
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 92
MORPHOLINE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1995
This is a companion volume to Environmental Health Criteria 179:
Morpholine
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Health and safety guide for Morpholine.
(Health and safety guide ; no. 92)
1.Morpholines - adverse effects
2.Enviromental exposure I.Series
ISBN 92 4 151092 7 (NLM Classification: QD 399)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analysis
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Exposure
2.2. Uptake, metabolism, and excretion
2.3. Effects on organisms in the environment
2.4. Effects on experimental animals and in vitro test systems
2.5. Effects on humans
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Recommendations for the protection of human health
3.2. Recommendations for the protection of the environment
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Human health hazards, prevention and protection, first aid
4.1.1. Information for physicians
4.1.1.1 Signs and symptoms of exposure
4.1.1.2 First aid
4.1.1.3 Medical treatment
4.1.2. Health surveillance advice
4.1.2.1 Initial medical screening
4.1.2.2 Periodic medical examination
4.2. Explosion and fire hazards
4.2.1. Reactivity data
4.2.2. Explosion hazards
4.2.3. Fire hazards
4.2.4. Extinguishing media
4.2.5. Special firefighting procedures
4.3. Storage
4.4. Transport
4.5. Spillage
4.6. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.4.1. Labelling
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
CAS/IUPAC name: morpholine
Chemical formula: C4H9NO
Chemical structure: 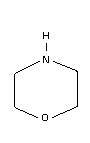 CAS registry number: 110-91-8
EC number: 613-028-00-9
EINECS number: 2038151
UN number: 2054
Synonyms: 1-oxa-4-azacyclohexane;
tetrahydro-2H-1,4-oxazine;
tetrahydro-1,4-oxazine;
tetrahydro-1,4-isoxazine; diethylene oximide;
diethyleneimide oxide; diethylene imidoxide
Relative molecular 87.12
mass:
Morpholine is distributed as an anhydrous liquid and as 40% and 88%
solutions with water. It is marketed as a product with approximately
99% purity.
1.2 Physical and Chemical Properties
Morpholine is a colourless, oily, hygroscopic, volatile liquid with a
characteristic amine odour. It is heavier than air and, as a result,
the vapour can travel a significant distance to a source of ignition
and "flash back".
Morpholine can undergo a diversity of chemical reactions. It is an
amino ether. The ether function of the molecule is typically inert and
most of the reactions involve the secondary amine group.
The physical and chemical properties of morpholine are given in
section 6.
1.3 Analysis
Methods suitable for measuring trace levels of morpholine include ion
chromatography (IC), gas chromatography (GC) with packed and also
capillary columns, high-performance liquid chromatography (HPLC)
usually using reverse phase (RP) columns.
The poor UV absorptivity of morpholine necessitates chemical
derivatization to detect trace amounts. Detection methods include
UV-detectors (HPLC) and flame ionisation (FID following GC) as well as
thermal energy analysers (TEA). Photochemical methods are not specific
for morpholine.
1.4 Production and Uses
At least 25 thousand tonnes of morpholine are produced throughout the
world each year. The main process used (in the western world) is the
reaction of diethylene glycol with ammonia in the presence of hydrogen
and catalysts. Morpholine is a versatile chemical. It is important as
a chemical intermediate in the rubber industry, as a corrosion
inhibitor, and in the synthesis of optical brighteners, pharmaceutical
products, crop protection agents, and dyes. Morpholine itself is a
solvent for a large variety of organic materials, including resins,
dyes, and waxes. It can be used as a catalyst. Morpholine is still
used in some countries in toiletry and cosmetic products as a
surfactant and emulsifier, at concentrations of up to 5%. It is used
in some countries in several direct and indirect food additive
applications.
2. SUMMARY AND EVALUATION
2.1 Exposure
The general population can be exposed to morpholine in food,
cosmetics, and tobacco. There is ample evidence that this substance
can be nitrosated to the carcinogenic N-nitrosomorpholine (NMOR) by
reactions outside, or within, the human body.
Food can become contaminated with morpholine in several ways: a)
through direct treatment of fruit with waxes containing morpholine for
conservation purposes; b) through steam treatment during processing,
and c) by use of packaging material containing morpholine.
Morpholine is used in some countries in cosmetic preparations,
particularly mascara eye makeup. NMOR has been found in some toiletry
articles and rubber articles including babies' pacifiers and bottle
nipples.
Morpholine and NMOR, probably from contact with waxed packaging and
containers, have been detected in various tobaccos. Neither of these
compounds has been detected in samples marketed in other packaging
materials.
Most countries recommend an 8-h TLV-TWA of 70 mg/m3 (skin notation)
for occupational exposure to morpholine, and a 15-min, short-term,
exposure limit (STEL) of 105 mg/m3.
2.2 Uptake, Metabolism, and Excretion
Morpholine is well absorbed after oral and dermal administration and
inhalation. Distribution studies showed that, in rabbits, following
inhalation or injection, the highest concentrations of morpholine were
found in the kidney, whereas, in rats, high concentrations were found
in muscle.
In mice, rats, hamsters, guinea-pigs, and rabbits, almost all ingested
or intravenously or intraperitoneally injected morpholine was excreted
unchanged in the urine. In guinea-pigs, the metabolite
N-methylmorpholine- N-oxide was identified. Only 0.5% was
eliminated as CO2.
NMOR may be formed following concomitant administration of morpholine
and nitrite or nitrous oxide under physiological conditions.
2.3 Effects on Organisms in the Environment
Morpholine has antibacterial and antimycotic properties, as has been
demonstrated on pathogenic organisms. The toxicity threshold of
morpholine for aerobic bacteria (after 16 h) was 310 mg/litre and for
green-blue algae (after 193 h), 1.7 mg/litre. Inhibition of protozoa
started at a morpholine concentration of 12 mg/litre, and growth of
algae was inhibited from 4.1 mg/litre.
The 24-h EC50 values for magna ranged from 100 to 119 mg/litre.
The EC0 values ranged from 16 to 68 mg/litre. The lowest LC50 for
freshwater fish (rainbow trout) was 180 mg/litre; the LC50 value for
a marine fish (tidewater silversides) was 400 mg/litre.
2.4 Effects on Experimental Animals and in vitro Test Systems
Morpholine administered orally resulted in LD50 values of 1-2 g/kg
body weight (rat) and 0.9 g/kg body weight (guinea-pig). Rats
receiving neutralized morpholine (1 g/kg body weight) survived.
