
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 48
BENTAZONE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1990
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Bentazone: health and safety guide
(Health and safety guide ; no. 48)
1.Benzothiadiazines - standards I.Series
ISBN 92 4 151048 X (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Human exposure to bentazone
2.2. Uptake, metabolism, and excretion
2.3. Effects on animals
2.4. Effects on human beings
2.5. Effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Prevention and protection
4.1.2. First aid
4.2. Advice to physicians
4.3. Explosion and fire hazards
4.4. Storage and transport
4.5. Spillage and disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Exposure limit values
6.2. Specific restrictions
6.3. Transport and labelling
INTRODUCTION
This Health and Safety Guide is not based on an existing Environmental
Health Criteria document, but on critical national reviews. The hazard
evaluation in the Health and Safety Guide was made on the basis of
carefully selected studies, after scrutiny of the original
publications.
In order to assist the peer-review process of the present Health and
Safety Guide, a background companion document was prepared by the IPCS
and can be obtained from the Manager on request; the IPCS does not
intend that the background document should be published.
The first three sections of this Health and Safety Guide present
essential technical information and the hazard evaluation. Section 4
includes advice on preventive and protective measures and emergency
action; health workers should be thoroughly familiar with the medical
information to ensure that they can act efficiently in an emergency.
The section on regulatory information has been extracted from the
legal file of the International Register of Potentially Toxic
Chemicals (IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name Bentazone
Chemical formula: C10H12N2O3S
Chemical structure:
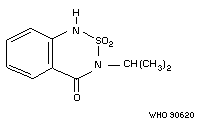 Common trade Bas 351-H; Basagran; Bendioxide;
names (including Bentazon
formulations):
CAS chemical name: 3-(1-methylethyl)-1H-2,1,3-benzothiadiazin-
4(3H)-one, 2,2-dioxide
Synonyms: 1H-2,1,3-benzothiadiazin-4(3H)-one-2,2-
dioxide, 3-isopropyl-; 3-isopropyl-
2,1,3-benzothiadiazinon-(4)-2,2-dioxide;
3-isopropyl-1H-2,1,3-benzothiadiazin-
4(3H)-one-2,2-
CAS registry 25057-89-0
number: 50723-80-3 (bentazone, sodium)
RTECS registry
number: DK 9900000
The technical product is about 93-96% pure. Main impurities are
anthranilic acid, N-isopropylsulfamoyl anthranilic acid, and sodium
chloride.
Almost all end-use products contain sodium bentazone as the active
ingredient.
The usual carrier is water, but, under certain conditions, an oil
concentrate is used.
1.2 Physical and Chemical Properties
Technical bentazone is a yellow-white crystalline powder without a
noticeable odour (vapour pressure < 10 nPa). A melting point range of
137-139°C has been reported for the technical product. It is slightly
soluble in water (0.5 g/litre), moderately soluble in benzene
(33 g/litre) and chloroform (180 g/litre), and readily soluble in
acetone (1.51 kg/litre) and ethanol (861 g/litre). Sodium bentazone is
considerably more soluble in water than the parent compound, with a
solubility of 2300 g/litre. Bentazone is stable in acid as well as
basic solutions; it decomposes at about 200°C.
The relative molecular mass is 240.3.
1.3 Analytical Methods
Residues are determined using gas chromatography with an N-specific
detector, after conversion to the 1-methyl derivatives. The minimum
detection limit varies according to the substrate.
1.4 Production and Uses
In 1982, 5 kilotonnes of bentazone/year were sold in the USA. It is
used as a contact herbicide for the control of broadleaf weeds and
sedges in crops, such as corn, rice, sorghum, soybeans, peanuts, corn,
peas, and established ornamental turf. It is applied, from the ground
or from the air, as a broadcast foliar spray, after crop and weeds
have emerged from the soil.
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Bentazone
No investigations dealing with the quantification of human exposure to
bentazone have been found.
2.2 Uptake, Metabolism, and Excretion
The uptake, metabolism, and excretion of bentazone have been studied
in rats and rabbits. The substance is rapidly absorbed from the
gastrointestinal tract and distributed to various organs and tissues.
