
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 41
Prepared by:
The 50th meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
IMIDOCARB
First draft prepared by
Dr E.A.M. Good
Veterinary Medicines Directorate
Addlestone, Surrey, United Kingdom
1 Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.2 Biotransformation
2.2 Toxicological studies
2.2.1 Acute toxicity
2.2.2 Short term toxicity
2.2.3 Long-term toxicity and carcinogenicity
2.2.4 Genotoxicity
2.2.5 Reproductive toxicity
2.2.6 Special studies to investigate serum prolactin
concentrations
2.2.7 Special studies on microbiological activity
2.2.8 Special studies on pharmacodynamic effects
2.3 Observations in humans
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Imidocarb is a carbanilide derivative that has been used for more
than 20 years for the treatment of certain protozoal diseases,
including babesiosis and anaplasmosis, in cattle, horses, sheep, and
dogs. Imidocarb has not previously been reviewed by the Committee.
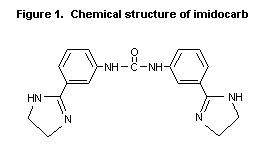
The chemical name of imidocarb is
N,N'-bis[3-(4,5-dihydro-1 H-imidazol-2-yl)phenylurea. The structure
of imidocarb is shown in Figure 1.
Most of the biological and toxicological studies that are
reviewed below were carried out using imidocarb dipropionate with a
purity close to 100%.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Studies in mice, rats, dogs, monkeys, and cattle have been
reported. Most of them were carried out during the 1960s and 1970s
according to the standards of those days, and important details are
missing from some of the reports. In contrast, the study in cattle was
certified for compliance with GLP.
Mice
Mice were injected into the tail vein with of 1 mg/kg bw
imidocarb dihydrochloride radiolabelled with 14C in the carbonyl
group. No radiolabel was detected in expired air. Excretion in the
urine was initially very rapid but quickly slowed and continued
throughout the observation period of 96 h. Faecal excretion followed a
similar pattern. Of the recovered radiolabel (90% of the administered
dose), about two-thirds was found in the urine and one-third in the
faeces (Wander, 1968a).
Rats
Wistar rats were dosed orally with 14C-imidocarb dipropionate
or 14C-imidocarb dihydrochloride and killed at various intervals up
to 48 h after treatment. Another rat was dosed subcutaneously with the
hydrochloride. Whole-body autoradiography indicated poor oral
absorption of both salts, and radiolabel was detectable only in the
liver, kidney, and gut. After subcutaneous treatment, a significant
amount of radiolabel remained at the injection site (Farebrother,
1973). In another study with various dose regimes and routes of
administration (oral, intraperitoneal, subcutaneous), the highest
concentrations of residues were found in the liver and kidney.
Residues were still detectable in these organs 55 days after
subcutaneous treatment with 5 mg/kg bw of the test substance.
Excretion was slow in rats treated subcutaneously, and only 20% of the
administered dose was recovered in excreta during the 78 h of
treatment (Nimmo-Smith, 1968). In a preliminary study to investigate
tissue retention, Wistar rats were given either a single oral dose or
30 daily oral doses of 5 mg/kg bw imidocarb dipropionate. The rats
were killed 24 h after the last dose, and residues in selected tissues
were determined by an unspecified analytical method. The highest
concentrations were found in kidney; surprisingly, the concentrations
in liver and brain were higher after the single-dose regime (Thomson,
1975a).
Dogs
Male beagle dogs were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and killed 24 h after the last
dose. The residues were determined in selected tissues by an
unspecified method. The highest concentrations were found in liver and
then in kidney; no residues were detected in muscle or brain (< 500
µg/kg) (Chesher et al., 1976). In another study, mongrel dogs were
given an intravenous bolus of 4 mg/kg bw of imidocarb dipropionate.
The pharmacokinetics were biphasic and indicated a large volume of
distribution. The mean terminal half-life was 207 min (Salam & Baggot,
1983).
Monkeys
Female patas monkeys were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and were killed some time after the
last dose (probably 24 h, although this is not stated). The residues
were determined in selected tissues by an unspecified method. The
highest concentrations were found in kidney and liver; none were
detected in muscle or brain (limit of detection, 500 µg/kg) (Thomson,
1975b).
Cattle
In a study which complied with the principles of GLP, six
lactating cows and eight calves were given a single subcutaneous
injection of 3 mg/kg bw 14C-imidocarb dipropionate with a specific
activity of 22 mCi/g. The test substance was formulated as the
proprietary product Imizol Injection. A mean peak blood concentration
of 1300 µg-equivalents/kg was attained 1 h after treatment, and the
concentration remained at this level for about 4 h, when 72œ91% of the
radiolabel was bound to plasma proteins. Excretion was very slow: over
the first 10 days, only 53% of the dose was recovered in excreta, with
38% in the faeces and 15% in the urine. The residues in tissues were
very persistent, particularly in liver where mean residues of 2200
µg-equivalents/kg were found 90 days after treatment (Ferguson, 1996).
2.1.2 Biotransformation
In bovine liver preparations in vitro, there was no evidence
for metabolism of imidocarb (Coldham et al., 1995).
Mice
Mice injected with 14C-imidocarb into the tail vein excreted
37% of the administered dose in the urine within 2 h. Of the
radiolabel in the urine, 95% was unmetabolized imidocarb. The mice
were killed 3.5 h after treatment, and 29% of the dose was found in
the liver, 6.8% in the kidney, and less than 1% in the gall-bladder.
More than 90% of the radiolabel in these organs was unmetabolized
imidocarb (Wander, 1968b).
Cattle
In the study described above in which cattle were treated
subcutaneously, the main component of the residues in liver, kidney,
muscle, and fat for up to 90 days after treatment was unmetabolized
imidocarb. Minor components, accounting for not more than 10% of the
total residues, were also present but were not identified. Imidicarb
was also the major component of cows' milk, urine, and faeces
(Ferguson, 1996).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of imidocarb are
summarized in Table 1. None of the studies complied with GLP, and the
reports fall short of modern standards. The signs of toxicity were
generally consistent with the anticholinesterase activity of imidocarb
and included lethargy, salivation, lachrymation, muscle fasciculation,
ataxia, tremors, and convulsions. Congestion of the lung and kidneys
and mottling of the liver were frequent post-mortem findings in the
rats that died.
In a published report, the signs of toxicity in groups of goats
given a single intramuscular dose in the range 12-24 mg/kg bw included
salivation, frequent urination and defaecation, weakness of the legs,
abnormal posture, and recumbency. There were dose-related decreases in
haemoglobin and erythrocyte counts and packed cell volume and
dose-related increases in asparate aminotransferase activity and in
creatinine and bilirubin concentrations. Serum cholinesterase activity
was reduced in a dose-related manner. No significant effects were
observed at 6 mg/kg bw. The effects were ameliorated by pretreatment
with atropine sulfate intramuscularly at a dose of 1 mg per animal
(Ali et al., 1985).
Whole-blood cholinesterase activity was significantly reduced in
calves given an intramuscular injection of 3.3 mg/kg bw imidocarb
dipropionate. Maximum depression occurred about 30 min after
treatment, and significant recovery was observed within 6 h. The
cholinesterase depression did not correlate with the intensity of the
clinical response (Michell et al., 1986).
2.2.2 Short-term toxicity
Mice
A study in mice, intended as a range-finding probe for a study of
dominant lethal mutations, was not carried out in accordance with GLP.
Groups of six male Evans No. 1 mice were given daily intramuscular
doses of 0 (distilled water), 7.8, 16, 31, 63, or 130 mg/kg bw per day
of imidocarb dipropionate (expressed as base) for five days. Signs of
toxicity were recorded for up to 14 days after the last dose. No blood
chemistry or pathological examinations were carried out. All of the
Table 1. Results of studies of acute toxicity with imidocarb
Species Route Vehicle Sex LD50 Salt Reference
(strain) (mg/kg bw) administered
Mouse (ICR) Oral gavage Sterile distilled water M 723a Dipropionate Szot (1973)
Mouse (ICR) Oral gavage Sterile distilled water F 646a Dipropionate Szot (1973)
Mouse (SAS) Oral gavage 0.9% saline F 544a Dihydrochloride Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 863a Diacetate Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 702a Dipropionate Follenfant & Green (1971a)
Mouse (Gambles) Oral gavage 0.9% saline M 1400 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline F 1500 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline M 1652a Dihydrochloride Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1216a Diacetate Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1251a Dipropionate Follenfant & Green (1971a)
Rat (Long Evans) Oral gavage Sterile distilled water M 454a Dipropionate Szot (1973)
Rat (Long Evans) Oral gavage Sterile distilled water F 650a Dipropionate Szot (1973)
Rabbit (New Zealand Oral gavage Water M + F 317 Dipropionate (27% Piercy & James (1975)
white) aqueous solution)
Rat (Long Evans) Intravenous 0.9% saline M 32.7a Dipropionate Szot (1973)
Mouse (ICR) Intravenous 0.9% saline M 22.3a Dipropionate Szot (1973)
Mouse (Gambles) Intravenous 0.9% saline M 16 Hydrochloride Green & Hughes (1969)
Mouse (SAS) Subcutaneous 0.9% saline F 78.6a Dihydrochloride Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 94.5a Diacetate Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 92.1a Dipropionate Follenfant & Green (1971b)
Mouse (Gambles) Subcutaneous 0.9% saline M 140 Hydrochloride Green & Hughes (1969)
Rat (Wistar) Subcutaneous 0.9% saline F 150 Hydrochloride Green & Hughes (1969)
Rat (Sprague-Dawley) Oral gavage Water F > 631a Dipropionate (10% Harper et al. (1997a)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M > 1000a Dipropionate (10% Harper et al. (1997b)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water F 1011.5a Dipropionate (10% Harper et al. (1997c)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M 1998a Dipropionate (10% Harper et al. (1997d)
aqueous solution)
a The LD50 values found in these studies are for the base. In the remaining studies, the results are believed to be quoted as the salt,
although this is not clear from the report in all cases.
animals at 63 and 130 mg/kg bw and 50% of those at 31 mg/kg bw died
three to four days after the start of treatment. The signs of toxicity
were slow respiration and lethargy. There was no NOEL (Harper & James,
1977).
Rats
In a preliminary study designed primarily to investigate
distribution in tissues, groups of five female Wistar rats were given
either a single oral dose of 5 mg/kg bw imidocarb dipropionate
dissolved in distilled water, or daily oral doses of 5 mg/kg bw per
day for 30 days. Five additional rats formed a control group. Blood
samples were taken for haematological and clinical chemical testing,
and electrocardiographic, ophthalmoscopic, and urinary measurements
were made before treatment and at termination; no histopathological
examination was performed. The rats were killed 24 h after the last
dose, and residues were determined in selected tissues. There were no
treatment-related effects on most of the parameters investigated. The
significant changes in blood parameters included increased total
leukocyte counts in the treated group; however, the toxicological
significance of these effects is doubtful since satisfactory blood
samples were obtained from only three treated rats (Thomson, 1975a).
In a study that did not conform to GLP, groups of 10 Wistar rats
of each sex were given daily oral doses of imidocarb dihydrochloride
for three months. The study was carried out in two phases. In phase 1,
the doses were 0 (distilled water), 125, 250, or 500 mg/kg bw per day;
in phase 2, the doses were 0, 500, 750, or 1500 mg/kg bw per day. It
was not clear whether the doses were expressed as the hydrochloride or
as the the base, and the solutions were not analysed. The rats were
weighed and examined daily. Blood samples for haematological and
clinical chemical determinations were taken just before termination.
Urinalysis was not carried out.
All rats given 1500 mg/kg bw died. The body-weight gain of all
treated rats was significantly lower than that of controls in phase 2.
There were no significant changes in haematological or clinical
chemical values, but changes were seen in the weights of a number of
organs in animals at 500 or 750 mg/kg bw, including a significant
reduction in absolute (but not relative) prostate weight, increased
relative adrenal and thyroid weights in males, and increased relative
adrenal weight in females. Pathological changes described as cloudy
swelling were reported in the livers of 5/9 male and 7/10 female rats
given 125 mg/kg bw and in all males and 6/10 females given 250 mg/kg
bw, but in none of the controls. The lesion was reported to be
reversible, but no evidence was provided. No histopathological
examinations were carried out on rats at 500 or 750 mg/kg bw. No NOEL
was identified (Bushby, 1970).
Groups of Charles River rats were fed diets containing 0, 630,
1900, or 9400 mg/kg imidocarb dipropionate for up to 90 days,
according to the schedule shown in Table 2. The purpose was to
identify the appropriate doses for a long-term study of toxicity. The
study did not comply with the principles of GLP. The animals were
checked daily for deaths, and body weight and food consumption were
recorded weekly. Haematological and clinical chemical determinations
were carried out on five rats of each sex per dose before treatment
and during weeks 4 and 13. Five rats of each sex from the control
group and those at the highest dose were killed after seven weeks and
the remaining rats after 13 weeks. At both 7 and 13 weeks,
acetylcholinesterase activity was measured in one-half of the brain of
each of five rats of each sex in the control group and that at the
highest dose At termination, each rat was examined grossly.
Histopathological examinations were performed on 17 organs or tissues
from five rats of each sex in the control group and that at the
highest dose and on the livers, kidneys, and grossly abnormal tissues
from the other rats.
Table 2. Achieved dosages in a 90-day study with imidocarb in Charles River rats
Concentration in Males Females
feed (mg/kg)
No. rats Achieved dose No. rats Achieved dose
(mg/kg bw (mg/kg bw
imidocarb base) imidocarb base)
0 15 0 15 0
630 10 26 10 32
1900 10 75 10 100
9400 15 420 15 550
One male given 9400 mg/kg feed died during week 2, and a control
female died during week 6. No signs of overt toxicity were observed.
Body-weight gain was significantly reduced towards the end of the
study in animals of each sex given 9400 mg/kg feed. There were no
significant effects on haematological, clinical chemical, or urinary
parameters or on brain acetylcholinesterase activity. Pathological
changes attributable to treatment were limited to the livers of rats
given 9400 mg/kg feed; the changes consisted of mild stasis of the
bile in the canaliculi in one male and four females. The NOEL was 1900
mg/kg feed, equal to 75 mg/kg bw per day (Hart, 1973a).
Another study was conducted, which did not comply with the
principles of GLP and was intended to be a two-year study of toxicity,
but in which 117 rats died as a result of overheating of the animal
rooms during week 37. The study was terminated during week 44, when
all surviving rats were necropsied. In this study, groups of
50 Sprague-Dawley rats of each sex were to have received diets
containing the equivalent of 0, 75, 225, or 750 mg/kg bw per day of
imidocarb dipropionate. The rats were F1a weanlings from groups at
the corresponding doses in a study of reproductive toxicity; however,
the highest dose was fetotoxic, so that there were insufficient
weanlings available to form this group, and additional rats were
purchased. No data were reported on this group.
Until the overheating episode, there were no significant effects
on body weight, food consumption, or haematological, clinical
chemical, or urinary parameters. During the last five weeks, the food
intake of females at 225 mg/kg bw was increased, but they showed no
change in body weight. At week 44, the mean thyroid:body weight ratio
was increased in females at this dose. There were no significant
pathological findings. No firm conclusions could be drawn from this
study (Hart, 1974a).
Dogs
In a study that did not comply with GLP, a group of five male
beagle dogs, described as 'substandard', was given daily oral doses of
5 mg/kg bw imidocarb dipropionate (expressed as base) in distilled
water for 30 days. There was no untreated control group. No signs of
toxicity were observed and there appeared to be no adverse effects on
body weight, food or water intake, or haematological or clinical
chemical parameters. The dogs were killed 24 h after the last dose,
and urine was taken for determination of pH, protein, glucose, ketone,
and blood. Selected organs were weighed and a gross pathological
examination was carried out on each dog. There were no obvious
substance-related changes (Chesher et al., 1976).
In another study that did not comply with GLP, groups of four
beagle dogs of each sex were given daily oral doses of 0, 5, 20, or 80
mg/kg bw per day imidocarb dipropionate (expressed as base) in gelatin
capsules, for 90 days. The animals were weighed twice weekly, and each
dog was subjected to an ophthalmoscopic examination before treatment
and on days 36 and 76. Blood samples were taken from all dogs for
haematological and some clinical chemical investigations before
treatment and on days 7, 28, 56, 71, and 90; cholinesterase activity
was not monitored. Urine samples were collected at necropsy. About 25
tissues from each animal were examined microscopically.
All four males and two of the females given 80 mg/kg bw per day
died or were killed in extremis after having reduced food intake and
weight loss for about two weeks before death. The signs of toxicity in
this group included weakness, recumbency, salivation, muscle
fasiculation, ataxia, and splayed hind legs, probably indicating an
anticholinesterase effect. The food intake of dogs at 20 mg/kg bw was
transiently reduced. There were no treatment-related ophthalmoscopic
changes. Eosinophilia was observed in some dogs given 20 or 80 mg/kg
bw per day, and those at the highest dose showed a trend towards
increased serum alanine and aspartate aminotransferase activity and
bilirubin concentration, with occasional, transient increases in those
at 20 mg/kg bw per day. The absolute and relative weights of the
pituitary were decreased in dogs at 20 but not at 80 mg/kg bw per day,
but animals at 80 mg/kg bw per day showed significant increases in the
absolute and relative weights of the kidney, adrenal, and thyroid.
Gross examination revealed fatty changes and mottling in the livers,
particularly in dogs given 20 or 80 mg/kg bw per day.
Histological changes were observed in a range of tissues from
dogs at 80 mg/kg bw per day, particularly in the thymus, spleen, lymph
nodes, and stroma of the villi of the small intestine (pyknosis and
karyorrhexis), and fatty changes were observed in the thick section of
the loop of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty changes, granularity, or vacuolation of
the hepatocytes. Similar though less severe changes were observed in
the livers of dogs given 20 mg/kg bw per day. The NOEL was 5 mg/kg bw
per day (Reynolds et al., 1977).
Monkeys
In a preliminary study designed to investigate distribution to
tissues, five female patas monkeys were given daily oral doses of
5 mg/kg bw per day imidocarb dipropionate dissolved in distilled water
for 30 days. A control group of five females was left untreated. Blood
samples were taken before treatment, seven and 14 days after the start
of treatment, and at termination. Some time after the last dose (not
stated), the treated monkeys were killed and examined for gross
changes; the control monkeys were not autopsied. There were no signs
of toxicity in the treated animals, and no effects were observed on
haematological or clinical chemical values or gross appearance
(Thomson, 1975b).
2.2.3 Long-term toxicity and carcinogenicity
Rats
In a study that did not conform to GLP, groups of 50 Wistar rats
of each sex were fed diets calculated to provide intakes of 0, 15, 60,
or 240 mg/kg bw per day imidocarb dipropionate (expressed as base) for
104 weeks. Satellite groups of 15 rats of each sex per dose were used
for collection of blood and urine before treatment and at regular
intervals up to termination. Ophthalmoscopic examinations were carried
out at intervals throughout the study on controls and animals at the
highest dose. At termination, about 25 tissues were selected for
histopathological examination from 10 controls of each sex and from
four males and 19 females at the highest dose; eight or nine tissues
were examined from the remaining animals, comprising lymph nodes,
liver, spleen, kidneys, ovaries, adrenals, thyroids, pituitaries, and
blood films. Gross lesions from all animals were also examined
microscopically.
Survival of animals at the highest dose was adversely affected,
only 9/65 males surviving to termination. The rats in this group
became emaciated, their body-weight gain being significantly reduced
and food consumption significantly increased; females were less
severely affected than males. In the last weeks of the study, the food
consumption of the males exceeded that of the controls by about 40%. A
slight reduction in body-weight gain was also observed in females
given 60 mg/kg bw per day. Males at 240 mg/kg bw per day also had an
increased incidence of corneal opacity and evidence of mild anaemia,
with significant reductions in erythrocyte and haemoglobin counts and
packed cell volume.
Changes in clinical chemistry were seen mainly in rats at 240
mg/kg bw per day and appeared to be transient. In males, the changes
included significantly increased aspartate aminotransferase activity
during weeks 13 and 26, increased alkaline phosphatase activity during
week 8, and increased blood urea nitrogen concentration during week 52
and 78. The cholesterol concentration was significantly increased in
all treated groups during week 52 but not at other times. Females had
significant increases in aspartate aminotransferase activity during
weeks 13 and 78, in alanine aminotransferase activity during week 13
(but significant reductions in weeks 4, 52, and 78), in alkaline
phosphatase activity in week 26, and in cholesterol concentration in
week 8. Alkaline phosphatase activity was also increased in females
given 60 mg/kg bw per day during week 26. Males given 60 mg/kg bw per
day and rats of each sex given 240 mg/kg bw per day had significantly
greater water intake than the controls. Polyuria was observed in males
given 240 mg/kg bw per day, and a slight increase in urinary output
was noted in females at this dose. The specific gravity of urine
samples from the males given 240 mg/kg bw per day was consistently
lower than that of the controls.
At termination, the mean group kidney weight of males given 240
mg/kg bw per day was significantly increased. Treatment-related
microscopic changes were confined to the rats at 60 or 240 mg/kg bw
per day and included deposition of brown pigment in macrophages and
related cells in the liver, spleen, lymph nodes, and bone marrow, and
cystic distension of the renal tubules and glomeruli, with dystrophic
mineralization of the renal medulla. Muscle sections were not taken
for microscopic examination, but evidence of increased atrophy was
found in rats at the highest dose in muscle samples attached to the
sternum. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, some clinical chemical changes,
histopathological changes in the kidney, and skeletal muscle atrophy
at higher doses. The incidence of mammary fibroadenomas was increased
in females given 240 mg/kg bw per day, and many of them had multiple
tumours. Males at this dose had an increased incidence of cutaneous
fibromas (Brown, 1979).
In a separate report, the original histological data from the
study described above were re-tabulated and reanalysed. A number of
assumptions had to be made, since it was not always clear which
tissues had been examined from which animal, which lesions had been
reported, and what terminology had been used. The following overall
conclusions were drawn. Females given 240 mg/kg bw per day had a
significantly increased incidence of multiple fibroadenomas and a
decreased incidence of single fibroadenomas in the mammary gland as
compared with the controls; there was no significant difference in the
combined incidence of multiple and single fibroadenomas. Males at this
dose had a significantly increased incidence of multiple subcutaneous
fibromas in comparison with the controls; an apparent decrease in the
incidence of single fibromas was not significant, and there was no
significant difference in the combined incidence of multiple and
single fibroadenomas. Rats of each sex given 240 mg/kg bw per day had
a nonsignificant increase in the incidence of fibrosarcomas (Finch,
1993).
2.2.4 Genotoxicity
The results of studies of the genotoxicity of imidocarb are
summarized in Table 3. The concentrations and doses are expressed as
imidocarb base, except when stated otherwise. Negative results were
obtained in asays for gene mutation in bacteria and in mammalian cells
in vitro. In two separate experiments, imidocarb induced polyploidy
in human lymphocytes in vitro; however, there was no induction of
aneuploidy. Negative results were obtained in a test for micronucleus
formation in mouse bone marrow, in a metaphase analysis in bone marrow
of rats in vivo, and in an inadequately conducted assay for dominant
lethal mutation.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A multigeneration study was carried out in rats as part of a
larger study designed to investigate all aspects of reproduction,
including teratogenicity, and to provide weanling rats for a two-year
study. Groups of 15 Sprague-Dawley rats of each sex were maintained on
diets containing 0, 630, 1900, or 9000 mg/kg imidocarb dipropionate
(presumably expressed as salt, although this is not clear from the
report) for 60 days before mating. The diets were stated to provide an
equivalent of 0, 45, 130, or 760 mg/kg bw of imidocarb dipropionate
per day. The numbers of pups in both the F1a and F1b litters were
significantly reduced at the highest dose; thus, only three females
receiving 9000 mg/kg produced F1a litters. There was a similar
reduction in the F1b litters receiving the intermediate dose. F2
litters were bred only from the animals at the low dose, and the study
was terminated early owing to failure of the temperature control
mechanism in the animal rooms. No firm conclusions could be drawn from
this study (Hart, 1974b).
Table 3. Results of genotoxicity studies on imidocarb
End-point Test object Concentration S9 Result GLP Reference
In vitro
Gene mutation S. typhimurium TA1535, TA1537, < 1000 µg/plate + Negativea No Moore (1997)
TA1538, TA98, TA100 < 250 µg/plate - Negativea
Gene mutation S. typhimurium TA1535, TA1537, 0.2-1000 µg/plate + Negativea No Moore & Chatfield
TA1538, TA98, TA100 - Negativea (1983)
Gene mutation S. typhimurium TA98, TA100 4-2650 µg/plate + Negative Yes Tait & Clare
TA1535, TA1537, E. coli WP2 33-530 µg/plate - Negativeb (1991a)
pKM101, WP2uvrA- pKM101 53-265 µg/plate
(with strain WP2uvrA- pKM101 only)
Gene mutation hprt locus of L5178Y mouse 0.55-1749 µg/ml + Negative Yes Tait & Clare
lymphoma cells 54.7-1749 µg/ml - Negative (1991b)
Chromosomal Human peripheral blood 3.5-35 µg/ml (as imidocarb - Negative No Whitaker &
aberration lumphocytes dipropionate); higher doses were Bonhoff (1983)
toxic
Chromosomal Human peripheral blood 20 h -S9; 144-294 µg/ml; + Positivec Yes Tait & McEnaney
aberration lymphocytes 20 h +S9; 857-1749 µg/ml (expt 1) (1991)
20 h -S9; 235-367 µg/ml;
20 h + S9; 1119-1749 µg/ml;
44 h -S9; 235 µg/ml; 44 h +S9;
1119 µg/ml (expt 2)
44 h + S9; 366.8-1119 µg/ml (expt 3)
Micronucleus Human peripheral blood 366.8-1120 µg/ml (for frequency of + Negativee Yes Marshall (1993)
formation lymphocytes micronuclei)
895.5 µg/ml (for polyploidy)
Table 3. (continued)
End-point Test object Concentration S9 Result GLP Reference
Host-mediated Male ICR mice (10/group) Five daily oral doses of 15, 45 or Negative No Sibinovic (1986)
assay S. typhimurium TA1530, G-46 150 mg/kg bw; test organism given
intraperitoneally 30 min after last
dosea
In vivo
Bone marrow Sprague-Dawley rats (5/dose); Five consecutive daily Negative No Fabrizio (1986)
metaphase bone marrow harvested 5 days oral doses of 10, 30, or
analysis after treatment with imidocarb; 100 mg/kg bwa
40-50 metaphase spreads/rat
analysed
Micronucleus CD-1 mouse (5/sex per dose); Single intraperitoneal injection of Negative Yes Tait & Marshall
formation marrow harvested 24, 48, 72h 8, 5, 17, or 34 mg/kg bw (1991)
after treatment
Dominant Male Evans mice Five daily intramuscular injections Negatived No Harper et al.
lethal of 4 or 16 mg/kg bw followed by a (1977)
mutation six-week mating schedule
S9, 9000 × g fraction of rat liver; GLP, conformity with good laboratory practice
a No information on whether concentrations or doses reported in terms of imidocarb base or diproprionate salt
b Although there was a statistically significant increase in the number of revertants with strain WP2uvrA- pKM101 in the presence
of S9, the increase was less than double the value for the solvent control, there was no concentration-related response, and the
values were within the range for contemporary historical controls.
c Treatment in the absence of S9 resulted in significant increases in the frequencies of cells with structural aberrations; it was
stated that the frequencies were within the range in historical controls, but the Committee did not consider the historical
controls to be appropriate. Statistically significant increases in the frequency of cells with numerical aberrations were observed
in cultures treated in the presence of S9 and sampled at 44 h, in both experiments 2 and 3.
d Although there were statistically significant increases in the percentage of dead implants in the group receiving 6 mg/kg bw during
week 2, there was no dose-response relationship, and the incidence in the control group was unusually low. The treatment and mating
schedules used in this study did not comply with modern guidelines.
e There was no increase in the incidence of micronuclei at any concentration; however, an increased frequency of polypoid cells was
observed at the single concentration tested.
