
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
COMMISSION OF THE EUROPEAN COMMUNITIES
ENVIRONMENTAL HEALTH CRITERIA 59
PRINCIPLES FOR EVALUATING RISKS FROM
CHEMICALS DURING INFANCY AND EARLY
CHILDHOOD: THE NEED FOR A SPECIAL APPROACH
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, the World Health Organization, or the Commission
of the European Communities and persons acting on its behalf.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization, and on behalf of
the Commission of the European Communities
World Health Orgnization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154259 4
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1986
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
PRINCIPLES FOR EVALUATING HEALTH RISKS FROM CHEMICALS DURING
INFANCY AND EARLY CHILDHOOD: THE NEED FOR A SPECIAL APPROACH
PREFACE
SUMMARY OF THE REPORT
SUMMARY OF RECOMMENDATIONS
1. INTRODUCTION
1.1. Objective
1.2. Definitions
1.2.1. Infant and young child
1.2.2. Chemical
1.3. Physiological basis for concern
1.3.1. Small size and large surface area in relation to
weight
1.3.2. The higher metabolic rate and hence higher
consumption of oxygen and intake of air per unit
body weight in the infant compared with the adult
1.3.3. Rapid growth
1.3.4. Different body composition from adult
1.3.5. Functional immaturity of the organs and systems of
the body
1.3.6. Breast milk, infant formulae, and water as sources
of chemicals
1.4. Applicability of data on experimental animals to
human infants and young children
1.4.1. Some similarities in growth and development
between man and experimental animals
1.4.2. Some differences in growth and development
between man and experimental animals
1.5. Conclusions
2. PATHWAYS OF EXPOSURE
2.1. Introduction
2.2. The alimentary tract
2.2.1. Milk
2.2.1.1 Human milk
2.2.1.2 Other types of milk
2.2.1.3 Infant feeds
2.2.1.4 Water
2.3. The respiratory tract
2.4. The skin
2.5. Conclusions
3. KINETICS OF ABSORPTION, BIOTRANSFORMATION, AND ELIMINATION
3.1. Introduction
3.2. Absorption
3.2.1. Absorption from the gastrointestinal tract
3.2.2. Absorption from the lungs
3.3. Tissue distribution
3.3.1. Binding of chemicals to proteins
3.3.2. Distribution within the body
3.4. Biotransformation of organic chemicals
3.5. Elimination from the body
3.5.1. Elimination by the kidneys
3.5.2. Elimination by the liver
3.5.3. Elimination by other routes
3.6. Conclusions
4. EFFECTS OF CHEMICALS IN THE BODY
4.1. Introduction
4.2. Effects of chemicals on general growth and development
4.3. Effects of chemicals on some organs and systems
4.3.1. Nervous system
4.3.2. Kidneys
4.3.3. Liver
4.3.4. Lungs
4.3.5. Haematopoietic system
4.3.6. Immune system
4.3.7. Endocrine system
4.3.8. Skin
4.3.9. Bones and teeth
4.4. Carcinogenesis
4.5. Conclusions
5. MODIFYING FACTORS
5.1. Nutrition
5.2. State of health
5.3. Social and cultural way of life
5.4. Conclusion
6. GENERAL CONCLUSIONS
7. RECOMMENDATIONS
REFERENCES
APPENDIX I
1. Introduction
2. Assessment of the importance of chemicals in breast milk
2.1. Source of chemicals
2.2. Chemicals within the mother's body and their secretion in
milk
2.3. Development and limits of methods for measuring chemicals
in milk
2.4. Risks to the infant
2.5. Assessment of contamination of breast milk following
maternal exposure
2.5.1. Method of collection of breast milk
3. Conclusions
REFERENCES TO APPENDIX I
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
PARTICIPANTS OF MEETINGS
c,d Dr L. Amin-Zaki, Abu Dhabi, United Arab Emirates
(formerly Professor of Paediatrics, University of
Baghdad, Iraq)
c Dr V.N. Anisimov, Laboratory of Experimental Tumours,
N.N. Petrov Research Institute of Oncology,
Leningrad, USSR
b,c Professor E.A. Bababunmi, Laboratory of Biomembrane
Research, Department of Biochemistry, University of
Ibadan, College of Medicine, Ibadan, Nigeria
a,b,c Professor D. Barltrop, Westminster Children's
Hospital,London, United Kingdom (Rapporteur)
a Dr G. Becking, Health and Welfare, Ottawa, Canada (on
temporary assignment to WHO Regional Office for
Europe, Copenhagen, Denmark)
a Dr A. Bernard, Unit of Industrial and Medical
Toxicology, Faculty of Medicine, Catholic University
of Louvain, Brussels, Belgium
a Professor B. Clayton, Department of Biochemistry,
University of Southampton, Southampton, United
Kingdom
d Dr R.L. Dixon, Laboratory of Reproductive and
Developmental Toxicology, National Institute of
Environmental Health Sciences, Research Triangle
Park, North Carolina, USA
a Dr O.P. Ghai, Department of Paediatrics, All India
Institute of Medical Sciences, New Delhi, India
b Dr A.A. Jensen, National Institute of Occupational
Health, Hellerup, Denmark
a,c Dr J.H.P. Jonxis, Groningen University, Groningen, The
Netherlands
a Dr S.K. Kashyap, National Institute of Occupational
Health, Gujarat, India
a,b Professor F.H. Kemper, Institute of Pharmacology and
Toxicology, University of Münster, Münster, Federal
Republic of Germany
b,c Dr R.D. Kimbrough, Toxicology Branch, Clinical
Chemistry Division, Centre for Disease Control,
Atlanta, Georgia, USA
b,c Professor W. Klinger, Institute of Pharmacology and
Toxicology, Jena, German Democratic Republic
Participants (contd.)
a Dr B.A. Kolygin, Department of Oncopaediatrics, Petrov
Research Institute of Oncology, Leningrad, USSR
b,c Professor W. Koransky, Institute of Toxicology, Philipps
University, Marburg, Federal Republic of Germany
a,b,c Professor K. Kostial, Institute of Medical Research
and Occupational Health, Zagreb, Yugoslavia (Vice-
Chairman)
c Dr N. Lery, Centre de Toxicovigilance, Hospital E.
Herriot, Lyons, France
a,c Dr L. Macho, Institute of Experimental Endocrinology,
Slovak Academy of Sciences, Bratislava, Czechoslovakia
a,c Dr R.A. McCance, University of Cambridge, Cambridge,
United Kingdom
b Professor D. Neubert, Institute of Toxicology and
Embryo-Pharmacology of the University of Berlin,
Berlin (West)
a,b Dr S. Ostrowski, European Association for Studies on
Nutrition and Child Development, Paris, France
c Dr R. Parini, Department of Paediatrics, University
of Milan Medical School, Milan, Italy
b Dr P.K. Ray, Industrial Toxicology Research Centre,
Lucknow, India
a Dr H.H. Sandstead, US Department of Agriculture,
Agricultural Research Service, North Central Region,
Human Nutrition Research Centre, Grand Forks, North
Dakota, USA
b Dr L. Sann, Debrosse Hospital, Lyons, France
a Professor F. Sereni, Department of Paediatrics,
University of Milan Medical School, Milan, Italy
b,c Dr R.J. Smialowicz, Health Effects Research Laboratory,
US Environmental Protection Agency, Research
Triangle Park, North Carolina, USA
a Dr E.M. Smith, Albright & Wilson Ltd., London, United
Kingdom
c Dr H. Sourgens, Institute of Pharmacology and
Toxicology, University of Münster, Münster, Federal
Republic of Germany
Participants (contd.)
a Dr A.G. Spinola, Department of Preventive Medicine
UFBa, Bahia, Brazil
b Dr A. Ueki, Pharmaceuticals and Chemicals Safety
Division, Pharmaceutical Affairs Bureau, Ministry of
Health and Welfare, Tokyo, Japan
a Dr D. Wassermann, Department of Occupational Health,
Hebrew University, Hadassah Medical School,
Jerusalem, Israel
a,b,c Dr E. Widdowson, Department of Medicine, Addenbrooke's
Hospital, Cambridge, United Kingdom (Chairman)
b,c Dr S. Wood, Department of Labour, Dublin, Ireland
a,c Professor T. Yoshimura, University of Occupational and
Environmental Health, Department of Clinical
Epidemiology, Kitakyushu, Japan
Representatives of other Organizations
c Dr V. Morgenroth, Chemicals Division, Environment
Directorate, Organisation for Economic Cooperation
and Development, Paris, France
Joint Secretariat
b Dr M. Belsey, Maternal and Child Health, World
Health Organization, Geneva, Switzerland
a,b,c Dr A. Berlin, Health and Safety Directorate,
Commission of the European Communities, Luxembourg
(Co-Secretary)
b,c Dr J. Facer, Department of Health and Social Security,
London, United Kingdom
a Dr B. MacGibbon, Department of Health and Social
Security, London, United Kingdom
b Dr M. Mercier, International Programme on Chemical
Safety, World Health Organization, Geneva,
Switzerland
a,b,c Dr J. Parizek, International Programme on Chemical
Safety, World Health Organization, Geneva,
Switzerland (Co-Secretary)
a Dr P.M. Shah, Maternal and Child Health, World Health
Organization, Geneva, Switzerland
Joint Secretariat (contd.)
b,c Dr S. Tarkowski, Regional Officer for Toxicology, WHO
Regional Office for Europe, Copenhagen, Denmark
---------------------------------------------------------------------------
a Attended the IPCS Working Group/CEC Workshop in Cambridge,
20-24 June 1983.
b Attended the IPCS/CEC Follow-up Consultation in
Luxembourg, 13-17 December 1984.
c Attended the IPCS/CEC Task Group Meeting in Cambridge,
19-23 March 1985.
d Prepared a background paper for the IPCS Working Group/CEC
Workshop in Cambridge, 20-24 June 1983, but was unable to
attend.
PREFACE
The preparation of this document was initiated and organized
jointly by the UNEP/ILO/WHO International Programme on Chemical
Safety (IPCS) and the Commission of the European Communities (CEC)
with the active support of the Department of Health and Social
Security (DHSS) as part of the United Kingdom's contribution to the
IPCS.
The IPCS and CEC, concerned with the need to identify
approaches and to develop methodologies for the protection of
sensitive segments of the population from the health risks due to
exposure to chemicals, agreed to convene an IPCS Working Group/CEC
Workshop to discuss the principles for evaluating health risks from
chemicals during infancy and early childhood. DR E. WIDDOWSON,
FRS, Cambridge made the local arrangements for the first IPCS/CEC
meeting in Cambridge at Sidney Sussex College, 20-24 June 1983.
Twelve background papers were prepared by the participants for the
meeting.
At this meeting, the aims were to assess the importance of the
problem; develop interim conclusions; draw up an outline for a
publication; and establish a workplan for its preparation. The
meeting was opened by Dr J. Parizek on behalf of the IPCS and by
Dr A. Berlin on behalf of the CEC. Dr B. MacGibbon welcomed the
participants on behalf of the DHSS. Dr E. Widdowson was elected
Chairman and Professor K. Kostial Vice-Chairman. The Chairman
designated Professor D. Barltrop as the Rapporteur for the final
publication to be prepared after the meeting, and Dr H. Sandstead
as the Rapporteur for the interim conclusions. DR E. WIDDOWSON,
FRS, agreed to provide scientific guidance throughout the project.
The individual sections of the document prepared by the
participants on the basis of the discussions at the above meeting
were reviewed and completed at an IPCS/CEC follow-up Consultation
held at the invitation of the Health and Safety Directorate of the
Commission of the European Communities (CEC), in Luxembourg, 13-17
December 1984.
The complete text prepared by the Chairman, DR E. WIDDOWSON,
FRS, and the Rapporteur, PROFESSOR D. BARLTROP, was then considered
and finalized at the IPCS/CEC Task Group meeting held in Cambridge
at Sidney Sussex College, 19-23 March 1985. This meeting was again
hosted and financially supported by the Department of Health and
Social Security (DHSS) as part of the United Kingdom's contribution
to the IPCS.
The work of all the participants, in particular that of DR E.
WIDDOWSON, FRS, and PROFESSOR D. BARLTROP, is highly appreciated,
and the financial support of the Department of Health and Social
Security is gratefully aknowledged.
SUMMARY OF THE REPORT
1. The infant and young child have different structural and
functional characteristics from those of the older child and adult.
These represent stages in normal growth and development and may
affect their vulnerability when exposed to chemicals. They
include:
- larger body surface area in relation to weight;
- higher metabolic rate and oxygen consumption and
hence greater intake of air per unit body weight;
- different body composition;
- greater energy and fluid requirements per unit body
weight;
- special dietary needs, including the dependence of
the infant on milk.
2. Chemicals may enter the body of the infant and young child
through the alimentary tract, the respiratory tract, or the skin.
Those that traverse the mammary gland are potentially important
contaminants of human and artificial milks. Water used in the
preparation of infant formulae is also a possible source of
chemicals for the young infant. Special behaviour characteristics
of young children are important in determining exposure to
chemicals.
3. Generally speaking, chemicals, both organic and inorganic, are
absorbed more readily by the infant than by the adult. The organic
compounds undergo biotransformation less readily in the infant, and
the kidneys are immature and less able than those of the adult to
excrete chemicals, whether inorganic or the polar products of
biotransformation. Thus, a greater proportion of a similar dose of
a chemical per unit body weight is likely to accumulate in the body
of the infant compared with that in the older child or adult.
4. Exposure to chemicals during early postnatal development can be
associated with dose-effect and dose-response relationships, which
differ from those resulting from exposure in later years.
The exposure of the infant to chemicals can give rise, not only
to immediate effects, but also to manifestations due to the
disturbed maturation of organ systems and their altered response to
other environmental influences.
5. Variations that exist in the health and nutritional status
of children reared in different social and cultural environments
may influence exposure and modify response to chemicals in the
environment. Recognition of this will assist in the identification
of children at risk and enable appropriate preventive and remedial
measures to be adopted.
SUMMARY OF RECOMMENDATIONS
1. When health risks from chemicals are evaluated, the special
characteristics of infants and young children must be recognized.
Health care workers should be made aware of these characteristics.
2. The development of a strategy relating to chemicals detected in
breast milk requires a thorough appraisal of the merits and
disadvantages of the available options. The risks of continued
exposure to a chemical through breast feeding have to be balanced
against the risks of infection or nutritional deprivation, where
breast feeding is curtailed or discontinued.
3. Clinical and epidemiological data should be collected following
the inadvertent exposure of infants and young children to
chemicals.
4. Developmental toxicology should be promoted and the methodology
improved.
1. INTRODUCTION
1.1 Objective
The main objective of the Group concerned in the preparation of
this report was to investigate the need for specific approaches in
evaluating the health risks associated with exposure to chemicals
(both organic and inorganic) during infancy and childhood, on the
basis of a review of postnatal physiology, metabolism, and special
characteristics of exposure.
1.2 Definitions
1.2.1 Infant and young child
It was agreed to follow the recognized definitions for infant
and young child. The neonatal period extends from birth to 4
weeks, infancy up to one year, and young childhood from 1 to 5
years. It was recognized, however, that account should also be
taken of the physiological stages of development and of the mode
of nutrition, exclusive liquid feeding (breast or bottle) or mixed
feeding. The age of transition from one type of feeding to the
other depends significantly on social customs.
1.2.2 Chemical
In the context of this report, a chemical is taken to mean any
substance, whether organic or inorganic, irrespective of toxicity,
other than well recognized nutrients or drugs, that may enter the
infant's or child's body.
1.3 Physiological Basis for Concern
There are many ways in which infants and young children differ
from older children and adults, and these differences may affect
their vulnerability, when exposed to chemicals.
1.3.1 Small size and large surface area in relation to weight
A new-born infant weighing 3.5 kg is one-twentieth of the
weight of a 70-kg man, but the surface area of the infant is one-
eighth as great. Thus, the area of skin that could be exposed to a
chemical is 2.5 times as great per unit body weight in a naked
child as in a naked adult.
1.3.2 The higher metabolic rate and hence higher consumption of
oxygen and intake of air per unit body weight in the infant
compared with the adult
Because infants and young children have a larger cooling
surface per unit body weight than adults, and because they are
growing rapidly, they have a higher resting metabolic rate and rate
of oxygen consumption per unit body weight. The oxygen consumption
of an infant aged between one week and one year, at rest, in a
thermoneutral environment, is about 7 ml/kg body weight per min
(Hill, 1964); that of an adult, under the same conditions, is 3.5
ml/kg per min. Thus, the volume of air passing through the lungs
of the resting infant is twice that of the resting adult per unit
body weight and therefore twice as much of any chemical in the
atmosphere would reach the lungs of the resting infant compared
with that reaching the lungs of the adult per unit body weight, in
the same period of time.
The ability of an infant to increase its metabolic rate and
consumption of oxygen and air is limited to 2 - 3 times the basal
level (Hill, 1964). The metabolic rate in an adult increases much
more during heavy exercise, but this degree of activity is not
usually continued for long periods of time. An adult weighing 70 kg
and expending energy equivalent to 3000 kcal in 24 h consumes
oxygen at the rate of 6.2 ml/kg body weight per min, which is 1.8
times the adult's basal rate, but less than the infant's basal
level. An environ-mental temperature below the thermoneutral one
increases the metabolic rate and the requirement for oxygen. Since
the thermoneutral temperature is higher for infants than for
adults, the same moderately low environmental temperature will
cause an increase in the oxygen consumption of the infant before
that of the adult, and, in fact, cold and crying are the main
causes of an increase in oxygen consumption above the basal level
in young infants. As the infants become older and more active,
muscular activity plays a more important part.
1.3.3 Rapid growth
The infant gains weight more rapidly during the first 4 - 6
months after birth than during the rest of its life. This is
illustrated in Fig. 1 and 2, where the body weights published by
Tanner et al. (1966) have been used for constructing the curves.
Fig. 1 shows the increment in weight per month between birth and 18
years, and illustrates the fact that a male infant gains more
weight per month during the first 4 - 6 months than a boy does at
the height of the puberty growth spurt. The increment in weight of
the female infant is essentially similar, but the gain per month
during adolescence is less for a girl than for a boy. This greater
increment in weight per month in infancy is being added to a much
smaller body than the lesser increment in the adolescent and when
the gains in weight per month are expressed as g/kg weight at the
beginning of the month (Fig. 2), the very high rate of growth of
the infant is even more striking. The organs as well as the major
tissues of the body participate in this rapid growth (Table 1).
Chemicals reaching the body by any route, and capable of
accumulating in the body during infancy, may, in some instances, be
incorporated into the body tissue in greater amounts than later on
in childhood, when growth is slower. This is particularly true if
the chemical is taken in by an infant on a long-term basis. It may
be argued that a single dose of a chemical that is not acutely
toxic clinically may be less harmful for the infant than the same
dose per unit body weight would be for the older child or adult,
because the chemical would be more rapidly diluted. Clearly, the
ability to eliminate substances from the body is also important,
and this is discussed in section 3. There is also the possibility
that the chemical within the body may itself hinder growth.
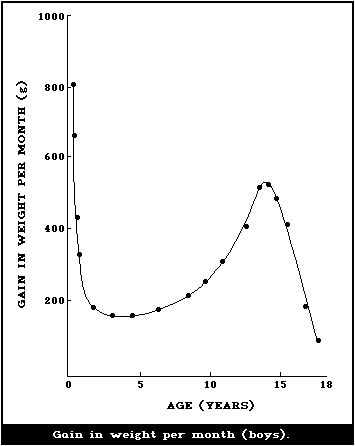
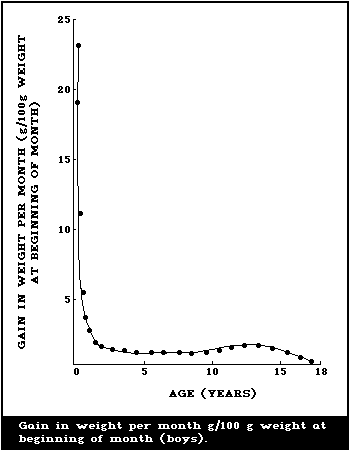 Table 1. Increase in weight of body and
organs during the first 9 months after
birtha
-----------------------------------------
Body/organ Increase
(g/kg weight at birth)
-----------------------------------------
Body 1810
Brain 1480
Liver 1420
Kidneys 1580
Heart 1160
Lungs 1500
-----------------------------------------
a Based on: values given in Diem & Lentner (1970).
The constituents of some organs and tissues have a slower rate
of turnover than those of others. The lipids, for example, once
laid down, have a slow rate of turnover compared with protein, in
all parts of the body including the brain. Fat-soluble chemicals,
which might be incorporated into brain lipids during the rapid
growth of the brain in infancy (Dobbing & Sands, 1973), would not
enter the tissue in significant amounts after the growth of the
brain has ceased. The fatty acid composition of brain lipids of
rats and guinea-pigs can be appreciably altered, early in life, by
the nature of the fat in the diet of the mother during pregnancy
and lactation (Svennerholm et al., 1972; Pavey & Widdowson, 1980).
Information on man is lacking.
1.3.4 Different body composition from adult
Infants have a different body composition from adults. As in
adults, the contribution of fat to the body weight varies
considerably between one individual and another in infants, but the
average is about 15% in full-term new-born infants and in adult
men. However, infants have a higher percentage of water and a
smaller percentage of solids in the lean body tissue. The infant,
at term, has 82% of water in its lean tissue compared with 72% in
the adult (Widdowson & Dickerson, 1964). The additional water in
the infant is mainly extracellular, the volume of extracellular
fluid per unit body weight in the young infant being about twice
that in an adult (Widdowson & Dickerson, 1964). Thus, any chemical
that is confined to the extracellular compartment of the body will
have a greater volume in which to distribute itself per unit body
weight. The advantage of this for the infant could be offset by
the slower rate of excretion by the kidneys (section 3).
The percentage of water in the organs and tissues, and thus in
the body as a whole, decreases with age. The levels of water in
some organs at birth, at 6 - 12 months where figures are available,
and in the adult (Widdowson & Dickerson, 1964) are shown in Table
2. The decrease in water in the liver, kidneys, heart, and lungs
is due primarily to an increase in protein; in the brain, it is due
to an increase in myelin.
Table 2. Water in organs and tissues (g/kg)a
-------------------------------------------------
Organ/tissue At birth 6 - 12 Adult
months
-------------------------------------------------
Whole body (fat-free 820 780 720
basis)
Brain 900 830 770
Liver 780 760 710
Kidneys 840 - 810
Heart 840 830 830
Lungs 860 - 790
Skeletal muscle
Total 800 780 790
Extracellular 350 290 180
Intracellular 450 490 610
-------------------------------------------------
a From: Widdowson & Dickerson (1964).
Another difference between infants and adults is that most of
the cells of the organs and tissues of infants are smaller than
those of adults (Widdowson et al., 1972). Small cells, like small
bodies, have a larger surface area in relation to mass than bigger
cells and bodies, and this in itself may have important
implications for chemicals that may enter the cells.
The bones of an infant contain more water and less fat,
protein, and mineral than adult bones. This is illustrated in
Table 3 for the whole femur (Dickerson, 1962b). Unlike soft
tissues, the chemical maturation of the bone, as evidenced by the
decrease in percentage of water and increase in degree of
calcification, mainly takes place after 1 - 2 years. From the
values in Table 3, it can be calculated that the increment per
month of calcium in one femur is approximately 0.2 g between birth
and 18 months, 0.48 g between 18 months and 11 years, and 1.0 g
between 11 and 18 years. The femur may not be completely typical
of all parts of the skeleton, but it does seem likely that the rate
of deposition of chemicals taken up by the bone along with calcium,
and indeed with magnesium, sodium, and other metals present in
bone, is likely to be greater in later childhood than in infancy.
However, the bones of infants are smaller, and the rate of
deposition of calcium per kg of bone per month is greater in
infancy, being 7.8 g/kg bone between birth and 3 months, 6.8 g/kg
between 3 and 18 months, and 1.9 g/kg bone between 18 months and 18
years. Thus, whether the bones of infants are considered likely to
take up more or less of a chemical than those of older children
depends partly on the method of expression of the rate of
deposition.
Table 3. Composition of the whole femur of the infant, child, and adulta
---------------------------------------------------------------------
At birth 3 months 1 - 2 11 - 12 Adult
(full- (milk years years (pre-
term) feeding) (weaned) adolescent)
---------------------------------------------------------------------
Weight femur g 16.6 26.4 63.7 326 646
Fat g/kg fresh bone 1.4 6.5 75 26 31
Composition g/kg
fresh fat-free bone
Water 639 641 554 364 227
Collagen 93 107 141 163 173
Ca 61 54 70 138 194
P 28 27 33 63 83
---------------------------------------------------------------------
a From: Dickerson (1962b).
1.3.5 Functional immaturity of the organs and systems of the body
Most organs and systems of the body have not reached structural
or functional maturity at birth. Development of the nervous system
continues in postnatal life, much of the myelination of the brain
takes place after birth and continues until adolescence (Hoar &
Monie, 1981). The structural development of the lung also
continues postnatally with an increase in alveolar surface area
(Langston, 1983). Several components of the immune system are not
fully developed at birth, for example, the lymphocytes responsible
for producing antibodies, and it has been suggested that this is
responsible for the greater susceptibility of the new-born infant
to certain bacterial infections (Andersson et al., 1981). The
gastrointestinal, endocrine, and reproductive systems, and also
renal function, are all immature at birth. The kidneys are less
able than those of older children and adults to eliminate excessive
amounts of substances normally present in milk, for example, sodium
and phosphate (Dean & McCance, 1947a, 1948; Barltrop & Oppé, 1970),
and this may also apply to extraneous chemicals present in, and
absorbed from, milk. Moreover, the activities of many liver enzyme
systems that metabolize endogenous and exogenous substances may be
low in the new-born infant. These topics are covered in more
detail in section 3.
1.3.6 Breast milk, infant formulae, and water as sources of
chemicals
The young infant lives on a single food, milk, either from the
breast or the bottle. Thus, the presence of a chemical in breast
milk, or in the powder or water used to make up an infant formula,
is likely to have greater implications for the infant than for the
older child having mixed feeding, where milk forms only part of the
daily food. Furthermore, infants take in a much larger amount of
fluid per unit body weight than older children and adults. An
infant living on milk takes about one-seventh of its own weight of
water each day, which would correspond to 10 litres for a 70-kg
man. This large intake is necessary for the infant, because it
loses more water per kg body weight through the lungs, owing to the
greater passage of air through them, the skin, because of the
larger surface area, and the kidneys, because of the inability to
concentrate the urine to the same extent as the older child or
adult. Any chemical present in the water used to make up infant
formulae will, therefore, be taken in by an infant in larger
quantities per unit body weight than by an older child or adult
using the same water supply. This is discussed in more detail in
section 2.
Nowadays, it is common practice among manufacturers to add
vitamins and trace elements to their preparations. Occasionally,
these have been added in such large amounts that they have been
reported to be toxic, even though in smaller, physiological amounts
they are essential. Examples are vitamin D (British Paediatric
Association, 1956) and manganese (Collipp et al., 1983).
1.4 Applicability of Data on Experimental Animals to Human Infants
and Young Children
Data on the toxic effects of chemicals in man are frequently
incomplete or even absent, and this is particularly true for
infants and young children. Some insight can be gained from
observations on young animals, but any assessment of human risk on
the basis of such studies should take into account the differences
that exist between the species in their reactions to chemicals.
Moreover, the conditions under which the animal data were obtained
may not reflect the human circumstances under consideration.
Animal data must be considered, therefore, in the context of the
similarities and differences that are known to exist between man
and experimental animals, and conclusions need to incorporate a
margin of safety to accommodate known, and possibly unknown,
species differences.
1.4.1 Some similarities in growth and development between
man and experimental animals
In many ways, the differences between the young infant and
adult are the same in animals as they are in man. Thus, the infant
animal is always smaller than the adult and, thus, it has a larger
surface area in relation to its body weight. All animals that are
homoeothermic from the time of birth have a higher consumption of
air per unit body weight than the adult of the same species, at the
same environmental temperature. The young of all animals species
grow rapidly, often far more rapidly than the human infant, and
their body composition differs from that of the adult in the same
way that that of the human infant differs from that of the human
adult. The functions of the organs and systems are immature in
early life, and most mammalian species depend on mother's milk for
a period after birth.
The anatomical, physiological, and chemical differences between
the infant and adult of an animal species are therefore in the same
direction as they are in the human species, and, in general, the
effects of chemicals on the young of experimental animals can be
expected to differ from those on the adult of the species just as
they do in man. However, the differences between species make it
unwise to assume that the results obtained on a young animal will
necessarily apply to the human infant.
1.4.2 Some differences in growth and development between man and
experimental animals
Different animals are born at different stages of their
anatomical, physiological, and chemical development and, although
the qualitative differences between the infant and adult are
similar in man and experimental animals, the quantitative
differences are by no means the same. The rat is a widely used
experimental animal in studies on the effects of chemicals on the
infant body. Some stages of development that take place after
birth in the rat have already occurred in the human fetus before
birth. Examples are the anatomical and functional maturation of
the hypothalamus (Dörner & Staudt, 1972) and the deposition of body
fat. Some aspects of development, however, only occur after birth
in all species, for example, respiration of air and the full
functioning of the gastrointestinal tract.
The new-born rat is smaller than the new-born human infant in
comparison with the weight of the adult, and, during infancy, it
grows at a much more rapid rate. Thus, the discussion in section
1.3.3 about the human infant applies with even greater force to the
infant rat. The rat at birth has a less mature chemical
composition than the human infant, as evidenced by the higher
percentage of water in its lean body tissue (Widdowson & Dickerson,
1964). The greater degree of immaturity of the body composition of
the rat at birth is well illustrated by reference to the bones.
Characteristically, rat bones have very little fatty marrow, but,
apart from this, the composition of the adult femur is not very
different in the 2 species. At birth, however, the rat femur has
less than half the concentration of calcium that there is in the
human femur, more water, and considerably less collagen, which
makes up the matrix on which the bone mineral is deposited
(Dickerson, 1962a,b). In the rat, as in man, the major changes in
the composition of bone do not take place until the period of
infancy is over. The composition of bone, and of the body as a
whole, though less mature at the time of birth in the rat than in
man, changes much more rapidly. All this must be taken into
account in considering the effects of a chemical on the bones or
bodies of young rats compared with the effects on those of human
infants.
The new-born rat is less mature physiologically than the human
infant, and it matures more rapidly. This may limit the
application of results obtained on the young rat to the human
infant. This is true of the renal function (McCance, 1948), so
that the new-born rat is less able than the adult to excrete
chemicals administered to it (section 3.5.1).
The activity of the hepatic enzymes is low in both new-born
experimental animals and new-born human infants, but the whole
subject of enzyme activity in this context is complex and is
considered in more detail in section 3.
One difference between the young of man and some animal species
is the ability to absorb macromolecules from the intestine. In the
human infant, the passage of macromolecules across the intestinal
mucosa is very limited, but in the young of such species as the
pig, cow, sheep, and horse, macromolecules are transferred from
the milk into the circulation in quite large amounts for a limited
period of hours or days. In the rat, the transfer goes on all
through the suckling period (Walker, 1979). This may have a
bearing on the interpretation of the results of studies on the
intestinal absorption of certain chemicals by the new-born
experimental animal in relation to the human infant.
1.5 Conclusions
The infant and young child have different structural and
functional characteristics from those of the older child and adult,
which represent stages in normal growth and development. These
include a larger surface area in relation to weight, different body
composition, a higher metabolic rate and oxygen consumption and,
hence greater intake of air per unit body weight, rapid growth and
development of the body as a whole and of the organs and tissues,
particularly during the first 6 months after birth. Many organs
and tissues are functionally immature at the time of birth, and
they mature at different rates. Moreover, infants have greater
energy and fluid requirements per unit body weight than older
children and adults. All these characteristics must be taken into
account in considering principles for evaluating health risks from
chemicals during infancy and childhood.
The structural and functional differences between the infant
and adult animal tend to follow the same direction as in the human
species, but there are important quantitative differences in, for
example, the stage of development at birth, the rate of growth, and
the intestinal absorption of macromolecules. The age of the infant
rat or other animal corresponding to an age in the human infant
will differ according to the aspect of development under
consideration.
2. PATHWAYS OF EXPOSURE
2.1 Introduction
As with adults, the potential for exposure to chemicals for
infants and young children is through ingestion, inhalation, and
percutaneous absorption. Both voluntary and inadvertent exposures
may occur, but their nature and extent are modified by the
physiological, behavioural, and other characteristics peculiar to
this age group. Thus, the methods of nurture, the social,
cultural, and occupational proclivities of the family, the quality
of care and supervision, and the inherent behavioural patterns of
the child, may all be significant factors for determining or
modifying exposure.
The new-born infant may already have encountered toxic chemical
agents in utero by the placental route; for example, the sequelae
of maternal exposure to methylmercury (Clarkson et al., 1976),
ethanol (Jones & Smith, 1975), tobacco (US DHEW, 1979), and
narcotics (Barr et al., 1984) are well recognized. Moreover, the
young infant is an obligate milk feeder, and chemicals that
traverse the mammary gland in lactation are potentially important
contaminants of both human and artificial milk.
The use of appropriate cleaning and antiseptic chemicals for
the routine care of the skin, umbilicus, and clothing of the new-
born infant have all been associated with toxicity (Armstrong et
al., 1969). Similarly, effects from atmospheric contaminants have
been encountered in neonatal units from agents used on the clothing
or for the disinfection of apparatus and operational areas.
Development from infancy to childhood is marked by increasing
mobility and exploration of the immediate surroundings. Oral
exploration is commonplace towards the end of the first year of
life in which the mouth is used to sample the taste and texture of
the objects and materials that are encountered. Oral exploration
may be accompanied by the ingestion of a wide variety of substances
not normally regarded as food (pica) including soils, dusts, and
surface coatings in the vicinity of the home. Although mouthing
and pica diminish with continuing development after the first year
of life, hand to mouth activity tends to persist (Barltrop, 1966).
As a consequence of increasing mobility and diminishing
supervision, poisoning due to the ingestion of toxic substances is
very common in early childhood. For example, in England and Wales,
one-fifth of all hospital admissions for suspected poisoning
involved children under the age of 5 years, amounting to
approximately 20 000 admissions per annum. Household,
horticultural, agricultural, and industrial chemicals were all
implicated, though medicaments and therapeutic compounds were the
principal agents (Vale & Meredith, 1981). It is unlikely that all
acute ingestions are reported, and their prevalence is not known
with certainty. The quality of available supervision is an
important determinant of accidental poisoning and will itself be
modified by social, cultural, and economic factors.
Infants and children will also be subject to chemical exposure
resulting from contamination of the natural and domestic
environments that they share with adults. However, the provision
of foodstuffs, beverages, clothing, furniture, toys, and
medicaments, specifically manufactured or adapted for the young,
may modify the route of exposure for certain chemicals; efforts are
usually made to minimize the contamination of such materials with
chemicals that might be harmful.
2.2 The Alimentary Tract
2.2.1 Milk
Milk may be regarded as one of the routes of excretion of
foreign compounds that have entered the body of the lactating
mammal. The degree to which a particular chemical traverses the
mammary gland depends on several factors, including its own
physical and chemical properties, such as the degree of ionization
and binding in biological fluids, the solubility characteristics,
and the maternal plasma concentration achieved. Passive diffusion,
active transport, and reverse pinocytosis across the apical
membrane of the mammary alveolar cell may all be involved, but the
milk/plasma ratio cannot always be predicted for a given chemical
in a particular species. In general, transfer from plasma to milk
is enhanced by high solubility in lipids, low relative molecular
mass, and weak binding to plasma-proteins (Giacoia & Catz, 1979).
2.2.1.1 Human milk
Nearly all chemicals to which the mother is exposed may, to
some extent, be excreted in human milk (Jensen, 1983, 1985).
Contamination of human milk may result from the maternal diet,
environment, occupation, addiction, and medical treatment. Marked
variation in the concentrations of chemicals in milk, both between
and within individuals, is characteristic and may reflect the stage
of lactation, the time and method of sample collection, and the
age, weight, and parity of the mother. Chemical residues most
frequently reported in human milk are the organochlorine pesticides
(DDT) and industrial chemicals (PCBs and metabolites, HCH isomers,
and cyclodienes such as dieldrin). Lactation is a route of
excretion for some of these chemicals, and adverse health effects
have been noted in breast-fed infants when the concen-tration of
chemicals was extremely high, as in accidental poisoning outbreaks,
and in some cases of occupational exposure. During the Iraqi
outbreak of methylmercury poisoning of 1971-72 (Amin-Zaki et al.,
1974), mothers who consumed bread made from seed grains treated
with a methylmercury fungicide were found to have milk containing
8.6% of the concentration of mercury in maternal blood collected at
the same time (Amin-Zaki et al., 1976). Infants who were born
before their mothers ate the contaminated bread and who received
methylmercury only through suckling, were found to have blood-
mercury values of up to 1.0 g/litre. Follow-up assessment over 5
years showed that some of the children suffered impaired
neurological and mental development (Amin-Zaki et al., 1981).
In Turkey, bread prepared from seed grains treated with
hexachlorobenzene between 1955-59 also affected infants (Peters,
1976). In some villages, almost all children under the age of 2
years, who had been breast-fed by mothers who had eaten the
contaminated wheat, died of the condition known as "pembe yara"
(Turkish for pink sore), which included symptoms of weakness,
convulsions, and localized cutaneous annular erythema.
Reports of such episodes have been rare. However, if
confronted with such poisoning outbreaks, exposure of infants
through human milk should be considered.
2.2.1.2 Other types of milk
Cow's milk, and the milk of other species used for infant
feeding, are subject to contamination by many of the chemicals
found in human milk, together with others that reflect local
techniques of animal husbandry and veterinary practice. Infants fed
milk derived from a particular animal or herd may be at greater or
lesser risk of exposure to a given contaminant than those fed
artificial or manufactured milk derived from the pooled secretions
of multiple herds. Conversely, the pooling of milk during the
manufacture of infant feeds will tend to increase the number of
potential contaminants in the end-product, but may diminish their
concentrations.
The food sources of lactating animals may be contaminated by
local industrial wastes, by the application of pesticides, by
naturally-occurring substances, such as mycotoxins, or by storage
in containers or buildings previously contaminated with pesticides,
disinfectants, preservatives, and other chemicals. The
organochlorine pesticides are well recognized fodder contaminants.
Numerous examples are known of milk containing chemicals derived
from animal feeds including DDT, hexachlorocyclohexane (HCH)
(Miyabe et al., 1971), heptachlorepoxide (Smith, 1982), and
hexachlorobenzene (Goursaud et al., 1972). Chemicals in milk
derived from containers of contaminated animal feed include PCBs
(Villett & Hess, 1975), pentachlorophenol (PCP) (Haering &
Schefer, 1980), and dieldrin (Epps et al., 1974). The inadvertent
substitution of a polybrominated biphenyl (PBB) product for
magnesium oxide in prepared cattle feeds resulted in contamination
of the milk (Isleib & Whitehead, 1975).
Several examples of milk contamination occurring after
collection have been reported. Thus, iodophors and other
disinfectants (Kroger, 1972), dieldrin (Tadjer & Dore, 1971) and
chlordane (Landsberg et al., 1977) have all been identified in milk
stored in vessels that had been inadvertently rinsed or cleaned
with, or fabricated from, previously contaminated materials. The
inadvertent addition of toxic chemicals to infant milk during
manufacture is rare, though arsenic poisoning resulting from the
use of a contaminated sodium phosphate stabilizer has been reported
(Kitamura et al., 1953).
Pesticides and drugs for the control of parasitic infection and
other diseases in lactating animals are liable to be absorbed and
excreted in the milk, even after topical application. Milk
contamination with antibiotics and hormones, given for economic
rather than therapeutic or preventative indications, may also
occur.
2.2.1.3 Infant feeds
Foods and beverages, other than milk, which have been
specifically prepared or manufactured for infants and young
children, may be diverse in composition and comprise only a
proportion of the total dietary intake. However, they are subject
to the same sources of chemical contamination as foods intended for
adults, during producion, manufacture, and packaging. The
potential significance of contaminants is enhanced when the dietary
range is restricted.
Numerous examples of contamination at source have been
described in common with those for human and other milk. Thus,
residues of pesticides, antibiotics, fungicides, and heavy metals
have all been encountered as a result of agricultural techniques,
veterinary practice, and the use of contaminated land and water.
An additional factor with regard to meat products is, for example,
the use of anabolic agents, such as diethylstilboestrol, in animal
husbandry (Umberger, 1975). Thus, in one study, one-third of 450
samples of baby food containing veal were found to have detectable
estrogen activity (Loizzo et al., 1984).
Many chemical or non-nutritional substances are used in food
manufacture as preservatives, anti-oxidants, stabilizers,
emulsifiers, and thickening agents, with the intention of ensuring
that the food is wholesome and palatable, when consumed. Numerous
chemical colouring agents are potentially available, and their use
in infant foods has attracted controversy, since marketing rather
than nutritional considerations are involved.
Manufactured infant and junior foods are characteristically
distributed in appropriately small containers which, therefore,
have a relatively large surface area/volume ratio. The degree to
which contaminants derived from the container material, seams, or
internal surface coatings may affect the contents is inversely
proportional to the container volume. The degree to which the
container design and manufacture may influence food contamination
is indicated by the reduction of dietary lead intake observed in
infants in the USA during the last decade, when progressive
replacement of lead soldered side-seamed cans by welded or seamless
containers resulted in a 47% reduction in lead intake by infants
aged 0 - 5 months (Mahaffey, 1983).
Contamination of foods during preparation in the home may
result from the use of inappropriate vessels, culinary agents such
as cooking oils, and cleaning materials, as well as from water.
Thus, excessive intake of copper derived from domestic cooking
utensils has been implicated in Indian childhood cirrhosis (Marwha
et al., 1981), and contaminated edible oils were involved in an
outbreak of Yusho disease (Kuratsune, 1980; Japan/US Cooperative
Science Program, 1983).
The use of detergent chemicals for the cleaning of cooking and
eating utensils is inevitably associated with ingestion of the
chemicals by all members of the household, but, in normal usage, it
is unlikely that toxic intakes will be encountered. However,
intakes are likely to be enhanced when rinsing or wiping is
omitted.
2.2.1.4 Water
Water for domestic consumption may be contaminated by chemicals
at source, during treatment and transport, or in the home from
domestic plumbing, cisterns, or culinary utensils. The use of
recycled water, particularly during drought, will tend to enhance
the concentration of some pollutants. The direct ingestion of
water used in the preparation of foods and beverages will involve
children of all ages, depending on local climatic conditions and
cultural and dietary practices. However, the use of water to
reconstitute powdered artificial milk is a special case,
particularly where the milk is the sole nutrient source.
The potential contaminants of drinking-water are numerous and
include a wide range of metals and other elements; nitrates,
cyanide, thiocyanate, and ferrocyanate radicals; organic compounds
including pesticides; organic solvents; and anionic, cationic, and
non-ionic detergents. Asbestos and polyaromatic hydrocarbons have
been detected. The chlorination of water for disinfection,
particularly in the presence of humic material, results in the
formation of a number of haloforms or C-chlorinated compounds
including chloroform, bromodichloromethane, and
dibromochloromethane (Rook, 1974).
The significance of lead in drinking-water has been
increasingly appreciated, particularly in areas where lead pipes
and plumbosolvent water supplies co-exist. Studies in a population
in Scotland showed that there was a curvilinear relationship
between the concentration of lead in water and that in the blood of
artificially fed infants; 36% had blood-lead values in excess of
350 µg/litre (Sherlock et al., 1982). Similarly, lead intakes of
up to 3.4 mg/week were reported from Scotland in artificially fed
infants, whose food was measured and analysed, with correspondingly
marked increases in blood-lead values (United Kingdom Central
Directorate on Environmental Pollution, 1982).
Nitrate in drinking-water is not itself hazardous, but may be
converted to nitrite by nitrate-reducing bacteria in the
gastrointestinal tract of infants under appropriate conditions.
Nitrites promote the formation of methaemoglobin from haemoglobin,
thus impairing the transport of oxygen and causing cyanosis.
Methaemoglobin is especially associated with the consumption of
well water with a high nitrate content, and infants appear to be
more susceptible to it than older children and adults (American
Academy of Pediatrics Committee on Nutrition, 1970).
2.3 The Respiratory Tract
Although infants and children may inhale chemical compounds,
information concerning the structure and function of the
respiratory tract in this context is limited. It is known that the
architecture of the respiratory tract continues to develop, at
least until the age of 18 months. Anatomically, 3 processes have
been identified including:
(a) centripetal alveolization giving rise to an extra
generation of respiratory bronchioli;
(b) transformation of some distal respiratory bronchioli
into alveolar ducts; and
(c) further branching of 1 or 2 alveolar duct generations.
After the age of 18 months, the respiratory structures increase
in size with a progressive increase in elastic fibre bundles in the
alveolar wall up to the age of 18 years. The length/diameter
relationship of each respiratory unit remains relatively constant
throughout development. The effects of these architectural changes
is thought to be minimal in the case of inhaled aerosols, but the
position concerning suspended particulates of various sizes has
not been well studied in childhood (Hislop & Reid, 1981).
Numerous chemical substances are found in the atmosphere, and
over 1600 have been identified. Not all of these are man-made, and
many may be derived from a single source (Graedel, 1978). The bulk
of atmospheric pollution is provided by carbon monoxide,
particulates and oxides of sulfur, hydrocarbons, and oxides of
nitrogen. Not all pollutants act directly; for example, the oxides
of nitrogen may exert their effects indirectly, by virtue of their
role in photochemical smog formation (National Research Council,
Subcommittee on Airborne Particles, 1979).
The exposure of infants and children to atmospheric pollutants
may be enhanced compared with that of adults, if the pollutants are
emitted close to the ground or, in the case of aerosols, are gases
or vapours of high density.
2.4 The Skin
Percutaneous absorption of chemical substances is known to
occur through normal skin in both adults and children. There is
little evidence that the rate of absorption varies with age, since
the limiting factor would appear to be the thickness of the
stratum corneum, which is relatively constant throughout postnatal
development after a full-term birth. Different processes of
absorption are thought to exist, but the transcutaneous flux cannot
be predicted with certainty, since marked variations in absorption
rates have been observed between closely related chemical
compounds. While absorption through normal skin does not vary with
age, absorption is greatly enhanced when the skin barrier is
damaged or when the substance is applied under an occlusive
dressing. A problem specific to infants is that napkins (diapers)
serve as an occlusive dressing, which may promote the absorption of
chemicals applied to the buttocks and increase the likelihood of a
systemic effect. Absorption may be further enhanced by skin
reactions in the napkin area.
The prematurely born infant may constitute a special case,
since studies on the development of the stratum corneum in fetal
life suggest that the permeability barrier is incomplete until just
before term (Singer et al., 1971). Thus, pre-term infants bathed
with a solution of hexachlorophene showed greater peak blood
concentrations of the compound than full-term infants subjected to
the same procedure (Greaves et al., 1975).
A pertinent factor in infants is the relatively large surface
area to body weight ratio; thus, compounds absorbed through a given
part of the skin surface will result in greater tissue
concentrations than in an adult (Wester & Maibach, 1982).
2.5 Conclusions
Infants and young children may be exposed to chemicals by the
gastrointestinal, respiratory, and percutaneous routes. The
relative importance of these routes will vary with age according to
the nature of the diet, the behavioural characteristics, and the
maturation of the system involved.
Since infants are obligate milk feeders, they will ingest
chemicals that cross the mammary gland during lactation. These are
potentially important contaminants of both human milk and infant
formulae containing the milk of other species. Similarly, chemicals
contaminating the water used for the reconstitution of powdered
milks will contribute to the ingested burden. Foods and beverages
specially manufactured for infants and young children are also
potentially important sources, especially when the dietary range is
restricted. Oral exploration, hand to mouth activity, and pica
contribute to the risk of ingestion of chemicals encountered in the
domestic environment.
Limited information is available concerning the absorption of
inhaled chemical atmospheric pollutants in relation to the
continuing development of the respiratory tract. However, exposure
of infants and children to atmospheric pollutants may be enhanced
compared with that of adults, when the source of emission is close
to the ground, and under circumstances in which gases or vapour of
high density are involved.
Chemicals can be absorbed through normal skin, but, in infants
and children, the relatively large surface area to body weight
ratio may result in greater tissue concentrations than in the
adult. Percutaneous absorption is enhanced when the skin is
damaged or macerated, or when the substance is applied under an
occlusive dressing such as a napkin (diaper). The prematurely born
infant may constitute a special case, since the permeability of the
skin is greater, because of the incomplete development of the
stratum corneum.
3. KINETICS OF ABSORPTION, BIOTRANSFORMATION, AND ELIMINATION
3.1 Introduction
Because of the morphological, biochemical, and physiological
characteristics of the infant and young child described in section
1, the kinetics of chemicals gaining access to their bodies need
special consideration, particularly with regard to the timing of
the maturation processes governing absorption, distribution,
biotransformation, and elimination from the body.
3.2 Absorption
A chemical may be absorbed through an external (skin) or
internal (gastrointestinal and respiratory tract) surface.
Absorption depends on the physical and chemical characteristics of
the chemical, its concentration gradient, the conditions at the
site of absorption, and the biological characteristics of the
absorptive surface. The conditions at the site of absorption and
the characteristics of the absorptive surface can be expected to
change with age; for example, postnatal changes in pH in different
parts of the gastrointestinal tract and skin, and alterations in
the thickness and structure of membranes of the gastrointestinal
tract and lungs (Hoffmann, 1982) cause changes in perfusion, which
in turn influence the concentration gradient.
3.2.1 Absorption from the gastrointestinal tract
Absorption from the gastrointestinal tract is an important way
in which chemicals enter the body of the infant and young child.
It is influenced by pH, total mucosal surface area, blood supply,
perfusion rate, and period of contact of the chemical with the
absorptive surface, that is the gastric emptying and intestinal
transit time. All these change with postnatal development.
The absorption of some chemicals by infant animals is greater
than that by young adults of the same species. This can be
explained by several factors, including immaturity of the
gastrointestinal tract, the characteristics of the infant's diet,
and the greater proportion of nutrients absorbed. This is of
benefit for growth, but is a disadvantage as far as non-nutritive
chemicals are concerned. Much of the evidence for this comes from
studies on experimental animals exposed to metals. Thus, the adult
rat absorbs 1% or less of lead added to its diet compared with 40 -
50% for the infant animal (Kostial et al., 1971, 1978; Forbes &
Reina, 1972a,b). According to Alexander et al. (1974) and Ziegler
et al. (1978), infants and young children absorb substantially more
lead than adults, but Barltrop & Strehlow (1978) did not find any
age-related differences. The intake of lead by the infants studied
by Alexander et al. (1974) and Ziegler et al. (1978) was greater
than that of the children in the study of Barltrop & Strehlow
(1978), and this may explain the different result.
Methylmercury is well absorbed in the gastrointestinal tract of
infants and young children, as it is in adults (Berlin, 1983).
Some methylmercury is evidently re-excreted into the intestine and,
if this is demethylated by the intestinal bacteria, the mercuric
mercury from it is excreted in the faeces. In mice, there is an
increase in intestinal demethylation at weaning, and the results of
in vitro studies have shown that the microflora in faeces from
young infants receiving milk have negligible demethylating ability
compared with those of weaned children and adults (Rowland et al.,
1983). Thus, it seems likely that more of a dose of methylmercury
will be retained within the body of young infants than of older
children and adults.
3.2.2 Absorption from the lungs
Chemicals permeate the air-blood barrier by simple dif fusion,
but the thickness of the membrane, its lipid composition, surface
area, and porosity determine pulmonary absorption. Lipid-soluble
chemicals are absorbed at similar rates by the lungs of new-born
and adult rats, but hydrophilic chemicals are absorbed at a more
rapid rate by young animals (Hoffmann, 1982).
3.3 Tissue Distribution
3.3.1 Binding of chemicals to proteins
Most of chemicals bind to plasma-proteins. The amount of
binding depends mainly on the concentration and structure of the
binding proteins, the affinity constant, and the presence of other
compounds capable of modifying the chemical-protein interaction.
In the new-born infant, several factors contribute to a lower
plasma-protein binding than in the adult. The concentration of
albumin in the plasma of neonates, and hence the number of binding
sites, is low. Moreover, these binding sites are occupied by
endogenous substances such as fatty acids, steroids, and bilirubin.
