
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 94
BROMADIOLONE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1995
This is a companion volume to Environmental Health Criteria 175:
Anticoagulant Rodenticides
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Health and safety guide for Bromadiolone
(Health and safety guide ; no. 94)
1.Rodenticides 2.Anticoagulants
3.4-Hydroxycoumarins - toxicity 4.Environmental exposure I.Series
ISBN 92 4 151094 3 (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Identity, physical and chemical properties, and analytical
methods
2.2. Sources of human and environmental exposure
2.3. Environmental transport, distribution, and transformation
2.4. Environmental levels and human exposure
2.5. Kinetics and metabolism in laboratory animals and humans
2.6. Effects on laboratory mammals and in vitro test systems
2.7. Effects on humans
2.8. Effects on other organisms in the laboratory and field
2.9. Evaluation of human health risks and effects on the
environment
2.9.1. Evaluation of human health risks
2.9.2. Evaluation of effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations for the protection of human health and the
environment
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Advice to physicians
4.1.2. Health surveillance advice
4.2. Explosion and fire hazards
4.3. Storage
4.4. Transport
4.5. Spillage
4.6. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: bromadiolone
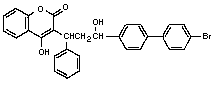
Chemical formula: C30H23BrO4
Chemical structure:
 Common synonyms: broprodifacoum
Trade names: Apobas; Bromard; Bromatrol; Bromorat;
Contrac; Deadline; Hurex; Lanirat; Maki;
Morfaron; Musal; Ramortal; Ratimon; Rodine-c;
Slaymore; Super-caid; Toidon
CAS chemical name: 3-[3[(4'-bromo-[1,1'-biphnyl]-4-yl)-3-
hydroxy-1-phenylpropyl]-4-hydroxy-2H-1-
benzopyran-2 one (9CI)
3-[-[ p-( p-bromophenyl)-hydroxyphenethyl]-
benzyl-4-hydroxycoumarin](8CI)
IUPAC chemical name: 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-
phenylpropyl]-4-hydroxycoumarin
CAS registry number: 28772-56-7
RTECS registry number: GN4934700
1.2 Physical and Chemical Properties
Bromadiolone is a white to off-white powder. Its solubility in water
is very low (less than 20 mg/litre at 20°C). It is slightly soluble in
ethanol and ethyl acetate, and soluble in dimethylformamide. The
flash-point temperature is 218°C.
Further physical and chemical properties of bromadiolone are given in
the "Summary of Chemical Safety Information" (section 6).
1.3 Analytical Methods
The determination of bromadiolone is based on high-performance liquid
chromatography with a detection limit of 0.01 mg/kg.
1.4 Production and Uses
The rodenticidal properties of bromadiolone were reported in 1976. It
is an anticoagulant that is effective against rats and mice, including
those resistant to first generation anticoagulants. It is used in the
form of ready-to-use baits of low concentration containing 0.005%
bromadiolone.
2. SUMMARY AND EVALUATION
2.1 Identity, Physical and Chemical Properties, and Analytical
Methods
Bromadiolone is a white to off-white powder. It is stable at room
temperature and has a melting point of 200-210°C. Its solubility in
water is very low. It is slightly soluble in ethanol and ethyl
acetate, and soluble in dimethylformamide. The determination of
bromadiolone is based on high-performance liquid chromatography.
2.2 Sources of Human and Environmental Exposure
Bromadiolone does not occur naturally. It is used as a rodenticide in
urban and farm rodent control and acts by disrupting the normal blood
clotting mechanisms causing an increased tendency to bleed.
2.3 Environmental Transport, Distribution, and Transformation
Bromadiolone is unlikely to enter the atmosphere, because of its low
volatility. It is practically insoluble in water. Bromadiolone is
readily adsorbed on soils rich in clay and organic compounds, with no
leaching. Degradation in soil is significant with half-lives ranging
from 1.8 to 7.4 days.
2.4 Environmental Levels and Human Exposure
Bromadiolone is not intended for direct application to growing crops
and never for use as a food additive.
No information is available on concentrations in air, water, and soil.
2.5 Kinetics and Metabolism in Laboratory Animals and Humans
Bromadiolone is absorbed through the gastrointestinal tract, skin, and
respiratory system. The major route of elimination in different
species after oral administration is via the faeces. The liver is the
main organ of accumulation and storage. Bromadiolone has been found
in the liver as the unchanged parent compound. Elimination from the
liver is biphasic with an initial rapid phase of 2-8 days and a slower
phase with a half-life of 170 days. No data are available on the
kinetics and metabolism of bromadiolone in humans.
2.6 Effects on Laboratory Mammals and in vitro Test Systems
Bromadiolone has a high, acute oral toxicity (LD50 of 1-3 mg/kg) for
various species including rodents and non-rodents. The dermal
toxicity is also high (LD50 of 9.4 mg/kg in rabbits). Signs of
poisoning are those associated with an increased tendency to bleed.
