
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 47
ATRAZINE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1990
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Health and safety guide for Atrazine
(Health and safety guide ; no. 47)
1.Atrazine - standards I.Series
ISBN 92 4 151047 1 (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Human exposure to atrazine
2.2. Uptake, metabolism, and excretion
2.3. Effects on animals
2.4. Effects on human beings
2.5. Effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Prevention and protection
4.1.2. First aid
4.2. Advice to physicians
4.3. Explosion and fire hazards
4.4. Storage and transport
4.5. Spillage and disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Exposure limit values
6.2. Specific restrictions
6.3. Transport and labelling
INTRODUCTION
This Health and Safety Guide is not based on an existing Environmental
Health Criteria document, but on critical national reviews. The hazard
evaluation in the Health and Safety Guide was made on the basis of
carefully selected studies, after scrutiny of the original
publications.
In order to assist the peer-review process of the present Health and
Safety Guide, a background companion document was prepared by the IPCS
and can be obtained from the Manager on request; the IPCS does not
intend that the background document should be published.
The first three sections of this Health and Safety Guide present
essential technical information and the hazard evaluation. Section 4
includes advice on preventive and protective measures and emergency
action; health workers should be thoroughly familiar with the medical
information to ensure that they can act efficiently in an emergency.
The section on regulatory information has been extracted from the
legal file of the International Register of Potentially Toxic
Chemicals (IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: atrazine
Chemical formula: C8H14ClN5
Chemical structure: 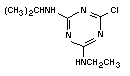 Relative molecular 215.7
mass:
Common trade A 361; Aatrex; Aatrex 4L; Aatrex 80W; Aatrex
names (including Nine-O; Aktikon; Aktikon PK; Aktinit A;
formulations): Aktinit PK Argezin; Atazinax; Atranex;
Atrasine; Atratol A; Atrazin; Atred; Atrex;
Candex; Cekuzina-T; Crisatrina; Crisazine;
Cyazin; Farmco Atrazine; Fenamin; Fenamine;
Fenatrol; G 30027; Geigy 30,027; Gesaprim;
Gesoprim; Griffex; Hungazin; Hungazin PK;
Inakor; Oleogesaprim; Primatol; Primatol A;
Primaze; Radazin; Radizine; Strazine;
Triazine A 1294; Vectal; Vectal SC; Weedex A;
Wonuk; Zeazin; Zeazine
CAS chemical name: 2-chloro-4-ethylamine-6-isopropylamino- S-
triazine
Synonyms: S-triazine, 2-chloro-4-ethylamino-
6-isopropylamino-; 1,3,5-triazine-2,4-
diamine, 6-chloro- N-ethyl- N'-
(1-methylethyl)-; 2-aethylamino-4-
isopropylamino-6-chlor- 1,3,5-triazin;
2-chloro-4-ethylamineiso-propyl-
amine- S-triazine; 1-Chloro-3-ethylamino-5-
isopropyl-amino-2,4,6-triazine;
1-chloro-3-ethylamino-5-isopropyl-amino- S-
triazine; 2-chloro-4-ethylamino-6-isopropyl-
amino- 1,3,5-triazine;
2-chloro-4-ethylamino-6-isopropyl-
amino- S-triazine 6-chloro- N-ethyl-
N'-(1-methylethyl)-1,3,5-triazine-
2,4-diamine; 2-chloro-4-(2-propylamino)-
6-ethylamino- S-triazine
CAS registry
number: 1912-24-9
RTECS registry
number: XY5600000
According to FAO specifications, commercial atrazine should be at
least 92% pure, and most products are about 95% pure. Common
impurities are sodium chloride and other symmetric triazines, such as
simazine and propazine. It is often formulated as wettable powders,
granules, and as flowable suspensions.
Wettable powder formulations contain 450-800 g atrazine/kg (less if
mixed with other pesticides), and liquid formulations (including
suspension concentrate), 40-650 g/litre.
1.2 Physical and Chemical Properties
Technical atrazine is a colourless crystalline powder with low vapour
pressure (40 nPa at 20°C). A melting point range of 175-177°C has been
reported for the technical product. It is readily soluble in dimethyl
sulfoxide (183 g/litre), moderately soluble in methanol (18 g/litre),
diethyl ether (12 g/litre), chloroform (52 g/litre), and ethyl acetate
(28 g/litre), and very slightly soluble in water (30 mg/litre). It is
stable in the dry state, but is hydrolysed to the herbicidally
inactive 2-hydroxy analogue in acid or in alkaline solutions and more
slowly in neutral aqueous solutions, even at elevated temperatures.