In another study that did not comply with GLP, groups of 20
Wistar rats of each sex were fed diets calculated to provide 0, 15,
45, or 135 mg/kg bw per day imidocarb dipropionate. Treatment
commenced when the rats were six weeks old, 60 days before the first
mating, and continued throughout the breeding of three succesive
generations. At 21 days post partum, the F1a, F2a, and F2b
litters were killed and examined internally. The F1b and F2b
litters were mated to provide the subsequent generations. The F3b
fetuses were removed on day 20 of gestation and examined for
malformations.
During the premating period, females of the parental generation
showed a small but dose-related decrease in body-weight gain; there
were no clear dose-related effects on body weight in subsequent
generations. The numbers of live births among females receiving 135
mg/kg bw per day were reduced after the first mating of the F0
generation, and the numbers of dead or missing fetuses were increased.
A similar trend was observed after the first mating of the F1
generation. Maternal body-weight gain was reduced on day 17 of
gestation after the second mating of the F1 generation. No evidence
of teratogenicity was found on examination of the F3b fetuses;
however, there was a small, nonsignificant increase in the incidence
of fetuses with bifid sternebrae. The NOEL was 45 mg/kg bw per day for
both fetal toxicity and maternal toxicity (James, 1977).
Dogs
Five female dogs were given subcutaneous injections of 14 mg/kg
bw imidocarb dipropionate (expressed as free base) on the first day of
pro-oestrus and again immediately after successful mating. Six
untreated dogs were used as controls. The dogs were allowed to litter
naturally. After the first whelping, all the treated dogs were dosed
and bred a second time. There were no signs of toxicity and no effects
on on fertility, gestation, or appearance of pups (Szot, 1972).
(ii) Developmental toxicity
Rats
Teratogenicity was studied in rats within a multigeneration
study, which did not comply with the principles of GLP. Groups of 15
female rats were fed imidocarb dipropionate continuously in the diet
for 60 days before mating, achieving intakes of about 0, 47, 140, or
760 mg/kg bw per day. They were killed on day 19 of gestation, and the
uterine contents were examined. Two-thirds of the fetuses were then
processed for staining with Alizarin Red S for skeletal evaluation,
and the remaining one-third were fixed in Bouin's solution and
sectioned for evaluation of visceral abnormalities.
Maternal body-weight gain was significantly reduced at the doses
of 140 and 760 mg/kg bw per day and the incidence of resorptions was
significant-ly increased in those at 760 mg/kg bw per day with a
consequent reduction in the numbers of live fetuses. Animals at 140
mg/kg bw per day also showed a small increase in the resorption rate.
Fetal body weight and length were reduced at 760 mg/kg bw per day.
There was no evidence of teratogenicity at any dose (Hart, 1973b).
In a second study, which was not carried out in compliance with
the principles of GLP, groups of 27-31 mated female Wistar rats were
given daily oral doses of 0 (untreated), 0 (distilled water, solvent),
19, 76, or 300 mg/kg bw imidocarb dipropionate (expressed as base) by
gavage on days 6-16 of gestation. Groups of 20-25 dams were killed on
day 20 of gestation and the uterine contents examined. One-third of
the fetuses were examined by open dissection, one-third were processed
by Wilson's section technique for examination of thick serial
sections, and one-third were processed for staining with Alizarin Red
S for skeletal evaluation. The remaining six dams in each group were
allowed to deliver naturally and rear the offspring to weaning on day
21 post partum.
Maternal body-weight gain was significantly reduced during
treatment with 300 mg/kg bw per day. There was no evidence of
teratogenicity, but the numbers of fetuses with bifid or H-shaped
sternebrae were increased at doses of 76 and 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day (James, 1976a).
Rabbits
Groups of 16-27 female Dutch rabbits were given daily doses of 0
(water), 20, 80, or 320 mg/kg bw per day of imidocarb dipropionate
(doses expressed as base) by oral gavage on days 8-19 of gestation.
The study was not carried out in compliance with the principles of
GLP. All 16 rabbits given 320 mg/kg bw per day and one out of 27 given
80 mg/kg bw per day died. The remaining dams showed signs of stress
(nervous behaviour, diarrhoea, weight loss), which were attributed to
their close proximity to a dog colony. Five to six dams in each group
were allowed to deliver naturally, but most of the dams killed their
offspring. There was no evidence of teratogenicity. The study was
inadequate for establishing a NOEL (James, 1976b).
In another study, which was not carried out in accordance with
the principles of GLP, groups of 10-20 female New Zealand white
rabbits were given doses of 0 (distilled water), 5, 10, 20, 60, or 180
mg/kg bw per day of imidocarb dipropionate by oral gavage on days 6-18
of gestation. The surviving does were killed on day 29 of gestation
and the uterine contents examined. The general appearance of each
fetus and the internal organs of the neck, thorax, and abdomen were
examined grossly. All fetuses were then eviscerated, preserved, and
stained for skeletal examination.
All 10 does given 180 mg/kg bw per day and 12 out of 15 given
60 mg/kg bw per day died. The does in these groups displayed signs of
gastrointestinal disturbance, salivation, nasal exudation, and weight
loss before death. The incidence of post-implantation loss was
increased in animals at 60 mg/kg bw per day, and only 12 fetuses
remained for examination. Fetal weights appeared to be reduced, and
the incidence of delayed ossification appeared to be increased. There
was no evidence of teratogenicity at any dose. The NOEL for both
maternal toxicity and fetotoxicity was 20 mg/kg bw per day (Tesh et
al., 1977).
2.2.6 Special studies to investigate serum prolactin concentrations
A study which did not comply with GLP was carried out to
investigate the mechanism of the induction of mammary tumours in the
two-year study in rats. Groups of 10 male Wistar rats were fed diets
calculated to provide 0, 15, 60, or 240 mg/kg bw per day of imidocarb
(as base) for 28 days. Another group was given 160 mg/kg bw per day of
sulpiride. Serum prolactin concentrations were measured before
treatment and 2, 5, 8, 14, and 22 days after the first dose. The mean
prolactin concentrations were significantly (p < 0.001) increased
in the group receiving sulpiride from day 2 onwards and were slightly
increased in the group given 240 mg/kg bw per day imidocarb, although
they never approached the values found in the group given sulpiride
(Clampitt et al., 1982).
2.2.7 Special studies on microbiological activity
In a study that did not conform to GLP, MIC values in vitro
were determined for imidocarb against 20 bacterial isolates
representing seven genera. MIC values > 16 µg/ml were obtained for 18
isolates, and values of 4 and 8 µg/ml were obtained for two strains of
Pasteurella (Darby & Pelham, 1989).
2.2.8 Special studies on pharmacodynamic effects
It is generally accepted in the published literature that
imidocarb has anticholinesterase activity and that the toxic signs
observed after treatment can be alleviated with atropine (Ali et al.,
1985; Michell et al., 1986; McDougald & Roberson, 1988). The
pharmacodynamic effects of imidocarb were investigated in a screening
study in which mice were given single subcutaneous doses of the
dihydrochloride salt. Myosis was observed at 50 mg/kg bw, but
mydriasis occurred at 150 mg/kg bw (Green, 1966). In another study,
cardiovascular and neuromuscular effects were observed after
intravenous administration of imidocarb dihydrochloride to
anaesthetized cats and dogs, which were attributed in part to the
anticholinesterase effect. No effect on the pupil was observed after
instillation of a solution of 10 mg/ml imidocarb dihydrochloride into
a cat's eye (Green & Hughes, 1969). No conclusions could be drawn
about anticholinesterase potency from these data.
The mode of action of imidocarb as an antiprotozoal agent is
uncertain, although two mechanisms have been proposed:
* As the effect of imidocarb on Trypanosoma brucei is antagonized
by excess polyamines, it is has been suggested that imidocarb
interferes with their production and/or use (Bacchi et al.,
1981).
* Imidocarb blocks the entry of inositol into erythrocytes
containing Babesia, resulting in 'starvation' of the parasite
(McHardy et al., 1986).
2.3 Observations in humans
It is believed that imidocarb has been used occasionally in human
medicine, but no details of doses or adverse effects were available to
the Committee.
3. COMMENTS
The Committee considered data from studies of the
pharmacokinetics, metabolism, acute, and short-term toxicity,
long-term toxicity and carcino-genicity, reproductive toxicity and
genotoxicity of imidocarb and some special studies. Most of the
studies were carried out with the dipropionate salt, and the doses
used were expressed as imidocarb base. Except for some of the assays
for genotoxicity, the studies were not carried out to contemporary
standards for study protocol and conduct but were reported in
sufficient detail and were considered adequate for assessment.
The pharmacokinetics of imidocarb was investigated in mice, rats,
dogs, monkeys, and cattle. The dipropionate and dihydrochloride salts
of imidocarb appeared to be poorly absorbed after oral administration
to rats, but the extent of the oral bioavailability could not be
estimated from the data available. In cattle dosed subcutaneously,
imidocarb was bound to plasma proteins. The drug was excreted in both
urine and the faeces for several days in rodents and for at least 28
days in cattle.
In mice dosed intravenously with radiolabelled imidocarb and
killed 3.5 h later, more than 90% of the residues in liver and kidney
were unmetabolized imidocarb. The parent compound also accounted for
95% of the radiolabelled material in mouse urine. In cattle dosed
subcutaneously with radiolabelled imidocarb, the parent compound
accounted for most of the residues in urine and faeces and 70-90% of
the residues in edible tissues and milk. No evidence of metabolism was
found in a study of bovine liver slices, isolated hepatocytes, or
microsomal fractions in vitro.
Imidocarb dipropionate is moderately hazardous, with oral LD50
values in the range of 650-720 mg/kg bw in mice and 450-1200 mg/kg bw
in rats. The signs were generally consistent with anticholinesterase
activity.
Serum cholinesterase activity was depressed in a dose-related
manner in goats given single intramuscular doses of 12-24 mg/kg bw.
Whole-blood cholinesterase activity was decreased in calves given
single intramuscular injections of 3.3 mg/kg bw.
When rats were given daily oral doses of 0, 125, 250, 500, 750,
or 1500 mg/kg bw per day of imidocarb dihydrochloride for three
months, all rats at the highest dose died. Cloudy swelling in the
liver was observed in rats given 125 and 250 mg/kg bw per day.
Histopathological examinations were not performed on rats at higher
doses. No NOEL was identified.
In a study conducted to identify appropriate doses for a
long-term study of toxicity, rats were fed diets calculated to provide
doses of imidocarb dipropionate equal to 0, 26, 75, or 420 mg/kg bw
per day for 90 days. Body-weight gain was reduced in animals of each
sex given 420 mg/kg bw per day. Acetylcholinesterase activity,
measured in one-half of the brain from each of 10 rats given 420 mg/kg
bw per day and killed after 7 and 13 weeks, was not significantly
different from that in controls. Pathological changes attributable to
treatment were limited to mild stasis of the bile in the canaliculi of
the liver in one male and four females given 420 mg/kg bw per day. The
NOEL was 75 mg/kg bw per day on the basis of reduced body-weight gain
and hepatic toxicity at higher doses.
Groups of four dogs of each sex per dose were given oral doses of
0, 5, 20, or 80 mg/kg bw per day imidocarb dipropionate in gelatin
capsules for 90 days. The signs of toxicity in the dogs given 80 mg/kg
bw per day included recumbency, salivation, muscle fasciculation,
ataxia, and splayed legs. All males and two of four females given this
dose died or were killed. Blood eosinophilia was also observed at 80
mg/kg bw per day, with increased serum alanine aminotransferase and
aspartate aminotransferase activity and increased bilirubin
concentration. Similar but less severe changes were observed in the
dogs given 20 mg/kg bw per day. The weights of the kidney, thyroid,
and adrenal glands were increased in animals at 80 mg/kg bw per day,
and histopathological changes were found in a range of tissues. In the
kidney, these included fatty changes in the thick section of the loop
of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty change, and microgranularity or
vacuolation of hepatocytes. Similar but less severe hepatocellular
changes were found in the livers of dogs given 20 mg/kg bw per day.
The NOEL was 5 mg/kg bw per day on the basis of minor changes in
haematological and clinical chemical parameters and hepatocellular
changes at higher doses.
In a long-term study of toxicity and carcinogenicity, rats were
fed diets calculated to provide intakes of 0, 15, 60, or 240 mg/kg bw
per day of imidocarb as dipropionate for two years. Survival was
adversely affected at the highest dose. Body-weight gain was
significantly reduced in animals of each sex given 240 mg/kg bw per
day and was slightly reduced in females given 60 mg/kg bw per day.
Males at 240 mg/kg bw per day showed evidence of anaemia. Changes in
clinical chemical parameters were transient and occurred mainly in the
animals given the highest dose. Rats given 60 or 240 mg/kg bw per day
drank considerably more water than the controls, and polyuria was
observed in males at the highest dose. At termination, the weights of
the kidneys of males given 240 mg/kg bw per day were significantly
increased. Dose-related histopathological changes were observed at 60
and 240 mg/kg bw per day, which included cystic distension of the
renal tubules and glomeruli and dystrophic mineralization in the renal
medulla. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, changes in some clinical chemical
parameters, and histopathological changes in the kidney. All animals
were examined grossly, and all gross lesions were examined
microscopically; however, comprehensive pathological examinations was
made on only 10 control rats of each sex and 19 females and four males
at the highest dose at termination of the study. A limited range of
tissues from the other animals was examined, and mammary glands were
examined only when a gross lesion was found. At the highest dose, a
significantly increased incidence of multiple fibroadenomas of the
mammary gland was found in females and of multiple subcutaneous
fibromas in males. Because of the excessive toxicity at this dose, the
poor survival, and limited histopathological examination, the
significance of these findings could not be evaluated. The incidences
of tumour at other sites were not increased.
The genotoxic properties of imidocarb dipropionate were
investigated in a range of assays in vitro and in vivo. Negative
results were obtained for gene mutation in Salmonella typhimurium
and in mammalian cells in vitro and in a host-mediated assay in male
mice. In two separate experiments, imidocarb induced polyploidy in
human peripheral blood lymphocytes in vitro in the presence of
metabolic activation; however, aneuploidy was not induced. Micronuclei
were not induced in mouse bone marrow, and no chromosomal aberrations
were induced in rat bone marrow in vivo. Dominant lethal mutations
were not found in mice, although the dosing and mating schedules used
were not in accordance with contemporary guidelines. The Committee
concluded that imidocarb is unlikely to be genotoxic. In a
three-generation study of reproductive toxicity, rats were fed diets
calculated to provide intakes of 0, 15, 45, or 135 mg/kg bw per day
imidocarb as dipropionate. The body-weight gain of dams at 135 mg/kg
bw per day was decreased, and the number of live births was reduced.
The NOEL was 45 mg/kg bw per day for both fetal toxicity and maternal
toxicity.
In a study of developmental toxicity in rats, there was no
evidence of teratogenicity after administration of imidocarb
dipropionate by gavage at doses of 0, 19, 76, or 300 mg/kg bw per day
on days 6œ16 of gestation. Groups of 20-25 dams were killed on day 20
of gestation and the uterine contents examined; the remaining six dams
in each group were allowed to deliver naturally and rear the
offspring. Maternal body-weight gain was reduced at 300 mg/kg bw per
day. The number of fetuses with bifid or H-shaped sternebrae was
increased in groups of rats receiving 76 or 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day. In a study of developmental toxicity in rabbits, there was no
evidence of teratogenicity after administration of 0, 5, 10, 20, 60,
or 180 mg/kg bw per day of imidocarb as dipropionate by gavage on days
6œ18 of gestation. Severe maternal toxicity was observed at 60 and 180
mg/kg bw per day, and all dams given 180 mg/kg bw per day and 12 of 15
given 60 mg/kg bw per day died. The frequency of post-implantation
losses was increased and fetal weights were reduced in the group given
60 mg/kg bw per day. The NOEL for maternal and fetotoxicity was 20
mg/kg bw per day.
4. EVALUATION
An ADI of 0-10 µg/kg bw per day was established on the basis of
the NOEL of 5 mg/kg bw per day in the 90-day study in dogs. A safety
factor of 500 was used to compensate for the limited pathological and
clinical chemical investigations in this study, the absence of
information about potential inhibition of erythrocyte and regional
brain acetylcholinesterase activity in this species, the lack of data
on neurotoxicity, and the limited data on carcinogenic potential.
5. REFERENCES
Ali, B.H., Hassan, T., Suliman, H.B. & Abdelsalam, E.B. (1985) Some
effects of imidocarb in goats. Vet. Human Toxicol., 27, 477-480.
Bacchi, C.J., Nathan, H.C., Hutner, S.H., Duch, D.S. & Nichol, C.A.
(1981) Prevention by polyamines of the curative effect of amicarbalide
and imidocarb for Trypanosoma brucei infections in mice. Biochem.
Pharmacol., 30, 883-886.
Brown, D. (1979) A two year feeding study in the rat with compound 4A.
Unpublished report No. XZIG 86-C1 from Hazleton Laboratories Europe
Ltd, Harrogate, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Bushby, S.R.M. (1970) Prolonged toxicity examination of 4A65 di Hcl in
the rat. Unpublished report No. HZIG 76-2 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Chesher, B.C., Clampitt, R.B. & Malone, J.C. (1976) Imidocarb
dipropionate, 30 day toxicity/residue study in the dog. Unpublished
report No. HZIG 76-2 from Wellcome Research Laboratories, Berkhamsted,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Clampitt, R.B., Taylor, P.E. & MacPherson, I.S. (1982) An experiment
to study the effect of imidocarb dipropionate (4A65) on circulating
prolactin concentration in mature male rats. Unpublished report No.
HZIG 82-C3 from the Wellcome Foundation Ltd, Berkhamsted Hill, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Coldham, N. G., Moore, A.S., Dave, M., Graham, P.J.,
Sivapathasundaram, S., Lake, B.G. & Sauer, M.J. (1995) Imidocarb
residues in edible bovine tissues and in vitro assessment of imidocarb
metabolism and cytotoxicity. Drug Metab. Disposition, 23, 501-505.
Darby, I.H. & Pelham, P.E. (1989) Investigation into the antimicrobial
activiy of three anti-protozoal compounds against a range of aerobic
bacteria. Unpublished report No. CYSE 89-15 from Coopers Animal
Health, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Fabrizio, P.A. (1986) Final report. Cytogenetic study. Compound 4A65
imidocarb dipropionate. Unpublished report No. XZIG 86-6 from Litton
Bionetics Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Farebrother, D.A. (1973) 4A65 Wholebody autoradiographical study in
male rats. Unpublished report No.BPAT 73/8 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Ferguson, E. (1996) (14C)-Imidocarb: Absorption, distribution,
metabolism and excretion in cattle following a single subcutaneous
injection of Imizol. Unpublished report No. S8107-001-R from Corning
Hazleton (Europe). Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Finch, J.M. (1993) Preparation of pathology tables from report XZIG
86-C1 (1299R - 187/1). A two year feeding study in rats with
Imidocarb. Unpublished report No. R-93-070 from Inveresk Research
International, Tranent, Scotland, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Follefant, R. & Green, A.F. (1971a) Imidocarb i.e. 4A65. Acute oral
toxicity in mice and rats: Comparison of the dihydrochloride,
diacetate and dipropionate. Unpublished report No. BPHR/71/86 from
Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Follefant, R & Green, A.F. (1971b) Imidocarb i.e. 4A65. Comparative
toxicity in mice. Unpublished report No BPHR/71/79 from Wellcome
Research Laboratories, Beckenham, Kent, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. (1966) Preliminary assessment of acute pharmacodynamic
effects. Unpublished report No.BPHR 66-58 from Wellcome Research
Laboratories, Beckenham, Kent, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. & Hughes, R. (1969) Pharmacodynamics and acute toxicity.
Unpublished report No. BPHR 69-8 from Wellcome Research Laboratories,
Beckenham, Kent, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. & James, J.A. (1977) Imidocarb dipropionate: Multiple
dosing intramuscular toxicity in male mice (range finder study for
dominant lethal test). Unpublished report No. HZIG 77-1 from Wellcome
Research Laboratories, Berkhamsted and Beckenham, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977a) Imidocarb
dipropionate: Acute oral toxicity in the female rat (10% solution).
Unpublished report No. HZIG 77/2 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977b) Imidocarb
dipropionate: Acute oral toxicity in the male rat (10% solution).
Unpublished report No. HZIG 77/3 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977c) Imidocarb
dipropionate: Acute oral LD50 in the female rat (30% solution).
Unpublished report No. HZIG 77/5 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977d) Imidocarb
dipropionate: Acute oral LD50 in the male rat (30% solution).
Unpublished report No. HZIG 77/6 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. James, J.A. & Parker, M.J. (1977e) Imidocarb
dipropionate: Dominant lethal study in male mice. Unpublished report
No. HZIG 77-4 from Wellcome Research Laboratories, Beckenham, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Hart, E.R. (1973a) 90-day feeding study in rats. Imidicarb
dipropionate. Revised final report. Unpublished report No. XZIG 86-4
from Litton Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1973b) Teratology phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-7 from Litton Bionetics, Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinkrodt.
Hart, E.R. (1974a) Chronic toxicity phase. Imidicarb dipropionate.
Final report. Unpublished report No. XZIG 86-5 from Litton Bionetics,
Inc., Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1974b) Reproduction phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-9 from Litton Bionetics Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976a) Foetal toxicity study of 4A65 (imidocarb
dipropionate) in rats. Unpublished report No. BPAT 76/9 from Wellcome
Research Laboratories, Beckenham, Kent, UNited Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976b) An account of attempted foetal toxicity study of
4A65 (imidocarb dipropionate) in the rabbit. Unpublished report No.
BPAT 76/20 from Wellcome Research Laboratories, Beckenham, Kent,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
James, D.A. (1977) Multigeneration reproduction study of 4A65
(imidocarb dipropionate) in the rat. Unpublished report No. BPAT 77/24
from Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Marshall, R.R. (1993) Study to evaluate the clastogenic potential of
imidocarb by its effects on the frequency of micronuclei in cultured
human peripheral blood lymphocytes. Unpublished report No. R-93-015
from Hazleton Microtest, Harrogate, United Kingdom. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
McDougald, L.R. & Roberson, E.L. (1988) Antiprotozoan drugs. In:
Veterinary Pharmacology and Therapeutics, 6th Ed., Ames, Iowa State
University Press, pp. 950-968.
McHardy, N., Woollon, R.M., Clampitt, R.B., James, J.A. & Crawley,
R.J. (1986) Efficacy, toxicity and metabolism of imidocarb
dipropionate in the treatment of Babesia ovis infection in sheep.
Res. Vet. Sci., 41, 14-20.
Michell, A.R., White, D.G., Higgins, A.J., Moss, P. & Lees, P. (1986)
Effect of induced hypomagnesemia on the toxicity of imidocarb in
calves. Res. Vet. Sci., 40, 264-270.
Moore, W.B. (1977) Mutagenic activity of imidocarb dipropionate.
Unpublished report No. BEMU/77/3 from Wellcome Research Laboratories,
Beckenham, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Moore, W.B. & Chatfield, S.N. (1983) Mutagenicity: Evaluation of
imidocarb dipropionate using a method based on the Yahagi modification
of the Ames Salmonella/microsome incorporation test. Unpublished
report No. BEMU/83/1 from Wellcome Research Laboratories, Beckenham,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Nimmo-Smith, R.H. (1968) Distribution, excretion and tissue levels of
compound 4A65 (HR 2073) in the rat. Unpublished report No. 3BT68 from
Wellcome Research Laboratories, Berkhamsted, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Piercy, D.W.T. & James, J.A. (1976) Imidocarb dipropionate oral LD50
in rabbits. Unpublished report No. HZIG 76-1 from Wellcome Research &
Development, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Reynolds, J., Clampitt, R.B., Piercy, D.W.T., James, J.A. & Skarpa, M.
(1977) Subacute 90 day oral toxicity of imidocarb dipropionate in the
dog. Unpublished report No. HZIG 77-11 from Wellcome Research
Laboratories, Berkhamsted and Beckenham, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Salam, A.A. & Baggot, J.D. (1983) Adverse effects of imidocarb
dipropionate (Imizol) in a dog. J. Vet. Pharmacol. Ther., 5,
131-135.
Sibinovic, K.H. (1986) In vitro and subacute in vivo host-mediated
assay for mutagenesis. Unpublished report No. XZIG from Litton
Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1973a) Oral and intravenous acute toxicity studies of
imidocarb dipropionate (4A65) in mice and rats. Unpublished report no.
T-TEP-73-4 from Department of Toxicology-Experimental Pathology,
Research Triangle Park, North Carolina, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1972b) Fertility study in dogs given imidocarb
dipropionate. Unpublished report No. TTEP-72-11 from the Department of
Toxicology - Experimental Pathology, Research Triangle Park, North
Carolina, USA. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991a) Study to evaluate the ability of
imidocarb to induce mutation in four histidine-requiring strains of
Salmonella typhimurium and two tryptophan requiring strains of
E. coli. Unpublished report No. RRS-91-22 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991b) Study to determine the ability of
imidocarb to induce mutations to 6-thioguanine resistance in mouse
lymphoma L5179Y cells using a fluctuation assay. Unpublished report
No. RRS-91-15 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tait, A.J. & Marshall, R.R. (1991) Study to evaluate the potential of
imidocarb to induce micronuclei in the polychromatic erythrocytes of
CD-1 mice. Unpublished report No. RRS-91-23 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & McEnaney, S. (1991) Study to evaluate the chromosome
damaging potential of imidocarb by its effect on cultured human
lymphocytes using an in vitro cytogenetics assay. Unpublished report
No. RRS-91-32 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tesh, J.M., Ross, F.W. & Tesh, S.A. (1977) 4A: Effects of oral
administration upon pregnancy in the rabbit. Unpublished report No.
77/WRL2/109 from Life Science Research, Stock, Essex, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975a) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the rat. Unpublished report No. BPAT75/17
from Wellcome Research Laboratories, Berkhamsted, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975b) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the monkey. Unpublished report No.
