
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
ETHYL p-HYDROXYBENZOATE
Synonyms Ethyl p-oxybenzoate; Ethylparaben
Chemical name Ethyl p-hydroxybenzoate; ethyl ester of
p-hydroxybenzoic acid
Empirical formula C9H10O3
Structural formula
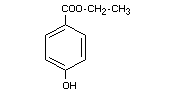 Molecular weight 166.18
Definition Ethyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C9H10O3.
Description Ethyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The metabolism of the ethyl ester is similar to that of the
methyl ester. Dogs given 1000 mg/kg body-weight orally or 50 mg/kg
i.v. excreted 66-70 per cent. in the urine. Very low plasma levels,
just detectable, were found two hours after oral administration or at
5 and 15 minutes after i.v. injection (Sokol, 1952).
Human studies on six subjects involved administration of 10 or 20
mg/kg body-weight of ester orally. After 60, 135 and 255 minutes,
n-hydroxybenzoate but not the ester, was detectable in serum. The
maximum serum level attained was 4.5 µg/ml (Heim, 1960-1).
Acute Toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 2 000-2 400 Heyden, 1939
Rabbit oral 5 000 Sabalitschka & Neufeld-
Crzellitzer, 1954
Dog oral 5 000 Sabalitschka &
Neufeld-Crzellitzer, 1954
Short-term studies
Rabbit. Suspensions of 0.5 and 7.5 per cent. ethyl
p-hydroxybenzoate had the same local anaesthetic effect on the cornea
as 0.12 and 0.27 per cent. solutions of cocaine hydrochloride.
Therefore, the local anaesthetic activity of the ester is 3-4 times
less than that of cocaine and twice that of procaine (Truhaut, 1962).
Using the same method of Regnier & Quevauviller (1939), Adler-Hradecky
and Kelentey (1960) found no local anaesthetic effect on the cornea by
0.25-0.30 per cent. solutions of methyl, ethyl, propyl or butyl
p-hydroxybenzoates.
Long-term studies
Rat. Forty rats were fed 15 mg/kg body-weight, 20 animals 150
mg/kg body-weight and 20 animals 1500 mg/kg body-weight of a mixture
of 40 per cent. ethyl ester and 60 per cent. propyl ester as the
sodium salts for 18 months in the diet. The rate of weight gain showed
some growth stimulation at the 15 and 150 mg/kg body-weight levels.
The group on 1500 mg/kg body-weight showed initial retardation
followed later by normal growth. Mortality rate and pathological
examination of the major organs in all treated groups showed no
significant difference from the control group (Heyden, 1940; Heyden,
1942).
A group of 65 rats (35 males and 30 females) was fed a diet
containing 2 per cent. of the ethyl ester for the life span, with 50
animals as the control group. All animals were autopsied on death. No
adverse effects could be detected on weight gain, except a small
retardation during the first month, and the mortality rate,
haematology, tumour incidence and histopathology of major organs did
not differ from the controls (Truhaut, 1962).
A group of 39 rats (19 males and 20 females) was injected weekly
with 1 ml of an aqueous solution of 10 per cent. sodium ethyl
p-hydroxybenzoate for their life span. The 27 controls (16 males and
11 females) were injected with 1 ml of a 3 per cent. sodium chloride
solution. Because of irritation by the high p.H of the ester solution,
the frequency of injection had to be reduced to 1 in 2 weeks from the
4th to the 10th month and later to 1 injection a month up to the end
of the experiment. No effect on mortality and tumour incidence could
be detected (Truhaut, 1962).
Comments
The long-term studies in rats are adequate for assessment, taking
into consideration the data on methyl and propyl p-hydroxybenzoates.
However, further biochemical studies in man and animals are desirable
and further studies on the local anaesthetic action should also be
undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B (1960) Arch. int. Pharmacodyn.,
128, 135
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser., 228
Heim, F. (1960-1) Sitzber. Physik-Med. Soz. Erlangen, 81, 14
Heyden,-. (1939) Unpublished Report from Applied Research Inc., MX-124
Heyden, -. (1940) Unpublished Report from Applied Research Inc.,
MX-185
Heyden, -. (1942) Unpublished Report from Applied Research Inc.,
MX-185
Regnier, M. & Quevauviller, A. (1939) Arch. exp. Path. Pharm.,
193, 48
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch.,4, 575
Schübel, K. & Manger, J. (1929) Arch. exp. Path. Pharmak., 146,
208
Sokol, H. (1952) Drug Stand., 20, 89
Truhaut, R. (1962) Estratto dai Rendiconti dell'Instituto Superiore di
Sanità (Document submitted to WHO in 1964)
Molecular weight 166.18
Definition Ethyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C9H10O3.