Bromadiolone is non-irritant to the skin. It is a slight irritant for
the eye.
In feeding studies on rats, the only effect found has been that
associated with anticoagulant action. In a 12-week feeding study on
rats, the maximum tolerated dose was 10 µg/kg body weight per day.
Mutagenicity and teratogenicity studies have not shown any mutagenic,
embryotoxic, or teratogenic effects.
2.7 Effects on Humans
Symptoms of acute intoxication by bromadiolone include an increased
tendency to bleed in less severe cases of poisoning, and massive
haemorrhaging in more severe cases. The signs of poisoning develop
with a delay of one to several days after ingestion.
Incidents of poisoning have been reported.
2.8 Effects on Other Organisms in the Laboratory and Field
Bromadiolone has shown toxicity for aquatic organisms. The LC50
(96-h) for various fish species ranged from 1.4 to more than
3 mg/litre.
Bird species appear to be less susceptible to bromadiolone than
mammals with a reported acute, oral LD50 of at 138 mg/kg.
Secondary poisoning through the consumption of rats and mice killed
with bromadiolone may occur in dogs and cats in urban situations, but
more likely in farm situations.
2.9 Evaluation of Human Health Risks and Effects on the Environment
2.9.1 Evaluation of human health risks
As bromadiolone is mainly used in urban rodent control in the form of
low-concentration baits, increased levels in air are unlikely.
Furthermore, as it is only slightly soluble in water, its use cannot
be a significant source of water contamination. Bromadiolone is not
intended for direct application to growing crops and no residues in
plant food-stuffs are expected. Occupational exposure may occur
during manufacture, formulation, and bait application, but data
concerning the levels of exposure are not available. Bromadiolone may
be absorbed through the gastrointestinal tract and also through the
skin. The major route of elimination is via the faeces. The liver is
the major organ for the accumulation of bromadiolone, which has mainly
been found as the unchanged parent compound. Elimination from the
liver is slow.
As a technical material, bromadiolone is extremely toxic for many
mammalian species. Signs of poisoning in all species, including
humans, are associated with an increased tendency to bleed.
Incidents of poisoning have been reported.
The level of prothrombin time is a satisfactory guide to the severity
of acute intoxication, and also the effectiveness and duration of the
therapy.
The specific antidote is vitamin K1 in both animals and man (see
section 4.1.1).
2.9.2 Evaluation of effects on the environment
Bromadiolone is applied to discrete sites in the form of
low-concentration baits and is stable under normal conditions.
Bromadiolone is poorly soluble in water and, in a bait formulation, it
is is unlikely to be a source of water pollution. As a technical
material, it is toxic for aquatic organisms.
Bromadiolone is readily adsorbed on soil, rich in clay and organic
compounds, with no leaching; degradation in soil is significant.
Non-target organisms are potentially at risk from direct consumption
of baits (primary hazard) and through eating poisoned rodents
(secondary hazard).
Whole-grain baits are highly attractive to birds. Bird species appear
to be less susceptible to bromadiolone than rodents.
The primary hazard is usually expressed by the amount of finished bait
that must be consumed to approach the lethal dose. To reach the toxic
or lethal dose, the non-target species must consume comparatively
large amounts of bait with a concentration of 0.005% active
ingredient.
Some secondary toxicity laboratory studies on wildlife have shown that
captive predators could be intoxicated by no-choice feeding of
bromadiolone-poisoned or dosed prey. The significance of these
results in terms of hazards under field conditions is difficult to
assess, because the predators would not be expected to eat only
poisoned animals. However, predators may take poisoned small mammals
that are still alive, preferentially. In areas close to baiting,
poisoned rodents may represent a high proportion of the diet for
individual birds. However, only few individuals will be affected,
unless there is very widespread and constant use of the baits.
Therefore, some kills of owls can be expected, but there will be no
severe population effects. This ties in with small numbers of
poisoned owls observed in the field.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Exposure of the general population to bromadiolone through air,
drinking-water, or food is unlikely and does not constitute a
significant health hazard. Poisoning incidents may occur in cases of
massive intentional, or unintentional, ingestion, or prolonged
exposure during manufacture and formulation.
Bromadiolone is relatively persistent in the environment, but its
specific use as low-concentration bait formulations cannot be a
significant source of air, water, soil, and food contamination.
Direct and secondary poisoning of birds, domestic and farm animals,
and wildlife may occur.
3.2 Recommendations for the Protection of Human Health and the
Environment
Potentially exposed workers should receive appropriate biomonitoring
and health evaluation.
To prevent primary poisoning, baits should be placed where they cannot
be readily available to non-target species, e.g., in bait stations.
