
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
SAFETY EVALUATIONS OF GROUPS OF RELATED SUBSTANCES BY THE PROCEDURE
FOR THE SAFETY EVALUATION OF FLAVOURING AGENTS
INTRODUCTION
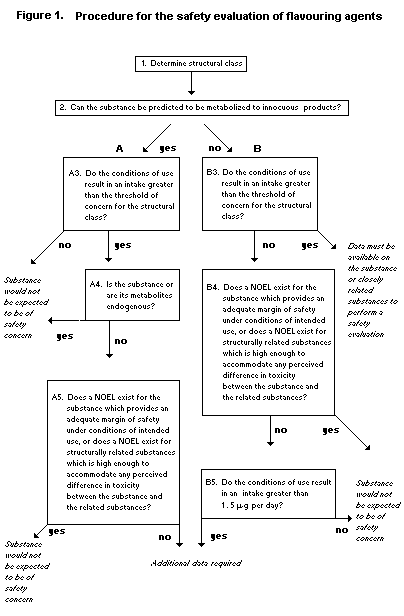
Seven groups of flavouring agents were evaluated by the Procedure
for the Safety Evaluation of Flavouring Agents outlined in Figure 1
(Annex 1, references 116, 122, and 131).
The Committee noted that in applying the Procedure, the substance
is first assigned to one of the structural classes identified at the
forty-sixth meeting of the Committee (Annex 1, reference 122):
* Class I. Substances that have simple chemical structures and
efficient modes of metabolism that would suggest a low order of
toxicity when given by the oral route
* Class II. Substances that have structural features that are less
innocuous than those of substances in Class I but are not
suggestive of toxicity. Substances in this class may contain
reactive functional groups.
* Class III. Substances that have structural features that permit
no strong initial presumption of safety or may even suggest
significant toxicity.
A key element of the Procedure involves determining whether a
flavouring agent and the product(s) of its metabolism are innocuous
and/or endogenous. For the purposes of evaluation, the Committee used
the following definitions, adapted from the report of its forty-sixth
meeting:
Innocuous metabolic products are defined as products that are
known or readily predicted to be harmless to humans at the estimated
intake of the flavouring agent.
Endogenous substances are intermediary metabolites normally
present in human tissues and fluids, whether free or conjugated;
hormones and other substances with biochemical or physiological
regulatory functions are not included. The estimated intake of a
flavouring agent that is, or is metabolized to, an endogenous
substance should not give rise to perturbations outside the
physiological range.
Intake
Intakes were estimated from surveys carried out in Europe and the
United States. Estimates of the intake of flavouring agents by
populations typically involve the acquisition of data on the amounts
used in food. In Europe, a survey was conducted in 1995 by the
International Organization of the Flavor Industry, in which flavour
manufacturers reported the total amount of each flavouring agent
incorporated into food sold in the European Union during the previous
year. Manufacturers were requested to exclude use of flavouring agents
in pharmaceutical, tobacco, and cosmetic products. In the United
States, a series of surveys was conducted between 1970 and 1987 by the
National Academy of Sciences National Research Council (under contract
to the Food and Drug Administration) in which information was obtained
from ingredient manufacturers and food processers on the amount of
each substance destined for addition to the food supply and on the
usual and maximal levels at which each substance was added to a number
of broad food categories.
In using data from these surveys to estimate the intakes of
flavouring agents, it was assumed that only 60% of the total amount
used is reported and that the total amount used in food is consumed by
only 10% of the population.
Intake annual volume of production (kg) × 109 (µg/kg)
=
(µg/person per day) population of consumers × 0.6 × 365 days
The population of consumers was assumed to be 31 × 106 in Europe and
24 × 106 in the United States.
General aspects of metabolism
The flavouring agents evaluated at the present meeting share a
number of functional groups, e.g. linear, branched, alicyclic, and
unsaturated alkyl chains and alcohol, ester, and ketone groups.
Therefore, they have a number of common and inter-related metabolic
features, and data on one or more members of a group being evaluated
can be used to predict the metabolic fate of analogues for which there
were no data. Metabolic pathways common to the groups of flavouring
agents evaluated at the present meeting are:
(i) hydrolysis of esters;
(ii) oxidation of alcohols and aldehydes;
(iii) conjugation of alcohols;
(iv) reduction of ketones;
(v) reduction of double bonds;
(vi) oxidation of side-chains ;
(vii) oxidation of alicyclics; and
(vii) conjugation with glutathione.
(i) Ester hydrolysis
The hydrolysis of ethyl, isoamyl, and allyl esters was considered
at the forty-sixth meeting of the Committee (Annex 1, reference 122).
Most esters are hydrolysed rapidly by enzymes present in the gut
lumen, intestinal wall, and liver to yield the corresponding acids and
alcohols.
 A wide range of enzymes (carboxyesterases and carboxylic acid
hydrolases) can hydrolyse esters into their constituent alcohols and
acids. Carboxyesterases are found in many tissues, and there is strong
esterase activity in the intestinal tract, blood, liver, kidney, lung,
brain, and pancreas. The multiplicity of esterases and their wide
tissue distribution result in rapid hydrolysis of esters in vivo,
and this extensive metabolic activity is the basis for the development
by the pharmaceutical industry of pro-drugs, which are carboxylic acid
esters of therapeutically active agents. The esters considered at the
present meeting contain larger, more complex alkyl constituents in
both the alcohol and the acid moieties. There was only limited
information on the hydrolysis of these esters in vitro, but the data
were comparable to those considered in the forty-sixth report of the
Committee. Isopropyl phenylacetate and isopropyl butyrate underwent
40% hydrolysis after 2 h with pancreatin (Grundschober, 1977), a value
similar to that for some of the esters considered at the previous
meeting. Essentially complete hydrolysis of isopropyl palmitate,
isopropyl oleate, and isopropyl stearate radiolabelled in the fatty
acid moiety was demonstrated in vivo by the detection of > 95% of
the radiolabel in the triglyceride fraction of rat blood (Savary &
Constantin, 1970). n-Octyl esters would be expected to undergo very
rapid hydrolysis on the basis of the relationships between increased
chain length (in the range C1-C8), increased Vmax, and decreased Km
(considered at the forty sixth meeting). The hydrolysis of branched-
chain octyl and larger esters would probably be slower than that of
the corresponding n-alkyl analogues, and absorption of the intact
ester is possible (see below).
The hydrolysis of esters with larger alcohol substituents, such
as are present in the linalyl, terpinyl, carvyl, and menthyl esters
evaluated at the present meeting, has been the subject of only limited
study. Linalyl acetate was hydrolysed rapidly in water and simulated
gastric and pancreatic fluids: the mean half-lives for hydrolysis were
5.5 min in gastric fluid and 52.5 min in pancreatic fluid (R.L. Hall,
personal communication to the Flavor and Extract Manufacturers'
Association of the United States), values comparable to those
considered at the forty-sixth meeting. Allyl tiglate and benzyl
tiglate were not hydrolysed by these simulated fluids but were
hydrolysed extensively on incubation with intestinal tissue
(Grundschober, 1977) and would be predicted to be completely
hydrolysed by the liver, both in vitro and in vivo. (-)-Menthol
ethylene glycol carbonate and (-)-menthol propylene glycol carbonate
were hydrolysed slowly on incubation with rat liver homogenate for 4
h, with 85% hydrolysis to menthol for (-)-menthol ethylene glycol
carbonate and 75% for (-)-menthol propylene glycol carbonate
(Emberger, personal communication to the Flavor and Extract
Manufacturers' Association of the United States). The structurally
related agent bornyl acetate was excreted largely as the glucuronic
acid conjugate of borneol after oral administration to rabbits
(Williams, 1959), consistent with extensive hydrolysis in vivo. More
than 80% of radiolabelled cyclandelate, a trimethyl cyclohexyl ester,
was hydrolysed after 20 min of incubation with rat hepatic microsomes
(White et al., 1990). Methyl cinnamate was hydrolysed only slowly in
the rat intestinal lumen in vivo after oral administration but
underwent essentially complete first-pass hydrolysis, because none of
the parent compound could be detected in the systemic circulation
(Fahelbum & James, 1977). In contrast, propyl anthranilate was not
completely hydrolysed during absorption, and the intact ester was
detected in peripheral blood and urine (Fahelbum & James, 1979). These
findings are similar to those concerning hydrolysis considered at the
forty-sixth meeting.
The results indicate that hydrolysis is the major metabolic
pathway for all of the esters evaluated at the present meeting;
however, some of the esters in some groups may undergo a low rate of
hydrolysis, resulting in incomplete pre-systemic metabolism to the
corresponding alcohol and acid. In general, esters that reach the
general circulation intact would be expected to be hydrolysed
extensively by tissue esterases into the acid and alcohol
constituents.
(ii) Oxidation of alcohols and aldehydes
(a) Primary alcohols and aldehydes
Primary aliphatic alcohols (attached to linear, branched, or
unsaturated alkyl chains) and the corresponding aldehydes are
efficiently oxidized to the corresponding carboxylic acids by
high-capacity enzyme pathways. NAD+/NADH-dependent alcohol
dehydrogenase catalyses the oxidation of primary aliphatic saturated
and unsaturated alcohols to the corresponding aldehyde (Pietruszko et
al., 1973). A comparison of the alcohol structure with the enzyme
binding affinity of alcohol dehydrogenase indicates that increased
binding (lower Km) occurs with increasing chain length (C1-C6) of
the substrate and the presence of unsaturation. The maximum rates of
oxidation were essentially constant, regardless of the alcohol
structure, suggesting that alcohol-enzyme binding is not the
rate-limiting step for oxidation; the activity of the enzyme appears
to be dependent on the lipophilic character of the alcohol substrate
(Klesov et al., 1977). The three classes of alcohol dehydrogenase
present in human liver (Eklund et al., 1990) show decreased Km with
increasing chain length from C2 to C8; the alcohol groups of
12-hydroxydodecanoic and 16-hydroxyhexadecanoic acid show Km values
similar to that of hexanol, indicating rapid oxidation of alcohols
arising from omega-oxidation reactions (see later).
Similarly, aldehyde dehydrogenase, which is present predominantly
in hepatic cytosol, has a broad substrate specificity for the
oxidation of aldehydes to carboxylic acids (Feldman & Weiner, 1972).
This enzyme is more active on aldehydes of higher relative molecular
mass (Nakayasu et al., 1978). Three isoenzymes of aldehyde
dehydrogenase, with overlapping substrate specificities, are present
in human liver, which oxidize a range of naturally occurring
aldehydes, such as gamma-butyraldehyde, 3,4-dihydroxyphenyl-
acetaldehyde, 5-hydroxyindoleacetaldehyde, and acrolein. Alkyl
aldehydes (C1-C6) have Km values similar to those of these natural
substrates, and the Km decreases with increasing chain length
(Ambroziak & Pietruszko, 1991). Xanthine oxidase and aldehyde oxidase
also catalyse the oxidation of a wide range of aldehydes to the
corresponding unsaturated acids (Beedham, 1988). Before oxidation to
the corresponding acid, the aldehyde may be conjugated with sulfhydryl
groups such as glutathione to yield thiohemiacetals. Oxidation of
aldehydes with low relative molecular masses requires glutathione
(Eckfeldt & Yonetani, 1982), which implies that the substrate for
aldehyde dehydroge-nase-mediated oxidation may be the thiohemiacetal
(Brabec, 1993).
Branched-chain aliphatic primary alcohols and aldehydes are
substrates for alcohol dehydrogenase and aldehyde dehydrogenase,
although the rates of oxidation are lower than those for linear
primary alcohols and aldehydes (Blair & Bodley, 1969; Hedlund &
Kiessling, 1969; Albro, 1975; Saito, 1975; Ambroziak & Pietruszko,
1991). Unsaturated, linear and branched-chain primary alcohols are
better substrates than the saturated analogues, but the reverse is
true of aldehydes (Pietruszko et al., 1973). Citral, a mixture of two
unsaturated branched-chain aldehydes (neral and geranial), was not
oxidized to the corresponding acid by aldehyde dehydrogenase in rat
liver preparations in vitro but was reduced by alcohol dehydrogenase
in the presence of NADH (Boyer & Petersen, 1990); oxidation is a major
metabolic pathway in vivo, resulting in acid metabolites.
