-ionized ammonia concentrations, based on more recent
aqueous ammonia equilibrium tables, shows that the concentrations
at 6.1 °C were 0.003 - 0.006 mg NH3/litre and, at 13.9 °C, were
0.005 - 0.011 mg NH3/litre. At both temperatures, and at all
ammonia concentrations, some fish showed excessive proliferation
and clubbing of the gill filaments. The proliferation was
progressive for the first 4 weeks, after which no measurable
increase was observed. After 3 weeks in fresh water, examination
of fish exposed at 6.1 °C indicated that recovery from the
extensive proliferation had not taken place. In the study on larger
fish at 13.9 °C, a marked recovery from hyperplasia was noted after
the 3 weeks in fresh water. In the first study, the proliferated
areas had consolidated; in the second, they had not. It was
postulated that continuous ammonia exposure is a precursor of
bacterial gill disease.
Duplicate groups (90 fish each) of hatchery-reared coho salmon
were exposed for 91 days to "river-water" solutions of ammonium
chloride at concentrations of 0.019 - 0.33 mg NH3/litre. Control
groups were reared at 0.002 mg/litre. Haemoglobin content and
haematocrit readings were slightly, but significantly, reduced in
fish exposed to the highest concentration tested, and there was
also a greater percentage of immature erythrocytes. Blood-ammonia
and -urea concentrations were not significantly different after 91
days, regardless of the concentration of ammonia to which the fish
were exposed (Buckley et al., 1979). Rankin (1979) exposed embryos
of sockeye salmon, Oncorhynchus nerka, to ammonia from fertilization
to hatching. Total embryo lethality occurred at concentrations of
0.49 - 4.9 mg NH3/litre. The times required to achieve 50%
mortality at these concentrations were 40 - 26 days. Mortality of
the embryos exposed to 0.12 mg NH3/litre was 30%, and time to 50%
mortality was 66 days.
Two full life-cycle ammonia toxicity tests, each lasting
approximately 1 year, were conducted on fathead minnows (Thurston
et al., in press). These tests began with newly hatched fry and
were continued through their growth, maturation and spawning
stages; progeny were exposed from hatching through growth to 60
days of age. While no statistically-significant differences were
observed in survival, growth, egg production, and egg viability, at
concentrations up to 0.4 mg NH3/litre, effects were seen at 0.4 mg
NH3/litre.
Tissues from fathead minnows subjected to prolonged (up to 304
days) ammonia exposure were examined (Smith, 1984). Growths, some
massive, were observed on the heads of several fish exposed to
concentrations of 1.25 or 2.17 mg NH3/litre, and swollen darkened
areas were observed on the heads of several fish exposed to 0.639 -
1.07 mg NH3/litre. Thurston et al. (in press) also reported
lesions at concentrations below those at which other effects were
observed. Brain lesions were common at concentrations of 0.21 mg
NH3/litre and higher. Grossly and histologically, the severity of
the lesions, which varied from mild to severe, was positively
correlated with ammonia concentration. The lesions appeared to be
of a cell type originating from the meninx primativa covering the
brain. The hyperplastic tissue often completely surrounded the
brain but was not observed around the spinal cord.
An early life-stage test initiated at the blastula stage of
embryogenesis and extending through 39 days post-hatching was
conducted on green sunfish (McCormick et al., 1984). Retardation
of growth was found in green sunfish exposed from embryo through
juvenile life stages to concentrations of 0.489 mg NH3/litre or
more, but not at 0.219 mg NH3/litre. In a long-term test on green
sunfish, Jude (1973) reported that, at levels higher than 0.17 mg
NH3/litre, mean fish weight increased less rapidly than that of the
controls on the 4 days following the introduction of ammonia.
Thereafter, fish exposed to 0.26 and 0.35 mg NH3/litre grew at an
increasing rate, while fish exposed to 0.68 and 0.64 mg NH3/litre
remained the same for 12 days before increases in growth occurred.
Four simultaneous early life-stage ammonia tests with
smallmouth bass were carried out at 4 different pH levels, ranging
from 6.6 to 8.7, in order to examine the effect of pH on chronic
ammonia toxicity. Exposure to ammonium chloride solutions began on
2- to 3-day old embryos and lasted for 32 days. The end-point
observed was growth, and ammonia was found to have a greater effect
on growth at lower pH levels than at high. Concentrations found to
retard growth ranged from 0.056 mg/litre at pH 6.60, to 0.865
mg/litre at pH 8.68 (Broderius et al., in press).
In early life-stage tests (29 - 31 days' exposure) on channel
catfish and white sucker, no significant effects on percent hatch
or larval survival were observed for channel catfish exposed to
ammonium chloride at concentrations as high as 0.583 mg NH3/litre
and for white sucker at concentrations as high as 0.239 mg
NH3/litre. Significant retardation of growth, however, occurred in
channel catfish at concentrations of 0.392 mg NH3/litre or more and
in white sucker at 0.070 mg NH3/litre and higher. A delay in time
to swim-up stage was also observed for both species at elevated
(0.06 - 0.07 mg/litre) ammonia concentrations (Reinbold &
Pescitelli, 1982a).
In cultured channel catfish fingerlings, exposed for periods
of approximately 1 month to concentrations of 0.01 - 0.16 mg
NH3/litre, growth at 0.01 and 0.07 mg NH3/litre was not
significantly different from that of control fish, but growth
retardation at 0.15 and 0.16 mg NH3/litre was statistically
significant (Robinette, 1976). Colt (1978) and Colt &
Tchobanoglous (1978) reported retardation of growth of juvenile
channel catfish during a 31-day period of exposure to
concentrations ranging from 0.058 to 1.2 mg NH3/litre. Growth
rate was reduced by 50% at 0.63 mg NH3/litre, and no growth
occurred at 1.2 mg NH3/litre.
6.5. Wild and Domesticated Animals
6.5.1. Wildlife
Although ammonia has been known to be toxic for nearly a
century (Hahn et al., 1983), studies describing the toxicological
effects of ammonia on wildlife are very limited. Normally,
atmospheric ammonia does not appear to be a problem for wild
animals, but concentrations of ammonia could reach harmful levels
in accidents during transport near forests and remote areas. NRC
(1979) has reported 2 types of observations in relation to this
topic: (a) the use of anhydrous ammonia to exterminate wild birds
and mice in farm buildings; and (b) the tolerance of bats to
atmospheric ammonia.
The use of anhydrous ammonia has been recommended for
exterminating wild birds and mice from farm buildings by Day et al.
(1965). The technique is simple, economical, and does not leave
any harmful residue. The farm buildings, after removal of the
livestock, were sealed and treated with anhydrous ammonia at 1600
mg/m3 (2285 ppm) for 7 min and then reopened. Ammonia fumes were
fatal for the wild inhabitants, particularly for wild birds.
Within 0.5 h, dead starlings, sparrows, pigeons, and mice were
removed from the barns. Farm animals were allowed to enter the
barns within 1 h of their reopening. According to laboratory
studies, the mouse appears to be more sensitive than other animal
species such as the rat, rabbit, and guinea-pig. When mice were
exposed for 10 min to ammonia at 6140 - 9060 mg/m3 (8770 - 12 940
ppm), death with convulsion began after 5 min of exposure, and over
50% of the mice died before the study was completed. The surviving
animals appeared to recover rapidly, but another 4% died between
the 6th and 10th days after exposure (Underwriters Laboratories,
1933).
Large colonies of Guano bats (Tadarida brasiliensis) frequently
inhabit caves or other areas, producing large amounts of guano,
which, on bacterial decomposition, results in a very high
concentration of ammonia in the atmosphere. Although high ammonia
concentrations, together with high relative humidity in caves,
discoloured the pelage of bats (Eads et al., 1955; Constantine,
1958; Mitchell, 1964), no other adverse physiological effects were
observed in these mammals. This apparent adaptation to inhaled
ammonia prompted laboratory studies relating to the physiological
mechanisms involved in ammonia tolerance in different species of
bats (Mitchell, 1963; Studier, 1966; Studier et al., 1967).
California leaf-nosed bats (Macrotus californicus) can
tolerate exposure to 2100 mg/m3 (3000 ppm) for up to 9 h (Mitchell,
1963).
Ammonia toxicity at lethal doses was manifested by corrosion
of the skin and mucous membranes, pulmonary oedema, and distinct
visceral damage. The blood-non-protein nitrogen in the exposed
bats was significantly elevated without any increase in urinary-
urea or -ammonia.
Studier et al. (1967) studied the effects of increasing
concentrations of atmospheric ammonia on ammonia tolerance and
metabolic rates in rats, mice, and 3 species of bat. Rats, mice,
and 2 species of bat (Myotis lucifugus and Eptesicus fuscus)
tended to show increased oxygen utilization, when exposed to
increased ammonia levels. However, the guano bat (Tadarida
brasiliensis) exhibited a large decrease in oxygen utilization
with increasing ammonia concentration (i.e., 74% depression) when
exposed to air containing 4900 mg/m3 (7000 ppm) of gaseous ammonia.
Earlier, Studier (1966) had shown that ~35% of gaseous ammonia
filtered through the mucous linings in the respiratory passage in
guano bats, when they were exposed to 2100 mg/m3 (3000 ppm) of
ammonia. There was no change in their normal blood pH during
exposures to high ammonia concentrations. The animals, however,
exhaled measurable amounts of ammonia when transferred to normal
air.
6.5.2. Domesticated animals
6.5.2.1. Oral exposure
(a) Ruminants
The use of urea as a partial source of nitrogen in ruminant
nutrition is limited by its toxicity, which results from its
metabolism to ammonia.
The toxic effects of urea in ruminants are related to a high
ammonia content in the blood. Urea itself is not as toxic as the
ammonia, which is rapidly released in the rumen by the action of
bacterial urease (EC 3.5.1.5) on ingested urea (Bloomfield et al.,
1960). The absorption of this excess ammonia has been shown to
depend on the pH of the ruminal contents.
Hogan (1961) examined the effects of pH on the absorption of
ammonia from the rumen in sheep. When an ammonia-containing buffer
at pH 6.5 was placed in the rumen, absorption increased with the
concentration gradient. At a pH of 4.5, however, the concentration
of ammonia in the rumen did not affect the absorption across the
epithelium. The net loss of ammonia-nitrogen from the rumen at pH
6.5 was more than 3 times the loss at a pH of 4.5.
Additional support for the effect of pH on ammonia absorption
across the ruminal epithelium in sheep has been presented by
Bloomfield et al., (1962). As the pH of the ruminal contents
increased from 6.21 to 6.45, no ammonia was absorbed; however, as
the pH increased to 7.59, the absorption rate was 16 mmol/litre per
h. One sheep with a ruminal pH of 7.7 died of ammonia toxicity
within 30 min. These data support the hypothesis that the non-
ionized ammonia, which increases at higher pH, penetrates the lipid
layers of the ruminal epithelium more effectively than the charged
ammonium ion (Coombe et al., 1960).
Toxic signs become apparent as the blood-ammonia-nitrogen
increases to 10 mg/litre; tetanic spasms occur between 10 and
20 mg/litre and are followed by death (Repp et al., 1955; McBarron
& McInnes, 1968; Kirkpatrick et al., 1972, 1973; Webb et al.,
1972). Wilson et al. (1968a) attributed the cause of death to the
cardiotoxic effects of ammonia produced from the urea. However,
Singer & McCarty (1971) observed that only one sheep died of
ventricular fibrillation and the remainder of respiratory failure.
More recently, Edjtehadi et al. (1978) reported the arrest of
respiration, and not cardiovascular collapse, as the cause of death
in sheep.
Certain aspects of the blood chemistry have been described for
sheep with urea poisoning (Kirkpatrick et al., 1973; Edjtehadi et
al., 1978). In general, during the initial stages of urea
toxicosis, an alkalosis is induced, followed by systemic acidosis
due to hyperventilation prior to death (Edjtehadi et al., 1978).
In addition to increases in blood-ammonia and blood-urea levels,
there is a marked increase in the blood-glucose level, with no
change in ketone concentrations in the body (Singer & McCarty,
1971). The following changes have been recorded at death: red-
cell count and haemoglobin concentration increased by 7.9%; white-
cell count decreased by 27.5%; and packed-cell volume increased by
11.4%. Mean corpuscular volume, mean corpuscular haemoglobin, and
mean corpuscular haemoglobin concentration were not substantially
changed (Kirkpatrick et al., 1973).
Pathological effects of ammonia toxicity in sheep have been
described by Singer & McCarty (1971). The changes were similar
when sheep received intraruminal injections of ammonium chloride,
ammonium sulfate, or a mixture of ammonium chloride, carbonate,
phosphate, and sulfate. General passive hyperaemia and numerous
petechial and ecchymotic haemorrhages in the musculature, heart,
thymus, and lungs were found. The lungs were distended and
severely congested. On microscopic examination, the pulmonary
lesions included severe hyperaemia, haemorrhage, alveolar oedema,
and alveolar emphysema. In the thymus, there was degeneration and
necrosis of Hassall's corpuscles and centrilobular haemorrhages.
Lesions in kidneys included severe generalized cloudy swellings and
multiple foci of early coagulative necrosis of the proximal
convoluted tubules, general hyperaemia of the glomerular tufts, and
degeneration of the glomerular tuft cells.
In Marschang & Crainiceanu's (1971) study on the effects of
ammonia in the air of calf stables, ammonia concentrations
reportedly ranged from 0.7 to 140 mg/m3 (1 - 200 ppm). During
these periods of high ammonia concentration, high mortality rates
were observed among the calves. The authors suggested that the
high ammonia content weakened the resistance of the animals and
thus created conditions for the development of secondary
infections. Deaths were mainly caused by respiratory diseases.
Autopsy indicated various types of change in the lungs, chiefly
inflammation.
Air-ammonia concentrations in 3 cattle-fattening facilities in
Romania were measured by Marschang & Petre (1971), who found
concentrations ranging from 2 to 1400 mg/m3 (3 - 2000 ppm).
Morbidity (mainly from respiratory disease) and mortality rates
increased with ammonia concentrations in the stalls and decreased
as some of the toxic gas levels decreased to admissible
concentrations. The authors suggested that ammonia is the most
important environmental factor in producing disease in cattle-
fattening stalls. They did not refer to the growth rate of the
cattle; however, in an additional report, Marschang (1972) observed
a marked decrease in the growth rate of fattening cattle, when the
ammonia content of the stable was high.
(b) Monogastric animals
Monogastric animals are considered relatively tolerant to
dietary urea, since they lack the large amounts of bacterial urease
present in the rumen of ruminants. Horses may frequently consume
cattle rations that contain urea or other non-protein-nitrogen
sources.
Hintz et al. (1970) found that the urease activity in the
caecal fluid from ponies was 17 - 25% of that reported for bovine
rumen fluid. They subjected 8 ponies to oral doses of urea at 3.3
- 3.6 g/kg body weight, to study the toxic effects of urea
overdosage. Seven of the ponies died of ammonia toxicity, 3 - 12 h
after treatment. Clinical signs of toxicosis were characteristic
of severe central nervous system derangement. These signs were
similar to those previously reported for ruminants, with the
exception of head pressing against a fixed object prior to loss of
coordination. No significant gross lesions were observed on
necropsy. Blood-ammonia increased linearly until death. Blood-
alpha-keto-glutarate decreased initially, reached minimal values
at about 30 min and then increased to 3 times the zero time value.
Blood-glucose remained constant for the first 2.5 h and then
increased to about 3 times the initial value. Blood-pyruvate
decreased during the first 3.5 h and then increased to 10 times
the initial values.
6.5.2.2. Inhalation exposure
(a) Swine
The acute inhalation effects of ammonia in swine are given in
Table 15.
(b) Poultry
As discussed previously, poultry are exposed to ammonia in the
atmosphere of poultry houses; this ammonia is released from the
action of bacteria on poultry wastes. The toxic effects of this
exposure are primarily seen in the eyes and respiratory tract.
An idiopathic ocular disorder in young chicks, designated
keratoconjunctivitis, was first described by Bullis et al. (1950),
who attributed it to environmental factors in the rearing
facilities.
Anderson et al. (1964a) reported that chickens exposed
continuously to ammonia at 14 mg/m3 (20 ppm) showed some signs
of discomfort, including rubbing of the eyes, slight lachrymation,
anorexia, and, later, weight loss. Chickens exposed to ammonia at
14 mg/m3 for as little as 72 h were more susceptible to aerosol
infection with Newcastle disease virus. Gross and microscopic
damage to the respiratory tract could be detected after 6 weeks of
continuous exposure to ammonia at 14 mg/m3. Valentine (1964)
reported tracheitis in chicks exposed to ammonia at 42 - 49 mg/m3
(60 - 70 ppm). The breathing of the birds was audible as moist
rales with bubbling sounds. At post-mortem examination, some of
the birds had slight congestion of the lungs with excess mucous in
the respiratory tract. The mucous membranes of the trachea were
much thicker than in the control birds, and there was leukocytic
infiltration of the tissue. It was suggested that this tracheitis
may predispose the affected birds to respiratory diseases with the
added risks of secondary infections.
Charles & Payne (1966a) reported that exposure to atmospheric
ammonia at 70 mg/m3 (100 ppm) caused a reduction in carbon dioxide
production and depth of respiration and a 7 - 24% decrease in the
respiration rate of laying hens. The authors also observed that
broilers reared to 28 days of age in atmospheres containing high
concentrations of ammonia consumed less food and grew more slowly
than unexposed chickens. Pullets reared in high-ammonia
atmospheres matured up to 2 weeks later than pullets reared in
ammonia-free atmospheres.
Airsacculitis, one of many respiratory diseases in poultry, has
been associated with high ammonia concentrations in poultry houses
(Ernst, 1968). High concentrations of dust were also noted during
periods of winter confinement, when high ammonia concentrations
were observed. The incidence and severity of air-sac lesions in
turkeys increased signficantly with high concentrations of dust
(0.6 - 1.0 mg/m3 or 21 - 35 mg/m3) in the atmosphere. Flocks with
a high rate (47%) or a low rate (2%) of infection with Mycoplasma
meleagridis were similarly affected. No significant interaction
between dust and ammonia concentrations (up to 21 mg/m3 or 30 ppm)
with regard to effects on the development of air-sac lesions was
found. Mortality rate and feed conversion were not significantly
affected by exposure to dust and ammonia. There was considerable
loss of cilia from the epithelium of the tracheal lumen and an
increase in mucous-secreting goblet cells in turkeys exposed to
high concentrations of dust and ammonia. Areas of consolidation
and inflammation were frequently observed in the lungs of these
turkeys. The air-sac lesions ranged from mild (lymphocytic
infiltration) to severe (masses of gaseous material).
Airsacculitis has also been experimentally induced in chickens
exposed to atmospheric ammonia and the stress of infectious
bronchitis vaccination (Kling & Quarles, 1974). Eighty Leghorn
male chicks were maintained in 12 controlled-environment chambers.
Ammonia at 0, 17.5, or 35 mg/m3 (0, 25, or 50 ppm) was introduced
into the chambers from the 4th to 8th weeks of age. An infectious
bronchitis vaccination was administered to all chicks at 5 weeks of
age. Body weights and feed efficiencies were determined at 4, 6,
and 8 weeks. At 4, 5, 6, and 8 weeks, lung and bursae of Fabricius
weights, haematocrits, and air-sac scores were determined. Body
weights and feed efficiencies were significantly reduced in the
ammonia chambers. The bursae of Fabricius in the ammonia-stressed
chickens were significantly larger than those of controls at 5
weeks of age and significantly smaller at 8 weeks of age. Chickens
grown in ammoniated environments had significantly larger lungs at
8 weeks. Haematocrits were not significantly different among
treatments. Total air-sac scores were significantly higher in the
ammonia-stressed chickens at 8 weeks. The results indicated that
chickens were stressed by the ammonia at 17.5 or 35 mg/m3, and by
the infective bronchitis vaccination. In a similar study (Quarles
& Kling, 1974), exposure of broiler chicks to 17.5 or 35 mg/m3 (25
or 50 ppm) from the 4th to the 6th week resulted in the observation
of severe airsacculitis at 6 and 8 weeks of age. During the test,
airborne bacterial counts were significantly higher in chambers
with ammonia than in control chambers.
Table 15. Acute inhalation effects of ammonia in swine
---------------------------------------------------------------------------------------------------------
Ammonia concentration Dose Effects Reference
---------------------------------------------------------------------------------------------------------
196 mg/m3 (280 ppm) single frothing of the mouth and excessive Stombaugh et al. (1969)
secretion; after 36 h, convulsions
occurred, and breathing was extremely
short and irregular; the effects
ceased after a few h
7, 35, 70, or 105 mg/mg3 5 weeks high concentrations (70 and 105 mg/m3) Stombaugh et al. (1969)
(10, 50, 100, or 150 ppm) appeared to cause excessive nasal,
lachrymal, and mouth secretion after
3 - 4 days of exposure at 35 mg/m3,
the secretory rate was only slightly
higher than that in control animals;
after 1 - 2 weeks of exposure, the
signs noted appeared to lessen
gradually; examination of respiratory
tract did not reveal any significant
gross- or microscopic differences
related to ammonia exposure
0, 35, 70, or 105 mg/m3 4 weeks decrease in pig growth was noted at Drummond et al. (1980)
(0, 50, 100, or 105 ppm) all concentrations; at 35 or 70 mg/m3,
pigs converted feed to body weight
gain more efficiently than either
controls or pigs exposed to 105 mg/m3;
an acute imflammatory reaction in the
tracheal epithelium and a mild-to-
heavy exudate in the turbinate lumen
were observed at 70 and 105 mg/m3,
only
0 and 70 mg/m3 (100 ppm) 2 - 6 conjunctival irritation after the Doig & Willoughby (1971)
(1 - 7 weeks old) weeks first day which persisted for 1 week;
0 + dust dust alone had no effect; histopatho-
(100 ppm + dust) logical changes were limited to the
nasal and tracheal epithelium; there
was no evidence of structural damage
in the bronchial epithelium or alveoli
---------------------------------------------------------------------------------------------------------
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The authors suggested that the quality of eggs left all day in hen
houses containing high concentrations of ammonia might be adversely
affected.
7. KINETICS AND METABOLISM
Ammonia, a by-product of protein and nucleic acid metabolism
and a minor component of the diet, is in a state of flux in the
body, though it is present in low steady-state concentrations in
body fluids. In animals, metabolically-produced ammonia is
conjugated and excreted. Toxicity will only occur if these
conjugation and excretion mechanisms are defective, or if they are
overwhelmed by excessive exposure.
7.1. Absorption
7.1.1. Respiratory tract
Egle (1973) studied the retention, over a short period of time,
of inhaled ammonia in air at concentrations in the range 150 -
500 mg/m3 (214 - 714 ppm), in mongrel dogs of both sexes (7 - 37
per study). Retention was not materially affected by respiratory
rate, tidal volume, or concentration. Retention by the whole
respiratory tract averaged 78%, but the complexity of the dynamics
of this retention is illustrated by the fact that when respiration
was via an endotracheal tube, limiting exposure to the lower
respiratory tract, and when the upper respiratory tract (muzzle to
tracheal bifurcation) was perfused tidally, the retention was 78%
in each case. However, unidirectional perfusion of the upper
respiratory tract with ammonia in air produced a higher mean
retention of 89%.
Schaerdel et al. (1983) exposed 4 groups of rats, 8 per group,
to average ammonia concentrations of 11, 23, 220, or 826 mg/m3
(15, 32, 310, or 1157 ppm) for 24 h. Ammonia in blood was measured
at 0, 8, 12, and 24 h. At the 2 lowest concentrations, there was
no increase in blood-ammonia. However, after 8 h at 220 and
826 mg/m3, significant increases of 0.192 and 0.244 mmol (3.26 and
4.18 mg/litre) were noted. After 12 and 24 h, the increases were
not so marked, indicating an increase in ammonia metabolism.
In a study by Silverman et al. (1949), 7 male volunteers were
exposed to 350 mg/m3 (500 ppm) for 30 min. Initial ammonia
retention was not reported for all subjects, but, in one instance,
was around 75%. Retention decreased progressively until at
equilibrium it was 23% (range 4 - 30%); equilibrium was reached in
10 - 27 min. Some irritation was noted in the nose and throat,
leading to the suggestion that ammonia at this concentration was
primarily absorbed by the upper respiratory tract. Levels of
blood-urea-nitrogen (BUN), non-protein nitrogen, urinary-urea, and
urinary-ammonia remained normal.
In another study (Kustov, 1967), exposure of human volunteers
to ammonia for a longer duration (14 mg/m3 (20 ppm) for 8 h) was
accompanied by a statistically-significant increase in BUN from
23.9 to 30 mg%.
An early study was conducted by Landahl & Herrmann (1950) on
the retention of gases by the human nose and lung. At ammonia
concentrations of between 40 and 350 mg/m3 (57 and 500 ppm) and a
mean minute volume of 6 - 7 litre/min, for short durations (< 2
min), they found that approximately 92% ± 2% was retained in the
respiratory system (i.e., mouth, lungs, etc.) in 2 male volunteers,
tested 4 times. Differences in concentrations of ammonia did not
affect retention values. In a separate study, about 83% was
retained in the nasopharnyx at a flow rate of 18 litre/min, but
only 63 - 71% was retained when the flow rate was tripled. These
data are consistent with, though somewhat higher than, those
reported by Egle (1973) for exposure in dogs.
It should be noted that experimental animals kept in cages may
be exposed to relatively high concentrations of ammonia, even
exceeding 100 mg/m3, due to the degradation of urea in urine and
faeces (Flynn, 1968; Schaerdel et al., 1983).
Because ammonia is very water soluble, and thus absorbed by the
mucous coating in the upper respiratory tract, the lungs are
protected from the effects of exposure to low concentrations of
ammonia (Haggard, 1924; Boyd et al., 1944). At the levels of
ammonia associated with ambient air (i.e., 1 - 200 µg/m3), very
little, if any, is absorbed through the lungs.
If a person breathes an ammonia concentration in air of
18 mg/m3 (a common occupational exposure limit) at 1 m3/h and,
if all the ammonia is retained, then 18/60 = 0.3 mg ammonia/min
would require to be cleared by hepatic blood flow (say 1
litre/min). The rise in systemic blood-ammonia would be calculated
at 0.3 mg/litre or 0.018 mmol. If the more realistic assumption of
30% retention were used, the corresponding increase in blood-
ammonia concentration would be 0.09 mg/litre, about µmol. An
arterial fasting ammonia concentration of 1.05 mg/litre has been
reported in healthy subjects (Conn, 1972), so the calculated rise
is only 10% over fasting levels.
7.1.2. Gastrointestinal tract
Ammonia is a trace compound in foods. Ammonia that is absorbed
from the intestinal tract arises primarily from the bacterial
degradation in the intestine of amino and nucleic acids from
ingested food, endogenous epithelial debris, and mucosal cell
luminal secretions, or from the hydrolysis of urea diffusing from
the systemic circulation into the intestinal tract. The estimated
ammonia production from various substrates in the human intestines
ranges from 10 mg/day in the duodenum to 3080 mg/day in the colon
and faecal contents. Nearly all the ammonia formed is absorbed
(about 99% or 4000 mg). In healthy individuals, absorbed ammonia
is mainly catabolized rapidly in the liver to urea; therefore,
relatively small amounts reach the systemic circulation after
absorption from the gastrointestinal tract as a consequence of this
"first pass effect" (Summerskill & Wolpert, 1970).
Castell & Moore (1971) have shown that ammonia uptake from the
human colon, the major site of ammonia production, increases with
increased pH of the luminal contents. A similar effect of pH has
been shown for the absorption of ammonia from the rumen of sheep
(Hogan, 1961; Bloomfield et al., 1962). Since an increase in pH
increases the proportion of non-ionized ammonia, the authors
concluded that simple non-ionic diffusion was responsible for the
majority of ammonia transport. Evidence also exists for the active
transport of the ammonium ion from the intestinal tract. Castell &
Moore (1971) showed that ammonia transport by the human colon,
though greatly diminished, still occurred when the luminal pH was
reduced to 5, at which value non-ionized ammonia would be virtually
absent. Mossberg & Ross (1967) and Mossberg (1967) studied the
absorption of ammonia from isolated intestinal loops of the golden
hamster and found that the ileal movement of ammonia against a
concentration gradient was inhibited by cyanide, dinitrophenol, and
anaerobiosis. This suggested that an energy-dependent transport
system was operable in the ileum, where ammonia was absorbed
preferentially, but not in the jejunum.
Membrane transport of the ammonium ion by the human erythrocyte
has been demonstrated (Post & Jolly, 1957).
7.1.3. Skin and eye
Ammonia is highly mobile in all tissues and the ammonium ion
readily penetrates the corneal epithelium. Within 5 seconds,
traces are present in the anterior chamber of the eye (Siegrist,
1920). However, systemic and intra-ocular absorption by these
routes are not quantitatively important.
7.2. Distribution
The ammonia normally present in all tissues in the body
constitutes a dynamic pool throughout which absorbed ammonia is
distributed. The distribution of total ammonia between body
compartments is strongly influenced by pH. The non-ionized NH3 is
freely diffusible, whereas NH4+ is less diffusible and relatively
confined in compartments. The lower the pH of a compartment, the
greater its total ammonia content (NRC, 1979).
The fate of absorbed ammonia molecules has been studied in man,
by measurement of blood constituents, and, in experimental animals,
by following the distribution of 15N after the administration of
15NH3 and compounds.
7.2.1. Human studies
In human beings, inhalation of ammonia (350 mg/m3; 500 ppm) for
30 min did not have any effect on blood-nitrogen levels (Silverman
et al., 1949). In another study, exposure of human subjects to
14 mg ammonia/m3 (20 ppm), for a duration of 8 h, revealed a
statistically-significant increase in BUN (from 23.9 to 30 mg%)
(Kustov, 1967). However, this is unlikely to have represented the
metabolic conversion of absorbed ammonia, since the increase was
far greater than could have been accounted for by the quantity of
ammonia inhaled.
Administration of 9 mg NH4Cl/kg body weight, orally, to 20
healthy adult male and female volunteers caused a transient
increase in ammonia concentrations in arterial blood in
approximately half of the subjects. Concentrations peaked (mean,
1.4 mg NH3/litre) at 15 min and returned to fasting levels (mean,
1.05 mg NH3/litre) by 30 min. However, in 50 male patients with
cirrhosis of the liver, blood-ammonia levels increased from already
elevated fasting levels (mean, 1.56 mg NH3/litre) to much higher
peak concentrations (mean, 3.7 mg NH3/litre) at 15 min, followed by
a slow decrease reflecting impaired hepatic urea synthesis. Blood-
ammonia levels, before and after administration of ammonium
chloride, were significantly higher among cirrhotic patients with
portacaval anastomoses than among patients lacking such shunts
(Conn, 1972).
7.2.2. Animal studies
The distribution, as well as the metabolic fate of ammonia,
depends on the route of administration. After intestinal
absorption, ammonium ions are primarily transformed by the liver
to urea, and subsequently excreted in the urine. In contrast,
intravenously-administered ammonium salts are more available as
non-essential nitrogen for protein synthesis (Furst et al., 1969).
However, some orally-administered ammonia, has been found to be
incorporated into tissue proteins. Incorporation of 15N was higher
in serum globulins than in albumin after intravenous dosing with
15N-ammonium salts, but this order was reversed after oral
administration (Furst et al., 1970). The amount incorporated into
protein by this route was greater, when protein intake was
restricted (Richards et al., 1968).
Duda & Handler (1958) analysed the tissue of rats, 15 min after
an intravenous injection of 15N-ammonium lactate, and found that
its major metabolites, glutamine, and urea, were quickly
distributed throughout the body. The highest levels of labelled
urea (in µmoles 15N/g tissue) were found in the kidney (0.0217) and
liver (0.0159), while lesser amounts were found in the heart
(0.0086), spleen (0.0067), brain (0.0029), testes (0.0027), and
carcass (0.0070). The highest levels of labelled glutamine (µmoles
15N/g tissue) were found in the heart (0.086) and liver (0.055) and
lesser amounts (0.005 to 0.032) in the brain, spleen, carcass,
kidney, and testes.
Vitti et al. (1964) examined the distribution of 15N from
ammonium citrate, administered by different routes, into the
proteins of various tissues of hypophysectomized rats. The liver,
kidney, and spleen contained greater concentrations of 15N
incorporated into proteins than heart or muscle fractions during
72 h following intragastric, intraperitoneal, and subcutaneous
administration of 15N-ammonium citrate. After the first 6 h,
during which the intragastric route gave higher values, the
quantity of 15N incorporated into liver-protein was not
substantially affected by the route of administration. In most of
the other tissues studied, however, 15N incorporation tended to be
least by the intragastric route, followed, in increasing order, by
the intraperitoneal and subcutaneous routes. By the last route,
more labelled ammonia was apparently made available to the widely
distributed glutamine-synthetase (EC 6.3.1.2) system (section
7.4.3).
7.3. Metabolic Transformation
Most organisms have mechanisms for conjugating ammonia into
non-toxic compounds for excretion. Terrestrial mammals synthesize
urea, which requires the concerted action of several enzymes of the
Krebs-Henseleit (urea) cycle. One of these enzymes, glutamine
synthetase (EC 6.3.1.2), was present in the brains of all
vertebrate species examined. Glutamine synthetase was also present
at significant levels in the liver in all organisms examined (Brown
et al., 1957).
Exogenous ammonia, administered intravenously as an ammonium
compound, is metabolized to glutamine as the major early product
(Duda & Handler, 1958). The ammonia fixed in glutamine may
eventually end up in amino acids, purines, pyrimidines, or other
nitrogen-containing compounds. Ingested ammonium chloride or
endogenous ammonia is absorbed into the portal vein and converted
in the liver to urea (Furst et al., 1969; Goodman & Gilman, 1970;
Pitts, 1971).
Results of studies on the metabolic fate of dietary ammonium
citrate (Foster et al., 1939) and intravenously-administered
ammonium lactate (Duda & Handler, 1958) in rats showed that urea
synthesis represented a nearly constant fraction of the
administered ammonia over a large concentration range. Besides
glutamine and urea, labelled nitrogen also appeared in creatine,
glycine, alanine, proline, histidine, arginine, glutamic acid, and
aspartic acid. Vitti et al. (1964) examined the incorporation of
15N from ammonium citrate into proteins of liver, heart, kidney,
spleen, and muscle fractions of untreated and growth hormone-
treated, hypophysectomized rats, and found differences in the
metabolic fate, depending on the route of administration.
Subcutaneous injection facilitated the labelling of amide nitrogen,
indicating extensive disposition via glutamine synthesis. In
contrast, intragastric or intraperitoneal administration resulted
in the labelling of arginine, glutamic acid, and other alpha-amino
acids of the liver. Amide-nitrogen was labelled to a much lesser
extent than by the subcutaneous route. The tissue distribution of
the label also differed according to the route of entry (section
7.2.2).
7.4. Reaction with Body Components
Ammonia-nitrogen is central in nitrogen metabolism and
therefore becomes incorporated in all proteins and nitrogen-
containing components in the course of metabolic turnover. Ammonia
does not react with body components in the manner of alkylating
agents or compounds that modify haemoglobin.
7.5. Elimination and Excretion
7.5.1. Expired air
Ammonia may be excreted through expired air. Hunt (1977)
reported human expired air levels of ammonia of between 105 and
2219 µg/m3; Larson (1977) reported values of between 196 and
1162 µg/m3, during mouth breathing. These values are higher than
those expected from equilibration with plasma- and lung-parenchyma-
ammonia levels (28 - 49 µg/m3). This is most likely due to the
synthesis of ammonia from salivary urea by oral microflora (Biswas
& Kleinberg, 1971). Measurable amounts of free ammonia were also
found in air expired by dogs given ammonium acetate intravenously
(Robin et al., 1959), and normal dogs and human beings with
hepatic-induced ammonia toxicity (Jacquez et al., 1957, 1959).
Bloomfield et al. (1962) reported the presence of free ammonia in
expired air from sheep during experimentally-induced urea toxicity.
Normal levels of ammonia in the expired air of the rat have been
reported to range from 7 to 247.1 µg/m3, with a mean of 54.6 µg/m3
in nose-breathing animals and 23.8 - 520.8 µg/m3, with a mean of
200.2 µg/m3 in tracheal-cannulated animals (Barrow & Steinhagen,
1980). The presence of ammonia in the expired air of human beings
and experimental animals suggests that reaction products may be
formed with a variety of airborne chemicals, thereby altering their
toxicity.
7.5.2. Urine and faeces
Free ammonia is excreted by ammonotelic organisms (e.g., fish),
uric acid by uricotelic animals (e.g., birds), and urea by
ureotelic animals (e.g., mammals). Mammals may also secrete
ammonia directly into the urine. Glutaminase (EC 3.5.1.2)
catalyses the release of ammonia in the kidney tubular epithelium,
where it serves as an acceptor of H+ and regulates the acid-base
balance (Van Slyke et al., 1943; White et al., 1973). In acidosis,
the renal concentration of glutaminase increases over several
days, paralleling the increased excretion of ammonium ions (Davies
& Yudkin, 1952; Muntwyler et al., 1956; Kamin & Handler, 1957);
two-thirds of the urinary-ammonia is contributed by this pathway
(Van Slyke et al., 1943), and approximately one-third by protein
metabolism and ammonia clearance from the plasma by the kidney.
Oral and intravenous administration of ammonium lactate to
healthy human volunteers produced different patterns of excretion,
reflecting the effective barrier of the liver in preventing
ingested ammonia from gaining access to peripheral circulation by
converting most of the ammonia load to urea. Urinary-ammonia
excretion was increased 8-fold and urea excretion was reduced by
one-half after intravenous injection, as opposed to oral
administration (Gay et al., 1969), probably due to the anabolism
involved in the "first pass" effect after oral administration.
Less than 1% of the 4 g total ammonia produced in the human
intestinal tract, per day, is excreted in the faeces (Summerskill &
Wolpert, 1970).
7.6. Retention and Turnover
Some nitrogen derived from absorbed ammonia is incorporated in
amino acids and proteins. The rate of ammonia-derived nitrogen
turnover is rapid, but depends on the nutritional state. Thus,
when 15NH4Cl had been administered orally to healthy male
volunteers, for one week, 70% 15N was excreted by those on a 70-g
protein/day diet, while only about 35% 15N was excreted by those on
20 g protein/day (Richards et al., 1968, 1975).
7.7. Uptake and Metabolism in Plants
Ammonia is used by many plants and preferentially by a few.
However, ammonia is toxic, and its uptake in large quantities may
put a severe strain on the carbohydrate metabolism of the plant in
the provision of carbon skeletons for its detoxification. The
absorption of ammonium usually is coupled with the exchange of
cations as H+. Ammonia-nitrogen functions as a nitrogen source
for the synthesis of amino acids, which are incorporated in
proteins. Plants that are able to absorb it in large amounts
include many acid plants, such as Rumex, which are able to detoxify
ammonia by forming ammonium salts of organic acids. "Amide
plants", such as beet, spinach, and squash, are able to form large
amounts of the amides, glutamine, and asparagine and can withstand
quite high concentrations of ammonium salts by detoxifying the
ammonia. Certain plants, such as rice, which live in water-logged
anaerobic soils, require NH3 or reduced organic nitrogen
fertilizer, alone.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Inhalation exposure
LC50 studies and studies to determine the threshold for
irritating effects on the respiratory system for the rat and mouse
are summarized in Tables 16 and 17, respectively.
Table 16. Lethal concentrations (1-h exposure) of
ammonia for rats and micea
----------------------------------------------------
Measured Species Mortality Mean weight gain
concentration ratiob of survivors at
(mg/m3) 14 days (g)
----------------------------------------------------
4347 rat 0/10 3.5
5474 rat 8/10 -c
6888 rat 9/10 -
controls rat - 21.4
2520 mouse 0/10 -0.2
3185 mouse 3/10 -0.7
4004 mouse 9/10 -
controls mouse - 1.6
----------------------------------------------------
a Adapted from: MacEwen & Vernot (1972).
b Number dead/number exposed.
c Not enough survivors for comparison.
The acute lethal dose of ammonia by inhalation has been
determined for both the rat and the mouse (MacEwen & Vernot, 1972).
The results are summarized in Table 16. Male CFE rats ranging in
weight from 200 to 300 g and male CF1 mice weighing from 20 to 30 g
(ICR derived) were exposed for 1 h to several concentrations of
ammonia. Inhalation of ammonia gas produced immediate nasal and
eye irritation followed by laboured breathing and gasping in all
test groups. In addition, convulsions were seen in mice.
Surviving rats necropsied after 14 days showed moderate mottling of
the liver, regarded as probable fatty infiltration, at the 5474 and
6888 mg/m3 (7820 and 9840 ppm) dose levels. Mice surviving the 2
highest dose levels of 3185 and 4004 mg/m3 (4550 and 5720 ppm)
showed mild congestion of the liver. Pathological lesions were not
seen in rats exposed to 4347 mg/m3 (6210 ppm) or mice exposed to
2520 mg/m3 (3600 ppm). The calculated 1-h LC50 values for the rat
and the mouse were 5137 and 3386 mg/m3 (7338 and 4837 ppm),
respectively.
In another inhalation study, an LC50 value for the rat, with a
2-h exposure, was 7600 mg/m3 (10 860 ppm) (Alpatov, 1964). In a
further study, the threshold for acute effects (depression, then
hyperactivity and convulsions) for a 2-h exposure was 85 mg/m3 (121
ppm) (Alpatov & Mikhailov, 1963).
Table 17. Single-dose inhalation studies (LC50)
----------------------------------------------------------
Species Exposure time LC50 Reference
(min) (mg/m3)
----------------------------------------------------------
rat 120 7600 Alpatov (1964)
rat 60 5137 MacEwen & Vernot (1972)
rat 5 18 693 Prokop'eva et al. (1973)
rat 15 12 160 Prokop'eva et al. (1973)
rat 30 7035 Prokop'eva et al. (1973)
rat 60 7939 Prokop'eva et al. (1973)
rat 10 31 612 Appelman et al. (1982)
rat 60 11 620 Appelman et al. (1982)
mouse 10 7060 Silver & McGrath (1948)
mouse 60 3386 MacEwen & Vernot (1972)
mouse 60 2960 Kapeghian et al. (1982)
----------------------------------------------------------
Kapeghian et al. (1982) reported an acute inhalation toxicity
study on male ICR mice, in which the 1-h LC50 with a 14-day
observation period was calculated to be 2960 mg/m3 (4230 ppm).
Lungs of mice that died during exposure were diffusely
haemorrhagic. Histology revealed acute vascular congestion and
diffuse intra-alveolar haemorrhage. A mild to moderate degree of
chronic focal pneumonitis was also seen. Focal atelectasis was
evident in survivors sacrificed after the observation period.
Liver damage was also seen in these mice. There was evidence of
swelling and increased cytoplasmic granularity of hepatocytes at
2408 mg/m3 (3440 ppm) and scattered foci of frank cellular necrosis
at 2954 mg/m3 (4220 ppm). At 3402 mg/m3 (4860 ppm), necrosis was
increased. The liver lesions may have resulted from the
compromised nutritional state of the mice. Follicular hyperplasia
in the spleen was also seen in surviving animals, but this was
absent in animals that died during exposure.
The acute LC50 in male and female Wistar rats was 31 612 mg/m3
(40 300 ppm) for a 10-min exposure, 20 017 mg/m3 (28 595 ppm) for a
20-min exposure, 14 210 mg/m3 (20 300 ppm) for a 40-min exposure,
and 11 620 mg/m3 (16 600 ppm) for a 60-min exposure (Appelman et
al., 1982). Survivors were observed for 14 days. Clinical signs
of restlessness, eye irritation, nasal discharge, mouth breathing,
and laboured respiration were seen during exposure. Gross necropsy
revealed haemorrhagic lungs in animals that died during the study
as well as in survivors. No histopathology was performed.
Prokop'eva et al. (1973) reported that white rats exposed to
high concentrations of ammonia (6000, 3000, 1000 mg/m3 or 6814,
4307, 1436 ppm) for periods of 5, 15, 30, and 60 min exhibited
dyspnoea, irritation of the respiratory tract and eyes, cyanosis of
the extremities, and increased excitability. The LC50 values for
inhalation exposures of 5 and 15 min were 18 693 mg/m3 (26 704 ppm)
and 12 160 mg/m3 (17 372 ppm), respectively, while for 30 and 60
min, the values were 7035 mg/m3 (10 050 ppm) and 7939 mg/m3 (11 342
ppm), respectively. Inhalation of ammonia at concentrations of
3000, 1000, or 300 mg/m3 (4307, 1436, 431 ppm) resulted in a drop
in static muscular tension, leukocytosis, prolongation of the
latent reflex time, increase in total protein and blood sugar,
increased oxygen consumption, and a rise in the level of residual
nitrogen. No changes were observed in rats exposed to a
concentration of ammonia of 100 mg/m3 (144 ppm), for 5, 15, 30, and
60 min. Animals exposed to high concentrations of ammonia (exact
concentration not specified) developed pneumonia (Prokop'eva et
al., 1973).
When exposed to toxic levels of ammonia, within 1 min, mice
exhibited excitement, closing their eyes immediately and gasping
(Silver & McGrath, 1948). Groups of 20 mice were exposed to
ammonia gas for 10 min at 9 concentrations ranging from 6100 to
9000 mg/m3 (8758 to 12 921 ppm). The median lethal concentration,
calculated from the mortality at 10 days, was 7060 ± 320 mg/m3
(10 152 ± 460 ppm). Most (93%) deaths occurred rapidly, due to
convulsions after 5 min of exposure. Survivors usually recovered
within 10 min.
The LC50 values for the rat and the mouse are summarized in
Table 17.
Dose-dependent ultrastructural changes in the terminal airways
of mice exposed for 3 - 60 min to ammonia (concentration
unspecified) included oedema of the alveolar epithelium,
development of intracapillary platelet thrombosis, increased
secretions by the Clara cells, presumed to be phospholipids, and an
increase in the number of empty lamellar bodies in the large
alveolar cells (Niden, 1968).
Twenty cats were anaesthetized and exposed to 700 mg/m3 (1000
ppm) for 10 min via endotracheal tube and then observed for up to
35 days (Dodd & Gross, 1980). All cats had severe dyspnoea,
anorexia, and dehydration, 24 h after exposure. Several measures
of pulmonary function were impaired. Gross pathology of the lungs
showed various degrees of congestion, haemorrhage, oedema,
interstitial emphysema, and collapse, all non-specific for any
post-exposure day. Bronchopneumonia was common, 7 days after
exposure.
Barrow et al. (1978) exposed male Swiss-Webster mice to
concentrations of ammonia ranging from 70 to 560 mg/m3 (100 -
1000 ppm) for 30 min. The average respiratory rate depression in
4 mice for each of 4 exposure levels was evaluated. The maximum
depression in respiratory rate at each exposure level occurred
within the first 2 min. The concentration expected to elicit a 50%
decrease in respiratory rate (RD50) in mice, calculated by a
regression equation for ammonia, was 212 mg/m3 (303 ppm) with 95%
confidence limits of 132 - 343 mg/m3 (188 - 490 ppm). Effects of
ammonia exposure such as bradycardia and peripheral
vasoconstriction accompanied respiratory rate depression at the
RD50 and above.
A concentration of 350 mg/m3 (500 ppm) was reported by Wood
(1979) to be the level at which unrestrained mice did not
consistently adopt avoidance measures on inhalation of ammonia.
Both this author and Barrow et al. (1978) claimed to offer more
sensitive end-points for assessing ammonia irritation. However,
the no-observed-adverse-effect level reported by Wood (1979) was
higher than the concentration determined by Barrow et al. (1978) to
elicit 50% depression of respiratory rates in mice.
Dalhamn & Sjoholm (1963) tested in vitro preparations of the
tracheas of 8 rabbits. Arrested ciliary activity was observed
after 5 min of exposure to ammonia at 350 - 700 mg/m3 (500 -
1000 ppm). In in vivo studies on rabbits, Dalhamn (1963) showed
that the level of ammonia entering the nasal cavity, necessary to
cause small changes in the rate of tracheal ciliary beating was
1400 mg/m3 (2000 ppm). This corresponds to a tracheal
concentration of approximately 70 mg/m3 (100 ppm).
When anaesthetized male rabbits were exposed to 8 ammonia
concentrations ranging from 700 to 14 000 mg/m3 (1000 - 20 000 ppm)
(Richard et al., 1978b), bradycardia appeared at 1750 mg/m3 (2500
ppm). Hypertension, cardiac arrhythmia, macroscopic lung changes,
and EEG abnormalities were also reported. Signs appeared more
rapidly at higher concentrations with the complete syndrome
appearing at 3500 mg/m3 (5000 ppm).
Mayan & Merilan (1972) exposed 16 adult female New Zealand
white rabbits to ammonia concentrations of 35 and 75 mg/m3 (50 and
100 ppm) for 2.5 h. The average decreases in respiratory rate,
which were 34 and 32.7%, respectively, were significantly ( P <
0.01) less than control values. There were no histopathological
changes in lung, liver, spleen, or kidneys.
Enzymatic alterations in rats following inhalation of low
levels of ammonia have also been reported. At concentrations of
approximately 20 - 121 mg/m3 (29 - 173 ppm), there was a
decrease in the activities of liver succinic dehydrogenase (EC
1.3.99.1), lactate dehydrogenase (EC 1.1.1.28), glucose-6-phosphate
dehydrogenase (EC 1.1.1.49), and adenosine triphosphatase (EC
3.6.1.3). Liver acid phosphatase (EC 3.1.3.2) activity was
increased (Zlateva et al., 1974).
When 180 mice were exposed for 10 min to ammonia at 6139 -
9058 mg/m3 (8770 - 12 940 ppm), death with convulsions began to
occur 5 min after exposure. One hundred mice died during the
exposure. The surviving animals (80) recovered rapidly; however,
7 died between the sixth and tenth days following exposure (NRC,
1979).
8.1.2. Oral exposure
The effects of ammonia and its compounds are mainly of 2 types.
The first is the effect of ammonia itself. The second is the
effect of the anion bound to the ammonium ion. Ammonium chloride,
especially, will mainly exert its effects in the mammalian body due
to the formation of hydrogen chloride. Most of the experimental
work concerning the oral route of administration has centred on
ammonium chloride, which has been used extensively in the study of
metabolic acidosis. There have been few studies in which attempts
have been made to identify the role of the ammonium ion and ammonia
in the effects (Table 18).
Median lethal doses (LD50s) of 4400 and 3100 mg/kg body weight
have been reported for ammonium sulfamate in the rat and the mouse,
respectively. (Vinokurova & Mal'kova, 1963). A similar figure for
the oral LD50 in the rat of 4520 (4070 - 5020) mg/kg was reported
by Bukhovskaya et al. (1966). Frank (1948) reported a lethal dose
for ammonium sulfate of between 3000 and 4000 mg/kg body weight in
the rat.
8.1.2.1. Effects of metabolic acidosis induced by ammonium chloride
The ingestion of ammonium chloride in doses of around 500 -
1000 mg/kg body weight per day, for periods ranging from 1 to 8
days, has induced metabolic acidosis in mice, guinea-pigs, rats,
rabbits, and dogs. However, Boyd & Seymour (1946) did not report
any toxic effects at doses of up to 1 g/kg body weight in rats,
rabbits, guinea-pigs, and cats (50 animals per group).
Clinical signs, depending on the severity of the acidosis,
include: a decrease in plasma- and urinary-pH; decreased appetite;
decreased carbon dioxide-combining power; an increase in BUN and
chlorides; an increase in plasma proteins; an increase in
haematocrit (haemoconcentration); increased gluconeogenesis;
increased phosphoenolpyruvate carboxykinase (EC 4.1.1.49) activity;
increased urinary ammonium; increased urea, sodium, chloride,
calcium, and titratable acid excretion; an increase in malate and
oxaloacetate concentrations in renal tissue; and decreased
concentrations of glutamine, glutamate, and alpha-ketoglutarate in
the kidney. Pulmonary oedema, central nervous system dysfunction,
and renal changes are reported to have occurred after ingestion of
ammonium chloride.
Susceptibility to ammonium chloride differs among species. For
instance, pulmonary oedema is produced in cats, but not in rabbits;
yet cats have been shown to be more resistant to oral poisoning by
ammonium chloride than other animals studied.
Age is an important factor in the response of rats to oral
doses of ammonium salts. Benyajati & Goldstein (1975) found that
the administration of a single dose of 5 mmol ammonium chloride/kg
body weight (267.5 mg/kg) by gavage to 7- to 12-day-old rats (39
animals) increased ammonia excretion by about 64%, within 4 h,
compared to an increase of 155% in 20 adult rats after similar
treatment.
Table 18. Selected studies on the acute oral effects of ammonia compounds in rats
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Urea drinking- female, Holtz- 6 concentration no renal hypertrophy Lotspeich (1965)
water man, 200 - of N equivalent
250 g to that of 0.28
mol/litre NH4Cl
for 7 days ad
lib
Citrate drinking- female, Holtz- 6 0.28 mol/litre no renal hypertrophy Lotspeich (1965)
water man, 200 - solution for
250 g 7 days ad lib
Chloride drinking- female, Holtz- 9 0.28 mol/litre metabolic acidosis; increase Lotspeich (1965)
water man, 200 - for 7 days, ad in kidney weight and total
250 g lib (about 1000 N; increased capacity to
mg/kg per day) produce ammonia from
glutamine in kidney; renal
hypertrophy; unilateral
nephrectomy plus acidosis
induced by NH4Cl caused a
greater hypertrophy than
either alone
------------------------------------------------------------------------------------------------------------
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Chloride diet female, 150 g 125 all on low- consumption of food inversely Motyl & Debski
protein diet; related to dosage of NH4Cl; (1977)
Group I: no dosage-related decrement in
NH4; Group II: weight gain; increase in LD50
1% NH4Cl (300 value for ip dose of 2.7%
mg/kg body NH4Cl in Groups II and III,
weight per indicating an increased
day); Group adaptive capacity for ammonia
III: 2% NH4Cl detoxification; LD50 value of
(380 mg/kg Group IV same as Group III;
body weight per LD50 value of Group V lower
day); Group IV: than Group I; dose-dependent
4% NH4Cl (560 decrease in blood-glucose
mg/kg body levels; decrease of muscle
weight per and liver glycogen; dose-
day); Group V: dependent increase in delta-
8% NH4Cl (540 ornithine transaminase and
mg/kg body ornithine transcarbamylase;
weight per day, increase in adenosine
for 7 days triphosphate concentration in
blood
------------------------------------------------------------------------------------------------------------
8.1.2.2. Organ effects following oral administration
(a) Lung and central nervous system
Toxic doses of ammonium salts induced acute pulmonary oedema in
rats, guinea-pigs, and cats, but not in rabbits given ammonium
chloride (6% aqueous solution) intraperitoneally or by gavage,
though the doses were sufficient to cause death. To induce similar
effects in cats, a larger dose (unspecified) of ammonium chloride
and a longer latency period (from 1 to 3 h) were required,
indicating a greater resistance of this species to ammonium
chloride (Koenig & Koenig, 1949).
In a limited study to assess the role of the ammonium ion in
producing pulmonary oedema, Koenig & Koenig (1949) administered, by
gavage, to 5 different guinea-pigs, 6 ml of 20% ammonium nitrate;
7 ml of 6.4% ammonium acetate; 7 ml of 10% ammonium bromide; 7 ml
of 6% ammonium chloride; or 7 ml of 7.4% ammonium sulfate,
respectively. The last 4 solutions contained approximately
equivalent concentrations of ammonium. All 5 animals died of acute
pulmonary oedema; the lungs of a control animal that had received
water by gavage remained normal. The pulmonary oedema could not be
attributed to the induced acidosis, since guinea-pigs and cats,
made severely acidotic by gavage with sodium lactate or dilute
hydrochloric acid, did not show lung oedema (Koenig & Koenig,
1949).
Progressive signs of ammonium poisoning caused by ammonium
salts, given by gavage, indicative of both pulmonary and nervous
system dysfunction, were reported in less than 30 min in guinea-
pigs and rats (Koenig & Koenig, 1949) including:
(i) a rapid increase in the rate and depth of respiration;
(ii) weakness and difficulty in locomotion;
(iii) hyperexcitability for tactile, auditory, and painful
stimuli; and
(iv) muscle fasciculations over most of the body followed
by generalized tonic convulsions and then coma.
The respiratory rate was greatly reduced but the depth of
respiration increased and was accompanied by gasping and stridor.
Histological changes occurred in both the lung and brain, while
oedema, congestion, and haemorrhage were found principally in the
lung (Koenig & Koenig, 1949; Cameron & Shiekh, 1951).
The addition of ammonium chloride to rat feed resulted in
reduced dietary consumption (Motyl & Debski, 1977). The results of
studies by Noda & Chikamori (1976) pointed to a direct effect of
the ammonium ion on the brain area that regulates feeding. Rats
with bilateral lesions in the prepyriform cortical area of the
brain consumed as much diet containing 3% ammonium chloride as
basal diet. A unilateral injection of 10 mg/litre of 2% NH4Cl/kg
body weight into prepyriform cortical areas, in contrast to an
injection into other areas of the brain, or an injection of sodium
chloride, significantly reduced the food intake of 6 adult male
Wistar rats. The results of these studies suggest that ammonium
ions directly influence appetite by their effects on prepyriform
cortical areas.
(b) Kidney
Lotspeich (1965) reported that ingestion for 7 days of
0.28 mol/litre (1.5%) ammonium chloride in water (ad lib) produced
renal hypertrophy with new cell formation and an enlargement of
existing cells. Some animals from each of the acidotic and control
groups were subjected to unilateral nephrectomy. Highly
significant increases in kidney wet weight, dry weight, and total
nitrogen were observed in the acidotic group. Similar changes were
produced by unilateral nephrectomy. In unilaterally nephrectomized
rats made acidotic by the ingestion of ammonium chloride, the
remaining kidney was larger than that seen in animals with
unilateral nephrectomy without induced acidosis, suggesting an
additive effect of unilateral nephrectomy and ammonium chloride
intake.
The relationship between the renal hypertrophy and the ammonium
ion, acidosis, non-specific intake of nitrogen, or increased solute
load was examined (Lotspeich, 1965). Isomolar (0.28 mol/litre)
solutions of sodium chloride and ammonium chloride produced renal
hypertrophy. The increase due to sodium chloride was less marked
(it was noted that the sodium chloride group drank 3 or 4 times as
much solution each day as the ammonium chloride group). Isomolar
(0.2 mol/litre) solutions of sodium bicarbonate and ammonium
citrate did not induce renal hypertrophy. When 3 groups of 6 rats
were given drinking-water containing 0.28 mol/litre sodium
chloride, 0.28 ammonium chloride, or a urea solution containing
nitrogen equivalent to that in the 0.28 mol/litre ammonium
chloride, for 7 days, only the ammonium chloride solution induced
acidosis. The rats in both the sodium chloride and ammonium
chloride groups had larger kidneys than the urea-drinking rats,
though the kidneys of the sodium chloride-drinking rats were not as
large as those of the ammonium chloride-drinking rats. Since renal
hypertrophy was produced by solutions containing the chloride ion,
while ammonium citrate and urea did not cause any hypertrophy, the
evidence does not support the hypothesis that renal hypertrophy is
due to the ammonium ion.
Thompson & Halliburton (1966) also reported renal hypertrophy
in rats that ingested 3% ammonium chloride in the diet for 6 days.
The ingestion of ammonium citrate or sodium chloride in amounts
that were equivalent to that of the ammonium chloride did not cause
renal hypertrophy.
Janicki (1970) examined the kidneys of rabbits that had been
administered 16.2 g ammonium chloride, by gavage, over a period of
2 days and found the epithelium of the convoluted tubules swollen,
vacuolated, and completely filling the lumen; nuclei exhibited
karyolysis. Findings were similar in 4 rabbits administered 5 -
7 g ammonium chloride, by gavage, over a period of 3 - 7 days.
However, hyperaemia of the renal cortex of rabbits was noted
after the administration, by gavage, of single doses of 0.8 and 1 g
ammonium carbonate/kg body weight (Yoshida et al., 1957). As the
carbonate ion does not have an acidifying effect, this suggests
that the ammonium ion produces some effects on the kidney.
8.1.2.3. Influence of diet on the effects of ammonia
In 6 mongrel dogs, blood-urea levels were increased by a higher
level of protein intake (6 g protein/kg per day) (Bressani &
Braham, 1977). Levels of ~30 mg BUN/litre were found 8 h after the
daily feed. When the protein intake was low (4 mg protein/kg per
day), BUN levels of 14 mg/litre were reported 8 h after the daily
feed. The frequency of protein intake did not affect the maximum
value of blood-urea levels when the protein intake was low (4 mg
protein/kg per day). This suggests that high levels of urea and
ammonia in the blood might occur in animals that suffer from liver
insufficiency and are fed high-protein diets.
Kulasek et al. (1975) studied the effects of nitrogen in the
diet on ammonia detoxification, using 210 rats and 6 diets
containing 50, 150, and 700 g casein/kg (low-, optimal-, or high-
protein diet, respectively), with or without the addition of
ammonium chloride at 20 g/kg. After 8 or 9 days of feeding, the
LD50 from an intraperitoneal injection of a 2.7% solution of
ammonium chloride was determined. The LD50 increased in proportion
to the amount of nitrogen in the diet. The data suggest that
higher doses of exogenous ammonia were tolerated by rats on
protein-rich diets or diets containing an ammonium salt as an
additive. An adaptive capacity for ammonia detoxification was
further demonstrated by Motyl & Debski (1977) in a study using rats
fed low-protein diets with the addition of NH4Cl. The LD50 values
for the ip injection of 2.7% NH4Cl were higher in animals fed low-
protein diets supplemented with NH4Cl (Table 18). Stevens et al.
(1975) raised weanling rats for 3 - 6 weeks on a protein-deficient
diet. Subsequent challenge by a large, intraperitoneally-injected
dose of ammonium chloride indicated that severe protein deprivation
increased vulnerability to ammonia poisoning compared with that of
control groups not prefed protein-deficient diets. Thus, with the
exception of animals suffering from liver dysfunction, animals on
high-protein diets seem able to tolerate a higher oral intake of an
ammonium salt.
A group of eight, 6- to 8-month-old cats, given a single meal
of a complete amino acid diet without arginine, developed
hyperammonaemia with elevated plasma levels of glucose and ammonia
and showed clinical signs of ammonia toxicity within 2 h. One cat
died 4.5 h after ingesting only 8 g of the diet. Five of the
surviving cats given a single meal of complete amino acids, in
which arginine was replaced with an equivalent amount of ornithine,
did not show any unusual signs. This finding indicates that the
cat is unable to synthesize ornithine at the rate required by the
urea cycle to dispose of ammonia from amino acid catabolism;
however, the dietary requirements for cats may differ from those of
other adult animals, including human beings, because the cat has a
much higher protein requirement, i.e., it requires approximately
20% of dietary calories as protein, as opposed to only 4 - 8%
required by the rat, dog, sheep, and man (Morris & Rogers, 1978).
The lethal effects of ammonia poisoning have been prevented by
the amino acids, ornithine and aspartic acid. To test whether this
was mediated by a sparing action of adenosine triphosphate (ATP) on
ammonia metabolism, 20 dogs with chronic Eck's fistula were
injected in the duodenum with ammonium acetate at 4.1 mmol/kg body
weight (~219.4 mg/kg). Half of the group was given 2 mg ATP/kg,
1 h prior to administration of the ammonium acetate. In 9 of the
10 dogs, ATP prevented a rise in levels of ammonia in venous blood.
Grossi et al. (1968) administered ATP (2 mg/kg) intravenously to 6
dogs with chronic Eck's fistula and on high-protein diets. Within
1 h, high blood-ammonia levels returned to normal. In chronic
liver disease, there may be insufficient ATP available for ammonia
detoxification, resulting in hyperammonaemia.
8.1.3. Dermal exposure
No data are available regarding any systemic effects of dermal
exposure to ammonia or ammonium compounds.
8.1.4. Effects due to parenteral routes of exposure
The parenteral toxicity of ammonia and ammonium compounds has
been studied extensively. Toxicity is influenced by the route of
administration, e.g., with oral administration, there is the
capacity for detoxification of exogenous ammonia by the liver.
8.1.4.1. Lethality
The intravenous (iv) and intraperitoneal (ip) LD50 values for a
number of ammonium compounds in various species are summarized in
Table 19. The toxic syndrome was similar in all species studied.
The signs, after iv injection, were characterized by immediate
hyperventilation and clonic convulsions followed by either fatal
tonic extensor convulsion or the onset of coma in 3 - 5 min. The
animals remained comatose for approximately 30 - 45 min. At this
stage, tonic convulsions and death can occur at any time, but
animals that survive usually recover rapidly and completely
(Warren, 1958; Warren & Schenker, 1964; Wilson et al., 1968a,b).
After intraperitoneal injection, the signs did not appear until
15 - 20 min after administration (Greenstein et al., 1956; Wilson
et al., 1968b).
Table 19. Toxicity of several ammonium compounds in selected species
-----------------------------------------------------------------------------------------------------------
Ammonium Species Intravenous dose Intraperitoneal dose
compound (mmol/kg of body weight) (mmol/kg of body weight)
(LD50) (LD50) Reference
-----------------------------------------------------------------------------------------------------------
Acetate rat - 8.20 Greenstein et al. (1956)
mouse 6.23 - Warren (1958)
mouse 5.64 10.84 Wilson et al. (1968a)
chick 2.27 10.44 Wilson et al. (1968a)
Bicarbonate mouse 5.05 - Warren (1958)
mouse 3.10 - Wilson et al. (1968b)
Carbamate mouse 0.99 - Wilson et al. (1968b)
Carbonate mouse 4.47 - Warren (1958)
mouse 1.02 - Wilson et al. (1968b)
Chloride mouse 6.75 - Warren (1958)
mouse (38.8 °C)a 6.6 - Warren & Schenker (1964)
mouse (40.4 °C)a 5.17 - Warren & Schenker (1964)
mouse (27.9 °C)a 10.21 - Warren & Schenker (1964)
Hydroxide mouse 2.53 - Warren (1958)
-----------------------------------------------------------------------------------------------------------
a Body temperature.
8.1.4.2. Central nervous system effects
Navazio et al. (1961) observed that characteristic toxic signs
were not observed in rats until the ammonia concentration in the
blood was double that of basal values (attained within 8 - 10 min
of an ip injection of 601 mg ammonium acetate/kg body weight). No
substantial increase in brain-ammonia was observed. However, when
the blood-ammonia concentration reached more than 20 times the
basal value, there was a sudden rise in the ammonia concentration
in the brain, which reached a maximum of approximately 100 mg
ammonia-nitrogen/kg (wet weight), between 10 and 26 min after
injection. Muscular contractions with occasional tetanic spasms,
and then coma occurred, when the concentration of ammonia in the
brain reached approximately 50 mg/kg. Although the animals started
to recover from the comatose state approximately 70 min after
onset, blood- and brain-ammonia concentrations did not return to
basal levels until 2 h after the injection of ammonium acetate.
However, other authors observed an immediate increase in brain-
ammonia after ip injection of ammonium acetate (Torda, 1953; du
Ruisseau et al., 1957; Salvatore et al., 1963). These authors
found dramatic increases in the brain-ammonia content, 2 - 5 min
after the injection of ammonium acetate. Salvatore et al. (1963)
suggested that there was no critical blood-ammonia concentration
for diffusion through the blood-brain barrier.
Ammonia has been shown to be more highly toxic at elevated body
temperature, whereas hypothermia affords marked protection
(Schenker & Warren, 1962). The LD50 values for ammonium chloride
in the mouse, at various body temperatures, are shown in Table 19.
The increased toxicity of ammonia at elevated body temperature was
suggested to be due to a direct metabolic effect of hyperthermia on
the brain, unrelated to dehydration or stress.
8.1.4.3. Effects on the heart
Intravenous LD50 values for ammonium carbamate, ammonium
carbonate, and ammonium bicarbonate have been determined in mice by
Wilson et al. (1968a,b) (Table 19). The physiological effects of
the injected ammonium compounds in dogs and sheep were also
investigated. Electrocardiograms recorded during the toxic
syndrome indicated that the animals died from ventricular
fibrillation due to a direct effect of ammonia on the heart. These
findings were in agreement with the effects noted by Berl et al.
(1962) during the iv infusion of ammonium chloride in cats when
electrocardiograms were altered in a complex manner. However,
Warren & Nathan (1958) were unable to demonstrate any cardiotoxic
effects of the ammonium compounds in mice and concluded that the
toxicity syndrome was due primarily to a cerebral effect and not a
direct effect on cardiac or skeletal muscle.
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
When 48 rats were continuously exposed to 127 mg ammonia/m3
(181 ppm) for 90 days, no abnormalities were found in organs or
tissues. Inhalation of 262 mg ammonia/m3 (374 ppm) for 90 days
induced mild nasal irritation in about 25% of 49 rats and a mild
leukocytosis in 4 of the rats. Continuous exposure to 455 mg/m3
(650 ppm) resulted in the death of 50 out of 51 rats by the 65th
day of exposure. All animals exhibited mild nasal discharge and
laboured breathing. In a second study, rats, guinea-pigs, rabbits,
and dogs were continuously exposed to 470 mg/m3 (671 ppm) for 90
days. Thirteen out of 15 rats and 4 out of 15 guinea-pigs died.
Marked eye irritation was noted in rabbits and dogs, with corneal
opacities in about one-third of the rabbits. At autopsy, all test
animals examined had more extensive focal or diffuse interstitial
inflammatory processes in the lungs than the controls (Coon et al.,
1970).
White rats were exposed to ammonia, by inhalation, at
concentrations of 100 and 30 mg/m3 (143 and 43 ppm) for 25 or
60 min, every 48 h, for a period of 3 months. Rats exposed to a
concentration of 100 mg/m3 (143 ppm) showed only a mild
leukocytosis, with no significant differences from the control
group with regard to oxygen consumption, neuromuscular excitation
threshold, heart rate, blood-sugar, blood-residual nitrogen, and
total serum-protein (Prokop'eva & Yushkov, 1975). In another study
by Alpatov & Mikhailov (1963), the threshold level for toxic
effects in a 2-month exposure was 40 mg/m3 (57 ppm). Histological
changes were seen in the lungs of the animals exposed to a
concentration of 100 mg/m3 (143 ppm), including small areas of
interstitial pneumonia with signs of peribronchitis and
perivasculitis. No changes were seen in other organs compared with
those in the control group.
Broderson et al. (1976) exposed Sherman and Fisher rats to
ammonia from natural sources, at an average concentration of
105 mg/m3 (150 ppm) for 75 days, and to purified ammonia at
175 mg/m3 (250 ppm) for 35 days. Histological changes in the
olfactory and respiratory epithelia of the nasal cavity were
similar in all the exposed rats, showing increased thickness,
pyknotic nuclei, and hyperplasia. The submucosa was oedematous
with marked dilation of small vessels. Lesions decreased
posteriorly.
Exposure to ammonia at concentrations of 17 - 175 mg/m3 (25 -
250 ppm) increased the infectious effects of Pasteurella multocida
in mice and Mycoplasma pulmonis in rats; this effect increased
with the concentration of ammonia (Broderson et al., 1976; Richard
et al., 1978a).
Richard et al. (1978a) selected a concentration of 350 mg/m3
(500 ppm) for a study of effects of continuous exposure to ammonia
after noting that general toxic effects, particularly on growth
rate, were not found at 175 - 210 mg/m3 (250 - 300 ppm). Young
male specific-pathogen-free rats were age- and weight-matched with
controls (27 per group) and exposed for up to 8 weeks. Nasal
irritation began on the fourth day. After 3 weeks, exposed rats
showed nasal irritation and inflammation of the upper respiratory
tract, but no effects were observed on the bronchioles and alveoli.
The number of pulmonary alveolar macrophages was similar to that in
the controls. After 8 weeks, none of these inflammatory lesions
were present.
In a study by Coon et al. (1970), 15 rats, 15 guinea-pigs, 3
rabbits, 2 dogs, and 3 monkeys were exposed to an ammonia
concentration of 155 mg/m3 (221 ppm) for 8 h/day, 5 days a week,
for 6 weeks. Pathological effects were not observed in any of the
species except for evidence of focal pneumonitis in the lungs of
one of the monkeys. Exposure to 770 mg/m3 (1100 ppm) for the same
duration induced mild to moderate eye irritation and laboured
breathing in the rabbits and dogs at the beginning of exposure, but
these signs disappeared by the second week and no other signs of
irritation or toxicity were noted. Autopsy findings included non-
specific inflammatory changes in the lungs of the rats and guinea-
pigs.
Male Sprague Dawley rats were studied to determine whether
ammonia was absorbed through the lungs into the blood, and the
subsequent effects on the blood pH, blood gases, and hepatic drug-
metabolizing enzymes (Schaerdel et al., 1983). Rats were exposed
to ammonia at 7 - 840 mg/m3 (10 - 1200 ppm) for 1, 3, or 7 days.
No significant changes were found in blood pH, pCO2, or in the
histological appearance of the lungs or trachea. Liver microsomal
enzymes (ethyl-morphine- N-demethylase and cytochrome P-450) showed
only minor changes. Blood-ammonia levels increased in a linear
fashion with increasing ammonia concentrations in air. A
concentration of 70 mg/m3 (100 ppm) or less produced only very
small changes in blood-ammonia levels and had no measureable
effects on any of the parameters studied.
8.2.2. Oral exposure
8.2.2.1. Histopathological effects
Rats given ammonium salts, for periods ranging up to 90 days,
did not appear to sustain renal damage (Freedman & Beeson, 1961;
Gupta et al., 1979). Twelve adult male Sprague Dawley rats were
given ammonium chloride in the drinking-water at 16 g/litre, for up
to 3 weeks. Urinalysis did not reveal any evidence of renal
injury, and no gross or histological "abnormalities" of the kidney
were seen at autopsy (Freedman & Beeson, 1961). Glutaminase
activity per kg of kidney increased with duration of exposure; this
is a physiological adaptation to acidosis. Another group of 10
rats, similarly treated and then given ammonium chloride in the
drinking-water at 10 g/litre, for an additional 2.5 months also did
not show any gross or microscopic renal abnormalities. Assuming
the rats weighed 250 g and consumed 25 ml of water per day, they
could have ingested as much as 1.6 g/kg body weight per day while
drinking the ammonium chloride at 16 g/litre and 1.0 g/kg body
weight per day while drinking a level of 10 g/litre. However,
actual intake may have been lower, because ammonium chloride is
known to affect the appetite and may render the water less
palatable. When ammonium chloride was given at 20 g/litre, food
and water consumption were drastically reduced.
In Table 20, selected studies are presented on ammonium salts
other than the chloride. In general, at low doses, no detrimental
effects were observed when rats and pigs were exposed to the NH4
salts listed.
In a 90-day study by Gupta et al. (1979) on rats, there was not
any evidence of renal damage. Ammonium sulfamate (NH4SO3NH2) as a
100 g/litre solution was given orally at rates of 100, 250, or
5000 mg/kg body weight per day, 6 days a week for 30, 60, or 90
days to adult female rats (ITRC colony-bred albino) and to weanling
male and female rats (20/sex per age per dose level). Under
certain conditions, the sulfamate ion is hydrolysed to bisulfate
ion and ammonia; however, it is unclear whether, and to what
extent, hydrolysis occurs in the rat intestine. Equivalent doses,
listed in Table 20, were calculated on the assumption that no
hydrolysis occurs. The effects of the anion, sulfamate and/or
sulfate, on the action of ammonium is a matter of conjecture.
Ammonium sulfamate would be expected to produce metabolic acidosis
on the basis of its structure, but this has not been verified
experimentally. The general health of both treated and control
rats was good, and there were no significant differences in mean
body weights throughout the study, except for a slight depression
in the highest-dose adult females at 60 and 90 days. Food and
water consumption were unaffected, except in the highest dose
weanlings of both sexes, which consumed less food and drank more
water than control weanlings. Interim sacrifice of 6 animals/sex
per dosage group was performed for haematological and histological
examination. There were no significant differences in
haematological values (packed cell volume, haemoglobin
concentration, total red cell count, total and differential white
cell count). Relative organ weights in all treated groups did not
differ significantly from those in the controls. Histological
examination of the kidney, liver, lung, stomach, heart, spleen,
thyroid, adrenal, gonads, intestine, and lymph nodes did not reveal
any abnormalities, except slight hepatic fatty degeneration in one
adult female at 90 days.
Table 20. Dose-response data for short-term oral administration of ammonium compounds
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Sulfamate rat, albino, orally as 10% 30, 60, 100; 15.0; no effects on body Gupta et
20 adult solution; not 90 days 250 37.3 weight, food, and al. (1979)
female, 20 specified whether water consumption,
weanling given by gavage haematological values
male, 20 or organ weights; no
weanling histological
female per abnormalities in
dose organs (including
kidneys)
500 74.8 slight decrease in
body weight in adult
females; slightly
lower food and higher
water consumption in
weanlings of both
sexes; no effects on
haematological values
or organ weights; no
histological
abnormalities in
organs (including
kidneys)
------------------------------------------------------------------------------------------------------------------- kidneys)
Table 20. (contd.)
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Diammonium pig, male 3.75% diammonium 28 days 1820 274 body weight gain Kagota et
citrate 22.5 kg, citrate in diet increased but % meat al. (1979)
3-4/group containing 6.4% crude and fat of carcass
protein unaffected; plasma-
and urine-urea-
nitrogen increased
but blood-ammonia-
nitrogen level
unaffected; no effect
on haematological
values; no gross
abnormalities or
lesions
Diammonium pig, 27.3 kg basal diet contained 81 days 820 161 (10% no effect on weight Wehrbein
phosphate 3 males and 16% protein; 0,5,10, replacement) gain, BUN, or food et al.
and 3 females/ or 20% of dietary N consumption (no (1970)
diammonium group was replaced by N effect on these
citrate as from equimolar parameters at 5%
equimolar mixture of designated 81 days 1600 322 (20% either) slight
mixture NH4+ salts replacement) decrease in weight
gain, BUN and food
consumption
-------------------------------------------------------------------------------------------------------------------
a Estimated for all studies except Gupta et al. (1979).
b Equivalent intake expressed as ammonia (NH3).
Treatment of virgin female rabbits with ammonium carbonate,
chloride, hydrophosphate, sulfate, or hydroxide at 0.1 - 0.2 g/kg
body weight, orally, on alternate days, for periods of 3 weeks
separated by 1-week intervals of no treatment, was associated with
enlargement of the ovaries, follicle maturation, and formation of
corpora lutea (Fazekas, 1949). There was also enlargement of the
uterus, hypertrophy of the teats, and secretion of milk.
Rabbits (10 - 80 per group) were given 0.1 - 0.2 g/kg body
weight of an ammonium salt in 100 - 150 ml of drinking-water, twice
daily. One hundred and sixty rabbits (96 female, 64 male)
received, twice daily, 50 - 80 ml of a 0.5% ammonium hydroxide
solution (~135 - 210 mg/kg body weight), in gradually increasing
doses. The chemicals were given for periods of 3 weeks separated
by 1-week intervals of no treatment. In a related study, similar
treatment of rabbits with ammonium chloride or ammonium sulfate
resulted in fluctuations in serum-calcium and -phosphorus levels
(Fazekas, 1954a).
8.2.2.2. Effects of ammonium as a dietary nitrogen supplement
Ammonia from ammonium salts can stimulate the growth of animals
on diets deficient in non-essential amino acids or restricted in
protein content. Weanling rats, given an artificial diet
comprising essential amino acids, B vitamins, vitamin C, salts, and
glucose for 21 days, grew poorly. Rats given a similar diet in
which some of the glucose was replaced with 8.6% ammonium acetate
(equivalent to 15.7 g nitrogen/kg diet) showed a dramatically
improved weight gain (Birnbaum et al., 1957). The food
consumption/weight gain ratio (feed/gain ratio) was also improved
in animals receiving the ammonium salt.
Similar results were obtained with young male pigs given a more
natural basal diet restricted in non-essential amino acids and
containing the minimum requirement of essential amino acids (Kagota
et al., 1979). The crude protein level of this basal diet was
6.4%. Three pigs fed the basal diet supplemented with 3.75%
diammonium citrate had a significantly greater weight gain and a
slightly lower feed/gain ratio than 4 pigs fed the basal diet (the
diets were made isocaloric by adjusting carbohydrate). No
significant differences in the packed cell volume or ammonia-
nitrogen level of the blood or in total protein concentration of
plasma were found between the 2 groups. Plasma- and urine-urea-
nitrogen were increased in the pigs fed the diet supplemented with
diammonium citrate. Autopsy of the pigs after 28 days on the basal
or ammonium citrate diets did not reveal any lesions or
abnormalities or any differences in the percentage of carcass meat
and fat. The average daily food intake for pigs receiving
diammonium citrate was 1.44 kg, which corresponds to 54 g
diammonium citrate per day. Using a body weight midway between the
initial (22.5 kg) and final (36.7 kg) body weights, the mean intake
of diammonium citrate can be estimated to be 1.82 g/kg body weight,
per day, or 16.1 mEq NH4+/kg body weight, per day.
Addition of ammonium salts to diets with a higher protein
content (10% crude protein) did not produce significant changes in
weight gain in rats or pigs (Clawson & Armstrong, 1981).
Similarly, no significant changes in weight gain occurred in pigs,
when up to 10% of the nitrogen from crude protein (diet = 16% crude
protein) was replaced with nitrogen from ammonium salts (Wehrbein
et al., 1970). Replacement of 20% of the dietary nitrogen with
ammonium salts slightly decreased body weight gain in the pigs;
food consumption and BUN also decreased. The 20% replacement diet
contained 1.54% diammonium phosphate and 2.07% diammonium citrate
for a total of 3.61% ammonium salts and was fed for 81 days. The
estimated mean intake was 0.70 g diammonium phosphate and 0.94 g
diammonium citrate/kg body weight per day, giving a total of
18.9 mEq NH4+/kg body weight per day.
8.2.3. Dermal exposure
There is no information regarding systemic toxicity from short-
term dermal exposure to ammonia or ammonium compounds.
8.3. Skin and Eye Irritation; Sensitization
Ammonia in the form of a gas, an anhydrous liquid boiling at
low temperatures, or an aqueous solution is a recognized skin and
eye irritant. Most of the information is human clinical data and
is described in section 9.
Ammonia, partly because of its lipid solubility, penetrates
the corneal membrane rapidly (NRC, 1979). In rabbits, conjunctival
oedema with ischaemia and segmentation of limbal vessels were noted
within 30 min. By 24 h, there was a reduction in the
mucopolysaccharide contents of the corneal stroma, and extensive
polymorphonuclear infiltration and anterior lens opacities were
apparent. Aqueous levels of glucose and ascorbate and intraocular
pressure were depressed (NRC, 1979). In rabbits with corneal
burns, neovascularization occurred after 1 week, but it was delayed
in animals with corneal and limbal burns. Complications of severe
burns included symblepharon, pannus, pseudopterygia, progressive or
recurrent corneal ulcerations leading to perforations, permanent
corneal opacity, corneal staphyloma, persistent iritis, phthisis
bulbi, secondary glaucoma, and dry eye (NRC, 1979).
Ammonium persulfate is a recognized skin sensitizer for human
beings. There are no data on its sensitization potential in animal
models.
8.4. Long-Term Exposures
8.4.1. Inhalation exposure
Weatherby (1952) exposed 12 guinea-pigs to an ammonia
concentration of about 119 mg/m3 (170 ppm). Chamber concentrations
ranged from 98 to 140 mg/m3 (140 to 200 ppm) for 6 h/day, 5 days a
week, for up to 18 weeks. There were no significant findings at
autopsy in animals sacrificed after 6 or 12 weeks of exposure. In
animals sacrificed after 18 weeks of exposure, there was congestion
of the liver, spleen, and kidneys, with early degenerative changes
in the adrenal glands. Increased erythrocyte destruction was
suggested by increased quantities of haemosiderin in the spleen.
In the proximal tubules of the kidneys, there was cloudy swelling
of the epithelium and precipitated albumin in the lumen with some
casts. The cells of the adrenal glands were swollen and the
cytoplasm in some areas had lost its normal granular structure.
Coon et al. (1970) conducted studies in which rats, guinea-
pigs, rabbits, dogs, and monkeys were continuously exposed
(24 h/day, 7 days per week) to ammonia. No signs of toxicity were
seen in any species following continuous exposure to ammonia at a
concentration of 40 mg/m3 (57 ppm) for 114 days, and gross and
microscopic examination did not reveal any lung abnormalities.
8.4.2. Oral exposure
The administration of ammonium carbonate, chloride, sulfate,
hydrophosphate, acetate, lactate, or hydroxide to a total of 296
rabbits for 3 - 16 months resulted in enlargement of the
parathyroids (Fazekas, 1954a). Administration of sodium
dihydrophosphate, sodium ammonium phosphate, calcium chloride,
hydrochloric acid, acetate acid, or lactic acid gave similar
results (Fazekas, 1954a).
Rabbits given 0.1 g ammonium hydroxide/kg body weight (as a
0.5 - 1.0% solution) by gavage, initially, on alternate days and
then daily for up to 17 months, had enlarged adrenal glands
(Fazekas, 1939). An initial fall in blood pressure of 2.67 -
4.00 kPa (20 - 30 mmHg) was followed by a gradual rise to 1.33 -
4.00 kPa (10 - 30 mmHg) above the baseline, after several months
of treatment.
The results of studies on rats, rabbits, and dogs indicate that
long-term administration of ammonium chloride can induce
osteoporosis (Seegal, 1927; Bodansky et al., 1932; Jaffe et al.,
1932a,b; Barzel & Jowsey, 1969; Barzel, 1975). During prolonged
metabolic acidosis, the release of bone mineral by resorption is
thought to provide additional buffering capacity, sparing
bicarbonate.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The interaction of ammonium chloride with other teratogenic
agents has been investigated in 2 studies. Goldman & Yakovac
(1964) used ammonium chloride to investigate the role of metabolic
acidosis in salicylate-induced teratogenesis in Sprague Dawley
rats. Beginning on day 7 of gestation, rats received either
0.17 mol/litre (0.9%) ammonium chloride in the drinking-water, a
single subcutaneous injection of salicylate, or both. Ammonium
chloride alone inhibited fetal growth, but was not teratogenic.
However, when administered with salicylate, ammonium chloride
significantly increased maternal and fetal mortality and the fetal
anomaly rate (dorsal midline, ventral midline, and eye defects)
compared with that due to salicylate alone. These effects were
attributed to acidosis and not to ammonia. However, it has been
shown by Miller (1973) that the addition of 0.5% ammonium chloride
to the 5%-glucose drinking-water of fasting, pregnant CFW mice
significantly reduced the incidence of fast-induced cleft palate in
the progeny.
8.6. Mutagenicity
Ammonium sulfate was reported to be non-mutagenic in the
Salmonella and Saccharomyces systems (Litton Bionetics, Inc.,
1975). Demerec et al. (1951) tested ammonia for its ability to
induce back-mutations from streptomycin dependence to non-
dependence in Escherichia coli and found it to be mutagenic, but
only in treatments that left less than 2% survivors. Iwaoka et al.
(1981a,b) showed that extraction of ingredients from fried
hamburger and refrigerated biscuit products with ammonium hydroxide
or ammonium sulfate increased the mutagenic activity in
S. typhimurium TA98 and TA1538, compared with sodium sulfate
extraction. This suggests that ammonium salts may, in some way,
influence the mutagenic activity of some agents, or may themselves
be responsible for false positive findings (Iwaoka et al., 1981a).
However, it is also possible that ammonium salts extract mutagenic
components from foods more efficiently.
Lobashov & Smirnov (1934) and Lobashov (1937) found ammonia to
have a mutagenic effect on Drosophila. Ammonia showed slight
mutagenic activity in studies in which survival of Drosophila was
lower than 2% after treatment. In studies by Auerbach & Robson
(1947), when Drosophila was exposed to ammonia vapour in small
glass containers, 0.5% sex-linked lethals were observed.
It was reported by Rosenfeld (1932) that ammonia induced
clumping of chromosomes, arrest spindle formation, and induce
polyploidy in chick fibroblasts in vitro.
There are no data to show that ammonia is mutagenic in mammals.
8.7. Carcinogenicity
There is no evidence indicating that ammonia is carcinogenic.
Gibson et al. (1971) observed that animals treated with ammonia
developed inflammatory lesions of the colon. It has been suggested
that cell proliferation may increase errors in DNA replication
(Zimber, 1970; Zimber & Visek, 1972), activate oncogenic agents
present in sub-threshold doses (Anderson et al., 1964), and even
unmask latent changes in the genetic material caused by mutagenic
agents (Visek et al., 1978).
In a study on male Sprague Dawley rats (Topping & Visek, 1976),
there was no evidence that ammonia increased the incidence of
tumours with increased protein intake. Development of lung
tumours was observed in CFLP mice treated intra-gastrically with
diethyl pyrocarbonate and ammonia, neither of which induces cancer
independently in animals. However, this may have resulted from a
carcinogenic substance, possibly urethane, formed in vivo from
diethyl pyrocarbonate in the presence of ammonia (Uzvolgyi & Bojan,
1980).
Life-long ingestion of ammonium hydroxide in the drinking-water
by Swiss and C3H mice did not produce any carcinogenic effects, and
had no effect on the spontaneous development of adenocarcinoma of
the breast in C3H females, a characteristic of this strain (Toth,
1972).
Ammonium chloride and ammonium tungstoantimonate and related
compounds have been shown to have an inhibitory effect on malignant
cells (Phillips, 1970; Anghileri, 1975; Flaks & Clayson, 1975;
Flaks et al., 1973; Sof'ina et al., 1978).
8.8. Factors Modifying Effects
8.8.1. Synergistic effects
Stevens et al. (1975) raised weanling rats for 3 - 6 weeks on a
protein-deficient diet. Subsequent challenge by a large,
intraperitoneally-injected dose of ammonium chloride indicated that
severe protein deprivation increases vulnerability to ammonia
poisoning in comparison with control groups not prefed protein-
deficient diets.
Dalhamn (1963) employed a technique in rabbits in which an air
pollutant mixture was drawn into the nostrils and out through a
tracheal cannula. The effects of ammonia alone and ammonia mixed
with (and presumably adsorbed on) carbon particles, on the beating
rate of tracheal cilia was examined. Ammonia alone had to be
administered at a high concentration (1400 mg/m3 or 2000 ppm) to
produce a tracheal concentration of 70 mg/m3 (100 ppm). The
reduction in beating rate was not substantially altered by the
addition of carbon particles at 2 mg/m3.
Simultaneous injection of ammonium salts and a fatty acid into
rats or cats produced coma at lower plasma levels of ammonia and
free fatty acids than a single injection of either compound (Zieve
et al., 1974).
Impaired handling of an ammonium load has been observed in
animals with hepatic dysfunction. Elevated levels of ammonia or
urea have been reported in dogs with experimentally-bypassed livers
(section 7.1.2). The importance of the kidney in detoxification of
an ammonium load is apparent in the studies of Lotspeich (1965)
(section 8.1.2.2), where an additive hypertrophic effect of
ammonium chloride and unilateral nephrectomy was observed on the
remaining kidney. Increased sensitivity to ammonia toxicity was
found in young rats (Benyajati & Goldstein, 1975) and in rats that
had been castrated, adrenalectomized, or thymectomized (Paik et
al., 1975).
8.8.2. Antagonistic effects
L-arginine and the related amino acids, ornithine and aspartic
acid, substrates from urea synthesis, have been reported to exert a
protective effect against acute ammonia poisoning in rats, dogs,
and cats (Morris & Rogers, 1978; NRC, 1979).
High-protein diets exert an antagonistic effect on the toxicity
of ammonium salts, unless liver dysfunction is present (section
7.1.2.4). In addition, an increase in toxic response has been
observed with increase in age due to the adaptive response of
glutaminase activity in rats to ammonia detoxication during
acidosis.
8.9. Mechanisms of Toxicity
Hypotheses proposed for the mechanism of ammonia toxicity
include impaired decarboxylation of pyruvic acid (McKhann & Tower,
1961), NADH depletion slowing down the generation of ATP (Worcel &
Erecinska, 1962), depletion of alpha-ketoglutarate resulting in
impairment of the Krebs cycle (Bessman & Bessman, 1955; Fazekas et
al., 1956; Warren & Schenker, 1964); depletion of ATP due to
glutamine formation by the glutamine synthetase (EC 6.3.1.2) system
(Warren & Schenker, 1964; Nakazawa & Quastel, 1968), stimulation of
membrane ATPase (EC 3.6.1.8) producing increased nerve cell
excitability and activity (Hawkins et al., 1973), and depletion of
ATP causing a decrease in cerebral acetylcholine (Braganca et al.,
1953; Ulshafer, 1958).
9. EFFECTS ON MAN
9.1. Organoleptic Aspects
9.1.1. Taste
Campbell et al. (1958) determined the taste threshold
concentration for ammonia in redistilled water with 21 - 22
subjects in "difference tests of the triangle type". At ammonia
concentrations of 26, 52, and 105 mg/litre, the percentages of
correct identifications were 61.9, 71.4, and 85.7, respectively.
Defining the threshold concentration as the level at which
correct identification is 50% greater than that expected by
chance, the taste threshold of ammonia was determined to be
around 35 mg/litre. However, this definition of the threshold
concentration seems to be somewhat arbitrary and McBride & Laing
(1979) have reported significant positional bias in using the
triangle test to determine taste thresholds. Furthermore, the
triangle test is not intended to mimic environmental exposures
in which the taste thresholds could be substantially higher. It
seems reasonable to conclude only that the palatability of water
is not likely to be significantly affected by total ammonia levels
of < 35 mg/litre (as NH3), but will be affected at higher levels.
9.1.2. Odour
Odour thresholds reported in the literature may vary according
to the definition of odour response, mode of presentation of the
stimulus, chemical purity of the agent used, and the number of
subjects and trials in the study. The detection threshold for
ammonia, defined as the concentration that produces the first
detectable difference in odour over background, was reported to be
37 mg/m3 (DallaValle & Dudley, 1939). This reference, though often
cited, gives no information on the study design from which this
number was derived.
The considerable variability in threshold data prompted work by
Leonardos et al. (1969), who used a standardized procedure to
determine recognition thresholds rather than detection thresholds
for 53 chemicals. The odour threshold was defined as the first
concentration at which all four panel members (trained odour
analysts) were able to recognize the characteristic odour of the
chemical. The panel tested only one chemical per day.
Concentrations examined were multiples by 10 of 0.7, 1.47, and
3.3 mg/m3 (1, 2.1, and 4.6 ppm). The recognition threshold for the
odour of ammonia was 32.6 mg/m3 (46.8 ppm).
The results of several other studies suggest that human beings
can detect ammonia at much lower levels. Stephens (1971) reported
2.7 mg/m3 (3.9 ppm) as the lowest concentration producing an odour
response, when a contaminated air stream and a reference stream
were compared by sniffing (number of subjects was not reported). A
report by Saifutdinov (1966), of an olfactory threshold of 0.55 -
0.50 mg/m3 for the most sensitive of 22 subjects, did not include
sufficient detail to evaluate this excessively low estimate.
Carpenter et al. (1948) stated that "a group of 8 persons found
that the least odour they could detect on entering a room
containing various concentrations" was 0.7 mg/m3 (1 ppm) ammonia.
Again, no other details were given.
The best estimates of the thresholds at which ammonia can be
expected to be detected by taste and odour are 35 mg/litre (as NH3)
and 35 mg/m3, respectively. Sensitive individuals may detect
concentrations an order of magnitude lower.
9.2. Clinical and Controlled Human Studies
9.2.1. Inhalation exposure
The severe effects resulting from acute exposure to ammonia
have been described in case reports of accidents involving groups
of people or individuals. There are no data on actual levels of
ammonia in air during such accidents, but estimates have been made
(Yahagi et al., 1959; Takahashi et al., 1984; NRC, 1979).
Exposure to an ammonia concentration of 280 mg/m3 (400 ppm) has
been reported to produce immediate throat irritation; 1200 mg/m3
(1700 ppm) to produce cough; 1700 mg/m3 (2400 ppm) to be life-
threatening, and 3500 - 7000 mg/m3 (500 - 10 000 ppm) to cause a
high mortality rate (Patty, 1963; Helmers et al., 1971).
A burning sensation in the eyes, nose, and throat, as well as
respiratory distress accompanied by lachrymation, coughing, and an
increase in respiratory rate are some of the irritant effects of
ammonia (Caplin, 1941). Chest X-rays are generally normal in such
mild cases (Watson, 1973; Close et al., 1980). More severe
respiratory effects include laryngeal and pulmonary oedema and
bronchopneumonia (Slot, 1938; Caplin, 1941; Levy et al., 1964;
Taplin et al., 1976; Flury et al., 1983). The signs and symptoms
are generally reversible, but chronic bronchitis and bronchiectasis
have been reported (Slot, 1938; Sugiyama et al., 1968; Taplin et
al., 1976; Close et al., 1980).
In cases with a lethal outcome, the cause of death has been
severe lung damage and secondary cardiovascular effects (Slot,
1938; Mulder & van der Zalm, 1967).
There have also been some studies on volunteers exposed to
ammonia under laboratory conditions. Some of these studies are
summarized in Table 21.
Silverman et al. (1949) reported on 7 human volunteers exposed
to a concentration of 300 mg/m3 (500 ppm) ammonia for 30 min using
an oral-nasal mask. All 7 experienced upper respiratory
irritation, which lasted up to 24 h in 2 of the volunteers. Two
subjects experienced marked lachrymation, in spite of the exposure
being by oro-nasal mask. The average respiratory minute volume
increased markedly compared with control values, and in the 5
subjects in which minute-by-minute expired volumes were measured,
there was a marked cyclical variation in minute volume with a
period of 4 - 7 min. No coughing was noted.
After exposure, respiratory minute volumes fell to levels below
the pre-exposure rate, but returned to pre-exposure values within 5
min of exposure. Ammonia retention decreased progressively until
an equilibrium of 24% retention (ranging from 4 to 30%) was reached
at approximately 19 min (range 10 - 27 min). The indices of
nitrogen metabolism (BUN, NPN, urine-urea, and urine-ammonia)
remained normal. The carbon dioxide combining power did not
change.
Verbeck (1977) assessed respiratory function in 16 volunteers
following exposure to 35, 56, 77, and 98 mg ammonia/m3 (50, 80,
110, and 140 ppm) for 0.5, 1, and 2 h. The respiratory variables of
vital capacity (VC), forced expiratory volume (FEV), and forced
inspiratory volume (FIV), measured before and after exposure, did
not decrease by more than 10%. Subjective variables, including
smell, taste, irritation of the eyes, nose, or throat, urge to
cough, headache, and general discomfort were monitored every 15 min
and ranked by 8 experts and 8 non-experts (students) on a scale
from 0 to 5. Subjective responses were ranked higher by the non-
experts. A concentration of 77 mg/m3 (110 ppm) was tolerated for
2 h, but at 98 mg/m3 (140 ppm), all the subjects left the chamber
because the exposure was intolerable.
Respiratory responses to ammonia during exercise were examined
by Cole et al. (1977). Eighteen males, aged 18 - 39 years, had 2
periods of exercise in 3 consecutive half-day sessions. Mean
exposure levels were 71, 144, 106, and 235 mg/m3 (101, 206, 151,
and 336 ppm). Ventilation minute volume and total volume
decreased, and mean respiratory frequency increased at exposure
levels > 106 mg/m3 (151 ppm).
In a study by MacEwen et al. (1970), 6 volunteers were exposed
to ammonia concentrations of 21 and 35 mg/m3 (30 and 50 ppm) for 10
min. The irritation was rated subjectively on a scale of 0 - 4.
At 35 mg/m3 (50 ppm), irritation was rated as "moderate" by 4 of
the volunteers, while 1 individual reported no detectable
irritation. None of the volunteers found the irritation at
35 mg/m3 (50 ppm) to be "discomforting" or "painful".
The irritation threshold for ammonia was examined in 10 human
volunteers by Industrial Bio-Test Laboratories, Inc. (1973). The
subjects were exposed to 4 different concentrations of 22, 35, 50,
and 94 mg/m3 (32, 50, 72, and 134 ppm) for 5 min. The frequency of
positive findings for the 10 subjects was as follows: at 22 mg/m3,
1 subject complained of dryness of the nose; at 35 mg/m3, 2
subjects experienced dryness of the nose; at 50 mg/m3, 3 subjects
had eye irritation, 2 had nasal irritation, and 3 had throat
irritation; at 94 mg/m3, 5 subjects demonstrated signs of
lachrymation, 5 had eye irritation, 7 had nasal irritation, 8 had
throat irritation, and 1 complained of chest irritation.
Table 21. Effects of inhaled ammonia in human volunteers
--------------------------------------------------------------------------------------------
Number Concentration Duration Effects and response Reference
(mg/m3) (min)
--------------------------------------------------------------------------------------------
7 300 30 irritation of upper respiratory Silverman et al. (1949)
tract (7/7); increase in minute
volume; no change in blood
chemistry
16 35 - 98 30 - 120 10% increase in VC, FEV, and Verbeck (1977)
FIV; 98 mg/m3 was not tolerated;
77 mg/m3 was tolerated for 2 h
18 71 - 235 8 - 11 no effects noted during exercise Cole et al. (1977)
at 71 mg/m3 except slight
irritation; decrease in
ventilation minute volume and
tidal volume and increase in
respiratory frequency > 106
mg/m3
6 35 10 "moderate irritation" (4/5) MacEwen et al. (1970)
10 22 5 dryness of the nose (1/10) Industrial Biotest
10 35 dryness of the nose (2/10) Laboratories (1973)
10 50 eye irritation (3/10)
10 94 throat irritation (8/10)
eye irritation (5/10)
6 17.5, 35, 2 - 6 h, increase in FEV1, with Ferguson et al. (1977)
or 70+ 5 days/ increasing NH3; adaptation
week, for to irritating effects
6 weeks
--------------------------------------------------------------------------------------------
A controlled study on human volunteers was conducted by
Ferguson et al. (1977) to evaluate the responses to inhaled ammonia
at concentrations of 17.5, 35, and 70 mg/m3 (25, 50, and 100 ppm).
Six adults, not acclimatized to ammonia exposure, were divided into
3 groups, which were exposed for 2 - 6 h each day, 5 days per week,
for 6 weeks. One pair of subjects was exposed only to 35 mg
ammonia/m3 for 6-h periods throughout the test. Subjects were
examined daily for irritation of the eyes, nose, or throat, and
periodically for pulse rate, respiration rate, pulmonary function
(forced vital capacity (FVC) and forced expiratory volume in 1
second (FEV1)), blood pressure, neurological responses, and
interference in task-performance ability. A statistical analysis
of the results demonstrated that the only significant change among
the vital functions measured was an increase in forced expiratory
volume in 1 second (FEV1) with increasing ammonia concentration.
In addition, the rate of mild eye, nose, or throat irritations over
the last 3 weeks of the test was significantly less than during the
first 2 weeks. This indicated that an acclimatization process had
occurred, with increased tolerance to the irritant effects of
ammonia developing with increasing time of exposure. Overall, the
ammonia exposure produced 2 incidents of mild irritation (78
observations made) at 17.5 mg/m3 (25 ppm), 22 incidents (198
observations made) at 35 mg/m3 (50 ppm), and 11 incidents (84
observations made) at 70 mg/m3 (100 ppm). Among control subjects,
4 irritation incidents were recorded during 45 observations. When
ammonia concentrations exceeded 105 mg/m3 (150 ppm), all subjects
experienced lachrymation accompanied by dryness of the nose and
throat.
In an inhalation study, the threshold for effects on
respiration, skin electric potential, and the electroencephalogram
was found to be 22 mg/m3 (31 ppm) (Alpatov & Mikhailov, 1963;
Alpatov, 1964).
Reports from industries with ammonia exposure indicate that
irritative effects have appeared over a wide range of ammonia
concentrations (Elkins, 1950; Vigliani & Zurlo, 1955; Mangold,
1971). A concentration of 88 mg/m3 was called "definitely
irritating" (Elkins, 1950), and "barely noticeable" eye irritation
was reported at 3 mg/m3 (Mangold, 1971).
Giguz (1968), in a study involving 140 subjects exposed to
ammonia and nitrogen oxides, at concentrations not exceeding the
"maximum permissible concentration" (20 mg/m3), 3 h per day during
2 - 3 years of vocational training, demonstrated increased
incidences of upper respiratory tract disease, compared with those
in a control group of unexposed subjects.
9.2.2. Oral exposure
9.2.2.1. Effects of acute oral exposure
Cases of the ingestion of large doses of ammonia have been
reported. When solutions of ammonia were ingested orally, a
tissue-destructive caustic effect was noted for concentrated
solutions, owing to their high pH. A solution of ammonia at a
concentration of 100 g/litre, for example, has a pH of 12.5.
Oesophageal burns were reported in 25 cases of accidental
ammonia ingestion (Hawkins et al., 1980). One child suffered a
mild burn. Four adults had mild burns limited to the mucosa and
nine adults had oesophageal burns that were moderate or more severe
in nature. One adult female suffered from a complication of airway
obstruction from supraglottic oedema. Oesophageal stricture from
the ingestion of ammonia (100 g/litre) was reported by Vancura et
al. (1980) (Table 19).
A fatal outcome after ingestion of a solution containing 24 g
ammonia/litre was reported by Klendshoj & Rejent (1966). Autopsy
showed haemorrhagic inflammatory changes in the oesophagus,
stomach, and small intestine.
There are many reports on the effects of ammonium chloride, but
since acidosis is caused by the chloride, such studies have little
relevance for evaluating ammonia toxicity.
There are no data on acute effects of ingestion of ammonium
compounds, other than the chloride.
9.2.3. Endogenous hyperammonaemia
9.2.3.1. Inborn errors of metabolism
These affect the uptake of ammonia rather than its rate of
production.
Congenital deficiency of carbamyl phosphate synthetase I (EC
6.3.4.1.6), and, to a lesser extent, of other enzymes of the
ornithine cycle, and several other metabolic disorders may lead to
hyperammonaemia and various abnormal urinary constitutents.
Hyperammonaemia may be lethal in new-born infants, may cause severe
symptoms in infancy, or may cause chronic remittent symptoms in
older children or adults (Hsia, 1974).
Clinical features in neonates may resemble those of hepatic
coma and may be precipitated by protein-rich milk feeds. In older
children, episodic vomiting, neurological disorders (including
seizures) or coma are precipitated by high-protein foods.
9.2.3.2. Hepatic features
Varied and complex functions of the liver may fail
progressively in chronic liver disease or rapidly in acute
disorders. The syndromes differ in the extent to which portal
blood from the intestine is shunted into the systemic venous
circulation either by cirrhotic changes in hepatic vascular
resistance or by surgical procedures to correct portal hypertension
resulting from it.
In either case, a syndrome is recognised that comprises a
spectrum of neurological features from irritability via
inappropriate behaviour, tremors, hyperreflexia and generalised
muscular rigidity, to delirium, stupor, convulsions and coma.
There is disagreement regarding the extent to which this
syndrome is an expression of ammonia toxicity. On the one hand,
over 90% of persons with the disorder have elevated levels of
ammonia in the blood or cerebrospinal fluid; the condition may be
induced in those with marginal liver function by the administration
of ammonium salts or an intestinal haemorrhage (which leads to
intestinal ammonia production greater than that from an equivalent
amount of meat) and the condition may be treated by reducing
intestinal ammonia production, by the administration of antibiotics
to eliminate ammonia-producing flora. On the other hand, there is
an imperfect correlation between the clinical state and the blood-
ammonia level and the condition may occur in the presence of normal
blood-ammonia levels.
It is unlikely that the syndrome resulting from the failure of
an organ as complicated as the liver should be explicable in terms
of a single metabolic component, but the similarity between hepatic
failure and certain expressions of congenital hyperammonaemias
suggests an important role of ammonia toxicity in the pathogenesis
of the syndrome.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Atmospheric Exposure and Effects
10.1.1. General population exposure
Background levels of ammonia in community air are low in
comparison with levels that have been established as safe in
occupational settings, but there is considerable variabilty
according to the type of land use. In general, levels in areas
with intensive livestock husbandry or high rates of manure
application are in the range of 100 - 200 µg/m3, levels in urban
areas range from approximately 5 to 40 µg/m3, while those in rural
areas, without intensive manure production or use, range up to
10 µg/m3. This is in contrast to odour thresholds of the order
10 000 µg/m3, thresholds for irritation of the order 20 000 -
50 000 µg/m3, and the estimated LC for man of 5000 - 10 000 mg/m3.
The LC50 estimation for the rat was 7 600 000 µg/m3, for a 2-h
exposure. In an occupational setting, workers did not voluntarily
use respiratory protective devices at concentrations below about
500 000 µg/m3. General ambient atmospheric levels are therefore
of no concern in respect of discomfort or acute toxicity.
Ammonia is not mutagenic and long-term studies on both
laboratory and farm animals have not shown any pathological effects
at levels below 35 000 µg/m3. Long-term toxic effects are
unlikely, even at levels much higher than ambient levels, both
generally and in the neighbourhood of ammonia-emitting systems.
There is a lack of evidence regarding recent trends in global
atmospheric ammonia concentrations.
10.1.2. Occupational exposure
Occupational problems are predominantly those of accidental
exposure.
Though for certain groups in, for example, agriculture, the
chemical industry, waste disposal and transport, occupational
exposure levels may be very much higher than general, there is
historical evidence that, even at levels significantly in excess of
current occupational exposure limits, there was a low prevalence of
adverse health effects.
The distribution kinetics of absorbed atmospheric ammonia
suggest that the rise in blood-ammonia at a typical occupational
exposure limit will be within the normal range of variation.
Thus, there is both historical and theoretical evidence that
most recommended exposure limits are acceptable.
10.2. Exposure Through Water and Food
Ammonia will have a toxic effect, only if intake exceeds the
capacity of mammals to detoxify ammonia. Unfortunately, there are
no data permitting the evaluation of this capacity in healthy human
beings or other terrestrial mammals. In addition, there is some
evidence that the mode of intake may be a factor in the capacity of
individuals to detoxify ammonia. Parenteral administration of
ammonia results in patterns of metabolism and elimination that are
markedly different from those seen in oral administration. Thus,
the considerable body of data on the parenteral toxicity of ammonia
is not of direct relevance to criteria for oral exposure.
Similarly, because of the different kinetic patterns between oral
and inhalation exposure to ammonia, as well as the highly irritant
effects of ammonia on the lung, the available inhalation data on
ammonia are not applicable to the estimation of an acceptable daily
intake (ADI) for ingestion. However, it would be possible to
define a clearly undesirable level of oral exposure to total
ammonia (NH3 and NH4+) as well as a level that is clearly
tolerable. The range between these 2 levels would exceed the range
within which an ADI could be established.
The amount of excess ammonia (i.e., over and above the amount
normally produced in the body) that can be safely ingested and
assimilated is difficult to define. In short-term (28 - 90 days)
studies carried out on rats and pigs, no adverse effects were
reported at higher levels of ammonia intake (75 - 545 mg NH3/kg
body weight per day) in the form of sulfamate, phosphate, citrate,
or chloride.
The effects attributed directly to elevated ammonium ion levels
are acute pulmonary oedema and central nervous system (CNS)
toxicity, depression of appetite due to a direct effect of the
ammonium ion on the brain, and promotion of growth via the use of
ammonium salts as a source of non-essential nitrogen under certain
circumstances.
Some effects (such as renal growth and demineralization of
bone) arising from the administration of ammonium chloride seem to
be secondary effects of acidosis.
Surveys of total ammonia (NH3 + NH4+) concentrations in surface
waters indicate an average of < 0.18 mg/litre in most areas, and
0.5 mg/litre in waters near large metropolitan areas (Wolaver,
1972; US EPA, 1979a). Levels in ground water are usually low,
since ammonia is generally immobile in soil. Ammonia is practically
absent when drinking-water is chlorinated.
Ammonia is a negligible natural constituent of food, but
ammonium compounds are added in small amounts (< 0.001 - 3.2%) to
various foods as stabilizers, leavening agents, flavourings, or for
other purposes. The daily human intake from these sources is
estimated to be 18 mg as NH3.
10.3. Ocular and Dermal Exposure
Ammonia in aqueous solution or in contact with body fluids is
alkaline and causes burns or inflammation of eyes or skin. The
ocular irritation commonly experienced at atmospheric ammonia
concentrations of > 20 mg/m3 (MacEwen et al., 1970; Keplinger et
al., 1973; Verberk, 1977) is readily reversible when exposure
ceases, and may also be reduced by acclimatization (Ferguson et
al., 1977).
Serious ocular damage normally occurs, only with a direct blast
or splash contact with anhydrous or aqueous ammonia (Grant, 1974).
Skin damage is reported to occur at concentrations of ~7000 mg/m3
(NRC, 1979).
10.4. Accidental Exposure
High gaseous ammonia concentrations may be encountered locally,
both in domestic and work-place environments, as a result of gaseous
emissions and/or spillages of concentrated solutions, and
respiratory (and skin and eye) injury may result. On a larger
scale, spillage from stock or transport tanks or refrigeration
plant of concentrated ammonia liquor or anhydrous ammonia would
constitute a severe environmental insult and would cause serious
injury to the people, animals, and plants in the vicinity. Because
of its low density and short biopersistence, major spillages would
be expected to disperse rapidly and not to persist in the
environment.
10.5. Evaluation of Risks for the Environment
Environments that receive more ammonia than can be used may be
acidified and nitrogen-enriched. As a consequence of these
physical-chemical changes, the structure and functioning of the
ecosystem will be disturbed.
Ammonia plays an important role in the metabolism of all
organisms as a nutrient at low concentrations, but becomes toxic at
higher concentrations. For example, microorganisms both assimilate
and generate ammonia as a part of natural nutrient cycling
processes. High levels of ammonia may inhibit growth or survival
of microbial organisms, including, at higher levels, nitrifying
organisms.
10.5.1. The aquatic environment
Ammonia concentrations in the aquatic environment are variable,
reflecting the proximity and nature of sources (section 4).
Where there are large numbers of people and animals in relation
to the volume of surface waters and drainage flow, the load of
nitrogen added to surface waters from sewage and industrial
effluent is the predominant source and may lead to ammonia
concentrations that constitute a significant local and/or regional
environmental problem.
Otherwise, ammonia deposition contributes a major input. In
surface waters that are poorly buffered, poor in nutrients, and
hydrologically dependent on rainfall and/or snow melt, this may
result in acidification and nitrogen enrichment resulting in marked
changes in plant community structure with concommitant changes in
the animal population structure.
The toxic effects on aquatic organisms are attributed to non-
ionized ammonia (NH3) rather than to ammonium ion levels. This is
because non-ionized ammonia easily penetrates biological membranes,
whereas ammonium ions require specialized transport processes.
There are similarities between the modes of the acute toxic
action of ammonia in mammals and in fish: in the latter, however,
environmental conditions (such as pH, ionic composition and
concentration, temperature, and oxygen availability), which are
more variable in water than in air, have a marked modulating
effect.
Aquatic animals have a limited ability to detoxify ammonia and,
therefore, the body load is dependent on ammonia concentrations in
the water. Except in open oceans, exposure to environmental levels
produces many chronic effects (including reduced growth, decreased
survival, impaired reproduction) and may increase susceptibility to
disease and also cause histopathological changes.
High levels of ammonia in aquatic systems are also toxic for
plants. The detoxification of excessive ammonia places a severe
strain on the carbohydrate metabolism of the plant which
subsequently results in foliar injury, and growth effects, and thus
may modify plant community composition.
Where the dominant species, be it fish or plant, is also
sensitive to ammonia the effects on the whole ecosystem will be
marked.
10.5.2. The terrestrial environment
The most common effect of exposure of plants to atmospheric
ammonia is foliar injury. Prolonged exposure to high ambient
concentrations of about 75 µg/mg, such as occur in the vicinity of
intensive livestock farms, adversely affects more susceptible
species such as pine trees. The observed damage is the result of
both direct and indirect effects due to, changes in soil, and
secondarily, to increased susceptibility to disease and
meteorological stress.
The data on ammonia toxicity for wildlife are very limited.
There is no evidence that wildlife populations, in general, are at
risk from ammonia, but, there may be secondary effects associated
with changes in plant communities. Certain species of bats are
able to withstand the very high ammonia levels found in caves where
they live.
10.6. Conclusions
The major groups of organisms at risk from elevated ammonia
levels are aquatic animals and terrestrial plants. There appears
to be little danger for terrestrial animals, including man, except
from acute accidental exposure.
10.6.1. General population
There are no data suggesting that present environmental levels
of ammonia are hazardous for the general population. Only high-
level accidental exposures from domestic sources and transportation
and storage accidents pose an occasional acute health hazard.
10.6.2. Sub-populations at special risk
Groups likely to exhibit ammonia toxicity include those with
hepatic or renal impairment, though, even in these cases, levels of
exogenous ammonia are insignificant in comparison with endogeneous
levels, so that, in the absence of any environmental exposure, such
persons would still be affected. The mechanism is different in the
two cases. Hepatic impairment limits the conversion of ammonia to
urea, and renal failure, by increasing urea concentrations and its
intestinal secretion, leads to increased endogeneous intestinal
ammonia production.
There have been few studies on the chronic effects of ammonia
inhalation. It can be speculated that subpopulations that have
been found to be hyperreactive to other respiratory irritants
(e.g., sulfur dioxide, particulates, ozone) may also be
hyperreactive to ammonia. These subpopulations may include
children, elderly persons with pre-existing cardiorespiratory
symptoms, individuals with asthma or bronchitis, and those engaged
in vigorous physical exercise (Calabrese, 1978). However, there is
also some indication that previous exposure to low levels of
ammonia may cause inurement to its effects (Ferguson et al., 1977).
10.6.3. Occupational exposure
Accidental exposures are the predominant problem (section
10.1.1.3). Otherwise, occupational exposure can be controlled by
the application of most current occupational exposure limits and
proper industrial hygiene.
10.6.4. Farm animals
Farm animals may be at risk, because of continous exposure
under confined housing conditions resulting in high atmospheric
levels of ammonia within the confinement areas; this applies
particularly to cattle, swine, and poultry. Available reported
data provide a range of measured exposure levels of from 2 to
1400 mg/m3. In winter months in colder climates, most of the
measured concentrations exceeded the admissible upper limit of
35 mg/m3 (50 ppm).
10.6.5. Environment
Environments with a low buffer capacity and poor in nutrients
are susceptible to acidification and nitrogen enrichment by
elevated ammonia loading; prolonged high ammonia-loading results in
changes in both the structure and function of plant and animal
communities. Levels of atmospheric ammonia necessary for the onset
of these changes have not been established; however, changes in the
structure of these communities have been observed where ammonia
levels in the atmosphere were possibly up to 100 µg/m3.
Plants use ammonia as a nutrient, but high levels can be toxic.
Terrestrial plants show a susceptibility to reduced growth and
reduced vitality, when exposed to levels as low as 75 µg NH3/m3 in
the atmosphere.
Aquatic animals have a low capacity to detoxify ammonia. Acute
effects on some fish have been demonstrated in laboratories at
concentrations as low as 0.1 mg NH3/litre and chronic effects at
concentrations as low as 0.02 mg NH3/litre. Thus, as ammonia
levels in some waters are often similar to those shown to cause
chronic effects in some fish, it would appear that these animals
are at risk. Aquatic invertebrates are, in general, less sensitive
to elevated ammonia levels in water.
Aquatic animals are at risk because of increases in ammonia
concentrations in water systems, whereas some plant communities
appear to be at risk from elevations in atmospheric ammonia
loading.
11. RECOMMENDATIONS
11.1. Research Needs
1. Long-term monitoring of ammonia and other pollutants in
water systems with different aquatic ecosystems.
2. Studies of the long-term effects of ammonia on terrestrial
vegetation.
3. Studies of the global nitrogen balance to identify long-
term trends.
4. Ecotoxicological studies to elucidate environmental
effects.
5. Epidemiological studies in relation to ammonia exposure in
order to make better hygiene recommendations.
6. Long-term experimental animal studies to establish a no-
observed-adverse-effect level of exposure.
7. Studies on the role of ammonia in modifying physical and
chemical conditions of soil and water systems.
8. More data are needed to assess accurately the relative
contributions of various point and non-point sources of
ammonia for surface waters.
9. Research into methods and their application directed
towards reducing emissions from point sources.
10. Additional acute toxicity tests with salt-water fish and
invertebrate species.
11. Life-cycle and early-life-stage tests with representative
fresh-water and salt-water organisms from different
families, with investigation of pH effects on chronic
toxicity.
12. Fluctuating or intermittent exposure tests under a variety
of exposure patterns on additional species.
13. Both acute and long-term tests at cold-water temperatures.
14. Studies on the effects of dissolved and suspended solids
on acute and chronic toxicity.
15. More histopathological and histochemical research with
fish, which would provide a rapid means of identifying and
quantifying sublethal ammonia effects.
16. In fish, the relative concentration limits for both
acclimatization and subsequent acute response need better
definition and a more complete explanation.
REFERENCES
ABBAS, R. & TANNER, R.L. (1981) Continuous determination of
gaseous ammonia in the ambient atmosphere using fluorescence
derivatization. Atmos. Environ., 15: 277-281.
ABELOVICH, A. & AZOV, Y. (1976) Toxicity of ammonia to algae
in sewage oxidation ponds. Appl. environ. Microbiol., 31(6):
801-806.
ADMIRAAL, W. (1977) Tolerance of estuarine benthic diatoms
to high concentrations of ammonia, nitrite ion, nitrate ion
and orthophosphate. Mar. Biol., 43(4): 307-315.
AGAMI, M., LITAV, M., & WAISEL, Y. (1976) The effects of
various components of water pollution on the behaviour of some
aquatic macrophytes of the coastal rivers of Israel. Aquat.
Bot., 2(3): 203-213.
ALABASTER, J.S. & HERBERT, D.W.M. (1954) Influence of carbon
dioxide on the toxicity of ammonia. Nature (Lond.), 174(4426):
404.
ALABASTER, J.S., SHURBEN, D.G., & KNOWLES, G. (1979) The
effect of dissolved oxygen and salinity on the toxicity of
ammonia to smolts of salmon, Salmo salar L. J. Fish Biol.,
15(6): 705-712.
ALLAN, I.R.H. (1955) Effects of pollution on fisheries.
Verh. Int. Ver. Theor. Agnew Limnol., 12: 804-810.
ALLAN, I.R.H., HERBERT, D.W.M., & ALABASTER, J.S. (1958) A
field and laboratory investigation of fish in a sewage
effluent. Fish. Invest., 6(2): 1-76.
ALPATOV, I.M. (1964) [A study of gazeous ammonia toxicity.]
Gig. Tr. prof. Zabol., 2: 14-18 (in Russian).
ALPATOV, I.M. & MIKHAILOV, V.I. (1963) [Inquires into the
gaseous ammonia toxicity. Communication 1.] Gig. Tr. prof.
Zabol., 12: 51-53 (in Russian)
ANALYTICAL QUALITY CONTROL (HARMONISED MONITORING) COMMITTTEE
(1982) Accuracy of determination of ammoniacal nitrogen in
river waters: analytical quality control in the harmonised
monitoring scheme. Analyst, 107(June): 680-688.
ANDERSON, B.G. (1948) The apparent thresholds of toxicity to
Daphnia magna for chlorides of various metals when added to
Lake Erie water. Trans. Am. Fish Soc., 78: 96-113.
ANDERSON, D.P., BEARD, C.W., & HANSON, R.P. (1964a) The
adverse effects of ammonia on chickens including resistance to
infections with Newcastle disease virus. Avian Dis., 8:
369-379.
ANDERSON, D.P., CHERMS, F.L., & HANSON, R.P. (1964b) Studies
on measuring the environment of turkeys raised in confinement.
Poult. Sci., 43: 305-318.
ANDERSON, K.B., SPARKS, R.E., & PAPARO, A.A. (1978) Rapid
assessment of water quality, using the fingernail clam,
Musculium transversum, Urbana, Illinois, Water Resources
Center, University of Illinois, 115 pp (WRC Research Report
No. 133).
ANGHILERI, L.G. (1975) Tumour growth inhibition by ammonium
chloride-induced acidosis. Int. J. clin. Pharmacol., 12: 320.
ANTHONISEN, A.C., LOEHR, R.C., PRAKASAM, T.B.S., & SRINATH,
E.G. (1976) Inhibition of nitrification by ammonia and
nitrous acid. J. Water Poll. Control Fed., 48: 835-852.
APHA (1976) Standard methods for the examination of water
and wastewater, 14th ed., Washington DC, American Public
Health Association, American Water Works Association, Water
Pollution Control Federation, 1193 pp.
API (1981) The sources, chemistry, fate, and effects of
ammonia in aquatic environments, Washington DC, American
Petroleum Institute, 145 pp.
APPELMAN, L.M., TEN BERGE, W.F., & REUZEL, P.G.J. (1982)
Acute inhalation toxicity study of ammonia in rats with
variable exposure periods. Am. Ind. Hyg. Assoc. J., 43(9):
662-665.
ARMSTRONG, D.A., CHIPPENDALE, D., KNIGHT, A.W., & COLT, J.E.
(1978) Interaction of ionized and un-ionized ammonia on
short-term survival and growth of prawn larvae, Macrobrachium
rosenbergii. Biol. Bull., 154(1): 15-31.
ARNDT, F.L., DAY, D.L., & HATFIELD, E. (1979) Processing and
Handling of animal excreta for refeeding. J. anim. Sci., 48:
157.
ASTM (1980) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
AUERBACH, C. & ROBSON, J.M. (1947) Tests of chemical
substances for mutagenic action. Proc. R. Soc. Edinburgh Sec.
Biol., 62B: 284-291.
AVOT, YU.K., KANEP, V.V., SLUTSKER, D.S., & SHAFRAN, L.M.
(1977) [Medical and sanitary aspects of sailors' work,] Riga,
Zvaigzne, 132 pp (in Russian).
AXELROD, H.D. & GREENBERG, J.P. (1976) Determination of
trace concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 10: 495-496.
BAIRD, R., BOTTOMLEY, J., & TAITZ, H. (1979) Ammonia
toxicity and pH control in fish toxicity bioassays of treated
wastewaters. Water Res., 13(2): 181-184.
BALL, I.R. (1967) The relative susceptibilities of some
species of fresh-water fish to poisons. I. Ammonia. Water
Res., 1(11/12): 767-775.
BARROW, C.S. & STEINHAGEN, W.H. (1980) NH3 concentrations in
the expired air of the rat: Importance to inhalation
toxicology. Toxicol. appl. Pharmacol., 53: 116-121.
BARROW, C.S., ALARIE, Y., & STOCK, M.F. (1978) Sensory
irritation and incapacitation evoked by thermal decomposition
products of polymers and comparisons with known sensory
irritants. Arch. environ. Health, 33(2): 79-88.
BARTON, L.V. (1940) Toxicity of ammonia, chlorine, hydrogen
cyanide, hydrogen sulphide and sulphur dioxide gases. IV.
Seeds. Contrib. Boyce Thompson Inst., 11: 357-363.
BARZEL, U.S. (1975) Effect of chronic ammonium chloride
ingestion on parathyroid-hormone function. Nephron, 14(15):
339-346.
BARZEL, U.S. & JOWSEY, J. (1969) Effects of chronic acid and
alkali administration on bone turnover in adult rats. Clin.
Sci., 36(3): 517-524.
BENEDICT, H.M. & BREEN, W.H. (1955) The use of weeds as a
means of evaluating vegetation damage caused by air pollution.
In: Proceedings of the Third National Air Pollution
Symposium, Pasadena, California, April 1955, pp. 177-190.
BENNETT, W.F., PESEK, J., & HANWAY, J.J. (1964) Effects of
nitrate and ammonium on growth of corn in nutrient solution
sand culture. Agric. J., 56: 342-345.
BENYAJATI, S. & GOLDSTEIN, L. (1975) Renal glutaminase
adaptation and ammonia excretion in infant rats.
Am. J. Physiol., 228(3): 693-698.
BERL, S., TAKAGAKI, G., SCHWERIN, P., & WAELSCH, H. (1962)
Metabolic compartments in vivo: Ammonia and glutamic acid
metabolism in brain and liver. J. biol. Chem., 237: 2562-2569.
BESSMAN, S.P. & BESSMAN, A.N. (1955) The cerebral and
peripheral uptake of ammonia in liver disease with an
hypothesis for the mechanism of hepatic coma. J. clin.
Invest., 34: 622-628.
BHATTACHARYA, A.N. & TAYLOR, J.C. (1975) Recycling animal
waste as a feedstuff: A review. J. anim. Sci., 44: 1438.
BIDWELL, R.G.B. (1974) Plant physiology, New York,
MacMillan, 197 pp.
BINSTOCK, L. & LECAR, H. (1969) Ammonium ion currents in the
squid giant axon. J. gen. Physiol., 53: 342-361.
BIRNBAUM, S.M., WINTZ, M., & GREENSTEIN, J.P. (1957)
Quantitative nutritional studies with water-soluble,
chemically defined diets. III. Individual amino acids as
sources of "non-essential" nitrogen. Arch. Biochem. Biophys.,
72(2): 428-436.
BISWAS, S.D. & KLEINBERG, I. (1971) Effect of urea
concentration on its utilisation, on the pH and the formation
of ammonia and carbon dioxide in a human salivary sediment
system. Arch. oral Biol., 16: 759-780.
BLOOMFIELD, R.A., GARNER, G.B., & MUHRER, M.E. (1960)
Kinetics of urea metabolism in sheep. J. anim. Sci., 19: 1248.
BLOOMFIELD, R.A., KEARLEY, E.O., CREACH, D.O., & MUHRER, M.E.
(1962) Ruminal pH and absorption of ammonia and VFA. J. anim.
Sci., 22: 833 (Abstract).
BOCK, F., MICHELSON, I., BROSS, I.D., & PRIORE, R.L. (1974)
Carcinogenic activity of smoke condensate from cigarettes with
ammonium sulfamate-treated paper. Cancer, 33: 1010.
BODANSKY, A., JAFFE, M.L., & CHANDLER, J.P. (1932) Serum
phosphatase changes in ammonium chloride osteoporosis. Proc.
Soc. Exp. Biol. Med., 29: 871-875.
BOER, W.M.J., DEN & BASTIAENS H. (1984) [Effects of
acidification on vegetation,] Ministry of Agriculture and
Fisheries, Ministry of Housing, Physical Planning and
Environment, 82 pp (Report No. 84(2)) (in Dutch).
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1973)
Ammonia kills spoilage moulds in corn. J. Dairy Sci., 56: 241.
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1975a)
Effect of pH and substrate on growth. Eur. J. appl. Microbiol,
1: 55-56.
BOTHAST, R.J., ADAMS, G.H., HATFIELD, E.E., & LANCASTER, E.B.
(1975b) Preservation of high-moisture corn: a microbiological
evaluation J. Dairy Sci., 58: 386-391.
BOYD, E.M. & SEYMOUR, K.G.W. (1946) Ethylenediamine
dihydrochloride or "chlorethamine". II. Untoward and toxic
reactions. Exp. Med. Surg., : 223-227.
BOYD, E.M., McLACHLAN, M.L., & PERRY, W.F. (1944)
Experimental ammonia gas poisoning in rabbits and cats.
J. ind. Hyg. Toxicol., 26: 29-34.
BRAGANGA, B.M., FAULKNER, P., & QUASTEL, J.M. (1953) Effects
of inhibitors of glutamine synthesis in brain slices by
ammonium ions. Biochim. Biophys. Acta, 10: 83-88.
BREEDING, R.J., LODGE, J.P., Jr, PATE, J.B., SHEESLEY, D.C.,
KLONIS, H.B., FOGLE, B., ANDERSON, J.A., ENGLERT, T.R.,
HAAGENSON, P.L., MCBETH, R.B., MORRIS, A.L., POGUE, R., &
WARTBURG, A.F. (1973) Background trace concentrations in the
central United States. J. geophys. Res., 78: 7057-7064.
BREEDING, R.J., KLONIS, H.B., LODGE, J.P., Jr, PATE, J.B.,
SHEESLEY, D.C., ENGLERT, T.R., & SEARS, D.R. (1976)
Measurements of atmospheric pollutants in the St. Louis area.
Atmos. Environ., 10: 181-184.
BRENNAN, E., LEONE, I.A., & DAINES, R.H. (1962) Ammonia
injury to applies and peaches in storage. Plant Dis. Rep., 46:
792-795.
BRESSANI, R. & BRAHAM, J.E. (1977) Effect of frequency of
feeding on blood serum urea changes in dogs. J. Nutr. Rep.
Int., 16(3): 305-316.
BRETT, J.R. & ZALA, C.A. (1975) Daily pattern of nitrogen
excretion and oxygen consumption of sockeye salmon
(Oncorhyncus nerka) under controlled conditions. J. Fish. Res.
Board Can., 32: 2479-2486.
BRETTHAUER, R. (1978) Some ecological limits of tolerance to
Ochromonas sociabilis. Verh. Internat. Verein. Limnol., 20(3):
1850-1854.
BROCKWAY, D.R. (1950) Metabolic products and their effects.
Prog. Fish-Cult., 12(3): 127-129.
BRODERIUS, S.J. & SMITH, L.L., Jr (1979) Lethal and
sublethal effects of binary mixtures of cyanide and hexavalent
chromium, zinc, or ammonia to the fathead minnow Pimephales
promelas and rainbow trout Salmo gairdneri. J. Fish. Res.
Board Can., 36(2): 164-172.
BRODERIUS, S.J., SMITH, L.L., Jr, & LIND, D.T. (1977)
Relative toxicity of free cyanide and dissolved sulfide forms
to the fathead minnow. Pimephales promelas. J. Fish. Res.
Board Can., 34(12): 2323-2332.
BRODERIUS, S.J., DRUMMOND, R.A., FIANDT, J.T., & RUSSOM, C.L.
(in press) Toxicity of ammonia to smallmouth bass,
Micropterus dolomieui, as related to pH. Environ. Toxicol.
Chem..
BRODERSON, J.R., LINDSEY, J.R., & CRAWFORD, J.E. (1976) The
role of environmental ammonia in respiratory mycoplasmosis of
rats. Am. J. Pathol., 85: 115-130.
BROSSET, C., ANDREASSON, K., & FERM, M. (1975) The nature
and possible origin of acid particles observed at the Swedish
west coast. Atmos. Environ., 9: 631-642.
BROWN, A.C. (1974) Observation on the effect of ammonium
nitrate solutions on some common marine animals from Table
Bay. Trans. R. Soc. S. Afr., 41(2): 217-223.
BROWN, V.M. (1968) The calculation of the acute toxicity of
mixtures of poisons to rainbow trout. Water Res., 2(10):
723-733.
BROWN, R.H., DUDA, G.D., KORKES, S., & HANDLER, P. (1957) A
colorimetric micromethod for determination of ammonia: the
ammonia content of rat tissues and human plasma. Arch.
Biochem. Biophys., 66: 301-309.
BROWN, V.M., JORDAN, D.H.M., & TILLER, B.A. (1969) The acute
toxicity to rainbow trout of fluctuating concentrations and
mixtures of ammonia, phenol and zinc. J. Fish Biol., 1(1): 1-9.
BUCKLEY, J.A. (1978) Acute toxicity of unionized ammonia to
fingerling coho salmon. Prog. Fish-Cult., 40(1): 30-32.
BUCKLEY, J.A., WHITMORE, C.M., & LIMING, B.D. (1979) Effects
of prolonged exposure to ammonia on the blood and liver
glycogen of coho salmon (Oncorhynchus kisutch). Comp. Biochem.
Physiol., 63C(2): 297-303.
BUIKEMA, A.I., Jr, CAIRNS, J., Jr, & SULLIVAN, G.W. (1974)
Evaluation of Philodina acuticornis (Rotifera) as a bioassay
organism for heavy metals. Water Res. Bull., 10(4): 648-661.
BUKHOVSKAYA, M.S., GINZBURG, S.L., & KHALIZOVA, O. (1966)
[Methods for determination of harmful substances in air. A
practical guide,] 2nd ed., Moscow, Meditaina (in Russian).
BULLIS, K.L., SNOEYENBOS, G.H., & VAN ROEKEL, H. (1950) A
keratoconjunctivitis in chickens. Poult. Sci., 29: 386-389.
BURKHALTER, D.E. & KAYA, C.M. (1977) Effects of prolonged
exposure to ammonia on fertilized eggs and sac fry of rainbow
trout (Salmo gairdneri). Trans. Am. Fish Soc., 106(5): 470-475.
BURROWS, R.E. (1964) Effects of acclimated excretory
products on hatchery-reared salmonids, Washington DC, Fish and
Wildlife Service, US Department of the Interior, 12 pp
(Research Report 66).
BUTTERWORTH, B. (1979) Nutritionally improved straw: the
machines do the job. Agric. Eng., 60(7): 41-42.
BUYSMAN, E. (1984) [Ammonia emissions in the Netherlands,]
Ministry of Housing, Physical Planning, and Environment,
Directorate Air (Report No. 22) (in Dutch).
BUYSMAN, E., MAAS, J.F.M., & ASMAN, W.A.M. (1985) Ammonia
emission in Europe, Utrecht, the Netherlands, Institute for
Meteorology and Oceanography, State University Utrecht.
BYERRUM, R.U. & BENSON, A.A. (1975) Effect of ammonia on
photosynthetic rate and photosynthate release by Amphidinium
carterae (Dinophyceae). J. Phycol., 11: 449-452.
CALABRESE, E.J. (1978) Pollutants and high risk groups,
New York, Wiley-Interscience Publications.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1977) [Effect of
prolonged treatments with ammonia on stages of development of
Salmo gairdneri.] Nuovi Ann. Ig. Microbiol., 28(5): 333-345.
(in Italian).
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1981) Effects of
long-term exposure to ammonia on the developmental stages of
rainbow trout ( Salmo gairdnerii Richardson). Rapp. P.-V. Réun.
Cons. Int. Explor. Mer, 178: 81-86.
CAMERON, J.N. (1979) Excretion of CO2 in water-breathing
animals. Marine Biol. Lett., 1: 3-13.
CAMERON, J.N. & HEISLER, N. (1983) Studies of ammonia in the
rainbow trout physio-chemical parameter, acid-base behavior,
and respiratory clearance. J. exp. Biol., 105:: 107-125.
CAMERON, G.R. & SHEIKH, A.H. (1951) Experimental production
of pulmonary edema with ammonium salts, together with
classification of lung oedemas. J. Pathol. Bacteriol., 63:
609-617.
CAMPBELL, C.L., DAWES, R.K., DEOLALKAR, S., & MERRITT, M.C.
(1958) Effect of certain chemicals in water on the flavor of
brewed coffee. Food Res., 23: 575-579.
CAPLIN, M. (1941) Ammonia gas poisoning. Forty-seven cases
in a London shelter. Lancet, 2: 95-96.
CAPUCO, A.V. (1977) Ammonia, a modulator of 3T3 cell growth,
Ithaca, New York, Cornell University (Ph.D Thesis).
CARPENTER, C.P., SMYTH, H.S., Jr, & SHAFFER, C.B. (1948) The
acute toxicity of ethylene imine to small animals. J. ind.
Hyg. Toxicol., 30: 2-6.
CARY, G.A. (1976) A report on the assessment of aquatic
environmental impact of Union Carbide's Uravan operations:
on-site toxicity bioassays, Tarrytown, New York, Aquatic
Environmental Sciences, Union Carbide Corporation, 67 pp
(Report prepared for Metals Division, Union Carbide
Corporation, Uravan, Colorado).
CASTELL, D.O. & MOORE, E.W. (1971) Ammonia absorption from
the human colon. Gastroenterology, 60(1): 33-42.
CATEDRAL, F.F., COLOSO, R., VALERA, N., CASALMIR, C.M., &
QUIBUYEN, A.T. (1977a) Effect of some physico-chemical
factors on the survival and growth of Penaeus monodon
postlarvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian Fish.
Dev. Cent., 1(3): 13-16.
CATEDRAL, F.F., GEROCHI, D.D., QUIBUYEN, A.T., & CASALMIR,
C.M. (1977b) Effect of nitrite, ammonia, and temperature on
P. monodon larvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian
Fish. Dev. Cent., 1(3): 9-12.
CHAMP, M.A., LOCK, J.T., BJORK, C.D., KLUSSMANN, W.G., &
MCCULLOUGH, J.D., Jr (1973) Effects of anhydrous ammonia on
a central Texas pond and a review of previous research with
ammonia in fisheries management. Trans. Am. Fish. Soc.,
102(1): 73-82.
CHARLES, D.R. & PAYNE, C.G. (1966a) The influence of graded
levels of atmospheric ammonia on chickens. I. Effects on
respiration and on the performance of broilers and replacement
growing stock. Br. Poult. Sci., 7: 177-187.
CHARLES, D.R. & PAYNE, C.G. (1966b) The influence of graded
levels of atmospheric ammonia on chickens. II. Effects on the
performance of laying hens. Br. Poult. Sci., 7: 189-198.
CHEMICAL AND ENGINEERING NEWS (1980) C and EN's top fifty
chemical products. Am. Chem. Soc., : 16.
CHIPMAN, W.A., Jr (1934) The role of pH in determining the
toxicity of ammonium compounds, Columbia, Missouri, University
of Missouri, 153 pp (Ph.D. Thesis).
CLAIBORNE, J.B., EVANS, D.H., & GOLDSTEIN, L. (1982) Fish
branchial Na+-K+-activated ATPase. J. exp. Biol., 96: 431-434.
CLAIBORNE, J.B. & HEISLER, N. (1984) Acid-base regulation in
the carp (Cyprinus carpio) during and after exposure to
environmental hypercapnia. J. exp. Biol., 108: 25-43.
CLAWSON, A.J. & ARMSTRONG, W.D. (1981) Ammonium
polyphosphate as a source of phosphorous and nonprotein
nitrogen for monogastrics (in corn-soybean meal diets for
growing rats and growing finishing [G-F] pigs). J. anim. Sci.,
52(1): 1-7.
CLOSE, L.G., CATLIN, F.I., & COHN, A.M. (1980) Acute and
chronic effects of ammonia burns of the respiratory tract.
Arch. Otolaryngol., 106: 151-158.
COLE, T.J., COTES, J.E., JOHNSON, G.R., & MARTIN, H. (1977)
Ventilation cardiac frequency and pattern of breathing during
exercise in men exposed to o-chlorobenzylidene malonitril and
ammonia gas in low concentrations. Q. J. exp. Physiol., 62:
341-351.
COLT, J.E. (1978) The effects of ammonia on the growth of
channel catfish, Ictalurus punctatus, Davis, California,
University of California, 185 pp (Ph.D. Thesis).
COLT, J.E. & TCHOBANOGLOUS, G. (1976) Evaluation of the
short-term toxicity of nitrogenous compounds to channel
catfish, Ictalurus punctatus. Aquaculture, 8(3): 209-224.
COLT, J.E. & TCHOBANOGLOUS, G. (1978) Chronic exposure of
channel catfish, Ictalurus punctatus to ammonia: effects on
growth and survival. Aquaculture, 15(4): 353-372.
CONN, H.O. (1972) Studies of the source and significance of
blood ammonia. IV. Early ammonia peaks after ingestion of
ammonium salts. Yale J. Biol. Med., 45: 543-549.
CONSTANTINE, D.G. (1958) Bleaching of hair pigment in bats
by the atmosphere in caves. J. Mammal, 39: 513-520.
COOMBE, J.B., TRIBE, D.E., & MORRISON, J.W. (1960) Some
experimental observations on the toxicity of urea to sheep.
Aust. J. agric. Res., 11: 247-256.
COON, R.A., JONES, R.A., JENKINS, L.J., Jr, & SIEGEL, J.
(1970) Animal inhalation studies on ammonia, ethylene glycol,
formaldehyde, dimethylamine, and ethanol. Toxicol. appl.
Pharmacol., 16: 646-655.
COTTERILL, O.J. & NORDSKOG, A.W. (1954) Influence of ammonia
on egg white quality. Poult. Sci., 33: 432-434.
CRIBB, G.S. (1964) Nitrogen. In: Mellor's comprehensive
treatise on inorganic and theoretical chemistry, London,
Longmans, Vol. 8, Suppl. I, pp. 276-327.
CURTIS, S.E. (1972) Air environment and animal performance.
J. anim. Sci., 35: 628-634.
DALHAMN, T. (1963) Effect of ammonia alone and combined with
carbon particles on ciliary activity in the rabbit trachea in
vivo, with studies of the absorption capacity of the nasal
cavity. Int. J. Air Water Pollut., 7: 531-539.
DALHAMN, T. & SJOHOLM, J. (1961) Studies of SO2, NO2, and
NH3: effect on ciliary activity in the rabbit trachea of
single in vitro exposure and resorption in rabbit nasal
cavity. Acta physiol. Scand., 58: 287-291.
DALLAVALLE, J.M. & DUDLEY, H.C. (1939) Evaluation of odor
nuisance in the manufacture of kraft paper. Public Health
Rep., 54(1): 35-43.
DANECKER, E. (1964) [The jauche poisoning of fish: an
ammonia poisoning.] Osterr. Fischerei., 3/4: 55-68. (in
German).
DAVIES, B.M.A. & YUDKIN, J. (1952) Studies in biochemical
adaptation. The origin of urinary ammonia as indicated by the
effect of chronic acidosis and alkalosis on some renal enzymes
in the rat. Biochem. J., 52: 407-412.
DAY, D.L., HANSEN, E.L., & ANDERSON, S. (1965) Gases and
odors in confinement swine buildings. Trans. Am. Soc. Agric.
Eng., 8: 118-121.
DEAL, P.H., SOUZA, K.A., & MACK, H.M. (1975) High pH,
ammonia toxicity, and the search for life on the Jovian
planets. Origins Life, 6(4): 561-573.
DEAN, J.A., ed. (1979) Lange's handbook of chemistry, 12th
ed., New York, McGraw-Hill Book Company.
DEGRAEVE, G.M., OVERCAST, R.L., & BERGMAN, H.L. (1980)
Toxicity of underground coal gasification condenser water and
selected constitutents to aquatic biota. Arch. environ.
Contam. Toxicol., 9(5): 543-555.
DELISTRATY, D.A., CARLBERG, J.M., VAN OLST, J.C., & FORD,
R.F. (1977) Ammonia toxicity in cultured larvae of the
American lobster (Homarus americanus). In: Proceedings of the
8th Annual Meeting of the World Mariculture Society, San Jose,
Costa Rica, pp. 647-673.
DEMEREC, M., BERTANI, G., & FLINT, J. (1951) A survey of
chemicals for mutagenic action on E. coli. Am. Nat., 85: 119.
DEMUYNCK, M., RAHN, K.A., JANSSENS, M., & DAMS, R. (1976)
Chemical analysis of airborne particulate matter during a
period of unusually high pollution. Atmos. Environ., 10: 21-26.
DE VOOYS, G.G.N. (1968) Formation and excretion of ammonia
in Teleostei. I. Excretion of ammonia through the gills. Arch.
int. Physiol. Biochimie., 76: 268-272.
DODD, K.T. & GROSS, D.R. (1980) Ammonia inhalation toxicity
in cats. Arch. environ. Health, 35: 6-14.
DOIG, P.A. & WILLOUGHBY, R.A. (1971) Response of swine to
atmospheric ammonia and organic dust. J. Am. Vet. Med. Assoc.,
159: 1353-1361.
DOWDEN, B.F. & BENNETT, H.J. (1965) Toxicity of selected
chemicals to certain animals. J. Water Pollut. Control. Fed.,
37(9): 1308-1316.
DOWNING, K.M. & MERKENS, J.C. (1955) The influence of
dissolved-oxygen concentration on the toxicity of un-ionized
ammonia to rainbow trout ( Salmo gairdneri Richardson).
Ann. appl. Biol., 43(2): 243-246.
DREWES, D.R. & HALES, J.M. (1980) Removal of pollutants from
power plant plumes by precipitation, Richland, Washington,
Battelle-Northwest, US Electric Power Research Institute
(Report to the EPRI).
DRIEDZIC, W.R. & HOCHACHKA, P.W. (1976) Control of energy
metabolism in fish white muscle. Am. J. Physiol., 230: 579-582.
DRUMMOND, J.G., CURTIS, S.E., SIMON, J., & NORTON, H.W.
(1980) Effects of aerial ammonia on growth and health of
young pigs. J. anim. Sci., 50: 1085-1091.
DUDA, G.D. & HANDLER, P. (1958) Kinetics of ammonia
metabolism in vivo. J. biol. Chem., 232: 303-314.
DU RUISSEAU, J.P., GREENSTEIN, J.P., WINITZ, M., & BIRNBAUM,
S.M. (1957) Studies on the metabolism of amino acids and
related compounds in vivo. VI. Free amino acid levels in the
tissues of rats protected against ammonia toxicity. Arch.
Biochem. Biophys., 68: 161-171.
EADS, R.B., WISEMAN, J.S., GRIMES, J.E., & MENZIES, G.C.
(1955) Wildlife rabies in Texas. Public Health Rep., 70:
995-1000.
ECKERT, J.W. (1967) Application and use of postharvest
fungicides. In: Torgeson, D.C., ed. Fungicides, New York,
Academic Press, pp. 341.
ECKERT, J.W., KOLBEZEN, M.J., & GARBER, M.J. (1963)
Evaluation of ammonia-generating formations for control of
citrus fruit decay. Phytopathology, 53: 140-148.
ECOLOGICAL ANALYSTS, INC. (1981) The sources, chemistry,
fate, and effects of ammonia in aquatic environments,
Washington DC, American Petroleum Institute.
EDELMANN, C.M., Jr, BIOCHIS, H., SORIANO, J.R., & STARK, H.
(1967) The renal response of children to acute ammonium
chloride acidosis. Pediat. Res., 1: 452-460.
EDJTEHADI, M., SZABUNIEWICZ, M., & EMMANUEL, B. (1978) Acute
urea toxicity in sheep. Can. J. Comp. Med., 42: 63-68.
EDWARDS, J.G. & CONDORELLI, L. (1928) Studies on aglomerular
and glomerular kidneys. II. Physiological. Am.J. Physiol., 86:
383-398.
EGGELTON, A.E.J. (1969) The chemical composition of
atmospheric aerosols on Tees-side and its relation to
visibility. Atmos. Environ., 3: 355-372.
EGLE, J.L., Jr (1973) Retention of inhaled acetone and
ammonia in the dog. Am. Ind. Hyg. Assoc. J., 34(12): 533-539.
ELKINS, H.B. (1950) The chemistry of industrial toxicology,
New York, John Wiley and Sons, 84 pp.
ELKINS, H.B. (1959) Ammonia, NH3. In: The chemistry of
industrial toxicology, 2nd ed., New York, John Wiley and Sons,
86 pp.
EMERSON, K., RUSSO, R.C., LUND, R.E., & THURSTON, R.V.
(1975) Aqueous ammonia equilibrium calculations: effect of pH
and temperature. J. Fish. Res. Board Can., 32: 2379-2383.
EMERY, R.M. & WELCH, E.B. (1969) The toxicity of alkaline
solutions of ammonia to juvenile bluegill sunfish (Lepomis
macrochirus Raf.), Chattanooga, Tennessee, Water Quality
Branch, Division of Health and Safety, Tennessee Valley
Authority, 31 pp (Mimeo).
EPEL, D., STEINHARDT, R., HUMPHREYS, T., & MAZIA, D. (1974)
An analysis of the partial metabolic derepression of sea
urchin eggs by ammonia; the existence of independent pathways.
Dev. Biol., 40: 245-255.
EPIFANO, C.E. & SRNA, R.F. (1975) Toxicity of ammonia,
nitrite ion, nitrate ion, and orthophosphate to Mercenaria
mercenaria and Crassostrea virginica. Mar. Biol., 33(3):
241-246.
ERNST, R.A. (1968) The effect of ammonia on poultry.
Feedstuffs, 40(32): 40.
EUROPEAN INLAND FISHERIES ADVISORY COMMISSION (1970) Water
quality criteria for European freshwater fish. Report on
ammonia and inland fisheries, pp. 12 (EIFAC Technical Paper
No. 11) (Also in: Water Res., 7(7): 1011-1022 (1973)).
EVANS, D.H. (1977) Further evidence for Na/NH4 exchange in
marine teleost fish. J. exp. Biol., 70: 213-220.
EVANS, D.H. (1980) Osmotic and ionic regulation by
freshwater and marine fishes. In: Ali, M.A. ed. Environmental
Physiology of Fishes, New York, Plenum Publishing Co.,
pp. 93-122.
EVANS, D.H., KORMANIK, G.A., & KRASNY, E.J. (1979)
Mechanisms of ammonia and acid extrusion by the little skate,
Raja erinacea. J. exp. Zool., 208: 431-437.
EVANS, J.W. (1979) The construction and use of a
continuous-flow bioassay apparatus to determine a preliminary
un-ionized ammonia 96-h LC50 for the crayfish, Orconectes
nais, Lawrence, Kansas, University of Kansas, 76 pp (M.S.
Thesis).
FARBER, R.J. & ROSSANO, A.T. (1975) A new approach for
measuring atmospheric concentrations of ammonia. Paper
presented at Annual Meeting of the Pacific Northwest
International Section of the Air Pollution Control
Association, Vancouver, British Columbia, November 19-21,
36 pp.
FASEB (1974) Evaluation of the health aspects of certain
ammonium salts as food ingredients, Springfield, Virginia,
Federation of American Societies for Experimental Biology,
21 pp (Prepared for the US Food and Drug Administration under
Contract No. FDA 72-85) (US NTIS PPB-254-532).
FAUCONNEAU, B. & LUQUET, P. (1979) Influence d'une élévation
de température sur l'évolution de l'aminoacidémie et de
l'ammoniémie après le repas chez la truite arc en ciel (Salmo
gairdneri R.). Ann. Biol. anim. Biochim. Biophys., 19(4A):
1063-1079.
FAZEKAS, I.G. (1939) Experimental suprarenal hypertrophy
induced by ammonium hydroxide. Endokrinologie, 21: 315-337.
FAZEKAS, I.G. (1949) [Experimental data on influencing
ovarian functions by simple compounds.] Orv. Hetil., 90:
777-781 (in German).
FAZEKAS, I.G. (1954a) [Enlargement of the parathyroids by
treatment with simple acidotic compounds.] Kirchow's Arch.
Pathol. Anat. Physiol., 324: 531-542 (in German).
FAZEKAS, J.F., TICKTIN, H.E., EHRMENTRAUT, W.R., & ALMAN,
R.W. (1956) Cerebral metabolism in hepatic insufficiency.
Am. J. Med., 21: 843-849.
FEPCC (1972) [Investigation of air pollution in the city of
Tsuruga.] Annu. Rep. Fukui Environ. Pollut. Control Centre, 1:
88-116 (in Japanese).
FERGUSON, W.S., KOCH, W.C., WEBSTER, L.B., & GOULD, J.R.
(1977) Human physiological response and adaptation to
ammonia. J. occup. Med., 19: 319-326.
FETH, J.H. (1966) Nitrogen compounds in natural water. A
review. Water Resour. Res., 2: 41-58.
FISHER, D.W., GAMBELL, A.W., LIKENS, G.E., & BURMANN, F.H.
(1968) Atmospheric contributions to water quality of streams
in the Hubbard Brook Experimental Forest, New Hampshire. Water
Resour. Res., 4: 1115-1126.
FLAKS, A. & CLAYSON, D.B. (1975) The influence of ammonium
chloride on the induction of bladder tumors by 4-ethyl-
sulfonylnaphthalene-1-sulfonamide. Br. J. Cancer, 31: 585.
FLAKS, A., HAMILTON, J.M., & CLAYSON, D.B. (1973) Effect of
ammonium chloride on incidence of bladder tumours induced by
4-ethylsulfonylnapthalene-1-sulfonamide. J. Natl Cancer Inst.,
51: 2007.
FLORKIN, M. & DUCHATEAU, G. (1943) Les formes du système
enzymatique de l'uricalyse et l'évolution du catabolisme
purique chez les animaux. Arch. int. Physiol., 53: 267-307.
FLURY, K.E., DINES, D.E., RODARTE, J.R., & RODGERS, R.
(1983) Airway obstruction due to inhalation of ammonia. Mayo
Clin. Proc., 58: 389-393.
FLYNN, R.J. (1968) A new cage cover as an aid to laboratory
rodent disease control. Proc. Soc. Exp. Biol. Med., 129:
714-717.
FORSTER, R.P. & GOLDSTEIN, L. (1969) Formation of excretory
products. In: Hoar, W.S. & Randall, D.J. ed. Fish Physiology,
New York, Academic Press, Vol. 1, pp. 313-350.
FOSTER, G.L., SCHOENHEIMER, R., & RITTENBERG, D. (1939)
Studies in protein metabolism. V. The utilization of ammonia
for amino acid and creatine formation in animals. J. biol.
Chem., 127: 319-327.
FRANK, J.F. (1948) The toxicity of sodium chlorate
herbicides. Can. J. comp. Med., 12: 216-218.
FRASER, D.I., DYER, W.J., WEINSTEIN, H.M., DINGLE, J.R., &
HINES, J.A. (1966) Glycolytic metabolites and their
distribution at death in the white and red muscle of cod
following various degrees of antemortem muscular activity.
Can. J. Biochem., 44: 1015-1033.
FREEDMAN, L.R. & BEESON, P.B. (1961) Experimental
pyelonephritis. VIII. The effect of acidifying agents on
susceptibility to infection. Yale J. Biol. Med., 33: 318.
FRIZZOLA, J.A. & BAIER, J.H. (1975) Contaminants in
rainwater and their relation to water quality. Water Sewage
Works, 122: 94-95.
FROMM, P.O. (1963) Studies on renal and extra-renal
excretion in a freshwater teleost, Salmo gairdneri. Comp.
Biochem. Physiol., 10: 121-128.
FROMM, P.O. (1970) Effect of ammonia on trout and goldfish.
In: Toxic action of water soluble pollutants on freshwater
fish, Washington DC, US Environmental Protection Agency,
pp. 9-22 (EPA Water Pollution Control Research Series No. 18050
DST 12/70).
FROMM, P.O. & GILLETTE, J.R. (1968) Effect of ambient
ammonia on blood ammonia and nitrogen excretion of rainbow
trout (Salmo gairdneri). Comp. Biochem. Physiol., 26: 887-896.
FUHRER, J. (1985) Formation of secondary airpollutants and
their occurrence in Europe. Experientia (Basel), 41: 286-301.
FURST, P., JOSEPHSON, B., MASCHIO, G., & VINNARS, E. (1969)
Nitrogen balance after intravenous and oral administration of
ammonium salts to man. J. appl. Physiol., 26(1): 13-22.
FURST, P., JONSSON, A., JOSEPHSON, B., & VINNARS, E. (1970)
Distribution in muscle and liver vein protein of 15N
administered as ammonium acidosis to man. J. appl. Physiol.,
29(3): 307-312.
GALL, W.K. (1980) Effect of cow faeces on the ecology of
laboratory streams, Madison, Wisconsin, University of
Wisconsin, pp. 73-83 (M.S. Thesis).
GAY, W.M.B., CRANE, C.W., & STONE, W.D. (1969) The
metabolism of ammonia in liver disease: a comparison of
urinary data following oral and intravenous loading of [15N]
ammonium lactate. Clin. Sci., 37: 815-823.
