
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES 40
Prepared by:
The forty-ninth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
ANNEX 1
Reports and other documents resulting from previous meetings of the
Joint FAO/WHO Expert Committee on Food Additives
1. General principles governing the use of food additives (First
report of the Joint FAO/WHO Expert Committee on Food Additives).
FAO Nutrition Meetings Report Series, No. 15, 1957; WHO Technical
Report Series, No. 129, 1957 (out of print).
2. Procedures for the testing of intentional food additives to
establish their safety for use (Second report of the Joint FAO/WHO
Expert Committee on Food Additives). FAO Nutrition Meetings Report
Series, No. 17, 1958; WHO Technical Report Series, No. 144, 1958
(out of print).
3. Specifications for identity and purity of food additives
(antimicrobial preservatives and antioxidants) (Third report of the
Joint FAO/WHO Expert Committee on Food Additives). These
specifications were subsequently revised and published as
Specifications for identity and purity of food additives, Vol. I.
Antimicrobial preservatives and antioxidants, Rome, Food and
Agriculture Organization of the United Nations, 1962 (out of
print).
4. Specifications for identity and purity of food additives (food
colours) (Fourth report of the Joint FAO/WHO Expert Committee on
Food Additives). These specifications were subsequently revised and
published as Specifications for identity and purity of food
additives, Vol. II. Food colours, Rome, Food and Agriculture
Organization of the United Nations, 1963 (out of print).
5. Evaluation of the carcinogenic hazards of food additives (Fifth
report of the Joint FAO/WHO Expert Committee on Food Additives).
FAO Nutrition Meetings Report Series, No. 29, 1961; WHO Technical
Report Series, No. 220, 1961 (out of print).
6. Evaluation of the toxicity of a number of antimicrobials and
antioxidants (Sixth report of the Joint FAO/WHO Expert Committee on
Food Additives). FAO Nutrition Meetings Report Series, No. 31,
1962; WHO Technical Report Series, No. 228, 1962 (out of print).
7. Specifications for the identity and purity of food additives and
their toxicological evaluation: emulsifiers, stabilizers, bleaching
and maturing agents (Seventh report of the Joint FAO/WHO Expert
Committee on Food Additives). FAO Nutrition Meetings Report Series,
No. 35, 1964; WHO Technical Report Series, No. 281, 1964 (out of
print).
8. Specifications for the identity and purity of food additives and
their toxicological evaluation: food colours and some
antimicrobials and antioxidants (Eighth report of the Joint FAO/WHO
Expert Committee on Food Additives). FAO Nutrition Meetings Report
Series, No. 38, 1965; WHO Technical Report Series, No. 309, 1965
(out of print).
9. Specifications for identity and purity and toxicological
evaluation of some antimicrobials and antioxidants. FAO Nutrition
Meetings Report Series, No. 38A, 1965; WHO/Food Add/24.65 (out of
print).
10. Specifications for identity and purity and toxicological
evaluation of food colours. FAO Nutrition Meetings Report Series,
No. 38B, 1966; WHO/Food Add/66.25 (out of print).
11. Specifications for the identity and purity of food additives
and their toxicological evaluation: some antimicrobials,
antioxidants, emulsifiers, stabilizers, flour-treatment agents,
acids, and bases (Ninth report of the Joint FAO/WHO Expert
Committee on Food Additives). FAO Nutrition Meetings Report Series,
No. 40, 1966; WHO Technical Report Series, No. 339, 1966 (out of
print).
12. Toxicological evaluation of some antimicrobials, antioxidants,
emulsifiers, stabilizers, flour-treatment agents, acids, and bases.
FAO Nutrition Meetings Report Series, No. 40A, B, C; WHO/Food
Add/67.29 (out of print).
13. Specifications for the identity and purity of food additives
and their toxicological evaluation: some emulsifiers and
stabilizers and certain other substances (Tenth report of the Joint
FAO/WHO Expert Committee on Food Additives). FAO Nutrition Meetings
Report Series, No. 43, 1967; WHO Technical Report Series, No. 373,
1967 (out of print).
14. Specifications for the identity and purity of food additives
and their toxicological evaluation: some flavouring substances and
non-nutritive sweetening agents (Eleventh report of the Joint
FAO/WHO Expert Committee on Food Additives). FAO Nutrition Meetings
Report Series, No. 44, 1968; WHO Technical Report Series, No. 383,
1968 (out of print).
15. Toxicological evaluation of some flavouring substances and
non-nutritive sweetening agents. FAO Nutrition Meetings Report
Series, No. 44A, 1968; WHO/Food Add/68.33 (out of print).
16. Specifications and criteria for identity and purity of some
flavouring substances and non-nutritive sweetening agents. FAO
Nutrition Meetings Report Series, No. 44B, 1969; WHO/Food Add/69.31
(out of print).
17. Specifications for the identity and purity of food additives
and their toxicological evaluation: some antibiotics (Twelfth
report of the Joint FAO/WHO Expert Committee on Food Additives).
FAO Nutrition Meetings Report Series, No. 45, 1969; WHO Technical
Report Series, No. 430, 1969.
18. Specifications for the identity and purity of some antibiotics.
FAO Nutrition Meetings Report Series, No. 45A, 1969; WHO/Food
Add/69.34 (out of print).
19. Specifications for the identity and purity of food additives
and their toxicological evaluation: some food colours, emulsifiers,
stabilizers, anticaking agents, and certain other substances
(Thirteenth report of the Joint FAO/WHO Expert Committee on Food
Additives). FAO Nutrition Meetings Report Series, No. 46, 1970; WHO
Technical Report Series, No. 445, 1970.
20. Toxicological evaluation of some food colours, emulsifiers,
stabilizers, anticaking agents, and certain other substances. FAO
Nutrition Meetings Report Series, No. 46A, 1970; WHO/Food Add/70.36
(out of print).
21. Specifications for the identity and purity of some food
colours, emulsifiers, stabilizers, anticaking agents, and certain
other food additives. FAO Nutrition Meetings Report Series, No.
46B, 1970; WHO/Food Add/70.37 (out of print).
22. Evaluation of food additives: specifications for the identity
and purity of food additives and their toxicological evaluation:
some extraction solvents and certain other substances; and a review
of the technological efficacy of some antimicrobial agents.
(Fourteenth report of the Joint FAO/WHO Expert Committee on Food
Additives). FAO Nutrition Meetings Report Series, No. 48, 1971; WHO
Technical Report Series, No. 462, 1971.
23. Toxicological evaluation of some extraction solvents and
certain other substances. FAO Nutrition Meetings Report Series, No.
48A, 1971; WHO/Food Add/70.39 (out of print).
24. Specifications for the identity and purity of some extraction
solvents and certain other substances. FAO Nutrition Meetings
Report Series, No. 48B, 1971; WHO/Food Add/70.40 (out of print).
25. A review of the technological efficacy of some antimicrobial
agents. FAO Nutrition Meetings Report Series, No. 48C, 1971;
WHO/Food Add/70.41 (out of print).
26. Evaluation of food additives: some enzymes, modified starches,
and certain other substances: Toxicological evaluations and
specifications and a review of the technological efficacy of some
antioxidants (Fifteenth report of the Joint FAO/WHO Expert
Committee on Food Additives). FAO Nutrition Meetings Report Series,
No. 50, 1972; WHO Technical Report Series, No. 488, 1972.
27. Toxicological evaluation of some enzymes, modified starches,
and certain other substances. FAO Nutrition Meetings Report Series,
No. 50A, 1972; WHO Food Additives Series, No. 1, 1972.
28. Specifications for the identity and purity of some enzymes and
certain other substances. FAO Nutrition Meetings Report Series, No.
50B, 1972; WHO Food Additives Series, No. 2, 1972.
29. A review of the technological efficacy of some antioxidants and
synergists. FAO Nutrition Meetings Report Series, No. 50C, 1972;
WHO Food Additives Series, No. 3, 1972.
30. Evaluation of certain food additives and the contaminants
mercury, lead, and cadmium (Sixteenth report of the Joint FAO/WHO
Expert Committee on Food Additives). FAO Nutrition Meetings Report
Series, No. 51, 1972; WHO Technical Report Series, No. 505, 1972,
and corrigendum.
31. Evaluation of mercury, lead, cadmium and the food additives
amaranth, diethylpyrocarbonate, and octyl gallate. FAO Nutrition
Meetings Report Series, No. 51A, 1972; WHO Food Additives Series,
No. 4, 1972.
32. Toxicological evaluation of certain food additives with a
review of general principles and of specifications (Seventeenth
report of the Joint FAO/WHO Expert Committee on Food Additives).
FAO Nutrition Meetings Report Series, No. 53, 1974; WHO Technical
Report Series, No. 539, 1974, and corrigendum (out of print).
33. Toxicological evaluation of some food additives including
anticaking agents, antimicrobials, antioxidants, emulsifiers, and
thickening agents. FAO Nutrition Meetings Report Series, No. 53A,
1974; WHO Food Additives Series, No. 5, 1974.
34. Specifications for identity and purity of thickening agents,
anticaking agents, antimicrobials, antioxidants and emulsifiers.
FAO Food and Nutrition Paper, No. 4, 1978.
35. Evaluation of certain food additives (Eighteenth report of the
Joint FAO/WHO Expert Committee on Food Additives). FAO Nutrition
Meetings Report Series, No. 54, 1974; WHO Technical Report Series,
No. 557, 1974, and corrigendum.
36. Toxicological evaluation of some food colours, enzymes, flavour
enhancers, thickening agents, and certain other food additives. FAO
Nutrition Meetings Report Series, No. 54A, 1975; WHO Food Additives
Series, No. 6, 1975.
37. Specifications for the identity and purity of some food colours
enhancers, thickening agents, and certain food flavour additives.
FAO Nutrition Meetings Report Series, No. 54B, 1975; WHO Food
Additives Series, No. 7, 1975.
38. Evaluation of certain food additives: some food colours,
thickening agents, smoke condensates, and certain other substances.
(Nineteenth report of the Joint FAO/WHO Expert Committee on Food
Additives). FAO Nutrition Meetings Report Series, No. 55, 1975; WHO
Technical Report Series, No. 576, 1975.
39. Toxicological evaluation of some food colours, thickening
agents, and certain other substances. FAO Nutrition Meetings Report
Series, No. 55A, 1975; WHO Food Additives Series, No. 8, 1975.
40. Specifications for the identity and purity of certain food
additives. FAO Nutrition Meetings Report Series, No. 55B, 1976; WHO
Food Additives Series, No. 9, 1976.
41. Evaluation of certain food additives (Twentieth report of the
Joint FAO/WHO Expert Committee on Food Additives). FAO Food and
Nutrition Meetings Series, No. 1, 1976; WHO Technical Report
Series, No. 599, 1976.
42. Toxicological evaluation of certain food additives. WHO Food
Additives Series, No. 10, 1976.
43. Specifications for the identity and purity of some food
additives. FAO Food and Nutrition Series, No. 1B, 1977; WHO Food
Additives Series, No. 11, 1977.
44. Evaluation of certain food additives (Twenty-first report of
the Joint FAO/WHO Expert Committee on Food Additives). WHO
Technical Report Series, No. 617, 1978.
45. Summary of toxicological data of certain food additives. WHO
Food Additives Series, No. 12, 1977.
46. Specifications for identity and purity of some food additives,
including antioxidants, food colours, thickeners, and others. FAO
Nutrition Meetings Report Series, No. 57, 1977.
47. Evaluation of certain food additives and contaminants
(Twenty-second report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 631, 1978.
48. Summary of toxicological data of certain food additives and
contaminants. WHO Food Additives Series, No. 13, 1978.
49. Specifications for the identity and purity of certain food
additives. FAO Food and Nutrition Paper, No. 7, 1978.
50. Evaluation of certain food additives (Twenty-third report of
the Joint FAO/WHO Expert Committee on Food Additives). WHO
Technical Report Series, No. 648, 1980, and corrigenda.
51. Toxicological evaluation of certain food additives. WHO Food
Additives Series, No. 14, 1980.
52. Specifications for identity and purity of food colours,
flavouring agents, and other food additives. FAO Food and Nutrition
Paper, No. 12, 1979.
53. Evaluation of certain food additives (Twenty-fourth report of
the Joint FAO/WHO Expert Committee on Food Additives). WHO
Technical Report Series, No. 653, 1980.
54. Toxicological evaluation of certain food additives. WHO Food
Additives Series, No. 15, 1980.
55. Specifications for identity and purity of food additives
(sweetening agents, emulsifying agents, and other food additives).
FAO Food and Nutrition Paper, No. 17, 1980.
56. Evaluation of certain food additives (Twenty-fifth report of
the Joint FAO/WHO Expert Committee on Food Additives). WHO
Technical Report Series, No. 669, 1981.
57. Toxicological evaluation of certain food additives. WHO Food
Additives Series, No. 16, 1981.
58. Specifications for identity and purity of food additives
(carrier solvents, emulsifiers and stabilizers, enzyme
preparations, flavouring agents, food colours, sweetening agents,
and other food additives). FAO Food and Nutrition Paper, No. 19,
1981.
59. Evaluation of certain food additives and contaminants
(Twenty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 683, 1982.
60. Toxicological evaluation of certain food additives. WHO Food
Additives Series, No. 17, 1982.
61. Specifications for the identity and purity of certain food
additives. FAO Food and Nutrition Paper, No. 25, 1982.
62. Evaluation of certain food additives and contaminants
(Twenty-seventh report of the Joint FAO/WHO Expert Committee on
Food Additives). WHO Technical Report Series, No. 696, 1983, and
corrigenda.
63. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 18, 1983.
64. Specifications for the identity and purity of certain food
additives. FAO Food and Nutrition Paper, No. 28, 1983.
65. Guide to specifications--General notices, general methods,
identification tests, test solutions, and other reference
materials. FAO Food and Nutrition Paper, No. 5, Rev. 1, 1983.
66. Evaluation of certain food additives and contaminants
(Twenty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 710, 1984, and
corrigendum.
67. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 19, 1984.
68. Specifications for the identity and purity of food colours. FAO
Food and Nutrition Paper, No. 31/1, 1984.
69. Specifications for the identity and purity of food additives.
FAO Food and nutrition Paper, No. 31/2, 1984.
70. Evaluation of certain food additives and contaminants
(Twenty-ninth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 733, 1986, and
corrigendum.
71. Specifications for the identity and purity of certain food
additives. FAO Food and nutrition Paper, No. 34, 1986.
72. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 20. Cambridge
University Press, 1987.
73. Evaluation of certain food additives and contaminants
(Thirtieth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 751, 1987.
74. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 21. Cambridge
University Press, 1987.
75. Specifications for the identity and purity of certain food
additives. FAO Food and Nutrition Paper, No. 37, 1986.
76. Principles for the safety assessment of food additives and
contaminants in food. WHO Environmental Health Criteria, No. 70.
Geneva, World Health Organization, 1987.
77. Evaluation of certain food additives and contaminants
(Thirty-first report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 759, 1987, and
corrigendum.
78. Toxicological evaluation of certain food additives. WHO Food
Additives Series, No. 22. Cambridge University Press, 1988.
79. Specifications for the identity and purity of certain food
additives. FAO Food and Nutrition Paper, No. 38, 1988.
80. Evaluation of certain veterinary drug residues in food
(Thirty-second report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 763, 1988.
81. Toxicological evaluation of certain veterinary drug residues in
food. WHO Food Additives Series, No. 23. Cambridge University
Press, 1988.
82. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition paper, No. 41, 1988 (out of print).
83. Evaluation of certain food additives and contaminants
(Thirty-third report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 776, 1989.
84. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 24. Cambridge
University Press, 1989.
85. Evaluation of certain veterinary drug residues in food
(Thirty-fourth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 788, 1989.
86. Toxicological evaluation of certain veterinary drug residues in
food. WHO Food Additives Series, No. 25, 1990.
87. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/2, 1990.
88. Evaluation of certain food additives and contaminants
(Thirty-fifth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 789, 1990, and
corrigenda.
89. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 26, 1990.
90. Specifications for identity and purity of certain food
additives. FAO Food and Nutrition Paper, No. 49, 1990.
91. Evaluation of certain veterinary drug residues in food
(Thirty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 799, 1990.
92. Toxicological evaluation of certain veterinary drug residues in
food. WHO Food Additives Series, No. 27, 1991.
93. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/3, 1991.
94. Evaluation of certain food additives and contaminants
(Thirty-seventh report of the Joint FAO/WHO Expert Committee on
Food Additives). WHO Technical Report Series, No. 806, 1991, and
corrigenda.
95. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 28, 1991.
96. Compendium of Food Additive Specifications. Joint FAO/WHO
Expert Committee on Food Additives (JECFA). Combined specifications
from 1st through the 37th Meetings, 1956-1990. FAO, 1992 (2
volumes).
97. Evaluation of certain veterinary drug residues in food
(Thirty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 815, 1991.
98. Toxicological evaluation of certain veterinary residues in
food. WHO Food Additives Series, No. 29, 1991.
99. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/4, 1991.
100. Guide to specifications - General notices, general analytical
techniques, identification tests, test solutions, and other
reference materials. FAO Food and Nutrition Paper, No. 5, Ref. 2,
1991.
101. Evaluation of certain food additives and naturally occurring
toxicants (Thirty-ninth report of the Joint FAO/WHO Expert
Committee on Food Additives). WHO Technical Report Series No. 828,
1992.
102. Toxicological evaluation of certain food additives and
naturally occurring toxicants. WHO Food Additive Series, No. 30,
1993.
103. Compendium of food additive specifications: Addendum 1. FAO
Food and Nutrition Paper, No. 52, 1992.
104. Evaluation of certain veterinary drug residues in food
(Fortieth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 832, 1993.
105. Toxicological evaluation of certain veterinary drug residues
in food. WHO Food Additives Series, No. 31, 1993.
106. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/5, 1993.
107. Evaluation of certain food additives and contaminants
(Forty-first report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 837, 1993.
108. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 32, 1993.
109. Compendium of food additive specifications, addendum 2. FAO
Food and Nutrition Paper, No. 52, Add. 2, 1993.
110. Evaluation of certain veterinary drug residues in food
(Forty-second report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 851, 1995.
111. Toxicological evaluation of certain veterinary drug residues
in food. WHO Food Additives Series, No. 33, 1994.
112. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/6, 1994.
113. Evaluation of certain veterinary drug residues in food
(Forty-third report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 855, 1995, and
corrigendum.
114. Toxicological evaluation of certain veterinary drug residues
in food. WHO Food Additives Series, No. 34, 1995.
115. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/7, 1995.
116. Evaluation of certain food additives and contaminants
(Forty-fourth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 859, 1995.
117. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 35, 1996.
118. Compendium of food additive specifications, addendum 3. FAO
Food and Nutrition Paper, No. 52, Add. 3, 1995.
119. Evaluation of certain veterinary drug residues in food
(Forty-fifth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 864, 1996.
120. Toxicological evaluation of certain veterinary drug residues
in food. WHO Food Additives Series, No. 36, 1996.
121. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/8, 1996.
122. Evaluation of certain food additives and contaminants
(Forty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 868, 1997.
123. Toxicological evaluation of certain food additives and
contaminants. WHO Food Additives Series, No. 37, 1996.
124. Compendium of food additives specifications, addendum 4. FAO
Food and Nutrition Paper, No. 52, Add. 4, 1996.
125. Evaluation of certain veterinary drug residues in food
(Forty-seventh report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, No. 876, in press.
126. Toxicological evaluation of certain veterinary drug residues
in food. WHO Food Additives Series, No. 38, 1996.
127. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/9, 1997.
128. Evaluation of certain veterinary drug residues in food
(Forty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, in press.
129. Toxicological evaluation of certain veterinary drug residues
in food. WHO Food Additives Series, No. 39, 1997.
130. Residues of some veterinary drugs in animals and foods. FAO
Food and Nutrition Paper, No. 41/10, in press.
131. Evaluation of certain food additives and contaminants
(Forty-ninth report of the Joint FAO/WHO Expert Committee on Food
Additives). WHO Technical Report Series, in preparation.
ANNEX 2
ABBREVIATIONS USED IN THE MONOGRAPHS
ADH alcohol dehydrogenase
ADI acceptable daily intake
ALD aldehyde dehydrogenase
ALDC alpha-acetolactate decarboxylase
ALT alanine transferase
AP alkaline phosphatase
ASAT aspartate aminotransferase
BHA butylated hydroxyanisole
BSP bromosulfophthalein
BUN blood urea nitrogen
bw body weight
CHO Chinese hamster ovary
DMSO dimethyl sulfoxide
fm femtomole (10-15 mole)
GC gas chromatography
GGT gamma-glutamyltranspeptidase
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
GSH glutathione-SH
Hb haemoglobin
HDL high density lipoprotein
HSH hydrogenated starch hydrolysates
Ht haematocrit
HPLC high performance liquid chromatography
i.p. intraperitoneal
JECFA Joint FAO/WHO Committee on Food Additives
LD50 median lethal dose
LDH lactate dehydrogenase
MCH mean corpuscular haemoglobin
MCHC mean corpuscular haemoglobin concentration
MCV mean corpuscular volume
MNNG N-methyl- N'-nitro- N-nitrosoguanidine
MRL maximum residue limit
MS mass spectrometry
NOEL no-observed-effect level
NR not reported
OCT ornithine carbamoyltransferase
PCV packed cell volume
RBC red blood cell
s.c. subcutaneous
SCE sister chromatid exchange
SD Sprague-Dawley
SOD superoxide dismutase
TBHQ tert-butylhydroquinone
TBQ tert-butylquinone
TOS total organic solids
UDS unscheduled DNA synthesis
VLDL very low density lipoprotein
WBC white blood cell
ANNEX 3
49TH JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES
Rome, 17-26 June 1997
Members
Mrs J. Baines, Nutritionist, Australia New Zealand Food Authority
(ANZFA), Barton, ACT, Australia (Rapporteur)
Dr Junshi Chen, Deputy Director, Institute of Nutrition and Food
Hygiene, Chinese Academy of Preventive Medicine, Beijing, China
(Vice-chairman)
Dr S.M. Dagher, Associate Professor, American University of Beirut,
Beirut, Lebanon
Dr C.E. Fisher, Head, Risk Assessment and Management Branch,
Ministry of Agriculture, Fisheries and Food (MAFF), London, England
Dr W. Grunow, Director, Professor, Head of Food Toxicology
Division, Federal Institute for Health Protection of Consumers and
Veterinary Medicine (BgVV), Berlin, Germany
Dr D.G. Hattan, Director, Division of Health Effects Evaluation,
Center for Food Safety and Applied Nutrition, Food and Drug
Administration, Washington, DC, USA (Rapporteur)
Professor J.H. Hotchkiss, Professor, Department of Food Science,
Cornell University, Ithaca, NY, USA
Dr F.N. Johnson, Project Director, Food Chemicals Codex, National
Academy of Sciences, Washington, DC, USA
Dr Y. Kawamura, Section Chief, Division of Food Additives, National
Institute of Health Sciences, Tokyo, Japan
Dr A.G.A.C. Knaap, Centre for Substances and Risk Assessment,
National Institute of Public Health and the Environment, Bilthoven,
Netherlands
Dr P. Kuznesof, Acting Deputy Director, Division of Product
Manufacture and Use, Office of Pre-Market Approval, Center for Food
Safety and Applied Nutrition, Food and Drug Administration,
Washington, DC, USA (Chairman)
Dr J. C. Larsen, Head of Department, Department of Biochemical and
Molecular Toxicology, Institute of Toxicology, Danish Veterinary
and Food Administration, Ministry of Food, Agriculture and
Fisheries, Sœborg, Denmark
Mrs I. Meyland, Senior Scientific Adviser, Danish Veterinary and
Food Administration, Ministry of Food, Agriculture and Fisheries,
Sœborg, Denmark (Rapporteur)
Mrs J. Mutamba, Deputy Director, Department of Nutrition, Ministry
of Health, Harare, Zimbabwe
Dr A. M. Pacin, Researcher, Scientific Research Commission of
Buenos Aires Province, Lujan, Argentina
Dr G. Pascal, Chairman of the Scientific Committee for Food of the
European Union, National Centre for Scientific Research, Ministry
of Higher Education and Research, Paris, France
Dr B. Petersen, President, Novigen Sciences Inc., Washington, DC,
USA
Mrs M. Riordan, Senior Advisor, Food and Nutrition Section Ministry
of Health, Wellington, New Zealand
Dr P.J.P. Verger, Director, Foch Research Centre, University René
Descartes-Paris V, Paris, France
Dr R. Vilu, Professor of Biochemistry, Department of Biochemistry,
Tallinn Technical University, Tallinn, Estonia
Professor R. Walker, Professor of Food Science, School of
Biological Sciences, University of Surrey, Guildford, Surrey,
England (Vice-chairman)
Mrs H. Wallin, Senior Research Scientist, VTT Biotechnology and
Food Research, Espoo, Finland
Dr D. B. Whitehouse, Bowdon, Cheshire, England
Dr J. Wilson, Senior Fellow, Center for Risk Management, Resources
for the Future, Washington, DC, USA
Secretariat
Dr P.J. Abbott, Australia New Zealand Food Authority (ANZFA),
Canberra, ACT, Australia (WHO Temporary Adviser)
Dr H. Blumenthal, Silver Spring, MD, USA WHO (WHO Temporary
Adviser)
Dr X. Bosch, Chief, Department of Epidemiology and Cancer Registry,
Hospital Duran i Reynals, Barcelona, Spain (WHO Temporary
Adviser)
Dr R. Clarke, Agroindustries and Post-Harvest Management Service,
Agricultural Support Systems Division, Food and Agriculture
Organization of the United Nations, Rome, Italy
Dr J. Greig, Department of Health, London, England (WHO Temporary
Adviser)
Dr J. Gry, Institute of Toxicology, Danish Veterinary and Food
Administration, Ministry of Food, Agriculture and Fisheries,
Sœborg, Denmark (WHO Temporary Adviser)
Mr E.F.F. Hecker, Chairman, Codex Committee on Food Additives and
Contaminants and Senior Coordinator Risk, Substances and Nutrition
Division, Department of Environment, Quality and Health, Ministry
of Agriculture, Nature Management & Fisheries The Hague,
Netherlands (WHO Temporary Adviser)
Dr S. Henry, Office of Plant and Dairy Foods and Beverages (HFS-
308), Center for Food Safety and Applied Nutrition, US Food and
Drug Administration, Washington, DC, USA (WHO Temporary Adviser)
Dr J.L. Herrman, Assessment of Risk and Methodologies,
International Programme on Chemical Safety , World Health
Organization, Geneva, Switzerland (Joint Secretary)
Dr P.G. Jenkins, Editor, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (editor)
Professor R. Kroes, Director, Research Institute Technology
(RITOX), Utrech University, Utrecht, Netherlands (WHO Temporary
Adviser)
Dr J. Lupton, Professor, Faculty of Nutrition, Texas A&M
University, College Station, TX, USA (WHO Temporary Adviser)
Dr A. Mattia, Division of Product Policy, Office of PreMarket
Approval (HFS-206), Center for Food Safety and Applied Nutrition,
US Food and Drug Administration, Washington, DC, USA (WHO
Temporary Adviser)
Dr G. Moy, Division of Food and Nutrition, Food Safety, World
Health Organization, Geneva, Switzerland
Dr I.C. Munro, CanTox Inc., Mississauga, Ontario, Canada (WHO
Temporary Adviser)
Dr A. Nishikawa, Division of Pathology, Biological Safety Research
Center, National Institute of Health Sciences, Kamiyoga, Setagaya,
Tokyo, Japan (WHO Temporary Adviser)
Dr J. Paakkanen, Nutrition Officer, Food and Nutrition Division,
Food and Agriculture Organization of the United Nations, Rome,
Italy (Joint Secretary)
Dr C.J. Portier, Chief, Laboratory of Computal Biology and Risk
Analysis, Division of Intramural Research, National Institute of
Environmental Health Sciences, Research Triangle Park, NC, USA
(WHO Temporary Adviser)
Dr A.G. Renwick, Clinical Pharmacology Group, University of
Southampton, Medical & Biological Sciences Building, Southampton,
UK (WHO Temporary Adviser)
Dr G. Semino, Institute of Pharmacological Sciences, University of
Milan, Milano, Italy (WHO Temporary Adviser)
Professor P. Shubik, Green College, Oxford, UK (WHO Temporary
Adviser)
Dr G.J.A. Speijers, Head of the Section Public Health of the Centre
for Substances and Risk Assessment, National Institute of Public
Health and Environmental Protection, Bilthoven, Netherlands
(WHO Temporary Adviser)
Ms E. Vavasour, Chemical Health Hazard Assessment Division, Bureau
of Chemical Safety, Food Directorate, Health Protection Branch,
Health Canada, Ottawa, Ontario, Canada (WHO Temporary Adviser)
ANNEX 4
Acceptable Daily Intakes, other toxicological information, and
information on specifications
1. Food additives and food ingredients
Substance Specifications1 Acceptable Daily
Intake (ADI) and other
toxicological
recommendations
Antioxidant
tert-Butylhydroquinone R 0-0.7 mg/kg bw
(TBHQ)
Emulsifiers
Microcrystalline cellulose R ADI "not specified"2
Sucrose esters of fatty acids R ) 0-30 mg/kg bw3
and sucroglycerides R )
Enzyme preparations
alpha-Acetolactate decarboxylase N,T Temporary ADI "not
specified"4
Maltogenic amylase N,T Temporary ADI "not
specified"4
Flavouring agent
rans-Anethole R 0-0.6 mg/kg bw5
Glazing agent
Hydrogenated poly-1-decene N No ADI allocated6
Sweetening agent
Maltitol syrup R ADI "not specified"2
Miscellaneous substance
Salatrim (short- and long-chain N An adequate basis was
acyl triglyceride molecules) not available for
evaluating its safety
and nutritional
effects7
1 N, new specfications prepared; O, no specifications prepared; R,
existing specifications revised; S, specifications exist, revision not
considered or required; T, the existing, new or revised specifications
are tentative and comments are invited; W, existing specifications
withdrawn; NR, specifications not reviewed.
2 Applies to the product conforming to the revised specifications.
3 Group ADI for sucrose esters of fatty acids and sucroglycerides.
4 Temporary pending consideration of the "tentative" designation
for the specifications. The "tentative" designation for Appendix B to
Annex 1 (general specifications for enzyme preparations used in food
processing) of the Compendium of food additive specifications (1992)
will be reviewed in 1998.
5 Temporary ADI extended to 1998 to review studies underway that
were requested by earlier Committees.
6 Data were insufficient for establishing an ADI.
7 The Committee recommended that additional appropriately designed
studies be performed to assess fully both the toxicological and
nutritional consequences of ingestion of salatrim.
