
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 41
Prepared by:
The 50th meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
GENTAMICIN (addendum)
First draft prepared by
Dr P. Olsen & Dr L. Dragsted
Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration, Copenhagen, Denmark
1. Explanation
2. Biological data
2.1 Toxicological studies
2.2.1 Special studies on human intestinal flora
2.2.2 Special studies on genotoxicity
2.2 Structural similarities with known carcinogens
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Gentamicin is an aminoglycoside antibiotic. This compound was
previously evaluated by the Committee at its forty-third meeting
(Annex 1, reference 113), when a temporary ADI of 0-4 µg/kg bw was
allocated. The Committee recommended temporary maximum residue limits
(MRLs) of 100 µg/kg for muscle and fat, 200 µg/kg for liver, and 1000
µg/kg for kidney, in both cattle and pigs, and 100 µg/L for cow's
milk, all of the values being expressed as parent compound.
The Committee required the following information for evaluation
in 1997: (i) results of studies on the effects of gentamicin on
specific genera of microorganisms obtained from the human intestine;
(ii) additional data to assist in the assessment of carcinogenic
potential, which should include the results of assays for gene
mutations in mammalian cells and chromosomal aberrations in vitro
and in vivo, and details of an investigation on possible structural
similarities between gentamicin and known carcinogens; and (iii) a
validated chemical analytical method with a limit of quantification
below the MRL recommended for milk. These results were not available
to the Committee at its forty-eight meeting (Annex 1, reference 128),
and the temporary acceptable daily intake established at the
forty-third meeting of the Committee (Annex 1, reference 113) and the
temporary MRLs recommended at that meeting were extended until 1998.
This information has now become available and is summarized and
discussed in this monograph addendum.
2. BIOLOGICAL DATA
2.1 Toxicological studies
2.1.1 Special studies on human intestinal flora
Studies on the effects of gentamicin on bacterial isolates from
human intestinal microflora in vitro were available; studies have
not been performed in vivo. The minimal inhibitory concentration
(MIC) of gentamicin was determined for anaerobic and aerobic bacteria
isolated from fresh stools of 25 healthy adult volunteers. All tests
were performed at the department of Medical Microbiology of St Radboud
University Hospital in Nijmegen, The Netherlands. No statement was
made about accordance with GLP procedures. Ten isolates each of
Enterococcus spp., coliforms, Proteus spp., Bacteroides spp.,
Lactobacillus spp, Bifidobacterium spp, Prevotella spp,
Eubacterium spp., Clostridium spp., Fusobacterium spp., and
anaerobic gram-positive cocci were tested, including five control
strains. The range of species tested was considered to be
representative of the human gut bacterial flora, mainly governed by
anaerobes.
The anaerobic bacteria were tested by the agar dilution method
recommended by the National Council for Clinical Laboratory Standards.
Brucella agar base supplemented with haemin and vitamin K was used,
and gentamicin was tested in serial double dilutions. A density of
1 × 106 bacterial cells per spot was used, and incubation was for
48 h at 37°C under anaerobic conditions. The control strains used were
Bacteroides fragilis (ATCC 10584) and Peptostreptococcus
anaerobius (ATCC 27337). Facultative anaerobic bacteria was tested
by a broth dilution technique on microtitre plates, as recommended by
the National Council for Clinical Laboratory Standards. Isosensitest
Broth (Oxoid CM 491) was used as the medium, and gentamicin was tested
in serial double dilutions. Bacterial cells were tested at a density
of 1 × 104 per well, and bacterial growth was determined after 24
and 48 h of incubation. The control strains used were Escherichia
coli (ATCC 25922), Enterococcus faecalis (ATCC 29212), and
Proteus mirabilis (ATCC 14273). The MIC value was defined as the
lowest concentration of anaerobic or facultative anaerobic bacteria
that prevented visible growth in the test medium. The MIC values
determined are shown in Tables 1 and 2. The sensitivity of human
intestinal bacteria to gentamicin was presented as the geometric mean
MIC value for each genus and for the overall population of strains.
The geometric mean MIC values of gentamicin ranged from 0.04 µg/ml for
Proteus spp. to > 128 µg/ml for Prevotella and Bacteroides spp.
Among the anaerobes, the most sensitive to gentamicin were
Eubacterium spp., with MIC values ranging from 2 to 32 µg/ml and a
geometric mean MIC value of 6.06 µg/ml. The overall geometric mean MIC
value for gentamicin for all genera tested was 8.86 µg/ml (Lohuis &
Aerts, 1996).