BPAT75/18 from Wellcome Research Laboratories, Berkhamsted, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Wander, A. (1968a) Distribution and excretion of (14C) labelled HR
2073 after intravenous administration of a single dose (1 mg/kg) in
the mouse. Translation of unpublished report No. 94/2555. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Wander, A. (1968b) Investigation of the activity in the urine, liver,
gall bladder and kidneys of the mouse after intravenous administration
of a single dose of HR 2073 C-14. Translation of unpublished report
No. 94/2554. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Whitaker, A.M. & Bonhoff, A.J. (1983) Cytogenetic study of the effect
of imidocarb dipropionate (3 ',3 '-di(2-imidazolin-2-yl)carbanilide
dipropionate) on peripheral human lymphocytes in vitro. Unpublished
report No. BNCD/83/7 from Wellcome Biotechnology Ltd, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Studies in mice, rats, dogs, monkeys, and cattle have been
reported. Most of them were carried out during the 1960s and 1970s
according to the standards of those days, and important details are
missing from some of the reports. In contrast, the study in cattle was
certified for compliance with GLP.
Mice
Mice were injected into the tail vein with of 1 mg/kg bw
imidocarb dihydrochloride radiolabelled with 14C in the carbonyl
group. No radiolabel was detected in expired air. Excretion in the
urine was initially very rapid but quickly slowed and continued
throughout the observation period of 96 h. Faecal excretion followed a
similar pattern. Of the recovered radiolabel (90% of the administered
dose), about two-thirds was found in the urine and one-third in the
faeces (Wander, 1968a).
Rats
Wistar rats were dosed orally with 14C-imidocarb dipropionate
or 14C-imidocarb dihydrochloride and killed at various intervals up
to 48 h after treatment. Another rat was dosed subcutaneously with the
hydrochloride. Whole-body autoradiography indicated poor oral
absorption of both salts, and radiolabel was detectable only in the
liver, kidney, and gut. After subcutaneous treatment, a significant
amount of radiolabel remained at the injection site (Farebrother,
1973). In another study with various dose regimes and routes of
administration (oral, intraperitoneal, subcutaneous), the highest
concentrations of residues were found in the liver and kidney.
Residues were still detectable in these organs 55 days after
subcutaneous treatment with 5 mg/kg bw of the test substance.
Excretion was slow in rats treated subcutaneously, and only 20% of the
administered dose was recovered in excreta during the 78 h of
treatment (Nimmo-Smith, 1968). In a preliminary study to investigate
tissue retention, Wistar rats were given either a single oral dose or
30 daily oral doses of 5 mg/kg bw imidocarb dipropionate. The rats
were killed 24 h after the last dose, and residues in selected tissues
were determined by an unspecified analytical method. The highest
concentrations were found in kidney; surprisingly, the concentrations
in liver and brain were higher after the single-dose regime (Thomson,
1975a).
Dogs
Male beagle dogs were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and killed 24 h after the last
dose. The residues were determined in selected tissues by an
unspecified method. The highest concentrations were found in liver and
then in kidney; no residues were detected in muscle or brain (< 500
µg/kg) (Chesher et al., 1976). In another study, mongrel dogs were
given an intravenous bolus of 4 mg/kg bw of imidocarb dipropionate.
The pharmacokinetics were biphasic and indicated a large volume of
distribution. The mean terminal half-life was 207 min (Salam & Baggot,
1983).
Monkeys
Female patas monkeys were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and were killed some time after the
last dose (probably 24 h, although this is not stated). The residues
were determined in selected tissues by an unspecified method. The
highest concentrations were found in kidney and liver; none were
detected in muscle or brain (limit of detection, 500 µg/kg) (Thomson,
1975b).
Cattle
In a study which complied with the principles of GLP, six
lactating cows and eight calves were given a single subcutaneous
injection of 3 mg/kg bw 14C-imidocarb dipropionate with a specific
activity of 22 mCi/g. The test substance was formulated as the
proprietary product Imizol Injection. A mean peak blood concentration
of 1300 µg-equivalents/kg was attained 1 h after treatment, and the
concentration remained at this level for about 4 h, when 72œ91% of the
radiolabel was bound to plasma proteins. Excretion was very slow: over
the first 10 days, only 53% of the dose was recovered in excreta, with
38% in the faeces and 15% in the urine. The residues in tissues were
very persistent, particularly in liver where mean residues of 2200
µg-equivalents/kg were found 90 days after treatment (Ferguson, 1996).
2.1.2 Biotransformation
In bovine liver preparations in vitro, there was no evidence
for metabolism of imidocarb (Coldham et al., 1995).
Mice
Mice injected with 14C-imidocarb into the tail vein excreted
37% of the administered dose in the urine within 2 h. Of the
radiolabel in the urine, 95% was unmetabolized imidocarb. The mice
were killed 3.5 h after treatment, and 29% of the dose was found in
the liver, 6.8% in the kidney, and less than 1% in the gall-bladder.
More than 90% of the radiolabel in these organs was unmetabolized
imidocarb (Wander, 1968b).
Cattle
In the study described above in which cattle were treated
subcutaneously, the main component of the residues in liver, kidney,
muscle, and fat for up to 90 days after treatment was unmetabolized
imidocarb. Minor components, accounting for not more than 10% of the
total residues, were also present but were not identified. Imidicarb
was also the major component of cows' milk, urine, and faeces
(Ferguson, 1996).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of imidocarb are
summarized in Table 1. None of the studies complied with GLP, and the
reports fall short of modern standards. The signs of toxicity were
generally consistent with the anticholinesterase activity of imidocarb
and included lethargy, salivation, lachrymation, muscle fasciculation,
ataxia, tremors, and convulsions. Congestion of the lung and kidneys
and mottling of the liver were frequent post-mortem findings in the
rats that died.
In a published report, the signs of toxicity in groups of goats
given a single intramuscular dose in the range 12-24 mg/kg bw included
salivation, frequent urination and defaecation, weakness of the legs,
abnormal posture, and recumbency. There were dose-related decreases in
haemoglobin and erythrocyte counts and packed cell volume and
dose-related increases in asparate aminotransferase activity and in
creatinine and bilirubin concentrations. Serum cholinesterase activity
was reduced in a dose-related manner. No significant effects were
observed at 6 mg/kg bw. The effects were ameliorated by pretreatment
with atropine sulfate intramuscularly at a dose of 1 mg per animal
(Ali et al., 1985).
Whole-blood cholinesterase activity was significantly reduced in
calves given an intramuscular injection of 3.3 mg/kg bw imidocarb
dipropionate. Maximum depression occurred about 30 min after
treatment, and significant recovery was observed within 6 h. The
cholinesterase depression did not correlate with the intensity of the
clinical response (Michell et al., 1986).
2.2.2 Short-term toxicity
Mice
A study in mice, intended as a range-finding probe for a study of
dominant lethal mutations, was not carried out in accordance with GLP.
Groups of six male Evans No. 1 mice were given daily intramuscular
doses of 0 (distilled water), 7.8, 16, 31, 63, or 130 mg/kg bw per day
of imidocarb dipropionate (expressed as base) for five days. Signs of
toxicity were recorded for up to 14 days after the last dose. No blood
chemistry or pathological examinations were carried out. All of the
Table 1. Results of studies of acute toxicity with imidocarb
Species Route Vehicle Sex LD50 Salt Reference
(strain) (mg/kg bw) administered
Mouse (ICR) Oral gavage Sterile distilled water M 723a Dipropionate Szot (1973)
Mouse (ICR) Oral gavage Sterile distilled water F 646a Dipropionate Szot (1973)
Mouse (SAS) Oral gavage 0.9% saline F 544a Dihydrochloride Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 863a Diacetate Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 702a Dipropionate Follenfant & Green (1971a)
Mouse (Gambles) Oral gavage 0.9% saline M 1400 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline F 1500 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline M 1652a Dihydrochloride Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1216a Diacetate Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1251a Dipropionate Follenfant & Green (1971a)
Rat (Long Evans) Oral gavage Sterile distilled water M 454a Dipropionate Szot (1973)
Rat (Long Evans) Oral gavage Sterile distilled water F 650a Dipropionate Szot (1973)
Rabbit (New Zealand Oral gavage Water M + F 317 Dipropionate (27% Piercy & James (1975)
white) aqueous solution)
Rat (Long Evans) Intravenous 0.9% saline M 32.7a Dipropionate Szot (1973)
Mouse (ICR) Intravenous 0.9% saline M 22.3a Dipropionate Szot (1973)
Mouse (Gambles) Intravenous 0.9% saline M 16 Hydrochloride Green & Hughes (1969)
Mouse (SAS) Subcutaneous 0.9% saline F 78.6a Dihydrochloride Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 94.5a Diacetate Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 92.1a Dipropionate Follenfant & Green (1971b)
Mouse (Gambles) Subcutaneous 0.9% saline M 140 Hydrochloride Green & Hughes (1969)
Rat (Wistar) Subcutaneous 0.9% saline F 150 Hydrochloride Green & Hughes (1969)
Rat (Sprague-Dawley) Oral gavage Water F > 631a Dipropionate (10% Harper et al. (1997a)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M > 1000a Dipropionate (10% Harper et al. (1997b)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water F 1011.5a Dipropionate (10% Harper et al. (1997c)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M 1998a Dipropionate (10% Harper et al. (1997d)
aqueous solution)
a The LD50 values found in these studies are for the base. In the remaining studies, the results are believed to be quoted as the salt,
although this is not clear from the report in all cases.
animals at 63 and 130 mg/kg bw and 50% of those at 31 mg/kg bw died
three to four days after the start of treatment. The signs of toxicity
were slow respiration and lethargy. There was no NOEL (Harper & James,
1977).
Rats
In a preliminary study designed primarily to investigate
distribution in tissues, groups of five female Wistar rats were given
either a single oral dose of 5 mg/kg bw imidocarb dipropionate
dissolved in distilled water, or daily oral doses of 5 mg/kg bw per
day for 30 days. Five additional rats formed a control group. Blood
samples were taken for haematological and clinical chemical testing,
and electrocardiographic, ophthalmoscopic, and urinary measurements
were made before treatment and at termination; no histopathological
examination was performed. The rats were killed 24 h after the last
dose, and residues were determined in selected tissues. There were no
treatment-related effects on most of the parameters investigated. The
significant changes in blood parameters included increased total
leukocyte counts in the treated group; however, the toxicological
significance of these effects is doubtful since satisfactory blood
samples were obtained from only three treated rats (Thomson, 1975a).
In a study that did not conform to GLP, groups of 10 Wistar rats
of each sex were given daily oral doses of imidocarb dihydrochloride
for three months. The study was carried out in two phases. In phase 1,
the doses were 0 (distilled water), 125, 250, or 500 mg/kg bw per day;
in phase 2, the doses were 0, 500, 750, or 1500 mg/kg bw per day. It
was not clear whether the doses were expressed as the hydrochloride or
as the the base, and the solutions were not analysed. The rats were
weighed and examined daily. Blood samples for haematological and
clinical chemical determinations were taken just before termination.
Urinalysis was not carried out.
All rats given 1500 mg/kg bw died. The body-weight gain of all
treated rats was significantly lower than that of controls in phase 2.
There were no significant changes in haematological or clinical
chemical values, but changes were seen in the weights of a number of
organs in animals at 500 or 750 mg/kg bw, including a significant
reduction in absolute (but not relative) prostate weight, increased
relative adrenal and thyroid weights in males, and increased relative
adrenal weight in females. Pathological changes described as cloudy
swelling were reported in the livers of 5/9 male and 7/10 female rats
given 125 mg/kg bw and in all males and 6/10 females given 250 mg/kg
bw, but in none of the controls. The lesion was reported to be
reversible, but no evidence was provided. No histopathological
examinations were carried out on rats at 500 or 750 mg/kg bw. No NOEL
was identified (Bushby, 1970).
Groups of Charles River rats were fed diets containing 0, 630,
1900, or 9400 mg/kg imidocarb dipropionate for up to 90 days,
according to the schedule shown in Table 2. The purpose was to
identify the appropriate doses for a long-term study of toxicity. The
study did not comply with the principles of GLP. The animals were
checked daily for deaths, and body weight and food consumption were
recorded weekly. Haematological and clinical chemical determinations
were carried out on five rats of each sex per dose before treatment
and during weeks 4 and 13. Five rats of each sex from the control
group and those at the highest dose were killed after seven weeks and
the remaining rats after 13 weeks. At both 7 and 13 weeks,
acetylcholinesterase activity was measured in one-half of the brain of
each of five rats of each sex in the control group and that at the
highest dose At termination, each rat was examined grossly.
Histopathological examinations were performed on 17 organs or tissues
from five rats of each sex in the control group and that at the
highest dose and on the livers, kidneys, and grossly abnormal tissues
from the other rats.
Table 2. Achieved dosages in a 90-day study with imidocarb in Charles River rats
Concentration in Males Females
feed (mg/kg)
No. rats Achieved dose No. rats Achieved dose
(mg/kg bw (mg/kg bw
imidocarb base) imidocarb base)
0 15 0 15 0
630 10 26 10 32
1900 10 75 10 100
9400 15 420 15 550
One male given 9400 mg/kg feed died during week 2, and a control
female died during week 6. No signs of overt toxicity were observed.
Body-weight gain was significantly reduced towards the end of the
study in animals of each sex given 9400 mg/kg feed. There were no
significant effects on haematological, clinical chemical, or urinary
parameters or on brain acetylcholinesterase activity. Pathological
changes attributable to treatment were limited to the livers of rats
given 9400 mg/kg feed; the changes consisted of mild stasis of the
bile in the canaliculi in one male and four females. The NOEL was 1900
mg/kg feed, equal to 75 mg/kg bw per day (Hart, 1973a).
Another study was conducted, which did not comply with the
principles of GLP and was intended to be a two-year study of toxicity,
but in which 117 rats died as a result of overheating of the animal
rooms during week 37. The study was terminated during week 44, when
all surviving rats were necropsied. In this study, groups of
50 Sprague-Dawley rats of each sex were to have received diets
containing the equivalent of 0, 75, 225, or 750 mg/kg bw per day of
imidocarb dipropionate. The rats were F1a weanlings from groups at
the corresponding doses in a study of reproductive toxicity; however,
the highest dose was fetotoxic, so that there were insufficient
weanlings available to form this group, and additional rats were
purchased. No data were reported on this group.
Until the overheating episode, there were no significant effects
on body weight, food consumption, or haematological, clinical
chemical, or urinary parameters. During the last five weeks, the food
intake of females at 225 mg/kg bw was increased, but they showed no
change in body weight. At week 44, the mean thyroid:body weight ratio
was increased in females at this dose. There were no significant
pathological findings. No firm conclusions could be drawn from this
study (Hart, 1974a).
Dogs
In a study that did not comply with GLP, a group of five male
beagle dogs, described as 'substandard', was given daily oral doses of
5 mg/kg bw imidocarb dipropionate (expressed as base) in distilled
water for 30 days. There was no untreated control group. No signs of
toxicity were observed and there appeared to be no adverse effects on
body weight, food or water intake, or haematological or clinical
chemical parameters. The dogs were killed 24 h after the last dose,
and urine was taken for determination of pH, protein, glucose, ketone,
and blood. Selected organs were weighed and a gross pathological
examination was carried out on each dog. There were no obvious
substance-related changes (Chesher et al., 1976).
In another study that did not comply with GLP, groups of four
beagle dogs of each sex were given daily oral doses of 0, 5, 20, or 80
mg/kg bw per day imidocarb dipropionate (expressed as base) in gelatin
capsules, for 90 days. The animals were weighed twice weekly, and each
dog was subjected to an ophthalmoscopic examination before treatment
and on days 36 and 76. Blood samples were taken from all dogs for
haematological and some clinical chemical investigations before
treatment and on days 7, 28, 56, 71, and 90; cholinesterase activity
was not monitored. Urine samples were collected at necropsy. About 25
tissues from each animal were examined microscopically.
All four males and two of the females given 80 mg/kg bw per day
died or were killed in extremis after having reduced food intake and
weight loss for about two weeks before death. The signs of toxicity in
this group included weakness, recumbency, salivation, muscle
fasiculation, ataxia, and splayed hind legs, probably indicating an
anticholinesterase effect. The food intake of dogs at 20 mg/kg bw was
transiently reduced. There were no treatment-related ophthalmoscopic
changes. Eosinophilia was observed in some dogs given 20 or 80 mg/kg
bw per day, and those at the highest dose showed a trend towards
increased serum alanine and aspartate aminotransferase activity and
bilirubin concentration, with occasional, transient increases in those
at 20 mg/kg bw per day. The absolute and relative weights of the
pituitary were decreased in dogs at 20 but not at 80 mg/kg bw per day,
but animals at 80 mg/kg bw per day showed significant increases in the
absolute and relative weights of the kidney, adrenal, and thyroid.
Gross examination revealed fatty changes and mottling in the livers,
particularly in dogs given 20 or 80 mg/kg bw per day.
Histological changes were observed in a range of tissues from
dogs at 80 mg/kg bw per day, particularly in the thymus, spleen, lymph
nodes, and stroma of the villi of the small intestine (pyknosis and
karyorrhexis), and fatty changes were observed in the thick section of
the loop of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty changes, granularity, or vacuolation of
the hepatocytes. Similar though less severe changes were observed in
the livers of dogs given 20 mg/kg bw per day. The NOEL was 5 mg/kg bw
per day (Reynolds et al., 1977).
Monkeys
In a preliminary study designed to investigate distribution to
tissues, five female patas monkeys were given daily oral doses of
5 mg/kg bw per day imidocarb dipropionate dissolved in distilled water
for 30 days. A control group of five females was left untreated. Blood
samples were taken before treatment, seven and 14 days after the start
of treatment, and at termination. Some time after the last dose (not
stated), the treated monkeys were killed and examined for gross
changes; the control monkeys were not autopsied. There were no signs
of toxicity in the treated animals, and no effects were observed on
haematological or clinical chemical values or gross appearance
(Thomson, 1975b).
2.2.3 Long-term toxicity and carcinogenicity
Rats
In a study that did not conform to GLP, groups of 50 Wistar rats
of each sex were fed diets calculated to provide intakes of 0, 15, 60,
or 240 mg/kg bw per day imidocarb dipropionate (expressed as base) for
104 weeks. Satellite groups of 15 rats of each sex per dose were used
for collection of blood and urine before treatment and at regular
intervals up to termination. Ophthalmoscopic examinations were carried
out at intervals throughout the study on controls and animals at the
highest dose. At termination, about 25 tissues were selected for
histopathological examination from 10 controls of each sex and from
four males and 19 females at the highest dose; eight or nine tissues
were examined from the remaining animals, comprising lymph nodes,
liver, spleen, kidneys, ovaries, adrenals, thyroids, pituitaries, and
blood films. Gross lesions from all animals were also examined
microscopically.
Survival of animals at the highest dose was adversely affected,
only 9/65 males surviving to termination. The rats in this group
became emaciated, their body-weight gain being significantly reduced
and food consumption significantly increased; females were less
severely affected than males. In the last weeks of the study, the food
consumption of the males exceeded that of the controls by about 40%. A
slight reduction in body-weight gain was also observed in females
given 60 mg/kg bw per day. Males at 240 mg/kg bw per day also had an
increased incidence of corneal opacity and evidence of mild anaemia,
with significant reductions in erythrocyte and haemoglobin counts and
packed cell volume.
Changes in clinical chemistry were seen mainly in rats at 240
mg/kg bw per day and appeared to be transient. In males, the changes
included significantly increased aspartate aminotransferase activity
during weeks 13 and 26, increased alkaline phosphatase activity during
week 8, and increased blood urea nitrogen concentration during week 52
and 78. The cholesterol concentration was significantly increased in
all treated groups during week 52 but not at other times. Females had
significant increases in aspartate aminotransferase activity during
weeks 13 and 78, in alanine aminotransferase activity during week 13
(but significant reductions in weeks 4, 52, and 78), in alkaline
phosphatase activity in week 26, and in cholesterol concentration in
week 8. Alkaline phosphatase activity was also increased in females
given 60 mg/kg bw per day during week 26. Males given 60 mg/kg bw per
day and rats of each sex given 240 mg/kg bw per day had significantly
greater water intake than the controls. Polyuria was observed in males
given 240 mg/kg bw per day, and a slight increase in urinary output
was noted in females at this dose. The specific gravity of urine
samples from the males given 240 mg/kg bw per day was consistently
lower than that of the controls.
At termination, the mean group kidney weight of males given 240
mg/kg bw per day was significantly increased. Treatment-related
microscopic changes were confined to the rats at 60 or 240 mg/kg bw
per day and included deposition of brown pigment in macrophages and
related cells in the liver, spleen, lymph nodes, and bone marrow, and
cystic distension of the renal tubules and glomeruli, with dystrophic
mineralization of the renal medulla. Muscle sections were not taken
for microscopic examination, but evidence of increased atrophy was
found in rats at the highest dose in muscle samples attached to the
sternum. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, some clinical chemical changes,
histopathological changes in the kidney, and skeletal muscle atrophy
at higher doses. The incidence of mammary fibroadenomas was increased
in females given 240 mg/kg bw per day, and many of them had multiple
tumours. Males at this dose had an increased incidence of cutaneous
fibromas (Brown, 1979).
In a separate report, the original histological data from the
study described above were re-tabulated and reanalysed. A number of
assumptions had to be made, since it was not always clear which
tissues had been examined from which animal, which lesions had been
reported, and what terminology had been used. The following overall
conclusions were drawn. Females given 240 mg/kg bw per day had a
significantly increased incidence of multiple fibroadenomas and a
decreased incidence of single fibroadenomas in the mammary gland as
compared with the controls; there was no significant difference in the
combined incidence of multiple and single fibroadenomas. Males at this
dose had a significantly increased incidence of multiple subcutaneous
fibromas in comparison with the controls; an apparent decrease in the
incidence of single fibromas was not significant, and there was no
significant difference in the combined incidence of multiple and
single fibroadenomas. Rats of each sex given 240 mg/kg bw per day had
a nonsignificant increase in the incidence of fibrosarcomas (Finch,
1993).
2.2.4 Genotoxicity
The results of studies of the genotoxicity of imidocarb are
summarized in Table 3. The concentrations and doses are expressed as
imidocarb base, except when stated otherwise. Negative results were
obtained in asays for gene mutation in bacteria and in mammalian cells
in vitro. In two separate experiments, imidocarb induced polyploidy
in human lymphocytes in vitro; however, there was no induction of
aneuploidy. Negative results were obtained in a test for micronucleus
formation in mouse bone marrow, in a metaphase analysis in bone marrow
of rats in vivo, and in an inadequately conducted assay for dominant
lethal mutation.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A multigeneration study was carried out in rats as part of a
larger study designed to investigate all aspects of reproduction,
including teratogenicity, and to provide weanling rats for a two-year
study. Groups of 15 Sprague-Dawley rats of each sex were maintained on
diets containing 0, 630, 1900, or 9000 mg/kg imidocarb dipropionate
(presumably expressed as salt, although this is not clear from the
report) for 60 days before mating. The diets were stated to provide an
equivalent of 0, 45, 130, or 760 mg/kg bw of imidocarb dipropionate
per day. The numbers of pups in both the F1a and F1b litters were
significantly reduced at the highest dose; thus, only three females
receiving 9000 mg/kg produced F1a litters. There was a similar
reduction in the F1b litters receiving the intermediate dose. F2
litters were bred only from the animals at the low dose, and the study
was terminated early owing to failure of the temperature control
mechanism in the animal rooms. No firm conclusions could be drawn from
this study (Hart, 1974b).
Table 3. Results of genotoxicity studies on imidocarb
End-point Test object Concentration S9 Result GLP Reference
In vitro
Gene mutation S. typhimurium TA1535, TA1537, < 1000 µg/plate + Negativea No Moore (1997)
TA1538, TA98, TA100 < 250 µg/plate - Negativea
Gene mutation S. typhimurium TA1535, TA1537, 0.2-1000 µg/plate + Negativea No Moore & Chatfield
TA1538, TA98, TA100 - Negativea (1983)
Gene mutation S. typhimurium TA98, TA100 4-2650 µg/plate + Negative Yes Tait & Clare
TA1535, TA1537, E. coli WP2 33-530 µg/plate - Negativeb (1991a)
pKM101, WP2uvrA- pKM101 53-265 µg/plate
(with strain WP2uvrA- pKM101 only)
Gene mutation hprt locus of L5178Y mouse 0.55-1749 µg/ml + Negative Yes Tait & Clare
lymphoma cells 54.7-1749 µg/ml - Negative (1991b)
Chromosomal Human peripheral blood 3.5-35 µg/ml (as imidocarb - Negative No Whitaker &
aberration lumphocytes dipropionate); higher doses were Bonhoff (1983)
toxic
Chromosomal Human peripheral blood 20 h -S9; 144-294 µg/ml; + Positivec Yes Tait & McEnaney
aberration lymphocytes 20 h +S9; 857-1749 µg/ml (expt 1) (1991)
20 h -S9; 235-367 µg/ml;
20 h + S9; 1119-1749 µg/ml;
44 h -S9; 235 µg/ml; 44 h +S9;
1119 µg/ml (expt 2)
44 h + S9; 366.8-1119 µg/ml (expt 3)
Micronucleus Human peripheral blood 366.8-1120 µg/ml (for frequency of + Negativee Yes Marshall (1993)
formation lymphocytes micronuclei)
895.5 µg/ml (for polyploidy)
Table 3. (continued)
End-point Test object Concentration S9 Result GLP Reference
Host-mediated Male ICR mice (10/group) Five daily oral doses of 15, 45 or Negative No Sibinovic (1986)
assay S. typhimurium TA1530, G-46 150 mg/kg bw; test organism given
intraperitoneally 30 min after last
dosea
In vivo
Bone marrow Sprague-Dawley rats (5/dose); Five consecutive daily Negative No Fabrizio (1986)
metaphase bone marrow harvested 5 days oral doses of 10, 30, or
analysis after treatment with imidocarb; 100 mg/kg bwa
40-50 metaphase spreads/rat
analysed
Micronucleus CD-1 mouse (5/sex per dose); Single intraperitoneal injection of Negative Yes Tait & Marshall
formation marrow harvested 24, 48, 72h 8, 5, 17, or 34 mg/kg bw (1991)
after treatment
Dominant Male Evans mice Five daily intramuscular injections Negatived No Harper et al.
lethal of 4 or 16 mg/kg bw followed by a (1977)
mutation six-week mating schedule
S9, 9000 × g fraction of rat liver; GLP, conformity with good laboratory practice
a No information on whether concentrations or doses reported in terms of imidocarb base or diproprionate salt
b Although there was a statistically significant increase in the number of revertants with strain WP2uvrA- pKM101 in the presence
of S9, the increase was less than double the value for the solvent control, there was no concentration-related response, and the
values were within the range for contemporary historical controls.
c Treatment in the absence of S9 resulted in significant increases in the frequencies of cells with structural aberrations; it was
stated that the frequencies were within the range in historical controls, but the Committee did not consider the historical
controls to be appropriate. Statistically significant increases in the frequency of cells with numerical aberrations were observed
in cultures treated in the presence of S9 and sampled at 44 h, in both experiments 2 and 3.
d Although there were statistically significant increases in the percentage of dead implants in the group receiving 6 mg/kg bw during
week 2, there was no dose-response relationship, and the incidence in the control group was unusually low. The treatment and mating
schedules used in this study did not comply with modern guidelines.
e There was no increase in the incidence of micronuclei at any concentration; however, an increased frequency of polypoid cells was
observed at the single concentration tested.
