
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
POLYVINYLPYRROLIDONE
Synonyms Polyvidone; PVP
Empirical formula (C6H9ON)n
Structural formula
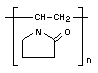 Molecular weight of unit: 111.1
Average molecular weight range of the
polymer between 11 500 and 25 000.
Definition Polyvinylpyrrolidone contains not less
than 95 per cent. (C6H9ON)n
Description Fine white or light yellow powder,
odourless, tasteless and slightly
hygroscopic.
Uses Clarifying agent.
Biological Data
Biochemical aspects
Polyvinylpyrrolidone (PVP) is a macromolecular polymer of
N-vinylpyrrolidone. It is metabolically inert in rat, dog and man as
shown by experiments using 14C- or 131I-labelled PVP (Ravin et al.,
1952). It has been widely used as plasma expander. The excretion of
PVP is inversely related to increasing molecular weight. The
glomerulus can excrete all PVP of molecular weight 40 000 or below
within a few days (Ravin et al., 1952). Low molecular weight PVP
adsorbs various substances, e.g. bacterial toxins, inorganic poisons.
barbiturates, vitamins and hormones in the blood, either reducing
their toxicity or prolonging their activity (Weese, 1944). In blood it
is mainly attached to the upsilon globulins (Bennhold & Schubert,
1944). The reticuloendothelial system retains PVP with a molecular
weight in excess of about 100 000 for a long time (Ravin et al., 1952;
Heinrich et al., 1966; Weese & Fresen, 1952).PVP is also accumulated
in the mitochondria of the kidneys (Traenckner, 1954), In the
PVP-storing cells it is surrounded by carbohydrates and lipids in a
capsule-like manner, perhaps due to coacervation processes (Hübner,
1960). The problem of whether the extremely long storage of PVP in the
body produces toxic effects is open to discussion (Altemeir et al.,
1954; Ammon & Miller, 1949).
Transfer of intravenously injected PVP to the brain or through
the placenta was not observed (Ravin et al, 1952). I.v. injection of
131I-labelled PVP has been clinically used to detect gastrointestinal
protein losses.
PVP of m.w. 11 500 is not absorbed from the intestinal tract by
man or by rat (Angerwall & Berntsson, 1961). PVP of m.w. 16 000 is not
absorbed from the gastrointestinal tract by guinea-pigs (Scheffner,
1955).
Acute toxicity
Animal Route Molecular weight LD50 References
(mg/kg
body-weight)
Rat oral 10 000-30 000 >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
Mouse oral >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
i.p. 12 000-15 000 Angerwall & Berntsson,
1961
Rat oral 40 000 100 000 Burnette, 1962
Shelanski et al., 1954
Guinea-pig oral 40 000 100 000 Burnette, 1962
Shelanski et al, 1954
Oral PVP (m.w, up to 40 000) in higher doses causes diarrhoea,
the minimal effective dose being 0.5 g/kg body-weight for cats, and
1-2 g/kg body-weight for dogs (Scheffner, 1955)
Short-term studies
PVP molecular weight up to 40 000
Dog. Four groups of 4 beagle dogs were, fed 0, 2, 5, and 10 per
cent. PVP (m.w. 38 000) for 2 years. There were no differences in
weight gain, food consumption and results of the of blood and urine.
At the end of the 2-year period all animals were in good health and
histological examination disclosed no specific changes, except for
swollen RES cells in the mesenteric and other lymph nodes of all test
groups, especially at the 10 per cent. level. No PVP was detected in
the urine. No malignant tumours were detected (Burnette, 1962). In 2
similar feeding experiments using a total of 32 dogs and lasting for 1
year no adverse effects could be detected. The intestines, spleens and
livers of all animals ware shown to be free of PVP, but PVP was
demonstrated in the mesenteric lymph nodes of all animals, including
the controls (Burnette, 1962).
Several other short-term studies in rat, cat and dog showed no
toxic effects (Scheffner, 1955).