GEORGII, H.W. (1963) Oxides of nitrogen and ammonia in the
atmosphere. J. geophys. Res., 68: 963-970.
GEORGII, H.W. & MULLER, W.J. (1974) On the distribution of
ammonia in the middle and lower trophosphere. Tellus, 26:
180-184.
GIBBS, M. & CALO, N. (1959) Factors affecting light induced
fixations of carbon dioxide by isolated spinach chloroplasts.
Plant Physiol., 34: 318-323.
GIBSON, G.E., ZIMBER, A., KROOK, L., & VISEK, W.J. (1971)
Nucleic acids and brain and intestinal lesions in ammonia
intoxicated mice. Fed. Proc., 30: 578.
GIGUZ, T.L. (1968) Effect of low concentrations of ammonia
and nitrogen oxides on adolescents undergoing vocational
training in the chemical industry. Hyg. Sanit., 33(7-9):
431-434.
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity tests.
Sewage ind. Wastes, 24(11): 1397-1401.
GILLIAM, J.W., DANIELS, R.B., & LUTZ, J.F. (1974) Nitrogen
content of shallow ground water in North Carolina Coastal
Plain. J. environ. Qual., 3: 147-151.
GIRARD, J.O. & PAYAN, P. (1980) Ion exchanges through
respiratory and chloride cells in freshwater and seawater
adapted teleosteans. Am. J. Physiol., 238: R260-R268
(Regulatory Integrative Comp. Physiol. 7).
GOLDHABER, M.B. & KAPLAN, I.R. (1974) The sulfur cycle. In:
Goldberg, D., ed. The sea: ideas and observations on progress
in the study of the seas, New York, John Wiley and Sons,
Vol. 5, pp. 569-655.
GOLDMAN, A.S. & YAKOVAC, W.C. (1964) Salicylate
intoxication and congenital anomalies. Arch. environ.
Health, 8: 648-656.
GOLDSTEIN, L., CLAIRBORNE, J.B., & EVANS, D.E. (1982)
Ammonia excretion by the gills of two marine teleost fish: the
importance of NH4+ permeance. J. exp. Zool., 219: 395-397.
GOLDSTEIN, L. & FORSTER, R.P. (1961) Source of ammonia
excreted by the gills of the marine teleost, Myoxocephalus
scropius. Am. J. Physiol., 200(5): 1116-1118.
GOLYAKOVA, L.P. (1971) [Present-day state of industrial
hygiene problems in hydrometallurgical production of tungsten
and molybdenum salts and oxides.] Gig. Tr. Prof. Zabol.,
15(2): 4-8 (in Russian with English summary).
GOODMAN, L.S. & GILMAN, A. (1970) The pharmacological basis
of therapeutics, 4th ed., New York, The Macmillan Company,
1794 pp.
GORDON, R.J. & BRYAN, R. (1973) Ammonium nitrate in airborne
particles in Los Angeles. Environ. Sci. Technol., 7: 645-647.
GORDON, H.H., MCNAMARA, H., & BENJAMIN, H.R. (1948) Response
of young infants to ingestion. Pediatrics, 2: 290-302.
GRANT, W.M. (1974) Toxicology of the eye: drugs, chemicals,
plant venoms, 2nd ed., Springfield, Illinois, C.C. Thomas,
pp. 96-101, 121-128.
GREENSTEIN, J.P., WINITZ, M., GULLINO, P., BIRNBAUM, S.M., &
OTEY, M.C. (1956) Studies on the metabolism of amino acids
and related compounds in vivo. III. Prevention of ammonia
toxicity by arginine and related compounds. Arch. Biochem.
Biophys., 64: 342-354.
GREGORY, R.B. (1977) Synthesis and total excretion of waste
nitrogen by fish of the Periopthalmus (mudskipper) and
Scartelass families. Comp. Biochem. Physiol., 51A: 33-36.
GRINDLEY, J. (1946) Toxicity to rainbow trout and minnows of
some substances known to be present in waste water discharged
to rivers. Ann. appl. Biol., 33(1): 103-112.
GROLLMAN, A. (1929) The urine of the goosefish (Lophius
piscatorius): its nitrogenous constituents with special
reference to the presence in it of trimethylamine oxide.
J. Biol. Chem., 81: 267-278.
GROSJEAN, D., DOYLE, G.J., MISCHKE, T.M., POE, M.P., FITZ,
D.R., SMITH, J.P., & PITTS, J.N., Jr (1976) The
concentration, size distribution, and modes of formation of
particulate nitrate, sulfate, and ammonium compounds in the
eastern part of the Los Angeles Basin. Presented at: The 69th
Annual Meeting of the Air Pollution Control Association,
Portland, Oregon, June 27 - July 1 1976, 14 pp (Paper 76-20.3).
GROSSI, C.E., PRYTZ, B., & ROUSSELOT, L.M. (1968) Adenosine
triphosphate in prevention of ammonia intoxication in dogs.
Surg. Forum, 19: 363-369.
GRUBE, H.J. (1973) [Load and loading capacity of submerged
macrophytes in running water in southern Lower Saxony.]
Verh. Ges. Okol., Saarbrücken: 103-109 (in German with English
summary).
GUERIN-ANCEY, O. (1976a) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
II. Effets du jeune sur l'excretion d'ammoniac et d'uree.
Aquaculture, 9(2): 187-294.
GUERIN-ANCEY, O. (1976b) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
III. Effets du volume et de la concentration initiale en
ammoniac sur l'excretion d'ammoniac et d'uree. Aquaculture,
9(2): 253-258.
GUPTA, B.N., KHANNA, R.N., & DATTA, K.K. (1979) Toxicological
studies of ammonium sulfamate in rat after repeated oral
administration. Toxicology, 13(1): 45-49.
GYORE, K. & OLAH, J. (1980) Ammonia tolerance of Moina
rectirostris Leydig (Cladocera). Aquaculture Hungarica
(Szarvas), 2: 50-54.
HAGGARD, H.W. (1924) Action of irritant gases upon the
respiratory tract. J. ind. Hyg., 5: 390-398.
HAHN, M., MASSEN, O., NENCKI, N., & PAWLOW, J. (1983) [The
connection between the vena cava inferior and the portal vein
and its consequences for the organism.] Arch. Exp. Path.
Pharmakol., 32: 161-210 (in German).
HALL, L.W., Jr, BUIKEMA, A.L., Jr, & CAIRNS, J., Jr (1978)
The effects of a stimulated refinery effluent on the grass
shrimp, Palaemonetes pugio. Arch. environ. Contam. Toxicol.,
7(1) 23-35.
HANTZSCH, S. & LAHMANN, E. (1970) [Ammonia determinations in
city air.] Schriftner. Ver. Wasser- Boden- Lufthyg. (Berlin-
Dahlem), 33: 35-39 (in German).
HARADER, R.R. & ALLEN, G.H. (1983) Ammonia toxicity to
chinook salmon parr: reduction in saline water. Trans Am.
Fish. Soc., 112: 834-837.
HARDING, A.J. (1959) Ammonia manufacture and uses, London,
Oxford University Press.
HAYNES, R.J. & GOH, K.M. (1977) Evaluation of potting media
for commercial nursery production of container grown plants.
II. Effects of media, fertilizer nitrogen and nitrification
inhibitor on yield and nitrogen uptake of Callistephus
chinensis (L.) nees "Pink Princess". N.Z. J. Agric. Res., 20:
371-381.
HAWKINS, R.A., MILLER, A.L., NIELSEN, R.C., & VEECH, R.L.
(1973) The acute action of ammonia on rat brain metabolism in
vivo. Biochem. J., 134: 1001-1008.
HAWKINS, D.B., DEMETER, M.J., & BARNETT, T.E. (1980) Caustic
ingestion: Controversies in management. A review of 14 cases.
Laryngoscope, 90: 98-109.
HAZEL, C.R., THOMSEN, W., & MEITH, S.J. (1971) Sensitivity
of striped bass and stickleback to ammonia in relation to
temperature and salinity. Calif. Fish Game, 57(3): 138-153.
HAZEL, R.H., BURKHEAD, C.E., & HUGGINS, D.G. (1979) The
development of water quality criteria for ammonia and total
residual chlorine for the protection of aquatic life in two
Johnson County, Kansas streams, Washington DC, Office of Water
Research and Technology, US Department of the Interior, 146 pp.
HEALY, T.V. (1974) Ammonia and related pollutants at
Harwell. Atmos. Environ., 8: 81-83.
HEALY, T.V., MCKAY, H.A.C., PILBEAM, A., & SCARGILL, D.
(1970) Ammonia and ammonium sulfate in the troposphere over
the United Kingdom. J. geophys. Res., 75: 2317-2321.
HEIL, G.W. & DIEMONT, W.M. (1983) Raised nutrient levels
change heathland into grassland. Vegetatio, 53: 113-120.
HEISLER, N. (1984) Acid-base regulation in fishes. In: Hoar,
W. & Randall, D.J., ed. Fish physiology, New York, Academic
Press, Vol. 10A.
HELMERS, S., TOP, F.H., & KNAPP, L.W., Jr (1971) Ammonia
injuries in agriculture. J. Iowa Med. Soc., 61: 271-280.
HEMENS, J. (1966) The toxicity of ammonia solutions to the
mosquito fish ( Gambusia affinis Baird & Girard). J. Proc.
Inst. Sewage Purif., 65: 265-271.
HENIS, Y. & CHET, I. (1967) Mode of action of ammonia on
Sclerotium rolfsii. Phytopathology, 57: 425-427.
HERBERT, D.W.M. (1956) La toxicité d'un effluent d'eau
d'égout. Bull. Cent. belge Etude Doc. Eaux, 32: 115-120.
HERBERT, D.W.M. (1962) The toxicity of rainbow trout of
spent still liquors from the distillation of coal. Ann. appl.
Biol., 50(4): 755-777.
HERBERT, D.W.M. & SHURBEN, D.S. (1965) The susceptibility of
salmonid fish to poisons under estuarine conditions. II.
Ammonium chloride. Int. J. Air Water Pollut., 9: 89-91.
HIDY, G.M. (1974) Characterization of aerosols in California
(ACHEX), Thousand Oaks, California, Rockwell International,
Vol. 1-4 (Final Report prepared for California Air Resources
Board).
HILLABY, B.A. & RANDALL, D.J. (1979) Acute ammonia toxicity
and ammonia excretion in rainbow trout (Salmo gairdneri). J.
Fish. Res. Board Can., 36(6): 621-629.
HINTZ, H.F., LOWE, J.E., CLIFFORD, A.J. ET AL. (1970)
Ammonia intoxication resulting from urea ingestion by ponies.
J. Am. Vet. Med. Assoc., 157: 963-966.
HOEFT, R.G., KENNEY, D.R., & WALSH, L.M. (1972) Nitrogen and
sulfur in precipitation and sulfur dioxide in the atmosphere
in Wisconsin. J. environ. Qual., 1: 203-208.
HOGAN, J.P. (1961) The absorption of ammonia through the
rumen of sheep. Aust. J. biol. Sci., 14: 448-460.
HOLETON, G.F., NEUMANN, P., & HEISLER, N. (1983) Branchial
ion exchange and acid-base regulation after strenous exercise
in rainbow trout. Resp. Physiol., 51: 303-318.
HOLLAND, G.A., LASATER, J.E., NEUMANN, E.D., & ELDRIDGE, W.E.
(1960) Experiments with inorganic pollutants - ammonia. Toxic
effects of organic and inorganic pollutants on young salmon
and trout, State of Washington, Department of Fisheries,
pp. 183-187 (Research Bulletin No. 5).
HOLT, G.J. & ARNOLD, C.R. (1983) Effects of ammonia and
nitrite on growth and survival of red drum eggs and larvae.
Trans. Am. Fish. Soc., 112: 314-318.
HOLT, J.D. & MALCOLM, S.A. (1979) Evaluation of fish loading
rates in regulatory static bioassays. Tech. Rep. -Fish. Mar.
Serv. (Can.)., 862: 146-160.
HORNE, F.R. & MCINTOSH, S. (1979) Factors influencing
distribution of mussels in the Blanco River of central Texas.
Nautilus, 94(4): 119-133.
HSIA, Y.E. (1974) Inherited hyperammonemic syndromes.
Gastroenterology, 67: 347-374.
HUNGER, W. (1978) [Dying off of old spruce trees in the
vicinity of a pig fattening station.] Beitrage
Forstwirtschaft, 4: 188-189 (in German).
HUNT, R.D. (1977) Measurement of partial pressure of ammonia
in breath and arterial blood. Evidence for secretion of
ammonia into expired gas by metabolism of monoamines in the
lung. Diss. Abstr. Int. Ser. B, 37: 3279.
ILO (1970) Permissible levels of toxic substances in the
working environment. In: Proceedings of the 6th Session of the
Joint ILO/WHO Committee on Occupational Health, Geneva, 4-10
June 1968, Geneva, International Labour Office, 405 pp.
INDUSTRIAL BIO-TEST LABORATORIES, INC. (1973) Irritation
threshold evaluation study with ammonia, Industrial Bio-Test
Laboratories, Inc. (Report to International Institute of
Ammonia Refrigeration, Publication No. 663-03161).
IWAOKA, W.T., OKRONE, C.A., SULLIVAN, J.J., & JOHNSON, C.A.
(1981a) Effect of pH and ammonium ions on mutagenic activity
in cooked beef. Cancer Lett., 12: 335.
IWAOKA, W.T., URONE, C.A., SULLIVAN, J.J., MEAKER, E.H., &
JOHNSON, C.A. (1981b) A source of error in mutagen testing
of foods. Cancer Lett., 11: 225.
JACOBS, M.H. (1940) Some aspects of cell permeability to
weak electrolytes. Cold Spring Harbour Sym. Quant. Biol., 8:
30-39.
JACQUEZ, J.A., POPPELL, J.W., LAWRENCE, W., Jr, & ROBERTS,
K.E. (1957) Clinical significance of the partial pressure of
ammonia (pNH3) in patients with ammonium toxicity. Clin. Res.
Proc., 5: 20 (Abstract).
JACQUEZ, J.A., POPPELL, J.W., & JELTSCH, R. (1959) Partial
pressure of ammonia in aveolar air. Science, 129: 269-270.
JAFFE, H.L., BODANSKY, A., & BLAIR, J.E. (1932a) Effects of
parathormone (parathyroid preparation) and ammonium chloride
on bones of rabbits. J. exp. Med., 55: 695-701.
JAFFE, H.L., BODANSKY, A., & CHANDLER, J.P. (1932b) Ammonium
chloride decalcification, as modified by calcium intake: the
relation between generalized osteoporosis and osteitis
fibrosa. J. exp. Med., 56: 823-834.
JANICKI, R.H. (1970) Renal adaptation during chronic NH4Cl
acidosis in the rat: no role for hyperplasia. Am. J. Physiol.,
219(3): 613-618.
JANSSEN, Th. W. (1982) [Intensive animal husbandry in
relation to space and environment,] Utrecht, The Netherlands,
Dutch State Forest Service (in Dutch).
JOHANNING, G.L., MUHRER, M.E., & GADDY, J.H. (1978)
Nutritional evaluation of chlorinated-ammoniated corn stover.
Paper presented at: The Joint Meeting of the American Dairy
Science Association and the American Society of Animal
Science, 11 July 1978.
JOHNSON, J.D., EPEL, D., & PAUL, M. (1976) Intracellular pH
and activation of sea urchin eggs after fertilization. Nature
(Lond.), 262(5570): 661-664.
JONES, K. (1973) Ammonia. In: Bailar, J.C., Jr, Emeleus,
H.J., Nyholm, R., & Trotman-Dickenson, A.F., ed. Comprehensive
inorganic chemistry, New York, Pergammon Press, Vol. 2, pp.
199-227.
JONES, R.D. & HOOD, M.A. (1980) Effects of temperature, pH,
salinity and inorganic nitrogen on the rate of ammonium
oxidation by nitrifiers isolated from wetland environments.
Microb. Ecol., 6: 339-347.
JUDE, D.J. (1973) Sublethal effects of ammonia and cadmium
on growth of green sunfish, East Lansing, Michigan, Michigan
State University, 193 pp (Ph.D. Thesis).
KADOWAKI, S. (1976) Size distribution of atmospheric total
aerosols, sulfate, ammonium and nitrate particulates in the
Nagoya area. Atmos. Environ., 10: 39-43.
KAGOTA, K., IWASE, T., KOJIMA, T., NIIYAMA, M., & NAMIOKA, S.
(1979) Diammonium citrate addition to a diet restricted
non-essential amino acids for young pigs. Jap. J. vet. Sci.,
41: 131-138.
KAMIN, H. & HANDLER, P. (1957) Amino acid and protein
metabolism. Annu. Rev. Biochem., 26: 419-490.
KAPEGHIAN, J.C., MINCER, H.H., JONES, A.B., VERLANGIERI, A.J.,
& WATERS, I.W. (1982) Acute inhalation toxicity of ammonia
in mice. Bull. environ. Contam. Toxicol., 29: 371-378.
KARNAKY, K.J., Jr, KINTER, L.B., KINTER, W.B., & STIRLING,
C.E. (1976) Teleost chloride cell. II. Autoradiographic
localization of gill Na, K-ATPase in killifish Fundulus
heterclitus adapted to low and high salinity environments.
J. cell Biol., 70: 157-177.
KATZ, M. & PIERRO, R.A. (1967) Estimates of the acute
toxicity of ammonia-urea plant wastes to coho salmon,
Oncorhynchus kisutch, Seattle, Washington, Fisheries Research
Institute, College of Fisheries, University of Washington,
15 pp (Final report).
KEESEE, R.G., HOPF, S.B., & MOYERS, J.L. (1975) The
distribution and characteristics of particulate sulfates in
the southwest desert atmosphere. Presented at: The
International Conference on Environmental Sensing and
Assessment, Las Vegas, Nevada, 14-19 September, 1975, Vol. 2,
5 pp (Paper 23-6).
KEMPINSKA, H. (1968) [The effect of ammonia fertilizers on
fish.] Gospod. Rybn., 20: 3-5 (in Russian).
KEPLINGER, M.L., SCHADEBERG, K.J., GOODE, J.W., & CALANDRA,
J.C. (1973) Irritation threshold evaluation study with
ammonia. In: Report to the Institute of Ammonia Refrigeration,
Northbrook, Illinois, Industrial Bio-Test Laboratories, Inc.
KERN, D.M. (1960) The hydration of carbon dioxide. J. Chem.
Ed., 37: 14-23.
KERSTETTER, T.H., KIRSCHNER, L.B., & RAFUSE, D.D. (1970) On
the mechanisms of sodium ion transport by the irrigated gills
of rainbow. J. gen. Physiol., 56: 342-359.
KEYES, W.F. (1975) Nitrogen. In: Mineral facts and problems,
Washington DC, US Department of the Interior, pp. 749-760
(Bureau of Mines Bulletin No. 667).
KHOLDEBARIN, B. & OERTLI, J.J. (1977) Effect of pH and
ammonia on the rate of nitrification of surface water.
J. Water Pollut. Control Fed., 49: 1693-1697.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.N. (1972)
Serum- and tissue-ammonia nitrogen and tissue-water values in
ammonia-intoxicated sheep. Am. J. Vet. Res., 33: 1187-1190.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.M. (1973)
Hemogram of sheep acutely intoxicated with ammonia.
Am. J. Vet. Res., 34: 587-589.
KIRSCHNER, L.B., GREENWALD, L., & KERSTETTER, T.H. (1973)
Effect of amiloride on sodium transport across body surfaces
of freshwater animals. Am. J. Physiol., 224: 832-837.
KLEMM, K. (1967) The effect of N source on the yield of
various crop plants. Bodenkultur, 18: 210-218.
KLENDSHOJ, N.C. & REJENT, T.A. (1966) Tissue levels of some
poisoning agents less frequently encountered. J. Forensic
Sci., 11: 75-80.
KLING, H.F. & QUARLES, C.L. (1974) Effect of atmospheric
ammonia and the stress of infectious bronchitis vaccination on
Leghorn males. Poult. Sci., 53: 1161-1167.
KLUESENER, J.W. & LEE, G.F. (1974) Nutrient loading from a
separate storm sewer in Madison, Wisconsin. J. Water Pollut.
Control Fed., 46(5): 920-936.
KNEPP, G.L. & ARKIN, G.F. (1973) Ammonia toxicity levels and
nitrate tolerance of channel catfish. Prog. Fish-Cult., 35(4):
221-224.
KNOWLES, G., DOWNING, A.L., & BARRETT, M.J. (1965)
Determination of kinetic constants for nitrifying bacteria in
mixed culture, with the aid of an electronic computer.
J. gen. Microbiol., 38: 263.
KOENIG, H. & KOENIG, R. (1949) Production of acute pulmonary
edema by ammonium salts. Proc. Soc. Exp. Biol. Med., 70:
375-380.
KOPP, A., FELLER, U., & ERISMANN, K.H. (1974) [Studies on
the regulation of nitrogen assimilation by Lemna minor in
transition from ammonium to nitrate feeding or from nitrate to
ammonium feeding under conditions of photosynthesis.]
Z. Pflanzen physiol., 73: 456-460 (in German).
KORMANIK, G.A. & CAMERON, J.N. (1981) Ammonia excretion in
the FW catfish: the role of diffusion. Am. Soc. Zool., 21(4):
1042.
KORTING, W. (1969a) [The harmful effect of ammonia on fish.]
Munch. Beitr. Abwasser-. Fisch.-Flussbiol., 16: 38-48 (in
German).
KORTING, W. (1969b) [Investigations on the effects of
ammonia on the blood of fish.] Wasser Abwasser Forsch., 4:
154-159 (in German).
KRYSTAL, G.W. & POCCIA, D. (1979) Control of chromosome
condensation in the sea urchin egg. Exp. cell Res., 123(2):
207-219.
KULASEK, G., SIWECKA, B., OWCZARCZYK, B., RYNCA, J., & BAREJ,
W. (1975) Effect of protein level and synthesize chloride
addition to diet on ammonia detoxification in rats. Zentralbl.
Veterinaermed., 22(3): 215-223.
KUSHNIR, J.J., MALKIN, H.I., MOHNEN, V.A., JENCHA, A.J., &
MCLAREN, E.H. (1970) Non-photochemical aerosol formation
from the anhydrous reaction between ammonia and sulfur dioxide
gases. Mechanism for the formation of ammonium sulfate in the
atmosphere, Albany, New York, Department of Chemistry and
Atmospheric Sciences Research Center, State University of New
York (Report 165 of National Air Pollution Control
Administration Research Grant 5RO1A).
KUSTOV, V.V. (1967) [Means of determining the maximum
allowable concentration of toxic products of natural human
metabolism.] In: General questions of industrial toxicology,
Moscow, pp. 63-65. (National Aeronautics and Space
Administration Technical Translation NASA TT F-11,358) (in
Russian).
LANDAHL, H.D. & HERRMANN, R.G. (1950) Retention of vapors
and gases in the human nose and lung. Arch. ind. Hyg. occup.
Med., 1: 36-45.
LANGOWSKA, I. & MOSKAL, J. (1974) Effect of ammonia and urea
on nitrobacteria in water environment. Pol. Arch. Hydrobiol.,
21(1): 119-123.
LARSON, T.V., COVERT, D.S., FRANK, R., & CHARLSON, R.J.
(1977) Ammonia in the human airways: neutralization of
inspired acid sulfate aerosols. Science, 197: 161-163.
LECHTENBERG, V.L., BUETTNER, M.R., HOLT, D.A., RICHEY, C.B., &
PARSONS, S.D. (1977) Hay preservation by anhydrous ammonia
treatment. In: Grain and Forage Harvesting. Proceedings of the
First International Grain and Forage Conference, St. Joseph,
American Society of Agricultural Engineers, pp. 327-328, 338.
LEE, J.A., (1985) Effects of NO2 on aquatic ecosystems. In:
Study on the need of NO2 long term limit value for the
protection of terrestrial and aquatic ecosystems (Final Report
Feb. 1985).
LEE, R.E., Jr & GORANSON, S. (1976) National air
surveillance cascade impact network. III. Variations in size
of airborne particulate matter over a three-year period.
Environ. Sci. Technol., 10: 1022-1027.
LEITHE, W. (1971) The analysis of air pollutants, Ann Arbor,
Michigan, Ann Arbor Science Publishers.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odour
threshold determination of 52 odorant chemicals. J. Air
Pollut. Control Assoc., 19: 91-95.
LEUVEN, R.S.E.W. & SCHUURKES, J.A.A.R. (1984) [Effects of
acid precipitation containing sulfur and nitrogen on weakly
buffered waters low in nutrients] The Netherlands, Laboratory
of Aquatic Ecology, University of Nymegen, and Ministry of
Housing, Physical Planning and Environment, 131 pp (in Dutch).
LEVI, G., MORISI, G., COLETTI, A., & CATANZARO, R. (1974)
Free amino acids in fish brain: normal levels and changes upon
exposure to high ammonia concentrations in vivo, and upon
incubation of brain slices. Comp. Biochem. Physiol., 49A:
623-636.
LEVY, D.M., DIVERTIE, M.B., LITZOW, T.J., & HENDERSON, J.W.
(1964) Ammonia burns of the face and respiratory tract.
J. Am. Med. Assoc., 190: 873-876.
LIEBHARDT, W.C., GOLT, C., & TUPIN, J. (1979) Nitrate and
ammonium concentrations of ground water resulting from poultry
manure applications. J. environ. Qual., 8: 211-215.
LIECHTI, P.M. & HUGGINS, D.G. (1980) Effects of a 24-h
ammonia injection on stream drift and benthic standing crop.
Trans. Kansas Acad. Sci., 83(3): 140.
LIETZKE, M.H. (1978) A kinetic model for predicting the
composition of chlorinated water discharged from power plant
cooling systems. In: Water chlorination environmental impact
and health effects, Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., Vol. 2, pp. 707-716.
LILLIE, R.J. (1970) Ammonia. In: Air pollutants affecting
the performance of domestic animals. A literature review,
Washington DC (Agriculture Handbook No 380).
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide
formulations against two brackish water organisms, the bleak
(Alburnus alburnus) and the harpacticoid (Nitocra spinipes).
Chemosphere, 11(12): 843-851.
LINZON, S.N. (1971) Effects of air pollutants on vegetation.
In: McCormac, B.M., ed. Introduction to the scientific study
of atmospheric pollution, Dordrecht, Holland, D. Reidel
Publishing Company, pp. 131-151.
LITAV, M. & AGAMI, M. (1976) Relationship between water
pollution and the flora of two coastal rivers of Israel.
Aquat. Biol., 2(1): 23-41.
LITAV, M. & LEHRER, Y. (1978) The effects of ammonium in
water on Potamageton lucens. Aquat. Biol., 5(2): 127-138.
LITTON BIONETICS, INC. (1975) Mutagenic evaluation of
compound FDA 73-42 ammonium sulfate, granular, food grade,
Washington DC, Litton Bionetics (Prepared for US Food and Drug
Administration) (NTIS PB-245 506).
LLOYD, R. (1961) Effect of dissolved oxygen concentrations
on the toxicity of several poisons to rainbow trout ( Salmo
gairdnerii Richardson). J. exp. Biol., 38: 447-455.
LLOYD, R. & HERBERT, D.W.M. (1960) The influence of carbon
dioxide on the toxicity of un-ionized ammonia to rainbow trout
( Salmo gairdnerii Richardson). Ann. appl. Biol., 48(2):
399-404.
LLOYD, R. & ORR, L.D. (1969) The diuretic response by
rainbow trout to sub-lethal concentrations of ammonia. Water
Res., 3(5): 335-344.
LOBASHOV, M.E. (1937) [Nature of the effects of chemical
agents on the mutation process of Drosphila melanogaster.]
Genetica, 19: 200-241 (in German).
LOBASHOV, M.E. & SMIRNOV, F.A. (1934) [Effect of acetic acid
on nondisjunction and mutations in Drosphila melanogaster.]
Dokl. Acad. Sci. USSR N.S., 2: 307 (in Russian with German
summary).
LODGE, J.P., Jr, MACHADO, P.A., PATE, J.B., SHEESLEY, D.C., &
WARTBURG, A.F. (1974) Atmospheric trace chemistry in the
American humid tropics. Tellus, 26: 250-253.
LOEHR, R.C. (1974) Characteristics and comparative magnitude
of non-point sources. J. Water Pollut. Control Fed., 46(8):
1849-1872.
LOSADA, M. & ARNON, D.I. (1963) Selective inhibitors of
photosynthesis. In: Hochester, R. & Quastel, J.H., ed.
Metabolic inhibitors, New York, Academic Press, Vol. 2, p. 559.
LOSADA, M., HERRERA, J., MALDONADO, J.M., & PANEQUE, A.
(1973) Mechanism of nitrate reductase reversible inactivation
by ammonia in Chlamydomonas. Plant Sci. Lett., 1: 31-37.
LOTSPEICH, W.D. (1965) Renal hypertrophy in metabolic
acidosis and its relation to ammonia excretion.
Am. J. Physiol., 208(6): 1135-1142.
LUBINSKI, K.S. (1979) Monitoring bluegill swimming behaviour
and the effects of sublethal ammonium chloride gradients,
Blacksburg, Virginia, Virginia Polytechnic Institute and State
University, 99 pp (Ph.D. Thesis).
LUBINSKI, K.S., SPARKS, R.E., & JAHN, L.A. (1974) The
development of toxicity indices for assessing the quality of
the Illinois River, Urbana, Illinois, Water Resources Center,
University of Illinois, 46 pp (Research Report No. 96).
LUBINSKI, K.S., DICKSON, K.L., & CAIRNS, J., Jr (1980)
Effects of abrupt sublethal gradients of ammonium chloride on
the activity level, turning, and preference-avoidance
behaviour of bluegills, In: Eaton, J.G., Parrish, P.R., &
Hendricks, A.C. ed. Aquatic toxicology, Philadelphia,
Pennsylvania, American Society for Testing and Materials,
pp. 328-340 (ASTM STP 707).
MACEWEN, J.D. & VERNOT, E.H. (1972) Toxic hazards research
unit annual technical report (NTIS AD 755-358).
MACEWEN, J.D., THEODORE, J., & VERNOT, E.H. (1970) Human
exposure to EEL concentrations of monomethylhydrazine. In:
Proceedings of the 1st Annual Conference on Environmental
Toxicology, Ohio, Wright-Patterson Air Force Base,
9-11 September, 1970, Aerospace Medical Research Laboratory,
pp. 355-363 (AMRL-TR-70-102, Paper No. 23).
MCBARRON, E.J. & MCINNES, P. (1968) Observations on urea
toxicity in sheep. Aust. Vet. J., 44: 90-96.
MCBEAN, R.L., NEPPEL, M.J., & GOLDSTEIN, L. (1966)
Glutaminate dehydrogenase and ammonia production in the eel
(Anguilla rostrata). Comp. Biochem. Physiol., 18: 909-920.
MCBRIDE, R.L. & LAING, D.G. (1979) Threshold determination
by triangle testing: Effects of judgmental procedure,
positional bias and incidental training. Chem. Senses Flavor.,
4(4): 319-328.
MCCALLAN, S.E.A. & SETTERSTROM, C. (1940) Toxicity of
ammonia, chlorine, hydrogen cyanide, hydrogen sulphide, and
sulphur dioxide gases. I. General methods and correlations.
Contrib. Boyce Thompson Inst., 11: 325-330.
MCCAY, C.M. & VARS, H.M. (1931) Studies upon fish blood and
its relation to water pollution. In: A biological survey of
the St. Lawrence Watershed. Supplement to the 20th Annual
Report, 1930, Albany, New York, New York Conservation
Department, pp. 230-233 (Biol. Survey (1930), No. V).
MCCORMICK, J.H., BRODERIUS, S.J., & FIANDT, J.T. (1984)
Toxicity of ammonia to early life stages of the green sunfish
Lepomis cyanellus. Environ. Pollut. Ser. A., 36: 147-163.
Erratum: Environ. Pollut. Ser. A. (in press).
MCDONALD, D.G. & WOOD, C.M. (1981) Branchial and renal acid
and ion fluxes in the rainbow trout at low environmental pH.
J. exp. Biol., 93: 101-118.
MCKAY, H.A.C. (1969) Ammonia and air pollution. Chem. Ind.,
34: 1162-1165.
MCKHANN, G.M. & TOWER, D.B. (1961) Ammonia toxicity in
cerebral oxidative metabolism. Am. J. Physiol., 200: 420-424.
MAETZ, J. (1972) Branchial sodium exchange and ammonia
excretion in the goldfish Carassius auratus. Effects of
ammonia-loading and temperature changes. J. exp. Biol., 56:
601-620.
MAETZ, J. (1973) Na+/NH4+, Na+/H+ exchanges and NH3 movement
across the gill of Carassius auratus. J. exp. Biol., 58:
255-275.
MAETZ, J. & GARCIA-ROMEU, F. (1964) The mechanism of sodium
and chloride uptake by the gills of a freshwater fish,
Carassius auratus. II Evidence for NH4+/Na+ and HCO3-/Cl-
exchanges. J. gen. Physiol., 47: 1209-1227.
MAGALHAES BASTOS, J.A. (1954) [Importance of ammonia as an
ichthyotoxic substance,] Brazil, Serv. Piscicultura, Dept.
nacl. obras contra secas, Ministerio viacao e obras publicas,
pp. 115-132 (Publ. Ser. 1-C, No. 159) (in Portuguese).
MAI, K.L. (1977) Energy and petrochemical raw materials
through 1990. Chem. Eng., 84: 122-128.
MALACEA, I. (1966) [Contributions to knowledge of the
cyanide, ammonia, mercury and arsenic toxic action on some
species of fishes and on Daphnia.] Stud. Prot. Epurarea Apelor
Inst. Stud. Cercet Hidrotehnice, 7(2: 751-792 (English
summary) Biol. Abstr., 50: 92715 (1969).
MALACEA, I. (1968) [Studies on the acclimatization of fish
to high concentrations of toxic substances.] Arch. Hydrobiol.,
65(1): 74-95 (in German with English summary).
MALLERY, C.H. (1979) Ammonium stimulated properties of a
K+-dependent ATPase in Opsanus beta, a marine teleost with an
NH4+/Na+ exchange pump. Am. Zool., 19: 944.
MANGOLD, C.A. (1971) Investigation of occupational exposure
to ammonia, Washington, Puget Sound Naval Shipyard, Industrial
Hygiene Division of Investigation.
MANGUM, C.P., SILVERTHORN, S.U., HARRIS, J.L., TOWLE, D.W., &
KRALL, A.R. (1976) The relationship between blood pH,
ammonia excretion and adaptation to low salinity in the blue
crab Callinectes sapidus. J. exp. Zool., 195(1): 129-136.
MANGUM, C.P., DYKENS, J.A., HENRY, R.P., & POLITES, G.
(1978) The excretion of NH4+ and its ouabain sensitivity in
aquatic annelids and molluscs. J. exp. Zool., 203: 151-157.
MARSCHANG, F. (1972) [The NH3 content of air in stables and
the fattening capacity of cows.] Dtsch. Tieraerztl.
Wochenschr., 79: 214-216 (in German).
MARSCHANG, F. & CRAINICEANU, E. (1971) [Investigations on
the connections between cowshed climate and calf mortality.]
Berlin München Tieraerztl. Wochenschr., 84: 417-420 (in
German).
MARSCHANG, F. & PETRE, C. (1971) [The NH3 content of cowshed
air and its influence on morbidity and animal lossess in
cattle-fattening pens.] Zentralbl. Veterinaermed., 18: 646-654
(in German).
MATASA, C. & MATASA, E. (1968) L'industrie moderne des
produits azotés, Paris, Dunod, pp. 63-208.
MATSUMOTO, H., OKAMURA, K., & TAKAHASHI, E. (1976) Changes
in activity and isoenzymes of intervase in cucumber leaves due
to nitrogen status. Plant Cell Physiol., 17: 867-874.
MATUSIAK, K. (1976) Studies on the purification of
wastewater from the nitrogen fertilizer industry by intensive
algal cultures. Acta microbiol. Pol., 25(3): 233-242.
MAYAN, M.H. & MERILAN, C.P. (1972) Effects of ammonia
inhalation on respiratory rate of rabbits. J. anim. Sci., 34:
448-452.
MAYNARD, D.N. & BARKER, A.V. (1969) Studies on the tolerance
of the plants to ammonium nutrition. J. Am. Soc. Hortic. Sci.,
94: 235-239.
MEARNS, A.J. (1981) Ecological effects on ocean sewage
outfalls: observations and lessons. Oceanus, 24: 45-54.
MERKENS, J.C. & DOWNING, K.M. (1957) The effect of tension
of dissolved oxygen on the toxicity of un-ionized ammonia to
several species of fish. Ann. appl. Biol., 45(3): 521-527.
METCALF & EDDY, INC. (1972) Wastewater engineering, New
York, McGraw-Hill.
MILES, F.D. (1961) Nitric acid manufacture and uses, London,
Oxford University Press.
MILLER, T.J. (1973) Cleft palate formation: The effects of
fasting and iodoacetic acid on mice. Teratology, 7: 177-182.
MILLER, G.R., SHIMP, G.F., ANDREWS, H.O., NIMMO, D.W., & ILEY,
E.S. (1981) Effect of domestic wastewater on trout in Dillon
Reservoir, Colorado. Paper presented at: The ASTM Symposium on
Aquatic Toxicology, St. Louis, Missouri, 13-14 October 1981,
Philadelphia, Pennsylvania, American Society for Testing
Materials.
MINER, J.R. & HAZEN, T.E. (1969) Ammonia and amines:
components of swine-building odour. Trans. Am. Soc. Agric.
Eng., 12: 772-774.
MITCHELL, H.A. (1963) Ammonia tolerance of the California
leaf-nosed bat. J. Mammal, 44: 543-551.
MITCHELL, H.A. (1964) Investigations of the cave atmosphere
of a Mexican bat colony. J. Mammal, 45: 568-577.
MORII, H., NISHIKATA, K., & TAMURA, O. (1978) Nitrogen
excretion of mudskipper fish Periophthalmus cantonensis and
Boleophthalmus pectinirostris in water and on land. Comp.
Biochem. Physiol., 60A(2): 189-193.
MORRIS, J.C. (1967) Kinetics of reactions between aqueous
chlorine and nitrogenous compounds. In: Faust, S.D. & Hunter,
J.V., ed. Principles and applications of water chemistry,
New York, John Wiley and Sons, pp. 23-53.
MORRIS, J.C. (1978) Chemistry of aqueous chlorine in
relation to water chlorination. In: Jolley, R.L., ed. Water
chlorination: Environmental impact and health effects,
Ann Arbor, Michigan, Ann Arbor Science Publishers, Vol. 1,
pp. 21-36.
MORRIS, J.C. & ROGERS, Q.R. (1978) Ammonia intoxication in
near-adult cat as a result of a dietary deficiency of
arginine. Science, 199(4327): 431-432.
MOSIER, A.R. (1978) Inhibition of photosynthesis and
nitrogen fixation in algae by volatile nitrogen bases.
J. environ. Qual., 7(2): 237-240.
MOSSBERG, S.M. (1967) Ammonia absorption in hamster iluem.
Effect of pH and total CO2 on transport in everted sacs.
Am. J. Physiol., 213: 1327-1330.
MOSSBERG, S.M. & ROSS, G. (1967) Ammonia movement in the
small intestine: Preferential transport by the ileum. J. clin.
Invest., 46: 490-498.
MOTYL, T. & DEBSKI, B. (1977) The influence of increasing
amounts of NH4Cl in the diet on ammonia detoxification in
rats. Zbl. Vet. Med., 24: 333-342.
MULDER, J.S. & VAN DER ZALM, H.O. (1967) [A fatal case of
ammonia poisoning.] Tijdschr. Soc. Geneeskd., 45: 458-460 (in
Dutch).
MULLER, G. & GRUHN, M. (1969) [The effect of water-free
ammonia fertilizer on soil microorganisms.] Zentralbl.
Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 2 Naturw.,
123: 667-676 (in German with English summary).
MUNTWYLER, E., IACOBELLIS, M., & GRIFFIN, G.E. (1956) Kidney
glutaminase and carbonic anhydrase activities and renal
electrolyte excretion in rats. Am. J. Physiol., 184: 83-90.
NAKAZAWA, S. & QUASTEL, J.H. (1968) Inhibitory effects on
ammonium ions and some amino acids on stimulated brain
respiration and cerebral amino acid transport. Can. J.
Biochem., 46: 543-548.
NATARAJAN, K.V. (1970) Toxicity of ammonia to marine
diatoms. J. Water Pollut. Control Fed., 42(5, part 2):
R184-R190.
NAVAZIO, F., GERRITSEN, T., & WRIGHT, G.J. (1961)
Relationship of ammonia intoxication to convulsions and coma
in rats. J. Neurochem., 8: 146-151.
NEHRING, D. (1963) [The toxic effect of urea solutions
containing urease on various fish species.] Z. Fischerei,
11(7/8): 539-547 (in German).
NEUFELD, R.D., HILL, A.J., & ADEKOYA, D.O. (1980) Phenol and
free ammonia inhibition to Nitrosomonas activity. Water Res.,
14(12): 1695-1703.
NIDEN, A.H. (1968) Effects of ammonia inhalation of the
terminal airways. In: Proceedings of the 11th Aspen Emphysema
Conference, Vol. 11, pp. 41-44.
NIHLGARD, B. (1970) Precipitation, its chemical composition
and effects on soil water and beech and spruce forests in
South-Sweden. Oikos., 21: 208
NIHLGARD, B. (1985) The ammonium hypothesis: an additional
explanation to the forest dieback in Europe. Ambio, 14(1): 1-8.
NISHIOKA, D.J. (1976) The initiation of cell cycle events in
ammonia-treated eggs and merogons of the sea urchin, Berkeley,
California, University of California, 107 pp (Ph.D. Thesis).
NODA, K. & CHIKAMORI, K. (1976) Effect of ammonia via
prepyriform cortex on regulation of food intake in the rat.
Am. J. Physiol., 231: 1263-1266.
NRC (1979) Ammonia. Subcommittee on Ammonia. Committee on
Medical and Biologic Effects of Environmental Pollutants.
Division of Medical Sciences, Assembly of Life Sciences,
National Research Council, Baltimore, Maryland, University
Park Press (NTIS No. PB 278-027).
OKITA, T. & KANAMORI, S. (1971) Determination of trace
concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 5: 621-627.
OVERREIN, L.N., SEIP, H.M., & TOLLAN, A., ed. (1980) Acid
precipitation: effects on forest and fish, Oslo (Final report
SNSF Project 1972-80).
PAIK, W.K., LAWSON, D., & KIM, S. (1975) Effect of endocrine
glands on the protection of rat from ammonia intoxication.
Life Sci., 15: 1189-1196.
PARKHURST, B.R., BRADSHAW, A.S., FORTE, J.L., & WRIGHT, G.P.
(1979) An evaluation of the acute toxicity to aquatic biota
of a coal conversion effluent and its major components.
Bull. environ. Contam. Toxicol., 23: 349-356.
PARKHURST, B.R., MEYER, J.S., DEGRAEVE, G.M., & BERGMAN, H.L.
(1981) A re-evaluation of the toxicity of coal conversion
process waters. Bull. environ. Contam. Toxicol., 26: 9-15.
PAUL, M., JOHNSON, J.D., & EPEL, D. (1976) Fertilization
acid of sea urchin eggs is not a consequence of cortical
granule exocytosis. J. exp. Zool., 197(1): 127-133.
PAUL, M. & JOHNSTON, R.N. (1978) Absence of a Ca response
following ammonia activation of sea urchin eggs. Dev. Biol.,
67(2): 330-335.
PAYAN, P. (1978) A study of the Na+/NH4+ exchange across the
gill of the perfused head of the trout (Salmo gairdneri). J.
comp. Physiol., 124: 181-188.
PAYAN, P. & MAETZ, J. (1973) Branchial sodium transport
mechanisms in Scyliorhinis canicula: evidence for Na+/NH4+
and Na+/H+ exchanges and for a role of carbonic anhydrase.
J. exp. Biol., 58: 487-502.
PAYAN, P. & MATTY, A.J. (1975) The characteristics of
ammonia excretion by a perfused isolated head of trout
(Salmo gairdneri): effect of temperature and CO2-free ringer.
J. comp. Physiol., 96: 167-184.
PAYAN, P., MATTY, A.J., & MAETZ, J. (1975) A study of the
sodium pump in the perfused head preparation of the trout,
Salmo gairdneri in freshwater. J. comp. Physiol., 104: 33-48.
PEPLINSKI, A.J., BREKKE, O.L., BOTHAST, R.J., & BLACK, L.T.
(1978) High moisture corn: an extended preservation trial
with ammonia. Trans. Am. Soc. Agricul. Eng., 21: 773-776, 781.
PEQUIN, L. & SERFATY, A. (1963) L'excrétion ammoniacale chez
un téléostéen dulcicole Cyprinus carpio. J. comp. Biochem.
Physiol., 10: 315-324.
PERRY, J.H., CHILTON, C.H., & KIRKPATRICK, S.D., ed. (1963)
Chemical engineers' handbook, 4th ed., New York, McGraw-Hill,
pp. 3-151.
PHILLIPS, A.M. (1950) The effect of metabolic products on
brook trout, Cortland, New York, New York State Conservation
Department, pp. 14-16 (Fisheries Research Bulletin 14)
(Cortland Hatcherry Report 19).
PHILLIPS, R.W. (1970) Further clinical studies of
trimethylpuringyl ammonium chloride (NSA-51095) in malignant
disease with special emphasis on cancer of urinary bladder.
Cancer Chemother. Rep., 54: 181.
PIERPONT, R.A. & MINOTTI, P.L. (1977) Effects of calcium
carbonate on ammonium assimilation by tomato seedlings.
J. Am. Soc. Hortic. Sci., 102: 20-23.
PINTER, I.J. & PROVASOLI, L. (1963) Nutritional characteristics
of some chrysomonads. In: Oppenheimer, C.M., ed. Symposium on
Marine Microbiology, Springfield, Massachusetts, Thomas
Publishers, pp. 114-121.
PITTS, R.F. (1971) The role of ammonia production and
excretion in regulation of acid-base balance. New Engl. J.
Med., 284: 32-38.
PITTS, J.N., Jr & GROSJEAN, D. (1976) Detailed
characterization of gaseous and size-resolved particulate
pollutants at a south coast air basin smog receptor site:
levels and modes of formation of sulfate, nitrate and organic
particulates and their implications for control strategies,
Riverside, California, Statewide Air Pollution Research
Center, University of California (California Air Resources
Board Contract 5-384, Six Month Progress Report, November 17,
1975 to May 16, 1976).
POLIAK, V.E. (1981) [Air pollution by ammonia around swine-
breeding complexes.] Gig. i. Sanit., 6: 67 (in Russian).
POST, R.L. & JOLLY, P.C. (1957) The linkage of sodium,
potassium and ammonium active transport across the human
erythrocyte membrane. Biochem. Biophys. Acta, 25: 118-128.
POWERS, E.B. (1920) Influence of temperature and concentration
on the toxicity of salts to fishes. Ecology, 1: 95-112.
POWERS, W.L., TERRY, R.V., & MURPHY, L.S. (1977) Fate of
nitrogen from manure disposal. In: Proceedings of the National
Conference on Disposal of Residues on Land, pp. 156-160.
PROPKOPIEVA, A.S. & YUSHKOV, G.G. (1975) [Toxicology of
ammonia in animals repeatedly exposed for short lengths of
time.] Gig. Tr. Prof. Zabol., 9: 54-55 (in Russian).
PROPKOPIEVA, A.S., YUSHKOV, G.G., & UBASHEEV, I.O. (1973)
[Materials for a toxicological characteristic of the one-term
effect of ammonia on the organisms of animals after brief
exposure.] Gig. Tr. Prof. Zabol., 6: 56-57 (in Russian).
PROVASOLI, L. & MCLAUGHLIN, J.J.A. (1963) Limited
heterotrophy of some photosynthetic dinoflagellates. In:
Oppenheimer, C.M., ed. Symposium on Marine Microbiology,
Springfield, MA, Thomas Publishers, pp. 105-113.
PRZYTOCKA-JUSIAK, M. (1976) Growth and survival of Chlorella
vulgaris in high concentrations of nitrogen. Acta microbiol.
Pol., 25(3): 287-289.
PRZYTOCKA-JUSIAK, M., MLYNARCZYK, A., KULESZA, M., &
MYCIELSKI, R. (1977) Properties of Chlorella vulgaris strain
adapted to high concentrations of ammonium nitrogen. Acta
microbiol. Pol., 26(2): 185-197.
QUARLES, C.L. & KLING, H.F. (1974) Evaluation of ammonia and
infectious bronchitis vaccination stress on broiler
performance and carcass quality. Poult. Sci., 53: 1592-1596.
RAJAGOPAL, R. (1978) Impact of land use on ground water
quality in the Grand Traverse Bay region of Michigan.
J. environ. Qual., 7: 93-98.
RAMACHANDRAN, V. & RAMAPRABHU, T. (1976) Use of ammonia as
herbicide: A Review. In: Varshney, C.K. & Rzoska, J., ed.
Aquatic weeds in South East Asia, The Hague, B.V. Publishers,
pp. 293-298.
RAMACHANDRAN, V., RAMAPRABHU, T., & REDDY, P.V.G.K. (1975)
Observations on the use of ammonia for the control of Pistia
stratiotes Linn. J. Int. Fish. Soc. India, 7: 124-130.
RAMSEY, G.B. (1953) Mechanical and chemical injuries. In:
The yearbook of agriculture 1953, Washington DC, US Department
of Agriculture, Plant Diseases, pp. 835-837.
RANDALL, D.J. (1970) Gas exchange in fish. In: Hoar, W.S. &
Randall, D.J., ed. Fish physiology, New York, Academic Press,
Vol. IV., pp. 253-292.
RANKIN, D.P. (1979) The influence of un-ionized ammonia on
the long-term survival of sockeye salmon eggs. Fish. Mar.
Serv. Tech. Rep., 912: 17.
RAO, T.S., RAO, M.S., & PRASAD, S.B.S.K. (1975) Median
tolerance limits of some chemicals to the fresh water fish
"Cyprinus carpio". Indian J. environ. Health, 17(2): 140-146.
REDNER, B.D. & STICKNEY, R.R. (1979) Acclimatization to
ammonia by Tilapia aurea. Trans. Am. Fish Soc., 108(4):
383-388.
REICHENBACH-KLINKE, H.-H. (1967) [Investigations on the
influence of the ammonia content on the fish organism.] Arch.
Fischereiwiss., 17(2): 122-132. (in German).
REINBOLD, K.A. & PESCITELLI, S.M. (1982a) Effects of
exposure to ammonia on sensitive life stages of aquatic
organisms, Champaign, Illinois, Illinois Natural History
Survey (Project Report: Contract No. 68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982b) Effects of cold
temperature on toxicity of ammonia to rainbow trout, blue
gills, and fathead minnows, Champaign, Illinois, Illinois
Natural History Survey (Project Report: Contract No.
68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982c) Acute toxicity of
ammonia to the white sucker, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. 2W-3946 NAEX).
REINBOLD, K.A. & PESCITELLI, S.M. (1982d) Acute toxicity of
ammonia to channel catfish, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. J 2482 NAEX).
REITER, R., SLADKOVIC, R., & POTZL, K. (1976) Chemical
components of aerosol particles in the lower troposphere above
central Europe measured under pure-air conditions. Atmos.
Environ., 10: 841-853.
REMPEL, B. (1975) Use ammonia to stop corn deterioration.
Crops Soils, 27: 13-14.
REPP, W.W., HALE, W.H., CHENG, E.W., & BURROUGHS, W. (1955)
The influence of oral administration of non-protein nitrogen
feeding compounds upon blood ammonia and urea levels in lambs.
J. anim. Sci., 14: 118-131.
RICE, S.D. & BAILEY, J.E. (1980) Survival, size, and
emergence of pink salmon, Oncorhynchus gorbuscha, alevins
after short- and long-term exposures to ammonia. Fish Bull.,
78(3): 641-648.
RICHARD, D., BOULEY, G., & BOUDENE, C. (1978a) Effets de
l'inhalation continue d'ammoniac chez le rat et la souris.
Bull. eur. Physiopathol. resp., 14: 573-582.
RICHARD, D., JOUARY, J., & BOUDENE, C. (1978b) Toxicité
aiguë par voie aérienne du gaz ammoniac chez le lapin.
C.R. Acad. X. Paris, 287(Série D): 375-378.
RICHARDS, B.O. & FROMM, P.O. (1970) Sodium uptake by
isolated perfused gills of rainbow trout (Salmo gairdneri).
Comp. Biochem. Physiol., 33: 303-310.
RICHARDS, P., HOUGHTON, B.J., METCALFE-GIBSON, A., WARD, E.E.,
& WRONG, O. (1968) Incorporation of orally administered
ammonia into tissue proteins in man; the influence of diet and
ureamia. In: Nutrition and renal disease, Edinburgh, E. and S.
Livingstone, pp. 93-98.
RICHARDS, P., BROWN, C.L., HOUGHTON, B.J., & WRONG, D.M.
(1975) The incorporation of ammonia nitrogen into albumin in
man: The effects of diet, uremia and growth hormone.
Clin. Nephrol., 3(5): 172-179.
RINGER, R.K. (1971) Adaptation of poultry to confinement
rearing systems. J. anim. Sci., 3: 590-598.
ROBIN, E.D., TRAVIS, D.M., BROMBERG, P.A., FORKNER, C.E., Jr,
& TYLER, J.M. (1959) Ammonia excretion by mammalian lung.
Science, 129: 270-271.
ROBINETTE, H.R. (1976) Effect of selected sublethal levels
of ammonia on the growth of channel catfish (Ictalurus
punctatus). Prog. Fish-Cult., 38(1): 26-29.
ROBINSON, E. & ROBBINS, R.C. (1968) Sources, abundance and
fate of gaseous atmospheric pollutants, Menlo Park, Stanford
Research Institute, California, 123 pp (prepared for the
American Petroleum Institute).
ROBINSON, E. & ROBBINS, R.C. (1971) Emissions, concentrations,
and fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, New York, John Wiley and Sons, Part 2,
pp. 1-93.
ROBINSON-WILSON, E.F. & SEIM, W.K. (1975) The lethal and
sublethal effects of a zirconium process effluent on juvenile
salmonids. Water Resour. Bull., 11(5): 975-986.
ROELOFS, J.G.M., SCHUURKES, J.A.A.R., & SMITS, A.J.M. (1984)
Impact of acidification and eutrophication on macrophyte
communities in soft waters in the Netherlands. II. Experimental
studies. Aquat. Bot., 18: 389-411.
ROELOFS, J.G.M. (in press) The effect of sulfur and nitrogen
deposition on aquatic and terrestrial heathland vegetation.
Experientia (Basel).
ROELOFS, J.G.M., KEMPERS, A.J., HOUDYK, A.L.F.M., & JANSEN,
J. (1985) The effect of airborne ammonium sulphate on Pinus
nigra var maritima, in the Netherlands. Plant Soil, 84: 45-56.
ROSAGE, T.F., SCHUTSKY, R.M., & RAPP, K.M. (1979) Toxicity
of un-ionized ammonia to the spotfin shiner (Notropis
spilopterus). Proc. Pa. Acad. Sci., 53: 39-42.
ROSEBOOM, D.P. & RICHEY, D.L. (1977) Acute toxicity of
residual chlorine and ammonia to some native Illinois fishes,
Urbana, Illinois, Illinois State Water Survey, 42 pp (Report
of Investigation 85).
ROSENFELD, M. (1932) Experimental modification of mitosis by
ammonia. Arch. exp. Zellforsch. Gewebezuecht., 14: 1.
RUBIN, A.J. & ELMARAGHY, M.A. (1976) Studies on the toxicity
of ammonia, nitrate, and their mixtures to the common guppy,
Columbus, Ohio, Water Resources Center, Ohio State University,
47 pp (Project No. A-033-OHIO).
RUBIN, A.J. & ELMARAGHY, G.A. (1977) Studies on the toxicity
of ammonia, nitrate and their mixtures to guppy fry. Water
Res., 11(10): 927-935.
SALVATORE, F., BOCCHINI, V., & CIMINO, F. (1963) Ammonia
intoxication and its effects on brain and blood ammonia
levels. Biochem. Pharmacol., 12: 1-6.
SAIFUTDINOV, M.M. (1966) Maximum permissible concentration
of ammonia in the atmosphere. Hyg. Sanit., 176: 171-175.
SAMYLIN, A.F. (1969) [Effect of ammonium carbonate on early
stages of development of salmon.] Uchen. Zap. Leningr. Gos.
Pedagog. Inst. Im. A. I. Gertsena, 422: 47-62 (in Russian).
SCHAERDEL, A.D., WHITE, W.J., LANG, C.M., DVORCHIK, B.H., &
BOHNER, K. (1983) Localized and systemic effects of
environmental ammonia in rats. Lab. anim. Sci, 33(1): 40-45.
SCHENKER, S. & WARREN, K.S. (1962) Effect of temperature
variation on toxicity and metabolism of ammonia in mice.
J. Lab. clin. Med., 60: 291-301.
SCHULZE-WIEHENBRAUCK, H. (1976) Effects of sublethal ammonia
concentrations on metabolism in juvenile rainbow trout ( Salmo
gairdneri Richardson). Ber. Dtsch. Wiss. Komm. Meeresforsch.,
24(4): 234-250.
SCHUMAN, G.E. & BURWELL, R.E. (1974) Precipitation nitrogen
contribution relative to surface runoff discharges.
J. environ. Qual., 3: 366-369.
SCHUURKES, J.A.A.R. (in press) Ammonium sulfate deposition
and its role in the acidification and nitrogen enrichment of
poorly-buffered aquatic systems. Experientia (Basel).
SCHUURKES, J.A.A.R., HECK, I., HESEN, P., LEUVEN, R.S.E.W., &
ROELOFS, J.G.M. (1985) Effects of acid and acidifying
deposition on water composition and macrophyte composition of
simulated poorly buffered aquatic systems. In: Proceedings of
the Muskoka Conference, Toronto, Canada, September 1985.
SEEGAL, B.C. (1927) Chronic acidosis in rabbits and in dogs,
with relation to kidney pathologic change. Arch. intern. Med.,
39(4): 550-563.
SELESI, D. & VAMOS, R. (1976) [Factors affecting the lethal
concentration of ammonia in fish ponds.] Ichthyologia, 8(1):
115-121 (in Serbocroation with English summary).
SERVANT, J. & DELAPART, M. (1983) Concentrations and origins
of NO3- and NH4+ in oceanic air. In: CACGP Symposium on
Tropospheric Chemistry with Emphasis on Sulphur and Nitrogen
Cycles and the Chemistry of Clouds and Precipitation
(Abstracts), 28 August - 3 September 1983, Oxford, England,
Oxford, New York, Sydney, Paris, Frankfurt, Pergamon Press,
57 pp (Fifth International Congress of the Commission on
Atmoshperic Chemistry and Global Pollution).
SHAW, J. (1960) The absorption of sodium ions by the cray-
fish Astacus pallipes Lereboullet. III. The effect of other
cations in the external solution. J. exp. Biol., 37(3):
548-556.
SHEN, S.S. & STEINHARDT, R.A. (1978) Direct measurement of
intracellular pH during metabolic derepression of the sea
urchin egg. Nature (Lond.), 272(5650): 253-254.
SHERWOOD, T.K. (1925) Solubilities of sulfur dioxide and
ammonia in water. Ind. Eng. Chem., 17: 745-747.
SHILO, M. & SHILO, M. (1953) Conditions which determine the
efficiency of ammonium sulphate in the control of Prymnesium
parvum in fish breeding ponds. Appl. Microbiol., 1: 330-333.
SHILO, M. & SHILO, M. (1955) Control of the phytoflagellate
Prymnesium parvum. Verh. Int. Ver. Limnol., 12: 233-240.
SHOLKOVITZ, E. (1973) Interstitial water chemistry of the
Santa Barbara Basin sediments. Geochim. Cosmochim. Acta, 37:
2043-2073.
SHUTTLEWORTH, T.J. & FREEMAN, R.F. (1974) Factors affecting
the net fluxes of ions in the isolated perfused gills of
freshwater Anguilla dieffenbachii. J. comp. Physiol., 94:
297-307.
SIEGRIST, A. (1920) [Concentrated effect of alkali and acid
on the eye.] Z. Augenheilkd., 43: 176-194 (in German).
SILVER, S.D. & MCGRATH, F.P. (1948) A comparison of acute
toxicities of ethylene amine and ammonia to mice. J. ind. Hyg.
Toxicol., 30: 7-9.
SILVERMAN, L., WHITTENBERGER, J.L., & MULLER, J. (1949)
Physiological response of man to ammonia in low
concentrations. J. ind. Hyg. Toxicol., 31: 74-78.
SINGER, R.H. & MCCARTY, R.T. (1971) Pathologic changes
resulting from acute ammonium salt poisoning in sheep.
Am. J. Vet. Res., 32: 1239-1246.
SLOAN, C.H. & MORIE, G.P. (1974) Determination of ammonia in
tobacco and tobacco smoke with the ammonia electrode. Anal.
Chim. Acta, 69: 243-247.
SLOT, G.M.J. (1938) Ammonia gas burns: an account of six
cases. Lancet, 2: 1356-1357.
SMART, G. (1976) The effect of ammonia exposure on gill
structure of the rainbow trout (Salmo gairdneri). J. Fish
Biol., 8(6): 471-475.
SMITH, C.E. (1972) Effects of metabolic products on the
quality of rainbow trout. Am. Fishes US Trout News, 17(3):
7-8, 21.
SMITH, C.E. (1984) Hyperplastic lesions of the primitive
meninx of fathead minnows, Pimephales promelas, induced by
ammonia: species potential for carcinogen testing. Natl Cancer
Inst. Monogr., 65: 119-125.
SMITH, C.E. & PIPER, R.G. (1975) Lesions associated with
chronic exposure to ammonia. In: Ribelin, W.E. & Migaki, G.,
ed. The pathology of fishes, Madison, Wisconsin, University of
Wisconsin Press, pp. 497-514.
SMITH, L.W. & WHEELER, W.E. (1979) Nutritional and economic
value of animal excreta. J. anim. Sci., 48: 144.
SODERLUND, R. & SVENSSON, B.H. (1976) The global nitrogen
cycle. In: Svensson, B.H. & Söderlund, R., ed. Nitrogen,
phosphorus, and sulfur: global sources, Stockholm, pp. 23-76
(SCOPE Report No. 7) (Ecology Bulletin No. 22).
SOF'INA, E.P., VORONKOV, M.G., D'IAKOV, V.W., SOMENOVA, N.V.,
PLATONOVA, G.N., PERETOLCHINA, N.M., LESNAIA, N.A., &
SMETANKINA, O.Z. (1978) Tris (2-oxy-ethyl) ammonium salts of
some synthetic phytohormones and their antitumor activity.
Khim Farm. 2h, 12: 74.
SOLOMONSON, L.P. (1969) Effects of ammonia and some of its
derivatives on photosynthesis in the blue-green alga,
Plectonema boryanum, Chicago, Illinois, University of Chicago,
68 pp (Ph.D. Thesis).
SOUSA, R.J., MEADE, T.K., & FELBECK, G.T., Jr (1977) The
exposure of ammonia to the photodissociated haemoglobin of the
marine bloodworm, Glycera dibranchiata. Comp. Biochem.
Physiol., 58A: 19-22.
SPARKS, R.E. (1975) The acute, lethal effects of ammonia on
channel catfish (Ictalurus punctatus), bluegills (Lepomis
macrochirus), and fathead minnows (Pimephales promelas),
Chicago, Illinois, 15 pp (report to Illinois Institute for
Environmental Quality, project No. 20.060).
SPARKS, R.E. & SANDUSKY, M.J. (1981) Identification of
factors responsible for decreased production of fish food
organisms in the Illinois and Mississippi Rivers, Havana,
Illinois, River Research Laboratory, Illinois Natural History
Survey, 63 pp (Project No. 3-291-R).
SPICER, C.W. (1977) The fate of nitrogen compounds in the
atmosphere. In: Pitts, J.N., Jr, Metcalf, R.L., & Lloyd, A.C.,
ed. Advances in environmental sciences and technology, New
York, John Wiley and Sons, Vol. 7, pp. 163-261.
SPINAZZOLA, A., MARRACCINI, L., DEVOTO, G., & ZEDDA, S.
(1966) [Study of the behaviour of some volatile toxic
substances as an index of air pollution in the cith of
Cagliari. Note III. Ammonia.] Folia Med. (Naples), 49: 641-648
(in Italian).
STAMMER, H.A. (1953) [The effect of hydrogen sulfide and
ammonia on characteristic animal forms in the saprobiotic
system.] Vom Wasser, 20: 34-71 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil (Myriophylum spicatum). Arch. environ.
Contam. Toxicol., 2(4): 331-341.
STEINHARDT, R.A. & MAZIA, D. (1973) Development of
K+-conductance and membrane potentials in unfertilized sea
urchin eggs after exposure to NH4OH. Nature (Lond.),
241(5389): 400-401.
STEPHENS, E.R. (1971) Identification of odors from cattle
feedlots. Calif. Agric., 25: 10-11.
STEVENS, C., KENNAWAY, N.G., & FELLMAN, J.H. (1975) Ammonia
intoxicaton: A hazard during rehabilitation of protein-
deprived rats. J. Nutr., i105: 1384-1390.
STEVENSON, T.J. (1977) The effects of ammonia, pH and
salinity on the white perch, Morone americana, Kingston, Rhode
Island, University of Rhode Island, 154 pp (Ph.D. Thesis).
STEWART, B.A., VIETS, F.G., Jr, HUTCHINSON, G.L., & KEMPER,
W.D. (1967) Nitrates and other water pollutants under fields
and feedlots. Environ. Sci. Technol., 1(9): 736-739.
STRUZEWSKI, E.J. (1971) Current status on control and
treatment of storm and combined sewer overflow, Edison Water
Quality Laboratory (EPA Technology Transfer).
STUDIER, E.H. (1966) Studies on the mechanisms of ammonia
tolerance of the guano bat. J. exp. Zool., 163: 79-85.
STUDIER, E.H., BECK, L.R., & LINDBORG, R.G. (1967) Tolerance
and initial metabolic response to ammonia intoxication in
selected bats and rodents. J. Mammal., 48: 564-572.
SUGIYAMA, K., YOSHIDA, M., & OYAMADA, M. (1968) [A case of
chemical bronchiectasia induced by inhalation of ammonia gaz.]
Nippon Kyohbu Shikkan Gakkai Zasshi, 27: 797-803 (in Japanese).
SUKUMARAN, N. & KUTTY, M.N. (1977) Oxygen consumption and
ammonia excretion in the catfish Mystus armatus, with special
reference to swimming speed and ambient oxygen. Proc. Indian
Acad. Sci., 86B: 195-206.
SUMMERSKILL, W.H.J. & WOLPERT, E. (1970) Ammonia metabolism
in the gut. Am. J. clin. Nutr., 23: 633-639.
SUYAMA, M., KOIKE, J., & SUZUKI, K. (1960) Studies on the
glycolysis and the formation of ammonia in the muscle and
blood of elasmobranchs. J. Tokyo Univ. Fish., 46: 51-60.
SWIGERT, J.P. & SPACIE, A. (1983) Survival and growth of
warmwater fishes exposed to ammonia under low flow conditions,
Springfield, Virginia, National Technical Information Service
(NTIS PB83-257535).
TABATA, K. (1962) [Toxicity of ammonia to aquatic animals
with reference to the effect of pH and carbon dioxide.]
Hokoku, 34: 67-74 (in Japanese).
TAIGANIDES, E.P. & WHITE, R.K. (1969) The menace of noxious
gases in animal units. Trans. Am. Soc. Agric. Eng., 12:
359-362.
TAKAHASHI, H., WAKAI, K., & OHUCHI, K. (1984) [Experiences
of chemical burns induced by ammonia gaz.] Nippon Saigai
Igakkai Kaishi, 32: 741-749 (in Japanese).
TAPLIN, G.V., CHOPRA, S., YANDA, R., & ELAM, D. (1976)
Radionuclidic lung-imaging procedures in the assessment of
injury due to ammonia inhalation. Chest, 69: 5.
TAYLOR, A.W., EDWARDS, W.M., & SIMPSON, E.C. (1971)
Nutrients in streams draining woodland and farmland near
Coshocton, Ohio. Water Resour. Res., 7: 81-89.
TAYLOR, J.E. (1973) Water quality and bioassay study from
Crawford National Fish Hatchery. Trans. Nebr. Acad. Sci., 2:
176-181.
THE NETHERLANDS MINISTRY OF HOUSING, PHYSICAL PLANNING AND
ENVIRONMENT (1984) Acidification in the Netherlands: effects
and policies. In: Proceedings of a Conference on Air Pollution
in Europe, Munich, 24-27 June, 1984, The Netherlands Ministry
of Housing, Physical Planning and Environment, pp. 9.
THOMAS, W.H., HASTINGS, J., & FUJITA, M. (1980) Ammonium
input to the sea via large sewage outfalls. Part 2. Effects of
ammonium on growth and photosynthesis of southern California
phytoplankton cultures. Mar. environ. Res., 3(4): 291-296.
THOMPSON, R.Y. & HALLIBURTON, I.W. (1966) Effect of diet on
the composition of the kidney. Biochem. J., 99(3): 44.
THORNTON, N.C. & SETTERSTROM, C. (1940) Toxicity of ammonia,
chlorine, hydrogen cyanide, hydrogen sulphide, and sulphur
dioxide gases. III. Green plants. Contrib. Boyce Thompson
Inst., 11: 343-356.
THURSTON, R.V. (1981) Acute toxicity of ammonia to the
pumpkinseed (Lepomis gibbosus), Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research
Laboratory.
THURSTON, R.V. & MEYN, E.L. (1984) Acute toxicity of ammonia
to five fish species from the northwest United States,
Bozeman, Montana Fisheries Bioassay Laboratory, Montana State
University, 13 pp (Technical Report Vol. 84, No. 4).
THURSTON, R.V. & RUSSO, R.C. (1981) Acute toxicity of
ammonia to golden trout (Salmo aguabonita) and mottled sculpin
(Cottus bairdi), Bozeman, Montana, Fisheries Bioassay
Laboratory, Montana State University, 10 pp (Technical Report
Vol. 81, No. 1).
THURSTON, R.V. & RUSSO, R.C. (1983) Acute toxicity of
ammonia to rainbow trout. Trans. Am. Fish. Soc., 112: 696-704.
THURSTON, R.V., RUSSO, R.C., & SMITH, C.E. (1978) Acute
toxicity of ammonia and nitrite to cutthroat trout fry. Trans.
Am. Fish. Soc., 107(2): 361-368.
THURSTON, R.V., CHAKOUMAKOS, C., & RUSSO, R.C. (1981a)
Effect of fluctuating exposures on the acute toxicity of
ammonia to rainbow trout (Salmo gairdneri) and cutthroat trout
(S. clarki). Water Res., 15(7): 911-917.
THURSTON, R.V., PHILLIPS, G.R., RUSSO, R.C., & HINKINS, S.M.
(1981b) Increased toxicity of ammonia to rainbow trout (Salmo
gairdneri) resulting from reduced concentrations of dissolved
oxygen. Can. J. Fish. aquat. Sci., 38(8): 983-988.
THURSTON, R.V., RUSSO, R.C., & VINOGRADOV, G.A. (1981c)
Ammonia toxicity to fishes: Effect of pH on the toxicity of
the un-ionized ammonia species. Environ. Sci. Technol., 15(7):
837-840.
THURSTON, R.V., RUSSO, R.C., & PHILLIPS, G.R. (1983) Acute
toxicity of ammonia to fathead minnows. Trans. Am. Fish. Soc.,
112: 705-711.
THURSTON, R.V., LUEDTKE, R.J., & RUSSO, R.C. (1984a)
Toxicity of ammonia to fresh-water insects of three families,
Bozeman, Montana, Fisheries Bioassay Laboratory, Montana State
University, 26 pp (Technical Report Vol. 84, No. 2).
THURSTON, R.V., RUSSO, R.C., LUEDTKE, R.J., SMITH, C.E., MEYN,
E.L., CHAKOUMAKOS, C., WANG, K.C., & BROWN, C.J.D. (1984b)
Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish.
Soc., 113(1): 56-73.
THURSTON, R.V., MEYN, E.L., ZAJDEL, R.K., & RUSSO, R.C.
(submitted) Chronic toxicity of ammonia to fathead minnows
(Pimephales promelas). Submitted to Trans. Am. Fish. Soc.
TMRI (1971) [Atmosphere division. On survey of air
pollutants peculiar to factory-clustered area.] Tokyo
Meteorol. Res. Inst. Environ. Prot., 2: 109-111 (Annual
Report) (in Japanese).
TOMASSO, J.R., GOUDIE, C.A., SIMCO, B.A., & DAVIS, K.B.
(1980) Effects of environmental pH and calcium on ammonia
toxicity in channel catfish. Trans. Am. Fish. Soc., 109(2):
229-234.
TOPPING, D.C. & VISEK, W.J. (1976) Nitrogen intake and
tumourigenesis in rats injected with 1,2-dimethylhydrazine.
J. Nutr., 106: 1583.
TORDA, C. (1953) Ammonium ion content and electrical
activity of the brain during the preconvulsive and convulsive
phases induced by various convulsants. J. Pharmacol. exp.
Ther., 107: 197-203.
TOTH, B. (1972) Hydrazine, methylhydrazine, and
methylhydrazine sulfate carcinogenesis in Swiss mice. Failure
of ammonium hydroxice to interfere in the development of
tumors. Int. J. Cancer, 9: 109.
TOWLE, D.W., PALMER, G.E., & HARRIS, J.L. III (1976) Role of
gill Na+ + K+-dependent ATPase in acclimation of blue crabs
(Callinectes sapidus) to low salinity. J. exp. Biol., 196:
315-322.
TOWLE, D.W. & TAYLOR, D.D. (1976) Effect of NH4+ and K+ on
Na+-transport ATPase activity of blue crab gill Am. Zool., 16:
224.
TRESHOW, M. (1970) Ammonia. In: Environment and plant
response, New York, McGraw-Hill Book Company, pp. 362-363.
TURNBULL, H., DEMANN, J.G., & WESTON, R.F. (1954) Toxicity
of various refinery materials to fresh water fish. Ind. Eng.
Chem., 46(2): 324-333.
ULRICH, B. (1983) Soil acidity and its relation to acid
deposition. In: Effects of accumulation of air pollutants in
forest ecosystems, Ulrich and Pankrasth, The Netherlands,
D. Reidel Publishing Company, pp. 127-146.
ULSHAFER, T.R. (1958) The measurement of changes in
acetylcholine level (ACh) in rat brain following ammonium ion
intoxication and its possible bearing on the problem of
hepatic coma. J. lab. clin. Med., 52: 718-723.
ULTSCH, G.R., JACKSON, D.C., & MOALLI, R. (1981) Metabolic
oxygen conformity among lower vertebrates: the toadfish
revisited. J. comp. Physiol., 142: 439-443.
UNDERWRITERS LABORATORIES (1933) Report on the comparative
life, fire, and explosion hazards of common refrigerants.
Miscellaneous Hazard No. 2375, Chicago, Illinois, Underwriters
Laboratories, pp. 26-28.
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1963) Effects of
pollution on fish; toxicity of mixutures of zinc, sulfate, and
ammonium chloride; toxicity of sewage effluents; the toxicity
of poisons under estuarine conditions, London, United Kingdom
Ministry of Technology, pp. 76-83 (Water Pollution Research
1962).
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1968) Effects of
pollution on fish, London, United Kingdom Ministry of
Technology, pp. 56-65 (Water Pollution Research 1967).
US EPA (1972) Air quality data for 1968 from the National
Air Surveillance Networks and contributing state and local
networks, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Programs, 231
pp (Report No. APTD-0978).
US EPA (1979a) Ammonia study using STORET ambient monitoring
data, Washington DC, US Environmental Protection Agency,
Monitoring and Data Support Division, Office of Water Planning
and Standards.
US EPA (1979b) Methods for chemical analysis of water and
wastes, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring and Support Laboratory (Report No.
EPA 600/4-79-020).
US EPA (1981) Multimedia health assessment document on
ammonia, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (Report No.
ECAO/82-DO10).
US EPA (1985) Ambient water quality criteria for ammonia -
1984, Duluth, Minnesota, US Environmental Protection Agency,
Office of Research and Development.
US NIOSH (1974) Criteria for a recommended standard.
Occupational exposure to ammonia, Washington DC, National
Institute for Occupational Safety and Health, pp. 74-136 (US
DHEW Publication No. 246-699).
US NIOSH (1977) NIOSH manual of analytical methods. Part I.
NIOSH monitoring methods, Washington DC, National Institute
for Occupational Safety and Health, Vol. 1, pp. 205.1-205.3
(US DHEW Publication No. 77-157-A).
UZVOLGYI, E. & BOJAN, F. (1980) Possible in vivo formation
of a carcinogenic substance from diethyl pyrocarbonate and
ammonia. J. Cancer Res. clin. Oncol., 97: 205.
VALENTINE, H. (1964) A study of the effect of different
ventilation rates on the ammonia concentrations in the
atmosphere of broiler houses. Br. Poult. Sci., 5: 149-159.
VAMOS, R. (1963) Ammonia poisoning in carp. Acta. biol.
Szeged., 9(1-4): 291-297.
VAMOS, R. & TASNADI, R. (1967) Ammonia poisoning in carp. 3.
The oxygen content as a factor influencing the toxic limit of
ammonia. Acta. biol. Szeged., 13(3-4): 99-105.
VAN AALST, R.M. (1984) [Atmospheric process and deposition,]
Ministry of Agriculture and Fisheries, Ministry of Housing,
Physical Planning and Environment, 76 pp (Report No. 84(2))
(in Dutch).
VAN BREEMEN, N. & JORDENS, E.R. (1983) Effects of
atmospheric ammonium sulfate on calcareous and non-calcareous
soils of woodlands in the Netherlands. In: Ulrich, B. &
Panlerath, J., ed. Effects of accumulation of air pollutants
in forest ecosystems, The Netherlands, Reidel Publishing
Company, pp. 171-182.
VANCURA, E.M., CLINTON, J.E., RUIZ, E., & KRENZELOK, E.P.
(1980) Toxicity of alkaline solutions. Ann. emergency Med.,
9(3): 118-122.
VAN DER EERDEN, L.J.M. (1982) Toxicity of ammonia to plants.
Agric. Environ., 7: 223-235
VAN SLYKE, D.D., PHILLIPS, R.A., HAMILTON, P.B., ARCHIBALD,
R.M., FUTCHER, P.H., & MILLER, A. (1943) Glutamine as source
material of urinary ammonia. J. Biol. Chem., 150: 481-482.
VAUGHN, R.E. & SIMCO, B.A. (1977) Effects of ammonia on
channel catfish, Ictalurus punctatus. ASB Bull., 24(2): 92.
VENKATARAMIAK, A., LAKSHMI, G.J., BEST, C., GUNTER, G.,
HARTWIG, E., & WILDE, P. (1981) Studies on toxicity of OTEC
plant components on marine animals from the Gulf of Mexico,
Springfield, Virginia, National Technical Information Service
(DE81030167).
VERBERK, M.M. (1977) Effects of ammonia in volunteers.
Int. Arch. occup. environ. Health, 39: 73 -81.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experiences of the
Clinica del Lavoro with their own maximal exposure limits for
industrial chemicals.] Arch. Gewerbepathol. Gewerbehyg., 13:
528-534 (in German).
VINES, H.M. & WEDDING, R.T. (1960) Some effects of ammonia on
plant metabolism and a possible mechanism for ammonia
toxicity. Plant Physiol., 35: 820-825.
VINOKUROVA, M.K. & MAL'KOVA, V.B. (1963) [Toxicology of the
selective herbicide ammonium sulfamate.] Gig. Trud. Prof.
Zabol., 7(5): 56-57 (in Russian).
VISEK, W.J., KOLONDY, G.M., & GROSS, P.R. (1972) Ammonia
effects in cultures of normal and transformed 3T3 cells.
J. cell Physiol., 80: 373.
VISEK, W.J., CLINTON, S.K., & TRUEX, C.R. (1978) Nutrition
and experimental carcinogenesis. Cornell Vet., 68: 3.
VITTI, T.G., VUKMIROVICH, R., & GAEBLER, O.H. (1964)
Utilization of ammonia nitrogen, administered by intragastric,
intraperitoneal and subcutaneous roues: Effects of growth
hormone. Arch. Biochem. Biophys., 106: 475-482.
WALLEN, I.E., GREER, W.C., & LASATER, R. (1957) Toxicity to
Gambusia affinis of certain pure chemicals in turbid waters.
Sewage Ind. Wastes, 29(6): 695-711.
WALLINGFORD, G.W. (1977) Fate of nitrogen from fertilizer
practices. In: Disposal of residues on land, Rockville,
Maryland, Information Transfer, Inc., pp. 152-155.
WALSH, L.M. (1977) Updating the nitrogen cycle. In: Disposal
of residues on land, Rockville, Maryland, Information
Transfer, Inc., pp. 146-151.
WALTON, M.J. & COWEY, C.B. (1977) Aspects of ammoniogenesis
in rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol.,
57B: 143-149.
WALUGA, D. & FLIS, J. (1971) [Changes in peripheral blood in
carp ( Cyprinus carpio L.) under the influence of ammonia
solution.] Rocz. Nauk Roln. Ser. H, 93(2): 87-94 (in Russian).
WARNCKE, D.D. & BARBER, S.A. (1973) Ammonium and nitrate
uptake by corn ( Zea mays L.) as influenced by nitrogen
concentration and NH4-/NO3-ratio. Agric. J., 65: 950-953.
WARREN, K.S. (1958) The differential toxicity of ammonium
salts. J. clin. Invest., 37: 497-501.
WARREN, K.S. & NATHAN, D.G. (1958) The passage of ammonia
across the blood, brain, barrier and its relation to blood pH.
J. clin. Invest., 37: 1724-1728.
WARREN, K.S. & SCHENKER, S. (1964) Effect of an inhibitor of
glutamine synthesis (methionine sulfoximine) on ammonia
toxicity and metabolism. J. lab. clin. Med., 64: 442-449.
WARREN SPRING LABORATORY (1982) Acidity of rainfall in the
United Kingdom.
WATTS, R.L. & WATTS, D.C. (1974) Nitrogen metabolism in
fishes. In: Florkin, M. & Scheer, B.T. ed. Chemical Zoology,
New York, Academic Press, Vol. III, pp. 369-446.
WEAST, R.C., ed. (1979) CRC handbook on chemistry and
physics, 59th ed., Boca Raton, Florida, CRC Press Inc.
WEATHERBY, J.H. (1952) Chronic toxicity of ammonia fumes by
inhalation. Proc. Soc. Exp. Biol. Med., 81: 300-301.
WEBB, D.W., BARTLEY, E.E., & MEYER, R.M. (1972) A comparison
of nitrogen metabolism and ammonia toxicity from ammonium
acetate and urea in cattle. J. anim. Sci., 35: 1263-1270.
WEBB, J.T. & BROWN, G.W., Jr (1976) Some properties and
occurrence of glutamine synthetase in fish. Comp. Biochem.
Physiol., 54B: 171-175.
WEDDING, R.T. & VINES, H.M. (1959) Inhibition of reduced
diphosphopyridine nucleotide oxidation by ammonia. Nature
(Lond.), 184: 1226-1227.
WEEKS, M.E. (1968) Discovery of the elements, 7th ed.,
Washington DC, Journal of Chemical Education, p. 197.
WEHRBEIN, M.F., VIPPERMAN, P.E., Jr, PEO, E.R., Jr, &
CUNNINGHAM, P.J. (1970) Diammonium citrate and diammonium
phosphate as sources of dietary nitrogen for growing-finishing
swine. J. anim. Sci., 31: 327-332.
WHITE, A., HANDLER, P., & SMITH, E.L. (1973) Renal function
and the composition of urine. In: Principles of biochemistry,
5th ed., New York, McGraw Hill Book Co., pp. 604-704 and
928-948.
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series No.
539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
WICKINS, J.F. (1976) The tolerance of warm-water prawns to
recirculated water. Aquaculture, 9(1): 19-37.
WILKIN, D.C. & FLEMAL, R.C. (1980) Feasibility of water
quality improvement in three Illinois rivers. J. Water Poll.
Control Fed., 52(2): 293.
WILKINSON, R.H., THOMAS, W.J., HANSEN, C.M., & SHIELDS, E.A.
(1978) Forage preservation by timed-slow release of NH3 (ASAE
Paper No. 78-1523).
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968a) Toxicologic effects of ammonium carbamate and related
compounds. Am. J. vet. Res., 29: 987-906.
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968b) Comparative ammonia toxicity. Comp. Biochem.
Physiol., 25: 295-301.
WILT, F.H. & MAZIA, D. (1974) The stimulation of cytoplasmic
polyadenylyation in sea urchin eggs by ammonia. Dev. Biol.,
37: 422-424.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) Merck Index, 9th ed., Rahway, New Jersey, Merck
and Co. Inc., p. 69.
WINKLER, M.M. & GRAINGER, J.L. (1978) Mechanisms of action
of NH4Cl and other weak bases in the activation of sea urchin
eggs. Nature (Lond.), 273(5663): 536-538.
WOKER, H. & WUHRMANN, K. (1950) [The sensitivity of
different species of fish to ammonia, hydrocyanic acid and
phenol.] Rev. Suisse Zool., 57(3): 548-553 (in German).
WOLAVER, T.C. (1972) Distribution of natural and
anthropogenic elements and levels in precipitation across the
US: theory and quantitative models, Chapel Hill, North
Carolina, University of North Carolina, 75 pp (Prepared for US
EPA).
WOOD, R.W. (1979) Behavioral evaluation of sensory
irritation evoked by ammonia. Toxicol. appl. Pharmacol., 50:
157-162.
WORCEL, A. & ERECINSKA, M. (1962) Mechanism of inhibitory
action of ammonia on the respiration of rat liver
mitochondria. Biochim. Biophys. Acta, 65: 27-33.
WRIGHT, P.A. & WOOD, C.M. (1985) An analysis of branchial
ammonia excretion in the freshwater rainbow trout: effects of
environmental pH change and sodium uptake blockage.
J. exp. Biol., 114: 329-353.
WUHRMANN, K. & WOKER, H. (1948) [Contributions to the
toxicology of fishes. II. Experimental investigations on
ammonia and hydrocyanic acid poisoning.] Schweiz. Z. Hydrol.,
11: 210-244 (in German).
WUHRMANN, K., ZEHENDER, F., & WOKER, H. (1947) [Biological
significance for fisheries of ammonium-ion and ammonia content
of flowing bodies of water.] Vierteljahrsschr. Naturforsch.
Ges. Zurich, 92: 198-204 (in German).
YAHAGI, H., SUZUKI, K., & MATSUI, Y. (1959) [Fourteen cases
of ammonia gas poisoning.] Shuhkan Igaku Tsuhshin, 601: 26-30
(in Japanese).
YECK, R.G., SMITH, L.W., & CALVERT, C.C. (1975) Recovery of
nutrients from animal wastes. An overview of existing options
and potentials for use in feed. In: Managing livestock waste:
Proceedings of the Third International Symposium on Livestock
Wastes, St. Joseph, Michigan, American Society of Agricultural
Engineers, pp. 192-196.
YOSHIDA, J., NAKAME, K., & NAKAMURA, R. (1957) Toxicity of
urea and its control. II. Toxicity of ammonium salts and urea
in rabbits and goats. Nippon Chikusangaku Kaiho, 28: 185-191.
ZIEVE, L., DOIZAKI, W.M., & ZIEVC, F.J. (1974) Synergism
between mercaptans and ammonia or fatty acids in the
production of coma. A possible role for mercaptans in the
pathogenesis of hepatic coma. J. lab. clin. Med., 83: 16-28.
ZIMBER, A. (1970) Studies on the effect of ammonia on DNA
synthesis and on cellular proliferation and renewal in tissues
of the mouse and in Ehrlich ascites tumor cells, Ithaca,
New York, Cornell University (Ph.D. Thesis).
ZIMBER, A. & VISEK, W.J. (1972) Effect of urease injection
on DNA synthesis in mice. Am. J. Physiol., 223: 1004.
ZIMMERMAN, P.W. (1949) Impurities in the air and their
influence on plant life. In: Proceedings of the First National
Air Pollution Symposium, Pasadena, California, 10-11 November,
1949, pp. 135-141 (Sponsored by Stanford Research Institute).
ZLATEVA, M., VALCEVA, V., & ANTOV, G. (1974) Toxic organic
changes in laboratory animals placed in an ammonia-rich
working atmosphere. Tr. Inst. Hig. Ohrana Tr. Prof. Zabol.,
22(1): 147-154.
Annex I. Ammonium salts evaluations prepared by the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)a
------------------------------------------------------------------------------------------------------
Ammonium salt Functional use Evaluation status Reference
(ADI, MTDI)b,c
------------------------------------------------------------------------------------------------------
acetate pH-adjusting agent not specifiedd (grouped with WHO (1982a)
other ammonium salts and acetate)
alginate thickening agent, stabilizer 0 - 50 (grouped with alginic acid WHO (1974a,b)
and calcium, potassium, and
sodium alginate)
bicarbonate leavening agent, buffer not specified WHO (1982b)
(hydrogen carbonate) neutralizing agent, alkali
carbonate
chloride dough conditioner, yeast, food not specified (grouped with WHO (1980)
hydrochloric acid and magnesium
and potassium chlorides)
hydrogen phosphate buffering agent, dough MTDI:, 70 expressed as phosphorus WHO (1982a,b)
phosphate dibasic conditioner, leavening agent, (grouped with phosphates and
yeast polyphosphates, including
phosphates occurring naturally in
food)
hydroxide (strong alkali not specified WHO (1966)
ammonia solution)
lactate buffer, dough conditioner not specified WHO (1974a,b)
persulfate flour-treatment agent no ADI set WHO (1966)
------------------------------------------------------------------------------------------------------
a Queries concerning updated information should be addressed to: Joint WHO Secretary of the Joint
FAO/WHO Expert Committee on Food Additives, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland.
b ADI = Acceptable daily intake for man expressed as mg/kg body weight.
c MTDI = Maximum tolerable daily intake for man, expressed as mg/kg body weight.
d ADI not specified = the total intake of the substance arising from its uses at the levels necessary
to achieve the desired effect does not represent a hazard to health.
REFERENCES TO ANNEX I
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series
No. 539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
ANNEX II: TREATMENT OF EXCESSIVE EXPOSURE TO AMMONIA
Ammonia (gas and liquid) is an extremely irritant chemical
affecting the skin, eyes, and the respiratory tracts. Ammonia gas
can produce burning of the eyes, lachrymation, and severe eye
damage. When inhaled it can produce coughing, laryngitis,
bronchitis, chest pains, and severe respiratory problems. Contact
with liquid ammonia can result in severe eye and skin burns due
both to its irritant properties and chilling effect.
Those working with ammonia should be trained in its safe use
including the dangers of improper handling, the use of protective
equipment, and the avoidance of unnecessary inhalation of the gas
and direct contact with liquid ammonia. After handling liquid
ammonia, the hands should be washed thoroughly before eating or
smoking.
The provision of protective clothing and equipment is not an
adequate substitute for safe working conditions. However, where
exposure cannot be adequately controlled, workers should be
provided with suitable impervious clothing, boots and gloves, and,
depending on the severity of the conditions, a face shield or
safety goggles and a mask or self contained breathing apparatus.
In places where very high gaseous ammonia concentrations are
expected, complete gas suits should be used.
Emergency showers and eye wash or water sprays should be
provided in all areas where ammonia is handled and where leaks,
spills or splashes may occur. Clothes contaminated with ammonia
should be discarded immediately and not worn again until thoroughly
cleansed.
First aid
If excessive exposure has occurred first aid treatment should
be promptly initiated and medical advice obtained as soon as
possible.
Ammonia in the eye (gas, liquid, or liquor)
Ammonia in the eye may cause severe injury and must be treated
immediately by irrigation for at least 15 min with flowing water or
sterile buffered eye irrigation solution.
Ammonia on the skin (liquid or liquor)
Drench the affected area with water and remove contaminated
clothing and footwear. Wash the affected area continuously for 5 -
10 min or until pain ceases.
Ingestion (liquid or liquor)
If the patient is conscious large quantities of water may be
given to dilute the chemical in the stomach. No attempt should be
made to induce vomiting.
Inhalation of gas/vapour
1. Remove from exposure, secure airway and place in semiprone
recovery position if unconscious. Give artificial
respiration if not breathing.
2. If heartbeat is absent give external cardiac massage.
3. If there is cyanosis (blueness of lips) or air-hunger
administer oxygen by facemask.
4. A conscious patient may be given water to drink.
Further treatment
Ammonia in the eyes
Corneal damage is probable. Use local anaesthetics and
cycloplegics to enable thorough irrigation and examination. If the
cornea is damaged, administer topical antibiotics. Refer to a
specialist centre.
Ammonia on the skin
Treat as a chemical burn. Liquid ammonia may produce deep
burns that may require grafting. Refer deep or extensive burns to
a specialist centre.
Inhalation of gas/vapour
1. Ammonia is irritant to the respiratory tract causing:
(a) bronchial oedema, spasm, and hypersecretion
resulting in chest tightness, wheeze, and cough,
which may progress to severe dyspnoea; and
(b) lower airway inflammation with exudative
pulmonary oedema and impaired gaseous
diffusion. Symptoms may be delayed 24 h or
more. Resolution may be by fibrosis producing a
restrictive defect.
2. Treat hypoxia with oxygen, ventilation, and bronchial
lavage, as appropriate.
3. Consider administration of steroids by multiple metered
doses of topical aerosol, by inhalation, and/or by
injection. Early prophylactic use may be indicated.
4. Administer bronchodilators by inhalation or injection, as
indicated. Maintain with oral treatment.
5. Keep under medical surveillance for at least 48 h. Treat
symptomatically.
6. Observe for secondary respiratory infection and treat as
necessary.
Manifestations of ammonia toxicity can be traced to several
metabolic disturbances. Both photosynthetic and respiratory
pathways are affected adversely by ammonia. There is a direct
relationship between ammonia concentration and respiratory
metabolism, including oxygen uptake, glycolysis, and the
tricarboxylic acid cycle (Matsumoto et al., 1976). Ammonium ions
may restrict photosynthesis through the uncoupling of noncyclic
photophosphorylation in isolated chloroplasts (Gibbs & Calo, 1959;
Losada & Arnon, 1963), though the mechanism of action is not known
(Losada et al., 1973). It is well documented that uncoupling leads
to an increase in reducing power in the cell. There is evidence
that 1 - 3 mmol ammonium inhibits respiration in plants (Wedding &
Vines, 1959; Vines & Wedding, 1960). It is suspected that similar
or identical inhibition occurs in ammonium toxicity in animals.
The site of toxicity is thought to be in the electron-transport
system; specifically, at the oxidation of NADH to NAD. This may
have a mechanism similar to the interference by ammonium ion with
electron transfer in the photosynthetic reaction.
High atmospheric levels may have direct toxic effects. Plant
growth is inhibited by a decrease in the carbohydrate production as
a consequence of inhibition of photosynthesis (Losada & Arnon,
1963). Ammonium increases the permeability of the cell membrane
causing plasmolysis and necrosis. Furthermore, the flexibility of
cells may be decreased, which results in an increased sensitivity
to frost. Excess ammonia can be detoxified as long as amino acids
are converted in the presence of carbohydrates (Fig. 1) (Van der
Eerden, 1982).
Most data on the toxic exposure of plants to ammonia have been
derived from controlled fumigation studies. Such experiments have
provided the following relative susceptibilities to ammonia:
leaves > stems, fungi, and bacteria < seeds, sclerotia, and
animals (McCallan & Setterstrom, 1940).
An exposure of 175 mg/m3 (250 ppm) for 4 min produced 50%
foliar necrosis in tomatoes, whereas the same foliar injury was
only produced in buckwheat and tobacco with exposure to 700 mg/m3
(1000 ppm) for 5 and 8 min, respectively (Thornton & Setterstrom,
1940). Zimmerman (1949) reported that fumigation with ammonia at
28 mg/m3 (40 ppm) for 60 min injured tomato, sunflower, and coleus
completely, and a concentration of 2.1 mg/m3 (3 ppm) severely
injured mustard (Benedict & Breen, 1955). Other plant parts are
more resistant to ammonia injury than the foliage. Thornton &
Setterstrom (1940) found 50% injury in tomato stems after exposure
to 700 mg/m3 (1000 ppm) for 60 min. Barton (1940) observed that
moist spring rye seeds were killed in a 4-h exposure to 700 mg
ammonia/m3 (100 ppm), whereas moist radish seeds were still viable
after 16 h. Exposure to a concentration of 175 mg/m3 (250 ppm) for
16 h reduced germination of rye seeds by half, but had no effect on
radish seeds.
Foliar injury is the most common toxic effect of anhydrous
ammonia on vegetation (Linzon, 1971). In broad-leaved woody plants
exposed to high concentrations of ammonia, the injury begins as
large, dark green water-soaked areas on leaves, which darken into
black or brownish-gray bifacial necrotic lesions, widely
distributed over the leaf surface. In lightly injured leaves,
these symptoms occur mainly on the upper surface. Conifer foliage
injured by ammonia exposure darkens to shades of gray-brown,
purple, or black. The entire needle is usually affected.
Abscission of severely damaged leaves is often seen in broad-leaved
and conifer species. On trees or shrubs with crowded leaves,
injury may be confined to particular sections of the leaf.
Occasionally, the foliage of woody species turns a variety of
colours, mimicking autumn colours. Symptoms of injury in
herbaceous plants are more variable, ranging from irregular,
bleached, bifacial, necrotic lesions to dark upper-surface
discolouration (Treshow, 1970). Grasses and cereal grains develop
tan to reddish-brown marginal or interveinal necrosis, and broad-
leaved weeds show red-brown to dark-brown upper surface
discolouration on terminal or marginal portions of the leaf
(Benedict & Breen, 1955). Coleus leaves lose their brilliant
colour after exposure to ammonia and appear green (Zimmerman,
1949). Injury to flowers by ammonia is rarely seen in the field,
but the development of small necrotic spots on azalea flowers has
been reported following ammonia exposure (Treshow, 1970).
Although parts of plants, other than the foliage, are less
susceptible to the injurious effects of ammonia (McCallan &
Setterstrom, 1940), injury to apples, peaches, and other fruits and
vegetables, accidentally exposed during cold storage, has been
reported (Ramsey, 1953). Apparently, ammonia entered the fruit and
turned red-pigmented tissues black, or brown, and yellow tissues,
dark-brown. Immediately on exposure to ammonia, the outer skins of
red onions became greenish-black, and the skin of yellow and brown
onions became dark brown. These colour changes were usually
permanent. Fumigation of fruit with ammonia also caused overall
darkening of the skin. Peaches and apples showed such symptoms at
140 mg/m3 (200 ppm) and 210 mg/m3 (300 ppm), respectively (Brennan
et al., 1962). These symptoms of injury were similar to those seen
on fruits injured by the accidental release of ammonia.
In studies in the Netherlands, several vegetables and trees
showed leaf damage by necrosis, growth reduction, and increased
frost senstivity at concentrations of 75 µg/m3 (annual average),
600 µg/m3 (during 24 h), and 10 000 µg/m3 (during 1 h) (Van der
Eerden, 1982). The sensitivity of pine trees to exposure to
ammonia differed between species (den Boer & Bastiaens, 1984). A
survey is given in Table 14. Airborne ammonium sulfate deposits
damaged the needles of pine trees. Owing to the enhanced uptake of
ammonium, the excretion of potassium, magnesium, and calcium
increases, often resulting in potassium and/or magnesium
deficiency, which may lead to premature shedding of needles
(Roelofs et al., 1985).
Effects of ammonia on the root system have generally been
studied by exposing the roots to different aqueous ammonium
solutions.
Table 14. Relative sensitivity of some pine trees to NH3a
-----------------------------------------------------------------
High sensitivity Moderate sensitivity Low sensitivity
-----------------------------------------------------------------
Picea abies Picea omorika Pinus sylvestris
Picea sitchensis Pinus nigra var. Pinus nigra nigra
Taxus maritima Tsuga canadensis
Cupressus leylandii Taxus baccata Taxus media
Pseutsuga menziesii Pinus mugo var. mughus
-----------------------------------------------------------------
a From: den Boer & Bastiaens (1984).
Exposure of tomato seedlings to ammonium solutions as the sole
nitrogen source showed reduced growth. The root system was
sparsely branched and discoloured and the stem was easily bruised.
There was considerable wilting of leaves, which developed marginal
necrosis (Pierpont & Minotti, 1977). Earlier, Maynard & Barker
(1969), using cucumber (C. sativus), bean (P. vulgaris), and pea
(Pisum sativum L.) in sand culture, demonstrated that ammonium
toxicity was generally characterized by an immediate reduction in
growth rate, wilting, marginal necrosis, interveinal chlorosis of
terminal leaves and, finally, death of the entire plant. However,
these symptoms did not occur in the ammonium medium with added
calcium carbonate (CaCO3) (Maynard & Barker, 1969; Pierpont &
Minotti, 1977). Several studies have shown that an increase in the
ammonium concentration is more deleterious to root than to shoot
growth (Bennett et al., 1964; Haynes & Goh, 1977). Warncke &
Barber (1973) observed that the ratio of root dry weight to shoot
dry weight decreased significantly with increasing concentrations
of nitrogen, but was not affected by the ammonium-to-nitrate ratio.
The roots appeared darker and less branched. The authors
attributed the observed decrease in root production to greater
acidity around the roots of ammonium-fed plants (Klemm, 1967;
Warncke & Barber, 1973).
In the Netherlands, field observations and experiments have
shown effects of ammonia and ammonium on pine forests, and
heathland vegetation (Heil & Diemont, 1983; Van Breemen & Jordens,
1983; den Boer & Bastiaens, 1984; Roelofs, in press; Schuurkes, in
press). In the United Kingdom, ombrotrophic mires (upland bogs
deriving water only from rain) are affected by eutrophication (Lee,
1985). Forest ecosystems in the Federal Republic of Germany may
also suffer from ammonia exposure (Hunger, 1978; Ulrich, 1983).
Although only little information is available for other countries,
it is expected that similar effects may occur in Belgium, Denmark,
and the northwestern part of France, where high ammonia emissions
occur as a consequence of the production of animal manure (Buysman
et al., 1985).
The condition of forests may deteriorate as a consequence of
direct exposure to ammonia. Pine trees in areas near intensive
livestock farms are particularly affected (Hunger, 1978; Janssen,
1982). In the Netherlands, there is a clear correlation between
ammonia emission and forest condition (Van Aalst, 1984). The
damage observed is the result of both direct and indirect effects,
and it is often difficult to distinguish between the two.
Field observations and experiments have shown deleterious
effects on pine trees, namely:
- decreased vitality (growth reduction and necrosis);
- higher susceptibility to fungal diseases and attack
by insects; and
- increased sensitivity to meteorological stress
factors, e.g., hard frost.
Most of these phenomena can be explained in terms of disturbed
nutrient budgets in trees. Enhanced ammonium sulfate uptake
results in the excretion of essential nutrients, such as potassium
and magnesium, by the needles, and the uptake of these ions by the
root system is inhibited as a result of ammonium accumulation and
cation leaching in soils (den Boer & Bastiaens, 1984; Roelofs, in
press). Increased ammonium uptake ultimately leads to enhanced
nitrogen levels in the leaves of beech (Nihlgard, 1970) and pine
tress (Roelofs et al., 1985).
More attention is being paid to the role of ammonium in the
forest dieback in Europe (Nihlgard, 1985).
Heather communities on poorly-buffered, slightly-acidic, and
nutrient-poor heathland soils are also disturbed as a result of the
deposition of airborne ammonia compounds, the number of plant
species declining in acidified and nitrogen-enriched heathland
soils. A succession from heather-dominated to grass-dominated
heathlands has been observed (Heil & Diemont, 1983; Roelofs, in
press). The same effects can be expected in other western European
countries where similar plant communities are present.
6.2.2. Aquatic plants
In the aquatic environment, nitrogen plays an important role in
determining the composition of phytoplankton and vascular plant
communities; in some cases, it can act as a limiting factor in
primary production. Ammonia is important in nitrogen metabolism,
because it functions as a nitrogen source in the synthesis of amino
acids.
Most species can use either ammonium or nitrate as the sole
nitrogen source, though, when both forms are available, ammonium is
used first. Uptake takes place through both roots and leaves. The
relative importance of ammonium as a nutrient depends on the
absolute concentration and the ratio of ammonium to nitrate.
Assimilation of ammonium is less expensive in terms of energy than
nitrate, because the first metabolic step in which nitrate is
reduced is not needed. Although ammonia is an important nutrient,
it appears to be toxic at higher concentrations. Its uptake in
large quantities may put a severe strain on the carbohydrate
metabolism of the species, because carbon skeletons are used for
detoxification (Bidwell, 1974). Increasing ammonia levels within
the cell inhibit the utilization of nitrate. Ammonia solutions
seem to be more toxic at high than at low pH, indicating that
toxicity is probably due primarily to NH3 rather than to NH4+.
Surface waters that are poorly buffered, nutrient poor, and
hydrologically dependent on rainfall and/or snow melt are most
sensitive to ammonia. Deposition of ammonia and other nitrogen
compounds may contribute significantly to the nitrogen enrichment
of susceptible waters. Ombrotrophic mire plant communities can be
altered by this atmospheric pollutant (Lee, 1985). In these
Sphagnum-dominated wetlands, seed plants become more dominant. In
small, poorly-buffered and nutrient-poor clear water lakes, both
water composition and macrophyte composition are altered.
Atmospheric ammonia deposition plays a major role in the
acidification and nitrogen enrichment of surface waters in the
Netherlands (Schuurkes, in press). Typical plant species belonging
to the Littorellion alliance disappear from acidified waters.
These species may be supressed by the luxuriant growth of Sphagnum
species and Juncus bulbosus. Ammonium enrichment enables the last
two species to form extremely high biomasses (Roelofs et al., 1984;
Schuurkes, in press; Schuurkes et al., 1985). It should be noted
that these changes in the plant community also influence the
structure of the animal population.
6.2.3. Fresh-water plants
Experimental data concerning the toxicity of ammonia for fresh-
water phytoplankton are limited. Przytocka-Jusiak (1976) reported
the effects of ammonia on the growth of Chlorella vulgaris. A 50%
inhibition was seen in 5 days of exposure to a concentration of
2.4 mg NH3/litre; complete growth inhibition occurred in 5 days
at 5.5 mg/litre. The NH3 concentration resulting in 50% survival
of C. vulgaris after 5 days was found to be 9.8 mg/litre. A C.
vulgaris strain in which the tolerance to elevated ammonia
concentrations was enhanced by prolonged incubation of the alga in
ammonium carbonate solutions was isolated by Przytocka-Jusiak et
al. (1977). C. vulgaris was reported to grow well in solutions
containing 4.4 mg NH3/litre, but growth was inhibited at 7.4
mg/litre (Matusiak, 1976). Tolerance to elevated concentrations of
NH3 seemed to show a slight increase, when other forms of nitrogen
were available to the alga, rather than when ammonia was the only
form of nitrogen in the medium. Bretthauer (1978) found that a
concentration (assuming pH 6.5 and 30 °C) of 0.6 mg NH3/litre
killed Ochromonas sociabilis, and that, at 0.3 mg/litre,
development of the population was reduced. Concentrations of 0.06
to 0.15 mg NH3/litre had an insignificant effect on growth, and
concentrations of 0.015 to 0.03 mg/litre enhanced growth.
Ammonia at concentrations exceeding 2.5 mg NH3/litre inhibited
photosynthesis and growth in the algal species Scenedesmus obliquus
and inhibited photosynthesis in the algae Chlorella pyrenoidosa,
Anacystis nidulans, and Plectonema boryanum (Abelovich & Azov,
1976). Mosier (1978) reported that NH3 concentrations causing a
50% reduction in oxygen production by the green alga Chlorella
ellipsoidea and blue-green alga Anabaena subcylindrica were
16.0 x 10-8 and 251.0 x 10-8 µg NH3-N/cell, respectively.
The rate of photosynthesis in the blue-green alga P. boryanum
was stimulated by NH4+, but inhibited by NH3 (Solomonson 1969); the
magnitude of these effects was dependent on the sodium-potassium
composition of the suspension media. Inhibition of photosynthesis
by NH3 was associated with a conversion of inorganic polyphosphate,
stored in the cells, to orthophosphate.
Champ et al. (1973) treated a small natural water pond with
ammonia to achieve a mean concentration of 25.6 mg NH3/litre. A
diverse population of dinoflagellates, diatoms, desmids, and blue-
green algae was present before ammonia treatment. Twenty-four
hours after treatment, the mean number of phytoplankton cells/litre
was reduced by 84%. By the end of 2 weeks (3.6 mg NH3/litre), the
original concentration of cells had been reduced by 95%.
Some research has been carried out to investigate the possible
use of ammonia as an aquatic herbicide. Champ et al. (1973)
reported virtually complete eradication of rooted aquatic
vegetation (water shield, Brasenia schreberi, and American lotus,
Nelumbo sp.). The NH3 concentration was 25.6 mg/litre 24 h after
ammonia addition, and 3.6 mg/litre, 2 weeks later. The use of high
concentrations of ammonia to eradicate aquatic vegetation has been
described by Ramachandran & Ramaprabhu (1976) and Ramachandran et
al. (1975).
In experiments with Potamogeton lucens, Litav & Lehrer (1978)
observed that ammonia caused appreciable injury to detached
branches. Ammonia inhibition of the growth of Eurasian
watermilfoil (Myriophyllum spicatum) affected both the length and
weight of roots and shoots (Stanley, 1974).
Grube (1973) found that Sium erectum was slightly injured at
15 mg NH3-N/litre, and completely eradicated at 35 mg/litre after a
10-week exposure. Callitriche sp. showed slight injury at 5 mg
NH3-N/litre, and the lethal dose was between 10 and 15 mg/litre.
Injury was estimated from the amount of black colouring and the
death of leaves. Roelofs et al. (1984) reported that exposure of
the isoebid Littorella uniflora to 50 µmol NH4+/litre for 10 weeks
resulted in excretion of an equivalent amount of potassium, which
may lead to discolouration and starvation.
Changes in the vegetation in 2 rivers subject to increased
pollution from agricultural fertilizers, urban sewage, and
industrial wastes, were studied by Litav & Agami (1976), who
attributed the changes in the composition of the plant species
primarily to a combination of ammonia and detergents. Agami et al.
(1976) transplanted 7 species of "clean water" macrophytes to
various sections of a river, and found that ammonia affected only
Nymphaea caerulea.
6.2.4. Salt-water plants
A concentration of 0.24 mg NH3/litre retarded the growth of
most of 10 species of benthic diatoms cultured for 10 days by
Admiraal (1977). Pinter & Provasoli (1963) found that Coccolithus
huxleyi was the most sensitive, and Pavlova gyrans and Hymenomonas
sp. the most tolerant to ammonium sulfate with intermediate tolerance
exhibited by Syracosphaera sp. and Ochrosphaera neapolitana.
Shilo & Shilo (1953, 1955) reported that the euryhaline alga
Prymnesium parvum was effectively controlled by applications of
ammonium sulfate, which exerted a lytic effect that decreased with
increasing pH, indicating that NH3 and not NH4+ is responsible for
the lytic activity of ammonium sulfate on P. parvum. It was
reported by Byerrum & Benson (1975) that added ammonium ion at
concentrations found to stimulate the photosynthetic rate also
caused the alga Amphidinium carterae to release up to 60% of fixed
14CO2 to the medium.
Natarajan (1970) found that the concentrations of fertilizer
plant effluent that were toxic for natural phytoplankton
(predominantly diatoms) ranged between 1.1 and 11 mg NH3/litre.
Thomas et al. (1980) concluded that increased ammonium
concentrations found near sewage outlets would not be inhibiting to
phytoplankton in the vicinity. Provasoli & McLaughlin (1963)
reported that ammonium sulfate was toxic for some marine
dinoflagellates, but only at concentrations far exceeding those in
sea water.
6.3. Aquatic Invertebrates
The toxicity of ammonia has been less extensively studied in
invertebrates than in fish. Most of the available invertebrate
data consists of studies on arthropods, primarily crustaceans and
insects. Many of these studies are laboratory tests in which test
animals were exposed to known concentrations of a toxic agent for
specified periods of time. Results may be expressed as a median
lethal concentration (LC50) for a given time period (e.g., 48-h
LC50) or, occasionally, as an effective concentration (EC), that
is, the concentration at which the test animal is completely
immobilized, though it might still be respiring (e.g., 48-h EC).
Another method of reporting test results is as the estimated time
required to kill 50% of a test population (LT50) at a given
concentration of toxin (e.g., LT50 = 250 min).
6.3.1. Fresh-water invertebrates: acute toxicity
The acute toxicity of ammonia for Daphnia magna has been
studied by Parkhurst et al. (1979, 1981) and Reinbold & Pescitelli
(1982a) with reported 48-h LC50s values of 2.08 and 4.94 mg
NH3/litre. DeGraeve et al. (1980) reported a similar 48-h LC50
value for Daphnia pulicaria of 1.16 mg NH3/litre. A threshold
toxicity value for D. magna of 2.4 - 3.6 mg NH3/litre lake water
was reported by Anderson (1948). Threshold concentration was taken
as the highest concentration that would just fail to immobilize the
test animals, under conditions of prolonged exposure (Anderson,
1948). A minimum lethal concentration of 0.55 mg NH3/litre was
reported for D. magna by Malacea (1966), and a 24-h LC50 value of
1.50 mg NH3/litre was reported by Györe & Oláh (1980) for Moina
rectirostris.
Buikema et al. (1974) reported an EC50 for NH3 toxicity in the
rotifer, Philodina acuticornis, to be 2.9 - 9.1 mg NH3/litre
(calculated using reported pH values of 7.4 - 7.9). Tests of
ammonia toxicity for the flatworm, Dendrocoelum lacteum (Procotyla
fluviatilis), and a tubificid worm (Tubifex tubifex) gave LC50
values of 1.4 and 2.7 mg NH3/litre, respectively (Stammer, 1953).
Thurston et al. (1984a) conducted 25 flow-through toxicity
tests with 3 mayfly, 2 stonefly, 1 caddisfly, and 1 isopod species;
all tests were conducted with water of similar chemical
composition. The 96-h LC50 values ranged from 1.8 to 5.9 mg
NH3/litre. The results also indicated that a 96-h test is not long
enough to determine the acutely lethal effects of ammonia on the
species tested, because an asymptotic LC50 was not always obtained
within 96 h. Percentage survival data were reported for some
mayfly, stonefly, and caddisfly tests in which LC50 values were not
obtained; there was 60 - 100% survival at test concentrations
ranging from 1.5 to 7.5 mg NH3/litre. Gall (1980) tested NH4Cl
with Ephemerella sp. (near excrucians). Organisms were exposed
to ammonia for 24 h, followed by 72 h in ammonia-free water;
mortality observations were made at the end of the overall 96-h
period. An EC50 value of 4.7 mg NH3/litre was obtained. Hazel et
al. (1979) reported a LC50 value of 8.0 mg NH3/litre for the beetle
Stenelmis sexlineata.
No deaths occurred in ammonia toxicity tests conducted on scud,
Gammarus lacustris, or D. magna, using dilution water from a
river, after a 96-h exposure to 0.08 mg NH3/litre. In a second
test, using river water buffered with sodium bicarbonate, 13%
mortality occurred with scud at the several concentrations tested,
including the highest and lowest of 0.77 and 0.12 mg NH3/litre,
respectively; 7 and 13% mortality occurred with D. magna at the
same concentrations (Miller et al., 1981).
Five fresh-water mussel species, Amblema p. plicata, Anodonta
imbecillis, Corbicula manilensis, Cyrtonaias tampicoensis, and
Toxolasma texasensis, were exposed for 165 h to a concentration of
0.32 mg NH3/litre; T. texasensis was most tolerant to ammonia, and
A. p. plicata was most sensitive (Horne & McIntosh, 1979). During
the tests, the more tolerant species generally had their shells
tightly shut, whereas the least tolerant species continued
siphoning or had their mantles exposed. In 2 studies, acute
exposure of the fresh-water crayfish, Orconectes nais, to ammonium
chloride gave LC50 values of 3.15 and 3.82 mg NH3/litre,
respectively (Evans, 1979; Hazel et al., 1979).
6.3.2. Fresh-water invertebrates: chronic toxicity
Few studies have been conducted on the long-term exposure of
fresh-water invertebrates to ammonia. In a long-term test
conducted by Reinbold & Pescitelli (1982a), reproduction and growth
of D. magna were affected at a concentration of 1.6 mg NH3/litre.
Two tests lasting 42 days were conducted by Anderson et al.
(1978) on the effects of ammonium chloride on the fingernail clam,
Musculium transversum. Significant mortality (67 and 72%) occurred
in both tests at a concentration of 0.7 mg NH3/litre. In one of
the studies, significant reduction in growth was observed after 14
days of exposure to 0.41 mg NH3/litre. Sparks & Sandusky (1981)
reported that fingernail clams exposed to 0.23 and 0.63 mg
NH3/litre showed 36 and 23% mortality, respectively, in 4 weeks;
after 6 weeks, there was 47% mortality at 0.073 mg NH3/litre, and
83% mortality at 0.23 and 0.63 mg NH3/litre. After 6 weeks there
was no growth in any test chamber (concentrations of 0.036 mg
NH3/litre and higher), other than in the controls.
Two partial tests, lasting 24 and 30 days, respectively, were
conducted by Thurston et al. (1984a) on the stonefly Pteronarcella
badia. Adult stonefly emergence was delayed with increasing
ammonia concentration, and little or no emergence occurred at
concentrations exceeding 3.4 mg NH3/litre. There was no significant
relationship between the food consumption rates of nymphs and
concentrations up to 6.9 mg NH3/litre. LC50 values for 24- and
30-day exposures were 1.45 and 4.57 mg NH3/litre, respectively.
The effects of ammonia on the ciliary beating rate of clam
gills were investigated by Anderson et al. (1978). Concentrations
of 0.036 - 0.11 mg NH3/litre caused a reduction in the ciliary
beating rate of fingernail clams; the effects ranged from a 50%
reduction in beating rate to complete inhibition. Adult clams (> 5
mm) were more sensitive than juveniles (< -5 mm); adults were also
slightly more sensitive than the unionid mussel, Elliptio
complanata, and the Asiatic clam, C. manilensis. Shaw (1960)
investigated the effects of ammonium chloride on sodium influx in
the fresh-water crayfish, Astacus pallipes. Ammonia produced an
inhibition of sodium influx; a concentration of 18 mg NH4+/litre
reduced the influx to about 20% of its normal value, and influx
reduction was related to increasing ammonia concentration. This
effect was attributed to NH4+ ions and not to any toxic effect
exerted on the transporting cells by non-ionized ammonia. NH4+ did
not affect chloride influx nor the rate of sodium loss.
Ammonia was added to a stream at a 24-h average concentration
of 1.4 mg NH3/litre, and a 24-h drift net sampling was conducted
(Liechti & Huggins, 1980). No change in diel drift pattern was
observed, but there was an increase in the magnitude of drift, a
shift in the kinds of organisms present, and changes in benthic
standing crop estimates; this ammonia concentration was non-lethal.
6.3.3. Salt-water invertebrates: acute and chronic toxicity
Data on the acute toxicity of ammonia for salt-water
invertebrate species are very limited. A 96-h LC50 value of 1.5 mg
NH3/litre has been reported for the copepod, Nitocra spinipes
(Linden et al., 1979). Lethal effects of ammonium chloride on the
quahog clam, Mercenaria mercenaria, and eastern oyster,
Crassostrea virginica, were studied by Epifano & Srna (1975).
There were no observed differences in susceptibility between
juveniles and adults of the 2 species. Armstrong et al. (1978)
conducted acute toxicity tests (6 days) on ammonium chloride using
prawn larvae, Macrobrachium rosenbergii. LC50 values were highly
pH-dependent. The acute toxicity of ammonium chloride for penaeid
shrimp was reported as a 48-h composite LC50 value of 1.6 mg
NH3/litre for 7 species pooled, including the resident species
Penaeus setiferus (Wickins, 1976). The acute toxicity of ammonium
chloride for the caridean prawn, M. rosenbergii, was reported
(Wickins, 1976) as LT50 values of 1700 - 560 min at concentrations
of 1.74 - 3.41 mg NH3/litre. From the data of Hall et al. (1978),
48-h LC50 values of 0.34 - 0.53 were estimated for grass shrimp,
Palaemonetes pugio. Catedral et al. (1977a,b) investigated the
effects of ammonium chloride on the survival and growth of Penaeus
monodon; larvae had a lower tolerance to ammonia compared with
post-larvae. Brown (1974) reported a time to 50% mortality of
106 min for the nemertine worm, Cerebratulus fuscus, at a
concentration of 2.3 mg NH3/litre.
Effects of ammonium chloride solutions on the American lobster,
Homarus americanus, were studied by Delistraty et al. (1977).
Their studies were performed on fourth-stage larvae, which was
considered to be the most sensitive life stage. A 96-h LC50 value
of 2.2 mg NH3/litre and an incipient LC50 value of 1.7 mg NH3/litre
were reported. A "safe" concentration of 0.17 mg NH3/litre was
tentatively recommended.
The sublethal toxicity of ammonium chloride for the quahog clam
and eastern oyster was studied by Epifano & Srna (1975) who
measured the effect of a 20-h exposure to ammonia on the rate of
removal of the alga, Isochrysis galbana, from suspension (clearing
rate) by the clams and oysters. Concentrations of 0.06 - 0.2 mg
NH3/litre affected removal; no differences were observed between
juveniles and adults. The effect of ammonia on the ciliary beating
rate of the mussel, Mytilus edulis, was studied by Anderson et al.
(1978). Concentrations of 0.097 - 0.12 mg NH3/litre resulted in a
reduction in the ciliary beating rate ranging from 50% to complete
inhibition.
Exposure of unfertilized sea urchin, Lytechinus pictus, eggs to
ammonium chloride resulted in stimulation of the initial rate of
protein synthesis, an event that normally follows fertilization
(Winkler & Grainger, 1978). Exposure of unfertilized eggs of
Strongylocentrotus purpurpatus, L. pictus, and Strongylocentrotus
drobachiensis to ammonium chloride (Johnson et al., 1976; Paul et
al., 1976) caused "fertilization acid" to be released more rapidly
and in greater amounts than after insemination. Activation of
unfertilized L. pictus eggs by ammonium chloride exposure was
also evidenced by an increase in intracellular pH (Steinhardt &
Mazia, 1973; Shen & Steinhardt, 1978). Ammonia treatment has also
been reported to activate phosphorylation of thymidine and
synthesis of histones in unfertilized eggs of the sea urchin,
S. purpuratus, (Nishioka, 1976). Premature chromosome
condensation was induced by ammonia treatment of eggs of L. pictus
and S. purpuratus (Epel et al., 1974; Wilt & Mazia, 1974; Krystal
& Poccia, 1979). Treatment of S. purpuratus and S. drobachiensis
fertilized eggs with ammonia resulted in an absence of the normal
uptake of calcium following insemination. However, calcium uptake
was not inhibited when ammonia treatment preceded insemination
(Paul & Johnston, 1978).
The polychetous annelid, Nereis succinea, the channelled whelk,
Busycon canaliculatum, and the brackish-water clam, Rangia
cuneata, were exposed to concentrations of 0.85, 0.37, and 0.27 mg
NH3/litre and the ammonia excretion measured (Mangum et al., 1978).
Excretion was inhibited by non-lethal concentrations of ammonia,
and the authors concluded that ammonia crosses the excretory
epithelium in the ionized form, and that the process is linked to
the activity of the Na++K+ ATPases. When blue crabs, Callinectes
sapidus, were moved from water of 28 parts per thousand salinity
to water of 5 parts per thousand, a doubling of the ammonia
excretion rate occurred; addition of excess ammonium chloride to
the low-salinity water inhibited ammonia excretion and decreased
net acid output (Mangum et al., 1976). The effect of gaseous NH3
on haemoglobin from the blood of the common marine bloodworm,
Glycera dibrachiata, was examined by Sousa et al. (1977) in an
attempt to determine whether there was competition between NH3 and
oxygen in binding to haemoglobin; such an NH3/O2 relationship was
not found.
6.4. Fish
Ammonia is highly toxic for fish, and, because of its
occurrence at high concentrations in some water systems, it can
present a major pollution problem. It enters aquatic environments
from several sources, including sewage effluent, deposition of
human wastes without treatment, industrial discharges, and runoff
from animal culture and agricultural operations. It is also a
metabolic waste product of fish and, therefore, can be a problem in
facilities involved with intensive fish culture.
Elevated ammonium ion (NH4+) concentrations within the bodies
of fish, as with other vertebrates, cause convulsions and death.
The concentration of non-ionized ammonia (NH3) in the environment
of the fish is important, because ammonia is transferred between
the water and fish largely in this form. Thus, while NH3 is the
more toxic chemical species in the water, within the fish, toxicity
is related to the NH4 concentration.
Research by Chipman (1934), Wuhrman et al. (1947), Wuhrman &
Woker (1948), and Tabata (1962) implicated NH3 as the ammonia
species in water that is mainly toxic for fish, and reported that
NH4+ was non-toxic or considerably less toxic. More recent
research by Robinson-Wilson & Seim (1975), Armstrong et al. (1978),
and Thurston et al. (1981c) has demonstrated that the role of water
pH in the toxicity of ammonia for fish is more than the regulation
of the NH3/NH4+ equilibrium. NH3 is considerably more toxic in
water when pH values are lower than 7 - 9, and there is some
evidence that the toxicity of NH3 is also increased above this
range. Temperature and dissolved oxygen and the ionic composition
of the background water all play a role in the toxicity of NH3 for
some fish.
In a comprehensive analysis of the data, it was concluded that
a number of fish species that are phylogenetically similar are also
similar in their sensitivity to the toxicity of ammonia, and that
many of the factors that affect the toxicity of ammonia similarly
affect all the species that have been studied (US EPA, 1985).
It was formerly considered that fish of the family Salmonidae
were among the most sensitive to the effects of many pollutants,
whereas other fish species, which have evolved in warm-water, low
oxygen, or more turbid aquatic environments, may be less sensitive
to many naturally-occuring pollutants, such as ammonia. However,
other fish species, including some that are frequently referred to
as "warm water" fishes, are of comparable sensitivity (US EPA,
1985). In the "resident-species" approach for establishing water
quality criteria for a toxic agent in a given water body, the
tolerance of selected fish and invertebrate species, naturally
resident in the water body, is used. It must be borne in mind
that, if these selected species are less sensitive than other
species in different water bodies, the standards may be
inappropriate for other water bodies.
Because of variation among different background test water
conditions in different laboratories, as well as differences in the
genetic pools of the same species, results of a single test on a
given species may not be as meaningful as composite results from
several tests conducted at different laboratories.
Much of the evidence on ammonia toxicity is empirical, so, for
a more complete understanding of the biological actions of this
chemical, the results of toxicity tests must be integrated with a
knowledge of ammonia metabolism in fish.
6.4.1. Ammonia metabolism in fish
6.4.1.1. Ammonia production and utilization
The major pathway for the production of ammonia in fish, as in
other vertebrates, is through the transamination of various amino
acids (Forster & Goldstein, 1969; Watts & Watts, 1974). The
primary site for ammonia production is probably the liver (Pequin &
Serfaty, 1963), but the necessary enzymes have also been located in
the kidneys, gills, and skeletal muscle tissue (Goldstein &
Forster, 1961; McBean et al., 1966; Walton & Cowey, 1977). Ammonia
is also produced by the deamination of adenylates in fish muscle
(Driedzic & Hochachka, 1976). The quantitative importance of
muscle ammoniogenesis in total ammonia excretion depends on the
level of activity of the animal, and increases with increasing
workload (Suyama et al., 1960; Fraser et al., 1966; Driedzic &
Hochachka, 1976).
Ammonia toxicity can be ameliorated by the formation of less
toxic compounds, namely glutamine and urea. Levi et al. (1974)
recorded high levels of glutamine in the brain of goldfish,
Carrasius auratus, and found that brain-glutamine levels increased
with ambient ammonia concentrations. Webb & Brown (1976) found
high glutamine synthetase (EC 6.3.1.2) activity in the brains of
teleosts and elasmobranchs, and this may be important in protecting
the brain from sudden surges in ammonia concentration. Walton &
Cowey (1977) were able to detect glutaminase activity in the gills
of rainbow trout, but were unable to measure any in vivo
utilization of glutamine by the gills.
Ammonia can be converted, through carbamyl phosphate, to urea
either via purines (uricolysis) or via the ornithine cycle. The
enzymes required for uricolysis have been found in most fish
studied (Forster & Goldstein, 1969; Watts & Watts, 1974), but
Florkin & Duchateau (1943) were unable to detect any activity of
uricolytic enzymes in the cyclostome, Lampetra. The ratio of urea
production via the ornithine cycle to production via uricolysis is
about 100 to 1 in elasmobranchs and dipnoi, whereas in teleosts
most of the urea is formed via uricolysis (Gregory, 1977).
6.4.1.2. Ammonia excretion
The gills are the major site of ammonia excretion in fish, but
smaller quantities of ammonia may also be eliminated by the kidneys
(Edwards & Condorelli, 1928; Grollman, 1929; Fromm, 1963; Maetz,
1972) and skin (Morii et al., 1978). Although the majority of
branchial ammonia excretion represents clearance from the blood,
gill metabolism may contribute between 20% (Payan & Matty, 1975)
and 5 - 8% (Cameron & Heisler, 1983) of the net ammonia excretion.
The excretion of ammonia by fish is variable, depending on the
state of the animal, the environmental conditions, and the species.
Ammonia excretion tripled in sockeye salmon, Oncorhynchus nerka,
following daily feeding (Brett & Zala, 1975) but remained low and
unchanging during starvation (Brett & Zala, 1975; Guerin-Ancey,
1976a). In fresh-water fish, ammonia excretion increases in
response to exercise (Sukumaran & Kutty, 1977; Holeton et al.,
1983), long-term acid exposure (McDonald & Wood, 1981; Ultsch et
al., 1981), hypercapnia (Claiborne & Heisler, 1984), and NH4Cl
infusion (Hillaby & Randall, 1979). In contrast, increased levels
of environmental ammonia (Guerin-Ancey, 1976b) and short-term
exposure to acid or alkaline water (Wright & Wood, 1985) cause a
decrease in ammonia excretion. It is not known if these changes in
excretion reflect change in the rate of ammonia production or in
the ammonia content of the body. The ammonia content of fish is
likely to be the equivalent of the ammonia excreted in about 2 h,
most of the ammonia being in the tissues with a lower pH, such as
muscle. Blood levels are around 0.2 to 0.3 mmol, but muscle at a
lower pH may contain levels of up to 1 mmol; thus, a 1-kg fish may
contain about 0.5 to 0.7 mmol of ammonia and have an excretion rate
of about 0.3 mmol/h. There is increased ammonia production in
muscle during exercise (Driedzic & Hochachka, 1976). Ammonia
excretion by the spiny dogfish, Squalus acanthias, in sea water is
unaffected by temperature change, exercise, hyperoxia, hypercapnia,
or the infusion of either HCl or NaHCO3 or anything that induces
acid-base stress (Heisler, 1984). This is surprising, because many
of these changes affect pH and therefore would be expected to alter
the ammonia content of body compartments and consequently ammonia
excretion.
There is an elevation in blood-ammonia during starvation (Morii
et al., 1978; Hillaby & Randall, 1979), even though ammonia
excretion does not change (Brett & Zala, 1975). Blood-ammonia
concentrations also rise with increases in both temperature
(Fauconneau & Luquet, 1979) and ammonia concentrations in the water
(Fromm & Gillette, 1968; Thurston et al., 1984b). Exposure of fish
to either air (Gordon, 1970) or increased ammonia levels in water
(Fromm, 1970; Guerin-Ancey, 1976b), raises blood-ammonia levels and
reduces ammonia excretion; this is associated with a rise in urea
production in many, but not all fish. Unlike the authors of the
above studies, Buckley et al. (1979) did not find any change in
blood-total ammonia when coho salmon, Oncorhynchus kisutch, were
exposed to elevated ammonia levels in the environment. However, a
significant rise in plasma-sodium, indicating some coupling between
sodium uptake and ammonia excretion, was observed.
The study of ammonia movement is complicated by the fact that,
with present analytical techniques, it is impossible to distinguish
between the transfer of a molecule of NH3 plus a H+ ion from the
transfer of an NH4+ ion. Thus, only indirect evidence can be
obtained regarding the relative gas and ion movements across the
gill epithelium.
Three possible mechanisms of ammonia excretion have received
the most attention: passive NH3 flux, ionic exchange of NH4+ for
Na+, and passive NH4+ flux. There seems to be little doubt that a
significant pathway for branchial ammonia excretion is by the
passive diffusion of NH3 down its partial pressure gradient.
Changes in the NH3 partial pressure gradient are positively
correlated with changes in net ammonia excretion in the channel
catfish, Ictalurus punctatus, (Kormanik & Cameron, 1981), and
rainbow trout (Cameron & Heisler, 1983; Wright & Wood, 1985).
Ammonia entry into the fish has also been shown to be dependent on
the NH3 gradient (Wuhrmann et al., 1947; Wuhrmann & Woker, 1948;
Fromm & Gillette, 1968).
The excretion of NH4+ is strongly coupled with the movement
of other ions. Many studies have attempted to link the
transepithelial exchange of Na+ uptake to NH4+ efflux. Although
there is considerable indirect evidence for the presence of a
coupled ionic exchange mechanism under certain conditions (Maetz &
Garcéa-Romeu, 1964; Maetz, 1973; Payan & Maetz, 1973; Evans, 1977,
1980; Payan, 1978; Girard & Payan, 1980; Wright & Wood, 1985), the
ubiquity and stoichiometry of this exchange remain controversial.
While Na+ influx can be monitored with isotopes, it is difficult to
determine NH4+ efflux. Investigators have attempted to quantify
the relationship between Na+ uptake and NH4+ excretion by
manipulating Na+ levels in the environmental water, by
pharmaceutical inhibition of the Na+ influx mechanism, or by
loading the fish with ammonia.
In goldfish (Maetz, 1973), and in irrigated rainbow trout gills
(Kirschner et al., 1973), Na+ influx was best correlated with the
sum of H+ and NH4+ ion efflux. The possibility of a Na+ uptake
carrier coupled to either NH4+ or H+ appears likely in other fish
as well (Kerstetter et al., 1970; Payan & Maetz, 1973; Evans,
1977). In perfused heads of trout, Na+ uptake was tightly coupled
with NH4+ efflux (Payan et al., 1975; Payan, 1978). Wright & Wood
(1985) demonstrated that, in intact trout, the rate of ion exchange
was influenced by external water pH, increasing from no exchange at
pH 4 to maximal rates at pH 8. The relationship between Na+ and
NH4+ was one-to-one at a pH of less than 8. However, Payan et al.
(1975) and Wright & Wood (1985) found that the majority of the
ammonia was eliminated by gaseous diffusion, and only when NH3 was
subtracted from total ammonia efflux was the NH4+/Na+ exchange
evident. In common carp, Cyprinus carpio (de Vooys, 1968), and
little skate, Raja erinacea (Evans et al., 1979), ammonia
excretion was unaffected by a reduction in environmental Na+
levels. Cameron & Heisler (1983) found that, under resting
conditions, diffusive movement of NH3 could account for ammonia
excretion in trout, but when the ammonia gradient was reversed and
directed inwards, an NH4+/Na+ exchange could counter-balance the
diffusive uptake of NH3 from the water. If this hypothesis is
correct, then it would explain the unchanging blood-ammonia levels
and increased Na+ levels in coho salmon exposed to elevated
concentrations of ammonia in the water (Buckley et al., 1979).
The Na+/NH4+ (H+) exchange is probably located on the
epithelial apical membrane. Either acid conditions or amiloride in
the water inhibits Na+ influx across the gills and both these
conditions result in a reduction in ammonia excretion (Wright &
Wood, 1985).
The ammonium ion can displace potassium in many membrane
processes in, for example, the giant axon of squid, Loligo pealei
(Binstock & Lecar, 1969), and this is the probable reason that
elevated ammonia causes convulsions in so many vertebrates. In
various aquatic animals, it is possible that NH4+ can substitute
for potassium in oubain-sensitive sodium/potassium exchange (Payan
et al., 1975; Towle & Taylor, 1976; Towle et al., 1976; Mallery,
1979; Girard & Payan, 1980). NH4+ ions will substitute for K+ ions
across the epithelial basolateral border (Richards & Fromm, 1970;
Shuttleworth & Freeman, 1974; Karnaky et al., 1976), but the
importance to net ammonia transfer in fresh-water fish is unknown.
The passive movement of NH4+ down its electrochemical gradient
may also contribute to net ammonia excretion (Claiborne et al.,
1982; Goldstein et al., 1982). Lipid membranes are relatively
impermeable to cations (Jacobs, 1940) and, because respiratory
epithelial cells of fresh-water fishes are joined by tight
junctions (Girard & Payan, 1980), it appears unlikely that NH4+
diffusion is of quantitative importance (Kormanik & Cameron, 1981).
Indeed, Wright & Wood (1985) found a negative correlation between
ammonia excretion and the NH4+ concentration gradient in rainbow
trout exposed to 5 different water pH regimes. Although it appears
that NH4+ diffusion may be of minor importance, simultaneous
measurements of the electrical and chemical gradient have not been
made and are necessary before conclusions can be drawn.
6.4.2. Fish: acute toxicity
The acute toxicity of ammonia for rainbow trout has been
studied by many investigators, with reported 96-h LC50 values
ranging from 0.16 to 1.1 mg NH3/litre. Thurston & Russo (1983)
conducted 71 toxicity tests on rainbow trout ranging in size from
sac fry (< 0.1 g) to 4-year-old adults (2.6 kg), in water of
uniform chemical composition. LC50 values ranged from 0.16 to
1.1 mg NH3/litre for 96-h exposures. Fish susceptibility to NH3
decreased with increasing weight over the range 0.06 - 2.0 g, but
gradually increased above that weight range. LC50 values for 12-
and 35-day exposures did not differ greatly from 96-h values. No
statistically-significant differences in results were observed when
different ammonium salts [NH4Cl, NH4HCO3, (NH4)2HPO4, (NH4)2SO4]
were used. Grindley (1946) also reported that there were no
appreciable differences in toxicity between toxic solutions of
NH4Cl and (NH4)2SO4 in rainbow trout tests. However, Calamari et
al. (1977, 1981) reported that embryos and fingerlings were less
sensitive than the other life stages studied. LC50 values (96-h)
ranging from 0.16 to 1.02 mg NH3/litre for rainbow trout exposed to
ammonia were reported by Calamari et al. (1977, 1981), Broderius &
Smith (1979), Holt & Malcolm (1979), DeGraeve et al. (1980), and
Reinbold & Pescitelli (1982b).
Although acute toxicity studies with salmonids have mainly been
conducted on rainbow trout, some data are available for a few other
salmonid species. Thurston et al. (1978) investigated the
toxicity of ammonia for cutthroat trout, Salmo clarki, and
reported 96-h LC50 values of 0.52 - 0.80 mg NH3/litre. Thurston &
Russo (1981) reported a 96-h LC50 value of 0.76 mg NH3/litre for
golden trout, Salmo aguabonita. Brown trout, Salmo trutta, were
exposed to 0.15 mg NH3/litre for 18 h, resulting in a 36%
mortality; when returned to ammonia-free water, the test fish
recovered after 24 h (Taylor, 1973). No mortality occurred during
the 96-h exposure at 0.090 mg NH3/litre, although fish would not
feed. Exposure to 0.8 mg NH3/litre was not acutely toxic for brown
trout according to Woker & Wuhrmann (1950). However, Thurston &
Meyn (1984) reported 96-h LC50 values of 0.60 - 0.70 mg NH3/litre,
and Miller et al. (1981) reported a 96-h LC50 value of 0.47 mg
NH3/litre for brown trout using test dilution river water. Brook
trout, Salvelinus fontinalis, showed distress within 1.75 h at a
concentration of 3.25 mg NH3/litre and within 2.5 h at 5.5 mg/litre
(Phillips, 1950). Thurston & Meyn (1984) reported 96-h LC50 values
of 0.96 - 1.05 mg NH3/litre for brook trout, 0.40 - 0.48 mg
NH3/litre for chinook salmon, Oncorhynchus tshawytscha, and 0.14 -
0.47 mg NH3/litre for mountain whitefish, Prosopium williamsoni.
Toxicity tests with (NH4)2SO4 on pink salmon, Oncorhynchus
gorbuscha, at different early stages of development (Rice & Baily,
1980) showed that late alevins near swim-up stage were the most
sensitive (96-h LC50 = 0.083 mg NH3/litre), and eyed embryos were
the most tolerant, surviving 96 h at > 1.5 mg NH3/litre. Buckley
(1978) reported a 96-h LC50 value of 0.55 mg NH3/litre for
fingerling coho salmon, Oncorhynchus kisutch, and Herbert &
Shurben (1965) reported a 24-h LC50 value of 0.28 mg NH3/litre for
Atlantic salmon, Salmo salar.
There are acute toxicity data for ammonia in a variety of non-
salmonid fish species. Thurston et al. (1983) studied the toxicity
of ammonia for fathead minnows, Pimephales promelas, ranging from
0.1 to 2.3 g in weight and found that 96-h LC50 values in 29 tests
ranged from 0.75 to 3.4 mg NH3/litre; toxicity was not dependent on
the test fish size or source. LC50 values ranging from 0.73 to
2.35 mg NH3/litre for fathead minnows were also reported by Sparks
(1975), DeGraeve et al. (1980), Reinbold & Pescitelli (1982b),
Swigert & Spacie (1983). LC50 values for white sucker, Catostomus
commersoni, exposed to ammonium chloride solutions for 96 h
(Reinbold & Pescitelli 1982c) were 1.40 and 1.35 mg/litre NH3,
though a somewhat lower 96-h LC50 of 0.79 mg NH3/litre was
determined by Swigbert & Spacie (1983). Thurston & Meyn (1984)
reported 96-h LC50 values of 0.67 - 0.82 mg NH3/litre for the
mountain sucker, Caststomus platyrhynchus.
Reported LC50 values for 96-h exposures of bluegill, Lepomis
macrochirus, ranged from 0.26 to 4.60 mg NH3/litre (Emery & Welch,
1969; Lubinski et al., 1974; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982b; Swigert & Spacie, 1983). LC50 values (96-h) of
0.7 - 1.8 mg NH3/litre for smallmouth bass, Micropterus dolomieui,
and 1.0 - 1.7 mg NH3/litre for largemouth bass, Micropterus
salmoides, were reported by Broderius et al. (in press) and
Roseboom & Richey (1977), respectively. Sparks (1975) reported
48-h LC50 values for bluegill of 2.30 mg NH3/litre, and for channel
catfish of 2.92 mg NH3/litre. For goldfish, Carassius auratus,
Dowden & Bennett (1965) reported a 24-h LC50 of 7.2 mg NH3/litre,
and Chipman (1934) reported lethal threshold values of 0.97 -
3.8 mg NH3/litre. Turnbull et al. (1954) reported a 48-h LC50 for
bluegill to be within the range 0.024 - 0.093 mg NH3/litre; during
the exposure, they observed that the fish exhibited a lack of
ability to avoid objects.
Reported 96-h LC50 values for channel catfish, Ictalurus
punctatus, ranged from 1.5 to 4.2 mg NH3/litre (Colt &
Tchobanoglous, 1976; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982d; Swigert & Spacie, 1983). Vaughn & Simco (1977)
reported a 48-h LC50 for channel catfish of 1.24 - 1.96 mg
NH3/litre, and Knepp & Arkin (1973) reported 1-week LC50 values of
0.97 - 2.0 mg NH3/litre. The results of studies on bluegill,
channel catfish, and largemouth bass (Roseboom & Richey, 1977)
showed that bluegill susceptibility was dependent on fish weight,
fish weighing 0.07 g being slightly more sensitive than those
weighing either 0.22 or 0.65 g; size had little effect on the
susceptibility of channel catfish or bass.
Hazel et al. (1979) reported 96-h LC50 values of 0.90 and
1.07 mg NH3/litre for the orangethroat darter, Etheostoma
spectabile, and red shiner, Notropis lutrensis. Largemouth bass,
channel catfish, and bluegill were also exposed for 96 h to a
concentration of 0.21 mg NH3/litre resulting in zero mortality for
bluegill and channel catfish and one death (6%) among the
largemouth bass tested. A 96-h LC50 value for walleye,
Stizostedion vitreum, of 0.85 mg/litre NH3 was reported by
Reinbold & Pescitelli (1982a).
LC50 values ranging from 2.4 to 3.2 mg NH3/litre for
(NH4)2CO3, NH4Cl, NH4C2H3O2, and NH4OH, in 96-h exposures of
mosquitofish, Gambusia affinis, in waters with suspended solids
ranging from < 25 to 1400 mg/litre were reported by Wallen et al.
(1957). Susceptibility of mosquito fish to ammonia was studied by
Hemens (1966) who reported a 17-h LC50 of 1.3 mg NH3/litre; he also
observed that male fish were more susceptible than females. Rubin
& Elmaraghy (1976, 1977) tested guppy, Poecilia reticulata, fry
and reported 96-h LC50 values averaging 1.50 mg NH3/litre; mature
guppy males were more tolerant, with 100% survival for 96 h at
concentrations of 0.17 - 1.58 mg NH3/litre. LC50 values (96-h) of
0.15 and 0.20 mg NH3/litre at pH 6.0, and of 0.52 and 2.13 mg
NH3/litre at pH 8.0, were reported by Stevenson (1977) for white
perch, Morone americana. LC50 values (96-h) of 1.20 and 1.62 mg
NH3/litre for spotfin shiner, Notropis spilopterus, were reported
by Rosage et al. (1979), and of 1.20 mg NH3/litre for golden
shiner, Notemigonus crysoleucas, by Baird et al. (1979). Swigert
& Spacie (1983) reported 96-h LC50 values of 0.72 mg NH3/litre for
golden shiner, 1.35 mg NH3/litre for spotfin shiner, 1.25 mg
NH3/litre for steelcolour shiner, Notropis whipplei, and 1.72 mg
NH3/litre for stoneroller, Campostoma anomalum.
Jude (1973), Reinbold & Pescitelli (1982a), and McCormick et
al. (1984) reported 96-h LC50 values ranging from 0.6 to 2.1 mg
NH3/litre for green sunfish, Lepomis cyanellus. In studies on the
pumpkinseed sunfish, Lepomis gibbosus, by Jude (1973) and Thurston
(1981), 96-h LC50 values ranged from 0.14 to 0.86 mg NH3/litre.
Mottled sculpin, Cottus bairdi, were tested by Thurston & Russo
(1981), yielding a 96-h LC50 value of 1.39 mg NH3/litre. An
asymptotic (6-day) LC50 of 0.44 mg NH3/litre was determined for
rudd, Scardinius erythropthalmus (Ball, 1967).
Rao et al. (1975) reported a 96-h LC50 value for the common
carp, Cyprinus carpio, of 1.1 mg NH3/litre. Carp exposed to
0.24 mg NH3/litre exhibited no adverse effects in 18 h (Vámos
1963). However, exposure to 0.67 mg NH3/litre caused gasping and
equilibrium disturbance within 18 min, frenetic swimming activity
at 25 min, then sinking to the tank bottom after 60 min; after
75 min, the fish were placed in ammonia-free water and all revived.
Kempinska (1968) reported a lethal concentration of 7.5 mg
NH3/litre for carp. Studies on the acute exposure of bitterling,
Rhodeus sericeus, and carp to ammonium sulfate revealed minimum
lethal concentrations of 0.76 mg NH3/litre for bitterling and
1.4 mg NH3/litre for carp (Malacea, 1966). Nehring (1963) reported
survival times for carp at concentrations of 9.7 and 2.1 mg
NH3/litre of 2.4 and 6.0 h, respectively. The survival time for
tench, Tinca tinca, was reported to be 20 - 24 h at 2.5 mg
NH3/litre by Danecker (1964). In a 24-h exposure of creek chub,
Semotilus atromaculatus, to ammonium hydroxide solution, the
"critical range" below which all test fish lived and above which
all died was reported to be 0.26 - 1.2 mg NH3/litre (Gillette et
al., 1952).
In static exposures lasting 9 - 24 h, with a gradual increase
in NH3 content, mortalities occurred in oscar, Astronutus
ocellatus, at 0.50 mg NH3/litre (4%) to 1.8 mg/litre (100%)
(Magalhaes Bastos, 1954). Tests on oscar of two different sizes
showed no difference in susceptibility, in relation to size. A
72-h LC50 value of 2.85 mg NH3/litre was reported by Redner &
Stickney (1979) for blue tilapia, Tilapia aurea.
6.4.2.1. Saltwater fish
Very few acute toxicity data are available for salt-water fish
species. Holland et al. (1960) reported the critical level for
chinook salmon, Oncorhynchus tshawytscha, to be between 0.04 and
0.11 mg NH3/litre and for coho salmon to be 0.134 mg NH3/litre. A
static test with coho salmon provided a 48-h LC50 value of 0.50 mg
NH3/litre (Katz & Pierro, 1967). Atlantic salmon smolts and
yearling rainbow trout exposed for 24 h in 50 and 75% saltwater
solutions exhibited similar sensitivities to ammonia (United
Kingdom Ministry of Technology, 1963). Holt & Arnold (1983)
reported a 96-h LC50 value of 0.47 mg NH3/litre for red drum,
Sciaenops ocellatus. LC50 values (96-h) of 1.2 - 2.4 mg NH3/litre
were reported by Venkataramiak et al. (1981) for striped mullet,
Mugil caphalus, and 0.69 mg NH3/litre for planehead filefish,
Monacanthus hispidus.
6.4.3. Factors affecting acute toxicity
A number of factors can affect the toxicity of ammonia for
aquatic organisms. These include the effects of pH, temperature,
dissolved oxygen concentration, previous acclimatization to
ammonia, fluctuating or intermittent exposures, carbon dioxide
concentration, salinity, and the presence of other toxicants.
Almost all studies of factors affecting ammonia toxicity have been
carried out using only acute exposures.
6.4.3.1. pH
The toxicity for fish of aqueous solutions of ammonia and
ammonium compounds has been attributed to the non-ionized
(undissociated) ammonia present in the solution. The earliest
reported thorough study of the pH dependence of ammonia toxicity
was that of Chipman (1934), who concluded from studies on goldfish,
amphipods, and cladocerans that ammonia toxicity was a function of
pH and therefore of the concentration of undissociated ammonia in
the solution. Downing & Merkens (1955) tested rainbow trout at
different concentrations of ammonia at both pH 7 and pH 8. The
results were consistent when ammonia concentration was expressed as
NH3. Tabata (1962) conducted 24-h tests on the toxicity of ammonia
for Daphnia (species not specified) and guppy at different pH
values and calculated the relative toxicity of NH3/NH4+ to be 48
for Daphnia and 190 for guppy (i.e., NH3 is 190 times more toxic
than NH4+).
More recently, Robinson-Wilson & Seim (1975) studied the
toxicity of ammonium chloride for juvenile coho salmon in flow-
through bioassays within the pH range 7.0 - 8.5; the reported
96-h LC50 for NH3 was approximately 60% less at pH 7.0 than at pH
8.5. The toxicity of ammonium chloride for larvae of prawn,
Macrobrachium rosenbergii, was studied by Armstrong et al. (1978)
in 6-day tests within the pH range 6.8 - 8.3 with test solutions
being renewed every 24 h. The 96-h LC50 for NH3 at pH 6.83 was
approximately 70% less than that at pH 8.34. It was concluded that
the toxicity of ammonia was not due solely to the NH3 molecule and
that in solutions of different pH, but equal NH3 concentrations,
survival was greatly reduced as NH4+ levels increased. Tomasso et
al. (1980) studied the toxicity of ammonia (NH3) for channel
catfish at pH values of 7, 8, and 9 and reported that 24-h LC50
values were significantly higher at pH 8 than at pH 7 or pH 9.
Thurston et al. (1981c) tested the toxicity of ammonia for
rainbow trout and fathead minnows in 96-h flow-through tests at
different pH levels within the range 6.5 - 9.0. Results showed
that the toxicity of ammonia, in terms of NH3, increased at lower
pH values, and could also increase at higher pH values. It was
concluded that NH4+ exerts some measure of toxicity, and/or that
increased H+ concentration increases the toxicity of NH3. Acute
(96-h) exposures of green sunfish and smallmouth bass at 4
different pH levels over the range 6.5 - 8.7 showed that, for both
species, NH3 toxicity increased markedly with a decrease in pH,
with LC50 values at the lowest pH tested (6.6 for sunfish, 6.5 for
bass) being 3.6 (sunfish) and 2.6 (bass) times smaller than those
at the highest pH (8.7) tested (McCormick et al., 1984; Broderius
et al., in press).
It is concluded that NH3 is more toxic for fish at lower pH
values than within the pH range 7 - 9; the toxicity of NH3 may
increase again above this range.
6.4.3.2. Temperature
Information in the literature on the effects of temperature on
ammonia toxicity is varied. The concentration of NH3 increases
with increasing temperature. Several researchers have reported an
effect of temperature on the toxicity of the non-ionized ammonia
species, independent of the effect of temperature on the aqueous
ammonia equilibrium.
McCay & Vars (1931) reported that it took three times as long
for the brown bullhead, Ictalurus nebulosus, to succumb to the
toxicity of ammonia in water at 10 - 13 °C than at 26 °C. The pH
of the tested water was not reported but with the probable range
tested (pH 7 - 8), the percent NH3 at the higher test temperature
would have been approximately three times that at the mean lower
temperature. The toxicity of ammonium chloride for goldfish,
bluntnose minnow, Pimephales notatus, and the straw-coloured
minnow or river shiner, Notropis blennius, was reported (Powers,
1920) to be greater at high temperatures than at low, but no
consideration was given to the increase in the relative
concentration of NH3 as the temperature increased. Herbert (1962)
suggested that the effects of temperature on the susceptibility of
rainbow trout to NH3 toxicity was only slightly, if at all,
affected by temperature change. In studies on striped bass, Morone
saxtilis, and stickleback, Gasterosteus aculeatus, Hazel et al.
(1971) found a slight difference in toxicity between 15° and 23 °C
in fresh water, with both fish species being slightly more
resistant at the lower temperature.
However, there are other studies in which the toxicity of NH3
decreased with increasing temperature over the ranges studied. The
toxicity of NH3 for rainbow trout has been reported to be much
higher at 5 °C than at 18 °C (United Kingdom Ministry of
Technology, 1968). Brown (1968) reported that the 48-h LC50 for
rainbow trout increased with increase in temperature over the range
3 °C - 18 °C; the reported increase in tolerance between ~12 °C -
~18 °C was considerably less than that between ~3 °C - ~12 °C. A
relationship between temperature and 96-h LC50 was reported for
rainbow trout over the temperature range 12 °C - 19 °C with ammonia
toxicity decreasing with increasing temperature (Thurston & Russo,
1983).
Thurston et al. (1983) reported that the acute toxicity of NH3
for fathead minnows decreased with a rise in temperature over the
range 12 °C - 22 °C. Bluegill and fathead minnow were tested at
low and high temperatures of 4.0 °C - 4.6 °C and 23.9 °C - 25.2 °C,
respectively, and rainbow trout were tested at 3 °C and 14 °C
(Reinbold & Pescitelli, 1982b). All three species were more
sensitive to NH3 at the low temperatures, with toxicity being 1.5 -
5 times higher in the colder water. Bluegill appeared to be the
most sensitive of the three species to the effects of low
temperature on ammonia toxicity. Colt & Tchobanoglous (1976)
reported that the toxicity of NH3 for channel catfish decreased
with increasing temperature over the range 22 °C - 30 °C. LC50
values for bluegill, channel catfish, and largemouth bass at 28 °C
- 30 °C were approximately twice those at 22 °C (Roseboom & Richey,
1977). An effluent containing ammonia as the principal toxic
component showed a marked decrease in toxicity for channel catfish
over the temperature range 4.6 °C - 21.3 °C (Cary, 1976).
Lloyd & Orr (1969) investigated the effects of temperature
(range 10 - 20 °C) on urine flow rates in rainbow trout exposed to
0.30 mg NH3/litre, and did not find any apparent temperature effect
on the total diuretic response of the fish, though the relative
increase in urine production was less at higher temperatures. From
a study of the behavioural response of bluegill to gradients of
ammonium chloride, it was hypothesized that low temperatures
increased the sensitivity of the bluegill and interfered with the
ability, either to detect ammonia after a certain period of
exposure, or, to compensate behaviourally for physiological stress
caused by ammonia gradients (Lubinski, 1979; Lubinski et al.,
1980).
The European Inland Fisheries Advisory Commission (1970)
has stated that, at temperatures below 5 °C, the toxic effects of
non-ionized ammonia may be greater than at above 5 °C, though the
basis for this is not clearly documented. The evidence that
temperature, independent of its role in the aqueous ammonia
equilibrium, affects the toxicity of NH3 for fish argues for
further consideration of the temperature/ammonia toxicity
relationship.
6.4.3.3. Salinity
Herbert & Shurben (1965) reported that the resistance of
yearling rainbow trout to ammonium chloride increased with
increasing salinity up to levels of 30 - 40% sea water; above this
level, resistance appeared to decrease. Fingerling coho salmon
were tested at salinity levels of 20 - 30 parts per thousand (57 -
86% salt water), and it was found that the toxicity of an ammonia-
ammonium waste increased as salinity increased (Katz & Pierro,
1967). These findings are in agreement, at the levels tested, with
those of Herbert & Shurben (1965). Atlantic salmon were exposed to
ammonium chloride solutions for 24 h under both fresh-water and 30%
salt-water conditions; LC50 values were 0.15 and 0.3 mg NH3/litre,
respectively, in the 2 different waters (Alabaster et al., 1979).
Harader & Allen (1983) reported that the resistance to ammonia of
chinook salmon parr increased by about 500%, as salinity increased
to almost 30% sea water, but declined as salinity increased beyond
that.
There is a slight decrease in the NH3 fraction of total ammonia
as ionic strength increases in dilute saline solutions, but the
relative changes in NH3 toxicity, as salinity increases, are more
directly attributable to changes in the rate of exchange of NH3 and
NH4+ across the fish gill membranes.
6.4.3.4. Dissolved oxygen
A decrease in dissolved oxygen concentration in the water can
increase ammonia toxicity. There is a reduction in fish blood
oxygen-carrying capacity following ammonia exposure (Brockway,
1950; Danecker, 1964; Reichenback-Klinke, 1967; Körting, 1969a,b;
Waluga & Flis, 1971). Hypoxia would further exacerbate problems of
oxygen delivery and could lead to the early demise of the fish.