In another study that did not comply with GLP, groups of 20
Wistar rats of each sex were fed diets calculated to provide 0, 15,
45, or 135 mg/kg bw per day imidocarb dipropionate. Treatment
commenced when the rats were six weeks old, 60 days before the first
mating, and continued throughout the breeding of three succesive
generations. At 21 days post partum, the F1a, F2a, and F2b
litters were killed and examined internally. The F1b and F2b
litters were mated to provide the subsequent generations. The F3b
fetuses were removed on day 20 of gestation and examined for
malformations.
During the premating period, females of the parental generation
showed a small but dose-related decrease in body-weight gain; there
were no clear dose-related effects on body weight in subsequent
generations. The numbers of live births among females receiving 135
mg/kg bw per day were reduced after the first mating of the F0
generation, and the numbers of dead or missing fetuses were increased.
A similar trend was observed after the first mating of the F1
generation. Maternal body-weight gain was reduced on day 17 of
gestation after the second mating of the F1 generation. No evidence
of teratogenicity was found on examination of the F3b fetuses;
however, there was a small, nonsignificant increase in the incidence
of fetuses with bifid sternebrae. The NOEL was 45 mg/kg bw per day for
both fetal toxicity and maternal toxicity (James, 1977).
Dogs
Five female dogs were given subcutaneous injections of 14 mg/kg
bw imidocarb dipropionate (expressed as free base) on the first day of
pro-oestrus and again immediately after successful mating. Six
untreated dogs were used as controls. The dogs were allowed to litter
naturally. After the first whelping, all the treated dogs were dosed
and bred a second time. There were no signs of toxicity and no effects
on on fertility, gestation, or appearance of pups (Szot, 1972).
(ii) Developmental toxicity
Rats
Teratogenicity was studied in rats within a multigeneration
study, which did not comply with the principles of GLP. Groups of 15
female rats were fed imidocarb dipropionate continuously in the diet
for 60 days before mating, achieving intakes of about 0, 47, 140, or
760 mg/kg bw per day. They were killed on day 19 of gestation, and the
uterine contents were examined. Two-thirds of the fetuses were then
processed for staining with Alizarin Red S for skeletal evaluation,
and the remaining one-third were fixed in Bouin's solution and
sectioned for evaluation of visceral abnormalities.
Maternal body-weight gain was significantly reduced at the doses
of 140 and 760 mg/kg bw per day and the incidence of resorptions was
significant-ly increased in those at 760 mg/kg bw per day with a
consequent reduction in the numbers of live fetuses. Animals at 140
mg/kg bw per day also showed a small increase in the resorption rate.
Fetal body weight and length were reduced at 760 mg/kg bw per day.
There was no evidence of teratogenicity at any dose (Hart, 1973b).
In a second study, which was not carried out in compliance with
the principles of GLP, groups of 27-31 mated female Wistar rats were
given daily oral doses of 0 (untreated), 0 (distilled water, solvent),
19, 76, or 300 mg/kg bw imidocarb dipropionate (expressed as base) by
gavage on days 6-16 of gestation. Groups of 20-25 dams were killed on
day 20 of gestation and the uterine contents examined. One-third of
the fetuses were examined by open dissection, one-third were processed
by Wilson's section technique for examination of thick serial
sections, and one-third were processed for staining with Alizarin Red
S for skeletal evaluation. The remaining six dams in each group were
allowed to deliver naturally and rear the offspring to weaning on day
21 post partum.
Maternal body-weight gain was significantly reduced during
treatment with 300 mg/kg bw per day. There was no evidence of
teratogenicity, but the numbers of fetuses with bifid or H-shaped
sternebrae were increased at doses of 76 and 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day (James, 1976a).
Rabbits
Groups of 16-27 female Dutch rabbits were given daily doses of 0
(water), 20, 80, or 320 mg/kg bw per day of imidocarb dipropionate
(doses expressed as base) by oral gavage on days 8-19 of gestation.
The study was not carried out in compliance with the principles of
GLP. All 16 rabbits given 320 mg/kg bw per day and one out of 27 given
80 mg/kg bw per day died. The remaining dams showed signs of stress
(nervous behaviour, diarrhoea, weight loss), which were attributed to
their close proximity to a dog colony. Five to six dams in each group
were allowed to deliver naturally, but most of the dams killed their
offspring. There was no evidence of teratogenicity. The study was
inadequate for establishing a NOEL (James, 1976b).
In another study, which was not carried out in accordance with
the principles of GLP, groups of 10-20 female New Zealand white
rabbits were given doses of 0 (distilled water), 5, 10, 20, 60, or 180
mg/kg bw per day of imidocarb dipropionate by oral gavage on days 6-18
of gestation. The surviving does were killed on day 29 of gestation
and the uterine contents examined. The general appearance of each
fetus and the internal organs of the neck, thorax, and abdomen were
examined grossly. All fetuses were then eviscerated, preserved, and
stained for skeletal examination.
All 10 does given 180 mg/kg bw per day and 12 out of 15 given
60 mg/kg bw per day died. The does in these groups displayed signs of
gastrointestinal disturbance, salivation, nasal exudation, and weight
loss before death. The incidence of post-implantation loss was
increased in animals at 60 mg/kg bw per day, and only 12 fetuses
remained for examination. Fetal weights appeared to be reduced, and
the incidence of delayed ossification appeared to be increased. There
was no evidence of teratogenicity at any dose. The NOEL for both
maternal toxicity and fetotoxicity was 20 mg/kg bw per day (Tesh et
al., 1977).
2.2.6 Special studies to investigate serum prolactin concentrations
A study which did not comply with GLP was carried out to
investigate the mechanism of the induction of mammary tumours in the
two-year study in rats. Groups of 10 male Wistar rats were fed diets
calculated to provide 0, 15, 60, or 240 mg/kg bw per day of imidocarb
(as base) for 28 days. Another group was given 160 mg/kg bw per day of
sulpiride. Serum prolactin concentrations were measured before
treatment and 2, 5, 8, 14, and 22 days after the first dose. The mean
prolactin concentrations were significantly (p < 0.001) increased
in the group receiving sulpiride from day 2 onwards and were slightly
increased in the group given 240 mg/kg bw per day imidocarb, although
they never approached the values found in the group given sulpiride
(Clampitt et al., 1982).
2.2.7 Special studies on microbiological activity
In a study that did not conform to GLP, MIC values in vitro
were determined for imidocarb against 20 bacterial isolates
representing seven genera. MIC values > 16 µg/ml were obtained for 18
isolates, and values of 4 and 8 µg/ml were obtained for two strains of
Pasteurella (Darby & Pelham, 1989).
2.2.8 Special studies on pharmacodynamic effects
It is generally accepted in the published literature that
imidocarb has anticholinesterase activity and that the toxic signs
observed after treatment can be alleviated with atropine (Ali et al.,
1985; Michell et al., 1986; McDougald & Roberson, 1988). The
pharmacodynamic effects of imidocarb were investigated in a screening
study in which mice were given single subcutaneous doses of the
dihydrochloride salt. Myosis was observed at 50 mg/kg bw, but
mydriasis occurred at 150 mg/kg bw (Green, 1966). In another study,
cardiovascular and neuromuscular effects were observed after
intravenous administration of imidocarb dihydrochloride to
anaesthetized cats and dogs, which were attributed in part to the
anticholinesterase effect. No effect on the pupil was observed after
instillation of a solution of 10 mg/ml imidocarb dihydrochloride into
a cat's eye (Green & Hughes, 1969). No conclusions could be drawn
about anticholinesterase potency from these data.
The mode of action of imidocarb as an antiprotozoal agent is
uncertain, although two mechanisms have been proposed:
* As the effect of imidocarb on Trypanosoma brucei is antagonized
by excess polyamines, it is has been suggested that imidocarb
interferes with their production and/or use (Bacchi et al.,
1981).
* Imidocarb blocks the entry of inositol into erythrocytes
containing Babesia, resulting in 'starvation' of the parasite
(McHardy et al., 1986).
2.3 Observations in humans
It is believed that imidocarb has been used occasionally in human
medicine, but no details of doses or adverse effects were available to
the Committee.
3. COMMENTS
The Committee considered data from studies of the
pharmacokinetics, metabolism, acute, and short-term toxicity,
long-term toxicity and carcino-genicity, reproductive toxicity and
genotoxicity of imidocarb and some special studies. Most of the
studies were carried out with the dipropionate salt, and the doses
used were expressed as imidocarb base. Except for some of the assays
for genotoxicity, the studies were not carried out to contemporary
standards for study protocol and conduct but were reported in
sufficient detail and were considered adequate for assessment.
The pharmacokinetics of imidocarb was investigated in mice, rats,
dogs, monkeys, and cattle. The dipropionate and dihydrochloride salts
of imidocarb appeared to be poorly absorbed after oral administration
to rats, but the extent of the oral bioavailability could not be
estimated from the data available. In cattle dosed subcutaneously,
imidocarb was bound to plasma proteins. The drug was excreted in both
urine and the faeces for several days in rodents and for at least 28
days in cattle.
In mice dosed intravenously with radiolabelled imidocarb and
killed 3.5 h later, more than 90% of the residues in liver and kidney
were unmetabolized imidocarb. The parent compound also accounted for
95% of the radiolabelled material in mouse urine. In cattle dosed
subcutaneously with radiolabelled imidocarb, the parent compound
accounted for most of the residues in urine and faeces and 70-90% of
the residues in edible tissues and milk. No evidence of metabolism was
found in a study of bovine liver slices, isolated hepatocytes, or
microsomal fractions in vitro.
Imidocarb dipropionate is moderately hazardous, with oral LD50
values in the range of 650-720 mg/kg bw in mice and 450-1200 mg/kg bw
in rats. The signs were generally consistent with anticholinesterase
activity.
Serum cholinesterase activity was depressed in a dose-related
manner in goats given single intramuscular doses of 12-24 mg/kg bw.
Whole-blood cholinesterase activity was decreased in calves given
single intramuscular injections of 3.3 mg/kg bw.
When rats were given daily oral doses of 0, 125, 250, 500, 750,
or 1500 mg/kg bw per day of imidocarb dihydrochloride for three
months, all rats at the highest dose died. Cloudy swelling in the
liver was observed in rats given 125 and 250 mg/kg bw per day.
Histopathological examinations were not performed on rats at higher
doses. No NOEL was identified.
In a study conducted to identify appropriate doses for a
long-term study of toxicity, rats were fed diets calculated to provide
doses of imidocarb dipropionate equal to 0, 26, 75, or 420 mg/kg bw
per day for 90 days. Body-weight gain was reduced in animals of each
sex given 420 mg/kg bw per day. Acetylcholinesterase activity,
measured in one-half of the brain from each of 10 rats given 420 mg/kg
bw per day and killed after 7 and 13 weeks, was not significantly
different from that in controls. Pathological changes attributable to
treatment were limited to mild stasis of the bile in the canaliculi of
the liver in one male and four females given 420 mg/kg bw per day. The
NOEL was 75 mg/kg bw per day on the basis of reduced body-weight gain
and hepatic toxicity at higher doses.
Groups of four dogs of each sex per dose were given oral doses of
0, 5, 20, or 80 mg/kg bw per day imidocarb dipropionate in gelatin
capsules for 90 days. The signs of toxicity in the dogs given 80 mg/kg
bw per day included recumbency, salivation, muscle fasciculation,
ataxia, and splayed legs. All males and two of four females given this
dose died or were killed. Blood eosinophilia was also observed at 80
mg/kg bw per day, with increased serum alanine aminotransferase and
aspartate aminotransferase activity and increased bilirubin
concentration. Similar but less severe changes were observed in the
dogs given 20 mg/kg bw per day. The weights of the kidney, thyroid,
and adrenal glands were increased in animals at 80 mg/kg bw per day,
and histopathological changes were found in a range of tissues. In the
kidney, these included fatty changes in the thick section of the loop
of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty change, and microgranularity or
vacuolation of hepatocytes. Similar but less severe hepatocellular
changes were found in the livers of dogs given 20 mg/kg bw per day.
The NOEL was 5 mg/kg bw per day on the basis of minor changes in
haematological and clinical chemical parameters and hepatocellular
changes at higher doses.
In a long-term study of toxicity and carcinogenicity, rats were
fed diets calculated to provide intakes of 0, 15, 60, or 240 mg/kg bw
per day of imidocarb as dipropionate for two years. Survival was
adversely affected at the highest dose. Body-weight gain was
significantly reduced in animals of each sex given 240 mg/kg bw per
day and was slightly reduced in females given 60 mg/kg bw per day.
Males at 240 mg/kg bw per day showed evidence of anaemia. Changes in
clinical chemical parameters were transient and occurred mainly in the
animals given the highest dose. Rats given 60 or 240 mg/kg bw per day
drank considerably more water than the controls, and polyuria was
observed in males at the highest dose. At termination, the weights of
the kidneys of males given 240 mg/kg bw per day were significantly
increased. Dose-related histopathological changes were observed at 60
and 240 mg/kg bw per day, which included cystic distension of the
renal tubules and glomeruli and dystrophic mineralization in the renal
medulla. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, changes in some clinical chemical
parameters, and histopathological changes in the kidney. All animals
were examined grossly, and all gross lesions were examined
microscopically; however, comprehensive pathological examinations was
made on only 10 control rats of each sex and 19 females and four males
at the highest dose at termination of the study. A limited range of
tissues from the other animals was examined, and mammary glands were
examined only when a gross lesion was found. At the highest dose, a
significantly increased incidence of multiple fibroadenomas of the
mammary gland was found in females and of multiple subcutaneous
fibromas in males. Because of the excessive toxicity at this dose, the
poor survival, and limited histopathological examination, the
significance of these findings could not be evaluated. The incidences
of tumour at other sites were not increased.
The genotoxic properties of imidocarb dipropionate were
investigated in a range of assays in vitro and in vivo. Negative
results were obtained for gene mutation in Salmonella typhimurium
and in mammalian cells in vitro and in a host-mediated assay in male
mice. In two separate experiments, imidocarb induced polyploidy in
human peripheral blood lymphocytes in vitro in the presence of
metabolic activation; however, aneuploidy was not induced. Micronuclei
were not induced in mouse bone marrow, and no chromosomal aberrations
were induced in rat bone marrow in vivo. Dominant lethal mutations
were not found in mice, although the dosing and mating schedules used
were not in accordance with contemporary guidelines. The Committee
concluded that imidocarb is unlikely to be genotoxic. In a
three-generation study of reproductive toxicity, rats were fed diets
calculated to provide intakes of 0, 15, 45, or 135 mg/kg bw per day
imidocarb as dipropionate. The body-weight gain of dams at 135 mg/kg
bw per day was decreased, and the number of live births was reduced.
The NOEL was 45 mg/kg bw per day for both fetal toxicity and maternal
toxicity.
In a study of developmental toxicity in rats, there was no
evidence of teratogenicity after administration of imidocarb
dipropionate by gavage at doses of 0, 19, 76, or 300 mg/kg bw per day
on days 6œ16 of gestation. Groups of 20-25 dams were killed on day 20
of gestation and the uterine contents examined; the remaining six dams
in each group were allowed to deliver naturally and rear the
offspring. Maternal body-weight gain was reduced at 300 mg/kg bw per
day. The number of fetuses with bifid or H-shaped sternebrae was
increased in groups of rats receiving 76 or 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day. In a study of developmental toxicity in rabbits, there was no
evidence of teratogenicity after administration of 0, 5, 10, 20, 60,
or 180 mg/kg bw per day of imidocarb as dipropionate by gavage on days
6œ18 of gestation. Severe maternal toxicity was observed at 60 and 180
mg/kg bw per day, and all dams given 180 mg/kg bw per day and 12 of 15
given 60 mg/kg bw per day died. The frequency of post-implantation
losses was increased and fetal weights were reduced in the group given
60 mg/kg bw per day. The NOEL for maternal and fetotoxicity was 20
mg/kg bw per day.
4. EVALUATION
An ADI of 0-10 µg/kg bw per day was established on the basis of
the NOEL of 5 mg/kg bw per day in the 90-day study in dogs. A safety
factor of 500 was used to compensate for the limited pathological and
clinical chemical investigations in this study, the absence of
information about potential inhibition of erythrocyte and regional
brain acetylcholinesterase activity in this species, the lack of data
on neurotoxicity, and the limited data on carcinogenic potential.
5. REFERENCES
Ali, B.H., Hassan, T., Suliman, H.B. & Abdelsalam, E.B. (1985) Some
effects of imidocarb in goats. Vet. Human Toxicol., 27, 477-480.
Bacchi, C.J., Nathan, H.C., Hutner, S.H., Duch, D.S. & Nichol, C.A.
(1981) Prevention by polyamines of the curative effect of amicarbalide
and imidocarb for Trypanosoma brucei infections in mice. Biochem.
Pharmacol., 30, 883-886.
Brown, D. (1979) A two year feeding study in the rat with compound 4A.
Unpublished report No. XZIG 86-C1 from Hazleton Laboratories Europe
Ltd, Harrogate, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Bushby, S.R.M. (1970) Prolonged toxicity examination of 4A65 di Hcl in
the rat. Unpublished report No. HZIG 76-2 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Chesher, B.C., Clampitt, R.B. & Malone, J.C. (1976) Imidocarb
dipropionate, 30 day toxicity/residue study in the dog. Unpublished
report No. HZIG 76-2 from Wellcome Research Laboratories, Berkhamsted,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Clampitt, R.B., Taylor, P.E. & MacPherson, I.S. (1982) An experiment
to study the effect of imidocarb dipropionate (4A65) on circulating
prolactin concentration in mature male rats. Unpublished report No.
HZIG 82-C3 from the Wellcome Foundation Ltd, Berkhamsted Hill, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Coldham, N. G., Moore, A.S., Dave, M., Graham, P.J.,
Sivapathasundaram, S., Lake, B.G. & Sauer, M.J. (1995) Imidocarb
residues in edible bovine tissues and in vitro assessment of imidocarb
metabolism and cytotoxicity. Drug Metab. Disposition, 23, 501-505.
Darby, I.H. & Pelham, P.E. (1989) Investigation into the antimicrobial
activiy of three anti-protozoal compounds against a range of aerobic
bacteria. Unpublished report No. CYSE 89-15 from Coopers Animal
Health, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Fabrizio, P.A. (1986) Final report. Cytogenetic study. Compound 4A65
imidocarb dipropionate. Unpublished report No. XZIG 86-6 from Litton
Bionetics Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Farebrother, D.A. (1973) 4A65 Wholebody autoradiographical study in
male rats. Unpublished report No.BPAT 73/8 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Ferguson, E. (1996) (14C)-Imidocarb: Absorption, distribution,
metabolism and excretion in cattle following a single subcutaneous
injection of Imizol. Unpublished report No. S8107-001-R from Corning
Hazleton (Europe). Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Finch, J.M. (1993) Preparation of pathology tables from report XZIG
86-C1 (1299R - 187/1). A two year feeding study in rats with
Imidocarb. Unpublished report No. R-93-070 from Inveresk Research
International, Tranent, Scotland, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Follefant, R. & Green, A.F. (1971a) Imidocarb i.e. 4A65. Acute oral
toxicity in mice and rats: Comparison of the dihydrochloride,
diacetate and dipropionate. Unpublished report No. BPHR/71/86 from
Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Follefant, R & Green, A.F. (1971b) Imidocarb i.e. 4A65. Comparative
toxicity in mice. Unpublished report No BPHR/71/79 from Wellcome
Research Laboratories, Beckenham, Kent, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. (1966) Preliminary assessment of acute pharmacodynamic
effects. Unpublished report No.BPHR 66-58 from Wellcome Research
Laboratories, Beckenham, Kent, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. & Hughes, R. (1969) Pharmacodynamics and acute toxicity.
Unpublished report No. BPHR 69-8 from Wellcome Research Laboratories,
Beckenham, Kent, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. & James, J.A. (1977) Imidocarb dipropionate: Multiple
dosing intramuscular toxicity in male mice (range finder study for
dominant lethal test). Unpublished report No. HZIG 77-1 from Wellcome
Research Laboratories, Berkhamsted and Beckenham, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977a) Imidocarb
dipropionate: Acute oral toxicity in the female rat (10% solution).
Unpublished report No. HZIG 77/2 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977b) Imidocarb
dipropionate: Acute oral toxicity in the male rat (10% solution).
Unpublished report No. HZIG 77/3 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977c) Imidocarb
dipropionate: Acute oral LD50 in the female rat (30% solution).
Unpublished report No. HZIG 77/5 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977d) Imidocarb
dipropionate: Acute oral LD50 in the male rat (30% solution).
Unpublished report No. HZIG 77/6 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. James, J.A. & Parker, M.J. (1977e) Imidocarb
dipropionate: Dominant lethal study in male mice. Unpublished report
No. HZIG 77-4 from Wellcome Research Laboratories, Beckenham, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Hart, E.R. (1973a) 90-day feeding study in rats. Imidicarb
dipropionate. Revised final report. Unpublished report No. XZIG 86-4
from Litton Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1973b) Teratology phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-7 from Litton Bionetics, Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinkrodt.
Hart, E.R. (1974a) Chronic toxicity phase. Imidicarb dipropionate.
Final report. Unpublished report No. XZIG 86-5 from Litton Bionetics,
Inc., Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1974b) Reproduction phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-9 from Litton Bionetics Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976a) Foetal toxicity study of 4A65 (imidocarb
dipropionate) in rats. Unpublished report No. BPAT 76/9 from Wellcome
Research Laboratories, Beckenham, Kent, UNited Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976b) An account of attempted foetal toxicity study of
4A65 (imidocarb dipropionate) in the rabbit. Unpublished report No.
BPAT 76/20 from Wellcome Research Laboratories, Beckenham, Kent,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
James, D.A. (1977) Multigeneration reproduction study of 4A65
(imidocarb dipropionate) in the rat. Unpublished report No. BPAT 77/24
from Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Marshall, R.R. (1993) Study to evaluate the clastogenic potential of
imidocarb by its effects on the frequency of micronuclei in cultured
human peripheral blood lymphocytes. Unpublished report No. R-93-015
from Hazleton Microtest, Harrogate, United Kingdom. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
McDougald, L.R. & Roberson, E.L. (1988) Antiprotozoan drugs. In:
Veterinary Pharmacology and Therapeutics, 6th Ed., Ames, Iowa State
University Press, pp. 950-968.
McHardy, N., Woollon, R.M., Clampitt, R.B., James, J.A. & Crawley,
R.J. (1986) Efficacy, toxicity and metabolism of imidocarb
dipropionate in the treatment of Babesia ovis infection in sheep.
Res. Vet. Sci., 41, 14-20.
Michell, A.R., White, D.G., Higgins, A.J., Moss, P. & Lees, P. (1986)
Effect of induced hypomagnesemia on the toxicity of imidocarb in
calves. Res. Vet. Sci., 40, 264-270.
Moore, W.B. (1977) Mutagenic activity of imidocarb dipropionate.
Unpublished report No. BEMU/77/3 from Wellcome Research Laboratories,
Beckenham, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Moore, W.B. & Chatfield, S.N. (1983) Mutagenicity: Evaluation of
imidocarb dipropionate using a method based on the Yahagi modification
of the Ames Salmonella/microsome incorporation test. Unpublished
report No. BEMU/83/1 from Wellcome Research Laboratories, Beckenham,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Nimmo-Smith, R.H. (1968) Distribution, excretion and tissue levels of
compound 4A65 (HR 2073) in the rat. Unpublished report No. 3BT68 from
Wellcome Research Laboratories, Berkhamsted, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Piercy, D.W.T. & James, J.A. (1976) Imidocarb dipropionate oral LD50
in rabbits. Unpublished report No. HZIG 76-1 from Wellcome Research &
Development, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Reynolds, J., Clampitt, R.B., Piercy, D.W.T., James, J.A. & Skarpa, M.
(1977) Subacute 90 day oral toxicity of imidocarb dipropionate in the
dog. Unpublished report No. HZIG 77-11 from Wellcome Research
Laboratories, Berkhamsted and Beckenham, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Salam, A.A. & Baggot, J.D. (1983) Adverse effects of imidocarb
dipropionate (Imizol) in a dog. J. Vet. Pharmacol. Ther., 5,
131-135.
Sibinovic, K.H. (1986) In vitro and subacute in vivo host-mediated
assay for mutagenesis. Unpublished report No. XZIG from Litton
Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1973a) Oral and intravenous acute toxicity studies of
imidocarb dipropionate (4A65) in mice and rats. Unpublished report no.
T-TEP-73-4 from Department of Toxicology-Experimental Pathology,
Research Triangle Park, North Carolina, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1972b) Fertility study in dogs given imidocarb
dipropionate. Unpublished report No. TTEP-72-11 from the Department of
Toxicology - Experimental Pathology, Research Triangle Park, North
Carolina, USA. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991a) Study to evaluate the ability of
imidocarb to induce mutation in four histidine-requiring strains of
Salmonella typhimurium and two tryptophan requiring strains of
E. coli. Unpublished report No. RRS-91-22 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991b) Study to determine the ability of
imidocarb to induce mutations to 6-thioguanine resistance in mouse
lymphoma L5179Y cells using a fluctuation assay. Unpublished report
No. RRS-91-15 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tait, A.J. & Marshall, R.R. (1991) Study to evaluate the potential of
imidocarb to induce micronuclei in the polychromatic erythrocytes of
CD-1 mice. Unpublished report No. RRS-91-23 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & McEnaney, S. (1991) Study to evaluate the chromosome
damaging potential of imidocarb by its effect on cultured human
lymphocytes using an in vitro cytogenetics assay. Unpublished report
No. RRS-91-32 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tesh, J.M., Ross, F.W. & Tesh, S.A. (1977) 4A: Effects of oral
administration upon pregnancy in the rabbit. Unpublished report No.
77/WRL2/109 from Life Science Research, Stock, Essex, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975a) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the rat. Unpublished report No. BPAT75/17
from Wellcome Research Laboratories, Berkhamsted, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975b) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the monkey. Unpublished report No.
BPAT75/18 from Wellcome Research Laboratories, Berkhamsted, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Wander, A. (1968a) Distribution and excretion of (14C) labelled HR
2073 after intravenous administration of a single dose (1 mg/kg) in
the mouse. Translation of unpublished report No. 94/2555. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Wander, A. (1968b) Investigation of the activity in the urine, liver,
gall bladder and kidneys of the mouse after intravenous administration
of a single dose of HR 2073 C-14. Translation of unpublished report
No. 94/2554. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Whitaker, A.M. & Bonhoff, A.J. (1983) Cytogenetic study of the effect
of imidocarb dipropionate (3 ',3 '-di(2-imidazolin-2-yl)carbanilide
dipropionate) on peripheral human lymphocytes in vitro. Unpublished
report No. BNCD/83/7 from Wellcome Biotechnology Ltd, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Studies in mice, rats, dogs, monkeys, and cattle have been
reported. Most of them were carried out during the 1960s and 1970s
according to the standards of those days, and important details are
missing from some of the reports. In contrast, the study in cattle was
certified for compliance with GLP.
Mice
Mice were injected into the tail vein with of 1 mg/kg bw
imidocarb dihydrochloride radiolabelled with 14C in the carbonyl
group. No radiolabel was detected in expired air. Excretion in the
urine was initially very rapid but quickly slowed and continued
throughout the observation period of 96 h. Faecal excretion followed a
similar pattern. Of the recovered radiolabel (90% of the administered
dose), about two-thirds was found in the urine and one-third in the
faeces (Wander, 1968a).
