
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
FURFURAL
First draft prepared by
R. Kroes
Research Institute of Toxicology, Utrecht University,
Utrecht, Netherlands
Explanation
Biological data
Biochemical aspects
Absorption, distribution, biotransformation, and
excretion
Aldehyde reactivity
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Special studies on carcinogenicity
Genotoxicity
Reproductive toxicity
Human intake
Comments
Evaluation
References
1. EXPLANATION
Furfural was evaluated previously at the thirty-ninth meeting of
the Committee (Annex I, reference 101), but an ADI was not established
because of evidence of its genotoxicity and carcinogenicity. At that
time, the Committee considered that its direct addition as a flavour
was not appropriate, that its use as a solvent should be restricted to
situations in which alternatives are not available, and that its
transfer into food should be reduced to the lowest extent technically
feasible. At the present Meeting, the Committee considered furfural as
a flavouring agent.
Furfural is used as a flavouring agent in a variety of food
products and alcoholic and non-alcoholic beverages. Furfural and many
of its derivatives occur widely as natural constituents of the food
supply.
Since the last evaluation, additional data, including those from
studies of the metabolism of furfural, its potential genotoxicity, and
effects in the liver have become available.
This consolidated monograph includes relevant information from
the previous monograph and the results of studies reviewed for the
first time at the present Meeting.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, biotransformation, and excretion
Laboratory animals
Furfural is well absorbed after administration by any route. In
rats, 85% of 14C-furfural administered by gavage in corn oil was
recovered in urine within 72 h (National Toxicology Program, 1987).
Similar results were found for rats and mice (Nomeir et al., 1992;
Parkash & Caldwell, 1994). After oral administration, furfural is
rapidly absorbed from the gastrointestinal tract and distributed to
the tissues, principally the liver and kidney. The tissue
concentrations are generally proportional to the dose.
Furfural is metabolized primarily by oxidation of the aldehyde
function in rats (Paul et al., 1949; Rice, 1972; Nomeir et al., 1992;
Parkash & Caldwell, 1994) and mice (Parkash & Caldwell, 1994).
Oxidation yields furoic acid, which, as the coenzyme A (CoA)
thioester, is either conjugated with glycine and excreted or condensed
with acetyl CoA to form the chain-lengthened metabolite
2-furanacryloyl CoA (Figure 1). 2-Furanacryloyl CoA conjugates with
glycine and is excreted primarily in the urine. In rats and mice,
furoic acid appears to decarboxylate to a very minor extent (~1%)
via oxidation of the furan ring to yield carbon dioxide; the
mechanism is unknown. The absorption (83-90%), tissue distribution
(primarily liver and kidney), relative amounts of metabolites (urine,
76-105%; faeces, 1-7%; breath, 4-7%; tissues, 1%), and pattern of
excretion in mice and rats were linear over the range of doses
investigated (0.1-200 mg/kg bw of furfural) (Nomeir et al., 1992;
Parkash & Caldwell, 1994).
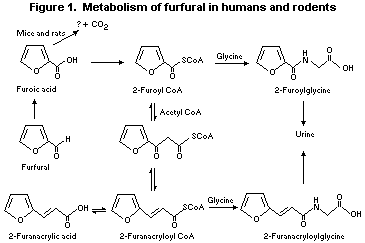 In animals, the condensation reaction of 2-furoyl CoA with acetyl
CoA to yield furanacryloyl CoA is reversible, favouring formation of
2-furoyl CoA (Parkash & Caldwell, 1994). The observation that furoic
acid is excreted in the urine of dogs given furanacrylic acid
(Friedmann, 1911) supports this conclusion. Analogous reversible
reactions between the CoA thioesters of benzoic acid and cinnamic acid
in vivo favour benzoic acid (Nutley et al., 1994). Excretion of
unconjugated furoic acid and furanacrylic acid at higher doses
suggests that glycine conjugation in laboratory animals is limited,
probably by the supply of endogenous glycine (Gregus et al., 1993). As
mentioned earlier, the principal metabolite in rodents, furoic acid,
may also be metabolized to a very minor extent via oxidation of the
heteroaromatic ring. Since heteroaromatic and aromatic carboxylic
acids do not normally undergo decarboxylation in vivo (Caldwell,
1982), it can be assumed that oxidation of the furan ring system of
furoic acid precedes the loss of carbon dioxide. On the basis of this
assumption, epoxidation (Ramsdell & Eaton, 1990) or hydroxylation
(Ravindranath & Boyd, 1985; Koenig & Andreesen, 1990) of the furan
ring may yield reactive intermediates (e.g. furfural-2,3-epoxide,
acetylacrolein, and alpha-ketoglutaric acid) which readily undergo
decarboxylation. Biochemical changes in the lungs and livers of
animals exposed to furfural indicate that ring oxidation may be
catalysed by a cytochrome P450b isoenzyme, yielding an intermediate
which subsequently conjugates with glutathione (Gupta et al., 1991;
Mishra et al., 1991).
Humans
As in laboratory animals, the predominant pathway for
detoxification of furfural in humans is oxidation of the aldehyde to
yield furoic acid, which either conjugates with amino acids or
condenses with acetyl CoA to produce furanacrylic acid.
After inhalation or dermal absorption, furfural is efficiently
and rapidly absorbed ithrough the lungs and skin, with 20-30% of the
amount absorbed by the lungs (Flek & Sedivec, 1978). When six
volunteers were exposed to 30 mg/m3 for 8 h, the mean pulmonary
retention was 78% (range, 75-82%). Retention was independent of the
concentration of furfural vapour and the length of exposure. An
average 8-h exposure maximum was equivalent to a dose of 1.9 mg/kg bw.
The biological half-life of absorbed furfural in humans is only about
2 h. Essentially all of the absorbed furfural could be accounted for:
97% (range, 93-100%) was oxidized to 2-furoic acid and excreted as the
glycine conjugate, 0.5-5% was excreted as furanacrylic acid, and less
than 1% was exhaled unchanged.
2.1.2 Aldehyde reactivity
Furfural contains a heteroaromatic furan ring with a reactive
aldehyde functional group at the 2 position. The reactivity of the
aldehyde function of aldehydes of low molecular mass, like furfural,
suggests that such compounds are not absorbed intact at doses that do
not saturate the oxidation or condensation reactions associated with
the aldehyde function in digestive fluids. Likewise, when furfural
reaches the body fluids, it is likely to react before entering the
cell. The reactivity of the aldehyde group has been demonstrated, for
example, for acetaldehyde and formaldehyde, both normal metabolic
intermediates, which may have toxic effects including the induction of
tumours when administered under non-physiological conditions at high
doses (Til et al., 1989).
Aldehydes of low molecular mass have been reported to bind with
soluble proteins, protein components of cell membranes, and
thiol-containing molecules to form unstable and stable adducts.
Formation of adducts with albumin (Nagasawa et al., 1980; Donahue et
al., 1983; Tuma et al., 1984), haemoglobin (Peterson & Nguyen, 1985),
and erythrocyte membranes (Gaines et al., 1977) has been demonstrated
in vitro and in vivo. In membranes, binding is usually with the
protein component (Nomura & Lieber, 1981). Aldehydes also bind
glutathione (Videlia et al., 1982). Aldehyde dehydrogenase-catalysed
oxidation of low-molecular-mass aldehydes requires glutathione
(Eckfeldt & Yonetani, 1982), suggesting that free aldehyde is rapidly
conjugated with glutathione in vivo to form a thiohemiacetal which
is subsequently oxidized to the corresponding acid (Brabec, 1981).
Thus, the reactivity of the aldehyde function will significantly
curtail the intracellular concentration of free aldehyde.
When an aldehyde is present in a cell, it undergoes rapid
oxidation, with conjugation of the resulting carboxylic acid (Gregus
et al., 1993). The various metabolic processes, i.e. oxidation,
conjugation, and condensation (see section 2.1.1) effectively
eliminate the reactive aldehyde functional group when not saturated by
high, non-physiological doses.
2.2 Toxicological studies
2.2.1 Acute toxicity
On the basis of the oral LD50 values in various species
(Table 1), furfural is acutely toxic at lower doses in rats than in
mice.
Wistar/Slc rats given a single dose of 50 mg/kg bw furfural by
gavage had sporadic eosinophilic degeneration of hepatic cells with
nuclear pyknosis and eosinophilic necrosis and increased hepatocyte
mitosis. No massive or zonal necrosis was observed. Since the control
rats were killed at the beginning of the experiment, no appropriate
controls were available (Shimizu & Kanisawa, 1986). Decreased
activities of succinic dehydrogenase and ATPase and increased
activities of acid phosphatase and acid DNase II were reported in male
Wistar rats injected intraperitoneally with 20 or 50 mg/kg bw (Jonek
et al., 1975).
Table 1. Acute toxicity of furfural
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Rat Oral 127 Jenner et al. (1964)
Rat Intraperitoneal 120 Tiunov et al. (1970)
Mouse Oral 333 Boyland (1940)
Mouse Subcutaneous 200 Tiunov et al. (1970)
Mouse Subcutaneous 223 (1-day survival) Castellino et al.(1963)
119 (10-day survival)
Rabbit Dermal > 310 Moreno (1976)
Hamster Inhalation 2500 Kruysse (1972)
Bird Oral > 98 Schafer et al. (1983)
2.2.2 Short-term studies of toxicity
Rats
In rats receiving 0.05, 2.5, or 25 mg/kg bw per day furfural for
35 days, no hepatic impairment was seen (Kuznetsov, 1966). When male
rats were injected intraperitoneally with furfural in increasing doses
from 29 to 58 mg/kg bw for 30 days, damage and increased intracellular
catabolic processes were seen in liver and kidney cells (Kaminska,
1977).
In a 16-day study, groups of five male and five female Fischer
344/N rats were given furfural at doses of 0, 15, 30, 60, 120, or
240 mg/kg bw per day in corn oil by gavage, five days per week for a
total of 12 doses. At the end of the study, the surviving animals were
killed, necropsied, and examined histopathologically. No
treatment-related abnormalities were found. Eight of ten rats at the
highest dose had died by the third day due to gavaging accidents.
Animals at 120 mg/kg bw appeared to be less active. The body weights
of rats given doses < 120 mg/kg bw per day did not differ
significantly from those of controls.
In a 16-day study, groups of five male and five female B6C3F1
mice were given furfural at doses of 0, 25, 50, 100, 200, or 400 mg/kg
bw per day in corn oil by gavage on five days per week for a total of
12 doses. At the end of the study, the surviving animals were killed,
necropsied, and examined histopathologically. The mean body weights of
the treated mice did not differ significantly from those of controls.
One male at the highest dose had died by day five, and two female mice
died from gavaging accidents. No treatment-related abnormalities were
found.
In a 13-week study, groups of 10 male and 10 female B6C3F1 mice
were given furfural at doses of 0, 75, 150, 300, 600, or 1200 mg/kg bw
per day in corn oil by gavage on five days per week. Necropsies were
performed on all survivors, and histopathological examinations were
performed on those at the two highest doses and the controls. All the
mice at the two highest doses died before the end of the study, except
for one male and one female at 600 mg/kg bw per day. The body weights
of the males treated with doses > 150 mg/kg bw per day were
slightly lower than those of controls. Female mice at 75-300 mg/kg bw
per day and male mice at 300 mg/kg bw per day showed significantly
increased relative liver weights as compared with controls.
Centrilobular coagulative necrosis of hepatocytes was observed in most
male mice at the two highest doses and in one male per group treated
with 150 or 300 mg/kg bw per day. Among the females, only two at the
highest dose showed similar lesions. Animals with coagulative necrosis
also had inflammation of the liver, with minimal to mild mononuclear
inflammatory cell infiltrate (National Toxicology Program, 1990).
A group of 48 male, six-week-old Wistar/Slc rats was fed furfural
at a concentration of 20 ml/kg diet (equivalent to approximately 1200
mg/kg bw per day) for one week, 30 ml/kg diet for one week, and then
40 ml/kg diet on days 15-90; on days 90-120, the rats were given the
diet containing 40 ml/kg for only five days per week to prevent
reduced weight gain. The concentration of furfural in the feed was not
analysed after preparation of the diet. A control group of 40 male
rats of the same age was fed the basal diet.
Treated rats sacrificed after 90 days had marked
cholangiofibrosis, with areas of increased density containing red
nodules. The nodules showed fibrous widening of Glisson's sheath,
bile-duct proliferation, and destruction of the limiting plates. The
parenchymal damage consisted of bridging necrosis and hydropic
degeneration of hepatocytes. The parenchyma often showed no cirrhotic
changes in areas other than those with fibrosis, suggesting a
regenerative process in the liver. Increased numbers of cells
undergoing mitosis were seen. In rats killed on day 120 of the study,
similar but more marked findings were reported. The incidence of
hepatic fibrosis was increased in the furfural-treated rats. No
atypical hepatocyte growth was noted. Furfural did not cause
hepatocellular hyperplastic changes (Shimizu & Kanisawa, 1986).
In a study designed to examine the mechanism of furfural-induced
hepatocytic injury, six groups of six male Wistar/Slc rats were
maintained on diets containing furfural at doses calculated to result
in average daily intakes of 20 ml/kg diet (approximately 1200 mg/kg bw
per day) for the first 30 days and 30 ml/kg diet (approximately
1700 mg/kg bw per day) thereafter. Each group also included four
control animals. The furfural-containing diets were replaced after 15,
30, 60, 90, 120, or 150 days, and animals were killed 14 days after
termination of furfural administration. Increased duration of exposure
to furfural was accompanied by increased numbers and size of foci
positive for the placental form of glutathione- S-transferase in
hepatocytes (Shimizu et al., 1989). Such foci have been reported to be
a marker for early detection of preneoplastic or neoplastic cells
(Pickett & Lu, 1988).
In a 13-week study, groups of 10 male and 10 female Fischer 344/N
rats were given furfural at doses of 0, 11, 22, 45, 90, or 180 mg/kg
bw per day in corn oil by gavage on five days per week. All survivors
were necropsied, and histopathological examinations were performed on
those at the two highest doses and the controls. Only one male rat at
the highest dose survived to the end of the study; four of the deaths
were considered to be related to gavage, as were two deaths among
controls and four among rats at the second highest dose. The mean body
weights of treated rats did not differ from those of controls. The
relative and absolute weights of the livers and kidneys of males given
90 and 180 mg/kg bw per day were significantly higher than those of
controls. An increased incidence of cytoplasmic vacuolization in
hepatocytes was observed in all groups of treated males: control,
4/10; 11 mg/kg bw per day, 10/10; 22 mg/kg bw per day, 10/10; 45 mg/kg
bw per day, 10/10; 90 mg/kg bw per day, 9/10. No lesions were reported
in the kidneys of these rats (National Toxicology Program, 1990).
Hamsters
Groups of 10 Syrian golden hamsters of each sex were exposed to
furfural vapour at 0, 77, 448, or 2165 mg/m3 for 6 h per day, five
days per week over 13 weeks. The main findings were mild growth
retardation, irritation of the eyes and nose, and hyperplastic atrophy
of the nasal epithelium, all at the highest dose. The NOEL was 77
mg/m3, since mild nasal epithelial degeneration was observed at 448
mg/m3 (Feron & Kruysse, 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a two-year study, 50 male and 50 female B6C3F1 mice were
given furfural at doses of 0, 50, 100, or 175 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed weekly
for the first 13 weeks and monthly thereafter until the conclusion of
the study, when surviving animals were killed. All animals were
necropsied and examined histologically. Furfural had no significant
effect on body weight or survival. Microscopic examination revealed
lesions in the liver, kidneys, and forestomach. In the liver,
pigmentation, necrosis, and chronic inflammation were seen in males at
the intermediate and high doses and in females at the high dose (Table
2). Hepatocellular adenomas and carcinomas were seen in all groups,
including controls, and the increased incidence was statistically
significant only in males at the high dose. Carcinomas occurred at the
same incidence in females at the high dose as in controls.
Table 2. Incidences of liver lesions and hepatocellular adenomas and carcinomas in male and
female B6C3F1 mice given furfural by gavage
Lesion Dose (mg/kg bw)
0 50 100 175
M F M F M F M F
Pigmentation 0/50 0/50 0/50 0/50 8/49 0/50 18/50 11/50
Necrosis, focal and multifocal 3/50 2/50 2/50 1/50 7/49 3/50 10/50 2/50
Chronic Inflammation 0/50 0/50 0/50 0/50 8/49 1/50 18/50 8/50
Hepatocellular adenoma 9/50 1/50 13/50 3/50 11/49 5/50 19/50 8/50
Hepatocellular carcinoma 7/50 4/50 12/50 0/50 6/49 2/50 21/50 4/50
Combined rates 16/50 5/50 22/50 3/50 17/49 7/50 32/50 12/50
From National Toxicology Program (1990)
M, male; F, female
Hyperplasia of the renal tubules was found in one male at the low
dose. One male at the intermediate dose, one at the high dose, and one
female at the low dose had a cortical adenoma, and one male at the low
dose had a cortical carcinoma. Owing to the low incidence and lack of
dose-response relationship, these tumours were not considered as
evidence for carcino-genicity. Marginal increases in the incidences of
hyperplasia and papillomas in the forestomach of female mice were
considered to be of no biological significance and were probably
related to the irritating effects of chronic administration of the
compound by gavage or the method of gavage itself. None of the animals
showed evidence of malignant forestomach lesions (National Toxicology
Program, 1990).
Rats
In a two-year study, 50 male and 50 female Fischer 344/N rats
were given furfural at doses of 0, 30, or 60 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed at
specific intervals, and the survivors were killed at the end of the
study, necropsied, and examined histologically. Overall survival was
slightly reduced for female rats at the highest dose (78% at week 97),
due to accidental gavage-related deaths. Furfural had no effect on
body weight or survival. Congestion and foreign bodies were observed
in a dose-related manner in the lungs of female rats. A small increase
in the incidence of squamous-cell carcinomas and papillomas in the
forestomachs of treated rats was observed but was considered unrelated
to treatment. Mild centrilobular hepatocellular necrosis was observed
in all groups (vehicle control: males, 3/50; females, 10/50; low dose:
males, 9/50; females, 9/50; high dose: males, 12/50; females, 4/50).
Bile-duct hyperplasia occurred in 45/50 control males, 36/50 control
females; 41/50 males and 28/50 females at the low dose; and 44/50
males and 24/50 females at the high dose. Focal bile-duct dysplasia
was noted in one male at the intermediate dose, and bile-duct
hyperplasia accompanied by fibrosis (cholangiofibrosis) was seen in
two males at the high dose. One control male had a hepatocellular
adenoma, and two males at the high dose had a cholangiocarcinoma. The
incidence of the latter lesion was not statistically significant, but
some evidence of carcinogenicity was consluded since
cholangiocarcinomas were rare in historical controls (3/2145)
(National Toxicology Program, 1990).
2.2.3.1 Special studies of carcinogenicity
The aldehydes citral and heptaldehyde inhibit the growth of
spontaneous mammary tumours in mice; however, furfural and its
furfuracrylic acid metabolite at a daily dose of 2.5 mg had no effect
on the growth of spontaneous mammary tumours in mice, although they
were weakly active against sarcomata (Boyland, 1940).
Studies have been designed to evaluate the effect of furfural on
the carcinogenic potential of known carcinogens. In the first study,
three groups of 35 male and 35 female Syrian golden hamsters were
provided with 0.2 ml of 1.5% furfural in physiological saline (about
25 mg/kg bw), 0.5% benzo [a]pyrene (8.3 mg/kg bw), or 1.5% furfural
plus 0.5% benzo [a]pyrene intratracheally once weekly for 36 weeks.
Furfural in combination with benzo [a]pyrene led to earlier
development of hyper- and metaplastic changes in the tracheobronchial
epithelium, a shorter latency for tracheobronchial tumours, a few more
bronchial and peripheral squamous-cell carcinomas, and a substantially
reduced number of tracheal squamous-cell carcinomas. The incidence of
peritracheal sarcomas was increased, which may indicate a
co-carcinogenic effect of furfural. Irritation but no tumorigenic
effect was observed after administration of furfural alone (Feron,
1972).
In a subsequent study, groups of 126 Syrian golden hamsters of
each sex were exposed to atmospheres containing 400 ppm (1600 mg/m3)
furfural for 7 h per day, five days per week for nine weeks, then 330
ppm (1300 mg/m3) for 11 weeks, and 250 ppm (970 mg/m3) for an
additional 32 weeks. The effects on the respiratory tract included
atrophy and downward growth of the olfactory epithelium, degenerative
changes in Bowman's glands, and the appearance of cyst-like structures
in the lamina propria beneath the olfactory epithelium. Although
furfural was irritating to the olfactory epithelium, treatment did not
result in hepatic toxicity or carcinogenicity. Furfural did not
potentiate the carcinogenic effect of benzo [a]pyrene or
N-nitrosodiethylamine (Feron & Kruysse, 1978).
Localized irritation of the nasal cavity and respiratory tract,
with no hepatic effects, were also observed in B6C3F1 mice and
Fischer 344/N rats exposed to atmospheres containing 0, 2, 4, 8, 16,
or 32 ppm of the metabolic precursor furfuryl alcohol for 13 weeks
(Mellick et al., 1991).
SPF Wistar rats were given furfural at doses of 20 ml
(approximately equal to 1200 mg/kg bw per day) for one week, 30 ml for
the second week, and 40 ml for the following 10 weeks to produce
hepatic cirrhosis. Half of the remaining rats in this study (38
treated and 32 controls) were fed a diet containing 0.03%
N-2-fluorenylacetamide for three weeks, followed by one week of
normal diet; the other half received only the normal diet. All
surviving rats were killed 12 weeks later, and their livers were
examined. Rats that had received furfural only had no hyperplastic
changes in the liver but did have cirrhosis. Rats that had been
treated with N-2-fluorenylacetamide developed multiple hyperplastic
nodules which stained for alpha-fetoprotein, and this response was
markedly potentiated in the rats previously treated with furfural. The
authors concluded that furfural-induced hepatic cirrhosis increases
the susceptibility of rats to potent hepatocarcinogens (Shimizu,
1986).
Samples of mouse liver tumours from the National Toxicology
Program carcinogenesis bioassay programme were assessed for
transforming gene activity by Southern blot analysis for H-, K-, and
N- ras oncogens. The pattern of mutations in the oncogenes from liver
tumours that occurred spontaneously differed from that which occurred
in some furfural-treated animals: The activated ras genes were
H- ras in 15 of 17 spontaneous tumours, with one raf and one
unknown oncogene; activating point mutations occurred at codon 61 in
six tumours, codon 13 in two tumours, and codon 117 in one tumour. The
authors concluded that the spectrum of activating mutations in the
H- ras gene and the pattern of ras gene activation in liver tumours
derived from furfural-treated mice differed from those in liver
tumours derived from untreated animals, and indicates a direct
genotoxic effect of furfural. An alternative mechanism-induction of
mutations by an indirect, secondary genotoxic pathway as a result of a
cytotoxic event -- was mentioned but excluded by the authors on the
basis of the absence of cytotoxic lesions in the livers of similar
mice after 90 days of treatment with the same dose of furfural. The
authors do not mention, however, that necrosis, pigmentation, and
chronic inflammation were seen regularly in mice at the intermediate
and high doses in the carcinogenicity experiment (see Table 2). It can
be concluded that the effect was either due to a direct genotoxic
event or occurred via an indirect, secondary genotoxic pathway
(Reynolds et al., 1987).
2.2.4 Genotoxicity
Studies of the genotoxicity of furfural are summarized in
Table 3. Testing of furfural for mutagenicity in vitro at
concentrations up to those causing cytotoxicity provided no evidence
that it is mutagenic to Salmonella typhimurium strains TA98, TA102,
TA1535, or TA1537 or ito two strains of Escherichia coli, with or
without metabolic activation (Sasaki & Endo, 1978; Zdzienicka et al.,
1978; McMahon et al., 1979; Loquet et al., 1981; Marnett et al., 1985;
Mortelmans et al., 1986; Shinohara et al., 1986; Nakamura et al.,
1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al., 1989;
National Toxicology Program, 1990; Dillon et al., 1992). The majority
of these studies also showed negative results for S. typhimurium
TA104, although a single positive result was reported (Shane et al.,
1988). Furfural was not mutagenic when incubated with
S. typhimurium TA100 in most reports (Sasaki & Endo, 1978; McMahon
et al., 1979; Mortelmans et al., 1986; Shinohara et al., 1986; Kim et
al., 1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al.,
1989; National Toxicology Program, 1990; Eder et al., 1991; Dillon et
al., 1992) but was weakly mutagenic in other studies (Zdzienicka et
al., 1978; Loquet et al., 1981; Shane et al., 1988). When fufural was
co-incubated with benzo [a]pyrene and S. typhimurium TA100, it did
not alter the mutagenic activity of benzo [a]pyrene in this strain
(Zdzienicka et al., 1978). Furfural showed no mutagenic potential in
two rec assays with Bacillus subtilis by a direct streaking method
(Osawa & Namiki, 1982; Matsui et al., 1989) but also gave positive
results in this test system (Shinohara et al., 1986).
Table 3. Results of assays for the genotoxicity of furfural
End-point Test object Concentration Result Reference
In vitro
Reverse mutation S. typhimurium TA100 NR Negative Eder et al. (1991)
Reverse mutation S. typhimurium TA100, TA102, and NR Negativea Dillon et al. (1992)
TA104
Reverse mutation S. typhimurium TA100, TA98, and < 36 µmol/plate Weakly positive (TA100)b Loquet et al. (1981)
TA1535 60 µmol/plate Negativec
Reverse mutation S. typhimurium TA100, TA98, and < 1.2 mmol/plate Negative Aeschbacher et al. (1989)
TA102
Reverse mutation S. typhimurium TA100 and TA98 0.165-0.660 µmol/plate Negativea Shinohara et al. (1986)
Reverse mutation S. typhimurium TA100, TA102, and 5-500 µg/plate Positive (TA104) Shane et al. (1988)
TA104
Reverse mutation S. typhimurium TA100, TA98, and NR Negativea Kato et al. (1989)
TA104; E. coli WP2uvrA/PKM101
Reverse mutation S. typhimurium TA104 and TA102 1 µmol/plate Negative Marnett et al. (1985)
Reverse mutation S. typhimurium TA98, TA100, < 6667 µg/plate Negativea Mortelmans et al. (1986)
TA1535, and TA1537
Reverse mutation S. typhimurium TA100 and TA98 7 µl/plate Positivea (TA100) Zdzienicka et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, < 20 µl/plate Negativea McMahon et al. (1979)
TA1537, TA1538, TA98, G46;
E. coli WP2uvrA
Reverse mutation S. typhimurium TA100 and TA98 NR Negativea Sasaki & Endo (1978)
Reverse mutation S. typhimurium TA100 4.44 µmol/plate Negativea Kim et al. (1987)
umu gene expression S. typhimurium TA1535/pSK/002 1932 µg/ml Negativea Nakamura et al. (1987)
Reverse mutation S. typhimurium TA100 < 1 mg Negativea Osawa & Namiki (1982)
Reverse mutation S.typhimurium TA98, TA100, 33-6666 µg/plate Negativea National Toxicology Program
TA1535, and TA1537 (1990a)
Reverse mutation S.typhimurium TA98, TA100, and 33-6666 µg/plate Negativea National Toxicology Program
TA1535 (TA100 equivocal) (1990b)
rec Gene mutation B. subtilis H17 and M45 < 1 mg Negativea Osawa & Namiki (1982)
rec Gene mutation B. subtilis H17 and M45 1.7-17 mg/disk Positivea Shinohara et al. (1986)
rec Gene mutation B. subtilis H17 and M45 0.6 ml Negativea Matsui et al. (1989)
Table 3. (continued)
End-point Test object Concentration Result Reference
Chromosomal Chinese hamster ovary cells < 40 mmol/L Positivea Stich (1981a)
aberration
Chromosomal Chinese hamster ovary cells 3 mg/ml Positive Stich (1981b)
aberration
Forward mutation L5178Y tk+/- mouse lymphoma cells 25-400 µg/ml Positiveb McGregor et al. (1988)
Sister chromatid Chinese hamster ovary cells < 1170 µg/ml Positivea National Toxicology Prohgram
exchange/Chromosomal (1990)
aberration
Sister chromatid Human lymphocytes < 0.14 mmol/L Positive Gomez-Arroyo & Souza (1985)
exchange
Unscheduled DNA Human liver slices 0-25 mmol/L Negative Lake et al. (1998)
synthesis
In vivo
Sex-linked recessive D. melanogaster 1000 ppm in diet Negative Woodruff et al. (1985)
lethal mutation
Sex-linked recessive D. melanogaster 100 ppm by injection Positive Woodruff et al. (1985)
lethal mutation
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Negative Rodriguez-Arnaiz et al. (1992)
repair-proficient females < 33% lethal dose
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Positive only after Rodriguez-Arnaiz et al. (1992)
repair-deficient females < 33% lethal dose injection
Sex-chromosome loss D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
injection
Sex-linked recessive D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
lethal mutation injection
Reciprocal D. melanogaster 100 ppm by injection Negative Woodruff et al. (1985)
translocation
Sister chromatid B6C3F1 mouse bone-marrow cells 50-200 mg/kg by Negative National Toxicology Program
exchange/Chromosomal injection (1990)
aberration
Table 3. (continued)
End-point Test object Concentration Result Reference
Somatic chromosomal Swiss albino mouse bone-marrow < 4000 ppm for 5 days Negatived Subramanyam et al. (1989)
mutation cells
Sperm-head Swiss albino mouse < 4000 ppm for 5 Negatived Subramanyam et al. (1989)
abnormalities weeks
Unscheduled DNA Fischer 344 rat hepatocytes 5.0, 16.7, or 50 mg/kg Negative Phillips et al. (1997)
synthesis bw orally
NR, not reported
a With and without metabolic activation
b Without metabolic activation
c With metabolic activation
d Abstract only: reported to have positive effects at 4000 ppm, but no details available
Furfural produced a dose-dependent increase in the frequency of
chromatid breaks and exchanges in Chinese hamster ovary cells with and
without metabolic activation (Stich et al., 1981a,b; National
Toxicology Program, 1990) and induced chromosomal aberration in these
cells at a cytotoxic concentration (National Toxicology Program,
1990). An increased frequency of sister chromatid exchange was
reported in human lymphocytes incubated with furfural at a
concentration of 0.07 or 0.14 mmol/L, but not at 0.035 mmol/L
(Gomez-Arroyo & Souza, 1985). In this study, furfuryl alcohol did not
increase the frequency of sister chromatid exchange at a concentration
of 3.3, 6.6, or 9.9 mmol/L.