Thus, new-born infants have a lower capacity for binding chemicals
to plasma-albumin, and competition at the binding site may lead to
the release of, for example, bilirubin, resulting in kernicterus.
Moreover, hypoxaemia and acidosis may also decrease binding
capacity.
3.3.2 Distribution within the body
From early fetal to adult life, the fluid compartments of the
body change continuously. The larger extracellular space in
infancy (section 1.3.4) provides a larger volume for the
distribution of water-soluble chemicals that do not penetrate the
cells, and plasma concentrations of these chemicals may be lower in
infants than in adults, even if the total body burden per unit body
weight is greater.
Alterations in the morphology, physiology, and biochemistry of
the organs, as well as in their weight relative to the body as a
whole, may affect the distribution of chemicals. The brain is
proportionately larger in the infant than in the adult, and rapid
myelination during the first year after birth may favour the uptake
of lipophilic compounds. Metals, too, are retained in the brain
more readily in infancy than in adulthood. Momcilovic & Kostial
(1974), for example, found that a far greater percentage of the
intake of lead was retained in the brain of the suckling rat than
in that of the adult, and that the rate of removal was slower.
Similarly, Kostial et al. (1978) and Wong & Klaassen (1980) found
that a greater percentage of the intake of cadmium was retained in
the brain of the suckling rat than in that of the adult; Jugo
(1980) made a similar observation for mercury. There is little
direct evidence about the human brain. By analogy with animals, it
seems likely that the concentration of lead in the brains of
infants exposed to lead would rise more rapidly than the
concentration in the brains of older children or adults exposed to
a similar dose.
Methylmercury is known to accumulate in brain tissues,
predominantly in grey matter, but whether the brains of infants and
young children accumulate more than those of adults is not known.
Other tissues take up metals more readily in the early period
of life, when the animal is growing rapidly, than when growth has
slowed down or ceased; this particularly applies to the bones. The
concentration of lead in human bones doubles between infancy and
the late teen years (Barry, 1975).
The liver and kidney also accumulate metals. At birth, the
concentrations of cadmium and mercury are low. Henke et al.
(1970), using neutron activation analysis, determined the cadmium
concentrations in the liver and kidneys of 41 children and adults
and found that they increased 200 fold during the first 3 years
after birth. The total increase in cadmium in the liver and kidney
was greater than appears from the concentration, because both
organs increased considerably in weight.
3.4 Biotransformation of Organic Chemicals
The kinetic behaviour of organic chemicals in living organisms
is generally characterized by several processes modifying the
duration of their action. The limiting factors are either
excretion through the kidneys, bile, or gastrointestinal tract, or
biotransformation mechanisms in the liver or other organs. The
products of biotransformation are generally more polar than the
original compound and, thus, are more readily excreted.
Biotransformation is brought about by enzyme activity, either
associated with microsomal systems in the liver or with enzymes in
the plasma or other tissues.
The biochemical processes involved in the biotransformation of
organic chemicals can be divided into 2 phases:
Phase 1. hydroxylation, dealkylation, dehydrogenation,
reduction, hydrolysis, and other reactions
producing a conjugable grouping; and
Phase 2. biosynthetic reactions, whereby conjugates are
formed with endogenous polar molecules; these
conjugates are, generally, readily excreted.
Both Phase 1 and Phase 2 reactions are generally slower in the
new-born infant than in the adult. Consequently, blood-plasma
half-lives of chemicals in which the degradation and elimination
are dependent on one or the other or both of these types of
reactions are generally appreciably longer in neonates than in
adults.
The rate of maturation of processes concerned with the
biotransformation of organic chemicals varies from one reaction,
and from one chemical, to another. Highest activities are reached
in children aged 12 - 16 years, after which there is generally a
decline. A detailed description, with full documentation, has been
published by Klinger (1982).
3.5 Elimination from the Body
3.5.1 Elimination by the kidneys
The renal function of the full-term infant at birth is immature
by adult standards, and this applies both to glomerular and
tubular function (McCance & Young, 1940; Dean & McCance, 1947b).
The rapid growth of the young infant (section 1.3.3) involves the
retention of nitrogen and minerals, so that the equivalent of only
part of the nitrogen and minerals absorbed from the food requires
to be excreted by the kideys. The breast-fed infant or the infant
fed on a formula similar in composition to breast milk is able to
do this satisfactorily, and so maintain the composition of the body
fluids at their proper level (McCance & Widdowson, 1957a).
However, excessive intakes of nutrients, such as protein
(nitrogen), phosphorus, and sodium, are not excreted completely by
the kidneys of the new-born infant or animal, and the
concentrations in the plasma rise (McCance & Widdowson, 1956,
1957b). Retention of chemicals introduced into the body of the
infant may be due to both renal immaturity and insufficient
biotransformation.
The function of the renal tubules at full-term birth is less
mature than that of the glomeruli, but it matures more rapidly
(Edelmann & Spitzer, 1976). This is important in the young infant
for the chemicals that depend on tubular secretion for their
elimination from the body. Moreover, the reabsorption of chemicals
from the tubular lumen into the tubular cells also varies with age
and depends, in part, on the pH of the urine. Weak organic acids
are reabsorbed more readily by the infant, but, more important, the
low capacity for biotransformation by the infant's liver results in
organic chemicals reaching the kidneys in their original lipophilic
form, and these are not excreted, but reabsorbed into the
circulation (for reviews, see Scheler, 1980; Braunlich, 1981).
Some metals depend on the kidneys for their elimination from
the body, and the results of animal studies suggest that suckling
rats excrete less of their body burden of cadmium, mercury, and
manganese than adults (Kostial et al., 1978). Infants appear to
excrete a smaller proportion of absorbed lead by the renal route
than adults (Ziegler et al., 1978).
3.5.2 Elimination by the liver
The excretion of chemicals in the bile is an important means of
elimination, though some of the chemicals excreted through the bile
may be reabsorbed in the gut and re-enter the body, thus resulting
in their enterohepatic circulation. They cannot penetrate the
epithelium of the biliary duct. Many chemicals are excreted by the
liver as glucuronides in the bile, but the formation of
glucuronides is limited in the new-born infants (section 3.4).
3.5.3 Elimination by other routes
Other routes of excretion include the gastrointestinal tract,
the respiratory tract, and the skin. They are non-regulatory and,
while they may be important for some chemicals, information is
limited about age-related differences in elimination.
3.6 Conclusions
Generally speaking, both organic and inorganic chemicals are
absorbed more readily by the infant than by the adult, but the
organic chemicals undergo biotransformation less readily. For some
chemicals, alternative biochemical pathways may exist.
The kidneys of the neonate are immature and less able than
those of the older child or adult to excrete chemicals, whether
inorganic or the polar products of biotransformations. A greater
proportion of a similar dose of a chemical per unit body weight is
therefore likely to accumulate in the body of the infant, and the
concentration of the chemical in the blood and tissues of the
infant will be higher. The possible effects of this are discussed
in section 4.
4. EFFECTS OF CHEMICALS IN THE BODY
4.1 Introduction
Differences in kinetics can enhance the toxicity of a chemical
in the infant but, in some cases, may result in a qualitative
difference of response or in a lower toxicity, depending on the
intermediary metabolite. In addition, the qualitative and
quantitative response of the target tissues to the same internal
dose of a chemical may be different at various stages of postnatal
development because of the gradual development of characteristics
of the target tissues. A third aspect of developmental toxicology,
which should be recognized, is the fact that a chemical introduced
into the body of an infant or young child may affect the structural
and functional development of a particular organ or system, which
may interfere with the growth and development of the body as a
whole. Animal studies have shown that the toxic effects of certain
chemicals are different at different stages of development.
Moreover, chemicals reaching the body early in life may produce
delayed effects in later years. The response of the growing infant
may therefore be different from that of the fully grown adult.
The problems of extrapolating quantitatively from animals to
man and reaching conclusions on the implications of evidence from
animal studies for human health are well known. These problems are
greater during the new-born period, since the stage of development
at the time of birth varies considerably between species (section
1.4.2). Information from clinical experience of exposure is
limited, and systematic epidemiological data from studies on
infants are sparse, so that the results of studies on animals may
provide the only information available. When information
concerning human infants is available, it should be given
preference.
4.2 Effects of Chemicals on General Growth and Development
General growth and development are possible indicators of the
effects of chemicals on infants and children, whether the exposure
occurred prenatally or postnatally. The principles for evaluating
the effects of prenatal exposure have been considered in an earlier
Environmental Health Criteria publication (WHO, 1984). Factual
information about the effects of postnatal exposure is scarce. In
a study on the infants of mothers who had consumed bread
contaminated with a methylmercury fungicide during lactation, Amin-
Zaki et al. (1981) found abnormal neurological signs in some
infants at the first examination and in more infants on subsequent
occasions. Delayed motor and other aspects of development,
including intelligence, were not detected when the infants were
first seen, but were observed at follow-up examinations during the
next 5 years.
4.3 Effects of Chemicals on Some Organs and Systems
4.3.1 Nervous system
The brain of the infant at birth is not fully developed. The
full number of neurones is not reached until about 2 years of age
(Dobbing & Sands, 1973); myelination is not complete until
adolescence (Hoar & Monie, 1981).
Brain development involves changes in the proportions of myelin
and non-myelinated tissue. Shuman et al. (1975) observed status
spongiosus in the myelin sheaths of the reticular formation of
premature infants, following exposure to hexachlorophene, but no
lesions were present in the non-myelinated part of the brain.
Brain lesions were not produced during the first 8 - 10 days after
birth in the rat. However, between 10 and 25 days, when myelination
takes place, the suckling rats became sensitive to hexachlorophene
(Nieminers et al., 1973; Ulsamer et al., 1975).
There are changes in the sensitivity of the brain of the rat to
hormones or drugs during development, and this has been correlated
with the development of the cell receptors (Macho et al., 1972;
Valcana & Timiras, 1978). On the basis of experimental data, Czaba
(1984) concluded that, during maturation, cell receptors in several
organs require reinforcement by the appropriate hormone at a
critical stage of development. This process can be hindered or
prevented by the presence of molecules analagous to the hormone, or
by a drug or other chemical that binds to the receptor and so
prevents reinforcement by the hormone and hence normal development.
Many confounding factors and co-variables such as nutrition,
socio-economic status, and mental stimulation have to be taken into
account when evaluating the effects of a chemical on the
functioning of the human brain. Only when good information on
these factors is available will it be possible to make a valid
interpretation of the observations on brain function after exposure
to a chemical. This has been discussed most extensively in the
case of lead (United Kingdom Department of Health and Social
Security, 1980; MRC, 1984) (section 3.3.2).
4.3.2 Kidneys
The functional immaturity of the infant's kidneys has already
been discussed (section 3.5.1). Specific reports of their
susceptibility to chemicals are not available, but results of
animal studies suggest that some compounds are less toxic to the
immature kidney than to the mature organ. For example, the same
dose of mercuric chloride per unit body weight that produced renal
failure in adult rats did not have any effect on renal function in
new-born rats (Kavlock & Gray, 1983). Information is lacking on
the effects on renal function of the accumulation of chemicals in
the human kidney during the early years of life (section 3.3.2).
4.3.3 Liver
The pre-term infant may still have foci of extra-medullary
haematopoiesis in its liver, but the histological appearance of the
liver at term is similar to that of the adult. The activity of
enzymes responsible for the biotransformation of chemicals in the
liver of the infant is not equal to that of the adult (section
3.4). However, it appears to be possible to induce the activity of
microsomal enzymes in the liver of neonates to some extent. If
infants have been exposed to phenobarbital, either during
intrauterine life, or immediately after birth, the synthesis of
components of certain microsomal enzyme systems in the liver is
accelerated. The metabolism and urinary excretion of bilirubin is
increased (Maurer et al., 1968; Trolle, 1968; Ramboer et al.,
1969). Increased activities of some drug-metabolizing enzymes have
also been found in new-born infants exposed to phenobarbital
before, or immediately after, birth. Such infants were able to
metabolize drugs such as diazepam more readily than infants not
exposed to phenobarbital, and their urinary excretion of hydroxy
derivatives was greater (Morselli, 1976).
4.3.4 Lungs
Animal studies indicate that some chemicals, such as nitrofen,
retard the development of the lung in the rat fetus (Kimbrough et
al., 1974), but there do not appear to be any reports of effects of
chemicals on the lung specific to infants. It seems that the
response of the infant's lungs to ingested or inhaled chemicals has
not been investigated sufficiently. A greater degree of impairment
of respiratory function might be expected in infants compared with
adults for a given level of exposure, particularly to particles of
substances that result in obstructive symptoms. An excess
mortality rate was found in infants aged less than 12 months
compared with older children and young adults in a population
exposed to atmospheric pollution with sulfur dioxide (Lawther &
Waller, 1975).
4.3.5 Haematopoietic system
The maturation and differentiation of haematopoietic cells in
the bone marrow are processes that continue throughout life, and
represent one of the most rapidly renewing cell populations.
Although the haematopoietic system and the blood are well known to
be the target of many chemical agents, there is little information,
at present, on any specific vulnerability of the developing bone
marrow. An example of the vulnerability of the red cells in young
children is the occurrence of methaemoglobinaemia after exposure
to very low doses of methaemoglobin-forming compounds, e.g.,
nitrates (Kiese, 1974).
4.3.6 Immune system
Studies of immune ontogenesis have revealed that new-born
mammals do not possess fully competent immune defence systems.
While human infants have more highly developed immune systems than
some other mammals at birth, the human infant is still
immunologically immature.
The development of the immune system in mammals involves a
sequence of steps that begins early in gestation. Perturbation or
abrogation of this developmental sequence of events can lead to
immune dysfunctions that may be life-threatening. Defects in the
development of the immune system, due to heritable changes in the
lymphoid elements, have provided clinical and experimental examples
of the consequences of impaired immune development (for review, see
Heise, 1982).
The most profound effects of chemicals that modulate the immune
system of animals occur when dosing of the compound begins during
the development of the lymphoid system (Vos, 1977). Although there
are no data on the immunological effects resulting from perinatal
exposure of infants to chemicals, the potential for such effects
should not be ignored.
The extent of adverse effects on the immune system resulting
from exposure of adult animals to a wide variety of environmental
chemicals has only recently begun to be appreciated (Vos, 1977).
Even less is known about the magnitude of the immunological effects
resulting from fetal and neonatal exposure to environmental
chemicals. It would be expected that disruption of the normal
sequence of events in the development of the immune system by
chemicals would result in alterations in the competence of the
system. Such alterations may not only lessen the host's ability
to combat infection and neoplasia but may also lead to
hypersensitivity or to a failure in the control of homeostasis,
which may lead to autoimmunity. The immunological effects of
perinatal exposure to chemicals in animals have been reviewed by
Roberts & Chapman (1981) and Schmidt (1984). Exposure during
gestation and/or early in postnatal life to chemicals such as
2,3,7,8-tetrachlorodibenzodioxin (Vos & Moore, 1974; Faith & Moore,
1977; Luster et al., 1980), lead (Luster et al., 1978; Faith et
al., 1979), hexachlorobenzene (Vos et al., 1979), and
benzo( a )pyrene (Urso & Gengozian, 1980) has been shown to affect
the developing immune system of animals. However, no specific data
are available for human infants.
4.3.7 Endocrine system
Chemicals may affect the developing endocrine system directly,
or they may interact with some step of the regulating axis
controlled by the pituitary, hypothalamus, or other part of the
brain. The interaction between a chemical and the neuroendocrine
organs can also affect the reproductive and other systems. Some
pesticides have been shown to possess estrogenic activity
(McLachlan et al., 1981). Exposure of experimental animals in the
early postnatal period to chemicals with androgenic or estrogenic
activity induced disturbances of sex differentiation in the
hypothalamus and acyclic ovarian function in females (Barraclough,
1961, 1966; Takewaki, 1968; Flerko, 1975).
Chemicals that give rise to premature onsent of puberty in
children are of particular importance. Premature thelarche in
large numbers of young children in Puerto Rico was attributed to
estrogens in infant foods containing meat (Saenz de Rodriguez &
Toro-Sola, 1982); the cause of a similar outbreak in an Italian
school (Scaglioni et al., 1978) was not discovered. Exposure to
chemicals with androgenic or estrogenic activity may also affect
growth and final stature.
The development of the regulatory function of the adrenal
glands of experimental animals is altered by the administration of
compounds such as steroids and diazepam to the new-born animal
(Hcik 1969a, 1970; Erdosova et al., 1977), and results in changes
in adrenal function and in the reaction of the pituitary-adrenal
axis to stress stimuli later in life (Levine & Mullins, 1967; Hcik,
1969b, 1970; Libertun & Lau, 1972).
A special risk for new-born infants is an excessive intake of
iodine, leading to an abnormal rate of accumulation in the thyroid
gland. Exposure to high concentrations of iodine or goitrogenic
compounds can alter the function of the thyroid gland, resulting in
a deficient production of thyroid hormones at the time when
appropriate levels of the hormones are important for the maturation
of functions of the brain, liver, and other organs. Premature
infants are particularly prone to the development of hypothyroidism
as a result of iodine overdose (Chabrolle & Rossier, 1978; Malpuech
et al., 1978; L'Allemand et al., 1983).
4.3.8 Skin
The skin is a complex structure, the functional development of
which is incomplete until after puberty. Clinically, the cutaneous
manifestations of disease vary with age, and some disorders are
confined to infants and children. Thus, it would be expected that
age-related differences in the response of the skin to chemicals
would be encountered. Chloracne was found to occur principally in
young children rather than adults in a population exposed to dioxin
(TCDD, 2,3,7,8-tetrachlorodibenzo-para-dioxin), suggesting the
greater susceptibility of the child (Fara et al., 1982).
Chemicals applied to the skin may be absorbed and induce
systemic effects. New-born infants whose skin was treated with an
antibacterial preparation containing 3% hexachlorophene developed
blood levels of the compound similar to those known to cause brain
damage in animals. Similarly, some 40 deaths in French infants in
1972 were attributed to the use of baby powder accidentally
contaminated with 6.6% hexachlorophene (Marzulli & Maibach, 1975).
Some environmental pollutants or contaminants are known to be
readily absorbed through the skin, and this subject has been of
particular interest in paediatric therapeutics. Thus, both fatal
and non-fatal exposures occurred when pentachlorophenol was used in
a laundry room resulting in contamination of napkins used in the
hospital nursery (Armstrong et al., 1969).
4.3.9 Bones and teeth
The bones undergo considerable change during postnatal
development with regard to growth, change in shape, and
composition. Immature bones contain more water and less collagen
in the matrix than mature bones, and the degree of mineralization
is much lower (section 1.3.4) (Table 3). Minerals, including
strontium, caesium, and lead, tend to be deposited in the bones,
together with calcium, during the process of mineralization. Lead
may affect the conversion of 25-hydroxy vitamin D to the 1.25-
hydroxy metabolite in the kidney (for review, see Mahaffey, 1981).
Interference with skeletal development may result from the
action of lead in altering the balance of osteoblastic and
osteoclastic activity, and from the direct action of chemicals such
as fluoride.
The discoloration of developing teeth after administration of
tetracycline is an example of the particular propensity for
accumulation or fixation of chemicals in hard tissues. The results
of studies on primates suggested that excessive exposure to
selenium during the development of the teeth might increase
susceptibility to caries (Bowen, 1972).
4.4 Carcinogenesis
Maternal exposure to carcinogens may lead to exposure of the
fetus through the placenta and to exposure of the infant through
breast milk. The transfer of chemicals to the fetus depends on the
relative molecular mass and solubility of the chemical, non-ionized
and lipid chemicals entering the fetus most readily. Lipophilic
chemicals may be retained in the tissues of the fetus and have been
found in tissues of still-born infants (Curley et al., 1969).
Transplacental transfer of carcinogens has recently been reviewed
(WHO, 1984).
So far, no epidemiological observations on human infants have
been reported in which the occurrence of cancer was associated
solely with exposure to carcinogens in the early postnatal period.
The few existing data come from animal studies and, in most of
these, the route of exposure was parenteral and not through the
mother's milk. For example, the injection of aflatoxin resulted in
liver cancer in mice, but only when administered to new-born
animals (Vesselinovitch et al., 1972).
Within the past decade, it has been increasingly recognized
that not all carcinogens act by the same mechanism. Many of the
environmental chemicals, particularly the halocarbons, are though
to be promoters of carcinogenesis rather than initiators. Many of
these types of chemicals are persistent in the organism and are
secreted in breast milk (section 2.2.1.1) (Appendix I). The
mechanism of action of promoters is not well understood at present,
and effects on health at the low doses received by infants and
young children are not clear.
An intrinsic problem in studying prenatal and postnatal
exposure to carcinogens is the capacity for metabolic activation in
the tissues of the fetal or new-born organism, compared with that
of the adult (section 3.4). In this respect, pronounced species
variations have been shown between rodents and primates, including
man. The perinatal development of different organ systems varies
considerably among species. All these variables make extrapolation
from animals to man extremely difficult.
The significance for human health of early postnatal exposure
to tumour growth promoters or co-carcinogens is not known.
4.5 Conclusions
The structural and functional characteristics of infants and
young children described in sections 1, 2, and 3 might make them
more or less vulnerable than older children and adults, when they
are exposed to chemicals at a similar dose per unit body weight.
However, it should be emphasized that reports of specific effects
are rare. This may be because such specific effects do not exist
or because they have passed largely unnoticed. The examples quoted
here show that exposure to certain chemicals during early postnatal
development can be associated with dose-effect and dose-response
relationships that differ from those resulting from exposure in
later years.
Exposure to chemicals during early postnatal development can
give rise, not only to immediate effects on health, but also to
manifestations resulting from the disturbed maturation of organ
systems and their altered response to other environmental
influences. Depending on the chemical concerned, the vulnerability
of the developing organ systems can be higher or lower than that of
the more mature systems.
It is recommended that, if laboratory tests suggest that the
body burden of a chemical is a cause for concern, follow-up studies
should be made. Children who have been accidentally exposed to
chemicals that might affect the reproductive organs should be
followed prospectively to determine possible effects on puberty and
reproductive capacity. Similarly, if other organ systems are
likely to have been affected, prospective studies should, if
possible, be made to assess functional development, morbidity, and
mortality.
5. MODIFYING FACTORS
Considerable variations exist in the health and nutritional
status of children reared in different social and cultural
environments. The response of children exposed to chemicals may
therefore be modified in certain sections of a population to a
degree that cannot readily be predicted. Although analogous data
for the severity of response to infections, such as measles, are
well documented, information concerning chemical exposure and its
sequelae is limited.
5.1 Nutrition
The intake of chemicals contaminating foods and beverages will
vary with the amount of food consumed, the range and variety of
dietary items selected or available, and the methods of
preparation. Children with high intakes of marine fish will, for
example, have a greater intake of methylmercury from polluted
sources than those in which fish constitutes only a small
proportion of the diet (Turner et al., 1980). Conversely, some
pesticide residues in milk are decreased by heating, drying,
evaporation, or pasteurization, so that less of these substances
will occur in children consuming manufactured or processed milk, as
opposed to the raw product. The duration of milk feeding in
infancy, the method of weaning, and the interval before a normal
adult diet is introduced are important factors that vary greatly
between social, cultural, and ethnic groups.
Specific dietary components may interact with ingested
chemicals, thus modifying their availability for absorption. The
relative solubility of the chemical in the lipid or aqueous
fractions of the diet, and the potential for physical interaction
or adsorption to non-adsorptive mechanisms at the enteral surface
of the gut mucosa, may all have a role in determining the
bioavailability of the ingested chemical. Thus, the absorption of
lead, consumed simultaneously with the diet, is markedly lower
compared with its absorption from aqueous solutions, partly due to
interaction with other dietary components. In animals, and
possibly in man, lead absorption is reduced by diets rich in
calcium and phosphorus, but is increased when the intake of these
nutrients is restricted (Barltrop & Khoo, 1975).
The consequences of nutritional deficiency for the absorption
of chemicals cannot be predicted with certainty. In kwashiorkor,
both the digestive and absorptive functions of the gut may be
impaired and the activation of chemicals during conjugation
reactions reduced, particularly acetylation with amino acids
(Thabrew et al., 1982). On the other hand, specific mineral
deficiencies, for example, a lack of calcium, may stimulate
absorptive mechanisms shared by other, potentially toxic, divalent
cations (Barltrop, 1979). Iron deficiency in children has been
related to enhanced absorption and retention of lead, but the
mechanism involved is uncertain. Interactions between essential
and toxic metals after they have been absorbed into the body have
been described. Zinc, for example, competes with lead for
receptors on the enzyme aminolaevulinic acid dehydratase (Nordberg,
1979), but the significance of this in infants and children is
uncertain.
5.2 State of Health
Several diseases in addition to nutritional disorders may
modify the exposure and response of infants and children to
chemicals. Ill health associated with diminished activity or
mobility of the child will, for example, alter the balance between
exposures related to the indoor as opposed to the external
environment. Conditions requiring the long-term administration of
oral therapeutic agents or modified diets will inevitably alter the
intestinal milieu and hence the absorption of ingested chemicals.
The absorptive ability of the gut is modified in a wide range
of diseases, including the enteropathies and chronic diarrhoeal
states encountered in childhood arising, for example, from acquired
or inherited food intolerance. The steatorrhoea associated with
childhood malabsorptive disorders such as coeliac disease is known
to impair the absorption of lipid-soluble substances such as
cholecalciferol and alpha-tocopherol (Harrison, 1980), and would
therefore be expected to have a similar effect on the absorption of
other lipid-soluble chemical compounds.
Children with chronic renal or hepatic dysfunction will have an
impaired ability to excrete, metabolize, and, in some cases, store
chemicals that have been absorbed, which may therefore accumulate
in the body. Compromised integrity of the dermal or respiratory
epithelium by disease will enhance the potential for the absorption
of chemicals by these routes.
Some children have genetically determined variations in their
ability to metabolize or respond to certain chemicals. Thus,
deficiency of the enzyme glucose-6-phosphate dehydrogenase (EC
1.1.1.49), which is encountered in some sub-populations, is
characterized by a haemolytic reaction in response to various
triggering agents. Such reactions in childhood have been reported
after exposure to chemicals including naphthalene, aniline dyes,
and nitrofurantoin (Willoughby, 1984).
5.3 Social and Cultural Way of Life
There are many complex interactions between environmental
chemicals and the social and cultural ways of life. The effects of
place of residence, mobility, state of health, nutritional status,
and family income cannot always be distinguished without resort to
sophisticated epidemiological methods. Cultural and social
attitudes to child care and supervision differ between
subpopulations and are clearly important in relation to acute or
short-term exposures. The mechanisms whereby class and cultural
differences modify exposure are not always apparent. For example,
the different blood-lead values found in Negro versus Hispanic
children in New York and in Asian versus Northern European children
in the United Kingdom have yet to be adequately explained
(Barltrop, 1982). Family practices with regard to personal
hygiene, and especially handwashing before meals, may be important
determinants of exposure resulting from hand to mouth activity in
children.
5.4 Conclusion
Recognition of social and cultural factors that influence
exposure and modify response will assist in the identification of
children at risk and enable appropriate preventive and remedial
measures to be adopted.
6. GENERAL CONCLUSIONS
1. Infants and young children differ from older children and
adults in some of their morphological, physiological, biochemical,
metabolic, and behavioural characteristics, and in their
nutritional requirements.
2. The response of infants and young children to chemicals may
differ from that of adults by virtue of their biological
characteristics. Moreover, a feature of these responses is that
the processes of development may themselves be modified by exposure
to chemicals.
3. The effects of chemical exposure and their relationship to dose
may vary with age. The expression of the effects of chemicals may
be greater or lesser than in the adult, depending on the chemical
species concerned; the kinetics of its absorption,
biotransformation, and elimination; and the degree of maturation of
target organs and tissues.
4. The characteristics of infants and children are such that they
determine the pattern of exposure to chemicals. The pathways
involved are related to development and vary with the environment
of the child. Thus, skin contact, the consumption of breast milk
or its substitutes, the ingestion of substances other than food,
may each be predominant routes at different stages of development.
5. The exposure, uptake, and effects of chemicals may be modified
by factors that are characteristically encountered in early life,
including the mode of nutrition, social, economic, and cultural
conditions, and disease.
6. The expression of chemical effects may not be immediate, but
may be delayed until a later age. The health of adults may thus be
compromised by chemical exposures in early life.
7. The development of a strategy relating to chemicals detected in
breast milk requires a thorough appraisal of the merits and
disadvantages of the available options. Any risk of continued
exposure to a chemical through breast-feeding has to be balanced
against the risk of infection or nutritional deprivation, should
breast-feeding be curtailed or discontinued.
7. RECOMMENDATIONS
1. When health risks from chemicals are evaluated, the special
characteristics of infants and young children must be recognized.
An appropriate methodology based on the knowledge of these
differences should be used.
2. Preventive measures should take account of the special
conditions of exposure to chemicals characteristic for infants and
young children, including exposure through breast milk.
3. Health care workers should be made aware of the special risks
related to chemical exposure during infancy and childhood.
4. Any increased incidence of a disease possibly related to
chemical exposure should be reported and investigated. Awareness of
this among health care workers should be improved. The
establishment of an appropriate surveillance system should be
considered.
5. Clinical and epidemiological data should be collected following
the inadvertent exposure of infants and young children to
chemicals.
6. Developmental toxicology should be promoted and its methodology
improved in order to increase the predictive power of experimental
animal and other laboratory studies.
7. Studies are urgently needed to evaluate the risk associated
with exposure during infancy and early childhood to the initiators
and promoters of carcinogenesis.
REFERENCES
ALEXANDER, F.W., CLAYTON, B.E., & DELVES, H.T. (1974) Mineral and
trace metal balances in children receiving normal and synthetic
diets. Q. J. Med., 43: 89-111.
AMERICAN ACADEMY OF PEDIATRICS COMMITTEE ON NUTRITION (1970)
Infant methaemoglobinaemia: the role of dietary nitrate.
Pediatrics, 46: 475-478.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., & GREENWOOD, M.R. (1974) Studies of infants
postnatally exposed to methylmercury. J. Pediat., 85: 81-84.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., GREENWOOD, M.R., & GIOVANOLI-JAKUBCZAK, T.
(1976) Perinatal methylmercury poisoning in Iraq. Am. J. Dis.
Child., 130: 1070-1076.
AMIN-ZAKI, L., MAJEED, M.A., GREENWOOD, M.R., EL HASSANI,
S.B., CLARKSON, T.W., & DOHERTY, R.A. (1981) Am. J. Dis.
Chil., 130: 1070-1076.
ANDERSSON, U., BIRD, A.G., BRITTON, S., & PALACIOS, R.
(1981) Humoral and cellular immunity in humans studied at the
cell level from birth to two years of age. Immunolog. Rev.,
57: 5-38.
ARMSTRONG, R.W., EICHNER, E.R., KLEIN, D.E., BARTHEL, W.F., ET
AL. (1969) Pentachlorophenol poisoning in a nursery for
new-born infants. II. Epidemiological and toxicological
studies. J. Pediat., 75: 317-325.
BARLTROP, D. (1966) The prevalence of pica. Am. J. Dis.
Child., 112: 116-123.
BARLTROP, D. (1979) Metal-nutrient interactions in lead
absorption. In: Di Ferrante, E., ed. Trace metals: exposure
and health effects, Oxford, CEC/Pergamon Press, pp. 158-161.
BARLTROP, D. (1982) Nutritional and maturational factors
modifying the absorption of inorganic lead from the
gastrointestinal tract. In: Environmental factors in human
growth and development, New York, Cold Spring Harbor
Laboratory, pp. 35-41 (Banbury Report No. 11).
BARLTROP, D. & KHOO, H.F. (1975) The influence of
nutritional factors on lead absorption. Postgrad. Med. J., 51:
795-800.
BARLTROP, D. & OPPE, T.E. (1970) Dietary factors in neonatal
calcium homeostasis. Lancet, 2: 1333-1335.
BARLTROP, D. & STREHLOW, C.D. (1978) The absorption of lead
by children. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals, Munich, Technical University of
Munich, Freising-Weihenstephan, pp. 332-334.
BARR, H.M., STREISSGUTH, A.P., MARTIN, D.C., & HERMAN, C.S. (1984)
Infant size at 8 months of age: relationship to maternal use of
alcohol, nicotine, and caffeine during pregnancy. Paediatrics, 74:
336-341.
BARRACLOUGH, C.A. (1961) Production of anovulatory sterile rats
by single injection of testosterone propionate. Endocrinology, 68:
62-67.
BARRACLOUGH, C.A. (1966) Modification in the CNS regulation
of reproduction after exposure of prepubertal rats to steroid
hormones. Recent. Progr. Horm. Res., 22: 503-539.
BARRY, P.S.I. (1975) A comparison of concentrations of lead
in human tissues. Br. J. ind. Med., 32: 119-139.
BERLIN, M. (1983) Toxicokinetics of mercury. In: Schmidt,
E.H.F. & Hildebrandt, A.G., ed. Health evaluation of heavy
metals in infant formula and junior food, Berlin, Springer
Verlag, pp. 147-161.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRAUNLICH, H. (1981) Excretion of drugs during postnatal
development. Pharmac. Ther., 12: 299-320.
BRITISH PAEDIATRIC ASSOCIATION (1956) Hypercalcaemia in
infants and vitamin D. Br. med. J., 2: 149.
CHABROLLE, J.P. & ROSSIER, A. (1978) Goitre and
hypothyroidism in the newborn after cutaneous absorption of
iodine. Arch. Dis. Child., 53: 495-498.
CLARKSON, T.W., AMIN-ZAKI, L., & AL-TIKRITI, S.K. (1976) An
outbreak of methylmercury poisoning due to consumption of
contaminated grain. Fed. Proc., 35: 2394-2399.
COLLIPP, P.J., CHEN, S.Y., & MAITINSKY, S. (1983) Manganese
in infant formulas and learning disability. Ann. Nutr. Metab.,
27: 488-494.
CURLEY, A., COPELAND, R., & KIMBROUGH, R.D. (1969)
Chlorinated hydrocarbon insecticides in organs of stillborn
and blood of new-born babies. Arch. environ. Health, 19:
628-632.
CZABA, G. (1984) The present state in the phylogeny and
ontogeny of hormone receptors. Horm. Metab. Res., 16: 329-335.
DEAN, R.F.A. & MCCANCE, R.A. (1947a) Response of new-born
children to hypertonic solutions of sodium chloride and urea.
Nature (Lond.), 160: 904-906.
DEAN, R.F.A. & MCCANCE, R.A. (1947b) Inulin, diodone,
creatinine, and urea clearances in new-born infants. J.
Physiol., 106: 431-439.
DEAN, R.F.A. & MCCANCE, R.A. (1948) Phosphate clearances in
infants and adults. J. Physiol., 107: 182-186.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1984) [Residues and
contaminants in breastmilk,] Weinheim, Verlag Chemie (in
German).
DICKERSON, J.W.T. (1962a) The effect of development on the
composition of a long bone of the pig, rat, and fowl. Biochem.
J., 82: 47-55.
DICKERSON, J.W.T. (1962b) Changes in the composition of the
human femur during growth. Biochem. J., 82: 56-61.
DIEM, K. & LENTNER, C., ed. (1970) Scientific tables, 7th
ed., Basel, J.R. Geigy A.G.
DOBBING, J. & SANDS, J. (1973) The quantitative growth and
development of the human brain. Arch. Dis. Child., 48: 757-767.
DORNER, G. & STAUDT, J. (1972) [Comparative morphology of
the hypothalamus: differences between the rat and man.]
Endokrinologie, 59: 152-155 (in German).
EDELMANN, C.M., Jr & SPITZER, A. (1976) The kidney. In:
Smith, C.M. & Nelson, N.M., ed. The physiology of the new-born
infant, 4th ed., Springfield, Illinois, pp. 416-458.
EPPS, E.A., Jr, BONNER, F.L., & COWART, R.P. (1974) Dieldrin
contamination of milk by use of second-hand bags for feed.
Bull. environ. Contam. Toxicol., 12: 209-211.
ERDOSOVA, R., KRAUS, M., & REHULKA, J. (1977) Corticosterone
synthesis and serum levels at the end of the perinatal period:
a study of the effects of stress, diazepam, and polyethylene
glycol treatments. Physiol. bohemoslov., 26: 297-302.
FAITH, R.E. & MOORE, J.A. (1977) Impairment of thymus-
dependent immune functions by exposure of the developing
immune system to 2,3,7,8-tetrachlorobenzeno-p-dioxin (TCDD).
J. Toxicol. environ. Health, 3: 451-464.
FAITH, R.E., LUSTER, M.I., & KIMMEL, C.A. (1979) Effect of
chronic developmental lead exposure on cell-mediated immune
functions. Clin. exp. Immunol., 35: 413-420.
FARA, G.M., DEL CORNO, G., BONETTI, F., CARAMASCHI, L.,
DARDANONI, L., FAVARETTI, C., JIAMBELLUCA, S.E., MARNI, E.,
MOCARELLI, P., MONTESARCHIO, E., PUCCINELLI, V., & VOLPATO,
C. (1982) Chloracne after release of TCDD at Seveso, Italy.
In: Hutzinger, O., Frei, R.W., Merian, E., & Pocchiari, F.,
ed. Chlorinated dioxins and related compounds, Oxford,
Pergamon Press, pp. 545-560.
FLERKO, B. (1975) Perinatal androgen action and the
differentiation of the hypothalamus. In: Brazier, M.A.B., ed.
Growth and development of the brain, New York, Raven Press,
pp. 117-137.
FORBES, G.B. & REINA, J.C. (1972a) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. Health
Phys., 22: 169-175.
FORBES, G.B. & REINA, J.C. (1972b) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. J. Nutr.,
102: 647-652.
GIACOIA, G.P. & CATZ, C.S. (1979) Drugs and pollutants in
breast milk. In: Clinics in perinatology, Vol. 6, pp. 181-196.
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Contamination of milk with hexachlorobenzene residues.
Ind. Aliment. Agric., 89: 31-35.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
London, Academic Press.
GREAVES, S.J., FERRY, D.G., MCQUEEN, E.G., MALCOLM, D.S., &
BUCKFIELD, P.M. (1975) Serial hexachlorophene blood levels
in the premature infant. N.Z. med. J., 81: 334-336.
HACIK, T. (1969a) Effect of estradiol on adrenal and gonadal
functions during postnatal development in female rats. Endocr.
Exper., 3: 147-153.
HACIK, T. (1969b) Postnatal development of androgenized
female rats. Physiol. bohemoslov., 18: 263-269.
HACIK, T. (1970) Effect of estrogens on adrenal function and
body growth during postnatal period in male rats. Endocr.
Exper., 4: 31-38.
HAERING, M. & SCHEFER, W. (1980) Quantitative analysis of
biocide residues. Chimia, 34: 397-400.
HARRISON, H.E. (1980) Vitamin and mineral deficiencies in
intestinal malabsorption. In: Lifshitz, F., ed. Clinical
disorders in pediatric gastorenterology and nutrition, New
York, Dekker, pp. 371-375.
HEISE, E.R. (1982) Diseases associated with immuno-
suppression. Environ. Health Perspect., 43: 9-19.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Determination of
cadmium in liver and kidneys of children and adolescents by
neutron activation analysis.] Arch. Toxicol., 26: 8-16 (in
German).
HILL, J.R. (1964) The development of thermal stability in
the new-born baby. In: Jonxis, J.H.P., Visser, H.K.A., &
Troelstra, J.A., ed. The adaptation of the newborn to
extrauterine life, Netherlands, H.E. Stenfert Kroese NV, pp.
223-228.
HISLOP, A. & REID, L. (1981) Growth and development of the
respiratory system. In: Davis, J.A. & Dobbing, J., ed.
Paediatrics, 2nd ed., London, Heinemann, pp. 390-432.
HOAR, R.M. & MONIE, I.W. (1981) Comparative development of
specific organ systems. In: Kimmel, C.A. & Buelke-Sam, J., ed.
Developmental toxicology, New York, Raven Press, pp. 13-33.
HOFFMANN, H. (1982) Absorption of drugs and other
xenobiotics during development in experimental animals.
Pharmac. Ther., 16: 247-260.
ISLEIB, D.R. & WHITEHEAD, G.L. (1975) Polybrominated
biphenyls: an agricultural accident and its consequences. I.
The agricultural effects of exposure. In: Hemphill, D.D., ed.
Trace substances in environmental Health. IX, pp. 47-55.
JAPAN/US COOPERATIVE SCIENCE PROGRAM (1983) Toxicity of
chlorinated biphenyls, dibenzofurans, dibenzodioxins, and
related compounds. In: Proceedings of a Joint Seminar,
Fukvoka, Japan, pp. 25-28.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Res. Rev., 89: 1-128.
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
JONES, K.L. & SMITH, D.W. (1975) The fetal alcohol syndrome.
Teratology, 12: 11-26.
JUGO, S. (1980) Chelatable fraction of 203Pb in blood of
young and adult rats. Environ. Res., 21: 336-342.
KAVLOCK, R.J. & GRAY, J.A. (1983) Morphometric, biochemical,
and physiological assessment of perinatally induced renal
dysfunction. J. Toxicol. environ. Health, 11: 1-3.
KIESE, M. (1974) Methaemoglobinaemia: a comprehensive
treatise, Ohio, CRC Press, pp. 17-19.
KIMBROUGH, R.D., TAINES, T.V., & LINDER, R.E. (1974) The
effect of 2,4-dichlorophenyl- p -nitrophenyl ether on the rat
fetus. Arch. environ. Health, 28: 316-320.
KITAMURA, Y., MIYAO, M., & KAWAZOE, K., ET AL. (1953) In:
Friberg, L., ed. Toxic metals and their implications for human
health. Acta med., 8: 205-225.
KLINGER, W. (1982) Biotransformation of drugs and other
xenobiotics during postnatal development. Pharmac. Ther., 16:
377-429.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
KOSTIAL, K., SIMONOVIC, I., & PISONIC, M. (1971) Lead
absorption from the intestine in new-born rats. Nature
(Lond.), 233: 564.
KOSTIAL, K., KELLO, D., JUGO, S., RABAR, I., & MALJKOVIC, T.
(1978) The influence of age on metal metabolism and toxicity.
Environ. Health Perspect., 25: 81-86.
KROGER, M. (1972) Insecticide residues in human milk.
Pediatrics (Springfield), 80: 401-405.
KURATSUNE, M. (1980) "Yusho". In: Kimbrough, R.D., ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzo-
dioxins, and related products, Amsterdam, Elsevier/North
Holland Biomedical Press, pp. 287-302 (Topics in Environmental
Health No. 4).
L'ALLEMAND, D., GRUTERS, A., HEIDEMANN, P., & SCHURNBRAND, P.
(1983) Iodine-induced alterations of thyroid function in
new-born infants after prenatal and perinatal exposure to
povidone iodine. J. Pediatr., 102: 935-938.
LANDSBERG, J.D., BODYFELT, F.W., & MORGAN, M.E. (1977)
Retention of chemical contaminants by glass, polyethylene, and
polycarbonate multi-use containers. J. Food Protect., 40:
772-777.
LANGSTON, C. (1983) Normal and abnormal structural
development of the human lung. In: Abnormal functional
development of the heart, lungs, and kidneys: approaches to
functional teratology, New York, Alan R. Liss, pp. 75-91.
LAWTHER, P.J. & WALLER, R.E. (1975) Physical hazards. In:
Barltrop, D., ed. Paediatrics and the environment. Fellowship
of Postgraduate Medicine/Unigate Paediatric Workshop No. 2,
London, pp. 5-9.
LEVINE, S. & MULLINS, R. (1967) Neonatal androgen or
estrogen treatment and the adrenal cortical response to stress
in adult rats. Endocrinology, 80: 1177-1179.
LIBERTUN, C. & LAU, C. (1972) Adrenocortical function in
prepuberal rats: neonatal effects of testosterone. J. Endocr.,
55: 221-222.
LOIZZO, A., GATTI, G.L., MACRI, A., MORRETTI, G., ORTOLANI,
E., & PALAZZESI, S. (1984) Italian baby food containing
diethylstilboestrol: three years later. Lancet, 1: 1014-1015.
LUSTER, M.I., FAITH, R.E., & KIMMEL, D.A. (1978) Depression
of humoral immunity in rats following chronic developmental
lead exposure. J. environ. Pathol. Toxicol., 1: 397-402.
LUSTER, M.I., BOORMAN, G.A., DEAN, J.H., HARRIS, M.W., LUEBKE,
R.W., PADARATHSINGH, M.L., & MOORE, J.A. (1980) Examination
of bone marrows, immunological parameters, and host suscept-
ibilities following pre- and postnatal exposure to 2,3,7,8-
tetrachlorodibenzo- p -dioxin (TCDD). Int. J. Immunopharmacol.,
2: 301-310.
MCCANCE, R.A. (1948) Renal function in early life. Physiol.
Rev., 28: 331-348.
MCCANCE, R.A. & WIDDOWSON, E.M. (1956) Metabolism, growth,
and renal function of piglets in the first days of life. J.
Physiol., 133: 373-384.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957a) New thoughts on
renal function in the early days of life. Br. med. Bull., 13:
3-6.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957b) Hypertonic expansion
of the extracellular fluids. Acta paediatr., 46: 337-353.
MCCANCE, R.A. & YOUNG, W.F. (1940) The secretion of urine by
new-born infants. J. Physiol., 99: 265-282.
MCLACHLAN, J.A., NEWBOLD, R.R., KORACH, K.S., LAMB, J.C., &
SUZUKI, Y. (1981) Transplacental toxicology: prenatal
factors influencing postnatal fertility. In: Kimmel, C.A. &
Buelke-Sam, J., ed. Developmental toxicology, New York, Raven
Press, pp. 213-232.
MACHO, L., STRBAK, V., & HROMADOVA, M. (1972) The effect of
thyroxine on amino acid incorporation into protein during
ontogenesis in rats. Hormones, 3: 354-360.
MAHAFFEY, K.R. (1981) Nutritional factors in lead poisoning.
Nutrit. Rev., 39: 353-362.
MAHAFFEY, K.R. (1983) Absorption of lead by infants and
young children. In: Schmidt, E.H.F. & Hildebrandt, A.G., ed.
Health evaluation of heavy metals in infant formula and junior
food, Berlin, Springer Verlag, pp. 69-85.
MALPUECH, G., GAILLARD, G., GAULME, J., DOLY, M., BESSE, G.,
GOUMY, P., & RAYNAUD, E.J. (1978) Hypothyroidie transitoire
chez huit nouveau-nes de petit poids de naissance. Arch.
Franc. Ped., 35: 620-630.
MARWHA, H., NAYAK, N.C., ROY, S., KALRA, V., & GHAI, O.P.
(1981) The role of excess hepatic copper in the evaluation of
Indian childhood cirrhosis. Indian J. med. Res., 73: 395-403.
MARZULLI, F.N. & MAIBACH, H.I. (1975) Relevance of animal
models: the hexachlorophene story. In: Maibach, H., ed. Animal
models in dermatology, New York, Churchill Livingstone, pp.
156-167.
MAURER, H.M., WOLFF, J.A., FINSTER, M., POPPERS, P.J.,
PANTUCK, E., KUNTZMAN, R., & CONNEY, A.H. (1968) Reduction
in concentration of total serum-bilirubin in offspring of
women treated with phenobarbitone during pregnancy. Lancet, 2:
122-124.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
MIYABE, M., TSUBOUCHI, H., & SAKABE, Y. (1971) Studies on
pesticide residues in foods. II. Residues of BHC in milk. Rep.
Nagoya Munic. Pub. Health Lab., 18: 37-41.
MOMCILOVIC, B. & KOSTIAL, K. (1974) Kinetics of lead
retention and distribution in suckling and adult rats.
Environ. Res., 8: 214-220.
MORSELLI, P.L. (1976) Clinical pharmacokinetics in neonates.
Clin. Pharmacokin., 1: 81-98.
MRC (1984) The neuropsychological effects of lead in
children: a review of recent research, (1979-83), London,
Medical Research Council.
NATIONAL RESEARCH COUNCIL, SUBCOMMITTEE ON AIRBORNE PARTICLES
(1979) Epidemiologic studies on the effects of airborne
particles on human health. In: Airborne particles, Baltimore,
Maryland, University Park Press, pp. 167-198.
NIEMINERS, L., BJORNDAHL, K., & MOTTONEN, M. (1973) Effect
of hexachlorophene on the rat brain during organogenesis. Food
cosmet. Toxicol., 11: 635-639.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
NORDBERG, G.F. (1979) Factors influencing metabolism and
toxicity of metals. In: Di Ferrante, E., ed. Trace metals:
exposure and health effects, Oxford, CEC/Pergamon Press, pp.
157-158.
PAVEY, D.E. & WIDDOWSON, E.M. (1980) Body lipids of
guinea-pigs exposed to different dietary fats from
mid-gestation to 3 months of age. V. The fatty acid
composition of brain lipids at birth. Nutr. Metab., 24:
357-366.
PETERS, H.A. (1976) Hexachlorobenzene poisoning in Turkey.
Fed. Proc., 35: 2400-2403.
RAMBOER, C., THOMPSON, R.P.H., & WILLIAMS, R. (1969)
Controlled trials of phenobarbitone therapy in neonatal
jaundice. Lancet, 1: 966-968.
ROBERTS, D.W. & CHAPMAN, J.R. (1981) Concepts essential to
the assessment of toxicity to the developing immune system.
In: Kimmel, C.A. & Buelke-Sam, J., ed. Developmental
toxicology, New York, Raven Press, pp. 167-189.
ROGAN, W. & GLADEN, B. (1982) Duration of breast-feeding and
environmental contaminants in milk. Am. J. Epidemiol., 116:
565A.
ROOK, J.J. (1974) Formation of halogens during clorination
of natural waters. J. Water Treat. Exam., 23: 234-243.
ROWLAND, I.R., ROBINSON, R.D., DOHERTY, R.A., & LANDRY, T.D.
(1983) Are developmental changes in methylmercury metabolism
and excretion mediated by the intestinal microflora? In:
Clarkson, T.W., Nordberg, G.F., & Sager, P.R., ed.
Reproductive and developmental toxicity of metals, New York,
Plenum Press, pp. 745-758.
SAENZ DE RODRIGUEZ, C.A. & TORO-SOLA, M.A. (1982) Anabolic
steroids in meat and premature telarche. Lancet, 1: 1300.
SCAGLIONI, S., DI PIETRO, C., BIGATELLO, A., & CHIUMELLO, G.
(1978) Breast enlargement at an Italian school. Lancet, 1:
551-552.
SCHELER, W. (1980) [The basis of general pharmacology,] 2nd
ed., Stuttgart, Gustav Fischer Verlag (in German).
SCHMIDT, R.R. (1984) Altered development of immunocompetence
following prenatal or combined perinatal-postnatal insult: a
timely review. J. Am. Coll. Toxicol., 3: 57-72.
SHERLOCK, J.C., SMART, G., FORBES, G.I., MOORE, M.J.,
PATTERSON, W.J., RICHARDS, W.N., & WILSON, T.S. (1982)
Assessment of lead intakes and dose response for a population
in Ayr exposed to a plumbosolvent water supply. Hum. Toxicol.,
1: 115-122.
SHUMAN, R.M., LEECH, R.W., & ALVORD, E.C., Jr (1975)
Neurotoxicity of hexachlorophene in humans. II. A clinico-
pathological study of 46 premature infants. Arch. Neurol., 32:
320-325.
SINGER, E.J., WEGMANN, P.C., LEHMAN, M.D., CHRISTENSEN, M.S.,
& VINSON, L.J. (1971) Barrier development, ultrastructure,
and sulfhydryl content of the fetal epidermis. J. Soc. Cosmet.
Chem., 22: 119-137.
SMITH, R.J. (1982) Hawaiian milk contamination. Science
N.Y., 217: 137-140.
SVENNERHOLM, L., ALLING, C., BRUCE, A., KARLSSON, I., & SAPIA,
O. (1972) Effects on offspring of maternal malnutrition in
the rat. In: Elliott, K. & Knight, J., ed. Lipids,
malnutrition, and the brain, Amsterdam, Associated Scientific
Publishers, pp. 141-157.
TADJER, G.S. & DORE; I. (1971) Possibility of contamination
of bottles used by food industries by insecticides. Harefuah,
81: 385.
TAKEWAKI, K. (1968) Reproductive organs and anterior
hypophysis of neonatally androgenized female rats. Sci. Rep.
Tokyo Women's Christ. Coll., 1-6: 31-47.