Penetration across the blood-brain barrier does not occur. Bentazone
is rapidly eliminated from the mammalian organism, mainly via the
urine as unchanged bentazone.
2.3 Effects on Animals
Bentazone has a moderate to low oral acute toxicity (oral LD50,
1100 mg/kg in the rat). Inhalation toxicity appears to be low.
After repeated administration of high doses, bentazone has
demonstrated a potential for inducing toxicity involving the kidneys
and liver of experimental animals. Under these conditions, it also
seems to interfere with blood coagulation.
Although there was no apparent indication of carcinogenicity in rats,
a slight increase in proliferative lesions was observed in female
mice. The biological significance of this is not clear. No indications
of genotoxicity were found in several in vivo and in vitro assays.
Bentazone does not appear to be tetratogenic.
2.4 Effects on Human Beings
No information is available.
2.5 Effects on the Environment
Based on acute and short-term dietary tests, bentazone is practically
non-toxic to birds. Avian reproduction testing did not reveal any
significant effects up to the highest dietary levels tested.
Furthermore, technical bentazone is practically non-toxic for
freshwater fish, and only slightly toxic for aquatic invertebrates.
Bentazone appears to be stable to hydrolysis, but photodegrades in
water with a half-life of < 24 h. It is also photochemically degraded
in soil. Under aerobic conditions, bentazone degrades with a half-life
ranging from < 7 days to 14 weeks, depending on soil types and
conditions.
Bentazone is very mobile in soil and, therefore, has the potential to
contaminate surface water.
Although degradation is relatively rapid, bentazone, or its major
metabolite, could contaminate ground water.
Bentazone has a low potential for bioaccumulation, with a measured
bioconcentration factor of < 10 in crayfish.
3. CONCLUSIONS AND RECOMMENDATIONS
The acute toxicity of bentazone in animals is low to moderate. After
long-term administration of high doses, various manifestations of
toxicity were observed in rodents and dogs, including hepatic and
renal toxicity. Although there was no apparent indication of
oncogenicity in rats, a slight increase in proliferative lesions was
observed in the liver in female mice. The biological significance of
this increase is not clear. No indications of genotoxicity were found
in several in vivo and in vitro assays. Although bentazone does
not appear to present a teratogenic hazard for human beings,
additional reproductive toxicity testing is needed.
There is no indication that bentazone poses a health hazard for the
general population or exposed workers.
Bentazone is practically non-toxic for birds and fish and is slightly
toxic for aquatic invertebrates. Bentazone is highly mobile in soils
and may not be readily degradable, depending on soil type and
conditions. Thus, adequate precautions must be taken to avoid
contamination of surface and ground water.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The toxicity of technical bentazone for human beings is thought to be
low, and no adverse health effects from exposure to this herbicide
have been reported. Nevertheless, proper care should be taken during
occupational use to avoid excessive inhalation of dust or spray
particles and to prevent accidental contamination of food products and
water.
4.1.1 Prevention and Protection
The following precautions should be observed during handling and use,
in order to reduce the risk of accidental contamination:
(a) Avoid contact with the skin and eyes.
(b) Do not smoke, drink, or eat in the workplace. Wash hands and any
exposed skin before eating, drinking, smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable powder
formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear protective PVC or
neoprene gloves.
(f) When handling leaking containers, or when dealing with leaks and
spills, wear overalls, PVC or neoprene gloves, boots and eye/face
protection. If overalls become contaminated, change and wash them
thoroughly before re-use.
(g) Store products in closed original containers, out of reach of
children, and away from food, drink, and animal feed.
4.1.2 First Aid
Acute poisoning by bentazone is unlikely unless large amounts have
been ingested. In case of over-exposure, apply routine first-aid
measures. If material has been spilled on the skin, immediately remove
the patient from the source of contamination, remove all contaminated
clothing, and wash affected areas with soap and running water. If the
material is in the eyes, flush with clean water for at least 15
minutes. In case of ingestion of significant quantities, if the
patient is conscious, give several glasses of water. Do not induce
vomiting. In serious cases, medical attention should be sought.
