
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 4
2-BUTANOL
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1987
This is a companion volume to Environmental Health Criteria 65:
Butanols -- Four Isomers: 1-Butanol, 2-Butanol, tert-Butanol,
Isobutanol
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
ISBN 92 4 154465 1
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1987
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
IPCS
HEALTH AND SAFETY GUIDE FOR 2-BUTANOL
INTRODUCTION
HOW TO USE THE GUIDE
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Uses
2. SUMMARY AND EVALUATION
2.1. Exposure to 2-butanol
2.2. Uptake, metabolism, and excretion
2.3. Effects on organisms in the environment
2.4. Effects on animals
2.5. Effects on human beings
3. CONCLUSIONS AND RECOMMENDATIONS
4. HEALTH HAZARDS FOR MAN, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main hazards for man, prevention and protection,
first aid
4.2. Advice to physicians
4.3. Health surveillance advice
4.4. Explosion and fire hazards
4.4.1. Explosion hazards
4.4.2. Fire hazards
4.4.3. Fire-extinguishing agents
4.5. Storage
4.6. Transport
4.7. Spillage and disposal
4.7.1. Spillage
4.7.1.1 Small spillage
4.7.1.2 Large spillage
4.7.2. Disposal
5. INTERNATIONAL CHEMICAL SAFETY CARD
6. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Exposure limit values
7.2. Labelling, packaging, and transport
INTRODUCTION
The International Programme on Chemical Safety is responsible for the
publication of a series of Environmental Health Criteria documents,
each of which assesses the existing information on the relationship
between exposure to a specific chemical, mixture of chemicals, or
combination of chemicals and physical and biological agents, and man's
health and the integrity of the environment. The documents provide
guidelines for setting exposure limits consistent with the protection
of human health and the environment.
To facilitate the application of these guidelines in national chemical
safety programmes, "Health and Safety Guides" are being prepared,
highlighting the information contained in the documents for those who
need to know the health and environmental issues involved, but not the
scientific details. The Guides include advice on preventive and
protective measures and emergency action.
Review and revision of the information in this Health and Safety Guide
will take place in due course, and the eventual aim is to use
standardized terminology. We should be grateful if you would help by
telling us of any difficulties encountered in using the information in
this guide.
Comments please, addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
HOW TO USE THE GUIDE
All people in the work-place environment should be given the relevant
written information in this book, supplemented by a clear, personal
explanation to ensure that they are fully aware of the dangers and the
current courses of protective and emergency action.
The International Chemical Safety Card should be displayed as directed
and its contents clearly explained to all working personnel.
Medical staff should be fully conversant with the medical information
to ensure they can act rapidly and efficiently in an emergency.
Posters should be used to give impact to basic safety measures.
Further copies of the Health and Safety Guide, and, for those
requiring more detailed scientific information, the relevant
Environmental Health Criteria publication, are available to order.
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical formula: C4H10O
Chemical structure: CH3-CHOH-CH2-CH3
Primary constituent: 2-butanol
Common synonyms: sec-butyl alcohol, secondary butyl
alcohol, butylene hydrate, 2-hydroxy
butane, methyl ethyl carbinol,
butan-2-ol, sec-butanol, SBA
CAS registry number: 78-92-2
Conversion factors
1 mg/m3 = 0.325 ppm
1 ppm = 3.078 mg/m3
1.2 Physical and Chemical Properties
2-Butanol is a flammable, colourless liquid with a characteristic
sweet odour. Some physical and chemical properties of 2-butanol are
given in the Sample International Chemical Safety Card.
1.3 Uses
2-Butanol occurs naturally as a product of fermentation of
carbohydrates. It is used for the extraction of fish meal to produce
fish protein concentrate. It is also used for the production of fruit
essences, as a flavouring in food, and as a solvent.
2. SUMMARY AND EVALUATION
2.1 Exposure to 2-Butanol
Human exposure to 2-butanol is mainly occupational. Exposure of the
general population will be through its natural occurrence in foods and
beverages, and its use as a flavouring agent. Exposure may also result
from industrial emissions. Data are not available on the above
exposure levels.
2.2 Uptake, Metabolism, and Excretion
In animals, 2-butanol is absorbed through the lungs and
gastrointestinal tract. No information is available regarding dermal
absorption. In animals, approximately 97% of the dose of 2-butanol is
converted by alcohol dehydrogenase to the corresponding ketone, which
is either excreted in the breath and urine or further metabolized.