1.3 Analytical Methods
Gas chromatography with a nitrogen-phosphorus detector (NPD) is used
for the determination of residues and the analysis of environmental
samples. The minimum detection limit varies according to the
substrate.
1.4 Production and Uses
Atrazine was introduced in 1958. In 1987, total worldwide production
was estimated to be 70 000 tonnes.
Atrazine is a selective pre- and post-emergence herbicide used for the
control of weeds in crops, such as asparagus, corn, sorghum, sugar
cane, and pineapple. It is also used in forestry and, at higher
application rates, for non-selective weed control in non-crop areas.
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Atrazine
Highest human exposure to atrazine is associated with its production
and its use in agriculture. Widespread, low-level exposure of the
general population may occur through contaminated drinking-water.
2.2 Uptake, Metabolism, and Excretion
Atrazine is readily absorbed from the gastrointestinal tract, but only
penetrates the skin to a very limited extent. The absorbed herbicide
is rapidly eliminated. In the rat, the whole-body half-life is about
1.3 days, and 95% of the dose is eliminated within 7 days. The highest
concentration of atrazine and/or its metabolites is found in the red
blood cells, to which the triazines bind effectively. In rats given
low, oral doses, daily, levels were found in the tissues in the
following decreasing order: erythrocytes, liver, spleen, kidney. The
primary route of elimination in rodents is via the urine (about 75%);
approximately 20% is eliminated in the faeces.
Dealkylation at the C-4 and C-6 positions of the atrazine molecule are
the principal metabolic degradation reactions. Dechlorination at the
C-2 position also occurs, to some extent. In the rat, the major
urinary metabolites include the two mono- N-dealkylated metabolites
and the fully dealkylated derivative, 2-chloro-4,6-diamino- S-triazine.
2.3 Effects on Animals
The acute oral toxicity and the dermal toxicity of atrazine in rodents
are both low. Acute oral studies conducted on different rodent species
have indicated LD50 values in the range of 1700-4000 mg/kg. Young
rats are much less sensitive to atrazine than adults. Ruminants seem
to be much more sensitive to the acute toxic action of atrazine than
rodents. In one study, 2 doses of 250 mg/kg caused death in both sheep
and cattle.
The irritant action of atrazine is only slight on rabbit skin, but is
moderate for the rabbit eye.
Atrazine has been subjected to extensive long-term testing on rats,
mice, and dogs. Significant cardiac toxicity was observed in dogs
after long-term oral administration of atrazine at doses of 5 mg/kg
per day or more. In rats and mice, reduced food intake, decreased
weight gain, and toxic effects on other organs were detected after
long-term administration of atrazine at high dose levels (> 25 mg/kg
per day). These effects included muscle degeneration, retinal
degeneration, necrosis of the liver, and haematological effects in
rats and in mice. An increase in mammary tumours was observed in rats,
but not in mice. Taking into account the very high background level of
mammary tumours in untreated animals, the compound's apparent lack of
genotoxicity, and the fact that the maximum tolerated dose was
exceeded, these findings do not represent convincing evidence of
carcinogenicity.
Atrazine exerts a fetotoxic action at, or close to, dose levels that
have a deleterious action on the mothers, but does not seem to have
any significant teratogenic action in rats, mice, or rabbits.
2.4 Effects on Human Beings
There are no substantiated indications that atrazine causes any health
or safety hazards for the general population or for exposed workers.
2.5 Effects on the Environment
On the basis of acute and short-term dietary studies, the toxicity of
atrazine for birds is low, with no mortality at 10 000 mg/kg diet.
Atrazine is virtually non-toxic for bees, but is moderately toxic for
aquatic organisms (96-h LC50 range from 0.5 to 15 mg/litre). The
herbicide is degradable and has little tendency to bioaccumulate,
thereby limiting possible long-term adverse effects on wildlife and
fish. As it is an effective herbicide, its phytotoxicity may
constitute a problem in the case of uncontrolled applications.
However, the main concern is its relative persistence (half-life of
125 days in sandy soils) and mobility in some types of soils (related
to rainfall), resulting in contamination of surface and ground waters.
