
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
METHYL p-HYDROXYBENZOATE
Synonyms Methyl p-oxybenzoate; Methylparaben
Chemical Name Methyl p-hydroxybenzoate; methyl ester
of p-hydrobenzoic acid
Empirical formula C8H8O3
Structural formula
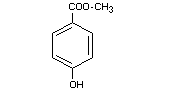 Molecular weight 152.15
Definition Methyl p-hydroxybenzoate, after dying
for 2 hours at 80, contains not less
than 99 per cent. of C8H8O3
Description Methyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in their Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The alkyl esters of p-hydroxybenzoic acid are well absorbed after
oral administration to dogs. In dogs the diglucuronide of
p-hydroxybenzoic acid has been shown to be the main metabolite
excreted in the urine. Man excreted free p-hydroxybenzoic acid and
p-hydroxyhippuric acid in approximately equal proportions (Quick,
1932). The urine of rats receiving p-hydroxybenzoic acid or its
methyl, ethyl or propyl esters contained the following metabolites:
p-hydroxybenzoic acid 40 per cent., p-hydroxyhippuric acid 23.5 per
cent., ether sulfate 5 per cent., ester glucuronides 23 per cent.
ether glucuronides 1.2 per cent. and 5 per of an unidentified
substance (Derache & Gourdon, 1963).
Dogs given 50 mg/kg body-weight intravenously or orally, excreted
85-89 per cent. in the urine within 48 hours. After intravenous
injection significant levels were found in the plasma only immediately
afterwards. When dogs were infused at the rate of 2 mg/kg/minute until
a total of 100 mg/kg body-weight was given, the levels in most organs
were below the plasma level. Appreciable amounts were only present in
liver and kidneys. No accumulation was noted in a dog given 1 mg/kg
body-weight of the methyl ester orally every day for a period of one
year. This dog excreted 96 per cent. of the daily dose within 24 hours
in the urine (Sokol, 1952).
Special studies
The blocking effect of a 0.1 per cent. solution of the methyl
ester on nervous conduction when applied directly to the spinal roots
or to the cervical vagus and sympathetics was found to be similar to
that of a 0.05 per cent. solution of procaine (Nathan & Smears, 1961).
The local anaesthetic effect of the esters rose with increasing number
of C-atoms and the toxicity decreased (Adler-Hradecky & Kelentey,
1960).
Acute toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 3 000-3 600 Heyden, 1939
Rabbit oral 6 000 Sabalitscheka & Neufeld
Crzellitzer, 1954
Dog oral 6 000 Sabalitschka & Neufeld
Crzellitzer, 1954
Short-term studies
Rat. Groups of 10 animals on a vitamin A deficient diet given
7.575 mg/kg body-weight of mixed methyl and propyl ester for 30 days
showed no additional pathological changes (Cremer, 1935).
Man. Two grams of the ester taken daily for 1 month produced
no ill effects, (Sabalitschka & Dietrich, 1924).
Long-term studies
Rat. When the methyl and propyl esters were fed to rats over
an 18-month period at a level of 150 mg/kg body-weight, no ill effects
were observed. There was some evidence of growth stimulation. When fed
at 1500 mg/kg body-weight, there was a decrease in growth rate, but no
pathological changes could be found (Sokol, 1952).
Comments
The long-term studies in rats are adequate for an assessment when
taken in conjunction with the evidence from the feeding experiment
lasting for a year with dogs. However, further biochemical studies in
man and animals are desirable and further studies on local anaesthetic
activity should also be undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int, Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt. Untersuch., 70, 136
Derache, R. & Gourdon, J. (1963) Food Cosmet. Toxicol., 1, 189
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser.,228
Heyden, (1939) Unpublished report submitted by Applied Research
Incorporated
Nathan, P. W. & Sears, T. A. (1961) Nature (Lond.), 192, 668
Quick, A. J. (1932) J. biol. Chem., 97, 403
Sabalitschka, T. & Dietrich. K. R. (1924) Pharmaz. Monatshefte,
5, 235
Sabalitachka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch, 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
Molecular weight 152.15
Definition Methyl p-hydroxybenzoate, after dying
for 2 hours at 80, contains not less
than 99 per cent. of C8H8O3
Description Methyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in their Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The alkyl esters of p-hydroxybenzoic acid are well absorbed after
oral administration to dogs. In dogs the diglucuronide of
p-hydroxybenzoic acid has been shown to be the main metabolite
excreted in the urine. Man excreted free p-hydroxybenzoic acid and
p-hydroxyhippuric acid in approximately equal proportions (Quick,
1932). The urine of rats receiving p-hydroxybenzoic acid or its
methyl, ethyl or propyl esters contained the following metabolites:
p-hydroxybenzoic acid 40 per cent., p-hydroxyhippuric acid 23.5 per
cent., ether sulfate 5 per cent., ester glucuronides 23 per cent.
ether glucuronides 1.2 per cent. and 5 per of an unidentified
substance (Derache & Gourdon, 1963).
