
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 41
Prepared by:
The 50th meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
NICARBAZIN
First draft prepared by
Dr G. Roberts
Chemical Products Assessment Section
Commonwealth Department of Health and Family Services
Canberra, Australia
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.2 Toxicological studies
2.2.1 Acute toxicity
2.2.2 Short-term toxicity
2.2.3 Long-term toxicity and carcinogenicity
2.2.4 Genotoxicity
2.2.5 Reproductive toxicity
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Nicarbazin has been used in starter rations for several decades
as an aid in the prevention of faecal and intestinal coccidiosis in
broiler chickens. It may be used in combination with ionophore
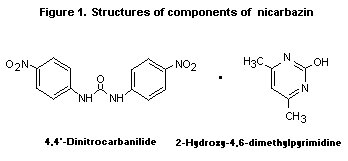
coccidiostatics. Chemically, it is an equimolar complex of
1,3- N,N'-bis(4-nitrophenyl)urea and 4,6-dimethyl-2(1 H)-pyrimidone.
These compounds are also known as 4,4 '-dinitrocarbanilide and
2-hydroxy-4,6-dimethylpyrimidine, respectively (see Figure 1).
Nicarbazin is described as an electron donor-acceptor molecular
complex; the sites of the interaction are the electron-poor NH amide
groups of the acceptor phenylurea and the electron-rich lone pairs of
the nitrogen in the pyrimidone donor ring.
Nicarbazin has not previously been evaluated by the Committee.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
Very little information was available on the absorption,
distribution, biotransformation, and excretion of nicarbazin in
laboratory animals. The available data were presented with few or no
methodological details, and the results were given in summary form
only, providing no opportunity for independent confirmation of the
conclusions.
2.1.1 Absorption, distribution, and excretion
Rats received single oral doses of 1, 5, or 10 mg/kg bw
nicarbazin; one animal at each dose was killed 6 or 18 h after
treatment, and the blood concentrations of the phenylurea and
pyrimidone components were determined. Low concentrations of the
phenylurea component were detected at 6 h, but it was not detected at
18 h. The pyrimidone component was found at considerably higher
concentrations, which increased between 6 and 18 h. Qualitatively
similar findings were obtained in rats given oral doses of 0.1, 1, or
5 mg/kg bw per day for eight days and killed 4 or 24 h after the last
dose. The blood concentrations of the pyrimidone component were
dose-related, while those of the phenylurea component showed a flat
response. In the latter experiment, urine collected for 5 h after the
last dose contained dose-related concentrations of each component of
nicarbazin, although the concentrations of the pyrimidone were an
order of magnitude higher than those of the phenylurea component
(Kuna, 1955).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies on the short-term toxicity of nicarbazin
and its components are shown in Table 1.
2.2.2 Short-term toxicity
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used, limited data on toxicological
findings, and were often in the form of progress reports. The
summaries reported kidney damage in the form of crystalline deposits
in the collecting tubules in rats at oral doses of 500 mg/kg bw per
day and more. In dogs, bile-duct proliferation was the principal
finding after an oral dose of 1600 mg/kg bw per day (Kuna, 1955).
Table 1. Acute toxicity of nicarbazin, 4,4'-dinitrocarbanilide
(DNC) and 2-hydroxy-4,6-dimethylpyrimidine (HDP) after oral
administration
Species Sex Substance LD50
(mg/kg bw)
Mouse Unspecified Nicarbazin > 25 000
HDP approx. 4 000
DNC > 18 000
Rat Unspecified Nicarbazin > 10 000
From Kuna (1955)
Dogs
Groups of five male and five female beagle dogs were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) in a ratio of 3:1 on six days per week for two years. The
actual intakes were 0, 60, 180, or 600 mg/kg bw per day of the
phenylurea component and 0, 20, 60, or 200 mg/kg bw per day of the
pyrimidone component. Two animals of each sex per group were killed
after one year. Clinical observations were made daily, and body
weight, food consumption, and reflexes were determined weekly; water
intake and urinary output were measured monthly. Haematological,
clinical chemical, and urinary parameters were determined before
treatment and in months 3, 6, 12, 18, and 24. A wide range of tissues
from all dogs was examined grossly and microscopically. The study was
conducted before the development of guidelines for the conduct of
toxicological studies.
No abnormal behaviour or physical signs were seen; however, one
male at the intermediate dose died of unknown causes during week 44. A
green-to-yellowish hue was seen in the excreta of all treated dogs.
Body-weight gain, food intake, and haematological and urinary
parameters were unaffected by treatment. Serum alanine
aminotransferase activity was increased in several dogs at the highest
dose and in one dog at each of the lower doses, but in most cases the
effects were transitory. The highest values were observed at about 12
months, and elevated activity persisted in only two animals at the
high dose. Organ weights and gross pathological appearance revealed no
treatment-related changes. The histopathological appearance was
unremarkable, apart from slight bile-duct proliferation in one dog
killed after one year of treatment with the high dose. This animal had
been found to have elevated serum alanine aminotransferase activity.
Although the relationship between the hepatic findings and treatment
was unclear, the conservative NOEL in this study is 240 mg/kg bw day
(Vogin, 1969a).
2.2.3 Long-term toxicity and carcinogenicity
Rats
Groups of FDRL rats were fed diets containing the phenylurea and
the pyrimidone components (purity unspecified) for two years at
concentrations calculated to give doses of 0, 50, 150, or 300 mg/kg bw
per day of the phenylurea component and 0, 17, 50, or 100 mg/kg bw per
day of the pyrimidone component. The groups consisted of 50 males and
50 females for control and the high doses and 40 males and 40 females
for the low and intermediate doses. Five rats of each sex from the
control and high-dose groups were killed during months 6 and 18, and
10 rats of each sex per group were killed in week 56. The animals were
observed daily for behaviour, physical appearance, and survival. Food
consumption and the efficiency of food use were assessed weekly for
the first 12 weeks and then periodically on 15 animals of each sex per
group. Body weight was recorded weekly for the first 12 weeks, then
biweekly until week 26 and monthly thereafter. Water intake and
urinary output were measured on 10 rats of each sex per group during
one week per month for the first three months. Limited haematological,
clinical chemical and urinary parameters were examined on 10 rats of
each sex per group at 3, 6, 9, 12, 18, and 24 months. A wide range of
tissues from all rats was examined grossly and microscopically. The
study was conducted before the development of guidelines for the
conduct of toxicological studies.
No abnormal behaviour was noted, and mortality was unaffected by
treatment. Food and water intake and body-weight gain were similar in
all groups. There were no treatment-related effects on haemoglobin,
haematocrit, leukocytes, blood urea nitrogen, serum alanine
aminotransferase activity, blood glucose, urinary parameters, organ
weights, or gross pathological appearance. Changes in the kidney, such
as calcareous material in the tubules, calcification in the renal
pelvis, tubules, glomeruli, or medulla, and calculi, were more
frequent in treated groups at 56 weeks, but the overall incidence was
similar in all groups at the end of the study. The incidence of
testicular atrophy was slightly elevated in some treated males at 104
weeks, but significance was not attained. Tumour incidences were
unaffected. The NOEL was 400 mg/kg bw per day of the 3:1 mixture
(Vogin, 1969b).
2.2.4 Genotoxicity
The results of assays for the genotoxicity of nicarbazine and its
components are shown in Table 2.