Furfural increased trifluorothymidine resistance when incubated
with L5178Y tk+/- mouse lymphoma cells at a concentration of 200 or
400 mg/ml without metabolic activation, but showed no mutagenic
activity at 25, 50, or 100 mg/ml (McGregor et al., 1988).
Concentrations of 400 and 800 mg/ml were cytotoxic in separate trials.
In vivo, furfural did not induce sex-linked recessive lethal
mutations or reciprocal translocations in male Drosophila
melanogaster when incorporated into their diet at 1000 ppm.
Mutations were induced when furfural was administered by injection at
a concentration of 100 ppm, but no reciprocal translocations occurred.
A similar pattern of responses was reported for other simple aldehydes
(e.g. acetaldehyde and trans-cinnamaldehyde) when given via the
intraperitoneal (positive) and oral (negative) routes of
administration (Woodruff et al., 1985). No sex-chromosome loss was
observed when male D. melanogaster were fed or injected with
furfural at concentrations equivalent to 25-33% of the lethal dose of
furfural and then mated with repair-proficient females
(Rodrigues-Arnaiz et al., 1992), but sex-chromosome loss was reported
by these authors when treated males were mated with repair-deficient
females. No sex-linked recessive lethal mutation or sex-chromosome
loss was reported when male D. melanogaster were injected with
furfuryl alcohol (Rodrigues-Arnaiz et al., 1989).
Tests of mammals treated in vivo with furfural have produced
mainly negative results. Furfural did not induce sister chromatid
exchange or chromosomal aberrations in B6C3F1 mouse bone-marrow cells
after intraperitoneal injections of furfural at doses of 50-200 mg/kg
bw (National Toxicology Program, 1990). No genotoxic effects or
spermhead abnormalities were reported in mice given up to 4000 ppm
furfural daily for five weeks, and only the highest dose tested was
associated with an increased frequency of somatic (liver)-cell
chromosomal mutations when given for 24 or 48 h but not after 72 h
(Subramanyam et al., 1989). Furfural administered by gavage to male
Fischer 344/N rats in corn oil at doses of 5, 16.7, or 50 mg/kg bw did
not induce unscheduled DNA synthesis in hepatocytes (Phillips et al.,
1997).
Chromosomal aberrations were not observed in lymphocytes taken
from workers occupationally exposed to furfural or furfuryl alcohol
(Gomez-Arroyo & Souza, 1985).
Liver slices prepared from samples from four human donors were
cultured in medium containing 3H-thymidine and 0-25 mmol/L furfural
for 24 h. In a preliminary experiment, furfural was markedly toxic at
concentrations > 10 mmol/L. As positive controls, liver slices were
also cultured with three known genotoxic agents,
2-acetylaminofluorene, aflatoxin B1, and
2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP). Unscheduled
DNA synthesis was quantified as the changes in mean nuclear and
cytoplasmic grain counts relative to controls, net grain count (i.e.
mean nuclear - mean cytoplasmic grain counts), and the percentage of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains. In
comparison with control liver slice cultures (in dimethylsulfoxide),
those treated with 0.02 or 0.05 mmol/L 2-acetylamino-fluorene, 0.002
or 0.02 mmol/L aflatoxin B1, and 0.003 or 0.05 mmol/L PhIP had
significantly higher net grain counts in centrilobular hepatocytes,
due primarily to large increases in mean nuclear grain counts. The
three genotoxic agents also significantly increased the number of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains.
Treatment with 0.005-0.5 mol/L furfural had no significant effect
on net grain count or mean nuclear or cytoplasmic grain counts. A
small but statistically significant increase in net grain counts
observed at 2, 5, and 10 mmol/L furfural was the result of a
significant concentration-dependent decrease in mean cytoplasmic grain
counts accompanied by a smaller increase in nuclear grain counts.
Furfural had no significant effect on the percentage of hepatocyte
nuclei with > 5 or > 10 net grain counts, except at a concentration
of 2 mmol/L and only in liver cultures in which marked toxicity was
noted at 10 mmol/L. The decreases in mean cytoplasmic and nuclear
grain counts and the correlation between cytotoxicity and changes in
net grains count and nuclei with > 5 net grains support the
conclusion that furfural at 2, 5, or 10 mmol/L induces a cytotoxic
response in cultured human liver slices. In comparison with the three
genotoxins tested, furfural did not induce unscheduled DNA synthesis
in cultured human liver slices (Lake et al., 1998).
Furfural was reported to increase the frequency of single-strand
DNA breaks in isolated calf-thymus duplex DNA (Hadi et al., 1989).
When incubated with plasmid DNA, furfural decreased the transformation
capacity of Escherichia coli host cells; however, the damaged
plasmids were repaired after propagation in the host (Khan & Hadi,
1993).
Thus, negative results were obtained with furfural in most tests
for genotoxicity in vitro. Positive results were reported at
relatively high doses in three of 16 assays for reverse mutation in
S. typhimurium and in one of three assays for gene mutation in
B. subtilis. Weakly positive results were obtained in tests for
chromosomal aberrations at relatively high doses. Positive results
were found in two assays for sister chromatid exchange and one for
forward mutation in mouse lymphoma cells. The results of all tests for
genotoxicity in vivo were negative, except in D. melanogaster
injected with furfural.
2.2.5 Reproductive toxicity
Rats
A summary report of an investigation to define the doses of
furfural for a study of developmental toxicity was submitted to the
Toxic Substances Control Administration of the US Environmental
Protection Agency. Five groups of eight Crl:CD(SD)BR rats received
furfural in water by gavage at doses of 10, 50, 100, 500, or 1000
mg/kg bw per day during gestation days 6-15. Because of excessive
deaths at 500 and 1000 mg/kg bw per day, the breeding phase of the
study was re-initiated to characterize the potential maternal and
developmental toxicity at 150, 250, and 350 mg/kg bw per day. Two
concurrent control groups of eight rats received only the vehicle
(reverse osmosis-treated water) by gastric intubation. Clinical
observations, body weights, and food consumption were recorded. On
gestation day 20, laparohysterectomy was performed on all surviving
animals; the uteri and ovaries were examined, and the numbers of
fetuses, early and late resorptions, total implantation, and corpora
lutea were recorded. The uteri and fetuses were weighed and examined
for external malformations, variations, and sex.
Because of excessive mortality on day 6 after dosing, all females
at 250, 350, 500, and 1000 mg/kg bw per day were killed. The clinical
signs in these animals included lethargy, effects on respiration, and
whole-body tremor. The maternal animals at other doses survived,
except for one female at 150 mg/kg bw per day group, which died within
I h of dosing. The surviving females at 150 mg/kg bw per day had
reduced body-weight gain and reduced food consumption; however, the
mean body weight, net body weight, net body-weight gain, and gravid
uterine weight were unaffected. Body weight and food consumption of
animals at 10, 50, and 100 mg/kg bw per day were unaffected.
Intrauterine growth and survival were not affected at 10, 50, 100, or
150 mg/kg bw per day. The only external malformation noted
(omphalocele) occurred in offspring of rats at 10 mg/kg bw per day. No
developmental variations were noted (US Environmental Protection
Agency, 1997).
3. HUMAN INTAKE
On the basis of the most recently reported annual volumes, 3600
kg in Europe (International Organization of the Flavor Industry, 1995)
and 590 kg in the United States (National Academy of Sciences, 1987),
the estimated daily per capita intake of 'eaters only' of furfural
from use as a flavouring substance is approximately 9 µg/kg bw per day
in Europe and 2 µg/kg bw per day in the United States. Furfural and
the corresponding furfuryl alcohol are virtually ubiquitous in nature
(Table 4). They are formed from the acid hydrolysis or heating of
Table 4. Natural occurrence of furfural
Food item Concentration of
furfural (ppm)
Apple (raw), apple juice, apricot (Prunus armeniaca L.), sweet 0.02-0.05
cherry (Prunus avium L.), sour cherry (Prunus cerasus L.)
Orange juice (Citrus sinensis L. Osbeck) Trace
Orange peel oil, grapefruit juice (Citrus paradisi) 0.34
Bilberry (Vaccinium myrtillus L.) 0.02
American cranberry (Vaccinium macrocarpon Ait.) 0.1-0.3
Lingonberry (Vaccinium vitis idaea L.) 0.02
Black currents, berries, guava (Psidium guajava L.) 0.0014-0.19
Grape (dried, sultana), peach (Prunus persica L.), pineapple 0.01
(Ananas comosus), raspberry (Rubus idaeus L.), strawberry
(Fragaria species), asparagus (raw), asparagus (cooked)
Carrot (Daucus carota L.), celery leaves (raw), onion (roasted), 0.005
leek (heated), potato (raw), potato (cooked), bell pepper
(Capsicum annuum)
Wheaten bread, sauerkraut, tomato (Lycopersicon 0.8-26
esculentum Mill.), cinnamon (Cinnamomum zeylanicum Blume),
cloves (Eugenia caryophyllata Thunberg), Mentha species
Crispbread, bread, other types, blue cheeses, parmesan, 0.02
butter, yogurt, milk, chicken and turkey (raw), beef (boiled/
cooked), beef (grilled/roasted)
Lamb and mutton, pork (heated), hop oil, beer 0-0.3
Cognac 0.6-33
Armagnac 2
Weinbrand 0.2-4.3
Grape brandy, other types, rum (all categories), rum (category I: 22
total volatiles > 3600 ppm)
Rum (category II: total volatiles 1100-3600 ppm) Trace-25
Rum (category III: total volatiles 240-1100 ppm) Trace
Bourbon whiskey 2-11.6
Irish whiskey 0.8-13.6
Malt whisky 10-37
Scotch blended whisky 1.1-30
Canadian whiskey 0.3-0.8
Japanese whiskey 0.5-4.5
Cider, sherry, white wine Trace-10.3
Red wine 0.005-0.05
Rose wine, port wine 2-34
Special wine, botrytized wine 0.13
Cocoa, coffee 55-255
Black tea 2-7
Green tea 0.1
Microbial fermented tea, tea (brewed) 0.3-0.8
Table 4. (Continued)
Food item Concentration of
furfural (ppm)
Barley (roasted), filbert (roasted, Corylus avellano), peanut 0.08-0.2
(roasted, Arachis hypogea), pecan (roasted), popcorn, potato
chips (American)
Oat flakes (toasted), honey, soybean, arctic bramble (Rubus Trace
arcticus L., Rubus stell.), cloudberry (Rubus chamaemorus L.)
Passion fruit juice (yellow), passion fruit (yellow), plum (raw), 2.58
plum (salted and pickled)
Beans (Phaseolus vulgaris L.), mushroom (raw) 0.05
Trassi (cooked), plum brandy, almond (roasted, Prunus 9
amygdalus)
Macadamia nut (roasted, Macadamia integrifolia), sesame 0-0.1
seed (roasted), mango (raw)
Mango (canned), cauliflower (cooked), tamarind (Tamarindus 7
indica), pear brandy
Apple brandy, beetroot (cooked),artichoke (cooked, Cynarus < 0.01
scolymus L.), gin, rice bran, traditional rice (cooked), quince
(Cydonia oblonga), radish (fermented), shoyu (fermented soya
hydrolysate), bacuri (Platonia insignis), cupuacu (Theobroma
grandiflora), muruci (Brysonima crassifolia)
Potato (sweet, heated), sukiyaki, licorice (Glycyrrhiza glabra L.), < 0.01
matsutake (Tricholoma matsutake), strawberry wine, pumpkin
(Cucurbita pepo L.), sake, oat groats, maize, cashew apple
(Anacardium occidentale), basil (Ocimum basilicum), malt,
peated malt, wort, bonito (dried, katsuobishi), elderberry
(Sambucus nigra L.), mangosteen (Garcinia mangostana),
cherimoya (Annona cherimola), bilberry wine, buchu oil,
vanilla, mountain papaya (Carica pubescens)
Wild rice (Zizania aquatica), chicory (Cichorium intrybus L.), 0-0.2
endive (Cichorium endivia L.), ouzo
Sapodilla fruit (Achras sapota L.) Trace
Aubergine (Solanum melongena L.), pistachio nut (roasted, 17.2
Pistachia vera), arrack
Nectarine < 0.01
From Maarse & Visscher (1994)
Intake (µg/kg bw per day) calculated as follows:
{[(annual volume, kg) × (1 × 109 mg/kg) × (1/60 kg bw)]/[population × 0.6 ×
365 days]}, where population (10%, 'eaters only') = 32 × 106 for Europe and
24 × 106 for the United States; 0.6 represents the assumption that only 60%
of the flavour volume was reported in the survey (National Academy of Sciences,
1987; International Organization of the Flavor Industry, 1995). Slight
variations may occur from rounding off.
polysaccharides which contain pentose and hexose fragments. Furfural
has been detected in a broad range of fruits and fruit juices, wines,
whiskeys, coffee, and tea (Maarse et al., 1994). The highest
concentrations of furfural in foods have been reported in cocoa and
coffee (55-255 ppm), alcoholic beverages (1-33 ppm), and whole-grain
bread (26 ppm). Furfuryl alcohol, which is readily converted to
furfural in vivo (Rice, 1972; Nomeir et al., 1992), has been found
in the highest concentrations in heated skim milk (230 ppm) and coffee
(90-881 ppm). The total potential daily per capita intake of
furfural and precursors of furfural (i.e. furfuryl alcohol and
furfuryl esters) from consumption of foods in which they occur
naturally (Stofberg & Grundschober, 1987) is approximately 0.3 mg/kg
bw per day (i.e. about 300 µg/kg bw per day) in the United States.
Thus, the intake of furfural and furfuryl derivatives from use as
flavouring substances represent 1-3% of the total intake.
4. COMMENTS
In both humans and rodents, furfural is efficiently metabolized
by oxidation of the aldehyde function to furoic acid, most of which
is conjugated with glycine and excreted. A minor proportion (< 5%)
of furoic acid is condensed with acetyl coenzyme A to form
furanacryloyl coenzyme A; the resulting furanacryloic acid is
conjugated with glycine and excreted in the urine. About 5% of
[carboxyl-14C]-labelled furfural is eliminated by rats and mice as
14C-carbon dioxide. The metabolic pathway, which could involve direct
decarboxylation or epoxidation and ring opening, has not been defined.
In short-term studies, furfural was clearly hepatotoxic at doses
> 90 mg/kg bw per day in male rats and > 150 mg/kg per day in
mice. Minor changes reported in the livers of male rats given furfural
at lower doses by gavage in corn oil for 13 weeks were also present to
a lesser extent in vehicle controls. In a study of developmental
toxicity, furfural was not toxic to rats at 150 mg/kg bw per day, the
highest dose tolerated.
Furfural, like other aldehydes such as endogenous acetaldehyde,
is a reactive aldehyde; it is reported to bind to soluble proteins and
protein components of cell membranes. Various metabolic processes
(i.e. oxidation, conjugation, and condensation) effectively eliminate
the reactive aldehyde functional group when these metabolic pathways
are not saturated by high, unphysiological doses.
Furfural was not genotoxic in most tests in vitro. Positive
results were reported at relatively high concentrations in only three
of 16 assays for reverse mutation in Salmonella typhimurium and in
one of three rec assays in B. subtilis. A few chromosomal
aberrations were seen in Chinese hamster ovary cells exposed to
relatively high concentrations. Sister chromatid exchanges and forward
mutations were induced in mouse lymphoma cells. This weak activity of
furfural in vitro in some tests for genotoxicity might be explained
by its aldehyde reactivity. Recent studies of unscheduled DNA
synthesis in hepatocytes of rats treated in vivo and in human liver
slices gave negative results. Negative results were found in tests for
genotoxicity in vivo, except in Drosophila injected with furfural.
In a two-year study of carcinogenicity in rats given furfural in
corn oil by gavage at doses of 30 or 60 mg/kg bw per day, bile-duct
hyperplasia and cholangiofibrosis were seen in essentially all rats,
the controls having the highest incidence. Mild hepatocellular
necrosis was seen in all groups, however, at higher rates in males in
all treated groups. Two cholangio-carcinomas were observed in males at
the high dose, but the incidence was not statistically significant.
In a study of carcinogenicity in mice, the combined incidence of
adenomas and carcinomas of the liver (64%) was significantly
(p < 0.01) increased in males at the high dose (175 mg/kg bw per
day) but not in females. Hepatocellular adenomas and carcinomas also
occurred in the controls and in animals at the low (50 mg/kg bw per
day) and intermediate (100 mg/kg bw per day) doses, at incidences of
34-44% in males and 6-14% in females. The incidences in historical
controls were 38% (14-50%) in males and 6.2% (0-30%) in females.
Hepatotoxicity, manifested by features such as focal and multifocal
necrosis and chronic inflammation, was seen in all groups, including
the controls, but was considerably more frequent in mice at the high
dose.
Studies of oncogene activation in samples of liver tumours from
treated mice in the study of carcinogenicity revealed some differences
in the pattern of mutations from those in liver tumours of controls.
As it was not possible to identify the animals from which the tumours
originated and since hepatoxicity was seen at the intermediate and
high doses, it was not possible to determine whether a direct
genotoxic event or a secondary genotoxic pathway is involved.
5. EVALUATION
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL was identified for hepatotoxicity
in rats, the Committee was unable to allocate an ADI. Before reviewing
the substance again, the Committee would wish to review the results of
studies of DNA binding or adduct formation in vivo to clarify
whether furfural interacts with DNA in mice and of a 90-day study of
toxicity in rats to identify a NOEL for hepatotoxicity.
6. REFERENCES
Aeschbacher, H., Wolleb, U., Löliger, J., Spadone, J.C. & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Boyland, E. (1940) Experiments on the chemotherapy of cancer. 4.
Further experiments with aldehydes and their derivatives. Biochem.
J., 34, 1196-1201.
Brabec, M.J. (1981) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, Third
revised Ed., New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Caldwell, J. (1982) Conjugation reactions in foreign-compound
metabolism: Definition, consequences, and species variations.
J. Drug Metab. Rev., 13, 745-777.
Castellino, N., Elmino, O. & Rozera, G. (1963) Experimental research
on the toxicity of furfural. Arch. Environ. Health, 7, 574-582.
Dillon, D.M., McGregor, D.B., Combes, R.D. & Zeiger, E. (1992)
Detection of mutagenicity in Salmonella of some aldehydes and
peroxides. Environ. Mol. Mutag. 17, 15.
Donahue, T.M., Tuma, D.J. & Sorrell, M.F. (1983) Acetaldehyde adducts
with proteins: Binding of [14C]acetaldehyde to serum albumin.
Arch. Biochem. Biophys., 220, 239-246.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Clayton, G.D. & Clayton, F.E.,
eds, Patty's Industrial Hygiene and Toxicology, Third revised Ed.,
New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Eder, E., Deininger, C. & Muth, D. (1991) Genotoxicity of
p-nitrocinnamaldehyde and related alpha,ß-unsaturated carbonyl
compounds in two bacterial assays. Mutagenesis, 6, 261-269.
Feron, V.J. (1972) Respiratory tract tumors in hamsters after
intratracheal instillations of benzo(a)pyrene alone and with furfural.
Cancer Res., 32, 28-36.
Feron, V.J. & Kruysse, A. (1978) Effects of exposure to furfural
vapour in hamsters simultaneously treated with benzo(a)pyrene or
diethylnitrosamine. Toxicology, 11, 127-144.
Flek, J. & Sedivec, V. (1978) The absorption, metabolism and excretion
of furfural in man. Arch. Occup. Environ. Health, 41, 159-168.
Friedmann, E. (1911) [Fate of furanacrylic acid and furoyl acid in the
animal body.] Biochem. Z., 35, 40-48 (in German).
Gaines, K.C., Salany, J.M., Tuma, D.J. & Sorrell, M.F. (1977) Reaction
of acetaldehyde with human erythrocyte membrane proteins. FEBS
Lett., 75, 115-119.
Gomez-Arroyo, S. & Souza, V.S. (1985) In vitro and occupational
induction of sister-chromatid exchanges in human lymphocytes with
furfuryl alcohol and furfural. Mutat. Res., 156, 233-238.
Gregus, Z., Fekete, T., Varga, F. & Klaassen, C.D. (1993) Dependence
of glycine conjugation on availability of glycine: Role of the glycine
cleavage system. Xenobiotica, 23, 141-153.
Gupta, G.D., Misra, A. & Agarwal, D.K. (1991) Inhalation toxicity of
furfural vapours: An assessment of biochemical response in rat lungs.
J. Appl. Toxicol., 11, 343-347.
Hadi, S.M., Rehman, S. & Rehman, A. (1989) Specificity of the
interaction of furfural with DNA. Mutat. Res., 225, 101-106.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Jonek, J.J., Konecki, J. & Kaminski, M. (1975) Histoenzymatic changes
in liver in acute poisoning with furfural. J. Morphol. Embryol., 21,
47-51.
Kaminska, O. (1977) Morphological and histochemical investigations of
liver and kidneys in different stage of furfural poisoning. Folio
Histochem. Cytochem., 15, 150-151.
Kato, F., Araki, A., Nozaki, K. & Matsushima, T. (1989) Mutagenicity
of aldehydes and diketones. Mutat. Res., 216, 366.
Khan, Q.A. & Hadi, S.M. (1993) Effect of furfural on plasmid DNA.
Biochem. Mol. Biol. Int., 29, 1153-1160.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxy-carbonyl compounds against
mutagenic heterocyclic amines. Mutat. Res., 177, 9-15.
Koenig, K. & Andreesen, J. R. (1990) Xanthine dehydrogenase and
2-furoyl-coenzyme A dehydrogenase from Pseudomonas p. Fu1; two
molybenum-containing dehydrogenases of novel structural composition.
J. Bacteriol., 172, 5999-6009.
Kruysse, A. (1972). Acute inhalation toxicity of furfural in hamsters.
Unpublished report No. R 3853 from Central Institute for Nutrition and
Food Research TNO, Zeist, Netherlands.
Kuznetsov, P.I. (1966) Toxicological characteristics of furfural. II.
Effect of furfural on certain functions of the animal under conditions
of sub-acute poisoning. Nauch. Trud. Omskii Med. Inst. Imeni M.I.
Kalinina, 69, 41-44.
Lake, B.G., Adams, T.B., Beamad, J.A., Price, R.J., Ford, R.A. &
Goodman, J.I. (1998) An investigation of the effect of furfural on the
unscheduled DNA synthesis in cultured human liver slices. Unpublished
report to the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Loquet, C., Toussant, G. & LeTalaer, J.Y. (1981) Studies on mutagenic
constituents of apple brandy and various alcoholic beverages collected
in western France, a high incidence area for oesophageal cancer.
Mutat. Res., 88, 155-164.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148,
25-34.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D. &
Caspary, W.J. (1988) Responses of the L5178Y tk+/- mouse lymphoma
cell forward mutation assay. II: 18 coded chemicals. Environ. Mol.
Mutag., 11, 91-118.
McMahon, R.E., Cline, J.C. & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39, 682-693.
Mellick, P.W., Leach, C.L., Chou, B.J., Irwin, R.D. & Roycroft, J.H.
(1991) Nasal toxicity in Fischer 344 rats inhaling furfuryl alcohol
for 2 or 13 weeks. Toxicologist, 11, 183.
Mishra, A., Dwivedi, P.D., Verma, A.S., Sinha, M., Mishra, J., Lal,
K., Pandya, K.P. & Dutta, K.K. (1991) Pathological and biochemical
alterations induced by inhalation of furfural vapor in rat lung.
J. Bull. Environ. Contam. Toxicol., 47, 668-674.
Moreno, O. (1976) Dermal toxicity in rabbits. Report No. MB 75-941
from MB Research Laboratories Inc. Submitted to WHO by the Flavor &
Extract Manufacturers' Association of the United States, Washington
DC, United States
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Trainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from
the testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nagasawa, H.T., Goon, D.J.W., Conastotino, N.V. & Alexander, C.S.
(1980) Diversion of ethanol metabolism by sulfhydryl amino acids.
D-Penicillamine-directed excretion of
2,5,5-trimethyl-D-thiazolidine-4-carboxylic acid in the urine of rats
after ethanol administration. Life Sci., 17, 707-714.
Nakamura, S.-I., Oda, Y., Shimada, T., Oki, I. & Sugimoto, K. (1987)
SOS-inducing activity of chemical carcinogens and mutagens in
Salmonella typhimurium TA1535/pSK1002: Examination with 151
chemicals. Mutat. Res., 192, 239-246.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
National Toxicology Program (1987) Studies of chemical disposition in
mammals: The effect of dose on the chemical disposition of furfural in
rats after oral administration. NIEHS Contract No. N01-ES-66138 to
Arthur D. Little, Inc, Research Triangle Park, North Carolina, United
States.
National Toxicology Program (1990) Toxicology and carcinogenesis
studies of furfural (CAS No. 98-01-1) in Fischer 344/N rats and
B6C3F1 mice (gavage studies). National Toxicology Program Technical
Report Series No. 382. Research Triangle Park, North Carolina, United
States.
Nomeir, A.A., Silveria, D.M., McComish, M.F. & Chadwick, M. (1992)
Comparative metabolism and disposition of furfural and furfuryl
alcohol in rats. Drug Metab. Disposition, 20, 198-204.
Nomura, F. & Lieber,C.S. (1981) Binding of acetaldehyde to rat liver
microsomes: Enhancement after chronic alcohol consumption. Biochem.
Biophys. Res. Commun., 100, 131-137.
Nutley, B.P., Farmer, P. & Caldwell, J. (1994) Metabolism of
trans-cinnamic acid in the rat and the mouse and its variation with
dose. J. Food Chem. Toxicol., 32, 877-886.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Agric.
Biol. Chem., 45, 2299-2304.
Parkash M.K. & Caldwell J. (1994) Metabolism and excretion of
[14C]-furfural in the rat and the mouse. Food Chem. Toxicol., 32,
887-895.
Paul, H.E., Austin, F.L., Paul, M.F. & Ells, U.R. (1949) Metabolism of
the nitrofurans. I. Ultraviolet absorption studies of the urinary
products after oral administration. J. Biol. Chem., 180, 345-363.
Peterson, C.M. & Nguyen, L.B. (1985) Clinical implications of
acetaldehyde adducts with hemoglobin. In: Aldehyde Adducts in
Alcoholism, pp. 19-30.
Phillips, B.J., Jackson, L.I., Tate, B., Price, R.J., Adams, T.B.,
Ford, R.A., Goodman, J.I. & Lake, B.J. (1997) Furfural does not induce
unscheduled DNA synthesis (UDS) in the in vivo rat hepatocyte DNA
repair assay (Abstract). In Proceedings of the Society of Toxicology
Annual Meeting, 1997, Cincinnati, Ohio, New York, Academic Press.
Pickett, C.B. & Lu, A.Y.H. (1988) The structure, genetics and
regulation of soluble glutathione S-transferases. In: Sies, H. &
Ketterer, B., eds, Glutathione Conjugation: Mechanisms and
Biological Significance, New York, Academic Press, p. 147. A
Ramsdell, H.S. & Eaton, D.L. (1990) Species susceptibility to
aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal
biotransformation Cancer Res., 50, 615.
Ravindranath, A. & Boyd, M.R. (1985) Metabolic activiation of
2-methylfuran by rat microsomal systems. Toxicol. Appl. Pharmacol.,
78, 370-376.
Reynolds, S.H., Stowers, S.J., Patterson, R.M., Maronpot, R.R.,
Aaronson, S.A. & Anderson, M.W. (1987) Activated oncogenes in B6C3F1
mouse liver tumors: Implications for risk assessment. Science, 237,
1309-1316.
Rice, W.W. (1972) Furfural: Exogenous precursor of certain urinary
furans and possible toxicologic agent in humans. Clin. Chem., 18,
1550-1551.
Rodriguez-Arnaiz, R., Morales, P.R. & Zimmering, S. (1992) Evaluation
in Drosophila melanogaster of the mutagenic potential of furfural in
the mei-9(a) test for chromosome loss in germ-line cells. Mutat.
Res., 280, 75-80.
Rodriguez-Arnaiz, R., Morales, P.R., Moctezuma, R.V. & Salas, R.M.B.
(1989) Evidence for the absence of mutagenic activity of furfuryl
alcohol in tests of germ cells in Drosophila melanogaster. Mutat.
Res., 223, 309-311.
Sasaki, Y. & Endo, R. (1978) Mutagenicity of aldehydes in
Salmonella. Mutat. Res., 54, 251-252.
Schafer, E.W., Bowles, W.A. & Hurlbut, J. (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch. Environ. Contam.
Toxicol., 12, 355-382.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102, and TA104. Environ. Mol. Mutag., 11,
195-206.
Shimizu, A. (1986) Experimental studies on hepatic cirrhosis and
hepatocarcinogenesis. II. Influence of cirrhotic liver on 2-FAA
hepatocarcinogenesis in rats. Acta Pathol Jpn., 36,1039-1048.
Shimizu, A. & Kanisawa, M. (1986) Experimental studies on hepatic
cirrhosis and hepatocarcinogenesis. I. Production of hepatic cirrhosis
by furfural administration. Acta Pathol. Jpn., 36, 1027-1038.
Shimizu, A., Yoshiyasu, N., Harada, M., Ono, T., Sato, K., Inoue, T. &
Kanisawa, M. (1989) Positive foci of glutathione S-transferase
placental form in the liver of rats given furfural by oral
administration. Jpn. J. Cancer Res., 80, 608-611.
Shinohara, K., Kim, E. & Omura, H. (1986) Furans as the mutagens
formed by amino-carbonyl reactions. In: Fujimaki, M., Namiki, M. &
Kato, H., eds, Amino-Carbonyl Reactions in Food and Biological
Systems, New York, Elsevier.