2. Substances evaluated using the Procedure for the Safety Evaluation
of Flavouring Agents
A. Flavouring agents evaluated toxicologically
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0021 Allyl 2-furoate N,T No safety concern
Saturated aliphatic acyclic
linear primary alcohols,
aldehydes, and acids
0079 Formic acid R )
0080 Acetaldehyde N )
0081 Acetic acid R )No safety concern
0082 Propyl alcohol R )
0083 Propionaldehyde N )
0084 Propionic acid N )
0085 Butyl alcohol R )
0086 Butyraldehyde N )No safety concern
0087 Butyric acid N )
0088 Amyl alcohol N )
0089 Valeraldehyde N )
0090 Valeric acid N )
0091 Hexyl alcohol N )No safety concern
0092 Hexanal N )
0093 Hexanoic acid N )
0094 Heptyl alcohol N )
0095 Heptanal N )
0096 Heptanoic acid N )No safety concern
0097 1-Octanol N )
0098 Octanal R )
0099 Octanoic acid N )
0100 Nonyl alcohol N )
0101 Nonanal R )No safety concern
0102 Nonanoic acid N )
0103 1-Decanol N )
0104 Decanal N )
0105 Decanoic acid N )
0106 Undecyl alcohol N )No safety concern
0107 Undecanal N )
0108 Undecanoic acid N )
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0109 Lauryl alcohol N )
0110 Lauric aldehyde N )
0111 Lauric acid N )No safety concern
0112 Myristaldehyde N )
0113 Myristic acid N )
0114 1-Hexadecanol N )
0115 Palmitic acid N )No safety concern
0116 Stearic acid N )
Saturated aliphatic acyclic
branched-chain primary alcohols,
aldehydes, and acids
A. Structural class I
flavouring agents:
0251 Isobutyl alcohol NR )
0252 Isobutyraldehyde NR )
0253 Isobutyric acid NR )No safety concern
0254 2-Methylbutyraldehyde NR )
0255 2-Methylbutyric acid NR )
0258 3-Methylbutyraldehyde NR )
0259 Isovaleric acid NR )
0260 2-Methylpentanal NR )No safety concern
0261 2-Methylvaleric acid NR )
0262 3-Methylpentanoic acid NR )
0263 3-Methyl-1-pentanol NR )
0264 4-Methylpentanoic acid NR )
0265 2-Methylhexanoic acid NR )No safety concern
0266 5-Methylhexanoic acid NR )
0268 3,5,5-Trimethyl-1-hexanol NR )
0269 3,5,5-Trimethylhexanal NR
0270 2-Methyloctanal NR
0271 4-Methyloctanoic acid NR
0272 3,7-Dimethyl-1-octanol NR
0273 2,6-Dimethyloctanal NR
0274 4-Methylnonanoic acid NR )
0275 2-Methylundecanal NR )No safety concern
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
B. Structural class II
flavouring agents:
0256 2-Ethylbutyraldehyde NR )
0257 2-Ethylbutyric acid NR )No safety concern
0267 2-Ethyl-1-hexanol NR )
Aliphatic lactones
A. Structural class I
flavouring agents:
0219 4-Hydroxybutyric acid lactone
(gamma-butyrolactone) NR )
0220 gamma-Valerolactone NR )
0223 gamma-Hexalactone NR )No safety concern
0224 delta-Hexalactone NR )
0225 gamma-Heptalactone NR )
0226 gamma-Octalactone NR )
0228 delta-Octalactone NR )
0229 gamma-Nonalactone NR )No safety concern
0230 Hydroxynonanoic acid
delta-lactone NR )
0231 gamma-Decalactone NR )
0232 delta-Decalactone NR )
0241 epsilon-Decalactone NR )
0233 gamma-Undecalactone NR )No safety concern
0234 5-Hydroxyundecanoic acid
delta-lactone NR )
0235 gamma-Dodecalactone NR )
0236 delta-Dodecalactone NR )
0242 epsilon-Dodecalactone NR )
0238 delta-Tetradecalactone NR )No safety concern
0239 omega-Pentadecalactone NR )
0221 4-Hydroxy-3-pentenoic
acid lactone NR )
0247 5-Hydroxy-7-decenoic acid
delta-lactone NR )
0248 5-Hydroxy-8-undecenoic acid
delta-lactone NR )
0249 1,4-Dodec-6-enolactone NR )No safety concern
0240 omega-6-Hexadecenlactone NR )
0227 4,4,-Dibutyl-gamma-
butyrolactone NR )
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0244 3-Heptyldihydro-5-methyl-
2(3H)-furanone NR )
---- 4-Hydroxy-3-methyloctanoic
acid gamma-lactone NR )No safety concern
0237 6-Hydroxy-3,7-dimethyloctanoic
acid lactone NR )
0250 gamma-Methyldecalactone NR )
B. Structural class III
flavouring agents;
0246 5-Hydroxy-2-decenoic acid
delta-lactone NR )
0245 5-Hydroxy-2,4-decadienoic
acid delta-lactone NR )Not evaluated;
--- Mixture of 5-hydroxy-2- evaluation
decenoic acid delta-lactone, deferred pending
5-hydroxy-2-dodecenoic acid consideration of
delta-lactone, and 5-hydroxy- other alpha, beta-
2-tetradecenoic acid unsaturated
delta-lactone NR )compounds
--- 5-Hydroxy-2-dodecenoic
acid delta-lactone NR )
0222 5-Ethyl-3-hydroxy-4-methyl-
2(5H)-furanone NR )No safety concern
0243 4,5-Dimethyl-3-hydroxy-2,5-
dihydrofuran-2-one NR )
Esters of aliphatic acyclic primary
alcohols with branched-chain
aliphatic acyclic acids
0185 Methyl isobutyrate N )
0186 Ethyl isobutyrate N )
0187 Propyl isobutyrate N )No safety concern
0188 Butyl isobutyrate N )
0189 Hexyl isobutyrate N )
0190 Heptyl isobutyrate N )
0191 trans-3-Heptenyl
2-methylpropanoate N )
0192 Octyl isobutyrate N )No safety concern
0193 Dodecyl isobutyrate N )
0194 Isobutyl isobutyrate N )
0195 Methyl isovalerate N )
0196 Ethyl isovalerate R )
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0197 Propyl isovalerate N )
0198 Butyl isovalerate N )No safety concern
0199 Hexyl 3-methylbutanoate N )
0200 Octyl isovalerate N )
0201 Nonyl isovalerate N )
0202 3-Hexenyl 3-methylbutanoate N )No safety concern
0203 2-Methylpropyl 3-
methylbutyrate N )
0204 2-Methylbutyl 3-
methylbutanoate N,T )
0205 Methyl 2-methylbutyrate N )
0206 Ethyl 2-methylbutyrate N )
0207 n-Butyl 2-methylbutyrate N )No safety concern
0208 Hexyl 2-methylbutanoate N )
0209 Octyl 2-methylbutyrate N )
0210 Isopropyl 2-methylbutyrate N,T )
0211 3-Hexenyl 2-methylbutanoate N,T )
0212 2-Methylbutyl 2-
methylbutyrate N )No safety concern
0213 Methyl 2-methylpentanoate N,T )
0214 Ethyl 2-methylpentanoate N )
0215 Ethyl 3-methylpentanoate N,T )No safety concern
0216 Methyl 4-methylvalerate N )
Esters of aliphatic acyclic
primary alcohols with aliphatic
linear saturated carboxylic acids
0117 Propyl formate N )
0118 Butyl formate N )
0119 n-Amyl formate N )No safety concern
0120 Hexyl formate N )
0121 Heptyl formate N,T )
0122 Octyl formate N )
0123 cis-3-Hexenyl formate N )
0124 Isobutyl formate N )No safety concern
0125 Methyl acetate N )
0126 Propyl acetate N )
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0127 Butyl acetate R )
0128 Hexyl acetate N )
0129 Heptyl acetate N )No safety concern
0130 Octyl acetate N )
0131 Nonyl acetate N )
0132 Decyl acetate N )
0133 Lauryl acetate N )
0134 cis-3-Hexenyl acetate N )
0135 trans-3-Heptenyl acetate N )
0136 10-Undecen-1-yl acetate N )No safety concern
0137 Isobutyl acetate N )
0138 2-Methylbutyl acetate N )
0140 2-Ethylbutyl acetate N,T )
0141 Methyl propionate N )
0142 Propyl propionate N )
0143 Butyl propionate N )
0144 Hexyl propionate N )No safety concern
0145 Octyl propionate N )
0146 Decyl propionate N )
0147 cis-3- & trans-2-Hexenyl
propionate N Postponed2
0148 Isobutyl propionate N )
0149 Methyl butyrate N )
0150 Propyl butyrate N )No safety concern
0151 Butyl butyrate N )
0152 n-Amyl butyrate N )
0153 Hexyl butyrate N )
0154 Heptyl butyrate N,T )
0155 Octyl butyrate N,T )No safety concern
0156 Decyl butyrate N,T )
0157 cis-3-Hexenyl butyrate N )
0158 Isobutyl butyrate N )
0159 Methyl valerate N )
0160 Butyl valerate N )No safety concern
0161 Propyl hexanoate N )
0162 Butyl hexanoate N )
0163 n-Amyl hexanoate N )
0164 Hexyl hexanoate N )
0165 cis-3-Hexenyl hexanoate N,T )No safety concern
0166 Isobutyl hexanoate N,T )
0167 Methyl heptanoate N )
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0168 Propyl heptanoate N,T )
0169 Butyl heptanoate N,T )
0170 n-Amyl heptanoate N )No safety concern
0171 Octyl heptanoate N,T )
0172 Isobutyl heptanoate N,T )
0173 Methyl octanoate N )
0174 n-Amyl octanoate N )
0175 Hexyl octanoate N )No safety concern
0176 Heptyl octanoate N,T )
0177 Octyl octanoate N,T )
0178 Nonyl octanoate N,T )
0179 Methyl nonanoate N )
0180 Methyl laurate N )No safety concern
0181 Butyl laurate N )
0182 Isoamyl laurate N,T )
0183 Methyl myristate N )No safety concern
0184 Butyl stearate N,T )
Esters derived from branched-chain
terpenoid alcohols and aliphatic
acyclic carboxylic acids
0053 Citronellyl formate N )
0054 Geranyl formate N )
0055 Neryl formate N )No safety concern
0056 Rhodinyl formate N )
0057 Citronellyl acetate N )
0058 Geranyl acetate R )
0059 Neryl acetate N )
0060 Rhodinyl acetate N )No safety concern
0061 Citronellyl propionate N )
0062 Geranyl propionate N )
0063 Neryl propionate N )
0064 Rhodinyl propionate N,T )
0065 Citronellyl butyrate N )No safety concern
0066 Geranyl butyrate N )
0067 Neryl butyrate N )
No. Flavouring agent Specifications1 Conclusion based
on current levels
of intake
0068 Rhodinyl butyrate N )
0069 Citronellyl valerate N,T )
0070 Geranyl hexanoate N,T )No safety concern
0071 Citronellyl isobutyrate N )
0072 Geranyl isobutyrate N,T )
0073 Neryl isobutyrate N )
0074 Rhodinyl isobutyrate N )
0075 Geranyl isovalerate N,T )No safety concern
0076 Neryl isovalerate N )
0077 Rhodinyl isovalerate N,T )
0078 3,7-Dimethyl-2,6-octadien-
1-yl 2-ethylbutanoate N,T No safety concern
B. Flavouring agent considered for specifications only
No. Flavouring agent Specifications1
0218 Citric acid R
3. Contaminants
Contaminant Conclusions
Aflatoxins B, G, and M Potencies were estimated as
described in the report
4. Food additives considered for specifications only
Food additive Specifications
Agar R
Alginic acid R
Aluminium powder R
Ammonium alginate R
Anoxomer W
Food additive Specifications
Calcium alginate R
Calcium propionate R
Carbon dioxide R
Carthamus red R,T
Carthamus yellow R
Citric acid R
Diacetyltartaric and fatty
acid esters of glycerol (DATEM) R
Enzyme-hydrolyzed
carboxymethyl cellulose N,T
Enzyme-treated starches W
Ethyl hydroxyethyl cellulose R
Gellan gum R
Gum arabic R
Isoamyl acetate (amyl acetate) R
Microcrystalline wax R
Mixed carotenoids R
Modified starches R
Petroleum jelly R,T
Potassium alginate R
Potassium propionate R
Propionic acid R,T
Propylene glycol R
Propylene glycol alginate R
Propylene glycol esters of
fatty acids R
Sodium alginate R
Sodium propionate R
Sulfur dioxide R
Talc R
Tartaric, acetic and fatty acid
esters of glycerol, mixed S,T
Turmeric W
1 N, new specifications prepared; O, no specifications prepared; R,
existing specifications revised; S, specifications exist, revision not
considered or not required; T, the existing, new or revised
specifications are tentative and comments are invited; W, existing
specifications withdrawn; NR, specifications not reviewed.
2 Evaluation deferred pending consideration of other alpha, beta-
unsaturated carbonyl compounds. One of the predicted main metabolites
of trans-2-hexenyl propionate is trans-2-hexenol, which should be
oxidized to the alpha, beta-unsaturated substance, trans-2-hexenal.
ANNEX 5
APPLICATION OF A THRESHOLD OF TOXICOLOGICAL
CONCERN IN THE SAFETY EVALUATION OF
CERTAIN FLAVOURING SUBSTANCES
Ian C. Munro, PhD, FRCPath
CanTox Inc.
Mississauga, Ontario
Canada
Professor Robert Kroes
Prins Hendriklaan 63,
3721 AP Bilthoven
The Netherlands
This paper was considered at the forty-ninth meeting of the Joint
FAO/WHO Expert Committee on Food Additives. The conclusions of the
Committee relating to its consideration of the paper are provided in
the report, which has been published in the WHO Technical Report
Series. The opinions expressed herein are those of the authors.
ANNEX 5
APPLICATION OF A THRESHOLD OF TOXICOLOGICAL CONCERN IN
THE SAFETY EVALUATION OF CERTAIN FLAVOURING SUBSTANCES
Table of Contents
1. EXPLANATION
2. THE CONCEPT OF A THRESHOLD OF TOXICOLOGICAL CONCERN AND ITS
APPLICATION IN SAFETY EVALUATION OF FLAVOURING SUBSTANCES
2.1 The practical problem
2.2 Concepts in the derivation and application of a Threshold
Value
2.3 The scientific basis for the Threshold Value of 1.5 µg/person
per day specified at Step B5
3. APPLICATION OF A THRESHOLD OF TOXICOLOGICAL CONCERN TO FLAVOURING
SUBSTANCES
3.1 Additional factors that reduce the theoretical risk
4. SUMMARY
5. REFERENCES
APPENDIX I
1. EXPLANATION
At its forty-sixth meeting (JECFA, 1997) the Joint FAO/WHO Expert
Committee on Food Additives (JECFA) further considered the proposed
safety evaluation procedure for flavouring substances published in
Annex 5 of WHO Food Additives Series 35 (JECFA, 1996) "A Procedure for
the Safety Evaluation of Flavouring Substances" and applied this
procedure to the evaluation of 47 flavouring substances. The Committee
noted that one step in the safety evaluation procedure (Step B5;
Figure 1 (the steps have been renumbered since the original
publications)) involved the application of a decision criterion, based
on the concept of a threshold of toxicological concern, to flavouring
substances lacking sufficient toxicity or metabolic data to be
evaluated using other steps in the procedure. This decision criterion
is intended to be applied to flavouring agents that lack such data but
that have a daily intake equal to or less than the threshold intake
value of 1.5 µg/person per day specified at Step B5. At the
forty-sixth meeting, one substance, allyl-2-furoate, which lacked
sufficient hydrolysis and toxicity data and was thus eligible for
evaluation using Step B5, was not evaluated. The Committee deferred
application of the decision criterion pending a complete evaluation of
the scientific basis for the 1.5 µg/person per day threshold value.
The Committee recommended that the scientific issues involved in the
development and application of Step B5 of the safety evaluation
procedure be considered as soon as possible and that allyl-2-furoate
be considered for evaluation using this step.
2. THE CONCEPT OF A THRESHOLD OF TOXICOLOGICAL CONCERN AND
ITS APPLICATION IN SAFETY EVALUATION OF FLAVOURING SUBSTANCES
2.1 The practical problem
There are approximately 2500 chemically defined flavouring substances
in use in Europe and the USA. Of these substances, approximately 1500
have been evaluated by FEMA's Expert Panel and are recognized by the
US Food and Drug Administration (FDA) to be Generally Recognized As
Safe (GRAS) substances, meaning they are considered safe for their
intended use. Without exception, flavouring substances are volatile
organic chemicals. The vast majority of flavouring ingredients have
simple, well-characterized structures with a single functional group
and low relative molecular mass (<300 g/mol). More than 700 of the
1323 chemically defined flavouring substances used in food in the USA
are simple aliphatic acyclic and alicyclic alcohols, aldehydes,
ketones, carboxylic acids and related esters, lactones, ketals, and
acetals. Other structural categories include aromatic (e.g.,
cinnamaldehydes and anthranilates), heteroaromatic (e.g., pyrazines
and pyrroles) and heterocyclic (e.g., furanones and alicyclic
sulfides) substances with characteristic organoleptic properties. For
most flavouring substances, the structural differences within chemical
classes are small. Incremental changes in carbon chain length and the
position of a functional group or hydrocarbon chain typically describe
the structural variation in groups of related flavouring substances.
�Figure 1;V040JE14.BMP
These systematic changes in structure provide the basis for
understanding the effect of structure on the chemical and biological
properties of a substance.
Within structural groups of flavouring substances, many substances
have considerable toxicology data; subchronic studies exist for many
substances or their metabolic products, and several representative
members of structural groups have chronic toxicity studies. At the
forty-sixth meeting, the Committee was able to use information on
metabolism, toxicity and intake on individual substances within a
group to conclude, using all but Steps B5 of the safety evaluation
procedure outlined in Figure 1, that esters of ethyl alcohol and
isoamyl alcohol do not pose a safety concern at current levels of
intake. In addition, the Committee considered that the allyl ester
flavouring substances (with the exception of allyl-2-furoate which was
not evaluated) likewise do not pose a safety concern. Allyl-2-furoate
is one of a few flavouring substances currently in commercial use that
lacks toxicity and metabolic data and, because the Committee expressed
the view that evidence also was lacking for its rapid and complete
hydrolysis to allyl alcohol and furoic acid, it was not evaluated at
Step B4 along with the other allyl esters. However, allyl-2-furoate is
a candidate substance for consideration using Step B5 because it has
an exposure well below the 1.5 µg/person per day decision criterion
specified at Step B5 of the safety evaluation procedure. This paper
elaborates the scientific principles underpinning the decision
criterion specified at Step B5 and discusses the implications of
applying the threshold of toxicological concern concept to the safety
evaluation of flavouring substances.
2.2 Concepts in the derivation and application of a threshold value
With the possible exception of so-called genotoxic carcinogens,
the concept of a threshold in toxicological responses is universally
accepted and endorsed by the World Health Organization (IPCS, 1987,
1994). In fact, the threshold concept forms the basis for the process
of establishing Acceptable Daily Intakes (ADIs) that carry with them
no appreciable risk to human health (IPCS, 1987; SCF, 1996). The idea
that a generic threshold value or range of values might be established
that would preclude the need for toxicity data on chemicals having
human intakes below these thresholds was proposed over 30 years ago by
Frawley (1967). This approach provides an attractive alternative to
the conventional regulatory philosophy of rigorously testing each new
chemical substance regardless of expense or level of human exposure.
Two factors, pragmatism and scientific knowledge, have influenced the
evolution of the threshold concept. The scientific information base is
now sufficiently mature to consider application of a threshold of
toxicological concern as a concept that is both practical and
scientifically defensible. On a purely pragmatic level, it is
recognized that humans are exposed to thousands of substances through
the food supply and the number of substances increases logarithmically
with declining concentration (Hall, 1975).
It is neither practical nor scientifically defensible to test all
these substances by conventional toxicological procedures, and to
insist this be done would create a resource problem of immense
proportions. Another more important factor that justifies an approach
using a threshold of toxicological concern, is that in the past 10 to
15 years a great deal of knowledge has accumulated about the potential
human risks from chemicals in general and especially for those which
are carcinogenic. On the basis of accumulated knowledge, it is
theoretically possible to establish a range of threshold values
representing the full spectrum of toxicological end-points including
both carcinogenic and non-carcinogenic effects. In regard to
non-carcinogenic responses, Munro et al. (1996) have developed a
database of over 600 chemicals arranged into three chemical structural
groups from which they were able to plot the distribution of
no-observed-effect levels (NOELs) and estimate the 5th centile NOELs
for each structural class. From these 5th centile NOELs, generic human
exposure thresholds were calculated for each of the structural
classes. It was proposed by Munro et al. (1996) that these human
exposure threshold values could be used along with data on intake,
metabolism and, as required, toxicity data, to evaluate the safety of
flavouring substances. This procedure is outlined in the report of the
forty-fourth meeting of JECFA (JECFA, 1995) and was further discussed
and applied to the safety evaluation of flavours during the
forty-sixth meeting of the Committee (JECFA, 1997). Step B5 of the
safety evaluation procedure proposes the application of a threshold
value of 1.5 µg/person per day which is much lower than the threshold
values developed by Munro et al. (1996). Table 1 presents the 5th
centile NOELs and corresponding human exposure thresholds that were
derived from the Munro et al. (1996) database for the three
structural classes referred to in Annex 5 to WHO Food Additives Series
35 (JECFA, 1996). It should be noted that the 1.5 µg/person per day
threshold value is 60 times lower than the human exposure threshold
for structural Class III, the class with the highest presumptive
toxicity. That is because the 1.5 µg/person per day threshold value,
proposed at Step B5, is derived from the distribution of risks for
carcinogenic chemicals calculated using linear extrapolation models.
This methodology is considerably more conservative than that used to
derive the human exposure threshold values for non-carcinogenic
end-points (see footnote to Table 1). Since Step B5 is intended to be
applied to substances that lack adequate toxicity and metabolic data
to be evaluated at previous steps in the safety evaluation procedure,
the additional conservatism used in selecting this lower value is
justified.
Because of concerns, raised by others (SCF, 1996), that the 5th
centile NOELs for specific toxicological end-points such as
reproductive effects, neurotoxicity and immunotoxicity might result in
human exposure thresholds lower than 1.5 µg/person per day, this
matter was examined. Table 1 presents the NOELs and corresponding
human exposure thresholds for 100 substances reported in the RTECS
database (RTECS, 1987) to cause developmental abnormalities. The human
exposure threshold for this group of substances is 2076 µg/person per
day, 1384 times higher than the 1.5 µg/person per day value. In
addition, the 5th centile NOEL for 31 neurotoxic organophosphate
insecticides included in structural class III of the Munro et al.
(1996) database produced a corresponding human exposure threshold of
18 µg/person per day, 12 times greater than the threshold value
proposed at Step B5. It might be expected that such neurotoxic
compounds would have a low human exposure threshold because they are
specifically designed to be highly potent toxins. Moreover, the
measure of neurotoxicity selected to establish the NOELs in most cases
was cholinesterase inhibition, an extremely sensitive end-point. Most
importantly, organophosphates would not be used as flavouring
substances. A list of the 100 substances reported to cause
developmental abnormalities and of the 31 neurotoxic organophosphates,
along with their NOELs, is given in Annex I.
Table 1. Comparison of various human exposure threshold values
Category 5th Centile NOEL Human exposure threshold1
(mg/kg bw/day) (µg/person/day)
Structural Class I 3.0 1800
Structural Class II 0.91 540
Structural Class III 0.15 90
Developmental
abnormalities2 3.46 2076
Neurotoxic compounds3 0.03 18
Step B5 Decision Criterion4 1.5 µg/person/day
1 The human exposure threshold was calculated by multiplying the
5th centile NOEL by 60 (assuming a 60 kg individual) dividing by a
safety factor of 100, and multiplying by 1000 to convert from
milligrams to micrograms. (Munro et al., 1996)
2 Substances are from the RTECS database and were indicated to
cause developmental abnormalities. The NOELs were presented by
RTECs as the TDLo which is defined as the lowest dose of a
substance reported to produce any non-significant adverse effects.
(RTECS, 1987)
3 For organophosphates, end-point measured was typically
cholinesterase inhibition.
4 See text for details.
For the evaluation of the sensitivity of immunotoxicity as an
end-point, the data of Luster et al. (1992, 1993) were used to
conduct a comparison of NOELs and LOELs based on immunotoxic
end-points with corresponding NOELs and LOELs based on non-immunotoxic
end-points. Twenty-four substances meeting the criteria of
immunotoxicity used by Luster et al. (1992, 1993) were identified
that also had corresponding non-immunotoxic end-point NOELs or LOELs.
A list of these substances is provided in Appendix I. Six of these
substances had immunotoxic NOELs with corresponding non-immunotoxic
NOELs. Twelve of these substances had no immunotoxic NOELs but had
immunotoxic LOELs with corresponding non-immunotoxic LOELs. Five
additional substances had immunotoxic NOELs with corresponding
non-immunotoxic LOELs and one substance had an immunotoxic LOEL with a
corresponding non-immunotoxic NOEL. For these last 6 substances, NOELs
were compared with LOELs divided by a conservative factor of 10 to
adjust for differences between NOELs and LOELs (Dourson et al.,
1996). For example, tetraethyl lead has an immune end-point NOEL of
0.5 mg/kg bw per day and a non-immune end-point LOEL of 0.0012 mg/kg
bw per day. The LOEL was divided by 10 (0.00012 mg/kg bw per day) for
comparison with the NOEL. In order to perform the comparison of
immunotoxic end-point sensitivity with non-immunotoxic end-point
sensitivity, the immunotoxic NOEL/LOEL was divided by the
corresponding non-immunotoxic NOEL/LOEL resulting in a ratio. The
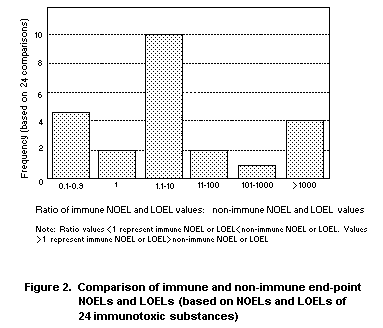
resulting ratios of the 24 comparisons are shown graphically in Figure
2. The majority of the substances (17/24) had non-immunotoxic NOELs or
LOELs that were lower (i.e., more sensitive) than the corresponding
immunotoxic NOELs or LOELs. Two substances had similar NOELS/LOELS and
5 substances had immunotoxic NOELs or LOELs that were less than
10-fold lower than their non-immuntoxic counterparts. These data
demonstrate that immunotoxicity is not a more sensitive end-point than
other forms of toxicity.
Overall, it may be considered that the threshold of toxicological
concern concept is highly conservative since the decision criterion of
1.5 µg/person per day, shown in Table 1, is 1200-fold below the 5th
centile NOEL for the most toxic threshold NOEL (for neurotoxic
chemicals) and 6000 times lower than the 5th centile for Class III
substances.
 2.3 The scientific basis for the threshold value of 1.5
µg/person per day specified at Step B5
Over the past several years, an immense amount of information has
accumulated on the range of carcinogenic potencies for chemicals that
have been tested in animals. For these chemicals, the distribution of
potencies in experimental animals and projected human risk (calculated
using linear risk assessment models) are well established and highly
unlikely to be altered by further cancer bioassays (Krewski et al.,
1990). In fact, the Carcinogenic Potency Database (CPD) compiled by
Gold et al. (1984) that was used to derive the decision criterion of
1.5 µg per day specified at Step B5, now contains nearly 500
substances reported to be carcinogenic in animals. It is reasonable to
assume that the addition to that data-base of several more "genotoxic"
carcinogens, should they be discovered, would be unlikely to alter the
distribution of known risks for identified carcinogens. Scientists may
never be prepared to say they know all they would like to know about
the distribution of risks of existing animal carcinogens. On the other
hand, it can be estimated with considerable confidence, based on data
available today, that a substance which has not been tested for
carcinogenicity and that is consumed in an amount below the threshold
value specified at Step B5 will not present a risk of human cancer
greater than one in one million (10-6).
With these thoughts in mind, it is now important to look at the
theoretical and practical aspects of the concept of threshold of
concern and how it can be applied to flavouring substances in the
context of JECFA safety evaluations.
The Carcinogen Potency Database (CPD) contains data on
approximately 3700 long-term animal studies of 975 chemicals (Gold
et al. 1986a,b,c; 1989). These include studies conducted by the US
National Toxicology Program as well as studies conducted in other
laboratories that have been published in the literature. Of the 975
chemicals tested, 955 were tested in rats and/or mice and 492 produced
an increase in tumour incidence (342 in rats and 278 in mice). Gold
et al. have put an enormous effort into compiling this database and
ensuring its quality. The reader is referred to a series of papers by
Gold et al. and others, published in Environmental Health
Perspectives, which document the validity of this database (Gold
et al., 1984, 1986a,b,c, 1989; Peto et al., 1984; Sawyer et
al., 1984).
For each compound, the CPD may include experiments with different
species, strains, sexes, dosing regimens, routes of administration, or
other experimental conditions (Gold et al., 1984). In most
experiments, two or more dose levels were used in addition to an
unexposed control; in some cases, however, only a single exposed group
was employed. Although a rigorous evaluation of the quality of
individual experiments is not possible, the CPD does include
information on the original investigators' conclusions regarding the
overall strength of evidence for carcinogenicity.
The CPD also contains a measure of carcinogenic potency (the
TD50) computed as described by Peto et al. (1984) and Sawyer
et al. (1984). In order to obtain some degree of comparability among
different studies, all TD50 values are expressed in units of mg/kg bw
per day and are adjusted to a 2-year standard rodent lifetime. In
cases where exposure is not constant throughout the study period, a
time-weighted average dose is used for purposes of modelling
dose-response. When individual animal data are available, the TD50
values are adjusted for intercurrent mortality; otherwise, the crude
proportions of animals with tumours are used to estimate carcinogenic
potency without adjusting for mortality from other causes. Finally,
the TD50 is estimated on the basis of an essentially linear one-hit
dose-response model.
The CPD represents an extremely useful source of information on
experiments with chemical carcinogens. The database includes
experiments on highly potent rodent carcinogens such as
2,3,7,8-tetrachlorodibenzo- p-dioxin and aflatoxin B1, as well as
less potent agents such as metronidazole and DDT. Gold et al. (1984)
noted that TD50 values included in the CPD vary by 10 million-fold.