Rats
Wistar rats were dosed orally with 14C-imidocarb dipropionate
or 14C-imidocarb dihydrochloride and killed at various intervals up
to 48 h after treatment. Another rat was dosed subcutaneously with the
hydrochloride. Whole-body autoradiography indicated poor oral
absorption of both salts, and radiolabel was detectable only in the
liver, kidney, and gut. After subcutaneous treatment, a significant
amount of radiolabel remained at the injection site (Farebrother,
1973). In another study with various dose regimes and routes of
administration (oral, intraperitoneal, subcutaneous), the highest
concentrations of residues were found in the liver and kidney.
Residues were still detectable in these organs 55 days after
subcutaneous treatment with 5 mg/kg bw of the test substance.
Excretion was slow in rats treated subcutaneously, and only 20% of the
administered dose was recovered in excreta during the 78 h of
treatment (Nimmo-Smith, 1968). In a preliminary study to investigate
tissue retention, Wistar rats were given either a single oral dose or
30 daily oral doses of 5 mg/kg bw imidocarb dipropionate. The rats
were killed 24 h after the last dose, and residues in selected tissues
were determined by an unspecified analytical method. The highest
concentrations were found in kidney; surprisingly, the concentrations
in liver and brain were higher after the single-dose regime (Thomson,
1975a).
Dogs
Male beagle dogs were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and killed 24 h after the last
dose. The residues were determined in selected tissues by an
unspecified method. The highest concentrations were found in liver and
then in kidney; no residues were detected in muscle or brain (< 500
µg/kg) (Chesher et al., 1976). In another study, mongrel dogs were
given an intravenous bolus of 4 mg/kg bw of imidocarb dipropionate.
The pharmacokinetics were biphasic and indicated a large volume of
distribution. The mean terminal half-life was 207 min (Salam & Baggot,
1983).
Monkeys
Female patas monkeys were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and were killed some time after the
last dose (probably 24 h, although this is not stated). The residues
were determined in selected tissues by an unspecified method. The
highest concentrations were found in kidney and liver; none were
detected in muscle or brain (limit of detection, 500 µg/kg) (Thomson,
1975b).
Cattle
In a study which complied with the principles of GLP, six
lactating cows and eight calves were given a single subcutaneous
injection of 3 mg/kg bw 14C-imidocarb dipropionate with a specific
activity of 22 mCi/g. The test substance was formulated as the
proprietary product Imizol Injection. A mean peak blood concentration
of 1300 µg-equivalents/kg was attained 1 h after treatment, and the
concentration remained at this level for about 4 h, when 72œ91% of the
radiolabel was bound to plasma proteins. Excretion was very slow: over
the first 10 days, only 53% of the dose was recovered in excreta, with
38% in the faeces and 15% in the urine. The residues in tissues were
very persistent, particularly in liver where mean residues of 2200
µg-equivalents/kg were found 90 days after treatment (Ferguson, 1996).
2.1.2 Biotransformation
In bovine liver preparations in vitro, there was no evidence
for metabolism of imidocarb (Coldham et al., 1995).
Mice
Mice injected with 14C-imidocarb into the tail vein excreted
37% of the administered dose in the urine within 2 h. Of the
radiolabel in the urine, 95% was unmetabolized imidocarb. The mice
were killed 3.5 h after treatment, and 29% of the dose was found in
the liver, 6.8% in the kidney, and less than 1% in the gall-bladder.
More than 90% of the radiolabel in these organs was unmetabolized
imidocarb (Wander, 1968b).
Cattle
In the study described above in which cattle were treated
subcutaneously, the main component of the residues in liver, kidney,
muscle, and fat for up to 90 days after treatment was unmetabolized
imidocarb. Minor components, accounting for not more than 10% of the
total residues, were also present but were not identified. Imidicarb
was also the major component of cows' milk, urine, and faeces
(Ferguson, 1996).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of imidocarb are
summarized in Table 1. None of the studies complied with GLP, and the
reports fall short of modern standards. The signs of toxicity were
generally consistent with the anticholinesterase activity of imidocarb
and included lethargy, salivation, lachrymation, muscle fasciculation,
ataxia, tremors, and convulsions. Congestion of the lung and kidneys
and mottling of the liver were frequent post-mortem findings in the
rats that died.
In a published report, the signs of toxicity in groups of goats
given a single intramuscular dose in the range 12-24 mg/kg bw included
salivation, frequent urination and defaecation, weakness of the legs,
abnormal posture, and recumbency. There were dose-related decreases in
haemoglobin and erythrocyte counts and packed cell volume and
dose-related increases in asparate aminotransferase activity and in
creatinine and bilirubin concentrations. Serum cholinesterase activity
was reduced in a dose-related manner. No significant effects were
observed at 6 mg/kg bw. The effects were ameliorated by pretreatment
with atropine sulfate intramuscularly at a dose of 1 mg per animal
(Ali et al., 1985).
Whole-blood cholinesterase activity was significantly reduced in
calves given an intramuscular injection of 3.3 mg/kg bw imidocarb
dipropionate. Maximum depression occurred about 30 min after
treatment, and significant recovery was observed within 6 h. The
cholinesterase depression did not correlate with the intensity of the
clinical response (Michell et al., 1986).
2.2.2 Short-term toxicity
Mice
A study in mice, intended as a range-finding probe for a study of
dominant lethal mutations, was not carried out in accordance with GLP.
Groups of six male Evans No. 1 mice were given daily intramuscular
doses of 0 (distilled water), 7.8, 16, 31, 63, or 130 mg/kg bw per day
of imidocarb dipropionate (expressed as base) for five days. Signs of
toxicity were recorded for up to 14 days after the last dose. No blood
chemistry or pathological examinations were carried out. All of the
Table 1. Results of studies of acute toxicity with imidocarb
Species Route Vehicle Sex LD50 Salt Reference
(strain) (mg/kg bw) administered
Mouse (ICR) Oral gavage Sterile distilled water M 723a Dipropionate Szot (1973)
Mouse (ICR) Oral gavage Sterile distilled water F 646a Dipropionate Szot (1973)
Mouse (SAS) Oral gavage 0.9% saline F 544a Dihydrochloride Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 863a Diacetate Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 702a Dipropionate Follenfant & Green (1971a)
Mouse (Gambles) Oral gavage 0.9% saline M 1400 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline F 1500 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline M 1652a Dihydrochloride Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1216a Diacetate Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1251a Dipropionate Follenfant & Green (1971a)
Rat (Long Evans) Oral gavage Sterile distilled water M 454a Dipropionate Szot (1973)
Rat (Long Evans) Oral gavage Sterile distilled water F 650a Dipropionate Szot (1973)
Rabbit (New Zealand Oral gavage Water M + F 317 Dipropionate (27% Piercy & James (1975)
white) aqueous solution)
Rat (Long Evans) Intravenous 0.9% saline M 32.7a Dipropionate Szot (1973)
Mouse (ICR) Intravenous 0.9% saline M 22.3a Dipropionate Szot (1973)
Mouse (Gambles) Intravenous 0.9% saline M 16 Hydrochloride Green & Hughes (1969)
Mouse (SAS) Subcutaneous 0.9% saline F 78.6a Dihydrochloride Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 94.5a Diacetate Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 92.1a Dipropionate Follenfant & Green (1971b)
Mouse (Gambles) Subcutaneous 0.9% saline M 140 Hydrochloride Green & Hughes (1969)
Rat (Wistar) Subcutaneous 0.9% saline F 150 Hydrochloride Green & Hughes (1969)
Rat (Sprague-Dawley) Oral gavage Water F > 631a Dipropionate (10% Harper et al. (1997a)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M > 1000a Dipropionate (10% Harper et al. (1997b)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water F 1011.5a Dipropionate (10% Harper et al. (1997c)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M 1998a Dipropionate (10% Harper et al. (1997d)
aqueous solution)
a The LD50 values found in these studies are for the base. In the remaining studies, the results are believed to be quoted as the salt,
although this is not clear from the report in all cases.
animals at 63 and 130 mg/kg bw and 50% of those at 31 mg/kg bw died
three to four days after the start of treatment. The signs of toxicity
were slow respiration and lethargy. There was no NOEL (Harper & James,
1977).
Rats
In a preliminary study designed primarily to investigate
distribution in tissues, groups of five female Wistar rats were given
either a single oral dose of 5 mg/kg bw imidocarb dipropionate
dissolved in distilled water, or daily oral doses of 5 mg/kg bw per
day for 30 days. Five additional rats formed a control group. Blood
samples were taken for haematological and clinical chemical testing,
and electrocardiographic, ophthalmoscopic, and urinary measurements
were made before treatment and at termination; no histopathological
examination was performed. The rats were killed 24 h after the last
dose, and residues were determined in selected tissues. There were no
treatment-related effects on most of the parameters investigated. The
significant changes in blood parameters included increased total
leukocyte counts in the treated group; however, the toxicological
significance of these effects is doubtful since satisfactory blood
samples were obtained from only three treated rats (Thomson, 1975a).
In a study that did not conform to GLP, groups of 10 Wistar rats
of each sex were given daily oral doses of imidocarb dihydrochloride
for three months. The study was carried out in two phases. In phase 1,
the doses were 0 (distilled water), 125, 250, or 500 mg/kg bw per day;
in phase 2, the doses were 0, 500, 750, or 1500 mg/kg bw per day. It
was not clear whether the doses were expressed as the hydrochloride or
as the the base, and the solutions were not analysed. The rats were
weighed and examined daily. Blood samples for haematological and
clinical chemical determinations were taken just before termination.
Urinalysis was not carried out.
All rats given 1500 mg/kg bw died. The body-weight gain of all
treated rats was significantly lower than that of controls in phase 2.
There were no significant changes in haematological or clinical
chemical values, but changes were seen in the weights of a number of
organs in animals at 500 or 750 mg/kg bw, including a significant
reduction in absolute (but not relative) prostate weight, increased
relative adrenal and thyroid weights in males, and increased relative
adrenal weight in females. Pathological changes described as cloudy
swelling were reported in the livers of 5/9 male and 7/10 female rats
given 125 mg/kg bw and in all males and 6/10 females given 250 mg/kg
bw, but in none of the controls. The lesion was reported to be
reversible, but no evidence was provided. No histopathological
examinations were carried out on rats at 500 or 750 mg/kg bw. No NOEL
was identified (Bushby, 1970).
Groups of Charles River rats were fed diets containing 0, 630,
1900, or 9400 mg/kg imidocarb dipropionate for up to 90 days,
according to the schedule shown in Table 2. The purpose was to
identify the appropriate doses for a long-term study of toxicity. The
study did not comply with the principles of GLP. The animals were
checked daily for deaths, and body weight and food consumption were
recorded weekly. Haematological and clinical chemical determinations
were carried out on five rats of each sex per dose before treatment
and during weeks 4 and 13. Five rats of each sex from the control
group and those at the highest dose were killed after seven weeks and
the remaining rats after 13 weeks. At both 7 and 13 weeks,
acetylcholinesterase activity was measured in one-half of the brain of
each of five rats of each sex in the control group and that at the
highest dose At termination, each rat was examined grossly.
Histopathological examinations were performed on 17 organs or tissues
from five rats of each sex in the control group and that at the
highest dose and on the livers, kidneys, and grossly abnormal tissues
from the other rats.
Table 2. Achieved dosages in a 90-day study with imidocarb in Charles River rats
Concentration in Males Females
feed (mg/kg)
No. rats Achieved dose No. rats Achieved dose
(mg/kg bw (mg/kg bw
imidocarb base) imidocarb base)
0 15 0 15 0
630 10 26 10 32
1900 10 75 10 100
9400 15 420 15 550
One male given 9400 mg/kg feed died during week 2, and a control
female died during week 6. No signs of overt toxicity were observed.
Body-weight gain was significantly reduced towards the end of the
study in animals of each sex given 9400 mg/kg feed. There were no
significant effects on haematological, clinical chemical, or urinary
parameters or on brain acetylcholinesterase activity. Pathological
changes attributable to treatment were limited to the livers of rats
given 9400 mg/kg feed; the changes consisted of mild stasis of the
bile in the canaliculi in one male and four females. The NOEL was 1900
mg/kg feed, equal to 75 mg/kg bw per day (Hart, 1973a).
Another study was conducted, which did not comply with the
principles of GLP and was intended to be a two-year study of toxicity,
but in which 117 rats died as a result of overheating of the animal
rooms during week 37. The study was terminated during week 44, when
all surviving rats were necropsied. In this study, groups of
50 Sprague-Dawley rats of each sex were to have received diets
containing the equivalent of 0, 75, 225, or 750 mg/kg bw per day of
imidocarb dipropionate. The rats were F1a weanlings from groups at
the corresponding doses in a study of reproductive toxicity; however,
the highest dose was fetotoxic, so that there were insufficient
weanlings available to form this group, and additional rats were
purchased. No data were reported on this group.
Until the overheating episode, there were no significant effects
on body weight, food consumption, or haematological, clinical
chemical, or urinary parameters. During the last five weeks, the food
intake of females at 225 mg/kg bw was increased, but they showed no
change in body weight. At week 44, the mean thyroid:body weight ratio
was increased in females at this dose. There were no significant
pathological findings. No firm conclusions could be drawn from this
study (Hart, 1974a).
Dogs
In a study that did not comply with GLP, a group of five male
beagle dogs, described as 'substandard', was given daily oral doses of
5 mg/kg bw imidocarb dipropionate (expressed as base) in distilled
water for 30 days. There was no untreated control group. No signs of
toxicity were observed and there appeared to be no adverse effects on
body weight, food or water intake, or haematological or clinical
chemical parameters. The dogs were killed 24 h after the last dose,
and urine was taken for determination of pH, protein, glucose, ketone,
and blood. Selected organs were weighed and a gross pathological
examination was carried out on each dog. There were no obvious
substance-related changes (Chesher et al., 1976).
In another study that did not comply with GLP, groups of four
beagle dogs of each sex were given daily oral doses of 0, 5, 20, or 80
mg/kg bw per day imidocarb dipropionate (expressed as base) in gelatin
capsules, for 90 days. The animals were weighed twice weekly, and each
dog was subjected to an ophthalmoscopic examination before treatment
and on days 36 and 76. Blood samples were taken from all dogs for
haematological and some clinical chemical investigations before
treatment and on days 7, 28, 56, 71, and 90; cholinesterase activity
was not monitored. Urine samples were collected at necropsy. About 25
tissues from each animal were examined microscopically.
All four males and two of the females given 80 mg/kg bw per day
died or were killed in extremis after having reduced food intake and
weight loss for about two weeks before death. The signs of toxicity in
this group included weakness, recumbency, salivation, muscle
fasiculation, ataxia, and splayed hind legs, probably indicating an
anticholinesterase effect. The food intake of dogs at 20 mg/kg bw was
transiently reduced. There were no treatment-related ophthalmoscopic
changes. Eosinophilia was observed in some dogs given 20 or 80 mg/kg
bw per day, and those at the highest dose showed a trend towards
increased serum alanine and aspartate aminotransferase activity and
bilirubin concentration, with occasional, transient increases in those
at 20 mg/kg bw per day. The absolute and relative weights of the
pituitary were decreased in dogs at 20 but not at 80 mg/kg bw per day,
but animals at 80 mg/kg bw per day showed significant increases in the
absolute and relative weights of the kidney, adrenal, and thyroid.
Gross examination revealed fatty changes and mottling in the livers,
particularly in dogs given 20 or 80 mg/kg bw per day.
Histological changes were observed in a range of tissues from
dogs at 80 mg/kg bw per day, particularly in the thymus, spleen, lymph
nodes, and stroma of the villi of the small intestine (pyknosis and
karyorrhexis), and fatty changes were observed in the thick section of
the loop of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty changes, granularity, or vacuolation of
the hepatocytes. Similar though less severe changes were observed in
the livers of dogs given 20 mg/kg bw per day. The NOEL was 5 mg/kg bw
per day (Reynolds et al., 1977).
Monkeys
In a preliminary study designed to investigate distribution to
tissues, five female patas monkeys were given daily oral doses of
5 mg/kg bw per day imidocarb dipropionate dissolved in distilled water
for 30 days. A control group of five females was left untreated. Blood
samples were taken before treatment, seven and 14 days after the start
of treatment, and at termination. Some time after the last dose (not
stated), the treated monkeys were killed and examined for gross
changes; the control monkeys were not autopsied. There were no signs
of toxicity in the treated animals, and no effects were observed on
haematological or clinical chemical values or gross appearance
(Thomson, 1975b).
2.2.3 Long-term toxicity and carcinogenicity
Rats
In a study that did not conform to GLP, groups of 50 Wistar rats
of each sex were fed diets calculated to provide intakes of 0, 15, 60,
or 240 mg/kg bw per day imidocarb dipropionate (expressed as base) for
104 weeks. Satellite groups of 15 rats of each sex per dose were used
for collection of blood and urine before treatment and at regular
intervals up to termination. Ophthalmoscopic examinations were carried
out at intervals throughout the study on controls and animals at the
highest dose. At termination, about 25 tissues were selected for
histopathological examination from 10 controls of each sex and from
four males and 19 females at the highest dose; eight or nine tissues
were examined from the remaining animals, comprising lymph nodes,
liver, spleen, kidneys, ovaries, adrenals, thyroids, pituitaries, and
blood films. Gross lesions from all animals were also examined
microscopically.
Survival of animals at the highest dose was adversely affected,
only 9/65 males surviving to termination. The rats in this group
became emaciated, their body-weight gain being significantly reduced
and food consumption significantly increased; females were less
severely affected than males. In the last weeks of the study, the food
consumption of the males exceeded that of the controls by about 40%. A
slight reduction in body-weight gain was also observed in females
given 60 mg/kg bw per day. Males at 240 mg/kg bw per day also had an
increased incidence of corneal opacity and evidence of mild anaemia,
with significant reductions in erythrocyte and haemoglobin counts and
packed cell volume.
Changes in clinical chemistry were seen mainly in rats at 240
mg/kg bw per day and appeared to be transient. In males, the changes
included significantly increased aspartate aminotransferase activity
during weeks 13 and 26, increased alkaline phosphatase activity during
week 8, and increased blood urea nitrogen concentration during week 52
and 78. The cholesterol concentration was significantly increased in
all treated groups during week 52 but not at other times. Females had
significant increases in aspartate aminotransferase activity during
weeks 13 and 78, in alanine aminotransferase activity during week 13
(but significant reductions in weeks 4, 52, and 78), in alkaline
phosphatase activity in week 26, and in cholesterol concentration in
week 8. Alkaline phosphatase activity was also increased in females
given 60 mg/kg bw per day during week 26. Males given 60 mg/kg bw per
day and rats of each sex given 240 mg/kg bw per day had significantly
greater water intake than the controls. Polyuria was observed in males
given 240 mg/kg bw per day, and a slight increase in urinary output
was noted in females at this dose. The specific gravity of urine
samples from the males given 240 mg/kg bw per day was consistently
lower than that of the controls.
At termination, the mean group kidney weight of males given 240
mg/kg bw per day was significantly increased. Treatment-related
microscopic changes were confined to the rats at 60 or 240 mg/kg bw
per day and included deposition of brown pigment in macrophages and
related cells in the liver, spleen, lymph nodes, and bone marrow, and
cystic distension of the renal tubules and glomeruli, with dystrophic
mineralization of the renal medulla. Muscle sections were not taken
for microscopic examination, but evidence of increased atrophy was
found in rats at the highest dose in muscle samples attached to the
sternum. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, some clinical chemical changes,
histopathological changes in the kidney, and skeletal muscle atrophy
at higher doses. The incidence of mammary fibroadenomas was increased
in females given 240 mg/kg bw per day, and many of them had multiple
tumours. Males at this dose had an increased incidence of cutaneous
fibromas (Brown, 1979).
In a separate report, the original histological data from the
study described above were re-tabulated and reanalysed. A number of
assumptions had to be made, since it was not always clear which
tissues had been examined from which animal, which lesions had been
reported, and what terminology had been used. The following overall
conclusions were drawn. Females given 240 mg/kg bw per day had a
significantly increased incidence of multiple fibroadenomas and a
decreased incidence of single fibroadenomas in the mammary gland as
compared with the controls; there was no significant difference in the
combined incidence of multiple and single fibroadenomas. Males at this
dose had a significantly increased incidence of multiple subcutaneous
fibromas in comparison with the controls; an apparent decrease in the
incidence of single fibromas was not significant, and there was no
significant difference in the combined incidence of multiple and
single fibroadenomas. Rats of each sex given 240 mg/kg bw per day had
a nonsignificant increase in the incidence of fibrosarcomas (Finch,
1993).
2.2.4 Genotoxicity
The results of studies of the genotoxicity of imidocarb are
summarized in Table 3. The concentrations and doses are expressed as
imidocarb base, except when stated otherwise. Negative results were
obtained in asays for gene mutation in bacteria and in mammalian cells
in vitro. In two separate experiments, imidocarb induced polyploidy
in human lymphocytes in vitro; however, there was no induction of
aneuploidy. Negative results were obtained in a test for micronucleus
formation in mouse bone marrow, in a metaphase analysis in bone marrow
of rats in vivo, and in an inadequately conducted assay for dominant
lethal mutation.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A multigeneration study was carried out in rats as part of a
larger study designed to investigate all aspects of reproduction,
including teratogenicity, and to provide weanling rats for a two-year
study. Groups of 15 Sprague-Dawley rats of each sex were maintained on
diets containing 0, 630, 1900, or 9000 mg/kg imidocarb dipropionate
(presumably expressed as salt, although this is not clear from the
report) for 60 days before mating. The diets were stated to provide an
equivalent of 0, 45, 130, or 760 mg/kg bw of imidocarb dipropionate
per day. The numbers of pups in both the F1a and F1b litters were
significantly reduced at the highest dose; thus, only three females
receiving 9000 mg/kg produced F1a litters. There was a similar
reduction in the F1b litters receiving the intermediate dose. F2
litters were bred only from the animals at the low dose, and the study
was terminated early owing to failure of the temperature control
mechanism in the animal rooms. No firm conclusions could be drawn from
this study (Hart, 1974b).
Table 3. Results of genotoxicity studies on imidocarb
End-point Test object Concentration S9 Result GLP Reference
In vitro
Gene mutation S. typhimurium TA1535, TA1537, < 1000 µg/plate + Negativea No Moore (1997)
TA1538, TA98, TA100 < 250 µg/plate - Negativea
Gene mutation S. typhimurium TA1535, TA1537, 0.2-1000 µg/plate + Negativea No Moore & Chatfield
TA1538, TA98, TA100 - Negativea (1983)
Gene mutation S. typhimurium TA98, TA100 4-2650 µg/plate + Negative Yes Tait & Clare
TA1535, TA1537, E. coli WP2 33-530 µg/plate - Negativeb (1991a)
pKM101, WP2uvrA- pKM101 53-265 µg/plate
(with strain WP2uvrA- pKM101 only)
Gene mutation hprt locus of L5178Y mouse 0.55-1749 µg/ml + Negative Yes Tait & Clare
lymphoma cells 54.7-1749 µg/ml - Negative (1991b)
Chromosomal Human peripheral blood 3.5-35 µg/ml (as imidocarb - Negative No Whitaker &
aberration lumphocytes dipropionate); higher doses were Bonhoff (1983)
toxic
Chromosomal Human peripheral blood 20 h -S9; 144-294 µg/ml; + Positivec Yes Tait & McEnaney
aberration lymphocytes 20 h +S9; 857-1749 µg/ml (expt 1) (1991)
20 h -S9; 235-367 µg/ml;
20 h + S9; 1119-1749 µg/ml;
44 h -S9; 235 µg/ml; 44 h +S9;
1119 µg/ml (expt 2)
44 h + S9; 366.8-1119 µg/ml (expt 3)
Micronucleus Human peripheral blood 366.8-1120 µg/ml (for frequency of + Negativee Yes Marshall (1993)
formation lymphocytes micronuclei)
895.5 µg/ml (for polyploidy)
Table 3. (continued)
End-point Test object Concentration S9 Result GLP Reference
Host-mediated Male ICR mice (10/group) Five daily oral doses of 15, 45 or Negative No Sibinovic (1986)
assay S. typhimurium TA1530, G-46 150 mg/kg bw; test organism given
intraperitoneally 30 min after last
dosea
In vivo
Bone marrow Sprague-Dawley rats (5/dose); Five consecutive daily Negative No Fabrizio (1986)
metaphase bone marrow harvested 5 days oral doses of 10, 30, or
analysis after treatment with imidocarb; 100 mg/kg bwa
40-50 metaphase spreads/rat
analysed
Micronucleus CD-1 mouse (5/sex per dose); Single intraperitoneal injection of Negative Yes Tait & Marshall
formation marrow harvested 24, 48, 72h 8, 5, 17, or 34 mg/kg bw (1991)
after treatment
Dominant Male Evans mice Five daily intramuscular injections Negatived No Harper et al.
lethal of 4 or 16 mg/kg bw followed by a (1977)
mutation six-week mating schedule
S9, 9000 × g fraction of rat liver; GLP, conformity with good laboratory practice
a No information on whether concentrations or doses reported in terms of imidocarb base or diproprionate salt
b Although there was a statistically significant increase in the number of revertants with strain WP2uvrA- pKM101 in the presence
of S9, the increase was less than double the value for the solvent control, there was no concentration-related response, and the
values were within the range for contemporary historical controls.
c Treatment in the absence of S9 resulted in significant increases in the frequencies of cells with structural aberrations; it was
stated that the frequencies were within the range in historical controls, but the Committee did not consider the historical
controls to be appropriate. Statistically significant increases in the frequency of cells with numerical aberrations were observed
in cultures treated in the presence of S9 and sampled at 44 h, in both experiments 2 and 3.
d Although there were statistically significant increases in the percentage of dead implants in the group receiving 6 mg/kg bw during
week 2, there was no dose-response relationship, and the incidence in the control group was unusually low. The treatment and mating
schedules used in this study did not comply with modern guidelines.
e There was no increase in the incidence of micronuclei at any concentration; however, an increased frequency of polypoid cells was
observed at the single concentration tested.
In another study that did not comply with GLP, groups of 20
Wistar rats of each sex were fed diets calculated to provide 0, 15,
45, or 135 mg/kg bw per day imidocarb dipropionate. Treatment
commenced when the rats were six weeks old, 60 days before the first
mating, and continued throughout the breeding of three succesive
generations. At 21 days post partum, the F1a, F2a, and F2b
litters were killed and examined internally. The F1b and F2b
litters were mated to provide the subsequent generations. The F3b
fetuses were removed on day 20 of gestation and examined for
malformations.
During the premating period, females of the parental generation
showed a small but dose-related decrease in body-weight gain; there
were no clear dose-related effects on body weight in subsequent
generations. The numbers of live births among females receiving 135
mg/kg bw per day were reduced after the first mating of the F0
generation, and the numbers of dead or missing fetuses were increased.