PVP molecular weight 220 000-1 500 000
Dog. Two dogs were given oral doses of 5 g/kg body-weight PVP
(m.w. 220 000 and 1 500 000) for 1-1/2 weeks and 2 weeks respectively
without any abnormal findings (Scheffner, 1955).
Long-term studies
Rat. Groups of rats were fed diets containing 0, 1 and 10 per
cent. PVP (m.w. 38 000) for 2 years. No toxic effects or gross or
histological changes were noted which could be attributed to the test
compound. There was no evidence of absorption of PVP from the
intestinal tract (Badische Anilin and Sodafabrik, 1958; Burnette,
1962).
Comments
There is a large amount of experience available on the parenteral
administration of PVP to man. I.v. injected PVP having a molecular
weight exceeding 40 000 is stored in the body for a long time, mainly
in the RES. Orally administered PVP is not absorbed from the
Intestinal tract except perhaps in small quantities that may enter
the intestinal lymph nodes. Further feeding experiments in order to
clarify the problem of possible storage or PVP intestinal lymph nodes
and studies on the possible effects of PVP on the absorption of
nutrients are required within the next 2 years.
Evaluation
The animal data provided do not allow the use of the normal
procedure for arriving at an acceptable daily intake for man. The
evaluation is based provisionally on the large clinical experience in
man and the known metabolic inertness of this substance. No
unconditional acceptance can be given because of probable accumulation
in the lymph nodes.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Further feeding experiments in order to clarify the problem of a
possible storage of PVP in the intestinal lymph nodes. Studies on the
possible effects of PVP on the absorption of nutrients.
REFERENCES
Altemeir, W. A,, Schiff, L., Gall, E. A., Gluseffi, J., Freiman, D.,
Mindrum, G. & Braunstein, H. (1954) Amer. Med. Assoc. Arch. Surg.,
69), 300
Ammon, R. & Müller, W. (1949) Dtsch. Med. Wsch., 15,
Angerwall, L. & Berntsson, S. (1961) J. Inst. brwnig., 67, 353
Badische Anilin und Sodafabrik A. G. (1958) Unpublished report
submitted to WHO
Bennhold, H. & Schubert, R. (1944) Klin. Wschr., 23, 30
Burnette, L. W. (1962) Proc. Sci. Sect. Toilet Goods Assoc., 38, 1
Heinrich, H. C., Gabbe, E. E., Nass, W. P. & Becker, K. (1966) Klin.
Wschr., 44, 488
Hübner, G. (1960) Virchows Arch. Path. Anat., 333, 29
Ravin, H. A., Seligman, A. M. & Fine, J. (1952) New England. J.
med., 247, 921
Scheffner, D. (1955) Thesis, University of Heidelberg, summary
submitted to WHO
Shelanski, A, A., Shelanski, M. V. & Cantor, A. (1954) J. Soc. Cosm.
Chem., 5 129
Traenckner, K. (1954) Z. ges. exp. Med., 123, 101
Weese, H. (1944) Med. Z., 1, 19
Weese, H. & Fresen, O. (1952) Zieglers Beitr., 112, 44
Molecular weight of unit: 111.1
Average molecular weight range of the
polymer between 11 500 and 25 000.
Definition Polyvinylpyrrolidone contains not less
than 95 per cent. (C6H9ON)n
Description Fine white or light yellow powder,
odourless, tasteless and slightly
hygroscopic.
Uses Clarifying agent.
