
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
HYDROXYPROPYL METHYLCELLULOSE
Chemical names Propylene glycol ether of
methylcellulose
Chemical formula
[C6H7O2(OH)z(OCH3) x (OCH2CHOHCH3)y]n
where x : 1.12-2.03 )
y : 0.07-0.34 ) : degrees of substitution
z : 3-(x+y)
Structural formula
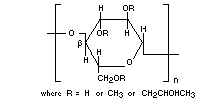 Molecular weight Unsubstituted structural unit: 162.14
Structural unit with 1.19 degree of
substitution: approx. 180
Structural unit with 2.37 degree of
substitution: approx. 210
Low polymers (n about 70): approx.
13 000
High polymers (n about 1000): approx.
208 000
Definition Hydroxypropyl methylcellulose is a
methylcellulose modified with a small
amount of propylene glycol ether groups
attached to the anhydroglucose of the
cellulose. The dry product contains 19
per cent. to 30 per cent. of methoxyl
(-OCH3) groups and 3 per cent. to 12
per cent. of hydroxypropyl
(-OCH2CHOHCH3) groups.
Description Hydroxylpropyl methylcellulose is a
white fibrous powder or as granules
(1,2).
Uses As a thickening agent or stabilizer.
Biological Data
Biochemical aspects
Twenty-five young human adults ingested doses ranging from 0.6 to
8.9 g of type B on three separate occasions. Only a mild laxative or
constipating effect was noted in several cases. About 97 per cent. of
the dose, determined as methoxy groups, was recovered from faeces
(Knight et al., 1952).
Acute toxicity of type B
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 5 000 Hodge et al., 1950
Rat i.p. 5 000 Hodge et al., 1950
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 0, 2, 10 and 25 per cent. type B for 30 days. Only in
the highest dose were interference with body-weight gain and diarrhoea
observed. There no histological lesions nor were there abnormal
findings in urine and blood (Hodge et al., 1950).
Groups of 10 and 10 female young rats were fed 0, 1, 3, 10 and 30
per cent. of type A for 121 days. Body-weight gain was markedly
retarded at the 30 per cent. level, with 50 per cent. mortality
attributed to under-nutrition. Only the male rats showed slight
body-weight gain retardation at the 10 per cent. dietary level, while
the weight-gain was normal at the lower levels. Histological
examination of internal organs revealed no abnormalities in any of the
5 groups (McCollister & Oyen, 1954).
Groups of 10 male and 10 female young rats were fed diets
containing 0, 0.3, 1, 10 and 20 per cent. of type C. for 90 days. At
the 20 per cent. level both sexes showed marked retardation of
body-weight gain, with 30 per cent. mortality. At the 10 per cent.
level male rats only showed slight but significant weight gain
retardation. At the lower levels there were no adverse effects. The
microscopic appearance of tissues was normal at all levels
(McCollister et al., 1961).
Groups of 10 male and 10 female young rats were fed 0, 0.3, 1, 3,
10 and 20 per cent. of type D for 84 days. No adverse effects were
noted with female rats at all levels. Male rats showed a definite
retardation of bodyweight gain at 20 per cent. level and a slight
retardation at 10 per cent. Organ weights and gross and microscopic
examination revealed no adverse effects (McCollister et al., 1961).
Rabbit. Groups of 6 rabbits were fed diets containing 0, 10 and
25 per cent. type B for 30 days. The group on the highest dose
maintained, but did not increase, their body-weight. Normal results
were obtained from urine and blood analyses, comparison of organ
weights and histological examination (Hodge et al., 1950).
Dog. Groups of 2 dogs were fed for one year 0.1, 0.3, 1.0 and
3.0 g/kg body-weight daily of type B without effect on body and organ
weights, urine, blood and microscopic appearance of internal organs.
One dog fed 25 g/kg body-weight daily for 30 days suffered no ill
effects. Another dog fed 50 g/kg body-weight daily for 30 days
exhibited some diarrhoea, slight weight loss and slight depression of
red blood cell count without any histological changes (Hodge et al.,
1950).
