
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 71
TRIMELLITIC ANHYDRIDE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1992
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the
United Nations Environment Programme, the International Labour
Organisation, and the World Health Organization)
This report contains the collective views of an international group
of experts and does not necessarily represent the decisions or the
stated policy of the United Nations Environment Programme, the
International Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Trimellitic anhydride : health and safety guide.
(Health and safety guide ; no. 71)
1. Phthalic anhydrides - adverse effects
2. Phthalic anhydrides - standards
3. Phthalic anhydrides - toxicity
4. Accidents, Occupational - prevention & control
5. Environmental exposure
6. Occupational diseases - chemically induced
I.Series
ISBN 92 4 151071 4 (NLM Classification: QD 341.A2)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Composition
1.4. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Exposure to trimellitic anhydride
2.2. Absorption, metabolism, and excretion
2.3. Fate in the environment and effects on organisms
2.4. Effects on animals
2.4.1. Acute toxicity
2.4.2. Short-term toxicity
2.4.3. Long-term toxicity
2.5. Effects on human beings
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Surveillance
4.1.2. First aid
4.2. Advice to physicians
4.3. Explosion and fire hazards
4.4. Storage and transport
4.5. Spillage
4.6. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Transport and labelling
BIBLIOGRAPHY
INTRODUCTION
This Health and Safety Guide is not based on an existing
Environmental Health Criteria document, but on critical national
reviews. The hazard evaluation in the Health and Safety Guide was
made on the basis of carefully selected studies, after scrutiny of
the original publications.
In order to assist the peer-review process of the present Health and
Safety Guide, a background companion document was prepared by the
IPCS and can be obtained from the Director on request; the IPCS
does not intend that the background document should be published.
The first three sections of this Health and Safety Guide present
essential technical information and the hazard evaluation. Section
4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in
an emergency. The section on regulatory information has been
extracted from the legal file of the International Register of
Potentially Toxic Chemicals (IRPTC) and from other United Nations
sources.
The target readership includes occupational health services, those
in ministries, governmental agencies, industry, and trade unions who
are involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING
POINT TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical formula: C9H4O5
Chemical structure:
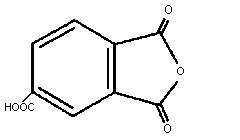 Common name: trimellitic anhydride
Common synonyms: anhydro trimellitic acid;
1,2,4-benzenetricarboxylic acid
1,2-anhydride; 1,2,4-benzenetricarboxylic
anhydride; 4-carboxyphthalic anhydride;
1,3-dioxo-5-phthalancarboxylic acid;
5-phthalancarboxylic acid, 1,3-dioxo-TMAN;
trimellitic acid 1,2-anhydride; TMA;a
TMAN
IUPAC systematic name: 1,3-dihydro-1,3-dioxo-5-isobenzofuran-
carboxylic acid
CAS registry number: 552-30-7
RTECS registry number: DC2050000
Conversion factor: 1 mg/m3 aprox. 0.13 ppm
Relative molecular mass: 192.12
a TMA is also an acronym for i.a. trimethylamine.
1.2 Physical and chemical properties
Trimellitic anhydride is a white solid in the form of flakes. It is
readily hydrolysed in water to trimellitic acid, which is moderately
soluble in water. Trimellitic anhydride is readily soluble in
acetone, cyclohexanone, 2-butane, ethyl acetate, and dimethyl
formamide (from 49.6 to 15.5% w/w at 25 °C).
Trimellitic anhydride reacts with alcohols forming the corresponding
esters. Reactions with ammonia yield amides, imides, and
amide-imides.
Flaked or molten trimellitic anhydride will burn if ignited,
producing an explosive dust. Similarly, the vapour from molten
trimellitic anhydride forms dangerously explosive mixtures with air.
Some additional physical and chemical properties are given in the
Summary of Chemical Safety Information (see section 6).
1.3 Composition
The purity of commercial trimellitic anhydride is greater than 99%.
1.4 Analytical methods
Airborne trimellitic anhydride can be sampled on various adsorbents,
such as cellulose ester membrane filter, glass fibre filter, or PVC
membrane. It can be determined by high performance liquid
chromatography (HPLC), gas chromatography, or polarography. HPLC
allows the accurate measurement of as little as 0.1 µg trimellitic
anhydride.
1.5 Production and uses
Trimellitic anhydride has been produced since the 1960s. Production
was estimated to be 50 000 tonnes per annum in 1990.
The preparation of trimellitic anhydride is based on the
liquid-phase air-oxidation of pseudocumene to form trimellitic acid,
this being subsequently dehydrated. An alternative process consists
of heating crude trimellitic acid with vanadium pentoxide.
Trimellitic anhydride is a very reactive chemical of low relative
molecular mass. Many of its industrial uses depend on the
reactivity of the anhydride group. It is mainly used in the
synthesis of trimellitate esters. These esters are used as
plasticizers for polyvinyl chloride, especially when temperature
stability is required, e.g., in wire and cable coatings.
Trimellitic anhydride esters are less volatile and less water
soluble than corresponding phthalates, and have begun to replace
them in some applications, e.g., car interior linings. An important
use for trimellitic anhydride polymers is in the production of the
wire enamels used to coat magnetic wire or for other applications
where high thermal resistance is required.
Trimellitic anhydride is also used in the production of polyester
resins for water-based and conventional solvent-based coatings and
paints, to make resins for electrodeposition and powder coatings,
and as a binder for glass fibres, sand, and other aggregates.
In addition, trimellitic anhydride is used as an embossing agent for
foam-backed vinyl flooring and as a curing agent for epoxy and other
resins. It is also used as a plasticizer in materials used to store
and cover food and in the synthesis of various anticorrosive surface
coatings, agricultural chemicals, and pharmaceutical products.
2. SUMMARY AND EVALUATION
2.1 Exposure to trimellitic anhydride
Exposure to trimellitic anhydride occurs in many different
occupational settings; 20 000 workers are known to be exposed
through manufacturing and use in the USA and the worldwide total is
considerably greater. Exposure occurs mainly by inhalation of dust
or fumes. Since trimellitic anhydride has a very low vapour pressure
at room temperature, extensive exposure occurs mainly when dust is
generated from the powder, by mixing or spraying, or by inhalation
of the fumes generated during processes requiring high temperature,
e.g., oven curing. Exposure to very high levels of trimellitic
anhydride fumes occurs when heated metal surfaces are sprayed with
anticorrosive materials containing trimellitic anhydride-based
compounds. High levels of trimellitic anhydride dust are found in
bagging areas.
Exposure of the general population to trimellitic anhydride is
possible, for example, around industrial plants, through its use in
food storage and protection materials, from car interior linings,
and during transportation of the compound. However, the likelihood
of exposure from these sources is very low. When trimellitate
plasticizers are used, trimellitic anhydride itself is not present,
but rather its ester derivatives; these are stable and do not
migrate. There are no reports of populations being affected in the
vicinity of industrial sites.
2.2 Absorption, metabolism, and excretion
In a distribution study, rats were exposed through inhalation to
950 µg 14C-labelled trimellitic anhydride/m3 for 45 minutes.
Treated rats were sacrificed at intervals ranging from 3 hours to 32
days. Maximum concentrations of 14C in all tissues occurred 3 hours
after exposure; these levels then declined in all tissues, except
for the lymph nodes associated with the lung, where there was an
initial decline followed by a second increase, peaking at the eighth
day and then declining.
There is no information on metabolism. Trimellitic anhydride reacts
with the free amino groups on proteins to form conjugates.
2.3 Fate in the environment and effects on organisms
There is very limited information on the fate and effects of
trimellitic anhydride in the environment. However, exposure is
expected to be very low, except from spills and accidents.