2.1.2 Special studies on genotoxicity
Gentamicin was tested in vitro for its ability to induce
forward gene mutation in Chinese hamster ovary cells at concentrations
of 128ś5000 µg/ml and chromosomal aberrations in these cells at
concentrations of 800-5000 µg/ml, both with and without metabolic
activation. It was also tested in vivo for its ability to induce
nuclear anomalies in CD-1 mouse bone-marrow cells at intravenous doses
of 20ś80 mg/kg bw, the highest dose being the maximum tolerated dose
(Table 3). All of the studies were performed according to appropriate
standards for study protocol and conduct. There was no indication of
mutagenic activity. Two additional studies on chromosomal aberrations
in vitro and in vivo (Won, 1996a,b) were received for evaluation,
but they were not taken into consideration because of shortcomings in
the study protocol and reporting.
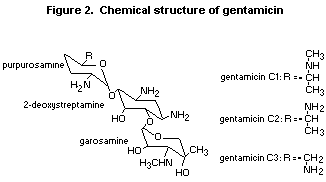
2.2 Structural similarities with known carcinogens
The structural relationship of gentamicin with known carcinogens
was analysed by investigating the chemical structure of gentamicin for
features commonly found to be associated with mutagenic and/or
carcinogenic potential ((structural alerts). Structurally, gentamicin
comprises three aliphatic six-membered rings, two tetrahydropyrane
rings (purpurosamine and garosamine), and one cyclohexane ring
(2-deoxystreptamine). The latter is bound to the tetrahydropyrane
rings through ether linkages in the form of glucosidic bonds. The ring
system is substituted with amino groups, hydroxyl groups, aliphatic
side chains, and aliphatic side chains containing primary or secondary
amino groups. The three analogues of gentamicin vary with respect to
an aliphatic side chain containing a primary or a secondary amino
group (Figure 1). Two compilations of structural alerts exist in the
literature. The first contains a list of 10 structural features that
are considered to indicate an increased probability of carcinogenic
activity in animals (US Food and Drug Administration, 1994). The
second is based on several comparisons of the chemical structure,
mutagenicity to Salmonella typhimurium, and carcinogenicity of 522
rodent carcinogens and 55 human carcinogens. The electrophilicity,
mutagenicity, and carcinogenicity of the tested chemicals was compared
on the basis of this data bank, and a list of 19 molecular structural
alerts (mainly for genotoxic carcinogens) was drawn up (Tennant &
Ashby, 1991; Ashby & Paton, 1993), many of which were similar to those
in the list of the US Food and Drug Administration.
Amino groups are considered as alerts in both lists, but only
when they are attached to an aromatic ring system; none of the amino
groups in gentamicin is attached to aromatic rings. None of the other
structural features listed in the two compilations was found in the
chemical structure of gentamicin. The author concluded that gentamicin
contains none of the structural alerts associated with potential
carcinogenic or mutagenic activity (Inveresk Research, 1997).
Table 1. Antimicrobial activity of gentamicin in vitro on
110 strains of facultative anaerobic and anaerobic bacteria
(10 isolates per strain) isolated from human stools
Species Geometric mean
MIC (µg/ml) Range
Facultative anaerobes
Enterococcus faecium and 0.87 0.06-2
Enterococcus faecalis.
Coliforms: Escherichia coli, 0.05 0.03-0.13
Citrobacter freundii,
Klebsiella pneumoniae and
Klebsiella oxytoca
Proteus mirabilis and 0.04 0.03-0.13
Proteus vulgaris
Anaerobesa
Bacteroides spp. > 128 > 128
Lactobacillus spp. 29.86 16-64
Bifidobacterium spp. 19.70 4-126
Prevotella spp. > 128 > 128
Eubacterium spp. 6.06 2-32
Clostridium spp. 111.4 64-> 128
Fusobacterium spp. 78.79 4-> 128
Anaerobic gram-positive cocci 27.86 8-126
From Lohuis & Aerts (1996)
a Species not specified
Table 2. Antimicrobial activity of gentamicin in vitro on some
strains used for quality control
Species MIC (µg/ml)
Aerobic incubation Anaerobic incubation
Enterococcus faecalis 4 -
(ATCC 29212)
Escherichia coli 0.125 -
(ATCC 25922)
Proteus mirabilis < 0.0625 -
(ATCC 14273)
Bacteroides fragilis - > 128
(ATCC 10584)
Peptostreptococcus
anaerobius - -
(ATCC 27337)
From Lohuis & Aerts (1996)
Table 3. Genotoxity of gentamicin sulfate
Test system Test object Concentration Result Reference
In vitro
Forward Chinese hamster 128, 320, 800, Negativea,b Poul (1997a)
mutation ovary cells (hprt locus) 2000, 5000 µg/ml
Chromosomal Chinese hamster 800, 2000, 5000 negativea,c Poul (1997b)
aberration ovary cells (CHO-K1) µg/ml
In vivo
Micronucleus Bone marrow of 20, 40, 80 mg/, Negatived Holmstrom &
formation CD-1 mice kg bw intravenously Innes (1997)
a With and without metabolic activation (S)
b Benzo[a]pyrene (+S9 mix) and ethyl methanesulfonate (-S9 mix) used as positive controls; in the
absence of metabolic activation the mutation frequency was twice the control value (unusually low
mutation frequency) at concentrations of 5000, 2000, and 125 µg/ml in one assay but not in the
second assay.
