INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES 40
Prepared by:
The forty-ninth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
SUBSTANCES EVALUATED USING THE PROCEDURE FOR THE SAFETY EVALUATION
OF FLAVOURING AGENTS
INTRODUCTION
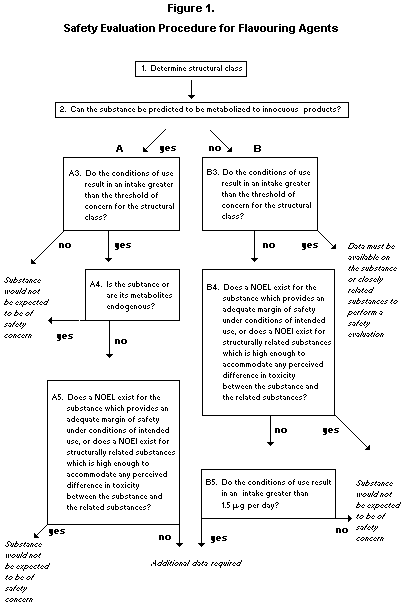
Six groups of flavouring agents were evaluated using the Procedure
for the Safety Evaluation of Flavouring Agents as modified at the
present meeting (Figure 1).
The Committee noted that in applying the Procedure, the substance
is first assigned to a structural class as identified at the
forty-sixth meeting of the Committee (Annex 1, reference 122). The
structural classes are as follows:
- Class I. Substances with simple chemical structures and efficient
modes of metabolism which would suggest a low order of toxicity by
the oral route.
- Class II. Substances with structural features that are less
innocuous than those of substances in Class I but are not
suggestive of toxicity. Substances in this class may contain
reactive functional groups.
- Class III. Substances with structural features that permit no
strong initial presumption of safety, or may even suggest
significant toxicity.
A key element of the procedure involves determining whether a
flavouring agent and the product(s) of its metabolism are innocuous
and/or endogenous substances. For the purpose of the evaluations, the
following definitions were used, which have been adapted from the
report of the forty-sixth meeting of the Committee:
Innocuous metabolic products are defined as products that are
known or readily predicted to be harmless to humans at the estimated
intake of the flavouring agent.
Endogenous substances are intermediary metabolites normally
present in human tissues and fluids, whether free or conjugated;
hormones and other substances with biochemical or physiological
regulatory functions are not included; the estimated intake should be
judged not to give rise to perturbations outside the physiological
range.
The Committee first considered the metabolic pathways common to
the flavouring agents evaluated at the present meeting.
a) Hydrolysis of esters
Linear alkyl esters are hydrolysed rapidly to their component
alcohols and carboxylic acids in the intestinal tract, blood and liver
and most tissues throughout the body. Hydrolysis is catalysed by
classes of enzymes recognized as carboxylesterases or esterases. For
simple linear esters, as considered at this meeting, the rate of
hydrolysis increases with increase in chain length of either the acid
or alcohol component. The rate of hydrolysis of straight-chain esters
is approximately 100 times faster than the rate of hydrolysis of
branched-chain esters. The rates of hydrolysis of the alkenyl esters
citronellyl acetate and citronellyl phenylacetate, by artificial
pancreatic juice, were similar to the rates for simple branched-chain
esters.
b) Oxidation of alkyl primary alcohols and aldehydes
Most linear and branched-chain, saturated and unsaturated primary
alcohols are oxidized rapidly to their corresponding aldehydes by
alcohol dehydrogenase. The rate of oxidation increases with increase
in chain length and with the presence of a double bond.
The subsequent oxidation of the aldehydes to the corresponding
acid is catalysed by dehydrogenase and oxidase enzymes. Most active is
a NAD+/NADH-dependent aldehyde dehydrogenase present in the cytosol,
the activity of which increases with increasing relative molecular
mass of the aldehyde substrate. Aldehydes may also be reduced to
alcohols or conjugated with sulfhydryl-containing substances, such as
glutathione. The oxidation of aldehydes with low relative molecular
mass by aldehyde dehydrogenase requires glutathione, which suggests
that the free aldehyde may be conjugated rapidly with glutathione
in vivo to form a thiohemiacetal that is subsequently oxidized to
the corresponding acid. Branched-chain aldehydes are excellent
substrates for aldehyde dehydrogenase, and the rate of oxidation of
2-methylpropanal is similar to that of acetaldehyde.
c) Oxidation of linear saturated carboxylic acids
Aliphatic linear saturated carboxylic acids are metabolized in the
fatty acid ß-oxidation pathway, the tricarboxylic acid cycle, or the
C1-tetrahydrofolate pathway. Oxidation of formic acid to carbon
dioxide and water occurs primarily in the liver and is catalysed by
tetrahydrofolate in humans and other primates.
Other carboxylic acids are condensed with coenzyme A (CoA) to
yield thioesters that undergo ß-cleavage to acetyl CoA. Even-numbered
carboxylic acids give acetyl CoA, whereas odd-numbered carboxylic
acids yield acetyl CoA and propionyl CoA. Acetyl CoA enters the citric
acid cycle directly. Propionyl CoA is converted to succinyl CoA before
entering the citric acid cycle.
d) Oxidation of branched saturated carboxylic acids
Short-chain (< C6) branched aliphatic acids undergo
ß-oxidation preferentially in the longer chain followed by cleavage to
yield linear acid fragments that are metabolized via the fatty acid
pathway or tricarboxylic acid cycle. Isobutyric acid, isovaleric acid
and 2-methylbutyric are formed during the oxidative deamination of
endogenous branched-chain amino acids and are metabolized by normal
pathways of intermediary metabolism. At high dose levels, longer
branched-chain acids may undergo omega-oxidation to yield diacids that
undergo further oxidation and cleavage.
Acids with a methyl substituent are extensively metabolized to
CO2 via ß-oxidation, unless the methyl group is located at the
ß-position (e.g., 3-methyl-pentanoic acid), in which case
alpha-oxidation occurs, yielding short-chain acid fragments capable of
being completely metabolized.
The presence of a 2-ethyl substituent prevents the ß-oxidation of
aliphatic carboxylic acids, and these compounds undergo omega-and
omega-1 oxidation to yield polar metabolites that are excreted
primarily in the urine. Saturation of this omega-oxidation pathway may
lead to formation of the 2-substituted carboxylic acid that may be
excreted as the glucuronic acid conjugate.