Description Ethyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The metabolism of the ethyl ester is similar to that of the
methyl ester. Dogs given 1000 mg/kg body-weight orally or 50 mg/kg
i.v. excreted 66-70 per cent. in the urine. Very low plasma levels,
just detectable, were found two hours after oral administration or at
5 and 15 minutes after i.v. injection (Sokol, 1952).
Human studies on six subjects involved administration of 10 or 20
mg/kg body-weight of ester orally. After 60, 135 and 255 minutes,
n-hydroxybenzoate but not the ester, was detectable in serum. The
maximum serum level attained was 4.5 µg/ml (Heim, 1960-1).
Acute Toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 2 000-2 400 Heyden, 1939
Rabbit oral 5 000 Sabalitschka & Neufeld-
Crzellitzer, 1954
Dog oral 5 000 Sabalitschka &
Neufeld-Crzellitzer, 1954
Short-term studies
Rabbit. Suspensions of 0.5 and 7.5 per cent. ethyl
p-hydroxybenzoate had the same local anaesthetic effect on the cornea
as 0.12 and 0.27 per cent. solutions of cocaine hydrochloride.
Therefore, the local anaesthetic activity of the ester is 3-4 times
less than that of cocaine and twice that of procaine (Truhaut, 1962).
Using the same method of Regnier & Quevauviller (1939), Adler-Hradecky
and Kelentey (1960) found no local anaesthetic effect on the cornea by
0.25-0.30 per cent. solutions of methyl, ethyl, propyl or butyl
p-hydroxybenzoates.
Long-term studies
Rat. Forty rats were fed 15 mg/kg body-weight, 20 animals 150
mg/kg body-weight and 20 animals 1500 mg/kg body-weight of a mixture
of 40 per cent. ethyl ester and 60 per cent. propyl ester as the
sodium salts for 18 months in the diet. The rate of weight gain showed
some growth stimulation at the 15 and 150 mg/kg body-weight levels.
The group on 1500 mg/kg body-weight showed initial retardation
followed later by normal growth. Mortality rate and pathological
examination of the major organs in all treated groups showed no
significant difference from the control group (Heyden, 1940; Heyden,
1942).
A group of 65 rats (35 males and 30 females) was fed a diet
containing 2 per cent. of the ethyl ester for the life span, with 50
animals as the control group. All animals were autopsied on death. No
adverse effects could be detected on weight gain, except a small
retardation during the first month, and the mortality rate,
haematology, tumour incidence and histopathology of major organs did
not differ from the controls (Truhaut, 1962).
A group of 39 rats (19 males and 20 females) was injected weekly
with 1 ml of an aqueous solution of 10 per cent. sodium ethyl
p-hydroxybenzoate for their life span. The 27 controls (16 males and
11 females) were injected with 1 ml of a 3 per cent. sodium chloride
solution. Because of irritation by the high p.H of the ester solution,
the frequency of injection had to be reduced to 1 in 2 weeks from the
4th to the 10th month and later to 1 injection a month up to the end
of the experiment. No effect on mortality and tumour incidence could
be detected (Truhaut, 1962).
Comments
The long-term studies in rats are adequate for assessment, taking
into consideration the data on methyl and propyl p-hydroxybenzoates.
However, further biochemical studies in man and animals are desirable
and further studies on the local anaesthetic action should also be
undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B (1960) Arch. int. Pharmacodyn.,
128, 135
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser., 228
Heim, F. (1960-1) Sitzber. Physik-Med. Soz. Erlangen, 81, 14
Heyden,-. (1939) Unpublished Report from Applied Research Inc., MX-124
Heyden, -. (1940) Unpublished Report from Applied Research Inc.,
MX-185
Heyden, -. (1942) Unpublished Report from Applied Research Inc.,
MX-185
Regnier, M. & Quevauviller, A. (1939) Arch. exp. Path. Pharm.,
193, 48
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch.,4, 575
Schübel, K. & Manger, J. (1929) Arch. exp. Path. Pharmak., 146,
208
Sokol, H. (1952) Drug Stand., 20, 89
Truhaut, R. (1962) Estratto dai Rendiconti dell'Instituto Superiore di
Sanità (Document submitted to WHO in 1964)
 Molecular weight 166.18
Definition Ethyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C9H10O3.