Table 2. Results of assays for genotoxicity with nicarbazine, 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine (HDP)
End-point Test object Substance Concentration S9 Results Reference
(µg/plate)
Reverse S. typhimurium Nicarbazine 100-500a +/- Negative Bradley &
mutation TA98, TA100, DNC 100-300a +/- Negative Cook (1980)
TA1535, TA1537 HDP 200-2000 +/- Negative
Reverse S. typhimurium Nicarbazine 0-10 000 +/- Weakly Ohta et al.
mutation TA98, TA1538 positive (1980)
TA100, TA1535, NR +/- Negative
TA1537
E coli WP2 hcr trp NR +/- Negative
DNA H17Rec+ Nicarbazine NR +/- Negative Ohta et al.
damage M45Rec- (1980)
S9, 9000 × g fraction of rat liver; NR, not reported
a Higher concentrations precipitated.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 12 male and 12 female FDRL rats were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) at concentrations calculated to achieve doses of 0, 50,
150, or 300 mg/kg bw per day of the phenylurea and 0, 17, 50, or 100
mg/kg bw per day of the pyrimidone. Treatment was administered
continuously during the production of two litters per generation for
three successive generations. The initial groups of animals were
paired 10 weeks after the start of treatment to produce the F1a
litter and were paired again seven days after weaning of the first
litter to produce the F1b litter. At four weeks of age, 12 male and
12 female F1b offspring were mated according to the above schedule
to produce the F2a and F2b litters, and the F2b offspring were
used to produce the F3a and F3b litters. The animals were examined
daily for survival, behaviour, and appearance. Body weight and food
consumption were measured weekly in adults. After birth, each litter
was limited to eight pups, which were weighed at birth and on
postnatal days 4, 12, and 21. After the offspring had been weaned, the
adult rats were autopsied, and the testes of F2 males in the control
and high-dose groups were examined histopathologically. The liver,
kidneys, heart, urinary bladder, and gonads from five male and five
female F3b weaned pups from each group were also examined
microscopically. The study was performed before the development of
guidelines for the conduct of toxicological studies.
Adult rats of each generation showed no effects on survival,
body-weight gain, or food intake, and the results of gross autopsy and
testicular examinations were unremarkable. The pregnancy rates and
duration of gestation were unaffected. The body-weight gain of F1b
pups at the high dose was slightly depressed during lactation, but a
similar effect was not found in any other litter. In subsequent
generations, the F2a and F3a litters at the high dose had slightly
fewer pups, but the effect was not reproduced in the F2b or F3b
litters. Histopathological examination of limited organs from F3b
pups revealed no abnormalities attributable to treatment, and it was
considered that there were no significant effects on reproduction. The
NOEL was the highest dose tested, 400 mg/kg bw per day of the 3:1
mixture (Kirschner & Vogin, 1970).
(ii) Developmental toxicity
Rats
Groups of 24-25 CD/CRJ pregnant rats were given nicarbazin
(equimolar complex; purity unspecified) as a suspension in 1%
carboxymethyl cellulose by gavage at doses of 0, 70, 200, or 600 mg/kg
bw per day on gestation days 7-17. Details of the method were not
given in the report, but the tabulated results indicate that food and
water intake and body weights were recorded daily on gestation days
7œ21. The times of sacrifice of dams and examination of fetuses were
not indicated. Fetuses were subjected to external, visceral, and
skeletal examinations. Since the protocol was not provided, it is not
known whether a recognized test guideline was followed or if quality
assurance was undertaken, and the adequacy of the study could not be
determined.
Seven rats at 600 mg/kg bw per day died, mostly during the
treatment period. The food intake and body-weight gain of dams at 600
mg/kg bw per day were depressed from gestation day 8. Implantations
and in-utero survival were similar in all groups, while fetal body
weight was lower at 600 mg/kg bw per day. The fetuses of the dams at
600 mg/kg bw per day had delayed ossification, hyperplastic and bent
ribs (four fetuses), sacralization of the 6th or 7th lumbar vertebrae
(two fetuses), cleft palate (two in same litter), subcutaneous oedema
(three in same litter), hydronephrosis (five fetuses), cryptorchismus
(one fetus), and remained Merkel's diverticulum (one fetus). The NOEL
for maternal and fetal toxicity was 200 mg/kg bw per day (Tajima,
1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, acute, short-term, and long-term toxicity,
genotoxicity, reproductive toxicity, and developmental toxicity of
nicarbazin. The studies were performed before the establishment of
guidelines for the conduct of toxicological studies. Some of the
reports were presented in sufficient detail for independent assessment
and were considered of acceptable quality. Additionally, an expert
report was available for consideration (Fitzpatrick, 1997).
After oral administration of nicarbazin to rats, low
concentrations of the phenylurea component and high concentrations of
the pyrimidone were found in blood. The urinary excretion of the
latter was considerably higher than that of the phenylurea. The
pyrimidone portion is therefore probably absorbed to a greater extent,
and most of the phenylurea was excreted in faeces without absorption.
No data were available on the metabolism of nicarbazin.
The acute oral toxicity of nicarbazin in rodents was low, the
LD50 values being > 25 000 mg/kg bw in mice and > 10 000 mg/kg bw
in rats. The individual components also had low acute toxicity, the
oral LD50 values in mice being 4000 mg/kg bw for the pyrimidone and
> 18 000 for the phenylurea component.
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used and limited data on
toxicological findings and were often in the form of progress reports.
The summaries reported kidney damage in the form of crystalline
deposits in the collecting tubules in rats at oral doses of 500 mg/kg
bw per day and more. In dogs, bile-duct proliferation was the
principal finding after an oral dose of 1600 mg/kg bw per day.
The highest dose chosen in the two-year study of toxicity and in
the study of reproductive toxicity in rats was 400 mg/kg bw per day.
This dose was selected because of the formation of acetylated
phenylurea, with resulting precipitation of crystals in the kidneys,
in rats given oral doses of 500 mg/kg bw per day. In some
toxicological studies, the test species were given phenylurea and
pyrimidone components in a ratio of 3:1, since it was claimed that
phenylurea and pyrimidone are present in that ratio in the muscle of
treated chickens. More recent data suggest a ratio as high as 8:1 for
the phenylurea to the pyrimidone residue. Dogs were fed diets
containing phenylurea and pyrimidone components in a ratio of 3:1 for
two years. The actual drug intakes were 0, 80, 240, or 800 mg/kg bw
per day. Serum alanine aminotransferase activity was increased in
several dogs, and slight bile-duct proliferation was observed in one
dog at 800 mg/kg bw per day. No other treatment-related effect was
observed. The NOEL was 240 mg/kg bw per day of the 3:1 mixture of
phenylurea and pyrimidone. In the two-year study in rats, doses of 0,
67, 200, or 400 mg/kg bw per day phenylurea and pyrimidone components
in a ratio of 3:1 were given in the diet. There was no
treatment-related toxicity, and tumour incidences were unaffected. The
NOEL was the highest dose, 400 mg/kg bw per day.
Nicarbazin slightly increased the mutation frequency in
Salmonella strains TA98 and TA1538 in one study but not in another.
Mutations were not detected in Salmonella strains TA100, TA1535, or
TA1537 or in E. coli WP2, and DNA damage was not induced in the rec
assay. No other end-points were investigated. The examination of
genotoxic potential was considered to be inadequate, since studies
were carried out only in bacteria.
A three-generation study of reproductive toxicity was conducted
in rats given a 3:1 ratio of phenylurea and pyrimidone components in
the diet at doses were 0, 67, 200, or 400 mg/kg bw per day. There were
isolated occurrences of slightly reduced litter size at birth or
depressed body-weight gain during lactation at the highest dose, but
these findings were not replicated in most litters and showed no
progression over the duration of the study. Therefore, the Committee
concluded that nicarbazin did not have significant effects on
reproduction. The NOEL was the highest dose tested, 400 mg/kg bw per
day.
Developmental toxicity was studied in rats given 0, 70, 200, or
600 mg/kg bw per day of nicarbazin, at an equimolar ratio of
phenylurea: pyrimidone, by gavage on gestation days 7-17. At 600 mg/kg
bw per day, maternal food intake and body weight were depressed, and
seven of 25 animals died. At this dose, the finding of lowered fetal
body weight and reduced ossification suggested retarded fetal
development, and a number of abnormalities were observed, in
particular hydronephrosis and hyperplastic and bent ribs. The NOEL was
200 mg/kg bw per day on the basis of maternal and fetal toxicity; no
teratogenic effects were observed.
4. EVALUATION
The Committee noted the absence of certain toxicological studies
in support of nicarbazin; however, the other data available provided
sufficient information to overcome most of these deficiencies. It was
noted that nicarbazin has been used in veterinary medicine in many
countries for over 40 years. On the basis of this long history of use
and the fact that use is restricted to starter rations in broiler
chickens, the Committee considered that an ADI could be supported.
The Committee established an ADI of 0-400 µg/kg bw on the basis
of the NOEL of 200 mg/kg bw per day in the study of developmental
toxicity in rats and using a safety factor of 500, chosen to account
for the limitations in the database.