Rulis (1986) used the Gold et al. (1984, 1986a,b,c, 1989)
database when he and others at FDA derived a threshold of regulation
for packaging materials. What was done, in essence, was to transform
the distribution of lowest TD50 for each carcinogen that was tested
by the oral route to a distribution of 10-6 risks. While numerous
mathematical models including the linearized multistage model could
have been used to perform this transformation, Rulis (1986) used the
slope (2TD50)-1 of a straight line joining the TD50 and the origin
as an estimator of the slope in the low-dose region. Although the
linearized multistage model could have been used since it has the
advantage of allowing for curvature in the dose-response curve, linear
extrapolation from the TD50 is computationally simple, extremely
conservative, and requires only published potency values from the CPD.
To illustrate the differences between these two approaches,
Krewski et al. (1990) considered the data on bladder tumours in mice
exposed to the experimental carcinogen 2-acetyl-aminofluorene (2-AAF),
shown in Figure 3. As indicated in Table 2, the TD50 for bladder
tumours based on the fitted multistage model is 17.2 mg/kg bw per day,
leading to a slope of (2TD50)-1 = 0.0291 (mg/kg per day) -1. Because
over 3300 animals were involved in this experiment, the 95% lower
confidence limit on the TD50 and the corresponding upper confidence
limit on the slope are close to the best estimates obtained from the
fitted model. Due to the high degree of curvature in the dose-response
curve for bladder tumours, however, the upper confidence limit on the
slope based on linear extrapolation from the lower confidence limit on
the TD50 is more than 75-fold greater than the slope derived from the
linearized multistage model.
2.3 The scientific basis for the threshold value of 1.5
µg/person per day specified at Step B5
Over the past several years, an immense amount of information has
accumulated on the range of carcinogenic potencies for chemicals that
have been tested in animals. For these chemicals, the distribution of
potencies in experimental animals and projected human risk (calculated
using linear risk assessment models) are well established and highly
unlikely to be altered by further cancer bioassays (Krewski et al.,
1990). In fact, the Carcinogenic Potency Database (CPD) compiled by
Gold et al. (1984) that was used to derive the decision criterion of
1.5 µg per day specified at Step B5, now contains nearly 500
substances reported to be carcinogenic in animals. It is reasonable to
assume that the addition to that data-base of several more "genotoxic"
carcinogens, should they be discovered, would be unlikely to alter the
distribution of known risks for identified carcinogens. Scientists may
never be prepared to say they know all they would like to know about
the distribution of risks of existing animal carcinogens. On the other
hand, it can be estimated with considerable confidence, based on data
available today, that a substance which has not been tested for
carcinogenicity and that is consumed in an amount below the threshold
value specified at Step B5 will not present a risk of human cancer
greater than one in one million (10-6).
With these thoughts in mind, it is now important to look at the
theoretical and practical aspects of the concept of threshold of
concern and how it can be applied to flavouring substances in the
context of JECFA safety evaluations.
The Carcinogen Potency Database (CPD) contains data on
approximately 3700 long-term animal studies of 975 chemicals (Gold
et al. 1986a,b,c; 1989). These include studies conducted by the US
National Toxicology Program as well as studies conducted in other
laboratories that have been published in the literature. Of the 975
chemicals tested, 955 were tested in rats and/or mice and 492 produced
an increase in tumour incidence (342 in rats and 278 in mice). Gold
et al. have put an enormous effort into compiling this database and
ensuring its quality. The reader is referred to a series of papers by
Gold et al. and others, published in Environmental Health
Perspectives, which document the validity of this database (Gold
et al., 1984, 1986a,b,c, 1989; Peto et al., 1984; Sawyer et
al., 1984).
For each compound, the CPD may include experiments with different
species, strains, sexes, dosing regimens, routes of administration, or
other experimental conditions (Gold et al., 1984). In most
experiments, two or more dose levels were used in addition to an
unexposed control; in some cases, however, only a single exposed group
was employed. Although a rigorous evaluation of the quality of
individual experiments is not possible, the CPD does include
information on the original investigators' conclusions regarding the
overall strength of evidence for carcinogenicity.
The CPD also contains a measure of carcinogenic potency (the
TD50) computed as described by Peto et al. (1984) and Sawyer
et al. (1984). In order to obtain some degree of comparability among
different studies, all TD50 values are expressed in units of mg/kg bw
per day and are adjusted to a 2-year standard rodent lifetime. In
cases where exposure is not constant throughout the study period, a
time-weighted average dose is used for purposes of modelling
dose-response. When individual animal data are available, the TD50
values are adjusted for intercurrent mortality; otherwise, the crude
proportions of animals with tumours are used to estimate carcinogenic
potency without adjusting for mortality from other causes. Finally,
the TD50 is estimated on the basis of an essentially linear one-hit
dose-response model.
The CPD represents an extremely useful source of information on
experiments with chemical carcinogens. The database includes
experiments on highly potent rodent carcinogens such as
2,3,7,8-tetrachlorodibenzo- p-dioxin and aflatoxin B1, as well as
less potent agents such as metronidazole and DDT. Gold et al. (1984)
noted that TD50 values included in the CPD vary by 10 million-fold.
Rulis (1986) used the Gold et al. (1984, 1986a,b,c, 1989)
database when he and others at FDA derived a threshold of regulation
for packaging materials. What was done, in essence, was to transform
the distribution of lowest TD50 for each carcinogen that was tested
by the oral route to a distribution of 10-6 risks. While numerous
mathematical models including the linearized multistage model could
have been used to perform this transformation, Rulis (1986) used the
slope (2TD50)-1 of a straight line joining the TD50 and the origin
as an estimator of the slope in the low-dose region. Although the
linearized multistage model could have been used since it has the
advantage of allowing for curvature in the dose-response curve, linear
extrapolation from the TD50 is computationally simple, extremely
conservative, and requires only published potency values from the CPD.
To illustrate the differences between these two approaches,
Krewski et al. (1990) considered the data on bladder tumours in mice
exposed to the experimental carcinogen 2-acetyl-aminofluorene (2-AAF),
shown in Figure 3. As indicated in Table 2, the TD50 for bladder
tumours based on the fitted multistage model is 17.2 mg/kg bw per day,
leading to a slope of (2TD50)-1 = 0.0291 (mg/kg per day) -1. Because
over 3300 animals were involved in this experiment, the 95% lower
confidence limit on the TD50 and the corresponding upper confidence
limit on the slope are close to the best estimates obtained from the
fitted model. Due to the high degree of curvature in the dose-response
curve for bladder tumours, however, the upper confidence limit on the
slope based on linear extrapolation from the lower confidence limit on
the TD50 is more than 75-fold greater than the slope derived from the
linearized multistage model.
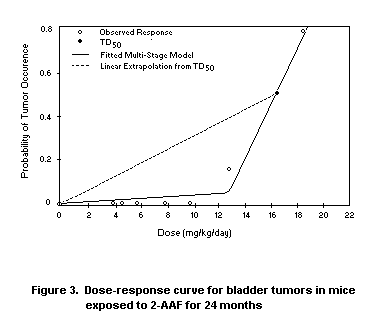 Table 2. Low-dose slopes for bladder tumors in mice exposed to
2-AAF for 24 months based on linear extrapolation from the TD50
Source of TD50 TD50 Slope of (2TD50)-1
(mg/kg/day) (mg/kg/day)
Fitted multistage model
Best estimate 17.2 0.0291
95% Confidence limit 16.71 0.02982
Fitted one-hit model
Best estimate 96.0 0.0052
95% Confidence limit 77.42 0.00652
95% Confidence limit on
the linear term in
multistage model (q1*) 0.00042
1 Lower confidence limit
2 Upper confidence limit
These data show that when there is significant upward curvature in
the dose-response curve, the methodology employed by Rulis (1986) to
calculate a dose associated with a 10-6 risk will produce a value
substantially lower than when these risk-specific doses are calculated
using the linearized multistage model. This point also has been made
by Hoel & Portier (1994), who noted that examination of the shape of
the dose-response curve for 315 chemicals found to produce cancer in
the NCI/NTP program indicates that tumour site data were more often
consistent with a quadratic response than a linear response,
suggesting that the use of linear dose-response models will often
overestimate risk. It may therefore be concluded that the methodology
used by Rulis (1986) (Federal Register, 1993, 1995) to estimate the
distribution of 10-6 risks for carcinogens in the Gold et al. CPD
was appropriately conservative.
The next step in the process of establishing a threshold value
involves the selection of an appropriate exposure, which is based on
the distribution of 10-6 risks. This value must be both highly
protective of human health and of sufficient practical value to reduce
the number of compounds requiring formal toxicological testing. Two
steps need to be considered here: the first involves the selection of
an appropriate risk standard that will ensure that any threshold value
selected will have an acceptably high probability of health
protection. The second step involves being pragmatic enough in the
selection of a threshold value that it still is of sufficient
magnitude to be of practical value. Of course, the protection of human
health is of greater concern than the practical value.
Initially Rulis (1986, 1989) proposed, for illustration, a
threshold value of 0.15 µg/person per day. Based on the distribution
of 10-6 risks from the CPD, this value would intersect the
distribution at the 85th centile, meaning that only 15% of carcinogens
in the database would present a risk greater than 10-6 at an intake
of 0.15 µg/day. This analysis indicates that, at an intake of 0.15
µg/person per day, 85% chemicals in the CPD known to induce cancer in
rodents would fail to show a significant increase in risk for the
exposed population. This is demonstrated graphically in Figure 4,
modified from Rulis (1989).
Table 2. Low-dose slopes for bladder tumors in mice exposed to
2-AAF for 24 months based on linear extrapolation from the TD50
Source of TD50 TD50 Slope of (2TD50)-1
(mg/kg/day) (mg/kg/day)
Fitted multistage model
Best estimate 17.2 0.0291
95% Confidence limit 16.71 0.02982
Fitted one-hit model
Best estimate 96.0 0.0052
95% Confidence limit 77.42 0.00652
95% Confidence limit on
the linear term in
multistage model (q1*) 0.00042
1 Lower confidence limit
2 Upper confidence limit
These data show that when there is significant upward curvature in
the dose-response curve, the methodology employed by Rulis (1986) to
calculate a dose associated with a 10-6 risk will produce a value
substantially lower than when these risk-specific doses are calculated
using the linearized multistage model. This point also has been made
by Hoel & Portier (1994), who noted that examination of the shape of
the dose-response curve for 315 chemicals found to produce cancer in
the NCI/NTP program indicates that tumour site data were more often
consistent with a quadratic response than a linear response,
suggesting that the use of linear dose-response models will often
overestimate risk. It may therefore be concluded that the methodology
used by Rulis (1986) (Federal Register, 1993, 1995) to estimate the
distribution of 10-6 risks for carcinogens in the Gold et al. CPD
was appropriately conservative.
The next step in the process of establishing a threshold value
involves the selection of an appropriate exposure, which is based on
the distribution of 10-6 risks. This value must be both highly
protective of human health and of sufficient practical value to reduce
the number of compounds requiring formal toxicological testing. Two
steps need to be considered here: the first involves the selection of
an appropriate risk standard that will ensure that any threshold value
selected will have an acceptably high probability of health
protection. The second step involves being pragmatic enough in the
selection of a threshold value that it still is of sufficient
magnitude to be of practical value. Of course, the protection of human
health is of greater concern than the practical value.
Initially Rulis (1986, 1989) proposed, for illustration, a
threshold value of 0.15 µg/person per day. Based on the distribution
of 10-6 risks from the CPD, this value would intersect the
distribution at the 85th centile, meaning that only 15% of carcinogens
in the database would present a risk greater than 10-6 at an intake
of 0.15 µg/day. This analysis indicates that, at an intake of 0.15
µg/person per day, 85% chemicals in the CPD known to induce cancer in
rodents would fail to show a significant increase in risk for the
exposed population. This is demonstrated graphically in Figure 4,
modified from Rulis (1989).
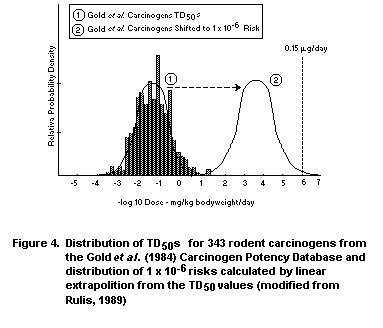 Subsequent to the report of Rulis (1989), Munro (1990) held a
workshop to evaluate factors that influence the selection of an
appropriate threshold value. The workshop first reanalysed the Gold
et al. (1984) database using the original database of 343 rodent
carcinogens and confirmed the observations of Rulis (1986, 1989) that
a dietary intake of 0.15 µg/day intersected the distribution of 10-6
risks at approximately the 85th centile. In addition, the workshop
extended the analysis to include additional carcinogens added to the
original Gold et al. (1984) database, bringing the total to 492
rodent carcinogens (Gold et al., 1989). This reanalysis with a
broader set of data produced essentially the same distribution of
1 × 10-6 risks as was originally published by Rulis (1986, 1989). The
workshop participants also noted that inherent in the acceptance of
any threshold value was the assumption that every new untested
substance was a likely carcinogen and could be as potent as the most
potent 15% of carcinogens in the CPD.
Recognizing that not every new substance would turn out to be a
carcinogen, the workshop (Munro, 1990) constructed a table of risk
avoidance probabilities (Table 3).
This table shows the effect of various assumptions regarding the
proportion of chemicals that are presumed carcinogens on the
probability that a 10-6 risk standard will not be exceeded. It should
be noted that as this proportion decreases, the probability of not
exceeding a specific risk standard increases dramatically. Thus, for
example, while there is a 63% chance that the risk will not exceed
10-6 with a value of 1.5 µg/person per day when 100% of new chemicals
are assumed to be carcinogenic, the probability that the risk will be
less than 10-6 is 96% when only 10% of new chemicals are assumed to
be carcinogenic. Moreover, if one invokes a less conservative risk
standard of 10-5 (Table 3, right side), then the probability of not
exceeding that risk at a threshold value of 1.5 µg/person per day
exceeds 96% even if it is assumed that 50% of new chemicals are
potential carcinogens. It also should be remembered that, in theory,
the probability of an untested substance having a potency greater than
the median of the distribution of TD50s from the CPD (Gold et al.,
1989) is 50%. In reality, however, it is most unlikely that a
genotoxic carcinogen with a potency equal to or greater than the
median carcinogen in the Gold et al. (1989) database would be
discovered from the existing inventory of flavouring substances, given
existing knowledge of structure-activity relationships in
carcinogenesis.
Table 3. Probability of a target risk not being exceeded at various threshold values
Threshold value Percentage of chemicals presumed carcinogenic
(µg/day) 100% 50% 20% 10% 100% 50% 20% 10%
10-6 Target risk 10-5 Target level
0.15 86 93 97 99 96 98 99 >99
0.3 80 90 96 98 94 97 99 99
0.6 74 87 95 97 91 96 98 99
1.5 63 82 93 96 86 96 97 99
3.0 55 77 91 95 80 90 96 98
6.0 46 73 89 95 74 87 95 97
(Modified from Munro, 1990).
Taking the above factors into consideration and keeping in mind
that the calculated 10-6 risks based on the Gold et al. (1989)
database were derived using a highly conservative methodology, Rulis
(1989) reexamined his previous selection criteria for a threshold
value and those of Munro (1990) and concluded that a threshold value
of 1.5 µg/person per day would provide a high degree of health
protection. This threshold value was subsequently adopted by FDA as
the threshold of regulation (Federal Register, 1993, 1995) and FDA
noted that such an exposure level would result in a negligible risk
even in the event that a substance of unknown toxicity was later shown
to be a carcinogen.
3. APPLICATION OF A THRESHOLD OF TOXICOLOGICAL CONCERN TO
FLAVOURING SUBSTANCES
The Scientific Committee for Food (SCF, 1996) has made the point
that the decision to accept any particular threshold value is both a
scientific and a risk management decision. The role of the scientist
is to ensure that risk managers are provided with the full range of
uncertainties surrounding selection of any threshold value. In the
foregoing sections it was pointed out that the threshold concept
should not be interpreted as providing absolute certainty of no risk.
Threshold of toxicological concern is a probabilistic methodology that
involves acceptance of a negligible risk standard. Such a standard is
commonly used by toxicologists in the establishment of ADIs, and, in
fact, IPCS (1987) has defined the ADI as "an estimate by JECFA of the
amount of a food additive, expressed on a body weight basis, that can
be ingested daily over a lifetime without appreciable health risk".
JECFA has noted that it uses the risk assessment process when setting
an ADI, i.e., the level of "no apparent risk" is set on the basis of
quantitative extrapolation from animal data to human beings typically
using a NOEL from the animal studies divided by a 100-fold safety
factor (IPCS, 1987). When ADIs (or such similar limits e.g., TLVs,
RfD, etc.) are established, there is a residual, usually
unquantifiable, element of risk (Purchase & Auton, 1995; Baird
et al., 1996; SCF, 1996; Sielken & Valdez-Flores, 1996). This is a
reflection of the inability to determine precisely the NOEL from
empirical data, statistical uncertainties associated with the
sensitivity of experimental models, completeness of data, or the
magnitude of safety factors needed to account for any residual
uncertainty (Dourson & Stara, 1983).
The threshold of toxicological concern likewise does not carry
with it the absolute certainty that an untested chemical present in
food below the decision criterion specified at Step B5 will present a
less than 10-6 risk. Rather there is a high probability (i.e. about
95%) that the cancer risk from such a chemical will be less than
10-6. It is this residue of uncertainty that has produced a concern
about the possibility, albeit remote, that a highly potent genotoxic
carcinogen might inadvertently be considered acceptable using the
threshold concept (SCF, 1996).
3.1 Additional factors that reduce the theoretical risk
When the threshold concept is applied to flavouring substances,
two additional factors significantly reduce the probability of risk of
cancer below one in one million. The first of these relates to very
low levels of exposure to flavouring substances. As exposure
decreases, the probability of not exceeding a 10-6 risk substantially
increases, approaching 97 to 99% at an order of magnitude below the
1.5 µg/person per day value (Table 3). Therefore, application of a
threshold of toxicological concern to substances having very low
exposures (i.e., less or much less than the threshold value) carries
with it a much higher probability of no appreciable risk. It also must
be kept in mind that exposure to the vast majority of flavouring
substances tends to be overestimated because these materials are
volatile and appreciable amounts are lost during food preparation,
storage, etc. These issues regarding exposure to flavouring substances
are discussed in Annex 5 of WHO Food Additives Series 35 (JECFA,
1996).
The second factor involves a consideration of chemical structure.
The use of chemical structure for predicting toxicity for food
chemicals, especially flavouring substances, has long been recognized
by JECFA (IPCS, 1987), and JECFA has noted that use of
structure-activity is most highly developed in the area of
carcinogenesis. The use of structural alerts in combination with a
knowledge of chemistry and metabolism offers a way of identifying
potential carcinogens (Ashby & Tennant 1988, 1991; Williams, 1990;
Tennant et al., 1990; Klopman & Rosenkranz, 1994). The examination
of many chemicals for genotoxic, mutagenic and carcinogenic activities
has led to the preparation of a series of structural alerts that
provide the basis for potential reaction with DNA and possible
carcinogenic potential of the substance. The existence of reactive
moieties on known rodent carcinogens implies that potential mutagenic
activity and, in many cases, the carcinogenic activity of untested
chemicals might be identified by an examination of structure (Ashby &
Tennant, 1988, 1991; Tennant & Ashby, 1991).
Structure-activity relationships have been successfully applied to
congeneric substances (i.e., individual substances within a
structurally related group of substances) for which no toxicity data
are available (Klopman & Rosenkranz, 1994). Congeners that are
potential human carcinogens and mutagens possess electrophilic
functional groups with the ability to react directly with DNA. These
electrophilic sites may be reactive functional groups on the congener
or those formed during metabolic activation. Conversely, these
functional groups may be lost during metabolic detoxication of the
substance. Although the carcinogenic and mutagenic potency of
congeneric substances may differ, structural alerts within the group
of congeners are indicative of carcinogenic or mutagenic potential
(Klopman & Rosenkranz, 1994). A list of the functional groups
identified by Ashby & Tennant (1988, 1991) and Tennant et al. (1990)
as structural alerts is given in Table 4.
Table 4. A list of functional groups identified by Ashby & Tennant
(1988, 1991) and Tennant et al. (1990) as structural alerts for DNA
reactivity
a) alkyl esters of phosphonic or sulfonic acids
b) aromatic nitro-groups
c) aromatic azo-groups (reduction to amine)
d) aromatic ring N-oxides
e) aromatic mono- and di-alkyl amino groups
f) alkyl hydrazines
g) alkyl aldehydes
h) N-methylol derivatives
i) monohaloalkanes
j) N and S mustards, beta-haloethyl-
k) N-chloramines
l) propiolactones and propiosulfones
m) aromatic and aliphatic aziridinyl derivatives
n) aromatic and aliphatic substituted primary alkyl halides
o) urethane derivatives (carbamates)
p) alkyl N-nitrosamines
q) aromatic amines and N-hydroxy derivatives
r) aliphatic epoxides and aromatic oxides
s) center of Michael reactivity
t) halogenated methanes (C(X)4)
u) aliphatic nitro groups
Most flavouring agents are simple aliphatic and aromatic
substances containing functional groups that are efficiently
metabolized via detoxication pathways, and very few flavouring agents
and/or their in vivo metabolites contain structural alerts. The
absence of structural alerts in a flavouring agent provides added
assurance that it will not present an appreciable risk at or below the
decision criterion of 1.5 µg/person per day. For those that do contain
significant structural alerts, such as aliphatic epoxides, additional
data have been generated to facilitate evaluation.
4. SUMMARY
The application of the decision criterion at Step B5 of the safety
evaluation procedure provides a means of evaluating those flavouring
substances that have extremely low exposures but lack metabolic and
toxicity data. It is recognized that application of a threshold of
toxicological concern is a departure from traditional toxicological
evaluation but, nonetheless, is based on highly conservative
methodology and assumptions which, taken together, ensure that
flavouring substances meeting the proposed criteria will present, at
most, an insignificant risk.
The following factors taken together ensure the absence of any
appreciable theoretical risk from the use of flavouring substances
evaluated using this procedure:
1. The decision criterion is based on carcinogenesis data, an
extremely sensitive end-point in susceptible animal species with
accepted relevance to humans.
2. The CPD presents a worse case situation since chemicals were
generally tested over a lifetime by daily administration at the
maximum tolerated dose (MTD), and the procedures used by Gold et
al. (1984) to establish the TD50s involved numerous conservative
assumptions.
3. The methods used by Rulis (1986, 1989) and others (Krewski et
al., 1990; Munro, 1990) to calculate the distribution of 10-6
risks, upon which the threshold value of 1.5 µg/person per day is
based, are highly conservative since they involved the use of linear
extrapolation from the lowest TD50 for each substance in the database.
4. It is unlikely that any untested flavouring agent would turn out to
be a genotoxic carcinogen, and the possibility that a carcinogen would
be accepted using the threshold concept can be substantially reduced
by the application of structural alert methodology.
5. Many flavouring substances are consumed in amounts considerably
below the threshold value of 1.5 µg/person per day and this
substantially increases the probability, already in the range of 90 to
95%, that they will not present any significant theoretical risk.
6. Toxicity end-points such as developmental toxicity, neurotoxicity
and immunotoxicity demonstrate considerably higher human exposure
thresholds than the threshold value of 1.5 µg/person per day specified
at Step B5 making it highly unlikely that these non-cancer end-points
are a relevant concern in applying the threshold concept.
Taken together, these factors provide a sound basis for concluding
that flavouring substances with intakes below the 1.5 µg/person per
day threshold of toxicological concern specified at Step B5 can be
safely consumed.
ACKNOWLEDGEMENT
The authors are deeply indebted to Dr. Alan Rulis for reviewing this
paper and providing many useful suggestions.
5. REFERENCES
Ashby, J. & Tennant, R.W. (1988) Chemical structure, Salmonella
mutagenicity and extent of carcinogenicity as indicators of genotoxic
carcinogenesis among 222 chemicals tested in rodents by the US
NCI/NTP. Mutat. Res., 204: 17-115.
Ashby, J. & Tennant, R.W. (1991) Definitive relationships among
chemical structure, carcinogenicity and mutagenicity for 301 chemicals
tested by the US NTP. Mutat. Res., 257: 229-306.
Baird, S.J.S., Cohen, J.T., Graham, J.D., Shlyakhter, A.I., & Evans,
J.S. (1996) Noncancer risk assessment: A probabilistic alternative to
current practice. Hum. Ecol. Risk Assess., 2(1): 79-102.
Dourson, M.L. & Stara, J.F. (1983) Regulatory history and
experimental support of uncertainty (safety) factors. Regul.
Toxicol. Pharmacol., 3: 224-228.
Dourson, M.L., Felter, S.P., & Robinson, D. (1996) Evolution of
science-based uncertainty factors in noncancer risk assessment.
Regul. Toxicol. Pharmacol., 24: 108-120.
Federal Register (1993) Food additives: Threshold of regulation for
substances used in food-contact articles (Proposed rule). Fed. Reg.,
58(195): 52719-52729.
Federal Register (1995) Food additives: Threshold of regulation for
substances used in food-contact articles (Final rule). Fed. Reg.,
60(136): 36582-36596.
Frawley, J.P. (1967) Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet. Toxicol., 5: 293-308.
Gold, L.S., Sawyer, C.B., Magaw, R., Backman, G.M., de Veciana, M.,
Levison, R., Hooper, N.K., Havender, W.R., Bernstein, L., Peto, R.,
Pike, M., & Ames, B.N. (1984) A carcinogenic potency database of the
standardized results of animal bioassays. Environ. Health Perspect.,
58: 9-319.
Gold, L.S., de Veciana, M., Backman, G.M., Magaw, R., Lopipero, P.
Smith, M., Blumenthal, M., Levinson, R., Bernstein, L., & Ames. B.N.
(1986a) Chronological supplement to the carcinogenic potency database:
Standardized results of animal bioassays published through December
1982. Environ. Health Perspect., 67: 161-200.
Gold, L.S., Bernstein, L., Kaldor, J., Backman, G., & Hoel, D. (1986b)
An empirical comparison of methods used to estimate carcinogenic
potency in long-term animal bioassays: Lifetable vs summary incidence
data. Fundam. Appl. Toxicol., 6: 263-269.
Gold, L.S., Ward, J.M., Bernstein, L., & Stern B. (1986c) Association
between carcinogenic potency and tumor pathology in rodent
carcinogenesis bioassays. Fundam. Appl. Toxicol., 6: 677-690.
Gold, L.S., Slone, T.H., & Bernstein, L. (1989) Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79:
259-272.
Hall, R.L. (1975) Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey -- Phase III, and to the FEMA.
Hoel, D.G. & Portier, C.J. (1994) Nonlinearity of dose-response
functions for carcinogenicity. Environ. Health Perspect., 102(1):
109-113.
IPCS (1987) Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. World
Health Organization, International Programme on Chemical Safety,
Geneva.
IPCS (1994) Environmental Health Criteria 170: Assessing human health
risk of chemicals: Derivation of guidance values for health-based
exposure limits. World Health Organization, International Programme on
Chemical Safety, Geneva.
JECFA (1995) Evaluation of certain food additives and contaminants.
Forty-fourth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 859).
JECFA (1996) Toxicological evaluation of certain food additives and
contaminants. World Health Organization, Geneva (WHO Food Additives
Series 35).
JECFA (1997) Evaluation of certain food additives and contaminants.
Forty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 868).
Klopman, G. & Rosenkranz, H.S. (1994) Approaches to SAR in
carcinogenesis and mutagenesis. Prediction of
carcinogenicity/mutagenicity using MULTI-CASE. Mutat. Res., 305:
33-46.
Krewski, D., Szyszkowicz, M., & Rosenkranz, H. (1990) Quantitative
factors in chemical carcinogenesis: Variation in carcinogenic potency.
Regul. Toxicol. Pharmacol., 12: 13-29.
Luster, M.I., Portier, C., Pait, D.G., White,K.L. Jr, Gennings, C.,
Munson, A.E., & Rosenthal, G.J. (1992) Risk assessment in
immunotoxicology: I. Sensitivity and predictability of immune tests.
Fundam. Appl. Toxicol., 18(2): 200-210.
Luster, M.I., Portier, C., Pait, D.G., Rosenthal, G.J., Germolec,
D.R., Gorsini, E., Blaylock, B.L., Pollock, P., Kouchi, Y., Craig, W.,
White, K.L., Munso, A.E., & Comment, C.E. (1993) Risk assessment in
Immunotoxicology: II. Relationships between immune and host
resistance tests. Fundam. Appl. Toxicol., 21(1): 71-82.
Munro, I.C. (1990) Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
Munro, I.C., Ford, R.A. Kennepohl, E., & Sprenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: A
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Peto, R., Pike, M.C., Bernstein, L., Gold, L.S., & Ames, B.N. (1984)
The TD50: A proposed general convention for the numerical description
of the carcinogenic potency of chemicals in chronic-exposure animal
experiments. Environ. Health Perspect., 5: 1-8.
Purchase, I.F.H. & Auton, T.R. (1995) Thresholds in chemical
carcinogenesis. Regul. Toxicol. Pharmacol., 22: 199-205.
RTECS (1987) Registry of toxic effects of chemical substances, 1985-86
ed. - Volume 1. US Department of Health and Human Services, Public
Health Service, Washington, D.C.
Rulis, A.M. (1986) De minimis and the threshold of regulation. In:
Felix, C.W. ed. Food protection technology. Lewis Publishers Inc.,
Chelsea, MI. pp. 29-37.
Rulis, A.M. (1989) Establishing a threshold of concern. In: Bonin,
J.J. & Stevenson, D.E. ed. Risk assessment in setting national
priorities. Plenum Press, New York, vol. 7, pp. 271-278.
Sawyer, C., Peto, R., Bernstein, L., & Pike, M.C. (1984) Calculation
of carcinogenic potency from long-term animal carcinogenesis
experiments. Biometrics, 40: 27-40.
SCF (1996) Opinion on response to request from the Commission for SCF
opinion on the scientific basis of the concept of threshold of
regulation in relation to food contact materials. European
Commission, Scientific Committee for Food, Brussels, Belgium.
Sielken, R.L. & Valdez-Flores, C. (1996) Comprehensive realism's
weight-of-evidence based distributional dose-response
characterization. Hum. Ecol. Risk Assess., 2(1): 175-193.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity
of a further 39 chemicals tested for carcinogenicity by the US
National Toxicology Program. Mutat. Res., 257: 209-227.
Tennant, R.W., Spalding, J., Stasiewicz, S., & Ashby, J. (1990)
Prediction of the outcome of rodent carcinogenicity bioassays
currently being conducted on 44 chemicals by the National Toxicology
Program. Mutagenesis, 5: 3-14.
Williams, G.M. (1990) Screening procedures for evaluating the
potential carcinogenicity of food-packaging chemicals. Regul.
Toxicol. Pharmacol., 12: 30-40.