Killed rodents should be burned or buried to prevent secondary
poisoning in predators.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
The oral toxicity of bromadiolone for mammals is extremely high (rat
oral LD50, 1.12 mg/kg; rabbit oral LD50, 1 mg/kg). The dermal
toxicity is also very high (rabbit LD50 9.4 mg/kg). No definite
toxic dose has been established for humans, because of the limited
clinical reports available.
The main features of bromadiolone poisoning in less severe cases are
excessive bruising, nose and gum bleeding, and blood in the urine and
faeces. Bleeding from several organs within the body leading to shock
and possibly death occurs in more severe cases. The onset of the
signs of poisoning may not be evident until one to several days after
ingestion.
Bromadiolone is non-irritant to the skin, but is slightly irritant to
the eye.
Bromadiolone is slowly metabolized by mammals and may accumulate in
the liver reaching toxic levels with repeated exposure.
Handling of technical material or powder concentrates will require the
use of full air-fed protection and an impervious suit, suitable for
wash-down. In operations with liquid concentrates, PVC or
nitrile-rubber gloves, armlets, and an apron should be worn together
with a face shield and rubber boots.
All persons who are bleeding must obtain medical attention.
4.1.1 Advice to physicians
If poisoning has occurred recently (within a few hours), gastric
lavage and the administration of charcoal in repeated doses is
recommended.
A venous blood sample should be taken to measure the haemoglobin
level, prothrombin time, blood grouping, and cross-matching.
If a patient is bleeding severely, 25 mg of vitamin K1
(phytomenadione) should be given by slow intravenous injection. The
patient should be transfused with whole blood or plasma. Fresh,
frozen plasma may be given. Prothrombin time should be checked at 3-h
intervals and injections of vitamin K1 repeated, if no improvement
occurs. Administration of factor concentrate may be considered to
avoid volume overload.
In less severe cases of poisoning, vitamin K1 may be given in lower
doses together with fresh, frozen plasma for rapid restoration of
blood clotting factors. Prothrombin time should be checked after
8-10 h and vitamin K1 administration repeated, if necessary.
Once the prothrombin time has stabilized, treatment with oral vitamin
K1 (10 mg) should be continued four times daily. Oral treatment may
be sufficient in minor cases.
The patient should be kept in hospital until the prothrombin time has
remained normal for three days.
The patient should be discharged from hospital with the following
treatment: oral vitamin K1 (10 mg) twice daily for up to 60 days
with close monitoring of the prothrombin time. It may be possible to
reduce the length of treatment.
4.1.2 Health surveillance advice
Workers handling concentrates must undergo periodic determination of
the potential disturbances of the clotting mechanisms, using the most
appropriate method, i.e., by measuring circulating descarboxy-
prothrombin, prothrombin concentration, or prothrombin time.
4.2 Explosion and Fire Hazards
High temperature decomposition or burning in air will lead to the
formation of toxic gases, which may include carbon monoxide and traces
of bromine and hydrogen bromide, as well as fumes of unchanged
rodenticide; breathing apparatus must be worn in fire fighting.
Heating of containers will cause a pressure rise, with the risk of
bursting and subsequent ignition. Fire-exposed containers should be
kept cool by spraying with water.
Extinguishers recommended for small fires are carbon dioxide or dry
powder; foam or water fog are recommended for larger fires. A water
jet should not be used.
Run-off water from the fire should be prevented from entering
surface-water drains or water sources.
4.3 Storage
Technical material and formulations should be stored in sealed
containers in locked, well-ventilated, dry areas, away from frost,
direct sunlight, and sources of heat and ignition. Keep products out
of reach of children and unauthorized personnel. Do not store near
food or animal feed.
4.4 Transport
Comply with local regulations regarding the movement of hazardous
goods. Before despatch, ensure that the containers are sound and that
labels are securely fixed and undamaged.
4.5 Spillage
During decontamination, the operator must wear protective clothing,
PVC gloves, a face shield, and rubber boots.
Dry spillages should be collected at once, by suction, and disposed of
as toxic waste according to local legislation.
Liquid spillages should be adsorbed onto vermiculite or other inert
adsorbent and treated similarly.
Contaminated areas should be washed down with cold water containing
surfactant; the washings must be prevented from entering surface-water
drains.
4.6 Disposal
Disposal should be carried out according to national regulations.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Bromadiolone is stable but rapidly binds on the soil with very slow
desorption and without leaching. Bromadiolone is slightly soluble in
water and, in the form of bait-formulations, it is unlikely to be a
source of water contamination.
Do not place baits where domestic or farm animals and birds can reach
them. Burn or bury any uneaten bait. Do not dump it in water. Look
for dead rats and mice and burn or bury them.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, bromadiolone. It should be displayed at,
or near, entrances to areas where there is potential exposure to
bromadiolone, and on processing equipment and containers. The summary
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
in the summary clearly explained.