Description Ethyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The metabolism of the ethyl ester is similar to that of the
methyl ester. Dogs given 1000 mg/kg body-weight orally or 50 mg/kg
i.v. excreted 66-70 per cent. in the urine. Very low plasma levels,
just detectable, were found two hours after oral administration or at
5 and 15 minutes after i.v. injection (Sokol, 1952).
Human studies on six subjects involved administration of 10 or 20
mg/kg body-weight of ester orally. After 60, 135 and 255 minutes,
n-hydroxybenzoate but not the ester, was detectable in serum. The
maximum serum level attained was 4.5 µg/ml (Heim, 1960-1).
Acute Toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 2 000-2 400 Heyden, 1939
Rabbit oral 5 000 Sabalitschka & Neufeld-
Crzellitzer, 1954
Dog oral 5 000 Sabalitschka &
Neufeld-Crzellitzer, 1954
Short-term studies
Rabbit. Suspensions of 0.5 and 7.5 per cent. ethyl
p-hydroxybenzoate had the same local anaesthetic effect on the cornea
as 0.12 and 0.27 per cent. solutions of cocaine hydrochloride.
Therefore, the local anaesthetic activity of the ester is 3-4 times
less than that of cocaine and twice that of procaine (Truhaut, 1962).
Using the same method of Regnier & Quevauviller (1939), Adler-Hradecky
and Kelentey (1960) found no local anaesthetic effect on the cornea by
0.25-0.30 per cent. solutions of methyl, ethyl, propyl or butyl
p-hydroxybenzoates.
Long-term studies
Rat. Forty rats were fed 15 mg/kg body-weight, 20 animals 150
mg/kg body-weight and 20 animals 1500 mg/kg body-weight of a mixture
of 40 per cent. ethyl ester and 60 per cent. propyl ester as the
sodium salts for 18 months in the diet. The rate of weight gain showed
some growth stimulation at the 15 and 150 mg/kg body-weight levels.
The group on 1500 mg/kg body-weight showed initial retardation
followed later by normal growth. Mortality rate and pathological
examination of the major organs in all treated groups showed no
significant difference from the control group (Heyden, 1940; Heyden,
1942).
A group of 65 rats (35 males and 30 females) was fed a diet
containing 2 per cent. of the ethyl ester for the life span, with 50
animals as the control group. All animals were autopsied on death. No
adverse effects could be detected on weight gain, except a small
retardation during the first month, and the mortality rate,
haematology, tumour incidence and histopathology of major organs did
not differ from the controls (Truhaut, 1962).
A group of 39 rats (19 males and 20 females) was injected weekly
with 1 ml of an aqueous solution of 10 per cent. sodium ethyl
p-hydroxybenzoate for their life span. The 27 controls (16 males and
11 females) were injected with 1 ml of a 3 per cent. sodium chloride
solution. Because of irritation by the high p.H of the ester solution,
the frequency of injection had to be reduced to 1 in 2 weeks from the
4th to the 10th month and later to 1 injection a month up to the end
of the experiment. No effect on mortality and tumour incidence could
be detected (Truhaut, 1962).
Comments
The long-term studies in rats are adequate for assessment, taking
into consideration the data on methyl and propyl p-hydroxybenzoates.
However, further biochemical studies in man and animals are desirable
and further studies on the local anaesthetic action should also be
undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B (1960) Arch. int. Pharmacodyn.,
128, 135
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser., 228
Heim, F. (1960-1) Sitzber. Physik-Med. Soz. Erlangen, 81, 14
Heyden,-. (1939) Unpublished Report from Applied Research Inc., MX-124
Heyden, -. (1940) Unpublished Report from Applied Research Inc.,
MX-185
Heyden, -. (1942) Unpublished Report from Applied Research Inc.,
MX-185
Regnier, M. & Quevauviller, A. (1939) Arch. exp. Path. Pharm.,
193, 48
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch.,4, 575
Schübel, K. & Manger, J. (1929) Arch. exp. Path. Pharmak., 146,
208
Sokol, H. (1952) Drug Stand., 20, 89
Truhaut, R. (1962) Estratto dai Rendiconti dell'Instituto Superiore di
Sanità (Document submitted to WHO in 1964)
Molecular weight 166.18
Definition Ethyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C9H10O3.