TANNER, J.M., WHITEHOUSE, R.H., & TAKAISHI, M. (1966)
Standards from birth to maturity for height, weight, height
velocity, and weight velocity. Part II. British children,
1965. Arch. Dis. Child., 41: 613-635.
THABREW, M.W., OLORUNSOGO, O.O., OLOWOOKERE, J.O., &
BABABUNMI, E.A. (1982) Possible defect in xenobiotic
activation before glycine conjugation in protein-energy
malnutrition. Xenobiotica, 12: 849-853.
TROLLE, D. (1968) Phenobarbitone and neonatal icterus.
Lancet, 1: 251-252.
TURNER, M.D., MARSH, D.O., SMITH, J.C., INGLIS, J.B.,
CLARKSON, T.W., RUBIO, C.E., CHIRIBOGA, J., & CHIRIBOGA, C.C.
(1980) Methylmercury in populations eating large quantities
of marine fish. I. Northern Peru. Arch. environ. Health,
35(6): 367-378.
ULSAMER, A.G., YODER, P.D., KIMBROUGH, R.D., & MARZULLI, F.N.
(1975) The effect of hexachlorophene on developing rats:
toxicity tissue concentrations and biochemistry. Food cosmet.
Toxicol., 13: 69-80.
UMBERGER, E.J. (1975) Products marketed to promote growth in
food-producing animals: steroid and hormone products.
Toxicology, 3: 3-21.
UNITED KINGDOM CENTRAL DIRECTORATE ON ENVIRONMENTAL POLLUTION
(1982) The Glasgow duplicate diet study (1979/80), London,
Her Majesty's Stationery Office (Pollution Report No. 11).
UNITED KINGDOM DEPARTMENT OF HEALTH AND SOCIAL SECURITY
(1980) Lead and health, London, Her Majesty's Stationery
Office (Report of a DHSS Working Party on Lead in the
Environment).
URSO, P. & GENGOZIAN, N. (1980) Depressed humoral immunity
and increased tumour incidence in mice following in utero
exposure to benzo( a )pyrene. J. Toxicol. environ. Health, 6:
569-576.
US DHEW (1979) Smoking and health: a report of the Surgeon
General, Washington DC, US Department of Health, Education and
Welfare (Section No. 8).
VALCANA, T. & TIMIRAS, P.S. (1978) Nuclear triiodothyronine
receptors in the developing rat brain. Mol. Cell. Endocrinol.,
11: 31-41.
VALE, J.A. & MEREDITH, T.J. (1981) Epidemiology of poisoning
in the UK. In: Vale, J.A. & Meredith, T.J., ed. Poisoning
diagnosis and treatment, 1st ed., London, Update Books, pp.
1-8.
VESSELINOVITCH, S.D., MIHAILOVICH, N., WOGAN, G.N., LOMBARD,
L.S., & RAO, K.V.N. (1972) Aflatoxin B1, a hepato-
carcinogen in the infant mouse. Cancer Res., 32: 2289-2291.
VILLETT, L.B. & HESS, J.F., Jr (1975) Polychlorinated
biphenyl residues in silos in the US. Residue Res., 55:
135-147.
VOS, J.G. (1977) Immune suppression as related to
toxicology. CRC Crit. Rev. Toxicol., 5: 67-101.
VOS, J.G. & MOORE, J.A. (1974) Suppression of cellular
immunity in rats and mice by maternal treatment with
2,3,7,8-tetrachlorodibenzo- p -dioxin. Int. Arch. Allergy appl.
Immunol., 47: 777-794.
VOS, J.G., VAN DOGTEN, M.J., KREEFTENBERG, J.G., STEERENBERG,
P.A., & KRUIZINGA, W. (1979) Effect of hexachlorobenzene on
the immune system of rats following combined pre- and
postnatal exposure. Drug Chem. Toxicol., 2: 61-76.
WALKER, W.A. (1979) Gastrointestinal host defence:
importance of gut closure in control of macromolecular
transport. In: Elliott, K. & Whelan, J., ed. Development of
mammalian absorptive processes, Amsterdam, Excerpta Medica,
pp. 201-216 (Ciba Foundation Symposium No. 70 (new series)).
WESTER, R.C. & MAIBACH, H.I. (1982) Percutaneous absorption:
neonate compared to the adult. In: Hunt, V.R., Smith, M.K., &
Worth, D., ed. Environmental factors in human growth and
development, New York, Cold Spring Harbor Laboratory (Banbury
Report No. 2).
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1984) Environmental Health Criteria 30: Principles for
evaluating health risks to progeny associated with exposure to
chemicals during pregnancy, Geneva, World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WIDDOWSON, E.M. & DICKERSON, J.W.T. (1964) Chemical
composition of the body. In: Comar, C.L. & Bronner, F., ed.
Mineral metabolism, New York, Academic Press, Vol. 2A, pp.
1-247.
WIDDOWSON, E.M., CRABB, D.E., & MILNER, R.D.G. (1972)
Cellular development of some human organs before birth. Arch.
Dis. Child., 47: 652-655.
WILLOUGHBY, M.L.N. (1984) Disorders of the blood and
reticuloendothelial system. In: Forfar, J.O. & Arneil, G.C., ed.
Textbook of paediatrics, 3rd ed., Edinburgh, Churchill
Livingstone, Vol. 1, pp. 935-982.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
WONG, K.L. & KLAASSEN, C. (1980) Tissue distribution and
retention of cadmium in rats during postnatal development:
minimal role of hepatic metallothionein. Toxicol. appl.
Pharmacol., 53: 343-353.
ZIEGLER, E.E., EDWARDS, B.B., JENSEN, R.L., MAHAFFEY, K.R., &
FOMON, S.J. (1978) Absorption and retention of lead by
infants. Pediatr. Res., 12: 29-34.
APPENDIX I: BREAST MILK
1. Introduction
The breast-fed infant requires special consideration in
circumstances in which chemical contamination of the mother's milk
is suspected, or has been confirmed. Young infants are
characteristically dependent on milk, and the structural and
functional development of their organs and tissues is adapted to
the milk of their own species. The potential risks of exposure to
chemicals in breast milk must therefore be assessed, taking into
account the benefits of breast-feeding together with the
disadvantages of feeding an alternative milk, in terms of
developmental toxicology.
The benefits of breast-feeding, particularly in the developing
countries, are now universally acknowledged. In these societies,
nearly all women of all classes breast-feed for 12 months or more.
The decline in breast-feeding in many developed countries has
recently been reversed; interestingly, both the decline in, and
return to, breast-feeding began among the most socially advantaged
women (WHO, 1981). National and international authorities have
been concerned that, in view of the health risks involved in the
preparation and provision of breast milk substitutes in developing
societies, mothers in these countries should not go through the
changes in infant feeding practice that have occurred in the
developed world. Thus, the Member States of the World Health
Organization have committed themselves to the protection and
promotion of breast-feeding as the first, and one of the most
critical, steps in sound infant nutrition policies (WHO/UNICEF,
1981).
This Appendix is concerned with principles for evaluating the
possible consequences to health of chemical contaminants in breast
milk. The evaluation of risks potentially associated with these
chemicals in the quantities that may be consumed must be carefully
weighed against the assessment of the risks of alternative methods
of infant feeding.
The breast milk of a well-nourished mother has an optimal
composition to meet the nutritional needs of the full-term infant
in early life, and there are associated immunological,
psychological, and economic advantages. In view of the importance
of breast milk for the nutrition and health of the infant, evidence
of any harmful effects of a foreign chemical that it may contain
must be carefully considered before recommendations are made that
breast-feeding should be curtailed or discontinued.
2. Assessment of the Importance of Chemicals in Breast Milk
2.1 Source of chemicals
Women may be exposed to chemical substances from many different
sources, including air, food, water, drugs, cosmetics, and the
occupational environment.
2.2 Chemicals within the mother's body and their secretion in milk
Chemicals that are easily metabolized and are eliminated from
the mother's body in the faeces and urine are only secreted in
significant quantities in breast milk following recent high-level
chemical exposure (Wolff, 1983; Jensen, 1985).
Other chemicals, such as heavy metals and persistent
halocarbons, are less readily removed from the body. Some metals
accumulate mainly in the liver, kidney, and bone, whereas
persistent halocarbons accumulate in the lipids of the body. Long-
term exposure, even to low concentrations of such chemicals, may
build up considerable body stores. On the other hand, a short-term
change of diet is not likely to alter the concentration of
halocarbon in the mother's fatty tissues.
A dynamic equilibrium for persistent lipophilic chemicals
exists between different tissue compartments. The concentrations
of these chemicals in adipose tissue, blood, and milk is in
proportion to the fat content; thus, the concentration in blood is
far lower than that in whole milk, and the concentration in milk is
far lower than that in adipose tissue. There is a relatively good
correlation between concentrations of organic halocarbons in the
lipids of blood, adipose tissue, and milk. This is illustrated for
DDT in the lipids of milk and adipose tissue in Fig. I.1.
Table 1. Increase in weight of body and
organs during the first 9 months after
birtha
-----------------------------------------
Body/organ Increase
(g/kg weight at birth)
-----------------------------------------
Body 1810
Brain 1480
Liver 1420
Kidneys 1580
Heart 1160
Lungs 1500
-----------------------------------------
a Based on: values given in Diem & Lentner (1970).
The constituents of some organs and tissues have a slower rate
of turnover than those of others. The lipids, for example, once
laid down, have a slow rate of turnover compared with protein, in
all parts of the body including the brain. Fat-soluble chemicals,
which might be incorporated into brain lipids during the rapid
growth of the brain in infancy (Dobbing & Sands, 1973), would not
enter the tissue in significant amounts after the growth of the
brain has ceased. The fatty acid composition of brain lipids of
rats and guinea-pigs can be appreciably altered, early in life, by
the nature of the fat in the diet of the mother during pregnancy
and lactation (Svennerholm et al., 1972; Pavey & Widdowson, 1980).
Information on man is lacking.
1.3.4 Different body composition from adult
Infants have a different body composition from adults. As in
adults, the contribution of fat to the body weight varies
considerably between one individual and another in infants, but the
average is about 15% in full-term new-born infants and in adult
men. However, infants have a higher percentage of water and a
smaller percentage of solids in the lean body tissue. The infant,
at term, has 82% of water in its lean tissue compared with 72% in
the adult (Widdowson & Dickerson, 1964). The additional water in
the infant is mainly extracellular, the volume of extracellular
fluid per unit body weight in the young infant being about twice
that in an adult (Widdowson & Dickerson, 1964). Thus, any chemical
that is confined to the extracellular compartment of the body will
have a greater volume in which to distribute itself per unit body
weight. The advantage of this for the infant could be offset by
the slower rate of excretion by the kidneys (section 3).
The percentage of water in the organs and tissues, and thus in
the body as a whole, decreases with age. The levels of water in
some organs at birth, at 6 - 12 months where figures are available,
and in the adult (Widdowson & Dickerson, 1964) are shown in Table
2. The decrease in water in the liver, kidneys, heart, and lungs
is due primarily to an increase in protein; in the brain, it is due
to an increase in myelin.
Table 2. Water in organs and tissues (g/kg)a
-------------------------------------------------
Organ/tissue At birth 6 - 12 Adult
months
-------------------------------------------------
Whole body (fat-free 820 780 720
basis)
Brain 900 830 770
Liver 780 760 710
Kidneys 840 - 810
Heart 840 830 830
Lungs 860 - 790
Skeletal muscle
Total 800 780 790
Extracellular 350 290 180
Intracellular 450 490 610
-------------------------------------------------
a From: Widdowson & Dickerson (1964).
Another difference between infants and adults is that most of
the cells of the organs and tissues of infants are smaller than
those of adults (Widdowson et al., 1972). Small cells, like small
bodies, have a larger surface area in relation to mass than bigger
cells and bodies, and this in itself may have important
implications for chemicals that may enter the cells.
The bones of an infant contain more water and less fat,
protein, and mineral than adult bones. This is illustrated in
Table 3 for the whole femur (Dickerson, 1962b). Unlike soft
tissues, the chemical maturation of the bone, as evidenced by the
decrease in percentage of water and increase in degree of
calcification, mainly takes place after 1 - 2 years. From the
values in Table 3, it can be calculated that the increment per
month of calcium in one femur is approximately 0.2 g between birth
and 18 months, 0.48 g between 18 months and 11 years, and 1.0 g
between 11 and 18 years. The femur may not be completely typical
of all parts of the skeleton, but it does seem likely that the rate
of deposition of chemicals taken up by the bone along with calcium,
and indeed with magnesium, sodium, and other metals present in
bone, is likely to be greater in later childhood than in infancy.
However, the bones of infants are smaller, and the rate of
deposition of calcium per kg of bone per month is greater in
infancy, being 7.8 g/kg bone between birth and 3 months, 6.8 g/kg
between 3 and 18 months, and 1.9 g/kg bone between 18 months and 18
years. Thus, whether the bones of infants are considered likely to
take up more or less of a chemical than those of older children
depends partly on the method of expression of the rate of
deposition.
Table 3. Composition of the whole femur of the infant, child, and adulta
---------------------------------------------------------------------
At birth 3 months 1 - 2 11 - 12 Adult
(full- (milk years years (pre-
term) feeding) (weaned) adolescent)
---------------------------------------------------------------------
Weight femur g 16.6 26.4 63.7 326 646
Fat g/kg fresh bone 1.4 6.5 75 26 31
Composition g/kg
fresh fat-free bone
Water 639 641 554 364 227
Collagen 93 107 141 163 173
Ca 61 54 70 138 194
P 28 27 33 63 83
---------------------------------------------------------------------
a From: Dickerson (1962b).
1.3.5 Functional immaturity of the organs and systems of the body
Most organs and systems of the body have not reached structural
or functional maturity at birth. Development of the nervous system
continues in postnatal life, much of the myelination of the brain
takes place after birth and continues until adolescence (Hoar &
Monie, 1981). The structural development of the lung also
continues postnatally with an increase in alveolar surface area
(Langston, 1983). Several components of the immune system are not
fully developed at birth, for example, the lymphocytes responsible
for producing antibodies, and it has been suggested that this is
responsible for the greater susceptibility of the new-born infant
to certain bacterial infections (Andersson et al., 1981). The
gastrointestinal, endocrine, and reproductive systems, and also
renal function, are all immature at birth. The kidneys are less
able than those of older children and adults to eliminate excessive
amounts of substances normally present in milk, for example, sodium
and phosphate (Dean & McCance, 1947a, 1948; Barltrop & Oppé, 1970),
and this may also apply to extraneous chemicals present in, and
absorbed from, milk. Moreover, the activities of many liver enzyme
systems that metabolize endogenous and exogenous substances may be
low in the new-born infant. These topics are covered in more
detail in section 3.
1.3.6 Breast milk, infant formulae, and water as sources of
chemicals
The young infant lives on a single food, milk, either from the
breast or the bottle. Thus, the presence of a chemical in breast
milk, or in the powder or water used to make up an infant formula,
is likely to have greater implications for the infant than for the
older child having mixed feeding, where milk forms only part of the
daily food. Furthermore, infants take in a much larger amount of
fluid per unit body weight than older children and adults. An
infant living on milk takes about one-seventh of its own weight of
water each day, which would correspond to 10 litres for a 70-kg
man. This large intake is necessary for the infant, because it
loses more water per kg body weight through the lungs, owing to the
greater passage of air through them, the skin, because of the
larger surface area, and the kidneys, because of the inability to
concentrate the urine to the same extent as the older child or
adult. Any chemical present in the water used to make up infant
formulae will, therefore, be taken in by an infant in larger
quantities per unit body weight than by an older child or adult
using the same water supply. This is discussed in more detail in
section 2.
Nowadays, it is common practice among manufacturers to add
vitamins and trace elements to their preparations. Occasionally,
these have been added in such large amounts that they have been
reported to be toxic, even though in smaller, physiological amounts
they are essential. Examples are vitamin D (British Paediatric
Association, 1956) and manganese (Collipp et al., 1983).
1.4 Applicability of Data on Experimental Animals to Human Infants
and Young Children
Data on the toxic effects of chemicals in man are frequently
incomplete or even absent, and this is particularly true for
infants and young children. Some insight can be gained from
observations on young animals, but any assessment of human risk on
the basis of such studies should take into account the differences
that exist between the species in their reactions to chemicals.
Moreover, the conditions under which the animal data were obtained
may not reflect the human circumstances under consideration.
Animal data must be considered, therefore, in the context of the
similarities and differences that are known to exist between man
and experimental animals, and conclusions need to incorporate a
margin of safety to accommodate known, and possibly unknown,
species differences.
1.4.1 Some similarities in growth and development between
man and experimental animals
In many ways, the differences between the young infant and
adult are the same in animals as they are in man. Thus, the infant
animal is always smaller than the adult and, thus, it has a larger
surface area in relation to its body weight. All animals that are
homoeothermic from the time of birth have a higher consumption of
air per unit body weight than the adult of the same species, at the
same environmental temperature. The young of all animals species
grow rapidly, often far more rapidly than the human infant, and
their body composition differs from that of the adult in the same
way that that of the human infant differs from that of the human
adult. The functions of the organs and systems are immature in
early life, and most mammalian species depend on mother's milk for
a period after birth.
The anatomical, physiological, and chemical differences between
the infant and adult of an animal species are therefore in the same
direction as they are in the human species, and, in general, the
effects of chemicals on the young of experimental animals can be
expected to differ from those on the adult of the species just as
they do in man. However, the differences between species make it
unwise to assume that the results obtained on a young animal will
necessarily apply to the human infant.
1.4.2 Some differences in growth and development between man and
experimental animals
Different animals are born at different stages of their
anatomical, physiological, and chemical development and, although
the qualitative differences between the infant and adult are
similar in man and experimental animals, the quantitative
differences are by no means the same. The rat is a widely used
experimental animal in studies on the effects of chemicals on the
infant body. Some stages of development that take place after
birth in the rat have already occurred in the human fetus before
birth. Examples are the anatomical and functional maturation of
the hypothalamus (Dörner & Staudt, 1972) and the deposition of body
fat. Some aspects of development, however, only occur after birth
in all species, for example, respiration of air and the full
functioning of the gastrointestinal tract.
The new-born rat is smaller than the new-born human infant in
comparison with the weight of the adult, and, during infancy, it
grows at a much more rapid rate. Thus, the discussion in section
1.3.3 about the human infant applies with even greater force to the
infant rat. The rat at birth has a less mature chemical
composition than the human infant, as evidenced by the higher
percentage of water in its lean body tissue (Widdowson & Dickerson,
1964). The greater degree of immaturity of the body composition of
the rat at birth is well illustrated by reference to the bones.
Characteristically, rat bones have very little fatty marrow, but,
apart from this, the composition of the adult femur is not very
different in the 2 species. At birth, however, the rat femur has
less than half the concentration of calcium that there is in the
human femur, more water, and considerably less collagen, which
makes up the matrix on which the bone mineral is deposited
(Dickerson, 1962a,b). In the rat, as in man, the major changes in
the composition of bone do not take place until the period of
infancy is over. The composition of bone, and of the body as a
whole, though less mature at the time of birth in the rat than in
man, changes much more rapidly. All this must be taken into
account in considering the effects of a chemical on the bones or
bodies of young rats compared with the effects on those of human
infants.
The new-born rat is less mature physiologically than the human
infant, and it matures more rapidly. This may limit the
application of results obtained on the young rat to the human
infant. This is true of the renal function (McCance, 1948), so
that the new-born rat is less able than the adult to excrete
chemicals administered to it (section 3.5.1).
The activity of the hepatic enzymes is low in both new-born
experimental animals and new-born human infants, but the whole
subject of enzyme activity in this context is complex and is
considered in more detail in section 3.
One difference between the young of man and some animal species
is the ability to absorb macromolecules from the intestine. In the
human infant, the passage of macromolecules across the intestinal
mucosa is very limited, but in the young of such species as the
pig, cow, sheep, and horse, macromolecules are transferred from
the milk into the circulation in quite large amounts for a limited
period of hours or days. In the rat, the transfer goes on all
through the suckling period (Walker, 1979). This may have a
bearing on the interpretation of the results of studies on the
intestinal absorption of certain chemicals by the new-born
experimental animal in relation to the human infant.
1.5 Conclusions
The infant and young child have different structural and
functional characteristics from those of the older child and adult,
which represent stages in normal growth and development. These
include a larger surface area in relation to weight, different body
composition, a higher metabolic rate and oxygen consumption and,
hence greater intake of air per unit body weight, rapid growth and
development of the body as a whole and of the organs and tissues,
particularly during the first 6 months after birth. Many organs
and tissues are functionally immature at the time of birth, and
they mature at different rates. Moreover, infants have greater
energy and fluid requirements per unit body weight than older
children and adults. All these characteristics must be taken into
account in considering principles for evaluating health risks from
chemicals during infancy and childhood.
The structural and functional differences between the infant
and adult animal tend to follow the same direction as in the human
species, but there are important quantitative differences in, for
example, the stage of development at birth, the rate of growth, and
the intestinal absorption of macromolecules. The age of the infant
rat or other animal corresponding to an age in the human infant
will differ according to the aspect of development under
consideration.
2. PATHWAYS OF EXPOSURE
2.1 Introduction
As with adults, the potential for exposure to chemicals for
infants and young children is through ingestion, inhalation, and
percutaneous absorption. Both voluntary and inadvertent exposures
may occur, but their nature and extent are modified by the
physiological, behavioural, and other characteristics peculiar to
this age group. Thus, the methods of nurture, the social,
cultural, and occupational proclivities of the family, the quality
of care and supervision, and the inherent behavioural patterns of
the child, may all be significant factors for determining or
modifying exposure.
The new-born infant may already have encountered toxic chemical
agents in utero by the placental route; for example, the sequelae
of maternal exposure to methylmercury (Clarkson et al., 1976),
ethanol (Jones & Smith, 1975), tobacco (US DHEW, 1979), and
narcotics (Barr et al., 1984) are well recognized. Moreover, the
young infant is an obligate milk feeder, and chemicals that
traverse the mammary gland in lactation are potentially important
contaminants of both human and artificial milk.
The use of appropriate cleaning and antiseptic chemicals for
the routine care of the skin, umbilicus, and clothing of the new-
born infant have all been associated with toxicity (Armstrong et
al., 1969). Similarly, effects from atmospheric contaminants have
been encountered in neonatal units from agents used on the clothing
or for the disinfection of apparatus and operational areas.
Development from infancy to childhood is marked by increasing
mobility and exploration of the immediate surroundings. Oral
exploration is commonplace towards the end of the first year of
life in which the mouth is used to sample the taste and texture of
the objects and materials that are encountered. Oral exploration
may be accompanied by the ingestion of a wide variety of substances
not normally regarded as food (pica) including soils, dusts, and
surface coatings in the vicinity of the home. Although mouthing
and pica diminish with continuing development after the first year
of life, hand to mouth activity tends to persist (Barltrop, 1966).
As a consequence of increasing mobility and diminishing
supervision, poisoning due to the ingestion of toxic substances is
very common in early childhood. For example, in England and Wales,
one-fifth of all hospital admissions for suspected poisoning
involved children under the age of 5 years, amounting to
approximately 20 000 admissions per annum. Household,
horticultural, agricultural, and industrial chemicals were all
implicated, though medicaments and therapeutic compounds were the
principal agents (Vale & Meredith, 1981). It is unlikely that all
acute ingestions are reported, and their prevalence is not known
with certainty. The quality of available supervision is an
important determinant of accidental poisoning and will itself be
modified by social, cultural, and economic factors.
Infants and children will also be subject to chemical exposure
resulting from contamination of the natural and domestic
environments that they share with adults. However, the provision
of foodstuffs, beverages, clothing, furniture, toys, and
medicaments, specifically manufactured or adapted for the young,
may modify the route of exposure for certain chemicals; efforts are
usually made to minimize the contamination of such materials with
chemicals that might be harmful.
2.2 The Alimentary Tract
2.2.1 Milk
Milk may be regarded as one of the routes of excretion of
foreign compounds that have entered the body of the lactating
mammal. The degree to which a particular chemical traverses the
mammary gland depends on several factors, including its own
physical and chemical properties, such as the degree of ionization
and binding in biological fluids, the solubility characteristics,
and the maternal plasma concentration achieved. Passive diffusion,
active transport, and reverse pinocytosis across the apical
membrane of the mammary alveolar cell may all be involved, but the
milk/plasma ratio cannot always be predicted for a given chemical
in a particular species. In general, transfer from plasma to milk
is enhanced by high solubility in lipids, low relative molecular
mass, and weak binding to plasma-proteins (Giacoia & Catz, 1979).
2.2.1.1 Human milk
Nearly all chemicals to which the mother is exposed may, to
some extent, be excreted in human milk (Jensen, 1983, 1985).
Contamination of human milk may result from the maternal diet,
environment, occupation, addiction, and medical treatment. Marked
variation in the concentrations of chemicals in milk, both between
and within individuals, is characteristic and may reflect the stage
of lactation, the time and method of sample collection, and the
age, weight, and parity of the mother. Chemical residues most
frequently reported in human milk are the organochlorine pesticides
(DDT) and industrial chemicals (PCBs and metabolites, HCH isomers,
and cyclodienes such as dieldrin). Lactation is a route of
excretion for some of these chemicals, and adverse health effects
have been noted in breast-fed infants when the concen-tration of
chemicals was extremely high, as in accidental poisoning outbreaks,
and in some cases of occupational exposure. During the Iraqi
outbreak of methylmercury poisoning of 1971-72 (Amin-Zaki et al.,
1974), mothers who consumed bread made from seed grains treated
with a methylmercury fungicide were found to have milk containing
8.6% of the concentration of mercury in maternal blood collected at
the same time (Amin-Zaki et al., 1976). Infants who were born
before their mothers ate the contaminated bread and who received
methylmercury only through suckling, were found to have blood-
mercury values of up to 1.0 g/litre. Follow-up assessment over 5
years showed that some of the children suffered impaired
neurological and mental development (Amin-Zaki et al., 1981).
In Turkey, bread prepared from seed grains treated with
hexachlorobenzene between 1955-59 also affected infants (Peters,
1976). In some villages, almost all children under the age of 2
years, who had been breast-fed by mothers who had eaten the
contaminated wheat, died of the condition known as "pembe yara"
(Turkish for pink sore), which included symptoms of weakness,
convulsions, and localized cutaneous annular erythema.
Reports of such episodes have been rare. However, if
confronted with such poisoning outbreaks, exposure of infants
through human milk should be considered.
2.2.1.2 Other types of milk
Cow's milk, and the milk of other species used for infant
feeding, are subject to contamination by many of the chemicals
found in human milk, together with others that reflect local
techniques of animal husbandry and veterinary practice. Infants fed
milk derived from a particular animal or herd may be at greater or
lesser risk of exposure to a given contaminant than those fed
artificial or manufactured milk derived from the pooled secretions
of multiple herds. Conversely, the pooling of milk during the
manufacture of infant feeds will tend to increase the number of
potential contaminants in the end-product, but may diminish their
concentrations.
The food sources of lactating animals may be contaminated by
local industrial wastes, by the application of pesticides, by
naturally-occurring substances, such as mycotoxins, or by storage
in containers or buildings previously contaminated with pesticides,
disinfectants, preservatives, and other chemicals. The
organochlorine pesticides are well recognized fodder contaminants.
Numerous examples are known of milk containing chemicals derived
from animal feeds including DDT, hexachlorocyclohexane (HCH)
(Miyabe et al., 1971), heptachlorepoxide (Smith, 1982), and
hexachlorobenzene (Goursaud et al., 1972). Chemicals in milk
derived from containers of contaminated animal feed include PCBs
(Villett & Hess, 1975), pentachlorophenol (PCP) (Haering &
Schefer, 1980), and dieldrin (Epps et al., 1974). The inadvertent
substitution of a polybrominated biphenyl (PBB) product for
magnesium oxide in prepared cattle feeds resulted in contamination
of the milk (Isleib & Whitehead, 1975).
Several examples of milk contamination occurring after
collection have been reported. Thus, iodophors and other
disinfectants (Kroger, 1972), dieldrin (Tadjer & Dore, 1971) and
chlordane (Landsberg et al., 1977) have all been identified in milk
stored in vessels that had been inadvertently rinsed or cleaned
with, or fabricated from, previously contaminated materials. The
inadvertent addition of toxic chemicals to infant milk during
manufacture is rare, though arsenic poisoning resulting from the
use of a contaminated sodium phosphate stabilizer has been reported
(Kitamura et al., 1953).
Pesticides and drugs for the control of parasitic infection and
other diseases in lactating animals are liable to be absorbed and
excreted in the milk, even after topical application. Milk
contamination with antibiotics and hormones, given for economic
rather than therapeutic or preventative indications, may also
occur.
2.2.1.3 Infant feeds
Foods and beverages, other than milk, which have been
specifically prepared or manufactured for infants and young
children, may be diverse in composition and comprise only a
proportion of the total dietary intake. However, they are subject
to the same sources of chemical contamination as foods intended for
adults, during producion, manufacture, and packaging. The
potential significance of contaminants is enhanced when the dietary
range is restricted.
Numerous examples of contamination at source have been
described in common with those for human and other milk. Thus,
residues of pesticides, antibiotics, fungicides, and heavy metals
have all been encountered as a result of agricultural techniques,
veterinary practice, and the use of contaminated land and water.
An additional factor with regard to meat products is, for example,
the use of anabolic agents, such as diethylstilboestrol, in animal
husbandry (Umberger, 1975). Thus, in one study, one-third of 450
samples of baby food containing veal were found to have detectable
estrogen activity (Loizzo et al., 1984).
Many chemical or non-nutritional substances are used in food
manufacture as preservatives, anti-oxidants, stabilizers,
emulsifiers, and thickening agents, with the intention of ensuring
that the food is wholesome and palatable, when consumed. Numerous
chemical colouring agents are potentially available, and their use
in infant foods has attracted controversy, since marketing rather
than nutritional considerations are involved.
Manufactured infant and junior foods are characteristically
distributed in appropriately small containers which, therefore,
have a relatively large surface area/volume ratio. The degree to
which contaminants derived from the container material, seams, or
internal surface coatings may affect the contents is inversely
proportional to the container volume. The degree to which the
container design and manufacture may influence food contamination
is indicated by the reduction of dietary lead intake observed in
infants in the USA during the last decade, when progressive
replacement of lead soldered side-seamed cans by welded or seamless
containers resulted in a 47% reduction in lead intake by infants
aged 0 - 5 months (Mahaffey, 1983).
Contamination of foods during preparation in the home may
result from the use of inappropriate vessels, culinary agents such
as cooking oils, and cleaning materials, as well as from water.
Thus, excessive intake of copper derived from domestic cooking
utensils has been implicated in Indian childhood cirrhosis (Marwha
et al., 1981), and contaminated edible oils were involved in an
outbreak of Yusho disease (Kuratsune, 1980; Japan/US Cooperative
Science Program, 1983).
The use of detergent chemicals for the cleaning of cooking and
eating utensils is inevitably associated with ingestion of the
chemicals by all members of the household, but, in normal usage, it
is unlikely that toxic intakes will be encountered. However,
intakes are likely to be enhanced when rinsing or wiping is
omitted.
2.2.1.4 Water
Water for domestic consumption may be contaminated by chemicals
at source, during treatment and transport, or in the home from
domestic plumbing, cisterns, or culinary utensils. The use of
recycled water, particularly during drought, will tend to enhance
the concentration of some pollutants. The direct ingestion of
water used in the preparation of foods and beverages will involve
children of all ages, depending on local climatic conditions and
cultural and dietary practices. However, the use of water to
reconstitute powdered artificial milk is a special case,
particularly where the milk is the sole nutrient source.
The potential contaminants of drinking-water are numerous and
include a wide range of metals and other elements; nitrates,
cyanide, thiocyanate, and ferrocyanate radicals; organic compounds
including pesticides; organic solvents; and anionic, cationic, and
non-ionic detergents. Asbestos and polyaromatic hydrocarbons have
been detected. The chlorination of water for disinfection,
particularly in the presence of humic material, results in the
formation of a number of haloforms or C-chlorinated compounds
including chloroform, bromodichloromethane, and
dibromochloromethane (Rook, 1974).
The significance of lead in drinking-water has been
increasingly appreciated, particularly in areas where lead pipes
and plumbosolvent water supplies co-exist. Studies in a population
in Scotland showed that there was a curvilinear relationship
between the concentration of lead in water and that in the blood of
artificially fed infants; 36% had blood-lead values in excess of
350 µg/litre (Sherlock et al., 1982). Similarly, lead intakes of
up to 3.4 mg/week were reported from Scotland in artificially fed
infants, whose food was measured and analysed, with correspondingly
marked increases in blood-lead values (United Kingdom Central
Directorate on Environmental Pollution, 1982).
Nitrate in drinking-water is not itself hazardous, but may be
converted to nitrite by nitrate-reducing bacteria in the
gastrointestinal tract of infants under appropriate conditions.
Nitrites promote the formation of methaemoglobin from haemoglobin,
thus impairing the transport of oxygen and causing cyanosis.
Methaemoglobin is especially associated with the consumption of
well water with a high nitrate content, and infants appear to be
more susceptible to it than older children and adults (American
Academy of Pediatrics Committee on Nutrition, 1970).
2.3 The Respiratory Tract
Although infants and children may inhale chemical compounds,
information concerning the structure and function of the
respiratory tract in this context is limited. It is known that the
architecture of the respiratory tract continues to develop, at
least until the age of 18 months. Anatomically, 3 processes have
been identified including:
(a) centripetal alveolization giving rise to an extra
generation of respiratory bronchioli;
(b) transformation of some distal respiratory bronchioli
into alveolar ducts; and
(c) further branching of 1 or 2 alveolar duct generations.
After the age of 18 months, the respiratory structures increase
in size with a progressive increase in elastic fibre bundles in the
alveolar wall up to the age of 18 years. The length/diameter
relationship of each respiratory unit remains relatively constant
throughout development. The effects of these architectural changes
is thought to be minimal in the case of inhaled aerosols, but the
position concerning suspended particulates of various sizes has
not been well studied in childhood (Hislop & Reid, 1981).
Numerous chemical substances are found in the atmosphere, and
over 1600 have been identified. Not all of these are man-made, and
many may be derived from a single source (Graedel, 1978). The bulk
of atmospheric pollution is provided by carbon monoxide,
particulates and oxides of sulfur, hydrocarbons, and oxides of
nitrogen. Not all pollutants act directly; for example, the oxides
of nitrogen may exert their effects indirectly, by virtue of their
role in photochemical smog formation (National Research Council,
Subcommittee on Airborne Particles, 1979).
The exposure of infants and children to atmospheric pollutants
may be enhanced compared with that of adults, if the pollutants are
emitted close to the ground or, in the case of aerosols, are gases
or vapours of high density.
2.4 The Skin
Percutaneous absorption of chemical substances is known to
occur through normal skin in both adults and children. There is
little evidence that the rate of absorption varies with age, since
the limiting factor would appear to be the thickness of the
stratum corneum, which is relatively constant throughout postnatal
development after a full-term birth. Different processes of
absorption are thought to exist, but the transcutaneous flux cannot
be predicted with certainty, since marked variations in absorption
rates have been observed between closely related chemical
compounds. While absorption through normal skin does not vary with
age, absorption is greatly enhanced when the skin barrier is
damaged or when the substance is applied under an occlusive
dressing. A problem specific to infants is that napkins (diapers)
serve as an occlusive dressing, which may promote the absorption of
chemicals applied to the buttocks and increase the likelihood of a
systemic effect. Absorption may be further enhanced by skin
reactions in the napkin area.
The prematurely born infant may constitute a special case,
since studies on the development of the stratum corneum in fetal
life suggest that the permeability barrier is incomplete until just
before term (Singer et al., 1971). Thus, pre-term infants bathed
with a solution of hexachlorophene showed greater peak blood
concentrations of the compound than full-term infants subjected to
the same procedure (Greaves et al., 1975).
A pertinent factor in infants is the relatively large surface
area to body weight ratio; thus, compounds absorbed through a given
part of the skin surface will result in greater tissue
concentrations than in an adult (Wester & Maibach, 1982).
2.5 Conclusions
Infants and young children may be exposed to chemicals by the
gastrointestinal, respiratory, and percutaneous routes. The
relative importance of these routes will vary with age according to
the nature of the diet, the behavioural characteristics, and the
maturation of the system involved.
Since infants are obligate milk feeders, they will ingest
chemicals that cross the mammary gland during lactation. These are
potentially important contaminants of both human milk and infant
formulae containing the milk of other species. Similarly, chemicals
contaminating the water used for the reconstitution of powdered
milks will contribute to the ingested burden. Foods and beverages
specially manufactured for infants and young children are also
potentially important sources, especially when the dietary range is
restricted. Oral exploration, hand to mouth activity, and pica
contribute to the risk of ingestion of chemicals encountered in the
domestic environment.
Limited information is available concerning the absorption of
inhaled chemical atmospheric pollutants in relation to the
continuing development of the respiratory tract. However, exposure
of infants and children to atmospheric pollutants may be enhanced
compared with that of adults, when the source of emission is close
to the ground, and under circumstances in which gases or vapour of
high density are involved.
Chemicals can be absorbed through normal skin, but, in infants
and children, the relatively large surface area to body weight
ratio may result in greater tissue concentrations than in the
adult. Percutaneous absorption is enhanced when the skin is
damaged or macerated, or when the substance is applied under an
occlusive dressing such as a napkin (diaper). The prematurely born
infant may constitute a special case, since the permeability of the
skin is greater, because of the incomplete development of the
stratum corneum.
3. KINETICS OF ABSORPTION, BIOTRANSFORMATION, AND ELIMINATION
3.1 Introduction
Because of the morphological, biochemical, and physiological
characteristics of the infant and young child described in section
1, the kinetics of chemicals gaining access to their bodies need
special consideration, particularly with regard to the timing of
the maturation processes governing absorption, distribution,
biotransformation, and elimination from the body.
3.2 Absorption
A chemical may be absorbed through an external (skin) or
internal (gastrointestinal and respiratory tract) surface.
Absorption depends on the physical and chemical characteristics of
the chemical, its concentration gradient, the conditions at the
site of absorption, and the biological characteristics of the
absorptive surface. The conditions at the site of absorption and
the characteristics of the absorptive surface can be expected to
change with age; for example, postnatal changes in pH in different
parts of the gastrointestinal tract and skin, and alterations in
the thickness and structure of membranes of the gastrointestinal
tract and lungs (Hoffmann, 1982) cause changes in perfusion, which
in turn influence the concentration gradient.
3.2.1 Absorption from the gastrointestinal tract
Absorption from the gastrointestinal tract is an important way
in which chemicals enter the body of the infant and young child.
It is influenced by pH, total mucosal surface area, blood supply,
perfusion rate, and period of contact of the chemical with the
absorptive surface, that is the gastric emptying and intestinal
transit time. All these change with postnatal development.
The absorption of some chemicals by infant animals is greater
than that by young adults of the same species. This can be
explained by several factors, including immaturity of the
gastrointestinal tract, the characteristics of the infant's diet,
and the greater proportion of nutrients absorbed. This is of
benefit for growth, but is a disadvantage as far as non-nutritive
chemicals are concerned. Much of the evidence for this comes from
studies on experimental animals exposed to metals. Thus, the adult
rat absorbs 1% or less of lead added to its diet compared with 40 -
50% for the infant animal (Kostial et al., 1971, 1978; Forbes &
Reina, 1972a,b). According to Alexander et al. (1974) and Ziegler
et al. (1978), infants and young children absorb substantially more
lead than adults, but Barltrop & Strehlow (1978) did not find any
age-related differences. The intake of lead by the infants studied
by Alexander et al. (1974) and Ziegler et al. (1978) was greater
than that of the children in the study of Barltrop & Strehlow
(1978), and this may explain the different result.
Methylmercury is well absorbed in the gastrointestinal tract of
infants and young children, as it is in adults (Berlin, 1983).
Some methylmercury is evidently re-excreted into the intestine and,
if this is demethylated by the intestinal bacteria, the mercuric
mercury from it is excreted in the faeces. In mice, there is an
increase in intestinal demethylation at weaning, and the results of
in vitro studies have shown that the microflora in faeces from
young infants receiving milk have negligible demethylating ability
compared with those of weaned children and adults (Rowland et al.,
1983). Thus, it seems likely that more of a dose of methylmercury
will be retained within the body of young infants than of older
children and adults.
3.2.2 Absorption from the lungs
Chemicals permeate the air-blood barrier by simple dif fusion,
but the thickness of the membrane, its lipid composition, surface
area, and porosity determine pulmonary absorption. Lipid-soluble
chemicals are absorbed at similar rates by the lungs of new-born
and adult rats, but hydrophilic chemicals are absorbed at a more
rapid rate by young animals (Hoffmann, 1982).
3.3 Tissue Distribution
3.3.1 Binding of chemicals to proteins
Most of chemicals bind to plasma-proteins. The amount of
binding depends mainly on the concentration and structure of the
binding proteins, the affinity constant, and the presence of other
compounds capable of modifying the chemical-protein interaction.
In the new-born infant, several factors contribute to a lower
plasma-protein binding than in the adult. The concentration of
albumin in the plasma of neonates, and hence the number of binding
sites, is low. Moreover, these binding sites are occupied by
endogenous substances such as fatty acids, steroids, and bilirubin.
Thus, new-born infants have a lower capacity for binding chemicals
to plasma-albumin, and competition at the binding site may lead to
the release of, for example, bilirubin, resulting in kernicterus.
Moreover, hypoxaemia and acidosis may also decrease binding
capacity.
3.3.2 Distribution within the body
From early fetal to adult life, the fluid compartments of the
body change continuously. The larger extracellular space in
infancy (section 1.3.4) provides a larger volume for the
distribution of water-soluble chemicals that do not penetrate the
cells, and plasma concentrations of these chemicals may be lower in
infants than in adults, even if the total body burden per unit body
weight is greater.
Alterations in the morphology, physiology, and biochemistry of
the organs, as well as in their weight relative to the body as a
whole, may affect the distribution of chemicals. The brain is
proportionately larger in the infant than in the adult, and rapid
myelination during the first year after birth may favour the uptake
of lipophilic compounds. Metals, too, are retained in the brain
more readily in infancy than in adulthood. Momcilovic & Kostial
(1974), for example, found that a far greater percentage of the
intake of lead was retained in the brain of the suckling rat than
in that of the adult, and that the rate of removal was slower.
Similarly, Kostial et al. (1978) and Wong & Klaassen (1980) found
that a greater percentage of the intake of cadmium was retained in
the brain of the suckling rat than in that of the adult; Jugo
(1980) made a similar observation for mercury. There is little
direct evidence about the human brain. By analogy with animals, it
seems likely that the concentration of lead in the brains of
infants exposed to lead would rise more rapidly than the
concentration in the brains of older children or adults exposed to
a similar dose.
Methylmercury is known to accumulate in brain tissues,
predominantly in grey matter, but whether the brains of infants and
young children accumulate more than those of adults is not known.
Other tissues take up metals more readily in the early period
of life, when the animal is growing rapidly, than when growth has
slowed down or ceased; this particularly applies to the bones. The
concentration of lead in human bones doubles between infancy and
the late teen years (Barry, 1975).
The liver and kidney also accumulate metals. At birth, the
concentrations of cadmium and mercury are low. Henke et al.
(1970), using neutron activation analysis, determined the cadmium
concentrations in the liver and kidneys of 41 children and adults
and found that they increased 200 fold during the first 3 years
after birth. The total increase in cadmium in the liver and kidney
was greater than appears from the concentration, because both
organs increased considerably in weight.
3.4 Biotransformation of Organic Chemicals
The kinetic behaviour of organic chemicals in living organisms
is generally characterized by several processes modifying the
duration of their action. The limiting factors are either
excretion through the kidneys, bile, or gastrointestinal tract, or
biotransformation mechanisms in the liver or other organs. The
products of biotransformation are generally more polar than the
original compound and, thus, are more readily excreted.
Biotransformation is brought about by enzyme activity, either
associated with microsomal systems in the liver or with enzymes in
the plasma or other tissues.
The biochemical processes involved in the biotransformation of
organic chemicals can be divided into 2 phases:
Phase 1. hydroxylation, dealkylation, dehydrogenation,
reduction, hydrolysis, and other reactions
producing a conjugable grouping; and
Phase 2. biosynthetic reactions, whereby conjugates are
formed with endogenous polar molecules; these
conjugates are, generally, readily excreted.
Both Phase 1 and Phase 2 reactions are generally slower in the
new-born infant than in the adult. Consequently, blood-plasma
half-lives of chemicals in which the degradation and elimination
are dependent on one or the other or both of these types of
reactions are generally appreciably longer in neonates than in
adults.
The rate of maturation of processes concerned with the
biotransformation of organic chemicals varies from one reaction,
and from one chemical, to another. Highest activities are reached
in children aged 12 - 16 years, after which there is generally a
decline. A detailed description, with full documentation, has been
published by Klinger (1982).
3.5 Elimination from the Body
3.5.1 Elimination by the kidneys
The renal function of the full-term infant at birth is immature
by adult standards, and this applies both to glomerular and
tubular function (McCance & Young, 1940; Dean & McCance, 1947b).
The rapid growth of the young infant (section 1.3.3) involves the
retention of nitrogen and minerals, so that the equivalent of only
part of the nitrogen and minerals absorbed from the food requires
to be excreted by the kideys. The breast-fed infant or the infant
fed on a formula similar in composition to breast milk is able to
do this satisfactorily, and so maintain the composition of the body
fluids at their proper level (McCance & Widdowson, 1957a).
However, excessive intakes of nutrients, such as protein
(nitrogen), phosphorus, and sodium, are not excreted completely by
the kidneys of the new-born infant or animal, and the
concentrations in the plasma rise (McCance & Widdowson, 1956,
1957b). Retention of chemicals introduced into the body of the
infant may be due to both renal immaturity and insufficient
biotransformation.
The function of the renal tubules at full-term birth is less
mature than that of the glomeruli, but it matures more rapidly
(Edelmann & Spitzer, 1976). This is important in the young infant
for the chemicals that depend on tubular secretion for their
elimination from the body. Moreover, the reabsorption of chemicals
from the tubular lumen into the tubular cells also varies with age
and depends, in part, on the pH of the urine. Weak organic acids
are reabsorbed more readily by the infant, but, more important, the
low capacity for biotransformation by the infant's liver results in
organic chemicals reaching the kidneys in their original lipophilic
form, and these are not excreted, but reabsorbed into the
circulation (for reviews, see Scheler, 1980; Braunlich, 1981).
Some metals depend on the kidneys for their elimination from
the body, and the results of animal studies suggest that suckling
rats excrete less of their body burden of cadmium, mercury, and
manganese than adults (Kostial et al., 1978). Infants appear to
excrete a smaller proportion of absorbed lead by the renal route
than adults (Ziegler et al., 1978).
3.5.2 Elimination by the liver
The excretion of chemicals in the bile is an important means of
elimination, though some of the chemicals excreted through the bile
may be reabsorbed in the gut and re-enter the body, thus resulting
in their enterohepatic circulation. They cannot penetrate the
epithelium of the biliary duct. Many chemicals are excreted by the
liver as glucuronides in the bile, but the formation of
glucuronides is limited in the new-born infants (section 3.4).
3.5.3 Elimination by other routes
Other routes of excretion include the gastrointestinal tract,
the respiratory tract, and the skin. They are non-regulatory and,
while they may be important for some chemicals, information is
limited about age-related differences in elimination.
3.6 Conclusions
Generally speaking, both organic and inorganic chemicals are
absorbed more readily by the infant than by the adult, but the
organic chemicals undergo biotransformation less readily. For some
chemicals, alternative biochemical pathways may exist.
The kidneys of the neonate are immature and less able than
those of the older child or adult to excrete chemicals, whether
inorganic or the polar products of biotransformations. A greater
proportion of a similar dose of a chemical per unit body weight is
therefore likely to accumulate in the body of the infant, and the
concentration of the chemical in the blood and tissues of the
infant will be higher. The possible effects of this are discussed
in section 4.
4. EFFECTS OF CHEMICALS IN THE BODY
4.1 Introduction
Differences in kinetics can enhance the toxicity of a chemical
in the infant but, in some cases, may result in a qualitative
difference of response or in a lower toxicity, depending on the
intermediary metabolite. In addition, the qualitative and
quantitative response of the target tissues to the same internal
dose of a chemical may be different at various stages of postnatal
development because of the gradual development of characteristics
of the target tissues. A third aspect of developmental toxicology,
which should be recognized, is the fact that a chemical introduced
into the body of an infant or young child may affect the structural
and functional development of a particular organ or system, which
may interfere with the growth and development of the body as a
whole. Animal studies have shown that the toxic effects of certain
chemicals are different at different stages of development.
Moreover, chemicals reaching the body early in life may produce
delayed effects in later years. The response of the growing infant
may therefore be different from that of the fully grown adult.
The problems of extrapolating quantitatively from animals to
man and reaching conclusions on the implications of evidence from
animal studies for human health are well known. These problems are
greater during the new-born period, since the stage of development
at the time of birth varies considerably between species (section
1.4.2). Information from clinical experience of exposure is
limited, and systematic epidemiological data from studies on
infants are sparse, so that the results of studies on animals may
provide the only information available. When information
concerning human infants is available, it should be given
preference.
4.2 Effects of Chemicals on General Growth and Development
General growth and development are possible indicators of the
effects of chemicals on infants and children, whether the exposure
occurred prenatally or postnatally. The principles for evaluating
the effects of prenatal exposure have been considered in an earlier
Environmental Health Criteria publication (WHO, 1984). Factual
information about the effects of postnatal exposure is scarce. In
a study on the infants of mothers who had consumed bread
contaminated with a methylmercury fungicide during lactation, Amin-
Zaki et al. (1981) found abnormal neurological signs in some
infants at the first examination and in more infants on subsequent
occasions. Delayed motor and other aspects of development,
including intelligence, were not detected when the infants were
first seen, but were observed at follow-up examinations during the
next 5 years.
4.3 Effects of Chemicals on Some Organs and Systems
4.3.1 Nervous system
The brain of the infant at birth is not fully developed. The
full number of neurones is not reached until about 2 years of age
(Dobbing & Sands, 1973); myelination is not complete until
adolescence (Hoar & Monie, 1981).
Brain development involves changes in the proportions of myelin
and non-myelinated tissue. Shuman et al. (1975) observed status
spongiosus in the myelin sheaths of the reticular formation of
premature infants, following exposure to hexachlorophene, but no
lesions were present in the non-myelinated part of the brain.
Brain lesions were not produced during the first 8 - 10 days after
birth in the rat. However, between 10 and 25 days, when myelination
takes place, the suckling rats became sensitive to hexachlorophene
(Nieminers et al., 1973; Ulsamer et al., 1975).
There are changes in the sensitivity of the brain of the rat to
hormones or drugs during development, and this has been correlated
with the development of the cell receptors (Macho et al., 1972;
Valcana & Timiras, 1978). On the basis of experimental data, Czaba
(1984) concluded that, during maturation, cell receptors in several
organs require reinforcement by the appropriate hormone at a
critical stage of development. This process can be hindered or
prevented by the presence of molecules analagous to the hormone, or
by a drug or other chemical that binds to the receptor and so
prevents reinforcement by the hormone and hence normal development.
Many confounding factors and co-variables such as nutrition,
socio-economic status, and mental stimulation have to be taken into
account when evaluating the effects of a chemical on the
functioning of the human brain. Only when good information on
these factors is available will it be possible to make a valid
interpretation of the observations on brain function after exposure
to a chemical. This has been discussed most extensively in the
case of lead (United Kingdom Department of Health and Social
Security, 1980; MRC, 1984) (section 3.3.2).
4.3.2 Kidneys
The functional immaturity of the infant's kidneys has already
been discussed (section 3.5.1). Specific reports of their
susceptibility to chemicals are not available, but results of
animal studies suggest that some compounds are less toxic to the
immature kidney than to the mature organ. For example, the same
dose of mercuric chloride per unit body weight that produced renal
failure in adult rats did not have any effect on renal function in
new-born rats (Kavlock & Gray, 1983). Information is lacking on
the effects on renal function of the accumulation of chemicals in
the human kidney during the early years of life (section 3.3.2).