A similar trend was observed after the first mating of the F1
generation. Maternal body-weight gain was reduced on day 17 of
gestation after the second mating of the F1 generation. No evidence
of teratogenicity was found on examination of the F3b fetuses;
however, there was a small, nonsignificant increase in the incidence
of fetuses with bifid sternebrae. The NOEL was 45 mg/kg bw per day for
both fetal toxicity and maternal toxicity (James, 1977).
Dogs
Five female dogs were given subcutaneous injections of 14 mg/kg
bw imidocarb dipropionate (expressed as free base) on the first day of
pro-oestrus and again immediately after successful mating. Six
untreated dogs were used as controls. The dogs were allowed to litter
naturally. After the first whelping, all the treated dogs were dosed
and bred a second time. There were no signs of toxicity and no effects
on on fertility, gestation, or appearance of pups (Szot, 1972).
(ii) Developmental toxicity
Rats
Teratogenicity was studied in rats within a multigeneration
study, which did not comply with the principles of GLP. Groups of 15
female rats were fed imidocarb dipropionate continuously in the diet
for 60 days before mating, achieving intakes of about 0, 47, 140, or
760 mg/kg bw per day. They were killed on day 19 of gestation, and the
uterine contents were examined. Two-thirds of the fetuses were then
processed for staining with Alizarin Red S for skeletal evaluation,
and the remaining one-third were fixed in Bouin's solution and
sectioned for evaluation of visceral abnormalities.
Maternal body-weight gain was significantly reduced at the doses
of 140 and 760 mg/kg bw per day and the incidence of resorptions was
significant-ly increased in those at 760 mg/kg bw per day with a
consequent reduction in the numbers of live fetuses. Animals at 140
mg/kg bw per day also showed a small increase in the resorption rate.
Fetal body weight and length were reduced at 760 mg/kg bw per day.
There was no evidence of teratogenicity at any dose (Hart, 1973b).
In a second study, which was not carried out in compliance with
the principles of GLP, groups of 27-31 mated female Wistar rats were
given daily oral doses of 0 (untreated), 0 (distilled water, solvent),
19, 76, or 300 mg/kg bw imidocarb dipropionate (expressed as base) by
gavage on days 6-16 of gestation. Groups of 20-25 dams were killed on
day 20 of gestation and the uterine contents examined. One-third of
the fetuses were examined by open dissection, one-third were processed
by Wilson's section technique for examination of thick serial
sections, and one-third were processed for staining with Alizarin Red
S for skeletal evaluation. The remaining six dams in each group were
allowed to deliver naturally and rear the offspring to weaning on day
21 post partum.
Maternal body-weight gain was significantly reduced during
treatment with 300 mg/kg bw per day. There was no evidence of
teratogenicity, but the numbers of fetuses with bifid or H-shaped
sternebrae were increased at doses of 76 and 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day (James, 1976a).
Rabbits
Groups of 16-27 female Dutch rabbits were given daily doses of 0
(water), 20, 80, or 320 mg/kg bw per day of imidocarb dipropionate
(doses expressed as base) by oral gavage on days 8-19 of gestation.
The study was not carried out in compliance with the principles of
GLP. All 16 rabbits given 320 mg/kg bw per day and one out of 27 given
80 mg/kg bw per day died. The remaining dams showed signs of stress
(nervous behaviour, diarrhoea, weight loss), which were attributed to
their close proximity to a dog colony. Five to six dams in each group
were allowed to deliver naturally, but most of the dams killed their
offspring. There was no evidence of teratogenicity. The study was
inadequate for establishing a NOEL (James, 1976b).
In another study, which was not carried out in accordance with
the principles of GLP, groups of 10-20 female New Zealand white
rabbits were given doses of 0 (distilled water), 5, 10, 20, 60, or 180
mg/kg bw per day of imidocarb dipropionate by oral gavage on days 6-18
of gestation. The surviving does were killed on day 29 of gestation
and the uterine contents examined. The general appearance of each
fetus and the internal organs of the neck, thorax, and abdomen were
examined grossly. All fetuses were then eviscerated, preserved, and
stained for skeletal examination.
All 10 does given 180 mg/kg bw per day and 12 out of 15 given
60 mg/kg bw per day died. The does in these groups displayed signs of
gastrointestinal disturbance, salivation, nasal exudation, and weight
loss before death. The incidence of post-implantation loss was
increased in animals at 60 mg/kg bw per day, and only 12 fetuses
remained for examination. Fetal weights appeared to be reduced, and
the incidence of delayed ossification appeared to be increased. There
was no evidence of teratogenicity at any dose. The NOEL for both
maternal toxicity and fetotoxicity was 20 mg/kg bw per day (Tesh et
al., 1977).
2.2.6 Special studies to investigate serum prolactin concentrations
A study which did not comply with GLP was carried out to
investigate the mechanism of the induction of mammary tumours in the
two-year study in rats. Groups of 10 male Wistar rats were fed diets
calculated to provide 0, 15, 60, or 240 mg/kg bw per day of imidocarb
(as base) for 28 days. Another group was given 160 mg/kg bw per day of
sulpiride. Serum prolactin concentrations were measured before
treatment and 2, 5, 8, 14, and 22 days after the first dose. The mean
prolactin concentrations were significantly (p < 0.001) increased
in the group receiving sulpiride from day 2 onwards and were slightly
increased in the group given 240 mg/kg bw per day imidocarb, although
they never approached the values found in the group given sulpiride
(Clampitt et al., 1982).
2.2.7 Special studies on microbiological activity
In a study that did not conform to GLP, MIC values in vitro
were determined for imidocarb against 20 bacterial isolates
representing seven genera. MIC values > 16 µg/ml were obtained for 18
isolates, and values of 4 and 8 µg/ml were obtained for two strains of
Pasteurella (Darby & Pelham, 1989).
2.2.8 Special studies on pharmacodynamic effects
It is generally accepted in the published literature that
imidocarb has anticholinesterase activity and that the toxic signs
observed after treatment can be alleviated with atropine (Ali et al.,
1985; Michell et al., 1986; McDougald & Roberson, 1988). The
pharmacodynamic effects of imidocarb were investigated in a screening
study in which mice were given single subcutaneous doses of the
dihydrochloride salt. Myosis was observed at 50 mg/kg bw, but
mydriasis occurred at 150 mg/kg bw (Green, 1966). In another study,
cardiovascular and neuromuscular effects were observed after
intravenous administration of imidocarb dihydrochloride to
anaesthetized cats and dogs, which were attributed in part to the
anticholinesterase effect. No effect on the pupil was observed after
instillation of a solution of 10 mg/ml imidocarb dihydrochloride into
a cat's eye (Green & Hughes, 1969). No conclusions could be drawn
about anticholinesterase potency from these data.
The mode of action of imidocarb as an antiprotozoal agent is
uncertain, although two mechanisms have been proposed:
* As the effect of imidocarb on Trypanosoma brucei is antagonized
by excess polyamines, it is has been suggested that imidocarb
interferes with their production and/or use (Bacchi et al.,
1981).
* Imidocarb blocks the entry of inositol into erythrocytes
containing Babesia, resulting in 'starvation' of the parasite
(McHardy et al., 1986).
2.3 Observations in humans
It is believed that imidocarb has been used occasionally in human
medicine, but no details of doses or adverse effects were available to
the Committee.
3. COMMENTS
The Committee considered data from studies of the
pharmacokinetics, metabolism, acute, and short-term toxicity,
long-term toxicity and carcino-genicity, reproductive toxicity and
genotoxicity of imidocarb and some special studies. Most of the
studies were carried out with the dipropionate salt, and the doses
used were expressed as imidocarb base. Except for some of the assays
for genotoxicity, the studies were not carried out to contemporary
standards for study protocol and conduct but were reported in
sufficient detail and were considered adequate for assessment.
The pharmacokinetics of imidocarb was investigated in mice, rats,
dogs, monkeys, and cattle. The dipropionate and dihydrochloride salts
of imidocarb appeared to be poorly absorbed after oral administration
to rats, but the extent of the oral bioavailability could not be
estimated from the data available. In cattle dosed subcutaneously,
imidocarb was bound to plasma proteins. The drug was excreted in both
urine and the faeces for several days in rodents and for at least 28
days in cattle.
In mice dosed intravenously with radiolabelled imidocarb and
killed 3.5 h later, more than 90% of the residues in liver and kidney
were unmetabolized imidocarb. The parent compound also accounted for
95% of the radiolabelled material in mouse urine. In cattle dosed
subcutaneously with radiolabelled imidocarb, the parent compound
accounted for most of the residues in urine and faeces and 70-90% of
the residues in edible tissues and milk. No evidence of metabolism was
found in a study of bovine liver slices, isolated hepatocytes, or
microsomal fractions in vitro.
Imidocarb dipropionate is moderately hazardous, with oral LD50
values in the range of 650-720 mg/kg bw in mice and 450-1200 mg/kg bw
in rats. The signs were generally consistent with anticholinesterase
activity.
Serum cholinesterase activity was depressed in a dose-related
manner in goats given single intramuscular doses of 12-24 mg/kg bw.
Whole-blood cholinesterase activity was decreased in calves given
single intramuscular injections of 3.3 mg/kg bw.
When rats were given daily oral doses of 0, 125, 250, 500, 750,
or 1500 mg/kg bw per day of imidocarb dihydrochloride for three
months, all rats at the highest dose died. Cloudy swelling in the
liver was observed in rats given 125 and 250 mg/kg bw per day.
Histopathological examinations were not performed on rats at higher
doses. No NOEL was identified.
In a study conducted to identify appropriate doses for a
long-term study of toxicity, rats were fed diets calculated to provide
doses of imidocarb dipropionate equal to 0, 26, 75, or 420 mg/kg bw
per day for 90 days. Body-weight gain was reduced in animals of each
sex given 420 mg/kg bw per day. Acetylcholinesterase activity,
measured in one-half of the brain from each of 10 rats given 420 mg/kg
bw per day and killed after 7 and 13 weeks, was not significantly
different from that in controls. Pathological changes attributable to
treatment were limited to mild stasis of the bile in the canaliculi of
the liver in one male and four females given 420 mg/kg bw per day. The
NOEL was 75 mg/kg bw per day on the basis of reduced body-weight gain
and hepatic toxicity at higher doses.
Groups of four dogs of each sex per dose were given oral doses of
0, 5, 20, or 80 mg/kg bw per day imidocarb dipropionate in gelatin
capsules for 90 days. The signs of toxicity in the dogs given 80 mg/kg
bw per day included recumbency, salivation, muscle fasciculation,
ataxia, and splayed legs. All males and two of four females given this
dose died or were killed. Blood eosinophilia was also observed at 80
mg/kg bw per day, with increased serum alanine aminotransferase and
aspartate aminotransferase activity and increased bilirubin
concentration. Similar but less severe changes were observed in the
dogs given 20 mg/kg bw per day. The weights of the kidney, thyroid,
and adrenal glands were increased in animals at 80 mg/kg bw per day,
and histopathological changes were found in a range of tissues. In the
kidney, these included fatty changes in the thick section of the loop
of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty change, and microgranularity or
vacuolation of hepatocytes. Similar but less severe hepatocellular
changes were found in the livers of dogs given 20 mg/kg bw per day.
The NOEL was 5 mg/kg bw per day on the basis of minor changes in
haematological and clinical chemical parameters and hepatocellular
changes at higher doses.
In a long-term study of toxicity and carcinogenicity, rats were
fed diets calculated to provide intakes of 0, 15, 60, or 240 mg/kg bw
per day of imidocarb as dipropionate for two years. Survival was
adversely affected at the highest dose. Body-weight gain was
significantly reduced in animals of each sex given 240 mg/kg bw per
day and was slightly reduced in females given 60 mg/kg bw per day.
Males at 240 mg/kg bw per day showed evidence of anaemia. Changes in
clinical chemical parameters were transient and occurred mainly in the
animals given the highest dose. Rats given 60 or 240 mg/kg bw per day
drank considerably more water than the controls, and polyuria was
observed in males at the highest dose. At termination, the weights of
the kidneys of males given 240 mg/kg bw per day were significantly
increased. Dose-related histopathological changes were observed at 60
and 240 mg/kg bw per day, which included cystic distension of the
renal tubules and glomeruli and dystrophic mineralization in the renal
medulla. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, changes in some clinical chemical
parameters, and histopathological changes in the kidney. All animals
were examined grossly, and all gross lesions were examined
microscopically; however, comprehensive pathological examinations was
made on only 10 control rats of each sex and 19 females and four males
at the highest dose at termination of the study. A limited range of
tissues from the other animals was examined, and mammary glands were
examined only when a gross lesion was found. At the highest dose, a
significantly increased incidence of multiple fibroadenomas of the
mammary gland was found in females and of multiple subcutaneous
fibromas in males. Because of the excessive toxicity at this dose, the
poor survival, and limited histopathological examination, the
significance of these findings could not be evaluated. The incidences
of tumour at other sites were not increased.
The genotoxic properties of imidocarb dipropionate were
investigated in a range of assays in vitro and in vivo. Negative
results were obtained for gene mutation in Salmonella typhimurium
and in mammalian cells in vitro and in a host-mediated assay in male
mice. In two separate experiments, imidocarb induced polyploidy in
human peripheral blood lymphocytes in vitro in the presence of
metabolic activation; however, aneuploidy was not induced. Micronuclei
were not induced in mouse bone marrow, and no chromosomal aberrations
were induced in rat bone marrow in vivo. Dominant lethal mutations
were not found in mice, although the dosing and mating schedules used
were not in accordance with contemporary guidelines. The Committee
concluded that imidocarb is unlikely to be genotoxic. In a
three-generation study of reproductive toxicity, rats were fed diets
calculated to provide intakes of 0, 15, 45, or 135 mg/kg bw per day
imidocarb as dipropionate. The body-weight gain of dams at 135 mg/kg
bw per day was decreased, and the number of live births was reduced.
The NOEL was 45 mg/kg bw per day for both fetal toxicity and maternal
toxicity.
In a study of developmental toxicity in rats, there was no
evidence of teratogenicity after administration of imidocarb
dipropionate by gavage at doses of 0, 19, 76, or 300 mg/kg bw per day
on days 6œ16 of gestation. Groups of 20-25 dams were killed on day 20
of gestation and the uterine contents examined; the remaining six dams
in each group were allowed to deliver naturally and rear the
offspring. Maternal body-weight gain was reduced at 300 mg/kg bw per
day. The number of fetuses with bifid or H-shaped sternebrae was
increased in groups of rats receiving 76 or 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day. In a study of developmental toxicity in rabbits, there was no
evidence of teratogenicity after administration of 0, 5, 10, 20, 60,
or 180 mg/kg bw per day of imidocarb as dipropionate by gavage on days
6œ18 of gestation. Severe maternal toxicity was observed at 60 and 180
mg/kg bw per day, and all dams given 180 mg/kg bw per day and 12 of 15
given 60 mg/kg bw per day died. The frequency of post-implantation
losses was increased and fetal weights were reduced in the group given
60 mg/kg bw per day. The NOEL for maternal and fetotoxicity was 20
mg/kg bw per day.
4. EVALUATION
An ADI of 0-10 µg/kg bw per day was established on the basis of
the NOEL of 5 mg/kg bw per day in the 90-day study in dogs. A safety
factor of 500 was used to compensate for the limited pathological and
clinical chemical investigations in this study, the absence of
information about potential inhibition of erythrocyte and regional
brain acetylcholinesterase activity in this species, the lack of data
on neurotoxicity, and the limited data on carcinogenic potential.
5. REFERENCES
Ali, B.H., Hassan, T., Suliman, H.B. & Abdelsalam, E.B. (1985) Some
effects of imidocarb in goats. Vet. Human Toxicol., 27, 477-480.
Bacchi, C.J., Nathan, H.C., Hutner, S.H., Duch, D.S. & Nichol, C.A.
(1981) Prevention by polyamines of the curative effect of amicarbalide
and imidocarb for Trypanosoma brucei infections in mice. Biochem.
Pharmacol., 30, 883-886.
Brown, D. (1979) A two year feeding study in the rat with compound 4A.
Unpublished report No. XZIG 86-C1 from Hazleton Laboratories Europe
Ltd, Harrogate, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Bushby, S.R.M. (1970) Prolonged toxicity examination of 4A65 di Hcl in
the rat. Unpublished report No. HZIG 76-2 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Chesher, B.C., Clampitt, R.B. & Malone, J.C. (1976) Imidocarb
dipropionate, 30 day toxicity/residue study in the dog. Unpublished
report No. HZIG 76-2 from Wellcome Research Laboratories, Berkhamsted,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Clampitt, R.B., Taylor, P.E. & MacPherson, I.S. (1982) An experiment
to study the effect of imidocarb dipropionate (4A65) on circulating
prolactin concentration in mature male rats. Unpublished report No.
HZIG 82-C3 from the Wellcome Foundation Ltd, Berkhamsted Hill, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Coldham, N. G., Moore, A.S., Dave, M., Graham, P.J.,
Sivapathasundaram, S., Lake, B.G. & Sauer, M.J. (1995) Imidocarb
residues in edible bovine tissues and in vitro assessment of imidocarb
metabolism and cytotoxicity. Drug Metab. Disposition, 23, 501-505.
Darby, I.H. & Pelham, P.E. (1989) Investigation into the antimicrobial
activiy of three anti-protozoal compounds against a range of aerobic
bacteria. Unpublished report No. CYSE 89-15 from Coopers Animal
Health, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Fabrizio, P.A. (1986) Final report. Cytogenetic study. Compound 4A65
imidocarb dipropionate. Unpublished report No. XZIG 86-6 from Litton
Bionetics Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Farebrother, D.A. (1973) 4A65 Wholebody autoradiographical study in
male rats. Unpublished report No.BPAT 73/8 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Ferguson, E. (1996) (14C)-Imidocarb: Absorption, distribution,
metabolism and excretion in cattle following a single subcutaneous
injection of Imizol. Unpublished report No. S8107-001-R from Corning
Hazleton (Europe). Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Finch, J.M. (1993) Preparation of pathology tables from report XZIG
86-C1 (1299R - 187/1). A two year feeding study in rats with
Imidocarb. Unpublished report No. R-93-070 from Inveresk Research
International, Tranent, Scotland, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Follefant, R. & Green, A.F. (1971a) Imidocarb i.e. 4A65. Acute oral
toxicity in mice and rats: Comparison of the dihydrochloride,
diacetate and dipropionate. Unpublished report No. BPHR/71/86 from
Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Follefant, R & Green, A.F. (1971b) Imidocarb i.e. 4A65. Comparative
toxicity in mice. Unpublished report No BPHR/71/79 from Wellcome
Research Laboratories, Beckenham, Kent, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. (1966) Preliminary assessment of acute pharmacodynamic
effects. Unpublished report No.BPHR 66-58 from Wellcome Research
Laboratories, Beckenham, Kent, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. & Hughes, R. (1969) Pharmacodynamics and acute toxicity.
Unpublished report No. BPHR 69-8 from Wellcome Research Laboratories,
Beckenham, Kent, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. & James, J.A. (1977) Imidocarb dipropionate: Multiple
dosing intramuscular toxicity in male mice (range finder study for
dominant lethal test). Unpublished report No. HZIG 77-1 from Wellcome
Research Laboratories, Berkhamsted and Beckenham, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977a) Imidocarb
dipropionate: Acute oral toxicity in the female rat (10% solution).
Unpublished report No. HZIG 77/2 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977b) Imidocarb
dipropionate: Acute oral toxicity in the male rat (10% solution).
Unpublished report No. HZIG 77/3 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977c) Imidocarb
dipropionate: Acute oral LD50 in the female rat (30% solution).
Unpublished report No. HZIG 77/5 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977d) Imidocarb
dipropionate: Acute oral LD50 in the male rat (30% solution).
Unpublished report No. HZIG 77/6 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. James, J.A. & Parker, M.J. (1977e) Imidocarb
dipropionate: Dominant lethal study in male mice. Unpublished report
No. HZIG 77-4 from Wellcome Research Laboratories, Beckenham, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Hart, E.R. (1973a) 90-day feeding study in rats. Imidicarb
dipropionate. Revised final report. Unpublished report No. XZIG 86-4
from Litton Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1973b) Teratology phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-7 from Litton Bionetics, Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinkrodt.
Hart, E.R. (1974a) Chronic toxicity phase. Imidicarb dipropionate.
Final report. Unpublished report No. XZIG 86-5 from Litton Bionetics,
Inc., Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1974b) Reproduction phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-9 from Litton Bionetics Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976a) Foetal toxicity study of 4A65 (imidocarb
dipropionate) in rats. Unpublished report No. BPAT 76/9 from Wellcome
Research Laboratories, Beckenham, Kent, UNited Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976b) An account of attempted foetal toxicity study of
4A65 (imidocarb dipropionate) in the rabbit. Unpublished report No.
BPAT 76/20 from Wellcome Research Laboratories, Beckenham, Kent,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
James, D.A. (1977) Multigeneration reproduction study of 4A65
(imidocarb dipropionate) in the rat. Unpublished report No. BPAT 77/24
from Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Marshall, R.R. (1993) Study to evaluate the clastogenic potential of
imidocarb by its effects on the frequency of micronuclei in cultured
human peripheral blood lymphocytes. Unpublished report No. R-93-015
from Hazleton Microtest, Harrogate, United Kingdom. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
McDougald, L.R. & Roberson, E.L. (1988) Antiprotozoan drugs. In:
Veterinary Pharmacology and Therapeutics, 6th Ed., Ames, Iowa State
University Press, pp. 950-968.
McHardy, N., Woollon, R.M., Clampitt, R.B., James, J.A. & Crawley,
R.J. (1986) Efficacy, toxicity and metabolism of imidocarb
dipropionate in the treatment of Babesia ovis infection in sheep.
Res. Vet. Sci., 41, 14-20.
Michell, A.R., White, D.G., Higgins, A.J., Moss, P. & Lees, P. (1986)
Effect of induced hypomagnesemia on the toxicity of imidocarb in
calves. Res. Vet. Sci., 40, 264-270.
Moore, W.B. (1977) Mutagenic activity of imidocarb dipropionate.
Unpublished report No. BEMU/77/3 from Wellcome Research Laboratories,
Beckenham, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Moore, W.B. & Chatfield, S.N. (1983) Mutagenicity: Evaluation of
imidocarb dipropionate using a method based on the Yahagi modification
of the Ames Salmonella/microsome incorporation test. Unpublished
report No. BEMU/83/1 from Wellcome Research Laboratories, Beckenham,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Nimmo-Smith, R.H. (1968) Distribution, excretion and tissue levels of
compound 4A65 (HR 2073) in the rat. Unpublished report No. 3BT68 from
Wellcome Research Laboratories, Berkhamsted, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Piercy, D.W.T. & James, J.A. (1976) Imidocarb dipropionate oral LD50
in rabbits. Unpublished report No. HZIG 76-1 from Wellcome Research &
Development, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Reynolds, J., Clampitt, R.B., Piercy, D.W.T., James, J.A. & Skarpa, M.
(1977) Subacute 90 day oral toxicity of imidocarb dipropionate in the
dog. Unpublished report No. HZIG 77-11 from Wellcome Research
Laboratories, Berkhamsted and Beckenham, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Salam, A.A. & Baggot, J.D. (1983) Adverse effects of imidocarb
dipropionate (Imizol) in a dog. J. Vet. Pharmacol. Ther., 5,
131-135.
Sibinovic, K.H. (1986) In vitro and subacute in vivo host-mediated
assay for mutagenesis. Unpublished report No. XZIG from Litton
Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1973a) Oral and intravenous acute toxicity studies of
imidocarb dipropionate (4A65) in mice and rats. Unpublished report no.
T-TEP-73-4 from Department of Toxicology-Experimental Pathology,
Research Triangle Park, North Carolina, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1972b) Fertility study in dogs given imidocarb
dipropionate. Unpublished report No. TTEP-72-11 from the Department of
Toxicology - Experimental Pathology, Research Triangle Park, North
Carolina, USA. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991a) Study to evaluate the ability of
imidocarb to induce mutation in four histidine-requiring strains of
Salmonella typhimurium and two tryptophan requiring strains of
E. coli. Unpublished report No. RRS-91-22 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991b) Study to determine the ability of
imidocarb to induce mutations to 6-thioguanine resistance in mouse
lymphoma L5179Y cells using a fluctuation assay. Unpublished report
No. RRS-91-15 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tait, A.J. & Marshall, R.R. (1991) Study to evaluate the potential of
imidocarb to induce micronuclei in the polychromatic erythrocytes of
CD-1 mice. Unpublished report No. RRS-91-23 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & McEnaney, S. (1991) Study to evaluate the chromosome
damaging potential of imidocarb by its effect on cultured human
lymphocytes using an in vitro cytogenetics assay. Unpublished report
No. RRS-91-32 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tesh, J.M., Ross, F.W. & Tesh, S.A. (1977) 4A: Effects of oral
administration upon pregnancy in the rabbit. Unpublished report No.
77/WRL2/109 from Life Science Research, Stock, Essex, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975a) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the rat. Unpublished report No. BPAT75/17
from Wellcome Research Laboratories, Berkhamsted, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975b) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the monkey. Unpublished report No.
BPAT75/18 from Wellcome Research Laboratories, Berkhamsted, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Wander, A. (1968a) Distribution and excretion of (14C) labelled HR
2073 after intravenous administration of a single dose (1 mg/kg) in
the mouse. Translation of unpublished report No. 94/2555. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Wander, A. (1968b) Investigation of the activity in the urine, liver,
gall bladder and kidneys of the mouse after intravenous administration
of a single dose of HR 2073 C-14. Translation of unpublished report
No. 94/2554. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Whitaker, A.M. & Bonhoff, A.J. (1983) Cytogenetic study of the effect
of imidocarb dipropionate (3 ',3 '-di(2-imidazolin-2-yl)carbanilide
dipropionate) on peripheral human lymphocytes in vitro. Unpublished
report No. BNCD/83/7 from Wellcome Biotechnology Ltd, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Studies in mice, rats, dogs, monkeys, and cattle have been
reported. Most of them were carried out during the 1960s and 1970s
according to the standards of those days, and important details are
missing from some of the reports. In contrast, the study in cattle was
certified for compliance with GLP.
Mice
Mice were injected into the tail vein with of 1 mg/kg bw
imidocarb dihydrochloride radiolabelled with 14C in the carbonyl
group. No radiolabel was detected in expired air. Excretion in the
urine was initially very rapid but quickly slowed and continued
throughout the observation period of 96 h. Faecal excretion followed a
similar pattern. Of the recovered radiolabel (90% of the administered
dose), about two-thirds was found in the urine and one-third in the
faeces (Wander, 1968a).
Rats
Wistar rats were dosed orally with 14C-imidocarb dipropionate
or 14C-imidocarb dihydrochloride and killed at various intervals up
to 48 h after treatment. Another rat was dosed subcutaneously with the
hydrochloride. Whole-body autoradiography indicated poor oral
absorption of both salts, and radiolabel was detectable only in the
liver, kidney, and gut. After subcutaneous treatment, a significant
amount of radiolabel remained at the injection site (Farebrother,
1973). In another study with various dose regimes and routes of
administration (oral, intraperitoneal, subcutaneous), the highest
concentrations of residues were found in the liver and kidney.
Residues were still detectable in these organs 55 days after
subcutaneous treatment with 5 mg/kg bw of the test substance.
