
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
SORBIC ACID
Chemical names Sorbic acid;
Trans, trans-2,4-hexadienoic acid
Empirical formula C6H8O2
Structural formula
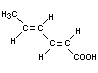 Molecular weight 112.13
Definition Sorbic acid after drying for 4 hours in
a vacuum desiccator over sulfuric acid,
contains not less than 99 per cent. of
C6H8O2.
Description Sorbic acid is a white, crystalline
solid with a mildly acrid odour.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee on
Food Additives in its Sixth and Eighth Reports (FAO/WHO, 1962;
FAO/WHO, 1965). Since their publication some new experimental work has
been carried out on this compound. This and other work not included in
the previous reports is presented in this monograph.
Biochemical aspects
The metabolism of 1-14C-sorbic acid has been studied by
Fingerhut et al. (1962); 85 per cent. of the activity was found in the
expired CO2, 0.4 per cent. in the faeces, 2 per cent. in the urine as
urea and CO2, 3 per cent. in internal organs, 3 per cent. in the
skeletal muscles and 6.6 per cent. in the other parts of the carcass.
No glycogen was formed from sorbic acid. Most of the activity was
found in the subcutaneous fat deposits and in the lipids of the
organs. There was a linear relationship between dose and oxidation
rate; the half-life, of the oxidation was 40-110 minutes in the dose
range from 60-1200 mg/kg body-weight. In a similar experiment on mice,
also using 1-14 C-sorbic acid, these results were confirmed; 81 ± 10
per cent. of the sorbic acid was oxidised to CO2, the dose given
ranging from 40-3000 mg/kg body-weight. About 7 per cent. of the
activity was excreted as sorbic acid and 0.4 per cent. as trans,
trans-muconic acid (Westöö, 1964).
Long-term studies
The feeding experiment on groups of 100 rats (50 males and 50
females) given 0 and 5 per cent. sorbic acid was extended to the whole
life-span of the first generation. The average life-span of the group
receiving sorbic acid was 811 days for the males and 789 days for the
females. In the control group the life-span of the males was 709 days
and for the females 804 days, possibly suggesting protection by sorbic
acid against lung infection. Autopsies were performed on all rats of
the first generation that died during the experiment. There were no
differences in the organ weight of the individual groups nor in the
distribution of the causes of death. In each group (5 per cent. sorbic
acid and controls) only 2 tumours were found. The animals of the
second generation were sacrificed after 250 days of feeding sorbic
acid. Examination of liver, kidney, heart and testes showed no
abnormalities (Lang, 1962; Long et al., 1967).
Evaluation
(See potassium Sorbate)
REFERENCES
FAO/WHO (1962) FAO Nutrition Meetings Report Series No 31 Wld
Hlth Org. techn. Rep. Ser., 228
FAO/WHO (1965) FAO Nutrition Meetings Report Series No. 38;
Wld Hlth Org. techn. Rep. Ser., 309
Fingerhut, M., Schmidt, B. & Lang, K.(1962) Biochem. Z., 335, 118
Lang, K. (1962) Arzneimittelforsch., 10, 997
Lang, K. et al. (1967) In preparation
Westöö, E. (1964) Acta chem. scand., 18, 1373
Molecular weight 112.13
Definition Sorbic acid after drying for 4 hours in
a vacuum desiccator over sulfuric acid,
contains not less than 99 per cent. of
C6H8O2.
Description Sorbic acid is a white, crystalline
solid with a mildly acrid odour.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee on
Food Additives in its Sixth and Eighth Reports (FAO/WHO, 1962;
FAO/WHO, 1965). Since their publication some new experimental work has
been carried out on this compound. This and other work not included in
the previous reports is presented in this monograph.
Biochemical aspects
The metabolism of 1-14C-sorbic acid has been studied by
Fingerhut et al. (1962); 85 per cent. of the activity was found in the
expired CO2, 0.4 per cent. in the faeces, 2 per cent. in the urine as
urea and CO2, 3 per cent. in internal organs, 3 per cent. in the
skeletal muscles and 6.6 per cent. in the other parts of the carcass.