Table 1. NOELS for organophosphate insecticides1
Agent Species End-point observed NOEL (mg/kg bw per day)
Acephate rat decreased body weight gain (parents and pups) 2.5
Azinphos methyl rat inhibition of plasma ChE activity 0.18
Coumaphos rat inhibition of RBC and plasma ChE activity 0.4
Crufomate rat inhibition of RBC ChE activity 3
Diazinon mouse decreased body weight gain 722
Dichlorvos rat inhibition of ChE activity (specific end-point
not indicated) 0.23
Dimethoate rat inhibition of brain, RBC and plasma ChE activity 0.05
Disulfoton rat inhibition of brain, RBC and plasma ChE activity 0.05
Ethephon rat inhibition of plasma and RBC ChE activity 15
Ethion rat inhibition of plasma ChE activity in females 0.2
Ethyl-p-nitrophenylphenyl
phosphorothioate rat inhibition of brain, RBC and plasma ChE activity 0.25
Express rat decreased body weight gain 1
Fenamiphos rabbit decreased maternal body weight gain 0.1
Fenchlorphos rat inhibition of ChE (form not specified) 15
Fonofos rat inhibition of RBC and plasma ChE activity 0.5
Glufosinate ammonium rat increased absolute and relative kidney
weight in males 0.42
Glyphosate rat increased incidence of renal tubular
dilation in F3b pups 10
Malathion rat inhibition of brain ChE activity 5
Merphos rat inhibition of RBC ChE activity in females 0.1
Merphos oxide rat inhibition of brain ChE activity 0.25
Methamidophos rat clinical signs typical of ChE inhibition 1
Methidithion rat inhibition of brain and RBC ChE activity 0.2
Methyl parathion rat decreased haemoglobin, haematocrit and RBCs 0.025
Naled rat decreased body weight gain 0.2
Parathion rat decreased body weight gain 1.8
Phosmet rat inhibition of RBC and plasma ChE activity 2
Phosphamidon rat decreased body weight gains 6.2
Pirimiphos-methyl rat inhibition of plasma ChE activity 0.5
Table 1. Continued...
Agent Species End-point observed NOEL (mg/kg bw per day)
Quinalphos mouse inhibition of plasma ChE activity 0.03
Tetrachlorvinphos rat inhibition of RBC ChE activity 6
Tetraethyl dithio
pyrophosphate rat inhibition of RBC and plasma ChE activity 0.5
1 Data taken from the EPA (IRIS) database and WHO 1969 Integrated Risk Information System
2 NOEL divided by a factor of 3 (see Munro et al., 1996 for explanation)
Table 2. Substances reported to cause developmental abnormalities (from RTECS)
Chemical Species Dose (TDLo)1
mg/kg bw
1 1,3,4-Thiadiazole, 2,2'-(methylenediimino)bis- rat 1
2 1-6-Hexanediamine rat 1840
3 1-Piperazinepropanol, 4-(6-((6-methoxy-8-quinolyl)amino)hexyl)-alpha-methyl-, maleate (1:2) rat 90
4 11H-Pyrido(2,1-b)quinazolone-2-carboxylic acid, 11-oxo- rat 4400
5 1H-Indazole-3-carboxylic acid, 1-(2,4-dichlorobenzyl)- rat 175
6 1H-Isoindole-1,3(2H)-dione,4,5,6,7-tetrahydro-2-(7-fluoro-3,4-dihydro-3-oxo-4-;
(2-propynyl)-2H-1,4-benzoxazin-6-yl)- rat 300
7 2,7-Naphthalenedisulfonic acid, 3,3'((3,3'-dimethyl-4,4'biphenylylene)bis(azo))
bis(5-; amino-4-hydroxy-, tetrasodium salt) rat 150
8 2-Propanone, 1,1,3,3-tetrachloro- rabbit 130
9 2-Pyridinemethanol,alpha-(3-(2,6-dimethyl-1-piperidinyl)propyl)-alpha-phenyl-,;
monohydrochloride, Z-(+-)- rabbit 650
10 3-Biphenylcarboxylic acid, 2',4'-difluoro-4-hydroxy- rabbit 520
11 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-((aminophenylacetyl)amino)-;
3,3-dimethyl-7-oxo-,(2,2-dimethyl-1-oxopropoxy)methyl ester,hydrochloride mouse 1200
12 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-(2-amino-2-phenylacetamido)-;
3,3-dimethyl-7-oxo-,trihydrate,D-(-)- rat 2800
13 4H-Pyrido(1,2-a)pyrimidin-4-one,9-methyl-3-(1H-tetrazol-5-yl)-, potassium salt rat 2750
14 4H-s-Triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine,6-(o-chlorophenyl)-8-ethyl-1-methyl- rabbit 13
15 5-Isoxazoleacetic acid, 3,4-bis(4-methoxyphenyl)- rat 1650
16 7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid,2,3-dihydro-9-fluoro-3-methyl-;
10-(4-methyl-1-piperazinyl)-7-oxo-,hemihydrate,(S)- rat 8910
17 9H-Purine-6-thiol, 9-beta-D-ribofuranosyl- rat 87.5
18 Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-(methylsulfonyl)phenethyl)-,;
D-threo-(+) rat 150
19 Acetamide, N,N-dimethyl- rabbit 3900
20 Acetic acid, (2,4-dichlorophenoxy)- rat 0.22
21 Acetic acid, (3,5,6-trichloro-2-pyridyloxy)- rat 2000
22 Acetic acid, oxo((3-(1H-tetrazol-5-yl)phenyl)amino)-,butyl ester rat 24000
23 Acetonitrile, amino-, bisulfate rat 200
24 Acridine, 9,9-dimethyl-10-(3-(N,N-dimethylamino)propyl)-,tartrate mouse 175
25 Alanine, 3-(3,4-dihydroxyphenyl)-, L- rat 500
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
26 Alanine, N-((5-chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-,;
sodium salt, (-)- rat 5
27 Alosenn rat 5500
28 Anthranilic acid, N-(2,3-xylyl)- mouse 8
29 Arsine oxide, dimethylhydroxy- rat 300
30 Benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-, monoacetate rabbit 390
31 Benzenesulfonamide, 4-amino-N-(4,5-dimethyl-2-oxazolyl)-,mixt. with 5-((3,4,5-;
trimethoxyphenyl)methyl)-2,4-pyrimidinediamine rat 3360
32 Benzenesulfonamide, 4-amino-N-(4,6-dimethoxy-2-pyrimidinyl)- rat500
33 Benzenesulfonic acid, thio-,S,S'-(2-(dimethylamino)trimethylene) ester rat 660
34 Benzhydrol, 2-chloro-alpha-(2-(dimethylamino)ethyl)-,hydrochloride mouse 120
35 Benzoic acid, 3,4,5-trimethoxy-beta-(dimethylamino)-beta-ethylphenethyl ester,; maleate (1:1) rabbit 6500
36 Benzyl alcohol, 4-amino-alpha-((tert-butylamino)methyl)-3,5-dichloro-,monohydrochloride rat 4.4
37 Biphenyl, 3,3',4,4'-tetramethyl- mouse 640
38 Butyric acid, 4-(p-bis(2-chloroethyl)aminophenyl)- mouse 3
39 Butyrophenone, 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluoro- rat 5.04
40 Cadmium rat 23
41 Carbazic acid, 3-(1-phthalazinyl)-, ethyl ester, monohydrochloride mouse 70
42 Chlordane rat 880
43 Cortisone mouse 500
44 Dibenzo(b,e)(1,4)dioxin, 2,3,7,8-tetrabromo- mouse 0.216
45 Dibenzo-p-dioxin, 2,7-dichloro- rat 5
46 Disulfide, bis(thiocarbamoyl) mouse 105
47 Ethane, 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)- rat 2000
48 Ethane, 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1,1-trichloro- rat 250
49 Ethanone, 1-(7-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-2-benzofuranyl)-, hydrochloride rat 1400
50 Ethanone, 2-((4-(2,4,dichloro-3-methylbenzoyl)-1,3-dimethyl-1H-pyrazol-5-yl)oxy)-;
1-(4-methylphenyl)- rat 2000
51 Folic acid, methyl- rat 500
52 Gallic acid, propyl ester rat 45000
53 Glutamic acid, N-(p-((1-(2-amino-4-hydroxy-6-pteridinyl)ethyl)amino)benzoyl)-L- rat 20
54 Gossypol acetic acid mouse 480
55 Hydrocinnamic acid, alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, L- rat 2100
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
56 Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl- rat 1
57 Isonicotinamide, 2-ethylthio- mouse 450
58 Isothiocyanic acid, butenyl ester rat 800
59 L-Glutamic acid, magnesium salt (1:1), hydrobromide rat 6000
60 L-Tyrosine rat 3500
61 L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo- rat 26.25
62 Linoleic acid (oxidized) rat 166000
63 Lysine, L- rat 81000
64 Manganese, (ethylenebis(dithiocarbamato))- and zinc acetate (50:1) rat 765
65 Mannitol, 1,6-dibromo-1,6-dideoxy-, D- mouse 150
66 Methanol, 1,3,4-thiadiazol-2-ylminodi- rat 5
67 Molybdenum rat 5.8
68 Morpholine, 4-(3,4,5-trimethoxybenzoyl)- rat 700
69 Norleucine, 6-amidino-, monohydrochloride, hydrate rat 2000
70 Oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one, 10-chloro-11b-(o-chlorophenyl)-2,3,7,11b- ;
tetrahydro mouse 1800
71 Phenol, p-amino- rat 2500
72 Phenothiazine-2-acetic acid, 10-methyl mouse 180
73 Phosphonic acid, (1,2-epoxypropyl)-, calcium salt (1:1), (1R,2S)-(-)- rat 15400
74 Phosphorodithioic acid, O,O-dimethyl ester, S-ester with 2-mercapto-N-methylacetamide rat 120
75 Phthalic acid, di(methoxyethyl)ester rat 593
76 Piperazine, 1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)- rat 320
77 Piperazine,1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)-,dihydrochloride rat 360
78 Piperidine, 3-((4-methoxyphenoxy)methyl)-1-methyl-4-phenyl-, hydrochloride, (3R-trans)- rat 210
79 Piperidine, 1-methyl-4-(N-2-thenylanilino)-, tartrate rat 157
80 Polychlorinated biphenyl (aroclor 1254) rat 90
81 Pregn-4-ene-3,20-dione,9-fluoro-11-beta,17,21-trihydroxy- rabbit 2
82 Pregna-,4-diene-2,20-dione,9-fluoro-11-beta,16-alpha,17,21-tetrahydroxy-,16,21-diacetate mouse 3.2
83 Propionic acid,2-(2,4,5-trichlorophenoxy)- mouse 1617
84 Pyrimidine, 2,4-diamino-6-methyl-5-phenyl- rat 100
85 Retinoic acid, 4-oxo-,13-cis- mouse 100
86 Retinoic acid, all-trans- mouse 15
87 Rowachol rat 9600
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
88 Stannane, diacetoxydibutyl- rat 15.2
89 Sulfanilamide, N(sup 1)-(6-methoxy-2-methyl-4-pyrimidinyl)- mouse 3000
90 Toluene, alpha-(2-(2-butoxyethoxy)ethoxy)-4,5-(methylenedioxy)-2-propyl- rat 2130
91 Tryptophan, N-acetyl-,L- rat 27500
92 Urea, (alpha-(2-methylhydrazino)-p-toluoyl)-, monohydrobromide rabbit 50
93 Urea, 1-butyl-3-(p-tolylsulfonyl)- mouse 1700
94 Urea, 1-butyl-3-sulfanilyl- rat 1000
95 Uridine, 5'-deoxy-5-fluoro- rat 550
96 ZZL-0820 rabbit 325
97 beta-Escin mouse 36
98 m-Propionotoluidide,2-methyl-4'-nitro-alpha,alpha,alpha-triflouro- rat 1050
99 p-Acetophenetidide rat 6000
100 p-Cresol, 2,6-di-tert-butyl- mouse 1200
1 TDLo = the lowest dose of a substance reported to produce any non-significant adverse effect (=NOEL).
Table 3. Substances with immunotoxic NOELs
Substance Immune Non-immune Non-immune Reference
NOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day)
ORAL ADMINISTRATION
1 p-nitrotoluene 400 200 hepatic, splenic Burns et al., 1994
2 pentachlorophenol 10 3 hepatic, renal Schwetz et al., 1978
3 o-phenylphenol 100 10 blood (red blood cells) Luster et al., 1981
4 hexachlorodibenzo-p-dioxin 0.056 1E-05 reproductive Murray et al., 1979
5 DPH 150 50 teratogenic McClain & Langhoff, 1979
6 tetraethyl lead 0.5 0.0012 hepatic,thymus Schepers, 1964
7 benzidine 11 2.7 neural, hepatic Littlefield et al., 1983
8 nitrobenzene 30 60 reproductive Kawashima et al., 1995
NON-ORAL ADMINISTRATION
1 indomethacin (subcutaneous) 2 1.6 reproductive/vascular
permeability(subcutaneous) Hoos & Hoffman, 1983
2 TPA (subcutaneous) 20 0.32 reproductive (subcutaneous) Nagasawa et al., 1980
3 ethyl carbamate
(intraperitoneal) 2 15 reproductive (subcutaneous) NTIS, 1968
Table 4. Substances with immunotoxic LOELs
Substance Immune Non-immune Non-immune Reference
LOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day
ORAL ADMINISTRATION
1 2,3,7,8-TCDD 0.086 1E-05 reproductive Murray et al., 1979
2 lithium carbonate 50 98 reproductive, body weight,
hepatic, renal Ibrahim & Canolty, 1990
3 m-nitrotoluene 200 40 hepatic (female) NTP, 1992
4 2,4-diaminotoluene 25 24 reproductive Thysen et al., 1985
5 dimethylvinyl chloride 50 125 splenic NTP, 1986
6 ethylene dibromide 125 30 mortality Teramoto et al., 1980
7 4,4-thiobis
(6-t-butyl-m-cresol) 10 45 hepatic NTP, 1994
NON-ORAL ADMINISTRATION
1 azathioprine
(intraperitoneal) 10 1 reproductive (intraperitoneal) Scott, 1977
2 benzo(a)pyrene
(subcutaneous) 50 50 reproductive (subcutaneous) Bui et al., 1986
3 diethylstilbestrol
(subcutaneous) 0.2 1E-05 reproductive (subcutaneous) McLachlan, 1977
4 DMB(a)A (subcutaneous) 5 1.25 reproductive (oral) Davis et al., 1978
5 N-nitroso dimethylamine
(intraperitoneal) 1.5 5 reproductive (intraperitoneal) Chaube, 1973
6 ochratoxin A
(intraperitoneal) 3.4 0.0625 renal (gavage) NTP, 1989
REFERENCES
Bui, Q.Q., Tran, M.B., & West, W.L. (1986) A comparative study of the
reproductive effects of methadone and benzo(a)pyrene in the pregnant
and pseudopregnant rat. Toxicology, 42(2/3): 195-204.
Burns, L.A., Bradley, S.G., White, K.L., McCay, J.A., Fuchs, B.A.,
Stern, M., Brown, R.D., Musgrove, D.L., Holsapple, M.P., Luster, M.I.,
& Munson, A.E. (1994) Immunotoxicity of mono-nitrotoluenes in female
B6C3F1 mice: I. Para-nitrotoluene. Drug Chem. Toxicol., 17(3):
317-358.
Chaube, S. (1973) Protective effects of thymidine,
5-aminoimidazolecarboxamide, and riboflavin against fetal
abnormalities produced in rats by 5-(3,3-dimethyl-1-triazene)
imidazole-4-carboxamide. Cancer Res., 33(10): 2231-2240.
Davis, G.J., McLachlan, J.A., & Lucier, G.W. (1978) The effect of
7,12-dimethylbenz(a)anthracene (DMBA) on the prenatal development of
gonads in mice. Teratology, 17: 33A (Abstract).
Hoos, P.C. & Hoffman, L.H. (1983) Effect of histamine receptor
antagonists and indomethacin in implantation in the rabbit. Biol.
Reprod., 29(4): 833-840.
Ibrahim, H.S. & Canolty, N.L. (1990) Effects of dietary lithium on
pregnant and lactating rats and the progeny. Nutr. Res., 10:
315-324.
Kawashima, K., Usami, M., Sakemi, K., & Ohno, Y. (1995) Studies on the
establishment of appropriate spermatogenic endpoints for male
fertility disturbance in rodent induced by drugs and chemicals: I.
Nitrobenzene. J. Toxicol. Sci., 20(1): 15-22.
Littlefield, N.A., Nelson, C.J., & Frith, C.H. (1983) Benzidine
dihydrochloride: Toxicological assessment in mice during chronic
exposures. J. Toxicol. Environ. Health, 12(4-6): 671-685.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A., & Wilson, R.E. (1981) The effect of
orthophenylphenol, tris(2,3-dichloropropyl) phosphate, and
cyclophosphamide on the immune system and host susceptibility of mice
following subchronic exposure. Toxicol. Appl. Pharmacol., 58(2):
252-261.
McClain, R.M. & Langhoff, L. (1979) Teratogenicity of
diphenylhydantoin in New Zealand rabbits. Toxicol. Appl.
Pharmacol., 48(1-Pt. 2): A32.
McLachlan, J.A. (1977) Prenatal exposure to diethylstilbestrol in
mice: Toxicological studies. J. Toxicol. Environ. Health, 2:(3)
527-537.
Murray, F.J., Smith, F.A., Nitschke, K.D., Humiston, C.G., Kociba,
R.J., & Schwetz, B.A. (1979) Three-generation reproduction study of
rats given 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) in the diet.
Toxicol. Appl. Pharmacol., 50(2): 241-252.
Nagasawa, H., Yanai, R., & Nakajima, Y. (1980) Suppression of
lactation by tumor promoters in mice. Proc. Soc. Exp. Biol. Med.,
165: 394-397.
NTIS (1968) Evaluation of carcinogenic, teratogenic, and mutagenic
activities of selected pesticides and industrial chemicals - Volume I:
Carcinogenic study and Volume II: Teratogenic study in mice and rats.
National Cancer Institute, Bethesda, MD (Bionetics Res. Lab).
NTP (1986) Toxicology and carcinogenesis studies of dimethylvinyl
chloride (1-chloro-2-methylpropene) (CAS No. 513-37-1) in F344/N rats
and B6C3F1 mice (Gavage studies). National Toxicology Program,
Research Triangle Park, NC (NTP Technical Report Series No. 316).
NTP (1989) Toxicology and carcinogenesis studies of ochratoxin A (CAS
No. 303-47-9) in F344/N rats (Gavage studies). National Toxicology
Program, Research Triangle Park, NC (NTP Technical Report Series No.
358).
NTP (1992) NTP Technical report on toxicity studies of o-, m-, and
p-nitrotoluenes (CAS Nos. 88-72-2, 99-08-1, 99-99-0) administered in
dosed feed to F344/N rats and B6C3F1 mice. National Toxicology
Program, Research Triangle Park, NC (NTP Toxicity Report Series No.
23).
NTP (1994) Toxicology and carcinogenesis studies of
4,4'-thiobis(6-t-butyl-m- cresol) (CAS No. 96-69-5) in F344/N rats and
B6C3F1 mice (Feed studies). National Toxicology Program, Research
Triangle Park, NC (NTP Technical Report Series No. 435).
Schepers, G.W. (1964) Tetraethyl lead and tetramethyl lead. Arch.
Environ. Health, 8(2): 277-295.
Schwetz, B.A., Quast, J.F., Keeley, P.A., Humiston, C.G., & Kociba,
R.J. (1978) Results of 2-year toxicity and reproduction studies on
pentachlorophenol in rats. In: Rao, K.R. ed. Pentachlorophenol:
Chemistry, pharmacology and environmental toxicology. Proceedings of
the EPA and University of West Florida Symposium, Pensacola, FL,
27-29 June 1977. Plenum Press, New York, pp. 301-309.
Scott, J.R. (1977) Fetal growth retardation associated with maternal
administration of immunosuppressive drugs. Am. J. Obstet. Gynecol.,
128(6): 668-676.
Teramoto, S., Saito, R., Aoyama, H., & Shirasu, Y. (1980) Dominant
lethal mutation induced in male rats by 1,2-dibromo-3-chloropropane
(DBCP). Mutat. Res., 77(1): 71-78.
Thysen, B., Bloch, E., & Varma, S.K. (1985) Reproductive toxicity of
2,4-toluenediamine in the rat: 2. Spermatogenic and hormonal effects.
J. Toxicol. Environ. Health, 16(6): 763-769.
Subsequent to the report of Rulis (1989), Munro (1990) held a
workshop to evaluate factors that influence the selection of an
appropriate threshold value. The workshop first reanalysed the Gold
et al. (1984) database using the original database of 343 rodent
carcinogens and confirmed the observations of Rulis (1986, 1989) that
a dietary intake of 0.15 µg/day intersected the distribution of 10-6
risks at approximately the 85th centile. In addition, the workshop
extended the analysis to include additional carcinogens added to the
original Gold et al. (1984) database, bringing the total to 492
rodent carcinogens (Gold et al., 1989). This reanalysis with a
broader set of data produced essentially the same distribution of
1 × 10-6 risks as was originally published by Rulis (1986, 1989). The
workshop participants also noted that inherent in the acceptance of
any threshold value was the assumption that every new untested
substance was a likely carcinogen and could be as potent as the most
potent 15% of carcinogens in the CPD.
Recognizing that not every new substance would turn out to be a
carcinogen, the workshop (Munro, 1990) constructed a table of risk
avoidance probabilities (Table 3).
This table shows the effect of various assumptions regarding the
proportion of chemicals that are presumed carcinogens on the
probability that a 10-6 risk standard will not be exceeded. It should
be noted that as this proportion decreases, the probability of not
exceeding a specific risk standard increases dramatically. Thus, for
example, while there is a 63% chance that the risk will not exceed
10-6 with a value of 1.5 µg/person per day when 100% of new chemicals
are assumed to be carcinogenic, the probability that the risk will be
less than 10-6 is 96% when only 10% of new chemicals are assumed to
be carcinogenic. Moreover, if one invokes a less conservative risk
standard of 10-5 (Table 3, right side), then the probability of not
exceeding that risk at a threshold value of 1.5 µg/person per day
exceeds 96% even if it is assumed that 50% of new chemicals are
potential carcinogens. It also should be remembered that, in theory,
the probability of an untested substance having a potency greater than
the median of the distribution of TD50s from the CPD (Gold et al.,
1989) is 50%. In reality, however, it is most unlikely that a
genotoxic carcinogen with a potency equal to or greater than the
median carcinogen in the Gold et al. (1989) database would be
discovered from the existing inventory of flavouring substances, given
existing knowledge of structure-activity relationships in
carcinogenesis.
Table 3. Probability of a target risk not being exceeded at various threshold values
Threshold value Percentage of chemicals presumed carcinogenic
(µg/day) 100% 50% 20% 10% 100% 50% 20% 10%
10-6 Target risk 10-5 Target level
0.15 86 93 97 99 96 98 99 >99
0.3 80 90 96 98 94 97 99 99
0.6 74 87 95 97 91 96 98 99
1.5 63 82 93 96 86 96 97 99
3.0 55 77 91 95 80 90 96 98
6.0 46 73 89 95 74 87 95 97
(Modified from Munro, 1990).
Taking the above factors into consideration and keeping in mind
that the calculated 10-6 risks based on the Gold et al. (1989)
database were derived using a highly conservative methodology, Rulis
(1989) reexamined his previous selection criteria for a threshold
value and those of Munro (1990) and concluded that a threshold value
of 1.5 µg/person per day would provide a high degree of health
protection. This threshold value was subsequently adopted by FDA as
the threshold of regulation (Federal Register, 1993, 1995) and FDA
noted that such an exposure level would result in a negligible risk
even in the event that a substance of unknown toxicity was later shown
to be a carcinogen.
3. APPLICATION OF A THRESHOLD OF TOXICOLOGICAL CONCERN TO
FLAVOURING SUBSTANCES
The Scientific Committee for Food (SCF, 1996) has made the point
that the decision to accept any particular threshold value is both a
scientific and a risk management decision. The role of the scientist
is to ensure that risk managers are provided with the full range of
uncertainties surrounding selection of any threshold value. In the
foregoing sections it was pointed out that the threshold concept
should not be interpreted as providing absolute certainty of no risk.
Threshold of toxicological concern is a probabilistic methodology that
involves acceptance of a negligible risk standard. Such a standard is
commonly used by toxicologists in the establishment of ADIs, and, in
fact, IPCS (1987) has defined the ADI as "an estimate by JECFA of the
amount of a food additive, expressed on a body weight basis, that can
be ingested daily over a lifetime without appreciable health risk".
JECFA has noted that it uses the risk assessment process when setting
an ADI, i.e., the level of "no apparent risk" is set on the basis of
quantitative extrapolation from animal data to human beings typically
using a NOEL from the animal studies divided by a 100-fold safety
factor (IPCS, 1987). When ADIs (or such similar limits e.g., TLVs,
RfD, etc.) are established, there is a residual, usually
unquantifiable, element of risk (Purchase & Auton, 1995; Baird
et al., 1996; SCF, 1996; Sielken & Valdez-Flores, 1996). This is a
reflection of the inability to determine precisely the NOEL from
empirical data, statistical uncertainties associated with the
sensitivity of experimental models, completeness of data, or the
magnitude of safety factors needed to account for any residual
uncertainty (Dourson & Stara, 1983).
The threshold of toxicological concern likewise does not carry
with it the absolute certainty that an untested chemical present in
food below the decision criterion specified at Step B5 will present a
less than 10-6 risk. Rather there is a high probability (i.e. about
95%) that the cancer risk from such a chemical will be less than
10-6. It is this residue of uncertainty that has produced a concern
about the possibility, albeit remote, that a highly potent genotoxic
carcinogen might inadvertently be considered acceptable using the
threshold concept (SCF, 1996).
3.1 Additional factors that reduce the theoretical risk
When the threshold concept is applied to flavouring substances,
two additional factors significantly reduce the probability of risk of
cancer below one in one million. The first of these relates to very
low levels of exposure to flavouring substances. As exposure
decreases, the probability of not exceeding a 10-6 risk substantially
increases, approaching 97 to 99% at an order of magnitude below the
1.5 µg/person per day value (Table 3). Therefore, application of a
threshold of toxicological concern to substances having very low
exposures (i.e., less or much less than the threshold value) carries
with it a much higher probability of no appreciable risk. It also must
be kept in mind that exposure to the vast majority of flavouring
substances tends to be overestimated because these materials are
volatile and appreciable amounts are lost during food preparation,
storage, etc. These issues regarding exposure to flavouring substances
are discussed in Annex 5 of WHO Food Additives Series 35 (JECFA,
1996).
The second factor involves a consideration of chemical structure.
The use of chemical structure for predicting toxicity for food
chemicals, especially flavouring substances, has long been recognized
by JECFA (IPCS, 1987), and JECFA has noted that use of
structure-activity is most highly developed in the area of
carcinogenesis. The use of structural alerts in combination with a
knowledge of chemistry and metabolism offers a way of identifying
potential carcinogens (Ashby & Tennant 1988, 1991; Williams, 1990;
Tennant et al., 1990; Klopman & Rosenkranz, 1994). The examination
of many chemicals for genotoxic, mutagenic and carcinogenic activities
has led to the preparation of a series of structural alerts that
provide the basis for potential reaction with DNA and possible
carcinogenic potential of the substance. The existence of reactive
moieties on known rodent carcinogens implies that potential mutagenic
activity and, in many cases, the carcinogenic activity of untested
chemicals might be identified by an examination of structure (Ashby &
Tennant, 1988, 1991; Tennant & Ashby, 1991).
Structure-activity relationships have been successfully applied to
congeneric substances (i.e., individual substances within a
structurally related group of substances) for which no toxicity data
are available (Klopman & Rosenkranz, 1994). Congeners that are
potential human carcinogens and mutagens possess electrophilic
functional groups with the ability to react directly with DNA. These
electrophilic sites may be reactive functional groups on the congener
or those formed during metabolic activation. Conversely, these
functional groups may be lost during metabolic detoxication of the
substance. Although the carcinogenic and mutagenic potency of
congeneric substances may differ, structural alerts within the group
of congeners are indicative of carcinogenic or mutagenic potential
(Klopman & Rosenkranz, 1994). A list of the functional groups
identified by Ashby & Tennant (1988, 1991) and Tennant et al. (1990)
as structural alerts is given in Table 4.
Table 4. A list of functional groups identified by Ashby & Tennant
(1988, 1991) and Tennant et al. (1990) as structural alerts for DNA
reactivity
a) alkyl esters of phosphonic or sulfonic acids
b) aromatic nitro-groups
c) aromatic azo-groups (reduction to amine)
d) aromatic ring N-oxides
e) aromatic mono- and di-alkyl amino groups
f) alkyl hydrazines
g) alkyl aldehydes
h) N-methylol derivatives
i) monohaloalkanes
j) N and S mustards, beta-haloethyl-
k) N-chloramines
l) propiolactones and propiosulfones
m) aromatic and aliphatic aziridinyl derivatives
n) aromatic and aliphatic substituted primary alkyl halides
o) urethane derivatives (carbamates)
p) alkyl N-nitrosamines
q) aromatic amines and N-hydroxy derivatives
r) aliphatic epoxides and aromatic oxides
s) center of Michael reactivity
t) halogenated methanes (C(X)4)
u) aliphatic nitro groups
Most flavouring agents are simple aliphatic and aromatic
substances containing functional groups that are efficiently
metabolized via detoxication pathways, and very few flavouring agents
and/or their in vivo metabolites contain structural alerts. The
absence of structural alerts in a flavouring agent provides added
assurance that it will not present an appreciable risk at or below the
decision criterion of 1.5 µg/person per day. For those that do contain
significant structural alerts, such as aliphatic epoxides, additional
data have been generated to facilitate evaluation.
4. SUMMARY
The application of the decision criterion at Step B5 of the safety
evaluation procedure provides a means of evaluating those flavouring
substances that have extremely low exposures but lack metabolic and
toxicity data. It is recognized that application of a threshold of
toxicological concern is a departure from traditional toxicological
evaluation but, nonetheless, is based on highly conservative
methodology and assumptions which, taken together, ensure that
flavouring substances meeting the proposed criteria will present, at
most, an insignificant risk.
The following factors taken together ensure the absence of any
appreciable theoretical risk from the use of flavouring substances
evaluated using this procedure:
1. The decision criterion is based on carcinogenesis data, an
extremely sensitive end-point in susceptible animal species with
accepted relevance to humans.
2. The CPD presents a worse case situation since chemicals were
generally tested over a lifetime by daily administration at the
maximum tolerated dose (MTD), and the procedures used by Gold et
al. (1984) to establish the TD50s involved numerous conservative
assumptions.