Biological Data
Biochemical aspects
Polyvinylpyrrolidone (PVP) is a macromolecular polymer of
N-vinylpyrrolidone. It is metabolically inert in rat, dog and man as
shown by experiments using 14C- or 131I-labelled PVP (Ravin et al.,
1952). It has been widely used as plasma expander. The excretion of
PVP is inversely related to increasing molecular weight. The
glomerulus can excrete all PVP of molecular weight 40 000 or below
within a few days (Ravin et al., 1952). Low molecular weight PVP
adsorbs various substances, e.g. bacterial toxins, inorganic poisons.
barbiturates, vitamins and hormones in the blood, either reducing
their toxicity or prolonging their activity (Weese, 1944). In blood it
is mainly attached to the upsilon globulins (Bennhold & Schubert,
1944). The reticuloendothelial system retains PVP with a molecular
weight in excess of about 100 000 for a long time (Ravin et al., 1952;
Heinrich et al., 1966; Weese & Fresen, 1952).PVP is also accumulated
in the mitochondria of the kidneys (Traenckner, 1954), In the
PVP-storing cells it is surrounded by carbohydrates and lipids in a
capsule-like manner, perhaps due to coacervation processes (Hübner,
1960). The problem of whether the extremely long storage of PVP in the
body produces toxic effects is open to discussion (Altemeir et al.,
1954; Ammon & Miller, 1949).
Transfer of intravenously injected PVP to the brain or through
the placenta was not observed (Ravin et al, 1952). I.v. injection of
131I-labelled PVP has been clinically used to detect gastrointestinal
protein losses.
PVP of m.w. 11 500 is not absorbed from the intestinal tract by
man or by rat (Angerwall & Berntsson, 1961). PVP of m.w. 16 000 is not
absorbed from the gastrointestinal tract by guinea-pigs (Scheffner,
1955).
Acute toxicity
Animal Route Molecular weight LD50 References
(mg/kg
body-weight)
Rat oral 10 000-30 000 >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
Mouse oral >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
i.p. 12 000-15 000 Angerwall & Berntsson,
1961
Rat oral 40 000 100 000 Burnette, 1962
Shelanski et al., 1954
Guinea-pig oral 40 000 100 000 Burnette, 1962
Shelanski et al, 1954
Oral PVP (m.w, up to 40 000) in higher doses causes diarrhoea,
the minimal effective dose being 0.5 g/kg body-weight for cats, and
1-2 g/kg body-weight for dogs (Scheffner, 1955)
Short-term studies
PVP molecular weight up to 40 000
Dog. Four groups of 4 beagle dogs were, fed 0, 2, 5, and 10 per
cent. PVP (m.w. 38 000) for 2 years. There were no differences in
weight gain, food consumption and results of the of blood and urine.
At the end of the 2-year period all animals were in good health and
histological examination disclosed no specific changes, except for
swollen RES cells in the mesenteric and other lymph nodes of all test
groups, especially at the 10 per cent. level. No PVP was detected in
the urine. No malignant tumours were detected (Burnette, 1962). In 2
similar feeding experiments using a total of 32 dogs and lasting for 1
year no adverse effects could be detected. The intestines, spleens and
livers of all animals ware shown to be free of PVP, but PVP was
demonstrated in the mesenteric lymph nodes of all animals, including
the controls (Burnette, 1962).
Several other short-term studies in rat, cat and dog showed no
toxic effects (Scheffner, 1955).
PVP molecular weight 220 000-1 500 000
Dog. Two dogs were given oral doses of 5 g/kg body-weight PVP
(m.w. 220 000 and 1 500 000) for 1-1/2 weeks and 2 weeks respectively
without any abnormal findings (Scheffner, 1955).
Long-term studies
Rat. Groups of rats were fed diets containing 0, 1 and 10 per
cent. PVP (m.w. 38 000) for 2 years. No toxic effects or gross or
histological changes were noted which could be attributed to the test
compound. There was no evidence of absorption of PVP from the
intestinal tract (Badische Anilin and Sodafabrik, 1958; Burnette,
1962).
Comments
There is a large amount of experience available on the parenteral
administration of PVP to man. I.v. injected PVP having a molecular
weight exceeding 40 000 is stored in the body for a long time, mainly
in the RES. Orally administered PVP is not absorbed from the
Intestinal tract except perhaps in small quantities that may enter
the intestinal lymph nodes. Further feeding experiments in order to
clarify the problem of possible storage or PVP intestinal lymph nodes
and studies on the possible effects of PVP on the absorption of
nutrients are required within the next 2 years.
