
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
PROPYL p-HYDROXYBENZOATE
Synonyms Propylparaben
Chemical name Propyl p-hydroxybenzoate; n-propyl ester
of p-hydroxybenzoic acid
Empirical formula C10H12O3
Structural formula
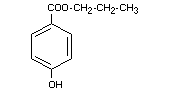 Molecular weight 180.21
Definition Propyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C10H12O3.
Description Propyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
Dogs fed 1000 mg/kg body-weight excreted 58 per cent. in the
urine and dogs given 50 mg/kg body-weight i.v. excreted 94 per cent.
The ester was detectable in the plasma only soon after administration
(Sokol, 1952; Jones et al., 1956).
Two grams propyl ester was given daily to human volunteers for 50
days. No unhydrolyzed ester could be detected in the urine
(Sabalitschka & Neufeld-Crzellitzer, 1954).
At high doses, propyl p-hydroxybenzoate was found to be a useful
anaesthetic for frogs and tadpoles (Kopsch, 1949).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Mouse (free ester) 400 Sokol, 1952
Short-term studies
Rat. Feeding experiments with n mixture of 40 per cent. ethyl
ester and 60 per cent. propyl ester were reported under ethyl
p-hydroxybenzoate (Heyden, 1939; Heyden, 1940). Rats ware fed on a
vitamin A deficient diet with a mixture of propyl ester and methyl
ester added at various concentrations. No additional pathological
effects were noted (Cremer, 1935).
Guinea-pig. Mixed propyl and methyl esters were fed to
animals on scorbutic diets. No additional pathological effects were noted
(Cremer, 1935). For details see the methyl ester.
Dog. Dogs were fed 0.7 g/kg body-weight for 90 days without
ill effects or macroscopic changes (Ghirardi, 1940).
Rabbit. 0.25-0.30 per cent. solutions had no local
anaesthetic effect on the cornea (Adler-Hradecky & Kelentey, 1960).
Man. The propyl ester has been used therapeutically at doses
of 800 mg/kg body-weight over 3 days for the treatment of moniliasis
(Rossier & Wegmann, 1953).
Long-term studies
Rat. Experiments were conducted with a mixture consisting of
40 per cent. ethyl ester and 60 per cent. propyl ester. For details and
results see ethyl p-hydroxybenzoate. It was concluded that the
mixture, when fed at a level of 1500 mg/kg body-weight, did not
produce any pathological changes, with the exception of a significant
decrease in growth rate from the 4th to the 8th month. No influence in
weight gain was seen at the 150 mg/kg body-weight level (Heyden,
1942).
Comments
The long-term studies in rats are adequate for an assessment.
However, further biochemical studies in man and animals are desirable
and further studies on local anaesthetic activity should also be
undertaken.
Evaluation
Applicable to the methyl, ethyl and propyl esters.
Level causing no toxicological effect
Rat. 20 000 ppm in the diet, equivalent to 1000 mg/kg
body-weight/day
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-2
Conditional acceptance 2-7
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int. Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt-Untersuch., 70, 136
Ghirardi, G, E. (1940) Arch, ital. Sci. farmcol., 9., 282
Heyden, -. (1939) Unpublished report from Applied Research
Incorporated
Heyden, -. (1940) Unpublished report from Applied Research
Incorporated
Heyden, -. (1942) Unpublished report from Applied Research
Incorporated
1 Sum of ethyl, methyl and propyl esters of p-hydroxybenzoic acid.
Jones, P. S., Thigpen, D., Morrison, J. L. & Richardson, A. P. (1956)
J. Amer. pharm. Ass,. sci. Ed., 45, 270
Kopsch, P. (1949) Anat. Anz.,97, 158
Rossier P. H. & Wegmann, T. (1953) Wien. Med. Wschr., 19/20, 358
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimittel-
Forsch., 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
Molecular weight 180.21
Definition Propyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C10H12O3.
Description Propyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
Dogs fed 1000 mg/kg body-weight excreted 58 per cent. in the
urine and dogs given 50 mg/kg body-weight i.v. excreted 94 per cent.
The ester was detectable in the plasma only soon after administration
(Sokol, 1952; Jones et al., 1956).
Two grams propyl ester was given daily to human volunteers for 50
days. No unhydrolyzed ester could be detected in the urine
(Sabalitschka & Neufeld-Crzellitzer, 1954).
At high doses, propyl p-hydroxybenzoate was found to be a useful
anaesthetic for frogs and tadpoles (Kopsch, 1949).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Mouse (free ester) 400 Sokol, 1952
Short-term studies
Rat. Feeding experiments with n mixture of 40 per cent. ethyl
ester and 60 per cent. propyl ester were reported under ethyl
p-hydroxybenzoate (Heyden, 1939; Heyden, 1940). Rats ware fed on a
vitamin A deficient diet with a mixture of propyl ester and methyl
ester added at various concentrations. No additional pathological
effects were noted (Cremer, 1935).
Guinea-pig. Mixed propyl and methyl esters were fed to
animals on scorbutic diets. No additional pathological effects were noted
(Cremer, 1935). For details see the methyl ester.
Dog. Dogs were fed 0.7 g/kg body-weight for 90 days without
ill effects or macroscopic changes (Ghirardi, 1940).
Rabbit. 0.25-0.30 per cent. solutions had no local
anaesthetic effect on the cornea (Adler-Hradecky & Kelentey, 1960).
Man. The propyl ester has been used therapeutically at doses
of 800 mg/kg body-weight over 3 days for the treatment of moniliasis
(Rossier & Wegmann, 1953).
Long-term studies
Rat. Experiments were conducted with a mixture consisting of
40 per cent. ethyl ester and 60 per cent. propyl ester. For details and
results see ethyl p-hydroxybenzoate. It was concluded that the
mixture, when fed at a level of 1500 mg/kg body-weight, did not
produce any pathological changes, with the exception of a significant
decrease in growth rate from the 4th to the 8th month. No influence in
weight gain was seen at the 150 mg/kg body-weight level (Heyden,
1942).
Comments
The long-term studies in rats are adequate for an assessment.
However, further biochemical studies in man and animals are desirable
and further studies on local anaesthetic activity should also be
undertaken.
Evaluation
Applicable to the methyl, ethyl and propyl esters.
Level causing no toxicological effect
Rat. 20 000 ppm in the diet, equivalent to 1000 mg/kg
body-weight/day
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-2
Conditional acceptance 2-7
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int. Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt-Untersuch., 70, 136
Ghirardi, G, E. (1940) Arch, ital. Sci. farmcol., 9., 282
Heyden, -. (1939) Unpublished report from Applied Research
Incorporated
Heyden, -. (1940) Unpublished report from Applied Research
Incorporated
Heyden, -. (1942) Unpublished report from Applied Research
Incorporated
1 Sum of ethyl, methyl and propyl esters of p-hydroxybenzoic acid.
Jones, P. S., Thigpen, D., Morrison, J. L. & Richardson, A. P. (1956)
J. Amer. pharm. Ass,. sci. Ed., 45, 270
Kopsch, P. (1949) Anat. Anz.,97, 158
Rossier P. H. & Wegmann, T. (1953) Wien. Med. Wschr., 19/20, 358
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimittel-
Forsch., 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
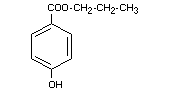 Molecular weight 180.21
Definition Propyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C10H12O3.
Description Propyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
Dogs fed 1000 mg/kg body-weight excreted 58 per cent. in the
urine and dogs given 50 mg/kg body-weight i.v. excreted 94 per cent.
The ester was detectable in the plasma only soon after administration
(Sokol, 1952; Jones et al., 1956).
Two grams propyl ester was given daily to human volunteers for 50
days. No unhydrolyzed ester could be detected in the urine
(Sabalitschka & Neufeld-Crzellitzer, 1954).
At high doses, propyl p-hydroxybenzoate was found to be a useful
anaesthetic for frogs and tadpoles (Kopsch, 1949).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Mouse (free ester) 400 Sokol, 1952
Short-term studies
Rat. Feeding experiments with n mixture of 40 per cent. ethyl
ester and 60 per cent. propyl ester were reported under ethyl
p-hydroxybenzoate (Heyden, 1939; Heyden, 1940). Rats ware fed on a
vitamin A deficient diet with a mixture of propyl ester and methyl
ester added at various concentrations. No additional pathological
effects were noted (Cremer, 1935).
Guinea-pig. Mixed propyl and methyl esters were fed to
animals on scorbutic diets. No additional pathological effects were noted
(Cremer, 1935). For details see the methyl ester.
Dog. Dogs were fed 0.7 g/kg body-weight for 90 days without
ill effects or macroscopic changes (Ghirardi, 1940).
Rabbit. 0.25-0.30 per cent. solutions had no local
anaesthetic effect on the cornea (Adler-Hradecky & Kelentey, 1960).
Man. The propyl ester has been used therapeutically at doses
of 800 mg/kg body-weight over 3 days for the treatment of moniliasis
(Rossier & Wegmann, 1953).
Long-term studies
Rat. Experiments were conducted with a mixture consisting of
40 per cent. ethyl ester and 60 per cent. propyl ester. For details and
results see ethyl p-hydroxybenzoate. It was concluded that the
mixture, when fed at a level of 1500 mg/kg body-weight, did not
produce any pathological changes, with the exception of a significant
decrease in growth rate from the 4th to the 8th month. No influence in
weight gain was seen at the 150 mg/kg body-weight level (Heyden,
1942).
Comments
The long-term studies in rats are adequate for an assessment.
However, further biochemical studies in man and animals are desirable
and further studies on local anaesthetic activity should also be
undertaken.
Evaluation
Applicable to the methyl, ethyl and propyl esters.