Space is available for insertion of the National Occupational Exposure
Limit, the address and telephone number of the National Poison Control
Centre, and local trade names.
BROMADIOLONE
Chemical formula: C30H23BrO4
CAS chemical name:3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxy-2H-1-benzopyran-2 one (9CI)
IUPAC chemical name: 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxycoumarin
CAS registry number: 28772-56-7
RTECS number: GN4934700
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Physical state powder Bromadiolone is an anticoagulant rodenticide; it is
Colour off-white formulated as low-concentration baits (usually 0.005%
Relative molecular mass 527.4 active ingredient)
Melting point (°C) 200-210
Vapour pressure (20°C) 2 × 10-6 Pa
Solubility in water at 20°C 19 mg/litre
Solubility in
dimethylformamide 730 g/litre
ethanol 8.2 g/litre
ethyl acetate 25 g/litre
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Readily absorbed following Avoid exposure Obtain medical attention; antidote - vitamin K1
ingestion or inhalation, or through
the skin; if absorbed, effects
may range from an increased tendency
to bleed to massive haemorrhaging
SKIN: Non-irritant; skin absorption Wear gloves when handling concentrate Wash with soap and water; seek medical
may occur from liquid concentrate attention
EYES: Slight irritant Use face shield when handling Flush eyes with water for at least 15 min
concentrate
INHALATION: Significant vapour Avoid inhaling concentrate aerosols or Obtain medical attention, if necessary
exposure unlikely bait dust
INGESTION: An unlikely Wash hands before eating, drinking, Transfer to hospital immediately; rinse out
occupational hazard or smoking the mouth with water
Accidental or intentional ingestion Keep out of reach of children, under
may lead to poisoning in several lock and key
hours or days
SPILLAGE STORAGE FIRE/EXPLOSION
Wear protective clothing during Store in sealed containers in a dry, Combustible solid;burning in air will lead to
decontamination; dry spillage - ventilated, and locked storeroom, away the formation of toxic gases; for small fires,
collect and dispose of as toxic from children, unauthorized persons, use carbon dioxide, halons, or dry powder;
waste; liquid spillage - absorb and domestic animals, food, and animal for larger fires, use foam or water fog; keep
on vermiculite or other inert feed containers cool by spraying with water
absorbent and treat similarly;
do not contaminate surface-water
drains
WASTE DISPOSAL NATIONAL INFORMATION
Proper incineration is the method
of choice
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1 Previous Evaluations by International Bodies
Bromadiolone (technical) has been classified by WHO in Class Ia -
Extremely Hazardous, based on the acute oral LD50 of 1.12 mg/kg for
rats.
7.2 Exposure Limit Values
No information is available.
7.3 Specific Restrictions
Bromadiolone has been approved for use as a rodenticide in many
countries. In some countries, specific uses are defined, as well as
limitations and precautions.
7.4 Labelling, Packaging, and Transport
The European Economic Community legislation requires labelling of
technical brodifacoum as very toxic with a hazard symbol T+ and the
following pictogram:
Common synonyms: broprodifacoum
Trade names: Apobas; Bromard; Bromatrol; Bromorat;
Contrac; Deadline; Hurex; Lanirat; Maki;
Morfaron; Musal; Ramortal; Ratimon; Rodine-c;
Slaymore; Super-caid; Toidon
CAS chemical name: 3-[3[(4'-bromo-[1,1'-biphnyl]-4-yl)-3-
hydroxy-1-phenylpropyl]-4-hydroxy-2H-1-
benzopyran-2 one (9CI)
3-[-[ p-( p-bromophenyl)-hydroxyphenethyl]-
benzyl-4-hydroxycoumarin](8CI)
IUPAC chemical name: 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-
phenylpropyl]-4-hydroxycoumarin
CAS registry number: 28772-56-7
RTECS registry number: GN4934700
1.2 Physical and Chemical Properties
Bromadiolone is a white to off-white powder. Its solubility in water
is very low (less than 20 mg/litre at 20°C). It is slightly soluble in
ethanol and ethyl acetate, and soluble in dimethylformamide. The
flash-point temperature is 218°C.
Further physical and chemical properties of bromadiolone are given in
the "Summary of Chemical Safety Information" (section 6).
1.3 Analytical Methods
The determination of bromadiolone is based on high-performance liquid
chromatography with a detection limit of 0.01 mg/kg.
1.4 Production and Uses
The rodenticidal properties of bromadiolone were reported in 1976. It
is an anticoagulant that is effective against rats and mice, including
those resistant to first generation anticoagulants. It is used in the
form of ready-to-use baits of low concentration containing 0.005%
bromadiolone.