(b) Secondary and tertiary alcohols
Secondary alcohol groups, such as those present in carveol and
ß-ionol, undergo reversible oxidation to the corresponding ketone (see
'ketone reduction', below). The alcohol is the more important form
in vivo because of its removal from the equilibrium by conjugation
with glucuronic acid. Tertiary alcohol groups, such as those present
in linalool and terpineol, do not undergo oxidative metabolism, and
tertiary alcohols are eliminated by conjugation.
(iii) Conjugation of alcohols
Conjugation represents the major pathway of metabolism for both
secondary and tertiary alcohols (Kamil et al., 1953). Extensive
conjugation with glucuronic acid is followed by excretion primarily in
the urine or bile, depending on the relative molecular mass and the
animal species. Glucuronic acid conjugation is a major phase-2
metabolic reaction, and both the liver and intestinal wall have a high
capacity for glucuronidation of a wide range of substrates. Sulfate
conjugation of alcohols occurs in many tissues, especially the liver,
and results in the formation of highly polar, water-soluble excretory
products. The urine is the main route of elimination of sulfate
conjugates of alcohols resulting from the metabolism of the flavouring
agents evaluated at the present meeting.
Glucuronic acid conjugation and excretion represent the primary
route of elimination of linalool, and conjugation with glucuronic acid
is the major pathway of metabolism of menthol. In rodents, the
glucuronic acid conjugates of linalool (Parke et al., 1974) and
menthol (Yamaguchi et al., 1994) are excreted primarily via the bile
into the intestine, where they may be hydrolysed to yield the free
alcohol, which undergoes reabsorption and subsequent oxidative
metabolism. The metabolites of menthol are eliminated in the urine and
faeces either unchanged or conjugated with glucuronic acid (Yamaguchi
et al., 1994). Bicyclic tertiary alcohols are relatively stable
in vivo, and in rabbits thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo[2.2.1]heptan-2-ol) are extensively conjugated
with glucuronic acid (Williams, 1938).
(iv) Reduction of ketones
Aliphatic ketones are metabolized primarily via reduction to
the corresponding secondary alcohol (Leibman, 1971; Felsted & Bachur,
1980; Bosron & Li, 1980). Reduction of aliphatic ketones is mediated
by alcohol dehydrogenases and NADH/NADPH-dependent cytosolic carbonyl
reductases (Bosron & Li, 1980). The reaction catalysed by carbonyl
reductase is stereoselective and favours formation of the S enantiomer
of the alcohol. The reaction is reversible under physiological
conditions, and oxidation of the secondary alcohol may lead to the
formation of the corresponding ketone in vivo (Felsted & Bachur,
1980). Ketones have been shown to undergo reduction to alcohols in
human hepatic microsomes (Leibman, 1971). Three reductases have been
purified from human liver cytosol: two aldehyde reductases, which can
reduce aliphatic aldehydes, alicyclic ketones, and alpha-diketones,
and a carbonyl reductase which is active with a broad range of
aldehyde and ketone substrates (Nakayama et al., 1985). Carbonyl
reductase activity in human liver shows limited interindividual
variability, and the enzyme reduces both 4-nitrobenzaldehyde and
4-nitroacetophenone (Iwata et al., 1993).
Alicyclic monoketones, such as cyclohexanone, are reduced to the
corresponding alcohols or undergo alpha-hydroxylation and reduction to
yield diols, which are excreted as the glucuronic acid conjugates.
Unsaturated ketones, such as menthone, dihydrocarvone, and
carvone, undergo extensive reduction of the ketone function to yield
the corresponding secondary alcohols (pulegone, dihydrocarveol, and
carveol), which are conjugated mainly with glucuronic acid and then
excreted in the urine (Hamalainen, 1912; Duterte-Catella et al., 1978;
Madyastha & Raj, 1993) or bile. In rodents, but probably not in
humans, the conjugate is excreted primarily into the bile and may then
be hydrolysed to yield the free alcohol (Matthews, 1994), which may
undergo enterohepatic recirculation and finally be excreted by the
kidney (Tamura et al., 1962). Conjugates with relative molecular
masses below about 500, such as the glucuronides of menthan-2-ol,
dihydrocarveol, and carveol, would be eliminated by humans in the
urine; the absence of significant biliary excretion would reduce the
extent of secondary oxidative metabolism.
In general, alicyclic alpha-diketones are metabolized via the
reduction pathway. Methyl-substituted diketones may be reduced to the
corresponding hydroxyketones and diols, which are excreted in the
urine as glucuronic acid conjugates. alpha-Hydroxyketones and their
diol metabolites may be excreted as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain-length ketones. If the carbonyl function is located
elsewhere on the chain, detoxification occurs predominantly by
reduction.
(v) Reduction of double bonds
Reduction of endocyclic and exocyclic double bonds occurs in some
of the flavouring agents evaluated at the present meeting. For
example, the endocyclic double bond of carvone is reduced to form
dihydrocarvone and dihydrocarveol, and the exocyclic double bond in
ß-ionone is reduced to give dihydro-ß-ionol (Ide & Toki, 1970). The
enzymes involved in these reactions have not been characterized; the
intestinal microflora may be involved, either before absorption or
after biliary excretion.
(vi) Oxidation of side-chains
The position and size of substituents in carboxylic acids
influences the route of metabolism. As chain length and lipophilicity
increase, omega-oxidation competes favourably with ß-oxidative
cleavage.
(a) ß -Oxidation
Linear and branched-chain saturated carboxylic acids are
substrates for ß cleavage to yield acetyl coenzyme A (acetyl CoA) and
a new CoA thioester of the carboxylic acid that has been reduced by
two carbon atoms. This process continues, to give complete oxidation
or until a branch point is reached. Acids with an even number of
carbon atoms continue to be cleaved to acetyl CoA, while acids with an
odd number of carbon atoms yield acetyl CoA and propionyl CoA. Acetyl
CoA enters the citric acid cycle directly, whereas propionyl CoA is
transformed into succinyl CoA which then enters the citric acid cycle.
Acids with a methyl substituent located at an even-numbered carbon
(e.g. 2-methylpentanoic acid and 4-methyldecanoic acid) are
metabolized to carbon dioxide via ß-oxidative cleavage in the fatty
acid pathway. If the methyl group is located at the 3 position,
ß-oxidation is inhibited and omega-oxidation predominates, leading
primarily to polar diacid metabolites that are excreted in the urine.
Ethyl or propyl substituents located at the alpha or ß position in
relation to the carboxylic acid group inhibit metabolism to carbon
dioxide (Deuel, 1957), in which case there is direct conjugation of
the acid with glucuronic acid or omega-oxidation followed by
conjugation.
Linear unsaturated carboxylic acids participate in normal fatty
acid metabolism. In this pathway, the saturated fatty acid is
condensed with CoA, which then undergoes catalytic dehydrogenation
mediated by acyl CoA dehydrogenase (Voet & Voet, 1990). The resulting
trans-2,3-unsaturated ester (trans-Delta2-enoyl CoA) is converted
to the 3-keto-thioester, which undergoes ß cleavage to yield an acetyl
CoA fragment and a new thioester reduced by two carbons. Cleavage of
acetyl CoA units continues along the carbon chain until the position
of unsaturation is reached. If unsaturation begins at an even-numbered
carbon, acetyl CoA fragmentation yields a Delta2-enoyl CoA product
which is a substrate for further fatty acid oxidation. If unsaturation
begins at an odd-numbered carbon, acetyl CoA fragmentation will
eventually yield Delta3-enoyl CoA, which cannot enter the fatty acid
cycle until it is isomerized to the trans-Delta2-enoyl CoA by enoyl
CoA isomerase. If the stereochemistry of the double bond is cis, it
is isomerized to the trans double bond by the action of
3-hydroxyacyl CoA epimerase before entering the fatty acid oxidation
pathway. Short-chain acids containing terminal unsaturation may be
metabolized via desaturation to yield a substrate which may
participate in the fatty acid pathway. For example, 4-pentenoic acid
is converted into the CoA thioester; this is dehydrogenated to yield
trans-2,4-pentadienoyl CoA, which undergoes NADPH-dependent
enzyme-catalysed reduction of the Delta2-alkene to
trans-2-pentenoic acid, which then participates in normal fatty acid
oxidation (Schulz, 1983). A second, more minor pathway involves
ß-oxidation to yield 3-keto-4-pentenoyl CoA.
(b) omega- and (omega-1)-Oxidation
omega- and (omega-1)-Oxidation reactions are important in the
elimination of compounds with long and/or complex alkyl chains,
because they eventually yield polar acid metabolites that are
eliminated in the urine (Horning et al., 1976; Ventura et al., 1985;
Madyastha & Srivatsan, 1988a). omega-Oxidation results in the
formation of a primary alcohol, which may undergo further oxidation to
the corresponding carboxylic acid, which may then be excreted in the
urine. For example, linalool undergoes cytochrome P450-mediated
oxidation to form 8-hydroxylinalool and 8-carboxylinalool, whereas
geraniol is oxidized to 8-hydroxygeraniol, 8-carboxygeraniol, and the
dibasic acid, Hildebrandt's acid (Chadha & Madyastha, 1984). Citral,
the aldehyde analogue of geraniol, undergoes oxidation of the aldehyde
group in vivo followed by omega-oxidation to yield a mixture of
diacids and hydroxy acids (Diliberto et al., 1990). Menthol undergoes
omega-oxidation of the side-chain substituents to yield various
polyols and hydroxy acids (Madyastha & Srivatsan, 1988b; Yamaguchi et
al., 1994); the (-) isomer undergoes more extensive oxidation than the
(+) isomer (Williams, 1939).
Short-chain ketones (< C4, e.g. butanone) that contain a
carbonyl function at the 2 position, and alpha-diketones, such as
acetoin, may undergo omega-oxidation of the terminal methyl group to
give a diketocarboxylic acid, which would undergo decarboxylation to
yield an alpha-ketocarboxylic acid. As intermediary metabolites,
alpha-ketoacids undergo oxidative decarboxylation to yield carbon
dioxide and a simple aliphatic carboxylic acid, which may be
completely metabolized via the fatty acid pathway and citric acid
cycle. Almost one-half of a dose of radiolabelled acetoin,
administered by intravenous injection to rats, was eliminated as
carbon dioxide in expired air (Gabriel et al., 1972); about 20% was
eliminated as unidentified urinary metabolites. trans-Methyl styryl
ketone undergoes essentially complete metabolism of the methyl group
after oral administration to rats; the glycine conjugates of
phenylacetic and benzoic acid were the major metabolites in the urine,
while the intermediate methyl styryl carbinol was detected in plasma
(Sauer et al., 1997).
omega-Oxidation of ketones yields hydroxyketones which may be
further reduced to diols and excreted in the urine as glucuronic acid
conjugates. Participation in these pathways depends on chain length,
the position of the carbonyl function, and the dose (Dietz et al.,
1981; Topping et al., 1994). Longer-chain aliphatic ketones (i.e.
carbon chain length > C5) are metabolized primarily via
reduction, but omega- and/or (omega-1)-oxidation are competing
pathways at high concentrations. At high doses, aliphatic ketones may
undergo omega- and/or (omega-1)-oxidation to yield ketoacids and
diketones, respectively.
A number of the flavouring agents evaluated at the present
meeting are alicyclic ketones containing an alkyl or alkenyl side
chain; these undergo oxidation of the side chain (probably by
cytochrome P450: Madyastha & Raj, 1990) to form polar metabolites that
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Williams, 1959; Ishida et al.,
1989).
(c) Oxidation of double bonds
Oxidation of double bonds via an epoxide intermediate to form
diols is probably only a minor pathway of metabolism for a few of the
flavouring agents considered at the present meeting. For example,
alpha-terpineol is oxidized by cytochrome P450 in rats, via an epoxide
intermediate, to a 1,2-dihydroxy metabolite (Madyastha & Srivatsan,
1988a); this diol metabolite has been detected in humans after
ingestion of alpha-terpineol (Horning et al., 1976).