Vámos & Tasnádi (1967) observed deaths of carp in ponds at
ammonia concentrations lower than would normally be lethal, and
attributed this to periodic low concentrations of oxygen. On the
basis of research in warm-water (20 °C - 22 °C) fish ponds, Selesi
& Vámos (1976) projected a "lethal line", relating acute ammonia
toxicity and dissolved oxygen, below which carp died. The line ran
between 0.2 mg NH3/litre at 5 mg dissolved oxygen/litre and 1.2 mg
NH3/litre at 10 mg dissolved oxygen/litre. Thurston et al. (1983)
compared the acute toxicity of ammonia for fathead minnows at
reduced and normal dissolved oxygen concentrations; seven 96-h
tests were conducted within the range 2.6 - 4.9 mg dissolved
oxygen/ litre, and 3 between 8.7 and 8.9 mg/litre. There was a
slight positive trend between 96-h LC50 values and dissolved
oxygen, though it was not shown to be statistically significant.
Atlantic salmon smolts were tested in both fresh water and 30% salt
water at 9.6 - 9.5 and 3.5 - 3.1 mg dissolved oxygen/litre. The
reported 24-h LC50 values at the higher oxygen concentrations were
about twice those at the lower (Alabaster et al., 1979).
Several studies have been reported on rainbow trout. Allan
(1955) reported that below 0.12 mg NH3/litre and at about 30%
oxygen saturation, the median survival time was greater than 24 h,
but at the same concentration with oxygen saturation below 30%, the
median survival time was less than 24 h. In studies by Downing &
Merkens (1955), fingerling rainbow trout were tested at 3 different
concentrations of NH3 at 5 different levels of dissolved oxygen.
In tests lasting up to 17 h, decreasing the oxygen level from 8.5
to 1.5 mg/litre shortened the period of survival at all ammonia
concentrations, and a decrease in survival time produced by a given
decrease in oxygen was greatest at the lowest concentration of NH3.
Merkens & Downing (1957), in tests lasting up to 13 days, also
reported that the effects of low concentrations of dissolved oxygen
on the survival of rainbow trout were more pronounced at low
concentrations of NH3. Ammonia (NH3) was found to be up to 2.5
times more toxic when the dissolved oxygen concentration was
reduced from 100 to about 40% saturation (Lloyd, 1961). It was
reported by Danecker (1964) that the toxicity of ammonia increased
rapidly when the oxygen concentration decreased below two-thirds of
the saturation value. Thurston et al. (1981b) conducted 15, 96-h
acute toxicity tests on rainbow trout over the dissolved oxygen
range 2.6 - 8.6 mg/litre. A positive linear correlation between
96-h LC50 and dissolved oxygen was reported over the entire range
tested.
When rainbow trout were treated in a channel receiving sewage
discharge containing 0.05 - 0.06 mg NH3/litre, it was found that,
at 25 - 35% dissolved oxygen saturation, more than 50% of the fish
died within 24 h, compared with 50% mortality of test fish in the
laboratory, at 15% dissolved oxygen saturation (Herbert, 1956).
The difference was attributed to unfavourable water conditions
below the sewage outflow, including ammonia, which increased the
sensitivity of the fish to the lack of oxygen.
6.4.3.5. Carbon dioxide
An increase in carbon dioxide (CO2) concentrations up to
30 mg/litre decreased total ammonia toxicity (Alabaster & Herbert,
1954; Allan et al., 1958). Carbon dioxide causes a decrease in pH,
thereby decreasing the proportion of non-ionized ammonia in
solution. However, Lloyd & Herbert (1960) found that, though total
ammonia toxicity was reduced at elevated CO2 levels, the inverse
was true when considering non-ionized ammonia alone; more NH3 was
required in low CO2, high pH water to exert the toxic effect seen
in fish in high CO2, low pH water. The explanation presented by
Lloyd & Herbert (1960) for the decreased toxicity of NH3 in low CO2
water was that CO2 excretion across the gills would reduce the pH
and, therefore, the NH3 concentration, in water flowing over the
gills. A basic flaw in this hypothesis has been discussed by
Broderius et al. (1977). Carbon dioxide will only form protons
very slowly in water at the tested temperature. The uncatalysed
CO2 hydration reaction has a half-time of seconds or even min
(e.g., at pH 8: 25 seconds at 25 °C; 300 seconds at 0 °C) (Kern
1960), and water does not remain in the opercular cavity for more
than a few seconds, and at the surface of a gill lamella for about
0.5 - 1 second (Randall, 1970; Cameron, 1979). Thus, the
liberation of CO2 across the gills will have little, if any, effect
on water pH or NH3 levels and the NH3 gradient across the gills
between water and blood.
6.4.3.6. Prior acclimatization to ammonia
The question of whether fishes can acquire an increased
tolerance to ammonia by acclimatization to low ammonia
concentrations is an important one. If fish were able to develop
such tolerance, they might be able to survive what would otherwise
be lethal ammonia concentrations.
Observations by McCay & Vars (1931) indicated that brown
bullheads subjected to several successive exposures to ammonia,
alternating with recovery in fresh water, did not acquire
tolerance. However, a number of research workers have reported
that previous exposure of fish to low concentrations of ammonia
increases their resistance to lethal concentrations. Vámos (1963)
reported that carp exposed to 0.67 or 0.52 mg NH3/litre for 75 min,
then transferred to fresh water for 12 h, followed by a solution
containing 0.7 mg NH3/litre, exhibited symptoms of ammonia toxicity
in 60 - 85 min, whereas control fish, exposed initially to
0.7 mg/litre NH3, developed symptoms within 20 min. Blue tilapia
acclimatized for 35 days to 0.52 - 0.64 mg NH3/litre subsequently
survived 48 h at 4.1 mg/litre, compared with the 48-h value for
unacclimatized fish of 2.9 mg/litre (Redner & Stickney, 1979).
Malacea (1968) studied the effects on bitterling of acclimatization
to ammonium sulfate solutions. A group of 10 fish was held in an
acclimatization solution of 0.26 mg NH3/litre for 94 h, after which
the fish were exposed to a 5.1 mg NH3/litre solution for 240 min.
A control group of 10 bitterling received identical treatment,
except that the acclimatization aquarium did not contain added
(NH4)2SO4. The ratio of the mean survival times of "adapted" to
"unadapted" fish was 1:13, indicating a slightly higher ammonia
tolerance for the adapted fish.
Schulze-Wiehenbrauck (1976) subjected 2 groups of rainbow trout
(mean weights 56 g and 110 g) that had been held for at least 3
weeks at sublethal ammonia concentrations, to lethal ammonia
concentrations. In the study on the 110-g fish, the
acclimatization concentrations were 0.007, 0.131, and 0.167 mg
NH3/litre. The fish were then subjected for 8.5 h to
concentrations of 0.45, 0.42 and 0.47 mg NH3/litre, respectively.
Fish from the 2 higher sublethal concentrations showed 100%
survival after 8.5 h in the 0.42 and 0.47 mg NH3/litre solutions,
whereas fish from the 0.007 mg NH3/litre concentration showed only
50% survival in 0.45 mg NH3/litre. In the study on the 56-g fish,
the acclimatization concentrations were 0.004 mg NH3/litre and
0.159 mg NH3/litre; these fish were placed for 10.25 h in NH3
concentrations of 0.515 and 0.523 mg/litre, respectively. There
was 100% survival in the acclimatized fish, and 85% survival in the
fish acclimatized to 0.004 mg/litre. The results of these studies
showed an increase in resistance of trout to high ammonia levels
after prior exposure to sublethal ammonia levels.
Alabaster et al. (1979) determined 24-h LC50 values of NH3 for
Atlantic salmon smolts under reduced dissolved oxygen test
conditions. Fish acclimatized to ammonia before oxygen reduction
had LC50 values 38% and 79% higher than fish without prior ammonia
acclimatization.
In studies by Brown et al. (1969), rainbow trout were tested by
moving back and forth between tanks in which the ammonia
concentrations were 0.5 and 2.5 times a previously determined 48-h
LC50 value. If fish were transferred on an hourly basis, the
median period of survival for the fluctuating exposure was reported
to be the same as that for constant exposure (> 700 min). When
the fish were transferred at 2-h intervals, the median survival
time for the fluctuating exposure was reported to be less (370
min), indicating that the toxic effects from exposure to the
fluctuating concentrations of ammonia were greater than those from
exposure to the constant concentration. Thurston et al. (1981a)
conducted acute toxicity tests in which rainbow trout and cutthroat
trout were exposed to short-term cyclical fluctuations of ammonia.
Companion tests were conducted in which test fish were subjected to
ammonia at constant concentrations. The LC50 values for both
average and peak concentrations of ammonia for the fluctuating
concentration tests were compared with the LC50 values for the
constant concentration tests. Comparison of total exposures showed
that fish were more tolerant to constant, than to fluctuating
concentrations of ammonia. Fish subjected to fluctuating
concentrations of ammonia at levels below those acutely toxic were
better able to withstand subsequent exposure to high fluctuating
concentrations than unacclimatized fish. There is reasonable
evidence that fish with a history of prior exposure to sublethal
concentrations of ammonia are better able to withstand an acutely
lethal concentration for a period of hours and possibly days.
Limited data on fluctuating exposures indicate that fish are more
susceptible to fluctuating than to constant exposure with the same
average NH3 concentrations.
6.4.4. Fish: chronic toxicity
"Full-chronic" tests cover the entire life cycle of the test
animal, beginning at a given stage of development of one generation
(frequently as the fertilized egg) and continuing through to this
same stage in the next generation. Common end-points for measuring
toxicity are survival, growth, and reproductive success, though
recent research on ammonia toxicity for fish has demonstrated the
desirability of also conducting histological examinations.
"Partial-chronic" tests on fish most frequently cover a period
of 30 days or longer, from the egg incubation stage to the free-
swimming stage; for many toxins it has been demonstrated that
these stages are the most sensitive. However, in the case of
ammonia it has been demonstrated that older, mature rainbow trout,
Salmo gairdneri, are potentially as susceptible to the effects of
ammonia as newly-hatched larvae.
Long-term ammonia exposure of fishes, including complete life-
cycle tests on rainbow trout and fathead minnows with several end-
points, including effects on spawning and egg incubation, growth,
survival and tissues, have been studied. The effects of prolonged
exposure to ammonia (up to 61 days) on the early life stages of of
pink salmon were studied by Rice & Bailey (1980). Three series of
exposures were carried out, beginning at selected times after
hatching. These were for 21 days prior to completion of yolk
absorption, for 40 days up to 21 days before yolk absorption, and
for 61 days up to yolk absorption. All test fish were sampled for
size when the controls had completed yolk absorption. Test
concentrations ranged from zero up to 0.004 mg NH3/litre. For fry
at the highest concentration of 0.004 mg NH3/litre, significant
decreases in weight were observed in all 3 exposed groups. At a
concentration of 0.0024 mg NH3/litre, the groups of fry exposed for
40 and 61 days were significantly smaller, whereas a concentration
of 0.0012 mg/litre had no significant effect on growth. Effects
were consistently more marked for the 61-day-exposed fish.
In a 3-generation, 5-year laboratory study, rainbow trout
exposed for 5 months to concentrations of ammonia ranging from 0.01
to 0.07 mg NH3/litre, spawned of their own volition. There was no
correlation between ammonia concentration and numbers of egg lots
spawned, total numbers of eggs produced, or numbers of eggs
subsequently hatched. Parental fish were exposed for 11 months,
the first filial generation (F1) for 4 years, and the second filial
generation (F2) for 5 months. Pathological lesions were observed in
both parental and F1 fish, when ammonia concentrations reached and
exceeded 0.04 mg NH3/litre. Measurements of blood-ammonia
concentrations in 4-year-old F1 fish showed an increase when test
water concentrations reached or exceeded 0.04 mg NH3/litre. The F1
fish exposed for 52 months from day of hatching showed no
relationship between growth and concentration at 10, 15, 21, and 52
months (Thurston et al., 1984b).
Burkhalter & Kaya (1977) tested ammonia at concentrations
ranging from 0.06 to 0.45 mg/litre on fertilized eggs and the
resultant sac fry of rainbow trout. Eggs were incubated at 12 °C
for 25 days in one test and at 10 °C for 33 days in another; fry
were maintained for 42 days. No concentration response was seen in
egg mortality or incubation time in either test. Retardation in
early growth and development occurred at 0.06 mg NH3/litre, the
lowest concentration tested. Fish exposed to 0.12 mg NH3/litre
required 1 week longer than the controls to achieve a free-swimming
state; fish at 0.34 and 0.45 mg NH3/litre did not achieve a free-
swimming state during a 42-day test period. A 21-day LC50 value of
0.30 mg NH3/litre was obtained. For sac fry exposed for 42 days
after hatching, hypertrophy of secondary gill lamellae epithelium
occurred at 0.23 mg NH3/litre, and karyolysis and karyorrhexis in
the secondary gill lamellae were observed after 28 days at 0.34 mg
NH3/litre and higher.
Calamari et al. (1977, 1981) exposed rainbow trout to
ammonium chloride solutions for 71 days, beginning 1 day after
fertilization and ending when fry had been feeding for 30 days.
A 72-day LC50 of 0.056 mg NH3/litre was calculated; 23% mortality
occurred at a concentration of 0.025 mg/litre. Examination of 986
rainbow trout embryos at the hatching stage after exposure to
concentrations of 0.010 - 0.193 mg NH3/litre for 24 days showed
an increase in gross malformations with increasing ammonia
concentration. The deformities observed were various degrees
of curvature from the median body axis, and various kinds of
malformations in the head region with a number of cases of double
heads. At the highest concentration tested, 0.193 mg NH3/litre,
60% of the observed fish were malformed. Microscopic examination,
at hatching, of 128 larvae from the same exposure showed
abnormalities of the epidermis and pronephros, which were
correlated with ammonia concentrations. The epidermis was
thickened with an irregular arrangement of the various layers of
cells and an increase in the number and dimensions of mucous cells.
The pronephros showed widespread vacuolization of the tubule cells,
together with a thickening of the wall. Increasing abnormalities
were observed after exposure to concentrations exceeding 0.025 mg
NH3/litre for the epidermis and 0.063 mg/litre for the pronephros.
Four 4-week-old rainbow trout fry were exposed for 30 days to
concentrations of ammonia (reported graphically) ranging from
~0.06 to 0.31 mg NH3/litre. Growth rate at ~0.06 mg NH3/litre was
comparable with that of controls, but, above ~0.10 mg NH3/litre,
growth rate decreased, in correlation with increased NH3
concentration. Survival at 0.32 mg NH3/litre was 70% of that of
the controls (Broderius & Smith, 1979). Schulze-Wiehenbrauck
(1976) tested juvenile rainbow trout of different sizes, for
periods of time ranging from 2 to 7 weeks, and at ammonia
concentrations ranging from 0.012 to 0.17 mg/litre. He concluded
that a concentration of 0.05 mg NH3/litre caused a slight decrease
in growth during the first 14-day interval in non-acclimatized
fish, but that the decrease was completely compensated for in the
next growth interval. Exposure to 0.13 mg NH3/litre (apparently
for 3 or 4 weeks) did not affect growth, food consumption, or food
conversion.
Young rainbow trout were reared in 3 concentrations of ammonia
(averaging 0.006, 0.012, and 0.017 mg/litre) for a period of 1
year. At 4 months, there was no significant difference in fish
growth at the 3 concentrations. At 11 months, there was a
difference with the fish at 0.012 and 0.017 mg NH3/litre, which
weighed 9% and 38% less, respectively, than the fish at 0.006 mg
NH3/litre. Microscopic examination of tissues from fish exposed to
the highest concentration, examined at 6, 9, and 12 months, showed
severe pathological changes in gill and liver tissues. Gills
showed extensive proliferation of the epithelium, which resulted in
severe fusion of gill lamellae preventing normal respiration.
Livers showed reduced glycogen storage and scattered areas of dead
cells; these became more extensive with increase in exposure time
(Smith, 1972; Smith & Piper, 1975).
Rainbow trout were exposed for 3 months to concentrations of
0.069, 0.14, and 0.28 mg NH3/litre. The cumulative mortality of a
control group (0.005 mg NH3/litre) was ~2%; cumulative mortality at
0.069 and 0.14 mg/litre was ~5%, and that at 0.28 mg/litre was ~15%
(United Kingdom Ministry of Technology, 1968). Reichenbach-Klinke
(1967) performed a series of 1-week tests on 240 fish of 9 species
(including rainbow trout, goldfish, northern pike, Esox lucius,
carp, and tench) at concentrations of 0.1 - 0.4 mg NH3/litre.
Swelling of, and diminution of the number of, red blood cells,
inflammation, and hyperplasia were observed. Irreversible blood
damage occurred in rainbow trout fry at concentrations above
0.27 mg NH3/litre. Low NH3 concentrations also inhibited the
growth of young trout and lessened their resistance to disease.
In rainbow trout exposed to 0.30 to 0.36 mg NH3/litre, 81%
mortality occurred over the 36-day duration of the test, with most
deaths occurring between days 14 and 21. Microscopic examination
of the gills revealed some thickening of the lamellar epithelium
and an increased mucous production. The most characteristic
feature was a large proportion of swollen, rounded secondary
lamellae in which the pillar system was broken down and the
epithelium enclosed a disorganized mass of pillar cells and
erythrocytes. Gill hyperplasia was not a characteristic
observation (Smart, 1976).
In rainbow trout exposed to < 0.0005 or 0.005 mg NH3/litre for
8 weeks, examination of the gill lamellae of fish from the lower
concentration showed them to be long and slender with no
significant pathology. Fish exposed to 0.005 mg NH3/litre had
shorter and thicker gill lamellae with bulbous ends, and some
consolidation of lamellae was noticed. Many filaments showed a
definite hyperplasia of the epithelial layer, evidenced by an
increase in the number of cell nuclei (Fromm, 1970).
Thurston et al. (1978) studied the toxicity of ammonia for
cutthroat trout fry in tests that lasted up to 36 days. Results of
duplicate tests on 1-g fish showed 29- and 36-day LC50 values of
0.56 mg NH3/litre. Duplicate tests on 3-g fish provided 29-day
LC50 values of 0.37 and 0.34 mg NH3/litre, slightly less than those
of the 1-g fish. The heart, gastrointestinal tract, and thymus of
cutthroat trout fry exposed to 0.34 mg NH3/litre for 29 days were
comparable with those of control fish, but the gills and kidneys
showed degenerative changes. The gills showed hypertrophy of
epithelium, some necrosis of epithelial cells, and separation of
epithelium due to oedema. The kidneys had mild hydropic
degeneration and accumulation of hyaline droplets in the renal
tubular epithelium. Reduced vacuolation was observed in livers.
Samylin (1969) studied the effects of ammonium carbonate on the
early stages of development of Atlantic salmon. The first set of
studies, at 13 °C, lasted 53 days and was conducted within the
range 0.001 to > 6.6 mg NH3/litre beginning with the "formed
embryo" stage. Accelerated hatching was observed with increasing
(NH4)2CO3 concentrations, but concentrations of > 0.16 mg
NH3/litre were lethal for emerging larvae within 12 - 36 h.
Because (NH4)2CO3 was used as the toxin, the pH in the test aquaria
increased from 6.7 to 7.6 with increasing NH3 concentration. Growth
inhibition was observed at 0.07 mg NH3/litre. Tissue changes were
observed in eyes, brains, fins, and blood of Atlantic salmon
embryos and larvae exposed to concentrations ranging from 0.16
to > 6.6 mg NH3/litre, with more marked changes at higher ammonia
concentrations. The effects observed included erosion of membranes
of the eyes and shedding of the crystalline lens, dilatation of
blood vessels in the liver and brain, accumulation of blood in the
occipital region and in the intestines. Reaction to light and
mechanical stimulation gradually disappeared with increased ammonia
concentration, and the heart rate slowed. Morphological
differences in development between experimental and control larvae
were observed from the tenth day of exposure, including a lag in
yolk resorption, decrease in growth of the skin fold, and
contraction of skin pigment cells causing the skin colour to become
paler than it was after hatching. At concentrations up to 0.07 mg
NH3/litre, no significant morphological differences were observed.
A second series of studies, at 16.5 °C, was carried out in
the 0.001 - 0.32 mg NH3/litre concentration range, beginning
with larval salmon (Samylin 1969). Concentrations of > 0.21
mg/litre were lethal and caused weight loss in fry; 0.001 -
0.09 mg NH3/litre caused a decrease in weight gain, though there
were no differences in feeding activity, behaviour, or development
at these concentrations compared with controls. Dissolved oxygen
concentrations in this second series of studies dropped as low as
3.5 mg/litre.
Burrows (1964) tested fingerling chinook salmon for 6 weeks in
outdoor water channels into which ammonium hydroxide was
introduced. Two studies were conducted, one at 6.1 °C and the
other at 13.9 °C, both at pH 7.8. The fish were then maintained in
fresh water for an additional 3 weeks. A recalculation of the
reported non-ionized ammonia concentrations, based on more recent
aqueous ammonia equilibrium tables, shows that the concentrations
at 6.1 °C were 0.003 - 0.006 mg NH3/litre and, at 13.9 °C, were
0.005 - 0.011 mg NH3/litre. At both temperatures, and at all
ammonia concentrations, some fish showed excessive proliferation
and clubbing of the gill filaments. The proliferation was
progressive for the first 4 weeks, after which no measurable
increase was observed. After 3 weeks in fresh water, examination
of fish exposed at 6.1 °C indicated that recovery from the
extensive proliferation had not taken place. In the study on larger
fish at 13.9 °C, a marked recovery from hyperplasia was noted after
the 3 weeks in fresh water. In the first study, the proliferated
areas had consolidated; in the second, they had not. It was
postulated that continuous ammonia exposure is a precursor of
bacterial gill disease.
Duplicate groups (90 fish each) of hatchery-reared coho salmon
were exposed for 91 days to "river-water" solutions of ammonium
chloride at concentrations of 0.019 - 0.33 mg NH3/litre. Control
groups were reared at 0.002 mg/litre. Haemoglobin content and
haematocrit readings were slightly, but significantly, reduced in
fish exposed to the highest concentration tested, and there was
also a greater percentage of immature erythrocytes. Blood-ammonia
and -urea concentrations were not significantly different after 91
days, regardless of the concentration of ammonia to which the fish
were exposed (Buckley et al., 1979). Rankin (1979) exposed embryos
of sockeye salmon, Oncorhynchus nerka, to ammonia from fertilization
to hatching. Total embryo lethality occurred at concentrations of
0.49 - 4.9 mg NH3/litre. The times required to achieve 50%
mortality at these concentrations were 40 - 26 days. Mortality of
the embryos exposed to 0.12 mg NH3/litre was 30%, and time to 50%
mortality was 66 days.
Two full life-cycle ammonia toxicity tests, each lasting
approximately 1 year, were conducted on fathead minnows (Thurston
et al., in press). These tests began with newly hatched fry and
were continued through their growth, maturation and spawning
stages; progeny were exposed from hatching through growth to 60
days of age. While no statistically-significant differences were
observed in survival, growth, egg production, and egg viability, at
concentrations up to 0.4 mg NH3/litre, effects were seen at 0.4 mg
NH3/litre.
Tissues from fathead minnows subjected to prolonged (up to 304
days) ammonia exposure were examined (Smith, 1984). Growths, some
massive, were observed on the heads of several fish exposed to
concentrations of 1.25 or 2.17 mg NH3/litre, and swollen darkened
areas were observed on the heads of several fish exposed to 0.639 -
1.07 mg NH3/litre. Thurston et al. (in press) also reported
lesions at concentrations below those at which other effects were
observed. Brain lesions were common at concentrations of 0.21 mg
NH3/litre and higher. Grossly and histologically, the severity of
the lesions, which varied from mild to severe, was positively
correlated with ammonia concentration. The lesions appeared to be
of a cell type originating from the meninx primativa covering the
brain. The hyperplastic tissue often completely surrounded the
brain but was not observed around the spinal cord.
An early life-stage test initiated at the blastula stage of
embryogenesis and extending through 39 days post-hatching was
conducted on green sunfish (McCormick et al., 1984). Retardation
of growth was found in green sunfish exposed from embryo through
juvenile life stages to concentrations of 0.489 mg NH3/litre or
more, but not at 0.219 mg NH3/litre. In a long-term test on green
sunfish, Jude (1973) reported that, at levels higher than 0.17 mg
NH3/litre, mean fish weight increased less rapidly than that of the
controls on the 4 days following the introduction of ammonia.
Thereafter, fish exposed to 0.26 and 0.35 mg NH3/litre grew at an
increasing rate, while fish exposed to 0.68 and 0.64 mg NH3/litre
remained the same for 12 days before increases in growth occurred.
Four simultaneous early life-stage ammonia tests with
smallmouth bass were carried out at 4 different pH levels, ranging
from 6.6 to 8.7, in order to examine the effect of pH on chronic
ammonia toxicity. Exposure to ammonium chloride solutions began on
2- to 3-day old embryos and lasted for 32 days. The end-point
observed was growth, and ammonia was found to have a greater effect
on growth at lower pH levels than at high. Concentrations found to
retard growth ranged from 0.056 mg/litre at pH 6.60, to 0.865
mg/litre at pH 8.68 (Broderius et al., in press).
In early life-stage tests (29 - 31 days' exposure) on channel
catfish and white sucker, no significant effects on percent hatch
or larval survival were observed for channel catfish exposed to
ammonium chloride at concentrations as high as 0.583 mg NH3/litre
and for white sucker at concentrations as high as 0.239 mg
NH3/litre. Significant retardation of growth, however, occurred in
channel catfish at concentrations of 0.392 mg NH3/litre or more and
in white sucker at 0.070 mg NH3/litre and higher. A delay in time
to swim-up stage was also observed for both species at elevated
(0.06 - 0.07 mg/litre) ammonia concentrations (Reinbold &
Pescitelli, 1982a).
In cultured channel catfish fingerlings, exposed for periods
of approximately 1 month to concentrations of 0.01 - 0.16 mg
NH3/litre, growth at 0.01 and 0.07 mg NH3/litre was not
significantly different from that of control fish, but growth
retardation at 0.15 and 0.16 mg NH3/litre was statistically
significant (Robinette, 1976). Colt (1978) and Colt &
Tchobanoglous (1978) reported retardation of growth of juvenile
channel catfish during a 31-day period of exposure to
concentrations ranging from 0.058 to 1.2 mg NH3/litre. Growth
rate was reduced by 50% at 0.63 mg NH3/litre, and no growth
occurred at 1.2 mg NH3/litre.
6.5. Wild and Domesticated Animals
6.5.1. Wildlife
Although ammonia has been known to be toxic for nearly a
century (Hahn et al., 1983), studies describing the toxicological
effects of ammonia on wildlife are very limited. Normally,
atmospheric ammonia does not appear to be a problem for wild
animals, but concentrations of ammonia could reach harmful levels
in accidents during transport near forests and remote areas. NRC
(1979) has reported 2 types of observations in relation to this
topic: (a) the use of anhydrous ammonia to exterminate wild birds
and mice in farm buildings; and (b) the tolerance of bats to
atmospheric ammonia.
The use of anhydrous ammonia has been recommended for
exterminating wild birds and mice from farm buildings by Day et al.
(1965). The technique is simple, economical, and does not leave
any harmful residue. The farm buildings, after removal of the
livestock, were sealed and treated with anhydrous ammonia at 1600
mg/m3 (2285 ppm) for 7 min and then reopened. Ammonia fumes were
fatal for the wild inhabitants, particularly for wild birds.
Within 0.5 h, dead starlings, sparrows, pigeons, and mice were
removed from the barns. Farm animals were allowed to enter the
barns within 1 h of their reopening. According to laboratory
studies, the mouse appears to be more sensitive than other animal
species such as the rat, rabbit, and guinea-pig. When mice were
exposed for 10 min to ammonia at 6140 - 9060 mg/m3 (8770 - 12 940
ppm), death with convulsion began after 5 min of exposure, and over
50% of the mice died before the study was completed. The surviving
animals appeared to recover rapidly, but another 4% died between
the 6th and 10th days after exposure (Underwriters Laboratories,
1933).
Large colonies of Guano bats (Tadarida brasiliensis) frequently
inhabit caves or other areas, producing large amounts of guano,
which, on bacterial decomposition, results in a very high
concentration of ammonia in the atmosphere. Although high ammonia
concentrations, together with high relative humidity in caves,
discoloured the pelage of bats (Eads et al., 1955; Constantine,
1958; Mitchell, 1964), no other adverse physiological effects were
observed in these mammals. This apparent adaptation to inhaled
ammonia prompted laboratory studies relating to the physiological
mechanisms involved in ammonia tolerance in different species of
bats (Mitchell, 1963; Studier, 1966; Studier et al., 1967).
California leaf-nosed bats (Macrotus californicus) can
tolerate exposure to 2100 mg/m3 (3000 ppm) for up to 9 h (Mitchell,
1963).
Ammonia toxicity at lethal doses was manifested by corrosion
of the skin and mucous membranes, pulmonary oedema, and distinct
visceral damage. The blood-non-protein nitrogen in the exposed
bats was significantly elevated without any increase in urinary-
urea or -ammonia.
Studier et al. (1967) studied the effects of increasing
concentrations of atmospheric ammonia on ammonia tolerance and
metabolic rates in rats, mice, and 3 species of bat. Rats, mice,
and 2 species of bat (Myotis lucifugus and Eptesicus fuscus)
tended to show increased oxygen utilization, when exposed to
increased ammonia levels. However, the guano bat (Tadarida
brasiliensis) exhibited a large decrease in oxygen utilization
with increasing ammonia concentration (i.e., 74% depression) when
exposed to air containing 4900 mg/m3 (7000 ppm) of gaseous ammonia.
Earlier, Studier (1966) had shown that ~35% of gaseous ammonia
filtered through the mucous linings in the respiratory passage in
guano bats, when they were exposed to 2100 mg/m3 (3000 ppm) of
ammonia. There was no change in their normal blood pH during
exposures to high ammonia concentrations. The animals, however,
exhaled measurable amounts of ammonia when transferred to normal
air.
6.5.2. Domesticated animals
6.5.2.1. Oral exposure
(a) Ruminants
The use of urea as a partial source of nitrogen in ruminant
nutrition is limited by its toxicity, which results from its
metabolism to ammonia.
The toxic effects of urea in ruminants are related to a high
ammonia content in the blood. Urea itself is not as toxic as the
ammonia, which is rapidly released in the rumen by the action of
bacterial urease (EC 3.5.1.5) on ingested urea (Bloomfield et al.,
1960). The absorption of this excess ammonia has been shown to
depend on the pH of the ruminal contents.
Hogan (1961) examined the effects of pH on the absorption of
ammonia from the rumen in sheep. When an ammonia-containing buffer
at pH 6.5 was placed in the rumen, absorption increased with the
concentration gradient. At a pH of 4.5, however, the concentration
of ammonia in the rumen did not affect the absorption across the
epithelium. The net loss of ammonia-nitrogen from the rumen at pH
6.5 was more than 3 times the loss at a pH of 4.5.
Additional support for the effect of pH on ammonia absorption
across the ruminal epithelium in sheep has been presented by
Bloomfield et al., (1962). As the pH of the ruminal contents
increased from 6.21 to 6.45, no ammonia was absorbed; however, as
the pH increased to 7.59, the absorption rate was 16 mmol/litre per
h. One sheep with a ruminal pH of 7.7 died of ammonia toxicity
within 30 min. These data support the hypothesis that the non-
ionized ammonia, which increases at higher pH, penetrates the lipid
layers of the ruminal epithelium more effectively than the charged
ammonium ion (Coombe et al., 1960).
Toxic signs become apparent as the blood-ammonia-nitrogen
increases to 10 mg/litre; tetanic spasms occur between 10 and
20 mg/litre and are followed by death (Repp et al., 1955; McBarron
& McInnes, 1968; Kirkpatrick et al., 1972, 1973; Webb et al.,
1972). Wilson et al. (1968a) attributed the cause of death to the
cardiotoxic effects of ammonia produced from the urea. However,
Singer & McCarty (1971) observed that only one sheep died of
ventricular fibrillation and the remainder of respiratory failure.
More recently, Edjtehadi et al. (1978) reported the arrest of
respiration, and not cardiovascular collapse, as the cause of death
in sheep.
Certain aspects of the blood chemistry have been described for
sheep with urea poisoning (Kirkpatrick et al., 1973; Edjtehadi et
al., 1978). In general, during the initial stages of urea
toxicosis, an alkalosis is induced, followed by systemic acidosis
due to hyperventilation prior to death (Edjtehadi et al., 1978).
In addition to increases in blood-ammonia and blood-urea levels,
there is a marked increase in the blood-glucose level, with no
change in ketone concentrations in the body (Singer & McCarty,
1971). The following changes have been recorded at death: red-
cell count and haemoglobin concentration increased by 7.9%; white-
cell count decreased by 27.5%; and packed-cell volume increased by
11.4%. Mean corpuscular volume, mean corpuscular haemoglobin, and
mean corpuscular haemoglobin concentration were not substantially
changed (Kirkpatrick et al., 1973).
Pathological effects of ammonia toxicity in sheep have been
described by Singer & McCarty (1971). The changes were similar
when sheep received intraruminal injections of ammonium chloride,
ammonium sulfate, or a mixture of ammonium chloride, carbonate,
phosphate, and sulfate. General passive hyperaemia and numerous
petechial and ecchymotic haemorrhages in the musculature, heart,
thymus, and lungs were found. The lungs were distended and
severely congested. On microscopic examination, the pulmonary
lesions included severe hyperaemia, haemorrhage, alveolar oedema,
and alveolar emphysema. In the thymus, there was degeneration and
necrosis of Hassall's corpuscles and centrilobular haemorrhages.
Lesions in kidneys included severe generalized cloudy swellings and
multiple foci of early coagulative necrosis of the proximal
convoluted tubules, general hyperaemia of the glomerular tufts, and
degeneration of the glomerular tuft cells.
In Marschang & Crainiceanu's (1971) study on the effects of
ammonia in the air of calf stables, ammonia concentrations
reportedly ranged from 0.7 to 140 mg/m3 (1 - 200 ppm). During
these periods of high ammonia concentration, high mortality rates
were observed among the calves. The authors suggested that the
high ammonia content weakened the resistance of the animals and
thus created conditions for the development of secondary
infections. Deaths were mainly caused by respiratory diseases.
Autopsy indicated various types of change in the lungs, chiefly
inflammation.
Air-ammonia concentrations in 3 cattle-fattening facilities in
Romania were measured by Marschang & Petre (1971), who found
concentrations ranging from 2 to 1400 mg/m3 (3 - 2000 ppm).
Morbidity (mainly from respiratory disease) and mortality rates
increased with ammonia concentrations in the stalls and decreased
as some of the toxic gas levels decreased to admissible
concentrations. The authors suggested that ammonia is the most
important environmental factor in producing disease in cattle-
fattening stalls. They did not refer to the growth rate of the
cattle; however, in an additional report, Marschang (1972) observed
a marked decrease in the growth rate of fattening cattle, when the
ammonia content of the stable was high.
(b) Monogastric animals
Monogastric animals are considered relatively tolerant to
dietary urea, since they lack the large amounts of bacterial urease
present in the rumen of ruminants. Horses may frequently consume
cattle rations that contain urea or other non-protein-nitrogen
sources.
Hintz et al. (1970) found that the urease activity in the
caecal fluid from ponies was 17 - 25% of that reported for bovine
rumen fluid. They subjected 8 ponies to oral doses of urea at 3.3
- 3.6 g/kg body weight, to study the toxic effects of urea
overdosage. Seven of the ponies died of ammonia toxicity, 3 - 12 h
after treatment. Clinical signs of toxicosis were characteristic
of severe central nervous system derangement. These signs were
similar to those previously reported for ruminants, with the
exception of head pressing against a fixed object prior to loss of
coordination. No significant gross lesions were observed on
necropsy. Blood-ammonia increased linearly until death. Blood-
alpha-keto-glutarate decreased initially, reached minimal values
at about 30 min and then increased to 3 times the zero time value.
Blood-glucose remained constant for the first 2.5 h and then
increased to about 3 times the initial value. Blood-pyruvate
decreased during the first 3.5 h and then increased to 10 times
the initial values.
6.5.2.2. Inhalation exposure
(a) Swine
The acute inhalation effects of ammonia in swine are given in
Table 15.
(b) Poultry
As discussed previously, poultry are exposed to ammonia in the
atmosphere of poultry houses; this ammonia is released from the
action of bacteria on poultry wastes. The toxic effects of this
exposure are primarily seen in the eyes and respiratory tract.
An idiopathic ocular disorder in young chicks, designated
keratoconjunctivitis, was first described by Bullis et al. (1950),
who attributed it to environmental factors in the rearing
facilities.
Anderson et al. (1964a) reported that chickens exposed
continuously to ammonia at 14 mg/m3 (20 ppm) showed some signs
of discomfort, including rubbing of the eyes, slight lachrymation,
anorexia, and, later, weight loss. Chickens exposed to ammonia at
14 mg/m3 for as little as 72 h were more susceptible to aerosol
infection with Newcastle disease virus. Gross and microscopic
damage to the respiratory tract could be detected after 6 weeks of
continuous exposure to ammonia at 14 mg/m3. Valentine (1964)
reported tracheitis in chicks exposed to ammonia at 42 - 49 mg/m3
(60 - 70 ppm). The breathing of the birds was audible as moist
rales with bubbling sounds. At post-mortem examination, some of
the birds had slight congestion of the lungs with excess mucous in
the respiratory tract. The mucous membranes of the trachea were
much thicker than in the control birds, and there was leukocytic
infiltration of the tissue. It was suggested that this tracheitis
may predispose the affected birds to respiratory diseases with the
added risks of secondary infections.
Charles & Payne (1966a) reported that exposure to atmospheric
ammonia at 70 mg/m3 (100 ppm) caused a reduction in carbon dioxide
production and depth of respiration and a 7 - 24% decrease in the
respiration rate of laying hens. The authors also observed that
broilers reared to 28 days of age in atmospheres containing high
concentrations of ammonia consumed less food and grew more slowly
than unexposed chickens. Pullets reared in high-ammonia
atmospheres matured up to 2 weeks later than pullets reared in
ammonia-free atmospheres.
Airsacculitis, one of many respiratory diseases in poultry, has
been associated with high ammonia concentrations in poultry houses
(Ernst, 1968). High concentrations of dust were also noted during
periods of winter confinement, when high ammonia concentrations
were observed. The incidence and severity of air-sac lesions in
turkeys increased signficantly with high concentrations of dust
(0.6 - 1.0 mg/m3 or 21 - 35 mg/m3) in the atmosphere. Flocks with
a high rate (47%) or a low rate (2%) of infection with Mycoplasma
meleagridis were similarly affected. No significant interaction
between dust and ammonia concentrations (up to 21 mg/m3 or 30 ppm)
with regard to effects on the development of air-sac lesions was
found. Mortality rate and feed conversion were not significantly
affected by exposure to dust and ammonia. There was considerable
loss of cilia from the epithelium of the tracheal lumen and an
increase in mucous-secreting goblet cells in turkeys exposed to
high concentrations of dust and ammonia. Areas of consolidation
and inflammation were frequently observed in the lungs of these
turkeys. The air-sac lesions ranged from mild (lymphocytic
infiltration) to severe (masses of gaseous material).
Airsacculitis has also been experimentally induced in chickens
exposed to atmospheric ammonia and the stress of infectious
bronchitis vaccination (Kling & Quarles, 1974). Eighty Leghorn
male chicks were maintained in 12 controlled-environment chambers.
Ammonia at 0, 17.5, or 35 mg/m3 (0, 25, or 50 ppm) was introduced
into the chambers from the 4th to 8th weeks of age. An infectious
bronchitis vaccination was administered to all chicks at 5 weeks of
age. Body weights and feed efficiencies were determined at 4, 6,
and 8 weeks. At 4, 5, 6, and 8 weeks, lung and bursae of Fabricius
weights, haematocrits, and air-sac scores were determined. Body
weights and feed efficiencies were significantly reduced in the
ammonia chambers. The bursae of Fabricius in the ammonia-stressed
chickens were significantly larger than those of controls at 5
weeks of age and significantly smaller at 8 weeks of age. Chickens
grown in ammoniated environments had significantly larger lungs at
8 weeks. Haematocrits were not significantly different among
treatments. Total air-sac scores were significantly higher in the
ammonia-stressed chickens at 8 weeks. The results indicated that
chickens were stressed by the ammonia at 17.5 or 35 mg/m3, and by
the infective bronchitis vaccination. In a similar study (Quarles
& Kling, 1974), exposure of broiler chicks to 17.5 or 35 mg/m3 (25
or 50 ppm) from the 4th to the 6th week resulted in the observation
of severe airsacculitis at 6 and 8 weeks of age. During the test,
airborne bacterial counts were significantly higher in chambers
with ammonia than in control chambers.
Table 15. Acute inhalation effects of ammonia in swine
---------------------------------------------------------------------------------------------------------
Ammonia concentration Dose Effects Reference
---------------------------------------------------------------------------------------------------------
196 mg/m3 (280 ppm) single frothing of the mouth and excessive Stombaugh et al. (1969)
secretion; after 36 h, convulsions
occurred, and breathing was extremely
short and irregular; the effects
ceased after a few h
7, 35, 70, or 105 mg/mg3 5 weeks high concentrations (70 and 105 mg/m3) Stombaugh et al. (1969)
(10, 50, 100, or 150 ppm) appeared to cause excessive nasal,
lachrymal, and mouth secretion after
3 - 4 days of exposure at 35 mg/m3,
the secretory rate was only slightly
higher than that in control animals;
after 1 - 2 weeks of exposure, the
signs noted appeared to lessen
gradually; examination of respiratory
tract did not reveal any significant
gross- or microscopic differences
related to ammonia exposure
0, 35, 70, or 105 mg/m3 4 weeks decrease in pig growth was noted at Drummond et al. (1980)
(0, 50, 100, or 105 ppm) all concentrations; at 35 or 70 mg/m3,
pigs converted feed to body weight
gain more efficiently than either
controls or pigs exposed to 105 mg/m3;
an acute imflammatory reaction in the
tracheal epithelium and a mild-to-
heavy exudate in the turbinate lumen
were observed at 70 and 105 mg/m3,
only
0 and 70 mg/m3 (100 ppm) 2 - 6 conjunctival irritation after the Doig & Willoughby (1971)
(1 - 7 weeks old) weeks first day which persisted for 1 week;
0 + dust dust alone had no effect; histopatho-
(100 ppm + dust) logical changes were limited to the
nasal and tracheal epithelium; there
was no evidence of structural damage
in the bronchial epithelium or alveoli
---------------------------------------------------------------------------------------------------------
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The authors suggested that the quality of eggs left all day in hen
houses containing high concentrations of ammonia might be adversely
affected.
7. KINETICS AND METABOLISM
Ammonia, a by-product of protein and nucleic acid metabolism
and a minor component of the diet, is in a state of flux in the
body, though it is present in low steady-state concentrations in
body fluids. In animals, metabolically-produced ammonia is
conjugated and excreted. Toxicity will only occur if these
conjugation and excretion mechanisms are defective, or if they are
overwhelmed by excessive exposure.
7.1. Absorption
7.1.1. Respiratory tract
Egle (1973) studied the retention, over a short period of time,
of inhaled ammonia in air at concentrations in the range 150 -
500 mg/m3 (214 - 714 ppm), in mongrel dogs of both sexes (7 - 37
per study). Retention was not materially affected by respiratory
rate, tidal volume, or concentration. Retention by the whole
respiratory tract averaged 78%, but the complexity of the dynamics
of this retention is illustrated by the fact that when respiration
was via an endotracheal tube, limiting exposure to the lower
respiratory tract, and when the upper respiratory tract (muzzle to
tracheal bifurcation) was perfused tidally, the retention was 78%
in each case. However, unidirectional perfusion of the upper
respiratory tract with ammonia in air produced a higher mean
retention of 89%.
Schaerdel et al. (1983) exposed 4 groups of rats, 8 per group,
to average ammonia concentrations of 11, 23, 220, or 826 mg/m3
(15, 32, 310, or 1157 ppm) for 24 h. Ammonia in blood was measured
at 0, 8, 12, and 24 h. At the 2 lowest concentrations, there was
no increase in blood-ammonia. However, after 8 h at 220 and
826 mg/m3, significant increases of 0.192 and 0.244 mmol (3.26 and
4.18 mg/litre) were noted. After 12 and 24 h, the increases were
not so marked, indicating an increase in ammonia metabolism.
In a study by Silverman et al. (1949), 7 male volunteers were
exposed to 350 mg/m3 (500 ppm) for 30 min. Initial ammonia
retention was not reported for all subjects, but, in one instance,
was around 75%. Retention decreased progressively until at
equilibrium it was 23% (range 4 - 30%); equilibrium was reached in
10 - 27 min. Some irritation was noted in the nose and throat,
leading to the suggestion that ammonia at this concentration was
primarily absorbed by the upper respiratory tract. Levels of
blood-urea-nitrogen (BUN), non-protein nitrogen, urinary-urea, and
urinary-ammonia remained normal.
In another study (Kustov, 1967), exposure of human volunteers
to ammonia for a longer duration (14 mg/m3 (20 ppm) for 8 h) was
accompanied by a statistically-significant increase in BUN from
23.9 to 30 mg%.
An early study was conducted by Landahl & Herrmann (1950) on
the retention of gases by the human nose and lung. At ammonia
concentrations of between 40 and 350 mg/m3 (57 and 500 ppm) and a
mean minute volume of 6 - 7 litre/min, for short durations (< 2
min), they found that approximately 92% ± 2% was retained in the
respiratory system (i.e., mouth, lungs, etc.) in 2 male volunteers,
tested 4 times. Differences in concentrations of ammonia did not
affect retention values. In a separate study, about 83% was
retained in the nasopharnyx at a flow rate of 18 litre/min, but
only 63 - 71% was retained when the flow rate was tripled. These
data are consistent with, though somewhat higher than, those
reported by Egle (1973) for exposure in dogs.
It should be noted that experimental animals kept in cages may
be exposed to relatively high concentrations of ammonia, even
exceeding 100 mg/m3, due to the degradation of urea in urine and
faeces (Flynn, 1968; Schaerdel et al., 1983).
Because ammonia is very water soluble, and thus absorbed by the
mucous coating in the upper respiratory tract, the lungs are
protected from the effects of exposure to low concentrations of
ammonia (Haggard, 1924; Boyd et al., 1944). At the levels of
ammonia associated with ambient air (i.e., 1 - 200 µg/m3), very
little, if any, is absorbed through the lungs.
If a person breathes an ammonia concentration in air of
18 mg/m3 (a common occupational exposure limit) at 1 m3/h and,
if all the ammonia is retained, then 18/60 = 0.3 mg ammonia/min
would require to be cleared by hepatic blood flow (say 1
litre/min). The rise in systemic blood-ammonia would be calculated
at 0.3 mg/litre or 0.018 mmol. If the more realistic assumption of
30% retention were used, the corresponding increase in blood-
ammonia concentration would be 0.09 mg/litre, about µmol. An
arterial fasting ammonia concentration of 1.05 mg/litre has been
reported in healthy subjects (Conn, 1972), so the calculated rise
is only 10% over fasting levels.
7.1.2. Gastrointestinal tract
Ammonia is a trace compound in foods. Ammonia that is absorbed
from the intestinal tract arises primarily from the bacterial
degradation in the intestine of amino and nucleic acids from
ingested food, endogenous epithelial debris, and mucosal cell
luminal secretions, or from the hydrolysis of urea diffusing from
the systemic circulation into the intestinal tract. The estimated
ammonia production from various substrates in the human intestines
ranges from 10 mg/day in the duodenum to 3080 mg/day in the colon
and faecal contents. Nearly all the ammonia formed is absorbed
(about 99% or 4000 mg). In healthy individuals, absorbed ammonia
is mainly catabolized rapidly in the liver to urea; therefore,
relatively small amounts reach the systemic circulation after
absorption from the gastrointestinal tract as a consequence of this
"first pass effect" (Summerskill & Wolpert, 1970).
Castell & Moore (1971) have shown that ammonia uptake from the
human colon, the major site of ammonia production, increases with
increased pH of the luminal contents. A similar effect of pH has
been shown for the absorption of ammonia from the rumen of sheep
(Hogan, 1961; Bloomfield et al., 1962). Since an increase in pH
increases the proportion of non-ionized ammonia, the authors
concluded that simple non-ionic diffusion was responsible for the
majority of ammonia transport. Evidence also exists for the active
transport of the ammonium ion from the intestinal tract. Castell &
Moore (1971) showed that ammonia transport by the human colon,
though greatly diminished, still occurred when the luminal pH was
reduced to 5, at which value non-ionized ammonia would be virtually
absent. Mossberg & Ross (1967) and Mossberg (1967) studied the
absorption of ammonia from isolated intestinal loops of the golden
hamster and found that the ileal movement of ammonia against a
concentration gradient was inhibited by cyanide, dinitrophenol, and
anaerobiosis. This suggested that an energy-dependent transport
system was operable in the ileum, where ammonia was absorbed
preferentially, but not in the jejunum.
Membrane transport of the ammonium ion by the human erythrocyte
has been demonstrated (Post & Jolly, 1957).
7.1.3. Skin and eye
Ammonia is highly mobile in all tissues and the ammonium ion
readily penetrates the corneal epithelium. Within 5 seconds,
traces are present in the anterior chamber of the eye (Siegrist,
1920). However, systemic and intra-ocular absorption by these
routes are not quantitatively important.
7.2. Distribution
The ammonia normally present in all tissues in the body
constitutes a dynamic pool throughout which absorbed ammonia is
distributed. The distribution of total ammonia between body
compartments is strongly influenced by pH. The non-ionized NH3 is
freely diffusible, whereas NH4+ is less diffusible and relatively
confined in compartments. The lower the pH of a compartment, the
greater its total ammonia content (NRC, 1979).
The fate of absorbed ammonia molecules has been studied in man,
by measurement of blood constituents, and, in experimental animals,
by following the distribution of 15N after the administration of
15NH3 and compounds.
7.2.1. Human studies
In human beings, inhalation of ammonia (350 mg/m3; 500 ppm) for
30 min did not have any effect on blood-nitrogen levels (Silverman
et al., 1949). In another study, exposure of human subjects to
14 mg ammonia/m3 (20 ppm), for a duration of 8 h, revealed a
statistically-significant increase in BUN (from 23.9 to 30 mg%)
(Kustov, 1967). However, this is unlikely to have represented the
metabolic conversion of absorbed ammonia, since the increase was
far greater than could have been accounted for by the quantity of
ammonia inhaled.
Administration of 9 mg NH4Cl/kg body weight, orally, to 20
healthy adult male and female volunteers caused a transient
increase in ammonia concentrations in arterial blood in
approximately half of the subjects. Concentrations peaked (mean,
1.4 mg NH3/litre) at 15 min and returned to fasting levels (mean,
1.05 mg NH3/litre) by 30 min. However, in 50 male patients with
cirrhosis of the liver, blood-ammonia levels increased from already
elevated fasting levels (mean, 1.56 mg NH3/litre) to much higher
peak concentrations (mean, 3.7 mg NH3/litre) at 15 min, followed by
a slow decrease reflecting impaired hepatic urea synthesis. Blood-
ammonia levels, before and after administration of ammonium
chloride, were significantly higher among cirrhotic patients with
portacaval anastomoses than among patients lacking such shunts
(Conn, 1972).
7.2.2. Animal studies
The distribution, as well as the metabolic fate of ammonia,
depends on the route of administration. After intestinal
absorption, ammonium ions are primarily transformed by the liver
to urea, and subsequently excreted in the urine. In contrast,
intravenously-administered ammonium salts are more available as
non-essential nitrogen for protein synthesis (Furst et al., 1969).
However, some orally-administered ammonia, has been found to be
incorporated into tissue proteins. Incorporation of 15N was higher
in serum globulins than in albumin after intravenous dosing with
15N-ammonium salts, but this order was reversed after oral
administration (Furst et al., 1970). The amount incorporated into
protein by this route was greater, when protein intake was
restricted (Richards et al., 1968).
Duda & Handler (1958) analysed the tissue of rats, 15 min after
an intravenous injection of 15N-ammonium lactate, and found that
its major metabolites, glutamine, and urea, were quickly
distributed throughout the body. The highest levels of labelled
urea (in µmoles 15N/g tissue) were found in the kidney (0.0217) and
liver (0.0159), while lesser amounts were found in the heart
(0.0086), spleen (0.0067), brain (0.0029), testes (0.0027), and
carcass (0.0070). The highest levels of labelled glutamine (µmoles
15N/g tissue) were found in the heart (0.086) and liver (0.055) and
lesser amounts (0.005 to 0.032) in the brain, spleen, carcass,
kidney, and testes.
Vitti et al. (1964) examined the distribution of 15N from
ammonium citrate, administered by different routes, into the
proteins of various tissues of hypophysectomized rats. The liver,
kidney, and spleen contained greater concentrations of 15N
incorporated into proteins than heart or muscle fractions during
72 h following intragastric, intraperitoneal, and subcutaneous
administration of 15N-ammonium citrate. After the first 6 h,
during which the intragastric route gave higher values, the
quantity of 15N incorporated into liver-protein was not
substantially affected by the route of administration. In most of
the other tissues studied, however, 15N incorporation tended to be
least by the intragastric route, followed, in increasing order, by
the intraperitoneal and subcutaneous routes. By the last route,
more labelled ammonia was apparently made available to the widely
distributed glutamine-synthetase (EC 6.3.1.2) system (section
7.4.3).
7.3. Metabolic Transformation
Most organisms have mechanisms for conjugating ammonia into
non-toxic compounds for excretion. Terrestrial mammals synthesize
urea, which requires the concerted action of several enzymes of the
Krebs-Henseleit (urea) cycle. One of these enzymes, glutamine
synthetase (EC 6.3.1.2), was present in the brains of all
vertebrate species examined. Glutamine synthetase was also present
at significant levels in the liver in all organisms examined (Brown
et al., 1957).
Exogenous ammonia, administered intravenously as an ammonium
compound, is metabolized to glutamine as the major early product
(Duda & Handler, 1958). The ammonia fixed in glutamine may
eventually end up in amino acids, purines, pyrimidines, or other
nitrogen-containing compounds. Ingested ammonium chloride or
endogenous ammonia is absorbed into the portal vein and converted
in the liver to urea (Furst et al., 1969; Goodman & Gilman, 1970;
Pitts, 1971).
Results of studies on the metabolic fate of dietary ammonium
citrate (Foster et al., 1939) and intravenously-administered
ammonium lactate (Duda & Handler, 1958) in rats showed that urea
synthesis represented a nearly constant fraction of the
administered ammonia over a large concentration range. Besides
glutamine and urea, labelled nitrogen also appeared in creatine,
glycine, alanine, proline, histidine, arginine, glutamic acid, and
aspartic acid. Vitti et al. (1964) examined the incorporation of
15N from ammonium citrate into proteins of liver, heart, kidney,
spleen, and muscle fractions of untreated and growth hormone-
treated, hypophysectomized rats, and found differences in the
metabolic fate, depending on the route of administration.
Subcutaneous injection facilitated the labelling of amide nitrogen,
indicating extensive disposition via glutamine synthesis. In
contrast, intragastric or intraperitoneal administration resulted
in the labelling of arginine, glutamic acid, and other alpha-amino
acids of the liver. Amide-nitrogen was labelled to a much lesser
extent than by the subcutaneous route. The tissue distribution of
the label also differed according to the route of entry (section
7.2.2).
7.4. Reaction with Body Components
Ammonia-nitrogen is central in nitrogen metabolism and
therefore becomes incorporated in all proteins and nitrogen-
containing components in the course of metabolic turnover. Ammonia
does not react with body components in the manner of alkylating
agents or compounds that modify haemoglobin.
7.5. Elimination and Excretion
7.5.1. Expired air
Ammonia may be excreted through expired air. Hunt (1977)
reported human expired air levels of ammonia of between 105 and
2219 µg/m3; Larson (1977) reported values of between 196 and
1162 µg/m3, during mouth breathing. These values are higher than
those expected from equilibration with plasma- and lung-parenchyma-
ammonia levels (28 - 49 µg/m3). This is most likely due to the
synthesis of ammonia from salivary urea by oral microflora (Biswas
& Kleinberg, 1971). Measurable amounts of free ammonia were also
found in air expired by dogs given ammonium acetate intravenously
(Robin et al., 1959), and normal dogs and human beings with
hepatic-induced ammonia toxicity (Jacquez et al., 1957, 1959).
Bloomfield et al. (1962) reported the presence of free ammonia in
expired air from sheep during experimentally-induced urea toxicity.
Normal levels of ammonia in the expired air of the rat have been
reported to range from 7 to 247.1 µg/m3, with a mean of 54.6 µg/m3
in nose-breathing animals and 23.8 - 520.8 µg/m3, with a mean of
200.2 µg/m3 in tracheal-cannulated animals (Barrow & Steinhagen,
1980). The presence of ammonia in the expired air of human beings
and experimental animals suggests that reaction products may be
formed with a variety of airborne chemicals, thereby altering their
toxicity.
7.5.2. Urine and faeces
Free ammonia is excreted by ammonotelic organisms (e.g., fish),
uric acid by uricotelic animals (e.g., birds), and urea by
ureotelic animals (e.g., mammals). Mammals may also secrete
ammonia directly into the urine. Glutaminase (EC 3.5.1.2)
catalyses the release of ammonia in the kidney tubular epithelium,
where it serves as an acceptor of H+ and regulates the acid-base
balance (Van Slyke et al., 1943; White et al., 1973). In acidosis,
the renal concentration of glutaminase increases over several
days, paralleling the increased excretion of ammonium ions (Davies
& Yudkin, 1952; Muntwyler et al., 1956; Kamin & Handler, 1957);
two-thirds of the urinary-ammonia is contributed by this pathway
(Van Slyke et al., 1943), and approximately one-third by protein
metabolism and ammonia clearance from the plasma by the kidney.
Oral and intravenous administration of ammonium lactate to
healthy human volunteers produced different patterns of excretion,
reflecting the effective barrier of the liver in preventing
ingested ammonia from gaining access to peripheral circulation by
converting most of the ammonia load to urea. Urinary-ammonia
excretion was increased 8-fold and urea excretion was reduced by
one-half after intravenous injection, as opposed to oral
administration (Gay et al., 1969), probably due to the anabolism
involved in the "first pass" effect after oral administration.
Less than 1% of the 4 g total ammonia produced in the human
intestinal tract, per day, is excreted in the faeces (Summerskill &
Wolpert, 1970).
7.6. Retention and Turnover
Some nitrogen derived from absorbed ammonia is incorporated in
amino acids and proteins. The rate of ammonia-derived nitrogen
turnover is rapid, but depends on the nutritional state. Thus,
when 15NH4Cl had been administered orally to healthy male
volunteers, for one week, 70% 15N was excreted by those on a 70-g
protein/day diet, while only about 35% 15N was excreted by those on
20 g protein/day (Richards et al., 1968, 1975).
7.7. Uptake and Metabolism in Plants
Ammonia is used by many plants and preferentially by a few.
However, ammonia is toxic, and its uptake in large quantities may
put a severe strain on the carbohydrate metabolism of the plant in
the provision of carbon skeletons for its detoxification. The
absorption of ammonium usually is coupled with the exchange of
cations as H+. Ammonia-nitrogen functions as a nitrogen source
for the synthesis of amino acids, which are incorporated in
proteins. Plants that are able to absorb it in large amounts
include many acid plants, such as Rumex, which are able to detoxify
ammonia by forming ammonium salts of organic acids. "Amide
plants", such as beet, spinach, and squash, are able to form large
amounts of the amides, glutamine, and asparagine and can withstand
quite high concentrations of ammonium salts by detoxifying the
ammonia. Certain plants, such as rice, which live in water-logged
anaerobic soils, require NH3 or reduced organic nitrogen
fertilizer, alone.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Inhalation exposure
LC50 studies and studies to determine the threshold for
irritating effects on the respiratory system for the rat and mouse
are summarized in Tables 16 and 17, respectively.
Table 16. Lethal concentrations (1-h exposure) of
ammonia for rats and micea
----------------------------------------------------
Measured Species Mortality Mean weight gain
concentration ratiob of survivors at
(mg/m3) 14 days (g)
----------------------------------------------------
4347 rat 0/10 3.5
5474 rat 8/10 -c
6888 rat 9/10 -
controls rat - 21.4
2520 mouse 0/10 -0.2
3185 mouse 3/10 -0.7
4004 mouse 9/10 -
controls mouse - 1.6
----------------------------------------------------
a Adapted from: MacEwen & Vernot (1972).
b Number dead/number exposed.
c Not enough survivors for comparison.
The acute lethal dose of ammonia by inhalation has been
determined for both the rat and the mouse (MacEwen & Vernot, 1972).
The results are summarized in Table 16. Male CFE rats ranging in
weight from 200 to 300 g and male CF1 mice weighing from 20 to 30 g
(ICR derived) were exposed for 1 h to several concentrations of
ammonia. Inhalation of ammonia gas produced immediate nasal and
eye irritation followed by laboured breathing and gasping in all
test groups. In addition, convulsions were seen in mice.
Surviving rats necropsied after 14 days showed moderate mottling of
the liver, regarded as probable fatty infiltration, at the 5474 and
6888 mg/m3 (7820 and 9840 ppm) dose levels. Mice surviving the 2
highest dose levels of 3185 and 4004 mg/m3 (4550 and 5720 ppm)
showed mild congestion of the liver. Pathological lesions were not
seen in rats exposed to 4347 mg/m3 (6210 ppm) or mice exposed to
2520 mg/m3 (3600 ppm). The calculated 1-h LC50 values for the rat
and the mouse were 5137 and 3386 mg/m3 (7338 and 4837 ppm),
respectively.
In another inhalation study, an LC50 value for the rat, with a
2-h exposure, was 7600 mg/m3 (10 860 ppm) (Alpatov, 1964). In a
further study, the threshold for acute effects (depression, then
hyperactivity and convulsions) for a 2-h exposure was 85 mg/m3 (121
ppm) (Alpatov & Mikhailov, 1963).
Table 17. Single-dose inhalation studies (LC50)
----------------------------------------------------------
Species Exposure time LC50 Reference
(min) (mg/m3)
----------------------------------------------------------
rat 120 7600 Alpatov (1964)
rat 60 5137 MacEwen & Vernot (1972)
rat 5 18 693 Prokop'eva et al. (1973)
rat 15 12 160 Prokop'eva et al. (1973)
rat 30 7035 Prokop'eva et al. (1973)
rat 60 7939 Prokop'eva et al. (1973)
rat 10 31 612 Appelman et al. (1982)
rat 60 11 620 Appelman et al. (1982)
mouse 10 7060 Silver & McGrath (1948)
mouse 60 3386 MacEwen & Vernot (1972)
mouse 60 2960 Kapeghian et al. (1982)
----------------------------------------------------------
Kapeghian et al. (1982) reported an acute inhalation toxicity
study on male ICR mice, in which the 1-h LC50 with a 14-day
observation period was calculated to be 2960 mg/m3 (4230 ppm).
Lungs of mice that died during exposure were diffusely
haemorrhagic. Histology revealed acute vascular congestion and
diffuse intra-alveolar haemorrhage. A mild to moderate degree of
chronic focal pneumonitis was also seen. Focal atelectasis was
evident in survivors sacrificed after the observation period.
Liver damage was also seen in these mice. There was evidence of
swelling and increased cytoplasmic granularity of hepatocytes at
2408 mg/m3 (3440 ppm) and scattered foci of frank cellular necrosis
at 2954 mg/m3 (4220 ppm). At 3402 mg/m3 (4860 ppm), necrosis was
increased. The liver lesions may have resulted from the
compromised nutritional state of the mice. Follicular hyperplasia
in the spleen was also seen in surviving animals, but this was
absent in animals that died during exposure.
The acute LC50 in male and female Wistar rats was 31 612 mg/m3
(40 300 ppm) for a 10-min exposure, 20 017 mg/m3 (28 595 ppm) for a
20-min exposure, 14 210 mg/m3 (20 300 ppm) for a 40-min exposure,
and 11 620 mg/m3 (16 600 ppm) for a 60-min exposure (Appelman et
al., 1982). Survivors were observed for 14 days. Clinical signs
of restlessness, eye irritation, nasal discharge, mouth breathing,
and laboured respiration were seen during exposure. Gross necropsy
revealed haemorrhagic lungs in animals that died during the study
as well as in survivors. No histopathology was performed.
Prokop'eva et al. (1973) reported that white rats exposed to
high concentrations of ammonia (6000, 3000, 1000 mg/m3 or 6814,
4307, 1436 ppm) for periods of 5, 15, 30, and 60 min exhibited
dyspnoea, irritation of the respiratory tract and eyes, cyanosis of
the extremities, and increased excitability. The LC50 values for
inhalation exposures of 5 and 15 min were 18 693 mg/m3 (26 704 ppm)
and 12 160 mg/m3 (17 372 ppm), respectively, while for 30 and 60
min, the values were 7035 mg/m3 (10 050 ppm) and 7939 mg/m3 (11 342
ppm), respectively. Inhalation of ammonia at concentrations of
3000, 1000, or 300 mg/m3 (4307, 1436, 431 ppm) resulted in a drop
in static muscular tension, leukocytosis, prolongation of the
latent reflex time, increase in total protein and blood sugar,
increased oxygen consumption, and a rise in the level of residual
nitrogen. No changes were observed in rats exposed to a
concentration of ammonia of 100 mg/m3 (144 ppm), for 5, 15, 30, and
60 min. Animals exposed to high concentrations of ammonia (exact
concentration not specified) developed pneumonia (Prokop'eva et
al., 1973).
When exposed to toxic levels of ammonia, within 1 min, mice
exhibited excitement, closing their eyes immediately and gasping
(Silver & McGrath, 1948). Groups of 20 mice were exposed to
ammonia gas for 10 min at 9 concentrations ranging from 6100 to
9000 mg/m3 (8758 to 12 921 ppm). The median lethal concentration,
calculated from the mortality at 10 days, was 7060 ± 320 mg/m3
(10 152 ± 460 ppm). Most (93%) deaths occurred rapidly, due to
convulsions after 5 min of exposure. Survivors usually recovered
within 10 min.
The LC50 values for the rat and the mouse are summarized in
Table 17.
Dose-dependent ultrastructural changes in the terminal airways
of mice exposed for 3 - 60 min to ammonia (concentration
unspecified) included oedema of the alveolar epithelium,
development of intracapillary platelet thrombosis, increased
secretions by the Clara cells, presumed to be phospholipids, and an
increase in the number of empty lamellar bodies in the large
alveolar cells (Niden, 1968).
Twenty cats were anaesthetized and exposed to 700 mg/m3 (1000
ppm) for 10 min via endotracheal tube and then observed for up to
35 days (Dodd & Gross, 1980). All cats had severe dyspnoea,
anorexia, and dehydration, 24 h after exposure. Several measures
of pulmonary function were impaired. Gross pathology of the lungs
showed various degrees of congestion, haemorrhage, oedema,
interstitial emphysema, and collapse, all non-specific for any
post-exposure day. Bronchopneumonia was common, 7 days after
exposure.
Barrow et al. (1978) exposed male Swiss-Webster mice to
concentrations of ammonia ranging from 70 to 560 mg/m3 (100 -
1000 ppm) for 30 min. The average respiratory rate depression in
4 mice for each of 4 exposure levels was evaluated. The maximum
depression in respiratory rate at each exposure level occurred
within the first 2 min. The concentration expected to elicit a 50%
decrease in respiratory rate (RD50) in mice, calculated by a
regression equation for ammonia, was 212 mg/m3 (303 ppm) with 95%
confidence limits of 132 - 343 mg/m3 (188 - 490 ppm). Effects of
ammonia exposure such as bradycardia and peripheral
vasoconstriction accompanied respiratory rate depression at the
RD50 and above.
A concentration of 350 mg/m3 (500 ppm) was reported by Wood
(1979) to be the level at which unrestrained mice did not
consistently adopt avoidance measures on inhalation of ammonia.
Both this author and Barrow et al. (1978) claimed to offer more
sensitive end-points for assessing ammonia irritation. However,
the no-observed-adverse-effect level reported by Wood (1979) was
higher than the concentration determined by Barrow et al. (1978) to
elicit 50% depression of respiratory rates in mice.
Dalhamn & Sjoholm (1963) tested in vitro preparations of the
tracheas of 8 rabbits. Arrested ciliary activity was observed
after 5 min of exposure to ammonia at 350 - 700 mg/m3 (500 -
1000 ppm). In in vivo studies on rabbits, Dalhamn (1963) showed
that the level of ammonia entering the nasal cavity, necessary to
cause small changes in the rate of tracheal ciliary beating was
1400 mg/m3 (2000 ppm). This corresponds to a tracheal
concentration of approximately 70 mg/m3 (100 ppm).
When anaesthetized male rabbits were exposed to 8 ammonia
concentrations ranging from 700 to 14 000 mg/m3 (1000 - 20 000 ppm)
(Richard et al., 1978b), bradycardia appeared at 1750 mg/m3 (2500
ppm). Hypertension, cardiac arrhythmia, macroscopic lung changes,
and EEG abnormalities were also reported. Signs appeared more
rapidly at higher concentrations with the complete syndrome
appearing at 3500 mg/m3 (5000 ppm).
Mayan & Merilan (1972) exposed 16 adult female New Zealand
white rabbits to ammonia concentrations of 35 and 75 mg/m3 (50 and
100 ppm) for 2.5 h. The average decreases in respiratory rate,
which were 34 and 32.7%, respectively, were significantly ( P <
0.01) less than control values. There were no histopathological
changes in lung, liver, spleen, or kidneys.
Enzymatic alterations in rats following inhalation of low
levels of ammonia have also been reported. At concentrations of
approximately 20 - 121 mg/m3 (29 - 173 ppm), there was a
decrease in the activities of liver succinic dehydrogenase (EC
1.3.99.1), lactate dehydrogenase (EC 1.1.1.28), glucose-6-phosphate
dehydrogenase (EC 1.1.1.49), and adenosine triphosphatase (EC
3.6.1.3). Liver acid phosphatase (EC 3.1.3.2) activity was
increased (Zlateva et al., 1974).
When 180 mice were exposed for 10 min to ammonia at 6139 -
9058 mg/m3 (8770 - 12 940 ppm), death with convulsions began to
occur 5 min after exposure. One hundred mice died during the
exposure. The surviving animals (80) recovered rapidly; however,
7 died between the sixth and tenth days following exposure (NRC,
1979).
8.1.2. Oral exposure
The effects of ammonia and its compounds are mainly of 2 types.
The first is the effect of ammonia itself. The second is the
effect of the anion bound to the ammonium ion. Ammonium chloride,
especially, will mainly exert its effects in the mammalian body due
to the formation of hydrogen chloride. Most of the experimental
work concerning the oral route of administration has centred on
ammonium chloride, which has been used extensively in the study of
metabolic acidosis. There have been few studies in which attempts
have been made to identify the role of the ammonium ion and ammonia
in the effects (Table 18).
Median lethal doses (LD50s) of 4400 and 3100 mg/kg body weight
have been reported for ammonium sulfamate in the rat and the mouse,
respectively. (Vinokurova & Mal'kova, 1963). A similar figure for
the oral LD50 in the rat of 4520 (4070 - 5020) mg/kg was reported
by Bukhovskaya et al. (1966). Frank (1948) reported a lethal dose
for ammonium sulfate of between 3000 and 4000 mg/kg body weight in
the rat.
8.1.2.1. Effects of metabolic acidosis induced by ammonium chloride
The ingestion of ammonium chloride in doses of around 500 -
1000 mg/kg body weight per day, for periods ranging from 1 to 8
days, has induced metabolic acidosis in mice, guinea-pigs, rats,
rabbits, and dogs. However, Boyd & Seymour (1946) did not report
any toxic effects at doses of up to 1 g/kg body weight in rats,
rabbits, guinea-pigs, and cats (50 animals per group).
Clinical signs, depending on the severity of the acidosis,
include: a decrease in plasma- and urinary-pH; decreased appetite;
decreased carbon dioxide-combining power; an increase in BUN and
chlorides; an increase in plasma proteins; an increase in
haematocrit (haemoconcentration); increased gluconeogenesis;
increased phosphoenolpyruvate carboxykinase (EC 4.1.1.49) activity;
increased urinary ammonium; increased urea, sodium, chloride,
calcium, and titratable acid excretion; an increase in malate and
oxaloacetate concentrations in renal tissue; and decreased
concentrations of glutamine, glutamate, and alpha-ketoglutarate in
the kidney. Pulmonary oedema, central nervous system dysfunction,
and renal changes are reported to have occurred after ingestion of
ammonium chloride.
Susceptibility to ammonium chloride differs among species. For
instance, pulmonary oedema is produced in cats, but not in rabbits;
yet cats have been shown to be more resistant to oral poisoning by
ammonium chloride than other animals studied.
Age is an important factor in the response of rats to oral
doses of ammonium salts. Benyajati & Goldstein (1975) found that
the administration of a single dose of 5 mmol ammonium chloride/kg
body weight (267.5 mg/kg) by gavage to 7- to 12-day-old rats (39
animals) increased ammonia excretion by about 64%, within 4 h,
compared to an increase of 155% in 20 adult rats after similar
treatment.
Table 18. Selected studies on the acute oral effects of ammonia compounds in rats
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Urea drinking- female, Holtz- 6 concentration no renal hypertrophy Lotspeich (1965)
water man, 200 - of N equivalent
250 g to that of 0.28
mol/litre NH4Cl
for 7 days ad
lib
Citrate drinking- female, Holtz- 6 0.28 mol/litre no renal hypertrophy Lotspeich (1965)
water man, 200 - solution for
250 g 7 days ad lib
Chloride drinking- female, Holtz- 9 0.28 mol/litre metabolic acidosis; increase Lotspeich (1965)
water man, 200 - for 7 days, ad in kidney weight and total
250 g lib (about 1000 N; increased capacity to
mg/kg per day) produce ammonia from
glutamine in kidney; renal
hypertrophy; unilateral
nephrectomy plus acidosis
induced by NH4Cl caused a
greater hypertrophy than
either alone
------------------------------------------------------------------------------------------------------------
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Chloride diet female, 150 g 125 all on low- consumption of food inversely Motyl & Debski
protein diet; related to dosage of NH4Cl; (1977)
Group I: no dosage-related decrement in
NH4; Group II: weight gain; increase in LD50
1% NH4Cl (300 value for ip dose of 2.7%
mg/kg body NH4Cl in Groups II and III,
weight per indicating an increased
day); Group adaptive capacity for ammonia
III: 2% NH4Cl detoxification; LD50 value of
(380 mg/kg Group IV same as Group III;
body weight per LD50 value of Group V lower
day); Group IV: than Group I; dose-dependent
4% NH4Cl (560 decrease in blood-glucose
mg/kg body levels; decrease of muscle
weight per and liver glycogen; dose-
day); Group V: dependent increase in delta-
8% NH4Cl (540 ornithine transaminase and
mg/kg body ornithine transcarbamylase;
weight per day, increase in adenosine
for 7 days triphosphate concentration in
blood
------------------------------------------------------------------------------------------------------------
8.1.2.2. Organ effects following oral administration
(a) Lung and central nervous system
Toxic doses of ammonium salts induced acute pulmonary oedema in
rats, guinea-pigs, and cats, but not in rabbits given ammonium
chloride (6% aqueous solution) intraperitoneally or by gavage,
though the doses were sufficient to cause death. To induce similar
effects in cats, a larger dose (unspecified) of ammonium chloride
and a longer latency period (from 1 to 3 h) were required,
indicating a greater resistance of this species to ammonium
chloride (Koenig & Koenig, 1949).
In a limited study to assess the role of the ammonium ion in
producing pulmonary oedema, Koenig & Koenig (1949) administered, by
gavage, to 5 different guinea-pigs, 6 ml of 20% ammonium nitrate;
7 ml of 6.4% ammonium acetate; 7 ml of 10% ammonium bromide; 7 ml
of 6% ammonium chloride; or 7 ml of 7.4% ammonium sulfate,
respectively. The last 4 solutions contained approximately
equivalent concentrations of ammonium. All 5 animals died of acute
pulmonary oedema; the lungs of a control animal that had received
water by gavage remained normal. The pulmonary oedema could not be
attributed to the induced acidosis, since guinea-pigs and cats,
made severely acidotic by gavage with sodium lactate or dilute
hydrochloric acid, did not show lung oedema (Koenig & Koenig,
1949).
Progressive signs of ammonium poisoning caused by ammonium
salts, given by gavage, indicative of both pulmonary and nervous
system dysfunction, were reported in less than 30 min in guinea-
pigs and rats (Koenig & Koenig, 1949) including:
(i) a rapid increase in the rate and depth of respiration;
(ii) weakness and difficulty in locomotion;
(iii) hyperexcitability for tactile, auditory, and painful
stimuli; and
(iv) muscle fasciculations over most of the body followed
by generalized tonic convulsions and then coma.
The respiratory rate was greatly reduced but the depth of
respiration increased and was accompanied by gasping and stridor.
Histological changes occurred in both the lung and brain, while
oedema, congestion, and haemorrhage were found principally in the
lung (Koenig & Koenig, 1949; Cameron & Shiekh, 1951).
The addition of ammonium chloride to rat feed resulted in
reduced dietary consumption (Motyl & Debski, 1977). The results of
studies by Noda & Chikamori (1976) pointed to a direct effect of
the ammonium ion on the brain area that regulates feeding. Rats
with bilateral lesions in the prepyriform cortical area of the
brain consumed as much diet containing 3% ammonium chloride as
basal diet. A unilateral injection of 10 mg/litre of 2% NH4Cl/kg
body weight into prepyriform cortical areas, in contrast to an
injection into other areas of the brain, or an injection of sodium
chloride, significantly reduced the food intake of 6 adult male
Wistar rats. The results of these studies suggest that ammonium
ions directly influence appetite by their effects on prepyriform
cortical areas.
(b) Kidney
Lotspeich (1965) reported that ingestion for 7 days of
0.28 mol/litre (1.5%) ammonium chloride in water (ad lib) produced
renal hypertrophy with new cell formation and an enlargement of
existing cells. Some animals from each of the acidotic and control
groups were subjected to unilateral nephrectomy. Highly
significant increases in kidney wet weight, dry weight, and total
nitrogen were observed in the acidotic group. Similar changes were
produced by unilateral nephrectomy. In unilaterally nephrectomized
rats made acidotic by the ingestion of ammonium chloride, the
remaining kidney was larger than that seen in animals with
unilateral nephrectomy without induced acidosis, suggesting an
additive effect of unilateral nephrectomy and ammonium chloride
intake.
The relationship between the renal hypertrophy and the ammonium
ion, acidosis, non-specific intake of nitrogen, or increased solute
load was examined (Lotspeich, 1965). Isomolar (0.28 mol/litre)
solutions of sodium chloride and ammonium chloride produced renal
hypertrophy. The increase due to sodium chloride was less marked
(it was noted that the sodium chloride group drank 3 or 4 times as
much solution each day as the ammonium chloride group). Isomolar
(0.2 mol/litre) solutions of sodium bicarbonate and ammonium
citrate did not induce renal hypertrophy. When 3 groups of 6 rats
were given drinking-water containing 0.28 mol/litre sodium
chloride, 0.28 ammonium chloride, or a urea solution containing
nitrogen equivalent to that in the 0.28 mol/litre ammonium
chloride, for 7 days, only the ammonium chloride solution induced
acidosis. The rats in both the sodium chloride and ammonium
chloride groups had larger kidneys than the urea-drinking rats,
though the kidneys of the sodium chloride-drinking rats were not as
large as those of the ammonium chloride-drinking rats. Since renal
hypertrophy was produced by solutions containing the chloride ion,
while ammonium citrate and urea did not cause any hypertrophy, the
evidence does not support the hypothesis that renal hypertrophy is
due to the ammonium ion.
Thompson & Halliburton (1966) also reported renal hypertrophy
in rats that ingested 3% ammonium chloride in the diet for 6 days.
The ingestion of ammonium citrate or sodium chloride in amounts
that were equivalent to that of the ammonium chloride did not cause
renal hypertrophy.
Janicki (1970) examined the kidneys of rabbits that had been
administered 16.2 g ammonium chloride, by gavage, over a period of
2 days and found the epithelium of the convoluted tubules swollen,
vacuolated, and completely filling the lumen; nuclei exhibited
karyolysis. Findings were similar in 4 rabbits administered 5 -
7 g ammonium chloride, by gavage, over a period of 3 - 7 days.
However, hyperaemia of the renal cortex of rabbits was noted
after the administration, by gavage, of single doses of 0.8 and 1 g
ammonium carbonate/kg body weight (Yoshida et al., 1957). As the
carbonate ion does not have an acidifying effect, this suggests
that the ammonium ion produces some effects on the kidney.
8.1.2.3. Influence of diet on the effects of ammonia
In 6 mongrel dogs, blood-urea levels were increased by a higher
level of protein intake (6 g protein/kg per day) (Bressani &
Braham, 1977). Levels of ~30 mg BUN/litre were found 8 h after the
daily feed. When the protein intake was low (4 mg protein/kg per
day), BUN levels of 14 mg/litre were reported 8 h after the daily
feed. The frequency of protein intake did not affect the maximum
value of blood-urea levels when the protein intake was low (4 mg
protein/kg per day). This suggests that high levels of urea and
ammonia in the blood might occur in animals that suffer from liver
insufficiency and are fed high-protein diets.
Kulasek et al. (1975) studied the effects of nitrogen in the
diet on ammonia detoxification, using 210 rats and 6 diets
containing 50, 150, and 700 g casein/kg (low-, optimal-, or high-
protein diet, respectively), with or without the addition of
ammonium chloride at 20 g/kg. After 8 or 9 days of feeding, the
LD50 from an intraperitoneal injection of a 2.7% solution of
ammonium chloride was determined. The LD50 increased in proportion
to the amount of nitrogen in the diet. The data suggest that
higher doses of exogenous ammonia were tolerated by rats on
protein-rich diets or diets containing an ammonium salt as an
additive. An adaptive capacity for ammonia detoxification was
further demonstrated by Motyl & Debski (1977) in a study using rats
fed low-protein diets with the addition of NH4Cl. The LD50 values
for the ip injection of 2.7% NH4Cl were higher in animals fed low-
protein diets supplemented with NH4Cl (Table 18). Stevens et al.
(1975) raised weanling rats for 3 - 6 weeks on a protein-deficient
diet. Subsequent challenge by a large, intraperitoneally-injected
dose of ammonium chloride indicated that severe protein deprivation
increased vulnerability to ammonia poisoning compared with that of
control groups not prefed protein-deficient diets. Thus, with the
exception of animals suffering from liver dysfunction, animals on
high-protein diets seem able to tolerate a higher oral intake of an
ammonium salt.
A group of eight, 6- to 8-month-old cats, given a single meal
of a complete amino acid diet without arginine, developed
hyperammonaemia with elevated plasma levels of glucose and ammonia
and showed clinical signs of ammonia toxicity within 2 h. One cat
died 4.5 h after ingesting only 8 g of the diet. Five of the
surviving cats given a single meal of complete amino acids, in
which arginine was replaced with an equivalent amount of ornithine,
did not show any unusual signs. This finding indicates that the
cat is unable to synthesize ornithine at the rate required by the
urea cycle to dispose of ammonia from amino acid catabolism;
however, the dietary requirements for cats may differ from those of
other adult animals, including human beings, because the cat has a
much higher protein requirement, i.e., it requires approximately
20% of dietary calories as protein, as opposed to only 4 - 8%
required by the rat, dog, sheep, and man (Morris & Rogers, 1978).
The lethal effects of ammonia poisoning have been prevented by
the amino acids, ornithine and aspartic acid. To test whether this
was mediated by a sparing action of adenosine triphosphate (ATP) on
ammonia metabolism, 20 dogs with chronic Eck's fistula were
injected in the duodenum with ammonium acetate at 4.1 mmol/kg body
weight (~219.4 mg/kg). Half of the group was given 2 mg ATP/kg,
1 h prior to administration of the ammonium acetate. In 9 of the
10 dogs, ATP prevented a rise in levels of ammonia in venous blood.
Grossi et al. (1968) administered ATP (2 mg/kg) intravenously to 6
dogs with chronic Eck's fistula and on high-protein diets. Within
1 h, high blood-ammonia levels returned to normal. In chronic
liver disease, there may be insufficient ATP available for ammonia
detoxification, resulting in hyperammonaemia.
8.1.3. Dermal exposure
No data are available regarding any systemic effects of dermal
exposure to ammonia or ammonium compounds.
8.1.4. Effects due to parenteral routes of exposure
The parenteral toxicity of ammonia and ammonium compounds has
been studied extensively. Toxicity is influenced by the route of
administration, e.g., with oral administration, there is the
capacity for detoxification of exogenous ammonia by the liver.
8.1.4.1. Lethality
The intravenous (iv) and intraperitoneal (ip) LD50 values for a
number of ammonium compounds in various species are summarized in
Table 19. The toxic syndrome was similar in all species studied.
The signs, after iv injection, were characterized by immediate
hyperventilation and clonic convulsions followed by either fatal
tonic extensor convulsion or the onset of coma in 3 - 5 min. The
animals remained comatose for approximately 30 - 45 min. At this
stage, tonic convulsions and death can occur at any time, but
animals that survive usually recover rapidly and completely
(Warren, 1958; Warren & Schenker, 1964; Wilson et al., 1968a,b).
After intraperitoneal injection, the signs did not appear until
15 - 20 min after administration (Greenstein et al., 1956; Wilson
et al., 1968b).
Table 19. Toxicity of several ammonium compounds in selected species
-----------------------------------------------------------------------------------------------------------
Ammonium Species Intravenous dose Intraperitoneal dose
compound (mmol/kg of body weight) (mmol/kg of body weight)
(LD50) (LD50) Reference
-----------------------------------------------------------------------------------------------------------
Acetate rat - 8.20 Greenstein et al. (1956)
mouse 6.23 - Warren (1958)
mouse 5.64 10.84 Wilson et al. (1968a)
chick 2.27 10.44 Wilson et al. (1968a)
Bicarbonate mouse 5.05 - Warren (1958)
mouse 3.10 - Wilson et al. (1968b)
Carbamate mouse 0.99 - Wilson et al. (1968b)
Carbonate mouse 4.47 - Warren (1958)
mouse 1.02 - Wilson et al. (1968b)
Chloride mouse 6.75 - Warren (1958)
mouse (38.8 °C)a 6.6 - Warren & Schenker (1964)
mouse (40.4 °C)a 5.17 - Warren & Schenker (1964)
mouse (27.9 °C)a 10.21 - Warren & Schenker (1964)
Hydroxide mouse 2.53 - Warren (1958)
-----------------------------------------------------------------------------------------------------------
a Body temperature.
8.1.4.2. Central nervous system effects
Navazio et al. (1961) observed that characteristic toxic signs
were not observed in rats until the ammonia concentration in the
blood was double that of basal values (attained within 8 - 10 min
of an ip injection of 601 mg ammonium acetate/kg body weight). No
substantial increase in brain-ammonia was observed. However, when
the blood-ammonia concentration reached more than 20 times the
basal value, there was a sudden rise in the ammonia concentration
in the brain, which reached a maximum of approximately 100 mg
ammonia-nitrogen/kg (wet weight), between 10 and 26 min after
injection. Muscular contractions with occasional tetanic spasms,
and then coma occurred, when the concentration of ammonia in the
brain reached approximately 50 mg/kg. Although the animals started
to recover from the comatose state approximately 70 min after
onset, blood- and brain-ammonia concentrations did not return to
basal levels until 2 h after the injection of ammonium acetate.
However, other authors observed an immediate increase in brain-
ammonia after ip injection of ammonium acetate (Torda, 1953; du
Ruisseau et al., 1957; Salvatore et al., 1963). These authors
found dramatic increases in the brain-ammonia content, 2 - 5 min
after the injection of ammonium acetate. Salvatore et al. (1963)
suggested that there was no critical blood-ammonia concentration
for diffusion through the blood-brain barrier.
Ammonia has been shown to be more highly toxic at elevated body
temperature, whereas hypothermia affords marked protection
(Schenker & Warren, 1962). The LD50 values for ammonium chloride
in the mouse, at various body temperatures, are shown in Table 19.
The increased toxicity of ammonia at elevated body temperature was
suggested to be due to a direct metabolic effect of hyperthermia on
the brain, unrelated to dehydration or stress.
8.1.4.3. Effects on the heart
Intravenous LD50 values for ammonium carbamate, ammonium
carbonate, and ammonium bicarbonate have been determined in mice by
Wilson et al. (1968a,b) (Table 19). The physiological effects of
the injected ammonium compounds in dogs and sheep were also
investigated. Electrocardiograms recorded during the toxic
syndrome indicated that the animals died from ventricular
fibrillation due to a direct effect of ammonia on the heart. These
findings were in agreement with the effects noted by Berl et al.
(1962) during the iv infusion of ammonium chloride in cats when
electrocardiograms were altered in a complex manner. However,
Warren & Nathan (1958) were unable to demonstrate any cardiotoxic
effects of the ammonium compounds in mice and concluded that the
toxicity syndrome was due primarily to a cerebral effect and not a
direct effect on cardiac or skeletal muscle.
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
When 48 rats were continuously exposed to 127 mg ammonia/m3
(181 ppm) for 90 days, no abnormalities were found in organs or
tissues. Inhalation of 262 mg ammonia/m3 (374 ppm) for 90 days
induced mild nasal irritation in about 25% of 49 rats and a mild
leukocytosis in 4 of the rats. Continuous exposure to 455 mg/m3
(650 ppm) resulted in the death of 50 out of 51 rats by the 65th
day of exposure. All animals exhibited mild nasal discharge and
laboured breathing. In a second study, rats, guinea-pigs, rabbits,
and dogs were continuously exposed to 470 mg/m3 (671 ppm) for 90
days. Thirteen out of 15 rats and 4 out of 15 guinea-pigs died.
Marked eye irritation was noted in rabbits and dogs, with corneal
opacities in about one-third of the rabbits. At autopsy, all test
animals examined had more extensive focal or diffuse interstitial
inflammatory processes in the lungs than the controls (Coon et al.,
1970).
White rats were exposed to ammonia, by inhalation, at
concentrations of 100 and 30 mg/m3 (143 and 43 ppm) for 25 or
60 min, every 48 h, for a period of 3 months. Rats exposed to a
concentration of 100 mg/m3 (143 ppm) showed only a mild
leukocytosis, with no significant differences from the control
group with regard to oxygen consumption, neuromuscular excitation
threshold, heart rate, blood-sugar, blood-residual nitrogen, and
total serum-protein (Prokop'eva & Yushkov, 1975). In another study
by Alpatov & Mikhailov (1963), the threshold level for toxic
effects in a 2-month exposure was 40 mg/m3 (57 ppm). Histological
changes were seen in the lungs of the animals exposed to a
concentration of 100 mg/m3 (143 ppm), including small areas of
interstitial pneumonia with signs of peribronchitis and
perivasculitis. No changes were seen in other organs compared with
those in the control group.
Broderson et al. (1976) exposed Sherman and Fisher rats to
ammonia from natural sources, at an average concentration of
105 mg/m3 (150 ppm) for 75 days, and to purified ammonia at
175 mg/m3 (250 ppm) for 35 days. Histological changes in the
olfactory and respiratory epithelia of the nasal cavity were
similar in all the exposed rats, showing increased thickness,
pyknotic nuclei, and hyperplasia. The submucosa was oedematous
with marked dilation of small vessels. Lesions decreased
posteriorly.
Exposure to ammonia at concentrations of 17 - 175 mg/m3 (25 -
250 ppm) increased the infectious effects of Pasteurella multocida
in mice and Mycoplasma pulmonis in rats; this effect increased
with the concentration of ammonia (Broderson et al., 1976; Richard
et al., 1978a).
Richard et al. (1978a) selected a concentration of 350 mg/m3
(500 ppm) for a study of effects of continuous exposure to ammonia
after noting that general toxic effects, particularly on growth
rate, were not found at 175 - 210 mg/m3 (250 - 300 ppm). Young
male specific-pathogen-free rats were age- and weight-matched with
controls (27 per group) and exposed for up to 8 weeks. Nasal
irritation began on the fourth day. After 3 weeks, exposed rats
showed nasal irritation and inflammation of the upper respiratory
tract, but no effects were observed on the bronchioles and alveoli.
The number of pulmonary alveolar macrophages was similar to that in
the controls. After 8 weeks, none of these inflammatory lesions
were present.
In a study by Coon et al. (1970), 15 rats, 15 guinea-pigs, 3
rabbits, 2 dogs, and 3 monkeys were exposed to an ammonia
concentration of 155 mg/m3 (221 ppm) for 8 h/day, 5 days a week,
for 6 weeks. Pathological effects were not observed in any of the
species except for evidence of focal pneumonitis in the lungs of
one of the monkeys. Exposure to 770 mg/m3 (1100 ppm) for the same
duration induced mild to moderate eye irritation and laboured
breathing in the rabbits and dogs at the beginning of exposure, but
these signs disappeared by the second week and no other signs of
irritation or toxicity were noted. Autopsy findings included non-
specific inflammatory changes in the lungs of the rats and guinea-
pigs.
Male Sprague Dawley rats were studied to determine whether
ammonia was absorbed through the lungs into the blood, and the
subsequent effects on the blood pH, blood gases, and hepatic drug-
metabolizing enzymes (Schaerdel et al., 1983). Rats were exposed
to ammonia at 7 - 840 mg/m3 (10 - 1200 ppm) for 1, 3, or 7 days.
No significant changes were found in blood pH, pCO2, or in the
histological appearance of the lungs or trachea. Liver microsomal
enzymes (ethyl-morphine- N-demethylase and cytochrome P-450) showed
only minor changes. Blood-ammonia levels increased in a linear
fashion with increasing ammonia concentrations in air. A
concentration of 70 mg/m3 (100 ppm) or less produced only very
small changes in blood-ammonia levels and had no measureable
effects on any of the parameters studied.
8.2.2. Oral exposure
8.2.2.1. Histopathological effects
Rats given ammonium salts, for periods ranging up to 90 days,
did not appear to sustain renal damage (Freedman & Beeson, 1961;
Gupta et al., 1979). Twelve adult male Sprague Dawley rats were
given ammonium chloride in the drinking-water at 16 g/litre, for up
to 3 weeks. Urinalysis did not reveal any evidence of renal
injury, and no gross or histological "abnormalities" of the kidney
were seen at autopsy (Freedman & Beeson, 1961). Glutaminase
activity per kg of kidney increased with duration of exposure; this
is a physiological adaptation to acidosis. Another group of 10
rats, similarly treated and then given ammonium chloride in the
drinking-water at 10 g/litre, for an additional 2.5 months also did
not show any gross or microscopic renal abnormalities. Assuming
the rats weighed 250 g and consumed 25 ml of water per day, they
could have ingested as much as 1.6 g/kg body weight per day while
drinking the ammonium chloride at 16 g/litre and 1.0 g/kg body
weight per day while drinking a level of 10 g/litre. However,
actual intake may have been lower, because ammonium chloride is
known to affect the appetite and may render the water less
palatable. When ammonium chloride was given at 20 g/litre, food
and water consumption were drastically reduced.
In Table 20, selected studies are presented on ammonium salts
other than the chloride. In general, at low doses, no detrimental
effects were observed when rats and pigs were exposed to the NH4
salts listed.
In a 90-day study by Gupta et al. (1979) on rats, there was not
any evidence of renal damage. Ammonium sulfamate (NH4SO3NH2) as a
100 g/litre solution was given orally at rates of 100, 250, or
5000 mg/kg body weight per day, 6 days a week for 30, 60, or 90
days to adult female rats (ITRC colony-bred albino) and to weanling
male and female rats (20/sex per age per dose level). Under
certain conditions, the sulfamate ion is hydrolysed to bisulfate
ion and ammonia; however, it is unclear whether, and to what
extent, hydrolysis occurs in the rat intestine. Equivalent doses,
listed in Table 20, were calculated on the assumption that no
hydrolysis occurs. The effects of the anion, sulfamate and/or
sulfate, on the action of ammonium is a matter of conjecture.
Ammonium sulfamate would be expected to produce metabolic acidosis
on the basis of its structure, but this has not been verified
experimentally. The general health of both treated and control
rats was good, and there were no significant differences in mean
body weights throughout the study, except for a slight depression
in the highest-dose adult females at 60 and 90 days. Food and
water consumption were unaffected, except in the highest dose
weanlings of both sexes, which consumed less food and drank more
water than control weanlings. Interim sacrifice of 6 animals/sex
per dosage group was performed for haematological and histological
examination. There were no significant differences in
haematological values (packed cell volume, haemoglobin
concentration, total red cell count, total and differential white
cell count). Relative organ weights in all treated groups did not
differ significantly from those in the controls. Histological
examination of the kidney, liver, lung, stomach, heart, spleen,
thyroid, adrenal, gonads, intestine, and lymph nodes did not reveal
any abnormalities, except slight hepatic fatty degeneration in one
adult female at 90 days.
Table 20. Dose-response data for short-term oral administration of ammonium compounds
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Sulfamate rat, albino, orally as 10% 30, 60, 100; 15.0; no effects on body Gupta et
20 adult solution; not 90 days 250 37.3 weight, food, and al. (1979)
female, 20 specified whether water consumption,
weanling given by gavage haematological values
male, 20 or organ weights; no
weanling histological
female per abnormalities in
dose organs (including
kidneys)
500 74.8 slight decrease in
body weight in adult
females; slightly
lower food and higher
water consumption in
weanlings of both
sexes; no effects on
haematological values
or organ weights; no
histological
abnormalities in
organs (including
kidneys)
------------------------------------------------------------------------------------------------------------------- kidneys)
Table 20. (contd.)
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Diammonium pig, male 3.75% diammonium 28 days 1820 274 body weight gain Kagota et
citrate 22.5 kg, citrate in diet increased but % meat al. (1979)
3-4/group containing 6.4% crude and fat of carcass
protein unaffected; plasma-
and urine-urea-
nitrogen increased
but blood-ammonia-
nitrogen level
unaffected; no effect
on haematological
values; no gross
abnormalities or
lesions
Diammonium pig, 27.3 kg basal diet contained 81 days 820 161 (10% no effect on weight Wehrbein
phosphate 3 males and 16% protein; 0,5,10, replacement) gain, BUN, or food et al.
and 3 females/ or 20% of dietary N consumption (no (1970)
diammonium group was replaced by N effect on these
citrate as from equimolar parameters at 5%
equimolar mixture of designated 81 days 1600 322 (20% either) slight
mixture NH4+ salts replacement) decrease in weight
gain, BUN and food
consumption
-------------------------------------------------------------------------------------------------------------------
a Estimated for all studies except Gupta et al. (1979).
b Equivalent intake expressed as ammonia (NH3).
Treatment of virgin female rabbits with ammonium carbonate,
chloride, hydrophosphate, sulfate, or hydroxide at 0.1 - 0.2 g/kg
body weight, orally, on alternate days, for periods of 3 weeks
separated by 1-week intervals of no treatment, was associated with
enlargement of the ovaries, follicle maturation, and formation of
corpora lutea (Fazekas, 1949). There was also enlargement of the
uterus, hypertrophy of the teats, and secretion of milk.
Rabbits (10 - 80 per group) were given 0.1 - 0.2 g/kg body
weight of an ammonium salt in 100 - 150 ml of drinking-water, twice
daily. One hundred and sixty rabbits (96 female, 64 male)
received, twice daily, 50 - 80 ml of a 0.5% ammonium hydroxide
solution (~135 - 210 mg/kg body weight), in gradually increasing
doses. The chemicals were given for periods of 3 weeks separated
by 1-week intervals of no treatment. In a related study, similar
treatment of rabbits with ammonium chloride or ammonium sulfate
resulted in fluctuations in serum-calcium and -phosphorus levels
(Fazekas, 1954a).
8.2.2.2. Effects of ammonium as a dietary nitrogen supplement
Ammonia from ammonium salts can stimulate the growth of animals
on diets deficient in non-essential amino acids or restricted in
protein content. Weanling rats, given an artificial diet
comprising essential amino acids, B vitamins, vitamin C, salts, and
glucose for 21 days, grew poorly. Rats given a similar diet in
which some of the glucose was replaced with 8.6% ammonium acetate
(equivalent to 15.7 g nitrogen/kg diet) showed a dramatically
improved weight gain (Birnbaum et al., 1957). The food
consumption/weight gain ratio (feed/gain ratio) was also improved
in animals receiving the ammonium salt.
Similar results were obtained with young male pigs given a more
natural basal diet restricted in non-essential amino acids and
containing the minimum requirement of essential amino acids (Kagota
et al., 1979). The crude protein level of this basal diet was
6.4%. Three pigs fed the basal diet supplemented with 3.75%
diammonium citrate had a significantly greater weight gain and a
slightly lower feed/gain ratio than 4 pigs fed the basal diet (the
diets were made isocaloric by adjusting carbohydrate). No
significant differences in the packed cell volume or ammonia-
nitrogen level of the blood or in total protein concentration of
plasma were found between the 2 groups. Plasma- and urine-urea-
nitrogen were increased in the pigs fed the diet supplemented with
diammonium citrate. Autopsy of the pigs after 28 days on the basal
or ammonium citrate diets did not reveal any lesions or
abnormalities or any differences in the percentage of carcass meat
and fat. The average daily food intake for pigs receiving
diammonium citrate was 1.44 kg, which corresponds to 54 g
diammonium citrate per day. Using a body weight midway between the
initial (22.5 kg) and final (36.7 kg) body weights, the mean intake
of diammonium citrate can be estimated to be 1.82 g/kg body weight,
per day, or 16.1 mEq NH4+/kg body weight, per day.
Addition of ammonium salts to diets with a higher protein
content (10% crude protein) did not produce significant changes in
weight gain in rats or pigs (Clawson & Armstrong, 1981).
Similarly, no significant changes in weight gain occurred in pigs,
when up to 10% of the nitrogen from crude protein (diet = 16% crude
protein) was replaced with nitrogen from ammonium salts (Wehrbein
et al., 1970). Replacement of 20% of the dietary nitrogen with
ammonium salts slightly decreased body weight gain in the pigs;
food consumption and BUN also decreased. The 20% replacement diet
contained 1.54% diammonium phosphate and 2.07% diammonium citrate
for a total of 3.61% ammonium salts and was fed for 81 days. The
estimated mean intake was 0.70 g diammonium phosphate and 0.94 g
diammonium citrate/kg body weight per day, giving a total of
18.9 mEq NH4+/kg body weight per day.
8.2.3. Dermal exposure
There is no information regarding systemic toxicity from short-
term dermal exposure to ammonia or ammonium compounds.
8.3. Skin and Eye Irritation; Sensitization
Ammonia in the form of a gas, an anhydrous liquid boiling at
low temperatures, or an aqueous solution is a recognized skin and
eye irritant. Most of the information is human clinical data and
is described in section 9.
Ammonia, partly because of its lipid solubility, penetrates
the corneal membrane rapidly (NRC, 1979). In rabbits, conjunctival
oedema with ischaemia and segmentation of limbal vessels were noted
within 30 min. By 24 h, there was a reduction in the
mucopolysaccharide contents of the corneal stroma, and extensive
polymorphonuclear infiltration and anterior lens opacities were
apparent. Aqueous levels of glucose and ascorbate and intraocular
pressure were depressed (NRC, 1979). In rabbits with corneal
burns, neovascularization occurred after 1 week, but it was delayed
in animals with corneal and limbal burns. Complications of severe
burns included symblepharon, pannus, pseudopterygia, progressive or
recurrent corneal ulcerations leading to perforations, permanent
corneal opacity, corneal staphyloma, persistent iritis, phthisis
bulbi, secondary glaucoma, and dry eye (NRC, 1979).
Ammonium persulfate is a recognized skin sensitizer for human
beings. There are no data on its sensitization potential in animal
models.
8.4. Long-Term Exposures
8.4.1. Inhalation exposure
Weatherby (1952) exposed 12 guinea-pigs to an ammonia
concentration of about 119 mg/m3 (170 ppm). Chamber concentrations
ranged from 98 to 140 mg/m3 (140 to 200 ppm) for 6 h/day, 5 days a
week, for up to 18 weeks. There were no significant findings at
autopsy in animals sacrificed after 6 or 12 weeks of exposure. In
animals sacrificed after 18 weeks of exposure, there was congestion
of the liver, spleen, and kidneys, with early degenerative changes
in the adrenal glands. Increased erythrocyte destruction was
suggested by increased quantities of haemosiderin in the spleen.
In the proximal tubules of the kidneys, there was cloudy swelling
of the epithelium and precipitated albumin in the lumen with some
casts. The cells of the adrenal glands were swollen and the
cytoplasm in some areas had lost its normal granular structure.
Coon et al. (1970) conducted studies in which rats, guinea-
pigs, rabbits, dogs, and monkeys were continuously exposed
(24 h/day, 7 days per week) to ammonia. No signs of toxicity were
seen in any species following continuous exposure to ammonia at a
concentration of 40 mg/m3 (57 ppm) for 114 days, and gross and
microscopic examination did not reveal any lung abnormalities.
8.4.2. Oral exposure
The administration of ammonium carbonate, chloride, sulfate,
hydrophosphate, acetate, lactate, or hydroxide to a total of 296
rabbits for 3 - 16 months resulted in enlargement of the
parathyroids (Fazekas, 1954a). Administration of sodium
dihydrophosphate, sodium ammonium phosphate, calcium chloride,
hydrochloric acid, acetate acid, or lactic acid gave similar
results (Fazekas, 1954a).
Rabbits given 0.1 g ammonium hydroxide/kg body weight (as a
0.5 - 1.0% solution) by gavage, initially, on alternate days and
then daily for up to 17 months, had enlarged adrenal glands
(Fazekas, 1939). An initial fall in blood pressure of 2.67 -
4.00 kPa (20 - 30 mmHg) was followed by a gradual rise to 1.33 -
4.00 kPa (10 - 30 mmHg) above the baseline, after several months
of treatment.
The results of studies on rats, rabbits, and dogs indicate that
long-term administration of ammonium chloride can induce
osteoporosis (Seegal, 1927; Bodansky et al., 1932; Jaffe et al.,
1932a,b; Barzel & Jowsey, 1969; Barzel, 1975). During prolonged
metabolic acidosis, the release of bone mineral by resorption is
thought to provide additional buffering capacity, sparing
bicarbonate.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The interaction of ammonium chloride with other teratogenic
agents has been investigated in 2 studies. Goldman & Yakovac
(1964) used ammonium chloride to investigate the role of metabolic
acidosis in salicylate-induced teratogenesis in Sprague Dawley
rats. Beginning on day 7 of gestation, rats received either
0.17 mol/litre (0.9%) ammonium chloride in the drinking-water, a
single subcutaneous injection of salicylate, or both. Ammonium
chloride alone inhibited fetal growth, but was not teratogenic.
However, when administered with salicylate, ammonium chloride
significantly increased maternal and fetal mortality and the fetal
anomaly rate (dorsal midline, ventral midline, and eye defects)
compared with that due to salicylate alone. These effects were
attributed to acidosis and not to ammonia. However, it has been
shown by Miller (1973) that the addition of 0.5% ammonium chloride
to the 5%-glucose drinking-water of fasting, pregnant CFW mice
significantly reduced the incidence of fast-induced cleft palate in
the progeny.
8.6. Mutagenicity
Ammonium sulfate was reported to be non-mutagenic in the
Salmonella and Saccharomyces systems (Litton Bionetics, Inc.,
1975). Demerec et al. (1951) tested ammonia for its ability to
induce back-mutations from streptomycin dependence to non-
dependence in Escherichia coli and found it to be mutagenic, but
only in treatments that left less than 2% survivors. Iwaoka et al.
(1981a,b) showed that extraction of ingredients from fried
hamburger and refrigerated biscuit products with ammonium hydroxide
or ammonium sulfate increased the mutagenic activity in
S. typhimurium TA98 and TA1538, compared with sodium sulfate
extraction. This suggests that ammonium salts may, in some way,
influence the mutagenic activity of some agents, or may themselves
be responsible for false positive findings (Iwaoka et al., 1981a).
However, it is also possible that ammonium salts extract mutagenic
components from foods more efficiently.
Lobashov & Smirnov (1934) and Lobashov (1937) found ammonia to
have a mutagenic effect on Drosophila. Ammonia showed slight
mutagenic activity in studies in which survival of Drosophila was
lower than 2% after treatment. In studies by Auerbach & Robson
(1947), when Drosophila was exposed to ammonia vapour in small
glass containers, 0.5% sex-linked lethals were observed.
It was reported by Rosenfeld (1932) that ammonia induced
clumping of chromosomes, arrest spindle formation, and induce
polyploidy in chick fibroblasts in vitro.
There are no data to show that ammonia is mutagenic in mammals.
8.7. Carcinogenicity
There is no evidence indicating that ammonia is carcinogenic.
Gibson et al. (1971) observed that animals treated with ammonia
developed inflammatory lesions of the colon. It has been suggested
that cell proliferation may increase errors in DNA replication
(Zimber, 1970; Zimber & Visek, 1972), activate oncogenic agents
present in sub-threshold doses (Anderson et al., 1964), and even
unmask latent changes in the genetic material caused by mutagenic
agents (Visek et al., 1978).
In a study on male Sprague Dawley rats (Topping & Visek, 1976),
there was no evidence that ammonia increased the incidence of
tumours with increased protein intake. Development of lung
tumours was observed in CFLP mice treated intra-gastrically with
diethyl pyrocarbonate and ammonia, neither of which induces cancer
independently in animals. However, this may have resulted from a
carcinogenic substance, possibly urethane, formed in vivo from
diethyl pyrocarbonate in the presence of ammonia (Uzvolgyi & Bojan,
1980).
Life-long ingestion of ammonium hydroxide in the drinking-water
by Swiss and C3H mice did not produce any carcinogenic effects, and
had no effect on the spontaneous development of adenocarcinoma of
the breast in C3H females, a characteristic of this strain (Toth,
1972).
Ammonium chloride and ammonium tungstoantimonate and related
compounds have been shown to have an inhibitory effect on malignant
cells (Phillips, 1970; Anghileri, 1975; Flaks & Clayson, 1975;
Flaks et al., 1973; Sof'ina et al., 1978).
8.8. Factors Modifying Effects
8.8.1. Synergistic effects
Stevens et al. (1975) raised weanling rats for 3 - 6 weeks on a
protein-deficient diet. Subsequent challenge by a large,
intraperitoneally-injected dose of ammonium chloride indicated that
severe protein deprivation increases vulnerability to ammonia
poisoning in comparison with control groups not prefed protein-
deficient diets.
Dalhamn (1963) employed a technique in rabbits in which an air
pollutant mixture was drawn into the nostrils and out through a
tracheal cannula. The effects of ammonia alone and ammonia mixed
with (and presumably adsorbed on) carbon particles, on the beating
rate of tracheal cilia was examined. Ammonia alone had to be
administered at a high concentration (1400 mg/m3 or 2000 ppm) to
produce a tracheal concentration of 70 mg/m3 (100 ppm). The
reduction in beating rate was not substantially altered by the
addition of carbon particles at 2 mg/m3.
Simultaneous injection of ammonium salts and a fatty acid into
rats or cats produced coma at lower plasma levels of ammonia and
free fatty acids than a single injection of either compound (Zieve
et al., 1974).
Impaired handling of an ammonium load has been observed in
animals with hepatic dysfunction. Elevated levels of ammonia or
urea have been reported in dogs with experimentally-bypassed livers
(section 7.1.2). The importance of the kidney in detoxification of
an ammonium load is apparent in the studies of Lotspeich (1965)
(section 8.1.2.2), where an additive hypertrophic effect of
ammonium chloride and unilateral nephrectomy was observed on the
remaining kidney. Increased sensitivity to ammonia toxicity was
found in young rats (Benyajati & Goldstein, 1975) and in rats that
had been castrated, adrenalectomized, or thymectomized (Paik et
al., 1975).
8.8.2. Antagonistic effects
L-arginine and the related amino acids, ornithine and aspartic
acid, substrates from urea synthesis, have been reported to exert a
protective effect against acute ammonia poisoning in rats, dogs,
and cats (Morris & Rogers, 1978; NRC, 1979).
High-protein diets exert an antagonistic effect on the toxicity
of ammonium salts, unless liver dysfunction is present (section
7.1.2.4). In addition, an increase in toxic response has been
observed with increase in age due to the adaptive response of
glutaminase activity in rats to ammonia detoxication during
acidosis.
8.9. Mechanisms of Toxicity
Hypotheses proposed for the mechanism of ammonia toxicity
include impaired decarboxylation of pyruvic acid (McKhann & Tower,
1961), NADH depletion slowing down the generation of ATP (Worcel &
Erecinska, 1962), depletion of alpha-ketoglutarate resulting in
impairment of the Krebs cycle (Bessman & Bessman, 1955; Fazekas et
al., 1956; Warren & Schenker, 1964); depletion of ATP due to
glutamine formation by the glutamine synthetase (EC 6.3.1.2) system
(Warren & Schenker, 1964; Nakazawa & Quastel, 1968), stimulation of
membrane ATPase (EC 3.6.1.8) producing increased nerve cell
excitability and activity (Hawkins et al., 1973), and depletion of
ATP causing a decrease in cerebral acetylcholine (Braganca et al.,
1953; Ulshafer, 1958).
9. EFFECTS ON MAN
9.1. Organoleptic Aspects
9.1.1. Taste
Campbell et al. (1958) determined the taste threshold
concentration for ammonia in redistilled water with 21 - 22
subjects in "difference tests of the triangle type". At ammonia
concentrations of 26, 52, and 105 mg/litre, the percentages of
correct identifications were 61.9, 71.4, and 85.7, respectively.
Defining the threshold concentration as the level at which
correct identification is 50% greater than that expected by
chance, the taste threshold of ammonia was determined to be
around 35 mg/litre. However, this definition of the threshold
concentration seems to be somewhat arbitrary and McBride & Laing
(1979) have reported significant positional bias in using the
triangle test to determine taste thresholds. Furthermore, the
triangle test is not intended to mimic environmental exposures
in which the taste thresholds could be substantially higher. It
seems reasonable to conclude only that the palatability of water
is not likely to be significantly affected by total ammonia levels
of < 35 mg/litre (as NH3), but will be affected at higher levels.
9.1.2. Odour
Odour thresholds reported in the literature may vary according
to the definition of odour response, mode of presentation of the
stimulus, chemical purity of the agent used, and the number of
subjects and trials in the study. The detection threshold for
ammonia, defined as the concentration that produces the first
detectable difference in odour over background, was reported to be
37 mg/m3 (DallaValle & Dudley, 1939). This reference, though often
cited, gives no information on the study design from which this
number was derived.
The considerable variability in threshold data prompted work by
Leonardos et al. (1969), who used a standardized procedure to
determine recognition thresholds rather than detection thresholds
for 53 chemicals. The odour threshold was defined as the first
concentration at which all four panel members (trained odour
analysts) were able to recognize the characteristic odour of the
chemical. The panel tested only one chemical per day.
Concentrations examined were multiples by 10 of 0.7, 1.47, and
3.3 mg/m3 (1, 2.1, and 4.6 ppm). The recognition threshold for the
odour of ammonia was 32.6 mg/m3 (46.8 ppm).
The results of several other studies suggest that human beings
can detect ammonia at much lower levels. Stephens (1971) reported
2.7 mg/m3 (3.9 ppm) as the lowest concentration producing an odour
response, when a contaminated air stream and a reference stream
were compared by sniffing (number of subjects was not reported). A
report by Saifutdinov (1966), of an olfactory threshold of 0.55 -
0.50 mg/m3 for the most sensitive of 22 subjects, did not include
sufficient detail to evaluate this excessively low estimate.
Carpenter et al. (1948) stated that "a group of 8 persons found
that the least odour they could detect on entering a room
containing various concentrations" was 0.7 mg/m3 (1 ppm) ammonia.
Again, no other details were given.
The best estimates of the thresholds at which ammonia can be
expected to be detected by taste and odour are 35 mg/litre (as NH3)
and 35 mg/m3, respectively. Sensitive individuals may detect
concentrations an order of magnitude lower.
9.2. Clinical and Controlled Human Studies
9.2.1. Inhalation exposure
The severe effects resulting from acute exposure to ammonia
have been described in case reports of accidents involving groups
of people or individuals. There are no data on actual levels of
ammonia in air during such accidents, but estimates have been made
(Yahagi et al., 1959; Takahashi et al., 1984; NRC, 1979).
Exposure to an ammonia concentration of 280 mg/m3 (400 ppm) has
been reported to produce immediate throat irritation; 1200 mg/m3
(1700 ppm) to produce cough; 1700 mg/m3 (2400 ppm) to be life-
threatening, and 3500 - 7000 mg/m3 (500 - 10 000 ppm) to cause a
high mortality rate (Patty, 1963; Helmers et al., 1971).
A burning sensation in the eyes, nose, and throat, as well as
respiratory distress accompanied by lachrymation, coughing, and an
increase in respiratory rate are some of the irritant effects of
ammonia (Caplin, 1941). Chest X-rays are generally normal in such
mild cases (Watson, 1973; Close et al., 1980). More severe
respiratory effects include laryngeal and pulmonary oedema and
bronchopneumonia (Slot, 1938; Caplin, 1941; Levy et al., 1964;
Taplin et al., 1976; Flury et al., 1983). The signs and symptoms
are generally reversible, but chronic bronchitis and bronchiectasis
have been reported (Slot, 1938; Sugiyama et al., 1968; Taplin et
al., 1976; Close et al., 1980).
In cases with a lethal outcome, the cause of death has been
severe lung damage and secondary cardiovascular effects (Slot,
1938; Mulder & van der Zalm, 1967).
There have also been some studies on volunteers exposed to
ammonia under laboratory conditions. Some of these studies are
summarized in Table 21.
Silverman et al. (1949) reported on 7 human volunteers exposed
to a concentration of 300 mg/m3 (500 ppm) ammonia for 30 min using
an oral-nasal mask. All 7 experienced upper respiratory
irritation, which lasted up to 24 h in 2 of the volunteers. Two
subjects experienced marked lachrymation, in spite of the exposure
being by oro-nasal mask. The average respiratory minute volume
increased markedly compared with control values, and in the 5
subjects in which minute-by-minute expired volumes were measured,
there was a marked cyclical variation in minute volume with a
period of 4 - 7 min. No coughing was noted.
After exposure, respiratory minute volumes fell to levels below
the pre-exposure rate, but returned to pre-exposure values within 5
min of exposure. Ammonia retention decreased progressively until
an equilibrium of 24% retention (ranging from 4 to 30%) was reached
at approximately 19 min (range 10 - 27 min). The indices of
nitrogen metabolism (BUN, NPN, urine-urea, and urine-ammonia)
remained normal. The carbon dioxide combining power did not
change.
Verbeck (1977) assessed respiratory function in 16 volunteers
following exposure to 35, 56, 77, and 98 mg ammonia/m3 (50, 80,
110, and 140 ppm) for 0.5, 1, and 2 h. The respiratory variables of
vital capacity (VC), forced expiratory volume (FEV), and forced
inspiratory volume (FIV), measured before and after exposure, did
not decrease by more than 10%. Subjective variables, including
smell, taste, irritation of the eyes, nose, or throat, urge to
cough, headache, and general discomfort were monitored every 15 min
and ranked by 8 experts and 8 non-experts (students) on a scale
from 0 to 5. Subjective responses were ranked higher by the non-
experts. A concentration of 77 mg/m3 (110 ppm) was tolerated for
2 h, but at 98 mg/m3 (140 ppm), all the subjects left the chamber
because the exposure was intolerable.
Respiratory responses to ammonia during exercise were examined
by Cole et al. (1977). Eighteen males, aged 18 - 39 years, had 2
periods of exercise in 3 consecutive half-day sessions. Mean
exposure levels were 71, 144, 106, and 235 mg/m3 (101, 206, 151,
and 336 ppm). Ventilation minute volume and total volume
decreased, and mean respiratory frequency increased at exposure
levels > 106 mg/m3 (151 ppm).
In a study by MacEwen et al. (1970), 6 volunteers were exposed
to ammonia concentrations of 21 and 35 mg/m3 (30 and 50 ppm) for 10
min. The irritation was rated subjectively on a scale of 0 - 4.
At 35 mg/m3 (50 ppm), irritation was rated as "moderate" by 4 of
the volunteers, while 1 individual reported no detectable
irritation. None of the volunteers found the irritation at
35 mg/m3 (50 ppm) to be "discomforting" or "painful".
The irritation threshold for ammonia was examined in 10 human
volunteers by Industrial Bio-Test Laboratories, Inc. (1973). The
subjects were exposed to 4 different concentrations of 22, 35, 50,
and 94 mg/m3 (32, 50, 72, and 134 ppm) for 5 min. The frequency of
positive findings for the 10 subjects was as follows: at 22 mg/m3,
1 subject complained of dryness of the nose; at 35 mg/m3, 2
subjects experienced dryness of the nose; at 50 mg/m3, 3 subjects
had eye irritation, 2 had nasal irritation, and 3 had throat
irritation; at 94 mg/m3, 5 subjects demonstrated signs of
lachrymation, 5 had eye irritation, 7 had nasal irritation, 8 had
throat irritation, and 1 complained of chest irritation.
Table 21. Effects of inhaled ammonia in human volunteers
--------------------------------------------------------------------------------------------
Number Concentration Duration Effects and response Reference
(mg/m3) (min)
--------------------------------------------------------------------------------------------
7 300 30 irritation of upper respiratory Silverman et al. (1949)
tract (7/7); increase in minute
volume; no change in blood
chemistry
16 35 - 98 30 - 120 10% increase in VC, FEV, and Verbeck (1977)
FIV; 98 mg/m3 was not tolerated;
77 mg/m3 was tolerated for 2 h
18 71 - 235 8 - 11 no effects noted during exercise Cole et al. (1977)
at 71 mg/m3 except slight
irritation; decrease in
ventilation minute volume and
tidal volume and increase in
respiratory frequency > 106
mg/m3
6 35 10 "moderate irritation" (4/5) MacEwen et al. (1970)
10 22 5 dryness of the nose (1/10) Industrial Biotest
10 35 dryness of the nose (2/10) Laboratories (1973)
10 50 eye irritation (3/10)
10 94 throat irritation (8/10)
eye irritation (5/10)
6 17.5, 35, 2 - 6 h, increase in FEV1, with Ferguson et al. (1977)
or 70+ 5 days/ increasing NH3; adaptation
week, for to irritating effects
6 weeks
--------------------------------------------------------------------------------------------
A controlled study on human volunteers was conducted by
Ferguson et al. (1977) to evaluate the responses to inhaled ammonia
at concentrations of 17.5, 35, and 70 mg/m3 (25, 50, and 100 ppm).
Six adults, not acclimatized to ammonia exposure, were divided into
3 groups, which were exposed for 2 - 6 h each day, 5 days per week,
for 6 weeks. One pair of subjects was exposed only to 35 mg
ammonia/m3 for 6-h periods throughout the test. Subjects were
examined daily for irritation of the eyes, nose, or throat, and
periodically for pulse rate, respiration rate, pulmonary function
(forced vital capacity (FVC) and forced expiratory volume in 1
second (FEV1)), blood pressure, neurological responses, and
interference in task-performance ability. A statistical analysis
of the results demonstrated that the only significant change among
the vital functions measured was an increase in forced expiratory
volume in 1 second (FEV1) with increasing ammonia concentration.
In addition, the rate of mild eye, nose, or throat irritations over
the last 3 weeks of the test was significantly less than during the
first 2 weeks. This indicated that an acclimatization process had
occurred, with increased tolerance to the irritant effects of
ammonia developing with increasing time of exposure. Overall, the
ammonia exposure produced 2 incidents of mild irritation (78
observations made) at 17.5 mg/m3 (25 ppm), 22 incidents (198
observations made) at 35 mg/m3 (50 ppm), and 11 incidents (84
observations made) at 70 mg/m3 (100 ppm). Among control subjects,
4 irritation incidents were recorded during 45 observations. When
ammonia concentrations exceeded 105 mg/m3 (150 ppm), all subjects
experienced lachrymation accompanied by dryness of the nose and
throat.
In an inhalation study, the threshold for effects on
respiration, skin electric potential, and the electroencephalogram
was found to be 22 mg/m3 (31 ppm) (Alpatov & Mikhailov, 1963;
Alpatov, 1964).
Reports from industries with ammonia exposure indicate that
irritative effects have appeared over a wide range of ammonia
concentrations (Elkins, 1950; Vigliani & Zurlo, 1955; Mangold,
1971). A concentration of 88 mg/m3 was called "definitely
irritating" (Elkins, 1950), and "barely noticeable" eye irritation
was reported at 3 mg/m3 (Mangold, 1971).
Giguz (1968), in a study involving 140 subjects exposed to
ammonia and nitrogen oxides, at concentrations not exceeding the
"maximum permissible concentration" (20 mg/m3), 3 h per day during
2 - 3 years of vocational training, demonstrated increased
incidences of upper respiratory tract disease, compared with those
in a control group of unexposed subjects.
9.2.2. Oral exposure
9.2.2.1. Effects of acute oral exposure
Cases of the ingestion of large doses of ammonia have been
reported. When solutions of ammonia were ingested orally, a
tissue-destructive caustic effect was noted for concentrated
solutions, owing to their high pH. A solution of ammonia at a
concentration of 100 g/litre, for example, has a pH of 12.5.
Oesophageal burns were reported in 25 cases of accidental
ammonia ingestion (Hawkins et al., 1980). One child suffered a
mild burn. Four adults had mild burns limited to the mucosa and
nine adults had oesophageal burns that were moderate or more severe
in nature. One adult female suffered from a complication of airway
obstruction from supraglottic oedema. Oesophageal stricture from
the ingestion of ammonia (100 g/litre) was reported by Vancura et
al. (1980) (Table 19).
A fatal outcome after ingestion of a solution containing 24 g
ammonia/litre was reported by Klendshoj & Rejent (1966). Autopsy
showed haemorrhagic inflammatory changes in the oesophagus,
stomach, and small intestine.
There are many reports on the effects of ammonium chloride, but
since acidosis is caused by the chloride, such studies have little
relevance for evaluating ammonia toxicity.
There are no data on acute effects of ingestion of ammonium
compounds, other than the chloride.
9.2.3. Endogenous hyperammonaemia
9.2.3.1. Inborn errors of metabolism
These affect the uptake of ammonia rather than its rate of
production.
Congenital deficiency of carbamyl phosphate synthetase I (EC
6.3.4.1.6), and, to a lesser extent, of other enzymes of the
ornithine cycle, and several other metabolic disorders may lead to
hyperammonaemia and various abnormal urinary constitutents.
Hyperammonaemia may be lethal in new-born infants, may cause severe
symptoms in infancy, or may cause chronic remittent symptoms in
older children or adults (Hsia, 1974).
Clinical features in neonates may resemble those of hepatic
coma and may be precipitated by protein-rich milk feeds. In older
children, episodic vomiting, neurological disorders (including
seizures) or coma are precipitated by high-protein foods.
9.2.3.2. Hepatic features
Varied and complex functions of the liver may fail
progressively in chronic liver disease or rapidly in acute
disorders. The syndromes differ in the extent to which portal
blood from the intestine is shunted into the systemic venous
circulation either by cirrhotic changes in hepatic vascular
resistance or by surgical procedures to correct portal hypertension
resulting from it.
In either case, a syndrome is recognised that comprises a
spectrum of neurological features from irritability via
inappropriate behaviour, tremors, hyperreflexia and generalised
muscular rigidity, to delirium, stupor, convulsions and coma.
There is disagreement regarding the extent to which this
syndrome is an expression of ammonia toxicity. On the one hand,
over 90% of persons with the disorder have elevated levels of
ammonia in the blood or cerebrospinal fluid; the condition may be
induced in those with marginal liver function by the administration
of ammonium salts or an intestinal haemorrhage (which leads to
intestinal ammonia production greater than that from an equivalent
amount of meat) and the condition may be treated by reducing
intestinal ammonia production, by the administration of antibiotics
to eliminate ammonia-producing flora. On the other hand, there is
an imperfect correlation between the clinical state and the blood-
ammonia level and the condition may occur in the presence of normal
blood-ammonia levels.
It is unlikely that the syndrome resulting from the failure of
an organ as complicated as the liver should be explicable in terms
of a single metabolic component, but the similarity between hepatic
failure and certain expressions of congenital hyperammonaemias
suggests an important role of ammonia toxicity in the pathogenesis
of the syndrome.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Atmospheric Exposure and Effects
10.1.1. General population exposure
Background levels of ammonia in community air are low in
comparison with levels that have been established as safe in
occupational settings, but there is considerable variabilty
according to the type of land use. In general, levels in areas
with intensive livestock husbandry or high rates of manure
application are in the range of 100 - 200 µg/m3, levels in urban
areas range from approximately 5 to 40 µg/m3, while those in rural
areas, without intensive manure production or use, range up to
10 µg/m3. This is in contrast to odour thresholds of the order
10 000 µg/m3, thresholds for irritation of the order 20 000 -
50 000 µg/m3, and the estimated LC for man of 5000 - 10 000 mg/m3.
The LC50 estimation for the rat was 7 600 000 µg/m3, for a 2-h
exposure. In an occupational setting, workers did not voluntarily
use respiratory protective devices at concentrations below about
500 000 µg/m3. General ambient atmospheric levels are therefore
of no concern in respect of discomfort or acute toxicity.
Ammonia is not mutagenic and long-term studies on both
laboratory and farm animals have not shown any pathological effects
at levels below 35 000 µg/m3. Long-term toxic effects are
unlikely, even at levels much higher than ambient levels, both
generally and in the neighbourhood of ammonia-emitting systems.
There is a lack of evidence regarding recent trends in global
atmospheric ammonia concentrations.
10.1.2. Occupational exposure
Occupational problems are predominantly those of accidental
exposure.
Though for certain groups in, for example, agriculture, the
chemical industry, waste disposal and transport, occupational
exposure levels may be very much higher than general, there is
historical evidence that, even at levels significantly in excess of
current occupational exposure limits, there was a low prevalence of
adverse health effects.
The distribution kinetics of absorbed atmospheric ammonia
suggest that the rise in blood-ammonia at a typical occupational
exposure limit will be within the normal range of variation.
Thus, there is both historical and theoretical evidence that
most recommended exposure limits are acceptable.
10.2. Exposure Through Water and Food
Ammonia will have a toxic effect, only if intake exceeds the
capacity of mammals to detoxify ammonia. Unfortunately, there are
no data permitting the evaluation of this capacity in healthy human
beings or other terrestrial mammals. In addition, there is some
evidence that the mode of intake may be a factor in the capacity of
individuals to detoxify ammonia. Parenteral administration of
ammonia results in patterns of metabolism and elimination that are
markedly different from those seen in oral administration. Thus,
the considerable body of data on the parenteral toxicity of ammonia
is not of direct relevance to criteria for oral exposure.
Similarly, because of the different kinetic patterns between oral
and inhalation exposure to ammonia, as well as the highly irritant
effects of ammonia on the lung, the available inhalation data on
ammonia are not applicable to the estimation of an acceptable daily
intake (ADI) for ingestion. However, it would be possible to
define a clearly undesirable level of oral exposure to total
ammonia (NH3 and NH4+) as well as a level that is clearly
tolerable. The range between these 2 levels would exceed the range
within which an ADI could be established.
The amount of excess ammonia (i.e., over and above the amount
normally produced in the body) that can be safely ingested and
assimilated is difficult to define. In short-term (28 - 90 days)
studies carried out on rats and pigs, no adverse effects were
reported at higher levels of ammonia intake (75 - 545 mg NH3/kg
body weight per day) in the form of sulfamate, phosphate, citrate,
or chloride.
The effects attributed directly to elevated ammonium ion levels
are acute pulmonary oedema and central nervous system (CNS)
toxicity, depression of appetite due to a direct effect of the
ammonium ion on the brain, and promotion of growth via the use of
ammonium salts as a source of non-essential nitrogen under certain
circumstances.
Some effects (such as renal growth and demineralization of
bone) arising from the administration of ammonium chloride seem to
be secondary effects of acidosis.
Surveys of total ammonia (NH3 + NH4+) concentrations in surface
waters indicate an average of < 0.18 mg/litre in most areas, and
0.5 mg/litre in waters near large metropolitan areas (Wolaver,
1972; US EPA, 1979a). Levels in ground water are usually low,
since ammonia is generally immobile in soil. Ammonia is practically
absent when drinking-water is chlorinated.
Ammonia is a negligible natural constituent of food, but
ammonium compounds are added in small amounts (< 0.001 - 3.2%) to
various foods as stabilizers, leavening agents, flavourings, or for
other purposes. The daily human intake from these sources is
estimated to be 18 mg as NH3.
10.3. Ocular and Dermal Exposure
Ammonia in aqueous solution or in contact with body fluids is
alkaline and causes burns or inflammation of eyes or skin. The
ocular irritation commonly experienced at atmospheric ammonia
concentrations of > 20 mg/m3 (MacEwen et al., 1970; Keplinger et
al., 1973; Verberk, 1977) is readily reversible when exposure
ceases, and may also be reduced by acclimatization (Ferguson et
al., 1977).
Serious ocular damage normally occurs, only with a direct blast
or splash contact with anhydrous or aqueous ammonia (Grant, 1974).
Skin damage is reported to occur at concentrations of ~7000 mg/m3
(NRC, 1979).
10.4. Accidental Exposure
High gaseous ammonia concentrations may be encountered locally,
both in domestic and work-place environments, as a result of gaseous
emissions and/or spillages of concentrated solutions, and
respiratory (and skin and eye) injury may result. On a larger
scale, spillage from stock or transport tanks or refrigeration
plant of concentrated ammonia liquor or anhydrous ammonia would
constitute a severe environmental insult and would cause serious
injury to the people, animals, and plants in the vicinity. Because
of its low density and short biopersistence, major spillages would
be expected to disperse rapidly and not to persist in the
environment.
10.5. Evaluation of Risks for the Environment
Environments that receive more ammonia than can be used may be
acidified and nitrogen-enriched. As a consequence of these
physical-chemical changes, the structure and functioning of the
ecosystem will be disturbed.
Ammonia plays an important role in the metabolism of all
organisms as a nutrient at low concentrations, but becomes toxic at
higher concentrations. For example, microorganisms both assimilate
and generate ammonia as a part of natural nutrient cycling
processes. High levels of ammonia may inhibit growth or survival
of microbial organisms, including, at higher levels, nitrifying
organisms.
10.5.1. The aquatic environment
Ammonia concentrations in the aquatic environment are variable,
reflecting the proximity and nature of sources (section 4).
Where there are large numbers of people and animals in relation
to the volume of surface waters and drainage flow, the load of
nitrogen added to surface waters from sewage and industrial
effluent is the predominant source and may lead to ammonia
concentrations that constitute a significant local and/or regional
environmental problem.
Otherwise, ammonia deposition contributes a major input. In
surface waters that are poorly buffered, poor in nutrients, and
hydrologically dependent on rainfall and/or snow melt, this may
result in acidification and nitrogen enrichment resulting in marked
changes in plant community structure with concommitant changes in
the animal population structure.
The toxic effects on aquatic organisms are attributed to non-
ionized ammonia (NH3) rather than to ammonium ion levels. This is
because non-ionized ammonia easily penetrates biological membranes,
whereas ammonium ions require specialized transport processes.
There are similarities between the modes of the acute toxic
action of ammonia in mammals and in fish: in the latter, however,
environmental conditions (such as pH, ionic composition and
concentration, temperature, and oxygen availability), which are
more variable in water than in air, have a marked modulating
effect.
Aquatic animals have a limited ability to detoxify ammonia and,
therefore, the body load is dependent on ammonia concentrations in
the water. Except in open oceans, exposure to environmental levels
produces many chronic effects (including reduced growth, decreased
survival, impaired reproduction) and may increase susceptibility to
disease and also cause histopathological changes.
High levels of ammonia in aquatic systems are also toxic for
plants. The detoxification of excessive ammonia places a severe
strain on the carbohydrate metabolism of the plant which
subsequently results in foliar injury, and growth effects, and thus
may modify plant community composition.
Where the dominant species, be it fish or plant, is also
sensitive to ammonia the effects on the whole ecosystem will be
marked.
10.5.2. The terrestrial environment
The most common effect of exposure of plants to atmospheric
ammonia is foliar injury. Prolonged exposure to high ambient
concentrations of about 75 µg/mg, such as occur in the vicinity of
intensive livestock farms, adversely affects more susceptible
species such as pine trees. The observed damage is the result of
both direct and indirect effects due to, changes in soil, and
secondarily, to increased susceptibility to disease and
meteorological stress.
The data on ammonia toxicity for wildlife are very limited.
There is no evidence that wildlife populations, in general, are at
risk from ammonia, but, there may be secondary effects associated
with changes in plant communities. Certain species of bats are
able to withstand the very high ammonia levels found in caves where
they live.
10.6. Conclusions
The major groups of organisms at risk from elevated ammonia
levels are aquatic animals and terrestrial plants. There appears
to be little danger for terrestrial animals, including man, except
from acute accidental exposure.
10.6.1. General population
There are no data suggesting that present environmental levels
of ammonia are hazardous for the general population. Only high-
level accidental exposures from domestic sources and transportation
and storage accidents pose an occasional acute health hazard.
10.6.2. Sub-populations at special risk
Groups likely to exhibit ammonia toxicity include those with
hepatic or renal impairment, though, even in these cases, levels of
exogenous ammonia are insignificant in comparison with endogeneous
levels, so that, in the absence of any environmental exposure, such
persons would still be affected. The mechanism is different in the
two cases. Hepatic impairment limits the conversion of ammonia to
urea, and renal failure, by increasing urea concentrations and its
intestinal secretion, leads to increased endogeneous intestinal
ammonia production.
There have been few studies on the chronic effects of ammonia
inhalation. It can be speculated that subpopulations that have
been found to be hyperreactive to other respiratory irritants
(e.g., sulfur dioxide, particulates, ozone) may also be
hyperreactive to ammonia. These subpopulations may include
children, elderly persons with pre-existing cardiorespiratory
symptoms, individuals with asthma or bronchitis, and those engaged
in vigorous physical exercise (Calabrese, 1978). However, there is
also some indication that previous exposure to low levels of
ammonia may cause inurement to its effects (Ferguson et al., 1977).
10.6.3. Occupational exposure
Accidental exposures are the predominant problem (section
10.1.1.3). Otherwise, occupational exposure can be controlled by
the application of most current occupational exposure limits and
proper industrial hygiene.
10.6.4. Farm animals
Farm animals may be at risk, because of continous exposure
under confined housing conditions resulting in high atmospheric
levels of ammonia within the confinement areas; this applies
particularly to cattle, swine, and poultry. Available reported
data provide a range of measured exposure levels of from 2 to
1400 mg/m3. In winter months in colder climates, most of the
measured concentrations exceeded the admissible upper limit of
35 mg/m3 (50 ppm).
10.6.5. Environment
Environments with a low buffer capacity and poor in nutrients
are susceptible to acidification and nitrogen enrichment by
elevated ammonia loading; prolonged high ammonia-loading results in
changes in both the structure and function of plant and animal
communities. Levels of atmospheric ammonia necessary for the onset
of these changes have not been established; however, changes in the
structure of these communities have been observed where ammonia
levels in the atmosphere were possibly up to 100 µg/m3.
Plants use ammonia as a nutrient, but high levels can be toxic.
Terrestrial plants show a susceptibility to reduced growth and
reduced vitality, when exposed to levels as low as 75 µg NH3/m3 in
the atmosphere.
Aquatic animals have a low capacity to detoxify ammonia. Acute
effects on some fish have been demonstrated in laboratories at
concentrations as low as 0.1 mg NH3/litre and chronic effects at
concentrations as low as 0.02 mg NH3/litre. Thus, as ammonia
levels in some waters are often similar to those shown to cause
chronic effects in some fish, it would appear that these animals
are at risk. Aquatic invertebrates are, in general, less sensitive
to elevated ammonia levels in water.
Aquatic animals are at risk because of increases in ammonia
concentrations in water systems, whereas some plant communities
appear to be at risk from elevations in atmospheric ammonia
loading.
11. RECOMMENDATIONS
11.1. Research Needs
1. Long-term monitoring of ammonia and other pollutants in
water systems with different aquatic ecosystems.
2. Studies of the long-term effects of ammonia on terrestrial
vegetation.
3. Studies of the global nitrogen balance to identify long-
term trends.
4. Ecotoxicological studies to elucidate environmental
effects.
5. Epidemiological studies in relation to ammonia exposure in
order to make better hygiene recommendations.
6. Long-term experimental animal studies to establish a no-
observed-adverse-effect level of exposure.
7. Studies on the role of ammonia in modifying physical and
chemical conditions of soil and water systems.
8. More data are needed to assess accurately the relative
contributions of various point and non-point sources of
ammonia for surface waters.
9. Research into methods and their application directed
towards reducing emissions from point sources.
10. Additional acute toxicity tests with salt-water fish and
invertebrate species.
11. Life-cycle and early-life-stage tests with representative
fresh-water and salt-water organisms from different
families, with investigation of pH effects on chronic
toxicity.
12. Fluctuating or intermittent exposure tests under a variety
of exposure patterns on additional species.
13. Both acute and long-term tests at cold-water temperatures.
14. Studies on the effects of dissolved and suspended solids
on acute and chronic toxicity.
15. More histopathological and histochemical research with
fish, which would provide a rapid means of identifying and
quantifying sublethal ammonia effects.
16. In fish, the relative concentration limits for both
acclimatization and subsequent acute response need better
definition and a more complete explanation.
REFERENCES
ABBAS, R. & TANNER, R.L. (1981) Continuous determination of
gaseous ammonia in the ambient atmosphere using fluorescence
derivatization. Atmos. Environ., 15: 277-281.
ABELOVICH, A. & AZOV, Y. (1976) Toxicity of ammonia to algae
in sewage oxidation ponds. Appl. environ. Microbiol., 31(6):
801-806.
ADMIRAAL, W. (1977) Tolerance of estuarine benthic diatoms
to high concentrations of ammonia, nitrite ion, nitrate ion
and orthophosphate. Mar. Biol., 43(4): 307-315.
AGAMI, M., LITAV, M., & WAISEL, Y. (1976) The effects of
various components of water pollution on the behaviour of some
aquatic macrophytes of the coastal rivers of Israel. Aquat.
Bot., 2(3): 203-213.
ALABASTER, J.S. & HERBERT, D.W.M. (1954) Influence of carbon
dioxide on the toxicity of ammonia. Nature (Lond.), 174(4426):
404.
ALABASTER, J.S., SHURBEN, D.G., & KNOWLES, G. (1979) The
effect of dissolved oxygen and salinity on the toxicity of
ammonia to smolts of salmon, Salmo salar L. J. Fish Biol.,
15(6): 705-712.
ALLAN, I.R.H. (1955) Effects of pollution on fisheries.
Verh. Int. Ver. Theor. Agnew Limnol., 12: 804-810.
ALLAN, I.R.H., HERBERT, D.W.M., & ALABASTER, J.S. (1958) A
field and laboratory investigation of fish in a sewage
effluent. Fish. Invest., 6(2): 1-76.
ALPATOV, I.M. (1964) [A study of gazeous ammonia toxicity.]
Gig. Tr. prof. Zabol., 2: 14-18 (in Russian).
ALPATOV, I.M. & MIKHAILOV, V.I. (1963) [Inquires into the
gaseous ammonia toxicity. Communication 1.] Gig. Tr. prof.
Zabol., 12: 51-53 (in Russian)
ANALYTICAL QUALITY CONTROL (HARMONISED MONITORING) COMMITTTEE
(1982) Accuracy of determination of ammoniacal nitrogen in
river waters: analytical quality control in the harmonised
monitoring scheme. Analyst, 107(June): 680-688.
ANDERSON, B.G. (1948) The apparent thresholds of toxicity to
Daphnia magna for chlorides of various metals when added to
Lake Erie water. Trans. Am. Fish Soc., 78: 96-113.
ANDERSON, D.P., BEARD, C.W., & HANSON, R.P. (1964a) The
adverse effects of ammonia on chickens including resistance to
infections with Newcastle disease virus. Avian Dis., 8:
369-379.
ANDERSON, D.P., CHERMS, F.L., & HANSON, R.P. (1964b) Studies
on measuring the environment of turkeys raised in confinement.
Poult. Sci., 43: 305-318.
ANDERSON, K.B., SPARKS, R.E., & PAPARO, A.A. (1978) Rapid
assessment of water quality, using the fingernail clam,
Musculium transversum, Urbana, Illinois, Water Resources
Center, University of Illinois, 115 pp (WRC Research Report
No. 133).
ANGHILERI, L.G. (1975) Tumour growth inhibition by ammonium
chloride-induced acidosis. Int. J. clin. Pharmacol., 12: 320.
ANTHONISEN, A.C., LOEHR, R.C., PRAKASAM, T.B.S., & SRINATH,
E.G. (1976) Inhibition of nitrification by ammonia and
nitrous acid. J. Water Poll. Control Fed., 48: 835-852.
APHA (1976) Standard methods for the examination of water
and wastewater, 14th ed., Washington DC, American Public
Health Association, American Water Works Association, Water
Pollution Control Federation, 1193 pp.
API (1981) The sources, chemistry, fate, and effects of
ammonia in aquatic environments, Washington DC, American
Petroleum Institute, 145 pp.
APPELMAN, L.M., TEN BERGE, W.F., & REUZEL, P.G.J. (1982)
Acute inhalation toxicity study of ammonia in rats with
variable exposure periods. Am. Ind. Hyg. Assoc. J., 43(9):
662-665.
ARMSTRONG, D.A., CHIPPENDALE, D., KNIGHT, A.W., & COLT, J.E.
(1978) Interaction of ionized and un-ionized ammonia on
short-term survival and growth of prawn larvae, Macrobrachium
rosenbergii. Biol. Bull., 154(1): 15-31.
ARNDT, F.L., DAY, D.L., & HATFIELD, E. (1979) Processing and
Handling of animal excreta for refeeding. J. anim. Sci., 48:
157.
ASTM (1980) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
AUERBACH, C. & ROBSON, J.M. (1947) Tests of chemical
substances for mutagenic action. Proc. R. Soc. Edinburgh Sec.
Biol., 62B: 284-291.
AVOT, YU.K., KANEP, V.V., SLUTSKER, D.S., & SHAFRAN, L.M.
(1977) [Medical and sanitary aspects of sailors' work,] Riga,
Zvaigzne, 132 pp (in Russian).
AXELROD, H.D. & GREENBERG, J.P. (1976) Determination of
trace concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 10: 495-496.
BAIRD, R., BOTTOMLEY, J., & TAITZ, H. (1979) Ammonia
toxicity and pH control in fish toxicity bioassays of treated
wastewaters. Water Res., 13(2): 181-184.
BALL, I.R. (1967) The relative susceptibilities of some
species of fresh-water fish to poisons. I. Ammonia. Water
Res., 1(11/12): 767-775.
BARROW, C.S. & STEINHAGEN, W.H. (1980) NH3 concentrations in
the expired air of the rat: Importance to inhalation
toxicology. Toxicol. appl. Pharmacol., 53: 116-121.
BARROW, C.S., ALARIE, Y., & STOCK, M.F. (1978) Sensory
irritation and incapacitation evoked by thermal decomposition
products of polymers and comparisons with known sensory
irritants. Arch. environ. Health, 33(2): 79-88.
BARTON, L.V. (1940) Toxicity of ammonia, chlorine, hydrogen
cyanide, hydrogen sulphide and sulphur dioxide gases. IV.
Seeds. Contrib. Boyce Thompson Inst., 11: 357-363.
BARZEL, U.S. (1975) Effect of chronic ammonium chloride
ingestion on parathyroid-hormone function. Nephron, 14(15):
339-346.
BARZEL, U.S. & JOWSEY, J. (1969) Effects of chronic acid and
alkali administration on bone turnover in adult rats. Clin.
Sci., 36(3): 517-524.
BENEDICT, H.M. & BREEN, W.H. (1955) The use of weeds as a
means of evaluating vegetation damage caused by air pollution.
In: Proceedings of the Third National Air Pollution
Symposium, Pasadena, California, April 1955, pp. 177-190.
BENNETT, W.F., PESEK, J., & HANWAY, J.J. (1964) Effects of
nitrate and ammonium on growth of corn in nutrient solution
sand culture. Agric. J., 56: 342-345.
BENYAJATI, S. & GOLDSTEIN, L. (1975) Renal glutaminase
adaptation and ammonia excretion in infant rats.
Am. J. Physiol., 228(3): 693-698.
BERL, S., TAKAGAKI, G., SCHWERIN, P., & WAELSCH, H. (1962)
Metabolic compartments in vivo: Ammonia and glutamic acid
metabolism in brain and liver. J. biol. Chem., 237: 2562-2569.
BESSMAN, S.P. & BESSMAN, A.N. (1955) The cerebral and
peripheral uptake of ammonia in liver disease with an
hypothesis for the mechanism of hepatic coma. J. clin.
Invest., 34: 622-628.
BHATTACHARYA, A.N. & TAYLOR, J.C. (1975) Recycling animal
waste as a feedstuff: A review. J. anim. Sci., 44: 1438.
BIDWELL, R.G.B. (1974) Plant physiology, New York,
MacMillan, 197 pp.
BINSTOCK, L. & LECAR, H. (1969) Ammonium ion currents in the
squid giant axon. J. gen. Physiol., 53: 342-361.
BIRNBAUM, S.M., WINTZ, M., & GREENSTEIN, J.P. (1957)
Quantitative nutritional studies with water-soluble,
chemically defined diets. III. Individual amino acids as
sources of "non-essential" nitrogen. Arch. Biochem. Biophys.,
72(2): 428-436.
BISWAS, S.D. & KLEINBERG, I. (1971) Effect of urea
concentration on its utilisation, on the pH and the formation
of ammonia and carbon dioxide in a human salivary sediment
system. Arch. oral Biol., 16: 759-780.
BLOOMFIELD, R.A., GARNER, G.B., & MUHRER, M.E. (1960)
Kinetics of urea metabolism in sheep. J. anim. Sci., 19: 1248.
BLOOMFIELD, R.A., KEARLEY, E.O., CREACH, D.O., & MUHRER, M.E.
(1962) Ruminal pH and absorption of ammonia and VFA. J. anim.
Sci., 22: 833 (Abstract).
BOCK, F., MICHELSON, I., BROSS, I.D., & PRIORE, R.L. (1974)
Carcinogenic activity of smoke condensate from cigarettes with
ammonium sulfamate-treated paper. Cancer, 33: 1010.
BODANSKY, A., JAFFE, M.L., & CHANDLER, J.P. (1932) Serum
phosphatase changes in ammonium chloride osteoporosis. Proc.
Soc. Exp. Biol. Med., 29: 871-875.
BOER, W.M.J., DEN & BASTIAENS H. (1984) [Effects of
acidification on vegetation,] Ministry of Agriculture and
Fisheries, Ministry of Housing, Physical Planning and
Environment, 82 pp (Report No. 84(2)) (in Dutch).
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1973)
Ammonia kills spoilage moulds in corn. J. Dairy Sci., 56: 241.
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1975a)
Effect of pH and substrate on growth. Eur. J. appl. Microbiol,
1: 55-56.
BOTHAST, R.J., ADAMS, G.H., HATFIELD, E.E., & LANCASTER, E.B.
(1975b) Preservation of high-moisture corn: a microbiological
evaluation J. Dairy Sci., 58: 386-391.
BOYD, E.M. & SEYMOUR, K.G.W. (1946) Ethylenediamine
dihydrochloride or "chlorethamine". II. Untoward and toxic
reactions. Exp. Med. Surg., : 223-227.
BOYD, E.M., McLACHLAN, M.L., & PERRY, W.F. (1944)
Experimental ammonia gas poisoning in rabbits and cats.
J. ind. Hyg. Toxicol., 26: 29-34.
BRAGANGA, B.M., FAULKNER, P., & QUASTEL, J.M. (1953) Effects
of inhibitors of glutamine synthesis in brain slices by
ammonium ions. Biochim. Biophys. Acta, 10: 83-88.
BREEDING, R.J., LODGE, J.P., Jr, PATE, J.B., SHEESLEY, D.C.,
KLONIS, H.B., FOGLE, B., ANDERSON, J.A., ENGLERT, T.R.,
HAAGENSON, P.L., MCBETH, R.B., MORRIS, A.L., POGUE, R., &
WARTBURG, A.F. (1973) Background trace concentrations in the
central United States. J. geophys. Res., 78: 7057-7064.
BREEDING, R.J., KLONIS, H.B., LODGE, J.P., Jr, PATE, J.B.,
SHEESLEY, D.C., ENGLERT, T.R., & SEARS, D.R. (1976)
Measurements of atmospheric pollutants in the St. Louis area.
Atmos. Environ., 10: 181-184.
BRENNAN, E., LEONE, I.A., & DAINES, R.H. (1962) Ammonia
injury to applies and peaches in storage. Plant Dis. Rep., 46:
792-795.
BRESSANI, R. & BRAHAM, J.E. (1977) Effect of frequency of
feeding on blood serum urea changes in dogs. J. Nutr. Rep.
Int., 16(3): 305-316.
BRETT, J.R. & ZALA, C.A. (1975) Daily pattern of nitrogen
excretion and oxygen consumption of sockeye salmon
(Oncorhyncus nerka) under controlled conditions. J. Fish. Res.
Board Can., 32: 2479-2486.
BRETTHAUER, R. (1978) Some ecological limits of tolerance to
Ochromonas sociabilis. Verh. Internat. Verein. Limnol., 20(3):
1850-1854.
BROCKWAY, D.R. (1950) Metabolic products and their effects.
Prog. Fish-Cult., 12(3): 127-129.
BRODERIUS, S.J. & SMITH, L.L., Jr (1979) Lethal and
sublethal effects of binary mixtures of cyanide and hexavalent
chromium, zinc, or ammonia to the fathead minnow Pimephales
promelas and rainbow trout Salmo gairdneri. J. Fish. Res.
Board Can., 36(2): 164-172.
BRODERIUS, S.J., SMITH, L.L., Jr, & LIND, D.T. (1977)
Relative toxicity of free cyanide and dissolved sulfide forms
to the fathead minnow. Pimephales promelas. J. Fish. Res.
Board Can., 34(12): 2323-2332.
BRODERIUS, S.J., DRUMMOND, R.A., FIANDT, J.T., & RUSSOM, C.L.
(in press) Toxicity of ammonia to smallmouth bass,
Micropterus dolomieui, as related to pH. Environ. Toxicol.
Chem..
BRODERSON, J.R., LINDSEY, J.R., & CRAWFORD, J.E. (1976) The
role of environmental ammonia in respiratory mycoplasmosis of
rats. Am. J. Pathol., 85: 115-130.
BROSSET, C., ANDREASSON, K., & FERM, M. (1975) The nature
and possible origin of acid particles observed at the Swedish
west coast. Atmos. Environ., 9: 631-642.
BROWN, A.C. (1974) Observation on the effect of ammonium
nitrate solutions on some common marine animals from Table
Bay. Trans. R. Soc. S. Afr., 41(2): 217-223.
BROWN, V.M. (1968) The calculation of the acute toxicity of
mixtures of poisons to rainbow trout. Water Res., 2(10):
723-733.
BROWN, R.H., DUDA, G.D., KORKES, S., & HANDLER, P. (1957) A
colorimetric micromethod for determination of ammonia: the
ammonia content of rat tissues and human plasma. Arch.
Biochem. Biophys., 66: 301-309.
BROWN, V.M., JORDAN, D.H.M., & TILLER, B.A. (1969) The acute
toxicity to rainbow trout of fluctuating concentrations and
mixtures of ammonia, phenol and zinc. J. Fish Biol., 1(1): 1-9.
BUCKLEY, J.A. (1978) Acute toxicity of unionized ammonia to
fingerling coho salmon. Prog. Fish-Cult., 40(1): 30-32.
BUCKLEY, J.A., WHITMORE, C.M., & LIMING, B.D. (1979) Effects
of prolonged exposure to ammonia on the blood and liver
glycogen of coho salmon (Oncorhynchus kisutch). Comp. Biochem.
Physiol., 63C(2): 297-303.
BUIKEMA, A.I., Jr, CAIRNS, J., Jr, & SULLIVAN, G.W. (1974)
Evaluation of Philodina acuticornis (Rotifera) as a bioassay
organism for heavy metals. Water Res. Bull., 10(4): 648-661.
BUKHOVSKAYA, M.S., GINZBURG, S.L., & KHALIZOVA, O. (1966)
[Methods for determination of harmful substances in air. A
practical guide,] 2nd ed., Moscow, Meditaina (in Russian).
BULLIS, K.L., SNOEYENBOS, G.H., & VAN ROEKEL, H. (1950) A
keratoconjunctivitis in chickens. Poult. Sci., 29: 386-389.
BURKHALTER, D.E. & KAYA, C.M. (1977) Effects of prolonged
exposure to ammonia on fertilized eggs and sac fry of rainbow
trout (Salmo gairdneri). Trans. Am. Fish Soc., 106(5): 470-475.
BURROWS, R.E. (1964) Effects of acclimated excretory
products on hatchery-reared salmonids, Washington DC, Fish and
Wildlife Service, US Department of the Interior, 12 pp
(Research Report 66).
BUTTERWORTH, B. (1979) Nutritionally improved straw: the
machines do the job. Agric. Eng., 60(7): 41-42.
BUYSMAN, E. (1984) [Ammonia emissions in the Netherlands,]
Ministry of Housing, Physical Planning, and Environment,
Directorate Air (Report No. 22) (in Dutch).
BUYSMAN, E., MAAS, J.F.M., & ASMAN, W.A.M. (1985) Ammonia
emission in Europe, Utrecht, the Netherlands, Institute for
Meteorology and Oceanography, State University Utrecht.
BYERRUM, R.U. & BENSON, A.A. (1975) Effect of ammonia on
photosynthetic rate and photosynthate release by Amphidinium
carterae (Dinophyceae). J. Phycol., 11: 449-452.
CALABRESE, E.J. (1978) Pollutants and high risk groups,
New York, Wiley-Interscience Publications.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1977) [Effect of
prolonged treatments with ammonia on stages of development of
Salmo gairdneri.] Nuovi Ann. Ig. Microbiol., 28(5): 333-345.
(in Italian).
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1981) Effects of
long-term exposure to ammonia on the developmental stages of
rainbow trout ( Salmo gairdnerii Richardson). Rapp. P.-V. Réun.
Cons. Int. Explor. Mer, 178: 81-86.
CAMERON, J.N. (1979) Excretion of CO2 in water-breathing
animals. Marine Biol. Lett., 1: 3-13.
CAMERON, J.N. & HEISLER, N. (1983) Studies of ammonia in the
rainbow trout physio-chemical parameter, acid-base behavior,
and respiratory clearance. J. exp. Biol., 105:: 107-125.
CAMERON, G.R. & SHEIKH, A.H. (1951) Experimental production
of pulmonary edema with ammonium salts, together with
classification of lung oedemas. J. Pathol. Bacteriol., 63:
609-617.
CAMPBELL, C.L., DAWES, R.K., DEOLALKAR, S., & MERRITT, M.C.
(1958) Effect of certain chemicals in water on the flavor of
brewed coffee. Food Res., 23: 575-579.
CAPLIN, M. (1941) Ammonia gas poisoning. Forty-seven cases
in a London shelter. Lancet, 2: 95-96.