Evaluation
The animal data provided do not allow the use of the normal
procedure for arriving at an acceptable daily intake for man. The
evaluation is based provisionally on the large clinical experience in
man and the known metabolic inertness of this substance. No
unconditional acceptance can be given because of probable accumulation
in the lymph nodes.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Further feeding experiments in order to clarify the problem of a
possible storage of PVP in the intestinal lymph nodes. Studies on the
possible effects of PVP on the absorption of nutrients.
REFERENCES
Altemeir, W. A,, Schiff, L., Gall, E. A., Gluseffi, J., Freiman, D.,
Mindrum, G. & Braunstein, H. (1954) Amer. Med. Assoc. Arch. Surg.,
69), 300
Ammon, R. & Müller, W. (1949) Dtsch. Med. Wsch., 15,
Angerwall, L. & Berntsson, S. (1961) J. Inst. brwnig., 67, 353
Badische Anilin und Sodafabrik A. G. (1958) Unpublished report
submitted to WHO
Bennhold, H. & Schubert, R. (1944) Klin. Wschr., 23, 30
Burnette, L. W. (1962) Proc. Sci. Sect. Toilet Goods Assoc., 38, 1
Heinrich, H. C., Gabbe, E. E., Nass, W. P. & Becker, K. (1966) Klin.
Wschr., 44, 488
Hübner, G. (1960) Virchows Arch. Path. Anat., 333, 29
Ravin, H. A., Seligman, A. M. & Fine, J. (1952) New England. J.
med., 247, 921
Scheffner, D. (1955) Thesis, University of Heidelberg, summary
submitted to WHO
Shelanski, A, A., Shelanski, M. V. & Cantor, A. (1954) J. Soc. Cosm.
Chem., 5 129
Traenckner, K. (1954) Z. ges. exp. Med., 123, 101
Weese, H. (1944) Med. Z., 1, 19
Weese, H. & Fresen, O. (1952) Zieglers Beitr., 112, 44
 Molecular weight of unit: 111.1
Average molecular weight range of the
polymer between 11 500 and 25 000.
Definition Polyvinylpyrrolidone contains not less
than 95 per cent. (C6H9ON)n
Description Fine white or light yellow powder,
odourless, tasteless and slightly
hygroscopic.
Uses Clarifying agent.
Biological Data
Biochemical aspects
Polyvinylpyrrolidone (PVP) is a macromolecular polymer of
N-vinylpyrrolidone. It is metabolically inert in rat, dog and man as
shown by experiments using 14C- or 131I-labelled PVP (Ravin et al.,
1952). It has been widely used as plasma expander. The excretion of
PVP is inversely related to increasing molecular weight. The
glomerulus can excrete all PVP of molecular weight 40 000 or below
within a few days (Ravin et al., 1952). Low molecular weight PVP
adsorbs various substances, e.g. bacterial toxins, inorganic poisons.
barbiturates, vitamins and hormones in the blood, either reducing
their toxicity or prolonging their activity (Weese, 1944). In blood it
is mainly attached to the upsilon globulins (Bennhold & Schubert,
1944). The reticuloendothelial system retains PVP with a molecular
weight in excess of about 100 000 for a long time (Ravin et al., 1952;
Heinrich et al., 1966; Weese & Fresen, 1952).PVP is also accumulated
in the mitochondria of the kidneys (Traenckner, 1954), In the
PVP-storing cells it is surrounded by carbohydrates and lipids in a
capsule-like manner, perhaps due to coacervation processes (Hübner,
1960). The problem of whether the extremely long storage of PVP in the
body produces toxic effects is open to discussion (Altemeir et al.,
1954; Ammon & Miller, 1949).
Transfer of intravenously injected PVP to the brain or through
the placenta was not observed (Ravin et al, 1952). I.v. injection of
131I-labelled PVP has been clinically used to detect gastrointestinal
protein losses.
