
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 41
Prepared by:
The 50th meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
RECOMBINANT BOVINE SOMATOTROPINS (addendum)
First draft prepared by
Professor F.R. Ungemach
Institute of Pharmacology, Pharmacy and Toxicology
Veterinary Faculty, University of Leipzig, Leipzig, Germany
and
Dr N.E. Weber
Food and Drug Administration, Center of Veterinary Medicine
Rockville, MD, USA
1. Explanation
2. Biological data
2.1 Use of antibiotics
2.2 Concentrations of bovine somatotropin and insulin-like
growth factor in tissues and milk
2.2.1 Tissues
2.2.2 Residues of insulin-like growth factor in milk
2.2.3 Assays
2.2.4 Bioavailability and bioactivity of insulin-like
growth factor residues in milk
2.3 Expression of lentiviruses and prion proteins
2.3.1 Somatotropins and the immune system
2.3.2 Effect of bovine somatotropin on expression of
retroviruses
2.3.3 Effect of recombinant bovine somatotropin on prion
proteins
2.4 Cow's milk and insulin-dependent type I diabetes mellitus in
childhood
3. Comments
4. Evaluation
5. References
1. EXPLANATION
The four analogues of bovine somatotropins, somagrebove,
sometribove, somavubove, and somidobove, that are produced by
recombinant DNA techniques were evaluated by the Committee at its
fortieth meeting (Annex 1, reference 104). At that time, the Committee
allocated an ADI 'not specified' for recombinant bovine somatotropins
(rbSTs) because of the lack of activity of orally administered rbSTs
and insulin-like growth factor I (IGF-I) and the low concentrations
and non-toxic nature of the residues of these compounds, even after
very high doses, resulting in an extremely large margin of safety for
humans consuming dairy products from rbST-treated cows. The Committee
concluded that use of these drugs according to good practice in
veterinary medicine did not represent a dietary hazard to human health
and that there was no need to specify a numerical ADI. Accordingly,
MRLs 'not specified' were recommended for milk and edible tissues of
cattle. The Codex Alimentarius Commission, when considering adoption
of these recommended MRLs at its twenty-second session in 1997,
postponed a decision pending re-evaluation of rbSTs by the Expert
Committee to consider scientific information that had become available
since its previous evaluation.
Information was submitted by organizations and individuals
relating to the following concerns:
* increased use of antibiotics, with a higher rate of violative
drug residues in milk due to a possible increase in the incidence
of mastitis in rbST-treated cows,
* the possibility that increased concentrations of IGF-I in the
milk of rbST-treated cows increase cell division and the growth
of tumours in humans,
* the potential effect of rbST on the expression of certain viruses
in cattle, particularly the retroviruses,
* the possibility that the incubation period of bovine spongiform
encephalopathy (BSE) is shortened by an IGF-I-induced increase in
the production of pathogenic prion proteins, and
* the possibility that early exposure of human newborns to milk
from rbST-treated cows increases the risk for developing
insulin-dependent diabetes mellitus.
2. BIOLOGICAL DATA
2.1 Use of antibiotics
The induction by rbST of an increased incidence of mastitis and
somatic-cell count in the milk of treated cows was not reviewed by the
Committee at its fortieth meeting, as these effects on animal health
were considered outside its terms of reference. At the present
meeting, the Committe considered results reported in the literature
and the results of a programme to monitor the effects of sometribove
(Posilac(R)) on mastitis and animal health in the United States. The
Committee confirmed that these effects of rbST on animal health and
the resulting treatment per animal with any medication are issues
outside the terms of reference of the Committee. The results of the
monitoring programme with regard to the percentage of milk discarded
because of drug residues above the approved limit, as a consequence of
antibiotic use, were, however, considered by the Committee. The
programme was initiated by the US Food and Drug Administration at the
time sometribove was approved, in November 1993 and began to be
commercialized, in February 1994. The objectives of this programme
were to determine whether the incidence of masitits and antibiotic use
were manageable under actual conditions of use and whether the
labelling of rbSTs was adequate (US Food and Drug Administration,
1996). The programme was designed to address the following areas
(Collier, 1996):
* the incidence of mastitis and responses related to herd health
(not within the terms of reference of the Committee),
* treatment of 28 herds with rbST-treated cows with any medication
(not within the terms of reference of the Committee), and
* the percent of milk discarded because of drug residues above the
allowed limit in key dairy states representing at least 50 % of
US milk production.
The programme was closely monitored by the US Food and Drug
Administration and performed according to the sponsor's standard
operating procedures for quality assurance. The US Food and Drug
Administration (1996) confirmed that the integrity of the findings was
acceptable and that the records and analyses showed excellent
fidelity.
The programme for tracking milk residues in key dairy states
before and after the approval of sometribove was designed to reveal
whether any increase in drug residues above the allowed limit in milk
was associated with an increased frequency of use of antibiotics for
mastitis and other health problems in rbST-treated herds of dairy cows
(Veenhuizen et al., 1996). The results of the milk monitoring
programme for the two years before commercial use of sometribove
(1992-93) were compared with those for the two years after its launch
on the market (1994-95). Residues in milk were traced according to the
National Drug Residue Milk Monitoring Program in which the contents of
all milk tanker trucks are sampled routinely. The data set represented
more than 50% of the total US milk supply. The data were analysed
quarterly by comparing the milk discarded before and after the launch
of sometribove.
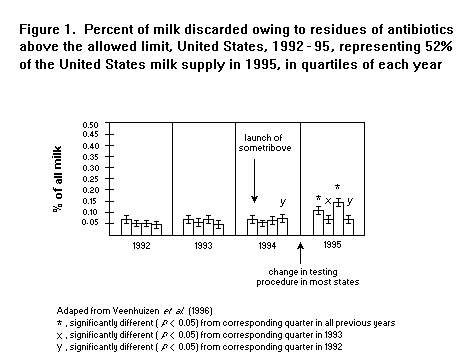
As seen in Figure 1, no change was observed in 1994 after
sometribove was approved. The average percent of milk discarded was
0.06% in 1992 and 1993 and 0.07% in 1994 after the launch; in 1995,
the number of violations increased slightly but significantly to
0.09%. This increase coincided, however, with a change in the
screening procedures in most states to include more sensitive tests.
Veenhuizen et al. (1996) submitted data to the US Food and Drug
Administration on 17 May 1996 showing that in New York State there had
been no significant change in the rate of discard of milk in the two
years after approval of Posilac in comparison with the two years
before approval, but that in that State the same testing protocol had
been used throughout the four-year period. The values were 0.062%
before approval and 0.064% afterwards. The company that launched
sometribove reported that rbST was purchased by nearly 37% of the
farms in the State, and these farms represented about 50% of the
State's cows. These reports indicate that:
* no product-related increase in residues above the approved limit
had occurred after commercialization of sometribove;
* the rate of positive results was even slightly lower than those
for antibiotics in grade A milk in the United States; and
* use of sometribove will have no effect on the safety of milk and
dairy products due to violative drug residues resulting from a
slightly higher rate of medication of rbST-treated animals, as
measured in the milk monitoring programme.
 It was concluded that use of rbST would not result in a higher risk to
human health due to the use of antibotics to treat mastitis and that
the increased potential for drug residues in milk could be managed by
practices currently in use by the dairy industry and by following the
directions for use (US Food & Drug Administration, 1996).
2.2 Concentrations of bovine somatotropin and insulin-like growth
factor in tissues and milk
2.2.1 Tissues
The concentrations of bST and IGF-I were measured in tissues of
cattle that had been treated with a 14-day sustained-release product
containing the natural variant of rbST, somavubove (Choi et al.,
1997), in two experiments. In the first experiment, three groups of 12
beef cattle (except at the low dose, to which only six animals were
exposed) with an average weight of 450 kg were treated by subcutaneous
injection for 20 weeks. The controls received no treatment or vehicle,
one group received 250 mg rbST each week, and another received 500 mg
rbST at two-week intervals. The controls and animals at the high dose
were further divided into groups receiving low- and high-energy feed.
The stated total doses were 5 and 10 g rbST, respectively; however,
the dose for both groups calculated from the stated regimens would be
5 g. Two weeks after the final treatment, the animals were slaughtered
and muscle samples were obtained and stored at -20°C for analysis. In
the second experiment, four groups of beef cattle were used: a control
group which received no drug or vehicle, a group receiving a
sustained-release low dose (0.42 mg/kg bw; 0.03 mg/kg bw per day), a
group receiving an intermediate dose (0.84 mg/kg bw; 0.06 kg bw per
day), and one receiving a high dose (1.26 mg/kg bw; 0.09 mg/kg bw per
day). The treated groups were given the drug by subcutaneous injection
every two weeks for 24 weeks, to give total doses of 2.3, 4.5, and 6.8
g, respectively. Two weeks after the final treatment, the animals were
slaughtered, and samples of muscle, kidney, liver, and fat were taken
and stored at -20°C.
The frozen samples were assayed for bST and IGF-I residues by
radioimmunoassay (RIA) in which 5 g of tissue were extracted in
acidethanol for muscle and acetic acid for kidney, liver, and fat.
The RIA procedures involved standard double-antibody techniques and
iodinated tracers. The detection limit for the assays (the amount that
could be distinguished from zero concentration with 95% confidence)
was 0.17 ng/g bST and 0.61 ng/g IGF-I. The coefficients of variation
for the two assays were 6% or less, and the recoveries from liver,
kidney, and fat were 64% for bST and 84% for IGF-I; similar recoveries
were obtained in muscle samples. A summary of the results is given in
Table 1. The authors concluded that two weeks after administration of
two doses of rbST for extended times, the tissue concentrations of bST
and IGF-I were not significantly different from those in untreated
animals.
2.2.2 Residues of insulin-like growth factor in milk
Extensive information on the residues of bST and IGF-I in the
milk of rbST-treated cows was evaluated by the Committee at its
fortieth meeting. IGF-I is a normal but highly variable constituent of
bovine milk, the concentration depending on the state of lactation,
nutritional status, and age. Over an entire lactation, the IGF-I
concentrations range from 1 to 30 ng/ml, with the highest
concentrations in colostrum and a constant decline thereafter.
Multiparous animals have higher concentrations of IGF-I in milk than
primoparous cows (Burton et al., 1994). Bulk milk from cows not
given rbST had IGF-I concentrations of 19 ng/ml (Juskevich & Guyer,
1990), and the fortieth Meeting cited an average value of 3.7 ng/ml
for untreated cows. In milk from rbST-treated cows, the concentrations
of IGF-I ranged from 1 to 13 ng/ml in most studies and were about
25-70% greater than those in untreated animals (Burton et al.,
1994). The fortieth meeting reported an average IGF-I concentration of
5.9 ng/ml, and the increase was significant, even though most of the
concentrations were < 10 ng/ml.
Table 1. Concentrations of bovine somatotropin (bST) and insulin-like growth factor (IGF-I) in tissues after treatment with
recombinant bovine somatotropin
First experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose High dose
bST IGF-I bST IGF-I bST IGF-I
Muscle 12 1.9 ± 1.8 88 ± 21 1.5 ± 1.6 130 ± 25 3.3 ± 2.2 110 ± 32
Second experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose Intermediate dose High dose
bST IGF-I bST IGF-I bST IGF-I bST IGF-I
Muscle 5 3.4 ± 1.5 45 ± 6.5 4.9 ± 1.5 35 ± 15 3.8 ± 2.0 40 ± 5.1 1.5 ± 0.86 55 ± 19
Fat 4 5.1 ± 1.7 210 ± 85 9.3 ± 5.2 200 ± 65 4.8 ± 1.9 200 ± 53 11 ± 12 340 ± 230
Liver 4 5.2 ± 0.59 350 ± 23 3.6 ± 1.7 390 ± 130 5.4 ± 1.2 380 ± 170 4.6 ± 2.0 290 ± 88
Kidney 4 3.6 ± 1.1 910 ± 130 4.5 ± 1.6 1000 ± 140 4.5 ± 1.8 820 ± 120 3.9 ± 0.94 980 ± 220
Since the original work was reviewed, few additional data have
appeared in the literature or in reports made available by the
sponsors. The manufacturer of Posilac(R) - previously identified as
sometribove, which is a form of rbST approved in a number of countries
- submitted additional information. In a study designed to determine
the concentrations of IGF-I in retail milk samples, the concentrations
in milk specifically labelled as coming from cows that had not been
treated with bST were compared with those in unlabelled milk. While
the sponsor assumed that all of the unlabelled milk was from cows that
had been treated with bST, the extent to which this was true was not
ascertained. The study was conducted under US Food and Drug
Administration GLP regulations (21CFR 58). The labelled and unlabelled
status was determined of 127 of 129 retail milk samples collected as
2% fat cow's milk in 51 retail outlets in 34 cities in Wisconsin,
Minnesota, and Iowa, USA, from four goats and 125 cows, and the
samples were analysed by RIA for IGF-I. For 78 samples, the farmer had
certified that rbST had not been used. As rbST is not approved for use
in goats in the United States, it is assumed that the goat-milk
samples were from untreated animals. The results are shown in Table 2
(Eppard et al., 1994). The values for IGF-I are not unlike those
reported in the monograph prepared at the fortieth meeting of the
Committee. Although the contribution of milk from rbST-treated cows to
the unlabelled milk is unknown, the results indicate that the IGF-I
concentrations in retail milk did not increase in the first year after
the launch of rbST.
Table 2. Concentrations of insulin-like growth factor in milk
certified by farmers as coming from cows not treated with
recombinant bovine somatotropin (labelled) and from unlabelled milk
Insulin-like growth factor (ng/ml)
Labelled Unlabelled
(n = 78) (n = 45)
Raw mean 4.3 ± 0.09 4.5 ± 0.12
Logea 1.47 ± 0.044 1.55 ± 0.031 p = 0.01769
Antilog (95% 4.4 (4.0-4.7) 4.7 (4.4-5.0)
confidence
interval)
a Least square means adjusted for state where purchased and dairy
2.2.3 Assays
Because of variations in the IGF-I values reported in different
studies, questions have been raised in submissions to the Committee
about their accuracy. Lower values were found in some studies because
the assay used involved acid-ethanol extraction; an assay involving
acidic gel filtration has been reported to allow better recovery. In a
comparison of the two assays for determining IGF-I in pre- and
post-partum mammary secretions, the acid-ethanol assay was found to
result in a 24 ± 6.6% underestimate in comparison with acid gel
filtration (Vega et al., 1991). Use of the acidethanol assay to
recover 125I-IGF-I in bovine serum and colostrum, which also contain
binding proteins, gave values of 86 ± 6% and 88 ± 7% recovery by the
antibody, respectively (Hadsell, 1991). The difference is due to the
fact that the binding protein with affinity for IGF-I competes with
the antibody used in the RIA procedure; however, gel filtration
removes the hormone that is extensively dissociated by the acid common
to both assays. The studies suggest that the acidethanol assay
probably results in 15-25% underestimates of the concentrations in
milk and plasma. The relative difference in milk will probably not
affect a decision, as the concentrations are low, although higher
control values may affect the inferences. Although incomplete removal
of IGF-binding proteins or differences in the source of standards
might affect the reported results, the Committee considered these
factors immaterial.
2.2.4 Bioavailability and bioactivity of insulin-like growth factor
residues in milk
At its fortieth meeting, the Committee concluded that many of the
physiological effects of rbSTs are mediated by bovine IGF-I, which is
structurally identical to human IGF-I. It noted that there is
substantial endogenous synthesis of IGF-I, mainly in the liver but
also in human milk, saliva, and pancreatic secretions. It further
concluded that IGF-I has no bioactivity when administered orally to
normal and hypophysectomized rats at doses up to 2 mg/kg bw per day,
as dietary IGF-I is degraded by digestive enzymes and is not active in
the upper gastrointestinal tract.
Concern has been expressed that widespread use of rbSTs in dairy
production would lead to a sustained increase in the concentrations of
IGF-I in bulk cow's milk and that, if IGF-I survives digestion, the
greater exposure of consumers would cause adverse health effects
(Hansen et al., 1997). For a quantitative assessment of risk, the
slight increases in the concentration of IGF-I in milk from
rbST-treated cows should be compared with the physiological variations
in the concentrations of this growth factor during lactation (see
section 2.2.2) and with the concentrations in human breast milk, in
the secretions of the gastrointestinal tract, and in serum.
The concentrations of IGF-I are 8-28 ng/ml in colostrum and
5-10 ng/ml thereafter (Zumkeller, 1992; Burton et al., 1994),
indicating that breast-fed newborns are usually exposed to IGF-I at
concentrations equal to or higher than those in milk from rbST-treated
cows. Assuming a daily intake of 1.5 L of milk from rbST-treated cows
with an average IGF-I concentration of 6 ng/ml, the amount of IGF-I
ingested would be 9000 ng/day. The additional daily ingestion of IGF-I
over that of people drinking milk from untreated animals, with an
average IGF-I concentration of 4 ng/ml or 6000 ng/1.5 L, would be 3000
ng. The slightly increased IGF-I concentrations would contribute to
the endogenous concentrations of IGF-I in the gastrointestinal tract
of consumers; however, the main site of IGF-I production in animals
and humans is the liver. It is also produced in the human
gastrointestinal mucosa and is found in saliva, bile, and pancreatic
juice (Olanrewaju et al., 1992). The average concentrations of IGF-I
in the five human gastrointestinal secretions were 0.9 nmol/L in
saliva, 3.5 nmol/L in gastric juice, 24.6 nmol/L in jejunal chyme, 3.6
nmol/L in pancreatic juice, and 0.9 nmol/L in bile (Chaurasia et
al., 1994). On the basis of a molecular mass of 7.5 kDa for IGF-I
(Zumkeller, 1992) and the volume of each of the fluids produced
(Vander et al., 1990), the total volume of IGF-I emptying into the
gastrointestinal tract is 383 000 ng/day. Table 3 shows that the
amount of endogenous IGF-I emptied into the gastrointestinal tract
daily is more than (383 000/9000) 42 times greater than the amount
present in 1.5 L of milk from rbST-treated cows. The 9000 ng value is
2.3% of the estimated daily gastrointestinal secretion of IGF-I in
adults. The additional daily ingestion of 3000 ng IGF-I over that in
milk from untreated animals thus represents 0.78% of the
gastrointestinal secretion.
Table 3. Concentration of insulin-like growth factor (IGF-I) in
gastrointestinal tract secretions
Secretion Volume Concentration Total IGF-I
(ml/day)a (average; ng/ml) secreted (ng)
Jejunal chyme 1500 184.5 276 750
Pancreatic juice 1500 27.0 40 500
Gastric juice 2000 26.2 52 400
Bile 500 6.8 3 400
Saliva 1500 6.8 10 200
Adapted from Baumann (1995)
a From Vander et al. (1990)
In contrast to the conclusion of the Committee at its fortieth
meeting, that IGF-I is completely and rapidly degraded in the
gastrointestinal tract, milk-borne IGF-I may escape digestion by
proteases and therefore be bioactive in the intestine (Hansen
et al., 1997) or even be absorbed as intact peptide into the
systemic circulation (Epstein, 1996). In a study designed to
investigate the potential therapeutic use of IGF-I and to determine
whether oral formulations were feasible, the degradation of
125I-IGF-I was determined in various segments of the
gastrointestinal tract of rats in vivo and in vitro (Xian
et al., 1995). The extent of degradation was measured by one of
three methods: receptor binding, immunoprecipitation, and
trichloracetic acid precipitation. Two segments of the duodenum,
ileum, or whole stomach and part of the colon were ligated in an
anaesthetized male Sprague-Dawley rat that had been fasted for 24 h. A
bolus of labelled IGF-I (8.6 ng/ml in 0.2% bovine serum albumin in
saline) was injected into each segment and incubated for various times
up to 1 h. The reactions were stopped, and the flushed luminal
contents were examined for intact IGF-I. In a parallel set of
experiments, the flushed luminal contents from each of the four gut
segments were used as a source of degredation enzymes in vitro. The
results are shown in Table 4. The most rapid degradation occurred in
the duodenum and ileum and in their contents in vitro, followed by
the stomach and then by the colon. In all cases the values seen
in vitro were equal to or greater than those seen in vivo.
The authors also examined the effectiveness of slowing the
degradation rate of IGF-I in the gut by protecting the molecule in
several ways. Casein at a concentration of 10 mg/ml conferred > 90%
protection on stomach contents in both the trichloracetic acid and
receptor assays; in duodenal flushings, casein conferred 80%
protection against IGF-I degradation in the trichloracetic acid assay
but only 36% protection in the receptor assay at a maximal casein
concentration of 40 mg/ml. The half-life of IGF-I in the upper
gastrointestinal tract in the receptor assay increased from 2-3 min in
the absence of casein to 35 min in its presence. While these results
appear to demonstrate significant protection by casein at a
concentration similar to those in milk, the authors acknowledge that
the observed effects could be explained by simple competition by the
additional proteins for degradation by proteases in the segments. The
results demonstrate that biological receptor-stimulating activity,
which is the best indicator of biological activity, is dramatically
reduced even with good protection from protease activity. The authors
noted with regard to milk residues of IGF-I that 'the protective
effect of casein makes irrelevant the argument that human saliva
contains IGF-I at concentrations greater than the quantities that
would be consumed in milk. As the IGF-I produced by salivary glands is
free IGF-I, without protective effect of casein, it is unlikely to
survive digestion.' (Hansen et al., 1997). That argumentation
neglects the following facts:
Table 4. Half-lives of intact 125I-insulin-like growth factor (IGF-I) in ligated Sprague-Dawley rat gut
segments in vivo and in the flushed contents in vitro
Test for Half-life (min)
intact IGF-I
Duodenum/ileum Stomach Colon
In vivo In vitroa In vivo In vitro In vivo In vitro
Trichloracetic 2 2 8 50 38 > 60
Antibody binding 2 5 33
Receptor binding 2 2 2.5 3 16 ND
ND, not determined
a Antibody and membrane receptor values reported as receptor binding
* Saliva is not the only source of IGF-I in the gastrointestinal
tract: most is secreted into the gut, and the high concentration
in intestinal chyme indicates that IGF-I is secreted in
substantial amounts by the mucosa throughout the gastrointestinal
tract (Olanrewaju et al., 1992; Chaurasia et al., 1994).
* Casein has a flexible structure and is readily degraded in the
stomach and small bowel (Xian et al., 1995). Thus, the
protective effect is present only in the upper gastrointestinal
tract.
* The half-life of IGF-I in the intestine in the presence of casein
is only 35 min (Xian et al., 1995). Therefore, less than 5% of
an initial dose of IGF-I will survive for more than 2 h during
passage through the upper gastrointestinal tract.
* In the presence of casein ingested with milk, endogenous IGF-I in
the gastrointestinal tract will also be protected.
Therefore, even if casein has a limited protective effect, the amount
of bioactive IGF-I ingested with milk from rbST-treated cows would
still be negligible.
Because of the protective effect of casein, some IGF-I might
escape digestive degradation and be absorbed intact. The absorption of
large (1 mg/kg) oral doses of 125I-labelled recombinant human IGF-I
was studied in fasted adult rats, with trichloracetic acid
precipitation of plasma proteins. The baseline bioavailability of the
administered IGF-I was 9.3% of the dose, but this was was increased by
co-administration of 4 mg/kg aprotinin (47%) and 10 mg/kg casein
(67%). RIA of the plasma confirmed the bioavailability of IGF-I in
this model, and the administered radiolabel was found in the form of
high-molecular-mass complexes (Kimura et al., 1997). It should be
noted, however, that the receptor assay, which is the most accurate,
was not used.
The relatively good bioavailability of intact IGF-I in this adult
rat model is in contrast to the lack of bioactivity of orally
administered IGF-I in adult animals (Annex 1, reference 104) and to
the results of studies with neonatal animals which have an incomplete
mucosal barrier and reduced intestinal proteolytic activity (Burrin,
1997). Studies in neonatal rats and piglets indicated that although
30% of an orally administered dose of 125I-IGF-I can be recovered in
the intestinal mucosa there is limited absorption into the peripheral
circulation (Phillips et al., 1995; Donovan et al., 1997). When
suckling transgenic rats ingested 1000-fold higher concentrations of
des(1,3) human IGF-I, no des(1,3)-IGF-I was detected in the plasma of
their pups (Burrin, 1997). Furthermore, in newborn calves and piglets
given large doses of IGF-I in milk replacers, no substantial increase
in the plasma concentration of this growth factor was found (Donovan
et al., 1997; Hammon & Blum, 1997; Houle et al., 1997). In one
study with newborn calves fed milk replacer, a small amount of orally
administered 125I-IGF-I was detected in plasma (Baumrucker et
al., 1992); however, the increase was observed only three days after
administration and in only three of six animals. Even in newborns,
therefore, IGF-I is absorbed to only a small extent, and absorption is
unlikely in adults.
Furthermore, the amount absorbed should be compared with the
normal concentrations of IGF-I in human serum, which show considerable
variation with age. The values are lowest in infants under two years
of age, then increase steadily to reach a maximum in late puberty, and
afterwards decrease to the adult values (Table 5). Assuming a blood
volume that is 5% of the body weight (Ganong, 1971), the serum load of
IGF-I can be calculated to be 50 000 ng in a 15-kg child, 714 000 ng
in a 60-kg adult, and 1 220 000 ng in a 50-kg teenager. The total
IGF-I production in adults has been estimated at 107 ng per day
(Guler et al., 1989). These amounts should be compared with the 9000
ng IGF-I in 1.5 L of milk, which constitutes only 0.09% of the daily
IGF-I production. Since only one-third of the concentrations in milk
can be attributed to IGF-I due to rbST treatment and only a small
amount if any will be absorbed, the milk-borne IGF-I that reaches the
systemic circulation is negligible and this small amount is
immediately sequestered by unsaturated binding proteins.
Table 5. Concentrations (ng/ml) of insulin-like growth factor (IGF-I)
in human plasma
Age Males Females
Mean Range Mean Range
0-2 years 42 14-98 56 14-238
3-5 years 56 59-210 84 21-322
6-10 years 98 28-308 182 56-364
Before puberty > 10 years 126 84-182 182 70-280
Early puberty 210 140-240 224 84-392
Late puberty 364 224-462 434 224-686
Adult > 23 yeras 112 42-266 140 56-308
From Schaff-Blass et al. (1984)
Concern has been expressed about the possible adverse effects on
the health of consumers exposed to increased concentrations of IGF-I
in milk from rbST-treated cows (Epstein, 1996; Hansen et al., 1997).
The most important potential adverse effects of IGF-I arise from the
fact that it is a mitogen for a number of cell types and has been
associated with the growth of tumours including those of the colon,
breast, and lung and osteosarcoma (Pines et al., 1985; Macaulay,
1992; National Institute of Health, 1995). The mitogenic effect could
also result in proliferative reactions locally in the gut. Thus,
orally administered IGF-I increased the cellularity of the intestinal
mucosa of rats in vivo (Olanrewaju et al., 1992) and increased the
rate of proliferation in cultures of human duodenal epithelial crypt
cells (Challacombe & Wheeler, 1994). Since IGF-I receptors can be
detected throughout the epithelium of the intestine, with a high
density in the colon (Laburthe et al., 1988), and the incidence of
colorectal cancer is increased in acromegalic patients who have
excessively high concentrations of free IGF-I in their plasma (Ezzat &
Melmed, 1991), concern has been expressed that increased
concentrations of milk-borne IGF-I may increase the risk for colon
cancer. Although the normal biological effects of IGF-I mean that it
could promote the growth of tumours, this hazard would become a risk
only if there were adequate exposure of consumers to IGF-I. Since
exposure to IGF-I in milk from rbST-treated cows is negligible when
compared with endogenous IGF-I production, it is extremely unlikely
that IGF-I residues cause any systemic or local mitogenic reaction.