(vii) Oxidation of alicyclics
Cyclohexane and cyclohexene rings, which are present in the
carvone and ionone groups considered at the present meeting, are
rapidly oxidized in vivo by ring hydroxylation, even when other
pathways of elimination are present. For example, the main pathways of
elimination of cyclohexane are exhalation unchanged in expired air and
excretion in urine as glucuronic acid conjugates of cyclohexanol and
cyclohexane-1,2-diol (Elliott et al., 1959). Cyclohexylamine is highly
water-soluble and is excreted in the urine as both the parent compound
and the glucuronides of ring-hydroxylated metabolites, together with
small amounts of cyclohexane-1,2-diol formed via deamination and
ring hydroxylation (Renwick & Williams, 1972). Ring oxidation, which
is catalysed by cytochrome P450 (CYP3A4), is a major pathway of
metabolism for the substituted cyclohexene ring systems that are
present in vitamin A and its metabolites, such as 13- cis- and
all- trans-retinoic acid, as well as the ionone group of flavouring
agents. Oxidation of the substituted cyclohexene rings present in
retinoids and ionones occurs on the carbon adjacent to the double
bond.
(vii) Conjugation with glutathione
The vast majority of the flavouring agents evaluated at the
present meeting are metabolized by the pathways described above.
Possible exceptions are carvone, carveol (and its esters), and some
members of the ionone group in which a ketone group, or a precursor
secondary alcohol group, is located adjacent to a double bond or
conjugated double bonds (alpha,ß-unsaturated ketone). Glutathione
conjugation is an important detoxification pathway, especially for
reactive compounds. The products of conjugation with glutathione are
usually eliminated in the bile as the glutathione conjugates per se
and in the urine as the N-acetylcysteine metabolites produced from
the glutathione conjugates. Glutathione conjugates may also be split
by C-S lyase (ß-lyase) in the kidney and intestinal microflora, to
give a thiol compound; this reaction represents a bioactivation
process for some chemicals. Only limited data were available on the
metabolism of the ionone group of flavouring agents, and glutathione
conjugation products have not been reported as urinary metabolites of
ionones or of carvone. Oral administration of 20 mg carvone, by gavage
in corn oil, to mice daily for three days caused a reduction in the
concentration of glutathione in the liver and an increase in
glutathione transferase activity (Zheng et al., 1992); however, these
effects may not have been due to the alpha,ß-unsaturated ketone
moiety, because the decrease in glutathione was not found with
alpha,ß-unsaturated carvone analogues lacking the exocyclic allyl
group, while the increase in glutathione transferase activity was
detected with compounds such as para-menthan-2-one which contain
neither an alpha,ß-unsaturated ketone moiety nor an exocyclic allyl
group.
The potential for non-enzymatic interaction with glutathione was
shown in vitro (Portoghese et al., 1989). The greatest reactivity
with glutathione was found with the simplest alpha,ß-unsaturated
ketone (butenone), which had a rate of reaction about 1000 times
greater than that of oct-2-ene-4-one. Damascenone, the 1,3-endocyclic
diene analogue of damascone, and carvone, which were evaluated at the
present meeting, showed rates of reaction that were 17 000, 11 000,
and 320 000 times slower than that of butenone, which indicates low
reactivity.
Overall, the flavouring agents with an alpha,ß-unsaturated ketone
moiety that were evaluated at the present meeting have low reactivity,
and conjugation with glutathione would not be a major route of
metabolism. The low predicted reactivity is supported by the
toxicological data for the ionones and carvone.
REFERENCES
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Ambroziak, W. & Pietruszko, R. (1991) Human aldehyde dehydrogenase:
Activity with aldehyde metabolites of monoamines, diamines, and
polyamines. J. Biol. Chem., 20, 13011-13018.
Beedham, C. (1988) Molybdenum hydroxylases. In: Gorrod, J.W.,
Oelschlager, H. & Caldwell, J., eds, Metabolism of Xenobiotics,
London, Taylor & Francis, pp. 51-58.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
Partial purification and properties. Can. J. Biochem., 47, 265-272.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Boyer, C.S. & Petersen, D.R. (1990) The metabolism of
3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and
cytosolic fractions. Drug Metab. Disposition, 16, 81-86.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, Vol. IIA, pp. 283-327.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Deuel, H.J., ed. (1957) The Lipids, Theit Chemistry and
Biochemistry, New York, Wiley Interscience, Vol. III.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1990) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Duterte-Catella, H., Nguyen, P., Dang, Q. & Truhaut, R. (1978)
Metabolic transformations of the trimethyl-3,5,5, cycloxene-2, one-1
(isophorone). Toxicol. Eur. Res., 1, 209-216.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogeanse from horse liver. In: Wood, W.A., ed., Methods in
Enzymology, New York, Academic Press, pp. 474-479.
Eklund, H., Müller-Wille, P., Horjales, E., Futer, O., Holmquist, B.,
Vallee, B.L., Höög, J.-O., Kaiser, R. & Jörnvall, H. (1990) Comparison
of three classes of human liver alcohol dehydrogenase: Emphasis on
different substrate binding pockets. Eur. J. Biochem., 193, 303-310.
Elliott, T.H., Parke, D.V. & Williams, R.T. (1959) Studies in
detoxication: 79. The metabolism of cyclo[14C]hexane and its
derivatives. Biochem. J., 72, 193-200.
Fahelbum, I.M.S. & James, S.P. (1977) The absorption and metabolism of
methyl cinnamate. Toxicology, 7, 123-132.
Fahelbum, I.M.S. & James, S.P. (1979) Absorption, distribution and
metabolism of propyl anthranilate. Toxicology, 12, 75-87.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York,
Academic Press, pp. 281-293.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Grundschober, F. (1977) Toxicological assessment of flavouring esters.
Toxicology, 8, 387-390.
Hamalainen, J. (1912) [Concerning the behaviour of alicyclic compounds
with glucuronic acid in organisms.] Skand. Arch. Physiol., 27,
141-226 (in German).
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. 1. The oxidation of some liver aliphatic fusel
alcohols and their effect on the mitochondrial oxidation of various
substrates. Acta Pharmacol. Toxicol., 27, 381-396.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.T. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxidediol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectrscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
Ishida, T., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(±)-7-hydroxycitronellal, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (±)-carvone in rabbits. Xenobiotica, 19,
843-855.
Iwata, N., Inazu, N., Hara, S., Yanase, T., Kano, S., Endo, T.,
Kuriiwa, F., Sato, Y. & Satoh, T. (1993) Interindividual variability
of carbonyl reductase levels in human livers. Biochem. Pharmacol.,
45, 1711-1714.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols: The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochemistry, 53,
129-136.
Klesov, A.A., Lange, L.G., Sytkowski, A.J. & Vallee, B.L. (1977)
[Unusual nature of the substrate specificity of alcohol dehydrogenase
of different origins.] Biorganich. Khim., 3, 1141-1144 (in Russian).
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Commun., 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone -- a hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Madyastha, K.M. & Srivatsan, V. (1988a) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Madyastha, K.M. & Srivatsan, V. (1988b) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Norwalk, Connecticut, Appleton &
Lange, pp. 177-192.
Nakayama, T., Hara, A., Yashiro, K. & Sawada, H. (1985) Reductases for
carbonyl compounds in human liver. Biochem. Pharmacol., 34, 107-117.
Nakayasu, H., Mihara, K. & Sato, R. (1978) Purification and properties
of membrane-bound aldehyde dehydrogenase from rat liver microsomes.
Biochem. Biophys. Res. Commun., 83, 697-703.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenases from human liver,
horse liver and yeast towards saturated and 2-enoic alcohols and
aldehydes. Arch. Biochem. Biophys., 159, 50-60.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Renwick, A.G. & Williams, R.T. (1972) The metabolites of
cyclohexylamine in man and certain animals. Biochem. J., 129,
857-867.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34, 569-585.
Sauer, J.-M., Smith, R.L., Bao, J., Kattnig, M.J., Kuester, R.K.,
McClure, T.D., Mayersohn, M. & Sipes, I.G. (1997) Oral and topical
absorption, disposition kinetics, and the metabolic fate of
trans-methyl styryl ketone in the male Fischer 344 rat. Drug
Metab. Disposition, 25, 732-739.
Savary, P. & Constantin, M.J. (1970) Intestinal hydrolysis and
lymphatic absorption of isopropyl asters of long chain fatty acids in
the rat. Biochem. Biophys. Acta, 218, 195.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. Biochemistry, 22,
1827-1832.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. I. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Fol.
Pharmacol. Jpn., 58, 323-336.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knight, S. &
Knight, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71-79.
Williams, R.T. (1938) Studies in detoxication. II. a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol in the rabbit. b) d-iso-Menthylglucuronide,
a new conjugated glucuronic acid. Biochem. J., 32, 1849-1855.
Williams, R.T. (1939) Studies in detoxication. III. The use of the
glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 33, 1519-1524.
Williams, R.T. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
A wide range of enzymes (carboxyesterases and carboxylic acid
hydrolases) can hydrolyse esters into their constituent alcohols and
acids. Carboxyesterases are found in many tissues, and there is strong
esterase activity in the intestinal tract, blood, liver, kidney, lung,
brain, and pancreas. The multiplicity of esterases and their wide
tissue distribution result in rapid hydrolysis of esters in vivo,
and this extensive metabolic activity is the basis for the development
by the pharmaceutical industry of pro-drugs, which are carboxylic acid
esters of therapeutically active agents. The esters considered at the
present meeting contain larger, more complex alkyl constituents in
both the alcohol and the acid moieties. There was only limited
information on the hydrolysis of these esters in vitro, but the data
were comparable to those considered in the forty-sixth report of the
Committee. Isopropyl phenylacetate and isopropyl butyrate underwent
40% hydrolysis after 2 h with pancreatin (Grundschober, 1977), a value
similar to that for some of the esters considered at the previous
meeting. Essentially complete hydrolysis of isopropyl palmitate,
isopropyl oleate, and isopropyl stearate radiolabelled in the fatty
acid moiety was demonstrated in vivo by the detection of > 95% of
the radiolabel in the triglyceride fraction of rat blood (Savary &
Constantin, 1970). n-Octyl esters would be expected to undergo very
rapid hydrolysis on the basis of the relationships between increased
chain length (in the range C1-C8), increased Vmax, and decreased Km
(considered at the forty sixth meeting). The hydrolysis of branched-
chain octyl and larger esters would probably be slower than that of
the corresponding n-alkyl analogues, and absorption of the intact
ester is possible (see below).
The hydrolysis of esters with larger alcohol substituents, such
as are present in the linalyl, terpinyl, carvyl, and menthyl esters
evaluated at the present meeting, has been the subject of only limited
study. Linalyl acetate was hydrolysed rapidly in water and simulated
gastric and pancreatic fluids: the mean half-lives for hydrolysis were
5.5 min in gastric fluid and 52.5 min in pancreatic fluid (R.L. Hall,
personal communication to the Flavor and Extract Manufacturers'
Association of the United States), values comparable to those
considered at the forty-sixth meeting. Allyl tiglate and benzyl
tiglate were not hydrolysed by these simulated fluids but were
hydrolysed extensively on incubation with intestinal tissue
(Grundschober, 1977) and would be predicted to be completely
hydrolysed by the liver, both in vitro and in vivo. (-)-Menthol
ethylene glycol carbonate and (-)-menthol propylene glycol carbonate
were hydrolysed slowly on incubation with rat liver homogenate for 4
h, with 85% hydrolysis to menthol for (-)-menthol ethylene glycol
carbonate and 75% for (-)-menthol propylene glycol carbonate
(Emberger, personal communication to the Flavor and Extract
Manufacturers' Association of the United States). The structurally
related agent bornyl acetate was excreted largely as the glucuronic
acid conjugate of borneol after oral administration to rabbits
(Williams, 1959), consistent with extensive hydrolysis in vivo. More
than 80% of radiolabelled cyclandelate, a trimethyl cyclohexyl ester,
was hydrolysed after 20 min of incubation with rat hepatic microsomes
(White et al., 1990). Methyl cinnamate was hydrolysed only slowly in
the rat intestinal lumen in vivo after oral administration but
underwent essentially complete first-pass hydrolysis, because none of
the parent compound could be detected in the systemic circulation
(Fahelbum & James, 1977). In contrast, propyl anthranilate was not
completely hydrolysed during absorption, and the intact ester was
detected in peripheral blood and urine (Fahelbum & James, 1979). These
findings are similar to those concerning hydrolysis considered at the
forty-sixth meeting.