CAPUCO, A.V. (1977) Ammonia, a modulator of 3T3 cell growth,
Ithaca, New York, Cornell University (Ph.D Thesis).
CARPENTER, C.P., SMYTH, H.S., Jr, & SHAFFER, C.B. (1948) The
acute toxicity of ethylene imine to small animals. J. ind.
Hyg. Toxicol., 30: 2-6.
CARY, G.A. (1976) A report on the assessment of aquatic
environmental impact of Union Carbide's Uravan operations:
on-site toxicity bioassays, Tarrytown, New York, Aquatic
Environmental Sciences, Union Carbide Corporation, 67 pp
(Report prepared for Metals Division, Union Carbide
Corporation, Uravan, Colorado).
CASTELL, D.O. & MOORE, E.W. (1971) Ammonia absorption from
the human colon. Gastroenterology, 60(1): 33-42.
CATEDRAL, F.F., COLOSO, R., VALERA, N., CASALMIR, C.M., &
QUIBUYEN, A.T. (1977a) Effect of some physico-chemical
factors on the survival and growth of Penaeus monodon
postlarvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian Fish.
Dev. Cent., 1(3): 13-16.
CATEDRAL, F.F., GEROCHI, D.D., QUIBUYEN, A.T., & CASALMIR,
C.M. (1977b) Effect of nitrite, ammonia, and temperature on
P. monodon larvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian
Fish. Dev. Cent., 1(3): 9-12.
CHAMP, M.A., LOCK, J.T., BJORK, C.D., KLUSSMANN, W.G., &
MCCULLOUGH, J.D., Jr (1973) Effects of anhydrous ammonia on
a central Texas pond and a review of previous research with
ammonia in fisheries management. Trans. Am. Fish. Soc.,
102(1): 73-82.
CHARLES, D.R. & PAYNE, C.G. (1966a) The influence of graded
levels of atmospheric ammonia on chickens. I. Effects on
respiration and on the performance of broilers and replacement
growing stock. Br. Poult. Sci., 7: 177-187.
CHARLES, D.R. & PAYNE, C.G. (1966b) The influence of graded
levels of atmospheric ammonia on chickens. II. Effects on the
performance of laying hens. Br. Poult. Sci., 7: 189-198.
CHEMICAL AND ENGINEERING NEWS (1980) C and EN's top fifty
chemical products. Am. Chem. Soc., : 16.
CHIPMAN, W.A., Jr (1934) The role of pH in determining the
toxicity of ammonium compounds, Columbia, Missouri, University
of Missouri, 153 pp (Ph.D. Thesis).
CLAIBORNE, J.B., EVANS, D.H., & GOLDSTEIN, L. (1982) Fish
branchial Na+-K+-activated ATPase. J. exp. Biol., 96: 431-434.
CLAIBORNE, J.B. & HEISLER, N. (1984) Acid-base regulation in
the carp (Cyprinus carpio) during and after exposure to
environmental hypercapnia. J. exp. Biol., 108: 25-43.
CLAWSON, A.J. & ARMSTRONG, W.D. (1981) Ammonium
polyphosphate as a source of phosphorous and nonprotein
nitrogen for monogastrics (in corn-soybean meal diets for
growing rats and growing finishing [G-F] pigs). J. anim. Sci.,
52(1): 1-7.
CLOSE, L.G., CATLIN, F.I., & COHN, A.M. (1980) Acute and
chronic effects of ammonia burns of the respiratory tract.
Arch. Otolaryngol., 106: 151-158.
COLE, T.J., COTES, J.E., JOHNSON, G.R., & MARTIN, H. (1977)
Ventilation cardiac frequency and pattern of breathing during
exercise in men exposed to o-chlorobenzylidene malonitril and
ammonia gas in low concentrations. Q. J. exp. Physiol., 62:
341-351.
COLT, J.E. (1978) The effects of ammonia on the growth of
channel catfish, Ictalurus punctatus, Davis, California,
University of California, 185 pp (Ph.D. Thesis).
COLT, J.E. & TCHOBANOGLOUS, G. (1976) Evaluation of the
short-term toxicity of nitrogenous compounds to channel
catfish, Ictalurus punctatus. Aquaculture, 8(3): 209-224.
COLT, J.E. & TCHOBANOGLOUS, G. (1978) Chronic exposure of
channel catfish, Ictalurus punctatus to ammonia: effects on
growth and survival. Aquaculture, 15(4): 353-372.
CONN, H.O. (1972) Studies of the source and significance of
blood ammonia. IV. Early ammonia peaks after ingestion of
ammonium salts. Yale J. Biol. Med., 45: 543-549.
CONSTANTINE, D.G. (1958) Bleaching of hair pigment in bats
by the atmosphere in caves. J. Mammal, 39: 513-520.
COOMBE, J.B., TRIBE, D.E., & MORRISON, J.W. (1960) Some
experimental observations on the toxicity of urea to sheep.
Aust. J. agric. Res., 11: 247-256.
COON, R.A., JONES, R.A., JENKINS, L.J., Jr, & SIEGEL, J.
(1970) Animal inhalation studies on ammonia, ethylene glycol,
formaldehyde, dimethylamine, and ethanol. Toxicol. appl.
Pharmacol., 16: 646-655.
COTTERILL, O.J. & NORDSKOG, A.W. (1954) Influence of ammonia
on egg white quality. Poult. Sci., 33: 432-434.
CRIBB, G.S. (1964) Nitrogen. In: Mellor's comprehensive
treatise on inorganic and theoretical chemistry, London,
Longmans, Vol. 8, Suppl. I, pp. 276-327.
CURTIS, S.E. (1972) Air environment and animal performance.
J. anim. Sci., 35: 628-634.
DALHAMN, T. (1963) Effect of ammonia alone and combined with
carbon particles on ciliary activity in the rabbit trachea in
vivo, with studies of the absorption capacity of the nasal
cavity. Int. J. Air Water Pollut., 7: 531-539.
DALHAMN, T. & SJOHOLM, J. (1961) Studies of SO2, NO2, and
NH3: effect on ciliary activity in the rabbit trachea of
single in vitro exposure and resorption in rabbit nasal
cavity. Acta physiol. Scand., 58: 287-291.
DALLAVALLE, J.M. & DUDLEY, H.C. (1939) Evaluation of odor
nuisance in the manufacture of kraft paper. Public Health
Rep., 54(1): 35-43.
DANECKER, E. (1964) [The jauche poisoning of fish: an
ammonia poisoning.] Osterr. Fischerei., 3/4: 55-68. (in
German).
DAVIES, B.M.A. & YUDKIN, J. (1952) Studies in biochemical
adaptation. The origin of urinary ammonia as indicated by the
effect of chronic acidosis and alkalosis on some renal enzymes
in the rat. Biochem. J., 52: 407-412.
DAY, D.L., HANSEN, E.L., & ANDERSON, S. (1965) Gases and
odors in confinement swine buildings. Trans. Am. Soc. Agric.
Eng., 8: 118-121.
DEAL, P.H., SOUZA, K.A., & MACK, H.M. (1975) High pH,
ammonia toxicity, and the search for life on the Jovian
planets. Origins Life, 6(4): 561-573.
DEAN, J.A., ed. (1979) Lange's handbook of chemistry, 12th
ed., New York, McGraw-Hill Book Company.
DEGRAEVE, G.M., OVERCAST, R.L., & BERGMAN, H.L. (1980)
Toxicity of underground coal gasification condenser water and
selected constitutents to aquatic biota. Arch. environ.
Contam. Toxicol., 9(5): 543-555.
DELISTRATY, D.A., CARLBERG, J.M., VAN OLST, J.C., & FORD,
R.F. (1977) Ammonia toxicity in cultured larvae of the
American lobster (Homarus americanus). In: Proceedings of the
8th Annual Meeting of the World Mariculture Society, San Jose,
Costa Rica, pp. 647-673.
DEMEREC, M., BERTANI, G., & FLINT, J. (1951) A survey of
chemicals for mutagenic action on E. coli. Am. Nat., 85: 119.
DEMUYNCK, M., RAHN, K.A., JANSSENS, M., & DAMS, R. (1976)
Chemical analysis of airborne particulate matter during a
period of unusually high pollution. Atmos. Environ., 10: 21-26.
DE VOOYS, G.G.N. (1968) Formation and excretion of ammonia
in Teleostei. I. Excretion of ammonia through the gills. Arch.
int. Physiol. Biochimie., 76: 268-272.
DODD, K.T. & GROSS, D.R. (1980) Ammonia inhalation toxicity
in cats. Arch. environ. Health, 35: 6-14.
DOIG, P.A. & WILLOUGHBY, R.A. (1971) Response of swine to
atmospheric ammonia and organic dust. J. Am. Vet. Med. Assoc.,
159: 1353-1361.
DOWDEN, B.F. & BENNETT, H.J. (1965) Toxicity of selected
chemicals to certain animals. J. Water Pollut. Control. Fed.,
37(9): 1308-1316.
DOWNING, K.M. & MERKENS, J.C. (1955) The influence of
dissolved-oxygen concentration on the toxicity of un-ionized
ammonia to rainbow trout ( Salmo gairdneri Richardson).
Ann. appl. Biol., 43(2): 243-246.
DREWES, D.R. & HALES, J.M. (1980) Removal of pollutants from
power plant plumes by precipitation, Richland, Washington,
Battelle-Northwest, US Electric Power Research Institute
(Report to the EPRI).
DRIEDZIC, W.R. & HOCHACHKA, P.W. (1976) Control of energy
metabolism in fish white muscle. Am. J. Physiol., 230: 579-582.
DRUMMOND, J.G., CURTIS, S.E., SIMON, J., & NORTON, H.W.
(1980) Effects of aerial ammonia on growth and health of
young pigs. J. anim. Sci., 50: 1085-1091.
DUDA, G.D. & HANDLER, P. (1958) Kinetics of ammonia
metabolism in vivo. J. biol. Chem., 232: 303-314.
DU RUISSEAU, J.P., GREENSTEIN, J.P., WINITZ, M., & BIRNBAUM,
S.M. (1957) Studies on the metabolism of amino acids and
related compounds in vivo. VI. Free amino acid levels in the
tissues of rats protected against ammonia toxicity. Arch.
Biochem. Biophys., 68: 161-171.
EADS, R.B., WISEMAN, J.S., GRIMES, J.E., & MENZIES, G.C.
(1955) Wildlife rabies in Texas. Public Health Rep., 70:
995-1000.
ECKERT, J.W. (1967) Application and use of postharvest
fungicides. In: Torgeson, D.C., ed. Fungicides, New York,
Academic Press, pp. 341.
ECKERT, J.W., KOLBEZEN, M.J., & GARBER, M.J. (1963)
Evaluation of ammonia-generating formations for control of
citrus fruit decay. Phytopathology, 53: 140-148.
ECOLOGICAL ANALYSTS, INC. (1981) The sources, chemistry,
fate, and effects of ammonia in aquatic environments,
Washington DC, American Petroleum Institute.
EDELMANN, C.M., Jr, BIOCHIS, H., SORIANO, J.R., & STARK, H.
(1967) The renal response of children to acute ammonium
chloride acidosis. Pediat. Res., 1: 452-460.
EDJTEHADI, M., SZABUNIEWICZ, M., & EMMANUEL, B. (1978) Acute
urea toxicity in sheep. Can. J. Comp. Med., 42: 63-68.
EDWARDS, J.G. & CONDORELLI, L. (1928) Studies on aglomerular
and glomerular kidneys. II. Physiological. Am.J. Physiol., 86:
383-398.
EGGELTON, A.E.J. (1969) The chemical composition of
atmospheric aerosols on Tees-side and its relation to
visibility. Atmos. Environ., 3: 355-372.
EGLE, J.L., Jr (1973) Retention of inhaled acetone and
ammonia in the dog. Am. Ind. Hyg. Assoc. J., 34(12): 533-539.
ELKINS, H.B. (1950) The chemistry of industrial toxicology,
New York, John Wiley and Sons, 84 pp.
ELKINS, H.B. (1959) Ammonia, NH3. In: The chemistry of
industrial toxicology, 2nd ed., New York, John Wiley and Sons,
86 pp.
EMERSON, K., RUSSO, R.C., LUND, R.E., & THURSTON, R.V.
(1975) Aqueous ammonia equilibrium calculations: effect of pH
and temperature. J. Fish. Res. Board Can., 32: 2379-2383.
EMERY, R.M. & WELCH, E.B. (1969) The toxicity of alkaline
solutions of ammonia to juvenile bluegill sunfish (Lepomis
macrochirus Raf.), Chattanooga, Tennessee, Water Quality
Branch, Division of Health and Safety, Tennessee Valley
Authority, 31 pp (Mimeo).
EPEL, D., STEINHARDT, R., HUMPHREYS, T., & MAZIA, D. (1974)
An analysis of the partial metabolic derepression of sea
urchin eggs by ammonia; the existence of independent pathways.
Dev. Biol., 40: 245-255.
EPIFANO, C.E. & SRNA, R.F. (1975) Toxicity of ammonia,
nitrite ion, nitrate ion, and orthophosphate to Mercenaria
mercenaria and Crassostrea virginica. Mar. Biol., 33(3):
241-246.
ERNST, R.A. (1968) The effect of ammonia on poultry.
Feedstuffs, 40(32): 40.
EUROPEAN INLAND FISHERIES ADVISORY COMMISSION (1970) Water
quality criteria for European freshwater fish. Report on
ammonia and inland fisheries, pp. 12 (EIFAC Technical Paper
No. 11) (Also in: Water Res., 7(7): 1011-1022 (1973)).
EVANS, D.H. (1977) Further evidence for Na/NH4 exchange in
marine teleost fish. J. exp. Biol., 70: 213-220.
EVANS, D.H. (1980) Osmotic and ionic regulation by
freshwater and marine fishes. In: Ali, M.A. ed. Environmental
Physiology of Fishes, New York, Plenum Publishing Co.,
pp. 93-122.
EVANS, D.H., KORMANIK, G.A., & KRASNY, E.J. (1979)
Mechanisms of ammonia and acid extrusion by the little skate,
Raja erinacea. J. exp. Zool., 208: 431-437.
EVANS, J.W. (1979) The construction and use of a
continuous-flow bioassay apparatus to determine a preliminary
un-ionized ammonia 96-h LC50 for the crayfish, Orconectes
nais, Lawrence, Kansas, University of Kansas, 76 pp (M.S.
Thesis).
FARBER, R.J. & ROSSANO, A.T. (1975) A new approach for
measuring atmospheric concentrations of ammonia. Paper
presented at Annual Meeting of the Pacific Northwest
International Section of the Air Pollution Control
Association, Vancouver, British Columbia, November 19-21,
36 pp.
FASEB (1974) Evaluation of the health aspects of certain
ammonium salts as food ingredients, Springfield, Virginia,
Federation of American Societies for Experimental Biology,
21 pp (Prepared for the US Food and Drug Administration under
Contract No. FDA 72-85) (US NTIS PPB-254-532).
FAUCONNEAU, B. & LUQUET, P. (1979) Influence d'une élévation
de température sur l'évolution de l'aminoacidémie et de
l'ammoniémie après le repas chez la truite arc en ciel (Salmo
gairdneri R.). Ann. Biol. anim. Biochim. Biophys., 19(4A):
1063-1079.
FAZEKAS, I.G. (1939) Experimental suprarenal hypertrophy
induced by ammonium hydroxide. Endokrinologie, 21: 315-337.
FAZEKAS, I.G. (1949) [Experimental data on influencing
ovarian functions by simple compounds.] Orv. Hetil., 90:
777-781 (in German).
FAZEKAS, I.G. (1954a) [Enlargement of the parathyroids by
treatment with simple acidotic compounds.] Kirchow's Arch.
Pathol. Anat. Physiol., 324: 531-542 (in German).
FAZEKAS, J.F., TICKTIN, H.E., EHRMENTRAUT, W.R., & ALMAN,
R.W. (1956) Cerebral metabolism in hepatic insufficiency.
Am. J. Med., 21: 843-849.
FEPCC (1972) [Investigation of air pollution in the city of
Tsuruga.] Annu. Rep. Fukui Environ. Pollut. Control Centre, 1:
88-116 (in Japanese).
FERGUSON, W.S., KOCH, W.C., WEBSTER, L.B., & GOULD, J.R.
(1977) Human physiological response and adaptation to
ammonia. J. occup. Med., 19: 319-326.
FETH, J.H. (1966) Nitrogen compounds in natural water. A
review. Water Resour. Res., 2: 41-58.
FISHER, D.W., GAMBELL, A.W., LIKENS, G.E., & BURMANN, F.H.
(1968) Atmospheric contributions to water quality of streams
in the Hubbard Brook Experimental Forest, New Hampshire. Water
Resour. Res., 4: 1115-1126.
FLAKS, A. & CLAYSON, D.B. (1975) The influence of ammonium
chloride on the induction of bladder tumors by 4-ethyl-
sulfonylnaphthalene-1-sulfonamide. Br. J. Cancer, 31: 585.
FLAKS, A., HAMILTON, J.M., & CLAYSON, D.B. (1973) Effect of
ammonium chloride on incidence of bladder tumours induced by
4-ethylsulfonylnapthalene-1-sulfonamide. J. Natl Cancer Inst.,
51: 2007.
FLORKIN, M. & DUCHATEAU, G. (1943) Les formes du système
enzymatique de l'uricalyse et l'évolution du catabolisme
purique chez les animaux. Arch. int. Physiol., 53: 267-307.
FLURY, K.E., DINES, D.E., RODARTE, J.R., & RODGERS, R.
(1983) Airway obstruction due to inhalation of ammonia. Mayo
Clin. Proc., 58: 389-393.
FLYNN, R.J. (1968) A new cage cover as an aid to laboratory
rodent disease control. Proc. Soc. Exp. Biol. Med., 129:
714-717.
FORSTER, R.P. & GOLDSTEIN, L. (1969) Formation of excretory
products. In: Hoar, W.S. & Randall, D.J. ed. Fish Physiology,
New York, Academic Press, Vol. 1, pp. 313-350.
FOSTER, G.L., SCHOENHEIMER, R., & RITTENBERG, D. (1939)
Studies in protein metabolism. V. The utilization of ammonia
for amino acid and creatine formation in animals. J. biol.
Chem., 127: 319-327.
FRANK, J.F. (1948) The toxicity of sodium chlorate
herbicides. Can. J. comp. Med., 12: 216-218.
FRASER, D.I., DYER, W.J., WEINSTEIN, H.M., DINGLE, J.R., &
HINES, J.A. (1966) Glycolytic metabolites and their
distribution at death in the white and red muscle of cod
following various degrees of antemortem muscular activity.
Can. J. Biochem., 44: 1015-1033.
FREEDMAN, L.R. & BEESON, P.B. (1961) Experimental
pyelonephritis. VIII. The effect of acidifying agents on
susceptibility to infection. Yale J. Biol. Med., 33: 318.
FRIZZOLA, J.A. & BAIER, J.H. (1975) Contaminants in
rainwater and their relation to water quality. Water Sewage
Works, 122: 94-95.
FROMM, P.O. (1963) Studies on renal and extra-renal
excretion in a freshwater teleost, Salmo gairdneri. Comp.
Biochem. Physiol., 10: 121-128.
FROMM, P.O. (1970) Effect of ammonia on trout and goldfish.
In: Toxic action of water soluble pollutants on freshwater
fish, Washington DC, US Environmental Protection Agency,
pp. 9-22 (EPA Water Pollution Control Research Series No. 18050
DST 12/70).
FROMM, P.O. & GILLETTE, J.R. (1968) Effect of ambient
ammonia on blood ammonia and nitrogen excretion of rainbow
trout (Salmo gairdneri). Comp. Biochem. Physiol., 26: 887-896.
FUHRER, J. (1985) Formation of secondary airpollutants and
their occurrence in Europe. Experientia (Basel), 41: 286-301.
FURST, P., JOSEPHSON, B., MASCHIO, G., & VINNARS, E. (1969)
Nitrogen balance after intravenous and oral administration of
ammonium salts to man. J. appl. Physiol., 26(1): 13-22.
FURST, P., JONSSON, A., JOSEPHSON, B., & VINNARS, E. (1970)
Distribution in muscle and liver vein protein of 15N
administered as ammonium acidosis to man. J. appl. Physiol.,
29(3): 307-312.
GALL, W.K. (1980) Effect of cow faeces on the ecology of
laboratory streams, Madison, Wisconsin, University of
Wisconsin, pp. 73-83 (M.S. Thesis).
GAY, W.M.B., CRANE, C.W., & STONE, W.D. (1969) The
metabolism of ammonia in liver disease: a comparison of
urinary data following oral and intravenous loading of [15N]
ammonium lactate. Clin. Sci., 37: 815-823.
GEORGII, H.W. (1963) Oxides of nitrogen and ammonia in the
atmosphere. J. geophys. Res., 68: 963-970.
GEORGII, H.W. & MULLER, W.J. (1974) On the distribution of
ammonia in the middle and lower trophosphere. Tellus, 26:
180-184.
GIBBS, M. & CALO, N. (1959) Factors affecting light induced
fixations of carbon dioxide by isolated spinach chloroplasts.
Plant Physiol., 34: 318-323.
GIBSON, G.E., ZIMBER, A., KROOK, L., & VISEK, W.J. (1971)
Nucleic acids and brain and intestinal lesions in ammonia
intoxicated mice. Fed. Proc., 30: 578.
GIGUZ, T.L. (1968) Effect of low concentrations of ammonia
and nitrogen oxides on adolescents undergoing vocational
training in the chemical industry. Hyg. Sanit., 33(7-9):
431-434.
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity tests.
Sewage ind. Wastes, 24(11): 1397-1401.
GILLIAM, J.W., DANIELS, R.B., & LUTZ, J.F. (1974) Nitrogen
content of shallow ground water in North Carolina Coastal
Plain. J. environ. Qual., 3: 147-151.
GIRARD, J.O. & PAYAN, P. (1980) Ion exchanges through
respiratory and chloride cells in freshwater and seawater
adapted teleosteans. Am. J. Physiol., 238: R260-R268
(Regulatory Integrative Comp. Physiol. 7).
GOLDHABER, M.B. & KAPLAN, I.R. (1974) The sulfur cycle. In:
Goldberg, D., ed. The sea: ideas and observations on progress
in the study of the seas, New York, John Wiley and Sons,
Vol. 5, pp. 569-655.
GOLDMAN, A.S. & YAKOVAC, W.C. (1964) Salicylate
intoxication and congenital anomalies. Arch. environ.
Health, 8: 648-656.
GOLDSTEIN, L., CLAIRBORNE, J.B., & EVANS, D.E. (1982)
Ammonia excretion by the gills of two marine teleost fish: the
importance of NH4+ permeance. J. exp. Zool., 219: 395-397.
GOLDSTEIN, L. & FORSTER, R.P. (1961) Source of ammonia
excreted by the gills of the marine teleost, Myoxocephalus
scropius. Am. J. Physiol., 200(5): 1116-1118.
GOLYAKOVA, L.P. (1971) [Present-day state of industrial
hygiene problems in hydrometallurgical production of tungsten
and molybdenum salts and oxides.] Gig. Tr. Prof. Zabol.,
15(2): 4-8 (in Russian with English summary).
GOODMAN, L.S. & GILMAN, A. (1970) The pharmacological basis
of therapeutics, 4th ed., New York, The Macmillan Company,
1794 pp.
GORDON, R.J. & BRYAN, R. (1973) Ammonium nitrate in airborne
particles in Los Angeles. Environ. Sci. Technol., 7: 645-647.
GORDON, H.H., MCNAMARA, H., & BENJAMIN, H.R. (1948) Response
of young infants to ingestion. Pediatrics, 2: 290-302.
GRANT, W.M. (1974) Toxicology of the eye: drugs, chemicals,
plant venoms, 2nd ed., Springfield, Illinois, C.C. Thomas,
pp. 96-101, 121-128.
GREENSTEIN, J.P., WINITZ, M., GULLINO, P., BIRNBAUM, S.M., &
OTEY, M.C. (1956) Studies on the metabolism of amino acids
and related compounds in vivo. III. Prevention of ammonia
toxicity by arginine and related compounds. Arch. Biochem.
Biophys., 64: 342-354.
GREGORY, R.B. (1977) Synthesis and total excretion of waste
nitrogen by fish of the Periopthalmus (mudskipper) and
Scartelass families. Comp. Biochem. Physiol., 51A: 33-36.
GRINDLEY, J. (1946) Toxicity to rainbow trout and minnows of
some substances known to be present in waste water discharged
to rivers. Ann. appl. Biol., 33(1): 103-112.
GROLLMAN, A. (1929) The urine of the goosefish (Lophius
piscatorius): its nitrogenous constituents with special
reference to the presence in it of trimethylamine oxide.
J. Biol. Chem., 81: 267-278.
GROSJEAN, D., DOYLE, G.J., MISCHKE, T.M., POE, M.P., FITZ,
D.R., SMITH, J.P., & PITTS, J.N., Jr (1976) The
concentration, size distribution, and modes of formation of
particulate nitrate, sulfate, and ammonium compounds in the
eastern part of the Los Angeles Basin. Presented at: The 69th
Annual Meeting of the Air Pollution Control Association,
Portland, Oregon, June 27 - July 1 1976, 14 pp (Paper 76-20.3).
GROSSI, C.E., PRYTZ, B., & ROUSSELOT, L.M. (1968) Adenosine
triphosphate in prevention of ammonia intoxication in dogs.
Surg. Forum, 19: 363-369.
GRUBE, H.J. (1973) [Load and loading capacity of submerged
macrophytes in running water in southern Lower Saxony.]
Verh. Ges. Okol., Saarbrücken: 103-109 (in German with English
summary).
GUERIN-ANCEY, O. (1976a) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
II. Effets du jeune sur l'excretion d'ammoniac et d'uree.
Aquaculture, 9(2): 187-294.
GUERIN-ANCEY, O. (1976b) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
III. Effets du volume et de la concentration initiale en
ammoniac sur l'excretion d'ammoniac et d'uree. Aquaculture,
9(2): 253-258.
GUPTA, B.N., KHANNA, R.N., & DATTA, K.K. (1979) Toxicological
studies of ammonium sulfamate in rat after repeated oral
administration. Toxicology, 13(1): 45-49.
GYORE, K. & OLAH, J. (1980) Ammonia tolerance of Moina
rectirostris Leydig (Cladocera). Aquaculture Hungarica
(Szarvas), 2: 50-54.
HAGGARD, H.W. (1924) Action of irritant gases upon the
respiratory tract. J. ind. Hyg., 5: 390-398.
HAHN, M., MASSEN, O., NENCKI, N., & PAWLOW, J. (1983) [The
connection between the vena cava inferior and the portal vein
and its consequences for the organism.] Arch. Exp. Path.
Pharmakol., 32: 161-210 (in German).
HALL, L.W., Jr, BUIKEMA, A.L., Jr, & CAIRNS, J., Jr (1978)
The effects of a stimulated refinery effluent on the grass
shrimp, Palaemonetes pugio. Arch. environ. Contam. Toxicol.,
7(1) 23-35.
HANTZSCH, S. & LAHMANN, E. (1970) [Ammonia determinations in
city air.] Schriftner. Ver. Wasser- Boden- Lufthyg. (Berlin-
Dahlem), 33: 35-39 (in German).
HARADER, R.R. & ALLEN, G.H. (1983) Ammonia toxicity to
chinook salmon parr: reduction in saline water. Trans Am.
Fish. Soc., 112: 834-837.
HARDING, A.J. (1959) Ammonia manufacture and uses, London,
Oxford University Press.
HAYNES, R.J. & GOH, K.M. (1977) Evaluation of potting media
for commercial nursery production of container grown plants.
II. Effects of media, fertilizer nitrogen and nitrification
inhibitor on yield and nitrogen uptake of Callistephus
chinensis (L.) nees "Pink Princess". N.Z. J. Agric. Res., 20:
371-381.
HAWKINS, R.A., MILLER, A.L., NIELSEN, R.C., & VEECH, R.L.
(1973) The acute action of ammonia on rat brain metabolism in
vivo. Biochem. J., 134: 1001-1008.
HAWKINS, D.B., DEMETER, M.J., & BARNETT, T.E. (1980) Caustic
ingestion: Controversies in management. A review of 14 cases.
Laryngoscope, 90: 98-109.
HAZEL, C.R., THOMSEN, W., & MEITH, S.J. (1971) Sensitivity
of striped bass and stickleback to ammonia in relation to
temperature and salinity. Calif. Fish Game, 57(3): 138-153.
HAZEL, R.H., BURKHEAD, C.E., & HUGGINS, D.G. (1979) The
development of water quality criteria for ammonia and total
residual chlorine for the protection of aquatic life in two
Johnson County, Kansas streams, Washington DC, Office of Water
Research and Technology, US Department of the Interior, 146 pp.
HEALY, T.V. (1974) Ammonia and related pollutants at
Harwell. Atmos. Environ., 8: 81-83.
HEALY, T.V., MCKAY, H.A.C., PILBEAM, A., & SCARGILL, D.
(1970) Ammonia and ammonium sulfate in the troposphere over
the United Kingdom. J. geophys. Res., 75: 2317-2321.
HEIL, G.W. & DIEMONT, W.M. (1983) Raised nutrient levels
change heathland into grassland. Vegetatio, 53: 113-120.
HEISLER, N. (1984) Acid-base regulation in fishes. In: Hoar,
W. & Randall, D.J., ed. Fish physiology, New York, Academic
Press, Vol. 10A.
HELMERS, S., TOP, F.H., & KNAPP, L.W., Jr (1971) Ammonia
injuries in agriculture. J. Iowa Med. Soc., 61: 271-280.
HEMENS, J. (1966) The toxicity of ammonia solutions to the
mosquito fish ( Gambusia affinis Baird & Girard). J. Proc.
Inst. Sewage Purif., 65: 265-271.
HENIS, Y. & CHET, I. (1967) Mode of action of ammonia on
Sclerotium rolfsii. Phytopathology, 57: 425-427.
HERBERT, D.W.M. (1956) La toxicité d'un effluent d'eau
d'égout. Bull. Cent. belge Etude Doc. Eaux, 32: 115-120.
HERBERT, D.W.M. (1962) The toxicity of rainbow trout of
spent still liquors from the distillation of coal. Ann. appl.
Biol., 50(4): 755-777.
HERBERT, D.W.M. & SHURBEN, D.S. (1965) The susceptibility of
salmonid fish to poisons under estuarine conditions. II.
Ammonium chloride. Int. J. Air Water Pollut., 9: 89-91.
HIDY, G.M. (1974) Characterization of aerosols in California
(ACHEX), Thousand Oaks, California, Rockwell International,
Vol. 1-4 (Final Report prepared for California Air Resources
Board).
HILLABY, B.A. & RANDALL, D.J. (1979) Acute ammonia toxicity
and ammonia excretion in rainbow trout (Salmo gairdneri). J.
Fish. Res. Board Can., 36(6): 621-629.
HINTZ, H.F., LOWE, J.E., CLIFFORD, A.J. ET AL. (1970)
Ammonia intoxication resulting from urea ingestion by ponies.
J. Am. Vet. Med. Assoc., 157: 963-966.
HOEFT, R.G., KENNEY, D.R., & WALSH, L.M. (1972) Nitrogen and
sulfur in precipitation and sulfur dioxide in the atmosphere
in Wisconsin. J. environ. Qual., 1: 203-208.
HOGAN, J.P. (1961) The absorption of ammonia through the
rumen of sheep. Aust. J. biol. Sci., 14: 448-460.
HOLETON, G.F., NEUMANN, P., & HEISLER, N. (1983) Branchial
ion exchange and acid-base regulation after strenous exercise
in rainbow trout. Resp. Physiol., 51: 303-318.
HOLLAND, G.A., LASATER, J.E., NEUMANN, E.D., & ELDRIDGE, W.E.
(1960) Experiments with inorganic pollutants - ammonia. Toxic
effects of organic and inorganic pollutants on young salmon
and trout, State of Washington, Department of Fisheries,
pp. 183-187 (Research Bulletin No. 5).
HOLT, G.J. & ARNOLD, C.R. (1983) Effects of ammonia and
nitrite on growth and survival of red drum eggs and larvae.
Trans. Am. Fish. Soc., 112: 314-318.
HOLT, J.D. & MALCOLM, S.A. (1979) Evaluation of fish loading
rates in regulatory static bioassays. Tech. Rep. -Fish. Mar.
Serv. (Can.)., 862: 146-160.
HORNE, F.R. & MCINTOSH, S. (1979) Factors influencing
distribution of mussels in the Blanco River of central Texas.
Nautilus, 94(4): 119-133.
HSIA, Y.E. (1974) Inherited hyperammonemic syndromes.
Gastroenterology, 67: 347-374.
HUNGER, W. (1978) [Dying off of old spruce trees in the
vicinity of a pig fattening station.] Beitrage
Forstwirtschaft, 4: 188-189 (in German).
HUNT, R.D. (1977) Measurement of partial pressure of ammonia
in breath and arterial blood. Evidence for secretion of
ammonia into expired gas by metabolism of monoamines in the
lung. Diss. Abstr. Int. Ser. B, 37: 3279.
ILO (1970) Permissible levels of toxic substances in the
working environment. In: Proceedings of the 6th Session of the
Joint ILO/WHO Committee on Occupational Health, Geneva, 4-10
June 1968, Geneva, International Labour Office, 405 pp.
INDUSTRIAL BIO-TEST LABORATORIES, INC. (1973) Irritation
threshold evaluation study with ammonia, Industrial Bio-Test
Laboratories, Inc. (Report to International Institute of
Ammonia Refrigeration, Publication No. 663-03161).
IWAOKA, W.T., OKRONE, C.A., SULLIVAN, J.J., & JOHNSON, C.A.
(1981a) Effect of pH and ammonium ions on mutagenic activity
in cooked beef. Cancer Lett., 12: 335.
IWAOKA, W.T., URONE, C.A., SULLIVAN, J.J., MEAKER, E.H., &
JOHNSON, C.A. (1981b) A source of error in mutagen testing
of foods. Cancer Lett., 11: 225.
JACOBS, M.H. (1940) Some aspects of cell permeability to
weak electrolytes. Cold Spring Harbour Sym. Quant. Biol., 8:
30-39.
JACQUEZ, J.A., POPPELL, J.W., LAWRENCE, W., Jr, & ROBERTS,
K.E. (1957) Clinical significance of the partial pressure of
ammonia (pNH3) in patients with ammonium toxicity. Clin. Res.
Proc., 5: 20 (Abstract).
JACQUEZ, J.A., POPPELL, J.W., & JELTSCH, R. (1959) Partial
pressure of ammonia in aveolar air. Science, 129: 269-270.
JAFFE, H.L., BODANSKY, A., & BLAIR, J.E. (1932a) Effects of
parathormone (parathyroid preparation) and ammonium chloride
on bones of rabbits. J. exp. Med., 55: 695-701.
JAFFE, H.L., BODANSKY, A., & CHANDLER, J.P. (1932b) Ammonium
chloride decalcification, as modified by calcium intake: the
relation between generalized osteoporosis and osteitis
fibrosa. J. exp. Med., 56: 823-834.
JANICKI, R.H. (1970) Renal adaptation during chronic NH4Cl
acidosis in the rat: no role for hyperplasia. Am. J. Physiol.,
219(3): 613-618.
JANSSEN, Th. W. (1982) [Intensive animal husbandry in
relation to space and environment,] Utrecht, The Netherlands,
Dutch State Forest Service (in Dutch).
JOHANNING, G.L., MUHRER, M.E., & GADDY, J.H. (1978)
Nutritional evaluation of chlorinated-ammoniated corn stover.
Paper presented at: The Joint Meeting of the American Dairy
Science Association and the American Society of Animal
Science, 11 July 1978.
JOHNSON, J.D., EPEL, D., & PAUL, M. (1976) Intracellular pH
and activation of sea urchin eggs after fertilization. Nature
(Lond.), 262(5570): 661-664.
JONES, K. (1973) Ammonia. In: Bailar, J.C., Jr, Emeleus,
H.J., Nyholm, R., & Trotman-Dickenson, A.F., ed. Comprehensive
inorganic chemistry, New York, Pergammon Press, Vol. 2, pp.
199-227.
JONES, R.D. & HOOD, M.A. (1980) Effects of temperature, pH,
salinity and inorganic nitrogen on the rate of ammonium
oxidation by nitrifiers isolated from wetland environments.
Microb. Ecol., 6: 339-347.
JUDE, D.J. (1973) Sublethal effects of ammonia and cadmium
on growth of green sunfish, East Lansing, Michigan, Michigan
State University, 193 pp (Ph.D. Thesis).
KADOWAKI, S. (1976) Size distribution of atmospheric total
aerosols, sulfate, ammonium and nitrate particulates in the
Nagoya area. Atmos. Environ., 10: 39-43.
KAGOTA, K., IWASE, T., KOJIMA, T., NIIYAMA, M., & NAMIOKA, S.
(1979) Diammonium citrate addition to a diet restricted
non-essential amino acids for young pigs. Jap. J. vet. Sci.,
41: 131-138.
KAMIN, H. & HANDLER, P. (1957) Amino acid and protein
metabolism. Annu. Rev. Biochem., 26: 419-490.
KAPEGHIAN, J.C., MINCER, H.H., JONES, A.B., VERLANGIERI, A.J.,
& WATERS, I.W. (1982) Acute inhalation toxicity of ammonia
in mice. Bull. environ. Contam. Toxicol., 29: 371-378.
KARNAKY, K.J., Jr, KINTER, L.B., KINTER, W.B., & STIRLING,
C.E. (1976) Teleost chloride cell. II. Autoradiographic
localization of gill Na, K-ATPase in killifish Fundulus
heterclitus adapted to low and high salinity environments.
J. cell Biol., 70: 157-177.
KATZ, M. & PIERRO, R.A. (1967) Estimates of the acute
toxicity of ammonia-urea plant wastes to coho salmon,
Oncorhynchus kisutch, Seattle, Washington, Fisheries Research
Institute, College of Fisheries, University of Washington,
15 pp (Final report).
KEESEE, R.G., HOPF, S.B., & MOYERS, J.L. (1975) The
distribution and characteristics of particulate sulfates in
the southwest desert atmosphere. Presented at: The
International Conference on Environmental Sensing and
Assessment, Las Vegas, Nevada, 14-19 September, 1975, Vol. 2,
5 pp (Paper 23-6).
KEMPINSKA, H. (1968) [The effect of ammonia fertilizers on
fish.] Gospod. Rybn., 20: 3-5 (in Russian).
KEPLINGER, M.L., SCHADEBERG, K.J., GOODE, J.W., & CALANDRA,
J.C. (1973) Irritation threshold evaluation study with
ammonia. In: Report to the Institute of Ammonia Refrigeration,
Northbrook, Illinois, Industrial Bio-Test Laboratories, Inc.
KERN, D.M. (1960) The hydration of carbon dioxide. J. Chem.
Ed., 37: 14-23.
KERSTETTER, T.H., KIRSCHNER, L.B., & RAFUSE, D.D. (1970) On
the mechanisms of sodium ion transport by the irrigated gills
of rainbow. J. gen. Physiol., 56: 342-359.
KEYES, W.F. (1975) Nitrogen. In: Mineral facts and problems,
Washington DC, US Department of the Interior, pp. 749-760
(Bureau of Mines Bulletin No. 667).
KHOLDEBARIN, B. & OERTLI, J.J. (1977) Effect of pH and
ammonia on the rate of nitrification of surface water.
J. Water Pollut. Control Fed., 49: 1693-1697.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.N. (1972)
Serum- and tissue-ammonia nitrogen and tissue-water values in
ammonia-intoxicated sheep. Am. J. Vet. Res., 33: 1187-1190.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.M. (1973)
Hemogram of sheep acutely intoxicated with ammonia.
Am. J. Vet. Res., 34: 587-589.
KIRSCHNER, L.B., GREENWALD, L., & KERSTETTER, T.H. (1973)
Effect of amiloride on sodium transport across body surfaces
of freshwater animals. Am. J. Physiol., 224: 832-837.
KLEMM, K. (1967) The effect of N source on the yield of
various crop plants. Bodenkultur, 18: 210-218.
KLENDSHOJ, N.C. & REJENT, T.A. (1966) Tissue levels of some
poisoning agents less frequently encountered. J. Forensic
Sci., 11: 75-80.
KLING, H.F. & QUARLES, C.L. (1974) Effect of atmospheric
ammonia and the stress of infectious bronchitis vaccination on
Leghorn males. Poult. Sci., 53: 1161-1167.
KLUESENER, J.W. & LEE, G.F. (1974) Nutrient loading from a
separate storm sewer in Madison, Wisconsin. J. Water Pollut.
Control Fed., 46(5): 920-936.
KNEPP, G.L. & ARKIN, G.F. (1973) Ammonia toxicity levels and
nitrate tolerance of channel catfish. Prog. Fish-Cult., 35(4):
221-224.
KNOWLES, G., DOWNING, A.L., & BARRETT, M.J. (1965)
Determination of kinetic constants for nitrifying bacteria in
mixed culture, with the aid of an electronic computer.
J. gen. Microbiol., 38: 263.
KOENIG, H. & KOENIG, R. (1949) Production of acute pulmonary
edema by ammonium salts. Proc. Soc. Exp. Biol. Med., 70:
375-380.
KOPP, A., FELLER, U., & ERISMANN, K.H. (1974) [Studies on
the regulation of nitrogen assimilation by Lemna minor in
transition from ammonium to nitrate feeding or from nitrate to
ammonium feeding under conditions of photosynthesis.]
Z. Pflanzen physiol., 73: 456-460 (in German).
KORMANIK, G.A. & CAMERON, J.N. (1981) Ammonia excretion in
the FW catfish: the role of diffusion. Am. Soc. Zool., 21(4):
1042.
KORTING, W. (1969a) [The harmful effect of ammonia on fish.]
Munch. Beitr. Abwasser-. Fisch.-Flussbiol., 16: 38-48 (in
German).
KORTING, W. (1969b) [Investigations on the effects of
ammonia on the blood of fish.] Wasser Abwasser Forsch., 4:
154-159 (in German).
KRYSTAL, G.W. & POCCIA, D. (1979) Control of chromosome
condensation in the sea urchin egg. Exp. cell Res., 123(2):
207-219.
KULASEK, G., SIWECKA, B., OWCZARCZYK, B., RYNCA, J., & BAREJ,
W. (1975) Effect of protein level and synthesize chloride
addition to diet on ammonia detoxification in rats. Zentralbl.
Veterinaermed., 22(3): 215-223.
KUSHNIR, J.J., MALKIN, H.I., MOHNEN, V.A., JENCHA, A.J., &
MCLAREN, E.H. (1970) Non-photochemical aerosol formation
from the anhydrous reaction between ammonia and sulfur dioxide
gases. Mechanism for the formation of ammonium sulfate in the
atmosphere, Albany, New York, Department of Chemistry and
Atmospheric Sciences Research Center, State University of New
York (Report 165 of National Air Pollution Control
Administration Research Grant 5RO1A).
KUSTOV, V.V. (1967) [Means of determining the maximum
allowable concentration of toxic products of natural human
metabolism.] In: General questions of industrial toxicology,
Moscow, pp. 63-65. (National Aeronautics and Space
Administration Technical Translation NASA TT F-11,358) (in
Russian).
LANDAHL, H.D. & HERRMANN, R.G. (1950) Retention of vapors
and gases in the human nose and lung. Arch. ind. Hyg. occup.
Med., 1: 36-45.
LANGOWSKA, I. & MOSKAL, J. (1974) Effect of ammonia and urea
on nitrobacteria in water environment. Pol. Arch. Hydrobiol.,
21(1): 119-123.
LARSON, T.V., COVERT, D.S., FRANK, R., & CHARLSON, R.J.
(1977) Ammonia in the human airways: neutralization of
inspired acid sulfate aerosols. Science, 197: 161-163.
LECHTENBERG, V.L., BUETTNER, M.R., HOLT, D.A., RICHEY, C.B., &
PARSONS, S.D. (1977) Hay preservation by anhydrous ammonia
treatment. In: Grain and Forage Harvesting. Proceedings of the
First International Grain and Forage Conference, St. Joseph,
American Society of Agricultural Engineers, pp. 327-328, 338.
LEE, J.A., (1985) Effects of NO2 on aquatic ecosystems. In:
Study on the need of NO2 long term limit value for the
protection of terrestrial and aquatic ecosystems (Final Report
Feb. 1985).
LEE, R.E., Jr & GORANSON, S. (1976) National air
surveillance cascade impact network. III. Variations in size
of airborne particulate matter over a three-year period.
Environ. Sci. Technol., 10: 1022-1027.
LEITHE, W. (1971) The analysis of air pollutants, Ann Arbor,
Michigan, Ann Arbor Science Publishers.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odour
threshold determination of 52 odorant chemicals. J. Air
Pollut. Control Assoc., 19: 91-95.
LEUVEN, R.S.E.W. & SCHUURKES, J.A.A.R. (1984) [Effects of
acid precipitation containing sulfur and nitrogen on weakly
buffered waters low in nutrients] The Netherlands, Laboratory
of Aquatic Ecology, University of Nymegen, and Ministry of
Housing, Physical Planning and Environment, 131 pp (in Dutch).
LEVI, G., MORISI, G., COLETTI, A., & CATANZARO, R. (1974)
Free amino acids in fish brain: normal levels and changes upon
exposure to high ammonia concentrations in vivo, and upon
incubation of brain slices. Comp. Biochem. Physiol., 49A:
623-636.
LEVY, D.M., DIVERTIE, M.B., LITZOW, T.J., & HENDERSON, J.W.
(1964) Ammonia burns of the face and respiratory tract.
J. Am. Med. Assoc., 190: 873-876.
LIEBHARDT, W.C., GOLT, C., & TUPIN, J. (1979) Nitrate and
ammonium concentrations of ground water resulting from poultry
manure applications. J. environ. Qual., 8: 211-215.
LIECHTI, P.M. & HUGGINS, D.G. (1980) Effects of a 24-h
ammonia injection on stream drift and benthic standing crop.
Trans. Kansas Acad. Sci., 83(3): 140.
LIETZKE, M.H. (1978) A kinetic model for predicting the
composition of chlorinated water discharged from power plant
cooling systems. In: Water chlorination environmental impact
and health effects, Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., Vol. 2, pp. 707-716.
LILLIE, R.J. (1970) Ammonia. In: Air pollutants affecting
the performance of domestic animals. A literature review,
Washington DC (Agriculture Handbook No 380).
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide
formulations against two brackish water organisms, the bleak
(Alburnus alburnus) and the harpacticoid (Nitocra spinipes).
Chemosphere, 11(12): 843-851.
LINZON, S.N. (1971) Effects of air pollutants on vegetation.
In: McCormac, B.M., ed. Introduction to the scientific study
of atmospheric pollution, Dordrecht, Holland, D. Reidel
Publishing Company, pp. 131-151.
LITAV, M. & AGAMI, M. (1976) Relationship between water
pollution and the flora of two coastal rivers of Israel.
Aquat. Biol., 2(1): 23-41.
LITAV, M. & LEHRER, Y. (1978) The effects of ammonium in
water on Potamageton lucens. Aquat. Biol., 5(2): 127-138.
LITTON BIONETICS, INC. (1975) Mutagenic evaluation of
compound FDA 73-42 ammonium sulfate, granular, food grade,
Washington DC, Litton Bionetics (Prepared for US Food and Drug
Administration) (NTIS PB-245 506).
LLOYD, R. (1961) Effect of dissolved oxygen concentrations
on the toxicity of several poisons to rainbow trout ( Salmo
gairdnerii Richardson). J. exp. Biol., 38: 447-455.
LLOYD, R. & HERBERT, D.W.M. (1960) The influence of carbon
dioxide on the toxicity of un-ionized ammonia to rainbow trout
( Salmo gairdnerii Richardson). Ann. appl. Biol., 48(2):
399-404.
LLOYD, R. & ORR, L.D. (1969) The diuretic response by
rainbow trout to sub-lethal concentrations of ammonia. Water
Res., 3(5): 335-344.
LOBASHOV, M.E. (1937) [Nature of the effects of chemical
agents on the mutation process of Drosphila melanogaster.]
Genetica, 19: 200-241 (in German).
LOBASHOV, M.E. & SMIRNOV, F.A. (1934) [Effect of acetic acid
on nondisjunction and mutations in Drosphila melanogaster.]
Dokl. Acad. Sci. USSR N.S., 2: 307 (in Russian with German
summary).
LODGE, J.P., Jr, MACHADO, P.A., PATE, J.B., SHEESLEY, D.C., &
WARTBURG, A.F. (1974) Atmospheric trace chemistry in the
American humid tropics. Tellus, 26: 250-253.
LOEHR, R.C. (1974) Characteristics and comparative magnitude
of non-point sources. J. Water Pollut. Control Fed., 46(8):
1849-1872.
LOSADA, M. & ARNON, D.I. (1963) Selective inhibitors of
photosynthesis. In: Hochester, R. & Quastel, J.H., ed.
Metabolic inhibitors, New York, Academic Press, Vol. 2, p. 559.
LOSADA, M., HERRERA, J., MALDONADO, J.M., & PANEQUE, A.
(1973) Mechanism of nitrate reductase reversible inactivation
by ammonia in Chlamydomonas. Plant Sci. Lett., 1: 31-37.
LOTSPEICH, W.D. (1965) Renal hypertrophy in metabolic
acidosis and its relation to ammonia excretion.
Am. J. Physiol., 208(6): 1135-1142.
LUBINSKI, K.S. (1979) Monitoring bluegill swimming behaviour
and the effects of sublethal ammonium chloride gradients,
Blacksburg, Virginia, Virginia Polytechnic Institute and State
University, 99 pp (Ph.D. Thesis).
LUBINSKI, K.S., SPARKS, R.E., & JAHN, L.A. (1974) The
development of toxicity indices for assessing the quality of
the Illinois River, Urbana, Illinois, Water Resources Center,
University of Illinois, 46 pp (Research Report No. 96).
LUBINSKI, K.S., DICKSON, K.L., & CAIRNS, J., Jr (1980)
Effects of abrupt sublethal gradients of ammonium chloride on
the activity level, turning, and preference-avoidance
behaviour of bluegills, In: Eaton, J.G., Parrish, P.R., &
Hendricks, A.C. ed. Aquatic toxicology, Philadelphia,
Pennsylvania, American Society for Testing and Materials,
pp. 328-340 (ASTM STP 707).
MACEWEN, J.D. & VERNOT, E.H. (1972) Toxic hazards research
unit annual technical report (NTIS AD 755-358).
MACEWEN, J.D., THEODORE, J., & VERNOT, E.H. (1970) Human
exposure to EEL concentrations of monomethylhydrazine. In:
Proceedings of the 1st Annual Conference on Environmental
Toxicology, Ohio, Wright-Patterson Air Force Base,
9-11 September, 1970, Aerospace Medical Research Laboratory,
pp. 355-363 (AMRL-TR-70-102, Paper No. 23).
MCBARRON, E.J. & MCINNES, P. (1968) Observations on urea
toxicity in sheep. Aust. Vet. J., 44: 90-96.
MCBEAN, R.L., NEPPEL, M.J., & GOLDSTEIN, L. (1966)
Glutaminate dehydrogenase and ammonia production in the eel
(Anguilla rostrata). Comp. Biochem. Physiol., 18: 909-920.
MCBRIDE, R.L. & LAING, D.G. (1979) Threshold determination
by triangle testing: Effects of judgmental procedure,
positional bias and incidental training. Chem. Senses Flavor.,
4(4): 319-328.
MCCALLAN, S.E.A. & SETTERSTROM, C. (1940) Toxicity of
ammonia, chlorine, hydrogen cyanide, hydrogen sulphide, and
sulphur dioxide gases. I. General methods and correlations.
Contrib. Boyce Thompson Inst., 11: 325-330.
MCCAY, C.M. & VARS, H.M. (1931) Studies upon fish blood and
its relation to water pollution. In: A biological survey of
the St. Lawrence Watershed. Supplement to the 20th Annual
Report, 1930, Albany, New York, New York Conservation
Department, pp. 230-233 (Biol. Survey (1930), No. V).
MCCORMICK, J.H., BRODERIUS, S.J., & FIANDT, J.T. (1984)
Toxicity of ammonia to early life stages of the green sunfish
Lepomis cyanellus. Environ. Pollut. Ser. A., 36: 147-163.
Erratum: Environ. Pollut. Ser. A. (in press).
MCDONALD, D.G. & WOOD, C.M. (1981) Branchial and renal acid
and ion fluxes in the rainbow trout at low environmental pH.
J. exp. Biol., 93: 101-118.
MCKAY, H.A.C. (1969) Ammonia and air pollution. Chem. Ind.,
34: 1162-1165.
MCKHANN, G.M. & TOWER, D.B. (1961) Ammonia toxicity in
cerebral oxidative metabolism. Am. J. Physiol., 200: 420-424.
MAETZ, J. (1972) Branchial sodium exchange and ammonia
excretion in the goldfish Carassius auratus. Effects of
ammonia-loading and temperature changes. J. exp. Biol., 56:
601-620.
MAETZ, J. (1973) Na+/NH4+, Na+/H+ exchanges and NH3 movement
across the gill of Carassius auratus. J. exp. Biol., 58:
255-275.
MAETZ, J. & GARCIA-ROMEU, F. (1964) The mechanism of sodium
and chloride uptake by the gills of a freshwater fish,
Carassius auratus. II Evidence for NH4+/Na+ and HCO3-/Cl-
exchanges. J. gen. Physiol., 47: 1209-1227.
MAGALHAES BASTOS, J.A. (1954) [Importance of ammonia as an
ichthyotoxic substance,] Brazil, Serv. Piscicultura, Dept.
nacl. obras contra secas, Ministerio viacao e obras publicas,
pp. 115-132 (Publ. Ser. 1-C, No. 159) (in Portuguese).
MAI, K.L. (1977) Energy and petrochemical raw materials
through 1990. Chem. Eng., 84: 122-128.
MALACEA, I. (1966) [Contributions to knowledge of the
cyanide, ammonia, mercury and arsenic toxic action on some
species of fishes and on Daphnia.] Stud. Prot. Epurarea Apelor
Inst. Stud. Cercet Hidrotehnice, 7(2: 751-792 (English
summary) Biol. Abstr., 50: 92715 (1969).
MALACEA, I. (1968) [Studies on the acclimatization of fish
to high concentrations of toxic substances.] Arch. Hydrobiol.,
65(1): 74-95 (in German with English summary).
MALLERY, C.H. (1979) Ammonium stimulated properties of a
K+-dependent ATPase in Opsanus beta, a marine teleost with an
NH4+/Na+ exchange pump. Am. Zool., 19: 944.
MANGOLD, C.A. (1971) Investigation of occupational exposure
to ammonia, Washington, Puget Sound Naval Shipyard, Industrial
Hygiene Division of Investigation.
MANGUM, C.P., SILVERTHORN, S.U., HARRIS, J.L., TOWLE, D.W., &
KRALL, A.R. (1976) The relationship between blood pH,
ammonia excretion and adaptation to low salinity in the blue
crab Callinectes sapidus. J. exp. Zool., 195(1): 129-136.
MANGUM, C.P., DYKENS, J.A., HENRY, R.P., & POLITES, G.
(1978) The excretion of NH4+ and its ouabain sensitivity in
aquatic annelids and molluscs. J. exp. Zool., 203: 151-157.
MARSCHANG, F. (1972) [The NH3 content of air in stables and
the fattening capacity of cows.] Dtsch. Tieraerztl.
Wochenschr., 79: 214-216 (in German).
MARSCHANG, F. & CRAINICEANU, E. (1971) [Investigations on
the connections between cowshed climate and calf mortality.]
Berlin München Tieraerztl. Wochenschr., 84: 417-420 (in
German).
MARSCHANG, F. & PETRE, C. (1971) [The NH3 content of cowshed
air and its influence on morbidity and animal lossess in
cattle-fattening pens.] Zentralbl. Veterinaermed., 18: 646-654
(in German).
MATASA, C. & MATASA, E. (1968) L'industrie moderne des
produits azotés, Paris, Dunod, pp. 63-208.
MATSUMOTO, H., OKAMURA, K., & TAKAHASHI, E. (1976) Changes
in activity and isoenzymes of intervase in cucumber leaves due
to nitrogen status. Plant Cell Physiol., 17: 867-874.
MATUSIAK, K. (1976) Studies on the purification of
wastewater from the nitrogen fertilizer industry by intensive
algal cultures. Acta microbiol. Pol., 25(3): 233-242.
MAYAN, M.H. & MERILAN, C.P. (1972) Effects of ammonia
inhalation on respiratory rate of rabbits. J. anim. Sci., 34:
448-452.
MAYNARD, D.N. & BARKER, A.V. (1969) Studies on the tolerance
of the plants to ammonium nutrition. J. Am. Soc. Hortic. Sci.,
94: 235-239.
MEARNS, A.J. (1981) Ecological effects on ocean sewage
outfalls: observations and lessons. Oceanus, 24: 45-54.
MERKENS, J.C. & DOWNING, K.M. (1957) The effect of tension
of dissolved oxygen on the toxicity of un-ionized ammonia to
several species of fish. Ann. appl. Biol., 45(3): 521-527.
METCALF & EDDY, INC. (1972) Wastewater engineering, New
York, McGraw-Hill.
MILES, F.D. (1961) Nitric acid manufacture and uses, London,
Oxford University Press.
MILLER, T.J. (1973) Cleft palate formation: The effects of
fasting and iodoacetic acid on mice. Teratology, 7: 177-182.
MILLER, G.R., SHIMP, G.F., ANDREWS, H.O., NIMMO, D.W., & ILEY,
E.S. (1981) Effect of domestic wastewater on trout in Dillon
Reservoir, Colorado. Paper presented at: The ASTM Symposium on
Aquatic Toxicology, St. Louis, Missouri, 13-14 October 1981,
Philadelphia, Pennsylvania, American Society for Testing
Materials.
MINER, J.R. & HAZEN, T.E. (1969) Ammonia and amines:
components of swine-building odour. Trans. Am. Soc. Agric.
Eng., 12: 772-774.
MITCHELL, H.A. (1963) Ammonia tolerance of the California
leaf-nosed bat. J. Mammal, 44: 543-551.
MITCHELL, H.A. (1964) Investigations of the cave atmosphere
of a Mexican bat colony. J. Mammal, 45: 568-577.
MORII, H., NISHIKATA, K., & TAMURA, O. (1978) Nitrogen
excretion of mudskipper fish Periophthalmus cantonensis and
Boleophthalmus pectinirostris in water and on land. Comp.
Biochem. Physiol., 60A(2): 189-193.
MORRIS, J.C. (1967) Kinetics of reactions between aqueous
chlorine and nitrogenous compounds. In: Faust, S.D. & Hunter,
J.V., ed. Principles and applications of water chemistry,
New York, John Wiley and Sons, pp. 23-53.
MORRIS, J.C. (1978) Chemistry of aqueous chlorine in
relation to water chlorination. In: Jolley, R.L., ed. Water
chlorination: Environmental impact and health effects,
Ann Arbor, Michigan, Ann Arbor Science Publishers, Vol. 1,
pp. 21-36.
MORRIS, J.C. & ROGERS, Q.R. (1978) Ammonia intoxication in
near-adult cat as a result of a dietary deficiency of
arginine. Science, 199(4327): 431-432.
MOSIER, A.R. (1978) Inhibition of photosynthesis and
nitrogen fixation in algae by volatile nitrogen bases.
J. environ. Qual., 7(2): 237-240.
MOSSBERG, S.M. (1967) Ammonia absorption in hamster iluem.
Effect of pH and total CO2 on transport in everted sacs.
Am. J. Physiol., 213: 1327-1330.
MOSSBERG, S.M. & ROSS, G. (1967) Ammonia movement in the
small intestine: Preferential transport by the ileum. J. clin.
Invest., 46: 490-498.
MOTYL, T. & DEBSKI, B. (1977) The influence of increasing
amounts of NH4Cl in the diet on ammonia detoxification in
rats. Zbl. Vet. Med., 24: 333-342.
MULDER, J.S. & VAN DER ZALM, H.O. (1967) [A fatal case of
ammonia poisoning.] Tijdschr. Soc. Geneeskd., 45: 458-460 (in
Dutch).
MULLER, G. & GRUHN, M. (1969) [The effect of water-free
ammonia fertilizer on soil microorganisms.] Zentralbl.
Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 2 Naturw.,
123: 667-676 (in German with English summary).
MUNTWYLER, E., IACOBELLIS, M., & GRIFFIN, G.E. (1956) Kidney
glutaminase and carbonic anhydrase activities and renal
electrolyte excretion in rats. Am. J. Physiol., 184: 83-90.
NAKAZAWA, S. & QUASTEL, J.H. (1968) Inhibitory effects on
ammonium ions and some amino acids on stimulated brain
respiration and cerebral amino acid transport. Can. J.
Biochem., 46: 543-548.
NATARAJAN, K.V. (1970) Toxicity of ammonia to marine
diatoms. J. Water Pollut. Control Fed., 42(5, part 2):
R184-R190.
NAVAZIO, F., GERRITSEN, T., & WRIGHT, G.J. (1961)
Relationship of ammonia intoxication to convulsions and coma
in rats. J. Neurochem., 8: 146-151.
NEHRING, D. (1963) [The toxic effect of urea solutions
containing urease on various fish species.] Z. Fischerei,
11(7/8): 539-547 (in German).
NEUFELD, R.D., HILL, A.J., & ADEKOYA, D.O. (1980) Phenol and
free ammonia inhibition to Nitrosomonas activity. Water Res.,
14(12): 1695-1703.
NIDEN, A.H. (1968) Effects of ammonia inhalation of the
terminal airways. In: Proceedings of the 11th Aspen Emphysema
Conference, Vol. 11, pp. 41-44.
NIHLGARD, B. (1970) Precipitation, its chemical composition
and effects on soil water and beech and spruce forests in
South-Sweden. Oikos., 21: 208
NIHLGARD, B. (1985) The ammonium hypothesis: an additional
explanation to the forest dieback in Europe. Ambio, 14(1): 1-8.
NISHIOKA, D.J. (1976) The initiation of cell cycle events in
ammonia-treated eggs and merogons of the sea urchin, Berkeley,
California, University of California, 107 pp (Ph.D. Thesis).
NODA, K. & CHIKAMORI, K. (1976) Effect of ammonia via
prepyriform cortex on regulation of food intake in the rat.
Am. J. Physiol., 231: 1263-1266.
NRC (1979) Ammonia. Subcommittee on Ammonia. Committee on
Medical and Biologic Effects of Environmental Pollutants.
Division of Medical Sciences, Assembly of Life Sciences,
National Research Council, Baltimore, Maryland, University
Park Press (NTIS No. PB 278-027).
OKITA, T. & KANAMORI, S. (1971) Determination of trace
concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 5: 621-627.
OVERREIN, L.N., SEIP, H.M., & TOLLAN, A., ed. (1980) Acid
precipitation: effects on forest and fish, Oslo (Final report
SNSF Project 1972-80).
PAIK, W.K., LAWSON, D., & KIM, S. (1975) Effect of endocrine
glands on the protection of rat from ammonia intoxication.
Life Sci., 15: 1189-1196.
PARKHURST, B.R., BRADSHAW, A.S., FORTE, J.L., & WRIGHT, G.P.
(1979) An evaluation of the acute toxicity to aquatic biota
of a coal conversion effluent and its major components.
Bull. environ. Contam. Toxicol., 23: 349-356.
PARKHURST, B.R., MEYER, J.S., DEGRAEVE, G.M., & BERGMAN, H.L.
(1981) A re-evaluation of the toxicity of coal conversion
process waters. Bull. environ. Contam. Toxicol., 26: 9-15.
PAUL, M., JOHNSON, J.D., & EPEL, D. (1976) Fertilization
acid of sea urchin eggs is not a consequence of cortical
granule exocytosis. J. exp. Zool., 197(1): 127-133.
PAUL, M. & JOHNSTON, R.N. (1978) Absence of a Ca response
following ammonia activation of sea urchin eggs. Dev. Biol.,
67(2): 330-335.
PAYAN, P. (1978) A study of the Na+/NH4+ exchange across the
gill of the perfused head of the trout (Salmo gairdneri). J.
comp. Physiol., 124: 181-188.
PAYAN, P. & MAETZ, J. (1973) Branchial sodium transport
mechanisms in Scyliorhinis canicula: evidence for Na+/NH4+
and Na+/H+ exchanges and for a role of carbonic anhydrase.
J. exp. Biol., 58: 487-502.
PAYAN, P. & MATTY, A.J. (1975) The characteristics of
ammonia excretion by a perfused isolated head of trout
(Salmo gairdneri): effect of temperature and CO2-free ringer.
J. comp. Physiol., 96: 167-184.
PAYAN, P., MATTY, A.J., & MAETZ, J. (1975) A study of the
sodium pump in the perfused head preparation of the trout,
Salmo gairdneri in freshwater. J. comp. Physiol., 104: 33-48.
PEPLINSKI, A.J., BREKKE, O.L., BOTHAST, R.J., & BLACK, L.T.
(1978) High moisture corn: an extended preservation trial
with ammonia. Trans. Am. Soc. Agricul. Eng., 21: 773-776, 781.
PEQUIN, L. & SERFATY, A. (1963) L'excrétion ammoniacale chez
un téléostéen dulcicole Cyprinus carpio. J. comp. Biochem.
Physiol., 10: 315-324.
PERRY, J.H., CHILTON, C.H., & KIRKPATRICK, S.D., ed. (1963)
Chemical engineers' handbook, 4th ed., New York, McGraw-Hill,
pp. 3-151.
PHILLIPS, A.M. (1950) The effect of metabolic products on
brook trout, Cortland, New York, New York State Conservation
Department, pp. 14-16 (Fisheries Research Bulletin 14)
(Cortland Hatcherry Report 19).
PHILLIPS, R.W. (1970) Further clinical studies of
trimethylpuringyl ammonium chloride (NSA-51095) in malignant
disease with special emphasis on cancer of urinary bladder.
Cancer Chemother. Rep., 54: 181.
PIERPONT, R.A. & MINOTTI, P.L. (1977) Effects of calcium
carbonate on ammonium assimilation by tomato seedlings.
J. Am. Soc. Hortic. Sci., 102: 20-23.
PINTER, I.J. & PROVASOLI, L. (1963) Nutritional characteristics
of some chrysomonads. In: Oppenheimer, C.M., ed. Symposium on
Marine Microbiology, Springfield, Massachusetts, Thomas
Publishers, pp. 114-121.
PITTS, R.F. (1971) The role of ammonia production and
excretion in regulation of acid-base balance. New Engl. J.
Med., 284: 32-38.
PITTS, J.N., Jr & GROSJEAN, D. (1976) Detailed
characterization of gaseous and size-resolved particulate
pollutants at a south coast air basin smog receptor site:
levels and modes of formation of sulfate, nitrate and organic
particulates and their implications for control strategies,
Riverside, California, Statewide Air Pollution Research
Center, University of California (California Air Resources
Board Contract 5-384, Six Month Progress Report, November 17,
1975 to May 16, 1976).
POLIAK, V.E. (1981) [Air pollution by ammonia around swine-
breeding complexes.] Gig. i. Sanit., 6: 67 (in Russian).
POST, R.L. & JOLLY, P.C. (1957) The linkage of sodium,
potassium and ammonium active transport across the human
erythrocyte membrane. Biochem. Biophys. Acta, 25: 118-128.
POWERS, E.B. (1920) Influence of temperature and concentration
on the toxicity of salts to fishes. Ecology, 1: 95-112.
POWERS, W.L., TERRY, R.V., & MURPHY, L.S. (1977) Fate of
nitrogen from manure disposal. In: Proceedings of the National
Conference on Disposal of Residues on Land, pp. 156-160.
PROPKOPIEVA, A.S. & YUSHKOV, G.G. (1975) [Toxicology of
ammonia in animals repeatedly exposed for short lengths of
time.] Gig. Tr. Prof. Zabol., 9: 54-55 (in Russian).
PROPKOPIEVA, A.S., YUSHKOV, G.G., & UBASHEEV, I.O. (1973)
[Materials for a toxicological characteristic of the one-term
effect of ammonia on the organisms of animals after brief
exposure.] Gig. Tr. Prof. Zabol., 6: 56-57 (in Russian).
PROVASOLI, L. & MCLAUGHLIN, J.J.A. (1963) Limited
heterotrophy of some photosynthetic dinoflagellates. In:
Oppenheimer, C.M., ed. Symposium on Marine Microbiology,
Springfield, MA, Thomas Publishers, pp. 105-113.
PRZYTOCKA-JUSIAK, M. (1976) Growth and survival of Chlorella
vulgaris in high concentrations of nitrogen. Acta microbiol.
Pol., 25(3): 287-289.
PRZYTOCKA-JUSIAK, M., MLYNARCZYK, A., KULESZA, M., &
MYCIELSKI, R. (1977) Properties of Chlorella vulgaris strain
adapted to high concentrations of ammonium nitrogen. Acta
microbiol. Pol., 26(2): 185-197.
QUARLES, C.L. & KLING, H.F. (1974) Evaluation of ammonia and
infectious bronchitis vaccination stress on broiler
performance and carcass quality. Poult. Sci., 53: 1592-1596.
RAJAGOPAL, R. (1978) Impact of land use on ground water
quality in the Grand Traverse Bay region of Michigan.
J. environ. Qual., 7: 93-98.
RAMACHANDRAN, V. & RAMAPRABHU, T. (1976) Use of ammonia as
herbicide: A Review. In: Varshney, C.K. & Rzoska, J., ed.
Aquatic weeds in South East Asia, The Hague, B.V. Publishers,
pp. 293-298.
RAMACHANDRAN, V., RAMAPRABHU, T., & REDDY, P.V.G.K. (1975)
Observations on the use of ammonia for the control of Pistia
stratiotes Linn. J. Int. Fish. Soc. India, 7: 124-130.
RAMSEY, G.B. (1953) Mechanical and chemical injuries. In:
The yearbook of agriculture 1953, Washington DC, US Department
of Agriculture, Plant Diseases, pp. 835-837.
RANDALL, D.J. (1970) Gas exchange in fish. In: Hoar, W.S. &
Randall, D.J., ed. Fish physiology, New York, Academic Press,
Vol. IV., pp. 253-292.
RANKIN, D.P. (1979) The influence of un-ionized ammonia on
the long-term survival of sockeye salmon eggs. Fish. Mar.
Serv. Tech. Rep., 912: 17.
RAO, T.S., RAO, M.S., & PRASAD, S.B.S.K. (1975) Median
tolerance limits of some chemicals to the fresh water fish
"Cyprinus carpio". Indian J. environ. Health, 17(2): 140-146.
REDNER, B.D. & STICKNEY, R.R. (1979) Acclimatization to
ammonia by Tilapia aurea. Trans. Am. Fish Soc., 108(4):
383-388.
REICHENBACH-KLINKE, H.-H. (1967) [Investigations on the
influence of the ammonia content on the fish organism.] Arch.
Fischereiwiss., 17(2): 122-132. (in German).
REINBOLD, K.A. & PESCITELLI, S.M. (1982a) Effects of
exposure to ammonia on sensitive life stages of aquatic
organisms, Champaign, Illinois, Illinois Natural History
Survey (Project Report: Contract No. 68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982b) Effects of cold
temperature on toxicity of ammonia to rainbow trout, blue
gills, and fathead minnows, Champaign, Illinois, Illinois
Natural History Survey (Project Report: Contract No.
68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982c) Acute toxicity of
ammonia to the white sucker, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. 2W-3946 NAEX).
REINBOLD, K.A. & PESCITELLI, S.M. (1982d) Acute toxicity of
ammonia to channel catfish, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. J 2482 NAEX).
REITER, R., SLADKOVIC, R., & POTZL, K. (1976) Chemical
components of aerosol particles in the lower troposphere above
central Europe measured under pure-air conditions. Atmos.
Environ., 10: 841-853.
REMPEL, B. (1975) Use ammonia to stop corn deterioration.
Crops Soils, 27: 13-14.
REPP, W.W., HALE, W.H., CHENG, E.W., & BURROUGHS, W. (1955)
The influence of oral administration of non-protein nitrogen
feeding compounds upon blood ammonia and urea levels in lambs.
J. anim. Sci., 14: 118-131.
RICE, S.D. & BAILEY, J.E. (1980) Survival, size, and
emergence of pink salmon, Oncorhynchus gorbuscha, alevins
after short- and long-term exposures to ammonia. Fish Bull.,
78(3): 641-648.
RICHARD, D., BOULEY, G., & BOUDENE, C. (1978a) Effets de
l'inhalation continue d'ammoniac chez le rat et la souris.
Bull. eur. Physiopathol. resp., 14: 573-582.
RICHARD, D., JOUARY, J., & BOUDENE, C. (1978b) Toxicité
aiguë par voie aérienne du gaz ammoniac chez le lapin.
C.R. Acad. X. Paris, 287(Série D): 375-378.
RICHARDS, B.O. & FROMM, P.O. (1970) Sodium uptake by
isolated perfused gills of rainbow trout (Salmo gairdneri).
Comp. Biochem. Physiol., 33: 303-310.
RICHARDS, P., HOUGHTON, B.J., METCALFE-GIBSON, A., WARD, E.E.,
& WRONG, O. (1968) Incorporation of orally administered
ammonia into tissue proteins in man; the influence of diet and
ureamia. In: Nutrition and renal disease, Edinburgh, E. and S.
Livingstone, pp. 93-98.
RICHARDS, P., BROWN, C.L., HOUGHTON, B.J., & WRONG, D.M.
(1975) The incorporation of ammonia nitrogen into albumin in
man: The effects of diet, uremia and growth hormone.
Clin. Nephrol., 3(5): 172-179.
RINGER, R.K. (1971) Adaptation of poultry to confinement
rearing systems. J. anim. Sci., 3: 590-598.
ROBIN, E.D., TRAVIS, D.M., BROMBERG, P.A., FORKNER, C.E., Jr,
& TYLER, J.M. (1959) Ammonia excretion by mammalian lung.
Science, 129: 270-271.
ROBINETTE, H.R. (1976) Effect of selected sublethal levels
of ammonia on the growth of channel catfish (Ictalurus
punctatus). Prog. Fish-Cult., 38(1): 26-29.
ROBINSON, E. & ROBBINS, R.C. (1968) Sources, abundance and
fate of gaseous atmospheric pollutants, Menlo Park, Stanford
Research Institute, California, 123 pp (prepared for the
American Petroleum Institute).
ROBINSON, E. & ROBBINS, R.C. (1971) Emissions, concentrations,
and fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, New York, John Wiley and Sons, Part 2,
pp. 1-93.
ROBINSON-WILSON, E.F. & SEIM, W.K. (1975) The lethal and
sublethal effects of a zirconium process effluent on juvenile
salmonids. Water Resour. Bull., 11(5): 975-986.
ROELOFS, J.G.M., SCHUURKES, J.A.A.R., & SMITS, A.J.M. (1984)
Impact of acidification and eutrophication on macrophyte
communities in soft waters in the Netherlands. II. Experimental
studies. Aquat. Bot., 18: 389-411.
ROELOFS, J.G.M. (in press) The effect of sulfur and nitrogen
deposition on aquatic and terrestrial heathland vegetation.
Experientia (Basel).
ROELOFS, J.G.M., KEMPERS, A.J., HOUDYK, A.L.F.M., & JANSEN,
J. (1985) The effect of airborne ammonium sulphate on Pinus
nigra var maritima, in the Netherlands. Plant Soil, 84: 45-56.
ROSAGE, T.F., SCHUTSKY, R.M., & RAPP, K.M. (1979) Toxicity
of un-ionized ammonia to the spotfin shiner (Notropis
spilopterus). Proc. Pa. Acad. Sci., 53: 39-42.
ROSEBOOM, D.P. & RICHEY, D.L. (1977) Acute toxicity of
residual chlorine and ammonia to some native Illinois fishes,
Urbana, Illinois, Illinois State Water Survey, 42 pp (Report
of Investigation 85).
ROSENFELD, M. (1932) Experimental modification of mitosis by
ammonia. Arch. exp. Zellforsch. Gewebezuecht., 14: 1.
RUBIN, A.J. & ELMARAGHY, M.A. (1976) Studies on the toxicity
of ammonia, nitrate, and their mixtures to the common guppy,
Columbus, Ohio, Water Resources Center, Ohio State University,
47 pp (Project No. A-033-OHIO).
RUBIN, A.J. & ELMARAGHY, G.A. (1977) Studies on the toxicity
of ammonia, nitrate and their mixtures to guppy fry. Water
Res., 11(10): 927-935.
SALVATORE, F., BOCCHINI, V., & CIMINO, F. (1963) Ammonia
intoxication and its effects on brain and blood ammonia
levels. Biochem. Pharmacol., 12: 1-6.
SAIFUTDINOV, M.M. (1966) Maximum permissible concentration
of ammonia in the atmosphere. Hyg. Sanit., 176: 171-175.
SAMYLIN, A.F. (1969) [Effect of ammonium carbonate on early
stages of development of salmon.] Uchen. Zap. Leningr. Gos.
Pedagog. Inst. Im. A. I. Gertsena, 422: 47-62 (in Russian).
SCHAERDEL, A.D., WHITE, W.J., LANG, C.M., DVORCHIK, B.H., &
BOHNER, K. (1983) Localized and systemic effects of
environmental ammonia in rats. Lab. anim. Sci, 33(1): 40-45.
SCHENKER, S. & WARREN, K.S. (1962) Effect of temperature
variation on toxicity and metabolism of ammonia in mice.
J. Lab. clin. Med., 60: 291-301.
SCHULZE-WIEHENBRAUCK, H. (1976) Effects of sublethal ammonia
concentrations on metabolism in juvenile rainbow trout ( Salmo
gairdneri Richardson). Ber. Dtsch. Wiss. Komm. Meeresforsch.,
24(4): 234-250.
SCHUMAN, G.E. & BURWELL, R.E. (1974) Precipitation nitrogen
contribution relative to surface runoff discharges.
J. environ. Qual., 3: 366-369.
SCHUURKES, J.A.A.R. (in press) Ammonium sulfate deposition
and its role in the acidification and nitrogen enrichment of
poorly-buffered aquatic systems. Experientia (Basel).
SCHUURKES, J.A.A.R., HECK, I., HESEN, P., LEUVEN, R.S.E.W., &
ROELOFS, J.G.M. (1985) Effects of acid and acidifying
deposition on water composition and macrophyte composition of
simulated poorly buffered aquatic systems. In: Proceedings of
the Muskoka Conference, Toronto, Canada, September 1985.
SEEGAL, B.C. (1927) Chronic acidosis in rabbits and in dogs,
with relation to kidney pathologic change. Arch. intern. Med.,
39(4): 550-563.
SELESI, D. & VAMOS, R. (1976) [Factors affecting the lethal
concentration of ammonia in fish ponds.] Ichthyologia, 8(1):
115-121 (in Serbocroation with English summary).
SERVANT, J. & DELAPART, M. (1983) Concentrations and origins
of NO3- and NH4+ in oceanic air. In: CACGP Symposium on
Tropospheric Chemistry with Emphasis on Sulphur and Nitrogen
Cycles and the Chemistry of Clouds and Precipitation
(Abstracts), 28 August - 3 September 1983, Oxford, England,
Oxford, New York, Sydney, Paris, Frankfurt, Pergamon Press,
57 pp (Fifth International Congress of the Commission on
Atmoshperic Chemistry and Global Pollution).
SHAW, J. (1960) The absorption of sodium ions by the cray-
fish Astacus pallipes Lereboullet. III. The effect of other
cations in the external solution. J. exp. Biol., 37(3):
548-556.
SHEN, S.S. & STEINHARDT, R.A. (1978) Direct measurement of
intracellular pH during metabolic derepression of the sea
urchin egg. Nature (Lond.), 272(5650): 253-254.
SHERWOOD, T.K. (1925) Solubilities of sulfur dioxide and
ammonia in water. Ind. Eng. Chem., 17: 745-747.
SHILO, M. & SHILO, M. (1953) Conditions which determine the
efficiency of ammonium sulphate in the control of Prymnesium
parvum in fish breeding ponds. Appl. Microbiol., 1: 330-333.
SHILO, M. & SHILO, M. (1955) Control of the phytoflagellate
Prymnesium parvum. Verh. Int. Ver. Limnol., 12: 233-240.
SHOLKOVITZ, E. (1973) Interstitial water chemistry of the
Santa Barbara Basin sediments. Geochim. Cosmochim. Acta, 37:
2043-2073.
SHUTTLEWORTH, T.J. & FREEMAN, R.F. (1974) Factors affecting
the net fluxes of ions in the isolated perfused gills of
freshwater Anguilla dieffenbachii. J. comp. Physiol., 94:
297-307.
SIEGRIST, A. (1920) [Concentrated effect of alkali and acid
on the eye.] Z. Augenheilkd., 43: 176-194 (in German).
SILVER, S.D. & MCGRATH, F.P. (1948) A comparison of acute
toxicities of ethylene amine and ammonia to mice. J. ind. Hyg.
Toxicol., 30: 7-9.
SILVERMAN, L., WHITTENBERGER, J.L., & MULLER, J. (1949)
Physiological response of man to ammonia in low
concentrations. J. ind. Hyg. Toxicol., 31: 74-78.
SINGER, R.H. & MCCARTY, R.T. (1971) Pathologic changes
resulting from acute ammonium salt poisoning in sheep.
Am. J. Vet. Res., 32: 1239-1246.
SLOAN, C.H. & MORIE, G.P. (1974) Determination of ammonia in
tobacco and tobacco smoke with the ammonia electrode. Anal.
Chim. Acta, 69: 243-247.
SLOT, G.M.J. (1938) Ammonia gas burns: an account of six
cases. Lancet, 2: 1356-1357.
SMART, G. (1976) The effect of ammonia exposure on gill
structure of the rainbow trout (Salmo gairdneri). J. Fish
Biol., 8(6): 471-475.
SMITH, C.E. (1972) Effects of metabolic products on the
quality of rainbow trout. Am. Fishes US Trout News, 17(3):
7-8, 21.
SMITH, C.E. (1984) Hyperplastic lesions of the primitive
meninx of fathead minnows, Pimephales promelas, induced by
ammonia: species potential for carcinogen testing. Natl Cancer
Inst. Monogr., 65: 119-125.
SMITH, C.E. & PIPER, R.G. (1975) Lesions associated with
chronic exposure to ammonia. In: Ribelin, W.E. & Migaki, G.,
ed. The pathology of fishes, Madison, Wisconsin, University of
Wisconsin Press, pp. 497-514.
SMITH, L.W. & WHEELER, W.E. (1979) Nutritional and economic
value of animal excreta. J. anim. Sci., 48: 144.
SODERLUND, R. & SVENSSON, B.H. (1976) The global nitrogen
cycle. In: Svensson, B.H. & Söderlund, R., ed. Nitrogen,
phosphorus, and sulfur: global sources, Stockholm, pp. 23-76
(SCOPE Report No. 7) (Ecology Bulletin No. 22).
SOF'INA, E.P., VORONKOV, M.G., D'IAKOV, V.W., SOMENOVA, N.V.,
PLATONOVA, G.N., PERETOLCHINA, N.M., LESNAIA, N.A., &
SMETANKINA, O.Z. (1978) Tris (2-oxy-ethyl) ammonium salts of
some synthetic phytohormones and their antitumor activity.
Khim Farm. 2h, 12: 74.
SOLOMONSON, L.P. (1969) Effects of ammonia and some of its
derivatives on photosynthesis in the blue-green alga,
Plectonema boryanum, Chicago, Illinois, University of Chicago,
68 pp (Ph.D. Thesis).
SOUSA, R.J., MEADE, T.K., & FELBECK, G.T., Jr (1977) The
exposure of ammonia to the photodissociated haemoglobin of the
marine bloodworm, Glycera dibranchiata. Comp. Biochem.
Physiol., 58A: 19-22.
SPARKS, R.E. (1975) The acute, lethal effects of ammonia on
channel catfish (Ictalurus punctatus), bluegills (Lepomis
macrochirus), and fathead minnows (Pimephales promelas),
Chicago, Illinois, 15 pp (report to Illinois Institute for
Environmental Quality, project No. 20.060).
SPARKS, R.E. & SANDUSKY, M.J. (1981) Identification of
factors responsible for decreased production of fish food
organisms in the Illinois and Mississippi Rivers, Havana,
Illinois, River Research Laboratory, Illinois Natural History
Survey, 63 pp (Project No. 3-291-R).
SPICER, C.W. (1977) The fate of nitrogen compounds in the
atmosphere. In: Pitts, J.N., Jr, Metcalf, R.L., & Lloyd, A.C.,
ed. Advances in environmental sciences and technology, New
York, John Wiley and Sons, Vol. 7, pp. 163-261.
SPINAZZOLA, A., MARRACCINI, L., DEVOTO, G., & ZEDDA, S.
(1966) [Study of the behaviour of some volatile toxic
substances as an index of air pollution in the cith of
Cagliari. Note III. Ammonia.] Folia Med. (Naples), 49: 641-648
(in Italian).
STAMMER, H.A. (1953) [The effect of hydrogen sulfide and
ammonia on characteristic animal forms in the saprobiotic
system.] Vom Wasser, 20: 34-71 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil (Myriophylum spicatum). Arch. environ.
Contam. Toxicol., 2(4): 331-341.
STEINHARDT, R.A. & MAZIA, D. (1973) Development of
K+-conductance and membrane potentials in unfertilized sea
urchin eggs after exposure to NH4OH. Nature (Lond.),
241(5389): 400-401.
STEPHENS, E.R. (1971) Identification of odors from cattle
feedlots. Calif. Agric., 25: 10-11.
STEVENS, C., KENNAWAY, N.G., & FELLMAN, J.H. (1975) Ammonia
intoxicaton: A hazard during rehabilitation of protein-
deprived rats. J. Nutr., i105: 1384-1390.
STEVENSON, T.J. (1977) The effects of ammonia, pH and
salinity on the white perch, Morone americana, Kingston, Rhode
Island, University of Rhode Island, 154 pp (Ph.D. Thesis).
STEWART, B.A., VIETS, F.G., Jr, HUTCHINSON, G.L., & KEMPER,
W.D. (1967) Nitrates and other water pollutants under fields
and feedlots. Environ. Sci. Technol., 1(9): 736-739.
STRUZEWSKI, E.J. (1971) Current status on control and
treatment of storm and combined sewer overflow, Edison Water
Quality Laboratory (EPA Technology Transfer).
STUDIER, E.H. (1966) Studies on the mechanisms of ammonia
tolerance of the guano bat. J. exp. Zool., 163: 79-85.
STUDIER, E.H., BECK, L.R., & LINDBORG, R.G. (1967) Tolerance
and initial metabolic response to ammonia intoxication in
selected bats and rodents. J. Mammal., 48: 564-572.
SUGIYAMA, K., YOSHIDA, M., & OYAMADA, M. (1968) [A case of
chemical bronchiectasia induced by inhalation of ammonia gaz.]
Nippon Kyohbu Shikkan Gakkai Zasshi, 27: 797-803 (in Japanese).
SUKUMARAN, N. & KUTTY, M.N. (1977) Oxygen consumption and
ammonia excretion in the catfish Mystus armatus, with special
reference to swimming speed and ambient oxygen. Proc. Indian
Acad. Sci., 86B: 195-206.
SUMMERSKILL, W.H.J. & WOLPERT, E. (1970) Ammonia metabolism
in the gut. Am. J. clin. Nutr., 23: 633-639.
SUYAMA, M., KOIKE, J., & SUZUKI, K. (1960) Studies on the
glycolysis and the formation of ammonia in the muscle and
blood of elasmobranchs. J. Tokyo Univ. Fish., 46: 51-60.
SWIGERT, J.P. & SPACIE, A. (1983) Survival and growth of
warmwater fishes exposed to ammonia under low flow conditions,
Springfield, Virginia, National Technical Information Service
(NTIS PB83-257535).
TABATA, K. (1962) [Toxicity of ammonia to aquatic animals
with reference to the effect of pH and carbon dioxide.]
Hokoku, 34: 67-74 (in Japanese).
TAIGANIDES, E.P. & WHITE, R.K. (1969) The menace of noxious
gases in animal units. Trans. Am. Soc. Agric. Eng., 12:
359-362.
TAKAHASHI, H., WAKAI, K., & OHUCHI, K. (1984) [Experiences
of chemical burns induced by ammonia gaz.] Nippon Saigai
Igakkai Kaishi, 32: 741-749 (in Japanese).
TAPLIN, G.V., CHOPRA, S., YANDA, R., & ELAM, D. (1976)
Radionuclidic lung-imaging procedures in the assessment of
injury due to ammonia inhalation. Chest, 69: 5.
TAYLOR, A.W., EDWARDS, W.M., & SIMPSON, E.C. (1971)
Nutrients in streams draining woodland and farmland near
Coshocton, Ohio. Water Resour. Res., 7: 81-89.
TAYLOR, J.E. (1973) Water quality and bioassay study from
Crawford National Fish Hatchery. Trans. Nebr. Acad. Sci., 2:
176-181.
THE NETHERLANDS MINISTRY OF HOUSING, PHYSICAL PLANNING AND
ENVIRONMENT (1984) Acidification in the Netherlands: effects
and policies. In: Proceedings of a Conference on Air Pollution
in Europe, Munich, 24-27 June, 1984, The Netherlands Ministry
of Housing, Physical Planning and Environment, pp. 9.
THOMAS, W.H., HASTINGS, J., & FUJITA, M. (1980) Ammonium
input to the sea via large sewage outfalls. Part 2. Effects of
ammonium on growth and photosynthesis of southern California
phytoplankton cultures. Mar. environ. Res., 3(4): 291-296.
THOMPSON, R.Y. & HALLIBURTON, I.W. (1966) Effect of diet on
the composition of the kidney. Biochem. J., 99(3): 44.
THORNTON, N.C. & SETTERSTROM, C. (1940) Toxicity of ammonia,
chlorine, hydrogen cyanide, hydrogen sulphide, and sulphur
dioxide gases. III. Green plants. Contrib. Boyce Thompson
Inst., 11: 343-356.
THURSTON, R.V. (1981) Acute toxicity of ammonia to the
pumpkinseed (Lepomis gibbosus), Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research
Laboratory.
THURSTON, R.V. & MEYN, E.L. (1984) Acute toxicity of ammonia
to five fish species from the northwest United States,
Bozeman, Montana Fisheries Bioassay Laboratory, Montana State
University, 13 pp (Technical Report Vol. 84, No. 4).
THURSTON, R.V. & RUSSO, R.C. (1981) Acute toxicity of
ammonia to golden trout (Salmo aguabonita) and mottled sculpin
(Cottus bairdi), Bozeman, Montana, Fisheries Bioassay
Laboratory, Montana State University, 10 pp (Technical Report
Vol. 81, No. 1).
THURSTON, R.V. & RUSSO, R.C. (1983) Acute toxicity of
ammonia to rainbow trout. Trans. Am. Fish. Soc., 112: 696-704.
THURSTON, R.V., RUSSO, R.C., & SMITH, C.E. (1978) Acute
toxicity of ammonia and nitrite to cutthroat trout fry. Trans.
Am. Fish. Soc., 107(2): 361-368.
THURSTON, R.V., CHAKOUMAKOS, C., & RUSSO, R.C. (1981a)
Effect of fluctuating exposures on the acute toxicity of
ammonia to rainbow trout (Salmo gairdneri) and cutthroat trout
(S. clarki). Water Res., 15(7): 911-917.
THURSTON, R.V., PHILLIPS, G.R., RUSSO, R.C., & HINKINS, S.M.
(1981b) Increased toxicity of ammonia to rainbow trout (Salmo
gairdneri) resulting from reduced concentrations of dissolved
oxygen. Can. J. Fish. aquat. Sci., 38(8): 983-988.
THURSTON, R.V., RUSSO, R.C., & VINOGRADOV, G.A. (1981c)
Ammonia toxicity to fishes: Effect of pH on the toxicity of
the un-ionized ammonia species. Environ. Sci. Technol., 15(7):
837-840.
THURSTON, R.V., RUSSO, R.C., & PHILLIPS, G.R. (1983) Acute
toxicity of ammonia to fathead minnows. Trans. Am. Fish. Soc.,
112: 705-711.
THURSTON, R.V., LUEDTKE, R.J., & RUSSO, R.C. (1984a)
Toxicity of ammonia to fresh-water insects of three families,
Bozeman, Montana, Fisheries Bioassay Laboratory, Montana State
University, 26 pp (Technical Report Vol. 84, No. 2).
THURSTON, R.V., RUSSO, R.C., LUEDTKE, R.J., SMITH, C.E., MEYN,
E.L., CHAKOUMAKOS, C., WANG, K.C., & BROWN, C.J.D. (1984b)
Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish.
Soc., 113(1): 56-73.
THURSTON, R.V., MEYN, E.L., ZAJDEL, R.K., & RUSSO, R.C.
(submitted) Chronic toxicity of ammonia to fathead minnows
(Pimephales promelas). Submitted to Trans. Am. Fish. Soc.
TMRI (1971) [Atmosphere division. On survey of air
pollutants peculiar to factory-clustered area.] Tokyo
Meteorol. Res. Inst. Environ. Prot., 2: 109-111 (Annual
Report) (in Japanese).
TOMASSO, J.R., GOUDIE, C.A., SIMCO, B.A., & DAVIS, K.B.
(1980) Effects of environmental pH and calcium on ammonia
toxicity in channel catfish. Trans. Am. Fish. Soc., 109(2):
229-234.
TOPPING, D.C. & VISEK, W.J. (1976) Nitrogen intake and
tumourigenesis in rats injected with 1,2-dimethylhydrazine.
J. Nutr., 106: 1583.
TORDA, C. (1953) Ammonium ion content and electrical
activity of the brain during the preconvulsive and convulsive
phases induced by various convulsants. J. Pharmacol. exp.
Ther., 107: 197-203.
TOTH, B. (1972) Hydrazine, methylhydrazine, and
methylhydrazine sulfate carcinogenesis in Swiss mice. Failure
of ammonium hydroxice to interfere in the development of
tumors. Int. J. Cancer, 9: 109.
TOWLE, D.W., PALMER, G.E., & HARRIS, J.L. III (1976) Role of
gill Na+ + K+-dependent ATPase in acclimation of blue crabs
(Callinectes sapidus) to low salinity. J. exp. Biol., 196:
315-322.
TOWLE, D.W. & TAYLOR, D.D. (1976) Effect of NH4+ and K+ on
Na+-transport ATPase activity of blue crab gill Am. Zool., 16:
224.
TRESHOW, M. (1970) Ammonia. In: Environment and plant
response, New York, McGraw-Hill Book Company, pp. 362-363.
TURNBULL, H., DEMANN, J.G., & WESTON, R.F. (1954) Toxicity
of various refinery materials to fresh water fish. Ind. Eng.
Chem., 46(2): 324-333.
ULRICH, B. (1983) Soil acidity and its relation to acid
deposition. In: Effects of accumulation of air pollutants in
forest ecosystems, Ulrich and Pankrasth, The Netherlands,
D. Reidel Publishing Company, pp. 127-146.
ULSHAFER, T.R. (1958) The measurement of changes in
acetylcholine level (ACh) in rat brain following ammonium ion
intoxication and its possible bearing on the problem of
hepatic coma. J. lab. clin. Med., 52: 718-723.
ULTSCH, G.R., JACKSON, D.C., & MOALLI, R. (1981) Metabolic
oxygen conformity among lower vertebrates: the toadfish
revisited. J. comp. Physiol., 142: 439-443.
UNDERWRITERS LABORATORIES (1933) Report on the comparative
life, fire, and explosion hazards of common refrigerants.
Miscellaneous Hazard No. 2375, Chicago, Illinois, Underwriters
Laboratories, pp. 26-28.
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1963) Effects of
pollution on fish; toxicity of mixutures of zinc, sulfate, and
ammonium chloride; toxicity of sewage effluents; the toxicity
of poisons under estuarine conditions, London, United Kingdom
Ministry of Technology, pp. 76-83 (Water Pollution Research
1962).
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1968) Effects of
pollution on fish, London, United Kingdom Ministry of
Technology, pp. 56-65 (Water Pollution Research 1967).
US EPA (1972) Air quality data for 1968 from the National
Air Surveillance Networks and contributing state and local
networks, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Programs, 231
pp (Report No. APTD-0978).
US EPA (1979a) Ammonia study using STORET ambient monitoring
data, Washington DC, US Environmental Protection Agency,
Monitoring and Data Support Division, Office of Water Planning
and Standards.
US EPA (1979b) Methods for chemical analysis of water and
wastes, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring and Support Laboratory (Report No.
EPA 600/4-79-020).
US EPA (1981) Multimedia health assessment document on
ammonia, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (Report No.
ECAO/82-DO10).
US EPA (1985) Ambient water quality criteria for ammonia -
1984, Duluth, Minnesota, US Environmental Protection Agency,
Office of Research and Development.
US NIOSH (1974) Criteria for a recommended standard.
Occupational exposure to ammonia, Washington DC, National
Institute for Occupational Safety and Health, pp. 74-136 (US
DHEW Publication No. 246-699).
US NIOSH (1977) NIOSH manual of analytical methods. Part I.
NIOSH monitoring methods, Washington DC, National Institute
for Occupational Safety and Health, Vol. 1, pp. 205.1-205.3
(US DHEW Publication No. 77-157-A).
UZVOLGYI, E. & BOJAN, F. (1980) Possible in vivo formation
of a carcinogenic substance from diethyl pyrocarbonate and
ammonia. J. Cancer Res. clin. Oncol., 97: 205.
VALENTINE, H. (1964) A study of the effect of different
ventilation rates on the ammonia concentrations in the
atmosphere of broiler houses. Br. Poult. Sci., 5: 149-159.
VAMOS, R. (1963) Ammonia poisoning in carp. Acta. biol.
Szeged., 9(1-4): 291-297.
VAMOS, R. & TASNADI, R. (1967) Ammonia poisoning in carp. 3.
The oxygen content as a factor influencing the toxic limit of
ammonia. Acta. biol. Szeged., 13(3-4): 99-105.
VAN AALST, R.M. (1984) [Atmospheric process and deposition,]
Ministry of Agriculture and Fisheries, Ministry of Housing,
Physical Planning and Environment, 76 pp (Report No. 84(2))
(in Dutch).
VAN BREEMEN, N. & JORDENS, E.R. (1983) Effects of
atmospheric ammonium sulfate on calcareous and non-calcareous
soils of woodlands in the Netherlands. In: Ulrich, B. &
Panlerath, J., ed. Effects of accumulation of air pollutants
in forest ecosystems, The Netherlands, Reidel Publishing
Company, pp. 171-182.
VANCURA, E.M., CLINTON, J.E., RUIZ, E., & KRENZELOK, E.P.
(1980) Toxicity of alkaline solutions. Ann. emergency Med.,
9(3): 118-122.
VAN DER EERDEN, L.J.M. (1982) Toxicity of ammonia to plants.
Agric. Environ., 7: 223-235
VAN SLYKE, D.D., PHILLIPS, R.A., HAMILTON, P.B., ARCHIBALD,
R.M., FUTCHER, P.H., & MILLER, A. (1943) Glutamine as source
material of urinary ammonia. J. Biol. Chem., 150: 481-482.
VAUGHN, R.E. & SIMCO, B.A. (1977) Effects of ammonia on
channel catfish, Ictalurus punctatus. ASB Bull., 24(2): 92.
VENKATARAMIAK, A., LAKSHMI, G.J., BEST, C., GUNTER, G.,
HARTWIG, E., & WILDE, P. (1981) Studies on toxicity of OTEC
plant components on marine animals from the Gulf of Mexico,
Springfield, Virginia, National Technical Information Service
(DE81030167).
VERBERK, M.M. (1977) Effects of ammonia in volunteers.
Int. Arch. occup. environ. Health, 39: 73 -81.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experiences of the
Clinica del Lavoro with their own maximal exposure limits for
industrial chemicals.] Arch. Gewerbepathol. Gewerbehyg., 13:
528-534 (in German).
VINES, H.M. & WEDDING, R.T. (1960) Some effects of ammonia on
plant metabolism and a possible mechanism for ammonia
toxicity. Plant Physiol., 35: 820-825.
VINOKUROVA, M.K. & MAL'KOVA, V.B. (1963) [Toxicology of the
selective herbicide ammonium sulfamate.] Gig. Trud. Prof.
Zabol., 7(5): 56-57 (in Russian).
VISEK, W.J., KOLONDY, G.M., & GROSS, P.R. (1972) Ammonia
effects in cultures of normal and transformed 3T3 cells.
J. cell Physiol., 80: 373.
VISEK, W.J., CLINTON, S.K., & TRUEX, C.R. (1978) Nutrition
and experimental carcinogenesis. Cornell Vet., 68: 3.
VITTI, T.G., VUKMIROVICH, R., & GAEBLER, O.H. (1964)
Utilization of ammonia nitrogen, administered by intragastric,
intraperitoneal and subcutaneous roues: Effects of growth
hormone. Arch. Biochem. Biophys., 106: 475-482.
WALLEN, I.E., GREER, W.C., & LASATER, R. (1957) Toxicity to
Gambusia affinis of certain pure chemicals in turbid waters.
Sewage Ind. Wastes, 29(6): 695-711.
WALLINGFORD, G.W. (1977) Fate of nitrogen from fertilizer
practices. In: Disposal of residues on land, Rockville,
Maryland, Information Transfer, Inc., pp. 152-155.
WALSH, L.M. (1977) Updating the nitrogen cycle. In: Disposal
of residues on land, Rockville, Maryland, Information
Transfer, Inc., pp. 146-151.
WALTON, M.J. & COWEY, C.B. (1977) Aspects of ammoniogenesis
in rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol.,
57B: 143-149.
WALUGA, D. & FLIS, J. (1971) [Changes in peripheral blood in
carp ( Cyprinus carpio L.) under the influence of ammonia
solution.] Rocz. Nauk Roln. Ser. H, 93(2): 87-94 (in Russian).
WARNCKE, D.D. & BARBER, S.A. (1973) Ammonium and nitrate
uptake by corn ( Zea mays L.) as influenced by nitrogen
concentration and NH4-/NO3-ratio. Agric. J., 65: 950-953.
WARREN, K.S. (1958) The differential toxicity of ammonium
salts. J. clin. Invest., 37: 497-501.
WARREN, K.S. & NATHAN, D.G. (1958) The passage of ammonia
across the blood, brain, barrier and its relation to blood pH.
J. clin. Invest., 37: 1724-1728.
WARREN, K.S. & SCHENKER, S. (1964) Effect of an inhibitor of
glutamine synthesis (methionine sulfoximine) on ammonia
toxicity and metabolism. J. lab. clin. Med., 64: 442-449.
WARREN SPRING LABORATORY (1982) Acidity of rainfall in the
United Kingdom.
WATTS, R.L. & WATTS, D.C. (1974) Nitrogen metabolism in
fishes. In: Florkin, M. & Scheer, B.T. ed. Chemical Zoology,
New York, Academic Press, Vol. III, pp. 369-446.
WEAST, R.C., ed. (1979) CRC handbook on chemistry and
physics, 59th ed., Boca Raton, Florida, CRC Press Inc.
WEATHERBY, J.H. (1952) Chronic toxicity of ammonia fumes by
inhalation. Proc. Soc. Exp. Biol. Med., 81: 300-301.
WEBB, D.W., BARTLEY, E.E., & MEYER, R.M. (1972) A comparison
of nitrogen metabolism and ammonia toxicity from ammonium
acetate and urea in cattle. J. anim. Sci., 35: 1263-1270.
WEBB, J.T. & BROWN, G.W., Jr (1976) Some properties and
occurrence of glutamine synthetase in fish. Comp. Biochem.
Physiol., 54B: 171-175.
WEDDING, R.T. & VINES, H.M. (1959) Inhibition of reduced
diphosphopyridine nucleotide oxidation by ammonia. Nature
(Lond.), 184: 1226-1227.
WEEKS, M.E. (1968) Discovery of the elements, 7th ed.,
Washington DC, Journal of Chemical Education, p. 197.
WEHRBEIN, M.F., VIPPERMAN, P.E., Jr, PEO, E.R., Jr, &
CUNNINGHAM, P.J. (1970) Diammonium citrate and diammonium
phosphate as sources of dietary nitrogen for growing-finishing
swine. J. anim. Sci., 31: 327-332.
WHITE, A., HANDLER, P., & SMITH, E.L. (1973) Renal function
and the composition of urine. In: Principles of biochemistry,
5th ed., New York, McGraw Hill Book Co., pp. 604-704 and
928-948.
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series No.
539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
WICKINS, J.F. (1976) The tolerance of warm-water prawns to
recirculated water. Aquaculture, 9(1): 19-37.
WILKIN, D.C. & FLEMAL, R.C. (1980) Feasibility of water
quality improvement in three Illinois rivers. J. Water Poll.
Control Fed., 52(2): 293.
WILKINSON, R.H., THOMAS, W.J., HANSEN, C.M., & SHIELDS, E.A.
(1978) Forage preservation by timed-slow release of NH3 (ASAE
Paper No. 78-1523).
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968a) Toxicologic effects of ammonium carbamate and related
compounds. Am. J. vet. Res., 29: 987-906.
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968b) Comparative ammonia toxicity. Comp. Biochem.
Physiol., 25: 295-301.
WILT, F.H. & MAZIA, D. (1974) The stimulation of cytoplasmic
polyadenylyation in sea urchin eggs by ammonia. Dev. Biol.,
37: 422-424.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) Merck Index, 9th ed., Rahway, New Jersey, Merck
and Co. Inc., p. 69.
WINKLER, M.M. & GRAINGER, J.L. (1978) Mechanisms of action
of NH4Cl and other weak bases in the activation of sea urchin
eggs. Nature (Lond.), 273(5663): 536-538.
WOKER, H. & WUHRMANN, K. (1950) [The sensitivity of
different species of fish to ammonia, hydrocyanic acid and
phenol.] Rev. Suisse Zool., 57(3): 548-553 (in German).
WOLAVER, T.C. (1972) Distribution of natural and
anthropogenic elements and levels in precipitation across the
US: theory and quantitative models, Chapel Hill, North
Carolina, University of North Carolina, 75 pp (Prepared for US
EPA).
WOOD, R.W. (1979) Behavioral evaluation of sensory
irritation evoked by ammonia. Toxicol. appl. Pharmacol., 50:
157-162.
WORCEL, A. & ERECINSKA, M. (1962) Mechanism of inhibitory
action of ammonia on the respiration of rat liver
mitochondria. Biochim. Biophys. Acta, 65: 27-33.
WRIGHT, P.A. & WOOD, C.M. (1985) An analysis of branchial
ammonia excretion in the freshwater rainbow trout: effects of
environmental pH change and sodium uptake blockage.
J. exp. Biol., 114: 329-353.
WUHRMANN, K. & WOKER, H. (1948) [Contributions to the
toxicology of fishes. II. Experimental investigations on
ammonia and hydrocyanic acid poisoning.] Schweiz. Z. Hydrol.,
11: 210-244 (in German).
WUHRMANN, K., ZEHENDER, F., & WOKER, H. (1947) [Biological
significance for fisheries of ammonium-ion and ammonia content
of flowing bodies of water.] Vierteljahrsschr. Naturforsch.
Ges. Zurich, 92: 198-204 (in German).
YAHAGI, H., SUZUKI, K., & MATSUI, Y. (1959) [Fourteen cases
of ammonia gas poisoning.] Shuhkan Igaku Tsuhshin, 601: 26-30
(in Japanese).
YECK, R.G., SMITH, L.W., & CALVERT, C.C. (1975) Recovery of
nutrients from animal wastes. An overview of existing options
and potentials for use in feed. In: Managing livestock waste:
Proceedings of the Third International Symposium on Livestock
Wastes, St. Joseph, Michigan, American Society of Agricultural
Engineers, pp. 192-196.
YOSHIDA, J., NAKAME, K., & NAKAMURA, R. (1957) Toxicity of
urea and its control. II. Toxicity of ammonium salts and urea
in rabbits and goats. Nippon Chikusangaku Kaiho, 28: 185-191.
ZIEVE, L., DOIZAKI, W.M., & ZIEVC, F.J. (1974) Synergism
between mercaptans and ammonia or fatty acids in the
production of coma. A possible role for mercaptans in the
pathogenesis of hepatic coma. J. lab. clin. Med., 83: 16-28.
ZIMBER, A. (1970) Studies on the effect of ammonia on DNA
synthesis and on cellular proliferation and renewal in tissues
of the mouse and in Ehrlich ascites tumor cells, Ithaca,
New York, Cornell University (Ph.D. Thesis).
ZIMBER, A. & VISEK, W.J. (1972) Effect of urease injection
on DNA synthesis in mice. Am. J. Physiol., 223: 1004.
ZIMMERMAN, P.W. (1949) Impurities in the air and their
influence on plant life. In: Proceedings of the First National
Air Pollution Symposium, Pasadena, California, 10-11 November,
1949, pp. 135-141 (Sponsored by Stanford Research Institute).
ZLATEVA, M., VALCEVA, V., & ANTOV, G. (1974) Toxic organic
changes in laboratory animals placed in an ammonia-rich
working atmosphere. Tr. Inst. Hig. Ohrana Tr. Prof. Zabol.,
22(1): 147-154.
Annex I. Ammonium salts evaluations prepared by the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)a
------------------------------------------------------------------------------------------------------
Ammonium salt Functional use Evaluation status Reference
(ADI, MTDI)b,c
------------------------------------------------------------------------------------------------------
acetate pH-adjusting agent not specifiedd (grouped with WHO (1982a)
other ammonium salts and acetate)
alginate thickening agent, stabilizer 0 - 50 (grouped with alginic acid WHO (1974a,b)
and calcium, potassium, and
sodium alginate)
bicarbonate leavening agent, buffer not specified WHO (1982b)
(hydrogen carbonate) neutralizing agent, alkali
carbonate
chloride dough conditioner, yeast, food not specified (grouped with WHO (1980)
hydrochloric acid and magnesium
and potassium chlorides)
hydrogen phosphate buffering agent, dough MTDI:, 70 expressed as phosphorus WHO (1982a,b)
phosphate dibasic conditioner, leavening agent, (grouped with phosphates and
yeast polyphosphates, including
phosphates occurring naturally in
food)
hydroxide (strong alkali not specified WHO (1966)
ammonia solution)
lactate buffer, dough conditioner not specified WHO (1974a,b)
persulfate flour-treatment agent no ADI set WHO (1966)
------------------------------------------------------------------------------------------------------
a Queries concerning updated information should be addressed to: Joint WHO Secretary of the Joint
FAO/WHO Expert Committee on Food Additives, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland.
b ADI = Acceptable daily intake for man expressed as mg/kg body weight.
c MTDI = Maximum tolerable daily intake for man, expressed as mg/kg body weight.
d ADI not specified = the total intake of the substance arising from its uses at the levels necessary
to achieve the desired effect does not represent a hazard to health.
REFERENCES TO ANNEX I
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series
No. 539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
ANNEX II: TREATMENT OF EXCESSIVE EXPOSURE TO AMMONIA
Ammonia (gas and liquid) is an extremely irritant chemical
affecting the skin, eyes, and the respiratory tracts. Ammonia gas
can produce burning of the eyes, lachrymation, and severe eye
damage. When inhaled it can produce coughing, laryngitis,
bronchitis, chest pains, and severe respiratory problems. Contact
with liquid ammonia can result in severe eye and skin burns due
both to its irritant properties and chilling effect.
Those working with ammonia should be trained in its safe use
including the dangers of improper handling, the use of protective
equipment, and the avoidance of unnecessary inhalation of the gas
and direct contact with liquid ammonia. After handling liquid
ammonia, the hands should be washed thoroughly before eating or
smoking.
The provision of protective clothing and equipment is not an
adequate substitute for safe working conditions. However, where
exposure cannot be adequately controlled, workers should be
provided with suitable impervious clothing, boots and gloves, and,
depending on the severity of the conditions, a face shield or
safety goggles and a mask or self contained breathing apparatus.
In places where very high gaseous ammonia concentrations are
expected, complete gas suits should be used.
Emergency showers and eye wash or water sprays should be
provided in all areas where ammonia is handled and where leaks,
spills or splashes may occur. Clothes contaminated with ammonia
should be discarded immediately and not worn again until thoroughly
cleansed.
First aid
If excessive exposure has occurred first aid treatment should
be promptly initiated and medical advice obtained as soon as
possible.
Ammonia in the eye (gas, liquid, or liquor)
Ammonia in the eye may cause severe injury and must be treated
immediately by irrigation for at least 15 min with flowing water or
sterile buffered eye irrigation solution.
Ammonia on the skin (liquid or liquor)
Drench the affected area with water and remove contaminated
clothing and footwear. Wash the affected area continuously for 5 -
10 min or until pain ceases.
Ingestion (liquid or liquor)
If the patient is conscious large quantities of water may be
given to dilute the chemical in the stomach. No attempt should be
made to induce vomiting.
Inhalation of gas/vapour
1. Remove from exposure, secure airway and place in semiprone
recovery position if unconscious. Give artificial
respiration if not breathing.
2. If heartbeat is absent give external cardiac massage.
3. If there is cyanosis (blueness of lips) or air-hunger
administer oxygen by facemask.
4. A conscious patient may be given water to drink.
Further treatment
Ammonia in the eyes
Corneal damage is probable. Use local anaesthetics and
cycloplegics to enable thorough irrigation and examination. If the
cornea is damaged, administer topical antibiotics. Refer to a
specialist centre.
Ammonia on the skin
Treat as a chemical burn. Liquid ammonia may produce deep
burns that may require grafting. Refer deep or extensive burns to
a specialist centre.
Inhalation of gas/vapour
1. Ammonia is irritant to the respiratory tract causing:
(a) bronchial oedema, spasm, and hypersecretion
resulting in chest tightness, wheeze, and cough,
which may progress to severe dyspnoea; and
(b) lower airway inflammation with exudative
pulmonary oedema and impaired gaseous
diffusion. Symptoms may be delayed 24 h or
more. Resolution may be by fibrosis producing a
restrictive defect.
2. Treat hypoxia with oxygen, ventilation, and bronchial
lavage, as appropriate.
3. Consider administration of steroids by multiple metered
doses of topical aerosol, by inhalation, and/or by
injection. Early prophylactic use may be indicated.
4. Administer bronchodilators by inhalation or injection, as
indicated. Maintain with oral treatment.
5. Keep under medical surveillance for at least 48 h. Treat
symptomatically.
6. Observe for secondary respiratory infection and treat as
necessary.
 Manifestations of ammonia toxicity can be traced to several
metabolic disturbances. Both photosynthetic and respiratory
pathways are affected adversely by ammonia. There is a direct
relationship between ammonia concentration and respiratory
metabolism, including oxygen uptake, glycolysis, and the
tricarboxylic acid cycle (Matsumoto et al., 1976). Ammonium ions
may restrict photosynthesis through the uncoupling of noncyclic
photophosphorylation in isolated chloroplasts (Gibbs & Calo, 1959;
Losada & Arnon, 1963), though the mechanism of action is not known
(Losada et al., 1973). It is well documented that uncoupling leads
to an increase in reducing power in the cell. There is evidence
that 1 - 3 mmol ammonium inhibits respiration in plants (Wedding &
Vines, 1959; Vines & Wedding, 1960). It is suspected that similar
or identical inhibition occurs in ammonium toxicity in animals.
The site of toxicity is thought to be in the electron-transport
system; specifically, at the oxidation of NADH to NAD. This may
have a mechanism similar to the interference by ammonium ion with
electron transfer in the photosynthetic reaction.
High atmospheric levels may have direct toxic effects. Plant
growth is inhibited by a decrease in the carbohydrate production as
a consequence of inhibition of photosynthesis (Losada & Arnon,
1963). Ammonium increases the permeability of the cell membrane
causing plasmolysis and necrosis. Furthermore, the flexibility of
cells may be decreased, which results in an increased sensitivity
to frost. Excess ammonia can be detoxified as long as amino acids
are converted in the presence of carbohydrates (Fig. 1) (Van der
Eerden, 1982).
Most data on the toxic exposure of plants to ammonia have been
derived from controlled fumigation studies. Such experiments have
provided the following relative susceptibilities to ammonia:
leaves > stems, fungi, and bacteria < seeds, sclerotia, and
animals (McCallan & Setterstrom, 1940).
An exposure of 175 mg/m3 (250 ppm) for 4 min produced 50%
foliar necrosis in tomatoes, whereas the same foliar injury was
only produced in buckwheat and tobacco with exposure to 700 mg/m3
(1000 ppm) for 5 and 8 min, respectively (Thornton & Setterstrom,
1940). Zimmerman (1949) reported that fumigation with ammonia at
28 mg/m3 (40 ppm) for 60 min injured tomato, sunflower, and coleus
completely, and a concentration of 2.1 mg/m3 (3 ppm) severely
injured mustard (Benedict & Breen, 1955). Other plant parts are
more resistant to ammonia injury than the foliage. Thornton &
Setterstrom (1940) found 50% injury in tomato stems after exposure
to 700 mg/m3 (1000 ppm) for 60 min. Barton (1940) observed that
moist spring rye seeds were killed in a 4-h exposure to 700 mg
ammonia/m3 (100 ppm), whereas moist radish seeds were still viable
after 16 h. Exposure to a concentration of 175 mg/m3 (250 ppm) for
16 h reduced germination of rye seeds by half, but had no effect on
radish seeds.
Foliar injury is the most common toxic effect of anhydrous
ammonia on vegetation (Linzon, 1971). In broad-leaved woody plants
exposed to high concentrations of ammonia, the injury begins as
large, dark green water-soaked areas on leaves, which darken into
black or brownish-gray bifacial necrotic lesions, widely
distributed over the leaf surface. In lightly injured leaves,
these symptoms occur mainly on the upper surface. Conifer foliage
injured by ammonia exposure darkens to shades of gray-brown,
purple, or black. The entire needle is usually affected.
Abscission of severely damaged leaves is often seen in broad-leaved
and conifer species. On trees or shrubs with crowded leaves,
injury may be confined to particular sections of the leaf.
Occasionally, the foliage of woody species turns a variety of
colours, mimicking autumn colours. Symptoms of injury in
herbaceous plants are more variable, ranging from irregular,
bleached, bifacial, necrotic lesions to dark upper-surface
discolouration (Treshow, 1970). Grasses and cereal grains develop
tan to reddish-brown marginal or interveinal necrosis, and broad-
leaved weeds show red-brown to dark-brown upper surface
discolouration on terminal or marginal portions of the leaf
(Benedict & Breen, 1955). Coleus leaves lose their brilliant
colour after exposure to ammonia and appear green (Zimmerman,
1949). Injury to flowers by ammonia is rarely seen in the field,
but the development of small necrotic spots on azalea flowers has
been reported following ammonia exposure (Treshow, 1970).
Although parts of plants, other than the foliage, are less
susceptible to the injurious effects of ammonia (McCallan &
Setterstrom, 1940), injury to apples, peaches, and other fruits and
vegetables, accidentally exposed during cold storage, has been
reported (Ramsey, 1953). Apparently, ammonia entered the fruit and
turned red-pigmented tissues black, or brown, and yellow tissues,
dark-brown. Immediately on exposure to ammonia, the outer skins of
red onions became greenish-black, and the skin of yellow and brown
onions became dark brown. These colour changes were usually
permanent. Fumigation of fruit with ammonia also caused overall
darkening of the skin. Peaches and apples showed such symptoms at
140 mg/m3 (200 ppm) and 210 mg/m3 (300 ppm), respectively (Brennan
et al., 1962). These symptoms of injury were similar to those seen
on fruits injured by the accidental release of ammonia.
In studies in the Netherlands, several vegetables and trees
showed leaf damage by necrosis, growth reduction, and increased
frost senstivity at concentrations of 75 µg/m3 (annual average),
600 µg/m3 (during 24 h), and 10 000 µg/m3 (during 1 h) (Van der
Eerden, 1982). The sensitivity of pine trees to exposure to
ammonia differed between species (den Boer & Bastiaens, 1984). A
survey is given in Table 14. Airborne ammonium sulfate deposits
damaged the needles of pine trees. Owing to the enhanced uptake of
ammonium, the excretion of potassium, magnesium, and calcium
increases, often resulting in potassium and/or magnesium
deficiency, which may lead to premature shedding of needles
(Roelofs et al., 1985).
Effects of ammonia on the root system have generally been
studied by exposing the roots to different aqueous ammonium
solutions.
Table 14. Relative sensitivity of some pine trees to NH3a
-----------------------------------------------------------------
High sensitivity Moderate sensitivity Low sensitivity
-----------------------------------------------------------------
Picea abies Picea omorika Pinus sylvestris
Picea sitchensis Pinus nigra var. Pinus nigra nigra
Taxus maritima Tsuga canadensis
Cupressus leylandii Taxus baccata Taxus media
Pseutsuga menziesii Pinus mugo var. mughus
-----------------------------------------------------------------
a From: den Boer & Bastiaens (1984).
Exposure of tomato seedlings to ammonium solutions as the sole
nitrogen source showed reduced growth. The root system was
sparsely branched and discoloured and the stem was easily bruised.
There was considerable wilting of leaves, which developed marginal
necrosis (Pierpont & Minotti, 1977). Earlier, Maynard & Barker
(1969), using cucumber (C. sativus), bean (P. vulgaris), and pea
(Pisum sativum L.) in sand culture, demonstrated that ammonium
toxicity was generally characterized by an immediate reduction in
growth rate, wilting, marginal necrosis, interveinal chlorosis of
terminal leaves and, finally, death of the entire plant. However,
these symptoms did not occur in the ammonium medium with added
calcium carbonate (CaCO3) (Maynard & Barker, 1969; Pierpont &
Minotti, 1977). Several studies have shown that an increase in the
ammonium concentration is more deleterious to root than to shoot
growth (Bennett et al., 1964; Haynes & Goh, 1977). Warncke &
Barber (1973) observed that the ratio of root dry weight to shoot
dry weight decreased significantly with increasing concentrations
of nitrogen, but was not affected by the ammonium-to-nitrate ratio.
The roots appeared darker and less branched. The authors
attributed the observed decrease in root production to greater
acidity around the roots of ammonium-fed plants (Klemm, 1967;
Warncke & Barber, 1973).
In the Netherlands, field observations and experiments have
shown effects of ammonia and ammonium on pine forests, and
heathland vegetation (Heil & Diemont, 1983; Van Breemen & Jordens,
1983; den Boer & Bastiaens, 1984; Roelofs, in press; Schuurkes, in
press). In the United Kingdom, ombrotrophic mires (upland bogs
deriving water only from rain) are affected by eutrophication (Lee,
1985). Forest ecosystems in the Federal Republic of Germany may
also suffer from ammonia exposure (Hunger, 1978; Ulrich, 1983).
Although only little information is available for other countries,
it is expected that similar effects may occur in Belgium, Denmark,
and the northwestern part of France, where high ammonia emissions
occur as a consequence of the production of animal manure (Buysman
et al., 1985).
The condition of forests may deteriorate as a consequence of
direct exposure to ammonia. Pine trees in areas near intensive
livestock farms are particularly affected (Hunger, 1978; Janssen,
1982). In the Netherlands, there is a clear correlation between
ammonia emission and forest condition (Van Aalst, 1984). The
damage observed is the result of both direct and indirect effects,
and it is often difficult to distinguish between the two.
Field observations and experiments have shown deleterious
effects on pine trees, namely:
- decreased vitality (growth reduction and necrosis);
- higher susceptibility to fungal diseases and attack
by insects; and
- increased sensitivity to meteorological stress
factors, e.g., hard frost.
Most of these phenomena can be explained in terms of disturbed
nutrient budgets in trees. Enhanced ammonium sulfate uptake
results in the excretion of essential nutrients, such as potassium
and magnesium, by the needles, and the uptake of these ions by the
root system is inhibited as a result of ammonium accumulation and
cation leaching in soils (den Boer & Bastiaens, 1984; Roelofs, in
press). Increased ammonium uptake ultimately leads to enhanced
nitrogen levels in the leaves of beech (Nihlgard, 1970) and pine
tress (Roelofs et al., 1985).
More attention is being paid to the role of ammonium in the
forest dieback in Europe (Nihlgard, 1985).
Heather communities on poorly-buffered, slightly-acidic, and
nutrient-poor heathland soils are also disturbed as a result of the
deposition of airborne ammonia compounds, the number of plant
species declining in acidified and nitrogen-enriched heathland
soils. A succession from heather-dominated to grass-dominated
heathlands has been observed (Heil & Diemont, 1983; Roelofs, in
press). The same effects can be expected in other western European
countries where similar plant communities are present.
6.2.2. Aquatic plants
In the aquatic environment, nitrogen plays an important role in
determining the composition of phytoplankton and vascular plant
communities; in some cases, it can act as a limiting factor in
primary production. Ammonia is important in nitrogen metabolism,
because it functions as a nitrogen source in the synthesis of amino
acids.
Most species can use either ammonium or nitrate as the sole
nitrogen source, though, when both forms are available, ammonium is
used first. Uptake takes place through both roots and leaves. The
relative importance of ammonium as a nutrient depends on the
absolute concentration and the ratio of ammonium to nitrate.
Assimilation of ammonium is less expensive in terms of energy than
nitrate, because the first metabolic step in which nitrate is
reduced is not needed. Although ammonia is an important nutrient,
it appears to be toxic at higher concentrations. Its uptake in
large quantities may put a severe strain on the carbohydrate
metabolism of the species, because carbon skeletons are used for
detoxification (Bidwell, 1974). Increasing ammonia levels within
the cell inhibit the utilization of nitrate. Ammonia solutions
seem to be more toxic at high than at low pH, indicating that
toxicity is probably due primarily to NH3 rather than to NH4+.
Surface waters that are poorly buffered, nutrient poor, and
hydrologically dependent on rainfall and/or snow melt are most
sensitive to ammonia. Deposition of ammonia and other nitrogen
compounds may contribute significantly to the nitrogen enrichment
of susceptible waters. Ombrotrophic mire plant communities can be
altered by this atmospheric pollutant (Lee, 1985). In these
Sphagnum-dominated wetlands, seed plants become more dominant. In
small, poorly-buffered and nutrient-poor clear water lakes, both
water composition and macrophyte composition are altered.
Atmospheric ammonia deposition plays a major role in the
acidification and nitrogen enrichment of surface waters in the
Netherlands (Schuurkes, in press). Typical plant species belonging
to the Littorellion alliance disappear from acidified waters.
These species may be supressed by the luxuriant growth of Sphagnum
species and Juncus bulbosus. Ammonium enrichment enables the last
two species to form extremely high biomasses (Roelofs et al., 1984;
Schuurkes, in press; Schuurkes et al., 1985). It should be noted
that these changes in the plant community also influence the
structure of the animal population.
6.2.3. Fresh-water plants
Experimental data concerning the toxicity of ammonia for fresh-
water phytoplankton are limited. Przytocka-Jusiak (1976) reported
the effects of ammonia on the growth of Chlorella vulgaris. A 50%
inhibition was seen in 5 days of exposure to a concentration of
2.4 mg NH3/litre; complete growth inhibition occurred in 5 days
at 5.5 mg/litre. The NH3 concentration resulting in 50% survival
of C. vulgaris after 5 days was found to be 9.8 mg/litre. A C.
vulgaris strain in which the tolerance to elevated ammonia
concentrations was enhanced by prolonged incubation of the alga in
ammonium carbonate solutions was isolated by Przytocka-Jusiak et
al. (1977). C. vulgaris was reported to grow well in solutions
containing 4.4 mg NH3/litre, but growth was inhibited at 7.4
mg/litre (Matusiak, 1976). Tolerance to elevated concentrations of
NH3 seemed to show a slight increase, when other forms of nitrogen
were available to the alga, rather than when ammonia was the only
form of nitrogen in the medium. Bretthauer (1978) found that a
concentration (assuming pH 6.5 and 30 °C) of 0.6 mg NH3/litre
killed Ochromonas sociabilis, and that, at 0.3 mg/litre,
development of the population was reduced. Concentrations of 0.06
to 0.15 mg NH3/litre had an insignificant effect on growth, and
concentrations of 0.015 to 0.03 mg/litre enhanced growth.
Ammonia at concentrations exceeding 2.5 mg NH3/litre inhibited
photosynthesis and growth in the algal species Scenedesmus obliquus
and inhibited photosynthesis in the algae Chlorella pyrenoidosa,
Anacystis nidulans, and Plectonema boryanum (Abelovich & Azov,
1976). Mosier (1978) reported that NH3 concentrations causing a
50% reduction in oxygen production by the green alga Chlorella
ellipsoidea and blue-green alga Anabaena subcylindrica were
16.0 x 10-8 and 251.0 x 10-8 µg NH3-N/cell, respectively.
The rate of photosynthesis in the blue-green alga P. boryanum
was stimulated by NH4+, but inhibited by NH3 (Solomonson 1969); the
magnitude of these effects was dependent on the sodium-potassium
composition of the suspension media. Inhibition of photosynthesis
by NH3 was associated with a conversion of inorganic polyphosphate,
stored in the cells, to orthophosphate.
Champ et al. (1973) treated a small natural water pond with
ammonia to achieve a mean concentration of 25.6 mg NH3/litre. A
diverse population of dinoflagellates, diatoms, desmids, and blue-
green algae was present before ammonia treatment. Twenty-four
hours after treatment, the mean number of phytoplankton cells/litre
was reduced by 84%. By the end of 2 weeks (3.6 mg NH3/litre), the
original concentration of cells had been reduced by 95%.
Some research has been carried out to investigate the possible
use of ammonia as an aquatic herbicide. Champ et al. (1973)
reported virtually complete eradication of rooted aquatic
vegetation (water shield, Brasenia schreberi, and American lotus,
Nelumbo sp.). The NH3 concentration was 25.6 mg/litre 24 h after
ammonia addition, and 3.6 mg/litre, 2 weeks later. The use of high
concentrations of ammonia to eradicate aquatic vegetation has been
described by Ramachandran & Ramaprabhu (1976) and Ramachandran et
al. (1975).
In experiments with Potamogeton lucens, Litav & Lehrer (1978)
observed that ammonia caused appreciable injury to detached
branches. Ammonia inhibition of the growth of Eurasian
watermilfoil (Myriophyllum spicatum) affected both the length and
weight of roots and shoots (Stanley, 1974).
Grube (1973) found that Sium erectum was slightly injured at
15 mg NH3-N/litre, and completely eradicated at 35 mg/litre after a
10-week exposure. Callitriche sp. showed slight injury at 5 mg
NH3-N/litre, and the lethal dose was between 10 and 15 mg/litre.
Injury was estimated from the amount of black colouring and the
death of leaves. Roelofs et al. (1984) reported that exposure of
the isoebid Littorella uniflora to 50 µmol NH4+/litre for 10 weeks
resulted in excretion of an equivalent amount of potassium, which
may lead to discolouration and starvation.
Changes in the vegetation in 2 rivers subject to increased
pollution from agricultural fertilizers, urban sewage, and
industrial wastes, were studied by Litav & Agami (1976), who
attributed the changes in the composition of the plant species
primarily to a combination of ammonia and detergents. Agami et al.
(1976) transplanted 7 species of "clean water" macrophytes to
various sections of a river, and found that ammonia affected only
Nymphaea caerulea.
6.2.4. Salt-water plants
A concentration of 0.24 mg NH3/litre retarded the growth of
most of 10 species of benthic diatoms cultured for 10 days by
Admiraal (1977). Pinter & Provasoli (1963) found that Coccolithus
huxleyi was the most sensitive, and Pavlova gyrans and Hymenomonas
sp. the most tolerant to ammonium sulfate with intermediate tolerance
exhibited by Syracosphaera sp. and Ochrosphaera neapolitana.
Shilo & Shilo (1953, 1955) reported that the euryhaline alga
Prymnesium parvum was effectively controlled by applications of
ammonium sulfate, which exerted a lytic effect that decreased with
increasing pH, indicating that NH3 and not NH4+ is responsible for
the lytic activity of ammonium sulfate on P. parvum. It was
reported by Byerrum & Benson (1975) that added ammonium ion at
concentrations found to stimulate the photosynthetic rate also
caused the alga Amphidinium carterae to release up to 60% of fixed
14CO2 to the medium.
Natarajan (1970) found that the concentrations of fertilizer
plant effluent that were toxic for natural phytoplankton
(predominantly diatoms) ranged between 1.1 and 11 mg NH3/litre.
Thomas et al. (1980) concluded that increased ammonium
concentrations found near sewage outlets would not be inhibiting to
phytoplankton in the vicinity. Provasoli & McLaughlin (1963)
reported that ammonium sulfate was toxic for some marine
dinoflagellates, but only at concentrations far exceeding those in
sea water.
6.3. Aquatic Invertebrates
The toxicity of ammonia has been less extensively studied in
invertebrates than in fish. Most of the available invertebrate
data consists of studies on arthropods, primarily crustaceans and
insects. Many of these studies are laboratory tests in which test
animals were exposed to known concentrations of a toxic agent for
specified periods of time. Results may be expressed as a median
lethal concentration (LC50) for a given time period (e.g., 48-h
LC50) or, occasionally, as an effective concentration (EC), that
is, the concentration at which the test animal is completely
immobilized, though it might still be respiring (e.g., 48-h EC).
Another method of reporting test results is as the estimated time
required to kill 50% of a test population (LT50) at a given
concentration of toxin (e.g., LT50 = 250 min).
6.3.1. Fresh-water invertebrates: acute toxicity
The acute toxicity of ammonia for Daphnia magna has been
studied by Parkhurst et al. (1979, 1981) and Reinbold & Pescitelli
(1982a) with reported 48-h LC50s values of 2.08 and 4.94 mg
NH3/litre. DeGraeve et al. (1980) reported a similar 48-h LC50
value for Daphnia pulicaria of 1.16 mg NH3/litre. A threshold
toxicity value for D. magna of 2.4 - 3.6 mg NH3/litre lake water
was reported by Anderson (1948). Threshold concentration was taken
as the highest concentration that would just fail to immobilize the
test animals, under conditions of prolonged exposure (Anderson,
1948). A minimum lethal concentration of 0.55 mg NH3/litre was
reported for D. magna by Malacea (1966), and a 24-h LC50 value of
1.50 mg NH3/litre was reported by Györe & Oláh (1980) for Moina
rectirostris.
Buikema et al. (1974) reported an EC50 for NH3 toxicity in the
rotifer, Philodina acuticornis, to be 2.9 - 9.1 mg NH3/litre
(calculated using reported pH values of 7.4 - 7.9). Tests of
ammonia toxicity for the flatworm, Dendrocoelum lacteum (Procotyla
fluviatilis), and a tubificid worm (Tubifex tubifex) gave LC50
values of 1.4 and 2.7 mg NH3/litre, respectively (Stammer, 1953).
Thurston et al. (1984a) conducted 25 flow-through toxicity
tests with 3 mayfly, 2 stonefly, 1 caddisfly, and 1 isopod species;
all tests were conducted with water of similar chemical
composition. The 96-h LC50 values ranged from 1.8 to 5.9 mg
NH3/litre. The results also indicated that a 96-h test is not long
enough to determine the acutely lethal effects of ammonia on the
species tested, because an asymptotic LC50 was not always obtained
within 96 h. Percentage survival data were reported for some
mayfly, stonefly, and caddisfly tests in which LC50 values were not
obtained; there was 60 - 100% survival at test concentrations
ranging from 1.5 to 7.5 mg NH3/litre. Gall (1980) tested NH4Cl
with Ephemerella sp. (near excrucians). Organisms were exposed
to ammonia for 24 h, followed by 72 h in ammonia-free water;
mortality observations were made at the end of the overall 96-h
period. An EC50 value of 4.7 mg NH3/litre was obtained. Hazel et
al. (1979) reported a LC50 value of 8.0 mg NH3/litre for the beetle
Stenelmis sexlineata.
No deaths occurred in ammonia toxicity tests conducted on scud,
Gammarus lacustris, or D. magna, using dilution water from a
river, after a 96-h exposure to 0.08 mg NH3/litre. In a second
test, using river water buffered with sodium bicarbonate, 13%
mortality occurred with scud at the several concentrations tested,
including the highest and lowest of 0.77 and 0.12 mg NH3/litre,
respectively; 7 and 13% mortality occurred with D. magna at the
same concentrations (Miller et al., 1981).
Five fresh-water mussel species, Amblema p. plicata, Anodonta
imbecillis, Corbicula manilensis, Cyrtonaias tampicoensis, and
Toxolasma texasensis, were exposed for 165 h to a concentration of
0.32 mg NH3/litre; T. texasensis was most tolerant to ammonia, and
A. p. plicata was most sensitive (Horne & McIntosh, 1979). During
the tests, the more tolerant species generally had their shells
tightly shut, whereas the least tolerant species continued
siphoning or had their mantles exposed. In 2 studies, acute
exposure of the fresh-water crayfish, Orconectes nais, to ammonium
chloride gave LC50 values of 3.15 and 3.82 mg NH3/litre,
respectively (Evans, 1979; Hazel et al., 1979).
6.3.2. Fresh-water invertebrates: chronic toxicity
Few studies have been conducted on the long-term exposure of
fresh-water invertebrates to ammonia. In a long-term test
conducted by Reinbold & Pescitelli (1982a), reproduction and growth
of D. magna were affected at a concentration of 1.6 mg NH3/litre.
Two tests lasting 42 days were conducted by Anderson et al.
(1978) on the effects of ammonium chloride on the fingernail clam,
Musculium transversum. Significant mortality (67 and 72%) occurred
in both tests at a concentration of 0.7 mg NH3/litre. In one of
the studies, significant reduction in growth was observed after 14
days of exposure to 0.41 mg NH3/litre. Sparks & Sandusky (1981)
reported that fingernail clams exposed to 0.23 and 0.63 mg
NH3/litre showed 36 and 23% mortality, respectively, in 4 weeks;
after 6 weeks, there was 47% mortality at 0.073 mg NH3/litre, and
83% mortality at 0.23 and 0.63 mg NH3/litre. After 6 weeks there
was no growth in any test chamber (concentrations of 0.036 mg
NH3/litre and higher), other than in the controls.
Two partial tests, lasting 24 and 30 days, respectively, were
conducted by Thurston et al. (1984a) on the stonefly Pteronarcella
badia. Adult stonefly emergence was delayed with increasing
ammonia concentration, and little or no emergence occurred at
concentrations exceeding 3.4 mg NH3/litre. There was no significant
relationship between the food consumption rates of nymphs and
concentrations up to 6.9 mg NH3/litre. LC50 values for 24- and
30-day exposures were 1.45 and 4.57 mg NH3/litre, respectively.
The effects of ammonia on the ciliary beating rate of clam
gills were investigated by Anderson et al. (1978). Concentrations
of 0.036 - 0.11 mg NH3/litre caused a reduction in the ciliary
beating rate of fingernail clams; the effects ranged from a 50%
reduction in beating rate to complete inhibition. Adult clams (> 5
mm) were more sensitive than juveniles (< -5 mm); adults were also
slightly more sensitive than the unionid mussel, Elliptio
complanata, and the Asiatic clam, C. manilensis. Shaw (1960)
investigated the effects of ammonium chloride on sodium influx in
the fresh-water crayfish, Astacus pallipes. Ammonia produced an
inhibition of sodium influx; a concentration of 18 mg NH4+/litre
reduced the influx to about 20% of its normal value, and influx
reduction was related to increasing ammonia concentration. This
effect was attributed to NH4+ ions and not to any toxic effect
exerted on the transporting cells by non-ionized ammonia. NH4+ did
not affect chloride influx nor the rate of sodium loss.
Ammonia was added to a stream at a 24-h average concentration
of 1.4 mg NH3/litre, and a 24-h drift net sampling was conducted
(Liechti & Huggins, 1980). No change in diel drift pattern was
observed, but there was an increase in the magnitude of drift, a
shift in the kinds of organisms present, and changes in benthic
standing crop estimates; this ammonia concentration was non-lethal.
6.3.3. Salt-water invertebrates: acute and chronic toxicity
Data on the acute toxicity of ammonia for salt-water
invertebrate species are very limited. A 96-h LC50 value of 1.5 mg
NH3/litre has been reported for the copepod, Nitocra spinipes
(Linden et al., 1979). Lethal effects of ammonium chloride on the
quahog clam, Mercenaria mercenaria, and eastern oyster,
Crassostrea virginica, were studied by Epifano & Srna (1975).
There were no observed differences in susceptibility between
juveniles and adults of the 2 species. Armstrong et al. (1978)
conducted acute toxicity tests (6 days) on ammonium chloride using
prawn larvae, Macrobrachium rosenbergii. LC50 values were highly
pH-dependent. The acute toxicity of ammonium chloride for penaeid
shrimp was reported as a 48-h composite LC50 value of 1.6 mg
NH3/litre for 7 species pooled, including the resident species
Penaeus setiferus (Wickins, 1976). The acute toxicity of ammonium
chloride for the caridean prawn, M. rosenbergii, was reported
(Wickins, 1976) as LT50 values of 1700 - 560 min at concentrations
of 1.74 - 3.41 mg NH3/litre. From the data of Hall et al. (1978),
48-h LC50 values of 0.34 - 0.53 were estimated for grass shrimp,
Palaemonetes pugio. Catedral et al. (1977a,b) investigated the
effects of ammonium chloride on the survival and growth of Penaeus
monodon; larvae had a lower tolerance to ammonia compared with
post-larvae. Brown (1974) reported a time to 50% mortality of
106 min for the nemertine worm, Cerebratulus fuscus, at a
concentration of 2.3 mg NH3/litre.
Effects of ammonium chloride solutions on the American lobster,
Homarus americanus, were studied by Delistraty et al. (1977).
Their studies were performed on fourth-stage larvae, which was
considered to be the most sensitive life stage. A 96-h LC50 value
of 2.2 mg NH3/litre and an incipient LC50 value of 1.7 mg NH3/litre
were reported. A "safe" concentration of 0.17 mg NH3/litre was
tentatively recommended.
The sublethal toxicity of ammonium chloride for the quahog clam
and eastern oyster was studied by Epifano & Srna (1975) who
measured the effect of a 20-h exposure to ammonia on the rate of
removal of the alga, Isochrysis galbana, from suspension (clearing
rate) by the clams and oysters. Concentrations of 0.06 - 0.2 mg
NH3/litre affected removal; no differences were observed between
juveniles and adults. The effect of ammonia on the ciliary beating
rate of the mussel, Mytilus edulis, was studied by Anderson et al.
(1978). Concentrations of 0.097 - 0.12 mg NH3/litre resulted in a
reduction in the ciliary beating rate ranging from 50% to complete
inhibition.
Exposure of unfertilized sea urchin, Lytechinus pictus, eggs to
ammonium chloride resulted in stimulation of the initial rate of
protein synthesis, an event that normally follows fertilization
(Winkler & Grainger, 1978). Exposure of unfertilized eggs of
Strongylocentrotus purpurpatus, L. pictus, and Strongylocentrotus
drobachiensis to ammonium chloride (Johnson et al., 1976; Paul et
al., 1976) caused "fertilization acid" to be released more rapidly
and in greater amounts than after insemination. Activation of
unfertilized L. pictus eggs by ammonium chloride exposure was
also evidenced by an increase in intracellular pH (Steinhardt &
Mazia, 1973; Shen & Steinhardt, 1978). Ammonia treatment has also
been reported to activate phosphorylation of thymidine and
synthesis of histones in unfertilized eggs of the sea urchin,
S. purpuratus, (Nishioka, 1976). Premature chromosome
condensation was induced by ammonia treatment of eggs of L. pictus
and S. purpuratus (Epel et al., 1974; Wilt & Mazia, 1974; Krystal
& Poccia, 1979). Treatment of S. purpuratus and S. drobachiensis
fertilized eggs with ammonia resulted in an absence of the normal
uptake of calcium following insemination. However, calcium uptake
was not inhibited when ammonia treatment preceded insemination
(Paul & Johnston, 1978).
The polychetous annelid, Nereis succinea, the channelled whelk,
Busycon canaliculatum, and the brackish-water clam, Rangia
cuneata, were exposed to concentrations of 0.85, 0.37, and 0.27 mg
NH3/litre and the ammonia excretion measured (Mangum et al., 1978).
Excretion was inhibited by non-lethal concentrations of ammonia,
and the authors concluded that ammonia crosses the excretory
epithelium in the ionized form, and that the process is linked to
the activity of the Na++K+ ATPases. When blue crabs, Callinectes
sapidus, were moved from water of 28 parts per thousand salinity
to water of 5 parts per thousand, a doubling of the ammonia
excretion rate occurred; addition of excess ammonium chloride to
the low-salinity water inhibited ammonia excretion and decreased
net acid output (Mangum et al., 1976). The effect of gaseous NH3
on haemoglobin from the blood of the common marine bloodworm,
Glycera dibrachiata, was examined by Sousa et al. (1977) in an
attempt to determine whether there was competition between NH3 and
oxygen in binding to haemoglobin; such an NH3/O2 relationship was
not found.
6.4. Fish
Ammonia is highly toxic for fish, and, because of its
occurrence at high concentrations in some water systems, it can
present a major pollution problem. It enters aquatic environments
from several sources, including sewage effluent, deposition of
human wastes without treatment, industrial discharges, and runoff
from animal culture and agricultural operations. It is also a
metabolic waste product of fish and, therefore, can be a problem in
facilities involved with intensive fish culture.
Elevated ammonium ion (NH4+) concentrations within the bodies
of fish, as with other vertebrates, cause convulsions and death.
The concentration of non-ionized ammonia (NH3) in the environment
of the fish is important, because ammonia is transferred between
the water and fish largely in this form. Thus, while NH3 is the
more toxic chemical species in the water, within the fish, toxicity
is related to the NH4 concentration.
Research by Chipman (1934), Wuhrman et al. (1947), Wuhrman &
Woker (1948), and Tabata (1962) implicated NH3 as the ammonia
species in water that is mainly toxic for fish, and reported that
NH4+ was non-toxic or considerably less toxic. More recent
research by Robinson-Wilson & Seim (1975), Armstrong et al. (1978),
and Thurston et al. (1981c) has demonstrated that the role of water
pH in the toxicity of ammonia for fish is more than the regulation
of the NH3/NH4+ equilibrium. NH3 is considerably more toxic in
water when pH values are lower than 7 - 9, and there is some
evidence that the toxicity of NH3 is also increased above this
range. Temperature and dissolved oxygen and the ionic composition
of the background water all play a role in the toxicity of NH3 for
some fish.
In a comprehensive analysis of the data, it was concluded that
a number of fish species that are phylogenetically similar are also
similar in their sensitivity to the toxicity of ammonia, and that
many of the factors that affect the toxicity of ammonia similarly
affect all the species that have been studied (US EPA, 1985).
It was formerly considered that fish of the family Salmonidae
were among the most sensitive to the effects of many pollutants,
whereas other fish species, which have evolved in warm-water, low
oxygen, or more turbid aquatic environments, may be less sensitive
to many naturally-occuring pollutants, such as ammonia. However,
other fish species, including some that are frequently referred to
as "warm water" fishes, are of comparable sensitivity (US EPA,
1985). In the "resident-species" approach for establishing water
quality criteria for a toxic agent in a given water body, the
tolerance of selected fish and invertebrate species, naturally
resident in the water body, is used. It must be borne in mind
that, if these selected species are less sensitive than other
species in different water bodies, the standards may be
inappropriate for other water bodies.
Because of variation among different background test water
conditions in different laboratories, as well as differences in the
genetic pools of the same species, results of a single test on a
given species may not be as meaningful as composite results from
several tests conducted at different laboratories.
Much of the evidence on ammonia toxicity is empirical, so, for
a more complete understanding of the biological actions of this
chemical, the results of toxicity tests must be integrated with a
knowledge of ammonia metabolism in fish.
6.4.1. Ammonia metabolism in fish
6.4.1.1. Ammonia production and utilization
The major pathway for the production of ammonia in fish, as in
other vertebrates, is through the transamination of various amino
acids (Forster & Goldstein, 1969; Watts & Watts, 1974). The
primary site for ammonia production is probably the liver (Pequin &
Serfaty, 1963), but the necessary enzymes have also been located in
the kidneys, gills, and skeletal muscle tissue (Goldstein &
Forster, 1961; McBean et al., 1966; Walton & Cowey, 1977). Ammonia
is also produced by the deamination of adenylates in fish muscle
(Driedzic & Hochachka, 1976). The quantitative importance of
muscle ammoniogenesis in total ammonia excretion depends on the
level of activity of the animal, and increases with increasing
workload (Suyama et al., 1960; Fraser et al., 1966; Driedzic &
Hochachka, 1976).
Ammonia toxicity can be ameliorated by the formation of less
toxic compounds, namely glutamine and urea. Levi et al. (1974)
recorded high levels of glutamine in the brain of goldfish,
Carrasius auratus, and found that brain-glutamine levels increased
with ambient ammonia concentrations. Webb & Brown (1976) found
high glutamine synthetase (EC 6.3.1.2) activity in the brains of
teleosts and elasmobranchs, and this may be important in protecting
the brain from sudden surges in ammonia concentration. Walton &
Cowey (1977) were able to detect glutaminase activity in the gills
of rainbow trout, but were unable to measure any in vivo
utilization of glutamine by the gills.
Ammonia can be converted, through carbamyl phosphate, to urea
either via purines (uricolysis) or via the ornithine cycle. The
enzymes required for uricolysis have been found in most fish
studied (Forster & Goldstein, 1969; Watts & Watts, 1974), but
Florkin & Duchateau (1943) were unable to detect any activity of
uricolytic enzymes in the cyclostome, Lampetra. The ratio of urea
production via the ornithine cycle to production via uricolysis is
about 100 to 1 in elasmobranchs and dipnoi, whereas in teleosts
most of the urea is formed via uricolysis (Gregory, 1977).
6.4.1.2. Ammonia excretion
The gills are the major site of ammonia excretion in fish, but
smaller quantities of ammonia may also be eliminated by the kidneys
(Edwards & Condorelli, 1928; Grollman, 1929; Fromm, 1963; Maetz,
1972) and skin (Morii et al., 1978). Although the majority of
branchial ammonia excretion represents clearance from the blood,
gill metabolism may contribute between 20% (Payan & Matty, 1975)
and 5 - 8% (Cameron & Heisler, 1983) of the net ammonia excretion.
The excretion of ammonia by fish is variable, depending on the
state of the animal, the environmental conditions, and the species.
Ammonia excretion tripled in sockeye salmon, Oncorhynchus nerka,
following daily feeding (Brett & Zala, 1975) but remained low and
unchanging during starvation (Brett & Zala, 1975; Guerin-Ancey,
1976a). In fresh-water fish, ammonia excretion increases in
response to exercise (Sukumaran & Kutty, 1977; Holeton et al.,
1983), long-term acid exposure (McDonald & Wood, 1981; Ultsch et
al., 1981), hypercapnia (Claiborne & Heisler, 1984), and NH4Cl
infusion (Hillaby & Randall, 1979). In contrast, increased levels
of environmental ammonia (Guerin-Ancey, 1976b) and short-term
exposure to acid or alkaline water (Wright & Wood, 1985) cause a
decrease in ammonia excretion. It is not known if these changes in
excretion reflect change in the rate of ammonia production or in
the ammonia content of the body. The ammonia content of fish is
likely to be the equivalent of the ammonia excreted in about 2 h,
most of the ammonia being in the tissues with a lower pH, such as
muscle. Blood levels are around 0.2 to 0.3 mmol, but muscle at a
lower pH may contain levels of up to 1 mmol; thus, a 1-kg fish may
contain about 0.5 to 0.7 mmol of ammonia and have an excretion rate
of about 0.3 mmol/h. There is increased ammonia production in
muscle during exercise (Driedzic & Hochachka, 1976). Ammonia
excretion by the spiny dogfish, Squalus acanthias, in sea water is
unaffected by temperature change, exercise, hyperoxia, hypercapnia,
or the infusion of either HCl or NaHCO3 or anything that induces
acid-base stress (Heisler, 1984). This is surprising, because many
of these changes affect pH and therefore would be expected to alter
the ammonia content of body compartments and consequently ammonia
excretion.
There is an elevation in blood-ammonia during starvation (Morii
et al., 1978; Hillaby & Randall, 1979), even though ammonia
excretion does not change (Brett & Zala, 1975). Blood-ammonia
concentrations also rise with increases in both temperature
(Fauconneau & Luquet, 1979) and ammonia concentrations in the water
(Fromm & Gillette, 1968; Thurston et al., 1984b). Exposure of fish
to either air (Gordon, 1970) or increased ammonia levels in water
(Fromm, 1970; Guerin-Ancey, 1976b), raises blood-ammonia levels and
reduces ammonia excretion; this is associated with a rise in urea
production in many, but not all fish. Unlike the authors of the
above studies, Buckley et al. (1979) did not find any change in
blood-total ammonia when coho salmon, Oncorhynchus kisutch, were
exposed to elevated ammonia levels in the environment. However, a
significant rise in plasma-sodium, indicating some coupling between
sodium uptake and ammonia excretion, was observed.
The study of ammonia movement is complicated by the fact that,
with present analytical techniques, it is impossible to distinguish
between the transfer of a molecule of NH3 plus a H+ ion from the
transfer of an NH4+ ion. Thus, only indirect evidence can be
obtained regarding the relative gas and ion movements across the
gill epithelium.
Three possible mechanisms of ammonia excretion have received
the most attention: passive NH3 flux, ionic exchange of NH4+ for
Na+, and passive NH4+ flux. There seems to be little doubt that a
significant pathway for branchial ammonia excretion is by the
passive diffusion of NH3 down its partial pressure gradient.
Changes in the NH3 partial pressure gradient are positively
correlated with changes in net ammonia excretion in the channel
catfish, Ictalurus punctatus, (Kormanik & Cameron, 1981), and
rainbow trout (Cameron & Heisler, 1983; Wright & Wood, 1985).
Ammonia entry into the fish has also been shown to be dependent on
the NH3 gradient (Wuhrmann et al., 1947; Wuhrmann & Woker, 1948;
Fromm & Gillette, 1968).
The excretion of NH4+ is strongly coupled with the movement
of other ions. Many studies have attempted to link the
transepithelial exchange of Na+ uptake to NH4+ efflux. Although
there is considerable indirect evidence for the presence of a
coupled ionic exchange mechanism under certain conditions (Maetz &
Garcéa-Romeu, 1964; Maetz, 1973; Payan & Maetz, 1973; Evans, 1977,
1980; Payan, 1978; Girard & Payan, 1980; Wright & Wood, 1985), the
ubiquity and stoichiometry of this exchange remain controversial.
While Na+ influx can be monitored with isotopes, it is difficult to
determine NH4+ efflux. Investigators have attempted to quantify
the relationship between Na+ uptake and NH4+ excretion by
manipulating Na+ levels in the environmental water, by
pharmaceutical inhibition of the Na+ influx mechanism, or by
loading the fish with ammonia.
In goldfish (Maetz, 1973), and in irrigated rainbow trout gills
(Kirschner et al., 1973), Na+ influx was best correlated with the
sum of H+ and NH4+ ion efflux. The possibility of a Na+ uptake
carrier coupled to either NH4+ or H+ appears likely in other fish
as well (Kerstetter et al., 1970; Payan & Maetz, 1973; Evans,
1977). In perfused heads of trout, Na+ uptake was tightly coupled
with NH4+ efflux (Payan et al., 1975; Payan, 1978). Wright & Wood
(1985) demonstrated that, in intact trout, the rate of ion exchange
was influenced by external water pH, increasing from no exchange at
pH 4 to maximal rates at pH 8. The relationship between Na+ and
NH4+ was one-to-one at a pH of less than 8. However, Payan et al.
(1975) and Wright & Wood (1985) found that the majority of the
ammonia was eliminated by gaseous diffusion, and only when NH3 was
subtracted from total ammonia efflux was the NH4+/Na+ exchange
evident. In common carp, Cyprinus carpio (de Vooys, 1968), and
little skate, Raja erinacea (Evans et al., 1979), ammonia
excretion was unaffected by a reduction in environmental Na+
levels. Cameron & Heisler (1983) found that, under resting
conditions, diffusive movement of NH3 could account for ammonia
excretion in trout, but when the ammonia gradient was reversed and
directed inwards, an NH4+/Na+ exchange could counter-balance the
diffusive uptake of NH3 from the water. If this hypothesis is
correct, then it would explain the unchanging blood-ammonia levels
and increased Na+ levels in coho salmon exposed to elevated
concentrations of ammonia in the water (Buckley et al., 1979).
The Na+/NH4+ (H+) exchange is probably located on the
epithelial apical membrane. Either acid conditions or amiloride in
the water inhibits Na+ influx across the gills and both these
conditions result in a reduction in ammonia excretion (Wright &
Wood, 1985).
The ammonium ion can displace potassium in many membrane
processes in, for example, the giant axon of squid, Loligo pealei
(Binstock & Lecar, 1969), and this is the probable reason that
elevated ammonia causes convulsions in so many vertebrates. In
various aquatic animals, it is possible that NH4+ can substitute
for potassium in oubain-sensitive sodium/potassium exchange (Payan
et al., 1975; Towle & Taylor, 1976; Towle et al., 1976; Mallery,
1979; Girard & Payan, 1980). NH4+ ions will substitute for K+ ions
across the epithelial basolateral border (Richards & Fromm, 1970;
Shuttleworth & Freeman, 1974; Karnaky et al., 1976), but the
importance to net ammonia transfer in fresh-water fish is unknown.
The passive movement of NH4+ down its electrochemical gradient
may also contribute to net ammonia excretion (Claiborne et al.,
1982; Goldstein et al., 1982). Lipid membranes are relatively
impermeable to cations (Jacobs, 1940) and, because respiratory
epithelial cells of fresh-water fishes are joined by tight
junctions (Girard & Payan, 1980), it appears unlikely that NH4+
diffusion is of quantitative importance (Kormanik & Cameron, 1981).
Indeed, Wright & Wood (1985) found a negative correlation between
ammonia excretion and the NH4+ concentration gradient in rainbow
trout exposed to 5 different water pH regimes. Although it appears
that NH4+ diffusion may be of minor importance, simultaneous
measurements of the electrical and chemical gradient have not been
made and are necessary before conclusions can be drawn.
6.4.2. Fish: acute toxicity
The acute toxicity of ammonia for rainbow trout has been
studied by many investigators, with reported 96-h LC50 values
ranging from 0.16 to 1.1 mg NH3/litre. Thurston & Russo (1983)
conducted 71 toxicity tests on rainbow trout ranging in size from
sac fry (< 0.1 g) to 4-year-old adults (2.6 kg), in water of
uniform chemical composition. LC50 values ranged from 0.16 to
1.1 mg NH3/litre for 96-h exposures. Fish susceptibility to NH3
decreased with increasing weight over the range 0.06 - 2.0 g, but
gradually increased above that weight range. LC50 values for 12-
and 35-day exposures did not differ greatly from 96-h values. No
statistically-significant differences in results were observed when
different ammonium salts [NH4Cl, NH4HCO3, (NH4)2HPO4, (NH4)2SO4]
were used. Grindley (1946) also reported that there were no
appreciable differences in toxicity between toxic solutions of
NH4Cl and (NH4)2SO4 in rainbow trout tests. However, Calamari et
al. (1977, 1981) reported that embryos and fingerlings were less
sensitive than the other life stages studied. LC50 values (96-h)
ranging from 0.16 to 1.02 mg NH3/litre for rainbow trout exposed to
ammonia were reported by Calamari et al. (1977, 1981), Broderius &
Smith (1979), Holt & Malcolm (1979), DeGraeve et al. (1980), and
Reinbold & Pescitelli (1982b).