2.3 Expression of lentiviruses and prion proteins
2.3.1 Somatotropins and the immune system
Somatotropin enhances the immune system in many species,
including cattle (Comens-Keller et al., 1995). The primary effect
appears to be altered responsiveness of the immune system, although
substantive evidence of this effect is lacking (Burton et al.,
1994), and information on changes in cytokine concentrations or
secretion and on their binding sites are needed in order to define the
nature of the immune-enhancing effects of somatotropins. The studies
reported in the literature differ with regard to the source of
somatotropin, the treatment schedule, and the age of the animals. The
differences between findings in vitro and in vivo may be due to
the release in vivo of mediators such as IGFs and cytokines, which
are not present in vitro (Kelley, 1989). A better understanding of
somatotropin-mediated immune enhancement as a homeorhetic regulator of
the overall health and disease resistance of animals is needed.
Lymphocytes from rbST-treated cows have a greater average maximum
lymphoblastogenic response to rbST than to other mitogens around the
time of parturition (Comens-Keller et al., 1995), and this effect
might prevent mastitis and the other infectious diseases that occur
during this period of immunosuppression.
2.3.2 Effect of bovine somatotropin on the expression of retroviruses
Concern has been expressed that the immunomodulatory effect of
bST might affect retroviral expression in treated animals and thus
cause resurgence of latent retroviral and lentiviral infections in the
ruminant population and the presence of these viruses in somatic cells
in milk. The concern is based largely on a review by Lerondelle et al.
(1994), who discussed the evidence for induction of these viruses in
small ruminants by steroid hormones and the evidence for induction of
lentiviruses in other species by hormones including growth hormone and
IGF-I, and an unpublished study by Lerondelle et al. (1996), who
investigated the effects of rbST on the expression of caprine
arthritis encephalitis virus (CAEV) in goats. This virus belongs to
the group of lentiviruses which, like maedivisna, can infect small
ruminants.
Ruminant lentiviruses are of interest for at least three reasons.
First, they may cause disease in persons who drink milk. Second, if
use of rbST increases the prevalence of ruminant viruses, by the
presence of rbST itself or by the action of IGF-I, the small
additional amounts of growth hormone in the milk of treated cows could
also affect the retroviruses that attack the human immune system,
HIV-1 and HIV-2. Finally, the severity or kinetics of expression of
the disease in ruminants might be increased.
The study of Lerondelle and coworkers (1996) attempted to address
the question of whether rbST increases the expression of CAEV. Viral
expression was measured by assaying reverse transcriptase activity in
milk cells, clinical examination of the udders and joints of the
animals at the beginning and end of the study, and evidence of
infection in an immunodiffusion assay. Twelve pregnant Saanen goats
that were seronegative for CAEV were given an intramammary injection
of monocytes infected in vitro with the Cork strain of CAEV at the
time of drying off. Seven weeks after giving birth, four goats were
treated daily with 5 mg/goat rbST (sometribove), a second group was
treated daily with 10 mg/goat thyroxine, and the control group was
untreated. The compounds were administered in suspension in sterile
water for 30 days, followed by a 45-day observation period. Milk
samples were taken to measure reverse transcriptase activity on days
7, 14, 21, and 28 of treatment and three times during the observation
period. Examination of the udders and joints and immunodiffusion tests
were carried out at the beginning and end of the study. Milk
production and milk-cell counts were evaluated every two days. The
occurrence of CAEV and the onset of effects are shown in Table 6. More
positive cultured cells were seen in the controls than after treatment
with either hormone. Perhaps the most striking effect is the lack of
increase in the rate of infectivity and even a suggestion of a
decrease after treatment with rbST, particularly after the first milk
sampling.
When the results were expressed as a ratio of transcriptase
activity to the number of cells considered to contain virus (Table 7),
there was no correlation between the effect of rbST or thyroxine and
the activity of reverse transcriptase in the milk samples. In fact,
there was no evidence of increased transcriptase activity in any
group. In particular, even though the group receiving rbST had a lower
initial rate of infection than the other two groups, including the
controls, the rate of infection was not increased, as measured by the
number of positive cultures or increased transcriptase activity. The
authors interpreted their results as showing a tendency to increased
viral expression with increased milk production. The results for rbST
were, however, biased by the fact that only two animals were included
in the final evaluation. The authors concluded that, owing to the
Table 6. Onset of appearance of cytopathic effect (in days) for each of 10 milk samplings from groups of four goats given no treatment or
thyroxine or recombinant bovine somatotropin (rbST), as a function of the period of hormonal treatment
Treatment Milk sampling
Before treatment During treatment After treatment
1 2 3 4 5 6 7 8 9
Control 4 8 6 10 6 6 6 8 10
4 6 6 6 8 6 6 6 -
4 6 4 6 4 4 6 4 4
6 8 10 6 10 6 - - -
Mean ± SD (n) 6 ± 1.91 (12) 6.4 ± 1.71 (15) 7 ± 2.39 (8)
Thyroxine
8 6 10 4 6 6 - - 6
6 4 4 4 4 4 4 4 ND
4 4 4 4 4 4 6 4 ND
4 8 6 4 4 4 6 4 ND
Mean ± SD (n) 5.67 ± 2.06 (12) 4.53 ± 0.92 (15) 4.25 ± 0.71 (8)
rbST 8 - - - - - - - -
4 8 8 - 8 - - - 4
ND - - - - - - - -
4 4 4 4 4 4 4 4 4
Mean ± SD (n) 5.71 ± 2.41 (7) 4.8 ± 1.79 (5) 4 (4)
ND, not determined
Table 7. Number of samples found to contain virus by the reverse
transcriptase-positive culture ratio in groups of four goats given
no treatment, thyroxine, or recombinant bovine somatotropin (rbST)
Treatment Before During After Total
treatment treatment treatment
Control 3/6 4/7 3/4 10/17
3/6 3/5 1/3 7/14
6/6 4/5 5/6 15/17
4/4 2/8 3/6 9/18
Total 16/22 13/25 12/19 41/66
Thyroxine 0/3 2/4 0/3 2/10
4/4 5/7 6/6 15/17
6/6 8/8 6/6 20/20
6/6 8/8 4/6 18/20
Total 16/19 23/27 16/21 55/67
rbST 2/4 0/6 0/1 2/11
6/6 7/8 2/5 15/19
0/2 0/4 0/3 0/8
4/4 4/5 5/6 12/14
Total 12/16 11/23 7/15 29/52
heterogeneity of the effects on milk production and the small number
of animals tested, the evidence for a promoting effect of rbST on
viral expression was insufficient.
These results provide no evidence that treatment of cows infected
with lentiviruses with rbST will cause resurgence of viral infections
or pose any risk to human health. Lentiviruses are retroviruses that
replicate only in activated cells of the immune system. They may stay
dormant for months or years before they gradually wear down the immune
system to the point of collapse. The phylogenetic tree of lentiviruses
includes the subfamily of bovine immunodeficiency virus (also called
bovine leukaemia virus, BLV) and the subfamily of HIV-1 and HIV-2
which are the causative agents of AIDS. BLV and HIV are widely
separated phylogenetically (Robertson, 1997). BLV is not known to
cause disease in humans although it can infect human cells in
vitro, where host defence mechanisms are not present (Georgiades
et al., 1978; van der Maaten & Miller, 1990); infection of human
cells in vivo could not be demonstrated, and all attempts to obtain
direct evidence of infection in exposed human populations have yielded
negative results (Straub, 1981; van der Maaten & Miller, 1990).
Failure to infect humans has also been shown for other ruminant
lentiviruses, such as CAEV and maedivisna (Straub, 1981). Excretion
of virus with milk somatic cells can infect the offspring of infected
cows. Such transmission can be effectively blocked by procedures
similar to pasteurization, which destroy the virus at 60°C within 30 s
(Abramova et al., 1974; Baumgartner et al., 1976; van der Maaten &
Miller, 1990). BLV therefore cannot induce disease in humans and is
completely inactivated by routine pasteurization. Furthermore, the
company that sells sometribove has reported that there is no
indication that the incidence of BLV infection has increased in cattle
after eight years of continuous use of rbST in Mexico and Brazil and
four years of use in the United States (Collier & Kowalczyk, 1998),
although this statement is not further qualified.
An increase in the expression of HIV in humans who ingest milk
from rbST-treated cows is extermely unlikely because of the negligible
residues of rbST and IGF-I. Treatment of AIDS patients for six weeks
with recombinant human growth hormone and IGF-I had no effect on the
HIV titres in peripheral blood mononuclear cells, on the CD3, CD4, or
CD8 counts in peripheral blood, or on serum HIV p24 antigen
concentrations (Waters et al., 1996).
2.3.3 Effect of recombinant bovine somatotropin on prion proteins
Concern has been expressed that rbST treatment could increase the
risk for BSE in dairy cows (Hansen et al., 1997). Little evidence is
available to support this concern, and that which has been provided is
indirect. It is currently thought that the infectious agent of BSE is
a prion protein (Prusiner, 1982). Prion proteins are found normally in
all animals and are encoded by a prion-protein gene. BSE is associated
with a post-translationally modified protease-resistant prion protein,
which differs in its three-dimensional structure from the normal
protease-sensitive prion protein. Normal prion proteins are bound to
membranes on the surface of all nerve cells, some lymphocytes, and
other tissues (Prusiner, 1991). To date, no function has been ascribed
to normal prion protein. The most widely accepted theory of BSE is the
conversion of normal prion protein to the abnormal protease-resistant
form, which in turn causes more normal prion protein to convert to
protease resistance. The mechanisms of the conversion to the
disease-causing protease-resistant prion protein are not clearly
understood. In contrast to the normal form, the protease-resistant
form cannot be turned over and builds up in cells, forming large
oligomers which are observed as plaques (amyloids) in the brains of
affected individuals (Gajdusek, 1993).
IGF-I increased the production of prion protein mRNA in vitro
in a rat phaeochromocytoma cell line (PC12 cells), with a rather flat
dose-response curve showing a 40% increase at 10 ng/ml and a doubling
at 100 ng/ml (Lasmézas et al., 1993). In transgenic mice that
harbour multiple copies of the prion protein gene, the progession of
scrapie was accelerated (Prusiner, 1991). It has been speculated that
the increased IGF-I concentrations in rbST-treated cows could increase
prion protein production and possibly speed up the progression of BSE;
however, no studies are available that directly address the question
of whether rbST or IGF-I increases the formation of normal prion
protein or its pathogenic protease-resistant mutant in the brains of
the cattle, and the possibility of a link between rbST treatment and
BSE is highly speculative.
2.4 Cow's milk and insulin-dependent type I diabetes mellitus in
childhood
Epidemiological studies have shown that environmental factors
such as chemicals, viral infections, a short duration of
breastfeeding, and early dietary exposure of newborns to cow's milk
increase the risk for insulin-dependent type I diabetes mellitus
(IDDM) by about 1.5 times (Scott, 1990; Dahlquist et al., 1991;
Jorgensen et al., 1991; Virtanen et al., 1993; Gerstein, 1994;
Verge et al., 1994; Virtanen et al., 1994). IDDM develops as a
consequence of autoimmune destruction of the insulin-producing b cells
of the pancreatic islets. The precise trigger of the autoimmune
reaction is unknown, but it is assumed to be a genetically acquired
immune defect in susceptible individuals (Gerstein, 1994). The
epidemiological evidence indicates that IDDM is geographically and
temporally related to neonatal feeding with cow's milk and that
avoidance of cow's milk during the first few month of life can protect
genetically predisposed individuals (Gerstein, 1994; Verge et al.,
1994).
Serological evidence supports the theory that the immune defect
is triggered by exposure to proteins in cow's milk (Gerstein, 1994).
It has been postulated that, in newborns, milk proteins cross the
immature gut wall, initiating an immune response that cross-reacts
with a beta-cell surface antigen (Verge et al., 1994). Children aged
five to nine, who have an intact intestinal barrier, are not at risk
of acquiring IDDM by drinking cow's milk (Dahlquist et al., 1991).
The possible triggering factors in cow's milk have not been
identified. Casein is unlikely to be involved, since replacement of
milk proteins by casein in the diets of rats susceptible to diabetes
completely prevented the disease (Jorgensen et al., 1991). Increased
concentrations of immunoglobulin A antibodies to cow's milk and
b-lactoglobulin appear to be associated with an increased risk for
IDDM (Dahlquist et al., 1991; Virtanen et al., 1994).
It is unlikely that exposure of human newborns to milk from
rbST-treated cows would increase the risk for IDDM for the following
reasons:
* The composition of milk from rbST-treated cows is well within the
normal variations observed during lactation,
* The slightly increased IGF-I concentrations can be excluded as a
triggering factor because bovine and human IGF-I are identical
and because the concentrations of IGF-I in breast milk are equal
to or initially higher than those found in cow's milk.
3. COMMENTS
Use of antibiotics
After reviewing the available information, the Committee
considered the risk for mastitis associated with use of rbST as an
issue of animal health that is not within its terms of reference;
however, the possibly increased use of antibiotics was considered. A
post-approval monitoring programme was established in the United
States to address the following issues:
* the incidence of mastitis and responses related to herd health
(not within the terms of reference of the Committee),
* treatment with any medications of 28 herds of rbST-treated cows
(not within the terms of reference of the Committee), and
* the percent of milk discarded because of violative drug residues
in key dairy states representing at least 50% of United States
milk production.
In New York State, the percentage of milk discarded because of the
presence of antibiotic residues was not significantly changed after
the introduction of rbST. In other states, however, a small but
statistically significant, increase was observed in 1995, which
coincided with a change to a more sensitive testing method in those
states. The Committee concluded that the use of rbST would not result
in a higher risk to human health due to the use of antibiotics to
treat mastitis and that the increased potential for drug residues in
milk could be managed by practices currently in use within the dairy
industry and by following the directions for use.
Concentrations of insulin-like growth factor in milk and tissues
Insulin-like growth factor (IGF-I) is a normal component of milk
and is found in abundance in a variety of body fluids (Table 8). The
presence and concentrations of IGF-I were the source of much of the
scientific discussion during the original review of bST undertaken at
the fortieth meeting of the Committee and in submissions to the
present one. The information that was reviewed is summarized in FAO
Food and Nutrition Paper No. 41/5 (Annex 1, reference 106). The
concentrations of IGF-I in milk are variable and have been shown to
depend on the state of lactation, nutrition, and age.
Methods for assaying IGF-I were considered by the Committee.
Although incomplete removal of IGF-binding proteins or differences in
the source of standards and extraction methods might affect the
reported values, these factors were considered not to alter any
conclusions materially. The relatively high values previously reported
in milk were considered to reflect inadequate extraction.
Table 8. Insulin-like growth factor in milk and body fluids
Medium Concentration
(ng/ml)
Milk
Human 5-10
Colostrum 8-28
Bovine (bulk milk)
Untreated 1-9
rbST-treated 1-13
Plasma
Child 17-250
Adolescent 180-780
Adult 120-460
Gastrointestinal secretions (human)
Saliva 6.8
Gastric juice 26
Pancreatic juice 27
Bile 6.8
Jejunal chyme 180
Daily production of adult humans 107 ng/d
Since the previous evaluation, few additional data on residues
have appeared in the literature or in reports provided by interested
parties; however, the manufacturer of sometribove submitted additional
information on the concentrations of IGF-I in retail milk after the
approval of rbST in the United States. The results showed no
difference in the IGF-I concentrations of milk certified as derived
from cows not treated with rbST and of unlabelled milk; however, the
percentage of the unlabelled milk that was derived from cows receiving
rbST was not specified.
Concern has been expressed that any rbST-induced increase in the
concentration of IGF-I in milk would contribute to the endogenous
levels of IGF-I in the gastrointestinal tract and serum if it were not
biodegraded and were absorbed. In rats, IGF-I is rapidly degraded in
the gastrointestinal tract; however, a protective effect of casein on
IGF-I was demonstrated in these studies. It was postulated that the
retarded degradation leads to increased serum levels of IGF-I (as
shown in one study in rats) and to prolonged exposure of the gut. The
Committee noted that seven days' oral administration of high doses of
IGF-I in milk replacer did not increase the circulating concentrations
of IGF-I in newborn calves and piglets, indicating that significant
absorption of IGF-I is unlikely to occur under physiological
conditions. In view of the decreased rate of degradation in the small
intestine of rats in the presence of casein, the concentrations of the
growth factor would probably decrease to less than 5% of their initial
values within 2 h, so that milk-borne IGF-I would not be expected to
contribute to the concentrations of IGF-I in the large intestine.
If 1.5 L of milk are ingested per day, the average intake of
IGF-I will be 6000 ng in milk from untreated cows containing an
assumed IGF-I concentration of 4 ng/ml and 9000 ng in milk from rbST-
treated cows with an approximate average concentration of 6 ng/ml. It
has been calculated that IGF-I in gastrointestinal secretions amounts
to about 380 000 ng/day. Therefore, the additional amount of IGF-I in
1.5 L of milk from rbST-treated cows would represent only about 0.8%
of the IGF-I secreted in the gastrointestinal tract. The total amount
of IGF-I in serum has been calculated to range from 50 000 to 1 220
000 ng, depending on age. The total daily IGF-I production in adult
humans has been estimated to be 107 ng. Therefore, the daily amount
of IGF-I ingested with milk from rbST-treated cows would represent
less than 0.09% of the daily production of adults. Even if all of the
milk-borne IGF-I were absorbed, the additional amount would be
negligible.
When sustained-release rbST was administered to cattle once every
two weeks for a total of 20 weeks, the tissue concentrations of rbST
and IGF-I two weeks after the final dose were not significantly
increased.
The Committee concluded that any increase in the concentration of
IGF-I in milk from rbST-treated cows is orders of magnitude lower than
the physiological amounts produced in the gastrointestinal tract and
in other parts of the body. Thus, the concentration of IGF-I would not
increase either locally in the gut or systemically, and the potential
for IGF-I to promote tumour growth would not increase when milk from
rbST-treated cows was consumed; there is thus no appreciable risk for
consumers.
Expression of retroviruses
Concern that treatment of cattle with rbST would increase the
expression of retroviruses, including BLV, were addressed in
experiments with caprine arthritis encephalitis virus in goats.
Infectivity was not increased when measured as numbers of infected
cells, and there was no evidence of increased reverse transcriptase
activity. These studies provide no evidence that rbST affects the
expression of BLV, a lentivirus in cattle. Furthermore it has been
shown that BLV is destroyed in simulated pasteurization conditions by
heating milk to 60 .C for 30 s. In addition, there is no evidence
of human susceptibility or response to ruminant retroviruses.
Expression of prion proteins
Concern has been expressed that treatment with rbST could shorten
the incubation period for BSE. This hypothesis is based on results
obtained with a neuronal cell line in vitro indicating increased
formation of prion protein mRNA in response to IGF-I. Furthermore,
increased formation of prion protein shortened the incubation period
for scrapie in transgenic mice harbouring multiple copies of the gene
that codes for prion proteins. No data were available, however, to
address directly the question of whether rbST or IGF-I increases the
formation of normal prion protein or its pathogenic protease-resistant
mutant in the brains of cattle. The Committee considered that the
possibility of a link between rbST treatment and BSE was highly
speculative.
Risk for insulin-dependent diabetes mellitus
Exposure of human newborns to cow's milk increases the risk for
insulin-dependent diabetes mellitus by about 1.5-fold. The Committee
considered whether exposure of newborns to milk from rbST-treated cows
further increases this risk. It concluded that, because of its
unchanged composition, the milk of rbST-treated cows would not pose an
additional risk to the development of insulin-dependent diabetes
mellitus.
4. EVALUATION
On the basis of the following:
* insignificant changes in the quantities of milk discarded after
testing for antibiotic residues following the introduction of
rbST into commercial use;
* low concentrations of rbST and IGF-I residues in milk;
* degradation of IGF-I in the gut and its abundance in gut
secretions;
* the extremely low concentrations of ingested IGF-I in comparison
with endogenous production;
* lack of evidence that rbST stimulates the expression of
retroviruses;
* lack of a direct link between rbST treatment and BSE; and
* the absence of significant changes in the composition of milk
from rbST-treated cows that could contribute to an additional
risk for the development of insulin-dependent diabetes mellitus,
the Committee concluded that rbST can be used without any appreciable
risk to the health of consumers. The Committee reaffirmed its previous
ADIs1 and MRLs2 'not specified' for somagrebove, sometribove,
somavubove, and somidobove.
5. REFERENCES
Abramova, E.M., Kondratev, V.S. & Sytinskii, I.A. (1974) The
biochemistry of leucosis in cattle. Vet. Bull., 44, 689-711.
Baumann, D.M. (1995) IGF-I Fact Sheet, Department of Animal Science,
Cornell University, Ithaca, NY, USA.
Baumgartner, L.E., Olson, C. & Onuma, M. (1976) Effect of
pasteurization and heat treatment on bovine leukemia virus. J. Am.
Vet. Med. Assoc., 169, 1189-1191.
Baumrucker, R.R., Hadsell, D.J., Skarr, T.C., Campbell, P.G. & Blum,
J.W. (1992) Insulin-like growth factors (IGFs) and IGF binding
proteins in mammary secretions: Origins and implications in neonatal
physiology. In: Picciano, M.F. & Lonnerdal, B., eds, Mechanisms
Regulating Lactation and Infant Nutrient Utilization, New York,
Wiley-Liss, pp. 285-308.
Burrin, D.G. (1997). Is milk-borne insulin-like growth factor-l
essential for neonatal development? J. Nutr., 127, 975S-979S.
Burton, J.L., McBride, B.W., Block, E., Glimm, D.R. & Kenelly, J.J.
(1994) A review of bovine growth hormone. Can. J. Anim. Sci., 74,
167-201.
Challacombe, D.N. & Wheeler, E.E. (1994) Safety of milk from cows
treated with bovine somatotropin. Lancet, 344, 815-816.
Chaurasia, O.P., Marcuard, S.P. & Seidel, E.R. (1994) Insulin-like
growth factor l in human gastrointestinal exocrine excretion.
Regul. Pept., 50, 113-119.
Choi, J., Choi, M.J., Kim, C., Ha, J., Hong, A., Ji, Y. & Chang, B.
(1997). The effect of recombinant bovine somatotropin (rbST)
administration on residual BST and insulin-like growth factor I levels
in various tissues of cattle. J. Food Hyg. Soc. Jpn, 38, 225-232.
Codex Alimentarius Commission (1997) Report of the Twenty-second
Session of the Codex Alimentarius Commission, Geneva, 23-28 June
1997 (FAO Document ALINORM 97/37), Rome, Food and Agriculture
Organization of the United Nations.
Collier, R.J. (1996) Post-approval evaluation of Posilac bovine
somatotropin in commercial dairy herds (100-USA-COW-RJC-93-051).
Unpublished report from Protiva, Monsanto Co. Submitted to WHO by
Protiva, Monsanto Co., St Louis, Missouri, USA.
Collier, R.J. & Kowalczyk, D.F. (1998) Human health risk of
retroviruses in cattle and bovine somatotropine. Unpublished report
from Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co.,
St Louis, Missouri, USA.
Comens-Keller, P.G., Eppard, P.J. & Collier, R.L. (1995). Evaluation
of somatotropin as a homeorhetic regulator of immunity. In: Ivan, M.,
ed., Animal Science Research and Development: Moving Toward a New
Century. Proceedings of the 75th Anniversary Meeting of the
Canadian Society of Animal Science, Ottawa, pp. 79-94.
Dahlquist, G., Blom, L. & Lönnberg, G. (1991) The Swedish childhood
diabetes study: A multivariate analysis of risk determinants for
diabetes in different age groups. Diabetologica, 34, 757-762.
Donovan, S.M., Chao, J.C., Zijlstra, R.T. & Odle, J. (1997) Oral
administered iodinated recombinant human insulin-like growth factor-I
(125I-rhIGF-I) is poorly absorbed by the neonatal piglet. J.
Pedriat. Gastroenterol. Nutr., 24, 174-182.
Eppard, P.J., Collier, R.J., Hintz, R.L., Veenhuizen, J.J. & Baile,
C.A. (1994) Survey of milk insulin-like growth factor in retail milk
samples. Unpublished report No. 100-USA-COW-RJC-94-074 from Protiva,
Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St Louis,
Missouri, USA.
Epstein, S.S. (1996) Unlabeled milk from cows treated with
biosynthetic growth hormones: A case of regulatory abdication. Int.
J. Health Serv., 26, 173-185.
Ezzat, H. & Melmed, S. (1991) Clinical review 18: Are patients with
acromegaly at increased risk for neoplasia? J. Clin. Endocrinol.
Metab., 72, 245-249.
Gajdusek, D.C. (1993) Genetic control of nucleation and polymerization
of host precursors to infectious amyloids in the transmissible
amyliodoses of brain. Br. Med. Bull., 49, 913-931.
Ganong, W.F., ed. (1971) Review of Medical Physiology, Los Altos,
Lange Medical Publications, p. 5.
Georgiades, J.A., Billiau, A. & Vanderschueren, B. (1978) Infection of
human cell cultures with bovine visna virus. J. Gen. Virol., 38,
375-381.
Gerstein, H.C. (1994) Cow's milk exposure and type I diabetes
mellitus. Diabetes Care, 17, 13-19.
Guler, H.P., Zapf, J., Schmid, C. &, Froesch, E.R. (1989) Insulin-like
growth factors I and II in healthy man. Estimations of half-lives and
production rates. Arch. Endocrinol., 121, 753-758.
Hadsell, D.L. (1991) Insulin-like Growth Factor (IGF) Receptors in
the Bovine Mammary Gland: Regulation, Mitogenic Action, and Role in
Mammary Secretion of IGF-I, PhD Thesis, Pennsylvania State
University, Philadelphia, Pennsylvania, pp. 108-114,
Hammon, H. & Blum, J.W. (1997) The somatotropic axis in neonatal
calves can be modulated by nutrition, growth hormone and
Long-R3-IGF-I. Am. J. Physiol., 273, E130-E138.
Hansen, M., Halloran, J.M., Groth, E., III & Lefferts, L.Y. (1997)
Potential public health impacts of the use of recombinant bovine
somatotropin in dairy production. Unpublished report. Submitted to WHO
by Consumer Policy Institute, Yonkers, New York, USA.
Houle, V.M., Sschroeder, E.A., Odle, J. & Donovan, S.M. (1997) Small
intestinal disaccharidase activity and ileal villus height are
increased in piglets consuming recombinant human insulin-like growth
factor-I. Pediatr. Res., 42, 78-86.
Jorgensen, K.D., Joner, G. & Hanssen, K.F. (1991) Relationship between
cows' milk consumption and incidence of childhood IDDM. Diabetes
Care, 14, 1081-1083.
Juskevich, J.C. & Guyer, C.G. (1990) Bovine growth hormone: Human food
safety evaluation. Science, 249, 875-884.
Kelley, K.W. (1989) Growth hormone, lymphocytes and macrophages.
Biochem. Pharmacol., 38, 705-713.
Kimura, T., Murakawa, Y., Ohno, M., Ohtani, S. & Higaki, K. (1997)
Gastrointestinal absorption of recombinant human insulin-like growth
factor-I in rats. J. Pharmacol. Exp. Ther., 283, 611-618.
Laburthe, M., Rouyer-Fessard, C. & Gammeltoft, S. (1988) Receptors for
insulin-like growth factors I and II in the rat gastrointestinasl
epithelium. Am. J. Physiol., 254, 457-462.
Lasmézas, C., Deslys, J.P. & Dormont, D. (1993) Recombinant human
growth hormone and insulin-like growth factor I induce PrP gene
expression in PC12 cells. Biochem. Biophys. Res. Commun., 196,
1163-1169.