Excretion was slow in rats treated subcutaneously, and only 20% of the
administered dose was recovered in excreta during the 78 h of
treatment (Nimmo-Smith, 1968). In a preliminary study to investigate
tissue retention, Wistar rats were given either a single oral dose or
30 daily oral doses of 5 mg/kg bw imidocarb dipropionate. The rats
were killed 24 h after the last dose, and residues in selected tissues
were determined by an unspecified analytical method. The highest
concentrations were found in kidney; surprisingly, the concentrations
in liver and brain were higher after the single-dose regime (Thomson,
1975a).
Dogs
Male beagle dogs were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and killed 24 h after the last
dose. The residues were determined in selected tissues by an
unspecified method. The highest concentrations were found in liver and
then in kidney; no residues were detected in muscle or brain (< 500
µg/kg) (Chesher et al., 1976). In another study, mongrel dogs were
given an intravenous bolus of 4 mg/kg bw of imidocarb dipropionate.
The pharmacokinetics were biphasic and indicated a large volume of
distribution. The mean terminal half-life was 207 min (Salam & Baggot,
1983).
Monkeys
Female patas monkeys were given daily oral doses of 5 mg/kg bw
imidocarb dipropionate for 30 days and were killed some time after the
last dose (probably 24 h, although this is not stated). The residues
were determined in selected tissues by an unspecified method. The
highest concentrations were found in kidney and liver; none were
detected in muscle or brain (limit of detection, 500 µg/kg) (Thomson,
1975b).
Cattle
In a study which complied with the principles of GLP, six
lactating cows and eight calves were given a single subcutaneous
injection of 3 mg/kg bw 14C-imidocarb dipropionate with a specific
activity of 22 mCi/g. The test substance was formulated as the
proprietary product Imizol Injection. A mean peak blood concentration
of 1300 µg-equivalents/kg was attained 1 h after treatment, and the
concentration remained at this level for about 4 h, when 72œ91% of the
radiolabel was bound to plasma proteins. Excretion was very slow: over
the first 10 days, only 53% of the dose was recovered in excreta, with
38% in the faeces and 15% in the urine. The residues in tissues were
very persistent, particularly in liver where mean residues of 2200
µg-equivalents/kg were found 90 days after treatment (Ferguson, 1996).
2.1.2 Biotransformation
In bovine liver preparations in vitro, there was no evidence
for metabolism of imidocarb (Coldham et al., 1995).
Mice
Mice injected with 14C-imidocarb into the tail vein excreted
37% of the administered dose in the urine within 2 h. Of the
radiolabel in the urine, 95% was unmetabolized imidocarb. The mice
were killed 3.5 h after treatment, and 29% of the dose was found in
the liver, 6.8% in the kidney, and less than 1% in the gall-bladder.
More than 90% of the radiolabel in these organs was unmetabolized
imidocarb (Wander, 1968b).
Cattle
In the study described above in which cattle were treated
subcutaneously, the main component of the residues in liver, kidney,
muscle, and fat for up to 90 days after treatment was unmetabolized
imidocarb. Minor components, accounting for not more than 10% of the
total residues, were also present but were not identified. Imidicarb
was also the major component of cows' milk, urine, and faeces
(Ferguson, 1996).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies of the acute toxicity of imidocarb are
summarized in Table 1. None of the studies complied with GLP, and the
reports fall short of modern standards. The signs of toxicity were
generally consistent with the anticholinesterase activity of imidocarb
and included lethargy, salivation, lachrymation, muscle fasciculation,
ataxia, tremors, and convulsions. Congestion of the lung and kidneys
and mottling of the liver were frequent post-mortem findings in the
rats that died.
In a published report, the signs of toxicity in groups of goats
given a single intramuscular dose in the range 12-24 mg/kg bw included
salivation, frequent urination and defaecation, weakness of the legs,
abnormal posture, and recumbency. There were dose-related decreases in
haemoglobin and erythrocyte counts and packed cell volume and
dose-related increases in asparate aminotransferase activity and in
creatinine and bilirubin concentrations. Serum cholinesterase activity
was reduced in a dose-related manner. No significant effects were
observed at 6 mg/kg bw. The effects were ameliorated by pretreatment
with atropine sulfate intramuscularly at a dose of 1 mg per animal
(Ali et al., 1985).
Whole-blood cholinesterase activity was significantly reduced in
calves given an intramuscular injection of 3.3 mg/kg bw imidocarb
dipropionate. Maximum depression occurred about 30 min after
treatment, and significant recovery was observed within 6 h. The
cholinesterase depression did not correlate with the intensity of the
clinical response (Michell et al., 1986).
2.2.2 Short-term toxicity
Mice
A study in mice, intended as a range-finding probe for a study of
dominant lethal mutations, was not carried out in accordance with GLP.
Groups of six male Evans No. 1 mice were given daily intramuscular
doses of 0 (distilled water), 7.8, 16, 31, 63, or 130 mg/kg bw per day
of imidocarb dipropionate (expressed as base) for five days. Signs of
toxicity were recorded for up to 14 days after the last dose. No blood
chemistry or pathological examinations were carried out. All of the
Table 1. Results of studies of acute toxicity with imidocarb
Species Route Vehicle Sex LD50 Salt Reference
(strain) (mg/kg bw) administered
Mouse (ICR) Oral gavage Sterile distilled water M 723a Dipropionate Szot (1973)
Mouse (ICR) Oral gavage Sterile distilled water F 646a Dipropionate Szot (1973)
Mouse (SAS) Oral gavage 0.9% saline F 544a Dihydrochloride Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 863a Diacetate Follenfant & Green (1971a)
Mouse (SAS) Oral gavage 0.9% saline F 702a Dipropionate Follenfant & Green (1971a)
Mouse (Gambles) Oral gavage 0.9% saline M 1400 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline F 1500 Dihydrochloride Green & Hughes (1969)
Rat (Wistar) Oral gavage 0.9% saline M 1652a Dihydrochloride Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1216a Diacetate Follenfant & Green (1971a)
Rat (Wistar) Oral gavage 0.9% saline M 1251a Dipropionate Follenfant & Green (1971a)
Rat (Long Evans) Oral gavage Sterile distilled water M 454a Dipropionate Szot (1973)
Rat (Long Evans) Oral gavage Sterile distilled water F 650a Dipropionate Szot (1973)
Rabbit (New Zealand Oral gavage Water M + F 317 Dipropionate (27% Piercy & James (1975)
white) aqueous solution)
Rat (Long Evans) Intravenous 0.9% saline M 32.7a Dipropionate Szot (1973)
Mouse (ICR) Intravenous 0.9% saline M 22.3a Dipropionate Szot (1973)
Mouse (Gambles) Intravenous 0.9% saline M 16 Hydrochloride Green & Hughes (1969)
Mouse (SAS) Subcutaneous 0.9% saline F 78.6a Dihydrochloride Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 94.5a Diacetate Follenfant & Green (1971b)
Mouse (SAS) Subcutaneous 0.9% saline F 92.1a Dipropionate Follenfant & Green (1971b)
Mouse (Gambles) Subcutaneous 0.9% saline M 140 Hydrochloride Green & Hughes (1969)
Rat (Wistar) Subcutaneous 0.9% saline F 150 Hydrochloride Green & Hughes (1969)
Rat (Sprague-Dawley) Oral gavage Water F > 631a Dipropionate (10% Harper et al. (1997a)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M > 1000a Dipropionate (10% Harper et al. (1997b)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water F 1011.5a Dipropionate (10% Harper et al. (1997c)
aqueous solution)
Rat (Sprague-Dawley) Oral gavage Water M 1998a Dipropionate (10% Harper et al. (1997d)
aqueous solution)
a The LD50 values found in these studies are for the base. In the remaining studies, the results are believed to be quoted as the salt,
although this is not clear from the report in all cases.
animals at 63 and 130 mg/kg bw and 50% of those at 31 mg/kg bw died
three to four days after the start of treatment. The signs of toxicity
were slow respiration and lethargy. There was no NOEL (Harper & James,
1977).
Rats
In a preliminary study designed primarily to investigate
distribution in tissues, groups of five female Wistar rats were given
either a single oral dose of 5 mg/kg bw imidocarb dipropionate
dissolved in distilled water, or daily oral doses of 5 mg/kg bw per
day for 30 days. Five additional rats formed a control group. Blood
samples were taken for haematological and clinical chemical testing,
and electrocardiographic, ophthalmoscopic, and urinary measurements
were made before treatment and at termination; no histopathological
examination was performed. The rats were killed 24 h after the last
dose, and residues were determined in selected tissues. There were no
treatment-related effects on most of the parameters investigated. The
significant changes in blood parameters included increased total
leukocyte counts in the treated group; however, the toxicological
significance of these effects is doubtful since satisfactory blood
samples were obtained from only three treated rats (Thomson, 1975a).
In a study that did not conform to GLP, groups of 10 Wistar rats
of each sex were given daily oral doses of imidocarb dihydrochloride
for three months. The study was carried out in two phases. In phase 1,
the doses were 0 (distilled water), 125, 250, or 500 mg/kg bw per day;
in phase 2, the doses were 0, 500, 750, or 1500 mg/kg bw per day. It
was not clear whether the doses were expressed as the hydrochloride or
as the the base, and the solutions were not analysed. The rats were
weighed and examined daily. Blood samples for haematological and
clinical chemical determinations were taken just before termination.
Urinalysis was not carried out.
All rats given 1500 mg/kg bw died. The body-weight gain of all
treated rats was significantly lower than that of controls in phase 2.
There were no significant changes in haematological or clinical
chemical values, but changes were seen in the weights of a number of
organs in animals at 500 or 750 mg/kg bw, including a significant
reduction in absolute (but not relative) prostate weight, increased
relative adrenal and thyroid weights in males, and increased relative
adrenal weight in females. Pathological changes described as cloudy
swelling were reported in the livers of 5/9 male and 7/10 female rats
given 125 mg/kg bw and in all males and 6/10 females given 250 mg/kg
bw, but in none of the controls. The lesion was reported to be
reversible, but no evidence was provided. No histopathological
examinations were carried out on rats at 500 or 750 mg/kg bw. No NOEL
was identified (Bushby, 1970).
Groups of Charles River rats were fed diets containing 0, 630,
1900, or 9400 mg/kg imidocarb dipropionate for up to 90 days,
according to the schedule shown in Table 2. The purpose was to
identify the appropriate doses for a long-term study of toxicity. The
study did not comply with the principles of GLP. The animals were
checked daily for deaths, and body weight and food consumption were
recorded weekly. Haematological and clinical chemical determinations
were carried out on five rats of each sex per dose before treatment
and during weeks 4 and 13. Five rats of each sex from the control
group and those at the highest dose were killed after seven weeks and
the remaining rats after 13 weeks. At both 7 and 13 weeks,
acetylcholinesterase activity was measured in one-half of the brain of
each of five rats of each sex in the control group and that at the
highest dose At termination, each rat was examined grossly.
Histopathological examinations were performed on 17 organs or tissues
from five rats of each sex in the control group and that at the
highest dose and on the livers, kidneys, and grossly abnormal tissues
from the other rats.
Table 2. Achieved dosages in a 90-day study with imidocarb in Charles River rats
Concentration in Males Females
feed (mg/kg)
No. rats Achieved dose No. rats Achieved dose
(mg/kg bw (mg/kg bw
imidocarb base) imidocarb base)
0 15 0 15 0
630 10 26 10 32
1900 10 75 10 100
9400 15 420 15 550
One male given 9400 mg/kg feed died during week 2, and a control
female died during week 6. No signs of overt toxicity were observed.
Body-weight gain was significantly reduced towards the end of the
study in animals of each sex given 9400 mg/kg feed. There were no
significant effects on haematological, clinical chemical, or urinary
parameters or on brain acetylcholinesterase activity. Pathological
changes attributable to treatment were limited to the livers of rats
given 9400 mg/kg feed; the changes consisted of mild stasis of the
bile in the canaliculi in one male and four females. The NOEL was 1900
mg/kg feed, equal to 75 mg/kg bw per day (Hart, 1973a).
Another study was conducted, which did not comply with the
principles of GLP and was intended to be a two-year study of toxicity,
but in which 117 rats died as a result of overheating of the animal
rooms during week 37. The study was terminated during week 44, when
all surviving rats were necropsied. In this study, groups of
50 Sprague-Dawley rats of each sex were to have received diets
containing the equivalent of 0, 75, 225, or 750 mg/kg bw per day of
imidocarb dipropionate. The rats were F1a weanlings from groups at
the corresponding doses in a study of reproductive toxicity; however,
the highest dose was fetotoxic, so that there were insufficient
weanlings available to form this group, and additional rats were
purchased. No data were reported on this group.
Until the overheating episode, there were no significant effects
on body weight, food consumption, or haematological, clinical
chemical, or urinary parameters. During the last five weeks, the food
intake of females at 225 mg/kg bw was increased, but they showed no
change in body weight. At week 44, the mean thyroid:body weight ratio
was increased in females at this dose. There were no significant
pathological findings. No firm conclusions could be drawn from this
study (Hart, 1974a).
Dogs
In a study that did not comply with GLP, a group of five male
beagle dogs, described as 'substandard', was given daily oral doses of
5 mg/kg bw imidocarb dipropionate (expressed as base) in distilled
water for 30 days. There was no untreated control group. No signs of
toxicity were observed and there appeared to be no adverse effects on
body weight, food or water intake, or haematological or clinical
chemical parameters. The dogs were killed 24 h after the last dose,
and urine was taken for determination of pH, protein, glucose, ketone,
and blood. Selected organs were weighed and a gross pathological
examination was carried out on each dog. There were no obvious
substance-related changes (Chesher et al., 1976).
In another study that did not comply with GLP, groups of four
beagle dogs of each sex were given daily oral doses of 0, 5, 20, or 80
mg/kg bw per day imidocarb dipropionate (expressed as base) in gelatin
capsules, for 90 days. The animals were weighed twice weekly, and each
dog was subjected to an ophthalmoscopic examination before treatment
and on days 36 and 76. Blood samples were taken from all dogs for
haematological and some clinical chemical investigations before
treatment and on days 7, 28, 56, 71, and 90; cholinesterase activity
was not monitored. Urine samples were collected at necropsy. About 25
tissues from each animal were examined microscopically.
All four males and two of the females given 80 mg/kg bw per day
died or were killed in extremis after having reduced food intake and
weight loss for about two weeks before death. The signs of toxicity in
this group included weakness, recumbency, salivation, muscle
fasiculation, ataxia, and splayed hind legs, probably indicating an
anticholinesterase effect. The food intake of dogs at 20 mg/kg bw was
transiently reduced. There were no treatment-related ophthalmoscopic
changes. Eosinophilia was observed in some dogs given 20 or 80 mg/kg
bw per day, and those at the highest dose showed a trend towards
increased serum alanine and aspartate aminotransferase activity and
bilirubin concentration, with occasional, transient increases in those
at 20 mg/kg bw per day. The absolute and relative weights of the
pituitary were decreased in dogs at 20 but not at 80 mg/kg bw per day,
but animals at 80 mg/kg bw per day showed significant increases in the
absolute and relative weights of the kidney, adrenal, and thyroid.
Gross examination revealed fatty changes and mottling in the livers,
particularly in dogs given 20 or 80 mg/kg bw per day.
Histological changes were observed in a range of tissues from
dogs at 80 mg/kg bw per day, particularly in the thymus, spleen, lymph
nodes, and stroma of the villi of the small intestine (pyknosis and
karyorrhexis), and fatty changes were observed in the thick section of
the loop of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty changes, granularity, or vacuolation of
the hepatocytes. Similar though less severe changes were observed in
the livers of dogs given 20 mg/kg bw per day. The NOEL was 5 mg/kg bw
per day (Reynolds et al., 1977).
Monkeys
In a preliminary study designed to investigate distribution to
tissues, five female patas monkeys were given daily oral doses of
5 mg/kg bw per day imidocarb dipropionate dissolved in distilled water
for 30 days. A control group of five females was left untreated. Blood
samples were taken before treatment, seven and 14 days after the start
of treatment, and at termination. Some time after the last dose (not
stated), the treated monkeys were killed and examined for gross
changes; the control monkeys were not autopsied. There were no signs
of toxicity in the treated animals, and no effects were observed on
haematological or clinical chemical values or gross appearance
(Thomson, 1975b).
2.2.3 Long-term toxicity and carcinogenicity
Rats
In a study that did not conform to GLP, groups of 50 Wistar rats
of each sex were fed diets calculated to provide intakes of 0, 15, 60,
or 240 mg/kg bw per day imidocarb dipropionate (expressed as base) for
104 weeks. Satellite groups of 15 rats of each sex per dose were used
for collection of blood and urine before treatment and at regular
intervals up to termination. Ophthalmoscopic examinations were carried
out at intervals throughout the study on controls and animals at the
highest dose. At termination, about 25 tissues were selected for
histopathological examination from 10 controls of each sex and from
four males and 19 females at the highest dose; eight or nine tissues
were examined from the remaining animals, comprising lymph nodes,
liver, spleen, kidneys, ovaries, adrenals, thyroids, pituitaries, and
blood films. Gross lesions from all animals were also examined
microscopically.
Survival of animals at the highest dose was adversely affected,
only 9/65 males surviving to termination. The rats in this group
became emaciated, their body-weight gain being significantly reduced
and food consumption significantly increased; females were less
severely affected than males. In the last weeks of the study, the food
consumption of the males exceeded that of the controls by about 40%. A
slight reduction in body-weight gain was also observed in females
given 60 mg/kg bw per day. Males at 240 mg/kg bw per day also had an
increased incidence of corneal opacity and evidence of mild anaemia,
with significant reductions in erythrocyte and haemoglobin counts and
packed cell volume.
Changes in clinical chemistry were seen mainly in rats at 240
mg/kg bw per day and appeared to be transient. In males, the changes
included significantly increased aspartate aminotransferase activity
during weeks 13 and 26, increased alkaline phosphatase activity during
week 8, and increased blood urea nitrogen concentration during week 52
and 78. The cholesterol concentration was significantly increased in
all treated groups during week 52 but not at other times. Females had
significant increases in aspartate aminotransferase activity during
weeks 13 and 78, in alanine aminotransferase activity during week 13
(but significant reductions in weeks 4, 52, and 78), in alkaline
phosphatase activity in week 26, and in cholesterol concentration in
week 8. Alkaline phosphatase activity was also increased in females
given 60 mg/kg bw per day during week 26. Males given 60 mg/kg bw per
day and rats of each sex given 240 mg/kg bw per day had significantly
greater water intake than the controls. Polyuria was observed in males
given 240 mg/kg bw per day, and a slight increase in urinary output
was noted in females at this dose. The specific gravity of urine
samples from the males given 240 mg/kg bw per day was consistently
lower than that of the controls.
At termination, the mean group kidney weight of males given 240
mg/kg bw per day was significantly increased. Treatment-related
microscopic changes were confined to the rats at 60 or 240 mg/kg bw
per day and included deposition of brown pigment in macrophages and
related cells in the liver, spleen, lymph nodes, and bone marrow, and
cystic distension of the renal tubules and glomeruli, with dystrophic
mineralization of the renal medulla. Muscle sections were not taken
for microscopic examination, but evidence of increased atrophy was
found in rats at the highest dose in muscle samples attached to the
sternum. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, some clinical chemical changes,
histopathological changes in the kidney, and skeletal muscle atrophy
at higher doses. The incidence of mammary fibroadenomas was increased
in females given 240 mg/kg bw per day, and many of them had multiple
tumours. Males at this dose had an increased incidence of cutaneous
fibromas (Brown, 1979).
In a separate report, the original histological data from the
study described above were re-tabulated and reanalysed. A number of
assumptions had to be made, since it was not always clear which
tissues had been examined from which animal, which lesions had been
reported, and what terminology had been used. The following overall
conclusions were drawn. Females given 240 mg/kg bw per day had a
significantly increased incidence of multiple fibroadenomas and a
decreased incidence of single fibroadenomas in the mammary gland as
compared with the controls; there was no significant difference in the
combined incidence of multiple and single fibroadenomas. Males at this
dose had a significantly increased incidence of multiple subcutaneous
fibromas in comparison with the controls; an apparent decrease in the
incidence of single fibromas was not significant, and there was no
significant difference in the combined incidence of multiple and
single fibroadenomas. Rats of each sex given 240 mg/kg bw per day had
a nonsignificant increase in the incidence of fibrosarcomas (Finch,
1993).
2.2.4 Genotoxicity
The results of studies of the genotoxicity of imidocarb are
summarized in Table 3. The concentrations and doses are expressed as
imidocarb base, except when stated otherwise. Negative results were
obtained in asays for gene mutation in bacteria and in mammalian cells
in vitro. In two separate experiments, imidocarb induced polyploidy
in human lymphocytes in vitro; however, there was no induction of
aneuploidy. Negative results were obtained in a test for micronucleus
formation in mouse bone marrow, in a metaphase analysis in bone marrow
of rats in vivo, and in an inadequately conducted assay for dominant
lethal mutation.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A multigeneration study was carried out in rats as part of a
larger study designed to investigate all aspects of reproduction,
including teratogenicity, and to provide weanling rats for a two-year
study. Groups of 15 Sprague-Dawley rats of each sex were maintained on
diets containing 0, 630, 1900, or 9000 mg/kg imidocarb dipropionate
(presumably expressed as salt, although this is not clear from the
report) for 60 days before mating. The diets were stated to provide an
equivalent of 0, 45, 130, or 760 mg/kg bw of imidocarb dipropionate
per day. The numbers of pups in both the F1a and F1b litters were
significantly reduced at the highest dose; thus, only three females
receiving 9000 mg/kg produced F1a litters. There was a similar
reduction in the F1b litters receiving the intermediate dose. F2
litters were bred only from the animals at the low dose, and the study
was terminated early owing to failure of the temperature control
mechanism in the animal rooms. No firm conclusions could be drawn from
this study (Hart, 1974b).
Table 3. Results of genotoxicity studies on imidocarb
End-point Test object Concentration S9 Result GLP Reference
In vitro
Gene mutation S. typhimurium TA1535, TA1537, < 1000 µg/plate + Negativea No Moore (1997)
TA1538, TA98, TA100 < 250 µg/plate - Negativea
Gene mutation S. typhimurium TA1535, TA1537, 0.2-1000 µg/plate + Negativea No Moore & Chatfield
TA1538, TA98, TA100 - Negativea (1983)
Gene mutation S. typhimurium TA98, TA100 4-2650 µg/plate + Negative Yes Tait & Clare
TA1535, TA1537, E. coli WP2 33-530 µg/plate - Negativeb (1991a)
pKM101, WP2uvrA- pKM101 53-265 µg/plate
(with strain WP2uvrA- pKM101 only)
Gene mutation hprt locus of L5178Y mouse 0.55-1749 µg/ml + Negative Yes Tait & Clare
lymphoma cells 54.7-1749 µg/ml - Negative (1991b)
Chromosomal Human peripheral blood 3.5-35 µg/ml (as imidocarb - Negative No Whitaker &
aberration lumphocytes dipropionate); higher doses were Bonhoff (1983)
toxic
Chromosomal Human peripheral blood 20 h -S9; 144-294 µg/ml; + Positivec Yes Tait & McEnaney
aberration lymphocytes 20 h +S9; 857-1749 µg/ml (expt 1) (1991)
20 h -S9; 235-367 µg/ml;
20 h + S9; 1119-1749 µg/ml;
44 h -S9; 235 µg/ml; 44 h +S9;
1119 µg/ml (expt 2)
44 h + S9; 366.8-1119 µg/ml (expt 3)
Micronucleus Human peripheral blood 366.8-1120 µg/ml (for frequency of + Negativee Yes Marshall (1993)
formation lymphocytes micronuclei)
895.5 µg/ml (for polyploidy)
Table 3. (continued)
End-point Test object Concentration S9 Result GLP Reference
Host-mediated Male ICR mice (10/group) Five daily oral doses of 15, 45 or Negative No Sibinovic (1986)
assay S. typhimurium TA1530, G-46 150 mg/kg bw; test organism given
intraperitoneally 30 min after last
dosea
In vivo
Bone marrow Sprague-Dawley rats (5/dose); Five consecutive daily Negative No Fabrizio (1986)
metaphase bone marrow harvested 5 days oral doses of 10, 30, or
analysis after treatment with imidocarb; 100 mg/kg bwa
40-50 metaphase spreads/rat
analysed
Micronucleus CD-1 mouse (5/sex per dose); Single intraperitoneal injection of Negative Yes Tait & Marshall
formation marrow harvested 24, 48, 72h 8, 5, 17, or 34 mg/kg bw (1991)
after treatment
Dominant Male Evans mice Five daily intramuscular injections Negatived No Harper et al.
lethal of 4 or 16 mg/kg bw followed by a (1977)
mutation six-week mating schedule
S9, 9000 × g fraction of rat liver; GLP, conformity with good laboratory practice
a No information on whether concentrations or doses reported in terms of imidocarb base or diproprionate salt
b Although there was a statistically significant increase in the number of revertants with strain WP2uvrA- pKM101 in the presence
of S9, the increase was less than double the value for the solvent control, there was no concentration-related response, and the
values were within the range for contemporary historical controls.
c Treatment in the absence of S9 resulted in significant increases in the frequencies of cells with structural aberrations; it was
stated that the frequencies were within the range in historical controls, but the Committee did not consider the historical
controls to be appropriate. Statistically significant increases in the frequency of cells with numerical aberrations were observed
in cultures treated in the presence of S9 and sampled at 44 h, in both experiments 2 and 3.
d Although there were statistically significant increases in the percentage of dead implants in the group receiving 6 mg/kg bw during
week 2, there was no dose-response relationship, and the incidence in the control group was unusually low. The treatment and mating
schedules used in this study did not comply with modern guidelines.
e There was no increase in the incidence of micronuclei at any concentration; however, an increased frequency of polypoid cells was
observed at the single concentration tested.
In another study that did not comply with GLP, groups of 20
Wistar rats of each sex were fed diets calculated to provide 0, 15,
45, or 135 mg/kg bw per day imidocarb dipropionate. Treatment
commenced when the rats were six weeks old, 60 days before the first
mating, and continued throughout the breeding of three succesive
generations. At 21 days post partum, the F1a, F2a, and F2b
litters were killed and examined internally. The F1b and F2b
litters were mated to provide the subsequent generations. The F3b
fetuses were removed on day 20 of gestation and examined for
malformations.
During the premating period, females of the parental generation
showed a small but dose-related decrease in body-weight gain; there
were no clear dose-related effects on body weight in subsequent
generations. The numbers of live births among females receiving 135
mg/kg bw per day were reduced after the first mating of the F0
generation, and the numbers of dead or missing fetuses were increased.
A similar trend was observed after the first mating of the F1
generation. Maternal body-weight gain was reduced on day 17 of
gestation after the second mating of the F1 generation. No evidence
of teratogenicity was found on examination of the F3b fetuses;
however, there was a small, nonsignificant increase in the incidence
of fetuses with bifid sternebrae. The NOEL was 45 mg/kg bw per day for
both fetal toxicity and maternal toxicity (James, 1977).