Although acute toxicity studies with salmonids have mainly been
conducted on rainbow trout, some data are available for a few other
salmonid species. Thurston et al. (1978) investigated the
toxicity of ammonia for cutthroat trout, Salmo clarki, and
reported 96-h LC50 values of 0.52 - 0.80 mg NH3/litre. Thurston &
Russo (1981) reported a 96-h LC50 value of 0.76 mg NH3/litre for
golden trout, Salmo aguabonita. Brown trout, Salmo trutta, were
exposed to 0.15 mg NH3/litre for 18 h, resulting in a 36%
mortality; when returned to ammonia-free water, the test fish
recovered after 24 h (Taylor, 1973). No mortality occurred during
the 96-h exposure at 0.090 mg NH3/litre, although fish would not
feed. Exposure to 0.8 mg NH3/litre was not acutely toxic for brown
trout according to Woker & Wuhrmann (1950). However, Thurston &
Meyn (1984) reported 96-h LC50 values of 0.60 - 0.70 mg NH3/litre,
and Miller et al. (1981) reported a 96-h LC50 value of 0.47 mg
NH3/litre for brown trout using test dilution river water. Brook
trout, Salvelinus fontinalis, showed distress within 1.75 h at a
concentration of 3.25 mg NH3/litre and within 2.5 h at 5.5 mg/litre
(Phillips, 1950). Thurston & Meyn (1984) reported 96-h LC50 values
of 0.96 - 1.05 mg NH3/litre for brook trout, 0.40 - 0.48 mg
NH3/litre for chinook salmon, Oncorhynchus tshawytscha, and 0.14 -
0.47 mg NH3/litre for mountain whitefish, Prosopium williamsoni.
Toxicity tests with (NH4)2SO4 on pink salmon, Oncorhynchus
gorbuscha, at different early stages of development (Rice & Baily,
1980) showed that late alevins near swim-up stage were the most
sensitive (96-h LC50 = 0.083 mg NH3/litre), and eyed embryos were
the most tolerant, surviving 96 h at > 1.5 mg NH3/litre. Buckley
(1978) reported a 96-h LC50 value of 0.55 mg NH3/litre for
fingerling coho salmon, Oncorhynchus kisutch, and Herbert &
Shurben (1965) reported a 24-h LC50 value of 0.28 mg NH3/litre for
Atlantic salmon, Salmo salar.
There are acute toxicity data for ammonia in a variety of non-
salmonid fish species. Thurston et al. (1983) studied the toxicity
of ammonia for fathead minnows, Pimephales promelas, ranging from
0.1 to 2.3 g in weight and found that 96-h LC50 values in 29 tests
ranged from 0.75 to 3.4 mg NH3/litre; toxicity was not dependent on
the test fish size or source. LC50 values ranging from 0.73 to
2.35 mg NH3/litre for fathead minnows were also reported by Sparks
(1975), DeGraeve et al. (1980), Reinbold & Pescitelli (1982b),
Swigert & Spacie (1983). LC50 values for white sucker, Catostomus
commersoni, exposed to ammonium chloride solutions for 96 h
(Reinbold & Pescitelli 1982c) were 1.40 and 1.35 mg/litre NH3,
though a somewhat lower 96-h LC50 of 0.79 mg NH3/litre was
determined by Swigbert & Spacie (1983). Thurston & Meyn (1984)
reported 96-h LC50 values of 0.67 - 0.82 mg NH3/litre for the
mountain sucker, Caststomus platyrhynchus.
Reported LC50 values for 96-h exposures of bluegill, Lepomis
macrochirus, ranged from 0.26 to 4.60 mg NH3/litre (Emery & Welch,
1969; Lubinski et al., 1974; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982b; Swigert & Spacie, 1983). LC50 values (96-h) of
0.7 - 1.8 mg NH3/litre for smallmouth bass, Micropterus dolomieui,
and 1.0 - 1.7 mg NH3/litre for largemouth bass, Micropterus
salmoides, were reported by Broderius et al. (in press) and
Roseboom & Richey (1977), respectively. Sparks (1975) reported
48-h LC50 values for bluegill of 2.30 mg NH3/litre, and for channel
catfish of 2.92 mg NH3/litre. For goldfish, Carassius auratus,
Dowden & Bennett (1965) reported a 24-h LC50 of 7.2 mg NH3/litre,
and Chipman (1934) reported lethal threshold values of 0.97 -
3.8 mg NH3/litre. Turnbull et al. (1954) reported a 48-h LC50 for
bluegill to be within the range 0.024 - 0.093 mg NH3/litre; during
the exposure, they observed that the fish exhibited a lack of
ability to avoid objects.
Reported 96-h LC50 values for channel catfish, Ictalurus
punctatus, ranged from 1.5 to 4.2 mg NH3/litre (Colt &
Tchobanoglous, 1976; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982d; Swigert & Spacie, 1983). Vaughn & Simco (1977)
reported a 48-h LC50 for channel catfish of 1.24 - 1.96 mg
NH3/litre, and Knepp & Arkin (1973) reported 1-week LC50 values of
0.97 - 2.0 mg NH3/litre. The results of studies on bluegill,
channel catfish, and largemouth bass (Roseboom & Richey, 1977)
showed that bluegill susceptibility was dependent on fish weight,
fish weighing 0.07 g being slightly more sensitive than those
weighing either 0.22 or 0.65 g; size had little effect on the
susceptibility of channel catfish or bass.
Hazel et al. (1979) reported 96-h LC50 values of 0.90 and
1.07 mg NH3/litre for the orangethroat darter, Etheostoma
spectabile, and red shiner, Notropis lutrensis. Largemouth bass,
channel catfish, and bluegill were also exposed for 96 h to a
concentration of 0.21 mg NH3/litre resulting in zero mortality for
bluegill and channel catfish and one death (6%) among the
largemouth bass tested. A 96-h LC50 value for walleye,
Stizostedion vitreum, of 0.85 mg/litre NH3 was reported by
Reinbold & Pescitelli (1982a).
LC50 values ranging from 2.4 to 3.2 mg NH3/litre for
(NH4)2CO3, NH4Cl, NH4C2H3O2, and NH4OH, in 96-h exposures of
mosquitofish, Gambusia affinis, in waters with suspended solids
ranging from < 25 to 1400 mg/litre were reported by Wallen et al.
(1957). Susceptibility of mosquito fish to ammonia was studied by
Hemens (1966) who reported a 17-h LC50 of 1.3 mg NH3/litre; he also
observed that male fish were more susceptible than females. Rubin
& Elmaraghy (1976, 1977) tested guppy, Poecilia reticulata, fry
and reported 96-h LC50 values averaging 1.50 mg NH3/litre; mature
guppy males were more tolerant, with 100% survival for 96 h at
concentrations of 0.17 - 1.58 mg NH3/litre. LC50 values (96-h) of
0.15 and 0.20 mg NH3/litre at pH 6.0, and of 0.52 and 2.13 mg
NH3/litre at pH 8.0, were reported by Stevenson (1977) for white
perch, Morone americana. LC50 values (96-h) of 1.20 and 1.62 mg
NH3/litre for spotfin shiner, Notropis spilopterus, were reported
by Rosage et al. (1979), and of 1.20 mg NH3/litre for golden
shiner, Notemigonus crysoleucas, by Baird et al. (1979). Swigert
& Spacie (1983) reported 96-h LC50 values of 0.72 mg NH3/litre for
golden shiner, 1.35 mg NH3/litre for spotfin shiner, 1.25 mg
NH3/litre for steelcolour shiner, Notropis whipplei, and 1.72 mg
NH3/litre for stoneroller, Campostoma anomalum.
Jude (1973), Reinbold & Pescitelli (1982a), and McCormick et
al. (1984) reported 96-h LC50 values ranging from 0.6 to 2.1 mg
NH3/litre for green sunfish, Lepomis cyanellus. In studies on the
pumpkinseed sunfish, Lepomis gibbosus, by Jude (1973) and Thurston
(1981), 96-h LC50 values ranged from 0.14 to 0.86 mg NH3/litre.
Mottled sculpin, Cottus bairdi, were tested by Thurston & Russo
(1981), yielding a 96-h LC50 value of 1.39 mg NH3/litre. An
asymptotic (6-day) LC50 of 0.44 mg NH3/litre was determined for
rudd, Scardinius erythropthalmus (Ball, 1967).
Rao et al. (1975) reported a 96-h LC50 value for the common
carp, Cyprinus carpio, of 1.1 mg NH3/litre. Carp exposed to
0.24 mg NH3/litre exhibited no adverse effects in 18 h (Vámos
1963). However, exposure to 0.67 mg NH3/litre caused gasping and
equilibrium disturbance within 18 min, frenetic swimming activity
at 25 min, then sinking to the tank bottom after 60 min; after
75 min, the fish were placed in ammonia-free water and all revived.
Kempinska (1968) reported a lethal concentration of 7.5 mg
NH3/litre for carp. Studies on the acute exposure of bitterling,
Rhodeus sericeus, and carp to ammonium sulfate revealed minimum
lethal concentrations of 0.76 mg NH3/litre for bitterling and
1.4 mg NH3/litre for carp (Malacea, 1966). Nehring (1963) reported
survival times for carp at concentrations of 9.7 and 2.1 mg
NH3/litre of 2.4 and 6.0 h, respectively. The survival time for
tench, Tinca tinca, was reported to be 20 - 24 h at 2.5 mg
NH3/litre by Danecker (1964). In a 24-h exposure of creek chub,
Semotilus atromaculatus, to ammonium hydroxide solution, the
"critical range" below which all test fish lived and above which
all died was reported to be 0.26 - 1.2 mg NH3/litre (Gillette et
al., 1952).
In static exposures lasting 9 - 24 h, with a gradual increase
in NH3 content, mortalities occurred in oscar, Astronutus
ocellatus, at 0.50 mg NH3/litre (4%) to 1.8 mg/litre (100%)
(Magalhaes Bastos, 1954). Tests on oscar of two different sizes
showed no difference in susceptibility, in relation to size. A
72-h LC50 value of 2.85 mg NH3/litre was reported by Redner &
Stickney (1979) for blue tilapia, Tilapia aurea.
6.4.2.1. Saltwater fish
Very few acute toxicity data are available for salt-water fish
species. Holland et al. (1960) reported the critical level for
chinook salmon, Oncorhynchus tshawytscha, to be between 0.04 and
0.11 mg NH3/litre and for coho salmon to be 0.134 mg NH3/litre. A
static test with coho salmon provided a 48-h LC50 value of 0.50 mg
NH3/litre (Katz & Pierro, 1967). Atlantic salmon smolts and
yearling rainbow trout exposed for 24 h in 50 and 75% saltwater
solutions exhibited similar sensitivities to ammonia (United
Kingdom Ministry of Technology, 1963). Holt & Arnold (1983)
reported a 96-h LC50 value of 0.47 mg NH3/litre for red drum,
Sciaenops ocellatus. LC50 values (96-h) of 1.2 - 2.4 mg NH3/litre
were reported by Venkataramiak et al. (1981) for striped mullet,
Mugil caphalus, and 0.69 mg NH3/litre for planehead filefish,
Monacanthus hispidus.
6.4.3. Factors affecting acute toxicity
A number of factors can affect the toxicity of ammonia for
aquatic organisms. These include the effects of pH, temperature,
dissolved oxygen concentration, previous acclimatization to
ammonia, fluctuating or intermittent exposures, carbon dioxide
concentration, salinity, and the presence of other toxicants.
Almost all studies of factors affecting ammonia toxicity have been
carried out using only acute exposures.
6.4.3.1. pH
The toxicity for fish of aqueous solutions of ammonia and
ammonium compounds has been attributed to the non-ionized
(undissociated) ammonia present in the solution. The earliest
reported thorough study of the pH dependence of ammonia toxicity
was that of Chipman (1934), who concluded from studies on goldfish,
amphipods, and cladocerans that ammonia toxicity was a function of
pH and therefore of the concentration of undissociated ammonia in
the solution. Downing & Merkens (1955) tested rainbow trout at
different concentrations of ammonia at both pH 7 and pH 8. The
results were consistent when ammonia concentration was expressed as
NH3. Tabata (1962) conducted 24-h tests on the toxicity of ammonia
for Daphnia (species not specified) and guppy at different pH
values and calculated the relative toxicity of NH3/NH4+ to be 48
for Daphnia and 190 for guppy (i.e., NH3 is 190 times more toxic
than NH4+).
More recently, Robinson-Wilson & Seim (1975) studied the
toxicity of ammonium chloride for juvenile coho salmon in flow-
through bioassays within the pH range 7.0 - 8.5; the reported
96-h LC50 for NH3 was approximately 60% less at pH 7.0 than at pH
8.5. The toxicity of ammonium chloride for larvae of prawn,
Macrobrachium rosenbergii, was studied by Armstrong et al. (1978)
in 6-day tests within the pH range 6.8 - 8.3 with test solutions
being renewed every 24 h. The 96-h LC50 for NH3 at pH 6.83 was
approximately 70% less than that at pH 8.34. It was concluded that
the toxicity of ammonia was not due solely to the NH3 molecule and
that in solutions of different pH, but equal NH3 concentrations,
survival was greatly reduced as NH4+ levels increased. Tomasso et
al. (1980) studied the toxicity of ammonia (NH3) for channel
catfish at pH values of 7, 8, and 9 and reported that 24-h LC50
values were significantly higher at pH 8 than at pH 7 or pH 9.
Thurston et al. (1981c) tested the toxicity of ammonia for
rainbow trout and fathead minnows in 96-h flow-through tests at
different pH levels within the range 6.5 - 9.0. Results showed
that the toxicity of ammonia, in terms of NH3, increased at lower
pH values, and could also increase at higher pH values. It was
concluded that NH4+ exerts some measure of toxicity, and/or that
increased H+ concentration increases the toxicity of NH3. Acute
(96-h) exposures of green sunfish and smallmouth bass at 4
different pH levels over the range 6.5 - 8.7 showed that, for both
species, NH3 toxicity increased markedly with a decrease in pH,
with LC50 values at the lowest pH tested (6.6 for sunfish, 6.5 for
bass) being 3.6 (sunfish) and 2.6 (bass) times smaller than those
at the highest pH (8.7) tested (McCormick et al., 1984; Broderius
et al., in press).
It is concluded that NH3 is more toxic for fish at lower pH
values than within the pH range 7 - 9; the toxicity of NH3 may
increase again above this range.
6.4.3.2. Temperature
Information in the literature on the effects of temperature on
ammonia toxicity is varied. The concentration of NH3 increases
with increasing temperature. Several researchers have reported an
effect of temperature on the toxicity of the non-ionized ammonia
species, independent of the effect of temperature on the aqueous
ammonia equilibrium.
McCay & Vars (1931) reported that it took three times as long
for the brown bullhead, Ictalurus nebulosus, to succumb to the
toxicity of ammonia in water at 10 - 13 °C than at 26 °C. The pH
of the tested water was not reported but with the probable range
tested (pH 7 - 8), the percent NH3 at the higher test temperature
would have been approximately three times that at the mean lower
temperature. The toxicity of ammonium chloride for goldfish,
bluntnose minnow, Pimephales notatus, and the straw-coloured
minnow or river shiner, Notropis blennius, was reported (Powers,
1920) to be greater at high temperatures than at low, but no
consideration was given to the increase in the relative
concentration of NH3 as the temperature increased. Herbert (1962)
suggested that the effects of temperature on the susceptibility of
rainbow trout to NH3 toxicity was only slightly, if at all,
affected by temperature change. In studies on striped bass, Morone
saxtilis, and stickleback, Gasterosteus aculeatus, Hazel et al.
(1971) found a slight difference in toxicity between 15° and 23 °C
in fresh water, with both fish species being slightly more
resistant at the lower temperature.
However, there are other studies in which the toxicity of NH3
decreased with increasing temperature over the ranges studied. The
toxicity of NH3 for rainbow trout has been reported to be much
higher at 5 °C than at 18 °C (United Kingdom Ministry of
Technology, 1968). Brown (1968) reported that the 48-h LC50 for
rainbow trout increased with increase in temperature over the range
3 °C - 18 °C; the reported increase in tolerance between ~12 °C -
~18 °C was considerably less than that between ~3 °C - ~12 °C. A
relationship between temperature and 96-h LC50 was reported for
rainbow trout over the temperature range 12 °C - 19 °C with ammonia
toxicity decreasing with increasing temperature (Thurston & Russo,
1983).
Thurston et al. (1983) reported that the acute toxicity of NH3
for fathead minnows decreased with a rise in temperature over the
range 12 °C - 22 °C. Bluegill and fathead minnow were tested at
low and high temperatures of 4.0 °C - 4.6 °C and 23.9 °C - 25.2 °C,
respectively, and rainbow trout were tested at 3 °C and 14 °C
(Reinbold & Pescitelli, 1982b). All three species were more
sensitive to NH3 at the low temperatures, with toxicity being 1.5 -
5 times higher in the colder water. Bluegill appeared to be the
most sensitive of the three species to the effects of low
temperature on ammonia toxicity. Colt & Tchobanoglous (1976)
reported that the toxicity of NH3 for channel catfish decreased
with increasing temperature over the range 22 °C - 30 °C. LC50
values for bluegill, channel catfish, and largemouth bass at 28 °C
- 30 °C were approximately twice those at 22 °C (Roseboom & Richey,
1977). An effluent containing ammonia as the principal toxic
component showed a marked decrease in toxicity for channel catfish
over the temperature range 4.6 °C - 21.3 °C (Cary, 1976).
Lloyd & Orr (1969) investigated the effects of temperature
(range 10 - 20 °C) on urine flow rates in rainbow trout exposed to
0.30 mg NH3/litre, and did not find any apparent temperature effect
on the total diuretic response of the fish, though the relative
increase in urine production was less at higher temperatures. From
a study of the behavioural response of bluegill to gradients of
ammonium chloride, it was hypothesized that low temperatures
increased the sensitivity of the bluegill and interfered with the
ability, either to detect ammonia after a certain period of
exposure, or, to compensate behaviourally for physiological stress
caused by ammonia gradients (Lubinski, 1979; Lubinski et al.,
1980).
The European Inland Fisheries Advisory Commission (1970)
has stated that, at temperatures below 5 °C, the toxic effects of
non-ionized ammonia may be greater than at above 5 °C, though the
basis for this is not clearly documented. The evidence that
temperature, independent of its role in the aqueous ammonia
equilibrium, affects the toxicity of NH3 for fish argues for
further consideration of the temperature/ammonia toxicity
relationship.
6.4.3.3. Salinity
Herbert & Shurben (1965) reported that the resistance of
yearling rainbow trout to ammonium chloride increased with
increasing salinity up to levels of 30 - 40% sea water; above this
level, resistance appeared to decrease. Fingerling coho salmon
were tested at salinity levels of 20 - 30 parts per thousand (57 -
86% salt water), and it was found that the toxicity of an ammonia-
ammonium waste increased as salinity increased (Katz & Pierro,
1967). These findings are in agreement, at the levels tested, with
those of Herbert & Shurben (1965). Atlantic salmon were exposed to
ammonium chloride solutions for 24 h under both fresh-water and 30%
salt-water conditions; LC50 values were 0.15 and 0.3 mg NH3/litre,
respectively, in the 2 different waters (Alabaster et al., 1979).
Harader & Allen (1983) reported that the resistance to ammonia of
chinook salmon parr increased by about 500%, as salinity increased
to almost 30% sea water, but declined as salinity increased beyond
that.
There is a slight decrease in the NH3 fraction of total ammonia
as ionic strength increases in dilute saline solutions, but the
relative changes in NH3 toxicity, as salinity increases, are more
directly attributable to changes in the rate of exchange of NH3 and
NH4+ across the fish gill membranes.
6.4.3.4. Dissolved oxygen
A decrease in dissolved oxygen concentration in the water can
increase ammonia toxicity. There is a reduction in fish blood
oxygen-carrying capacity following ammonia exposure (Brockway,
1950; Danecker, 1964; Reichenback-Klinke, 1967; Körting, 1969a,b;
Waluga & Flis, 1971). Hypoxia would further exacerbate problems of
oxygen delivery and could lead to the early demise of the fish.
Vámos & Tasnádi (1967) observed deaths of carp in ponds at
ammonia concentrations lower than would normally be lethal, and
attributed this to periodic low concentrations of oxygen. On the
basis of research in warm-water (20 °C - 22 °C) fish ponds, Selesi
& Vámos (1976) projected a "lethal line", relating acute ammonia
toxicity and dissolved oxygen, below which carp died. The line ran
between 0.2 mg NH3/litre at 5 mg dissolved oxygen/litre and 1.2 mg
NH3/litre at 10 mg dissolved oxygen/litre. Thurston et al. (1983)
compared the acute toxicity of ammonia for fathead minnows at
reduced and normal dissolved oxygen concentrations; seven 96-h
tests were conducted within the range 2.6 - 4.9 mg dissolved
oxygen/ litre, and 3 between 8.7 and 8.9 mg/litre. There was a
slight positive trend between 96-h LC50 values and dissolved
oxygen, though it was not shown to be statistically significant.
Atlantic salmon smolts were tested in both fresh water and 30% salt
water at 9.6 - 9.5 and 3.5 - 3.1 mg dissolved oxygen/litre. The
reported 24-h LC50 values at the higher oxygen concentrations were
about twice those at the lower (Alabaster et al., 1979).
Several studies have been reported on rainbow trout. Allan
(1955) reported that below 0.12 mg NH3/litre and at about 30%
oxygen saturation, the median survival time was greater than 24 h,
but at the same concentration with oxygen saturation below 30%, the
median survival time was less than 24 h. In studies by Downing &
Merkens (1955), fingerling rainbow trout were tested at 3 different
concentrations of NH3 at 5 different levels of dissolved oxygen.
In tests lasting up to 17 h, decreasing the oxygen level from 8.5
to 1.5 mg/litre shortened the period of survival at all ammonia
concentrations, and a decrease in survival time produced by a given
decrease in oxygen was greatest at the lowest concentration of NH3.
Merkens & Downing (1957), in tests lasting up to 13 days, also
reported that the effects of low concentrations of dissolved oxygen
on the survival of rainbow trout were more pronounced at low
concentrations of NH3. Ammonia (NH3) was found to be up to 2.5
times more toxic when the dissolved oxygen concentration was
reduced from 100 to about 40% saturation (Lloyd, 1961). It was
reported by Danecker (1964) that the toxicity of ammonia increased
rapidly when the oxygen concentration decreased below two-thirds of
the saturation value. Thurston et al. (1981b) conducted 15, 96-h
acute toxicity tests on rainbow trout over the dissolved oxygen
range 2.6 - 8.6 mg/litre. A positive linear correlation between
96-h LC50 and dissolved oxygen was reported over the entire range
tested.
When rainbow trout were treated in a channel receiving sewage
discharge containing 0.05 - 0.06 mg NH3/litre, it was found that,
at 25 - 35% dissolved oxygen saturation, more than 50% of the fish
died within 24 h, compared with 50% mortality of test fish in the
laboratory, at 15% dissolved oxygen saturation (Herbert, 1956).
The difference was attributed to unfavourable water conditions
below the sewage outflow, including ammonia, which increased the
sensitivity of the fish to the lack of oxygen.
6.4.3.5. Carbon dioxide
An increase in carbon dioxide (CO2) concentrations up to
30 mg/litre decreased total ammonia toxicity (Alabaster & Herbert,
1954; Allan et al., 1958). Carbon dioxide causes a decrease in pH,
thereby decreasing the proportion of non-ionized ammonia in
solution. However, Lloyd & Herbert (1960) found that, though total
ammonia toxicity was reduced at elevated CO2 levels, the inverse
was true when considering non-ionized ammonia alone; more NH3 was
required in low CO2, high pH water to exert the toxic effect seen
in fish in high CO2, low pH water. The explanation presented by
Lloyd & Herbert (1960) for the decreased toxicity of NH3 in low CO2
water was that CO2 excretion across the gills would reduce the pH
and, therefore, the NH3 concentration, in water flowing over the
gills. A basic flaw in this hypothesis has been discussed by
Broderius et al. (1977). Carbon dioxide will only form protons
very slowly in water at the tested temperature. The uncatalysed
CO2 hydration reaction has a half-time of seconds or even min
(e.g., at pH 8: 25 seconds at 25 °C; 300 seconds at 0 °C) (Kern
1960), and water does not remain in the opercular cavity for more
than a few seconds, and at the surface of a gill lamella for about
0.5 - 1 second (Randall, 1970; Cameron, 1979). Thus, the
liberation of CO2 across the gills will have little, if any, effect
on water pH or NH3 levels and the NH3 gradient across the gills
between water and blood.
6.4.3.6. Prior acclimatization to ammonia
The question of whether fishes can acquire an increased
tolerance to ammonia by acclimatization to low ammonia
concentrations is an important one. If fish were able to develop
such tolerance, they might be able to survive what would otherwise
be lethal ammonia concentrations.
Observations by McCay & Vars (1931) indicated that brown
bullheads subjected to several successive exposures to ammonia,
alternating with recovery in fresh water, did not acquire
tolerance. However, a number of research workers have reported
that previous exposure of fish to low concentrations of ammonia
increases their resistance to lethal concentrations. Vámos (1963)
reported that carp exposed to 0.67 or 0.52 mg NH3/litre for 75 min,
then transferred to fresh water for 12 h, followed by a solution
containing 0.7 mg NH3/litre, exhibited symptoms of ammonia toxicity
in 60 - 85 min, whereas control fish, exposed initially to
0.7 mg/litre NH3, developed symptoms within 20 min. Blue tilapia
acclimatized for 35 days to 0.52 - 0.64 mg NH3/litre subsequently
survived 48 h at 4.1 mg/litre, compared with the 48-h value for
unacclimatized fish of 2.9 mg/litre (Redner & Stickney, 1979).
Malacea (1968) studied the effects on bitterling of acclimatization
to ammonium sulfate solutions. A group of 10 fish was held in an
acclimatization solution of 0.26 mg NH3/litre for 94 h, after which
the fish were exposed to a 5.1 mg NH3/litre solution for 240 min.
A control group of 10 bitterling received identical treatment,
except that the acclimatization aquarium did not contain added
(NH4)2SO4. The ratio of the mean survival times of "adapted" to
"unadapted" fish was 1:13, indicating a slightly higher ammonia
tolerance for the adapted fish.
Schulze-Wiehenbrauck (1976) subjected 2 groups of rainbow trout
(mean weights 56 g and 110 g) that had been held for at least 3
weeks at sublethal ammonia concentrations, to lethal ammonia
concentrations. In the study on the 110-g fish, the
acclimatization concentrations were 0.007, 0.131, and 0.167 mg
NH3/litre. The fish were then subjected for 8.5 h to
concentrations of 0.45, 0.42 and 0.47 mg NH3/litre, respectively.
Fish from the 2 higher sublethal concentrations showed 100%
survival after 8.5 h in the 0.42 and 0.47 mg NH3/litre solutions,
whereas fish from the 0.007 mg NH3/litre concentration showed only
50% survival in 0.45 mg NH3/litre. In the study on the 56-g fish,
the acclimatization concentrations were 0.004 mg NH3/litre and
0.159 mg NH3/litre; these fish were placed for 10.25 h in NH3
concentrations of 0.515 and 0.523 mg/litre, respectively. There
was 100% survival in the acclimatized fish, and 85% survival in the
fish acclimatized to 0.004 mg/litre. The results of these studies
showed an increase in resistance of trout to high ammonia levels
after prior exposure to sublethal ammonia levels.
Alabaster et al. (1979) determined 24-h LC50 values of NH3 for
Atlantic salmon smolts under reduced dissolved oxygen test
conditions. Fish acclimatized to ammonia before oxygen reduction
had LC50 values 38% and 79% higher than fish without prior ammonia
acclimatization.
In studies by Brown et al. (1969), rainbow trout were tested by
moving back and forth between tanks in which the ammonia
concentrations were 0.5 and 2.5 times a previously determined 48-h
LC50 value. If fish were transferred on an hourly basis, the
median period of survival for the fluctuating exposure was reported
to be the same as that for constant exposure (> 700 min). When
the fish were transferred at 2-h intervals, the median survival
time for the fluctuating exposure was reported to be less (370
min), indicating that the toxic effects from exposure to the
fluctuating concentrations of ammonia were greater than those from
exposure to the constant concentration. Thurston et al. (1981a)
conducted acute toxicity tests in which rainbow trout and cutthroat
trout were exposed to short-term cyclical fluctuations of ammonia.
Companion tests were conducted in which test fish were subjected to
ammonia at constant concentrations. The LC50 values for both
average and peak concentrations of ammonia for the fluctuating
concentration tests were compared with the LC50 values for the
constant concentration tests. Comparison of total exposures showed
that fish were more tolerant to constant, than to fluctuating
concentrations of ammonia. Fish subjected to fluctuating
concentrations of ammonia at levels below those acutely toxic were
better able to withstand subsequent exposure to high fluctuating
concentrations than unacclimatized fish. There is reasonable
evidence that fish with a history of prior exposure to sublethal
concentrations of ammonia are better able to withstand an acutely
lethal concentration for a period of hours and possibly days.
Limited data on fluctuating exposures indicate that fish are more
susceptible to fluctuating than to constant exposure with the same
average NH3 concentrations.
6.4.4. Fish: chronic toxicity
"Full-chronic" tests cover the entire life cycle of the test
animal, beginning at a given stage of development of one generation
(frequently as the fertilized egg) and continuing through to this
same stage in the next generation. Common end-points for measuring
toxicity are survival, growth, and reproductive success, though
recent research on ammonia toxicity for fish has demonstrated the
desirability of also conducting histological examinations.
"Partial-chronic" tests on fish most frequently cover a period
of 30 days or longer, from the egg incubation stage to the free-
swimming stage; for many toxins it has been demonstrated that
these stages are the most sensitive. However, in the case of
ammonia it has been demonstrated that older, mature rainbow trout,
Salmo gairdneri, are potentially as susceptible to the effects of
ammonia as newly-hatched larvae.
Long-term ammonia exposure of fishes, including complete life-
cycle tests on rainbow trout and fathead minnows with several end-
points, including effects on spawning and egg incubation, growth,
survival and tissues, have been studied. The effects of prolonged
exposure to ammonia (up to 61 days) on the early life stages of of
pink salmon were studied by Rice & Bailey (1980). Three series of
exposures were carried out, beginning at selected times after
hatching. These were for 21 days prior to completion of yolk
absorption, for 40 days up to 21 days before yolk absorption, and
for 61 days up to yolk absorption. All test fish were sampled for
size when the controls had completed yolk absorption. Test
concentrations ranged from zero up to 0.004 mg NH3/litre. For fry
at the highest concentration of 0.004 mg NH3/litre, significant
decreases in weight were observed in all 3 exposed groups. At a
concentration of 0.0024 mg NH3/litre, the groups of fry exposed for
40 and 61 days were significantly smaller, whereas a concentration
of 0.0012 mg/litre had no significant effect on growth. Effects
were consistently more marked for the 61-day-exposed fish.
In a 3-generation, 5-year laboratory study, rainbow trout
exposed for 5 months to concentrations of ammonia ranging from 0.01
to 0.07 mg NH3/litre, spawned of their own volition. There was no
correlation between ammonia concentration and numbers of egg lots
spawned, total numbers of eggs produced, or numbers of eggs
subsequently hatched. Parental fish were exposed for 11 months,
the first filial generation (F1) for 4 years, and the second filial
generation (F2) for 5 months. Pathological lesions were observed in
both parental and F1 fish, when ammonia concentrations reached and
exceeded 0.04 mg NH3/litre. Measurements of blood-ammonia
concentrations in 4-year-old F1 fish showed an increase when test
water concentrations reached or exceeded 0.04 mg NH3/litre. The F1
fish exposed for 52 months from day of hatching showed no
relationship between growth and concentration at 10, 15, 21, and 52
months (Thurston et al., 1984b).
Burkhalter & Kaya (1977) tested ammonia at concentrations
ranging from 0.06 to 0.45 mg/litre on fertilized eggs and the
resultant sac fry of rainbow trout. Eggs were incubated at 12 °C
for 25 days in one test and at 10 °C for 33 days in another; fry
were maintained for 42 days. No concentration response was seen in
egg mortality or incubation time in either test. Retardation in
early growth and development occurred at 0.06 mg NH3/litre, the
lowest concentration tested. Fish exposed to 0.12 mg NH3/litre
required 1 week longer than the controls to achieve a free-swimming
state; fish at 0.34 and 0.45 mg NH3/litre did not achieve a free-
swimming state during a 42-day test period. A 21-day LC50 value of
0.30 mg NH3/litre was obtained. For sac fry exposed for 42 days
after hatching, hypertrophy of secondary gill lamellae epithelium
occurred at 0.23 mg NH3/litre, and karyolysis and karyorrhexis in
the secondary gill lamellae were observed after 28 days at 0.34 mg
NH3/litre and higher.
Calamari et al. (1977, 1981) exposed rainbow trout to
ammonium chloride solutions for 71 days, beginning 1 day after
fertilization and ending when fry had been feeding for 30 days.
A 72-day LC50 of 0.056 mg NH3/litre was calculated; 23% mortality
occurred at a concentration of 0.025 mg/litre. Examination of 986
rainbow trout embryos at the hatching stage after exposure to
concentrations of 0.010 - 0.193 mg NH3/litre for 24 days showed
an increase in gross malformations with increasing ammonia
concentration. The deformities observed were various degrees
of curvature from the median body axis, and various kinds of
malformations in the head region with a number of cases of double
heads. At the highest concentration tested, 0.193 mg NH3/litre,
60% of the observed fish were malformed. Microscopic examination,
at hatching, of 128 larvae from the same exposure showed
abnormalities of the epidermis and pronephros, which were
correlated with ammonia concentrations. The epidermis was
thickened with an irregular arrangement of the various layers of
cells and an increase in the number and dimensions of mucous cells.
The pronephros showed widespread vacuolization of the tubule cells,
together with a thickening of the wall. Increasing abnormalities
were observed after exposure to concentrations exceeding 0.025 mg
NH3/litre for the epidermis and 0.063 mg/litre for the pronephros.
Four 4-week-old rainbow trout fry were exposed for 30 days to
concentrations of ammonia (reported graphically) ranging from
~0.06 to 0.31 mg NH3/litre. Growth rate at ~0.06 mg NH3/litre was
comparable with that of controls, but, above ~0.10 mg NH3/litre,
growth rate decreased, in correlation with increased NH3
concentration. Survival at 0.32 mg NH3/litre was 70% of that of
the controls (Broderius & Smith, 1979). Schulze-Wiehenbrauck
(1976) tested juvenile rainbow trout of different sizes, for
periods of time ranging from 2 to 7 weeks, and at ammonia
concentrations ranging from 0.012 to 0.17 mg/litre. He concluded
that a concentration of 0.05 mg NH3/litre caused a slight decrease
in growth during the first 14-day interval in non-acclimatized
fish, but that the decrease was completely compensated for in the
next growth interval. Exposure to 0.13 mg NH3/litre (apparently
for 3 or 4 weeks) did not affect growth, food consumption, or food
conversion.
Young rainbow trout were reared in 3 concentrations of ammonia
(averaging 0.006, 0.012, and 0.017 mg/litre) for a period of 1
year. At 4 months, there was no significant difference in fish
growth at the 3 concentrations. At 11 months, there was a
difference with the fish at 0.012 and 0.017 mg NH3/litre, which
weighed 9% and 38% less, respectively, than the fish at 0.006 mg
NH3/litre. Microscopic examination of tissues from fish exposed to
the highest concentration, examined at 6, 9, and 12 months, showed
severe pathological changes in gill and liver tissues. Gills
showed extensive proliferation of the epithelium, which resulted in
severe fusion of gill lamellae preventing normal respiration.
Livers showed reduced glycogen storage and scattered areas of dead
cells; these became more extensive with increase in exposure time
(Smith, 1972; Smith & Piper, 1975).
Rainbow trout were exposed for 3 months to concentrations of
0.069, 0.14, and 0.28 mg NH3/litre. The cumulative mortality of a
control group (0.005 mg NH3/litre) was ~2%; cumulative mortality at
0.069 and 0.14 mg/litre was ~5%, and that at 0.28 mg/litre was ~15%
(United Kingdom Ministry of Technology, 1968). Reichenbach-Klinke
(1967) performed a series of 1-week tests on 240 fish of 9 species
(including rainbow trout, goldfish, northern pike, Esox lucius,
carp, and tench) at concentrations of 0.1 - 0.4 mg NH3/litre.
Swelling of, and diminution of the number of, red blood cells,
inflammation, and hyperplasia were observed. Irreversible blood
damage occurred in rainbow trout fry at concentrations above
0.27 mg NH3/litre. Low NH3 concentrations also inhibited the
growth of young trout and lessened their resistance to disease.
In rainbow trout exposed to 0.30 to 0.36 mg NH3/litre, 81%
mortality occurred over the 36-day duration of the test, with most
deaths occurring between days 14 and 21. Microscopic examination
of the gills revealed some thickening of the lamellar epithelium
and an increased mucous production. The most characteristic
feature was a large proportion of swollen, rounded secondary
lamellae in which the pillar system was broken down and the
epithelium enclosed a disorganized mass of pillar cells and
erythrocytes. Gill hyperplasia was not a characteristic
observation (Smart, 1976).
In rainbow trout exposed to < 0.0005 or 0.005 mg NH3/litre for
8 weeks, examination of the gill lamellae of fish from the lower
concentration showed them to be long and slender with no
significant pathology. Fish exposed to 0.005 mg NH3/litre had
shorter and thicker gill lamellae with bulbous ends, and some
consolidation of lamellae was noticed. Many filaments showed a
definite hyperplasia of the epithelial layer, evidenced by an
increase in the number of cell nuclei (Fromm, 1970).
Thurston et al. (1978) studied the toxicity of ammonia for
cutthroat trout fry in tests that lasted up to 36 days. Results of
duplicate tests on 1-g fish showed 29- and 36-day LC50 values of
0.56 mg NH3/litre. Duplicate tests on 3-g fish provided 29-day
LC50 values of 0.37 and 0.34 mg NH3/litre, slightly less than those
of the 1-g fish. The heart, gastrointestinal tract, and thymus of
cutthroat trout fry exposed to 0.34 mg NH3/litre for 29 days were
comparable with those of control fish, but the gills and kidneys
showed degenerative changes. The gills showed hypertrophy of
epithelium, some necrosis of epithelial cells, and separation of
epithelium due to oedema. The kidneys had mild hydropic
degeneration and accumulation of hyaline droplets in the renal
tubular epithelium. Reduced vacuolation was observed in livers.
Samylin (1969) studied the effects of ammonium carbonate on the
early stages of development of Atlantic salmon. The first set of
studies, at 13 °C, lasted 53 days and was conducted within the
range 0.001 to > 6.6 mg NH3/litre beginning with the "formed
embryo" stage. Accelerated hatching was observed with increasing
(NH4)2CO3 concentrations, but concentrations of > 0.16 mg
NH3/litre were lethal for emerging larvae within 12 - 36 h.
Because (NH4)2CO3 was used as the toxin, the pH in the test aquaria
increased from 6.7 to 7.6 with increasing NH3 concentration. Growth
inhibition was observed at 0.07 mg NH3/litre. Tissue changes were
observed in eyes, brains, fins, and blood of Atlantic salmon
embryos and larvae exposed to concentrations ranging from 0.16
to > 6.6 mg NH3/litre, with more marked changes at higher ammonia
concentrations. The effects observed included erosion of membranes
of the eyes and shedding of the crystalline lens, dilatation of
blood vessels in the liver and brain, accumulation of blood in the
occipital region and in the intestines. Reaction to light and
mechanical stimulation gradually disappeared with increased ammonia
concentration, and the heart rate slowed. Morphological
differences in development between experimental and control larvae
were observed from the tenth day of exposure, including a lag in
yolk resorption, decrease in growth of the skin fold, and
contraction of skin pigment cells causing the skin colour to become
paler than it was after hatching. At concentrations up to 0.07 mg
NH3/litre, no significant morphological differences were observed.
A second series of studies, at 16.5 °C, was carried out in
the 0.001 - 0.32 mg NH3/litre concentration range, beginning
with larval salmon (Samylin 1969). Concentrations of > 0.21
mg/litre were lethal and caused weight loss in fry; 0.001 -
0.09 mg NH3/litre caused a decrease in weight gain, though there
were no differences in feeding activity, behaviour, or development
at these concentrations compared with controls. Dissolved oxygen
concentrations in this second series of studies dropped as low as
3.5 mg/litre.
Burrows (1964) tested fingerling chinook salmon for 6 weeks in
outdoor water channels into which ammonium hydroxide was
introduced. Two studies were conducted, one at 6.1 °C and the
other at 13.9 °C, both at pH 7.8. The fish were then maintained in
fresh water for an additional 3 weeks. A recalculation of the
reported non-ionized ammonia concentrations, based on more recent
aqueous ammonia equilibrium tables, shows that the concentrations
at 6.1 °C were 0.003 - 0.006 mg NH3/litre and, at 13.9 °C, were
0.005 - 0.011 mg NH3/litre. At both temperatures, and at all
ammonia concentrations, some fish showed excessive proliferation
and clubbing of the gill filaments. The proliferation was
progressive for the first 4 weeks, after which no measurable
increase was observed. After 3 weeks in fresh water, examination
of fish exposed at 6.1 °C indicated that recovery from the
extensive proliferation had not taken place. In the study on larger
fish at 13.9 °C, a marked recovery from hyperplasia was noted after
the 3 weeks in fresh water. In the first study, the proliferated
areas had consolidated; in the second, they had not. It was
postulated that continuous ammonia exposure is a precursor of
bacterial gill disease.
Duplicate groups (90 fish each) of hatchery-reared coho salmon
were exposed for 91 days to "river-water" solutions of ammonium
chloride at concentrations of 0.019 - 0.33 mg NH3/litre. Control
groups were reared at 0.002 mg/litre. Haemoglobin content and
haematocrit readings were slightly, but significantly, reduced in
fish exposed to the highest concentration tested, and there was
also a greater percentage of immature erythrocytes. Blood-ammonia
and -urea concentrations were not significantly different after 91
days, regardless of the concentration of ammonia to which the fish
were exposed (Buckley et al., 1979). Rankin (1979) exposed embryos
of sockeye salmon, Oncorhynchus nerka, to ammonia from fertilization
to hatching. Total embryo lethality occurred at concentrations of
0.49 - 4.9 mg NH3/litre. The times required to achieve 50%
mortality at these concentrations were 40 - 26 days. Mortality of
the embryos exposed to 0.12 mg NH3/litre was 30%, and time to 50%
mortality was 66 days.
Two full life-cycle ammonia toxicity tests, each lasting
approximately 1 year, were conducted on fathead minnows (Thurston
et al., in press). These tests began with newly hatched fry and
were continued through their growth, maturation and spawning
stages; progeny were exposed from hatching through growth to 60
days of age. While no statistically-significant differences were
observed in survival, growth, egg production, and egg viability, at
concentrations up to 0.4 mg NH3/litre, effects were seen at 0.4 mg
NH3/litre.
Tissues from fathead minnows subjected to prolonged (up to 304
days) ammonia exposure were examined (Smith, 1984). Growths, some
massive, were observed on the heads of several fish exposed to
concentrations of 1.25 or 2.17 mg NH3/litre, and swollen darkened
areas were observed on the heads of several fish exposed to 0.639 -
1.07 mg NH3/litre. Thurston et al. (in press) also reported
lesions at concentrations below those at which other effects were
observed. Brain lesions were common at concentrations of 0.21 mg
NH3/litre and higher. Grossly and histologically, the severity of
the lesions, which varied from mild to severe, was positively
correlated with ammonia concentration. The lesions appeared to be
of a cell type originating from the meninx primativa covering the
brain. The hyperplastic tissue often completely surrounded the
brain but was not observed around the spinal cord.
An early life-stage test initiated at the blastula stage of
embryogenesis and extending through 39 days post-hatching was
conducted on green sunfish (McCormick et al., 1984). Retardation
of growth was found in green sunfish exposed from embryo through
juvenile life stages to concentrations of 0.489 mg NH3/litre or
more, but not at 0.219 mg NH3/litre. In a long-term test on green
sunfish, Jude (1973) reported that, at levels higher than 0.17 mg
NH3/litre, mean fish weight increased less rapidly than that of the
controls on the 4 days following the introduction of ammonia.
Thereafter, fish exposed to 0.26 and 0.35 mg NH3/litre grew at an
increasing rate, while fish exposed to 0.68 and 0.64 mg NH3/litre
remained the same for 12 days before increases in growth occurred.
Four simultaneous early life-stage ammonia tests with
smallmouth bass were carried out at 4 different pH levels, ranging
from 6.6 to 8.7, in order to examine the effect of pH on chronic
ammonia toxicity. Exposure to ammonium chloride solutions began on
2- to 3-day old embryos and lasted for 32 days. The end-point
observed was growth, and ammonia was found to have a greater effect
on growth at lower pH levels than at high. Concentrations found to
retard growth ranged from 0.056 mg/litre at pH 6.60, to 0.865
mg/litre at pH 8.68 (Broderius et al., in press).
In early life-stage tests (29 - 31 days' exposure) on channel
catfish and white sucker, no significant effects on percent hatch
or larval survival were observed for channel catfish exposed to
ammonium chloride at concentrations as high as 0.583 mg NH3/litre
and for white sucker at concentrations as high as 0.239 mg
NH3/litre. Significant retardation of growth, however, occurred in
channel catfish at concentrations of 0.392 mg NH3/litre or more and
in white sucker at 0.070 mg NH3/litre and higher. A delay in time
to swim-up stage was also observed for both species at elevated
(0.06 - 0.07 mg/litre) ammonia concentrations (Reinbold &
Pescitelli, 1982a).
In cultured channel catfish fingerlings, exposed for periods
of approximately 1 month to concentrations of 0.01 - 0.16 mg
NH3/litre, growth at 0.01 and 0.07 mg NH3/litre was not
significantly different from that of control fish, but growth
retardation at 0.15 and 0.16 mg NH3/litre was statistically
significant (Robinette, 1976). Colt (1978) and Colt &
Tchobanoglous (1978) reported retardation of growth of juvenile
channel catfish during a 31-day period of exposure to
concentrations ranging from 0.058 to 1.2 mg NH3/litre. Growth
rate was reduced by 50% at 0.63 mg NH3/litre, and no growth
occurred at 1.2 mg NH3/litre.
6.5. Wild and Domesticated Animals
6.5.1. Wildlife
Although ammonia has been known to be toxic for nearly a
century (Hahn et al., 1983), studies describing the toxicological
effects of ammonia on wildlife are very limited. Normally,
atmospheric ammonia does not appear to be a problem for wild
animals, but concentrations of ammonia could reach harmful levels
in accidents during transport near forests and remote areas. NRC
(1979) has reported 2 types of observations in relation to this
topic: (a) the use of anhydrous ammonia to exterminate wild birds
and mice in farm buildings; and (b) the tolerance of bats to
atmospheric ammonia.
The use of anhydrous ammonia has been recommended for
exterminating wild birds and mice from farm buildings by Day et al.
(1965). The technique is simple, economical, and does not leave
any harmful residue. The farm buildings, after removal of the
livestock, were sealed and treated with anhydrous ammonia at 1600
mg/m3 (2285 ppm) for 7 min and then reopened. Ammonia fumes were
fatal for the wild inhabitants, particularly for wild birds.
Within 0.5 h, dead starlings, sparrows, pigeons, and mice were
removed from the barns. Farm animals were allowed to enter the
barns within 1 h of their reopening. According to laboratory
studies, the mouse appears to be more sensitive than other animal
species such as the rat, rabbit, and guinea-pig. When mice were
exposed for 10 min to ammonia at 6140 - 9060 mg/m3 (8770 - 12 940
ppm), death with convulsion began after 5 min of exposure, and over
50% of the mice died before the study was completed. The surviving
animals appeared to recover rapidly, but another 4% died between
the 6th and 10th days after exposure (Underwriters Laboratories,
1933).
Large colonies of Guano bats (Tadarida brasiliensis) frequently
inhabit caves or other areas, producing large amounts of guano,
which, on bacterial decomposition, results in a very high
concentration of ammonia in the atmosphere. Although high ammonia
concentrations, together with high relative humidity in caves,
discoloured the pelage of bats (Eads et al., 1955; Constantine,
1958; Mitchell, 1964), no other adverse physiological effects were
observed in these mammals. This apparent adaptation to inhaled
ammonia prompted laboratory studies relating to the physiological
mechanisms involved in ammonia tolerance in different species of
bats (Mitchell, 1963; Studier, 1966; Studier et al., 1967).
California leaf-nosed bats (Macrotus californicus) can
tolerate exposure to 2100 mg/m3 (3000 ppm) for up to 9 h (Mitchell,
1963).
Ammonia toxicity at lethal doses was manifested by corrosion
of the skin and mucous membranes, pulmonary oedema, and distinct
visceral damage. The blood-non-protein nitrogen in the exposed
bats was significantly elevated without any increase in urinary-
urea or -ammonia.
Studier et al. (1967) studied the effects of increasing
concentrations of atmospheric ammonia on ammonia tolerance and
metabolic rates in rats, mice, and 3 species of bat. Rats, mice,
and 2 species of bat (Myotis lucifugus and Eptesicus fuscus)
tended to show increased oxygen utilization, when exposed to
increased ammonia levels. However, the guano bat (Tadarida
brasiliensis) exhibited a large decrease in oxygen utilization
with increasing ammonia concentration (i.e., 74% depression) when
exposed to air containing 4900 mg/m3 (7000 ppm) of gaseous ammonia.
Earlier, Studier (1966) had shown that ~35% of gaseous ammonia
filtered through the mucous linings in the respiratory passage in
guano bats, when they were exposed to 2100 mg/m3 (3000 ppm) of
ammonia. There was no change in their normal blood pH during
exposures to high ammonia concentrations. The animals, however,
exhaled measurable amounts of ammonia when transferred to normal
air.
6.5.2. Domesticated animals
6.5.2.1. Oral exposure
(a) Ruminants
The use of urea as a partial source of nitrogen in ruminant
nutrition is limited by its toxicity, which results from its
metabolism to ammonia.
The toxic effects of urea in ruminants are related to a high
ammonia content in the blood. Urea itself is not as toxic as the
ammonia, which is rapidly released in the rumen by the action of
bacterial urease (EC 3.5.1.5) on ingested urea (Bloomfield et al.,
1960). The absorption of this excess ammonia has been shown to
depend on the pH of the ruminal contents.
Hogan (1961) examined the effects of pH on the absorption of
ammonia from the rumen in sheep. When an ammonia-containing buffer
at pH 6.5 was placed in the rumen, absorption increased with the
concentration gradient. At a pH of 4.5, however, the concentration
of ammonia in the rumen did not affect the absorption across the
epithelium. The net loss of ammonia-nitrogen from the rumen at pH
6.5 was more than 3 times the loss at a pH of 4.5.
Additional support for the effect of pH on ammonia absorption
across the ruminal epithelium in sheep has been presented by
Bloomfield et al., (1962). As the pH of the ruminal contents
increased from 6.21 to 6.45, no ammonia was absorbed; however, as
the pH increased to 7.59, the absorption rate was 16 mmol/litre per
h. One sheep with a ruminal pH of 7.7 died of ammonia toxicity
within 30 min. These data support the hypothesis that the non-
ionized ammonia, which increases at higher pH, penetrates the lipid
layers of the ruminal epithelium more effectively than the charged
ammonium ion (Coombe et al., 1960).
Toxic signs become apparent as the blood-ammonia-nitrogen
increases to 10 mg/litre; tetanic spasms occur between 10 and
20 mg/litre and are followed by death (Repp et al., 1955; McBarron
& McInnes, 1968; Kirkpatrick et al., 1972, 1973; Webb et al.,
1972). Wilson et al. (1968a) attributed the cause of death to the
cardiotoxic effects of ammonia produced from the urea. However,
Singer & McCarty (1971) observed that only one sheep died of
ventricular fibrillation and the remainder of respiratory failure.
More recently, Edjtehadi et al. (1978) reported the arrest of
respiration, and not cardiovascular collapse, as the cause of death
in sheep.
Certain aspects of the blood chemistry have been described for
sheep with urea poisoning (Kirkpatrick et al., 1973; Edjtehadi et
al., 1978). In general, during the initial stages of urea
toxicosis, an alkalosis is induced, followed by systemic acidosis
due to hyperventilation prior to death (Edjtehadi et al., 1978).
In addition to increases in blood-ammonia and blood-urea levels,
there is a marked increase in the blood-glucose level, with no
change in ketone concentrations in the body (Singer & McCarty,
1971). The following changes have been recorded at death: red-
cell count and haemoglobin concentration increased by 7.9%; white-
cell count decreased by 27.5%; and packed-cell volume increased by
11.4%. Mean corpuscular volume, mean corpuscular haemoglobin, and
mean corpuscular haemoglobin concentration were not substantially
changed (Kirkpatrick et al., 1973).
Pathological effects of ammonia toxicity in sheep have been
described by Singer & McCarty (1971). The changes were similar
when sheep received intraruminal injections of ammonium chloride,
ammonium sulfate, or a mixture of ammonium chloride, carbonate,
phosphate, and sulfate. General passive hyperaemia and numerous
petechial and ecchymotic haemorrhages in the musculature, heart,
thymus, and lungs were found. The lungs were distended and
severely congested. On microscopic examination, the pulmonary
lesions included severe hyperaemia, haemorrhage, alveolar oedema,
and alveolar emphysema. In the thymus, there was degeneration and
necrosis of Hassall's corpuscles and centrilobular haemorrhages.
Lesions in kidneys included severe generalized cloudy swellings and
multiple foci of early coagulative necrosis of the proximal
convoluted tubules, general hyperaemia of the glomerular tufts, and
degeneration of the glomerular tuft cells.
In Marschang & Crainiceanu's (1971) study on the effects of
ammonia in the air of calf stables, ammonia concentrations
reportedly ranged from 0.7 to 140 mg/m3 (1 - 200 ppm). During
these periods of high ammonia concentration, high mortality rates
were observed among the calves. The authors suggested that the
high ammonia content weakened the resistance of the animals and
thus created conditions for the development of secondary
infections. Deaths were mainly caused by respiratory diseases.
Autopsy indicated various types of change in the lungs, chiefly
inflammation.
Air-ammonia concentrations in 3 cattle-fattening facilities in
Romania were measured by Marschang & Petre (1971), who found
concentrations ranging from 2 to 1400 mg/m3 (3 - 2000 ppm).
Morbidity (mainly from respiratory disease) and mortality rates
increased with ammonia concentrations in the stalls and decreased
as some of the toxic gas levels decreased to admissible
concentrations. The authors suggested that ammonia is the most
important environmental factor in producing disease in cattle-
fattening stalls. They did not refer to the growth rate of the
cattle; however, in an additional report, Marschang (1972) observed
a marked decrease in the growth rate of fattening cattle, when the
ammonia content of the stable was high.
(b) Monogastric animals
Monogastric animals are considered relatively tolerant to
dietary urea, since they lack the large amounts of bacterial urease
present in the rumen of ruminants. Horses may frequently consume
cattle rations that contain urea or other non-protein-nitrogen
sources.
Hintz et al. (1970) found that the urease activity in the
caecal fluid from ponies was 17 - 25% of that reported for bovine
rumen fluid. They subjected 8 ponies to oral doses of urea at 3.3
- 3.6 g/kg body weight, to study the toxic effects of urea
overdosage. Seven of the ponies died of ammonia toxicity, 3 - 12 h
after treatment. Clinical signs of toxicosis were characteristic
of severe central nervous system derangement. These signs were
similar to those previously reported for ruminants, with the
exception of head pressing against a fixed object prior to loss of
coordination. No significant gross lesions were observed on
necropsy. Blood-ammonia increased linearly until death. Blood-
alpha-keto-glutarate decreased initially, reached minimal values
at about 30 min and then increased to 3 times the zero time value.
Blood-glucose remained constant for the first 2.5 h and then
increased to about 3 times the initial value. Blood-pyruvate
decreased during the first 3.5 h and then increased to 10 times
the initial values.
6.5.2.2. Inhalation exposure
(a) Swine
The acute inhalation effects of ammonia in swine are given in
Table 15.
(b) Poultry
As discussed previously, poultry are exposed to ammonia in the
atmosphere of poultry houses; this ammonia is released from the
action of bacteria on poultry wastes. The toxic effects of this
exposure are primarily seen in the eyes and respiratory tract.
An idiopathic ocular disorder in young chicks, designated
keratoconjunctivitis, was first described by Bullis et al. (1950),
who attributed it to environmental factors in the rearing
facilities.
Anderson et al. (1964a) reported that chickens exposed
continuously to ammonia at 14 mg/m3 (20 ppm) showed some signs
of discomfort, including rubbing of the eyes, slight lachrymation,
anorexia, and, later, weight loss. Chickens exposed to ammonia at
14 mg/m3 for as little as 72 h were more susceptible to aerosol
infection with Newcastle disease virus. Gross and microscopic
damage to the respiratory tract could be detected after 6 weeks of
continuous exposure to ammonia at 14 mg/m3. Valentine (1964)
reported tracheitis in chicks exposed to ammonia at 42 - 49 mg/m3
(60 - 70 ppm). The breathing of the birds was audible as moist
rales with bubbling sounds. At post-mortem examination, some of
the birds had slight congestion of the lungs with excess mucous in
the respiratory tract. The mucous membranes of the trachea were
much thicker than in the control birds, and there was leukocytic
infiltration of the tissue. It was suggested that this tracheitis
may predispose the affected birds to respiratory diseases with the
added risks of secondary infections.
Charles & Payne (1966a) reported that exposure to atmospheric
ammonia at 70 mg/m3 (100 ppm) caused a reduction in carbon dioxide
production and depth of respiration and a 7 - 24% decrease in the
respiration rate of laying hens. The authors also observed that
broilers reared to 28 days of age in atmospheres containing high
concentrations of ammonia consumed less food and grew more slowly
than unexposed chickens. Pullets reared in high-ammonia
atmospheres matured up to 2 weeks later than pullets reared in
ammonia-free atmospheres.
Airsacculitis, one of many respiratory diseases in poultry, has
been associated with high ammonia concentrations in poultry houses
(Ernst, 1968). High concentrations of dust were also noted during
periods of winter confinement, when high ammonia concentrations
were observed. The incidence and severity of air-sac lesions in
turkeys increased signficantly with high concentrations of dust
(0.6 - 1.0 mg/m3 or 21 - 35 mg/m3) in the atmosphere. Flocks with
a high rate (47%) or a low rate (2%) of infection with Mycoplasma
meleagridis were similarly affected. No significant interaction
between dust and ammonia concentrations (up to 21 mg/m3 or 30 ppm)
with regard to effects on the development of air-sac lesions was
found. Mortality rate and feed conversion were not significantly
affected by exposure to dust and ammonia. There was considerable
loss of cilia from the epithelium of the tracheal lumen and an
increase in mucous-secreting goblet cells in turkeys exposed to
high concentrations of dust and ammonia. Areas of consolidation
and inflammation were frequently observed in the lungs of these
turkeys. The air-sac lesions ranged from mild (lymphocytic
infiltration) to severe (masses of gaseous material).
Airsacculitis has also been experimentally induced in chickens
exposed to atmospheric ammonia and the stress of infectious
bronchitis vaccination (Kling & Quarles, 1974). Eighty Leghorn
male chicks were maintained in 12 controlled-environment chambers.
Ammonia at 0, 17.5, or 35 mg/m3 (0, 25, or 50 ppm) was introduced
into the chambers from the 4th to 8th weeks of age. An infectious
bronchitis vaccination was administered to all chicks at 5 weeks of
age. Body weights and feed efficiencies were determined at 4, 6,
and 8 weeks. At 4, 5, 6, and 8 weeks, lung and bursae of Fabricius
weights, haematocrits, and air-sac scores were determined. Body
weights and feed efficiencies were significantly reduced in the
ammonia chambers. The bursae of Fabricius in the ammonia-stressed
chickens were significantly larger than those of controls at 5
weeks of age and significantly smaller at 8 weeks of age. Chickens
grown in ammoniated environments had significantly larger lungs at
8 weeks. Haematocrits were not significantly different among
treatments. Total air-sac scores were significantly higher in the
ammonia-stressed chickens at 8 weeks. The results indicated that
chickens were stressed by the ammonia at 17.5 or 35 mg/m3, and by
the infective bronchitis vaccination. In a similar study (Quarles
& Kling, 1974), exposure of broiler chicks to 17.5 or 35 mg/m3 (25
or 50 ppm) from the 4th to the 6th week resulted in the observation
of severe airsacculitis at 6 and 8 weeks of age. During the test,
airborne bacterial counts were significantly higher in chambers
with ammonia than in control chambers.
Table 15. Acute inhalation effects of ammonia in swine
---------------------------------------------------------------------------------------------------------
Ammonia concentration Dose Effects Reference
---------------------------------------------------------------------------------------------------------
196 mg/m3 (280 ppm) single frothing of the mouth and excessive Stombaugh et al. (1969)
secretion; after 36 h, convulsions
occurred, and breathing was extremely
short and irregular; the effects
ceased after a few h
7, 35, 70, or 105 mg/mg3 5 weeks high concentrations (70 and 105 mg/m3) Stombaugh et al. (1969)
(10, 50, 100, or 150 ppm) appeared to cause excessive nasal,
lachrymal, and mouth secretion after
3 - 4 days of exposure at 35 mg/m3,
the secretory rate was only slightly
higher than that in control animals;
after 1 - 2 weeks of exposure, the
signs noted appeared to lessen
gradually; examination of respiratory
tract did not reveal any significant
gross- or microscopic differences
related to ammonia exposure
0, 35, 70, or 105 mg/m3 4 weeks decrease in pig growth was noted at Drummond et al. (1980)
(0, 50, 100, or 105 ppm) all concentrations; at 35 or 70 mg/m3,
pigs converted feed to body weight
gain more efficiently than either
controls or pigs exposed to 105 mg/m3;
an acute imflammatory reaction in the
tracheal epithelium and a mild-to-
heavy exudate in the turbinate lumen
were observed at 70 and 105 mg/m3,
only
0 and 70 mg/m3 (100 ppm) 2 - 6 conjunctival irritation after the Doig & Willoughby (1971)
(1 - 7 weeks old) weeks first day which persisted for 1 week;
0 + dust dust alone had no effect; histopatho-
(100 ppm + dust) logical changes were limited to the
nasal and tracheal epithelium; there
was no evidence of structural damage
in the bronchial epithelium or alveoli
---------------------------------------------------------------------------------------------------------
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The authors suggested that the quality of eggs left all day in hen
houses containing high concentrations of ammonia might be adversely
affected.
7. KINETICS AND METABOLISM
Ammonia, a by-product of protein and nucleic acid metabolism
and a minor component of the diet, is in a state of flux in the
body, though it is present in low steady-state concentrations in
body fluids. In animals, metabolically-produced ammonia is
conjugated and excreted. Toxicity will only occur if these
conjugation and excretion mechanisms are defective, or if they are
overwhelmed by excessive exposure.
7.1. Absorption
7.1.1. Respiratory tract
Egle (1973) studied the retention, over a short period of time,
of inhaled ammonia in air at concentrations in the range 150 -
500 mg/m3 (214 - 714 ppm), in mongrel dogs of both sexes (7 - 37
per study). Retention was not materially affected by respiratory
rate, tidal volume, or concentration. Retention by the whole
respiratory tract averaged 78%, but the complexity of the dynamics
of this retention is illustrated by the fact that when respiration
was via an endotracheal tube, limiting exposure to the lower
respiratory tract, and when the upper respiratory tract (muzzle to
tracheal bifurcation) was perfused tidally, the retention was 78%
in each case. However, unidirectional perfusion of the upper
respiratory tract with ammonia in air produced a higher mean
retention of 89%.
Schaerdel et al. (1983) exposed 4 groups of rats, 8 per group,
to average ammonia concentrations of 11, 23, 220, or 826 mg/m3
(15, 32, 310, or 1157 ppm) for 24 h. Ammonia in blood was measured
at 0, 8, 12, and 24 h. At the 2 lowest concentrations, there was
no increase in blood-ammonia. However, after 8 h at 220 and
826 mg/m3, significant increases of 0.192 and 0.244 mmol (3.26 and
4.18 mg/litre) were noted. After 12 and 24 h, the increases were
not so marked, indicating an increase in ammonia metabolism.
In a study by Silverman et al. (1949), 7 male volunteers were
exposed to 350 mg/m3 (500 ppm) for 30 min. Initial ammonia
retention was not reported for all subjects, but, in one instance,
was around 75%. Retention decreased progressively until at
equilibrium it was 23% (range 4 - 30%); equilibrium was reached in
10 - 27 min. Some irritation was noted in the nose and throat,
leading to the suggestion that ammonia at this concentration was
primarily absorbed by the upper respiratory tract. Levels of
blood-urea-nitrogen (BUN), non-protein nitrogen, urinary-urea, and
urinary-ammonia remained normal.
In another study (Kustov, 1967), exposure of human volunteers
to ammonia for a longer duration (14 mg/m3 (20 ppm) for 8 h) was
accompanied by a statistically-significant increase in BUN from
23.9 to 30 mg%.
An early study was conducted by Landahl & Herrmann (1950) on
the retention of gases by the human nose and lung. At ammonia
concentrations of between 40 and 350 mg/m3 (57 and 500 ppm) and a
mean minute volume of 6 - 7 litre/min, for short durations (< 2
min), they found that approximately 92% ± 2% was retained in the
respiratory system (i.e., mouth, lungs, etc.) in 2 male volunteers,
tested 4 times. Differences in concentrations of ammonia did not
affect retention values. In a separate study, about 83% was
retained in the nasopharnyx at a flow rate of 18 litre/min, but
only 63 - 71% was retained when the flow rate was tripled. These
data are consistent with, though somewhat higher than, those
reported by Egle (1973) for exposure in dogs.
It should be noted that experimental animals kept in cages may
be exposed to relatively high concentrations of ammonia, even
exceeding 100 mg/m3, due to the degradation of urea in urine and
faeces (Flynn, 1968; Schaerdel et al., 1983).
Because ammonia is very water soluble, and thus absorbed by the
mucous coating in the upper respiratory tract, the lungs are
protected from the effects of exposure to low concentrations of
ammonia (Haggard, 1924; Boyd et al., 1944). At the levels of
ammonia associated with ambient air (i.e., 1 - 200 µg/m3), very
little, if any, is absorbed through the lungs.
If a person breathes an ammonia concentration in air of
18 mg/m3 (a common occupational exposure limit) at 1 m3/h and,
if all the ammonia is retained, then 18/60 = 0.3 mg ammonia/min
would require to be cleared by hepatic blood flow (say 1
litre/min). The rise in systemic blood-ammonia would be calculated
at 0.3 mg/litre or 0.018 mmol. If the more realistic assumption of
30% retention were used, the corresponding increase in blood-
ammonia concentration would be 0.09 mg/litre, about µmol. An
arterial fasting ammonia concentration of 1.05 mg/litre has been
reported in healthy subjects (Conn, 1972), so the calculated rise
is only 10% over fasting levels.
7.1.2. Gastrointestinal tract
Ammonia is a trace compound in foods. Ammonia that is absorbed
from the intestinal tract arises primarily from the bacterial
degradation in the intestine of amino and nucleic acids from
ingested food, endogenous epithelial debris, and mucosal cell
luminal secretions, or from the hydrolysis of urea diffusing from
the systemic circulation into the intestinal tract. The estimated
ammonia production from various substrates in the human intestines
ranges from 10 mg/day in the duodenum to 3080 mg/day in the colon
and faecal contents. Nearly all the ammonia formed is absorbed
(about 99% or 4000 mg). In healthy individuals, absorbed ammonia
is mainly catabolized rapidly in the liver to urea; therefore,
relatively small amounts reach the systemic circulation after
absorption from the gastrointestinal tract as a consequence of this
"first pass effect" (Summerskill & Wolpert, 1970).
Castell & Moore (1971) have shown that ammonia uptake from the
human colon, the major site of ammonia production, increases with
increased pH of the luminal contents. A similar effect of pH has
been shown for the absorption of ammonia from the rumen of sheep
(Hogan, 1961; Bloomfield et al., 1962). Since an increase in pH
increases the proportion of non-ionized ammonia, the authors
concluded that simple non-ionic diffusion was responsible for the
majority of ammonia transport. Evidence also exists for the active
transport of the ammonium ion from the intestinal tract. Castell &
Moore (1971) showed that ammonia transport by the human colon,
though greatly diminished, still occurred when the luminal pH was
reduced to 5, at which value non-ionized ammonia would be virtually
absent. Mossberg & Ross (1967) and Mossberg (1967) studied the
absorption of ammonia from isolated intestinal loops of the golden
hamster and found that the ileal movement of ammonia against a
concentration gradient was inhibited by cyanide, dinitrophenol, and
anaerobiosis. This suggested that an energy-dependent transport
system was operable in the ileum, where ammonia was absorbed
preferentially, but not in the jejunum.
Membrane transport of the ammonium ion by the human erythrocyte
has been demonstrated (Post & Jolly, 1957).
7.1.3. Skin and eye
Ammonia is highly mobile in all tissues and the ammonium ion
readily penetrates the corneal epithelium. Within 5 seconds,
traces are present in the anterior chamber of the eye (Siegrist,
1920). However, systemic and intra-ocular absorption by these
routes are not quantitatively important.
7.2. Distribution
The ammonia normally present in all tissues in the body
constitutes a dynamic pool throughout which absorbed ammonia is
distributed. The distribution of total ammonia between body
compartments is strongly influenced by pH. The non-ionized NH3 is
freely diffusible, whereas NH4+ is less diffusible and relatively
confined in compartments. The lower the pH of a compartment, the
greater its total ammonia content (NRC, 1979).
The fate of absorbed ammonia molecules has been studied in man,
by measurement of blood constituents, and, in experimental animals,
by following the distribution of 15N after the administration of
15NH3 and compounds.
7.2.1. Human studies
In human beings, inhalation of ammonia (350 mg/m3; 500 ppm) for
30 min did not have any effect on blood-nitrogen levels (Silverman
et al., 1949). In another study, exposure of human subjects to
14 mg ammonia/m3 (20 ppm), for a duration of 8 h, revealed a
statistically-significant increase in BUN (from 23.9 to 30 mg%)
(Kustov, 1967). However, this is unlikely to have represented the
metabolic conversion of absorbed ammonia, since the increase was
far greater than could have been accounted for by the quantity of
ammonia inhaled.
Administration of 9 mg NH4Cl/kg body weight, orally, to 20
healthy adult male and female volunteers caused a transient
increase in ammonia concentrations in arterial blood in
approximately half of the subjects. Concentrations peaked (mean,
1.4 mg NH3/litre) at 15 min and returned to fasting levels (mean,
1.05 mg NH3/litre) by 30 min. However, in 50 male patients with
cirrhosis of the liver, blood-ammonia levels increased from already
elevated fasting levels (mean, 1.56 mg NH3/litre) to much higher
peak concentrations (mean, 3.7 mg NH3/litre) at 15 min, followed by
a slow decrease reflecting impaired hepatic urea synthesis. Blood-
ammonia levels, before and after administration of ammonium
chloride, were significantly higher among cirrhotic patients with
portacaval anastomoses than among patients lacking such shunts
(Conn, 1972).
7.2.2. Animal studies
The distribution, as well as the metabolic fate of ammonia,
depends on the route of administration. After intestinal
absorption, ammonium ions are primarily transformed by the liver
to urea, and subsequently excreted in the urine. In contrast,
intravenously-administered ammonium salts are more available as
non-essential nitrogen for protein synthesis (Furst et al., 1969).
However, some orally-administered ammonia, has been found to be
incorporated into tissue proteins. Incorporation of 15N was higher
in serum globulins than in albumin after intravenous dosing with
15N-ammonium salts, but this order was reversed after oral
administration (Furst et al., 1970). The amount incorporated into
protein by this route was greater, when protein intake was
restricted (Richards et al., 1968).
Duda & Handler (1958) analysed the tissue of rats, 15 min after
an intravenous injection of 15N-ammonium lactate, and found that
its major metabolites, glutamine, and urea, were quickly
distributed throughout the body. The highest levels of labelled
urea (in µmoles 15N/g tissue) were found in the kidney (0.0217) and
liver (0.0159), while lesser amounts were found in the heart
(0.0086), spleen (0.0067), brain (0.0029), testes (0.0027), and
carcass (0.0070). The highest levels of labelled glutamine (µmoles
15N/g tissue) were found in the heart (0.086) and liver (0.055) and
lesser amounts (0.005 to 0.032) in the brain, spleen, carcass,
kidney, and testes.
Vitti et al. (1964) examined the distribution of 15N from
ammonium citrate, administered by different routes, into the
proteins of various tissues of hypophysectomized rats. The liver,
kidney, and spleen contained greater concentrations of 15N
incorporated into proteins than heart or muscle fractions during
72 h following intragastric, intraperitoneal, and subcutaneous
administration of 15N-ammonium citrate. After the first 6 h,
during which the intragastric route gave higher values, the
quantity of 15N incorporated into liver-protein was not
substantially affected by the route of administration. In most of
the other tissues studied, however, 15N incorporation tended to be
least by the intragastric route, followed, in increasing order, by
the intraperitoneal and subcutaneous routes. By the last route,
more labelled ammonia was apparently made available to the widely
distributed glutamine-synthetase (EC 6.3.1.2) system (section
7.4.3).
7.3. Metabolic Transformation
Most organisms have mechanisms for conjugating ammonia into
non-toxic compounds for excretion. Terrestrial mammals synthesize
urea, which requires the concerted action of several enzymes of the
Krebs-Henseleit (urea) cycle. One of these enzymes, glutamine
synthetase (EC 6.3.1.2), was present in the brains of all
vertebrate species examined. Glutamine synthetase was also present
at significant levels in the liver in all organisms examined (Brown
et al., 1957).
Exogenous ammonia, administered intravenously as an ammonium
compound, is metabolized to glutamine as the major early product
(Duda & Handler, 1958). The ammonia fixed in glutamine may
eventually end up in amino acids, purines, pyrimidines, or other
nitrogen-containing compounds. Ingested ammonium chloride or
endogenous ammonia is absorbed into the portal vein and converted
in the liver to urea (Furst et al., 1969; Goodman & Gilman, 1970;
Pitts, 1971).
Results of studies on the metabolic fate of dietary ammonium
citrate (Foster et al., 1939) and intravenously-administered
ammonium lactate (Duda & Handler, 1958) in rats showed that urea
synthesis represented a nearly constant fraction of the
administered ammonia over a large concentration range. Besides
glutamine and urea, labelled nitrogen also appeared in creatine,
glycine, alanine, proline, histidine, arginine, glutamic acid, and
aspartic acid. Vitti et al. (1964) examined the incorporation of
15N from ammonium citrate into proteins of liver, heart, kidney,
spleen, and muscle fractions of untreated and growth hormone-
treated, hypophysectomized rats, and found differences in the
metabolic fate, depending on the route of administration.
Subcutaneous injection facilitated the labelling of amide nitrogen,
indicating extensive disposition via glutamine synthesis. In
contrast, intragastric or intraperitoneal administration resulted
in the labelling of arginine, glutamic acid, and other alpha-amino
acids of the liver. Amide-nitrogen was labelled to a much lesser
extent than by the subcutaneous route. The tissue distribution of
the label also differed according to the route of entry (section
7.2.2).
7.4. Reaction with Body Components
Ammonia-nitrogen is central in nitrogen metabolism and
therefore becomes incorporated in all proteins and nitrogen-
containing components in the course of metabolic turnover. Ammonia
does not react with body components in the manner of alkylating
agents or compounds that modify haemoglobin.
7.5. Elimination and Excretion
7.5.1. Expired air
Ammonia may be excreted through expired air. Hunt (1977)
reported human expired air levels of ammonia of between 105 and
2219 µg/m3; Larson (1977) reported values of between 196 and
1162 µg/m3, during mouth breathing. These values are higher than
those expected from equilibration with plasma- and lung-parenchyma-
ammonia levels (28 - 49 µg/m3). This is most likely due to the
synthesis of ammonia from salivary urea by oral microflora (Biswas
& Kleinberg, 1971). Measurable amounts of free ammonia were also
found in air expired by dogs given ammonium acetate intravenously
(Robin et al., 1959), and normal dogs and human beings with
hepatic-induced ammonia toxicity (Jacquez et al., 1957, 1959).
Bloomfield et al. (1962) reported the presence of free ammonia in
expired air from sheep during experimentally-induced urea toxicity.
Normal levels of ammonia in the expired air of the rat have been
reported to range from 7 to 247.1 µg/m3, with a mean of 54.6 µg/m3
in nose-breathing animals and 23.8 - 520.8 µg/m3, with a mean of
200.2 µg/m3 in tracheal-cannulated animals (Barrow & Steinhagen,
1980). The presence of ammonia in the expired air of human beings
and experimental animals suggests that reaction products may be
formed with a variety of airborne chemicals, thereby altering their
toxicity.
7.5.2. Urine and faeces
Free ammonia is excreted by ammonotelic organisms (e.g., fish),
uric acid by uricotelic animals (e.g., birds), and urea by
ureotelic animals (e.g., mammals). Mammals may also secrete
ammonia directly into the urine. Glutaminase (EC 3.5.1.2)
catalyses the release of ammonia in the kidney tubular epithelium,
where it serves as an acceptor of H+ and regulates the acid-base
balance (Van Slyke et al., 1943; White et al., 1973). In acidosis,
the renal concentration of glutaminase increases over several
days, paralleling the increased excretion of ammonium ions (Davies
& Yudkin, 1952; Muntwyler et al., 1956; Kamin & Handler, 1957);
two-thirds of the urinary-ammonia is contributed by this pathway
(Van Slyke et al., 1943), and approximately one-third by protein
metabolism and ammonia clearance from the plasma by the kidney.
Oral and intravenous administration of ammonium lactate to
healthy human volunteers produced different patterns of excretion,
reflecting the effective barrier of the liver in preventing
ingested ammonia from gaining access to peripheral circulation by
converting most of the ammonia load to urea. Urinary-ammonia
excretion was increased 8-fold and urea excretion was reduced by
one-half after intravenous injection, as opposed to oral
administration (Gay et al., 1969), probably due to the anabolism
involved in the "first pass" effect after oral administration.
Less than 1% of the 4 g total ammonia produced in the human
intestinal tract, per day, is excreted in the faeces (Summerskill &
Wolpert, 1970).
7.6. Retention and Turnover
Some nitrogen derived from absorbed ammonia is incorporated in
amino acids and proteins. The rate of ammonia-derived nitrogen
turnover is rapid, but depends on the nutritional state. Thus,
when 15NH4Cl had been administered orally to healthy male
volunteers, for one week, 70% 15N was excreted by those on a 70-g
protein/day diet, while only about 35% 15N was excreted by those on
20 g protein/day (Richards et al., 1968, 1975).
7.7. Uptake and Metabolism in Plants
Ammonia is used by many plants and preferentially by a few.
However, ammonia is toxic, and its uptake in large quantities may
put a severe strain on the carbohydrate metabolism of the plant in
the provision of carbon skeletons for its detoxification. The
absorption of ammonium usually is coupled with the exchange of
cations as H+. Ammonia-nitrogen functions as a nitrogen source
for the synthesis of amino acids, which are incorporated in
proteins. Plants that are able to absorb it in large amounts
include many acid plants, such as Rumex, which are able to detoxify
ammonia by forming ammonium salts of organic acids. "Amide
plants", such as beet, spinach, and squash, are able to form large
amounts of the amides, glutamine, and asparagine and can withstand
quite high concentrations of ammonium salts by detoxifying the
ammonia. Certain plants, such as rice, which live in water-logged
anaerobic soils, require NH3 or reduced organic nitrogen
fertilizer, alone.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Inhalation exposure
LC50 studies and studies to determine the threshold for
irritating effects on the respiratory system for the rat and mouse
are summarized in Tables 16 and 17, respectively.
Table 16. Lethal concentrations (1-h exposure) of
ammonia for rats and micea
----------------------------------------------------
Measured Species Mortality Mean weight gain
concentration ratiob of survivors at
(mg/m3) 14 days (g)
----------------------------------------------------
4347 rat 0/10 3.5
5474 rat 8/10 -c
6888 rat 9/10 -
controls rat - 21.4
2520 mouse 0/10 -0.2
3185 mouse 3/10 -0.7
4004 mouse 9/10 -
controls mouse - 1.6
----------------------------------------------------
a Adapted from: MacEwen & Vernot (1972).
b Number dead/number exposed.
c Not enough survivors for comparison.
The acute lethal dose of ammonia by inhalation has been
determined for both the rat and the mouse (MacEwen & Vernot, 1972).
The results are summarized in Table 16. Male CFE rats ranging in
weight from 200 to 300 g and male CF1 mice weighing from 20 to 30 g
(ICR derived) were exposed for 1 h to several concentrations of
ammonia. Inhalation of ammonia gas produced immediate nasal and
eye irritation followed by laboured breathing and gasping in all
test groups. In addition, convulsions were seen in mice.
Surviving rats necropsied after 14 days showed moderate mottling of
the liver, regarded as probable fatty infiltration, at the 5474 and
6888 mg/m3 (7820 and 9840 ppm) dose levels. Mice surviving the 2
highest dose levels of 3185 and 4004 mg/m3 (4550 and 5720 ppm)
showed mild congestion of the liver. Pathological lesions were not
seen in rats exposed to 4347 mg/m3 (6210 ppm) or mice exposed to
2520 mg/m3 (3600 ppm). The calculated 1-h LC50 values for the rat
and the mouse were 5137 and 3386 mg/m3 (7338 and 4837 ppm),
respectively.
In another inhalation study, an LC50 value for the rat, with a
2-h exposure, was 7600 mg/m3 (10 860 ppm) (Alpatov, 1964). In a
further study, the threshold for acute effects (depression, then
hyperactivity and convulsions) for a 2-h exposure was 85 mg/m3 (121
ppm) (Alpatov & Mikhailov, 1963).
Table 17. Single-dose inhalation studies (LC50)
----------------------------------------------------------
Species Exposure time LC50 Reference
(min) (mg/m3)
----------------------------------------------------------
rat 120 7600 Alpatov (1964)
rat 60 5137 MacEwen & Vernot (1972)
rat 5 18 693 Prokop'eva et al. (1973)
rat 15 12 160 Prokop'eva et al. (1973)
rat 30 7035 Prokop'eva et al. (1973)
rat 60 7939 Prokop'eva et al. (1973)
rat 10 31 612 Appelman et al. (1982)
rat 60 11 620 Appelman et al. (1982)
mouse 10 7060 Silver & McGrath (1948)
mouse 60 3386 MacEwen & Vernot (1972)
mouse 60 2960 Kapeghian et al. (1982)
----------------------------------------------------------
Kapeghian et al. (1982) reported an acute inhalation toxicity
study on male ICR mice, in which the 1-h LC50 with a 14-day
observation period was calculated to be 2960 mg/m3 (4230 ppm).
Lungs of mice that died during exposure were diffusely
haemorrhagic. Histology revealed acute vascular congestion and
diffuse intra-alveolar haemorrhage. A mild to moderate degree of
chronic focal pneumonitis was also seen. Focal atelectasis was
evident in survivors sacrificed after the observation period.
Liver damage was also seen in these mice. There was evidence of
swelling and increased cytoplasmic granularity of hepatocytes at
2408 mg/m3 (3440 ppm) and scattered foci of frank cellular necrosis
at 2954 mg/m3 (4220 ppm). At 3402 mg/m3 (4860 ppm), necrosis was
increased. The liver lesions may have resulted from the
compromised nutritional state of the mice. Follicular hyperplasia
in the spleen was also seen in surviving animals, but this was
absent in animals that died during exposure.
The acute LC50 in male and female Wistar rats was 31 612 mg/m3
(40 300 ppm) for a 10-min exposure, 20 017 mg/m3 (28 595 ppm) for a
20-min exposure, 14 210 mg/m3 (20 300 ppm) for a 40-min exposure,
and 11 620 mg/m3 (16 600 ppm) for a 60-min exposure (Appelman et
al., 1982). Survivors were observed for 14 days. Clinical signs
of restlessness, eye irritation, nasal discharge, mouth breathing,
and laboured respiration were seen during exposure. Gross necropsy
revealed haemorrhagic lungs in animals that died during the study
as well as in survivors. No histopathology was performed.
Prokop'eva et al. (1973) reported that white rats exposed to
high concentrations of ammonia (6000, 3000, 1000 mg/m3 or 6814,
4307, 1436 ppm) for periods of 5, 15, 30, and 60 min exhibited
dyspnoea, irritation of the respiratory tract and eyes, cyanosis of
the extremities, and increased excitability. The LC50 values for
inhalation exposures of 5 and 15 min were 18 693 mg/m3 (26 704 ppm)
and 12 160 mg/m3 (17 372 ppm), respectively, while for 30 and 60
min, the values were 7035 mg/m3 (10 050 ppm) and 7939 mg/m3 (11 342
ppm), respectively. Inhalation of ammonia at concentrations of
3000, 1000, or 300 mg/m3 (4307, 1436, 431 ppm) resulted in a drop
in static muscular tension, leukocytosis, prolongation of the
latent reflex time, increase in total protein and blood sugar,
increased oxygen consumption, and a rise in the level of residual
nitrogen. No changes were observed in rats exposed to a
concentration of ammonia of 100 mg/m3 (144 ppm), for 5, 15, 30, and
60 min. Animals exposed to high concentrations of ammonia (exact
concentration not specified) developed pneumonia (Prokop'eva et
al., 1973).
When exposed to toxic levels of ammonia, within 1 min, mice
exhibited excitement, closing their eyes immediately and gasping
(Silver & McGrath, 1948). Groups of 20 mice were exposed to
ammonia gas for 10 min at 9 concentrations ranging from 6100 to
9000 mg/m3 (8758 to 12 921 ppm). The median lethal concentration,
calculated from the mortality at 10 days, was 7060 ± 320 mg/m3
(10 152 ± 460 ppm). Most (93%) deaths occurred rapidly, due to
convulsions after 5 min of exposure. Survivors usually recovered
within 10 min.
The LC50 values for the rat and the mouse are summarized in
Table 17.
Dose-dependent ultrastructural changes in the terminal airways
of mice exposed for 3 - 60 min to ammonia (concentration
unspecified) included oedema of the alveolar epithelium,
development of intracapillary platelet thrombosis, increased
secretions by the Clara cells, presumed to be phospholipids, and an
increase in the number of empty lamellar bodies in the large
alveolar cells (Niden, 1968).
Twenty cats were anaesthetized and exposed to 700 mg/m3 (1000
ppm) for 10 min via endotracheal tube and then observed for up to
35 days (Dodd & Gross, 1980). All cats had severe dyspnoea,
anorexia, and dehydration, 24 h after exposure. Several measures
of pulmonary function were impaired. Gross pathology of the lungs
showed various degrees of congestion, haemorrhage, oedema,
interstitial emphysema, and collapse, all non-specific for any
post-exposure day. Bronchopneumonia was common, 7 days after
exposure.
Barrow et al. (1978) exposed male Swiss-Webster mice to
concentrations of ammonia ranging from 70 to 560 mg/m3 (100 -
1000 ppm) for 30 min. The average respiratory rate depression in
4 mice for each of 4 exposure levels was evaluated. The maximum
depression in respiratory rate at each exposure level occurred
within the first 2 min. The concentration expected to elicit a 50%
decrease in respiratory rate (RD50) in mice, calculated by a
regression equation for ammonia, was 212 mg/m3 (303 ppm) with 95%
confidence limits of 132 - 343 mg/m3 (188 - 490 ppm). Effects of
ammonia exposure such as bradycardia and peripheral
vasoconstriction accompanied respiratory rate depression at the
RD50 and above.
A concentration of 350 mg/m3 (500 ppm) was reported by Wood
(1979) to be the level at which unrestrained mice did not
consistently adopt avoidance measures on inhalation of ammonia.
Both this author and Barrow et al. (1978) claimed to offer more
sensitive end-points for assessing ammonia irritation. However,
the no-observed-adverse-effect level reported by Wood (1979) was
higher than the concentration determined by Barrow et al. (1978) to
elicit 50% depression of respiratory rates in mice.
Dalhamn & Sjoholm (1963) tested in vitro preparations of the
tracheas of 8 rabbits. Arrested ciliary activity was observed
after 5 min of exposure to ammonia at 350 - 700 mg/m3 (500 -
1000 ppm). In in vivo studies on rabbits, Dalhamn (1963) showed
that the level of ammonia entering the nasal cavity, necessary to
cause small changes in the rate of tracheal ciliary beating was
1400 mg/m3 (2000 ppm). This corresponds to a tracheal
concentration of approximately 70 mg/m3 (100 ppm).
When anaesthetized male rabbits were exposed to 8 ammonia
concentrations ranging from 700 to 14 000 mg/m3 (1000 - 20 000 ppm)
(Richard et al., 1978b), bradycardia appeared at 1750 mg/m3 (2500
ppm). Hypertension, cardiac arrhythmia, macroscopic lung changes,
and EEG abnormalities were also reported. Signs appeared more
rapidly at higher concentrations with the complete syndrome
appearing at 3500 mg/m3 (5000 ppm).
Mayan & Merilan (1972) exposed 16 adult female New Zealand
white rabbits to ammonia concentrations of 35 and 75 mg/m3 (50 and
100 ppm) for 2.5 h. The average decreases in respiratory rate,
which were 34 and 32.7%, respectively, were significantly ( P <
0.01) less than control values. There were no histopathological
changes in lung, liver, spleen, or kidneys.
Enzymatic alterations in rats following inhalation of low
levels of ammonia have also been reported. At concentrations of
approximately 20 - 121 mg/m3 (29 - 173 ppm), there was a
decrease in the activities of liver succinic dehydrogenase (EC
1.3.99.1), lactate dehydrogenase (EC 1.1.1.28), glucose-6-phosphate
dehydrogenase (EC 1.1.1.49), and adenosine triphosphatase (EC
3.6.1.3). Liver acid phosphatase (EC 3.1.3.2) activity was
increased (Zlateva et al., 1974).
When 180 mice were exposed for 10 min to ammonia at 6139 -
9058 mg/m3 (8770 - 12 940 ppm), death with convulsions began to
occur 5 min after exposure. One hundred mice died during the
exposure. The surviving animals (80) recovered rapidly; however,
7 died between the sixth and tenth days following exposure (NRC,
1979).
8.1.2. Oral exposure
The effects of ammonia and its compounds are mainly of 2 types.
The first is the effect of ammonia itself. The second is the
effect of the anion bound to the ammonium ion. Ammonium chloride,
especially, will mainly exert its effects in the mammalian body due
to the formation of hydrogen chloride. Most of the experimental
work concerning the oral route of administration has centred on
ammonium chloride, which has been used extensively in the study of
metabolic acidosis. There have been few studies in which attempts
have been made to identify the role of the ammonium ion and ammonia
in the effects (Table 18).
Median lethal doses (LD50s) of 4400 and 3100 mg/kg body weight
have been reported for ammonium sulfamate in the rat and the mouse,
respectively. (Vinokurova & Mal'kova, 1963). A similar figure for
the oral LD50 in the rat of 4520 (4070 - 5020) mg/kg was reported
by Bukhovskaya et al. (1966). Frank (1948) reported a lethal dose
for ammonium sulfate of between 3000 and 4000 mg/kg body weight in
the rat.
8.1.2.1. Effects of metabolic acidosis induced by ammonium chloride
The ingestion of ammonium chloride in doses of around 500 -
1000 mg/kg body weight per day, for periods ranging from 1 to 8
days, has induced metabolic acidosis in mice, guinea-pigs, rats,
rabbits, and dogs. However, Boyd & Seymour (1946) did not report
any toxic effects at doses of up to 1 g/kg body weight in rats,
rabbits, guinea-pigs, and cats (50 animals per group).
Clinical signs, depending on the severity of the acidosis,
include: a decrease in plasma- and urinary-pH; decreased appetite;
decreased carbon dioxide-combining power; an increase in BUN and
chlorides; an increase in plasma proteins; an increase in
haematocrit (haemoconcentration); increased gluconeogenesis;
increased phosphoenolpyruvate carboxykinase (EC 4.1.1.49) activity;
increased urinary ammonium; increased urea, sodium, chloride,
calcium, and titratable acid excretion; an increase in malate and
oxaloacetate concentrations in renal tissue; and decreased
concentrations of glutamine, glutamate, and alpha-ketoglutarate in
the kidney. Pulmonary oedema, central nervous system dysfunction,
and renal changes are reported to have occurred after ingestion of
ammonium chloride.
Susceptibility to ammonium chloride differs among species. For
instance, pulmonary oedema is produced in cats, but not in rabbits;
yet cats have been shown to be more resistant to oral poisoning by
ammonium chloride than other animals studied.
Age is an important factor in the response of rats to oral
doses of ammonium salts. Benyajati & Goldstein (1975) found that
the administration of a single dose of 5 mmol ammonium chloride/kg
body weight (267.5 mg/kg) by gavage to 7- to 12-day-old rats (39
animals) increased ammonia excretion by about 64%, within 4 h,
compared to an increase of 155% in 20 adult rats after similar
treatment.
Table 18. Selected studies on the acute oral effects of ammonia compounds in rats
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Urea drinking- female, Holtz- 6 concentration no renal hypertrophy Lotspeich (1965)
water man, 200 - of N equivalent
250 g to that of 0.28
mol/litre NH4Cl
for 7 days ad
lib
Citrate drinking- female, Holtz- 6 0.28 mol/litre no renal hypertrophy Lotspeich (1965)
water man, 200 - solution for
250 g 7 days ad lib
Chloride drinking- female, Holtz- 9 0.28 mol/litre metabolic acidosis; increase Lotspeich (1965)
water man, 200 - for 7 days, ad in kidney weight and total
250 g lib (about 1000 N; increased capacity to
mg/kg per day) produce ammonia from
glutamine in kidney; renal
hypertrophy; unilateral
nephrectomy plus acidosis
induced by NH4Cl caused a
greater hypertrophy than
either alone
------------------------------------------------------------------------------------------------------------
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Chloride diet female, 150 g 125 all on low- consumption of food inversely Motyl & Debski
protein diet; related to dosage of NH4Cl; (1977)
Group I: no dosage-related decrement in
NH4; Group II: weight gain; increase in LD50
1% NH4Cl (300 value for ip dose of 2.7%
mg/kg body NH4Cl in Groups II and III,
weight per indicating an increased
day); Group adaptive capacity for ammonia
III: 2% NH4Cl detoxification; LD50 value of
(380 mg/kg Group IV same as Group III;
body weight per LD50 value of Group V lower
day); Group IV: than Group I; dose-dependent
4% NH4Cl (560 decrease in blood-glucose
mg/kg body levels; decrease of muscle
weight per and liver glycogen; dose-
day); Group V: dependent increase in delta-
8% NH4Cl (540 ornithine transaminase and
mg/kg body ornithine transcarbamylase;
weight per day, increase in adenosine
for 7 days triphosphate concentration in
blood
------------------------------------------------------------------------------------------------------------
8.1.2.2. Organ effects following oral administration
(a) Lung and central nervous system
Toxic doses of ammonium salts induced acute pulmonary oedema in
rats, guinea-pigs, and cats, but not in rabbits given ammonium
chloride (6% aqueous solution) intraperitoneally or by gavage,
though the doses were sufficient to cause death. To induce similar
effects in cats, a larger dose (unspecified) of ammonium chloride
and a longer latency period (from 1 to 3 h) were required,
indicating a greater resistance of this species to ammonium
chloride (Koenig & Koenig, 1949).
In a limited study to assess the role of the ammonium ion in
producing pulmonary oedema, Koenig & Koenig (1949) administered, by
gavage, to 5 different guinea-pigs, 6 ml of 20% ammonium nitrate;
7 ml of 6.4% ammonium acetate; 7 ml of 10% ammonium bromide; 7 ml
of 6% ammonium chloride; or 7 ml of 7.4% ammonium sulfate,
respectively. The last 4 solutions contained approximately
equivalent concentrations of ammonium. All 5 animals died of acute
pulmonary oedema; the lungs of a control animal that had received
water by gavage remained normal. The pulmonary oedema could not be
attributed to the induced acidosis, since guinea-pigs and cats,
made severely acidotic by gavage with sodium lactate or dilute
hydrochloric acid, did not show lung oedema (Koenig & Koenig,
1949).
Progressive signs of ammonium poisoning caused by ammonium
salts, given by gavage, indicative of both pulmonary and nervous
system dysfunction, were reported in less than 30 min in guinea-
pigs and rats (Koenig & Koenig, 1949) including:
(i) a rapid increase in the rate and depth of respiration;
(ii) weakness and difficulty in locomotion;
(iii) hyperexcitability for tactile, auditory, and painful
stimuli; and
(iv) muscle fasciculations over most of the body followed
by generalized tonic convulsions and then coma.
The respiratory rate was greatly reduced but the depth of
respiration increased and was accompanied by gasping and stridor.
Histological changes occurred in both the lung and brain, while
oedema, congestion, and haemorrhage were found principally in the
lung (Koenig & Koenig, 1949; Cameron & Shiekh, 1951).
The addition of ammonium chloride to rat feed resulted in
reduced dietary consumption (Motyl & Debski, 1977). The results of
studies by Noda & Chikamori (1976) pointed to a direct effect of
the ammonium ion on the brain area that regulates feeding. Rats
with bilateral lesions in the prepyriform cortical area of the
brain consumed as much diet containing 3% ammonium chloride as
basal diet. A unilateral injection of 10 mg/litre of 2% NH4Cl/kg
body weight into prepyriform cortical areas, in contrast to an
injection into other areas of the brain, or an injection of sodium
chloride, significantly reduced the food intake of 6 adult male
Wistar rats. The results of these studies suggest that ammonium
ions directly influence appetite by their effects on prepyriform
cortical areas.
(b) Kidney
Lotspeich (1965) reported that ingestion for 7 days of
0.28 mol/litre (1.5%) ammonium chloride in water (ad lib) produced
renal hypertrophy with new cell formation and an enlargement of
existing cells. Some animals from each of the acidotic and control
groups were subjected to unilateral nephrectomy. Highly
significant increases in kidney wet weight, dry weight, and total
nitrogen were observed in the acidotic group. Similar changes were
produced by unilateral nephrectomy. In unilaterally nephrectomized
rats made acidotic by the ingestion of ammonium chloride, the
remaining kidney was larger than that seen in animals with
unilateral nephrectomy without induced acidosis, suggesting an
additive effect of unilateral nephrectomy and ammonium chloride
intake.
The relationship between the renal hypertrophy and the ammonium
ion, acidosis, non-specific intake of nitrogen, or increased solute
load was examined (Lotspeich, 1965). Isomolar (0.28 mol/litre)
solutions of sodium chloride and ammonium chloride produced renal
hypertrophy. The increase due to sodium chloride was less marked
(it was noted that the sodium chloride group drank 3 or 4 times as
much solution each day as the ammonium chloride group). Isomolar
(0.2 mol/litre) solutions of sodium bicarbonate and ammonium
citrate did not induce renal hypertrophy. When 3 groups of 6 rats
were given drinking-water containing 0.28 mol/litre sodium
chloride, 0.28 ammonium chloride, or a urea solution containing
nitrogen equivalent to that in the 0.28 mol/litre ammonium
chloride, for 7 days, only the ammonium chloride solution induced
acidosis. The rats in both the sodium chloride and ammonium
chloride groups had larger kidneys than the urea-drinking rats,
though the kidneys of the sodium chloride-drinking rats were not as
large as those of the ammonium chloride-drinking rats. Since renal
hypertrophy was produced by solutions containing the chloride ion,
while ammonium citrate and urea did not cause any hypertrophy, the
evidence does not support the hypothesis that renal hypertrophy is
due to the ammonium ion.
Thompson & Halliburton (1966) also reported renal hypertrophy
in rats that ingested 3% ammonium chloride in the diet for 6 days.
The ingestion of ammonium citrate or sodium chloride in amounts
that were equivalent to that of the ammonium chloride did not cause
renal hypertrophy.
Janicki (1970) examined the kidneys of rabbits that had been
administered 16.2 g ammonium chloride, by gavage, over a period of
2 days and found the epithelium of the convoluted tubules swollen,
vacuolated, and completely filling the lumen; nuclei exhibited
karyolysis. Findings were similar in 4 rabbits administered 5 -
7 g ammonium chloride, by gavage, over a period of 3 - 7 days.
However, hyperaemia of the renal cortex of rabbits was noted
after the administration, by gavage, of single doses of 0.8 and 1 g
ammonium carbonate/kg body weight (Yoshida et al., 1957). As the
carbonate ion does not have an acidifying effect, this suggests
that the ammonium ion produces some effects on the kidney.
8.1.2.3. Influence of diet on the effects of ammonia
In 6 mongrel dogs, blood-urea levels were increased by a higher
level of protein intake (6 g protein/kg per day) (Bressani &
Braham, 1977). Levels of ~30 mg BUN/litre were found 8 h after the
daily feed. When the protein intake was low (4 mg protein/kg per
day), BUN levels of 14 mg/litre were reported 8 h after the daily
feed. The frequency of protein intake did not affect the maximum
value of blood-urea levels when the protein intake was low (4 mg
protein/kg per day). This suggests that high levels of urea and
ammonia in the blood might occur in animals that suffer from liver
insufficiency and are fed high-protein diets.
Kulasek et al. (1975) studied the effects of nitrogen in the
diet on ammonia detoxification, using 210 rats and 6 diets
containing 50, 150, and 700 g casein/kg (low-, optimal-, or high-
protein diet, respectively), with or without the addition of
ammonium chloride at 20 g/kg. After 8 or 9 days of feeding, the
LD50 from an intraperitoneal injection of a 2.7% solution of
ammonium chloride was determined. The LD50 increased in proportion
to the amount of nitrogen in the diet. The data suggest that
higher doses of exogenous ammonia were tolerated by rats on
protein-rich diets or diets containing an ammonium salt as an
additive. An adaptive capacity for ammonia detoxification was
further demonstrated by Motyl & Debski (1977) in a study using rats
fed low-protein diets with the addition of NH4Cl. The LD50 values
for the ip injection of 2.7% NH4Cl were higher in animals fed low-
protein diets supplemented with NH4Cl (Table 18). Stevens et al.
(1975) raised weanling rats for 3 - 6 weeks on a protein-deficient
diet. Subsequent challenge by a large, intraperitoneally-injected
dose of ammonium chloride indicated that severe protein deprivation
increased vulnerability to ammonia poisoning compared with that of
control groups not prefed protein-deficient diets. Thus, with the
exception of animals suffering from liver dysfunction, animals on
high-protein diets seem able to tolerate a higher oral intake of an
ammonium salt.
A group of eight, 6- to 8-month-old cats, given a single meal
of a complete amino acid diet without arginine, developed
hyperammonaemia with elevated plasma levels of glucose and ammonia
and showed clinical signs of ammonia toxicity within 2 h. One cat
died 4.5 h after ingesting only 8 g of the diet. Five of the
surviving cats given a single meal of complete amino acids, in
which arginine was replaced with an equivalent amount of ornithine,
did not show any unusual signs. This finding indicates that the
cat is unable to synthesize ornithine at the rate required by the
urea cycle to dispose of ammonia from amino acid catabolism;
however, the dietary requirements for cats may differ from those of
other adult animals, including human beings, because the cat has a
much higher protein requirement, i.e., it requires approximately
20% of dietary calories as protein, as opposed to only 4 - 8%
required by the rat, dog, sheep, and man (Morris & Rogers, 1978).
The lethal effects of ammonia poisoning have been prevented by
the amino acids, ornithine and aspartic acid. To test whether this
was mediated by a sparing action of adenosine triphosphate (ATP) on
ammonia metabolism, 20 dogs with chronic Eck's fistula were
injected in the duodenum with ammonium acetate at 4.1 mmol/kg body
weight (~219.4 mg/kg). Half of the group was given 2 mg ATP/kg,
1 h prior to administration of the ammonium acetate. In 9 of the
10 dogs, ATP prevented a rise in levels of ammonia in venous blood.
Grossi et al. (1968) administered ATP (2 mg/kg) intravenously to 6
dogs with chronic Eck's fistula and on high-protein diets. Within
1 h, high blood-ammonia levels returned to normal. In chronic
liver disease, there may be insufficient ATP available for ammonia
detoxification, resulting in hyperammonaemia.
8.1.3. Dermal exposure
No data are available regarding any systemic effects of dermal
exposure to ammonia or ammonium compounds.
8.1.4. Effects due to parenteral routes of exposure
The parenteral toxicity of ammonia and ammonium compounds has
been studied extensively. Toxicity is influenced by the route of
administration, e.g., with oral administration, there is the
capacity for detoxification of exogenous ammonia by the liver.
8.1.4.1. Lethality
The intravenous (iv) and intraperitoneal (ip) LD50 values for a
number of ammonium compounds in various species are summarized in
Table 19. The toxic syndrome was similar in all species studied.
The signs, after iv injection, were characterized by immediate
hyperventilation and clonic convulsions followed by either fatal
tonic extensor convulsion or the onset of coma in 3 - 5 min. The
animals remained comatose for approximately 30 - 45 min. At this
stage, tonic convulsions and death can occur at any time, but
animals that survive usually recover rapidly and completely
(Warren, 1958; Warren & Schenker, 1964; Wilson et al., 1968a,b).
After intraperitoneal injection, the signs did not appear until
15 - 20 min after administration (Greenstein et al., 1956; Wilson
et al., 1968b).
Table 19. Toxicity of several ammonium compounds in selected species
-----------------------------------------------------------------------------------------------------------
Ammonium Species Intravenous dose Intraperitoneal dose
compound (mmol/kg of body weight) (mmol/kg of body weight)
(LD50) (LD50) Reference
-----------------------------------------------------------------------------------------------------------
Acetate rat - 8.20 Greenstein et al. (1956)
mouse 6.23 - Warren (1958)
mouse 5.64 10.84 Wilson et al. (1968a)
chick 2.27 10.44 Wilson et al. (1968a)
Bicarbonate mouse 5.05 - Warren (1958)
mouse 3.10 - Wilson et al. (1968b)
Carbamate mouse 0.99 - Wilson et al. (1968b)
Carbonate mouse 4.47 - Warren (1958)
mouse 1.02 - Wilson et al. (1968b)
Chloride mouse 6.75 - Warren (1958)
mouse (38.8 °C)a 6.6 - Warren & Schenker (1964)
mouse (40.4 °C)a 5.17 - Warren & Schenker (1964)
mouse (27.9 °C)a 10.21 - Warren & Schenker (1964)
Hydroxide mouse 2.53 - Warren (1958)
-----------------------------------------------------------------------------------------------------------
a Body temperature.
8.1.4.2. Central nervous system effects
Navazio et al. (1961) observed that characteristic toxic signs
were not observed in rats until the ammonia concentration in the
blood was double that of basal values (attained within 8 - 10 min
of an ip injection of 601 mg ammonium acetate/kg body weight). No
substantial increase in brain-ammonia was observed. However, when
the blood-ammonia concentration reached more than 20 times the
basal value, there was a sudden rise in the ammonia concentration
in the brain, which reached a maximum of approximately 100 mg
ammonia-nitrogen/kg (wet weight), between 10 and 26 min after
injection. Muscular contractions with occasional tetanic spasms,
and then coma occurred, when the concentration of ammonia in the
brain reached approximately 50 mg/kg. Although the animals started
to recover from the comatose state approximately 70 min after
onset, blood- and brain-ammonia concentrations did not return to
basal levels until 2 h after the injection of ammonium acetate.
However, other authors observed an immediate increase in brain-
ammonia after ip injection of ammonium acetate (Torda, 1953; du
Ruisseau et al., 1957; Salvatore et al., 1963). These authors
found dramatic increases in the brain-ammonia content, 2 - 5 min
after the injection of ammonium acetate. Salvatore et al. (1963)
suggested that there was no critical blood-ammonia concentration
for diffusion through the blood-brain barrier.
Ammonia has been shown to be more highly toxic at elevated body
temperature, whereas hypothermia affords marked protection
(Schenker & Warren, 1962). The LD50 values for ammonium chloride
in the mouse, at various body temperatures, are shown in Table 19.
The increased toxicity of ammonia at elevated body temperature was
suggested to be due to a direct metabolic effect of hyperthermia on
the brain, unrelated to dehydration or stress.
8.1.4.3. Effects on the heart
Intravenous LD50 values for ammonium carbamate, ammonium
carbonate, and ammonium bicarbonate have been determined in mice by
Wilson et al. (1968a,b) (Table 19). The physiological effects of
the injected ammonium compounds in dogs and sheep were also
investigated. Electrocardiograms recorded during the toxic
syndrome indicated that the animals died from ventricular
fibrillation due to a direct effect of ammonia on the heart. These
findings were in agreement with the effects noted by Berl et al.
(1962) during the iv infusion of ammonium chloride in cats when
electrocardiograms were altered in a complex manner. However,
Warren & Nathan (1958) were unable to demonstrate any cardiotoxic
effects of the ammonium compounds in mice and concluded that the
toxicity syndrome was due primarily to a cerebral effect and not a
direct effect on cardiac or skeletal muscle.
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
When 48 rats were continuously exposed to 127 mg ammonia/m3
(181 ppm) for 90 days, no abnormalities were found in organs or
tissues. Inhalation of 262 mg ammonia/m3 (374 ppm) for 90 days
induced mild nasal irritation in about 25% of 49 rats and a mild
leukocytosis in 4 of the rats. Continuous exposure to 455 mg/m3
(650 ppm) resulted in the death of 50 out of 51 rats by the 65th
day of exposure. All animals exhibited mild nasal discharge and
laboured breathing. In a second study, rats, guinea-pigs, rabbits,
and dogs were continuously exposed to 470 mg/m3 (671 ppm) for 90
days. Thirteen out of 15 rats and 4 out of 15 guinea-pigs died.
Marked eye irritation was noted in rabbits and dogs, with corneal
opacities in about one-third of the rabbits. At autopsy, all test
animals examined had more extensive focal or diffuse interstitial
inflammatory processes in the lungs than the controls (Coon et al.,
1970).
White rats were exposed to ammonia, by inhalation, at
concentrations of 100 and 30 mg/m3 (143 and 43 ppm) for 25 or
60 min, every 48 h, for a period of 3 months. Rats exposed to a
concentration of 100 mg/m3 (143 ppm) showed only a mild
leukocytosis, with no significant differences from the control
group with regard to oxygen consumption, neuromuscular excitation
threshold, heart rate, blood-sugar, blood-residual nitrogen, and
total serum-protein (Prokop'eva & Yushkov, 1975). In another study
by Alpatov & Mikhailov (1963), the threshold level for toxic
effects in a 2-month exposure was 40 mg/m3 (57 ppm). Histological
changes were seen in the lungs of the animals exposed to a
concentration of 100 mg/m3 (143 ppm), including small areas of
interstitial pneumonia with signs of peribronchitis and
perivasculitis. No changes were seen in other organs compared with
those in the control group.
Broderson et al. (1976) exposed Sherman and Fisher rats to
ammonia from natural sources, at an average concentration of
105 mg/m3 (150 ppm) for 75 days, and to purified ammonia at
175 mg/m3 (250 ppm) for 35 days. Histological changes in the
olfactory and respiratory epithelia of the nasal cavity were
similar in all the exposed rats, showing increased thickness,
pyknotic nuclei, and hyperplasia. The submucosa was oedematous
with marked dilation of small vessels. Lesions decreased
posteriorly.
Exposure to ammonia at concentrations of 17 - 175 mg/m3 (25 -
250 ppm) increased the infectious effects of Pasteurella multocida
in mice and Mycoplasma pulmonis in rats; this effect increased
with the concentration of ammonia (Broderson et al., 1976; Richard
et al., 1978a).
Richard et al. (1978a) selected a concentration of 350 mg/m3
(500 ppm) for a study of effects of continuous exposure to ammonia
after noting that general toxic effects, particularly on growth
rate, were not found at 175 - 210 mg/m3 (250 - 300 ppm). Young
male specific-pathogen-free rats were age- and weight-matched with
controls (27 per group) and exposed for up to 8 weeks. Nasal
irritation began on the fourth day. After 3 weeks, exposed rats
showed nasal irritation and inflammation of the upper respiratory
tract, but no effects were observed on the bronchioles and alveoli.
The number of pulmonary alveolar macrophages was similar to that in
the controls. After 8 weeks, none of these inflammatory lesions
were present.
In a study by Coon et al. (1970), 15 rats, 15 guinea-pigs, 3
rabbits, 2 dogs, and 3 monkeys were exposed to an ammonia
concentration of 155 mg/m3 (221 ppm) for 8 h/day, 5 days a week,
for 6 weeks. Pathological effects were not observed in any of the
species except for evidence of focal pneumonitis in the lungs of
one of the monkeys. Exposure to 770 mg/m3 (1100 ppm) for the same
duration induced mild to moderate eye irritation and laboured
breathing in the rabbits and dogs at the beginning of exposure, but
these signs disappeared by the second week and no other signs of
irritation or toxicity were noted. Autopsy findings included non-
specific inflammatory changes in the lungs of the rats and guinea-
pigs.
Male Sprague Dawley rats were studied to determine whether
ammonia was absorbed through the lungs into the blood, and the
subsequent effects on the blood pH, blood gases, and hepatic drug-
metabolizing enzymes (Schaerdel et al., 1983). Rats were exposed
to ammonia at 7 - 840 mg/m3 (10 - 1200 ppm) for 1, 3, or 7 days.
No significant changes were found in blood pH, pCO2, or in the
histological appearance of the lungs or trachea. Liver microsomal
enzymes (ethyl-morphine- N-demethylase and cytochrome P-450) showed
only minor changes. Blood-ammonia levels increased in a linear
fashion with increasing ammonia concentrations in air. A
concentration of 70 mg/m3 (100 ppm) or less produced only very
small changes in blood-ammonia levels and had no measureable
effects on any of the parameters studied.
8.2.2. Oral exposure
8.2.2.1. Histopathological effects
Rats given ammonium salts, for periods ranging up to 90 days,
did not appear to sustain renal damage (Freedman & Beeson, 1961;
Gupta et al., 1979). Twelve adult male Sprague Dawley rats were
given ammonium chloride in the drinking-water at 16 g/litre, for up
to 3 weeks. Urinalysis did not reveal any evidence of renal
injury, and no gross or histological "abnormalities" of the kidney
were seen at autopsy (Freedman & Beeson, 1961). Glutaminase
activity per kg of kidney increased with duration of exposure; this
is a physiological adaptation to acidosis. Another group of 10
rats, similarly treated and then given ammonium chloride in the
drinking-water at 10 g/litre, for an additional 2.5 months also did
not show any gross or microscopic renal abnormalities. Assuming
the rats weighed 250 g and consumed 25 ml of water per day, they
could have ingested as much as 1.6 g/kg body weight per day while
drinking the ammonium chloride at 16 g/litre and 1.0 g/kg body
weight per day while drinking a level of 10 g/litre. However,
actual intake may have been lower, because ammonium chloride is
known to affect the appetite and may render the water less
palatable. When ammonium chloride was given at 20 g/litre, food
and water consumption were drastically reduced.
In Table 20, selected studies are presented on ammonium salts
other than the chloride. In general, at low doses, no detrimental
effects were observed when rats and pigs were exposed to the NH4
salts listed.
In a 90-day study by Gupta et al. (1979) on rats, there was not
any evidence of renal damage. Ammonium sulfamate (NH4SO3NH2) as a
100 g/litre solution was given orally at rates of 100, 250, or
5000 mg/kg body weight per day, 6 days a week for 30, 60, or 90
days to adult female rats (ITRC colony-bred albino) and to weanling
male and female rats (20/sex per age per dose level). Under
certain conditions, the sulfamate ion is hydrolysed to bisulfate
ion and ammonia; however, it is unclear whether, and to what
extent, hydrolysis occurs in the rat intestine. Equivalent doses,
listed in Table 20, were calculated on the assumption that no
hydrolysis occurs. The effects of the anion, sulfamate and/or
sulfate, on the action of ammonium is a matter of conjecture.
Ammonium sulfamate would be expected to produce metabolic acidosis
on the basis of its structure, but this has not been verified
experimentally. The general health of both treated and control
rats was good, and there were no significant differences in mean
body weights throughout the study, except for a slight depression
in the highest-dose adult females at 60 and 90 days. Food and
water consumption were unaffected, except in the highest dose
weanlings of both sexes, which consumed less food and drank more
water than control weanlings. Interim sacrifice of 6 animals/sex
per dosage group was performed for haematological and histological
examination. There were no significant differences in
haematological values (packed cell volume, haemoglobin
concentration, total red cell count, total and differential white
cell count). Relative organ weights in all treated groups did not
differ significantly from those in the controls. Histological
examination of the kidney, liver, lung, stomach, heart, spleen,
thyroid, adrenal, gonads, intestine, and lymph nodes did not reveal
any abnormalities, except slight hepatic fatty degeneration in one
adult female at 90 days.
Table 20. Dose-response data for short-term oral administration of ammonium compounds
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Sulfamate rat, albino, orally as 10% 30, 60, 100; 15.0; no effects on body Gupta et
20 adult solution; not 90 days 250 37.3 weight, food, and al. (1979)
female, 20 specified whether water consumption,
weanling given by gavage haematological values
male, 20 or organ weights; no
weanling histological
female per abnormalities in
dose organs (including
kidneys)
500 74.8 slight decrease in
body weight in adult
females; slightly
lower food and higher
water consumption in
weanlings of both
sexes; no effects on
haematological values
or organ weights; no
histological
abnormalities in
organs (including
kidneys)
------------------------------------------------------------------------------------------------------------------- kidneys)
Table 20. (contd.)
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Diammonium pig, male 3.75% diammonium 28 days 1820 274 body weight gain Kagota et
citrate 22.5 kg, citrate in diet increased but % meat al. (1979)
3-4/group containing 6.4% crude and fat of carcass
protein unaffected; plasma-
and urine-urea-
nitrogen increased
but blood-ammonia-
nitrogen level
unaffected; no effect
on haematological
values; no gross
abnormalities or
lesions
Diammonium pig, 27.3 kg basal diet contained 81 days 820 161 (10% no effect on weight Wehrbein
phosphate 3 males and 16% protein; 0,5,10, replacement) gain, BUN, or food et al.
and 3 females/ or 20% of dietary N consumption (no (1970)
diammonium group was replaced by N effect on these
citrate as from equimolar parameters at 5%
equimolar mixture of designated 81 days 1600 322 (20% either) slight
mixture NH4+ salts replacement) decrease in weight
gain, BUN and food
consumption
-------------------------------------------------------------------------------------------------------------------
a Estimated for all studies except Gupta et al. (1979).
b Equivalent intake expressed as ammonia (NH3).
Treatment of virgin female rabbits with ammonium carbonate,
chloride, hydrophosphate, sulfate, or hydroxide at 0.1 - 0.2 g/kg
body weight, orally, on alternate days, for periods of 3 weeks
separated by 1-week intervals of no treatment, was associated with
enlargement of the ovaries, follicle maturation, and formation of
corpora lutea (Fazekas, 1949). There was also enlargement of the
uterus, hypertrophy of the teats, and secretion of milk.
Rabbits (10 - 80 per group) were given 0.1 - 0.2 g/kg body
weight of an ammonium salt in 100 - 150 ml of drinking-water, twice
daily. One hundred and sixty rabbits (96 female, 64 male)
received, twice daily, 50 - 80 ml of a 0.5% ammonium hydroxide
solution (~135 - 210 mg/kg body weight), in gradually increasing
doses. The chemicals were given for periods of 3 weeks separated
by 1-week intervals of no treatment. In a related study, similar
treatment of rabbits with ammonium chloride or ammonium sulfate
resulted in fluctuations in serum-calcium and -phosphorus levels
(Fazekas, 1954a).
8.2.2.2. Effects of ammonium as a dietary nitrogen supplement
Ammonia from ammonium salts can stimulate the growth of animals
on diets deficient in non-essential amino acids or restricted in
protein content. Weanling rats, given an artificial diet
comprising essential amino acids, B vitamins, vitamin C, salts, and
glucose for 21 days, grew poorly. Rats given a similar diet in
which some of the glucose was replaced with 8.6% ammonium acetate
(equivalent to 15.7 g nitrogen/kg diet) showed a dramatically
improved weight gain (Birnbaum et al., 1957). The food
consumption/weight gain ratio (feed/gain ratio) was also improved
in animals receiving the ammonium salt.
Similar results were obtained with young male pigs given a more
natural basal diet restricted in non-essential amino acids and
containing the minimum requirement of essential amino acids (Kagota
et al., 1979). The crude protein level of this basal diet was
6.4%. Three pigs fed the basal diet supplemented with 3.75%
diammonium citrate had a significantly greater weight gain and a
slightly lower feed/gain ratio than 4 pigs fed the basal diet (the
diets were made isocaloric by adjusting carbohydrate). No
significant differences in the packed cell volume or ammonia-
nitrogen level of the blood or in total protein concentration of
plasma were found between the 2 groups. Plasma- and urine-urea-
nitrogen were increased in the pigs fed the diet supplemented with
diammonium citrate. Autopsy of the pigs after 28 days on the basal
or ammonium citrate diets did not reveal any lesions or
abnormalities or any differences in the percentage of carcass meat
and fat. The average daily food intake for pigs receiving
diammonium citrate was 1.44 kg, which corresponds to 54 g
diammonium citrate per day. Using a body weight midway between the
initial (22.5 kg) and final (36.7 kg) body weights, the mean intake
of diammonium citrate can be estimated to be 1.82 g/kg body weight,
per day, or 16.1 mEq NH4+/kg body weight, per day.
Addition of ammonium salts to diets with a higher protein
content (10% crude protein) did not produce significant changes in
weight gain in rats or pigs (Clawson & Armstrong, 1981).
Similarly, no significant changes in weight gain occurred in pigs,
when up to 10% of the nitrogen from crude protein (diet = 16% crude
protein) was replaced with nitrogen from ammonium salts (Wehrbein
et al., 1970). Replacement of 20% of the dietary nitrogen with
ammonium salts slightly decreased body weight gain in the pigs;
food consumption and BUN also decreased. The 20% replacement diet
contained 1.54% diammonium phosphate and 2.07% diammonium citrate
for a total of 3.61% ammonium salts and was fed for 81 days. The
estimated mean intake was 0.70 g diammonium phosphate and 0.94 g
diammonium citrate/kg body weight per day, giving a total of
18.9 mEq NH4+/kg body weight per day.
8.2.3. Dermal exposure
There is no information regarding systemic toxicity from short-
term dermal exposure to ammonia or ammonium compounds.
8.3. Skin and Eye Irritation; Sensitization
Ammonia in the form of a gas, an anhydrous liquid boiling at
low temperatures, or an aqueous solution is a recognized skin and
eye irritant. Most of the information is human clinical data and
is described in section 9.
Ammonia, partly because of its lipid solubility, penetrates
the corneal membrane rapidly (NRC, 1979). In rabbits, conjunctival
oedema with ischaemia and segmentation of limbal vessels were noted
within 30 min. By 24 h, there was a reduction in the
mucopolysaccharide contents of the corneal stroma, and extensive
polymorphonuclear infiltration and anterior lens opacities were
apparent. Aqueous levels of glucose and ascorbate and intraocular
pressure were depressed (NRC, 1979). In rabbits with corneal
burns, neovascularization occurred after 1 week, but it was delayed
in animals with corneal and limbal burns. Complications of severe
burns included symblepharon, pannus, pseudopterygia, progressive or
recurrent corneal ulcerations leading to perforations, permanent
corneal opacity, corneal staphyloma, persistent iritis, phthisis
bulbi, secondary glaucoma, and dry eye (NRC, 1979).
Ammonium persulfate is a recognized skin sensitizer for human
beings. There are no data on its sensitization potential in animal
models.
8.4. Long-Term Exposures
8.4.1. Inhalation exposure
Weatherby (1952) exposed 12 guinea-pigs to an ammonia
concentration of about 119 mg/m3 (170 ppm). Chamber concentrations
ranged from 98 to 140 mg/m3 (140 to 200 ppm) for 6 h/day, 5 days a
week, for up to 18 weeks. There were no significant findings at
autopsy in animals sacrificed after 6 or 12 weeks of exposure. In
animals sacrificed after 18 weeks of exposure, there was congestion
of the liver, spleen, and kidneys, with early degenerative changes
in the adrenal glands. Increased erythrocyte destruction was
suggested by increased quantities of haemosiderin in the spleen.
In the proximal tubules of the kidneys, there was cloudy swelling
of the epithelium and precipitated albumin in the lumen with some
casts. The cells of the adrenal glands were swollen and the
cytoplasm in some areas had lost its normal granular structure.
Coon et al. (1970) conducted studies in which rats, guinea-
pigs, rabbits, dogs, and monkeys were continuously exposed
(24 h/day, 7 days per week) to ammonia. No signs of toxicity were
seen in any species following continuous exposure to ammonia at a
concentration of 40 mg/m3 (57 ppm) for 114 days, and gross and
microscopic examination did not reveal any lung abnormalities.
8.4.2. Oral exposure
The administration of ammonium carbonate, chloride, sulfate,
hydrophosphate, acetate, lactate, or hydroxide to a total of 296
rabbits for 3 - 16 months resulted in enlargement of the
parathyroids (Fazekas, 1954a). Administration of sodium
dihydrophosphate, sodium ammonium phosphate, calcium chloride,
hydrochloric acid, acetate acid, or lactic acid gave similar
results (Fazekas, 1954a).
Rabbits given 0.1 g ammonium hydroxide/kg body weight (as a
0.5 - 1.0% solution) by gavage, initially, on alternate days and
then daily for up to 17 months, had enlarged adrenal glands
(Fazekas, 1939). An initial fall in blood pressure of 2.67 -
4.00 kPa (20 - 30 mmHg) was followed by a gradual rise to 1.33 -
4.00 kPa (10 - 30 mmHg) above the baseline, after several months
of treatment.
The results of studies on rats, rabbits, and dogs indicate that
long-term administration of ammonium chloride can induce
osteoporosis (Seegal, 1927; Bodansky et al., 1932; Jaffe et al.,
1932a,b; Barzel & Jowsey, 1969; Barzel, 1975). During prolonged
metabolic acidosis, the release of bone mineral by resorption is
thought to provide additional buffering capacity, sparing
bicarbonate.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The interaction of ammonium chloride with other teratogenic
agents has been investigated in 2 studies. Goldman & Yakovac
(1964) used ammonium chloride to investigate the role of metabolic
acidosis in salicylate-induced teratogenesis in Sprague Dawley
rats. Beginning on day 7 of gestation, rats received either
0.17 mol/litre (0.9%) ammonium chloride in the drinking-water, a
single subcutaneous injection of salicylate, or both. Ammonium
chloride alone inhibited fetal growth, but was not teratogenic.
However, when administered with salicylate, ammonium chloride
significantly increased maternal and fetal mortality and the fetal
anomaly rate (dorsal midline, ventral midline, and eye defects)
compared with that due to salicylate alone. These effects were
attributed to acidosis and not to ammonia. However, it has been
shown by Miller (1973) that the addition of 0.5% ammonium chloride
to the 5%-glucose drinking-water of fasting, pregnant CFW mice
significantly reduced the incidence of fast-induced cleft palate in
the progeny.
8.6. Mutagenicity
Ammonium sulfate was reported to be non-mutagenic in the
Salmonella and Saccharomyces systems (Litton Bionetics, Inc.,
1975). Demerec et al. (1951) tested ammonia for its ability to
induce back-mutations from streptomycin dependence to non-
dependence in Escherichia coli and found it to be mutagenic, but
only in treatments that left less than 2% survivors. Iwaoka et al.
(1981a,b) showed that extraction of ingredients from fried
hamburger and refrigerated biscuit products with ammonium hydroxide
or ammonium sulfate increased the mutagenic activity in
S. typhimurium TA98 and TA1538, compared with sodium sulfate
extraction. This suggests that ammonium salts may, in some way,
influence the mutagenic activity of some agents, or may themselves
be responsible for false positive findings (Iwaoka et al., 1981a).
However, it is also possible that ammonium salts extract mutagenic
components from foods more efficiently.
Lobashov & Smirnov (1934) and Lobashov (1937) found ammonia to
have a mutagenic effect on Drosophila. Ammonia showed slight
mutagenic activity in studies in which survival of Drosophila was
lower than 2% after treatment. In studies by Auerbach & Robson
(1947), when Drosophila was exposed to ammonia vapour in small
glass containers, 0.5% sex-linked lethals were observed.
It was reported by Rosenfeld (1932) that ammonia induced
clumping of chromosomes, arrest spindle formation, and induce
polyploidy in chick fibroblasts in vitro.
There are no data to show that ammonia is mutagenic in mammals.
8.7. Carcinogenicity
There is no evidence indicating that ammonia is carcinogenic.
Gibson et al. (1971) observed that animals treated with ammonia
developed inflammatory lesions of the colon. It has been suggested
that cell proliferation may increase errors in DNA replication
(Zimber, 1970; Zimber & Visek, 1972), activate oncogenic agents
present in sub-threshold doses (Anderson et al., 1964), and even
unmask latent changes in the genetic material caused by mutagenic
agents (Visek et al., 1978).
In a study on male Sprague Dawley rats (Topping & Visek, 1976),
there was no evidence that ammonia increased the incidence of
tumours with increased protein intake. Development of lung
tumours was observed in CFLP mice treated intra-gastrically with
diethyl pyrocarbonate and ammonia, neither of which induces cancer
independently in animals. However, this may have resulted from a
carcinogenic substance, possibly urethane, formed in vivo from
diethyl pyrocarbonate in the presence of ammonia (Uzvolgyi & Bojan,
1980).
Life-long ingestion of ammonium hydroxide in the drinking-water
by Swiss and C3H mice did not produce any carcinogenic effects, and
had no effect on the spontaneous development of adenocarcinoma of
the breast in C3H females, a characteristic of this strain (Toth,
1972).
Ammonium chloride and ammonium tungstoantimonate and related
compounds have been shown to have an inhibitory effect on malignant
cells (Phillips, 1970; Anghileri, 1975; Flaks & Clayson, 1975;
Flaks et al., 1973; Sof'ina et al., 1978).
8.8. Factors Modifying Effects
8.8.1. Synergistic effects
Stevens et al. (1975) raised weanling rats for 3 - 6 weeks on a
protein-deficient diet. Subsequent challenge by a large,
intraperitoneally-injected dose of ammonium chloride indicated that
severe protein deprivation increases vulnerability to ammonia
poisoning in comparison with control groups not prefed protein-
deficient diets.
Dalhamn (1963) employed a technique in rabbits in which an air
pollutant mixture was drawn into the nostrils and out through a
tracheal cannula. The effects of ammonia alone and ammonia mixed
with (and presumably adsorbed on) carbon particles, on the beating
rate of tracheal cilia was examined. Ammonia alone had to be
administered at a high concentration (1400 mg/m3 or 2000 ppm) to
produce a tracheal concentration of 70 mg/m3 (100 ppm). The
reduction in beating rate was not substantially altered by the
addition of carbon particles at 2 mg/m3.
Simultaneous injection of ammonium salts and a fatty acid into
rats or cats produced coma at lower plasma levels of ammonia and
free fatty acids than a single injection of either compound (Zieve
et al., 1974).
Impaired handling of an ammonium load has been observed in
animals with hepatic dysfunction. Elevated levels of ammonia or
urea have been reported in dogs with experimentally-bypassed livers
(section 7.1.2). The importance of the kidney in detoxification of
an ammonium load is apparent in the studies of Lotspeich (1965)
(section 8.1.2.2), where an additive hypertrophic effect of
ammonium chloride and unilateral nephrectomy was observed on the
remaining kidney. Increased sensitivity to ammonia toxicity was
found in young rats (Benyajati & Goldstein, 1975) and in rats that
had been castrated, adrenalectomized, or thymectomized (Paik et
al., 1975).
8.8.2. Antagonistic effects
L-arginine and the related amino acids, ornithine and aspartic
acid, substrates from urea synthesis, have been reported to exert a
protective effect against acute ammonia poisoning in rats, dogs,
and cats (Morris & Rogers, 1978; NRC, 1979).
High-protein diets exert an antagonistic effect on the toxicity
of ammonium salts, unless liver dysfunction is present (section
7.1.2.4). In addition, an increase in toxic response has been
observed with increase in age due to the adaptive response of
glutaminase activity in rats to ammonia detoxication during
acidosis.
8.9. Mechanisms of Toxicity
Hypotheses proposed for the mechanism of ammonia toxicity
include impaired decarboxylation of pyruvic acid (McKhann & Tower,
1961), NADH depletion slowing down the generation of ATP (Worcel &
Erecinska, 1962), depletion of alpha-ketoglutarate resulting in
impairment of the Krebs cycle (Bessman & Bessman, 1955; Fazekas et
al., 1956; Warren & Schenker, 1964); depletion of ATP due to
glutamine formation by the glutamine synthetase (EC 6.3.1.2) system
(Warren & Schenker, 1964; Nakazawa & Quastel, 1968), stimulation of
membrane ATPase (EC 3.6.1.8) producing increased nerve cell
excitability and activity (Hawkins et al., 1973), and depletion of
ATP causing a decrease in cerebral acetylcholine (Braganca et al.,
1953; Ulshafer, 1958).
9. EFFECTS ON MAN
9.1. Organoleptic Aspects
9.1.1. Taste
Campbell et al. (1958) determined the taste threshold
concentration for ammonia in redistilled water with 21 - 22
subjects in "difference tests of the triangle type". At ammonia
concentrations of 26, 52, and 105 mg/litre, the percentages of
correct identifications were 61.9, 71.4, and 85.7, respectively.
Defining the threshold concentration as the level at which
correct identification is 50% greater than that expected by
chance, the taste threshold of ammonia was determined to be
around 35 mg/litre. However, this definition of the threshold
concentration seems to be somewhat arbitrary and McBride & Laing
(1979) have reported significant positional bias in using the
triangle test to determine taste thresholds. Furthermore, the
triangle test is not intended to mimic environmental exposures
in which the taste thresholds could be substantially higher. It
seems reasonable to conclude only that the palatability of water
is not likely to be significantly affected by total ammonia levels
of < 35 mg/litre (as NH3), but will be affected at higher levels.
9.1.2. Odour
Odour thresholds reported in the literature may vary according
to the definition of odour response, mode of presentation of the
stimulus, chemical purity of the agent used, and the number of
subjects and trials in the study. The detection threshold for
ammonia, defined as the concentration that produces the first
detectable difference in odour over background, was reported to be
37 mg/m3 (DallaValle & Dudley, 1939). This reference, though often
cited, gives no information on the study design from which this
number was derived.
The considerable variability in threshold data prompted work by
Leonardos et al. (1969), who used a standardized procedure to
determine recognition thresholds rather than detection thresholds
for 53 chemicals. The odour threshold was defined as the first
concentration at which all four panel members (trained odour
analysts) were able to recognize the characteristic odour of the
chemical. The panel tested only one chemical per day.
Concentrations examined were multiples by 10 of 0.7, 1.47, and
3.3 mg/m3 (1, 2.1, and 4.6 ppm). The recognition threshold for the
odour of ammonia was 32.6 mg/m3 (46.8 ppm).
The results of several other studies suggest that human beings
can detect ammonia at much lower levels. Stephens (1971) reported
2.7 mg/m3 (3.9 ppm) as the lowest concentration producing an odour
response, when a contaminated air stream and a reference stream
were compared by sniffing (number of subjects was not reported). A
report by Saifutdinov (1966), of an olfactory threshold of 0.55 -
0.50 mg/m3 for the most sensitive of 22 subjects, did not include
sufficient detail to evaluate this excessively low estimate.
Carpenter et al. (1948) stated that "a group of 8 persons found
that the least odour they could detect on entering a room
containing various concentrations" was 0.7 mg/m3 (1 ppm) ammonia.
Again, no other details were given.
The best estimates of the thresholds at which ammonia can be
expected to be detected by taste and odour are 35 mg/litre (as NH3)
and 35 mg/m3, respectively. Sensitive individuals may detect
concentrations an order of magnitude lower.
9.2. Clinical and Controlled Human Studies
9.2.1. Inhalation exposure
The severe effects resulting from acute exposure to ammonia
have been described in case reports of accidents involving groups
of people or individuals. There are no data on actual levels of
ammonia in air during such accidents, but estimates have been made
(Yahagi et al., 1959; Takahashi et al., 1984; NRC, 1979).
Exposure to an ammonia concentration of 280 mg/m3 (400 ppm) has
been reported to produce immediate throat irritation; 1200 mg/m3
(1700 ppm) to produce cough; 1700 mg/m3 (2400 ppm) to be life-
threatening, and 3500 - 7000 mg/m3 (500 - 10 000 ppm) to cause a
high mortality rate (Patty, 1963; Helmers et al., 1971).
A burning sensation in the eyes, nose, and throat, as well as
respiratory distress accompanied by lachrymation, coughing, and an
increase in respiratory rate are some of the irritant effects of
ammonia (Caplin, 1941). Chest X-rays are generally normal in such
mild cases (Watson, 1973; Close et al., 1980). More severe
respiratory effects include laryngeal and pulmonary oedema and
bronchopneumonia (Slot, 1938; Caplin, 1941; Levy et al., 1964;
Taplin et al., 1976; Flury et al., 1983). The signs and symptoms
are generally reversible, but chronic bronchitis and bronchiectasis
have been reported (Slot, 1938; Sugiyama et al., 1968; Taplin et
al., 1976; Close et al., 1980).
In cases with a lethal outcome, the cause of death has been
severe lung damage and secondary cardiovascular effects (Slot,
1938; Mulder & van der Zalm, 1967).
There have also been some studies on volunteers exposed to
ammonia under laboratory conditions. Some of these studies are
summarized in Table 21.
Silverman et al. (1949) reported on 7 human volunteers exposed
to a concentration of 300 mg/m3 (500 ppm) ammonia for 30 min using
an oral-nasal mask. All 7 experienced upper respiratory
irritation, which lasted up to 24 h in 2 of the volunteers. Two
subjects experienced marked lachrymation, in spite of the exposure
being by oro-nasal mask. The average respiratory minute volume
increased markedly compared with control values, and in the 5
subjects in which minute-by-minute expired volumes were measured,
there was a marked cyclical variation in minute volume with a
period of 4 - 7 min. No coughing was noted.
After exposure, respiratory minute volumes fell to levels below
the pre-exposure rate, but returned to pre-exposure values within 5
min of exposure. Ammonia retention decreased progressively until
an equilibrium of 24% retention (ranging from 4 to 30%) was reached
at approximately 19 min (range 10 - 27 min). The indices of
nitrogen metabolism (BUN, NPN, urine-urea, and urine-ammonia)
remained normal. The carbon dioxide combining power did not
change.
Verbeck (1977) assessed respiratory function in 16 volunteers
following exposure to 35, 56, 77, and 98 mg ammonia/m3 (50, 80,
110, and 140 ppm) for 0.5, 1, and 2 h. The respiratory variables of
vital capacity (VC), forced expiratory volume (FEV), and forced
inspiratory volume (FIV), measured before and after exposure, did
not decrease by more than 10%. Subjective variables, including
smell, taste, irritation of the eyes, nose, or throat, urge to
cough, headache, and general discomfort were monitored every 15 min
and ranked by 8 experts and 8 non-experts (students) on a scale
from 0 to 5. Subjective responses were ranked higher by the non-
experts. A concentration of 77 mg/m3 (110 ppm) was tolerated for
2 h, but at 98 mg/m3 (140 ppm), all the subjects left the chamber
because the exposure was intolerable.
Respiratory responses to ammonia during exercise were examined
by Cole et al. (1977). Eighteen males, aged 18 - 39 years, had 2
periods of exercise in 3 consecutive half-day sessions. Mean
exposure levels were 71, 144, 106, and 235 mg/m3 (101, 206, 151,
and 336 ppm). Ventilation minute volume and total volume
decreased, and mean respiratory frequency increased at exposure
levels > 106 mg/m3 (151 ppm).
In a study by MacEwen et al. (1970), 6 volunteers were exposed
to ammonia concentrations of 21 and 35 mg/m3 (30 and 50 ppm) for 10
min. The irritation was rated subjectively on a scale of 0 - 4.
At 35 mg/m3 (50 ppm), irritation was rated as "moderate" by 4 of
the volunteers, while 1 individual reported no detectable
irritation. None of the volunteers found the irritation at
35 mg/m3 (50 ppm) to be "discomforting" or "painful".
The irritation threshold for ammonia was examined in 10 human
volunteers by Industrial Bio-Test Laboratories, Inc. (1973). The
subjects were exposed to 4 different concentrations of 22, 35, 50,
and 94 mg/m3 (32, 50, 72, and 134 ppm) for 5 min. The frequency of
positive findings for the 10 subjects was as follows: at 22 mg/m3,
1 subject complained of dryness of the nose; at 35 mg/m3, 2
subjects experienced dryness of the nose; at 50 mg/m3, 3 subjects
had eye irritation, 2 had nasal irritation, and 3 had throat
irritation; at 94 mg/m3, 5 subjects demonstrated signs of
lachrymation, 5 had eye irritation, 7 had nasal irritation, 8 had
throat irritation, and 1 complained of chest irritation.
Table 21. Effects of inhaled ammonia in human volunteers
--------------------------------------------------------------------------------------------
Number Concentration Duration Effects and response Reference
(mg/m3) (min)
--------------------------------------------------------------------------------------------
7 300 30 irritation of upper respiratory Silverman et al. (1949)
tract (7/7); increase in minute
volume; no change in blood
chemistry
16 35 - 98 30 - 120 10% increase in VC, FEV, and Verbeck (1977)
FIV; 98 mg/m3 was not tolerated;
77 mg/m3 was tolerated for 2 h
18 71 - 235 8 - 11 no effects noted during exercise Cole et al. (1977)
at 71 mg/m3 except slight
irritation; decrease in
ventilation minute volume and
tidal volume and increase in
respiratory frequency > 106
mg/m3
6 35 10 "moderate irritation" (4/5) MacEwen et al. (1970)
10 22 5 dryness of the nose (1/10) Industrial Biotest
10 35 dryness of the nose (2/10) Laboratories (1973)
10 50 eye irritation (3/10)
10 94 throat irritation (8/10)
eye irritation (5/10)
6 17.5, 35, 2 - 6 h, increase in FEV1, with Ferguson et al. (1977)
or 70+ 5 days/ increasing NH3; adaptation
week, for to irritating effects
6 weeks
--------------------------------------------------------------------------------------------
A controlled study on human volunteers was conducted by
Ferguson et al. (1977) to evaluate the responses to inhaled ammonia
at concentrations of 17.5, 35, and 70 mg/m3 (25, 50, and 100 ppm).
Six adults, not acclimatized to ammonia exposure, were divided into
3 groups, which were exposed for 2 - 6 h each day, 5 days per week,
for 6 weeks. One pair of subjects was exposed only to 35 mg
ammonia/m3 for 6-h periods throughout the test. Subjects were
examined daily for irritation of the eyes, nose, or throat, and
periodically for pulse rate, respiration rate, pulmonary function
(forced vital capacity (FVC) and forced expiratory volume in 1
second (FEV1)), blood pressure, neurological responses, and
interference in task-performance ability. A statistical analysis
of the results demonstrated that the only significant change among
the vital functions measured was an increase in forced expiratory
volume in 1 second (FEV1) with increasing ammonia concentration.
In addition, the rate of mild eye, nose, or throat irritations over
the last 3 weeks of the test was significantly less than during the
first 2 weeks. This indicated that an acclimatization process had
occurred, with increased tolerance to the irritant effects of
ammonia developing with increasing time of exposure. Overall, the
ammonia exposure produced 2 incidents of mild irritation (78
observations made) at 17.5 mg/m3 (25 ppm), 22 incidents (198
observations made) at 35 mg/m3 (50 ppm), and 11 incidents (84
observations made) at 70 mg/m3 (100 ppm). Among control subjects,
4 irritation incidents were recorded during 45 observations. When
ammonia concentrations exceeded 105 mg/m3 (150 ppm), all subjects
experienced lachrymation accompanied by dryness of the nose and
throat.
In an inhalation study, the threshold for effects on
respiration, skin electric potential, and the electroencephalogram
was found to be 22 mg/m3 (31 ppm) (Alpatov & Mikhailov, 1963;
Alpatov, 1964).
Reports from industries with ammonia exposure indicate that
irritative effects have appeared over a wide range of ammonia
concentrations (Elkins, 1950; Vigliani & Zurlo, 1955; Mangold,
1971). A concentration of 88 mg/m3 was called "definitely
irritating" (Elkins, 1950), and "barely noticeable" eye irritation
was reported at 3 mg/m3 (Mangold, 1971).
Giguz (1968), in a study involving 140 subjects exposed to
ammonia and nitrogen oxides, at concentrations not exceeding the
"maximum permissible concentration" (20 mg/m3), 3 h per day during
2 - 3 years of vocational training, demonstrated increased
incidences of upper respiratory tract disease, compared with those
in a control group of unexposed subjects.
9.2.2. Oral exposure
9.2.2.1. Effects of acute oral exposure
Cases of the ingestion of large doses of ammonia have been
reported. When solutions of ammonia were ingested orally, a
tissue-destructive caustic effect was noted for concentrated
solutions, owing to their high pH. A solution of ammonia at a
concentration of 100 g/litre, for example, has a pH of 12.5.
Oesophageal burns were reported in 25 cases of accidental
ammonia ingestion (Hawkins et al., 1980). One child suffered a
mild burn. Four adults had mild burns limited to the mucosa and
nine adults had oesophageal burns that were moderate or more severe
in nature. One adult female suffered from a complication of airway
obstruction from supraglottic oedema. Oesophageal stricture from
the ingestion of ammonia (100 g/litre) was reported by Vancura et
al. (1980) (Table 19).
A fatal outcome after ingestion of a solution containing 24 g
ammonia/litre was reported by Klendshoj & Rejent (1966). Autopsy
showed haemorrhagic inflammatory changes in the oesophagus,
stomach, and small intestine.
There are many reports on the effects of ammonium chloride, but
since acidosis is caused by the chloride, such studies have little
relevance for evaluating ammonia toxicity.
There are no data on acute effects of ingestion of ammonium
compounds, other than the chloride.
9.2.3. Endogenous hyperammonaemia
9.2.3.1. Inborn errors of metabolism
These affect the uptake of ammonia rather than its rate of
production.
Congenital deficiency of carbamyl phosphate synthetase I (EC
6.3.4.1.6), and, to a lesser extent, of other enzymes of the
ornithine cycle, and several other metabolic disorders may lead to
hyperammonaemia and various abnormal urinary constitutents.
Hyperammonaemia may be lethal in new-born infants, may cause severe
symptoms in infancy, or may cause chronic remittent symptoms in
older children or adults (Hsia, 1974).
Clinical features in neonates may resemble those of hepatic
coma and may be precipitated by protein-rich milk feeds. In older
children, episodic vomiting, neurological disorders (including
seizures) or coma are precipitated by high-protein foods.
9.2.3.2. Hepatic features
Varied and complex functions of the liver may fail
progressively in chronic liver disease or rapidly in acute
disorders. The syndromes differ in the extent to which portal
blood from the intestine is shunted into the systemic venous
circulation either by cirrhotic changes in hepatic vascular
resistance or by surgical procedures to correct portal hypertension
resulting from it.
In either case, a syndrome is recognised that comprises a
spectrum of neurological features from irritability via
inappropriate behaviour, tremors, hyperreflexia and generalised
muscular rigidity, to delirium, stupor, convulsions and coma.
There is disagreement regarding the extent to which this
syndrome is an expression of ammonia toxicity. On the one hand,
over 90% of persons with the disorder have elevated levels of
ammonia in the blood or cerebrospinal fluid; the condition may be
induced in those with marginal liver function by the administration
of ammonium salts or an intestinal haemorrhage (which leads to
intestinal ammonia production greater than that from an equivalent
amount of meat) and the condition may be treated by reducing
intestinal ammonia production, by the administration of antibiotics
to eliminate ammonia-producing flora. On the other hand, there is
an imperfect correlation between the clinical state and the blood-
ammonia level and the condition may occur in the presence of normal
blood-ammonia levels.
It is unlikely that the syndrome resulting from the failure of
an organ as complicated as the liver should be explicable in terms
of a single metabolic component, but the similarity between hepatic
failure and certain expressions of congenital hyperammonaemias
suggests an important role of ammonia toxicity in the pathogenesis
of the syndrome.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Atmospheric Exposure and Effects
10.1.1. General population exposure
Background levels of ammonia in community air are low in
comparison with levels that have been established as safe in
occupational settings, but there is considerable variabilty
according to the type of land use. In general, levels in areas
with intensive livestock husbandry or high rates of manure
application are in the range of 100 - 200 µg/m3, levels in urban
areas range from approximately 5 to 40 µg/m3, while those in rural
areas, without intensive manure production or use, range up to
10 µg/m3. This is in contrast to odour thresholds of the order
10 000 µg/m3, thresholds for irritation of the order 20 000 -
50 000 µg/m3, and the estimated LC for man of 5000 - 10 000 mg/m3.
The LC50 estimation for the rat was 7 600 000 µg/m3, for a 2-h
exposure. In an occupational setting, workers did not voluntarily
use respiratory protective devices at concentrations below about
500 000 µg/m3. General ambient atmospheric levels are therefore
of no concern in respect of discomfort or acute toxicity.
Ammonia is not mutagenic and long-term studies on both
laboratory and farm animals have not shown any pathological effects
at levels below 35 000 µg/m3. Long-term toxic effects are
unlikely, even at levels much higher than ambient levels, both
generally and in the neighbourhood of ammonia-emitting systems.
There is a lack of evidence regarding recent trends in global
atmospheric ammonia concentrations.
10.1.2. Occupational exposure
Occupational problems are predominantly those of accidental
exposure.
Though for certain groups in, for example, agriculture, the
chemical industry, waste disposal and transport, occupational
exposure levels may be very much higher than general, there is
historical evidence that, even at levels significantly in excess of
current occupational exposure limits, there was a low prevalence of
adverse health effects.
The distribution kinetics of absorbed atmospheric ammonia
suggest that the rise in blood-ammonia at a typical occupational
exposure limit will be within the normal range of variation.
Thus, there is both historical and theoretical evidence that
most recommended exposure limits are acceptable.
10.2. Exposure Through Water and Food
Ammonia will have a toxic effect, only if intake exceeds the
capacity of mammals to detoxify ammonia. Unfortunately, there are
no data permitting the evaluation of this capacity in healthy human
beings or other terrestrial mammals. In addition, there is some
evidence that the mode of intake may be a factor in the capacity of
individuals to detoxify ammonia. Parenteral administration of
ammonia results in patterns of metabolism and elimination that are
markedly different from those seen in oral administration. Thus,
the considerable body of data on the parenteral toxicity of ammonia
is not of direct relevance to criteria for oral exposure.
Similarly, because of the different kinetic patterns between oral
and inhalation exposure to ammonia, as well as the highly irritant
effects of ammonia on the lung, the available inhalation data on
ammonia are not applicable to the estimation of an acceptable daily
intake (ADI) for ingestion. However, it would be possible to
define a clearly undesirable level of oral exposure to total
ammonia (NH3 and NH4+) as well as a level that is clearly
tolerable. The range between these 2 levels would exceed the range
within which an ADI could be established.
The amount of excess ammonia (i.e., over and above the amount
normally produced in the body) that can be safely ingested and
assimilated is difficult to define. In short-term (28 - 90 days)
studies carried out on rats and pigs, no adverse effects were
reported at higher levels of ammonia intake (75 - 545 mg NH3/kg
body weight per day) in the form of sulfamate, phosphate, citrate,
or chloride.
The effects attributed directly to elevated ammonium ion levels
are acute pulmonary oedema and central nervous system (CNS)
toxicity, depression of appetite due to a direct effect of the
ammonium ion on the brain, and promotion of growth via the use of
ammonium salts as a source of non-essential nitrogen under certain
circumstances.
Some effects (such as renal growth and demineralization of
bone) arising from the administration of ammonium chloride seem to
be secondary effects of acidosis.
Surveys of total ammonia (NH3 + NH4+) concentrations in surface
waters indicate an average of < 0.18 mg/litre in most areas, and
0.5 mg/litre in waters near large metropolitan areas (Wolaver,
1972; US EPA, 1979a). Levels in ground water are usually low,
since ammonia is generally immobile in soil. Ammonia is practically
absent when drinking-water is chlorinated.
Ammonia is a negligible natural constituent of food, but
ammonium compounds are added in small amounts (< 0.001 - 3.2%) to
various foods as stabilizers, leavening agents, flavourings, or for
other purposes. The daily human intake from these sources is
estimated to be 18 mg as NH3.
10.3. Ocular and Dermal Exposure
Ammonia in aqueous solution or in contact with body fluids is
alkaline and causes burns or inflammation of eyes or skin. The
ocular irritation commonly experienced at atmospheric ammonia
concentrations of > 20 mg/m3 (MacEwen et al., 1970; Keplinger et
al., 1973; Verberk, 1977) is readily reversible when exposure
ceases, and may also be reduced by acclimatization (Ferguson et
al., 1977).
Serious ocular damage normally occurs, only with a direct blast
or splash contact with anhydrous or aqueous ammonia (Grant, 1974).
Skin damage is reported to occur at concentrations of ~7000 mg/m3
(NRC, 1979).
10.4. Accidental Exposure
High gaseous ammonia concentrations may be encountered locally,
both in domestic and work-place environments, as a result of gaseous
emissions and/or spillages of concentrated solutions, and
respiratory (and skin and eye) injury may result. On a larger
scale, spillage from stock or transport tanks or refrigeration
plant of concentrated ammonia liquor or anhydrous ammonia would
constitute a severe environmental insult and would cause serious
injury to the people, animals, and plants in the vicinity. Because
of its low density and short biopersistence, major spillages would
be expected to disperse rapidly and not to persist in the
environment.
10.5. Evaluation of Risks for the Environment
Environments that receive more ammonia than can be used may be
acidified and nitrogen-enriched. As a consequence of these
physical-chemical changes, the structure and functioning of the
ecosystem will be disturbed.
Ammonia plays an important role in the metabolism of all
organisms as a nutrient at low concentrations, but becomes toxic at
higher concentrations. For example, microorganisms both assimilate
and generate ammonia as a part of natural nutrient cycling
processes. High levels of ammonia may inhibit growth or survival
of microbial organisms, including, at higher levels, nitrifying
organisms.
10.5.1. The aquatic environment
Ammonia concentrations in the aquatic environment are variable,
reflecting the proximity and nature of sources (section 4).
Where there are large numbers of people and animals in relation
to the volume of surface waters and drainage flow, the load of
nitrogen added to surface waters from sewage and industrial
effluent is the predominant source and may lead to ammonia
concentrations that constitute a significant local and/or regional
environmental problem.
Otherwise, ammonia deposition contributes a major input. In
surface waters that are poorly buffered, poor in nutrients, and
hydrologically dependent on rainfall and/or snow melt, this may
result in acidification and nitrogen enrichment resulting in marked
changes in plant community structure with concommitant changes in
the animal population structure.
The toxic effects on aquatic organisms are attributed to non-
ionized ammonia (NH3) rather than to ammonium ion levels. This is
because non-ionized ammonia easily penetrates biological membranes,
whereas ammonium ions require specialized transport processes.
There are similarities between the modes of the acute toxic
action of ammonia in mammals and in fish: in the latter, however,
environmental conditions (such as pH, ionic composition and
concentration, temperature, and oxygen availability), which are
more variable in water than in air, have a marked modulating
effect.
Aquatic animals have a limited ability to detoxify ammonia and,
therefore, the body load is dependent on ammonia concentrations in
the water. Except in open oceans, exposure to environmental levels
produces many chronic effects (including reduced growth, decreased
survival, impaired reproduction) and may increase susceptibility to
disease and also cause histopathological changes.
High levels of ammonia in aquatic systems are also toxic for
plants. The detoxification of excessive ammonia places a severe
strain on the carbohydrate metabolism of the plant which
subsequently results in foliar injury, and growth effects, and thus
may modify plant community composition.
Where the dominant species, be it fish or plant, is also
sensitive to ammonia the effects on the whole ecosystem will be
marked.
10.5.2. The terrestrial environment
The most common effect of exposure of plants to atmospheric
ammonia is foliar injury. Prolonged exposure to high ambient
concentrations of about 75 µg/mg, such as occur in the vicinity of
intensive livestock farms, adversely affects more susceptible
species such as pine trees. The observed damage is the result of
both direct and indirect effects due to, changes in soil, and
secondarily, to increased susceptibility to disease and
meteorological stress.
The data on ammonia toxicity for wildlife are very limited.
There is no evidence that wildlife populations, in general, are at
risk from ammonia, but, there may be secondary effects associated
with changes in plant communities. Certain species of bats are
able to withstand the very high ammonia levels found in caves where
they live.
10.6. Conclusions
The major groups of organisms at risk from elevated ammonia
levels are aquatic animals and terrestrial plants. There appears
to be little danger for terrestrial animals, including man, except
from acute accidental exposure.
10.6.1. General population
There are no data suggesting that present environmental levels
of ammonia are hazardous for the general population. Only high-
level accidental exposures from domestic sources and transportation
and storage accidents pose an occasional acute health hazard.
10.6.2. Sub-populations at special risk
Groups likely to exhibit ammonia toxicity include those with
hepatic or renal impairment, though, even in these cases, levels of
exogenous ammonia are insignificant in comparison with endogeneous
levels, so that, in the absence of any environmental exposure, such
persons would still be affected. The mechanism is different in the
two cases. Hepatic impairment limits the conversion of ammonia to
urea, and renal failure, by increasing urea concentrations and its
intestinal secretion, leads to increased endogeneous intestinal
ammonia production.
There have been few studies on the chronic effects of ammonia
inhalation. It can be speculated that subpopulations that have
been found to be hyperreactive to other respiratory irritants
(e.g., sulfur dioxide, particulates, ozone) may also be
hyperreactive to ammonia. These subpopulations may include
children, elderly persons with pre-existing cardiorespiratory
symptoms, individuals with asthma or bronchitis, and those engaged
in vigorous physical exercise (Calabrese, 1978). However, there is
also some indication that previous exposure to low levels of
ammonia may cause inurement to its effects (Ferguson et al., 1977).
10.6.3. Occupational exposure
Accidental exposures are the predominant problem (section
10.1.1.3). Otherwise, occupational exposure can be controlled by
the application of most current occupational exposure limits and
proper industrial hygiene.
10.6.4. Farm animals
Farm animals may be at risk, because of continous exposure
under confined housing conditions resulting in high atmospheric
levels of ammonia within the confinement areas; this applies
particularly to cattle, swine, and poultry. Available reported
data provide a range of measured exposure levels of from 2 to
1400 mg/m3. In winter months in colder climates, most of the
measured concentrations exceeded the admissible upper limit of
35 mg/m3 (50 ppm).