3. The methods used by Rulis (1986, 1989) and others (Krewski et
al., 1990; Munro, 1990) to calculate the distribution of 10-6
risks, upon which the threshold value of 1.5 µg/person per day is
based, are highly conservative since they involved the use of linear
extrapolation from the lowest TD50 for each substance in the database.
4. It is unlikely that any untested flavouring agent would turn out to
be a genotoxic carcinogen, and the possibility that a carcinogen would
be accepted using the threshold concept can be substantially reduced
by the application of structural alert methodology.
5. Many flavouring substances are consumed in amounts considerably
below the threshold value of 1.5 µg/person per day and this
substantially increases the probability, already in the range of 90 to
95%, that they will not present any significant theoretical risk.
6. Toxicity end-points such as developmental toxicity, neurotoxicity
and immunotoxicity demonstrate considerably higher human exposure
thresholds than the threshold value of 1.5 µg/person per day specified
at Step B5 making it highly unlikely that these non-cancer end-points
are a relevant concern in applying the threshold concept.
Taken together, these factors provide a sound basis for concluding
that flavouring substances with intakes below the 1.5 µg/person per
day threshold of toxicological concern specified at Step B5 can be
safely consumed.
ACKNOWLEDGEMENT
The authors are deeply indebted to Dr. Alan Rulis for reviewing this
paper and providing many useful suggestions.
5. REFERENCES
Ashby, J. & Tennant, R.W. (1988) Chemical structure, Salmonella
mutagenicity and extent of carcinogenicity as indicators of genotoxic
carcinogenesis among 222 chemicals tested in rodents by the US
NCI/NTP. Mutat. Res., 204: 17-115.
Ashby, J. & Tennant, R.W. (1991) Definitive relationships among
chemical structure, carcinogenicity and mutagenicity for 301 chemicals
tested by the US NTP. Mutat. Res., 257: 229-306.
Baird, S.J.S., Cohen, J.T., Graham, J.D., Shlyakhter, A.I., & Evans,
J.S. (1996) Noncancer risk assessment: A probabilistic alternative to
current practice. Hum. Ecol. Risk Assess., 2(1): 79-102.
Dourson, M.L. & Stara, J.F. (1983) Regulatory history and
experimental support of uncertainty (safety) factors. Regul.
Toxicol. Pharmacol., 3: 224-228.
Dourson, M.L., Felter, S.P., & Robinson, D. (1996) Evolution of
science-based uncertainty factors in noncancer risk assessment.
Regul. Toxicol. Pharmacol., 24: 108-120.
Federal Register (1993) Food additives: Threshold of regulation for
substances used in food-contact articles (Proposed rule). Fed. Reg.,
58(195): 52719-52729.
Federal Register (1995) Food additives: Threshold of regulation for
substances used in food-contact articles (Final rule). Fed. Reg.,
60(136): 36582-36596.
Frawley, J.P. (1967) Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet. Toxicol., 5: 293-308.
Gold, L.S., Sawyer, C.B., Magaw, R., Backman, G.M., de Veciana, M.,
Levison, R., Hooper, N.K., Havender, W.R., Bernstein, L., Peto, R.,
Pike, M., & Ames, B.N. (1984) A carcinogenic potency database of the
standardized results of animal bioassays. Environ. Health Perspect.,
58: 9-319.
Gold, L.S., de Veciana, M., Backman, G.M., Magaw, R., Lopipero, P.
Smith, M., Blumenthal, M., Levinson, R., Bernstein, L., & Ames. B.N.
(1986a) Chronological supplement to the carcinogenic potency database:
Standardized results of animal bioassays published through December
1982. Environ. Health Perspect., 67: 161-200.
Gold, L.S., Bernstein, L., Kaldor, J., Backman, G., & Hoel, D. (1986b)
An empirical comparison of methods used to estimate carcinogenic
potency in long-term animal bioassays: Lifetable vs summary incidence
data. Fundam. Appl. Toxicol., 6: 263-269.
Gold, L.S., Ward, J.M., Bernstein, L., & Stern B. (1986c) Association
between carcinogenic potency and tumor pathology in rodent
carcinogenesis bioassays. Fundam. Appl. Toxicol., 6: 677-690.
Gold, L.S., Slone, T.H., & Bernstein, L. (1989) Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79:
259-272.
Hall, R.L. (1975) Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey -- Phase III, and to the FEMA.
Hoel, D.G. & Portier, C.J. (1994) Nonlinearity of dose-response
functions for carcinogenicity. Environ. Health Perspect., 102(1):
109-113.
IPCS (1987) Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. World
Health Organization, International Programme on Chemical Safety,
Geneva.
IPCS (1994) Environmental Health Criteria 170: Assessing human health
risk of chemicals: Derivation of guidance values for health-based
exposure limits. World Health Organization, International Programme on
Chemical Safety, Geneva.
JECFA (1995) Evaluation of certain food additives and contaminants.
Forty-fourth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 859).
JECFA (1996) Toxicological evaluation of certain food additives and
contaminants. World Health Organization, Geneva (WHO Food Additives
Series 35).
JECFA (1997) Evaluation of certain food additives and contaminants.
Forty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 868).
Klopman, G. & Rosenkranz, H.S. (1994) Approaches to SAR in
carcinogenesis and mutagenesis. Prediction of
carcinogenicity/mutagenicity using MULTI-CASE. Mutat. Res., 305:
33-46.
Krewski, D., Szyszkowicz, M., & Rosenkranz, H. (1990) Quantitative
factors in chemical carcinogenesis: Variation in carcinogenic potency.
Regul. Toxicol. Pharmacol., 12: 13-29.
Luster, M.I., Portier, C., Pait, D.G., White,K.L. Jr, Gennings, C.,
Munson, A.E., & Rosenthal, G.J. (1992) Risk assessment in
immunotoxicology: I. Sensitivity and predictability of immune tests.
Fundam. Appl. Toxicol., 18(2): 200-210.
Luster, M.I., Portier, C., Pait, D.G., Rosenthal, G.J., Germolec,
D.R., Gorsini, E., Blaylock, B.L., Pollock, P., Kouchi, Y., Craig, W.,
White, K.L., Munso, A.E., & Comment, C.E. (1993) Risk assessment in
Immunotoxicology: II. Relationships between immune and host
resistance tests. Fundam. Appl. Toxicol., 21(1): 71-82.
Munro, I.C. (1990) Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
Munro, I.C., Ford, R.A. Kennepohl, E., & Sprenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: A
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Peto, R., Pike, M.C., Bernstein, L., Gold, L.S., & Ames, B.N. (1984)
The TD50: A proposed general convention for the numerical description
of the carcinogenic potency of chemicals in chronic-exposure animal
experiments. Environ. Health Perspect., 5: 1-8.
Purchase, I.F.H. & Auton, T.R. (1995) Thresholds in chemical
carcinogenesis. Regul. Toxicol. Pharmacol., 22: 199-205.
RTECS (1987) Registry of toxic effects of chemical substances, 1985-86
ed. - Volume 1. US Department of Health and Human Services, Public
Health Service, Washington, D.C.
Rulis, A.M. (1986) De minimis and the threshold of regulation. In:
Felix, C.W. ed. Food protection technology. Lewis Publishers Inc.,
Chelsea, MI. pp. 29-37.
Rulis, A.M. (1989) Establishing a threshold of concern. In: Bonin,
J.J. & Stevenson, D.E. ed. Risk assessment in setting national
priorities. Plenum Press, New York, vol. 7, pp. 271-278.
Sawyer, C., Peto, R., Bernstein, L., & Pike, M.C. (1984) Calculation
of carcinogenic potency from long-term animal carcinogenesis
experiments. Biometrics, 40: 27-40.
SCF (1996) Opinion on response to request from the Commission for SCF
opinion on the scientific basis of the concept of threshold of
regulation in relation to food contact materials. European
Commission, Scientific Committee for Food, Brussels, Belgium.
Sielken, R.L. & Valdez-Flores, C. (1996) Comprehensive realism's
weight-of-evidence based distributional dose-response
characterization. Hum. Ecol. Risk Assess., 2(1): 175-193.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity
of a further 39 chemicals tested for carcinogenicity by the US
National Toxicology Program. Mutat. Res., 257: 209-227.
Tennant, R.W., Spalding, J., Stasiewicz, S., & Ashby, J. (1990)
Prediction of the outcome of rodent carcinogenicity bioassays
currently being conducted on 44 chemicals by the National Toxicology
Program. Mutagenesis, 5: 3-14.
Williams, G.M. (1990) Screening procedures for evaluating the
potential carcinogenicity of food-packaging chemicals. Regul.
Toxicol. Pharmacol., 12: 30-40.
Table 1. NOELS for organophosphate insecticides1
Agent Species End-point observed NOEL (mg/kg bw per day)
Acephate rat decreased body weight gain (parents and pups) 2.5
Azinphos methyl rat inhibition of plasma ChE activity 0.18
Coumaphos rat inhibition of RBC and plasma ChE activity 0.4
Crufomate rat inhibition of RBC ChE activity 3
Diazinon mouse decreased body weight gain 722
Dichlorvos rat inhibition of ChE activity (specific end-point
not indicated) 0.23
Dimethoate rat inhibition of brain, RBC and plasma ChE activity 0.05
Disulfoton rat inhibition of brain, RBC and plasma ChE activity 0.05
Ethephon rat inhibition of plasma and RBC ChE activity 15
Ethion rat inhibition of plasma ChE activity in females 0.2
Ethyl-p-nitrophenylphenyl
phosphorothioate rat inhibition of brain, RBC and plasma ChE activity 0.25
Express rat decreased body weight gain 1
Fenamiphos rabbit decreased maternal body weight gain 0.1
Fenchlorphos rat inhibition of ChE (form not specified) 15
Fonofos rat inhibition of RBC and plasma ChE activity 0.5
Glufosinate ammonium rat increased absolute and relative kidney
weight in males 0.42
Glyphosate rat increased incidence of renal tubular
dilation in F3b pups 10
Malathion rat inhibition of brain ChE activity 5
Merphos rat inhibition of RBC ChE activity in females 0.1
Merphos oxide rat inhibition of brain ChE activity 0.25
Methamidophos rat clinical signs typical of ChE inhibition 1
Methidithion rat inhibition of brain and RBC ChE activity 0.2
Methyl parathion rat decreased haemoglobin, haematocrit and RBCs 0.025
Naled rat decreased body weight gain 0.2
Parathion rat decreased body weight gain 1.8
Phosmet rat inhibition of RBC and plasma ChE activity 2
Phosphamidon rat decreased body weight gains 6.2
Pirimiphos-methyl rat inhibition of plasma ChE activity 0.5
Table 1. Continued...
Agent Species End-point observed NOEL (mg/kg bw per day)
Quinalphos mouse inhibition of plasma ChE activity 0.03
Tetrachlorvinphos rat inhibition of RBC ChE activity 6
Tetraethyl dithio
pyrophosphate rat inhibition of RBC and plasma ChE activity 0.5
1 Data taken from the EPA (IRIS) database and WHO 1969 Integrated Risk Information System
2 NOEL divided by a factor of 3 (see Munro et al., 1996 for explanation)
Table 2. Substances reported to cause developmental abnormalities (from RTECS)
Chemical Species Dose (TDLo)1
mg/kg bw
1 1,3,4-Thiadiazole, 2,2'-(methylenediimino)bis- rat 1
2 1-6-Hexanediamine rat 1840
3 1-Piperazinepropanol, 4-(6-((6-methoxy-8-quinolyl)amino)hexyl)-alpha-methyl-, maleate (1:2) rat 90
4 11H-Pyrido(2,1-b)quinazolone-2-carboxylic acid, 11-oxo- rat 4400
5 1H-Indazole-3-carboxylic acid, 1-(2,4-dichlorobenzyl)- rat 175
6 1H-Isoindole-1,3(2H)-dione,4,5,6,7-tetrahydro-2-(7-fluoro-3,4-dihydro-3-oxo-4-;
(2-propynyl)-2H-1,4-benzoxazin-6-yl)- rat 300
7 2,7-Naphthalenedisulfonic acid, 3,3'((3,3'-dimethyl-4,4'biphenylylene)bis(azo))
bis(5-; amino-4-hydroxy-, tetrasodium salt) rat 150
8 2-Propanone, 1,1,3,3-tetrachloro- rabbit 130
9 2-Pyridinemethanol,alpha-(3-(2,6-dimethyl-1-piperidinyl)propyl)-alpha-phenyl-,;
monohydrochloride, Z-(+-)- rabbit 650
10 3-Biphenylcarboxylic acid, 2',4'-difluoro-4-hydroxy- rabbit 520
11 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-((aminophenylacetyl)amino)-;
3,3-dimethyl-7-oxo-,(2,2-dimethyl-1-oxopropoxy)methyl ester,hydrochloride mouse 1200
12 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-(2-amino-2-phenylacetamido)-;
3,3-dimethyl-7-oxo-,trihydrate,D-(-)- rat 2800
13 4H-Pyrido(1,2-a)pyrimidin-4-one,9-methyl-3-(1H-tetrazol-5-yl)-, potassium salt rat 2750
14 4H-s-Triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine,6-(o-chlorophenyl)-8-ethyl-1-methyl- rabbit 13
15 5-Isoxazoleacetic acid, 3,4-bis(4-methoxyphenyl)- rat 1650
16 7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid,2,3-dihydro-9-fluoro-3-methyl-;
10-(4-methyl-1-piperazinyl)-7-oxo-,hemihydrate,(S)- rat 8910
17 9H-Purine-6-thiol, 9-beta-D-ribofuranosyl- rat 87.5
18 Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-(methylsulfonyl)phenethyl)-,;
D-threo-(+) rat 150
19 Acetamide, N,N-dimethyl- rabbit 3900
20 Acetic acid, (2,4-dichlorophenoxy)- rat 0.22
21 Acetic acid, (3,5,6-trichloro-2-pyridyloxy)- rat 2000
22 Acetic acid, oxo((3-(1H-tetrazol-5-yl)phenyl)amino)-,butyl ester rat 24000
23 Acetonitrile, amino-, bisulfate rat 200
24 Acridine, 9,9-dimethyl-10-(3-(N,N-dimethylamino)propyl)-,tartrate mouse 175
25 Alanine, 3-(3,4-dihydroxyphenyl)-, L- rat 500
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
26 Alanine, N-((5-chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-,;
sodium salt, (-)- rat 5
27 Alosenn rat 5500
28 Anthranilic acid, N-(2,3-xylyl)- mouse 8
29 Arsine oxide, dimethylhydroxy- rat 300
30 Benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-, monoacetate rabbit 390
31 Benzenesulfonamide, 4-amino-N-(4,5-dimethyl-2-oxazolyl)-,mixt. with 5-((3,4,5-;
trimethoxyphenyl)methyl)-2,4-pyrimidinediamine rat 3360
32 Benzenesulfonamide, 4-amino-N-(4,6-dimethoxy-2-pyrimidinyl)- rat500
33 Benzenesulfonic acid, thio-,S,S'-(2-(dimethylamino)trimethylene) ester rat 660
34 Benzhydrol, 2-chloro-alpha-(2-(dimethylamino)ethyl)-,hydrochloride mouse 120
35 Benzoic acid, 3,4,5-trimethoxy-beta-(dimethylamino)-beta-ethylphenethyl ester,; maleate (1:1) rabbit 6500
36 Benzyl alcohol, 4-amino-alpha-((tert-butylamino)methyl)-3,5-dichloro-,monohydrochloride rat 4.4
37 Biphenyl, 3,3',4,4'-tetramethyl- mouse 640
38 Butyric acid, 4-(p-bis(2-chloroethyl)aminophenyl)- mouse 3
39 Butyrophenone, 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluoro- rat 5.04
40 Cadmium rat 23
41 Carbazic acid, 3-(1-phthalazinyl)-, ethyl ester, monohydrochloride mouse 70
42 Chlordane rat 880
43 Cortisone mouse 500
44 Dibenzo(b,e)(1,4)dioxin, 2,3,7,8-tetrabromo- mouse 0.216
45 Dibenzo-p-dioxin, 2,7-dichloro- rat 5
46 Disulfide, bis(thiocarbamoyl) mouse 105
47 Ethane, 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)- rat 2000
48 Ethane, 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1,1-trichloro- rat 250
49 Ethanone, 1-(7-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-2-benzofuranyl)-, hydrochloride rat 1400
50 Ethanone, 2-((4-(2,4,dichloro-3-methylbenzoyl)-1,3-dimethyl-1H-pyrazol-5-yl)oxy)-;
1-(4-methylphenyl)- rat 2000
51 Folic acid, methyl- rat 500
52 Gallic acid, propyl ester rat 45000
53 Glutamic acid, N-(p-((1-(2-amino-4-hydroxy-6-pteridinyl)ethyl)amino)benzoyl)-L- rat 20
54 Gossypol acetic acid mouse 480
55 Hydrocinnamic acid, alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, L- rat 2100
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
56 Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl- rat 1
57 Isonicotinamide, 2-ethylthio- mouse 450
58 Isothiocyanic acid, butenyl ester rat 800
59 L-Glutamic acid, magnesium salt (1:1), hydrobromide rat 6000
60 L-Tyrosine rat 3500
61 L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo- rat 26.25
62 Linoleic acid (oxidized) rat 166000
63 Lysine, L- rat 81000
64 Manganese, (ethylenebis(dithiocarbamato))- and zinc acetate (50:1) rat 765
65 Mannitol, 1,6-dibromo-1,6-dideoxy-, D- mouse 150
66 Methanol, 1,3,4-thiadiazol-2-ylminodi- rat 5
67 Molybdenum rat 5.8
68 Morpholine, 4-(3,4,5-trimethoxybenzoyl)- rat 700
69 Norleucine, 6-amidino-, monohydrochloride, hydrate rat 2000
70 Oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one, 10-chloro-11b-(o-chlorophenyl)-2,3,7,11b- ;
tetrahydro mouse 1800
71 Phenol, p-amino- rat 2500
72 Phenothiazine-2-acetic acid, 10-methyl mouse 180
73 Phosphonic acid, (1,2-epoxypropyl)-, calcium salt (1:1), (1R,2S)-(-)- rat 15400
74 Phosphorodithioic acid, O,O-dimethyl ester, S-ester with 2-mercapto-N-methylacetamide rat 120
75 Phthalic acid, di(methoxyethyl)ester rat 593
76 Piperazine, 1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)- rat 320
77 Piperazine,1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)-,dihydrochloride rat 360
78 Piperidine, 3-((4-methoxyphenoxy)methyl)-1-methyl-4-phenyl-, hydrochloride, (3R-trans)- rat 210
79 Piperidine, 1-methyl-4-(N-2-thenylanilino)-, tartrate rat 157
80 Polychlorinated biphenyl (aroclor 1254) rat 90
81 Pregn-4-ene-3,20-dione,9-fluoro-11-beta,17,21-trihydroxy- rabbit 2
82 Pregna-,4-diene-2,20-dione,9-fluoro-11-beta,16-alpha,17,21-tetrahydroxy-,16,21-diacetate mouse 3.2
83 Propionic acid,2-(2,4,5-trichlorophenoxy)- mouse 1617
84 Pyrimidine, 2,4-diamino-6-methyl-5-phenyl- rat 100
85 Retinoic acid, 4-oxo-,13-cis- mouse 100
86 Retinoic acid, all-trans- mouse 15
87 Rowachol rat 9600
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
88 Stannane, diacetoxydibutyl- rat 15.2
89 Sulfanilamide, N(sup 1)-(6-methoxy-2-methyl-4-pyrimidinyl)- mouse 3000
90 Toluene, alpha-(2-(2-butoxyethoxy)ethoxy)-4,5-(methylenedioxy)-2-propyl- rat 2130
91 Tryptophan, N-acetyl-,L- rat 27500
92 Urea, (alpha-(2-methylhydrazino)-p-toluoyl)-, monohydrobromide rabbit 50
93 Urea, 1-butyl-3-(p-tolylsulfonyl)- mouse 1700
94 Urea, 1-butyl-3-sulfanilyl- rat 1000
95 Uridine, 5'-deoxy-5-fluoro- rat 550
96 ZZL-0820 rabbit 325
97 beta-Escin mouse 36
98 m-Propionotoluidide,2-methyl-4'-nitro-alpha,alpha,alpha-triflouro- rat 1050
99 p-Acetophenetidide rat 6000
100 p-Cresol, 2,6-di-tert-butyl- mouse 1200
1 TDLo = the lowest dose of a substance reported to produce any non-significant adverse effect (=NOEL).
Table 3. Substances with immunotoxic NOELs
Substance Immune Non-immune Non-immune Reference
NOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day)
ORAL ADMINISTRATION
1 p-nitrotoluene 400 200 hepatic, splenic Burns et al., 1994
2 pentachlorophenol 10 3 hepatic, renal Schwetz et al., 1978
3 o-phenylphenol 100 10 blood (red blood cells) Luster et al., 1981
4 hexachlorodibenzo-p-dioxin 0.056 1E-05 reproductive Murray et al., 1979
5 DPH 150 50 teratogenic McClain & Langhoff, 1979
6 tetraethyl lead 0.5 0.0012 hepatic,thymus Schepers, 1964
7 benzidine 11 2.7 neural, hepatic Littlefield et al., 1983
8 nitrobenzene 30 60 reproductive Kawashima et al., 1995
NON-ORAL ADMINISTRATION
1 indomethacin (subcutaneous) 2 1.6 reproductive/vascular
permeability(subcutaneous) Hoos & Hoffman, 1983
2 TPA (subcutaneous) 20 0.32 reproductive (subcutaneous) Nagasawa et al., 1980
3 ethyl carbamate
(intraperitoneal) 2 15 reproductive (subcutaneous) NTIS, 1968
Table 4. Substances with immunotoxic LOELs
Substance Immune Non-immune Non-immune Reference
LOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day
ORAL ADMINISTRATION
1 2,3,7,8-TCDD 0.086 1E-05 reproductive Murray et al., 1979
2 lithium carbonate 50 98 reproductive, body weight,
hepatic, renal Ibrahim & Canolty, 1990
3 m-nitrotoluene 200 40 hepatic (female) NTP, 1992
4 2,4-diaminotoluene 25 24 reproductive Thysen et al., 1985
5 dimethylvinyl chloride 50 125 splenic NTP, 1986
6 ethylene dibromide 125 30 mortality Teramoto et al., 1980
7 4,4-thiobis
(6-t-butyl-m-cresol) 10 45 hepatic NTP, 1994
NON-ORAL ADMINISTRATION
1 azathioprine
(intraperitoneal) 10 1 reproductive (intraperitoneal) Scott, 1977
2 benzo(a)pyrene
(subcutaneous) 50 50 reproductive (subcutaneous) Bui et al., 1986
3 diethylstilbestrol
(subcutaneous) 0.2 1E-05 reproductive (subcutaneous) McLachlan, 1977
4 DMB(a)A (subcutaneous) 5 1.25 reproductive (oral) Davis et al., 1978
5 N-nitroso dimethylamine
(intraperitoneal) 1.5 5 reproductive (intraperitoneal) Chaube, 1973
6 ochratoxin A
(intraperitoneal) 3.4 0.0625 renal (gavage) NTP, 1989
REFERENCES
Bui, Q.Q., Tran, M.B., & West, W.L. (1986) A comparative study of the
reproductive effects of methadone and benzo(a)pyrene in the pregnant
and pseudopregnant rat. Toxicology, 42(2/3): 195-204.
Burns, L.A., Bradley, S.G., White, K.L., McCay, J.A., Fuchs, B.A.,
Stern, M., Brown, R.D., Musgrove, D.L., Holsapple, M.P., Luster, M.I.,
& Munson, A.E. (1994) Immunotoxicity of mono-nitrotoluenes in female
B6C3F1 mice: I. Para-nitrotoluene. Drug Chem. Toxicol., 17(3):
317-358.
Chaube, S. (1973) Protective effects of thymidine,
5-aminoimidazolecarboxamide, and riboflavin against fetal
abnormalities produced in rats by 5-(3,3-dimethyl-1-triazene)
imidazole-4-carboxamide. Cancer Res., 33(10): 2231-2240.
Davis, G.J., McLachlan, J.A., & Lucier, G.W. (1978) The effect of
7,12-dimethylbenz(a)anthracene (DMBA) on the prenatal development of
gonads in mice. Teratology, 17: 33A (Abstract).
Hoos, P.C. & Hoffman, L.H. (1983) Effect of histamine receptor
antagonists and indomethacin in implantation in the rabbit. Biol.
Reprod., 29(4): 833-840.
Ibrahim, H.S. & Canolty, N.L. (1990) Effects of dietary lithium on
pregnant and lactating rats and the progeny. Nutr. Res., 10:
315-324.
Kawashima, K., Usami, M., Sakemi, K., & Ohno, Y. (1995) Studies on the
establishment of appropriate spermatogenic endpoints for male
fertility disturbance in rodent induced by drugs and chemicals: I.
Nitrobenzene. J. Toxicol. Sci., 20(1): 15-22.
Littlefield, N.A., Nelson, C.J., & Frith, C.H. (1983) Benzidine
dihydrochloride: Toxicological assessment in mice during chronic
exposures. J. Toxicol. Environ. Health, 12(4-6): 671-685.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A., & Wilson, R.E. (1981) The effect of
orthophenylphenol, tris(2,3-dichloropropyl) phosphate, and
cyclophosphamide on the immune system and host susceptibility of mice
following subchronic exposure. Toxicol. Appl. Pharmacol., 58(2):
252-261.
McClain, R.M. & Langhoff, L. (1979) Teratogenicity of
diphenylhydantoin in New Zealand rabbits. Toxicol. Appl.
Pharmacol., 48(1-Pt. 2): A32.
McLachlan, J.A. (1977) Prenatal exposure to diethylstilbestrol in
mice: Toxicological studies. J. Toxicol. Environ. Health, 2:(3)
527-537.
Murray, F.J., Smith, F.A., Nitschke, K.D., Humiston, C.G., Kociba,
R.J., & Schwetz, B.A. (1979) Three-generation reproduction study of
rats given 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) in the diet.
Toxicol. Appl. Pharmacol., 50(2): 241-252.
Nagasawa, H., Yanai, R., & Nakajima, Y. (1980) Suppression of
lactation by tumor promoters in mice. Proc. Soc. Exp. Biol. Med.,
165: 394-397.
NTIS (1968) Evaluation of carcinogenic, teratogenic, and mutagenic
activities of selected pesticides and industrial chemicals - Volume I:
Carcinogenic study and Volume II: Teratogenic study in mice and rats.
National Cancer Institute, Bethesda, MD (Bionetics Res. Lab).
NTP (1986) Toxicology and carcinogenesis studies of dimethylvinyl
chloride (1-chloro-2-methylpropene) (CAS No. 513-37-1) in F344/N rats
and B6C3F1 mice (Gavage studies). National Toxicology Program,
Research Triangle Park, NC (NTP Technical Report Series No. 316).
NTP (1989) Toxicology and carcinogenesis studies of ochratoxin A (CAS
No. 303-47-9) in F344/N rats (Gavage studies). National Toxicology
Program, Research Triangle Park, NC (NTP Technical Report Series No.
358).
NTP (1992) NTP Technical report on toxicity studies of o-, m-, and
p-nitrotoluenes (CAS Nos. 88-72-2, 99-08-1, 99-99-0) administered in
dosed feed to F344/N rats and B6C3F1 mice. National Toxicology
Program, Research Triangle Park, NC (NTP Toxicity Report Series No.
23).
NTP (1994) Toxicology and carcinogenesis studies of
4,4'-thiobis(6-t-butyl-m- cresol) (CAS No. 96-69-5) in F344/N rats and
B6C3F1 mice (Feed studies). National Toxicology Program, Research
Triangle Park, NC (NTP Technical Report Series No. 435).
Schepers, G.W. (1964) Tetraethyl lead and tetramethyl lead. Arch.
Environ. Health, 8(2): 277-295.
Schwetz, B.A., Quast, J.F., Keeley, P.A., Humiston, C.G., & Kociba,
R.J. (1978) Results of 2-year toxicity and reproduction studies on
pentachlorophenol in rats. In: Rao, K.R. ed. Pentachlorophenol:
Chemistry, pharmacology and environmental toxicology. Proceedings of
the EPA and University of West Florida Symposium, Pensacola, FL,
27-29 June 1977. Plenum Press, New York, pp. 301-309.
Scott, J.R. (1977) Fetal growth retardation associated with maternal
administration of immunosuppressive drugs. Am. J. Obstet. Gynecol.,
128(6): 668-676.
Teramoto, S., Saito, R., Aoyama, H., & Shirasu, Y. (1980) Dominant
lethal mutation induced in male rats by 1,2-dibromo-3-chloropropane
(DBCP). Mutat. Res., 77(1): 71-78.
Thysen, B., Bloch, E., & Varma, S.K. (1985) Reproductive toxicity of
2,4-toluenediamine in the rat: 2. Spermatogenic and hormonal effects.
J. Toxicol. Environ. Health, 16(6): 763-769.
 2.3 The scientific basis for the threshold value of 1.5
µg/person per day specified at Step B5
Over the past several years, an immense amount of information has
accumulated on the range of carcinogenic potencies for chemicals that
have been tested in animals. For these chemicals, the distribution of
potencies in experimental animals and projected human risk (calculated
using linear risk assessment models) are well established and highly
unlikely to be altered by further cancer bioassays (Krewski et al.,
1990). In fact, the Carcinogenic Potency Database (CPD) compiled by
Gold et al. (1984) that was used to derive the decision criterion of
1.5 µg per day specified at Step B5, now contains nearly 500
substances reported to be carcinogenic in animals. It is reasonable to
assume that the addition to that data-base of several more "genotoxic"
carcinogens, should they be discovered, would be unlikely to alter the
distribution of known risks for identified carcinogens. Scientists may
never be prepared to say they know all they would like to know about
the distribution of risks of existing animal carcinogens. On the other
hand, it can be estimated with considerable confidence, based on data
available today, that a substance which has not been tested for
carcinogenicity and that is consumed in an amount below the threshold
value specified at Step B5 will not present a risk of human cancer
greater than one in one million (10-6).
With these thoughts in mind, it is now important to look at the
theoretical and practical aspects of the concept of threshold of
concern and how it can be applied to flavouring substances in the
context of JECFA safety evaluations.
The Carcinogen Potency Database (CPD) contains data on
approximately 3700 long-term animal studies of 975 chemicals (Gold
et al. 1986a,b,c; 1989). These include studies conducted by the US
National Toxicology Program as well as studies conducted in other
laboratories that have been published in the literature. Of the 975
chemicals tested, 955 were tested in rats and/or mice and 492 produced
an increase in tumour incidence (342 in rats and 278 in mice). Gold
et al. have put an enormous effort into compiling this database and
ensuring its quality. The reader is referred to a series of papers by
Gold et al. and others, published in Environmental Health
Perspectives, which document the validity of this database (Gold
et al., 1984, 1986a,b,c, 1989; Peto et al., 1984; Sawyer et
al., 1984).