2.3 Effects on Organisms in the Environment
No quantitative data on the levels in the general environment are
available, but, because 2-butanol is readily biodegradable,
substantial concentrations are only likely to occur locally in cases
of major spillage. It does not bioaccumulate.
At background concentrations likely to occur in the environment,
2-butanol is not toxic for aquatic animals, algae, protozoa, or
bacteria. However, it poses an indirect hazard for the aquatic
environment, because of its ready biodegradability, which may lead to
oxygen depletion. It should be managed in the environment as a
slightly toxic compound.
2.4 Effects on Animals
The acute oral LD50 for 2-butanol in the rat is 6.5 g/kg body
weight; therefore, it is practically non-toxic, according the scale of
Hodge & Sterner. The toxic effects from acute exposure are ataxia and
narcosis. The potency of 2-butanol for intoxication is approximately 4
times that of ethanol. 2-Butanol is irritating to the eyes and
non-irritating to the skin. It is not possible to determined a
no-observed-adverse-effect level on the basis of available animal
studies. No adequate data are available on mutagenicity,
carcinogenicity, teratogenicity, or effects on reproduction.
2.5 Effects on Human Beings
In man, the most likely acute effect of 2-butanol is alcoholic
intoxication. No published data are available concerning other effects
on man.
3. CONCLUSIONS AND RECOMMENDATIONS
1. On the available data, the Task Group was unable to make an
assessment of the health risks of 2-butanol for the general
population; however, it was considered unlikely to pose a serious
hazard under normal exposure conditions.
2. The Task Group was of the opinion that sufficient data were not
available to establish guidelines for setting occupational exposure
limits. In line with good manufacturing practice, exposure to
2-butanol should be minimized.
3. The available ecotoxicological data indicate that the impact of
background concentrations of 2-butanol on the aquatic environment can
be expected to be minimal.
From: Environmental Health Criteria 65: Butanols - Four Isomers:
1-Butanol, 2-Butanol, tert-Butanol, Isobutanol
4. HEALTH HAZARDS FOR MAN, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Hazards for Man, Prevention and Protection, First Aid
The vapour of 2-butanol can irritate the respiratory system, the skin,
and the eyes. The liquid is irritating to the eyes and the skin.
Narcosis may follow the ingestion of 2-butanol or inhalation of high
concentrations of its vapours.
The human health hazards associated with certain types of exposure to
2-butanol, together with preventive and protective measures are listed
in the following table.
GOLDEN RULES
1. Do not smoke, drink, or eat in the work-place.
2. In case of overexposure, the victim should leave, or be removed
from, the contaminated area to fresh air as rapidly as possible.
3. Remove contaminated clothing and shoes and wash with plenty of
water and soap.
4. Flush affected eye(s) with water for at least 15 minutes.
4.2 Advice to Physicians
Absorption of 2-butanol may be confirmed by identification of the
alcohol or its metabolites in the blood. Because of its toxicity,
emptying of the stomach should be considered in cases of ingestion. If
this is not performed within 4 h, it is unlikely to be of benefit.
4.3 Health Surveillance Advice
No specific measures are indicated.
4.4 Explosion and Fire Hazards
4.4.1 Explosion hazards
Above 23°C, explosive vapour-air mixtures may be formed.
4.4.2 Fire hazards
2-Butanol reacts with strong oxidizing agents and alkali metals to
form a combustible gas (hydrogen). It is classified as flammable.
Keep stored drums cool by spraying with water.
ROUTE HEALTH HAZARDS PREVENTION AND PROTECTION FIRST AID
SINGLE EXPOSURE
SKIN Moderately irritating Wear protective clothing Remove contaminated clothing;
flush immediately and
thoroughly with water; seek
medical attention
EYES Moderately irritating as liquid Wear goggles or face shield Flush eyes immediately with
or vapour water for at least 15 min; seek
medical attention
INHALATION Potentially irritating; at high Minimize exposure by ensuring Fresh air; if breathing has
concentrations, symptoms of alcohol adequate ventilation or using stopped, apply artificial
intoxication and narcosis may suitable respiratory protection respiration; seek medical
occur attention immediately
INGESTION Unlikely occupational hazard; it Use normal hygienic practices Do not induce vomiting; seek
may be absorbed and cause medical attention immediately
systemic effects such as alcohol
poisoning and narcosis
REPEATED EXPOSURE
INHALATION
As for single exposure; no Use normal hygienic practices
long-term adverse health effects
INGESTION have been reported in man
4.4.3 Fire-extinguishing agents
For a small fire, use carbon dioxide, dry chemical powder,
alcohol-resistant foam, sand, earth, or water fog. For a large fire,
use alcohol-resistant foam or water fog.