Stich, H.F., Rosin, M.P., Wu, C.H. & Powrie, W.D. (1981a)
Clastogenicity of furans found in food. Cancer Lett., 13, 89-95.
Stich, H.F., Rosin, M.P., San, R.H., Wu, C.H. & Powrie, W.D. (1981b)
Intake, formation, and release of mutagens by man. In:
Gastrointestinal Cancer (Banbury Report 7), Cold Spring Harbor, NY,
CSH Press, pp. 247-266.
Stofberg J. & Grundschober F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Subramanyam, S., Sailaja, D. & Rathnaprabha, D. (1989) Genotoxic assay
of two dietary furans by some in vivo cytogenetic parameters.
Environ. Mol. Mutag., 14, 1-252.
Til, H.P., Woutersen, R.A., Feron, V.J., Hollanders, V.H.M., Falke,
H.E. & Clary, J.J. (1989) Two-year drinking-water study of
formaldehyde in rats. Food Chem. Toxicol., 27, 77-78.
Tiunov, L.A., Vasilev, G.A. & Ivanova, L.V. (1970) Effect of frequency
of injection on the toxic action of furfural. Bull. Exp. Biol.
Med., 70, 999-1000.
Tuma, D.J., Donahue, T.M., Medina, V.A. & Sorrell, M.F. (1984)
Enhancement of acetaldehyde-protein adduct formation by L-ascorbate.
Arch. Biochem. Biophys., 234, 377-381.
Videlia, L.A., Fernandez, V., deMarinis, A., Fernandez, N. &
Valenzuala, A. (1982) Liver lipoperoxidative pressure and glutathione
status following acetaldehyde and aliphatic alcohols pretreatments in
the rat. Biochem. Biophys. Res. Commun., 104, 965-970.
US Environmental Protection Agency (1997) Summary report: A dose range
finding development toxicity study of furfural in rats (TSCA
submission 8EHQ-1097-14026), Washington DC.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
Zdzienicka, M., Tudek, B., Zielenska, M. & Szymczyk, T. (1978)
Mutagenic activity of furfural in Salmonella typhimurium TA100.
Mutat. Res., 58, 205-209.
In animals, the condensation reaction of 2-furoyl CoA with acetyl
CoA to yield furanacryloyl CoA is reversible, favouring formation of
2-furoyl CoA (Parkash & Caldwell, 1994). The observation that furoic
acid is excreted in the urine of dogs given furanacrylic acid
(Friedmann, 1911) supports this conclusion. Analogous reversible
reactions between the CoA thioesters of benzoic acid and cinnamic acid
in vivo favour benzoic acid (Nutley et al., 1994). Excretion of
unconjugated furoic acid and furanacrylic acid at higher doses
suggests that glycine conjugation in laboratory animals is limited,
probably by the supply of endogenous glycine (Gregus et al., 1993). As
mentioned earlier, the principal metabolite in rodents, furoic acid,
may also be metabolized to a very minor extent via oxidation of the
heteroaromatic ring. Since heteroaromatic and aromatic carboxylic
acids do not normally undergo decarboxylation in vivo (Caldwell,
1982), it can be assumed that oxidation of the furan ring system of
furoic acid precedes the loss of carbon dioxide. On the basis of this
assumption, epoxidation (Ramsdell & Eaton, 1990) or hydroxylation
(Ravindranath & Boyd, 1985; Koenig & Andreesen, 1990) of the furan
ring may yield reactive intermediates (e.g. furfural-2,3-epoxide,
acetylacrolein, and alpha-ketoglutaric acid) which readily undergo
decarboxylation. Biochemical changes in the lungs and livers of
animals exposed to furfural indicate that ring oxidation may be
catalysed by a cytochrome P450b isoenzyme, yielding an intermediate
which subsequently conjugates with glutathione (Gupta et al., 1991;
Mishra et al., 1991).
Humans
As in laboratory animals, the predominant pathway for
detoxification of furfural in humans is oxidation of the aldehyde to
yield furoic acid, which either conjugates with amino acids or
condenses with acetyl CoA to produce furanacrylic acid.
After inhalation or dermal absorption, furfural is efficiently
and rapidly absorbed ithrough the lungs and skin, with 20-30% of the
amount absorbed by the lungs (Flek & Sedivec, 1978). When six
volunteers were exposed to 30 mg/m3 for 8 h, the mean pulmonary
retention was 78% (range, 75-82%). Retention was independent of the
concentration of furfural vapour and the length of exposure. An
average 8-h exposure maximum was equivalent to a dose of 1.9 mg/kg bw.
The biological half-life of absorbed furfural in humans is only about
2 h. Essentially all of the absorbed furfural could be accounted for:
97% (range, 93-100%) was oxidized to 2-furoic acid and excreted as the
glycine conjugate, 0.5-5% was excreted as furanacrylic acid, and less
than 1% was exhaled unchanged.
2.1.2 Aldehyde reactivity
Furfural contains a heteroaromatic furan ring with a reactive
aldehyde functional group at the 2 position. The reactivity of the
aldehyde function of aldehydes of low molecular mass, like furfural,
suggests that such compounds are not absorbed intact at doses that do
not saturate the oxidation or condensation reactions associated with
the aldehyde function in digestive fluids. Likewise, when furfural
reaches the body fluids, it is likely to react before entering the
cell. The reactivity of the aldehyde group has been demonstrated, for
example, for acetaldehyde and formaldehyde, both normal metabolic
intermediates, which may have toxic effects including the induction of
tumours when administered under non-physiological conditions at high
doses (Til et al., 1989).
Aldehydes of low molecular mass have been reported to bind with
soluble proteins, protein components of cell membranes, and
thiol-containing molecules to form unstable and stable adducts.
Formation of adducts with albumin (Nagasawa et al., 1980; Donahue et
al., 1983; Tuma et al., 1984), haemoglobin (Peterson & Nguyen, 1985),
and erythrocyte membranes (Gaines et al., 1977) has been demonstrated
in vitro and in vivo. In membranes, binding is usually with the
protein component (Nomura & Lieber, 1981). Aldehydes also bind
glutathione (Videlia et al., 1982). Aldehyde dehydrogenase-catalysed
oxidation of low-molecular-mass aldehydes requires glutathione
(Eckfeldt & Yonetani, 1982), suggesting that free aldehyde is rapidly
conjugated with glutathione in vivo to form a thiohemiacetal which
is subsequently oxidized to the corresponding acid (Brabec, 1981).
Thus, the reactivity of the aldehyde function will significantly
curtail the intracellular concentration of free aldehyde.
When an aldehyde is present in a cell, it undergoes rapid
oxidation, with conjugation of the resulting carboxylic acid (Gregus
et al., 1993). The various metabolic processes, i.e. oxidation,
conjugation, and condensation (see section 2.1.1) effectively
eliminate the reactive aldehyde functional group when not saturated by
high, non-physiological doses.
2.2 Toxicological studies
2.2.1 Acute toxicity
On the basis of the oral LD50 values in various species
(Table 1), furfural is acutely toxic at lower doses in rats than in
mice.
Wistar/Slc rats given a single dose of 50 mg/kg bw furfural by
gavage had sporadic eosinophilic degeneration of hepatic cells with
nuclear pyknosis and eosinophilic necrosis and increased hepatocyte
mitosis. No massive or zonal necrosis was observed. Since the control
rats were killed at the beginning of the experiment, no appropriate
controls were available (Shimizu & Kanisawa, 1986). Decreased
activities of succinic dehydrogenase and ATPase and increased
activities of acid phosphatase and acid DNase II were reported in male
Wistar rats injected intraperitoneally with 20 or 50 mg/kg bw (Jonek
et al., 1975).
Table 1. Acute toxicity of furfural
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Rat Oral 127 Jenner et al. (1964)
Rat Intraperitoneal 120 Tiunov et al. (1970)
Mouse Oral 333 Boyland (1940)
Mouse Subcutaneous 200 Tiunov et al. (1970)
Mouse Subcutaneous 223 (1-day survival) Castellino et al.(1963)
119 (10-day survival)
Rabbit Dermal > 310 Moreno (1976)
Hamster Inhalation 2500 Kruysse (1972)
Bird Oral > 98 Schafer et al. (1983)
2.2.2 Short-term studies of toxicity
Rats
In rats receiving 0.05, 2.5, or 25 mg/kg bw per day furfural for
35 days, no hepatic impairment was seen (Kuznetsov, 1966). When male
rats were injected intraperitoneally with furfural in increasing doses
from 29 to 58 mg/kg bw for 30 days, damage and increased intracellular
catabolic processes were seen in liver and kidney cells (Kaminska,
1977).
In a 16-day study, groups of five male and five female Fischer
344/N rats were given furfural at doses of 0, 15, 30, 60, 120, or
240 mg/kg bw per day in corn oil by gavage, five days per week for a
total of 12 doses. At the end of the study, the surviving animals were
killed, necropsied, and examined histopathologically. No
treatment-related abnormalities were found. Eight of ten rats at the
highest dose had died by the third day due to gavaging accidents.
Animals at 120 mg/kg bw appeared to be less active. The body weights
of rats given doses < 120 mg/kg bw per day did not differ
significantly from those of controls.
In a 16-day study, groups of five male and five female B6C3F1
mice were given furfural at doses of 0, 25, 50, 100, 200, or 400 mg/kg
bw per day in corn oil by gavage on five days per week for a total of
12 doses. At the end of the study, the surviving animals were killed,
necropsied, and examined histopathologically. The mean body weights of
the treated mice did not differ significantly from those of controls.
One male at the highest dose had died by day five, and two female mice
died from gavaging accidents. No treatment-related abnormalities were
found.
In a 13-week study, groups of 10 male and 10 female B6C3F1 mice
were given furfural at doses of 0, 75, 150, 300, 600, or 1200 mg/kg bw
per day in corn oil by gavage on five days per week. Necropsies were
performed on all survivors, and histopathological examinations were
performed on those at the two highest doses and the controls. All the
mice at the two highest doses died before the end of the study, except
for one male and one female at 600 mg/kg bw per day. The body weights
of the males treated with doses > 150 mg/kg bw per day were
slightly lower than those of controls. Female mice at 75-300 mg/kg bw
per day and male mice at 300 mg/kg bw per day showed significantly
increased relative liver weights as compared with controls.
Centrilobular coagulative necrosis of hepatocytes was observed in most
male mice at the two highest doses and in one male per group treated
with 150 or 300 mg/kg bw per day. Among the females, only two at the
highest dose showed similar lesions. Animals with coagulative necrosis
also had inflammation of the liver, with minimal to mild mononuclear
inflammatory cell infiltrate (National Toxicology Program, 1990).
A group of 48 male, six-week-old Wistar/Slc rats was fed furfural
at a concentration of 20 ml/kg diet (equivalent to approximately 1200
mg/kg bw per day) for one week, 30 ml/kg diet for one week, and then
40 ml/kg diet on days 15-90; on days 90-120, the rats were given the
diet containing 40 ml/kg for only five days per week to prevent
reduced weight gain. The concentration of furfural in the feed was not
analysed after preparation of the diet. A control group of 40 male
rats of the same age was fed the basal diet.
Treated rats sacrificed after 90 days had marked
cholangiofibrosis, with areas of increased density containing red
nodules. The nodules showed fibrous widening of Glisson's sheath,
bile-duct proliferation, and destruction of the limiting plates. The
parenchymal damage consisted of bridging necrosis and hydropic
degeneration of hepatocytes. The parenchyma often showed no cirrhotic
changes in areas other than those with fibrosis, suggesting a
regenerative process in the liver. Increased numbers of cells
undergoing mitosis were seen. In rats killed on day 120 of the study,
similar but more marked findings were reported. The incidence of
hepatic fibrosis was increased in the furfural-treated rats. No
atypical hepatocyte growth was noted. Furfural did not cause
hepatocellular hyperplastic changes (Shimizu & Kanisawa, 1986).
In a study designed to examine the mechanism of furfural-induced
hepatocytic injury, six groups of six male Wistar/Slc rats were
maintained on diets containing furfural at doses calculated to result
in average daily intakes of 20 ml/kg diet (approximately 1200 mg/kg bw
per day) for the first 30 days and 30 ml/kg diet (approximately
1700 mg/kg bw per day) thereafter. Each group also included four
control animals. The furfural-containing diets were replaced after 15,
30, 60, 90, 120, or 150 days, and animals were killed 14 days after
termination of furfural administration. Increased duration of exposure
to furfural was accompanied by increased numbers and size of foci
positive for the placental form of glutathione- S-transferase in
hepatocytes (Shimizu et al., 1989). Such foci have been reported to be
a marker for early detection of preneoplastic or neoplastic cells
(Pickett & Lu, 1988).
In a 13-week study, groups of 10 male and 10 female Fischer 344/N
rats were given furfural at doses of 0, 11, 22, 45, 90, or 180 mg/kg
bw per day in corn oil by gavage on five days per week. All survivors
were necropsied, and histopathological examinations were performed on
those at the two highest doses and the controls. Only one male rat at
the highest dose survived to the end of the study; four of the deaths
were considered to be related to gavage, as were two deaths among
controls and four among rats at the second highest dose. The mean body
weights of treated rats did not differ from those of controls. The
relative and absolute weights of the livers and kidneys of males given
90 and 180 mg/kg bw per day were significantly higher than those of
controls. An increased incidence of cytoplasmic vacuolization in
hepatocytes was observed in all groups of treated males: control,
4/10; 11 mg/kg bw per day, 10/10; 22 mg/kg bw per day, 10/10; 45 mg/kg
bw per day, 10/10; 90 mg/kg bw per day, 9/10. No lesions were reported
in the kidneys of these rats (National Toxicology Program, 1990).
Hamsters
Groups of 10 Syrian golden hamsters of each sex were exposed to
furfural vapour at 0, 77, 448, or 2165 mg/m3 for 6 h per day, five
days per week over 13 weeks. The main findings were mild growth
retardation, irritation of the eyes and nose, and hyperplastic atrophy
of the nasal epithelium, all at the highest dose. The NOEL was 77
mg/m3, since mild nasal epithelial degeneration was observed at 448
mg/m3 (Feron & Kruysse, 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a two-year study, 50 male and 50 female B6C3F1 mice were
given furfural at doses of 0, 50, 100, or 175 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed weekly
for the first 13 weeks and monthly thereafter until the conclusion of
the study, when surviving animals were killed. All animals were
necropsied and examined histologically. Furfural had no significant
effect on body weight or survival. Microscopic examination revealed
lesions in the liver, kidneys, and forestomach. In the liver,
pigmentation, necrosis, and chronic inflammation were seen in males at
the intermediate and high doses and in females at the high dose (Table
2). Hepatocellular adenomas and carcinomas were seen in all groups,
including controls, and the increased incidence was statistically
significant only in males at the high dose. Carcinomas occurred at the
same incidence in females at the high dose as in controls.
Table 2. Incidences of liver lesions and hepatocellular adenomas and carcinomas in male and
female B6C3F1 mice given furfural by gavage
Lesion Dose (mg/kg bw)
0 50 100 175
M F M F M F M F
Pigmentation 0/50 0/50 0/50 0/50 8/49 0/50 18/50 11/50
Necrosis, focal and multifocal 3/50 2/50 2/50 1/50 7/49 3/50 10/50 2/50
Chronic Inflammation 0/50 0/50 0/50 0/50 8/49 1/50 18/50 8/50
Hepatocellular adenoma 9/50 1/50 13/50 3/50 11/49 5/50 19/50 8/50
Hepatocellular carcinoma 7/50 4/50 12/50 0/50 6/49 2/50 21/50 4/50
Combined rates 16/50 5/50 22/50 3/50 17/49 7/50 32/50 12/50
From National Toxicology Program (1990)
M, male; F, female
Hyperplasia of the renal tubules was found in one male at the low
dose. One male at the intermediate dose, one at the high dose, and one
female at the low dose had a cortical adenoma, and one male at the low
dose had a cortical carcinoma. Owing to the low incidence and lack of
dose-response relationship, these tumours were not considered as
evidence for carcino-genicity. Marginal increases in the incidences of
hyperplasia and papillomas in the forestomach of female mice were
considered to be of no biological significance and were probably
related to the irritating effects of chronic administration of the
compound by gavage or the method of gavage itself. None of the animals
showed evidence of malignant forestomach lesions (National Toxicology
Program, 1990).
Rats
In a two-year study, 50 male and 50 female Fischer 344/N rats
were given furfural at doses of 0, 30, or 60 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed at
specific intervals, and the survivors were killed at the end of the
study, necropsied, and examined histologically. Overall survival was
slightly reduced for female rats at the highest dose (78% at week 97),
due to accidental gavage-related deaths. Furfural had no effect on
body weight or survival. Congestion and foreign bodies were observed
in a dose-related manner in the lungs of female rats. A small increase
in the incidence of squamous-cell carcinomas and papillomas in the
forestomachs of treated rats was observed but was considered unrelated
to treatment. Mild centrilobular hepatocellular necrosis was observed
in all groups (vehicle control: males, 3/50; females, 10/50; low dose:
males, 9/50; females, 9/50; high dose: males, 12/50; females, 4/50).
Bile-duct hyperplasia occurred in 45/50 control males, 36/50 control
females; 41/50 males and 28/50 females at the low dose; and 44/50
males and 24/50 females at the high dose. Focal bile-duct dysplasia
was noted in one male at the intermediate dose, and bile-duct
hyperplasia accompanied by fibrosis (cholangiofibrosis) was seen in
two males at the high dose. One control male had a hepatocellular
adenoma, and two males at the high dose had a cholangiocarcinoma. The
incidence of the latter lesion was not statistically significant, but
some evidence of carcinogenicity was consluded since
cholangiocarcinomas were rare in historical controls (3/2145)
(National Toxicology Program, 1990).
2.2.3.1 Special studies of carcinogenicity
The aldehydes citral and heptaldehyde inhibit the growth of
spontaneous mammary tumours in mice; however, furfural and its
furfuracrylic acid metabolite at a daily dose of 2.5 mg had no effect
on the growth of spontaneous mammary tumours in mice, although they
were weakly active against sarcomata (Boyland, 1940).
Studies have been designed to evaluate the effect of furfural on
the carcinogenic potential of known carcinogens. In the first study,
three groups of 35 male and 35 female Syrian golden hamsters were
provided with 0.2 ml of 1.5% furfural in physiological saline (about
25 mg/kg bw), 0.5% benzo [a]pyrene (8.3 mg/kg bw), or 1.5% furfural
plus 0.5% benzo [a]pyrene intratracheally once weekly for 36 weeks.
Furfural in combination with benzo [a]pyrene led to earlier
development of hyper- and metaplastic changes in the tracheobronchial
epithelium, a shorter latency for tracheobronchial tumours, a few more
bronchial and peripheral squamous-cell carcinomas, and a substantially
reduced number of tracheal squamous-cell carcinomas. The incidence of
peritracheal sarcomas was increased, which may indicate a
co-carcinogenic effect of furfural. Irritation but no tumorigenic
effect was observed after administration of furfural alone (Feron,
1972).
In a subsequent study, groups of 126 Syrian golden hamsters of
each sex were exposed to atmospheres containing 400 ppm (1600 mg/m3)
furfural for 7 h per day, five days per week for nine weeks, then 330
ppm (1300 mg/m3) for 11 weeks, and 250 ppm (970 mg/m3) for an
additional 32 weeks. The effects on the respiratory tract included
atrophy and downward growth of the olfactory epithelium, degenerative
changes in Bowman's glands, and the appearance of cyst-like structures
in the lamina propria beneath the olfactory epithelium. Although
furfural was irritating to the olfactory epithelium, treatment did not
result in hepatic toxicity or carcinogenicity. Furfural did not
potentiate the carcinogenic effect of benzo [a]pyrene or
N-nitrosodiethylamine (Feron & Kruysse, 1978).
Localized irritation of the nasal cavity and respiratory tract,
with no hepatic effects, were also observed in B6C3F1 mice and
Fischer 344/N rats exposed to atmospheres containing 0, 2, 4, 8, 16,
or 32 ppm of the metabolic precursor furfuryl alcohol for 13 weeks
(Mellick et al., 1991).
SPF Wistar rats were given furfural at doses of 20 ml
(approximately equal to 1200 mg/kg bw per day) for one week, 30 ml for
the second week, and 40 ml for the following 10 weeks to produce
hepatic cirrhosis. Half of the remaining rats in this study (38
treated and 32 controls) were fed a diet containing 0.03%
N-2-fluorenylacetamide for three weeks, followed by one week of
normal diet; the other half received only the normal diet. All
surviving rats were killed 12 weeks later, and their livers were
examined. Rats that had received furfural only had no hyperplastic
changes in the liver but did have cirrhosis. Rats that had been
treated with N-2-fluorenylacetamide developed multiple hyperplastic
nodules which stained for alpha-fetoprotein, and this response was
markedly potentiated in the rats previously treated with furfural. The
authors concluded that furfural-induced hepatic cirrhosis increases
the susceptibility of rats to potent hepatocarcinogens (Shimizu,
1986).
Samples of mouse liver tumours from the National Toxicology
Program carcinogenesis bioassay programme were assessed for
transforming gene activity by Southern blot analysis for H-, K-, and
N- ras oncogens. The pattern of mutations in the oncogenes from liver
tumours that occurred spontaneously differed from that which occurred
in some furfural-treated animals: The activated ras genes were
H- ras in 15 of 17 spontaneous tumours, with one raf and one
unknown oncogene; activating point mutations occurred at codon 61 in
six tumours, codon 13 in two tumours, and codon 117 in one tumour. The
authors concluded that the spectrum of activating mutations in the
H- ras gene and the pattern of ras gene activation in liver tumours
derived from furfural-treated mice differed from those in liver
tumours derived from untreated animals, and indicates a direct
genotoxic effect of furfural. An alternative mechanism-induction of
mutations by an indirect, secondary genotoxic pathway as a result of a
cytotoxic event -- was mentioned but excluded by the authors on the
basis of the absence of cytotoxic lesions in the livers of similar
mice after 90 days of treatment with the same dose of furfural. The
authors do not mention, however, that necrosis, pigmentation, and
chronic inflammation were seen regularly in mice at the intermediate
and high doses in the carcinogenicity experiment (see Table 2). It can
be concluded that the effect was either due to a direct genotoxic
event or occurred via an indirect, secondary genotoxic pathway
(Reynolds et al., 1987).
2.2.4 Genotoxicity
Studies of the genotoxicity of furfural are summarized in
Table 3. Testing of furfural for mutagenicity in vitro at
concentrations up to those causing cytotoxicity provided no evidence
that it is mutagenic to Salmonella typhimurium strains TA98, TA102,
TA1535, or TA1537 or ito two strains of Escherichia coli, with or
without metabolic activation (Sasaki & Endo, 1978; Zdzienicka et al.,
1978; McMahon et al., 1979; Loquet et al., 1981; Marnett et al., 1985;
Mortelmans et al., 1986; Shinohara et al., 1986; Nakamura et al.,
1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al., 1989;
National Toxicology Program, 1990; Dillon et al., 1992). The majority
of these studies also showed negative results for S. typhimurium
TA104, although a single positive result was reported (Shane et al.,
1988). Furfural was not mutagenic when incubated with
S. typhimurium TA100 in most reports (Sasaki & Endo, 1978; McMahon
et al., 1979; Mortelmans et al., 1986; Shinohara et al., 1986; Kim et
al., 1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al.,
1989; National Toxicology Program, 1990; Eder et al., 1991; Dillon et
al., 1992) but was weakly mutagenic in other studies (Zdzienicka et
al., 1978; Loquet et al., 1981; Shane et al., 1988). When fufural was
co-incubated with benzo [a]pyrene and S. typhimurium TA100, it did
not alter the mutagenic activity of benzo [a]pyrene in this strain
(Zdzienicka et al., 1978). Furfural showed no mutagenic potential in
two rec assays with Bacillus subtilis by a direct streaking method
(Osawa & Namiki, 1982; Matsui et al., 1989) but also gave positive
results in this test system (Shinohara et al., 1986).
Table 3. Results of assays for the genotoxicity of furfural
End-point Test object Concentration Result Reference
In vitro
Reverse mutation S. typhimurium TA100 NR Negative Eder et al. (1991)
Reverse mutation S. typhimurium TA100, TA102, and NR Negativea Dillon et al. (1992)
TA104
Reverse mutation S. typhimurium TA100, TA98, and < 36 µmol/plate Weakly positive (TA100)b Loquet et al. (1981)
TA1535 60 µmol/plate Negativec
Reverse mutation S. typhimurium TA100, TA98, and < 1.2 mmol/plate Negative Aeschbacher et al. (1989)
TA102
Reverse mutation S. typhimurium TA100 and TA98 0.165-0.660 µmol/plate Negativea Shinohara et al. (1986)
Reverse mutation S. typhimurium TA100, TA102, and 5-500 µg/plate Positive (TA104) Shane et al. (1988)
TA104
Reverse mutation S. typhimurium TA100, TA98, and NR Negativea Kato et al. (1989)
TA104; E. coli WP2uvrA/PKM101
Reverse mutation S. typhimurium TA104 and TA102 1 µmol/plate Negative Marnett et al. (1985)
Reverse mutation S. typhimurium TA98, TA100, < 6667 µg/plate Negativea Mortelmans et al. (1986)
TA1535, and TA1537
Reverse mutation S. typhimurium TA100 and TA98 7 µl/plate Positivea (TA100) Zdzienicka et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, < 20 µl/plate Negativea McMahon et al. (1979)
TA1537, TA1538, TA98, G46;
E. coli WP2uvrA
Reverse mutation S. typhimurium TA100 and TA98 NR Negativea Sasaki & Endo (1978)
Reverse mutation S. typhimurium TA100 4.44 µmol/plate Negativea Kim et al. (1987)
umu gene expression S. typhimurium TA1535/pSK/002 1932 µg/ml Negativea Nakamura et al. (1987)
Reverse mutation S. typhimurium TA100 < 1 mg Negativea Osawa & Namiki (1982)
Reverse mutation S.typhimurium TA98, TA100, 33-6666 µg/plate Negativea National Toxicology Program
TA1535, and TA1537 (1990a)
Reverse mutation S.typhimurium TA98, TA100, and 33-6666 µg/plate Negativea National Toxicology Program
TA1535 (TA100 equivocal) (1990b)
rec Gene mutation B. subtilis H17 and M45 < 1 mg Negativea Osawa & Namiki (1982)
rec Gene mutation B. subtilis H17 and M45 1.7-17 mg/disk Positivea Shinohara et al. (1986)
rec Gene mutation B. subtilis H17 and M45 0.6 ml Negativea Matsui et al. (1989)
Table 3. (continued)
End-point Test object Concentration Result Reference
Chromosomal Chinese hamster ovary cells < 40 mmol/L Positivea Stich (1981a)
aberration
Chromosomal Chinese hamster ovary cells 3 mg/ml Positive Stich (1981b)
aberration
Forward mutation L5178Y tk+/- mouse lymphoma cells 25-400 µg/ml Positiveb McGregor et al. (1988)
Sister chromatid Chinese hamster ovary cells < 1170 µg/ml Positivea National Toxicology Prohgram
exchange/Chromosomal (1990)
aberration
Sister chromatid Human lymphocytes < 0.14 mmol/L Positive Gomez-Arroyo & Souza (1985)
exchange
Unscheduled DNA Human liver slices 0-25 mmol/L Negative Lake et al. (1998)
synthesis
In vivo
Sex-linked recessive D. melanogaster 1000 ppm in diet Negative Woodruff et al. (1985)
lethal mutation
Sex-linked recessive D. melanogaster 100 ppm by injection Positive Woodruff et al. (1985)
lethal mutation
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Negative Rodriguez-Arnaiz et al. (1992)
repair-proficient females < 33% lethal dose
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Positive only after Rodriguez-Arnaiz et al. (1992)
repair-deficient females < 33% lethal dose injection
Sex-chromosome loss D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
injection
Sex-linked recessive D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
lethal mutation injection
Reciprocal D. melanogaster 100 ppm by injection Negative Woodruff et al. (1985)
translocation
Sister chromatid B6C3F1 mouse bone-marrow cells 50-200 mg/kg by Negative National Toxicology Program
exchange/Chromosomal injection (1990)
aberration
Table 3. (continued)
End-point Test object Concentration Result Reference
Somatic chromosomal Swiss albino mouse bone-marrow < 4000 ppm for 5 days Negatived Subramanyam et al. (1989)
mutation cells
Sperm-head Swiss albino mouse < 4000 ppm for 5 Negatived Subramanyam et al. (1989)
abnormalities weeks
Unscheduled DNA Fischer 344 rat hepatocytes 5.0, 16.7, or 50 mg/kg Negative Phillips et al. (1997)
synthesis bw orally
NR, not reported
a With and without metabolic activation
b Without metabolic activation
c With metabolic activation
d Abstract only: reported to have positive effects at 4000 ppm, but no details available
Furfural produced a dose-dependent increase in the frequency of
chromatid breaks and exchanges in Chinese hamster ovary cells with and
without metabolic activation (Stich et al., 1981a,b; National
Toxicology Program, 1990) and induced chromosomal aberration in these
cells at a cytotoxic concentration (National Toxicology Program,
1990). An increased frequency of sister chromatid exchange was
reported in human lymphocytes incubated with furfural at a
concentration of 0.07 or 0.14 mmol/L, but not at 0.035 mmol/L
(Gomez-Arroyo & Souza, 1985). In this study, furfuryl alcohol did not
increase the frequency of sister chromatid exchange at a concentration
of 3.3, 6.6, or 9.9 mmol/L.
Furfural increased trifluorothymidine resistance when incubated
with L5178Y tk+/- mouse lymphoma cells at a concentration of 200 or
400 mg/ml without metabolic activation, but showed no mutagenic
activity at 25, 50, or 100 mg/ml (McGregor et al., 1988).
Concentrations of 400 and 800 mg/ml were cytotoxic in separate trials.
In vivo, furfural did not induce sex-linked recessive lethal
mutations or reciprocal translocations in male Drosophila
melanogaster when incorporated into their diet at 1000 ppm.