c Methyl methanesulfonate (+S9 mix) and cyclophosphamide (-S9 mix) used as positive controls
d Cyclophosphamide used as positive control
 It was noted that existing long-term studies on the
aminoglucosides neomycin, dihydrostreptomycin, and aminosidine in
experimental animals were not included in the databases on known
carcinogenes, which formed the basis for tabulating the structural
alerts. Consequently, the lists may have limited value for predicting
the possible carcinogenic potential of the gentamicin structure. Since
no carcinogenic effect has been observed with the aminoglycosides that
have been tested in long-term studies (Annex 1, reference 111;
Committee for Veterinary Medicinal Products, 1996), however, it is
reasonable to assume that inclusion of these studies in the databases
would not have altered the conclusion about structureśactivity
relationships.
3. COMMENTS
The Committee considered the results of studies of mutagenicity
in vitro and in vivo, analyses of the structural similarities of
gentamicin to known carcinogens, and the effect of gentamicin on
specific bacterial species obtained from the human gut. The studies
were carried out according to appropriate standards for study protocol
and conduct.
Gentamicin was tested in vitro for its ability to cause gene
mutations and chromosomal aberrations in Chinese hamster ovary cells
and in vivo for its ability to induce nuclear anomalies in mouse
bone-marrow cells at doses of 20ś80 mg/kg bw by intravenous
administration. The results of these tests were negative, and the
Committee concluded that gentamicin is unlikely to be genotoxic.
Possible structural similarities between gentamicin and known
carcinogens were analysed. None of the structural features listed in
the available databases for carcinogenicity were found within the
chemical structure of gentamicin. In view of this finding and since
the aminoglycosides that have been tested (neomycin,
dihydrostreptomycin, and aminosidine) do not elicit a tumorigenic
effect in rats, the Committee considered that gentamicin is unlikely
to have carcinogenic activity. This conclusion is supported by the
results of the tests for genotoxicity in mammalian cells in vitro
and in vivo.
The effect of gentamicin on the growth of 110 bacterial strains
obtained from the human gastrointestinal tract was evaluated after
incubation in vitro. MIC values were determined for 80 isolates of
the eight predominant groups of human intestinal anaerobic microflora,
Bacteroides spp., Lactobacillus spp., Bifidobacterium spp.,
Prevotella spp., Eubacterium spp., Clostridium spp.,
Fusobacterium spp., and anaerobic gram-positive cocci. In addition,
data were provided for 30 isolates of the facultative anaerobes
Enterococci spp., Proteus spp., and coliforms. The MIC values for
these isolates ranged from 0.06 to > 128 µg/ml. Although the
facultative anaerobic bacteria were the most sensitive organisms, the
Committee agreed that they should not be used in the calculation of
the ADI because they are not predominant species in the human
intestine. Instead, the Committee used the MIC for the most sensitive
relevant genera isolated from the human gastrointestinal tract, in
this case Eubacterium spp. The geometric mean for this strain was
6 µg/ml at an inoculum density of 106 colony-forming units.
Eubacterium spp. were also used in the evaluation at the forty-third
meeting, when an MIC value of 0.8 µg/ml was used for establishing the
temporary ADI. Although the MIC values identified at the forty-third
and the present meeting were different, the Committee concluded that
the value obtained using isolates from the human gut flora was more
relevant for establishing an ADI.
In calculating an ADI based on the antimicrobial activity of
gentamicin, the Committee used the formula described on p. 28, as
follows:
Upper limit 6 µg/ga × 220 g
=
of ADI 1b × 1c × 60 kg
= 22 µg/kg bw
a For the purpose of this evaluation, the MIC50 value is the
geometric mean MIC for gentamicin against the 10 strains of the
most sensitive relevant genus isolated from the human intestinal
tract, in this case Eubacterium spp.
b Absorption of gentamicin after oral administration is poor;
therefore, a factor of 1 was used to represent 100% availability
in the gastrointestinal tract.
c A safety factor of 1 was used because sufficient, relevant
microbiological data were available.
4. EVALUATION
The Committee established an ADI of 0-20 µg/kg bw on the basis of
a microbiological end-point. The Committee noted that the lowest NOEL
identified at the forty-third meeting in toxicological studies was 10
mg per kg bw, which is 500 times the microbiological ADI.
5. REFERENCES
Ashby, J. & Paton, D. (1993) The influence of chemical structure on
the extent and sites of carcinogenesis for 522 rodent carcinogens and
55 different human carcinogen exposures. Mutat. Res., 286, 3-74.
Committee for Veterinary Medicinal Products (1996) Aminosidine. Status
report (EMEA/MRL/050/95-Final), http://www.eudora.org/emea.html. The
European Agency for the Evaluation of Medicinal Products, London,
United Kingdom.