3. CONCLUSIONS AND RECOMMENDATIONS
The acute oral and dermal toxicities of atrazine for most mammalian
species are low. Atrazine has no significant teratogenic action in
rats, mice, or rabbits. There is no convincing evidence of
carcinogenicity in rats or mice. Toxic effects, including ocular,
cardiac, haematological, and hepatic effects, have been observed in
both rats and mice after long-term administration.
On the basis of acute and short-term dietary studies, the toxicity of
atrazine is low for birds and moderate for aquatic organisms. It has a
low capacity for bioconcentration.
The relative persistence and mobility of atrazine in some types of
soils can result in contamination of ground and surface waters. It is
recommended that adequate precautions should be taken to prevent this
contamination.
A few gaps in data have been identified that indicate a need for
further mutagenicity testing and studies of the mechanism of tumour
induction in rats.
Atrazine is moderately irritating to the eye. No significant health
effects on either the general population or exposed workers are
expected.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The acute toxicity of technical atrazine for human beings is thought
to be low, and no adverse health effects from exposure to this
herbicide have been reported. In view of the toxicity induced in
experimental animals on repeated exposure, proper care should be taken
during occupational use to avoid excessive inhalation of dust or spray
particles, and to prevent accidental contamination of food products
and water.
4.1.1 Prevention and Protection
In order to reduce the risk of accidental contamination, the following
precautions should be observed during handling and use:
(a) Avoid contact with the skin and eyes.
(b) Do not smoke, drink, or eat in the work-place. Wash hands and any
exposed skin before eating, drinking, or smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable powder
formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear protective PVC or
neoprene gloves.
(f) When handling leaking containers, or when dealing with leaks and
spills, wear overalls and PVC or neoprene gloves and boots. If
overalls become contaminated, change and wash them thoroughly before
re-use.
(g) Store products in dosed original containers, out of reach of
children, and away from food, drink, and animal feed.
4.1.2 First Aid
Poisoning by atrazine is unlikely, unless large amounts have been
ingested. In case of over-exposure, apply routine first-aid measures.
If material has been spilled on the skin, immediately remove the
patient from the source of contamination, remove all contaminated
clothing, and wash affected areas with soap and running water. If the
material is in the eyes, flush with clean water for at least 15
minutes. In case of ingestion of significant quantities, and, if the
patient is conscious, give a glass of water and subsequently
administer activated charcoal. Do not induce vomiting. In serious
cases, medical attention should be sought.
4.2 Advice to Physicians
The acute toxicity of atrazine for human beings is believed to be low.
There is no specific antidote. Treat symptomatically when required.
When large amounts have been ingested, gastric lavage may be
indicated.
4.3 Explosion and Fire Hazards
Technical atrazine is not flammable but, on heating, it decomposes to
form toxic fumes containing oxides of nitrogen (NOx) and hydrogen
chloride (HCl). Extinguish small fires with carbon dioxide, dry
powder, or alcohol-resistant foam. Water spray can be used for larger
fires and the cooling of unaffected stock, but avoid the accumulation
of polluted run-off from the site.
Beware: Some liquid formulations may be highly flammable and require
alcohol-resistant foam as an extinguishing agent.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
Keep spectators away from any leakage. Prevent contamination of other
goods or cargo, or nearby vegetation and waterways.
Absorb spilled liquid products with sawdust or sand, sweep up and
place in separate container.
Empty any product remaining in damaged or leaking containers into a
clean empty container, which should be suitably labelled.
Sweep up any spilled powder with damp earth or sand or other suitable
absorbent, such as sawdust, taking care not to raise a dust cloud (use
a vacuum cleaner). Place in separate container for subsequent
disposal. Contaminated absorbents, used containers, surplus product,
etc., should be burnt in an incinerator, preferably designed for
pesticide disposal. Hydrolysis under alkaline conditions (10% w/v
sodium hydroxide) is a suitable method to dispose of small quantities
of atrazine. Heating speeds the process. After hydrolysis, dilute and
dispose of via the sewage system. Atrazine is relatively stable and
characterized by high mobility in some soils and should not be buried
in dump sites, etc. Comply with any local legislation applying to
waste disposal.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Triazines may be ingested by domestic animals. In view of the
relatively high toxicity of atrazine for ruminants, exposure to
atrazine of sheep and cows should be avoided. Because of its relative
persistence and mobility in some types of soils, atrazine has a
significant potential for contamination of ground and surface waters.
The contamination of ponds, waterways, and ditches should be avoided.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Exposure Limit Values
Exposure limit values for atrazine are given in the following table.