2. SUMMARY AND EVALUATION
2.1 Identity, Physical and Chemical Properties, and Analytical
Methods
Bromadiolone is a white to off-white powder. It is stable at room
temperature and has a melting point of 200-210°C. Its solubility in
water is very low. It is slightly soluble in ethanol and ethyl
acetate, and soluble in dimethylformamide. The determination of
bromadiolone is based on high-performance liquid chromatography.
2.2 Sources of Human and Environmental Exposure
Bromadiolone does not occur naturally. It is used as a rodenticide in
urban and farm rodent control and acts by disrupting the normal blood
clotting mechanisms causing an increased tendency to bleed.
2.3 Environmental Transport, Distribution, and Transformation
Bromadiolone is unlikely to enter the atmosphere, because of its low
volatility. It is practically insoluble in water. Bromadiolone is
readily adsorbed on soils rich in clay and organic compounds, with no
leaching. Degradation in soil is significant with half-lives ranging
from 1.8 to 7.4 days.
2.4 Environmental Levels and Human Exposure
Bromadiolone is not intended for direct application to growing crops
and never for use as a food additive.
No information is available on concentrations in air, water, and soil.
2.5 Kinetics and Metabolism in Laboratory Animals and Humans
Bromadiolone is absorbed through the gastrointestinal tract, skin, and
respiratory system. The major route of elimination in different
species after oral administration is via the faeces. The liver is the
main organ of accumulation and storage. Bromadiolone has been found
in the liver as the unchanged parent compound. Elimination from the
liver is biphasic with an initial rapid phase of 2-8 days and a slower
phase with a half-life of 170 days. No data are available on the
kinetics and metabolism of bromadiolone in humans.
2.6 Effects on Laboratory Mammals and in vitro Test Systems
Bromadiolone has a high, acute oral toxicity (LD50 of 1-3 mg/kg) for
various species including rodents and non-rodents. The dermal
toxicity is also high (LD50 of 9.4 mg/kg in rabbits). Signs of
poisoning are those associated with an increased tendency to bleed.
Bromadiolone is non-irritant to the skin. It is a slight irritant for
the eye.
In feeding studies on rats, the only effect found has been that
associated with anticoagulant action. In a 12-week feeding study on
rats, the maximum tolerated dose was 10 µg/kg body weight per day.
Mutagenicity and teratogenicity studies have not shown any mutagenic,
embryotoxic, or teratogenic effects.
2.7 Effects on Humans
Symptoms of acute intoxication by bromadiolone include an increased
tendency to bleed in less severe cases of poisoning, and massive
haemorrhaging in more severe cases. The signs of poisoning develop
with a delay of one to several days after ingestion.
Incidents of poisoning have been reported.
2.8 Effects on Other Organisms in the Laboratory and Field
Bromadiolone has shown toxicity for aquatic organisms. The LC50
(96-h) for various fish species ranged from 1.4 to more than
3 mg/litre.
Bird species appear to be less susceptible to bromadiolone than
mammals with a reported acute, oral LD50 of at 138 mg/kg.
Secondary poisoning through the consumption of rats and mice killed
with bromadiolone may occur in dogs and cats in urban situations, but
more likely in farm situations.
2.9 Evaluation of Human Health Risks and Effects on the Environment
2.9.1 Evaluation of human health risks
As bromadiolone is mainly used in urban rodent control in the form of
low-concentration baits, increased levels in air are unlikely.
Furthermore, as it is only slightly soluble in water, its use cannot
be a significant source of water contamination. Bromadiolone is not
intended for direct application to growing crops and no residues in
plant food-stuffs are expected. Occupational exposure may occur
during manufacture, formulation, and bait application, but data
concerning the levels of exposure are not available. Bromadiolone may
be absorbed through the gastrointestinal tract and also through the
skin. The major route of elimination is via the faeces. The liver is
the major organ for the accumulation of bromadiolone, which has mainly
been found as the unchanged parent compound. Elimination from the
liver is slow.
As a technical material, bromadiolone is extremely toxic for many
mammalian species. Signs of poisoning in all species, including
humans, are associated with an increased tendency to bleed.
Incidents of poisoning have been reported.
The level of prothrombin time is a satisfactory guide to the severity
of acute intoxication, and also the effectiveness and duration of the
therapy.
The specific antidote is vitamin K1 in both animals and man (see
section 4.1.1).
2.9.2 Evaluation of effects on the environment
Bromadiolone is applied to discrete sites in the form of
low-concentration baits and is stable under normal conditions.
Bromadiolone is poorly soluble in water and, in a bait formulation, it
is is unlikely to be a source of water pollution. As a technical
material, it is toxic for aquatic organisms.
Bromadiolone is readily adsorbed on soil, rich in clay and organic
compounds, with no leaching; degradation in soil is significant.
Non-target organisms are potentially at risk from direct consumption
of baits (primary hazard) and through eating poisoned rodents
(secondary hazard).