5. REFERENCES
Bradley, M.O. & Cook, M.M. (1980) Nicarbazin: Microbial mutagen tests.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Fitzpatrick, S.C. (1997) Nicarbazin: Evaluation of available
toxicology data on nicarbazin: Rewrite format as outlined in WHO
Technical Report 832, Veterinary Drugs with a Long History of Use.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Kirschner, S.L. & Vogin, E.E. (1970) Multigeneration reproduction and
lactation studies with 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine dihydrate (HDP). Unpublished report
from Food and Drug Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Kuna, S. (1955) Tolerance studies in mammals. Unpublished report.
Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Ohta, T., Moriya, M., Kaneda, Y., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Tajima, M. (1979) Teratogenicity test of nicarbazin with rats by oral
administration. Unpublished report from Nisseiken (NIBS). Submitted to
WHO by Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969a) Two-year chronic toxicity studies with components
of nicarbazin in dogs. Unpublished report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969b) Chronic toxicity studies with nicarbazin
formulation in rats. Unpublished Report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, CA, USA.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
Very little information was available on the absorption,
distribution, biotransformation, and excretion of nicarbazin in
laboratory animals. The available data were presented with few or no
methodological details, and the results were given in summary form
only, providing no opportunity for independent confirmation of the
conclusions.
2.1.1 Absorption, distribution, and excretion
Rats received single oral doses of 1, 5, or 10 mg/kg bw
nicarbazin; one animal at each dose was killed 6 or 18 h after
treatment, and the blood concentrations of the phenylurea and
pyrimidone components were determined. Low concentrations of the
phenylurea component were detected at 6 h, but it was not detected at
18 h. The pyrimidone component was found at considerably higher
concentrations, which increased between 6 and 18 h. Qualitatively
similar findings were obtained in rats given oral doses of 0.1, 1, or
5 mg/kg bw per day for eight days and killed 4 or 24 h after the last
dose. The blood concentrations of the pyrimidone component were
dose-related, while those of the phenylurea component showed a flat
response. In the latter experiment, urine collected for 5 h after the
last dose contained dose-related concentrations of each component of
nicarbazin, although the concentrations of the pyrimidone were an
order of magnitude higher than those of the phenylurea component
(Kuna, 1955).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies on the short-term toxicity of nicarbazin
and its components are shown in Table 1.
2.2.2 Short-term toxicity
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used, limited data on toxicological
findings, and were often in the form of progress reports. The
summaries reported kidney damage in the form of crystalline deposits
in the collecting tubules in rats at oral doses of 500 mg/kg bw per
day and more. In dogs, bile-duct proliferation was the principal
finding after an oral dose of 1600 mg/kg bw per day (Kuna, 1955).
Table 1. Acute toxicity of nicarbazin, 4,4'-dinitrocarbanilide
(DNC) and 2-hydroxy-4,6-dimethylpyrimidine (HDP) after oral
administration
Species Sex Substance LD50
(mg/kg bw)
Mouse Unspecified Nicarbazin > 25 000
HDP approx. 4 000
DNC > 18 000
Rat Unspecified Nicarbazin > 10 000
From Kuna (1955)
Dogs
Groups of five male and five female beagle dogs were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) in a ratio of 3:1 on six days per week for two years. The
actual intakes were 0, 60, 180, or 600 mg/kg bw per day of the
phenylurea component and 0, 20, 60, or 200 mg/kg bw per day of the
pyrimidone component. Two animals of each sex per group were killed
after one year. Clinical observations were made daily, and body
weight, food consumption, and reflexes were determined weekly; water
intake and urinary output were measured monthly. Haematological,
clinical chemical, and urinary parameters were determined before
treatment and in months 3, 6, 12, 18, and 24. A wide range of tissues
from all dogs was examined grossly and microscopically. The study was
conducted before the development of guidelines for the conduct of
toxicological studies.
No abnormal behaviour or physical signs were seen; however, one
male at the intermediate dose died of unknown causes during week 44. A
green-to-yellowish hue was seen in the excreta of all treated dogs.
Body-weight gain, food intake, and haematological and urinary
parameters were unaffected by treatment. Serum alanine
aminotransferase activity was increased in several dogs at the highest
dose and in one dog at each of the lower doses, but in most cases the
effects were transitory. The highest values were observed at about 12
months, and elevated activity persisted in only two animals at the
high dose. Organ weights and gross pathological appearance revealed no
treatment-related changes. The histopathological appearance was
unremarkable, apart from slight bile-duct proliferation in one dog
killed after one year of treatment with the high dose. This animal had
been found to have elevated serum alanine aminotransferase activity.
Although the relationship between the hepatic findings and treatment
was unclear, the conservative NOEL in this study is 240 mg/kg bw day
(Vogin, 1969a).
2.2.3 Long-term toxicity and carcinogenicity
Rats
Groups of FDRL rats were fed diets containing the phenylurea and
the pyrimidone components (purity unspecified) for two years at
concentrations calculated to give doses of 0, 50, 150, or 300 mg/kg bw
per day of the phenylurea component and 0, 17, 50, or 100 mg/kg bw per
day of the pyrimidone component. The groups consisted of 50 males and
50 females for control and the high doses and 40 males and 40 females
for the low and intermediate doses. Five rats of each sex from the
control and high-dose groups were killed during months 6 and 18, and
10 rats of each sex per group were killed in week 56. The animals were
observed daily for behaviour, physical appearance, and survival. Food
consumption and the efficiency of food use were assessed weekly for
the first 12 weeks and then periodically on 15 animals of each sex per
group. Body weight was recorded weekly for the first 12 weeks, then
biweekly until week 26 and monthly thereafter. Water intake and
urinary output were measured on 10 rats of each sex per group during
one week per month for the first three months. Limited haematological,
clinical chemical and urinary parameters were examined on 10 rats of
each sex per group at 3, 6, 9, 12, 18, and 24 months. A wide range of
tissues from all rats was examined grossly and microscopically. The
study was conducted before the development of guidelines for the
conduct of toxicological studies.
No abnormal behaviour was noted, and mortality was unaffected by
treatment. Food and water intake and body-weight gain were similar in
all groups. There were no treatment-related effects on haemoglobin,
haematocrit, leukocytes, blood urea nitrogen, serum alanine
aminotransferase activity, blood glucose, urinary parameters, organ
weights, or gross pathological appearance. Changes in the kidney, such
as calcareous material in the tubules, calcification in the renal
pelvis, tubules, glomeruli, or medulla, and calculi, were more
frequent in treated groups at 56 weeks, but the overall incidence was
similar in all groups at the end of the study. The incidence of
testicular atrophy was slightly elevated in some treated males at 104
weeks, but significance was not attained. Tumour incidences were
unaffected. The NOEL was 400 mg/kg bw per day of the 3:1 mixture
(Vogin, 1969b).
2.2.4 Genotoxicity
The results of assays for the genotoxicity of nicarbazine and its
components are shown in Table 2.
Table 2. Results of assays for genotoxicity with nicarbazine, 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine (HDP)
End-point Test object Substance Concentration S9 Results Reference
(µg/plate)
Reverse S. typhimurium Nicarbazine 100-500a +/- Negative Bradley &
mutation TA98, TA100, DNC 100-300a +/- Negative Cook (1980)
TA1535, TA1537 HDP 200-2000 +/- Negative
Reverse S. typhimurium Nicarbazine 0-10 000 +/- Weakly Ohta et al.
mutation TA98, TA1538 positive (1980)
TA100, TA1535, NR +/- Negative
TA1537
E coli WP2 hcr trp NR +/- Negative
DNA H17Rec+ Nicarbazine NR +/- Negative Ohta et al.
damage M45Rec- (1980)
S9, 9000 × g fraction of rat liver; NR, not reported
a Higher concentrations precipitated.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 12 male and 12 female FDRL rats were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) at concentrations calculated to achieve doses of 0, 50,
150, or 300 mg/kg bw per day of the phenylurea and 0, 17, 50, or 100
mg/kg bw per day of the pyrimidone. Treatment was administered
continuously during the production of two litters per generation for
three successive generations. The initial groups of animals were
paired 10 weeks after the start of treatment to produce the F1a
litter and were paired again seven days after weaning of the first
litter to produce the F1b litter. At four weeks of age, 12 male and
12 female F1b offspring were mated according to the above schedule
to produce the F2a and F2b litters, and the F2b offspring were
used to produce the F3a and F3b litters. The animals were examined
daily for survival, behaviour, and appearance. Body weight and food
consumption were measured weekly in adults. After birth, each litter
was limited to eight pups, which were weighed at birth and on
postnatal days 4, 12, and 21. After the offspring had been weaned, the
adult rats were autopsied, and the testes of F2 males in the control
and high-dose groups were examined histopathologically. The liver,
kidneys, heart, urinary bladder, and gonads from five male and five
female F3b weaned pups from each group were also examined
microscopically. The study was performed before the development of
guidelines for the conduct of toxicological studies.