10.6.5. Environment
Environments with a low buffer capacity and poor in nutrients
are susceptible to acidification and nitrogen enrichment by
elevated ammonia loading; prolonged high ammonia-loading results in
changes in both the structure and function of plant and animal
communities. Levels of atmospheric ammonia necessary for the onset
of these changes have not been established; however, changes in the
structure of these communities have been observed where ammonia
levels in the atmosphere were possibly up to 100 µg/m3.
Plants use ammonia as a nutrient, but high levels can be toxic.
Terrestrial plants show a susceptibility to reduced growth and
reduced vitality, when exposed to levels as low as 75 µg NH3/m3 in
the atmosphere.
Aquatic animals have a low capacity to detoxify ammonia. Acute
effects on some fish have been demonstrated in laboratories at
concentrations as low as 0.1 mg NH3/litre and chronic effects at
concentrations as low as 0.02 mg NH3/litre. Thus, as ammonia
levels in some waters are often similar to those shown to cause
chronic effects in some fish, it would appear that these animals
are at risk. Aquatic invertebrates are, in general, less sensitive
to elevated ammonia levels in water.
Aquatic animals are at risk because of increases in ammonia
concentrations in water systems, whereas some plant communities
appear to be at risk from elevations in atmospheric ammonia
loading.
11. RECOMMENDATIONS
11.1. Research Needs
1. Long-term monitoring of ammonia and other pollutants in
water systems with different aquatic ecosystems.
2. Studies of the long-term effects of ammonia on terrestrial
vegetation.
3. Studies of the global nitrogen balance to identify long-
term trends.
4. Ecotoxicological studies to elucidate environmental
effects.
5. Epidemiological studies in relation to ammonia exposure in
order to make better hygiene recommendations.
6. Long-term experimental animal studies to establish a no-
observed-adverse-effect level of exposure.
7. Studies on the role of ammonia in modifying physical and
chemical conditions of soil and water systems.
8. More data are needed to assess accurately the relative
contributions of various point and non-point sources of
ammonia for surface waters.
9. Research into methods and their application directed
towards reducing emissions from point sources.
10. Additional acute toxicity tests with salt-water fish and
invertebrate species.
11. Life-cycle and early-life-stage tests with representative
fresh-water and salt-water organisms from different
families, with investigation of pH effects on chronic
toxicity.
12. Fluctuating or intermittent exposure tests under a variety
of exposure patterns on additional species.
13. Both acute and long-term tests at cold-water temperatures.
14. Studies on the effects of dissolved and suspended solids
on acute and chronic toxicity.
15. More histopathological and histochemical research with
fish, which would provide a rapid means of identifying and
quantifying sublethal ammonia effects.
16. In fish, the relative concentration limits for both
acclimatization and subsequent acute response need better
definition and a more complete explanation.
REFERENCES
ABBAS, R. & TANNER, R.L. (1981) Continuous determination of
gaseous ammonia in the ambient atmosphere using fluorescence
derivatization. Atmos. Environ., 15: 277-281.
ABELOVICH, A. & AZOV, Y. (1976) Toxicity of ammonia to algae
in sewage oxidation ponds. Appl. environ. Microbiol., 31(6):
801-806.
ADMIRAAL, W. (1977) Tolerance of estuarine benthic diatoms
to high concentrations of ammonia, nitrite ion, nitrate ion
and orthophosphate. Mar. Biol., 43(4): 307-315.
AGAMI, M., LITAV, M., & WAISEL, Y. (1976) The effects of
various components of water pollution on the behaviour of some
aquatic macrophytes of the coastal rivers of Israel. Aquat.
Bot., 2(3): 203-213.
ALABASTER, J.S. & HERBERT, D.W.M. (1954) Influence of carbon
dioxide on the toxicity of ammonia. Nature (Lond.), 174(4426):
404.
ALABASTER, J.S., SHURBEN, D.G., & KNOWLES, G. (1979) The
effect of dissolved oxygen and salinity on the toxicity of
ammonia to smolts of salmon, Salmo salar L. J. Fish Biol.,
15(6): 705-712.
ALLAN, I.R.H. (1955) Effects of pollution on fisheries.
Verh. Int. Ver. Theor. Agnew Limnol., 12: 804-810.
ALLAN, I.R.H., HERBERT, D.W.M., & ALABASTER, J.S. (1958) A
field and laboratory investigation of fish in a sewage
effluent. Fish. Invest., 6(2): 1-76.
ALPATOV, I.M. (1964) [A study of gazeous ammonia toxicity.]
Gig. Tr. prof. Zabol., 2: 14-18 (in Russian).
ALPATOV, I.M. & MIKHAILOV, V.I. (1963) [Inquires into the
gaseous ammonia toxicity. Communication 1.] Gig. Tr. prof.
Zabol., 12: 51-53 (in Russian)
ANALYTICAL QUALITY CONTROL (HARMONISED MONITORING) COMMITTTEE
(1982) Accuracy of determination of ammoniacal nitrogen in
river waters: analytical quality control in the harmonised
monitoring scheme. Analyst, 107(June): 680-688.
ANDERSON, B.G. (1948) The apparent thresholds of toxicity to
Daphnia magna for chlorides of various metals when added to
Lake Erie water. Trans. Am. Fish Soc., 78: 96-113.
ANDERSON, D.P., BEARD, C.W., & HANSON, R.P. (1964a) The
adverse effects of ammonia on chickens including resistance to
infections with Newcastle disease virus. Avian Dis., 8:
369-379.
ANDERSON, D.P., CHERMS, F.L., & HANSON, R.P. (1964b) Studies
on measuring the environment of turkeys raised in confinement.
Poult. Sci., 43: 305-318.
ANDERSON, K.B., SPARKS, R.E., & PAPARO, A.A. (1978) Rapid
assessment of water quality, using the fingernail clam,
Musculium transversum, Urbana, Illinois, Water Resources
Center, University of Illinois, 115 pp (WRC Research Report
No. 133).
ANGHILERI, L.G. (1975) Tumour growth inhibition by ammonium
chloride-induced acidosis. Int. J. clin. Pharmacol., 12: 320.
ANTHONISEN, A.C., LOEHR, R.C., PRAKASAM, T.B.S., & SRINATH,
E.G. (1976) Inhibition of nitrification by ammonia and
nitrous acid. J. Water Poll. Control Fed., 48: 835-852.
APHA (1976) Standard methods for the examination of water
and wastewater, 14th ed., Washington DC, American Public
Health Association, American Water Works Association, Water
Pollution Control Federation, 1193 pp.
API (1981) The sources, chemistry, fate, and effects of
ammonia in aquatic environments, Washington DC, American
Petroleum Institute, 145 pp.
APPELMAN, L.M., TEN BERGE, W.F., & REUZEL, P.G.J. (1982)
Acute inhalation toxicity study of ammonia in rats with
variable exposure periods. Am. Ind. Hyg. Assoc. J., 43(9):
662-665.
ARMSTRONG, D.A., CHIPPENDALE, D., KNIGHT, A.W., & COLT, J.E.
(1978) Interaction of ionized and un-ionized ammonia on
short-term survival and growth of prawn larvae, Macrobrachium
rosenbergii. Biol. Bull., 154(1): 15-31.
ARNDT, F.L., DAY, D.L., & HATFIELD, E. (1979) Processing and
Handling of animal excreta for refeeding. J. anim. Sci., 48:
157.
ASTM (1980) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
AUERBACH, C. & ROBSON, J.M. (1947) Tests of chemical
substances for mutagenic action. Proc. R. Soc. Edinburgh Sec.
Biol., 62B: 284-291.
AVOT, YU.K., KANEP, V.V., SLUTSKER, D.S., & SHAFRAN, L.M.
(1977) [Medical and sanitary aspects of sailors' work,] Riga,
Zvaigzne, 132 pp (in Russian).
AXELROD, H.D. & GREENBERG, J.P. (1976) Determination of
trace concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 10: 495-496.
BAIRD, R., BOTTOMLEY, J., & TAITZ, H. (1979) Ammonia
toxicity and pH control in fish toxicity bioassays of treated
wastewaters. Water Res., 13(2): 181-184.
BALL, I.R. (1967) The relative susceptibilities of some
species of fresh-water fish to poisons. I. Ammonia. Water
Res., 1(11/12): 767-775.
BARROW, C.S. & STEINHAGEN, W.H. (1980) NH3 concentrations in
the expired air of the rat: Importance to inhalation
toxicology. Toxicol. appl. Pharmacol., 53: 116-121.
BARROW, C.S., ALARIE, Y., & STOCK, M.F. (1978) Sensory
irritation and incapacitation evoked by thermal decomposition
products of polymers and comparisons with known sensory
irritants. Arch. environ. Health, 33(2): 79-88.
BARTON, L.V. (1940) Toxicity of ammonia, chlorine, hydrogen
cyanide, hydrogen sulphide and sulphur dioxide gases. IV.
Seeds. Contrib. Boyce Thompson Inst., 11: 357-363.
BARZEL, U.S. (1975) Effect of chronic ammonium chloride
ingestion on parathyroid-hormone function. Nephron, 14(15):
339-346.
BARZEL, U.S. & JOWSEY, J. (1969) Effects of chronic acid and
alkali administration on bone turnover in adult rats. Clin.
Sci., 36(3): 517-524.
BENEDICT, H.M. & BREEN, W.H. (1955) The use of weeds as a
means of evaluating vegetation damage caused by air pollution.
In: Proceedings of the Third National Air Pollution
Symposium, Pasadena, California, April 1955, pp. 177-190.
BENNETT, W.F., PESEK, J., & HANWAY, J.J. (1964) Effects of
nitrate and ammonium on growth of corn in nutrient solution
sand culture. Agric. J., 56: 342-345.
BENYAJATI, S. & GOLDSTEIN, L. (1975) Renal glutaminase
adaptation and ammonia excretion in infant rats.
Am. J. Physiol., 228(3): 693-698.
BERL, S., TAKAGAKI, G., SCHWERIN, P., & WAELSCH, H. (1962)
Metabolic compartments in vivo: Ammonia and glutamic acid
metabolism in brain and liver. J. biol. Chem., 237: 2562-2569.
BESSMAN, S.P. & BESSMAN, A.N. (1955) The cerebral and
peripheral uptake of ammonia in liver disease with an
hypothesis for the mechanism of hepatic coma. J. clin.
Invest., 34: 622-628.
BHATTACHARYA, A.N. & TAYLOR, J.C. (1975) Recycling animal
waste as a feedstuff: A review. J. anim. Sci., 44: 1438.
BIDWELL, R.G.B. (1974) Plant physiology, New York,
MacMillan, 197 pp.
BINSTOCK, L. & LECAR, H. (1969) Ammonium ion currents in the
squid giant axon. J. gen. Physiol., 53: 342-361.
BIRNBAUM, S.M., WINTZ, M., & GREENSTEIN, J.P. (1957)
Quantitative nutritional studies with water-soluble,
chemically defined diets. III. Individual amino acids as
sources of "non-essential" nitrogen. Arch. Biochem. Biophys.,
72(2): 428-436.
BISWAS, S.D. & KLEINBERG, I. (1971) Effect of urea
concentration on its utilisation, on the pH and the formation
of ammonia and carbon dioxide in a human salivary sediment
system. Arch. oral Biol., 16: 759-780.
BLOOMFIELD, R.A., GARNER, G.B., & MUHRER, M.E. (1960)
Kinetics of urea metabolism in sheep. J. anim. Sci., 19: 1248.
BLOOMFIELD, R.A., KEARLEY, E.O., CREACH, D.O., & MUHRER, M.E.
(1962) Ruminal pH and absorption of ammonia and VFA. J. anim.
Sci., 22: 833 (Abstract).
BOCK, F., MICHELSON, I., BROSS, I.D., & PRIORE, R.L. (1974)
Carcinogenic activity of smoke condensate from cigarettes with
ammonium sulfamate-treated paper. Cancer, 33: 1010.
BODANSKY, A., JAFFE, M.L., & CHANDLER, J.P. (1932) Serum
phosphatase changes in ammonium chloride osteoporosis. Proc.
Soc. Exp. Biol. Med., 29: 871-875.
BOER, W.M.J., DEN & BASTIAENS H. (1984) [Effects of
acidification on vegetation,] Ministry of Agriculture and
Fisheries, Ministry of Housing, Physical Planning and
Environment, 82 pp (Report No. 84(2)) (in Dutch).
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1973)
Ammonia kills spoilage moulds in corn. J. Dairy Sci., 56: 241.
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1975a)
Effect of pH and substrate on growth. Eur. J. appl. Microbiol,
1: 55-56.
BOTHAST, R.J., ADAMS, G.H., HATFIELD, E.E., & LANCASTER, E.B.
(1975b) Preservation of high-moisture corn: a microbiological
evaluation J. Dairy Sci., 58: 386-391.
BOYD, E.M. & SEYMOUR, K.G.W. (1946) Ethylenediamine
dihydrochloride or "chlorethamine". II. Untoward and toxic
reactions. Exp. Med. Surg., : 223-227.
BOYD, E.M., McLACHLAN, M.L., & PERRY, W.F. (1944)
Experimental ammonia gas poisoning in rabbits and cats.
J. ind. Hyg. Toxicol., 26: 29-34.
BRAGANGA, B.M., FAULKNER, P., & QUASTEL, J.M. (1953) Effects
of inhibitors of glutamine synthesis in brain slices by
ammonium ions. Biochim. Biophys. Acta, 10: 83-88.
BREEDING, R.J., LODGE, J.P., Jr, PATE, J.B., SHEESLEY, D.C.,
KLONIS, H.B., FOGLE, B., ANDERSON, J.A., ENGLERT, T.R.,
HAAGENSON, P.L., MCBETH, R.B., MORRIS, A.L., POGUE, R., &
WARTBURG, A.F. (1973) Background trace concentrations in the
central United States. J. geophys. Res., 78: 7057-7064.
BREEDING, R.J., KLONIS, H.B., LODGE, J.P., Jr, PATE, J.B.,
SHEESLEY, D.C., ENGLERT, T.R., & SEARS, D.R. (1976)
Measurements of atmospheric pollutants in the St. Louis area.
Atmos. Environ., 10: 181-184.
BRENNAN, E., LEONE, I.A., & DAINES, R.H. (1962) Ammonia
injury to applies and peaches in storage. Plant Dis. Rep., 46:
792-795.
BRESSANI, R. & BRAHAM, J.E. (1977) Effect of frequency of
feeding on blood serum urea changes in dogs. J. Nutr. Rep.
Int., 16(3): 305-316.
BRETT, J.R. & ZALA, C.A. (1975) Daily pattern of nitrogen
excretion and oxygen consumption of sockeye salmon
(Oncorhyncus nerka) under controlled conditions. J. Fish. Res.
Board Can., 32: 2479-2486.
BRETTHAUER, R. (1978) Some ecological limits of tolerance to
Ochromonas sociabilis. Verh. Internat. Verein. Limnol., 20(3):
1850-1854.
BROCKWAY, D.R. (1950) Metabolic products and their effects.
Prog. Fish-Cult., 12(3): 127-129.
BRODERIUS, S.J. & SMITH, L.L., Jr (1979) Lethal and
sublethal effects of binary mixtures of cyanide and hexavalent
chromium, zinc, or ammonia to the fathead minnow Pimephales
promelas and rainbow trout Salmo gairdneri. J. Fish. Res.
Board Can., 36(2): 164-172.
BRODERIUS, S.J., SMITH, L.L., Jr, & LIND, D.T. (1977)
Relative toxicity of free cyanide and dissolved sulfide forms
to the fathead minnow. Pimephales promelas. J. Fish. Res.
Board Can., 34(12): 2323-2332.
BRODERIUS, S.J., DRUMMOND, R.A., FIANDT, J.T., & RUSSOM, C.L.
(in press) Toxicity of ammonia to smallmouth bass,
Micropterus dolomieui, as related to pH. Environ. Toxicol.
Chem..
BRODERSON, J.R., LINDSEY, J.R., & CRAWFORD, J.E. (1976) The
role of environmental ammonia in respiratory mycoplasmosis of
rats. Am. J. Pathol., 85: 115-130.
BROSSET, C., ANDREASSON, K., & FERM, M. (1975) The nature
and possible origin of acid particles observed at the Swedish
west coast. Atmos. Environ., 9: 631-642.
BROWN, A.C. (1974) Observation on the effect of ammonium
nitrate solutions on some common marine animals from Table
Bay. Trans. R. Soc. S. Afr., 41(2): 217-223.
BROWN, V.M. (1968) The calculation of the acute toxicity of
mixtures of poisons to rainbow trout. Water Res., 2(10):
723-733.
BROWN, R.H., DUDA, G.D., KORKES, S., & HANDLER, P. (1957) A
colorimetric micromethod for determination of ammonia: the
ammonia content of rat tissues and human plasma. Arch.
Biochem. Biophys., 66: 301-309.
BROWN, V.M., JORDAN, D.H.M., & TILLER, B.A. (1969) The acute
toxicity to rainbow trout of fluctuating concentrations and
mixtures of ammonia, phenol and zinc. J. Fish Biol., 1(1): 1-9.
BUCKLEY, J.A. (1978) Acute toxicity of unionized ammonia to
fingerling coho salmon. Prog. Fish-Cult., 40(1): 30-32.
BUCKLEY, J.A., WHITMORE, C.M., & LIMING, B.D. (1979) Effects
of prolonged exposure to ammonia on the blood and liver
glycogen of coho salmon (Oncorhynchus kisutch). Comp. Biochem.
Physiol., 63C(2): 297-303.
BUIKEMA, A.I., Jr, CAIRNS, J., Jr, & SULLIVAN, G.W. (1974)
Evaluation of Philodina acuticornis (Rotifera) as a bioassay
organism for heavy metals. Water Res. Bull., 10(4): 648-661.
BUKHOVSKAYA, M.S., GINZBURG, S.L., & KHALIZOVA, O. (1966)
[Methods for determination of harmful substances in air. A
practical guide,] 2nd ed., Moscow, Meditaina (in Russian).
BULLIS, K.L., SNOEYENBOS, G.H., & VAN ROEKEL, H. (1950) A
keratoconjunctivitis in chickens. Poult. Sci., 29: 386-389.
BURKHALTER, D.E. & KAYA, C.M. (1977) Effects of prolonged
exposure to ammonia on fertilized eggs and sac fry of rainbow
trout (Salmo gairdneri). Trans. Am. Fish Soc., 106(5): 470-475.
BURROWS, R.E. (1964) Effects of acclimated excretory
products on hatchery-reared salmonids, Washington DC, Fish and
Wildlife Service, US Department of the Interior, 12 pp
(Research Report 66).
BUTTERWORTH, B. (1979) Nutritionally improved straw: the
machines do the job. Agric. Eng., 60(7): 41-42.
BUYSMAN, E. (1984) [Ammonia emissions in the Netherlands,]
Ministry of Housing, Physical Planning, and Environment,
Directorate Air (Report No. 22) (in Dutch).
BUYSMAN, E., MAAS, J.F.M., & ASMAN, W.A.M. (1985) Ammonia
emission in Europe, Utrecht, the Netherlands, Institute for
Meteorology and Oceanography, State University Utrecht.
BYERRUM, R.U. & BENSON, A.A. (1975) Effect of ammonia on
photosynthetic rate and photosynthate release by Amphidinium
carterae (Dinophyceae). J. Phycol., 11: 449-452.
CALABRESE, E.J. (1978) Pollutants and high risk groups,
New York, Wiley-Interscience Publications.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1977) [Effect of
prolonged treatments with ammonia on stages of development of
Salmo gairdneri.] Nuovi Ann. Ig. Microbiol., 28(5): 333-345.
(in Italian).
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1981) Effects of
long-term exposure to ammonia on the developmental stages of
rainbow trout ( Salmo gairdnerii Richardson). Rapp. P.-V. Réun.
Cons. Int. Explor. Mer, 178: 81-86.
CAMERON, J.N. (1979) Excretion of CO2 in water-breathing
animals. Marine Biol. Lett., 1: 3-13.
CAMERON, J.N. & HEISLER, N. (1983) Studies of ammonia in the
rainbow trout physio-chemical parameter, acid-base behavior,
and respiratory clearance. J. exp. Biol., 105:: 107-125.
CAMERON, G.R. & SHEIKH, A.H. (1951) Experimental production
of pulmonary edema with ammonium salts, together with
classification of lung oedemas. J. Pathol. Bacteriol., 63:
609-617.
CAMPBELL, C.L., DAWES, R.K., DEOLALKAR, S., & MERRITT, M.C.
(1958) Effect of certain chemicals in water on the flavor of
brewed coffee. Food Res., 23: 575-579.
CAPLIN, M. (1941) Ammonia gas poisoning. Forty-seven cases
in a London shelter. Lancet, 2: 95-96.
CAPUCO, A.V. (1977) Ammonia, a modulator of 3T3 cell growth,
Ithaca, New York, Cornell University (Ph.D Thesis).
CARPENTER, C.P., SMYTH, H.S., Jr, & SHAFFER, C.B. (1948) The
acute toxicity of ethylene imine to small animals. J. ind.
Hyg. Toxicol., 30: 2-6.
CARY, G.A. (1976) A report on the assessment of aquatic
environmental impact of Union Carbide's Uravan operations:
on-site toxicity bioassays, Tarrytown, New York, Aquatic
Environmental Sciences, Union Carbide Corporation, 67 pp
(Report prepared for Metals Division, Union Carbide
Corporation, Uravan, Colorado).
CASTELL, D.O. & MOORE, E.W. (1971) Ammonia absorption from
the human colon. Gastroenterology, 60(1): 33-42.
CATEDRAL, F.F., COLOSO, R., VALERA, N., CASALMIR, C.M., &
QUIBUYEN, A.T. (1977a) Effect of some physico-chemical
factors on the survival and growth of Penaeus monodon
postlarvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian Fish.
Dev. Cent., 1(3): 13-16.
CATEDRAL, F.F., GEROCHI, D.D., QUIBUYEN, A.T., & CASALMIR,
C.M. (1977b) Effect of nitrite, ammonia, and temperature on
P. monodon larvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian
Fish. Dev. Cent., 1(3): 9-12.
CHAMP, M.A., LOCK, J.T., BJORK, C.D., KLUSSMANN, W.G., &
MCCULLOUGH, J.D., Jr (1973) Effects of anhydrous ammonia on
a central Texas pond and a review of previous research with
ammonia in fisheries management. Trans. Am. Fish. Soc.,
102(1): 73-82.
CHARLES, D.R. & PAYNE, C.G. (1966a) The influence of graded
levels of atmospheric ammonia on chickens. I. Effects on
respiration and on the performance of broilers and replacement
growing stock. Br. Poult. Sci., 7: 177-187.
CHARLES, D.R. & PAYNE, C.G. (1966b) The influence of graded
levels of atmospheric ammonia on chickens. II. Effects on the
performance of laying hens. Br. Poult. Sci., 7: 189-198.
CHEMICAL AND ENGINEERING NEWS (1980) C and EN's top fifty
chemical products. Am. Chem. Soc., : 16.
CHIPMAN, W.A., Jr (1934) The role of pH in determining the
toxicity of ammonium compounds, Columbia, Missouri, University
of Missouri, 153 pp (Ph.D. Thesis).
CLAIBORNE, J.B., EVANS, D.H., & GOLDSTEIN, L. (1982) Fish
branchial Na+-K+-activated ATPase. J. exp. Biol., 96: 431-434.
CLAIBORNE, J.B. & HEISLER, N. (1984) Acid-base regulation in
the carp (Cyprinus carpio) during and after exposure to
environmental hypercapnia. J. exp. Biol., 108: 25-43.
CLAWSON, A.J. & ARMSTRONG, W.D. (1981) Ammonium
polyphosphate as a source of phosphorous and nonprotein
nitrogen for monogastrics (in corn-soybean meal diets for
growing rats and growing finishing [G-F] pigs). J. anim. Sci.,
52(1): 1-7.
CLOSE, L.G., CATLIN, F.I., & COHN, A.M. (1980) Acute and
chronic effects of ammonia burns of the respiratory tract.
Arch. Otolaryngol., 106: 151-158.
COLE, T.J., COTES, J.E., JOHNSON, G.R., & MARTIN, H. (1977)
Ventilation cardiac frequency and pattern of breathing during
exercise in men exposed to o-chlorobenzylidene malonitril and
ammonia gas in low concentrations. Q. J. exp. Physiol., 62:
341-351.
COLT, J.E. (1978) The effects of ammonia on the growth of
channel catfish, Ictalurus punctatus, Davis, California,
University of California, 185 pp (Ph.D. Thesis).
COLT, J.E. & TCHOBANOGLOUS, G. (1976) Evaluation of the
short-term toxicity of nitrogenous compounds to channel
catfish, Ictalurus punctatus. Aquaculture, 8(3): 209-224.
COLT, J.E. & TCHOBANOGLOUS, G. (1978) Chronic exposure of
channel catfish, Ictalurus punctatus to ammonia: effects on
growth and survival. Aquaculture, 15(4): 353-372.
CONN, H.O. (1972) Studies of the source and significance of
blood ammonia. IV. Early ammonia peaks after ingestion of
ammonium salts. Yale J. Biol. Med., 45: 543-549.
CONSTANTINE, D.G. (1958) Bleaching of hair pigment in bats
by the atmosphere in caves. J. Mammal, 39: 513-520.
COOMBE, J.B., TRIBE, D.E., & MORRISON, J.W. (1960) Some
experimental observations on the toxicity of urea to sheep.
Aust. J. agric. Res., 11: 247-256.
COON, R.A., JONES, R.A., JENKINS, L.J., Jr, & SIEGEL, J.
(1970) Animal inhalation studies on ammonia, ethylene glycol,
formaldehyde, dimethylamine, and ethanol. Toxicol. appl.
Pharmacol., 16: 646-655.
COTTERILL, O.J. & NORDSKOG, A.W. (1954) Influence of ammonia
on egg white quality. Poult. Sci., 33: 432-434.
CRIBB, G.S. (1964) Nitrogen. In: Mellor's comprehensive
treatise on inorganic and theoretical chemistry, London,
Longmans, Vol. 8, Suppl. I, pp. 276-327.
CURTIS, S.E. (1972) Air environment and animal performance.
J. anim. Sci., 35: 628-634.
DALHAMN, T. (1963) Effect of ammonia alone and combined with
carbon particles on ciliary activity in the rabbit trachea in
vivo, with studies of the absorption capacity of the nasal
cavity. Int. J. Air Water Pollut., 7: 531-539.
DALHAMN, T. & SJOHOLM, J. (1961) Studies of SO2, NO2, and
NH3: effect on ciliary activity in the rabbit trachea of
single in vitro exposure and resorption in rabbit nasal
cavity. Acta physiol. Scand., 58: 287-291.
DALLAVALLE, J.M. & DUDLEY, H.C. (1939) Evaluation of odor
nuisance in the manufacture of kraft paper. Public Health
Rep., 54(1): 35-43.
DANECKER, E. (1964) [The jauche poisoning of fish: an
ammonia poisoning.] Osterr. Fischerei., 3/4: 55-68. (in
German).
DAVIES, B.M.A. & YUDKIN, J. (1952) Studies in biochemical
adaptation. The origin of urinary ammonia as indicated by the
effect of chronic acidosis and alkalosis on some renal enzymes
in the rat. Biochem. J., 52: 407-412.
DAY, D.L., HANSEN, E.L., & ANDERSON, S. (1965) Gases and
odors in confinement swine buildings. Trans. Am. Soc. Agric.
Eng., 8: 118-121.
DEAL, P.H., SOUZA, K.A., & MACK, H.M. (1975) High pH,
ammonia toxicity, and the search for life on the Jovian
planets. Origins Life, 6(4): 561-573.
DEAN, J.A., ed. (1979) Lange's handbook of chemistry, 12th
ed., New York, McGraw-Hill Book Company.
DEGRAEVE, G.M., OVERCAST, R.L., & BERGMAN, H.L. (1980)
Toxicity of underground coal gasification condenser water and
selected constitutents to aquatic biota. Arch. environ.
Contam. Toxicol., 9(5): 543-555.
DELISTRATY, D.A., CARLBERG, J.M., VAN OLST, J.C., & FORD,
R.F. (1977) Ammonia toxicity in cultured larvae of the
American lobster (Homarus americanus). In: Proceedings of the
8th Annual Meeting of the World Mariculture Society, San Jose,
Costa Rica, pp. 647-673.
DEMEREC, M., BERTANI, G., & FLINT, J. (1951) A survey of
chemicals for mutagenic action on E. coli. Am. Nat., 85: 119.
DEMUYNCK, M., RAHN, K.A., JANSSENS, M., & DAMS, R. (1976)
Chemical analysis of airborne particulate matter during a
period of unusually high pollution. Atmos. Environ., 10: 21-26.
DE VOOYS, G.G.N. (1968) Formation and excretion of ammonia
in Teleostei. I. Excretion of ammonia through the gills. Arch.
int. Physiol. Biochimie., 76: 268-272.
DODD, K.T. & GROSS, D.R. (1980) Ammonia inhalation toxicity
in cats. Arch. environ. Health, 35: 6-14.
DOIG, P.A. & WILLOUGHBY, R.A. (1971) Response of swine to
atmospheric ammonia and organic dust. J. Am. Vet. Med. Assoc.,
159: 1353-1361.
DOWDEN, B.F. & BENNETT, H.J. (1965) Toxicity of selected
chemicals to certain animals. J. Water Pollut. Control. Fed.,
37(9): 1308-1316.
DOWNING, K.M. & MERKENS, J.C. (1955) The influence of
dissolved-oxygen concentration on the toxicity of un-ionized
ammonia to rainbow trout ( Salmo gairdneri Richardson).
Ann. appl. Biol., 43(2): 243-246.
DREWES, D.R. & HALES, J.M. (1980) Removal of pollutants from
power plant plumes by precipitation, Richland, Washington,
Battelle-Northwest, US Electric Power Research Institute
(Report to the EPRI).
DRIEDZIC, W.R. & HOCHACHKA, P.W. (1976) Control of energy
metabolism in fish white muscle. Am. J. Physiol., 230: 579-582.
DRUMMOND, J.G., CURTIS, S.E., SIMON, J., & NORTON, H.W.
(1980) Effects of aerial ammonia on growth and health of
young pigs. J. anim. Sci., 50: 1085-1091.
DUDA, G.D. & HANDLER, P. (1958) Kinetics of ammonia
metabolism in vivo. J. biol. Chem., 232: 303-314.
DU RUISSEAU, J.P., GREENSTEIN, J.P., WINITZ, M., & BIRNBAUM,
S.M. (1957) Studies on the metabolism of amino acids and
related compounds in vivo. VI. Free amino acid levels in the
tissues of rats protected against ammonia toxicity. Arch.
Biochem. Biophys., 68: 161-171.
EADS, R.B., WISEMAN, J.S., GRIMES, J.E., & MENZIES, G.C.
(1955) Wildlife rabies in Texas. Public Health Rep., 70:
995-1000.
ECKERT, J.W. (1967) Application and use of postharvest
fungicides. In: Torgeson, D.C., ed. Fungicides, New York,
Academic Press, pp. 341.
ECKERT, J.W., KOLBEZEN, M.J., & GARBER, M.J. (1963)
Evaluation of ammonia-generating formations for control of
citrus fruit decay. Phytopathology, 53: 140-148.
ECOLOGICAL ANALYSTS, INC. (1981) The sources, chemistry,
fate, and effects of ammonia in aquatic environments,
Washington DC, American Petroleum Institute.
EDELMANN, C.M., Jr, BIOCHIS, H., SORIANO, J.R., & STARK, H.
(1967) The renal response of children to acute ammonium
chloride acidosis. Pediat. Res., 1: 452-460.
EDJTEHADI, M., SZABUNIEWICZ, M., & EMMANUEL, B. (1978) Acute
urea toxicity in sheep. Can. J. Comp. Med., 42: 63-68.
EDWARDS, J.G. & CONDORELLI, L. (1928) Studies on aglomerular
and glomerular kidneys. II. Physiological. Am.J. Physiol., 86:
383-398.
EGGELTON, A.E.J. (1969) The chemical composition of
atmospheric aerosols on Tees-side and its relation to
visibility. Atmos. Environ., 3: 355-372.
EGLE, J.L., Jr (1973) Retention of inhaled acetone and
ammonia in the dog. Am. Ind. Hyg. Assoc. J., 34(12): 533-539.
ELKINS, H.B. (1950) The chemistry of industrial toxicology,
New York, John Wiley and Sons, 84 pp.
ELKINS, H.B. (1959) Ammonia, NH3. In: The chemistry of
industrial toxicology, 2nd ed., New York, John Wiley and Sons,
86 pp.
EMERSON, K., RUSSO, R.C., LUND, R.E., & THURSTON, R.V.
(1975) Aqueous ammonia equilibrium calculations: effect of pH
and temperature. J. Fish. Res. Board Can., 32: 2379-2383.
EMERY, R.M. & WELCH, E.B. (1969) The toxicity of alkaline
solutions of ammonia to juvenile bluegill sunfish (Lepomis
macrochirus Raf.), Chattanooga, Tennessee, Water Quality
Branch, Division of Health and Safety, Tennessee Valley
Authority, 31 pp (Mimeo).
EPEL, D., STEINHARDT, R., HUMPHREYS, T., & MAZIA, D. (1974)
An analysis of the partial metabolic derepression of sea
urchin eggs by ammonia; the existence of independent pathways.
Dev. Biol., 40: 245-255.
EPIFANO, C.E. & SRNA, R.F. (1975) Toxicity of ammonia,
nitrite ion, nitrate ion, and orthophosphate to Mercenaria
mercenaria and Crassostrea virginica. Mar. Biol., 33(3):
241-246.
ERNST, R.A. (1968) The effect of ammonia on poultry.
Feedstuffs, 40(32): 40.
EUROPEAN INLAND FISHERIES ADVISORY COMMISSION (1970) Water
quality criteria for European freshwater fish. Report on
ammonia and inland fisheries, pp. 12 (EIFAC Technical Paper
No. 11) (Also in: Water Res., 7(7): 1011-1022 (1973)).
EVANS, D.H. (1977) Further evidence for Na/NH4 exchange in
marine teleost fish. J. exp. Biol., 70: 213-220.
EVANS, D.H. (1980) Osmotic and ionic regulation by
freshwater and marine fishes. In: Ali, M.A. ed. Environmental
Physiology of Fishes, New York, Plenum Publishing Co.,
pp. 93-122.
EVANS, D.H., KORMANIK, G.A., & KRASNY, E.J. (1979)
Mechanisms of ammonia and acid extrusion by the little skate,
Raja erinacea. J. exp. Zool., 208: 431-437.
EVANS, J.W. (1979) The construction and use of a
continuous-flow bioassay apparatus to determine a preliminary
un-ionized ammonia 96-h LC50 for the crayfish, Orconectes
nais, Lawrence, Kansas, University of Kansas, 76 pp (M.S.
Thesis).
FARBER, R.J. & ROSSANO, A.T. (1975) A new approach for
measuring atmospheric concentrations of ammonia. Paper
presented at Annual Meeting of the Pacific Northwest
International Section of the Air Pollution Control
Association, Vancouver, British Columbia, November 19-21,
36 pp.
FASEB (1974) Evaluation of the health aspects of certain
ammonium salts as food ingredients, Springfield, Virginia,
Federation of American Societies for Experimental Biology,
21 pp (Prepared for the US Food and Drug Administration under
Contract No. FDA 72-85) (US NTIS PPB-254-532).
FAUCONNEAU, B. & LUQUET, P. (1979) Influence d'une élévation
de température sur l'évolution de l'aminoacidémie et de
l'ammoniémie après le repas chez la truite arc en ciel (Salmo
gairdneri R.). Ann. Biol. anim. Biochim. Biophys., 19(4A):
1063-1079.
FAZEKAS, I.G. (1939) Experimental suprarenal hypertrophy
induced by ammonium hydroxide. Endokrinologie, 21: 315-337.
FAZEKAS, I.G. (1949) [Experimental data on influencing
ovarian functions by simple compounds.] Orv. Hetil., 90:
777-781 (in German).
FAZEKAS, I.G. (1954a) [Enlargement of the parathyroids by
treatment with simple acidotic compounds.] Kirchow's Arch.
Pathol. Anat. Physiol., 324: 531-542 (in German).
FAZEKAS, J.F., TICKTIN, H.E., EHRMENTRAUT, W.R., & ALMAN,
R.W. (1956) Cerebral metabolism in hepatic insufficiency.
Am. J. Med., 21: 843-849.
FEPCC (1972) [Investigation of air pollution in the city of
Tsuruga.] Annu. Rep. Fukui Environ. Pollut. Control Centre, 1:
88-116 (in Japanese).
FERGUSON, W.S., KOCH, W.C., WEBSTER, L.B., & GOULD, J.R.
(1977) Human physiological response and adaptation to
ammonia. J. occup. Med., 19: 319-326.
FETH, J.H. (1966) Nitrogen compounds in natural water. A
review. Water Resour. Res., 2: 41-58.
FISHER, D.W., GAMBELL, A.W., LIKENS, G.E., & BURMANN, F.H.
(1968) Atmospheric contributions to water quality of streams
in the Hubbard Brook Experimental Forest, New Hampshire. Water
Resour. Res., 4: 1115-1126.
FLAKS, A. & CLAYSON, D.B. (1975) The influence of ammonium
chloride on the induction of bladder tumors by 4-ethyl-
sulfonylnaphthalene-1-sulfonamide. Br. J. Cancer, 31: 585.
FLAKS, A., HAMILTON, J.M., & CLAYSON, D.B. (1973) Effect of
ammonium chloride on incidence of bladder tumours induced by
4-ethylsulfonylnapthalene-1-sulfonamide. J. Natl Cancer Inst.,
51: 2007.
FLORKIN, M. & DUCHATEAU, G. (1943) Les formes du système
enzymatique de l'uricalyse et l'évolution du catabolisme
purique chez les animaux. Arch. int. Physiol., 53: 267-307.
FLURY, K.E., DINES, D.E., RODARTE, J.R., & RODGERS, R.
(1983) Airway obstruction due to inhalation of ammonia. Mayo
Clin. Proc., 58: 389-393.
FLYNN, R.J. (1968) A new cage cover as an aid to laboratory
rodent disease control. Proc. Soc. Exp. Biol. Med., 129:
714-717.
FORSTER, R.P. & GOLDSTEIN, L. (1969) Formation of excretory
products. In: Hoar, W.S. & Randall, D.J. ed. Fish Physiology,
New York, Academic Press, Vol. 1, pp. 313-350.
FOSTER, G.L., SCHOENHEIMER, R., & RITTENBERG, D. (1939)
Studies in protein metabolism. V. The utilization of ammonia
for amino acid and creatine formation in animals. J. biol.
Chem., 127: 319-327.
FRANK, J.F. (1948) The toxicity of sodium chlorate
herbicides. Can. J. comp. Med., 12: 216-218.
FRASER, D.I., DYER, W.J., WEINSTEIN, H.M., DINGLE, J.R., &
HINES, J.A. (1966) Glycolytic metabolites and their
distribution at death in the white and red muscle of cod
following various degrees of antemortem muscular activity.
Can. J. Biochem., 44: 1015-1033.
FREEDMAN, L.R. & BEESON, P.B. (1961) Experimental
pyelonephritis. VIII. The effect of acidifying agents on
susceptibility to infection. Yale J. Biol. Med., 33: 318.
FRIZZOLA, J.A. & BAIER, J.H. (1975) Contaminants in
rainwater and their relation to water quality. Water Sewage
Works, 122: 94-95.
FROMM, P.O. (1963) Studies on renal and extra-renal
excretion in a freshwater teleost, Salmo gairdneri. Comp.
Biochem. Physiol., 10: 121-128.
FROMM, P.O. (1970) Effect of ammonia on trout and goldfish.
In: Toxic action of water soluble pollutants on freshwater
fish, Washington DC, US Environmental Protection Agency,
pp. 9-22 (EPA Water Pollution Control Research Series No. 18050
DST 12/70).
FROMM, P.O. & GILLETTE, J.R. (1968) Effect of ambient
ammonia on blood ammonia and nitrogen excretion of rainbow
trout (Salmo gairdneri). Comp. Biochem. Physiol., 26: 887-896.
FUHRER, J. (1985) Formation of secondary airpollutants and
their occurrence in Europe. Experientia (Basel), 41: 286-301.
FURST, P., JOSEPHSON, B., MASCHIO, G., & VINNARS, E. (1969)
Nitrogen balance after intravenous and oral administration of
ammonium salts to man. J. appl. Physiol., 26(1): 13-22.
FURST, P., JONSSON, A., JOSEPHSON, B., & VINNARS, E. (1970)
Distribution in muscle and liver vein protein of 15N
administered as ammonium acidosis to man. J. appl. Physiol.,
29(3): 307-312.
GALL, W.K. (1980) Effect of cow faeces on the ecology of
laboratory streams, Madison, Wisconsin, University of
Wisconsin, pp. 73-83 (M.S. Thesis).
GAY, W.M.B., CRANE, C.W., & STONE, W.D. (1969) The
metabolism of ammonia in liver disease: a comparison of
urinary data following oral and intravenous loading of [15N]
ammonium lactate. Clin. Sci., 37: 815-823.
GEORGII, H.W. (1963) Oxides of nitrogen and ammonia in the
atmosphere. J. geophys. Res., 68: 963-970.
GEORGII, H.W. & MULLER, W.J. (1974) On the distribution of
ammonia in the middle and lower trophosphere. Tellus, 26:
180-184.
GIBBS, M. & CALO, N. (1959) Factors affecting light induced
fixations of carbon dioxide by isolated spinach chloroplasts.
Plant Physiol., 34: 318-323.
GIBSON, G.E., ZIMBER, A., KROOK, L., & VISEK, W.J. (1971)
Nucleic acids and brain and intestinal lesions in ammonia
intoxicated mice. Fed. Proc., 30: 578.
GIGUZ, T.L. (1968) Effect of low concentrations of ammonia
and nitrogen oxides on adolescents undergoing vocational
training in the chemical industry. Hyg. Sanit., 33(7-9):
431-434.
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity tests.
Sewage ind. Wastes, 24(11): 1397-1401.
GILLIAM, J.W., DANIELS, R.B., & LUTZ, J.F. (1974) Nitrogen
content of shallow ground water in North Carolina Coastal
Plain. J. environ. Qual., 3: 147-151.
GIRARD, J.O. & PAYAN, P. (1980) Ion exchanges through
respiratory and chloride cells in freshwater and seawater
adapted teleosteans. Am. J. Physiol., 238: R260-R268
(Regulatory Integrative Comp. Physiol. 7).
GOLDHABER, M.B. & KAPLAN, I.R. (1974) The sulfur cycle. In:
Goldberg, D., ed. The sea: ideas and observations on progress
in the study of the seas, New York, John Wiley and Sons,
Vol. 5, pp. 569-655.
GOLDMAN, A.S. & YAKOVAC, W.C. (1964) Salicylate
intoxication and congenital anomalies. Arch. environ.
Health, 8: 648-656.
GOLDSTEIN, L., CLAIRBORNE, J.B., & EVANS, D.E. (1982)
Ammonia excretion by the gills of two marine teleost fish: the
importance of NH4+ permeance. J. exp. Zool., 219: 395-397.
GOLDSTEIN, L. & FORSTER, R.P. (1961) Source of ammonia
excreted by the gills of the marine teleost, Myoxocephalus
scropius. Am. J. Physiol., 200(5): 1116-1118.
GOLYAKOVA, L.P. (1971) [Present-day state of industrial
hygiene problems in hydrometallurgical production of tungsten
and molybdenum salts and oxides.] Gig. Tr. Prof. Zabol.,
15(2): 4-8 (in Russian with English summary).
GOODMAN, L.S. & GILMAN, A. (1970) The pharmacological basis
of therapeutics, 4th ed., New York, The Macmillan Company,
1794 pp.
GORDON, R.J. & BRYAN, R. (1973) Ammonium nitrate in airborne
particles in Los Angeles. Environ. Sci. Technol., 7: 645-647.
GORDON, H.H., MCNAMARA, H., & BENJAMIN, H.R. (1948) Response
of young infants to ingestion. Pediatrics, 2: 290-302.
GRANT, W.M. (1974) Toxicology of the eye: drugs, chemicals,
plant venoms, 2nd ed., Springfield, Illinois, C.C. Thomas,
pp. 96-101, 121-128.
GREENSTEIN, J.P., WINITZ, M., GULLINO, P., BIRNBAUM, S.M., &
OTEY, M.C. (1956) Studies on the metabolism of amino acids
and related compounds in vivo. III. Prevention of ammonia
toxicity by arginine and related compounds. Arch. Biochem.
Biophys., 64: 342-354.
GREGORY, R.B. (1977) Synthesis and total excretion of waste
nitrogen by fish of the Periopthalmus (mudskipper) and
Scartelass families. Comp. Biochem. Physiol., 51A: 33-36.
GRINDLEY, J. (1946) Toxicity to rainbow trout and minnows of
some substances known to be present in waste water discharged
to rivers. Ann. appl. Biol., 33(1): 103-112.
GROLLMAN, A. (1929) The urine of the goosefish (Lophius
piscatorius): its nitrogenous constituents with special
reference to the presence in it of trimethylamine oxide.
J. Biol. Chem., 81: 267-278.
GROSJEAN, D., DOYLE, G.J., MISCHKE, T.M., POE, M.P., FITZ,
D.R., SMITH, J.P., & PITTS, J.N., Jr (1976) The
concentration, size distribution, and modes of formation of
particulate nitrate, sulfate, and ammonium compounds in the
eastern part of the Los Angeles Basin. Presented at: The 69th
Annual Meeting of the Air Pollution Control Association,
Portland, Oregon, June 27 - July 1 1976, 14 pp (Paper 76-20.3).
GROSSI, C.E., PRYTZ, B., & ROUSSELOT, L.M. (1968) Adenosine
triphosphate in prevention of ammonia intoxication in dogs.
Surg. Forum, 19: 363-369.
GRUBE, H.J. (1973) [Load and loading capacity of submerged
macrophytes in running water in southern Lower Saxony.]
Verh. Ges. Okol., Saarbrücken: 103-109 (in German with English
summary).
GUERIN-ANCEY, O. (1976a) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
II. Effets du jeune sur l'excretion d'ammoniac et d'uree.
Aquaculture, 9(2): 187-294.
GUERIN-ANCEY, O. (1976b) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
III. Effets du volume et de la concentration initiale en
ammoniac sur l'excretion d'ammoniac et d'uree. Aquaculture,
9(2): 253-258.
GUPTA, B.N., KHANNA, R.N., & DATTA, K.K. (1979) Toxicological
studies of ammonium sulfamate in rat after repeated oral
administration. Toxicology, 13(1): 45-49.
GYORE, K. & OLAH, J. (1980) Ammonia tolerance of Moina
rectirostris Leydig (Cladocera). Aquaculture Hungarica
(Szarvas), 2: 50-54.
HAGGARD, H.W. (1924) Action of irritant gases upon the
respiratory tract. J. ind. Hyg., 5: 390-398.
HAHN, M., MASSEN, O., NENCKI, N., & PAWLOW, J. (1983) [The
connection between the vena cava inferior and the portal vein
and its consequences for the organism.] Arch. Exp. Path.
Pharmakol., 32: 161-210 (in German).
HALL, L.W., Jr, BUIKEMA, A.L., Jr, & CAIRNS, J., Jr (1978)
The effects of a stimulated refinery effluent on the grass
shrimp, Palaemonetes pugio. Arch. environ. Contam. Toxicol.,
7(1) 23-35.
HANTZSCH, S. & LAHMANN, E. (1970) [Ammonia determinations in
city air.] Schriftner. Ver. Wasser- Boden- Lufthyg. (Berlin-
Dahlem), 33: 35-39 (in German).
HARADER, R.R. & ALLEN, G.H. (1983) Ammonia toxicity to
chinook salmon parr: reduction in saline water. Trans Am.
Fish. Soc., 112: 834-837.
HARDING, A.J. (1959) Ammonia manufacture and uses, London,
Oxford University Press.
HAYNES, R.J. & GOH, K.M. (1977) Evaluation of potting media
for commercial nursery production of container grown plants.
II. Effects of media, fertilizer nitrogen and nitrification
inhibitor on yield and nitrogen uptake of Callistephus
chinensis (L.) nees "Pink Princess". N.Z. J. Agric. Res., 20:
371-381.
HAWKINS, R.A., MILLER, A.L., NIELSEN, R.C., & VEECH, R.L.
(1973) The acute action of ammonia on rat brain metabolism in
vivo. Biochem. J., 134: 1001-1008.
HAWKINS, D.B., DEMETER, M.J., & BARNETT, T.E. (1980) Caustic
ingestion: Controversies in management. A review of 14 cases.
Laryngoscope, 90: 98-109.
HAZEL, C.R., THOMSEN, W., & MEITH, S.J. (1971) Sensitivity
of striped bass and stickleback to ammonia in relation to
temperature and salinity. Calif. Fish Game, 57(3): 138-153.
HAZEL, R.H., BURKHEAD, C.E., & HUGGINS, D.G. (1979) The
development of water quality criteria for ammonia and total
residual chlorine for the protection of aquatic life in two
Johnson County, Kansas streams, Washington DC, Office of Water
Research and Technology, US Department of the Interior, 146 pp.
HEALY, T.V. (1974) Ammonia and related pollutants at
Harwell. Atmos. Environ., 8: 81-83.
HEALY, T.V., MCKAY, H.A.C., PILBEAM, A., & SCARGILL, D.
(1970) Ammonia and ammonium sulfate in the troposphere over
the United Kingdom. J. geophys. Res., 75: 2317-2321.
HEIL, G.W. & DIEMONT, W.M. (1983) Raised nutrient levels
change heathland into grassland. Vegetatio, 53: 113-120.
HEISLER, N. (1984) Acid-base regulation in fishes. In: Hoar,
W. & Randall, D.J., ed. Fish physiology, New York, Academic
Press, Vol. 10A.
HELMERS, S., TOP, F.H., & KNAPP, L.W., Jr (1971) Ammonia
injuries in agriculture. J. Iowa Med. Soc., 61: 271-280.
HEMENS, J. (1966) The toxicity of ammonia solutions to the
mosquito fish ( Gambusia affinis Baird & Girard). J. Proc.
Inst. Sewage Purif., 65: 265-271.
HENIS, Y. & CHET, I. (1967) Mode of action of ammonia on
Sclerotium rolfsii. Phytopathology, 57: 425-427.
HERBERT, D.W.M. (1956) La toxicité d'un effluent d'eau
d'égout. Bull. Cent. belge Etude Doc. Eaux, 32: 115-120.
HERBERT, D.W.M. (1962) The toxicity of rainbow trout of
spent still liquors from the distillation of coal. Ann. appl.
Biol., 50(4): 755-777.
HERBERT, D.W.M. & SHURBEN, D.S. (1965) The susceptibility of
salmonid fish to poisons under estuarine conditions. II.
Ammonium chloride. Int. J. Air Water Pollut., 9: 89-91.
HIDY, G.M. (1974) Characterization of aerosols in California
(ACHEX), Thousand Oaks, California, Rockwell International,
Vol. 1-4 (Final Report prepared for California Air Resources
Board).
HILLABY, B.A. & RANDALL, D.J. (1979) Acute ammonia toxicity
and ammonia excretion in rainbow trout (Salmo gairdneri). J.
Fish. Res. Board Can., 36(6): 621-629.
HINTZ, H.F., LOWE, J.E., CLIFFORD, A.J. ET AL. (1970)
Ammonia intoxication resulting from urea ingestion by ponies.
J. Am. Vet. Med. Assoc., 157: 963-966.
HOEFT, R.G., KENNEY, D.R., & WALSH, L.M. (1972) Nitrogen and
sulfur in precipitation and sulfur dioxide in the atmosphere
in Wisconsin. J. environ. Qual., 1: 203-208.
HOGAN, J.P. (1961) The absorption of ammonia through the
rumen of sheep. Aust. J. biol. Sci., 14: 448-460.
HOLETON, G.F., NEUMANN, P., & HEISLER, N. (1983) Branchial
ion exchange and acid-base regulation after strenous exercise
in rainbow trout. Resp. Physiol., 51: 303-318.
HOLLAND, G.A., LASATER, J.E., NEUMANN, E.D., & ELDRIDGE, W.E.
(1960) Experiments with inorganic pollutants - ammonia. Toxic
effects of organic and inorganic pollutants on young salmon
and trout, State of Washington, Department of Fisheries,
pp. 183-187 (Research Bulletin No. 5).
HOLT, G.J. & ARNOLD, C.R. (1983) Effects of ammonia and
nitrite on growth and survival of red drum eggs and larvae.
Trans. Am. Fish. Soc., 112: 314-318.
HOLT, J.D. & MALCOLM, S.A. (1979) Evaluation of fish loading
rates in regulatory static bioassays. Tech. Rep. -Fish. Mar.
Serv. (Can.)., 862: 146-160.
HORNE, F.R. & MCINTOSH, S. (1979) Factors influencing
distribution of mussels in the Blanco River of central Texas.
Nautilus, 94(4): 119-133.
HSIA, Y.E. (1974) Inherited hyperammonemic syndromes.
Gastroenterology, 67: 347-374.
HUNGER, W. (1978) [Dying off of old spruce trees in the
vicinity of a pig fattening station.] Beitrage
Forstwirtschaft, 4: 188-189 (in German).
HUNT, R.D. (1977) Measurement of partial pressure of ammonia
in breath and arterial blood. Evidence for secretion of
ammonia into expired gas by metabolism of monoamines in the
lung. Diss. Abstr. Int. Ser. B, 37: 3279.
ILO (1970) Permissible levels of toxic substances in the
working environment. In: Proceedings of the 6th Session of the
Joint ILO/WHO Committee on Occupational Health, Geneva, 4-10
June 1968, Geneva, International Labour Office, 405 pp.
INDUSTRIAL BIO-TEST LABORATORIES, INC. (1973) Irritation
threshold evaluation study with ammonia, Industrial Bio-Test
Laboratories, Inc. (Report to International Institute of
Ammonia Refrigeration, Publication No. 663-03161).
IWAOKA, W.T., OKRONE, C.A., SULLIVAN, J.J., & JOHNSON, C.A.
(1981a) Effect of pH and ammonium ions on mutagenic activity
in cooked beef. Cancer Lett., 12: 335.
IWAOKA, W.T., URONE, C.A., SULLIVAN, J.J., MEAKER, E.H., &
JOHNSON, C.A. (1981b) A source of error in mutagen testing
of foods. Cancer Lett., 11: 225.
JACOBS, M.H. (1940) Some aspects of cell permeability to
weak electrolytes. Cold Spring Harbour Sym. Quant. Biol., 8:
30-39.
JACQUEZ, J.A., POPPELL, J.W., LAWRENCE, W., Jr, & ROBERTS,
K.E. (1957) Clinical significance of the partial pressure of
ammonia (pNH3) in patients with ammonium toxicity. Clin. Res.
Proc., 5: 20 (Abstract).
JACQUEZ, J.A., POPPELL, J.W., & JELTSCH, R. (1959) Partial
pressure of ammonia in aveolar air. Science, 129: 269-270.
JAFFE, H.L., BODANSKY, A., & BLAIR, J.E. (1932a) Effects of
parathormone (parathyroid preparation) and ammonium chloride
on bones of rabbits. J. exp. Med., 55: 695-701.
JAFFE, H.L., BODANSKY, A., & CHANDLER, J.P. (1932b) Ammonium
chloride decalcification, as modified by calcium intake: the
relation between generalized osteoporosis and osteitis
fibrosa. J. exp. Med., 56: 823-834.
JANICKI, R.H. (1970) Renal adaptation during chronic NH4Cl
acidosis in the rat: no role for hyperplasia. Am. J. Physiol.,
219(3): 613-618.
JANSSEN, Th. W. (1982) [Intensive animal husbandry in
relation to space and environment,] Utrecht, The Netherlands,
Dutch State Forest Service (in Dutch).
JOHANNING, G.L., MUHRER, M.E., & GADDY, J.H. (1978)
Nutritional evaluation of chlorinated-ammoniated corn stover.
Paper presented at: The Joint Meeting of the American Dairy
Science Association and the American Society of Animal
Science, 11 July 1978.
JOHNSON, J.D., EPEL, D., & PAUL, M. (1976) Intracellular pH
and activation of sea urchin eggs after fertilization. Nature
(Lond.), 262(5570): 661-664.
JONES, K. (1973) Ammonia. In: Bailar, J.C., Jr, Emeleus,
H.J., Nyholm, R., & Trotman-Dickenson, A.F., ed. Comprehensive
inorganic chemistry, New York, Pergammon Press, Vol. 2, pp.
199-227.
JONES, R.D. & HOOD, M.A. (1980) Effects of temperature, pH,
salinity and inorganic nitrogen on the rate of ammonium
oxidation by nitrifiers isolated from wetland environments.
Microb. Ecol., 6: 339-347.
JUDE, D.J. (1973) Sublethal effects of ammonia and cadmium
on growth of green sunfish, East Lansing, Michigan, Michigan
State University, 193 pp (Ph.D. Thesis).
KADOWAKI, S. (1976) Size distribution of atmospheric total
aerosols, sulfate, ammonium and nitrate particulates in the
Nagoya area. Atmos. Environ., 10: 39-43.
KAGOTA, K., IWASE, T., KOJIMA, T., NIIYAMA, M., & NAMIOKA, S.
(1979) Diammonium citrate addition to a diet restricted
non-essential amino acids for young pigs. Jap. J. vet. Sci.,
41: 131-138.
KAMIN, H. & HANDLER, P. (1957) Amino acid and protein
metabolism. Annu. Rev. Biochem., 26: 419-490.
KAPEGHIAN, J.C., MINCER, H.H., JONES, A.B., VERLANGIERI, A.J.,
& WATERS, I.W. (1982) Acute inhalation toxicity of ammonia
in mice. Bull. environ. Contam. Toxicol., 29: 371-378.
KARNAKY, K.J., Jr, KINTER, L.B., KINTER, W.B., & STIRLING,
C.E. (1976) Teleost chloride cell. II. Autoradiographic
localization of gill Na, K-ATPase in killifish Fundulus
heterclitus adapted to low and high salinity environments.
J. cell Biol., 70: 157-177.
KATZ, M. & PIERRO, R.A. (1967) Estimates of the acute
toxicity of ammonia-urea plant wastes to coho salmon,
Oncorhynchus kisutch, Seattle, Washington, Fisheries Research
Institute, College of Fisheries, University of Washington,
15 pp (Final report).
KEESEE, R.G., HOPF, S.B., & MOYERS, J.L. (1975) The
distribution and characteristics of particulate sulfates in
the southwest desert atmosphere. Presented at: The
International Conference on Environmental Sensing and
Assessment, Las Vegas, Nevada, 14-19 September, 1975, Vol. 2,
5 pp (Paper 23-6).
KEMPINSKA, H. (1968) [The effect of ammonia fertilizers on
fish.] Gospod. Rybn., 20: 3-5 (in Russian).
KEPLINGER, M.L., SCHADEBERG, K.J., GOODE, J.W., & CALANDRA,
J.C. (1973) Irritation threshold evaluation study with
ammonia. In: Report to the Institute of Ammonia Refrigeration,
Northbrook, Illinois, Industrial Bio-Test Laboratories, Inc.
KERN, D.M. (1960) The hydration of carbon dioxide. J. Chem.
Ed., 37: 14-23.
KERSTETTER, T.H., KIRSCHNER, L.B., & RAFUSE, D.D. (1970) On
the mechanisms of sodium ion transport by the irrigated gills
of rainbow. J. gen. Physiol., 56: 342-359.
KEYES, W.F. (1975) Nitrogen. In: Mineral facts and problems,
Washington DC, US Department of the Interior, pp. 749-760
(Bureau of Mines Bulletin No. 667).
KHOLDEBARIN, B. & OERTLI, J.J. (1977) Effect of pH and
ammonia on the rate of nitrification of surface water.
J. Water Pollut. Control Fed., 49: 1693-1697.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.N. (1972)
Serum- and tissue-ammonia nitrogen and tissue-water values in
ammonia-intoxicated sheep. Am. J. Vet. Res., 33: 1187-1190.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.M. (1973)
Hemogram of sheep acutely intoxicated with ammonia.
Am. J. Vet. Res., 34: 587-589.
KIRSCHNER, L.B., GREENWALD, L., & KERSTETTER, T.H. (1973)
Effect of amiloride on sodium transport across body surfaces
of freshwater animals. Am. J. Physiol., 224: 832-837.
KLEMM, K. (1967) The effect of N source on the yield of
various crop plants. Bodenkultur, 18: 210-218.
KLENDSHOJ, N.C. & REJENT, T.A. (1966) Tissue levels of some
poisoning agents less frequently encountered. J. Forensic
Sci., 11: 75-80.
KLING, H.F. & QUARLES, C.L. (1974) Effect of atmospheric
ammonia and the stress of infectious bronchitis vaccination on
Leghorn males. Poult. Sci., 53: 1161-1167.
KLUESENER, J.W. & LEE, G.F. (1974) Nutrient loading from a
separate storm sewer in Madison, Wisconsin. J. Water Pollut.
Control Fed., 46(5): 920-936.
KNEPP, G.L. & ARKIN, G.F. (1973) Ammonia toxicity levels and
nitrate tolerance of channel catfish. Prog. Fish-Cult., 35(4):
221-224.
KNOWLES, G., DOWNING, A.L., & BARRETT, M.J. (1965)
Determination of kinetic constants for nitrifying bacteria in
mixed culture, with the aid of an electronic computer.
J. gen. Microbiol., 38: 263.
KOENIG, H. & KOENIG, R. (1949) Production of acute pulmonary
edema by ammonium salts. Proc. Soc. Exp. Biol. Med., 70:
375-380.
KOPP, A., FELLER, U., & ERISMANN, K.H. (1974) [Studies on
the regulation of nitrogen assimilation by Lemna minor in
transition from ammonium to nitrate feeding or from nitrate to
ammonium feeding under conditions of photosynthesis.]
Z. Pflanzen physiol., 73: 456-460 (in German).
KORMANIK, G.A. & CAMERON, J.N. (1981) Ammonia excretion in
the FW catfish: the role of diffusion. Am. Soc. Zool., 21(4):
1042.
KORTING, W. (1969a) [The harmful effect of ammonia on fish.]
Munch. Beitr. Abwasser-. Fisch.-Flussbiol., 16: 38-48 (in
German).
KORTING, W. (1969b) [Investigations on the effects of
ammonia on the blood of fish.] Wasser Abwasser Forsch., 4:
154-159 (in German).
KRYSTAL, G.W. & POCCIA, D. (1979) Control of chromosome
condensation in the sea urchin egg. Exp. cell Res., 123(2):
207-219.
KULASEK, G., SIWECKA, B., OWCZARCZYK, B., RYNCA, J., & BAREJ,
W. (1975) Effect of protein level and synthesize chloride
addition to diet on ammonia detoxification in rats. Zentralbl.
Veterinaermed., 22(3): 215-223.
KUSHNIR, J.J., MALKIN, H.I., MOHNEN, V.A., JENCHA, A.J., &
MCLAREN, E.H. (1970) Non-photochemical aerosol formation
from the anhydrous reaction between ammonia and sulfur dioxide
gases. Mechanism for the formation of ammonium sulfate in the
atmosphere, Albany, New York, Department of Chemistry and
Atmospheric Sciences Research Center, State University of New
York (Report 165 of National Air Pollution Control
Administration Research Grant 5RO1A).
KUSTOV, V.V. (1967) [Means of determining the maximum
allowable concentration of toxic products of natural human
metabolism.] In: General questions of industrial toxicology,
Moscow, pp. 63-65. (National Aeronautics and Space
Administration Technical Translation NASA TT F-11,358) (in
Russian).
LANDAHL, H.D. & HERRMANN, R.G. (1950) Retention of vapors
and gases in the human nose and lung. Arch. ind. Hyg. occup.
Med., 1: 36-45.
LANGOWSKA, I. & MOSKAL, J. (1974) Effect of ammonia and urea
on nitrobacteria in water environment. Pol. Arch. Hydrobiol.,
21(1): 119-123.
LARSON, T.V., COVERT, D.S., FRANK, R., & CHARLSON, R.J.
(1977) Ammonia in the human airways: neutralization of
inspired acid sulfate aerosols. Science, 197: 161-163.
LECHTENBERG, V.L., BUETTNER, M.R., HOLT, D.A., RICHEY, C.B., &
PARSONS, S.D. (1977) Hay preservation by anhydrous ammonia
treatment. In: Grain and Forage Harvesting. Proceedings of the
First International Grain and Forage Conference, St. Joseph,
American Society of Agricultural Engineers, pp. 327-328, 338.
LEE, J.A., (1985) Effects of NO2 on aquatic ecosystems. In:
Study on the need of NO2 long term limit value for the
protection of terrestrial and aquatic ecosystems (Final Report
Feb. 1985).
LEE, R.E., Jr & GORANSON, S. (1976) National air
surveillance cascade impact network. III. Variations in size
of airborne particulate matter over a three-year period.
Environ. Sci. Technol., 10: 1022-1027.
LEITHE, W. (1971) The analysis of air pollutants, Ann Arbor,
Michigan, Ann Arbor Science Publishers.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odour
threshold determination of 52 odorant chemicals. J. Air
Pollut. Control Assoc., 19: 91-95.
LEUVEN, R.S.E.W. & SCHUURKES, J.A.A.R. (1984) [Effects of
acid precipitation containing sulfur and nitrogen on weakly
buffered waters low in nutrients] The Netherlands, Laboratory
of Aquatic Ecology, University of Nymegen, and Ministry of
Housing, Physical Planning and Environment, 131 pp (in Dutch).
LEVI, G., MORISI, G., COLETTI, A., & CATANZARO, R. (1974)
Free amino acids in fish brain: normal levels and changes upon
exposure to high ammonia concentrations in vivo, and upon
incubation of brain slices. Comp. Biochem. Physiol., 49A:
623-636.
LEVY, D.M., DIVERTIE, M.B., LITZOW, T.J., & HENDERSON, J.W.
(1964) Ammonia burns of the face and respiratory tract.
J. Am. Med. Assoc., 190: 873-876.
LIEBHARDT, W.C., GOLT, C., & TUPIN, J. (1979) Nitrate and
ammonium concentrations of ground water resulting from poultry
manure applications. J. environ. Qual., 8: 211-215.
LIECHTI, P.M. & HUGGINS, D.G. (1980) Effects of a 24-h
ammonia injection on stream drift and benthic standing crop.
Trans. Kansas Acad. Sci., 83(3): 140.
LIETZKE, M.H. (1978) A kinetic model for predicting the
composition of chlorinated water discharged from power plant
cooling systems. In: Water chlorination environmental impact
and health effects, Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., Vol. 2, pp. 707-716.
LILLIE, R.J. (1970) Ammonia. In: Air pollutants affecting
the performance of domestic animals. A literature review,
Washington DC (Agriculture Handbook No 380).
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide
formulations against two brackish water organisms, the bleak
(Alburnus alburnus) and the harpacticoid (Nitocra spinipes).
Chemosphere, 11(12): 843-851.
LINZON, S.N. (1971) Effects of air pollutants on vegetation.
In: McCormac, B.M., ed. Introduction to the scientific study
of atmospheric pollution, Dordrecht, Holland, D. Reidel
Publishing Company, pp. 131-151.
LITAV, M. & AGAMI, M. (1976) Relationship between water
pollution and the flora of two coastal rivers of Israel.
Aquat. Biol., 2(1): 23-41.
LITAV, M. & LEHRER, Y. (1978) The effects of ammonium in
water on Potamageton lucens. Aquat. Biol., 5(2): 127-138.
LITTON BIONETICS, INC. (1975) Mutagenic evaluation of
compound FDA 73-42 ammonium sulfate, granular, food grade,
Washington DC, Litton Bionetics (Prepared for US Food and Drug
Administration) (NTIS PB-245 506).
LLOYD, R. (1961) Effect of dissolved oxygen concentrations
on the toxicity of several poisons to rainbow trout ( Salmo
gairdnerii Richardson). J. exp. Biol., 38: 447-455.
LLOYD, R. & HERBERT, D.W.M. (1960) The influence of carbon
dioxide on the toxicity of un-ionized ammonia to rainbow trout
( Salmo gairdnerii Richardson). Ann. appl. Biol., 48(2):
399-404.
LLOYD, R. & ORR, L.D. (1969) The diuretic response by
rainbow trout to sub-lethal concentrations of ammonia. Water
Res., 3(5): 335-344.
LOBASHOV, M.E. (1937) [Nature of the effects of chemical
agents on the mutation process of Drosphila melanogaster.]
Genetica, 19: 200-241 (in German).
LOBASHOV, M.E. & SMIRNOV, F.A. (1934) [Effect of acetic acid
on nondisjunction and mutations in Drosphila melanogaster.]
Dokl. Acad. Sci. USSR N.S., 2: 307 (in Russian with German
summary).
LODGE, J.P., Jr, MACHADO, P.A., PATE, J.B., SHEESLEY, D.C., &
WARTBURG, A.F. (1974) Atmospheric trace chemistry in the
American humid tropics. Tellus, 26: 250-253.
LOEHR, R.C. (1974) Characteristics and comparative magnitude
of non-point sources. J. Water Pollut. Control Fed., 46(8):
1849-1872.
LOSADA, M. & ARNON, D.I. (1963) Selective inhibitors of
photosynthesis. In: Hochester, R. & Quastel, J.H., ed.
Metabolic inhibitors, New York, Academic Press, Vol. 2, p. 559.
LOSADA, M., HERRERA, J., MALDONADO, J.M., & PANEQUE, A.
(1973) Mechanism of nitrate reductase reversible inactivation
by ammonia in Chlamydomonas. Plant Sci. Lett., 1: 31-37.
LOTSPEICH, W.D. (1965) Renal hypertrophy in metabolic
acidosis and its relation to ammonia excretion.
Am. J. Physiol., 208(6): 1135-1142.
LUBINSKI, K.S. (1979) Monitoring bluegill swimming behaviour
and the effects of sublethal ammonium chloride gradients,
Blacksburg, Virginia, Virginia Polytechnic Institute and State
University, 99 pp (Ph.D. Thesis).
LUBINSKI, K.S., SPARKS, R.E., & JAHN, L.A. (1974) The
development of toxicity indices for assessing the quality of
the Illinois River, Urbana, Illinois, Water Resources Center,
University of Illinois, 46 pp (Research Report No. 96).
LUBINSKI, K.S., DICKSON, K.L., & CAIRNS, J., Jr (1980)
Effects of abrupt sublethal gradients of ammonium chloride on
the activity level, turning, and preference-avoidance
behaviour of bluegills, In: Eaton, J.G., Parrish, P.R., &
Hendricks, A.C. ed. Aquatic toxicology, Philadelphia,
Pennsylvania, American Society for Testing and Materials,
pp. 328-340 (ASTM STP 707).
MACEWEN, J.D. & VERNOT, E.H. (1972) Toxic hazards research
unit annual technical report (NTIS AD 755-358).
MACEWEN, J.D., THEODORE, J., & VERNOT, E.H. (1970) Human
exposure to EEL concentrations of monomethylhydrazine. In:
Proceedings of the 1st Annual Conference on Environmental
Toxicology, Ohio, Wright-Patterson Air Force Base,
9-11 September, 1970, Aerospace Medical Research Laboratory,
pp. 355-363 (AMRL-TR-70-102, Paper No. 23).
MCBARRON, E.J. & MCINNES, P. (1968) Observations on urea
toxicity in sheep. Aust. Vet. J., 44: 90-96.
MCBEAN, R.L., NEPPEL, M.J., & GOLDSTEIN, L. (1966)
Glutaminate dehydrogenase and ammonia production in the eel
(Anguilla rostrata). Comp. Biochem. Physiol., 18: 909-920.
MCBRIDE, R.L. & LAING, D.G. (1979) Threshold determination
by triangle testing: Effects of judgmental procedure,
positional bias and incidental training. Chem. Senses Flavor.,
4(4): 319-328.
MCCALLAN, S.E.A. & SETTERSTROM, C. (1940) Toxicity of
ammonia, chlorine, hydrogen cyanide, hydrogen sulphide, and
sulphur dioxide gases. I. General methods and correlations.
Contrib. Boyce Thompson Inst., 11: 325-330.
MCCAY, C.M. & VARS, H.M. (1931) Studies upon fish blood and
its relation to water pollution. In: A biological survey of
the St. Lawrence Watershed. Supplement to the 20th Annual
Report, 1930, Albany, New York, New York Conservation
Department, pp. 230-233 (Biol. Survey (1930), No. V).
MCCORMICK, J.H., BRODERIUS, S.J., & FIANDT, J.T. (1984)
Toxicity of ammonia to early life stages of the green sunfish
Lepomis cyanellus. Environ. Pollut. Ser. A., 36: 147-163.
Erratum: Environ. Pollut. Ser. A. (in press).
MCDONALD, D.G. & WOOD, C.M. (1981) Branchial and renal acid
and ion fluxes in the rainbow trout at low environmental pH.
J. exp. Biol., 93: 101-118.
MCKAY, H.A.C. (1969) Ammonia and air pollution. Chem. Ind.,
34: 1162-1165.
MCKHANN, G.M. & TOWER, D.B. (1961) Ammonia toxicity in
cerebral oxidative metabolism. Am. J. Physiol., 200: 420-424.
MAETZ, J. (1972) Branchial sodium exchange and ammonia
excretion in the goldfish Carassius auratus. Effects of
ammonia-loading and temperature changes. J. exp. Biol., 56:
601-620.
MAETZ, J. (1973) Na+/NH4+, Na+/H+ exchanges and NH3 movement
across the gill of Carassius auratus. J. exp. Biol., 58:
255-275.
MAETZ, J. & GARCIA-ROMEU, F. (1964) The mechanism of sodium
and chloride uptake by the gills of a freshwater fish,
Carassius auratus. II Evidence for NH4+/Na+ and HCO3-/Cl-
exchanges. J. gen. Physiol., 47: 1209-1227.
MAGALHAES BASTOS, J.A. (1954) [Importance of ammonia as an
ichthyotoxic substance,] Brazil, Serv. Piscicultura, Dept.
nacl. obras contra secas, Ministerio viacao e obras publicas,
pp. 115-132 (Publ. Ser. 1-C, No. 159) (in Portuguese).
MAI, K.L. (1977) Energy and petrochemical raw materials
through 1990. Chem. Eng., 84: 122-128.
MALACEA, I. (1966) [Contributions to knowledge of the
cyanide, ammonia, mercury and arsenic toxic action on some
species of fishes and on Daphnia.] Stud. Prot. Epurarea Apelor
Inst. Stud. Cercet Hidrotehnice, 7(2: 751-792 (English
summary) Biol. Abstr., 50: 92715 (1969).
MALACEA, I. (1968) [Studies on the acclimatization of fish
to high concentrations of toxic substances.] Arch. Hydrobiol.,
65(1): 74-95 (in German with English summary).
MALLERY, C.H. (1979) Ammonium stimulated properties of a
K+-dependent ATPase in Opsanus beta, a marine teleost with an
NH4+/Na+ exchange pump. Am. Zool., 19: 944.
MANGOLD, C.A. (1971) Investigation of occupational exposure
to ammonia, Washington, Puget Sound Naval Shipyard, Industrial
Hygiene Division of Investigation.
MANGUM, C.P., SILVERTHORN, S.U., HARRIS, J.L., TOWLE, D.W., &
KRALL, A.R. (1976) The relationship between blood pH,
ammonia excretion and adaptation to low salinity in the blue
crab Callinectes sapidus. J. exp. Zool., 195(1): 129-136.
MANGUM, C.P., DYKENS, J.A., HENRY, R.P., & POLITES, G.
(1978) The excretion of NH4+ and its ouabain sensitivity in
aquatic annelids and molluscs. J. exp. Zool., 203: 151-157.
MARSCHANG, F. (1972) [The NH3 content of air in stables and
the fattening capacity of cows.] Dtsch. Tieraerztl.
Wochenschr., 79: 214-216 (in German).
MARSCHANG, F. & CRAINICEANU, E. (1971) [Investigations on
the connections between cowshed climate and calf mortality.]
Berlin München Tieraerztl. Wochenschr., 84: 417-420 (in
German).
MARSCHANG, F. & PETRE, C. (1971) [The NH3 content of cowshed
air and its influence on morbidity and animal lossess in
cattle-fattening pens.] Zentralbl. Veterinaermed., 18: 646-654
(in German).
MATASA, C. & MATASA, E. (1968) L'industrie moderne des
produits azotés, Paris, Dunod, pp. 63-208.
MATSUMOTO, H., OKAMURA, K., & TAKAHASHI, E. (1976) Changes
in activity and isoenzymes of intervase in cucumber leaves due
to nitrogen status. Plant Cell Physiol., 17: 867-874.
MATUSIAK, K. (1976) Studies on the purification of
wastewater from the nitrogen fertilizer industry by intensive
algal cultures. Acta microbiol. Pol., 25(3): 233-242.
MAYAN, M.H. & MERILAN, C.P. (1972) Effects of ammonia
inhalation on respiratory rate of rabbits. J. anim. Sci., 34:
448-452.
MAYNARD, D.N. & BARKER, A.V. (1969) Studies on the tolerance
of the plants to ammonium nutrition. J. Am. Soc. Hortic. Sci.,
94: 235-239.
MEARNS, A.J. (1981) Ecological effects on ocean sewage
outfalls: observations and lessons. Oceanus, 24: 45-54.
MERKENS, J.C. & DOWNING, K.M. (1957) The effect of tension
of dissolved oxygen on the toxicity of un-ionized ammonia to
several species of fish. Ann. appl. Biol., 45(3): 521-527.
METCALF & EDDY, INC. (1972) Wastewater engineering, New
York, McGraw-Hill.
MILES, F.D. (1961) Nitric acid manufacture and uses, London,
Oxford University Press.
MILLER, T.J. (1973) Cleft palate formation: The effects of
fasting and iodoacetic acid on mice. Teratology, 7: 177-182.
MILLER, G.R., SHIMP, G.F., ANDREWS, H.O., NIMMO, D.W., & ILEY,
E.S. (1981) Effect of domestic wastewater on trout in Dillon
Reservoir, Colorado. Paper presented at: The ASTM Symposium on
Aquatic Toxicology, St. Louis, Missouri, 13-14 October 1981,
Philadelphia, Pennsylvania, American Society for Testing
Materials.
MINER, J.R. & HAZEN, T.E. (1969) Ammonia and amines:
components of swine-building odour. Trans. Am. Soc. Agric.
Eng., 12: 772-774.
MITCHELL, H.A. (1963) Ammonia tolerance of the California
leaf-nosed bat. J. Mammal, 44: 543-551.
MITCHELL, H.A. (1964) Investigations of the cave atmosphere
of a Mexican bat colony. J. Mammal, 45: 568-577.
MORII, H., NISHIKATA, K., & TAMURA, O. (1978) Nitrogen
excretion of mudskipper fish Periophthalmus cantonensis and
Boleophthalmus pectinirostris in water and on land. Comp.
Biochem. Physiol., 60A(2): 189-193.
MORRIS, J.C. (1967) Kinetics of reactions between aqueous
chlorine and nitrogenous compounds. In: Faust, S.D. & Hunter,
J.V., ed. Principles and applications of water chemistry,
New York, John Wiley and Sons, pp. 23-53.
MORRIS, J.C. (1978) Chemistry of aqueous chlorine in
relation to water chlorination. In: Jolley, R.L., ed. Water
chlorination: Environmental impact and health effects,
Ann Arbor, Michigan, Ann Arbor Science Publishers, Vol. 1,
pp. 21-36.
MORRIS, J.C. & ROGERS, Q.R. (1978) Ammonia intoxication in
near-adult cat as a result of a dietary deficiency of
arginine. Science, 199(4327): 431-432.
MOSIER, A.R. (1978) Inhibition of photosynthesis and
nitrogen fixation in algae by volatile nitrogen bases.
J. environ. Qual., 7(2): 237-240.
MOSSBERG, S.M. (1967) Ammonia absorption in hamster iluem.
Effect of pH and total CO2 on transport in everted sacs.
Am. J. Physiol., 213: 1327-1330.
MOSSBERG, S.M. & ROSS, G. (1967) Ammonia movement in the
small intestine: Preferential transport by the ileum. J. clin.
Invest., 46: 490-498.
MOTYL, T. & DEBSKI, B. (1977) The influence of increasing
amounts of NH4Cl in the diet on ammonia detoxification in
rats. Zbl. Vet. Med., 24: 333-342.
MULDER, J.S. & VAN DER ZALM, H.O. (1967) [A fatal case of
ammonia poisoning.] Tijdschr. Soc. Geneeskd., 45: 458-460 (in
Dutch).
MULLER, G. & GRUHN, M. (1969) [The effect of water-free
ammonia fertilizer on soil microorganisms.] Zentralbl.
Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 2 Naturw.,
123: 667-676 (in German with English summary).
MUNTWYLER, E., IACOBELLIS, M., & GRIFFIN, G.E. (1956) Kidney
glutaminase and carbonic anhydrase activities and renal
electrolyte excretion in rats. Am. J. Physiol., 184: 83-90.
NAKAZAWA, S. & QUASTEL, J.H. (1968) Inhibitory effects on
ammonium ions and some amino acids on stimulated brain
respiration and cerebral amino acid transport. Can. J.
Biochem., 46: 543-548.
NATARAJAN, K.V. (1970) Toxicity of ammonia to marine
diatoms. J. Water Pollut. Control Fed., 42(5, part 2):
R184-R190.
NAVAZIO, F., GERRITSEN, T., & WRIGHT, G.J. (1961)
Relationship of ammonia intoxication to convulsions and coma
in rats. J. Neurochem., 8: 146-151.
NEHRING, D. (1963) [The toxic effect of urea solutions
containing urease on various fish species.] Z. Fischerei,
11(7/8): 539-547 (in German).
NEUFELD, R.D., HILL, A.J., & ADEKOYA, D.O. (1980) Phenol and
free ammonia inhibition to Nitrosomonas activity. Water Res.,
14(12): 1695-1703.
NIDEN, A.H. (1968) Effects of ammonia inhalation of the
terminal airways. In: Proceedings of the 11th Aspen Emphysema
Conference, Vol. 11, pp. 41-44.
NIHLGARD, B. (1970) Precipitation, its chemical composition
and effects on soil water and beech and spruce forests in
South-Sweden. Oikos., 21: 208
NIHLGARD, B. (1985) The ammonium hypothesis: an additional
explanation to the forest dieback in Europe. Ambio, 14(1): 1-8.
NISHIOKA, D.J. (1976) The initiation of cell cycle events in
ammonia-treated eggs and merogons of the sea urchin, Berkeley,
California, University of California, 107 pp (Ph.D. Thesis).
NODA, K. & CHIKAMORI, K. (1976) Effect of ammonia via
prepyriform cortex on regulation of food intake in the rat.
Am. J. Physiol., 231: 1263-1266.
NRC (1979) Ammonia. Subcommittee on Ammonia. Committee on
Medical and Biologic Effects of Environmental Pollutants.
Division of Medical Sciences, Assembly of Life Sciences,
National Research Council, Baltimore, Maryland, University
Park Press (NTIS No. PB 278-027).
OKITA, T. & KANAMORI, S. (1971) Determination of trace
concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 5: 621-627.
OVERREIN, L.N., SEIP, H.M., & TOLLAN, A., ed. (1980) Acid
precipitation: effects on forest and fish, Oslo (Final report
SNSF Project 1972-80).
PAIK, W.K., LAWSON, D., & KIM, S. (1975) Effect of endocrine
glands on the protection of rat from ammonia intoxication.
Life Sci., 15: 1189-1196.
PARKHURST, B.R., BRADSHAW, A.S., FORTE, J.L., & WRIGHT, G.P.
(1979) An evaluation of the acute toxicity to aquatic biota
of a coal conversion effluent and its major components.
Bull. environ. Contam. Toxicol., 23: 349-356.
PARKHURST, B.R., MEYER, J.S., DEGRAEVE, G.M., & BERGMAN, H.L.
(1981) A re-evaluation of the toxicity of coal conversion
process waters. Bull. environ. Contam. Toxicol., 26: 9-15.
PAUL, M., JOHNSON, J.D., & EPEL, D. (1976) Fertilization
acid of sea urchin eggs is not a consequence of cortical
granule exocytosis. J. exp. Zool., 197(1): 127-133.
PAUL, M. & JOHNSTON, R.N. (1978) Absence of a Ca response
following ammonia activation of sea urchin eggs. Dev. Biol.,
67(2): 330-335.
PAYAN, P. (1978) A study of the Na+/NH4+ exchange across the
gill of the perfused head of the trout (Salmo gairdneri). J.
comp. Physiol., 124: 181-188.
PAYAN, P. & MAETZ, J. (1973) Branchial sodium transport
mechanisms in Scyliorhinis canicula: evidence for Na+/NH4+
and Na+/H+ exchanges and for a role of carbonic anhydrase.
J. exp. Biol., 58: 487-502.
PAYAN, P. & MATTY, A.J. (1975) The characteristics of
ammonia excretion by a perfused isolated head of trout
(Salmo gairdneri): effect of temperature and CO2-free ringer.
J. comp. Physiol., 96: 167-184.
PAYAN, P., MATTY, A.J., & MAETZ, J. (1975) A study of the
sodium pump in the perfused head preparation of the trout,
Salmo gairdneri in freshwater. J. comp. Physiol., 104: 33-48.
PEPLINSKI, A.J., BREKKE, O.L., BOTHAST, R.J., & BLACK, L.T.
(1978) High moisture corn: an extended preservation trial
with ammonia. Trans. Am. Soc. Agricul. Eng., 21: 773-776, 781.
PEQUIN, L. & SERFATY, A. (1963) L'excrétion ammoniacale chez
un téléostéen dulcicole Cyprinus carpio. J. comp. Biochem.
Physiol., 10: 315-324.
PERRY, J.H., CHILTON, C.H., & KIRKPATRICK, S.D., ed. (1963)
Chemical engineers' handbook, 4th ed., New York, McGraw-Hill,
pp. 3-151.
PHILLIPS, A.M. (1950) The effect of metabolic products on
brook trout, Cortland, New York, New York State Conservation
Department, pp. 14-16 (Fisheries Research Bulletin 14)
(Cortland Hatcherry Report 19).
PHILLIPS, R.W. (1970) Further clinical studies of
trimethylpuringyl ammonium chloride (NSA-51095) in malignant
disease with special emphasis on cancer of urinary bladder.
Cancer Chemother. Rep., 54: 181.
PIERPONT, R.A. & MINOTTI, P.L. (1977) Effects of calcium
carbonate on ammonium assimilation by tomato seedlings.
J. Am. Soc. Hortic. Sci., 102: 20-23.
PINTER, I.J. & PROVASOLI, L. (1963) Nutritional characteristics
of some chrysomonads. In: Oppenheimer, C.M., ed. Symposium on
Marine Microbiology, Springfield, Massachusetts, Thomas
Publishers, pp. 114-121.
PITTS, R.F. (1971) The role of ammonia production and
excretion in regulation of acid-base balance. New Engl. J.
Med., 284: 32-38.
PITTS, J.N., Jr & GROSJEAN, D. (1976) Detailed
characterization of gaseous and size-resolved particulate
pollutants at a south coast air basin smog receptor site:
levels and modes of formation of sulfate, nitrate and organic
particulates and their implications for control strategies,
Riverside, California, Statewide Air Pollution Research
Center, University of California (California Air Resources
Board Contract 5-384, Six Month Progress Report, November 17,
1975 to May 16, 1976).
POLIAK, V.E. (1981) [Air pollution by ammonia around swine-
breeding complexes.] Gig. i. Sanit., 6: 67 (in Russian).
POST, R.L. & JOLLY, P.C. (1957) The linkage of sodium,
potassium and ammonium active transport across the human
erythrocyte membrane. Biochem. Biophys. Acta, 25: 118-128.
POWERS, E.B. (1920) Influence of temperature and concentration
on the toxicity of salts to fishes. Ecology, 1: 95-112.
POWERS, W.L., TERRY, R.V., & MURPHY, L.S. (1977) Fate of
nitrogen from manure disposal. In: Proceedings of the National
Conference on Disposal of Residues on Land, pp. 156-160.
PROPKOPIEVA, A.S. & YUSHKOV, G.G. (1975) [Toxicology of
ammonia in animals repeatedly exposed for short lengths of
time.] Gig. Tr. Prof. Zabol., 9: 54-55 (in Russian).
PROPKOPIEVA, A.S., YUSHKOV, G.G., & UBASHEEV, I.O. (1973)
[Materials for a toxicological characteristic of the one-term
effect of ammonia on the organisms of animals after brief
exposure.] Gig. Tr. Prof. Zabol., 6: 56-57 (in Russian).
PROVASOLI, L. & MCLAUGHLIN, J.J.A. (1963) Limited
heterotrophy of some photosynthetic dinoflagellates. In:
Oppenheimer, C.M., ed. Symposium on Marine Microbiology,
Springfield, MA, Thomas Publishers, pp. 105-113.
PRZYTOCKA-JUSIAK, M. (1976) Growth and survival of Chlorella
vulgaris in high concentrations of nitrogen. Acta microbiol.
Pol., 25(3): 287-289.
PRZYTOCKA-JUSIAK, M., MLYNARCZYK, A., KULESZA, M., &
MYCIELSKI, R. (1977) Properties of Chlorella vulgaris strain
adapted to high concentrations of ammonium nitrogen. Acta
microbiol. Pol., 26(2): 185-197.
QUARLES, C.L. & KLING, H.F. (1974) Evaluation of ammonia and
infectious bronchitis vaccination stress on broiler
performance and carcass quality. Poult. Sci., 53: 1592-1596.
RAJAGOPAL, R. (1978) Impact of land use on ground water
quality in the Grand Traverse Bay region of Michigan.
J. environ. Qual., 7: 93-98.
RAMACHANDRAN, V. & RAMAPRABHU, T. (1976) Use of ammonia as
herbicide: A Review. In: Varshney, C.K. & Rzoska, J., ed.
Aquatic weeds in South East Asia, The Hague, B.V. Publishers,
pp. 293-298.
RAMACHANDRAN, V., RAMAPRABHU, T., & REDDY, P.V.G.K. (1975)
Observations on the use of ammonia for the control of Pistia
stratiotes Linn. J. Int. Fish. Soc. India, 7: 124-130.
RAMSEY, G.B. (1953) Mechanical and chemical injuries. In:
The yearbook of agriculture 1953, Washington DC, US Department
of Agriculture, Plant Diseases, pp. 835-837.
RANDALL, D.J. (1970) Gas exchange in fish. In: Hoar, W.S. &
Randall, D.J., ed. Fish physiology, New York, Academic Press,
Vol. IV., pp. 253-292.
RANKIN, D.P. (1979) The influence of un-ionized ammonia on
the long-term survival of sockeye salmon eggs. Fish. Mar.
Serv. Tech. Rep., 912: 17.
RAO, T.S., RAO, M.S., & PRASAD, S.B.S.K. (1975) Median
tolerance limits of some chemicals to the fresh water fish
"Cyprinus carpio". Indian J. environ. Health, 17(2): 140-146.
REDNER, B.D. & STICKNEY, R.R. (1979) Acclimatization to
ammonia by Tilapia aurea. Trans. Am. Fish Soc., 108(4):
383-388.
REICHENBACH-KLINKE, H.-H. (1967) [Investigations on the
influence of the ammonia content on the fish organism.] Arch.
Fischereiwiss., 17(2): 122-132. (in German).
REINBOLD, K.A. & PESCITELLI, S.M. (1982a) Effects of
exposure to ammonia on sensitive life stages of aquatic
organisms, Champaign, Illinois, Illinois Natural History
Survey (Project Report: Contract No. 68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982b) Effects of cold
temperature on toxicity of ammonia to rainbow trout, blue
gills, and fathead minnows, Champaign, Illinois, Illinois
Natural History Survey (Project Report: Contract No.
68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982c) Acute toxicity of
ammonia to the white sucker, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. 2W-3946 NAEX).
REINBOLD, K.A. & PESCITELLI, S.M. (1982d) Acute toxicity of
ammonia to channel catfish, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. J 2482 NAEX).
REITER, R., SLADKOVIC, R., & POTZL, K. (1976) Chemical
components of aerosol particles in the lower troposphere above
central Europe measured under pure-air conditions. Atmos.
Environ., 10: 841-853.
REMPEL, B. (1975) Use ammonia to stop corn deterioration.
Crops Soils, 27: 13-14.
REPP, W.W., HALE, W.H., CHENG, E.W., & BURROUGHS, W. (1955)
The influence of oral administration of non-protein nitrogen
feeding compounds upon blood ammonia and urea levels in lambs.
J. anim. Sci., 14: 118-131.
RICE, S.D. & BAILEY, J.E. (1980) Survival, size, and
emergence of pink salmon, Oncorhynchus gorbuscha, alevins
after short- and long-term exposures to ammonia. Fish Bull.,
78(3): 641-648.
RICHARD, D., BOULEY, G., & BOUDENE, C. (1978a) Effets de
l'inhalation continue d'ammoniac chez le rat et la souris.
Bull. eur. Physiopathol. resp., 14: 573-582.
RICHARD, D., JOUARY, J., & BOUDENE, C. (1978b) Toxicité
aiguë par voie aérienne du gaz ammoniac chez le lapin.
C.R. Acad. X. Paris, 287(Série D): 375-378.
RICHARDS, B.O. & FROMM, P.O. (1970) Sodium uptake by
isolated perfused gills of rainbow trout (Salmo gairdneri).
Comp. Biochem. Physiol., 33: 303-310.
RICHARDS, P., HOUGHTON, B.J., METCALFE-GIBSON, A., WARD, E.E.,
& WRONG, O. (1968) Incorporation of orally administered
ammonia into tissue proteins in man; the influence of diet and
ureamia. In: Nutrition and renal disease, Edinburgh, E. and S.
Livingstone, pp. 93-98.
RICHARDS, P., BROWN, C.L., HOUGHTON, B.J., & WRONG, D.M.
(1975) The incorporation of ammonia nitrogen into albumin in
man: The effects of diet, uremia and growth hormone.
Clin. Nephrol., 3(5): 172-179.
RINGER, R.K. (1971) Adaptation of poultry to confinement
rearing systems. J. anim. Sci., 3: 590-598.
ROBIN, E.D., TRAVIS, D.M., BROMBERG, P.A., FORKNER, C.E., Jr,
& TYLER, J.M. (1959) Ammonia excretion by mammalian lung.
Science, 129: 270-271.
ROBINETTE, H.R. (1976) Effect of selected sublethal levels
of ammonia on the growth of channel catfish (Ictalurus
punctatus). Prog. Fish-Cult., 38(1): 26-29.
ROBINSON, E. & ROBBINS, R.C. (1968) Sources, abundance and
fate of gaseous atmospheric pollutants, Menlo Park, Stanford
Research Institute, California, 123 pp (prepared for the
American Petroleum Institute).
ROBINSON, E. & ROBBINS, R.C. (1971) Emissions, concentrations,
and fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, New York, John Wiley and Sons, Part 2,
pp. 1-93.
ROBINSON-WILSON, E.F. & SEIM, W.K. (1975) The lethal and
sublethal effects of a zirconium process effluent on juvenile
salmonids. Water Resour. Bull., 11(5): 975-986.
ROELOFS, J.G.M., SCHUURKES, J.A.A.R., & SMITS, A.J.M. (1984)
Impact of acidification and eutrophication on macrophyte
communities in soft waters in the Netherlands. II. Experimental
studies. Aquat. Bot., 18: 389-411.
ROELOFS, J.G.M. (in press) The effect of sulfur and nitrogen
deposition on aquatic and terrestrial heathland vegetation.
Experientia (Basel).
ROELOFS, J.G.M., KEMPERS, A.J., HOUDYK, A.L.F.M., & JANSEN,
J. (1985) The effect of airborne ammonium sulphate on Pinus
nigra var maritima, in the Netherlands. Plant Soil, 84: 45-56.
ROSAGE, T.F., SCHUTSKY, R.M., & RAPP, K.M. (1979) Toxicity
of un-ionized ammonia to the spotfin shiner (Notropis
spilopterus). Proc. Pa. Acad. Sci., 53: 39-42.
ROSEBOOM, D.P. & RICHEY, D.L. (1977) Acute toxicity of
residual chlorine and ammonia to some native Illinois fishes,
Urbana, Illinois, Illinois State Water Survey, 42 pp (Report
of Investigation 85).
ROSENFELD, M. (1932) Experimental modification of mitosis by
ammonia. Arch. exp. Zellforsch. Gewebezuecht., 14: 1.
RUBIN, A.J. & ELMARAGHY, M.A. (1976) Studies on the toxicity
of ammonia, nitrate, and their mixtures to the common guppy,
Columbus, Ohio, Water Resources Center, Ohio State University,
47 pp (Project No. A-033-OHIO).
RUBIN, A.J. & ELMARAGHY, G.A. (1977) Studies on the toxicity
of ammonia, nitrate and their mixtures to guppy fry. Water
Res., 11(10): 927-935.
SALVATORE, F., BOCCHINI, V., & CIMINO, F. (1963) Ammonia
intoxication and its effects on brain and blood ammonia
levels. Biochem. Pharmacol., 12: 1-6.
SAIFUTDINOV, M.M. (1966) Maximum permissible concentration
of ammonia in the atmosphere. Hyg. Sanit., 176: 171-175.
SAMYLIN, A.F. (1969) [Effect of ammonium carbonate on early
stages of development of salmon.] Uchen. Zap. Leningr. Gos.
Pedagog. Inst. Im. A. I. Gertsena, 422: 47-62 (in Russian).
SCHAERDEL, A.D., WHITE, W.J., LANG, C.M., DVORCHIK, B.H., &
BOHNER, K. (1983) Localized and systemic effects of
environmental ammonia in rats. Lab. anim. Sci, 33(1): 40-45.
SCHENKER, S. & WARREN, K.S. (1962) Effect of temperature
variation on toxicity and metabolism of ammonia in mice.
J. Lab. clin. Med., 60: 291-301.
SCHULZE-WIEHENBRAUCK, H. (1976) Effects of sublethal ammonia
concentrations on metabolism in juvenile rainbow trout ( Salmo
gairdneri Richardson). Ber. Dtsch. Wiss. Komm. Meeresforsch.,
24(4): 234-250.
SCHUMAN, G.E. & BURWELL, R.E. (1974) Precipitation nitrogen
contribution relative to surface runoff discharges.
J. environ. Qual., 3: 366-369.
SCHUURKES, J.A.A.R. (in press) Ammonium sulfate deposition
and its role in the acidification and nitrogen enrichment of
poorly-buffered aquatic systems. Experientia (Basel).
SCHUURKES, J.A.A.R., HECK, I., HESEN, P., LEUVEN, R.S.E.W., &
ROELOFS, J.G.M. (1985) Effects of acid and acidifying
deposition on water composition and macrophyte composition of
simulated poorly buffered aquatic systems. In: Proceedings of
the Muskoka Conference, Toronto, Canada, September 1985.
SEEGAL, B.C. (1927) Chronic acidosis in rabbits and in dogs,
with relation to kidney pathologic change. Arch. intern. Med.,
39(4): 550-563.
SELESI, D. & VAMOS, R. (1976) [Factors affecting the lethal
concentration of ammonia in fish ponds.] Ichthyologia, 8(1):
115-121 (in Serbocroation with English summary).
SERVANT, J. & DELAPART, M. (1983) Concentrations and origins
of NO3- and NH4+ in oceanic air. In: CACGP Symposium on
Tropospheric Chemistry with Emphasis on Sulphur and Nitrogen
Cycles and the Chemistry of Clouds and Precipitation
(Abstracts), 28 August - 3 September 1983, Oxford, England,
Oxford, New York, Sydney, Paris, Frankfurt, Pergamon Press,
57 pp (Fifth International Congress of the Commission on
Atmoshperic Chemistry and Global Pollution).
SHAW, J. (1960) The absorption of sodium ions by the cray-
fish Astacus pallipes Lereboullet. III. The effect of other
cations in the external solution. J. exp. Biol., 37(3):
548-556.
SHEN, S.S. & STEINHARDT, R.A. (1978) Direct measurement of
intracellular pH during metabolic derepression of the sea
urchin egg. Nature (Lond.), 272(5650): 253-254.
SHERWOOD, T.K. (1925) Solubilities of sulfur dioxide and
ammonia in water. Ind. Eng. Chem., 17: 745-747.
SHILO, M. & SHILO, M. (1953) Conditions which determine the
efficiency of ammonium sulphate in the control of Prymnesium
parvum in fish breeding ponds. Appl. Microbiol., 1: 330-333.
SHILO, M. & SHILO, M. (1955) Control of the phytoflagellate
Prymnesium parvum. Verh. Int. Ver. Limnol., 12: 233-240.
SHOLKOVITZ, E. (1973) Interstitial water chemistry of the
Santa Barbara Basin sediments. Geochim. Cosmochim. Acta, 37:
2043-2073.
SHUTTLEWORTH, T.J. & FREEMAN, R.F. (1974) Factors affecting
the net fluxes of ions in the isolated perfused gills of
freshwater Anguilla dieffenbachii. J. comp. Physiol., 94:
297-307.
SIEGRIST, A. (1920) [Concentrated effect of alkali and acid
on the eye.] Z. Augenheilkd., 43: 176-194 (in German).
SILVER, S.D. & MCGRATH, F.P. (1948) A comparison of acute
toxicities of ethylene amine and ammonia to mice. J. ind. Hyg.
Toxicol., 30: 7-9.
SILVERMAN, L., WHITTENBERGER, J.L., & MULLER, J. (1949)
Physiological response of man to ammonia in low
concentrations. J. ind. Hyg. Toxicol., 31: 74-78.
SINGER, R.H. & MCCARTY, R.T. (1971) Pathologic changes
resulting from acute ammonium salt poisoning in sheep.
Am. J. Vet. Res., 32: 1239-1246.
SLOAN, C.H. & MORIE, G.P. (1974) Determination of ammonia in
tobacco and tobacco smoke with the ammonia electrode. Anal.
Chim. Acta, 69: 243-247.
SLOT, G.M.J. (1938) Ammonia gas burns: an account of six
cases. Lancet, 2: 1356-1357.
SMART, G. (1976) The effect of ammonia exposure on gill
structure of the rainbow trout (Salmo gairdneri). J. Fish
Biol., 8(6): 471-475.
SMITH, C.E. (1972) Effects of metabolic products on the
quality of rainbow trout. Am. Fishes US Trout News, 17(3):
7-8, 21.
SMITH, C.E. (1984) Hyperplastic lesions of the primitive
meninx of fathead minnows, Pimephales promelas, induced by
ammonia: species potential for carcinogen testing. Natl Cancer
Inst. Monogr., 65: 119-125.
SMITH, C.E. & PIPER, R.G. (1975) Lesions associated with
chronic exposure to ammonia. In: Ribelin, W.E. & Migaki, G.,
ed. The pathology of fishes, Madison, Wisconsin, University of
Wisconsin Press, pp. 497-514.
SMITH, L.W. & WHEELER, W.E. (1979) Nutritional and economic
value of animal excreta. J. anim. Sci., 48: 144.
SODERLUND, R. & SVENSSON, B.H. (1976) The global nitrogen
cycle. In: Svensson, B.H. & Söderlund, R., ed. Nitrogen,
phosphorus, and sulfur: global sources, Stockholm, pp. 23-76
(SCOPE Report No. 7) (Ecology Bulletin No. 22).
SOF'INA, E.P., VORONKOV, M.G., D'IAKOV, V.W., SOMENOVA, N.V.,
PLATONOVA, G.N., PERETOLCHINA, N.M., LESNAIA, N.A., &
SMETANKINA, O.Z. (1978) Tris (2-oxy-ethyl) ammonium salts of
some synthetic phytohormones and their antitumor activity.
Khim Farm. 2h, 12: 74.
SOLOMONSON, L.P. (1969) Effects of ammonia and some of its
derivatives on photosynthesis in the blue-green alga,
Plectonema boryanum, Chicago, Illinois, University of Chicago,
68 pp (Ph.D. Thesis).
SOUSA, R.J., MEADE, T.K., & FELBECK, G.T., Jr (1977) The
exposure of ammonia to the photodissociated haemoglobin of the
marine bloodworm, Glycera dibranchiata. Comp. Biochem.
Physiol., 58A: 19-22.
SPARKS, R.E. (1975) The acute, lethal effects of ammonia on
channel catfish (Ictalurus punctatus), bluegills (Lepomis
macrochirus), and fathead minnows (Pimephales promelas),
Chicago, Illinois, 15 pp (report to Illinois Institute for
Environmental Quality, project No. 20.060).
SPARKS, R.E. & SANDUSKY, M.J. (1981) Identification of
factors responsible for decreased production of fish food
organisms in the Illinois and Mississippi Rivers, Havana,
Illinois, River Research Laboratory, Illinois Natural History
Survey, 63 pp (Project No. 3-291-R).
SPICER, C.W. (1977) The fate of nitrogen compounds in the
atmosphere. In: Pitts, J.N., Jr, Metcalf, R.L., & Lloyd, A.C.,
ed. Advances in environmental sciences and technology, New
York, John Wiley and Sons, Vol. 7, pp. 163-261.
SPINAZZOLA, A., MARRACCINI, L., DEVOTO, G., & ZEDDA, S.
(1966) [Study of the behaviour of some volatile toxic
substances as an index of air pollution in the cith of
Cagliari. Note III. Ammonia.] Folia Med. (Naples), 49: 641-648
(in Italian).
STAMMER, H.A. (1953) [The effect of hydrogen sulfide and
ammonia on characteristic animal forms in the saprobiotic
system.] Vom Wasser, 20: 34-71 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil (Myriophylum spicatum). Arch. environ.
Contam. Toxicol., 2(4): 331-341.
STEINHARDT, R.A. & MAZIA, D. (1973) Development of
K+-conductance and membrane potentials in unfertilized sea
urchin eggs after exposure to NH4OH. Nature (Lond.),
241(5389): 400-401.
STEPHENS, E.R. (1971) Identification of odors from cattle
feedlots. Calif. Agric., 25: 10-11.
STEVENS, C., KENNAWAY, N.G., & FELLMAN, J.H. (1975) Ammonia
intoxicaton: A hazard during rehabilitation of protein-
deprived rats. J. Nutr., i105: 1384-1390.
STEVENSON, T.J. (1977) The effects of ammonia, pH and
salinity on the white perch, Morone americana, Kingston, Rhode
Island, University of Rhode Island, 154 pp (Ph.D. Thesis).
STEWART, B.A., VIETS, F.G., Jr, HUTCHINSON, G.L., & KEMPER,
W.D. (1967) Nitrates and other water pollutants under fields
and feedlots. Environ. Sci. Technol., 1(9): 736-739.
STRUZEWSKI, E.J. (1971) Current status on control and
treatment of storm and combined sewer overflow, Edison Water
Quality Laboratory (EPA Technology Transfer).
STUDIER, E.H. (1966) Studies on the mechanisms of ammonia
tolerance of the guano bat. J. exp. Zool., 163: 79-85.
STUDIER, E.H., BECK, L.R., & LINDBORG, R.G. (1967) Tolerance
and initial metabolic response to ammonia intoxication in
selected bats and rodents. J. Mammal., 48: 564-572.
SUGIYAMA, K., YOSHIDA, M., & OYAMADA, M. (1968) [A case of
chemical bronchiectasia induced by inhalation of ammonia gaz.]
Nippon Kyohbu Shikkan Gakkai Zasshi, 27: 797-803 (in Japanese).
SUKUMARAN, N. & KUTTY, M.N. (1977) Oxygen consumption and
ammonia excretion in the catfish Mystus armatus, with special
reference to swimming speed and ambient oxygen. Proc. Indian
Acad. Sci., 86B: 195-206.
SUMMERSKILL, W.H.J. & WOLPERT, E. (1970) Ammonia metabolism
in the gut. Am. J. clin. Nutr., 23: 633-639.
SUYAMA, M., KOIKE, J., & SUZUKI, K. (1960) Studies on the
glycolysis and the formation of ammonia in the muscle and
blood of elasmobranchs. J. Tokyo Univ. Fish., 46: 51-60.
SWIGERT, J.P. & SPACIE, A. (1983) Survival and growth of
warmwater fishes exposed to ammonia under low flow conditions,
Springfield, Virginia, National Technical Information Service
(NTIS PB83-257535).
TABATA, K. (1962) [Toxicity of ammonia to aquatic animals
with reference to the effect of pH and carbon dioxide.]
Hokoku, 34: 67-74 (in Japanese).
TAIGANIDES, E.P. & WHITE, R.K. (1969) The menace of noxious
gases in animal units. Trans. Am. Soc. Agric. Eng., 12:
359-362.
TAKAHASHI, H., WAKAI, K., & OHUCHI, K. (1984) [Experiences
of chemical burns induced by ammonia gaz.] Nippon Saigai
Igakkai Kaishi, 32: 741-749 (in Japanese).
TAPLIN, G.V., CHOPRA, S., YANDA, R., & ELAM, D. (1976)
Radionuclidic lung-imaging procedures in the assessment of
injury due to ammonia inhalation. Chest, 69: 5.
TAYLOR, A.W., EDWARDS, W.M., & SIMPSON, E.C. (1971)
Nutrients in streams draining woodland and farmland near
Coshocton, Ohio. Water Resour. Res., 7: 81-89.
TAYLOR, J.E. (1973) Water quality and bioassay study from
Crawford National Fish Hatchery. Trans. Nebr. Acad. Sci., 2:
176-181.
THE NETHERLANDS MINISTRY OF HOUSING, PHYSICAL PLANNING AND
ENVIRONMENT (1984) Acidification in the Netherlands: effects
and policies. In: Proceedings of a Conference on Air Pollution
in Europe, Munich, 24-27 June, 1984, The Netherlands Ministry
of Housing, Physical Planning and Environment, pp. 9.
THOMAS, W.H., HASTINGS, J., & FUJITA, M. (1980) Ammonium
input to the sea via large sewage outfalls. Part 2. Effects of
ammonium on growth and photosynthesis of southern California
phytoplankton cultures. Mar. environ. Res., 3(4): 291-296.
THOMPSON, R.Y. & HALLIBURTON, I.W. (1966) Effect of diet on
the composition of the kidney. Biochem. J., 99(3): 44.
THORNTON, N.C. & SETTERSTROM, C. (1940) Toxicity of ammonia,
chlorine, hydrogen cyanide, hydrogen sulphide, and sulphur
dioxide gases. III. Green plants. Contrib. Boyce Thompson
Inst., 11: 343-356.
THURSTON, R.V. (1981) Acute toxicity of ammonia to the
pumpkinseed (Lepomis gibbosus), Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research
Laboratory.
THURSTON, R.V. & MEYN, E.L. (1984) Acute toxicity of ammonia
to five fish species from the northwest United States,
Bozeman, Montana Fisheries Bioassay Laboratory, Montana State
University, 13 pp (Technical Report Vol. 84, No. 4).
THURSTON, R.V. & RUSSO, R.C. (1981) Acute toxicity of
ammonia to golden trout (Salmo aguabonita) and mottled sculpin
(Cottus bairdi), Bozeman, Montana, Fisheries Bioassay
Laboratory, Montana State University, 10 pp (Technical Report
Vol. 81, No. 1).
THURSTON, R.V. & RUSSO, R.C. (1983) Acute toxicity of
ammonia to rainbow trout. Trans. Am. Fish. Soc., 112: 696-704.
THURSTON, R.V., RUSSO, R.C., & SMITH, C.E. (1978) Acute
toxicity of ammonia and nitrite to cutthroat trout fry. Trans.
Am. Fish. Soc., 107(2): 361-368.
THURSTON, R.V., CHAKOUMAKOS, C., & RUSSO, R.C. (1981a)
Effect of fluctuating exposures on the acute toxicity of
ammonia to rainbow trout (Salmo gairdneri) and cutthroat trout
(S. clarki). Water Res., 15(7): 911-917.
THURSTON, R.V., PHILLIPS, G.R., RUSSO, R.C., & HINKINS, S.M.
(1981b) Increased toxicity of ammonia to rainbow trout (Salmo
gairdneri) resulting from reduced concentrations of dissolved
oxygen. Can. J. Fish. aquat. Sci., 38(8): 983-988.
THURSTON, R.V., RUSSO, R.C., & VINOGRADOV, G.A. (1981c)
Ammonia toxicity to fishes: Effect of pH on the toxicity of
the un-ionized ammonia species. Environ. Sci. Technol., 15(7):
837-840.
THURSTON, R.V., RUSSO, R.C., & PHILLIPS, G.R. (1983) Acute
toxicity of ammonia to fathead minnows. Trans. Am. Fish. Soc.,
112: 705-711.
THURSTON, R.V., LUEDTKE, R.J., & RUSSO, R.C. (1984a)
Toxicity of ammonia to fresh-water insects of three families,
Bozeman, Montana, Fisheries Bioassay Laboratory, Montana State
University, 26 pp (Technical Report Vol. 84, No. 2).
THURSTON, R.V., RUSSO, R.C., LUEDTKE, R.J., SMITH, C.E., MEYN,
E.L., CHAKOUMAKOS, C., WANG, K.C., & BROWN, C.J.D. (1984b)
Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish.
Soc., 113(1): 56-73.
THURSTON, R.V., MEYN, E.L., ZAJDEL, R.K., & RUSSO, R.C.
(submitted) Chronic toxicity of ammonia to fathead minnows
(Pimephales promelas). Submitted to Trans. Am. Fish. Soc.
TMRI (1971) [Atmosphere division. On survey of air
pollutants peculiar to factory-clustered area.] Tokyo
Meteorol. Res. Inst. Environ. Prot., 2: 109-111 (Annual
Report) (in Japanese).
TOMASSO, J.R., GOUDIE, C.A., SIMCO, B.A., & DAVIS, K.B.
(1980) Effects of environmental pH and calcium on ammonia
toxicity in channel catfish. Trans. Am. Fish. Soc., 109(2):
229-234.
TOPPING, D.C. & VISEK, W.J. (1976) Nitrogen intake and
tumourigenesis in rats injected with 1,2-dimethylhydrazine.
J. Nutr., 106: 1583.
TORDA, C. (1953) Ammonium ion content and electrical
activity of the brain during the preconvulsive and convulsive
phases induced by various convulsants. J. Pharmacol. exp.
Ther., 107: 197-203.
TOTH, B. (1972) Hydrazine, methylhydrazine, and
methylhydrazine sulfate carcinogenesis in Swiss mice. Failure
of ammonium hydroxice to interfere in the development of
tumors. Int. J. Cancer, 9: 109.
TOWLE, D.W., PALMER, G.E., & HARRIS, J.L. III (1976) Role of
gill Na+ + K+-dependent ATPase in acclimation of blue crabs
(Callinectes sapidus) to low salinity. J. exp. Biol., 196:
315-322.
TOWLE, D.W. & TAYLOR, D.D. (1976) Effect of NH4+ and K+ on
Na+-transport ATPase activity of blue crab gill Am. Zool., 16:
224.
TRESHOW, M. (1970) Ammonia. In: Environment and plant
response, New York, McGraw-Hill Book Company, pp. 362-363.
TURNBULL, H., DEMANN, J.G., & WESTON, R.F. (1954) Toxicity
of various refinery materials to fresh water fish. Ind. Eng.
Chem., 46(2): 324-333.
ULRICH, B. (1983) Soil acidity and its relation to acid
deposition. In: Effects of accumulation of air pollutants in
forest ecosystems, Ulrich and Pankrasth, The Netherlands,
D. Reidel Publishing Company, pp. 127-146.
ULSHAFER, T.R. (1958) The measurement of changes in
acetylcholine level (ACh) in rat brain following ammonium ion
intoxication and its possible bearing on the problem of
hepatic coma. J. lab. clin. Med., 52: 718-723.
ULTSCH, G.R., JACKSON, D.C., & MOALLI, R. (1981) Metabolic
oxygen conformity among lower vertebrates: the toadfish
revisited. J. comp. Physiol., 142: 439-443.
UNDERWRITERS LABORATORIES (1933) Report on the comparative
life, fire, and explosion hazards of common refrigerants.
Miscellaneous Hazard No. 2375, Chicago, Illinois, Underwriters
Laboratories, pp. 26-28.
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1963) Effects of
pollution on fish; toxicity of mixutures of zinc, sulfate, and
ammonium chloride; toxicity of sewage effluents; the toxicity
of poisons under estuarine conditions, London, United Kingdom
Ministry of Technology, pp. 76-83 (Water Pollution Research
1962).
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1968) Effects of
pollution on fish, London, United Kingdom Ministry of
Technology, pp. 56-65 (Water Pollution Research 1967).
US EPA (1972) Air quality data for 1968 from the National
Air Surveillance Networks and contributing state and local
networks, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Programs, 231
pp (Report No. APTD-0978).
US EPA (1979a) Ammonia study using STORET ambient monitoring
data, Washington DC, US Environmental Protection Agency,
Monitoring and Data Support Division, Office of Water Planning
and Standards.
US EPA (1979b) Methods for chemical analysis of water and
wastes, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring and Support Laboratory (Report No.
EPA 600/4-79-020).
US EPA (1981) Multimedia health assessment document on
ammonia, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (Report No.
ECAO/82-DO10).
US EPA (1985) Ambient water quality criteria for ammonia -
1984, Duluth, Minnesota, US Environmental Protection Agency,
Office of Research and Development.
US NIOSH (1974) Criteria for a recommended standard.
Occupational exposure to ammonia, Washington DC, National
Institute for Occupational Safety and Health, pp. 74-136 (US
DHEW Publication No. 246-699).
US NIOSH (1977) NIOSH manual of analytical methods. Part I.
NIOSH monitoring methods, Washington DC, National Institute
for Occupational Safety and Health, Vol. 1, pp. 205.1-205.3
(US DHEW Publication No. 77-157-A).
UZVOLGYI, E. & BOJAN, F. (1980) Possible in vivo formation
of a carcinogenic substance from diethyl pyrocarbonate and
ammonia. J. Cancer Res. clin. Oncol., 97: 205.
VALENTINE, H. (1964) A study of the effect of different
ventilation rates on the ammonia concentrations in the
atmosphere of broiler houses. Br. Poult. Sci., 5: 149-159.
VAMOS, R. (1963) Ammonia poisoning in carp. Acta. biol.
Szeged., 9(1-4): 291-297.
VAMOS, R. & TASNADI, R. (1967) Ammonia poisoning in carp. 3.
The oxygen content as a factor influencing the toxic limit of
ammonia. Acta. biol. Szeged., 13(3-4): 99-105.
VAN AALST, R.M. (1984) [Atmospheric process and deposition,]
Ministry of Agriculture and Fisheries, Ministry of Housing,
Physical Planning and Environment, 76 pp (Report No. 84(2))
(in Dutch).
VAN BREEMEN, N. & JORDENS, E.R. (1983) Effects of
atmospheric ammonium sulfate on calcareous and non-calcareous
soils of woodlands in the Netherlands. In: Ulrich, B. &
Panlerath, J., ed. Effects of accumulation of air pollutants
in forest ecosystems, The Netherlands, Reidel Publishing
Company, pp. 171-182.
VANCURA, E.M., CLINTON, J.E., RUIZ, E., & KRENZELOK, E.P.
(1980) Toxicity of alkaline solutions. Ann. emergency Med.,
9(3): 118-122.
VAN DER EERDEN, L.J.M. (1982) Toxicity of ammonia to plants.
Agric. Environ., 7: 223-235
VAN SLYKE, D.D., PHILLIPS, R.A., HAMILTON, P.B., ARCHIBALD,
R.M., FUTCHER, P.H., & MILLER, A. (1943) Glutamine as source
material of urinary ammonia. J. Biol. Chem., 150: 481-482.
VAUGHN, R.E. & SIMCO, B.A. (1977) Effects of ammonia on
channel catfish, Ictalurus punctatus. ASB Bull., 24(2): 92.
VENKATARAMIAK, A., LAKSHMI, G.J., BEST, C., GUNTER, G.,
HARTWIG, E., & WILDE, P. (1981) Studies on toxicity of OTEC
plant components on marine animals from the Gulf of Mexico,
Springfield, Virginia, National Technical Information Service
(DE81030167).
VERBERK, M.M. (1977) Effects of ammonia in volunteers.
Int. Arch. occup. environ. Health, 39: 73 -81.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experiences of the
Clinica del Lavoro with their own maximal exposure limits for
industrial chemicals.] Arch. Gewerbepathol. Gewerbehyg., 13:
528-534 (in German).
VINES, H.M. & WEDDING, R.T. (1960) Some effects of ammonia on
plant metabolism and a possible mechanism for ammonia
toxicity. Plant Physiol., 35: 820-825.
VINOKUROVA, M.K. & MAL'KOVA, V.B. (1963) [Toxicology of the
selective herbicide ammonium sulfamate.] Gig. Trud. Prof.
Zabol., 7(5): 56-57 (in Russian).
VISEK, W.J., KOLONDY, G.M., & GROSS, P.R. (1972) Ammonia
effects in cultures of normal and transformed 3T3 cells.
J. cell Physiol., 80: 373.
VISEK, W.J., CLINTON, S.K., & TRUEX, C.R. (1978) Nutrition
and experimental carcinogenesis. Cornell Vet., 68: 3.
VITTI, T.G., VUKMIROVICH, R., & GAEBLER, O.H. (1964)
Utilization of ammonia nitrogen, administered by intragastric,
intraperitoneal and subcutaneous roues: Effects of growth
hormone. Arch. Biochem. Biophys., 106: 475-482.
WALLEN, I.E., GREER, W.C., & LASATER, R. (1957) Toxicity to
Gambusia affinis of certain pure chemicals in turbid waters.
Sewage Ind. Wastes, 29(6): 695-711.
WALLINGFORD, G.W. (1977) Fate of nitrogen from fertilizer
practices. In: Disposal of residues on land, Rockville,
Maryland, Information Transfer, Inc., pp. 152-155.
WALSH, L.M. (1977) Updating the nitrogen cycle. In: Disposal
of residues on land, Rockville, Maryland, Information
Transfer, Inc., pp. 146-151.
WALTON, M.J. & COWEY, C.B. (1977) Aspects of ammoniogenesis
in rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol.,
57B: 143-149.
WALUGA, D. & FLIS, J. (1971) [Changes in peripheral blood in
carp ( Cyprinus carpio L.) under the influence of ammonia
solution.] Rocz. Nauk Roln. Ser. H, 93(2): 87-94 (in Russian).
WARNCKE, D.D. & BARBER, S.A. (1973) Ammonium and nitrate
uptake by corn ( Zea mays L.) as influenced by nitrogen
concentration and NH4-/NO3-ratio. Agric. J., 65: 950-953.
WARREN, K.S. (1958) The differential toxicity of ammonium
salts. J. clin. Invest., 37: 497-501.
WARREN, K.S. & NATHAN, D.G. (1958) The passage of ammonia
across the blood, brain, barrier and its relation to blood pH.
J. clin. Invest., 37: 1724-1728.
WARREN, K.S. & SCHENKER, S. (1964) Effect of an inhibitor of
glutamine synthesis (methionine sulfoximine) on ammonia
toxicity and metabolism. J. lab. clin. Med., 64: 442-449.
WARREN SPRING LABORATORY (1982) Acidity of rainfall in the
United Kingdom.
WATTS, R.L. & WATTS, D.C. (1974) Nitrogen metabolism in
fishes. In: Florkin, M. & Scheer, B.T. ed. Chemical Zoology,
New York, Academic Press, Vol. III, pp. 369-446.
WEAST, R.C., ed. (1979) CRC handbook on chemistry and
physics, 59th ed., Boca Raton, Florida, CRC Press Inc.
WEATHERBY, J.H. (1952) Chronic toxicity of ammonia fumes by
inhalation. Proc. Soc. Exp. Biol. Med., 81: 300-301.
WEBB, D.W., BARTLEY, E.E., & MEYER, R.M. (1972) A comparison
of nitrogen metabolism and ammonia toxicity from ammonium
acetate and urea in cattle. J. anim. Sci., 35: 1263-1270.
WEBB, J.T. & BROWN, G.W., Jr (1976) Some properties and
occurrence of glutamine synthetase in fish. Comp. Biochem.
Physiol., 54B: 171-175.
WEDDING, R.T. & VINES, H.M. (1959) Inhibition of reduced
diphosphopyridine nucleotide oxidation by ammonia. Nature
(Lond.), 184: 1226-1227.
WEEKS, M.E. (1968) Discovery of the elements, 7th ed.,
Washington DC, Journal of Chemical Education, p. 197.
WEHRBEIN, M.F., VIPPERMAN, P.E., Jr, PEO, E.R., Jr, &
CUNNINGHAM, P.J. (1970) Diammonium citrate and diammonium
phosphate as sources of dietary nitrogen for growing-finishing
swine. J. anim. Sci., 31: 327-332.
WHITE, A., HANDLER, P., & SMITH, E.L. (1973) Renal function
and the composition of urine. In: Principles of biochemistry,
5th ed., New York, McGraw Hill Book Co., pp. 604-704 and
928-948.
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series No.
539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
WICKINS, J.F. (1976) The tolerance of warm-water prawns to
recirculated water. Aquaculture, 9(1): 19-37.
WILKIN, D.C. & FLEMAL, R.C. (1980) Feasibility of water
quality improvement in three Illinois rivers. J. Water Poll.
Control Fed., 52(2): 293.
WILKINSON, R.H., THOMAS, W.J., HANSEN, C.M., & SHIELDS, E.A.
(1978) Forage preservation by timed-slow release of NH3 (ASAE
Paper No. 78-1523).
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968a) Toxicologic effects of ammonium carbamate and related
compounds. Am. J. vet. Res., 29: 987-906.
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968b) Comparative ammonia toxicity. Comp. Biochem.
Physiol., 25: 295-301.
WILT, F.H. & MAZIA, D. (1974) The stimulation of cytoplasmic
polyadenylyation in sea urchin eggs by ammonia. Dev. Biol.,
37: 422-424.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) Merck Index, 9th ed., Rahway, New Jersey, Merck
and Co. Inc., p. 69.
WINKLER, M.M. & GRAINGER, J.L. (1978) Mechanisms of action
of NH4Cl and other weak bases in the activation of sea urchin
eggs. Nature (Lond.), 273(5663): 536-538.
WOKER, H. & WUHRMANN, K. (1950) [The sensitivity of
different species of fish to ammonia, hydrocyanic acid and
phenol.] Rev. Suisse Zool., 57(3): 548-553 (in German).
WOLAVER, T.C. (1972) Distribution of natural and
anthropogenic elements and levels in precipitation across the
US: theory and quantitative models, Chapel Hill, North
Carolina, University of North Carolina, 75 pp (Prepared for US
EPA).
WOOD, R.W. (1979) Behavioral evaluation of sensory
irritation evoked by ammonia. Toxicol. appl. Pharmacol., 50:
157-162.
WORCEL, A. & ERECINSKA, M. (1962) Mechanism of inhibitory
action of ammonia on the respiration of rat liver
mitochondria. Biochim. Biophys. Acta, 65: 27-33.
WRIGHT, P.A. & WOOD, C.M. (1985) An analysis of branchial
ammonia excretion in the freshwater rainbow trout: effects of
environmental pH change and sodium uptake blockage.
J. exp. Biol., 114: 329-353.
WUHRMANN, K. & WOKER, H. (1948) [Contributions to the
toxicology of fishes. II. Experimental investigations on
ammonia and hydrocyanic acid poisoning.] Schweiz. Z. Hydrol.,
11: 210-244 (in German).
WUHRMANN, K., ZEHENDER, F., & WOKER, H. (1947) [Biological
significance for fisheries of ammonium-ion and ammonia content
of flowing bodies of water.] Vierteljahrsschr. Naturforsch.
Ges. Zurich, 92: 198-204 (in German).
YAHAGI, H., SUZUKI, K., & MATSUI, Y. (1959) [Fourteen cases
of ammonia gas poisoning.] Shuhkan Igaku Tsuhshin, 601: 26-30
(in Japanese).
YECK, R.G., SMITH, L.W., & CALVERT, C.C. (1975) Recovery of
nutrients from animal wastes. An overview of existing options
and potentials for use in feed. In: Managing livestock waste:
Proceedings of the Third International Symposium on Livestock
Wastes, St. Joseph, Michigan, American Society of Agricultural
Engineers, pp. 192-196.
YOSHIDA, J., NAKAME, K., & NAKAMURA, R. (1957) Toxicity of
urea and its control. II. Toxicity of ammonium salts and urea
in rabbits and goats. Nippon Chikusangaku Kaiho, 28: 185-191.
ZIEVE, L., DOIZAKI, W.M., & ZIEVC, F.J. (1974) Synergism
between mercaptans and ammonia or fatty acids in the
production of coma. A possible role for mercaptans in the
pathogenesis of hepatic coma. J. lab. clin. Med., 83: 16-28.
ZIMBER, A. (1970) Studies on the effect of ammonia on DNA
synthesis and on cellular proliferation and renewal in tissues
of the mouse and in Ehrlich ascites tumor cells, Ithaca,
New York, Cornell University (Ph.D. Thesis).
ZIMBER, A. & VISEK, W.J. (1972) Effect of urease injection
on DNA synthesis in mice. Am. J. Physiol., 223: 1004.
ZIMMERMAN, P.W. (1949) Impurities in the air and their
influence on plant life. In: Proceedings of the First National
Air Pollution Symposium, Pasadena, California, 10-11 November,
1949, pp. 135-141 (Sponsored by Stanford Research Institute).
ZLATEVA, M., VALCEVA, V., & ANTOV, G. (1974) Toxic organic
changes in laboratory animals placed in an ammonia-rich
working atmosphere. Tr. Inst. Hig. Ohrana Tr. Prof. Zabol.,
22(1): 147-154.
Annex I. Ammonium salts evaluations prepared by the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)a
------------------------------------------------------------------------------------------------------
Ammonium salt Functional use Evaluation status Reference
(ADI, MTDI)b,c
------------------------------------------------------------------------------------------------------
acetate pH-adjusting agent not specifiedd (grouped with WHO (1982a)
other ammonium salts and acetate)
alginate thickening agent, stabilizer 0 - 50 (grouped with alginic acid WHO (1974a,b)
and calcium, potassium, and
sodium alginate)
bicarbonate leavening agent, buffer not specified WHO (1982b)
(hydrogen carbonate) neutralizing agent, alkali
carbonate
chloride dough conditioner, yeast, food not specified (grouped with WHO (1980)
hydrochloric acid and magnesium
and potassium chlorides)
hydrogen phosphate buffering agent, dough MTDI:, 70 expressed as phosphorus WHO (1982a,b)
phosphate dibasic conditioner, leavening agent, (grouped with phosphates and
yeast polyphosphates, including
phosphates occurring naturally in
food)
hydroxide (strong alkali not specified WHO (1966)
ammonia solution)
lactate buffer, dough conditioner not specified WHO (1974a,b)
persulfate flour-treatment agent no ADI set WHO (1966)
------------------------------------------------------------------------------------------------------
a Queries concerning updated information should be addressed to: Joint WHO Secretary of the Joint
FAO/WHO Expert Committee on Food Additives, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland.
b ADI = Acceptable daily intake for man expressed as mg/kg body weight.
c MTDI = Maximum tolerable daily intake for man, expressed as mg/kg body weight.
d ADI not specified = the total intake of the substance arising from its uses at the levels necessary
to achieve the desired effect does not represent a hazard to health.
REFERENCES TO ANNEX I
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series
No. 539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
ANNEX II: TREATMENT OF EXCESSIVE EXPOSURE TO AMMONIA
Ammonia (gas and liquid) is an extremely irritant chemical
affecting the skin, eyes, and the respiratory tracts. Ammonia gas
can produce burning of the eyes, lachrymation, and severe eye
damage. When inhaled it can produce coughing, laryngitis,
bronchitis, chest pains, and severe respiratory problems. Contact
with liquid ammonia can result in severe eye and skin burns due
both to its irritant properties and chilling effect.
Those working with ammonia should be trained in its safe use
including the dangers of improper handling, the use of protective
equipment, and the avoidance of unnecessary inhalation of the gas
and direct contact with liquid ammonia. After handling liquid
ammonia, the hands should be washed thoroughly before eating or
smoking.
The provision of protective clothing and equipment is not an
adequate substitute for safe working conditions. However, where
exposure cannot be adequately controlled, workers should be
provided with suitable impervious clothing, boots and gloves, and,
depending on the severity of the conditions, a face shield or
safety goggles and a mask or self contained breathing apparatus.
In places where very high gaseous ammonia concentrations are
expected, complete gas suits should be used.
Emergency showers and eye wash or water sprays should be
provided in all areas where ammonia is handled and where leaks,
spills or splashes may occur. Clothes contaminated with ammonia
should be discarded immediately and not worn again until thoroughly
cleansed.
First aid
If excessive exposure has occurred first aid treatment should
be promptly initiated and medical advice obtained as soon as
possible.
Ammonia in the eye (gas, liquid, or liquor)
Ammonia in the eye may cause severe injury and must be treated
immediately by irrigation for at least 15 min with flowing water or
sterile buffered eye irrigation solution.
Ammonia on the skin (liquid or liquor)
Drench the affected area with water and remove contaminated
clothing and footwear. Wash the affected area continuously for 5 -
10 min or until pain ceases.
Ingestion (liquid or liquor)
If the patient is conscious large quantities of water may be
given to dilute the chemical in the stomach. No attempt should be
made to induce vomiting.
Inhalation of gas/vapour
1. Remove from exposure, secure airway and place in semiprone
recovery position if unconscious. Give artificial
respiration if not breathing.
2. If heartbeat is absent give external cardiac massage.
3. If there is cyanosis (blueness of lips) or air-hunger
administer oxygen by facemask.
4. A conscious patient may be given water to drink.
Further treatment
Ammonia in the eyes
Corneal damage is probable. Use local anaesthetics and
cycloplegics to enable thorough irrigation and examination. If the
cornea is damaged, administer topical antibiotics. Refer to a
specialist centre.
Ammonia on the skin
Treat as a chemical burn. Liquid ammonia may produce deep
burns that may require grafting. Refer deep or extensive burns to
a specialist centre.
Inhalation of gas/vapour
1. Ammonia is irritant to the respiratory tract causing:
(a) bronchial oedema, spasm, and hypersecretion
resulting in chest tightness, wheeze, and cough,
which may progress to severe dyspnoea; and
(b) lower airway inflammation with exudative
pulmonary oedema and impaired gaseous
diffusion. Symptoms may be delayed 24 h or
more. Resolution may be by fibrosis producing a
restrictive defect.
2. Treat hypoxia with oxygen, ventilation, and bronchial
lavage, as appropriate.
3. Consider administration of steroids by multiple metered
doses of topical aerosol, by inhalation, and/or by
injection. Early prophylactic use may be indicated.
4. Administer bronchodilators by inhalation or injection, as
indicated. Maintain with oral treatment.
5. Keep under medical surveillance for at least 48 h. Treat
symptomatically.
6. Observe for secondary respiratory infection and treat as
necessary.
Manifestations of ammonia toxicity can be traced to several
metabolic disturbances. Both photosynthetic and respiratory
pathways are affected adversely by ammonia. There is a direct
relationship between ammonia concentration and respiratory
metabolism, including oxygen uptake, glycolysis, and the
tricarboxylic acid cycle (Matsumoto et al., 1976). Ammonium ions
may restrict photosynthesis through the uncoupling of noncyclic
photophosphorylation in isolated chloroplasts (Gibbs & Calo, 1959;
Losada & Arnon, 1963), though the mechanism of action is not known
(Losada et al., 1973). It is well documented that uncoupling leads
to an increase in reducing power in the cell. There is evidence
that 1 - 3 mmol ammonium inhibits respiration in plants (Wedding &
Vines, 1959; Vines & Wedding, 1960). It is suspected that similar
or identical inhibition occurs in ammonium toxicity in animals.
The site of toxicity is thought to be in the electron-transport
system; specifically, at the oxidation of NADH to NAD. This may
have a mechanism similar to the interference by ammonium ion with
electron transfer in the photosynthetic reaction.
High atmospheric levels may have direct toxic effects. Plant
growth is inhibited by a decrease in the carbohydrate production as
a consequence of inhibition of photosynthesis (Losada & Arnon,
1963). Ammonium increases the permeability of the cell membrane
causing plasmolysis and necrosis. Furthermore, the flexibility of
cells may be decreased, which results in an increased sensitivity
to frost. Excess ammonia can be detoxified as long as amino acids
are converted in the presence of carbohydrates (Fig. 1) (Van der
Eerden, 1982).
Most data on the toxic exposure of plants to ammonia have been
derived from controlled fumigation studies. Such experiments have
provided the following relative susceptibilities to ammonia:
leaves > stems, fungi, and bacteria < seeds, sclerotia, and
animals (McCallan & Setterstrom, 1940).
An exposure of 175 mg/m3 (250 ppm) for 4 min produced 50%
foliar necrosis in tomatoes, whereas the same foliar injury was
only produced in buckwheat and tobacco with exposure to 700 mg/m3
(1000 ppm) for 5 and 8 min, respectively (Thornton & Setterstrom,
1940). Zimmerman (1949) reported that fumigation with ammonia at
28 mg/m3 (40 ppm) for 60 min injured tomato, sunflower, and coleus
completely, and a concentration of 2.1 mg/m3 (3 ppm) severely
injured mustard (Benedict & Breen, 1955). Other plant parts are
more resistant to ammonia injury than the foliage. Thornton &
Setterstrom (1940) found 50% injury in tomato stems after exposure
to 700 mg/m3 (1000 ppm) for 60 min. Barton (1940) observed that
moist spring rye seeds were killed in a 4-h exposure to 700 mg
ammonia/m3 (100 ppm), whereas moist radish seeds were still viable
after 16 h. Exposure to a concentration of 175 mg/m3 (250 ppm) for
16 h reduced germination of rye seeds by half, but had no effect on
radish seeds.
Foliar injury is the most common toxic effect of anhydrous
ammonia on vegetation (Linzon, 1971). In broad-leaved woody plants
exposed to high concentrations of ammonia, the injury begins as
large, dark green water-soaked areas on leaves, which darken into
black or brownish-gray bifacial necrotic lesions, widely
distributed over the leaf surface. In lightly injured leaves,
these symptoms occur mainly on the upper surface. Conifer foliage
injured by ammonia exposure darkens to shades of gray-brown,
purple, or black. The entire needle is usually affected.
Abscission of severely damaged leaves is often seen in broad-leaved
and conifer species. On trees or shrubs with crowded leaves,
injury may be confined to particular sections of the leaf.
Occasionally, the foliage of woody species turns a variety of
colours, mimicking autumn colours. Symptoms of injury in
herbaceous plants are more variable, ranging from irregular,
bleached, bifacial, necrotic lesions to dark upper-surface
discolouration (Treshow, 1970). Grasses and cereal grains develop
tan to reddish-brown marginal or interveinal necrosis, and broad-
leaved weeds show red-brown to dark-brown upper surface
discolouration on terminal or marginal portions of the leaf
(Benedict & Breen, 1955). Coleus leaves lose their brilliant
colour after exposure to ammonia and appear green (Zimmerman,
1949). Injury to flowers by ammonia is rarely seen in the field,
but the development of small necrotic spots on azalea flowers has
been reported following ammonia exposure (Treshow, 1970).
Although parts of plants, other than the foliage, are less
susceptible to the injurious effects of ammonia (McCallan &
Setterstrom, 1940), injury to apples, peaches, and other fruits and
vegetables, accidentally exposed during cold storage, has been
reported (Ramsey, 1953). Apparently, ammonia entered the fruit and
turned red-pigmented tissues black, or brown, and yellow tissues,
dark-brown. Immediately on exposure to ammonia, the outer skins of
red onions became greenish-black, and the skin of yellow and brown
onions became dark brown. These colour changes were usually
permanent. Fumigation of fruit with ammonia also caused overall
darkening of the skin. Peaches and apples showed such symptoms at
140 mg/m3 (200 ppm) and 210 mg/m3 (300 ppm), respectively (Brennan
et al., 1962). These symptoms of injury were similar to those seen
on fruits injured by the accidental release of ammonia.
In studies in the Netherlands, several vegetables and trees
showed leaf damage by necrosis, growth reduction, and increased
frost senstivity at concentrations of 75 µg/m3 (annual average),
600 µg/m3 (during 24 h), and 10 000 µg/m3 (during 1 h) (Van der
Eerden, 1982). The sensitivity of pine trees to exposure to
ammonia differed between species (den Boer & Bastiaens, 1984). A
survey is given in Table 14. Airborne ammonium sulfate deposits
damaged the needles of pine trees. Owing to the enhanced uptake of
ammonium, the excretion of potassium, magnesium, and calcium
increases, often resulting in potassium and/or magnesium
deficiency, which may lead to premature shedding of needles
(Roelofs et al., 1985).
Effects of ammonia on the root system have generally been
studied by exposing the roots to different aqueous ammonium
solutions.
Table 14. Relative sensitivity of some pine trees to NH3a
-----------------------------------------------------------------
High sensitivity Moderate sensitivity Low sensitivity
-----------------------------------------------------------------
Picea abies Picea omorika Pinus sylvestris
Picea sitchensis Pinus nigra var. Pinus nigra nigra
Taxus maritima Tsuga canadensis
Cupressus leylandii Taxus baccata Taxus media
Pseutsuga menziesii Pinus mugo var. mughus
-----------------------------------------------------------------
a From: den Boer & Bastiaens (1984).
Exposure of tomato seedlings to ammonium solutions as the sole
nitrogen source showed reduced growth. The root system was
sparsely branched and discoloured and the stem was easily bruised.
There was considerable wilting of leaves, which developed marginal
necrosis (Pierpont & Minotti, 1977). Earlier, Maynard & Barker
(1969), using cucumber (C. sativus), bean (P. vulgaris), and pea
(Pisum sativum L.) in sand culture, demonstrated that ammonium
toxicity was generally characterized by an immediate reduction in
growth rate, wilting, marginal necrosis, interveinal chlorosis of
terminal leaves and, finally, death of the entire plant. However,
these symptoms did not occur in the ammonium medium with added
calcium carbonate (CaCO3) (Maynard & Barker, 1969; Pierpont &
Minotti, 1977). Several studies have shown that an increase in the
ammonium concentration is more deleterious to root than to shoot
growth (Bennett et al., 1964; Haynes & Goh, 1977). Warncke &
Barber (1973) observed that the ratio of root dry weight to shoot
dry weight decreased significantly with increasing concentrations
of nitrogen, but was not affected by the ammonium-to-nitrate ratio.
The roots appeared darker and less branched. The authors
attributed the observed decrease in root production to greater
acidity around the roots of ammonium-fed plants (Klemm, 1967;
Warncke & Barber, 1973).
In the Netherlands, field observations and experiments have
shown effects of ammonia and ammonium on pine forests, and
heathland vegetation (Heil & Diemont, 1983; Van Breemen & Jordens,
1983; den Boer & Bastiaens, 1984; Roelofs, in press; Schuurkes, in
press). In the United Kingdom, ombrotrophic mires (upland bogs
deriving water only from rain) are affected by eutrophication (Lee,
1985). Forest ecosystems in the Federal Republic of Germany may
also suffer from ammonia exposure (Hunger, 1978; Ulrich, 1983).
Although only little information is available for other countries,
it is expected that similar effects may occur in Belgium, Denmark,
and the northwestern part of France, where high ammonia emissions
occur as a consequence of the production of animal manure (Buysman
et al., 1985).
The condition of forests may deteriorate as a consequence of
direct exposure to ammonia. Pine trees in areas near intensive
livestock farms are particularly affected (Hunger, 1978; Janssen,
1982). In the Netherlands, there is a clear correlation between
ammonia emission and forest condition (Van Aalst, 1984). The
damage observed is the result of both direct and indirect effects,
and it is often difficult to distinguish between the two.
Field observations and experiments have shown deleterious
effects on pine trees, namely:
- decreased vitality (growth reduction and necrosis);
- higher susceptibility to fungal diseases and attack
by insects; and
- increased sensitivity to meteorological stress
factors, e.g., hard frost.
Most of these phenomena can be explained in terms of disturbed
nutrient budgets in trees. Enhanced ammonium sulfate uptake
results in the excretion of essential nutrients, such as potassium
and magnesium, by the needles, and the uptake of these ions by the
root system is inhibited as a result of ammonium accumulation and
cation leaching in soils (den Boer & Bastiaens, 1984; Roelofs, in
press). Increased ammonium uptake ultimately leads to enhanced
nitrogen levels in the leaves of beech (Nihlgard, 1970) and pine
tress (Roelofs et al., 1985).
More attention is being paid to the role of ammonium in the
forest dieback in Europe (Nihlgard, 1985).
Heather communities on poorly-buffered, slightly-acidic, and
nutrient-poor heathland soils are also disturbed as a result of the
deposition of airborne ammonia compounds, the number of plant
species declining in acidified and nitrogen-enriched heathland
soils. A succession from heather-dominated to grass-dominated
heathlands has been observed (Heil & Diemont, 1983; Roelofs, in
press). The same effects can be expected in other western European
countries where similar plant communities are present.
6.2.2. Aquatic plants
In the aquatic environment, nitrogen plays an important role in
determining the composition of phytoplankton and vascular plant
communities; in some cases, it can act as a limiting factor in
primary production. Ammonia is important in nitrogen metabolism,
because it functions as a nitrogen source in the synthesis of amino
acids.
Most species can use either ammonium or nitrate as the sole
nitrogen source, though, when both forms are available, ammonium is
used first. Uptake takes place through both roots and leaves. The
relative importance of ammonium as a nutrient depends on the
absolute concentration and the ratio of ammonium to nitrate.
Assimilation of ammonium is less expensive in terms of energy than
nitrate, because the first metabolic step in which nitrate is
reduced is not needed. Although ammonia is an important nutrient,
it appears to be toxic at higher concentrations. Its uptake in
large quantities may put a severe strain on the carbohydrate
metabolism of the species, because carbon skeletons are used for
detoxification (Bidwell, 1974). Increasing ammonia levels within
the cell inhibit the utilization of nitrate. Ammonia solutions
seem to be more toxic at high than at low pH, indicating that
toxicity is probably due primarily to NH3 rather than to NH4+.
Surface waters that are poorly buffered, nutrient poor, and
hydrologically dependent on rainfall and/or snow melt are most
sensitive to ammonia. Deposition of ammonia and other nitrogen
compounds may contribute significantly to the nitrogen enrichment
of susceptible waters. Ombrotrophic mire plant communities can be
altered by this atmospheric pollutant (Lee, 1985). In these
Sphagnum-dominated wetlands, seed plants become more dominant. In
small, poorly-buffered and nutrient-poor clear water lakes, both
water composition and macrophyte composition are altered.
Atmospheric ammonia deposition plays a major role in the
acidification and nitrogen enrichment of surface waters in the
Netherlands (Schuurkes, in press). Typical plant species belonging
to the Littorellion alliance disappear from acidified waters.
These species may be supressed by the luxuriant growth of Sphagnum
species and Juncus bulbosus. Ammonium enrichment enables the last
two species to form extremely high biomasses (Roelofs et al., 1984;
Schuurkes, in press; Schuurkes et al., 1985). It should be noted
that these changes in the plant community also influence the
structure of the animal population.
6.2.3. Fresh-water plants
Experimental data concerning the toxicity of ammonia for fresh-
water phytoplankton are limited. Przytocka-Jusiak (1976) reported
the effects of ammonia on the growth of Chlorella vulgaris. A 50%
inhibition was seen in 5 days of exposure to a concentration of
2.4 mg NH3/litre; complete growth inhibition occurred in 5 days
at 5.5 mg/litre. The NH3 concentration resulting in 50% survival
of C. vulgaris after 5 days was found to be 9.8 mg/litre. A C.
vulgaris strain in which the tolerance to elevated ammonia
concentrations was enhanced by prolonged incubation of the alga in
ammonium carbonate solutions was isolated by Przytocka-Jusiak et
al. (1977). C. vulgaris was reported to grow well in solutions
containing 4.4 mg NH3/litre, but growth was inhibited at 7.4
mg/litre (Matusiak, 1976). Tolerance to elevated concentrations of
NH3 seemed to show a slight increase, when other forms of nitrogen
were available to the alga, rather than when ammonia was the only
form of nitrogen in the medium. Bretthauer (1978) found that a
concentration (assuming pH 6.5 and 30 °C) of 0.6 mg NH3/litre
killed Ochromonas sociabilis, and that, at 0.3 mg/litre,
development of the population was reduced. Concentrations of 0.06
to 0.15 mg NH3/litre had an insignificant effect on growth, and
concentrations of 0.015 to 0.03 mg/litre enhanced growth.
Ammonia at concentrations exceeding 2.5 mg NH3/litre inhibited
photosynthesis and growth in the algal species Scenedesmus obliquus
and inhibited photosynthesis in the algae Chlorella pyrenoidosa,
Anacystis nidulans, and Plectonema boryanum (Abelovich & Azov,
1976). Mosier (1978) reported that NH3 concentrations causing a
50% reduction in oxygen production by the green alga Chlorella
ellipsoidea and blue-green alga Anabaena subcylindrica were
16.0 x 10-8 and 251.0 x 10-8 µg NH3-N/cell, respectively.
The rate of photosynthesis in the blue-green alga P. boryanum
was stimulated by NH4+, but inhibited by NH3 (Solomonson 1969); the
magnitude of these effects was dependent on the sodium-potassium
composition of the suspension media. Inhibition of photosynthesis
by NH3 was associated with a conversion of inorganic polyphosphate,
stored in the cells, to orthophosphate.
Champ et al. (1973) treated a small natural water pond with
ammonia to achieve a mean concentration of 25.6 mg NH3/litre. A
diverse population of dinoflagellates, diatoms, desmids, and blue-
green algae was present before ammonia treatment. Twenty-four
hours after treatment, the mean number of phytoplankton cells/litre
was reduced by 84%. By the end of 2 weeks (3.6 mg NH3/litre), the
original concentration of cells had been reduced by 95%.
Some research has been carried out to investigate the possible
use of ammonia as an aquatic herbicide. Champ et al. (1973)
reported virtually complete eradication of rooted aquatic
vegetation (water shield, Brasenia schreberi, and American lotus,
Nelumbo sp.). The NH3 concentration was 25.6 mg/litre 24 h after
ammonia addition, and 3.6 mg/litre, 2 weeks later. The use of high
concentrations of ammonia to eradicate aquatic vegetation has been
described by Ramachandran & Ramaprabhu (1976) and Ramachandran et
al. (1975).
In experiments with Potamogeton lucens, Litav & Lehrer (1978)
observed that ammonia caused appreciable injury to detached
branches. Ammonia inhibition of the growth of Eurasian
watermilfoil (Myriophyllum spicatum) affected both the length and
weight of roots and shoots (Stanley, 1974).
Grube (1973) found that Sium erectum was slightly injured at
15 mg NH3-N/litre, and completely eradicated at 35 mg/litre after a
10-week exposure. Callitriche sp. showed slight injury at 5 mg
NH3-N/litre, and the lethal dose was between 10 and 15 mg/litre.
Injury was estimated from the amount of black colouring and the
death of leaves. Roelofs et al. (1984) reported that exposure of
the isoebid Littorella uniflora to 50 µmol NH4+/litre for 10 weeks
resulted in excretion of an equivalent amount of potassium, which
may lead to discolouration and starvation.
Changes in the vegetation in 2 rivers subject to increased
pollution from agricultural fertilizers, urban sewage, and
industrial wastes, were studied by Litav & Agami (1976), who
attributed the changes in the composition of the plant species
primarily to a combination of ammonia and detergents. Agami et al.
(1976) transplanted 7 species of "clean water" macrophytes to
various sections of a river, and found that ammonia affected only
Nymphaea caerulea.
6.2.4. Salt-water plants
A concentration of 0.24 mg NH3/litre retarded the growth of
most of 10 species of benthic diatoms cultured for 10 days by
Admiraal (1977). Pinter & Provasoli (1963) found that Coccolithus
huxleyi was the most sensitive, and Pavlova gyrans and Hymenomonas
sp. the most tolerant to ammonium sulfate with intermediate tolerance
exhibited by Syracosphaera sp. and Ochrosphaera neapolitana.
Shilo & Shilo (1953, 1955) reported that the euryhaline alga
Prymnesium parvum was effectively controlled by applications of
ammonium sulfate, which exerted a lytic effect that decreased with
increasing pH, indicating that NH3 and not NH4+ is responsible for
the lytic activity of ammonium sulfate on P. parvum. It was
reported by Byerrum & Benson (1975) that added ammonium ion at
concentrations found to stimulate the photosynthetic rate also
caused the alga Amphidinium carterae to release up to 60% of fixed
14CO2 to the medium.
Natarajan (1970) found that the concentrations of fertilizer
plant effluent that were toxic for natural phytoplankton
(predominantly diatoms) ranged between 1.1 and 11 mg NH3/litre.
Thomas et al. (1980) concluded that increased ammonium
concentrations found near sewage outlets would not be inhibiting to
phytoplankton in the vicinity. Provasoli & McLaughlin (1963)
reported that ammonium sulfate was toxic for some marine
dinoflagellates, but only at concentrations far exceeding those in
sea water.
6.3. Aquatic Invertebrates
The toxicity of ammonia has been less extensively studied in
invertebrates than in fish. Most of the available invertebrate
data consists of studies on arthropods, primarily crustaceans and
insects. Many of these studies are laboratory tests in which test
animals were exposed to known concentrations of a toxic agent for
specified periods of time. Results may be expressed as a median
lethal concentration (LC50) for a given time period (e.g., 48-h
LC50) or, occasionally, as an effective concentration (EC), that
is, the concentration at which the test animal is completely
immobilized, though it might still be respiring (e.g., 48-h EC).
Another method of reporting test results is as the estimated time
required to kill 50% of a test population (LT50) at a given
concentration of toxin (e.g., LT50 = 250 min).
6.3.1. Fresh-water invertebrates: acute toxicity
The acute toxicity of ammonia for Daphnia magna has been
studied by Parkhurst et al. (1979, 1981) and Reinbold & Pescitelli
(1982a) with reported 48-h LC50s values of 2.08 and 4.94 mg
NH3/litre. DeGraeve et al. (1980) reported a similar 48-h LC50
value for Daphnia pulicaria of 1.16 mg NH3/litre. A threshold
toxicity value for D. magna of 2.4 - 3.6 mg NH3/litre lake water
was reported by Anderson (1948). Threshold concentration was taken
as the highest concentration that would just fail to immobilize the
test animals, under conditions of prolonged exposure (Anderson,
1948). A minimum lethal concentration of 0.55 mg NH3/litre was
reported for D. magna by Malacea (1966), and a 24-h LC50 value of
1.50 mg NH3/litre was reported by Györe & Oláh (1980) for Moina
rectirostris.
Buikema et al. (1974) reported an EC50 for NH3 toxicity in the
rotifer, Philodina acuticornis, to be 2.9 - 9.1 mg NH3/litre
(calculated using reported pH values of 7.4 - 7.9). Tests of
ammonia toxicity for the flatworm, Dendrocoelum lacteum (Procotyla
fluviatilis), and a tubificid worm (Tubifex tubifex) gave LC50
values of 1.4 and 2.7 mg NH3/litre, respectively (Stammer, 1953).
Thurston et al. (1984a) conducted 25 flow-through toxicity
tests with 3 mayfly, 2 stonefly, 1 caddisfly, and 1 isopod species;
all tests were conducted with water of similar chemical
composition. The 96-h LC50 values ranged from 1.8 to 5.9 mg
NH3/litre. The results also indicated that a 96-h test is not long
enough to determine the acutely lethal effects of ammonia on the
species tested, because an asymptotic LC50 was not always obtained
within 96 h. Percentage survival data were reported for some
mayfly, stonefly, and caddisfly tests in which LC50 values were not
obtained; there was 60 - 100% survival at test concentrations
ranging from 1.5 to 7.5 mg NH3/litre. Gall (1980) tested NH4Cl
with Ephemerella sp. (near excrucians). Organisms were exposed
to ammonia for 24 h, followed by 72 h in ammonia-free water;
mortality observations were made at the end of the overall 96-h
period. An EC50 value of 4.7 mg NH3/litre was obtained. Hazel et
al. (1979) reported a LC50 value of 8.0 mg NH3/litre for the beetle
Stenelmis sexlineata.
No deaths occurred in ammonia toxicity tests conducted on scud,
Gammarus lacustris, or D. magna, using dilution water from a
river, after a 96-h exposure to 0.08 mg NH3/litre. In a second
test, using river water buffered with sodium bicarbonate, 13%
mortality occurred with scud at the several concentrations tested,
including the highest and lowest of 0.77 and 0.12 mg NH3/litre,
respectively; 7 and 13% mortality occurred with D. magna at the
same concentrations (Miller et al., 1981).
Five fresh-water mussel species, Amblema p. plicata, Anodonta
imbecillis, Corbicula manilensis, Cyrtonaias tampicoensis, and
Toxolasma texasensis, were exposed for 165 h to a concentration of
0.32 mg NH3/litre; T. texasensis was most tolerant to ammonia, and
A. p. plicata was most sensitive (Horne & McIntosh, 1979). During
the tests, the more tolerant species generally had their shells
tightly shut, whereas the least tolerant species continued
siphoning or had their mantles exposed. In 2 studies, acute
exposure of the fresh-water crayfish, Orconectes nais, to ammonium
chloride gave LC50 values of 3.15 and 3.82 mg NH3/litre,
respectively (Evans, 1979; Hazel et al., 1979).
6.3.2. Fresh-water invertebrates: chronic toxicity
Few studies have been conducted on the long-term exposure of
fresh-water invertebrates to ammonia. In a long-term test
conducted by Reinbold & Pescitelli (1982a), reproduction and growth
of D. magna were affected at a concentration of 1.6 mg NH3/litre.
Two tests lasting 42 days were conducted by Anderson et al.
(1978) on the effects of ammonium chloride on the fingernail clam,
Musculium transversum. Significant mortality (67 and 72%) occurred
in both tests at a concentration of 0.7 mg NH3/litre. In one of
the studies, significant reduction in growth was observed after 14
days of exposure to 0.41 mg NH3/litre. Sparks & Sandusky (1981)
reported that fingernail clams exposed to 0.23 and 0.63 mg
NH3/litre showed 36 and 23% mortality, respectively, in 4 weeks;
after 6 weeks, there was 47% mortality at 0.073 mg NH3/litre, and
83% mortality at 0.23 and 0.63 mg NH3/litre. After 6 weeks there
was no growth in any test chamber (concentrations of 0.036 mg
NH3/litre and higher), other than in the controls.
Two partial tests, lasting 24 and 30 days, respectively, were
conducted by Thurston et al. (1984a) on the stonefly Pteronarcella
badia. Adult stonefly emergence was delayed with increasing
ammonia concentration, and little or no emergence occurred at
concentrations exceeding 3.4 mg NH3/litre. There was no significant
relationship between the food consumption rates of nymphs and
concentrations up to 6.9 mg NH3/litre. LC50 values for 24- and
30-day exposures were 1.45 and 4.57 mg NH3/litre, respectively.