4.2 Advice to Physicians
The acute toxicity of bentazone for human beings is believed to be
low. There is no specific antidote. Treat symptomatically when
required. In cases of ingestion of large amounts, gastric lavage may
be indicated.
4.3 Explosion and Fire Hazards
Bentazone as such is not flammable, but on heating may produce toxic
fumes, such as sulfur dioxide and oxides of nitrogen.
Extinguish small fires with carbon dioxide, dry powder, or alcohol-
resistant foam. Water spray can be used for larger fires and for the
cooling of unaffected stock, but avoid the accumulation of polluted
run-off from the site.
Beware: Some liquid formulations may be highly flammable and require
alcohol-resistant foam as an extinguishing agent.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
Avoid contact with solid or dust. Keep spectators away from any
leakage. Prevent contamination of other goods or cargo, or nearby
vegetation and waterways.
Absorb spilled liquid products with earth or sand. If available,
sawdust, peat moss, and straw are also suitable absorbents. Sweep up
and place in a separate container. Empty any product remaining in
damaged or leaking containers into a clean empty container, which
should be suitably labelled. Sweep up any spilled powder with damp
sawdust taking care not to raise a dust cloud (use a vacuum cleaner).
Remove trapped material with suction hoses. Place in separate
container for subsequent disposal.
Bentazone is characterized by high mobility in soil and should not be
buried in dump sites. Incineration seems to offer the most acceptable
method for disposal of bentazone. Contaminated absorbents, used
containers, and surplus product should be burnt in an incinerator,
preferably designed for pesticide disposal. Comply with any local
legislation applying to waste disposal.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Bentazone is highly mobile in soil and may contaminate ground water.
Technical bentazone is practically non-toxic for birds, fish, and
Daphnia and no adverse environmental effects have been reported.
Contamination of ponds, waterways, and ditches with bentazone should
be avoided.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with appropriate regulatory authorities
before application.
6.1 Exposure Limit Values
Exposure limit values are presented in the table on page 18.
6.2 Specific Restrictions
In the USA (1985), no new uses of bentazone will be permitted until a
data base adequate to complete a hazard assessment has been obtained.
6.3 Transport and Labelling
Supply and use labelling
European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
Common trade Bas 351-H; Basagran; Bendioxide;
names (including Bentazon
formulations):
CAS chemical name: 3-(1-methylethyl)-1H-2,1,3-benzothiadiazin-
4(3H)-one, 2,2-dioxide
Synonyms: 1H-2,1,3-benzothiadiazin-4(3H)-one-2,2-
dioxide, 3-isopropyl-; 3-isopropyl-
2,1,3-benzothiadiazinon-(4)-2,2-dioxide;
3-isopropyl-1H-2,1,3-benzothiadiazin-
4(3H)-one-2,2-
CAS registry 25057-89-0
number: 50723-80-3 (bentazone, sodium)
RTECS registry
number: DK 9900000
The technical product is about 93-96% pure. Main impurities are
anthranilic acid, N-isopropylsulfamoyl anthranilic acid, and sodium
chloride.
Almost all end-use products contain sodium bentazone as the active
ingredient.
The usual carrier is water, but, under certain conditions, an oil
concentrate is used.
1.2 Physical and Chemical Properties
Technical bentazone is a yellow-white crystalline powder without a
noticeable odour (vapour pressure < 10 nPa). A melting point range of
137-139°C has been reported for the technical product. It is slightly
soluble in water (0.5 g/litre), moderately soluble in benzene
(33 g/litre) and chloroform (180 g/litre), and readily soluble in
acetone (1.51 kg/litre) and ethanol (861 g/litre). Sodium bentazone is
considerably more soluble in water than the parent compound, with a
solubility of 2300 g/litre. Bentazone is stable in acid as well as
basic solutions; it decomposes at about 200°C.
The relative molecular mass is 240.3.
1.3 Analytical Methods
Residues are determined using gas chromatography with an N-specific
detector, after conversion to the 1-methyl derivatives. The minimum
detection limit varies according to the substrate.