In a laboratory degradation study, more than 60% of the theoretical
quantity of carbon dioxide was generated in 10 days. Hydrolysis of
the anhydride was not checked during the study. Trimellitic
anhydride can thus be considered as readily degradable by by
bacteria. Data on aquatic toxicity, e.g., on fish species, are not
yet available
2.4 Effects on animals
2.4.1 Acute toxicity
The acute toxicity of trimellitic anhydride for experimental animals
is low. For the mouse, the most susceptible species, the oral LD50
was 1300 mg/kg body weight. Treated mice showed gastrointestinal
mucosal irritation, with hyperaemia and haemorrhage; in some cases,
perforations were noted. For the rat, the oral LD50 was 2730 mg/kg
body weight, and the dermal LD50 was estimated to be greater than
2000 mg/kg body weight.
A single application of trimellitic anhydride (TMA) to rabbit skin
resulted in mild dermatitis. TMA caused severe irritation to the
eyes of rabbits, leading to corrosive lesions and chemical burns in
the cornea.
2.4.2 Short-term toxicity
Animal inhalation studies, for 2-13 weeks, have shown a clear
correlation between TMA exposure and the occurrence of lung damage.
The development of lung injury is concurrent with a rise in antibody
levels in the products of broncho-alveolar lavage and in serum.
There are marked species differences in susceptibility to
trimellitic anhydride-induced respiratory sensitization. In one
recent study, the rat appeared to be the most sensitive experimental
animal. No sensitization response was observed on challenge 3 weeks
after a 6-hour exposure at 1.6 µg TMA/m3, but sensitization was
seen after exposure at 7.7 µg/m3. Repeated respiratory exposure to
TMA resulted in dose-related focal haemorrhagic areas in the lungs,
with oedema and lobular bronchopneumonia.
An unusual finding in a 13-week rat inhalation study was the
apparent development of tolerance to TMA during the study, as
indicated by reductions in antibody titres and the prevalence of
lung lesions. The results suggest adaptation in rats to TMA after
short-term continuous exposure (Leach et al. 1989).
Repeated skin applications of TMA in guinea-pigs induce significant
dermal sensitization reactions.
Conjugates formed between protein groups and trimellitic anhydride
are able to trigger an allergic response.
Data on genotoxicity and reproductive toxicity are limited; the only
results available on genetic toxicity are those from Ames tests,
which were negative. No developmental toxicity was recorded in a
teratology screeningstudy on pregnant mice. However, trimellitic
anhydride-specific antibody can be transferred from mother to fetus
in rats and guinea-pigs.
2.4.3 Long-term toxicity
There are no data on which the long-term toxicity or carcinogenicity
of TMA can be assessed.
2.5 Effects on human beings
Trimellitic anhydride dust or fumes are highly irritating to the
eyes and respiratory system. The dust may cause corrosive eye
damage, and frequent exposure can lead to respiratory sensitization,
with, in rare cases, quite severe symptoms. Trimellitic anhydride
can produce skin irritation following prolonged or repeated
exposure.
TMA is a potent respiratory sensitizer; it has caused
immunologically-mediated respiratory illness in 29% of workers,
exposed prior to 1979 to levels considerably above the present
threshold limit value. The period from 1979 to 1985 was
characterized by implementation of control measures to reduce
exposure to TMA in the workplace. These measures clearly resulted in
a marked decrease in both clinical symptoms and levels of TMA
antibody formation.
Four clinical syndromes have been identified. Three of these
syndromes are associated with immunological reactions. The first is
an immediate type airway response characterized by asthma or
rhinitis, or both. These symptoms only occur when individuals are
sensitized following a latent period of exposure, which may be weeks
or years. Once sensitization is acquired, symptoms occur within
seconds or minutes of exposure. The syndrome is associated with an
increase in antibody levels.
The second form, the late respiratory systemic syndrome, often
termed "TMA-flu", also requires a latent period of exposure for
sensitization. It is characterized by a delayed onset of symptoms
after exposure, such as coughing and wheezing that occur 4-12 hours
after exposure to trimellitic anhydride; however, the syndrome also
includes muscle and joint pains, and fever. High levels of
antibodies are also associated with this syndrome.
The most severe and rare reaction is the pulmonary-disease-anaemia
syndrome, which may lead to respiratory failure. No fatal cases
have been reported. This illness has appeared only after exposures
to high concentrations of trimellitic anhydride fumes, from the
heating of materials containing trimellitic anhydride, for
relatively short periods. The symptoms vary in severity and may
include cough, with blood-stained sputum, and breathlessness,
resulting in severe pathological changes in the lung. The disease
also requires a latent period of exposure before the onset of
symptoms and it is characterized by high serum antibody levels.
These symptoms are, however, reversible after trimellitic anhydride
exposure has ceased.
The fourth respiratory syndrome is a non-immunological irritant
reaction to trimellitic anhydride characterized by a transient
irritation in the upper airways, with lacrimation and rhinorrhoea.
The irritant symptoms are related to exposure level and can occur in
any worker after a single high-level exposure to trimellitic
anhydride powder or fumes.
Descriptions of the human syndromes are summarized in Table 1.
Table 1. Characteristics of syndromes in humans related to the inhalation of trimellitic anhydridea
Syndrome
Characteristic Rhinitis and immediate- Late respiratory systemic Pulmonary disease- Irritant syndrome
type asthma syndrome anaemia
Latent period (duration Months to years of Months to years of Weeks to months Occurs on first high-
of work exposure prior work exposure work exposure of work exposure level exposure
to onset of symptoms
Onset of symptoms Immediate (minutes) 4-12 hours Progressive with Variable, depending
after work exposure further work on exposure
exposure
Type/degree of exposure TMA dust or fumes/ TMA dust or fumes/ TMA fumes/high Fumes or dust/high
mild moderate
a From Zeiss et al. (1982).
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Trimellitic anhydride is extensively used in industry throughout the
world. It is hazardous to human health in the workplace. Exposure
may result in irritant and/or sensitization respiratory effects,
ranging from mild to severe. It is a mild irritant and sensitizer to
the skin, and corrosive to the eye. Eye damage is frequently
irreversible.
Trimellitic anhydride-related immune response and illness can be
reduced with proper workplace control.
Flaked or molten trimellitic anhydride will burn if ignited,
producing an explosive dust. The vapour from molten trimellitic
anhydride forms dangerously explosive mixtures with air.
3.2 Recommendations
(a) This compound should be handled strictly in accordance with safe
work and good-housekeeping practices. If these are not feasible, the
use of less hazardous alternatives to TMA should be considered.
(b) Since trimellitic anhydride is a respiratory sensitizer, some
response in previously sensitized workers may occur even at the
recommended threshold limit value (TLV); exposure to TMA in the
workplace should, therefore, be kept as low as possible.
(c) Workers with a previous history of asthma or allergies should
not be exposed to trimellitic anhydride. It is advisable to have a
pre-employment chest X-ray.
(d) Pre-employment and subsequent periodic medical surveillance
should be carried out, including the determination of antibody
levels and careful examination of eyes, respiratory tract, and skin.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main human health hazards, prevention and protection, first aid
Since the packaging and handling of trimellitic anhydride can result
in exposure, procedures for diminishing worker exposures to
trimellitic anhydride are essential. In the work area, closed
systems with continuous ventilation should be used, or, if not
feasible, mechanical ventilation and local exhaust should be
installed. If ventilation is insufficient to maintain exposures
below the recommended limit, a full face-piece, cartridge respirator
is required as the minimum protection. A combination type cartridge
may be used for dust or fume concentrations up to 50 times the
recommended exposure limit. For higher concentrations, an
air-supplied, full face-piece respirator is required. Overalls or
other appropriate clothing, chemical goggles, and gloves must be
worn by persons engaged in work that results in direct contact with
trimellitic anhydride. Contaminated clothing should be removed and
thoroughly cleaned and dried before reuse. High dust concentration
may form a flammable or explosive mixture with air and should be
avoided. Dry dust can be charged electrostatically by turbulence,
pneumatic transport, pouring, and in exhaust ducts and during
transportation. Build-up of electrostatic charges should be
prevented by grounding.
Users of TMA should consult the manufacturers with regard to
appropriate and effective exhaust ventilation, and precautions to be
followed when using the compound.