4.5 Storage
Store away from direct sunlight and other sources of heat.
4.6 Transport
Treat as for flammable liquids; otherwise, no special measures
indicated.
4.7 Spillage and Disposal
4.7.1 Spillage
Extinguish naked flames. Do not smoke. Avoid sparks. Avoid contact
with the skin, eyes, and clothing. Wear rubber gloves, goggles or face
shield, and boots. Avoid breathing the vapour. If necessary, wear a
respiratora containing a canister, such as BSI Type CC (colour black
with a grey stripe) or DIN Type A (colour brown), or self-contained
breathing apparatus.
4.7.1.1 Small spillage
Absorb the liquid with sand, earth, sawdust, or other suitable
absorbant material. Shovel up and remove all material to safe place
for subsequent disposal by burning. Flush the contaminated area with
plenty of water.
4.7.1.2 Large spillage
Prevent the spilt liquid from spreading by the use of sand or earth.
Transfer the liquid to a salvage tank, if possible. Otherwise, treat
as for a small spillage. Inform the local authorities (particularly
the fire service) at once, if the spilt liquid enters the surface
water drains, since a potential explosive and toxic hazard will be
created.
4.7.2 Disposal
Recommended disposal practices include incineration as the major
disposal method. The waste 2-butanol should be sprayed into the
firebox of an incinerator. Combustion can be improved by mixing with a
more flammable solvent.
a A respirator is not sufficient protection in air containing a
high concentration of vapour or in enclosed spaces (e.g., storage
tanks) where the air is deficient in oxygen.
5. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, 2-butanol. It should be displayed at, or near,
entrances to areas where there is potential exposure to 2-butanol, and
on processing equipment and containers. The card should be translated
into the appropriate language(s).
All persons potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
SAMPLE INTERNATIONAL CHEMICAL SAFETY CARD
2-BUTANOL
(sec-butanol, sec-butyl alcohol) (CH3CH2CHOHCH3)
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point (°C) 99 Colourless liquid with
Melting point (°C) -89 characteristic odour; reacts with
Flash point (°C) 24 strong oxidizing agents and alkali
Autoignition temperature (°C) 390 metals to form combustible gas
Relative density (water = 1) 0.8 (hydrogen); attacks many plastics
Relative vapour density (air = 1) 2.6
Vapour pressure in mbar (20°C) 17.3
Solubility in water (g/litre at 20°C) 125
Explosive limits (vol. % in air) 1.7-9.8
Relative molecular mass 74.1
HAZARDS/SYMPTOMS PREVENTION FIRST AID
INHALATION: Sore throat, Minimize exposure by ensuring Fresh air; rest in half upright position;
coughing, shortness of breath, ventilation, local exhaust, or seek medical help, if necessary
dullness using breathing protection
SKIN: May be absorbed; redness Wear protective gloves Remove contaminated clothing; rinse skin
with plenty of water or shower
EYES: Redness, pain, blurred vision Wear safety goggles First rinse with plenty of water; then seek
medical help
SAMPLE INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
HAZARDS/SYMPTOMS PREVENTION FIRST AID
INGESTION: Abdominal pain, Rinse mouth; give plenty of water to
vomiting, diarrhoea drink; seek medical help or transport to
hospital
GENERAL: Alcoholic beverages
may enhance toxic effects
SPILLAGE STORAGE FIRE AND EXPLOSION
Collect leaking liquid in sealable Fireproof Flammable; above 24°C, explosive
containers, absorb spilled liquid in vapour-air mixtures may be formed; no
sand or inert absorbent and open flames, no sparks, and no smoking;
remove to safe place use closed systems, ventilation, and
explosion-proof electrical equipment; in
case of fire, keep tanks or drums cool by
spraying with water; extinguish fire with
powder, AFFF, foam, halons, or carbon
dioxide
SAMPLE INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
WASTE DISPOSAL
National Occupational UN: 1121
Exposure Limit:
National Poison
Control Centre:
 Adapted from: Handling Chemicals Safely (1980). Published by the Dutch Association of Safety Experts, the Dutch Chemical Industry
Association, and the Dutch Safety Institute, The Hague.
6. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
2-Butanol should be managed in the environment as a compound that is
practically non-toxic but poses an indirect hazard for the aquatic
environment, because ready biodegradation may lead to oxygen
depletion. Thus, in the case of spillage into surface water, consider
dilution or (artificial) reaeration.