1.4 Production and Uses
In 1982, 5 kilotonnes of bentazone/year were sold in the USA. It is
used as a contact herbicide for the control of broadleaf weeds and
sedges in crops, such as corn, rice, sorghum, soybeans, peanuts, corn,
peas, and established ornamental turf. It is applied, from the ground
or from the air, as a broadcast foliar spray, after crop and weeds
have emerged from the soil.
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Bentazone
No investigations dealing with the quantification of human exposure to
bentazone have been found.
2.2 Uptake, Metabolism, and Excretion
The uptake, metabolism, and excretion of bentazone have been studied
in rats and rabbits. The substance is rapidly absorbed from the
gastrointestinal tract and distributed to various organs and tissues.
Penetration across the blood-brain barrier does not occur. Bentazone
is rapidly eliminated from the mammalian organism, mainly via the
urine as unchanged bentazone.
2.3 Effects on Animals
Bentazone has a moderate to low oral acute toxicity (oral LD50,
1100 mg/kg in the rat). Inhalation toxicity appears to be low.
After repeated administration of high doses, bentazone has
demonstrated a potential for inducing toxicity involving the kidneys
and liver of experimental animals. Under these conditions, it also
seems to interfere with blood coagulation.
Although there was no apparent indication of carcinogenicity in rats,
a slight increase in proliferative lesions was observed in female
mice. The biological significance of this is not clear. No indications
of genotoxicity were found in several in vivo and in vitro assays.
Bentazone does not appear to be tetratogenic.
2.4 Effects on Human Beings
No information is available.
2.5 Effects on the Environment
Based on acute and short-term dietary tests, bentazone is practically
non-toxic to birds. Avian reproduction testing did not reveal any
significant effects up to the highest dietary levels tested.
Furthermore, technical bentazone is practically non-toxic for
freshwater fish, and only slightly toxic for aquatic invertebrates.
Bentazone appears to be stable to hydrolysis, but photodegrades in
water with a half-life of < 24 h. It is also photochemically degraded
in soil. Under aerobic conditions, bentazone degrades with a half-life
ranging from < 7 days to 14 weeks, depending on soil types and
conditions.
Bentazone is very mobile in soil and, therefore, has the potential to
contaminate surface water.
Although degradation is relatively rapid, bentazone, or its major
metabolite, could contaminate ground water.
Bentazone has a low potential for bioaccumulation, with a measured
bioconcentration factor of < 10 in crayfish.
3. CONCLUSIONS AND RECOMMENDATIONS
The acute toxicity of bentazone in animals is low to moderate. After
long-term administration of high doses, various manifestations of
toxicity were observed in rodents and dogs, including hepatic and
renal toxicity. Although there was no apparent indication of
oncogenicity in rats, a slight increase in proliferative lesions was
observed in the liver in female mice. The biological significance of
this increase is not clear. No indications of genotoxicity were found
in several in vivo and in vitro assays. Although bentazone does
not appear to present a teratogenic hazard for human beings,
additional reproductive toxicity testing is needed.
There is no indication that bentazone poses a health hazard for the
general population or exposed workers.
Bentazone is practically non-toxic for birds and fish and is slightly
toxic for aquatic invertebrates. Bentazone is highly mobile in soils
and may not be readily degradable, depending on soil type and
conditions. Thus, adequate precautions must be taken to avoid
contamination of surface and ground water.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The toxicity of technical bentazone for human beings is thought to be
low, and no adverse health effects from exposure to this herbicide
have been reported. Nevertheless, proper care should be taken during
occupational use to avoid excessive inhalation of dust or spray
particles and to prevent accidental contamination of food products and
water.
4.1.1 Prevention and Protection
The following precautions should be observed during handling and use,
in order to reduce the risk of accidental contamination:
(a) Avoid contact with the skin and eyes.
(b) Do not smoke, drink, or eat in the workplace. Wash hands and any
exposed skin before eating, drinking, smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable powder
formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear protective PVC or
neoprene gloves.
(f) When handling leaking containers, or when dealing with leaks and
spills, wear overalls, PVC or neoprene gloves, boots and eye/face
protection. If overalls become contaminated, change and wash them
thoroughly before re-use.
(g) Store products in closed original containers, out of reach of
children, and away from food, drink, and animal feed.