Holmstrom, L.M. & Innes, D.C. (1997) Gentamicin, micronucleus test in
bone marrow of CD-1 mice. Unpublished report No. 14888 from Inveresk
Research, Tranent, Scotland, United Kingdom. Submitted to WHO by
Inveresk Research, Tranent, Scotland, United Kingdom.
Inveresk Research (1997) Gentamicin: Structureśactivity relationships.
Unpublished report submitted to WHO by Inveresk Research, Tranent,
Scotland, United Kingdom.
Lohuis, J. & Aerts, R. (1996) Susceptibility of anaerobic and aerobic
isolates from human faeces towards gentamicin. Unpublished report No.
96R/0478 from Intervet, Boxmeer R & D Laboratories. Submitted to WHO
by Inveresk Research, Tranent, Scotland, United Kingdom.
Poul, J.M. (1997a) Evaluation of gentamicin sulfate in the Chinese
hamster ovary cell/hypoxanthine-guanine-phosphoribosyl-transferase
(CHO/HGPRT) forward mutation assay. Unpublished report No. RTOX9703
from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Poul, J.M. (1997b) Evaluation of gentamicin sulfate in the in vitro
chromosome aberration assay using CHO-K1 cells. Unpublished report No.
RTOX9702 from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity of
a further 39 chemicals tested for carcinogenicity by the US National
Toxicology Program. Mutat. Res., 257, 209-227.
US Food and Drug Administration (1994) General Principles for
Evaluating the Safety of Compounds Used in Food-producing Animals,
Rockville, Maryland, USA, Center for Veterinary Medicine.
Won, H. (1996a) Evaluation of gentamicin for the induction of
chromosomal aberrations using Chinese hamster lung cells (CHL)
in vitro. Unpublished report from Department of Genetic Toxicology,
Toxicology Research Institute, Korea Food and Drug Administration,
Seoul, 122-020, Republic of Korea. Submitted to WHO by Dr G. Roberts,
Chemical Product Assessment Section, Commonwealth Department of Health
& Family Services, Woden, ACT, Australia.
Won, H. (1996b) Evaluation of gentamicin for the induction of
chromosomal aberrations using ddY male mouse in vivo. Unpublished
report from Department of Genetic Toxicology, Toxicology Research
Institute, Korea Food and Drug Administration, Seoul, 122-020,
Republic of Korea. Submitted to WHO by Dr G. Roberts, Chemical Product
Assessment Section, Commonwealth Department of Health & Family
Services, Woden, ACT, Australia.
It was noted that existing long-term studies on the
aminoglucosides neomycin, dihydrostreptomycin, and aminosidine in
experimental animals were not included in the databases on known
carcinogenes, which formed the basis for tabulating the structural
alerts. Consequently, the lists may have limited value for predicting
the possible carcinogenic potential of the gentamicin structure. Since
no carcinogenic effect has been observed with the aminoglycosides that
have been tested in long-term studies (Annex 1, reference 111;
Committee for Veterinary Medicinal Products, 1996), however, it is
reasonable to assume that inclusion of these studies in the databases
would not have altered the conclusion about structureśactivity
relationships.
3. COMMENTS
The Committee considered the results of studies of mutagenicity
in vitro and in vivo, analyses of the structural similarities of
gentamicin to known carcinogens, and the effect of gentamicin on
specific bacterial species obtained from the human gut. The studies
were carried out according to appropriate standards for study protocol
and conduct.
Gentamicin was tested in vitro for its ability to cause gene
mutations and chromosomal aberrations in Chinese hamster ovary cells
and in vivo for its ability to induce nuclear anomalies in mouse
bone-marrow cells at doses of 20ś80 mg/kg bw by intravenous
administration. The results of these tests were negative, and the
Committee concluded that gentamicin is unlikely to be genotoxic.
Possible structural similarities between gentamicin and known
carcinogens were analysed. None of the structural features listed in
the available databases for carcinogenicity were found within the
chemical structure of gentamicin. In view of this finding and since
the aminoglycosides that have been tested (neomycin,
dihydrostreptomycin, and aminosidine) do not elicit a tumorigenic
effect in rats, the Committee considered that gentamicin is unlikely
to have carcinogenic activity. This conclusion is supported by the
results of the tests for genotoxicity in mammalian cells in vitro
and in vivo.
