
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
CALCIUM SORBATE
Chemical name Calcium sorbate, Calcium salt of
trans,trans-2,4- hexadienoic acid
Empirical formula C12H14Cao4
Structural formula
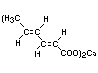 Molecular weight 262.32
Definition Calcium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of C12H14CaO4
after drying.
Description Calcium sorbate occurs as a fine white
crystalline powder.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
(See Potassium Sorbate)
POTASSIUM SORBATE
Chemical names Potassium sorbate; Potassium salt of
trans,trans-2,4- hexadienoic acid
Empirical formula C6H7O2K
Structural formula
Molecular weight 262.32
Definition Calcium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of C12H14CaO4
after drying.
Description Calcium sorbate occurs as a fine white
crystalline powder.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
(See Potassium Sorbate)
POTASSIUM SORBATE
Chemical names Potassium sorbate; Potassium salt of
trans,trans-2,4- hexadienoic acid
Empirical formula C6H7O2K
Structural formula
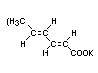 Molecular weight 150.22
Definition Potassium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of
C6H7O2K.
Description Potassium sorbate occurs as white or yellowish-
white crystals or crystalline powder.
Uses As an antimicrobial and fungistatic agent.
Biological Data
Biochemical aspects
The studies on the metabolism of 14C-labelled sorbic acid were
performed by using either sorbic acid neutralized with sodium
hydroxide (Fingerhut et al., 1962) or the potassium salt (West÷÷,
1964).
Acute toxicity
Substance Animal Route LD50 References
(mg/kg
body-weight)
Sodium sorbate rat oral 7 160 Smyth & Carpenter, 1948
Sodium sorbate rat oral 4 000 and 5 940 Deuel et al., 1954
Potassium sorbate rat oral 4 920 Mellon Institute, 1954
(solid isomer)
Potassium sorbate rat oral 6 170 Mellon Institute, 1954
(mixed isomer)
Sodium sorbate mouse i.p. 2 500 Rh˘ne-Poulenc, 1965
Potassium sorbate mouse i.p. 1 300 Rh˘ne-Poulenc, 1965
Short-term studies
Rat. Groups of 10 rats (5 male and 5 female) were fed potassium
sorbate (solid or mixed isomers) at levels of 0, 1, 2, 5 and 10 per
cent. of the diet for 3 months. Body-weight gain was initially
depressed at 10 per cent., and, to a lesser degree, in the 5 per cent.
female group. There are no figures given for the food consumption
during this time. At the end of the experiment, the weights of the
rats receiving 10 per cent. of either isomer were slightly depressed,
but the food consumption of these animals was smaller. The food
efficiency (weight gain/g food) was practically the same as in the
control group. Relative liver weights were the same in all groups.
Kidney weights were increased at the 10 per cent. level, probably due
to the high potassium load. This was also noted to a lesser degree at
the 5 per cent. level. Gross pathological examination showed no
abnormalities, even in the 10 per cent. level groups (Mellon
Institute, 1954).
Dog. Eight dogs received 1 per cent. and 8 dogs 2 per cent. of
the solid or mixed isomers of potassium sorbate in the diet for 3
months; 4 dogs were used as controls. There were no differences in
weight gain. Gross examination on autopsy showed no evidence of a
deleterious affect attributable to the sorbates (Mellon Institute,
1954).
Comments
The level of 10 per cent. and even per cent. potassium sorbate
causes a very high potassium intake. The food consumption of 15
g/rat/day results in a daily intake of 12 meq. K for the 10 per cent.
level group. The normal daily allowance for the mature rat is 1.5 meq.
K.
When considering the results of the short-term studies,
allowance, has been made for the adverse effects of high potassium
intake. Accordingly it was concluded that the same no-effect level
could be accepted for evaluation of sorbic acid and its Ca and K
salts.
It is desirable that long-term studies be carried out on animals
and metabolic investigations in man using the Ca or K salts.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/k
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg body-weight1
Unconditional acceptance 0-12.5
Conditional acceptance 12.5-25
1 As the sum of sorbic acid and Ca and K sorbates (calculated as
sorbic acid).