Avoid gross contamination of soil. Spilled material should be
contained and removed, as far as possible. Final traces can be
dispersed with water.
Methods of disposal should meet the requirements of operative
legislation. In the absence of such legislation, handle responsibly;
if deemed necessary, consult local authorities.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file.
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. A full reference to the
original national document from which the information was extracted
can be obtained from the IRPTC.a When no effective date appears in
the IRPTC legal file, the year of the reference from which the data
are taken is shown, indicated by (r).
7.1 Exposure Limit Values
For some exposure limit values, see the following table.
7.2 Labelling, Packaging, and Transport
2-Butanol is classified as a flammable liquid (Hazard Class 3) by the
United Nations Committee of Experts on the Transport of Dangerous
Goods, and as a flammable liquid by the International Maritime
Organization (Hazard Class 3.2). The following symbol should be used:
Adapted from: Handling Chemicals Safely (1980). Published by the Dutch Association of Safety Experts, the Dutch Chemical Industry
Association, and the Dutch Safety Institute, The Hague.
6. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
2-Butanol should be managed in the environment as a compound that is
practically non-toxic but poses an indirect hazard for the aquatic
environment, because ready biodegradation may lead to oxygen
depletion. Thus, in the case of spillage into surface water, consider
dilution or (artificial) reaeration.
Avoid gross contamination of soil. Spilled material should be
contained and removed, as far as possible. Final traces can be
dispersed with water.
Methods of disposal should meet the requirements of operative
legislation. In the absence of such legislation, handle responsibly;
if deemed necessary, consult local authorities.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file.
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. A full reference to the
original national document from which the information was extracted
can be obtained from the IRPTC.a When no effective date appears in
the IRPTC legal file, the year of the reference from which the data
are taken is shown, indicated by (r).
7.1 Exposure Limit Values
For some exposure limit values, see the following table.
7.2 Labelling, Packaging, and Transport
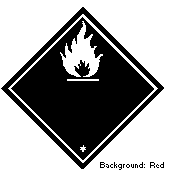
2-Butanol is classified as a flammable liquid (Hazard Class 3) by the
United Nations Committee of Experts on the Transport of Dangerous
Goods, and as a flammable liquid by the International Maritime
Organization (Hazard Class 3.2). The following symbol should be used:
 a International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 988400-985850).
The European Community legislation requires labelling as dangerous
substance using the symbol:
a International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 988400-985850).
The European Community legislation requires labelling as dangerous
substance using the symbol:
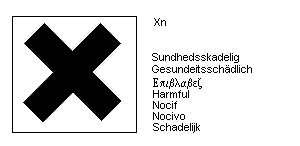 The label must read: flammable - harmful by inhalation; keep away from
sources of ignition - no smoking.
The European Community legislation on labelling of solvant
preparations classifies 2-butanol in Class II d for the purpose of
determining the label for preparations containing 2-butanol and other
active ingredients (1980).
SOME EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Occupational Argentina Maximum permissible concentration (MPC) 1979
-- Time-weighted average 450 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
Australia Threshold limit value (TLV) 1983 (r)
-- Time-weighted average 450 mg/m3
Belgium Threshold limit value (TLV) 450 mg/m3
Germany, Federal Maximum work-site concentration (MAK) 1985 (r)
Republic of -- 8-h time-weighted average 300 mg/m3
-- Short-term exposure limit (STEL) (30 min, 600 mg/m3
4 × per shift) (average value)
Italy Threshold limit value (TLV) 250 mg/m3
Netherlands Maximum limit 1985 (r)
-- Time-weighted average 450 mg/m3
Sweden Hygienic limit value (HLV) 1985
-- One-day time-weighted average 150 mg/m3a
-- Short-term exposure limit (STEL) 250 mg/m3
(15-min time-weighted average)
Switzerland Maximum work-site concentration (MAK) 1984 (r)
-- Time-weighted average 300 mg/m3
SOME EXPOSURE LIMIT VALUES (cont'd).
Medium Specification Country/ Exposure limit description Value Effective
organization date
United Recommended limit 1985 (r)
Kingdom -- 8-h time-weighted average 450 mg/m3
-- Intended 8-h time weighted average 300 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
(10 min time-weighted average)
USA (ACGIH) Threshold limit value (TLV) 1984 (r)
-- Time-weighted average 305 mg/m3
Short-term exposure limit (STEL) 455 mg/m3
USA (OSHA) Permissible exposure limit (PEL) 1981 (r)
-- Time-weighted average 450 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.2 mg/litre 1983 (r)
a Absorption through the skin is indicated as a potentially hazardous route in the regulatory documents of Sweden.