Dogs given 50 mg/kg body-weight intravenously or orally, excreted
85-89 per cent. in the urine within 48 hours. After intravenous
injection significant levels were found in the plasma only immediately
afterwards. When dogs were infused at the rate of 2 mg/kg/minute until
a total of 100 mg/kg body-weight was given, the levels in most organs
were below the plasma level. Appreciable amounts were only present in
liver and kidneys. No accumulation was noted in a dog given 1 mg/kg
body-weight of the methyl ester orally every day for a period of one
year. This dog excreted 96 per cent. of the daily dose within 24 hours
in the urine (Sokol, 1952).
Special studies
The blocking effect of a 0.1 per cent. solution of the methyl
ester on nervous conduction when applied directly to the spinal roots
or to the cervical vagus and sympathetics was found to be similar to
that of a 0.05 per cent. solution of procaine (Nathan & Smears, 1961).
The local anaesthetic effect of the esters rose with increasing number
of C-atoms and the toxicity decreased (Adler-Hradecky & Kelentey,
1960).
Acute toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 3 000-3 600 Heyden, 1939
Rabbit oral 6 000 Sabalitscheka & Neufeld
Crzellitzer, 1954
Dog oral 6 000 Sabalitschka & Neufeld
Crzellitzer, 1954
Short-term studies
Rat. Groups of 10 animals on a vitamin A deficient diet given
7.575 mg/kg body-weight of mixed methyl and propyl ester for 30 days
showed no additional pathological changes (Cremer, 1935).
Man. Two grams of the ester taken daily for 1 month produced
no ill effects, (Sabalitschka & Dietrich, 1924).
Long-term studies
Rat. When the methyl and propyl esters were fed to rats over
an 18-month period at a level of 150 mg/kg body-weight, no ill effects
were observed. There was some evidence of growth stimulation. When fed
at 1500 mg/kg body-weight, there was a decrease in growth rate, but no
pathological changes could be found (Sokol, 1952).
Comments
The long-term studies in rats are adequate for an assessment when
taken in conjunction with the evidence from the feeding experiment
lasting for a year with dogs. However, further biochemical studies in
man and animals are desirable and further studies on local anaesthetic
activity should also be undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int, Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt. Untersuch., 70, 136
Derache, R. & Gourdon, J. (1963) Food Cosmet. Toxicol., 1, 189
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser.,228
Heyden, (1939) Unpublished report submitted by Applied Research
Incorporated
Nathan, P. W. & Sears, T. A. (1961) Nature (Lond.), 192, 668
Quick, A. J. (1932) J. biol. Chem., 97, 403
Sabalitschka, T. & Dietrich. K. R. (1924) Pharmaz. Monatshefte,
5, 235
Sabalitachka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch, 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
 Molecular weight 152.15
Definition Methyl p-hydroxybenzoate, after dying
for 2 hours at 80, contains not less
than 99 per cent. of C8H8O3
Description Methyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in their Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The alkyl esters of p-hydroxybenzoic acid are well absorbed after
oral administration to dogs. In dogs the diglucuronide of
p-hydroxybenzoic acid has been shown to be the main metabolite
excreted in the urine. Man excreted free p-hydroxybenzoic acid and
p-hydroxyhippuric acid in approximately equal proportions (Quick,
1932). The urine of rats receiving p-hydroxybenzoic acid or its
methyl, ethyl or propyl esters contained the following metabolites:
p-hydroxybenzoic acid 40 per cent., p-hydroxyhippuric acid 23.5 per
cent., ether sulfate 5 per cent., ester glucuronides 23 per cent.
ether glucuronides 1.2 per cent. and 5 per of an unidentified
substance (Derache & Gourdon, 1963).
Dogs given 50 mg/kg body-weight intravenously or orally, excreted
85-89 per cent. in the urine within 48 hours. After intravenous
injection significant levels were found in the plasma only immediately
afterwards. When dogs were infused at the rate of 2 mg/kg/minute until
a total of 100 mg/kg body-weight was given, the levels in most organs
were below the plasma level. Appreciable amounts were only present in
liver and kidneys. No accumulation was noted in a dog given 1 mg/kg
body-weight of the methyl ester orally every day for a period of one
year. This dog excreted 96 per cent. of the daily dose within 24 hours
in the urine (Sokol, 1952).
Special studies
The blocking effect of a 0.1 per cent. solution of the methyl
ester on nervous conduction when applied directly to the spinal roots
or to the cervical vagus and sympathetics was found to be similar to
that of a 0.05 per cent. solution of procaine (Nathan & Smears, 1961).
The local anaesthetic effect of the esters rose with increasing number
of C-atoms and the toxicity decreased (Adler-Hradecky & Kelentey,
1960).
Acute toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 3 000-3 600 Heyden, 1939
Rabbit oral 6 000 Sabalitscheka & Neufeld
Crzellitzer, 1954
Dog oral 6 000 Sabalitschka & Neufeld
Crzellitzer, 1954
Short-term studies
Rat. Groups of 10 animals on a vitamin A deficient diet given
7.575 mg/kg body-weight of mixed methyl and propyl ester for 30 days
showed no additional pathological changes (Cremer, 1935).
Man. Two grams of the ester taken daily for 1 month produced
no ill effects, (Sabalitschka & Dietrich, 1924).
Long-term studies
Rat. When the methyl and propyl esters were fed to rats over
an 18-month period at a level of 150 mg/kg body-weight, no ill effects
were observed. There was some evidence of growth stimulation. When fed
at 1500 mg/kg body-weight, there was a decrease in growth rate, but no
pathological changes could be found (Sokol, 1952).
Comments
The long-term studies in rats are adequate for an assessment when
taken in conjunction with the evidence from the feeding experiment
lasting for a year with dogs. However, further biochemical studies in
man and animals are desirable and further studies on local anaesthetic
activity should also be undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int, Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt. Untersuch., 70, 136
Derache, R. & Gourdon, J. (1963) Food Cosmet. Toxicol., 1, 189
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser.,228
Heyden, (1939) Unpublished report submitted by Applied Research
Incorporated
Nathan, P. W. & Sears, T. A. (1961) Nature (Lond.), 192, 668
Quick, A. J. (1932) J. biol. Chem., 97, 403
Sabalitschka, T. & Dietrich. K. R. (1924) Pharmaz. Monatshefte,
5, 235
Sabalitachka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch, 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
Molecular weight 152.15
Definition Methyl p-hydroxybenzoate, after dying
for 2 hours at 80, contains not less
than 99 per cent. of C8H8O3
Description Methyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in their Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
The alkyl esters of p-hydroxybenzoic acid are well absorbed after
oral administration to dogs. In dogs the diglucuronide of
p-hydroxybenzoic acid has been shown to be the main metabolite
excreted in the urine. Man excreted free p-hydroxybenzoic acid and
p-hydroxyhippuric acid in approximately equal proportions (Quick,
1932). The urine of rats receiving p-hydroxybenzoic acid or its
methyl, ethyl or propyl esters contained the following metabolites:
p-hydroxybenzoic acid 40 per cent., p-hydroxyhippuric acid 23.5 per
cent., ether sulfate 5 per cent., ester glucuronides 23 per cent.
ether glucuronides 1.2 per cent. and 5 per of an unidentified
substance (Derache & Gourdon, 1963).
Dogs given 50 mg/kg body-weight intravenously or orally, excreted
85-89 per cent. in the urine within 48 hours. After intravenous
injection significant levels were found in the plasma only immediately
afterwards. When dogs were infused at the rate of 2 mg/kg/minute until
a total of 100 mg/kg body-weight was given, the levels in most organs
were below the plasma level. Appreciable amounts were only present in
liver and kidneys. No accumulation was noted in a dog given 1 mg/kg
body-weight of the methyl ester orally every day for a period of one
year. This dog excreted 96 per cent. of the daily dose within 24 hours
in the urine (Sokol, 1952).
Special studies
The blocking effect of a 0.1 per cent. solution of the methyl
ester on nervous conduction when applied directly to the spinal roots
or to the cervical vagus and sympathetics was found to be similar to
that of a 0.05 per cent. solution of procaine (Nathan & Smears, 1961).
The local anaesthetic effect of the esters rose with increasing number
of C-atoms and the toxicity decreased (Adler-Hradecky & Kelentey,
1960).
Acute toxicity
Animal Route LD 50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Guinea-pig oral 3 000-3 600 Heyden, 1939
Rabbit oral 6 000 Sabalitscheka & Neufeld
Crzellitzer, 1954
Dog oral 6 000 Sabalitschka & Neufeld
Crzellitzer, 1954
Short-term studies
Rat. Groups of 10 animals on a vitamin A deficient diet given
7.575 mg/kg body-weight of mixed methyl and propyl ester for 30 days
showed no additional pathological changes (Cremer, 1935).
Man. Two grams of the ester taken daily for 1 month produced
no ill effects, (Sabalitschka & Dietrich, 1924).
Long-term studies
Rat. When the methyl and propyl esters were fed to rats over
an 18-month period at a level of 150 mg/kg body-weight, no ill effects
were observed. There was some evidence of growth stimulation. When fed
at 1500 mg/kg body-weight, there was a decrease in growth rate, but no
pathological changes could be found (Sokol, 1952).
Comments
The long-term studies in rats are adequate for an assessment when
taken in conjunction with the evidence from the feeding experiment
lasting for a year with dogs. However, further biochemical studies in
man and animals are desirable and further studies on local anaesthetic
activity should also be undertaken.
Evaluation
(See propyl p-hydroxybenzoate)
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int, Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt. Untersuch., 70, 136
Derache, R. & Gourdon, J. (1963) Food Cosmet. Toxicol., 1, 189
FAO/WHO (1962) FAO Nutrition Meetings Report Series, No. 31;
Wld Hlth Org. techn. Rep. Ser.,228
Heyden, (1939) Unpublished report submitted by Applied Research
Incorporated
Nathan, P. W. & Sears, T. A. (1961) Nature (Lond.), 192, 668
Quick, A. J. (1932) J. biol. Chem., 97, 403
Sabalitschka, T. & Dietrich. K. R. (1924) Pharmaz. Monatshefte,
5, 235
Sabalitachka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimitt.-
Forsch, 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