The results indicate that hydrolysis is the major metabolic
pathway for all of the esters evaluated at the present meeting;
however, some of the esters in some groups may undergo a low rate of
hydrolysis, resulting in incomplete pre-systemic metabolism to the
corresponding alcohol and acid. In general, esters that reach the
general circulation intact would be expected to be hydrolysed
extensively by tissue esterases into the acid and alcohol
constituents.
(ii) Oxidation of alcohols and aldehydes
(a) Primary alcohols and aldehydes
Primary aliphatic alcohols (attached to linear, branched, or
unsaturated alkyl chains) and the corresponding aldehydes are
efficiently oxidized to the corresponding carboxylic acids by
high-capacity enzyme pathways. NAD+/NADH-dependent alcohol
dehydrogenase catalyses the oxidation of primary aliphatic saturated
and unsaturated alcohols to the corresponding aldehyde (Pietruszko et
al., 1973). A comparison of the alcohol structure with the enzyme
binding affinity of alcohol dehydrogenase indicates that increased
binding (lower Km) occurs with increasing chain length (C1-C6) of
the substrate and the presence of unsaturation. The maximum rates of
oxidation were essentially constant, regardless of the alcohol
structure, suggesting that alcohol-enzyme binding is not the
rate-limiting step for oxidation; the activity of the enzyme appears
to be dependent on the lipophilic character of the alcohol substrate
(Klesov et al., 1977). The three classes of alcohol dehydrogenase
present in human liver (Eklund et al., 1990) show decreased Km with
increasing chain length from C2 to C8; the alcohol groups of
12-hydroxydodecanoic and 16-hydroxyhexadecanoic acid show Km values
similar to that of hexanol, indicating rapid oxidation of alcohols
arising from omega-oxidation reactions (see later).
Similarly, aldehyde dehydrogenase, which is present predominantly
in hepatic cytosol, has a broad substrate specificity for the
oxidation of aldehydes to carboxylic acids (Feldman & Weiner, 1972).
This enzyme is more active on aldehydes of higher relative molecular
mass (Nakayasu et al., 1978). Three isoenzymes of aldehyde
dehydrogenase, with overlapping substrate specificities, are present
in human liver, which oxidize a range of naturally occurring
aldehydes, such as gamma-butyraldehyde, 3,4-dihydroxyphenyl-
acetaldehyde, 5-hydroxyindoleacetaldehyde, and acrolein. Alkyl
aldehydes (C1-C6) have Km values similar to those of these natural
substrates, and the Km decreases with increasing chain length
(Ambroziak & Pietruszko, 1991). Xanthine oxidase and aldehyde oxidase
also catalyse the oxidation of a wide range of aldehydes to the
corresponding unsaturated acids (Beedham, 1988). Before oxidation to
the corresponding acid, the aldehyde may be conjugated with sulfhydryl
groups such as glutathione to yield thiohemiacetals. Oxidation of
aldehydes with low relative molecular masses requires glutathione
(Eckfeldt & Yonetani, 1982), which implies that the substrate for
aldehyde dehydroge-nase-mediated oxidation may be the thiohemiacetal
(Brabec, 1993).
Branched-chain aliphatic primary alcohols and aldehydes are
substrates for alcohol dehydrogenase and aldehyde dehydrogenase,
although the rates of oxidation are lower than those for linear
primary alcohols and aldehydes (Blair & Bodley, 1969; Hedlund &
Kiessling, 1969; Albro, 1975; Saito, 1975; Ambroziak & Pietruszko,
1991). Unsaturated, linear and branched-chain primary alcohols are
better substrates than the saturated analogues, but the reverse is
true of aldehydes (Pietruszko et al., 1973). Citral, a mixture of two
unsaturated branched-chain aldehydes (neral and geranial), was not
oxidized to the corresponding acid by aldehyde dehydrogenase in rat
liver preparations in vitro but was reduced by alcohol dehydrogenase
in the presence of NADH (Boyer & Petersen, 1990); oxidation is a major
metabolic pathway in vivo, resulting in acid metabolites.
(b) Secondary and tertiary alcohols
Secondary alcohol groups, such as those present in carveol and
ß-ionol, undergo reversible oxidation to the corresponding ketone (see
'ketone reduction', below). The alcohol is the more important form
in vivo because of its removal from the equilibrium by conjugation
with glucuronic acid. Tertiary alcohol groups, such as those present
in linalool and terpineol, do not undergo oxidative metabolism, and
tertiary alcohols are eliminated by conjugation.
(iii) Conjugation of alcohols
Conjugation represents the major pathway of metabolism for both
secondary and tertiary alcohols (Kamil et al., 1953). Extensive
conjugation with glucuronic acid is followed by excretion primarily in
the urine or bile, depending on the relative molecular mass and the
animal species. Glucuronic acid conjugation is a major phase-2
metabolic reaction, and both the liver and intestinal wall have a high
capacity for glucuronidation of a wide range of substrates. Sulfate
conjugation of alcohols occurs in many tissues, especially the liver,
and results in the formation of highly polar, water-soluble excretory
products. The urine is the main route of elimination of sulfate
conjugates of alcohols resulting from the metabolism of the flavouring
agents evaluated at the present meeting.
Glucuronic acid conjugation and excretion represent the primary
route of elimination of linalool, and conjugation with glucuronic acid
is the major pathway of metabolism of menthol. In rodents, the
glucuronic acid conjugates of linalool (Parke et al., 1974) and
menthol (Yamaguchi et al., 1994) are excreted primarily via the bile
into the intestine, where they may be hydrolysed to yield the free
alcohol, which undergoes reabsorption and subsequent oxidative
metabolism. The metabolites of menthol are eliminated in the urine and
faeces either unchanged or conjugated with glucuronic acid (Yamaguchi
et al., 1994). Bicyclic tertiary alcohols are relatively stable
in vivo, and in rabbits thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo[2.2.1]heptan-2-ol) are extensively conjugated
with glucuronic acid (Williams, 1938).
(iv) Reduction of ketones
Aliphatic ketones are metabolized primarily via reduction to
the corresponding secondary alcohol (Leibman, 1971; Felsted & Bachur,
1980; Bosron & Li, 1980). Reduction of aliphatic ketones is mediated
by alcohol dehydrogenases and NADH/NADPH-dependent cytosolic carbonyl
reductases (Bosron & Li, 1980). The reaction catalysed by carbonyl
reductase is stereoselective and favours formation of the S enantiomer
of the alcohol. The reaction is reversible under physiological
conditions, and oxidation of the secondary alcohol may lead to the
formation of the corresponding ketone in vivo (Felsted & Bachur,
1980). Ketones have been shown to undergo reduction to alcohols in
human hepatic microsomes (Leibman, 1971). Three reductases have been
purified from human liver cytosol: two aldehyde reductases, which can
reduce aliphatic aldehydes, alicyclic ketones, and alpha-diketones,
and a carbonyl reductase which is active with a broad range of
aldehyde and ketone substrates (Nakayama et al., 1985). Carbonyl
reductase activity in human liver shows limited interindividual
variability, and the enzyme reduces both 4-nitrobenzaldehyde and
4-nitroacetophenone (Iwata et al., 1993).
Alicyclic monoketones, such as cyclohexanone, are reduced to the
corresponding alcohols or undergo alpha-hydroxylation and reduction to
yield diols, which are excreted as the glucuronic acid conjugates.
Unsaturated ketones, such as menthone, dihydrocarvone, and
carvone, undergo extensive reduction of the ketone function to yield
the corresponding secondary alcohols (pulegone, dihydrocarveol, and
carveol), which are conjugated mainly with glucuronic acid and then
excreted in the urine (Hamalainen, 1912; Duterte-Catella et al., 1978;
Madyastha & Raj, 1993) or bile. In rodents, but probably not in
humans, the conjugate is excreted primarily into the bile and may then
be hydrolysed to yield the free alcohol (Matthews, 1994), which may
undergo enterohepatic recirculation and finally be excreted by the
kidney (Tamura et al., 1962). Conjugates with relative molecular
masses below about 500, such as the glucuronides of menthan-2-ol,
dihydrocarveol, and carveol, would be eliminated by humans in the
urine; the absence of significant biliary excretion would reduce the
extent of secondary oxidative metabolism.
In general, alicyclic alpha-diketones are metabolized via the
reduction pathway. Methyl-substituted diketones may be reduced to the
corresponding hydroxyketones and diols, which are excreted in the
urine as glucuronic acid conjugates. alpha-Hydroxyketones and their
diol metabolites may be excreted as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain-length ketones. If the carbonyl function is located
elsewhere on the chain, detoxification occurs predominantly by
reduction.
(v) Reduction of double bonds
Reduction of endocyclic and exocyclic double bonds occurs in some
of the flavouring agents evaluated at the present meeting. For
example, the endocyclic double bond of carvone is reduced to form
dihydrocarvone and dihydrocarveol, and the exocyclic double bond in
ß-ionone is reduced to give dihydro-ß-ionol (Ide & Toki, 1970). The
enzymes involved in these reactions have not been characterized; the
intestinal microflora may be involved, either before absorption or
after biliary excretion.
(vi) Oxidation of side-chains
The position and size of substituents in carboxylic acids
influences the route of metabolism. As chain length and lipophilicity
increase, omega-oxidation competes favourably with ß-oxidative
cleavage.
(a) ß -Oxidation
Linear and branched-chain saturated carboxylic acids are
substrates for ß cleavage to yield acetyl coenzyme A (acetyl CoA) and
a new CoA thioester of the carboxylic acid that has been reduced by
two carbon atoms. This process continues, to give complete oxidation
or until a branch point is reached. Acids with an even number of
carbon atoms continue to be cleaved to acetyl CoA, while acids with an
odd number of carbon atoms yield acetyl CoA and propionyl CoA. Acetyl
CoA enters the citric acid cycle directly, whereas propionyl CoA is
transformed into succinyl CoA which then enters the citric acid cycle.
Acids with a methyl substituent located at an even-numbered carbon
(e.g. 2-methylpentanoic acid and 4-methyldecanoic acid) are
metabolized to carbon dioxide via ß-oxidative cleavage in the fatty
acid pathway. If the methyl group is located at the 3 position,
ß-oxidation is inhibited and omega-oxidation predominates, leading
primarily to polar diacid metabolites that are excreted in the urine.
Ethyl or propyl substituents located at the alpha or ß position in
relation to the carboxylic acid group inhibit metabolism to carbon
dioxide (Deuel, 1957), in which case there is direct conjugation of
the acid with glucuronic acid or omega-oxidation followed by
conjugation.
Linear unsaturated carboxylic acids participate in normal fatty
acid metabolism. In this pathway, the saturated fatty acid is
condensed with CoA, which then undergoes catalytic dehydrogenation
mediated by acyl CoA dehydrogenase (Voet & Voet, 1990). The resulting
trans-2,3-unsaturated ester (trans-Delta2-enoyl CoA) is converted
to the 3-keto-thioester, which undergoes ß cleavage to yield an acetyl
CoA fragment and a new thioester reduced by two carbons. Cleavage of
acetyl CoA units continues along the carbon chain until the position
of unsaturation is reached. If unsaturation begins at an even-numbered
carbon, acetyl CoA fragmentation yields a Delta2-enoyl CoA product
which is a substrate for further fatty acid oxidation. If unsaturation
begins at an odd-numbered carbon, acetyl CoA fragmentation will
eventually yield Delta3-enoyl CoA, which cannot enter the fatty acid
cycle until it is isomerized to the trans-Delta2-enoyl CoA by enoyl
CoA isomerase. If the stereochemistry of the double bond is cis, it
is isomerized to the trans double bond by the action of
3-hydroxyacyl CoA epimerase before entering the fatty acid oxidation
pathway. Short-chain acids containing terminal unsaturation may be
metabolized via desaturation to yield a substrate which may
participate in the fatty acid pathway. For example, 4-pentenoic acid
is converted into the CoA thioester; this is dehydrogenated to yield
trans-2,4-pentadienoyl CoA, which undergoes NADPH-dependent
enzyme-catalysed reduction of the Delta2-alkene to
trans-2-pentenoic acid, which then participates in normal fatty acid
oxidation (Schulz, 1983). A second, more minor pathway involves
ß-oxidation to yield 3-keto-4-pentenoyl CoA.