Mutations were induced when furfural was administered by injection at
a concentration of 100 ppm, but no reciprocal translocations occurred.
A similar pattern of responses was reported for other simple aldehydes
(e.g. acetaldehyde and trans-cinnamaldehyde) when given via the
intraperitoneal (positive) and oral (negative) routes of
administration (Woodruff et al., 1985). No sex-chromosome loss was
observed when male D. melanogaster were fed or injected with
furfural at concentrations equivalent to 25-33% of the lethal dose of
furfural and then mated with repair-proficient females
(Rodrigues-Arnaiz et al., 1992), but sex-chromosome loss was reported
by these authors when treated males were mated with repair-deficient
females. No sex-linked recessive lethal mutation or sex-chromosome
loss was reported when male D. melanogaster were injected with
furfuryl alcohol (Rodrigues-Arnaiz et al., 1989).
Tests of mammals treated in vivo with furfural have produced
mainly negative results. Furfural did not induce sister chromatid
exchange or chromosomal aberrations in B6C3F1 mouse bone-marrow cells
after intraperitoneal injections of furfural at doses of 50-200 mg/kg
bw (National Toxicology Program, 1990). No genotoxic effects or
spermhead abnormalities were reported in mice given up to 4000 ppm
furfural daily for five weeks, and only the highest dose tested was
associated with an increased frequency of somatic (liver)-cell
chromosomal mutations when given for 24 or 48 h but not after 72 h
(Subramanyam et al., 1989). Furfural administered by gavage to male
Fischer 344/N rats in corn oil at doses of 5, 16.7, or 50 mg/kg bw did
not induce unscheduled DNA synthesis in hepatocytes (Phillips et al.,
1997).
Chromosomal aberrations were not observed in lymphocytes taken
from workers occupationally exposed to furfural or furfuryl alcohol
(Gomez-Arroyo & Souza, 1985).
Liver slices prepared from samples from four human donors were
cultured in medium containing 3H-thymidine and 0-25 mmol/L furfural
for 24 h. In a preliminary experiment, furfural was markedly toxic at
concentrations > 10 mmol/L. As positive controls, liver slices were
also cultured with three known genotoxic agents,
2-acetylaminofluorene, aflatoxin B1, and
2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP). Unscheduled
DNA synthesis was quantified as the changes in mean nuclear and
cytoplasmic grain counts relative to controls, net grain count (i.e.
mean nuclear - mean cytoplasmic grain counts), and the percentage of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains. In
comparison with control liver slice cultures (in dimethylsulfoxide),
those treated with 0.02 or 0.05 mmol/L 2-acetylamino-fluorene, 0.002
or 0.02 mmol/L aflatoxin B1, and 0.003 or 0.05 mmol/L PhIP had
significantly higher net grain counts in centrilobular hepatocytes,
due primarily to large increases in mean nuclear grain counts. The
three genotoxic agents also significantly increased the number of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains.
Treatment with 0.005-0.5 mol/L furfural had no significant effect
on net grain count or mean nuclear or cytoplasmic grain counts. A
small but statistically significant increase in net grain counts
observed at 2, 5, and 10 mmol/L furfural was the result of a
significant concentration-dependent decrease in mean cytoplasmic grain
counts accompanied by a smaller increase in nuclear grain counts.
Furfural had no significant effect on the percentage of hepatocyte
nuclei with > 5 or > 10 net grain counts, except at a concentration
of 2 mmol/L and only in liver cultures in which marked toxicity was
noted at 10 mmol/L. The decreases in mean cytoplasmic and nuclear
grain counts and the correlation between cytotoxicity and changes in
net grains count and nuclei with > 5 net grains support the
conclusion that furfural at 2, 5, or 10 mmol/L induces a cytotoxic
response in cultured human liver slices. In comparison with the three
genotoxins tested, furfural did not induce unscheduled DNA synthesis
in cultured human liver slices (Lake et al., 1998).
Furfural was reported to increase the frequency of single-strand
DNA breaks in isolated calf-thymus duplex DNA (Hadi et al., 1989).
When incubated with plasmid DNA, furfural decreased the transformation
capacity of Escherichia coli host cells; however, the damaged
plasmids were repaired after propagation in the host (Khan & Hadi,
1993).
Thus, negative results were obtained with furfural in most tests
for genotoxicity in vitro. Positive results were reported at
relatively high doses in three of 16 assays for reverse mutation in
S. typhimurium and in one of three assays for gene mutation in
B. subtilis. Weakly positive results were obtained in tests for
chromosomal aberrations at relatively high doses. Positive results
were found in two assays for sister chromatid exchange and one for
forward mutation in mouse lymphoma cells. The results of all tests for
genotoxicity in vivo were negative, except in D. melanogaster
injected with furfural.
2.2.5 Reproductive toxicity
Rats
A summary report of an investigation to define the doses of
furfural for a study of developmental toxicity was submitted to the
Toxic Substances Control Administration of the US Environmental
Protection Agency. Five groups of eight Crl:CD(SD)BR rats received
furfural in water by gavage at doses of 10, 50, 100, 500, or 1000
mg/kg bw per day during gestation days 6-15. Because of excessive
deaths at 500 and 1000 mg/kg bw per day, the breeding phase of the
study was re-initiated to characterize the potential maternal and
developmental toxicity at 150, 250, and 350 mg/kg bw per day. Two
concurrent control groups of eight rats received only the vehicle
(reverse osmosis-treated water) by gastric intubation. Clinical
observations, body weights, and food consumption were recorded. On
gestation day 20, laparohysterectomy was performed on all surviving
animals; the uteri and ovaries were examined, and the numbers of
fetuses, early and late resorptions, total implantation, and corpora
lutea were recorded. The uteri and fetuses were weighed and examined
for external malformations, variations, and sex.
Because of excessive mortality on day 6 after dosing, all females
at 250, 350, 500, and 1000 mg/kg bw per day were killed. The clinical
signs in these animals included lethargy, effects on respiration, and
whole-body tremor. The maternal animals at other doses survived,
except for one female at 150 mg/kg bw per day group, which died within
I h of dosing. The surviving females at 150 mg/kg bw per day had
reduced body-weight gain and reduced food consumption; however, the
mean body weight, net body weight, net body-weight gain, and gravid
uterine weight were unaffected. Body weight and food consumption of
animals at 10, 50, and 100 mg/kg bw per day were unaffected.
Intrauterine growth and survival were not affected at 10, 50, 100, or
150 mg/kg bw per day. The only external malformation noted
(omphalocele) occurred in offspring of rats at 10 mg/kg bw per day. No
developmental variations were noted (US Environmental Protection
Agency, 1997).
3. HUMAN INTAKE
On the basis of the most recently reported annual volumes, 3600
kg in Europe (International Organization of the Flavor Industry, 1995)
and 590 kg in the United States (National Academy of Sciences, 1987),
the estimated daily per capita intake of 'eaters only' of furfural
from use as a flavouring substance is approximately 9 µg/kg bw per day
in Europe and 2 µg/kg bw per day in the United States. Furfural and
the corresponding furfuryl alcohol are virtually ubiquitous in nature
(Table 4). They are formed from the acid hydrolysis or heating of
Table 4. Natural occurrence of furfural
Food item Concentration of
furfural (ppm)
Apple (raw), apple juice, apricot (Prunus armeniaca L.), sweet 0.02-0.05
cherry (Prunus avium L.), sour cherry (Prunus cerasus L.)
Orange juice (Citrus sinensis L. Osbeck) Trace
Orange peel oil, grapefruit juice (Citrus paradisi) 0.34
Bilberry (Vaccinium myrtillus L.) 0.02
American cranberry (Vaccinium macrocarpon Ait.) 0.1-0.3
Lingonberry (Vaccinium vitis idaea L.) 0.02
Black currents, berries, guava (Psidium guajava L.) 0.0014-0.19
Grape (dried, sultana), peach (Prunus persica L.), pineapple 0.01
(Ananas comosus), raspberry (Rubus idaeus L.), strawberry
(Fragaria species), asparagus (raw), asparagus (cooked)
Carrot (Daucus carota L.), celery leaves (raw), onion (roasted), 0.005
leek (heated), potato (raw), potato (cooked), bell pepper
(Capsicum annuum)
Wheaten bread, sauerkraut, tomato (Lycopersicon 0.8-26
esculentum Mill.), cinnamon (Cinnamomum zeylanicum Blume),
cloves (Eugenia caryophyllata Thunberg), Mentha species
Crispbread, bread, other types, blue cheeses, parmesan, 0.02
butter, yogurt, milk, chicken and turkey (raw), beef (boiled/
cooked), beef (grilled/roasted)
Lamb and mutton, pork (heated), hop oil, beer 0-0.3
Cognac 0.6-33
Armagnac 2
Weinbrand 0.2-4.3
Grape brandy, other types, rum (all categories), rum (category I: 22
total volatiles > 3600 ppm)
Rum (category II: total volatiles 1100-3600 ppm) Trace-25
Rum (category III: total volatiles 240-1100 ppm) Trace
Bourbon whiskey 2-11.6
Irish whiskey 0.8-13.6
Malt whisky 10-37
Scotch blended whisky 1.1-30
Canadian whiskey 0.3-0.8
Japanese whiskey 0.5-4.5
Cider, sherry, white wine Trace-10.3
Red wine 0.005-0.05
Rose wine, port wine 2-34
Special wine, botrytized wine 0.13
Cocoa, coffee 55-255
Black tea 2-7
Green tea 0.1
Microbial fermented tea, tea (brewed) 0.3-0.8
Table 4. (Continued)
Food item Concentration of
furfural (ppm)
Barley (roasted), filbert (roasted, Corylus avellano), peanut 0.08-0.2
(roasted, Arachis hypogea), pecan (roasted), popcorn, potato
chips (American)
Oat flakes (toasted), honey, soybean, arctic bramble (Rubus Trace
arcticus L., Rubus stell.), cloudberry (Rubus chamaemorus L.)
Passion fruit juice (yellow), passion fruit (yellow), plum (raw), 2.58
plum (salted and pickled)
Beans (Phaseolus vulgaris L.), mushroom (raw) 0.05
Trassi (cooked), plum brandy, almond (roasted, Prunus 9
amygdalus)
Macadamia nut (roasted, Macadamia integrifolia), sesame 0-0.1
seed (roasted), mango (raw)
Mango (canned), cauliflower (cooked), tamarind (Tamarindus 7
indica), pear brandy
Apple brandy, beetroot (cooked),artichoke (cooked, Cynarus < 0.01
scolymus L.), gin, rice bran, traditional rice (cooked), quince
(Cydonia oblonga), radish (fermented), shoyu (fermented soya
hydrolysate), bacuri (Platonia insignis), cupuacu (Theobroma
grandiflora), muruci (Brysonima crassifolia)
Potato (sweet, heated), sukiyaki, licorice (Glycyrrhiza glabra L.), < 0.01
matsutake (Tricholoma matsutake), strawberry wine, pumpkin
(Cucurbita pepo L.), sake, oat groats, maize, cashew apple
(Anacardium occidentale), basil (Ocimum basilicum), malt,
peated malt, wort, bonito (dried, katsuobishi), elderberry
(Sambucus nigra L.), mangosteen (Garcinia mangostana),
cherimoya (Annona cherimola), bilberry wine, buchu oil,
vanilla, mountain papaya (Carica pubescens)
Wild rice (Zizania aquatica), chicory (Cichorium intrybus L.), 0-0.2
endive (Cichorium endivia L.), ouzo
Sapodilla fruit (Achras sapota L.) Trace
Aubergine (Solanum melongena L.), pistachio nut (roasted, 17.2
Pistachia vera), arrack
Nectarine < 0.01
From Maarse & Visscher (1994)
Intake (µg/kg bw per day) calculated as follows:
{[(annual volume, kg) × (1 × 109 mg/kg) × (1/60 kg bw)]/[population × 0.6 ×
365 days]}, where population (10%, 'eaters only') = 32 × 106 for Europe and
24 × 106 for the United States; 0.6 represents the assumption that only 60%
of the flavour volume was reported in the survey (National Academy of Sciences,
1987; International Organization of the Flavor Industry, 1995). Slight
variations may occur from rounding off.
polysaccharides which contain pentose and hexose fragments. Furfural
has been detected in a broad range of fruits and fruit juices, wines,
whiskeys, coffee, and tea (Maarse et al., 1994). The highest
concentrations of furfural in foods have been reported in cocoa and
coffee (55-255 ppm), alcoholic beverages (1-33 ppm), and whole-grain
bread (26 ppm). Furfuryl alcohol, which is readily converted to
furfural in vivo (Rice, 1972; Nomeir et al., 1992), has been found
in the highest concentrations in heated skim milk (230 ppm) and coffee
(90-881 ppm). The total potential daily per capita intake of
furfural and precursors of furfural (i.e. furfuryl alcohol and
furfuryl esters) from consumption of foods in which they occur
naturally (Stofberg & Grundschober, 1987) is approximately 0.3 mg/kg
bw per day (i.e. about 300 µg/kg bw per day) in the United States.
Thus, the intake of furfural and furfuryl derivatives from use as
flavouring substances represent 1-3% of the total intake.
4. COMMENTS
In both humans and rodents, furfural is efficiently metabolized
by oxidation of the aldehyde function to furoic acid, most of which
is conjugated with glycine and excreted. A minor proportion (< 5%)
of furoic acid is condensed with acetyl coenzyme A to form
furanacryloyl coenzyme A; the resulting furanacryloic acid is
conjugated with glycine and excreted in the urine. About 5% of
[carboxyl-14C]-labelled furfural is eliminated by rats and mice as
14C-carbon dioxide. The metabolic pathway, which could involve direct
decarboxylation or epoxidation and ring opening, has not been defined.
In short-term studies, furfural was clearly hepatotoxic at doses
> 90 mg/kg bw per day in male rats and > 150 mg/kg per day in
mice. Minor changes reported in the livers of male rats given furfural
at lower doses by gavage in corn oil for 13 weeks were also present to
a lesser extent in vehicle controls. In a study of developmental
toxicity, furfural was not toxic to rats at 150 mg/kg bw per day, the
highest dose tolerated.
Furfural, like other aldehydes such as endogenous acetaldehyde,
is a reactive aldehyde; it is reported to bind to soluble proteins and
protein components of cell membranes. Various metabolic processes
(i.e. oxidation, conjugation, and condensation) effectively eliminate
the reactive aldehyde functional group when these metabolic pathways
are not saturated by high, unphysiological doses.
Furfural was not genotoxic in most tests in vitro. Positive
results were reported at relatively high concentrations in only three
of 16 assays for reverse mutation in Salmonella typhimurium and in
one of three rec assays in B. subtilis. A few chromosomal
aberrations were seen in Chinese hamster ovary cells exposed to
relatively high concentrations. Sister chromatid exchanges and forward
mutations were induced in mouse lymphoma cells. This weak activity of
furfural in vitro in some tests for genotoxicity might be explained
by its aldehyde reactivity. Recent studies of unscheduled DNA
synthesis in hepatocytes of rats treated in vivo and in human liver
slices gave negative results. Negative results were found in tests for
genotoxicity in vivo, except in Drosophila injected with furfural.
In a two-year study of carcinogenicity in rats given furfural in
corn oil by gavage at doses of 30 or 60 mg/kg bw per day, bile-duct
hyperplasia and cholangiofibrosis were seen in essentially all rats,
the controls having the highest incidence. Mild hepatocellular
necrosis was seen in all groups, however, at higher rates in males in
all treated groups. Two cholangio-carcinomas were observed in males at
the high dose, but the incidence was not statistically significant.
In a study of carcinogenicity in mice, the combined incidence of
adenomas and carcinomas of the liver (64%) was significantly
(p < 0.01) increased in males at the high dose (175 mg/kg bw per
day) but not in females. Hepatocellular adenomas and carcinomas also
occurred in the controls and in animals at the low (50 mg/kg bw per
day) and intermediate (100 mg/kg bw per day) doses, at incidences of
34-44% in males and 6-14% in females. The incidences in historical
controls were 38% (14-50%) in males and 6.2% (0-30%) in females.
Hepatotoxicity, manifested by features such as focal and multifocal
necrosis and chronic inflammation, was seen in all groups, including
the controls, but was considerably more frequent in mice at the high
dose.
Studies of oncogene activation in samples of liver tumours from
treated mice in the study of carcinogenicity revealed some differences
in the pattern of mutations from those in liver tumours of controls.
As it was not possible to identify the animals from which the tumours
originated and since hepatoxicity was seen at the intermediate and
high doses, it was not possible to determine whether a direct
genotoxic event or a secondary genotoxic pathway is involved.
5. EVALUATION
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL was identified for hepatotoxicity
in rats, the Committee was unable to allocate an ADI. Before reviewing
the substance again, the Committee would wish to review the results of
studies of DNA binding or adduct formation in vivo to clarify
whether furfural interacts with DNA in mice and of a 90-day study of
toxicity in rats to identify a NOEL for hepatotoxicity.
6. REFERENCES
Aeschbacher, H., Wolleb, U., Löliger, J., Spadone, J.C. & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Boyland, E. (1940) Experiments on the chemotherapy of cancer. 4.
Further experiments with aldehydes and their derivatives. Biochem.
J., 34, 1196-1201.
Brabec, M.J. (1981) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, Third
revised Ed., New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Caldwell, J. (1982) Conjugation reactions in foreign-compound
metabolism: Definition, consequences, and species variations.
J. Drug Metab. Rev., 13, 745-777.
Castellino, N., Elmino, O. & Rozera, G. (1963) Experimental research
on the toxicity of furfural. Arch. Environ. Health, 7, 574-582.
Dillon, D.M., McGregor, D.B., Combes, R.D. & Zeiger, E. (1992)
Detection of mutagenicity in Salmonella of some aldehydes and
peroxides. Environ. Mol. Mutag. 17, 15.
Donahue, T.M., Tuma, D.J. & Sorrell, M.F. (1983) Acetaldehyde adducts
with proteins: Binding of [14C]acetaldehyde to serum albumin.
Arch. Biochem. Biophys., 220, 239-246.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Clayton, G.D. & Clayton, F.E.,
eds, Patty's Industrial Hygiene and Toxicology, Third revised Ed.,
New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Eder, E., Deininger, C. & Muth, D. (1991) Genotoxicity of
p-nitrocinnamaldehyde and related alpha,ß-unsaturated carbonyl
compounds in two bacterial assays. Mutagenesis, 6, 261-269.
Feron, V.J. (1972) Respiratory tract tumors in hamsters after
intratracheal instillations of benzo(a)pyrene alone and with furfural.
Cancer Res., 32, 28-36.
Feron, V.J. & Kruysse, A. (1978) Effects of exposure to furfural
vapour in hamsters simultaneously treated with benzo(a)pyrene or
diethylnitrosamine. Toxicology, 11, 127-144.
Flek, J. & Sedivec, V. (1978) The absorption, metabolism and excretion
of furfural in man. Arch. Occup. Environ. Health, 41, 159-168.
Friedmann, E. (1911) [Fate of furanacrylic acid and furoyl acid in the
animal body.] Biochem. Z., 35, 40-48 (in German).
Gaines, K.C., Salany, J.M., Tuma, D.J. & Sorrell, M.F. (1977) Reaction
of acetaldehyde with human erythrocyte membrane proteins. FEBS
Lett., 75, 115-119.
Gomez-Arroyo, S. & Souza, V.S. (1985) In vitro and occupational
induction of sister-chromatid exchanges in human lymphocytes with
furfuryl alcohol and furfural. Mutat. Res., 156, 233-238.
Gregus, Z., Fekete, T., Varga, F. & Klaassen, C.D. (1993) Dependence
of glycine conjugation on availability of glycine: Role of the glycine
cleavage system. Xenobiotica, 23, 141-153.
Gupta, G.D., Misra, A. & Agarwal, D.K. (1991) Inhalation toxicity of
furfural vapours: An assessment of biochemical response in rat lungs.
J. Appl. Toxicol., 11, 343-347.
Hadi, S.M., Rehman, S. & Rehman, A. (1989) Specificity of the
interaction of furfural with DNA. Mutat. Res., 225, 101-106.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Jonek, J.J., Konecki, J. & Kaminski, M. (1975) Histoenzymatic changes
in liver in acute poisoning with furfural. J. Morphol. Embryol., 21,
47-51.
Kaminska, O. (1977) Morphological and histochemical investigations of
liver and kidneys in different stage of furfural poisoning. Folio
Histochem. Cytochem., 15, 150-151.
Kato, F., Araki, A., Nozaki, K. & Matsushima, T. (1989) Mutagenicity
of aldehydes and diketones. Mutat. Res., 216, 366.
Khan, Q.A. & Hadi, S.M. (1993) Effect of furfural on plasmid DNA.
Biochem. Mol. Biol. Int., 29, 1153-1160.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxy-carbonyl compounds against
mutagenic heterocyclic amines. Mutat. Res., 177, 9-15.
Koenig, K. & Andreesen, J. R. (1990) Xanthine dehydrogenase and
2-furoyl-coenzyme A dehydrogenase from Pseudomonas p. Fu1; two
molybenum-containing dehydrogenases of novel structural composition.
J. Bacteriol., 172, 5999-6009.
Kruysse, A. (1972). Acute inhalation toxicity of furfural in hamsters.
Unpublished report No. R 3853 from Central Institute for Nutrition and
Food Research TNO, Zeist, Netherlands.
Kuznetsov, P.I. (1966) Toxicological characteristics of furfural. II.
Effect of furfural on certain functions of the animal under conditions
of sub-acute poisoning. Nauch. Trud. Omskii Med. Inst. Imeni M.I.
Kalinina, 69, 41-44.
Lake, B.G., Adams, T.B., Beamad, J.A., Price, R.J., Ford, R.A. &
Goodman, J.I. (1998) An investigation of the effect of furfural on the
unscheduled DNA synthesis in cultured human liver slices. Unpublished
report to the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Loquet, C., Toussant, G. & LeTalaer, J.Y. (1981) Studies on mutagenic
constituents of apple brandy and various alcoholic beverages collected
in western France, a high incidence area for oesophageal cancer.
Mutat. Res., 88, 155-164.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148,
25-34.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D. &
Caspary, W.J. (1988) Responses of the L5178Y tk+/- mouse lymphoma
cell forward mutation assay. II: 18 coded chemicals. Environ. Mol.
Mutag., 11, 91-118.
McMahon, R.E., Cline, J.C. & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39, 682-693.
Mellick, P.W., Leach, C.L., Chou, B.J., Irwin, R.D. & Roycroft, J.H.
(1991) Nasal toxicity in Fischer 344 rats inhaling furfuryl alcohol
for 2 or 13 weeks. Toxicologist, 11, 183.
Mishra, A., Dwivedi, P.D., Verma, A.S., Sinha, M., Mishra, J., Lal,
K., Pandya, K.P. & Dutta, K.K. (1991) Pathological and biochemical
alterations induced by inhalation of furfural vapor in rat lung.
J. Bull. Environ. Contam. Toxicol., 47, 668-674.
Moreno, O. (1976) Dermal toxicity in rabbits. Report No. MB 75-941
from MB Research Laboratories Inc. Submitted to WHO by the Flavor &
Extract Manufacturers' Association of the United States, Washington
DC, United States
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Trainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from
the testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nagasawa, H.T., Goon, D.J.W., Conastotino, N.V. & Alexander, C.S.
(1980) Diversion of ethanol metabolism by sulfhydryl amino acids.
D-Penicillamine-directed excretion of
2,5,5-trimethyl-D-thiazolidine-4-carboxylic acid in the urine of rats
after ethanol administration. Life Sci., 17, 707-714.
Nakamura, S.-I., Oda, Y., Shimada, T., Oki, I. & Sugimoto, K. (1987)
SOS-inducing activity of chemical carcinogens and mutagens in
Salmonella typhimurium TA1535/pSK1002: Examination with 151
chemicals. Mutat. Res., 192, 239-246.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
National Toxicology Program (1987) Studies of chemical disposition in
mammals: The effect of dose on the chemical disposition of furfural in
rats after oral administration. NIEHS Contract No. N01-ES-66138 to
Arthur D. Little, Inc, Research Triangle Park, North Carolina, United
States.
National Toxicology Program (1990) Toxicology and carcinogenesis
studies of furfural (CAS No. 98-01-1) in Fischer 344/N rats and
B6C3F1 mice (gavage studies). National Toxicology Program Technical
Report Series No. 382. Research Triangle Park, North Carolina, United
States.
Nomeir, A.A., Silveria, D.M., McComish, M.F. & Chadwick, M. (1992)
Comparative metabolism and disposition of furfural and furfuryl
alcohol in rats. Drug Metab. Disposition, 20, 198-204.
Nomura, F. & Lieber,C.S. (1981) Binding of acetaldehyde to rat liver
microsomes: Enhancement after chronic alcohol consumption. Biochem.
Biophys. Res. Commun., 100, 131-137.
Nutley, B.P., Farmer, P. & Caldwell, J. (1994) Metabolism of
trans-cinnamic acid in the rat and the mouse and its variation with
dose. J. Food Chem. Toxicol., 32, 877-886.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Agric.
Biol. Chem., 45, 2299-2304.
Parkash M.K. & Caldwell J. (1994) Metabolism and excretion of
[14C]-furfural in the rat and the mouse. Food Chem. Toxicol., 32,
887-895.
Paul, H.E., Austin, F.L., Paul, M.F. & Ells, U.R. (1949) Metabolism of
the nitrofurans. I. Ultraviolet absorption studies of the urinary
products after oral administration. J. Biol. Chem., 180, 345-363.
Peterson, C.M. & Nguyen, L.B. (1985) Clinical implications of
acetaldehyde adducts with hemoglobin. In: Aldehyde Adducts in
Alcoholism, pp. 19-30.
Phillips, B.J., Jackson, L.I., Tate, B., Price, R.J., Adams, T.B.,
Ford, R.A., Goodman, J.I. & Lake, B.J. (1997) Furfural does not induce
unscheduled DNA synthesis (UDS) in the in vivo rat hepatocyte DNA
repair assay (Abstract). In Proceedings of the Society of Toxicology
Annual Meeting, 1997, Cincinnati, Ohio, New York, Academic Press.
Pickett, C.B. & Lu, A.Y.H. (1988) The structure, genetics and
regulation of soluble glutathione S-transferases. In: Sies, H. &
Ketterer, B., eds, Glutathione Conjugation: Mechanisms and
Biological Significance, New York, Academic Press, p. 147. A
Ramsdell, H.S. & Eaton, D.L. (1990) Species susceptibility to
aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal
biotransformation Cancer Res., 50, 615.
Ravindranath, A. & Boyd, M.R. (1985) Metabolic activiation of
2-methylfuran by rat microsomal systems. Toxicol. Appl. Pharmacol.,
78, 370-376.
Reynolds, S.H., Stowers, S.J., Patterson, R.M., Maronpot, R.R.,
Aaronson, S.A. & Anderson, M.W. (1987) Activated oncogenes in B6C3F1
mouse liver tumors: Implications for risk assessment. Science, 237,
1309-1316.
Rice, W.W. (1972) Furfural: Exogenous precursor of certain urinary
furans and possible toxicologic agent in humans. Clin. Chem., 18,
1550-1551.
Rodriguez-Arnaiz, R., Morales, P.R. & Zimmering, S. (1992) Evaluation
in Drosophila melanogaster of the mutagenic potential of furfural in
the mei-9(a) test for chromosome loss in germ-line cells. Mutat.
Res., 280, 75-80.
Rodriguez-Arnaiz, R., Morales, P.R., Moctezuma, R.V. & Salas, R.M.B.
(1989) Evidence for the absence of mutagenic activity of furfuryl
alcohol in tests of germ cells in Drosophila melanogaster. Mutat.
Res., 223, 309-311.
Sasaki, Y. & Endo, R. (1978) Mutagenicity of aldehydes in
Salmonella. Mutat. Res., 54, 251-252.
Schafer, E.W., Bowles, W.A. & Hurlbut, J. (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch. Environ. Contam.
Toxicol., 12, 355-382.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102, and TA104. Environ. Mol. Mutag., 11,
195-206.
Shimizu, A. (1986) Experimental studies on hepatic cirrhosis and
hepatocarcinogenesis. II. Influence of cirrhotic liver on 2-FAA
hepatocarcinogenesis in rats. Acta Pathol Jpn., 36,1039-1048.
Shimizu, A. & Kanisawa, M. (1986) Experimental studies on hepatic
cirrhosis and hepatocarcinogenesis. I. Production of hepatic cirrhosis
by furfural administration. Acta Pathol. Jpn., 36, 1027-1038.
Shimizu, A., Yoshiyasu, N., Harada, M., Ono, T., Sato, K., Inoue, T. &
Kanisawa, M. (1989) Positive foci of glutathione S-transferase
placental form in the liver of rats given furfural by oral
administration. Jpn. J. Cancer Res., 80, 608-611.
Shinohara, K., Kim, E. & Omura, H. (1986) Furans as the mutagens
formed by amino-carbonyl reactions. In: Fujimaki, M., Namiki, M. &
Kato, H., eds, Amino-Carbonyl Reactions in Food and Biological
Systems, New York, Elsevier.
Stich, H.F., Rosin, M.P., Wu, C.H. & Powrie, W.D. (1981a)
Clastogenicity of furans found in food. Cancer Lett., 13, 89-95.
Stich, H.F., Rosin, M.P., San, R.H., Wu, C.H. & Powrie, W.D. (1981b)
Intake, formation, and release of mutagens by man. In:
Gastrointestinal Cancer (Banbury Report 7), Cold Spring Harbor, NY,
CSH Press, pp. 247-266.
Stofberg J. & Grundschober F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Subramanyam, S., Sailaja, D. & Rathnaprabha, D. (1989) Genotoxic assay
of two dietary furans by some in vivo cytogenetic parameters.
Environ. Mol. Mutag., 14, 1-252.
Til, H.P., Woutersen, R.A., Feron, V.J., Hollanders, V.H.M., Falke,
H.E. & Clary, J.J. (1989) Two-year drinking-water study of
formaldehyde in rats. Food Chem. Toxicol., 27, 77-78.