6.2 Specific Restrictions
The National Swedish Chemicals Inspectorate has recently announced its
intention to prohibit the use of atrazine and other triazine
herbicides for weed elimination on gravel roads and similar surfaces.
Atrazine constitutes a contaminant required to be regulated by the US
Safe Water Drinking Act of 1986, including the promulgation of a MCLG
(Maximum Concentration Limit Goal).
6.3 Transport and Labelling
The United Nations Committee of Experts on Transportation of Dangerous
Goods classifies atrazine in:
- Hazard Class 6.1: poisonous substances;
- Packing group III: substance presenting a relatively low risk of
poisoning in transport.
EXPOSURE LIMIT VALUESa
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Occupational Australia Time-weighted average (TWA) 5 mg/m3 1983
Belgium Time-weighted average (TWA) 5 mg/m3 1988
Germany, Federal Time-weighted average (TWA) 2 mg/m3 1988
Republic of, - Maximum worksite concentration 2 mg/m3
Switzerland Time-weighted average (TWA) 10 mg/m3 1987
United Kingdom Maximum allowable concentration 2 mg/m3 1977
USSR (Ceiling value)
WATER Drinking- Canada Interim maximum acceptable 0.06 mg/litre 1987
concentration
EEC Maximum allowable concentration 0.1 µg/litre
Switzerland Maximum residue limit 0.1 µg/litre 1980
WHO/EURO Guideline value 2.0 µg/litre 1987
WATER Oral intake WHO/EURO Acceptable daily intake (ADI) 0.7 mg/g 1987
body weight
FOOD Plant (general) EEC Maximum residue limit 0.1 mg/kg
FOOD Plant (specified) Brazil Acceptable limit 0.1-0.2 mg/kg 1984
EXPOSURE LIMIT VALUESa (cont'd).
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant (general) Germany, Federal Maximum residue limit 0.1 mg/kg 1984
Republic of,
Plant (specified) Germany, Federal Maximum residue limit 0.5-10 mg/kg 1984
Republic of,
FOOD Specified food Kenya Maximum limit 0.02-0.25 mg/kg
products
FOOD Raw agricultural USA Tolerance (Acceptable residue limit) 0.02-15 mg/kg 1982
products
(specified plant
and animal
products)
Oral intake USA Reference dose 0.005 mg/kg
body weight
FOOD Specified food USSR Acceptable daily intake (ADI) 0.004 mg/kg 1983
products
FOOD Oral intake USA Tolerance 0.005 mg/kg
GOODS Brazil Use in domestic sanitation not
authorized
a For other regulatory data see complete legal file of IRC data profile.
The label should be as follows:
Relative molecular 215.7
mass:
Common trade A 361; Aatrex; Aatrex 4L; Aatrex 80W; Aatrex
names (including Nine-O; Aktikon; Aktikon PK; Aktinit A;
formulations): Aktinit PK Argezin; Atazinax; Atranex;
Atrasine; Atratol A; Atrazin; Atred; Atrex;
Candex; Cekuzina-T; Crisatrina; Crisazine;
Cyazin; Farmco Atrazine; Fenamin; Fenamine;
Fenatrol; G 30027; Geigy 30,027; Gesaprim;
Gesoprim; Griffex; Hungazin; Hungazin PK;
Inakor; Oleogesaprim; Primatol; Primatol A;
Primaze; Radazin; Radizine; Strazine;
Triazine A 1294; Vectal; Vectal SC; Weedex A;
Wonuk; Zeazin; Zeazine
CAS chemical name: 2-chloro-4-ethylamine-6-isopropylamino- S-
triazine
Synonyms: S-triazine, 2-chloro-4-ethylamino-
6-isopropylamino-; 1,3,5-triazine-2,4-
diamine, 6-chloro- N-ethyl- N'-
(1-methylethyl)-; 2-aethylamino-4-
isopropylamino-6-chlor- 1,3,5-triazin;
2-chloro-4-ethylamineiso-propyl-
amine- S-triazine; 1-Chloro-3-ethylamino-5-
isopropyl-amino-2,4,6-triazine;
1-chloro-3-ethylamino-5-isopropyl-amino- S-
triazine; 2-chloro-4-ethylamino-6-isopropyl-
amino- 1,3,5-triazine;
2-chloro-4-ethylamino-6-isopropyl-
amino- S-triazine 6-chloro- N-ethyl-
N'-(1-methylethyl)-1,3,5-triazine-
2,4-diamine; 2-chloro-4-(2-propylamino)-
6-ethylamino- S-triazine
CAS registry
number: 1912-24-9
RTECS registry
number: XY5600000
According to FAO specifications, commercial atrazine should be at
least 92% pure, and most products are about 95% pure. Common
impurities are sodium chloride and other symmetric triazines, such as
simazine and propazine. It is often formulated as wettable powders,
granules, and as flowable suspensions.