4.3.3 Liver
The pre-term infant may still have foci of extra-medullary
haematopoiesis in its liver, but the histological appearance of the
liver at term is similar to that of the adult. The activity of
enzymes responsible for the biotransformation of chemicals in the
liver of the infant is not equal to that of the adult (section
3.4). However, it appears to be possible to induce the activity of
microsomal enzymes in the liver of neonates to some extent. If
infants have been exposed to phenobarbital, either during
intrauterine life, or immediately after birth, the synthesis of
components of certain microsomal enzyme systems in the liver is
accelerated. The metabolism and urinary excretion of bilirubin is
increased (Maurer et al., 1968; Trolle, 1968; Ramboer et al.,
1969). Increased activities of some drug-metabolizing enzymes have
also been found in new-born infants exposed to phenobarbital
before, or immediately after, birth. Such infants were able to
metabolize drugs such as diazepam more readily than infants not
exposed to phenobarbital, and their urinary excretion of hydroxy
derivatives was greater (Morselli, 1976).
4.3.4 Lungs
Animal studies indicate that some chemicals, such as nitrofen,
retard the development of the lung in the rat fetus (Kimbrough et
al., 1974), but there do not appear to be any reports of effects of
chemicals on the lung specific to infants. It seems that the
response of the infant's lungs to ingested or inhaled chemicals has
not been investigated sufficiently. A greater degree of impairment
of respiratory function might be expected in infants compared with
adults for a given level of exposure, particularly to particles of
substances that result in obstructive symptoms. An excess
mortality rate was found in infants aged less than 12 months
compared with older children and young adults in a population
exposed to atmospheric pollution with sulfur dioxide (Lawther &
Waller, 1975).
4.3.5 Haematopoietic system
The maturation and differentiation of haematopoietic cells in
the bone marrow are processes that continue throughout life, and
represent one of the most rapidly renewing cell populations.
Although the haematopoietic system and the blood are well known to
be the target of many chemical agents, there is little information,
at present, on any specific vulnerability of the developing bone
marrow. An example of the vulnerability of the red cells in young
children is the occurrence of methaemoglobinaemia after exposure
to very low doses of methaemoglobin-forming compounds, e.g.,
nitrates (Kiese, 1974).
4.3.6 Immune system
Studies of immune ontogenesis have revealed that new-born
mammals do not possess fully competent immune defence systems.
While human infants have more highly developed immune systems than
some other mammals at birth, the human infant is still
immunologically immature.
The development of the immune system in mammals involves a
sequence of steps that begins early in gestation. Perturbation or
abrogation of this developmental sequence of events can lead to
immune dysfunctions that may be life-threatening. Defects in the
development of the immune system, due to heritable changes in the
lymphoid elements, have provided clinical and experimental examples
of the consequences of impaired immune development (for review, see
Heise, 1982).
The most profound effects of chemicals that modulate the immune
system of animals occur when dosing of the compound begins during
the development of the lymphoid system (Vos, 1977). Although there
are no data on the immunological effects resulting from perinatal
exposure of infants to chemicals, the potential for such effects
should not be ignored.
The extent of adverse effects on the immune system resulting
from exposure of adult animals to a wide variety of environmental
chemicals has only recently begun to be appreciated (Vos, 1977).
Even less is known about the magnitude of the immunological effects
resulting from fetal and neonatal exposure to environmental
chemicals. It would be expected that disruption of the normal
sequence of events in the development of the immune system by
chemicals would result in alterations in the competence of the
system. Such alterations may not only lessen the host's ability
to combat infection and neoplasia but may also lead to
hypersensitivity or to a failure in the control of homeostasis,
which may lead to autoimmunity. The immunological effects of
perinatal exposure to chemicals in animals have been reviewed by
Roberts & Chapman (1981) and Schmidt (1984). Exposure during
gestation and/or early in postnatal life to chemicals such as
2,3,7,8-tetrachlorodibenzodioxin (Vos & Moore, 1974; Faith & Moore,
1977; Luster et al., 1980), lead (Luster et al., 1978; Faith et
al., 1979), hexachlorobenzene (Vos et al., 1979), and
benzo( a )pyrene (Urso & Gengozian, 1980) has been shown to affect
the developing immune system of animals. However, no specific data
are available for human infants.
4.3.7 Endocrine system
Chemicals may affect the developing endocrine system directly,
or they may interact with some step of the regulating axis
controlled by the pituitary, hypothalamus, or other part of the
brain. The interaction between a chemical and the neuroendocrine
organs can also affect the reproductive and other systems. Some
pesticides have been shown to possess estrogenic activity
(McLachlan et al., 1981). Exposure of experimental animals in the
early postnatal period to chemicals with androgenic or estrogenic
activity induced disturbances of sex differentiation in the
hypothalamus and acyclic ovarian function in females (Barraclough,
1961, 1966; Takewaki, 1968; Flerko, 1975).
Chemicals that give rise to premature onsent of puberty in
children are of particular importance. Premature thelarche in
large numbers of young children in Puerto Rico was attributed to
estrogens in infant foods containing meat (Saenz de Rodriguez &
Toro-Sola, 1982); the cause of a similar outbreak in an Italian
school (Scaglioni et al., 1978) was not discovered. Exposure to
chemicals with androgenic or estrogenic activity may also affect
growth and final stature.
The development of the regulatory function of the adrenal
glands of experimental animals is altered by the administration of
compounds such as steroids and diazepam to the new-born animal
(Hcik 1969a, 1970; Erdosova et al., 1977), and results in changes
in adrenal function and in the reaction of the pituitary-adrenal
axis to stress stimuli later in life (Levine & Mullins, 1967; Hcik,
1969b, 1970; Libertun & Lau, 1972).
A special risk for new-born infants is an excessive intake of
iodine, leading to an abnormal rate of accumulation in the thyroid
gland. Exposure to high concentrations of iodine or goitrogenic
compounds can alter the function of the thyroid gland, resulting in
a deficient production of thyroid hormones at the time when
appropriate levels of the hormones are important for the maturation
of functions of the brain, liver, and other organs. Premature
infants are particularly prone to the development of hypothyroidism
as a result of iodine overdose (Chabrolle & Rossier, 1978; Malpuech
et al., 1978; L'Allemand et al., 1983).
4.3.8 Skin
The skin is a complex structure, the functional development of
which is incomplete until after puberty. Clinically, the cutaneous
manifestations of disease vary with age, and some disorders are
confined to infants and children. Thus, it would be expected that
age-related differences in the response of the skin to chemicals
would be encountered. Chloracne was found to occur principally in
young children rather than adults in a population exposed to dioxin
(TCDD, 2,3,7,8-tetrachlorodibenzo-para-dioxin), suggesting the
greater susceptibility of the child (Fara et al., 1982).
Chemicals applied to the skin may be absorbed and induce
systemic effects. New-born infants whose skin was treated with an
antibacterial preparation containing 3% hexachlorophene developed
blood levels of the compound similar to those known to cause brain
damage in animals. Similarly, some 40 deaths in French infants in
1972 were attributed to the use of baby powder accidentally
contaminated with 6.6% hexachlorophene (Marzulli & Maibach, 1975).
Some environmental pollutants or contaminants are known to be
readily absorbed through the skin, and this subject has been of
particular interest in paediatric therapeutics. Thus, both fatal
and non-fatal exposures occurred when pentachlorophenol was used in
a laundry room resulting in contamination of napkins used in the
hospital nursery (Armstrong et al., 1969).
4.3.9 Bones and teeth
The bones undergo considerable change during postnatal
development with regard to growth, change in shape, and
composition. Immature bones contain more water and less collagen
in the matrix than mature bones, and the degree of mineralization
is much lower (section 1.3.4) (Table 3). Minerals, including
strontium, caesium, and lead, tend to be deposited in the bones,
together with calcium, during the process of mineralization. Lead
may affect the conversion of 25-hydroxy vitamin D to the 1.25-
hydroxy metabolite in the kidney (for review, see Mahaffey, 1981).
Interference with skeletal development may result from the
action of lead in altering the balance of osteoblastic and
osteoclastic activity, and from the direct action of chemicals such
as fluoride.
The discoloration of developing teeth after administration of
tetracycline is an example of the particular propensity for
accumulation or fixation of chemicals in hard tissues. The results
of studies on primates suggested that excessive exposure to
selenium during the development of the teeth might increase
susceptibility to caries (Bowen, 1972).
4.4 Carcinogenesis
Maternal exposure to carcinogens may lead to exposure of the
fetus through the placenta and to exposure of the infant through
breast milk. The transfer of chemicals to the fetus depends on the
relative molecular mass and solubility of the chemical, non-ionized
and lipid chemicals entering the fetus most readily. Lipophilic
chemicals may be retained in the tissues of the fetus and have been
found in tissues of still-born infants (Curley et al., 1969).
Transplacental transfer of carcinogens has recently been reviewed
(WHO, 1984).
So far, no epidemiological observations on human infants have
been reported in which the occurrence of cancer was associated
solely with exposure to carcinogens in the early postnatal period.
The few existing data come from animal studies and, in most of
these, the route of exposure was parenteral and not through the
mother's milk. For example, the injection of aflatoxin resulted in
liver cancer in mice, but only when administered to new-born
animals (Vesselinovitch et al., 1972).
Within the past decade, it has been increasingly recognized
that not all carcinogens act by the same mechanism. Many of the
environmental chemicals, particularly the halocarbons, are though
to be promoters of carcinogenesis rather than initiators. Many of
these types of chemicals are persistent in the organism and are
secreted in breast milk (section 2.2.1.1) (Appendix I). The
mechanism of action of promoters is not well understood at present,
and effects on health at the low doses received by infants and
young children are not clear.
An intrinsic problem in studying prenatal and postnatal
exposure to carcinogens is the capacity for metabolic activation in
the tissues of the fetal or new-born organism, compared with that
of the adult (section 3.4). In this respect, pronounced species
variations have been shown between rodents and primates, including
man. The perinatal development of different organ systems varies
considerably among species. All these variables make extrapolation
from animals to man extremely difficult.
The significance for human health of early postnatal exposure
to tumour growth promoters or co-carcinogens is not known.
4.5 Conclusions
The structural and functional characteristics of infants and
young children described in sections 1, 2, and 3 might make them
more or less vulnerable than older children and adults, when they
are exposed to chemicals at a similar dose per unit body weight.
However, it should be emphasized that reports of specific effects
are rare. This may be because such specific effects do not exist
or because they have passed largely unnoticed. The examples quoted
here show that exposure to certain chemicals during early postnatal
development can be associated with dose-effect and dose-response
relationships that differ from those resulting from exposure in
later years.
Exposure to chemicals during early postnatal development can
give rise, not only to immediate effects on health, but also to
manifestations resulting from the disturbed maturation of organ
systems and their altered response to other environmental
influences. Depending on the chemical concerned, the vulnerability
of the developing organ systems can be higher or lower than that of
the more mature systems.
It is recommended that, if laboratory tests suggest that the
body burden of a chemical is a cause for concern, follow-up studies
should be made. Children who have been accidentally exposed to
chemicals that might affect the reproductive organs should be
followed prospectively to determine possible effects on puberty and
reproductive capacity. Similarly, if other organ systems are
likely to have been affected, prospective studies should, if
possible, be made to assess functional development, morbidity, and
mortality.
5. MODIFYING FACTORS
Considerable variations exist in the health and nutritional
status of children reared in different social and cultural
environments. The response of children exposed to chemicals may
therefore be modified in certain sections of a population to a
degree that cannot readily be predicted. Although analogous data
for the severity of response to infections, such as measles, are
well documented, information concerning chemical exposure and its
sequelae is limited.
5.1 Nutrition
The intake of chemicals contaminating foods and beverages will
vary with the amount of food consumed, the range and variety of
dietary items selected or available, and the methods of
preparation. Children with high intakes of marine fish will, for
example, have a greater intake of methylmercury from polluted
sources than those in which fish constitutes only a small
proportion of the diet (Turner et al., 1980). Conversely, some
pesticide residues in milk are decreased by heating, drying,
evaporation, or pasteurization, so that less of these substances
will occur in children consuming manufactured or processed milk, as
opposed to the raw product. The duration of milk feeding in
infancy, the method of weaning, and the interval before a normal
adult diet is introduced are important factors that vary greatly
between social, cultural, and ethnic groups.
Specific dietary components may interact with ingested
chemicals, thus modifying their availability for absorption. The
relative solubility of the chemical in the lipid or aqueous
fractions of the diet, and the potential for physical interaction
or adsorption to non-adsorptive mechanisms at the enteral surface
of the gut mucosa, may all have a role in determining the
bioavailability of the ingested chemical. Thus, the absorption of
lead, consumed simultaneously with the diet, is markedly lower
compared with its absorption from aqueous solutions, partly due to
interaction with other dietary components. In animals, and
possibly in man, lead absorption is reduced by diets rich in
calcium and phosphorus, but is increased when the intake of these
nutrients is restricted (Barltrop & Khoo, 1975).
The consequences of nutritional deficiency for the absorption
of chemicals cannot be predicted with certainty. In kwashiorkor,
both the digestive and absorptive functions of the gut may be
impaired and the activation of chemicals during conjugation
reactions reduced, particularly acetylation with amino acids
(Thabrew et al., 1982). On the other hand, specific mineral
deficiencies, for example, a lack of calcium, may stimulate
absorptive mechanisms shared by other, potentially toxic, divalent
cations (Barltrop, 1979). Iron deficiency in children has been
related to enhanced absorption and retention of lead, but the
mechanism involved is uncertain. Interactions between essential
and toxic metals after they have been absorbed into the body have
been described. Zinc, for example, competes with lead for
receptors on the enzyme aminolaevulinic acid dehydratase (Nordberg,
1979), but the significance of this in infants and children is
uncertain.
5.2 State of Health
Several diseases in addition to nutritional disorders may
modify the exposure and response of infants and children to
chemicals. Ill health associated with diminished activity or
mobility of the child will, for example, alter the balance between
exposures related to the indoor as opposed to the external
environment. Conditions requiring the long-term administration of
oral therapeutic agents or modified diets will inevitably alter the
intestinal milieu and hence the absorption of ingested chemicals.
The absorptive ability of the gut is modified in a wide range
of diseases, including the enteropathies and chronic diarrhoeal
states encountered in childhood arising, for example, from acquired
or inherited food intolerance. The steatorrhoea associated with
childhood malabsorptive disorders such as coeliac disease is known
to impair the absorption of lipid-soluble substances such as
cholecalciferol and alpha-tocopherol (Harrison, 1980), and would
therefore be expected to have a similar effect on the absorption of
other lipid-soluble chemical compounds.
Children with chronic renal or hepatic dysfunction will have an
impaired ability to excrete, metabolize, and, in some cases, store
chemicals that have been absorbed, which may therefore accumulate
in the body. Compromised integrity of the dermal or respiratory
epithelium by disease will enhance the potential for the absorption
of chemicals by these routes.
Some children have genetically determined variations in their
ability to metabolize or respond to certain chemicals. Thus,
deficiency of the enzyme glucose-6-phosphate dehydrogenase (EC
1.1.1.49), which is encountered in some sub-populations, is
characterized by a haemolytic reaction in response to various
triggering agents. Such reactions in childhood have been reported
after exposure to chemicals including naphthalene, aniline dyes,
and nitrofurantoin (Willoughby, 1984).
5.3 Social and Cultural Way of Life
There are many complex interactions between environmental
chemicals and the social and cultural ways of life. The effects of
place of residence, mobility, state of health, nutritional status,
and family income cannot always be distinguished without resort to
sophisticated epidemiological methods. Cultural and social
attitudes to child care and supervision differ between
subpopulations and are clearly important in relation to acute or
short-term exposures. The mechanisms whereby class and cultural
differences modify exposure are not always apparent. For example,
the different blood-lead values found in Negro versus Hispanic
children in New York and in Asian versus Northern European children
in the United Kingdom have yet to be adequately explained
(Barltrop, 1982). Family practices with regard to personal
hygiene, and especially handwashing before meals, may be important
determinants of exposure resulting from hand to mouth activity in
children.
5.4 Conclusion
Recognition of social and cultural factors that influence
exposure and modify response will assist in the identification of
children at risk and enable appropriate preventive and remedial
measures to be adopted.
6. GENERAL CONCLUSIONS
1. Infants and young children differ from older children and
adults in some of their morphological, physiological, biochemical,
metabolic, and behavioural characteristics, and in their
nutritional requirements.
2. The response of infants and young children to chemicals may
differ from that of adults by virtue of their biological
characteristics. Moreover, a feature of these responses is that
the processes of development may themselves be modified by exposure
to chemicals.
3. The effects of chemical exposure and their relationship to dose
may vary with age. The expression of the effects of chemicals may
be greater or lesser than in the adult, depending on the chemical
species concerned; the kinetics of its absorption,
biotransformation, and elimination; and the degree of maturation of
target organs and tissues.
4. The characteristics of infants and children are such that they
determine the pattern of exposure to chemicals. The pathways
involved are related to development and vary with the environment
of the child. Thus, skin contact, the consumption of breast milk
or its substitutes, the ingestion of substances other than food,
may each be predominant routes at different stages of development.
5. The exposure, uptake, and effects of chemicals may be modified
by factors that are characteristically encountered in early life,
including the mode of nutrition, social, economic, and cultural
conditions, and disease.
6. The expression of chemical effects may not be immediate, but
may be delayed until a later age. The health of adults may thus be
compromised by chemical exposures in early life.
7. The development of a strategy relating to chemicals detected in
breast milk requires a thorough appraisal of the merits and
disadvantages of the available options. Any risk of continued
exposure to a chemical through breast-feeding has to be balanced
against the risk of infection or nutritional deprivation, should
breast-feeding be curtailed or discontinued.
7. RECOMMENDATIONS
1. When health risks from chemicals are evaluated, the special
characteristics of infants and young children must be recognized.
An appropriate methodology based on the knowledge of these
differences should be used.
2. Preventive measures should take account of the special
conditions of exposure to chemicals characteristic for infants and
young children, including exposure through breast milk.
3. Health care workers should be made aware of the special risks
related to chemical exposure during infancy and childhood.
4. Any increased incidence of a disease possibly related to
chemical exposure should be reported and investigated. Awareness of
this among health care workers should be improved. The
establishment of an appropriate surveillance system should be
considered.
5. Clinical and epidemiological data should be collected following
the inadvertent exposure of infants and young children to
chemicals.
6. Developmental toxicology should be promoted and its methodology
improved in order to increase the predictive power of experimental
animal and other laboratory studies.
7. Studies are urgently needed to evaluate the risk associated
with exposure during infancy and early childhood to the initiators
and promoters of carcinogenesis.
REFERENCES
ALEXANDER, F.W., CLAYTON, B.E., & DELVES, H.T. (1974) Mineral and
trace metal balances in children receiving normal and synthetic
diets. Q. J. Med., 43: 89-111.
AMERICAN ACADEMY OF PEDIATRICS COMMITTEE ON NUTRITION (1970)
Infant methaemoglobinaemia: the role of dietary nitrate.
Pediatrics, 46: 475-478.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., & GREENWOOD, M.R. (1974) Studies of infants
postnatally exposed to methylmercury. J. Pediat., 85: 81-84.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., GREENWOOD, M.R., & GIOVANOLI-JAKUBCZAK, T.
(1976) Perinatal methylmercury poisoning in Iraq. Am. J. Dis.
Child., 130: 1070-1076.
AMIN-ZAKI, L., MAJEED, M.A., GREENWOOD, M.R., EL HASSANI,
S.B., CLARKSON, T.W., & DOHERTY, R.A. (1981) Am. J. Dis.
Chil., 130: 1070-1076.
ANDERSSON, U., BIRD, A.G., BRITTON, S., & PALACIOS, R.
(1981) Humoral and cellular immunity in humans studied at the
cell level from birth to two years of age. Immunolog. Rev.,
57: 5-38.
ARMSTRONG, R.W., EICHNER, E.R., KLEIN, D.E., BARTHEL, W.F., ET
AL. (1969) Pentachlorophenol poisoning in a nursery for
new-born infants. II. Epidemiological and toxicological
studies. J. Pediat., 75: 317-325.
BARLTROP, D. (1966) The prevalence of pica. Am. J. Dis.
Child., 112: 116-123.
BARLTROP, D. (1979) Metal-nutrient interactions in lead
absorption. In: Di Ferrante, E., ed. Trace metals: exposure
and health effects, Oxford, CEC/Pergamon Press, pp. 158-161.
BARLTROP, D. (1982) Nutritional and maturational factors
modifying the absorption of inorganic lead from the
gastrointestinal tract. In: Environmental factors in human
growth and development, New York, Cold Spring Harbor
Laboratory, pp. 35-41 (Banbury Report No. 11).
BARLTROP, D. & KHOO, H.F. (1975) The influence of
nutritional factors on lead absorption. Postgrad. Med. J., 51:
795-800.
BARLTROP, D. & OPPE, T.E. (1970) Dietary factors in neonatal
calcium homeostasis. Lancet, 2: 1333-1335.
BARLTROP, D. & STREHLOW, C.D. (1978) The absorption of lead
by children. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals, Munich, Technical University of
Munich, Freising-Weihenstephan, pp. 332-334.
BARR, H.M., STREISSGUTH, A.P., MARTIN, D.C., & HERMAN, C.S. (1984)
Infant size at 8 months of age: relationship to maternal use of
alcohol, nicotine, and caffeine during pregnancy. Paediatrics, 74:
336-341.
BARRACLOUGH, C.A. (1961) Production of anovulatory sterile rats
by single injection of testosterone propionate. Endocrinology, 68:
62-67.
BARRACLOUGH, C.A. (1966) Modification in the CNS regulation
of reproduction after exposure of prepubertal rats to steroid
hormones. Recent. Progr. Horm. Res., 22: 503-539.
BARRY, P.S.I. (1975) A comparison of concentrations of lead
in human tissues. Br. J. ind. Med., 32: 119-139.
BERLIN, M. (1983) Toxicokinetics of mercury. In: Schmidt,
E.H.F. & Hildebrandt, A.G., ed. Health evaluation of heavy
metals in infant formula and junior food, Berlin, Springer
Verlag, pp. 147-161.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRAUNLICH, H. (1981) Excretion of drugs during postnatal
development. Pharmac. Ther., 12: 299-320.
BRITISH PAEDIATRIC ASSOCIATION (1956) Hypercalcaemia in
infants and vitamin D. Br. med. J., 2: 149.
CHABROLLE, J.P. & ROSSIER, A. (1978) Goitre and
hypothyroidism in the newborn after cutaneous absorption of
iodine. Arch. Dis. Child., 53: 495-498.
CLARKSON, T.W., AMIN-ZAKI, L., & AL-TIKRITI, S.K. (1976) An
outbreak of methylmercury poisoning due to consumption of
contaminated grain. Fed. Proc., 35: 2394-2399.
COLLIPP, P.J., CHEN, S.Y., & MAITINSKY, S. (1983) Manganese
in infant formulas and learning disability. Ann. Nutr. Metab.,
27: 488-494.
CURLEY, A., COPELAND, R., & KIMBROUGH, R.D. (1969)
Chlorinated hydrocarbon insecticides in organs of stillborn
and blood of new-born babies. Arch. environ. Health, 19:
628-632.
CZABA, G. (1984) The present state in the phylogeny and
ontogeny of hormone receptors. Horm. Metab. Res., 16: 329-335.
DEAN, R.F.A. & MCCANCE, R.A. (1947a) Response of new-born
children to hypertonic solutions of sodium chloride and urea.
Nature (Lond.), 160: 904-906.
DEAN, R.F.A. & MCCANCE, R.A. (1947b) Inulin, diodone,
creatinine, and urea clearances in new-born infants. J.
Physiol., 106: 431-439.
DEAN, R.F.A. & MCCANCE, R.A. (1948) Phosphate clearances in
infants and adults. J. Physiol., 107: 182-186.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1984) [Residues and
contaminants in breastmilk,] Weinheim, Verlag Chemie (in
German).
DICKERSON, J.W.T. (1962a) The effect of development on the
composition of a long bone of the pig, rat, and fowl. Biochem.
J., 82: 47-55.
DICKERSON, J.W.T. (1962b) Changes in the composition of the
human femur during growth. Biochem. J., 82: 56-61.
DIEM, K. & LENTNER, C., ed. (1970) Scientific tables, 7th
ed., Basel, J.R. Geigy A.G.
DOBBING, J. & SANDS, J. (1973) The quantitative growth and
development of the human brain. Arch. Dis. Child., 48: 757-767.
DORNER, G. & STAUDT, J. (1972) [Comparative morphology of
the hypothalamus: differences between the rat and man.]
Endokrinologie, 59: 152-155 (in German).
EDELMANN, C.M., Jr & SPITZER, A. (1976) The kidney. In:
Smith, C.M. & Nelson, N.M., ed. The physiology of the new-born
infant, 4th ed., Springfield, Illinois, pp. 416-458.
EPPS, E.A., Jr, BONNER, F.L., & COWART, R.P. (1974) Dieldrin
contamination of milk by use of second-hand bags for feed.
Bull. environ. Contam. Toxicol., 12: 209-211.
ERDOSOVA, R., KRAUS, M., & REHULKA, J. (1977) Corticosterone
synthesis and serum levels at the end of the perinatal period:
a study of the effects of stress, diazepam, and polyethylene
glycol treatments. Physiol. bohemoslov., 26: 297-302.
FAITH, R.E. & MOORE, J.A. (1977) Impairment of thymus-
dependent immune functions by exposure of the developing
immune system to 2,3,7,8-tetrachlorobenzeno-p-dioxin (TCDD).
J. Toxicol. environ. Health, 3: 451-464.
FAITH, R.E., LUSTER, M.I., & KIMMEL, C.A. (1979) Effect of
chronic developmental lead exposure on cell-mediated immune
functions. Clin. exp. Immunol., 35: 413-420.
FARA, G.M., DEL CORNO, G., BONETTI, F., CARAMASCHI, L.,
DARDANONI, L., FAVARETTI, C., JIAMBELLUCA, S.E., MARNI, E.,
MOCARELLI, P., MONTESARCHIO, E., PUCCINELLI, V., & VOLPATO,
C. (1982) Chloracne after release of TCDD at Seveso, Italy.
In: Hutzinger, O., Frei, R.W., Merian, E., & Pocchiari, F.,
ed. Chlorinated dioxins and related compounds, Oxford,
Pergamon Press, pp. 545-560.
FLERKO, B. (1975) Perinatal androgen action and the
differentiation of the hypothalamus. In: Brazier, M.A.B., ed.
Growth and development of the brain, New York, Raven Press,
pp. 117-137.
FORBES, G.B. & REINA, J.C. (1972a) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. Health
Phys., 22: 169-175.
FORBES, G.B. & REINA, J.C. (1972b) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. J. Nutr.,
102: 647-652.
GIACOIA, G.P. & CATZ, C.S. (1979) Drugs and pollutants in
breast milk. In: Clinics in perinatology, Vol. 6, pp. 181-196.
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Contamination of milk with hexachlorobenzene residues.
Ind. Aliment. Agric., 89: 31-35.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
London, Academic Press.
GREAVES, S.J., FERRY, D.G., MCQUEEN, E.G., MALCOLM, D.S., &
BUCKFIELD, P.M. (1975) Serial hexachlorophene blood levels
in the premature infant. N.Z. med. J., 81: 334-336.
HACIK, T. (1969a) Effect of estradiol on adrenal and gonadal
functions during postnatal development in female rats. Endocr.
Exper., 3: 147-153.
HACIK, T. (1969b) Postnatal development of androgenized
female rats. Physiol. bohemoslov., 18: 263-269.
HACIK, T. (1970) Effect of estrogens on adrenal function and
body growth during postnatal period in male rats. Endocr.
Exper., 4: 31-38.
HAERING, M. & SCHEFER, W. (1980) Quantitative analysis of
biocide residues. Chimia, 34: 397-400.
HARRISON, H.E. (1980) Vitamin and mineral deficiencies in
intestinal malabsorption. In: Lifshitz, F., ed. Clinical
disorders in pediatric gastorenterology and nutrition, New
York, Dekker, pp. 371-375.
HEISE, E.R. (1982) Diseases associated with immuno-
suppression. Environ. Health Perspect., 43: 9-19.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Determination of
cadmium in liver and kidneys of children and adolescents by
neutron activation analysis.] Arch. Toxicol., 26: 8-16 (in
German).
HILL, J.R. (1964) The development of thermal stability in
the new-born baby. In: Jonxis, J.H.P., Visser, H.K.A., &
Troelstra, J.A., ed. The adaptation of the newborn to
extrauterine life, Netherlands, H.E. Stenfert Kroese NV, pp.
223-228.
HISLOP, A. & REID, L. (1981) Growth and development of the
respiratory system. In: Davis, J.A. & Dobbing, J., ed.
Paediatrics, 2nd ed., London, Heinemann, pp. 390-432.
HOAR, R.M. & MONIE, I.W. (1981) Comparative development of
specific organ systems. In: Kimmel, C.A. & Buelke-Sam, J., ed.
Developmental toxicology, New York, Raven Press, pp. 13-33.
HOFFMANN, H. (1982) Absorption of drugs and other
xenobiotics during development in experimental animals.
Pharmac. Ther., 16: 247-260.
ISLEIB, D.R. & WHITEHEAD, G.L. (1975) Polybrominated
biphenyls: an agricultural accident and its consequences. I.
The agricultural effects of exposure. In: Hemphill, D.D., ed.
Trace substances in environmental Health. IX, pp. 47-55.
JAPAN/US COOPERATIVE SCIENCE PROGRAM (1983) Toxicity of
chlorinated biphenyls, dibenzofurans, dibenzodioxins, and
related compounds. In: Proceedings of a Joint Seminar,
Fukvoka, Japan, pp. 25-28.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Res. Rev., 89: 1-128.
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
JONES, K.L. & SMITH, D.W. (1975) The fetal alcohol syndrome.
Teratology, 12: 11-26.
JUGO, S. (1980) Chelatable fraction of 203Pb in blood of
young and adult rats. Environ. Res., 21: 336-342.
KAVLOCK, R.J. & GRAY, J.A. (1983) Morphometric, biochemical,
and physiological assessment of perinatally induced renal
dysfunction. J. Toxicol. environ. Health, 11: 1-3.
KIESE, M. (1974) Methaemoglobinaemia: a comprehensive
treatise, Ohio, CRC Press, pp. 17-19.
KIMBROUGH, R.D., TAINES, T.V., & LINDER, R.E. (1974) The
effect of 2,4-dichlorophenyl- p -nitrophenyl ether on the rat
fetus. Arch. environ. Health, 28: 316-320.
KITAMURA, Y., MIYAO, M., & KAWAZOE, K., ET AL. (1953) In:
Friberg, L., ed. Toxic metals and their implications for human
health. Acta med., 8: 205-225.
KLINGER, W. (1982) Biotransformation of drugs and other
xenobiotics during postnatal development. Pharmac. Ther., 16:
377-429.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
KOSTIAL, K., SIMONOVIC, I., & PISONIC, M. (1971) Lead
absorption from the intestine in new-born rats. Nature
(Lond.), 233: 564.
KOSTIAL, K., KELLO, D., JUGO, S., RABAR, I., & MALJKOVIC, T.
(1978) The influence of age on metal metabolism and toxicity.
Environ. Health Perspect., 25: 81-86.
KROGER, M. (1972) Insecticide residues in human milk.
Pediatrics (Springfield), 80: 401-405.
KURATSUNE, M. (1980) "Yusho". In: Kimbrough, R.D., ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzo-
dioxins, and related products, Amsterdam, Elsevier/North
Holland Biomedical Press, pp. 287-302 (Topics in Environmental
Health No. 4).
L'ALLEMAND, D., GRUTERS, A., HEIDEMANN, P., & SCHURNBRAND, P.
(1983) Iodine-induced alterations of thyroid function in
new-born infants after prenatal and perinatal exposure to
povidone iodine. J. Pediatr., 102: 935-938.
LANDSBERG, J.D., BODYFELT, F.W., & MORGAN, M.E. (1977)
Retention of chemical contaminants by glass, polyethylene, and
polycarbonate multi-use containers. J. Food Protect., 40:
772-777.
LANGSTON, C. (1983) Normal and abnormal structural
development of the human lung. In: Abnormal functional
development of the heart, lungs, and kidneys: approaches to
functional teratology, New York, Alan R. Liss, pp. 75-91.
LAWTHER, P.J. & WALLER, R.E. (1975) Physical hazards. In:
Barltrop, D., ed. Paediatrics and the environment. Fellowship
of Postgraduate Medicine/Unigate Paediatric Workshop No. 2,
London, pp. 5-9.
LEVINE, S. & MULLINS, R. (1967) Neonatal androgen or
estrogen treatment and the adrenal cortical response to stress
in adult rats. Endocrinology, 80: 1177-1179.
LIBERTUN, C. & LAU, C. (1972) Adrenocortical function in
prepuberal rats: neonatal effects of testosterone. J. Endocr.,
55: 221-222.
LOIZZO, A., GATTI, G.L., MACRI, A., MORRETTI, G., ORTOLANI,
E., & PALAZZESI, S. (1984) Italian baby food containing
diethylstilboestrol: three years later. Lancet, 1: 1014-1015.
LUSTER, M.I., FAITH, R.E., & KIMMEL, D.A. (1978) Depression
of humoral immunity in rats following chronic developmental
lead exposure. J. environ. Pathol. Toxicol., 1: 397-402.
LUSTER, M.I., BOORMAN, G.A., DEAN, J.H., HARRIS, M.W., LUEBKE,
R.W., PADARATHSINGH, M.L., & MOORE, J.A. (1980) Examination
of bone marrows, immunological parameters, and host suscept-
ibilities following pre- and postnatal exposure to 2,3,7,8-
tetrachlorodibenzo- p -dioxin (TCDD). Int. J. Immunopharmacol.,
2: 301-310.
MCCANCE, R.A. (1948) Renal function in early life. Physiol.
Rev., 28: 331-348.
MCCANCE, R.A. & WIDDOWSON, E.M. (1956) Metabolism, growth,
and renal function of piglets in the first days of life. J.
Physiol., 133: 373-384.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957a) New thoughts on
renal function in the early days of life. Br. med. Bull., 13:
3-6.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957b) Hypertonic expansion
of the extracellular fluids. Acta paediatr., 46: 337-353.
MCCANCE, R.A. & YOUNG, W.F. (1940) The secretion of urine by
new-born infants. J. Physiol., 99: 265-282.
MCLACHLAN, J.A., NEWBOLD, R.R., KORACH, K.S., LAMB, J.C., &
SUZUKI, Y. (1981) Transplacental toxicology: prenatal
factors influencing postnatal fertility. In: Kimmel, C.A. &
Buelke-Sam, J., ed. Developmental toxicology, New York, Raven
Press, pp. 213-232.
MACHO, L., STRBAK, V., & HROMADOVA, M. (1972) The effect of
thyroxine on amino acid incorporation into protein during
ontogenesis in rats. Hormones, 3: 354-360.
MAHAFFEY, K.R. (1981) Nutritional factors in lead poisoning.
Nutrit. Rev., 39: 353-362.
MAHAFFEY, K.R. (1983) Absorption of lead by infants and
young children. In: Schmidt, E.H.F. & Hildebrandt, A.G., ed.
Health evaluation of heavy metals in infant formula and junior
food, Berlin, Springer Verlag, pp. 69-85.
MALPUECH, G., GAILLARD, G., GAULME, J., DOLY, M., BESSE, G.,
GOUMY, P., & RAYNAUD, E.J. (1978) Hypothyroidie transitoire
chez huit nouveau-nes de petit poids de naissance. Arch.
Franc. Ped., 35: 620-630.
MARWHA, H., NAYAK, N.C., ROY, S., KALRA, V., & GHAI, O.P.
(1981) The role of excess hepatic copper in the evaluation of
Indian childhood cirrhosis. Indian J. med. Res., 73: 395-403.
MARZULLI, F.N. & MAIBACH, H.I. (1975) Relevance of animal
models: the hexachlorophene story. In: Maibach, H., ed. Animal
models in dermatology, New York, Churchill Livingstone, pp.
156-167.
MAURER, H.M., WOLFF, J.A., FINSTER, M., POPPERS, P.J.,
PANTUCK, E., KUNTZMAN, R., & CONNEY, A.H. (1968) Reduction
in concentration of total serum-bilirubin in offspring of
women treated with phenobarbitone during pregnancy. Lancet, 2:
122-124.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
MIYABE, M., TSUBOUCHI, H., & SAKABE, Y. (1971) Studies on
pesticide residues in foods. II. Residues of BHC in milk. Rep.
Nagoya Munic. Pub. Health Lab., 18: 37-41.
MOMCILOVIC, B. & KOSTIAL, K. (1974) Kinetics of lead
retention and distribution in suckling and adult rats.
Environ. Res., 8: 214-220.
MORSELLI, P.L. (1976) Clinical pharmacokinetics in neonates.
Clin. Pharmacokin., 1: 81-98.
MRC (1984) The neuropsychological effects of lead in
children: a review of recent research, (1979-83), London,
Medical Research Council.
NATIONAL RESEARCH COUNCIL, SUBCOMMITTEE ON AIRBORNE PARTICLES
(1979) Epidemiologic studies on the effects of airborne
particles on human health. In: Airborne particles, Baltimore,
Maryland, University Park Press, pp. 167-198.
NIEMINERS, L., BJORNDAHL, K., & MOTTONEN, M. (1973) Effect
of hexachlorophene on the rat brain during organogenesis. Food
cosmet. Toxicol., 11: 635-639.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
NORDBERG, G.F. (1979) Factors influencing metabolism and
toxicity of metals. In: Di Ferrante, E., ed. Trace metals:
exposure and health effects, Oxford, CEC/Pergamon Press, pp.
157-158.
PAVEY, D.E. & WIDDOWSON, E.M. (1980) Body lipids of
guinea-pigs exposed to different dietary fats from
mid-gestation to 3 months of age. V. The fatty acid
composition of brain lipids at birth. Nutr. Metab., 24:
357-366.
PETERS, H.A. (1976) Hexachlorobenzene poisoning in Turkey.
Fed. Proc., 35: 2400-2403.
RAMBOER, C., THOMPSON, R.P.H., & WILLIAMS, R. (1969)
Controlled trials of phenobarbitone therapy in neonatal
jaundice. Lancet, 1: 966-968.
ROBERTS, D.W. & CHAPMAN, J.R. (1981) Concepts essential to
the assessment of toxicity to the developing immune system.
In: Kimmel, C.A. & Buelke-Sam, J., ed. Developmental
toxicology, New York, Raven Press, pp. 167-189.
ROGAN, W. & GLADEN, B. (1982) Duration of breast-feeding and
environmental contaminants in milk. Am. J. Epidemiol., 116:
565A.
ROOK, J.J. (1974) Formation of halogens during clorination
of natural waters. J. Water Treat. Exam., 23: 234-243.
ROWLAND, I.R., ROBINSON, R.D., DOHERTY, R.A., & LANDRY, T.D.
(1983) Are developmental changes in methylmercury metabolism
and excretion mediated by the intestinal microflora? In:
Clarkson, T.W., Nordberg, G.F., & Sager, P.R., ed.
Reproductive and developmental toxicity of metals, New York,
Plenum Press, pp. 745-758.
SAENZ DE RODRIGUEZ, C.A. & TORO-SOLA, M.A. (1982) Anabolic
steroids in meat and premature telarche. Lancet, 1: 1300.
SCAGLIONI, S., DI PIETRO, C., BIGATELLO, A., & CHIUMELLO, G.
(1978) Breast enlargement at an Italian school. Lancet, 1:
551-552.
SCHELER, W. (1980) [The basis of general pharmacology,] 2nd
ed., Stuttgart, Gustav Fischer Verlag (in German).
SCHMIDT, R.R. (1984) Altered development of immunocompetence
following prenatal or combined perinatal-postnatal insult: a
timely review. J. Am. Coll. Toxicol., 3: 57-72.
SHERLOCK, J.C., SMART, G., FORBES, G.I., MOORE, M.J.,
PATTERSON, W.J., RICHARDS, W.N., & WILSON, T.S. (1982)
Assessment of lead intakes and dose response for a population
in Ayr exposed to a plumbosolvent water supply. Hum. Toxicol.,
1: 115-122.
SHUMAN, R.M., LEECH, R.W., & ALVORD, E.C., Jr (1975)
Neurotoxicity of hexachlorophene in humans. II. A clinico-
pathological study of 46 premature infants. Arch. Neurol., 32:
320-325.
SINGER, E.J., WEGMANN, P.C., LEHMAN, M.D., CHRISTENSEN, M.S.,
& VINSON, L.J. (1971) Barrier development, ultrastructure,
and sulfhydryl content of the fetal epidermis. J. Soc. Cosmet.
Chem., 22: 119-137.
SMITH, R.J. (1982) Hawaiian milk contamination. Science
N.Y., 217: 137-140.
SVENNERHOLM, L., ALLING, C., BRUCE, A., KARLSSON, I., & SAPIA,
O. (1972) Effects on offspring of maternal malnutrition in
the rat. In: Elliott, K. & Knight, J., ed. Lipids,
malnutrition, and the brain, Amsterdam, Associated Scientific
Publishers, pp. 141-157.
TADJER, G.S. & DORE; I. (1971) Possibility of contamination
of bottles used by food industries by insecticides. Harefuah,
81: 385.
TAKEWAKI, K. (1968) Reproductive organs and anterior
hypophysis of neonatally androgenized female rats. Sci. Rep.
Tokyo Women's Christ. Coll., 1-6: 31-47.
TANNER, J.M., WHITEHOUSE, R.H., & TAKAISHI, M. (1966)
Standards from birth to maturity for height, weight, height
velocity, and weight velocity. Part II. British children,
1965. Arch. Dis. Child., 41: 613-635.
THABREW, M.W., OLORUNSOGO, O.O., OLOWOOKERE, J.O., &
BABABUNMI, E.A. (1982) Possible defect in xenobiotic
activation before glycine conjugation in protein-energy
malnutrition. Xenobiotica, 12: 849-853.
TROLLE, D. (1968) Phenobarbitone and neonatal icterus.
Lancet, 1: 251-252.
TURNER, M.D., MARSH, D.O., SMITH, J.C., INGLIS, J.B.,
CLARKSON, T.W., RUBIO, C.E., CHIRIBOGA, J., & CHIRIBOGA, C.C.
(1980) Methylmercury in populations eating large quantities
of marine fish. I. Northern Peru. Arch. environ. Health,
35(6): 367-378.
ULSAMER, A.G., YODER, P.D., KIMBROUGH, R.D., & MARZULLI, F.N.
(1975) The effect of hexachlorophene on developing rats:
toxicity tissue concentrations and biochemistry. Food cosmet.
Toxicol., 13: 69-80.
UMBERGER, E.J. (1975) Products marketed to promote growth in
food-producing animals: steroid and hormone products.
Toxicology, 3: 3-21.
UNITED KINGDOM CENTRAL DIRECTORATE ON ENVIRONMENTAL POLLUTION
(1982) The Glasgow duplicate diet study (1979/80), London,
Her Majesty's Stationery Office (Pollution Report No. 11).
UNITED KINGDOM DEPARTMENT OF HEALTH AND SOCIAL SECURITY
(1980) Lead and health, London, Her Majesty's Stationery
Office (Report of a DHSS Working Party on Lead in the
Environment).
URSO, P. & GENGOZIAN, N. (1980) Depressed humoral immunity
and increased tumour incidence in mice following in utero
exposure to benzo( a )pyrene. J. Toxicol. environ. Health, 6:
569-576.
US DHEW (1979) Smoking and health: a report of the Surgeon
General, Washington DC, US Department of Health, Education and
Welfare (Section No. 8).
VALCANA, T. & TIMIRAS, P.S. (1978) Nuclear triiodothyronine
receptors in the developing rat brain. Mol. Cell. Endocrinol.,
11: 31-41.
VALE, J.A. & MEREDITH, T.J. (1981) Epidemiology of poisoning
in the UK. In: Vale, J.A. & Meredith, T.J., ed. Poisoning
diagnosis and treatment, 1st ed., London, Update Books, pp.
1-8.
VESSELINOVITCH, S.D., MIHAILOVICH, N., WOGAN, G.N., LOMBARD,
L.S., & RAO, K.V.N. (1972) Aflatoxin B1, a hepato-
carcinogen in the infant mouse. Cancer Res., 32: 2289-2291.
VILLETT, L.B. & HESS, J.F., Jr (1975) Polychlorinated
biphenyl residues in silos in the US. Residue Res., 55:
135-147.
VOS, J.G. (1977) Immune suppression as related to
toxicology. CRC Crit. Rev. Toxicol., 5: 67-101.
VOS, J.G. & MOORE, J.A. (1974) Suppression of cellular
immunity in rats and mice by maternal treatment with
2,3,7,8-tetrachlorodibenzo- p -dioxin. Int. Arch. Allergy appl.
Immunol., 47: 777-794.
VOS, J.G., VAN DOGTEN, M.J., KREEFTENBERG, J.G., STEERENBERG,
P.A., & KRUIZINGA, W. (1979) Effect of hexachlorobenzene on
the immune system of rats following combined pre- and
postnatal exposure. Drug Chem. Toxicol., 2: 61-76.
WALKER, W.A. (1979) Gastrointestinal host defence:
importance of gut closure in control of macromolecular
transport. In: Elliott, K. & Whelan, J., ed. Development of
mammalian absorptive processes, Amsterdam, Excerpta Medica,
pp. 201-216 (Ciba Foundation Symposium No. 70 (new series)).
WESTER, R.C. & MAIBACH, H.I. (1982) Percutaneous absorption:
neonate compared to the adult. In: Hunt, V.R., Smith, M.K., &
Worth, D., ed. Environmental factors in human growth and
development, New York, Cold Spring Harbor Laboratory (Banbury
Report No. 2).
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1984) Environmental Health Criteria 30: Principles for
evaluating health risks to progeny associated with exposure to
chemicals during pregnancy, Geneva, World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WIDDOWSON, E.M. & DICKERSON, J.W.T. (1964) Chemical
composition of the body. In: Comar, C.L. & Bronner, F., ed.
Mineral metabolism, New York, Academic Press, Vol. 2A, pp.
1-247.
WIDDOWSON, E.M., CRABB, D.E., & MILNER, R.D.G. (1972)
Cellular development of some human organs before birth. Arch.
Dis. Child., 47: 652-655.
WILLOUGHBY, M.L.N. (1984) Disorders of the blood and
reticuloendothelial system. In: Forfar, J.O. & Arneil, G.C., ed.
Textbook of paediatrics, 3rd ed., Edinburgh, Churchill
Livingstone, Vol. 1, pp. 935-982.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
WONG, K.L. & KLAASSEN, C. (1980) Tissue distribution and
retention of cadmium in rats during postnatal development:
minimal role of hepatic metallothionein. Toxicol. appl.
Pharmacol., 53: 343-353.
ZIEGLER, E.E., EDWARDS, B.B., JENSEN, R.L., MAHAFFEY, K.R., &
FOMON, S.J. (1978) Absorption and retention of lead by
infants. Pediatr. Res., 12: 29-34.
APPENDIX I: BREAST MILK
1. Introduction
The breast-fed infant requires special consideration in
circumstances in which chemical contamination of the mother's milk
is suspected, or has been confirmed. Young infants are
characteristically dependent on milk, and the structural and
functional development of their organs and tissues is adapted to
the milk of their own species. The potential risks of exposure to
chemicals in breast milk must therefore be assessed, taking into
account the benefits of breast-feeding together with the
disadvantages of feeding an alternative milk, in terms of
developmental toxicology.
The benefits of breast-feeding, particularly in the developing
countries, are now universally acknowledged. In these societies,
nearly all women of all classes breast-feed for 12 months or more.
The decline in breast-feeding in many developed countries has
recently been reversed; interestingly, both the decline in, and
return to, breast-feeding began among the most socially advantaged
women (WHO, 1981). National and international authorities have
been concerned that, in view of the health risks involved in the
preparation and provision of breast milk substitutes in developing
societies, mothers in these countries should not go through the
changes in infant feeding practice that have occurred in the
developed world. Thus, the Member States of the World Health
Organization have committed themselves to the protection and
promotion of breast-feeding as the first, and one of the most
critical, steps in sound infant nutrition policies (WHO/UNICEF,
1981).
This Appendix is concerned with principles for evaluating the
possible consequences to health of chemical contaminants in breast
milk. The evaluation of risks potentially associated with these
chemicals in the quantities that may be consumed must be carefully
weighed against the assessment of the risks of alternative methods
of infant feeding.
The breast milk of a well-nourished mother has an optimal
composition to meet the nutritional needs of the full-term infant
in early life, and there are associated immunological,
psychological, and economic advantages. In view of the importance
of breast milk for the nutrition and health of the infant, evidence
of any harmful effects of a foreign chemical that it may contain
must be carefully considered before recommendations are made that
breast-feeding should be curtailed or discontinued.
2. Assessment of the Importance of Chemicals in Breast Milk
2.1 Source of chemicals
Women may be exposed to chemical substances from many different
sources, including air, food, water, drugs, cosmetics, and the
occupational environment.
2.2 Chemicals within the mother's body and their secretion in milk
Chemicals that are easily metabolized and are eliminated from
the mother's body in the faeces and urine are only secreted in
significant quantities in breast milk following recent high-level
chemical exposure (Wolff, 1983; Jensen, 1985).
Other chemicals, such as heavy metals and persistent
halocarbons, are less readily removed from the body. Some metals
accumulate mainly in the liver, kidney, and bone, whereas
persistent halocarbons accumulate in the lipids of the body. Long-
term exposure, even to low concentrations of such chemicals, may
build up considerable body stores. On the other hand, a short-term
change of diet is not likely to alter the concentration of
halocarbon in the mother's fatty tissues.
A dynamic equilibrium for persistent lipophilic chemicals
exists between different tissue compartments. The concentrations
of these chemicals in adipose tissue, blood, and milk is in
proportion to the fat content; thus, the concentration in blood is
far lower than that in whole milk, and the concentration in milk is
far lower than that in adipose tissue. There is a relatively good
correlation between concentrations of organic halocarbons in the
lipids of blood, adipose tissue, and milk. This is illustrated for
DDT in the lipids of milk and adipose tissue in Fig. I.1.
 2.3 Development and limits of methods for measuring chemicals in
milk
During the 1960s and 1970s, techniques such as gas
chromatography were developed, which increased the scope and
accuracy of analytical methods for the determination of chemicals
in breast milk. Studies were made in various countries, and many
different halocarbons were detected. The sensitivity of the
methods was greatly increased with the introduction of glass
capillary columns in gas chromatography, high resolution liquid
chromatography, and mass spectrometry, and it is now possible to
detect some environmental chemicals in human milk fat at the ng/kg
level (Jensen, 1985). Similarly, the sensitivity of methods for
measuring heavy metals has also greatly increased in recent years
with the introduction of electrothermal atomic absorption
spectrophotometry, mass spectrometry, and neutron activation
techniques.
These newly developed methods require specialized laboratory
staff and facilities, as well as quality assessment programmes, to
ensure the accuracy and precision of results. The analyses are
often time-consuming and costly to perform. This limits the number
that can be made, and this must be taken into account when
developing strategies and requirements for the determination of
chemicals in breast milk.
2.4 Risks to the infant
The presence of chemicals in breast milk must be seen in
perspective. Because a chemical has been detected and measured, it
does not mean that it is necessarily harmful to the infant in the
quantities consumed.
The magnitude of chemical burdens derived from breast milk
cannot be assessed in isolation but must be related to those
obtained from other routes of exposure. Additional contributions
to the uptake of chemicals by the infant may occur through the
percutaneous and respiratory pathways and, in some cases, there is
a pre-existing burden sustained in utero from placental transfer.
The evaluation of the risks associated with a given chemical in
breast milk requires knowledge of:
(a) intake of the chemical by the infant;
(b) availability for absorption from the infant
intestinal tract;
(c) kinetics of uptake, transport, distribution,
metabolism, excretion, and net retention by the
infant body;
(d) effects on developing organs, tissues, and systems of
the young infant; and
(e) long-term sequelae in later childhood and adult life.
These considerations are discussed in sections 3 and 4 of this
publication. Information about most of them is incomplete.
In breast-fed infants, the concentrations of halocarbons in the
blood, for example, polychlorobiphenyls (PCBs), were found to
increase after birth and reached the maternal levels after 3
months; the increase continued until breast feeding was stopped;
thereafter, the concentrations of halocarbons decreased (Kodama &
Ota, 1980). An association between breast-feeding and high
concentrations of persistent halocarbons in the body fat of infants
has also been observed (Niessen et al., 1984), and it has been
calculated that, within a few months of birth, the concentration of
halocarbons in the fat of the infant may be equal to that in the
maternal fat (Wickizer et al., 1981; Mes et al., 1984). Although
the period of breast-feeding is short in relation to the life span,
the chemicals accumulated in the body during these months may be
retained for many years (Jensen, 1985).