The effect of gentamicin on the growth of 110 bacterial strains
obtained from the human gastrointestinal tract was evaluated after
incubation in vitro. MIC values were determined for 80 isolates of
the eight predominant groups of human intestinal anaerobic microflora,
Bacteroides spp., Lactobacillus spp., Bifidobacterium spp.,
Prevotella spp., Eubacterium spp., Clostridium spp.,
Fusobacterium spp., and anaerobic gram-positive cocci. In addition,
data were provided for 30 isolates of the facultative anaerobes
Enterococci spp., Proteus spp., and coliforms. The MIC values for
these isolates ranged from 0.06 to > 128 µg/ml. Although the
facultative anaerobic bacteria were the most sensitive organisms, the
Committee agreed that they should not be used in the calculation of
the ADI because they are not predominant species in the human
intestine. Instead, the Committee used the MIC for the most sensitive
relevant genera isolated from the human gastrointestinal tract, in
this case Eubacterium spp. The geometric mean for this strain was
6 µg/ml at an inoculum density of 106 colony-forming units.
Eubacterium spp. were also used in the evaluation at the forty-third
meeting, when an MIC value of 0.8 µg/ml was used for establishing the
temporary ADI. Although the MIC values identified at the forty-third
and the present meeting were different, the Committee concluded that
the value obtained using isolates from the human gut flora was more
relevant for establishing an ADI.
In calculating an ADI based on the antimicrobial activity of
gentamicin, the Committee used the formula described on p. 28, as
follows:
Upper limit 6 µg/ga × 220 g
=
of ADI 1b × 1c × 60 kg
= 22 µg/kg bw
a For the purpose of this evaluation, the MIC50 value is the
geometric mean MIC for gentamicin against the 10 strains of the
most sensitive relevant genus isolated from the human intestinal
tract, in this case Eubacterium spp.
b Absorption of gentamicin after oral administration is poor;
therefore, a factor of 1 was used to represent 100% availability
in the gastrointestinal tract.
c A safety factor of 1 was used because sufficient, relevant
microbiological data were available.
4. EVALUATION
The Committee established an ADI of 0-20 µg/kg bw on the basis of
a microbiological end-point. The Committee noted that the lowest NOEL
identified at the forty-third meeting in toxicological studies was 10
mg per kg bw, which is 500 times the microbiological ADI.
5. REFERENCES
Ashby, J. & Paton, D. (1993) The influence of chemical structure on
the extent and sites of carcinogenesis for 522 rodent carcinogens and
55 different human carcinogen exposures. Mutat. Res., 286, 3-74.
Committee for Veterinary Medicinal Products (1996) Aminosidine. Status
report (EMEA/MRL/050/95-Final), http://www.eudora.org/emea.html. The
European Agency for the Evaluation of Medicinal Products, London,
United Kingdom.
Holmstrom, L.M. & Innes, D.C. (1997) Gentamicin, micronucleus test in
bone marrow of CD-1 mice. Unpublished report No. 14888 from Inveresk
Research, Tranent, Scotland, United Kingdom. Submitted to WHO by
Inveresk Research, Tranent, Scotland, United Kingdom.
Inveresk Research (1997) Gentamicin: Structureśactivity relationships.
Unpublished report submitted to WHO by Inveresk Research, Tranent,
Scotland, United Kingdom.
Lohuis, J. & Aerts, R. (1996) Susceptibility of anaerobic and aerobic
isolates from human faeces towards gentamicin. Unpublished report No.
96R/0478 from Intervet, Boxmeer R & D Laboratories. Submitted to WHO
by Inveresk Research, Tranent, Scotland, United Kingdom.
Poul, J.M. (1997a) Evaluation of gentamicin sulfate in the Chinese
hamster ovary cell/hypoxanthine-guanine-phosphoribosyl-transferase
(CHO/HGPRT) forward mutation assay. Unpublished report No. RTOX9703
from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Poul, J.M. (1997b) Evaluation of gentamicin sulfate in the in vitro
chromosome aberration assay using CHO-K1 cells. Unpublished report No.
RTOX9702 from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity of
a further 39 chemicals tested for carcinogenicity by the US National
Toxicology Program. Mutat. Res., 257, 209-227.
US Food and Drug Administration (1994) General Principles for
Evaluating the Safety of Compounds Used in Food-producing Animals,
Rockville, Maryland, USA, Center for Veterinary Medicine.
Won, H. (1996a) Evaluation of gentamicin for the induction of
chromosomal aberrations using Chinese hamster lung cells (CHL)
in vitro. Unpublished report from Department of Genetic Toxicology,
Toxicology Research Institute, Korea Food and Drug Administration,
Seoul, 122-020, Republic of Korea. Submitted to WHO by Dr G. Roberts,
Chemical Product Assessment Section, Commonwealth Department of Health
& Family Services, Woden, ACT, Australia.
Won, H. (1996b) Evaluation of gentamicin for the induction of
chromosomal aberrations using ddY male mouse in vivo. Unpublished
report from Department of Genetic Toxicology, Toxicology Research
Institute, Korea Food and Drug Administration, Seoul, 122-020,
Republic of Korea. Submitted to WHO by Dr G. Roberts, Chemical Product
Assessment Section, Commonwealth Department of Health & Family
Services, Woden, ACT, Australia.