PVP of m.w. 11 500 is not absorbed from the intestinal tract by
man or by rat (Angerwall & Berntsson, 1961). PVP of m.w. 16 000 is not
absorbed from the gastrointestinal tract by guinea-pigs (Scheffner,
1955).
Acute toxicity
Animal Route Molecular weight LD50 References
(mg/kg
body-weight)
Rat oral 10 000-30 000 >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
Mouse oral >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
i.p. 12 000-15 000 Angerwall & Berntsson,
1961
Rat oral 40 000 100 000 Burnette, 1962
Shelanski et al., 1954
Guinea-pig oral 40 000 100 000 Burnette, 1962
Shelanski et al, 1954
Oral PVP (m.w, up to 40 000) in higher doses causes diarrhoea,
the minimal effective dose being 0.5 g/kg body-weight for cats, and
1-2 g/kg body-weight for dogs (Scheffner, 1955)
Short-term studies
PVP molecular weight up to 40 000
Dog. Four groups of 4 beagle dogs were, fed 0, 2, 5, and 10 per
cent. PVP (m.w. 38 000) for 2 years. There were no differences in
weight gain, food consumption and results of the of blood and urine.
At the end of the 2-year period all animals were in good health and
histological examination disclosed no specific changes, except for
swollen RES cells in the mesenteric and other lymph nodes of all test
groups, especially at the 10 per cent. level. No PVP was detected in
the urine. No malignant tumours were detected (Burnette, 1962). In 2
similar feeding experiments using a total of 32 dogs and lasting for 1
year no adverse effects could be detected. The intestines, spleens and
livers of all animals ware shown to be free of PVP, but PVP was
demonstrated in the mesenteric lymph nodes of all animals, including
the controls (Burnette, 1962).
Several other short-term studies in rat, cat and dog showed no
toxic effects (Scheffner, 1955).
PVP molecular weight 220 000-1 500 000
Dog. Two dogs were given oral doses of 5 g/kg body-weight PVP
(m.w. 220 000 and 1 500 000) for 1-1/2 weeks and 2 weeks respectively
without any abnormal findings (Scheffner, 1955).
Long-term studies
Rat. Groups of rats were fed diets containing 0, 1 and 10 per
cent. PVP (m.w. 38 000) for 2 years. No toxic effects or gross or
histological changes were noted which could be attributed to the test
compound. There was no evidence of absorption of PVP from the
intestinal tract (Badische Anilin and Sodafabrik, 1958; Burnette,
1962).
Comments
There is a large amount of experience available on the parenteral
administration of PVP to man. I.v. injected PVP having a molecular
weight exceeding 40 000 is stored in the body for a long time, mainly
in the RES. Orally administered PVP is not absorbed from the
Intestinal tract except perhaps in small quantities that may enter
the intestinal lymph nodes. Further feeding experiments in order to
clarify the problem of possible storage or PVP intestinal lymph nodes
and studies on the possible effects of PVP on the absorption of
nutrients are required within the next 2 years.
Evaluation
The animal data provided do not allow the use of the normal
procedure for arriving at an acceptable daily intake for man. The
evaluation is based provisionally on the large clinical experience in
man and the known metabolic inertness of this substance. No
unconditional acceptance can be given because of probable accumulation
in the lymph nodes.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Further feeding experiments in order to clarify the problem of a
possible storage of PVP in the intestinal lymph nodes. Studies on the
possible effects of PVP on the absorption of nutrients.
REFERENCES
Altemeir, W. A,, Schiff, L., Gall, E. A., Gluseffi, J., Freiman, D.,
Mindrum, G. & Braunstein, H. (1954) Amer. Med. Assoc. Arch. Surg.,
69), 300
Ammon, R. & Müller, W. (1949) Dtsch. Med. Wsch., 15,
Angerwall, L. & Berntsson, S. (1961) J. Inst. brwnig., 67, 353
Badische Anilin und Sodafabrik A. G. (1958) Unpublished report
submitted to WHO
Bennhold, H. & Schubert, R. (1944) Klin. Wschr., 23, 30
Burnette, L. W. (1962) Proc. Sci. Sect. Toilet Goods Assoc., 38, 1
Heinrich, H. C., Gabbe, E. E., Nass, W. P. & Becker, K. (1966) Klin.