4.1.1 Surveillance
Antibody titres should be determined in workers exposed to
trimellitic anhydride every 6 months.
4.1.2 First aid
In the case of exposure to TMA, medical attention should be sought
immediately; in the meantime, render first aid. Move the patient to
fresh air, remove contaminated clothing. Wash contaminated skin with
soap and water and provide artificial respiration if necessary. In
the case of splashes in the eyes, irrigate the eyes with plenty of
water for 15 minutes. If large amounts are ingested, vomiting should
not be in induced, to avoid the potential complication of aspiration
pneumonitis.
4.2 Advice to physicians
No specific antidote is known. General symptomatic and supportive
therapy is indicated, depending on the system affected: respiratory
system, eyes, or skin.
4.3 Explosion and fire hazards
Trimellitic anhydride is not readily combustible. However, high
dust concentrations have a potential for combustion or explosion.
Trimellitic anhydride forms an explosive mixture with air; the lower
and upper explosive limits are 1% and 7%, respectively. Care should
be taken to keep dust concentrations low and to prevent ignition.
High-voltage static electricity build-up is possible when
significant quantities of dust are present.
In the event of a fire, use water fog, foam, or dry chemical.
Personnel should wear full protective clothing and breathing
apparatus.
4.4 Storage and transport
Containers of trimellitic anhydride should be protected from
physical damage to prevent any leakage of the contents. The
substance should be stored in well-ventilated areas.
During transport, containers should be sound and well sealed to
prevent loss of contents.
4.5 Spillage
Spills should be carefully swept or gathered up to avoid producing
dust and transferred to a sealable container. Alternatively, an
approved high-efficiency vacuum may be used, fitted with
high-efficiency (HEPA) filters, so that very fine dust will not be
blown through the filters and become airborne. Subsequently, flush
the area with water. Liquid spills should be absorbed with earth or
sand and collected for disposal. Persons involved in cleaning up
spills should be adequately protected against eye contact and
inhalation of the powder. The fire brigade should be called to deal
with a large spill.
4.6 Disposal
Waste should be disposed of in an approved landfill site or in a
suitable incinerator, unless directed otherwise by local
authorities.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Exposure of organisms in the environment to trimellitic anhydride is
expected to be very low, except in the case of accidents or spills.
A laboratory study suggests that trimellitic anhydride is readily
degraded by bacteria. Data on aquatic toxicity, e.g., on fish
species, are not yet available.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, trimellitic anhydride. It should
be displayed at, or near, entrances to areas where there is
potential exposure to trimellitic anhydride, and on processing
equipment and containers. The summary should be translated
into the appropriate language(s). All persons potentially
exposed to the chemical should also have the instructions in
the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the
National Poison Control Centre, and local trade names.
SUMMARY OF CHEMICAL SAFETY INFORMATION
TRIMELLITIC ANHYDRIDE
(1,2,4-benzenetricarboxylic acid 1,2-anhydride;
4-carboxyphthalic anhydride; 1,3-dioxo-5-phthalancarboxylic acid; TMA)
C9H4O5
IUPAC systematic name: 1,3-dihydro-1,3-dioxo-5-isobenzofurancarboxylic acid
CAS registry number: 552-30-7
RTECS registry number: DC2050000
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 192.12 White flakes, musty acid odour; fine dust can be released
Boiling point (°C) 390 during handling; flaked or molten trimellitic anhydride
Melting point (°C) 165 will burn if ignited, producing an explosive dust; the vapour
Solubility (g/100 g)(25 °C) from molten trimellitic anhydride forms dangerously
acetone 49.6 explosive mixtures with air
cyclohexanone 38.4
2-butane 36.5
ethyl acetate 21.6
dimethylformamide 15.5
mixed xylenes 0.4
ligroin 0.06
carbon tetrachloride 0.002
Water solubility moderate after hydrolysis
to trimellitic acid
Specific density (20 °C) 1.54
Relative vapour density 6.6
(air=1)
Vapour pressure below 1.1 × 10-7
(mmHg) (25 °C)
Flash point (°C) (open cup) 227
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Mildly irritating to skin; can Avoid skin contact; wear suitable Wash exposed skin with soap and water;
cause skin sensitization protective clothing and gloves remove contaminated clothing and thoroughly
clean and dry all garments before reuse
EYES: Causes eye burns Avoid exposure; wear chemical Immediately flush eyes with plenty of water
goggles for at least 15 minutes; seek immediate
medical advice
INHALATION: Causes irritation of Avoid exposure; use exhaust venti- Remove patient to uncontaminated area;
respiratory tract; may cause sensi- lation and breathing protectiona seek medical advice
tization by inhalation, with effects
such as pulmonary oedema, cough
with blood-stained sputum, anaemia,
and fever
INGESTION: May cause oral and Unlikely occupational hazard Give plenty of water to drink; do not induce
gastrointestinal irritation vomiting; seek medical attention
Other effects: Children of women Avoid exposure
sensitized to trimellitic anhydride
may also be sensitized
a If ventilation is inadequate, use respirator that will protect against organic vapour/particulates.
If dust concentration exceeds assigned protection limit for air-purifying respirator, use supplied-air respirator.
SPILLAGE STORAGE FIRE AND EXPLOSION
Spills must be carefully swept or Protect container from physical High dust concentration may form a
gathered up; alternatively vacuum damage; provide adequate flammable or explosive mixture; its explosive
promptly, with high efficiency filters, ventilation (8-10 air changes limits are 1-7%; in case of fire, use dry
into a dust-tight container; creation of per hour) chemical, carbon dioxide, water fog, or foam
dust is to be avoided; subsequently,
flush the area with water; for personal
protection use protective clothing and
equipment to prevent inhalation, and
eye or skin contact
WASTE DISPOSAL NATIONAL INFORMATION
Deposit in an approved landfill site or National occupational exposure limit:
incinerate in an approved facility,
unless directed otherwise by local
authorities
National poison control centre:
Local trade names:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1 Previous evaluations by international bodies
There have not been any previous evaluations by international
bodies.
7.2 Exposure limit values
Some exposure limit values are given in the table on the page
opposite. Some of these values are incorporated in regulations and
some are recommendations. These have been taken from entries in the
International Register of Potentially Toxic Chemicals (IRPTC) Legal
File.
7.3 Specific restrictions
The United States of America permits trimellitic anhydride to be
used as a cross-linking agent for epoxy resins intended for use with
food (effective 1983) with limits of 15% by weight of the resin. The
European Economic Community (EEC) has included TMA in Section B of
Directive 90/128/EEC (List of substances that may still be used at
national level, pending a decision on inclusion in Section A
(Community list)). The Scientific Committee for Food has not yet
evaluated TMA, because of the lack of technical and toxicological
data.
Restrictions on the handling of trimellitic anhydride by pre-adults,
pregnant women, and nursing mothers (effective 1980) are operative
in Germany.
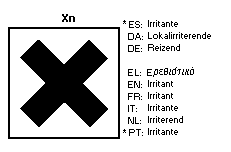
7.4 Transport and labelling
The European Community legislation requires labelling as a dangerous
substance using the symbol:
Common name: trimellitic anhydride
Common synonyms: anhydro trimellitic acid;
1,2,4-benzenetricarboxylic acid
1,2-anhydride; 1,2,4-benzenetricarboxylic
anhydride; 4-carboxyphthalic anhydride;
1,3-dioxo-5-phthalancarboxylic acid;
5-phthalancarboxylic acid, 1,3-dioxo-TMAN;
trimellitic acid 1,2-anhydride; TMA;a
TMAN
IUPAC systematic name: 1,3-dihydro-1,3-dioxo-5-isobenzofuran-
carboxylic acid
CAS registry number: 552-30-7
RTECS registry number: DC2050000
Conversion factor: 1 mg/m3 aprox. 0.13 ppm
Relative molecular mass: 192.12
a TMA is also an acronym for i.a. trimethylamine.