REFERENCES
Deuel, H. J. jr et al. (1954)Food Res., 19, 1
Fingerhut, M., Schmidt, B. & Lang, K. (1962) Biochem. Z., 366, 118
Mellon Institute (1954) Unpublished report
Rh˘ne-Poulenc (1965) Unpublished report
Smyth, H. F. jr & Carpenter, C. P. (1948) J industr. Hyg. Toxicol.,
30, 63
West÷÷, E. (1964) Acta chem. scand., 18, 1373
Molecular weight 150.22
Definition Potassium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of
C6H7O2K.
Description Potassium sorbate occurs as white or yellowish-
white crystals or crystalline powder.
Uses As an antimicrobial and fungistatic agent.
Biological Data
Biochemical aspects
The studies on the metabolism of 14C-labelled sorbic acid were
performed by using either sorbic acid neutralized with sodium
hydroxide (Fingerhut et al., 1962) or the potassium salt (West÷÷,
1964).
Acute toxicity
Substance Animal Route LD50 References
(mg/kg
body-weight)
Sodium sorbate rat oral 7 160 Smyth & Carpenter, 1948
Sodium sorbate rat oral 4 000 and 5 940 Deuel et al., 1954
Potassium sorbate rat oral 4 920 Mellon Institute, 1954
(solid isomer)
Potassium sorbate rat oral 6 170 Mellon Institute, 1954
(mixed isomer)
Sodium sorbate mouse i.p. 2 500 Rh˘ne-Poulenc, 1965
Potassium sorbate mouse i.p. 1 300 Rh˘ne-Poulenc, 1965
Short-term studies
Rat. Groups of 10 rats (5 male and 5 female) were fed potassium
sorbate (solid or mixed isomers) at levels of 0, 1, 2, 5 and 10 per
cent. of the diet for 3 months. Body-weight gain was initially
depressed at 10 per cent., and, to a lesser degree, in the 5 per cent.
female group. There are no figures given for the food consumption
during this time. At the end of the experiment, the weights of the
rats receiving 10 per cent. of either isomer were slightly depressed,
but the food consumption of these animals was smaller. The food
efficiency (weight gain/g food) was practically the same as in the
control group. Relative liver weights were the same in all groups.
Kidney weights were increased at the 10 per cent. level, probably due
to the high potassium load. This was also noted to a lesser degree at
the 5 per cent. level. Gross pathological examination showed no
abnormalities, even in the 10 per cent. level groups (Mellon
Institute, 1954).
Dog. Eight dogs received 1 per cent. and 8 dogs 2 per cent. of
the solid or mixed isomers of potassium sorbate in the diet for 3
months; 4 dogs were used as controls. There were no differences in
weight gain. Gross examination on autopsy showed no evidence of a
deleterious affect attributable to the sorbates (Mellon Institute,
1954).
Comments
The level of 10 per cent. and even per cent. potassium sorbate
causes a very high potassium intake. The food consumption of 15
g/rat/day results in a daily intake of 12 meq. K for the 10 per cent.
level group. The normal daily allowance for the mature rat is 1.5 meq.
K.
When considering the results of the short-term studies,
allowance, has been made for the adverse effects of high potassium
intake. Accordingly it was concluded that the same no-effect level
could be accepted for evaluation of sorbic acid and its Ca and K
salts.
It is desirable that long-term studies be carried out on animals
and metabolic investigations in man using the Ca or K salts.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/k
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg body-weight1
Unconditional acceptance 0-12.5
Conditional acceptance 12.5-25
1 As the sum of sorbic acid and Ca and K sorbates (calculated as
sorbic acid).
REFERENCES
Deuel, H. J. jr et al. (1954)Food Res., 19, 1
Fingerhut, M., Schmidt, B. & Lang, K. (1962) Biochem. Z., 366, 118
Mellon Institute (1954) Unpublished report
Rh˘ne-Poulenc (1965) Unpublished report
Smyth, H. F. jr & Carpenter, C. P. (1948) J industr. Hyg. Toxicol.,
30, 63
West÷÷, E. (1964) Acta chem. scand., 18, 1373
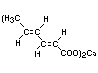 Molecular weight 262.32
Definition Calcium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of C12H14CaO4
after drying.