Long-term studies
Rat. Groups of 50 male and 50 female rats were fed for 2 years
on diets containing 0, 1, 5 and 20 per cent. of type B. There was a
slight retardation of body-weight gain in the male group at the
highest dose. Mortality ranged from 60-84 per cent. with no
significant difference between the groups. Tumour incidence was the
same in the experimental groups as in controls (Hodge et al., 1950).
Comments
No significant amounts appear to be absorbed from the digestive
tract in man. There are no acute oral toxicity data, and only one
long-term study has been done on one particular type. Short-term
studies have been done with four slightly different, types, but gave
consistent results.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-301
Conditional acceptance : Higher levels may be used for
dietetic or calorie control
purposes
REFERENCES
Hodge, H. C., Maynard, E. A., Wilt, W. G. jr, Blanchett, H. J. jr &
Hyatt, R. E. (1950) J. Pharmacol. exp. Ther., 99, 112
Knight, H. F., Hodge, H. C., Samsel, E. P., Delap, R. E. &
McCollister, D. D. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 427
McCollister, D. D. & Oyen, F. (1954) J. Amer, pharm. Ass., sci.
Ed.,, 43, 664
McCollister, D. D., Oyen, F. & Greminger, G. K. jr (1961) J. Pharm.
Sci., 50, 615
1 As sum of total cellulose derivatives.
Molecular weight Unsubstituted structural unit: 162.14
Structural unit with 1.19 degree of
substitution: approx. 180
Structural unit with 2.37 degree of
substitution: approx. 210
Low polymers (n about 70): approx.
13 000
High polymers (n about 1000): approx.
208 000
Definition Hydroxypropyl methylcellulose is a
methylcellulose modified with a small
amount of propylene glycol ether groups
attached to the anhydroglucose of the
cellulose. The dry product contains 19
per cent. to 30 per cent. of methoxyl
(-OCH3) groups and 3 per cent. to 12
per cent. of hydroxypropyl
(-OCH2CHOHCH3) groups.
Description Hydroxylpropyl methylcellulose is a
white fibrous powder or as granules
(1,2).
Uses As a thickening agent or stabilizer.
Biological Data
Biochemical aspects
Twenty-five young human adults ingested doses ranging from 0.6 to
8.9 g of type B on three separate occasions. Only a mild laxative or
constipating effect was noted in several cases. About 97 per cent. of
the dose, determined as methoxy groups, was recovered from faeces
(Knight et al., 1952).
Acute toxicity of type B
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 5 000 Hodge et al., 1950
Rat i.p. 5 000 Hodge et al., 1950
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 0, 2, 10 and 25 per cent. type B for 30 days. Only in
the highest dose were interference with body-weight gain and diarrhoea
observed. There no histological lesions nor were there abnormal
findings in urine and blood (Hodge et al., 1950).
Groups of 10 and 10 female young rats were fed 0, 1, 3, 10 and 30
per cent. of type A for 121 days. Body-weight gain was markedly
retarded at the 30 per cent. level, with 50 per cent. mortality
attributed to under-nutrition. Only the male rats showed slight
body-weight gain retardation at the 10 per cent. dietary level, while
the weight-gain was normal at the lower levels. Histological
examination of internal organs revealed no abnormalities in any of the
5 groups (McCollister & Oyen, 1954).
Groups of 10 male and 10 female young rats were fed diets
containing 0, 0.3, 1, 10 and 20 per cent. of type C. for 90 days. At
the 20 per cent. level both sexes showed marked retardation of
body-weight gain, with 30 per cent. mortality. At the 10 per cent.
level male rats only showed slight but significant weight gain
retardation. At the lower levels there were no adverse effects. The
microscopic appearance of tissues was normal at all levels
(McCollister et al., 1961).
Groups of 10 male and 10 female young rats were fed 0, 0.3, 1, 3,
10 and 20 per cent. of type D for 84 days. No adverse effects were
noted with female rats at all levels. Male rats showed a definite
retardation of bodyweight gain at 20 per cent. level and a slight
retardation at 10 per cent. Organ weights and gross and microscopic
examination revealed no adverse effects (McCollister et al., 1961).