Wettable powder formulations contain 450-800 g atrazine/kg (less if
mixed with other pesticides), and liquid formulations (including
suspension concentrate), 40-650 g/litre.
1.2 Physical and Chemical Properties
Technical atrazine is a colourless crystalline powder with low vapour
pressure (40 nPa at 20°C). A melting point range of 175-177°C has been
reported for the technical product. It is readily soluble in dimethyl
sulfoxide (183 g/litre), moderately soluble in methanol (18 g/litre),
diethyl ether (12 g/litre), chloroform (52 g/litre), and ethyl acetate
(28 g/litre), and very slightly soluble in water (30 mg/litre). It is
stable in the dry state, but is hydrolysed to the herbicidally
inactive 2-hydroxy analogue in acid or in alkaline solutions and more
slowly in neutral aqueous solutions, even at elevated temperatures.
1.3 Analytical Methods
Gas chromatography with a nitrogen-phosphorus detector (NPD) is used
for the determination of residues and the analysis of environmental
samples. The minimum detection limit varies according to the
substrate.
1.4 Production and Uses
Atrazine was introduced in 1958. In 1987, total worldwide production
was estimated to be 70 000 tonnes.
Atrazine is a selective pre- and post-emergence herbicide used for the
control of weeds in crops, such as asparagus, corn, sorghum, sugar
cane, and pineapple. It is also used in forestry and, at higher
application rates, for non-selective weed control in non-crop areas.
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Atrazine
Highest human exposure to atrazine is associated with its production
and its use in agriculture. Widespread, low-level exposure of the
general population may occur through contaminated drinking-water.
2.2 Uptake, Metabolism, and Excretion
Atrazine is readily absorbed from the gastrointestinal tract, but only
penetrates the skin to a very limited extent. The absorbed herbicide
is rapidly eliminated. In the rat, the whole-body half-life is about
1.3 days, and 95% of the dose is eliminated within 7 days. The highest
concentration of atrazine and/or its metabolites is found in the red
blood cells, to which the triazines bind effectively. In rats given
low, oral doses, daily, levels were found in the tissues in the
following decreasing order: erythrocytes, liver, spleen, kidney. The
primary route of elimination in rodents is via the urine (about 75%);
approximately 20% is eliminated in the faeces.
Dealkylation at the C-4 and C-6 positions of the atrazine molecule are
the principal metabolic degradation reactions. Dechlorination at the
C-2 position also occurs, to some extent. In the rat, the major
urinary metabolites include the two mono- N-dealkylated metabolites
and the fully dealkylated derivative, 2-chloro-4,6-diamino- S-triazine.
2.3 Effects on Animals
The acute oral toxicity and the dermal toxicity of atrazine in rodents
are both low. Acute oral studies conducted on different rodent species
have indicated LD50 values in the range of 1700-4000 mg/kg. Young
rats are much less sensitive to atrazine than adults. Ruminants seem
to be much more sensitive to the acute toxic action of atrazine than
rodents. In one study, 2 doses of 250 mg/kg caused death in both sheep
and cattle.
The irritant action of atrazine is only slight on rabbit skin, but is
moderate for the rabbit eye.
Atrazine has been subjected to extensive long-term testing on rats,
mice, and dogs. Significant cardiac toxicity was observed in dogs
after long-term oral administration of atrazine at doses of 5 mg/kg
per day or more. In rats and mice, reduced food intake, decreased
weight gain, and toxic effects on other organs were detected after
long-term administration of atrazine at high dose levels (> 25 mg/kg
per day). These effects included muscle degeneration, retinal
degeneration, necrosis of the liver, and haematological effects in
rats and in mice. An increase in mammary tumours was observed in rats,
but not in mice. Taking into account the very high background level of
mammary tumours in untreated animals, the compound's apparent lack of
genotoxicity, and the fact that the maximum tolerated dose was
exceeded, these findings do not represent convincing evidence of
carcinogenicity.