At present, there appears to be only one preliminary report of
a prospective study in which 850 breast-fed infants were
periodically examined, and the milk was analysed for halocarbons
(Rogan & Gladen, 1982). The main result at the time the report was
written was that the mothers with the greatest concentration of
2,2 bis(4-chlorophenol)-1,1-dichloroethylene (DDE) in their milk
did not breast-feed for as long as those with the least, and they
gave their infants formula at an earlier age.
2.5 Assessment of contamination of breast milk following
maternal exposure
The purpose of investigating the chemical contamination of
breast milk in the context of this publication is to assess the
possible harm to infants if they continue to take milk from exposed
mothers. The daily intake of a contaminant depends on the volume
of breast milk taken and on the concentration of the chemical in
the milk. At all ages, the intake of milk by a full-term breast-
fed infant varies considerably between individuals. This depends
partly on body weight; 150 ml/kg body weight per day can be taken
as a representative intake, after the first week. This gradually
falls after 2 months to about 120 ml/kg per day at 4 - 6 months.
The concentration of lipophilic chemicals in milk is clearly
related to its fat content, and this varies with the stage of
lactation, the maternal diet, the time of day, and during the
course of a single feed. There is also a variation in
concentration of protein-bound and water-soluble chemicals at
different stages of lactation. Thus, the infant's intake of
chemicals is not constant from day to day, or within one day, or
even within a particular feed. How then should samples of breast
milk be collected for analysis to obtain a reasonable figure
representing the infant's intake of the chemical in question? If
the chemical is lipid-soluble, much of the difficulty is removed if
the percentage of fat is also determined and the concentration of
the chemical expressed per unit weight fat. Then, the following
equation can be applied:
TD = C x F x M
where,
TD = daily intake of contaminant (units per kg body weight
per day)
C = average concentration of contaminant in milk fat
(units per g fat)
F = average concentration of fat in milk (g/g milk)
M = average consumption of milk (g/kg per day).
2.5.1 Method of collection of breast milk
Methods for collecting breast milk have already been described
in the report of a WHO Collaborative Study on Breast-Feeding (WHO,
1985).
3. Conclusions
Because of the importance of breast-feeding to the health and
development of the infant, a strategy related to chemicals detected
in breast milk requires a thorough appraisal of the advantages and
disadvantages of the available options. In evaluating the risk of
continued exposure to a chemical through breast-feeding, the risk
of nutritional deprivation or infection, if breast-feeding is
curtailed, must be assessed simultaneously before a decision is
made.
For this reason, studies are required to establish a
relationship between levels of environmental exposure of mothers
(and hence subsequent milk contamination) and effects on the health
of breast-fed infants. Surveys of concentrations of chemicals in
breast milk should be undertaken as part of the strategy for infant
nutrition. The finding of chemicals in breast milk should lead to
an evaluation of the implications for the health of the infant.
REFERENCES TO APPENDIX I
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
ROGAN, W.H. & GLADEN, B.C. (1982) Duration of breast-feeding
and environmental contaminants in milk. Am. J. Epidemiol.,
116: 565A.
ROGAN, W.J. & GLADEN, B.C. (1985) Study of human lactation
for effects of environmental contaminants: the North Carolina
breast milk and formula project and some other ideas. Environ.
Health Perspect., 60: 215-221.
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
2.3 Development and limits of methods for measuring chemicals in
milk
During the 1960s and 1970s, techniques such as gas
chromatography were developed, which increased the scope and
accuracy of analytical methods for the determination of chemicals
in breast milk. Studies were made in various countries, and many
different halocarbons were detected. The sensitivity of the
methods was greatly increased with the introduction of glass
capillary columns in gas chromatography, high resolution liquid
chromatography, and mass spectrometry, and it is now possible to
detect some environmental chemicals in human milk fat at the ng/kg
level (Jensen, 1985). Similarly, the sensitivity of methods for
measuring heavy metals has also greatly increased in recent years
with the introduction of electrothermal atomic absorption
spectrophotometry, mass spectrometry, and neutron activation
techniques.
These newly developed methods require specialized laboratory
staff and facilities, as well as quality assessment programmes, to
ensure the accuracy and precision of results. The analyses are
often time-consuming and costly to perform. This limits the number
that can be made, and this must be taken into account when
developing strategies and requirements for the determination of
chemicals in breast milk.
2.4 Risks to the infant
The presence of chemicals in breast milk must be seen in
perspective. Because a chemical has been detected and measured, it
does not mean that it is necessarily harmful to the infant in the
quantities consumed.
The magnitude of chemical burdens derived from breast milk
cannot be assessed in isolation but must be related to those
obtained from other routes of exposure. Additional contributions
to the uptake of chemicals by the infant may occur through the
percutaneous and respiratory pathways and, in some cases, there is
a pre-existing burden sustained in utero from placental transfer.
The evaluation of the risks associated with a given chemical in
breast milk requires knowledge of:
(a) intake of the chemical by the infant;
(b) availability for absorption from the infant
intestinal tract;
(c) kinetics of uptake, transport, distribution,
metabolism, excretion, and net retention by the
infant body;
(d) effects on developing organs, tissues, and systems of
the young infant; and
(e) long-term sequelae in later childhood and adult life.
These considerations are discussed in sections 3 and 4 of this
publication. Information about most of them is incomplete.
In breast-fed infants, the concentrations of halocarbons in the
blood, for example, polychlorobiphenyls (PCBs), were found to
increase after birth and reached the maternal levels after 3
months; the increase continued until breast feeding was stopped;
thereafter, the concentrations of halocarbons decreased (Kodama &
Ota, 1980). An association between breast-feeding and high
concentrations of persistent halocarbons in the body fat of infants
has also been observed (Niessen et al., 1984), and it has been
calculated that, within a few months of birth, the concentration of
halocarbons in the fat of the infant may be equal to that in the
maternal fat (Wickizer et al., 1981; Mes et al., 1984). Although
the period of breast-feeding is short in relation to the life span,
the chemicals accumulated in the body during these months may be
retained for many years (Jensen, 1985).
At present, there appears to be only one preliminary report of
a prospective study in which 850 breast-fed infants were
periodically examined, and the milk was analysed for halocarbons
(Rogan & Gladen, 1982). The main result at the time the report was
written was that the mothers with the greatest concentration of
2,2 bis(4-chlorophenol)-1,1-dichloroethylene (DDE) in their milk
did not breast-feed for as long as those with the least, and they
gave their infants formula at an earlier age.
2.5 Assessment of contamination of breast milk following
maternal exposure
The purpose of investigating the chemical contamination of
breast milk in the context of this publication is to assess the
possible harm to infants if they continue to take milk from exposed
mothers. The daily intake of a contaminant depends on the volume
of breast milk taken and on the concentration of the chemical in
the milk. At all ages, the intake of milk by a full-term breast-
fed infant varies considerably between individuals. This depends
partly on body weight; 150 ml/kg body weight per day can be taken
as a representative intake, after the first week. This gradually
falls after 2 months to about 120 ml/kg per day at 4 - 6 months.
The concentration of lipophilic chemicals in milk is clearly
related to its fat content, and this varies with the stage of
lactation, the maternal diet, the time of day, and during the
course of a single feed. There is also a variation in
concentration of protein-bound and water-soluble chemicals at
different stages of lactation. Thus, the infant's intake of
chemicals is not constant from day to day, or within one day, or
even within a particular feed. How then should samples of breast
milk be collected for analysis to obtain a reasonable figure
representing the infant's intake of the chemical in question? If
the chemical is lipid-soluble, much of the difficulty is removed if
the percentage of fat is also determined and the concentration of
the chemical expressed per unit weight fat. Then, the following
equation can be applied:
TD = C x F x M
where,
TD = daily intake of contaminant (units per kg body weight
per day)
C = average concentration of contaminant in milk fat
(units per g fat)
F = average concentration of fat in milk (g/g milk)
M = average consumption of milk (g/kg per day).
2.5.1 Method of collection of breast milk
Methods for collecting breast milk have already been described
in the report of a WHO Collaborative Study on Breast-Feeding (WHO,
1985).
3. Conclusions
Because of the importance of breast-feeding to the health and
development of the infant, a strategy related to chemicals detected
in breast milk requires a thorough appraisal of the advantages and
disadvantages of the available options. In evaluating the risk of
continued exposure to a chemical through breast-feeding, the risk
of nutritional deprivation or infection, if breast-feeding is
curtailed, must be assessed simultaneously before a decision is
made.
For this reason, studies are required to establish a
relationship between levels of environmental exposure of mothers
(and hence subsequent milk contamination) and effects on the health
of breast-fed infants. Surveys of concentrations of chemicals in
breast milk should be undertaken as part of the strategy for infant
nutrition. The finding of chemicals in breast milk should lead to
an evaluation of the implications for the health of the infant.
REFERENCES TO APPENDIX I
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
ROGAN, W.H. & GLADEN, B.C. (1982) Duration of breast-feeding
and environmental contaminants in milk. Am. J. Epidemiol.,
116: 565A.
ROGAN, W.J. & GLADEN, B.C. (1985) Study of human lactation
for effects of environmental contaminants: the North Carolina
breast milk and formula project and some other ideas. Environ.
Health Perspect., 60: 215-221.
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.

 Table 1. Increase in weight of body and
organs during the first 9 months after
birtha
-----------------------------------------
Body/organ Increase
(g/kg weight at birth)
-----------------------------------------
Body 1810
Brain 1480
Liver 1420
Kidneys 1580
Heart 1160
Lungs 1500
-----------------------------------------
a Based on: values given in Diem & Lentner (1970).
The constituents of some organs and tissues have a slower rate
of turnover than those of others. The lipids, for example, once
laid down, have a slow rate of turnover compared with protein, in
all parts of the body including the brain. Fat-soluble chemicals,
which might be incorporated into brain lipids during the rapid
growth of the brain in infancy (Dobbing & Sands, 1973), would not
enter the tissue in significant amounts after the growth of the
brain has ceased. The fatty acid composition of brain lipids of
rats and guinea-pigs can be appreciably altered, early in life, by
the nature of the fat in the diet of the mother during pregnancy
and lactation (Svennerholm et al., 1972; Pavey & Widdowson, 1980).
Information on man is lacking.
1.3.4 Different body composition from adult
Infants have a different body composition from adults. As in
adults, the contribution of fat to the body weight varies
considerably between one individual and another in infants, but the
average is about 15% in full-term new-born infants and in adult
men. However, infants have a higher percentage of water and a
smaller percentage of solids in the lean body tissue. The infant,
at term, has 82% of water in its lean tissue compared with 72% in
the adult (Widdowson & Dickerson, 1964). The additional water in
the infant is mainly extracellular, the volume of extracellular
fluid per unit body weight in the young infant being about twice
that in an adult (Widdowson & Dickerson, 1964). Thus, any chemical
that is confined to the extracellular compartment of the body will
have a greater volume in which to distribute itself per unit body
weight. The advantage of this for the infant could be offset by
the slower rate of excretion by the kidneys (section 3).
The percentage of water in the organs and tissues, and thus in
the body as a whole, decreases with age. The levels of water in
some organs at birth, at 6 - 12 months where figures are available,
and in the adult (Widdowson & Dickerson, 1964) are shown in Table
2. The decrease in water in the liver, kidneys, heart, and lungs
is due primarily to an increase in protein; in the brain, it is due
to an increase in myelin.
Table 2. Water in organs and tissues (g/kg)a
-------------------------------------------------
Organ/tissue At birth 6 - 12 Adult
months
-------------------------------------------------
Whole body (fat-free 820 780 720
basis)
Brain 900 830 770
Liver 780 760 710
Kidneys 840 - 810
Heart 840 830 830
Lungs 860 - 790
Skeletal muscle
Total 800 780 790
Extracellular 350 290 180
Intracellular 450 490 610
-------------------------------------------------
a From: Widdowson & Dickerson (1964).
Another difference between infants and adults is that most of
the cells of the organs and tissues of infants are smaller than
those of adults (Widdowson et al., 1972). Small cells, like small
bodies, have a larger surface area in relation to mass than bigger
cells and bodies, and this in itself may have important
implications for chemicals that may enter the cells.
The bones of an infant contain more water and less fat,
protein, and mineral than adult bones. This is illustrated in
Table 3 for the whole femur (Dickerson, 1962b). Unlike soft
tissues, the chemical maturation of the bone, as evidenced by the
decrease in percentage of water and increase in degree of
calcification, mainly takes place after 1 - 2 years. From the
values in Table 3, it can be calculated that the increment per
month of calcium in one femur is approximately 0.2 g between birth
and 18 months, 0.48 g between 18 months and 11 years, and 1.0 g
between 11 and 18 years. The femur may not be completely typical
of all parts of the skeleton, but it does seem likely that the rate
of deposition of chemicals taken up by the bone along with calcium,
and indeed with magnesium, sodium, and other metals present in
bone, is likely to be greater in later childhood than in infancy.
However, the bones of infants are smaller, and the rate of
deposition of calcium per kg of bone per month is greater in
infancy, being 7.8 g/kg bone between birth and 3 months, 6.8 g/kg
between 3 and 18 months, and 1.9 g/kg bone between 18 months and 18
years. Thus, whether the bones of infants are considered likely to
take up more or less of a chemical than those of older children
depends partly on the method of expression of the rate of
deposition.
Table 3. Composition of the whole femur of the infant, child, and adulta
---------------------------------------------------------------------
At birth 3 months 1 - 2 11 - 12 Adult
(full- (milk years years (pre-
term) feeding) (weaned) adolescent)
---------------------------------------------------------------------
Weight femur g 16.6 26.4 63.7 326 646
Fat g/kg fresh bone 1.4 6.5 75 26 31
Composition g/kg
fresh fat-free bone
Water 639 641 554 364 227
Collagen 93 107 141 163 173
Ca 61 54 70 138 194
P 28 27 33 63 83
---------------------------------------------------------------------
a From: Dickerson (1962b).
1.3.5 Functional immaturity of the organs and systems of the body
Most organs and systems of the body have not reached structural
or functional maturity at birth. Development of the nervous system
continues in postnatal life, much of the myelination of the brain
takes place after birth and continues until adolescence (Hoar &
Monie, 1981). The structural development of the lung also
continues postnatally with an increase in alveolar surface area
(Langston, 1983). Several components of the immune system are not
fully developed at birth, for example, the lymphocytes responsible
for producing antibodies, and it has been suggested that this is
responsible for the greater susceptibility of the new-born infant
to certain bacterial infections (Andersson et al., 1981). The
gastrointestinal, endocrine, and reproductive systems, and also
renal function, are all immature at birth. The kidneys are less
able than those of older children and adults to eliminate excessive
amounts of substances normally present in milk, for example, sodium
and phosphate (Dean & McCance, 1947a, 1948; Barltrop & Oppé, 1970),
and this may also apply to extraneous chemicals present in, and
absorbed from, milk. Moreover, the activities of many liver enzyme
systems that metabolize endogenous and exogenous substances may be
low in the new-born infant. These topics are covered in more
detail in section 3.
1.3.6 Breast milk, infant formulae, and water as sources of
chemicals
The young infant lives on a single food, milk, either from the
breast or the bottle. Thus, the presence of a chemical in breast
milk, or in the powder or water used to make up an infant formula,
is likely to have greater implications for the infant than for the
older child having mixed feeding, where milk forms only part of the
daily food. Furthermore, infants take in a much larger amount of
fluid per unit body weight than older children and adults. An
infant living on milk takes about one-seventh of its own weight of
water each day, which would correspond to 10 litres for a 70-kg
man. This large intake is necessary for the infant, because it
loses more water per kg body weight through the lungs, owing to the
greater passage of air through them, the skin, because of the
larger surface area, and the kidneys, because of the inability to
concentrate the urine to the same extent as the older child or
adult. Any chemical present in the water used to make up infant
formulae will, therefore, be taken in by an infant in larger
quantities per unit body weight than by an older child or adult
using the same water supply. This is discussed in more detail in
section 2.
Nowadays, it is common practice among manufacturers to add
vitamins and trace elements to their preparations. Occasionally,
these have been added in such large amounts that they have been
reported to be toxic, even though in smaller, physiological amounts
they are essential. Examples are vitamin D (British Paediatric
Association, 1956) and manganese (Collipp et al., 1983).
1.4 Applicability of Data on Experimental Animals to Human Infants
and Young Children
Data on the toxic effects of chemicals in man are frequently
incomplete or even absent, and this is particularly true for
infants and young children. Some insight can be gained from
observations on young animals, but any assessment of human risk on
the basis of such studies should take into account the differences
that exist between the species in their reactions to chemicals.
Moreover, the conditions under which the animal data were obtained
may not reflect the human circumstances under consideration.
Animal data must be considered, therefore, in the context of the
similarities and differences that are known to exist between man
and experimental animals, and conclusions need to incorporate a
margin of safety to accommodate known, and possibly unknown,
species differences.
1.4.1 Some similarities in growth and development between
man and experimental animals
In many ways, the differences between the young infant and
adult are the same in animals as they are in man. Thus, the infant
animal is always smaller than the adult and, thus, it has a larger
surface area in relation to its body weight. All animals that are
homoeothermic from the time of birth have a higher consumption of
air per unit body weight than the adult of the same species, at the
same environmental temperature. The young of all animals species
grow rapidly, often far more rapidly than the human infant, and
their body composition differs from that of the adult in the same
way that that of the human infant differs from that of the human
adult. The functions of the organs and systems are immature in
early life, and most mammalian species depend on mother's milk for
a period after birth.
The anatomical, physiological, and chemical differences between
the infant and adult of an animal species are therefore in the same
direction as they are in the human species, and, in general, the
effects of chemicals on the young of experimental animals can be
expected to differ from those on the adult of the species just as
they do in man. However, the differences between species make it
unwise to assume that the results obtained on a young animal will
necessarily apply to the human infant.
1.4.2 Some differences in growth and development between man and
experimental animals
Different animals are born at different stages of their
anatomical, physiological, and chemical development and, although
the qualitative differences between the infant and adult are
similar in man and experimental animals, the quantitative
differences are by no means the same. The rat is a widely used
experimental animal in studies on the effects of chemicals on the
infant body. Some stages of development that take place after
birth in the rat have already occurred in the human fetus before
birth. Examples are the anatomical and functional maturation of
the hypothalamus (Dörner & Staudt, 1972) and the deposition of body
fat. Some aspects of development, however, only occur after birth
in all species, for example, respiration of air and the full
functioning of the gastrointestinal tract.
The new-born rat is smaller than the new-born human infant in
comparison with the weight of the adult, and, during infancy, it
grows at a much more rapid rate. Thus, the discussion in section
1.3.3 about the human infant applies with even greater force to the
infant rat. The rat at birth has a less mature chemical
composition than the human infant, as evidenced by the higher
percentage of water in its lean body tissue (Widdowson & Dickerson,
1964). The greater degree of immaturity of the body composition of
the rat at birth is well illustrated by reference to the bones.
Characteristically, rat bones have very little fatty marrow, but,
apart from this, the composition of the adult femur is not very
different in the 2 species. At birth, however, the rat femur has
less than half the concentration of calcium that there is in the
human femur, more water, and considerably less collagen, which
makes up the matrix on which the bone mineral is deposited
(Dickerson, 1962a,b). In the rat, as in man, the major changes in
the composition of bone do not take place until the period of
infancy is over. The composition of bone, and of the body as a
whole, though less mature at the time of birth in the rat than in
man, changes much more rapidly. All this must be taken into
account in considering the effects of a chemical on the bones or
bodies of young rats compared with the effects on those of human
infants.
The new-born rat is less mature physiologically than the human
infant, and it matures more rapidly. This may limit the
application of results obtained on the young rat to the human
infant. This is true of the renal function (McCance, 1948), so
that the new-born rat is less able than the adult to excrete
chemicals administered to it (section 3.5.1).
The activity of the hepatic enzymes is low in both new-born
experimental animals and new-born human infants, but the whole
subject of enzyme activity in this context is complex and is
considered in more detail in section 3.
One difference between the young of man and some animal species
is the ability to absorb macromolecules from the intestine. In the
human infant, the passage of macromolecules across the intestinal
mucosa is very limited, but in the young of such species as the
pig, cow, sheep, and horse, macromolecules are transferred from
the milk into the circulation in quite large amounts for a limited
period of hours or days. In the rat, the transfer goes on all
through the suckling period (Walker, 1979). This may have a
bearing on the interpretation of the results of studies on the
intestinal absorption of certain chemicals by the new-born
experimental animal in relation to the human infant.
1.5 Conclusions
The infant and young child have different structural and
functional characteristics from those of the older child and adult,
which represent stages in normal growth and development. These
include a larger surface area in relation to weight, different body
composition, a higher metabolic rate and oxygen consumption and,
hence greater intake of air per unit body weight, rapid growth and
development of the body as a whole and of the organs and tissues,
particularly during the first 6 months after birth. Many organs
and tissues are functionally immature at the time of birth, and
they mature at different rates. Moreover, infants have greater
energy and fluid requirements per unit body weight than older
children and adults. All these characteristics must be taken into
account in considering principles for evaluating health risks from
chemicals during infancy and childhood.
The structural and functional differences between the infant
and adult animal tend to follow the same direction as in the human
species, but there are important quantitative differences in, for
example, the stage of development at birth, the rate of growth, and
the intestinal absorption of macromolecules. The age of the infant
rat or other animal corresponding to an age in the human infant
will differ according to the aspect of development under
consideration.
2. PATHWAYS OF EXPOSURE
2.1 Introduction
As with adults, the potential for exposure to chemicals for
infants and young children is through ingestion, inhalation, and
percutaneous absorption. Both voluntary and inadvertent exposures
may occur, but their nature and extent are modified by the
physiological, behavioural, and other characteristics peculiar to
this age group. Thus, the methods of nurture, the social,
cultural, and occupational proclivities of the family, the quality
of care and supervision, and the inherent behavioural patterns of
the child, may all be significant factors for determining or
modifying exposure.
The new-born infant may already have encountered toxic chemical
agents in utero by the placental route; for example, the sequelae
of maternal exposure to methylmercury (Clarkson et al., 1976),
ethanol (Jones & Smith, 1975), tobacco (US DHEW, 1979), and
narcotics (Barr et al., 1984) are well recognized. Moreover, the
young infant is an obligate milk feeder, and chemicals that
traverse the mammary gland in lactation are potentially important
contaminants of both human and artificial milk.
The use of appropriate cleaning and antiseptic chemicals for
the routine care of the skin, umbilicus, and clothing of the new-
born infant have all been associated with toxicity (Armstrong et
al., 1969). Similarly, effects from atmospheric contaminants have
been encountered in neonatal units from agents used on the clothing
or for the disinfection of apparatus and operational areas.
Development from infancy to childhood is marked by increasing
mobility and exploration of the immediate surroundings. Oral
exploration is commonplace towards the end of the first year of
life in which the mouth is used to sample the taste and texture of
the objects and materials that are encountered. Oral exploration
may be accompanied by the ingestion of a wide variety of substances
not normally regarded as food (pica) including soils, dusts, and
surface coatings in the vicinity of the home. Although mouthing
and pica diminish with continuing development after the first year
of life, hand to mouth activity tends to persist (Barltrop, 1966).
As a consequence of increasing mobility and diminishing
supervision, poisoning due to the ingestion of toxic substances is
very common in early childhood. For example, in England and Wales,
one-fifth of all hospital admissions for suspected poisoning
involved children under the age of 5 years, amounting to
approximately 20 000 admissions per annum. Household,
horticultural, agricultural, and industrial chemicals were all
implicated, though medicaments and therapeutic compounds were the
principal agents (Vale & Meredith, 1981). It is unlikely that all
acute ingestions are reported, and their prevalence is not known
with certainty. The quality of available supervision is an
important determinant of accidental poisoning and will itself be
modified by social, cultural, and economic factors.
Infants and children will also be subject to chemical exposure
resulting from contamination of the natural and domestic
environments that they share with adults. However, the provision
of foodstuffs, beverages, clothing, furniture, toys, and
medicaments, specifically manufactured or adapted for the young,
may modify the route of exposure for certain chemicals; efforts are
usually made to minimize the contamination of such materials with
chemicals that might be harmful.
2.2 The Alimentary Tract
2.2.1 Milk
Milk may be regarded as one of the routes of excretion of
foreign compounds that have entered the body of the lactating
mammal. The degree to which a particular chemical traverses the
mammary gland depends on several factors, including its own
physical and chemical properties, such as the degree of ionization
and binding in biological fluids, the solubility characteristics,
and the maternal plasma concentration achieved. Passive diffusion,
active transport, and reverse pinocytosis across the apical
membrane of the mammary alveolar cell may all be involved, but the
milk/plasma ratio cannot always be predicted for a given chemical
in a particular species. In general, transfer from plasma to milk
is enhanced by high solubility in lipids, low relative molecular
mass, and weak binding to plasma-proteins (Giacoia & Catz, 1979).
2.2.1.1 Human milk
Nearly all chemicals to which the mother is exposed may, to
some extent, be excreted in human milk (Jensen, 1983, 1985).
Contamination of human milk may result from the maternal diet,
environment, occupation, addiction, and medical treatment. Marked
variation in the concentrations of chemicals in milk, both between
and within individuals, is characteristic and may reflect the stage
of lactation, the time and method of sample collection, and the
age, weight, and parity of the mother. Chemical residues most
frequently reported in human milk are the organochlorine pesticides
(DDT) and industrial chemicals (PCBs and metabolites, HCH isomers,
and cyclodienes such as dieldrin). Lactation is a route of
excretion for some of these chemicals, and adverse health effects
have been noted in breast-fed infants when the concen-tration of
chemicals was extremely high, as in accidental poisoning outbreaks,
and in some cases of occupational exposure. During the Iraqi
outbreak of methylmercury poisoning of 1971-72 (Amin-Zaki et al.,
1974), mothers who consumed bread made from seed grains treated
with a methylmercury fungicide were found to have milk containing
8.6% of the concentration of mercury in maternal blood collected at
the same time (Amin-Zaki et al., 1976). Infants who were born
before their mothers ate the contaminated bread and who received
methylmercury only through suckling, were found to have blood-
mercury values of up to 1.0 g/litre. Follow-up assessment over 5
years showed that some of the children suffered impaired
neurological and mental development (Amin-Zaki et al., 1981).
In Turkey, bread prepared from seed grains treated with
hexachlorobenzene between 1955-59 also affected infants (Peters,
1976). In some villages, almost all children under the age of 2
years, who had been breast-fed by mothers who had eaten the
contaminated wheat, died of the condition known as "pembe yara"
(Turkish for pink sore), which included symptoms of weakness,
convulsions, and localized cutaneous annular erythema.
Reports of such episodes have been rare. However, if
confronted with such poisoning outbreaks, exposure of infants
through human milk should be considered.
2.2.1.2 Other types of milk
Cow's milk, and the milk of other species used for infant
feeding, are subject to contamination by many of the chemicals
found in human milk, together with others that reflect local
techniques of animal husbandry and veterinary practice. Infants fed
milk derived from a particular animal or herd may be at greater or
lesser risk of exposure to a given contaminant than those fed
artificial or manufactured milk derived from the pooled secretions
of multiple herds. Conversely, the pooling of milk during the
manufacture of infant feeds will tend to increase the number of
potential contaminants in the end-product, but may diminish their
concentrations.
The food sources of lactating animals may be contaminated by
local industrial wastes, by the application of pesticides, by
naturally-occurring substances, such as mycotoxins, or by storage
in containers or buildings previously contaminated with pesticides,
disinfectants, preservatives, and other chemicals. The
organochlorine pesticides are well recognized fodder contaminants.
Numerous examples are known of milk containing chemicals derived
from animal feeds including DDT, hexachlorocyclohexane (HCH)
(Miyabe et al., 1971), heptachlorepoxide (Smith, 1982), and
hexachlorobenzene (Goursaud et al., 1972). Chemicals in milk
derived from containers of contaminated animal feed include PCBs
(Villett & Hess, 1975), pentachlorophenol (PCP) (Haering &
Schefer, 1980), and dieldrin (Epps et al., 1974). The inadvertent
substitution of a polybrominated biphenyl (PBB) product for
magnesium oxide in prepared cattle feeds resulted in contamination
of the milk (Isleib & Whitehead, 1975).
Several examples of milk contamination occurring after
collection have been reported. Thus, iodophors and other
disinfectants (Kroger, 1972), dieldrin (Tadjer & Dore, 1971) and
chlordane (Landsberg et al., 1977) have all been identified in milk
stored in vessels that had been inadvertently rinsed or cleaned
with, or fabricated from, previously contaminated materials. The
inadvertent addition of toxic chemicals to infant milk during
manufacture is rare, though arsenic poisoning resulting from the
use of a contaminated sodium phosphate stabilizer has been reported
(Kitamura et al., 1953).
Pesticides and drugs for the control of parasitic infection and
other diseases in lactating animals are liable to be absorbed and
excreted in the milk, even after topical application. Milk
contamination with antibiotics and hormones, given for economic
rather than therapeutic or preventative indications, may also
occur.
2.2.1.3 Infant feeds
Foods and beverages, other than milk, which have been
specifically prepared or manufactured for infants and young
children, may be diverse in composition and comprise only a
proportion of the total dietary intake. However, they are subject
to the same sources of chemical contamination as foods intended for
adults, during producion, manufacture, and packaging. The
potential significance of contaminants is enhanced when the dietary
range is restricted.
Numerous examples of contamination at source have been
described in common with those for human and other milk. Thus,
residues of pesticides, antibiotics, fungicides, and heavy metals
have all been encountered as a result of agricultural techniques,
veterinary practice, and the use of contaminated land and water.
An additional factor with regard to meat products is, for example,
the use of anabolic agents, such as diethylstilboestrol, in animal
husbandry (Umberger, 1975). Thus, in one study, one-third of 450
samples of baby food containing veal were found to have detectable
estrogen activity (Loizzo et al., 1984).
Many chemical or non-nutritional substances are used in food
manufacture as preservatives, anti-oxidants, stabilizers,
emulsifiers, and thickening agents, with the intention of ensuring
that the food is wholesome and palatable, when consumed. Numerous
chemical colouring agents are potentially available, and their use
in infant foods has attracted controversy, since marketing rather
than nutritional considerations are involved.
Manufactured infant and junior foods are characteristically
distributed in appropriately small containers which, therefore,
have a relatively large surface area/volume ratio. The degree to
which contaminants derived from the container material, seams, or
internal surface coatings may affect the contents is inversely
proportional to the container volume. The degree to which the
container design and manufacture may influence food contamination
is indicated by the reduction of dietary lead intake observed in
infants in the USA during the last decade, when progressive
replacement of lead soldered side-seamed cans by welded or seamless
containers resulted in a 47% reduction in lead intake by infants
aged 0 - 5 months (Mahaffey, 1983).
Contamination of foods during preparation in the home may
result from the use of inappropriate vessels, culinary agents such
as cooking oils, and cleaning materials, as well as from water.
Thus, excessive intake of copper derived from domestic cooking
utensils has been implicated in Indian childhood cirrhosis (Marwha
et al., 1981), and contaminated edible oils were involved in an
outbreak of Yusho disease (Kuratsune, 1980; Japan/US Cooperative
Science Program, 1983).
The use of detergent chemicals for the cleaning of cooking and
eating utensils is inevitably associated with ingestion of the
chemicals by all members of the household, but, in normal usage, it
is unlikely that toxic intakes will be encountered. However,
intakes are likely to be enhanced when rinsing or wiping is
omitted.
2.2.1.4 Water
Water for domestic consumption may be contaminated by chemicals
at source, during treatment and transport, or in the home from
domestic plumbing, cisterns, or culinary utensils. The use of
recycled water, particularly during drought, will tend to enhance
the concentration of some pollutants. The direct ingestion of
water used in the preparation of foods and beverages will involve
children of all ages, depending on local climatic conditions and
cultural and dietary practices. However, the use of water to
reconstitute powdered artificial milk is a special case,
particularly where the milk is the sole nutrient source.
The potential contaminants of drinking-water are numerous and
include a wide range of metals and other elements; nitrates,
cyanide, thiocyanate, and ferrocyanate radicals; organic compounds
including pesticides; organic solvents; and anionic, cationic, and
non-ionic detergents. Asbestos and polyaromatic hydrocarbons have
been detected. The chlorination of water for disinfection,
particularly in the presence of humic material, results in the
formation of a number of haloforms or C-chlorinated compounds
including chloroform, bromodichloromethane, and
dibromochloromethane (Rook, 1974).
The significance of lead in drinking-water has been
increasingly appreciated, particularly in areas where lead pipes
and plumbosolvent water supplies co-exist. Studies in a population
in Scotland showed that there was a curvilinear relationship
between the concentration of lead in water and that in the blood of
artificially fed infants; 36% had blood-lead values in excess of
350 µg/litre (Sherlock et al., 1982). Similarly, lead intakes of
up to 3.4 mg/week were reported from Scotland in artificially fed
infants, whose food was measured and analysed, with correspondingly
marked increases in blood-lead values (United Kingdom Central
Directorate on Environmental Pollution, 1982).
Nitrate in drinking-water is not itself hazardous, but may be
converted to nitrite by nitrate-reducing bacteria in the
gastrointestinal tract of infants under appropriate conditions.
Nitrites promote the formation of methaemoglobin from haemoglobin,
thus impairing the transport of oxygen and causing cyanosis.
Methaemoglobin is especially associated with the consumption of
well water with a high nitrate content, and infants appear to be
more susceptible to it than older children and adults (American
Academy of Pediatrics Committee on Nutrition, 1970).
2.3 The Respiratory Tract
Although infants and children may inhale chemical compounds,
information concerning the structure and function of the
respiratory tract in this context is limited. It is known that the
architecture of the respiratory tract continues to develop, at
least until the age of 18 months. Anatomically, 3 processes have
been identified including:
(a) centripetal alveolization giving rise to an extra
generation of respiratory bronchioli;
(b) transformation of some distal respiratory bronchioli
into alveolar ducts; and
(c) further branching of 1 or 2 alveolar duct generations.
After the age of 18 months, the respiratory structures increase
in size with a progressive increase in elastic fibre bundles in the
alveolar wall up to the age of 18 years. The length/diameter
relationship of each respiratory unit remains relatively constant
throughout development. The effects of these architectural changes
is thought to be minimal in the case of inhaled aerosols, but the
position concerning suspended particulates of various sizes has
not been well studied in childhood (Hislop & Reid, 1981).
Numerous chemical substances are found in the atmosphere, and
over 1600 have been identified. Not all of these are man-made, and
many may be derived from a single source (Graedel, 1978). The bulk
of atmospheric pollution is provided by carbon monoxide,
particulates and oxides of sulfur, hydrocarbons, and oxides of
nitrogen. Not all pollutants act directly; for example, the oxides
of nitrogen may exert their effects indirectly, by virtue of their
role in photochemical smog formation (National Research Council,
Subcommittee on Airborne Particles, 1979).
The exposure of infants and children to atmospheric pollutants
may be enhanced compared with that of adults, if the pollutants are
emitted close to the ground or, in the case of aerosols, are gases
or vapours of high density.
2.4 The Skin
Percutaneous absorption of chemical substances is known to
occur through normal skin in both adults and children. There is
little evidence that the rate of absorption varies with age, since
the limiting factor would appear to be the thickness of the
stratum corneum, which is relatively constant throughout postnatal
development after a full-term birth. Different processes of
absorption are thought to exist, but the transcutaneous flux cannot
be predicted with certainty, since marked variations in absorption
rates have been observed between closely related chemical
compounds. While absorption through normal skin does not vary with
age, absorption is greatly enhanced when the skin barrier is
damaged or when the substance is applied under an occlusive
dressing. A problem specific to infants is that napkins (diapers)
serve as an occlusive dressing, which may promote the absorption of
chemicals applied to the buttocks and increase the likelihood of a
systemic effect. Absorption may be further enhanced by skin
reactions in the napkin area.
The prematurely born infant may constitute a special case,
since studies on the development of the stratum corneum in fetal
life suggest that the permeability barrier is incomplete until just
before term (Singer et al., 1971). Thus, pre-term infants bathed
with a solution of hexachlorophene showed greater peak blood
concentrations of the compound than full-term infants subjected to
the same procedure (Greaves et al., 1975).
A pertinent factor in infants is the relatively large surface
area to body weight ratio; thus, compounds absorbed through a given
part of the skin surface will result in greater tissue
concentrations than in an adult (Wester & Maibach, 1982).
2.5 Conclusions
Infants and young children may be exposed to chemicals by the
gastrointestinal, respiratory, and percutaneous routes. The
relative importance of these routes will vary with age according to
the nature of the diet, the behavioural characteristics, and the
maturation of the system involved.
Since infants are obligate milk feeders, they will ingest
chemicals that cross the mammary gland during lactation. These are
potentially important contaminants of both human milk and infant
formulae containing the milk of other species. Similarly, chemicals
contaminating the water used for the reconstitution of powdered
milks will contribute to the ingested burden. Foods and beverages
specially manufactured for infants and young children are also
potentially important sources, especially when the dietary range is
restricted. Oral exploration, hand to mouth activity, and pica
contribute to the risk of ingestion of chemicals encountered in the
domestic environment.
Limited information is available concerning the absorption of
inhaled chemical atmospheric pollutants in relation to the
continuing development of the respiratory tract. However, exposure
of infants and children to atmospheric pollutants may be enhanced
compared with that of adults, when the source of emission is close
to the ground, and under circumstances in which gases or vapour of
high density are involved.
Chemicals can be absorbed through normal skin, but, in infants
and children, the relatively large surface area to body weight
ratio may result in greater tissue concentrations than in the
adult. Percutaneous absorption is enhanced when the skin is
damaged or macerated, or when the substance is applied under an
occlusive dressing such as a napkin (diaper). The prematurely born
infant may constitute a special case, since the permeability of the
skin is greater, because of the incomplete development of the
stratum corneum.
3. KINETICS OF ABSORPTION, BIOTRANSFORMATION, AND ELIMINATION
3.1 Introduction
Because of the morphological, biochemical, and physiological
characteristics of the infant and young child described in section
1, the kinetics of chemicals gaining access to their bodies need
special consideration, particularly with regard to the timing of
the maturation processes governing absorption, distribution,
biotransformation, and elimination from the body.
3.2 Absorption
A chemical may be absorbed through an external (skin) or
internal (gastrointestinal and respiratory tract) surface.
Absorption depends on the physical and chemical characteristics of
the chemical, its concentration gradient, the conditions at the
site of absorption, and the biological characteristics of the
absorptive surface. The conditions at the site of absorption and
the characteristics of the absorptive surface can be expected to
change with age; for example, postnatal changes in pH in different
parts of the gastrointestinal tract and skin, and alterations in
the thickness and structure of membranes of the gastrointestinal
tract and lungs (Hoffmann, 1982) cause changes in perfusion, which
in turn influence the concentration gradient.
3.2.1 Absorption from the gastrointestinal tract
Absorption from the gastrointestinal tract is an important way
in which chemicals enter the body of the infant and young child.
It is influenced by pH, total mucosal surface area, blood supply,
perfusion rate, and period of contact of the chemical with the
absorptive surface, that is the gastric emptying and intestinal
transit time. All these change with postnatal development.
The absorption of some chemicals by infant animals is greater
than that by young adults of the same species. This can be
explained by several factors, including immaturity of the
gastrointestinal tract, the characteristics of the infant's diet,
and the greater proportion of nutrients absorbed. This is of
benefit for growth, but is a disadvantage as far as non-nutritive
chemicals are concerned. Much of the evidence for this comes from
studies on experimental animals exposed to metals. Thus, the adult
rat absorbs 1% or less of lead added to its diet compared with 40 -
50% for the infant animal (Kostial et al., 1971, 1978; Forbes &
Reina, 1972a,b). According to Alexander et al. (1974) and Ziegler
et al. (1978), infants and young children absorb substantially more
lead than adults, but Barltrop & Strehlow (1978) did not find any
age-related differences. The intake of lead by the infants studied
by Alexander et al. (1974) and Ziegler et al. (1978) was greater
than that of the children in the study of Barltrop & Strehlow
(1978), and this may explain the different result.
Methylmercury is well absorbed in the gastrointestinal tract of
infants and young children, as it is in adults (Berlin, 1983).
Some methylmercury is evidently re-excreted into the intestine and,
if this is demethylated by the intestinal bacteria, the mercuric
mercury from it is excreted in the faeces. In mice, there is an
increase in intestinal demethylation at weaning, and the results of
in vitro studies have shown that the microflora in faeces from
young infants receiving milk have negligible demethylating ability
compared with those of weaned children and adults (Rowland et al.,
1983). Thus, it seems likely that more of a dose of methylmercury
will be retained within the body of young infants than of older
children and adults.
3.2.2 Absorption from the lungs
Chemicals permeate the air-blood barrier by simple dif fusion,
but the thickness of the membrane, its lipid composition, surface
area, and porosity determine pulmonary absorption. Lipid-soluble
chemicals are absorbed at similar rates by the lungs of new-born
and adult rats, but hydrophilic chemicals are absorbed at a more
rapid rate by young animals (Hoffmann, 1982).
3.3 Tissue Distribution
3.3.1 Binding of chemicals to proteins
Most of chemicals bind to plasma-proteins. The amount of
binding depends mainly on the concentration and structure of the
binding proteins, the affinity constant, and the presence of other
compounds capable of modifying the chemical-protein interaction.
In the new-born infant, several factors contribute to a lower
plasma-protein binding than in the adult. The concentration of
albumin in the plasma of neonates, and hence the number of binding
sites, is low. Moreover, these binding sites are occupied by
endogenous substances such as fatty acids, steroids, and bilirubin.
Thus, new-born infants have a lower capacity for binding chemicals
to plasma-albumin, and competition at the binding site may lead to
the release of, for example, bilirubin, resulting in kernicterus.
Moreover, hypoxaemia and acidosis may also decrease binding
capacity.
3.3.2 Distribution within the body
From early fetal to adult life, the fluid compartments of the
body change continuously. The larger extracellular space in
infancy (section 1.3.4) provides a larger volume for the
distribution of water-soluble chemicals that do not penetrate the
cells, and plasma concentrations of these chemicals may be lower in
infants than in adults, even if the total body burden per unit body
weight is greater.
Alterations in the morphology, physiology, and biochemistry of
the organs, as well as in their weight relative to the body as a
whole, may affect the distribution of chemicals. The brain is
proportionately larger in the infant than in the adult, and rapid
myelination during the first year after birth may favour the uptake
of lipophilic compounds. Metals, too, are retained in the brain
more readily in infancy than in adulthood. Momcilovic & Kostial
(1974), for example, found that a far greater percentage of the
intake of lead was retained in the brain of the suckling rat than
in that of the adult, and that the rate of removal was slower.
Similarly, Kostial et al. (1978) and Wong & Klaassen (1980) found
that a greater percentage of the intake of cadmium was retained in
the brain of the suckling rat than in that of the adult; Jugo
(1980) made a similar observation for mercury. There is little
direct evidence about the human brain. By analogy with animals, it
seems likely that the concentration of lead in the brains of
infants exposed to lead would rise more rapidly than the
concentration in the brains of older children or adults exposed to
a similar dose.
Methylmercury is known to accumulate in brain tissues,
predominantly in grey matter, but whether the brains of infants and
young children accumulate more than those of adults is not known.
Other tissues take up metals more readily in the early period
of life, when the animal is growing rapidly, than when growth has
slowed down or ceased; this particularly applies to the bones. The
concentration of lead in human bones doubles between infancy and
the late teen years (Barry, 1975).
The liver and kidney also accumulate metals. At birth, the
concentrations of cadmium and mercury are low. Henke et al.
(1970), using neutron activation analysis, determined the cadmium
concentrations in the liver and kidneys of 41 children and adults
and found that they increased 200 fold during the first 3 years
after birth. The total increase in cadmium in the liver and kidney
was greater than appears from the concentration, because both
organs increased considerably in weight.
3.4 Biotransformation of Organic Chemicals
The kinetic behaviour of organic chemicals in living organisms
is generally characterized by several processes modifying the
duration of their action. The limiting factors are either
excretion through the kidneys, bile, or gastrointestinal tract, or
biotransformation mechanisms in the liver or other organs. The
products of biotransformation are generally more polar than the
original compound and, thus, are more readily excreted.
Biotransformation is brought about by enzyme activity, either
associated with microsomal systems in the liver or with enzymes in
the plasma or other tissues.
The biochemical processes involved in the biotransformation of
organic chemicals can be divided into 2 phases:
Phase 1. hydroxylation, dealkylation, dehydrogenation,
reduction, hydrolysis, and other reactions
producing a conjugable grouping; and
Phase 2. biosynthetic reactions, whereby conjugates are
formed with endogenous polar molecules; these
conjugates are, generally, readily excreted.
Both Phase 1 and Phase 2 reactions are generally slower in the
new-born infant than in the adult. Consequently, blood-plasma
half-lives of chemicals in which the degradation and elimination
are dependent on one or the other or both of these types of
reactions are generally appreciably longer in neonates than in
adults.
The rate of maturation of processes concerned with the
biotransformation of organic chemicals varies from one reaction,
and from one chemical, to another. Highest activities are reached
in children aged 12 - 16 years, after which there is generally a
decline. A detailed description, with full documentation, has been
published by Klinger (1982).
3.5 Elimination from the Body
3.5.1 Elimination by the kidneys
The renal function of the full-term infant at birth is immature
by adult standards, and this applies both to glomerular and
tubular function (McCance & Young, 1940; Dean & McCance, 1947b).
The rapid growth of the young infant (section 1.3.3) involves the
retention of nitrogen and minerals, so that the equivalent of only
part of the nitrogen and minerals absorbed from the food requires
to be excreted by the kideys. The breast-fed infant or the infant
fed on a formula similar in composition to breast milk is able to
do this satisfactorily, and so maintain the composition of the body
fluids at their proper level (McCance & Widdowson, 1957a).
However, excessive intakes of nutrients, such as protein
(nitrogen), phosphorus, and sodium, are not excreted completely by
the kidneys of the new-born infant or animal, and the
concentrations in the plasma rise (McCance & Widdowson, 1956,
1957b). Retention of chemicals introduced into the body of the
infant may be due to both renal immaturity and insufficient
biotransformation.
The function of the renal tubules at full-term birth is less
mature than that of the glomeruli, but it matures more rapidly
(Edelmann & Spitzer, 1976). This is important in the young infant
for the chemicals that depend on tubular secretion for their
elimination from the body. Moreover, the reabsorption of chemicals
from the tubular lumen into the tubular cells also varies with age
and depends, in part, on the pH of the urine. Weak organic acids
are reabsorbed more readily by the infant, but, more important, the
low capacity for biotransformation by the infant's liver results in
organic chemicals reaching the kidneys in their original lipophilic
form, and these are not excreted, but reabsorbed into the
circulation (for reviews, see Scheler, 1980; Braunlich, 1981).
Some metals depend on the kidneys for their elimination from
the body, and the results of animal studies suggest that suckling
rats excrete less of their body burden of cadmium, mercury, and
manganese than adults (Kostial et al., 1978). Infants appear to
excrete a smaller proportion of absorbed lead by the renal route
than adults (Ziegler et al., 1978).
3.5.2 Elimination by the liver
The excretion of chemicals in the bile is an important means of
elimination, though some of the chemicals excreted through the bile
may be reabsorbed in the gut and re-enter the body, thus resulting
in their enterohepatic circulation. They cannot penetrate the
epithelium of the biliary duct. Many chemicals are excreted by the
liver as glucuronides in the bile, but the formation of
glucuronides is limited in the new-born infants (section 3.4).
3.5.3 Elimination by other routes
Other routes of excretion include the gastrointestinal tract,
the respiratory tract, and the skin. They are non-regulatory and,
while they may be important for some chemicals, information is
limited about age-related differences in elimination.
3.6 Conclusions
Generally speaking, both organic and inorganic chemicals are
absorbed more readily by the infant than by the adult, but the
organic chemicals undergo biotransformation less readily. For some
chemicals, alternative biochemical pathways may exist.
The kidneys of the neonate are immature and less able than
those of the older child or adult to excrete chemicals, whether
inorganic or the polar products of biotransformations. A greater
proportion of a similar dose of a chemical per unit body weight is
therefore likely to accumulate in the body of the infant, and the
concentration of the chemical in the blood and tissues of the
infant will be higher. The possible effects of this are discussed
in section 4.
4. EFFECTS OF CHEMICALS IN THE BODY
4.1 Introduction
Differences in kinetics can enhance the toxicity of a chemical
in the infant but, in some cases, may result in a qualitative
difference of response or in a lower toxicity, depending on the
intermediary metabolite. In addition, the qualitative and
quantitative response of the target tissues to the same internal
dose of a chemical may be different at various stages of postnatal
development because of the gradual development of characteristics
of the target tissues. A third aspect of developmental toxicology,
which should be recognized, is the fact that a chemical introduced
into the body of an infant or young child may affect the structural
and functional development of a particular organ or system, which
may interfere with the growth and development of the body as a
whole. Animal studies have shown that the toxic effects of certain
chemicals are different at different stages of development.
Moreover, chemicals reaching the body early in life may produce
delayed effects in later years. The response of the growing infant
may therefore be different from that of the fully grown adult.
The problems of extrapolating quantitatively from animals to
man and reaching conclusions on the implications of evidence from
animal studies for human health are well known. These problems are
greater during the new-born period, since the stage of development
at the time of birth varies considerably between species (section
1.4.2). Information from clinical experience of exposure is
limited, and systematic epidemiological data from studies on
infants are sparse, so that the results of studies on animals may
provide the only information available. When information
concerning human infants is available, it should be given
preference.
4.2 Effects of Chemicals on General Growth and Development
General growth and development are possible indicators of the
effects of chemicals on infants and children, whether the exposure
occurred prenatally or postnatally. The principles for evaluating
the effects of prenatal exposure have been considered in an earlier
Environmental Health Criteria publication (WHO, 1984). Factual
information about the effects of postnatal exposure is scarce. In
a study on the infants of mothers who had consumed bread
contaminated with a methylmercury fungicide during lactation, Amin-
Zaki et al. (1981) found abnormal neurological signs in some
infants at the first examination and in more infants on subsequent
occasions. Delayed motor and other aspects of development,
including intelligence, were not detected when the infants were
first seen, but were observed at follow-up examinations during the
next 5 years.
4.3 Effects of Chemicals on Some Organs and Systems
4.3.1 Nervous system
The brain of the infant at birth is not fully developed. The
full number of neurones is not reached until about 2 years of age
(Dobbing & Sands, 1973); myelination is not complete until
adolescence (Hoar & Monie, 1981).
Brain development involves changes in the proportions of myelin
and non-myelinated tissue. Shuman et al. (1975) observed status
spongiosus in the myelin sheaths of the reticular formation of
premature infants, following exposure to hexachlorophene, but no
lesions were present in the non-myelinated part of the brain.
Brain lesions were not produced during the first 8 - 10 days after
birth in the rat. However, between 10 and 25 days, when myelination
takes place, the suckling rats became sensitive to hexachlorophene
(Nieminers et al., 1973; Ulsamer et al., 1975).
There are changes in the sensitivity of the brain of the rat to
hormones or drugs during development, and this has been correlated
with the development of the cell receptors (Macho et al., 1972;
Valcana & Timiras, 1978). On the basis of experimental data, Czaba
(1984) concluded that, during maturation, cell receptors in several
organs require reinforcement by the appropriate hormone at a
critical stage of development. This process can be hindered or
prevented by the presence of molecules analagous to the hormone, or
by a drug or other chemical that binds to the receptor and so
prevents reinforcement by the hormone and hence normal development.
Many confounding factors and co-variables such as nutrition,
socio-economic status, and mental stimulation have to be taken into
account when evaluating the effects of a chemical on the
functioning of the human brain. Only when good information on
these factors is available will it be possible to make a valid
interpretation of the observations on brain function after exposure
to a chemical. This has been discussed most extensively in the
case of lead (United Kingdom Department of Health and Social
Security, 1980; MRC, 1984) (section 3.3.2).
4.3.2 Kidneys
The functional immaturity of the infant's kidneys has already
been discussed (section 3.5.1). Specific reports of their
susceptibility to chemicals are not available, but results of
animal studies suggest that some compounds are less toxic to the
immature kidney than to the mature organ. For example, the same
dose of mercuric chloride per unit body weight that produced renal
failure in adult rats did not have any effect on renal function in
new-born rats (Kavlock & Gray, 1983). Information is lacking on
the effects on renal function of the accumulation of chemicals in
the human kidney during the early years of life (section 3.3.2).