Rabbit. Groups of 6 rabbits were fed diets containing 0, 10 and
25 per cent. type B for 30 days. The group on the highest dose
maintained, but did not increase, their body-weight. Normal results
were obtained from urine and blood analyses, comparison of organ
weights and histological examination (Hodge et al., 1950).
Dog. Groups of 2 dogs were fed for one year 0.1, 0.3, 1.0 and
3.0 g/kg body-weight daily of type B without effect on body and organ
weights, urine, blood and microscopic appearance of internal organs.
One dog fed 25 g/kg body-weight daily for 30 days suffered no ill
effects. Another dog fed 50 g/kg body-weight daily for 30 days
exhibited some diarrhoea, slight weight loss and slight depression of
red blood cell count without any histological changes (Hodge et al.,
1950).
Long-term studies
Rat. Groups of 50 male and 50 female rats were fed for 2 years
on diets containing 0, 1, 5 and 20 per cent. of type B. There was a
slight retardation of body-weight gain in the male group at the
highest dose. Mortality ranged from 60-84 per cent. with no
significant difference between the groups. Tumour incidence was the
same in the experimental groups as in controls (Hodge et al., 1950).
Comments
No significant amounts appear to be absorbed from the digestive
tract in man. There are no acute oral toxicity data, and only one
long-term study has been done on one particular type. Short-term
studies have been done with four slightly different, types, but gave
consistent results.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-301
Conditional acceptance : Higher levels may be used for
dietetic or calorie control
purposes
REFERENCES
Hodge, H. C., Maynard, E. A., Wilt, W. G. jr, Blanchett, H. J. jr &
Hyatt, R. E. (1950) J. Pharmacol. exp. Ther., 99, 112
Knight, H. F., Hodge, H. C., Samsel, E. P., Delap, R. E. &
McCollister, D. D. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 427
McCollister, D. D. & Oyen, F. (1954) J. Amer, pharm. Ass., sci.
Ed.,, 43, 664
McCollister, D. D., Oyen, F. & Greminger, G. K. jr (1961) J. Pharm.
Sci., 50, 615
1 As sum of total cellulose derivatives.
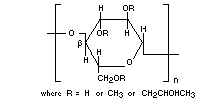 Molecular weight Unsubstituted structural unit: 162.14
Structural unit with 1.19 degree of
substitution: approx. 180
Structural unit with 2.37 degree of
substitution: approx. 210
Low polymers (n about 70): approx.
13 000
High polymers (n about 1000): approx.
208 000
Definition Hydroxypropyl methylcellulose is a
methylcellulose modified with a small
amount of propylene glycol ether groups
attached to the anhydroglucose of the
cellulose. The dry product contains 19
per cent. to 30 per cent. of methoxyl
(-OCH3) groups and 3 per cent. to 12
per cent. of hydroxypropyl
(-OCH2CHOHCH3) groups.
Description Hydroxylpropyl methylcellulose is a
white fibrous powder or as granules
(1,2).
Uses As a thickening agent or stabilizer.
Biological Data
Biochemical aspects
Twenty-five young human adults ingested doses ranging from 0.6 to
8.9 g of type B on three separate occasions. Only a mild laxative or
constipating effect was noted in several cases. About 97 per cent. of
the dose, determined as methoxy groups, was recovered from faeces
(Knight et al., 1952).
Acute toxicity of type B
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 5 000 Hodge et al., 1950
Rat i.p. 5 000 Hodge et al., 1950
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 0, 2, 10 and 25 per cent. type B for 30 days. Only in
the highest dose were interference with body-weight gain and diarrhoea
observed. There no histological lesions nor were there abnormal
findings in urine and blood (Hodge et al., 1950).
Groups of 10 and 10 female young rats were fed 0, 1, 3, 10 and 30
per cent. of type A for 121 days. Body-weight gain was markedly
retarded at the 30 per cent. level, with 50 per cent. mortality
attributed to under-nutrition. Only the male rats showed slight
body-weight gain retardation at the 10 per cent. dietary level, while
the weight-gain was normal at the lower levels. Histological
examination of internal organs revealed no abnormalities in any of the
5 groups (McCollister & Oyen, 1954).