Description Calcium sorbate occurs as a fine white
crystalline powder.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
(See Potassium Sorbate)
POTASSIUM SORBATE
Chemical names Potassium sorbate; Potassium salt of
trans,trans-2,4- hexadienoic acid
Empirical formula C6H7O2K
Structural formula
Molecular weight 262.32
Definition Calcium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of C12H14CaO4
after drying.
Description Calcium sorbate occurs as a fine white
crystalline powder.
Uses As an antimicrobial and fungistatic
agent.
Biological Data
(See Potassium Sorbate)
POTASSIUM SORBATE
Chemical names Potassium sorbate; Potassium salt of
trans,trans-2,4- hexadienoic acid
Empirical formula C6H7O2K
Structural formula
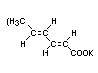 Molecular weight 150.22
Definition Potassium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of
C6H7O2K.
Description Potassium sorbate occurs as white or yellowish-
white crystals or crystalline powder.
Uses As an antimicrobial and fungistatic agent.
Biological Data
Biochemical aspects
The studies on the metabolism of 14C-labelled sorbic acid were
performed by using either sorbic acid neutralized with sodium
hydroxide (Fingerhut et al., 1962) or the potassium salt (West÷÷,
1964).
Acute toxicity
Substance Animal Route LD50 References
(mg/kg
body-weight)
Sodium sorbate rat oral 7 160 Smyth & Carpenter, 1948
Sodium sorbate rat oral 4 000 and 5 940 Deuel et al., 1954
Potassium sorbate rat oral 4 920 Mellon Institute, 1954
(solid isomer)
Potassium sorbate rat oral 6 170 Mellon Institute, 1954
(mixed isomer)
Sodium sorbate mouse i.p. 2 500 Rh˘ne-Poulenc, 1965
Potassium sorbate mouse i.p. 1 300 Rh˘ne-Poulenc, 1965
Short-term studies
Rat. Groups of 10 rats (5 male and 5 female) were fed potassium
sorbate (solid or mixed isomers) at levels of 0, 1, 2, 5 and 10 per
cent. of the diet for 3 months. Body-weight gain was initially
depressed at 10 per cent., and, to a lesser degree, in the 5 per cent.
female group. There are no figures given for the food consumption
during this time. At the end of the experiment, the weights of the
rats receiving 10 per cent. of either isomer were slightly depressed,
but the food consumption of these animals was smaller. The food
efficiency (weight gain/g food) was practically the same as in the
control group. Relative liver weights were the same in all groups.
Kidney weights were increased at the 10 per cent. level, probably due
to the high potassium load. This was also noted to a lesser degree at
the 5 per cent. level. Gross pathological examination showed no
abnormalities, even in the 10 per cent. level groups (Mellon
Institute, 1954).
Dog. Eight dogs received 1 per cent. and 8 dogs 2 per cent. of
the solid or mixed isomers of potassium sorbate in the diet for 3
months; 4 dogs were used as controls. There were no differences in
weight gain. Gross examination on autopsy showed no evidence of a
deleterious affect attributable to the sorbates (Mellon Institute,
1954).
Comments
The level of 10 per cent. and even per cent. potassium sorbate
causes a very high potassium intake. The food consumption of 15
g/rat/day results in a daily intake of 12 meq. K for the 10 per cent.
level group. The normal daily allowance for the mature rat is 1.5 meq.
K.
When considering the results of the short-term studies,
allowance, has been made for the adverse effects of high potassium
intake. Accordingly it was concluded that the same no-effect level
could be accepted for evaluation of sorbic acid and its Ca and K
salts.
It is desirable that long-term studies be carried out on animals
and metabolic investigations in man using the Ca or K salts.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/k
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg body-weight1
Unconditional acceptance 0-12.5
Conditional acceptance 12.5-25
1 As the sum of sorbic acid and Ca and K sorbates (calculated as
sorbic acid).