In short-term oral administration of doses of half of the LD50 to
rats and guinea-pigs, nearly all the animals died before 30 days, the
principal symptoms being severe damage to the secreting tubules of the
kidney, fatty degeneration of the liver, and necrosis of the stomach
glandular epithelium. Long-term oral exposure to morpholine caused
fatty degeneration of the liver in rats.
Feeding up to 2.5% morpholine oleic acid salt (MOAS) resulted in
reduced body weight and renal malfunction in the highest-dose group;
otherwise, no effects were seen on physical appearance and general
behaviour, and there were no changes in biochemical, pathological, and
histological observations.
Discrepancies in the reports of morpholine vapour saturation
concentrations make an evaluation of inhalation data difficult.
Probable LC50 values are 8 g/m3 for rats and 5-7 g/m3 for mice.
In short-term inhalation studies, lung haemorrhage, and damage to the
kidneys and liver were described. At low concentrations (1.8 g/m3;
6 h/day; 9 days), weight loss and eye irritation were reported.
Long-term inhalation studies (up to 0.54 g morpholine/m3; 104 weeks)
showed increased incidences of inflammation of the cornea, and
inflammation and necrosis of the nasal cavity in rats.
In rats exposed to morpholine levels of up to 0.5 g/m3, keratitis,
oedema, abrasion, scarring, ulceration with or without
neovascularization, and corneal endothelial hyperplasia were observed.
The irritative action of morpholine was reduced on neutralization.
In studies on rabbits treated with a mascara composite containing 1%
morpholine, a slight redness of the conjunctiva was noted throughout
the study, but this disappeared 24 h after treatment.
No data are available on reproductive toxicity, embryotoxicity, and
teratogenicity.
Morpholine was reported to be non-mutagenic in microbial (Salmonella
typhimurium, Escherichia coli) gene mutation assays, with, and
without, metabolic activation (with an exception at a relatively high
concentration where there was also cytotoxicity), and in the
host-mediated assay. In a DNA damage and repair assay on primary rat
hepatocytes, no repair synthesis was induced. It did not produce point
or chromosomal mutations in hamster embryos exposed in utero.
Morpholine did not cause a meaningful increase in sister chromatid
exchanges in Chinese hamster ovary cells. It did not induce
unscheduled DNA synthesis.
Morpholine was considered to be weakly mutagenic in the L5178Y mouse
lymphoma assay. It induced increases in type III foci in the Balb/3T3
malignant cell transformation assay, but, when this was conducted at a
neutral pH, there was no significant induction of transformed foci.
Morpholine can be easily nitrosated to form N-nitrosomorpholine
(NMOR), the mutagenicity of which is well documented. The intake of
ascorbic acid can prevent the formation of nitrosamines. Morpholine,
given sequentially with nitrite or nitrate, was mutagenic in a
host-mediated mutagenicity assay using Salmonella typhimurium TA1950
as a genetic indicator system.
In a long-term inhalation study for carcinogenicity in rats, there was
no increase in the incidence of tumours at concentrations of
morpholine of up to 0.54 g/m3.
Similarly, in a long-term oral study on mice, administration of
morpholine oleate in the drinking-water did not result in any increase
in the incidence of tumours. The results of a long-term study in
which morpholine (1000 mg/kg) was fed in the diet showed a marginal
increase in tumours of the liver and lung in rats, but not in
hamsters. Morpholine fed to rats at a dietary concentration of
1000 mg/kg caused an increase in tumours (mostly hepatocellular
carcinomas and sarcomas of the liver and lungs), probably because of
the endogenous formation of NMOR.
2.5 Effects on Humans
There are no data on acute toxicity or the effects of short- or
long-term exposure to morpholine in the general public.
The phenomenon known as blue vision or glaucopsia, as well as some
instances of skin and respiratory tract irritation, have been
described in older reports of occupational exposure to morpholine.
When applied to human finger tips, undiluted morpholine causes
cracking of the eponychium and hyponychium about the nail. The intense
stinging sensation makes it impossible to tolerate it on the fingers
for a sufficient length of time to allow absorption. Diluted
morpholine (1:40) is also a mild irritant.
In a cytogenetic analysis of the lymphocytes in the peripheral blood,
24 workers (16 men, 8 women) with 3-10 years exposure to morpholine
(0.54-0.93 mg/m3, the maximum single concentration being
0.74-2.14 mg/m3) during production were compared with a control
group of the same size, from the same town, having no contact with
chemicals at work. There was no significant increase in the number of
cells with chromosome aberrations.
3. CONCLUSIONS AND RECOMMENDATIONS
Morpholine does not present a toxic risk for humans at the usual
levels of exposure, but its potential for conversion to the
carcinogenic compound N-nitrosomorpholine should be noted.
There is no evidence that, at present levels of exposure, morpholine
poses a substantial risk for biota in the environment.
3.1 Recommendations for the Protection of Human Health
Human exposure to morpholine should be avoided as far as possible.
Contamination of food through food packaging and food processing
should be avoided.
Morpholine should not be used in rubber products intended for direct
contact with humans.
Morpholine should not be used in toiletry or cosmetic preparations.
Industrial effluents should be rigorously treated to avoid entry of
morpholine into drinking-water.
In the light of the formation of carcinogenic N-nitrosomorpholine,
the present occupational exposure limits should be reconsidered.
3.2 Recommendations for the Protection of the Environment
The addition of spills and shock loads to effluent-treatment plants
should be avoided.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
Morpholine is a corrosive liquid and is a severe irritant for the
skin, eyes, and respiratory tract. It is toxic through skin absorption
and via inhalation. Thus, it is essential that the correct precautions
should be observed during handling and use.
4.1.1 Information for physicians
4.1.1.1 Signs and symptoms of exposure
Contact of undiluted product with eyes or skin causes severe
irritation and pain and may cause burns, necrosis, and permanent
injury. Burns of the eye may cause blindness. Morpholine is readily
absorbed and can cause malaise, discomfort, injury, and death, unless
treated promptly.
The vapour in low concentrations can cause lacrimation,
conjunctivitis, and corneal oedema. Inhalation of vapour causes
irritation of the respiratory tract; there may be coughing and chest
pain.
Repeated and/or prolonged exposure to morpholine at low levels may
damage the respiratory tract, produce adverse skin effects, or blue
vision (glaucopsia).