For each compound, the CPD may include experiments with different
species, strains, sexes, dosing regimens, routes of administration, or
other experimental conditions (Gold et al., 1984). In most
experiments, two or more dose levels were used in addition to an
unexposed control; in some cases, however, only a single exposed group
was employed. Although a rigorous evaluation of the quality of
individual experiments is not possible, the CPD does include
information on the original investigators' conclusions regarding the
overall strength of evidence for carcinogenicity.
The CPD also contains a measure of carcinogenic potency (the
TD50) computed as described by Peto et al. (1984) and Sawyer
et al. (1984). In order to obtain some degree of comparability among
different studies, all TD50 values are expressed in units of mg/kg bw
per day and are adjusted to a 2-year standard rodent lifetime. In
cases where exposure is not constant throughout the study period, a
time-weighted average dose is used for purposes of modelling
dose-response. When individual animal data are available, the TD50
values are adjusted for intercurrent mortality; otherwise, the crude
proportions of animals with tumours are used to estimate carcinogenic
potency without adjusting for mortality from other causes. Finally,
the TD50 is estimated on the basis of an essentially linear one-hit
dose-response model.
The CPD represents an extremely useful source of information on
experiments with chemical carcinogens. The database includes
experiments on highly potent rodent carcinogens such as
2,3,7,8-tetrachlorodibenzo- p-dioxin and aflatoxin B1, as well as
less potent agents such as metronidazole and DDT. Gold et al. (1984)
noted that TD50 values included in the CPD vary by 10 million-fold.
Rulis (1986) used the Gold et al. (1984, 1986a,b,c, 1989)
database when he and others at FDA derived a threshold of regulation
for packaging materials. What was done, in essence, was to transform
the distribution of lowest TD50 for each carcinogen that was tested
by the oral route to a distribution of 10-6 risks. While numerous
mathematical models including the linearized multistage model could
have been used to perform this transformation, Rulis (1986) used the
slope (2TD50)-1 of a straight line joining the TD50 and the origin
as an estimator of the slope in the low-dose region. Although the
linearized multistage model could have been used since it has the
advantage of allowing for curvature in the dose-response curve, linear
extrapolation from the TD50 is computationally simple, extremely
conservative, and requires only published potency values from the CPD.
To illustrate the differences between these two approaches,
Krewski et al. (1990) considered the data on bladder tumours in mice
exposed to the experimental carcinogen 2-acetyl-aminofluorene (2-AAF),
shown in Figure 3. As indicated in Table 2, the TD50 for bladder
tumours based on the fitted multistage model is 17.2 mg/kg bw per day,
leading to a slope of (2TD50)-1 = 0.0291 (mg/kg per day) -1. Because
over 3300 animals were involved in this experiment, the 95% lower
confidence limit on the TD50 and the corresponding upper confidence
limit on the slope are close to the best estimates obtained from the
fitted model. Due to the high degree of curvature in the dose-response
curve for bladder tumours, however, the upper confidence limit on the
slope based on linear extrapolation from the lower confidence limit on
the TD50 is more than 75-fold greater than the slope derived from the
linearized multistage model.
2.3 The scientific basis for the threshold value of 1.5
µg/person per day specified at Step B5
Over the past several years, an immense amount of information has
accumulated on the range of carcinogenic potencies for chemicals that
have been tested in animals. For these chemicals, the distribution of
potencies in experimental animals and projected human risk (calculated
using linear risk assessment models) are well established and highly
unlikely to be altered by further cancer bioassays (Krewski et al.,
1990). In fact, the Carcinogenic Potency Database (CPD) compiled by
Gold et al. (1984) that was used to derive the decision criterion of
1.5 µg per day specified at Step B5, now contains nearly 500
substances reported to be carcinogenic in animals. It is reasonable to
assume that the addition to that data-base of several more "genotoxic"
carcinogens, should they be discovered, would be unlikely to alter the
distribution of known risks for identified carcinogens. Scientists may
never be prepared to say they know all they would like to know about
the distribution of risks of existing animal carcinogens. On the other
hand, it can be estimated with considerable confidence, based on data
available today, that a substance which has not been tested for
carcinogenicity and that is consumed in an amount below the threshold
value specified at Step B5 will not present a risk of human cancer
greater than one in one million (10-6).
With these thoughts in mind, it is now important to look at the
theoretical and practical aspects of the concept of threshold of
concern and how it can be applied to flavouring substances in the
context of JECFA safety evaluations.
The Carcinogen Potency Database (CPD) contains data on
approximately 3700 long-term animal studies of 975 chemicals (Gold
et al. 1986a,b,c; 1989). These include studies conducted by the US
National Toxicology Program as well as studies conducted in other
laboratories that have been published in the literature. Of the 975
chemicals tested, 955 were tested in rats and/or mice and 492 produced
an increase in tumour incidence (342 in rats and 278 in mice). Gold
et al. have put an enormous effort into compiling this database and
ensuring its quality. The reader is referred to a series of papers by
Gold et al. and others, published in Environmental Health
Perspectives, which document the validity of this database (Gold
et al., 1984, 1986a,b,c, 1989; Peto et al., 1984; Sawyer et
al., 1984).
For each compound, the CPD may include experiments with different
species, strains, sexes, dosing regimens, routes of administration, or
other experimental conditions (Gold et al., 1984). In most
experiments, two or more dose levels were used in addition to an
unexposed control; in some cases, however, only a single exposed group
was employed. Although a rigorous evaluation of the quality of
individual experiments is not possible, the CPD does include
information on the original investigators' conclusions regarding the
overall strength of evidence for carcinogenicity.
The CPD also contains a measure of carcinogenic potency (the
TD50) computed as described by Peto et al. (1984) and Sawyer
et al. (1984). In order to obtain some degree of comparability among
different studies, all TD50 values are expressed in units of mg/kg bw
per day and are adjusted to a 2-year standard rodent lifetime. In
cases where exposure is not constant throughout the study period, a
time-weighted average dose is used for purposes of modelling
dose-response. When individual animal data are available, the TD50
values are adjusted for intercurrent mortality; otherwise, the crude
proportions of animals with tumours are used to estimate carcinogenic
potency without adjusting for mortality from other causes. Finally,
the TD50 is estimated on the basis of an essentially linear one-hit
dose-response model.
The CPD represents an extremely useful source of information on
experiments with chemical carcinogens. The database includes
experiments on highly potent rodent carcinogens such as
2,3,7,8-tetrachlorodibenzo- p-dioxin and aflatoxin B1, as well as
less potent agents such as metronidazole and DDT. Gold et al. (1984)
noted that TD50 values included in the CPD vary by 10 million-fold.
Rulis (1986) used the Gold et al. (1984, 1986a,b,c, 1989)
database when he and others at FDA derived a threshold of regulation
for packaging materials. What was done, in essence, was to transform
the distribution of lowest TD50 for each carcinogen that was tested
by the oral route to a distribution of 10-6 risks. While numerous
mathematical models including the linearized multistage model could
have been used to perform this transformation, Rulis (1986) used the
slope (2TD50)-1 of a straight line joining the TD50 and the origin
as an estimator of the slope in the low-dose region. Although the
linearized multistage model could have been used since it has the
advantage of allowing for curvature in the dose-response curve, linear
extrapolation from the TD50 is computationally simple, extremely
conservative, and requires only published potency values from the CPD.
To illustrate the differences between these two approaches,
Krewski et al. (1990) considered the data on bladder tumours in mice
exposed to the experimental carcinogen 2-acetyl-aminofluorene (2-AAF),
shown in Figure 3. As indicated in Table 2, the TD50 for bladder
tumours based on the fitted multistage model is 17.2 mg/kg bw per day,
leading to a slope of (2TD50)-1 = 0.0291 (mg/kg per day) -1. Because
over 3300 animals were involved in this experiment, the 95% lower
confidence limit on the TD50 and the corresponding upper confidence
limit on the slope are close to the best estimates obtained from the
fitted model. Due to the high degree of curvature in the dose-response
curve for bladder tumours, however, the upper confidence limit on the
slope based on linear extrapolation from the lower confidence limit on
the TD50 is more than 75-fold greater than the slope derived from the
linearized multistage model.
 Table 2. Low-dose slopes for bladder tumors in mice exposed to
2-AAF for 24 months based on linear extrapolation from the TD50
Source of TD50 TD50 Slope of (2TD50)-1
(mg/kg/day) (mg/kg/day)
Fitted multistage model
Best estimate 17.2 0.0291
95% Confidence limit 16.71 0.02982
Fitted one-hit model
Best estimate 96.0 0.0052
95% Confidence limit 77.42 0.00652
95% Confidence limit on
the linear term in
multistage model (q1*) 0.00042
1 Lower confidence limit
2 Upper confidence limit
These data show that when there is significant upward curvature in
the dose-response curve, the methodology employed by Rulis (1986) to
calculate a dose associated with a 10-6 risk will produce a value
substantially lower than when these risk-specific doses are calculated
using the linearized multistage model. This point also has been made
by Hoel & Portier (1994), who noted that examination of the shape of
the dose-response curve for 315 chemicals found to produce cancer in
the NCI/NTP program indicates that tumour site data were more often
consistent with a quadratic response than a linear response,
suggesting that the use of linear dose-response models will often
overestimate risk. It may therefore be concluded that the methodology
used by Rulis (1986) (Federal Register, 1993, 1995) to estimate the
distribution of 10-6 risks for carcinogens in the Gold et al. CPD
was appropriately conservative.
The next step in the process of establishing a threshold value
involves the selection of an appropriate exposure, which is based on
the distribution of 10-6 risks. This value must be both highly
protective of human health and of sufficient practical value to reduce
the number of compounds requiring formal toxicological testing. Two
steps need to be considered here: the first involves the selection of
an appropriate risk standard that will ensure that any threshold value
selected will have an acceptably high probability of health
protection. The second step involves being pragmatic enough in the
selection of a threshold value that it still is of sufficient
magnitude to be of practical value. Of course, the protection of human
health is of greater concern than the practical value.
Initially Rulis (1986, 1989) proposed, for illustration, a
threshold value of 0.15 µg/person per day. Based on the distribution
of 10-6 risks from the CPD, this value would intersect the
distribution at the 85th centile, meaning that only 15% of carcinogens
in the database would present a risk greater than 10-6 at an intake
of 0.15 µg/day. This analysis indicates that, at an intake of 0.15
µg/person per day, 85% chemicals in the CPD known to induce cancer in
rodents would fail to show a significant increase in risk for the
exposed population. This is demonstrated graphically in Figure 4,
modified from Rulis (1989).
Table 2. Low-dose slopes for bladder tumors in mice exposed to
2-AAF for 24 months based on linear extrapolation from the TD50
Source of TD50 TD50 Slope of (2TD50)-1
(mg/kg/day) (mg/kg/day)
Fitted multistage model
Best estimate 17.2 0.0291
95% Confidence limit 16.71 0.02982
Fitted one-hit model
Best estimate 96.0 0.0052
95% Confidence limit 77.42 0.00652
95% Confidence limit on
the linear term in
multistage model (q1*) 0.00042
1 Lower confidence limit
2 Upper confidence limit
These data show that when there is significant upward curvature in
the dose-response curve, the methodology employed by Rulis (1986) to
calculate a dose associated with a 10-6 risk will produce a value
substantially lower than when these risk-specific doses are calculated
using the linearized multistage model. This point also has been made
by Hoel & Portier (1994), who noted that examination of the shape of
the dose-response curve for 315 chemicals found to produce cancer in
the NCI/NTP program indicates that tumour site data were more often
consistent with a quadratic response than a linear response,
suggesting that the use of linear dose-response models will often
overestimate risk. It may therefore be concluded that the methodology
used by Rulis (1986) (Federal Register, 1993, 1995) to estimate the
distribution of 10-6 risks for carcinogens in the Gold et al. CPD
was appropriately conservative.
The next step in the process of establishing a threshold value
involves the selection of an appropriate exposure, which is based on
the distribution of 10-6 risks. This value must be both highly
protective of human health and of sufficient practical value to reduce
the number of compounds requiring formal toxicological testing. Two
steps need to be considered here: the first involves the selection of
an appropriate risk standard that will ensure that any threshold value
selected will have an acceptably high probability of health
protection. The second step involves being pragmatic enough in the
selection of a threshold value that it still is of sufficient
magnitude to be of practical value. Of course, the protection of human
health is of greater concern than the practical value.
Initially Rulis (1986, 1989) proposed, for illustration, a
threshold value of 0.15 µg/person per day. Based on the distribution
of 10-6 risks from the CPD, this value would intersect the
distribution at the 85th centile, meaning that only 15% of carcinogens
in the database would present a risk greater than 10-6 at an intake
of 0.15 µg/day. This analysis indicates that, at an intake of 0.15
µg/person per day, 85% chemicals in the CPD known to induce cancer in
rodents would fail to show a significant increase in risk for the
exposed population. This is demonstrated graphically in Figure 4,
modified from Rulis (1989).
 Subsequent to the report of Rulis (1989), Munro (1990) held a
workshop to evaluate factors that influence the selection of an
appropriate threshold value. The workshop first reanalysed the Gold
et al. (1984) database using the original database of 343 rodent
carcinogens and confirmed the observations of Rulis (1986, 1989) that
a dietary intake of 0.15 µg/day intersected the distribution of 10-6
risks at approximately the 85th centile. In addition, the workshop
extended the analysis to include additional carcinogens added to the
original Gold et al. (1984) database, bringing the total to 492
rodent carcinogens (Gold et al., 1989). This reanalysis with a
broader set of data produced essentially the same distribution of
1 × 10-6 risks as was originally published by Rulis (1986, 1989). The
workshop participants also noted that inherent in the acceptance of
any threshold value was the assumption that every new untested
substance was a likely carcinogen and could be as potent as the most
potent 15% of carcinogens in the CPD.
Recognizing that not every new substance would turn out to be a
carcinogen, the workshop (Munro, 1990) constructed a table of risk
avoidance probabilities (Table 3).
This table shows the effect of various assumptions regarding the
proportion of chemicals that are presumed carcinogens on the
probability that a 10-6 risk standard will not be exceeded. It should
be noted that as this proportion decreases, the probability of not
exceeding a specific risk standard increases dramatically. Thus, for
example, while there is a 63% chance that the risk will not exceed
10-6 with a value of 1.5 µg/person per day when 100% of new chemicals
are assumed to be carcinogenic, the probability that the risk will be
less than 10-6 is 96% when only 10% of new chemicals are assumed to
be carcinogenic. Moreover, if one invokes a less conservative risk
standard of 10-5 (Table 3, right side), then the probability of not
exceeding that risk at a threshold value of 1.5 µg/person per day
exceeds 96% even if it is assumed that 50% of new chemicals are
potential carcinogens. It also should be remembered that, in theory,
the probability of an untested substance having a potency greater than
the median of the distribution of TD50s from the CPD (Gold et al.,
1989) is 50%. In reality, however, it is most unlikely that a
genotoxic carcinogen with a potency equal to or greater than the
median carcinogen in the Gold et al. (1989) database would be
discovered from the existing inventory of flavouring substances, given
existing knowledge of structure-activity relationships in
carcinogenesis.
Table 3. Probability of a target risk not being exceeded at various threshold values
Threshold value Percentage of chemicals presumed carcinogenic
(µg/day) 100% 50% 20% 10% 100% 50% 20% 10%
10-6 Target risk 10-5 Target level
0.15 86 93 97 99 96 98 99 >99
0.3 80 90 96 98 94 97 99 99
0.6 74 87 95 97 91 96 98 99
1.5 63 82 93 96 86 96 97 99
3.0 55 77 91 95 80 90 96 98
6.0 46 73 89 95 74 87 95 97
(Modified from Munro, 1990).
Taking the above factors into consideration and keeping in mind
that the calculated 10-6 risks based on the Gold et al. (1989)
database were derived using a highly conservative methodology, Rulis
(1989) reexamined his previous selection criteria for a threshold
value and those of Munro (1990) and concluded that a threshold value
of 1.5 µg/person per day would provide a high degree of health
protection. This threshold value was subsequently adopted by FDA as
the threshold of regulation (Federal Register, 1993, 1995) and FDA
noted that such an exposure level would result in a negligible risk
even in the event that a substance of unknown toxicity was later shown
to be a carcinogen.
3. APPLICATION OF A THRESHOLD OF TOXICOLOGICAL CONCERN TO
FLAVOURING SUBSTANCES
The Scientific Committee for Food (SCF, 1996) has made the point
that the decision to accept any particular threshold value is both a
scientific and a risk management decision. The role of the scientist
is to ensure that risk managers are provided with the full range of
uncertainties surrounding selection of any threshold value. In the
foregoing sections it was pointed out that the threshold concept
should not be interpreted as providing absolute certainty of no risk.
Threshold of toxicological concern is a probabilistic methodology that
involves acceptance of a negligible risk standard. Such a standard is
commonly used by toxicologists in the establishment of ADIs, and, in
fact, IPCS (1987) has defined the ADI as "an estimate by JECFA of the
amount of a food additive, expressed on a body weight basis, that can
be ingested daily over a lifetime without appreciable health risk".
JECFA has noted that it uses the risk assessment process when setting
an ADI, i.e., the level of "no apparent risk" is set on the basis of
quantitative extrapolation from animal data to human beings typically
using a NOEL from the animal studies divided by a 100-fold safety
factor (IPCS, 1987). When ADIs (or such similar limits e.g., TLVs,
RfD, etc.) are established, there is a residual, usually
unquantifiable, element of risk (Purchase & Auton, 1995; Baird
et al., 1996; SCF, 1996; Sielken & Valdez-Flores, 1996). This is a
reflection of the inability to determine precisely the NOEL from
empirical data, statistical uncertainties associated with the
sensitivity of experimental models, completeness of data, or the
magnitude of safety factors needed to account for any residual
uncertainty (Dourson & Stara, 1983).
The threshold of toxicological concern likewise does not carry
with it the absolute certainty that an untested chemical present in
food below the decision criterion specified at Step B5 will present a
less than 10-6 risk. Rather there is a high probability (i.e. about
95%) that the cancer risk from such a chemical will be less than
10-6. It is this residue of uncertainty that has produced a concern
about the possibility, albeit remote, that a highly potent genotoxic
carcinogen might inadvertently be considered acceptable using the
threshold concept (SCF, 1996).
3.1 Additional factors that reduce the theoretical risk
When the threshold concept is applied to flavouring substances,
two additional factors significantly reduce the probability of risk of
cancer below one in one million. The first of these relates to very
low levels of exposure to flavouring substances. As exposure
decreases, the probability of not exceeding a 10-6 risk substantially
increases, approaching 97 to 99% at an order of magnitude below the
1.5 µg/person per day value (Table 3). Therefore, application of a
threshold of toxicological concern to substances having very low
exposures (i.e., less or much less than the threshold value) carries
with it a much higher probability of no appreciable risk. It also must
be kept in mind that exposure to the vast majority of flavouring
substances tends to be overestimated because these materials are
volatile and appreciable amounts are lost during food preparation,
storage, etc. These issues regarding exposure to flavouring substances
are discussed in Annex 5 of WHO Food Additives Series 35 (JECFA,
1996).
The second factor involves a consideration of chemical structure.
The use of chemical structure for predicting toxicity for food
chemicals, especially flavouring substances, has long been recognized
by JECFA (IPCS, 1987), and JECFA has noted that use of
structure-activity is most highly developed in the area of
carcinogenesis. The use of structural alerts in combination with a
knowledge of chemistry and metabolism offers a way of identifying
potential carcinogens (Ashby & Tennant 1988, 1991; Williams, 1990;
Tennant et al., 1990; Klopman & Rosenkranz, 1994). The examination
of many chemicals for genotoxic, mutagenic and carcinogenic activities
has led to the preparation of a series of structural alerts that
provide the basis for potential reaction with DNA and possible
carcinogenic potential of the substance. The existence of reactive
moieties on known rodent carcinogens implies that potential mutagenic
activity and, in many cases, the carcinogenic activity of untested
chemicals might be identified by an examination of structure (Ashby &
Tennant, 1988, 1991; Tennant & Ashby, 1991).
Structure-activity relationships have been successfully applied to
congeneric substances (i.e., individual substances within a
structurally related group of substances) for which no toxicity data
are available (Klopman & Rosenkranz, 1994). Congeners that are
potential human carcinogens and mutagens possess electrophilic
functional groups with the ability to react directly with DNA. These
electrophilic sites may be reactive functional groups on the congener
or those formed during metabolic activation. Conversely, these
functional groups may be lost during metabolic detoxication of the
substance. Although the carcinogenic and mutagenic potency of
congeneric substances may differ, structural alerts within the group
of congeners are indicative of carcinogenic or mutagenic potential
(Klopman & Rosenkranz, 1994). A list of the functional groups
identified by Ashby & Tennant (1988, 1991) and Tennant et al. (1990)
as structural alerts is given in Table 4.
Table 4. A list of functional groups identified by Ashby & Tennant
(1988, 1991) and Tennant et al. (1990) as structural alerts for DNA
reactivity
a) alkyl esters of phosphonic or sulfonic acids
b) aromatic nitro-groups
c) aromatic azo-groups (reduction to amine)
d) aromatic ring N-oxides
e) aromatic mono- and di-alkyl amino groups
f) alkyl hydrazines
g) alkyl aldehydes
h) N-methylol derivatives
i) monohaloalkanes
j) N and S mustards, beta-haloethyl-
k) N-chloramines
l) propiolactones and propiosulfones
m) aromatic and aliphatic aziridinyl derivatives
n) aromatic and aliphatic substituted primary alkyl halides
o) urethane derivatives (carbamates)
p) alkyl N-nitrosamines
q) aromatic amines and N-hydroxy derivatives
r) aliphatic epoxides and aromatic oxides
s) center of Michael reactivity
t) halogenated methanes (C(X)4)
u) aliphatic nitro groups
Most flavouring agents are simple aliphatic and aromatic
substances containing functional groups that are efficiently
metabolized via detoxication pathways, and very few flavouring agents
and/or their in vivo metabolites contain structural alerts. The
absence of structural alerts in a flavouring agent provides added
assurance that it will not present an appreciable risk at or below the
decision criterion of 1.5 µg/person per day. For those that do contain
significant structural alerts, such as aliphatic epoxides, additional
data have been generated to facilitate evaluation.
4. SUMMARY
The application of the decision criterion at Step B5 of the safety
evaluation procedure provides a means of evaluating those flavouring
substances that have extremely low exposures but lack metabolic and
toxicity data. It is recognized that application of a threshold of
toxicological concern is a departure from traditional toxicological
evaluation but, nonetheless, is based on highly conservative
methodology and assumptions which, taken together, ensure that
flavouring substances meeting the proposed criteria will present, at
most, an insignificant risk.
The following factors taken together ensure the absence of any
appreciable theoretical risk from the use of flavouring substances
evaluated using this procedure:
1. The decision criterion is based on carcinogenesis data, an
extremely sensitive end-point in susceptible animal species with
accepted relevance to humans.
2. The CPD presents a worse case situation since chemicals were
generally tested over a lifetime by daily administration at the
maximum tolerated dose (MTD), and the procedures used by Gold et
al. (1984) to establish the TD50s involved numerous conservative
assumptions.
3. The methods used by Rulis (1986, 1989) and others (Krewski et
al., 1990; Munro, 1990) to calculate the distribution of 10-6
risks, upon which the threshold value of 1.5 µg/person per day is
based, are highly conservative since they involved the use of linear
extrapolation from the lowest TD50 for each substance in the database.
4. It is unlikely that any untested flavouring agent would turn out to
be a genotoxic carcinogen, and the possibility that a carcinogen would
be accepted using the threshold concept can be substantially reduced
by the application of structural alert methodology.
5. Many flavouring substances are consumed in amounts considerably
below the threshold value of 1.5 µg/person per day and this
substantially increases the probability, already in the range of 90 to
95%, that they will not present any significant theoretical risk.
6. Toxicity end-points such as developmental toxicity, neurotoxicity
and immunotoxicity demonstrate considerably higher human exposure
thresholds than the threshold value of 1.5 µg/person per day specified
at Step B5 making it highly unlikely that these non-cancer end-points
are a relevant concern in applying the threshold concept.
Taken together, these factors provide a sound basis for concluding
that flavouring substances with intakes below the 1.5 µg/person per
day threshold of toxicological concern specified at Step B5 can be
safely consumed.
ACKNOWLEDGEMENT
The authors are deeply indebted to Dr. Alan Rulis for reviewing this
paper and providing many useful suggestions.
5. REFERENCES
Ashby, J. & Tennant, R.W. (1988) Chemical structure, Salmonella
mutagenicity and extent of carcinogenicity as indicators of genotoxic
carcinogenesis among 222 chemicals tested in rodents by the US
NCI/NTP. Mutat. Res., 204: 17-115.
Ashby, J. & Tennant, R.W. (1991) Definitive relationships among
chemical structure, carcinogenicity and mutagenicity for 301 chemicals
tested by the US NTP. Mutat. Res., 257: 229-306.
Baird, S.J.S., Cohen, J.T., Graham, J.D., Shlyakhter, A.I., & Evans,
J.S. (1996) Noncancer risk assessment: A probabilistic alternative to
current practice. Hum. Ecol. Risk Assess., 2(1): 79-102.
Dourson, M.L. & Stara, J.F. (1983) Regulatory history and
experimental support of uncertainty (safety) factors. Regul.
Toxicol. Pharmacol., 3: 224-228.
Dourson, M.L., Felter, S.P., & Robinson, D. (1996) Evolution of
science-based uncertainty factors in noncancer risk assessment.
Regul. Toxicol. Pharmacol., 24: 108-120.
Federal Register (1993) Food additives: Threshold of regulation for
substances used in food-contact articles (Proposed rule). Fed. Reg.,
58(195): 52719-52729.
Federal Register (1995) Food additives: Threshold of regulation for
substances used in food-contact articles (Final rule). Fed. Reg.,
60(136): 36582-36596.
Frawley, J.P. (1967) Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet. Toxicol., 5: 293-308.
Gold, L.S., Sawyer, C.B., Magaw, R., Backman, G.M., de Veciana, M.,
Levison, R., Hooper, N.K., Havender, W.R., Bernstein, L., Peto, R.,
Pike, M., & Ames, B.N. (1984) A carcinogenic potency database of the
standardized results of animal bioassays. Environ. Health Perspect.,
58: 9-319.
Gold, L.S., de Veciana, M., Backman, G.M., Magaw, R., Lopipero, P.
Smith, M., Blumenthal, M., Levinson, R., Bernstein, L., & Ames. B.N.
(1986a) Chronological supplement to the carcinogenic potency database:
Standardized results of animal bioassays published through December
1982. Environ. Health Perspect., 67: 161-200.
Gold, L.S., Bernstein, L., Kaldor, J., Backman, G., & Hoel, D. (1986b)
An empirical comparison of methods used to estimate carcinogenic
potency in long-term animal bioassays: Lifetable vs summary incidence
data. Fundam. Appl. Toxicol., 6: 263-269.
Gold, L.S., Ward, J.M., Bernstein, L., & Stern B. (1986c) Association
between carcinogenic potency and tumor pathology in rodent
carcinogenesis bioassays. Fundam. Appl. Toxicol., 6: 677-690.
Gold, L.S., Slone, T.H., & Bernstein, L. (1989) Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79:
259-272.
Hall, R.L. (1975) Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey -- Phase III, and to the FEMA.
Hoel, D.G. & Portier, C.J. (1994) Nonlinearity of dose-response
functions for carcinogenicity. Environ. Health Perspect., 102(1):
109-113.
IPCS (1987) Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. World
Health Organization, International Programme on Chemical Safety,
Geneva.
IPCS (1994) Environmental Health Criteria 170: Assessing human health
risk of chemicals: Derivation of guidance values for health-based
exposure limits. World Health Organization, International Programme on
Chemical Safety, Geneva.
JECFA (1995) Evaluation of certain food additives and contaminants.
Forty-fourth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 859).
JECFA (1996) Toxicological evaluation of certain food additives and
contaminants. World Health Organization, Geneva (WHO Food Additives
Series 35).
JECFA (1997) Evaluation of certain food additives and contaminants.
Forty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 868).
Klopman, G. & Rosenkranz, H.S. (1994) Approaches to SAR in
carcinogenesis and mutagenesis. Prediction of
carcinogenicity/mutagenicity using MULTI-CASE. Mutat. Res., 305:
33-46.
Krewski, D., Szyszkowicz, M., & Rosenkranz, H. (1990) Quantitative
factors in chemical carcinogenesis: Variation in carcinogenic potency.
Regul. Toxicol. Pharmacol., 12: 13-29.
Luster, M.I., Portier, C., Pait, D.G., White,K.L. Jr, Gennings, C.,
Munson, A.E., & Rosenthal, G.J. (1992) Risk assessment in
immunotoxicology: I. Sensitivity and predictability of immune tests.
Fundam. Appl. Toxicol., 18(2): 200-210.
Luster, M.I., Portier, C., Pait, D.G., Rosenthal, G.J., Germolec,
D.R., Gorsini, E., Blaylock, B.L., Pollock, P., Kouchi, Y., Craig, W.,
White, K.L., Munso, A.E., & Comment, C.E. (1993) Risk assessment in
Immunotoxicology: II. Relationships between immune and host
resistance tests. Fundam. Appl. Toxicol., 21(1): 71-82.
Munro, I.C. (1990) Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
Munro, I.C., Ford, R.A. Kennepohl, E., & Sprenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: A
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Peto, R., Pike, M.C., Bernstein, L., Gold, L.S., & Ames, B.N. (1984)
The TD50: A proposed general convention for the numerical description
of the carcinogenic potency of chemicals in chronic-exposure animal
experiments. Environ. Health Perspect., 5: 1-8.
Purchase, I.F.H. & Auton, T.R. (1995) Thresholds in chemical
carcinogenesis. Regul. Toxicol. Pharmacol., 22: 199-205.
RTECS (1987) Registry of toxic effects of chemical substances, 1985-86
ed. - Volume 1. US Department of Health and Human Services, Public
Health Service, Washington, D.C.
Rulis, A.M. (1986) De minimis and the threshold of regulation. In:
Felix, C.W. ed. Food protection technology. Lewis Publishers Inc.,
Chelsea, MI. pp. 29-37.
Rulis, A.M. (1989) Establishing a threshold of concern. In: Bonin,
J.J. & Stevenson, D.E. ed. Risk assessment in setting national
priorities. Plenum Press, New York, vol. 7, pp. 271-278.
Sawyer, C., Peto, R., Bernstein, L., & Pike, M.C. (1984) Calculation
of carcinogenic potency from long-term animal carcinogenesis
experiments. Biometrics, 40: 27-40.