 It was noted that existing long-term studies on the
aminoglucosides neomycin, dihydrostreptomycin, and aminosidine in
experimental animals were not included in the databases on known
carcinogenes, which formed the basis for tabulating the structural
alerts. Consequently, the lists may have limited value for predicting
the possible carcinogenic potential of the gentamicin structure. Since
no carcinogenic effect has been observed with the aminoglycosides that
have been tested in long-term studies (Annex 1, reference 111;
Committee for Veterinary Medicinal Products, 1996), however, it is
reasonable to assume that inclusion of these studies in the databases
would not have altered the conclusion about structureśactivity
relationships.
3. COMMENTS
The Committee considered the results of studies of mutagenicity
in vitro and in vivo, analyses of the structural similarities of
gentamicin to known carcinogens, and the effect of gentamicin on
specific bacterial species obtained from the human gut. The studies
were carried out according to appropriate standards for study protocol
and conduct.
Gentamicin was tested in vitro for its ability to cause gene
mutations and chromosomal aberrations in Chinese hamster ovary cells
and in vivo for its ability to induce nuclear anomalies in mouse
bone-marrow cells at doses of 20ś80 mg/kg bw by intravenous
administration. The results of these tests were negative, and the
Committee concluded that gentamicin is unlikely to be genotoxic.
Possible structural similarities between gentamicin and known
carcinogens were analysed. None of the structural features listed in
the available databases for carcinogenicity were found within the
chemical structure of gentamicin. In view of this finding and since
the aminoglycosides that have been tested (neomycin,
dihydrostreptomycin, and aminosidine) do not elicit a tumorigenic
effect in rats, the Committee considered that gentamicin is unlikely
to have carcinogenic activity. This conclusion is supported by the
results of the tests for genotoxicity in mammalian cells in vitro
and in vivo.
The effect of gentamicin on the growth of 110 bacterial strains
obtained from the human gastrointestinal tract was evaluated after
incubation in vitro. MIC values were determined for 80 isolates of
the eight predominant groups of human intestinal anaerobic microflora,
Bacteroides spp., Lactobacillus spp., Bifidobacterium spp.,
Prevotella spp., Eubacterium spp., Clostridium spp.,
Fusobacterium spp., and anaerobic gram-positive cocci. In addition,
data were provided for 30 isolates of the facultative anaerobes
Enterococci spp., Proteus spp., and coliforms. The MIC values for
these isolates ranged from 0.06 to > 128 µg/ml. Although the
facultative anaerobic bacteria were the most sensitive organisms, the
Committee agreed that they should not be used in the calculation of
the ADI because they are not predominant species in the human
intestine. Instead, the Committee used the MIC for the most sensitive
relevant genera isolated from the human gastrointestinal tract, in
this case Eubacterium spp. The geometric mean for this strain was
6 µg/ml at an inoculum density of 106 colony-forming units.
Eubacterium spp. were also used in the evaluation at the forty-third
meeting, when an MIC value of 0.8 µg/ml was used for establishing the
temporary ADI. Although the MIC values identified at the forty-third
and the present meeting were different, the Committee concluded that
the value obtained using isolates from the human gut flora was more
relevant for establishing an ADI.
In calculating an ADI based on the antimicrobial activity of
gentamicin, the Committee used the formula described on p. 28, as
follows:
Upper limit 6 µg/ga × 220 g
=
of ADI 1b × 1c × 60 kg
= 22 µg/kg bw
a For the purpose of this evaluation, the MIC50 value is the
geometric mean MIC for gentamicin against the 10 strains of the
most sensitive relevant genus isolated from the human intestinal
tract, in this case Eubacterium spp.
b Absorption of gentamicin after oral administration is poor;
therefore, a factor of 1 was used to represent 100% availability
in the gastrointestinal tract.
c A safety factor of 1 was used because sufficient, relevant
microbiological data were available.
4. EVALUATION
The Committee established an ADI of 0-20 µg/kg bw on the basis of
a microbiological end-point. The Committee noted that the lowest NOEL
identified at the forty-third meeting in toxicological studies was 10
mg per kg bw, which is 500 times the microbiological ADI.
5. REFERENCES
Ashby, J. & Paton, D. (1993) The influence of chemical structure on
the extent and sites of carcinogenesis for 522 rodent carcinogens and
55 different human carcinogen exposures. Mutat. Res., 286, 3-74.
Committee for Veterinary Medicinal Products (1996) Aminosidine. Status
report (EMEA/MRL/050/95-Final), http://www.eudora.org/emea.html. The
European Agency for the Evaluation of Medicinal Products, London,
United Kingdom.
Holmstrom, L.M. & Innes, D.C. (1997) Gentamicin, micronucleus test in
bone marrow of CD-1 mice. Unpublished report No. 14888 from Inveresk
Research, Tranent, Scotland, United Kingdom. Submitted to WHO by
Inveresk Research, Tranent, Scotland, United Kingdom.
Inveresk Research (1997) Gentamicin: Structureśactivity relationships.
Unpublished report submitted to WHO by Inveresk Research, Tranent,
Scotland, United Kingdom.
Lohuis, J. & Aerts, R. (1996) Susceptibility of anaerobic and aerobic
isolates from human faeces towards gentamicin. Unpublished report No.
96R/0478 from Intervet, Boxmeer R & D Laboratories. Submitted to WHO
by Inveresk Research, Tranent, Scotland, United Kingdom.
Poul, J.M. (1997a) Evaluation of gentamicin sulfate in the Chinese
hamster ovary cell/hypoxanthine-guanine-phosphoribosyl-transferase
(CHO/HGPRT) forward mutation assay. Unpublished report No. RTOX9703
from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Poul, J.M. (1997b) Evaluation of gentamicin sulfate in the in vitro
chromosome aberration assay using CHO-K1 cells. Unpublished report No.
RTOX9702 from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity of
a further 39 chemicals tested for carcinogenicity by the US National
Toxicology Program. Mutat. Res., 257, 209-227.
US Food and Drug Administration (1994) General Principles for
Evaluating the Safety of Compounds Used in Food-producing Animals,
Rockville, Maryland, USA, Center for Veterinary Medicine.
Won, H. (1996a) Evaluation of gentamicin for the induction of
chromosomal aberrations using Chinese hamster lung cells (CHL)
in vitro. Unpublished report from Department of Genetic Toxicology,
Toxicology Research Institute, Korea Food and Drug Administration,
Seoul, 122-020, Republic of Korea. Submitted to WHO by Dr G. Roberts,
Chemical Product Assessment Section, Commonwealth Department of Health
& Family Services, Woden, ACT, Australia.
Won, H. (1996b) Evaluation of gentamicin for the induction of
chromosomal aberrations using ddY male mouse in vivo. Unpublished
report from Department of Genetic Toxicology, Toxicology Research
Institute, Korea Food and Drug Administration, Seoul, 122-020,
Republic of Korea. Submitted to WHO by Dr G. Roberts, Chemical Product
Assessment Section, Commonwealth Department of Health & Family
Services, Woden, ACT, Australia.
It was noted that existing long-term studies on the
aminoglucosides neomycin, dihydrostreptomycin, and aminosidine in
experimental animals were not included in the databases on known
carcinogenes, which formed the basis for tabulating the structural
alerts. Consequently, the lists may have limited value for predicting
the possible carcinogenic potential of the gentamicin structure. Since
no carcinogenic effect has been observed with the aminoglycosides that
have been tested in long-term studies (Annex 1, reference 111;
Committee for Veterinary Medicinal Products, 1996), however, it is
reasonable to assume that inclusion of these studies in the databases
would not have altered the conclusion about structureśactivity
relationships.
3. COMMENTS
The Committee considered the results of studies of mutagenicity
in vitro and in vivo, analyses of the structural similarities of
gentamicin to known carcinogens, and the effect of gentamicin on
specific bacterial species obtained from the human gut. The studies
were carried out according to appropriate standards for study protocol
and conduct.
Gentamicin was tested in vitro for its ability to cause gene
mutations and chromosomal aberrations in Chinese hamster ovary cells
and in vivo for its ability to induce nuclear anomalies in mouse
bone-marrow cells at doses of 20ś80 mg/kg bw by intravenous
administration. The results of these tests were negative, and the
Committee concluded that gentamicin is unlikely to be genotoxic.
Possible structural similarities between gentamicin and known
carcinogens were analysed. None of the structural features listed in
the available databases for carcinogenicity were found within the
chemical structure of gentamicin. In view of this finding and since
the aminoglycosides that have been tested (neomycin,
dihydrostreptomycin, and aminosidine) do not elicit a tumorigenic
effect in rats, the Committee considered that gentamicin is unlikely
to have carcinogenic activity. This conclusion is supported by the
results of the tests for genotoxicity in mammalian cells in vitro
and in vivo.
The effect of gentamicin on the growth of 110 bacterial strains
obtained from the human gastrointestinal tract was evaluated after
incubation in vitro. MIC values were determined for 80 isolates of
the eight predominant groups of human intestinal anaerobic microflora,
Bacteroides spp., Lactobacillus spp., Bifidobacterium spp.,
Prevotella spp., Eubacterium spp., Clostridium spp.,
Fusobacterium spp., and anaerobic gram-positive cocci. In addition,
data were provided for 30 isolates of the facultative anaerobes
Enterococci spp., Proteus spp., and coliforms. The MIC values for
these isolates ranged from 0.06 to > 128 µg/ml. Although the
facultative anaerobic bacteria were the most sensitive organisms, the
Committee agreed that they should not be used in the calculation of
the ADI because they are not predominant species in the human
intestine. Instead, the Committee used the MIC for the most sensitive
relevant genera isolated from the human gastrointestinal tract, in
this case Eubacterium spp. The geometric mean for this strain was
6 µg/ml at an inoculum density of 106 colony-forming units.
Eubacterium spp. were also used in the evaluation at the forty-third
meeting, when an MIC value of 0.8 µg/ml was used for establishing the
temporary ADI. Although the MIC values identified at the forty-third
and the present meeting were different, the Committee concluded that
the value obtained using isolates from the human gut flora was more
relevant for establishing an ADI.
In calculating an ADI based on the antimicrobial activity of
gentamicin, the Committee used the formula described on p. 28, as
follows:
Upper limit 6 µg/ga × 220 g
=
of ADI 1b × 1c × 60 kg
= 22 µg/kg bw
a For the purpose of this evaluation, the MIC50 value is the
geometric mean MIC for gentamicin against the 10 strains of the
most sensitive relevant genus isolated from the human intestinal
tract, in this case Eubacterium spp.
b Absorption of gentamicin after oral administration is poor;
therefore, a factor of 1 was used to represent 100% availability
in the gastrointestinal tract.
c A safety factor of 1 was used because sufficient, relevant
microbiological data were available.
4. EVALUATION
The Committee established an ADI of 0-20 µg/kg bw on the basis of
a microbiological end-point. The Committee noted that the lowest NOEL
identified at the forty-third meeting in toxicological studies was 10
mg per kg bw, which is 500 times the microbiological ADI.
5. REFERENCES
Ashby, J. & Paton, D. (1993) The influence of chemical structure on
the extent and sites of carcinogenesis for 522 rodent carcinogens and
55 different human carcinogen exposures. Mutat. Res., 286, 3-74.
Committee for Veterinary Medicinal Products (1996) Aminosidine. Status
report (EMEA/MRL/050/95-Final), http://www.eudora.org/emea.html. The
European Agency for the Evaluation of Medicinal Products, London,
United Kingdom.
Holmstrom, L.M. & Innes, D.C. (1997) Gentamicin, micronucleus test in
bone marrow of CD-1 mice. Unpublished report No. 14888 from Inveresk
Research, Tranent, Scotland, United Kingdom. Submitted to WHO by
Inveresk Research, Tranent, Scotland, United Kingdom.
Inveresk Research (1997) Gentamicin: Structureśactivity relationships.
Unpublished report submitted to WHO by Inveresk Research, Tranent,
Scotland, United Kingdom.
Lohuis, J. & Aerts, R. (1996) Susceptibility of anaerobic and aerobic
isolates from human faeces towards gentamicin. Unpublished report No.
96R/0478 from Intervet, Boxmeer R & D Laboratories. Submitted to WHO
by Inveresk Research, Tranent, Scotland, United Kingdom.
Poul, J.M. (1997a) Evaluation of gentamicin sulfate in the Chinese
hamster ovary cell/hypoxanthine-guanine-phosphoribosyl-transferase
(CHO/HGPRT) forward mutation assay. Unpublished report No. RTOX9703
from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Poul, J.M. (1997b) Evaluation of gentamicin sulfate in the in vitro
chromosome aberration assay using CHO-K1 cells. Unpublished report No.
RTOX9702 from Centre National d'Etudes Vétérinaires et Alimentaires,
Laboratoire des médicaments vétérinaires, Unité de toxicologie,
F-35133 Fougčres, France. Submitted to WHO by Inveresk Research,
Tranent, Scotland, United Kingdom.
Tennant, R.W. & Ashby, J. (1991) Classification according to chemical
structure, mutagenicity to Salmonella and level of carcinogenicity of
a further 39 chemicals tested for carcinogenicity by the US National
Toxicology Program. Mutat. Res., 257, 209-227.
US Food and Drug Administration (1994) General Principles for
Evaluating the Safety of Compounds Used in Food-producing Animals,
Rockville, Maryland, USA, Center for Veterinary Medicine.
Won, H. (1996a) Evaluation of gentamicin for the induction of
chromosomal aberrations using Chinese hamster lung cells (CHL)
in vitro. Unpublished report from Department of Genetic Toxicology,
Toxicology Research Institute, Korea Food and Drug Administration,
Seoul, 122-020, Republic of Korea. Submitted to WHO by Dr G. Roberts,
Chemical Product Assessment Section, Commonwealth Department of Health
& Family Services, Woden, ACT, Australia.
Won, H. (1996b) Evaluation of gentamicin for the induction of
chromosomal aberrations using ddY male mouse in vivo. Unpublished
report from Department of Genetic Toxicology, Toxicology Research
Institute, Korea Food and Drug Administration, Seoul, 122-020,
Republic of Korea. Submitted to WHO by Dr G. Roberts, Chemical Product
Assessment Section, Commonwealth Department of Health & Family
Services, Woden, ACT, Australia.