Medical conditions generally aggravated by exposure to irritant
agents, including morpholine, are:
- asthma
- skin disorders and allergies
- chronic respiratory disease, e.g., bronchitis, emphysema
- eye disease.
4.1.1.2 First aid
Eye contact: Eyes should be flushed immediately and gently with
large volumes of water for 15 min. Prompt medical assistance is
necessary.
Skin contact: The affected area should be flushed promptly with
large quantities of water for 15 min. With the exception of minor
cases, the affected area should be covered with a sterile dressing or
clean sheeting and the patient transported for medical care. Greases
or ointments should not be applied. When necessary, treat for shock.
Clothes should be laundered prior to reuse and contaminated leather
wear discarded.
Inhalation: The affected person should be moved to an
uncontaminated atmosphere. If breathing has stopped or is impaired,
assisted respiration (e.g., mouth to mouth) and/or supplemental oxygen
should be given. The air passages should be kept free of vomited
material and mucous. Medical attention should be sought.
Ingestion: In the event of ingestion, vomiting should not be
induced. Medical care and hospital treatment are essential, as soon
as possible.
4.1.1.3 Medical treatment
The main health effects of morpholine are similar to those of strongly
irritating agents, i.e., it is injurious to tissues. Chemical
pneumonitis, pulmonary oedema, laryngeal oedema, and delayed scarring
of the airways or other affected organs may occur following exposure.
There is no specific treatment. Clinical management is based upon
supportive treatment, which is similar to that for thermal burns.
Victims with major skin contact should be kept under medical
observation for at least 24 h.
4.1.2 Health surveillance advice
Each employee exposed to morpholine at potentially hazardous levels
should undergo an initial medical screening for history of certain
medical conditions (listed below) that might place the employee at
increased risk from morpholine exposure.
4.1.2.1 Initial medical screening
(a) Chronic respiratory disease: Morpholine causes respiratory tract
irritation. In persons with impaired pulmonary function, especially
those with obstructive airway diseases, the breathing of morpholine
may cause exacerbation of symptoms, because of its irritant
properties.
(b) Liver/kidney disease: Morpholine causes liver and kidney damage
in animals. This should therefore be considered before exposing
persons with impaired liver or renal function.
(c) Eye disease: Morpholine is an eye irritant and has caused
corneal oedema in exposed workers. Persons with pre-existing eye
disorders may be more susceptible to the effects of this agent.
(d) Skin disease: Morpholine is a primary skin irritant. Persons
with pre-existing skin disorders may be more susceptible to the
effects of this agent.
4.1.2.2 Periodic medical examination
Any employee developing the above-listed conditions should be referred
for further medical examination.
4.2 Explosion and Fire Hazards
4.2.1 Reactivity data
The product is chemically stable and insensitive to light.
However, violent reaction and fire may result when the product is
mixed with oxidizing agents, such as perchlorates, nitrates,
permanganates, chromates, nitric acid, halogens, peroxides, and some
cleaning solutions containing acids.
A reaction accompanied by great heat release occurs when the product
is mixed with acids. The heat generated may be sufficient to cause
vigorous boiling, creating a hazard because of the splashing or
splattering of hot material.
The product corrodes copper, aluminium, zinc, and galvanized surfaces.
Materials for containment should be constructed of iron or steel.
The combustion of morpholine in the presence of sufficient oxygen may
result in harmful concentrations of carbon monoxide, carbon dioxide,
and nitrogen oxide gases. Nitrogen oxide can react with water vapours
to yield corrosive nitric acid.
Combustion of morpholine under oxygen-starved conditions can be
expected to produce numerous toxic products including carbon monoxide,
hydrogen cyanide, nitriles, cyanic acid, isocyanates, cyanogens,
nitrosamines, amides, and carbamates.
Liquid morpholine attacks some forms of plastic and rubber.
Caution: N-nitrosomorpholine, known to be a potent carcinogen in
experimental animals, may be formed when the product comes in contact
with nitrous acid, nitrites, or atmospheres with high nitrogen oxide
concentrations.
4.2.2 Explosion hazards
If the product is heated to temperatures above 35°C, explosive
air-vapour mixtures may form.
4.2.3 Fire hazards
Flash-point (anhydrous): 35°C (closed cup); 38°C (open cup)
88% solution: 42°C
Flammability limits (%) (anhydrous):
Lower: 1.8%
Upper: 10.8%
Ignition temperature: 310°C
(anhydrous)
4.2.4 Extinguishing media
Water spray, carbon dioxide, dry chemical, and "alcohol" foam are
recommended as extinguishing agents. Water may be ineffective on
flames, but should be used to cool fire-exposed containers and to
disperse vapours.
4.2.5 Special firefighting procedures
Firefighters should wear self-contained breathing apparatus to prevent
inhalation of toxic ammonia, carbon monoxide, or nitrogen oxide gases,
which may form when the product is burned or is heated.
Suits, gloves, and boots, impervious to chemicals (e.g., butyl
rubber), should be worn.
4.3 Storage
Morpholine should be protected from atmospheric moisture and carbon
dioxide. As morpholine corrodes copper, aluminium, zinc, and
galvanized surfaces, the compound should be stored in iron or steel
containers, preferably located outdoors, above ground, and surrounded
by dykes to contain spills or leaks.
4.4 Transport
Local requirements regarding movements of hazardous goods should be
complied with.
UN Classification: Class 3; Number 2054
4.5 Spillage
All sources of ignition should be removed and, if possible, the leak
stopped. Spills should be contained and covered with sodium bisulfate
to neutralize the product. The spillage should then be sprayed with
water and scooped up into steel containers for proper disposal.
The spilled product must be prevented from entering drinking-water
supplies or streams. Morpholine is completely miscible with water.
4.6 Disposal
Incineration is acceptable and the preferred method of disposal;
however, nitrogen oxide emission controls may be required to meet
environmental regulations. Morpholine is also broken down by
activated sludge and this is a possible method of disposal under
controlled conditions.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1 Hazards
As morpholine is an important industrial chemical with a wide range of
applications, the presence of the compound or its derivatives is to be
expected in many industrial effluents.
Morpholine has a very low bioaccumulation potential. The compound is
inherently biodegradable. Biotic decomposition is possible in adapted
industrial treatment plants. In natural waters, biodegradation of
morpholine is probably not significant. It is acutely toxic for
aquatic organisms from 1.7 mg/litre (see section 2.3).