1.2 Physical and chemical properties
Trimellitic anhydride is a white solid in the form of flakes. It is
readily hydrolysed in water to trimellitic acid, which is moderately
soluble in water. Trimellitic anhydride is readily soluble in
acetone, cyclohexanone, 2-butane, ethyl acetate, and dimethyl
formamide (from 49.6 to 15.5% w/w at 25 °C).
Trimellitic anhydride reacts with alcohols forming the corresponding
esters. Reactions with ammonia yield amides, imides, and
amide-imides.
Flaked or molten trimellitic anhydride will burn if ignited,
producing an explosive dust. Similarly, the vapour from molten
trimellitic anhydride forms dangerously explosive mixtures with air.
Some additional physical and chemical properties are given in the
Summary of Chemical Safety Information (see section 6).
1.3 Composition
The purity of commercial trimellitic anhydride is greater than 99%.
1.4 Analytical methods
Airborne trimellitic anhydride can be sampled on various adsorbents,
such as cellulose ester membrane filter, glass fibre filter, or PVC
membrane. It can be determined by high performance liquid
chromatography (HPLC), gas chromatography, or polarography. HPLC
allows the accurate measurement of as little as 0.1 µg trimellitic
anhydride.
1.5 Production and uses
Trimellitic anhydride has been produced since the 1960s. Production
was estimated to be 50 000 tonnes per annum in 1990.
The preparation of trimellitic anhydride is based on the
liquid-phase air-oxidation of pseudocumene to form trimellitic acid,
this being subsequently dehydrated. An alternative process consists
of heating crude trimellitic acid with vanadium pentoxide.
Trimellitic anhydride is a very reactive chemical of low relative
molecular mass. Many of its industrial uses depend on the
reactivity of the anhydride group. It is mainly used in the
synthesis of trimellitate esters. These esters are used as
plasticizers for polyvinyl chloride, especially when temperature
stability is required, e.g., in wire and cable coatings.
Trimellitic anhydride esters are less volatile and less water
soluble than corresponding phthalates, and have begun to replace
them in some applications, e.g., car interior linings. An important
use for trimellitic anhydride polymers is in the production of the
wire enamels used to coat magnetic wire or for other applications
where high thermal resistance is required.
Trimellitic anhydride is also used in the production of polyester
resins for water-based and conventional solvent-based coatings and
paints, to make resins for electrodeposition and powder coatings,
and as a binder for glass fibres, sand, and other aggregates.
In addition, trimellitic anhydride is used as an embossing agent for
foam-backed vinyl flooring and as a curing agent for epoxy and other
resins. It is also used as a plasticizer in materials used to store
and cover food and in the synthesis of various anticorrosive surface
coatings, agricultural chemicals, and pharmaceutical products.
2. SUMMARY AND EVALUATION
2.1 Exposure to trimellitic anhydride
Exposure to trimellitic anhydride occurs in many different
occupational settings; 20 000 workers are known to be exposed
through manufacturing and use in the USA and the worldwide total is
considerably greater. Exposure occurs mainly by inhalation of dust
or fumes. Since trimellitic anhydride has a very low vapour pressure
at room temperature, extensive exposure occurs mainly when dust is
generated from the powder, by mixing or spraying, or by inhalation
of the fumes generated during processes requiring high temperature,
e.g., oven curing. Exposure to very high levels of trimellitic
anhydride fumes occurs when heated metal surfaces are sprayed with
anticorrosive materials containing trimellitic anhydride-based
compounds. High levels of trimellitic anhydride dust are found in
bagging areas.
Exposure of the general population to trimellitic anhydride is
possible, for example, around industrial plants, through its use in
food storage and protection materials, from car interior linings,
and during transportation of the compound. However, the likelihood
of exposure from these sources is very low. When trimellitate
plasticizers are used, trimellitic anhydride itself is not present,
but rather its ester derivatives; these are stable and do not
migrate. There are no reports of populations being affected in the
vicinity of industrial sites.
2.2 Absorption, metabolism, and excretion
In a distribution study, rats were exposed through inhalation to
950 µg 14C-labelled trimellitic anhydride/m3 for 45 minutes.
Treated rats were sacrificed at intervals ranging from 3 hours to 32
days. Maximum concentrations of 14C in all tissues occurred 3 hours
after exposure; these levels then declined in all tissues, except
for the lymph nodes associated with the lung, where there was an
initial decline followed by a second increase, peaking at the eighth
day and then declining.
There is no information on metabolism. Trimellitic anhydride reacts
with the free amino groups on proteins to form conjugates.
2.3 Fate in the environment and effects on organisms
There is very limited information on the fate and effects of
trimellitic anhydride in the environment. However, exposure is
expected to be very low, except from spills and accidents.
In a laboratory degradation study, more than 60% of the theoretical
quantity of carbon dioxide was generated in 10 days. Hydrolysis of
the anhydride was not checked during the study. Trimellitic
anhydride can thus be considered as readily degradable by by
bacteria. Data on aquatic toxicity, e.g., on fish species, are not
yet available
2.4 Effects on animals
2.4.1 Acute toxicity
The acute toxicity of trimellitic anhydride for experimental animals
is low. For the mouse, the most susceptible species, the oral LD50
was 1300 mg/kg body weight. Treated mice showed gastrointestinal
mucosal irritation, with hyperaemia and haemorrhage; in some cases,
perforations were noted. For the rat, the oral LD50 was 2730 mg/kg
body weight, and the dermal LD50 was estimated to be greater than
2000 mg/kg body weight.
A single application of trimellitic anhydride (TMA) to rabbit skin
resulted in mild dermatitis. TMA caused severe irritation to the
eyes of rabbits, leading to corrosive lesions and chemical burns in
the cornea.
2.4.2 Short-term toxicity
Animal inhalation studies, for 2-13 weeks, have shown a clear
correlation between TMA exposure and the occurrence of lung damage.
The development of lung injury is concurrent with a rise in antibody
levels in the products of broncho-alveolar lavage and in serum.
There are marked species differences in susceptibility to
trimellitic anhydride-induced respiratory sensitization. In one
recent study, the rat appeared to be the most sensitive experimental
animal. No sensitization response was observed on challenge 3 weeks
after a 6-hour exposure at 1.6 µg TMA/m3, but sensitization was
seen after exposure at 7.7 µg/m3. Repeated respiratory exposure to
TMA resulted in dose-related focal haemorrhagic areas in the lungs,
with oedema and lobular bronchopneumonia.
An unusual finding in a 13-week rat inhalation study was the
apparent development of tolerance to TMA during the study, as
indicated by reductions in antibody titres and the prevalence of
lung lesions. The results suggest adaptation in rats to TMA after
short-term continuous exposure (Leach et al. 1989).
Repeated skin applications of TMA in guinea-pigs induce significant
dermal sensitization reactions.
Conjugates formed between protein groups and trimellitic anhydride
are able to trigger an allergic response.
Data on genotoxicity and reproductive toxicity are limited; the only
results available on genetic toxicity are those from Ames tests,
which were negative. No developmental toxicity was recorded in a
teratology screeningstudy on pregnant mice. However, trimellitic
anhydride-specific antibody can be transferred from mother to fetus
in rats and guinea-pigs.
2.4.3 Long-term toxicity
There are no data on which the long-term toxicity or carcinogenicity
of TMA can be assessed.
2.5 Effects on human beings
Trimellitic anhydride dust or fumes are highly irritating to the
eyes and respiratory system. The dust may cause corrosive eye
damage, and frequent exposure can lead to respiratory sensitization,
with, in rare cases, quite severe symptoms. Trimellitic anhydride
can produce skin irritation following prolonged or repeated
exposure.
TMA is a potent respiratory sensitizer; it has caused
immunologically-mediated respiratory illness in 29% of workers,
exposed prior to 1979 to levels considerably above the present
threshold limit value. The period from 1979 to 1985 was
characterized by implementation of control measures to reduce
exposure to TMA in the workplace. These measures clearly resulted in
a marked decrease in both clinical symptoms and levels of TMA
antibody formation.