Dogs
Five female dogs were given subcutaneous injections of 14 mg/kg
bw imidocarb dipropionate (expressed as free base) on the first day of
pro-oestrus and again immediately after successful mating. Six
untreated dogs were used as controls. The dogs were allowed to litter
naturally. After the first whelping, all the treated dogs were dosed
and bred a second time. There were no signs of toxicity and no effects
on on fertility, gestation, or appearance of pups (Szot, 1972).
(ii) Developmental toxicity
Rats
Teratogenicity was studied in rats within a multigeneration
study, which did not comply with the principles of GLP. Groups of 15
female rats were fed imidocarb dipropionate continuously in the diet
for 60 days before mating, achieving intakes of about 0, 47, 140, or
760 mg/kg bw per day. They were killed on day 19 of gestation, and the
uterine contents were examined. Two-thirds of the fetuses were then
processed for staining with Alizarin Red S for skeletal evaluation,
and the remaining one-third were fixed in Bouin's solution and
sectioned for evaluation of visceral abnormalities.
Maternal body-weight gain was significantly reduced at the doses
of 140 and 760 mg/kg bw per day and the incidence of resorptions was
significant-ly increased in those at 760 mg/kg bw per day with a
consequent reduction in the numbers of live fetuses. Animals at 140
mg/kg bw per day also showed a small increase in the resorption rate.
Fetal body weight and length were reduced at 760 mg/kg bw per day.
There was no evidence of teratogenicity at any dose (Hart, 1973b).
In a second study, which was not carried out in compliance with
the principles of GLP, groups of 27-31 mated female Wistar rats were
given daily oral doses of 0 (untreated), 0 (distilled water, solvent),
19, 76, or 300 mg/kg bw imidocarb dipropionate (expressed as base) by
gavage on days 6-16 of gestation. Groups of 20-25 dams were killed on
day 20 of gestation and the uterine contents examined. One-third of
the fetuses were examined by open dissection, one-third were processed
by Wilson's section technique for examination of thick serial
sections, and one-third were processed for staining with Alizarin Red
S for skeletal evaluation. The remaining six dams in each group were
allowed to deliver naturally and rear the offspring to weaning on day
21 post partum.
Maternal body-weight gain was significantly reduced during
treatment with 300 mg/kg bw per day. There was no evidence of
teratogenicity, but the numbers of fetuses with bifid or H-shaped
sternebrae were increased at doses of 76 and 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day (James, 1976a).
Rabbits
Groups of 16-27 female Dutch rabbits were given daily doses of 0
(water), 20, 80, or 320 mg/kg bw per day of imidocarb dipropionate
(doses expressed as base) by oral gavage on days 8-19 of gestation.
The study was not carried out in compliance with the principles of
GLP. All 16 rabbits given 320 mg/kg bw per day and one out of 27 given
80 mg/kg bw per day died. The remaining dams showed signs of stress
(nervous behaviour, diarrhoea, weight loss), which were attributed to
their close proximity to a dog colony. Five to six dams in each group
were allowed to deliver naturally, but most of the dams killed their
offspring. There was no evidence of teratogenicity. The study was
inadequate for establishing a NOEL (James, 1976b).
In another study, which was not carried out in accordance with
the principles of GLP, groups of 10-20 female New Zealand white
rabbits were given doses of 0 (distilled water), 5, 10, 20, 60, or 180
mg/kg bw per day of imidocarb dipropionate by oral gavage on days 6-18
of gestation. The surviving does were killed on day 29 of gestation
and the uterine contents examined. The general appearance of each
fetus and the internal organs of the neck, thorax, and abdomen were
examined grossly. All fetuses were then eviscerated, preserved, and
stained for skeletal examination.
All 10 does given 180 mg/kg bw per day and 12 out of 15 given
60 mg/kg bw per day died. The does in these groups displayed signs of
gastrointestinal disturbance, salivation, nasal exudation, and weight
loss before death. The incidence of post-implantation loss was
increased in animals at 60 mg/kg bw per day, and only 12 fetuses
remained for examination. Fetal weights appeared to be reduced, and
the incidence of delayed ossification appeared to be increased. There
was no evidence of teratogenicity at any dose. The NOEL for both
maternal toxicity and fetotoxicity was 20 mg/kg bw per day (Tesh et
al., 1977).
2.2.6 Special studies to investigate serum prolactin concentrations
A study which did not comply with GLP was carried out to
investigate the mechanism of the induction of mammary tumours in the
two-year study in rats. Groups of 10 male Wistar rats were fed diets
calculated to provide 0, 15, 60, or 240 mg/kg bw per day of imidocarb
(as base) for 28 days. Another group was given 160 mg/kg bw per day of
sulpiride. Serum prolactin concentrations were measured before
treatment and 2, 5, 8, 14, and 22 days after the first dose. The mean
prolactin concentrations were significantly (p < 0.001) increased
in the group receiving sulpiride from day 2 onwards and were slightly
increased in the group given 240 mg/kg bw per day imidocarb, although
they never approached the values found in the group given sulpiride
(Clampitt et al., 1982).
2.2.7 Special studies on microbiological activity
In a study that did not conform to GLP, MIC values in vitro
were determined for imidocarb against 20 bacterial isolates
representing seven genera. MIC values > 16 µg/ml were obtained for 18
isolates, and values of 4 and 8 µg/ml were obtained for two strains of
Pasteurella (Darby & Pelham, 1989).
2.2.8 Special studies on pharmacodynamic effects
It is generally accepted in the published literature that
imidocarb has anticholinesterase activity and that the toxic signs
observed after treatment can be alleviated with atropine (Ali et al.,
1985; Michell et al., 1986; McDougald & Roberson, 1988). The
pharmacodynamic effects of imidocarb were investigated in a screening
study in which mice were given single subcutaneous doses of the
dihydrochloride salt. Myosis was observed at 50 mg/kg bw, but
mydriasis occurred at 150 mg/kg bw (Green, 1966). In another study,
cardiovascular and neuromuscular effects were observed after
intravenous administration of imidocarb dihydrochloride to
anaesthetized cats and dogs, which were attributed in part to the
anticholinesterase effect. No effect on the pupil was observed after
instillation of a solution of 10 mg/ml imidocarb dihydrochloride into
a cat's eye (Green & Hughes, 1969). No conclusions could be drawn
about anticholinesterase potency from these data.
The mode of action of imidocarb as an antiprotozoal agent is
uncertain, although two mechanisms have been proposed:
* As the effect of imidocarb on Trypanosoma brucei is antagonized
by excess polyamines, it is has been suggested that imidocarb
interferes with their production and/or use (Bacchi et al.,
1981).
* Imidocarb blocks the entry of inositol into erythrocytes
containing Babesia, resulting in 'starvation' of the parasite
(McHardy et al., 1986).
2.3 Observations in humans
It is believed that imidocarb has been used occasionally in human
medicine, but no details of doses or adverse effects were available to
the Committee.
3. COMMENTS
The Committee considered data from studies of the
pharmacokinetics, metabolism, acute, and short-term toxicity,
long-term toxicity and carcino-genicity, reproductive toxicity and
genotoxicity of imidocarb and some special studies. Most of the
studies were carried out with the dipropionate salt, and the doses
used were expressed as imidocarb base. Except for some of the assays
for genotoxicity, the studies were not carried out to contemporary
standards for study protocol and conduct but were reported in
sufficient detail and were considered adequate for assessment.
The pharmacokinetics of imidocarb was investigated in mice, rats,
dogs, monkeys, and cattle. The dipropionate and dihydrochloride salts
of imidocarb appeared to be poorly absorbed after oral administration
to rats, but the extent of the oral bioavailability could not be
estimated from the data available. In cattle dosed subcutaneously,
imidocarb was bound to plasma proteins. The drug was excreted in both
urine and the faeces for several days in rodents and for at least 28
days in cattle.
In mice dosed intravenously with radiolabelled imidocarb and
killed 3.5 h later, more than 90% of the residues in liver and kidney
were unmetabolized imidocarb. The parent compound also accounted for
95% of the radiolabelled material in mouse urine. In cattle dosed
subcutaneously with radiolabelled imidocarb, the parent compound
accounted for most of the residues in urine and faeces and 70-90% of
the residues in edible tissues and milk. No evidence of metabolism was
found in a study of bovine liver slices, isolated hepatocytes, or
microsomal fractions in vitro.
Imidocarb dipropionate is moderately hazardous, with oral LD50
values in the range of 650-720 mg/kg bw in mice and 450-1200 mg/kg bw
in rats. The signs were generally consistent with anticholinesterase
activity.
Serum cholinesterase activity was depressed in a dose-related
manner in goats given single intramuscular doses of 12-24 mg/kg bw.
Whole-blood cholinesterase activity was decreased in calves given
single intramuscular injections of 3.3 mg/kg bw.
When rats were given daily oral doses of 0, 125, 250, 500, 750,
or 1500 mg/kg bw per day of imidocarb dihydrochloride for three
months, all rats at the highest dose died. Cloudy swelling in the
liver was observed in rats given 125 and 250 mg/kg bw per day.
Histopathological examinations were not performed on rats at higher
doses. No NOEL was identified.
In a study conducted to identify appropriate doses for a
long-term study of toxicity, rats were fed diets calculated to provide
doses of imidocarb dipropionate equal to 0, 26, 75, or 420 mg/kg bw
per day for 90 days. Body-weight gain was reduced in animals of each
sex given 420 mg/kg bw per day. Acetylcholinesterase activity,
measured in one-half of the brain from each of 10 rats given 420 mg/kg
bw per day and killed after 7 and 13 weeks, was not significantly
different from that in controls. Pathological changes attributable to
treatment were limited to mild stasis of the bile in the canaliculi of
the liver in one male and four females given 420 mg/kg bw per day. The
NOEL was 75 mg/kg bw per day on the basis of reduced body-weight gain
and hepatic toxicity at higher doses.
Groups of four dogs of each sex per dose were given oral doses of
0, 5, 20, or 80 mg/kg bw per day imidocarb dipropionate in gelatin
capsules for 90 days. The signs of toxicity in the dogs given 80 mg/kg
bw per day included recumbency, salivation, muscle fasciculation,
ataxia, and splayed legs. All males and two of four females given this
dose died or were killed. Blood eosinophilia was also observed at 80
mg/kg bw per day, with increased serum alanine aminotransferase and
aspartate aminotransferase activity and increased bilirubin
concentration. Similar but less severe changes were observed in the
dogs given 20 mg/kg bw per day. The weights of the kidney, thyroid,
and adrenal glands were increased in animals at 80 mg/kg bw per day,
and histopathological changes were found in a range of tissues. In the
kidney, these included fatty changes in the thick section of the loop
of Henle and the distal convoluted tubules. The liver showed
haemorrhagic necrosis, fatty change, and microgranularity or
vacuolation of hepatocytes. Similar but less severe hepatocellular
changes were found in the livers of dogs given 20 mg/kg bw per day.
The NOEL was 5 mg/kg bw per day on the basis of minor changes in
haematological and clinical chemical parameters and hepatocellular
changes at higher doses.
In a long-term study of toxicity and carcinogenicity, rats were
fed diets calculated to provide intakes of 0, 15, 60, or 240 mg/kg bw
per day of imidocarb as dipropionate for two years. Survival was
adversely affected at the highest dose. Body-weight gain was
significantly reduced in animals of each sex given 240 mg/kg bw per
day and was slightly reduced in females given 60 mg/kg bw per day.
Males at 240 mg/kg bw per day showed evidence of anaemia. Changes in
clinical chemical parameters were transient and occurred mainly in the
animals given the highest dose. Rats given 60 or 240 mg/kg bw per day
drank considerably more water than the controls, and polyuria was
observed in males at the highest dose. At termination, the weights of
the kidneys of males given 240 mg/kg bw per day were significantly
increased. Dose-related histopathological changes were observed at 60
and 240 mg/kg bw per day, which included cystic distension of the
renal tubules and glomeruli and dystrophic mineralization in the renal
medulla. The NOEL for toxicity was 15 mg/kg bw per day on the basis of
reduced body-weight gain, changes in some clinical chemical
parameters, and histopathological changes in the kidney. All animals
were examined grossly, and all gross lesions were examined
microscopically; however, comprehensive pathological examinations was
made on only 10 control rats of each sex and 19 females and four males
at the highest dose at termination of the study. A limited range of
tissues from the other animals was examined, and mammary glands were
examined only when a gross lesion was found. At the highest dose, a
significantly increased incidence of multiple fibroadenomas of the
mammary gland was found in females and of multiple subcutaneous
fibromas in males. Because of the excessive toxicity at this dose, the
poor survival, and limited histopathological examination, the
significance of these findings could not be evaluated. The incidences
of tumour at other sites were not increased.
The genotoxic properties of imidocarb dipropionate were
investigated in a range of assays in vitro and in vivo. Negative
results were obtained for gene mutation in Salmonella typhimurium
and in mammalian cells in vitro and in a host-mediated assay in male
mice. In two separate experiments, imidocarb induced polyploidy in
human peripheral blood lymphocytes in vitro in the presence of
metabolic activation; however, aneuploidy was not induced. Micronuclei
were not induced in mouse bone marrow, and no chromosomal aberrations
were induced in rat bone marrow in vivo. Dominant lethal mutations
were not found in mice, although the dosing and mating schedules used
were not in accordance with contemporary guidelines. The Committee
concluded that imidocarb is unlikely to be genotoxic. In a
three-generation study of reproductive toxicity, rats were fed diets
calculated to provide intakes of 0, 15, 45, or 135 mg/kg bw per day
imidocarb as dipropionate. The body-weight gain of dams at 135 mg/kg
bw per day was decreased, and the number of live births was reduced.
The NOEL was 45 mg/kg bw per day for both fetal toxicity and maternal
toxicity.
In a study of developmental toxicity in rats, there was no
evidence of teratogenicity after administration of imidocarb
dipropionate by gavage at doses of 0, 19, 76, or 300 mg/kg bw per day
on days 6œ16 of gestation. Groups of 20-25 dams were killed on day 20
of gestation and the uterine contents examined; the remaining six dams
in each group were allowed to deliver naturally and rear the
offspring. Maternal body-weight gain was reduced at 300 mg/kg bw per
day. The number of fetuses with bifid or H-shaped sternebrae was
increased in groups of rats receiving 76 or 300 mg/kg bw per day.
There were no treatment-related effects on the growth or survival of
the offspring post partum. The NOEL for fetotoxicity was 19 mg/kg bw
per day. In a study of developmental toxicity in rabbits, there was no
evidence of teratogenicity after administration of 0, 5, 10, 20, 60,
or 180 mg/kg bw per day of imidocarb as dipropionate by gavage on days
6œ18 of gestation. Severe maternal toxicity was observed at 60 and 180
mg/kg bw per day, and all dams given 180 mg/kg bw per day and 12 of 15
given 60 mg/kg bw per day died. The frequency of post-implantation
losses was increased and fetal weights were reduced in the group given
60 mg/kg bw per day. The NOEL for maternal and fetotoxicity was 20
mg/kg bw per day.
4. EVALUATION
An ADI of 0-10 µg/kg bw per day was established on the basis of
the NOEL of 5 mg/kg bw per day in the 90-day study in dogs. A safety
factor of 500 was used to compensate for the limited pathological and
clinical chemical investigations in this study, the absence of
information about potential inhibition of erythrocyte and regional
brain acetylcholinesterase activity in this species, the lack of data
on neurotoxicity, and the limited data on carcinogenic potential.
5. REFERENCES
Ali, B.H., Hassan, T., Suliman, H.B. & Abdelsalam, E.B. (1985) Some
effects of imidocarb in goats. Vet. Human Toxicol., 27, 477-480.
Bacchi, C.J., Nathan, H.C., Hutner, S.H., Duch, D.S. & Nichol, C.A.
(1981) Prevention by polyamines of the curative effect of amicarbalide
and imidocarb for Trypanosoma brucei infections in mice. Biochem.
Pharmacol., 30, 883-886.
Brown, D. (1979) A two year feeding study in the rat with compound 4A.
Unpublished report No. XZIG 86-C1 from Hazleton Laboratories Europe
Ltd, Harrogate, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Bushby, S.R.M. (1970) Prolonged toxicity examination of 4A65 di Hcl in
the rat. Unpublished report No. HZIG 76-2 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Chesher, B.C., Clampitt, R.B. & Malone, J.C. (1976) Imidocarb
dipropionate, 30 day toxicity/residue study in the dog. Unpublished
report No. HZIG 76-2 from Wellcome Research Laboratories, Berkhamsted,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Clampitt, R.B., Taylor, P.E. & MacPherson, I.S. (1982) An experiment
to study the effect of imidocarb dipropionate (4A65) on circulating
prolactin concentration in mature male rats. Unpublished report No.
HZIG 82-C3 from the Wellcome Foundation Ltd, Berkhamsted Hill, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Coldham, N. G., Moore, A.S., Dave, M., Graham, P.J.,
Sivapathasundaram, S., Lake, B.G. & Sauer, M.J. (1995) Imidocarb
residues in edible bovine tissues and in vitro assessment of imidocarb
metabolism and cytotoxicity. Drug Metab. Disposition, 23, 501-505.
Darby, I.H. & Pelham, P.E. (1989) Investigation into the antimicrobial
activiy of three anti-protozoal compounds against a range of aerobic
bacteria. Unpublished report No. CYSE 89-15 from Coopers Animal
Health, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Fabrizio, P.A. (1986) Final report. Cytogenetic study. Compound 4A65
imidocarb dipropionate. Unpublished report No. XZIG 86-6 from Litton
Bionetics Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Farebrother, D.A. (1973) 4A65 Wholebody autoradiographical study in
male rats. Unpublished report No.BPAT 73/8 from Wellcome Research
Laboratories, Berkhamsted, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Ferguson, E. (1996) (14C)-Imidocarb: Absorption, distribution,
metabolism and excretion in cattle following a single subcutaneous
injection of Imizol. Unpublished report No. S8107-001-R from Corning
Hazleton (Europe). Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Finch, J.M. (1993) Preparation of pathology tables from report XZIG
86-C1 (1299R - 187/1). A two year feeding study in rats with
Imidocarb. Unpublished report No. R-93-070 from Inveresk Research
International, Tranent, Scotland, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Follefant, R. & Green, A.F. (1971a) Imidocarb i.e. 4A65. Acute oral
toxicity in mice and rats: Comparison of the dihydrochloride,
diacetate and dipropionate. Unpublished report No. BPHR/71/86 from
Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Follefant, R & Green, A.F. (1971b) Imidocarb i.e. 4A65. Comparative
toxicity in mice. Unpublished report No BPHR/71/79 from Wellcome
Research Laboratories, Beckenham, Kent, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. (1966) Preliminary assessment of acute pharmacodynamic
effects. Unpublished report No.BPHR 66-58 from Wellcome Research
Laboratories, Beckenham, Kent, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Green, A.F. & Hughes, R. (1969) Pharmacodynamics and acute toxicity.
Unpublished report No. BPHR 69-8 from Wellcome Research Laboratories,
Beckenham, Kent, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. & James, J.A. (1977) Imidocarb dipropionate: Multiple
dosing intramuscular toxicity in male mice (range finder study for
dominant lethal test). Unpublished report No. HZIG 77-1 from Wellcome
Research Laboratories, Berkhamsted and Beckenham, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977a) Imidocarb
dipropionate: Acute oral toxicity in the female rat (10% solution).
Unpublished report No. HZIG 77/2 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977b) Imidocarb
dipropionate: Acute oral toxicity in the male rat (10% solution).
Unpublished report No. HZIG 77/3 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977c) Imidocarb
dipropionate: Acute oral LD50 in the female rat (30% solution).
Unpublished report No. HZIG 77/5 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W., Piercy, D.W.T. & James, J.A. (1977d) Imidocarb
dipropionate: Acute oral LD50 in the male rat (30% solution).
Unpublished report No. HZIG 77/6 from Wellcome Research & Development,
Berkhamsted Hill, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Harper, D.W. James, J.A. & Parker, M.J. (1977e) Imidocarb
dipropionate: Dominant lethal study in male mice. Unpublished report
No. HZIG 77-4 from Wellcome Research Laboratories, Beckenham, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Hart, E.R. (1973a) 90-day feeding study in rats. Imidicarb
dipropionate. Revised final report. Unpublished report No. XZIG 86-4
from Litton Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1973b) Teratology phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-7 from Litton Bionetics, Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinkrodt.
Hart, E.R. (1974a) Chronic toxicity phase. Imidicarb dipropionate.
Final report. Unpublished report No. XZIG 86-5 from Litton Bionetics,
Inc., Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Hart, E.R. (1974b) Reproduction phase. Imidocarb dipropionate. Final
report. Unpublished report No. XZIG 86-9 from Litton Bionetics Inc.,
Bethesda, Maryland, USA. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976a) Foetal toxicity study of 4A65 (imidocarb
dipropionate) in rats. Unpublished report No. BPAT 76/9 from Wellcome
Research Laboratories, Beckenham, Kent, UNited Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
James, D.A. (1976b) An account of attempted foetal toxicity study of
4A65 (imidocarb dipropionate) in the rabbit. Unpublished report No.
BPAT 76/20 from Wellcome Research Laboratories, Beckenham, Kent,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
James, D.A. (1977) Multigeneration reproduction study of 4A65
(imidocarb dipropionate) in the rat. Unpublished report No. BPAT 77/24
from Wellcome Research Laboratories, Beckenham, Kent, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Marshall, R.R. (1993) Study to evaluate the clastogenic potential of
imidocarb by its effects on the frequency of micronuclei in cultured
human peripheral blood lymphocytes. Unpublished report No. R-93-015
from Hazleton Microtest, Harrogate, United Kingdom. Submitted to WHO
by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
McDougald, L.R. & Roberson, E.L. (1988) Antiprotozoan drugs. In:
Veterinary Pharmacology and Therapeutics, 6th Ed., Ames, Iowa State
University Press, pp. 950-968.
McHardy, N., Woollon, R.M., Clampitt, R.B., James, J.A. & Crawley,
R.J. (1986) Efficacy, toxicity and metabolism of imidocarb
dipropionate in the treatment of Babesia ovis infection in sheep.
Res. Vet. Sci., 41, 14-20.
Michell, A.R., White, D.G., Higgins, A.J., Moss, P. & Lees, P. (1986)
Effect of induced hypomagnesemia on the toxicity of imidocarb in
calves. Res. Vet. Sci., 40, 264-270.
Moore, W.B. (1977) Mutagenic activity of imidocarb dipropionate.
Unpublished report No. BEMU/77/3 from Wellcome Research Laboratories,
Beckenham, United Kingdom. Submitted to WHO by Mallinckrodt,
Harefield, Uxbridge, Middlesex, United Kingdom.
Moore, W.B. & Chatfield, S.N. (1983) Mutagenicity: Evaluation of
imidocarb dipropionate using a method based on the Yahagi modification
of the Ames Salmonella/microsome incorporation test. Unpublished
report No. BEMU/83/1 from Wellcome Research Laboratories, Beckenham,
United Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Nimmo-Smith, R.H. (1968) Distribution, excretion and tissue levels of
compound 4A65 (HR 2073) in the rat. Unpublished report No. 3BT68 from
Wellcome Research Laboratories, Berkhamsted, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Piercy, D.W.T. & James, J.A. (1976) Imidocarb dipropionate oral LD50
in rabbits. Unpublished report No. HZIG 76-1 from Wellcome Research &
Development, Berkhamsted Hill, United Kingdom. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Reynolds, J., Clampitt, R.B., Piercy, D.W.T., James, J.A. & Skarpa, M.
(1977) Subacute 90 day oral toxicity of imidocarb dipropionate in the
dog. Unpublished report No. HZIG 77-11 from Wellcome Research
Laboratories, Berkhamsted and Beckenham, United Kingdom. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Salam, A.A. & Baggot, J.D. (1983) Adverse effects of imidocarb
dipropionate (Imizol) in a dog. J. Vet. Pharmacol. Ther., 5,
131-135.
Sibinovic, K.H. (1986) In vitro and subacute in vivo host-mediated
assay for mutagenesis. Unpublished report No. XZIG from Litton
Bionetics, Inc., Bethesda, Maryland, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1973a) Oral and intravenous acute toxicity studies of
imidocarb dipropionate (4A65) in mice and rats. Unpublished report no.
T-TEP-73-4 from Department of Toxicology-Experimental Pathology,
Research Triangle Park, North Carolina, USA. Submitted to WHO by
Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Szot, R.J. (1972b) Fertility study in dogs given imidocarb
dipropionate. Unpublished report No. TTEP-72-11 from the Department of
Toxicology - Experimental Pathology, Research Triangle Park, North
Carolina, USA. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991a) Study to evaluate the ability of
imidocarb to induce mutation in four histidine-requiring strains of
Salmonella typhimurium and two tryptophan requiring strains of
E. coli. Unpublished report No. RRS-91-22 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & Clare, C.B. (1991b) Study to determine the ability of
imidocarb to induce mutations to 6-thioguanine resistance in mouse
lymphoma L5179Y cells using a fluctuation assay. Unpublished report
No. RRS-91-15 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tait, A.J. & Marshall, R.R. (1991) Study to evaluate the potential of
imidocarb to induce micronuclei in the polychromatic erythrocytes of
CD-1 mice. Unpublished report No. RRS-91-23 from Hazleton Microtest,
York, United Kingdom. Submitted to WHO by Mallinckrodt, Harefield,
Uxbridge, Middlesex, United Kingdom.
Tait, A.J. & McEnaney, S. (1991) Study to evaluate the chromosome
damaging potential of imidocarb by its effect on cultured human
lymphocytes using an in vitro cytogenetics assay. Unpublished report
No. RRS-91-32 from Hazleton Microtest, York, United Kingdom. Submitted
to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United
Kingdom.
Tesh, J.M., Ross, F.W. & Tesh, S.A. (1977) 4A: Effects of oral
administration upon pregnancy in the rabbit. Unpublished report No.
77/WRL2/109 from Life Science Research, Stock, Essex, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975a) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the rat. Unpublished report No. BPAT75/17
from Wellcome Research Laboratories, Berkhamsted, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
Thomson, P.M. (1975b) Preliminary study of oral imidocarb dipropionate
to reveal its retention in the monkey. Unpublished report No.
BPAT75/18 from Wellcome Research Laboratories, Berkhamsted, United
Kingdom. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Wander, A. (1968a) Distribution and excretion of (14C) labelled HR
2073 after intravenous administration of a single dose (1 mg/kg) in
the mouse. Translation of unpublished report No. 94/2555. Submitted to
WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex, United Kingdom.
Wander, A. (1968b) Investigation of the activity in the urine, liver,
gall bladder and kidneys of the mouse after intravenous administration
of a single dose of HR 2073 C-14. Translation of unpublished report
No. 94/2554. Submitted to WHO by Mallinckrodt, Harefield, Uxbridge,
Middlesex, United Kingdom.
Whitaker, A.M. & Bonhoff, A.J. (1983) Cytogenetic study of the effect
of imidocarb dipropionate (3 ',3 '-di(2-imidazolin-2-yl)carbanilide
dipropionate) on peripheral human lymphocytes in vitro. Unpublished
report No. BNCD/83/7 from Wellcome Biotechnology Ltd, United Kingdom.
Submitted to WHO by Mallinckrodt, Harefield, Uxbridge, Middlesex,
United Kingdom.