(b) omega- and (omega-1)-Oxidation
omega- and (omega-1)-Oxidation reactions are important in the
elimination of compounds with long and/or complex alkyl chains,
because they eventually yield polar acid metabolites that are
eliminated in the urine (Horning et al., 1976; Ventura et al., 1985;
Madyastha & Srivatsan, 1988a). omega-Oxidation results in the
formation of a primary alcohol, which may undergo further oxidation to
the corresponding carboxylic acid, which may then be excreted in the
urine. For example, linalool undergoes cytochrome P450-mediated
oxidation to form 8-hydroxylinalool and 8-carboxylinalool, whereas
geraniol is oxidized to 8-hydroxygeraniol, 8-carboxygeraniol, and the
dibasic acid, Hildebrandt's acid (Chadha & Madyastha, 1984). Citral,
the aldehyde analogue of geraniol, undergoes oxidation of the aldehyde
group in vivo followed by omega-oxidation to yield a mixture of
diacids and hydroxy acids (Diliberto et al., 1990). Menthol undergoes
omega-oxidation of the side-chain substituents to yield various
polyols and hydroxy acids (Madyastha & Srivatsan, 1988b; Yamaguchi et
al., 1994); the (-) isomer undergoes more extensive oxidation than the
(+) isomer (Williams, 1939).
Short-chain ketones (< C4, e.g. butanone) that contain a
carbonyl function at the 2 position, and alpha-diketones, such as
acetoin, may undergo omega-oxidation of the terminal methyl group to
give a diketocarboxylic acid, which would undergo decarboxylation to
yield an alpha-ketocarboxylic acid. As intermediary metabolites,
alpha-ketoacids undergo oxidative decarboxylation to yield carbon
dioxide and a simple aliphatic carboxylic acid, which may be
completely metabolized via the fatty acid pathway and citric acid
cycle. Almost one-half of a dose of radiolabelled acetoin,
administered by intravenous injection to rats, was eliminated as
carbon dioxide in expired air (Gabriel et al., 1972); about 20% was
eliminated as unidentified urinary metabolites. trans-Methyl styryl
ketone undergoes essentially complete metabolism of the methyl group
after oral administration to rats; the glycine conjugates of
phenylacetic and benzoic acid were the major metabolites in the urine,
while the intermediate methyl styryl carbinol was detected in plasma
(Sauer et al., 1997).
omega-Oxidation of ketones yields hydroxyketones which may be
further reduced to diols and excreted in the urine as glucuronic acid
conjugates. Participation in these pathways depends on chain length,
the position of the carbonyl function, and the dose (Dietz et al.,
1981; Topping et al., 1994). Longer-chain aliphatic ketones (i.e.
carbon chain length > C5) are metabolized primarily via
reduction, but omega- and/or (omega-1)-oxidation are competing
pathways at high concentrations. At high doses, aliphatic ketones may
undergo omega- and/or (omega-1)-oxidation to yield ketoacids and
diketones, respectively.
A number of the flavouring agents evaluated at the present
meeting are alicyclic ketones containing an alkyl or alkenyl side
chain; these undergo oxidation of the side chain (probably by
cytochrome P450: Madyastha & Raj, 1990) to form polar metabolites that
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Williams, 1959; Ishida et al.,
1989).
(c) Oxidation of double bonds
Oxidation of double bonds via an epoxide intermediate to form
diols is probably only a minor pathway of metabolism for a few of the
flavouring agents considered at the present meeting. For example,
alpha-terpineol is oxidized by cytochrome P450 in rats, via an epoxide
intermediate, to a 1,2-dihydroxy metabolite (Madyastha & Srivatsan,
1988a); this diol metabolite has been detected in humans after
ingestion of alpha-terpineol (Horning et al., 1976).
(vii) Oxidation of alicyclics
Cyclohexane and cyclohexene rings, which are present in the
carvone and ionone groups considered at the present meeting, are
rapidly oxidized in vivo by ring hydroxylation, even when other
pathways of elimination are present. For example, the main pathways of
elimination of cyclohexane are exhalation unchanged in expired air and
excretion in urine as glucuronic acid conjugates of cyclohexanol and
cyclohexane-1,2-diol (Elliott et al., 1959). Cyclohexylamine is highly
water-soluble and is excreted in the urine as both the parent compound
and the glucuronides of ring-hydroxylated metabolites, together with
small amounts of cyclohexane-1,2-diol formed via deamination and
ring hydroxylation (Renwick & Williams, 1972). Ring oxidation, which
is catalysed by cytochrome P450 (CYP3A4), is a major pathway of
metabolism for the substituted cyclohexene ring systems that are
present in vitamin A and its metabolites, such as 13- cis- and
all- trans-retinoic acid, as well as the ionone group of flavouring
agents. Oxidation of the substituted cyclohexene rings present in
retinoids and ionones occurs on the carbon adjacent to the double
bond.
(vii) Conjugation with glutathione
The vast majority of the flavouring agents evaluated at the
present meeting are metabolized by the pathways described above.
Possible exceptions are carvone, carveol (and its esters), and some
members of the ionone group in which a ketone group, or a precursor
secondary alcohol group, is located adjacent to a double bond or
conjugated double bonds (alpha,ß-unsaturated ketone). Glutathione
conjugation is an important detoxification pathway, especially for
reactive compounds. The products of conjugation with glutathione are
usually eliminated in the bile as the glutathione conjugates per se
and in the urine as the N-acetylcysteine metabolites produced from
the glutathione conjugates. Glutathione conjugates may also be split
by C-S lyase (ß-lyase) in the kidney and intestinal microflora, to
give a thiol compound; this reaction represents a bioactivation
process for some chemicals. Only limited data were available on the
metabolism of the ionone group of flavouring agents, and glutathione
conjugation products have not been reported as urinary metabolites of
ionones or of carvone. Oral administration of 20 mg carvone, by gavage
in corn oil, to mice daily for three days caused a reduction in the
concentration of glutathione in the liver and an increase in
glutathione transferase activity (Zheng et al., 1992); however, these
effects may not have been due to the alpha,ß-unsaturated ketone
moiety, because the decrease in glutathione was not found with
alpha,ß-unsaturated carvone analogues lacking the exocyclic allyl
group, while the increase in glutathione transferase activity was
detected with compounds such as para-menthan-2-one which contain
neither an alpha,ß-unsaturated ketone moiety nor an exocyclic allyl
group.
The potential for non-enzymatic interaction with glutathione was
shown in vitro (Portoghese et al., 1989). The greatest reactivity
with glutathione was found with the simplest alpha,ß-unsaturated
ketone (butenone), which had a rate of reaction about 1000 times
greater than that of oct-2-ene-4-one. Damascenone, the 1,3-endocyclic
diene analogue of damascone, and carvone, which were evaluated at the
present meeting, showed rates of reaction that were 17 000, 11 000,
and 320 000 times slower than that of butenone, which indicates low
reactivity.
Overall, the flavouring agents with an alpha,ß-unsaturated ketone
moiety that were evaluated at the present meeting have low reactivity,
and conjugation with glutathione would not be a major route of
metabolism. The low predicted reactivity is supported by the
toxicological data for the ionones and carvone.
REFERENCES
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Ambroziak, W. & Pietruszko, R. (1991) Human aldehyde dehydrogenase:
Activity with aldehyde metabolites of monoamines, diamines, and
polyamines. J. Biol. Chem., 20, 13011-13018.
Beedham, C. (1988) Molybdenum hydroxylases. In: Gorrod, J.W.,
Oelschlager, H. & Caldwell, J., eds, Metabolism of Xenobiotics,
London, Taylor & Francis, pp. 51-58.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
Partial purification and properties. Can. J. Biochem., 47, 265-272.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Boyer, C.S. & Petersen, D.R. (1990) The metabolism of
3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and
cytosolic fractions. Drug Metab. Disposition, 16, 81-86.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, Vol. IIA, pp. 283-327.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Deuel, H.J., ed. (1957) The Lipids, Theit Chemistry and
Biochemistry, New York, Wiley Interscience, Vol. III.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1990) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Duterte-Catella, H., Nguyen, P., Dang, Q. & Truhaut, R. (1978)
Metabolic transformations of the trimethyl-3,5,5, cycloxene-2, one-1
(isophorone). Toxicol. Eur. Res., 1, 209-216.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogeanse from horse liver. In: Wood, W.A., ed., Methods in
Enzymology, New York, Academic Press, pp. 474-479.
Eklund, H., Müller-Wille, P., Horjales, E., Futer, O., Holmquist, B.,
Vallee, B.L., Höög, J.-O., Kaiser, R. & Jörnvall, H. (1990) Comparison
of three classes of human liver alcohol dehydrogenase: Emphasis on
different substrate binding pockets. Eur. J. Biochem., 193, 303-310.
Elliott, T.H., Parke, D.V. & Williams, R.T. (1959) Studies in
detoxication: 79. The metabolism of cyclo[14C]hexane and its
derivatives. Biochem. J., 72, 193-200.
Fahelbum, I.M.S. & James, S.P. (1977) The absorption and metabolism of
methyl cinnamate. Toxicology, 7, 123-132.
Fahelbum, I.M.S. & James, S.P. (1979) Absorption, distribution and
metabolism of propyl anthranilate. Toxicology, 12, 75-87.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York,
Academic Press, pp. 281-293.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Grundschober, F. (1977) Toxicological assessment of flavouring esters.
Toxicology, 8, 387-390.
Hamalainen, J. (1912) [Concerning the behaviour of alicyclic compounds
with glucuronic acid in organisms.] Skand. Arch. Physiol., 27,
141-226 (in German).
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. 1. The oxidation of some liver aliphatic fusel
alcohols and their effect on the mitochondrial oxidation of various
substrates. Acta Pharmacol. Toxicol., 27, 381-396.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.T. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxidediol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectrscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
Ishida, T., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(±)-7-hydroxycitronellal, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (±)-carvone in rabbits. Xenobiotica, 19,
843-855.
Iwata, N., Inazu, N., Hara, S., Yanase, T., Kano, S., Endo, T.,
Kuriiwa, F., Sato, Y. & Satoh, T. (1993) Interindividual variability
of carbonyl reductase levels in human livers. Biochem. Pharmacol.,
45, 1711-1714.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols: The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochemistry, 53,
129-136.
Klesov, A.A., Lange, L.G., Sytkowski, A.J. & Vallee, B.L. (1977)
[Unusual nature of the substrate specificity of alcohol dehydrogenase
of different origins.] Biorganich. Khim., 3, 1141-1144 (in Russian).
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Commun., 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone -- a hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Madyastha, K.M. & Srivatsan, V. (1988a) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Madyastha, K.M. & Srivatsan, V. (1988b) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Norwalk, Connecticut, Appleton &
Lange, pp. 177-192.
Nakayama, T., Hara, A., Yashiro, K. & Sawada, H. (1985) Reductases for
carbonyl compounds in human liver. Biochem. Pharmacol., 34, 107-117.
Nakayasu, H., Mihara, K. & Sato, R. (1978) Purification and properties
of membrane-bound aldehyde dehydrogenase from rat liver microsomes.
Biochem. Biophys. Res. Commun., 83, 697-703.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenases from human liver,
horse liver and yeast towards saturated and 2-enoic alcohols and
aldehydes. Arch. Biochem. Biophys., 159, 50-60.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Renwick, A.G. & Williams, R.T. (1972) The metabolites of
cyclohexylamine in man and certain animals. Biochem. J., 129,
857-867.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34, 569-585.