4.3.3 Liver
The pre-term infant may still have foci of extra-medullary
haematopoiesis in its liver, but the histological appearance of the
liver at term is similar to that of the adult. The activity of
enzymes responsible for the biotransformation of chemicals in the
liver of the infant is not equal to that of the adult (section
3.4). However, it appears to be possible to induce the activity of
microsomal enzymes in the liver of neonates to some extent. If
infants have been exposed to phenobarbital, either during
intrauterine life, or immediately after birth, the synthesis of
components of certain microsomal enzyme systems in the liver is
accelerated. The metabolism and urinary excretion of bilirubin is
increased (Maurer et al., 1968; Trolle, 1968; Ramboer et al.,
1969). Increased activities of some drug-metabolizing enzymes have
also been found in new-born infants exposed to phenobarbital
before, or immediately after, birth. Such infants were able to
metabolize drugs such as diazepam more readily than infants not
exposed to phenobarbital, and their urinary excretion of hydroxy
derivatives was greater (Morselli, 1976).
4.3.4 Lungs
Animal studies indicate that some chemicals, such as nitrofen,
retard the development of the lung in the rat fetus (Kimbrough et
al., 1974), but there do not appear to be any reports of effects of
chemicals on the lung specific to infants. It seems that the
response of the infant's lungs to ingested or inhaled chemicals has
not been investigated sufficiently. A greater degree of impairment
of respiratory function might be expected in infants compared with
adults for a given level of exposure, particularly to particles of
substances that result in obstructive symptoms. An excess
mortality rate was found in infants aged less than 12 months
compared with older children and young adults in a population
exposed to atmospheric pollution with sulfur dioxide (Lawther &
Waller, 1975).
4.3.5 Haematopoietic system
The maturation and differentiation of haematopoietic cells in
the bone marrow are processes that continue throughout life, and
represent one of the most rapidly renewing cell populations.
Although the haematopoietic system and the blood are well known to
be the target of many chemical agents, there is little information,
at present, on any specific vulnerability of the developing bone
marrow. An example of the vulnerability of the red cells in young
children is the occurrence of methaemoglobinaemia after exposure
to very low doses of methaemoglobin-forming compounds, e.g.,
nitrates (Kiese, 1974).
4.3.6 Immune system
Studies of immune ontogenesis have revealed that new-born
mammals do not possess fully competent immune defence systems.
While human infants have more highly developed immune systems than
some other mammals at birth, the human infant is still
immunologically immature.
The development of the immune system in mammals involves a
sequence of steps that begins early in gestation. Perturbation or
abrogation of this developmental sequence of events can lead to
immune dysfunctions that may be life-threatening. Defects in the
development of the immune system, due to heritable changes in the
lymphoid elements, have provided clinical and experimental examples
of the consequences of impaired immune development (for review, see
Heise, 1982).
The most profound effects of chemicals that modulate the immune
system of animals occur when dosing of the compound begins during
the development of the lymphoid system (Vos, 1977). Although there
are no data on the immunological effects resulting from perinatal
exposure of infants to chemicals, the potential for such effects
should not be ignored.
The extent of adverse effects on the immune system resulting
from exposure of adult animals to a wide variety of environmental
chemicals has only recently begun to be appreciated (Vos, 1977).
Even less is known about the magnitude of the immunological effects
resulting from fetal and neonatal exposure to environmental
chemicals. It would be expected that disruption of the normal
sequence of events in the development of the immune system by
chemicals would result in alterations in the competence of the
system. Such alterations may not only lessen the host's ability
to combat infection and neoplasia but may also lead to
hypersensitivity or to a failure in the control of homeostasis,
which may lead to autoimmunity. The immunological effects of
perinatal exposure to chemicals in animals have been reviewed by
Roberts & Chapman (1981) and Schmidt (1984). Exposure during
gestation and/or early in postnatal life to chemicals such as
2,3,7,8-tetrachlorodibenzodioxin (Vos & Moore, 1974; Faith & Moore,
1977; Luster et al., 1980), lead (Luster et al., 1978; Faith et
al., 1979), hexachlorobenzene (Vos et al., 1979), and
benzo( a )pyrene (Urso & Gengozian, 1980) has been shown to affect
the developing immune system of animals. However, no specific data
are available for human infants.
4.3.7 Endocrine system
Chemicals may affect the developing endocrine system directly,
or they may interact with some step of the regulating axis
controlled by the pituitary, hypothalamus, or other part of the
brain. The interaction between a chemical and the neuroendocrine
organs can also affect the reproductive and other systems. Some
pesticides have been shown to possess estrogenic activity
(McLachlan et al., 1981). Exposure of experimental animals in the
early postnatal period to chemicals with androgenic or estrogenic
activity induced disturbances of sex differentiation in the
hypothalamus and acyclic ovarian function in females (Barraclough,
1961, 1966; Takewaki, 1968; Flerko, 1975).
Chemicals that give rise to premature onsent of puberty in
children are of particular importance. Premature thelarche in
large numbers of young children in Puerto Rico was attributed to
estrogens in infant foods containing meat (Saenz de Rodriguez &
Toro-Sola, 1982); the cause of a similar outbreak in an Italian
school (Scaglioni et al., 1978) was not discovered. Exposure to
chemicals with androgenic or estrogenic activity may also affect
growth and final stature.
The development of the regulatory function of the adrenal
glands of experimental animals is altered by the administration of
compounds such as steroids and diazepam to the new-born animal
(Hcik 1969a, 1970; Erdosova et al., 1977), and results in changes
in adrenal function and in the reaction of the pituitary-adrenal
axis to stress stimuli later in life (Levine & Mullins, 1967; Hcik,
1969b, 1970; Libertun & Lau, 1972).
A special risk for new-born infants is an excessive intake of
iodine, leading to an abnormal rate of accumulation in the thyroid
gland. Exposure to high concentrations of iodine or goitrogenic
compounds can alter the function of the thyroid gland, resulting in
a deficient production of thyroid hormones at the time when
appropriate levels of the hormones are important for the maturation
of functions of the brain, liver, and other organs. Premature
infants are particularly prone to the development of hypothyroidism
as a result of iodine overdose (Chabrolle & Rossier, 1978; Malpuech
et al., 1978; L'Allemand et al., 1983).
4.3.8 Skin
The skin is a complex structure, the functional development of
which is incomplete until after puberty. Clinically, the cutaneous
manifestations of disease vary with age, and some disorders are
confined to infants and children. Thus, it would be expected that
age-related differences in the response of the skin to chemicals
would be encountered. Chloracne was found to occur principally in
young children rather than adults in a population exposed to dioxin
(TCDD, 2,3,7,8-tetrachlorodibenzo-para-dioxin), suggesting the
greater susceptibility of the child (Fara et al., 1982).
Chemicals applied to the skin may be absorbed and induce
systemic effects. New-born infants whose skin was treated with an
antibacterial preparation containing 3% hexachlorophene developed
blood levels of the compound similar to those known to cause brain
damage in animals. Similarly, some 40 deaths in French infants in
1972 were attributed to the use of baby powder accidentally
contaminated with 6.6% hexachlorophene (Marzulli & Maibach, 1975).
Some environmental pollutants or contaminants are known to be
readily absorbed through the skin, and this subject has been of
particular interest in paediatric therapeutics. Thus, both fatal
and non-fatal exposures occurred when pentachlorophenol was used in
a laundry room resulting in contamination of napkins used in the
hospital nursery (Armstrong et al., 1969).
4.3.9 Bones and teeth
The bones undergo considerable change during postnatal
development with regard to growth, change in shape, and
composition. Immature bones contain more water and less collagen
in the matrix than mature bones, and the degree of mineralization
is much lower (section 1.3.4) (Table 3). Minerals, including
strontium, caesium, and lead, tend to be deposited in the bones,
together with calcium, during the process of mineralization. Lead
may affect the conversion of 25-hydroxy vitamin D to the 1.25-
hydroxy metabolite in the kidney (for review, see Mahaffey, 1981).
Interference with skeletal development may result from the
action of lead in altering the balance of osteoblastic and
osteoclastic activity, and from the direct action of chemicals such
as fluoride.
The discoloration of developing teeth after administration of
tetracycline is an example of the particular propensity for
accumulation or fixation of chemicals in hard tissues. The results
of studies on primates suggested that excessive exposure to
selenium during the development of the teeth might increase
susceptibility to caries (Bowen, 1972).
4.4 Carcinogenesis
Maternal exposure to carcinogens may lead to exposure of the
fetus through the placenta and to exposure of the infant through
breast milk. The transfer of chemicals to the fetus depends on the
relative molecular mass and solubility of the chemical, non-ionized
and lipid chemicals entering the fetus most readily. Lipophilic
chemicals may be retained in the tissues of the fetus and have been
found in tissues of still-born infants (Curley et al., 1969).
Transplacental transfer of carcinogens has recently been reviewed
(WHO, 1984).
So far, no epidemiological observations on human infants have
been reported in which the occurrence of cancer was associated
solely with exposure to carcinogens in the early postnatal period.
The few existing data come from animal studies and, in most of
these, the route of exposure was parenteral and not through the
mother's milk. For example, the injection of aflatoxin resulted in
liver cancer in mice, but only when administered to new-born
animals (Vesselinovitch et al., 1972).
Within the past decade, it has been increasingly recognized
that not all carcinogens act by the same mechanism. Many of the
environmental chemicals, particularly the halocarbons, are though
to be promoters of carcinogenesis rather than initiators. Many of
these types of chemicals are persistent in the organism and are
secreted in breast milk (section 2.2.1.1) (Appendix I). The
mechanism of action of promoters is not well understood at present,
and effects on health at the low doses received by infants and
young children are not clear.
An intrinsic problem in studying prenatal and postnatal
exposure to carcinogens is the capacity for metabolic activation in
the tissues of the fetal or new-born organism, compared with that
of the adult (section 3.4). In this respect, pronounced species
variations have been shown between rodents and primates, including
man. The perinatal development of different organ systems varies
considerably among species. All these variables make extrapolation
from animals to man extremely difficult.
The significance for human health of early postnatal exposure
to tumour growth promoters or co-carcinogens is not known.
4.5 Conclusions
The structural and functional characteristics of infants and
young children described in sections 1, 2, and 3 might make them
more or less vulnerable than older children and adults, when they
are exposed to chemicals at a similar dose per unit body weight.
However, it should be emphasized that reports of specific effects
are rare. This may be because such specific effects do not exist
or because they have passed largely unnoticed. The examples quoted
here show that exposure to certain chemicals during early postnatal
development can be associated with dose-effect and dose-response
relationships that differ from those resulting from exposure in
later years.
Exposure to chemicals during early postnatal development can
give rise, not only to immediate effects on health, but also to
manifestations resulting from the disturbed maturation of organ
systems and their altered response to other environmental
influences. Depending on the chemical concerned, the vulnerability
of the developing organ systems can be higher or lower than that of
the more mature systems.
It is recommended that, if laboratory tests suggest that the
body burden of a chemical is a cause for concern, follow-up studies
should be made. Children who have been accidentally exposed to
chemicals that might affect the reproductive organs should be
followed prospectively to determine possible effects on puberty and
reproductive capacity. Similarly, if other organ systems are
likely to have been affected, prospective studies should, if
possible, be made to assess functional development, morbidity, and
mortality.
5. MODIFYING FACTORS
Considerable variations exist in the health and nutritional
status of children reared in different social and cultural
environments. The response of children exposed to chemicals may
therefore be modified in certain sections of a population to a
degree that cannot readily be predicted. Although analogous data
for the severity of response to infections, such as measles, are
well documented, information concerning chemical exposure and its
sequelae is limited.
5.1 Nutrition
The intake of chemicals contaminating foods and beverages will
vary with the amount of food consumed, the range and variety of
dietary items selected or available, and the methods of
preparation. Children with high intakes of marine fish will, for
example, have a greater intake of methylmercury from polluted
sources than those in which fish constitutes only a small
proportion of the diet (Turner et al., 1980). Conversely, some
pesticide residues in milk are decreased by heating, drying,
evaporation, or pasteurization, so that less of these substances
will occur in children consuming manufactured or processed milk, as
opposed to the raw product. The duration of milk feeding in
infancy, the method of weaning, and the interval before a normal
adult diet is introduced are important factors that vary greatly
between social, cultural, and ethnic groups.
Specific dietary components may interact with ingested
chemicals, thus modifying their availability for absorption. The
relative solubility of the chemical in the lipid or aqueous
fractions of the diet, and the potential for physical interaction
or adsorption to non-adsorptive mechanisms at the enteral surface
of the gut mucosa, may all have a role in determining the
bioavailability of the ingested chemical. Thus, the absorption of
lead, consumed simultaneously with the diet, is markedly lower
compared with its absorption from aqueous solutions, partly due to
interaction with other dietary components. In animals, and
possibly in man, lead absorption is reduced by diets rich in
calcium and phosphorus, but is increased when the intake of these
nutrients is restricted (Barltrop & Khoo, 1975).
The consequences of nutritional deficiency for the absorption
of chemicals cannot be predicted with certainty. In kwashiorkor,
both the digestive and absorptive functions of the gut may be
impaired and the activation of chemicals during conjugation
reactions reduced, particularly acetylation with amino acids
(Thabrew et al., 1982). On the other hand, specific mineral
deficiencies, for example, a lack of calcium, may stimulate
absorptive mechanisms shared by other, potentially toxic, divalent
cations (Barltrop, 1979). Iron deficiency in children has been
related to enhanced absorption and retention of lead, but the
mechanism involved is uncertain. Interactions between essential
and toxic metals after they have been absorbed into the body have
been described. Zinc, for example, competes with lead for
receptors on the enzyme aminolaevulinic acid dehydratase (Nordberg,
1979), but the significance of this in infants and children is
uncertain.
5.2 State of Health
Several diseases in addition to nutritional disorders may
modify the exposure and response of infants and children to
chemicals. Ill health associated with diminished activity or
mobility of the child will, for example, alter the balance between
exposures related to the indoor as opposed to the external
environment. Conditions requiring the long-term administration of
oral therapeutic agents or modified diets will inevitably alter the
intestinal milieu and hence the absorption of ingested chemicals.
The absorptive ability of the gut is modified in a wide range
of diseases, including the enteropathies and chronic diarrhoeal
states encountered in childhood arising, for example, from acquired
or inherited food intolerance. The steatorrhoea associated with
childhood malabsorptive disorders such as coeliac disease is known
to impair the absorption of lipid-soluble substances such as
cholecalciferol and alpha-tocopherol (Harrison, 1980), and would
therefore be expected to have a similar effect on the absorption of
other lipid-soluble chemical compounds.
Children with chronic renal or hepatic dysfunction will have an
impaired ability to excrete, metabolize, and, in some cases, store
chemicals that have been absorbed, which may therefore accumulate
in the body. Compromised integrity of the dermal or respiratory
epithelium by disease will enhance the potential for the absorption
of chemicals by these routes.
Some children have genetically determined variations in their
ability to metabolize or respond to certain chemicals. Thus,
deficiency of the enzyme glucose-6-phosphate dehydrogenase (EC
1.1.1.49), which is encountered in some sub-populations, is
characterized by a haemolytic reaction in response to various
triggering agents. Such reactions in childhood have been reported
after exposure to chemicals including naphthalene, aniline dyes,
and nitrofurantoin (Willoughby, 1984).
5.3 Social and Cultural Way of Life
There are many complex interactions between environmental
chemicals and the social and cultural ways of life. The effects of
place of residence, mobility, state of health, nutritional status,
and family income cannot always be distinguished without resort to
sophisticated epidemiological methods. Cultural and social
attitudes to child care and supervision differ between
subpopulations and are clearly important in relation to acute or
short-term exposures. The mechanisms whereby class and cultural
differences modify exposure are not always apparent. For example,
the different blood-lead values found in Negro versus Hispanic
children in New York and in Asian versus Northern European children
in the United Kingdom have yet to be adequately explained
(Barltrop, 1982). Family practices with regard to personal
hygiene, and especially handwashing before meals, may be important
determinants of exposure resulting from hand to mouth activity in
children.
5.4 Conclusion
Recognition of social and cultural factors that influence
exposure and modify response will assist in the identification of
children at risk and enable appropriate preventive and remedial
measures to be adopted.
6. GENERAL CONCLUSIONS
1. Infants and young children differ from older children and
adults in some of their morphological, physiological, biochemical,
metabolic, and behavioural characteristics, and in their
nutritional requirements.
2. The response of infants and young children to chemicals may
differ from that of adults by virtue of their biological
characteristics. Moreover, a feature of these responses is that
the processes of development may themselves be modified by exposure
to chemicals.
3. The effects of chemical exposure and their relationship to dose
may vary with age. The expression of the effects of chemicals may
be greater or lesser than in the adult, depending on the chemical
species concerned; the kinetics of its absorption,
biotransformation, and elimination; and the degree of maturation of
target organs and tissues.
4. The characteristics of infants and children are such that they
determine the pattern of exposure to chemicals. The pathways
involved are related to development and vary with the environment
of the child. Thus, skin contact, the consumption of breast milk
or its substitutes, the ingestion of substances other than food,
may each be predominant routes at different stages of development.
5. The exposure, uptake, and effects of chemicals may be modified
by factors that are characteristically encountered in early life,
including the mode of nutrition, social, economic, and cultural
conditions, and disease.
6. The expression of chemical effects may not be immediate, but
may be delayed until a later age. The health of adults may thus be
compromised by chemical exposures in early life.
7. The development of a strategy relating to chemicals detected in
breast milk requires a thorough appraisal of the merits and
disadvantages of the available options. Any risk of continued
exposure to a chemical through breast-feeding has to be balanced
against the risk of infection or nutritional deprivation, should
breast-feeding be curtailed or discontinued.
7. RECOMMENDATIONS
1. When health risks from chemicals are evaluated, the special
characteristics of infants and young children must be recognized.
An appropriate methodology based on the knowledge of these
differences should be used.
2. Preventive measures should take account of the special
conditions of exposure to chemicals characteristic for infants and
young children, including exposure through breast milk.
3. Health care workers should be made aware of the special risks
related to chemical exposure during infancy and childhood.
4. Any increased incidence of a disease possibly related to
chemical exposure should be reported and investigated. Awareness of
this among health care workers should be improved. The
establishment of an appropriate surveillance system should be
considered.
5. Clinical and epidemiological data should be collected following
the inadvertent exposure of infants and young children to
chemicals.
6. Developmental toxicology should be promoted and its methodology
improved in order to increase the predictive power of experimental
animal and other laboratory studies.
7. Studies are urgently needed to evaluate the risk associated
with exposure during infancy and early childhood to the initiators
and promoters of carcinogenesis.
REFERENCES
ALEXANDER, F.W., CLAYTON, B.E., & DELVES, H.T. (1974) Mineral and
trace metal balances in children receiving normal and synthetic
diets. Q. J. Med., 43: 89-111.
AMERICAN ACADEMY OF PEDIATRICS COMMITTEE ON NUTRITION (1970)
Infant methaemoglobinaemia: the role of dietary nitrate.
Pediatrics, 46: 475-478.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., & GREENWOOD, M.R. (1974) Studies of infants
postnatally exposed to methylmercury. J. Pediat., 85: 81-84.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., GREENWOOD, M.R., & GIOVANOLI-JAKUBCZAK, T.
(1976) Perinatal methylmercury poisoning in Iraq. Am. J. Dis.
Child., 130: 1070-1076.
AMIN-ZAKI, L., MAJEED, M.A., GREENWOOD, M.R., EL HASSANI,
S.B., CLARKSON, T.W., & DOHERTY, R.A. (1981) Am. J. Dis.
Chil., 130: 1070-1076.
ANDERSSON, U., BIRD, A.G., BRITTON, S., & PALACIOS, R.
(1981) Humoral and cellular immunity in humans studied at the
cell level from birth to two years of age. Immunolog. Rev.,
57: 5-38.
ARMSTRONG, R.W., EICHNER, E.R., KLEIN, D.E., BARTHEL, W.F., ET
AL. (1969) Pentachlorophenol poisoning in a nursery for
new-born infants. II. Epidemiological and toxicological
studies. J. Pediat., 75: 317-325.
BARLTROP, D. (1966) The prevalence of pica. Am. J. Dis.
Child., 112: 116-123.
BARLTROP, D. (1979) Metal-nutrient interactions in lead
absorption. In: Di Ferrante, E., ed. Trace metals: exposure
and health effects, Oxford, CEC/Pergamon Press, pp. 158-161.
BARLTROP, D. (1982) Nutritional and maturational factors
modifying the absorption of inorganic lead from the
gastrointestinal tract. In: Environmental factors in human
growth and development, New York, Cold Spring Harbor
Laboratory, pp. 35-41 (Banbury Report No. 11).
BARLTROP, D. & KHOO, H.F. (1975) The influence of
nutritional factors on lead absorption. Postgrad. Med. J., 51:
795-800.
BARLTROP, D. & OPPE, T.E. (1970) Dietary factors in neonatal
calcium homeostasis. Lancet, 2: 1333-1335.
BARLTROP, D. & STREHLOW, C.D. (1978) The absorption of lead
by children. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals, Munich, Technical University of
Munich, Freising-Weihenstephan, pp. 332-334.
BARR, H.M., STREISSGUTH, A.P., MARTIN, D.C., & HERMAN, C.S. (1984)
Infant size at 8 months of age: relationship to maternal use of
alcohol, nicotine, and caffeine during pregnancy. Paediatrics, 74:
336-341.
BARRACLOUGH, C.A. (1961) Production of anovulatory sterile rats
by single injection of testosterone propionate. Endocrinology, 68:
62-67.
BARRACLOUGH, C.A. (1966) Modification in the CNS regulation
of reproduction after exposure of prepubertal rats to steroid
hormones. Recent. Progr. Horm. Res., 22: 503-539.
BARRY, P.S.I. (1975) A comparison of concentrations of lead
in human tissues. Br. J. ind. Med., 32: 119-139.
BERLIN, M. (1983) Toxicokinetics of mercury. In: Schmidt,
E.H.F. & Hildebrandt, A.G., ed. Health evaluation of heavy
metals in infant formula and junior food, Berlin, Springer
Verlag, pp. 147-161.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRAUNLICH, H. (1981) Excretion of drugs during postnatal
development. Pharmac. Ther., 12: 299-320.
BRITISH PAEDIATRIC ASSOCIATION (1956) Hypercalcaemia in
infants and vitamin D. Br. med. J., 2: 149.
CHABROLLE, J.P. & ROSSIER, A. (1978) Goitre and
hypothyroidism in the newborn after cutaneous absorption of
iodine. Arch. Dis. Child., 53: 495-498.
CLARKSON, T.W., AMIN-ZAKI, L., & AL-TIKRITI, S.K. (1976) An
outbreak of methylmercury poisoning due to consumption of
contaminated grain. Fed. Proc., 35: 2394-2399.
COLLIPP, P.J., CHEN, S.Y., & MAITINSKY, S. (1983) Manganese
in infant formulas and learning disability. Ann. Nutr. Metab.,
27: 488-494.
CURLEY, A., COPELAND, R., & KIMBROUGH, R.D. (1969)
Chlorinated hydrocarbon insecticides in organs of stillborn
and blood of new-born babies. Arch. environ. Health, 19:
628-632.
CZABA, G. (1984) The present state in the phylogeny and
ontogeny of hormone receptors. Horm. Metab. Res., 16: 329-335.
DEAN, R.F.A. & MCCANCE, R.A. (1947a) Response of new-born
children to hypertonic solutions of sodium chloride and urea.
Nature (Lond.), 160: 904-906.
DEAN, R.F.A. & MCCANCE, R.A. (1947b) Inulin, diodone,
creatinine, and urea clearances in new-born infants. J.
Physiol., 106: 431-439.
DEAN, R.F.A. & MCCANCE, R.A. (1948) Phosphate clearances in
infants and adults. J. Physiol., 107: 182-186.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1984) [Residues and
contaminants in breastmilk,] Weinheim, Verlag Chemie (in
German).
DICKERSON, J.W.T. (1962a) The effect of development on the
composition of a long bone of the pig, rat, and fowl. Biochem.
J., 82: 47-55.
DICKERSON, J.W.T. (1962b) Changes in the composition of the
human femur during growth. Biochem. J., 82: 56-61.
DIEM, K. & LENTNER, C., ed. (1970) Scientific tables, 7th
ed., Basel, J.R. Geigy A.G.
DOBBING, J. & SANDS, J. (1973) The quantitative growth and
development of the human brain. Arch. Dis. Child., 48: 757-767.
DORNER, G. & STAUDT, J. (1972) [Comparative morphology of
the hypothalamus: differences between the rat and man.]
Endokrinologie, 59: 152-155 (in German).
EDELMANN, C.M., Jr & SPITZER, A. (1976) The kidney. In:
Smith, C.M. & Nelson, N.M., ed. The physiology of the new-born
infant, 4th ed., Springfield, Illinois, pp. 416-458.
EPPS, E.A., Jr, BONNER, F.L., & COWART, R.P. (1974) Dieldrin
contamination of milk by use of second-hand bags for feed.
Bull. environ. Contam. Toxicol., 12: 209-211.
ERDOSOVA, R., KRAUS, M., & REHULKA, J. (1977) Corticosterone
synthesis and serum levels at the end of the perinatal period:
a study of the effects of stress, diazepam, and polyethylene
glycol treatments. Physiol. bohemoslov., 26: 297-302.
FAITH, R.E. & MOORE, J.A. (1977) Impairment of thymus-
dependent immune functions by exposure of the developing
immune system to 2,3,7,8-tetrachlorobenzeno-p-dioxin (TCDD).
J. Toxicol. environ. Health, 3: 451-464.
FAITH, R.E., LUSTER, M.I., & KIMMEL, C.A. (1979) Effect of
chronic developmental lead exposure on cell-mediated immune
functions. Clin. exp. Immunol., 35: 413-420.
FARA, G.M., DEL CORNO, G., BONETTI, F., CARAMASCHI, L.,
DARDANONI, L., FAVARETTI, C., JIAMBELLUCA, S.E., MARNI, E.,
MOCARELLI, P., MONTESARCHIO, E., PUCCINELLI, V., & VOLPATO,
C. (1982) Chloracne after release of TCDD at Seveso, Italy.
In: Hutzinger, O., Frei, R.W., Merian, E., & Pocchiari, F.,
ed. Chlorinated dioxins and related compounds, Oxford,
Pergamon Press, pp. 545-560.
FLERKO, B. (1975) Perinatal androgen action and the
differentiation of the hypothalamus. In: Brazier, M.A.B., ed.
Growth and development of the brain, New York, Raven Press,
pp. 117-137.
FORBES, G.B. & REINA, J.C. (1972a) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. Health
Phys., 22: 169-175.
FORBES, G.B. & REINA, J.C. (1972b) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. J. Nutr.,
102: 647-652.
GIACOIA, G.P. & CATZ, C.S. (1979) Drugs and pollutants in
breast milk. In: Clinics in perinatology, Vol. 6, pp. 181-196.
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Contamination of milk with hexachlorobenzene residues.
Ind. Aliment. Agric., 89: 31-35.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
London, Academic Press.
GREAVES, S.J., FERRY, D.G., MCQUEEN, E.G., MALCOLM, D.S., &
BUCKFIELD, P.M. (1975) Serial hexachlorophene blood levels
in the premature infant. N.Z. med. J., 81: 334-336.
HACIK, T. (1969a) Effect of estradiol on adrenal and gonadal
functions during postnatal development in female rats. Endocr.
Exper., 3: 147-153.
HACIK, T. (1969b) Postnatal development of androgenized
female rats. Physiol. bohemoslov., 18: 263-269.
HACIK, T. (1970) Effect of estrogens on adrenal function and
body growth during postnatal period in male rats. Endocr.
Exper., 4: 31-38.
HAERING, M. & SCHEFER, W. (1980) Quantitative analysis of
biocide residues. Chimia, 34: 397-400.
HARRISON, H.E. (1980) Vitamin and mineral deficiencies in
intestinal malabsorption. In: Lifshitz, F., ed. Clinical
disorders in pediatric gastorenterology and nutrition, New
York, Dekker, pp. 371-375.
HEISE, E.R. (1982) Diseases associated with immuno-
suppression. Environ. Health Perspect., 43: 9-19.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Determination of
cadmium in liver and kidneys of children and adolescents by
neutron activation analysis.] Arch. Toxicol., 26: 8-16 (in
German).
HILL, J.R. (1964) The development of thermal stability in
the new-born baby. In: Jonxis, J.H.P., Visser, H.K.A., &
Troelstra, J.A., ed. The adaptation of the newborn to
extrauterine life, Netherlands, H.E. Stenfert Kroese NV, pp.
223-228.
HISLOP, A. & REID, L. (1981) Growth and development of the
respiratory system. In: Davis, J.A. & Dobbing, J., ed.
Paediatrics, 2nd ed., London, Heinemann, pp. 390-432.
HOAR, R.M. & MONIE, I.W. (1981) Comparative development of
specific organ systems. In: Kimmel, C.A. & Buelke-Sam, J., ed.
Developmental toxicology, New York, Raven Press, pp. 13-33.
HOFFMANN, H. (1982) Absorption of drugs and other
xenobiotics during development in experimental animals.
Pharmac. Ther., 16: 247-260.
ISLEIB, D.R. & WHITEHEAD, G.L. (1975) Polybrominated
biphenyls: an agricultural accident and its consequences. I.
The agricultural effects of exposure. In: Hemphill, D.D., ed.
Trace substances in environmental Health. IX, pp. 47-55.
JAPAN/US COOPERATIVE SCIENCE PROGRAM (1983) Toxicity of
chlorinated biphenyls, dibenzofurans, dibenzodioxins, and
related compounds. In: Proceedings of a Joint Seminar,
Fukvoka, Japan, pp. 25-28.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Res. Rev., 89: 1-128.
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
JONES, K.L. & SMITH, D.W. (1975) The fetal alcohol syndrome.
Teratology, 12: 11-26.
JUGO, S. (1980) Chelatable fraction of 203Pb in blood of
young and adult rats. Environ. Res., 21: 336-342.
KAVLOCK, R.J. & GRAY, J.A. (1983) Morphometric, biochemical,
and physiological assessment of perinatally induced renal
dysfunction. J. Toxicol. environ. Health, 11: 1-3.
KIESE, M. (1974) Methaemoglobinaemia: a comprehensive
treatise, Ohio, CRC Press, pp. 17-19.
KIMBROUGH, R.D., TAINES, T.V., & LINDER, R.E. (1974) The
effect of 2,4-dichlorophenyl- p -nitrophenyl ether on the rat
fetus. Arch. environ. Health, 28: 316-320.
KITAMURA, Y., MIYAO, M., & KAWAZOE, K., ET AL. (1953) In:
Friberg, L., ed. Toxic metals and their implications for human
health. Acta med., 8: 205-225.
KLINGER, W. (1982) Biotransformation of drugs and other
xenobiotics during postnatal development. Pharmac. Ther., 16:
377-429.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
KOSTIAL, K., SIMONOVIC, I., & PISONIC, M. (1971) Lead
absorption from the intestine in new-born rats. Nature
(Lond.), 233: 564.
KOSTIAL, K., KELLO, D., JUGO, S., RABAR, I., & MALJKOVIC, T.
(1978) The influence of age on metal metabolism and toxicity.
Environ. Health Perspect., 25: 81-86.
KROGER, M. (1972) Insecticide residues in human milk.
Pediatrics (Springfield), 80: 401-405.
KURATSUNE, M. (1980) "Yusho". In: Kimbrough, R.D., ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzo-
dioxins, and related products, Amsterdam, Elsevier/North
Holland Biomedical Press, pp. 287-302 (Topics in Environmental
Health No. 4).
L'ALLEMAND, D., GRUTERS, A., HEIDEMANN, P., & SCHURNBRAND, P.
(1983) Iodine-induced alterations of thyroid function in
new-born infants after prenatal and perinatal exposure to
povidone iodine. J. Pediatr., 102: 935-938.
LANDSBERG, J.D., BODYFELT, F.W., & MORGAN, M.E. (1977)
Retention of chemical contaminants by glass, polyethylene, and
polycarbonate multi-use containers. J. Food Protect., 40:
772-777.
LANGSTON, C. (1983) Normal and abnormal structural
development of the human lung. In: Abnormal functional
development of the heart, lungs, and kidneys: approaches to
functional teratology, New York, Alan R. Liss, pp. 75-91.
LAWTHER, P.J. & WALLER, R.E. (1975) Physical hazards. In:
Barltrop, D., ed. Paediatrics and the environment. Fellowship
of Postgraduate Medicine/Unigate Paediatric Workshop No. 2,
London, pp. 5-9.
LEVINE, S. & MULLINS, R. (1967) Neonatal androgen or
estrogen treatment and the adrenal cortical response to stress
in adult rats. Endocrinology, 80: 1177-1179.
LIBERTUN, C. & LAU, C. (1972) Adrenocortical function in
prepuberal rats: neonatal effects of testosterone. J. Endocr.,
55: 221-222.
LOIZZO, A., GATTI, G.L., MACRI, A., MORRETTI, G., ORTOLANI,
E., & PALAZZESI, S. (1984) Italian baby food containing
diethylstilboestrol: three years later. Lancet, 1: 1014-1015.
LUSTER, M.I., FAITH, R.E., & KIMMEL, D.A. (1978) Depression
of humoral immunity in rats following chronic developmental
lead exposure. J. environ. Pathol. Toxicol., 1: 397-402.
LUSTER, M.I., BOORMAN, G.A., DEAN, J.H., HARRIS, M.W., LUEBKE,
R.W., PADARATHSINGH, M.L., & MOORE, J.A. (1980) Examination
of bone marrows, immunological parameters, and host suscept-
ibilities following pre- and postnatal exposure to 2,3,7,8-
tetrachlorodibenzo- p -dioxin (TCDD). Int. J. Immunopharmacol.,
2: 301-310.
MCCANCE, R.A. (1948) Renal function in early life. Physiol.
Rev., 28: 331-348.
MCCANCE, R.A. & WIDDOWSON, E.M. (1956) Metabolism, growth,
and renal function of piglets in the first days of life. J.
Physiol., 133: 373-384.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957a) New thoughts on
renal function in the early days of life. Br. med. Bull., 13:
3-6.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957b) Hypertonic expansion
of the extracellular fluids. Acta paediatr., 46: 337-353.
MCCANCE, R.A. & YOUNG, W.F. (1940) The secretion of urine by
new-born infants. J. Physiol., 99: 265-282.
MCLACHLAN, J.A., NEWBOLD, R.R., KORACH, K.S., LAMB, J.C., &
SUZUKI, Y. (1981) Transplacental toxicology: prenatal
factors influencing postnatal fertility. In: Kimmel, C.A. &
Buelke-Sam, J., ed. Developmental toxicology, New York, Raven
Press, pp. 213-232.
MACHO, L., STRBAK, V., & HROMADOVA, M. (1972) The effect of
thyroxine on amino acid incorporation into protein during
ontogenesis in rats. Hormones, 3: 354-360.
MAHAFFEY, K.R. (1981) Nutritional factors in lead poisoning.
Nutrit. Rev., 39: 353-362.
MAHAFFEY, K.R. (1983) Absorption of lead by infants and
young children. In: Schmidt, E.H.F. & Hildebrandt, A.G., ed.
Health evaluation of heavy metals in infant formula and junior
food, Berlin, Springer Verlag, pp. 69-85.
MALPUECH, G., GAILLARD, G., GAULME, J., DOLY, M., BESSE, G.,
GOUMY, P., & RAYNAUD, E.J. (1978) Hypothyroidie transitoire
chez huit nouveau-nes de petit poids de naissance. Arch.
Franc. Ped., 35: 620-630.
MARWHA, H., NAYAK, N.C., ROY, S., KALRA, V., & GHAI, O.P.
(1981) The role of excess hepatic copper in the evaluation of
Indian childhood cirrhosis. Indian J. med. Res., 73: 395-403.
MARZULLI, F.N. & MAIBACH, H.I. (1975) Relevance of animal
models: the hexachlorophene story. In: Maibach, H., ed. Animal
models in dermatology, New York, Churchill Livingstone, pp.
156-167.
MAURER, H.M., WOLFF, J.A., FINSTER, M., POPPERS, P.J.,
PANTUCK, E., KUNTZMAN, R., & CONNEY, A.H. (1968) Reduction
in concentration of total serum-bilirubin in offspring of
women treated with phenobarbitone during pregnancy. Lancet, 2:
122-124.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
MIYABE, M., TSUBOUCHI, H., & SAKABE, Y. (1971) Studies on
pesticide residues in foods. II. Residues of BHC in milk. Rep.
Nagoya Munic. Pub. Health Lab., 18: 37-41.
MOMCILOVIC, B. & KOSTIAL, K. (1974) Kinetics of lead
retention and distribution in suckling and adult rats.
Environ. Res., 8: 214-220.
MORSELLI, P.L. (1976) Clinical pharmacokinetics in neonates.
Clin. Pharmacokin., 1: 81-98.
MRC (1984) The neuropsychological effects of lead in
children: a review of recent research, (1979-83), London,
Medical Research Council.
NATIONAL RESEARCH COUNCIL, SUBCOMMITTEE ON AIRBORNE PARTICLES
(1979) Epidemiologic studies on the effects of airborne
particles on human health. In: Airborne particles, Baltimore,
Maryland, University Park Press, pp. 167-198.
NIEMINERS, L., BJORNDAHL, K., & MOTTONEN, M. (1973) Effect
of hexachlorophene on the rat brain during organogenesis. Food
cosmet. Toxicol., 11: 635-639.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
NORDBERG, G.F. (1979) Factors influencing metabolism and
toxicity of metals. In: Di Ferrante, E., ed. Trace metals:
exposure and health effects, Oxford, CEC/Pergamon Press, pp.
157-158.
PAVEY, D.E. & WIDDOWSON, E.M. (1980) Body lipids of
guinea-pigs exposed to different dietary fats from
mid-gestation to 3 months of age. V. The fatty acid
composition of brain lipids at birth. Nutr. Metab., 24:
357-366.
PETERS, H.A. (1976) Hexachlorobenzene poisoning in Turkey.
Fed. Proc., 35: 2400-2403.
RAMBOER, C., THOMPSON, R.P.H., & WILLIAMS, R. (1969)
Controlled trials of phenobarbitone therapy in neonatal
jaundice. Lancet, 1: 966-968.
ROBERTS, D.W. & CHAPMAN, J.R. (1981) Concepts essential to
the assessment of toxicity to the developing immune system.
In: Kimmel, C.A. & Buelke-Sam, J., ed. Developmental
toxicology, New York, Raven Press, pp. 167-189.
ROGAN, W. & GLADEN, B. (1982) Duration of breast-feeding and
environmental contaminants in milk. Am. J. Epidemiol., 116:
565A.
ROOK, J.J. (1974) Formation of halogens during clorination
of natural waters. J. Water Treat. Exam., 23: 234-243.
ROWLAND, I.R., ROBINSON, R.D., DOHERTY, R.A., & LANDRY, T.D.
(1983) Are developmental changes in methylmercury metabolism
and excretion mediated by the intestinal microflora? In:
Clarkson, T.W., Nordberg, G.F., & Sager, P.R., ed.
Reproductive and developmental toxicity of metals, New York,
Plenum Press, pp. 745-758.
SAENZ DE RODRIGUEZ, C.A. & TORO-SOLA, M.A. (1982) Anabolic
steroids in meat and premature telarche. Lancet, 1: 1300.
SCAGLIONI, S., DI PIETRO, C., BIGATELLO, A., & CHIUMELLO, G.
(1978) Breast enlargement at an Italian school. Lancet, 1:
551-552.
SCHELER, W. (1980) [The basis of general pharmacology,] 2nd
ed., Stuttgart, Gustav Fischer Verlag (in German).
SCHMIDT, R.R. (1984) Altered development of immunocompetence
following prenatal or combined perinatal-postnatal insult: a
timely review. J. Am. Coll. Toxicol., 3: 57-72.
SHERLOCK, J.C., SMART, G., FORBES, G.I., MOORE, M.J.,
PATTERSON, W.J., RICHARDS, W.N., & WILSON, T.S. (1982)
Assessment of lead intakes and dose response for a population
in Ayr exposed to a plumbosolvent water supply. Hum. Toxicol.,
1: 115-122.
SHUMAN, R.M., LEECH, R.W., & ALVORD, E.C., Jr (1975)
Neurotoxicity of hexachlorophene in humans. II. A clinico-
pathological study of 46 premature infants. Arch. Neurol., 32:
320-325.
SINGER, E.J., WEGMANN, P.C., LEHMAN, M.D., CHRISTENSEN, M.S.,
& VINSON, L.J. (1971) Barrier development, ultrastructure,
and sulfhydryl content of the fetal epidermis. J. Soc. Cosmet.
Chem., 22: 119-137.
SMITH, R.J. (1982) Hawaiian milk contamination. Science
N.Y., 217: 137-140.
SVENNERHOLM, L., ALLING, C., BRUCE, A., KARLSSON, I., & SAPIA,
O. (1972) Effects on offspring of maternal malnutrition in
the rat. In: Elliott, K. & Knight, J., ed. Lipids,
malnutrition, and the brain, Amsterdam, Associated Scientific
Publishers, pp. 141-157.
TADJER, G.S. & DORE; I. (1971) Possibility of contamination
of bottles used by food industries by insecticides. Harefuah,
81: 385.
TAKEWAKI, K. (1968) Reproductive organs and anterior
hypophysis of neonatally androgenized female rats. Sci. Rep.
Tokyo Women's Christ. Coll., 1-6: 31-47.
TANNER, J.M., WHITEHOUSE, R.H., & TAKAISHI, M. (1966)
Standards from birth to maturity for height, weight, height
velocity, and weight velocity. Part II. British children,
1965. Arch. Dis. Child., 41: 613-635.
THABREW, M.W., OLORUNSOGO, O.O., OLOWOOKERE, J.O., &
BABABUNMI, E.A. (1982) Possible defect in xenobiotic
activation before glycine conjugation in protein-energy
malnutrition. Xenobiotica, 12: 849-853.
TROLLE, D. (1968) Phenobarbitone and neonatal icterus.
Lancet, 1: 251-252.
TURNER, M.D., MARSH, D.O., SMITH, J.C., INGLIS, J.B.,
CLARKSON, T.W., RUBIO, C.E., CHIRIBOGA, J., & CHIRIBOGA, C.C.
(1980) Methylmercury in populations eating large quantities
of marine fish. I. Northern Peru. Arch. environ. Health,
35(6): 367-378.
ULSAMER, A.G., YODER, P.D., KIMBROUGH, R.D., & MARZULLI, F.N.
(1975) The effect of hexachlorophene on developing rats:
toxicity tissue concentrations and biochemistry. Food cosmet.
Toxicol., 13: 69-80.
UMBERGER, E.J. (1975) Products marketed to promote growth in
food-producing animals: steroid and hormone products.
Toxicology, 3: 3-21.
UNITED KINGDOM CENTRAL DIRECTORATE ON ENVIRONMENTAL POLLUTION
(1982) The Glasgow duplicate diet study (1979/80), London,
Her Majesty's Stationery Office (Pollution Report No. 11).
UNITED KINGDOM DEPARTMENT OF HEALTH AND SOCIAL SECURITY
(1980) Lead and health, London, Her Majesty's Stationery
Office (Report of a DHSS Working Party on Lead in the
Environment).
URSO, P. & GENGOZIAN, N. (1980) Depressed humoral immunity
and increased tumour incidence in mice following in utero
exposure to benzo( a )pyrene. J. Toxicol. environ. Health, 6:
569-576.
US DHEW (1979) Smoking and health: a report of the Surgeon
General, Washington DC, US Department of Health, Education and
Welfare (Section No. 8).
VALCANA, T. & TIMIRAS, P.S. (1978) Nuclear triiodothyronine
receptors in the developing rat brain. Mol. Cell. Endocrinol.,
11: 31-41.
VALE, J.A. & MEREDITH, T.J. (1981) Epidemiology of poisoning
in the UK. In: Vale, J.A. & Meredith, T.J., ed. Poisoning
diagnosis and treatment, 1st ed., London, Update Books, pp.
1-8.
VESSELINOVITCH, S.D., MIHAILOVICH, N., WOGAN, G.N., LOMBARD,
L.S., & RAO, K.V.N. (1972) Aflatoxin B1, a hepato-
carcinogen in the infant mouse. Cancer Res., 32: 2289-2291.
VILLETT, L.B. & HESS, J.F., Jr (1975) Polychlorinated
biphenyl residues in silos in the US. Residue Res., 55:
135-147.
VOS, J.G. (1977) Immune suppression as related to
toxicology. CRC Crit. Rev. Toxicol., 5: 67-101.
VOS, J.G. & MOORE, J.A. (1974) Suppression of cellular
immunity in rats and mice by maternal treatment with
2,3,7,8-tetrachlorodibenzo- p -dioxin. Int. Arch. Allergy appl.
Immunol., 47: 777-794.
VOS, J.G., VAN DOGTEN, M.J., KREEFTENBERG, J.G., STEERENBERG,
P.A., & KRUIZINGA, W. (1979) Effect of hexachlorobenzene on
the immune system of rats following combined pre- and
postnatal exposure. Drug Chem. Toxicol., 2: 61-76.
WALKER, W.A. (1979) Gastrointestinal host defence:
importance of gut closure in control of macromolecular
transport. In: Elliott, K. & Whelan, J., ed. Development of
mammalian absorptive processes, Amsterdam, Excerpta Medica,
pp. 201-216 (Ciba Foundation Symposium No. 70 (new series)).
WESTER, R.C. & MAIBACH, H.I. (1982) Percutaneous absorption:
neonate compared to the adult. In: Hunt, V.R., Smith, M.K., &
Worth, D., ed. Environmental factors in human growth and
development, New York, Cold Spring Harbor Laboratory (Banbury
Report No. 2).
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1984) Environmental Health Criteria 30: Principles for
evaluating health risks to progeny associated with exposure to
chemicals during pregnancy, Geneva, World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WIDDOWSON, E.M. & DICKERSON, J.W.T. (1964) Chemical
composition of the body. In: Comar, C.L. & Bronner, F., ed.
Mineral metabolism, New York, Academic Press, Vol. 2A, pp.
1-247.
WIDDOWSON, E.M., CRABB, D.E., & MILNER, R.D.G. (1972)
Cellular development of some human organs before birth. Arch.
Dis. Child., 47: 652-655.
WILLOUGHBY, M.L.N. (1984) Disorders of the blood and
reticuloendothelial system. In: Forfar, J.O. & Arneil, G.C., ed.
Textbook of paediatrics, 3rd ed., Edinburgh, Churchill
Livingstone, Vol. 1, pp. 935-982.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
WONG, K.L. & KLAASSEN, C. (1980) Tissue distribution and
retention of cadmium in rats during postnatal development:
minimal role of hepatic metallothionein. Toxicol. appl.
Pharmacol., 53: 343-353.
ZIEGLER, E.E., EDWARDS, B.B., JENSEN, R.L., MAHAFFEY, K.R., &
FOMON, S.J. (1978) Absorption and retention of lead by
infants. Pediatr. Res., 12: 29-34.
APPENDIX I: BREAST MILK
1. Introduction
The breast-fed infant requires special consideration in
circumstances in which chemical contamination of the mother's milk
is suspected, or has been confirmed. Young infants are
characteristically dependent on milk, and the structural and
functional development of their organs and tissues is adapted to
the milk of their own species. The potential risks of exposure to
chemicals in breast milk must therefore be assessed, taking into
account the benefits of breast-feeding together with the
disadvantages of feeding an alternative milk, in terms of
developmental toxicology.
The benefits of breast-feeding, particularly in the developing
countries, are now universally acknowledged. In these societies,
nearly all women of all classes breast-feed for 12 months or more.
The decline in breast-feeding in many developed countries has
recently been reversed; interestingly, both the decline in, and
return to, breast-feeding began among the most socially advantaged
women (WHO, 1981). National and international authorities have
been concerned that, in view of the health risks involved in the
preparation and provision of breast milk substitutes in developing
societies, mothers in these countries should not go through the
changes in infant feeding practice that have occurred in the
developed world. Thus, the Member States of the World Health
Organization have committed themselves to the protection and
promotion of breast-feeding as the first, and one of the most
critical, steps in sound infant nutrition policies (WHO/UNICEF,
1981).
This Appendix is concerned with principles for evaluating the
possible consequences to health of chemical contaminants in breast
milk. The evaluation of risks potentially associated with these
chemicals in the quantities that may be consumed must be carefully
weighed against the assessment of the risks of alternative methods
of infant feeding.
The breast milk of a well-nourished mother has an optimal
composition to meet the nutritional needs of the full-term infant
in early life, and there are associated immunological,
psychological, and economic advantages. In view of the importance
of breast milk for the nutrition and health of the infant, evidence
of any harmful effects of a foreign chemical that it may contain
must be carefully considered before recommendations are made that
breast-feeding should be curtailed or discontinued.
2. Assessment of the Importance of Chemicals in Breast Milk
2.1 Source of chemicals
Women may be exposed to chemical substances from many different
sources, including air, food, water, drugs, cosmetics, and the
occupational environment.
2.2 Chemicals within the mother's body and their secretion in milk
Chemicals that are easily metabolized and are eliminated from
the mother's body in the faeces and urine are only secreted in
significant quantities in breast milk following recent high-level
chemical exposure (Wolff, 1983; Jensen, 1985).
Other chemicals, such as heavy metals and persistent
halocarbons, are less readily removed from the body. Some metals
accumulate mainly in the liver, kidney, and bone, whereas
persistent halocarbons accumulate in the lipids of the body. Long-
term exposure, even to low concentrations of such chemicals, may
build up considerable body stores. On the other hand, a short-term
change of diet is not likely to alter the concentration of
halocarbon in the mother's fatty tissues.
A dynamic equilibrium for persistent lipophilic chemicals
exists between different tissue compartments. The concentrations
of these chemicals in adipose tissue, blood, and milk is in
proportion to the fat content; thus, the concentration in blood is
far lower than that in whole milk, and the concentration in milk is
far lower than that in adipose tissue. There is a relatively good
correlation between concentrations of organic halocarbons in the
lipids of blood, adipose tissue, and milk. This is illustrated for
DDT in the lipids of milk and adipose tissue in Fig. I.1.
Table 1. Increase in weight of body and
organs during the first 9 months after
birtha
-----------------------------------------
Body/organ Increase
(g/kg weight at birth)
-----------------------------------------
Body 1810
Brain 1480
Liver 1420
Kidneys 1580
Heart 1160
Lungs 1500
-----------------------------------------
a Based on: values given in Diem & Lentner (1970).
The constituents of some organs and tissues have a slower rate
of turnover than those of others. The lipids, for example, once
laid down, have a slow rate of turnover compared with protein, in
all parts of the body including the brain. Fat-soluble chemicals,
which might be incorporated into brain lipids during the rapid
growth of the brain in infancy (Dobbing & Sands, 1973), would not
enter the tissue in significant amounts after the growth of the
brain has ceased. The fatty acid composition of brain lipids of
rats and guinea-pigs can be appreciably altered, early in life, by
the nature of the fat in the diet of the mother during pregnancy
and lactation (Svennerholm et al., 1972; Pavey & Widdowson, 1980).
Information on man is lacking.
1.3.4 Different body composition from adult
Infants have a different body composition from adults. As in
adults, the contribution of fat to the body weight varies
considerably between one individual and another in infants, but the
average is about 15% in full-term new-born infants and in adult
men. However, infants have a higher percentage of water and a
smaller percentage of solids in the lean body tissue. The infant,
at term, has 82% of water in its lean tissue compared with 72% in
the adult (Widdowson & Dickerson, 1964). The additional water in
the infant is mainly extracellular, the volume of extracellular
fluid per unit body weight in the young infant being about twice
that in an adult (Widdowson & Dickerson, 1964). Thus, any chemical
that is confined to the extracellular compartment of the body will
have a greater volume in which to distribute itself per unit body
weight. The advantage of this for the infant could be offset by
the slower rate of excretion by the kidneys (section 3).
The percentage of water in the organs and tissues, and thus in
the body as a whole, decreases with age. The levels of water in
some organs at birth, at 6 - 12 months where figures are available,
and in the adult (Widdowson & Dickerson, 1964) are shown in Table
2. The decrease in water in the liver, kidneys, heart, and lungs
is due primarily to an increase in protein; in the brain, it is due
to an increase in myelin.