Atrazine exerts a fetotoxic action at, or close to, dose levels that
have a deleterious action on the mothers, but does not seem to have
any significant teratogenic action in rats, mice, or rabbits.
2.4 Effects on Human Beings
There are no substantiated indications that atrazine causes any health
or safety hazards for the general population or for exposed workers.
2.5 Effects on the Environment
On the basis of acute and short-term dietary studies, the toxicity of
atrazine for birds is low, with no mortality at 10 000 mg/kg diet.
Atrazine is virtually non-toxic for bees, but is moderately toxic for
aquatic organisms (96-h LC50 range from 0.5 to 15 mg/litre). The
herbicide is degradable and has little tendency to bioaccumulate,
thereby limiting possible long-term adverse effects on wildlife and
fish. As it is an effective herbicide, its phytotoxicity may
constitute a problem in the case of uncontrolled applications.
However, the main concern is its relative persistence (half-life of
125 days in sandy soils) and mobility in some types of soils (related
to rainfall), resulting in contamination of surface and ground waters.
3. CONCLUSIONS AND RECOMMENDATIONS
The acute oral and dermal toxicities of atrazine for most mammalian
species are low. Atrazine has no significant teratogenic action in
rats, mice, or rabbits. There is no convincing evidence of
carcinogenicity in rats or mice. Toxic effects, including ocular,
cardiac, haematological, and hepatic effects, have been observed in
both rats and mice after long-term administration.
On the basis of acute and short-term dietary studies, the toxicity of
atrazine is low for birds and moderate for aquatic organisms. It has a
low capacity for bioconcentration.
The relative persistence and mobility of atrazine in some types of
soils can result in contamination of ground and surface waters. It is
recommended that adequate precautions should be taken to prevent this
contamination.
A few gaps in data have been identified that indicate a need for
further mutagenicity testing and studies of the mechanism of tumour
induction in rats.
Atrazine is moderately irritating to the eye. No significant health
effects on either the general population or exposed workers are
expected.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The acute toxicity of technical atrazine for human beings is thought
to be low, and no adverse health effects from exposure to this
herbicide have been reported. In view of the toxicity induced in
experimental animals on repeated exposure, proper care should be taken
during occupational use to avoid excessive inhalation of dust or spray
particles, and to prevent accidental contamination of food products
and water.
4.1.1 Prevention and Protection
In order to reduce the risk of accidental contamination, the following
precautions should be observed during handling and use:
(a) Avoid contact with the skin and eyes.
(b) Do not smoke, drink, or eat in the work-place. Wash hands and any
exposed skin before eating, drinking, or smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable powder
formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear protective PVC or
neoprene gloves.
(f) When handling leaking containers, or when dealing with leaks and
spills, wear overalls and PVC or neoprene gloves and boots. If
overalls become contaminated, change and wash them thoroughly before
re-use.
(g) Store products in dosed original containers, out of reach of
children, and away from food, drink, and animal feed.
4.1.2 First Aid
Poisoning by atrazine is unlikely, unless large amounts have been
ingested. In case of over-exposure, apply routine first-aid measures.
If material has been spilled on the skin, immediately remove the
patient from the source of contamination, remove all contaminated
clothing, and wash affected areas with soap and running water. If the
material is in the eyes, flush with clean water for at least 15
minutes. In case of ingestion of significant quantities, and, if the
patient is conscious, give a glass of water and subsequently
administer activated charcoal. Do not induce vomiting. In serious
cases, medical attention should be sought.
4.2 Advice to Physicians
The acute toxicity of atrazine for human beings is believed to be low.
There is no specific antidote. Treat symptomatically when required.
When large amounts have been ingested, gastric lavage may be
indicated.
4.3 Explosion and Fire Hazards
Technical atrazine is not flammable but, on heating, it decomposes to
form toxic fumes containing oxides of nitrogen (NOx) and hydrogen
chloride (HCl). Extinguish small fires with carbon dioxide, dry
powder, or alcohol-resistant foam. Water spray can be used for larger
fires and the cooling of unaffected stock, but avoid the accumulation
of polluted run-off from the site.
Beware: Some liquid formulations may be highly flammable and require
alcohol-resistant foam as an extinguishing agent.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
Keep spectators away from any leakage. Prevent contamination of other
goods or cargo, or nearby vegetation and waterways.
Absorb spilled liquid products with sawdust or sand, sweep up and
place in separate container.
Empty any product remaining in damaged or leaking containers into a
clean empty container, which should be suitably labelled.