Sauer, J.-M., Smith, R.L., Bao, J., Kattnig, M.J., Kuester, R.K.,
McClure, T.D., Mayersohn, M. & Sipes, I.G. (1997) Oral and topical
absorption, disposition kinetics, and the metabolic fate of
trans-methyl styryl ketone in the male Fischer 344 rat. Drug
Metab. Disposition, 25, 732-739.
Savary, P. & Constantin, M.J. (1970) Intestinal hydrolysis and
lymphatic absorption of isopropyl asters of long chain fatty acids in
the rat. Biochem. Biophys. Acta, 218, 195.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. Biochemistry, 22,
1827-1832.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. I. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Fol.
Pharmacol. Jpn., 58, 323-336.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knight, S. &
Knight, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71-79.
Williams, R.T. (1938) Studies in detoxication. II. a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol in the rabbit. b) d-iso-Menthylglucuronide,
a new conjugated glucuronic acid. Biochem. J., 32, 1849-1855.
Williams, R.T. (1939) Studies in detoxication. III. The use of the
glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 33, 1519-1524.
Williams, R.T. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
 A wide range of enzymes (carboxyesterases and carboxylic acid
hydrolases) can hydrolyse esters into their constituent alcohols and
acids. Carboxyesterases are found in many tissues, and there is strong
esterase activity in the intestinal tract, blood, liver, kidney, lung,
brain, and pancreas. The multiplicity of esterases and their wide
tissue distribution result in rapid hydrolysis of esters in vivo,
and this extensive metabolic activity is the basis for the development
by the pharmaceutical industry of pro-drugs, which are carboxylic acid
esters of therapeutically active agents. The esters considered at the
present meeting contain larger, more complex alkyl constituents in
both the alcohol and the acid moieties. There was only limited
information on the hydrolysis of these esters in vitro, but the data
were comparable to those considered in the forty-sixth report of the
Committee. Isopropyl phenylacetate and isopropyl butyrate underwent
40% hydrolysis after 2 h with pancreatin (Grundschober, 1977), a value
similar to that for some of the esters considered at the previous
meeting. Essentially complete hydrolysis of isopropyl palmitate,
isopropyl oleate, and isopropyl stearate radiolabelled in the fatty
acid moiety was demonstrated in vivo by the detection of > 95% of
the radiolabel in the triglyceride fraction of rat blood (Savary &
Constantin, 1970). n-Octyl esters would be expected to undergo very
rapid hydrolysis on the basis of the relationships between increased
chain length (in the range C1-C8), increased Vmax, and decreased Km
(considered at the forty sixth meeting). The hydrolysis of branched-
chain octyl and larger esters would probably be slower than that of
the corresponding n-alkyl analogues, and absorption of the intact
ester is possible (see below).
The hydrolysis of esters with larger alcohol substituents, such
as are present in the linalyl, terpinyl, carvyl, and menthyl esters
evaluated at the present meeting, has been the subject of only limited
study. Linalyl acetate was hydrolysed rapidly in water and simulated
gastric and pancreatic fluids: the mean half-lives for hydrolysis were
5.5 min in gastric fluid and 52.5 min in pancreatic fluid (R.L. Hall,
personal communication to the Flavor and Extract Manufacturers'
Association of the United States), values comparable to those
considered at the forty-sixth meeting. Allyl tiglate and benzyl
tiglate were not hydrolysed by these simulated fluids but were
hydrolysed extensively on incubation with intestinal tissue
(Grundschober, 1977) and would be predicted to be completely
hydrolysed by the liver, both in vitro and in vivo. (-)-Menthol
ethylene glycol carbonate and (-)-menthol propylene glycol carbonate
were hydrolysed slowly on incubation with rat liver homogenate for 4
h, with 85% hydrolysis to menthol for (-)-menthol ethylene glycol
carbonate and 75% for (-)-menthol propylene glycol carbonate
(Emberger, personal communication to the Flavor and Extract
Manufacturers' Association of the United States). The structurally
related agent bornyl acetate was excreted largely as the glucuronic
acid conjugate of borneol after oral administration to rabbits
(Williams, 1959), consistent with extensive hydrolysis in vivo. More
than 80% of radiolabelled cyclandelate, a trimethyl cyclohexyl ester,
was hydrolysed after 20 min of incubation with rat hepatic microsomes
(White et al., 1990). Methyl cinnamate was hydrolysed only slowly in
the rat intestinal lumen in vivo after oral administration but
underwent essentially complete first-pass hydrolysis, because none of
the parent compound could be detected in the systemic circulation
(Fahelbum & James, 1977). In contrast, propyl anthranilate was not
completely hydrolysed during absorption, and the intact ester was
detected in peripheral blood and urine (Fahelbum & James, 1979). These
findings are similar to those concerning hydrolysis considered at the
forty-sixth meeting.
The results indicate that hydrolysis is the major metabolic
pathway for all of the esters evaluated at the present meeting;
however, some of the esters in some groups may undergo a low rate of
hydrolysis, resulting in incomplete pre-systemic metabolism to the
corresponding alcohol and acid. In general, esters that reach the
general circulation intact would be expected to be hydrolysed
extensively by tissue esterases into the acid and alcohol
constituents.
(ii) Oxidation of alcohols and aldehydes
(a) Primary alcohols and aldehydes
Primary aliphatic alcohols (attached to linear, branched, or
unsaturated alkyl chains) and the corresponding aldehydes are
efficiently oxidized to the corresponding carboxylic acids by
high-capacity enzyme pathways. NAD+/NADH-dependent alcohol
dehydrogenase catalyses the oxidation of primary aliphatic saturated
and unsaturated alcohols to the corresponding aldehyde (Pietruszko et
al., 1973). A comparison of the alcohol structure with the enzyme
binding affinity of alcohol dehydrogenase indicates that increased
binding (lower Km) occurs with increasing chain length (C1-C6) of
the substrate and the presence of unsaturation. The maximum rates of
oxidation were essentially constant, regardless of the alcohol
structure, suggesting that alcohol-enzyme binding is not the
rate-limiting step for oxidation; the activity of the enzyme appears
to be dependent on the lipophilic character of the alcohol substrate
(Klesov et al., 1977). The three classes of alcohol dehydrogenase
present in human liver (Eklund et al., 1990) show decreased Km with
increasing chain length from C2 to C8; the alcohol groups of
12-hydroxydodecanoic and 16-hydroxyhexadecanoic acid show Km values
similar to that of hexanol, indicating rapid oxidation of alcohols
arising from omega-oxidation reactions (see later).
Similarly, aldehyde dehydrogenase, which is present predominantly
in hepatic cytosol, has a broad substrate specificity for the
oxidation of aldehydes to carboxylic acids (Feldman & Weiner, 1972).
This enzyme is more active on aldehydes of higher relative molecular
mass (Nakayasu et al., 1978). Three isoenzymes of aldehyde
dehydrogenase, with overlapping substrate specificities, are present
in human liver, which oxidize a range of naturally occurring
aldehydes, such as gamma-butyraldehyde, 3,4-dihydroxyphenyl-
acetaldehyde, 5-hydroxyindoleacetaldehyde, and acrolein. Alkyl
aldehydes (C1-C6) have Km values similar to those of these natural
substrates, and the Km decreases with increasing chain length
(Ambroziak & Pietruszko, 1991). Xanthine oxidase and aldehyde oxidase
also catalyse the oxidation of a wide range of aldehydes to the
corresponding unsaturated acids (Beedham, 1988). Before oxidation to
the corresponding acid, the aldehyde may be conjugated with sulfhydryl
groups such as glutathione to yield thiohemiacetals. Oxidation of
aldehydes with low relative molecular masses requires glutathione
(Eckfeldt & Yonetani, 1982), which implies that the substrate for
aldehyde dehydroge-nase-mediated oxidation may be the thiohemiacetal
(Brabec, 1993).
Branched-chain aliphatic primary alcohols and aldehydes are
substrates for alcohol dehydrogenase and aldehyde dehydrogenase,
although the rates of oxidation are lower than those for linear
primary alcohols and aldehydes (Blair & Bodley, 1969; Hedlund &
Kiessling, 1969; Albro, 1975; Saito, 1975; Ambroziak & Pietruszko,
1991). Unsaturated, linear and branched-chain primary alcohols are
better substrates than the saturated analogues, but the reverse is
true of aldehydes (Pietruszko et al., 1973). Citral, a mixture of two
unsaturated branched-chain aldehydes (neral and geranial), was not
oxidized to the corresponding acid by aldehyde dehydrogenase in rat
liver preparations in vitro but was reduced by alcohol dehydrogenase
in the presence of NADH (Boyer & Petersen, 1990); oxidation is a major
metabolic pathway in vivo, resulting in acid metabolites.
(b) Secondary and tertiary alcohols
Secondary alcohol groups, such as those present in carveol and
ß-ionol, undergo reversible oxidation to the corresponding ketone (see
'ketone reduction', below). The alcohol is the more important form
in vivo because of its removal from the equilibrium by conjugation
with glucuronic acid. Tertiary alcohol groups, such as those present
in linalool and terpineol, do not undergo oxidative metabolism, and
tertiary alcohols are eliminated by conjugation.
(iii) Conjugation of alcohols
Conjugation represents the major pathway of metabolism for both
secondary and tertiary alcohols (Kamil et al., 1953). Extensive
conjugation with glucuronic acid is followed by excretion primarily in
the urine or bile, depending on the relative molecular mass and the
animal species. Glucuronic acid conjugation is a major phase-2
metabolic reaction, and both the liver and intestinal wall have a high
capacity for glucuronidation of a wide range of substrates. Sulfate
conjugation of alcohols occurs in many tissues, especially the liver,
and results in the formation of highly polar, water-soluble excretory
products. The urine is the main route of elimination of sulfate
conjugates of alcohols resulting from the metabolism of the flavouring
agents evaluated at the present meeting.
Glucuronic acid conjugation and excretion represent the primary
route of elimination of linalool, and conjugation with glucuronic acid
is the major pathway of metabolism of menthol. In rodents, the
glucuronic acid conjugates of linalool (Parke et al., 1974) and
menthol (Yamaguchi et al., 1994) are excreted primarily via the bile
into the intestine, where they may be hydrolysed to yield the free
alcohol, which undergoes reabsorption and subsequent oxidative
metabolism. The metabolites of menthol are eliminated in the urine and
faeces either unchanged or conjugated with glucuronic acid (Yamaguchi
et al., 1994). Bicyclic tertiary alcohols are relatively stable
in vivo, and in rabbits thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo[2.2.1]heptan-2-ol) are extensively conjugated
with glucuronic acid (Williams, 1938).
(iv) Reduction of ketones
Aliphatic ketones are metabolized primarily via reduction to
the corresponding secondary alcohol (Leibman, 1971; Felsted & Bachur,
1980; Bosron & Li, 1980). Reduction of aliphatic ketones is mediated
by alcohol dehydrogenases and NADH/NADPH-dependent cytosolic carbonyl
reductases (Bosron & Li, 1980). The reaction catalysed by carbonyl
reductase is stereoselective and favours formation of the S enantiomer
of the alcohol. The reaction is reversible under physiological
conditions, and oxidation of the secondary alcohol may lead to the
formation of the corresponding ketone in vivo (Felsted & Bachur,
1980). Ketones have been shown to undergo reduction to alcohols in
human hepatic microsomes (Leibman, 1971). Three reductases have been
purified from human liver cytosol: two aldehyde reductases, which can
reduce aliphatic aldehydes, alicyclic ketones, and alpha-diketones,
and a carbonyl reductase which is active with a broad range of
aldehyde and ketone substrates (Nakayama et al., 1985). Carbonyl
reductase activity in human liver shows limited interindividual
variability, and the enzyme reduces both 4-nitrobenzaldehyde and
4-nitroacetophenone (Iwata et al., 1993).
Alicyclic monoketones, such as cyclohexanone, are reduced to the
corresponding alcohols or undergo alpha-hydroxylation and reduction to
yield diols, which are excreted as the glucuronic acid conjugates.