Table 2. Water in organs and tissues (g/kg)a
-------------------------------------------------
Organ/tissue At birth 6 - 12 Adult
months
-------------------------------------------------
Whole body (fat-free 820 780 720
basis)
Brain 900 830 770
Liver 780 760 710
Kidneys 840 - 810
Heart 840 830 830
Lungs 860 - 790
Skeletal muscle
Total 800 780 790
Extracellular 350 290 180
Intracellular 450 490 610
-------------------------------------------------
a From: Widdowson & Dickerson (1964).
Another difference between infants and adults is that most of
the cells of the organs and tissues of infants are smaller than
those of adults (Widdowson et al., 1972). Small cells, like small
bodies, have a larger surface area in relation to mass than bigger
cells and bodies, and this in itself may have important
implications for chemicals that may enter the cells.
The bones of an infant contain more water and less fat,
protein, and mineral than adult bones. This is illustrated in
Table 3 for the whole femur (Dickerson, 1962b). Unlike soft
tissues, the chemical maturation of the bone, as evidenced by the
decrease in percentage of water and increase in degree of
calcification, mainly takes place after 1 - 2 years. From the
values in Table 3, it can be calculated that the increment per
month of calcium in one femur is approximately 0.2 g between birth
and 18 months, 0.48 g between 18 months and 11 years, and 1.0 g
between 11 and 18 years. The femur may not be completely typical
of all parts of the skeleton, but it does seem likely that the rate
of deposition of chemicals taken up by the bone along with calcium,
and indeed with magnesium, sodium, and other metals present in
bone, is likely to be greater in later childhood than in infancy.
However, the bones of infants are smaller, and the rate of
deposition of calcium per kg of bone per month is greater in
infancy, being 7.8 g/kg bone between birth and 3 months, 6.8 g/kg
between 3 and 18 months, and 1.9 g/kg bone between 18 months and 18
years. Thus, whether the bones of infants are considered likely to
take up more or less of a chemical than those of older children
depends partly on the method of expression of the rate of
deposition.
Table 3. Composition of the whole femur of the infant, child, and adulta
---------------------------------------------------------------------
At birth 3 months 1 - 2 11 - 12 Adult
(full- (milk years years (pre-
term) feeding) (weaned) adolescent)
---------------------------------------------------------------------
Weight femur g 16.6 26.4 63.7 326 646
Fat g/kg fresh bone 1.4 6.5 75 26 31
Composition g/kg
fresh fat-free bone
Water 639 641 554 364 227
Collagen 93 107 141 163 173
Ca 61 54 70 138 194
P 28 27 33 63 83
---------------------------------------------------------------------
a From: Dickerson (1962b).
1.3.5 Functional immaturity of the organs and systems of the body
Most organs and systems of the body have not reached structural
or functional maturity at birth. Development of the nervous system
continues in postnatal life, much of the myelination of the brain
takes place after birth and continues until adolescence (Hoar &
Monie, 1981). The structural development of the lung also
continues postnatally with an increase in alveolar surface area
(Langston, 1983). Several components of the immune system are not
fully developed at birth, for example, the lymphocytes responsible
for producing antibodies, and it has been suggested that this is
responsible for the greater susceptibility of the new-born infant
to certain bacterial infections (Andersson et al., 1981). The
gastrointestinal, endocrine, and reproductive systems, and also
renal function, are all immature at birth. The kidneys are less
able than those of older children and adults to eliminate excessive
amounts of substances normally present in milk, for example, sodium
and phosphate (Dean & McCance, 1947a, 1948; Barltrop & Oppé, 1970),
and this may also apply to extraneous chemicals present in, and
absorbed from, milk. Moreover, the activities of many liver enzyme
systems that metabolize endogenous and exogenous substances may be
low in the new-born infant. These topics are covered in more
detail in section 3.
1.3.6 Breast milk, infant formulae, and water as sources of
chemicals
The young infant lives on a single food, milk, either from the
breast or the bottle. Thus, the presence of a chemical in breast
milk, or in the powder or water used to make up an infant formula,
is likely to have greater implications for the infant than for the
older child having mixed feeding, where milk forms only part of the
daily food. Furthermore, infants take in a much larger amount of
fluid per unit body weight than older children and adults. An
infant living on milk takes about one-seventh of its own weight of
water each day, which would correspond to 10 litres for a 70-kg
man. This large intake is necessary for the infant, because it
loses more water per kg body weight through the lungs, owing to the
greater passage of air through them, the skin, because of the
larger surface area, and the kidneys, because of the inability to
concentrate the urine to the same extent as the older child or
adult. Any chemical present in the water used to make up infant
formulae will, therefore, be taken in by an infant in larger
quantities per unit body weight than by an older child or adult
using the same water supply. This is discussed in more detail in
section 2.
Nowadays, it is common practice among manufacturers to add
vitamins and trace elements to their preparations. Occasionally,
these have been added in such large amounts that they have been
reported to be toxic, even though in smaller, physiological amounts
they are essential. Examples are vitamin D (British Paediatric
Association, 1956) and manganese (Collipp et al., 1983).
1.4 Applicability of Data on Experimental Animals to Human Infants
and Young Children
Data on the toxic effects of chemicals in man are frequently
incomplete or even absent, and this is particularly true for
infants and young children. Some insight can be gained from
observations on young animals, but any assessment of human risk on
the basis of such studies should take into account the differences
that exist between the species in their reactions to chemicals.
Moreover, the conditions under which the animal data were obtained
may not reflect the human circumstances under consideration.
Animal data must be considered, therefore, in the context of the
similarities and differences that are known to exist between man
and experimental animals, and conclusions need to incorporate a
margin of safety to accommodate known, and possibly unknown,
species differences.
1.4.1 Some similarities in growth and development between
man and experimental animals
In many ways, the differences between the young infant and
adult are the same in animals as they are in man. Thus, the infant
animal is always smaller than the adult and, thus, it has a larger
surface area in relation to its body weight. All animals that are
homoeothermic from the time of birth have a higher consumption of
air per unit body weight than the adult of the same species, at the
same environmental temperature. The young of all animals species
grow rapidly, often far more rapidly than the human infant, and
their body composition differs from that of the adult in the same
way that that of the human infant differs from that of the human
adult. The functions of the organs and systems are immature in
early life, and most mammalian species depend on mother's milk for
a period after birth.
The anatomical, physiological, and chemical differences between
the infant and adult of an animal species are therefore in the same
direction as they are in the human species, and, in general, the
effects of chemicals on the young of experimental animals can be
expected to differ from those on the adult of the species just as
they do in man. However, the differences between species make it
unwise to assume that the results obtained on a young animal will
necessarily apply to the human infant.
1.4.2 Some differences in growth and development between man and
experimental animals
Different animals are born at different stages of their
anatomical, physiological, and chemical development and, although
the qualitative differences between the infant and adult are
similar in man and experimental animals, the quantitative
differences are by no means the same. The rat is a widely used
experimental animal in studies on the effects of chemicals on the
infant body. Some stages of development that take place after
birth in the rat have already occurred in the human fetus before
birth. Examples are the anatomical and functional maturation of
the hypothalamus (Dörner & Staudt, 1972) and the deposition of body
fat. Some aspects of development, however, only occur after birth
in all species, for example, respiration of air and the full
functioning of the gastrointestinal tract.
The new-born rat is smaller than the new-born human infant in
comparison with the weight of the adult, and, during infancy, it
grows at a much more rapid rate. Thus, the discussion in section
1.3.3 about the human infant applies with even greater force to the
infant rat. The rat at birth has a less mature chemical
composition than the human infant, as evidenced by the higher
percentage of water in its lean body tissue (Widdowson & Dickerson,
1964). The greater degree of immaturity of the body composition of
the rat at birth is well illustrated by reference to the bones.
Characteristically, rat bones have very little fatty marrow, but,
apart from this, the composition of the adult femur is not very
different in the 2 species. At birth, however, the rat femur has
less than half the concentration of calcium that there is in the
human femur, more water, and considerably less collagen, which
makes up the matrix on which the bone mineral is deposited
(Dickerson, 1962a,b). In the rat, as in man, the major changes in
the composition of bone do not take place until the period of
infancy is over. The composition of bone, and of the body as a
whole, though less mature at the time of birth in the rat than in
man, changes much more rapidly. All this must be taken into
account in considering the effects of a chemical on the bones or
bodies of young rats compared with the effects on those of human
infants.
The new-born rat is less mature physiologically than the human
infant, and it matures more rapidly. This may limit the
application of results obtained on the young rat to the human
infant. This is true of the renal function (McCance, 1948), so
that the new-born rat is less able than the adult to excrete
chemicals administered to it (section 3.5.1).
The activity of the hepatic enzymes is low in both new-born
experimental animals and new-born human infants, but the whole
subject of enzyme activity in this context is complex and is
considered in more detail in section 3.
One difference between the young of man and some animal species
is the ability to absorb macromolecules from the intestine. In the
human infant, the passage of macromolecules across the intestinal
mucosa is very limited, but in the young of such species as the
pig, cow, sheep, and horse, macromolecules are transferred from
the milk into the circulation in quite large amounts for a limited
period of hours or days. In the rat, the transfer goes on all
through the suckling period (Walker, 1979). This may have a
bearing on the interpretation of the results of studies on the
intestinal absorption of certain chemicals by the new-born
experimental animal in relation to the human infant.
1.5 Conclusions
The infant and young child have different structural and
functional characteristics from those of the older child and adult,
which represent stages in normal growth and development. These
include a larger surface area in relation to weight, different body
composition, a higher metabolic rate and oxygen consumption and,
hence greater intake of air per unit body weight, rapid growth and
development of the body as a whole and of the organs and tissues,
particularly during the first 6 months after birth. Many organs
and tissues are functionally immature at the time of birth, and
they mature at different rates. Moreover, infants have greater
energy and fluid requirements per unit body weight than older
children and adults. All these characteristics must be taken into
account in considering principles for evaluating health risks from
chemicals during infancy and childhood.
The structural and functional differences between the infant
and adult animal tend to follow the same direction as in the human
species, but there are important quantitative differences in, for
example, the stage of development at birth, the rate of growth, and
the intestinal absorption of macromolecules. The age of the infant
rat or other animal corresponding to an age in the human infant
will differ according to the aspect of development under
consideration.
2. PATHWAYS OF EXPOSURE
2.1 Introduction
As with adults, the potential for exposure to chemicals for
infants and young children is through ingestion, inhalation, and
percutaneous absorption. Both voluntary and inadvertent exposures
may occur, but their nature and extent are modified by the
physiological, behavioural, and other characteristics peculiar to
this age group. Thus, the methods of nurture, the social,
cultural, and occupational proclivities of the family, the quality
of care and supervision, and the inherent behavioural patterns of
the child, may all be significant factors for determining or
modifying exposure.
The new-born infant may already have encountered toxic chemical
agents in utero by the placental route; for example, the sequelae
of maternal exposure to methylmercury (Clarkson et al., 1976),
ethanol (Jones & Smith, 1975), tobacco (US DHEW, 1979), and
narcotics (Barr et al., 1984) are well recognized. Moreover, the
young infant is an obligate milk feeder, and chemicals that
traverse the mammary gland in lactation are potentially important
contaminants of both human and artificial milk.
The use of appropriate cleaning and antiseptic chemicals for
the routine care of the skin, umbilicus, and clothing of the new-
born infant have all been associated with toxicity (Armstrong et
al., 1969). Similarly, effects from atmospheric contaminants have
been encountered in neonatal units from agents used on the clothing
or for the disinfection of apparatus and operational areas.
Development from infancy to childhood is marked by increasing
mobility and exploration of the immediate surroundings. Oral
exploration is commonplace towards the end of the first year of
life in which the mouth is used to sample the taste and texture of
the objects and materials that are encountered. Oral exploration
may be accompanied by the ingestion of a wide variety of substances
not normally regarded as food (pica) including soils, dusts, and
surface coatings in the vicinity of the home. Although mouthing
and pica diminish with continuing development after the first year
of life, hand to mouth activity tends to persist (Barltrop, 1966).
As a consequence of increasing mobility and diminishing
supervision, poisoning due to the ingestion of toxic substances is
very common in early childhood. For example, in England and Wales,
one-fifth of all hospital admissions for suspected poisoning
involved children under the age of 5 years, amounting to
approximately 20 000 admissions per annum. Household,
horticultural, agricultural, and industrial chemicals were all
implicated, though medicaments and therapeutic compounds were the
principal agents (Vale & Meredith, 1981). It is unlikely that all
acute ingestions are reported, and their prevalence is not known
with certainty. The quality of available supervision is an
important determinant of accidental poisoning and will itself be
modified by social, cultural, and economic factors.
Infants and children will also be subject to chemical exposure
resulting from contamination of the natural and domestic
environments that they share with adults. However, the provision
of foodstuffs, beverages, clothing, furniture, toys, and
medicaments, specifically manufactured or adapted for the young,
may modify the route of exposure for certain chemicals; efforts are
usually made to minimize the contamination of such materials with
chemicals that might be harmful.
2.2 The Alimentary Tract
2.2.1 Milk
Milk may be regarded as one of the routes of excretion of
foreign compounds that have entered the body of the lactating
mammal. The degree to which a particular chemical traverses the
mammary gland depends on several factors, including its own
physical and chemical properties, such as the degree of ionization
and binding in biological fluids, the solubility characteristics,
and the maternal plasma concentration achieved. Passive diffusion,
active transport, and reverse pinocytosis across the apical
membrane of the mammary alveolar cell may all be involved, but the
milk/plasma ratio cannot always be predicted for a given chemical
in a particular species. In general, transfer from plasma to milk
is enhanced by high solubility in lipids, low relative molecular
mass, and weak binding to plasma-proteins (Giacoia & Catz, 1979).
2.2.1.1 Human milk
Nearly all chemicals to which the mother is exposed may, to
some extent, be excreted in human milk (Jensen, 1983, 1985).
Contamination of human milk may result from the maternal diet,
environment, occupation, addiction, and medical treatment. Marked
variation in the concentrations of chemicals in milk, both between
and within individuals, is characteristic and may reflect the stage
of lactation, the time and method of sample collection, and the
age, weight, and parity of the mother. Chemical residues most
frequently reported in human milk are the organochlorine pesticides
(DDT) and industrial chemicals (PCBs and metabolites, HCH isomers,
and cyclodienes such as dieldrin). Lactation is a route of
excretion for some of these chemicals, and adverse health effects
have been noted in breast-fed infants when the concen-tration of
chemicals was extremely high, as in accidental poisoning outbreaks,
and in some cases of occupational exposure. During the Iraqi
outbreak of methylmercury poisoning of 1971-72 (Amin-Zaki et al.,
1974), mothers who consumed bread made from seed grains treated
with a methylmercury fungicide were found to have milk containing
8.6% of the concentration of mercury in maternal blood collected at
the same time (Amin-Zaki et al., 1976). Infants who were born
before their mothers ate the contaminated bread and who received
methylmercury only through suckling, were found to have blood-
mercury values of up to 1.0 g/litre. Follow-up assessment over 5
years showed that some of the children suffered impaired
neurological and mental development (Amin-Zaki et al., 1981).
In Turkey, bread prepared from seed grains treated with
hexachlorobenzene between 1955-59 also affected infants (Peters,
1976). In some villages, almost all children under the age of 2
years, who had been breast-fed by mothers who had eaten the
contaminated wheat, died of the condition known as "pembe yara"
(Turkish for pink sore), which included symptoms of weakness,
convulsions, and localized cutaneous annular erythema.
Reports of such episodes have been rare. However, if
confronted with such poisoning outbreaks, exposure of infants
through human milk should be considered.
2.2.1.2 Other types of milk
Cow's milk, and the milk of other species used for infant
feeding, are subject to contamination by many of the chemicals
found in human milk, together with others that reflect local
techniques of animal husbandry and veterinary practice. Infants fed
milk derived from a particular animal or herd may be at greater or
lesser risk of exposure to a given contaminant than those fed
artificial or manufactured milk derived from the pooled secretions
of multiple herds. Conversely, the pooling of milk during the
manufacture of infant feeds will tend to increase the number of
potential contaminants in the end-product, but may diminish their
concentrations.
The food sources of lactating animals may be contaminated by
local industrial wastes, by the application of pesticides, by
naturally-occurring substances, such as mycotoxins, or by storage
in containers or buildings previously contaminated with pesticides,
disinfectants, preservatives, and other chemicals. The
organochlorine pesticides are well recognized fodder contaminants.
Numerous examples are known of milk containing chemicals derived
from animal feeds including DDT, hexachlorocyclohexane (HCH)
(Miyabe et al., 1971), heptachlorepoxide (Smith, 1982), and
hexachlorobenzene (Goursaud et al., 1972). Chemicals in milk
derived from containers of contaminated animal feed include PCBs
(Villett & Hess, 1975), pentachlorophenol (PCP) (Haering &
Schefer, 1980), and dieldrin (Epps et al., 1974). The inadvertent
substitution of a polybrominated biphenyl (PBB) product for
magnesium oxide in prepared cattle feeds resulted in contamination
of the milk (Isleib & Whitehead, 1975).
Several examples of milk contamination occurring after
collection have been reported. Thus, iodophors and other
disinfectants (Kroger, 1972), dieldrin (Tadjer & Dore, 1971) and
chlordane (Landsberg et al., 1977) have all been identified in milk
stored in vessels that had been inadvertently rinsed or cleaned
with, or fabricated from, previously contaminated materials. The
inadvertent addition of toxic chemicals to infant milk during
manufacture is rare, though arsenic poisoning resulting from the
use of a contaminated sodium phosphate stabilizer has been reported
(Kitamura et al., 1953).
Pesticides and drugs for the control of parasitic infection and
other diseases in lactating animals are liable to be absorbed and
excreted in the milk, even after topical application. Milk
contamination with antibiotics and hormones, given for economic
rather than therapeutic or preventative indications, may also
occur.
2.2.1.3 Infant feeds
Foods and beverages, other than milk, which have been
specifically prepared or manufactured for infants and young
children, may be diverse in composition and comprise only a
proportion of the total dietary intake. However, they are subject
to the same sources of chemical contamination as foods intended for
adults, during producion, manufacture, and packaging. The
potential significance of contaminants is enhanced when the dietary
range is restricted.
Numerous examples of contamination at source have been
described in common with those for human and other milk. Thus,
residues of pesticides, antibiotics, fungicides, and heavy metals
have all been encountered as a result of agricultural techniques,
veterinary practice, and the use of contaminated land and water.
An additional factor with regard to meat products is, for example,
the use of anabolic agents, such as diethylstilboestrol, in animal
husbandry (Umberger, 1975). Thus, in one study, one-third of 450
samples of baby food containing veal were found to have detectable
estrogen activity (Loizzo et al., 1984).
Many chemical or non-nutritional substances are used in food
manufacture as preservatives, anti-oxidants, stabilizers,
emulsifiers, and thickening agents, with the intention of ensuring
that the food is wholesome and palatable, when consumed. Numerous
chemical colouring agents are potentially available, and their use
in infant foods has attracted controversy, since marketing rather
than nutritional considerations are involved.
Manufactured infant and junior foods are characteristically
distributed in appropriately small containers which, therefore,
have a relatively large surface area/volume ratio. The degree to
which contaminants derived from the container material, seams, or
internal surface coatings may affect the contents is inversely
proportional to the container volume. The degree to which the
container design and manufacture may influence food contamination
is indicated by the reduction of dietary lead intake observed in
infants in the USA during the last decade, when progressive
replacement of lead soldered side-seamed cans by welded or seamless
containers resulted in a 47% reduction in lead intake by infants
aged 0 - 5 months (Mahaffey, 1983).
Contamination of foods during preparation in the home may
result from the use of inappropriate vessels, culinary agents such
as cooking oils, and cleaning materials, as well as from water.
Thus, excessive intake of copper derived from domestic cooking
utensils has been implicated in Indian childhood cirrhosis (Marwha
et al., 1981), and contaminated edible oils were involved in an
outbreak of Yusho disease (Kuratsune, 1980; Japan/US Cooperative
Science Program, 1983).
The use of detergent chemicals for the cleaning of cooking and
eating utensils is inevitably associated with ingestion of the
chemicals by all members of the household, but, in normal usage, it
is unlikely that toxic intakes will be encountered. However,
intakes are likely to be enhanced when rinsing or wiping is
omitted.
2.2.1.4 Water
Water for domestic consumption may be contaminated by chemicals
at source, during treatment and transport, or in the home from
domestic plumbing, cisterns, or culinary utensils. The use of
recycled water, particularly during drought, will tend to enhance
the concentration of some pollutants. The direct ingestion of
water used in the preparation of foods and beverages will involve
children of all ages, depending on local climatic conditions and
cultural and dietary practices. However, the use of water to
reconstitute powdered artificial milk is a special case,
particularly where the milk is the sole nutrient source.
The potential contaminants of drinking-water are numerous and
include a wide range of metals and other elements; nitrates,
cyanide, thiocyanate, and ferrocyanate radicals; organic compounds
including pesticides; organic solvents; and anionic, cationic, and
non-ionic detergents. Asbestos and polyaromatic hydrocarbons have
been detected. The chlorination of water for disinfection,
particularly in the presence of humic material, results in the
formation of a number of haloforms or C-chlorinated compounds
including chloroform, bromodichloromethane, and
dibromochloromethane (Rook, 1974).
The significance of lead in drinking-water has been
increasingly appreciated, particularly in areas where lead pipes
and plumbosolvent water supplies co-exist. Studies in a population
in Scotland showed that there was a curvilinear relationship
between the concentration of lead in water and that in the blood of
artificially fed infants; 36% had blood-lead values in excess of
350 µg/litre (Sherlock et al., 1982). Similarly, lead intakes of
up to 3.4 mg/week were reported from Scotland in artificially fed
infants, whose food was measured and analysed, with correspondingly
marked increases in blood-lead values (United Kingdom Central
Directorate on Environmental Pollution, 1982).
Nitrate in drinking-water is not itself hazardous, but may be
converted to nitrite by nitrate-reducing bacteria in the
gastrointestinal tract of infants under appropriate conditions.
Nitrites promote the formation of methaemoglobin from haemoglobin,
thus impairing the transport of oxygen and causing cyanosis.
Methaemoglobin is especially associated with the consumption of
well water with a high nitrate content, and infants appear to be
more susceptible to it than older children and adults (American
Academy of Pediatrics Committee on Nutrition, 1970).
2.3 The Respiratory Tract
Although infants and children may inhale chemical compounds,
information concerning the structure and function of the
respiratory tract in this context is limited. It is known that the
architecture of the respiratory tract continues to develop, at
least until the age of 18 months. Anatomically, 3 processes have
been identified including:
(a) centripetal alveolization giving rise to an extra
generation of respiratory bronchioli;
(b) transformation of some distal respiratory bronchioli
into alveolar ducts; and
(c) further branching of 1 or 2 alveolar duct generations.
After the age of 18 months, the respiratory structures increase
in size with a progressive increase in elastic fibre bundles in the
alveolar wall up to the age of 18 years. The length/diameter
relationship of each respiratory unit remains relatively constant
throughout development. The effects of these architectural changes
is thought to be minimal in the case of inhaled aerosols, but the
position concerning suspended particulates of various sizes has
not been well studied in childhood (Hislop & Reid, 1981).
Numerous chemical substances are found in the atmosphere, and
over 1600 have been identified. Not all of these are man-made, and
many may be derived from a single source (Graedel, 1978). The bulk
of atmospheric pollution is provided by carbon monoxide,
particulates and oxides of sulfur, hydrocarbons, and oxides of
nitrogen. Not all pollutants act directly; for example, the oxides
of nitrogen may exert their effects indirectly, by virtue of their
role in photochemical smog formation (National Research Council,
Subcommittee on Airborne Particles, 1979).
The exposure of infants and children to atmospheric pollutants
may be enhanced compared with that of adults, if the pollutants are
emitted close to the ground or, in the case of aerosols, are gases
or vapours of high density.
2.4 The Skin
Percutaneous absorption of chemical substances is known to
occur through normal skin in both adults and children. There is
little evidence that the rate of absorption varies with age, since
the limiting factor would appear to be the thickness of the
stratum corneum, which is relatively constant throughout postnatal
development after a full-term birth. Different processes of
absorption are thought to exist, but the transcutaneous flux cannot
be predicted with certainty, since marked variations in absorption
rates have been observed between closely related chemical
compounds. While absorption through normal skin does not vary with
age, absorption is greatly enhanced when the skin barrier is
damaged or when the substance is applied under an occlusive
dressing. A problem specific to infants is that napkins (diapers)
serve as an occlusive dressing, which may promote the absorption of
chemicals applied to the buttocks and increase the likelihood of a
systemic effect. Absorption may be further enhanced by skin
reactions in the napkin area.
The prematurely born infant may constitute a special case,
since studies on the development of the stratum corneum in fetal
life suggest that the permeability barrier is incomplete until just
before term (Singer et al., 1971). Thus, pre-term infants bathed
with a solution of hexachlorophene showed greater peak blood
concentrations of the compound than full-term infants subjected to
the same procedure (Greaves et al., 1975).
A pertinent factor in infants is the relatively large surface
area to body weight ratio; thus, compounds absorbed through a given
part of the skin surface will result in greater tissue
concentrations than in an adult (Wester & Maibach, 1982).
2.5 Conclusions
Infants and young children may be exposed to chemicals by the
gastrointestinal, respiratory, and percutaneous routes. The
relative importance of these routes will vary with age according to
the nature of the diet, the behavioural characteristics, and the
maturation of the system involved.
Since infants are obligate milk feeders, they will ingest
chemicals that cross the mammary gland during lactation. These are
potentially important contaminants of both human milk and infant
formulae containing the milk of other species. Similarly, chemicals
contaminating the water used for the reconstitution of powdered
milks will contribute to the ingested burden. Foods and beverages
specially manufactured for infants and young children are also
potentially important sources, especially when the dietary range is
restricted. Oral exploration, hand to mouth activity, and pica
contribute to the risk of ingestion of chemicals encountered in the
domestic environment.
Limited information is available concerning the absorption of
inhaled chemical atmospheric pollutants in relation to the
continuing development of the respiratory tract. However, exposure
of infants and children to atmospheric pollutants may be enhanced
compared with that of adults, when the source of emission is close
to the ground, and under circumstances in which gases or vapour of
high density are involved.
Chemicals can be absorbed through normal skin, but, in infants
and children, the relatively large surface area to body weight
ratio may result in greater tissue concentrations than in the
adult. Percutaneous absorption is enhanced when the skin is
damaged or macerated, or when the substance is applied under an
occlusive dressing such as a napkin (diaper). The prematurely born
infant may constitute a special case, since the permeability of the
skin is greater, because of the incomplete development of the
stratum corneum.
3. KINETICS OF ABSORPTION, BIOTRANSFORMATION, AND ELIMINATION
3.1 Introduction
Because of the morphological, biochemical, and physiological
characteristics of the infant and young child described in section
1, the kinetics of chemicals gaining access to their bodies need
special consideration, particularly with regard to the timing of
the maturation processes governing absorption, distribution,
biotransformation, and elimination from the body.
3.2 Absorption
A chemical may be absorbed through an external (skin) or
internal (gastrointestinal and respiratory tract) surface.
Absorption depends on the physical and chemical characteristics of
the chemical, its concentration gradient, the conditions at the
site of absorption, and the biological characteristics of the
absorptive surface. The conditions at the site of absorption and
the characteristics of the absorptive surface can be expected to
change with age; for example, postnatal changes in pH in different
parts of the gastrointestinal tract and skin, and alterations in
the thickness and structure of membranes of the gastrointestinal
tract and lungs (Hoffmann, 1982) cause changes in perfusion, which
in turn influence the concentration gradient.
3.2.1 Absorption from the gastrointestinal tract
Absorption from the gastrointestinal tract is an important way
in which chemicals enter the body of the infant and young child.
It is influenced by pH, total mucosal surface area, blood supply,
perfusion rate, and period of contact of the chemical with the
absorptive surface, that is the gastric emptying and intestinal
transit time. All these change with postnatal development.
The absorption of some chemicals by infant animals is greater
than that by young adults of the same species. This can be
explained by several factors, including immaturity of the
gastrointestinal tract, the characteristics of the infant's diet,
and the greater proportion of nutrients absorbed. This is of
benefit for growth, but is a disadvantage as far as non-nutritive
chemicals are concerned. Much of the evidence for this comes from
studies on experimental animals exposed to metals. Thus, the adult
rat absorbs 1% or less of lead added to its diet compared with 40 -
50% for the infant animal (Kostial et al., 1971, 1978; Forbes &
Reina, 1972a,b). According to Alexander et al. (1974) and Ziegler
et al. (1978), infants and young children absorb substantially more
lead than adults, but Barltrop & Strehlow (1978) did not find any
age-related differences. The intake of lead by the infants studied
by Alexander et al. (1974) and Ziegler et al. (1978) was greater
than that of the children in the study of Barltrop & Strehlow
(1978), and this may explain the different result.
Methylmercury is well absorbed in the gastrointestinal tract of
infants and young children, as it is in adults (Berlin, 1983).
Some methylmercury is evidently re-excreted into the intestine and,
if this is demethylated by the intestinal bacteria, the mercuric
mercury from it is excreted in the faeces. In mice, there is an
increase in intestinal demethylation at weaning, and the results of
in vitro studies have shown that the microflora in faeces from
young infants receiving milk have negligible demethylating ability
compared with those of weaned children and adults (Rowland et al.,
1983). Thus, it seems likely that more of a dose of methylmercury
will be retained within the body of young infants than of older
children and adults.
3.2.2 Absorption from the lungs
Chemicals permeate the air-blood barrier by simple dif fusion,
but the thickness of the membrane, its lipid composition, surface
area, and porosity determine pulmonary absorption. Lipid-soluble
chemicals are absorbed at similar rates by the lungs of new-born
and adult rats, but hydrophilic chemicals are absorbed at a more
rapid rate by young animals (Hoffmann, 1982).
3.3 Tissue Distribution
3.3.1 Binding of chemicals to proteins
Most of chemicals bind to plasma-proteins. The amount of
binding depends mainly on the concentration and structure of the
binding proteins, the affinity constant, and the presence of other
compounds capable of modifying the chemical-protein interaction.
In the new-born infant, several factors contribute to a lower
plasma-protein binding than in the adult. The concentration of
albumin in the plasma of neonates, and hence the number of binding
sites, is low. Moreover, these binding sites are occupied by
endogenous substances such as fatty acids, steroids, and bilirubin.
Thus, new-born infants have a lower capacity for binding chemicals
to plasma-albumin, and competition at the binding site may lead to
the release of, for example, bilirubin, resulting in kernicterus.
Moreover, hypoxaemia and acidosis may also decrease binding
capacity.
3.3.2 Distribution within the body
From early fetal to adult life, the fluid compartments of the
body change continuously. The larger extracellular space in
infancy (section 1.3.4) provides a larger volume for the
distribution of water-soluble chemicals that do not penetrate the
cells, and plasma concentrations of these chemicals may be lower in
infants than in adults, even if the total body burden per unit body
weight is greater.
Alterations in the morphology, physiology, and biochemistry of
the organs, as well as in their weight relative to the body as a
whole, may affect the distribution of chemicals. The brain is
proportionately larger in the infant than in the adult, and rapid
myelination during the first year after birth may favour the uptake
of lipophilic compounds. Metals, too, are retained in the brain
more readily in infancy than in adulthood. Momcilovic & Kostial
(1974), for example, found that a far greater percentage of the
intake of lead was retained in the brain of the suckling rat than
in that of the adult, and that the rate of removal was slower.
Similarly, Kostial et al. (1978) and Wong & Klaassen (1980) found
that a greater percentage of the intake of cadmium was retained in
the brain of the suckling rat than in that of the adult; Jugo
(1980) made a similar observation for mercury. There is little
direct evidence about the human brain. By analogy with animals, it
seems likely that the concentration of lead in the brains of
infants exposed to lead would rise more rapidly than the
concentration in the brains of older children or adults exposed to
a similar dose.
Methylmercury is known to accumulate in brain tissues,
predominantly in grey matter, but whether the brains of infants and
young children accumulate more than those of adults is not known.
Other tissues take up metals more readily in the early period
of life, when the animal is growing rapidly, than when growth has
slowed down or ceased; this particularly applies to the bones. The
concentration of lead in human bones doubles between infancy and
the late teen years (Barry, 1975).
The liver and kidney also accumulate metals. At birth, the
concentrations of cadmium and mercury are low. Henke et al.
(1970), using neutron activation analysis, determined the cadmium
concentrations in the liver and kidneys of 41 children and adults
and found that they increased 200 fold during the first 3 years
after birth. The total increase in cadmium in the liver and kidney
was greater than appears from the concentration, because both
organs increased considerably in weight.
3.4 Biotransformation of Organic Chemicals
The kinetic behaviour of organic chemicals in living organisms
is generally characterized by several processes modifying the
duration of their action. The limiting factors are either
excretion through the kidneys, bile, or gastrointestinal tract, or
biotransformation mechanisms in the liver or other organs. The
products of biotransformation are generally more polar than the
original compound and, thus, are more readily excreted.
Biotransformation is brought about by enzyme activity, either
associated with microsomal systems in the liver or with enzymes in
the plasma or other tissues.
The biochemical processes involved in the biotransformation of
organic chemicals can be divided into 2 phases:
Phase 1. hydroxylation, dealkylation, dehydrogenation,
reduction, hydrolysis, and other reactions
producing a conjugable grouping; and
Phase 2. biosynthetic reactions, whereby conjugates are
formed with endogenous polar molecules; these
conjugates are, generally, readily excreted.
Both Phase 1 and Phase 2 reactions are generally slower in the
new-born infant than in the adult. Consequently, blood-plasma
half-lives of chemicals in which the degradation and elimination
are dependent on one or the other or both of these types of
reactions are generally appreciably longer in neonates than in
adults.
The rate of maturation of processes concerned with the
biotransformation of organic chemicals varies from one reaction,
and from one chemical, to another. Highest activities are reached
in children aged 12 - 16 years, after which there is generally a
decline. A detailed description, with full documentation, has been
published by Klinger (1982).
3.5 Elimination from the Body
3.5.1 Elimination by the kidneys
The renal function of the full-term infant at birth is immature
by adult standards, and this applies both to glomerular and
tubular function (McCance & Young, 1940; Dean & McCance, 1947b).
The rapid growth of the young infant (section 1.3.3) involves the
retention of nitrogen and minerals, so that the equivalent of only
part of the nitrogen and minerals absorbed from the food requires
to be excreted by the kideys. The breast-fed infant or the infant
fed on a formula similar in composition to breast milk is able to
do this satisfactorily, and so maintain the composition of the body
fluids at their proper level (McCance & Widdowson, 1957a).
However, excessive intakes of nutrients, such as protein
(nitrogen), phosphorus, and sodium, are not excreted completely by
the kidneys of the new-born infant or animal, and the
concentrations in the plasma rise (McCance & Widdowson, 1956,
1957b). Retention of chemicals introduced into the body of the
infant may be due to both renal immaturity and insufficient
biotransformation.
The function of the renal tubules at full-term birth is less
mature than that of the glomeruli, but it matures more rapidly
(Edelmann & Spitzer, 1976). This is important in the young infant
for the chemicals that depend on tubular secretion for their
elimination from the body. Moreover, the reabsorption of chemicals
from the tubular lumen into the tubular cells also varies with age
and depends, in part, on the pH of the urine. Weak organic acids
are reabsorbed more readily by the infant, but, more important, the
low capacity for biotransformation by the infant's liver results in
organic chemicals reaching the kidneys in their original lipophilic
form, and these are not excreted, but reabsorbed into the
circulation (for reviews, see Scheler, 1980; Braunlich, 1981).
Some metals depend on the kidneys for their elimination from
the body, and the results of animal studies suggest that suckling
rats excrete less of their body burden of cadmium, mercury, and
manganese than adults (Kostial et al., 1978). Infants appear to
excrete a smaller proportion of absorbed lead by the renal route
than adults (Ziegler et al., 1978).
3.5.2 Elimination by the liver
The excretion of chemicals in the bile is an important means of
elimination, though some of the chemicals excreted through the bile
may be reabsorbed in the gut and re-enter the body, thus resulting
in their enterohepatic circulation. They cannot penetrate the
epithelium of the biliary duct. Many chemicals are excreted by the
liver as glucuronides in the bile, but the formation of
glucuronides is limited in the new-born infants (section 3.4).
3.5.3 Elimination by other routes
Other routes of excretion include the gastrointestinal tract,
the respiratory tract, and the skin. They are non-regulatory and,
while they may be important for some chemicals, information is
limited about age-related differences in elimination.
3.6 Conclusions
Generally speaking, both organic and inorganic chemicals are
absorbed more readily by the infant than by the adult, but the
organic chemicals undergo biotransformation less readily. For some
chemicals, alternative biochemical pathways may exist.
The kidneys of the neonate are immature and less able than
those of the older child or adult to excrete chemicals, whether
inorganic or the polar products of biotransformations. A greater
proportion of a similar dose of a chemical per unit body weight is
therefore likely to accumulate in the body of the infant, and the
concentration of the chemical in the blood and tissues of the
infant will be higher. The possible effects of this are discussed
in section 4.
4. EFFECTS OF CHEMICALS IN THE BODY
4.1 Introduction
Differences in kinetics can enhance the toxicity of a chemical
in the infant but, in some cases, may result in a qualitative
difference of response or in a lower toxicity, depending on the
intermediary metabolite. In addition, the qualitative and
quantitative response of the target tissues to the same internal
dose of a chemical may be different at various stages of postnatal
development because of the gradual development of characteristics
of the target tissues. A third aspect of developmental toxicology,
which should be recognized, is the fact that a chemical introduced
into the body of an infant or young child may affect the structural
and functional development of a particular organ or system, which
may interfere with the growth and development of the body as a
whole. Animal studies have shown that the toxic effects of certain
chemicals are different at different stages of development.
Moreover, chemicals reaching the body early in life may produce
delayed effects in later years. The response of the growing infant
may therefore be different from that of the fully grown adult.
The problems of extrapolating quantitatively from animals to
man and reaching conclusions on the implications of evidence from
animal studies for human health are well known. These problems are
greater during the new-born period, since the stage of development
at the time of birth varies considerably between species (section
1.4.2). Information from clinical experience of exposure is
limited, and systematic epidemiological data from studies on
infants are sparse, so that the results of studies on animals may
provide the only information available. When information
concerning human infants is available, it should be given
preference.
4.2 Effects of Chemicals on General Growth and Development
General growth and development are possible indicators of the
effects of chemicals on infants and children, whether the exposure
occurred prenatally or postnatally. The principles for evaluating
the effects of prenatal exposure have been considered in an earlier
Environmental Health Criteria publication (WHO, 1984). Factual
information about the effects of postnatal exposure is scarce. In
a study on the infants of mothers who had consumed bread
contaminated with a methylmercury fungicide during lactation, Amin-
Zaki et al. (1981) found abnormal neurological signs in some
infants at the first examination and in more infants on subsequent
occasions. Delayed motor and other aspects of development,
including intelligence, were not detected when the infants were
first seen, but were observed at follow-up examinations during the
next 5 years.
4.3 Effects of Chemicals on Some Organs and Systems
4.3.1 Nervous system
The brain of the infant at birth is not fully developed. The
full number of neurones is not reached until about 2 years of age
(Dobbing & Sands, 1973); myelination is not complete until
adolescence (Hoar & Monie, 1981).
Brain development involves changes in the proportions of myelin
and non-myelinated tissue. Shuman et al. (1975) observed status
spongiosus in the myelin sheaths of the reticular formation of
premature infants, following exposure to hexachlorophene, but no
lesions were present in the non-myelinated part of the brain.
Brain lesions were not produced during the first 8 - 10 days after
birth in the rat. However, between 10 and 25 days, when myelination
takes place, the suckling rats became sensitive to hexachlorophene
(Nieminers et al., 1973; Ulsamer et al., 1975).
There are changes in the sensitivity of the brain of the rat to
hormones or drugs during development, and this has been correlated
with the development of the cell receptors (Macho et al., 1972;
Valcana & Timiras, 1978). On the basis of experimental data, Czaba
(1984) concluded that, during maturation, cell receptors in several
organs require reinforcement by the appropriate hormone at a
critical stage of development. This process can be hindered or
prevented by the presence of molecules analagous to the hormone, or
by a drug or other chemical that binds to the receptor and so
prevents reinforcement by the hormone and hence normal development.
Many confounding factors and co-variables such as nutrition,
socio-economic status, and mental stimulation have to be taken into
account when evaluating the effects of a chemical on the
functioning of the human brain. Only when good information on
these factors is available will it be possible to make a valid
interpretation of the observations on brain function after exposure
to a chemical. This has been discussed most extensively in the
case of lead (United Kingdom Department of Health and Social
Security, 1980; MRC, 1984) (section 3.3.2).
4.3.2 Kidneys
The functional immaturity of the infant's kidneys has already
been discussed (section 3.5.1). Specific reports of their
susceptibility to chemicals are not available, but results of
animal studies suggest that some compounds are less toxic to the
immature kidney than to the mature organ. For example, the same
dose of mercuric chloride per unit body weight that produced renal
failure in adult rats did not have any effect on renal function in
new-born rats (Kavlock & Gray, 1983). Information is lacking on
the effects on renal function of the accumulation of chemicals in
the human kidney during the early years of life (section 3.3.2).
4.3.3 Liver
The pre-term infant may still have foci of extra-medullary
haematopoiesis in its liver, but the histological appearance of the
liver at term is similar to that of the adult. The activity of
enzymes responsible for the biotransformation of chemicals in the
liver of the infant is not equal to that of the adult (section
3.4). However, it appears to be possible to induce the activity of
microsomal enzymes in the liver of neonates to some extent. If
infants have been exposed to phenobarbital, either during
intrauterine life, or immediately after birth, the synthesis of
components of certain microsomal enzyme systems in the liver is
accelerated. The metabolism and urinary excretion of bilirubin is
increased (Maurer et al., 1968; Trolle, 1968; Ramboer et al.,
1969). Increased activities of some drug-metabolizing enzymes have
also been found in new-born infants exposed to phenobarbital
before, or immediately after, birth. Such infants were able to
metabolize drugs such as diazepam more readily than infants not
exposed to phenobarbital, and their urinary excretion of hydroxy
derivatives was greater (Morselli, 1976).
4.3.4 Lungs
Animal studies indicate that some chemicals, such as nitrofen,
retard the development of the lung in the rat fetus (Kimbrough et
al., 1974), but there do not appear to be any reports of effects of
chemicals on the lung specific to infants. It seems that the
response of the infant's lungs to ingested or inhaled chemicals has
not been investigated sufficiently. A greater degree of impairment
of respiratory function might be expected in infants compared with
adults for a given level of exposure, particularly to particles of
substances that result in obstructive symptoms. An excess
mortality rate was found in infants aged less than 12 months
compared with older children and young adults in a population
exposed to atmospheric pollution with sulfur dioxide (Lawther &
Waller, 1975).
4.3.5 Haematopoietic system
The maturation and differentiation of haematopoietic cells in
the bone marrow are processes that continue throughout life, and
represent one of the most rapidly renewing cell populations.
Although the haematopoietic system and the blood are well known to
be the target of many chemical agents, there is little information,
at present, on any specific vulnerability of the developing bone
marrow. An example of the vulnerability of the red cells in young
children is the occurrence of methaemoglobinaemia after exposure
to very low doses of methaemoglobin-forming compounds, e.g.,
nitrates (Kiese, 1974).
4.3.6 Immune system
Studies of immune ontogenesis have revealed that new-born
mammals do not possess fully competent immune defence systems.
While human infants have more highly developed immune systems than
some other mammals at birth, the human infant is still
immunologically immature.
The development of the immune system in mammals involves a
sequence of steps that begins early in gestation. Perturbation or
abrogation of this developmental sequence of events can lead to
immune dysfunctions that may be life-threatening. Defects in the
development of the immune system, due to heritable changes in the
lymphoid elements, have provided clinical and experimental examples
of the consequences of impaired immune development (for review, see
Heise, 1982).
The most profound effects of chemicals that modulate the immune
system of animals occur when dosing of the compound begins during
the development of the lymphoid system (Vos, 1977). Although there
are no data on the immunological effects resulting from perinatal
exposure of infants to chemicals, the potential for such effects
should not be ignored.
The extent of adverse effects on the immune system resulting
from exposure of adult animals to a wide variety of environmental
chemicals has only recently begun to be appreciated (Vos, 1977).
Even less is known about the magnitude of the immunological effects
resulting from fetal and neonatal exposure to environmental
chemicals. It would be expected that disruption of the normal
sequence of events in the development of the immune system by
chemicals would result in alterations in the competence of the
system. Such alterations may not only lessen the host's ability
to combat infection and neoplasia but may also lead to
hypersensitivity or to a failure in the control of homeostasis,
which may lead to autoimmunity. The immunological effects of
perinatal exposure to chemicals in animals have been reviewed by
Roberts & Chapman (1981) and Schmidt (1984). Exposure during
gestation and/or early in postnatal life to chemicals such as
2,3,7,8-tetrachlorodibenzodioxin (Vos & Moore, 1974; Faith & Moore,
1977; Luster et al., 1980), lead (Luster et al., 1978; Faith et
al., 1979), hexachlorobenzene (Vos et al., 1979), and
benzo( a )pyrene (Urso & Gengozian, 1980) has been shown to affect
the developing immune system of animals. However, no specific data
are available for human infants.
4.3.7 Endocrine system
Chemicals may affect the developing endocrine system directly,
or they may interact with some step of the regulating axis
controlled by the pituitary, hypothalamus, or other part of the
brain. The interaction between a chemical and the neuroendocrine
organs can also affect the reproductive and other systems. Some
pesticides have been shown to possess estrogenic activity
(McLachlan et al., 1981). Exposure of experimental animals in the
early postnatal period to chemicals with androgenic or estrogenic
activity induced disturbances of sex differentiation in the
hypothalamus and acyclic ovarian function in females (Barraclough,
1961, 1966; Takewaki, 1968; Flerko, 1975).
Chemicals that give rise to premature onsent of puberty in
children are of particular importance. Premature thelarche in
large numbers of young children in Puerto Rico was attributed to
estrogens in infant foods containing meat (Saenz de Rodriguez &
Toro-Sola, 1982); the cause of a similar outbreak in an Italian
school (Scaglioni et al., 1978) was not discovered. Exposure to
chemicals with androgenic or estrogenic activity may also affect
growth and final stature.
The development of the regulatory function of the adrenal
glands of experimental animals is altered by the administration of
compounds such as steroids and diazepam to the new-born animal
(Hcik 1969a, 1970; Erdosova et al., 1977), and results in changes
in adrenal function and in the reaction of the pituitary-adrenal
axis to stress stimuli later in life (Levine & Mullins, 1967; Hcik,
1969b, 1970; Libertun & Lau, 1972).
A special risk for new-born infants is an excessive intake of
iodine, leading to an abnormal rate of accumulation in the thyroid
gland. Exposure to high concentrations of iodine or goitrogenic
compounds can alter the function of the thyroid gland, resulting in
a deficient production of thyroid hormones at the time when
appropriate levels of the hormones are important for the maturation
of functions of the brain, liver, and other organs. Premature
infants are particularly prone to the development of hypothyroidism
as a result of iodine overdose (Chabrolle & Rossier, 1978; Malpuech
et al., 1978; L'Allemand et al., 1983).
4.3.8 Skin
The skin is a complex structure, the functional development of
which is incomplete until after puberty. Clinically, the cutaneous
manifestations of disease vary with age, and some disorders are
confined to infants and children. Thus, it would be expected that
age-related differences in the response of the skin to chemicals
would be encountered. Chloracne was found to occur principally in
young children rather than adults in a population exposed to dioxin
(TCDD, 2,3,7,8-tetrachlorodibenzo-para-dioxin), suggesting the
greater susceptibility of the child (Fara et al., 1982).
Chemicals applied to the skin may be absorbed and induce
systemic effects. New-born infants whose skin was treated with an
antibacterial preparation containing 3% hexachlorophene developed
blood levels of the compound similar to those known to cause brain
damage in animals. Similarly, some 40 deaths in French infants in
1972 were attributed to the use of baby powder accidentally
contaminated with 6.6% hexachlorophene (Marzulli & Maibach, 1975).
Some environmental pollutants or contaminants are known to be
readily absorbed through the skin, and this subject has been of
particular interest in paediatric therapeutics. Thus, both fatal
and non-fatal exposures occurred when pentachlorophenol was used in
a laundry room resulting in contamination of napkins used in the
hospital nursery (Armstrong et al., 1969).
4.3.9 Bones and teeth
The bones undergo considerable change during postnatal
development with regard to growth, change in shape, and
composition. Immature bones contain more water and less collagen
in the matrix than mature bones, and the degree of mineralization
is much lower (section 1.3.4) (Table 3). Minerals, including
strontium, caesium, and lead, tend to be deposited in the bones,
together with calcium, during the process of mineralization. Lead
may affect the conversion of 25-hydroxy vitamin D to the 1.25-
hydroxy metabolite in the kidney (for review, see Mahaffey, 1981).
Interference with skeletal development may result from the
action of lead in altering the balance of osteoblastic and
osteoclastic activity, and from the direct action of chemicals such
as fluoride.
The discoloration of developing teeth after administration of
tetracycline is an example of the particular propensity for
accumulation or fixation of chemicals in hard tissues. The results
of studies on primates suggested that excessive exposure to
selenium during the development of the teeth might increase
susceptibility to caries (Bowen, 1972).
4.4 Carcinogenesis
Maternal exposure to carcinogens may lead to exposure of the
fetus through the placenta and to exposure of the infant through
breast milk. The transfer of chemicals to the fetus depends on the
relative molecular mass and solubility of the chemical, non-ionized
and lipid chemicals entering the fetus most readily. Lipophilic
chemicals may be retained in the tissues of the fetus and have been
found in tissues of still-born infants (Curley et al., 1969).
Transplacental transfer of carcinogens has recently been reviewed
(WHO, 1984).
So far, no epidemiological observations on human infants have
been reported in which the occurrence of cancer was associated
solely with exposure to carcinogens in the early postnatal period.
The few existing data come from animal studies and, in most of
these, the route of exposure was parenteral and not through the
mother's milk. For example, the injection of aflatoxin resulted in
liver cancer in mice, but only when administered to new-born
animals (Vesselinovitch et al., 1972).
Within the past decade, it has been increasingly recognized
that not all carcinogens act by the same mechanism. Many of the
environmental chemicals, particularly the halocarbons, are though
to be promoters of carcinogenesis rather than initiators. Many of
these types of chemicals are persistent in the organism and are
secreted in breast milk (section 2.2.1.1) (Appendix I). The
mechanism of action of promoters is not well understood at present,
and effects on health at the low doses received by infants and
young children are not clear.
An intrinsic problem in studying prenatal and postnatal
exposure to carcinogens is the capacity for metabolic activation in
the tissues of the fetal or new-born organism, compared with that
of the adult (section 3.4). In this respect, pronounced species
variations have been shown between rodents and primates, including
man. The perinatal development of different organ systems varies
considerably among species. All these variables make extrapolation
from animals to man extremely difficult.
The significance for human health of early postnatal exposure
to tumour growth promoters or co-carcinogens is not known.
4.5 Conclusions
The structural and functional characteristics of infants and
young children described in sections 1, 2, and 3 might make them
more or less vulnerable than older children and adults, when they
are exposed to chemicals at a similar dose per unit body weight.
However, it should be emphasized that reports of specific effects
are rare. This may be because such specific effects do not exist
or because they have passed largely unnoticed. The examples quoted
here show that exposure to certain chemicals during early postnatal
development can be associated with dose-effect and dose-response
relationships that differ from those resulting from exposure in
later years.
Exposure to chemicals during early postnatal development can
give rise, not only to immediate effects on health, but also to
manifestations resulting from the disturbed maturation of organ
systems and their altered response to other environmental
influences. Depending on the chemical concerned, the vulnerability
of the developing organ systems can be higher or lower than that of
the more mature systems.
It is recommended that, if laboratory tests suggest that the
body burden of a chemical is a cause for concern, follow-up studies
should be made. Children who have been accidentally exposed to
chemicals that might affect the reproductive organs should be
followed prospectively to determine possible effects on puberty and
reproductive capacity. Similarly, if other organ systems are
likely to have been affected, prospective studies should, if
possible, be made to assess functional development, morbidity, and
mortality.