Tiunov, L.A., Vasilev, G.A. & Ivanova, L.V. (1970) Effect of frequency
of injection on the toxic action of furfural. Bull. Exp. Biol.
Med., 70, 999-1000.
Tuma, D.J., Donahue, T.M., Medina, V.A. & Sorrell, M.F. (1984)
Enhancement of acetaldehyde-protein adduct formation by L-ascorbate.
Arch. Biochem. Biophys., 234, 377-381.
Videlia, L.A., Fernandez, V., deMarinis, A., Fernandez, N. &
Valenzuala, A. (1982) Liver lipoperoxidative pressure and glutathione
status following acetaldehyde and aliphatic alcohols pretreatments in
the rat. Biochem. Biophys. Res. Commun., 104, 965-970.
US Environmental Protection Agency (1997) Summary report: A dose range
finding development toxicity study of furfural in rats (TSCA
submission 8EHQ-1097-14026), Washington DC.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
Zdzienicka, M., Tudek, B., Zielenska, M. & Szymczyk, T. (1978)
Mutagenic activity of furfural in Salmonella typhimurium TA100.
Mutat. Res., 58, 205-209.
 In animals, the condensation reaction of 2-furoyl CoA with acetyl
CoA to yield furanacryloyl CoA is reversible, favouring formation of
2-furoyl CoA (Parkash & Caldwell, 1994). The observation that furoic
acid is excreted in the urine of dogs given furanacrylic acid
(Friedmann, 1911) supports this conclusion. Analogous reversible
reactions between the CoA thioesters of benzoic acid and cinnamic acid
in vivo favour benzoic acid (Nutley et al., 1994). Excretion of
unconjugated furoic acid and furanacrylic acid at higher doses
suggests that glycine conjugation in laboratory animals is limited,
probably by the supply of endogenous glycine (Gregus et al., 1993). As
mentioned earlier, the principal metabolite in rodents, furoic acid,
may also be metabolized to a very minor extent via oxidation of the
heteroaromatic ring. Since heteroaromatic and aromatic carboxylic
acids do not normally undergo decarboxylation in vivo (Caldwell,
1982), it can be assumed that oxidation of the furan ring system of
furoic acid precedes the loss of carbon dioxide. On the basis of this
assumption, epoxidation (Ramsdell & Eaton, 1990) or hydroxylation
(Ravindranath & Boyd, 1985; Koenig & Andreesen, 1990) of the furan
ring may yield reactive intermediates (e.g. furfural-2,3-epoxide,
acetylacrolein, and alpha-ketoglutaric acid) which readily undergo
decarboxylation. Biochemical changes in the lungs and livers of
animals exposed to furfural indicate that ring oxidation may be
catalysed by a cytochrome P450b isoenzyme, yielding an intermediate
which subsequently conjugates with glutathione (Gupta et al., 1991;
Mishra et al., 1991).
Humans
As in laboratory animals, the predominant pathway for
detoxification of furfural in humans is oxidation of the aldehyde to
yield furoic acid, which either conjugates with amino acids or
condenses with acetyl CoA to produce furanacrylic acid.
After inhalation or dermal absorption, furfural is efficiently
and rapidly absorbed ithrough the lungs and skin, with 20-30% of the
amount absorbed by the lungs (Flek & Sedivec, 1978). When six
volunteers were exposed to 30 mg/m3 for 8 h, the mean pulmonary
retention was 78% (range, 75-82%). Retention was independent of the
concentration of furfural vapour and the length of exposure. An
average 8-h exposure maximum was equivalent to a dose of 1.9 mg/kg bw.
The biological half-life of absorbed furfural in humans is only about
2 h. Essentially all of the absorbed furfural could be accounted for:
97% (range, 93-100%) was oxidized to 2-furoic acid and excreted as the
glycine conjugate, 0.5-5% was excreted as furanacrylic acid, and less
than 1% was exhaled unchanged.
2.1.2 Aldehyde reactivity
Furfural contains a heteroaromatic furan ring with a reactive
aldehyde functional group at the 2 position. The reactivity of the
aldehyde function of aldehydes of low molecular mass, like furfural,
suggests that such compounds are not absorbed intact at doses that do
not saturate the oxidation or condensation reactions associated with
the aldehyde function in digestive fluids. Likewise, when furfural
reaches the body fluids, it is likely to react before entering the
cell. The reactivity of the aldehyde group has been demonstrated, for
example, for acetaldehyde and formaldehyde, both normal metabolic
intermediates, which may have toxic effects including the induction of
tumours when administered under non-physiological conditions at high
doses (Til et al., 1989).
Aldehydes of low molecular mass have been reported to bind with
soluble proteins, protein components of cell membranes, and
thiol-containing molecules to form unstable and stable adducts.
Formation of adducts with albumin (Nagasawa et al., 1980; Donahue et
al., 1983; Tuma et al., 1984), haemoglobin (Peterson & Nguyen, 1985),
and erythrocyte membranes (Gaines et al., 1977) has been demonstrated
in vitro and in vivo. In membranes, binding is usually with the
protein component (Nomura & Lieber, 1981). Aldehydes also bind
glutathione (Videlia et al., 1982). Aldehyde dehydrogenase-catalysed
oxidation of low-molecular-mass aldehydes requires glutathione
(Eckfeldt & Yonetani, 1982), suggesting that free aldehyde is rapidly
conjugated with glutathione in vivo to form a thiohemiacetal which
is subsequently oxidized to the corresponding acid (Brabec, 1981).
Thus, the reactivity of the aldehyde function will significantly
curtail the intracellular concentration of free aldehyde.
When an aldehyde is present in a cell, it undergoes rapid
oxidation, with conjugation of the resulting carboxylic acid (Gregus
et al., 1993). The various metabolic processes, i.e. oxidation,
conjugation, and condensation (see section 2.1.1) effectively
eliminate the reactive aldehyde functional group when not saturated by
high, non-physiological doses.
2.2 Toxicological studies
2.2.1 Acute toxicity
On the basis of the oral LD50 values in various species
(Table 1), furfural is acutely toxic at lower doses in rats than in
mice.
Wistar/Slc rats given a single dose of 50 mg/kg bw furfural by
gavage had sporadic eosinophilic degeneration of hepatic cells with
nuclear pyknosis and eosinophilic necrosis and increased hepatocyte
mitosis. No massive or zonal necrosis was observed. Since the control
rats were killed at the beginning of the experiment, no appropriate
controls were available (Shimizu & Kanisawa, 1986). Decreased
activities of succinic dehydrogenase and ATPase and increased
activities of acid phosphatase and acid DNase II were reported in male
Wistar rats injected intraperitoneally with 20 or 50 mg/kg bw (Jonek
et al., 1975).
Table 1. Acute toxicity of furfural
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Rat Oral 127 Jenner et al. (1964)
Rat Intraperitoneal 120 Tiunov et al. (1970)
Mouse Oral 333 Boyland (1940)
Mouse Subcutaneous 200 Tiunov et al. (1970)
Mouse Subcutaneous 223 (1-day survival) Castellino et al.(1963)
119 (10-day survival)
Rabbit Dermal > 310 Moreno (1976)
Hamster Inhalation 2500 Kruysse (1972)
Bird Oral > 98 Schafer et al. (1983)
2.2.2 Short-term studies of toxicity
Rats
In rats receiving 0.05, 2.5, or 25 mg/kg bw per day furfural for
35 days, no hepatic impairment was seen (Kuznetsov, 1966). When male
rats were injected intraperitoneally with furfural in increasing doses
from 29 to 58 mg/kg bw for 30 days, damage and increased intracellular
catabolic processes were seen in liver and kidney cells (Kaminska,
1977).
In a 16-day study, groups of five male and five female Fischer
344/N rats were given furfural at doses of 0, 15, 30, 60, 120, or
240 mg/kg bw per day in corn oil by gavage, five days per week for a
total of 12 doses. At the end of the study, the surviving animals were
killed, necropsied, and examined histopathologically. No
treatment-related abnormalities were found. Eight of ten rats at the
highest dose had died by the third day due to gavaging accidents.
Animals at 120 mg/kg bw appeared to be less active. The body weights
of rats given doses < 120 mg/kg bw per day did not differ
significantly from those of controls.
In a 16-day study, groups of five male and five female B6C3F1
mice were given furfural at doses of 0, 25, 50, 100, 200, or 400 mg/kg
bw per day in corn oil by gavage on five days per week for a total of
12 doses. At the end of the study, the surviving animals were killed,
necropsied, and examined histopathologically. The mean body weights of
the treated mice did not differ significantly from those of controls.
One male at the highest dose had died by day five, and two female mice
died from gavaging accidents. No treatment-related abnormalities were
found.
In a 13-week study, groups of 10 male and 10 female B6C3F1 mice
were given furfural at doses of 0, 75, 150, 300, 600, or 1200 mg/kg bw
per day in corn oil by gavage on five days per week. Necropsies were
performed on all survivors, and histopathological examinations were
performed on those at the two highest doses and the controls. All the
mice at the two highest doses died before the end of the study, except
for one male and one female at 600 mg/kg bw per day. The body weights
of the males treated with doses > 150 mg/kg bw per day were
slightly lower than those of controls. Female mice at 75-300 mg/kg bw
per day and male mice at 300 mg/kg bw per day showed significantly
increased relative liver weights as compared with controls.
Centrilobular coagulative necrosis of hepatocytes was observed in most
male mice at the two highest doses and in one male per group treated
with 150 or 300 mg/kg bw per day. Among the females, only two at the
highest dose showed similar lesions. Animals with coagulative necrosis
also had inflammation of the liver, with minimal to mild mononuclear
inflammatory cell infiltrate (National Toxicology Program, 1990).
A group of 48 male, six-week-old Wistar/Slc rats was fed furfural
at a concentration of 20 ml/kg diet (equivalent to approximately 1200
mg/kg bw per day) for one week, 30 ml/kg diet for one week, and then
40 ml/kg diet on days 15-90; on days 90-120, the rats were given the
diet containing 40 ml/kg for only five days per week to prevent
reduced weight gain. The concentration of furfural in the feed was not
analysed after preparation of the diet. A control group of 40 male
rats of the same age was fed the basal diet.
Treated rats sacrificed after 90 days had marked
cholangiofibrosis, with areas of increased density containing red
nodules. The nodules showed fibrous widening of Glisson's sheath,
bile-duct proliferation, and destruction of the limiting plates. The
parenchymal damage consisted of bridging necrosis and hydropic
degeneration of hepatocytes. The parenchyma often showed no cirrhotic
changes in areas other than those with fibrosis, suggesting a
regenerative process in the liver. Increased numbers of cells
undergoing mitosis were seen. In rats killed on day 120 of the study,
similar but more marked findings were reported. The incidence of
hepatic fibrosis was increased in the furfural-treated rats. No
atypical hepatocyte growth was noted. Furfural did not cause
hepatocellular hyperplastic changes (Shimizu & Kanisawa, 1986).
In a study designed to examine the mechanism of furfural-induced
hepatocytic injury, six groups of six male Wistar/Slc rats were
maintained on diets containing furfural at doses calculated to result
in average daily intakes of 20 ml/kg diet (approximately 1200 mg/kg bw
per day) for the first 30 days and 30 ml/kg diet (approximately
1700 mg/kg bw per day) thereafter. Each group also included four
control animals. The furfural-containing diets were replaced after 15,
30, 60, 90, 120, or 150 days, and animals were killed 14 days after
termination of furfural administration. Increased duration of exposure
to furfural was accompanied by increased numbers and size of foci
positive for the placental form of glutathione- S-transferase in
hepatocytes (Shimizu et al., 1989). Such foci have been reported to be
a marker for early detection of preneoplastic or neoplastic cells
(Pickett & Lu, 1988).
In a 13-week study, groups of 10 male and 10 female Fischer 344/N
rats were given furfural at doses of 0, 11, 22, 45, 90, or 180 mg/kg
bw per day in corn oil by gavage on five days per week. All survivors
were necropsied, and histopathological examinations were performed on
those at the two highest doses and the controls. Only one male rat at
the highest dose survived to the end of the study; four of the deaths
were considered to be related to gavage, as were two deaths among
controls and four among rats at the second highest dose. The mean body
weights of treated rats did not differ from those of controls. The
relative and absolute weights of the livers and kidneys of males given
90 and 180 mg/kg bw per day were significantly higher than those of
controls. An increased incidence of cytoplasmic vacuolization in
hepatocytes was observed in all groups of treated males: control,
4/10; 11 mg/kg bw per day, 10/10; 22 mg/kg bw per day, 10/10; 45 mg/kg
bw per day, 10/10; 90 mg/kg bw per day, 9/10. No lesions were reported
in the kidneys of these rats (National Toxicology Program, 1990).
Hamsters
Groups of 10 Syrian golden hamsters of each sex were exposed to
furfural vapour at 0, 77, 448, or 2165 mg/m3 for 6 h per day, five
days per week over 13 weeks. The main findings were mild growth
retardation, irritation of the eyes and nose, and hyperplastic atrophy
of the nasal epithelium, all at the highest dose. The NOEL was 77
mg/m3, since mild nasal epithelial degeneration was observed at 448
mg/m3 (Feron & Kruysse, 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a two-year study, 50 male and 50 female B6C3F1 mice were
given furfural at doses of 0, 50, 100, or 175 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed weekly
for the first 13 weeks and monthly thereafter until the conclusion of
the study, when surviving animals were killed. All animals were
necropsied and examined histologically. Furfural had no significant
effect on body weight or survival. Microscopic examination revealed
lesions in the liver, kidneys, and forestomach. In the liver,
pigmentation, necrosis, and chronic inflammation were seen in males at
the intermediate and high doses and in females at the high dose (Table
2). Hepatocellular adenomas and carcinomas were seen in all groups,
including controls, and the increased incidence was statistically
significant only in males at the high dose. Carcinomas occurred at the
same incidence in females at the high dose as in controls.
Table 2. Incidences of liver lesions and hepatocellular adenomas and carcinomas in male and
female B6C3F1 mice given furfural by gavage
Lesion Dose (mg/kg bw)
0 50 100 175
M F M F M F M F
Pigmentation 0/50 0/50 0/50 0/50 8/49 0/50 18/50 11/50
Necrosis, focal and multifocal 3/50 2/50 2/50 1/50 7/49 3/50 10/50 2/50
Chronic Inflammation 0/50 0/50 0/50 0/50 8/49 1/50 18/50 8/50
Hepatocellular adenoma 9/50 1/50 13/50 3/50 11/49 5/50 19/50 8/50
Hepatocellular carcinoma 7/50 4/50 12/50 0/50 6/49 2/50 21/50 4/50
Combined rates 16/50 5/50 22/50 3/50 17/49 7/50 32/50 12/50
From National Toxicology Program (1990)
M, male; F, female
Hyperplasia of the renal tubules was found in one male at the low
dose. One male at the intermediate dose, one at the high dose, and one
female at the low dose had a cortical adenoma, and one male at the low
dose had a cortical carcinoma. Owing to the low incidence and lack of
dose-response relationship, these tumours were not considered as
evidence for carcino-genicity. Marginal increases in the incidences of
hyperplasia and papillomas in the forestomach of female mice were
considered to be of no biological significance and were probably
related to the irritating effects of chronic administration of the
compound by gavage or the method of gavage itself. None of the animals
showed evidence of malignant forestomach lesions (National Toxicology
Program, 1990).
Rats
In a two-year study, 50 male and 50 female Fischer 344/N rats
were given furfural at doses of 0, 30, or 60 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed at
specific intervals, and the survivors were killed at the end of the
study, necropsied, and examined histologically. Overall survival was
slightly reduced for female rats at the highest dose (78% at week 97),
due to accidental gavage-related deaths. Furfural had no effect on
body weight or survival. Congestion and foreign bodies were observed
in a dose-related manner in the lungs of female rats. A small increase
in the incidence of squamous-cell carcinomas and papillomas in the
forestomachs of treated rats was observed but was considered unrelated
to treatment. Mild centrilobular hepatocellular necrosis was observed
in all groups (vehicle control: males, 3/50; females, 10/50; low dose:
males, 9/50; females, 9/50; high dose: males, 12/50; females, 4/50).
Bile-duct hyperplasia occurred in 45/50 control males, 36/50 control
females; 41/50 males and 28/50 females at the low dose; and 44/50
males and 24/50 females at the high dose. Focal bile-duct dysplasia
was noted in one male at the intermediate dose, and bile-duct
hyperplasia accompanied by fibrosis (cholangiofibrosis) was seen in
two males at the high dose. One control male had a hepatocellular
adenoma, and two males at the high dose had a cholangiocarcinoma. The
incidence of the latter lesion was not statistically significant, but
some evidence of carcinogenicity was consluded since
cholangiocarcinomas were rare in historical controls (3/2145)
(National Toxicology Program, 1990).
2.2.3.1 Special studies of carcinogenicity
The aldehydes citral and heptaldehyde inhibit the growth of
spontaneous mammary tumours in mice; however, furfural and its
furfuracrylic acid metabolite at a daily dose of 2.5 mg had no effect
on the growth of spontaneous mammary tumours in mice, although they
were weakly active against sarcomata (Boyland, 1940).
Studies have been designed to evaluate the effect of furfural on
the carcinogenic potential of known carcinogens. In the first study,
three groups of 35 male and 35 female Syrian golden hamsters were
provided with 0.2 ml of 1.5% furfural in physiological saline (about
25 mg/kg bw), 0.5% benzo [a]pyrene (8.3 mg/kg bw), or 1.5% furfural
plus 0.5% benzo [a]pyrene intratracheally once weekly for 36 weeks.
Furfural in combination with benzo [a]pyrene led to earlier
development of hyper- and metaplastic changes in the tracheobronchial
epithelium, a shorter latency for tracheobronchial tumours, a few more
bronchial and peripheral squamous-cell carcinomas, and a substantially
reduced number of tracheal squamous-cell carcinomas. The incidence of
peritracheal sarcomas was increased, which may indicate a
co-carcinogenic effect of furfural. Irritation but no tumorigenic
effect was observed after administration of furfural alone (Feron,
1972).
In a subsequent study, groups of 126 Syrian golden hamsters of
each sex were exposed to atmospheres containing 400 ppm (1600 mg/m3)
furfural for 7 h per day, five days per week for nine weeks, then 330
ppm (1300 mg/m3) for 11 weeks, and 250 ppm (970 mg/m3) for an
additional 32 weeks. The effects on the respiratory tract included
atrophy and downward growth of the olfactory epithelium, degenerative
changes in Bowman's glands, and the appearance of cyst-like structures
in the lamina propria beneath the olfactory epithelium. Although
furfural was irritating to the olfactory epithelium, treatment did not
result in hepatic toxicity or carcinogenicity. Furfural did not
potentiate the carcinogenic effect of benzo [a]pyrene or
N-nitrosodiethylamine (Feron & Kruysse, 1978).
Localized irritation of the nasal cavity and respiratory tract,
with no hepatic effects, were also observed in B6C3F1 mice and
Fischer 344/N rats exposed to atmospheres containing 0, 2, 4, 8, 16,
or 32 ppm of the metabolic precursor furfuryl alcohol for 13 weeks
(Mellick et al., 1991).
SPF Wistar rats were given furfural at doses of 20 ml
(approximately equal to 1200 mg/kg bw per day) for one week, 30 ml for
the second week, and 40 ml for the following 10 weeks to produce
hepatic cirrhosis. Half of the remaining rats in this study (38
treated and 32 controls) were fed a diet containing 0.03%
N-2-fluorenylacetamide for three weeks, followed by one week of
normal diet; the other half received only the normal diet. All
surviving rats were killed 12 weeks later, and their livers were
examined. Rats that had received furfural only had no hyperplastic
changes in the liver but did have cirrhosis. Rats that had been
treated with N-2-fluorenylacetamide developed multiple hyperplastic
nodules which stained for alpha-fetoprotein, and this response was
markedly potentiated in the rats previously treated with furfural. The
authors concluded that furfural-induced hepatic cirrhosis increases
the susceptibility of rats to potent hepatocarcinogens (Shimizu,
1986).
Samples of mouse liver tumours from the National Toxicology
Program carcinogenesis bioassay programme were assessed for
transforming gene activity by Southern blot analysis for H-, K-, and
N- ras oncogens. The pattern of mutations in the oncogenes from liver
tumours that occurred spontaneously differed from that which occurred
in some furfural-treated animals: The activated ras genes were
H- ras in 15 of 17 spontaneous tumours, with one raf and one
unknown oncogene; activating point mutations occurred at codon 61 in
six tumours, codon 13 in two tumours, and codon 117 in one tumour. The
authors concluded that the spectrum of activating mutations in the
H- ras gene and the pattern of ras gene activation in liver tumours
derived from furfural-treated mice differed from those in liver
tumours derived from untreated animals, and indicates a direct
genotoxic effect of furfural. An alternative mechanism-induction of
mutations by an indirect, secondary genotoxic pathway as a result of a
cytotoxic event -- was mentioned but excluded by the authors on the
basis of the absence of cytotoxic lesions in the livers of similar
mice after 90 days of treatment with the same dose of furfural. The
authors do not mention, however, that necrosis, pigmentation, and
chronic inflammation were seen regularly in mice at the intermediate
and high doses in the carcinogenicity experiment (see Table 2). It can
be concluded that the effect was either due to a direct genotoxic
event or occurred via an indirect, secondary genotoxic pathway
(Reynolds et al., 1987).
2.2.4 Genotoxicity
Studies of the genotoxicity of furfural are summarized in
Table 3. Testing of furfural for mutagenicity in vitro at
concentrations up to those causing cytotoxicity provided no evidence
that it is mutagenic to Salmonella typhimurium strains TA98, TA102,
TA1535, or TA1537 or ito two strains of Escherichia coli, with or
without metabolic activation (Sasaki & Endo, 1978; Zdzienicka et al.,
1978; McMahon et al., 1979; Loquet et al., 1981; Marnett et al., 1985;
Mortelmans et al., 1986; Shinohara et al., 1986; Nakamura et al.,
1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al., 1989;
National Toxicology Program, 1990; Dillon et al., 1992). The majority
of these studies also showed negative results for S. typhimurium
TA104, although a single positive result was reported (Shane et al.,
1988). Furfural was not mutagenic when incubated with
S. typhimurium TA100 in most reports (Sasaki & Endo, 1978; McMahon
et al., 1979; Mortelmans et al., 1986; Shinohara et al., 1986; Kim et
al., 1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al.,
1989; National Toxicology Program, 1990; Eder et al., 1991; Dillon et
al., 1992) but was weakly mutagenic in other studies (Zdzienicka et
al., 1978; Loquet et al., 1981; Shane et al., 1988). When fufural was
co-incubated with benzo [a]pyrene and S. typhimurium TA100, it did
not alter the mutagenic activity of benzo [a]pyrene in this strain
(Zdzienicka et al., 1978). Furfural showed no mutagenic potential in
two rec assays with Bacillus subtilis by a direct streaking method
(Osawa & Namiki, 1982; Matsui et al., 1989) but also gave positive
results in this test system (Shinohara et al., 1986).
Table 3. Results of assays for the genotoxicity of furfural
End-point Test object Concentration Result Reference
In vitro
Reverse mutation S. typhimurium TA100 NR Negative Eder et al. (1991)
Reverse mutation S. typhimurium TA100, TA102, and NR Negativea Dillon et al. (1992)
TA104
Reverse mutation S. typhimurium TA100, TA98, and < 36 µmol/plate Weakly positive (TA100)b Loquet et al. (1981)
TA1535 60 µmol/plate Negativec
Reverse mutation S. typhimurium TA100, TA98, and < 1.2 mmol/plate Negative Aeschbacher et al. (1989)
TA102
Reverse mutation S. typhimurium TA100 and TA98 0.165-0.660 µmol/plate Negativea Shinohara et al. (1986)
Reverse mutation S. typhimurium TA100, TA102, and 5-500 µg/plate Positive (TA104) Shane et al. (1988)
TA104
Reverse mutation S. typhimurium TA100, TA98, and NR Negativea Kato et al. (1989)
TA104; E. coli WP2uvrA/PKM101
Reverse mutation S. typhimurium TA104 and TA102 1 µmol/plate Negative Marnett et al. (1985)
Reverse mutation S. typhimurium TA98, TA100, < 6667 µg/plate Negativea Mortelmans et al. (1986)
TA1535, and TA1537
Reverse mutation S. typhimurium TA100 and TA98 7 µl/plate Positivea (TA100) Zdzienicka et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, < 20 µl/plate Negativea McMahon et al. (1979)
TA1537, TA1538, TA98, G46;
E. coli WP2uvrA
Reverse mutation S. typhimurium TA100 and TA98 NR Negativea Sasaki & Endo (1978)
Reverse mutation S. typhimurium TA100 4.44 µmol/plate Negativea Kim et al. (1987)
umu gene expression S. typhimurium TA1535/pSK/002 1932 µg/ml Negativea Nakamura et al. (1987)
Reverse mutation S. typhimurium TA100 < 1 mg Negativea Osawa & Namiki (1982)
Reverse mutation S.typhimurium TA98, TA100, 33-6666 µg/plate Negativea National Toxicology Program
TA1535, and TA1537 (1990a)
Reverse mutation S.typhimurium TA98, TA100, and 33-6666 µg/plate Negativea National Toxicology Program
TA1535 (TA100 equivocal) (1990b)
rec Gene mutation B. subtilis H17 and M45 < 1 mg Negativea Osawa & Namiki (1982)
rec Gene mutation B. subtilis H17 and M45 1.7-17 mg/disk Positivea Shinohara et al. (1986)
rec Gene mutation B. subtilis H17 and M45 0.6 ml Negativea Matsui et al. (1989)
Table 3. (continued)
End-point Test object Concentration Result Reference
Chromosomal Chinese hamster ovary cells < 40 mmol/L Positivea Stich (1981a)
aberration
Chromosomal Chinese hamster ovary cells 3 mg/ml Positive Stich (1981b)
aberration
Forward mutation L5178Y tk+/- mouse lymphoma cells 25-400 µg/ml Positiveb McGregor et al. (1988)
Sister chromatid Chinese hamster ovary cells < 1170 µg/ml Positivea National Toxicology Prohgram
exchange/Chromosomal (1990)
aberration
Sister chromatid Human lymphocytes < 0.14 mmol/L Positive Gomez-Arroyo & Souza (1985)
exchange
Unscheduled DNA Human liver slices 0-25 mmol/L Negative Lake et al. (1998)
synthesis
In vivo
Sex-linked recessive D. melanogaster 1000 ppm in diet Negative Woodruff et al. (1985)
lethal mutation
Sex-linked recessive D. melanogaster 100 ppm by injection Positive Woodruff et al. (1985)
lethal mutation
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Negative Rodriguez-Arnaiz et al. (1992)
repair-proficient females < 33% lethal dose
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Positive only after Rodriguez-Arnaiz et al. (1992)
repair-deficient females < 33% lethal dose injection
Sex-chromosome loss D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
injection
Sex-linked recessive D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
lethal mutation injection
Reciprocal D. melanogaster 100 ppm by injection Negative Woodruff et al. (1985)
translocation
Sister chromatid B6C3F1 mouse bone-marrow cells 50-200 mg/kg by Negative National Toxicology Program
exchange/Chromosomal injection (1990)
aberration
Table 3. (continued)
End-point Test object Concentration Result Reference
Somatic chromosomal Swiss albino mouse bone-marrow < 4000 ppm for 5 days Negatived Subramanyam et al. (1989)
mutation cells
Sperm-head Swiss albino mouse < 4000 ppm for 5 Negatived Subramanyam et al. (1989)
abnormalities weeks
Unscheduled DNA Fischer 344 rat hepatocytes 5.0, 16.7, or 50 mg/kg Negative Phillips et al. (1997)
synthesis bw orally
NR, not reported
a With and without metabolic activation
b Without metabolic activation
c With metabolic activation
d Abstract only: reported to have positive effects at 4000 ppm, but no details available
Furfural produced a dose-dependent increase in the frequency of
chromatid breaks and exchanges in Chinese hamster ovary cells with and
without metabolic activation (Stich et al., 1981a,b; National
Toxicology Program, 1990) and induced chromosomal aberration in these
cells at a cytotoxic concentration (National Toxicology Program,
1990). An increased frequency of sister chromatid exchange was
reported in human lymphocytes incubated with furfural at a
concentration of 0.07 or 0.14 mmol/L, but not at 0.035 mmol/L
(Gomez-Arroyo & Souza, 1985). In this study, furfuryl alcohol did not
increase the frequency of sister chromatid exchange at a concentration
of 3.3, 6.6, or 9.9 mmol/L.
Furfural increased trifluorothymidine resistance when incubated
with L5178Y tk+/- mouse lymphoma cells at a concentration of 200 or
400 mg/ml without metabolic activation, but showed no mutagenic
activity at 25, 50, or 100 mg/ml (McGregor et al., 1988).
Concentrations of 400 and 800 mg/ml were cytotoxic in separate trials.
In vivo, furfural did not induce sex-linked recessive lethal
mutations or reciprocal translocations in male Drosophila
melanogaster when incorporated into their diet at 1000 ppm.
Mutations were induced when furfural was administered by injection at
a concentration of 100 ppm, but no reciprocal translocations occurred.
A similar pattern of responses was reported for other simple aldehydes
(e.g. acetaldehyde and trans-cinnamaldehyde) when given via the
intraperitoneal (positive) and oral (negative) routes of
administration (Woodruff et al., 1985). No sex-chromosome loss was
observed when male D. melanogaster were fed or injected with
furfural at concentrations equivalent to 25-33% of the lethal dose of
furfural and then mated with repair-proficient females
(Rodrigues-Arnaiz et al., 1992), but sex-chromosome loss was reported
by these authors when treated males were mated with repair-deficient
females. No sex-linked recessive lethal mutation or sex-chromosome
loss was reported when male D. melanogaster were injected with
furfuryl alcohol (Rodrigues-Arnaiz et al., 1989).