Sweep up any spilled powder with damp earth or sand or other suitable
absorbent, such as sawdust, taking care not to raise a dust cloud (use
a vacuum cleaner). Place in separate container for subsequent
disposal. Contaminated absorbents, used containers, surplus product,
etc., should be burnt in an incinerator, preferably designed for
pesticide disposal. Hydrolysis under alkaline conditions (10% w/v
sodium hydroxide) is a suitable method to dispose of small quantities
of atrazine. Heating speeds the process. After hydrolysis, dilute and
dispose of via the sewage system. Atrazine is relatively stable and
characterized by high mobility in some soils and should not be buried
in dump sites, etc. Comply with any local legislation applying to
waste disposal.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Triazines may be ingested by domestic animals. In view of the
relatively high toxicity of atrazine for ruminants, exposure to
atrazine of sheep and cows should be avoided. Because of its relative
persistence and mobility in some types of soils, atrazine has a
significant potential for contamination of ground and surface waters.
The contamination of ponds, waterways, and ditches should be avoided.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Exposure Limit Values
Exposure limit values for atrazine are given in the following table.
6.2 Specific Restrictions
The National Swedish Chemicals Inspectorate has recently announced its
intention to prohibit the use of atrazine and other triazine
herbicides for weed elimination on gravel roads and similar surfaces.
Atrazine constitutes a contaminant required to be regulated by the US
Safe Water Drinking Act of 1986, including the promulgation of a MCLG
(Maximum Concentration Limit Goal).
6.3 Transport and Labelling
The United Nations Committee of Experts on Transportation of Dangerous
Goods classifies atrazine in:
- Hazard Class 6.1: poisonous substances;
- Packing group III: substance presenting a relatively low risk of
poisoning in transport.
EXPOSURE LIMIT VALUESa
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Occupational Australia Time-weighted average (TWA) 5 mg/m3 1983
Belgium Time-weighted average (TWA) 5 mg/m3 1988
Germany, Federal Time-weighted average (TWA) 2 mg/m3 1988
Republic of, - Maximum worksite concentration 2 mg/m3
Switzerland Time-weighted average (TWA) 10 mg/m3 1987
United Kingdom Maximum allowable concentration 2 mg/m3 1977
USSR (Ceiling value)
WATER Drinking- Canada Interim maximum acceptable 0.06 mg/litre 1987
concentration
EEC Maximum allowable concentration 0.1 µg/litre
Switzerland Maximum residue limit 0.1 µg/litre 1980
WHO/EURO Guideline value 2.0 µg/litre 1987
WATER Oral intake WHO/EURO Acceptable daily intake (ADI) 0.7 mg/g 1987
body weight
FOOD Plant (general) EEC Maximum residue limit 0.1 mg/kg
FOOD Plant (specified) Brazil Acceptable limit 0.1-0.2 mg/kg 1984
EXPOSURE LIMIT VALUESa (cont'd).
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant (general) Germany, Federal Maximum residue limit 0.1 mg/kg 1984
Republic of,
Plant (specified) Germany, Federal Maximum residue limit 0.5-10 mg/kg 1984
Republic of,
FOOD Specified food Kenya Maximum limit 0.02-0.25 mg/kg
products
FOOD Raw agricultural USA Tolerance (Acceptable residue limit) 0.02-15 mg/kg 1982
products
(specified plant
and animal
products)
Oral intake USA Reference dose 0.005 mg/kg
body weight
FOOD Specified food USSR Acceptable daily intake (ADI) 0.004 mg/kg 1983
products
FOOD Oral intake USA Tolerance 0.005 mg/kg
GOODS Brazil Use in domestic sanitation not
authorized
a For other regulatory data see complete legal file of IRC data profile.
The label should be as follows:
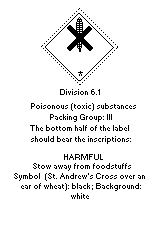 The European Economic Community legislation requires labelling as a
dangerous substance, using the symbol:
The European Economic Community legislation requires labelling as a
dangerous substance, using the symbol:
 The label must read:
Harmful by inhalation, in contact with the skin, and if swallowed;
keep out of reach of children; keep away from food drink; and animal
feed.
The label must read:
Harmful by inhalation, in contact with the skin, and if swallowed;
keep out of reach of children; keep away from food drink; and animal
feed.