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, morpholine. It should be displayed at,
or near, entrances to areas where there is potential exposure to
morpholine, and on processing equipment and containers. The summary
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
in the summary clearly explained.
Space is available for insertion of the National Occupational Exposure
Limit, the address and telephone number of the National Poison Control
Centre, and local trade names.
MORPHOLINE
CAS chemical name: morpholine
Synonyms: tetrahydro-1,4-oxazine; diethylene oximide
CAS registry number: 110-91-8: RTECS registry number: QD6475000
UN number: 2054; EC number: 613-028-00-9
Chemical formula: C4H9NO; Relative molecular mass: 87.1
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point at 101.3 kPa 129°C Morpholine is a colourless hygroscopic liquid, with characteristic
Melting point -5°C odour; the substance decomposes on heating, producing toxic
Relative density (water=1) 1.0 gases (nitrous oxides); it is a base, reacts violently with acid and is
Solubility in water miscible corrosive to aluminium, zinc and copper; morpholine reacts with
Vapour pressure, kPa at 20°C 1.06 oxidants and nitrites; the carcinogenic N-nitrosomorpholine can
Relative vapour density (air=1) 3.00 be formed from nitrosation of morpholine
Relative density of the vapour/
air mixture at 20°C (air=1) 1.01
Flash point (open cup) 38-43°C
Autoignition temperature 310°C
Explosive limits, vol% in air 1.8-15.2
Octanol/water partition
coefficient as log Pow -0.86 (at pH 5)
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: The substance is strongly
irritant to the eyes, the skin, and the
respiratory tract; inhalation of the
substance may cause lung oedema;
exposure to high concentrations may
result in death; long-term exposure
may affect the liver and kidneys
SKIN: MAY BE ABSORBED; Wear protective gloves, Remove contaminated clothes, rinse skin with
redness, skin burns, pain protective clothing plenty of water or shower, and refer for
medical attention
EYES: Redness, pain, blurred Face shield First rinse with plenty of water for several
vision minutes (remove contact lenses if easily
possible); refer for medical attention
INHALATION: Cough, laboured PREVENT GENERATION Fresh air, rest, half-upright position, artificial
breathing, shortness of breath, OF MISTS; use ventilation, local respiration, if indicated, and refer for medical
sore throat exhaust, or breathing protection attention
INGESTION: Abdominal pain, Do not eat, drink, or Rinse mouth, give large amounts of water to
cough, diarrhoea, vomiting smoke during work drink, and refer for medical attention
ENVIRONMENT: This substance may be hazardous for the environment; special attention should be given to water
SPILLAGE STORAGE FIRE AND EXPLOSION
Consult an expert, collect leaking Dry, fireproof storage is necessary; Morpholine is flammable; above 38°C
and spilled liquid in sealable keep separate from oxidants, explosive vapour/air mixtures may be formed;
containers as far as possible, absorb nitrites, and, acids above 38°C use closed system, ventilation,
remaining liquid in sand or inert and explosion-proof electrical equipment; in
absorbent and remove to safe place case of fire, use powder, alcohol-resistant
(extra personal protection: foam, water spray, carbon dioxide; keep
self-contained breathing apparatus) drums, etc., cool by spraying with water
WASTE DISPOSAL NATIONAL INFORMATION
Incineration National Occupational National Poison Control Centre:
Controlled activated sludge Exposure Limit: Local trade names:
treatment
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1 Previous Evaluations by International Bodies
The carcinogenic risks were evaluated by an International Agency for
Research on Cancer ad hoc expert group in 1989. It was concluded
that there was inadequate evidence for assessing the carcinogenicity
of morpholine in experimental animals. No data were available from
studies on humans on the carcinogenicity of morpholine.
The overall evaluation was that morpholine was not classifiable as to
its carcinogenicity to humans (Group 3).
7.2 Exposure Limit Values
Most countries recommend an 8-h TLV-TWA of 70 mg/m3 for skin, and a
15-min, short-term, exposure limit (STEL) of 105 mg/m3 for
occupational exposure to MORP (ILO, 1991).
7.3 Specific Restrictions
The use of morpholine in cosmetics has been forbidden in EU countries
since 1986. In Germany, the use of morpholine in water-repellent food
packaging material is forbidden (BUA, 1991).
7.4 Labelling, Packaging, and Transport
7.4.1 Labelling
The United Nations Committee of Experts on the Transport of Dangerous
Goods classifies morpholine as:
Hazard - Class 3 - Flammable
Packing Group III
UN Number 2054
The European Union legislation on classification and labelling
requires supply labelling as a corrosive substance and gives the
following risk and safety phrases:
R 10: Flammable
R 20/21/22: Harmful by inhalation, in contact with skin, and
if swallowed
R 34: Causes burns
S 23: Do not breathe fumes/vapour
S 36 Wear suitable protective clothing
The US Department of Transportation (DOT) specifies the Proper
Shipping Name for anhydrous morpholine as Morpholine, Flammable
Liquid, UN 2054. The DOT Proper Shipping Name for morpholine solutions
is Morpholine, aqueous mixture, Corrosive Material, NA 1760.
The US Occupational Safety and Health Agency (OSHA) Hazard
Communication Standard (29CFR1910.1200) hazard classification for
anhydrous morpholine is:
-toxic by absorption through skin and inhalation,
-corrosive,
-flammable.
The US Environmental Protection Agency (EPA) Title III hazard class
is:
-fire hazard
-immediate health hazard.
BIBLIOGRAPHY
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices. Cincinnati, American Conference of
Governmental Industrial Hygienists Inc. (ACGIH), p. 417.
Air Products and Chemicals Inc. (1989) Material safety data sheet.
Allentown, 10 pp.
BUA (1991) GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance. Morpholine. Weinheim, VCH, 181 pp.
(BUA Report 56) (in German).
CEC (1987) Legislation on dangerous substances. Classification and
labelling in the European Communities, Vol. 2. Brussels, Commission
of the European Communities.
CEC/IPCS (1991) International chemical safety cards. Industrial Health
and Safety EUR 12561/6 Fifth Series ILO.
Clayton GD & Clayton FE, ed. (1982) Patty's industrial hygiene and
toxicology, 3rd. revised ed. Volume 2A, New York, Wiley-Interscience
Publications, pp. 2276, 2693-2696.
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed., Geneva, International Labour Office, pp. 282-283
(Occupational safety and health series, No. 37).
IPCS (in preparation) Environmental Health Criteria: Morpholine.