4.1.2 First Aid
Acute poisoning by bentazone is unlikely unless large amounts have
been ingested. In case of over-exposure, apply routine first-aid
measures. If material has been spilled on the skin, immediately remove
the patient from the source of contamination, remove all contaminated
clothing, and wash affected areas with soap and running water. If the
material is in the eyes, flush with clean water for at least 15
minutes. In case of ingestion of significant quantities, if the
patient is conscious, give several glasses of water. Do not induce
vomiting. In serious cases, medical attention should be sought.
4.2 Advice to Physicians
The acute toxicity of bentazone for human beings is believed to be
low. There is no specific antidote. Treat symptomatically when
required. In cases of ingestion of large amounts, gastric lavage may
be indicated.
4.3 Explosion and Fire Hazards
Bentazone as such is not flammable, but on heating may produce toxic
fumes, such as sulfur dioxide and oxides of nitrogen.
Extinguish small fires with carbon dioxide, dry powder, or alcohol-
resistant foam. Water spray can be used for larger fires and for the
cooling of unaffected stock, but avoid the accumulation of polluted
run-off from the site.
Beware: Some liquid formulations may be highly flammable and require
alcohol-resistant foam as an extinguishing agent.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
Avoid contact with solid or dust. Keep spectators away from any
leakage. Prevent contamination of other goods or cargo, or nearby
vegetation and waterways.
Absorb spilled liquid products with earth or sand. If available,
sawdust, peat moss, and straw are also suitable absorbents. Sweep up
and place in a separate container. Empty any product remaining in
damaged or leaking containers into a clean empty container, which
should be suitably labelled. Sweep up any spilled powder with damp
sawdust taking care not to raise a dust cloud (use a vacuum cleaner).
Remove trapped material with suction hoses. Place in separate
container for subsequent disposal.
Bentazone is characterized by high mobility in soil and should not be
buried in dump sites. Incineration seems to offer the most acceptable
method for disposal of bentazone. Contaminated absorbents, used
containers, and surplus product should be burnt in an incinerator,
preferably designed for pesticide disposal. Comply with any local
legislation applying to waste disposal.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Bentazone is highly mobile in soil and may contaminate ground water.
Technical bentazone is practically non-toxic for birds, fish, and
Daphnia and no adverse environmental effects have been reported.
Contamination of ponds, waterways, and ditches with bentazone should
be avoided.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with appropriate regulatory authorities
before application.
6.1 Exposure Limit Values
Exposure limit values are presented in the table on page 18.
6.2 Specific Restrictions
In the USA (1985), no new uses of bentazone will be permitted until a
data base adequate to complete a hazard assessment has been obtained.
6.3 Transport and Labelling
Supply and use labelling
European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
 The label must read:
R20 Harmful by inhalation
R21 Harmful in contact with skin
R22 Harmful if swallowed
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feed.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
EXPOSURE LIMIT VALUESa
Medium Specification Country/ Exposure limit description Value Effective
organization date
WATER Drinking- USA Long-term maximum concentration: 0.06 mg/litre 1987
for a 10-kg child 0.25 mg/litre
for a 70-kg adult 0.875 mg/litre
Life-time health advisory value 87.5 µg/litre 1987
WATER Oral intake WHO/ WHO-EURO/guideline value 2.0 µg/litre 1987
EURO ADI 0.0075 mg/kg 1987
FOOD Plant (specified) Germany, Federal Tolerance 0.1 mg/kg 1984
Republic of
FOOD Animal feed USA Tolerance 4 mg/kg
FOOD Raw specified USA Tolerance 0.05-3.0 mg/kg 1984
plants
Raw specified USA Tolerance 0.02-0.05 mg/kg 1984
animal products
a For further details of other regulatory data, see IRPTC legal file.