Unsaturated ketones, such as menthone, dihydrocarvone, and
carvone, undergo extensive reduction of the ketone function to yield
the corresponding secondary alcohols (pulegone, dihydrocarveol, and
carveol), which are conjugated mainly with glucuronic acid and then
excreted in the urine (Hamalainen, 1912; Duterte-Catella et al., 1978;
Madyastha & Raj, 1993) or bile. In rodents, but probably not in
humans, the conjugate is excreted primarily into the bile and may then
be hydrolysed to yield the free alcohol (Matthews, 1994), which may
undergo enterohepatic recirculation and finally be excreted by the
kidney (Tamura et al., 1962). Conjugates with relative molecular
masses below about 500, such as the glucuronides of menthan-2-ol,
dihydrocarveol, and carveol, would be eliminated by humans in the
urine; the absence of significant biliary excretion would reduce the
extent of secondary oxidative metabolism.
In general, alicyclic alpha-diketones are metabolized via the
reduction pathway. Methyl-substituted diketones may be reduced to the
corresponding hydroxyketones and diols, which are excreted in the
urine as glucuronic acid conjugates. alpha-Hydroxyketones and their
diol metabolites may be excreted as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain-length ketones. If the carbonyl function is located
elsewhere on the chain, detoxification occurs predominantly by
reduction.
(v) Reduction of double bonds
Reduction of endocyclic and exocyclic double bonds occurs in some
of the flavouring agents evaluated at the present meeting. For
example, the endocyclic double bond of carvone is reduced to form
dihydrocarvone and dihydrocarveol, and the exocyclic double bond in
ß-ionone is reduced to give dihydro-ß-ionol (Ide & Toki, 1970). The
enzymes involved in these reactions have not been characterized; the
intestinal microflora may be involved, either before absorption or
after biliary excretion.
(vi) Oxidation of side-chains
The position and size of substituents in carboxylic acids
influences the route of metabolism. As chain length and lipophilicity
increase, omega-oxidation competes favourably with ß-oxidative
cleavage.
(a) ß -Oxidation
Linear and branched-chain saturated carboxylic acids are
substrates for ß cleavage to yield acetyl coenzyme A (acetyl CoA) and
a new CoA thioester of the carboxylic acid that has been reduced by
two carbon atoms. This process continues, to give complete oxidation
or until a branch point is reached. Acids with an even number of
carbon atoms continue to be cleaved to acetyl CoA, while acids with an
odd number of carbon atoms yield acetyl CoA and propionyl CoA. Acetyl
CoA enters the citric acid cycle directly, whereas propionyl CoA is
transformed into succinyl CoA which then enters the citric acid cycle.
Acids with a methyl substituent located at an even-numbered carbon
(e.g. 2-methylpentanoic acid and 4-methyldecanoic acid) are
metabolized to carbon dioxide via ß-oxidative cleavage in the fatty
acid pathway. If the methyl group is located at the 3 position,
ß-oxidation is inhibited and omega-oxidation predominates, leading
primarily to polar diacid metabolites that are excreted in the urine.
Ethyl or propyl substituents located at the alpha or ß position in
relation to the carboxylic acid group inhibit metabolism to carbon
dioxide (Deuel, 1957), in which case there is direct conjugation of
the acid with glucuronic acid or omega-oxidation followed by
conjugation.
Linear unsaturated carboxylic acids participate in normal fatty
acid metabolism. In this pathway, the saturated fatty acid is
condensed with CoA, which then undergoes catalytic dehydrogenation
mediated by acyl CoA dehydrogenase (Voet & Voet, 1990). The resulting
trans-2,3-unsaturated ester (trans-Delta2-enoyl CoA) is converted
to the 3-keto-thioester, which undergoes ß cleavage to yield an acetyl
CoA fragment and a new thioester reduced by two carbons. Cleavage of
acetyl CoA units continues along the carbon chain until the position
of unsaturation is reached. If unsaturation begins at an even-numbered
carbon, acetyl CoA fragmentation yields a Delta2-enoyl CoA product
which is a substrate for further fatty acid oxidation. If unsaturation
begins at an odd-numbered carbon, acetyl CoA fragmentation will
eventually yield Delta3-enoyl CoA, which cannot enter the fatty acid
cycle until it is isomerized to the trans-Delta2-enoyl CoA by enoyl
CoA isomerase. If the stereochemistry of the double bond is cis, it
is isomerized to the trans double bond by the action of
3-hydroxyacyl CoA epimerase before entering the fatty acid oxidation
pathway. Short-chain acids containing terminal unsaturation may be
metabolized via desaturation to yield a substrate which may
participate in the fatty acid pathway. For example, 4-pentenoic acid
is converted into the CoA thioester; this is dehydrogenated to yield
trans-2,4-pentadienoyl CoA, which undergoes NADPH-dependent
enzyme-catalysed reduction of the Delta2-alkene to
trans-2-pentenoic acid, which then participates in normal fatty acid
oxidation (Schulz, 1983). A second, more minor pathway involves
ß-oxidation to yield 3-keto-4-pentenoyl CoA.
(b) omega- and (omega-1)-Oxidation
omega- and (omega-1)-Oxidation reactions are important in the
elimination of compounds with long and/or complex alkyl chains,
because they eventually yield polar acid metabolites that are
eliminated in the urine (Horning et al., 1976; Ventura et al., 1985;
Madyastha & Srivatsan, 1988a). omega-Oxidation results in the
formation of a primary alcohol, which may undergo further oxidation to
the corresponding carboxylic acid, which may then be excreted in the
urine. For example, linalool undergoes cytochrome P450-mediated
oxidation to form 8-hydroxylinalool and 8-carboxylinalool, whereas
geraniol is oxidized to 8-hydroxygeraniol, 8-carboxygeraniol, and the
dibasic acid, Hildebrandt's acid (Chadha & Madyastha, 1984). Citral,
the aldehyde analogue of geraniol, undergoes oxidation of the aldehyde
group in vivo followed by omega-oxidation to yield a mixture of
diacids and hydroxy acids (Diliberto et al., 1990). Menthol undergoes
omega-oxidation of the side-chain substituents to yield various
polyols and hydroxy acids (Madyastha & Srivatsan, 1988b; Yamaguchi et
al., 1994); the (-) isomer undergoes more extensive oxidation than the
(+) isomer (Williams, 1939).
Short-chain ketones (< C4, e.g. butanone) that contain a
carbonyl function at the 2 position, and alpha-diketones, such as
acetoin, may undergo omega-oxidation of the terminal methyl group to
give a diketocarboxylic acid, which would undergo decarboxylation to
yield an alpha-ketocarboxylic acid. As intermediary metabolites,
alpha-ketoacids undergo oxidative decarboxylation to yield carbon
dioxide and a simple aliphatic carboxylic acid, which may be
completely metabolized via the fatty acid pathway and citric acid
cycle. Almost one-half of a dose of radiolabelled acetoin,
administered by intravenous injection to rats, was eliminated as
carbon dioxide in expired air (Gabriel et al., 1972); about 20% was
eliminated as unidentified urinary metabolites. trans-Methyl styryl
ketone undergoes essentially complete metabolism of the methyl group
after oral administration to rats; the glycine conjugates of
phenylacetic and benzoic acid were the major metabolites in the urine,
while the intermediate methyl styryl carbinol was detected in plasma
(Sauer et al., 1997).
omega-Oxidation of ketones yields hydroxyketones which may be
further reduced to diols and excreted in the urine as glucuronic acid
conjugates. Participation in these pathways depends on chain length,
the position of the carbonyl function, and the dose (Dietz et al.,
1981; Topping et al., 1994). Longer-chain aliphatic ketones (i.e.
carbon chain length > C5) are metabolized primarily via
reduction, but omega- and/or (omega-1)-oxidation are competing
pathways at high concentrations. At high doses, aliphatic ketones may
undergo omega- and/or (omega-1)-oxidation to yield ketoacids and
diketones, respectively.
A number of the flavouring agents evaluated at the present
meeting are alicyclic ketones containing an alkyl or alkenyl side
chain; these undergo oxidation of the side chain (probably by
cytochrome P450: Madyastha & Raj, 1990) to form polar metabolites that
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Williams, 1959; Ishida et al.,
1989).
(c) Oxidation of double bonds
Oxidation of double bonds via an epoxide intermediate to form
diols is probably only a minor pathway of metabolism for a few of the
flavouring agents considered at the present meeting. For example,
alpha-terpineol is oxidized by cytochrome P450 in rats, via an epoxide
intermediate, to a 1,2-dihydroxy metabolite (Madyastha & Srivatsan,
1988a); this diol metabolite has been detected in humans after
ingestion of alpha-terpineol (Horning et al., 1976).
(vii) Oxidation of alicyclics
Cyclohexane and cyclohexene rings, which are present in the
carvone and ionone groups considered at the present meeting, are
rapidly oxidized in vivo by ring hydroxylation, even when other
pathways of elimination are present. For example, the main pathways of
elimination of cyclohexane are exhalation unchanged in expired air and
excretion in urine as glucuronic acid conjugates of cyclohexanol and
cyclohexane-1,2-diol (Elliott et al., 1959). Cyclohexylamine is highly
water-soluble and is excreted in the urine as both the parent compound
and the glucuronides of ring-hydroxylated metabolites, together with
small amounts of cyclohexane-1,2-diol formed via deamination and
ring hydroxylation (Renwick & Williams, 1972). Ring oxidation, which
is catalysed by cytochrome P450 (CYP3A4), is a major pathway of
metabolism for the substituted cyclohexene ring systems that are
present in vitamin A and its metabolites, such as 13- cis- and
all- trans-retinoic acid, as well as the ionone group of flavouring
agents. Oxidation of the substituted cyclohexene rings present in
retinoids and ionones occurs on the carbon adjacent to the double
bond.
(vii) Conjugation with glutathione
The vast majority of the flavouring agents evaluated at the
present meeting are metabolized by the pathways described above.
Possible exceptions are carvone, carveol (and its esters), and some
members of the ionone group in which a ketone group, or a precursor
secondary alcohol group, is located adjacent to a double bond or
conjugated double bonds (alpha,ß-unsaturated ketone). Glutathione
conjugation is an important detoxification pathway, especially for
reactive compounds. The products of conjugation with glutathione are
usually eliminated in the bile as the glutathione conjugates per se
and in the urine as the N-acetylcysteine metabolites produced from
the glutathione conjugates. Glutathione conjugates may also be split
by C-S lyase (ß-lyase) in the kidney and intestinal microflora, to
give a thiol compound; this reaction represents a bioactivation
process for some chemicals. Only limited data were available on the
metabolism of the ionone group of flavouring agents, and glutathione
conjugation products have not been reported as urinary metabolites of
ionones or of carvone. Oral administration of 20 mg carvone, by gavage
in corn oil, to mice daily for three days caused a reduction in the
concentration of glutathione in the liver and an increase in
glutathione transferase activity (Zheng et al., 1992); however, these
effects may not have been due to the alpha,ß-unsaturated ketone
moiety, because the decrease in glutathione was not found with
alpha,ß-unsaturated carvone analogues lacking the exocyclic allyl
group, while the increase in glutathione transferase activity was
detected with compounds such as para-menthan-2-one which contain
neither an alpha,ß-unsaturated ketone moiety nor an exocyclic allyl
group.
The potential for non-enzymatic interaction with glutathione was
shown in vitro (Portoghese et al., 1989). The greatest reactivity
with glutathione was found with the simplest alpha,ß-unsaturated
ketone (butenone), which had a rate of reaction about 1000 times
greater than that of oct-2-ene-4-one. Damascenone, the 1,3-endocyclic
diene analogue of damascone, and carvone, which were evaluated at the
present meeting, showed rates of reaction that were 17 000, 11 000,
and 320 000 times slower than that of butenone, which indicates low
reactivity.
Overall, the flavouring agents with an alpha,ß-unsaturated ketone
moiety that were evaluated at the present meeting have low reactivity,
and conjugation with glutathione would not be a major route of
metabolism. The low predicted reactivity is supported by the
toxicological data for the ionones and carvone.