Geneva, World Health Organization.
IRPTC (1991) Data profile on morpholine. International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
OSHA (1981) Occupational health guidelines for chemical hazards:
Occupational health guideline for morpholine. Cincinnati, Ohio, Hazard
Evaluation and Technical Assistance Branch, Occupational Safety and
Health Agency, pp. 962-966.
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, New Jersey, Noyes Publications,
pp. 626-627.
SORBE (1990) Sicherheitstechnische Kenndaten. Gefahrenindex chemischer
Stoffe. Ecomed-Verlag (in German).
United Nations (1989) Recommendations on the transport of dangerous
goods, 6th rev. ed. New York, United Nations, p. 2054.
CAS registry number: 110-91-8
EC number: 613-028-00-9
EINECS number: 2038151
UN number: 2054
Synonyms: 1-oxa-4-azacyclohexane;
tetrahydro-2H-1,4-oxazine;
tetrahydro-1,4-oxazine;
tetrahydro-1,4-isoxazine; diethylene oximide;
diethyleneimide oxide; diethylene imidoxide
Relative molecular 87.12
mass:
Morpholine is distributed as an anhydrous liquid and as 40% and 88%
solutions with water. It is marketed as a product with approximately
99% purity.
1.2 Physical and Chemical Properties
Morpholine is a colourless, oily, hygroscopic, volatile liquid with a
characteristic amine odour. It is heavier than air and, as a result,
the vapour can travel a significant distance to a source of ignition
and "flash back".
Morpholine can undergo a diversity of chemical reactions. It is an
amino ether. The ether function of the molecule is typically inert and
most of the reactions involve the secondary amine group.
The physical and chemical properties of morpholine are given in
section 6.
1.3 Analysis
Methods suitable for measuring trace levels of morpholine include ion
chromatography (IC), gas chromatography (GC) with packed and also
capillary columns, high-performance liquid chromatography (HPLC)
usually using reverse phase (RP) columns.
The poor UV absorptivity of morpholine necessitates chemical
derivatization to detect trace amounts. Detection methods include
UV-detectors (HPLC) and flame ionisation (FID following GC) as well as
thermal energy analysers (TEA). Photochemical methods are not specific
for morpholine.
1.4 Production and Uses
At least 25 thousand tonnes of morpholine are produced throughout the
world each year. The main process used (in the western world) is the
reaction of diethylene glycol with ammonia in the presence of hydrogen
and catalysts. Morpholine is a versatile chemical. It is important as
a chemical intermediate in the rubber industry, as a corrosion
inhibitor, and in the synthesis of optical brighteners, pharmaceutical
products, crop protection agents, and dyes. Morpholine itself is a
solvent for a large variety of organic materials, including resins,
dyes, and waxes. It can be used as a catalyst. Morpholine is still
used in some countries in toiletry and cosmetic products as a
surfactant and emulsifier, at concentrations of up to 5%. It is used
in some countries in several direct and indirect food additive
applications.
2. SUMMARY AND EVALUATION
2.1 Exposure
The general population can be exposed to morpholine in food,
cosmetics, and tobacco. There is ample evidence that this substance
can be nitrosated to the carcinogenic N-nitrosomorpholine (NMOR) by
reactions outside, or within, the human body.
Food can become contaminated with morpholine in several ways: a)
through direct treatment of fruit with waxes containing morpholine for
conservation purposes; b) through steam treatment during processing,
and c) by use of packaging material containing morpholine.
Morpholine is used in some countries in cosmetic preparations,
particularly mascara eye makeup. NMOR has been found in some toiletry
articles and rubber articles including babies' pacifiers and bottle
nipples.
Morpholine and NMOR, probably from contact with waxed packaging and
containers, have been detected in various tobaccos. Neither of these
compounds has been detected in samples marketed in other packaging
materials.
Most countries recommend an 8-h TLV-TWA of 70 mg/m3 (skin notation)
for occupational exposure to morpholine, and a 15-min, short-term,
exposure limit (STEL) of 105 mg/m3.
2.2 Uptake, Metabolism, and Excretion
Morpholine is well absorbed after oral and dermal administration and
inhalation. Distribution studies showed that, in rabbits, following
inhalation or injection, the highest concentrations of morpholine were
found in the kidney, whereas, in rats, high concentrations were found
in muscle.
In mice, rats, hamsters, guinea-pigs, and rabbits, almost all ingested
or intravenously or intraperitoneally injected morpholine was excreted
unchanged in the urine. In guinea-pigs, the metabolite
N-methylmorpholine- N-oxide was identified. Only 0.5% was
eliminated as CO2.
NMOR may be formed following concomitant administration of morpholine
and nitrite or nitrous oxide under physiological conditions.
2.3 Effects on Organisms in the Environment
Morpholine has antibacterial and antimycotic properties, as has been
demonstrated on pathogenic organisms. The toxicity threshold of
morpholine for aerobic bacteria (after 16 h) was 310 mg/litre and for
green-blue algae (after 193 h), 1.7 mg/litre. Inhibition of protozoa
started at a morpholine concentration of 12 mg/litre, and growth of
algae was inhibited from 4.1 mg/litre.
The 24-h EC50 values for magna ranged from 100 to 119 mg/litre.
The EC0 values ranged from 16 to 68 mg/litre. The lowest LC50 for
freshwater fish (rainbow trout) was 180 mg/litre; the LC50 value for
a marine fish (tidewater silversides) was 400 mg/litre.
2.4 Effects on Experimental Animals and in vitro Test Systems
Morpholine administered orally resulted in LD50 values of 1-2 g/kg
body weight (rat) and 0.9 g/kg body weight (guinea-pig). Rats
receiving neutralized morpholine (1 g/kg body weight) survived.
In short-term oral administration of doses of half of the LD50 to
rats and guinea-pigs, nearly all the animals died before 30 days, the
principal symptoms being severe damage to the secreting tubules of the
kidney, fatty degeneration of the liver, and necrosis of the stomach
glandular epithelium. Long-term oral exposure to morpholine caused
fatty degeneration of the liver in rats.
Feeding up to 2.5% morpholine oleic acid salt (MOAS) resulted in
reduced body weight and renal malfunction in the highest-dose group;
otherwise, no effects were seen on physical appearance and general
behaviour, and there were no changes in biochemical, pathological, and
histological observations.
Discrepancies in the reports of morpholine vapour saturation
concentrations make an evaluation of inhalation data difficult.