The label must read:
R20 Harmful by inhalation
R21 Harmful in contact with skin
R22 Harmful if swallowed
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feed.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
EXPOSURE LIMIT VALUESa
Medium Specification Country/ Exposure limit description Value Effective
organization date
WATER Drinking- USA Long-term maximum concentration: 0.06 mg/litre 1987
for a 10-kg child 0.25 mg/litre
for a 70-kg adult 0.875 mg/litre
Life-time health advisory value 87.5 µg/litre 1987
WATER Oral intake WHO/ WHO-EURO/guideline value 2.0 µg/litre 1987
EURO ADI 0.0075 mg/kg 1987
FOOD Plant (specified) Germany, Federal Tolerance 0.1 mg/kg 1984
Republic of
FOOD Animal feed USA Tolerance 4 mg/kg
FOOD Raw specified USA Tolerance 0.05-3.0 mg/kg 1984
plants
Raw specified USA Tolerance 0.02-0.05 mg/kg 1984
animal products
a For further details of other regulatory data, see IRPTC legal file.
See Also:
Toxicological Abbreviations
Bentazone (ICSC)
Bentazone (Pesticide residues in food: 1991 evaluations Part II Toxicology)
 Common trade Bas 351-H; Basagran; Bendioxide;
names (including Bentazon
formulations):
CAS chemical name: 3-(1-methylethyl)-1H-2,1,3-benzothiadiazin-
4(3H)-one, 2,2-dioxide
Synonyms: 1H-2,1,3-benzothiadiazin-4(3H)-one-2,2-
dioxide, 3-isopropyl-; 3-isopropyl-
2,1,3-benzothiadiazinon-(4)-2,2-dioxide;
3-isopropyl-1H-2,1,3-benzothiadiazin-
4(3H)-one-2,2-
CAS registry
Common trade Bas 351-H; Basagran; Bendioxide;
names (including Bentazon
formulations):
CAS chemical name: 3-(1-methylethyl)-1H-2,1,3-benzothiadiazin-
4(3H)-one, 2,2-dioxide
Synonyms: 1H-2,1,3-benzothiadiazin-4(3H)-one-2,2-
dioxide, 3-isopropyl-; 3-isopropyl-
2,1,3-benzothiadiazinon-(4)-2,2-dioxide;
3-isopropyl-1H-2,1,3-benzothiadiazin-
4(3H)-one-2,2-
CAS registry  The label must read:
R20 Harmful by inhalation
R21 Harmful in contact with skin
R22 Harmful if swallowed
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feed.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
EXPOSURE LIMIT VALUESa
Medium Specification Country/ Exposure limit description Value Effective
organization date
WATER Drinking- USA Long-term maximum concentration: 0.06 mg/litre 1987
for a 10-kg child 0.25 mg/litre
for a 70-kg adult 0.875 mg/litre
Life-time health advisory value 87.5 µg/litre 1987
WATER Oral intake WHO/ WHO-EURO/guideline value 2.0 µg/litre 1987
EURO ADI 0.0075 mg/kg 1987
FOOD Plant (specified) Germany, Federal Tolerance 0.1 mg/kg 1984
Republic of
FOOD Animal feed USA Tolerance 4 mg/kg
FOOD Raw specified USA Tolerance 0.05-3.0 mg/kg 1984
plants
Raw specified USA Tolerance 0.02-0.05 mg/kg 1984
animal products
a For further details of other regulatory data, see IRPTC legal file.
The label must read:
R20 Harmful by inhalation
R21 Harmful in contact with skin
R22 Harmful if swallowed
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feed.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
EXPOSURE LIMIT VALUESa
Medium Specification Country/ Exposure limit description Value Effective
organization date
WATER Drinking- USA Long-term maximum concentration: 0.06 mg/litre 1987
for a 10-kg child 0.25 mg/litre
for a 70-kg adult 0.875 mg/litre
Life-time health advisory value 87.5 µg/litre 1987
WATER Oral intake WHO/ WHO-EURO/guideline value 2.0 µg/litre 1987
EURO ADI 0.0075 mg/kg 1987
FOOD Plant (specified) Germany, Federal Tolerance 0.1 mg/kg 1984
Republic of
FOOD Animal feed USA Tolerance 4 mg/kg
FOOD Raw specified USA Tolerance 0.05-3.0 mg/kg 1984
plants
Raw specified USA Tolerance 0.02-0.05 mg/kg 1984
animal products
a For further details of other regulatory data, see IRPTC legal file.