No glycogen was formed from sorbic acid. Most of the activity was
found in the subcutaneous fat deposits and in the lipids of the
organs. There was a linear relationship between dose and oxidation
rate; the half-life, of the oxidation was 40-110 minutes in the dose
range from 60-1200 mg/kg body-weight. In a similar experiment on mice,
also using 1-14 C-sorbic acid, these results were confirmed; 81 ± 10
per cent. of the sorbic acid was oxidised to CO2, the dose given
ranging from 40-3000 mg/kg body-weight. About 7 per cent. of the
activity was excreted as sorbic acid and 0.4 per cent. as trans,
trans-muconic acid (Westöö, 1964).
Long-term studies
The feeding experiment on groups of 100 rats (50 males and 50
females) given 0 and 5 per cent. sorbic acid was extended to the whole
life-span of the first generation. The average life-span of the group
receiving sorbic acid was 811 days for the males and 789 days for the
females. In the control group the life-span of the males was 709 days
and for the females 804 days, possibly suggesting protection by sorbic
acid against lung infection. Autopsies were performed on all rats of
the first generation that died during the experiment. There were no
differences in the organ weight of the individual groups nor in the
distribution of the causes of death. In each group (5 per cent. sorbic
acid and controls) only 2 tumours were found. The animals of the
second generation were sacrificed after 250 days of feeding sorbic
acid. Examination of liver, kidney, heart and testes showed no
abnormalities (Lang, 1962; Long et al., 1967).
Evaluation
(See potassium Sorbate)
REFERENCES
FAO/WHO (1962) FAO Nutrition Meetings Report Series No 31 Wld
Hlth Org. techn. Rep. Ser., 228
FAO/WHO (1965) FAO Nutrition Meetings Report Series No. 38;
Wld Hlth Org. techn. Rep. Ser., 309
Fingerhut, M., Schmidt, B. & Lang, K.(1962) Biochem. Z., 335, 118
Lang, K. (1962) Arzneimittelforsch., 10, 997
Lang, K. et al. (1967) In preparation
Westöö, E. (1964) Acta chem. scand., 18, 1373
 Molecular weight 112.13
Definition Sorbic acid after drying for 4 hours in
a vacuum desiccator over sulfuric acid,
contains not less than 99 per cent. of
C6H8O2.
Description Sorbic acid is a white, crystalline
solid with a mildly acrid odour.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee on
Food Additives in its Sixth and Eighth Reports (FAO/WHO, 1962;
FAO/WHO, 1965). Since their publication some new experimental work has
been carried out on this compound. This and other work not included in
the previous reports is presented in this monograph.
Biochemical aspects
The metabolism of 1-14C-sorbic acid has been studied by
Fingerhut et al. (1962); 85 per cent. of the activity was found in the
expired CO2, 0.4 per cent. in the faeces, 2 per cent. in the urine as
urea and CO2, 3 per cent. in internal organs, 3 per cent. in the
skeletal muscles and 6.6 per cent. in the other parts of the carcass.
No glycogen was formed from sorbic acid. Most of the activity was
found in the subcutaneous fat deposits and in the lipids of the
organs. There was a linear relationship between dose and oxidation
rate; the half-life, of the oxidation was 40-110 minutes in the dose
range from 60-1200 mg/kg body-weight. In a similar experiment on mice,
also using 1-14 C-sorbic acid, these results were confirmed; 81 ± 10
per cent. of the sorbic acid was oxidised to CO2, the dose given
ranging from 40-3000 mg/kg body-weight. About 7 per cent. of the
activity was excreted as sorbic acid and 0.4 per cent. as trans,
trans-muconic acid (Westöö, 1964).
Long-term studies
The feeding experiment on groups of 100 rats (50 males and 50
females) given 0 and 5 per cent. sorbic acid was extended to the whole
life-span of the first generation. The average life-span of the group
receiving sorbic acid was 811 days for the males and 789 days for the
females. In the control group the life-span of the males was 709 days
and for the females 804 days, possibly suggesting protection by sorbic
acid against lung infection. Autopsies were performed on all rats of
the first generation that died during the experiment. There were no
differences in the organ weight of the individual groups nor in the
distribution of the causes of death. In each group (5 per cent. sorbic
acid and controls) only 2 tumours were found. The animals of the
second generation were sacrificed after 250 days of feeding sorbic
acid. Examination of liver, kidney, heart and testes showed no
abnormalities (Lang, 1962; Long et al., 1967).