Wschr., 44, 488
Hübner, G. (1960) Virchows Arch. Path. Anat., 333, 29
Ravin, H. A., Seligman, A. M. & Fine, J. (1952) New England. J.
med., 247, 921
Scheffner, D. (1955) Thesis, University of Heidelberg, summary
submitted to WHO
Shelanski, A, A., Shelanski, M. V. & Cantor, A. (1954) J. Soc. Cosm.
Chem., 5 129
Traenckner, K. (1954) Z. ges. exp. Med., 123, 101
Weese, H. (1944) Med. Z., 1, 19
Weese, H. & Fresen, O. (1952) Zieglers Beitr., 112, 44
Molecular weight of unit: 111.1
Average molecular weight range of the
polymer between 11 500 and 25 000.
Definition Polyvinylpyrrolidone contains not less
than 95 per cent. (C6H9ON)n
Description Fine white or light yellow powder,
odourless, tasteless and slightly
hygroscopic.
Uses Clarifying agent.
Biological Data
Biochemical aspects
Polyvinylpyrrolidone (PVP) is a macromolecular polymer of
N-vinylpyrrolidone. It is metabolically inert in rat, dog and man as
shown by experiments using 14C- or 131I-labelled PVP (Ravin et al.,
1952). It has been widely used as plasma expander. The excretion of
PVP is inversely related to increasing molecular weight. The
glomerulus can excrete all PVP of molecular weight 40 000 or below
within a few days (Ravin et al., 1952). Low molecular weight PVP
adsorbs various substances, e.g. bacterial toxins, inorganic poisons.
barbiturates, vitamins and hormones in the blood, either reducing
their toxicity or prolonging their activity (Weese, 1944). In blood it
is mainly attached to the upsilon globulins (Bennhold & Schubert,
1944). The reticuloendothelial system retains PVP with a molecular
weight in excess of about 100 000 for a long time (Ravin et al., 1952;
Heinrich et al., 1966; Weese & Fresen, 1952).PVP is also accumulated
in the mitochondria of the kidneys (Traenckner, 1954), In the
PVP-storing cells it is surrounded by carbohydrates and lipids in a
capsule-like manner, perhaps due to coacervation processes (Hübner,
1960). The problem of whether the extremely long storage of PVP in the
body produces toxic effects is open to discussion (Altemeir et al.,
1954; Ammon & Miller, 1949).
Transfer of intravenously injected PVP to the brain or through
the placenta was not observed (Ravin et al, 1952). I.v. injection of
131I-labelled PVP has been clinically used to detect gastrointestinal
protein losses.
PVP of m.w. 11 500 is not absorbed from the intestinal tract by
man or by rat (Angerwall & Berntsson, 1961). PVP of m.w. 16 000 is not
absorbed from the gastrointestinal tract by guinea-pigs (Scheffner,
1955).
Acute toxicity
Animal Route Molecular weight LD50 References
(mg/kg
body-weight)
Rat oral 10 000-30 000 >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
Mouse oral >40 000 Scheffner, 1955
Badische Anilin und
Sodafabrik, 1958
i.p. 12 000-15 000 Angerwall & Berntsson,
1961
Rat oral 40 000 100 000 Burnette, 1962
Shelanski et al., 1954
Guinea-pig oral 40 000 100 000 Burnette, 1962
Shelanski et al, 1954
Oral PVP (m.w, up to 40 000) in higher doses causes diarrhoea,
the minimal effective dose being 0.5 g/kg body-weight for cats, and
1-2 g/kg body-weight for dogs (Scheffner, 1955)
Short-term studies
PVP molecular weight up to 40 000
Dog. Four groups of 4 beagle dogs were, fed 0, 2, 5, and 10 per
cent. PVP (m.w. 38 000) for 2 years. There were no differences in
weight gain, food consumption and results of the of blood and urine.
At the end of the 2-year period all animals were in good health and
histological examination disclosed no specific changes, except for
swollen RES cells in the mesenteric and other lymph nodes of all test
groups, especially at the 10 per cent. level. No PVP was detected in
the urine. No malignant tumours were detected (Burnette, 1962). In 2
similar feeding experiments using a total of 32 dogs and lasting for 1
year no adverse effects could be detected. The intestines, spleens and
livers of all animals ware shown to be free of PVP, but PVP was
demonstrated in the mesenteric lymph nodes of all animals, including
the controls (Burnette, 1962).
Several other short-term studies in rat, cat and dog showed no
toxic effects (Scheffner, 1955).
PVP molecular weight 220 000-1 500 000
Dog. Two dogs were given oral doses of 5 g/kg body-weight PVP
(m.w. 220 000 and 1 500 000) for 1-1/2 weeks and 2 weeks respectively
without any abnormal findings (Scheffner, 1955).
Long-term studies
Rat. Groups of rats were fed diets containing 0, 1 and 10 per
cent. PVP (m.w. 38 000) for 2 years. No toxic effects or gross or
histological changes were noted which could be attributed to the test
compound. There was no evidence of absorption of PVP from the
intestinal tract (Badische Anilin and Sodafabrik, 1958; Burnette,
1962).
Comments
There is a large amount of experience available on the parenteral
administration of PVP to man. I.v. injected PVP having a molecular
weight exceeding 40 000 is stored in the body for a long time, mainly
in the RES. Orally administered PVP is not absorbed from the
Intestinal tract except perhaps in small quantities that may enter
the intestinal lymph nodes. Further feeding experiments in order to
clarify the problem of possible storage or PVP intestinal lymph nodes
and studies on the possible effects of PVP on the absorption of
nutrients are required within the next 2 years.
Evaluation
The animal data provided do not allow the use of the normal
procedure for arriving at an acceptable daily intake for man. The
evaluation is based provisionally on the large clinical experience in
man and the known metabolic inertness of this substance. No
unconditional acceptance can be given because of probable accumulation
in the lymph nodes.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Further feeding experiments in order to clarify the problem of a
possible storage of PVP in the intestinal lymph nodes. Studies on the
possible effects of PVP on the absorption of nutrients.
REFERENCES
Altemeir, W. A,, Schiff, L., Gall, E. A., Gluseffi, J., Freiman, D.,
Mindrum, G. & Braunstein, H. (1954) Amer. Med. Assoc. Arch. Surg.,
69), 300
Ammon, R. & Müller, W. (1949) Dtsch. Med. Wsch., 15,
Angerwall, L. & Berntsson, S. (1961) J. Inst. brwnig., 67, 353
Badische Anilin und Sodafabrik A. G. (1958) Unpublished report
submitted to WHO
Bennhold, H. & Schubert, R. (1944) Klin. Wschr., 23, 30
Burnette, L. W. (1962) Proc. Sci. Sect. Toilet Goods Assoc., 38, 1
Heinrich, H. C., Gabbe, E. E., Nass, W. P. & Becker, K. (1966) Klin.
Wschr., 44, 488
Hübner, G. (1960) Virchows Arch. Path. Anat., 333, 29
Ravin, H. A., Seligman, A. M. & Fine, J. (1952) New England. J.
med., 247, 921
Scheffner, D. (1955) Thesis, University of Heidelberg, summary
submitted to WHO
Shelanski, A, A., Shelanski, M. V. & Cantor, A. (1954) J. Soc. Cosm.
Chem., 5 129
Traenckner, K. (1954) Z. ges. exp. Med., 123, 101
Weese, H. (1944) Med. Z., 1, 19
Weese, H. & Fresen, O. (1952) Zieglers Beitr., 112, 44