Adult rats of each generation showed no effects on survival,
body-weight gain, or food intake, and the results of gross autopsy and
testicular examinations were unremarkable. The pregnancy rates and
duration of gestation were unaffected. The body-weight gain of F1b
pups at the high dose was slightly depressed during lactation, but a
similar effect was not found in any other litter. In subsequent
generations, the F2a and F3a litters at the high dose had slightly
fewer pups, but the effect was not reproduced in the F2b or F3b
litters. Histopathological examination of limited organs from F3b
pups revealed no abnormalities attributable to treatment, and it was
considered that there were no significant effects on reproduction. The
NOEL was the highest dose tested, 400 mg/kg bw per day of the 3:1
mixture (Kirschner & Vogin, 1970).
(ii) Developmental toxicity
Rats
Groups of 24-25 CD/CRJ pregnant rats were given nicarbazin
(equimolar complex; purity unspecified) as a suspension in 1%
carboxymethyl cellulose by gavage at doses of 0, 70, 200, or 600 mg/kg
bw per day on gestation days 7-17. Details of the method were not
given in the report, but the tabulated results indicate that food and
water intake and body weights were recorded daily on gestation days
7œ21. The times of sacrifice of dams and examination of fetuses were
not indicated. Fetuses were subjected to external, visceral, and
skeletal examinations. Since the protocol was not provided, it is not
known whether a recognized test guideline was followed or if quality
assurance was undertaken, and the adequacy of the study could not be
determined.
Seven rats at 600 mg/kg bw per day died, mostly during the
treatment period. The food intake and body-weight gain of dams at 600
mg/kg bw per day were depressed from gestation day 8. Implantations
and in-utero survival were similar in all groups, while fetal body
weight was lower at 600 mg/kg bw per day. The fetuses of the dams at
600 mg/kg bw per day had delayed ossification, hyperplastic and bent
ribs (four fetuses), sacralization of the 6th or 7th lumbar vertebrae
(two fetuses), cleft palate (two in same litter), subcutaneous oedema
(three in same litter), hydronephrosis (five fetuses), cryptorchismus
(one fetus), and remained Merkel's diverticulum (one fetus). The NOEL
for maternal and fetal toxicity was 200 mg/kg bw per day (Tajima,
1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, acute, short-term, and long-term toxicity,
genotoxicity, reproductive toxicity, and developmental toxicity of
nicarbazin. The studies were performed before the establishment of
guidelines for the conduct of toxicological studies. Some of the
reports were presented in sufficient detail for independent assessment
and were considered of acceptable quality. Additionally, an expert
report was available for consideration (Fitzpatrick, 1997).
After oral administration of nicarbazin to rats, low
concentrations of the phenylurea component and high concentrations of
the pyrimidone were found in blood. The urinary excretion of the
latter was considerably higher than that of the phenylurea. The
pyrimidone portion is therefore probably absorbed to a greater extent,
and most of the phenylurea was excreted in faeces without absorption.
No data were available on the metabolism of nicarbazin.
The acute oral toxicity of nicarbazin in rodents was low, the
LD50 values being > 25 000 mg/kg bw in mice and > 10 000 mg/kg bw
in rats. The individual components also had low acute toxicity, the
oral LD50 values in mice being 4000 mg/kg bw for the pyrimidone and
> 18 000 for the phenylurea component.
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used and limited data on
toxicological findings and were often in the form of progress reports.
The summaries reported kidney damage in the form of crystalline
deposits in the collecting tubules in rats at oral doses of 500 mg/kg
bw per day and more. In dogs, bile-duct proliferation was the
principal finding after an oral dose of 1600 mg/kg bw per day.
The highest dose chosen in the two-year study of toxicity and in
the study of reproductive toxicity in rats was 400 mg/kg bw per day.
This dose was selected because of the formation of acetylated
phenylurea, with resulting precipitation of crystals in the kidneys,
in rats given oral doses of 500 mg/kg bw per day. In some
toxicological studies, the test species were given phenylurea and
pyrimidone components in a ratio of 3:1, since it was claimed that
phenylurea and pyrimidone are present in that ratio in the muscle of
treated chickens. More recent data suggest a ratio as high as 8:1 for
the phenylurea to the pyrimidone residue. Dogs were fed diets
containing phenylurea and pyrimidone components in a ratio of 3:1 for
two years. The actual drug intakes were 0, 80, 240, or 800 mg/kg bw
per day. Serum alanine aminotransferase activity was increased in
several dogs, and slight bile-duct proliferation was observed in one
dog at 800 mg/kg bw per day. No other treatment-related effect was
observed. The NOEL was 240 mg/kg bw per day of the 3:1 mixture of
phenylurea and pyrimidone. In the two-year study in rats, doses of 0,
67, 200, or 400 mg/kg bw per day phenylurea and pyrimidone components
in a ratio of 3:1 were given in the diet. There was no
treatment-related toxicity, and tumour incidences were unaffected. The
NOEL was the highest dose, 400 mg/kg bw per day.
Nicarbazin slightly increased the mutation frequency in
Salmonella strains TA98 and TA1538 in one study but not in another.
Mutations were not detected in Salmonella strains TA100, TA1535, or
TA1537 or in E. coli WP2, and DNA damage was not induced in the rec
assay. No other end-points were investigated. The examination of
genotoxic potential was considered to be inadequate, since studies
were carried out only in bacteria.
A three-generation study of reproductive toxicity was conducted
in rats given a 3:1 ratio of phenylurea and pyrimidone components in
the diet at doses were 0, 67, 200, or 400 mg/kg bw per day. There were
isolated occurrences of slightly reduced litter size at birth or
depressed body-weight gain during lactation at the highest dose, but
these findings were not replicated in most litters and showed no
progression over the duration of the study. Therefore, the Committee
concluded that nicarbazin did not have significant effects on
reproduction. The NOEL was the highest dose tested, 400 mg/kg bw per
day.
Developmental toxicity was studied in rats given 0, 70, 200, or
600 mg/kg bw per day of nicarbazin, at an equimolar ratio of
phenylurea: pyrimidone, by gavage on gestation days 7-17. At 600 mg/kg
bw per day, maternal food intake and body weight were depressed, and
seven of 25 animals died. At this dose, the finding of lowered fetal
body weight and reduced ossification suggested retarded fetal
development, and a number of abnormalities were observed, in
particular hydronephrosis and hyperplastic and bent ribs. The NOEL was
200 mg/kg bw per day on the basis of maternal and fetal toxicity; no
teratogenic effects were observed.
4. EVALUATION
The Committee noted the absence of certain toxicological studies
in support of nicarbazin; however, the other data available provided
sufficient information to overcome most of these deficiencies. It was
noted that nicarbazin has been used in veterinary medicine in many
countries for over 40 years. On the basis of this long history of use
and the fact that use is restricted to starter rations in broiler
chickens, the Committee considered that an ADI could be supported.
The Committee established an ADI of 0-400 µg/kg bw on the basis
of the NOEL of 200 mg/kg bw per day in the study of developmental
toxicity in rats and using a safety factor of 500, chosen to account
for the limitations in the database.
5. REFERENCES
Bradley, M.O. & Cook, M.M. (1980) Nicarbazin: Microbial mutagen tests.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Fitzpatrick, S.C. (1997) Nicarbazin: Evaluation of available
toxicology data on nicarbazin: Rewrite format as outlined in WHO
Technical Report 832, Veterinary Drugs with a Long History of Use.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Kirschner, S.L. & Vogin, E.E. (1970) Multigeneration reproduction and
lactation studies with 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine dihydrate (HDP). Unpublished report
from Food and Drug Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Kuna, S. (1955) Tolerance studies in mammals. Unpublished report.
Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Ohta, T., Moriya, M., Kaneda, Y., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Tajima, M. (1979) Teratogenicity test of nicarbazin with rats by oral
administration. Unpublished report from Nisseiken (NIBS). Submitted to
WHO by Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969a) Two-year chronic toxicity studies with components
of nicarbazin in dogs. Unpublished report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969b) Chronic toxicity studies with nicarbazin
formulation in rats. Unpublished Report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, CA, USA.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
Very little information was available on the absorption,
distribution, biotransformation, and excretion of nicarbazin in
laboratory animals. The available data were presented with few or no
methodological details, and the results were given in summary form
only, providing no opportunity for independent confirmation of the
conclusions.
2.1.1 Absorption, distribution, and excretion
Rats received single oral doses of 1, 5, or 10 mg/kg bw
nicarbazin; one animal at each dose was killed 6 or 18 h after
treatment, and the blood concentrations of the phenylurea and
pyrimidone components were determined. Low concentrations of the
phenylurea component were detected at 6 h, but it was not detected at
18 h. The pyrimidone component was found at considerably higher
concentrations, which increased between 6 and 18 h. Qualitatively
similar findings were obtained in rats given oral doses of 0.1, 1, or
5 mg/kg bw per day for eight days and killed 4 or 24 h after the last
dose. The blood concentrations of the pyrimidone component were
dose-related, while those of the phenylurea component showed a flat
response. In the latter experiment, urine collected for 5 h after the
last dose contained dose-related concentrations of each component of
nicarbazin, although the concentrations of the pyrimidone were an
order of magnitude higher than those of the phenylurea component
(Kuna, 1955).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies on the short-term toxicity of nicarbazin
and its components are shown in Table 1.
2.2.2 Short-term toxicity
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used, limited data on toxicological
findings, and were often in the form of progress reports. The
summaries reported kidney damage in the form of crystalline deposits
in the collecting tubules in rats at oral doses of 500 mg/kg bw per
day and more. In dogs, bile-duct proliferation was the principal
finding after an oral dose of 1600 mg/kg bw per day (Kuna, 1955).
Table 1. Acute toxicity of nicarbazin, 4,4'-dinitrocarbanilide
(DNC) and 2-hydroxy-4,6-dimethylpyrimidine (HDP) after oral
administration
Species Sex Substance LD50
(mg/kg bw)
Mouse Unspecified Nicarbazin > 25 000
HDP approx. 4 000
DNC > 18 000
Rat Unspecified Nicarbazin > 10 000
From Kuna (1955)
Dogs
Groups of five male and five female beagle dogs were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) in a ratio of 3:1 on six days per week for two years. The
actual intakes were 0, 60, 180, or 600 mg/kg bw per day of the
phenylurea component and 0, 20, 60, or 200 mg/kg bw per day of the
pyrimidone component. Two animals of each sex per group were killed
after one year. Clinical observations were made daily, and body
weight, food consumption, and reflexes were determined weekly; water
intake and urinary output were measured monthly. Haematological,
clinical chemical, and urinary parameters were determined before
treatment and in months 3, 6, 12, 18, and 24. A wide range of tissues
from all dogs was examined grossly and microscopically. The study was
conducted before the development of guidelines for the conduct of
toxicological studies.
No abnormal behaviour or physical signs were seen; however, one
male at the intermediate dose died of unknown causes during week 44. A
green-to-yellowish hue was seen in the excreta of all treated dogs.
Body-weight gain, food intake, and haematological and urinary
parameters were unaffected by treatment. Serum alanine
aminotransferase activity was increased in several dogs at the highest
dose and in one dog at each of the lower doses, but in most cases the
effects were transitory. The highest values were observed at about 12
months, and elevated activity persisted in only two animals at the
high dose. Organ weights and gross pathological appearance revealed no
treatment-related changes. The histopathological appearance was
unremarkable, apart from slight bile-duct proliferation in one dog
killed after one year of treatment with the high dose. This animal had
been found to have elevated serum alanine aminotransferase activity.
Although the relationship between the hepatic findings and treatment
was unclear, the conservative NOEL in this study is 240 mg/kg bw day
(Vogin, 1969a).
2.2.3 Long-term toxicity and carcinogenicity
Rats
Groups of FDRL rats were fed diets containing the phenylurea and
the pyrimidone components (purity unspecified) for two years at
concentrations calculated to give doses of 0, 50, 150, or 300 mg/kg bw
per day of the phenylurea component and 0, 17, 50, or 100 mg/kg bw per
day of the pyrimidone component. The groups consisted of 50 males and
50 females for control and the high doses and 40 males and 40 females
for the low and intermediate doses. Five rats of each sex from the
control and high-dose groups were killed during months 6 and 18, and
10 rats of each sex per group were killed in week 56. The animals were
observed daily for behaviour, physical appearance, and survival. Food
consumption and the efficiency of food use were assessed weekly for
the first 12 weeks and then periodically on 15 animals of each sex per
group. Body weight was recorded weekly for the first 12 weeks, then
biweekly until week 26 and monthly thereafter. Water intake and
urinary output were measured on 10 rats of each sex per group during
one week per month for the first three months. Limited haematological,
clinical chemical and urinary parameters were examined on 10 rats of
each sex per group at 3, 6, 9, 12, 18, and 24 months. A wide range of
tissues from all rats was examined grossly and microscopically. The
study was conducted before the development of guidelines for the
conduct of toxicological studies.
No abnormal behaviour was noted, and mortality was unaffected by
treatment. Food and water intake and body-weight gain were similar in
all groups. There were no treatment-related effects on haemoglobin,
haematocrit, leukocytes, blood urea nitrogen, serum alanine
aminotransferase activity, blood glucose, urinary parameters, organ
weights, or gross pathological appearance. Changes in the kidney, such
as calcareous material in the tubules, calcification in the renal
pelvis, tubules, glomeruli, or medulla, and calculi, were more
frequent in treated groups at 56 weeks, but the overall incidence was
similar in all groups at the end of the study. The incidence of
testicular atrophy was slightly elevated in some treated males at 104
weeks, but significance was not attained. Tumour incidences were
unaffected. The NOEL was 400 mg/kg bw per day of the 3:1 mixture
(Vogin, 1969b).
2.2.4 Genotoxicity
The results of assays for the genotoxicity of nicarbazine and its
components are shown in Table 2.
Table 2. Results of assays for genotoxicity with nicarbazine, 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine (HDP)
End-point Test object Substance Concentration S9 Results Reference
(µg/plate)
Reverse S. typhimurium Nicarbazine 100-500a +/- Negative Bradley &
mutation TA98, TA100, DNC 100-300a +/- Negative Cook (1980)
TA1535, TA1537 HDP 200-2000 +/- Negative
Reverse S. typhimurium Nicarbazine 0-10 000 +/- Weakly Ohta et al.
mutation TA98, TA1538 positive (1980)
TA100, TA1535, NR +/- Negative
TA1537
E coli WP2 hcr trp NR +/- Negative
DNA H17Rec+ Nicarbazine NR +/- Negative Ohta et al.
damage M45Rec- (1980)
S9, 9000 × g fraction of rat liver; NR, not reported
a Higher concentrations precipitated.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 12 male and 12 female FDRL rats were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) at concentrations calculated to achieve doses of 0, 50,
150, or 300 mg/kg bw per day of the phenylurea and 0, 17, 50, or 100
mg/kg bw per day of the pyrimidone. Treatment was administered
continuously during the production of two litters per generation for
three successive generations. The initial groups of animals were
paired 10 weeks after the start of treatment to produce the F1a
litter and were paired again seven days after weaning of the first
litter to produce the F1b litter. At four weeks of age, 12 male and
12 female F1b offspring were mated according to the above schedule
to produce the F2a and F2b litters, and the F2b offspring were
used to produce the F3a and F3b litters. The animals were examined
daily for survival, behaviour, and appearance. Body weight and food
consumption were measured weekly in adults. After birth, each litter
was limited to eight pups, which were weighed at birth and on
postnatal days 4, 12, and 21. After the offspring had been weaned, the
adult rats were autopsied, and the testes of F2 males in the control
and high-dose groups were examined histopathologically. The liver,
kidneys, heart, urinary bladder, and gonads from five male and five
female F3b weaned pups from each group were also examined
microscopically. The study was performed before the development of
guidelines for the conduct of toxicological studies.