Four clinical syndromes have been identified. Three of these
syndromes are associated with immunological reactions. The first is
an immediate type airway response characterized by asthma or
rhinitis, or both. These symptoms only occur when individuals are
sensitized following a latent period of exposure, which may be weeks
or years. Once sensitization is acquired, symptoms occur within
seconds or minutes of exposure. The syndrome is associated with an
increase in antibody levels.
The second form, the late respiratory systemic syndrome, often
termed "TMA-flu", also requires a latent period of exposure for
sensitization. It is characterized by a delayed onset of symptoms
after exposure, such as coughing and wheezing that occur 4-12 hours
after exposure to trimellitic anhydride; however, the syndrome also
includes muscle and joint pains, and fever. High levels of
antibodies are also associated with this syndrome.
The most severe and rare reaction is the pulmonary-disease-anaemia
syndrome, which may lead to respiratory failure. No fatal cases
have been reported. This illness has appeared only after exposures
to high concentrations of trimellitic anhydride fumes, from the
heating of materials containing trimellitic anhydride, for
relatively short periods. The symptoms vary in severity and may
include cough, with blood-stained sputum, and breathlessness,
resulting in severe pathological changes in the lung. The disease
also requires a latent period of exposure before the onset of
symptoms and it is characterized by high serum antibody levels.
These symptoms are, however, reversible after trimellitic anhydride
exposure has ceased.
The fourth respiratory syndrome is a non-immunological irritant
reaction to trimellitic anhydride characterized by a transient
irritation in the upper airways, with lacrimation and rhinorrhoea.
The irritant symptoms are related to exposure level and can occur in
any worker after a single high-level exposure to trimellitic
anhydride powder or fumes.
Descriptions of the human syndromes are summarized in Table 1.
Table 1. Characteristics of syndromes in humans related to the inhalation of trimellitic anhydridea
Syndrome
Characteristic Rhinitis and immediate- Late respiratory systemic Pulmonary disease- Irritant syndrome
type asthma syndrome anaemia
Latent period (duration Months to years of Months to years of Weeks to months Occurs on first high-
of work exposure prior work exposure work exposure of work exposure level exposure
to onset of symptoms
Onset of symptoms Immediate (minutes) 4-12 hours Progressive with Variable, depending
after work exposure further work on exposure
exposure
Type/degree of exposure TMA dust or fumes/ TMA dust or fumes/ TMA fumes/high Fumes or dust/high
mild moderate
a From Zeiss et al. (1982).
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Trimellitic anhydride is extensively used in industry throughout the
world. It is hazardous to human health in the workplace. Exposure
may result in irritant and/or sensitization respiratory effects,
ranging from mild to severe. It is a mild irritant and sensitizer to
the skin, and corrosive to the eye. Eye damage is frequently
irreversible.
Trimellitic anhydride-related immune response and illness can be
reduced with proper workplace control.
Flaked or molten trimellitic anhydride will burn if ignited,
producing an explosive dust. The vapour from molten trimellitic
anhydride forms dangerously explosive mixtures with air.
3.2 Recommendations
(a) This compound should be handled strictly in accordance with safe
work and good-housekeeping practices. If these are not feasible, the
use of less hazardous alternatives to TMA should be considered.
(b) Since trimellitic anhydride is a respiratory sensitizer, some
response in previously sensitized workers may occur even at the
recommended threshold limit value (TLV); exposure to TMA in the
workplace should, therefore, be kept as low as possible.
(c) Workers with a previous history of asthma or allergies should
not be exposed to trimellitic anhydride. It is advisable to have a
pre-employment chest X-ray.
(d) Pre-employment and subsequent periodic medical surveillance
should be carried out, including the determination of antibody
levels and careful examination of eyes, respiratory tract, and skin.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main human health hazards, prevention and protection, first aid
Since the packaging and handling of trimellitic anhydride can result
in exposure, procedures for diminishing worker exposures to
trimellitic anhydride are essential. In the work area, closed
systems with continuous ventilation should be used, or, if not
feasible, mechanical ventilation and local exhaust should be
installed. If ventilation is insufficient to maintain exposures
below the recommended limit, a full face-piece, cartridge respirator
is required as the minimum protection. A combination type cartridge
may be used for dust or fume concentrations up to 50 times the
recommended exposure limit. For higher concentrations, an
air-supplied, full face-piece respirator is required. Overalls or
other appropriate clothing, chemical goggles, and gloves must be
worn by persons engaged in work that results in direct contact with
trimellitic anhydride. Contaminated clothing should be removed and
thoroughly cleaned and dried before reuse. High dust concentration
may form a flammable or explosive mixture with air and should be
avoided. Dry dust can be charged electrostatically by turbulence,
pneumatic transport, pouring, and in exhaust ducts and during
transportation. Build-up of electrostatic charges should be
prevented by grounding.
Users of TMA should consult the manufacturers with regard to
appropriate and effective exhaust ventilation, and precautions to be
followed when using the compound.
4.1.1 Surveillance
Antibody titres should be determined in workers exposed to
trimellitic anhydride every 6 months.
4.1.2 First aid
In the case of exposure to TMA, medical attention should be sought
immediately; in the meantime, render first aid. Move the patient to
fresh air, remove contaminated clothing. Wash contaminated skin with
soap and water and provide artificial respiration if necessary. In
the case of splashes in the eyes, irrigate the eyes with plenty of
water for 15 minutes. If large amounts are ingested, vomiting should
not be in induced, to avoid the potential complication of aspiration
pneumonitis.
4.2 Advice to physicians
No specific antidote is known. General symptomatic and supportive
therapy is indicated, depending on the system affected: respiratory
system, eyes, or skin.
4.3 Explosion and fire hazards
Trimellitic anhydride is not readily combustible. However, high
dust concentrations have a potential for combustion or explosion.
Trimellitic anhydride forms an explosive mixture with air; the lower
and upper explosive limits are 1% and 7%, respectively. Care should
be taken to keep dust concentrations low and to prevent ignition.
High-voltage static electricity build-up is possible when
significant quantities of dust are present.
In the event of a fire, use water fog, foam, or dry chemical.
Personnel should wear full protective clothing and breathing
apparatus.
4.4 Storage and transport
Containers of trimellitic anhydride should be protected from
physical damage to prevent any leakage of the contents. The
substance should be stored in well-ventilated areas.
During transport, containers should be sound and well sealed to
prevent loss of contents.
4.5 Spillage
Spills should be carefully swept or gathered up to avoid producing
dust and transferred to a sealable container. Alternatively, an
approved high-efficiency vacuum may be used, fitted with
high-efficiency (HEPA) filters, so that very fine dust will not be
blown through the filters and become airborne. Subsequently, flush
the area with water. Liquid spills should be absorbed with earth or
sand and collected for disposal. Persons involved in cleaning up
spills should be adequately protected against eye contact and
inhalation of the powder. The fire brigade should be called to deal
with a large spill.
4.6 Disposal
Waste should be disposed of in an approved landfill site or in a
suitable incinerator, unless directed otherwise by local
authorities.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Exposure of organisms in the environment to trimellitic anhydride is
expected to be very low, except in the case of accidents or spills.
A laboratory study suggests that trimellitic anhydride is readily
degraded by bacteria. Data on aquatic toxicity, e.g., on fish
species, are not yet available.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, trimellitic anhydride. It should
be displayed at, or near, entrances to areas where there is
potential exposure to trimellitic anhydride, and on processing
equipment and containers. The summary should be translated
into the appropriate language(s). All persons potentially
exposed to the chemical should also have the instructions in
the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the
National Poison Control Centre, and local trade names.