5. MODIFYING FACTORS
Considerable variations exist in the health and nutritional
status of children reared in different social and cultural
environments. The response of children exposed to chemicals may
therefore be modified in certain sections of a population to a
degree that cannot readily be predicted. Although analogous data
for the severity of response to infections, such as measles, are
well documented, information concerning chemical exposure and its
sequelae is limited.
5.1 Nutrition
The intake of chemicals contaminating foods and beverages will
vary with the amount of food consumed, the range and variety of
dietary items selected or available, and the methods of
preparation. Children with high intakes of marine fish will, for
example, have a greater intake of methylmercury from polluted
sources than those in which fish constitutes only a small
proportion of the diet (Turner et al., 1980). Conversely, some
pesticide residues in milk are decreased by heating, drying,
evaporation, or pasteurization, so that less of these substances
will occur in children consuming manufactured or processed milk, as
opposed to the raw product. The duration of milk feeding in
infancy, the method of weaning, and the interval before a normal
adult diet is introduced are important factors that vary greatly
between social, cultural, and ethnic groups.
Specific dietary components may interact with ingested
chemicals, thus modifying their availability for absorption. The
relative solubility of the chemical in the lipid or aqueous
fractions of the diet, and the potential for physical interaction
or adsorption to non-adsorptive mechanisms at the enteral surface
of the gut mucosa, may all have a role in determining the
bioavailability of the ingested chemical. Thus, the absorption of
lead, consumed simultaneously with the diet, is markedly lower
compared with its absorption from aqueous solutions, partly due to
interaction with other dietary components. In animals, and
possibly in man, lead absorption is reduced by diets rich in
calcium and phosphorus, but is increased when the intake of these
nutrients is restricted (Barltrop & Khoo, 1975).
The consequences of nutritional deficiency for the absorption
of chemicals cannot be predicted with certainty. In kwashiorkor,
both the digestive and absorptive functions of the gut may be
impaired and the activation of chemicals during conjugation
reactions reduced, particularly acetylation with amino acids
(Thabrew et al., 1982). On the other hand, specific mineral
deficiencies, for example, a lack of calcium, may stimulate
absorptive mechanisms shared by other, potentially toxic, divalent
cations (Barltrop, 1979). Iron deficiency in children has been
related to enhanced absorption and retention of lead, but the
mechanism involved is uncertain. Interactions between essential
and toxic metals after they have been absorbed into the body have
been described. Zinc, for example, competes with lead for
receptors on the enzyme aminolaevulinic acid dehydratase (Nordberg,
1979), but the significance of this in infants and children is
uncertain.
5.2 State of Health
Several diseases in addition to nutritional disorders may
modify the exposure and response of infants and children to
chemicals. Ill health associated with diminished activity or
mobility of the child will, for example, alter the balance between
exposures related to the indoor as opposed to the external
environment. Conditions requiring the long-term administration of
oral therapeutic agents or modified diets will inevitably alter the
intestinal milieu and hence the absorption of ingested chemicals.
The absorptive ability of the gut is modified in a wide range
of diseases, including the enteropathies and chronic diarrhoeal
states encountered in childhood arising, for example, from acquired
or inherited food intolerance. The steatorrhoea associated with
childhood malabsorptive disorders such as coeliac disease is known
to impair the absorption of lipid-soluble substances such as
cholecalciferol and alpha-tocopherol (Harrison, 1980), and would
therefore be expected to have a similar effect on the absorption of
other lipid-soluble chemical compounds.
Children with chronic renal or hepatic dysfunction will have an
impaired ability to excrete, metabolize, and, in some cases, store
chemicals that have been absorbed, which may therefore accumulate
in the body. Compromised integrity of the dermal or respiratory
epithelium by disease will enhance the potential for the absorption
of chemicals by these routes.
Some children have genetically determined variations in their
ability to metabolize or respond to certain chemicals. Thus,
deficiency of the enzyme glucose-6-phosphate dehydrogenase (EC
1.1.1.49), which is encountered in some sub-populations, is
characterized by a haemolytic reaction in response to various
triggering agents. Such reactions in childhood have been reported
after exposure to chemicals including naphthalene, aniline dyes,
and nitrofurantoin (Willoughby, 1984).
5.3 Social and Cultural Way of Life
There are many complex interactions between environmental
chemicals and the social and cultural ways of life. The effects of
place of residence, mobility, state of health, nutritional status,
and family income cannot always be distinguished without resort to
sophisticated epidemiological methods. Cultural and social
attitudes to child care and supervision differ between
subpopulations and are clearly important in relation to acute or
short-term exposures. The mechanisms whereby class and cultural
differences modify exposure are not always apparent. For example,
the different blood-lead values found in Negro versus Hispanic
children in New York and in Asian versus Northern European children
in the United Kingdom have yet to be adequately explained
(Barltrop, 1982). Family practices with regard to personal
hygiene, and especially handwashing before meals, may be important
determinants of exposure resulting from hand to mouth activity in
children.
5.4 Conclusion
Recognition of social and cultural factors that influence
exposure and modify response will assist in the identification of
children at risk and enable appropriate preventive and remedial
measures to be adopted.
6. GENERAL CONCLUSIONS
1. Infants and young children differ from older children and
adults in some of their morphological, physiological, biochemical,
metabolic, and behavioural characteristics, and in their
nutritional requirements.
2. The response of infants and young children to chemicals may
differ from that of adults by virtue of their biological
characteristics. Moreover, a feature of these responses is that
the processes of development may themselves be modified by exposure
to chemicals.
3. The effects of chemical exposure and their relationship to dose
may vary with age. The expression of the effects of chemicals may
be greater or lesser than in the adult, depending on the chemical
species concerned; the kinetics of its absorption,
biotransformation, and elimination; and the degree of maturation of
target organs and tissues.
4. The characteristics of infants and children are such that they
determine the pattern of exposure to chemicals. The pathways
involved are related to development and vary with the environment
of the child. Thus, skin contact, the consumption of breast milk
or its substitutes, the ingestion of substances other than food,
may each be predominant routes at different stages of development.
5. The exposure, uptake, and effects of chemicals may be modified
by factors that are characteristically encountered in early life,
including the mode of nutrition, social, economic, and cultural
conditions, and disease.
6. The expression of chemical effects may not be immediate, but
may be delayed until a later age. The health of adults may thus be
compromised by chemical exposures in early life.
7. The development of a strategy relating to chemicals detected in
breast milk requires a thorough appraisal of the merits and
disadvantages of the available options. Any risk of continued
exposure to a chemical through breast-feeding has to be balanced
against the risk of infection or nutritional deprivation, should
breast-feeding be curtailed or discontinued.
7. RECOMMENDATIONS
1. When health risks from chemicals are evaluated, the special
characteristics of infants and young children must be recognized.
An appropriate methodology based on the knowledge of these
differences should be used.
2. Preventive measures should take account of the special
conditions of exposure to chemicals characteristic for infants and
young children, including exposure through breast milk.
3. Health care workers should be made aware of the special risks
related to chemical exposure during infancy and childhood.
4. Any increased incidence of a disease possibly related to
chemical exposure should be reported and investigated. Awareness of
this among health care workers should be improved. The
establishment of an appropriate surveillance system should be
considered.
5. Clinical and epidemiological data should be collected following
the inadvertent exposure of infants and young children to
chemicals.
6. Developmental toxicology should be promoted and its methodology
improved in order to increase the predictive power of experimental
animal and other laboratory studies.
7. Studies are urgently needed to evaluate the risk associated
with exposure during infancy and early childhood to the initiators
and promoters of carcinogenesis.
REFERENCES
ALEXANDER, F.W., CLAYTON, B.E., & DELVES, H.T. (1974) Mineral and
trace metal balances in children receiving normal and synthetic
diets. Q. J. Med., 43: 89-111.
AMERICAN ACADEMY OF PEDIATRICS COMMITTEE ON NUTRITION (1970)
Infant methaemoglobinaemia: the role of dietary nitrate.
Pediatrics, 46: 475-478.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., & GREENWOOD, M.R. (1974) Studies of infants
postnatally exposed to methylmercury. J. Pediat., 85: 81-84.
AMIN-ZAKI, L., EL HASSANI, S.B., MAJEED, M.A., CLARKSON, T.W.,
DOHERTY, R.A., GREENWOOD, M.R., & GIOVANOLI-JAKUBCZAK, T.
(1976) Perinatal methylmercury poisoning in Iraq. Am. J. Dis.
Child., 130: 1070-1076.
AMIN-ZAKI, L., MAJEED, M.A., GREENWOOD, M.R., EL HASSANI,
S.B., CLARKSON, T.W., & DOHERTY, R.A. (1981) Am. J. Dis.
Chil., 130: 1070-1076.
ANDERSSON, U., BIRD, A.G., BRITTON, S., & PALACIOS, R.
(1981) Humoral and cellular immunity in humans studied at the
cell level from birth to two years of age. Immunolog. Rev.,
57: 5-38.
ARMSTRONG, R.W., EICHNER, E.R., KLEIN, D.E., BARTHEL, W.F., ET
AL. (1969) Pentachlorophenol poisoning in a nursery for
new-born infants. II. Epidemiological and toxicological
studies. J. Pediat., 75: 317-325.
BARLTROP, D. (1966) The prevalence of pica. Am. J. Dis.
Child., 112: 116-123.
BARLTROP, D. (1979) Metal-nutrient interactions in lead
absorption. In: Di Ferrante, E., ed. Trace metals: exposure
and health effects, Oxford, CEC/Pergamon Press, pp. 158-161.
BARLTROP, D. (1982) Nutritional and maturational factors
modifying the absorption of inorganic lead from the
gastrointestinal tract. In: Environmental factors in human
growth and development, New York, Cold Spring Harbor
Laboratory, pp. 35-41 (Banbury Report No. 11).
BARLTROP, D. & KHOO, H.F. (1975) The influence of
nutritional factors on lead absorption. Postgrad. Med. J., 51:
795-800.
BARLTROP, D. & OPPE, T.E. (1970) Dietary factors in neonatal
calcium homeostasis. Lancet, 2: 1333-1335.
BARLTROP, D. & STREHLOW, C.D. (1978) The absorption of lead
by children. In: Kirchgessner, M., ed. Trace element
metabolism in man and animals, Munich, Technical University of
Munich, Freising-Weihenstephan, pp. 332-334.
BARR, H.M., STREISSGUTH, A.P., MARTIN, D.C., & HERMAN, C.S. (1984)
Infant size at 8 months of age: relationship to maternal use of
alcohol, nicotine, and caffeine during pregnancy. Paediatrics, 74:
336-341.
BARRACLOUGH, C.A. (1961) Production of anovulatory sterile rats
by single injection of testosterone propionate. Endocrinology, 68:
62-67.
BARRACLOUGH, C.A. (1966) Modification in the CNS regulation
of reproduction after exposure of prepubertal rats to steroid
hormones. Recent. Progr. Horm. Res., 22: 503-539.
BARRY, P.S.I. (1975) A comparison of concentrations of lead
in human tissues. Br. J. ind. Med., 32: 119-139.
BERLIN, M. (1983) Toxicokinetics of mercury. In: Schmidt,
E.H.F. & Hildebrandt, A.G., ed. Health evaluation of heavy
metals in infant formula and junior food, Berlin, Springer
Verlag, pp. 147-161.
BOWEN, W.H. (1972) The effects of selenium and vanadium on
caries activity in monkeys (M. irus). J. Irish Dent. Assoc.,
18: 83-89.
BRAUNLICH, H. (1981) Excretion of drugs during postnatal
development. Pharmac. Ther., 12: 299-320.
BRITISH PAEDIATRIC ASSOCIATION (1956) Hypercalcaemia in
infants and vitamin D. Br. med. J., 2: 149.
CHABROLLE, J.P. & ROSSIER, A. (1978) Goitre and
hypothyroidism in the newborn after cutaneous absorption of
iodine. Arch. Dis. Child., 53: 495-498.
CLARKSON, T.W., AMIN-ZAKI, L., & AL-TIKRITI, S.K. (1976) An
outbreak of methylmercury poisoning due to consumption of
contaminated grain. Fed. Proc., 35: 2394-2399.
COLLIPP, P.J., CHEN, S.Y., & MAITINSKY, S. (1983) Manganese
in infant formulas and learning disability. Ann. Nutr. Metab.,
27: 488-494.
CURLEY, A., COPELAND, R., & KIMBROUGH, R.D. (1969)
Chlorinated hydrocarbon insecticides in organs of stillborn
and blood of new-born babies. Arch. environ. Health, 19:
628-632.
CZABA, G. (1984) The present state in the phylogeny and
ontogeny of hormone receptors. Horm. Metab. Res., 16: 329-335.
DEAN, R.F.A. & MCCANCE, R.A. (1947a) Response of new-born
children to hypertonic solutions of sodium chloride and urea.
Nature (Lond.), 160: 904-906.
DEAN, R.F.A. & MCCANCE, R.A. (1947b) Inulin, diodone,
creatinine, and urea clearances in new-born infants. J.
Physiol., 106: 431-439.
DEAN, R.F.A. & MCCANCE, R.A. (1948) Phosphate clearances in
infants and adults. J. Physiol., 107: 182-186.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1984) [Residues and
contaminants in breastmilk,] Weinheim, Verlag Chemie (in
German).
DICKERSON, J.W.T. (1962a) The effect of development on the
composition of a long bone of the pig, rat, and fowl. Biochem.
J., 82: 47-55.
DICKERSON, J.W.T. (1962b) Changes in the composition of the
human femur during growth. Biochem. J., 82: 56-61.
DIEM, K. & LENTNER, C., ed. (1970) Scientific tables, 7th
ed., Basel, J.R. Geigy A.G.
DOBBING, J. & SANDS, J. (1973) The quantitative growth and
development of the human brain. Arch. Dis. Child., 48: 757-767.
DORNER, G. & STAUDT, J. (1972) [Comparative morphology of
the hypothalamus: differences between the rat and man.]
Endokrinologie, 59: 152-155 (in German).
EDELMANN, C.M., Jr & SPITZER, A. (1976) The kidney. In:
Smith, C.M. & Nelson, N.M., ed. The physiology of the new-born
infant, 4th ed., Springfield, Illinois, pp. 416-458.
EPPS, E.A., Jr, BONNER, F.L., & COWART, R.P. (1974) Dieldrin
contamination of milk by use of second-hand bags for feed.
Bull. environ. Contam. Toxicol., 12: 209-211.
ERDOSOVA, R., KRAUS, M., & REHULKA, J. (1977) Corticosterone
synthesis and serum levels at the end of the perinatal period:
a study of the effects of stress, diazepam, and polyethylene
glycol treatments. Physiol. bohemoslov., 26: 297-302.
FAITH, R.E. & MOORE, J.A. (1977) Impairment of thymus-
dependent immune functions by exposure of the developing
immune system to 2,3,7,8-tetrachlorobenzeno-p-dioxin (TCDD).
J. Toxicol. environ. Health, 3: 451-464.
FAITH, R.E., LUSTER, M.I., & KIMMEL, C.A. (1979) Effect of
chronic developmental lead exposure on cell-mediated immune
functions. Clin. exp. Immunol., 35: 413-420.
FARA, G.M., DEL CORNO, G., BONETTI, F., CARAMASCHI, L.,
DARDANONI, L., FAVARETTI, C., JIAMBELLUCA, S.E., MARNI, E.,
MOCARELLI, P., MONTESARCHIO, E., PUCCINELLI, V., & VOLPATO,
C. (1982) Chloracne after release of TCDD at Seveso, Italy.
In: Hutzinger, O., Frei, R.W., Merian, E., & Pocchiari, F.,
ed. Chlorinated dioxins and related compounds, Oxford,
Pergamon Press, pp. 545-560.
FLERKO, B. (1975) Perinatal androgen action and the
differentiation of the hypothalamus. In: Brazier, M.A.B., ed.
Growth and development of the brain, New York, Raven Press,
pp. 117-137.
FORBES, G.B. & REINA, J.C. (1972a) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. Health
Phys., 22: 169-175.
FORBES, G.B. & REINA, J.C. (1972b) Effect of age on
gastrointestinal absorption (Fe, Sr, Pb) in the rat. J. Nutr.,
102: 647-652.
GIACOIA, G.P. & CATZ, C.S. (1979) Drugs and pollutants in
breast milk. In: Clinics in perinatology, Vol. 6, pp. 181-196.
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Contamination of milk with hexachlorobenzene residues.
Ind. Aliment. Agric., 89: 31-35.
GRAEDEL, T.E. (1978) Chemical compounds in the atmosphere,
London, Academic Press.
GREAVES, S.J., FERRY, D.G., MCQUEEN, E.G., MALCOLM, D.S., &
BUCKFIELD, P.M. (1975) Serial hexachlorophene blood levels
in the premature infant. N.Z. med. J., 81: 334-336.
HACIK, T. (1969a) Effect of estradiol on adrenal and gonadal
functions during postnatal development in female rats. Endocr.
Exper., 3: 147-153.
HACIK, T. (1969b) Postnatal development of androgenized
female rats. Physiol. bohemoslov., 18: 263-269.
HACIK, T. (1970) Effect of estrogens on adrenal function and
body growth during postnatal period in male rats. Endocr.
Exper., 4: 31-38.
HAERING, M. & SCHEFER, W. (1980) Quantitative analysis of
biocide residues. Chimia, 34: 397-400.
HARRISON, H.E. (1980) Vitamin and mineral deficiencies in
intestinal malabsorption. In: Lifshitz, F., ed. Clinical
disorders in pediatric gastorenterology and nutrition, New
York, Dekker, pp. 371-375.
HEISE, E.R. (1982) Diseases associated with immuno-
suppression. Environ. Health Perspect., 43: 9-19.
HENKE, G., SACHS, H.W., & BOHN, G. (1970) [Determination of
cadmium in liver and kidneys of children and adolescents by
neutron activation analysis.] Arch. Toxicol., 26: 8-16 (in
German).
HILL, J.R. (1964) The development of thermal stability in
the new-born baby. In: Jonxis, J.H.P., Visser, H.K.A., &
Troelstra, J.A., ed. The adaptation of the newborn to
extrauterine life, Netherlands, H.E. Stenfert Kroese NV, pp.
223-228.
HISLOP, A. & REID, L. (1981) Growth and development of the
respiratory system. In: Davis, J.A. & Dobbing, J., ed.
Paediatrics, 2nd ed., London, Heinemann, pp. 390-432.
HOAR, R.M. & MONIE, I.W. (1981) Comparative development of
specific organ systems. In: Kimmel, C.A. & Buelke-Sam, J., ed.
Developmental toxicology, New York, Raven Press, pp. 13-33.
HOFFMANN, H. (1982) Absorption of drugs and other
xenobiotics during development in experimental animals.
Pharmac. Ther., 16: 247-260.
ISLEIB, D.R. & WHITEHEAD, G.L. (1975) Polybrominated
biphenyls: an agricultural accident and its consequences. I.
The agricultural effects of exposure. In: Hemphill, D.D., ed.
Trace substances in environmental Health. IX, pp. 47-55.
JAPAN/US COOPERATIVE SCIENCE PROGRAM (1983) Toxicity of
chlorinated biphenyls, dibenzofurans, dibenzodioxins, and
related compounds. In: Proceedings of a Joint Seminar,
Fukvoka, Japan, pp. 25-28.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Res. Rev., 89: 1-128.
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
JONES, K.L. & SMITH, D.W. (1975) The fetal alcohol syndrome.
Teratology, 12: 11-26.
JUGO, S. (1980) Chelatable fraction of 203Pb in blood of
young and adult rats. Environ. Res., 21: 336-342.
KAVLOCK, R.J. & GRAY, J.A. (1983) Morphometric, biochemical,
and physiological assessment of perinatally induced renal
dysfunction. J. Toxicol. environ. Health, 11: 1-3.
KIESE, M. (1974) Methaemoglobinaemia: a comprehensive
treatise, Ohio, CRC Press, pp. 17-19.
KIMBROUGH, R.D., TAINES, T.V., & LINDER, R.E. (1974) The
effect of 2,4-dichlorophenyl- p -nitrophenyl ether on the rat
fetus. Arch. environ. Health, 28: 316-320.
KITAMURA, Y., MIYAO, M., & KAWAZOE, K., ET AL. (1953) In:
Friberg, L., ed. Toxic metals and their implications for human
health. Acta med., 8: 205-225.
KLINGER, W. (1982) Biotransformation of drugs and other
xenobiotics during postnatal development. Pharmac. Ther., 16:
377-429.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
KOSTIAL, K., SIMONOVIC, I., & PISONIC, M. (1971) Lead
absorption from the intestine in new-born rats. Nature
(Lond.), 233: 564.
KOSTIAL, K., KELLO, D., JUGO, S., RABAR, I., & MALJKOVIC, T.
(1978) The influence of age on metal metabolism and toxicity.
Environ. Health Perspect., 25: 81-86.
KROGER, M. (1972) Insecticide residues in human milk.
Pediatrics (Springfield), 80: 401-405.
KURATSUNE, M. (1980) "Yusho". In: Kimbrough, R.D., ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzo-
dioxins, and related products, Amsterdam, Elsevier/North
Holland Biomedical Press, pp. 287-302 (Topics in Environmental
Health No. 4).
L'ALLEMAND, D., GRUTERS, A., HEIDEMANN, P., & SCHURNBRAND, P.
(1983) Iodine-induced alterations of thyroid function in
new-born infants after prenatal and perinatal exposure to
povidone iodine. J. Pediatr., 102: 935-938.
LANDSBERG, J.D., BODYFELT, F.W., & MORGAN, M.E. (1977)
Retention of chemical contaminants by glass, polyethylene, and
polycarbonate multi-use containers. J. Food Protect., 40:
772-777.
LANGSTON, C. (1983) Normal and abnormal structural
development of the human lung. In: Abnormal functional
development of the heart, lungs, and kidneys: approaches to
functional teratology, New York, Alan R. Liss, pp. 75-91.
LAWTHER, P.J. & WALLER, R.E. (1975) Physical hazards. In:
Barltrop, D., ed. Paediatrics and the environment. Fellowship
of Postgraduate Medicine/Unigate Paediatric Workshop No. 2,
London, pp. 5-9.
LEVINE, S. & MULLINS, R. (1967) Neonatal androgen or
estrogen treatment and the adrenal cortical response to stress
in adult rats. Endocrinology, 80: 1177-1179.
LIBERTUN, C. & LAU, C. (1972) Adrenocortical function in
prepuberal rats: neonatal effects of testosterone. J. Endocr.,
55: 221-222.
LOIZZO, A., GATTI, G.L., MACRI, A., MORRETTI, G., ORTOLANI,
E., & PALAZZESI, S. (1984) Italian baby food containing
diethylstilboestrol: three years later. Lancet, 1: 1014-1015.
LUSTER, M.I., FAITH, R.E., & KIMMEL, D.A. (1978) Depression
of humoral immunity in rats following chronic developmental
lead exposure. J. environ. Pathol. Toxicol., 1: 397-402.
LUSTER, M.I., BOORMAN, G.A., DEAN, J.H., HARRIS, M.W., LUEBKE,
R.W., PADARATHSINGH, M.L., & MOORE, J.A. (1980) Examination
of bone marrows, immunological parameters, and host suscept-
ibilities following pre- and postnatal exposure to 2,3,7,8-
tetrachlorodibenzo- p -dioxin (TCDD). Int. J. Immunopharmacol.,
2: 301-310.
MCCANCE, R.A. (1948) Renal function in early life. Physiol.
Rev., 28: 331-348.
MCCANCE, R.A. & WIDDOWSON, E.M. (1956) Metabolism, growth,
and renal function of piglets in the first days of life. J.
Physiol., 133: 373-384.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957a) New thoughts on
renal function in the early days of life. Br. med. Bull., 13:
3-6.
MCCANCE, R.A. & WIDDOWSON, E.M. (1957b) Hypertonic expansion
of the extracellular fluids. Acta paediatr., 46: 337-353.
MCCANCE, R.A. & YOUNG, W.F. (1940) The secretion of urine by
new-born infants. J. Physiol., 99: 265-282.
MCLACHLAN, J.A., NEWBOLD, R.R., KORACH, K.S., LAMB, J.C., &
SUZUKI, Y. (1981) Transplacental toxicology: prenatal
factors influencing postnatal fertility. In: Kimmel, C.A. &
Buelke-Sam, J., ed. Developmental toxicology, New York, Raven
Press, pp. 213-232.
MACHO, L., STRBAK, V., & HROMADOVA, M. (1972) The effect of
thyroxine on amino acid incorporation into protein during
ontogenesis in rats. Hormones, 3: 354-360.
MAHAFFEY, K.R. (1981) Nutritional factors in lead poisoning.
Nutrit. Rev., 39: 353-362.
MAHAFFEY, K.R. (1983) Absorption of lead by infants and
young children. In: Schmidt, E.H.F. & Hildebrandt, A.G., ed.
Health evaluation of heavy metals in infant formula and junior
food, Berlin, Springer Verlag, pp. 69-85.
MALPUECH, G., GAILLARD, G., GAULME, J., DOLY, M., BESSE, G.,
GOUMY, P., & RAYNAUD, E.J. (1978) Hypothyroidie transitoire
chez huit nouveau-nes de petit poids de naissance. Arch.
Franc. Ped., 35: 620-630.
MARWHA, H., NAYAK, N.C., ROY, S., KALRA, V., & GHAI, O.P.
(1981) The role of excess hepatic copper in the evaluation of
Indian childhood cirrhosis. Indian J. med. Res., 73: 395-403.
MARZULLI, F.N. & MAIBACH, H.I. (1975) Relevance of animal
models: the hexachlorophene story. In: Maibach, H., ed. Animal
models in dermatology, New York, Churchill Livingstone, pp.
156-167.
MAURER, H.M., WOLFF, J.A., FINSTER, M., POPPERS, P.J.,
PANTUCK, E., KUNTZMAN, R., & CONNEY, A.H. (1968) Reduction
in concentration of total serum-bilirubin in offspring of
women treated with phenobarbitone during pregnancy. Lancet, 2:
122-124.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
MIYABE, M., TSUBOUCHI, H., & SAKABE, Y. (1971) Studies on
pesticide residues in foods. II. Residues of BHC in milk. Rep.
Nagoya Munic. Pub. Health Lab., 18: 37-41.
MOMCILOVIC, B. & KOSTIAL, K. (1974) Kinetics of lead
retention and distribution in suckling and adult rats.
Environ. Res., 8: 214-220.
MORSELLI, P.L. (1976) Clinical pharmacokinetics in neonates.
Clin. Pharmacokin., 1: 81-98.
MRC (1984) The neuropsychological effects of lead in
children: a review of recent research, (1979-83), London,
Medical Research Council.
NATIONAL RESEARCH COUNCIL, SUBCOMMITTEE ON AIRBORNE PARTICLES
(1979) Epidemiologic studies on the effects of airborne
particles on human health. In: Airborne particles, Baltimore,
Maryland, University Park Press, pp. 167-198.
NIEMINERS, L., BJORNDAHL, K., & MOTTONEN, M. (1973) Effect
of hexachlorophene on the rat brain during organogenesis. Food
cosmet. Toxicol., 11: 635-639.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
NORDBERG, G.F. (1979) Factors influencing metabolism and
toxicity of metals. In: Di Ferrante, E., ed. Trace metals:
exposure and health effects, Oxford, CEC/Pergamon Press, pp.
157-158.
PAVEY, D.E. & WIDDOWSON, E.M. (1980) Body lipids of
guinea-pigs exposed to different dietary fats from
mid-gestation to 3 months of age. V. The fatty acid
composition of brain lipids at birth. Nutr. Metab., 24:
357-366.
PETERS, H.A. (1976) Hexachlorobenzene poisoning in Turkey.
Fed. Proc., 35: 2400-2403.
RAMBOER, C., THOMPSON, R.P.H., & WILLIAMS, R. (1969)
Controlled trials of phenobarbitone therapy in neonatal
jaundice. Lancet, 1: 966-968.
ROBERTS, D.W. & CHAPMAN, J.R. (1981) Concepts essential to
the assessment of toxicity to the developing immune system.
In: Kimmel, C.A. & Buelke-Sam, J., ed. Developmental
toxicology, New York, Raven Press, pp. 167-189.
ROGAN, W. & GLADEN, B. (1982) Duration of breast-feeding and
environmental contaminants in milk. Am. J. Epidemiol., 116:
565A.
ROOK, J.J. (1974) Formation of halogens during clorination
of natural waters. J. Water Treat. Exam., 23: 234-243.
ROWLAND, I.R., ROBINSON, R.D., DOHERTY, R.A., & LANDRY, T.D.
(1983) Are developmental changes in methylmercury metabolism
and excretion mediated by the intestinal microflora? In:
Clarkson, T.W., Nordberg, G.F., & Sager, P.R., ed.
Reproductive and developmental toxicity of metals, New York,
Plenum Press, pp. 745-758.
SAENZ DE RODRIGUEZ, C.A. & TORO-SOLA, M.A. (1982) Anabolic
steroids in meat and premature telarche. Lancet, 1: 1300.
SCAGLIONI, S., DI PIETRO, C., BIGATELLO, A., & CHIUMELLO, G.
(1978) Breast enlargement at an Italian school. Lancet, 1:
551-552.
SCHELER, W. (1980) [The basis of general pharmacology,] 2nd
ed., Stuttgart, Gustav Fischer Verlag (in German).
SCHMIDT, R.R. (1984) Altered development of immunocompetence
following prenatal or combined perinatal-postnatal insult: a
timely review. J. Am. Coll. Toxicol., 3: 57-72.
SHERLOCK, J.C., SMART, G., FORBES, G.I., MOORE, M.J.,
PATTERSON, W.J., RICHARDS, W.N., & WILSON, T.S. (1982)
Assessment of lead intakes and dose response for a population
in Ayr exposed to a plumbosolvent water supply. Hum. Toxicol.,
1: 115-122.
SHUMAN, R.M., LEECH, R.W., & ALVORD, E.C., Jr (1975)
Neurotoxicity of hexachlorophene in humans. II. A clinico-
pathological study of 46 premature infants. Arch. Neurol., 32:
320-325.
SINGER, E.J., WEGMANN, P.C., LEHMAN, M.D., CHRISTENSEN, M.S.,
& VINSON, L.J. (1971) Barrier development, ultrastructure,
and sulfhydryl content of the fetal epidermis. J. Soc. Cosmet.
Chem., 22: 119-137.
SMITH, R.J. (1982) Hawaiian milk contamination. Science
N.Y., 217: 137-140.
SVENNERHOLM, L., ALLING, C., BRUCE, A., KARLSSON, I., & SAPIA,
O. (1972) Effects on offspring of maternal malnutrition in
the rat. In: Elliott, K. & Knight, J., ed. Lipids,
malnutrition, and the brain, Amsterdam, Associated Scientific
Publishers, pp. 141-157.
TADJER, G.S. & DORE; I. (1971) Possibility of contamination
of bottles used by food industries by insecticides. Harefuah,
81: 385.
TAKEWAKI, K. (1968) Reproductive organs and anterior
hypophysis of neonatally androgenized female rats. Sci. Rep.
Tokyo Women's Christ. Coll., 1-6: 31-47.
TANNER, J.M., WHITEHOUSE, R.H., & TAKAISHI, M. (1966)
Standards from birth to maturity for height, weight, height
velocity, and weight velocity. Part II. British children,
1965. Arch. Dis. Child., 41: 613-635.
THABREW, M.W., OLORUNSOGO, O.O., OLOWOOKERE, J.O., &
BABABUNMI, E.A. (1982) Possible defect in xenobiotic
activation before glycine conjugation in protein-energy
malnutrition. Xenobiotica, 12: 849-853.
TROLLE, D. (1968) Phenobarbitone and neonatal icterus.
Lancet, 1: 251-252.
TURNER, M.D., MARSH, D.O., SMITH, J.C., INGLIS, J.B.,
CLARKSON, T.W., RUBIO, C.E., CHIRIBOGA, J., & CHIRIBOGA, C.C.
(1980) Methylmercury in populations eating large quantities
of marine fish. I. Northern Peru. Arch. environ. Health,
35(6): 367-378.
ULSAMER, A.G., YODER, P.D., KIMBROUGH, R.D., & MARZULLI, F.N.
(1975) The effect of hexachlorophene on developing rats:
toxicity tissue concentrations and biochemistry. Food cosmet.
Toxicol., 13: 69-80.
UMBERGER, E.J. (1975) Products marketed to promote growth in
food-producing animals: steroid and hormone products.
Toxicology, 3: 3-21.
UNITED KINGDOM CENTRAL DIRECTORATE ON ENVIRONMENTAL POLLUTION
(1982) The Glasgow duplicate diet study (1979/80), London,
Her Majesty's Stationery Office (Pollution Report No. 11).
UNITED KINGDOM DEPARTMENT OF HEALTH AND SOCIAL SECURITY
(1980) Lead and health, London, Her Majesty's Stationery
Office (Report of a DHSS Working Party on Lead in the
Environment).
URSO, P. & GENGOZIAN, N. (1980) Depressed humoral immunity
and increased tumour incidence in mice following in utero
exposure to benzo( a )pyrene. J. Toxicol. environ. Health, 6:
569-576.
US DHEW (1979) Smoking and health: a report of the Surgeon
General, Washington DC, US Department of Health, Education and
Welfare (Section No. 8).
VALCANA, T. & TIMIRAS, P.S. (1978) Nuclear triiodothyronine
receptors in the developing rat brain. Mol. Cell. Endocrinol.,
11: 31-41.
VALE, J.A. & MEREDITH, T.J. (1981) Epidemiology of poisoning
in the UK. In: Vale, J.A. & Meredith, T.J., ed. Poisoning
diagnosis and treatment, 1st ed., London, Update Books, pp.
1-8.
VESSELINOVITCH, S.D., MIHAILOVICH, N., WOGAN, G.N., LOMBARD,
L.S., & RAO, K.V.N. (1972) Aflatoxin B1, a hepato-
carcinogen in the infant mouse. Cancer Res., 32: 2289-2291.
VILLETT, L.B. & HESS, J.F., Jr (1975) Polychlorinated
biphenyl residues in silos in the US. Residue Res., 55:
135-147.
VOS, J.G. (1977) Immune suppression as related to
toxicology. CRC Crit. Rev. Toxicol., 5: 67-101.
VOS, J.G. & MOORE, J.A. (1974) Suppression of cellular
immunity in rats and mice by maternal treatment with
2,3,7,8-tetrachlorodibenzo- p -dioxin. Int. Arch. Allergy appl.
Immunol., 47: 777-794.
VOS, J.G., VAN DOGTEN, M.J., KREEFTENBERG, J.G., STEERENBERG,
P.A., & KRUIZINGA, W. (1979) Effect of hexachlorobenzene on
the immune system of rats following combined pre- and
postnatal exposure. Drug Chem. Toxicol., 2: 61-76.
WALKER, W.A. (1979) Gastrointestinal host defence:
importance of gut closure in control of macromolecular
transport. In: Elliott, K. & Whelan, J., ed. Development of
mammalian absorptive processes, Amsterdam, Excerpta Medica,
pp. 201-216 (Ciba Foundation Symposium No. 70 (new series)).
WESTER, R.C. & MAIBACH, H.I. (1982) Percutaneous absorption:
neonate compared to the adult. In: Hunt, V.R., Smith, M.K., &
Worth, D., ed. Environmental factors in human growth and
development, New York, Cold Spring Harbor Laboratory (Banbury
Report No. 2).
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1984) Environmental Health Criteria 30: Principles for
evaluating health risks to progeny associated with exposure to
chemicals during pregnancy, Geneva, World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WIDDOWSON, E.M. & DICKERSON, J.W.T. (1964) Chemical
composition of the body. In: Comar, C.L. & Bronner, F., ed.
Mineral metabolism, New York, Academic Press, Vol. 2A, pp.
1-247.
WIDDOWSON, E.M., CRABB, D.E., & MILNER, R.D.G. (1972)
Cellular development of some human organs before birth. Arch.
Dis. Child., 47: 652-655.
WILLOUGHBY, M.L.N. (1984) Disorders of the blood and
reticuloendothelial system. In: Forfar, J.O. & Arneil, G.C., ed.
Textbook of paediatrics, 3rd ed., Edinburgh, Churchill
Livingstone, Vol. 1, pp. 935-982.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
WONG, K.L. & KLAASSEN, C. (1980) Tissue distribution and
retention of cadmium in rats during postnatal development:
minimal role of hepatic metallothionein. Toxicol. appl.
Pharmacol., 53: 343-353.
ZIEGLER, E.E., EDWARDS, B.B., JENSEN, R.L., MAHAFFEY, K.R., &
FOMON, S.J. (1978) Absorption and retention of lead by
infants. Pediatr. Res., 12: 29-34.
APPENDIX I: BREAST MILK
1. Introduction
The breast-fed infant requires special consideration in
circumstances in which chemical contamination of the mother's milk
is suspected, or has been confirmed. Young infants are
characteristically dependent on milk, and the structural and
functional development of their organs and tissues is adapted to
the milk of their own species. The potential risks of exposure to
chemicals in breast milk must therefore be assessed, taking into
account the benefits of breast-feeding together with the
disadvantages of feeding an alternative milk, in terms of
developmental toxicology.
The benefits of breast-feeding, particularly in the developing
countries, are now universally acknowledged. In these societies,
nearly all women of all classes breast-feed for 12 months or more.
The decline in breast-feeding in many developed countries has
recently been reversed; interestingly, both the decline in, and
return to, breast-feeding began among the most socially advantaged
women (WHO, 1981). National and international authorities have
been concerned that, in view of the health risks involved in the
preparation and provision of breast milk substitutes in developing
societies, mothers in these countries should not go through the
changes in infant feeding practice that have occurred in the
developed world. Thus, the Member States of the World Health
Organization have committed themselves to the protection and
promotion of breast-feeding as the first, and one of the most
critical, steps in sound infant nutrition policies (WHO/UNICEF,
1981).
This Appendix is concerned with principles for evaluating the
possible consequences to health of chemical contaminants in breast
milk. The evaluation of risks potentially associated with these
chemicals in the quantities that may be consumed must be carefully
weighed against the assessment of the risks of alternative methods
of infant feeding.
The breast milk of a well-nourished mother has an optimal
composition to meet the nutritional needs of the full-term infant
in early life, and there are associated immunological,
psychological, and economic advantages. In view of the importance
of breast milk for the nutrition and health of the infant, evidence
of any harmful effects of a foreign chemical that it may contain
must be carefully considered before recommendations are made that
breast-feeding should be curtailed or discontinued.
2. Assessment of the Importance of Chemicals in Breast Milk
2.1 Source of chemicals
Women may be exposed to chemical substances from many different
sources, including air, food, water, drugs, cosmetics, and the
occupational environment.
2.2 Chemicals within the mother's body and their secretion in milk
Chemicals that are easily metabolized and are eliminated from
the mother's body in the faeces and urine are only secreted in
significant quantities in breast milk following recent high-level
chemical exposure (Wolff, 1983; Jensen, 1985).
Other chemicals, such as heavy metals and persistent
halocarbons, are less readily removed from the body. Some metals
accumulate mainly in the liver, kidney, and bone, whereas
persistent halocarbons accumulate in the lipids of the body. Long-
term exposure, even to low concentrations of such chemicals, may
build up considerable body stores. On the other hand, a short-term
change of diet is not likely to alter the concentration of
halocarbon in the mother's fatty tissues.
A dynamic equilibrium for persistent lipophilic chemicals
exists between different tissue compartments. The concentrations
of these chemicals in adipose tissue, blood, and milk is in
proportion to the fat content; thus, the concentration in blood is
far lower than that in whole milk, and the concentration in milk is
far lower than that in adipose tissue. There is a relatively good
correlation between concentrations of organic halocarbons in the
lipids of blood, adipose tissue, and milk. This is illustrated for
DDT in the lipids of milk and adipose tissue in Fig. I.1.
 2.3 Development and limits of methods for measuring chemicals in
milk
During the 1960s and 1970s, techniques such as gas
chromatography were developed, which increased the scope and
accuracy of analytical methods for the determination of chemicals
in breast milk. Studies were made in various countries, and many
different halocarbons were detected. The sensitivity of the
methods was greatly increased with the introduction of glass
capillary columns in gas chromatography, high resolution liquid
chromatography, and mass spectrometry, and it is now possible to
detect some environmental chemicals in human milk fat at the ng/kg
level (Jensen, 1985). Similarly, the sensitivity of methods for
measuring heavy metals has also greatly increased in recent years
with the introduction of electrothermal atomic absorption
spectrophotometry, mass spectrometry, and neutron activation
techniques.
These newly developed methods require specialized laboratory
staff and facilities, as well as quality assessment programmes, to
ensure the accuracy and precision of results. The analyses are
often time-consuming and costly to perform. This limits the number
that can be made, and this must be taken into account when
developing strategies and requirements for the determination of
chemicals in breast milk.
2.4 Risks to the infant
The presence of chemicals in breast milk must be seen in
perspective. Because a chemical has been detected and measured, it
does not mean that it is necessarily harmful to the infant in the
quantities consumed.
The magnitude of chemical burdens derived from breast milk
cannot be assessed in isolation but must be related to those
obtained from other routes of exposure. Additional contributions
to the uptake of chemicals by the infant may occur through the
percutaneous and respiratory pathways and, in some cases, there is
a pre-existing burden sustained in utero from placental transfer.
The evaluation of the risks associated with a given chemical in
breast milk requires knowledge of:
(a) intake of the chemical by the infant;
(b) availability for absorption from the infant
intestinal tract;
(c) kinetics of uptake, transport, distribution,
metabolism, excretion, and net retention by the
infant body;
(d) effects on developing organs, tissues, and systems of
the young infant; and
(e) long-term sequelae in later childhood and adult life.
These considerations are discussed in sections 3 and 4 of this
publication. Information about most of them is incomplete.
In breast-fed infants, the concentrations of halocarbons in the
blood, for example, polychlorobiphenyls (PCBs), were found to
increase after birth and reached the maternal levels after 3
months; the increase continued until breast feeding was stopped;
thereafter, the concentrations of halocarbons decreased (Kodama &
Ota, 1980). An association between breast-feeding and high
concentrations of persistent halocarbons in the body fat of infants
has also been observed (Niessen et al., 1984), and it has been
calculated that, within a few months of birth, the concentration of
halocarbons in the fat of the infant may be equal to that in the
maternal fat (Wickizer et al., 1981; Mes et al., 1984). Although
the period of breast-feeding is short in relation to the life span,
the chemicals accumulated in the body during these months may be
retained for many years (Jensen, 1985).
At present, there appears to be only one preliminary report of
a prospective study in which 850 breast-fed infants were
periodically examined, and the milk was analysed for halocarbons
(Rogan & Gladen, 1982). The main result at the time the report was
written was that the mothers with the greatest concentration of
2,2 bis(4-chlorophenol)-1,1-dichloroethylene (DDE) in their milk
did not breast-feed for as long as those with the least, and they
gave their infants formula at an earlier age.
2.5 Assessment of contamination of breast milk following
maternal exposure
The purpose of investigating the chemical contamination of
breast milk in the context of this publication is to assess the
possible harm to infants if they continue to take milk from exposed
mothers. The daily intake of a contaminant depends on the volume
of breast milk taken and on the concentration of the chemical in
the milk. At all ages, the intake of milk by a full-term breast-
fed infant varies considerably between individuals. This depends
partly on body weight; 150 ml/kg body weight per day can be taken
as a representative intake, after the first week. This gradually
falls after 2 months to about 120 ml/kg per day at 4 - 6 months.
The concentration of lipophilic chemicals in milk is clearly
related to its fat content, and this varies with the stage of
lactation, the maternal diet, the time of day, and during the
course of a single feed. There is also a variation in
concentration of protein-bound and water-soluble chemicals at
different stages of lactation. Thus, the infant's intake of
chemicals is not constant from day to day, or within one day, or
even within a particular feed. How then should samples of breast
milk be collected for analysis to obtain a reasonable figure
representing the infant's intake of the chemical in question? If
the chemical is lipid-soluble, much of the difficulty is removed if
the percentage of fat is also determined and the concentration of
the chemical expressed per unit weight fat. Then, the following
equation can be applied:
TD = C x F x M
where,
TD = daily intake of contaminant (units per kg body weight
per day)
C = average concentration of contaminant in milk fat
(units per g fat)
F = average concentration of fat in milk (g/g milk)
M = average consumption of milk (g/kg per day).
2.5.1 Method of collection of breast milk
Methods for collecting breast milk have already been described
in the report of a WHO Collaborative Study on Breast-Feeding (WHO,
1985).
3. Conclusions
Because of the importance of breast-feeding to the health and
development of the infant, a strategy related to chemicals detected
in breast milk requires a thorough appraisal of the advantages and
disadvantages of the available options. In evaluating the risk of
continued exposure to a chemical through breast-feeding, the risk
of nutritional deprivation or infection, if breast-feeding is
curtailed, must be assessed simultaneously before a decision is
made.
For this reason, studies are required to establish a
relationship between levels of environmental exposure of mothers
(and hence subsequent milk contamination) and effects on the health
of breast-fed infants. Surveys of concentrations of chemicals in
breast milk should be undertaken as part of the strategy for infant
nutrition. The finding of chemicals in breast milk should lead to
an evaluation of the implications for the health of the infant.
REFERENCES TO APPENDIX I
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
ROGAN, W.H. & GLADEN, B.C. (1982) Duration of breast-feeding
and environmental contaminants in milk. Am. J. Epidemiol.,
116: 565A.
ROGAN, W.J. & GLADEN, B.C. (1985) Study of human lactation
for effects of environmental contaminants: the North Carolina
breast milk and formula project and some other ideas. Environ.
Health Perspect., 60: 215-221.
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
2.3 Development and limits of methods for measuring chemicals in
milk
During the 1960s and 1970s, techniques such as gas
chromatography were developed, which increased the scope and
accuracy of analytical methods for the determination of chemicals
in breast milk. Studies were made in various countries, and many
different halocarbons were detected. The sensitivity of the
methods was greatly increased with the introduction of glass
capillary columns in gas chromatography, high resolution liquid
chromatography, and mass spectrometry, and it is now possible to
detect some environmental chemicals in human milk fat at the ng/kg
level (Jensen, 1985). Similarly, the sensitivity of methods for
measuring heavy metals has also greatly increased in recent years
with the introduction of electrothermal atomic absorption
spectrophotometry, mass spectrometry, and neutron activation
techniques.
These newly developed methods require specialized laboratory
staff and facilities, as well as quality assessment programmes, to
ensure the accuracy and precision of results. The analyses are
often time-consuming and costly to perform. This limits the number
that can be made, and this must be taken into account when
developing strategies and requirements for the determination of
chemicals in breast milk.
2.4 Risks to the infant
The presence of chemicals in breast milk must be seen in
perspective. Because a chemical has been detected and measured, it
does not mean that it is necessarily harmful to the infant in the
quantities consumed.
The magnitude of chemical burdens derived from breast milk
cannot be assessed in isolation but must be related to those
obtained from other routes of exposure. Additional contributions
to the uptake of chemicals by the infant may occur through the
percutaneous and respiratory pathways and, in some cases, there is
a pre-existing burden sustained in utero from placental transfer.
The evaluation of the risks associated with a given chemical in
breast milk requires knowledge of:
(a) intake of the chemical by the infant;
(b) availability for absorption from the infant
intestinal tract;
(c) kinetics of uptake, transport, distribution,
metabolism, excretion, and net retention by the
infant body;
(d) effects on developing organs, tissues, and systems of
the young infant; and
(e) long-term sequelae in later childhood and adult life.
These considerations are discussed in sections 3 and 4 of this
publication. Information about most of them is incomplete.
In breast-fed infants, the concentrations of halocarbons in the
blood, for example, polychlorobiphenyls (PCBs), were found to
increase after birth and reached the maternal levels after 3
months; the increase continued until breast feeding was stopped;
thereafter, the concentrations of halocarbons decreased (Kodama &
Ota, 1980). An association between breast-feeding and high
concentrations of persistent halocarbons in the body fat of infants
has also been observed (Niessen et al., 1984), and it has been
calculated that, within a few months of birth, the concentration of
halocarbons in the fat of the infant may be equal to that in the
maternal fat (Wickizer et al., 1981; Mes et al., 1984). Although
the period of breast-feeding is short in relation to the life span,
the chemicals accumulated in the body during these months may be
retained for many years (Jensen, 1985).
At present, there appears to be only one preliminary report of
a prospective study in which 850 breast-fed infants were
periodically examined, and the milk was analysed for halocarbons
(Rogan & Gladen, 1982). The main result at the time the report was
written was that the mothers with the greatest concentration of
2,2 bis(4-chlorophenol)-1,1-dichloroethylene (DDE) in their milk
did not breast-feed for as long as those with the least, and they
gave their infants formula at an earlier age.
2.5 Assessment of contamination of breast milk following
maternal exposure
The purpose of investigating the chemical contamination of
breast milk in the context of this publication is to assess the
possible harm to infants if they continue to take milk from exposed
mothers. The daily intake of a contaminant depends on the volume
of breast milk taken and on the concentration of the chemical in
the milk. At all ages, the intake of milk by a full-term breast-
fed infant varies considerably between individuals. This depends
partly on body weight; 150 ml/kg body weight per day can be taken
as a representative intake, after the first week. This gradually
falls after 2 months to about 120 ml/kg per day at 4 - 6 months.
The concentration of lipophilic chemicals in milk is clearly
related to its fat content, and this varies with the stage of
lactation, the maternal diet, the time of day, and during the
course of a single feed. There is also a variation in
concentration of protein-bound and water-soluble chemicals at
different stages of lactation. Thus, the infant's intake of
chemicals is not constant from day to day, or within one day, or
even within a particular feed. How then should samples of breast
milk be collected for analysis to obtain a reasonable figure
representing the infant's intake of the chemical in question? If
the chemical is lipid-soluble, much of the difficulty is removed if
the percentage of fat is also determined and the concentration of
the chemical expressed per unit weight fat. Then, the following
equation can be applied:
TD = C x F x M
where,
TD = daily intake of contaminant (units per kg body weight
per day)
C = average concentration of contaminant in milk fat
(units per g fat)
F = average concentration of fat in milk (g/g milk)
M = average consumption of milk (g/kg per day).
2.5.1 Method of collection of breast milk
Methods for collecting breast milk have already been described
in the report of a WHO Collaborative Study on Breast-Feeding (WHO,
1985).
3. Conclusions
Because of the importance of breast-feeding to the health and
development of the infant, a strategy related to chemicals detected
in breast milk requires a thorough appraisal of the advantages and
disadvantages of the available options. In evaluating the risk of
continued exposure to a chemical through breast-feeding, the risk
of nutritional deprivation or infection, if breast-feeding is
curtailed, must be assessed simultaneously before a decision is
made.
For this reason, studies are required to establish a
relationship between levels of environmental exposure of mothers
(and hence subsequent milk contamination) and effects on the health
of breast-fed infants. Surveys of concentrations of chemicals in
breast milk should be undertaken as part of the strategy for infant
nutrition. The finding of chemicals in breast milk should lead to
an evaluation of the implications for the health of the infant.
REFERENCES TO APPENDIX I
JENSEN, A.A. (1985) Toxic substances in mother's milk,
Luxembourg, Commission of the European Communities.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated
biphenyls to infants from their mothers. Arch. environ.
Health, 35: 95-100.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine
pesticides in milk and blood of Canadian women during
lactation. Arch. environ. Contam. Toxicol., 13: 217-223.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRUGMANN, G., &
HOFMANN, U. (1984) Chlorinated hydrocarbons in adipose
tissue of infants and toddlers: inventory and studies on their
association with intake of mother's milk. Eur. J. Pediatr.,
142: 238-243.
ROGAN, W.H. & GLADEN, B.C. (1982) Duration of breast-feeding
and environmental contaminants in milk. Am. J. Epidemiol.,
116: 565A.
ROGAN, W.J. & GLADEN, B.C. (1985) Study of human lactation
for effects of environmental contaminants: the North Carolina
breast milk and formula project and some other ideas. Environ.
Health Perspect., 60: 215-221.
WHO (1981) Contemporary patterns of breast-feeding: report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO (1985) The quantity and quality of breast milk. Report
on the WHO Collaborative Study on Breast-Feeding, Geneva,
World Health Organization.
WHO/UNICEF (1981) Infant and young child feeding: current
issues, Geneva, World Health Organization.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R.
(1981) Polychlorinated biphenyl contamination of nursing
mothers' milk in Michigan. Am. J. public Health, 71: 132-137.
WOLFF, M.S. (1983) Occupationally derived chemicals in
breast milk. Am. J. ind. Med., 4: 259-281.