Description Ethyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The metabolism of the ethyl ester is similar to that of the
methyl ester. Dogs given 1000 mg/kg body-weight orally or 50 mg/kg
i.v. excreted 66-70 per cent. in the urine. Very low plasma levels,
just detectable, were found two hours after oral administration or at
5 and 15 minutes after i.v. injection (Sokol, 1952).
Human studies on six subjects involved administration of 10 or 20
mg/kg body-weight of ester orally. After 60, 135 and 255 minutes,
n-hydroxybenzoate but not the ester, was detectable in serum. The
maximum serum level attained was 4.5 µg/ml (Heim, 1960-1).
Acute Toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 2 000-2 400 Heyden, 1939
Rabbit oral 5 000 Sabalitschka & Neufeld-
Crzellitzer, 1954
Dog oral 5 000 Sabalitschka &
Neufeld-Crzellitzer, 1954
Short-term studies
Rabbit. Suspensions of 0.5 and 7.5 per cent. ethyl
p-hydroxybenzoate had the same local anaesthetic effect on the cornea
as 0.12 and 0.27 per cent. solutions of cocaine hydrochloride.
Therefore, the local anaesthetic activity of the ester is 3-4 times
less than that of cocaine and twice that of procaine (Truhaut, 1962).
Using the same method of Regnier & Quevauviller (1939), Adler-Hradecky
and Kelentey (1960) found no local anaesthetic effect on the cornea by
0.25-0.30 per cent. solutions of methyl, ethyl, propyl or butyl
p-hydroxybenzoates.
Long-term studies
Rat. Forty rats were fed 15 mg/kg body-weight, 20 animals 150
mg/kg body-weight and 20 animals 1500 mg/kg body-weight of a mixture
of 40 per cent. ethyl ester and 60 per cent. propyl ester as the
sodium salts for 18 months in the diet. The rate of weight gain showed
some growth stimulation at the 15 and 150 mg/kg body-weight levels.
The group on 1500 mg/kg body-weight showed initial retardation
followed later by normal growth. Mortality rate and pathological
examination of the major organs in all treated groups showed no
significant difference from the control group (Heyden, 1940; Heyden,
1942).
A group of 65 rats (35 males and 30 females) was fed a diet
containing 2 per cent. of the ethyl ester for the life span, with 50
animals as the control group. All animals were autopsied on death. No
adverse effects could be detected on weight gain, except a small
retardation during the first month, and the mortality rate,
haematology, tumour incidence and histopathology of major organs did
not differ from the controls (Truhaut, 1962).
A group of 39 rats (19 males and 20 females) was injected weekly
with 1 ml of an aqueous solution of 10 per cent. sodium ethyl
p-hydroxybenzoate for their life span. The 27 controls (16 males and
11 females) were injected with 1 ml of a 3 per cent. sodium chloride
solution. Because of irritation by the high p.H of the ester solution,
the frequency of injection had to be reduced to 1 in 2 weeks from the
4th to the 10th month and later to 1 injection a month up to the end
of the experiment. No effect on mortality and tumour incidence could
be detected (Truhaut, 1962).
Comments
The long-term studies in rats are adequate for assessment, taking
into consideration the data on methyl and propyl p-hydroxybenzoates.
However, further biochemical studies in man and animals are desirable
and further studies on the local anaesthetic action should also be
undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B (1960) Arch. int. Pharmacodyn.,
128, 135
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser., 228
Heim, F. (1960-1) Sitzber. Physik-Med. Soz. Erlangen, 81, 14
Heyden,-. (1939) Unpublished Report from Applied Research Inc., MX-124
Heyden, -. (1940) Unpublished Report from Applied Research Inc.,
MX-185
Heyden, -. (1942) Unpublished Report from Applied Research Inc.,
MX-185
Regnier, M. & Quevauviller, A. (1939) Arch. exp. Path. Pharm.,
193, 48
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch.,4, 575
Schübel, K. & Manger, J. (1929) Arch. exp. Path. Pharmak., 146,
208
Sokol, H. (1952) Drug Stand., 20, 89
Truhaut, R. (1962) Estratto dai Rendiconti dell'Instituto Superiore di
Sanità (Document submitted to WHO in 1964)