SUMMARY OF CHEMICAL SAFETY INFORMATION
TRIMELLITIC ANHYDRIDE
(1,2,4-benzenetricarboxylic acid 1,2-anhydride;
4-carboxyphthalic anhydride; 1,3-dioxo-5-phthalancarboxylic acid; TMA)
C9H4O5
IUPAC systematic name: 1,3-dihydro-1,3-dioxo-5-isobenzofurancarboxylic acid
CAS registry number: 552-30-7
RTECS registry number: DC2050000
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 192.12 White flakes, musty acid odour; fine dust can be released
Boiling point (°C) 390 during handling; flaked or molten trimellitic anhydride
Melting point (°C) 165 will burn if ignited, producing an explosive dust; the vapour
Solubility (g/100 g)(25 °C) from molten trimellitic anhydride forms dangerously
acetone 49.6 explosive mixtures with air
cyclohexanone 38.4
2-butane 36.5
ethyl acetate 21.6
dimethylformamide 15.5
mixed xylenes 0.4
ligroin 0.06
carbon tetrachloride 0.002
Water solubility moderate after hydrolysis
to trimellitic acid
Specific density (20 °C) 1.54
Relative vapour density 6.6
(air=1)
Vapour pressure below 1.1 × 10-7
(mmHg) (25 °C)
Flash point (°C) (open cup) 227
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Mildly irritating to skin; can Avoid skin contact; wear suitable Wash exposed skin with soap and water;
cause skin sensitization protective clothing and gloves remove contaminated clothing and thoroughly
clean and dry all garments before reuse
EYES: Causes eye burns Avoid exposure; wear chemical Immediately flush eyes with plenty of water
goggles for at least 15 minutes; seek immediate
medical advice
INHALATION: Causes irritation of Avoid exposure; use exhaust venti- Remove patient to uncontaminated area;
respiratory tract; may cause sensi- lation and breathing protectiona seek medical advice
tization by inhalation, with effects
such as pulmonary oedema, cough
with blood-stained sputum, anaemia,
and fever
INGESTION: May cause oral and Unlikely occupational hazard Give plenty of water to drink; do not induce
gastrointestinal irritation vomiting; seek medical attention
Other effects: Children of women Avoid exposure
sensitized to trimellitic anhydride
may also be sensitized
a If ventilation is inadequate, use respirator that will protect against organic vapour/particulates.
If dust concentration exceeds assigned protection limit for air-purifying respirator, use supplied-air respirator.
SPILLAGE STORAGE FIRE AND EXPLOSION
Spills must be carefully swept or Protect container from physical High dust concentration may form a
gathered up; alternatively vacuum damage; provide adequate flammable or explosive mixture; its explosive
promptly, with high efficiency filters, ventilation (8-10 air changes limits are 1-7%; in case of fire, use dry
into a dust-tight container; creation of per hour) chemical, carbon dioxide, water fog, or foam
dust is to be avoided; subsequently,
flush the area with water; for personal
protection use protective clothing and
equipment to prevent inhalation, and
eye or skin contact
WASTE DISPOSAL NATIONAL INFORMATION
Deposit in an approved landfill site or National occupational exposure limit:
incinerate in an approved facility,
unless directed otherwise by local
authorities
National poison control centre:
Local trade names:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1 Previous evaluations by international bodies
There have not been any previous evaluations by international
bodies.
7.2 Exposure limit values
Some exposure limit values are given in the table on the page
opposite. Some of these values are incorporated in regulations and
some are recommendations. These have been taken from entries in the
International Register of Potentially Toxic Chemicals (IRPTC) Legal
File.
7.3 Specific restrictions
The United States of America permits trimellitic anhydride to be
used as a cross-linking agent for epoxy resins intended for use with
food (effective 1983) with limits of 15% by weight of the resin. The
European Economic Community (EEC) has included TMA in Section B of
Directive 90/128/EEC (List of substances that may still be used at
national level, pending a decision on inclusion in Section A
(Community list)). The Scientific Committee for Food has not yet
evaluated TMA, because of the lack of technical and toxicological
data.
Restrictions on the handling of trimellitic anhydride by pre-adults,
pregnant women, and nursing mothers (effective 1980) are operative
in Germany.
7.4 Transport and labelling
The European Community legislation requires labelling as a dangerous
substance using the symbol:
 CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Tolerances and exposure limits for air in the workplace a
Country Exposure limit description Value Effective date
Australia Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Belgium Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Canada Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm) 1980
Czechoslovakia Maximum workplace concentration (MAK) 0.005 mg/m3 (fumes)
0.04 mg/m3 (fine dust)
Germany Maximum workplace concentration (MAK)
- Time-weighted average (8-h) (TWA) 0.04 mg/m3 (0.005 ppm)
- Short-term exposure limit (5 min) (STEL) 0.08 mg/m3 (0.01 ppm)
Netherlands Maximum workplace concentration (MAK) 0.04 mg/m3 (0.005 ppm)
Sweden Threshold limit value (TLV) 0.04 mg/m3 (0.005 ppm) 1988
United Kingdom Occupational exposure standard (OES) 0.04 mg/m3 (0.005 ppm) 1987
USA Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
a Where no effective date is given, the limits are recommendations, not regulations.
The label must read:
Irritating to eyes, respiratory system and skin; may cause
sensitization by inhalation; do not breathe gas/fumes/
vapour/spray (appropriate wording to be specified by the
manufacturer); after contact with skin, wash immediately with
plenty of ..... (to be specified by the manufacturer).
The WHO Task Group that reviewed this publication did not consider
that the above risk and safety expressions fully indicated the
potential risk of severe and irreversible eye damage that has been
reported for trimellitic anhydride.
BIBLIOGRAPHY
AMOCO CHEMICAL COMPANY (1990) Biodegradation of TMA. Chicago,
Illinois (personal communication).
AMOCO Trimellitic anhydride. TMA health and safety information.
Amoco Chemical Company, Chicago, Illinois, 11 pp. (Bulletin TM-131
c).
AMOCO (1990) Material safety data sheet. Amoco Chemical Company,
Chicago, Illinois, 6 pp. (MSDS. No. 08001260).
BEMIS, A.G., DINDORF, J.A., HARWOOD, B. & SAMANS, C. (1982) Phthalic
acids. In: Grayson, M. & Eckroth, D. ed. Kirk-Othmer encyclopedia
of chemical technology, 3rd edition. New York, John Wiley and
Sons, Inc., pp. 736-767.
GILLNER, M. (1989) Trimellitic anhydride (TMA) - A hazard analysis.
Solna, Sweden, The National Chemicals Inspectorate, 45 pp. (Kemi
Report Series No. 1/89).
LEACH, C.L., HATOUM, N.S., ZEISS, C.R. & GARVIN, P.J. (1989)
Immunologic tolerance in rats during 13 weeks of inhalation exposure
to trimellitic anhydride. Fundamental and applied toxicology, 12:
519-529.
NIOSH (1978) Trimellitic anhydride (TMA). Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (Current
intelligence bulletin 21, Publication No. 78-121 DHHS (NIOSH)).
NIOSH (1988) Registry of toxic effects of chemical substances
(1985-86). Washington, DC, US National Institute for Occupational
Safety and Health (Publication No. 87-111 DHHS (NIOSH)).
RYAN, B.M. (1988) Teratological evaluation of trimellitic
anhydride (TMA) in rats and guinea-pigs. Chicago, Illinois,
School of Advanced Studies of Illinois Institute of Technology.
SANTODONATO, J. BOSCH, S. MEYLAN, W., BECKER, J. & NEAL, M. (1985)
Monograph on human exposure to chemicals in the workplace:
Trimellitic anhydride. Springfield, VA, US Department of Commerce,
National Technical Information Service (PB-14932).
THRASHER, J.D., MADISON, R., BROUGHTON, A. & GARD, Z. (1989)
Building-related illness and antibodies to albumin conjugates of
formaldehyde, toluene diisocyanate, and trimellitic anhydride. Am.
J. ind. Med, 15: 187-195.
WINDHOLZ, M., ed. (1983) The Merck index. Rahway, NJ, Merck and
Co., Inc.