SCF (1996) Opinion on response to request from the Commission for SCF
opinion on the scientific basis of the concept of threshold of
regulation in relation to food contact materials. European
Commission, Scientific Committee for Food, Brussels, Belgium.
Sielken, R.L. & Valdez-Flores, C. (1996) Comprehensive realism's
weight-of-evidence based distributional dose-response
characterization. Hum. Ecol. Risk Assess., 2(1): 175-193.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity
of a further 39 chemicals tested for carcinogenicity by the US
National Toxicology Program. Mutat. Res., 257: 209-227.
Tennant, R.W., Spalding, J., Stasiewicz, S., & Ashby, J. (1990)
Prediction of the outcome of rodent carcinogenicity bioassays
currently being conducted on 44 chemicals by the National Toxicology
Program. Mutagenesis, 5: 3-14.
Williams, G.M. (1990) Screening procedures for evaluating the
potential carcinogenicity of food-packaging chemicals. Regul.
Toxicol. Pharmacol., 12: 30-40.
Table 1. NOELS for organophosphate insecticides1
Agent Species End-point observed NOEL (mg/kg bw per day)
Acephate rat decreased body weight gain (parents and pups) 2.5
Azinphos methyl rat inhibition of plasma ChE activity 0.18
Coumaphos rat inhibition of RBC and plasma ChE activity 0.4
Crufomate rat inhibition of RBC ChE activity 3
Diazinon mouse decreased body weight gain 722
Dichlorvos rat inhibition of ChE activity (specific end-point
not indicated) 0.23
Dimethoate rat inhibition of brain, RBC and plasma ChE activity 0.05
Disulfoton rat inhibition of brain, RBC and plasma ChE activity 0.05
Ethephon rat inhibition of plasma and RBC ChE activity 15
Ethion rat inhibition of plasma ChE activity in females 0.2
Ethyl-p-nitrophenylphenyl
phosphorothioate rat inhibition of brain, RBC and plasma ChE activity 0.25
Express rat decreased body weight gain 1
Fenamiphos rabbit decreased maternal body weight gain 0.1
Fenchlorphos rat inhibition of ChE (form not specified) 15
Fonofos rat inhibition of RBC and plasma ChE activity 0.5
Glufosinate ammonium rat increased absolute and relative kidney
weight in males 0.42
Glyphosate rat increased incidence of renal tubular
dilation in F3b pups 10
Malathion rat inhibition of brain ChE activity 5
Merphos rat inhibition of RBC ChE activity in females 0.1
Merphos oxide rat inhibition of brain ChE activity 0.25
Methamidophos rat clinical signs typical of ChE inhibition 1
Methidithion rat inhibition of brain and RBC ChE activity 0.2
Methyl parathion rat decreased haemoglobin, haematocrit and RBCs 0.025
Naled rat decreased body weight gain 0.2
Parathion rat decreased body weight gain 1.8
Phosmet rat inhibition of RBC and plasma ChE activity 2
Phosphamidon rat decreased body weight gains 6.2
Pirimiphos-methyl rat inhibition of plasma ChE activity 0.5
Table 1. Continued...
Agent Species End-point observed NOEL (mg/kg bw per day)
Quinalphos mouse inhibition of plasma ChE activity 0.03
Tetrachlorvinphos rat inhibition of RBC ChE activity 6
Tetraethyl dithio
pyrophosphate rat inhibition of RBC and plasma ChE activity 0.5
1 Data taken from the EPA (IRIS) database and WHO 1969 Integrated Risk Information System
2 NOEL divided by a factor of 3 (see Munro et al., 1996 for explanation)
Table 2. Substances reported to cause developmental abnormalities (from RTECS)
Chemical Species Dose (TDLo)1
mg/kg bw
1 1,3,4-Thiadiazole, 2,2'-(methylenediimino)bis- rat 1
2 1-6-Hexanediamine rat 1840
3 1-Piperazinepropanol, 4-(6-((6-methoxy-8-quinolyl)amino)hexyl)-alpha-methyl-, maleate (1:2) rat 90
4 11H-Pyrido(2,1-b)quinazolone-2-carboxylic acid, 11-oxo- rat 4400
5 1H-Indazole-3-carboxylic acid, 1-(2,4-dichlorobenzyl)- rat 175
6 1H-Isoindole-1,3(2H)-dione,4,5,6,7-tetrahydro-2-(7-fluoro-3,4-dihydro-3-oxo-4-;
(2-propynyl)-2H-1,4-benzoxazin-6-yl)- rat 300
7 2,7-Naphthalenedisulfonic acid, 3,3'((3,3'-dimethyl-4,4'biphenylylene)bis(azo))
bis(5-; amino-4-hydroxy-, tetrasodium salt) rat 150
8 2-Propanone, 1,1,3,3-tetrachloro- rabbit 130
9 2-Pyridinemethanol,alpha-(3-(2,6-dimethyl-1-piperidinyl)propyl)-alpha-phenyl-,;
monohydrochloride, Z-(+-)- rabbit 650
10 3-Biphenylcarboxylic acid, 2',4'-difluoro-4-hydroxy- rabbit 520
11 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-((aminophenylacetyl)amino)-;
3,3-dimethyl-7-oxo-,(2,2-dimethyl-1-oxopropoxy)methyl ester,hydrochloride mouse 1200
12 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-(2-amino-2-phenylacetamido)-;
3,3-dimethyl-7-oxo-,trihydrate,D-(-)- rat 2800
13 4H-Pyrido(1,2-a)pyrimidin-4-one,9-methyl-3-(1H-tetrazol-5-yl)-, potassium salt rat 2750
14 4H-s-Triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine,6-(o-chlorophenyl)-8-ethyl-1-methyl- rabbit 13
15 5-Isoxazoleacetic acid, 3,4-bis(4-methoxyphenyl)- rat 1650
16 7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid,2,3-dihydro-9-fluoro-3-methyl-;
10-(4-methyl-1-piperazinyl)-7-oxo-,hemihydrate,(S)- rat 8910
17 9H-Purine-6-thiol, 9-beta-D-ribofuranosyl- rat 87.5
18 Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-(methylsulfonyl)phenethyl)-,;
D-threo-(+) rat 150
19 Acetamide, N,N-dimethyl- rabbit 3900
20 Acetic acid, (2,4-dichlorophenoxy)- rat 0.22
21 Acetic acid, (3,5,6-trichloro-2-pyridyloxy)- rat 2000
22 Acetic acid, oxo((3-(1H-tetrazol-5-yl)phenyl)amino)-,butyl ester rat 24000
23 Acetonitrile, amino-, bisulfate rat 200
24 Acridine, 9,9-dimethyl-10-(3-(N,N-dimethylamino)propyl)-,tartrate mouse 175
25 Alanine, 3-(3,4-dihydroxyphenyl)-, L- rat 500
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
26 Alanine, N-((5-chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-,;
sodium salt, (-)- rat 5
27 Alosenn rat 5500
28 Anthranilic acid, N-(2,3-xylyl)- mouse 8
29 Arsine oxide, dimethylhydroxy- rat 300
30 Benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-, monoacetate rabbit 390
31 Benzenesulfonamide, 4-amino-N-(4,5-dimethyl-2-oxazolyl)-,mixt. with 5-((3,4,5-;
trimethoxyphenyl)methyl)-2,4-pyrimidinediamine rat 3360
32 Benzenesulfonamide, 4-amino-N-(4,6-dimethoxy-2-pyrimidinyl)- rat500
33 Benzenesulfonic acid, thio-,S,S'-(2-(dimethylamino)trimethylene) ester rat 660
34 Benzhydrol, 2-chloro-alpha-(2-(dimethylamino)ethyl)-,hydrochloride mouse 120
35 Benzoic acid, 3,4,5-trimethoxy-beta-(dimethylamino)-beta-ethylphenethyl ester,; maleate (1:1) rabbit 6500
36 Benzyl alcohol, 4-amino-alpha-((tert-butylamino)methyl)-3,5-dichloro-,monohydrochloride rat 4.4
37 Biphenyl, 3,3',4,4'-tetramethyl- mouse 640
38 Butyric acid, 4-(p-bis(2-chloroethyl)aminophenyl)- mouse 3
39 Butyrophenone, 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluoro- rat 5.04
40 Cadmium rat 23
41 Carbazic acid, 3-(1-phthalazinyl)-, ethyl ester, monohydrochloride mouse 70
42 Chlordane rat 880
43 Cortisone mouse 500
44 Dibenzo(b,e)(1,4)dioxin, 2,3,7,8-tetrabromo- mouse 0.216
45 Dibenzo-p-dioxin, 2,7-dichloro- rat 5
46 Disulfide, bis(thiocarbamoyl) mouse 105
47 Ethane, 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)- rat 2000
48 Ethane, 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1,1-trichloro- rat 250
49 Ethanone, 1-(7-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-2-benzofuranyl)-, hydrochloride rat 1400
50 Ethanone, 2-((4-(2,4,dichloro-3-methylbenzoyl)-1,3-dimethyl-1H-pyrazol-5-yl)oxy)-;
1-(4-methylphenyl)- rat 2000
51 Folic acid, methyl- rat 500
52 Gallic acid, propyl ester rat 45000
53 Glutamic acid, N-(p-((1-(2-amino-4-hydroxy-6-pteridinyl)ethyl)amino)benzoyl)-L- rat 20
54 Gossypol acetic acid mouse 480
55 Hydrocinnamic acid, alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, L- rat 2100
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
56 Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl- rat 1
57 Isonicotinamide, 2-ethylthio- mouse 450
58 Isothiocyanic acid, butenyl ester rat 800
59 L-Glutamic acid, magnesium salt (1:1), hydrobromide rat 6000
60 L-Tyrosine rat 3500
61 L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo- rat 26.25
62 Linoleic acid (oxidized) rat 166000
63 Lysine, L- rat 81000
64 Manganese, (ethylenebis(dithiocarbamato))- and zinc acetate (50:1) rat 765
65 Mannitol, 1,6-dibromo-1,6-dideoxy-, D- mouse 150
66 Methanol, 1,3,4-thiadiazol-2-ylminodi- rat 5
67 Molybdenum rat 5.8
68 Morpholine, 4-(3,4,5-trimethoxybenzoyl)- rat 700
69 Norleucine, 6-amidino-, monohydrochloride, hydrate rat 2000
70 Oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one, 10-chloro-11b-(o-chlorophenyl)-2,3,7,11b- ;
tetrahydro mouse 1800
71 Phenol, p-amino- rat 2500
72 Phenothiazine-2-acetic acid, 10-methyl mouse 180
73 Phosphonic acid, (1,2-epoxypropyl)-, calcium salt (1:1), (1R,2S)-(-)- rat 15400
74 Phosphorodithioic acid, O,O-dimethyl ester, S-ester with 2-mercapto-N-methylacetamide rat 120
75 Phthalic acid, di(methoxyethyl)ester rat 593
76 Piperazine, 1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)- rat 320
77 Piperazine,1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)-,dihydrochloride rat 360
78 Piperidine, 3-((4-methoxyphenoxy)methyl)-1-methyl-4-phenyl-, hydrochloride, (3R-trans)- rat 210
79 Piperidine, 1-methyl-4-(N-2-thenylanilino)-, tartrate rat 157
80 Polychlorinated biphenyl (aroclor 1254) rat 90
81 Pregn-4-ene-3,20-dione,9-fluoro-11-beta,17,21-trihydroxy- rabbit 2
82 Pregna-,4-diene-2,20-dione,9-fluoro-11-beta,16-alpha,17,21-tetrahydroxy-,16,21-diacetate mouse 3.2
83 Propionic acid,2-(2,4,5-trichlorophenoxy)- mouse 1617
84 Pyrimidine, 2,4-diamino-6-methyl-5-phenyl- rat 100
85 Retinoic acid, 4-oxo-,13-cis- mouse 100
86 Retinoic acid, all-trans- mouse 15
87 Rowachol rat 9600
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
88 Stannane, diacetoxydibutyl- rat 15.2
89 Sulfanilamide, N(sup 1)-(6-methoxy-2-methyl-4-pyrimidinyl)- mouse 3000
90 Toluene, alpha-(2-(2-butoxyethoxy)ethoxy)-4,5-(methylenedioxy)-2-propyl- rat 2130
91 Tryptophan, N-acetyl-,L- rat 27500
92 Urea, (alpha-(2-methylhydrazino)-p-toluoyl)-, monohydrobromide rabbit 50
93 Urea, 1-butyl-3-(p-tolylsulfonyl)- mouse 1700
94 Urea, 1-butyl-3-sulfanilyl- rat 1000
95 Uridine, 5'-deoxy-5-fluoro- rat 550
96 ZZL-0820 rabbit 325
97 beta-Escin mouse 36
98 m-Propionotoluidide,2-methyl-4'-nitro-alpha,alpha,alpha-triflouro- rat 1050
99 p-Acetophenetidide rat 6000
100 p-Cresol, 2,6-di-tert-butyl- mouse 1200
1 TDLo = the lowest dose of a substance reported to produce any non-significant adverse effect (=NOEL).
Table 3. Substances with immunotoxic NOELs
Substance Immune Non-immune Non-immune Reference
NOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day)
ORAL ADMINISTRATION
1 p-nitrotoluene 400 200 hepatic, splenic Burns et al., 1994
2 pentachlorophenol 10 3 hepatic, renal Schwetz et al., 1978
3 o-phenylphenol 100 10 blood (red blood cells) Luster et al., 1981
4 hexachlorodibenzo-p-dioxin 0.056 1E-05 reproductive Murray et al., 1979
5 DPH 150 50 teratogenic McClain & Langhoff, 1979
6 tetraethyl lead 0.5 0.0012 hepatic,thymus Schepers, 1964
7 benzidine 11 2.7 neural, hepatic Littlefield et al., 1983
8 nitrobenzene 30 60 reproductive Kawashima et al., 1995
NON-ORAL ADMINISTRATION
1 indomethacin (subcutaneous) 2 1.6 reproductive/vascular
permeability(subcutaneous) Hoos & Hoffman, 1983
2 TPA (subcutaneous) 20 0.32 reproductive (subcutaneous) Nagasawa et al., 1980
3 ethyl carbamate
(intraperitoneal) 2 15 reproductive (subcutaneous) NTIS, 1968
Table 4. Substances with immunotoxic LOELs
Substance Immune Non-immune Non-immune Reference
LOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day
ORAL ADMINISTRATION
1 2,3,7,8-TCDD 0.086 1E-05 reproductive Murray et al., 1979
2 lithium carbonate 50 98 reproductive, body weight,
hepatic, renal Ibrahim & Canolty, 1990
3 m-nitrotoluene 200 40 hepatic (female) NTP, 1992
4 2,4-diaminotoluene 25 24 reproductive Thysen et al., 1985
5 dimethylvinyl chloride 50 125 splenic NTP, 1986
6 ethylene dibromide 125 30 mortality Teramoto et al., 1980
7 4,4-thiobis
(6-t-butyl-m-cresol) 10 45 hepatic NTP, 1994
NON-ORAL ADMINISTRATION
1 azathioprine
(intraperitoneal) 10 1 reproductive (intraperitoneal) Scott, 1977
2 benzo(a)pyrene
(subcutaneous) 50 50 reproductive (subcutaneous) Bui et al., 1986
3 diethylstilbestrol
(subcutaneous) 0.2 1E-05 reproductive (subcutaneous) McLachlan, 1977
4 DMB(a)A (subcutaneous) 5 1.25 reproductive (oral) Davis et al., 1978
5 N-nitroso dimethylamine
(intraperitoneal) 1.5 5 reproductive (intraperitoneal) Chaube, 1973
6 ochratoxin A
(intraperitoneal) 3.4 0.0625 renal (gavage) NTP, 1989
REFERENCES
Bui, Q.Q., Tran, M.B., & West, W.L. (1986) A comparative study of the
reproductive effects of methadone and benzo(a)pyrene in the pregnant
and pseudopregnant rat. Toxicology, 42(2/3): 195-204.
Burns, L.A., Bradley, S.G., White, K.L., McCay, J.A., Fuchs, B.A.,
Stern, M., Brown, R.D., Musgrove, D.L., Holsapple, M.P., Luster, M.I.,
& Munson, A.E. (1994) Immunotoxicity of mono-nitrotoluenes in female
B6C3F1 mice: I. Para-nitrotoluene. Drug Chem. Toxicol., 17(3):
317-358.
Chaube, S. (1973) Protective effects of thymidine,
5-aminoimidazolecarboxamide, and riboflavin against fetal
abnormalities produced in rats by 5-(3,3-dimethyl-1-triazene)
imidazole-4-carboxamide. Cancer Res., 33(10): 2231-2240.
Davis, G.J., McLachlan, J.A., & Lucier, G.W. (1978) The effect of
7,12-dimethylbenz(a)anthracene (DMBA) on the prenatal development of
gonads in mice. Teratology, 17: 33A (Abstract).
Hoos, P.C. & Hoffman, L.H. (1983) Effect of histamine receptor
antagonists and indomethacin in implantation in the rabbit. Biol.
Reprod., 29(4): 833-840.
Ibrahim, H.S. & Canolty, N.L. (1990) Effects of dietary lithium on
pregnant and lactating rats and the progeny. Nutr. Res., 10:
315-324.
Kawashima, K., Usami, M., Sakemi, K., & Ohno, Y. (1995) Studies on the
establishment of appropriate spermatogenic endpoints for male
fertility disturbance in rodent induced by drugs and chemicals: I.
Nitrobenzene. J. Toxicol. Sci., 20(1): 15-22.
Littlefield, N.A., Nelson, C.J., & Frith, C.H. (1983) Benzidine
dihydrochloride: Toxicological assessment in mice during chronic
exposures. J. Toxicol. Environ. Health, 12(4-6): 671-685.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A., & Wilson, R.E. (1981) The effect of
orthophenylphenol, tris(2,3-dichloropropyl) phosphate, and
cyclophosphamide on the immune system and host susceptibility of mice
following subchronic exposure. Toxicol. Appl. Pharmacol., 58(2):
252-261.
McClain, R.M. & Langhoff, L. (1979) Teratogenicity of
diphenylhydantoin in New Zealand rabbits. Toxicol. Appl.
Pharmacol., 48(1-Pt. 2): A32.
McLachlan, J.A. (1977) Prenatal exposure to diethylstilbestrol in
mice: Toxicological studies. J. Toxicol. Environ. Health, 2:(3)
527-537.
Murray, F.J., Smith, F.A., Nitschke, K.D., Humiston, C.G., Kociba,
R.J., & Schwetz, B.A. (1979) Three-generation reproduction study of
rats given 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) in the diet.
Toxicol. Appl. Pharmacol., 50(2): 241-252.
Nagasawa, H., Yanai, R., & Nakajima, Y. (1980) Suppression of
lactation by tumor promoters in mice. Proc. Soc. Exp. Biol. Med.,
165: 394-397.
NTIS (1968) Evaluation of carcinogenic, teratogenic, and mutagenic
activities of selected pesticides and industrial chemicals - Volume I:
Carcinogenic study and Volume II: Teratogenic study in mice and rats.
National Cancer Institute, Bethesda, MD (Bionetics Res. Lab).
NTP (1986) Toxicology and carcinogenesis studies of dimethylvinyl
chloride (1-chloro-2-methylpropene) (CAS No. 513-37-1) in F344/N rats
and B6C3F1 mice (Gavage studies). National Toxicology Program,
Research Triangle Park, NC (NTP Technical Report Series No. 316).
NTP (1989) Toxicology and carcinogenesis studies of ochratoxin A (CAS
No. 303-47-9) in F344/N rats (Gavage studies). National Toxicology
Program, Research Triangle Park, NC (NTP Technical Report Series No.
358).
NTP (1992) NTP Technical report on toxicity studies of o-, m-, and
p-nitrotoluenes (CAS Nos. 88-72-2, 99-08-1, 99-99-0) administered in
dosed feed to F344/N rats and B6C3F1 mice. National Toxicology
Program, Research Triangle Park, NC (NTP Toxicity Report Series No.
23).
NTP (1994) Toxicology and carcinogenesis studies of
4,4'-thiobis(6-t-butyl-m- cresol) (CAS No. 96-69-5) in F344/N rats and
B6C3F1 mice (Feed studies). National Toxicology Program, Research
Triangle Park, NC (NTP Technical Report Series No. 435).
Schepers, G.W. (1964) Tetraethyl lead and tetramethyl lead. Arch.
Environ. Health, 8(2): 277-295.
Schwetz, B.A., Quast, J.F., Keeley, P.A., Humiston, C.G., & Kociba,
R.J. (1978) Results of 2-year toxicity and reproduction studies on
pentachlorophenol in rats. In: Rao, K.R. ed. Pentachlorophenol:
Chemistry, pharmacology and environmental toxicology. Proceedings of
the EPA and University of West Florida Symposium, Pensacola, FL,
27-29 June 1977. Plenum Press, New York, pp. 301-309.
Scott, J.R. (1977) Fetal growth retardation associated with maternal
administration of immunosuppressive drugs. Am. J. Obstet. Gynecol.,
128(6): 668-676.
Teramoto, S., Saito, R., Aoyama, H., & Shirasu, Y. (1980) Dominant
lethal mutation induced in male rats by 1,2-dibromo-3-chloropropane
(DBCP). Mutat. Res., 77(1): 71-78.
Thysen, B., Bloch, E., & Varma, S.K. (1985) Reproductive toxicity of
2,4-toluenediamine in the rat: 2. Spermatogenic and hormonal effects.
J. Toxicol. Environ. Health, 16(6): 763-769.
Subsequent to the report of Rulis (1989), Munro (1990) held a
workshop to evaluate factors that influence the selection of an
appropriate threshold value. The workshop first reanalysed the Gold
et al. (1984) database using the original database of 343 rodent
carcinogens and confirmed the observations of Rulis (1986, 1989) that
a dietary intake of 0.15 µg/day intersected the distribution of 10-6
risks at approximately the 85th centile. In addition, the workshop
extended the analysis to include additional carcinogens added to the
original Gold et al. (1984) database, bringing the total to 492
rodent carcinogens (Gold et al., 1989). This reanalysis with a
broader set of data produced essentially the same distribution of
1 × 10-6 risks as was originally published by Rulis (1986, 1989). The
workshop participants also noted that inherent in the acceptance of
any threshold value was the assumption that every new untested
substance was a likely carcinogen and could be as potent as the most
potent 15% of carcinogens in the CPD.
Recognizing that not every new substance would turn out to be a
carcinogen, the workshop (Munro, 1990) constructed a table of risk
avoidance probabilities (Table 3).
This table shows the effect of various assumptions regarding the
proportion of chemicals that are presumed carcinogens on the
probability that a 10-6 risk standard will not be exceeded. It should
be noted that as this proportion decreases, the probability of not
exceeding a specific risk standard increases dramatically. Thus, for
example, while there is a 63% chance that the risk will not exceed
10-6 with a value of 1.5 µg/person per day when 100% of new chemicals
are assumed to be carcinogenic, the probability that the risk will be
less than 10-6 is 96% when only 10% of new chemicals are assumed to
be carcinogenic. Moreover, if one invokes a less conservative risk
standard of 10-5 (Table 3, right side), then the probability of not
exceeding that risk at a threshold value of 1.5 µg/person per day
exceeds 96% even if it is assumed that 50% of new chemicals are
potential carcinogens. It also should be remembered that, in theory,
the probability of an untested substance having a potency greater than
the median of the distribution of TD50s from the CPD (Gold et al.,
1989) is 50%. In reality, however, it is most unlikely that a
genotoxic carcinogen with a potency equal to or greater than the
median carcinogen in the Gold et al. (1989) database would be
discovered from the existing inventory of flavouring substances, given
existing knowledge of structure-activity relationships in
carcinogenesis.
Table 3. Probability of a target risk not being exceeded at various threshold values
Threshold value Percentage of chemicals presumed carcinogenic
(µg/day) 100% 50% 20% 10% 100% 50% 20% 10%
10-6 Target risk 10-5 Target level
0.15 86 93 97 99 96 98 99 >99
0.3 80 90 96 98 94 97 99 99
0.6 74 87 95 97 91 96 98 99
1.5 63 82 93 96 86 96 97 99
3.0 55 77 91 95 80 90 96 98
6.0 46 73 89 95 74 87 95 97
(Modified from Munro, 1990).
Taking the above factors into consideration and keeping in mind
that the calculated 10-6 risks based on the Gold et al. (1989)
database were derived using a highly conservative methodology, Rulis
(1989) reexamined his previous selection criteria for a threshold
value and those of Munro (1990) and concluded that a threshold value
of 1.5 µg/person per day would provide a high degree of health
protection. This threshold value was subsequently adopted by FDA as
the threshold of regulation (Federal Register, 1993, 1995) and FDA
noted that such an exposure level would result in a negligible risk
even in the event that a substance of unknown toxicity was later shown
to be a carcinogen.
3. APPLICATION OF A THRESHOLD OF TOXICOLOGICAL CONCERN TO
FLAVOURING SUBSTANCES
The Scientific Committee for Food (SCF, 1996) has made the point
that the decision to accept any particular threshold value is both a
scientific and a risk management decision. The role of the scientist
is to ensure that risk managers are provided with the full range of
uncertainties surrounding selection of any threshold value. In the
foregoing sections it was pointed out that the threshold concept
should not be interpreted as providing absolute certainty of no risk.
Threshold of toxicological concern is a probabilistic methodology that
involves acceptance of a negligible risk standard. Such a standard is
commonly used by toxicologists in the establishment of ADIs, and, in
fact, IPCS (1987) has defined the ADI as "an estimate by JECFA of the
amount of a food additive, expressed on a body weight basis, that can
be ingested daily over a lifetime without appreciable health risk".
JECFA has noted that it uses the risk assessment process when setting
an ADI, i.e., the level of "no apparent risk" is set on the basis of
quantitative extrapolation from animal data to human beings typically
using a NOEL from the animal studies divided by a 100-fold safety
factor (IPCS, 1987). When ADIs (or such similar limits e.g., TLVs,
RfD, etc.) are established, there is a residual, usually
unquantifiable, element of risk (Purchase & Auton, 1995; Baird
et al., 1996; SCF, 1996; Sielken & Valdez-Flores, 1996). This is a
reflection of the inability to determine precisely the NOEL from
empirical data, statistical uncertainties associated with the
sensitivity of experimental models, completeness of data, or the
magnitude of safety factors needed to account for any residual
uncertainty (Dourson & Stara, 1983).
The threshold of toxicological concern likewise does not carry
with it the absolute certainty that an untested chemical present in
food below the decision criterion specified at Step B5 will present a
less than 10-6 risk. Rather there is a high probability (i.e. about
95%) that the cancer risk from such a chemical will be less than
10-6. It is this residue of uncertainty that has produced a concern
about the possibility, albeit remote, that a highly potent genotoxic
carcinogen might inadvertently be considered acceptable using the
threshold concept (SCF, 1996).
3.1 Additional factors that reduce the theoretical risk
When the threshold concept is applied to flavouring substances,
two additional factors significantly reduce the probability of risk of
cancer below one in one million. The first of these relates to very
low levels of exposure to flavouring substances. As exposure
decreases, the probability of not exceeding a 10-6 risk substantially
increases, approaching 97 to 99% at an order of magnitude below the
1.5 µg/person per day value (Table 3). Therefore, application of a
threshold of toxicological concern to substances having very low
exposures (i.e., less or much less than the threshold value) carries
with it a much higher probability of no appreciable risk. It also must
be kept in mind that exposure to the vast majority of flavouring
substances tends to be overestimated because these materials are
volatile and appreciable amounts are lost during food preparation,
storage, etc. These issues regarding exposure to flavouring substances
are discussed in Annex 5 of WHO Food Additives Series 35 (JECFA,
1996).
The second factor involves a consideration of chemical structure.
The use of chemical structure for predicting toxicity for food
chemicals, especially flavouring substances, has long been recognized
by JECFA (IPCS, 1987), and JECFA has noted that use of
structure-activity is most highly developed in the area of
carcinogenesis. The use of structural alerts in combination with a
knowledge of chemistry and metabolism offers a way of identifying
potential carcinogens (Ashby & Tennant 1988, 1991; Williams, 1990;
Tennant et al., 1990; Klopman & Rosenkranz, 1994). The examination
of many chemicals for genotoxic, mutagenic and carcinogenic activities
has led to the preparation of a series of structural alerts that
provide the basis for potential reaction with DNA and possible
carcinogenic potential of the substance. The existence of reactive
moieties on known rodent carcinogens implies that potential mutagenic
activity and, in many cases, the carcinogenic activity of untested
chemicals might be identified by an examination of structure (Ashby &
Tennant, 1988, 1991; Tennant & Ashby, 1991).
Structure-activity relationships have been successfully applied to
congeneric substances (i.e., individual substances within a
structurally related group of substances) for which no toxicity data
are available (Klopman & Rosenkranz, 1994). Congeners that are
potential human carcinogens and mutagens possess electrophilic
functional groups with the ability to react directly with DNA. These
electrophilic sites may be reactive functional groups on the congener
or those formed during metabolic activation. Conversely, these
functional groups may be lost during metabolic detoxication of the
substance. Although the carcinogenic and mutagenic potency of
congeneric substances may differ, structural alerts within the group
of congeners are indicative of carcinogenic or mutagenic potential
(Klopman & Rosenkranz, 1994). A list of the functional groups
identified by Ashby & Tennant (1988, 1991) and Tennant et al. (1990)
as structural alerts is given in Table 4.
Table 4. A list of functional groups identified by Ashby & Tennant
(1988, 1991) and Tennant et al. (1990) as structural alerts for DNA
reactivity
a) alkyl esters of phosphonic or sulfonic acids
b) aromatic nitro-groups
c) aromatic azo-groups (reduction to amine)
d) aromatic ring N-oxides
e) aromatic mono- and di-alkyl amino groups
f) alkyl hydrazines
g) alkyl aldehydes
h) N-methylol derivatives
i) monohaloalkanes
j) N and S mustards, beta-haloethyl-
k) N-chloramines
l) propiolactones and propiosulfones
m) aromatic and aliphatic aziridinyl derivatives
n) aromatic and aliphatic substituted primary alkyl halides
o) urethane derivatives (carbamates)
p) alkyl N-nitrosamines
q) aromatic amines and N-hydroxy derivatives
r) aliphatic epoxides and aromatic oxides
s) center of Michael reactivity
t) halogenated methanes (C(X)4)
u) aliphatic nitro groups
Most flavouring agents are simple aliphatic and aromatic
substances containing functional groups that are efficiently
metabolized via detoxication pathways, and very few flavouring agents
and/or their in vivo metabolites contain structural alerts. The
absence of structural alerts in a flavouring agent provides added
assurance that it will not present an appreciable risk at or below the
decision criterion of 1.5 µg/person per day. For those that do contain
significant structural alerts, such as aliphatic epoxides, additional
data have been generated to facilitate evaluation.