The label must read: flammable - harmful by inhalation; keep away from
sources of ignition - no smoking.
The European Community legislation on labelling of solvant
preparations classifies 2-butanol in Class II d for the purpose of
determining the label for preparations containing 2-butanol and other
active ingredients (1980).
SOME EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Occupational Argentina Maximum permissible concentration (MPC) 1979
-- Time-weighted average 450 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
Australia Threshold limit value (TLV) 1983 (r)
-- Time-weighted average 450 mg/m3
Belgium Threshold limit value (TLV) 450 mg/m3
Germany, Federal Maximum work-site concentration (MAK) 1985 (r)
Republic of -- 8-h time-weighted average 300 mg/m3
-- Short-term exposure limit (STEL) (30 min, 600 mg/m3
4 × per shift) (average value)
Italy Threshold limit value (TLV) 250 mg/m3
Netherlands Maximum limit 1985 (r)
-- Time-weighted average 450 mg/m3
Sweden Hygienic limit value (HLV) 1985
-- One-day time-weighted average 150 mg/m3a
-- Short-term exposure limit (STEL) 250 mg/m3
(15-min time-weighted average)
Switzerland Maximum work-site concentration (MAK) 1984 (r)
-- Time-weighted average 300 mg/m3
SOME EXPOSURE LIMIT VALUES (cont'd).
Medium Specification Country/ Exposure limit description Value Effective
organization date
United Recommended limit 1985 (r)
Kingdom -- 8-h time-weighted average 450 mg/m3
-- Intended 8-h time weighted average 300 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
(10 min time-weighted average)
USA (ACGIH) Threshold limit value (TLV) 1984 (r)
-- Time-weighted average 305 mg/m3
Short-term exposure limit (STEL) 455 mg/m3
USA (OSHA) Permissible exposure limit (PEL) 1981 (r)
-- Time-weighted average 450 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.2 mg/litre 1983 (r)
a Absorption through the skin is indicated as a potentially hazardous route in the regulatory documents of Sweden.
See Also:
Toxicological Abbreviations
 Adapted from: Handling Chemicals Safely (1980). Published by the Dutch Association of Safety Experts, the Dutch Chemical Industry
Association, and the Dutch Safety Institute, The Hague.
6. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
2-Butanol should be managed in the environment as a compound that is
practically non-toxic but poses an indirect hazard for the aquatic
environment, because ready biodegradation may lead to oxygen
depletion. Thus, in the case of spillage into surface water, consider
dilution or (artificial) reaeration.
Avoid gross contamination of soil. Spilled material should be
contained and removed, as far as possible. Final traces can be
dispersed with water.
Methods of disposal should meet the requirements of operative
legislation. In the absence of such legislation, handle responsibly;
if deemed necessary, consult local authorities.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file.
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. A full reference to the
original national document from which the information was extracted
can be obtained from the IRPTC.a When no effective date appears in
the IRPTC legal file, the year of the reference from which the data
are taken is shown, indicated by (r).
7.1 Exposure Limit Values
For some exposure limit values, see the following table.
7.2 Labelling, Packaging, and Transport
2-Butanol is classified as a flammable liquid (Hazard Class 3) by the
United Nations Committee of Experts on the Transport of Dangerous
Goods, and as a flammable liquid by the International Maritime
Organization (Hazard Class 3.2). The following symbol should be used:
Adapted from: Handling Chemicals Safely (1980). Published by the Dutch Association of Safety Experts, the Dutch Chemical Industry
Association, and the Dutch Safety Institute, The Hague.
6. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
2-Butanol should be managed in the environment as a compound that is
practically non-toxic but poses an indirect hazard for the aquatic
environment, because ready biodegradation may lead to oxygen
depletion. Thus, in the case of spillage into surface water, consider
dilution or (artificial) reaeration.
Avoid gross contamination of soil. Spilled material should be
contained and removed, as far as possible. Final traces can be
dispersed with water.
Methods of disposal should meet the requirements of operative
legislation. In the absence of such legislation, handle responsibly;
if deemed necessary, consult local authorities.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file.
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. A full reference to the
original national document from which the information was extracted
can be obtained from the IRPTC.a When no effective date appears in
the IRPTC legal file, the year of the reference from which the data
are taken is shown, indicated by (r).
7.1 Exposure Limit Values
For some exposure limit values, see the following table.