Groups of 10 male and 10 female young rats were fed diets
containing 0, 0.3, 1, 10 and 20 per cent. of type C. for 90 days. At
the 20 per cent. level both sexes showed marked retardation of
body-weight gain, with 30 per cent. mortality. At the 10 per cent.
level male rats only showed slight but significant weight gain
retardation. At the lower levels there were no adverse effects. The
microscopic appearance of tissues was normal at all levels
(McCollister et al., 1961).
Groups of 10 male and 10 female young rats were fed 0, 0.3, 1, 3,
10 and 20 per cent. of type D for 84 days. No adverse effects were
noted with female rats at all levels. Male rats showed a definite
retardation of bodyweight gain at 20 per cent. level and a slight
retardation at 10 per cent. Organ weights and gross and microscopic
examination revealed no adverse effects (McCollister et al., 1961).
Rabbit. Groups of 6 rabbits were fed diets containing 0, 10 and
25 per cent. type B for 30 days. The group on the highest dose
maintained, but did not increase, their body-weight. Normal results
were obtained from urine and blood analyses, comparison of organ
weights and histological examination (Hodge et al., 1950).
Dog. Groups of 2 dogs were fed for one year 0.1, 0.3, 1.0 and
3.0 g/kg body-weight daily of type B without effect on body and organ
weights, urine, blood and microscopic appearance of internal organs.
One dog fed 25 g/kg body-weight daily for 30 days suffered no ill
effects. Another dog fed 50 g/kg body-weight daily for 30 days
exhibited some diarrhoea, slight weight loss and slight depression of
red blood cell count without any histological changes (Hodge et al.,
1950).
Long-term studies
Rat. Groups of 50 male and 50 female rats were fed for 2 years
on diets containing 0, 1, 5 and 20 per cent. of type B. There was a
slight retardation of body-weight gain in the male group at the
highest dose. Mortality ranged from 60-84 per cent. with no
significant difference between the groups. Tumour incidence was the
same in the experimental groups as in controls (Hodge et al., 1950).
Comments
No significant amounts appear to be absorbed from the digestive
tract in man. There are no acute oral toxicity data, and only one
long-term study has been done on one particular type. Short-term
studies have been done with four slightly different, types, but gave
consistent results.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-301
Conditional acceptance : Higher levels may be used for
dietetic or calorie control
purposes
REFERENCES
Hodge, H. C., Maynard, E. A., Wilt, W. G. jr, Blanchett, H. J. jr &
Hyatt, R. E. (1950) J. Pharmacol. exp. Ther., 99, 112
Knight, H. F., Hodge, H. C., Samsel, E. P., Delap, R. E. &
McCollister, D. D. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 427
McCollister, D. D. & Oyen, F. (1954) J. Amer, pharm. Ass., sci.
Ed.,, 43, 664
McCollister, D. D., Oyen, F. & Greminger, G. K. jr (1961) J. Pharm.
Sci., 50, 615
1 As sum of total cellulose derivatives.
Molecular weight Unsubstituted structural unit: 162.14
Structural unit with 1.19 degree of
substitution: approx. 180
Structural unit with 2.37 degree of
substitution: approx. 210
Low polymers (n about 70): approx.
13 000
High polymers (n about 1000): approx.
208 000
Definition Hydroxypropyl methylcellulose is a
methylcellulose modified with a small
amount of propylene glycol ether groups
attached to the anhydroglucose of the
cellulose. The dry product contains 19
per cent. to 30 per cent. of methoxyl
(-OCH3) groups and 3 per cent. to 12
per cent. of hydroxypropyl
(-OCH2CHOHCH3) groups.
Description Hydroxylpropyl methylcellulose is a
white fibrous powder or as granules
(1,2).
Uses As a thickening agent or stabilizer.
Biological Data
Biochemical aspects
Twenty-five young human adults ingested doses ranging from 0.6 to
8.9 g of type B on three separate occasions. Only a mild laxative or
constipating effect was noted in several cases. About 97 per cent. of
the dose, determined as methoxy groups, was recovered from faeces
(Knight et al., 1952).