Lerondelle, C., Lena, P. & Mornex, J.F. (1994) [Hormonal control of
retroviral infections.] Renc. Rech. Ruminants, 1, 4550 (in French).
Lerondelle, C., Guiguen, F., Fornazero, C., Gounel, F., Leroux, C.,
Chastang, J., Pardo, E., Bolland, P., Bruyas, S., Cordier, G. &
Mornex, J.F. (1996) Effects of recombinant bovine somatotropin and of
thyroxine on the expression of lentiviruses in ruminants. Unpublished
report from INRA Veterinary School of Lyon and INSERM Hôpital Louis
Pradel, Lyon, France. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
van der Maaten, M.J. & Miller, J.M. (1990) Bovine leucosis. In:
Virus Infections of Ruminants, Amsterdam, Elsevier Science
Publishers, pp. 419-429.
Macaulay, V.M. (1992). Insulin-like growth factors and cancer. Br.
J. Cancer, 65, 311-320.
National Institute of Health (1995) NIH conference: Insulin-like
growth factors and cancer. Ann. Intern. Med., 122, 54-59.
Olanrewaju, H., Patel, L. & Seidel, E.R. (1992) Trophic action of
intraileal infusion of insulin-like growth factor I: Polyamine
dependence. Am. J. Physiol., 263, E282-E286.
Phillips, A.F., Rao, R., Anderson, G.G., McCracken, G.M., Lake, M. &
Koldovsky, O. (1995) Fate of insulin-like growth factors I and II
administered orogastrically to suckling rats. Pediatr. Res., 37,
586-592.
Pines, A., Rozen, M.B. & Gilat, T. (1985) Gastrointestinal tumors in
acromegalic patients. Am. J. Gastroenterol., 80, 286-289.
Prusiner, S.B. (1982) Molecular biology of prion diseases. Science,
216, 136.
Prusiner, S.B. (1991) Molecular biology of prion diseases. Science,
252, 1515-1522.
Robertson, D.L. (1997) The evolution of AIDS viruses. From the
Internet
Schaff-Blass, E., Burstein, S. & Rosenfield, R.L. (1984) Advances in
diagnosis and treatment of short stature, with special reference to
the role of growth hormone. J. Pediatr., 104, 801.
Scott, F.W. (1990) Cow milk and insulin-dependent diabetes mellitus:
Is there a relationship? Am. J. Clin. Nutr., 51, 489-491.
Straub, O.C. (1981) Enzootic bovie leucosis. In: Gibbs, E.P.J., ed.,
Virus Diseases of Food Animals, New York, Academic Press, pp.
683-718.
US Food and Drug Administration (1996) CVM Update: VMAC endorses
post-approval program for Posilac(R), 18 December 1996.
Vander A.J., Sherman, J.H. & Luciana, D.S., eds (1990) Human
Physiology, The Mechanisms of Body Function, 5th Ed., New York,
McGraw-Hill Publishing Co., p. 15.
Veenhuizen, J.J., Hintz, R.L., Hemenover, M.L., Kowalczyk, D.F. &
Collier, R.J. (1996) Post-approval monitoring program for Posilac
bovine somatotropin: Tracking program of milk discarded due to drug
residue violations. Unpublished report No. 100-USA-Cow-RLH-93-053 from
Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
Vega, J.R., Gibson, C.A., Skaar, T.C., Hadsell, D.L. & Baumrucker,
C.R. (1991) Insulin-like growth factor (IGF)-I and II and IGF binding
proteins in serum and mammary secretions during the prepartum period
and early lactation in dairy cows. J. Anim. Sci., 69, 2538-2547.
Verge, C.F., Howard, N.J., Irwig, L., Simpson, J.M., Mackerras, D. &
Silink, M. (1994) Environmental factors in childhood IDDM. Diabetes
Care, 17, 1391-1389.
Virtanen, S.M., Räsänen, L., Ylönen, K., Aro, A., Clayton, D.,
Langholz, B., Pitkäniemi, J., Savilahti, E., Lounamaa, R., Tuomolehto,
J. & Akerblom, H.K. (1993) Early introduction of dairy products
associated with increased risk of IDDM in Finnish children.
Diabetes, 42, 1786-1790.
Virtanen, S.M., Saukkonen, T., Savilahti, E., Ylönen, K., Räsänen, L.,
Aro, A., Knip, M., Tuomolehto, J. & Akerblom, H.K. (1994) Diet, cow's
milk protein antibodies and the risk of IDDM in Finnish children.
Diabetologica, 37, 381-387.
Waters, D., Danska, J., Hardy, K., Koster, F., Qualls, C., Nickell,
D., Nightingale, S., Gesundheit, N., Watson, D. & Schade, C. (1996)
Recombinant human growth hormone, insulin-like growth factor 1, and
combination therapy in AIDS-associated wasting. Ann. Intern. Med.,
125, 865-872.
Xian, C.L., Shoubridge, C.A. & Read, L.C. (1995) Degradation of IGF-I
in the adult rat gastrointestinal tract is limited by a specific
antiserum or the dietary protein casein. J. Endocrinol., 146,
215-225.
Zumkeller W. (1992) Relationship between insulin-like growth factor-I
and II and IGF-binding proteins in milk and the gastrointestinal
tract: Growth and development of the gut. J. Pediatr.
Gastroenterol., 15, 357-369.
It was concluded that use of rbST would not result in a higher risk to
human health due to the use of antibotics to treat mastitis and that
the increased potential for drug residues in milk could be managed by
practices currently in use by the dairy industry and by following the
directions for use (US Food & Drug Administration, 1996).
2.2 Concentrations of bovine somatotropin and insulin-like growth
factor in tissues and milk
2.2.1 Tissues
The concentrations of bST and IGF-I were measured in tissues of
cattle that had been treated with a 14-day sustained-release product
containing the natural variant of rbST, somavubove (Choi et al.,
1997), in two experiments. In the first experiment, three groups of 12
beef cattle (except at the low dose, to which only six animals were
exposed) with an average weight of 450 kg were treated by subcutaneous
injection for 20 weeks. The controls received no treatment or vehicle,
one group received 250 mg rbST each week, and another received 500 mg
rbST at two-week intervals. The controls and animals at the high dose
were further divided into groups receiving low- and high-energy feed.
The stated total doses were 5 and 10 g rbST, respectively; however,
the dose for both groups calculated from the stated regimens would be
5 g. Two weeks after the final treatment, the animals were slaughtered
and muscle samples were obtained and stored at -20°C for analysis. In
the second experiment, four groups of beef cattle were used: a control
group which received no drug or vehicle, a group receiving a
sustained-release low dose (0.42 mg/kg bw; 0.03 mg/kg bw per day), a
group receiving an intermediate dose (0.84 mg/kg bw; 0.06 kg bw per
day), and one receiving a high dose (1.26 mg/kg bw; 0.09 mg/kg bw per
day). The treated groups were given the drug by subcutaneous injection
every two weeks for 24 weeks, to give total doses of 2.3, 4.5, and 6.8
g, respectively. Two weeks after the final treatment, the animals were
slaughtered, and samples of muscle, kidney, liver, and fat were taken
and stored at -20°C.
The frozen samples were assayed for bST and IGF-I residues by
radioimmunoassay (RIA) in which 5 g of tissue were extracted in
acidethanol for muscle and acetic acid for kidney, liver, and fat.
The RIA procedures involved standard double-antibody techniques and
iodinated tracers. The detection limit for the assays (the amount that
could be distinguished from zero concentration with 95% confidence)
was 0.17 ng/g bST and 0.61 ng/g IGF-I. The coefficients of variation
for the two assays were 6% or less, and the recoveries from liver,
kidney, and fat were 64% for bST and 84% for IGF-I; similar recoveries
were obtained in muscle samples. A summary of the results is given in
Table 1. The authors concluded that two weeks after administration of
two doses of rbST for extended times, the tissue concentrations of bST
and IGF-I were not significantly different from those in untreated
animals.
2.2.2 Residues of insulin-like growth factor in milk
Extensive information on the residues of bST and IGF-I in the
milk of rbST-treated cows was evaluated by the Committee at its
fortieth meeting. IGF-I is a normal but highly variable constituent of
bovine milk, the concentration depending on the state of lactation,
nutritional status, and age. Over an entire lactation, the IGF-I
concentrations range from 1 to 30 ng/ml, with the highest
concentrations in colostrum and a constant decline thereafter.
Multiparous animals have higher concentrations of IGF-I in milk than
primoparous cows (Burton et al., 1994). Bulk milk from cows not
given rbST had IGF-I concentrations of 19 ng/ml (Juskevich & Guyer,
1990), and the fortieth Meeting cited an average value of 3.7 ng/ml
for untreated cows. In milk from rbST-treated cows, the concentrations
of IGF-I ranged from 1 to 13 ng/ml in most studies and were about
25-70% greater than those in untreated animals (Burton et al.,
1994). The fortieth meeting reported an average IGF-I concentration of
5.9 ng/ml, and the increase was significant, even though most of the
concentrations were < 10 ng/ml.
Table 1. Concentrations of bovine somatotropin (bST) and insulin-like growth factor (IGF-I) in tissues after treatment with
recombinant bovine somatotropin
First experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose High dose
bST IGF-I bST IGF-I bST IGF-I
Muscle 12 1.9 ± 1.8 88 ± 21 1.5 ± 1.6 130 ± 25 3.3 ± 2.2 110 ± 32
Second experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose Intermediate dose High dose
bST IGF-I bST IGF-I bST IGF-I bST IGF-I
Muscle 5 3.4 ± 1.5 45 ± 6.5 4.9 ± 1.5 35 ± 15 3.8 ± 2.0 40 ± 5.1 1.5 ± 0.86 55 ± 19
Fat 4 5.1 ± 1.7 210 ± 85 9.3 ± 5.2 200 ± 65 4.8 ± 1.9 200 ± 53 11 ± 12 340 ± 230
Liver 4 5.2 ± 0.59 350 ± 23 3.6 ± 1.7 390 ± 130 5.4 ± 1.2 380 ± 170 4.6 ± 2.0 290 ± 88
Kidney 4 3.6 ± 1.1 910 ± 130 4.5 ± 1.6 1000 ± 140 4.5 ± 1.8 820 ± 120 3.9 ± 0.94 980 ± 220
Since the original work was reviewed, few additional data have
appeared in the literature or in reports made available by the
sponsors. The manufacturer of Posilac(R) - previously identified as
sometribove, which is a form of rbST approved in a number of countries
- submitted additional information. In a study designed to determine
the concentrations of IGF-I in retail milk samples, the concentrations
in milk specifically labelled as coming from cows that had not been
treated with bST were compared with those in unlabelled milk. While
the sponsor assumed that all of the unlabelled milk was from cows that
had been treated with bST, the extent to which this was true was not
ascertained. The study was conducted under US Food and Drug
Administration GLP regulations (21CFR 58). The labelled and unlabelled
status was determined of 127 of 129 retail milk samples collected as
2% fat cow's milk in 51 retail outlets in 34 cities in Wisconsin,
Minnesota, and Iowa, USA, from four goats and 125 cows, and the
samples were analysed by RIA for IGF-I. For 78 samples, the farmer had
certified that rbST had not been used. As rbST is not approved for use
in goats in the United States, it is assumed that the goat-milk
samples were from untreated animals. The results are shown in Table 2
(Eppard et al., 1994). The values for IGF-I are not unlike those
reported in the monograph prepared at the fortieth meeting of the
Committee. Although the contribution of milk from rbST-treated cows to
the unlabelled milk is unknown, the results indicate that the IGF-I
concentrations in retail milk did not increase in the first year after
the launch of rbST.
Table 2. Concentrations of insulin-like growth factor in milk
certified by farmers as coming from cows not treated with
recombinant bovine somatotropin (labelled) and from unlabelled milk
Insulin-like growth factor (ng/ml)
Labelled Unlabelled
(n = 78) (n = 45)
Raw mean 4.3 ± 0.09 4.5 ± 0.12
Logea 1.47 ± 0.044 1.55 ± 0.031 p = 0.01769
Antilog (95% 4.4 (4.0-4.7) 4.7 (4.4-5.0)
confidence
interval)
a Least square means adjusted for state where purchased and dairy
2.2.3 Assays
Because of variations in the IGF-I values reported in different
studies, questions have been raised in submissions to the Committee
about their accuracy. Lower values were found in some studies because
the assay used involved acid-ethanol extraction; an assay involving
acidic gel filtration has been reported to allow better recovery. In a
comparison of the two assays for determining IGF-I in pre- and
post-partum mammary secretions, the acid-ethanol assay was found to
result in a 24 ± 6.6% underestimate in comparison with acid gel
filtration (Vega et al., 1991). Use of the acidethanol assay to
recover 125I-IGF-I in bovine serum and colostrum, which also contain
binding proteins, gave values of 86 ± 6% and 88 ± 7% recovery by the
antibody, respectively (Hadsell, 1991). The difference is due to the
fact that the binding protein with affinity for IGF-I competes with
the antibody used in the RIA procedure; however, gel filtration
removes the hormone that is extensively dissociated by the acid common
to both assays. The studies suggest that the acidethanol assay
probably results in 15-25% underestimates of the concentrations in
milk and plasma. The relative difference in milk will probably not
affect a decision, as the concentrations are low, although higher
control values may affect the inferences. Although incomplete removal
of IGF-binding proteins or differences in the source of standards
might affect the reported results, the Committee considered these
factors immaterial.
2.2.4 Bioavailability and bioactivity of insulin-like growth factor
residues in milk
At its fortieth meeting, the Committee concluded that many of the
physiological effects of rbSTs are mediated by bovine IGF-I, which is
structurally identical to human IGF-I. It noted that there is
substantial endogenous synthesis of IGF-I, mainly in the liver but
also in human milk, saliva, and pancreatic secretions. It further
concluded that IGF-I has no bioactivity when administered orally to
normal and hypophysectomized rats at doses up to 2 mg/kg bw per day,
as dietary IGF-I is degraded by digestive enzymes and is not active in
the upper gastrointestinal tract.
Concern has been expressed that widespread use of rbSTs in dairy
production would lead to a sustained increase in the concentrations of
IGF-I in bulk cow's milk and that, if IGF-I survives digestion, the
greater exposure of consumers would cause adverse health effects
(Hansen et al., 1997). For a quantitative assessment of risk, the
slight increases in the concentration of IGF-I in milk from
rbST-treated cows should be compared with the physiological variations
in the concentrations of this growth factor during lactation (see
section 2.2.2) and with the concentrations in human breast milk, in
the secretions of the gastrointestinal tract, and in serum.
The concentrations of IGF-I are 8-28 ng/ml in colostrum and
5-10 ng/ml thereafter (Zumkeller, 1992; Burton et al., 1994),
indicating that breast-fed newborns are usually exposed to IGF-I at
concentrations equal to or higher than those in milk from rbST-treated
cows. Assuming a daily intake of 1.5 L of milk from rbST-treated cows
with an average IGF-I concentration of 6 ng/ml, the amount of IGF-I
ingested would be 9000 ng/day. The additional daily ingestion of IGF-I
over that of people drinking milk from untreated animals, with an
average IGF-I concentration of 4 ng/ml or 6000 ng/1.5 L, would be 3000
ng. The slightly increased IGF-I concentrations would contribute to
the endogenous concentrations of IGF-I in the gastrointestinal tract
of consumers; however, the main site of IGF-I production in animals
and humans is the liver. It is also produced in the human
gastrointestinal mucosa and is found in saliva, bile, and pancreatic
juice (Olanrewaju et al., 1992). The average concentrations of IGF-I
in the five human gastrointestinal secretions were 0.9 nmol/L in
saliva, 3.5 nmol/L in gastric juice, 24.6 nmol/L in jejunal chyme, 3.6
nmol/L in pancreatic juice, and 0.9 nmol/L in bile (Chaurasia et
al., 1994). On the basis of a molecular mass of 7.5 kDa for IGF-I
(Zumkeller, 1992) and the volume of each of the fluids produced
(Vander et al., 1990), the total volume of IGF-I emptying into the
gastrointestinal tract is 383 000 ng/day. Table 3 shows that the
amount of endogenous IGF-I emptied into the gastrointestinal tract
daily is more than (383 000/9000) 42 times greater than the amount
present in 1.5 L of milk from rbST-treated cows. The 9000 ng value is
2.3% of the estimated daily gastrointestinal secretion of IGF-I in
adults. The additional daily ingestion of 3000 ng IGF-I over that in
milk from untreated animals thus represents 0.78% of the
gastrointestinal secretion.
Table 3. Concentration of insulin-like growth factor (IGF-I) in
gastrointestinal tract secretions
Secretion Volume Concentration Total IGF-I
(ml/day)a (average; ng/ml) secreted (ng)
Jejunal chyme 1500 184.5 276 750
Pancreatic juice 1500 27.0 40 500
Gastric juice 2000 26.2 52 400
Bile 500 6.8 3 400
Saliva 1500 6.8 10 200
Adapted from Baumann (1995)
a From Vander et al. (1990)
In contrast to the conclusion of the Committee at its fortieth
meeting, that IGF-I is completely and rapidly degraded in the
gastrointestinal tract, milk-borne IGF-I may escape digestion by
proteases and therefore be bioactive in the intestine (Hansen
et al., 1997) or even be absorbed as intact peptide into the
systemic circulation (Epstein, 1996). In a study designed to
investigate the potential therapeutic use of IGF-I and to determine
whether oral formulations were feasible, the degradation of
125I-IGF-I was determined in various segments of the
gastrointestinal tract of rats in vivo and in vitro (Xian
et al., 1995). The extent of degradation was measured by one of
three methods: receptor binding, immunoprecipitation, and
trichloracetic acid precipitation. Two segments of the duodenum,
ileum, or whole stomach and part of the colon were ligated in an
anaesthetized male Sprague-Dawley rat that had been fasted for 24 h. A
bolus of labelled IGF-I (8.6 ng/ml in 0.2% bovine serum albumin in
saline) was injected into each segment and incubated for various times
up to 1 h. The reactions were stopped, and the flushed luminal
contents were examined for intact IGF-I. In a parallel set of
experiments, the flushed luminal contents from each of the four gut
segments were used as a source of degredation enzymes in vitro. The
results are shown in Table 4. The most rapid degradation occurred in
the duodenum and ileum and in their contents in vitro, followed by
the stomach and then by the colon. In all cases the values seen
in vitro were equal to or greater than those seen in vivo.
The authors also examined the effectiveness of slowing the
degradation rate of IGF-I in the gut by protecting the molecule in
several ways. Casein at a concentration of 10 mg/ml conferred > 90%
protection on stomach contents in both the trichloracetic acid and
receptor assays; in duodenal flushings, casein conferred 80%
protection against IGF-I degradation in the trichloracetic acid assay
but only 36% protection in the receptor assay at a maximal casein
concentration of 40 mg/ml. The half-life of IGF-I in the upper
gastrointestinal tract in the receptor assay increased from 2-3 min in
the absence of casein to 35 min in its presence. While these results
appear to demonstrate significant protection by casein at a
concentration similar to those in milk, the authors acknowledge that
the observed effects could be explained by simple competition by the
additional proteins for degradation by proteases in the segments. The
results demonstrate that biological receptor-stimulating activity,
which is the best indicator of biological activity, is dramatically
reduced even with good protection from protease activity. The authors
noted with regard to milk residues of IGF-I that 'the protective
effect of casein makes irrelevant the argument that human saliva
contains IGF-I at concentrations greater than the quantities that
would be consumed in milk. As the IGF-I produced by salivary glands is
free IGF-I, without protective effect of casein, it is unlikely to
survive digestion.' (Hansen et al., 1997). That argumentation
neglects the following facts:
Table 4. Half-lives of intact 125I-insulin-like growth factor (IGF-I) in ligated Sprague-Dawley rat gut
segments in vivo and in the flushed contents in vitro
Test for Half-life (min)
intact IGF-I
Duodenum/ileum Stomach Colon
In vivo In vitroa In vivo In vitro In vivo In vitro
Trichloracetic 2 2 8 50 38 > 60
Antibody binding 2 5 33
Receptor binding 2 2 2.5 3 16 ND
ND, not determined
a Antibody and membrane receptor values reported as receptor binding
* Saliva is not the only source of IGF-I in the gastrointestinal
tract: most is secreted into the gut, and the high concentration
in intestinal chyme indicates that IGF-I is secreted in
substantial amounts by the mucosa throughout the gastrointestinal
tract (Olanrewaju et al., 1992; Chaurasia et al., 1994).
* Casein has a flexible structure and is readily degraded in the
stomach and small bowel (Xian et al., 1995). Thus, the
protective effect is present only in the upper gastrointestinal
tract.
* The half-life of IGF-I in the intestine in the presence of casein
is only 35 min (Xian et al., 1995). Therefore, less than 5% of
an initial dose of IGF-I will survive for more than 2 h during
passage through the upper gastrointestinal tract.
* In the presence of casein ingested with milk, endogenous IGF-I in
the gastrointestinal tract will also be protected.
Therefore, even if casein has a limited protective effect, the amount
of bioactive IGF-I ingested with milk from rbST-treated cows would
still be negligible.
Because of the protective effect of casein, some IGF-I might
escape digestive degradation and be absorbed intact. The absorption of
large (1 mg/kg) oral doses of 125I-labelled recombinant human IGF-I
was studied in fasted adult rats, with trichloracetic acid
precipitation of plasma proteins. The baseline bioavailability of the
administered IGF-I was 9.3% of the dose, but this was was increased by
co-administration of 4 mg/kg aprotinin (47%) and 10 mg/kg casein
(67%). RIA of the plasma confirmed the bioavailability of IGF-I in
this model, and the administered radiolabel was found in the form of
high-molecular-mass complexes (Kimura et al., 1997). It should be
noted, however, that the receptor assay, which is the most accurate,
was not used.
The relatively good bioavailability of intact IGF-I in this adult
rat model is in contrast to the lack of bioactivity of orally
administered IGF-I in adult animals (Annex 1, reference 104) and to
the results of studies with neonatal animals which have an incomplete
mucosal barrier and reduced intestinal proteolytic activity (Burrin,
1997). Studies in neonatal rats and piglets indicated that although
30% of an orally administered dose of 125I-IGF-I can be recovered in
the intestinal mucosa there is limited absorption into the peripheral
circulation (Phillips et al., 1995; Donovan et al., 1997). When
suckling transgenic rats ingested 1000-fold higher concentrations of
des(1,3) human IGF-I, no des(1,3)-IGF-I was detected in the plasma of
their pups (Burrin, 1997). Furthermore, in newborn calves and piglets
given large doses of IGF-I in milk replacers, no substantial increase
in the plasma concentration of this growth factor was found (Donovan
et al., 1997; Hammon & Blum, 1997; Houle et al., 1997). In one
study with newborn calves fed milk replacer, a small amount of orally
administered 125I-IGF-I was detected in plasma (Baumrucker et
al., 1992); however, the increase was observed only three days after
administration and in only three of six animals. Even in newborns,
therefore, IGF-I is absorbed to only a small extent, and absorption is
unlikely in adults.
Furthermore, the amount absorbed should be compared with the
normal concentrations of IGF-I in human serum, which show considerable
variation with age. The values are lowest in infants under two years
of age, then increase steadily to reach a maximum in late puberty, and
afterwards decrease to the adult values (Table 5). Assuming a blood
volume that is 5% of the body weight (Ganong, 1971), the serum load of
IGF-I can be calculated to be 50 000 ng in a 15-kg child, 714 000 ng
in a 60-kg adult, and 1 220 000 ng in a 50-kg teenager. The total
IGF-I production in adults has been estimated at 107 ng per day
(Guler et al., 1989). These amounts should be compared with the 9000
ng IGF-I in 1.5 L of milk, which constitutes only 0.09% of the daily
IGF-I production. Since only one-third of the concentrations in milk
can be attributed to IGF-I due to rbST treatment and only a small
amount if any will be absorbed, the milk-borne IGF-I that reaches the
systemic circulation is negligible and this small amount is
immediately sequestered by unsaturated binding proteins.
Table 5. Concentrations (ng/ml) of insulin-like growth factor (IGF-I)
in human plasma
Age Males Females
Mean Range Mean Range
0-2 years 42 14-98 56 14-238
3-5 years 56 59-210 84 21-322
6-10 years 98 28-308 182 56-364
Before puberty > 10 years 126 84-182 182 70-280
Early puberty 210 140-240 224 84-392
Late puberty 364 224-462 434 224-686
Adult > 23 yeras 112 42-266 140 56-308
From Schaff-Blass et al. (1984)
Concern has been expressed about the possible adverse effects on
the health of consumers exposed to increased concentrations of IGF-I
in milk from rbST-treated cows (Epstein, 1996; Hansen et al., 1997).
The most important potential adverse effects of IGF-I arise from the
fact that it is a mitogen for a number of cell types and has been
associated with the growth of tumours including those of the colon,
breast, and lung and osteosarcoma (Pines et al., 1985; Macaulay,
1992; National Institute of Health, 1995). The mitogenic effect could
also result in proliferative reactions locally in the gut. Thus,
orally administered IGF-I increased the cellularity of the intestinal
mucosa of rats in vivo (Olanrewaju et al., 1992) and increased the
rate of proliferation in cultures of human duodenal epithelial crypt
cells (Challacombe & Wheeler, 1994). Since IGF-I receptors can be
detected throughout the epithelium of the intestine, with a high
density in the colon (Laburthe et al., 1988), and the incidence of
colorectal cancer is increased in acromegalic patients who have
excessively high concentrations of free IGF-I in their plasma (Ezzat &
Melmed, 1991), concern has been expressed that increased
concentrations of milk-borne IGF-I may increase the risk for colon
cancer. Although the normal biological effects of IGF-I mean that it
could promote the growth of tumours, this hazard would become a risk
only if there were adequate exposure of consumers to IGF-I. Since
exposure to IGF-I in milk from rbST-treated cows is negligible when
compared with endogenous IGF-I production, it is extremely unlikely
that IGF-I residues cause any systemic or local mitogenic reaction.
2.3 Expression of lentiviruses and prion proteins
2.3.1 Somatotropins and the immune system
Somatotropin enhances the immune system in many species,
including cattle (Comens-Keller et al., 1995). The primary effect
appears to be altered responsiveness of the immune system, although
substantive evidence of this effect is lacking (Burton et al.,
1994), and information on changes in cytokine concentrations or
secretion and on their binding sites are needed in order to define the
nature of the immune-enhancing effects of somatotropins. The studies
reported in the literature differ with regard to the source of
somatotropin, the treatment schedule, and the age of the animals. The
differences between findings in vitro and in vivo may be due to
the release in vivo of mediators such as IGFs and cytokines, which
are not present in vitro (Kelley, 1989). A better understanding of
somatotropin-mediated immune enhancement as a homeorhetic regulator of
the overall health and disease resistance of animals is needed.
Lymphocytes from rbST-treated cows have a greater average maximum
lymphoblastogenic response to rbST than to other mitogens around the
time of parturition (Comens-Keller et al., 1995), and this effect
might prevent mastitis and the other infectious diseases that occur
during this period of immunosuppression.
2.3.2 Effect of bovine somatotropin on the expression of retroviruses
Concern has been expressed that the immunomodulatory effect of
bST might affect retroviral expression in treated animals and thus
cause resurgence of latent retroviral and lentiviral infections in the
ruminant population and the presence of these viruses in somatic cells
in milk. The concern is based largely on a review by Lerondelle et al.
(1994), who discussed the evidence for induction of these viruses in
small ruminants by steroid hormones and the evidence for induction of
lentiviruses in other species by hormones including growth hormone and
IGF-I, and an unpublished study by Lerondelle et al. (1996), who
investigated the effects of rbST on the expression of caprine
arthritis encephalitis virus (CAEV) in goats. This virus belongs to
the group of lentiviruses which, like maedivisna, can infect small
ruminants.