ZEISS, C.R., PATTERSON, R., PRUZANSKY, J.J., MILLER, M.M.,
ROSENBERG, M & LEVITZ, D. (1977) Trimellitic anhydride-induced
airway syndromes: Clinical and immunologic studies. J. Allergy
clin. Immunol., 60: 96-103.
ZEISS, C.R., GRAMMER, L. & PATTERSON, R. (1989) Amoco Report-Joliet
survey 1988-89. Chicago, Illinois, The Veterans Administration
Medical Center, 42 pp.
ZEISS, C.R., WOLKONSKY, P., PRUZANSKY, J.J., & PATTERSON, R.
(1982) Clinical and immunologic evaluation of trimellitic anhydride
workers in multiple industrial settings. J. Allergy clin.
Immunol., 70(1): 15-18.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Tolerances and exposure limits for air in the workplace a
Country Exposure limit description Value Effective date
Australia Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Belgium Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Canada Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm) 1980
Czechoslovakia Maximum workplace concentration (MAK) 0.005 mg/m3 (fumes)
0.04 mg/m3 (fine dust)
Germany Maximum workplace concentration (MAK)
- Time-weighted average (8-h) (TWA) 0.04 mg/m3 (0.005 ppm)
- Short-term exposure limit (5 min) (STEL) 0.08 mg/m3 (0.01 ppm)
Netherlands Maximum workplace concentration (MAK) 0.04 mg/m3 (0.005 ppm)
Sweden Threshold limit value (TLV) 0.04 mg/m3 (0.005 ppm) 1988
United Kingdom Occupational exposure standard (OES) 0.04 mg/m3 (0.005 ppm) 1987
USA Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
a Where no effective date is given, the limits are recommendations, not regulations.
The label must read:
Irritating to eyes, respiratory system and skin; may cause
sensitization by inhalation; do not breathe gas/fumes/
vapour/spray (appropriate wording to be specified by the
manufacturer); after contact with skin, wash immediately with
plenty of ..... (to be specified by the manufacturer).
The WHO Task Group that reviewed this publication did not consider
that the above risk and safety expressions fully indicated the
potential risk of severe and irreversible eye damage that has been
reported for trimellitic anhydride.
BIBLIOGRAPHY
AMOCO CHEMICAL COMPANY (1990) Biodegradation of TMA. Chicago,
Illinois (personal communication).
AMOCO Trimellitic anhydride. TMA health and safety information.
Amoco Chemical Company, Chicago, Illinois, 11 pp. (Bulletin TM-131
c).
AMOCO (1990) Material safety data sheet. Amoco Chemical Company,
Chicago, Illinois, 6 pp. (MSDS. No. 08001260).
BEMIS, A.G., DINDORF, J.A., HARWOOD, B. & SAMANS, C. (1982) Phthalic
acids. In: Grayson, M. & Eckroth, D. ed. Kirk-Othmer encyclopedia
of chemical technology, 3rd edition. New York, John Wiley and
Sons, Inc., pp. 736-767.
GILLNER, M. (1989) Trimellitic anhydride (TMA) - A hazard analysis.
Solna, Sweden, The National Chemicals Inspectorate, 45 pp. (Kemi
Report Series No. 1/89).
LEACH, C.L., HATOUM, N.S., ZEISS, C.R. & GARVIN, P.J. (1989)
Immunologic tolerance in rats during 13 weeks of inhalation exposure
to trimellitic anhydride. Fundamental and applied toxicology, 12:
519-529.
NIOSH (1978) Trimellitic anhydride (TMA). Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (Current
intelligence bulletin 21, Publication No. 78-121 DHHS (NIOSH)).
NIOSH (1988) Registry of toxic effects of chemical substances
(1985-86). Washington, DC, US National Institute for Occupational
Safety and Health (Publication No. 87-111 DHHS (NIOSH)).
RYAN, B.M. (1988) Teratological evaluation of trimellitic
anhydride (TMA) in rats and guinea-pigs. Chicago, Illinois,
School of Advanced Studies of Illinois Institute of Technology.
SANTODONATO, J. BOSCH, S. MEYLAN, W., BECKER, J. & NEAL, M. (1985)
Monograph on human exposure to chemicals in the workplace:
Trimellitic anhydride. Springfield, VA, US Department of Commerce,
National Technical Information Service (PB-14932).
THRASHER, J.D., MADISON, R., BROUGHTON, A. & GARD, Z. (1989)
Building-related illness and antibodies to albumin conjugates of
formaldehyde, toluene diisocyanate, and trimellitic anhydride. Am.
J. ind. Med, 15: 187-195.
WINDHOLZ, M., ed. (1983) The Merck index. Rahway, NJ, Merck and
Co., Inc.
ZEISS, C.R., PATTERSON, R., PRUZANSKY, J.J., MILLER, M.M.,
ROSENBERG, M & LEVITZ, D. (1977) Trimellitic anhydride-induced
airway syndromes: Clinical and immunologic studies. J. Allergy
clin. Immunol., 60: 96-103.
ZEISS, C.R., GRAMMER, L. & PATTERSON, R. (1989) Amoco Report-Joliet
survey 1988-89. Chicago, Illinois, The Veterans Administration
Medical Center, 42 pp.
ZEISS, C.R., WOLKONSKY, P., PRUZANSKY, J.J., & PATTERSON, R.
(1982) Clinical and immunologic evaluation of trimellitic anhydride
workers in multiple industrial settings. J. Allergy clin.
Immunol., 70(1): 15-18.
 Common name: trimellitic anhydride
Common synonyms: anhydro trimellitic acid;
1,2,4-benzenetricarboxylic acid
1,2-anhydride; 1,2,4-benzenetricarboxylic
anhydride; 4-carboxyphthalic anhydride;
1,3-dioxo-5-phthalancarboxylic acid;
5-phthalancarboxylic acid, 1,3-dioxo-TMAN;
trimellitic acid 1,2-anhydride; TMA;a
TMAN
IUPAC systematic name: 1,3-dihydro-1,3-dioxo-5-isobenzofuran-
carboxylic acid
CAS registry number:
Common name: trimellitic anhydride
Common synonyms: anhydro trimellitic acid;
1,2,4-benzenetricarboxylic acid
1,2-anhydride; 1,2,4-benzenetricarboxylic
anhydride; 4-carboxyphthalic anhydride;
1,3-dioxo-5-phthalancarboxylic acid;
5-phthalancarboxylic acid, 1,3-dioxo-TMAN;
trimellitic acid 1,2-anhydride; TMA;a
TMAN
IUPAC systematic name: 1,3-dihydro-1,3-dioxo-5-isobenzofuran-
carboxylic acid
CAS registry number:  CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Tolerances and exposure limits for air in the workplace a
Country Exposure limit description Value Effective date
Australia Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Belgium Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Canada Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm) 1980
Czechoslovakia Maximum workplace concentration (MAK) 0.005 mg/m3 (fumes)
0.04 mg/m3 (fine dust)
Germany Maximum workplace concentration (MAK)
- Time-weighted average (8-h) (TWA) 0.04 mg/m3 (0.005 ppm)
- Short-term exposure limit (5 min) (STEL) 0.08 mg/m3 (0.01 ppm)
Netherlands Maximum workplace concentration (MAK) 0.04 mg/m3 (0.005 ppm)
Sweden Threshold limit value (TLV) 0.04 mg/m3 (0.005 ppm) 1988
United Kingdom Occupational exposure standard (OES) 0.04 mg/m3 (0.005 ppm) 1987
USA Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
a Where no effective date is given, the limits are recommendations, not regulations.
The label must read:
Irritating to eyes, respiratory system and skin; may cause
sensitization by inhalation; do not breathe gas/fumes/
vapour/spray (appropriate wording to be specified by the
manufacturer); after contact with skin, wash immediately with
plenty of ..... (to be specified by the manufacturer).
The WHO Task Group that reviewed this publication did not consider
that the above risk and safety expressions fully indicated the
potential risk of severe and irreversible eye damage that has been
reported for trimellitic anhydride.
BIBLIOGRAPHY
AMOCO CHEMICAL COMPANY (1990) Biodegradation of TMA. Chicago,
Illinois (personal communication).
AMOCO Trimellitic anhydride. TMA health and safety information.
Amoco Chemical Company, Chicago, Illinois, 11 pp. (Bulletin TM-131
c).
AMOCO (1990) Material safety data sheet. Amoco Chemical Company,
Chicago, Illinois, 6 pp. (MSDS. No. 08001260).
BEMIS, A.G., DINDORF, J.A., HARWOOD, B. & SAMANS, C. (1982) Phthalic
acids. In: Grayson, M. & Eckroth, D. ed. Kirk-Othmer encyclopedia
of chemical technology, 3rd edition. New York, John Wiley and
Sons, Inc., pp. 736-767.
GILLNER, M. (1989) Trimellitic anhydride (TMA) - A hazard analysis.
Solna, Sweden, The National Chemicals Inspectorate, 45 pp. (Kemi
Report Series No. 1/89).
LEACH, C.L., HATOUM, N.S., ZEISS, C.R. & GARVIN, P.J. (1989)
Immunologic tolerance in rats during 13 weeks of inhalation exposure
to trimellitic anhydride. Fundamental and applied toxicology, 12:
519-529.
NIOSH (1978) Trimellitic anhydride (TMA). Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (Current
intelligence bulletin 21, Publication No. 78-121 DHHS (NIOSH)).
NIOSH (1988) Registry of toxic effects of chemical substances
(1985-86). Washington, DC, US National Institute for Occupational
Safety and Health (Publication No. 87-111 DHHS (NIOSH)).
RYAN, B.M. (1988) Teratological evaluation of trimellitic
anhydride (TMA) in rats and guinea-pigs. Chicago, Illinois,
School of Advanced Studies of Illinois Institute of Technology.
SANTODONATO, J. BOSCH, S. MEYLAN, W., BECKER, J. & NEAL, M. (1985)
Monograph on human exposure to chemicals in the workplace:
Trimellitic anhydride. Springfield, VA, US Department of Commerce,
National Technical Information Service (PB-14932).
THRASHER, J.D., MADISON, R., BROUGHTON, A. & GARD, Z. (1989)
Building-related illness and antibodies to albumin conjugates of
formaldehyde, toluene diisocyanate, and trimellitic anhydride. Am.
J. ind. Med, 15: 187-195.
WINDHOLZ, M., ed. (1983) The Merck index. Rahway, NJ, Merck and
Co., Inc.
ZEISS, C.R., PATTERSON, R., PRUZANSKY, J.J., MILLER, M.M.,
ROSENBERG, M & LEVITZ, D. (1977) Trimellitic anhydride-induced
airway syndromes: Clinical and immunologic studies. J. Allergy
clin. Immunol., 60: 96-103.
ZEISS, C.R., GRAMMER, L. & PATTERSON, R. (1989) Amoco Report-Joliet
survey 1988-89. Chicago, Illinois, The Veterans Administration
Medical Center, 42 pp.
ZEISS, C.R., WOLKONSKY, P., PRUZANSKY, J.J., & PATTERSON, R.
(1982) Clinical and immunologic evaluation of trimellitic anhydride
workers in multiple industrial settings. J. Allergy clin.
Immunol., 70(1): 15-18.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Tolerances and exposure limits for air in the workplace a
Country Exposure limit description Value Effective date
Australia Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Belgium Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
Canada Threshold limit value TLV
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm) 1980
Czechoslovakia Maximum workplace concentration (MAK) 0.005 mg/m3 (fumes)
0.04 mg/m3 (fine dust)
Germany Maximum workplace concentration (MAK)
- Time-weighted average (8-h) (TWA) 0.04 mg/m3 (0.005 ppm)
- Short-term exposure limit (5 min) (STEL) 0.08 mg/m3 (0.01 ppm)
Netherlands Maximum workplace concentration (MAK) 0.04 mg/m3 (0.005 ppm)
Sweden Threshold limit value (TLV) 0.04 mg/m3 (0.005 ppm) 1988
United Kingdom Occupational exposure standard (OES) 0.04 mg/m3 (0.005 ppm) 1987
USA Threshold limit value (TLV)
- Time-weighted average (TWA) 0.04 mg/m3 (0.005 ppm)
a Where no effective date is given, the limits are recommendations, not regulations.
The label must read:
Irritating to eyes, respiratory system and skin; may cause
sensitization by inhalation; do not breathe gas/fumes/
vapour/spray (appropriate wording to be specified by the
manufacturer); after contact with skin, wash immediately with
plenty of ..... (to be specified by the manufacturer).
The WHO Task Group that reviewed this publication did not consider
that the above risk and safety expressions fully indicated the
potential risk of severe and irreversible eye damage that has been
reported for trimellitic anhydride.
BIBLIOGRAPHY
AMOCO CHEMICAL COMPANY (1990) Biodegradation of TMA. Chicago,
Illinois (personal communication).
AMOCO Trimellitic anhydride. TMA health and safety information.
Amoco Chemical Company, Chicago, Illinois, 11 pp. (Bulletin TM-131
c).
AMOCO (1990) Material safety data sheet. Amoco Chemical Company,
Chicago, Illinois, 6 pp. (MSDS. No. 08001260).
BEMIS, A.G., DINDORF, J.A., HARWOOD, B. & SAMANS, C. (1982) Phthalic
acids. In: Grayson, M. & Eckroth, D. ed. Kirk-Othmer encyclopedia
of chemical technology, 3rd edition. New York, John Wiley and
Sons, Inc., pp. 736-767.
GILLNER, M. (1989) Trimellitic anhydride (TMA) - A hazard analysis.
Solna, Sweden, The National Chemicals Inspectorate, 45 pp. (Kemi
Report Series No. 1/89).
LEACH, C.L., HATOUM, N.S., ZEISS, C.R. & GARVIN, P.J. (1989)
Immunologic tolerance in rats during 13 weeks of inhalation exposure
to trimellitic anhydride. Fundamental and applied toxicology, 12:
519-529.
NIOSH (1978) Trimellitic anhydride (TMA). Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (Current
intelligence bulletin 21, Publication No. 78-121 DHHS (NIOSH)).
NIOSH (1988) Registry of toxic effects of chemical substances
(1985-86). Washington, DC, US National Institute for Occupational
Safety and Health (Publication No. 87-111 DHHS (NIOSH)).
RYAN, B.M. (1988) Teratological evaluation of trimellitic
anhydride (TMA) in rats and guinea-pigs. Chicago, Illinois,
School of Advanced Studies of Illinois Institute of Technology.
SANTODONATO, J. BOSCH, S. MEYLAN, W., BECKER, J. & NEAL, M. (1985)
Monograph on human exposure to chemicals in the workplace:
Trimellitic anhydride. Springfield, VA, US Department of Commerce,
National Technical Information Service (PB-14932).
THRASHER, J.D., MADISON, R., BROUGHTON, A. & GARD, Z. (1989)
Building-related illness and antibodies to albumin conjugates of
formaldehyde, toluene diisocyanate, and trimellitic anhydride. Am.
J. ind. Med, 15: 187-195.
WINDHOLZ, M., ed. (1983) The Merck index. Rahway, NJ, Merck and
Co., Inc.
ZEISS, C.R., PATTERSON, R., PRUZANSKY, J.J., MILLER, M.M.,
ROSENBERG, M & LEVITZ, D. (1977) Trimellitic anhydride-induced
airway syndromes: Clinical and immunologic studies. J. Allergy
clin. Immunol., 60: 96-103.
ZEISS, C.R., GRAMMER, L. & PATTERSON, R. (1989) Amoco Report-Joliet
survey 1988-89. Chicago, Illinois, The Veterans Administration
Medical Center, 42 pp.
ZEISS, C.R., WOLKONSKY, P., PRUZANSKY, J.J., & PATTERSON, R.
(1982) Clinical and immunologic evaluation of trimellitic anhydride
workers in multiple industrial settings. J. Allergy clin.
Immunol., 70(1): 15-18.