Adult rats of each generation showed no effects on survival,
body-weight gain, or food intake, and the results of gross autopsy and
testicular examinations were unremarkable. The pregnancy rates and
duration of gestation were unaffected. The body-weight gain of F1b
pups at the high dose was slightly depressed during lactation, but a
similar effect was not found in any other litter. In subsequent
generations, the F2a and F3a litters at the high dose had slightly
fewer pups, but the effect was not reproduced in the F2b or F3b
litters. Histopathological examination of limited organs from F3b
pups revealed no abnormalities attributable to treatment, and it was
considered that there were no significant effects on reproduction. The
NOEL was the highest dose tested, 400 mg/kg bw per day of the 3:1
mixture (Kirschner & Vogin, 1970).
(ii) Developmental toxicity
Rats
Groups of 24-25 CD/CRJ pregnant rats were given nicarbazin
(equimolar complex; purity unspecified) as a suspension in 1%
carboxymethyl cellulose by gavage at doses of 0, 70, 200, or 600 mg/kg
bw per day on gestation days 7-17. Details of the method were not
given in the report, but the tabulated results indicate that food and
water intake and body weights were recorded daily on gestation days
7œ21. The times of sacrifice of dams and examination of fetuses were
not indicated. Fetuses were subjected to external, visceral, and
skeletal examinations. Since the protocol was not provided, it is not
known whether a recognized test guideline was followed or if quality
assurance was undertaken, and the adequacy of the study could not be
determined.
Seven rats at 600 mg/kg bw per day died, mostly during the
treatment period. The food intake and body-weight gain of dams at 600
mg/kg bw per day were depressed from gestation day 8. Implantations
and in-utero survival were similar in all groups, while fetal body
weight was lower at 600 mg/kg bw per day. The fetuses of the dams at
600 mg/kg bw per day had delayed ossification, hyperplastic and bent
ribs (four fetuses), sacralization of the 6th or 7th lumbar vertebrae
(two fetuses), cleft palate (two in same litter), subcutaneous oedema
(three in same litter), hydronephrosis (five fetuses), cryptorchismus
(one fetus), and remained Merkel's diverticulum (one fetus). The NOEL
for maternal and fetal toxicity was 200 mg/kg bw per day (Tajima,
1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, acute, short-term, and long-term toxicity,
genotoxicity, reproductive toxicity, and developmental toxicity of
nicarbazin. The studies were performed before the establishment of
guidelines for the conduct of toxicological studies. Some of the
reports were presented in sufficient detail for independent assessment
and were considered of acceptable quality. Additionally, an expert
report was available for consideration (Fitzpatrick, 1997).
After oral administration of nicarbazin to rats, low
concentrations of the phenylurea component and high concentrations of
the pyrimidone were found in blood. The urinary excretion of the
latter was considerably higher than that of the phenylurea. The
pyrimidone portion is therefore probably absorbed to a greater extent,
and most of the phenylurea was excreted in faeces without absorption.
No data were available on the metabolism of nicarbazin.
The acute oral toxicity of nicarbazin in rodents was low, the
LD50 values being > 25 000 mg/kg bw in mice and > 10 000 mg/kg bw
in rats. The individual components also had low acute toxicity, the
oral LD50 values in mice being 4000 mg/kg bw for the pyrimidone and
> 18 000 for the phenylurea component.
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used and limited data on
toxicological findings and were often in the form of progress reports.
The summaries reported kidney damage in the form of crystalline
deposits in the collecting tubules in rats at oral doses of 500 mg/kg
bw per day and more. In dogs, bile-duct proliferation was the
principal finding after an oral dose of 1600 mg/kg bw per day.
The highest dose chosen in the two-year study of toxicity and in
the study of reproductive toxicity in rats was 400 mg/kg bw per day.
This dose was selected because of the formation of acetylated
phenylurea, with resulting precipitation of crystals in the kidneys,
in rats given oral doses of 500 mg/kg bw per day. In some
toxicological studies, the test species were given phenylurea and
pyrimidone components in a ratio of 3:1, since it was claimed that
phenylurea and pyrimidone are present in that ratio in the muscle of
treated chickens. More recent data suggest a ratio as high as 8:1 for
the phenylurea to the pyrimidone residue. Dogs were fed diets
containing phenylurea and pyrimidone components in a ratio of 3:1 for
two years. The actual drug intakes were 0, 80, 240, or 800 mg/kg bw
per day. Serum alanine aminotransferase activity was increased in
several dogs, and slight bile-duct proliferation was observed in one
dog at 800 mg/kg bw per day. No other treatment-related effect was
observed. The NOEL was 240 mg/kg bw per day of the 3:1 mixture of
phenylurea and pyrimidone. In the two-year study in rats, doses of 0,
67, 200, or 400 mg/kg bw per day phenylurea and pyrimidone components
in a ratio of 3:1 were given in the diet. There was no
treatment-related toxicity, and tumour incidences were unaffected. The
NOEL was the highest dose, 400 mg/kg bw per day.
Nicarbazin slightly increased the mutation frequency in
Salmonella strains TA98 and TA1538 in one study but not in another.
Mutations were not detected in Salmonella strains TA100, TA1535, or
TA1537 or in E. coli WP2, and DNA damage was not induced in the rec
assay. No other end-points were investigated. The examination of
genotoxic potential was considered to be inadequate, since studies
were carried out only in bacteria.
A three-generation study of reproductive toxicity was conducted
in rats given a 3:1 ratio of phenylurea and pyrimidone components in
the diet at doses were 0, 67, 200, or 400 mg/kg bw per day. There were
isolated occurrences of slightly reduced litter size at birth or
depressed body-weight gain during lactation at the highest dose, but
these findings were not replicated in most litters and showed no
progression over the duration of the study. Therefore, the Committee
concluded that nicarbazin did not have significant effects on
reproduction. The NOEL was the highest dose tested, 400 mg/kg bw per
day.
Developmental toxicity was studied in rats given 0, 70, 200, or
600 mg/kg bw per day of nicarbazin, at an equimolar ratio of
phenylurea: pyrimidone, by gavage on gestation days 7-17. At 600 mg/kg
bw per day, maternal food intake and body weight were depressed, and
seven of 25 animals died. At this dose, the finding of lowered fetal
body weight and reduced ossification suggested retarded fetal
development, and a number of abnormalities were observed, in
particular hydronephrosis and hyperplastic and bent ribs. The NOEL was
200 mg/kg bw per day on the basis of maternal and fetal toxicity; no
teratogenic effects were observed.
4. EVALUATION
The Committee noted the absence of certain toxicological studies
in support of nicarbazin; however, the other data available provided
sufficient information to overcome most of these deficiencies. It was
noted that nicarbazin has been used in veterinary medicine in many
countries for over 40 years. On the basis of this long history of use
and the fact that use is restricted to starter rations in broiler
chickens, the Committee considered that an ADI could be supported.
The Committee established an ADI of 0-400 µg/kg bw on the basis
of the NOEL of 200 mg/kg bw per day in the study of developmental
toxicity in rats and using a safety factor of 500, chosen to account
for the limitations in the database.
5. REFERENCES
Bradley, M.O. & Cook, M.M. (1980) Nicarbazin: Microbial mutagen tests.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Fitzpatrick, S.C. (1997) Nicarbazin: Evaluation of available
toxicology data on nicarbazin: Rewrite format as outlined in WHO
Technical Report 832, Veterinary Drugs with a Long History of Use.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Kirschner, S.L. & Vogin, E.E. (1970) Multigeneration reproduction and
lactation studies with 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine dihydrate (HDP). Unpublished report
from Food and Drug Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Kuna, S. (1955) Tolerance studies in mammals. Unpublished report.
Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Ohta, T., Moriya, M., Kaneda, Y., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Tajima, M. (1979) Teratogenicity test of nicarbazin with rats by oral
administration. Unpublished report from Nisseiken (NIBS). Submitted to
WHO by Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969a) Two-year chronic toxicity studies with components
of nicarbazin in dogs. Unpublished report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969b) Chronic toxicity studies with nicarbazin
formulation in rats. Unpublished Report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, CA, USA.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
Very little information was available on the absorption,
distribution, biotransformation, and excretion of nicarbazin in
laboratory animals. The available data were presented with few or no
methodological details, and the results were given in summary form
only, providing no opportunity for independent confirmation of the
conclusions.