Probable LC50 values are 8 g/m3 for rats and 5-7 g/m3 for mice.
In short-term inhalation studies, lung haemorrhage, and damage to the
kidneys and liver were described. At low concentrations (1.8 g/m3;
6 h/day; 9 days), weight loss and eye irritation were reported.
Long-term inhalation studies (up to 0.54 g morpholine/m3; 104 weeks)
showed increased incidences of inflammation of the cornea, and
inflammation and necrosis of the nasal cavity in rats.
In rats exposed to morpholine levels of up to 0.5 g/m3, keratitis,
oedema, abrasion, scarring, ulceration with or without
neovascularization, and corneal endothelial hyperplasia were observed.
The irritative action of morpholine was reduced on neutralization.
In studies on rabbits treated with a mascara composite containing 1%
morpholine, a slight redness of the conjunctiva was noted throughout
the study, but this disappeared 24 h after treatment.
No data are available on reproductive toxicity, embryotoxicity, and
teratogenicity.
Morpholine was reported to be non-mutagenic in microbial (Salmonella
typhimurium, Escherichia coli) gene mutation assays, with, and
without, metabolic activation (with an exception at a relatively high
concentration where there was also cytotoxicity), and in the
host-mediated assay. In a DNA damage and repair assay on primary rat
hepatocytes, no repair synthesis was induced. It did not produce point
or chromosomal mutations in hamster embryos exposed in utero.
Morpholine did not cause a meaningful increase in sister chromatid
exchanges in Chinese hamster ovary cells. It did not induce
unscheduled DNA synthesis.
Morpholine was considered to be weakly mutagenic in the L5178Y mouse
lymphoma assay. It induced increases in type III foci in the Balb/3T3
malignant cell transformation assay, but, when this was conducted at a
neutral pH, there was no significant induction of transformed foci.
Morpholine can be easily nitrosated to form N-nitrosomorpholine
(NMOR), the mutagenicity of which is well documented. The intake of
ascorbic acid can prevent the formation of nitrosamines. Morpholine,
given sequentially with nitrite or nitrate, was mutagenic in a
host-mediated mutagenicity assay using Salmonella typhimurium TA1950
as a genetic indicator system.
In a long-term inhalation study for carcinogenicity in rats, there was
no increase in the incidence of tumours at concentrations of
morpholine of up to 0.54 g/m3.
Similarly, in a long-term oral study on mice, administration of
morpholine oleate in the drinking-water did not result in any increase
in the incidence of tumours. The results of a long-term study in
which morpholine (1000 mg/kg) was fed in the diet showed a marginal
increase in tumours of the liver and lung in rats, but not in
hamsters. Morpholine fed to rats at a dietary concentration of
1000 mg/kg caused an increase in tumours (mostly hepatocellular
carcinomas and sarcomas of the liver and lungs), probably because of
the endogenous formation of NMOR.
2.5 Effects on Humans
There are no data on acute toxicity or the effects of short- or
long-term exposure to morpholine in the general public.
The phenomenon known as blue vision or glaucopsia, as well as some
instances of skin and respiratory tract irritation, have been
described in older reports of occupational exposure to morpholine.
When applied to human finger tips, undiluted morpholine causes
cracking of the eponychium and hyponychium about the nail. The intense
stinging sensation makes it impossible to tolerate it on the fingers
for a sufficient length of time to allow absorption. Diluted
morpholine (1:40) is also a mild irritant.
In a cytogenetic analysis of the lymphocytes in the peripheral blood,
24 workers (16 men, 8 women) with 3-10 years exposure to morpholine
(0.54-0.93 mg/m3, the maximum single concentration being
0.74-2.14 mg/m3) during production were compared with a control
group of the same size, from the same town, having no contact with
chemicals at work. There was no significant increase in the number of
cells with chromosome aberrations.
3. CONCLUSIONS AND RECOMMENDATIONS
Morpholine does not present a toxic risk for humans at the usual
levels of exposure, but its potential for conversion to the
carcinogenic compound N-nitrosomorpholine should be noted.
There is no evidence that, at present levels of exposure, morpholine
poses a substantial risk for biota in the environment.
3.1 Recommendations for the Protection of Human Health
Human exposure to morpholine should be avoided as far as possible.
Contamination of food through food packaging and food processing
should be avoided.
Morpholine should not be used in rubber products intended for direct
contact with humans.
Morpholine should not be used in toiletry or cosmetic preparations.
Industrial effluents should be rigorously treated to avoid entry of
morpholine into drinking-water.
In the light of the formation of carcinogenic N-nitrosomorpholine,
the present occupational exposure limits should be reconsidered.
3.2 Recommendations for the Protection of the Environment
The addition of spills and shock loads to effluent-treatment plants
should be avoided.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
Morpholine is a corrosive liquid and is a severe irritant for the
skin, eyes, and respiratory tract. It is toxic through skin absorption
and via inhalation. Thus, it is essential that the correct precautions
should be observed during handling and use.
4.1.1 Information for physicians
4.1.1.1 Signs and symptoms of exposure
Contact of undiluted product with eyes or skin causes severe
irritation and pain and may cause burns, necrosis, and permanent
injury. Burns of the eye may cause blindness. Morpholine is readily
absorbed and can cause malaise, discomfort, injury, and death, unless
treated promptly.
The vapour in low concentrations can cause lacrimation,
conjunctivitis, and corneal oedema. Inhalation of vapour causes
irritation of the respiratory tract; there may be coughing and chest
pain.
Repeated and/or prolonged exposure to morpholine at low levels may
damage the respiratory tract, produce adverse skin effects, or blue
vision (glaucopsia).
Medical conditions generally aggravated by exposure to irritant
agents, including morpholine, are:
- asthma
- skin disorders and allergies
- chronic respiratory disease, e.g., bronchitis, emphysema
- eye disease.
4.1.1.2 First aid
Eye contact: Eyes should be flushed immediately and gently with
large volumes of water for 15 min. Prompt medical assistance is
necessary.
Skin contact: The affected area should be flushed promptly with
large quantities of water for 15 min. With the exception of minor
cases, the affected area should be covered with a sterile dressing or
clean sheeting and the patient transported for medical care. Greases
or ointments should not be applied. When necessary, treat for shock.
Clothes should be laundered prior to reuse and contaminated leather
wear discarded.