Evaluation
(See potassium Sorbate)
REFERENCES
FAO/WHO (1962) FAO Nutrition Meetings Report Series No 31 Wld
Hlth Org. techn. Rep. Ser., 228
FAO/WHO (1965) FAO Nutrition Meetings Report Series No. 38;
Wld Hlth Org. techn. Rep. Ser., 309
Fingerhut, M., Schmidt, B. & Lang, K.(1962) Biochem. Z., 335, 118
Lang, K. (1962) Arzneimittelforsch., 10, 997
Lang, K. et al. (1967) In preparation
Westöö, E. (1964) Acta chem. scand., 18, 1373
Molecular weight 112.13
Definition Sorbic acid after drying for 4 hours in
a vacuum desiccator over sulfuric acid,
contains not less than 99 per cent. of
C6H8O2.
Description Sorbic acid is a white, crystalline
solid with a mildly acrid odour.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
This additive was evaluated by the Joint FAO/WHO Expert Committee on
Food Additives in its Sixth and Eighth Reports (FAO/WHO, 1962;
FAO/WHO, 1965). Since their publication some new experimental work has
been carried out on this compound. This and other work not included in
the previous reports is presented in this monograph.
Biochemical aspects
The metabolism of 1-14C-sorbic acid has been studied by
Fingerhut et al. (1962); 85 per cent. of the activity was found in the
expired CO2, 0.4 per cent. in the faeces, 2 per cent. in the urine as
urea and CO2, 3 per cent. in internal organs, 3 per cent. in the
skeletal muscles and 6.6 per cent. in the other parts of the carcass.
No glycogen was formed from sorbic acid. Most of the activity was
found in the subcutaneous fat deposits and in the lipids of the
organs. There was a linear relationship between dose and oxidation
rate; the half-life, of the oxidation was 40-110 minutes in the dose
range from 60-1200 mg/kg body-weight. In a similar experiment on mice,
also using 1-14 C-sorbic acid, these results were confirmed; 81 ± 10
per cent. of the sorbic acid was oxidised to CO2, the dose given
ranging from 40-3000 mg/kg body-weight. About 7 per cent. of the
activity was excreted as sorbic acid and 0.4 per cent. as trans,
trans-muconic acid (Westöö, 1964).
Long-term studies
The feeding experiment on groups of 100 rats (50 males and 50
females) given 0 and 5 per cent. sorbic acid was extended to the whole
life-span of the first generation. The average life-span of the group
receiving sorbic acid was 811 days for the males and 789 days for the
females. In the control group the life-span of the males was 709 days
and for the females 804 days, possibly suggesting protection by sorbic
acid against lung infection. Autopsies were performed on all rats of
the first generation that died during the experiment. There were no
differences in the organ weight of the individual groups nor in the
distribution of the causes of death. In each group (5 per cent. sorbic
acid and controls) only 2 tumours were found. The animals of the
second generation were sacrificed after 250 days of feeding sorbic
acid. Examination of liver, kidney, heart and testes showed no
abnormalities (Lang, 1962; Long et al., 1967).
Evaluation
(See potassium Sorbate)
REFERENCES
FAO/WHO (1962) FAO Nutrition Meetings Report Series No 31 Wld
Hlth Org. techn. Rep. Ser., 228
FAO/WHO (1965) FAO Nutrition Meetings Report Series No. 38;
Wld Hlth Org. techn. Rep. Ser., 309
Fingerhut, M., Schmidt, B. & Lang, K.(1962) Biochem. Z., 335, 118
Lang, K. (1962) Arzneimittelforsch., 10, 997
Lang, K. et al. (1967) In preparation
Westöö, E. (1964) Acta chem. scand., 18, 1373