2.1.1 Absorption, distribution, and excretion
Rats received single oral doses of 1, 5, or 10 mg/kg bw
nicarbazin; one animal at each dose was killed 6 or 18 h after
treatment, and the blood concentrations of the phenylurea and
pyrimidone components were determined. Low concentrations of the
phenylurea component were detected at 6 h, but it was not detected at
18 h. The pyrimidone component was found at considerably higher
concentrations, which increased between 6 and 18 h. Qualitatively
similar findings were obtained in rats given oral doses of 0.1, 1, or
5 mg/kg bw per day for eight days and killed 4 or 24 h after the last
dose. The blood concentrations of the pyrimidone component were
dose-related, while those of the phenylurea component showed a flat
response. In the latter experiment, urine collected for 5 h after the
last dose contained dose-related concentrations of each component of
nicarbazin, although the concentrations of the pyrimidone were an
order of magnitude higher than those of the phenylurea component
(Kuna, 1955).
2.2 Toxicological studies
2.2.1 Acute toxicity
The results of studies on the short-term toxicity of nicarbazin
and its components are shown in Table 1.
2.2.2 Short-term toxicity
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used, limited data on toxicological
findings, and were often in the form of progress reports. The
summaries reported kidney damage in the form of crystalline deposits
in the collecting tubules in rats at oral doses of 500 mg/kg bw per
day and more. In dogs, bile-duct proliferation was the principal
finding after an oral dose of 1600 mg/kg bw per day (Kuna, 1955).
Table 1. Acute toxicity of nicarbazin, 4,4'-dinitrocarbanilide
(DNC) and 2-hydroxy-4,6-dimethylpyrimidine (HDP) after oral
administration
Species Sex Substance LD50
(mg/kg bw)
Mouse Unspecified Nicarbazin > 25 000
HDP approx. 4 000
DNC > 18 000
Rat Unspecified Nicarbazin > 10 000
From Kuna (1955)
Dogs
Groups of five male and five female beagle dogs were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) in a ratio of 3:1 on six days per week for two years. The
actual intakes were 0, 60, 180, or 600 mg/kg bw per day of the
phenylurea component and 0, 20, 60, or 200 mg/kg bw per day of the
pyrimidone component. Two animals of each sex per group were killed
after one year. Clinical observations were made daily, and body
weight, food consumption, and reflexes were determined weekly; water
intake and urinary output were measured monthly. Haematological,
clinical chemical, and urinary parameters were determined before
treatment and in months 3, 6, 12, 18, and 24. A wide range of tissues
from all dogs was examined grossly and microscopically. The study was
conducted before the development of guidelines for the conduct of
toxicological studies.
No abnormal behaviour or physical signs were seen; however, one
male at the intermediate dose died of unknown causes during week 44. A
green-to-yellowish hue was seen in the excreta of all treated dogs.
Body-weight gain, food intake, and haematological and urinary
parameters were unaffected by treatment. Serum alanine
aminotransferase activity was increased in several dogs at the highest
dose and in one dog at each of the lower doses, but in most cases the
effects were transitory. The highest values were observed at about 12
months, and elevated activity persisted in only two animals at the
high dose. Organ weights and gross pathological appearance revealed no
treatment-related changes. The histopathological appearance was
unremarkable, apart from slight bile-duct proliferation in one dog
killed after one year of treatment with the high dose. This animal had
been found to have elevated serum alanine aminotransferase activity.
Although the relationship between the hepatic findings and treatment
was unclear, the conservative NOEL in this study is 240 mg/kg bw day
(Vogin, 1969a).
2.2.3 Long-term toxicity and carcinogenicity
Rats
Groups of FDRL rats were fed diets containing the phenylurea and
the pyrimidone components (purity unspecified) for two years at
concentrations calculated to give doses of 0, 50, 150, or 300 mg/kg bw
per day of the phenylurea component and 0, 17, 50, or 100 mg/kg bw per
day of the pyrimidone component. The groups consisted of 50 males and
50 females for control and the high doses and 40 males and 40 females
for the low and intermediate doses. Five rats of each sex from the
control and high-dose groups were killed during months 6 and 18, and
10 rats of each sex per group were killed in week 56. The animals were
observed daily for behaviour, physical appearance, and survival. Food
consumption and the efficiency of food use were assessed weekly for
the first 12 weeks and then periodically on 15 animals of each sex per
group. Body weight was recorded weekly for the first 12 weeks, then
biweekly until week 26 and monthly thereafter. Water intake and
urinary output were measured on 10 rats of each sex per group during
one week per month for the first three months. Limited haematological,
clinical chemical and urinary parameters were examined on 10 rats of
each sex per group at 3, 6, 9, 12, 18, and 24 months. A wide range of
tissues from all rats was examined grossly and microscopically. The
study was conducted before the development of guidelines for the
conduct of toxicological studies.
No abnormal behaviour was noted, and mortality was unaffected by
treatment. Food and water intake and body-weight gain were similar in
all groups. There were no treatment-related effects on haemoglobin,
haematocrit, leukocytes, blood urea nitrogen, serum alanine
aminotransferase activity, blood glucose, urinary parameters, organ
weights, or gross pathological appearance. Changes in the kidney, such
as calcareous material in the tubules, calcification in the renal
pelvis, tubules, glomeruli, or medulla, and calculi, were more
frequent in treated groups at 56 weeks, but the overall incidence was
similar in all groups at the end of the study. The incidence of
testicular atrophy was slightly elevated in some treated males at 104
weeks, but significance was not attained. Tumour incidences were
unaffected. The NOEL was 400 mg/kg bw per day of the 3:1 mixture
(Vogin, 1969b).
2.2.4 Genotoxicity
The results of assays for the genotoxicity of nicarbazine and its
components are shown in Table 2.
Table 2. Results of assays for genotoxicity with nicarbazine, 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine (HDP)
End-point Test object Substance Concentration S9 Results Reference
(µg/plate)
Reverse S. typhimurium Nicarbazine 100-500a +/- Negative Bradley &
mutation TA98, TA100, DNC 100-300a +/- Negative Cook (1980)
TA1535, TA1537 HDP 200-2000 +/- Negative
Reverse S. typhimurium Nicarbazine 0-10 000 +/- Weakly Ohta et al.
mutation TA98, TA1538 positive (1980)
TA100, TA1535, NR +/- Negative
TA1537
E coli WP2 hcr trp NR +/- Negative
DNA H17Rec+ Nicarbazine NR +/- Negative Ohta et al.
damage M45Rec- (1980)
S9, 9000 × g fraction of rat liver; NR, not reported
a Higher concentrations precipitated.
2.2.5 Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 12 male and 12 female FDRL rats were fed diets
containing the phenylurea and the pyrimidone components (purity
unspecified) at concentrations calculated to achieve doses of 0, 50,
150, or 300 mg/kg bw per day of the phenylurea and 0, 17, 50, or 100
mg/kg bw per day of the pyrimidone. Treatment was administered
continuously during the production of two litters per generation for
three successive generations. The initial groups of animals were
paired 10 weeks after the start of treatment to produce the F1a
litter and were paired again seven days after weaning of the first
litter to produce the F1b litter. At four weeks of age, 12 male and
12 female F1b offspring were mated according to the above schedule
to produce the F2a and F2b litters, and the F2b offspring were
used to produce the F3a and F3b litters. The animals were examined
daily for survival, behaviour, and appearance. Body weight and food
consumption were measured weekly in adults. After birth, each litter
was limited to eight pups, which were weighed at birth and on
postnatal days 4, 12, and 21. After the offspring had been weaned, the
adult rats were autopsied, and the testes of F2 males in the control
and high-dose groups were examined histopathologically. The liver,
kidneys, heart, urinary bladder, and gonads from five male and five
female F3b weaned pups from each group were also examined
microscopically. The study was performed before the development of
guidelines for the conduct of toxicological studies.
Adult rats of each generation showed no effects on survival,
body-weight gain, or food intake, and the results of gross autopsy and
testicular examinations were unremarkable. The pregnancy rates and
duration of gestation were unaffected. The body-weight gain of F1b
pups at the high dose was slightly depressed during lactation, but a
similar effect was not found in any other litter. In subsequent
generations, the F2a and F3a litters at the high dose had slightly
fewer pups, but the effect was not reproduced in the F2b or F3b
litters. Histopathological examination of limited organs from F3b
pups revealed no abnormalities attributable to treatment, and it was
considered that there were no significant effects on reproduction. The
NOEL was the highest dose tested, 400 mg/kg bw per day of the 3:1
mixture (Kirschner & Vogin, 1970).