The effects of ammonia on the ciliary beating rate of clam
gills were investigated by Anderson et al. (1978). Concentrations
of 0.036 - 0.11 mg NH3/litre caused a reduction in the ciliary
beating rate of fingernail clams; the effects ranged from a 50%
reduction in beating rate to complete inhibition. Adult clams (> 5
mm) were more sensitive than juveniles (< -5 mm); adults were also
slightly more sensitive than the unionid mussel, Elliptio
complanata, and the Asiatic clam, C. manilensis. Shaw (1960)
investigated the effects of ammonium chloride on sodium influx in
the fresh-water crayfish, Astacus pallipes. Ammonia produced an
inhibition of sodium influx; a concentration of 18 mg NH4+/litre
reduced the influx to about 20% of its normal value, and influx
reduction was related to increasing ammonia concentration. This
effect was attributed to NH4+ ions and not to any toxic effect
exerted on the transporting cells by non-ionized ammonia. NH4+ did
not affect chloride influx nor the rate of sodium loss.
Ammonia was added to a stream at a 24-h average concentration
of 1.4 mg NH3/litre, and a 24-h drift net sampling was conducted
(Liechti & Huggins, 1980). No change in diel drift pattern was
observed, but there was an increase in the magnitude of drift, a
shift in the kinds of organisms present, and changes in benthic
standing crop estimates; this ammonia concentration was non-lethal.
6.3.3. Salt-water invertebrates: acute and chronic toxicity
Data on the acute toxicity of ammonia for salt-water
invertebrate species are very limited. A 96-h LC50 value of 1.5 mg
NH3/litre has been reported for the copepod, Nitocra spinipes
(Linden et al., 1979). Lethal effects of ammonium chloride on the
quahog clam, Mercenaria mercenaria, and eastern oyster,
Crassostrea virginica, were studied by Epifano & Srna (1975).
There were no observed differences in susceptibility between
juveniles and adults of the 2 species. Armstrong et al. (1978)
conducted acute toxicity tests (6 days) on ammonium chloride using
prawn larvae, Macrobrachium rosenbergii. LC50 values were highly
pH-dependent. The acute toxicity of ammonium chloride for penaeid
shrimp was reported as a 48-h composite LC50 value of 1.6 mg
NH3/litre for 7 species pooled, including the resident species
Penaeus setiferus (Wickins, 1976). The acute toxicity of ammonium
chloride for the caridean prawn, M. rosenbergii, was reported
(Wickins, 1976) as LT50 values of 1700 - 560 min at concentrations
of 1.74 - 3.41 mg NH3/litre. From the data of Hall et al. (1978),
48-h LC50 values of 0.34 - 0.53 were estimated for grass shrimp,
Palaemonetes pugio. Catedral et al. (1977a,b) investigated the
effects of ammonium chloride on the survival and growth of Penaeus
monodon; larvae had a lower tolerance to ammonia compared with
post-larvae. Brown (1974) reported a time to 50% mortality of
106 min for the nemertine worm, Cerebratulus fuscus, at a
concentration of 2.3 mg NH3/litre.
Effects of ammonium chloride solutions on the American lobster,
Homarus americanus, were studied by Delistraty et al. (1977).
Their studies were performed on fourth-stage larvae, which was
considered to be the most sensitive life stage. A 96-h LC50 value
of 2.2 mg NH3/litre and an incipient LC50 value of 1.7 mg NH3/litre
were reported. A "safe" concentration of 0.17 mg NH3/litre was
tentatively recommended.
The sublethal toxicity of ammonium chloride for the quahog clam
and eastern oyster was studied by Epifano & Srna (1975) who
measured the effect of a 20-h exposure to ammonia on the rate of
removal of the alga, Isochrysis galbana, from suspension (clearing
rate) by the clams and oysters. Concentrations of 0.06 - 0.2 mg
NH3/litre affected removal; no differences were observed between
juveniles and adults. The effect of ammonia on the ciliary beating
rate of the mussel, Mytilus edulis, was studied by Anderson et al.
(1978). Concentrations of 0.097 - 0.12 mg NH3/litre resulted in a
reduction in the ciliary beating rate ranging from 50% to complete
inhibition.
Exposure of unfertilized sea urchin, Lytechinus pictus, eggs to
ammonium chloride resulted in stimulation of the initial rate of
protein synthesis, an event that normally follows fertilization
(Winkler & Grainger, 1978). Exposure of unfertilized eggs of
Strongylocentrotus purpurpatus, L. pictus, and Strongylocentrotus
drobachiensis to ammonium chloride (Johnson et al., 1976; Paul et
al., 1976) caused "fertilization acid" to be released more rapidly
and in greater amounts than after insemination. Activation of
unfertilized L. pictus eggs by ammonium chloride exposure was
also evidenced by an increase in intracellular pH (Steinhardt &
Mazia, 1973; Shen & Steinhardt, 1978). Ammonia treatment has also
been reported to activate phosphorylation of thymidine and
synthesis of histones in unfertilized eggs of the sea urchin,
S. purpuratus, (Nishioka, 1976). Premature chromosome
condensation was induced by ammonia treatment of eggs of L. pictus
and S. purpuratus (Epel et al., 1974; Wilt & Mazia, 1974; Krystal
& Poccia, 1979). Treatment of S. purpuratus and S. drobachiensis
fertilized eggs with ammonia resulted in an absence of the normal
uptake of calcium following insemination. However, calcium uptake
was not inhibited when ammonia treatment preceded insemination
(Paul & Johnston, 1978).
The polychetous annelid, Nereis succinea, the channelled whelk,
Busycon canaliculatum, and the brackish-water clam, Rangia
cuneata, were exposed to concentrations of 0.85, 0.37, and 0.27 mg
NH3/litre and the ammonia excretion measured (Mangum et al., 1978).
Excretion was inhibited by non-lethal concentrations of ammonia,
and the authors concluded that ammonia crosses the excretory
epithelium in the ionized form, and that the process is linked to
the activity of the Na++K+ ATPases. When blue crabs, Callinectes
sapidus, were moved from water of 28 parts per thousand salinity
to water of 5 parts per thousand, a doubling of the ammonia
excretion rate occurred; addition of excess ammonium chloride to
the low-salinity water inhibited ammonia excretion and decreased
net acid output (Mangum et al., 1976). The effect of gaseous NH3
on haemoglobin from the blood of the common marine bloodworm,
Glycera dibrachiata, was examined by Sousa et al. (1977) in an
attempt to determine whether there was competition between NH3 and
oxygen in binding to haemoglobin; such an NH3/O2 relationship was
not found.
6.4. Fish
Ammonia is highly toxic for fish, and, because of its
occurrence at high concentrations in some water systems, it can
present a major pollution problem. It enters aquatic environments
from several sources, including sewage effluent, deposition of
human wastes without treatment, industrial discharges, and runoff
from animal culture and agricultural operations. It is also a
metabolic waste product of fish and, therefore, can be a problem in
facilities involved with intensive fish culture.
Elevated ammonium ion (NH4+) concentrations within the bodies
of fish, as with other vertebrates, cause convulsions and death.
The concentration of non-ionized ammonia (NH3) in the environment
of the fish is important, because ammonia is transferred between
the water and fish largely in this form. Thus, while NH3 is the
more toxic chemical species in the water, within the fish, toxicity
is related to the NH4 concentration.
Research by Chipman (1934), Wuhrman et al. (1947), Wuhrman &
Woker (1948), and Tabata (1962) implicated NH3 as the ammonia
species in water that is mainly toxic for fish, and reported that
NH4+ was non-toxic or considerably less toxic. More recent
research by Robinson-Wilson & Seim (1975), Armstrong et al. (1978),
and Thurston et al. (1981c) has demonstrated that the role of water
pH in the toxicity of ammonia for fish is more than the regulation
of the NH3/NH4+ equilibrium. NH3 is considerably more toxic in
water when pH values are lower than 7 - 9, and there is some
evidence that the toxicity of NH3 is also increased above this
range. Temperature and dissolved oxygen and the ionic composition
of the background water all play a role in the toxicity of NH3 for
some fish.
In a comprehensive analysis of the data, it was concluded that
a number of fish species that are phylogenetically similar are also
similar in their sensitivity to the toxicity of ammonia, and that
many of the factors that affect the toxicity of ammonia similarly
affect all the species that have been studied (US EPA, 1985).
It was formerly considered that fish of the family Salmonidae
were among the most sensitive to the effects of many pollutants,
whereas other fish species, which have evolved in warm-water, low
oxygen, or more turbid aquatic environments, may be less sensitive
to many naturally-occuring pollutants, such as ammonia. However,
other fish species, including some that are frequently referred to
as "warm water" fishes, are of comparable sensitivity (US EPA,
1985). In the "resident-species" approach for establishing water
quality criteria for a toxic agent in a given water body, the
tolerance of selected fish and invertebrate species, naturally
resident in the water body, is used. It must be borne in mind
that, if these selected species are less sensitive than other
species in different water bodies, the standards may be
inappropriate for other water bodies.
Because of variation among different background test water
conditions in different laboratories, as well as differences in the
genetic pools of the same species, results of a single test on a
given species may not be as meaningful as composite results from
several tests conducted at different laboratories.
Much of the evidence on ammonia toxicity is empirical, so, for
a more complete understanding of the biological actions of this
chemical, the results of toxicity tests must be integrated with a
knowledge of ammonia metabolism in fish.
6.4.1. Ammonia metabolism in fish
6.4.1.1. Ammonia production and utilization
The major pathway for the production of ammonia in fish, as in
other vertebrates, is through the transamination of various amino
acids (Forster & Goldstein, 1969; Watts & Watts, 1974). The
primary site for ammonia production is probably the liver (Pequin &
Serfaty, 1963), but the necessary enzymes have also been located in
the kidneys, gills, and skeletal muscle tissue (Goldstein &
Forster, 1961; McBean et al., 1966; Walton & Cowey, 1977). Ammonia
is also produced by the deamination of adenylates in fish muscle
(Driedzic & Hochachka, 1976). The quantitative importance of
muscle ammoniogenesis in total ammonia excretion depends on the
level of activity of the animal, and increases with increasing
workload (Suyama et al., 1960; Fraser et al., 1966; Driedzic &
Hochachka, 1976).
Ammonia toxicity can be ameliorated by the formation of less
toxic compounds, namely glutamine and urea. Levi et al. (1974)
recorded high levels of glutamine in the brain of goldfish,
Carrasius auratus, and found that brain-glutamine levels increased
with ambient ammonia concentrations. Webb & Brown (1976) found
high glutamine synthetase (EC 6.3.1.2) activity in the brains of
teleosts and elasmobranchs, and this may be important in protecting
the brain from sudden surges in ammonia concentration. Walton &
Cowey (1977) were able to detect glutaminase activity in the gills
of rainbow trout, but were unable to measure any in vivo
utilization of glutamine by the gills.
Ammonia can be converted, through carbamyl phosphate, to urea
either via purines (uricolysis) or via the ornithine cycle. The
enzymes required for uricolysis have been found in most fish
studied (Forster & Goldstein, 1969; Watts & Watts, 1974), but
Florkin & Duchateau (1943) were unable to detect any activity of
uricolytic enzymes in the cyclostome, Lampetra. The ratio of urea
production via the ornithine cycle to production via uricolysis is
about 100 to 1 in elasmobranchs and dipnoi, whereas in teleosts
most of the urea is formed via uricolysis (Gregory, 1977).
6.4.1.2. Ammonia excretion
The gills are the major site of ammonia excretion in fish, but
smaller quantities of ammonia may also be eliminated by the kidneys
(Edwards & Condorelli, 1928; Grollman, 1929; Fromm, 1963; Maetz,
1972) and skin (Morii et al., 1978). Although the majority of
branchial ammonia excretion represents clearance from the blood,
gill metabolism may contribute between 20% (Payan & Matty, 1975)
and 5 - 8% (Cameron & Heisler, 1983) of the net ammonia excretion.
The excretion of ammonia by fish is variable, depending on the
state of the animal, the environmental conditions, and the species.
Ammonia excretion tripled in sockeye salmon, Oncorhynchus nerka,
following daily feeding (Brett & Zala, 1975) but remained low and
unchanging during starvation (Brett & Zala, 1975; Guerin-Ancey,
1976a). In fresh-water fish, ammonia excretion increases in
response to exercise (Sukumaran & Kutty, 1977; Holeton et al.,
1983), long-term acid exposure (McDonald & Wood, 1981; Ultsch et
al., 1981), hypercapnia (Claiborne & Heisler, 1984), and NH4Cl
infusion (Hillaby & Randall, 1979). In contrast, increased levels
of environmental ammonia (Guerin-Ancey, 1976b) and short-term
exposure to acid or alkaline water (Wright & Wood, 1985) cause a
decrease in ammonia excretion. It is not known if these changes in
excretion reflect change in the rate of ammonia production or in
the ammonia content of the body. The ammonia content of fish is
likely to be the equivalent of the ammonia excreted in about 2 h,
most of the ammonia being in the tissues with a lower pH, such as
muscle. Blood levels are around 0.2 to 0.3 mmol, but muscle at a
lower pH may contain levels of up to 1 mmol; thus, a 1-kg fish may
contain about 0.5 to 0.7 mmol of ammonia and have an excretion rate
of about 0.3 mmol/h. There is increased ammonia production in
muscle during exercise (Driedzic & Hochachka, 1976). Ammonia
excretion by the spiny dogfish, Squalus acanthias, in sea water is
unaffected by temperature change, exercise, hyperoxia, hypercapnia,
or the infusion of either HCl or NaHCO3 or anything that induces
acid-base stress (Heisler, 1984). This is surprising, because many
of these changes affect pH and therefore would be expected to alter
the ammonia content of body compartments and consequently ammonia
excretion.
There is an elevation in blood-ammonia during starvation (Morii
et al., 1978; Hillaby & Randall, 1979), even though ammonia
excretion does not change (Brett & Zala, 1975). Blood-ammonia
concentrations also rise with increases in both temperature
(Fauconneau & Luquet, 1979) and ammonia concentrations in the water
(Fromm & Gillette, 1968; Thurston et al., 1984b). Exposure of fish
to either air (Gordon, 1970) or increased ammonia levels in water
(Fromm, 1970; Guerin-Ancey, 1976b), raises blood-ammonia levels and
reduces ammonia excretion; this is associated with a rise in urea
production in many, but not all fish. Unlike the authors of the
above studies, Buckley et al. (1979) did not find any change in
blood-total ammonia when coho salmon, Oncorhynchus kisutch, were
exposed to elevated ammonia levels in the environment. However, a
significant rise in plasma-sodium, indicating some coupling between
sodium uptake and ammonia excretion, was observed.
The study of ammonia movement is complicated by the fact that,
with present analytical techniques, it is impossible to distinguish
between the transfer of a molecule of NH3 plus a H+ ion from the
transfer of an NH4+ ion. Thus, only indirect evidence can be
obtained regarding the relative gas and ion movements across the
gill epithelium.
Three possible mechanisms of ammonia excretion have received
the most attention: passive NH3 flux, ionic exchange of NH4+ for
Na+, and passive NH4+ flux. There seems to be little doubt that a
significant pathway for branchial ammonia excretion is by the
passive diffusion of NH3 down its partial pressure gradient.
Changes in the NH3 partial pressure gradient are positively
correlated with changes in net ammonia excretion in the channel
catfish, Ictalurus punctatus, (Kormanik & Cameron, 1981), and
rainbow trout (Cameron & Heisler, 1983; Wright & Wood, 1985).
Ammonia entry into the fish has also been shown to be dependent on
the NH3 gradient (Wuhrmann et al., 1947; Wuhrmann & Woker, 1948;
Fromm & Gillette, 1968).
The excretion of NH4+ is strongly coupled with the movement
of other ions. Many studies have attempted to link the
transepithelial exchange of Na+ uptake to NH4+ efflux. Although
there is considerable indirect evidence for the presence of a
coupled ionic exchange mechanism under certain conditions (Maetz &
Garcéa-Romeu, 1964; Maetz, 1973; Payan & Maetz, 1973; Evans, 1977,
1980; Payan, 1978; Girard & Payan, 1980; Wright & Wood, 1985), the
ubiquity and stoichiometry of this exchange remain controversial.
While Na+ influx can be monitored with isotopes, it is difficult to
determine NH4+ efflux. Investigators have attempted to quantify
the relationship between Na+ uptake and NH4+ excretion by
manipulating Na+ levels in the environmental water, by
pharmaceutical inhibition of the Na+ influx mechanism, or by
loading the fish with ammonia.
In goldfish (Maetz, 1973), and in irrigated rainbow trout gills
(Kirschner et al., 1973), Na+ influx was best correlated with the
sum of H+ and NH4+ ion efflux. The possibility of a Na+ uptake
carrier coupled to either NH4+ or H+ appears likely in other fish
as well (Kerstetter et al., 1970; Payan & Maetz, 1973; Evans,
1977). In perfused heads of trout, Na+ uptake was tightly coupled
with NH4+ efflux (Payan et al., 1975; Payan, 1978). Wright & Wood
(1985) demonstrated that, in intact trout, the rate of ion exchange
was influenced by external water pH, increasing from no exchange at
pH 4 to maximal rates at pH 8. The relationship between Na+ and
NH4+ was one-to-one at a pH of less than 8. However, Payan et al.
(1975) and Wright & Wood (1985) found that the majority of the
ammonia was eliminated by gaseous diffusion, and only when NH3 was
subtracted from total ammonia efflux was the NH4+/Na+ exchange
evident. In common carp, Cyprinus carpio (de Vooys, 1968), and
little skate, Raja erinacea (Evans et al., 1979), ammonia
excretion was unaffected by a reduction in environmental Na+
levels. Cameron & Heisler (1983) found that, under resting
conditions, diffusive movement of NH3 could account for ammonia
excretion in trout, but when the ammonia gradient was reversed and
directed inwards, an NH4+/Na+ exchange could counter-balance the
diffusive uptake of NH3 from the water. If this hypothesis is
correct, then it would explain the unchanging blood-ammonia levels
and increased Na+ levels in coho salmon exposed to elevated
concentrations of ammonia in the water (Buckley et al., 1979).
The Na+/NH4+ (H+) exchange is probably located on the
epithelial apical membrane. Either acid conditions or amiloride in
the water inhibits Na+ influx across the gills and both these
conditions result in a reduction in ammonia excretion (Wright &
Wood, 1985).
The ammonium ion can displace potassium in many membrane
processes in, for example, the giant axon of squid, Loligo pealei
(Binstock & Lecar, 1969), and this is the probable reason that
elevated ammonia causes convulsions in so many vertebrates. In
various aquatic animals, it is possible that NH4+ can substitute
for potassium in oubain-sensitive sodium/potassium exchange (Payan
et al., 1975; Towle & Taylor, 1976; Towle et al., 1976; Mallery,
1979; Girard & Payan, 1980). NH4+ ions will substitute for K+ ions
across the epithelial basolateral border (Richards & Fromm, 1970;
Shuttleworth & Freeman, 1974; Karnaky et al., 1976), but the
importance to net ammonia transfer in fresh-water fish is unknown.
The passive movement of NH4+ down its electrochemical gradient
may also contribute to net ammonia excretion (Claiborne et al.,
1982; Goldstein et al., 1982). Lipid membranes are relatively
impermeable to cations (Jacobs, 1940) and, because respiratory
epithelial cells of fresh-water fishes are joined by tight
junctions (Girard & Payan, 1980), it appears unlikely that NH4+
diffusion is of quantitative importance (Kormanik & Cameron, 1981).
Indeed, Wright & Wood (1985) found a negative correlation between
ammonia excretion and the NH4+ concentration gradient in rainbow
trout exposed to 5 different water pH regimes. Although it appears
that NH4+ diffusion may be of minor importance, simultaneous
measurements of the electrical and chemical gradient have not been
made and are necessary before conclusions can be drawn.
6.4.2. Fish: acute toxicity
The acute toxicity of ammonia for rainbow trout has been
studied by many investigators, with reported 96-h LC50 values
ranging from 0.16 to 1.1 mg NH3/litre. Thurston & Russo (1983)
conducted 71 toxicity tests on rainbow trout ranging in size from
sac fry (< 0.1 g) to 4-year-old adults (2.6 kg), in water of
uniform chemical composition. LC50 values ranged from 0.16 to
1.1 mg NH3/litre for 96-h exposures. Fish susceptibility to NH3
decreased with increasing weight over the range 0.06 - 2.0 g, but
gradually increased above that weight range. LC50 values for 12-
and 35-day exposures did not differ greatly from 96-h values. No
statistically-significant differences in results were observed when
different ammonium salts [NH4Cl, NH4HCO3, (NH4)2HPO4, (NH4)2SO4]
were used. Grindley (1946) also reported that there were no
appreciable differences in toxicity between toxic solutions of
NH4Cl and (NH4)2SO4 in rainbow trout tests. However, Calamari et
al. (1977, 1981) reported that embryos and fingerlings were less
sensitive than the other life stages studied. LC50 values (96-h)
ranging from 0.16 to 1.02 mg NH3/litre for rainbow trout exposed to
ammonia were reported by Calamari et al. (1977, 1981), Broderius &
Smith (1979), Holt & Malcolm (1979), DeGraeve et al. (1980), and
Reinbold & Pescitelli (1982b).
Although acute toxicity studies with salmonids have mainly been
conducted on rainbow trout, some data are available for a few other
salmonid species. Thurston et al. (1978) investigated the
toxicity of ammonia for cutthroat trout, Salmo clarki, and
reported 96-h LC50 values of 0.52 - 0.80 mg NH3/litre. Thurston &
Russo (1981) reported a 96-h LC50 value of 0.76 mg NH3/litre for
golden trout, Salmo aguabonita. Brown trout, Salmo trutta, were
exposed to 0.15 mg NH3/litre for 18 h, resulting in a 36%
mortality; when returned to ammonia-free water, the test fish
recovered after 24 h (Taylor, 1973). No mortality occurred during
the 96-h exposure at 0.090 mg NH3/litre, although fish would not
feed. Exposure to 0.8 mg NH3/litre was not acutely toxic for brown
trout according to Woker & Wuhrmann (1950). However, Thurston &
Meyn (1984) reported 96-h LC50 values of 0.60 - 0.70 mg NH3/litre,
and Miller et al. (1981) reported a 96-h LC50 value of 0.47 mg
NH3/litre for brown trout using test dilution river water. Brook
trout, Salvelinus fontinalis, showed distress within 1.75 h at a
concentration of 3.25 mg NH3/litre and within 2.5 h at 5.5 mg/litre
(Phillips, 1950). Thurston & Meyn (1984) reported 96-h LC50 values
of 0.96 - 1.05 mg NH3/litre for brook trout, 0.40 - 0.48 mg
NH3/litre for chinook salmon, Oncorhynchus tshawytscha, and 0.14 -
0.47 mg NH3/litre for mountain whitefish, Prosopium williamsoni.
Toxicity tests with (NH4)2SO4 on pink salmon, Oncorhynchus
gorbuscha, at different early stages of development (Rice & Baily,
1980) showed that late alevins near swim-up stage were the most
sensitive (96-h LC50 = 0.083 mg NH3/litre), and eyed embryos were
the most tolerant, surviving 96 h at > 1.5 mg NH3/litre. Buckley
(1978) reported a 96-h LC50 value of 0.55 mg NH3/litre for
fingerling coho salmon, Oncorhynchus kisutch, and Herbert &
Shurben (1965) reported a 24-h LC50 value of 0.28 mg NH3/litre for
Atlantic salmon, Salmo salar.
There are acute toxicity data for ammonia in a variety of non-
salmonid fish species. Thurston et al. (1983) studied the toxicity
of ammonia for fathead minnows, Pimephales promelas, ranging from
0.1 to 2.3 g in weight and found that 96-h LC50 values in 29 tests
ranged from 0.75 to 3.4 mg NH3/litre; toxicity was not dependent on
the test fish size or source. LC50 values ranging from 0.73 to
2.35 mg NH3/litre for fathead minnows were also reported by Sparks
(1975), DeGraeve et al. (1980), Reinbold & Pescitelli (1982b),
Swigert & Spacie (1983). LC50 values for white sucker, Catostomus
commersoni, exposed to ammonium chloride solutions for 96 h
(Reinbold & Pescitelli 1982c) were 1.40 and 1.35 mg/litre NH3,
though a somewhat lower 96-h LC50 of 0.79 mg NH3/litre was
determined by Swigbert & Spacie (1983). Thurston & Meyn (1984)
reported 96-h LC50 values of 0.67 - 0.82 mg NH3/litre for the
mountain sucker, Caststomus platyrhynchus.
Reported LC50 values for 96-h exposures of bluegill, Lepomis
macrochirus, ranged from 0.26 to 4.60 mg NH3/litre (Emery & Welch,
1969; Lubinski et al., 1974; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982b; Swigert & Spacie, 1983). LC50 values (96-h) of
0.7 - 1.8 mg NH3/litre for smallmouth bass, Micropterus dolomieui,
and 1.0 - 1.7 mg NH3/litre for largemouth bass, Micropterus
salmoides, were reported by Broderius et al. (in press) and
Roseboom & Richey (1977), respectively. Sparks (1975) reported
48-h LC50 values for bluegill of 2.30 mg NH3/litre, and for channel
catfish of 2.92 mg NH3/litre. For goldfish, Carassius auratus,
Dowden & Bennett (1965) reported a 24-h LC50 of 7.2 mg NH3/litre,
and Chipman (1934) reported lethal threshold values of 0.97 -
3.8 mg NH3/litre. Turnbull et al. (1954) reported a 48-h LC50 for
bluegill to be within the range 0.024 - 0.093 mg NH3/litre; during
the exposure, they observed that the fish exhibited a lack of
ability to avoid objects.
Reported 96-h LC50 values for channel catfish, Ictalurus
punctatus, ranged from 1.5 to 4.2 mg NH3/litre (Colt &
Tchobanoglous, 1976; Roseboom & Richey, 1977; Reinbold &
Pescitelli, 1982d; Swigert & Spacie, 1983). Vaughn & Simco (1977)
reported a 48-h LC50 for channel catfish of 1.24 - 1.96 mg
NH3/litre, and Knepp & Arkin (1973) reported 1-week LC50 values of
0.97 - 2.0 mg NH3/litre. The results of studies on bluegill,
channel catfish, and largemouth bass (Roseboom & Richey, 1977)
showed that bluegill susceptibility was dependent on fish weight,
fish weighing 0.07 g being slightly more sensitive than those
weighing either 0.22 or 0.65 g; size had little effect on the
susceptibility of channel catfish or bass.
Hazel et al. (1979) reported 96-h LC50 values of 0.90 and
1.07 mg NH3/litre for the orangethroat darter, Etheostoma
spectabile, and red shiner, Notropis lutrensis. Largemouth bass,
channel catfish, and bluegill were also exposed for 96 h to a
concentration of 0.21 mg NH3/litre resulting in zero mortality for
bluegill and channel catfish and one death (6%) among the
largemouth bass tested. A 96-h LC50 value for walleye,
Stizostedion vitreum, of 0.85 mg/litre NH3 was reported by
Reinbold & Pescitelli (1982a).
LC50 values ranging from 2.4 to 3.2 mg NH3/litre for
(NH4)2CO3, NH4Cl, NH4C2H3O2, and NH4OH, in 96-h exposures of
mosquitofish, Gambusia affinis, in waters with suspended solids
ranging from < 25 to 1400 mg/litre were reported by Wallen et al.
(1957). Susceptibility of mosquito fish to ammonia was studied by
Hemens (1966) who reported a 17-h LC50 of 1.3 mg NH3/litre; he also
observed that male fish were more susceptible than females. Rubin
& Elmaraghy (1976, 1977) tested guppy, Poecilia reticulata, fry
and reported 96-h LC50 values averaging 1.50 mg NH3/litre; mature
guppy males were more tolerant, with 100% survival for 96 h at
concentrations of 0.17 - 1.58 mg NH3/litre. LC50 values (96-h) of
0.15 and 0.20 mg NH3/litre at pH 6.0, and of 0.52 and 2.13 mg
NH3/litre at pH 8.0, were reported by Stevenson (1977) for white
perch, Morone americana. LC50 values (96-h) of 1.20 and 1.62 mg
NH3/litre for spotfin shiner, Notropis spilopterus, were reported
by Rosage et al. (1979), and of 1.20 mg NH3/litre for golden
shiner, Notemigonus crysoleucas, by Baird et al. (1979). Swigert
& Spacie (1983) reported 96-h LC50 values of 0.72 mg NH3/litre for
golden shiner, 1.35 mg NH3/litre for spotfin shiner, 1.25 mg
NH3/litre for steelcolour shiner, Notropis whipplei, and 1.72 mg
NH3/litre for stoneroller, Campostoma anomalum.
Jude (1973), Reinbold & Pescitelli (1982a), and McCormick et
al. (1984) reported 96-h LC50 values ranging from 0.6 to 2.1 mg
NH3/litre for green sunfish, Lepomis cyanellus. In studies on the
pumpkinseed sunfish, Lepomis gibbosus, by Jude (1973) and Thurston
(1981), 96-h LC50 values ranged from 0.14 to 0.86 mg NH3/litre.
Mottled sculpin, Cottus bairdi, were tested by Thurston & Russo
(1981), yielding a 96-h LC50 value of 1.39 mg NH3/litre. An
asymptotic (6-day) LC50 of 0.44 mg NH3/litre was determined for
rudd, Scardinius erythropthalmus (Ball, 1967).
Rao et al. (1975) reported a 96-h LC50 value for the common
carp, Cyprinus carpio, of 1.1 mg NH3/litre. Carp exposed to
0.24 mg NH3/litre exhibited no adverse effects in 18 h (Vámos
1963). However, exposure to 0.67 mg NH3/litre caused gasping and
equilibrium disturbance within 18 min, frenetic swimming activity
at 25 min, then sinking to the tank bottom after 60 min; after
75 min, the fish were placed in ammonia-free water and all revived.
Kempinska (1968) reported a lethal concentration of 7.5 mg
NH3/litre for carp. Studies on the acute exposure of bitterling,
Rhodeus sericeus, and carp to ammonium sulfate revealed minimum
lethal concentrations of 0.76 mg NH3/litre for bitterling and
1.4 mg NH3/litre for carp (Malacea, 1966). Nehring (1963) reported
survival times for carp at concentrations of 9.7 and 2.1 mg
NH3/litre of 2.4 and 6.0 h, respectively. The survival time for
tench, Tinca tinca, was reported to be 20 - 24 h at 2.5 mg
NH3/litre by Danecker (1964). In a 24-h exposure of creek chub,
Semotilus atromaculatus, to ammonium hydroxide solution, the
"critical range" below which all test fish lived and above which
all died was reported to be 0.26 - 1.2 mg NH3/litre (Gillette et
al., 1952).
In static exposures lasting 9 - 24 h, with a gradual increase
in NH3 content, mortalities occurred in oscar, Astronutus
ocellatus, at 0.50 mg NH3/litre (4%) to 1.8 mg/litre (100%)
(Magalhaes Bastos, 1954). Tests on oscar of two different sizes
showed no difference in susceptibility, in relation to size. A
72-h LC50 value of 2.85 mg NH3/litre was reported by Redner &
Stickney (1979) for blue tilapia, Tilapia aurea.
6.4.2.1. Saltwater fish
Very few acute toxicity data are available for salt-water fish
species. Holland et al. (1960) reported the critical level for
chinook salmon, Oncorhynchus tshawytscha, to be between 0.04 and
0.11 mg NH3/litre and for coho salmon to be 0.134 mg NH3/litre. A
static test with coho salmon provided a 48-h LC50 value of 0.50 mg
NH3/litre (Katz & Pierro, 1967). Atlantic salmon smolts and
yearling rainbow trout exposed for 24 h in 50 and 75% saltwater
solutions exhibited similar sensitivities to ammonia (United
Kingdom Ministry of Technology, 1963). Holt & Arnold (1983)
reported a 96-h LC50 value of 0.47 mg NH3/litre for red drum,
Sciaenops ocellatus. LC50 values (96-h) of 1.2 - 2.4 mg NH3/litre
were reported by Venkataramiak et al. (1981) for striped mullet,
Mugil caphalus, and 0.69 mg NH3/litre for planehead filefish,
Monacanthus hispidus.
6.4.3. Factors affecting acute toxicity
A number of factors can affect the toxicity of ammonia for
aquatic organisms. These include the effects of pH, temperature,
dissolved oxygen concentration, previous acclimatization to
ammonia, fluctuating or intermittent exposures, carbon dioxide
concentration, salinity, and the presence of other toxicants.
Almost all studies of factors affecting ammonia toxicity have been
carried out using only acute exposures.
6.4.3.1. pH
The toxicity for fish of aqueous solutions of ammonia and
ammonium compounds has been attributed to the non-ionized
(undissociated) ammonia present in the solution. The earliest
reported thorough study of the pH dependence of ammonia toxicity
was that of Chipman (1934), who concluded from studies on goldfish,
amphipods, and cladocerans that ammonia toxicity was a function of
pH and therefore of the concentration of undissociated ammonia in
the solution. Downing & Merkens (1955) tested rainbow trout at
different concentrations of ammonia at both pH 7 and pH 8. The
results were consistent when ammonia concentration was expressed as
NH3. Tabata (1962) conducted 24-h tests on the toxicity of ammonia
for Daphnia (species not specified) and guppy at different pH
values and calculated the relative toxicity of NH3/NH4+ to be 48
for Daphnia and 190 for guppy (i.e., NH3 is 190 times more toxic
than NH4+).
More recently, Robinson-Wilson & Seim (1975) studied the
toxicity of ammonium chloride for juvenile coho salmon in flow-
through bioassays within the pH range 7.0 - 8.5; the reported
96-h LC50 for NH3 was approximately 60% less at pH 7.0 than at pH
8.5. The toxicity of ammonium chloride for larvae of prawn,
Macrobrachium rosenbergii, was studied by Armstrong et al. (1978)
in 6-day tests within the pH range 6.8 - 8.3 with test solutions
being renewed every 24 h. The 96-h LC50 for NH3 at pH 6.83 was
approximately 70% less than that at pH 8.34. It was concluded that
the toxicity of ammonia was not due solely to the NH3 molecule and
that in solutions of different pH, but equal NH3 concentrations,
survival was greatly reduced as NH4+ levels increased. Tomasso et
al. (1980) studied the toxicity of ammonia (NH3) for channel
catfish at pH values of 7, 8, and 9 and reported that 24-h LC50
values were significantly higher at pH 8 than at pH 7 or pH 9.
Thurston et al. (1981c) tested the toxicity of ammonia for
rainbow trout and fathead minnows in 96-h flow-through tests at
different pH levels within the range 6.5 - 9.0. Results showed
that the toxicity of ammonia, in terms of NH3, increased at lower
pH values, and could also increase at higher pH values. It was
concluded that NH4+ exerts some measure of toxicity, and/or that
increased H+ concentration increases the toxicity of NH3. Acute
(96-h) exposures of green sunfish and smallmouth bass at 4
different pH levels over the range 6.5 - 8.7 showed that, for both
species, NH3 toxicity increased markedly with a decrease in pH,
with LC50 values at the lowest pH tested (6.6 for sunfish, 6.5 for
bass) being 3.6 (sunfish) and 2.6 (bass) times smaller than those
at the highest pH (8.7) tested (McCormick et al., 1984; Broderius
et al., in press).
It is concluded that NH3 is more toxic for fish at lower pH
values than within the pH range 7 - 9; the toxicity of NH3 may
increase again above this range.
6.4.3.2. Temperature
Information in the literature on the effects of temperature on
ammonia toxicity is varied. The concentration of NH3 increases
with increasing temperature. Several researchers have reported an
effect of temperature on the toxicity of the non-ionized ammonia
species, independent of the effect of temperature on the aqueous
ammonia equilibrium.
McCay & Vars (1931) reported that it took three times as long
for the brown bullhead, Ictalurus nebulosus, to succumb to the
toxicity of ammonia in water at 10 - 13 °C than at 26 °C. The pH
of the tested water was not reported but with the probable range
tested (pH 7 - 8), the percent NH3 at the higher test temperature
would have been approximately three times that at the mean lower
temperature. The toxicity of ammonium chloride for goldfish,
bluntnose minnow, Pimephales notatus, and the straw-coloured
minnow or river shiner, Notropis blennius, was reported (Powers,
1920) to be greater at high temperatures than at low, but no
consideration was given to the increase in the relative
concentration of NH3 as the temperature increased. Herbert (1962)
suggested that the effects of temperature on the susceptibility of
rainbow trout to NH3 toxicity was only slightly, if at all,
affected by temperature change. In studies on striped bass, Morone
saxtilis, and stickleback, Gasterosteus aculeatus, Hazel et al.
(1971) found a slight difference in toxicity between 15° and 23 °C
in fresh water, with both fish species being slightly more
resistant at the lower temperature.
However, there are other studies in which the toxicity of NH3
decreased with increasing temperature over the ranges studied. The
toxicity of NH3 for rainbow trout has been reported to be much
higher at 5 °C than at 18 °C (United Kingdom Ministry of
Technology, 1968). Brown (1968) reported that the 48-h LC50 for
rainbow trout increased with increase in temperature over the range
3 °C - 18 °C; the reported increase in tolerance between ~12 °C -
~18 °C was considerably less than that between ~3 °C - ~12 °C. A
relationship between temperature and 96-h LC50 was reported for
rainbow trout over the temperature range 12 °C - 19 °C with ammonia
toxicity decreasing with increasing temperature (Thurston & Russo,
1983).
Thurston et al. (1983) reported that the acute toxicity of NH3
for fathead minnows decreased with a rise in temperature over the
range 12 °C - 22 °C. Bluegill and fathead minnow were tested at
low and high temperatures of 4.0 °C - 4.6 °C and 23.9 °C - 25.2 °C,
respectively, and rainbow trout were tested at 3 °C and 14 °C
(Reinbold & Pescitelli, 1982b). All three species were more
sensitive to NH3 at the low temperatures, with toxicity being 1.5 -
5 times higher in the colder water. Bluegill appeared to be the
most sensitive of the three species to the effects of low
temperature on ammonia toxicity. Colt & Tchobanoglous (1976)
reported that the toxicity of NH3 for channel catfish decreased
with increasing temperature over the range 22 °C - 30 °C. LC50
values for bluegill, channel catfish, and largemouth bass at 28 °C
- 30 °C were approximately twice those at 22 °C (Roseboom & Richey,
1977). An effluent containing ammonia as the principal toxic
component showed a marked decrease in toxicity for channel catfish
over the temperature range 4.6 °C - 21.3 °C (Cary, 1976).
Lloyd & Orr (1969) investigated the effects of temperature
(range 10 - 20 °C) on urine flow rates in rainbow trout exposed to
0.30 mg NH3/litre, and did not find any apparent temperature effect
on the total diuretic response of the fish, though the relative
increase in urine production was less at higher temperatures. From
a study of the behavioural response of bluegill to gradients of
ammonium chloride, it was hypothesized that low temperatures
increased the sensitivity of the bluegill and interfered with the
ability, either to detect ammonia after a certain period of
exposure, or, to compensate behaviourally for physiological stress
caused by ammonia gradients (Lubinski, 1979; Lubinski et al.,
1980).
The European Inland Fisheries Advisory Commission (1970)
has stated that, at temperatures below 5 °C, the toxic effects of
non-ionized ammonia may be greater than at above 5 °C, though the
basis for this is not clearly documented. The evidence that
temperature, independent of its role in the aqueous ammonia
equilibrium, affects the toxicity of NH3 for fish argues for
further consideration of the temperature/ammonia toxicity
relationship.
6.4.3.3. Salinity
Herbert & Shurben (1965) reported that the resistance of
yearling rainbow trout to ammonium chloride increased with
increasing salinity up to levels of 30 - 40% sea water; above this
level, resistance appeared to decrease. Fingerling coho salmon
were tested at salinity levels of 20 - 30 parts per thousand (57 -
86% salt water), and it was found that the toxicity of an ammonia-
ammonium waste increased as salinity increased (Katz & Pierro,
1967). These findings are in agreement, at the levels tested, with
those of Herbert & Shurben (1965). Atlantic salmon were exposed to
ammonium chloride solutions for 24 h under both fresh-water and 30%
salt-water conditions; LC50 values were 0.15 and 0.3 mg NH3/litre,
respectively, in the 2 different waters (Alabaster et al., 1979).
Harader & Allen (1983) reported that the resistance to ammonia of
chinook salmon parr increased by about 500%, as salinity increased
to almost 30% sea water, but declined as salinity increased beyond
that.
There is a slight decrease in the NH3 fraction of total ammonia
as ionic strength increases in dilute saline solutions, but the
relative changes in NH3 toxicity, as salinity increases, are more
directly attributable to changes in the rate of exchange of NH3 and
NH4+ across the fish gill membranes.
6.4.3.4. Dissolved oxygen
A decrease in dissolved oxygen concentration in the water can
increase ammonia toxicity. There is a reduction in fish blood
oxygen-carrying capacity following ammonia exposure (Brockway,
1950; Danecker, 1964; Reichenback-Klinke, 1967; Körting, 1969a,b;
Waluga & Flis, 1971). Hypoxia would further exacerbate problems of
oxygen delivery and could lead to the early demise of the fish.
Vámos & Tasnádi (1967) observed deaths of carp in ponds at
ammonia concentrations lower than would normally be lethal, and
attributed this to periodic low concentrations of oxygen. On the
basis of research in warm-water (20 °C - 22 °C) fish ponds, Selesi
& Vámos (1976) projected a "lethal line", relating acute ammonia
toxicity and dissolved oxygen, below which carp died. The line ran
between 0.2 mg NH3/litre at 5 mg dissolved oxygen/litre and 1.2 mg
NH3/litre at 10 mg dissolved oxygen/litre. Thurston et al. (1983)
compared the acute toxicity of ammonia for fathead minnows at
reduced and normal dissolved oxygen concentrations; seven 96-h
tests were conducted within the range 2.6 - 4.9 mg dissolved
oxygen/ litre, and 3 between 8.7 and 8.9 mg/litre. There was a
slight positive trend between 96-h LC50 values and dissolved
oxygen, though it was not shown to be statistically significant.
Atlantic salmon smolts were tested in both fresh water and 30% salt
water at 9.6 - 9.5 and 3.5 - 3.1 mg dissolved oxygen/litre. The
reported 24-h LC50 values at the higher oxygen concentrations were
about twice those at the lower (Alabaster et al., 1979).
Several studies have been reported on rainbow trout. Allan
(1955) reported that below 0.12 mg NH3/litre and at about 30%
oxygen saturation, the median survival time was greater than 24 h,
but at the same concentration with oxygen saturation below 30%, the
median survival time was less than 24 h. In studies by Downing &
Merkens (1955), fingerling rainbow trout were tested at 3 different
concentrations of NH3 at 5 different levels of dissolved oxygen.
In tests lasting up to 17 h, decreasing the oxygen level from 8.5
to 1.5 mg/litre shortened the period of survival at all ammonia
concentrations, and a decrease in survival time produced by a given
decrease in oxygen was greatest at the lowest concentration of NH3.
Merkens & Downing (1957), in tests lasting up to 13 days, also
reported that the effects of low concentrations of dissolved oxygen
on the survival of rainbow trout were more pronounced at low
concentrations of NH3. Ammonia (NH3) was found to be up to 2.5
times more toxic when the dissolved oxygen concentration was
reduced from 100 to about 40% saturation (Lloyd, 1961). It was
reported by Danecker (1964) that the toxicity of ammonia increased
rapidly when the oxygen concentration decreased below two-thirds of
the saturation value. Thurston et al. (1981b) conducted 15, 96-h
acute toxicity tests on rainbow trout over the dissolved oxygen
range 2.6 - 8.6 mg/litre. A positive linear correlation between
96-h LC50 and dissolved oxygen was reported over the entire range
tested.
When rainbow trout were treated in a channel receiving sewage
discharge containing 0.05 - 0.06 mg NH3/litre, it was found that,
at 25 - 35% dissolved oxygen saturation, more than 50% of the fish
died within 24 h, compared with 50% mortality of test fish in the
laboratory, at 15% dissolved oxygen saturation (Herbert, 1956).
The difference was attributed to unfavourable water conditions
below the sewage outflow, including ammonia, which increased the
sensitivity of the fish to the lack of oxygen.
6.4.3.5. Carbon dioxide
An increase in carbon dioxide (CO2) concentrations up to
30 mg/litre decreased total ammonia toxicity (Alabaster & Herbert,
1954; Allan et al., 1958). Carbon dioxide causes a decrease in pH,
thereby decreasing the proportion of non-ionized ammonia in
solution. However, Lloyd & Herbert (1960) found that, though total
ammonia toxicity was reduced at elevated CO2 levels, the inverse
was true when considering non-ionized ammonia alone; more NH3 was
required in low CO2, high pH water to exert the toxic effect seen
in fish in high CO2, low pH water. The explanation presented by
Lloyd & Herbert (1960) for the decreased toxicity of NH3 in low CO2
water was that CO2 excretion across the gills would reduce the pH
and, therefore, the NH3 concentration, in water flowing over the
gills. A basic flaw in this hypothesis has been discussed by
Broderius et al. (1977). Carbon dioxide will only form protons
very slowly in water at the tested temperature. The uncatalysed
CO2 hydration reaction has a half-time of seconds or even min
(e.g., at pH 8: 25 seconds at 25 °C; 300 seconds at 0 °C) (Kern
1960), and water does not remain in the opercular cavity for more
than a few seconds, and at the surface of a gill lamella for about
0.5 - 1 second (Randall, 1970; Cameron, 1979). Thus, the
liberation of CO2 across the gills will have little, if any, effect
on water pH or NH3 levels and the NH3 gradient across the gills
between water and blood.
6.4.3.6. Prior acclimatization to ammonia
The question of whether fishes can acquire an increased
tolerance to ammonia by acclimatization to low ammonia
concentrations is an important one. If fish were able to develop
such tolerance, they might be able to survive what would otherwise
be lethal ammonia concentrations.
Observations by McCay & Vars (1931) indicated that brown
bullheads subjected to several successive exposures to ammonia,
alternating with recovery in fresh water, did not acquire
tolerance. However, a number of research workers have reported
that previous exposure of fish to low concentrations of ammonia
increases their resistance to lethal concentrations. Vámos (1963)
reported that carp exposed to 0.67 or 0.52 mg NH3/litre for 75 min,
then transferred to fresh water for 12 h, followed by a solution
containing 0.7 mg NH3/litre, exhibited symptoms of ammonia toxicity
in 60 - 85 min, whereas control fish, exposed initially to
0.7 mg/litre NH3, developed symptoms within 20 min. Blue tilapia
acclimatized for 35 days to 0.52 - 0.64 mg NH3/litre subsequently
survived 48 h at 4.1 mg/litre, compared with the 48-h value for
unacclimatized fish of 2.9 mg/litre (Redner & Stickney, 1979).
Malacea (1968) studied the effects on bitterling of acclimatization
to ammonium sulfate solutions. A group of 10 fish was held in an
acclimatization solution of 0.26 mg NH3/litre for 94 h, after which
the fish were exposed to a 5.1 mg NH3/litre solution for 240 min.
A control group of 10 bitterling received identical treatment,
except that the acclimatization aquarium did not contain added
(NH4)2SO4. The ratio of the mean survival times of "adapted" to
"unadapted" fish was 1:13, indicating a slightly higher ammonia
tolerance for the adapted fish.
Schulze-Wiehenbrauck (1976) subjected 2 groups of rainbow trout
(mean weights 56 g and 110 g) that had been held for at least 3
weeks at sublethal ammonia concentrations, to lethal ammonia
concentrations. In the study on the 110-g fish, the
acclimatization concentrations were 0.007, 0.131, and 0.167 mg
NH3/litre. The fish were then subjected for 8.5 h to
concentrations of 0.45, 0.42 and 0.47 mg NH3/litre, respectively.
Fish from the 2 higher sublethal concentrations showed 100%
survival after 8.5 h in the 0.42 and 0.47 mg NH3/litre solutions,
whereas fish from the 0.007 mg NH3/litre concentration showed only
50% survival in 0.45 mg NH3/litre. In the study on the 56-g fish,
the acclimatization concentrations were 0.004 mg NH3/litre and
0.159 mg NH3/litre; these fish were placed for 10.25 h in NH3
concentrations of 0.515 and 0.523 mg/litre, respectively. There
was 100% survival in the acclimatized fish, and 85% survival in the
fish acclimatized to 0.004 mg/litre. The results of these studies
showed an increase in resistance of trout to high ammonia levels
after prior exposure to sublethal ammonia levels.
Alabaster et al. (1979) determined 24-h LC50 values of NH3 for
Atlantic salmon smolts under reduced dissolved oxygen test
conditions. Fish acclimatized to ammonia before oxygen reduction
had LC50 values 38% and 79% higher than fish without prior ammonia
acclimatization.
In studies by Brown et al. (1969), rainbow trout were tested by
moving back and forth between tanks in which the ammonia
concentrations were 0.5 and 2.5 times a previously determined 48-h
LC50 value. If fish were transferred on an hourly basis, the
median period of survival for the fluctuating exposure was reported
to be the same as that for constant exposure (> 700 min). When
the fish were transferred at 2-h intervals, the median survival
time for the fluctuating exposure was reported to be less (370
min), indicating that the toxic effects from exposure to the
fluctuating concentrations of ammonia were greater than those from
exposure to the constant concentration. Thurston et al. (1981a)
conducted acute toxicity tests in which rainbow trout and cutthroat
trout were exposed to short-term cyclical fluctuations of ammonia.
Companion tests were conducted in which test fish were subjected to
ammonia at constant concentrations. The LC50 values for both
average and peak concentrations of ammonia for the fluctuating
concentration tests were compared with the LC50 values for the
constant concentration tests. Comparison of total exposures showed
that fish were more tolerant to constant, than to fluctuating
concentrations of ammonia. Fish subjected to fluctuating
concentrations of ammonia at levels below those acutely toxic were
better able to withstand subsequent exposure to high fluctuating
concentrations than unacclimatized fish. There is reasonable
evidence that fish with a history of prior exposure to sublethal
concentrations of ammonia are better able to withstand an acutely
lethal concentration for a period of hours and possibly days.
Limited data on fluctuating exposures indicate that fish are more
susceptible to fluctuating than to constant exposure with the same
average NH3 concentrations.
6.4.4. Fish: chronic toxicity
"Full-chronic" tests cover the entire life cycle of the test
animal, beginning at a given stage of development of one generation
(frequently as the fertilized egg) and continuing through to this
same stage in the next generation. Common end-points for measuring
toxicity are survival, growth, and reproductive success, though
recent research on ammonia toxicity for fish has demonstrated the
desirability of also conducting histological examinations.
"Partial-chronic" tests on fish most frequently cover a period
of 30 days or longer, from the egg incubation stage to the free-
swimming stage; for many toxins it has been demonstrated that
these stages are the most sensitive. However, in the case of
ammonia it has been demonstrated that older, mature rainbow trout,
Salmo gairdneri, are potentially as susceptible to the effects of
ammonia as newly-hatched larvae.
Long-term ammonia exposure of fishes, including complete life-
cycle tests on rainbow trout and fathead minnows with several end-
points, including effects on spawning and egg incubation, growth,
survival and tissues, have been studied. The effects of prolonged
exposure to ammonia (up to 61 days) on the early life stages of of
pink salmon were studied by Rice & Bailey (1980). Three series of
exposures were carried out, beginning at selected times after
hatching. These were for 21 days prior to completion of yolk
absorption, for 40 days up to 21 days before yolk absorption, and
for 61 days up to yolk absorption. All test fish were sampled for
size when the controls had completed yolk absorption. Test
concentrations ranged from zero up to 0.004 mg NH3/litre. For fry
at the highest concentration of 0.004 mg NH3/litre, significant
decreases in weight were observed in all 3 exposed groups. At a
concentration of 0.0024 mg NH3/litre, the groups of fry exposed for
40 and 61 days were significantly smaller, whereas a concentration
of 0.0012 mg/litre had no significant effect on growth. Effects
were consistently more marked for the 61-day-exposed fish.
In a 3-generation, 5-year laboratory study, rainbow trout
exposed for 5 months to concentrations of ammonia ranging from 0.01
to 0.07 mg NH3/litre, spawned of their own volition. There was no
correlation between ammonia concentration and numbers of egg lots
spawned, total numbers of eggs produced, or numbers of eggs
subsequently hatched. Parental fish were exposed for 11 months,
the first filial generation (F1) for 4 years, and the second filial
generation (F2) for 5 months. Pathological lesions were observed in
both parental and F1 fish, when ammonia concentrations reached and
exceeded 0.04 mg NH3/litre. Measurements of blood-ammonia
concentrations in 4-year-old F1 fish showed an increase when test
water concentrations reached or exceeded 0.04 mg NH3/litre. The F1
fish exposed for 52 months from day of hatching showed no
relationship between growth and concentration at 10, 15, 21, and 52
months (Thurston et al., 1984b).
Burkhalter & Kaya (1977) tested ammonia at concentrations
ranging from 0.06 to 0.45 mg/litre on fertilized eggs and the
resultant sac fry of rainbow trout. Eggs were incubated at 12 °C
for 25 days in one test and at 10 °C for 33 days in another; fry
were maintained for 42 days. No concentration response was seen in
egg mortality or incubation time in either test. Retardation in
early growth and development occurred at 0.06 mg NH3/litre, the
lowest concentration tested. Fish exposed to 0.12 mg NH3/litre
required 1 week longer than the controls to achieve a free-swimming
state; fish at 0.34 and 0.45 mg NH3/litre did not achieve a free-
swimming state during a 42-day test period. A 21-day LC50 value of
0.30 mg NH3/litre was obtained. For sac fry exposed for 42 days
after hatching, hypertrophy of secondary gill lamellae epithelium
occurred at 0.23 mg NH3/litre, and karyolysis and karyorrhexis in
the secondary gill lamellae were observed after 28 days at 0.34 mg
NH3/litre and higher.
Calamari et al. (1977, 1981) exposed rainbow trout to
ammonium chloride solutions for 71 days, beginning 1 day after
fertilization and ending when fry had been feeding for 30 days.
A 72-day LC50 of 0.056 mg NH3/litre was calculated; 23% mortality
occurred at a concentration of 0.025 mg/litre. Examination of 986
rainbow trout embryos at the hatching stage after exposure to
concentrations of 0.010 - 0.193 mg NH3/litre for 24 days showed
an increase in gross malformations with increasing ammonia
concentration. The deformities observed were various degrees
of curvature from the median body axis, and various kinds of
malformations in the head region with a number of cases of double
heads. At the highest concentration tested, 0.193 mg NH3/litre,
60% of the observed fish were malformed. Microscopic examination,
at hatching, of 128 larvae from the same exposure showed
abnormalities of the epidermis and pronephros, which were
correlated with ammonia concentrations. The epidermis was
thickened with an irregular arrangement of the various layers of
cells and an increase in the number and dimensions of mucous cells.
The pronephros showed widespread vacuolization of the tubule cells,
together with a thickening of the wall. Increasing abnormalities
were observed after exposure to concentrations exceeding 0.025 mg
NH3/litre for the epidermis and 0.063 mg/litre for the pronephros.
Four 4-week-old rainbow trout fry were exposed for 30 days to
concentrations of ammonia (reported graphically) ranging from
~0.06 to 0.31 mg NH3/litre. Growth rate at ~0.06 mg NH3/litre was
comparable with that of controls, but, above ~0.10 mg NH3/litre,
growth rate decreased, in correlation with increased NH3
concentration. Survival at 0.32 mg NH3/litre was 70% of that of
the controls (Broderius & Smith, 1979). Schulze-Wiehenbrauck
(1976) tested juvenile rainbow trout of different sizes, for
periods of time ranging from 2 to 7 weeks, and at ammonia
concentrations ranging from 0.012 to 0.17 mg/litre. He concluded
that a concentration of 0.05 mg NH3/litre caused a slight decrease
in growth during the first 14-day interval in non-acclimatized
fish, but that the decrease was completely compensated for in the
next growth interval. Exposure to 0.13 mg NH3/litre (apparently
for 3 or 4 weeks) did not affect growth, food consumption, or food
conversion.
Young rainbow trout were reared in 3 concentrations of ammonia
(averaging 0.006, 0.012, and 0.017 mg/litre) for a period of 1
year. At 4 months, there was no significant difference in fish
growth at the 3 concentrations. At 11 months, there was a
difference with the fish at 0.012 and 0.017 mg NH3/litre, which
weighed 9% and 38% less, respectively, than the fish at 0.006 mg
NH3/litre. Microscopic examination of tissues from fish exposed to
the highest concentration, examined at 6, 9, and 12 months, showed
severe pathological changes in gill and liver tissues. Gills
showed extensive proliferation of the epithelium, which resulted in
severe fusion of gill lamellae preventing normal respiration.
Livers showed reduced glycogen storage and scattered areas of dead
cells; these became more extensive with increase in exposure time
(Smith, 1972; Smith & Piper, 1975).
Rainbow trout were exposed for 3 months to concentrations of
0.069, 0.14, and 0.28 mg NH3/litre. The cumulative mortality of a
control group (0.005 mg NH3/litre) was ~2%; cumulative mortality at
0.069 and 0.14 mg/litre was ~5%, and that at 0.28 mg/litre was ~15%
(United Kingdom Ministry of Technology, 1968). Reichenbach-Klinke
(1967) performed a series of 1-week tests on 240 fish of 9 species
(including rainbow trout, goldfish, northern pike, Esox lucius,
carp, and tench) at concentrations of 0.1 - 0.4 mg NH3/litre.
Swelling of, and diminution of the number of, red blood cells,
inflammation, and hyperplasia were observed. Irreversible blood
damage occurred in rainbow trout fry at concentrations above
0.27 mg NH3/litre. Low NH3 concentrations also inhibited the
growth of young trout and lessened their resistance to disease.
In rainbow trout exposed to 0.30 to 0.36 mg NH3/litre, 81%
mortality occurred over the 36-day duration of the test, with most
deaths occurring between days 14 and 21. Microscopic examination
of the gills revealed some thickening of the lamellar epithelium
and an increased mucous production. The most characteristic
feature was a large proportion of swollen, rounded secondary
lamellae in which the pillar system was broken down and the
epithelium enclosed a disorganized mass of pillar cells and
erythrocytes. Gill hyperplasia was not a characteristic
observation (Smart, 1976).
In rainbow trout exposed to < 0.0005 or 0.005 mg NH3/litre for
8 weeks, examination of the gill lamellae of fish from the lower
concentration showed them to be long and slender with no
significant pathology. Fish exposed to 0.005 mg NH3/litre had
shorter and thicker gill lamellae with bulbous ends, and some
consolidation of lamellae was noticed. Many filaments showed a
definite hyperplasia of the epithelial layer, evidenced by an
increase in the number of cell nuclei (Fromm, 1970).
Thurston et al. (1978) studied the toxicity of ammonia for
cutthroat trout fry in tests that lasted up to 36 days. Results of
duplicate tests on 1-g fish showed 29- and 36-day LC50 values of
0.56 mg NH3/litre. Duplicate tests on 3-g fish provided 29-day
LC50 values of 0.37 and 0.34 mg NH3/litre, slightly less than those
of the 1-g fish. The heart, gastrointestinal tract, and thymus of
cutthroat trout fry exposed to 0.34 mg NH3/litre for 29 days were
comparable with those of control fish, but the gills and kidneys
showed degenerative changes. The gills showed hypertrophy of
epithelium, some necrosis of epithelial cells, and separation of
epithelium due to oedema. The kidneys had mild hydropic
degeneration and accumulation of hyaline droplets in the renal
tubular epithelium. Reduced vacuolation was observed in livers.
Samylin (1969) studied the effects of ammonium carbonate on the
early stages of development of Atlantic salmon. The first set of
studies, at 13 °C, lasted 53 days and was conducted within the
range 0.001 to > 6.6 mg NH3/litre beginning with the "formed
embryo" stage. Accelerated hatching was observed with increasing
(NH4)2CO3 concentrations, but concentrations of > 0.16 mg
NH3/litre were lethal for emerging larvae within 12 - 36 h.
Because (NH4)2CO3 was used as the toxin, the pH in the test aquaria
increased from 6.7 to 7.6 with increasing NH3 concentration. Growth
inhibition was observed at 0.07 mg NH3/litre. Tissue changes were
observed in eyes, brains, fins, and blood of Atlantic salmon
embryos and larvae exposed to concentrations ranging from 0.16
to > 6.6 mg NH3/litre, with more marked changes at higher ammonia
concentrations. The effects observed included erosion of membranes
of the eyes and shedding of the crystalline lens, dilatation of
blood vessels in the liver and brain, accumulation of blood in the
occipital region and in the intestines. Reaction to light and
mechanical stimulation gradually disappeared with increased ammonia
concentration, and the heart rate slowed. Morphological
differences in development between experimental and control larvae
were observed from the tenth day of exposure, including a lag in
yolk resorption, decrease in growth of the skin fold, and
contraction of skin pigment cells causing the skin colour to become
paler than it was after hatching. At concentrations up to 0.07 mg
NH3/litre, no significant morphological differences were observed.
A second series of studies, at 16.5 °C, was carried out in
the 0.001 - 0.32 mg NH3/litre concentration range, beginning
with larval salmon (Samylin 1969). Concentrations of > 0.21
mg/litre were lethal and caused weight loss in fry; 0.001 -
0.09 mg NH3/litre caused a decrease in weight gain, though there
were no differences in feeding activity, behaviour, or development
at these concentrations compared with controls. Dissolved oxygen
concentrations in this second series of studies dropped as low as
3.5 mg/litre.
Burrows (1964) tested fingerling chinook salmon for 6 weeks in
outdoor water channels into which ammonium hydroxide was
introduced. Two studies were conducted, one at 6.1 °C and the
other at 13.9 °C, both at pH 7.8. The fish were then maintained in
fresh water for an additional 3 weeks. A recalculation of the
reported non-ionized ammonia concentrations, based on more recent
aqueous ammonia equilibrium tables, shows that the concentrations
at 6.1 °C were 0.003 - 0.006 mg NH3/litre and, at 13.9 °C, were
0.005 - 0.011 mg NH3/litre. At both temperatures, and at all
ammonia concentrations, some fish showed excessive proliferation
and clubbing of the gill filaments. The proliferation was
progressive for the first 4 weeks, after which no measurable
increase was observed. After 3 weeks in fresh water, examination
of fish exposed at 6.1 °C indicated that recovery from the
extensive proliferation had not taken place. In the study on larger
fish at 13.9 °C, a marked recovery from hyperplasia was noted after
the 3 weeks in fresh water. In the first study, the proliferated
areas had consolidated; in the second, they had not. It was
postulated that continuous ammonia exposure is a precursor of
bacterial gill disease.
Duplicate groups (90 fish each) of hatchery-reared coho salmon
were exposed for 91 days to "river-water" solutions of ammonium
chloride at concentrations of 0.019 - 0.33 mg NH3/litre. Control
groups were reared at 0.002 mg/litre. Haemoglobin content and
haematocrit readings were slightly, but significantly, reduced in
fish exposed to the highest concentration tested, and there was
also a greater percentage of immature erythrocytes. Blood-ammonia
and -urea concentrations were not significantly different after 91
days, regardless of the concentration of ammonia to which the fish
were exposed (Buckley et al., 1979). Rankin (1979) exposed embryos
of sockeye salmon, Oncorhynchus nerka, to ammonia from fertilization
to hatching. Total embryo lethality occurred at concentrations of
0.49 - 4.9 mg NH3/litre. The times required to achieve 50%
mortality at these concentrations were 40 - 26 days. Mortality of
the embryos exposed to 0.12 mg NH3/litre was 30%, and time to 50%
mortality was 66 days.
Two full life-cycle ammonia toxicity tests, each lasting
approximately 1 year, were conducted on fathead minnows (Thurston
et al., in press). These tests began with newly hatched fry and
were continued through their growth, maturation and spawning
stages; progeny were exposed from hatching through growth to 60
days of age. While no statistically-significant differences were
observed in survival, growth, egg production, and egg viability, at
concentrations up to 0.4 mg NH3/litre, effects were seen at 0.4 mg
NH3/litre.
Tissues from fathead minnows subjected to prolonged (up to 304
days) ammonia exposure were examined (Smith, 1984). Growths, some
massive, were observed on the heads of several fish exposed to
concentrations of 1.25 or 2.17 mg NH3/litre, and swollen darkened
areas were observed on the heads of several fish exposed to 0.639 -
1.07 mg NH3/litre. Thurston et al. (in press) also reported
lesions at concentrations below those at which other effects were
observed. Brain lesions were common at concentrations of 0.21 mg
NH3/litre and higher. Grossly and histologically, the severity of
the lesions, which varied from mild to severe, was positively
correlated with ammonia concentration. The lesions appeared to be
of a cell type originating from the meninx primativa covering the
brain. The hyperplastic tissue often completely surrounded the
brain but was not observed around the spinal cord.
An early life-stage test initiated at the blastula stage of
embryogenesis and extending through 39 days post-hatching was
conducted on green sunfish (McCormick et al., 1984). Retardation
of growth was found in green sunfish exposed from embryo through
juvenile life stages to concentrations of 0.489 mg NH3/litre or
more, but not at 0.219 mg NH3/litre. In a long-term test on green
sunfish, Jude (1973) reported that, at levels higher than 0.17 mg
NH3/litre, mean fish weight increased less rapidly than that of the
controls on the 4 days following the introduction of ammonia.
Thereafter, fish exposed to 0.26 and 0.35 mg NH3/litre grew at an
increasing rate, while fish exposed to 0.68 and 0.64 mg NH3/litre
remained the same for 12 days before increases in growth occurred.
Four simultaneous early life-stage ammonia tests with
smallmouth bass were carried out at 4 different pH levels, ranging
from 6.6 to 8.7, in order to examine the effect of pH on chronic
ammonia toxicity. Exposure to ammonium chloride solutions began on
2- to 3-day old embryos and lasted for 32 days. The end-point
observed was growth, and ammonia was found to have a greater effect
on growth at lower pH levels than at high. Concentrations found to
retard growth ranged from 0.056 mg/litre at pH 6.60, to 0.865
mg/litre at pH 8.68 (Broderius et al., in press).
In early life-stage tests (29 - 31 days' exposure) on channel
catfish and white sucker, no significant effects on percent hatch
or larval survival were observed for channel catfish exposed to
ammonium chloride at concentrations as high as 0.583 mg NH3/litre
and for white sucker at concentrations as high as 0.239 mg
NH3/litre. Significant retardation of growth, however, occurred in
channel catfish at concentrations of 0.392 mg NH3/litre or more and
in white sucker at 0.070 mg NH3/litre and higher. A delay in time
to swim-up stage was also observed for both species at elevated
(0.06 - 0.07 mg/litre) ammonia concentrations (Reinbold &
Pescitelli, 1982a).
In cultured channel catfish fingerlings, exposed for periods
of approximately 1 month to concentrations of 0.01 - 0.16 mg
NH3/litre, growth at 0.01 and 0.07 mg NH3/litre was not
significantly different from that of control fish, but growth
retardation at 0.15 and 0.16 mg NH3/litre was statistically
significant (Robinette, 1976). Colt (1978) and Colt &
Tchobanoglous (1978) reported retardation of growth of juvenile
channel catfish during a 31-day period of exposure to
concentrations ranging from 0.058 to 1.2 mg NH3/litre. Growth
rate was reduced by 50% at 0.63 mg NH3/litre, and no growth
occurred at 1.2 mg NH3/litre.
6.5. Wild and Domesticated Animals
6.5.1. Wildlife
Although ammonia has been known to be toxic for nearly a
century (Hahn et al., 1983), studies describing the toxicological
effects of ammonia on wildlife are very limited. Normally,
atmospheric ammonia does not appear to be a problem for wild
animals, but concentrations of ammonia could reach harmful levels
in accidents during transport near forests and remote areas. NRC
(1979) has reported 2 types of observations in relation to this
topic: (a) the use of anhydrous ammonia to exterminate wild birds
and mice in farm buildings; and (b) the tolerance of bats to
atmospheric ammonia.
The use of anhydrous ammonia has been recommended for
exterminating wild birds and mice from farm buildings by Day et al.
(1965). The technique is simple, economical, and does not leave
any harmful residue. The farm buildings, after removal of the
livestock, were sealed and treated with anhydrous ammonia at 1600
mg/m3 (2285 ppm) for 7 min and then reopened. Ammonia fumes were
fatal for the wild inhabitants, particularly for wild birds.
Within 0.5 h, dead starlings, sparrows, pigeons, and mice were
removed from the barns. Farm animals were allowed to enter the
barns within 1 h of their reopening. According to laboratory
studies, the mouse appears to be more sensitive than other animal
species such as the rat, rabbit, and guinea-pig. When mice were
exposed for 10 min to ammonia at 6140 - 9060 mg/m3 (8770 - 12 940
ppm), death with convulsion began after 5 min of exposure, and over
50% of the mice died before the study was completed. The surviving
animals appeared to recover rapidly, but another 4% died between
the 6th and 10th days after exposure (Underwriters Laboratories,
1933).
Large colonies of Guano bats (Tadarida brasiliensis) frequently
inhabit caves or other areas, producing large amounts of guano,
which, on bacterial decomposition, results in a very high
concentration of ammonia in the atmosphere. Although high ammonia
concentrations, together with high relative humidity in caves,
discoloured the pelage of bats (Eads et al., 1955; Constantine,
1958; Mitchell, 1964), no other adverse physiological effects were
observed in these mammals. This apparent adaptation to inhaled
ammonia prompted laboratory studies relating to the physiological
mechanisms involved in ammonia tolerance in different species of
bats (Mitchell, 1963; Studier, 1966; Studier et al., 1967).
California leaf-nosed bats (Macrotus californicus) can
tolerate exposure to 2100 mg/m3 (3000 ppm) for up to 9 h (Mitchell,
1963).
Ammonia toxicity at lethal doses was manifested by corrosion
of the skin and mucous membranes, pulmonary oedema, and distinct
visceral damage. The blood-non-protein nitrogen in the exposed
bats was significantly elevated without any increase in urinary-
urea or -ammonia.
Studier et al. (1967) studied the effects of increasing
concentrations of atmospheric ammonia on ammonia tolerance and
metabolic rates in rats, mice, and 3 species of bat. Rats, mice,
and 2 species of bat (Myotis lucifugus and Eptesicus fuscus)
tended to show increased oxygen utilization, when exposed to
increased ammonia levels. However, the guano bat (Tadarida
brasiliensis) exhibited a large decrease in oxygen utilization
with increasing ammonia concentration (i.e., 74% depression) when
exposed to air containing 4900 mg/m3 (7000 ppm) of gaseous ammonia.
Earlier, Studier (1966) had shown that ~35% of gaseous ammonia
filtered through the mucous linings in the respiratory passage in
guano bats, when they were exposed to 2100 mg/m3 (3000 ppm) of
ammonia. There was no change in their normal blood pH during
exposures to high ammonia concentrations. The animals, however,
exhaled measurable amounts of ammonia when transferred to normal
air.
6.5.2. Domesticated animals
6.5.2.1. Oral exposure
(a) Ruminants
The use of urea as a partial source of nitrogen in ruminant
nutrition is limited by its toxicity, which results from its
metabolism to ammonia.
The toxic effects of urea in ruminants are related to a high
ammonia content in the blood. Urea itself is not as toxic as the
ammonia, which is rapidly released in the rumen by the action of
bacterial urease (EC 3.5.1.5) on ingested urea (Bloomfield et al.,
1960). The absorption of this excess ammonia has been shown to
depend on the pH of the ruminal contents.
Hogan (1961) examined the effects of pH on the absorption of
ammonia from the rumen in sheep. When an ammonia-containing buffer
at pH 6.5 was placed in the rumen, absorption increased with the
concentration gradient. At a pH of 4.5, however, the concentration
of ammonia in the rumen did not affect the absorption across the
epithelium. The net loss of ammonia-nitrogen from the rumen at pH
6.5 was more than 3 times the loss at a pH of 4.5.
Additional support for the effect of pH on ammonia absorption
across the ruminal epithelium in sheep has been presented by
Bloomfield et al., (1962). As the pH of the ruminal contents
increased from 6.21 to 6.45, no ammonia was absorbed; however, as
the pH increased to 7.59, the absorption rate was 16 mmol/litre per
h. One sheep with a ruminal pH of 7.7 died of ammonia toxicity
within 30 min. These data support the hypothesis that the non-
ionized ammonia, which increases at higher pH, penetrates the lipid
layers of the ruminal epithelium more effectively than the charged
ammonium ion (Coombe et al., 1960).
Toxic signs become apparent as the blood-ammonia-nitrogen
increases to 10 mg/litre; tetanic spasms occur between 10 and
20 mg/litre and are followed by death (Repp et al., 1955; McBarron
& McInnes, 1968; Kirkpatrick et al., 1972, 1973; Webb et al.,
1972). Wilson et al. (1968a) attributed the cause of death to the
cardiotoxic effects of ammonia produced from the urea. However,
Singer & McCarty (1971) observed that only one sheep died of
ventricular fibrillation and the remainder of respiratory failure.
More recently, Edjtehadi et al. (1978) reported the arrest of
respiration, and not cardiovascular collapse, as the cause of death
in sheep.
Certain aspects of the blood chemistry have been described for
sheep with urea poisoning (Kirkpatrick et al., 1973; Edjtehadi et
al., 1978). In general, during the initial stages of urea
toxicosis, an alkalosis is induced, followed by systemic acidosis
due to hyperventilation prior to death (Edjtehadi et al., 1978).
In addition to increases in blood-ammonia and blood-urea levels,
there is a marked increase in the blood-glucose level, with no
change in ketone concentrations in the body (Singer & McCarty,
1971). The following changes have been recorded at death: red-
cell count and haemoglobin concentration increased by 7.9%; white-
cell count decreased by 27.5%; and packed-cell volume increased by
11.4%. Mean corpuscular volume, mean corpuscular haemoglobin, and
mean corpuscular haemoglobin concentration were not substantially
changed (Kirkpatrick et al., 1973).
Pathological effects of ammonia toxicity in sheep have been
described by Singer & McCarty (1971). The changes were similar
when sheep received intraruminal injections of ammonium chloride,
ammonium sulfate, or a mixture of ammonium chloride, carbonate,
phosphate, and sulfate. General passive hyperaemia and numerous
petechial and ecchymotic haemorrhages in the musculature, heart,
thymus, and lungs were found. The lungs were distended and
severely congested. On microscopic examination, the pulmonary
lesions included severe hyperaemia, haemorrhage, alveolar oedema,
and alveolar emphysema. In the thymus, there was degeneration and
necrosis of Hassall's corpuscles and centrilobular haemorrhages.
Lesions in kidneys included severe generalized cloudy swellings and
multiple foci of early coagulative necrosis of the proximal
convoluted tubules, general hyperaemia of the glomerular tufts, and
degeneration of the glomerular tuft cells.
In Marschang & Crainiceanu's (1971) study on the effects of
ammonia in the air of calf stables, ammonia concentrations
reportedly ranged from 0.7 to 140 mg/m3 (1 - 200 ppm). During
these periods of high ammonia concentration, high mortality rates
were observed among the calves. The authors suggested that the
high ammonia content weakened the resistance of the animals and
thus created conditions for the development of secondary
infections. Deaths were mainly caused by respiratory diseases.
Autopsy indicated various types of change in the lungs, chiefly
inflammation.
Air-ammonia concentrations in 3 cattle-fattening facilities in
Romania were measured by Marschang & Petre (1971), who found
concentrations ranging from 2 to 1400 mg/m3 (3 - 2000 ppm).
Morbidity (mainly from respiratory disease) and mortality rates
increased with ammonia concentrations in the stalls and decreased
as some of the toxic gas levels decreased to admissible
concentrations. The authors suggested that ammonia is the most
important environmental factor in producing disease in cattle-
fattening stalls. They did not refer to the growth rate of the
cattle; however, in an additional report, Marschang (1972) observed
a marked decrease in the growth rate of fattening cattle, when the
ammonia content of the stable was high.
(b) Monogastric animals
Monogastric animals are considered relatively tolerant to
dietary urea, since they lack the large amounts of bacterial urease
present in the rumen of ruminants. Horses may frequently consume
cattle rations that contain urea or other non-protein-nitrogen
sources.
Hintz et al. (1970) found that the urease activity in the
caecal fluid from ponies was 17 - 25% of that reported for bovine
rumen fluid. They subjected 8 ponies to oral doses of urea at 3.3
- 3.6 g/kg body weight, to study the toxic effects of urea
overdosage. Seven of the ponies died of ammonia toxicity, 3 - 12 h
after treatment. Clinical signs of toxicosis were characteristic
of severe central nervous system derangement. These signs were
similar to those previously reported for ruminants, with the
exception of head pressing against a fixed object prior to loss of
coordination. No significant gross lesions were observed on
necropsy. Blood-ammonia increased linearly until death. Blood-
alpha-keto-glutarate decreased initially, reached minimal values
at about 30 min and then increased to 3 times the zero time value.
Blood-glucose remained constant for the first 2.5 h and then
increased to about 3 times the initial value. Blood-pyruvate
decreased during the first 3.5 h and then increased to 10 times
the initial values.
6.5.2.2. Inhalation exposure
(a) Swine
The acute inhalation effects of ammonia in swine are given in
Table 15.
(b) Poultry
As discussed previously, poultry are exposed to ammonia in the
atmosphere of poultry houses; this ammonia is released from the
action of bacteria on poultry wastes. The toxic effects of this
exposure are primarily seen in the eyes and respiratory tract.
An idiopathic ocular disorder in young chicks, designated
keratoconjunctivitis, was first described by Bullis et al. (1950),
who attributed it to environmental factors in the rearing
facilities.
Anderson et al. (1964a) reported that chickens exposed
continuously to ammonia at 14 mg/m3 (20 ppm) showed some signs
of discomfort, including rubbing of the eyes, slight lachrymation,
anorexia, and, later, weight loss. Chickens exposed to ammonia at
14 mg/m3 for as little as 72 h were more susceptible to aerosol
infection with Newcastle disease virus. Gross and microscopic
damage to the respiratory tract could be detected after 6 weeks of
continuous exposure to ammonia at 14 mg/m3. Valentine (1964)
reported tracheitis in chicks exposed to ammonia at 42 - 49 mg/m3
(60 - 70 ppm). The breathing of the birds was audible as moist
rales with bubbling sounds. At post-mortem examination, some of
the birds had slight congestion of the lungs with excess mucous in
the respiratory tract. The mucous membranes of the trachea were
much thicker than in the control birds, and there was leukocytic
infiltration of the tissue. It was suggested that this tracheitis
may predispose the affected birds to respiratory diseases with the
added risks of secondary infections.
Charles & Payne (1966a) reported that exposure to atmospheric
ammonia at 70 mg/m3 (100 ppm) caused a reduction in carbon dioxide
production and depth of respiration and a 7 - 24% decrease in the
respiration rate of laying hens. The authors also observed that
broilers reared to 28 days of age in atmospheres containing high
concentrations of ammonia consumed less food and grew more slowly
than unexposed chickens. Pullets reared in high-ammonia
atmospheres matured up to 2 weeks later than pullets reared in
ammonia-free atmospheres.
Airsacculitis, one of many respiratory diseases in poultry, has
been associated with high ammonia concentrations in poultry houses
(Ernst, 1968). High concentrations of dust were also noted during
periods of winter confinement, when high ammonia concentrations
were observed. The incidence and severity of air-sac lesions in
turkeys increased signficantly with high concentrations of dust
(0.6 - 1.0 mg/m3 or 21 - 35 mg/m3) in the atmosphere. Flocks with
a high rate (47%) or a low rate (2%) of infection with Mycoplasma
meleagridis were similarly affected. No significant interaction
between dust and ammonia concentrations (up to 21 mg/m3 or 30 ppm)
with regard to effects on the development of air-sac lesions was
found. Mortality rate and feed conversion were not significantly
affected by exposure to dust and ammonia. There was considerable
loss of cilia from the epithelium of the tracheal lumen and an
increase in mucous-secreting goblet cells in turkeys exposed to
high concentrations of dust and ammonia. Areas of consolidation
and inflammation were frequently observed in the lungs of these
turkeys. The air-sac lesions ranged from mild (lymphocytic
infiltration) to severe (masses of gaseous material).
Airsacculitis has also been experimentally induced in chickens
exposed to atmospheric ammonia and the stress of infectious
bronchitis vaccination (Kling & Quarles, 1974). Eighty Leghorn
male chicks were maintained in 12 controlled-environment chambers.
Ammonia at 0, 17.5, or 35 mg/m3 (0, 25, or 50 ppm) was introduced
into the chambers from the 4th to 8th weeks of age. An infectious
bronchitis vaccination was administered to all chicks at 5 weeks of
age. Body weights and feed efficiencies were determined at 4, 6,
and 8 weeks. At 4, 5, 6, and 8 weeks, lung and bursae of Fabricius
weights, haematocrits, and air-sac scores were determined. Body
weights and feed efficiencies were significantly reduced in the
ammonia chambers. The bursae of Fabricius in the ammonia-stressed
chickens were significantly larger than those of controls at 5
weeks of age and significantly smaller at 8 weeks of age. Chickens
grown in ammoniated environments had significantly larger lungs at
8 weeks. Haematocrits were not significantly different among
treatments. Total air-sac scores were significantly higher in the
ammonia-stressed chickens at 8 weeks. The results indicated that
chickens were stressed by the ammonia at 17.5 or 35 mg/m3, and by
the infective bronchitis vaccination. In a similar study (Quarles
& Kling, 1974), exposure of broiler chicks to 17.5 or 35 mg/m3 (25
or 50 ppm) from the 4th to the 6th week resulted in the observation
of severe airsacculitis at 6 and 8 weeks of age. During the test,
airborne bacterial counts were significantly higher in chambers
with ammonia than in control chambers.
Table 15. Acute inhalation effects of ammonia in swine
---------------------------------------------------------------------------------------------------------
Ammonia concentration Dose Effects Reference
---------------------------------------------------------------------------------------------------------
196 mg/m3 (280 ppm) single frothing of the mouth and excessive Stombaugh et al. (1969)
secretion; after 36 h, convulsions
occurred, and breathing was extremely
short and irregular; the effects
ceased after a few h
7, 35, 70, or 105 mg/mg3 5 weeks high concentrations (70 and 105 mg/m3) Stombaugh et al. (1969)
(10, 50, 100, or 150 ppm) appeared to cause excessive nasal,
lachrymal, and mouth secretion after
3 - 4 days of exposure at 35 mg/m3,
the secretory rate was only slightly
higher than that in control animals;
after 1 - 2 weeks of exposure, the
signs noted appeared to lessen
gradually; examination of respiratory
tract did not reveal any significant
gross- or microscopic differences
related to ammonia exposure
0, 35, 70, or 105 mg/m3 4 weeks decrease in pig growth was noted at Drummond et al. (1980)
(0, 50, 100, or 105 ppm) all concentrations; at 35 or 70 mg/m3,
pigs converted feed to body weight
gain more efficiently than either
controls or pigs exposed to 105 mg/m3;
an acute imflammatory reaction in the
tracheal epithelium and a mild-to-
heavy exudate in the turbinate lumen
were observed at 70 and 105 mg/m3,
only
0 and 70 mg/m3 (100 ppm) 2 - 6 conjunctival irritation after the Doig & Willoughby (1971)
(1 - 7 weeks old) weeks first day which persisted for 1 week;
0 + dust dust alone had no effect; histopatho-
(100 ppm + dust) logical changes were limited to the
nasal and tracheal epithelium; there
was no evidence of structural damage
in the bronchial epithelium or alveoli
---------------------------------------------------------------------------------------------------------
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The authors suggested that the quality of eggs left all day in hen
houses containing high concentrations of ammonia might be adversely
affected.
7. KINETICS AND METABOLISM
Ammonia, a by-product of protein and nucleic acid metabolism
and a minor component of the diet, is in a state of flux in the
body, though it is present in low steady-state concentrations in
body fluids. In animals, metabolically-produced ammonia is
conjugated and excreted. Toxicity will only occur if these
conjugation and excretion mechanisms are defective, or if they are
overwhelmed by excessive exposure.
7.1. Absorption
7.1.1. Respiratory tract
Egle (1973) studied the retention, over a short period of time,
of inhaled ammonia in air at concentrations in the range 150 -
500 mg/m3 (214 - 714 ppm), in mongrel dogs of both sexes (7 - 37
per study). Retention was not materially affected by respiratory
rate, tidal volume, or concentration. Retention by the whole
respiratory tract averaged 78%, but the complexity of the dynamics
of this retention is illustrated by the fact that when respiration
was via an endotracheal tube, limiting exposure to the lower
respiratory tract, and when the upper respiratory tract (muzzle to
tracheal bifurcation) was perfused tidally, the retention was 78%
in each case. However, unidirectional perfusion of the upper
respiratory tract with ammonia in air produced a higher mean
retention of 89%.
Schaerdel et al. (1983) exposed 4 groups of rats, 8 per group,
to average ammonia concentrations of 11, 23, 220, or 826 mg/m3
(15, 32, 310, or 1157 ppm) for 24 h. Ammonia in blood was measured
at 0, 8, 12, and 24 h. At the 2 lowest concentrations, there was
no increase in blood-ammonia. However, after 8 h at 220 and
826 mg/m3, significant increases of 0.192 and 0.244 mmol (3.26 and
4.18 mg/litre) were noted. After 12 and 24 h, the increases were
not so marked, indicating an increase in ammonia metabolism.
In a study by Silverman et al. (1949), 7 male volunteers were
exposed to 350 mg/m3 (500 ppm) for 30 min. Initial ammonia
retention was not reported for all subjects, but, in one instance,
was around 75%. Retention decreased progressively until at
equilibrium it was 23% (range 4 - 30%); equilibrium was reached in
10 - 27 min. Some irritation was noted in the nose and throat,
leading to the suggestion that ammonia at this concentration was
primarily absorbed by the upper respiratory tract. Levels of
blood-urea-nitrogen (BUN), non-protein nitrogen, urinary-urea, and
urinary-ammonia remained normal.
In another study (Kustov, 1967), exposure of human volunteers
to ammonia for a longer duration (14 mg/m3 (20 ppm) for 8 h) was
accompanied by a statistically-significant increase in BUN from
23.9 to 30 mg%.
An early study was conducted by Landahl & Herrmann (1950) on
the retention of gases by the human nose and lung. At ammonia
concentrations of between 40 and 350 mg/m3 (57 and 500 ppm) and a
mean minute volume of 6 - 7 litre/min, for short durations (< 2
min), they found that approximately 92% ± 2% was retained in the
respiratory system (i.e., mouth, lungs, etc.) in 2 male volunteers,
tested 4 times. Differences in concentrations of ammonia did not
affect retention values. In a separate study, about 83% was
retained in the nasopharnyx at a flow rate of 18 litre/min, but
only 63 - 71% was retained when the flow rate was tripled. These
data are consistent with, though somewhat higher than, those
reported by Egle (1973) for exposure in dogs.
It should be noted that experimental animals kept in cages may
be exposed to relatively high concentrations of ammonia, even
exceeding 100 mg/m3, due to the degradation of urea in urine and
faeces (Flynn, 1968; Schaerdel et al., 1983).
Because ammonia is very water soluble, and thus absorbed by the
mucous coating in the upper respiratory tract, the lungs are
protected from the effects of exposure to low concentrations of
ammonia (Haggard, 1924; Boyd et al., 1944). At the levels of
ammonia associated with ambient air (i.e., 1 - 200 µg/m3), very
little, if any, is absorbed through the lungs.
If a person breathes an ammonia concentration in air of
18 mg/m3 (a common occupational exposure limit) at 1 m3/h and,
if all the ammonia is retained, then 18/60 = 0.3 mg ammonia/min
would require to be cleared by hepatic blood flow (say 1
litre/min). The rise in systemic blood-ammonia would be calculated
at 0.3 mg/litre or 0.018 mmol. If the more realistic assumption of
30% retention were used, the corresponding increase in blood-
ammonia concentration would be 0.09 mg/litre, about µmol. An
arterial fasting ammonia concentration of 1.05 mg/litre has been
reported in healthy subjects (Conn, 1972), so the calculated rise
is only 10% over fasting levels.
7.1.2. Gastrointestinal tract
Ammonia is a trace compound in foods. Ammonia that is absorbed
from the intestinal tract arises primarily from the bacterial
degradation in the intestine of amino and nucleic acids from
ingested food, endogenous epithelial debris, and mucosal cell
luminal secretions, or from the hydrolysis of urea diffusing from
the systemic circulation into the intestinal tract. The estimated
ammonia production from various substrates in the human intestines
ranges from 10 mg/day in the duodenum to 3080 mg/day in the colon
and faecal contents. Nearly all the ammonia formed is absorbed
(about 99% or 4000 mg). In healthy individuals, absorbed ammonia
is mainly catabolized rapidly in the liver to urea; therefore,
relatively small amounts reach the systemic circulation after
absorption from the gastrointestinal tract as a consequence of this
"first pass effect" (Summerskill & Wolpert, 1970).
Castell & Moore (1971) have shown that ammonia uptake from the
human colon, the major site of ammonia production, increases with
increased pH of the luminal contents. A similar effect of pH has
been shown for the absorption of ammonia from the rumen of sheep
(Hogan, 1961; Bloomfield et al., 1962). Since an increase in pH
increases the proportion of non-ionized ammonia, the authors
concluded that simple non-ionic diffusion was responsible for the
majority of ammonia transport. Evidence also exists for the active
transport of the ammonium ion from the intestinal tract. Castell &
Moore (1971) showed that ammonia transport by the human colon,
though greatly diminished, still occurred when the luminal pH was
reduced to 5, at which value non-ionized ammonia would be virtually
absent. Mossberg & Ross (1967) and Mossberg (1967) studied the
absorption of ammonia from isolated intestinal loops of the golden
hamster and found that the ileal movement of ammonia against a
concentration gradient was inhibited by cyanide, dinitrophenol, and
anaerobiosis. This suggested that an energy-dependent transport
system was operable in the ileum, where ammonia was absorbed
preferentially, but not in the jejunum.
Membrane transport of the ammonium ion by the human erythrocyte
has been demonstrated (Post & Jolly, 1957).
7.1.3. Skin and eye
Ammonia is highly mobile in all tissues and the ammonium ion
readily penetrates the corneal epithelium. Within 5 seconds,
traces are present in the anterior chamber of the eye (Siegrist,
1920). However, systemic and intra-ocular absorption by these
routes are not quantitatively important.
7.2. Distribution
The ammonia normally present in all tissues in the body
constitutes a dynamic pool throughout which absorbed ammonia is
distributed. The distribution of total ammonia between body
compartments is strongly influenced by pH. The non-ionized NH3 is
freely diffusible, whereas NH4+ is less diffusible and relatively
confined in compartments. The lower the pH of a compartment, the
greater its total ammonia content (NRC, 1979).
The fate of absorbed ammonia molecules has been studied in man,
by measurement of blood constituents, and, in experimental animals,
by following the distribution of 15N after the administration of
15NH3 and compounds.
7.2.1. Human studies
In human beings, inhalation of ammonia (350 mg/m3; 500 ppm) for
30 min did not have any effect on blood-nitrogen levels (Silverman
et al., 1949). In another study, exposure of human subjects to
14 mg ammonia/m3 (20 ppm), for a duration of 8 h, revealed a
statistically-significant increase in BUN (from 23.9 to 30 mg%)
(Kustov, 1967). However, this is unlikely to have represented the
metabolic conversion of absorbed ammonia, since the increase was
far greater than could have been accounted for by the quantity of
ammonia inhaled.
Administration of 9 mg NH4Cl/kg body weight, orally, to 20
healthy adult male and female volunteers caused a transient
increase in ammonia concentrations in arterial blood in
approximately half of the subjects. Concentrations peaked (mean,
1.4 mg NH3/litre) at 15 min and returned to fasting levels (mean,
1.05 mg NH3/litre) by 30 min. However, in 50 male patients with
cirrhosis of the liver, blood-ammonia levels increased from already
elevated fasting levels (mean, 1.56 mg NH3/litre) to much higher
peak concentrations (mean, 3.7 mg NH3/litre) at 15 min, followed by
a slow decrease reflecting impaired hepatic urea synthesis. Blood-
ammonia levels, before and after administration of ammonium
chloride, were significantly higher among cirrhotic patients with
portacaval anastomoses than among patients lacking such shunts
(Conn, 1972).
7.2.2. Animal studies
The distribution, as well as the metabolic fate of ammonia,
depends on the route of administration. After intestinal
absorption, ammonium ions are primarily transformed by the liver
to urea, and subsequently excreted in the urine. In contrast,
intravenously-administered ammonium salts are more available as
non-essential nitrogen for protein synthesis (Furst et al., 1969).
However, some orally-administered ammonia, has been found to be
incorporated into tissue proteins. Incorporation of 15N was higher
in serum globulins than in albumin after intravenous dosing with
15N-ammonium salts, but this order was reversed after oral
administration (Furst et al., 1970). The amount incorporated into
protein by this route was greater, when protein intake was
restricted (Richards et al., 1968).
Duda & Handler (1958) analysed the tissue of rats, 15 min after
an intravenous injection of 15N-ammonium lactate, and found that
its major metabolites, glutamine, and urea, were quickly
distributed throughout the body. The highest levels of labelled
urea (in µmoles 15N/g tissue) were found in the kidney (0.0217) and
liver (0.0159), while lesser amounts were found in the heart
(0.0086), spleen (0.0067), brain (0.0029), testes (0.0027), and
carcass (0.0070). The highest levels of labelled glutamine (µmoles
15N/g tissue) were found in the heart (0.086) and liver (0.055) and
lesser amounts (0.005 to 0.032) in the brain, spleen, carcass,
kidney, and testes.
Vitti et al. (1964) examined the distribution of 15N from
ammonium citrate, administered by different routes, into the
proteins of various tissues of hypophysectomized rats. The liver,
kidney, and spleen contained greater concentrations of 15N
incorporated into proteins than heart or muscle fractions during
72 h following intragastric, intraperitoneal, and subcutaneous
administration of 15N-ammonium citrate. After the first 6 h,
during which the intragastric route gave higher values, the
quantity of 15N incorporated into liver-protein was not
substantially affected by the route of administration. In most of
the other tissues studied, however, 15N incorporation tended to be
least by the intragastric route, followed, in increasing order, by
the intraperitoneal and subcutaneous routes. By the last route,
more labelled ammonia was apparently made available to the widely
distributed glutamine-synthetase (EC 6.3.1.2) system (section
7.4.3).
7.3. Metabolic Transformation
Most organisms have mechanisms for conjugating ammonia into
non-toxic compounds for excretion. Terrestrial mammals synthesize
urea, which requires the concerted action of several enzymes of the
Krebs-Henseleit (urea) cycle. One of these enzymes, glutamine
synthetase (EC 6.3.1.2), was present in the brains of all
vertebrate species examined. Glutamine synthetase was also present
at significant levels in the liver in all organisms examined (Brown
et al., 1957).
Exogenous ammonia, administered intravenously as an ammonium
compound, is metabolized to glutamine as the major early product
(Duda & Handler, 1958). The ammonia fixed in glutamine may
eventually end up in amino acids, purines, pyrimidines, or other
nitrogen-containing compounds. Ingested ammonium chloride or
endogenous ammonia is absorbed into the portal vein and converted
in the liver to urea (Furst et al., 1969; Goodman & Gilman, 1970;
Pitts, 1971).
Results of studies on the metabolic fate of dietary ammonium
citrate (Foster et al., 1939) and intravenously-administered
ammonium lactate (Duda & Handler, 1958) in rats showed that urea
synthesis represented a nearly constant fraction of the
administered ammonia over a large concentration range. Besides
glutamine and urea, labelled nitrogen also appeared in creatine,
glycine, alanine, proline, histidine, arginine, glutamic acid, and
aspartic acid. Vitti et al. (1964) examined the incorporation of
15N from ammonium citrate into proteins of liver, heart, kidney,
spleen, and muscle fractions of untreated and growth hormone-
treated, hypophysectomized rats, and found differences in the
metabolic fate, depending on the route of administration.
Subcutaneous injection facilitated the labelling of amide nitrogen,
indicating extensive disposition via glutamine synthesis. In
contrast, intragastric or intraperitoneal administration resulted
in the labelling of arginine, glutamic acid, and other alpha-amino
acids of the liver. Amide-nitrogen was labelled to a much lesser
extent than by the subcutaneous route. The tissue distribution of
the label also differed according to the route of entry (section
7.2.2).
7.4. Reaction with Body Components
Ammonia-nitrogen is central in nitrogen metabolism and
therefore becomes incorporated in all proteins and nitrogen-
containing components in the course of metabolic turnover. Ammonia
does not react with body components in the manner of alkylating
agents or compounds that modify haemoglobin.
7.5. Elimination and Excretion
7.5.1. Expired air
Ammonia may be excreted through expired air. Hunt (1977)
reported human expired air levels of ammonia of between 105 and
2219 µg/m3; Larson (1977) reported values of between 196 and
1162 µg/m3, during mouth breathing. These values are higher than
those expected from equilibration with plasma- and lung-parenchyma-
ammonia levels (28 - 49 µg/m3). This is most likely due to the
synthesis of ammonia from salivary urea by oral microflora (Biswas
& Kleinberg, 1971). Measurable amounts of free ammonia were also
found in air expired by dogs given ammonium acetate intravenously
(Robin et al., 1959), and normal dogs and human beings with
hepatic-induced ammonia toxicity (Jacquez et al., 1957, 1959).
Bloomfield et al. (1962) reported the presence of free ammonia in
expired air from sheep during experimentally-induced urea toxicity.
Normal levels of ammonia in the expired air of the rat have been
reported to range from 7 to 247.1 µg/m3, with a mean of 54.6 µg/m3
in nose-breathing animals and 23.8 - 520.8 µg/m3, with a mean of
200.2 µg/m3 in tracheal-cannulated animals (Barrow & Steinhagen,
1980). The presence of ammonia in the expired air of human beings
and experimental animals suggests that reaction products may be
formed with a variety of airborne chemicals, thereby altering their
toxicity.
7.5.2. Urine and faeces
Free ammonia is excreted by ammonotelic organisms (e.g., fish),
uric acid by uricotelic animals (e.g., birds), and urea by
ureotelic animals (e.g., mammals). Mammals may also secrete
ammonia directly into the urine. Glutaminase (EC 3.5.1.2)
catalyses the release of ammonia in the kidney tubular epithelium,
where it serves as an acceptor of H+ and regulates the acid-base
balance (Van Slyke et al., 1943; White et al., 1973). In acidosis,
the renal concentration of glutaminase increases over several
days, paralleling the increased excretion of ammonium ions (Davies
& Yudkin, 1952; Muntwyler et al., 1956; Kamin & Handler, 1957);
two-thirds of the urinary-ammonia is contributed by this pathway
(Van Slyke et al., 1943), and approximately one-third by protein
metabolism and ammonia clearance from the plasma by the kidney.
Oral and intravenous administration of ammonium lactate to
healthy human volunteers produced different patterns of excretion,
reflecting the effective barrier of the liver in preventing
ingested ammonia from gaining access to peripheral circulation by
converting most of the ammonia load to urea. Urinary-ammonia
excretion was increased 8-fold and urea excretion was reduced by
one-half after intravenous injection, as opposed to oral
administration (Gay et al., 1969), probably due to the anabolism
involved in the "first pass" effect after oral administration.
Less than 1% of the 4 g total ammonia produced in the human
intestinal tract, per day, is excreted in the faeces (Summerskill &
Wolpert, 1970).
7.6. Retention and Turnover
Some nitrogen derived from absorbed ammonia is incorporated in
amino acids and proteins. The rate of ammonia-derived nitrogen
turnover is rapid, but depends on the nutritional state. Thus,
when 15NH4Cl had been administered orally to healthy male
volunteers, for one week, 70% 15N was excreted by those on a 70-g
protein/day diet, while only about 35% 15N was excreted by those on
20 g protein/day (Richards et al., 1968, 1975).
7.7. Uptake and Metabolism in Plants
Ammonia is used by many plants and preferentially by a few.
However, ammonia is toxic, and its uptake in large quantities may
put a severe strain on the carbohydrate metabolism of the plant in
the provision of carbon skeletons for its detoxification. The
absorption of ammonium usually is coupled with the exchange of
cations as H+. Ammonia-nitrogen functions as a nitrogen source
for the synthesis of amino acids, which are incorporated in
proteins. Plants that are able to absorb it in large amounts
include many acid plants, such as Rumex, which are able to detoxify
ammonia by forming ammonium salts of organic acids. "Amide
plants", such as beet, spinach, and squash, are able to form large
amounts of the amides, glutamine, and asparagine and can withstand
quite high concentrations of ammonium salts by detoxifying the
ammonia. Certain plants, such as rice, which live in water-logged
anaerobic soils, require NH3 or reduced organic nitrogen
fertilizer, alone.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Inhalation exposure
LC50 studies and studies to determine the threshold for
irritating effects on the respiratory system for the rat and mouse
are summarized in Tables 16 and 17, respectively.
Table 16. Lethal concentrations (1-h exposure) of
ammonia for rats and micea
----------------------------------------------------
Measured Species Mortality Mean weight gain
concentration ratiob of survivors at
(mg/m3) 14 days (g)
----------------------------------------------------
4347 rat 0/10 3.5
5474 rat 8/10 -c
6888 rat 9/10 -
controls rat - 21.4
2520 mouse 0/10 -0.2
3185 mouse 3/10 -0.7
4004 mouse 9/10 -
controls mouse - 1.6
----------------------------------------------------
a Adapted from: MacEwen & Vernot (1972).
b Number dead/number exposed.
c Not enough survivors for comparison.
The acute lethal dose of ammonia by inhalation has been
determined for both the rat and the mouse (MacEwen & Vernot, 1972).
The results are summarized in Table 16. Male CFE rats ranging in
weight from 200 to 300 g and male CF1 mice weighing from 20 to 30 g
(ICR derived) were exposed for 1 h to several concentrations of
ammonia. Inhalation of ammonia gas produced immediate nasal and
eye irritation followed by laboured breathing and gasping in all
test groups. In addition, convulsions were seen in mice.
Surviving rats necropsied after 14 days showed moderate mottling of
the liver, regarded as probable fatty infiltration, at the 5474 and
6888 mg/m3 (7820 and 9840 ppm) dose levels. Mice surviving the 2
highest dose levels of 3185 and 4004 mg/m3 (4550 and 5720 ppm)
showed mild congestion of the liver. Pathological lesions were not
seen in rats exposed to 4347 mg/m3 (6210 ppm) or mice exposed to
2520 mg/m3 (3600 ppm). The calculated 1-h LC50 values for the rat
and the mouse were 5137 and 3386 mg/m3 (7338 and 4837 ppm),
respectively.
In another inhalation study, an LC50 value for the rat, with a
2-h exposure, was 7600 mg/m3 (10 860 ppm) (Alpatov, 1964). In a
further study, the threshold for acute effects (depression, then
hyperactivity and convulsions) for a 2-h exposure was 85 mg/m3 (121
ppm) (Alpatov & Mikhailov, 1963).
Table 17. Single-dose inhalation studies (LC50)
----------------------------------------------------------
Species Exposure time LC50 Reference
(min) (mg/m3)
----------------------------------------------------------
rat 120 7600 Alpatov (1964)
rat 60 5137 MacEwen & Vernot (1972)
rat 5 18 693 Prokop'eva et al. (1973)
rat 15 12 160 Prokop'eva et al. (1973)
rat 30 7035 Prokop'eva et al. (1973)
rat 60 7939 Prokop'eva et al. (1973)
rat 10 31 612 Appelman et al. (1982)
rat 60 11 620 Appelman et al. (1982)
mouse 10 7060 Silver & McGrath (1948)
mouse 60 3386 MacEwen & Vernot (1972)
mouse 60 2960 Kapeghian et al. (1982)
----------------------------------------------------------
Kapeghian et al. (1982) reported an acute inhalation toxicity
study on male ICR mice, in which the 1-h LC50 with a 14-day
observation period was calculated to be 2960 mg/m3 (4230 ppm).
Lungs of mice that died during exposure were diffusely
haemorrhagic. Histology revealed acute vascular congestion and
diffuse intra-alveolar haemorrhage. A mild to moderate degree of
chronic focal pneumonitis was also seen. Focal atelectasis was
evident in survivors sacrificed after the observation period.
Liver damage was also seen in these mice. There was evidence of
swelling and increased cytoplasmic granularity of hepatocytes at
2408 mg/m3 (3440 ppm) and scattered foci of frank cellular necrosis
at 2954 mg/m3 (4220 ppm). At 3402 mg/m3 (4860 ppm), necrosis was
increased. The liver lesions may have resulted from the
compromised nutritional state of the mice. Follicular hyperplasia
in the spleen was also seen in surviving animals, but this was
absent in animals that died during exposure.
The acute LC50 in male and female Wistar rats was 31 612 mg/m3
(40 300 ppm) for a 10-min exposure, 20 017 mg/m3 (28 595 ppm) for a
20-min exposure, 14 210 mg/m3 (20 300 ppm) for a 40-min exposure,
and 11 620 mg/m3 (16 600 ppm) for a 60-min exposure (Appelman et
al., 1982). Survivors were observed for 14 days. Clinical signs
of restlessness, eye irritation, nasal discharge, mouth breathing,
and laboured respiration were seen during exposure. Gross necropsy
revealed haemorrhagic lungs in animals that died during the study
as well as in survivors. No histopathology was performed.
Prokop'eva et al. (1973) reported that white rats exposed to
high concentrations of ammonia (6000, 3000, 1000 mg/m3 or 6814,
4307, 1436 ppm) for periods of 5, 15, 30, and 60 min exhibited
dyspnoea, irritation of the respiratory tract and eyes, cyanosis of
the extremities, and increased excitability. The LC50 values for
inhalation exposures of 5 and 15 min were 18 693 mg/m3 (26 704 ppm)
and 12 160 mg/m3 (17 372 ppm), respectively, while for 30 and 60
min, the values were 7035 mg/m3 (10 050 ppm) and 7939 mg/m3 (11 342
ppm), respectively. Inhalation of ammonia at concentrations of
3000, 1000, or 300 mg/m3 (4307, 1436, 431 ppm) resulted in a drop
in static muscular tension, leukocytosis, prolongation of the
latent reflex time, increase in total protein and blood sugar,
increased oxygen consumption, and a rise in the level of residual
nitrogen. No changes were observed in rats exposed to a
concentration of ammonia of 100 mg/m3 (144 ppm), for 5, 15, 30, and
60 min. Animals exposed to high concentrations of ammonia (exact
concentration not specified) developed pneumonia (Prokop'eva et
al., 1973).
When exposed to toxic levels of ammonia, within 1 min, mice
exhibited excitement, closing their eyes immediately and gasping
(Silver & McGrath, 1948). Groups of 20 mice were exposed to
ammonia gas for 10 min at 9 concentrations ranging from 6100 to
9000 mg/m3 (8758 to 12 921 ppm). The median lethal concentration,
calculated from the mortality at 10 days, was 7060 ± 320 mg/m3
(10 152 ± 460 ppm). Most (93%) deaths occurred rapidly, due to
convulsions after 5 min of exposure. Survivors usually recovered
within 10 min.
The LC50 values for the rat and the mouse are summarized in
Table 17.
Dose-dependent ultrastructural changes in the terminal airways
of mice exposed for 3 - 60 min to ammonia (concentration
unspecified) included oedema of the alveolar epithelium,
development of intracapillary platelet thrombosis, increased
secretions by the Clara cells, presumed to be phospholipids, and an
increase in the number of empty lamellar bodies in the large
alveolar cells (Niden, 1968).
Twenty cats were anaesthetized and exposed to 700 mg/m3 (1000
ppm) for 10 min via endotracheal tube and then observed for up to
35 days (Dodd & Gross, 1980). All cats had severe dyspnoea,
anorexia, and dehydration, 24 h after exposure. Several measures
of pulmonary function were impaired. Gross pathology of the lungs
showed various degrees of congestion, haemorrhage, oedema,
interstitial emphysema, and collapse, all non-specific for any
post-exposure day. Bronchopneumonia was common, 7 days after
exposure.
Barrow et al. (1978) exposed male Swiss-Webster mice to
concentrations of ammonia ranging from 70 to 560 mg/m3 (100 -
1000 ppm) for 30 min. The average respiratory rate depression in
4 mice for each of 4 exposure levels was evaluated. The maximum
depression in respiratory rate at each exposure level occurred
within the first 2 min. The concentration expected to elicit a 50%
decrease in respiratory rate (RD50) in mice, calculated by a
regression equation for ammonia, was 212 mg/m3 (303 ppm) with 95%
confidence limits of 132 - 343 mg/m3 (188 - 490 ppm). Effects of
ammonia exposure such as bradycardia and peripheral
vasoconstriction accompanied respiratory rate depression at the
RD50 and above.
A concentration of 350 mg/m3 (500 ppm) was reported by Wood
(1979) to be the level at which unrestrained mice did not
consistently adopt avoidance measures on inhalation of ammonia.
Both this author and Barrow et al. (1978) claimed to offer more
sensitive end-points for assessing ammonia irritation. However,
the no-observed-adverse-effect level reported by Wood (1979) was
higher than the concentration determined by Barrow et al. (1978) to
elicit 50% depression of respiratory rates in mice.
Dalhamn & Sjoholm (1963) tested in vitro preparations of the
tracheas of 8 rabbits. Arrested ciliary activity was observed
after 5 min of exposure to ammonia at 350 - 700 mg/m3 (500 -
1000 ppm). In in vivo studies on rabbits, Dalhamn (1963) showed
that the level of ammonia entering the nasal cavity, necessary to
cause small changes in the rate of tracheal ciliary beating was
1400 mg/m3 (2000 ppm). This corresponds to a tracheal
concentration of approximately 70 mg/m3 (100 ppm).
When anaesthetized male rabbits were exposed to 8 ammonia
concentrations ranging from 700 to 14 000 mg/m3 (1000 - 20 000 ppm)
(Richard et al., 1978b), bradycardia appeared at 1750 mg/m3 (2500
ppm). Hypertension, cardiac arrhythmia, macroscopic lung changes,
and EEG abnormalities were also reported. Signs appeared more
rapidly at higher concentrations with the complete syndrome
appearing at 3500 mg/m3 (5000 ppm).
Mayan & Merilan (1972) exposed 16 adult female New Zealand
white rabbits to ammonia concentrations of 35 and 75 mg/m3 (50 and
100 ppm) for 2.5 h. The average decreases in respiratory rate,
which were 34 and 32.7%, respectively, were significantly ( P <
0.01) less than control values. There were no histopathological
changes in lung, liver, spleen, or kidneys.
Enzymatic alterations in rats following inhalation of low
levels of ammonia have also been reported. At concentrations of
approximately 20 - 121 mg/m3 (29 - 173 ppm), there was a
decrease in the activities of liver succinic dehydrogenase (EC
1.3.99.1), lactate dehydrogenase (EC 1.1.1.28), glucose-6-phosphate
dehydrogenase (EC 1.1.1.49), and adenosine triphosphatase (EC
3.6.1.3). Liver acid phosphatase (EC 3.1.3.2) activity was
increased (Zlateva et al., 1974).
When 180 mice were exposed for 10 min to ammonia at 6139 -
9058 mg/m3 (8770 - 12 940 ppm), death with convulsions began to
occur 5 min after exposure. One hundred mice died during the
exposure. The surviving animals (80) recovered rapidly; however,
7 died between the sixth and tenth days following exposure (NRC,
1979).
8.1.2. Oral exposure
The effects of ammonia and its compounds are mainly of 2 types.
The first is the effect of ammonia itself. The second is the
effect of the anion bound to the ammonium ion. Ammonium chloride,
especially, will mainly exert its effects in the mammalian body due
to the formation of hydrogen chloride. Most of the experimental
work concerning the oral route of administration has centred on
ammonium chloride, which has been used extensively in the study of
metabolic acidosis. There have been few studies in which attempts
have been made to identify the role of the ammonium ion and ammonia
in the effects (Table 18).
Median lethal doses (LD50s) of 4400 and 3100 mg/kg body weight
have been reported for ammonium sulfamate in the rat and the mouse,
respectively. (Vinokurova & Mal'kova, 1963). A similar figure for
the oral LD50 in the rat of 4520 (4070 - 5020) mg/kg was reported
by Bukhovskaya et al. (1966). Frank (1948) reported a lethal dose
for ammonium sulfate of between 3000 and 4000 mg/kg body weight in
the rat.
8.1.2.1. Effects of metabolic acidosis induced by ammonium chloride
The ingestion of ammonium chloride in doses of around 500 -
1000 mg/kg body weight per day, for periods ranging from 1 to 8
days, has induced metabolic acidosis in mice, guinea-pigs, rats,
rabbits, and dogs. However, Boyd & Seymour (1946) did not report
any toxic effects at doses of up to 1 g/kg body weight in rats,
rabbits, guinea-pigs, and cats (50 animals per group).
Clinical signs, depending on the severity of the acidosis,
include: a decrease in plasma- and urinary-pH; decreased appetite;
decreased carbon dioxide-combining power; an increase in BUN and
chlorides; an increase in plasma proteins; an increase in
haematocrit (haemoconcentration); increased gluconeogenesis;
increased phosphoenolpyruvate carboxykinase (EC 4.1.1.49) activity;
increased urinary ammonium; increased urea, sodium, chloride,
calcium, and titratable acid excretion; an increase in malate and
oxaloacetate concentrations in renal tissue; and decreased
concentrations of glutamine, glutamate, and alpha-ketoglutarate in
the kidney. Pulmonary oedema, central nervous system dysfunction,
and renal changes are reported to have occurred after ingestion of
ammonium chloride.
Susceptibility to ammonium chloride differs among species. For
instance, pulmonary oedema is produced in cats, but not in rabbits;
yet cats have been shown to be more resistant to oral poisoning by
ammonium chloride than other animals studied.
Age is an important factor in the response of rats to oral
doses of ammonium salts. Benyajati & Goldstein (1975) found that
the administration of a single dose of 5 mmol ammonium chloride/kg
body weight (267.5 mg/kg) by gavage to 7- to 12-day-old rats (39
animals) increased ammonia excretion by about 64%, within 4 h,
compared to an increase of 155% in 20 adult rats after similar
treatment.
Table 18. Selected studies on the acute oral effects of ammonia compounds in rats
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Urea drinking- female, Holtz- 6 concentration no renal hypertrophy Lotspeich (1965)
water man, 200 - of N equivalent
250 g to that of 0.28
mol/litre NH4Cl
for 7 days ad
lib
Citrate drinking- female, Holtz- 6 0.28 mol/litre no renal hypertrophy Lotspeich (1965)
water man, 200 - solution for
250 g 7 days ad lib
Chloride drinking- female, Holtz- 9 0.28 mol/litre metabolic acidosis; increase Lotspeich (1965)
water man, 200 - for 7 days, ad in kidney weight and total
250 g lib (about 1000 N; increased capacity to
mg/kg per day) produce ammonia from
glutamine in kidney; renal
hypertrophy; unilateral
nephrectomy plus acidosis
induced by NH4Cl caused a
greater hypertrophy than
either alone
------------------------------------------------------------------------------------------------------------
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Ammonium Mode of Sex, strain, Num- Dose Effect Reference
compound administ- age, or weight ber
ration
------------------------------------------------------------------------------------------------------------
Chloride diet female, 150 g 125 all on low- consumption of food inversely Motyl & Debski
protein diet; related to dosage of NH4Cl; (1977)
Group I: no dosage-related decrement in
NH4; Group II: weight gain; increase in LD50
1% NH4Cl (300 value for ip dose of 2.7%
mg/kg body NH4Cl in Groups II and III,
weight per indicating an increased
day); Group adaptive capacity for ammonia
III: 2% NH4Cl detoxification; LD50 value of
(380 mg/kg Group IV same as Group III;
body weight per LD50 value of Group V lower
day); Group IV: than Group I; dose-dependent
4% NH4Cl (560 decrease in blood-glucose
mg/kg body levels; decrease of muscle
weight per and liver glycogen; dose-
day); Group V: dependent increase in delta-
8% NH4Cl (540 ornithine transaminase and
mg/kg body ornithine transcarbamylase;
weight per day, increase in adenosine
for 7 days triphosphate concentration in
blood
------------------------------------------------------------------------------------------------------------
8.1.2.2. Organ effects following oral administration
(a) Lung and central nervous system
Toxic doses of ammonium salts induced acute pulmonary oedema in
rats, guinea-pigs, and cats, but not in rabbits given ammonium
chloride (6% aqueous solution) intraperitoneally or by gavage,
though the doses were sufficient to cause death. To induce similar
effects in cats, a larger dose (unspecified) of ammonium chloride
and a longer latency period (from 1 to 3 h) were required,
indicating a greater resistance of this species to ammonium
chloride (Koenig & Koenig, 1949).
In a limited study to assess the role of the ammonium ion in
producing pulmonary oedema, Koenig & Koenig (1949) administered, by
gavage, to 5 different guinea-pigs, 6 ml of 20% ammonium nitrate;
7 ml of 6.4% ammonium acetate; 7 ml of 10% ammonium bromide; 7 ml
of 6% ammonium chloride; or 7 ml of 7.4% ammonium sulfate,
respectively. The last 4 solutions contained approximately
equivalent concentrations of ammonium. All 5 animals died of acute
pulmonary oedema; the lungs of a control animal that had received
water by gavage remained normal. The pulmonary oedema could not be
attributed to the induced acidosis, since guinea-pigs and cats,
made severely acidotic by gavage with sodium lactate or dilute
hydrochloric acid, did not show lung oedema (Koenig & Koenig,
1949).
Progressive signs of ammonium poisoning caused by ammonium
salts, given by gavage, indicative of both pulmonary and nervous
system dysfunction, were reported in less than 30 min in guinea-
pigs and rats (Koenig & Koenig, 1949) including:
(i) a rapid increase in the rate and depth of respiration;
(ii) weakness and difficulty in locomotion;
(iii) hyperexcitability for tactile, auditory, and painful
stimuli; and
(iv) muscle fasciculations over most of the body followed
by generalized tonic convulsions and then coma.
The respiratory rate was greatly reduced but the depth of
respiration increased and was accompanied by gasping and stridor.
Histological changes occurred in both the lung and brain, while
oedema, congestion, and haemorrhage were found principally in the
lung (Koenig & Koenig, 1949; Cameron & Shiekh, 1951).
The addition of ammonium chloride to rat feed resulted in
reduced dietary consumption (Motyl & Debski, 1977). The results of
studies by Noda & Chikamori (1976) pointed to a direct effect of
the ammonium ion on the brain area that regulates feeding. Rats
with bilateral lesions in the prepyriform cortical area of the
brain consumed as much diet containing 3% ammonium chloride as
basal diet. A unilateral injection of 10 mg/litre of 2% NH4Cl/kg
body weight into prepyriform cortical areas, in contrast to an
injection into other areas of the brain, or an injection of sodium
chloride, significantly reduced the food intake of 6 adult male
Wistar rats. The results of these studies suggest that ammonium
ions directly influence appetite by their effects on prepyriform
cortical areas.
(b) Kidney
Lotspeich (1965) reported that ingestion for 7 days of
0.28 mol/litre (1.5%) ammonium chloride in water (ad lib) produced
renal hypertrophy with new cell formation and an enlargement of
existing cells. Some animals from each of the acidotic and control
groups were subjected to unilateral nephrectomy. Highly
significant increases in kidney wet weight, dry weight, and total
nitrogen were observed in the acidotic group. Similar changes were
produced by unilateral nephrectomy. In unilaterally nephrectomized
rats made acidotic by the ingestion of ammonium chloride, the
remaining kidney was larger than that seen in animals with
unilateral nephrectomy without induced acidosis, suggesting an
additive effect of unilateral nephrectomy and ammonium chloride
intake.
The relationship between the renal hypertrophy and the ammonium
ion, acidosis, non-specific intake of nitrogen, or increased solute
load was examined (Lotspeich, 1965). Isomolar (0.28 mol/litre)
solutions of sodium chloride and ammonium chloride produced renal
hypertrophy. The increase due to sodium chloride was less marked
(it was noted that the sodium chloride group drank 3 or 4 times as
much solution each day as the ammonium chloride group). Isomolar
(0.2 mol/litre) solutions of sodium bicarbonate and ammonium
citrate did not induce renal hypertrophy. When 3 groups of 6 rats
were given drinking-water containing 0.28 mol/litre sodium
chloride, 0.28 ammonium chloride, or a urea solution containing
nitrogen equivalent to that in the 0.28 mol/litre ammonium
chloride, for 7 days, only the ammonium chloride solution induced
acidosis. The rats in both the sodium chloride and ammonium
chloride groups had larger kidneys than the urea-drinking rats,
though the kidneys of the sodium chloride-drinking rats were not as
large as those of the ammonium chloride-drinking rats. Since renal
hypertrophy was produced by solutions containing the chloride ion,
while ammonium citrate and urea did not cause any hypertrophy, the
evidence does not support the hypothesis that renal hypertrophy is
due to the ammonium ion.
Thompson & Halliburton (1966) also reported renal hypertrophy
in rats that ingested 3% ammonium chloride in the diet for 6 days.
The ingestion of ammonium citrate or sodium chloride in amounts
that were equivalent to that of the ammonium chloride did not cause
renal hypertrophy.
Janicki (1970) examined the kidneys of rabbits that had been
administered 16.2 g ammonium chloride, by gavage, over a period of
2 days and found the epithelium of the convoluted tubules swollen,
vacuolated, and completely filling the lumen; nuclei exhibited
karyolysis. Findings were similar in 4 rabbits administered 5 -
7 g ammonium chloride, by gavage, over a period of 3 - 7 days.
However, hyperaemia of the renal cortex of rabbits was noted
after the administration, by gavage, of single doses of 0.8 and 1 g
ammonium carbonate/kg body weight (Yoshida et al., 1957). As the
carbonate ion does not have an acidifying effect, this suggests
that the ammonium ion produces some effects on the kidney.
8.1.2.3. Influence of diet on the effects of ammonia
In 6 mongrel dogs, blood-urea levels were increased by a higher
level of protein intake (6 g protein/kg per day) (Bressani &
Braham, 1977). Levels of ~30 mg BUN/litre were found 8 h after the
daily feed. When the protein intake was low (4 mg protein/kg per
day), BUN levels of 14 mg/litre were reported 8 h after the daily
feed. The frequency of protein intake did not affect the maximum
value of blood-urea levels when the protein intake was low (4 mg
protein/kg per day). This suggests that high levels of urea and
ammonia in the blood might occur in animals that suffer from liver
insufficiency and are fed high-protein diets.
Kulasek et al. (1975) studied the effects of nitrogen in the
diet on ammonia detoxification, using 210 rats and 6 diets
containing 50, 150, and 700 g casein/kg (low-, optimal-, or high-
protein diet, respectively), with or without the addition of
ammonium chloride at 20 g/kg. After 8 or 9 days of feeding, the
LD50 from an intraperitoneal injection of a 2.7% solution of
ammonium chloride was determined. The LD50 increased in proportion
to the amount of nitrogen in the diet. The data suggest that
higher doses of exogenous ammonia were tolerated by rats on
protein-rich diets or diets containing an ammonium salt as an
additive. An adaptive capacity for ammonia detoxification was
further demonstrated by Motyl & Debski (1977) in a study using rats
fed low-protein diets with the addition of NH4Cl. The LD50 values
for the ip injection of 2.7% NH4Cl were higher in animals fed low-
protein diets supplemented with NH4Cl (Table 18). Stevens et al.
(1975) raised weanling rats for 3 - 6 weeks on a protein-deficient
diet. Subsequent challenge by a large, intraperitoneally-injected
dose of ammonium chloride indicated that severe protein deprivation
increased vulnerability to ammonia poisoning compared with that of
control groups not prefed protein-deficient diets. Thus, with the
exception of animals suffering from liver dysfunction, animals on
high-protein diets seem able to tolerate a higher oral intake of an
ammonium salt.
A group of eight, 6- to 8-month-old cats, given a single meal
of a complete amino acid diet without arginine, developed
hyperammonaemia with elevated plasma levels of glucose and ammonia
and showed clinical signs of ammonia toxicity within 2 h. One cat
died 4.5 h after ingesting only 8 g of the diet. Five of the
surviving cats given a single meal of complete amino acids, in
which arginine was replaced with an equivalent amount of ornithine,
did not show any unusual signs. This finding indicates that the
cat is unable to synthesize ornithine at the rate required by the
urea cycle to dispose of ammonia from amino acid catabolism;
however, the dietary requirements for cats may differ from those of
other adult animals, including human beings, because the cat has a
much higher protein requirement, i.e., it requires approximately
20% of dietary calories as protein, as opposed to only 4 - 8%
required by the rat, dog, sheep, and man (Morris & Rogers, 1978).
The lethal effects of ammonia poisoning have been prevented by
the amino acids, ornithine and aspartic acid. To test whether this
was mediated by a sparing action of adenosine triphosphate (ATP) on
ammonia metabolism, 20 dogs with chronic Eck's fistula were
injected in the duodenum with ammonium acetate at 4.1 mmol/kg body
weight (~219.4 mg/kg). Half of the group was given 2 mg ATP/kg,
1 h prior to administration of the ammonium acetate. In 9 of the
10 dogs, ATP prevented a rise in levels of ammonia in venous blood.
Grossi et al. (1968) administered ATP (2 mg/kg) intravenously to 6
dogs with chronic Eck's fistula and on high-protein diets. Within
1 h, high blood-ammonia levels returned to normal. In chronic
liver disease, there may be insufficient ATP available for ammonia
detoxification, resulting in hyperammonaemia.
8.1.3. Dermal exposure
No data are available regarding any systemic effects of dermal
exposure to ammonia or ammonium compounds.
8.1.4. Effects due to parenteral routes of exposure
The parenteral toxicity of ammonia and ammonium compounds has
been studied extensively. Toxicity is influenced by the route of
administration, e.g., with oral administration, there is the
capacity for detoxification of exogenous ammonia by the liver.
8.1.4.1. Lethality
The intravenous (iv) and intraperitoneal (ip) LD50 values for a
number of ammonium compounds in various species are summarized in
Table 19. The toxic syndrome was similar in all species studied.
The signs, after iv injection, were characterized by immediate
hyperventilation and clonic convulsions followed by either fatal
tonic extensor convulsion or the onset of coma in 3 - 5 min. The
animals remained comatose for approximately 30 - 45 min. At this
stage, tonic convulsions and death can occur at any time, but
animals that survive usually recover rapidly and completely
(Warren, 1958; Warren & Schenker, 1964; Wilson et al., 1968a,b).
After intraperitoneal injection, the signs did not appear until
15 - 20 min after administration (Greenstein et al., 1956; Wilson
et al., 1968b).
Table 19. Toxicity of several ammonium compounds in selected species
-----------------------------------------------------------------------------------------------------------
Ammonium Species Intravenous dose Intraperitoneal dose
compound (mmol/kg of body weight) (mmol/kg of body weight)
(LD50) (LD50) Reference
-----------------------------------------------------------------------------------------------------------
Acetate rat - 8.20 Greenstein et al. (1956)
mouse 6.23 - Warren (1958)
mouse 5.64 10.84 Wilson et al. (1968a)
chick 2.27 10.44 Wilson et al. (1968a)
Bicarbonate mouse 5.05 - Warren (1958)
mouse 3.10 - Wilson et al. (1968b)
Carbamate mouse 0.99 - Wilson et al. (1968b)
Carbonate mouse 4.47 - Warren (1958)
mouse 1.02 - Wilson et al. (1968b)
Chloride mouse 6.75 - Warren (1958)
mouse (38.8 °C)a 6.6 - Warren & Schenker (1964)
mouse (40.4 °C)a 5.17 - Warren & Schenker (1964)
mouse (27.9 °C)a 10.21 - Warren & Schenker (1964)
Hydroxide mouse 2.53 - Warren (1958)
-----------------------------------------------------------------------------------------------------------
a Body temperature.
8.1.4.2. Central nervous system effects
Navazio et al. (1961) observed that characteristic toxic signs
were not observed in rats until the ammonia concentration in the
blood was double that of basal values (attained within 8 - 10 min
of an ip injection of 601 mg ammonium acetate/kg body weight). No
substantial increase in brain-ammonia was observed. However, when
the blood-ammonia concentration reached more than 20 times the
basal value, there was a sudden rise in the ammonia concentration
in the brain, which reached a maximum of approximately 100 mg
ammonia-nitrogen/kg (wet weight), between 10 and 26 min after
injection. Muscular contractions with occasional tetanic spasms,
and then coma occurred, when the concentration of ammonia in the
brain reached approximately 50 mg/kg. Although the animals started
to recover from the comatose state approximately 70 min after
onset, blood- and brain-ammonia concentrations did not return to
basal levels until 2 h after the injection of ammonium acetate.
However, other authors observed an immediate increase in brain-
ammonia after ip injection of ammonium acetate (Torda, 1953; du
Ruisseau et al., 1957; Salvatore et al., 1963). These authors
found dramatic increases in the brain-ammonia content, 2 - 5 min
after the injection of ammonium acetate. Salvatore et al. (1963)
suggested that there was no critical blood-ammonia concentration
for diffusion through the blood-brain barrier.
Ammonia has been shown to be more highly toxic at elevated body
temperature, whereas hypothermia affords marked protection
(Schenker & Warren, 1962). The LD50 values for ammonium chloride
in the mouse, at various body temperatures, are shown in Table 19.
The increased toxicity of ammonia at elevated body temperature was
suggested to be due to a direct metabolic effect of hyperthermia on
the brain, unrelated to dehydration or stress.
8.1.4.3. Effects on the heart
Intravenous LD50 values for ammonium carbamate, ammonium
carbonate, and ammonium bicarbonate have been determined in mice by
Wilson et al. (1968a,b) (Table 19). The physiological effects of
the injected ammonium compounds in dogs and sheep were also
investigated. Electrocardiograms recorded during the toxic
syndrome indicated that the animals died from ventricular
fibrillation due to a direct effect of ammonia on the heart. These
findings were in agreement with the effects noted by Berl et al.
(1962) during the iv infusion of ammonium chloride in cats when
electrocardiograms were altered in a complex manner. However,
Warren & Nathan (1958) were unable to demonstrate any cardiotoxic
effects of the ammonium compounds in mice and concluded that the
toxicity syndrome was due primarily to a cerebral effect and not a
direct effect on cardiac or skeletal muscle.
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
When 48 rats were continuously exposed to 127 mg ammonia/m3
(181 ppm) for 90 days, no abnormalities were found in organs or
tissues. Inhalation of 262 mg ammonia/m3 (374 ppm) for 90 days
induced mild nasal irritation in about 25% of 49 rats and a mild
leukocytosis in 4 of the rats. Continuous exposure to 455 mg/m3
(650 ppm) resulted in the death of 50 out of 51 rats by the 65th
day of exposure. All animals exhibited mild nasal discharge and
laboured breathing. In a second study, rats, guinea-pigs, rabbits,
and dogs were continuously exposed to 470 mg/m3 (671 ppm) for 90
days. Thirteen out of 15 rats and 4 out of 15 guinea-pigs died.
Marked eye irritation was noted in rabbits and dogs, with corneal
opacities in about one-third of the rabbits. At autopsy, all test
animals examined had more extensive focal or diffuse interstitial
inflammatory processes in the lungs than the controls (Coon et al.,
1970).
White rats were exposed to ammonia, by inhalation, at
concentrations of 100 and 30 mg/m3 (143 and 43 ppm) for 25 or
60 min, every 48 h, for a period of 3 months. Rats exposed to a
concentration of 100 mg/m3 (143 ppm) showed only a mild
leukocytosis, with no significant differences from the control
group with regard to oxygen consumption, neuromuscular excitation
threshold, heart rate, blood-sugar, blood-residual nitrogen, and
total serum-protein (Prokop'eva & Yushkov, 1975). In another study
by Alpatov & Mikhailov (1963), the threshold level for toxic
effects in a 2-month exposure was 40 mg/m3 (57 ppm). Histological
changes were seen in the lungs of the animals exposed to a
concentration of 100 mg/m3 (143 ppm), including small areas of
interstitial pneumonia with signs of peribronchitis and
perivasculitis. No changes were seen in other organs compared with
those in the control group.
Broderson et al. (1976) exposed Sherman and Fisher rats to
ammonia from natural sources, at an average concentration of
105 mg/m3 (150 ppm) for 75 days, and to purified ammonia at
175 mg/m3 (250 ppm) for 35 days. Histological changes in the
olfactory and respiratory epithelia of the nasal cavity were
similar in all the exposed rats, showing increased thickness,
pyknotic nuclei, and hyperplasia. The submucosa was oedematous
with marked dilation of small vessels. Lesions decreased
posteriorly.
Exposure to ammonia at concentrations of 17 - 175 mg/m3 (25 -
250 ppm) increased the infectious effects of Pasteurella multocida
in mice and Mycoplasma pulmonis in rats; this effect increased
with the concentration of ammonia (Broderson et al., 1976; Richard
et al., 1978a).
Richard et al. (1978a) selected a concentration of 350 mg/m3
(500 ppm) for a study of effects of continuous exposure to ammonia
after noting that general toxic effects, particularly on growth
rate, were not found at 175 - 210 mg/m3 (250 - 300 ppm). Young
male specific-pathogen-free rats were age- and weight-matched with
controls (27 per group) and exposed for up to 8 weeks. Nasal
irritation began on the fourth day. After 3 weeks, exposed rats
showed nasal irritation and inflammation of the upper respiratory
tract, but no effects were observed on the bronchioles and alveoli.
The number of pulmonary alveolar macrophages was similar to that in
the controls. After 8 weeks, none of these inflammatory lesions
were present.
In a study by Coon et al. (1970), 15 rats, 15 guinea-pigs, 3
rabbits, 2 dogs, and 3 monkeys were exposed to an ammonia
concentration of 155 mg/m3 (221 ppm) for 8 h/day, 5 days a week,
for 6 weeks. Pathological effects were not observed in any of the
species except for evidence of focal pneumonitis in the lungs of
one of the monkeys. Exposure to 770 mg/m3 (1100 ppm) for the same
duration induced mild to moderate eye irritation and laboured
breathing in the rabbits and dogs at the beginning of exposure, but
these signs disappeared by the second week and no other signs of
irritation or toxicity were noted. Autopsy findings included non-
specific inflammatory changes in the lungs of the rats and guinea-
pigs.
Male Sprague Dawley rats were studied to determine whether
ammonia was absorbed through the lungs into the blood, and the
subsequent effects on the blood pH, blood gases, and hepatic drug-
metabolizing enzymes (Schaerdel et al., 1983). Rats were exposed
to ammonia at 7 - 840 mg/m3 (10 - 1200 ppm) for 1, 3, or 7 days.
No significant changes were found in blood pH, pCO2, or in the
histological appearance of the lungs or trachea. Liver microsomal
enzymes (ethyl-morphine- N-demethylase and cytochrome P-450) showed
only minor changes. Blood-ammonia levels increased in a linear
fashion with increasing ammonia concentrations in air. A
concentration of 70 mg/m3 (100 ppm) or less produced only very
small changes in blood-ammonia levels and had no measureable
effects on any of the parameters studied.
8.2.2. Oral exposure
8.2.2.1. Histopathological effects
Rats given ammonium salts, for periods ranging up to 90 days,
did not appear to sustain renal damage (Freedman & Beeson, 1961;
Gupta et al., 1979). Twelve adult male Sprague Dawley rats were
given ammonium chloride in the drinking-water at 16 g/litre, for up
to 3 weeks. Urinalysis did not reveal any evidence of renal
injury, and no gross or histological "abnormalities" of the kidney
were seen at autopsy (Freedman & Beeson, 1961). Glutaminase
activity per kg of kidney increased with duration of exposure; this
is a physiological adaptation to acidosis. Another group of 10
rats, similarly treated and then given ammonium chloride in the
drinking-water at 10 g/litre, for an additional 2.5 months also did
not show any gross or microscopic renal abnormalities. Assuming
the rats weighed 250 g and consumed 25 ml of water per day, they
could have ingested as much as 1.6 g/kg body weight per day while
drinking the ammonium chloride at 16 g/litre and 1.0 g/kg body
weight per day while drinking a level of 10 g/litre. However,
actual intake may have been lower, because ammonium chloride is
known to affect the appetite and may render the water less
palatable. When ammonium chloride was given at 20 g/litre, food
and water consumption were drastically reduced.
In Table 20, selected studies are presented on ammonium salts
other than the chloride. In general, at low doses, no detrimental
effects were observed when rats and pigs were exposed to the NH4
salts listed.
In a 90-day study by Gupta et al. (1979) on rats, there was not
any evidence of renal damage. Ammonium sulfamate (NH4SO3NH2) as a
100 g/litre solution was given orally at rates of 100, 250, or
5000 mg/kg body weight per day, 6 days a week for 30, 60, or 90
days to adult female rats (ITRC colony-bred albino) and to weanling
male and female rats (20/sex per age per dose level). Under
certain conditions, the sulfamate ion is hydrolysed to bisulfate
ion and ammonia; however, it is unclear whether, and to what
extent, hydrolysis occurs in the rat intestine. Equivalent doses,
listed in Table 20, were calculated on the assumption that no
hydrolysis occurs. The effects of the anion, sulfamate and/or
sulfate, on the action of ammonium is a matter of conjecture.
Ammonium sulfamate would be expected to produce metabolic acidosis
on the basis of its structure, but this has not been verified
experimentally. The general health of both treated and control
rats was good, and there were no significant differences in mean
body weights throughout the study, except for a slight depression
in the highest-dose adult females at 60 and 90 days. Food and
water consumption were unaffected, except in the highest dose
weanlings of both sexes, which consumed less food and drank more
water than control weanlings. Interim sacrifice of 6 animals/sex
per dosage group was performed for haematological and histological
examination. There were no significant differences in
haematological values (packed cell volume, haemoglobin
concentration, total red cell count, total and differential white
cell count). Relative organ weights in all treated groups did not
differ significantly from those in the controls. Histological
examination of the kidney, liver, lung, stomach, heart, spleen,
thyroid, adrenal, gonads, intestine, and lymph nodes did not reveal
any abnormalities, except slight hepatic fatty degeneration in one
adult female at 90 days.
Table 20. Dose-response data for short-term oral administration of ammonium compounds
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Sulfamate rat, albino, orally as 10% 30, 60, 100; 15.0; no effects on body Gupta et
20 adult solution; not 90 days 250 37.3 weight, food, and al. (1979)
female, 20 specified whether water consumption,
weanling given by gavage haematological values
male, 20 or organ weights; no
weanling histological
female per abnormalities in
dose organs (including
kidneys)
500 74.8 slight decrease in
body weight in adult
females; slightly
lower food and higher
water consumption in
weanlings of both
sexes; no effects on
haematological values
or organ weights; no
histological
abnormalities in
organs (including
kidneys)
------------------------------------------------------------------------------------------------------------------- kidneys)
Table 20. (contd.)
-------------------------------------------------------------------------------------------------------------------
Ammonium Species, Mode of Duration Daily Intakea Response Reference
salt No/group, administration (mg/kg (mg NH3/kg
sex body body
weight) weight)b
-------------------------------------------------------------------------------------------------------------------
Diammonium pig, male 3.75% diammonium 28 days 1820 274 body weight gain Kagota et
citrate 22.5 kg, citrate in diet increased but % meat al. (1979)
3-4/group containing 6.4% crude and fat of carcass
protein unaffected; plasma-
and urine-urea-
nitrogen increased
but blood-ammonia-
nitrogen level
unaffected; no effect
on haematological
values; no gross
abnormalities or
lesions
Diammonium pig, 27.3 kg basal diet contained 81 days 820 161 (10% no effect on weight Wehrbein
phosphate 3 males and 16% protein; 0,5,10, replacement) gain, BUN, or food et al.
and 3 females/ or 20% of dietary N consumption (no (1970)
diammonium group was replaced by N effect on these
citrate as from equimolar parameters at 5%
equimolar mixture of designated 81 days 1600 322 (20% either) slight
mixture NH4+ salts replacement) decrease in weight
gain, BUN and food
consumption
-------------------------------------------------------------------------------------------------------------------
a Estimated for all studies except Gupta et al. (1979).
b Equivalent intake expressed as ammonia (NH3).
Treatment of virgin female rabbits with ammonium carbonate,
chloride, hydrophosphate, sulfate, or hydroxide at 0.1 - 0.2 g/kg
body weight, orally, on alternate days, for periods of 3 weeks
separated by 1-week intervals of no treatment, was associated with
enlargement of the ovaries, follicle maturation, and formation of
corpora lutea (Fazekas, 1949). There was also enlargement of the
uterus, hypertrophy of the teats, and secretion of milk.
Rabbits (10 - 80 per group) were given 0.1 - 0.2 g/kg body
weight of an ammonium salt in 100 - 150 ml of drinking-water, twice
daily. One hundred and sixty rabbits (96 female, 64 male)
received, twice daily, 50 - 80 ml of a 0.5% ammonium hydroxide
solution (~135 - 210 mg/kg body weight), in gradually increasing
doses. The chemicals were given for periods of 3 weeks separated
by 1-week intervals of no treatment. In a related study, similar
treatment of rabbits with ammonium chloride or ammonium sulfate
resulted in fluctuations in serum-calcium and -phosphorus levels
(Fazekas, 1954a).
8.2.2.2. Effects of ammonium as a dietary nitrogen supplement
Ammonia from ammonium salts can stimulate the growth of animals
on diets deficient in non-essential amino acids or restricted in
protein content. Weanling rats, given an artificial diet
comprising essential amino acids, B vitamins, vitamin C, salts, and
glucose for 21 days, grew poorly. Rats given a similar diet in
which some of the glucose was replaced with 8.6% ammonium acetate
(equivalent to 15.7 g nitrogen/kg diet) showed a dramatically
improved weight gain (Birnbaum et al., 1957). The food
consumption/weight gain ratio (feed/gain ratio) was also improved
in animals receiving the ammonium salt.
Similar results were obtained with young male pigs given a more
natural basal diet restricted in non-essential amino acids and
containing the minimum requirement of essential amino acids (Kagota
et al., 1979). The crude protein level of this basal diet was
6.4%. Three pigs fed the basal diet supplemented with 3.75%
diammonium citrate had a significantly greater weight gain and a
slightly lower feed/gain ratio than 4 pigs fed the basal diet (the
diets were made isocaloric by adjusting carbohydrate). No
significant differences in the packed cell volume or ammonia-
nitrogen level of the blood or in total protein concentration of
plasma were found between the 2 groups. Plasma- and urine-urea-
nitrogen were increased in the pigs fed the diet supplemented with
diammonium citrate. Autopsy of the pigs after 28 days on the basal
or ammonium citrate diets did not reveal any lesions or
abnormalities or any differences in the percentage of carcass meat
and fat. The average daily food intake for pigs receiving
diammonium citrate was 1.44 kg, which corresponds to 54 g
diammonium citrate per day. Using a body weight midway between the
initial (22.5 kg) and final (36.7 kg) body weights, the mean intake
of diammonium citrate can be estimated to be 1.82 g/kg body weight,
per day, or 16.1 mEq NH4+/kg body weight, per day.
Addition of ammonium salts to diets with a higher protein
content (10% crude protein) did not produce significant changes in
weight gain in rats or pigs (Clawson & Armstrong, 1981).
Similarly, no significant changes in weight gain occurred in pigs,
when up to 10% of the nitrogen from crude protein (diet = 16% crude
protein) was replaced with nitrogen from ammonium salts (Wehrbein
et al., 1970). Replacement of 20% of the dietary nitrogen with
ammonium salts slightly decreased body weight gain in the pigs;
food consumption and BUN also decreased. The 20% replacement diet
contained 1.54% diammonium phosphate and 2.07% diammonium citrate
for a total of 3.61% ammonium salts and was fed for 81 days. The
estimated mean intake was 0.70 g diammonium phosphate and 0.94 g
diammonium citrate/kg body weight per day, giving a total of
18.9 mEq NH4+/kg body weight per day.
8.2.3. Dermal exposure
There is no information regarding systemic toxicity from short-
term dermal exposure to ammonia or ammonium compounds.
8.3. Skin and Eye Irritation; Sensitization
Ammonia in the form of a gas, an anhydrous liquid boiling at
low temperatures, or an aqueous solution is a recognized skin and
eye irritant. Most of the information is human clinical data and
is described in section 9.
Ammonia, partly because of its lipid solubility, penetrates
the corneal membrane rapidly (NRC, 1979). In rabbits, conjunctival
oedema with ischaemia and segmentation of limbal vessels were noted
within 30 min. By 24 h, there was a reduction in the
mucopolysaccharide contents of the corneal stroma, and extensive
polymorphonuclear infiltration and anterior lens opacities were
apparent. Aqueous levels of glucose and ascorbate and intraocular
pressure were depressed (NRC, 1979). In rabbits with corneal
burns, neovascularization occurred after 1 week, but it was delayed
in animals with corneal and limbal burns. Complications of severe
burns included symblepharon, pannus, pseudopterygia, progressive or
recurrent corneal ulcerations leading to perforations, permanent
corneal opacity, corneal staphyloma, persistent iritis, phthisis
bulbi, secondary glaucoma, and dry eye (NRC, 1979).
Ammonium persulfate is a recognized skin sensitizer for human
beings. There are no data on its sensitization potential in animal
models.
8.4. Long-Term Exposures
8.4.1. Inhalation exposure
Weatherby (1952) exposed 12 guinea-pigs to an ammonia
concentration of about 119 mg/m3 (170 ppm). Chamber concentrations
ranged from 98 to 140 mg/m3 (140 to 200 ppm) for 6 h/day, 5 days a
week, for up to 18 weeks. There were no significant findings at
autopsy in animals sacrificed after 6 or 12 weeks of exposure. In
animals sacrificed after 18 weeks of exposure, there was congestion
of the liver, spleen, and kidneys, with early degenerative changes
in the adrenal glands. Increased erythrocyte destruction was
suggested by increased quantities of haemosiderin in the spleen.
In the proximal tubules of the kidneys, there was cloudy swelling
of the epithelium and precipitated albumin in the lumen with some
casts. The cells of the adrenal glands were swollen and the
cytoplasm in some areas had lost its normal granular structure.
Coon et al. (1970) conducted studies in which rats, guinea-
pigs, rabbits, dogs, and monkeys were continuously exposed
(24 h/day, 7 days per week) to ammonia. No signs of toxicity were
seen in any species following continuous exposure to ammonia at a
concentration of 40 mg/m3 (57 ppm) for 114 days, and gross and
microscopic examination did not reveal any lung abnormalities.
8.4.2. Oral exposure
The administration of ammonium carbonate, chloride, sulfate,
hydrophosphate, acetate, lactate, or hydroxide to a total of 296
rabbits for 3 - 16 months resulted in enlargement of the
parathyroids (Fazekas, 1954a). Administration of sodium
dihydrophosphate, sodium ammonium phosphate, calcium chloride,
hydrochloric acid, acetate acid, or lactic acid gave similar
results (Fazekas, 1954a).
Rabbits given 0.1 g ammonium hydroxide/kg body weight (as a
0.5 - 1.0% solution) by gavage, initially, on alternate days and
then daily for up to 17 months, had enlarged adrenal glands
(Fazekas, 1939). An initial fall in blood pressure of 2.67 -
4.00 kPa (20 - 30 mmHg) was followed by a gradual rise to 1.33 -
4.00 kPa (10 - 30 mmHg) above the baseline, after several months
of treatment.
The results of studies on rats, rabbits, and dogs indicate that
long-term administration of ammonium chloride can induce
osteoporosis (Seegal, 1927; Bodansky et al., 1932; Jaffe et al.,
1932a,b; Barzel & Jowsey, 1969; Barzel, 1975). During prolonged
metabolic acidosis, the release of bone mineral by resorption is
thought to provide additional buffering capacity, sparing
bicarbonate.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
Charles & Payne (1966b) studied the effects of graded
concentrations of atmospheric ammonia on the performance of laying
hens. At 18 °C, ammonia at 73.5 mg/m3 (105 ppm) significantly
reduced egg production, after 10 weeks of exposure. No effects
were observed on egg quality. Food intake was reduced and weight
gain was lower. No recovery in egg production occurred when the
treated groups were maintained for an additional 12 weeks in an
ammonia-free atmosphere. Similar results were observed at 28 °C,
under the same conditions. Earlier work had indicated that egg
quality could be affected by ammonia exposure (Cotterill &
Nordskog, 1954). Freshly-laid eggs were exposed to various
concentrations of ammonia in a desiccator for 14 h at room
temperature and then moved to normal atmosphere for another 32 h at
50 °C, before examination. There was evidence of absorption of
ammonia into the eggs and significant impairment of interior egg
quality, as measured by Haugh units, pH, and transmission of light.
The interaction of ammonium chloride with other teratogenic
agents has been investigated in 2 studies. Goldman & Yakovac
(1964) used ammonium chloride to investigate the role of metabolic
acidosis in salicylate-induced teratogenesis in Sprague Dawley
rats. Beginning on day 7 of gestation, rats received either
0.17 mol/litre (0.9%) ammonium chloride in the drinking-water, a
single subcutaneous injection of salicylate, or both. Ammonium
chloride alone inhibited fetal growth, but was not teratogenic.
However, when administered with salicylate, ammonium chloride
significantly increased maternal and fetal mortality and the fetal
anomaly rate (dorsal midline, ventral midline, and eye defects)
compared with that due to salicylate alone. These effects were
attributed to acidosis and not to ammonia. However, it has been
shown by Miller (1973) that the addition of 0.5% ammonium chloride
to the 5%-glucose drinking-water of fasting, pregnant CFW mice
significantly reduced the incidence of fast-induced cleft palate in
the progeny.
8.6. Mutagenicity
Ammonium sulfate was reported to be non-mutagenic in the
Salmonella and Saccharomyces systems (Litton Bionetics, Inc.,
1975). Demerec et al. (1951) tested ammonia for its ability to
induce back-mutations from streptomycin dependence to non-
dependence in Escherichia coli and found it to be mutagenic, but
only in treatments that left less than 2% survivors. Iwaoka et al.
(1981a,b) showed that extraction of ingredients from fried
hamburger and refrigerated biscuit products with ammonium hydroxide
or ammonium sulfate increased the mutagenic activity in
S. typhimurium TA98 and TA1538, compared with sodium sulfate
extraction. This suggests that ammonium salts may, in some way,
influence the mutagenic activity of some agents, or may themselves
be responsible for false positive findings (Iwaoka et al., 1981a).
However, it is also possible that ammonium salts extract mutagenic
components from foods more efficiently.
Lobashov & Smirnov (1934) and Lobashov (1937) found ammonia to
have a mutagenic effect on Drosophila. Ammonia showed slight
mutagenic activity in studies in which survival of Drosophila was
lower than 2% after treatment. In studies by Auerbach & Robson
(1947), when Drosophila was exposed to ammonia vapour in small
glass containers, 0.5% sex-linked lethals were observed.
It was reported by Rosenfeld (1932) that ammonia induced
clumping of chromosomes, arrest spindle formation, and induce
polyploidy in chick fibroblasts in vitro.
There are no data to show that ammonia is mutagenic in mammals.
8.7. Carcinogenicity
There is no evidence indicating that ammonia is carcinogenic.
Gibson et al. (1971) observed that animals treated with ammonia
developed inflammatory lesions of the colon. It has been suggested
that cell proliferation may increase errors in DNA replication
(Zimber, 1970; Zimber & Visek, 1972), activate oncogenic agents
present in sub-threshold doses (Anderson et al., 1964), and even
unmask latent changes in the genetic material caused by mutagenic
agents (Visek et al., 1978).
In a study on male Sprague Dawley rats (Topping & Visek, 1976),
there was no evidence that ammonia increased the incidence of
tumours with increased protein intake. Development of lung
tumours was observed in CFLP mice treated intra-gastrically with
diethyl pyrocarbonate and ammonia, neither of which induces cancer
independently in animals. However, this may have resulted from a
carcinogenic substance, possibly urethane, formed in vivo from
diethyl pyrocarbonate in the presence of ammonia (Uzvolgyi & Bojan,
1980).
Life-long ingestion of ammonium hydroxide in the drinking-water
by Swiss and C3H mice did not produce any carcinogenic effects, and
had no effect on the spontaneous development of adenocarcinoma of
the breast in C3H females, a characteristic of this strain (Toth,
1972).
Ammonium chloride and ammonium tungstoantimonate and related
compounds have been shown to have an inhibitory effect on malignant
cells (Phillips, 1970; Anghileri, 1975; Flaks & Clayson, 1975;
Flaks et al., 1973; Sof'ina et al., 1978).
8.8. Factors Modifying Effects
8.8.1. Synergistic effects
Stevens et al. (1975) raised weanling rats for 3 - 6 weeks on a
protein-deficient diet. Subsequent challenge by a large,
intraperitoneally-injected dose of ammonium chloride indicated that
severe protein deprivation increases vulnerability to ammonia
poisoning in comparison with control groups not prefed protein-
deficient diets.
Dalhamn (1963) employed a technique in rabbits in which an air
pollutant mixture was drawn into the nostrils and out through a
tracheal cannula. The effects of ammonia alone and ammonia mixed
with (and presumably adsorbed on) carbon particles, on the beating
rate of tracheal cilia was examined. Ammonia alone had to be
administered at a high concentration (1400 mg/m3 or 2000 ppm) to
produce a tracheal concentration of 70 mg/m3 (100 ppm). The
reduction in beating rate was not substantially altered by the
addition of carbon particles at 2 mg/m3.
Simultaneous injection of ammonium salts and a fatty acid into
rats or cats produced coma at lower plasma levels of ammonia and
free fatty acids than a single injection of either compound (Zieve
et al., 1974).
Impaired handling of an ammonium load has been observed in
animals with hepatic dysfunction. Elevated levels of ammonia or
urea have been reported in dogs with experimentally-bypassed livers
(section 7.1.2). The importance of the kidney in detoxification of
an ammonium load is apparent in the studies of Lotspeich (1965)
(section 8.1.2.2), where an additive hypertrophic effect of
ammonium chloride and unilateral nephrectomy was observed on the
remaining kidney. Increased sensitivity to ammonia toxicity was
found in young rats (Benyajati & Goldstein, 1975) and in rats that
had been castrated, adrenalectomized, or thymectomized (Paik et
al., 1975).
8.8.2. Antagonistic effects
L-arginine and the related amino acids, ornithine and aspartic
acid, substrates from urea synthesis, have been reported to exert a
protective effect against acute ammonia poisoning in rats, dogs,
and cats (Morris & Rogers, 1978; NRC, 1979).
High-protein diets exert an antagonistic effect on the toxicity
of ammonium salts, unless liver dysfunction is present (section
7.1.2.4). In addition, an increase in toxic response has been
observed with increase in age due to the adaptive response of
glutaminase activity in rats to ammonia detoxication during
acidosis.
8.9. Mechanisms of Toxicity
Hypotheses proposed for the mechanism of ammonia toxicity
include impaired decarboxylation of pyruvic acid (McKhann & Tower,
1961), NADH depletion slowing down the generation of ATP (Worcel &
Erecinska, 1962), depletion of alpha-ketoglutarate resulting in
impairment of the Krebs cycle (Bessman & Bessman, 1955; Fazekas et
al., 1956; Warren & Schenker, 1964); depletion of ATP due to
glutamine formation by the glutamine synthetase (EC 6.3.1.2) system
(Warren & Schenker, 1964; Nakazawa & Quastel, 1968), stimulation of
membrane ATPase (EC 3.6.1.8) producing increased nerve cell
excitability and activity (Hawkins et al., 1973), and depletion of
ATP causing a decrease in cerebral acetylcholine (Braganca et al.,
1953; Ulshafer, 1958).
9. EFFECTS ON MAN
9.1. Organoleptic Aspects
9.1.1. Taste
Campbell et al. (1958) determined the taste threshold
concentration for ammonia in redistilled water with 21 - 22
subjects in "difference tests of the triangle type". At ammonia
concentrations of 26, 52, and 105 mg/litre, the percentages of
correct identifications were 61.9, 71.4, and 85.7, respectively.
Defining the threshold concentration as the level at which
correct identification is 50% greater than that expected by
chance, the taste threshold of ammonia was determined to be
around 35 mg/litre. However, this definition of the threshold
concentration seems to be somewhat arbitrary and McBride & Laing
(1979) have reported significant positional bias in using the
triangle test to determine taste thresholds. Furthermore, the
triangle test is not intended to mimic environmental exposures
in which the taste thresholds could be substantially higher. It
seems reasonable to conclude only that the palatability of water
is not likely to be significantly affected by total ammonia levels
of < 35 mg/litre (as NH3), but will be affected at higher levels.
9.1.2. Odour
Odour thresholds reported in the literature may vary according
to the definition of odour response, mode of presentation of the
stimulus, chemical purity of the agent used, and the number of
subjects and trials in the study. The detection threshold for
ammonia, defined as the concentration that produces the first
detectable difference in odour over background, was reported to be
37 mg/m3 (DallaValle & Dudley, 1939). This reference, though often
cited, gives no information on the study design from which this
number was derived.
The considerable variability in threshold data prompted work by
Leonardos et al. (1969), who used a standardized procedure to
determine recognition thresholds rather than detection thresholds
for 53 chemicals. The odour threshold was defined as the first
concentration at which all four panel members (trained odour
analysts) were able to recognize the characteristic odour of the
chemical. The panel tested only one chemical per day.
Concentrations examined were multiples by 10 of 0.7, 1.47, and
3.3 mg/m3 (1, 2.1, and 4.6 ppm). The recognition threshold for the
odour of ammonia was 32.6 mg/m3 (46.8 ppm).
The results of several other studies suggest that human beings
can detect ammonia at much lower levels. Stephens (1971) reported
2.7 mg/m3 (3.9 ppm) as the lowest concentration producing an odour
response, when a contaminated air stream and a reference stream
were compared by sniffing (number of subjects was not reported). A
report by Saifutdinov (1966), of an olfactory threshold of 0.55 -
0.50 mg/m3 for the most sensitive of 22 subjects, did not include
sufficient detail to evaluate this excessively low estimate.
Carpenter et al. (1948) stated that "a group of 8 persons found
that the least odour they could detect on entering a room
containing various concentrations" was 0.7 mg/m3 (1 ppm) ammonia.
Again, no other details were given.
The best estimates of the thresholds at which ammonia can be
expected to be detected by taste and odour are 35 mg/litre (as NH3)
and 35 mg/m3, respectively. Sensitive individuals may detect
concentrations an order of magnitude lower.
9.2. Clinical and Controlled Human Studies
9.2.1. Inhalation exposure
The severe effects resulting from acute exposure to ammonia
have been described in case reports of accidents involving groups
of people or individuals. There are no data on actual levels of
ammonia in air during such accidents, but estimates have been made
(Yahagi et al., 1959; Takahashi et al., 1984; NRC, 1979).
Exposure to an ammonia concentration of 280 mg/m3 (400 ppm) has
been reported to produce immediate throat irritation; 1200 mg/m3
(1700 ppm) to produce cough; 1700 mg/m3 (2400 ppm) to be life-
threatening, and 3500 - 7000 mg/m3 (500 - 10 000 ppm) to cause a
high mortality rate (Patty, 1963; Helmers et al., 1971).