4. SUMMARY
The application of the decision criterion at Step B5 of the safety
evaluation procedure provides a means of evaluating those flavouring
substances that have extremely low exposures but lack metabolic and
toxicity data. It is recognized that application of a threshold of
toxicological concern is a departure from traditional toxicological
evaluation but, nonetheless, is based on highly conservative
methodology and assumptions which, taken together, ensure that
flavouring substances meeting the proposed criteria will present, at
most, an insignificant risk.
The following factors taken together ensure the absence of any
appreciable theoretical risk from the use of flavouring substances
evaluated using this procedure:
1. The decision criterion is based on carcinogenesis data, an
extremely sensitive end-point in susceptible animal species with
accepted relevance to humans.
2. The CPD presents a worse case situation since chemicals were
generally tested over a lifetime by daily administration at the
maximum tolerated dose (MTD), and the procedures used by Gold et
al. (1984) to establish the TD50s involved numerous conservative
assumptions.
3. The methods used by Rulis (1986, 1989) and others (Krewski et
al., 1990; Munro, 1990) to calculate the distribution of 10-6
risks, upon which the threshold value of 1.5 µg/person per day is
based, are highly conservative since they involved the use of linear
extrapolation from the lowest TD50 for each substance in the database.
4. It is unlikely that any untested flavouring agent would turn out to
be a genotoxic carcinogen, and the possibility that a carcinogen would
be accepted using the threshold concept can be substantially reduced
by the application of structural alert methodology.
5. Many flavouring substances are consumed in amounts considerably
below the threshold value of 1.5 µg/person per day and this
substantially increases the probability, already in the range of 90 to
95%, that they will not present any significant theoretical risk.
6. Toxicity end-points such as developmental toxicity, neurotoxicity
and immunotoxicity demonstrate considerably higher human exposure
thresholds than the threshold value of 1.5 µg/person per day specified
at Step B5 making it highly unlikely that these non-cancer end-points
are a relevant concern in applying the threshold concept.
Taken together, these factors provide a sound basis for concluding
that flavouring substances with intakes below the 1.5 µg/person per
day threshold of toxicological concern specified at Step B5 can be
safely consumed.
ACKNOWLEDGEMENT
The authors are deeply indebted to Dr. Alan Rulis for reviewing this
paper and providing many useful suggestions.
5. REFERENCES
Ashby, J. & Tennant, R.W. (1988) Chemical structure, Salmonella
mutagenicity and extent of carcinogenicity as indicators of genotoxic
carcinogenesis among 222 chemicals tested in rodents by the US
NCI/NTP. Mutat. Res., 204: 17-115.
Ashby, J. & Tennant, R.W. (1991) Definitive relationships among
chemical structure, carcinogenicity and mutagenicity for 301 chemicals
tested by the US NTP. Mutat. Res., 257: 229-306.
Baird, S.J.S., Cohen, J.T., Graham, J.D., Shlyakhter, A.I., & Evans,
J.S. (1996) Noncancer risk assessment: A probabilistic alternative to
current practice. Hum. Ecol. Risk Assess., 2(1): 79-102.
Dourson, M.L. & Stara, J.F. (1983) Regulatory history and
experimental support of uncertainty (safety) factors. Regul.
Toxicol. Pharmacol., 3: 224-228.
Dourson, M.L., Felter, S.P., & Robinson, D. (1996) Evolution of
science-based uncertainty factors in noncancer risk assessment.
Regul. Toxicol. Pharmacol., 24: 108-120.
Federal Register (1993) Food additives: Threshold of regulation for
substances used in food-contact articles (Proposed rule). Fed. Reg.,
58(195): 52719-52729.
Federal Register (1995) Food additives: Threshold of regulation for
substances used in food-contact articles (Final rule). Fed. Reg.,
60(136): 36582-36596.
Frawley, J.P. (1967) Scientific evidence and common sense as a basis
for food-packaging regulations. Food Cosmet. Toxicol., 5: 293-308.
Gold, L.S., Sawyer, C.B., Magaw, R., Backman, G.M., de Veciana, M.,
Levison, R., Hooper, N.K., Havender, W.R., Bernstein, L., Peto, R.,
Pike, M., & Ames, B.N. (1984) A carcinogenic potency database of the
standardized results of animal bioassays. Environ. Health Perspect.,
58: 9-319.
Gold, L.S., de Veciana, M., Backman, G.M., Magaw, R., Lopipero, P.
Smith, M., Blumenthal, M., Levinson, R., Bernstein, L., & Ames. B.N.
(1986a) Chronological supplement to the carcinogenic potency database:
Standardized results of animal bioassays published through December
1982. Environ. Health Perspect., 67: 161-200.
Gold, L.S., Bernstein, L., Kaldor, J., Backman, G., & Hoel, D. (1986b)
An empirical comparison of methods used to estimate carcinogenic
potency in long-term animal bioassays: Lifetable vs summary incidence
data. Fundam. Appl. Toxicol., 6: 263-269.
Gold, L.S., Ward, J.M., Bernstein, L., & Stern B. (1986c) Association
between carcinogenic potency and tumor pathology in rodent
carcinogenesis bioassays. Fundam. Appl. Toxicol., 6: 677-690.
Gold, L.S., Slone, T.H., & Bernstein, L. (1989) Summary of
carcinogenic potency and positivity for 492 rodent carcinogens in the
carcinogenic potency database. Environ. Health Perspect., 79:
259-272.
Hall, R.L. (1975) Unpublished data from personal communication to the
NAS/NRC Committee on GRAS List Survey -- Phase III, and to the FEMA.
Hoel, D.G. & Portier, C.J. (1994) Nonlinearity of dose-response
functions for carcinogenicity. Environ. Health Perspect., 102(1):
109-113.
IPCS (1987) Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food. World
Health Organization, International Programme on Chemical Safety,
Geneva.
IPCS (1994) Environmental Health Criteria 170: Assessing human health
risk of chemicals: Derivation of guidance values for health-based
exposure limits. World Health Organization, International Programme on
Chemical Safety, Geneva.
JECFA (1995) Evaluation of certain food additives and contaminants.
Forty-fourth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 859).
JECFA (1996) Toxicological evaluation of certain food additives and
contaminants. World Health Organization, Geneva (WHO Food Additives
Series 35).
JECFA (1997) Evaluation of certain food additives and contaminants.
Forty-sixth report of the Joint FAO/WHO Expert Committee on Food
Additives. World Health Organization, Geneva (WHO Technical Report
Series, No. 868).
Klopman, G. & Rosenkranz, H.S. (1994) Approaches to SAR in
carcinogenesis and mutagenesis. Prediction of
carcinogenicity/mutagenicity using MULTI-CASE. Mutat. Res., 305:
33-46.
Krewski, D., Szyszkowicz, M., & Rosenkranz, H. (1990) Quantitative
factors in chemical carcinogenesis: Variation in carcinogenic potency.
Regul. Toxicol. Pharmacol., 12: 13-29.
Luster, M.I., Portier, C., Pait, D.G., White,K.L. Jr, Gennings, C.,
Munson, A.E., & Rosenthal, G.J. (1992) Risk assessment in
immunotoxicology: I. Sensitivity and predictability of immune tests.
Fundam. Appl. Toxicol., 18(2): 200-210.
Luster, M.I., Portier, C., Pait, D.G., Rosenthal, G.J., Germolec,
D.R., Gorsini, E., Blaylock, B.L., Pollock, P., Kouchi, Y., Craig, W.,
White, K.L., Munso, A.E., & Comment, C.E. (1993) Risk assessment in
Immunotoxicology: II. Relationships between immune and host
resistance tests. Fundam. Appl. Toxicol., 21(1): 71-82.
Munro, I.C. (1990) Safety assessment procedures for indirect food
additives: An overview. Regul. Toxicol. Pharmacol., 12: 001-0011.
Munro, I.C., Ford, R.A. Kennepohl, E., & Sprenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: A
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Peto, R., Pike, M.C., Bernstein, L., Gold, L.S., & Ames, B.N. (1984)
The TD50: A proposed general convention for the numerical description
of the carcinogenic potency of chemicals in chronic-exposure animal
experiments. Environ. Health Perspect., 5: 1-8.
Purchase, I.F.H. & Auton, T.R. (1995) Thresholds in chemical
carcinogenesis. Regul. Toxicol. Pharmacol., 22: 199-205.
RTECS (1987) Registry of toxic effects of chemical substances, 1985-86
ed. - Volume 1. US Department of Health and Human Services, Public
Health Service, Washington, D.C.
Rulis, A.M. (1986) De minimis and the threshold of regulation. In:
Felix, C.W. ed. Food protection technology. Lewis Publishers Inc.,
Chelsea, MI. pp. 29-37.
Rulis, A.M. (1989) Establishing a threshold of concern. In: Bonin,
J.J. & Stevenson, D.E. ed. Risk assessment in setting national
priorities. Plenum Press, New York, vol. 7, pp. 271-278.
Sawyer, C., Peto, R., Bernstein, L., & Pike, M.C. (1984) Calculation
of carcinogenic potency from long-term animal carcinogenesis
experiments. Biometrics, 40: 27-40.
SCF (1996) Opinion on response to request from the Commission for SCF
opinion on the scientific basis of the concept of threshold of
regulation in relation to food contact materials. European
Commission, Scientific Committee for Food, Brussels, Belgium.
Sielken, R.L. & Valdez-Flores, C. (1996) Comprehensive realism's
weight-of-evidence based distributional dose-response
characterization. Hum. Ecol. Risk Assess., 2(1): 175-193.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity
of a further 39 chemicals tested for carcinogenicity by the US
National Toxicology Program. Mutat. Res., 257: 209-227.
Tennant, R.W., Spalding, J., Stasiewicz, S., & Ashby, J. (1990)
Prediction of the outcome of rodent carcinogenicity bioassays
currently being conducted on 44 chemicals by the National Toxicology
Program. Mutagenesis, 5: 3-14.
Williams, G.M. (1990) Screening procedures for evaluating the
potential carcinogenicity of food-packaging chemicals. Regul.
Toxicol. Pharmacol., 12: 30-40.
Table 1. NOELS for organophosphate insecticides1
Agent Species End-point observed NOEL (mg/kg bw per day)
Acephate rat decreased body weight gain (parents and pups) 2.5
Azinphos methyl rat inhibition of plasma ChE activity 0.18
Coumaphos rat inhibition of RBC and plasma ChE activity 0.4
Crufomate rat inhibition of RBC ChE activity 3
Diazinon mouse decreased body weight gain 722
Dichlorvos rat inhibition of ChE activity (specific end-point
not indicated) 0.23
Dimethoate rat inhibition of brain, RBC and plasma ChE activity 0.05
Disulfoton rat inhibition of brain, RBC and plasma ChE activity 0.05
Ethephon rat inhibition of plasma and RBC ChE activity 15
Ethion rat inhibition of plasma ChE activity in females 0.2
Ethyl-p-nitrophenylphenyl
phosphorothioate rat inhibition of brain, RBC and plasma ChE activity 0.25
Express rat decreased body weight gain 1
Fenamiphos rabbit decreased maternal body weight gain 0.1
Fenchlorphos rat inhibition of ChE (form not specified) 15
Fonofos rat inhibition of RBC and plasma ChE activity 0.5
Glufosinate ammonium rat increased absolute and relative kidney
weight in males 0.42
Glyphosate rat increased incidence of renal tubular
dilation in F3b pups 10
Malathion rat inhibition of brain ChE activity 5
Merphos rat inhibition of RBC ChE activity in females 0.1
Merphos oxide rat inhibition of brain ChE activity 0.25
Methamidophos rat clinical signs typical of ChE inhibition 1
Methidithion rat inhibition of brain and RBC ChE activity 0.2
Methyl parathion rat decreased haemoglobin, haematocrit and RBCs 0.025
Naled rat decreased body weight gain 0.2
Parathion rat decreased body weight gain 1.8
Phosmet rat inhibition of RBC and plasma ChE activity 2
Phosphamidon rat decreased body weight gains 6.2
Pirimiphos-methyl rat inhibition of plasma ChE activity 0.5
Table 1. Continued...
Agent Species End-point observed NOEL (mg/kg bw per day)
Quinalphos mouse inhibition of plasma ChE activity 0.03
Tetrachlorvinphos rat inhibition of RBC ChE activity 6
Tetraethyl dithio
pyrophosphate rat inhibition of RBC and plasma ChE activity 0.5
1 Data taken from the EPA (IRIS) database and WHO 1969 Integrated Risk Information System
2 NOEL divided by a factor of 3 (see Munro et al., 1996 for explanation)
Table 2. Substances reported to cause developmental abnormalities (from RTECS)
Chemical Species Dose (TDLo)1
mg/kg bw
1 1,3,4-Thiadiazole, 2,2'-(methylenediimino)bis- rat 1
2 1-6-Hexanediamine rat 1840
3 1-Piperazinepropanol, 4-(6-((6-methoxy-8-quinolyl)amino)hexyl)-alpha-methyl-, maleate (1:2) rat 90
4 11H-Pyrido(2,1-b)quinazolone-2-carboxylic acid, 11-oxo- rat 4400
5 1H-Indazole-3-carboxylic acid, 1-(2,4-dichlorobenzyl)- rat 175
6 1H-Isoindole-1,3(2H)-dione,4,5,6,7-tetrahydro-2-(7-fluoro-3,4-dihydro-3-oxo-4-;
(2-propynyl)-2H-1,4-benzoxazin-6-yl)- rat 300
7 2,7-Naphthalenedisulfonic acid, 3,3'((3,3'-dimethyl-4,4'biphenylylene)bis(azo))
bis(5-; amino-4-hydroxy-, tetrasodium salt) rat 150
8 2-Propanone, 1,1,3,3-tetrachloro- rabbit 130
9 2-Pyridinemethanol,alpha-(3-(2,6-dimethyl-1-piperidinyl)propyl)-alpha-phenyl-,;
monohydrochloride, Z-(+-)- rabbit 650
10 3-Biphenylcarboxylic acid, 2',4'-difluoro-4-hydroxy- rabbit 520
11 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-((aminophenylacetyl)amino)-;
3,3-dimethyl-7-oxo-,(2,2-dimethyl-1-oxopropoxy)methyl ester,hydrochloride mouse 1200
12 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid,6-(2-amino-2-phenylacetamido)-;
3,3-dimethyl-7-oxo-,trihydrate,D-(-)- rat 2800
13 4H-Pyrido(1,2-a)pyrimidin-4-one,9-methyl-3-(1H-tetrazol-5-yl)-, potassium salt rat 2750
14 4H-s-Triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine,6-(o-chlorophenyl)-8-ethyl-1-methyl- rabbit 13
15 5-Isoxazoleacetic acid, 3,4-bis(4-methoxyphenyl)- rat 1650
16 7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid,2,3-dihydro-9-fluoro-3-methyl-;
10-(4-methyl-1-piperazinyl)-7-oxo-,hemihydrate,(S)- rat 8910
17 9H-Purine-6-thiol, 9-beta-D-ribofuranosyl- rat 87.5
18 Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-(methylsulfonyl)phenethyl)-,;
D-threo-(+) rat 150
19 Acetamide, N,N-dimethyl- rabbit 3900
20 Acetic acid, (2,4-dichlorophenoxy)- rat 0.22
21 Acetic acid, (3,5,6-trichloro-2-pyridyloxy)- rat 2000
22 Acetic acid, oxo((3-(1H-tetrazol-5-yl)phenyl)amino)-,butyl ester rat 24000
23 Acetonitrile, amino-, bisulfate rat 200
24 Acridine, 9,9-dimethyl-10-(3-(N,N-dimethylamino)propyl)-,tartrate mouse 175
25 Alanine, 3-(3,4-dihydroxyphenyl)-, L- rat 500
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
26 Alanine, N-((5-chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-,;
sodium salt, (-)- rat 5
27 Alosenn rat 5500
28 Anthranilic acid, N-(2,3-xylyl)- mouse 8
29 Arsine oxide, dimethylhydroxy- rat 300
30 Benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-, monoacetate rabbit 390
31 Benzenesulfonamide, 4-amino-N-(4,5-dimethyl-2-oxazolyl)-,mixt. with 5-((3,4,5-;
trimethoxyphenyl)methyl)-2,4-pyrimidinediamine rat 3360
32 Benzenesulfonamide, 4-amino-N-(4,6-dimethoxy-2-pyrimidinyl)- rat500
33 Benzenesulfonic acid, thio-,S,S'-(2-(dimethylamino)trimethylene) ester rat 660
34 Benzhydrol, 2-chloro-alpha-(2-(dimethylamino)ethyl)-,hydrochloride mouse 120
35 Benzoic acid, 3,4,5-trimethoxy-beta-(dimethylamino)-beta-ethylphenethyl ester,; maleate (1:1) rabbit 6500
36 Benzyl alcohol, 4-amino-alpha-((tert-butylamino)methyl)-3,5-dichloro-,monohydrochloride rat 4.4
37 Biphenyl, 3,3',4,4'-tetramethyl- mouse 640
38 Butyric acid, 4-(p-bis(2-chloroethyl)aminophenyl)- mouse 3
39 Butyrophenone, 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluoro- rat 5.04
40 Cadmium rat 23
41 Carbazic acid, 3-(1-phthalazinyl)-, ethyl ester, monohydrochloride mouse 70
42 Chlordane rat 880
43 Cortisone mouse 500
44 Dibenzo(b,e)(1,4)dioxin, 2,3,7,8-tetrabromo- mouse 0.216
45 Dibenzo-p-dioxin, 2,7-dichloro- rat 5
46 Disulfide, bis(thiocarbamoyl) mouse 105
47 Ethane, 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)- rat 2000
48 Ethane, 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1,1-trichloro- rat 250
49 Ethanone, 1-(7-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-2-benzofuranyl)-, hydrochloride rat 1400
50 Ethanone, 2-((4-(2,4,dichloro-3-methylbenzoyl)-1,3-dimethyl-1H-pyrazol-5-yl)oxy)-;
1-(4-methylphenyl)- rat 2000
51 Folic acid, methyl- rat 500
52 Gallic acid, propyl ester rat 45000
53 Glutamic acid, N-(p-((1-(2-amino-4-hydroxy-6-pteridinyl)ethyl)amino)benzoyl)-L- rat 20
54 Gossypol acetic acid mouse 480
55 Hydrocinnamic acid, alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, L- rat 2100
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
56 Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl- rat 1
57 Isonicotinamide, 2-ethylthio- mouse 450
58 Isothiocyanic acid, butenyl ester rat 800
59 L-Glutamic acid, magnesium salt (1:1), hydrobromide rat 6000
60 L-Tyrosine rat 3500
61 L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo- rat 26.25
62 Linoleic acid (oxidized) rat 166000
63 Lysine, L- rat 81000
64 Manganese, (ethylenebis(dithiocarbamato))- and zinc acetate (50:1) rat 765
65 Mannitol, 1,6-dibromo-1,6-dideoxy-, D- mouse 150
66 Methanol, 1,3,4-thiadiazol-2-ylminodi- rat 5
67 Molybdenum rat 5.8
68 Morpholine, 4-(3,4,5-trimethoxybenzoyl)- rat 700
69 Norleucine, 6-amidino-, monohydrochloride, hydrate rat 2000
70 Oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one, 10-chloro-11b-(o-chlorophenyl)-2,3,7,11b- ;
tetrahydro mouse 1800
71 Phenol, p-amino- rat 2500
72 Phenothiazine-2-acetic acid, 10-methyl mouse 180
73 Phosphonic acid, (1,2-epoxypropyl)-, calcium salt (1:1), (1R,2S)-(-)- rat 15400
74 Phosphorodithioic acid, O,O-dimethyl ester, S-ester with 2-mercapto-N-methylacetamide rat 120
75 Phthalic acid, di(methoxyethyl)ester rat 593
76 Piperazine, 1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)- rat 320
77 Piperazine,1-(p-tert-butylbenzyl)-4-(p-chloro-alpha-phenylbenzyl)-,dihydrochloride rat 360
78 Piperidine, 3-((4-methoxyphenoxy)methyl)-1-methyl-4-phenyl-, hydrochloride, (3R-trans)- rat 210
79 Piperidine, 1-methyl-4-(N-2-thenylanilino)-, tartrate rat 157
80 Polychlorinated biphenyl (aroclor 1254) rat 90
81 Pregn-4-ene-3,20-dione,9-fluoro-11-beta,17,21-trihydroxy- rabbit 2
82 Pregna-,4-diene-2,20-dione,9-fluoro-11-beta,16-alpha,17,21-tetrahydroxy-,16,21-diacetate mouse 3.2
83 Propionic acid,2-(2,4,5-trichlorophenoxy)- mouse 1617
84 Pyrimidine, 2,4-diamino-6-methyl-5-phenyl- rat 100
85 Retinoic acid, 4-oxo-,13-cis- mouse 100
86 Retinoic acid, all-trans- mouse 15
87 Rowachol rat 9600
Table 2. Continued...
Chemical Species Dose (TDLo)1
mg/kg bw
88 Stannane, diacetoxydibutyl- rat 15.2
89 Sulfanilamide, N(sup 1)-(6-methoxy-2-methyl-4-pyrimidinyl)- mouse 3000
90 Toluene, alpha-(2-(2-butoxyethoxy)ethoxy)-4,5-(methylenedioxy)-2-propyl- rat 2130
91 Tryptophan, N-acetyl-,L- rat 27500
92 Urea, (alpha-(2-methylhydrazino)-p-toluoyl)-, monohydrobromide rabbit 50
93 Urea, 1-butyl-3-(p-tolylsulfonyl)- mouse 1700
94 Urea, 1-butyl-3-sulfanilyl- rat 1000
95 Uridine, 5'-deoxy-5-fluoro- rat 550
96 ZZL-0820 rabbit 325
97 beta-Escin mouse 36
98 m-Propionotoluidide,2-methyl-4'-nitro-alpha,alpha,alpha-triflouro- rat 1050
99 p-Acetophenetidide rat 6000
100 p-Cresol, 2,6-di-tert-butyl- mouse 1200
1 TDLo = the lowest dose of a substance reported to produce any non-significant adverse effect (=NOEL).
Table 3. Substances with immunotoxic NOELs
Substance Immune Non-immune Non-immune Reference
NOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day)
ORAL ADMINISTRATION
1 p-nitrotoluene 400 200 hepatic, splenic Burns et al., 1994
2 pentachlorophenol 10 3 hepatic, renal Schwetz et al., 1978
3 o-phenylphenol 100 10 blood (red blood cells) Luster et al., 1981
4 hexachlorodibenzo-p-dioxin 0.056 1E-05 reproductive Murray et al., 1979
5 DPH 150 50 teratogenic McClain & Langhoff, 1979
6 tetraethyl lead 0.5 0.0012 hepatic,thymus Schepers, 1964
7 benzidine 11 2.7 neural, hepatic Littlefield et al., 1983
8 nitrobenzene 30 60 reproductive Kawashima et al., 1995
NON-ORAL ADMINISTRATION
1 indomethacin (subcutaneous) 2 1.6 reproductive/vascular
permeability(subcutaneous) Hoos & Hoffman, 1983
2 TPA (subcutaneous) 20 0.32 reproductive (subcutaneous) Nagasawa et al., 1980
3 ethyl carbamate
(intraperitoneal) 2 15 reproductive (subcutaneous) NTIS, 1968
Table 4. Substances with immunotoxic LOELs
Substance Immune Non-immune Non-immune Reference
LOEL NOEL LOEL end-point
(mg/kg bw (mg/kg bw (mg/kg bw
per day) per day) per day
ORAL ADMINISTRATION
1 2,3,7,8-TCDD 0.086 1E-05 reproductive Murray et al., 1979
2 lithium carbonate 50 98 reproductive, body weight,
hepatic, renal Ibrahim & Canolty, 1990
3 m-nitrotoluene 200 40 hepatic (female) NTP, 1992
4 2,4-diaminotoluene 25 24 reproductive Thysen et al., 1985
5 dimethylvinyl chloride 50 125 splenic NTP, 1986
6 ethylene dibromide 125 30 mortality Teramoto et al., 1980
7 4,4-thiobis
(6-t-butyl-m-cresol) 10 45 hepatic NTP, 1994
NON-ORAL ADMINISTRATION
1 azathioprine
(intraperitoneal) 10 1 reproductive (intraperitoneal) Scott, 1977
2 benzo(a)pyrene
(subcutaneous) 50 50 reproductive (subcutaneous) Bui et al., 1986
3 diethylstilbestrol
(subcutaneous) 0.2 1E-05 reproductive (subcutaneous) McLachlan, 1977
4 DMB(a)A (subcutaneous) 5 1.25 reproductive (oral) Davis et al., 1978
5 N-nitroso dimethylamine
(intraperitoneal) 1.5 5 reproductive (intraperitoneal) Chaube, 1973
6 ochratoxin A
(intraperitoneal) 3.4 0.0625 renal (gavage) NTP, 1989
REFERENCES
Bui, Q.Q., Tran, M.B., & West, W.L. (1986) A comparative study of the
reproductive effects of methadone and benzo(a)pyrene in the pregnant
and pseudopregnant rat. Toxicology, 42(2/3): 195-204.
Burns, L.A., Bradley, S.G., White, K.L., McCay, J.A., Fuchs, B.A.,
Stern, M., Brown, R.D., Musgrove, D.L., Holsapple, M.P., Luster, M.I.,
& Munson, A.E. (1994) Immunotoxicity of mono-nitrotoluenes in female
B6C3F1 mice: I. Para-nitrotoluene. Drug Chem. Toxicol., 17(3):
317-358.
Chaube, S. (1973) Protective effects of thymidine,
5-aminoimidazolecarboxamide, and riboflavin against fetal
abnormalities produced in rats by 5-(3,3-dimethyl-1-triazene)
imidazole-4-carboxamide. Cancer Res., 33(10): 2231-2240.
Davis, G.J., McLachlan, J.A., & Lucier, G.W. (1978) The effect of
7,12-dimethylbenz(a)anthracene (DMBA) on the prenatal development of
gonads in mice. Teratology, 17: 33A (Abstract).
Hoos, P.C. & Hoffman, L.H. (1983) Effect of histamine receptor
antagonists and indomethacin in implantation in the rabbit. Biol.
Reprod., 29(4): 833-840.
Ibrahim, H.S. & Canolty, N.L. (1990) Effects of dietary lithium on
pregnant and lactating rats and the progeny. Nutr. Res., 10:
315-324.
Kawashima, K., Usami, M., Sakemi, K., & Ohno, Y. (1995) Studies on the
establishment of appropriate spermatogenic endpoints for male
fertility disturbance in rodent induced by drugs and chemicals: I.
Nitrobenzene. J. Toxicol. Sci., 20(1): 15-22.
Littlefield, N.A., Nelson, C.J., & Frith, C.H. (1983) Benzidine
dihydrochloride: Toxicological assessment in mice during chronic
exposures. J. Toxicol. Environ. Health, 12(4-6): 671-685.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A., & Wilson, R.E. (1981) The effect of
orthophenylphenol, tris(2,3-dichloropropyl) phosphate, and
cyclophosphamide on the immune system and host susceptibility of mice
following subchronic exposure. Toxicol. Appl. Pharmacol., 58(2):
252-261.
McClain, R.M. & Langhoff, L. (1979) Teratogenicity of
diphenylhydantoin in New Zealand rabbits. Toxicol. Appl.
Pharmacol., 48(1-Pt. 2): A32.
McLachlan, J.A. (1977) Prenatal exposure to diethylstilbestrol in
mice: Toxicological studies. J. Toxicol. Environ. Health, 2:(3)
527-537.
Murray, F.J., Smith, F.A., Nitschke, K.D., Humiston, C.G., Kociba,
R.J., & Schwetz, B.A. (1979) Three-generation reproduction study of
rats given 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) in the diet.
Toxicol. Appl. Pharmacol., 50(2): 241-252.
Nagasawa, H., Yanai, R., & Nakajima, Y. (1980) Suppression of
lactation by tumor promoters in mice. Proc. Soc. Exp. Biol. Med.,
165: 394-397.
NTIS (1968) Evaluation of carcinogenic, teratogenic, and mutagenic
activities of selected pesticides and industrial chemicals - Volume I:
Carcinogenic study and Volume II: Teratogenic study in mice and rats.
National Cancer Institute, Bethesda, MD (Bionetics Res. Lab).
NTP (1986) Toxicology and carcinogenesis studies of dimethylvinyl
chloride (1-chloro-2-methylpropene) (CAS No. 513-37-1) in F344/N rats
and B6C3F1 mice (Gavage studies). National Toxicology Program,
Research Triangle Park, NC (NTP Technical Report Series No. 316).
NTP (1989) Toxicology and carcinogenesis studies of ochratoxin A (CAS
No. 303-47-9) in F344/N rats (Gavage studies). National Toxicology
Program, Research Triangle Park, NC (NTP Technical Report Series No.
358).
NTP (1992) NTP Technical report on toxicity studies of o-, m-, and
p-nitrotoluenes (CAS Nos. 88-72-2, 99-08-1, 99-99-0) administered in
dosed feed to F344/N rats and B6C3F1 mice. National Toxicology
Program, Research Triangle Park, NC (NTP Toxicity Report Series No.
23).
NTP (1994) Toxicology and carcinogenesis studies of
4,4'-thiobis(6-t-butyl-m- cresol) (CAS No. 96-69-5) in F344/N rats and
B6C3F1 mice (Feed studies). National Toxicology Program, Research
Triangle Park, NC (NTP Technical Report Series No. 435).
Schepers, G.W. (1964) Tetraethyl lead and tetramethyl lead. Arch.
Environ. Health, 8(2): 277-295.
Schwetz, B.A., Quast, J.F., Keeley, P.A., Humiston, C.G., & Kociba,
R.J. (1978) Results of 2-year toxicity and reproduction studies on
pentachlorophenol in rats. In: Rao, K.R. ed. Pentachlorophenol:
Chemistry, pharmacology and environmental toxicology. Proceedings of
the EPA and University of West Florida Symposium, Pensacola, FL,
27-29 June 1977. Plenum Press, New York, pp. 301-309.
Scott, J.R. (1977) Fetal growth retardation associated with maternal
administration of immunosuppressive drugs. Am. J. Obstet. Gynecol.,
128(6): 668-676.
Teramoto, S., Saito, R., Aoyama, H., & Shirasu, Y. (1980) Dominant
lethal mutation induced in male rats by 1,2-dibromo-3-chloropropane
(DBCP). Mutat. Res., 77(1): 71-78.
Thysen, B., Bloch, E., & Varma, S.K. (1985) Reproductive toxicity of
2,4-toluenediamine in the rat: 2. Spermatogenic and hormonal effects.
J. Toxicol. Environ. Health, 16(6): 763-769.