(ii) Developmental toxicity
Rats
Groups of 24-25 CD/CRJ pregnant rats were given nicarbazin
(equimolar complex; purity unspecified) as a suspension in 1%
carboxymethyl cellulose by gavage at doses of 0, 70, 200, or 600 mg/kg
bw per day on gestation days 7-17. Details of the method were not
given in the report, but the tabulated results indicate that food and
water intake and body weights were recorded daily on gestation days
7œ21. The times of sacrifice of dams and examination of fetuses were
not indicated. Fetuses were subjected to external, visceral, and
skeletal examinations. Since the protocol was not provided, it is not
known whether a recognized test guideline was followed or if quality
assurance was undertaken, and the adequacy of the study could not be
determined.
Seven rats at 600 mg/kg bw per day died, mostly during the
treatment period. The food intake and body-weight gain of dams at 600
mg/kg bw per day were depressed from gestation day 8. Implantations
and in-utero survival were similar in all groups, while fetal body
weight was lower at 600 mg/kg bw per day. The fetuses of the dams at
600 mg/kg bw per day had delayed ossification, hyperplastic and bent
ribs (four fetuses), sacralization of the 6th or 7th lumbar vertebrae
(two fetuses), cleft palate (two in same litter), subcutaneous oedema
(three in same litter), hydronephrosis (five fetuses), cryptorchismus
(one fetus), and remained Merkel's diverticulum (one fetus). The NOEL
for maternal and fetal toxicity was 200 mg/kg bw per day (Tajima,
1979).
3. COMMENTS
The Committee considered the results of studies on the
toxicokinetics, acute, short-term, and long-term toxicity,
genotoxicity, reproductive toxicity, and developmental toxicity of
nicarbazin. The studies were performed before the establishment of
guidelines for the conduct of toxicological studies. Some of the
reports were presented in sufficient detail for independent assessment
and were considered of acceptable quality. Additionally, an expert
report was available for consideration (Fitzpatrick, 1997).
After oral administration of nicarbazin to rats, low
concentrations of the phenylurea component and high concentrations of
the pyrimidone were found in blood. The urinary excretion of the
latter was considerably higher than that of the phenylurea. The
pyrimidone portion is therefore probably absorbed to a greater extent,
and most of the phenylurea was excreted in faeces without absorption.
No data were available on the metabolism of nicarbazin.
The acute oral toxicity of nicarbazin in rodents was low, the
LD50 values being > 25 000 mg/kg bw in mice and > 10 000 mg/kg bw
in rats. The individual components also had low acute toxicity, the
oral LD50 values in mice being 4000 mg/kg bw for the pyrimidone and
> 18 000 for the phenylurea component.
A number of short-term studies of nicarbazin were available, but
the reports were inadequate for detailed evaluation as they contained
minimal details of the protocols used and limited data on
toxicological findings and were often in the form of progress reports.
The summaries reported kidney damage in the form of crystalline
deposits in the collecting tubules in rats at oral doses of 500 mg/kg
bw per day and more. In dogs, bile-duct proliferation was the
principal finding after an oral dose of 1600 mg/kg bw per day.
The highest dose chosen in the two-year study of toxicity and in
the study of reproductive toxicity in rats was 400 mg/kg bw per day.
This dose was selected because of the formation of acetylated
phenylurea, with resulting precipitation of crystals in the kidneys,
in rats given oral doses of 500 mg/kg bw per day. In some
toxicological studies, the test species were given phenylurea and
pyrimidone components in a ratio of 3:1, since it was claimed that
phenylurea and pyrimidone are present in that ratio in the muscle of
treated chickens. More recent data suggest a ratio as high as 8:1 for
the phenylurea to the pyrimidone residue. Dogs were fed diets
containing phenylurea and pyrimidone components in a ratio of 3:1 for
two years. The actual drug intakes were 0, 80, 240, or 800 mg/kg bw
per day. Serum alanine aminotransferase activity was increased in
several dogs, and slight bile-duct proliferation was observed in one
dog at 800 mg/kg bw per day. No other treatment-related effect was
observed. The NOEL was 240 mg/kg bw per day of the 3:1 mixture of
phenylurea and pyrimidone. In the two-year study in rats, doses of 0,
67, 200, or 400 mg/kg bw per day phenylurea and pyrimidone components
in a ratio of 3:1 were given in the diet. There was no
treatment-related toxicity, and tumour incidences were unaffected. The
NOEL was the highest dose, 400 mg/kg bw per day.
Nicarbazin slightly increased the mutation frequency in
Salmonella strains TA98 and TA1538 in one study but not in another.
Mutations were not detected in Salmonella strains TA100, TA1535, or
TA1537 or in E. coli WP2, and DNA damage was not induced in the rec
assay. No other end-points were investigated. The examination of
genotoxic potential was considered to be inadequate, since studies
were carried out only in bacteria.
A three-generation study of reproductive toxicity was conducted
in rats given a 3:1 ratio of phenylurea and pyrimidone components in
the diet at doses were 0, 67, 200, or 400 mg/kg bw per day. There were
isolated occurrences of slightly reduced litter size at birth or
depressed body-weight gain during lactation at the highest dose, but
these findings were not replicated in most litters and showed no
progression over the duration of the study. Therefore, the Committee
concluded that nicarbazin did not have significant effects on
reproduction. The NOEL was the highest dose tested, 400 mg/kg bw per
day.
Developmental toxicity was studied in rats given 0, 70, 200, or
600 mg/kg bw per day of nicarbazin, at an equimolar ratio of
phenylurea: pyrimidone, by gavage on gestation days 7-17. At 600 mg/kg
bw per day, maternal food intake and body weight were depressed, and
seven of 25 animals died. At this dose, the finding of lowered fetal
body weight and reduced ossification suggested retarded fetal
development, and a number of abnormalities were observed, in
particular hydronephrosis and hyperplastic and bent ribs. The NOEL was
200 mg/kg bw per day on the basis of maternal and fetal toxicity; no
teratogenic effects were observed.
4. EVALUATION
The Committee noted the absence of certain toxicological studies
in support of nicarbazin; however, the other data available provided
sufficient information to overcome most of these deficiencies. It was
noted that nicarbazin has been used in veterinary medicine in many
countries for over 40 years. On the basis of this long history of use
and the fact that use is restricted to starter rations in broiler
chickens, the Committee considered that an ADI could be supported.
The Committee established an ADI of 0-400 µg/kg bw on the basis
of the NOEL of 200 mg/kg bw per day in the study of developmental
toxicity in rats and using a safety factor of 500, chosen to account
for the limitations in the database.
5. REFERENCES
Bradley, M.O. & Cook, M.M. (1980) Nicarbazin: Microbial mutagen tests.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Fitzpatrick, S.C. (1997) Nicarbazin: Evaluation of available
toxicology data on nicarbazin: Rewrite format as outlined in WHO
Technical Report 832, Veterinary Drugs with a Long History of Use.
Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe,
California, USA.
Kirschner, S.L. & Vogin, E.E. (1970) Multigeneration reproduction and
lactation studies with 4,4'-dinitrocarbanilide (DNC) and
2-hydroxy-4,6-dimethylpyrimidine dihydrate (HDP). Unpublished report
from Food and Drug Research Laboratories, West Point, Pennsylvania,
USA. Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Kuna, S. (1955) Tolerance studies in mammals. Unpublished report.
Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA.
Ohta, T., Moriya, M., Kaneda, Y., Watanabe, K., Miyazawa, T.,
Sugiyama, F. & Shirasu, Y. (1980) Mutagenicity screening of feed
additives in the microbial system. Mutat. Res., 77, 21-30.
Tajima, M. (1979) Teratogenicity test of nicarbazin with rats by oral
administration. Unpublished report from Nisseiken (NIBS). Submitted to
WHO by Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969a) Two-year chronic toxicity studies with components
of nicarbazin in dogs. Unpublished report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, California, USA.
Vogin, E.E. (1969b) Chronic toxicity studies with nicarbazin
formulation in rats. Unpublished Report from Food and Drug Research
Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by
Koffolk, Rancho Santa Fe, CA, USA.