A burning sensation in the eyes, nose, and throat, as well as
respiratory distress accompanied by lachrymation, coughing, and an
increase in respiratory rate are some of the irritant effects of
ammonia (Caplin, 1941). Chest X-rays are generally normal in such
mild cases (Watson, 1973; Close et al., 1980). More severe
respiratory effects include laryngeal and pulmonary oedema and
bronchopneumonia (Slot, 1938; Caplin, 1941; Levy et al., 1964;
Taplin et al., 1976; Flury et al., 1983). The signs and symptoms
are generally reversible, but chronic bronchitis and bronchiectasis
have been reported (Slot, 1938; Sugiyama et al., 1968; Taplin et
al., 1976; Close et al., 1980).
In cases with a lethal outcome, the cause of death has been
severe lung damage and secondary cardiovascular effects (Slot,
1938; Mulder & van der Zalm, 1967).
There have also been some studies on volunteers exposed to
ammonia under laboratory conditions. Some of these studies are
summarized in Table 21.
Silverman et al. (1949) reported on 7 human volunteers exposed
to a concentration of 300 mg/m3 (500 ppm) ammonia for 30 min using
an oral-nasal mask. All 7 experienced upper respiratory
irritation, which lasted up to 24 h in 2 of the volunteers. Two
subjects experienced marked lachrymation, in spite of the exposure
being by oro-nasal mask. The average respiratory minute volume
increased markedly compared with control values, and in the 5
subjects in which minute-by-minute expired volumes were measured,
there was a marked cyclical variation in minute volume with a
period of 4 - 7 min. No coughing was noted.
After exposure, respiratory minute volumes fell to levels below
the pre-exposure rate, but returned to pre-exposure values within 5
min of exposure. Ammonia retention decreased progressively until
an equilibrium of 24% retention (ranging from 4 to 30%) was reached
at approximately 19 min (range 10 - 27 min). The indices of
nitrogen metabolism (BUN, NPN, urine-urea, and urine-ammonia)
remained normal. The carbon dioxide combining power did not
change.
Verbeck (1977) assessed respiratory function in 16 volunteers
following exposure to 35, 56, 77, and 98 mg ammonia/m3 (50, 80,
110, and 140 ppm) for 0.5, 1, and 2 h. The respiratory variables of
vital capacity (VC), forced expiratory volume (FEV), and forced
inspiratory volume (FIV), measured before and after exposure, did
not decrease by more than 10%. Subjective variables, including
smell, taste, irritation of the eyes, nose, or throat, urge to
cough, headache, and general discomfort were monitored every 15 min
and ranked by 8 experts and 8 non-experts (students) on a scale
from 0 to 5. Subjective responses were ranked higher by the non-
experts. A concentration of 77 mg/m3 (110 ppm) was tolerated for
2 h, but at 98 mg/m3 (140 ppm), all the subjects left the chamber
because the exposure was intolerable.
Respiratory responses to ammonia during exercise were examined
by Cole et al. (1977). Eighteen males, aged 18 - 39 years, had 2
periods of exercise in 3 consecutive half-day sessions. Mean
exposure levels were 71, 144, 106, and 235 mg/m3 (101, 206, 151,
and 336 ppm). Ventilation minute volume and total volume
decreased, and mean respiratory frequency increased at exposure
levels > 106 mg/m3 (151 ppm).
In a study by MacEwen et al. (1970), 6 volunteers were exposed
to ammonia concentrations of 21 and 35 mg/m3 (30 and 50 ppm) for 10
min. The irritation was rated subjectively on a scale of 0 - 4.
At 35 mg/m3 (50 ppm), irritation was rated as "moderate" by 4 of
the volunteers, while 1 individual reported no detectable
irritation. None of the volunteers found the irritation at
35 mg/m3 (50 ppm) to be "discomforting" or "painful".
The irritation threshold for ammonia was examined in 10 human
volunteers by Industrial Bio-Test Laboratories, Inc. (1973). The
subjects were exposed to 4 different concentrations of 22, 35, 50,
and 94 mg/m3 (32, 50, 72, and 134 ppm) for 5 min. The frequency of
positive findings for the 10 subjects was as follows: at 22 mg/m3,
1 subject complained of dryness of the nose; at 35 mg/m3, 2
subjects experienced dryness of the nose; at 50 mg/m3, 3 subjects
had eye irritation, 2 had nasal irritation, and 3 had throat
irritation; at 94 mg/m3, 5 subjects demonstrated signs of
lachrymation, 5 had eye irritation, 7 had nasal irritation, 8 had
throat irritation, and 1 complained of chest irritation.
Table 21. Effects of inhaled ammonia in human volunteers
--------------------------------------------------------------------------------------------
Number Concentration Duration Effects and response Reference
(mg/m3) (min)
--------------------------------------------------------------------------------------------
7 300 30 irritation of upper respiratory Silverman et al. (1949)
tract (7/7); increase in minute
volume; no change in blood
chemistry
16 35 - 98 30 - 120 10% increase in VC, FEV, and Verbeck (1977)
FIV; 98 mg/m3 was not tolerated;
77 mg/m3 was tolerated for 2 h
18 71 - 235 8 - 11 no effects noted during exercise Cole et al. (1977)
at 71 mg/m3 except slight
irritation; decrease in
ventilation minute volume and
tidal volume and increase in
respiratory frequency > 106
mg/m3
6 35 10 "moderate irritation" (4/5) MacEwen et al. (1970)
10 22 5 dryness of the nose (1/10) Industrial Biotest
10 35 dryness of the nose (2/10) Laboratories (1973)
10 50 eye irritation (3/10)
10 94 throat irritation (8/10)
eye irritation (5/10)
6 17.5, 35, 2 - 6 h, increase in FEV1, with Ferguson et al. (1977)
or 70+ 5 days/ increasing NH3; adaptation
week, for to irritating effects
6 weeks
--------------------------------------------------------------------------------------------
A controlled study on human volunteers was conducted by
Ferguson et al. (1977) to evaluate the responses to inhaled ammonia
at concentrations of 17.5, 35, and 70 mg/m3 (25, 50, and 100 ppm).
Six adults, not acclimatized to ammonia exposure, were divided into
3 groups, which were exposed for 2 - 6 h each day, 5 days per week,
for 6 weeks. One pair of subjects was exposed only to 35 mg
ammonia/m3 for 6-h periods throughout the test. Subjects were
examined daily for irritation of the eyes, nose, or throat, and
periodically for pulse rate, respiration rate, pulmonary function
(forced vital capacity (FVC) and forced expiratory volume in 1
second (FEV1)), blood pressure, neurological responses, and
interference in task-performance ability. A statistical analysis
of the results demonstrated that the only significant change among
the vital functions measured was an increase in forced expiratory
volume in 1 second (FEV1) with increasing ammonia concentration.
In addition, the rate of mild eye, nose, or throat irritations over
the last 3 weeks of the test was significantly less than during the
first 2 weeks. This indicated that an acclimatization process had
occurred, with increased tolerance to the irritant effects of
ammonia developing with increasing time of exposure. Overall, the
ammonia exposure produced 2 incidents of mild irritation (78
observations made) at 17.5 mg/m3 (25 ppm), 22 incidents (198
observations made) at 35 mg/m3 (50 ppm), and 11 incidents (84
observations made) at 70 mg/m3 (100 ppm). Among control subjects,
4 irritation incidents were recorded during 45 observations. When
ammonia concentrations exceeded 105 mg/m3 (150 ppm), all subjects
experienced lachrymation accompanied by dryness of the nose and
throat.
In an inhalation study, the threshold for effects on
respiration, skin electric potential, and the electroencephalogram
was found to be 22 mg/m3 (31 ppm) (Alpatov & Mikhailov, 1963;
Alpatov, 1964).
Reports from industries with ammonia exposure indicate that
irritative effects have appeared over a wide range of ammonia
concentrations (Elkins, 1950; Vigliani & Zurlo, 1955; Mangold,
1971). A concentration of 88 mg/m3 was called "definitely
irritating" (Elkins, 1950), and "barely noticeable" eye irritation
was reported at 3 mg/m3 (Mangold, 1971).
Giguz (1968), in a study involving 140 subjects exposed to
ammonia and nitrogen oxides, at concentrations not exceeding the
"maximum permissible concentration" (20 mg/m3), 3 h per day during
2 - 3 years of vocational training, demonstrated increased
incidences of upper respiratory tract disease, compared with those
in a control group of unexposed subjects.
9.2.2. Oral exposure
9.2.2.1. Effects of acute oral exposure
Cases of the ingestion of large doses of ammonia have been
reported. When solutions of ammonia were ingested orally, a
tissue-destructive caustic effect was noted for concentrated
solutions, owing to their high pH. A solution of ammonia at a
concentration of 100 g/litre, for example, has a pH of 12.5.
Oesophageal burns were reported in 25 cases of accidental
ammonia ingestion (Hawkins et al., 1980). One child suffered a
mild burn. Four adults had mild burns limited to the mucosa and
nine adults had oesophageal burns that were moderate or more severe
in nature. One adult female suffered from a complication of airway
obstruction from supraglottic oedema. Oesophageal stricture from
the ingestion of ammonia (100 g/litre) was reported by Vancura et
al. (1980) (Table 19).
A fatal outcome after ingestion of a solution containing 24 g
ammonia/litre was reported by Klendshoj & Rejent (1966). Autopsy
showed haemorrhagic inflammatory changes in the oesophagus,
stomach, and small intestine.
There are many reports on the effects of ammonium chloride, but
since acidosis is caused by the chloride, such studies have little
relevance for evaluating ammonia toxicity.
There are no data on acute effects of ingestion of ammonium
compounds, other than the chloride.
9.2.3. Endogenous hyperammonaemia
9.2.3.1. Inborn errors of metabolism
These affect the uptake of ammonia rather than its rate of
production.
Congenital deficiency of carbamyl phosphate synthetase I (EC
6.3.4.1.6), and, to a lesser extent, of other enzymes of the
ornithine cycle, and several other metabolic disorders may lead to
hyperammonaemia and various abnormal urinary constitutents.
Hyperammonaemia may be lethal in new-born infants, may cause severe
symptoms in infancy, or may cause chronic remittent symptoms in
older children or adults (Hsia, 1974).
Clinical features in neonates may resemble those of hepatic
coma and may be precipitated by protein-rich milk feeds. In older
children, episodic vomiting, neurological disorders (including
seizures) or coma are precipitated by high-protein foods.
9.2.3.2. Hepatic features
Varied and complex functions of the liver may fail
progressively in chronic liver disease or rapidly in acute
disorders. The syndromes differ in the extent to which portal
blood from the intestine is shunted into the systemic venous
circulation either by cirrhotic changes in hepatic vascular
resistance or by surgical procedures to correct portal hypertension
resulting from it.
In either case, a syndrome is recognised that comprises a
spectrum of neurological features from irritability via
inappropriate behaviour, tremors, hyperreflexia and generalised
muscular rigidity, to delirium, stupor, convulsions and coma.
There is disagreement regarding the extent to which this
syndrome is an expression of ammonia toxicity. On the one hand,
over 90% of persons with the disorder have elevated levels of
ammonia in the blood or cerebrospinal fluid; the condition may be
induced in those with marginal liver function by the administration
of ammonium salts or an intestinal haemorrhage (which leads to
intestinal ammonia production greater than that from an equivalent
amount of meat) and the condition may be treated by reducing
intestinal ammonia production, by the administration of antibiotics
to eliminate ammonia-producing flora. On the other hand, there is
an imperfect correlation between the clinical state and the blood-
ammonia level and the condition may occur in the presence of normal
blood-ammonia levels.
It is unlikely that the syndrome resulting from the failure of
an organ as complicated as the liver should be explicable in terms
of a single metabolic component, but the similarity between hepatic
failure and certain expressions of congenital hyperammonaemias
suggests an important role of ammonia toxicity in the pathogenesis
of the syndrome.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Atmospheric Exposure and Effects
10.1.1. General population exposure
Background levels of ammonia in community air are low in
comparison with levels that have been established as safe in
occupational settings, but there is considerable variabilty
according to the type of land use. In general, levels in areas
with intensive livestock husbandry or high rates of manure
application are in the range of 100 - 200 µg/m3, levels in urban
areas range from approximately 5 to 40 µg/m3, while those in rural
areas, without intensive manure production or use, range up to
10 µg/m3. This is in contrast to odour thresholds of the order
10 000 µg/m3, thresholds for irritation of the order 20 000 -
50 000 µg/m3, and the estimated LC for man of 5000 - 10 000 mg/m3.
The LC50 estimation for the rat was 7 600 000 µg/m3, for a 2-h
exposure. In an occupational setting, workers did not voluntarily
use respiratory protective devices at concentrations below about
500 000 µg/m3. General ambient atmospheric levels are therefore
of no concern in respect of discomfort or acute toxicity.
Ammonia is not mutagenic and long-term studies on both
laboratory and farm animals have not shown any pathological effects
at levels below 35 000 µg/m3. Long-term toxic effects are
unlikely, even at levels much higher than ambient levels, both
generally and in the neighbourhood of ammonia-emitting systems.
There is a lack of evidence regarding recent trends in global
atmospheric ammonia concentrations.
10.1.2. Occupational exposure
Occupational problems are predominantly those of accidental
exposure.
Though for certain groups in, for example, agriculture, the
chemical industry, waste disposal and transport, occupational
exposure levels may be very much higher than general, there is
historical evidence that, even at levels significantly in excess of
current occupational exposure limits, there was a low prevalence of
adverse health effects.
The distribution kinetics of absorbed atmospheric ammonia
suggest that the rise in blood-ammonia at a typical occupational
exposure limit will be within the normal range of variation.
Thus, there is both historical and theoretical evidence that
most recommended exposure limits are acceptable.
10.2. Exposure Through Water and Food
Ammonia will have a toxic effect, only if intake exceeds the
capacity of mammals to detoxify ammonia. Unfortunately, there are
no data permitting the evaluation of this capacity in healthy human
beings or other terrestrial mammals. In addition, there is some
evidence that the mode of intake may be a factor in the capacity of
individuals to detoxify ammonia. Parenteral administration of
ammonia results in patterns of metabolism and elimination that are
markedly different from those seen in oral administration. Thus,
the considerable body of data on the parenteral toxicity of ammonia
is not of direct relevance to criteria for oral exposure.
Similarly, because of the different kinetic patterns between oral
and inhalation exposure to ammonia, as well as the highly irritant
effects of ammonia on the lung, the available inhalation data on
ammonia are not applicable to the estimation of an acceptable daily
intake (ADI) for ingestion. However, it would be possible to
define a clearly undesirable level of oral exposure to total
ammonia (NH3 and NH4+) as well as a level that is clearly
tolerable. The range between these 2 levels would exceed the range
within which an ADI could be established.
The amount of excess ammonia (i.e., over and above the amount
normally produced in the body) that can be safely ingested and
assimilated is difficult to define. In short-term (28 - 90 days)
studies carried out on rats and pigs, no adverse effects were
reported at higher levels of ammonia intake (75 - 545 mg NH3/kg
body weight per day) in the form of sulfamate, phosphate, citrate,
or chloride.
The effects attributed directly to elevated ammonium ion levels
are acute pulmonary oedema and central nervous system (CNS)
toxicity, depression of appetite due to a direct effect of the
ammonium ion on the brain, and promotion of growth via the use of
ammonium salts as a source of non-essential nitrogen under certain
circumstances.
Some effects (such as renal growth and demineralization of
bone) arising from the administration of ammonium chloride seem to
be secondary effects of acidosis.
Surveys of total ammonia (NH3 + NH4+) concentrations in surface
waters indicate an average of < 0.18 mg/litre in most areas, and
0.5 mg/litre in waters near large metropolitan areas (Wolaver,
1972; US EPA, 1979a). Levels in ground water are usually low,
since ammonia is generally immobile in soil. Ammonia is practically
absent when drinking-water is chlorinated.
Ammonia is a negligible natural constituent of food, but
ammonium compounds are added in small amounts (< 0.001 - 3.2%) to
various foods as stabilizers, leavening agents, flavourings, or for
other purposes. The daily human intake from these sources is
estimated to be 18 mg as NH3.
10.3. Ocular and Dermal Exposure
Ammonia in aqueous solution or in contact with body fluids is
alkaline and causes burns or inflammation of eyes or skin. The
ocular irritation commonly experienced at atmospheric ammonia
concentrations of > 20 mg/m3 (MacEwen et al., 1970; Keplinger et
al., 1973; Verberk, 1977) is readily reversible when exposure
ceases, and may also be reduced by acclimatization (Ferguson et
al., 1977).
Serious ocular damage normally occurs, only with a direct blast
or splash contact with anhydrous or aqueous ammonia (Grant, 1974).
Skin damage is reported to occur at concentrations of ~7000 mg/m3
(NRC, 1979).
10.4. Accidental Exposure
High gaseous ammonia concentrations may be encountered locally,
both in domestic and work-place environments, as a result of gaseous
emissions and/or spillages of concentrated solutions, and
respiratory (and skin and eye) injury may result. On a larger
scale, spillage from stock or transport tanks or refrigeration
plant of concentrated ammonia liquor or anhydrous ammonia would
constitute a severe environmental insult and would cause serious
injury to the people, animals, and plants in the vicinity. Because
of its low density and short biopersistence, major spillages would
be expected to disperse rapidly and not to persist in the
environment.
10.5. Evaluation of Risks for the Environment
Environments that receive more ammonia than can be used may be
acidified and nitrogen-enriched. As a consequence of these
physical-chemical changes, the structure and functioning of the
ecosystem will be disturbed.
Ammonia plays an important role in the metabolism of all
organisms as a nutrient at low concentrations, but becomes toxic at
higher concentrations. For example, microorganisms both assimilate
and generate ammonia as a part of natural nutrient cycling
processes. High levels of ammonia may inhibit growth or survival
of microbial organisms, including, at higher levels, nitrifying
organisms.
10.5.1. The aquatic environment
Ammonia concentrations in the aquatic environment are variable,
reflecting the proximity and nature of sources (section 4).
Where there are large numbers of people and animals in relation
to the volume of surface waters and drainage flow, the load of
nitrogen added to surface waters from sewage and industrial
effluent is the predominant source and may lead to ammonia
concentrations that constitute a significant local and/or regional
environmental problem.
Otherwise, ammonia deposition contributes a major input. In
surface waters that are poorly buffered, poor in nutrients, and
hydrologically dependent on rainfall and/or snow melt, this may
result in acidification and nitrogen enrichment resulting in marked
changes in plant community structure with concommitant changes in
the animal population structure.
The toxic effects on aquatic organisms are attributed to non-
ionized ammonia (NH3) rather than to ammonium ion levels. This is
because non-ionized ammonia easily penetrates biological membranes,
whereas ammonium ions require specialized transport processes.
There are similarities between the modes of the acute toxic
action of ammonia in mammals and in fish: in the latter, however,
environmental conditions (such as pH, ionic composition and
concentration, temperature, and oxygen availability), which are
more variable in water than in air, have a marked modulating
effect.
Aquatic animals have a limited ability to detoxify ammonia and,
therefore, the body load is dependent on ammonia concentrations in
the water. Except in open oceans, exposure to environmental levels
produces many chronic effects (including reduced growth, decreased
survival, impaired reproduction) and may increase susceptibility to
disease and also cause histopathological changes.
High levels of ammonia in aquatic systems are also toxic for
plants. The detoxification of excessive ammonia places a severe
strain on the carbohydrate metabolism of the plant which
subsequently results in foliar injury, and growth effects, and thus
may modify plant community composition.
Where the dominant species, be it fish or plant, is also
sensitive to ammonia the effects on the whole ecosystem will be
marked.
10.5.2. The terrestrial environment
The most common effect of exposure of plants to atmospheric
ammonia is foliar injury. Prolonged exposure to high ambient
concentrations of about 75 µg/mg, such as occur in the vicinity of
intensive livestock farms, adversely affects more susceptible
species such as pine trees. The observed damage is the result of
both direct and indirect effects due to, changes in soil, and
secondarily, to increased susceptibility to disease and
meteorological stress.
The data on ammonia toxicity for wildlife are very limited.
There is no evidence that wildlife populations, in general, are at
risk from ammonia, but, there may be secondary effects associated
with changes in plant communities. Certain species of bats are
able to withstand the very high ammonia levels found in caves where
they live.
10.6. Conclusions
The major groups of organisms at risk from elevated ammonia
levels are aquatic animals and terrestrial plants. There appears
to be little danger for terrestrial animals, including man, except
from acute accidental exposure.
10.6.1. General population
There are no data suggesting that present environmental levels
of ammonia are hazardous for the general population. Only high-
level accidental exposures from domestic sources and transportation
and storage accidents pose an occasional acute health hazard.
10.6.2. Sub-populations at special risk
Groups likely to exhibit ammonia toxicity include those with
hepatic or renal impairment, though, even in these cases, levels of
exogenous ammonia are insignificant in comparison with endogeneous
levels, so that, in the absence of any environmental exposure, such
persons would still be affected. The mechanism is different in the
two cases. Hepatic impairment limits the conversion of ammonia to
urea, and renal failure, by increasing urea concentrations and its
intestinal secretion, leads to increased endogeneous intestinal
ammonia production.
There have been few studies on the chronic effects of ammonia
inhalation. It can be speculated that subpopulations that have
been found to be hyperreactive to other respiratory irritants
(e.g., sulfur dioxide, particulates, ozone) may also be
hyperreactive to ammonia. These subpopulations may include
children, elderly persons with pre-existing cardiorespiratory
symptoms, individuals with asthma or bronchitis, and those engaged
in vigorous physical exercise (Calabrese, 1978). However, there is
also some indication that previous exposure to low levels of
ammonia may cause inurement to its effects (Ferguson et al., 1977).
10.6.3. Occupational exposure
Accidental exposures are the predominant problem (section
10.1.1.3). Otherwise, occupational exposure can be controlled by
the application of most current occupational exposure limits and
proper industrial hygiene.
10.6.4. Farm animals
Farm animals may be at risk, because of continous exposure
under confined housing conditions resulting in high atmospheric
levels of ammonia within the confinement areas; this applies
particularly to cattle, swine, and poultry. Available reported
data provide a range of measured exposure levels of from 2 to
1400 mg/m3. In winter months in colder climates, most of the
measured concentrations exceeded the admissible upper limit of
35 mg/m3 (50 ppm).
10.6.5. Environment
Environments with a low buffer capacity and poor in nutrients
are susceptible to acidification and nitrogen enrichment by
elevated ammonia loading; prolonged high ammonia-loading results in
changes in both the structure and function of plant and animal
communities. Levels of atmospheric ammonia necessary for the onset
of these changes have not been established; however, changes in the
structure of these communities have been observed where ammonia
levels in the atmosphere were possibly up to 100 µg/m3.
Plants use ammonia as a nutrient, but high levels can be toxic.
Terrestrial plants show a susceptibility to reduced growth and
reduced vitality, when exposed to levels as low as 75 µg NH3/m3 in
the atmosphere.
Aquatic animals have a low capacity to detoxify ammonia. Acute
effects on some fish have been demonstrated in laboratories at
concentrations as low as 0.1 mg NH3/litre and chronic effects at
concentrations as low as 0.02 mg NH3/litre. Thus, as ammonia
levels in some waters are often similar to those shown to cause
chronic effects in some fish, it would appear that these animals
are at risk. Aquatic invertebrates are, in general, less sensitive
to elevated ammonia levels in water.
Aquatic animals are at risk because of increases in ammonia
concentrations in water systems, whereas some plant communities
appear to be at risk from elevations in atmospheric ammonia
loading.
11. RECOMMENDATIONS
11.1. Research Needs
1. Long-term monitoring of ammonia and other pollutants in
water systems with different aquatic ecosystems.
2. Studies of the long-term effects of ammonia on terrestrial
vegetation.
3. Studies of the global nitrogen balance to identify long-
term trends.
4. Ecotoxicological studies to elucidate environmental
effects.
5. Epidemiological studies in relation to ammonia exposure in
order to make better hygiene recommendations.
6. Long-term experimental animal studies to establish a no-
observed-adverse-effect level of exposure.
7. Studies on the role of ammonia in modifying physical and
chemical conditions of soil and water systems.
8. More data are needed to assess accurately the relative
contributions of various point and non-point sources of
ammonia for surface waters.
9. Research into methods and their application directed
towards reducing emissions from point sources.
10. Additional acute toxicity tests with salt-water fish and
invertebrate species.
11. Life-cycle and early-life-stage tests with representative
fresh-water and salt-water organisms from different
families, with investigation of pH effects on chronic
toxicity.
12. Fluctuating or intermittent exposure tests under a variety
of exposure patterns on additional species.
13. Both acute and long-term tests at cold-water temperatures.
14. Studies on the effects of dissolved and suspended solids
on acute and chronic toxicity.
15. More histopathological and histochemical research with
fish, which would provide a rapid means of identifying and
quantifying sublethal ammonia effects.
16. In fish, the relative concentration limits for both
acclimatization and subsequent acute response need better
definition and a more complete explanation.
REFERENCES
ABBAS, R. & TANNER, R.L. (1981) Continuous determination of
gaseous ammonia in the ambient atmosphere using fluorescence
derivatization. Atmos. Environ., 15: 277-281.
ABELOVICH, A. & AZOV, Y. (1976) Toxicity of ammonia to algae
in sewage oxidation ponds. Appl. environ. Microbiol., 31(6):
801-806.
ADMIRAAL, W. (1977) Tolerance of estuarine benthic diatoms
to high concentrations of ammonia, nitrite ion, nitrate ion
and orthophosphate. Mar. Biol., 43(4): 307-315.
AGAMI, M., LITAV, M., & WAISEL, Y. (1976) The effects of
various components of water pollution on the behaviour of some
aquatic macrophytes of the coastal rivers of Israel. Aquat.
Bot., 2(3): 203-213.
ALABASTER, J.S. & HERBERT, D.W.M. (1954) Influence of carbon
dioxide on the toxicity of ammonia. Nature (Lond.), 174(4426):
404.
ALABASTER, J.S., SHURBEN, D.G., & KNOWLES, G. (1979) The
effect of dissolved oxygen and salinity on the toxicity of
ammonia to smolts of salmon, Salmo salar L. J. Fish Biol.,
15(6): 705-712.
ALLAN, I.R.H. (1955) Effects of pollution on fisheries.
Verh. Int. Ver. Theor. Agnew Limnol., 12: 804-810.
ALLAN, I.R.H., HERBERT, D.W.M., & ALABASTER, J.S. (1958) A
field and laboratory investigation of fish in a sewage
effluent. Fish. Invest., 6(2): 1-76.
ALPATOV, I.M. (1964) [A study of gazeous ammonia toxicity.]
Gig. Tr. prof. Zabol., 2: 14-18 (in Russian).
ALPATOV, I.M. & MIKHAILOV, V.I. (1963) [Inquires into the
gaseous ammonia toxicity. Communication 1.] Gig. Tr. prof.
Zabol., 12: 51-53 (in Russian)
ANALYTICAL QUALITY CONTROL (HARMONISED MONITORING) COMMITTTEE
(1982) Accuracy of determination of ammoniacal nitrogen in
river waters: analytical quality control in the harmonised
monitoring scheme. Analyst, 107(June): 680-688.
ANDERSON, B.G. (1948) The apparent thresholds of toxicity to
Daphnia magna for chlorides of various metals when added to
Lake Erie water. Trans. Am. Fish Soc., 78: 96-113.
ANDERSON, D.P., BEARD, C.W., & HANSON, R.P. (1964a) The
adverse effects of ammonia on chickens including resistance to
infections with Newcastle disease virus. Avian Dis., 8:
369-379.
ANDERSON, D.P., CHERMS, F.L., & HANSON, R.P. (1964b) Studies
on measuring the environment of turkeys raised in confinement.
Poult. Sci., 43: 305-318.
ANDERSON, K.B., SPARKS, R.E., & PAPARO, A.A. (1978) Rapid
assessment of water quality, using the fingernail clam,
Musculium transversum, Urbana, Illinois, Water Resources
Center, University of Illinois, 115 pp (WRC Research Report
No. 133).
ANGHILERI, L.G. (1975) Tumour growth inhibition by ammonium
chloride-induced acidosis. Int. J. clin. Pharmacol., 12: 320.
ANTHONISEN, A.C., LOEHR, R.C., PRAKASAM, T.B.S., & SRINATH,
E.G. (1976) Inhibition of nitrification by ammonia and
nitrous acid. J. Water Poll. Control Fed., 48: 835-852.
APHA (1976) Standard methods for the examination of water
and wastewater, 14th ed., Washington DC, American Public
Health Association, American Water Works Association, Water
Pollution Control Federation, 1193 pp.
API (1981) The sources, chemistry, fate, and effects of
ammonia in aquatic environments, Washington DC, American
Petroleum Institute, 145 pp.
APPELMAN, L.M., TEN BERGE, W.F., & REUZEL, P.G.J. (1982)
Acute inhalation toxicity study of ammonia in rats with
variable exposure periods. Am. Ind. Hyg. Assoc. J., 43(9):
662-665.
ARMSTRONG, D.A., CHIPPENDALE, D., KNIGHT, A.W., & COLT, J.E.
(1978) Interaction of ionized and un-ionized ammonia on
short-term survival and growth of prawn larvae, Macrobrachium
rosenbergii. Biol. Bull., 154(1): 15-31.
ARNDT, F.L., DAY, D.L., & HATFIELD, E. (1979) Processing and
Handling of animal excreta for refeeding. J. anim. Sci., 48:
157.
ASTM (1980) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials.
AUERBACH, C. & ROBSON, J.M. (1947) Tests of chemical
substances for mutagenic action. Proc. R. Soc. Edinburgh Sec.
Biol., 62B: 284-291.
AVOT, YU.K., KANEP, V.V., SLUTSKER, D.S., & SHAFRAN, L.M.
(1977) [Medical and sanitary aspects of sailors' work,] Riga,
Zvaigzne, 132 pp (in Russian).
AXELROD, H.D. & GREENBERG, J.P. (1976) Determination of
trace concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 10: 495-496.
BAIRD, R., BOTTOMLEY, J., & TAITZ, H. (1979) Ammonia
toxicity and pH control in fish toxicity bioassays of treated
wastewaters. Water Res., 13(2): 181-184.
BALL, I.R. (1967) The relative susceptibilities of some
species of fresh-water fish to poisons. I. Ammonia. Water
Res., 1(11/12): 767-775.
BARROW, C.S. & STEINHAGEN, W.H. (1980) NH3 concentrations in
the expired air of the rat: Importance to inhalation
toxicology. Toxicol. appl. Pharmacol., 53: 116-121.
BARROW, C.S., ALARIE, Y., & STOCK, M.F. (1978) Sensory
irritation and incapacitation evoked by thermal decomposition
products of polymers and comparisons with known sensory
irritants. Arch. environ. Health, 33(2): 79-88.
BARTON, L.V. (1940) Toxicity of ammonia, chlorine, hydrogen
cyanide, hydrogen sulphide and sulphur dioxide gases. IV.
Seeds. Contrib. Boyce Thompson Inst., 11: 357-363.
BARZEL, U.S. (1975) Effect of chronic ammonium chloride
ingestion on parathyroid-hormone function. Nephron, 14(15):
339-346.
BARZEL, U.S. & JOWSEY, J. (1969) Effects of chronic acid and
alkali administration on bone turnover in adult rats. Clin.
Sci., 36(3): 517-524.
BENEDICT, H.M. & BREEN, W.H. (1955) The use of weeds as a
means of evaluating vegetation damage caused by air pollution.
In: Proceedings of the Third National Air Pollution
Symposium, Pasadena, California, April 1955, pp. 177-190.
BENNETT, W.F., PESEK, J., & HANWAY, J.J. (1964) Effects of
nitrate and ammonium on growth of corn in nutrient solution
sand culture. Agric. J., 56: 342-345.
BENYAJATI, S. & GOLDSTEIN, L. (1975) Renal glutaminase
adaptation and ammonia excretion in infant rats.
Am. J. Physiol., 228(3): 693-698.
BERL, S., TAKAGAKI, G., SCHWERIN, P., & WAELSCH, H. (1962)
Metabolic compartments in vivo: Ammonia and glutamic acid
metabolism in brain and liver. J. biol. Chem., 237: 2562-2569.
BESSMAN, S.P. & BESSMAN, A.N. (1955) The cerebral and
peripheral uptake of ammonia in liver disease with an
hypothesis for the mechanism of hepatic coma. J. clin.
Invest., 34: 622-628.
BHATTACHARYA, A.N. & TAYLOR, J.C. (1975) Recycling animal
waste as a feedstuff: A review. J. anim. Sci., 44: 1438.
BIDWELL, R.G.B. (1974) Plant physiology, New York,
MacMillan, 197 pp.
BINSTOCK, L. & LECAR, H. (1969) Ammonium ion currents in the
squid giant axon. J. gen. Physiol., 53: 342-361.
BIRNBAUM, S.M., WINTZ, M., & GREENSTEIN, J.P. (1957)
Quantitative nutritional studies with water-soluble,
chemically defined diets. III. Individual amino acids as
sources of "non-essential" nitrogen. Arch. Biochem. Biophys.,
72(2): 428-436.
BISWAS, S.D. & KLEINBERG, I. (1971) Effect of urea
concentration on its utilisation, on the pH and the formation
of ammonia and carbon dioxide in a human salivary sediment
system. Arch. oral Biol., 16: 759-780.
BLOOMFIELD, R.A., GARNER, G.B., & MUHRER, M.E. (1960)
Kinetics of urea metabolism in sheep. J. anim. Sci., 19: 1248.
BLOOMFIELD, R.A., KEARLEY, E.O., CREACH, D.O., & MUHRER, M.E.
(1962) Ruminal pH and absorption of ammonia and VFA. J. anim.
Sci., 22: 833 (Abstract).
BOCK, F., MICHELSON, I., BROSS, I.D., & PRIORE, R.L. (1974)
Carcinogenic activity of smoke condensate from cigarettes with
ammonium sulfamate-treated paper. Cancer, 33: 1010.
BODANSKY, A., JAFFE, M.L., & CHANDLER, J.P. (1932) Serum
phosphatase changes in ammonium chloride osteoporosis. Proc.
Soc. Exp. Biol. Med., 29: 871-875.
BOER, W.M.J., DEN & BASTIAENS H. (1984) [Effects of
acidification on vegetation,] Ministry of Agriculture and
Fisheries, Ministry of Housing, Physical Planning and
Environment, 82 pp (Report No. 84(2)) (in Dutch).
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1973)
Ammonia kills spoilage moulds in corn. J. Dairy Sci., 56: 241.
BOTHAST, R.J., LANCASTER, E.B., & HESSELTINE, C.W. (1975a)
Effect of pH and substrate on growth. Eur. J. appl. Microbiol,
1: 55-56.
BOTHAST, R.J., ADAMS, G.H., HATFIELD, E.E., & LANCASTER, E.B.
(1975b) Preservation of high-moisture corn: a microbiological
evaluation J. Dairy Sci., 58: 386-391.
BOYD, E.M. & SEYMOUR, K.G.W. (1946) Ethylenediamine
dihydrochloride or "chlorethamine". II. Untoward and toxic
reactions. Exp. Med. Surg., : 223-227.
BOYD, E.M., McLACHLAN, M.L., & PERRY, W.F. (1944)
Experimental ammonia gas poisoning in rabbits and cats.
J. ind. Hyg. Toxicol., 26: 29-34.
BRAGANGA, B.M., FAULKNER, P., & QUASTEL, J.M. (1953) Effects
of inhibitors of glutamine synthesis in brain slices by
ammonium ions. Biochim. Biophys. Acta, 10: 83-88.
BREEDING, R.J., LODGE, J.P., Jr, PATE, J.B., SHEESLEY, D.C.,
KLONIS, H.B., FOGLE, B., ANDERSON, J.A., ENGLERT, T.R.,
HAAGENSON, P.L., MCBETH, R.B., MORRIS, A.L., POGUE, R., &
WARTBURG, A.F. (1973) Background trace concentrations in the
central United States. J. geophys. Res., 78: 7057-7064.
BREEDING, R.J., KLONIS, H.B., LODGE, J.P., Jr, PATE, J.B.,
SHEESLEY, D.C., ENGLERT, T.R., & SEARS, D.R. (1976)
Measurements of atmospheric pollutants in the St. Louis area.
Atmos. Environ., 10: 181-184.
BRENNAN, E., LEONE, I.A., & DAINES, R.H. (1962) Ammonia
injury to applies and peaches in storage. Plant Dis. Rep., 46:
792-795.
BRESSANI, R. & BRAHAM, J.E. (1977) Effect of frequency of
feeding on blood serum urea changes in dogs. J. Nutr. Rep.
Int., 16(3): 305-316.
BRETT, J.R. & ZALA, C.A. (1975) Daily pattern of nitrogen
excretion and oxygen consumption of sockeye salmon
(Oncorhyncus nerka) under controlled conditions. J. Fish. Res.
Board Can., 32: 2479-2486.
BRETTHAUER, R. (1978) Some ecological limits of tolerance to
Ochromonas sociabilis. Verh. Internat. Verein. Limnol., 20(3):
1850-1854.
BROCKWAY, D.R. (1950) Metabolic products and their effects.
Prog. Fish-Cult., 12(3): 127-129.
BRODERIUS, S.J. & SMITH, L.L., Jr (1979) Lethal and
sublethal effects of binary mixtures of cyanide and hexavalent
chromium, zinc, or ammonia to the fathead minnow Pimephales
promelas and rainbow trout Salmo gairdneri. J. Fish. Res.
Board Can., 36(2): 164-172.
BRODERIUS, S.J., SMITH, L.L., Jr, & LIND, D.T. (1977)
Relative toxicity of free cyanide and dissolved sulfide forms
to the fathead minnow. Pimephales promelas. J. Fish. Res.
Board Can., 34(12): 2323-2332.
BRODERIUS, S.J., DRUMMOND, R.A., FIANDT, J.T., & RUSSOM, C.L.
(in press) Toxicity of ammonia to smallmouth bass,
Micropterus dolomieui, as related to pH. Environ. Toxicol.
Chem..
BRODERSON, J.R., LINDSEY, J.R., & CRAWFORD, J.E. (1976) The
role of environmental ammonia in respiratory mycoplasmosis of
rats. Am. J. Pathol., 85: 115-130.
BROSSET, C., ANDREASSON, K., & FERM, M. (1975) The nature
and possible origin of acid particles observed at the Swedish
west coast. Atmos. Environ., 9: 631-642.
BROWN, A.C. (1974) Observation on the effect of ammonium
nitrate solutions on some common marine animals from Table
Bay. Trans. R. Soc. S. Afr., 41(2): 217-223.
BROWN, V.M. (1968) The calculation of the acute toxicity of
mixtures of poisons to rainbow trout. Water Res., 2(10):
723-733.
BROWN, R.H., DUDA, G.D., KORKES, S., & HANDLER, P. (1957) A
colorimetric micromethod for determination of ammonia: the
ammonia content of rat tissues and human plasma. Arch.
Biochem. Biophys., 66: 301-309.
BROWN, V.M., JORDAN, D.H.M., & TILLER, B.A. (1969) The acute
toxicity to rainbow trout of fluctuating concentrations and
mixtures of ammonia, phenol and zinc. J. Fish Biol., 1(1): 1-9.
BUCKLEY, J.A. (1978) Acute toxicity of unionized ammonia to
fingerling coho salmon. Prog. Fish-Cult., 40(1): 30-32.
BUCKLEY, J.A., WHITMORE, C.M., & LIMING, B.D. (1979) Effects
of prolonged exposure to ammonia on the blood and liver
glycogen of coho salmon (Oncorhynchus kisutch). Comp. Biochem.
Physiol., 63C(2): 297-303.
BUIKEMA, A.I., Jr, CAIRNS, J., Jr, & SULLIVAN, G.W. (1974)
Evaluation of Philodina acuticornis (Rotifera) as a bioassay
organism for heavy metals. Water Res. Bull., 10(4): 648-661.
BUKHOVSKAYA, M.S., GINZBURG, S.L., & KHALIZOVA, O. (1966)
[Methods for determination of harmful substances in air. A
practical guide,] 2nd ed., Moscow, Meditaina (in Russian).
BULLIS, K.L., SNOEYENBOS, G.H., & VAN ROEKEL, H. (1950) A
keratoconjunctivitis in chickens. Poult. Sci., 29: 386-389.
BURKHALTER, D.E. & KAYA, C.M. (1977) Effects of prolonged
exposure to ammonia on fertilized eggs and sac fry of rainbow
trout (Salmo gairdneri). Trans. Am. Fish Soc., 106(5): 470-475.
BURROWS, R.E. (1964) Effects of acclimated excretory
products on hatchery-reared salmonids, Washington DC, Fish and
Wildlife Service, US Department of the Interior, 12 pp
(Research Report 66).
BUTTERWORTH, B. (1979) Nutritionally improved straw: the
machines do the job. Agric. Eng., 60(7): 41-42.
BUYSMAN, E. (1984) [Ammonia emissions in the Netherlands,]
Ministry of Housing, Physical Planning, and Environment,
Directorate Air (Report No. 22) (in Dutch).
BUYSMAN, E., MAAS, J.F.M., & ASMAN, W.A.M. (1985) Ammonia
emission in Europe, Utrecht, the Netherlands, Institute for
Meteorology and Oceanography, State University Utrecht.
BYERRUM, R.U. & BENSON, A.A. (1975) Effect of ammonia on
photosynthetic rate and photosynthate release by Amphidinium
carterae (Dinophyceae). J. Phycol., 11: 449-452.
CALABRESE, E.J. (1978) Pollutants and high risk groups,
New York, Wiley-Interscience Publications.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1977) [Effect of
prolonged treatments with ammonia on stages of development of
Salmo gairdneri.] Nuovi Ann. Ig. Microbiol., 28(5): 333-345.
(in Italian).
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1981) Effects of
long-term exposure to ammonia on the developmental stages of
rainbow trout ( Salmo gairdnerii Richardson). Rapp. P.-V. Réun.
Cons. Int. Explor. Mer, 178: 81-86.
CAMERON, J.N. (1979) Excretion of CO2 in water-breathing
animals. Marine Biol. Lett., 1: 3-13.
CAMERON, J.N. & HEISLER, N. (1983) Studies of ammonia in the
rainbow trout physio-chemical parameter, acid-base behavior,
and respiratory clearance. J. exp. Biol., 105:: 107-125.
CAMERON, G.R. & SHEIKH, A.H. (1951) Experimental production
of pulmonary edema with ammonium salts, together with
classification of lung oedemas. J. Pathol. Bacteriol., 63:
609-617.
CAMPBELL, C.L., DAWES, R.K., DEOLALKAR, S., & MERRITT, M.C.
(1958) Effect of certain chemicals in water on the flavor of
brewed coffee. Food Res., 23: 575-579.
CAPLIN, M. (1941) Ammonia gas poisoning. Forty-seven cases
in a London shelter. Lancet, 2: 95-96.
CAPUCO, A.V. (1977) Ammonia, a modulator of 3T3 cell growth,
Ithaca, New York, Cornell University (Ph.D Thesis).
CARPENTER, C.P., SMYTH, H.S., Jr, & SHAFFER, C.B. (1948) The
acute toxicity of ethylene imine to small animals. J. ind.
Hyg. Toxicol., 30: 2-6.
CARY, G.A. (1976) A report on the assessment of aquatic
environmental impact of Union Carbide's Uravan operations:
on-site toxicity bioassays, Tarrytown, New York, Aquatic
Environmental Sciences, Union Carbide Corporation, 67 pp
(Report prepared for Metals Division, Union Carbide
Corporation, Uravan, Colorado).
CASTELL, D.O. & MOORE, E.W. (1971) Ammonia absorption from
the human colon. Gastroenterology, 60(1): 33-42.
CATEDRAL, F.F., COLOSO, R., VALERA, N., CASALMIR, C.M., &
QUIBUYEN, A.T. (1977a) Effect of some physico-chemical
factors on the survival and growth of Penaeus monodon
postlarvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian Fish.
Dev. Cent., 1(3): 13-16.
CATEDRAL, F.F., GEROCHI, D.D., QUIBUYEN, A.T., & CASALMIR,
C.M. (1977b) Effect of nitrite, ammonia, and temperature on
P. monodon larvae. Q. Res. Rep. Aquacult. Dep. Southeast Asian
Fish. Dev. Cent., 1(3): 9-12.
CHAMP, M.A., LOCK, J.T., BJORK, C.D., KLUSSMANN, W.G., &
MCCULLOUGH, J.D., Jr (1973) Effects of anhydrous ammonia on
a central Texas pond and a review of previous research with
ammonia in fisheries management. Trans. Am. Fish. Soc.,
102(1): 73-82.
CHARLES, D.R. & PAYNE, C.G. (1966a) The influence of graded
levels of atmospheric ammonia on chickens. I. Effects on
respiration and on the performance of broilers and replacement
growing stock. Br. Poult. Sci., 7: 177-187.
CHARLES, D.R. & PAYNE, C.G. (1966b) The influence of graded
levels of atmospheric ammonia on chickens. II. Effects on the
performance of laying hens. Br. Poult. Sci., 7: 189-198.
CHEMICAL AND ENGINEERING NEWS (1980) C and EN's top fifty
chemical products. Am. Chem. Soc., : 16.
CHIPMAN, W.A., Jr (1934) The role of pH in determining the
toxicity of ammonium compounds, Columbia, Missouri, University
of Missouri, 153 pp (Ph.D. Thesis).
CLAIBORNE, J.B., EVANS, D.H., & GOLDSTEIN, L. (1982) Fish
branchial Na+-K+-activated ATPase. J. exp. Biol., 96: 431-434.
CLAIBORNE, J.B. & HEISLER, N. (1984) Acid-base regulation in
the carp (Cyprinus carpio) during and after exposure to
environmental hypercapnia. J. exp. Biol., 108: 25-43.
CLAWSON, A.J. & ARMSTRONG, W.D. (1981) Ammonium
polyphosphate as a source of phosphorous and nonprotein
nitrogen for monogastrics (in corn-soybean meal diets for
growing rats and growing finishing [G-F] pigs). J. anim. Sci.,
52(1): 1-7.
CLOSE, L.G., CATLIN, F.I., & COHN, A.M. (1980) Acute and
chronic effects of ammonia burns of the respiratory tract.
Arch. Otolaryngol., 106: 151-158.
COLE, T.J., COTES, J.E., JOHNSON, G.R., & MARTIN, H. (1977)
Ventilation cardiac frequency and pattern of breathing during
exercise in men exposed to o-chlorobenzylidene malonitril and
ammonia gas in low concentrations. Q. J. exp. Physiol., 62:
341-351.
COLT, J.E. (1978) The effects of ammonia on the growth of
channel catfish, Ictalurus punctatus, Davis, California,
University of California, 185 pp (Ph.D. Thesis).
COLT, J.E. & TCHOBANOGLOUS, G. (1976) Evaluation of the
short-term toxicity of nitrogenous compounds to channel
catfish, Ictalurus punctatus. Aquaculture, 8(3): 209-224.
COLT, J.E. & TCHOBANOGLOUS, G. (1978) Chronic exposure of
channel catfish, Ictalurus punctatus to ammonia: effects on
growth and survival. Aquaculture, 15(4): 353-372.
CONN, H.O. (1972) Studies of the source and significance of
blood ammonia. IV. Early ammonia peaks after ingestion of
ammonium salts. Yale J. Biol. Med., 45: 543-549.
CONSTANTINE, D.G. (1958) Bleaching of hair pigment in bats
by the atmosphere in caves. J. Mammal, 39: 513-520.
COOMBE, J.B., TRIBE, D.E., & MORRISON, J.W. (1960) Some
experimental observations on the toxicity of urea to sheep.
Aust. J. agric. Res., 11: 247-256.
COON, R.A., JONES, R.A., JENKINS, L.J., Jr, & SIEGEL, J.
(1970) Animal inhalation studies on ammonia, ethylene glycol,
formaldehyde, dimethylamine, and ethanol. Toxicol. appl.
Pharmacol., 16: 646-655.
COTTERILL, O.J. & NORDSKOG, A.W. (1954) Influence of ammonia
on egg white quality. Poult. Sci., 33: 432-434.
CRIBB, G.S. (1964) Nitrogen. In: Mellor's comprehensive
treatise on inorganic and theoretical chemistry, London,
Longmans, Vol. 8, Suppl. I, pp. 276-327.
CURTIS, S.E. (1972) Air environment and animal performance.
J. anim. Sci., 35: 628-634.
DALHAMN, T. (1963) Effect of ammonia alone and combined with
carbon particles on ciliary activity in the rabbit trachea in
vivo, with studies of the absorption capacity of the nasal
cavity. Int. J. Air Water Pollut., 7: 531-539.
DALHAMN, T. & SJOHOLM, J. (1961) Studies of SO2, NO2, and
NH3: effect on ciliary activity in the rabbit trachea of
single in vitro exposure and resorption in rabbit nasal
cavity. Acta physiol. Scand., 58: 287-291.
DALLAVALLE, J.M. & DUDLEY, H.C. (1939) Evaluation of odor
nuisance in the manufacture of kraft paper. Public Health
Rep., 54(1): 35-43.
DANECKER, E. (1964) [The jauche poisoning of fish: an
ammonia poisoning.] Osterr. Fischerei., 3/4: 55-68. (in
German).
DAVIES, B.M.A. & YUDKIN, J. (1952) Studies in biochemical
adaptation. The origin of urinary ammonia as indicated by the
effect of chronic acidosis and alkalosis on some renal enzymes
in the rat. Biochem. J., 52: 407-412.
DAY, D.L., HANSEN, E.L., & ANDERSON, S. (1965) Gases and
odors in confinement swine buildings. Trans. Am. Soc. Agric.
Eng., 8: 118-121.
DEAL, P.H., SOUZA, K.A., & MACK, H.M. (1975) High pH,
ammonia toxicity, and the search for life on the Jovian
planets. Origins Life, 6(4): 561-573.
DEAN, J.A., ed. (1979) Lange's handbook of chemistry, 12th
ed., New York, McGraw-Hill Book Company.
DEGRAEVE, G.M., OVERCAST, R.L., & BERGMAN, H.L. (1980)
Toxicity of underground coal gasification condenser water and
selected constitutents to aquatic biota. Arch. environ.
Contam. Toxicol., 9(5): 543-555.
DELISTRATY, D.A., CARLBERG, J.M., VAN OLST, J.C., & FORD,
R.F. (1977) Ammonia toxicity in cultured larvae of the
American lobster (Homarus americanus). In: Proceedings of the
8th Annual Meeting of the World Mariculture Society, San Jose,
Costa Rica, pp. 647-673.
DEMEREC, M., BERTANI, G., & FLINT, J. (1951) A survey of
chemicals for mutagenic action on E. coli. Am. Nat., 85: 119.
DEMUYNCK, M., RAHN, K.A., JANSSENS, M., & DAMS, R. (1976)
Chemical analysis of airborne particulate matter during a
period of unusually high pollution. Atmos. Environ., 10: 21-26.
DE VOOYS, G.G.N. (1968) Formation and excretion of ammonia
in Teleostei. I. Excretion of ammonia through the gills. Arch.
int. Physiol. Biochimie., 76: 268-272.
DODD, K.T. & GROSS, D.R. (1980) Ammonia inhalation toxicity
in cats. Arch. environ. Health, 35: 6-14.
DOIG, P.A. & WILLOUGHBY, R.A. (1971) Response of swine to
atmospheric ammonia and organic dust. J. Am. Vet. Med. Assoc.,
159: 1353-1361.
DOWDEN, B.F. & BENNETT, H.J. (1965) Toxicity of selected
chemicals to certain animals. J. Water Pollut. Control. Fed.,
37(9): 1308-1316.
DOWNING, K.M. & MERKENS, J.C. (1955) The influence of
dissolved-oxygen concentration on the toxicity of un-ionized
ammonia to rainbow trout ( Salmo gairdneri Richardson).
Ann. appl. Biol., 43(2): 243-246.
DREWES, D.R. & HALES, J.M. (1980) Removal of pollutants from
power plant plumes by precipitation, Richland, Washington,
Battelle-Northwest, US Electric Power Research Institute
(Report to the EPRI).
DRIEDZIC, W.R. & HOCHACHKA, P.W. (1976) Control of energy
metabolism in fish white muscle. Am. J. Physiol., 230: 579-582.
DRUMMOND, J.G., CURTIS, S.E., SIMON, J., & NORTON, H.W.
(1980) Effects of aerial ammonia on growth and health of
young pigs. J. anim. Sci., 50: 1085-1091.
DUDA, G.D. & HANDLER, P. (1958) Kinetics of ammonia
metabolism in vivo. J. biol. Chem., 232: 303-314.
DU RUISSEAU, J.P., GREENSTEIN, J.P., WINITZ, M., & BIRNBAUM,
S.M. (1957) Studies on the metabolism of amino acids and
related compounds in vivo. VI. Free amino acid levels in the
tissues of rats protected against ammonia toxicity. Arch.
Biochem. Biophys., 68: 161-171.
EADS, R.B., WISEMAN, J.S., GRIMES, J.E., & MENZIES, G.C.
(1955) Wildlife rabies in Texas. Public Health Rep., 70:
995-1000.
ECKERT, J.W. (1967) Application and use of postharvest
fungicides. In: Torgeson, D.C., ed. Fungicides, New York,
Academic Press, pp. 341.
ECKERT, J.W., KOLBEZEN, M.J., & GARBER, M.J. (1963)
Evaluation of ammonia-generating formations for control of
citrus fruit decay. Phytopathology, 53: 140-148.
ECOLOGICAL ANALYSTS, INC. (1981) The sources, chemistry,
fate, and effects of ammonia in aquatic environments,
Washington DC, American Petroleum Institute.
EDELMANN, C.M., Jr, BIOCHIS, H., SORIANO, J.R., & STARK, H.
(1967) The renal response of children to acute ammonium
chloride acidosis. Pediat. Res., 1: 452-460.
EDJTEHADI, M., SZABUNIEWICZ, M., & EMMANUEL, B. (1978) Acute
urea toxicity in sheep. Can. J. Comp. Med., 42: 63-68.
EDWARDS, J.G. & CONDORELLI, L. (1928) Studies on aglomerular
and glomerular kidneys. II. Physiological. Am.J. Physiol., 86:
383-398.
EGGELTON, A.E.J. (1969) The chemical composition of
atmospheric aerosols on Tees-side and its relation to
visibility. Atmos. Environ., 3: 355-372.
EGLE, J.L., Jr (1973) Retention of inhaled acetone and
ammonia in the dog. Am. Ind. Hyg. Assoc. J., 34(12): 533-539.
ELKINS, H.B. (1950) The chemistry of industrial toxicology,
New York, John Wiley and Sons, 84 pp.
ELKINS, H.B. (1959) Ammonia, NH3. In: The chemistry of
industrial toxicology, 2nd ed., New York, John Wiley and Sons,
86 pp.
EMERSON, K., RUSSO, R.C., LUND, R.E., & THURSTON, R.V.
(1975) Aqueous ammonia equilibrium calculations: effect of pH
and temperature. J. Fish. Res. Board Can., 32: 2379-2383.
EMERY, R.M. & WELCH, E.B. (1969) The toxicity of alkaline
solutions of ammonia to juvenile bluegill sunfish (Lepomis
macrochirus Raf.), Chattanooga, Tennessee, Water Quality
Branch, Division of Health and Safety, Tennessee Valley
Authority, 31 pp (Mimeo).
EPEL, D., STEINHARDT, R., HUMPHREYS, T., & MAZIA, D. (1974)
An analysis of the partial metabolic derepression of sea
urchin eggs by ammonia; the existence of independent pathways.
Dev. Biol., 40: 245-255.
EPIFANO, C.E. & SRNA, R.F. (1975) Toxicity of ammonia,
nitrite ion, nitrate ion, and orthophosphate to Mercenaria
mercenaria and Crassostrea virginica. Mar. Biol., 33(3):
241-246.
ERNST, R.A. (1968) The effect of ammonia on poultry.
Feedstuffs, 40(32): 40.
EUROPEAN INLAND FISHERIES ADVISORY COMMISSION (1970) Water
quality criteria for European freshwater fish. Report on
ammonia and inland fisheries, pp. 12 (EIFAC Technical Paper
No. 11) (Also in: Water Res., 7(7): 1011-1022 (1973)).
EVANS, D.H. (1977) Further evidence for Na/NH4 exchange in
marine teleost fish. J. exp. Biol., 70: 213-220.
EVANS, D.H. (1980) Osmotic and ionic regulation by
freshwater and marine fishes. In: Ali, M.A. ed. Environmental
Physiology of Fishes, New York, Plenum Publishing Co.,
pp. 93-122.
EVANS, D.H., KORMANIK, G.A., & KRASNY, E.J. (1979)
Mechanisms of ammonia and acid extrusion by the little skate,
Raja erinacea. J. exp. Zool., 208: 431-437.
EVANS, J.W. (1979) The construction and use of a
continuous-flow bioassay apparatus to determine a preliminary
un-ionized ammonia 96-h LC50 for the crayfish, Orconectes
nais, Lawrence, Kansas, University of Kansas, 76 pp (M.S.
Thesis).
FARBER, R.J. & ROSSANO, A.T. (1975) A new approach for
measuring atmospheric concentrations of ammonia. Paper
presented at Annual Meeting of the Pacific Northwest
International Section of the Air Pollution Control
Association, Vancouver, British Columbia, November 19-21,
36 pp.
FASEB (1974) Evaluation of the health aspects of certain
ammonium salts as food ingredients, Springfield, Virginia,
Federation of American Societies for Experimental Biology,
21 pp (Prepared for the US Food and Drug Administration under
Contract No. FDA 72-85) (US NTIS PPB-254-532).
FAUCONNEAU, B. & LUQUET, P. (1979) Influence d'une élévation
de température sur l'évolution de l'aminoacidémie et de
l'ammoniémie après le repas chez la truite arc en ciel (Salmo
gairdneri R.). Ann. Biol. anim. Biochim. Biophys., 19(4A):
1063-1079.
FAZEKAS, I.G. (1939) Experimental suprarenal hypertrophy
induced by ammonium hydroxide. Endokrinologie, 21: 315-337.
FAZEKAS, I.G. (1949) [Experimental data on influencing
ovarian functions by simple compounds.] Orv. Hetil., 90:
777-781 (in German).
FAZEKAS, I.G. (1954a) [Enlargement of the parathyroids by
treatment with simple acidotic compounds.] Kirchow's Arch.
Pathol. Anat. Physiol., 324: 531-542 (in German).
FAZEKAS, J.F., TICKTIN, H.E., EHRMENTRAUT, W.R., & ALMAN,
R.W. (1956) Cerebral metabolism in hepatic insufficiency.
Am. J. Med., 21: 843-849.
FEPCC (1972) [Investigation of air pollution in the city of
Tsuruga.] Annu. Rep. Fukui Environ. Pollut. Control Centre, 1:
88-116 (in Japanese).
FERGUSON, W.S., KOCH, W.C., WEBSTER, L.B., & GOULD, J.R.
(1977) Human physiological response and adaptation to
ammonia. J. occup. Med., 19: 319-326.
FETH, J.H. (1966) Nitrogen compounds in natural water. A
review. Water Resour. Res., 2: 41-58.
FISHER, D.W., GAMBELL, A.W., LIKENS, G.E., & BURMANN, F.H.
(1968) Atmospheric contributions to water quality of streams
in the Hubbard Brook Experimental Forest, New Hampshire. Water
Resour. Res., 4: 1115-1126.
FLAKS, A. & CLAYSON, D.B. (1975) The influence of ammonium
chloride on the induction of bladder tumors by 4-ethyl-
sulfonylnaphthalene-1-sulfonamide. Br. J. Cancer, 31: 585.
FLAKS, A., HAMILTON, J.M., & CLAYSON, D.B. (1973) Effect of
ammonium chloride on incidence of bladder tumours induced by
4-ethylsulfonylnapthalene-1-sulfonamide. J. Natl Cancer Inst.,
51: 2007.
FLORKIN, M. & DUCHATEAU, G. (1943) Les formes du système
enzymatique de l'uricalyse et l'évolution du catabolisme
purique chez les animaux. Arch. int. Physiol., 53: 267-307.
FLURY, K.E., DINES, D.E., RODARTE, J.R., & RODGERS, R.
(1983) Airway obstruction due to inhalation of ammonia. Mayo
Clin. Proc., 58: 389-393.
FLYNN, R.J. (1968) A new cage cover as an aid to laboratory
rodent disease control. Proc. Soc. Exp. Biol. Med., 129:
714-717.
FORSTER, R.P. & GOLDSTEIN, L. (1969) Formation of excretory
products. In: Hoar, W.S. & Randall, D.J. ed. Fish Physiology,
New York, Academic Press, Vol. 1, pp. 313-350.
FOSTER, G.L., SCHOENHEIMER, R., & RITTENBERG, D. (1939)
Studies in protein metabolism. V. The utilization of ammonia
for amino acid and creatine formation in animals. J. biol.
Chem., 127: 319-327.
FRANK, J.F. (1948) The toxicity of sodium chlorate
herbicides. Can. J. comp. Med., 12: 216-218.
FRASER, D.I., DYER, W.J., WEINSTEIN, H.M., DINGLE, J.R., &
HINES, J.A. (1966) Glycolytic metabolites and their
distribution at death in the white and red muscle of cod
following various degrees of antemortem muscular activity.
Can. J. Biochem., 44: 1015-1033.
FREEDMAN, L.R. & BEESON, P.B. (1961) Experimental
pyelonephritis. VIII. The effect of acidifying agents on
susceptibility to infection. Yale J. Biol. Med., 33: 318.
FRIZZOLA, J.A. & BAIER, J.H. (1975) Contaminants in
rainwater and their relation to water quality. Water Sewage
Works, 122: 94-95.
FROMM, P.O. (1963) Studies on renal and extra-renal
excretion in a freshwater teleost, Salmo gairdneri. Comp.
Biochem. Physiol., 10: 121-128.
FROMM, P.O. (1970) Effect of ammonia on trout and goldfish.
In: Toxic action of water soluble pollutants on freshwater
fish, Washington DC, US Environmental Protection Agency,
pp. 9-22 (EPA Water Pollution Control Research Series No. 18050
DST 12/70).
FROMM, P.O. & GILLETTE, J.R. (1968) Effect of ambient
ammonia on blood ammonia and nitrogen excretion of rainbow
trout (Salmo gairdneri). Comp. Biochem. Physiol., 26: 887-896.
FUHRER, J. (1985) Formation of secondary airpollutants and
their occurrence in Europe. Experientia (Basel), 41: 286-301.
FURST, P., JOSEPHSON, B., MASCHIO, G., & VINNARS, E. (1969)
Nitrogen balance after intravenous and oral administration of
ammonium salts to man. J. appl. Physiol., 26(1): 13-22.
FURST, P., JONSSON, A., JOSEPHSON, B., & VINNARS, E. (1970)
Distribution in muscle and liver vein protein of 15N
administered as ammonium acidosis to man. J. appl. Physiol.,
29(3): 307-312.
GALL, W.K. (1980) Effect of cow faeces on the ecology of
laboratory streams, Madison, Wisconsin, University of
Wisconsin, pp. 73-83 (M.S. Thesis).
GAY, W.M.B., CRANE, C.W., & STONE, W.D. (1969) The
metabolism of ammonia in liver disease: a comparison of
urinary data following oral and intravenous loading of [15N]
ammonium lactate. Clin. Sci., 37: 815-823.
GEORGII, H.W. (1963) Oxides of nitrogen and ammonia in the
atmosphere. J. geophys. Res., 68: 963-970.
GEORGII, H.W. & MULLER, W.J. (1974) On the distribution of
ammonia in the middle and lower trophosphere. Tellus, 26:
180-184.
GIBBS, M. & CALO, N. (1959) Factors affecting light induced
fixations of carbon dioxide by isolated spinach chloroplasts.
Plant Physiol., 34: 318-323.
GIBSON, G.E., ZIMBER, A., KROOK, L., & VISEK, W.J. (1971)
Nucleic acids and brain and intestinal lesions in ammonia
intoxicated mice. Fed. Proc., 30: 578.
GIGUZ, T.L. (1968) Effect of low concentrations of ammonia
and nitrogen oxides on adolescents undergoing vocational
training in the chemical industry. Hyg. Sanit., 33(7-9):
431-434.
GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity tests.
Sewage ind. Wastes, 24(11): 1397-1401.
GILLIAM, J.W., DANIELS, R.B., & LUTZ, J.F. (1974) Nitrogen
content of shallow ground water in North Carolina Coastal
Plain. J. environ. Qual., 3: 147-151.
GIRARD, J.O. & PAYAN, P. (1980) Ion exchanges through
respiratory and chloride cells in freshwater and seawater
adapted teleosteans. Am. J. Physiol., 238: R260-R268
(Regulatory Integrative Comp. Physiol. 7).
GOLDHABER, M.B. & KAPLAN, I.R. (1974) The sulfur cycle. In:
Goldberg, D., ed. The sea: ideas and observations on progress
in the study of the seas, New York, John Wiley and Sons,
Vol. 5, pp. 569-655.
GOLDMAN, A.S. & YAKOVAC, W.C. (1964) Salicylate
intoxication and congenital anomalies. Arch. environ.
Health, 8: 648-656.
GOLDSTEIN, L., CLAIRBORNE, J.B., & EVANS, D.E. (1982)
Ammonia excretion by the gills of two marine teleost fish: the
importance of NH4+ permeance. J. exp. Zool., 219: 395-397.
GOLDSTEIN, L. & FORSTER, R.P. (1961) Source of ammonia
excreted by the gills of the marine teleost, Myoxocephalus
scropius. Am. J. Physiol., 200(5): 1116-1118.
GOLYAKOVA, L.P. (1971) [Present-day state of industrial
hygiene problems in hydrometallurgical production of tungsten
and molybdenum salts and oxides.] Gig. Tr. Prof. Zabol.,
15(2): 4-8 (in Russian with English summary).
GOODMAN, L.S. & GILMAN, A. (1970) The pharmacological basis
of therapeutics, 4th ed., New York, The Macmillan Company,
1794 pp.
GORDON, R.J. & BRYAN, R. (1973) Ammonium nitrate in airborne
particles in Los Angeles. Environ. Sci. Technol., 7: 645-647.
GORDON, H.H., MCNAMARA, H., & BENJAMIN, H.R. (1948) Response
of young infants to ingestion. Pediatrics, 2: 290-302.
GRANT, W.M. (1974) Toxicology of the eye: drugs, chemicals,
plant venoms, 2nd ed., Springfield, Illinois, C.C. Thomas,
pp. 96-101, 121-128.
GREENSTEIN, J.P., WINITZ, M., GULLINO, P., BIRNBAUM, S.M., &
OTEY, M.C. (1956) Studies on the metabolism of amino acids
and related compounds in vivo. III. Prevention of ammonia
toxicity by arginine and related compounds. Arch. Biochem.
Biophys., 64: 342-354.
GREGORY, R.B. (1977) Synthesis and total excretion of waste
nitrogen by fish of the Periopthalmus (mudskipper) and
Scartelass families. Comp. Biochem. Physiol., 51A: 33-36.
GRINDLEY, J. (1946) Toxicity to rainbow trout and minnows of
some substances known to be present in waste water discharged
to rivers. Ann. appl. Biol., 33(1): 103-112.
GROLLMAN, A. (1929) The urine of the goosefish (Lophius
piscatorius): its nitrogenous constituents with special
reference to the presence in it of trimethylamine oxide.
J. Biol. Chem., 81: 267-278.
GROSJEAN, D., DOYLE, G.J., MISCHKE, T.M., POE, M.P., FITZ,
D.R., SMITH, J.P., & PITTS, J.N., Jr (1976) The
concentration, size distribution, and modes of formation of
particulate nitrate, sulfate, and ammonium compounds in the
eastern part of the Los Angeles Basin. Presented at: The 69th
Annual Meeting of the Air Pollution Control Association,
Portland, Oregon, June 27 - July 1 1976, 14 pp (Paper 76-20.3).
GROSSI, C.E., PRYTZ, B., & ROUSSELOT, L.M. (1968) Adenosine
triphosphate in prevention of ammonia intoxication in dogs.
Surg. Forum, 19: 363-369.
GRUBE, H.J. (1973) [Load and loading capacity of submerged
macrophytes in running water in southern Lower Saxony.]
Verh. Ges. Okol., Saarbrücken: 103-109 (in German with English
summary).
GUERIN-ANCEY, O. (1976a) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
II. Effets du jeune sur l'excretion d'ammoniac et d'uree.
Aquaculture, 9(2): 187-294.
GUERIN-ANCEY, O. (1976b) Etude experimentale de l'excretion
azotee du bar (Dicentrarchus labrax) en cours de croissance.
III. Effets du volume et de la concentration initiale en
ammoniac sur l'excretion d'ammoniac et d'uree. Aquaculture,
9(2): 253-258.
GUPTA, B.N., KHANNA, R.N., & DATTA, K.K. (1979) Toxicological
studies of ammonium sulfamate in rat after repeated oral
administration. Toxicology, 13(1): 45-49.
GYORE, K. & OLAH, J. (1980) Ammonia tolerance of Moina
rectirostris Leydig (Cladocera). Aquaculture Hungarica
(Szarvas), 2: 50-54.
HAGGARD, H.W. (1924) Action of irritant gases upon the
respiratory tract. J. ind. Hyg., 5: 390-398.
HAHN, M., MASSEN, O., NENCKI, N., & PAWLOW, J. (1983) [The
connection between the vena cava inferior and the portal vein
and its consequences for the organism.] Arch. Exp. Path.
Pharmakol., 32: 161-210 (in German).
HALL, L.W., Jr, BUIKEMA, A.L., Jr, & CAIRNS, J., Jr (1978)
The effects of a stimulated refinery effluent on the grass
shrimp, Palaemonetes pugio. Arch. environ. Contam. Toxicol.,
7(1) 23-35.
HANTZSCH, S. & LAHMANN, E. (1970) [Ammonia determinations in
city air.] Schriftner. Ver. Wasser- Boden- Lufthyg. (Berlin-
Dahlem), 33: 35-39 (in German).
HARADER, R.R. & ALLEN, G.H. (1983) Ammonia toxicity to
chinook salmon parr: reduction in saline water. Trans Am.
Fish. Soc., 112: 834-837.
HARDING, A.J. (1959) Ammonia manufacture and uses, London,
Oxford University Press.
HAYNES, R.J. & GOH, K.M. (1977) Evaluation of potting media
for commercial nursery production of container grown plants.
II. Effects of media, fertilizer nitrogen and nitrification
inhibitor on yield and nitrogen uptake of Callistephus
chinensis (L.) nees "Pink Princess". N.Z. J. Agric. Res., 20:
371-381.
HAWKINS, R.A., MILLER, A.L., NIELSEN, R.C., & VEECH, R.L.
(1973) The acute action of ammonia on rat brain metabolism in
vivo. Biochem. J., 134: 1001-1008.
HAWKINS, D.B., DEMETER, M.J., & BARNETT, T.E. (1980) Caustic
ingestion: Controversies in management. A review of 14 cases.
Laryngoscope, 90: 98-109.
HAZEL, C.R., THOMSEN, W., & MEITH, S.J. (1971) Sensitivity
of striped bass and stickleback to ammonia in relation to
temperature and salinity. Calif. Fish Game, 57(3): 138-153.
HAZEL, R.H., BURKHEAD, C.E., & HUGGINS, D.G. (1979) The
development of water quality criteria for ammonia and total
residual chlorine for the protection of aquatic life in two
Johnson County, Kansas streams, Washington DC, Office of Water
Research and Technology, US Department of the Interior, 146 pp.
HEALY, T.V. (1974) Ammonia and related pollutants at
Harwell. Atmos. Environ., 8: 81-83.
HEALY, T.V., MCKAY, H.A.C., PILBEAM, A., & SCARGILL, D.
(1970) Ammonia and ammonium sulfate in the troposphere over
the United Kingdom. J. geophys. Res., 75: 2317-2321.
HEIL, G.W. & DIEMONT, W.M. (1983) Raised nutrient levels
change heathland into grassland. Vegetatio, 53: 113-120.
HEISLER, N. (1984) Acid-base regulation in fishes. In: Hoar,
W. & Randall, D.J., ed. Fish physiology, New York, Academic
Press, Vol. 10A.
HELMERS, S., TOP, F.H., & KNAPP, L.W., Jr (1971) Ammonia
injuries in agriculture. J. Iowa Med. Soc., 61: 271-280.
HEMENS, J. (1966) The toxicity of ammonia solutions to the
mosquito fish ( Gambusia affinis Baird & Girard). J. Proc.
Inst. Sewage Purif., 65: 265-271.
HENIS, Y. & CHET, I. (1967) Mode of action of ammonia on
Sclerotium rolfsii. Phytopathology, 57: 425-427.
HERBERT, D.W.M. (1956) La toxicité d'un effluent d'eau
d'égout. Bull. Cent. belge Etude Doc. Eaux, 32: 115-120.
HERBERT, D.W.M. (1962) The toxicity of rainbow trout of
spent still liquors from the distillation of coal. Ann. appl.
Biol., 50(4): 755-777.
HERBERT, D.W.M. & SHURBEN, D.S. (1965) The susceptibility of
salmonid fish to poisons under estuarine conditions. II.
Ammonium chloride. Int. J. Air Water Pollut., 9: 89-91.
HIDY, G.M. (1974) Characterization of aerosols in California
(ACHEX), Thousand Oaks, California, Rockwell International,
Vol. 1-4 (Final Report prepared for California Air Resources
Board).
HILLABY, B.A. & RANDALL, D.J. (1979) Acute ammonia toxicity
and ammonia excretion in rainbow trout (Salmo gairdneri). J.
Fish. Res. Board Can., 36(6): 621-629.
HINTZ, H.F., LOWE, J.E., CLIFFORD, A.J. ET AL. (1970)
Ammonia intoxication resulting from urea ingestion by ponies.
J. Am. Vet. Med. Assoc., 157: 963-966.
HOEFT, R.G., KENNEY, D.R., & WALSH, L.M. (1972) Nitrogen and
sulfur in precipitation and sulfur dioxide in the atmosphere
in Wisconsin. J. environ. Qual., 1: 203-208.
HOGAN, J.P. (1961) The absorption of ammonia through the
rumen of sheep. Aust. J. biol. Sci., 14: 448-460.
HOLETON, G.F., NEUMANN, P., & HEISLER, N. (1983) Branchial
ion exchange and acid-base regulation after strenous exercise
in rainbow trout. Resp. Physiol., 51: 303-318.
HOLLAND, G.A., LASATER, J.E., NEUMANN, E.D., & ELDRIDGE, W.E.
(1960) Experiments with inorganic pollutants - ammonia. Toxic
effects of organic and inorganic pollutants on young salmon
and trout, State of Washington, Department of Fisheries,
pp. 183-187 (Research Bulletin No. 5).
HOLT, G.J. & ARNOLD, C.R. (1983) Effects of ammonia and
nitrite on growth and survival of red drum eggs and larvae.
Trans. Am. Fish. Soc., 112: 314-318.
HOLT, J.D. & MALCOLM, S.A. (1979) Evaluation of fish loading
rates in regulatory static bioassays. Tech. Rep. -Fish. Mar.
Serv. (Can.)., 862: 146-160.
HORNE, F.R. & MCINTOSH, S. (1979) Factors influencing
distribution of mussels in the Blanco River of central Texas.
Nautilus, 94(4): 119-133.
HSIA, Y.E. (1974) Inherited hyperammonemic syndromes.
Gastroenterology, 67: 347-374.
HUNGER, W. (1978) [Dying off of old spruce trees in the
vicinity of a pig fattening station.] Beitrage
Forstwirtschaft, 4: 188-189 (in German).
HUNT, R.D. (1977) Measurement of partial pressure of ammonia
in breath and arterial blood. Evidence for secretion of
ammonia into expired gas by metabolism of monoamines in the
lung. Diss. Abstr. Int. Ser. B, 37: 3279.
ILO (1970) Permissible levels of toxic substances in the
working environment. In: Proceedings of the 6th Session of the
Joint ILO/WHO Committee on Occupational Health, Geneva, 4-10
June 1968, Geneva, International Labour Office, 405 pp.
INDUSTRIAL BIO-TEST LABORATORIES, INC. (1973) Irritation
threshold evaluation study with ammonia, Industrial Bio-Test
Laboratories, Inc. (Report to International Institute of
Ammonia Refrigeration, Publication No. 663-03161).
IWAOKA, W.T., OKRONE, C.A., SULLIVAN, J.J., & JOHNSON, C.A.
(1981a) Effect of pH and ammonium ions on mutagenic activity
in cooked beef. Cancer Lett., 12: 335.
IWAOKA, W.T., URONE, C.A., SULLIVAN, J.J., MEAKER, E.H., &
JOHNSON, C.A. (1981b) A source of error in mutagen testing
of foods. Cancer Lett., 11: 225.
JACOBS, M.H. (1940) Some aspects of cell permeability to
weak electrolytes. Cold Spring Harbour Sym. Quant. Biol., 8:
30-39.
JACQUEZ, J.A., POPPELL, J.W., LAWRENCE, W., Jr, & ROBERTS,
K.E. (1957) Clinical significance of the partial pressure of
ammonia (pNH3) in patients with ammonium toxicity. Clin. Res.
Proc., 5: 20 (Abstract).
JACQUEZ, J.A., POPPELL, J.W., & JELTSCH, R. (1959) Partial
pressure of ammonia in aveolar air. Science, 129: 269-270.
JAFFE, H.L., BODANSKY, A., & BLAIR, J.E. (1932a) Effects of
parathormone (parathyroid preparation) and ammonium chloride
on bones of rabbits. J. exp. Med., 55: 695-701.
JAFFE, H.L., BODANSKY, A., & CHANDLER, J.P. (1932b) Ammonium
chloride decalcification, as modified by calcium intake: the
relation between generalized osteoporosis and osteitis
fibrosa. J. exp. Med., 56: 823-834.
JANICKI, R.H. (1970) Renal adaptation during chronic NH4Cl
acidosis in the rat: no role for hyperplasia. Am. J. Physiol.,
219(3): 613-618.
JANSSEN, Th. W. (1982) [Intensive animal husbandry in
relation to space and environment,] Utrecht, The Netherlands,
Dutch State Forest Service (in Dutch).
JOHANNING, G.L., MUHRER, M.E., & GADDY, J.H. (1978)
Nutritional evaluation of chlorinated-ammoniated corn stover.
Paper presented at: The Joint Meeting of the American Dairy
Science Association and the American Society of Animal
Science, 11 July 1978.
JOHNSON, J.D., EPEL, D., & PAUL, M. (1976) Intracellular pH
and activation of sea urchin eggs after fertilization. Nature
(Lond.), 262(5570): 661-664.
JONES, K. (1973) Ammonia. In: Bailar, J.C., Jr, Emeleus,
H.J., Nyholm, R., & Trotman-Dickenson, A.F., ed. Comprehensive
inorganic chemistry, New York, Pergammon Press, Vol. 2, pp.
199-227.
JONES, R.D. & HOOD, M.A. (1980) Effects of temperature, pH,
salinity and inorganic nitrogen on the rate of ammonium
oxidation by nitrifiers isolated from wetland environments.
Microb. Ecol., 6: 339-347.
JUDE, D.J. (1973) Sublethal effects of ammonia and cadmium
on growth of green sunfish, East Lansing, Michigan, Michigan
State University, 193 pp (Ph.D. Thesis).
KADOWAKI, S. (1976) Size distribution of atmospheric total
aerosols, sulfate, ammonium and nitrate particulates in the
Nagoya area. Atmos. Environ., 10: 39-43.
KAGOTA, K., IWASE, T., KOJIMA, T., NIIYAMA, M., & NAMIOKA, S.
(1979) Diammonium citrate addition to a diet restricted
non-essential amino acids for young pigs. Jap. J. vet. Sci.,
41: 131-138.
KAMIN, H. & HANDLER, P. (1957) Amino acid and protein
metabolism. Annu. Rev. Biochem., 26: 419-490.
KAPEGHIAN, J.C., MINCER, H.H., JONES, A.B., VERLANGIERI, A.J.,
& WATERS, I.W. (1982) Acute inhalation toxicity of ammonia
in mice. Bull. environ. Contam. Toxicol., 29: 371-378.
KARNAKY, K.J., Jr, KINTER, L.B., KINTER, W.B., & STIRLING,
C.E. (1976) Teleost chloride cell. II. Autoradiographic
localization of gill Na, K-ATPase in killifish Fundulus
heterclitus adapted to low and high salinity environments.
J. cell Biol., 70: 157-177.
KATZ, M. & PIERRO, R.A. (1967) Estimates of the acute
toxicity of ammonia-urea plant wastes to coho salmon,
Oncorhynchus kisutch, Seattle, Washington, Fisheries Research
Institute, College of Fisheries, University of Washington,
15 pp (Final report).
KEESEE, R.G., HOPF, S.B., & MOYERS, J.L. (1975) The
distribution and characteristics of particulate sulfates in
the southwest desert atmosphere. Presented at: The
International Conference on Environmental Sensing and
Assessment, Las Vegas, Nevada, 14-19 September, 1975, Vol. 2,
5 pp (Paper 23-6).
KEMPINSKA, H. (1968) [The effect of ammonia fertilizers on
fish.] Gospod. Rybn., 20: 3-5 (in Russian).
KEPLINGER, M.L., SCHADEBERG, K.J., GOODE, J.W., & CALANDRA,
J.C. (1973) Irritation threshold evaluation study with
ammonia. In: Report to the Institute of Ammonia Refrigeration,
Northbrook, Illinois, Industrial Bio-Test Laboratories, Inc.
KERN, D.M. (1960) The hydration of carbon dioxide. J. Chem.
Ed., 37: 14-23.
KERSTETTER, T.H., KIRSCHNER, L.B., & RAFUSE, D.D. (1970) On
the mechanisms of sodium ion transport by the irrigated gills
of rainbow. J. gen. Physiol., 56: 342-359.
KEYES, W.F. (1975) Nitrogen. In: Mineral facts and problems,
Washington DC, US Department of the Interior, pp. 749-760
(Bureau of Mines Bulletin No. 667).
KHOLDEBARIN, B. & OERTLI, J.J. (1977) Effect of pH and
ammonia on the rate of nitrification of surface water.
J. Water Pollut. Control Fed., 49: 1693-1697.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.N. (1972)
Serum- and tissue-ammonia nitrogen and tissue-water values in
ammonia-intoxicated sheep. Am. J. Vet. Res., 33: 1187-1190.
KIRKPATRICK, W.C., ROLLER, M.H., & SWANSON, R.M. (1973)
Hemogram of sheep acutely intoxicated with ammonia.
Am. J. Vet. Res., 34: 587-589.
KIRSCHNER, L.B., GREENWALD, L., & KERSTETTER, T.H. (1973)
Effect of amiloride on sodium transport across body surfaces
of freshwater animals. Am. J. Physiol., 224: 832-837.
KLEMM, K. (1967) The effect of N source on the yield of
various crop plants. Bodenkultur, 18: 210-218.
KLENDSHOJ, N.C. & REJENT, T.A. (1966) Tissue levels of some
poisoning agents less frequently encountered. J. Forensic
Sci., 11: 75-80.
KLING, H.F. & QUARLES, C.L. (1974) Effect of atmospheric
ammonia and the stress of infectious bronchitis vaccination on
Leghorn males. Poult. Sci., 53: 1161-1167.
KLUESENER, J.W. & LEE, G.F. (1974) Nutrient loading from a
separate storm sewer in Madison, Wisconsin. J. Water Pollut.
Control Fed., 46(5): 920-936.
KNEPP, G.L. & ARKIN, G.F. (1973) Ammonia toxicity levels and
nitrate tolerance of channel catfish. Prog. Fish-Cult., 35(4):
221-224.
KNOWLES, G., DOWNING, A.L., & BARRETT, M.J. (1965)
Determination of kinetic constants for nitrifying bacteria in
mixed culture, with the aid of an electronic computer.
J. gen. Microbiol., 38: 263.
KOENIG, H. & KOENIG, R. (1949) Production of acute pulmonary
edema by ammonium salts. Proc. Soc. Exp. Biol. Med., 70:
375-380.
KOPP, A., FELLER, U., & ERISMANN, K.H. (1974) [Studies on
the regulation of nitrogen assimilation by Lemna minor in
transition from ammonium to nitrate feeding or from nitrate to
ammonium feeding under conditions of photosynthesis.]
Z. Pflanzen physiol., 73: 456-460 (in German).
KORMANIK, G.A. & CAMERON, J.N. (1981) Ammonia excretion in
the FW catfish: the role of diffusion. Am. Soc. Zool., 21(4):
1042.
KORTING, W. (1969a) [The harmful effect of ammonia on fish.]
Munch. Beitr. Abwasser-. Fisch.-Flussbiol., 16: 38-48 (in
German).
KORTING, W. (1969b) [Investigations on the effects of
ammonia on the blood of fish.] Wasser Abwasser Forsch., 4:
154-159 (in German).
KRYSTAL, G.W. & POCCIA, D. (1979) Control of chromosome
condensation in the sea urchin egg. Exp. cell Res., 123(2):
207-219.
KULASEK, G., SIWECKA, B., OWCZARCZYK, B., RYNCA, J., & BAREJ,
W. (1975) Effect of protein level and synthesize chloride
addition to diet on ammonia detoxification in rats. Zentralbl.
Veterinaermed., 22(3): 215-223.
KUSHNIR, J.J., MALKIN, H.I., MOHNEN, V.A., JENCHA, A.J., &
MCLAREN, E.H. (1970) Non-photochemical aerosol formation
from the anhydrous reaction between ammonia and sulfur dioxide
gases. Mechanism for the formation of ammonium sulfate in the
atmosphere, Albany, New York, Department of Chemistry and
Atmospheric Sciences Research Center, State University of New
York (Report 165 of National Air Pollution Control
Administration Research Grant 5RO1A).
KUSTOV, V.V. (1967) [Means of determining the maximum
allowable concentration of toxic products of natural human
metabolism.] In: General questions of industrial toxicology,
Moscow, pp. 63-65. (National Aeronautics and Space
Administration Technical Translation NASA TT F-11,358) (in
Russian).
LANDAHL, H.D. & HERRMANN, R.G. (1950) Retention of vapors
and gases in the human nose and lung. Arch. ind. Hyg. occup.
Med., 1: 36-45.
LANGOWSKA, I. & MOSKAL, J. (1974) Effect of ammonia and urea
on nitrobacteria in water environment. Pol. Arch. Hydrobiol.,
21(1): 119-123.
LARSON, T.V., COVERT, D.S., FRANK, R., & CHARLSON, R.J.
(1977) Ammonia in the human airways: neutralization of
inspired acid sulfate aerosols. Science, 197: 161-163.
LECHTENBERG, V.L., BUETTNER, M.R., HOLT, D.A., RICHEY, C.B., &
PARSONS, S.D. (1977) Hay preservation by anhydrous ammonia
treatment. In: Grain and Forage Harvesting. Proceedings of the
First International Grain and Forage Conference, St. Joseph,
American Society of Agricultural Engineers, pp. 327-328, 338.
LEE, J.A., (1985) Effects of NO2 on aquatic ecosystems. In:
Study on the need of NO2 long term limit value for the
protection of terrestrial and aquatic ecosystems (Final Report
Feb. 1985).
LEE, R.E., Jr & GORANSON, S. (1976) National air
surveillance cascade impact network. III. Variations in size
of airborne particulate matter over a three-year period.
Environ. Sci. Technol., 10: 1022-1027.
LEITHE, W. (1971) The analysis of air pollutants, Ann Arbor,
Michigan, Ann Arbor Science Publishers.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odour
threshold determination of 52 odorant chemicals. J. Air
Pollut. Control Assoc., 19: 91-95.
LEUVEN, R.S.E.W. & SCHUURKES, J.A.A.R. (1984) [Effects of
acid precipitation containing sulfur and nitrogen on weakly
buffered waters low in nutrients] The Netherlands, Laboratory
of Aquatic Ecology, University of Nymegen, and Ministry of
Housing, Physical Planning and Environment, 131 pp (in Dutch).
LEVI, G., MORISI, G., COLETTI, A., & CATANZARO, R. (1974)
Free amino acids in fish brain: normal levels and changes upon
exposure to high ammonia concentrations in vivo, and upon
incubation of brain slices. Comp. Biochem. Physiol., 49A:
623-636.
LEVY, D.M., DIVERTIE, M.B., LITZOW, T.J., & HENDERSON, J.W.
(1964) Ammonia burns of the face and respiratory tract.
J. Am. Med. Assoc., 190: 873-876.
LIEBHARDT, W.C., GOLT, C., & TUPIN, J. (1979) Nitrate and
ammonium concentrations of ground water resulting from poultry
manure applications. J. environ. Qual., 8: 211-215.
LIECHTI, P.M. & HUGGINS, D.G. (1980) Effects of a 24-h
ammonia injection on stream drift and benthic standing crop.
Trans. Kansas Acad. Sci., 83(3): 140.
LIETZKE, M.H. (1978) A kinetic model for predicting the
composition of chlorinated water discharged from power plant
cooling systems. In: Water chlorination environmental impact
and health effects, Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., Vol. 2, pp. 707-716.
LILLIE, R.J. (1970) Ammonia. In: Air pollutants affecting
the performance of domestic animals. A literature review,
Washington DC (Agriculture Handbook No 380).
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide
formulations against two brackish water organisms, the bleak
(Alburnus alburnus) and the harpacticoid (Nitocra spinipes).
Chemosphere, 11(12): 843-851.
LINZON, S.N. (1971) Effects of air pollutants on vegetation.
In: McCormac, B.M., ed. Introduction to the scientific study
of atmospheric pollution, Dordrecht, Holland, D. Reidel
Publishing Company, pp. 131-151.
LITAV, M. & AGAMI, M. (1976) Relationship between water
pollution and the flora of two coastal rivers of Israel.
Aquat. Biol., 2(1): 23-41.
LITAV, M. & LEHRER, Y. (1978) The effects of ammonium in
water on Potamageton lucens. Aquat. Biol., 5(2): 127-138.
LITTON BIONETICS, INC. (1975) Mutagenic evaluation of
compound FDA 73-42 ammonium sulfate, granular, food grade,
Washington DC, Litton Bionetics (Prepared for US Food and Drug
Administration) (NTIS PB-245 506).
LLOYD, R. (1961) Effect of dissolved oxygen concentrations
on the toxicity of several poisons to rainbow trout ( Salmo
gairdnerii Richardson). J. exp. Biol., 38: 447-455.
LLOYD, R. & HERBERT, D.W.M. (1960) The influence of carbon
dioxide on the toxicity of un-ionized ammonia to rainbow trout
( Salmo gairdnerii Richardson). Ann. appl. Biol., 48(2):
399-404.
LLOYD, R. & ORR, L.D. (1969) The diuretic response by
rainbow trout to sub-lethal concentrations of ammonia. Water
Res., 3(5): 335-344.
LOBASHOV, M.E. (1937) [Nature of the effects of chemical
agents on the mutation process of Drosphila melanogaster.]
Genetica, 19: 200-241 (in German).
LOBASHOV, M.E. & SMIRNOV, F.A. (1934) [Effect of acetic acid
on nondisjunction and mutations in Drosphila melanogaster.]
Dokl. Acad. Sci. USSR N.S., 2: 307 (in Russian with German
summary).
LODGE, J.P., Jr, MACHADO, P.A., PATE, J.B., SHEESLEY, D.C., &
WARTBURG, A.F. (1974) Atmospheric trace chemistry in the
American humid tropics. Tellus, 26: 250-253.
LOEHR, R.C. (1974) Characteristics and comparative magnitude
of non-point sources. J. Water Pollut. Control Fed., 46(8):
1849-1872.
LOSADA, M. & ARNON, D.I. (1963) Selective inhibitors of
photosynthesis. In: Hochester, R. & Quastel, J.H., ed.
Metabolic inhibitors, New York, Academic Press, Vol. 2, p. 559.
LOSADA, M., HERRERA, J., MALDONADO, J.M., & PANEQUE, A.
(1973) Mechanism of nitrate reductase reversible inactivation
by ammonia in Chlamydomonas. Plant Sci. Lett., 1: 31-37.
LOTSPEICH, W.D. (1965) Renal hypertrophy in metabolic
acidosis and its relation to ammonia excretion.
Am. J. Physiol., 208(6): 1135-1142.
LUBINSKI, K.S. (1979) Monitoring bluegill swimming behaviour
and the effects of sublethal ammonium chloride gradients,
Blacksburg, Virginia, Virginia Polytechnic Institute and State
University, 99 pp (Ph.D. Thesis).
LUBINSKI, K.S., SPARKS, R.E., & JAHN, L.A. (1974) The
development of toxicity indices for assessing the quality of
the Illinois River, Urbana, Illinois, Water Resources Center,
University of Illinois, 46 pp (Research Report No. 96).
LUBINSKI, K.S., DICKSON, K.L., & CAIRNS, J., Jr (1980)
Effects of abrupt sublethal gradients of ammonium chloride on
the activity level, turning, and preference-avoidance
behaviour of bluegills, In: Eaton, J.G., Parrish, P.R., &
Hendricks, A.C. ed. Aquatic toxicology, Philadelphia,
Pennsylvania, American Society for Testing and Materials,
pp. 328-340 (ASTM STP 707).
MACEWEN, J.D. & VERNOT, E.H. (1972) Toxic hazards research
unit annual technical report (NTIS AD 755-358).
MACEWEN, J.D., THEODORE, J., & VERNOT, E.H. (1970) Human
exposure to EEL concentrations of monomethylhydrazine. In:
Proceedings of the 1st Annual Conference on Environmental
Toxicology, Ohio, Wright-Patterson Air Force Base,
9-11 September, 1970, Aerospace Medical Research Laboratory,
pp. 355-363 (AMRL-TR-70-102, Paper No. 23).
MCBARRON, E.J. & MCINNES, P. (1968) Observations on urea
toxicity in sheep. Aust. Vet. J., 44: 90-96.
MCBEAN, R.L., NEPPEL, M.J., & GOLDSTEIN, L. (1966)
Glutaminate dehydrogenase and ammonia production in the eel
(Anguilla rostrata). Comp. Biochem. Physiol., 18: 909-920.
MCBRIDE, R.L. & LAING, D.G. (1979) Threshold determination
by triangle testing: Effects of judgmental procedure,
positional bias and incidental training. Chem. Senses Flavor.,
4(4): 319-328.
MCCALLAN, S.E.A. & SETTERSTROM, C. (1940) Toxicity of
ammonia, chlorine, hydrogen cyanide, hydrogen sulphide, and
sulphur dioxide gases. I. General methods and correlations.
Contrib. Boyce Thompson Inst., 11: 325-330.
MCCAY, C.M. & VARS, H.M. (1931) Studies upon fish blood and
its relation to water pollution. In: A biological survey of
the St. Lawrence Watershed. Supplement to the 20th Annual
Report, 1930, Albany, New York, New York Conservation
Department, pp. 230-233 (Biol. Survey (1930), No. V).
MCCORMICK, J.H., BRODERIUS, S.J., & FIANDT, J.T. (1984)
Toxicity of ammonia to early life stages of the green sunfish
Lepomis cyanellus. Environ. Pollut. Ser. A., 36: 147-163.
Erratum: Environ. Pollut. Ser. A. (in press).
MCDONALD, D.G. & WOOD, C.M. (1981) Branchial and renal acid
and ion fluxes in the rainbow trout at low environmental pH.
J. exp. Biol., 93: 101-118.
MCKAY, H.A.C. (1969) Ammonia and air pollution. Chem. Ind.,
34: 1162-1165.
MCKHANN, G.M. & TOWER, D.B. (1961) Ammonia toxicity in
cerebral oxidative metabolism. Am. J. Physiol., 200: 420-424.
MAETZ, J. (1972) Branchial sodium exchange and ammonia
excretion in the goldfish Carassius auratus. Effects of
ammonia-loading and temperature changes. J. exp. Biol., 56:
601-620.
MAETZ, J. (1973) Na+/NH4+, Na+/H+ exchanges and NH3 movement
across the gill of Carassius auratus. J. exp. Biol., 58:
255-275.
MAETZ, J. & GARCIA-ROMEU, F. (1964) The mechanism of sodium
and chloride uptake by the gills of a freshwater fish,
Carassius auratus. II Evidence for NH4+/Na+ and HCO3-/Cl-
exchanges. J. gen. Physiol., 47: 1209-1227.
MAGALHAES BASTOS, J.A. (1954) [Importance of ammonia as an
ichthyotoxic substance,] Brazil, Serv. Piscicultura, Dept.
nacl. obras contra secas, Ministerio viacao e obras publicas,
pp. 115-132 (Publ. Ser. 1-C, No. 159) (in Portuguese).
MAI, K.L. (1977) Energy and petrochemical raw materials
through 1990. Chem. Eng., 84: 122-128.
MALACEA, I. (1966) [Contributions to knowledge of the
cyanide, ammonia, mercury and arsenic toxic action on some
species of fishes and on Daphnia.] Stud. Prot. Epurarea Apelor
Inst. Stud. Cercet Hidrotehnice, 7(2: 751-792 (English
summary) Biol. Abstr., 50: 92715 (1969).
MALACEA, I. (1968) [Studies on the acclimatization of fish
to high concentrations of toxic substances.] Arch. Hydrobiol.,
65(1): 74-95 (in German with English summary).
MALLERY, C.H. (1979) Ammonium stimulated properties of a
K+-dependent ATPase in Opsanus beta, a marine teleost with an
NH4+/Na+ exchange pump. Am. Zool., 19: 944.
MANGOLD, C.A. (1971) Investigation of occupational exposure
to ammonia, Washington, Puget Sound Naval Shipyard, Industrial
Hygiene Division of Investigation.
MANGUM, C.P., SILVERTHORN, S.U., HARRIS, J.L., TOWLE, D.W., &
KRALL, A.R. (1976) The relationship between blood pH,
ammonia excretion and adaptation to low salinity in the blue
crab Callinectes sapidus. J. exp. Zool., 195(1): 129-136.
MANGUM, C.P., DYKENS, J.A., HENRY, R.P., & POLITES, G.
(1978) The excretion of NH4+ and its ouabain sensitivity in
aquatic annelids and molluscs. J. exp. Zool., 203: 151-157.
MARSCHANG, F. (1972) [The NH3 content of air in stables and
the fattening capacity of cows.] Dtsch. Tieraerztl.
Wochenschr., 79: 214-216 (in German).
MARSCHANG, F. & CRAINICEANU, E. (1971) [Investigations on
the connections between cowshed climate and calf mortality.]
Berlin München Tieraerztl. Wochenschr., 84: 417-420 (in
German).
MARSCHANG, F. & PETRE, C. (1971) [The NH3 content of cowshed
air and its influence on morbidity and animal lossess in
cattle-fattening pens.] Zentralbl. Veterinaermed., 18: 646-654
(in German).
MATASA, C. & MATASA, E. (1968) L'industrie moderne des
produits azotés, Paris, Dunod, pp. 63-208.
MATSUMOTO, H., OKAMURA, K., & TAKAHASHI, E. (1976) Changes
in activity and isoenzymes of intervase in cucumber leaves due
to nitrogen status. Plant Cell Physiol., 17: 867-874.
MATUSIAK, K. (1976) Studies on the purification of
wastewater from the nitrogen fertilizer industry by intensive
algal cultures. Acta microbiol. Pol., 25(3): 233-242.
MAYAN, M.H. & MERILAN, C.P. (1972) Effects of ammonia
inhalation on respiratory rate of rabbits. J. anim. Sci., 34:
448-452.
MAYNARD, D.N. & BARKER, A.V. (1969) Studies on the tolerance
of the plants to ammonium nutrition. J. Am. Soc. Hortic. Sci.,
94: 235-239.
MEARNS, A.J. (1981) Ecological effects on ocean sewage
outfalls: observations and lessons. Oceanus, 24: 45-54.
MERKENS, J.C. & DOWNING, K.M. (1957) The effect of tension
of dissolved oxygen on the toxicity of un-ionized ammonia to
several species of fish. Ann. appl. Biol., 45(3): 521-527.
METCALF & EDDY, INC. (1972) Wastewater engineering, New
York, McGraw-Hill.
MILES, F.D. (1961) Nitric acid manufacture and uses, London,
Oxford University Press.
MILLER, T.J. (1973) Cleft palate formation: The effects of
fasting and iodoacetic acid on mice. Teratology, 7: 177-182.
MILLER, G.R., SHIMP, G.F., ANDREWS, H.O., NIMMO, D.W., & ILEY,
E.S. (1981) Effect of domestic wastewater on trout in Dillon
Reservoir, Colorado. Paper presented at: The ASTM Symposium on
Aquatic Toxicology, St. Louis, Missouri, 13-14 October 1981,
Philadelphia, Pennsylvania, American Society for Testing
Materials.
MINER, J.R. & HAZEN, T.E. (1969) Ammonia and amines:
components of swine-building odour. Trans. Am. Soc. Agric.
Eng., 12: 772-774.
MITCHELL, H.A. (1963) Ammonia tolerance of the California
leaf-nosed bat. J. Mammal, 44: 543-551.
MITCHELL, H.A. (1964) Investigations of the cave atmosphere
of a Mexican bat colony. J. Mammal, 45: 568-577.
MORII, H., NISHIKATA, K., & TAMURA, O. (1978) Nitrogen
excretion of mudskipper fish Periophthalmus cantonensis and
Boleophthalmus pectinirostris in water and on land. Comp.
Biochem. Physiol., 60A(2): 189-193.
MORRIS, J.C. (1967) Kinetics of reactions between aqueous
chlorine and nitrogenous compounds. In: Faust, S.D. & Hunter,
J.V., ed. Principles and applications of water chemistry,
New York, John Wiley and Sons, pp. 23-53.
MORRIS, J.C. (1978) Chemistry of aqueous chlorine in
relation to water chlorination. In: Jolley, R.L., ed. Water
chlorination: Environmental impact and health effects,
Ann Arbor, Michigan, Ann Arbor Science Publishers, Vol. 1,
pp. 21-36.
MORRIS, J.C. & ROGERS, Q.R. (1978) Ammonia intoxication in
near-adult cat as a result of a dietary deficiency of
arginine. Science, 199(4327): 431-432.
MOSIER, A.R. (1978) Inhibition of photosynthesis and
nitrogen fixation in algae by volatile nitrogen bases.
J. environ. Qual., 7(2): 237-240.
MOSSBERG, S.M. (1967) Ammonia absorption in hamster iluem.
Effect of pH and total CO2 on transport in everted sacs.
Am. J. Physiol., 213: 1327-1330.
MOSSBERG, S.M. & ROSS, G. (1967) Ammonia movement in the
small intestine: Preferential transport by the ileum. J. clin.
Invest., 46: 490-498.
MOTYL, T. & DEBSKI, B. (1977) The influence of increasing
amounts of NH4Cl in the diet on ammonia detoxification in
rats. Zbl. Vet. Med., 24: 333-342.
MULDER, J.S. & VAN DER ZALM, H.O. (1967) [A fatal case of
ammonia poisoning.] Tijdschr. Soc. Geneeskd., 45: 458-460 (in
Dutch).
MULLER, G. & GRUHN, M. (1969) [The effect of water-free
ammonia fertilizer on soil microorganisms.] Zentralbl.
Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 2 Naturw.,
123: 667-676 (in German with English summary).
MUNTWYLER, E., IACOBELLIS, M., & GRIFFIN, G.E. (1956) Kidney
glutaminase and carbonic anhydrase activities and renal
electrolyte excretion in rats. Am. J. Physiol., 184: 83-90.
NAKAZAWA, S. & QUASTEL, J.H. (1968) Inhibitory effects on
ammonium ions and some amino acids on stimulated brain
respiration and cerebral amino acid transport. Can. J.
Biochem., 46: 543-548.
NATARAJAN, K.V. (1970) Toxicity of ammonia to marine
diatoms. J. Water Pollut. Control Fed., 42(5, part 2):
R184-R190.
NAVAZIO, F., GERRITSEN, T., & WRIGHT, G.J. (1961)
Relationship of ammonia intoxication to convulsions and coma
in rats. J. Neurochem., 8: 146-151.
NEHRING, D. (1963) [The toxic effect of urea solutions
containing urease on various fish species.] Z. Fischerei,
11(7/8): 539-547 (in German).
NEUFELD, R.D., HILL, A.J., & ADEKOYA, D.O. (1980) Phenol and
free ammonia inhibition to Nitrosomonas activity. Water Res.,
14(12): 1695-1703.
NIDEN, A.H. (1968) Effects of ammonia inhalation of the
terminal airways. In: Proceedings of the 11th Aspen Emphysema
Conference, Vol. 11, pp. 41-44.
NIHLGARD, B. (1970) Precipitation, its chemical composition
and effects on soil water and beech and spruce forests in
South-Sweden. Oikos., 21: 208
NIHLGARD, B. (1985) The ammonium hypothesis: an additional
explanation to the forest dieback in Europe. Ambio, 14(1): 1-8.
NISHIOKA, D.J. (1976) The initiation of cell cycle events in
ammonia-treated eggs and merogons of the sea urchin, Berkeley,
California, University of California, 107 pp (Ph.D. Thesis).
NODA, K. & CHIKAMORI, K. (1976) Effect of ammonia via
prepyriform cortex on regulation of food intake in the rat.
Am. J. Physiol., 231: 1263-1266.
NRC (1979) Ammonia. Subcommittee on Ammonia. Committee on
Medical and Biologic Effects of Environmental Pollutants.
Division of Medical Sciences, Assembly of Life Sciences,
National Research Council, Baltimore, Maryland, University
Park Press (NTIS No. PB 278-027).
OKITA, T. & KANAMORI, S. (1971) Determination of trace
concentration of ammonia in the atmosphere using
pyridine-pyrazolone reagent. Atmos. Environ., 5: 621-627.
OVERREIN, L.N., SEIP, H.M., & TOLLAN, A., ed. (1980) Acid
precipitation: effects on forest and fish, Oslo (Final report
SNSF Project 1972-80).
PAIK, W.K., LAWSON, D., & KIM, S. (1975) Effect of endocrine
glands on the protection of rat from ammonia intoxication.
Life Sci., 15: 1189-1196.
PARKHURST, B.R., BRADSHAW, A.S., FORTE, J.L., & WRIGHT, G.P.
(1979) An evaluation of the acute toxicity to aquatic biota
of a coal conversion effluent and its major components.
Bull. environ. Contam. Toxicol., 23: 349-356.
PARKHURST, B.R., MEYER, J.S., DEGRAEVE, G.M., & BERGMAN, H.L.
(1981) A re-evaluation of the toxicity of coal conversion
process waters. Bull. environ. Contam. Toxicol., 26: 9-15.
PAUL, M., JOHNSON, J.D., & EPEL, D. (1976) Fertilization
acid of sea urchin eggs is not a consequence of cortical
granule exocytosis. J. exp. Zool., 197(1): 127-133.
PAUL, M. & JOHNSTON, R.N. (1978) Absence of a Ca response
following ammonia activation of sea urchin eggs. Dev. Biol.,
67(2): 330-335.
PAYAN, P. (1978) A study of the Na+/NH4+ exchange across the
gill of the perfused head of the trout (Salmo gairdneri). J.
comp. Physiol., 124: 181-188.
PAYAN, P. & MAETZ, J. (1973) Branchial sodium transport
mechanisms in Scyliorhinis canicula: evidence for Na+/NH4+
and Na+/H+ exchanges and for a role of carbonic anhydrase.
J. exp. Biol., 58: 487-502.
PAYAN, P. & MATTY, A.J. (1975) The characteristics of
ammonia excretion by a perfused isolated head of trout
(Salmo gairdneri): effect of temperature and CO2-free ringer.
J. comp. Physiol., 96: 167-184.
PAYAN, P., MATTY, A.J., & MAETZ, J. (1975) A study of the
sodium pump in the perfused head preparation of the trout,
Salmo gairdneri in freshwater. J. comp. Physiol., 104: 33-48.
PEPLINSKI, A.J., BREKKE, O.L., BOTHAST, R.J., & BLACK, L.T.
(1978) High moisture corn: an extended preservation trial
with ammonia. Trans. Am. Soc. Agricul. Eng., 21: 773-776, 781.
PEQUIN, L. & SERFATY, A. (1963) L'excrétion ammoniacale chez
un téléostéen dulcicole Cyprinus carpio. J. comp. Biochem.
Physiol., 10: 315-324.
PERRY, J.H., CHILTON, C.H., & KIRKPATRICK, S.D., ed. (1963)
Chemical engineers' handbook, 4th ed., New York, McGraw-Hill,
pp. 3-151.
PHILLIPS, A.M. (1950) The effect of metabolic products on
brook trout, Cortland, New York, New York State Conservation
Department, pp. 14-16 (Fisheries Research Bulletin 14)
(Cortland Hatcherry Report 19).
PHILLIPS, R.W. (1970) Further clinical studies of
trimethylpuringyl ammonium chloride (NSA-51095) in malignant
disease with special emphasis on cancer of urinary bladder.
Cancer Chemother. Rep., 54: 181.
PIERPONT, R.A. & MINOTTI, P.L. (1977) Effects of calcium
carbonate on ammonium assimilation by tomato seedlings.
J. Am. Soc. Hortic. Sci., 102: 20-23.
PINTER, I.J. & PROVASOLI, L. (1963) Nutritional characteristics
of some chrysomonads. In: Oppenheimer, C.M., ed. Symposium on
Marine Microbiology, Springfield, Massachusetts, Thomas
Publishers, pp. 114-121.
PITTS, R.F. (1971) The role of ammonia production and
excretion in regulation of acid-base balance. New Engl. J.
Med., 284: 32-38.
PITTS, J.N., Jr & GROSJEAN, D. (1976) Detailed
characterization of gaseous and size-resolved particulate
pollutants at a south coast air basin smog receptor site:
levels and modes of formation of sulfate, nitrate and organic
particulates and their implications for control strategies,
Riverside, California, Statewide Air Pollution Research
Center, University of California (California Air Resources
Board Contract 5-384, Six Month Progress Report, November 17,
1975 to May 16, 1976).
POLIAK, V.E. (1981) [Air pollution by ammonia around swine-
breeding complexes.] Gig. i. Sanit., 6: 67 (in Russian).
POST, R.L. & JOLLY, P.C. (1957) The linkage of sodium,
potassium and ammonium active transport across the human
erythrocyte membrane. Biochem. Biophys. Acta, 25: 118-128.
POWERS, E.B. (1920) Influence of temperature and concentration
on the toxicity of salts to fishes. Ecology, 1: 95-112.
POWERS, W.L., TERRY, R.V., & MURPHY, L.S. (1977) Fate of
nitrogen from manure disposal. In: Proceedings of the National
Conference on Disposal of Residues on Land, pp. 156-160.
PROPKOPIEVA, A.S. & YUSHKOV, G.G. (1975) [Toxicology of
ammonia in animals repeatedly exposed for short lengths of
time.] Gig. Tr. Prof. Zabol., 9: 54-55 (in Russian).
PROPKOPIEVA, A.S., YUSHKOV, G.G., & UBASHEEV, I.O. (1973)
[Materials for a toxicological characteristic of the one-term
effect of ammonia on the organisms of animals after brief
exposure.] Gig. Tr. Prof. Zabol., 6: 56-57 (in Russian).
PROVASOLI, L. & MCLAUGHLIN, J.J.A. (1963) Limited
heterotrophy of some photosynthetic dinoflagellates. In:
Oppenheimer, C.M., ed. Symposium on Marine Microbiology,
Springfield, MA, Thomas Publishers, pp. 105-113.
PRZYTOCKA-JUSIAK, M. (1976) Growth and survival of Chlorella
vulgaris in high concentrations of nitrogen. Acta microbiol.
Pol., 25(3): 287-289.
PRZYTOCKA-JUSIAK, M., MLYNARCZYK, A., KULESZA, M., &
MYCIELSKI, R. (1977) Properties of Chlorella vulgaris strain
adapted to high concentrations of ammonium nitrogen. Acta
microbiol. Pol., 26(2): 185-197.
QUARLES, C.L. & KLING, H.F. (1974) Evaluation of ammonia and
infectious bronchitis vaccination stress on broiler
performance and carcass quality. Poult. Sci., 53: 1592-1596.
RAJAGOPAL, R. (1978) Impact of land use on ground water
quality in the Grand Traverse Bay region of Michigan.
J. environ. Qual., 7: 93-98.
RAMACHANDRAN, V. & RAMAPRABHU, T. (1976) Use of ammonia as
herbicide: A Review. In: Varshney, C.K. & Rzoska, J., ed.
Aquatic weeds in South East Asia, The Hague, B.V. Publishers,
pp. 293-298.
RAMACHANDRAN, V., RAMAPRABHU, T., & REDDY, P.V.G.K. (1975)
Observations on the use of ammonia for the control of Pistia
stratiotes Linn. J. Int. Fish. Soc. India, 7: 124-130.
RAMSEY, G.B. (1953) Mechanical and chemical injuries. In:
The yearbook of agriculture 1953, Washington DC, US Department
of Agriculture, Plant Diseases, pp. 835-837.
RANDALL, D.J. (1970) Gas exchange in fish. In: Hoar, W.S. &
Randall, D.J., ed. Fish physiology, New York, Academic Press,
Vol. IV., pp. 253-292.
RANKIN, D.P. (1979) The influence of un-ionized ammonia on
the long-term survival of sockeye salmon eggs. Fish. Mar.
Serv. Tech. Rep., 912: 17.
RAO, T.S., RAO, M.S., & PRASAD, S.B.S.K. (1975) Median
tolerance limits of some chemicals to the fresh water fish
"Cyprinus carpio". Indian J. environ. Health, 17(2): 140-146.
REDNER, B.D. & STICKNEY, R.R. (1979) Acclimatization to
ammonia by Tilapia aurea. Trans. Am. Fish Soc., 108(4):
383-388.
REICHENBACH-KLINKE, H.-H. (1967) [Investigations on the
influence of the ammonia content on the fish organism.] Arch.
Fischereiwiss., 17(2): 122-132. (in German).
REINBOLD, K.A. & PESCITELLI, S.M. (1982a) Effects of
exposure to ammonia on sensitive life stages of aquatic
organisms, Champaign, Illinois, Illinois Natural History
Survey (Project Report: Contract No. 68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982b) Effects of cold
temperature on toxicity of ammonia to rainbow trout, blue
gills, and fathead minnows, Champaign, Illinois, Illinois
Natural History Survey (Project Report: Contract No.
68-01-5832).
REINBOLD, K.A. & PESCITELLI, S.M. (1982c) Acute toxicity of
ammonia to the white sucker, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. 2W-3946 NAEX).
REINBOLD, K.A. & PESCITELLI, S.M. (1982d) Acute toxicity of
ammonia to channel catfish, Champaign, Illinois, Illinois
Natural History Survey, 11 pp (EPA Contract No. J 2482 NAEX).
REITER, R., SLADKOVIC, R., & POTZL, K. (1976) Chemical
components of aerosol particles in the lower troposphere above
central Europe measured under pure-air conditions. Atmos.
Environ., 10: 841-853.
REMPEL, B. (1975) Use ammonia to stop corn deterioration.
Crops Soils, 27: 13-14.
REPP, W.W., HALE, W.H., CHENG, E.W., & BURROUGHS, W. (1955)
The influence of oral administration of non-protein nitrogen
feeding compounds upon blood ammonia and urea levels in lambs.
J. anim. Sci., 14: 118-131.
RICE, S.D. & BAILEY, J.E. (1980) Survival, size, and
emergence of pink salmon, Oncorhynchus gorbuscha, alevins
after short- and long-term exposures to ammonia. Fish Bull.,
78(3): 641-648.
RICHARD, D., BOULEY, G., & BOUDENE, C. (1978a) Effets de
l'inhalation continue d'ammoniac chez le rat et la souris.
Bull. eur. Physiopathol. resp., 14: 573-582.
RICHARD, D., JOUARY, J., & BOUDENE, C. (1978b) Toxicité
aiguë par voie aérienne du gaz ammoniac chez le lapin.
C.R. Acad. X. Paris, 287(Série D): 375-378.
RICHARDS, B.O. & FROMM, P.O. (1970) Sodium uptake by
isolated perfused gills of rainbow trout (Salmo gairdneri).
Comp. Biochem. Physiol., 33: 303-310.
RICHARDS, P., HOUGHTON, B.J., METCALFE-GIBSON, A., WARD, E.E.,
& WRONG, O. (1968) Incorporation of orally administered
ammonia into tissue proteins in man; the influence of diet and
ureamia. In: Nutrition and renal disease, Edinburgh, E. and S.
Livingstone, pp. 93-98.
RICHARDS, P., BROWN, C.L., HOUGHTON, B.J., & WRONG, D.M.
(1975) The incorporation of ammonia nitrogen into albumin in
man: The effects of diet, uremia and growth hormone.
Clin. Nephrol., 3(5): 172-179.
RINGER, R.K. (1971) Adaptation of poultry to confinement
rearing systems. J. anim. Sci., 3: 590-598.
ROBIN, E.D., TRAVIS, D.M., BROMBERG, P.A., FORKNER, C.E., Jr,
& TYLER, J.M. (1959) Ammonia excretion by mammalian lung.
Science, 129: 270-271.
ROBINETTE, H.R. (1976) Effect of selected sublethal levels
of ammonia on the growth of channel catfish (Ictalurus
punctatus). Prog. Fish-Cult., 38(1): 26-29.
ROBINSON, E. & ROBBINS, R.C. (1968) Sources, abundance and
fate of gaseous atmospheric pollutants, Menlo Park, Stanford
Research Institute, California, 123 pp (prepared for the
American Petroleum Institute).
ROBINSON, E. & ROBBINS, R.C. (1971) Emissions, concentrations,
and fate of gaseous atmospheric pollutants. In: Strauss, W., ed.
Air pollution control, New York, John Wiley and Sons, Part 2,
pp. 1-93.
ROBINSON-WILSON, E.F. & SEIM, W.K. (1975) The lethal and
sublethal effects of a zirconium process effluent on juvenile
salmonids. Water Resour. Bull., 11(5): 975-986.
ROELOFS, J.G.M., SCHUURKES, J.A.A.R., & SMITS, A.J.M. (1984)
Impact of acidification and eutrophication on macrophyte
communities in soft waters in the Netherlands. II. Experimental
studies. Aquat. Bot., 18: 389-411.
ROELOFS, J.G.M. (in press) The effect of sulfur and nitrogen
deposition on aquatic and terrestrial heathland vegetation.
Experientia (Basel).
ROELOFS, J.G.M., KEMPERS, A.J., HOUDYK, A.L.F.M., & JANSEN,
J. (1985) The effect of airborne ammonium sulphate on Pinus
nigra var maritima, in the Netherlands. Plant Soil, 84: 45-56.
ROSAGE, T.F., SCHUTSKY, R.M., & RAPP, K.M. (1979) Toxicity
of un-ionized ammonia to the spotfin shiner (Notropis
spilopterus). Proc. Pa. Acad. Sci., 53: 39-42.
ROSEBOOM, D.P. & RICHEY, D.L. (1977) Acute toxicity of
residual chlorine and ammonia to some native Illinois fishes,
Urbana, Illinois, Illinois State Water Survey, 42 pp (Report
of Investigation 85).
ROSENFELD, M. (1932) Experimental modification of mitosis by
ammonia. Arch. exp. Zellforsch. Gewebezuecht., 14: 1.
RUBIN, A.J. & ELMARAGHY, M.A. (1976) Studies on the toxicity
of ammonia, nitrate, and their mixtures to the common guppy,
Columbus, Ohio, Water Resources Center, Ohio State University,
47 pp (Project No. A-033-OHIO).
RUBIN, A.J. & ELMARAGHY, G.A. (1977) Studies on the toxicity
of ammonia, nitrate and their mixtures to guppy fry. Water
Res., 11(10): 927-935.
SALVATORE, F., BOCCHINI, V., & CIMINO, F. (1963) Ammonia
intoxication and its effects on brain and blood ammonia
levels. Biochem. Pharmacol., 12: 1-6.
SAIFUTDINOV, M.M. (1966) Maximum permissible concentration
of ammonia in the atmosphere. Hyg. Sanit., 176: 171-175.
SAMYLIN, A.F. (1969) [Effect of ammonium carbonate on early
stages of development of salmon.] Uchen. Zap. Leningr. Gos.
Pedagog. Inst. Im. A. I. Gertsena, 422: 47-62 (in Russian).
SCHAERDEL, A.D., WHITE, W.J., LANG, C.M., DVORCHIK, B.H., &
BOHNER, K. (1983) Localized and systemic effects of
environmental ammonia in rats. Lab. anim. Sci, 33(1): 40-45.
SCHENKER, S. & WARREN, K.S. (1962) Effect of temperature
variation on toxicity and metabolism of ammonia in mice.
J. Lab. clin. Med., 60: 291-301.
SCHULZE-WIEHENBRAUCK, H. (1976) Effects of sublethal ammonia
concentrations on metabolism in juvenile rainbow trout ( Salmo
gairdneri Richardson). Ber. Dtsch. Wiss. Komm. Meeresforsch.,
24(4): 234-250.
SCHUMAN, G.E. & BURWELL, R.E. (1974) Precipitation nitrogen
contribution relative to surface runoff discharges.
J. environ. Qual., 3: 366-369.
SCHUURKES, J.A.A.R. (in press) Ammonium sulfate deposition
and its role in the acidification and nitrogen enrichment of
poorly-buffered aquatic systems. Experientia (Basel).
SCHUURKES, J.A.A.R., HECK, I., HESEN, P., LEUVEN, R.S.E.W., &
ROELOFS, J.G.M. (1985) Effects of acid and acidifying
deposition on water composition and macrophyte composition of
simulated poorly buffered aquatic systems. In: Proceedings of
the Muskoka Conference, Toronto, Canada, September 1985.
SEEGAL, B.C. (1927) Chronic acidosis in rabbits and in dogs,
with relation to kidney pathologic change. Arch. intern. Med.,
39(4): 550-563.
SELESI, D. & VAMOS, R. (1976) [Factors affecting the lethal
concentration of ammonia in fish ponds.] Ichthyologia, 8(1):
115-121 (in Serbocroation with English summary).
SERVANT, J. & DELAPART, M. (1983) Concentrations and origins
of NO3- and NH4+ in oceanic air. In: CACGP Symposium on
Tropospheric Chemistry with Emphasis on Sulphur and Nitrogen
Cycles and the Chemistry of Clouds and Precipitation
(Abstracts), 28 August - 3 September 1983, Oxford, England,
Oxford, New York, Sydney, Paris, Frankfurt, Pergamon Press,
57 pp (Fifth International Congress of the Commission on
Atmoshperic Chemistry and Global Pollution).
SHAW, J. (1960) The absorption of sodium ions by the cray-
fish Astacus pallipes Lereboullet. III. The effect of other
cations in the external solution. J. exp. Biol., 37(3):
548-556.
SHEN, S.S. & STEINHARDT, R.A. (1978) Direct measurement of
intracellular pH during metabolic derepression of the sea
urchin egg. Nature (Lond.), 272(5650): 253-254.
SHERWOOD, T.K. (1925) Solubilities of sulfur dioxide and
ammonia in water. Ind. Eng. Chem., 17: 745-747.
SHILO, M. & SHILO, M. (1953) Conditions which determine the
efficiency of ammonium sulphate in the control of Prymnesium
parvum in fish breeding ponds. Appl. Microbiol., 1: 330-333.
SHILO, M. & SHILO, M. (1955) Control of the phytoflagellate
Prymnesium parvum. Verh. Int. Ver. Limnol., 12: 233-240.
SHOLKOVITZ, E. (1973) Interstitial water chemistry of the
Santa Barbara Basin sediments. Geochim. Cosmochim. Acta, 37:
2043-2073.
SHUTTLEWORTH, T.J. & FREEMAN, R.F. (1974) Factors affecting
the net fluxes of ions in the isolated perfused gills of
freshwater Anguilla dieffenbachii. J. comp. Physiol., 94:
297-307.
SIEGRIST, A. (1920) [Concentrated effect of alkali and acid
on the eye.] Z. Augenheilkd., 43: 176-194 (in German).
SILVER, S.D. & MCGRATH, F.P. (1948) A comparison of acute
toxicities of ethylene amine and ammonia to mice. J. ind. Hyg.
Toxicol., 30: 7-9.
SILVERMAN, L., WHITTENBERGER, J.L., & MULLER, J. (1949)
Physiological response of man to ammonia in low
concentrations. J. ind. Hyg. Toxicol., 31: 74-78.
SINGER, R.H. & MCCARTY, R.T. (1971) Pathologic changes
resulting from acute ammonium salt poisoning in sheep.
Am. J. Vet. Res., 32: 1239-1246.
SLOAN, C.H. & MORIE, G.P. (1974) Determination of ammonia in
tobacco and tobacco smoke with the ammonia electrode. Anal.
Chim. Acta, 69: 243-247.
SLOT, G.M.J. (1938) Ammonia gas burns: an account of six
cases. Lancet, 2: 1356-1357.
SMART, G. (1976) The effect of ammonia exposure on gill
structure of the rainbow trout (Salmo gairdneri). J. Fish
Biol., 8(6): 471-475.
SMITH, C.E. (1972) Effects of metabolic products on the
quality of rainbow trout. Am. Fishes US Trout News, 17(3):
7-8, 21.
SMITH, C.E. (1984) Hyperplastic lesions of the primitive
meninx of fathead minnows, Pimephales promelas, induced by
ammonia: species potential for carcinogen testing. Natl Cancer
Inst. Monogr., 65: 119-125.
SMITH, C.E. & PIPER, R.G. (1975) Lesions associated with
chronic exposure to ammonia. In: Ribelin, W.E. & Migaki, G.,
ed. The pathology of fishes, Madison, Wisconsin, University of
Wisconsin Press, pp. 497-514.
SMITH, L.W. & WHEELER, W.E. (1979) Nutritional and economic
value of animal excreta. J. anim. Sci., 48: 144.
SODERLUND, R. & SVENSSON, B.H. (1976) The global nitrogen
cycle. In: Svensson, B.H. & Söderlund, R., ed. Nitrogen,
phosphorus, and sulfur: global sources, Stockholm, pp. 23-76
(SCOPE Report No. 7) (Ecology Bulletin No. 22).
SOF'INA, E.P., VORONKOV, M.G., D'IAKOV, V.W., SOMENOVA, N.V.,
PLATONOVA, G.N., PERETOLCHINA, N.M., LESNAIA, N.A., &
SMETANKINA, O.Z. (1978) Tris (2-oxy-ethyl) ammonium salts of
some synthetic phytohormones and their antitumor activity.
Khim Farm. 2h, 12: 74.
SOLOMONSON, L.P. (1969) Effects of ammonia and some of its
derivatives on photosynthesis in the blue-green alga,
Plectonema boryanum, Chicago, Illinois, University of Chicago,
68 pp (Ph.D. Thesis).
SOUSA, R.J., MEADE, T.K., & FELBECK, G.T., Jr (1977) The
exposure of ammonia to the photodissociated haemoglobin of the
marine bloodworm, Glycera dibranchiata. Comp. Biochem.
Physiol., 58A: 19-22.
SPARKS, R.E. (1975) The acute, lethal effects of ammonia on
channel catfish (Ictalurus punctatus), bluegills (Lepomis
macrochirus), and fathead minnows (Pimephales promelas),
Chicago, Illinois, 15 pp (report to Illinois Institute for
Environmental Quality, project No. 20.060).
SPARKS, R.E. & SANDUSKY, M.J. (1981) Identification of
factors responsible for decreased production of fish food
organisms in the Illinois and Mississippi Rivers, Havana,
Illinois, River Research Laboratory, Illinois Natural History
Survey, 63 pp (Project No. 3-291-R).
SPICER, C.W. (1977) The fate of nitrogen compounds in the
atmosphere. In: Pitts, J.N., Jr, Metcalf, R.L., & Lloyd, A.C.,
ed. Advances in environmental sciences and technology, New
York, John Wiley and Sons, Vol. 7, pp. 163-261.
SPINAZZOLA, A., MARRACCINI, L., DEVOTO, G., & ZEDDA, S.
(1966) [Study of the behaviour of some volatile toxic
substances as an index of air pollution in the cith of
Cagliari. Note III. Ammonia.] Folia Med. (Naples), 49: 641-648
(in Italian).
STAMMER, H.A. (1953) [The effect of hydrogen sulfide and
ammonia on characteristic animal forms in the saprobiotic
system.] Vom Wasser, 20: 34-71 (in German).
STANLEY, R.A. (1974) Toxicity of heavy metals and salts to
Eurasian watermilfoil (Myriophylum spicatum). Arch. environ.
Contam. Toxicol., 2(4): 331-341.
STEINHARDT, R.A. & MAZIA, D. (1973) Development of
K+-conductance and membrane potentials in unfertilized sea
urchin eggs after exposure to NH4OH. Nature (Lond.),
241(5389): 400-401.
STEPHENS, E.R. (1971) Identification of odors from cattle
feedlots. Calif. Agric., 25: 10-11.
STEVENS, C., KENNAWAY, N.G., & FELLMAN, J.H. (1975) Ammonia
intoxicaton: A hazard during rehabilitation of protein-
deprived rats. J. Nutr., i105: 1384-1390.
STEVENSON, T.J. (1977) The effects of ammonia, pH and
salinity on the white perch, Morone americana, Kingston, Rhode
Island, University of Rhode Island, 154 pp (Ph.D. Thesis).
STEWART, B.A., VIETS, F.G., Jr, HUTCHINSON, G.L., & KEMPER,
W.D. (1967) Nitrates and other water pollutants under fields
and feedlots. Environ. Sci. Technol., 1(9): 736-739.
STRUZEWSKI, E.J. (1971) Current status on control and
treatment of storm and combined sewer overflow, Edison Water
Quality Laboratory (EPA Technology Transfer).
STUDIER, E.H. (1966) Studies on the mechanisms of ammonia
tolerance of the guano bat. J. exp. Zool., 163: 79-85.
STUDIER, E.H., BECK, L.R., & LINDBORG, R.G. (1967) Tolerance
and initial metabolic response to ammonia intoxication in
selected bats and rodents. J. Mammal., 48: 564-572.
SUGIYAMA, K., YOSHIDA, M., & OYAMADA, M. (1968) [A case of
chemical bronchiectasia induced by inhalation of ammonia gaz.]
Nippon Kyohbu Shikkan Gakkai Zasshi, 27: 797-803 (in Japanese).
SUKUMARAN, N. & KUTTY, M.N. (1977) Oxygen consumption and
ammonia excretion in the catfish Mystus armatus, with special
reference to swimming speed and ambient oxygen. Proc. Indian
Acad. Sci., 86B: 195-206.
SUMMERSKILL, W.H.J. & WOLPERT, E. (1970) Ammonia metabolism
in the gut. Am. J. clin. Nutr., 23: 633-639.
SUYAMA, M., KOIKE, J., & SUZUKI, K. (1960) Studies on the
glycolysis and the formation of ammonia in the muscle and
blood of elasmobranchs. J. Tokyo Univ. Fish., 46: 51-60.
SWIGERT, J.P. & SPACIE, A. (1983) Survival and growth of
warmwater fishes exposed to ammonia under low flow conditions,
Springfield, Virginia, National Technical Information Service
(NTIS PB83-257535).
TABATA, K. (1962) [Toxicity of ammonia to aquatic animals
with reference to the effect of pH and carbon dioxide.]
Hokoku, 34: 67-74 (in Japanese).
TAIGANIDES, E.P. & WHITE, R.K. (1969) The menace of noxious
gases in animal units. Trans. Am. Soc. Agric. Eng., 12:
359-362.
TAKAHASHI, H., WAKAI, K., & OHUCHI, K. (1984) [Experiences
of chemical burns induced by ammonia gaz.] Nippon Saigai
Igakkai Kaishi, 32: 741-749 (in Japanese).
TAPLIN, G.V., CHOPRA, S., YANDA, R., & ELAM, D. (1976)
Radionuclidic lung-imaging procedures in the assessment of
injury due to ammonia inhalation. Chest, 69: 5.
TAYLOR, A.W., EDWARDS, W.M., & SIMPSON, E.C. (1971)
Nutrients in streams draining woodland and farmland near
Coshocton, Ohio. Water Resour. Res., 7: 81-89.
TAYLOR, J.E. (1973) Water quality and bioassay study from
Crawford National Fish Hatchery. Trans. Nebr. Acad. Sci., 2:
176-181.
THE NETHERLANDS MINISTRY OF HOUSING, PHYSICAL PLANNING AND
ENVIRONMENT (1984) Acidification in the Netherlands: effects
and policies. In: Proceedings of a Conference on Air Pollution
in Europe, Munich, 24-27 June, 1984, The Netherlands Ministry
of Housing, Physical Planning and Environment, pp. 9.
THOMAS, W.H., HASTINGS, J., & FUJITA, M. (1980) Ammonium
input to the sea via large sewage outfalls. Part 2. Effects of
ammonium on growth and photosynthesis of southern California
phytoplankton cultures. Mar. environ. Res., 3(4): 291-296.
THOMPSON, R.Y. & HALLIBURTON, I.W. (1966) Effect of diet on
the composition of the kidney. Biochem. J., 99(3): 44.
THORNTON, N.C. & SETTERSTROM, C. (1940) Toxicity of ammonia,
chlorine, hydrogen cyanide, hydrogen sulphide, and sulphur
dioxide gases. III. Green plants. Contrib. Boyce Thompson
Inst., 11: 343-356.
THURSTON, R.V. (1981) Acute toxicity of ammonia to the
pumpkinseed (Lepomis gibbosus), Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research
Laboratory.
THURSTON, R.V. & MEYN, E.L. (1984) Acute toxicity of ammonia
to five fish species from the northwest United States,
Bozeman, Montana Fisheries Bioassay Laboratory, Montana State
University, 13 pp (Technical Report Vol. 84, No. 4).
THURSTON, R.V. & RUSSO, R.C. (1981) Acute toxicity of
ammonia to golden trout (Salmo aguabonita) and mottled sculpin
(Cottus bairdi), Bozeman, Montana, Fisheries Bioassay
Laboratory, Montana State University, 10 pp (Technical Report
Vol. 81, No. 1).
THURSTON, R.V. & RUSSO, R.C. (1983) Acute toxicity of
ammonia to rainbow trout. Trans. Am. Fish. Soc., 112: 696-704.
THURSTON, R.V., RUSSO, R.C., & SMITH, C.E. (1978) Acute
toxicity of ammonia and nitrite to cutthroat trout fry. Trans.
Am. Fish. Soc., 107(2): 361-368.
THURSTON, R.V., CHAKOUMAKOS, C., & RUSSO, R.C. (1981a)
Effect of fluctuating exposures on the acute toxicity of
ammonia to rainbow trout (Salmo gairdneri) and cutthroat trout
(S. clarki). Water Res., 15(7): 911-917.
THURSTON, R.V., PHILLIPS, G.R., RUSSO, R.C., & HINKINS, S.M.
(1981b) Increased toxicity of ammonia to rainbow trout (Salmo
gairdneri) resulting from reduced concentrations of dissolved
oxygen. Can. J. Fish. aquat. Sci., 38(8): 983-988.
THURSTON, R.V., RUSSO, R.C., & VINOGRADOV, G.A. (1981c)
Ammonia toxicity to fishes: Effect of pH on the toxicity of
the un-ionized ammonia species. Environ. Sci. Technol., 15(7):
837-840.
THURSTON, R.V., RUSSO, R.C., & PHILLIPS, G.R. (1983) Acute
toxicity of ammonia to fathead minnows. Trans. Am. Fish. Soc.,
112: 705-711.
THURSTON, R.V., LUEDTKE, R.J., & RUSSO, R.C. (1984a)
Toxicity of ammonia to fresh-water insects of three families,
Bozeman, Montana, Fisheries Bioassay Laboratory, Montana State
University, 26 pp (Technical Report Vol. 84, No. 2).
THURSTON, R.V., RUSSO, R.C., LUEDTKE, R.J., SMITH, C.E., MEYN,
E.L., CHAKOUMAKOS, C., WANG, K.C., & BROWN, C.J.D. (1984b)
Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish.
Soc., 113(1): 56-73.
THURSTON, R.V., MEYN, E.L., ZAJDEL, R.K., & RUSSO, R.C.
(submitted) Chronic toxicity of ammonia to fathead minnows
(Pimephales promelas). Submitted to Trans. Am. Fish. Soc.
TMRI (1971) [Atmosphere division. On survey of air
pollutants peculiar to factory-clustered area.] Tokyo
Meteorol. Res. Inst. Environ. Prot., 2: 109-111 (Annual
Report) (in Japanese).
TOMASSO, J.R., GOUDIE, C.A., SIMCO, B.A., & DAVIS, K.B.
(1980) Effects of environmental pH and calcium on ammonia
toxicity in channel catfish. Trans. Am. Fish. Soc., 109(2):
229-234.
TOPPING, D.C. & VISEK, W.J. (1976) Nitrogen intake and
tumourigenesis in rats injected with 1,2-dimethylhydrazine.
J. Nutr., 106: 1583.
TORDA, C. (1953) Ammonium ion content and electrical
activity of the brain during the preconvulsive and convulsive
phases induced by various convulsants. J. Pharmacol. exp.
Ther., 107: 197-203.
TOTH, B. (1972) Hydrazine, methylhydrazine, and
methylhydrazine sulfate carcinogenesis in Swiss mice. Failure
of ammonium hydroxice to interfere in the development of
tumors. Int. J. Cancer, 9: 109.
TOWLE, D.W., PALMER, G.E., & HARRIS, J.L. III (1976) Role of
gill Na+ + K+-dependent ATPase in acclimation of blue crabs
(Callinectes sapidus) to low salinity. J. exp. Biol., 196:
315-322.
TOWLE, D.W. & TAYLOR, D.D. (1976) Effect of NH4+ and K+ on
Na+-transport ATPase activity of blue crab gill Am. Zool., 16:
224.
TRESHOW, M. (1970) Ammonia. In: Environment and plant
response, New York, McGraw-Hill Book Company, pp. 362-363.
TURNBULL, H., DEMANN, J.G., & WESTON, R.F. (1954) Toxicity
of various refinery materials to fresh water fish. Ind. Eng.
Chem., 46(2): 324-333.
ULRICH, B. (1983) Soil acidity and its relation to acid
deposition. In: Effects of accumulation of air pollutants in
forest ecosystems, Ulrich and Pankrasth, The Netherlands,
D. Reidel Publishing Company, pp. 127-146.
ULSHAFER, T.R. (1958) The measurement of changes in
acetylcholine level (ACh) in rat brain following ammonium ion
intoxication and its possible bearing on the problem of
hepatic coma. J. lab. clin. Med., 52: 718-723.
ULTSCH, G.R., JACKSON, D.C., & MOALLI, R. (1981) Metabolic
oxygen conformity among lower vertebrates: the toadfish
revisited. J. comp. Physiol., 142: 439-443.
UNDERWRITERS LABORATORIES (1933) Report on the comparative
life, fire, and explosion hazards of common refrigerants.
Miscellaneous Hazard No. 2375, Chicago, Illinois, Underwriters
Laboratories, pp. 26-28.
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1963) Effects of
pollution on fish; toxicity of mixutures of zinc, sulfate, and
ammonium chloride; toxicity of sewage effluents; the toxicity
of poisons under estuarine conditions, London, United Kingdom
Ministry of Technology, pp. 76-83 (Water Pollution Research
1962).
UNITED KINGDOM MINISTRY OF TECHNOLOGY (1968) Effects of
pollution on fish, London, United Kingdom Ministry of
Technology, pp. 56-65 (Water Pollution Research 1967).
US EPA (1972) Air quality data for 1968 from the National
Air Surveillance Networks and contributing state and local
networks, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Office of Air Programs, 231
pp (Report No. APTD-0978).
US EPA (1979a) Ammonia study using STORET ambient monitoring
data, Washington DC, US Environmental Protection Agency,
Monitoring and Data Support Division, Office of Water Planning
and Standards.
US EPA (1979b) Methods for chemical analysis of water and
wastes, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring and Support Laboratory (Report No.
EPA 600/4-79-020).
US EPA (1981) Multimedia health assessment document on
ammonia, Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (Report No.
ECAO/82-DO10).
US EPA (1985) Ambient water quality criteria for ammonia -
1984, Duluth, Minnesota, US Environmental Protection Agency,
Office of Research and Development.
US NIOSH (1974) Criteria for a recommended standard.
Occupational exposure to ammonia, Washington DC, National
Institute for Occupational Safety and Health, pp. 74-136 (US
DHEW Publication No. 246-699).
US NIOSH (1977) NIOSH manual of analytical methods. Part I.
NIOSH monitoring methods, Washington DC, National Institute
for Occupational Safety and Health, Vol. 1, pp. 205.1-205.3
(US DHEW Publication No. 77-157-A).
UZVOLGYI, E. & BOJAN, F. (1980) Possible in vivo formation
of a carcinogenic substance from diethyl pyrocarbonate and
ammonia. J. Cancer Res. clin. Oncol., 97: 205.
VALENTINE, H. (1964) A study of the effect of different
ventilation rates on the ammonia concentrations in the
atmosphere of broiler houses. Br. Poult. Sci., 5: 149-159.
VAMOS, R. (1963) Ammonia poisoning in carp. Acta. biol.
Szeged., 9(1-4): 291-297.
VAMOS, R. & TASNADI, R. (1967) Ammonia poisoning in carp. 3.
The oxygen content as a factor influencing the toxic limit of
ammonia. Acta. biol. Szeged., 13(3-4): 99-105.
VAN AALST, R.M. (1984) [Atmospheric process and deposition,]
Ministry of Agriculture and Fisheries, Ministry of Housing,
Physical Planning and Environment, 76 pp (Report No. 84(2))
(in Dutch).
VAN BREEMEN, N. & JORDENS, E.R. (1983) Effects of
atmospheric ammonium sulfate on calcareous and non-calcareous
soils of woodlands in the Netherlands. In: Ulrich, B. &
Panlerath, J., ed. Effects of accumulation of air pollutants
in forest ecosystems, The Netherlands, Reidel Publishing
Company, pp. 171-182.
VANCURA, E.M., CLINTON, J.E., RUIZ, E., & KRENZELOK, E.P.
(1980) Toxicity of alkaline solutions. Ann. emergency Med.,
9(3): 118-122.
VAN DER EERDEN, L.J.M. (1982) Toxicity of ammonia to plants.
Agric. Environ., 7: 223-235
VAN SLYKE, D.D., PHILLIPS, R.A., HAMILTON, P.B., ARCHIBALD,
R.M., FUTCHER, P.H., & MILLER, A. (1943) Glutamine as source
material of urinary ammonia. J. Biol. Chem., 150: 481-482.
VAUGHN, R.E. & SIMCO, B.A. (1977) Effects of ammonia on
channel catfish, Ictalurus punctatus. ASB Bull., 24(2): 92.
VENKATARAMIAK, A., LAKSHMI, G.J., BEST, C., GUNTER, G.,
HARTWIG, E., & WILDE, P. (1981) Studies on toxicity of OTEC
plant components on marine animals from the Gulf of Mexico,
Springfield, Virginia, National Technical Information Service
(DE81030167).
VERBERK, M.M. (1977) Effects of ammonia in volunteers.
Int. Arch. occup. environ. Health, 39: 73 -81.
VIGLIANI, E.C. & ZURLO, N. (1955) [Experiences of the
Clinica del Lavoro with their own maximal exposure limits for
industrial chemicals.] Arch. Gewerbepathol. Gewerbehyg., 13:
528-534 (in German).
VINES, H.M. & WEDDING, R.T. (1960) Some effects of ammonia on
plant metabolism and a possible mechanism for ammonia
toxicity. Plant Physiol., 35: 820-825.
VINOKUROVA, M.K. & MAL'KOVA, V.B. (1963) [Toxicology of the
selective herbicide ammonium sulfamate.] Gig. Trud. Prof.
Zabol., 7(5): 56-57 (in Russian).
VISEK, W.J., KOLONDY, G.M., & GROSS, P.R. (1972) Ammonia
effects in cultures of normal and transformed 3T3 cells.
J. cell Physiol., 80: 373.
VISEK, W.J., CLINTON, S.K., & TRUEX, C.R. (1978) Nutrition
and experimental carcinogenesis. Cornell Vet., 68: 3.
VITTI, T.G., VUKMIROVICH, R., & GAEBLER, O.H. (1964)
Utilization of ammonia nitrogen, administered by intragastric,
intraperitoneal and subcutaneous roues: Effects of growth
hormone. Arch. Biochem. Biophys., 106: 475-482.
WALLEN, I.E., GREER, W.C., & LASATER, R. (1957) Toxicity to
Gambusia affinis of certain pure chemicals in turbid waters.
Sewage Ind. Wastes, 29(6): 695-711.
WALLINGFORD, G.W. (1977) Fate of nitrogen from fertilizer
practices. In: Disposal of residues on land, Rockville,
Maryland, Information Transfer, Inc., pp. 152-155.
WALSH, L.M. (1977) Updating the nitrogen cycle. In: Disposal
of residues on land, Rockville, Maryland, Information
Transfer, Inc., pp. 146-151.
WALTON, M.J. & COWEY, C.B. (1977) Aspects of ammoniogenesis
in rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol.,
57B: 143-149.
WALUGA, D. & FLIS, J. (1971) [Changes in peripheral blood in
carp ( Cyprinus carpio L.) under the influence of ammonia
solution.] Rocz. Nauk Roln. Ser. H, 93(2): 87-94 (in Russian).
WARNCKE, D.D. & BARBER, S.A. (1973) Ammonium and nitrate
uptake by corn ( Zea mays L.) as influenced by nitrogen
concentration and NH4-/NO3-ratio. Agric. J., 65: 950-953.
WARREN, K.S. (1958) The differential toxicity of ammonium
salts. J. clin. Invest., 37: 497-501.
WARREN, K.S. & NATHAN, D.G. (1958) The passage of ammonia
across the blood, brain, barrier and its relation to blood pH.
J. clin. Invest., 37: 1724-1728.
WARREN, K.S. & SCHENKER, S. (1964) Effect of an inhibitor of
glutamine synthesis (methionine sulfoximine) on ammonia
toxicity and metabolism. J. lab. clin. Med., 64: 442-449.
WARREN SPRING LABORATORY (1982) Acidity of rainfall in the
United Kingdom.
WATTS, R.L. & WATTS, D.C. (1974) Nitrogen metabolism in
fishes. In: Florkin, M. & Scheer, B.T. ed. Chemical Zoology,
New York, Academic Press, Vol. III, pp. 369-446.
WEAST, R.C., ed. (1979) CRC handbook on chemistry and
physics, 59th ed., Boca Raton, Florida, CRC Press Inc.
WEATHERBY, J.H. (1952) Chronic toxicity of ammonia fumes by
inhalation. Proc. Soc. Exp. Biol. Med., 81: 300-301.
WEBB, D.W., BARTLEY, E.E., & MEYER, R.M. (1972) A comparison
of nitrogen metabolism and ammonia toxicity from ammonium
acetate and urea in cattle. J. anim. Sci., 35: 1263-1270.
WEBB, J.T. & BROWN, G.W., Jr (1976) Some properties and
occurrence of glutamine synthetase in fish. Comp. Biochem.
Physiol., 54B: 171-175.
WEDDING, R.T. & VINES, H.M. (1959) Inhibition of reduced
diphosphopyridine nucleotide oxidation by ammonia. Nature
(Lond.), 184: 1226-1227.
WEEKS, M.E. (1968) Discovery of the elements, 7th ed.,
Washington DC, Journal of Chemical Education, p. 197.
WEHRBEIN, M.F., VIPPERMAN, P.E., Jr, PEO, E.R., Jr, &
CUNNINGHAM, P.J. (1970) Diammonium citrate and diammonium
phosphate as sources of dietary nitrogen for growing-finishing
swine. J. anim. Sci., 31: 327-332.
WHITE, A., HANDLER, P., & SMITH, E.L. (1973) Renal function
and the composition of urine. In: Principles of biochemistry,
5th ed., New York, McGraw Hill Book Co., pp. 604-704 and
928-948.
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series No.
539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
WICKINS, J.F. (1976) The tolerance of warm-water prawns to
recirculated water. Aquaculture, 9(1): 19-37.
WILKIN, D.C. & FLEMAL, R.C. (1980) Feasibility of water
quality improvement in three Illinois rivers. J. Water Poll.
Control Fed., 52(2): 293.
WILKINSON, R.H., THOMAS, W.J., HANSEN, C.M., & SHIELDS, E.A.
(1978) Forage preservation by timed-slow release of NH3 (ASAE
Paper No. 78-1523).
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968a) Toxicologic effects of ammonium carbamate and related
compounds. Am. J. vet. Res., 29: 987-906.
WILSON, R.P., DAVIS, L.E., HUMRER, M.E., & BLOOMFIELD, R.A.
(1968b) Comparative ammonia toxicity. Comp. Biochem.
Physiol., 25: 295-301.
WILT, F.H. & MAZIA, D. (1974) The stimulation of cytoplasmic
polyadenylyation in sea urchin eggs by ammonia. Dev. Biol.,
37: 422-424.
WINDHOLZ, M., BUDAVARI, S., STROUMTSOS, L.Y., & FERTIG, M.N.,
ed. (1976) Merck Index, 9th ed., Rahway, New Jersey, Merck
and Co. Inc., p. 69.
WINKLER, M.M. & GRAINGER, J.L. (1978) Mechanisms of action
of NH4Cl and other weak bases in the activation of sea urchin
eggs. Nature (Lond.), 273(5663): 536-538.
WOKER, H. & WUHRMANN, K. (1950) [The sensitivity of
different species of fish to ammonia, hydrocyanic acid and
phenol.] Rev. Suisse Zool., 57(3): 548-553 (in German).
WOLAVER, T.C. (1972) Distribution of natural and
anthropogenic elements and levels in precipitation across the
US: theory and quantitative models, Chapel Hill, North
Carolina, University of North Carolina, 75 pp (Prepared for US
EPA).
WOOD, R.W. (1979) Behavioral evaluation of sensory
irritation evoked by ammonia. Toxicol. appl. Pharmacol., 50:
157-162.
WORCEL, A. & ERECINSKA, M. (1962) Mechanism of inhibitory
action of ammonia on the respiration of rat liver
mitochondria. Biochim. Biophys. Acta, 65: 27-33.
WRIGHT, P.A. & WOOD, C.M. (1985) An analysis of branchial
ammonia excretion in the freshwater rainbow trout: effects of
environmental pH change and sodium uptake blockage.
J. exp. Biol., 114: 329-353.
WUHRMANN, K. & WOKER, H. (1948) [Contributions to the
toxicology of fishes. II. Experimental investigations on
ammonia and hydrocyanic acid poisoning.] Schweiz. Z. Hydrol.,
11: 210-244 (in German).
WUHRMANN, K., ZEHENDER, F., & WOKER, H. (1947) [Biological
significance for fisheries of ammonium-ion and ammonia content
of flowing bodies of water.] Vierteljahrsschr. Naturforsch.
Ges. Zurich, 92: 198-204 (in German).
YAHAGI, H., SUZUKI, K., & MATSUI, Y. (1959) [Fourteen cases
of ammonia gas poisoning.] Shuhkan Igaku Tsuhshin, 601: 26-30
(in Japanese).
YECK, R.G., SMITH, L.W., & CALVERT, C.C. (1975) Recovery of
nutrients from animal wastes. An overview of existing options
and potentials for use in feed. In: Managing livestock waste:
Proceedings of the Third International Symposium on Livestock
Wastes, St. Joseph, Michigan, American Society of Agricultural
Engineers, pp. 192-196.
YOSHIDA, J., NAKAME, K., & NAKAMURA, R. (1957) Toxicity of
urea and its control. II. Toxicity of ammonium salts and urea
in rabbits and goats. Nippon Chikusangaku Kaiho, 28: 185-191.
ZIEVE, L., DOIZAKI, W.M., & ZIEVC, F.J. (1974) Synergism
between mercaptans and ammonia or fatty acids in the
production of coma. A possible role for mercaptans in the
pathogenesis of hepatic coma. J. lab. clin. Med., 83: 16-28.
ZIMBER, A. (1970) Studies on the effect of ammonia on DNA
synthesis and on cellular proliferation and renewal in tissues
of the mouse and in Ehrlich ascites tumor cells, Ithaca,
New York, Cornell University (Ph.D. Thesis).
ZIMBER, A. & VISEK, W.J. (1972) Effect of urease injection
on DNA synthesis in mice. Am. J. Physiol., 223: 1004.
ZIMMERMAN, P.W. (1949) Impurities in the air and their
influence on plant life. In: Proceedings of the First National
Air Pollution Symposium, Pasadena, California, 10-11 November,
1949, pp. 135-141 (Sponsored by Stanford Research Institute).
ZLATEVA, M., VALCEVA, V., & ANTOV, G. (1974) Toxic organic
changes in laboratory animals placed in an ammonia-rich
working atmosphere. Tr. Inst. Hig. Ohrana Tr. Prof. Zabol.,
22(1): 147-154.
Annex I. Ammonium salts evaluations prepared by the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)a
------------------------------------------------------------------------------------------------------
Ammonium salt Functional use Evaluation status Reference
(ADI, MTDI)b,c
------------------------------------------------------------------------------------------------------
acetate pH-adjusting agent not specifiedd (grouped with WHO (1982a)
other ammonium salts and acetate)
alginate thickening agent, stabilizer 0 - 50 (grouped with alginic acid WHO (1974a,b)
and calcium, potassium, and
sodium alginate)
bicarbonate leavening agent, buffer not specified WHO (1982b)
(hydrogen carbonate) neutralizing agent, alkali
carbonate
chloride dough conditioner, yeast, food not specified (grouped with WHO (1980)
hydrochloric acid and magnesium
and potassium chlorides)
hydrogen phosphate buffering agent, dough MTDI:, 70 expressed as phosphorus WHO (1982a,b)
phosphate dibasic conditioner, leavening agent, (grouped with phosphates and
yeast polyphosphates, including
phosphates occurring naturally in
food)
hydroxide (strong alkali not specified WHO (1966)
ammonia solution)
lactate buffer, dough conditioner not specified WHO (1974a,b)
persulfate flour-treatment agent no ADI set WHO (1966)
------------------------------------------------------------------------------------------------------
a Queries concerning updated information should be addressed to: Joint WHO Secretary of the Joint
FAO/WHO Expert Committee on Food Additives, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland.
b ADI = Acceptable daily intake for man expressed as mg/kg body weight.
c MTDI = Maximum tolerable daily intake for man, expressed as mg/kg body weight.
d ADI not specified = the total intake of the substance arising from its uses at the levels necessary
to achieve the desired effect does not represent a hazard to health.
REFERENCES TO ANNEX I
WHO (1966) Specifications for the identity and purity of
food additives and their toxicological evaluation: some
antimicrobials, antioxidants, emulsifiers, stabilizers, flour-
treatment agents, acids, and bases. Ninth Report of the Expert
Committee, Geneva, World Health Organization (WHO Technical
Report Series No. 339).
WHO (1974a) Toxicological evaluation of certain food
additives including anticaking agents, antimicrobials,
antioxidants, emulsifiers, and thickening agents, Geneva,
World Health Organization (WHO Food Additives Series No. 5).
WHO (1974b) Toxicological evaluation of certain food
additives with a review of general principles and of
specification. 17th report of the Expert Committee, Geneva,
World Health Organization (WHO Technical Report Series
No. 539).
WHO (1980) Evaluation of certain food additives.
Twenty-third Report of the Expert Committee, Geneva, World
Health Organization (WHO Technical Report Series No. 648).
WHO (1982a) Evaluation of certain food additives and
contaminants. Twenty-sixth Report of the Expert Committee,
Geneva, World Health Organization (WHO Technical Report Series
No. 683).
WHO (1982b) Toxicological evaluation of certain food
additives, Geneva, World Health Organization (WHO Food
Additives Series No. 17).
ANNEX II: TREATMENT OF EXCESSIVE EXPOSURE TO AMMONIA
Ammonia (gas and liquid) is an extremely irritant chemical
affecting the skin, eyes, and the respiratory tracts. Ammonia gas
can produce burning of the eyes, lachrymation, and severe eye
damage. When inhaled it can produce coughing, laryngitis,
bronchitis, chest pains, and severe respiratory problems. Contact
with liquid ammonia can result in severe eye and skin burns due
both to its irritant properties and chilling effect.
Those working with ammonia should be trained in its safe use
including the dangers of improper handling, the use of protective
equipment, and the avoidance of unnecessary inhalation of the gas
and direct contact with liquid ammonia. After handling liquid
ammonia, the hands should be washed thoroughly before eating or
smoking.
The provision of protective clothing and equipment is not an
adequate substitute for safe working conditions. However, where
exposure cannot be adequately controlled, workers should be
provided with suitable impervious clothing, boots and gloves, and,
depending on the severity of the conditions, a face shield or
safety goggles and a mask or self contained breathing apparatus.
In places where very high gaseous ammonia concentrations are
expected, complete gas suits should be used.
Emergency showers and eye wash or water sprays should be
provided in all areas where ammonia is handled and where leaks,
spills or splashes may occur. Clothes contaminated with ammonia
should be discarded immediately and not worn again until thoroughly
cleansed.
First aid
If excessive exposure has occurred first aid treatment should
be promptly initiated and medical advice obtained as soon as
possible.
Ammonia in the eye (gas, liquid, or liquor)
Ammonia in the eye may cause severe injury and must be treated
immediately by irrigation for at least 15 min with flowing water or
sterile buffered eye irrigation solution.
Ammonia on the skin (liquid or liquor)
Drench the affected area with water and remove contaminated
clothing and footwear. Wash the affected area continuously for 5 -
10 min or until pain ceases.
Ingestion (liquid or liquor)
If the patient is conscious large quantities of water may be
given to dilute the chemical in the stomach. No attempt should be
made to induce vomiting.
Inhalation of gas/vapour
1. Remove from exposure, secure airway and place in semiprone
recovery position if unconscious. Give artificial
respiration if not breathing.
2. If heartbeat is absent give external cardiac massage.
3. If there is cyanosis (blueness of lips) or air-hunger
administer oxygen by facemask.
4. A conscious patient may be given water to drink.
Further treatment
Ammonia in the eyes
Corneal damage is probable. Use local anaesthetics and
cycloplegics to enable thorough irrigation and examination. If the
cornea is damaged, administer topical antibiotics. Refer to a
specialist centre.
Ammonia on the skin
Treat as a chemical burn. Liquid ammonia may produce deep
burns that may require grafting. Refer deep or extensive burns to
a specialist centre.
Inhalation of gas/vapour
1. Ammonia is irritant to the respiratory tract causing:
(a) bronchial oedema, spasm, and hypersecretion
resulting in chest tightness, wheeze, and cough,
which may progress to severe dyspnoea; and
(b) lower airway inflammation with exudative
pulmonary oedema and impaired gaseous
diffusion. Symptoms may be delayed 24 h or
more. Resolution may be by fibrosis producing a
restrictive defect.
2. Treat hypoxia with oxygen, ventilation, and bronchial
lavage, as appropriate.
3. Consider administration of steroids by multiple metered
doses of topical aerosol, by inhalation, and/or by
injection. Early prophylactic use may be indicated.
4. Administer bronchodilators by inhalation or injection, as
indicated. Maintain with oral treatment.
5. Keep under medical surveillance for at least 48 h. Treat
symptomatically.
6. Observe for secondary respiratory infection and treat as
necessary.