7.2 Labelling, Packaging, and Transport
2-Butanol is classified as a flammable liquid (Hazard Class 3) by the
United Nations Committee of Experts on the Transport of Dangerous
Goods, and as a flammable liquid by the International Maritime
Organization (Hazard Class 3.2). The following symbol should be used:
 a International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 988400-985850).
The European Community legislation requires labelling as dangerous
substance using the symbol:
a International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 988400-985850).
The European Community legislation requires labelling as dangerous
substance using the symbol:
 The label must read: flammable - harmful by inhalation; keep away from
sources of ignition - no smoking.
The European Community legislation on labelling of solvant
preparations classifies 2-butanol in Class II d for the purpose of
determining the label for preparations containing 2-butanol and other
active ingredients (1980).
SOME EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Occupational Argentina Maximum permissible concentration (MPC) 1979
-- Time-weighted average 450 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
Australia Threshold limit value (TLV) 1983 (r)
-- Time-weighted average 450 mg/m3
Belgium Threshold limit value (TLV) 450 mg/m3
Germany, Federal Maximum work-site concentration (MAK) 1985 (r)
Republic of -- 8-h time-weighted average 300 mg/m3
-- Short-term exposure limit (STEL) (30 min, 600 mg/m3
4 × per shift) (average value)
Italy Threshold limit value (TLV) 250 mg/m3
Netherlands Maximum limit 1985 (r)
-- Time-weighted average 450 mg/m3
Sweden Hygienic limit value (HLV) 1985
-- One-day time-weighted average 150 mg/m3a
-- Short-term exposure limit (STEL) 250 mg/m3
(15-min time-weighted average)
Switzerland Maximum work-site concentration (MAK) 1984 (r)
-- Time-weighted average 300 mg/m3
SOME EXPOSURE LIMIT VALUES (cont'd).
Medium Specification Country/ Exposure limit description Value Effective
organization date
United Recommended limit 1985 (r)
Kingdom -- 8-h time-weighted average 450 mg/m3
-- Intended 8-h time weighted average 300 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
(10 min time-weighted average)
USA (ACGIH) Threshold limit value (TLV) 1984 (r)
-- Time-weighted average 305 mg/m3
Short-term exposure limit (STEL) 455 mg/m3
USA (OSHA) Permissible exposure limit (PEL) 1981 (r)
-- Time-weighted average 450 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.2 mg/litre 1983 (r)
a Absorption through the skin is indicated as a potentially hazardous route in the regulatory documents of Sweden.
The label must read: flammable - harmful by inhalation; keep away from
sources of ignition - no smoking.
The European Community legislation on labelling of solvant
preparations classifies 2-butanol in Class II d for the purpose of
determining the label for preparations containing 2-butanol and other
active ingredients (1980).
SOME EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Occupational Argentina Maximum permissible concentration (MPC) 1979
-- Time-weighted average 450 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
Australia Threshold limit value (TLV) 1983 (r)
-- Time-weighted average 450 mg/m3
Belgium Threshold limit value (TLV) 450 mg/m3
Germany, Federal Maximum work-site concentration (MAK) 1985 (r)
Republic of -- 8-h time-weighted average 300 mg/m3
-- Short-term exposure limit (STEL) (30 min, 600 mg/m3
4 × per shift) (average value)
Italy Threshold limit value (TLV) 250 mg/m3
Netherlands Maximum limit 1985 (r)
-- Time-weighted average 450 mg/m3
Sweden Hygienic limit value (HLV) 1985
-- One-day time-weighted average 150 mg/m3a
-- Short-term exposure limit (STEL) 250 mg/m3
(15-min time-weighted average)
Switzerland Maximum work-site concentration (MAK) 1984 (r)
-- Time-weighted average 300 mg/m3
SOME EXPOSURE LIMIT VALUES (cont'd).
Medium Specification Country/ Exposure limit description Value Effective
organization date
United Recommended limit 1985 (r)
Kingdom -- 8-h time-weighted average 450 mg/m3
-- Intended 8-h time weighted average 300 mg/m3
-- Short-term exposure limit (STEL) 450 mg/m3
(10 min time-weighted average)
USA (ACGIH) Threshold limit value (TLV) 1984 (r)
-- Time-weighted average 305 mg/m3
Short-term exposure limit (STEL) 455 mg/m3
USA (OSHA) Permissible exposure limit (PEL) 1981 (r)
-- Time-weighted average 450 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.2 mg/litre 1983 (r)
a Absorption through the skin is indicated as a potentially hazardous route in the regulatory documents of Sweden.