Whole-grain baits are highly attractive to birds. Bird species appear
to be less susceptible to bromadiolone than rodents.
The primary hazard is usually expressed by the amount of finished bait
that must be consumed to approach the lethal dose. To reach the toxic
or lethal dose, the non-target species must consume comparatively
large amounts of bait with a concentration of 0.005% active
ingredient.
Some secondary toxicity laboratory studies on wildlife have shown that
captive predators could be intoxicated by no-choice feeding of
bromadiolone-poisoned or dosed prey. The significance of these
results in terms of hazards under field conditions is difficult to
assess, because the predators would not be expected to eat only
poisoned animals. However, predators may take poisoned small mammals
that are still alive, preferentially. In areas close to baiting,
poisoned rodents may represent a high proportion of the diet for
individual birds. However, only few individuals will be affected,
unless there is very widespread and constant use of the baits.
Therefore, some kills of owls can be expected, but there will be no
severe population effects. This ties in with small numbers of
poisoned owls observed in the field.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Exposure of the general population to bromadiolone through air,
drinking-water, or food is unlikely and does not constitute a
significant health hazard. Poisoning incidents may occur in cases of
massive intentional, or unintentional, ingestion, or prolonged
exposure during manufacture and formulation.
Bromadiolone is relatively persistent in the environment, but its
specific use as low-concentration bait formulations cannot be a
significant source of air, water, soil, and food contamination.
Direct and secondary poisoning of birds, domestic and farm animals,
and wildlife may occur.
3.2 Recommendations for the Protection of Human Health and the
Environment
Potentially exposed workers should receive appropriate biomonitoring
and health evaluation.
To prevent primary poisoning, baits should be placed where they cannot
be readily available to non-target species, e.g., in bait stations.
Killed rodents should be burned or buried to prevent secondary
poisoning in predators.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
The oral toxicity of bromadiolone for mammals is extremely high (rat
oral LD50, 1.12 mg/kg; rabbit oral LD50, 1 mg/kg). The dermal
toxicity is also very high (rabbit LD50 9.4 mg/kg). No definite
toxic dose has been established for humans, because of the limited
clinical reports available.
The main features of bromadiolone poisoning in less severe cases are
excessive bruising, nose and gum bleeding, and blood in the urine and
faeces. Bleeding from several organs within the body leading to shock
and possibly death occurs in more severe cases. The onset of the
signs of poisoning may not be evident until one to several days after
ingestion.
Bromadiolone is non-irritant to the skin, but is slightly irritant to
the eye.
Bromadiolone is slowly metabolized by mammals and may accumulate in
the liver reaching toxic levels with repeated exposure.
Handling of technical material or powder concentrates will require the
use of full air-fed protection and an impervious suit, suitable for
wash-down. In operations with liquid concentrates, PVC or
nitrile-rubber gloves, armlets, and an apron should be worn together
with a face shield and rubber boots.
All persons who are bleeding must obtain medical attention.
4.1.1 Advice to physicians
If poisoning has occurred recently (within a few hours), gastric
lavage and the administration of charcoal in repeated doses is
recommended.
A venous blood sample should be taken to measure the haemoglobin
level, prothrombin time, blood grouping, and cross-matching.
If a patient is bleeding severely, 25 mg of vitamin K1
(phytomenadione) should be given by slow intravenous injection. The
patient should be transfused with whole blood or plasma. Fresh,
frozen plasma may be given. Prothrombin time should be checked at 3-h
intervals and injections of vitamin K1 repeated, if no improvement
occurs. Administration of factor concentrate may be considered to
avoid volume overload.
In less severe cases of poisoning, vitamin K1 may be given in lower
doses together with fresh, frozen plasma for rapid restoration of
blood clotting factors. Prothrombin time should be checked after
8-10 h and vitamin K1 administration repeated, if necessary.
Once the prothrombin time has stabilized, treatment with oral vitamin
K1 (10 mg) should be continued four times daily. Oral treatment may
be sufficient in minor cases.
The patient should be kept in hospital until the prothrombin time has
remained normal for three days.
The patient should be discharged from hospital with the following
treatment: oral vitamin K1 (10 mg) twice daily for up to 60 days
with close monitoring of the prothrombin time. It may be possible to
reduce the length of treatment.
4.1.2 Health surveillance advice
Workers handling concentrates must undergo periodic determination of
the potential disturbances of the clotting mechanisms, using the most
appropriate method, i.e., by measuring circulating descarboxy-
prothrombin, prothrombin concentration, or prothrombin time.
4.2 Explosion and Fire Hazards
High temperature decomposition or burning in air will lead to the
formation of toxic gases, which may include carbon monoxide and traces
of bromine and hydrogen bromide, as well as fumes of unchanged
rodenticide; breathing apparatus must be worn in fire fighting.