Inhalation: The affected person should be moved to an
uncontaminated atmosphere. If breathing has stopped or is impaired,
assisted respiration (e.g., mouth to mouth) and/or supplemental oxygen
should be given. The air passages should be kept free of vomited
material and mucous. Medical attention should be sought.
Ingestion: In the event of ingestion, vomiting should not be
induced. Medical care and hospital treatment are essential, as soon
as possible.
4.1.1.3 Medical treatment
The main health effects of morpholine are similar to those of strongly
irritating agents, i.e., it is injurious to tissues. Chemical
pneumonitis, pulmonary oedema, laryngeal oedema, and delayed scarring
of the airways or other affected organs may occur following exposure.
There is no specific treatment. Clinical management is based upon
supportive treatment, which is similar to that for thermal burns.
Victims with major skin contact should be kept under medical
observation for at least 24 h.
4.1.2 Health surveillance advice
Each employee exposed to morpholine at potentially hazardous levels
should undergo an initial medical screening for history of certain
medical conditions (listed below) that might place the employee at
increased risk from morpholine exposure.
4.1.2.1 Initial medical screening
(a) Chronic respiratory disease: Morpholine causes respiratory tract
irritation. In persons with impaired pulmonary function, especially
those with obstructive airway diseases, the breathing of morpholine
may cause exacerbation of symptoms, because of its irritant
properties.
(b) Liver/kidney disease: Morpholine causes liver and kidney damage
in animals. This should therefore be considered before exposing
persons with impaired liver or renal function.
(c) Eye disease: Morpholine is an eye irritant and has caused
corneal oedema in exposed workers. Persons with pre-existing eye
disorders may be more susceptible to the effects of this agent.
(d) Skin disease: Morpholine is a primary skin irritant. Persons
with pre-existing skin disorders may be more susceptible to the
effects of this agent.
4.1.2.2 Periodic medical examination
Any employee developing the above-listed conditions should be referred
for further medical examination.
4.2 Explosion and Fire Hazards
4.2.1 Reactivity data
The product is chemically stable and insensitive to light.
However, violent reaction and fire may result when the product is
mixed with oxidizing agents, such as perchlorates, nitrates,
permanganates, chromates, nitric acid, halogens, peroxides, and some
cleaning solutions containing acids.
A reaction accompanied by great heat release occurs when the product
is mixed with acids. The heat generated may be sufficient to cause
vigorous boiling, creating a hazard because of the splashing or
splattering of hot material.
The product corrodes copper, aluminium, zinc, and galvanized surfaces.
Materials for containment should be constructed of iron or steel.
The combustion of morpholine in the presence of sufficient oxygen may
result in harmful concentrations of carbon monoxide, carbon dioxide,
and nitrogen oxide gases. Nitrogen oxide can react with water vapours
to yield corrosive nitric acid.
Combustion of morpholine under oxygen-starved conditions can be
expected to produce numerous toxic products including carbon monoxide,
hydrogen cyanide, nitriles, cyanic acid, isocyanates, cyanogens,
nitrosamines, amides, and carbamates.
Liquid morpholine attacks some forms of plastic and rubber.
Caution: N-nitrosomorpholine, known to be a potent carcinogen in
experimental animals, may be formed when the product comes in contact
with nitrous acid, nitrites, or atmospheres with high nitrogen oxide
concentrations.
4.2.2 Explosion hazards
If the product is heated to temperatures above 35°C, explosive
air-vapour mixtures may form.
4.2.3 Fire hazards
Flash-point (anhydrous): 35°C (closed cup); 38°C (open cup)
88% solution: 42°C
Flammability limits (%) (anhydrous):
Lower: 1.8%
Upper: 10.8%
Ignition temperature: 310°C
(anhydrous)
4.2.4 Extinguishing media
Water spray, carbon dioxide, dry chemical, and "alcohol" foam are
recommended as extinguishing agents. Water may be ineffective on
flames, but should be used to cool fire-exposed containers and to
disperse vapours.
4.2.5 Special firefighting procedures
Firefighters should wear self-contained breathing apparatus to prevent
inhalation of toxic ammonia, carbon monoxide, or nitrogen oxide gases,
which may form when the product is burned or is heated.
Suits, gloves, and boots, impervious to chemicals (e.g., butyl
rubber), should be worn.
4.3 Storage
Morpholine should be protected from atmospheric moisture and carbon
dioxide. As morpholine corrodes copper, aluminium, zinc, and
galvanized surfaces, the compound should be stored in iron or steel
containers, preferably located outdoors, above ground, and surrounded
by dykes to contain spills or leaks.
4.4 Transport
Local requirements regarding movements of hazardous goods should be
complied with.
UN Classification: Class 3; Number 2054
4.5 Spillage
All sources of ignition should be removed and, if possible, the leak
stopped. Spills should be contained and covered with sodium bisulfate
to neutralize the product. The spillage should then be sprayed with
water and scooped up into steel containers for proper disposal.
The spilled product must be prevented from entering drinking-water
supplies or streams. Morpholine is completely miscible with water.
4.6 Disposal
Incineration is acceptable and the preferred method of disposal;
however, nitrogen oxide emission controls may be required to meet
environmental regulations. Morpholine is also broken down by
activated sludge and this is a possible method of disposal under
controlled conditions.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1 Hazards
As morpholine is an important industrial chemical with a wide range of
applications, the presence of the compound or its derivatives is to be
expected in many industrial effluents.
Morpholine has a very low bioaccumulation potential. The compound is
inherently biodegradable. Biotic decomposition is possible in adapted
industrial treatment plants. In natural waters, biodegradation of
morpholine is probably not significant. It is acutely toxic for
aquatic organisms from 1.7 mg/litre (see section 2.3).
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, morpholine. It should be displayed at,
or near, entrances to areas where there is potential exposure to
morpholine, and on processing equipment and containers. The summary
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
in the summary clearly explained.
Space is available for insertion of the National Occupational Exposure
Limit, the address and telephone number of the National Poison Control
Centre, and local trade names.