Level causing no toxicological effect
Rat. 20 000 ppm in the diet, equivalent to 1000 mg/kg
body-weight/day
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-2
Conditional acceptance 2-7
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int. Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt-Untersuch., 70, 136
Ghirardi, G, E. (1940) Arch, ital. Sci. farmcol., 9., 282
Heyden, -. (1939) Unpublished report from Applied Research
Incorporated
Heyden, -. (1940) Unpublished report from Applied Research
Incorporated
Heyden, -. (1942) Unpublished report from Applied Research
Incorporated
1 Sum of ethyl, methyl and propyl esters of p-hydroxybenzoic acid.
Jones, P. S., Thigpen, D., Morrison, J. L. & Richardson, A. P. (1956)
J. Amer. pharm. Ass,. sci. Ed., 45, 270
Kopsch, P. (1949) Anat. Anz.,97, 158
Rossier P. H. & Wegmann, T. (1953) Wien. Med. Wschr., 19/20, 358
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimittel-
Forsch., 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
Molecular weight 180.21
Definition Propyl p-hydroxybenzoate, after drying
for 2 hours at 80°, contains not less
than 99 per cent. of C10H12O3.
Description Propyl p-hydroxybenzoate is a white,
almost odourless, crystalline solid.
Use As an antimicrobial agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee
on Food Additives in its Sixth Report (FAO/WHO, 1962). Since its
publication some new experimental work has been carried out on these
compounds. This and other work not included in the Sixth Report is
presented and discussed in this monograph.
Biochemical aspects
Dogs fed 1000 mg/kg body-weight excreted 58 per cent. in the
urine and dogs given 50 mg/kg body-weight i.v. excreted 94 per cent.
The ester was detectable in the plasma only soon after administration
(Sokol, 1952; Jones et al., 1956).
Two grams propyl ester was given daily to human volunteers for 50
days. No unhydrolyzed ester could be detected in the urine
(Sabalitschka & Neufeld-Crzellitzer, 1954).
At high doses, propyl p-hydroxybenzoate was found to be a useful
anaesthetic for frogs and tadpoles (Kopsch, 1949).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 8 000 Sokol, 1952
Mouse (free ester) 400 Sokol, 1952
Short-term studies
Rat. Feeding experiments with n mixture of 40 per cent. ethyl
ester and 60 per cent. propyl ester were reported under ethyl
p-hydroxybenzoate (Heyden, 1939; Heyden, 1940). Rats ware fed on a
vitamin A deficient diet with a mixture of propyl ester and methyl
ester added at various concentrations. No additional pathological
effects were noted (Cremer, 1935).
Guinea-pig. Mixed propyl and methyl esters were fed to
animals on scorbutic diets. No additional pathological effects were noted
(Cremer, 1935). For details see the methyl ester.
Dog. Dogs were fed 0.7 g/kg body-weight for 90 days without
ill effects or macroscopic changes (Ghirardi, 1940).
Rabbit. 0.25-0.30 per cent. solutions had no local
anaesthetic effect on the cornea (Adler-Hradecky & Kelentey, 1960).
Man. The propyl ester has been used therapeutically at doses
of 800 mg/kg body-weight over 3 days for the treatment of moniliasis
(Rossier & Wegmann, 1953).
Long-term studies
Rat. Experiments were conducted with a mixture consisting of
40 per cent. ethyl ester and 60 per cent. propyl ester. For details and
results see ethyl p-hydroxybenzoate. It was concluded that the
mixture, when fed at a level of 1500 mg/kg body-weight, did not
produce any pathological changes, with the exception of a significant
decrease in growth rate from the 4th to the 8th month. No influence in
weight gain was seen at the 150 mg/kg body-weight level (Heyden,
1942).
Comments
The long-term studies in rats are adequate for an assessment.
However, further biochemical studies in man and animals are desirable
and further studies on local anaesthetic activity should also be
undertaken.
Evaluation
Applicable to the methyl, ethyl and propyl esters.
Level causing no toxicological effect
Rat. 20 000 ppm in the diet, equivalent to 1000 mg/kg
body-weight/day
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-2
Conditional acceptance 2-7
REFERENCES
Adler-Hradecky, C. & Kelentey, B. (1960) Arch. int. Pharmacodyn.,
128, 135
Cremer, H. (1935) Z. Lebensmitt-Untersuch., 70, 136
Ghirardi, G, E. (1940) Arch, ital. Sci. farmcol., 9., 282
Heyden, -. (1939) Unpublished report from Applied Research
Incorporated
Heyden, -. (1940) Unpublished report from Applied Research
Incorporated
Heyden, -. (1942) Unpublished report from Applied Research
Incorporated
1 Sum of ethyl, methyl and propyl esters of p-hydroxybenzoic acid.
Jones, P. S., Thigpen, D., Morrison, J. L. & Richardson, A. P. (1956)
J. Amer. pharm. Ass,. sci. Ed., 45, 270
Kopsch, P. (1949) Anat. Anz.,97, 158
Rossier P. H. & Wegmann, T. (1953) Wien. Med. Wschr., 19/20, 358
Sabalitschka, T. & Neufeld-Crzellitzer, R. (1954) Arzneimittel-
Forsch., 4, 575
Sokol, H. (1952) Drug Stand., 20, 89