Heating of containers will cause a pressure rise, with the risk of
bursting and subsequent ignition. Fire-exposed containers should be
kept cool by spraying with water.
Extinguishers recommended for small fires are carbon dioxide or dry
powder; foam or water fog are recommended for larger fires. A water
jet should not be used.
Run-off water from the fire should be prevented from entering
surface-water drains or water sources.
4.3 Storage
Technical material and formulations should be stored in sealed
containers in locked, well-ventilated, dry areas, away from frost,
direct sunlight, and sources of heat and ignition. Keep products out
of reach of children and unauthorized personnel. Do not store near
food or animal feed.
4.4 Transport
Comply with local regulations regarding the movement of hazardous
goods. Before despatch, ensure that the containers are sound and that
labels are securely fixed and undamaged.
4.5 Spillage
During decontamination, the operator must wear protective clothing,
PVC gloves, a face shield, and rubber boots.
Dry spillages should be collected at once, by suction, and disposed of
as toxic waste according to local legislation.
Liquid spillages should be adsorbed onto vermiculite or other inert
adsorbent and treated similarly.
Contaminated areas should be washed down with cold water containing
surfactant; the washings must be prevented from entering surface-water
drains.
4.6 Disposal
Disposal should be carried out according to national regulations.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Bromadiolone is stable but rapidly binds on the soil with very slow
desorption and without leaching. Bromadiolone is slightly soluble in
water and, in the form of bait-formulations, it is unlikely to be a
source of water contamination.
Do not place baits where domestic or farm animals and birds can reach
them. Burn or bury any uneaten bait. Do not dump it in water. Look
for dead rats and mice and burn or bury them.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, bromadiolone. It should be displayed at,
or near, entrances to areas where there is potential exposure to
bromadiolone, and on processing equipment and containers. The summary
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
in the summary clearly explained.
Space is available for insertion of the National Occupational Exposure
Limit, the address and telephone number of the National Poison Control
Centre, and local trade names.
BROMADIOLONE
Chemical formula: C30H23BrO4
CAS chemical name:3-[3-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxy-2H-1-benzopyran-2 one (9CI)
IUPAC chemical name: 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxycoumarin
CAS registry number: 28772-56-7
RTECS number: GN4934700
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Physical state powder Bromadiolone is an anticoagulant rodenticide; it is
Colour off-white formulated as low-concentration baits (usually 0.005%
Relative molecular mass 527.4 active ingredient)
Melting point (°C) 200-210
Vapour pressure (20°C) 2 × 10-6 Pa
Solubility in water at 20°C 19 mg/litre
Solubility in
dimethylformamide 730 g/litre
ethanol 8.2 g/litre
ethyl acetate 25 g/litre
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Readily absorbed following Avoid exposure Obtain medical attention; antidote - vitamin K1
ingestion or inhalation, or through
the skin; if absorbed, effects
may range from an increased tendency
to bleed to massive haemorrhaging
SKIN: Non-irritant; skin absorption Wear gloves when handling concentrate Wash with soap and water; seek medical
may occur from liquid concentrate attention
EYES: Slight irritant Use face shield when handling Flush eyes with water for at least 15 min
concentrate
INHALATION: Significant vapour Avoid inhaling concentrate aerosols or Obtain medical attention, if necessary
exposure unlikely bait dust
INGESTION: An unlikely Wash hands before eating, drinking, Transfer to hospital immediately; rinse out
occupational hazard or smoking the mouth with water
Accidental or intentional ingestion Keep out of reach of children, under
may lead to poisoning in several lock and key
hours or days
SPILLAGE STORAGE FIRE/EXPLOSION
Wear protective clothing during Store in sealed containers in a dry, Combustible solid;burning in air will lead to
decontamination; dry spillage - ventilated, and locked storeroom, away the formation of toxic gases; for small fires,
collect and dispose of as toxic from children, unauthorized persons, use carbon dioxide, halons, or dry powder;
waste; liquid spillage - absorb and domestic animals, food, and animal for larger fires, use foam or water fog; keep
on vermiculite or other inert feed containers cool by spraying with water
absorbent and treat similarly;
do not contaminate surface-water
drains
WASTE DISPOSAL NATIONAL INFORMATION
Proper incineration is the method
of choice
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1 Previous Evaluations by International Bodies
Bromadiolone (technical) has been classified by WHO in Class Ia -
Extremely Hazardous, based on the acute oral LD50 of 1.12 mg/kg for
rats.
7.2 Exposure Limit Values
No information is available.
7.3 Specific Restrictions
Bromadiolone has been approved for use as a rodenticide in many
countries. In some countries, specific uses are defined, as well as
limitations and precautions.