MORPHOLINE
CAS chemical name: morpholine
Synonyms: tetrahydro-1,4-oxazine; diethylene oximide
CAS registry number: 110-91-8: RTECS registry number: QD6475000
UN number: 2054; EC number: 613-028-00-9
Chemical formula: C4H9NO; Relative molecular mass: 87.1
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point at 101.3 kPa 129°C Morpholine is a colourless hygroscopic liquid, with characteristic
Melting point -5°C odour; the substance decomposes on heating, producing toxic
Relative density (water=1) 1.0 gases (nitrous oxides); it is a base, reacts violently with acid and is
Solubility in water miscible corrosive to aluminium, zinc and copper; morpholine reacts with
Vapour pressure, kPa at 20°C 1.06 oxidants and nitrites; the carcinogenic N-nitrosomorpholine can
Relative vapour density (air=1) 3.00 be formed from nitrosation of morpholine
Relative density of the vapour/
air mixture at 20°C (air=1) 1.01
Flash point (open cup) 38-43°C
Autoignition temperature 310°C
Explosive limits, vol% in air 1.8-15.2
Octanol/water partition
coefficient as log Pow -0.86 (at pH 5)
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: The substance is strongly
irritant to the eyes, the skin, and the
respiratory tract; inhalation of the
substance may cause lung oedema;
exposure to high concentrations may
result in death; long-term exposure
may affect the liver and kidneys
SKIN: MAY BE ABSORBED; Wear protective gloves, Remove contaminated clothes, rinse skin with
redness, skin burns, pain protective clothing plenty of water or shower, and refer for
medical attention
EYES: Redness, pain, blurred Face shield First rinse with plenty of water for several
vision minutes (remove contact lenses if easily
possible); refer for medical attention
INHALATION: Cough, laboured PREVENT GENERATION Fresh air, rest, half-upright position, artificial
breathing, shortness of breath, OF MISTS; use ventilation, local respiration, if indicated, and refer for medical
sore throat exhaust, or breathing protection attention
INGESTION: Abdominal pain, Do not eat, drink, or Rinse mouth, give large amounts of water to
cough, diarrhoea, vomiting smoke during work drink, and refer for medical attention
ENVIRONMENT: This substance may be hazardous for the environment; special attention should be given to water
SPILLAGE STORAGE FIRE AND EXPLOSION
Consult an expert, collect leaking Dry, fireproof storage is necessary; Morpholine is flammable; above 38°C
and spilled liquid in sealable keep separate from oxidants, explosive vapour/air mixtures may be formed;
containers as far as possible, absorb nitrites, and, acids above 38°C use closed system, ventilation,
remaining liquid in sand or inert and explosion-proof electrical equipment; in
absorbent and remove to safe place case of fire, use powder, alcohol-resistant
(extra personal protection: foam, water spray, carbon dioxide; keep
self-contained breathing apparatus) drums, etc., cool by spraying with water
WASTE DISPOSAL NATIONAL INFORMATION
Incineration National Occupational National Poison Control Centre:
Controlled activated sludge Exposure Limit: Local trade names:
treatment
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1 Previous Evaluations by International Bodies
The carcinogenic risks were evaluated by an International Agency for
Research on Cancer ad hoc expert group in 1989. It was concluded
that there was inadequate evidence for assessing the carcinogenicity
of morpholine in experimental animals. No data were available from
studies on humans on the carcinogenicity of morpholine.
The overall evaluation was that morpholine was not classifiable as to
its carcinogenicity to humans (Group 3).
7.2 Exposure Limit Values
Most countries recommend an 8-h TLV-TWA of 70 mg/m3 for skin, and a
15-min, short-term, exposure limit (STEL) of 105 mg/m3 for
occupational exposure to MORP (ILO, 1991).
7.3 Specific Restrictions
The use of morpholine in cosmetics has been forbidden in EU countries
since 1986. In Germany, the use of morpholine in water-repellent food
packaging material is forbidden (BUA, 1991).
7.4 Labelling, Packaging, and Transport
7.4.1 Labelling
The United Nations Committee of Experts on the Transport of Dangerous
Goods classifies morpholine as:
Hazard - Class 3 - Flammable
Packing Group III
UN Number 2054
The European Union legislation on classification and labelling
requires supply labelling as a corrosive substance and gives the
following risk and safety phrases:
R 10: Flammable
R 20/21/22: Harmful by inhalation, in contact with skin, and
if swallowed
R 34: Causes burns
S 23: Do not breathe fumes/vapour
S 36 Wear suitable protective clothing
The US Department of Transportation (DOT) specifies the Proper
Shipping Name for anhydrous morpholine as Morpholine, Flammable
Liquid, UN 2054. The DOT Proper Shipping Name for morpholine solutions
is Morpholine, aqueous mixture, Corrosive Material, NA 1760.
The US Occupational Safety and Health Agency (OSHA) Hazard
Communication Standard (29CFR1910.1200) hazard classification for
anhydrous morpholine is:
-toxic by absorption through skin and inhalation,
-corrosive,
-flammable.
The US Environmental Protection Agency (EPA) Title III hazard class
is:
-fire hazard
-immediate health hazard.
BIBLIOGRAPHY
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices. Cincinnati, American Conference of
Governmental Industrial Hygienists Inc. (ACGIH), p. 417.
Air Products and Chemicals Inc. (1989) Material safety data sheet.
Allentown, 10 pp.
BUA (1991) GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance. Morpholine. Weinheim, VCH, 181 pp.
(BUA Report 56) (in German).
CEC (1987) Legislation on dangerous substances. Classification and
labelling in the European Communities, Vol. 2. Brussels, Commission
of the European Communities.
CEC/IPCS (1991) International chemical safety cards. Industrial Health
and Safety EUR 12561/6 Fifth Series ILO.
Clayton GD & Clayton FE, ed. (1982) Patty's industrial hygiene and
toxicology, 3rd. revised ed. Volume 2A, New York, Wiley-Interscience
Publications, pp. 2276, 2693-2696.
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed., Geneva, International Labour Office, pp. 282-283
(Occupational safety and health series, No. 37).
IPCS (in preparation) Environmental Health Criteria: Morpholine.
Geneva, World Health Organization.
IRPTC (1991) Data profile on morpholine. International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
OSHA (1981) Occupational health guidelines for chemical hazards:
Occupational health guideline for morpholine. Cincinnati, Ohio, Hazard
Evaluation and Technical Assistance Branch, Occupational Safety and
Health Agency, pp. 962-966.
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, New Jersey, Noyes Publications,
pp. 626-627.
SORBE (1990) Sicherheitstechnische Kenndaten. Gefahrenindex chemischer
Stoffe. Ecomed-Verlag (in German).
United Nations (1989) Recommendations on the transport of dangerous
goods, 6th rev. ed. New York, United Nations, p. 2054.
 CAS registry number:
CAS registry number: 
 CAS registry number:
CAS registry number: