
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
CALCIUM DISODIUM ETHYLENEDIAMINETETRAACETATE
Synonyms Calcium Disodium EDTA; Calcium Disodium
Edetate
Chemical names Calcium disodium ethylenediaminetetraacetate;
Calcium disodium
(ethylenedinitrilo)tetraacetate
Empirical formula C10H12CaN2Na2O8.2H2O
Structural formula
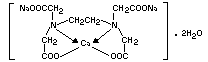 Molecular weight 410.31
Definition Calcium disodium ethylenediaminetetraacetate,
on the anhydrous basis, contains not less
than 97 per cent. and not more than the
equivalent of 102 per cent.
C10H12CaN2Na2O8.
Description Calcium disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder, slightly hygroscopic with a faint
saline taste.
Use As a sequestrant.
Biological Data
Biochemical aspects of ethylenediaminetetraacetic acid (EDTA) and
its salts
14C-labelled CaNa2EDTA, when fed to rats in doses of 50 mg/kg
body-weight, was absorbed only to an extent of 2-4 per cent.; 80-90
per cent. of the dose appeared in the faeces within 24 hours, and
absorption was still apparent at 48 hours. At the low pH of the
stomach the calcium chelate is dissociated with subsequent
precipitation of the free acid, and this is only slowly redissolved in
the intestines (Foreman et al., 1953).
Experiments in man also revealed poor absorption; only 2.5 per
cent. of a 3 g dose given was excreted in the urine (Srbova &
Teisinger, 1957). These authors also confirmed the dissociation of the
calcium chelate in the stomach. When 200 mg CaNa2EDTA was introduced
into the duodenum of rats, the authors found an absorption rate of
6.5-26 per cent. A dose of 1.5 mg of 14,C-labelled CaNa2EDTA given
in a gelatine capsule to normal healthy men was absorbed to an extent
of 5 per cent. (Foreman & Trujillo, 1954).
In feeding experiments, in rats receiving disodium EDTA at
dietary levels of 0.5, 1.0 and 5.0 per cent., the faeces contained
99.4, 98.2 and 97.5 per cent. of the excreted material (Yang, 1964;
Fellers et al., 1956)
Similar experiments conducted also in rats gave essentially the
same results. Thirty-two hours after a single dose of 95 mg disodium
EDTA/rat, 93 per cent. was recovered from the colon. After doses of
47.5, 95.0 and 142.5 mg disodium EDTA the amount of EDTA recovered in
the urine was directly proportional to the dose given, suggesting that
EDTA was absorbed from the gastrointestinal tract by passive
diffusion. The motility of the intestine was not affected by the
compound (Chan, 1964).
After parenteral administration to rats, 95-98 per cent. of
injected 14C-labelled CaNa2EDTA appeared in the urine within 6
hours. All the material passed through the body unchanged. Peak plasm
levels were found approximately 50 minutes after administration. Less
than 0.1 per cent. of the material was oxidized to 14CO2, and no
organs concentrated the substance. After i.v. injection, CaNa2EDTA
passed rapidly out of the vascular system to mix with approximately 90
per cent, of the body water, but did not pass into the red blood cells
and was cleared through the kidney by tubular excretion as well as by
glomerular filtration (Foreman et al., 1953), The same was also found
in man using 14C-labelled CaNa2EDTA. Three thousand milligrams were
given i.v. to 2 subjects and were almost entirely excreted within
12-16 hours (Srbova & Teisinger, 1957).
The maximum radioactivity in the urine after application of
14C-labelled CaNa2EDTA to the skin was only 10 ppm (Foreman &
Trujillo, 1954).
In biological systems, Ca ion will usually be most accessible to
EDTA. In general, zinc seems to be next most accessible. About 80 per
cent. of the zinc or liver is freely available to EDTA. The over-all
availability of the other physiologically important metals is probably
in the order: Cu>Fe>Mn >Co (Chenoweth, 1961). EDTA removes about
1.4 per cent. of the total iron from ferritin at pH 7.4 to form an
iron chelate (Westerfield, 1961). Transfer of Fe from Fe-transferrin
to EDTA in vitro occurs at a rate of less that 1 per cent. in 24
hours. in vivo studies in rabbits demonstrated transfer of Iron only
from FeEDTA to transferrin and not the reverse. It appeared that
tissue iron beams available to chelating agents including EDTA only
when an excess of iron was present (Cleton et al., 1963). Equal
distribution between a mixture of EDTA and siderophilin was obtained
only at EDTA : siderophilin ratios of 20-25 : 1 (Rubin, 1961). Human
iron deficiency anaemia was successfully treated with FeEDTA although
84 per cent. of labelled FeEDTA was excreted in the faeces and none
appeared in the urine. Red cells, however, contained labelled Fe and
reticulocytosis occurred. Since FeEDTA administered i.v. was almost
quantitatively excreted in the urine, it was concluded that FeEDTA was
degraded prior to absorption, when given orally (Lapinleimu &
Wegelius, 1959). Rabbits absorbed about 10 per cent. of oral FeEDTA,
and the rest was excreted in the faeces, while anaemic rats absorbed
50 per cent. of 6 mg/kg body-weight oral FeEDTA but only 25 per cent.
FeSO4 (Rubin & Princiotto, 1960). Addition of 1 per cent. Na2 EDTA
to a diet containing more than optimal amounts of iron and calcium
lowered the absorption and storage of iron in rats and increased the
amount present in plasma and urine. The metabolism calcium however,
was apparently unaffected (Larsen et al., 1960). A diet containing
0.15 mg of iron, 4.26 of calcium and 1 mg of EDTA per rat (equivalent
to 100 ppm in the diet) for 83 days had no influence on calcium and
iron metabolism, e.g. the iron content of liver and plasma (Hawkins,
et al., 1962).
CaNa2EDTA increased the excretion of zinc (Perry & Perry, 1959),
and was active in increasing the availability of zinc in
soybean-containing diets to poults (Kratzer et al., 1959). CaNa2EDTA
enhanced the excretion of Co, Hg, Mn, Ni, Pb, Tl and W (Foreman,
1961). The treatment of heavy metal poisoning with CaEDTA has become
so well established that its use for more commonly seen metal
poisonings, e.g. lead, is no longer reported in the literature
(Foreman, 1961). EDTA could not prevent the accumulation of 90Sr,
106Ru, 141Ba and 226ra in the skeleton. 91Y, 239Pu and 238U responded
fairly well to EDTA, the excretion being accelerated (Catsch, 1961).
EDTA had a lowering effect on serum cholesterol level when given
orally or intravenously. It may have acted by decreasing the capacity
of serum to transport cholesterol (Gould, 1961). Disodium EDTA had a
pyridoxin-like effect on the tryptophan metabolism of patients with
porphyria or scleroderma, due to a partial correction of imbalance of
polyvalent cations (Lelievre & Batz, 1961).
In vitro, 0.0033 M EDTA inhibited the respiration of liver
homogenates and of isolated mitochondria of liver and kidney (Lelievre
& Batz, 1961). The acetylation of sulfanilamide by a liver extract was
also inhibited (Lelievre, 1960). EDTA stimulated glucuronide synthesis
in rat liver, kidney and intestines but inhibited the process in
guinea-pig liver (Pogell & Leloir, 1961; Mittinen & Leskinen, 1962).
Of the heavy metal-containing enzymes, EDTA at a concentration of
about 10-3M inhibited aldehyde oxidase and homogentisinicase.
Succinic dehydrogenase, xanthine oxidase, NADF-cytochrome reductase,
and ceruloplasmin (oxidation of p-phenylenediamine) were not inhibited
(Westerfield, 1961). Disodium EDTA was found to be a strong inhibitor
for delta-aminolevulinic acid dehydrogenase, 5.5 × 10-6 M causing
50 per cent. inhibition (Gibson et al., 1955). The i.p. injection of
4.2 mmol/kg body-weight (equivalent to 1722 mg/kg body-weight) CaNa2
EDTA caused in rats an inhibition of the alkaline phosphatase of
liver, prostate and serum up to 4 days depending on the dose
administered; zinc restored the activity (Nigrovic, 1964).
In vitro, EDTA inhibited blood coagulation by chelating Ca2+.
The complete coagulation inhibition of human blood required 0.65-1.0
mg/ml. The i.v. injection of 79-200 mg EDTA/rabbit had no effect on
blood coagulation (Dyckerhoff et al., 1942).
I.v. injections of Na2EDTA and CaNa2EDTA had some
pharmacological effect on the blood pressure of cats; 0-20 mg/kg
body-wight CaNa2EDTA (as Ca) produce a slight rise; 20-50 mg/kg, a
biphasic response; and 50 mg/kg, a clear depression (Marquardt &
Schumacher, 1957).
One per cent. Na2EDTA enhances the absorption of 14C-labelled
acidic, neutral and basic compounds (mannitol, inulin, decamethonium
sulfanilic acid and EDTA itself) from isolated segments of rat
intestine, probably due to an increased permeability of the intestinal
wall (Schanker & Johnson, 1961).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat oral 10 000 ± 740 Oser et al., 1963
Rabbit oral 7 000 approx. Oser et al., 1963
i.p. 500 approx. Bauer et al, 1952
Dog oral 12 000 approx. Oser et al., 1964
The oral LD50 in rats is not affected by the presence of food in
the stomach or by pre-existing deficiency in Ca, Fe, Cu or Mn (Oser et
al., 1963).
Oral doses of over 250 mg/animal cause diarrhoea jr. rats
(foreman et al, 1953).
There are many reports in the literature on kidney damage by
parenteral over-dosage of CaEDTA. A review was given by Lachnit
(1961). Lesions simulating "versene nephrosis" in man have also been
produced in rats. Disodium EDTA in doses of 400-500 mg i.p. for 21
days caused severe hydropic degeneration of the proximal convoluted
tubules of the kidneys. CaNa2EDTA produced only minimal focal
hydropic changes in 58 per cent. of animals, disappearing almost 2
weeks after stopping the injections (Reuber & Schmieller, 1962).
Short-term studies
Rat. Groups of 5 male rats received 250 or 500 mg/kg
body-weight CaNa2EDTA i.p. daily for 3-21 days and some were observed
for an additional 2 weeks. Weight gain was satisfactory and histology
of lung, thymus, kidney, liver, spleen, adrenal, small gut and heart
was normal for mild to moderate renal hydropic change with focal
subcapsular swelling and proliferation in glomerular loops at the 500
mg level. There was very slight involvement with complete recovery at
the 250 mg level. Lesions were not more severe with simultaneous
cortisone administration (Reuber & Schmieller, 1962).
Groups of 3 male and 3 female rats were fed for 4 months or, low
mineral diet containing one-half the usual portion of salt
mixture(i.e. 1.25 per cent. instead of 2.50 per cent.) with the
addition of 0 per cent. and 1.5 per cent. CaNa2EDTA. The test group
showed a reduced weight gain, but there was no distinct difference in
general condition of the animals (Yang, 1964).
In another experiment, 3 groups of 8-13 male and female rats were
fed a low-mineral diet containing 0 per cent, 0.5 per cent. and 1 per
cent of CaNa2EDTA for 205 days. No significant differences from the
controls were shown regarding weight gain, mortality, gross pathology
of the organs and histopathology of liver, kidney and spleen except a
very slight dilatation of hepatic sinusoids. Blood coagulation time,
total bone ash and blood calcium level were unaffected. No significant
erosion of molars was noted. Basal metabolism was in the normal range
(Chan, 1964).
Dog. Four groups of 1 male and 3 female mongrels were fed diets
containing 0, 50, 100 and 250 mg/kg body-weight CaNa2EDTA daily for
12 months. All appeared in good health, without significant change in
blood cells, haemoglobin and urine (Ph, albumin, sugar sediment).
Blood sugar, non-protein nitrogen and prothrombin time, remained
normal. Radiographs of ribs and of long bones showed no adverse
changes at the 250 mg level. All dogs survived for 1 year. Gross and
microscopic findings were normal (Oser et al., 1963).
Long-term studies
Rat. Four groups of 25 male and 25 female rats ware fed diets
containing 0, 50, 125 and 250 mg/kg body-weight CaNa2EDTA for 2
years. Feeding was carried on through 4 successive generations. Rats
were mated after 12 weeks' feeding and allowed to lactate for 3 weeks
with 1 week's rest before producing a second litter. Ten male and 10
female rats of each group (F1 generation) and similar F2 and F3
generation groups were allowed to produce 2 litters. Of the second
litters of the F1, F2 and F3 generations only the control and the
250 mg/kg body-weight groups were kept until the end of 2 years' study
on the F0 generation. This scheme permitted terminal observation to
be made on rats receiving test diets for 0, 0.5, 1, 1.5 or 2 years in
the F3, F2, F1 and F0 generations, respectively. No significant
abnormalities in appearance and behaviour were noted during the 12
weeks of the post weaning period in all generations. The feeding
experiment showed no statistically significant differences in weight
gain, food efficiency, haemopoiesis, blood sugar, non-protein
nitrogen, serum calcium, urine, organ weights and histopathology of
liver, kidney, spleen, heart, adrenals, thyroid and gonads. Fertility,
lactation and weaning were not adversely affected for each mating.
Mortality and tumour incidence were unrelated to dosage level. The
prothrombin time was normal. There was no evidence of any chelate
effect on calcification of bone and teeth. Liver xanthine oxidase, and
blood carbonic anhydrase activities were unchanged (Oser et al.,
1963).
Comments
CaNa2EDTA is very poorly absorbed from the gut. The compound is
metabolically inert and no cumulation in the body has been found. A
vast clinical experience in its use in the treatment of metal
poisoning has demonstrated its safety in man. Long-term feeding
studies in rats and the one-year study in dogs gave no evidence of
interference with mineral metabolism in either species. Adverse
effects on mineral metabolism and nephrotoxicity were only seen after
parenteral administration of high doses.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 250 mg/kg
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg bodyweight1
Unconditional acceptance 0-1.25
Conditional acceptance 1.25-2.5
REFERENCES
Bauer, R. O., Rullo, F. R., Spooner, G. & Woodman, E. (1952) Fed.
Proc., 11, 321
Catsch, A. (1961) Fed. Proc., 20 (Suppl. 10), 206
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763-765
1 As calcium disodium salt
Chenoweth, M. B. (1961) Fed Proc., 20 (Suppl. 10), 125
Cleton F., Turnbull, A. & Finch, C. A. (1963) 42, 327
Dyckerhoff, H., Marx, R. & Ludwig, B, (1942) Z. ges. exp. Med.,
110, 412
Foreman, H. (1961) Fed. Proc., (Suppl. 10), 191
Foreman, H. & Trujillo, T. T. (1954) J. lab. clin. Med., 43, 566
Foreman, H., Vier, M. & Magee, M. (1953) J. biol. Chem., 203, 1045
Gibson, K. D., Neuberger, A. & Scott, J. C. (1955) Biochem. J.,
61, 618
Gould, R. G. (1961) Fed. Proc., 20 (Suppl. 10), 252
Hawkins, W. W., Leonhard, V. G., Maxwell, J. E. & Rastogi, K. S.
(1962) Canad. J. Biochem., 40, 391
Kratzer, F. H., Allred, J. A., Davis, P. N., Marshall, B. J. & Vohra,
P. (1959) J. Nutr., 68, 313
Lachnit, V. (1961) Arch. Gewerbepath. Gewerbehyg., 18, 495
Lapinleimu, K. & Wegelius, R. (1959) Antibiotic Med. Clin, Ther.
(Br. Edit.),6, 151
Larsen, B. A., Bidwell, R. G. S. & Hawkins, W. W. (1960) Canad. J.
Biochem., 38, 51
Lelièvre, P. (1960) C.R. Soc. Biol. (Paris), 154, 1890
Lelièvre, P. & Betz, E. H. (1961) C.R. Soc. Biol. (Paris), 155, 199
Marquardt, P. & Schumacher, H. (1957) Arzneimittelforsch., 7, 5
Miettinen, T. A. & Leskinen, E. (1962) Ans. Med. exp. Fenn., 40,
427
Nigrovic, V. (1964) Arch. exp. Pathol. Pharmacol., 249, 206
Oser, B. L., Oser, M. & Spencer, H. C. (1963) Toxincol. appl.
Pharmacol., 5, 142
Perry, H. M. & Perry, E. F. (1959) J. clin. Invest., 38, 1452
Pogell, B. M. & Leloir, L. F. (1961) J. biol. Chem., 236, 293
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Rubin, M. (1961) Fed. Proc., (Suppl. 10) 149
Rubin, M. & Princiotto, J. V. (1960) Ann. N.Y. Acad. Sci., 88, 450
Schanker, L. S. & Johnson, J. M. (1961) Biochem. Pharmacol., 421
Shibata, S. (1956) Folio pharmacol. Jan., 52, 113
Srbrova, J. & Teisinger, J. (1957) Arch. Gewerbepathol., 15, 572
Westerfeld, W. W. (1961) Fed Proc., (Suppl. 10), 158
Yang, S. S. (1964) Food Cosmet. Toxicol., 2, 763
Molecular weight 410.31
Definition Calcium disodium ethylenediaminetetraacetate,
on the anhydrous basis, contains not less
than 97 per cent. and not more than the
equivalent of 102 per cent.
C10H12CaN2Na2O8.
Description Calcium disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder, slightly hygroscopic with a faint
saline taste.
Use As a sequestrant.
Biological Data
Biochemical aspects of ethylenediaminetetraacetic acid (EDTA) and
its salts
14C-labelled CaNa2EDTA, when fed to rats in doses of 50 mg/kg
body-weight, was absorbed only to an extent of 2-4 per cent.; 80-90
per cent. of the dose appeared in the faeces within 24 hours, and
absorption was still apparent at 48 hours. At the low pH of the
stomach the calcium chelate is dissociated with subsequent
precipitation of the free acid, and this is only slowly redissolved in
the intestines (Foreman et al., 1953).
Experiments in man also revealed poor absorption; only 2.5 per
cent. of a 3 g dose given was excreted in the urine (Srbova &
Teisinger, 1957). These authors also confirmed the dissociation of the
calcium chelate in the stomach. When 200 mg CaNa2EDTA was introduced
into the duodenum of rats, the authors found an absorption rate of
6.5-26 per cent. A dose of 1.5 mg of 14,C-labelled CaNa2EDTA given
in a gelatine capsule to normal healthy men was absorbed to an extent
of 5 per cent. (Foreman & Trujillo, 1954).
In feeding experiments, in rats receiving disodium EDTA at
dietary levels of 0.5, 1.0 and 5.0 per cent., the faeces contained
99.4, 98.2 and 97.5 per cent. of the excreted material (Yang, 1964;
Fellers et al., 1956)
Similar experiments conducted also in rats gave essentially the
same results. Thirty-two hours after a single dose of 95 mg disodium
EDTA/rat, 93 per cent. was recovered from the colon. After doses of
47.5, 95.0 and 142.5 mg disodium EDTA the amount of EDTA recovered in
the urine was directly proportional to the dose given, suggesting that
EDTA was absorbed from the gastrointestinal tract by passive
diffusion. The motility of the intestine was not affected by the
compound (Chan, 1964).
After parenteral administration to rats, 95-98 per cent. of
injected 14C-labelled CaNa2EDTA appeared in the urine within 6
hours. All the material passed through the body unchanged. Peak plasm
levels were found approximately 50 minutes after administration. Less
than 0.1 per cent. of the material was oxidized to 14CO2, and no
organs concentrated the substance. After i.v. injection, CaNa2EDTA
passed rapidly out of the vascular system to mix with approximately 90
per cent, of the body water, but did not pass into the red blood cells
and was cleared through the kidney by tubular excretion as well as by
glomerular filtration (Foreman et al., 1953), The same was also found
in man using 14C-labelled CaNa2EDTA. Three thousand milligrams were
given i.v. to 2 subjects and were almost entirely excreted within
12-16 hours (Srbova & Teisinger, 1957).
The maximum radioactivity in the urine after application of
14C-labelled CaNa2EDTA to the skin was only 10 ppm (Foreman &
Trujillo, 1954).
In biological systems, Ca ion will usually be most accessible to
EDTA. In general, zinc seems to be next most accessible. About 80 per
cent. of the zinc or liver is freely available to EDTA. The over-all
availability of the other physiologically important metals is probably
in the order: Cu>Fe>Mn >Co (Chenoweth, 1961). EDTA removes about
1.4 per cent. of the total iron from ferritin at pH 7.4 to form an
iron chelate (Westerfield, 1961). Transfer of Fe from Fe-transferrin
to EDTA in vitro occurs at a rate of less that 1 per cent. in 24
hours. in vivo studies in rabbits demonstrated transfer of Iron only
from FeEDTA to transferrin and not the reverse. It appeared that
tissue iron beams available to chelating agents including EDTA only
when an excess of iron was present (Cleton et al., 1963). Equal
distribution between a mixture of EDTA and siderophilin was obtained
only at EDTA : siderophilin ratios of 20-25 : 1 (Rubin, 1961). Human
iron deficiency anaemia was successfully treated with FeEDTA although
84 per cent. of labelled FeEDTA was excreted in the faeces and none
appeared in the urine. Red cells, however, contained labelled Fe and
reticulocytosis occurred. Since FeEDTA administered i.v. was almost
quantitatively excreted in the urine, it was concluded that FeEDTA was
degraded prior to absorption, when given orally (Lapinleimu &
Wegelius, 1959). Rabbits absorbed about 10 per cent. of oral FeEDTA,
and the rest was excreted in the faeces, while anaemic rats absorbed
50 per cent. of 6 mg/kg body-weight oral FeEDTA but only 25 per cent.
FeSO4 (Rubin & Princiotto, 1960). Addition of 1 per cent. Na2 EDTA
to a diet containing more than optimal amounts of iron and calcium
lowered the absorption and storage of iron in rats and increased the
amount present in plasma and urine. The metabolism calcium however,
was apparently unaffected (Larsen et al., 1960). A diet containing
0.15 mg of iron, 4.26 of calcium and 1 mg of EDTA per rat (equivalent
to 100 ppm in the diet) for 83 days had no influence on calcium and
iron metabolism, e.g. the iron content of liver and plasma (Hawkins,
et al., 1962).
CaNa2EDTA increased the excretion of zinc (Perry & Perry, 1959),
and was active in increasing the availability of zinc in
soybean-containing diets to poults (Kratzer et al., 1959). CaNa2EDTA
enhanced the excretion of Co, Hg, Mn, Ni, Pb, Tl and W (Foreman,
1961). The treatment of heavy metal poisoning with CaEDTA has become
so well established that its use for more commonly seen metal
poisonings, e.g. lead, is no longer reported in the literature
(Foreman, 1961). EDTA could not prevent the accumulation of 90Sr,
106Ru, 141Ba and 226ra in the skeleton. 91Y, 239Pu and 238U responded
fairly well to EDTA, the excretion being accelerated (Catsch, 1961).
EDTA had a lowering effect on serum cholesterol level when given
orally or intravenously. It may have acted by decreasing the capacity
of serum to transport cholesterol (Gould, 1961). Disodium EDTA had a
pyridoxin-like effect on the tryptophan metabolism of patients with
porphyria or scleroderma, due to a partial correction of imbalance of
polyvalent cations (Lelievre & Batz, 1961).
In vitro, 0.0033 M EDTA inhibited the respiration of liver
homogenates and of isolated mitochondria of liver and kidney (Lelievre
& Batz, 1961). The acetylation of sulfanilamide by a liver extract was
also inhibited (Lelievre, 1960). EDTA stimulated glucuronide synthesis
in rat liver, kidney and intestines but inhibited the process in
guinea-pig liver (Pogell & Leloir, 1961; Mittinen & Leskinen, 1962).
Of the heavy metal-containing enzymes, EDTA at a concentration of
about 10-3M inhibited aldehyde oxidase and homogentisinicase.
Succinic dehydrogenase, xanthine oxidase, NADF-cytochrome reductase,
and ceruloplasmin (oxidation of p-phenylenediamine) were not inhibited
(Westerfield, 1961). Disodium EDTA was found to be a strong inhibitor
for delta-aminolevulinic acid dehydrogenase, 5.5 × 10-6 M causing
50 per cent. inhibition (Gibson et al., 1955). The i.p. injection of
4.2 mmol/kg body-weight (equivalent to 1722 mg/kg body-weight) CaNa2
EDTA caused in rats an inhibition of the alkaline phosphatase of
liver, prostate and serum up to 4 days depending on the dose
administered; zinc restored the activity (Nigrovic, 1964).
In vitro, EDTA inhibited blood coagulation by chelating Ca2+.
The complete coagulation inhibition of human blood required 0.65-1.0
mg/ml. The i.v. injection of 79-200 mg EDTA/rabbit had no effect on
blood coagulation (Dyckerhoff et al., 1942).
I.v. injections of Na2EDTA and CaNa2EDTA had some
pharmacological effect on the blood pressure of cats; 0-20 mg/kg
body-wight CaNa2EDTA (as Ca) produce a slight rise; 20-50 mg/kg, a
biphasic response; and 50 mg/kg, a clear depression (Marquardt &
Schumacher, 1957).
One per cent. Na2EDTA enhances the absorption of 14C-labelled
acidic, neutral and basic compounds (mannitol, inulin, decamethonium
sulfanilic acid and EDTA itself) from isolated segments of rat
intestine, probably due to an increased permeability of the intestinal
wall (Schanker & Johnson, 1961).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat oral 10 000 ± 740 Oser et al., 1963
Rabbit oral 7 000 approx. Oser et al., 1963
i.p. 500 approx. Bauer et al, 1952
Dog oral 12 000 approx. Oser et al., 1964
The oral LD50 in rats is not affected by the presence of food in
the stomach or by pre-existing deficiency in Ca, Fe, Cu or Mn (Oser et
al., 1963).
Oral doses of over 250 mg/animal cause diarrhoea jr. rats
(foreman et al, 1953).
There are many reports in the literature on kidney damage by
parenteral over-dosage of CaEDTA. A review was given by Lachnit
(1961). Lesions simulating "versene nephrosis" in man have also been
produced in rats. Disodium EDTA in doses of 400-500 mg i.p. for 21
days caused severe hydropic degeneration of the proximal convoluted
tubules of the kidneys. CaNa2EDTA produced only minimal focal
hydropic changes in 58 per cent. of animals, disappearing almost 2
weeks after stopping the injections (Reuber & Schmieller, 1962).
Short-term studies
Rat. Groups of 5 male rats received 250 or 500 mg/kg
body-weight CaNa2EDTA i.p. daily for 3-21 days and some were observed
for an additional 2 weeks. Weight gain was satisfactory and histology
of lung, thymus, kidney, liver, spleen, adrenal, small gut and heart
was normal for mild to moderate renal hydropic change with focal
subcapsular swelling and proliferation in glomerular loops at the 500
mg level. There was very slight involvement with complete recovery at
the 250 mg level. Lesions were not more severe with simultaneous
cortisone administration (Reuber & Schmieller, 1962).
Groups of 3 male and 3 female rats were fed for 4 months or, low
mineral diet containing one-half the usual portion of salt
mixture(i.e. 1.25 per cent. instead of 2.50 per cent.) with the
addition of 0 per cent. and 1.5 per cent. CaNa2EDTA. The test group
showed a reduced weight gain, but there was no distinct difference in
general condition of the animals (Yang, 1964).
In another experiment, 3 groups of 8-13 male and female rats were
fed a low-mineral diet containing 0 per cent, 0.5 per cent. and 1 per
cent of CaNa2EDTA for 205 days. No significant differences from the
controls were shown regarding weight gain, mortality, gross pathology
of the organs and histopathology of liver, kidney and spleen except a
very slight dilatation of hepatic sinusoids. Blood coagulation time,
total bone ash and blood calcium level were unaffected. No significant
erosion of molars was noted. Basal metabolism was in the normal range
(Chan, 1964).
Dog. Four groups of 1 male and 3 female mongrels were fed diets
containing 0, 50, 100 and 250 mg/kg body-weight CaNa2EDTA daily for
12 months. All appeared in good health, without significant change in
blood cells, haemoglobin and urine (Ph, albumin, sugar sediment).
Blood sugar, non-protein nitrogen and prothrombin time, remained
normal. Radiographs of ribs and of long bones showed no adverse
changes at the 250 mg level. All dogs survived for 1 year. Gross and
microscopic findings were normal (Oser et al., 1963).
Long-term studies
Rat. Four groups of 25 male and 25 female rats ware fed diets
containing 0, 50, 125 and 250 mg/kg body-weight CaNa2EDTA for 2
years. Feeding was carried on through 4 successive generations. Rats
were mated after 12 weeks' feeding and allowed to lactate for 3 weeks
with 1 week's rest before producing a second litter. Ten male and 10
female rats of each group (F1 generation) and similar F2 and F3
generation groups were allowed to produce 2 litters. Of the second
litters of the F1, F2 and F3 generations only the control and the
250 mg/kg body-weight groups were kept until the end of 2 years' study
on the F0 generation. This scheme permitted terminal observation to
be made on rats receiving test diets for 0, 0.5, 1, 1.5 or 2 years in
the F3, F2, F1 and F0 generations, respectively. No significant
abnormalities in appearance and behaviour were noted during the 12
weeks of the post weaning period in all generations. The feeding
experiment showed no statistically significant differences in weight
gain, food efficiency, haemopoiesis, blood sugar, non-protein
nitrogen, serum calcium, urine, organ weights and histopathology of
liver, kidney, spleen, heart, adrenals, thyroid and gonads. Fertility,
lactation and weaning were not adversely affected for each mating.
Mortality and tumour incidence were unrelated to dosage level. The
prothrombin time was normal. There was no evidence of any chelate
effect on calcification of bone and teeth. Liver xanthine oxidase, and
blood carbonic anhydrase activities were unchanged (Oser et al.,
1963).
Comments
CaNa2EDTA is very poorly absorbed from the gut. The compound is
metabolically inert and no cumulation in the body has been found. A
vast clinical experience in its use in the treatment of metal
poisoning has demonstrated its safety in man. Long-term feeding
studies in rats and the one-year study in dogs gave no evidence of
interference with mineral metabolism in either species. Adverse
effects on mineral metabolism and nephrotoxicity were only seen after
parenteral administration of high doses.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 250 mg/kg
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg bodyweight1
Unconditional acceptance 0-1.25
Conditional acceptance 1.25-2.5
REFERENCES
Bauer, R. O., Rullo, F. R., Spooner, G. & Woodman, E. (1952) Fed.
Proc., 11, 321
Catsch, A. (1961) Fed. Proc., 20 (Suppl. 10), 206
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763-765
1 As calcium disodium salt
Chenoweth, M. B. (1961) Fed Proc., 20 (Suppl. 10), 125
Cleton F., Turnbull, A. & Finch, C. A. (1963) 42, 327
Dyckerhoff, H., Marx, R. & Ludwig, B, (1942) Z. ges. exp. Med.,
110, 412
Foreman, H. (1961) Fed. Proc., (Suppl. 10), 191
Foreman, H. & Trujillo, T. T. (1954) J. lab. clin. Med., 43, 566
Foreman, H., Vier, M. & Magee, M. (1953) J. biol. Chem., 203, 1045
Gibson, K. D., Neuberger, A. & Scott, J. C. (1955) Biochem. J.,
61, 618
Gould, R. G. (1961) Fed. Proc., 20 (Suppl. 10), 252
Hawkins, W. W., Leonhard, V. G., Maxwell, J. E. & Rastogi, K. S.
(1962) Canad. J. Biochem., 40, 391
Kratzer, F. H., Allred, J. A., Davis, P. N., Marshall, B. J. & Vohra,
P. (1959) J. Nutr., 68, 313
Lachnit, V. (1961) Arch. Gewerbepath. Gewerbehyg., 18, 495
Lapinleimu, K. & Wegelius, R. (1959) Antibiotic Med. Clin, Ther.
(Br. Edit.),6, 151
Larsen, B. A., Bidwell, R. G. S. & Hawkins, W. W. (1960) Canad. J.
Biochem., 38, 51
Lelièvre, P. (1960) C.R. Soc. Biol. (Paris), 154, 1890
Lelièvre, P. & Betz, E. H. (1961) C.R. Soc. Biol. (Paris), 155, 199
Marquardt, P. & Schumacher, H. (1957) Arzneimittelforsch., 7, 5
Miettinen, T. A. & Leskinen, E. (1962) Ans. Med. exp. Fenn., 40,
427
Nigrovic, V. (1964) Arch. exp. Pathol. Pharmacol., 249, 206
Oser, B. L., Oser, M. & Spencer, H. C. (1963) Toxincol. appl.
Pharmacol., 5, 142
Perry, H. M. & Perry, E. F. (1959) J. clin. Invest., 38, 1452
Pogell, B. M. & Leloir, L. F. (1961) J. biol. Chem., 236, 293
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Rubin, M. (1961) Fed. Proc., (Suppl. 10) 149
Rubin, M. & Princiotto, J. V. (1960) Ann. N.Y. Acad. Sci., 88, 450
Schanker, L. S. & Johnson, J. M. (1961) Biochem. Pharmacol., 421
Shibata, S. (1956) Folio pharmacol. Jan., 52, 113
Srbrova, J. & Teisinger, J. (1957) Arch. Gewerbepathol., 15, 572
Westerfeld, W. W. (1961) Fed Proc., (Suppl. 10), 158
Yang, S. S. (1964) Food Cosmet. Toxicol., 2, 763
 Molecular weight 410.31
Definition Calcium disodium ethylenediaminetetraacetate,
on the anhydrous basis, contains not less
than 97 per cent. and not more than the
equivalent of 102 per cent.
C10H12CaN2Na2O8.
Description Calcium disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder, slightly hygroscopic with a faint
saline taste.
Use As a sequestrant.
Biological Data
Biochemical aspects of ethylenediaminetetraacetic acid (EDTA) and
its salts
14C-labelled CaNa2EDTA, when fed to rats in doses of 50 mg/kg
body-weight, was absorbed only to an extent of 2-4 per cent.; 80-90
per cent. of the dose appeared in the faeces within 24 hours, and
absorption was still apparent at 48 hours. At the low pH of the
stomach the calcium chelate is dissociated with subsequent
precipitation of the free acid, and this is only slowly redissolved in
the intestines (Foreman et al., 1953).
Experiments in man also revealed poor absorption; only 2.5 per
cent. of a 3 g dose given was excreted in the urine (Srbova &
Teisinger, 1957). These authors also confirmed the dissociation of the
calcium chelate in the stomach. When 200 mg CaNa2EDTA was introduced
into the duodenum of rats, the authors found an absorption rate of
6.5-26 per cent. A dose of 1.5 mg of 14,C-labelled CaNa2EDTA given
in a gelatine capsule to normal healthy men was absorbed to an extent
of 5 per cent. (Foreman & Trujillo, 1954).
In feeding experiments, in rats receiving disodium EDTA at
dietary levels of 0.5, 1.0 and 5.0 per cent., the faeces contained
99.4, 98.2 and 97.5 per cent. of the excreted material (Yang, 1964;
Fellers et al., 1956)
Similar experiments conducted also in rats gave essentially the
same results. Thirty-two hours after a single dose of 95 mg disodium
EDTA/rat, 93 per cent. was recovered from the colon. After doses of
47.5, 95.0 and 142.5 mg disodium EDTA the amount of EDTA recovered in
the urine was directly proportional to the dose given, suggesting that
EDTA was absorbed from the gastrointestinal tract by passive
diffusion. The motility of the intestine was not affected by the
compound (Chan, 1964).
After parenteral administration to rats, 95-98 per cent. of
injected 14C-labelled CaNa2EDTA appeared in the urine within 6
hours. All the material passed through the body unchanged. Peak plasm
levels were found approximately 50 minutes after administration. Less
than 0.1 per cent. of the material was oxidized to 14CO2, and no
organs concentrated the substance. After i.v. injection, CaNa2EDTA
passed rapidly out of the vascular system to mix with approximately 90
per cent, of the body water, but did not pass into the red blood cells
and was cleared through the kidney by tubular excretion as well as by
glomerular filtration (Foreman et al., 1953), The same was also found
in man using 14C-labelled CaNa2EDTA. Three thousand milligrams were
given i.v. to 2 subjects and were almost entirely excreted within
12-16 hours (Srbova & Teisinger, 1957).
The maximum radioactivity in the urine after application of
14C-labelled CaNa2EDTA to the skin was only 10 ppm (Foreman &
Trujillo, 1954).
In biological systems, Ca ion will usually be most accessible to
EDTA. In general, zinc seems to be next most accessible. About 80 per
cent. of the zinc or liver is freely available to EDTA. The over-all
availability of the other physiologically important metals is probably
in the order: Cu>Fe>Mn >Co (Chenoweth, 1961). EDTA removes about
1.4 per cent. of the total iron from ferritin at pH 7.4 to form an
iron chelate (Westerfield, 1961). Transfer of Fe from Fe-transferrin
to EDTA in vitro occurs at a rate of less that 1 per cent. in 24
hours. in vivo studies in rabbits demonstrated transfer of Iron only
from FeEDTA to transferrin and not the reverse. It appeared that
tissue iron beams available to chelating agents including EDTA only
when an excess of iron was present (Cleton et al., 1963). Equal
distribution between a mixture of EDTA and siderophilin was obtained
only at EDTA : siderophilin ratios of 20-25 : 1 (Rubin, 1961). Human
iron deficiency anaemia was successfully treated with FeEDTA although
84 per cent. of labelled FeEDTA was excreted in the faeces and none
appeared in the urine. Red cells, however, contained labelled Fe and
reticulocytosis occurred. Since FeEDTA administered i.v. was almost
quantitatively excreted in the urine, it was concluded that FeEDTA was
degraded prior to absorption, when given orally (Lapinleimu &
Wegelius, 1959). Rabbits absorbed about 10 per cent. of oral FeEDTA,
and the rest was excreted in the faeces, while anaemic rats absorbed
50 per cent. of 6 mg/kg body-weight oral FeEDTA but only 25 per cent.
FeSO4 (Rubin & Princiotto, 1960). Addition of 1 per cent. Na2 EDTA
to a diet containing more than optimal amounts of iron and calcium
lowered the absorption and storage of iron in rats and increased the
amount present in plasma and urine. The metabolism calcium however,
was apparently unaffected (Larsen et al., 1960). A diet containing
0.15 mg of iron, 4.26 of calcium and 1 mg of EDTA per rat (equivalent
to 100 ppm in the diet) for 83 days had no influence on calcium and
iron metabolism, e.g. the iron content of liver and plasma (Hawkins,
et al., 1962).
CaNa2EDTA increased the excretion of zinc (Perry & Perry, 1959),
and was active in increasing the availability of zinc in
soybean-containing diets to poults (Kratzer et al., 1959). CaNa2EDTA
enhanced the excretion of Co, Hg, Mn, Ni, Pb, Tl and W (Foreman,
1961). The treatment of heavy metal poisoning with CaEDTA has become
so well established that its use for more commonly seen metal
poisonings, e.g. lead, is no longer reported in the literature
(Foreman, 1961). EDTA could not prevent the accumulation of 90Sr,
106Ru, 141Ba and 226ra in the skeleton. 91Y, 239Pu and 238U responded
fairly well to EDTA, the excretion being accelerated (Catsch, 1961).
EDTA had a lowering effect on serum cholesterol level when given
orally or intravenously. It may have acted by decreasing the capacity
of serum to transport cholesterol (Gould, 1961). Disodium EDTA had a
pyridoxin-like effect on the tryptophan metabolism of patients with
porphyria or scleroderma, due to a partial correction of imbalance of
polyvalent cations (Lelievre & Batz, 1961).
In vitro, 0.0033 M EDTA inhibited the respiration of liver
homogenates and of isolated mitochondria of liver and kidney (Lelievre
& Batz, 1961). The acetylation of sulfanilamide by a liver extract was
also inhibited (Lelievre, 1960). EDTA stimulated glucuronide synthesis
in rat liver, kidney and intestines but inhibited the process in
guinea-pig liver (Pogell & Leloir, 1961; Mittinen & Leskinen, 1962).
Of the heavy metal-containing enzymes, EDTA at a concentration of
about 10-3M inhibited aldehyde oxidase and homogentisinicase.
Succinic dehydrogenase, xanthine oxidase, NADF-cytochrome reductase,
and ceruloplasmin (oxidation of p-phenylenediamine) were not inhibited
(Westerfield, 1961). Disodium EDTA was found to be a strong inhibitor
for delta-aminolevulinic acid dehydrogenase, 5.5 × 10-6 M causing
50 per cent. inhibition (Gibson et al., 1955). The i.p. injection of
4.2 mmol/kg body-weight (equivalent to 1722 mg/kg body-weight) CaNa2
EDTA caused in rats an inhibition of the alkaline phosphatase of
liver, prostate and serum up to 4 days depending on the dose
administered; zinc restored the activity (Nigrovic, 1964).
In vitro, EDTA inhibited blood coagulation by chelating Ca2+.
The complete coagulation inhibition of human blood required 0.65-1.0
mg/ml. The i.v. injection of 79-200 mg EDTA/rabbit had no effect on
blood coagulation (Dyckerhoff et al., 1942).
I.v. injections of Na2EDTA and CaNa2EDTA had some
pharmacological effect on the blood pressure of cats; 0-20 mg/kg
body-wight CaNa2EDTA (as Ca) produce a slight rise; 20-50 mg/kg, a
biphasic response; and 50 mg/kg, a clear depression (Marquardt &
Schumacher, 1957).
One per cent. Na2EDTA enhances the absorption of 14C-labelled
acidic, neutral and basic compounds (mannitol, inulin, decamethonium
sulfanilic acid and EDTA itself) from isolated segments of rat
intestine, probably due to an increased permeability of the intestinal
wall (Schanker & Johnson, 1961).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat oral 10 000 ± 740 Oser et al., 1963
Rabbit oral 7 000 approx. Oser et al., 1963
i.p. 500 approx. Bauer et al, 1952
Dog oral 12 000 approx. Oser et al., 1964
The oral LD50 in rats is not affected by the presence of food in
the stomach or by pre-existing deficiency in Ca, Fe, Cu or Mn (Oser et
al., 1963).
Oral doses of over 250 mg/animal cause diarrhoea jr. rats
(foreman et al, 1953).
There are many reports in the literature on kidney damage by
parenteral over-dosage of CaEDTA. A review was given by Lachnit
(1961). Lesions simulating "versene nephrosis" in man have also been
produced in rats. Disodium EDTA in doses of 400-500 mg i.p. for 21
days caused severe hydropic degeneration of the proximal convoluted
tubules of the kidneys. CaNa2EDTA produced only minimal focal
hydropic changes in 58 per cent. of animals, disappearing almost 2
weeks after stopping the injections (Reuber & Schmieller, 1962).
Short-term studies
Rat. Groups of 5 male rats received 250 or 500 mg/kg
body-weight CaNa2EDTA i.p. daily for 3-21 days and some were observed
for an additional 2 weeks. Weight gain was satisfactory and histology
of lung, thymus, kidney, liver, spleen, adrenal, small gut and heart
was normal for mild to moderate renal hydropic change with focal
subcapsular swelling and proliferation in glomerular loops at the 500
mg level. There was very slight involvement with complete recovery at
the 250 mg level. Lesions were not more severe with simultaneous
cortisone administration (Reuber & Schmieller, 1962).
Groups of 3 male and 3 female rats were fed for 4 months or, low
mineral diet containing one-half the usual portion of salt
mixture(i.e. 1.25 per cent. instead of 2.50 per cent.) with the
addition of 0 per cent. and 1.5 per cent. CaNa2EDTA. The test group
showed a reduced weight gain, but there was no distinct difference in
general condition of the animals (Yang, 1964).
In another experiment, 3 groups of 8-13 male and female rats were
fed a low-mineral diet containing 0 per cent, 0.5 per cent. and 1 per
cent of CaNa2EDTA for 205 days. No significant differences from the
controls were shown regarding weight gain, mortality, gross pathology
of the organs and histopathology of liver, kidney and spleen except a
very slight dilatation of hepatic sinusoids. Blood coagulation time,
total bone ash and blood calcium level were unaffected. No significant
erosion of molars was noted. Basal metabolism was in the normal range
(Chan, 1964).
Dog. Four groups of 1 male and 3 female mongrels were fed diets
containing 0, 50, 100 and 250 mg/kg body-weight CaNa2EDTA daily for
12 months. All appeared in good health, without significant change in
blood cells, haemoglobin and urine (Ph, albumin, sugar sediment).
Blood sugar, non-protein nitrogen and prothrombin time, remained
normal. Radiographs of ribs and of long bones showed no adverse
changes at the 250 mg level. All dogs survived for 1 year. Gross and
microscopic findings were normal (Oser et al., 1963).
Long-term studies
Rat. Four groups of 25 male and 25 female rats ware fed diets
containing 0, 50, 125 and 250 mg/kg body-weight CaNa2EDTA for 2
years. Feeding was carried on through 4 successive generations. Rats
were mated after 12 weeks' feeding and allowed to lactate for 3 weeks
with 1 week's rest before producing a second litter. Ten male and 10
female rats of each group (F1 generation) and similar F2 and F3
generation groups were allowed to produce 2 litters. Of the second
litters of the F1, F2 and F3 generations only the control and the
250 mg/kg body-weight groups were kept until the end of 2 years' study
on the F0 generation. This scheme permitted terminal observation to
be made on rats receiving test diets for 0, 0.5, 1, 1.5 or 2 years in
the F3, F2, F1 and F0 generations, respectively. No significant
abnormalities in appearance and behaviour were noted during the 12
weeks of the post weaning period in all generations. The feeding
experiment showed no statistically significant differences in weight
gain, food efficiency, haemopoiesis, blood sugar, non-protein
nitrogen, serum calcium, urine, organ weights and histopathology of
liver, kidney, spleen, heart, adrenals, thyroid and gonads. Fertility,
lactation and weaning were not adversely affected for each mating.
Mortality and tumour incidence were unrelated to dosage level. The
prothrombin time was normal. There was no evidence of any chelate
effect on calcification of bone and teeth. Liver xanthine oxidase, and
blood carbonic anhydrase activities were unchanged (Oser et al.,
1963).
Comments
CaNa2EDTA is very poorly absorbed from the gut. The compound is
metabolically inert and no cumulation in the body has been found. A
vast clinical experience in its use in the treatment of metal
poisoning has demonstrated its safety in man. Long-term feeding
studies in rats and the one-year study in dogs gave no evidence of
interference with mineral metabolism in either species. Adverse
effects on mineral metabolism and nephrotoxicity were only seen after
parenteral administration of high doses.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 250 mg/kg
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg bodyweight1
Unconditional acceptance 0-1.25
Conditional acceptance 1.25-2.5
REFERENCES
Bauer, R. O., Rullo, F. R., Spooner, G. & Woodman, E. (1952) Fed.
Proc., 11, 321
Catsch, A. (1961) Fed. Proc., 20 (Suppl. 10), 206
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763-765
1 As calcium disodium salt
Chenoweth, M. B. (1961) Fed Proc., 20 (Suppl. 10), 125
Cleton F., Turnbull, A. & Finch, C. A. (1963) 42, 327
Dyckerhoff, H., Marx, R. & Ludwig, B, (1942) Z. ges. exp. Med.,
110, 412
Foreman, H. (1961) Fed. Proc., (Suppl. 10), 191
Foreman, H. & Trujillo, T. T. (1954) J. lab. clin. Med., 43, 566
Foreman, H., Vier, M. & Magee, M. (1953) J. biol. Chem., 203, 1045
Gibson, K. D., Neuberger, A. & Scott, J. C. (1955) Biochem. J.,
61, 618
Gould, R. G. (1961) Fed. Proc., 20 (Suppl. 10), 252
Hawkins, W. W., Leonhard, V. G., Maxwell, J. E. & Rastogi, K. S.
(1962) Canad. J. Biochem., 40, 391
Kratzer, F. H., Allred, J. A., Davis, P. N., Marshall, B. J. & Vohra,
P. (1959) J. Nutr., 68, 313
Lachnit, V. (1961) Arch. Gewerbepath. Gewerbehyg., 18, 495
Lapinleimu, K. & Wegelius, R. (1959) Antibiotic Med. Clin, Ther.
(Br. Edit.),6, 151
Larsen, B. A., Bidwell, R. G. S. & Hawkins, W. W. (1960) Canad. J.
Biochem., 38, 51
Lelièvre, P. (1960) C.R. Soc. Biol. (Paris), 154, 1890
Lelièvre, P. & Betz, E. H. (1961) C.R. Soc. Biol. (Paris), 155, 199
Marquardt, P. & Schumacher, H. (1957) Arzneimittelforsch., 7, 5
Miettinen, T. A. & Leskinen, E. (1962) Ans. Med. exp. Fenn., 40,
427
Nigrovic, V. (1964) Arch. exp. Pathol. Pharmacol., 249, 206
Oser, B. L., Oser, M. & Spencer, H. C. (1963) Toxincol. appl.
Pharmacol., 5, 142
Perry, H. M. & Perry, E. F. (1959) J. clin. Invest., 38, 1452
Pogell, B. M. & Leloir, L. F. (1961) J. biol. Chem., 236, 293
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Rubin, M. (1961) Fed. Proc., (Suppl. 10) 149
Rubin, M. & Princiotto, J. V. (1960) Ann. N.Y. Acad. Sci., 88, 450
Schanker, L. S. & Johnson, J. M. (1961) Biochem. Pharmacol., 421
Shibata, S. (1956) Folio pharmacol. Jan., 52, 113
Srbrova, J. & Teisinger, J. (1957) Arch. Gewerbepathol., 15, 572
Westerfeld, W. W. (1961) Fed Proc., (Suppl. 10), 158
Yang, S. S. (1964) Food Cosmet. Toxicol., 2, 763
Molecular weight 410.31
Definition Calcium disodium ethylenediaminetetraacetate,
on the anhydrous basis, contains not less
than 97 per cent. and not more than the
equivalent of 102 per cent.
C10H12CaN2Na2O8.
Description Calcium disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder, slightly hygroscopic with a faint
saline taste.
Use As a sequestrant.
Biological Data
Biochemical aspects of ethylenediaminetetraacetic acid (EDTA) and
its salts
14C-labelled CaNa2EDTA, when fed to rats in doses of 50 mg/kg
body-weight, was absorbed only to an extent of 2-4 per cent.; 80-90
per cent. of the dose appeared in the faeces within 24 hours, and
absorption was still apparent at 48 hours. At the low pH of the
stomach the calcium chelate is dissociated with subsequent
precipitation of the free acid, and this is only slowly redissolved in
the intestines (Foreman et al., 1953).
Experiments in man also revealed poor absorption; only 2.5 per
cent. of a 3 g dose given was excreted in the urine (Srbova &
Teisinger, 1957). These authors also confirmed the dissociation of the
calcium chelate in the stomach. When 200 mg CaNa2EDTA was introduced
into the duodenum of rats, the authors found an absorption rate of
6.5-26 per cent. A dose of 1.5 mg of 14,C-labelled CaNa2EDTA given
in a gelatine capsule to normal healthy men was absorbed to an extent
of 5 per cent. (Foreman & Trujillo, 1954).
In feeding experiments, in rats receiving disodium EDTA at
dietary levels of 0.5, 1.0 and 5.0 per cent., the faeces contained
99.4, 98.2 and 97.5 per cent. of the excreted material (Yang, 1964;
Fellers et al., 1956)
Similar experiments conducted also in rats gave essentially the
same results. Thirty-two hours after a single dose of 95 mg disodium
EDTA/rat, 93 per cent. was recovered from the colon. After doses of
47.5, 95.0 and 142.5 mg disodium EDTA the amount of EDTA recovered in
the urine was directly proportional to the dose given, suggesting that
EDTA was absorbed from the gastrointestinal tract by passive
diffusion. The motility of the intestine was not affected by the
compound (Chan, 1964).
After parenteral administration to rats, 95-98 per cent. of
injected 14C-labelled CaNa2EDTA appeared in the urine within 6
hours. All the material passed through the body unchanged. Peak plasm
levels were found approximately 50 minutes after administration. Less
than 0.1 per cent. of the material was oxidized to 14CO2, and no
organs concentrated the substance. After i.v. injection, CaNa2EDTA
passed rapidly out of the vascular system to mix with approximately 90
per cent, of the body water, but did not pass into the red blood cells
and was cleared through the kidney by tubular excretion as well as by
glomerular filtration (Foreman et al., 1953), The same was also found
in man using 14C-labelled CaNa2EDTA. Three thousand milligrams were
given i.v. to 2 subjects and were almost entirely excreted within
12-16 hours (Srbova & Teisinger, 1957).
The maximum radioactivity in the urine after application of
14C-labelled CaNa2EDTA to the skin was only 10 ppm (Foreman &
Trujillo, 1954).
In biological systems, Ca ion will usually be most accessible to
EDTA. In general, zinc seems to be next most accessible. About 80 per
cent. of the zinc or liver is freely available to EDTA. The over-all
availability of the other physiologically important metals is probably
in the order: Cu>Fe>Mn >Co (Chenoweth, 1961). EDTA removes about
1.4 per cent. of the total iron from ferritin at pH 7.4 to form an
iron chelate (Westerfield, 1961). Transfer of Fe from Fe-transferrin
to EDTA in vitro occurs at a rate of less that 1 per cent. in 24
hours. in vivo studies in rabbits demonstrated transfer of Iron only
from FeEDTA to transferrin and not the reverse. It appeared that
tissue iron beams available to chelating agents including EDTA only
when an excess of iron was present (Cleton et al., 1963). Equal
distribution between a mixture of EDTA and siderophilin was obtained
only at EDTA : siderophilin ratios of 20-25 : 1 (Rubin, 1961). Human
iron deficiency anaemia was successfully treated with FeEDTA although
84 per cent. of labelled FeEDTA was excreted in the faeces and none
appeared in the urine. Red cells, however, contained labelled Fe and
reticulocytosis occurred. Since FeEDTA administered i.v. was almost
quantitatively excreted in the urine, it was concluded that FeEDTA was
degraded prior to absorption, when given orally (Lapinleimu &
Wegelius, 1959). Rabbits absorbed about 10 per cent. of oral FeEDTA,
and the rest was excreted in the faeces, while anaemic rats absorbed
50 per cent. of 6 mg/kg body-weight oral FeEDTA but only 25 per cent.
FeSO4 (Rubin & Princiotto, 1960). Addition of 1 per cent. Na2 EDTA
to a diet containing more than optimal amounts of iron and calcium
lowered the absorption and storage of iron in rats and increased the
amount present in plasma and urine. The metabolism calcium however,
was apparently unaffected (Larsen et al., 1960). A diet containing
0.15 mg of iron, 4.26 of calcium and 1 mg of EDTA per rat (equivalent
to 100 ppm in the diet) for 83 days had no influence on calcium and
iron metabolism, e.g. the iron content of liver and plasma (Hawkins,
et al., 1962).
CaNa2EDTA increased the excretion of zinc (Perry & Perry, 1959),
and was active in increasing the availability of zinc in
soybean-containing diets to poults (Kratzer et al., 1959). CaNa2EDTA
enhanced the excretion of Co, Hg, Mn, Ni, Pb, Tl and W (Foreman,
1961). The treatment of heavy metal poisoning with CaEDTA has become
so well established that its use for more commonly seen metal
poisonings, e.g. lead, is no longer reported in the literature
(Foreman, 1961). EDTA could not prevent the accumulation of 90Sr,
106Ru, 141Ba and 226ra in the skeleton. 91Y, 239Pu and 238U responded
fairly well to EDTA, the excretion being accelerated (Catsch, 1961).
EDTA had a lowering effect on serum cholesterol level when given
orally or intravenously. It may have acted by decreasing the capacity
of serum to transport cholesterol (Gould, 1961). Disodium EDTA had a
pyridoxin-like effect on the tryptophan metabolism of patients with
porphyria or scleroderma, due to a partial correction of imbalance of
polyvalent cations (Lelievre & Batz, 1961).
In vitro, 0.0033 M EDTA inhibited the respiration of liver
homogenates and of isolated mitochondria of liver and kidney (Lelievre
& Batz, 1961). The acetylation of sulfanilamide by a liver extract was
also inhibited (Lelievre, 1960). EDTA stimulated glucuronide synthesis
in rat liver, kidney and intestines but inhibited the process in
guinea-pig liver (Pogell & Leloir, 1961; Mittinen & Leskinen, 1962).
Of the heavy metal-containing enzymes, EDTA at a concentration of
about 10-3M inhibited aldehyde oxidase and homogentisinicase.
Succinic dehydrogenase, xanthine oxidase, NADF-cytochrome reductase,
and ceruloplasmin (oxidation of p-phenylenediamine) were not inhibited
(Westerfield, 1961). Disodium EDTA was found to be a strong inhibitor
for delta-aminolevulinic acid dehydrogenase, 5.5 × 10-6 M causing
50 per cent. inhibition (Gibson et al., 1955). The i.p. injection of
4.2 mmol/kg body-weight (equivalent to 1722 mg/kg body-weight) CaNa2
EDTA caused in rats an inhibition of the alkaline phosphatase of
liver, prostate and serum up to 4 days depending on the dose
administered; zinc restored the activity (Nigrovic, 1964).
In vitro, EDTA inhibited blood coagulation by chelating Ca2+.
The complete coagulation inhibition of human blood required 0.65-1.0
mg/ml. The i.v. injection of 79-200 mg EDTA/rabbit had no effect on
blood coagulation (Dyckerhoff et al., 1942).
I.v. injections of Na2EDTA and CaNa2EDTA had some
pharmacological effect on the blood pressure of cats; 0-20 mg/kg
body-wight CaNa2EDTA (as Ca) produce a slight rise; 20-50 mg/kg, a
biphasic response; and 50 mg/kg, a clear depression (Marquardt &
Schumacher, 1957).
One per cent. Na2EDTA enhances the absorption of 14C-labelled
acidic, neutral and basic compounds (mannitol, inulin, decamethonium
sulfanilic acid and EDTA itself) from isolated segments of rat
intestine, probably due to an increased permeability of the intestinal
wall (Schanker & Johnson, 1961).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat oral 10 000 ± 740 Oser et al., 1963
Rabbit oral 7 000 approx. Oser et al., 1963
i.p. 500 approx. Bauer et al, 1952
Dog oral 12 000 approx. Oser et al., 1964
The oral LD50 in rats is not affected by the presence of food in
the stomach or by pre-existing deficiency in Ca, Fe, Cu or Mn (Oser et
al., 1963).
Oral doses of over 250 mg/animal cause diarrhoea jr. rats
(foreman et al, 1953).
There are many reports in the literature on kidney damage by
parenteral over-dosage of CaEDTA. A review was given by Lachnit
(1961). Lesions simulating "versene nephrosis" in man have also been
produced in rats. Disodium EDTA in doses of 400-500 mg i.p. for 21
days caused severe hydropic degeneration of the proximal convoluted
tubules of the kidneys. CaNa2EDTA produced only minimal focal
hydropic changes in 58 per cent. of animals, disappearing almost 2
weeks after stopping the injections (Reuber & Schmieller, 1962).
Short-term studies
Rat. Groups of 5 male rats received 250 or 500 mg/kg
body-weight CaNa2EDTA i.p. daily for 3-21 days and some were observed
for an additional 2 weeks. Weight gain was satisfactory and histology
of lung, thymus, kidney, liver, spleen, adrenal, small gut and heart
was normal for mild to moderate renal hydropic change with focal
subcapsular swelling and proliferation in glomerular loops at the 500
mg level. There was very slight involvement with complete recovery at
the 250 mg level. Lesions were not more severe with simultaneous
cortisone administration (Reuber & Schmieller, 1962).
Groups of 3 male and 3 female rats were fed for 4 months or, low
mineral diet containing one-half the usual portion of salt
mixture(i.e. 1.25 per cent. instead of 2.50 per cent.) with the
addition of 0 per cent. and 1.5 per cent. CaNa2EDTA. The test group
showed a reduced weight gain, but there was no distinct difference in
general condition of the animals (Yang, 1964).
In another experiment, 3 groups of 8-13 male and female rats were
fed a low-mineral diet containing 0 per cent, 0.5 per cent. and 1 per
cent of CaNa2EDTA for 205 days. No significant differences from the
controls were shown regarding weight gain, mortality, gross pathology
of the organs and histopathology of liver, kidney and spleen except a
very slight dilatation of hepatic sinusoids. Blood coagulation time,
total bone ash and blood calcium level were unaffected. No significant
erosion of molars was noted. Basal metabolism was in the normal range
(Chan, 1964).
Dog. Four groups of 1 male and 3 female mongrels were fed diets
containing 0, 50, 100 and 250 mg/kg body-weight CaNa2EDTA daily for
12 months. All appeared in good health, without significant change in
blood cells, haemoglobin and urine (Ph, albumin, sugar sediment).
Blood sugar, non-protein nitrogen and prothrombin time, remained
normal. Radiographs of ribs and of long bones showed no adverse
changes at the 250 mg level. All dogs survived for 1 year. Gross and
microscopic findings were normal (Oser et al., 1963).
Long-term studies
Rat. Four groups of 25 male and 25 female rats ware fed diets
containing 0, 50, 125 and 250 mg/kg body-weight CaNa2EDTA for 2
years. Feeding was carried on through 4 successive generations. Rats
were mated after 12 weeks' feeding and allowed to lactate for 3 weeks
with 1 week's rest before producing a second litter. Ten male and 10
female rats of each group (F1 generation) and similar F2 and F3
generation groups were allowed to produce 2 litters. Of the second
litters of the F1, F2 and F3 generations only the control and the
250 mg/kg body-weight groups were kept until the end of 2 years' study
on the F0 generation. This scheme permitted terminal observation to
be made on rats receiving test diets for 0, 0.5, 1, 1.5 or 2 years in
the F3, F2, F1 and F0 generations, respectively. No significant
abnormalities in appearance and behaviour were noted during the 12
weeks of the post weaning period in all generations. The feeding
experiment showed no statistically significant differences in weight
gain, food efficiency, haemopoiesis, blood sugar, non-protein
nitrogen, serum calcium, urine, organ weights and histopathology of
liver, kidney, spleen, heart, adrenals, thyroid and gonads. Fertility,
lactation and weaning were not adversely affected for each mating.
Mortality and tumour incidence were unrelated to dosage level. The
prothrombin time was normal. There was no evidence of any chelate
effect on calcification of bone and teeth. Liver xanthine oxidase, and
blood carbonic anhydrase activities were unchanged (Oser et al.,
1963).
Comments
CaNa2EDTA is very poorly absorbed from the gut. The compound is
metabolically inert and no cumulation in the body has been found. A
vast clinical experience in its use in the treatment of metal
poisoning has demonstrated its safety in man. Long-term feeding
studies in rats and the one-year study in dogs gave no evidence of
interference with mineral metabolism in either species. Adverse
effects on mineral metabolism and nephrotoxicity were only seen after
parenteral administration of high doses.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 250 mg/kg
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg bodyweight1
Unconditional acceptance 0-1.25
Conditional acceptance 1.25-2.5
REFERENCES
Bauer, R. O., Rullo, F. R., Spooner, G. & Woodman, E. (1952) Fed.
Proc., 11, 321
Catsch, A. (1961) Fed. Proc., 20 (Suppl. 10), 206
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763-765
1 As calcium disodium salt
Chenoweth, M. B. (1961) Fed Proc., 20 (Suppl. 10), 125
Cleton F., Turnbull, A. & Finch, C. A. (1963) 42, 327
Dyckerhoff, H., Marx, R. & Ludwig, B, (1942) Z. ges. exp. Med.,
110, 412
Foreman, H. (1961) Fed. Proc., (Suppl. 10), 191
Foreman, H. & Trujillo, T. T. (1954) J. lab. clin. Med., 43, 566
Foreman, H., Vier, M. & Magee, M. (1953) J. biol. Chem., 203, 1045
Gibson, K. D., Neuberger, A. & Scott, J. C. (1955) Biochem. J.,
61, 618
Gould, R. G. (1961) Fed. Proc., 20 (Suppl. 10), 252
Hawkins, W. W., Leonhard, V. G., Maxwell, J. E. & Rastogi, K. S.
(1962) Canad. J. Biochem., 40, 391
Kratzer, F. H., Allred, J. A., Davis, P. N., Marshall, B. J. & Vohra,
P. (1959) J. Nutr., 68, 313
Lachnit, V. (1961) Arch. Gewerbepath. Gewerbehyg., 18, 495
Lapinleimu, K. & Wegelius, R. (1959) Antibiotic Med. Clin, Ther.
(Br. Edit.),6, 151
Larsen, B. A., Bidwell, R. G. S. & Hawkins, W. W. (1960) Canad. J.
Biochem., 38, 51
Lelièvre, P. (1960) C.R. Soc. Biol. (Paris), 154, 1890
Lelièvre, P. & Betz, E. H. (1961) C.R. Soc. Biol. (Paris), 155, 199
Marquardt, P. & Schumacher, H. (1957) Arzneimittelforsch., 7, 5
Miettinen, T. A. & Leskinen, E. (1962) Ans. Med. exp. Fenn., 40,
427
Nigrovic, V. (1964) Arch. exp. Pathol. Pharmacol., 249, 206
Oser, B. L., Oser, M. & Spencer, H. C. (1963) Toxincol. appl.
Pharmacol., 5, 142
Perry, H. M. & Perry, E. F. (1959) J. clin. Invest., 38, 1452
Pogell, B. M. & Leloir, L. F. (1961) J. biol. Chem., 236, 293
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Rubin, M. (1961) Fed. Proc., (Suppl. 10) 149
Rubin, M. & Princiotto, J. V. (1960) Ann. N.Y. Acad. Sci., 88, 450
Schanker, L. S. & Johnson, J. M. (1961) Biochem. Pharmacol., 421
Shibata, S. (1956) Folio pharmacol. Jan., 52, 113
Srbrova, J. & Teisinger, J. (1957) Arch. Gewerbepathol., 15, 572
Westerfeld, W. W. (1961) Fed Proc., (Suppl. 10), 158
Yang, S. S. (1964) Food Cosmet. Toxicol., 2, 763