7.4 Labelling, Packaging, and Transport
The European Economic Community legislation requires labelling of
technical brodifacoum as very toxic with a hazard symbol T+ and the
following pictogram:
 The United Nations in its Recommendations on the Transport of
Dangerous Goods classified bromadiolone in Category 6.1 as a poisonous
substance (No. 3027).
R26/27/28 Very toxic by inhalation, contact with the skin and by
ingestion.
R48 Risks of serious effects to health in case of prolonged
exposure.
7.5 Waste Disposal
No specific information is available.
BIBLIOGRAPHY
Hayes WJ Jr & Laws ER Jr (1991) Handbook of pesticide toxicology,
Vol. 3, New York, Academic Press.
IPCS (1995) Environmental Health Criteria 175: Anticoagulant
rodenticides, Geneva, World Health Organization.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995, Geneva (unpublished
document WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II), Clin Chem
33(11): 2074-2078.
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
The United Nations in its Recommendations on the Transport of
Dangerous Goods classified bromadiolone in Category 6.1 as a poisonous
substance (No. 3027).
R26/27/28 Very toxic by inhalation, contact with the skin and by
ingestion.
R48 Risks of serious effects to health in case of prolonged
exposure.
7.5 Waste Disposal
No specific information is available.
BIBLIOGRAPHY
Hayes WJ Jr & Laws ER Jr (1991) Handbook of pesticide toxicology,
Vol. 3, New York, Academic Press.
IPCS (1995) Environmental Health Criteria 175: Anticoagulant
rodenticides, Geneva, World Health Organization.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995, Geneva (unpublished
document WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II), Clin Chem
33(11): 2074-2078.
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
 Common synonyms: broprodifacoum
Trade names: Apobas; Bromard; Bromatrol; Bromorat;
Contrac; Deadline; Hurex; Lanirat; Maki;
Morfaron; Musal; Ramortal; Ratimon; Rodine-c;
Slaymore; Super-caid; Toidon
CAS chemical name: 3-[3[(4'-bromo-[1,1'-biphnyl]-4-yl)-3-
hydroxy-1-phenylpropyl]-4-hydroxy-2H-1-
benzopyran-2 one (9CI)
3-[-[ p-( p-bromophenyl)-hydroxyphenethyl]-
benzyl-4-hydroxycoumarin](8CI)
IUPAC chemical name: 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-
phenylpropyl]-4-hydroxycoumarin
CAS registry number:
Common synonyms: broprodifacoum
Trade names: Apobas; Bromard; Bromatrol; Bromorat;
Contrac; Deadline; Hurex; Lanirat; Maki;
Morfaron; Musal; Ramortal; Ratimon; Rodine-c;
Slaymore; Super-caid; Toidon
CAS chemical name: 3-[3[(4'-bromo-[1,1'-biphnyl]-4-yl)-3-
hydroxy-1-phenylpropyl]-4-hydroxy-2H-1-
benzopyran-2 one (9CI)
3-[-[ p-( p-bromophenyl)-hydroxyphenethyl]-
benzyl-4-hydroxycoumarin](8CI)
IUPAC chemical name: 3-[3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-
phenylpropyl]-4-hydroxycoumarin
CAS registry number:  The United Nations in its Recommendations on the Transport of
Dangerous Goods classified bromadiolone in Category 6.1 as a poisonous
substance (No. 3027).
R26/27/28 Very toxic by inhalation, contact with the skin and by
ingestion.
R48 Risks of serious effects to health in case of prolonged
exposure.
7.5 Waste Disposal
No specific information is available.
BIBLIOGRAPHY
Hayes WJ Jr & Laws ER Jr (1991) Handbook of pesticide toxicology,
Vol. 3, New York, Academic Press.
IPCS (1995) Environmental Health Criteria 175: Anticoagulant
rodenticides, Geneva, World Health Organization.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995, Geneva (unpublished
document WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II), Clin Chem
33(11): 2074-2078.
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
The United Nations in its Recommendations on the Transport of
Dangerous Goods classified bromadiolone in Category 6.1 as a poisonous
substance (No. 3027).
R26/27/28 Very toxic by inhalation, contact with the skin and by
ingestion.
R48 Risks of serious effects to health in case of prolonged
exposure.
7.5 Waste Disposal
No specific information is available.
BIBLIOGRAPHY
Hayes WJ Jr & Laws ER Jr (1991) Handbook of pesticide toxicology,
Vol. 3, New York, Academic Press.
IPCS (1995) Environmental Health Criteria 175: Anticoagulant
rodenticides, Geneva, World Health Organization.
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995, Geneva (unpublished
document WHO/PCS/94.2).
Widdershoven J, van Munster P, De Abreu R, Bosman H, van Lith Th, van
der Putten-van Meyel M, Motohara K, & Matsuda I (1987) Four methods
compared for measuring des-carboxy-prothrombin (PIVKA-II), Clin Chem
33(11): 2074-2078.
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