Acute toxicity of type B
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 5 000 Hodge et al., 1950
Rat i.p. 5 000 Hodge et al., 1950
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 0, 2, 10 and 25 per cent. type B for 30 days. Only in
the highest dose were interference with body-weight gain and diarrhoea
observed. There no histological lesions nor were there abnormal
findings in urine and blood (Hodge et al., 1950).
Groups of 10 and 10 female young rats were fed 0, 1, 3, 10 and 30
per cent. of type A for 121 days. Body-weight gain was markedly
retarded at the 30 per cent. level, with 50 per cent. mortality
attributed to under-nutrition. Only the male rats showed slight
body-weight gain retardation at the 10 per cent. dietary level, while
the weight-gain was normal at the lower levels. Histological
examination of internal organs revealed no abnormalities in any of the
5 groups (McCollister & Oyen, 1954).
Groups of 10 male and 10 female young rats were fed diets
containing 0, 0.3, 1, 10 and 20 per cent. of type C. for 90 days. At
the 20 per cent. level both sexes showed marked retardation of
body-weight gain, with 30 per cent. mortality. At the 10 per cent.
level male rats only showed slight but significant weight gain
retardation. At the lower levels there were no adverse effects. The
microscopic appearance of tissues was normal at all levels
(McCollister et al., 1961).
Groups of 10 male and 10 female young rats were fed 0, 0.3, 1, 3,
10 and 20 per cent. of type D for 84 days. No adverse effects were
noted with female rats at all levels. Male rats showed a definite
retardation of bodyweight gain at 20 per cent. level and a slight
retardation at 10 per cent. Organ weights and gross and microscopic
examination revealed no adverse effects (McCollister et al., 1961).
Rabbit. Groups of 6 rabbits were fed diets containing 0, 10 and
25 per cent. type B for 30 days. The group on the highest dose
maintained, but did not increase, their body-weight. Normal results
were obtained from urine and blood analyses, comparison of organ
weights and histological examination (Hodge et al., 1950).
Dog. Groups of 2 dogs were fed for one year 0.1, 0.3, 1.0 and
3.0 g/kg body-weight daily of type B without effect on body and organ
weights, urine, blood and microscopic appearance of internal organs.
One dog fed 25 g/kg body-weight daily for 30 days suffered no ill
effects. Another dog fed 50 g/kg body-weight daily for 30 days
exhibited some diarrhoea, slight weight loss and slight depression of
red blood cell count without any histological changes (Hodge et al.,
1950).
Long-term studies
Rat. Groups of 50 male and 50 female rats were fed for 2 years
on diets containing 0, 1, 5 and 20 per cent. of type B. There was a
slight retardation of body-weight gain in the male group at the
highest dose. Mortality ranged from 60-84 per cent. with no
significant difference between the groups. Tumour incidence was the
same in the experimental groups as in controls (Hodge et al., 1950).
Comments
No significant amounts appear to be absorbed from the digestive
tract in man. There are no acute oral toxicity data, and only one
long-term study has been done on one particular type. Short-term
studies have been done with four slightly different, types, but gave
consistent results.
Evaluation
Level causing no toxicological effect
Rat. 50 000 ppm in the diet, equivalent to 2500 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-301
Conditional acceptance : Higher levels may be used for
dietetic or calorie control
purposes
REFERENCES
Hodge, H. C., Maynard, E. A., Wilt, W. G. jr, Blanchett, H. J. jr &
Hyatt, R. E. (1950) J. Pharmacol. exp. Ther., 99, 112
Knight, H. F., Hodge, H. C., Samsel, E. P., Delap, R. E. &
McCollister, D. D. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 427
McCollister, D. D. & Oyen, F. (1954) J. Amer, pharm. Ass., sci.
Ed.,, 43, 664
McCollister, D. D., Oyen, F. & Greminger, G. K. jr (1961) J. Pharm.
Sci., 50, 615
1 As sum of total cellulose derivatives.