See Also:
Toxicological Abbreviations
Atrazine (ICSC)
Atrazine (IARC Summary & Evaluation, Volume 53, 1991)
Atrazine (IARC Summary & Evaluation, Volume 73, 1999)
 Relative molecular 215.7
mass:
Common trade A 361; Aatrex; Aatrex 4L; Aatrex 80W; Aatrex
names (including Nine-O; Aktikon; Aktikon PK; Aktinit A;
formulations): Aktinit PK Argezin; Atazinax; Atranex;
Atrasine; Atratol A; Atrazin; Atred; Atrex;
Candex; Cekuzina-T; Crisatrina; Crisazine;
Cyazin; Farmco Atrazine; Fenamin; Fenamine;
Fenatrol; G 30027; Geigy 30,027; Gesaprim;
Gesoprim; Griffex; Hungazin; Hungazin PK;
Inakor; Oleogesaprim; Primatol; Primatol A;
Primaze; Radazin; Radizine; Strazine;
Triazine A 1294; Vectal; Vectal SC; Weedex A;
Wonuk; Zeazin; Zeazine
CAS chemical name: 2-chloro-4-ethylamine-6-isopropylamino- S-
triazine
Synonyms: S-triazine, 2-chloro-4-ethylamino-
6-isopropylamino-; 1,3,5-triazine-2,4-
diamine, 6-chloro- N-ethyl- N'-
(1-methylethyl)-; 2-aethylamino-4-
isopropylamino-6-chlor- 1,3,5-triazin;
2-chloro-4-ethylamineiso-propyl-
amine- S-triazine; 1-Chloro-3-ethylamino-5-
isopropyl-amino-2,4,6-triazine;
1-chloro-3-ethylamino-5-isopropyl-amino- S-
triazine; 2-chloro-4-ethylamino-6-isopropyl-
amino- 1,3,5-triazine;
2-chloro-4-ethylamino-6-isopropyl-
amino- S-triazine 6-chloro- N-ethyl-
N'-(1-methylethyl)-1,3,5-triazine-
2,4-diamine; 2-chloro-4-(2-propylamino)-
6-ethylamino- S-triazine
CAS registry
number:
Relative molecular 215.7
mass:
Common trade A 361; Aatrex; Aatrex 4L; Aatrex 80W; Aatrex
names (including Nine-O; Aktikon; Aktikon PK; Aktinit A;
formulations): Aktinit PK Argezin; Atazinax; Atranex;
Atrasine; Atratol A; Atrazin; Atred; Atrex;
Candex; Cekuzina-T; Crisatrina; Crisazine;
Cyazin; Farmco Atrazine; Fenamin; Fenamine;
Fenatrol; G 30027; Geigy 30,027; Gesaprim;
Gesoprim; Griffex; Hungazin; Hungazin PK;
Inakor; Oleogesaprim; Primatol; Primatol A;
Primaze; Radazin; Radizine; Strazine;
Triazine A 1294; Vectal; Vectal SC; Weedex A;
Wonuk; Zeazin; Zeazine
CAS chemical name: 2-chloro-4-ethylamine-6-isopropylamino- S-
triazine
Synonyms: S-triazine, 2-chloro-4-ethylamino-
6-isopropylamino-; 1,3,5-triazine-2,4-
diamine, 6-chloro- N-ethyl- N'-
(1-methylethyl)-; 2-aethylamino-4-
isopropylamino-6-chlor- 1,3,5-triazin;
2-chloro-4-ethylamineiso-propyl-
amine- S-triazine; 1-Chloro-3-ethylamino-5-
isopropyl-amino-2,4,6-triazine;
1-chloro-3-ethylamino-5-isopropyl-amino- S-
triazine; 2-chloro-4-ethylamino-6-isopropyl-
amino- 1,3,5-triazine;
2-chloro-4-ethylamino-6-isopropyl-
amino- S-triazine 6-chloro- N-ethyl-
N'-(1-methylethyl)-1,3,5-triazine-
2,4-diamine; 2-chloro-4-(2-propylamino)-
6-ethylamino- S-triazine
CAS registry
number:  The European Economic Community legislation requires labelling as a
dangerous substance, using the symbol:
The European Economic Community legislation requires labelling as a
dangerous substance, using the symbol:
 The label must read:
Harmful by inhalation, in contact with the skin, and if swallowed;
keep out of reach of children; keep away from food drink; and animal
feed.
The label must read:
Harmful by inhalation, in contact with the skin, and if swallowed;
keep out of reach of children; keep away from food drink; and animal
feed.