Ruminant lentiviruses are of interest for at least three reasons.
First, they may cause disease in persons who drink milk. Second, if
use of rbST increases the prevalence of ruminant viruses, by the
presence of rbST itself or by the action of IGF-I, the small
additional amounts of growth hormone in the milk of treated cows could
also affect the retroviruses that attack the human immune system,
HIV-1 and HIV-2. Finally, the severity or kinetics of expression of
the disease in ruminants might be increased.
The study of Lerondelle and coworkers (1996) attempted to address
the question of whether rbST increases the expression of CAEV. Viral
expression was measured by assaying reverse transcriptase activity in
milk cells, clinical examination of the udders and joints of the
animals at the beginning and end of the study, and evidence of
infection in an immunodiffusion assay. Twelve pregnant Saanen goats
that were seronegative for CAEV were given an intramammary injection
of monocytes infected in vitro with the Cork strain of CAEV at the
time of drying off. Seven weeks after giving birth, four goats were
treated daily with 5 mg/goat rbST (sometribove), a second group was
treated daily with 10 mg/goat thyroxine, and the control group was
untreated. The compounds were administered in suspension in sterile
water for 30 days, followed by a 45-day observation period. Milk
samples were taken to measure reverse transcriptase activity on days
7, 14, 21, and 28 of treatment and three times during the observation
period. Examination of the udders and joints and immunodiffusion tests
were carried out at the beginning and end of the study. Milk
production and milk-cell counts were evaluated every two days. The
occurrence of CAEV and the onset of effects are shown in Table 6. More
positive cultured cells were seen in the controls than after treatment
with either hormone. Perhaps the most striking effect is the lack of
increase in the rate of infectivity and even a suggestion of a
decrease after treatment with rbST, particularly after the first milk
sampling.
When the results were expressed as a ratio of transcriptase
activity to the number of cells considered to contain virus (Table 7),
there was no correlation between the effect of rbST or thyroxine and
the activity of reverse transcriptase in the milk samples. In fact,
there was no evidence of increased transcriptase activity in any
group. In particular, even though the group receiving rbST had a lower
initial rate of infection than the other two groups, including the
controls, the rate of infection was not increased, as measured by the
number of positive cultures or increased transcriptase activity. The
authors interpreted their results as showing a tendency to increased
viral expression with increased milk production. The results for rbST
were, however, biased by the fact that only two animals were included
in the final evaluation. The authors concluded that, owing to the
Table 6. Onset of appearance of cytopathic effect (in days) for each of 10 milk samplings from groups of four goats given no treatment or
thyroxine or recombinant bovine somatotropin (rbST), as a function of the period of hormonal treatment
Treatment Milk sampling
Before treatment During treatment After treatment
1 2 3 4 5 6 7 8 9
Control 4 8 6 10 6 6 6 8 10
4 6 6 6 8 6 6 6 -
4 6 4 6 4 4 6 4 4
6 8 10 6 10 6 - - -
Mean ± SD (n) 6 ± 1.91 (12) 6.4 ± 1.71 (15) 7 ± 2.39 (8)
Thyroxine
8 6 10 4 6 6 - - 6
6 4 4 4 4 4 4 4 ND
4 4 4 4 4 4 6 4 ND
4 8 6 4 4 4 6 4 ND
Mean ± SD (n) 5.67 ± 2.06 (12) 4.53 ± 0.92 (15) 4.25 ± 0.71 (8)
rbST 8 - - - - - - - -
4 8 8 - 8 - - - 4
ND - - - - - - - -
4 4 4 4 4 4 4 4 4
Mean ± SD (n) 5.71 ± 2.41 (7) 4.8 ± 1.79 (5) 4 (4)
ND, not determined
Table 7. Number of samples found to contain virus by the reverse
transcriptase-positive culture ratio in groups of four goats given
no treatment, thyroxine, or recombinant bovine somatotropin (rbST)
Treatment Before During After Total
treatment treatment treatment
Control 3/6 4/7 3/4 10/17
3/6 3/5 1/3 7/14
6/6 4/5 5/6 15/17
4/4 2/8 3/6 9/18
Total 16/22 13/25 12/19 41/66
Thyroxine 0/3 2/4 0/3 2/10
4/4 5/7 6/6 15/17
6/6 8/8 6/6 20/20
6/6 8/8 4/6 18/20
Total 16/19 23/27 16/21 55/67
rbST 2/4 0/6 0/1 2/11
6/6 7/8 2/5 15/19
0/2 0/4 0/3 0/8
4/4 4/5 5/6 12/14
Total 12/16 11/23 7/15 29/52
heterogeneity of the effects on milk production and the small number
of animals tested, the evidence for a promoting effect of rbST on
viral expression was insufficient.
These results provide no evidence that treatment of cows infected
with lentiviruses with rbST will cause resurgence of viral infections
or pose any risk to human health. Lentiviruses are retroviruses that
replicate only in activated cells of the immune system. They may stay
dormant for months or years before they gradually wear down the immune
system to the point of collapse. The phylogenetic tree of lentiviruses
includes the subfamily of bovine immunodeficiency virus (also called
bovine leukaemia virus, BLV) and the subfamily of HIV-1 and HIV-2
which are the causative agents of AIDS. BLV and HIV are widely
separated phylogenetically (Robertson, 1997). BLV is not known to
cause disease in humans although it can infect human cells in
vitro, where host defence mechanisms are not present (Georgiades
et al., 1978; van der Maaten & Miller, 1990); infection of human
cells in vivo could not be demonstrated, and all attempts to obtain
direct evidence of infection in exposed human populations have yielded
negative results (Straub, 1981; van der Maaten & Miller, 1990).
Failure to infect humans has also been shown for other ruminant
lentiviruses, such as CAEV and maedivisna (Straub, 1981). Excretion
of virus with milk somatic cells can infect the offspring of infected
cows. Such transmission can be effectively blocked by procedures
similar to pasteurization, which destroy the virus at 60°C within 30 s
(Abramova et al., 1974; Baumgartner et al., 1976; van der Maaten &
Miller, 1990). BLV therefore cannot induce disease in humans and is
completely inactivated by routine pasteurization. Furthermore, the
company that sells sometribove has reported that there is no
indication that the incidence of BLV infection has increased in cattle
after eight years of continuous use of rbST in Mexico and Brazil and
four years of use in the United States (Collier & Kowalczyk, 1998),
although this statement is not further qualified.
An increase in the expression of HIV in humans who ingest milk
from rbST-treated cows is extermely unlikely because of the negligible
residues of rbST and IGF-I. Treatment of AIDS patients for six weeks
with recombinant human growth hormone and IGF-I had no effect on the
HIV titres in peripheral blood mononuclear cells, on the CD3, CD4, or
CD8 counts in peripheral blood, or on serum HIV p24 antigen
concentrations (Waters et al., 1996).
2.3.3 Effect of recombinant bovine somatotropin on prion proteins
Concern has been expressed that rbST treatment could increase the
risk for BSE in dairy cows (Hansen et al., 1997). Little evidence is
available to support this concern, and that which has been provided is
indirect. It is currently thought that the infectious agent of BSE is
a prion protein (Prusiner, 1982). Prion proteins are found normally in
all animals and are encoded by a prion-protein gene. BSE is associated
with a post-translationally modified protease-resistant prion protein,
which differs in its three-dimensional structure from the normal
protease-sensitive prion protein. Normal prion proteins are bound to
membranes on the surface of all nerve cells, some lymphocytes, and
other tissues (Prusiner, 1991). To date, no function has been ascribed
to normal prion protein. The most widely accepted theory of BSE is the
conversion of normal prion protein to the abnormal protease-resistant
form, which in turn causes more normal prion protein to convert to
protease resistance. The mechanisms of the conversion to the
disease-causing protease-resistant prion protein are not clearly
understood. In contrast to the normal form, the protease-resistant
form cannot be turned over and builds up in cells, forming large
oligomers which are observed as plaques (amyloids) in the brains of
affected individuals (Gajdusek, 1993).
IGF-I increased the production of prion protein mRNA in vitro
in a rat phaeochromocytoma cell line (PC12 cells), with a rather flat
dose-response curve showing a 40% increase at 10 ng/ml and a doubling
at 100 ng/ml (Lasmézas et al., 1993). In transgenic mice that
harbour multiple copies of the prion protein gene, the progession of
scrapie was accelerated (Prusiner, 1991). It has been speculated that
the increased IGF-I concentrations in rbST-treated cows could increase
prion protein production and possibly speed up the progression of BSE;
however, no studies are available that directly address the question
of whether rbST or IGF-I increases the formation of normal prion
protein or its pathogenic protease-resistant mutant in the brains of
the cattle, and the possibility of a link between rbST treatment and
BSE is highly speculative.
2.4 Cow's milk and insulin-dependent type I diabetes mellitus in
childhood
Epidemiological studies have shown that environmental factors
such as chemicals, viral infections, a short duration of
breastfeeding, and early dietary exposure of newborns to cow's milk
increase the risk for insulin-dependent type I diabetes mellitus
(IDDM) by about 1.5 times (Scott, 1990; Dahlquist et al., 1991;
Jorgensen et al., 1991; Virtanen et al., 1993; Gerstein, 1994;
Verge et al., 1994; Virtanen et al., 1994). IDDM develops as a
consequence of autoimmune destruction of the insulin-producing b cells
of the pancreatic islets. The precise trigger of the autoimmune
reaction is unknown, but it is assumed to be a genetically acquired
immune defect in susceptible individuals (Gerstein, 1994). The
epidemiological evidence indicates that IDDM is geographically and
temporally related to neonatal feeding with cow's milk and that
avoidance of cow's milk during the first few month of life can protect
genetically predisposed individuals (Gerstein, 1994; Verge et al.,
1994).
Serological evidence supports the theory that the immune defect
is triggered by exposure to proteins in cow's milk (Gerstein, 1994).
It has been postulated that, in newborns, milk proteins cross the
immature gut wall, initiating an immune response that cross-reacts
with a beta-cell surface antigen (Verge et al., 1994). Children aged
five to nine, who have an intact intestinal barrier, are not at risk
of acquiring IDDM by drinking cow's milk (Dahlquist et al., 1991).
The possible triggering factors in cow's milk have not been
identified. Casein is unlikely to be involved, since replacement of
milk proteins by casein in the diets of rats susceptible to diabetes
completely prevented the disease (Jorgensen et al., 1991). Increased
concentrations of immunoglobulin A antibodies to cow's milk and
b-lactoglobulin appear to be associated with an increased risk for
IDDM (Dahlquist et al., 1991; Virtanen et al., 1994).
It is unlikely that exposure of human newborns to milk from
rbST-treated cows would increase the risk for IDDM for the following
reasons:
* The composition of milk from rbST-treated cows is well within the
normal variations observed during lactation,
* The slightly increased IGF-I concentrations can be excluded as a
triggering factor because bovine and human IGF-I are identical
and because the concentrations of IGF-I in breast milk are equal
to or initially higher than those found in cow's milk.
3. COMMENTS
Use of antibiotics
After reviewing the available information, the Committee
considered the risk for mastitis associated with use of rbST as an
issue of animal health that is not within its terms of reference;
however, the possibly increased use of antibiotics was considered. A
post-approval monitoring programme was established in the United
States to address the following issues:
* the incidence of mastitis and responses related to herd health
(not within the terms of reference of the Committee),
* treatment with any medications of 28 herds of rbST-treated cows
(not within the terms of reference of the Committee), and
* the percent of milk discarded because of violative drug residues
in key dairy states representing at least 50% of United States
milk production.
In New York State, the percentage of milk discarded because of the
presence of antibiotic residues was not significantly changed after
the introduction of rbST. In other states, however, a small but
statistically significant, increase was observed in 1995, which
coincided with a change to a more sensitive testing method in those
states. The Committee concluded that the use of rbST would not result
in a higher risk to human health due to the use of antibiotics to
treat mastitis and that the increased potential for drug residues in
milk could be managed by practices currently in use within the dairy
industry and by following the directions for use.
Concentrations of insulin-like growth factor in milk and tissues
Insulin-like growth factor (IGF-I) is a normal component of milk
and is found in abundance in a variety of body fluids (Table 8). The
presence and concentrations of IGF-I were the source of much of the
scientific discussion during the original review of bST undertaken at
the fortieth meeting of the Committee and in submissions to the
present one. The information that was reviewed is summarized in FAO
Food and Nutrition Paper No. 41/5 (Annex 1, reference 106). The
concentrations of IGF-I in milk are variable and have been shown to
depend on the state of lactation, nutrition, and age.
Methods for assaying IGF-I were considered by the Committee.
Although incomplete removal of IGF-binding proteins or differences in
the source of standards and extraction methods might affect the
reported values, these factors were considered not to alter any
conclusions materially. The relatively high values previously reported
in milk were considered to reflect inadequate extraction.
Table 8. Insulin-like growth factor in milk and body fluids
Medium Concentration
(ng/ml)
Milk
Human 5-10
Colostrum 8-28
Bovine (bulk milk)
Untreated 1-9
rbST-treated 1-13
Plasma
Child 17-250
Adolescent 180-780
Adult 120-460
Gastrointestinal secretions (human)
Saliva 6.8
Gastric juice 26
Pancreatic juice 27
Bile 6.8
Jejunal chyme 180
Daily production of adult humans 107 ng/d
Since the previous evaluation, few additional data on residues
have appeared in the literature or in reports provided by interested
parties; however, the manufacturer of sometribove submitted additional
information on the concentrations of IGF-I in retail milk after the
approval of rbST in the United States. The results showed no
difference in the IGF-I concentrations of milk certified as derived
from cows not treated with rbST and of unlabelled milk; however, the
percentage of the unlabelled milk that was derived from cows receiving
rbST was not specified.
Concern has been expressed that any rbST-induced increase in the
concentration of IGF-I in milk would contribute to the endogenous
levels of IGF-I in the gastrointestinal tract and serum if it were not
biodegraded and were absorbed. In rats, IGF-I is rapidly degraded in
the gastrointestinal tract; however, a protective effect of casein on
IGF-I was demonstrated in these studies. It was postulated that the
retarded degradation leads to increased serum levels of IGF-I (as
shown in one study in rats) and to prolonged exposure of the gut. The
Committee noted that seven days' oral administration of high doses of
IGF-I in milk replacer did not increase the circulating concentrations
of IGF-I in newborn calves and piglets, indicating that significant
absorption of IGF-I is unlikely to occur under physiological
conditions. In view of the decreased rate of degradation in the small
intestine of rats in the presence of casein, the concentrations of the
growth factor would probably decrease to less than 5% of their initial
values within 2 h, so that milk-borne IGF-I would not be expected to
contribute to the concentrations of IGF-I in the large intestine.
If 1.5 L of milk are ingested per day, the average intake of
IGF-I will be 6000 ng in milk from untreated cows containing an
assumed IGF-I concentration of 4 ng/ml and 9000 ng in milk from rbST-
treated cows with an approximate average concentration of 6 ng/ml. It
has been calculated that IGF-I in gastrointestinal secretions amounts
to about 380 000 ng/day. Therefore, the additional amount of IGF-I in
1.5 L of milk from rbST-treated cows would represent only about 0.8%
of the IGF-I secreted in the gastrointestinal tract. The total amount
of IGF-I in serum has been calculated to range from 50 000 to 1 220
000 ng, depending on age. The total daily IGF-I production in adult
humans has been estimated to be 107 ng. Therefore, the daily amount
of IGF-I ingested with milk from rbST-treated cows would represent
less than 0.09% of the daily production of adults. Even if all of the
milk-borne IGF-I were absorbed, the additional amount would be
negligible.
When sustained-release rbST was administered to cattle once every
two weeks for a total of 20 weeks, the tissue concentrations of rbST
and IGF-I two weeks after the final dose were not significantly
increased.
The Committee concluded that any increase in the concentration of
IGF-I in milk from rbST-treated cows is orders of magnitude lower than
the physiological amounts produced in the gastrointestinal tract and
in other parts of the body. Thus, the concentration of IGF-I would not
increase either locally in the gut or systemically, and the potential
for IGF-I to promote tumour growth would not increase when milk from
rbST-treated cows was consumed; there is thus no appreciable risk for
consumers.
Expression of retroviruses
Concern that treatment of cattle with rbST would increase the
expression of retroviruses, including BLV, were addressed in
experiments with caprine arthritis encephalitis virus in goats.
Infectivity was not increased when measured as numbers of infected
cells, and there was no evidence of increased reverse transcriptase
activity. These studies provide no evidence that rbST affects the
expression of BLV, a lentivirus in cattle. Furthermore it has been
shown that BLV is destroyed in simulated pasteurization conditions by
heating milk to 60 .C for 30 s. In addition, there is no evidence
of human susceptibility or response to ruminant retroviruses.
Expression of prion proteins
Concern has been expressed that treatment with rbST could shorten
the incubation period for BSE. This hypothesis is based on results
obtained with a neuronal cell line in vitro indicating increased
formation of prion protein mRNA in response to IGF-I. Furthermore,
increased formation of prion protein shortened the incubation period
for scrapie in transgenic mice harbouring multiple copies of the gene
that codes for prion proteins. No data were available, however, to
address directly the question of whether rbST or IGF-I increases the
formation of normal prion protein or its pathogenic protease-resistant
mutant in the brains of cattle. The Committee considered that the
possibility of a link between rbST treatment and BSE was highly
speculative.
Risk for insulin-dependent diabetes mellitus
Exposure of human newborns to cow's milk increases the risk for
insulin-dependent diabetes mellitus by about 1.5-fold. The Committee
considered whether exposure of newborns to milk from rbST-treated cows
further increases this risk. It concluded that, because of its
unchanged composition, the milk of rbST-treated cows would not pose an
additional risk to the development of insulin-dependent diabetes
mellitus.
4. EVALUATION
On the basis of the following:
* insignificant changes in the quantities of milk discarded after
testing for antibiotic residues following the introduction of
rbST into commercial use;
* low concentrations of rbST and IGF-I residues in milk;
* degradation of IGF-I in the gut and its abundance in gut
secretions;
* the extremely low concentrations of ingested IGF-I in comparison
with endogenous production;
* lack of evidence that rbST stimulates the expression of
retroviruses;
* lack of a direct link between rbST treatment and BSE; and
* the absence of significant changes in the composition of milk
from rbST-treated cows that could contribute to an additional
risk for the development of insulin-dependent diabetes mellitus,
the Committee concluded that rbST can be used without any appreciable
risk to the health of consumers. The Committee reaffirmed its previous
ADIs1 and MRLs2 'not specified' for somagrebove, sometribove,
somavubove, and somidobove.
5. REFERENCES
Abramova, E.M., Kondratev, V.S. & Sytinskii, I.A. (1974) The
biochemistry of leucosis in cattle. Vet. Bull., 44, 689-711.
Baumann, D.M. (1995) IGF-I Fact Sheet, Department of Animal Science,
Cornell University, Ithaca, NY, USA.
Baumgartner, L.E., Olson, C. & Onuma, M. (1976) Effect of
pasteurization and heat treatment on bovine leukemia virus. J. Am.
Vet. Med. Assoc., 169, 1189-1191.
Baumrucker, R.R., Hadsell, D.J., Skarr, T.C., Campbell, P.G. & Blum,
J.W. (1992) Insulin-like growth factors (IGFs) and IGF binding
proteins in mammary secretions: Origins and implications in neonatal
physiology. In: Picciano, M.F. & Lonnerdal, B., eds, Mechanisms
Regulating Lactation and Infant Nutrient Utilization, New York,
Wiley-Liss, pp. 285-308.
Burrin, D.G. (1997). Is milk-borne insulin-like growth factor-l
essential for neonatal development? J. Nutr., 127, 975S-979S.
Burton, J.L., McBride, B.W., Block, E., Glimm, D.R. & Kenelly, J.J.
(1994) A review of bovine growth hormone. Can. J. Anim. Sci., 74,
167-201.
Challacombe, D.N. & Wheeler, E.E. (1994) Safety of milk from cows
treated with bovine somatotropin. Lancet, 344, 815-816.
Chaurasia, O.P., Marcuard, S.P. & Seidel, E.R. (1994) Insulin-like
growth factor l in human gastrointestinal exocrine excretion.
Regul. Pept., 50, 113-119.
Choi, J., Choi, M.J., Kim, C., Ha, J., Hong, A., Ji, Y. & Chang, B.
(1997). The effect of recombinant bovine somatotropin (rbST)
administration on residual BST and insulin-like growth factor I levels
in various tissues of cattle. J. Food Hyg. Soc. Jpn, 38, 225-232.
Codex Alimentarius Commission (1997) Report of the Twenty-second
Session of the Codex Alimentarius Commission, Geneva, 23-28 June
1997 (FAO Document ALINORM 97/37), Rome, Food and Agriculture
Organization of the United Nations.
Collier, R.J. (1996) Post-approval evaluation of Posilac bovine
somatotropin in commercial dairy herds (100-USA-COW-RJC-93-051).
Unpublished report from Protiva, Monsanto Co. Submitted to WHO by
Protiva, Monsanto Co., St Louis, Missouri, USA.
Collier, R.J. & Kowalczyk, D.F. (1998) Human health risk of
retroviruses in cattle and bovine somatotropine. Unpublished report
from Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co.,
St Louis, Missouri, USA.
Comens-Keller, P.G., Eppard, P.J. & Collier, R.L. (1995). Evaluation
of somatotropin as a homeorhetic regulator of immunity. In: Ivan, M.,
ed., Animal Science Research and Development: Moving Toward a New
Century. Proceedings of the 75th Anniversary Meeting of the
Canadian Society of Animal Science, Ottawa, pp. 79-94.
Dahlquist, G., Blom, L. & Lönnberg, G. (1991) The Swedish childhood
diabetes study: A multivariate analysis of risk determinants for
diabetes in different age groups. Diabetologica, 34, 757-762.
Donovan, S.M., Chao, J.C., Zijlstra, R.T. & Odle, J. (1997) Oral
administered iodinated recombinant human insulin-like growth factor-I
(125I-rhIGF-I) is poorly absorbed by the neonatal piglet. J.
Pedriat. Gastroenterol. Nutr., 24, 174-182.
Eppard, P.J., Collier, R.J., Hintz, R.L., Veenhuizen, J.J. & Baile,
C.A. (1994) Survey of milk insulin-like growth factor in retail milk
samples. Unpublished report No. 100-USA-COW-RJC-94-074 from Protiva,
Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St Louis,
Missouri, USA.
Epstein, S.S. (1996) Unlabeled milk from cows treated with
biosynthetic growth hormones: A case of regulatory abdication. Int.
J. Health Serv., 26, 173-185.
Ezzat, H. & Melmed, S. (1991) Clinical review 18: Are patients with
acromegaly at increased risk for neoplasia? J. Clin. Endocrinol.
Metab., 72, 245-249.
Gajdusek, D.C. (1993) Genetic control of nucleation and polymerization
of host precursors to infectious amyloids in the transmissible
amyliodoses of brain. Br. Med. Bull., 49, 913-931.
Ganong, W.F., ed. (1971) Review of Medical Physiology, Los Altos,
Lange Medical Publications, p. 5.
Georgiades, J.A., Billiau, A. & Vanderschueren, B. (1978) Infection of
human cell cultures with bovine visna virus. J. Gen. Virol., 38,
375-381.
Gerstein, H.C. (1994) Cow's milk exposure and type I diabetes
mellitus. Diabetes Care, 17, 13-19.
Guler, H.P., Zapf, J., Schmid, C. &, Froesch, E.R. (1989) Insulin-like
growth factors I and II in healthy man. Estimations of half-lives and
production rates. Arch. Endocrinol., 121, 753-758.
Hadsell, D.L. (1991) Insulin-like Growth Factor (IGF) Receptors in
the Bovine Mammary Gland: Regulation, Mitogenic Action, and Role in
Mammary Secretion of IGF-I, PhD Thesis, Pennsylvania State
University, Philadelphia, Pennsylvania, pp. 108-114,
Hammon, H. & Blum, J.W. (1997) The somatotropic axis in neonatal
calves can be modulated by nutrition, growth hormone and
Long-R3-IGF-I. Am. J. Physiol., 273, E130-E138.
Hansen, M., Halloran, J.M., Groth, E., III & Lefferts, L.Y. (1997)
Potential public health impacts of the use of recombinant bovine
somatotropin in dairy production. Unpublished report. Submitted to WHO
by Consumer Policy Institute, Yonkers, New York, USA.
Houle, V.M., Sschroeder, E.A., Odle, J. & Donovan, S.M. (1997) Small
intestinal disaccharidase activity and ileal villus height are
increased in piglets consuming recombinant human insulin-like growth
factor-I. Pediatr. Res., 42, 78-86.
Jorgensen, K.D., Joner, G. & Hanssen, K.F. (1991) Relationship between
cows' milk consumption and incidence of childhood IDDM. Diabetes
Care, 14, 1081-1083.
Juskevich, J.C. & Guyer, C.G. (1990) Bovine growth hormone: Human food
safety evaluation. Science, 249, 875-884.
Kelley, K.W. (1989) Growth hormone, lymphocytes and macrophages.
Biochem. Pharmacol., 38, 705-713.
Kimura, T., Murakawa, Y., Ohno, M., Ohtani, S. & Higaki, K. (1997)
Gastrointestinal absorption of recombinant human insulin-like growth
factor-I in rats. J. Pharmacol. Exp. Ther., 283, 611-618.
Laburthe, M., Rouyer-Fessard, C. & Gammeltoft, S. (1988) Receptors for
insulin-like growth factors I and II in the rat gastrointestinasl
epithelium. Am. J. Physiol., 254, 457-462.
Lasmézas, C., Deslys, J.P. & Dormont, D. (1993) Recombinant human
growth hormone and insulin-like growth factor I induce PrP gene
expression in PC12 cells. Biochem. Biophys. Res. Commun., 196,
1163-1169.
Lerondelle, C., Lena, P. & Mornex, J.F. (1994) [Hormonal control of
retroviral infections.] Renc. Rech. Ruminants, 1, 4550 (in French).
Lerondelle, C., Guiguen, F., Fornazero, C., Gounel, F., Leroux, C.,
Chastang, J., Pardo, E., Bolland, P., Bruyas, S., Cordier, G. &
Mornex, J.F. (1996) Effects of recombinant bovine somatotropin and of
thyroxine on the expression of lentiviruses in ruminants. Unpublished
report from INRA Veterinary School of Lyon and INSERM Hôpital Louis
Pradel, Lyon, France. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
van der Maaten, M.J. & Miller, J.M. (1990) Bovine leucosis. In:
Virus Infections of Ruminants, Amsterdam, Elsevier Science
Publishers, pp. 419-429.
Macaulay, V.M. (1992). Insulin-like growth factors and cancer. Br.
J. Cancer, 65, 311-320.
National Institute of Health (1995) NIH conference: Insulin-like
growth factors and cancer. Ann. Intern. Med., 122, 54-59.
Olanrewaju, H., Patel, L. & Seidel, E.R. (1992) Trophic action of
intraileal infusion of insulin-like growth factor I: Polyamine
dependence. Am. J. Physiol., 263, E282-E286.
Phillips, A.F., Rao, R., Anderson, G.G., McCracken, G.M., Lake, M. &
Koldovsky, O. (1995) Fate of insulin-like growth factors I and II
administered orogastrically to suckling rats. Pediatr. Res., 37,
586-592.
Pines, A., Rozen, M.B. & Gilat, T. (1985) Gastrointestinal tumors in
acromegalic patients. Am. J. Gastroenterol., 80, 286-289.
Prusiner, S.B. (1982) Molecular biology of prion diseases. Science,
216, 136.
Prusiner, S.B. (1991) Molecular biology of prion diseases. Science,
252, 1515-1522.
Robertson, D.L. (1997) The evolution of AIDS viruses. From the
Internet
Schaff-Blass, E., Burstein, S. & Rosenfield, R.L. (1984) Advances in
diagnosis and treatment of short stature, with special reference to
the role of growth hormone. J. Pediatr., 104, 801.
Scott, F.W. (1990) Cow milk and insulin-dependent diabetes mellitus:
Is there a relationship? Am. J. Clin. Nutr., 51, 489-491.
Straub, O.C. (1981) Enzootic bovie leucosis. In: Gibbs, E.P.J., ed.,
Virus Diseases of Food Animals, New York, Academic Press, pp.
683-718.
US Food and Drug Administration (1996) CVM Update: VMAC endorses
post-approval program for Posilac(R), 18 December 1996.
Vander A.J., Sherman, J.H. & Luciana, D.S., eds (1990) Human
Physiology, The Mechanisms of Body Function, 5th Ed., New York,
McGraw-Hill Publishing Co., p. 15.
Veenhuizen, J.J., Hintz, R.L., Hemenover, M.L., Kowalczyk, D.F. &
Collier, R.J. (1996) Post-approval monitoring program for Posilac
bovine somatotropin: Tracking program of milk discarded due to drug
residue violations. Unpublished report No. 100-USA-Cow-RLH-93-053 from
Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
Vega, J.R., Gibson, C.A., Skaar, T.C., Hadsell, D.L. & Baumrucker,
C.R. (1991) Insulin-like growth factor (IGF)-I and II and IGF binding
proteins in serum and mammary secretions during the prepartum period
and early lactation in dairy cows. J. Anim. Sci., 69, 2538-2547.
Verge, C.F., Howard, N.J., Irwig, L., Simpson, J.M., Mackerras, D. &
Silink, M. (1994) Environmental factors in childhood IDDM. Diabetes
Care, 17, 1391-1389.
Virtanen, S.M., Räsänen, L., Ylönen, K., Aro, A., Clayton, D.,
Langholz, B., Pitkäniemi, J., Savilahti, E., Lounamaa, R., Tuomolehto,
J. & Akerblom, H.K. (1993) Early introduction of dairy products
associated with increased risk of IDDM in Finnish children.
Diabetes, 42, 1786-1790.
Virtanen, S.M., Saukkonen, T., Savilahti, E., Ylönen, K., Räsänen, L.,
Aro, A., Knip, M., Tuomolehto, J. & Akerblom, H.K. (1994) Diet, cow's
milk protein antibodies and the risk of IDDM in Finnish children.
Diabetologica, 37, 381-387.
Waters, D., Danska, J., Hardy, K., Koster, F., Qualls, C., Nickell,
D., Nightingale, S., Gesundheit, N., Watson, D. & Schade, C. (1996)
Recombinant human growth hormone, insulin-like growth factor 1, and
combination therapy in AIDS-associated wasting. Ann. Intern. Med.,
125, 865-872.
Xian, C.L., Shoubridge, C.A. & Read, L.C. (1995) Degradation of IGF-I
in the adult rat gastrointestinal tract is limited by a specific
antiserum or the dietary protein casein. J. Endocrinol., 146,
215-225.
Zumkeller W. (1992) Relationship between insulin-like growth factor-I
and II and IGF-binding proteins in milk and the gastrointestinal
tract: Growth and development of the gut. J. Pediatr.
Gastroenterol., 15, 357-369.
 It was concluded that use of rbST would not result in a higher risk to
human health due to the use of antibotics to treat mastitis and that
the increased potential for drug residues in milk could be managed by
practices currently in use by the dairy industry and by following the
directions for use (US Food & Drug Administration, 1996).
2.2 Concentrations of bovine somatotropin and insulin-like growth
factor in tissues and milk
2.2.1 Tissues
The concentrations of bST and IGF-I were measured in tissues of
cattle that had been treated with a 14-day sustained-release product
containing the natural variant of rbST, somavubove (Choi et al.,
1997), in two experiments. In the first experiment, three groups of 12
beef cattle (except at the low dose, to which only six animals were
exposed) with an average weight of 450 kg were treated by subcutaneous
injection for 20 weeks. The controls received no treatment or vehicle,
one group received 250 mg rbST each week, and another received 500 mg
rbST at two-week intervals. The controls and animals at the high dose
were further divided into groups receiving low- and high-energy feed.
The stated total doses were 5 and 10 g rbST, respectively; however,
the dose for both groups calculated from the stated regimens would be
5 g. Two weeks after the final treatment, the animals were slaughtered
and muscle samples were obtained and stored at -20°C for analysis. In
the second experiment, four groups of beef cattle were used: a control
group which received no drug or vehicle, a group receiving a
sustained-release low dose (0.42 mg/kg bw; 0.03 mg/kg bw per day), a
group receiving an intermediate dose (0.84 mg/kg bw; 0.06 kg bw per
day), and one receiving a high dose (1.26 mg/kg bw; 0.09 mg/kg bw per
day). The treated groups were given the drug by subcutaneous injection
every two weeks for 24 weeks, to give total doses of 2.3, 4.5, and 6.8
g, respectively. Two weeks after the final treatment, the animals were
slaughtered, and samples of muscle, kidney, liver, and fat were taken
and stored at -20°C.
The frozen samples were assayed for bST and IGF-I residues by
radioimmunoassay (RIA) in which 5 g of tissue were extracted in
acidethanol for muscle and acetic acid for kidney, liver, and fat.
The RIA procedures involved standard double-antibody techniques and
iodinated tracers. The detection limit for the assays (the amount that
could be distinguished from zero concentration with 95% confidence)
was 0.17 ng/g bST and 0.61 ng/g IGF-I. The coefficients of variation
for the two assays were 6% or less, and the recoveries from liver,
kidney, and fat were 64% for bST and 84% for IGF-I; similar recoveries
were obtained in muscle samples. A summary of the results is given in
Table 1. The authors concluded that two weeks after administration of
two doses of rbST for extended times, the tissue concentrations of bST
and IGF-I were not significantly different from those in untreated
animals.
2.2.2 Residues of insulin-like growth factor in milk
Extensive information on the residues of bST and IGF-I in the
milk of rbST-treated cows was evaluated by the Committee at its
fortieth meeting. IGF-I is a normal but highly variable constituent of
bovine milk, the concentration depending on the state of lactation,
nutritional status, and age. Over an entire lactation, the IGF-I
concentrations range from 1 to 30 ng/ml, with the highest
concentrations in colostrum and a constant decline thereafter.
Multiparous animals have higher concentrations of IGF-I in milk than
primoparous cows (Burton et al., 1994). Bulk milk from cows not
given rbST had IGF-I concentrations of 19 ng/ml (Juskevich & Guyer,
1990), and the fortieth Meeting cited an average value of 3.7 ng/ml
for untreated cows. In milk from rbST-treated cows, the concentrations
of IGF-I ranged from 1 to 13 ng/ml in most studies and were about
25-70% greater than those in untreated animals (Burton et al.,
1994). The fortieth meeting reported an average IGF-I concentration of
5.9 ng/ml, and the increase was significant, even though most of the
concentrations were < 10 ng/ml.
Table 1. Concentrations of bovine somatotropin (bST) and insulin-like growth factor (IGF-I) in tissues after treatment with
recombinant bovine somatotropin
First experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose High dose
bST IGF-I bST IGF-I bST IGF-I
Muscle 12 1.9 ± 1.8 88 ± 21 1.5 ± 1.6 130 ± 25 3.3 ± 2.2 110 ± 32
Second experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose Intermediate dose High dose
bST IGF-I bST IGF-I bST IGF-I bST IGF-I
Muscle 5 3.4 ± 1.5 45 ± 6.5 4.9 ± 1.5 35 ± 15 3.8 ± 2.0 40 ± 5.1 1.5 ± 0.86 55 ± 19
Fat 4 5.1 ± 1.7 210 ± 85 9.3 ± 5.2 200 ± 65 4.8 ± 1.9 200 ± 53 11 ± 12 340 ± 230
Liver 4 5.2 ± 0.59 350 ± 23 3.6 ± 1.7 390 ± 130 5.4 ± 1.2 380 ± 170 4.6 ± 2.0 290 ± 88
Kidney 4 3.6 ± 1.1 910 ± 130 4.5 ± 1.6 1000 ± 140 4.5 ± 1.8 820 ± 120 3.9 ± 0.94 980 ± 220
Since the original work was reviewed, few additional data have
appeared in the literature or in reports made available by the
sponsors. The manufacturer of Posilac(R) - previously identified as
sometribove, which is a form of rbST approved in a number of countries
- submitted additional information. In a study designed to determine
the concentrations of IGF-I in retail milk samples, the concentrations
in milk specifically labelled as coming from cows that had not been
treated with bST were compared with those in unlabelled milk. While
the sponsor assumed that all of the unlabelled milk was from cows that
had been treated with bST, the extent to which this was true was not
ascertained. The study was conducted under US Food and Drug
Administration GLP regulations (21CFR 58). The labelled and unlabelled
status was determined of 127 of 129 retail milk samples collected as
2% fat cow's milk in 51 retail outlets in 34 cities in Wisconsin,
Minnesota, and Iowa, USA, from four goats and 125 cows, and the
samples were analysed by RIA for IGF-I. For 78 samples, the farmer had
certified that rbST had not been used. As rbST is not approved for use
in goats in the United States, it is assumed that the goat-milk
samples were from untreated animals. The results are shown in Table 2
(Eppard et al., 1994). The values for IGF-I are not unlike those
reported in the monograph prepared at the fortieth meeting of the
Committee. Although the contribution of milk from rbST-treated cows to
the unlabelled milk is unknown, the results indicate that the IGF-I
concentrations in retail milk did not increase in the first year after
the launch of rbST.
Table 2. Concentrations of insulin-like growth factor in milk
certified by farmers as coming from cows not treated with
recombinant bovine somatotropin (labelled) and from unlabelled milk
Insulin-like growth factor (ng/ml)
Labelled Unlabelled
(n = 78) (n = 45)
Raw mean 4.3 ± 0.09 4.5 ± 0.12
Logea 1.47 ± 0.044 1.55 ± 0.031 p = 0.01769
Antilog (95% 4.4 (4.0-4.7) 4.7 (4.4-5.0)
confidence
interval)
a Least square means adjusted for state where purchased and dairy
2.2.3 Assays
Because of variations in the IGF-I values reported in different
studies, questions have been raised in submissions to the Committee
about their accuracy. Lower values were found in some studies because
the assay used involved acid-ethanol extraction; an assay involving
acidic gel filtration has been reported to allow better recovery. In a
comparison of the two assays for determining IGF-I in pre- and
post-partum mammary secretions, the acid-ethanol assay was found to
result in a 24 ± 6.6% underestimate in comparison with acid gel
filtration (Vega et al., 1991). Use of the acidethanol assay to
recover 125I-IGF-I in bovine serum and colostrum, which also contain
binding proteins, gave values of 86 ± 6% and 88 ± 7% recovery by the
antibody, respectively (Hadsell, 1991). The difference is due to the
fact that the binding protein with affinity for IGF-I competes with
the antibody used in the RIA procedure; however, gel filtration
removes the hormone that is extensively dissociated by the acid common
to both assays. The studies suggest that the acidethanol assay
probably results in 15-25% underestimates of the concentrations in
milk and plasma. The relative difference in milk will probably not
affect a decision, as the concentrations are low, although higher
control values may affect the inferences. Although incomplete removal
of IGF-binding proteins or differences in the source of standards
might affect the reported results, the Committee considered these
factors immaterial.
2.2.4 Bioavailability and bioactivity of insulin-like growth factor
residues in milk
At its fortieth meeting, the Committee concluded that many of the
physiological effects of rbSTs are mediated by bovine IGF-I, which is
structurally identical to human IGF-I. It noted that there is
substantial endogenous synthesis of IGF-I, mainly in the liver but
also in human milk, saliva, and pancreatic secretions. It further
concluded that IGF-I has no bioactivity when administered orally to
normal and hypophysectomized rats at doses up to 2 mg/kg bw per day,
as dietary IGF-I is degraded by digestive enzymes and is not active in
the upper gastrointestinal tract.
Concern has been expressed that widespread use of rbSTs in dairy
production would lead to a sustained increase in the concentrations of
IGF-I in bulk cow's milk and that, if IGF-I survives digestion, the
greater exposure of consumers would cause adverse health effects
(Hansen et al., 1997). For a quantitative assessment of risk, the
slight increases in the concentration of IGF-I in milk from
rbST-treated cows should be compared with the physiological variations
in the concentrations of this growth factor during lactation (see
section 2.2.2) and with the concentrations in human breast milk, in
the secretions of the gastrointestinal tract, and in serum.
The concentrations of IGF-I are 8-28 ng/ml in colostrum and
5-10 ng/ml thereafter (Zumkeller, 1992; Burton et al., 1994),
indicating that breast-fed newborns are usually exposed to IGF-I at
concentrations equal to or higher than those in milk from rbST-treated
cows. Assuming a daily intake of 1.5 L of milk from rbST-treated cows
with an average IGF-I concentration of 6 ng/ml, the amount of IGF-I
ingested would be 9000 ng/day. The additional daily ingestion of IGF-I
over that of people drinking milk from untreated animals, with an
average IGF-I concentration of 4 ng/ml or 6000 ng/1.5 L, would be 3000
ng. The slightly increased IGF-I concentrations would contribute to
the endogenous concentrations of IGF-I in the gastrointestinal tract
of consumers; however, the main site of IGF-I production in animals
and humans is the liver. It is also produced in the human
gastrointestinal mucosa and is found in saliva, bile, and pancreatic
juice (Olanrewaju et al., 1992). The average concentrations of IGF-I
in the five human gastrointestinal secretions were 0.9 nmol/L in
saliva, 3.5 nmol/L in gastric juice, 24.6 nmol/L in jejunal chyme, 3.6
nmol/L in pancreatic juice, and 0.9 nmol/L in bile (Chaurasia et
al., 1994). On the basis of a molecular mass of 7.5 kDa for IGF-I
(Zumkeller, 1992) and the volume of each of the fluids produced
(Vander et al., 1990), the total volume of IGF-I emptying into the
gastrointestinal tract is 383 000 ng/day. Table 3 shows that the
amount of endogenous IGF-I emptied into the gastrointestinal tract
daily is more than (383 000/9000) 42 times greater than the amount
present in 1.5 L of milk from rbST-treated cows. The 9000 ng value is
2.3% of the estimated daily gastrointestinal secretion of IGF-I in
adults. The additional daily ingestion of 3000 ng IGF-I over that in
milk from untreated animals thus represents 0.78% of the
gastrointestinal secretion.
Table 3. Concentration of insulin-like growth factor (IGF-I) in
gastrointestinal tract secretions
Secretion Volume Concentration Total IGF-I
(ml/day)a (average; ng/ml) secreted (ng)
Jejunal chyme 1500 184.5 276 750
Pancreatic juice 1500 27.0 40 500
Gastric juice 2000 26.2 52 400
Bile 500 6.8 3 400
Saliva 1500 6.8 10 200
Adapted from Baumann (1995)
a From Vander et al. (1990)
In contrast to the conclusion of the Committee at its fortieth
meeting, that IGF-I is completely and rapidly degraded in the
gastrointestinal tract, milk-borne IGF-I may escape digestion by
proteases and therefore be bioactive in the intestine (Hansen
et al., 1997) or even be absorbed as intact peptide into the
systemic circulation (Epstein, 1996). In a study designed to
investigate the potential therapeutic use of IGF-I and to determine
whether oral formulations were feasible, the degradation of
125I-IGF-I was determined in various segments of the
gastrointestinal tract of rats in vivo and in vitro (Xian
et al., 1995). The extent of degradation was measured by one of
three methods: receptor binding, immunoprecipitation, and
trichloracetic acid precipitation. Two segments of the duodenum,
ileum, or whole stomach and part of the colon were ligated in an
anaesthetized male Sprague-Dawley rat that had been fasted for 24 h. A
bolus of labelled IGF-I (8.6 ng/ml in 0.2% bovine serum albumin in
saline) was injected into each segment and incubated for various times
up to 1 h. The reactions were stopped, and the flushed luminal
contents were examined for intact IGF-I. In a parallel set of
experiments, the flushed luminal contents from each of the four gut
segments were used as a source of degredation enzymes in vitro. The
results are shown in Table 4. The most rapid degradation occurred in
the duodenum and ileum and in their contents in vitro, followed by
the stomach and then by the colon. In all cases the values seen
in vitro were equal to or greater than those seen in vivo.
The authors also examined the effectiveness of slowing the
degradation rate of IGF-I in the gut by protecting the molecule in
several ways. Casein at a concentration of 10 mg/ml conferred > 90%
protection on stomach contents in both the trichloracetic acid and
receptor assays; in duodenal flushings, casein conferred 80%
protection against IGF-I degradation in the trichloracetic acid assay
but only 36% protection in the receptor assay at a maximal casein
concentration of 40 mg/ml. The half-life of IGF-I in the upper
gastrointestinal tract in the receptor assay increased from 2-3 min in
the absence of casein to 35 min in its presence. While these results
appear to demonstrate significant protection by casein at a
concentration similar to those in milk, the authors acknowledge that
the observed effects could be explained by simple competition by the
additional proteins for degradation by proteases in the segments. The
results demonstrate that biological receptor-stimulating activity,
which is the best indicator of biological activity, is dramatically
reduced even with good protection from protease activity. The authors
noted with regard to milk residues of IGF-I that 'the protective
effect of casein makes irrelevant the argument that human saliva
contains IGF-I at concentrations greater than the quantities that
would be consumed in milk. As the IGF-I produced by salivary glands is
free IGF-I, without protective effect of casein, it is unlikely to
survive digestion.' (Hansen et al., 1997). That argumentation
neglects the following facts:
Table 4. Half-lives of intact 125I-insulin-like growth factor (IGF-I) in ligated Sprague-Dawley rat gut
segments in vivo and in the flushed contents in vitro
Test for Half-life (min)
intact IGF-I
Duodenum/ileum Stomach Colon
In vivo In vitroa In vivo In vitro In vivo In vitro
Trichloracetic 2 2 8 50 38 > 60
Antibody binding 2 5 33
Receptor binding 2 2 2.5 3 16 ND
ND, not determined
a Antibody and membrane receptor values reported as receptor binding
* Saliva is not the only source of IGF-I in the gastrointestinal
tract: most is secreted into the gut, and the high concentration
in intestinal chyme indicates that IGF-I is secreted in
substantial amounts by the mucosa throughout the gastrointestinal
tract (Olanrewaju et al., 1992; Chaurasia et al., 1994).
* Casein has a flexible structure and is readily degraded in the
stomach and small bowel (Xian et al., 1995). Thus, the
protective effect is present only in the upper gastrointestinal
tract.
* The half-life of IGF-I in the intestine in the presence of casein
is only 35 min (Xian et al., 1995). Therefore, less than 5% of
an initial dose of IGF-I will survive for more than 2 h during
passage through the upper gastrointestinal tract.
* In the presence of casein ingested with milk, endogenous IGF-I in
the gastrointestinal tract will also be protected.
Therefore, even if casein has a limited protective effect, the amount
of bioactive IGF-I ingested with milk from rbST-treated cows would
still be negligible.
Because of the protective effect of casein, some IGF-I might
escape digestive degradation and be absorbed intact. The absorption of
large (1 mg/kg) oral doses of 125I-labelled recombinant human IGF-I
was studied in fasted adult rats, with trichloracetic acid
precipitation of plasma proteins. The baseline bioavailability of the
administered IGF-I was 9.3% of the dose, but this was was increased by
co-administration of 4 mg/kg aprotinin (47%) and 10 mg/kg casein
(67%). RIA of the plasma confirmed the bioavailability of IGF-I in
this model, and the administered radiolabel was found in the form of
high-molecular-mass complexes (Kimura et al., 1997). It should be
noted, however, that the receptor assay, which is the most accurate,
was not used.
The relatively good bioavailability of intact IGF-I in this adult
rat model is in contrast to the lack of bioactivity of orally
administered IGF-I in adult animals (Annex 1, reference 104) and to
the results of studies with neonatal animals which have an incomplete
mucosal barrier and reduced intestinal proteolytic activity (Burrin,
1997). Studies in neonatal rats and piglets indicated that although
30% of an orally administered dose of 125I-IGF-I can be recovered in
the intestinal mucosa there is limited absorption into the peripheral
circulation (Phillips et al., 1995; Donovan et al., 1997). When
suckling transgenic rats ingested 1000-fold higher concentrations of
des(1,3) human IGF-I, no des(1,3)-IGF-I was detected in the plasma of
their pups (Burrin, 1997). Furthermore, in newborn calves and piglets
given large doses of IGF-I in milk replacers, no substantial increase
in the plasma concentration of this growth factor was found (Donovan
et al., 1997; Hammon & Blum, 1997; Houle et al., 1997). In one
study with newborn calves fed milk replacer, a small amount of orally
administered 125I-IGF-I was detected in plasma (Baumrucker et
al., 1992); however, the increase was observed only three days after
administration and in only three of six animals. Even in newborns,
therefore, IGF-I is absorbed to only a small extent, and absorption is
unlikely in adults.
Furthermore, the amount absorbed should be compared with the
normal concentrations of IGF-I in human serum, which show considerable
variation with age. The values are lowest in infants under two years
of age, then increase steadily to reach a maximum in late puberty, and
afterwards decrease to the adult values (Table 5). Assuming a blood
volume that is 5% of the body weight (Ganong, 1971), the serum load of
IGF-I can be calculated to be 50 000 ng in a 15-kg child, 714 000 ng
in a 60-kg adult, and 1 220 000 ng in a 50-kg teenager. The total
IGF-I production in adults has been estimated at 107 ng per day
(Guler et al., 1989). These amounts should be compared with the 9000
ng IGF-I in 1.5 L of milk, which constitutes only 0.09% of the daily
IGF-I production. Since only one-third of the concentrations in milk
can be attributed to IGF-I due to rbST treatment and only a small
amount if any will be absorbed, the milk-borne IGF-I that reaches the
systemic circulation is negligible and this small amount is
immediately sequestered by unsaturated binding proteins.
Table 5. Concentrations (ng/ml) of insulin-like growth factor (IGF-I)
in human plasma
Age Males Females
Mean Range Mean Range
0-2 years 42 14-98 56 14-238
3-5 years 56 59-210 84 21-322
6-10 years 98 28-308 182 56-364
Before puberty > 10 years 126 84-182 182 70-280
Early puberty 210 140-240 224 84-392
Late puberty 364 224-462 434 224-686
Adult > 23 yeras 112 42-266 140 56-308
From Schaff-Blass et al. (1984)
Concern has been expressed about the possible adverse effects on
the health of consumers exposed to increased concentrations of IGF-I
in milk from rbST-treated cows (Epstein, 1996; Hansen et al., 1997).
The most important potential adverse effects of IGF-I arise from the
fact that it is a mitogen for a number of cell types and has been
associated with the growth of tumours including those of the colon,
breast, and lung and osteosarcoma (Pines et al., 1985; Macaulay,
1992; National Institute of Health, 1995). The mitogenic effect could
also result in proliferative reactions locally in the gut. Thus,
orally administered IGF-I increased the cellularity of the intestinal
mucosa of rats in vivo (Olanrewaju et al., 1992) and increased the
rate of proliferation in cultures of human duodenal epithelial crypt
cells (Challacombe & Wheeler, 1994). Since IGF-I receptors can be
detected throughout the epithelium of the intestine, with a high
density in the colon (Laburthe et al., 1988), and the incidence of
colorectal cancer is increased in acromegalic patients who have
excessively high concentrations of free IGF-I in their plasma (Ezzat &
Melmed, 1991), concern has been expressed that increased
concentrations of milk-borne IGF-I may increase the risk for colon
cancer. Although the normal biological effects of IGF-I mean that it
could promote the growth of tumours, this hazard would become a risk
only if there were adequate exposure of consumers to IGF-I. Since
exposure to IGF-I in milk from rbST-treated cows is negligible when
compared with endogenous IGF-I production, it is extremely unlikely
that IGF-I residues cause any systemic or local mitogenic reaction.
2.3 Expression of lentiviruses and prion proteins
2.3.1 Somatotropins and the immune system
Somatotropin enhances the immune system in many species,
including cattle (Comens-Keller et al., 1995). The primary effect
appears to be altered responsiveness of the immune system, although
substantive evidence of this effect is lacking (Burton et al.,
1994), and information on changes in cytokine concentrations or
secretion and on their binding sites are needed in order to define the
nature of the immune-enhancing effects of somatotropins. The studies
reported in the literature differ with regard to the source of
somatotropin, the treatment schedule, and the age of the animals. The
differences between findings in vitro and in vivo may be due to
the release in vivo of mediators such as IGFs and cytokines, which
are not present in vitro (Kelley, 1989). A better understanding of
somatotropin-mediated immune enhancement as a homeorhetic regulator of
the overall health and disease resistance of animals is needed.
Lymphocytes from rbST-treated cows have a greater average maximum
lymphoblastogenic response to rbST than to other mitogens around the
time of parturition (Comens-Keller et al., 1995), and this effect
might prevent mastitis and the other infectious diseases that occur
during this period of immunosuppression.
2.3.2 Effect of bovine somatotropin on the expression of retroviruses
Concern has been expressed that the immunomodulatory effect of
bST might affect retroviral expression in treated animals and thus
cause resurgence of latent retroviral and lentiviral infections in the
ruminant population and the presence of these viruses in somatic cells
in milk. The concern is based largely on a review by Lerondelle et al.
(1994), who discussed the evidence for induction of these viruses in
small ruminants by steroid hormones and the evidence for induction of
lentiviruses in other species by hormones including growth hormone and
IGF-I, and an unpublished study by Lerondelle et al. (1996), who
investigated the effects of rbST on the expression of caprine
arthritis encephalitis virus (CAEV) in goats. This virus belongs to
the group of lentiviruses which, like maedivisna, can infect small
ruminants.
Ruminant lentiviruses are of interest for at least three reasons.
First, they may cause disease in persons who drink milk. Second, if
use of rbST increases the prevalence of ruminant viruses, by the
presence of rbST itself or by the action of IGF-I, the small
additional amounts of growth hormone in the milk of treated cows could
also affect the retroviruses that attack the human immune system,
HIV-1 and HIV-2. Finally, the severity or kinetics of expression of
the disease in ruminants might be increased.
The study of Lerondelle and coworkers (1996) attempted to address
the question of whether rbST increases the expression of CAEV. Viral
expression was measured by assaying reverse transcriptase activity in
milk cells, clinical examination of the udders and joints of the
animals at the beginning and end of the study, and evidence of
infection in an immunodiffusion assay. Twelve pregnant Saanen goats
that were seronegative for CAEV were given an intramammary injection
of monocytes infected in vitro with the Cork strain of CAEV at the
time of drying off. Seven weeks after giving birth, four goats were
treated daily with 5 mg/goat rbST (sometribove), a second group was
treated daily with 10 mg/goat thyroxine, and the control group was
untreated. The compounds were administered in suspension in sterile
water for 30 days, followed by a 45-day observation period. Milk
samples were taken to measure reverse transcriptase activity on days
7, 14, 21, and 28 of treatment and three times during the observation
period. Examination of the udders and joints and immunodiffusion tests
were carried out at the beginning and end of the study. Milk
production and milk-cell counts were evaluated every two days. The
occurrence of CAEV and the onset of effects are shown in Table 6. More
positive cultured cells were seen in the controls than after treatment
with either hormone. Perhaps the most striking effect is the lack of
increase in the rate of infectivity and even a suggestion of a
decrease after treatment with rbST, particularly after the first milk
sampling.
When the results were expressed as a ratio of transcriptase
activity to the number of cells considered to contain virus (Table 7),
there was no correlation between the effect of rbST or thyroxine and
the activity of reverse transcriptase in the milk samples. In fact,
there was no evidence of increased transcriptase activity in any
group. In particular, even though the group receiving rbST had a lower
initial rate of infection than the other two groups, including the
controls, the rate of infection was not increased, as measured by the
number of positive cultures or increased transcriptase activity. The
authors interpreted their results as showing a tendency to increased
viral expression with increased milk production. The results for rbST
were, however, biased by the fact that only two animals were included
in the final evaluation. The authors concluded that, owing to the
Table 6. Onset of appearance of cytopathic effect (in days) for each of 10 milk samplings from groups of four goats given no treatment or
thyroxine or recombinant bovine somatotropin (rbST), as a function of the period of hormonal treatment
Treatment Milk sampling
Before treatment During treatment After treatment
1 2 3 4 5 6 7 8 9
Control 4 8 6 10 6 6 6 8 10
4 6 6 6 8 6 6 6 -
4 6 4 6 4 4 6 4 4
6 8 10 6 10 6 - - -
Mean ± SD (n) 6 ± 1.91 (12) 6.4 ± 1.71 (15) 7 ± 2.39 (8)
Thyroxine
8 6 10 4 6 6 - - 6
6 4 4 4 4 4 4 4 ND
4 4 4 4 4 4 6 4 ND
4 8 6 4 4 4 6 4 ND
Mean ± SD (n) 5.67 ± 2.06 (12) 4.53 ± 0.92 (15) 4.25 ± 0.71 (8)
rbST 8 - - - - - - - -
4 8 8 - 8 - - - 4
ND - - - - - - - -
4 4 4 4 4 4 4 4 4
Mean ± SD (n) 5.71 ± 2.41 (7) 4.8 ± 1.79 (5) 4 (4)
ND, not determined
Table 7. Number of samples found to contain virus by the reverse
transcriptase-positive culture ratio in groups of four goats given
no treatment, thyroxine, or recombinant bovine somatotropin (rbST)
Treatment Before During After Total
treatment treatment treatment
Control 3/6 4/7 3/4 10/17
3/6 3/5 1/3 7/14
6/6 4/5 5/6 15/17
4/4 2/8 3/6 9/18
Total 16/22 13/25 12/19 41/66
Thyroxine 0/3 2/4 0/3 2/10
4/4 5/7 6/6 15/17
6/6 8/8 6/6 20/20
6/6 8/8 4/6 18/20
Total 16/19 23/27 16/21 55/67
rbST 2/4 0/6 0/1 2/11
6/6 7/8 2/5 15/19
0/2 0/4 0/3 0/8
4/4 4/5 5/6 12/14
Total 12/16 11/23 7/15 29/52
heterogeneity of the effects on milk production and the small number
of animals tested, the evidence for a promoting effect of rbST on
viral expression was insufficient.
These results provide no evidence that treatment of cows infected
with lentiviruses with rbST will cause resurgence of viral infections
or pose any risk to human health. Lentiviruses are retroviruses that
replicate only in activated cells of the immune system. They may stay
dormant for months or years before they gradually wear down the immune
system to the point of collapse. The phylogenetic tree of lentiviruses
includes the subfamily of bovine immunodeficiency virus (also called
bovine leukaemia virus, BLV) and the subfamily of HIV-1 and HIV-2
which are the causative agents of AIDS. BLV and HIV are widely
separated phylogenetically (Robertson, 1997). BLV is not known to
cause disease in humans although it can infect human cells in
vitro, where host defence mechanisms are not present (Georgiades
et al., 1978; van der Maaten & Miller, 1990); infection of human
cells in vivo could not be demonstrated, and all attempts to obtain
direct evidence of infection in exposed human populations have yielded
negative results (Straub, 1981; van der Maaten & Miller, 1990).
Failure to infect humans has also been shown for other ruminant
lentiviruses, such as CAEV and maedivisna (Straub, 1981). Excretion
of virus with milk somatic cells can infect the offspring of infected
cows. Such transmission can be effectively blocked by procedures
similar to pasteurization, which destroy the virus at 60°C within 30 s
(Abramova et al., 1974; Baumgartner et al., 1976; van der Maaten &
Miller, 1990). BLV therefore cannot induce disease in humans and is
completely inactivated by routine pasteurization. Furthermore, the
company that sells sometribove has reported that there is no
indication that the incidence of BLV infection has increased in cattle
after eight years of continuous use of rbST in Mexico and Brazil and
four years of use in the United States (Collier & Kowalczyk, 1998),
although this statement is not further qualified.
An increase in the expression of HIV in humans who ingest milk
from rbST-treated cows is extermely unlikely because of the negligible
residues of rbST and IGF-I. Treatment of AIDS patients for six weeks
with recombinant human growth hormone and IGF-I had no effect on the
HIV titres in peripheral blood mononuclear cells, on the CD3, CD4, or
CD8 counts in peripheral blood, or on serum HIV p24 antigen
concentrations (Waters et al., 1996).
2.3.3 Effect of recombinant bovine somatotropin on prion proteins
Concern has been expressed that rbST treatment could increase the
risk for BSE in dairy cows (Hansen et al., 1997). Little evidence is
available to support this concern, and that which has been provided is
indirect. It is currently thought that the infectious agent of BSE is
a prion protein (Prusiner, 1982). Prion proteins are found normally in
all animals and are encoded by a prion-protein gene. BSE is associated
with a post-translationally modified protease-resistant prion protein,
which differs in its three-dimensional structure from the normal
protease-sensitive prion protein. Normal prion proteins are bound to
membranes on the surface of all nerve cells, some lymphocytes, and
other tissues (Prusiner, 1991). To date, no function has been ascribed
to normal prion protein. The most widely accepted theory of BSE is the
conversion of normal prion protein to the abnormal protease-resistant
form, which in turn causes more normal prion protein to convert to
protease resistance. The mechanisms of the conversion to the
disease-causing protease-resistant prion protein are not clearly
understood. In contrast to the normal form, the protease-resistant
form cannot be turned over and builds up in cells, forming large
oligomers which are observed as plaques (amyloids) in the brains of
affected individuals (Gajdusek, 1993).
IGF-I increased the production of prion protein mRNA in vitro
in a rat phaeochromocytoma cell line (PC12 cells), with a rather flat
dose-response curve showing a 40% increase at 10 ng/ml and a doubling
at 100 ng/ml (Lasmézas et al., 1993). In transgenic mice that
harbour multiple copies of the prion protein gene, the progession of
scrapie was accelerated (Prusiner, 1991). It has been speculated that
the increased IGF-I concentrations in rbST-treated cows could increase
prion protein production and possibly speed up the progression of BSE;
however, no studies are available that directly address the question
of whether rbST or IGF-I increases the formation of normal prion
protein or its pathogenic protease-resistant mutant in the brains of
the cattle, and the possibility of a link between rbST treatment and
BSE is highly speculative.
2.4 Cow's milk and insulin-dependent type I diabetes mellitus in
childhood
Epidemiological studies have shown that environmental factors
such as chemicals, viral infections, a short duration of
breastfeeding, and early dietary exposure of newborns to cow's milk
increase the risk for insulin-dependent type I diabetes mellitus
(IDDM) by about 1.5 times (Scott, 1990; Dahlquist et al., 1991;
Jorgensen et al., 1991; Virtanen et al., 1993; Gerstein, 1994;
Verge et al., 1994; Virtanen et al., 1994). IDDM develops as a
consequence of autoimmune destruction of the insulin-producing b cells
of the pancreatic islets. The precise trigger of the autoimmune
reaction is unknown, but it is assumed to be a genetically acquired
immune defect in susceptible individuals (Gerstein, 1994). The
epidemiological evidence indicates that IDDM is geographically and
temporally related to neonatal feeding with cow's milk and that
avoidance of cow's milk during the first few month of life can protect
genetically predisposed individuals (Gerstein, 1994; Verge et al.,
1994).
Serological evidence supports the theory that the immune defect
is triggered by exposure to proteins in cow's milk (Gerstein, 1994).
It has been postulated that, in newborns, milk proteins cross the
immature gut wall, initiating an immune response that cross-reacts
with a beta-cell surface antigen (Verge et al., 1994). Children aged
five to nine, who have an intact intestinal barrier, are not at risk
of acquiring IDDM by drinking cow's milk (Dahlquist et al., 1991).
The possible triggering factors in cow's milk have not been
identified. Casein is unlikely to be involved, since replacement of
milk proteins by casein in the diets of rats susceptible to diabetes
completely prevented the disease (Jorgensen et al., 1991). Increased
concentrations of immunoglobulin A antibodies to cow's milk and
b-lactoglobulin appear to be associated with an increased risk for
IDDM (Dahlquist et al., 1991; Virtanen et al., 1994).
It is unlikely that exposure of human newborns to milk from
rbST-treated cows would increase the risk for IDDM for the following
reasons:
* The composition of milk from rbST-treated cows is well within the
normal variations observed during lactation,
* The slightly increased IGF-I concentrations can be excluded as a
triggering factor because bovine and human IGF-I are identical
and because the concentrations of IGF-I in breast milk are equal
to or initially higher than those found in cow's milk.
3. COMMENTS
Use of antibiotics
After reviewing the available information, the Committee
considered the risk for mastitis associated with use of rbST as an
issue of animal health that is not within its terms of reference;
however, the possibly increased use of antibiotics was considered. A
post-approval monitoring programme was established in the United
States to address the following issues:
* the incidence of mastitis and responses related to herd health
(not within the terms of reference of the Committee),
* treatment with any medications of 28 herds of rbST-treated cows
(not within the terms of reference of the Committee), and
* the percent of milk discarded because of violative drug residues
in key dairy states representing at least 50% of United States
milk production.
In New York State, the percentage of milk discarded because of the
presence of antibiotic residues was not significantly changed after
the introduction of rbST. In other states, however, a small but
statistically significant, increase was observed in 1995, which
coincided with a change to a more sensitive testing method in those
states. The Committee concluded that the use of rbST would not result
in a higher risk to human health due to the use of antibiotics to
treat mastitis and that the increased potential for drug residues in
milk could be managed by practices currently in use within the dairy
industry and by following the directions for use.
Concentrations of insulin-like growth factor in milk and tissues
Insulin-like growth factor (IGF-I) is a normal component of milk
and is found in abundance in a variety of body fluids (Table 8). The
presence and concentrations of IGF-I were the source of much of the
scientific discussion during the original review of bST undertaken at
the fortieth meeting of the Committee and in submissions to the
present one. The information that was reviewed is summarized in FAO
Food and Nutrition Paper No. 41/5 (Annex 1, reference 106). The
concentrations of IGF-I in milk are variable and have been shown to
depend on the state of lactation, nutrition, and age.
Methods for assaying IGF-I were considered by the Committee.
Although incomplete removal of IGF-binding proteins or differences in
the source of standards and extraction methods might affect the
reported values, these factors were considered not to alter any
conclusions materially. The relatively high values previously reported
in milk were considered to reflect inadequate extraction.
Table 8. Insulin-like growth factor in milk and body fluids
Medium Concentration
(ng/ml)
Milk
Human 5-10
Colostrum 8-28
Bovine (bulk milk)
Untreated 1-9
rbST-treated 1-13
Plasma
Child 17-250
Adolescent 180-780
Adult 120-460
Gastrointestinal secretions (human)
Saliva 6.8
Gastric juice 26
Pancreatic juice 27
Bile 6.8
Jejunal chyme 180
Daily production of adult humans 107 ng/d
Since the previous evaluation, few additional data on residues
have appeared in the literature or in reports provided by interested
parties; however, the manufacturer of sometribove submitted additional
information on the concentrations of IGF-I in retail milk after the
approval of rbST in the United States. The results showed no
difference in the IGF-I concentrations of milk certified as derived
from cows not treated with rbST and of unlabelled milk; however, the
percentage of the unlabelled milk that was derived from cows receiving
rbST was not specified.
Concern has been expressed that any rbST-induced increase in the
concentration of IGF-I in milk would contribute to the endogenous
levels of IGF-I in the gastrointestinal tract and serum if it were not
biodegraded and were absorbed. In rats, IGF-I is rapidly degraded in
the gastrointestinal tract; however, a protective effect of casein on
IGF-I was demonstrated in these studies. It was postulated that the
retarded degradation leads to increased serum levels of IGF-I (as
shown in one study in rats) and to prolonged exposure of the gut. The
Committee noted that seven days' oral administration of high doses of
IGF-I in milk replacer did not increase the circulating concentrations
of IGF-I in newborn calves and piglets, indicating that significant
absorption of IGF-I is unlikely to occur under physiological
conditions. In view of the decreased rate of degradation in the small
intestine of rats in the presence of casein, the concentrations of the
growth factor would probably decrease to less than 5% of their initial
values within 2 h, so that milk-borne IGF-I would not be expected to
contribute to the concentrations of IGF-I in the large intestine.
If 1.5 L of milk are ingested per day, the average intake of
IGF-I will be 6000 ng in milk from untreated cows containing an
assumed IGF-I concentration of 4 ng/ml and 9000 ng in milk from rbST-
treated cows with an approximate average concentration of 6 ng/ml. It
has been calculated that IGF-I in gastrointestinal secretions amounts
to about 380 000 ng/day. Therefore, the additional amount of IGF-I in
1.5 L of milk from rbST-treated cows would represent only about 0.8%
of the IGF-I secreted in the gastrointestinal tract. The total amount
of IGF-I in serum has been calculated to range from 50 000 to 1 220
000 ng, depending on age. The total daily IGF-I production in adult
humans has been estimated to be 107 ng. Therefore, the daily amount
of IGF-I ingested with milk from rbST-treated cows would represent
less than 0.09% of the daily production of adults. Even if all of the
milk-borne IGF-I were absorbed, the additional amount would be
negligible.
When sustained-release rbST was administered to cattle once every
two weeks for a total of 20 weeks, the tissue concentrations of rbST
and IGF-I two weeks after the final dose were not significantly
increased.
The Committee concluded that any increase in the concentration of
IGF-I in milk from rbST-treated cows is orders of magnitude lower than
the physiological amounts produced in the gastrointestinal tract and
in other parts of the body. Thus, the concentration of IGF-I would not
increase either locally in the gut or systemically, and the potential
for IGF-I to promote tumour growth would not increase when milk from
rbST-treated cows was consumed; there is thus no appreciable risk for
consumers.
Expression of retroviruses
Concern that treatment of cattle with rbST would increase the
expression of retroviruses, including BLV, were addressed in
experiments with caprine arthritis encephalitis virus in goats.
Infectivity was not increased when measured as numbers of infected
cells, and there was no evidence of increased reverse transcriptase
activity. These studies provide no evidence that rbST affects the
expression of BLV, a lentivirus in cattle. Furthermore it has been
shown that BLV is destroyed in simulated pasteurization conditions by
heating milk to 60 .C for 30 s. In addition, there is no evidence
of human susceptibility or response to ruminant retroviruses.
Expression of prion proteins
Concern has been expressed that treatment with rbST could shorten
the incubation period for BSE. This hypothesis is based on results
obtained with a neuronal cell line in vitro indicating increased
formation of prion protein mRNA in response to IGF-I. Furthermore,
increased formation of prion protein shortened the incubation period
for scrapie in transgenic mice harbouring multiple copies of the gene
that codes for prion proteins. No data were available, however, to
address directly the question of whether rbST or IGF-I increases the
formation of normal prion protein or its pathogenic protease-resistant
mutant in the brains of cattle. The Committee considered that the
possibility of a link between rbST treatment and BSE was highly
speculative.
Risk for insulin-dependent diabetes mellitus
Exposure of human newborns to cow's milk increases the risk for
insulin-dependent diabetes mellitus by about 1.5-fold. The Committee
considered whether exposure of newborns to milk from rbST-treated cows
further increases this risk. It concluded that, because of its
unchanged composition, the milk of rbST-treated cows would not pose an
additional risk to the development of insulin-dependent diabetes
mellitus.
4. EVALUATION
On the basis of the following:
* insignificant changes in the quantities of milk discarded after
testing for antibiotic residues following the introduction of
rbST into commercial use;
* low concentrations of rbST and IGF-I residues in milk;
* degradation of IGF-I in the gut and its abundance in gut
secretions;
* the extremely low concentrations of ingested IGF-I in comparison
with endogenous production;
* lack of evidence that rbST stimulates the expression of
retroviruses;
* lack of a direct link between rbST treatment and BSE; and
* the absence of significant changes in the composition of milk
from rbST-treated cows that could contribute to an additional
risk for the development of insulin-dependent diabetes mellitus,
the Committee concluded that rbST can be used without any appreciable
risk to the health of consumers. The Committee reaffirmed its previous
ADIs1 and MRLs2 'not specified' for somagrebove, sometribove,
somavubove, and somidobove.
5. REFERENCES
Abramova, E.M., Kondratev, V.S. & Sytinskii, I.A. (1974) The
biochemistry of leucosis in cattle. Vet. Bull., 44, 689-711.
Baumann, D.M. (1995) IGF-I Fact Sheet, Department of Animal Science,
Cornell University, Ithaca, NY, USA.
Baumgartner, L.E., Olson, C. & Onuma, M. (1976) Effect of
pasteurization and heat treatment on bovine leukemia virus. J. Am.
Vet. Med. Assoc., 169, 1189-1191.
Baumrucker, R.R., Hadsell, D.J., Skarr, T.C., Campbell, P.G. & Blum,
J.W. (1992) Insulin-like growth factors (IGFs) and IGF binding
proteins in mammary secretions: Origins and implications in neonatal
physiology. In: Picciano, M.F. & Lonnerdal, B., eds, Mechanisms
Regulating Lactation and Infant Nutrient Utilization, New York,
Wiley-Liss, pp. 285-308.
Burrin, D.G. (1997). Is milk-borne insulin-like growth factor-l
essential for neonatal development? J. Nutr., 127, 975S-979S.
Burton, J.L., McBride, B.W., Block, E., Glimm, D.R. & Kenelly, J.J.
(1994) A review of bovine growth hormone. Can. J. Anim. Sci., 74,
167-201.
Challacombe, D.N. & Wheeler, E.E. (1994) Safety of milk from cows
treated with bovine somatotropin. Lancet, 344, 815-816.
Chaurasia, O.P., Marcuard, S.P. & Seidel, E.R. (1994) Insulin-like
growth factor l in human gastrointestinal exocrine excretion.
Regul. Pept., 50, 113-119.
Choi, J., Choi, M.J., Kim, C., Ha, J., Hong, A., Ji, Y. & Chang, B.
(1997). The effect of recombinant bovine somatotropin (rbST)
administration on residual BST and insulin-like growth factor I levels
in various tissues of cattle. J. Food Hyg. Soc. Jpn, 38, 225-232.
Codex Alimentarius Commission (1997) Report of the Twenty-second
Session of the Codex Alimentarius Commission, Geneva, 23-28 June
1997 (FAO Document ALINORM 97/37), Rome, Food and Agriculture
Organization of the United Nations.
Collier, R.J. (1996) Post-approval evaluation of Posilac bovine
somatotropin in commercial dairy herds (100-USA-COW-RJC-93-051).
Unpublished report from Protiva, Monsanto Co. Submitted to WHO by
Protiva, Monsanto Co., St Louis, Missouri, USA.
Collier, R.J. & Kowalczyk, D.F. (1998) Human health risk of
retroviruses in cattle and bovine somatotropine. Unpublished report
from Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co.,
St Louis, Missouri, USA.
Comens-Keller, P.G., Eppard, P.J. & Collier, R.L. (1995). Evaluation
of somatotropin as a homeorhetic regulator of immunity. In: Ivan, M.,
ed., Animal Science Research and Development: Moving Toward a New
Century. Proceedings of the 75th Anniversary Meeting of the
Canadian Society of Animal Science, Ottawa, pp. 79-94.
Dahlquist, G., Blom, L. & Lönnberg, G. (1991) The Swedish childhood
diabetes study: A multivariate analysis of risk determinants for
diabetes in different age groups. Diabetologica, 34, 757-762.
Donovan, S.M., Chao, J.C., Zijlstra, R.T. & Odle, J. (1997) Oral
administered iodinated recombinant human insulin-like growth factor-I
(125I-rhIGF-I) is poorly absorbed by the neonatal piglet. J.
Pedriat. Gastroenterol. Nutr., 24, 174-182.
Eppard, P.J., Collier, R.J., Hintz, R.L., Veenhuizen, J.J. & Baile,
C.A. (1994) Survey of milk insulin-like growth factor in retail milk
samples. Unpublished report No. 100-USA-COW-RJC-94-074 from Protiva,
Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St Louis,
Missouri, USA.
Epstein, S.S. (1996) Unlabeled milk from cows treated with
biosynthetic growth hormones: A case of regulatory abdication. Int.
J. Health Serv., 26, 173-185.
Ezzat, H. & Melmed, S. (1991) Clinical review 18: Are patients with
acromegaly at increased risk for neoplasia? J. Clin. Endocrinol.
Metab., 72, 245-249.
Gajdusek, D.C. (1993) Genetic control of nucleation and polymerization
of host precursors to infectious amyloids in the transmissible
amyliodoses of brain. Br. Med. Bull., 49, 913-931.
Ganong, W.F., ed. (1971) Review of Medical Physiology, Los Altos,
Lange Medical Publications, p. 5.
Georgiades, J.A., Billiau, A. & Vanderschueren, B. (1978) Infection of
human cell cultures with bovine visna virus. J. Gen. Virol., 38,
375-381.
Gerstein, H.C. (1994) Cow's milk exposure and type I diabetes
mellitus. Diabetes Care, 17, 13-19.
Guler, H.P., Zapf, J., Schmid, C. &, Froesch, E.R. (1989) Insulin-like
growth factors I and II in healthy man. Estimations of half-lives and
production rates. Arch. Endocrinol., 121, 753-758.
Hadsell, D.L. (1991) Insulin-like Growth Factor (IGF) Receptors in
the Bovine Mammary Gland: Regulation, Mitogenic Action, and Role in
Mammary Secretion of IGF-I, PhD Thesis, Pennsylvania State
University, Philadelphia, Pennsylvania, pp. 108-114,
Hammon, H. & Blum, J.W. (1997) The somatotropic axis in neonatal
calves can be modulated by nutrition, growth hormone and
Long-R3-IGF-I. Am. J. Physiol., 273, E130-E138.
Hansen, M., Halloran, J.M., Groth, E., III & Lefferts, L.Y. (1997)
Potential public health impacts of the use of recombinant bovine
somatotropin in dairy production. Unpublished report. Submitted to WHO
by Consumer Policy Institute, Yonkers, New York, USA.
Houle, V.M., Sschroeder, E.A., Odle, J. & Donovan, S.M. (1997) Small
intestinal disaccharidase activity and ileal villus height are
increased in piglets consuming recombinant human insulin-like growth
factor-I. Pediatr. Res., 42, 78-86.
Jorgensen, K.D., Joner, G. & Hanssen, K.F. (1991) Relationship between
cows' milk consumption and incidence of childhood IDDM. Diabetes
Care, 14, 1081-1083.
Juskevich, J.C. & Guyer, C.G. (1990) Bovine growth hormone: Human food
safety evaluation. Science, 249, 875-884.
Kelley, K.W. (1989) Growth hormone, lymphocytes and macrophages.
Biochem. Pharmacol., 38, 705-713.
Kimura, T., Murakawa, Y., Ohno, M., Ohtani, S. & Higaki, K. (1997)
Gastrointestinal absorption of recombinant human insulin-like growth
factor-I in rats. J. Pharmacol. Exp. Ther., 283, 611-618.
Laburthe, M., Rouyer-Fessard, C. & Gammeltoft, S. (1988) Receptors for
insulin-like growth factors I and II in the rat gastrointestinasl
epithelium. Am. J. Physiol., 254, 457-462.
Lasmézas, C., Deslys, J.P. & Dormont, D. (1993) Recombinant human
growth hormone and insulin-like growth factor I induce PrP gene
expression in PC12 cells. Biochem. Biophys. Res. Commun., 196,
1163-1169.
Lerondelle, C., Lena, P. & Mornex, J.F. (1994) [Hormonal control of
retroviral infections.] Renc. Rech. Ruminants, 1, 4550 (in French).
Lerondelle, C., Guiguen, F., Fornazero, C., Gounel, F., Leroux, C.,
Chastang, J., Pardo, E., Bolland, P., Bruyas, S., Cordier, G. &
Mornex, J.F. (1996) Effects of recombinant bovine somatotropin and of
thyroxine on the expression of lentiviruses in ruminants. Unpublished
report from INRA Veterinary School of Lyon and INSERM Hôpital Louis
Pradel, Lyon, France. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
van der Maaten, M.J. & Miller, J.M. (1990) Bovine leucosis. In:
Virus Infections of Ruminants, Amsterdam, Elsevier Science
Publishers, pp. 419-429.
Macaulay, V.M. (1992). Insulin-like growth factors and cancer. Br.
J. Cancer, 65, 311-320.
National Institute of Health (1995) NIH conference: Insulin-like
growth factors and cancer. Ann. Intern. Med., 122, 54-59.
Olanrewaju, H., Patel, L. & Seidel, E.R. (1992) Trophic action of
intraileal infusion of insulin-like growth factor I: Polyamine
dependence. Am. J. Physiol., 263, E282-E286.
Phillips, A.F., Rao, R., Anderson, G.G., McCracken, G.M., Lake, M. &
Koldovsky, O. (1995) Fate of insulin-like growth factors I and II
administered orogastrically to suckling rats. Pediatr. Res., 37,
586-592.
Pines, A., Rozen, M.B. & Gilat, T. (1985) Gastrointestinal tumors in
acromegalic patients. Am. J. Gastroenterol., 80, 286-289.
Prusiner, S.B. (1982) Molecular biology of prion diseases. Science,
216, 136.
Prusiner, S.B. (1991) Molecular biology of prion diseases. Science,
252, 1515-1522.
Robertson, D.L. (1997) The evolution of AIDS viruses. From the
Internet
Schaff-Blass, E., Burstein, S. & Rosenfield, R.L. (1984) Advances in
diagnosis and treatment of short stature, with special reference to
the role of growth hormone. J. Pediatr., 104, 801.
Scott, F.W. (1990) Cow milk and insulin-dependent diabetes mellitus:
Is there a relationship? Am. J. Clin. Nutr., 51, 489-491.
Straub, O.C. (1981) Enzootic bovie leucosis. In: Gibbs, E.P.J., ed.,
Virus Diseases of Food Animals, New York, Academic Press, pp.
683-718.
US Food and Drug Administration (1996) CVM Update: VMAC endorses
post-approval program for Posilac(R), 18 December 1996.
Vander A.J., Sherman, J.H. & Luciana, D.S., eds (1990) Human
Physiology, The Mechanisms of Body Function, 5th Ed., New York,
McGraw-Hill Publishing Co., p. 15.
Veenhuizen, J.J., Hintz, R.L., Hemenover, M.L., Kowalczyk, D.F. &
Collier, R.J. (1996) Post-approval monitoring program for Posilac
bovine somatotropin: Tracking program of milk discarded due to drug
residue violations. Unpublished report No. 100-USA-Cow-RLH-93-053 from
Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
Vega, J.R., Gibson, C.A., Skaar, T.C., Hadsell, D.L. & Baumrucker,
C.R. (1991) Insulin-like growth factor (IGF)-I and II and IGF binding
proteins in serum and mammary secretions during the prepartum period
and early lactation in dairy cows. J. Anim. Sci., 69, 2538-2547.
Verge, C.F., Howard, N.J., Irwig, L., Simpson, J.M., Mackerras, D. &
Silink, M. (1994) Environmental factors in childhood IDDM. Diabetes
Care, 17, 1391-1389.
Virtanen, S.M., Räsänen, L., Ylönen, K., Aro, A., Clayton, D.,
Langholz, B., Pitkäniemi, J., Savilahti, E., Lounamaa, R., Tuomolehto,
J. & Akerblom, H.K. (1993) Early introduction of dairy products
associated with increased risk of IDDM in Finnish children.
Diabetes, 42, 1786-1790.
Virtanen, S.M., Saukkonen, T., Savilahti, E., Ylönen, K., Räsänen, L.,
Aro, A., Knip, M., Tuomolehto, J. & Akerblom, H.K. (1994) Diet, cow's
milk protein antibodies and the risk of IDDM in Finnish children.
Diabetologica, 37, 381-387.
Waters, D., Danska, J., Hardy, K., Koster, F., Qualls, C., Nickell,
D., Nightingale, S., Gesundheit, N., Watson, D. & Schade, C. (1996)
Recombinant human growth hormone, insulin-like growth factor 1, and
combination therapy in AIDS-associated wasting. Ann. Intern. Med.,
125, 865-872.
Xian, C.L., Shoubridge, C.A. & Read, L.C. (1995) Degradation of IGF-I
in the adult rat gastrointestinal tract is limited by a specific
antiserum or the dietary protein casein. J. Endocrinol., 146,
215-225.
Zumkeller W. (1992) Relationship between insulin-like growth factor-I
and II and IGF-binding proteins in milk and the gastrointestinal
tract: Growth and development of the gut. J. Pediatr.
Gastroenterol., 15, 357-369.
It was concluded that use of rbST would not result in a higher risk to
human health due to the use of antibotics to treat mastitis and that
the increased potential for drug residues in milk could be managed by
practices currently in use by the dairy industry and by following the
directions for use (US Food & Drug Administration, 1996).
2.2 Concentrations of bovine somatotropin and insulin-like growth
factor in tissues and milk
2.2.1 Tissues
The concentrations of bST and IGF-I were measured in tissues of
cattle that had been treated with a 14-day sustained-release product
containing the natural variant of rbST, somavubove (Choi et al.,
1997), in two experiments. In the first experiment, three groups of 12
beef cattle (except at the low dose, to which only six animals were
exposed) with an average weight of 450 kg were treated by subcutaneous
injection for 20 weeks. The controls received no treatment or vehicle,
one group received 250 mg rbST each week, and another received 500 mg
rbST at two-week intervals. The controls and animals at the high dose
were further divided into groups receiving low- and high-energy feed.
The stated total doses were 5 and 10 g rbST, respectively; however,
the dose for both groups calculated from the stated regimens would be
5 g. Two weeks after the final treatment, the animals were slaughtered
and muscle samples were obtained and stored at -20°C for analysis. In
the second experiment, four groups of beef cattle were used: a control
group which received no drug or vehicle, a group receiving a
sustained-release low dose (0.42 mg/kg bw; 0.03 mg/kg bw per day), a
group receiving an intermediate dose (0.84 mg/kg bw; 0.06 kg bw per
day), and one receiving a high dose (1.26 mg/kg bw; 0.09 mg/kg bw per
day). The treated groups were given the drug by subcutaneous injection
every two weeks for 24 weeks, to give total doses of 2.3, 4.5, and 6.8
g, respectively. Two weeks after the final treatment, the animals were
slaughtered, and samples of muscle, kidney, liver, and fat were taken
and stored at -20°C.
The frozen samples were assayed for bST and IGF-I residues by
radioimmunoassay (RIA) in which 5 g of tissue were extracted in
acidethanol for muscle and acetic acid for kidney, liver, and fat.
The RIA procedures involved standard double-antibody techniques and
iodinated tracers. The detection limit for the assays (the amount that
could be distinguished from zero concentration with 95% confidence)
was 0.17 ng/g bST and 0.61 ng/g IGF-I. The coefficients of variation
for the two assays were 6% or less, and the recoveries from liver,
kidney, and fat were 64% for bST and 84% for IGF-I; similar recoveries
were obtained in muscle samples. A summary of the results is given in
Table 1. The authors concluded that two weeks after administration of
two doses of rbST for extended times, the tissue concentrations of bST
and IGF-I were not significantly different from those in untreated
animals.
2.2.2 Residues of insulin-like growth factor in milk
Extensive information on the residues of bST and IGF-I in the
milk of rbST-treated cows was evaluated by the Committee at its
fortieth meeting. IGF-I is a normal but highly variable constituent of
bovine milk, the concentration depending on the state of lactation,
nutritional status, and age. Over an entire lactation, the IGF-I
concentrations range from 1 to 30 ng/ml, with the highest
concentrations in colostrum and a constant decline thereafter.
Multiparous animals have higher concentrations of IGF-I in milk than
primoparous cows (Burton et al., 1994). Bulk milk from cows not
given rbST had IGF-I concentrations of 19 ng/ml (Juskevich & Guyer,
1990), and the fortieth Meeting cited an average value of 3.7 ng/ml
for untreated cows. In milk from rbST-treated cows, the concentrations
of IGF-I ranged from 1 to 13 ng/ml in most studies and were about
25-70% greater than those in untreated animals (Burton et al.,
1994). The fortieth meeting reported an average IGF-I concentration of
5.9 ng/ml, and the increase was significant, even though most of the
concentrations were < 10 ng/ml.
Table 1. Concentrations of bovine somatotropin (bST) and insulin-like growth factor (IGF-I) in tissues after treatment with
recombinant bovine somatotropin
First experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose High dose
bST IGF-I bST IGF-I bST IGF-I
Muscle 12 1.9 ± 1.8 88 ± 21 1.5 ± 1.6 130 ± 25 3.3 ± 2.2 110 ± 32
Second experiment
Tissue No. of Concentration (ng/g; mean ± SD)
samples
Controls Low dose Intermediate dose High dose
bST IGF-I bST IGF-I bST IGF-I bST IGF-I
Muscle 5 3.4 ± 1.5 45 ± 6.5 4.9 ± 1.5 35 ± 15 3.8 ± 2.0 40 ± 5.1 1.5 ± 0.86 55 ± 19
Fat 4 5.1 ± 1.7 210 ± 85 9.3 ± 5.2 200 ± 65 4.8 ± 1.9 200 ± 53 11 ± 12 340 ± 230
Liver 4 5.2 ± 0.59 350 ± 23 3.6 ± 1.7 390 ± 130 5.4 ± 1.2 380 ± 170 4.6 ± 2.0 290 ± 88
Kidney 4 3.6 ± 1.1 910 ± 130 4.5 ± 1.6 1000 ± 140 4.5 ± 1.8 820 ± 120 3.9 ± 0.94 980 ± 220
Since the original work was reviewed, few additional data have
appeared in the literature or in reports made available by the
sponsors. The manufacturer of Posilac(R) - previously identified as
sometribove, which is a form of rbST approved in a number of countries
- submitted additional information. In a study designed to determine
the concentrations of IGF-I in retail milk samples, the concentrations
in milk specifically labelled as coming from cows that had not been
treated with bST were compared with those in unlabelled milk. While
the sponsor assumed that all of the unlabelled milk was from cows that
had been treated with bST, the extent to which this was true was not
ascertained. The study was conducted under US Food and Drug
Administration GLP regulations (21CFR 58). The labelled and unlabelled
status was determined of 127 of 129 retail milk samples collected as
2% fat cow's milk in 51 retail outlets in 34 cities in Wisconsin,
Minnesota, and Iowa, USA, from four goats and 125 cows, and the
samples were analysed by RIA for IGF-I. For 78 samples, the farmer had
certified that rbST had not been used. As rbST is not approved for use
in goats in the United States, it is assumed that the goat-milk
samples were from untreated animals. The results are shown in Table 2
(Eppard et al., 1994). The values for IGF-I are not unlike those
reported in the monograph prepared at the fortieth meeting of the
Committee. Although the contribution of milk from rbST-treated cows to
the unlabelled milk is unknown, the results indicate that the IGF-I
concentrations in retail milk did not increase in the first year after
the launch of rbST.
Table 2. Concentrations of insulin-like growth factor in milk
certified by farmers as coming from cows not treated with
recombinant bovine somatotropin (labelled) and from unlabelled milk
Insulin-like growth factor (ng/ml)
Labelled Unlabelled
(n = 78) (n = 45)
Raw mean 4.3 ± 0.09 4.5 ± 0.12
Logea 1.47 ± 0.044 1.55 ± 0.031 p = 0.01769
Antilog (95% 4.4 (4.0-4.7) 4.7 (4.4-5.0)
confidence
interval)
a Least square means adjusted for state where purchased and dairy
2.2.3 Assays
Because of variations in the IGF-I values reported in different
studies, questions have been raised in submissions to the Committee
about their accuracy. Lower values were found in some studies because
the assay used involved acid-ethanol extraction; an assay involving
acidic gel filtration has been reported to allow better recovery. In a
comparison of the two assays for determining IGF-I in pre- and
post-partum mammary secretions, the acid-ethanol assay was found to
result in a 24 ± 6.6% underestimate in comparison with acid gel
filtration (Vega et al., 1991). Use of the acidethanol assay to
recover 125I-IGF-I in bovine serum and colostrum, which also contain
binding proteins, gave values of 86 ± 6% and 88 ± 7% recovery by the
antibody, respectively (Hadsell, 1991). The difference is due to the
fact that the binding protein with affinity for IGF-I competes with
the antibody used in the RIA procedure; however, gel filtration
removes the hormone that is extensively dissociated by the acid common
to both assays. The studies suggest that the acidethanol assay
probably results in 15-25% underestimates of the concentrations in
milk and plasma. The relative difference in milk will probably not
affect a decision, as the concentrations are low, although higher
control values may affect the inferences. Although incomplete removal
of IGF-binding proteins or differences in the source of standards
might affect the reported results, the Committee considered these
factors immaterial.
2.2.4 Bioavailability and bioactivity of insulin-like growth factor
residues in milk
At its fortieth meeting, the Committee concluded that many of the
physiological effects of rbSTs are mediated by bovine IGF-I, which is
structurally identical to human IGF-I. It noted that there is
substantial endogenous synthesis of IGF-I, mainly in the liver but
also in human milk, saliva, and pancreatic secretions. It further
concluded that IGF-I has no bioactivity when administered orally to
normal and hypophysectomized rats at doses up to 2 mg/kg bw per day,
as dietary IGF-I is degraded by digestive enzymes and is not active in
the upper gastrointestinal tract.
Concern has been expressed that widespread use of rbSTs in dairy
production would lead to a sustained increase in the concentrations of
IGF-I in bulk cow's milk and that, if IGF-I survives digestion, the
greater exposure of consumers would cause adverse health effects
(Hansen et al., 1997). For a quantitative assessment of risk, the
slight increases in the concentration of IGF-I in milk from
rbST-treated cows should be compared with the physiological variations
in the concentrations of this growth factor during lactation (see
section 2.2.2) and with the concentrations in human breast milk, in
the secretions of the gastrointestinal tract, and in serum.
The concentrations of IGF-I are 8-28 ng/ml in colostrum and
5-10 ng/ml thereafter (Zumkeller, 1992; Burton et al., 1994),
indicating that breast-fed newborns are usually exposed to IGF-I at
concentrations equal to or higher than those in milk from rbST-treated
cows. Assuming a daily intake of 1.5 L of milk from rbST-treated cows
with an average IGF-I concentration of 6 ng/ml, the amount of IGF-I
ingested would be 9000 ng/day. The additional daily ingestion of IGF-I
over that of people drinking milk from untreated animals, with an
average IGF-I concentration of 4 ng/ml or 6000 ng/1.5 L, would be 3000
ng. The slightly increased IGF-I concentrations would contribute to
the endogenous concentrations of IGF-I in the gastrointestinal tract
of consumers; however, the main site of IGF-I production in animals
and humans is the liver. It is also produced in the human
gastrointestinal mucosa and is found in saliva, bile, and pancreatic
juice (Olanrewaju et al., 1992). The average concentrations of IGF-I
in the five human gastrointestinal secretions were 0.9 nmol/L in
saliva, 3.5 nmol/L in gastric juice, 24.6 nmol/L in jejunal chyme, 3.6
nmol/L in pancreatic juice, and 0.9 nmol/L in bile (Chaurasia et
al., 1994). On the basis of a molecular mass of 7.5 kDa for IGF-I
(Zumkeller, 1992) and the volume of each of the fluids produced
(Vander et al., 1990), the total volume of IGF-I emptying into the
gastrointestinal tract is 383 000 ng/day. Table 3 shows that the
amount of endogenous IGF-I emptied into the gastrointestinal tract
daily is more than (383 000/9000) 42 times greater than the amount
present in 1.5 L of milk from rbST-treated cows. The 9000 ng value is
2.3% of the estimated daily gastrointestinal secretion of IGF-I in
adults. The additional daily ingestion of 3000 ng IGF-I over that in
milk from untreated animals thus represents 0.78% of the
gastrointestinal secretion.
Table 3. Concentration of insulin-like growth factor (IGF-I) in
gastrointestinal tract secretions
Secretion Volume Concentration Total IGF-I
(ml/day)a (average; ng/ml) secreted (ng)
Jejunal chyme 1500 184.5 276 750
Pancreatic juice 1500 27.0 40 500
Gastric juice 2000 26.2 52 400
Bile 500 6.8 3 400
Saliva 1500 6.8 10 200
Adapted from Baumann (1995)
a From Vander et al. (1990)
In contrast to the conclusion of the Committee at its fortieth
meeting, that IGF-I is completely and rapidly degraded in the
gastrointestinal tract, milk-borne IGF-I may escape digestion by
proteases and therefore be bioactive in the intestine (Hansen
et al., 1997) or even be absorbed as intact peptide into the
systemic circulation (Epstein, 1996). In a study designed to
investigate the potential therapeutic use of IGF-I and to determine
whether oral formulations were feasible, the degradation of
125I-IGF-I was determined in various segments of the
gastrointestinal tract of rats in vivo and in vitro (Xian
et al., 1995). The extent of degradation was measured by one of
three methods: receptor binding, immunoprecipitation, and
trichloracetic acid precipitation. Two segments of the duodenum,
ileum, or whole stomach and part of the colon were ligated in an
anaesthetized male Sprague-Dawley rat that had been fasted for 24 h. A
bolus of labelled IGF-I (8.6 ng/ml in 0.2% bovine serum albumin in
saline) was injected into each segment and incubated for various times
up to 1 h. The reactions were stopped, and the flushed luminal
contents were examined for intact IGF-I. In a parallel set of
experiments, the flushed luminal contents from each of the four gut
segments were used as a source of degredation enzymes in vitro. The
results are shown in Table 4. The most rapid degradation occurred in
the duodenum and ileum and in their contents in vitro, followed by
the stomach and then by the colon. In all cases the values seen
in vitro were equal to or greater than those seen in vivo.
The authors also examined the effectiveness of slowing the
degradation rate of IGF-I in the gut by protecting the molecule in
several ways. Casein at a concentration of 10 mg/ml conferred > 90%
protection on stomach contents in both the trichloracetic acid and
receptor assays; in duodenal flushings, casein conferred 80%
protection against IGF-I degradation in the trichloracetic acid assay
but only 36% protection in the receptor assay at a maximal casein
concentration of 40 mg/ml. The half-life of IGF-I in the upper
gastrointestinal tract in the receptor assay increased from 2-3 min in
the absence of casein to 35 min in its presence. While these results
appear to demonstrate significant protection by casein at a
concentration similar to those in milk, the authors acknowledge that
the observed effects could be explained by simple competition by the
additional proteins for degradation by proteases in the segments. The
results demonstrate that biological receptor-stimulating activity,
which is the best indicator of biological activity, is dramatically
reduced even with good protection from protease activity. The authors
noted with regard to milk residues of IGF-I that 'the protective
effect of casein makes irrelevant the argument that human saliva
contains IGF-I at concentrations greater than the quantities that
would be consumed in milk. As the IGF-I produced by salivary glands is
free IGF-I, without protective effect of casein, it is unlikely to
survive digestion.' (Hansen et al., 1997). That argumentation
neglects the following facts:
Table 4. Half-lives of intact 125I-insulin-like growth factor (IGF-I) in ligated Sprague-Dawley rat gut
segments in vivo and in the flushed contents in vitro
Test for Half-life (min)
intact IGF-I
Duodenum/ileum Stomach Colon
In vivo In vitroa In vivo In vitro In vivo In vitro
Trichloracetic 2 2 8 50 38 > 60
Antibody binding 2 5 33
Receptor binding 2 2 2.5 3 16 ND
ND, not determined
a Antibody and membrane receptor values reported as receptor binding
* Saliva is not the only source of IGF-I in the gastrointestinal
tract: most is secreted into the gut, and the high concentration
in intestinal chyme indicates that IGF-I is secreted in
substantial amounts by the mucosa throughout the gastrointestinal
tract (Olanrewaju et al., 1992; Chaurasia et al., 1994).
* Casein has a flexible structure and is readily degraded in the
stomach and small bowel (Xian et al., 1995). Thus, the
protective effect is present only in the upper gastrointestinal
tract.
* The half-life of IGF-I in the intestine in the presence of casein
is only 35 min (Xian et al., 1995). Therefore, less than 5% of
an initial dose of IGF-I will survive for more than 2 h during
passage through the upper gastrointestinal tract.
* In the presence of casein ingested with milk, endogenous IGF-I in
the gastrointestinal tract will also be protected.
Therefore, even if casein has a limited protective effect, the amount
of bioactive IGF-I ingested with milk from rbST-treated cows would
still be negligible.
Because of the protective effect of casein, some IGF-I might
escape digestive degradation and be absorbed intact. The absorption of
large (1 mg/kg) oral doses of 125I-labelled recombinant human IGF-I
was studied in fasted adult rats, with trichloracetic acid
precipitation of plasma proteins. The baseline bioavailability of the
administered IGF-I was 9.3% of the dose, but this was was increased by
co-administration of 4 mg/kg aprotinin (47%) and 10 mg/kg casein
(67%). RIA of the plasma confirmed the bioavailability of IGF-I in
this model, and the administered radiolabel was found in the form of
high-molecular-mass complexes (Kimura et al., 1997). It should be
noted, however, that the receptor assay, which is the most accurate,
was not used.
The relatively good bioavailability of intact IGF-I in this adult
rat model is in contrast to the lack of bioactivity of orally
administered IGF-I in adult animals (Annex 1, reference 104) and to
the results of studies with neonatal animals which have an incomplete
mucosal barrier and reduced intestinal proteolytic activity (Burrin,
1997). Studies in neonatal rats and piglets indicated that although
30% of an orally administered dose of 125I-IGF-I can be recovered in
the intestinal mucosa there is limited absorption into the peripheral
circulation (Phillips et al., 1995; Donovan et al., 1997). When
suckling transgenic rats ingested 1000-fold higher concentrations of
des(1,3) human IGF-I, no des(1,3)-IGF-I was detected in the plasma of
their pups (Burrin, 1997). Furthermore, in newborn calves and piglets
given large doses of IGF-I in milk replacers, no substantial increase
in the plasma concentration of this growth factor was found (Donovan
et al., 1997; Hammon & Blum, 1997; Houle et al., 1997). In one
study with newborn calves fed milk replacer, a small amount of orally
administered 125I-IGF-I was detected in plasma (Baumrucker et
al., 1992); however, the increase was observed only three days after
administration and in only three of six animals. Even in newborns,
therefore, IGF-I is absorbed to only a small extent, and absorption is
unlikely in adults.
Furthermore, the amount absorbed should be compared with the
normal concentrations of IGF-I in human serum, which show considerable
variation with age. The values are lowest in infants under two years
of age, then increase steadily to reach a maximum in late puberty, and
afterwards decrease to the adult values (Table 5). Assuming a blood
volume that is 5% of the body weight (Ganong, 1971), the serum load of
IGF-I can be calculated to be 50 000 ng in a 15-kg child, 714 000 ng
in a 60-kg adult, and 1 220 000 ng in a 50-kg teenager. The total
IGF-I production in adults has been estimated at 107 ng per day
(Guler et al., 1989). These amounts should be compared with the 9000
ng IGF-I in 1.5 L of milk, which constitutes only 0.09% of the daily
IGF-I production. Since only one-third of the concentrations in milk
can be attributed to IGF-I due to rbST treatment and only a small
amount if any will be absorbed, the milk-borne IGF-I that reaches the
systemic circulation is negligible and this small amount is
immediately sequestered by unsaturated binding proteins.
Table 5. Concentrations (ng/ml) of insulin-like growth factor (IGF-I)
in human plasma
Age Males Females
Mean Range Mean Range
0-2 years 42 14-98 56 14-238
3-5 years 56 59-210 84 21-322
6-10 years 98 28-308 182 56-364
Before puberty > 10 years 126 84-182 182 70-280
Early puberty 210 140-240 224 84-392
Late puberty 364 224-462 434 224-686
Adult > 23 yeras 112 42-266 140 56-308
From Schaff-Blass et al. (1984)
Concern has been expressed about the possible adverse effects on
the health of consumers exposed to increased concentrations of IGF-I
in milk from rbST-treated cows (Epstein, 1996; Hansen et al., 1997).
The most important potential adverse effects of IGF-I arise from the
fact that it is a mitogen for a number of cell types and has been
associated with the growth of tumours including those of the colon,
breast, and lung and osteosarcoma (Pines et al., 1985; Macaulay,
1992; National Institute of Health, 1995). The mitogenic effect could
also result in proliferative reactions locally in the gut. Thus,
orally administered IGF-I increased the cellularity of the intestinal
mucosa of rats in vivo (Olanrewaju et al., 1992) and increased the
rate of proliferation in cultures of human duodenal epithelial crypt
cells (Challacombe & Wheeler, 1994). Since IGF-I receptors can be
detected throughout the epithelium of the intestine, with a high
density in the colon (Laburthe et al., 1988), and the incidence of
colorectal cancer is increased in acromegalic patients who have
excessively high concentrations of free IGF-I in their plasma (Ezzat &
Melmed, 1991), concern has been expressed that increased
concentrations of milk-borne IGF-I may increase the risk for colon
cancer. Although the normal biological effects of IGF-I mean that it
could promote the growth of tumours, this hazard would become a risk
only if there were adequate exposure of consumers to IGF-I. Since
exposure to IGF-I in milk from rbST-treated cows is negligible when
compared with endogenous IGF-I production, it is extremely unlikely
that IGF-I residues cause any systemic or local mitogenic reaction.
2.3 Expression of lentiviruses and prion proteins
2.3.1 Somatotropins and the immune system
Somatotropin enhances the immune system in many species,
including cattle (Comens-Keller et al., 1995). The primary effect
appears to be altered responsiveness of the immune system, although
substantive evidence of this effect is lacking (Burton et al.,
1994), and information on changes in cytokine concentrations or
secretion and on their binding sites are needed in order to define the
nature of the immune-enhancing effects of somatotropins. The studies
reported in the literature differ with regard to the source of
somatotropin, the treatment schedule, and the age of the animals. The
differences between findings in vitro and in vivo may be due to
the release in vivo of mediators such as IGFs and cytokines, which
are not present in vitro (Kelley, 1989). A better understanding of
somatotropin-mediated immune enhancement as a homeorhetic regulator of
the overall health and disease resistance of animals is needed.
Lymphocytes from rbST-treated cows have a greater average maximum
lymphoblastogenic response to rbST than to other mitogens around the
time of parturition (Comens-Keller et al., 1995), and this effect
might prevent mastitis and the other infectious diseases that occur
during this period of immunosuppression.
2.3.2 Effect of bovine somatotropin on the expression of retroviruses
Concern has been expressed that the immunomodulatory effect of
bST might affect retroviral expression in treated animals and thus
cause resurgence of latent retroviral and lentiviral infections in the
ruminant population and the presence of these viruses in somatic cells
in milk. The concern is based largely on a review by Lerondelle et al.
(1994), who discussed the evidence for induction of these viruses in
small ruminants by steroid hormones and the evidence for induction of
lentiviruses in other species by hormones including growth hormone and
IGF-I, and an unpublished study by Lerondelle et al. (1996), who
investigated the effects of rbST on the expression of caprine
arthritis encephalitis virus (CAEV) in goats. This virus belongs to
the group of lentiviruses which, like maedivisna, can infect small
ruminants.
Ruminant lentiviruses are of interest for at least three reasons.
First, they may cause disease in persons who drink milk. Second, if
use of rbST increases the prevalence of ruminant viruses, by the
presence of rbST itself or by the action of IGF-I, the small
additional amounts of growth hormone in the milk of treated cows could
also affect the retroviruses that attack the human immune system,
HIV-1 and HIV-2. Finally, the severity or kinetics of expression of
the disease in ruminants might be increased.
The study of Lerondelle and coworkers (1996) attempted to address
the question of whether rbST increases the expression of CAEV. Viral
expression was measured by assaying reverse transcriptase activity in
milk cells, clinical examination of the udders and joints of the
animals at the beginning and end of the study, and evidence of
infection in an immunodiffusion assay. Twelve pregnant Saanen goats
that were seronegative for CAEV were given an intramammary injection
of monocytes infected in vitro with the Cork strain of CAEV at the
time of drying off. Seven weeks after giving birth, four goats were
treated daily with 5 mg/goat rbST (sometribove), a second group was
treated daily with 10 mg/goat thyroxine, and the control group was
untreated. The compounds were administered in suspension in sterile
water for 30 days, followed by a 45-day observation period. Milk
samples were taken to measure reverse transcriptase activity on days
7, 14, 21, and 28 of treatment and three times during the observation
period. Examination of the udders and joints and immunodiffusion tests
were carried out at the beginning and end of the study. Milk
production and milk-cell counts were evaluated every two days. The
occurrence of CAEV and the onset of effects are shown in Table 6. More
positive cultured cells were seen in the controls than after treatment
with either hormone. Perhaps the most striking effect is the lack of
increase in the rate of infectivity and even a suggestion of a
decrease after treatment with rbST, particularly after the first milk
sampling.
When the results were expressed as a ratio of transcriptase
activity to the number of cells considered to contain virus (Table 7),
there was no correlation between the effect of rbST or thyroxine and
the activity of reverse transcriptase in the milk samples. In fact,
there was no evidence of increased transcriptase activity in any
group. In particular, even though the group receiving rbST had a lower
initial rate of infection than the other two groups, including the
controls, the rate of infection was not increased, as measured by the
number of positive cultures or increased transcriptase activity. The
authors interpreted their results as showing a tendency to increased
viral expression with increased milk production. The results for rbST
were, however, biased by the fact that only two animals were included
in the final evaluation. The authors concluded that, owing to the
Table 6. Onset of appearance of cytopathic effect (in days) for each of 10 milk samplings from groups of four goats given no treatment or
thyroxine or recombinant bovine somatotropin (rbST), as a function of the period of hormonal treatment
Treatment Milk sampling
Before treatment During treatment After treatment
1 2 3 4 5 6 7 8 9
Control 4 8 6 10 6 6 6 8 10
4 6 6 6 8 6 6 6 -
4 6 4 6 4 4 6 4 4
6 8 10 6 10 6 - - -
Mean ± SD (n) 6 ± 1.91 (12) 6.4 ± 1.71 (15) 7 ± 2.39 (8)
Thyroxine
8 6 10 4 6 6 - - 6
6 4 4 4 4 4 4 4 ND
4 4 4 4 4 4 6 4 ND
4 8 6 4 4 4 6 4 ND
Mean ± SD (n) 5.67 ± 2.06 (12) 4.53 ± 0.92 (15) 4.25 ± 0.71 (8)
rbST 8 - - - - - - - -
4 8 8 - 8 - - - 4
ND - - - - - - - -
4 4 4 4 4 4 4 4 4
Mean ± SD (n) 5.71 ± 2.41 (7) 4.8 ± 1.79 (5) 4 (4)
ND, not determined
Table 7. Number of samples found to contain virus by the reverse
transcriptase-positive culture ratio in groups of four goats given
no treatment, thyroxine, or recombinant bovine somatotropin (rbST)
Treatment Before During After Total
treatment treatment treatment
Control 3/6 4/7 3/4 10/17
3/6 3/5 1/3 7/14
6/6 4/5 5/6 15/17
4/4 2/8 3/6 9/18
Total 16/22 13/25 12/19 41/66
Thyroxine 0/3 2/4 0/3 2/10
4/4 5/7 6/6 15/17
6/6 8/8 6/6 20/20
6/6 8/8 4/6 18/20
Total 16/19 23/27 16/21 55/67
rbST 2/4 0/6 0/1 2/11
6/6 7/8 2/5 15/19
0/2 0/4 0/3 0/8
4/4 4/5 5/6 12/14
Total 12/16 11/23 7/15 29/52
heterogeneity of the effects on milk production and the small number
of animals tested, the evidence for a promoting effect of rbST on
viral expression was insufficient.
These results provide no evidence that treatment of cows infected
with lentiviruses with rbST will cause resurgence of viral infections
or pose any risk to human health. Lentiviruses are retroviruses that
replicate only in activated cells of the immune system. They may stay
dormant for months or years before they gradually wear down the immune
system to the point of collapse. The phylogenetic tree of lentiviruses
includes the subfamily of bovine immunodeficiency virus (also called
bovine leukaemia virus, BLV) and the subfamily of HIV-1 and HIV-2
which are the causative agents of AIDS. BLV and HIV are widely
separated phylogenetically (Robertson, 1997). BLV is not known to
cause disease in humans although it can infect human cells in
vitro, where host defence mechanisms are not present (Georgiades
et al., 1978; van der Maaten & Miller, 1990); infection of human
cells in vivo could not be demonstrated, and all attempts to obtain
direct evidence of infection in exposed human populations have yielded
negative results (Straub, 1981; van der Maaten & Miller, 1990).
Failure to infect humans has also been shown for other ruminant
lentiviruses, such as CAEV and maedivisna (Straub, 1981). Excretion
of virus with milk somatic cells can infect the offspring of infected
cows. Such transmission can be effectively blocked by procedures
similar to pasteurization, which destroy the virus at 60°C within 30 s
(Abramova et al., 1974; Baumgartner et al., 1976; van der Maaten &
Miller, 1990). BLV therefore cannot induce disease in humans and is
completely inactivated by routine pasteurization. Furthermore, the
company that sells sometribove has reported that there is no
indication that the incidence of BLV infection has increased in cattle
after eight years of continuous use of rbST in Mexico and Brazil and
four years of use in the United States (Collier & Kowalczyk, 1998),
although this statement is not further qualified.
An increase in the expression of HIV in humans who ingest milk
from rbST-treated cows is extermely unlikely because of the negligible
residues of rbST and IGF-I. Treatment of AIDS patients for six weeks
with recombinant human growth hormone and IGF-I had no effect on the
HIV titres in peripheral blood mononuclear cells, on the CD3, CD4, or
CD8 counts in peripheral blood, or on serum HIV p24 antigen
concentrations (Waters et al., 1996).
2.3.3 Effect of recombinant bovine somatotropin on prion proteins
Concern has been expressed that rbST treatment could increase the
risk for BSE in dairy cows (Hansen et al., 1997). Little evidence is
available to support this concern, and that which has been provided is
indirect. It is currently thought that the infectious agent of BSE is
a prion protein (Prusiner, 1982). Prion proteins are found normally in
all animals and are encoded by a prion-protein gene. BSE is associated
with a post-translationally modified protease-resistant prion protein,
which differs in its three-dimensional structure from the normal
protease-sensitive prion protein. Normal prion proteins are bound to
membranes on the surface of all nerve cells, some lymphocytes, and
other tissues (Prusiner, 1991). To date, no function has been ascribed
to normal prion protein. The most widely accepted theory of BSE is the
conversion of normal prion protein to the abnormal protease-resistant
form, which in turn causes more normal prion protein to convert to
protease resistance. The mechanisms of the conversion to the
disease-causing protease-resistant prion protein are not clearly
understood. In contrast to the normal form, the protease-resistant
form cannot be turned over and builds up in cells, forming large
oligomers which are observed as plaques (amyloids) in the brains of
affected individuals (Gajdusek, 1993).
IGF-I increased the production of prion protein mRNA in vitro
in a rat phaeochromocytoma cell line (PC12 cells), with a rather flat
dose-response curve showing a 40% increase at 10 ng/ml and a doubling
at 100 ng/ml (Lasmézas et al., 1993). In transgenic mice that
harbour multiple copies of the prion protein gene, the progession of
scrapie was accelerated (Prusiner, 1991). It has been speculated that
the increased IGF-I concentrations in rbST-treated cows could increase
prion protein production and possibly speed up the progression of BSE;
however, no studies are available that directly address the question
of whether rbST or IGF-I increases the formation of normal prion
protein or its pathogenic protease-resistant mutant in the brains of
the cattle, and the possibility of a link between rbST treatment and
BSE is highly speculative.
2.4 Cow's milk and insulin-dependent type I diabetes mellitus in
childhood
Epidemiological studies have shown that environmental factors
such as chemicals, viral infections, a short duration of
breastfeeding, and early dietary exposure of newborns to cow's milk
increase the risk for insulin-dependent type I diabetes mellitus
(IDDM) by about 1.5 times (Scott, 1990; Dahlquist et al., 1991;
Jorgensen et al., 1991; Virtanen et al., 1993; Gerstein, 1994;
Verge et al., 1994; Virtanen et al., 1994). IDDM develops as a
consequence of autoimmune destruction of the insulin-producing b cells
of the pancreatic islets. The precise trigger of the autoimmune
reaction is unknown, but it is assumed to be a genetically acquired
immune defect in susceptible individuals (Gerstein, 1994). The
epidemiological evidence indicates that IDDM is geographically and
temporally related to neonatal feeding with cow's milk and that
avoidance of cow's milk during the first few month of life can protect
genetically predisposed individuals (Gerstein, 1994; Verge et al.,
1994).
Serological evidence supports the theory that the immune defect
is triggered by exposure to proteins in cow's milk (Gerstein, 1994).
It has been postulated that, in newborns, milk proteins cross the
immature gut wall, initiating an immune response that cross-reacts
with a beta-cell surface antigen (Verge et al., 1994). Children aged
five to nine, who have an intact intestinal barrier, are not at risk
of acquiring IDDM by drinking cow's milk (Dahlquist et al., 1991).
The possible triggering factors in cow's milk have not been
identified. Casein is unlikely to be involved, since replacement of
milk proteins by casein in the diets of rats susceptible to diabetes
completely prevented the disease (Jorgensen et al., 1991). Increased
concentrations of immunoglobulin A antibodies to cow's milk and
b-lactoglobulin appear to be associated with an increased risk for
IDDM (Dahlquist et al., 1991; Virtanen et al., 1994).
It is unlikely that exposure of human newborns to milk from
rbST-treated cows would increase the risk for IDDM for the following
reasons:
* The composition of milk from rbST-treated cows is well within the
normal variations observed during lactation,
* The slightly increased IGF-I concentrations can be excluded as a
triggering factor because bovine and human IGF-I are identical
and because the concentrations of IGF-I in breast milk are equal
to or initially higher than those found in cow's milk.
3. COMMENTS
Use of antibiotics
After reviewing the available information, the Committee
considered the risk for mastitis associated with use of rbST as an
issue of animal health that is not within its terms of reference;
however, the possibly increased use of antibiotics was considered. A
post-approval monitoring programme was established in the United
States to address the following issues:
* the incidence of mastitis and responses related to herd health
(not within the terms of reference of the Committee),
* treatment with any medications of 28 herds of rbST-treated cows
(not within the terms of reference of the Committee), and
* the percent of milk discarded because of violative drug residues
in key dairy states representing at least 50% of United States
milk production.
In New York State, the percentage of milk discarded because of the
presence of antibiotic residues was not significantly changed after
the introduction of rbST. In other states, however, a small but
statistically significant, increase was observed in 1995, which
coincided with a change to a more sensitive testing method in those
states. The Committee concluded that the use of rbST would not result
in a higher risk to human health due to the use of antibiotics to
treat mastitis and that the increased potential for drug residues in
milk could be managed by practices currently in use within the dairy
industry and by following the directions for use.
Concentrations of insulin-like growth factor in milk and tissues
Insulin-like growth factor (IGF-I) is a normal component of milk
and is found in abundance in a variety of body fluids (Table 8). The
presence and concentrations of IGF-I were the source of much of the
scientific discussion during the original review of bST undertaken at
the fortieth meeting of the Committee and in submissions to the
present one. The information that was reviewed is summarized in FAO
Food and Nutrition Paper No. 41/5 (Annex 1, reference 106). The
concentrations of IGF-I in milk are variable and have been shown to
depend on the state of lactation, nutrition, and age.
Methods for assaying IGF-I were considered by the Committee.
Although incomplete removal of IGF-binding proteins or differences in
the source of standards and extraction methods might affect the
reported values, these factors were considered not to alter any
conclusions materially. The relatively high values previously reported
in milk were considered to reflect inadequate extraction.
Table 8. Insulin-like growth factor in milk and body fluids
Medium Concentration
(ng/ml)
Milk
Human 5-10
Colostrum 8-28
Bovine (bulk milk)
Untreated 1-9
rbST-treated 1-13
Plasma
Child 17-250
Adolescent 180-780
Adult 120-460
Gastrointestinal secretions (human)
Saliva 6.8
Gastric juice 26
Pancreatic juice 27
Bile 6.8
Jejunal chyme 180
Daily production of adult humans 107 ng/d
Since the previous evaluation, few additional data on residues
have appeared in the literature or in reports provided by interested
parties; however, the manufacturer of sometribove submitted additional
information on the concentrations of IGF-I in retail milk after the
approval of rbST in the United States. The results showed no
difference in the IGF-I concentrations of milk certified as derived
from cows not treated with rbST and of unlabelled milk; however, the
percentage of the unlabelled milk that was derived from cows receiving
rbST was not specified.
Concern has been expressed that any rbST-induced increase in the
concentration of IGF-I in milk would contribute to the endogenous
levels of IGF-I in the gastrointestinal tract and serum if it were not
biodegraded and were absorbed. In rats, IGF-I is rapidly degraded in
the gastrointestinal tract; however, a protective effect of casein on
IGF-I was demonstrated in these studies. It was postulated that the
retarded degradation leads to increased serum levels of IGF-I (as
shown in one study in rats) and to prolonged exposure of the gut. The
Committee noted that seven days' oral administration of high doses of
IGF-I in milk replacer did not increase the circulating concentrations
of IGF-I in newborn calves and piglets, indicating that significant
absorption of IGF-I is unlikely to occur under physiological
conditions. In view of the decreased rate of degradation in the small
intestine of rats in the presence of casein, the concentrations of the
growth factor would probably decrease to less than 5% of their initial
values within 2 h, so that milk-borne IGF-I would not be expected to
contribute to the concentrations of IGF-I in the large intestine.
If 1.5 L of milk are ingested per day, the average intake of
IGF-I will be 6000 ng in milk from untreated cows containing an
assumed IGF-I concentration of 4 ng/ml and 9000 ng in milk from rbST-
treated cows with an approximate average concentration of 6 ng/ml. It
has been calculated that IGF-I in gastrointestinal secretions amounts
to about 380 000 ng/day. Therefore, the additional amount of IGF-I in
1.5 L of milk from rbST-treated cows would represent only about 0.8%
of the IGF-I secreted in the gastrointestinal tract. The total amount
of IGF-I in serum has been calculated to range from 50 000 to 1 220
000 ng, depending on age. The total daily IGF-I production in adult
humans has been estimated to be 107 ng. Therefore, the daily amount
of IGF-I ingested with milk from rbST-treated cows would represent
less than 0.09% of the daily production of adults. Even if all of the
milk-borne IGF-I were absorbed, the additional amount would be
negligible.
When sustained-release rbST was administered to cattle once every
two weeks for a total of 20 weeks, the tissue concentrations of rbST
and IGF-I two weeks after the final dose were not significantly
increased.
The Committee concluded that any increase in the concentration of
IGF-I in milk from rbST-treated cows is orders of magnitude lower than
the physiological amounts produced in the gastrointestinal tract and
in other parts of the body. Thus, the concentration of IGF-I would not
increase either locally in the gut or systemically, and the potential
for IGF-I to promote tumour growth would not increase when milk from
rbST-treated cows was consumed; there is thus no appreciable risk for
consumers.
Expression of retroviruses
Concern that treatment of cattle with rbST would increase the
expression of retroviruses, including BLV, were addressed in
experiments with caprine arthritis encephalitis virus in goats.
Infectivity was not increased when measured as numbers of infected
cells, and there was no evidence of increased reverse transcriptase
activity. These studies provide no evidence that rbST affects the
expression of BLV, a lentivirus in cattle. Furthermore it has been
shown that BLV is destroyed in simulated pasteurization conditions by
heating milk to 60 .C for 30 s. In addition, there is no evidence
of human susceptibility or response to ruminant retroviruses.
Expression of prion proteins
Concern has been expressed that treatment with rbST could shorten
the incubation period for BSE. This hypothesis is based on results
obtained with a neuronal cell line in vitro indicating increased
formation of prion protein mRNA in response to IGF-I. Furthermore,
increased formation of prion protein shortened the incubation period
for scrapie in transgenic mice harbouring multiple copies of the gene
that codes for prion proteins. No data were available, however, to
address directly the question of whether rbST or IGF-I increases the
formation of normal prion protein or its pathogenic protease-resistant
mutant in the brains of cattle. The Committee considered that the
possibility of a link between rbST treatment and BSE was highly
speculative.
Risk for insulin-dependent diabetes mellitus
Exposure of human newborns to cow's milk increases the risk for
insulin-dependent diabetes mellitus by about 1.5-fold. The Committee
considered whether exposure of newborns to milk from rbST-treated cows
further increases this risk. It concluded that, because of its
unchanged composition, the milk of rbST-treated cows would not pose an
additional risk to the development of insulin-dependent diabetes
mellitus.
4. EVALUATION
On the basis of the following:
* insignificant changes in the quantities of milk discarded after
testing for antibiotic residues following the introduction of
rbST into commercial use;
* low concentrations of rbST and IGF-I residues in milk;
* degradation of IGF-I in the gut and its abundance in gut
secretions;
* the extremely low concentrations of ingested IGF-I in comparison
with endogenous production;
* lack of evidence that rbST stimulates the expression of
retroviruses;
* lack of a direct link between rbST treatment and BSE; and
* the absence of significant changes in the composition of milk
from rbST-treated cows that could contribute to an additional
risk for the development of insulin-dependent diabetes mellitus,
the Committee concluded that rbST can be used without any appreciable
risk to the health of consumers. The Committee reaffirmed its previous
ADIs1 and MRLs2 'not specified' for somagrebove, sometribove,
somavubove, and somidobove.
5. REFERENCES
Abramova, E.M., Kondratev, V.S. & Sytinskii, I.A. (1974) The
biochemistry of leucosis in cattle. Vet. Bull., 44, 689-711.
Baumann, D.M. (1995) IGF-I Fact Sheet, Department of Animal Science,
Cornell University, Ithaca, NY, USA.
Baumgartner, L.E., Olson, C. & Onuma, M. (1976) Effect of
pasteurization and heat treatment on bovine leukemia virus. J. Am.
Vet. Med. Assoc., 169, 1189-1191.
Baumrucker, R.R., Hadsell, D.J., Skarr, T.C., Campbell, P.G. & Blum,
J.W. (1992) Insulin-like growth factors (IGFs) and IGF binding
proteins in mammary secretions: Origins and implications in neonatal
physiology. In: Picciano, M.F. & Lonnerdal, B., eds, Mechanisms
Regulating Lactation and Infant Nutrient Utilization, New York,
Wiley-Liss, pp. 285-308.
Burrin, D.G. (1997). Is milk-borne insulin-like growth factor-l
essential for neonatal development? J. Nutr., 127, 975S-979S.
Burton, J.L., McBride, B.W., Block, E., Glimm, D.R. & Kenelly, J.J.
(1994) A review of bovine growth hormone. Can. J. Anim. Sci., 74,
167-201.
Challacombe, D.N. & Wheeler, E.E. (1994) Safety of milk from cows
treated with bovine somatotropin. Lancet, 344, 815-816.
Chaurasia, O.P., Marcuard, S.P. & Seidel, E.R. (1994) Insulin-like
growth factor l in human gastrointestinal exocrine excretion.
Regul. Pept., 50, 113-119.
Choi, J., Choi, M.J., Kim, C., Ha, J., Hong, A., Ji, Y. & Chang, B.
(1997). The effect of recombinant bovine somatotropin (rbST)
administration on residual BST and insulin-like growth factor I levels
in various tissues of cattle. J. Food Hyg. Soc. Jpn, 38, 225-232.
Codex Alimentarius Commission (1997) Report of the Twenty-second
Session of the Codex Alimentarius Commission, Geneva, 23-28 June
1997 (FAO Document ALINORM 97/37), Rome, Food and Agriculture
Organization of the United Nations.
Collier, R.J. (1996) Post-approval evaluation of Posilac bovine
somatotropin in commercial dairy herds (100-USA-COW-RJC-93-051).
Unpublished report from Protiva, Monsanto Co. Submitted to WHO by
Protiva, Monsanto Co., St Louis, Missouri, USA.
Collier, R.J. & Kowalczyk, D.F. (1998) Human health risk of
retroviruses in cattle and bovine somatotropine. Unpublished report
from Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co.,
St Louis, Missouri, USA.
Comens-Keller, P.G., Eppard, P.J. & Collier, R.L. (1995). Evaluation
of somatotropin as a homeorhetic regulator of immunity. In: Ivan, M.,
ed., Animal Science Research and Development: Moving Toward a New
Century. Proceedings of the 75th Anniversary Meeting of the
Canadian Society of Animal Science, Ottawa, pp. 79-94.
Dahlquist, G., Blom, L. & Lönnberg, G. (1991) The Swedish childhood
diabetes study: A multivariate analysis of risk determinants for
diabetes in different age groups. Diabetologica, 34, 757-762.
Donovan, S.M., Chao, J.C., Zijlstra, R.T. & Odle, J. (1997) Oral
administered iodinated recombinant human insulin-like growth factor-I
(125I-rhIGF-I) is poorly absorbed by the neonatal piglet. J.
Pedriat. Gastroenterol. Nutr., 24, 174-182.
Eppard, P.J., Collier, R.J., Hintz, R.L., Veenhuizen, J.J. & Baile,
C.A. (1994) Survey of milk insulin-like growth factor in retail milk
samples. Unpublished report No. 100-USA-COW-RJC-94-074 from Protiva,
Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St Louis,
Missouri, USA.
Epstein, S.S. (1996) Unlabeled milk from cows treated with
biosynthetic growth hormones: A case of regulatory abdication. Int.
J. Health Serv., 26, 173-185.
Ezzat, H. & Melmed, S. (1991) Clinical review 18: Are patients with
acromegaly at increased risk for neoplasia? J. Clin. Endocrinol.
Metab., 72, 245-249.
Gajdusek, D.C. (1993) Genetic control of nucleation and polymerization
of host precursors to infectious amyloids in the transmissible
amyliodoses of brain. Br. Med. Bull., 49, 913-931.
Ganong, W.F., ed. (1971) Review of Medical Physiology, Los Altos,
Lange Medical Publications, p. 5.
Georgiades, J.A., Billiau, A. & Vanderschueren, B. (1978) Infection of
human cell cultures with bovine visna virus. J. Gen. Virol., 38,
375-381.
Gerstein, H.C. (1994) Cow's milk exposure and type I diabetes
mellitus. Diabetes Care, 17, 13-19.
Guler, H.P., Zapf, J., Schmid, C. &, Froesch, E.R. (1989) Insulin-like
growth factors I and II in healthy man. Estimations of half-lives and
production rates. Arch. Endocrinol., 121, 753-758.
Hadsell, D.L. (1991) Insulin-like Growth Factor (IGF) Receptors in
the Bovine Mammary Gland: Regulation, Mitogenic Action, and Role in
Mammary Secretion of IGF-I, PhD Thesis, Pennsylvania State
University, Philadelphia, Pennsylvania, pp. 108-114,
Hammon, H. & Blum, J.W. (1997) The somatotropic axis in neonatal
calves can be modulated by nutrition, growth hormone and
Long-R3-IGF-I. Am. J. Physiol., 273, E130-E138.
Hansen, M., Halloran, J.M., Groth, E., III & Lefferts, L.Y. (1997)
Potential public health impacts of the use of recombinant bovine
somatotropin in dairy production. Unpublished report. Submitted to WHO
by Consumer Policy Institute, Yonkers, New York, USA.
Houle, V.M., Sschroeder, E.A., Odle, J. & Donovan, S.M. (1997) Small
intestinal disaccharidase activity and ileal villus height are
increased in piglets consuming recombinant human insulin-like growth
factor-I. Pediatr. Res., 42, 78-86.
Jorgensen, K.D., Joner, G. & Hanssen, K.F. (1991) Relationship between
cows' milk consumption and incidence of childhood IDDM. Diabetes
Care, 14, 1081-1083.
Juskevich, J.C. & Guyer, C.G. (1990) Bovine growth hormone: Human food
safety evaluation. Science, 249, 875-884.
Kelley, K.W. (1989) Growth hormone, lymphocytes and macrophages.
Biochem. Pharmacol., 38, 705-713.
Kimura, T., Murakawa, Y., Ohno, M., Ohtani, S. & Higaki, K. (1997)
Gastrointestinal absorption of recombinant human insulin-like growth
factor-I in rats. J. Pharmacol. Exp. Ther., 283, 611-618.
Laburthe, M., Rouyer-Fessard, C. & Gammeltoft, S. (1988) Receptors for
insulin-like growth factors I and II in the rat gastrointestinasl
epithelium. Am. J. Physiol., 254, 457-462.
Lasmézas, C., Deslys, J.P. & Dormont, D. (1993) Recombinant human
growth hormone and insulin-like growth factor I induce PrP gene
expression in PC12 cells. Biochem. Biophys. Res. Commun., 196,
1163-1169.
Lerondelle, C., Lena, P. & Mornex, J.F. (1994) [Hormonal control of
retroviral infections.] Renc. Rech. Ruminants, 1, 4550 (in French).
Lerondelle, C., Guiguen, F., Fornazero, C., Gounel, F., Leroux, C.,
Chastang, J., Pardo, E., Bolland, P., Bruyas, S., Cordier, G. &
Mornex, J.F. (1996) Effects of recombinant bovine somatotropin and of
thyroxine on the expression of lentiviruses in ruminants. Unpublished
report from INRA Veterinary School of Lyon and INSERM Hôpital Louis
Pradel, Lyon, France. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
van der Maaten, M.J. & Miller, J.M. (1990) Bovine leucosis. In:
Virus Infections of Ruminants, Amsterdam, Elsevier Science
Publishers, pp. 419-429.
Macaulay, V.M. (1992). Insulin-like growth factors and cancer. Br.
J. Cancer, 65, 311-320.
National Institute of Health (1995) NIH conference: Insulin-like
growth factors and cancer. Ann. Intern. Med., 122, 54-59.
Olanrewaju, H., Patel, L. & Seidel, E.R. (1992) Trophic action of
intraileal infusion of insulin-like growth factor I: Polyamine
dependence. Am. J. Physiol., 263, E282-E286.
Phillips, A.F., Rao, R., Anderson, G.G., McCracken, G.M., Lake, M. &
Koldovsky, O. (1995) Fate of insulin-like growth factors I and II
administered orogastrically to suckling rats. Pediatr. Res., 37,
586-592.
Pines, A., Rozen, M.B. & Gilat, T. (1985) Gastrointestinal tumors in
acromegalic patients. Am. J. Gastroenterol., 80, 286-289.
Prusiner, S.B. (1982) Molecular biology of prion diseases. Science,
216, 136.
Prusiner, S.B. (1991) Molecular biology of prion diseases. Science,
252, 1515-1522.
Robertson, D.L. (1997) The evolution of AIDS viruses. From the
Internet
Schaff-Blass, E., Burstein, S. & Rosenfield, R.L. (1984) Advances in
diagnosis and treatment of short stature, with special reference to
the role of growth hormone. J. Pediatr., 104, 801.
Scott, F.W. (1990) Cow milk and insulin-dependent diabetes mellitus:
Is there a relationship? Am. J. Clin. Nutr., 51, 489-491.
Straub, O.C. (1981) Enzootic bovie leucosis. In: Gibbs, E.P.J., ed.,
Virus Diseases of Food Animals, New York, Academic Press, pp.
683-718.
US Food and Drug Administration (1996) CVM Update: VMAC endorses
post-approval program for Posilac(R), 18 December 1996.
Vander A.J., Sherman, J.H. & Luciana, D.S., eds (1990) Human
Physiology, The Mechanisms of Body Function, 5th Ed., New York,
McGraw-Hill Publishing Co., p. 15.
Veenhuizen, J.J., Hintz, R.L., Hemenover, M.L., Kowalczyk, D.F. &
Collier, R.J. (1996) Post-approval monitoring program for Posilac
bovine somatotropin: Tracking program of milk discarded due to drug
residue violations. Unpublished report No. 100-USA-Cow-RLH-93-053 from
Protiva, Monsanto Co. Submitted to WHO by Protiva, Monsanto Co., St
Louis, Missouri, USA.
Vega, J.R., Gibson, C.A., Skaar, T.C., Hadsell, D.L. & Baumrucker,
C.R. (1991) Insulin-like growth factor (IGF)-I and II and IGF binding
proteins in serum and mammary secretions during the prepartum period
and early lactation in dairy cows. J. Anim. Sci., 69, 2538-2547.
Verge, C.F., Howard, N.J., Irwig, L., Simpson, J.M., Mackerras, D. &
Silink, M. (1994) Environmental factors in childhood IDDM. Diabetes
Care, 17, 1391-1389.
Virtanen, S.M., Räsänen, L., Ylönen, K., Aro, A., Clayton, D.,
Langholz, B., Pitkäniemi, J., Savilahti, E., Lounamaa, R., Tuomolehto,
J. & Akerblom, H.K. (1993) Early introduction of dairy products
associated with increased risk of IDDM in Finnish children.
Diabetes, 42, 1786-1790.
Virtanen, S.M., Saukkonen, T., Savilahti, E., Ylönen, K., Räsänen, L.,
Aro, A., Knip, M., Tuomolehto, J. & Akerblom, H.K. (1994) Diet, cow's
milk protein antibodies and the risk of IDDM in Finnish children.
Diabetologica, 37, 381-387.
Waters, D., Danska, J., Hardy, K., Koster, F., Qualls, C., Nickell,
D., Nightingale, S., Gesundheit, N., Watson, D. & Schade, C. (1996)
Recombinant human growth hormone, insulin-like growth factor 1, and
combination therapy in AIDS-associated wasting. Ann. Intern. Med.,
125, 865-872.
Xian, C.L., Shoubridge, C.A. & Read, L.C. (1995) Degradation of IGF-I
in the adult rat gastrointestinal tract is limited by a specific
antiserum or the dietary protein casein. J. Endocrinol., 146,
215-225.
Zumkeller W. (1992) Relationship between insulin-like growth factor-I
and II and IGF-binding proteins in milk and the gastrointestinal
tract: Growth and development of the gut. J. Pediatr.
Gastroenterol., 15, 357-369.