REFERENCES
Deuel, H. J. jr et al. (1954)Food Res., 19, 1
Fingerhut, M., Schmidt, B. & Lang, K. (1962) Biochem. Z., 366, 118
Mellon Institute (1954) Unpublished report
Rh˘ne-Poulenc (1965) Unpublished report
Smyth, H. F. jr & Carpenter, C. P. (1948) J industr. Hyg. Toxicol.,
30, 63
West÷÷, E. (1964) Acta chem. scand., 18, 1373
Molecular weight 150.22
Definition Potassium sorbate contains not less than
98 per cent. and not more than the
equivalent of 102 per cent. of
C6H7O2K.
Description Potassium sorbate occurs as white or yellowish-
white crystals or crystalline powder.
Uses As an antimicrobial and fungistatic agent.
Biological Data
Biochemical aspects
The studies on the metabolism of 14C-labelled sorbic acid were
performed by using either sorbic acid neutralized with sodium
hydroxide (Fingerhut et al., 1962) or the potassium salt (West÷÷,
1964).
Acute toxicity
Substance Animal Route LD50 References
(mg/kg
body-weight)
Sodium sorbate rat oral 7 160 Smyth & Carpenter, 1948
Sodium sorbate rat oral 4 000 and 5 940 Deuel et al., 1954
Potassium sorbate rat oral 4 920 Mellon Institute, 1954
(solid isomer)
Potassium sorbate rat oral 6 170 Mellon Institute, 1954
(mixed isomer)
Sodium sorbate mouse i.p. 2 500 Rh˘ne-Poulenc, 1965
Potassium sorbate mouse i.p. 1 300 Rh˘ne-Poulenc, 1965
Short-term studies
Rat. Groups of 10 rats (5 male and 5 female) were fed potassium
sorbate (solid or mixed isomers) at levels of 0, 1, 2, 5 and 10 per
cent. of the diet for 3 months. Body-weight gain was initially
depressed at 10 per cent., and, to a lesser degree, in the 5 per cent.
female group. There are no figures given for the food consumption
during this time. At the end of the experiment, the weights of the
rats receiving 10 per cent. of either isomer were slightly depressed,
but the food consumption of these animals was smaller. The food
efficiency (weight gain/g food) was practically the same as in the
control group. Relative liver weights were the same in all groups.
Kidney weights were increased at the 10 per cent. level, probably due
to the high potassium load. This was also noted to a lesser degree at
the 5 per cent. level. Gross pathological examination showed no
abnormalities, even in the 10 per cent. level groups (Mellon
Institute, 1954).
Dog. Eight dogs received 1 per cent. and 8 dogs 2 per cent. of
the solid or mixed isomers of potassium sorbate in the diet for 3
months; 4 dogs were used as controls. There were no differences in
weight gain. Gross examination on autopsy showed no evidence of a
deleterious affect attributable to the sorbates (Mellon Institute,
1954).
Comments
The level of 10 per cent. and even per cent. potassium sorbate
causes a very high potassium intake. The food consumption of 15
g/rat/day results in a daily intake of 12 meq. K for the 10 per cent.
level group. The normal daily allowance for the mature rat is 1.5 meq.
K.
When considering the results of the short-term studies,
allowance, has been made for the adverse effects of high potassium
intake. Accordingly it was concluded that the same no-effect level
could be accepted for evaluation of sorbic acid and its Ca and K
salts.
It is desirable that long-term studies be carried out on animals
and metabolic investigations in man using the Ca or K salts.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/k
body-weight/day.
Estimate of acceptable daily intakes for man
mg/kg body-weight1
Unconditional acceptance 0-12.5
Conditional acceptance 12.5-25
1 As the sum of sorbic acid and Ca and K sorbates (calculated as
sorbic acid).
REFERENCES
Deuel, H. J. jr et al. (1954)Food Res., 19, 1
Fingerhut, M., Schmidt, B. & Lang, K. (1962) Biochem. Z., 366, 118
Mellon Institute (1954) Unpublished report
Rh˘ne-Poulenc (1965) Unpublished report
Smyth, H. F. jr & Carpenter, C. P. (1948) J industr. Hyg. Toxicol.,
30, 63
West÷÷, E. (1964) Acta chem. scand., 18, 1373