Tests of mammals treated in vivo with furfural have produced
mainly negative results. Furfural did not induce sister chromatid
exchange or chromosomal aberrations in B6C3F1 mouse bone-marrow cells
after intraperitoneal injections of furfural at doses of 50-200 mg/kg
bw (National Toxicology Program, 1990). No genotoxic effects or
spermhead abnormalities were reported in mice given up to 4000 ppm
furfural daily for five weeks, and only the highest dose tested was
associated with an increased frequency of somatic (liver)-cell
chromosomal mutations when given for 24 or 48 h but not after 72 h
(Subramanyam et al., 1989). Furfural administered by gavage to male
Fischer 344/N rats in corn oil at doses of 5, 16.7, or 50 mg/kg bw did
not induce unscheduled DNA synthesis in hepatocytes (Phillips et al.,
1997).
Chromosomal aberrations were not observed in lymphocytes taken
from workers occupationally exposed to furfural or furfuryl alcohol
(Gomez-Arroyo & Souza, 1985).
Liver slices prepared from samples from four human donors were
cultured in medium containing 3H-thymidine and 0-25 mmol/L furfural
for 24 h. In a preliminary experiment, furfural was markedly toxic at
concentrations > 10 mmol/L. As positive controls, liver slices were
also cultured with three known genotoxic agents,
2-acetylaminofluorene, aflatoxin B1, and
2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP). Unscheduled
DNA synthesis was quantified as the changes in mean nuclear and
cytoplasmic grain counts relative to controls, net grain count (i.e.
mean nuclear - mean cytoplasmic grain counts), and the percentage of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains. In
comparison with control liver slice cultures (in dimethylsulfoxide),
those treated with 0.02 or 0.05 mmol/L 2-acetylamino-fluorene, 0.002
or 0.02 mmol/L aflatoxin B1, and 0.003 or 0.05 mmol/L PhIP had
significantly higher net grain counts in centrilobular hepatocytes,
due primarily to large increases in mean nuclear grain counts. The
three genotoxic agents also significantly increased the number of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains.
Treatment with 0.005-0.5 mol/L furfural had no significant effect
on net grain count or mean nuclear or cytoplasmic grain counts. A
small but statistically significant increase in net grain counts
observed at 2, 5, and 10 mmol/L furfural was the result of a
significant concentration-dependent decrease in mean cytoplasmic grain
counts accompanied by a smaller increase in nuclear grain counts.
Furfural had no significant effect on the percentage of hepatocyte
nuclei with > 5 or > 10 net grain counts, except at a concentration
of 2 mmol/L and only in liver cultures in which marked toxicity was
noted at 10 mmol/L. The decreases in mean cytoplasmic and nuclear
grain counts and the correlation between cytotoxicity and changes in
net grains count and nuclei with > 5 net grains support the
conclusion that furfural at 2, 5, or 10 mmol/L induces a cytotoxic
response in cultured human liver slices. In comparison with the three
genotoxins tested, furfural did not induce unscheduled DNA synthesis
in cultured human liver slices (Lake et al., 1998).
Furfural was reported to increase the frequency of single-strand
DNA breaks in isolated calf-thymus duplex DNA (Hadi et al., 1989).
When incubated with plasmid DNA, furfural decreased the transformation
capacity of Escherichia coli host cells; however, the damaged
plasmids were repaired after propagation in the host (Khan & Hadi,
1993).
Thus, negative results were obtained with furfural in most tests
for genotoxicity in vitro. Positive results were reported at
relatively high doses in three of 16 assays for reverse mutation in
S. typhimurium and in one of three assays for gene mutation in
B. subtilis. Weakly positive results were obtained in tests for
chromosomal aberrations at relatively high doses. Positive results
were found in two assays for sister chromatid exchange and one for
forward mutation in mouse lymphoma cells. The results of all tests for
genotoxicity in vivo were negative, except in D. melanogaster
injected with furfural.
2.2.5 Reproductive toxicity
Rats
A summary report of an investigation to define the doses of
furfural for a study of developmental toxicity was submitted to the
Toxic Substances Control Administration of the US Environmental
Protection Agency. Five groups of eight Crl:CD(SD)BR rats received
furfural in water by gavage at doses of 10, 50, 100, 500, or 1000
mg/kg bw per day during gestation days 6-15. Because of excessive
deaths at 500 and 1000 mg/kg bw per day, the breeding phase of the
study was re-initiated to characterize the potential maternal and
developmental toxicity at 150, 250, and 350 mg/kg bw per day. Two
concurrent control groups of eight rats received only the vehicle
(reverse osmosis-treated water) by gastric intubation. Clinical
observations, body weights, and food consumption were recorded. On
gestation day 20, laparohysterectomy was performed on all surviving
animals; the uteri and ovaries were examined, and the numbers of
fetuses, early and late resorptions, total implantation, and corpora
lutea were recorded. The uteri and fetuses were weighed and examined
for external malformations, variations, and sex.
Because of excessive mortality on day 6 after dosing, all females
at 250, 350, 500, and 1000 mg/kg bw per day were killed. The clinical
signs in these animals included lethargy, effects on respiration, and
whole-body tremor. The maternal animals at other doses survived,
except for one female at 150 mg/kg bw per day group, which died within
I h of dosing. The surviving females at 150 mg/kg bw per day had
reduced body-weight gain and reduced food consumption; however, the
mean body weight, net body weight, net body-weight gain, and gravid
uterine weight were unaffected. Body weight and food consumption of
animals at 10, 50, and 100 mg/kg bw per day were unaffected.
Intrauterine growth and survival were not affected at 10, 50, 100, or
150 mg/kg bw per day. The only external malformation noted
(omphalocele) occurred in offspring of rats at 10 mg/kg bw per day. No
developmental variations were noted (US Environmental Protection
Agency, 1997).
3. HUMAN INTAKE
On the basis of the most recently reported annual volumes, 3600
kg in Europe (International Organization of the Flavor Industry, 1995)
and 590 kg in the United States (National Academy of Sciences, 1987),
the estimated daily per capita intake of 'eaters only' of furfural
from use as a flavouring substance is approximately 9 µg/kg bw per day
in Europe and 2 µg/kg bw per day in the United States. Furfural and
the corresponding furfuryl alcohol are virtually ubiquitous in nature
(Table 4). They are formed from the acid hydrolysis or heating of
Table 4. Natural occurrence of furfural
Food item Concentration of
furfural (ppm)
Apple (raw), apple juice, apricot (Prunus armeniaca L.), sweet 0.02-0.05
cherry (Prunus avium L.), sour cherry (Prunus cerasus L.)
Orange juice (Citrus sinensis L. Osbeck) Trace
Orange peel oil, grapefruit juice (Citrus paradisi) 0.34
Bilberry (Vaccinium myrtillus L.) 0.02
American cranberry (Vaccinium macrocarpon Ait.) 0.1-0.3
Lingonberry (Vaccinium vitis idaea L.) 0.02
Black currents, berries, guava (Psidium guajava L.) 0.0014-0.19
Grape (dried, sultana), peach (Prunus persica L.), pineapple 0.01
(Ananas comosus), raspberry (Rubus idaeus L.), strawberry
(Fragaria species), asparagus (raw), asparagus (cooked)
Carrot (Daucus carota L.), celery leaves (raw), onion (roasted), 0.005
leek (heated), potato (raw), potato (cooked), bell pepper
(Capsicum annuum)
Wheaten bread, sauerkraut, tomato (Lycopersicon 0.8-26
esculentum Mill.), cinnamon (Cinnamomum zeylanicum Blume),
cloves (Eugenia caryophyllata Thunberg), Mentha species
Crispbread, bread, other types, blue cheeses, parmesan, 0.02
butter, yogurt, milk, chicken and turkey (raw), beef (boiled/
cooked), beef (grilled/roasted)
Lamb and mutton, pork (heated), hop oil, beer 0-0.3
Cognac 0.6-33
Armagnac 2
Weinbrand 0.2-4.3
Grape brandy, other types, rum (all categories), rum (category I: 22
total volatiles > 3600 ppm)
Rum (category II: total volatiles 1100-3600 ppm) Trace-25
Rum (category III: total volatiles 240-1100 ppm) Trace
Bourbon whiskey 2-11.6
Irish whiskey 0.8-13.6
Malt whisky 10-37
Scotch blended whisky 1.1-30
Canadian whiskey 0.3-0.8
Japanese whiskey 0.5-4.5
Cider, sherry, white wine Trace-10.3
Red wine 0.005-0.05
Rose wine, port wine 2-34
Special wine, botrytized wine 0.13
Cocoa, coffee 55-255
Black tea 2-7
Green tea 0.1
Microbial fermented tea, tea (brewed) 0.3-0.8
Table 4. (Continued)
Food item Concentration of
furfural (ppm)
Barley (roasted), filbert (roasted, Corylus avellano), peanut 0.08-0.2
(roasted, Arachis hypogea), pecan (roasted), popcorn, potato
chips (American)
Oat flakes (toasted), honey, soybean, arctic bramble (Rubus Trace
arcticus L., Rubus stell.), cloudberry (Rubus chamaemorus L.)
Passion fruit juice (yellow), passion fruit (yellow), plum (raw), 2.58
plum (salted and pickled)
Beans (Phaseolus vulgaris L.), mushroom (raw) 0.05
Trassi (cooked), plum brandy, almond (roasted, Prunus 9
amygdalus)
Macadamia nut (roasted, Macadamia integrifolia), sesame 0-0.1
seed (roasted), mango (raw)
Mango (canned), cauliflower (cooked), tamarind (Tamarindus 7
indica), pear brandy
Apple brandy, beetroot (cooked),artichoke (cooked, Cynarus < 0.01
scolymus L.), gin, rice bran, traditional rice (cooked), quince
(Cydonia oblonga), radish (fermented), shoyu (fermented soya
hydrolysate), bacuri (Platonia insignis), cupuacu (Theobroma
grandiflora), muruci (Brysonima crassifolia)
Potato (sweet, heated), sukiyaki, licorice (Glycyrrhiza glabra L.), < 0.01
matsutake (Tricholoma matsutake), strawberry wine, pumpkin
(Cucurbita pepo L.), sake, oat groats, maize, cashew apple
(Anacardium occidentale), basil (Ocimum basilicum), malt,
peated malt, wort, bonito (dried, katsuobishi), elderberry
(Sambucus nigra L.), mangosteen (Garcinia mangostana),
cherimoya (Annona cherimola), bilberry wine, buchu oil,
vanilla, mountain papaya (Carica pubescens)
Wild rice (Zizania aquatica), chicory (Cichorium intrybus L.), 0-0.2
endive (Cichorium endivia L.), ouzo
Sapodilla fruit (Achras sapota L.) Trace
Aubergine (Solanum melongena L.), pistachio nut (roasted, 17.2
Pistachia vera), arrack
Nectarine < 0.01
From Maarse & Visscher (1994)
Intake (µg/kg bw per day) calculated as follows:
{[(annual volume, kg) × (1 × 109 mg/kg) × (1/60 kg bw)]/[population × 0.6 ×
365 days]}, where population (10%, 'eaters only') = 32 × 106 for Europe and
24 × 106 for the United States; 0.6 represents the assumption that only 60%
of the flavour volume was reported in the survey (National Academy of Sciences,
1987; International Organization of the Flavor Industry, 1995). Slight
variations may occur from rounding off.
polysaccharides which contain pentose and hexose fragments. Furfural
has been detected in a broad range of fruits and fruit juices, wines,
whiskeys, coffee, and tea (Maarse et al., 1994). The highest
concentrations of furfural in foods have been reported in cocoa and
coffee (55-255 ppm), alcoholic beverages (1-33 ppm), and whole-grain
bread (26 ppm). Furfuryl alcohol, which is readily converted to
furfural in vivo (Rice, 1972; Nomeir et al., 1992), has been found
in the highest concentrations in heated skim milk (230 ppm) and coffee
(90-881 ppm). The total potential daily per capita intake of
furfural and precursors of furfural (i.e. furfuryl alcohol and
furfuryl esters) from consumption of foods in which they occur
naturally (Stofberg & Grundschober, 1987) is approximately 0.3 mg/kg
bw per day (i.e. about 300 µg/kg bw per day) in the United States.
Thus, the intake of furfural and furfuryl derivatives from use as
flavouring substances represent 1-3% of the total intake.
4. COMMENTS
In both humans and rodents, furfural is efficiently metabolized
by oxidation of the aldehyde function to furoic acid, most of which
is conjugated with glycine and excreted. A minor proportion (< 5%)
of furoic acid is condensed with acetyl coenzyme A to form
furanacryloyl coenzyme A; the resulting furanacryloic acid is
conjugated with glycine and excreted in the urine. About 5% of
[carboxyl-14C]-labelled furfural is eliminated by rats and mice as
14C-carbon dioxide. The metabolic pathway, which could involve direct
decarboxylation or epoxidation and ring opening, has not been defined.
In short-term studies, furfural was clearly hepatotoxic at doses
> 90 mg/kg bw per day in male rats and > 150 mg/kg per day in
mice. Minor changes reported in the livers of male rats given furfural
at lower doses by gavage in corn oil for 13 weeks were also present to
a lesser extent in vehicle controls. In a study of developmental
toxicity, furfural was not toxic to rats at 150 mg/kg bw per day, the
highest dose tolerated.
Furfural, like other aldehydes such as endogenous acetaldehyde,
is a reactive aldehyde; it is reported to bind to soluble proteins and
protein components of cell membranes. Various metabolic processes
(i.e. oxidation, conjugation, and condensation) effectively eliminate
the reactive aldehyde functional group when these metabolic pathways
are not saturated by high, unphysiological doses.
Furfural was not genotoxic in most tests in vitro. Positive
results were reported at relatively high concentrations in only three
of 16 assays for reverse mutation in Salmonella typhimurium and in
one of three rec assays in B. subtilis. A few chromosomal
aberrations were seen in Chinese hamster ovary cells exposed to
relatively high concentrations. Sister chromatid exchanges and forward
mutations were induced in mouse lymphoma cells. This weak activity of
furfural in vitro in some tests for genotoxicity might be explained
by its aldehyde reactivity. Recent studies of unscheduled DNA
synthesis in hepatocytes of rats treated in vivo and in human liver
slices gave negative results. Negative results were found in tests for
genotoxicity in vivo, except in Drosophila injected with furfural.
In a two-year study of carcinogenicity in rats given furfural in
corn oil by gavage at doses of 30 or 60 mg/kg bw per day, bile-duct
hyperplasia and cholangiofibrosis were seen in essentially all rats,
the controls having the highest incidence. Mild hepatocellular
necrosis was seen in all groups, however, at higher rates in males in
all treated groups. Two cholangio-carcinomas were observed in males at
the high dose, but the incidence was not statistically significant.
In a study of carcinogenicity in mice, the combined incidence of
adenomas and carcinomas of the liver (64%) was significantly
(p < 0.01) increased in males at the high dose (175 mg/kg bw per
day) but not in females. Hepatocellular adenomas and carcinomas also
occurred in the controls and in animals at the low (50 mg/kg bw per
day) and intermediate (100 mg/kg bw per day) doses, at incidences of
34-44% in males and 6-14% in females. The incidences in historical
controls were 38% (14-50%) in males and 6.2% (0-30%) in females.
Hepatotoxicity, manifested by features such as focal and multifocal
necrosis and chronic inflammation, was seen in all groups, including
the controls, but was considerably more frequent in mice at the high
dose.
Studies of oncogene activation in samples of liver tumours from
treated mice in the study of carcinogenicity revealed some differences
in the pattern of mutations from those in liver tumours of controls.
As it was not possible to identify the animals from which the tumours
originated and since hepatoxicity was seen at the intermediate and
high doses, it was not possible to determine whether a direct
genotoxic event or a secondary genotoxic pathway is involved.
5. EVALUATION
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL was identified for hepatotoxicity
in rats, the Committee was unable to allocate an ADI. Before reviewing
the substance again, the Committee would wish to review the results of
studies of DNA binding or adduct formation in vivo to clarify
whether furfural interacts with DNA in mice and of a 90-day study of
toxicity in rats to identify a NOEL for hepatotoxicity.
6. REFERENCES
Aeschbacher, H., Wolleb, U., Löliger, J., Spadone, J.C. & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Boyland, E. (1940) Experiments on the chemotherapy of cancer. 4.
Further experiments with aldehydes and their derivatives. Biochem.
J., 34, 1196-1201.
Brabec, M.J. (1981) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, Third
revised Ed., New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Caldwell, J. (1982) Conjugation reactions in foreign-compound
metabolism: Definition, consequences, and species variations.
J. Drug Metab. Rev., 13, 745-777.
Castellino, N., Elmino, O. & Rozera, G. (1963) Experimental research
on the toxicity of furfural. Arch. Environ. Health, 7, 574-582.
Dillon, D.M., McGregor, D.B., Combes, R.D. & Zeiger, E. (1992)
Detection of mutagenicity in Salmonella of some aldehydes and
peroxides. Environ. Mol. Mutag. 17, 15.
Donahue, T.M., Tuma, D.J. & Sorrell, M.F. (1983) Acetaldehyde adducts
with proteins: Binding of [14C]acetaldehyde to serum albumin.
Arch. Biochem. Biophys., 220, 239-246.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Clayton, G.D. & Clayton, F.E.,
eds, Patty's Industrial Hygiene and Toxicology, Third revised Ed.,
New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Eder, E., Deininger, C. & Muth, D. (1991) Genotoxicity of
p-nitrocinnamaldehyde and related alpha,ß-unsaturated carbonyl
compounds in two bacterial assays. Mutagenesis, 6, 261-269.
Feron, V.J. (1972) Respiratory tract tumors in hamsters after
intratracheal instillations of benzo(a)pyrene alone and with furfural.
Cancer Res., 32, 28-36.
Feron, V.J. & Kruysse, A. (1978) Effects of exposure to furfural
vapour in hamsters simultaneously treated with benzo(a)pyrene or
diethylnitrosamine. Toxicology, 11, 127-144.
Flek, J. & Sedivec, V. (1978) The absorption, metabolism and excretion
of furfural in man. Arch. Occup. Environ. Health, 41, 159-168.
Friedmann, E. (1911) [Fate of furanacrylic acid and furoyl acid in the
animal body.] Biochem. Z., 35, 40-48 (in German).
Gaines, K.C., Salany, J.M., Tuma, D.J. & Sorrell, M.F. (1977) Reaction
of acetaldehyde with human erythrocyte membrane proteins. FEBS
Lett., 75, 115-119.
Gomez-Arroyo, S. & Souza, V.S. (1985) In vitro and occupational
induction of sister-chromatid exchanges in human lymphocytes with
furfuryl alcohol and furfural. Mutat. Res., 156, 233-238.
Gregus, Z., Fekete, T., Varga, F. & Klaassen, C.D. (1993) Dependence
of glycine conjugation on availability of glycine: Role of the glycine
cleavage system. Xenobiotica, 23, 141-153.
Gupta, G.D., Misra, A. & Agarwal, D.K. (1991) Inhalation toxicity of
furfural vapours: An assessment of biochemical response in rat lungs.
J. Appl. Toxicol., 11, 343-347.
Hadi, S.M., Rehman, S. & Rehman, A. (1989) Specificity of the
interaction of furfural with DNA. Mutat. Res., 225, 101-106.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Jonek, J.J., Konecki, J. & Kaminski, M. (1975) Histoenzymatic changes
in liver in acute poisoning with furfural. J. Morphol. Embryol., 21,
47-51.
Kaminska, O. (1977) Morphological and histochemical investigations of
liver and kidneys in different stage of furfural poisoning. Folio
Histochem. Cytochem., 15, 150-151.
Kato, F., Araki, A., Nozaki, K. & Matsushima, T. (1989) Mutagenicity
of aldehydes and diketones. Mutat. Res., 216, 366.
Khan, Q.A. & Hadi, S.M. (1993) Effect of furfural on plasmid DNA.
Biochem. Mol. Biol. Int., 29, 1153-1160.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxy-carbonyl compounds against
mutagenic heterocyclic amines. Mutat. Res., 177, 9-15.
Koenig, K. & Andreesen, J. R. (1990) Xanthine dehydrogenase and
2-furoyl-coenzyme A dehydrogenase from Pseudomonas p. Fu1; two
molybenum-containing dehydrogenases of novel structural composition.
J. Bacteriol., 172, 5999-6009.
Kruysse, A. (1972). Acute inhalation toxicity of furfural in hamsters.
Unpublished report No. R 3853 from Central Institute for Nutrition and
Food Research TNO, Zeist, Netherlands.
Kuznetsov, P.I. (1966) Toxicological characteristics of furfural. II.
Effect of furfural on certain functions of the animal under conditions
of sub-acute poisoning. Nauch. Trud. Omskii Med. Inst. Imeni M.I.
Kalinina, 69, 41-44.
Lake, B.G., Adams, T.B., Beamad, J.A., Price, R.J., Ford, R.A. &
Goodman, J.I. (1998) An investigation of the effect of furfural on the
unscheduled DNA synthesis in cultured human liver slices. Unpublished
report to the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Loquet, C., Toussant, G. & LeTalaer, J.Y. (1981) Studies on mutagenic
constituents of apple brandy and various alcoholic beverages collected
in western France, a high incidence area for oesophageal cancer.
Mutat. Res., 88, 155-164.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148,
25-34.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D. &
Caspary, W.J. (1988) Responses of the L5178Y tk+/- mouse lymphoma
cell forward mutation assay. II: 18 coded chemicals. Environ. Mol.
Mutag., 11, 91-118.
McMahon, R.E., Cline, J.C. & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39, 682-693.
Mellick, P.W., Leach, C.L., Chou, B.J., Irwin, R.D. & Roycroft, J.H.
(1991) Nasal toxicity in Fischer 344 rats inhaling furfuryl alcohol
for 2 or 13 weeks. Toxicologist, 11, 183.
Mishra, A., Dwivedi, P.D., Verma, A.S., Sinha, M., Mishra, J., Lal,
K., Pandya, K.P. & Dutta, K.K. (1991) Pathological and biochemical
alterations induced by inhalation of furfural vapor in rat lung.
J. Bull. Environ. Contam. Toxicol., 47, 668-674.
Moreno, O. (1976) Dermal toxicity in rabbits. Report No. MB 75-941
from MB Research Laboratories Inc. Submitted to WHO by the Flavor &
Extract Manufacturers' Association of the United States, Washington
DC, United States
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Trainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from
the testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nagasawa, H.T., Goon, D.J.W., Conastotino, N.V. & Alexander, C.S.
(1980) Diversion of ethanol metabolism by sulfhydryl amino acids.
D-Penicillamine-directed excretion of
2,5,5-trimethyl-D-thiazolidine-4-carboxylic acid in the urine of rats
after ethanol administration. Life Sci., 17, 707-714.
Nakamura, S.-I., Oda, Y., Shimada, T., Oki, I. & Sugimoto, K. (1987)
SOS-inducing activity of chemical carcinogens and mutagens in
Salmonella typhimurium TA1535/pSK1002: Examination with 151
chemicals. Mutat. Res., 192, 239-246.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
National Toxicology Program (1987) Studies of chemical disposition in
mammals: The effect of dose on the chemical disposition of furfural in
rats after oral administration. NIEHS Contract No. N01-ES-66138 to
Arthur D. Little, Inc, Research Triangle Park, North Carolina, United
States.
National Toxicology Program (1990) Toxicology and carcinogenesis
studies of furfural (CAS No. 98-01-1) in Fischer 344/N rats and
B6C3F1 mice (gavage studies). National Toxicology Program Technical
Report Series No. 382. Research Triangle Park, North Carolina, United
States.
Nomeir, A.A., Silveria, D.M., McComish, M.F. & Chadwick, M. (1992)
Comparative metabolism and disposition of furfural and furfuryl
alcohol in rats. Drug Metab. Disposition, 20, 198-204.
Nomura, F. & Lieber,C.S. (1981) Binding of acetaldehyde to rat liver
microsomes: Enhancement after chronic alcohol consumption. Biochem.
Biophys. Res. Commun., 100, 131-137.
Nutley, B.P., Farmer, P. & Caldwell, J. (1994) Metabolism of
trans-cinnamic acid in the rat and the mouse and its variation with
dose. J. Food Chem. Toxicol., 32, 877-886.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Agric.
Biol. Chem., 45, 2299-2304.
Parkash M.K. & Caldwell J. (1994) Metabolism and excretion of
[14C]-furfural in the rat and the mouse. Food Chem. Toxicol., 32,
887-895.
Paul, H.E., Austin, F.L., Paul, M.F. & Ells, U.R. (1949) Metabolism of
the nitrofurans. I. Ultraviolet absorption studies of the urinary
products after oral administration. J. Biol. Chem., 180, 345-363.
Peterson, C.M. & Nguyen, L.B. (1985) Clinical implications of
acetaldehyde adducts with hemoglobin. In: Aldehyde Adducts in
Alcoholism, pp. 19-30.
Phillips, B.J., Jackson, L.I., Tate, B., Price, R.J., Adams, T.B.,
Ford, R.A., Goodman, J.I. & Lake, B.J. (1997) Furfural does not induce
unscheduled DNA synthesis (UDS) in the in vivo rat hepatocyte DNA
repair assay (Abstract). In Proceedings of the Society of Toxicology
Annual Meeting, 1997, Cincinnati, Ohio, New York, Academic Press.
Pickett, C.B. & Lu, A.Y.H. (1988) The structure, genetics and
regulation of soluble glutathione S-transferases. In: Sies, H. &
Ketterer, B., eds, Glutathione Conjugation: Mechanisms and
Biological Significance, New York, Academic Press, p. 147. A
Ramsdell, H.S. & Eaton, D.L. (1990) Species susceptibility to
aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal
biotransformation Cancer Res., 50, 615.
Ravindranath, A. & Boyd, M.R. (1985) Metabolic activiation of
2-methylfuran by rat microsomal systems. Toxicol. Appl. Pharmacol.,
78, 370-376.
Reynolds, S.H., Stowers, S.J., Patterson, R.M., Maronpot, R.R.,
Aaronson, S.A. & Anderson, M.W. (1987) Activated oncogenes in B6C3F1
mouse liver tumors: Implications for risk assessment. Science, 237,
1309-1316.
Rice, W.W. (1972) Furfural: Exogenous precursor of certain urinary
furans and possible toxicologic agent in humans. Clin. Chem., 18,
1550-1551.
Rodriguez-Arnaiz, R., Morales, P.R. & Zimmering, S. (1992) Evaluation
in Drosophila melanogaster of the mutagenic potential of furfural in
the mei-9(a) test for chromosome loss in germ-line cells. Mutat.
Res., 280, 75-80.
Rodriguez-Arnaiz, R., Morales, P.R., Moctezuma, R.V. & Salas, R.M.B.
(1989) Evidence for the absence of mutagenic activity of furfuryl
alcohol in tests of germ cells in Drosophila melanogaster. Mutat.
Res., 223, 309-311.
Sasaki, Y. & Endo, R. (1978) Mutagenicity of aldehydes in
Salmonella. Mutat. Res., 54, 251-252.
Schafer, E.W., Bowles, W.A. & Hurlbut, J. (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch. Environ. Contam.
Toxicol., 12, 355-382.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102, and TA104. Environ. Mol. Mutag., 11,
195-206.
Shimizu, A. (1986) Experimental studies on hepatic cirrhosis and
hepatocarcinogenesis. II. Influence of cirrhotic liver on 2-FAA
hepatocarcinogenesis in rats. Acta Pathol Jpn., 36,1039-1048.
Shimizu, A. & Kanisawa, M. (1986) Experimental studies on hepatic
cirrhosis and hepatocarcinogenesis. I. Production of hepatic cirrhosis
by furfural administration. Acta Pathol. Jpn., 36, 1027-1038.
Shimizu, A., Yoshiyasu, N., Harada, M., Ono, T., Sato, K., Inoue, T. &
Kanisawa, M. (1989) Positive foci of glutathione S-transferase
placental form in the liver of rats given furfural by oral
administration. Jpn. J. Cancer Res., 80, 608-611.
Shinohara, K., Kim, E. & Omura, H. (1986) Furans as the mutagens
formed by amino-carbonyl reactions. In: Fujimaki, M., Namiki, M. &
Kato, H., eds, Amino-Carbonyl Reactions in Food and Biological
Systems, New York, Elsevier.
Stich, H.F., Rosin, M.P., Wu, C.H. & Powrie, W.D. (1981a)
Clastogenicity of furans found in food. Cancer Lett., 13, 89-95.
Stich, H.F., Rosin, M.P., San, R.H., Wu, C.H. & Powrie, W.D. (1981b)
Intake, formation, and release of mutagens by man. In:
Gastrointestinal Cancer (Banbury Report 7), Cold Spring Harbor, NY,
CSH Press, pp. 247-266.
Stofberg J. & Grundschober F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Subramanyam, S., Sailaja, D. & Rathnaprabha, D. (1989) Genotoxic assay
of two dietary furans by some in vivo cytogenetic parameters.
Environ. Mol. Mutag., 14, 1-252.
Til, H.P., Woutersen, R.A., Feron, V.J., Hollanders, V.H.M., Falke,
H.E. & Clary, J.J. (1989) Two-year drinking-water study of
formaldehyde in rats. Food Chem. Toxicol., 27, 77-78.
Tiunov, L.A., Vasilev, G.A. & Ivanova, L.V. (1970) Effect of frequency
of injection on the toxic action of furfural. Bull. Exp. Biol.
Med., 70, 999-1000.
Tuma, D.J., Donahue, T.M., Medina, V.A. & Sorrell, M.F. (1984)
Enhancement of acetaldehyde-protein adduct formation by L-ascorbate.
Arch. Biochem. Biophys., 234, 377-381.
Videlia, L.A., Fernandez, V., deMarinis, A., Fernandez, N. &
Valenzuala, A. (1982) Liver lipoperoxidative pressure and glutathione
status following acetaldehyde and aliphatic alcohols pretreatments in
the rat. Biochem. Biophys. Res. Commun., 104, 965-970.
US Environmental Protection Agency (1997) Summary report: A dose range
finding development toxicity study of furfural in rats (TSCA
submission 8EHQ-1097-14026), Washington DC.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
Zdzienicka, M., Tudek, B., Zielenska, M. & Szymczyk, T. (1978)
Mutagenic activity of furfural in Salmonella typhimurium TA100.
Mutat. Res., 58, 205-209.
In animals, the condensation reaction of 2-furoyl CoA with acetyl
CoA to yield furanacryloyl CoA is reversible, favouring formation of
2-furoyl CoA (Parkash & Caldwell, 1994). The observation that furoic
acid is excreted in the urine of dogs given furanacrylic acid
(Friedmann, 1911) supports this conclusion. Analogous reversible
reactions between the CoA thioesters of benzoic acid and cinnamic acid
in vivo favour benzoic acid (Nutley et al., 1994). Excretion of
unconjugated furoic acid and furanacrylic acid at higher doses
suggests that glycine conjugation in laboratory animals is limited,
probably by the supply of endogenous glycine (Gregus et al., 1993). As
mentioned earlier, the principal metabolite in rodents, furoic acid,
may also be metabolized to a very minor extent via oxidation of the
heteroaromatic ring. Since heteroaromatic and aromatic carboxylic
acids do not normally undergo decarboxylation in vivo (Caldwell,
1982), it can be assumed that oxidation of the furan ring system of
furoic acid precedes the loss of carbon dioxide. On the basis of this
assumption, epoxidation (Ramsdell & Eaton, 1990) or hydroxylation
(Ravindranath & Boyd, 1985; Koenig & Andreesen, 1990) of the furan
ring may yield reactive intermediates (e.g. furfural-2,3-epoxide,
acetylacrolein, and alpha-ketoglutaric acid) which readily undergo
decarboxylation. Biochemical changes in the lungs and livers of
animals exposed to furfural indicate that ring oxidation may be
catalysed by a cytochrome P450b isoenzyme, yielding an intermediate
which subsequently conjugates with glutathione (Gupta et al., 1991;
Mishra et al., 1991).
Humans
As in laboratory animals, the predominant pathway for
detoxification of furfural in humans is oxidation of the aldehyde to
yield furoic acid, which either conjugates with amino acids or
condenses with acetyl CoA to produce furanacrylic acid.
After inhalation or dermal absorption, furfural is efficiently
and rapidly absorbed ithrough the lungs and skin, with 20-30% of the
amount absorbed by the lungs (Flek & Sedivec, 1978). When six
volunteers were exposed to 30 mg/m3 for 8 h, the mean pulmonary
retention was 78% (range, 75-82%). Retention was independent of the
concentration of furfural vapour and the length of exposure. An
average 8-h exposure maximum was equivalent to a dose of 1.9 mg/kg bw.
The biological half-life of absorbed furfural in humans is only about
2 h. Essentially all of the absorbed furfural could be accounted for:
97% (range, 93-100%) was oxidized to 2-furoic acid and excreted as the
glycine conjugate, 0.5-5% was excreted as furanacrylic acid, and less
than 1% was exhaled unchanged.
2.1.2 Aldehyde reactivity
Furfural contains a heteroaromatic furan ring with a reactive
aldehyde functional group at the 2 position. The reactivity of the
aldehyde function of aldehydes of low molecular mass, like furfural,
suggests that such compounds are not absorbed intact at doses that do
not saturate the oxidation or condensation reactions associated with
the aldehyde function in digestive fluids. Likewise, when furfural
reaches the body fluids, it is likely to react before entering the
cell. The reactivity of the aldehyde group has been demonstrated, for
example, for acetaldehyde and formaldehyde, both normal metabolic
intermediates, which may have toxic effects including the induction of
tumours when administered under non-physiological conditions at high
doses (Til et al., 1989).
Aldehydes of low molecular mass have been reported to bind with
soluble proteins, protein components of cell membranes, and
thiol-containing molecules to form unstable and stable adducts.
Formation of adducts with albumin (Nagasawa et al., 1980; Donahue et
al., 1983; Tuma et al., 1984), haemoglobin (Peterson & Nguyen, 1985),
and erythrocyte membranes (Gaines et al., 1977) has been demonstrated
in vitro and in vivo. In membranes, binding is usually with the
protein component (Nomura & Lieber, 1981). Aldehydes also bind
glutathione (Videlia et al., 1982). Aldehyde dehydrogenase-catalysed
oxidation of low-molecular-mass aldehydes requires glutathione
(Eckfeldt & Yonetani, 1982), suggesting that free aldehyde is rapidly
conjugated with glutathione in vivo to form a thiohemiacetal which
is subsequently oxidized to the corresponding acid (Brabec, 1981).
Thus, the reactivity of the aldehyde function will significantly
curtail the intracellular concentration of free aldehyde.
When an aldehyde is present in a cell, it undergoes rapid
oxidation, with conjugation of the resulting carboxylic acid (Gregus
et al., 1993). The various metabolic processes, i.e. oxidation,
conjugation, and condensation (see section 2.1.1) effectively
eliminate the reactive aldehyde functional group when not saturated by
high, non-physiological doses.
2.2 Toxicological studies
2.2.1 Acute toxicity
On the basis of the oral LD50 values in various species
(Table 1), furfural is acutely toxic at lower doses in rats than in
mice.
Wistar/Slc rats given a single dose of 50 mg/kg bw furfural by
gavage had sporadic eosinophilic degeneration of hepatic cells with
nuclear pyknosis and eosinophilic necrosis and increased hepatocyte
mitosis. No massive or zonal necrosis was observed. Since the control
rats were killed at the beginning of the experiment, no appropriate
controls were available (Shimizu & Kanisawa, 1986). Decreased
activities of succinic dehydrogenase and ATPase and increased
activities of acid phosphatase and acid DNase II were reported in male
Wistar rats injected intraperitoneally with 20 or 50 mg/kg bw (Jonek
et al., 1975).
Table 1. Acute toxicity of furfural
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Rat Oral 127 Jenner et al. (1964)
Rat Intraperitoneal 120 Tiunov et al. (1970)
Mouse Oral 333 Boyland (1940)
Mouse Subcutaneous 200 Tiunov et al. (1970)
Mouse Subcutaneous 223 (1-day survival) Castellino et al.(1963)
119 (10-day survival)
Rabbit Dermal > 310 Moreno (1976)
Hamster Inhalation 2500 Kruysse (1972)
Bird Oral > 98 Schafer et al. (1983)
2.2.2 Short-term studies of toxicity
Rats
In rats receiving 0.05, 2.5, or 25 mg/kg bw per day furfural for
35 days, no hepatic impairment was seen (Kuznetsov, 1966). When male
rats were injected intraperitoneally with furfural in increasing doses
from 29 to 58 mg/kg bw for 30 days, damage and increased intracellular
catabolic processes were seen in liver and kidney cells (Kaminska,
1977).
In a 16-day study, groups of five male and five female Fischer
344/N rats were given furfural at doses of 0, 15, 30, 60, 120, or
240 mg/kg bw per day in corn oil by gavage, five days per week for a
total of 12 doses. At the end of the study, the surviving animals were
killed, necropsied, and examined histopathologically. No
treatment-related abnormalities were found. Eight of ten rats at the
highest dose had died by the third day due to gavaging accidents.
Animals at 120 mg/kg bw appeared to be less active. The body weights
of rats given doses < 120 mg/kg bw per day did not differ
significantly from those of controls.
In a 16-day study, groups of five male and five female B6C3F1
mice were given furfural at doses of 0, 25, 50, 100, 200, or 400 mg/kg
bw per day in corn oil by gavage on five days per week for a total of
12 doses. At the end of the study, the surviving animals were killed,
necropsied, and examined histopathologically. The mean body weights of
the treated mice did not differ significantly from those of controls.
One male at the highest dose had died by day five, and two female mice
died from gavaging accidents. No treatment-related abnormalities were
found.
In a 13-week study, groups of 10 male and 10 female B6C3F1 mice
were given furfural at doses of 0, 75, 150, 300, 600, or 1200 mg/kg bw
per day in corn oil by gavage on five days per week. Necropsies were
performed on all survivors, and histopathological examinations were
performed on those at the two highest doses and the controls. All the
mice at the two highest doses died before the end of the study, except
for one male and one female at 600 mg/kg bw per day. The body weights
of the males treated with doses > 150 mg/kg bw per day were
slightly lower than those of controls. Female mice at 75-300 mg/kg bw
per day and male mice at 300 mg/kg bw per day showed significantly
increased relative liver weights as compared with controls.
Centrilobular coagulative necrosis of hepatocytes was observed in most
male mice at the two highest doses and in one male per group treated
with 150 or 300 mg/kg bw per day. Among the females, only two at the
highest dose showed similar lesions. Animals with coagulative necrosis
also had inflammation of the liver, with minimal to mild mononuclear
inflammatory cell infiltrate (National Toxicology Program, 1990).
A group of 48 male, six-week-old Wistar/Slc rats was fed furfural
at a concentration of 20 ml/kg diet (equivalent to approximately 1200
mg/kg bw per day) for one week, 30 ml/kg diet for one week, and then
40 ml/kg diet on days 15-90; on days 90-120, the rats were given the
diet containing 40 ml/kg for only five days per week to prevent
reduced weight gain. The concentration of furfural in the feed was not
analysed after preparation of the diet. A control group of 40 male
rats of the same age was fed the basal diet.
Treated rats sacrificed after 90 days had marked
cholangiofibrosis, with areas of increased density containing red
nodules. The nodules showed fibrous widening of Glisson's sheath,
bile-duct proliferation, and destruction of the limiting plates. The
parenchymal damage consisted of bridging necrosis and hydropic
degeneration of hepatocytes. The parenchyma often showed no cirrhotic
changes in areas other than those with fibrosis, suggesting a
regenerative process in the liver. Increased numbers of cells
undergoing mitosis were seen. In rats killed on day 120 of the study,
similar but more marked findings were reported. The incidence of
hepatic fibrosis was increased in the furfural-treated rats. No
atypical hepatocyte growth was noted. Furfural did not cause
hepatocellular hyperplastic changes (Shimizu & Kanisawa, 1986).
In a study designed to examine the mechanism of furfural-induced
hepatocytic injury, six groups of six male Wistar/Slc rats were
maintained on diets containing furfural at doses calculated to result
in average daily intakes of 20 ml/kg diet (approximately 1200 mg/kg bw
per day) for the first 30 days and 30 ml/kg diet (approximately
1700 mg/kg bw per day) thereafter. Each group also included four
control animals. The furfural-containing diets were replaced after 15,
30, 60, 90, 120, or 150 days, and animals were killed 14 days after
termination of furfural administration. Increased duration of exposure
to furfural was accompanied by increased numbers and size of foci
positive for the placental form of glutathione- S-transferase in
hepatocytes (Shimizu et al., 1989). Such foci have been reported to be
a marker for early detection of preneoplastic or neoplastic cells
(Pickett & Lu, 1988).
In a 13-week study, groups of 10 male and 10 female Fischer 344/N
rats were given furfural at doses of 0, 11, 22, 45, 90, or 180 mg/kg
bw per day in corn oil by gavage on five days per week. All survivors
were necropsied, and histopathological examinations were performed on
those at the two highest doses and the controls. Only one male rat at
the highest dose survived to the end of the study; four of the deaths
were considered to be related to gavage, as were two deaths among
controls and four among rats at the second highest dose. The mean body
weights of treated rats did not differ from those of controls. The
relative and absolute weights of the livers and kidneys of males given
90 and 180 mg/kg bw per day were significantly higher than those of
controls. An increased incidence of cytoplasmic vacuolization in
hepatocytes was observed in all groups of treated males: control,
4/10; 11 mg/kg bw per day, 10/10; 22 mg/kg bw per day, 10/10; 45 mg/kg
bw per day, 10/10; 90 mg/kg bw per day, 9/10. No lesions were reported
in the kidneys of these rats (National Toxicology Program, 1990).
Hamsters
Groups of 10 Syrian golden hamsters of each sex were exposed to
furfural vapour at 0, 77, 448, or 2165 mg/m3 for 6 h per day, five
days per week over 13 weeks. The main findings were mild growth
retardation, irritation of the eyes and nose, and hyperplastic atrophy
of the nasal epithelium, all at the highest dose. The NOEL was 77
mg/m3, since mild nasal epithelial degeneration was observed at 448
mg/m3 (Feron & Kruysse, 1978).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a two-year study, 50 male and 50 female B6C3F1 mice were
given furfural at doses of 0, 50, 100, or 175 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed weekly
for the first 13 weeks and monthly thereafter until the conclusion of
the study, when surviving animals were killed. All animals were
necropsied and examined histologically. Furfural had no significant
effect on body weight or survival. Microscopic examination revealed
lesions in the liver, kidneys, and forestomach. In the liver,
pigmentation, necrosis, and chronic inflammation were seen in males at
the intermediate and high doses and in females at the high dose (Table
2). Hepatocellular adenomas and carcinomas were seen in all groups,
including controls, and the increased incidence was statistically
significant only in males at the high dose. Carcinomas occurred at the
same incidence in females at the high dose as in controls.
Table 2. Incidences of liver lesions and hepatocellular adenomas and carcinomas in male and
female B6C3F1 mice given furfural by gavage
Lesion Dose (mg/kg bw)
0 50 100 175
M F M F M F M F
Pigmentation 0/50 0/50 0/50 0/50 8/49 0/50 18/50 11/50
Necrosis, focal and multifocal 3/50 2/50 2/50 1/50 7/49 3/50 10/50 2/50
Chronic Inflammation 0/50 0/50 0/50 0/50 8/49 1/50 18/50 8/50
Hepatocellular adenoma 9/50 1/50 13/50 3/50 11/49 5/50 19/50 8/50
Hepatocellular carcinoma 7/50 4/50 12/50 0/50 6/49 2/50 21/50 4/50
Combined rates 16/50 5/50 22/50 3/50 17/49 7/50 32/50 12/50
From National Toxicology Program (1990)
M, male; F, female
Hyperplasia of the renal tubules was found in one male at the low
dose. One male at the intermediate dose, one at the high dose, and one
female at the low dose had a cortical adenoma, and one male at the low
dose had a cortical carcinoma. Owing to the low incidence and lack of
dose-response relationship, these tumours were not considered as
evidence for carcino-genicity. Marginal increases in the incidences of
hyperplasia and papillomas in the forestomach of female mice were
considered to be of no biological significance and were probably
related to the irritating effects of chronic administration of the
compound by gavage or the method of gavage itself. None of the animals
showed evidence of malignant forestomach lesions (National Toxicology
Program, 1990).
Rats
In a two-year study, 50 male and 50 female Fischer 344/N rats
were given furfural at doses of 0, 30, or 60 mg/kg bw per day in corn
oil by gavage on five days per week. The animals were weighed at
specific intervals, and the survivors were killed at the end of the
study, necropsied, and examined histologically. Overall survival was
slightly reduced for female rats at the highest dose (78% at week 97),
due to accidental gavage-related deaths. Furfural had no effect on
body weight or survival. Congestion and foreign bodies were observed
in a dose-related manner in the lungs of female rats. A small increase
in the incidence of squamous-cell carcinomas and papillomas in the
forestomachs of treated rats was observed but was considered unrelated
to treatment. Mild centrilobular hepatocellular necrosis was observed
in all groups (vehicle control: males, 3/50; females, 10/50; low dose:
males, 9/50; females, 9/50; high dose: males, 12/50; females, 4/50).
Bile-duct hyperplasia occurred in 45/50 control males, 36/50 control
females; 41/50 males and 28/50 females at the low dose; and 44/50
males and 24/50 females at the high dose. Focal bile-duct dysplasia
was noted in one male at the intermediate dose, and bile-duct
hyperplasia accompanied by fibrosis (cholangiofibrosis) was seen in
two males at the high dose. One control male had a hepatocellular
adenoma, and two males at the high dose had a cholangiocarcinoma. The
incidence of the latter lesion was not statistically significant, but
some evidence of carcinogenicity was consluded since
cholangiocarcinomas were rare in historical controls (3/2145)
(National Toxicology Program, 1990).
2.2.3.1 Special studies of carcinogenicity
The aldehydes citral and heptaldehyde inhibit the growth of
spontaneous mammary tumours in mice; however, furfural and its
furfuracrylic acid metabolite at a daily dose of 2.5 mg had no effect
on the growth of spontaneous mammary tumours in mice, although they
were weakly active against sarcomata (Boyland, 1940).
Studies have been designed to evaluate the effect of furfural on
the carcinogenic potential of known carcinogens. In the first study,
three groups of 35 male and 35 female Syrian golden hamsters were
provided with 0.2 ml of 1.5% furfural in physiological saline (about
25 mg/kg bw), 0.5% benzo [a]pyrene (8.3 mg/kg bw), or 1.5% furfural
plus 0.5% benzo [a]pyrene intratracheally once weekly for 36 weeks.
Furfural in combination with benzo [a]pyrene led to earlier
development of hyper- and metaplastic changes in the tracheobronchial
epithelium, a shorter latency for tracheobronchial tumours, a few more
bronchial and peripheral squamous-cell carcinomas, and a substantially
reduced number of tracheal squamous-cell carcinomas. The incidence of
peritracheal sarcomas was increased, which may indicate a
co-carcinogenic effect of furfural. Irritation but no tumorigenic
effect was observed after administration of furfural alone (Feron,
1972).
In a subsequent study, groups of 126 Syrian golden hamsters of
each sex were exposed to atmospheres containing 400 ppm (1600 mg/m3)
furfural for 7 h per day, five days per week for nine weeks, then 330
ppm (1300 mg/m3) for 11 weeks, and 250 ppm (970 mg/m3) for an
additional 32 weeks. The effects on the respiratory tract included
atrophy and downward growth of the olfactory epithelium, degenerative
changes in Bowman's glands, and the appearance of cyst-like structures
in the lamina propria beneath the olfactory epithelium. Although
furfural was irritating to the olfactory epithelium, treatment did not
result in hepatic toxicity or carcinogenicity. Furfural did not
potentiate the carcinogenic effect of benzo [a]pyrene or
N-nitrosodiethylamine (Feron & Kruysse, 1978).
Localized irritation of the nasal cavity and respiratory tract,
with no hepatic effects, were also observed in B6C3F1 mice and
Fischer 344/N rats exposed to atmospheres containing 0, 2, 4, 8, 16,
or 32 ppm of the metabolic precursor furfuryl alcohol for 13 weeks
(Mellick et al., 1991).
SPF Wistar rats were given furfural at doses of 20 ml
(approximately equal to 1200 mg/kg bw per day) for one week, 30 ml for
the second week, and 40 ml for the following 10 weeks to produce
hepatic cirrhosis. Half of the remaining rats in this study (38
treated and 32 controls) were fed a diet containing 0.03%
N-2-fluorenylacetamide for three weeks, followed by one week of
normal diet; the other half received only the normal diet. All
surviving rats were killed 12 weeks later, and their livers were
examined. Rats that had received furfural only had no hyperplastic
changes in the liver but did have cirrhosis. Rats that had been
treated with N-2-fluorenylacetamide developed multiple hyperplastic
nodules which stained for alpha-fetoprotein, and this response was
markedly potentiated in the rats previously treated with furfural. The
authors concluded that furfural-induced hepatic cirrhosis increases
the susceptibility of rats to potent hepatocarcinogens (Shimizu,
1986).
Samples of mouse liver tumours from the National Toxicology
Program carcinogenesis bioassay programme were assessed for
transforming gene activity by Southern blot analysis for H-, K-, and
N- ras oncogens. The pattern of mutations in the oncogenes from liver
tumours that occurred spontaneously differed from that which occurred
in some furfural-treated animals: The activated ras genes were
H- ras in 15 of 17 spontaneous tumours, with one raf and one
unknown oncogene; activating point mutations occurred at codon 61 in
six tumours, codon 13 in two tumours, and codon 117 in one tumour. The
authors concluded that the spectrum of activating mutations in the
H- ras gene and the pattern of ras gene activation in liver tumours
derived from furfural-treated mice differed from those in liver
tumours derived from untreated animals, and indicates a direct
genotoxic effect of furfural. An alternative mechanism-induction of
mutations by an indirect, secondary genotoxic pathway as a result of a
cytotoxic event -- was mentioned but excluded by the authors on the
basis of the absence of cytotoxic lesions in the livers of similar
mice after 90 days of treatment with the same dose of furfural. The
authors do not mention, however, that necrosis, pigmentation, and
chronic inflammation were seen regularly in mice at the intermediate
and high doses in the carcinogenicity experiment (see Table 2). It can
be concluded that the effect was either due to a direct genotoxic
event or occurred via an indirect, secondary genotoxic pathway
(Reynolds et al., 1987).
2.2.4 Genotoxicity
Studies of the genotoxicity of furfural are summarized in
Table 3. Testing of furfural for mutagenicity in vitro at
concentrations up to those causing cytotoxicity provided no evidence
that it is mutagenic to Salmonella typhimurium strains TA98, TA102,
TA1535, or TA1537 or ito two strains of Escherichia coli, with or
without metabolic activation (Sasaki & Endo, 1978; Zdzienicka et al.,
1978; McMahon et al., 1979; Loquet et al., 1981; Marnett et al., 1985;
Mortelmans et al., 1986; Shinohara et al., 1986; Nakamura et al.,
1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al., 1989;
National Toxicology Program, 1990; Dillon et al., 1992). The majority
of these studies also showed negative results for S. typhimurium
TA104, although a single positive result was reported (Shane et al.,
1988). Furfural was not mutagenic when incubated with
S. typhimurium TA100 in most reports (Sasaki & Endo, 1978; McMahon
et al., 1979; Mortelmans et al., 1986; Shinohara et al., 1986; Kim et
al., 1987; Shane et al., 1988; Aeschbacher et al., 1989; Kato et al.,
1989; National Toxicology Program, 1990; Eder et al., 1991; Dillon et
al., 1992) but was weakly mutagenic in other studies (Zdzienicka et
al., 1978; Loquet et al., 1981; Shane et al., 1988). When fufural was
co-incubated with benzo [a]pyrene and S. typhimurium TA100, it did
not alter the mutagenic activity of benzo [a]pyrene in this strain
(Zdzienicka et al., 1978). Furfural showed no mutagenic potential in
two rec assays with Bacillus subtilis by a direct streaking method
(Osawa & Namiki, 1982; Matsui et al., 1989) but also gave positive
results in this test system (Shinohara et al., 1986).
Table 3. Results of assays for the genotoxicity of furfural
End-point Test object Concentration Result Reference
In vitro
Reverse mutation S. typhimurium TA100 NR Negative Eder et al. (1991)
Reverse mutation S. typhimurium TA100, TA102, and NR Negativea Dillon et al. (1992)
TA104
Reverse mutation S. typhimurium TA100, TA98, and < 36 µmol/plate Weakly positive (TA100)b Loquet et al. (1981)
TA1535 60 µmol/plate Negativec
Reverse mutation S. typhimurium TA100, TA98, and < 1.2 mmol/plate Negative Aeschbacher et al. (1989)
TA102
Reverse mutation S. typhimurium TA100 and TA98 0.165-0.660 µmol/plate Negativea Shinohara et al. (1986)
Reverse mutation S. typhimurium TA100, TA102, and 5-500 µg/plate Positive (TA104) Shane et al. (1988)
TA104
Reverse mutation S. typhimurium TA100, TA98, and NR Negativea Kato et al. (1989)
TA104; E. coli WP2uvrA/PKM101
Reverse mutation S. typhimurium TA104 and TA102 1 µmol/plate Negative Marnett et al. (1985)
Reverse mutation S. typhimurium TA98, TA100, < 6667 µg/plate Negativea Mortelmans et al. (1986)
TA1535, and TA1537
Reverse mutation S. typhimurium TA100 and TA98 7 µl/plate Positivea (TA100) Zdzienicka et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, < 20 µl/plate Negativea McMahon et al. (1979)
TA1537, TA1538, TA98, G46;
E. coli WP2uvrA
Reverse mutation S. typhimurium TA100 and TA98 NR Negativea Sasaki & Endo (1978)
Reverse mutation S. typhimurium TA100 4.44 µmol/plate Negativea Kim et al. (1987)
umu gene expression S. typhimurium TA1535/pSK/002 1932 µg/ml Negativea Nakamura et al. (1987)
Reverse mutation S. typhimurium TA100 < 1 mg Negativea Osawa & Namiki (1982)
Reverse mutation S.typhimurium TA98, TA100, 33-6666 µg/plate Negativea National Toxicology Program
TA1535, and TA1537 (1990a)
Reverse mutation S.typhimurium TA98, TA100, and 33-6666 µg/plate Negativea National Toxicology Program
TA1535 (TA100 equivocal) (1990b)
rec Gene mutation B. subtilis H17 and M45 < 1 mg Negativea Osawa & Namiki (1982)
rec Gene mutation B. subtilis H17 and M45 1.7-17 mg/disk Positivea Shinohara et al. (1986)
rec Gene mutation B. subtilis H17 and M45 0.6 ml Negativea Matsui et al. (1989)
Table 3. (continued)
End-point Test object Concentration Result Reference
Chromosomal Chinese hamster ovary cells < 40 mmol/L Positivea Stich (1981a)
aberration
Chromosomal Chinese hamster ovary cells 3 mg/ml Positive Stich (1981b)
aberration
Forward mutation L5178Y tk+/- mouse lymphoma cells 25-400 µg/ml Positiveb McGregor et al. (1988)
Sister chromatid Chinese hamster ovary cells < 1170 µg/ml Positivea National Toxicology Prohgram
exchange/Chromosomal (1990)
aberration
Sister chromatid Human lymphocytes < 0.14 mmol/L Positive Gomez-Arroyo & Souza (1985)
exchange
Unscheduled DNA Human liver slices 0-25 mmol/L Negative Lake et al. (1998)
synthesis
In vivo
Sex-linked recessive D. melanogaster 1000 ppm in diet Negative Woodruff et al. (1985)
lethal mutation
Sex-linked recessive D. melanogaster 100 ppm by injection Positive Woodruff et al. (1985)
lethal mutation
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Negative Rodriguez-Arnaiz et al. (1992)
repair-proficient females < 33% lethal dose
Sex-chromosome loss D. melanogaster males mated with Fed or injected with Positive only after Rodriguez-Arnaiz et al. (1992)
repair-deficient females < 33% lethal dose injection
Sex-chromosome loss D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
injection
Sex-linked recessive D. melanogaster < 6500 ppm by Negative Rodriguez-Arnaiz et al. (1989)
lethal mutation injection
Reciprocal D. melanogaster 100 ppm by injection Negative Woodruff et al. (1985)
translocation
Sister chromatid B6C3F1 mouse bone-marrow cells 50-200 mg/kg by Negative National Toxicology Program
exchange/Chromosomal injection (1990)
aberration
Table 3. (continued)
End-point Test object Concentration Result Reference
Somatic chromosomal Swiss albino mouse bone-marrow < 4000 ppm for 5 days Negatived Subramanyam et al. (1989)
mutation cells
Sperm-head Swiss albino mouse < 4000 ppm for 5 Negatived Subramanyam et al. (1989)
abnormalities weeks
Unscheduled DNA Fischer 344 rat hepatocytes 5.0, 16.7, or 50 mg/kg Negative Phillips et al. (1997)
synthesis bw orally
NR, not reported
a With and without metabolic activation
b Without metabolic activation
c With metabolic activation
d Abstract only: reported to have positive effects at 4000 ppm, but no details available
Furfural produced a dose-dependent increase in the frequency of
chromatid breaks and exchanges in Chinese hamster ovary cells with and
without metabolic activation (Stich et al., 1981a,b; National
Toxicology Program, 1990) and induced chromosomal aberration in these
cells at a cytotoxic concentration (National Toxicology Program,
1990). An increased frequency of sister chromatid exchange was
reported in human lymphocytes incubated with furfural at a
concentration of 0.07 or 0.14 mmol/L, but not at 0.035 mmol/L
(Gomez-Arroyo & Souza, 1985). In this study, furfuryl alcohol did not
increase the frequency of sister chromatid exchange at a concentration
of 3.3, 6.6, or 9.9 mmol/L.
Furfural increased trifluorothymidine resistance when incubated
with L5178Y tk+/- mouse lymphoma cells at a concentration of 200 or
400 mg/ml without metabolic activation, but showed no mutagenic
activity at 25, 50, or 100 mg/ml (McGregor et al., 1988).
Concentrations of 400 and 800 mg/ml were cytotoxic in separate trials.
In vivo, furfural did not induce sex-linked recessive lethal
mutations or reciprocal translocations in male Drosophila
melanogaster when incorporated into their diet at 1000 ppm.
Mutations were induced when furfural was administered by injection at
a concentration of 100 ppm, but no reciprocal translocations occurred.
A similar pattern of responses was reported for other simple aldehydes
(e.g. acetaldehyde and trans-cinnamaldehyde) when given via the
intraperitoneal (positive) and oral (negative) routes of
administration (Woodruff et al., 1985). No sex-chromosome loss was
observed when male D. melanogaster were fed or injected with
furfural at concentrations equivalent to 25-33% of the lethal dose of
furfural and then mated with repair-proficient females
(Rodrigues-Arnaiz et al., 1992), but sex-chromosome loss was reported
by these authors when treated males were mated with repair-deficient
females. No sex-linked recessive lethal mutation or sex-chromosome
loss was reported when male D. melanogaster were injected with
furfuryl alcohol (Rodrigues-Arnaiz et al., 1989).
Tests of mammals treated in vivo with furfural have produced
mainly negative results. Furfural did not induce sister chromatid
exchange or chromosomal aberrations in B6C3F1 mouse bone-marrow cells
after intraperitoneal injections of furfural at doses of 50-200 mg/kg
bw (National Toxicology Program, 1990). No genotoxic effects or
spermhead abnormalities were reported in mice given up to 4000 ppm
furfural daily for five weeks, and only the highest dose tested was
associated with an increased frequency of somatic (liver)-cell
chromosomal mutations when given for 24 or 48 h but not after 72 h
(Subramanyam et al., 1989). Furfural administered by gavage to male
Fischer 344/N rats in corn oil at doses of 5, 16.7, or 50 mg/kg bw did
not induce unscheduled DNA synthesis in hepatocytes (Phillips et al.,
1997).
Chromosomal aberrations were not observed in lymphocytes taken
from workers occupationally exposed to furfural or furfuryl alcohol
(Gomez-Arroyo & Souza, 1985).
Liver slices prepared from samples from four human donors were
cultured in medium containing 3H-thymidine and 0-25 mmol/L furfural
for 24 h. In a preliminary experiment, furfural was markedly toxic at
concentrations > 10 mmol/L. As positive controls, liver slices were
also cultured with three known genotoxic agents,
2-acetylaminofluorene, aflatoxin B1, and
2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP). Unscheduled
DNA synthesis was quantified as the changes in mean nuclear and
cytoplasmic grain counts relative to controls, net grain count (i.e.
mean nuclear - mean cytoplasmic grain counts), and the percentage of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains. In
comparison with control liver slice cultures (in dimethylsulfoxide),
those treated with 0.02 or 0.05 mmol/L 2-acetylamino-fluorene, 0.002
or 0.02 mmol/L aflatoxin B1, and 0.003 or 0.05 mmol/L PhIP had
significantly higher net grain counts in centrilobular hepatocytes,
due primarily to large increases in mean nuclear grain counts. The
three genotoxic agents also significantly increased the number of
centrilobular hepatocyte nuclei with > 5 and > 10 net grains.
Treatment with 0.005-0.5 mol/L furfural had no significant effect
on net grain count or mean nuclear or cytoplasmic grain counts. A
small but statistically significant increase in net grain counts
observed at 2, 5, and 10 mmol/L furfural was the result of a
significant concentration-dependent decrease in mean cytoplasmic grain
counts accompanied by a smaller increase in nuclear grain counts.
Furfural had no significant effect on the percentage of hepatocyte
nuclei with > 5 or > 10 net grain counts, except at a concentration
of 2 mmol/L and only in liver cultures in which marked toxicity was
noted at 10 mmol/L. The decreases in mean cytoplasmic and nuclear
grain counts and the correlation between cytotoxicity and changes in
net grains count and nuclei with > 5 net grains support the
conclusion that furfural at 2, 5, or 10 mmol/L induces a cytotoxic
response in cultured human liver slices. In comparison with the three
genotoxins tested, furfural did not induce unscheduled DNA synthesis
in cultured human liver slices (Lake et al., 1998).
Furfural was reported to increase the frequency of single-strand
DNA breaks in isolated calf-thymus duplex DNA (Hadi et al., 1989).
When incubated with plasmid DNA, furfural decreased the transformation
capacity of Escherichia coli host cells; however, the damaged
plasmids were repaired after propagation in the host (Khan & Hadi,
1993).
Thus, negative results were obtained with furfural in most tests
for genotoxicity in vitro. Positive results were reported at
relatively high doses in three of 16 assays for reverse mutation in
S. typhimurium and in one of three assays for gene mutation in
B. subtilis. Weakly positive results were obtained in tests for
chromosomal aberrations at relatively high doses. Positive results
were found in two assays for sister chromatid exchange and one for
forward mutation in mouse lymphoma cells. The results of all tests for
genotoxicity in vivo were negative, except in D. melanogaster
injected with furfural.
2.2.5 Reproductive toxicity
Rats
A summary report of an investigation to define the doses of
furfural for a study of developmental toxicity was submitted to the
Toxic Substances Control Administration of the US Environmental
Protection Agency. Five groups of eight Crl:CD(SD)BR rats received
furfural in water by gavage at doses of 10, 50, 100, 500, or 1000
mg/kg bw per day during gestation days 6-15. Because of excessive
deaths at 500 and 1000 mg/kg bw per day, the breeding phase of the
study was re-initiated to characterize the potential maternal and
developmental toxicity at 150, 250, and 350 mg/kg bw per day. Two
concurrent control groups of eight rats received only the vehicle
(reverse osmosis-treated water) by gastric intubation. Clinical
observations, body weights, and food consumption were recorded. On
gestation day 20, laparohysterectomy was performed on all surviving
animals; the uteri and ovaries were examined, and the numbers of
fetuses, early and late resorptions, total implantation, and corpora
lutea were recorded. The uteri and fetuses were weighed and examined
for external malformations, variations, and sex.
Because of excessive mortality on day 6 after dosing, all females
at 250, 350, 500, and 1000 mg/kg bw per day were killed. The clinical
signs in these animals included lethargy, effects on respiration, and
whole-body tremor. The maternal animals at other doses survived,
except for one female at 150 mg/kg bw per day group, which died within
I h of dosing. The surviving females at 150 mg/kg bw per day had
reduced body-weight gain and reduced food consumption; however, the
mean body weight, net body weight, net body-weight gain, and gravid
uterine weight were unaffected. Body weight and food consumption of
animals at 10, 50, and 100 mg/kg bw per day were unaffected.
Intrauterine growth and survival were not affected at 10, 50, 100, or
150 mg/kg bw per day. The only external malformation noted
(omphalocele) occurred in offspring of rats at 10 mg/kg bw per day. No
developmental variations were noted (US Environmental Protection
Agency, 1997).
3. HUMAN INTAKE
On the basis of the most recently reported annual volumes, 3600
kg in Europe (International Organization of the Flavor Industry, 1995)
and 590 kg in the United States (National Academy of Sciences, 1987),
the estimated daily per capita intake of 'eaters only' of furfural
from use as a flavouring substance is approximately 9 µg/kg bw per day
in Europe and 2 µg/kg bw per day in the United States. Furfural and
the corresponding furfuryl alcohol are virtually ubiquitous in nature
(Table 4). They are formed from the acid hydrolysis or heating of
Table 4. Natural occurrence of furfural
Food item Concentration of
furfural (ppm)
Apple (raw), apple juice, apricot (Prunus armeniaca L.), sweet 0.02-0.05
cherry (Prunus avium L.), sour cherry (Prunus cerasus L.)
Orange juice (Citrus sinensis L. Osbeck) Trace
Orange peel oil, grapefruit juice (Citrus paradisi) 0.34
Bilberry (Vaccinium myrtillus L.) 0.02
American cranberry (Vaccinium macrocarpon Ait.) 0.1-0.3
Lingonberry (Vaccinium vitis idaea L.) 0.02
Black currents, berries, guava (Psidium guajava L.) 0.0014-0.19
Grape (dried, sultana), peach (Prunus persica L.), pineapple 0.01
(Ananas comosus), raspberry (Rubus idaeus L.), strawberry
(Fragaria species), asparagus (raw), asparagus (cooked)
Carrot (Daucus carota L.), celery leaves (raw), onion (roasted), 0.005
leek (heated), potato (raw), potato (cooked), bell pepper
(Capsicum annuum)
Wheaten bread, sauerkraut, tomato (Lycopersicon 0.8-26
esculentum Mill.), cinnamon (Cinnamomum zeylanicum Blume),
cloves (Eugenia caryophyllata Thunberg), Mentha species
Crispbread, bread, other types, blue cheeses, parmesan, 0.02
butter, yogurt, milk, chicken and turkey (raw), beef (boiled/
cooked), beef (grilled/roasted)
Lamb and mutton, pork (heated), hop oil, beer 0-0.3
Cognac 0.6-33
Armagnac 2
Weinbrand 0.2-4.3
Grape brandy, other types, rum (all categories), rum (category I: 22
total volatiles > 3600 ppm)
Rum (category II: total volatiles 1100-3600 ppm) Trace-25
Rum (category III: total volatiles 240-1100 ppm) Trace
Bourbon whiskey 2-11.6
Irish whiskey 0.8-13.6
Malt whisky 10-37
Scotch blended whisky 1.1-30
Canadian whiskey 0.3-0.8
Japanese whiskey 0.5-4.5
Cider, sherry, white wine Trace-10.3
Red wine 0.005-0.05
Rose wine, port wine 2-34
Special wine, botrytized wine 0.13
Cocoa, coffee 55-255
Black tea 2-7
Green tea 0.1
Microbial fermented tea, tea (brewed) 0.3-0.8
Table 4. (Continued)
Food item Concentration of
furfural (ppm)
Barley (roasted), filbert (roasted, Corylus avellano), peanut 0.08-0.2
(roasted, Arachis hypogea), pecan (roasted), popcorn, potato
chips (American)
Oat flakes (toasted), honey, soybean, arctic bramble (Rubus Trace
arcticus L., Rubus stell.), cloudberry (Rubus chamaemorus L.)
Passion fruit juice (yellow), passion fruit (yellow), plum (raw), 2.58
plum (salted and pickled)
Beans (Phaseolus vulgaris L.), mushroom (raw) 0.05
Trassi (cooked), plum brandy, almond (roasted, Prunus 9
amygdalus)
Macadamia nut (roasted, Macadamia integrifolia), sesame 0-0.1
seed (roasted), mango (raw)
Mango (canned), cauliflower (cooked), tamarind (Tamarindus 7
indica), pear brandy
Apple brandy, beetroot (cooked),artichoke (cooked, Cynarus < 0.01
scolymus L.), gin, rice bran, traditional rice (cooked), quince
(Cydonia oblonga), radish (fermented), shoyu (fermented soya
hydrolysate), bacuri (Platonia insignis), cupuacu (Theobroma
grandiflora), muruci (Brysonima crassifolia)
Potato (sweet, heated), sukiyaki, licorice (Glycyrrhiza glabra L.), < 0.01
matsutake (Tricholoma matsutake), strawberry wine, pumpkin
(Cucurbita pepo L.), sake, oat groats, maize, cashew apple
(Anacardium occidentale), basil (Ocimum basilicum), malt,
peated malt, wort, bonito (dried, katsuobishi), elderberry
(Sambucus nigra L.), mangosteen (Garcinia mangostana),
cherimoya (Annona cherimola), bilberry wine, buchu oil,
vanilla, mountain papaya (Carica pubescens)
Wild rice (Zizania aquatica), chicory (Cichorium intrybus L.), 0-0.2
endive (Cichorium endivia L.), ouzo
Sapodilla fruit (Achras sapota L.) Trace
Aubergine (Solanum melongena L.), pistachio nut (roasted, 17.2
Pistachia vera), arrack
Nectarine < 0.01
From Maarse & Visscher (1994)
Intake (µg/kg bw per day) calculated as follows:
{[(annual volume, kg) × (1 × 109 mg/kg) × (1/60 kg bw)]/[population × 0.6 ×
365 days]}, where population (10%, 'eaters only') = 32 × 106 for Europe and
24 × 106 for the United States; 0.6 represents the assumption that only 60%
of the flavour volume was reported in the survey (National Academy of Sciences,
1987; International Organization of the Flavor Industry, 1995). Slight
variations may occur from rounding off.
polysaccharides which contain pentose and hexose fragments. Furfural
has been detected in a broad range of fruits and fruit juices, wines,
whiskeys, coffee, and tea (Maarse et al., 1994). The highest
concentrations of furfural in foods have been reported in cocoa and
coffee (55-255 ppm), alcoholic beverages (1-33 ppm), and whole-grain
bread (26 ppm). Furfuryl alcohol, which is readily converted to
furfural in vivo (Rice, 1972; Nomeir et al., 1992), has been found
in the highest concentrations in heated skim milk (230 ppm) and coffee
(90-881 ppm). The total potential daily per capita intake of
furfural and precursors of furfural (i.e. furfuryl alcohol and
furfuryl esters) from consumption of foods in which they occur
naturally (Stofberg & Grundschober, 1987) is approximately 0.3 mg/kg
bw per day (i.e. about 300 µg/kg bw per day) in the United States.
Thus, the intake of furfural and furfuryl derivatives from use as
flavouring substances represent 1-3% of the total intake.
4. COMMENTS
In both humans and rodents, furfural is efficiently metabolized
by oxidation of the aldehyde function to furoic acid, most of which
is conjugated with glycine and excreted. A minor proportion (< 5%)
of furoic acid is condensed with acetyl coenzyme A to form
furanacryloyl coenzyme A; the resulting furanacryloic acid is
conjugated with glycine and excreted in the urine. About 5% of
[carboxyl-14C]-labelled furfural is eliminated by rats and mice as
14C-carbon dioxide. The metabolic pathway, which could involve direct
decarboxylation or epoxidation and ring opening, has not been defined.
In short-term studies, furfural was clearly hepatotoxic at doses
> 90 mg/kg bw per day in male rats and > 150 mg/kg per day in
mice. Minor changes reported in the livers of male rats given furfural
at lower doses by gavage in corn oil for 13 weeks were also present to
a lesser extent in vehicle controls. In a study of developmental
toxicity, furfural was not toxic to rats at 150 mg/kg bw per day, the
highest dose tolerated.
Furfural, like other aldehydes such as endogenous acetaldehyde,
is a reactive aldehyde; it is reported to bind to soluble proteins and
protein components of cell membranes. Various metabolic processes
(i.e. oxidation, conjugation, and condensation) effectively eliminate
the reactive aldehyde functional group when these metabolic pathways
are not saturated by high, unphysiological doses.
Furfural was not genotoxic in most tests in vitro. Positive
results were reported at relatively high concentrations in only three
of 16 assays for reverse mutation in Salmonella typhimurium and in
one of three rec assays in B. subtilis. A few chromosomal
aberrations were seen in Chinese hamster ovary cells exposed to
relatively high concentrations. Sister chromatid exchanges and forward
mutations were induced in mouse lymphoma cells. This weak activity of
furfural in vitro in some tests for genotoxicity might be explained
by its aldehyde reactivity. Recent studies of unscheduled DNA
synthesis in hepatocytes of rats treated in vivo and in human liver
slices gave negative results. Negative results were found in tests for
genotoxicity in vivo, except in Drosophila injected with furfural.
In a two-year study of carcinogenicity in rats given furfural in
corn oil by gavage at doses of 30 or 60 mg/kg bw per day, bile-duct
hyperplasia and cholangiofibrosis were seen in essentially all rats,
the controls having the highest incidence. Mild hepatocellular
necrosis was seen in all groups, however, at higher rates in males in
all treated groups. Two cholangio-carcinomas were observed in males at
the high dose, but the incidence was not statistically significant.
In a study of carcinogenicity in mice, the combined incidence of
adenomas and carcinomas of the liver (64%) was significantly
(p < 0.01) increased in males at the high dose (175 mg/kg bw per
day) but not in females. Hepatocellular adenomas and carcinomas also
occurred in the controls and in animals at the low (50 mg/kg bw per
day) and intermediate (100 mg/kg bw per day) doses, at incidences of
34-44% in males and 6-14% in females. The incidences in historical
controls were 38% (14-50%) in males and 6.2% (0-30%) in females.
Hepatotoxicity, manifested by features such as focal and multifocal
necrosis and chronic inflammation, was seen in all groups, including
the controls, but was considerably more frequent in mice at the high
dose.
Studies of oncogene activation in samples of liver tumours from
treated mice in the study of carcinogenicity revealed some differences
in the pattern of mutations from those in liver tumours of controls.
As it was not possible to identify the animals from which the tumours
originated and since hepatoxicity was seen at the intermediate and
high doses, it was not possible to determine whether a direct
genotoxic event or a secondary genotoxic pathway is involved.
5. EVALUATION
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL was identified for hepatotoxicity
in rats, the Committee was unable to allocate an ADI. Before reviewing
the substance again, the Committee would wish to review the results of
studies of DNA binding or adduct formation in vivo to clarify
whether furfural interacts with DNA in mice and of a 90-day study of
toxicity in rats to identify a NOEL for hepatotoxicity.
6. REFERENCES
Aeschbacher, H., Wolleb, U., Löliger, J., Spadone, J.C. & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Boyland, E. (1940) Experiments on the chemotherapy of cancer. 4.
Further experiments with aldehydes and their derivatives. Biochem.
J., 34, 1196-1201.
Brabec, M.J. (1981) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, Third
revised Ed., New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Caldwell, J. (1982) Conjugation reactions in foreign-compound
metabolism: Definition, consequences, and species variations.
J. Drug Metab. Rev., 13, 745-777.
Castellino, N., Elmino, O. & Rozera, G. (1963) Experimental research
on the toxicity of furfural. Arch. Environ. Health, 7, 574-582.
Dillon, D.M., McGregor, D.B., Combes, R.D. & Zeiger, E. (1992)
Detection of mutagenicity in Salmonella of some aldehydes and
peroxides. Environ. Mol. Mutag. 17, 15.
Donahue, T.M., Tuma, D.J. & Sorrell, M.F. (1983) Acetaldehyde adducts
with proteins: Binding of [14C]acetaldehyde to serum albumin.
Arch. Biochem. Biophys., 220, 239-246.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Clayton, G.D. & Clayton, F.E.,
eds, Patty's Industrial Hygiene and Toxicology, Third revised Ed.,
New York, John Wiley & Sons, Vol. IIB, pp. 2629-2669.
Eder, E., Deininger, C. & Muth, D. (1991) Genotoxicity of
p-nitrocinnamaldehyde and related alpha,ß-unsaturated carbonyl
compounds in two bacterial assays. Mutagenesis, 6, 261-269.
Feron, V.J. (1972) Respiratory tract tumors in hamsters after
intratracheal instillations of benzo(a)pyrene alone and with furfural.
Cancer Res., 32, 28-36.
Feron, V.J. & Kruysse, A. (1978) Effects of exposure to furfural
vapour in hamsters simultaneously treated with benzo(a)pyrene or
diethylnitrosamine. Toxicology, 11, 127-144.
Flek, J. & Sedivec, V. (1978) The absorption, metabolism and excretion
of furfural in man. Arch. Occup. Environ. Health, 41, 159-168.
Friedmann, E. (1911) [Fate of furanacrylic acid and furoyl acid in the
animal body.] Biochem. Z., 35, 40-48 (in German).
Gaines, K.C., Salany, J.M., Tuma, D.J. & Sorrell, M.F. (1977) Reaction
of acetaldehyde with human erythrocyte membrane proteins. FEBS
Lett., 75, 115-119.
Gomez-Arroyo, S. & Souza, V.S. (1985) In vitro and occupational
induction of sister-chromatid exchanges in human lymphocytes with
furfuryl alcohol and furfural. Mutat. Res., 156, 233-238.
Gregus, Z., Fekete, T., Varga, F. & Klaassen, C.D. (1993) Dependence
of glycine conjugation on availability of glycine: Role of the glycine
cleavage system. Xenobiotica, 23, 141-153.
Gupta, G.D., Misra, A. & Agarwal, D.K. (1991) Inhalation toxicity of
furfural vapours: An assessment of biochemical response in rat lungs.
J. Appl. Toxicol., 11, 343-347.
Hadi, S.M., Rehman, S. & Rehman, A. (1989) Specificity of the
interaction of furfural with DNA. Mutat. Res., 225, 101-106.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association of the United States, Washington
DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Jonek, J.J., Konecki, J. & Kaminski, M. (1975) Histoenzymatic changes
in liver in acute poisoning with furfural. J. Morphol. Embryol., 21,
47-51.
Kaminska, O. (1977) Morphological and histochemical investigations of
liver and kidneys in different stage of furfural poisoning. Folio
Histochem. Cytochem., 15, 150-151.
Kato, F., Araki, A., Nozaki, K. & Matsushima, T. (1989) Mutagenicity
of aldehydes and diketones. Mutat. Res., 216, 366.
Khan, Q.A. & Hadi, S.M. (1993) Effect of furfural on plasmid DNA.
Biochem. Mol. Biol. Int., 29, 1153-1160.
Kim, S.B., Hayase, F. & Kato, H. (1987) Desmutagenic effect of
alpha-dicarbonyl and alpha-hydroxy-carbonyl compounds against
mutagenic heterocyclic amines. Mutat. Res., 177, 9-15.
Koenig, K. & Andreesen, J. R. (1990) Xanthine dehydrogenase and
2-furoyl-coenzyme A dehydrogenase from Pseudomonas p. Fu1; two
molybenum-containing dehydrogenases of novel structural composition.
J. Bacteriol., 172, 5999-6009.
Kruysse, A. (1972). Acute inhalation toxicity of furfural in hamsters.
Unpublished report No. R 3853 from Central Institute for Nutrition and
Food Research TNO, Zeist, Netherlands.
Kuznetsov, P.I. (1966) Toxicological characteristics of furfural. II.
Effect of furfural on certain functions of the animal under conditions
of sub-acute poisoning. Nauch. Trud. Omskii Med. Inst. Imeni M.I.
Kalinina, 69, 41-44.
Lake, B.G., Adams, T.B., Beamad, J.A., Price, R.J., Ford, R.A. &
Goodman, J.I. (1998) An investigation of the effect of furfural on the
unscheduled DNA synthesis in cultured human liver slices. Unpublished
report to the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Loquet, C., Toussant, G. & LeTalaer, J.Y. (1981) Studies on mutagenic
constituents of apple brandy and various alcoholic beverages collected
in western France, a high incidence area for oesophageal cancer.
Mutat. Res., 88, 155-164.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148,
25-34.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D. &
Caspary, W.J. (1988) Responses of the L5178Y tk+/- mouse lymphoma
cell forward mutation assay. II: 18 coded chemicals. Environ. Mol.
Mutag., 11, 91-118.
McMahon, R.E., Cline, J.C. & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39, 682-693.
Mellick, P.W., Leach, C.L., Chou, B.J., Irwin, R.D. & Roycroft, J.H.
(1991) Nasal toxicity in Fischer 344 rats inhaling furfuryl alcohol
for 2 or 13 weeks. Toxicologist, 11, 183.
Mishra, A., Dwivedi, P.D., Verma, A.S., Sinha, M., Mishra, J., Lal,
K., Pandya, K.P. & Dutta, K.K. (1991) Pathological and biochemical
alterations induced by inhalation of furfural vapor in rat lung.
J. Bull. Environ. Contam. Toxicol., 47, 668-674.
Moreno, O. (1976) Dermal toxicity in rabbits. Report No. MB 75-941
from MB Research Laboratories Inc. Submitted to WHO by the Flavor &
Extract Manufacturers' Association of the United States, Washington
DC, United States
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Trainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from
the testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nagasawa, H.T., Goon, D.J.W., Conastotino, N.V. & Alexander, C.S.
(1980) Diversion of ethanol metabolism by sulfhydryl amino acids.
D-Penicillamine-directed excretion of
2,5,5-trimethyl-D-thiazolidine-4-carboxylic acid in the urine of rats
after ethanol administration. Life Sci., 17, 707-714.
Nakamura, S.-I., Oda, Y., Shimada, T., Oki, I. & Sugimoto, K. (1987)
SOS-inducing activity of chemical carcinogens and mutagens in
Salmonella typhimurium TA1535/pSK1002: Examination with 151
chemicals. Mutat. Res., 192, 239-246.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
National Toxicology Program (1987) Studies of chemical disposition in
mammals: The effect of dose on the chemical disposition of furfural in
rats after oral administration. NIEHS Contract No. N01-ES-66138 to
Arthur D. Little, Inc, Research Triangle Park, North Carolina, United
States.
National Toxicology Program (1990) Toxicology and carcinogenesis
studies of furfural (CAS No. 98-01-1) in Fischer 344/N rats and
B6C3F1 mice (gavage studies). National Toxicology Program Technical
Report Series No. 382. Research Triangle Park, North Carolina, United
States.
Nomeir, A.A., Silveria, D.M., McComish, M.F. & Chadwick, M. (1992)
Comparative metabolism and disposition of furfural and furfuryl
alcohol in rats. Drug Metab. Disposition, 20, 198-204.
Nomura, F. & Lieber,C.S. (1981) Binding of acetaldehyde to rat liver
microsomes: Enhancement after chronic alcohol consumption. Biochem.
Biophys. Res. Commun., 100, 131-137.
Nutley, B.P., Farmer, P. & Caldwell, J. (1994) Metabolism of
trans-cinnamic acid in the rat and the mouse and its variation with
dose. J. Food Chem. Toxicol., 32, 877-886.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Agric.
Biol. Chem., 45, 2299-2304.
Parkash M.K. & Caldwell J. (1994) Metabolism and excretion of
[14C]-furfural in the rat and the mouse. Food Chem. Toxicol., 32,
887-895.
Paul, H.E., Austin, F.L., Paul, M.F. & Ells, U.R. (1949) Metabolism of
the nitrofurans. I. Ultraviolet absorption studies of the urinary
products after oral administration. J. Biol. Chem., 180, 345-363.
Peterson, C.M. & Nguyen, L.B. (1985) Clinical implications of
acetaldehyde adducts with hemoglobin. In: Aldehyde Adducts in
Alcoholism, pp. 19-30.
Phillips, B.J., Jackson, L.I., Tate, B., Price, R.J., Adams, T.B.,
Ford, R.A., Goodman, J.I. & Lake, B.J. (1997) Furfural does not induce
unscheduled DNA synthesis (UDS) in the in vivo rat hepatocyte DNA
repair assay (Abstract). In Proceedings of the Society of Toxicology
Annual Meeting, 1997, Cincinnati, Ohio, New York, Academic Press.
Pickett, C.B. & Lu, A.Y.H. (1988) The structure, genetics and
regulation of soluble glutathione S-transferases. In: Sies, H. &
Ketterer, B., eds, Glutathione Conjugation: Mechanisms and
Biological Significance, New York, Academic Press, p. 147. A
Ramsdell, H.S. & Eaton, D.L. (1990) Species susceptibility to
aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal
biotransformation Cancer Res., 50, 615.
Ravindranath, A. & Boyd, M.R. (1985) Metabolic activiation of
2-methylfuran by rat microsomal systems. Toxicol. Appl. Pharmacol.,
78, 370-376.
Reynolds, S.H., Stowers, S.J., Patterson, R.M., Maronpot, R.R.,
Aaronson, S.A. & Anderson, M.W. (1987) Activated oncogenes in B6C3F1
mouse liver tumors: Implications for risk assessment. Science, 237,
1309-1316.
Rice, W.W. (1972) Furfural: Exogenous precursor of certain urinary
furans and possible toxicologic agent in humans. Clin. Chem., 18,
1550-1551.
Rodriguez-Arnaiz, R., Morales, P.R. & Zimmering, S. (1992) Evaluation
in Drosophila melanogaster of the mutagenic potential of furfural in
the mei-9(a) test for chromosome loss in germ-line cells. Mutat.
Res., 280, 75-80.
Rodriguez-Arnaiz, R., Morales, P.R., Moctezuma, R.V. & Salas, R.M.B.
(1989) Evidence for the absence of mutagenic activity of furfuryl
alcohol in tests of germ cells in Drosophila melanogaster. Mutat.
Res., 223, 309-311.
Sasaki, Y. & Endo, R. (1978) Mutagenicity of aldehydes in
Salmonella. Mutat. Res., 54, 251-252.
Schafer, E.W., Bowles, W.A. & Hurlbut, J. (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch. Environ. Contam.
Toxicol., 12, 355-382.
Shane, B.S., Troxclair, A.M., McMillin, D.J. & Henry, C.B. (1988)
Comparative mutagenicity of nine brands of coffee to Salmonella
typhimurium TA100, TA102, and TA104. Environ. Mol. Mutag., 11,
195-206.
Shimizu, A. (1986) Experimental studies on hepatic cirrhosis and
hepatocarcinogenesis. II. Influence of cirrhotic liver on 2-FAA
hepatocarcinogenesis in rats. Acta Pathol Jpn., 36,1039-1048.
Shimizu, A. & Kanisawa, M. (1986) Experimental studies on hepatic
cirrhosis and hepatocarcinogenesis. I. Production of hepatic cirrhosis
by furfural administration. Acta Pathol. Jpn., 36, 1027-1038.
Shimizu, A., Yoshiyasu, N., Harada, M., Ono, T., Sato, K., Inoue, T. &
Kanisawa, M. (1989) Positive foci of glutathione S-transferase
placental form in the liver of rats given furfural by oral
administration. Jpn. J. Cancer Res., 80, 608-611.
Shinohara, K., Kim, E. & Omura, H. (1986) Furans as the mutagens
formed by amino-carbonyl reactions. In: Fujimaki, M., Namiki, M. &
Kato, H., eds, Amino-Carbonyl Reactions in Food and Biological
Systems, New York, Elsevier.
Stich, H.F., Rosin, M.P., Wu, C.H. & Powrie, W.D. (1981a)
Clastogenicity of furans found in food. Cancer Lett., 13, 89-95.
Stich, H.F., Rosin, M.P., San, R.H., Wu, C.H. & Powrie, W.D. (1981b)
Intake, formation, and release of mutagens by man. In:
Gastrointestinal Cancer (Banbury Report 7), Cold Spring Harbor, NY,
CSH Press, pp. 247-266.
Stofberg J. & Grundschober F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Subramanyam, S., Sailaja, D. & Rathnaprabha, D. (1989) Genotoxic assay
of two dietary furans by some in vivo cytogenetic parameters.
Environ. Mol. Mutag., 14, 1-252.
Til, H.P., Woutersen, R.A., Feron, V.J., Hollanders, V.H.M., Falke,
H.E. & Clary, J.J. (1989) Two-year drinking-water study of
formaldehyde in rats. Food Chem. Toxicol., 27, 77-78.
Tiunov, L.A., Vasilev, G.A. & Ivanova, L.V. (1970) Effect of frequency
of injection on the toxic action of furfural. Bull. Exp. Biol.
Med., 70, 999-1000.
Tuma, D.J., Donahue, T.M., Medina, V.A. & Sorrell, M.F. (1984)
Enhancement of acetaldehyde-protein adduct formation by L-ascorbate.
Arch. Biochem. Biophys., 234, 377-381.
Videlia, L.A., Fernandez, V., deMarinis, A., Fernandez, N. &
Valenzuala, A. (1982) Liver lipoperoxidative pressure and glutathione
status following acetaldehyde and aliphatic alcohols pretreatments in
the rat. Biochem. Biophys. Res. Commun., 104, 965-970.
US Environmental Protection Agency (1997) Summary report: A dose range
finding development toxicity study of furfural in rats (TSCA
submission 8EHQ-1097-14026), Washington DC.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
Zdzienicka, M., Tudek, B., Zielenska, M. & Szymczyk, T. (1978)
Mutagenic activity of furfural in Salmonella typhimurium TA100.
Mutat. Res., 58, 205-209.