REFERENCES
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Ambroziak, W. & Pietruszko, R. (1991) Human aldehyde dehydrogenase:
Activity with aldehyde metabolites of monoamines, diamines, and
polyamines. J. Biol. Chem., 20, 13011-13018.
Beedham, C. (1988) Molybdenum hydroxylases. In: Gorrod, J.W.,
Oelschlager, H. & Caldwell, J., eds, Metabolism of Xenobiotics,
London, Taylor & Francis, pp. 51-58.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
Partial purification and properties. Can. J. Biochem., 47, 265-272.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Boyer, C.S. & Petersen, D.R. (1990) The metabolism of
3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and
cytosolic fractions. Drug Metab. Disposition, 16, 81-86.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, Vol. IIA, pp. 283-327.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Deuel, H.J., ed. (1957) The Lipids, Theit Chemistry and
Biochemistry, New York, Wiley Interscience, Vol. III.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1990) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Duterte-Catella, H., Nguyen, P., Dang, Q. & Truhaut, R. (1978)
Metabolic transformations of the trimethyl-3,5,5, cycloxene-2, one-1
(isophorone). Toxicol. Eur. Res., 1, 209-216.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogeanse from horse liver. In: Wood, W.A., ed., Methods in
Enzymology, New York, Academic Press, pp. 474-479.
Eklund, H., Müller-Wille, P., Horjales, E., Futer, O., Holmquist, B.,
Vallee, B.L., Höög, J.-O., Kaiser, R. & Jörnvall, H. (1990) Comparison
of three classes of human liver alcohol dehydrogenase: Emphasis on
different substrate binding pockets. Eur. J. Biochem., 193, 303-310.
Elliott, T.H., Parke, D.V. & Williams, R.T. (1959) Studies in
detoxication: 79. The metabolism of cyclo[14C]hexane and its
derivatives. Biochem. J., 72, 193-200.
Fahelbum, I.M.S. & James, S.P. (1977) The absorption and metabolism of
methyl cinnamate. Toxicology, 7, 123-132.
Fahelbum, I.M.S. & James, S.P. (1979) Absorption, distribution and
metabolism of propyl anthranilate. Toxicology, 12, 75-87.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York,
Academic Press, pp. 281-293.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Grundschober, F. (1977) Toxicological assessment of flavouring esters.
Toxicology, 8, 387-390.
Hamalainen, J. (1912) [Concerning the behaviour of alicyclic compounds
with glucuronic acid in organisms.] Skand. Arch. Physiol., 27,
141-226 (in German).
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. 1. The oxidation of some liver aliphatic fusel
alcohols and their effect on the mitochondrial oxidation of various
substrates. Acta Pharmacol. Toxicol., 27, 381-396.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.T. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxidediol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectrscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
Ishida, T., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(±)-7-hydroxycitronellal, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (±)-carvone in rabbits. Xenobiotica, 19,
843-855.
Iwata, N., Inazu, N., Hara, S., Yanase, T., Kano, S., Endo, T.,
Kuriiwa, F., Sato, Y. & Satoh, T. (1993) Interindividual variability
of carbonyl reductase levels in human livers. Biochem. Pharmacol.,
45, 1711-1714.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols: The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochemistry, 53,
129-136.
Klesov, A.A., Lange, L.G., Sytkowski, A.J. & Vallee, B.L. (1977)
[Unusual nature of the substrate specificity of alcohol dehydrogenase
of different origins.] Biorganich. Khim., 3, 1141-1144 (in Russian).
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Commun., 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone -- a hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Madyastha, K.M. & Srivatsan, V. (1988a) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Madyastha, K.M. & Srivatsan, V. (1988b) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Norwalk, Connecticut, Appleton &
Lange, pp. 177-192.
Nakayama, T., Hara, A., Yashiro, K. & Sawada, H. (1985) Reductases for
carbonyl compounds in human liver. Biochem. Pharmacol., 34, 107-117.
Nakayasu, H., Mihara, K. & Sato, R. (1978) Purification and properties
of membrane-bound aldehyde dehydrogenase from rat liver microsomes.
Biochem. Biophys. Res. Commun., 83, 697-703.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenases from human liver,
horse liver and yeast towards saturated and 2-enoic alcohols and
aldehydes. Arch. Biochem. Biophys., 159, 50-60.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Renwick, A.G. & Williams, R.T. (1972) The metabolites of
cyclohexylamine in man and certain animals. Biochem. J., 129,
857-867.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34, 569-585.
Sauer, J.-M., Smith, R.L., Bao, J., Kattnig, M.J., Kuester, R.K.,
McClure, T.D., Mayersohn, M. & Sipes, I.G. (1997) Oral and topical
absorption, disposition kinetics, and the metabolic fate of
trans-methyl styryl ketone in the male Fischer 344 rat. Drug
Metab. Disposition, 25, 732-739.
Savary, P. & Constantin, M.J. (1970) Intestinal hydrolysis and
lymphatic absorption of isopropyl asters of long chain fatty acids in
the rat. Biochem. Biophys. Acta, 218, 195.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. Biochemistry, 22,
1827-1832.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. I. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Fol.
Pharmacol. Jpn., 58, 323-336.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knight, S. &
Knight, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71-79.
Williams, R.T. (1938) Studies in detoxication. II. a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol in the rabbit. b) d-iso-Menthylglucuronide,
a new conjugated glucuronic acid. Biochem. J., 32, 1849-1855.
Williams, R.T. (1939) Studies in detoxication. III. The use of the
glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 33, 1519-1524.
Williams, R.T. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
A wide range of enzymes (carboxyesterases and carboxylic acid
hydrolases) can hydrolyse esters into their constituent alcohols and
acids. Carboxyesterases are found in many tissues, and there is strong
esterase activity in the intestinal tract, blood, liver, kidney, lung,
brain, and pancreas. The multiplicity of esterases and their wide
tissue distribution result in rapid hydrolysis of esters in vivo,
and this extensive metabolic activity is the basis for the development
by the pharmaceutical industry of pro-drugs, which are carboxylic acid
esters of therapeutically active agents. The esters considered at the
present meeting contain larger, more complex alkyl constituents in
both the alcohol and the acid moieties. There was only limited
information on the hydrolysis of these esters in vitro, but the data
were comparable to those considered in the forty-sixth report of the
Committee. Isopropyl phenylacetate and isopropyl butyrate underwent
40% hydrolysis after 2 h with pancreatin (Grundschober, 1977), a value
similar to that for some of the esters considered at the previous
meeting. Essentially complete hydrolysis of isopropyl palmitate,
isopropyl oleate, and isopropyl stearate radiolabelled in the fatty
acid moiety was demonstrated in vivo by the detection of > 95% of
the radiolabel in the triglyceride fraction of rat blood (Savary &
Constantin, 1970). n-Octyl esters would be expected to undergo very
rapid hydrolysis on the basis of the relationships between increased
chain length (in the range C1-C8), increased Vmax, and decreased Km
(considered at the forty sixth meeting). The hydrolysis of branched-
chain octyl and larger esters would probably be slower than that of
the corresponding n-alkyl analogues, and absorption of the intact
ester is possible (see below).
The hydrolysis of esters with larger alcohol substituents, such
as are present in the linalyl, terpinyl, carvyl, and menthyl esters
evaluated at the present meeting, has been the subject of only limited
study. Linalyl acetate was hydrolysed rapidly in water and simulated
gastric and pancreatic fluids: the mean half-lives for hydrolysis were
5.5 min in gastric fluid and 52.5 min in pancreatic fluid (R.L. Hall,
personal communication to the Flavor and Extract Manufacturers'
Association of the United States), values comparable to those
considered at the forty-sixth meeting. Allyl tiglate and benzyl
tiglate were not hydrolysed by these simulated fluids but were
hydrolysed extensively on incubation with intestinal tissue
(Grundschober, 1977) and would be predicted to be completely
hydrolysed by the liver, both in vitro and in vivo. (-)-Menthol
ethylene glycol carbonate and (-)-menthol propylene glycol carbonate
were hydrolysed slowly on incubation with rat liver homogenate for 4
h, with 85% hydrolysis to menthol for (-)-menthol ethylene glycol
carbonate and 75% for (-)-menthol propylene glycol carbonate
(Emberger, personal communication to the Flavor and Extract
Manufacturers' Association of the United States). The structurally
related agent bornyl acetate was excreted largely as the glucuronic
acid conjugate of borneol after oral administration to rabbits
(Williams, 1959), consistent with extensive hydrolysis in vivo. More
than 80% of radiolabelled cyclandelate, a trimethyl cyclohexyl ester,
was hydrolysed after 20 min of incubation with rat hepatic microsomes
(White et al., 1990). Methyl cinnamate was hydrolysed only slowly in
the rat intestinal lumen in vivo after oral administration but
underwent essentially complete first-pass hydrolysis, because none of
the parent compound could be detected in the systemic circulation
(Fahelbum & James, 1977). In contrast, propyl anthranilate was not
completely hydrolysed during absorption, and the intact ester was
detected in peripheral blood and urine (Fahelbum & James, 1979). These
findings are similar to those concerning hydrolysis considered at the
forty-sixth meeting.
The results indicate that hydrolysis is the major metabolic
pathway for all of the esters evaluated at the present meeting;
however, some of the esters in some groups may undergo a low rate of
hydrolysis, resulting in incomplete pre-systemic metabolism to the
corresponding alcohol and acid. In general, esters that reach the
general circulation intact would be expected to be hydrolysed
extensively by tissue esterases into the acid and alcohol
constituents.
(ii) Oxidation of alcohols and aldehydes
(a) Primary alcohols and aldehydes
Primary aliphatic alcohols (attached to linear, branched, or
unsaturated alkyl chains) and the corresponding aldehydes are
efficiently oxidized to the corresponding carboxylic acids by
high-capacity enzyme pathways. NAD+/NADH-dependent alcohol
dehydrogenase catalyses the oxidation of primary aliphatic saturated
and unsaturated alcohols to the corresponding aldehyde (Pietruszko et
al., 1973). A comparison of the alcohol structure with the enzyme
binding affinity of alcohol dehydrogenase indicates that increased
binding (lower Km) occurs with increasing chain length (C1-C6) of
the substrate and the presence of unsaturation. The maximum rates of
oxidation were essentially constant, regardless of the alcohol
structure, suggesting that alcohol-enzyme binding is not the
rate-limiting step for oxidation; the activity of the enzyme appears
to be dependent on the lipophilic character of the alcohol substrate
(Klesov et al., 1977). The three classes of alcohol dehydrogenase
present in human liver (Eklund et al., 1990) show decreased Km with
increasing chain length from C2 to C8; the alcohol groups of
12-hydroxydodecanoic and 16-hydroxyhexadecanoic acid show Km values
similar to that of hexanol, indicating rapid oxidation of alcohols
arising from omega-oxidation reactions (see later).
Similarly, aldehyde dehydrogenase, which is present predominantly
in hepatic cytosol, has a broad substrate specificity for the
oxidation of aldehydes to carboxylic acids (Feldman & Weiner, 1972).
This enzyme is more active on aldehydes of higher relative molecular
mass (Nakayasu et al., 1978). Three isoenzymes of aldehyde
dehydrogenase, with overlapping substrate specificities, are present
in human liver, which oxidize a range of naturally occurring
aldehydes, such as gamma-butyraldehyde, 3,4-dihydroxyphenyl-
acetaldehyde, 5-hydroxyindoleacetaldehyde, and acrolein. Alkyl
aldehydes (C1-C6) have Km values similar to those of these natural
substrates, and the Km decreases with increasing chain length
(Ambroziak & Pietruszko, 1991). Xanthine oxidase and aldehyde oxidase
also catalyse the oxidation of a wide range of aldehydes to the
corresponding unsaturated acids (Beedham, 1988). Before oxidation to
the corresponding acid, the aldehyde may be conjugated with sulfhydryl
groups such as glutathione to yield thiohemiacetals. Oxidation of
aldehydes with low relative molecular masses requires glutathione
(Eckfeldt & Yonetani, 1982), which implies that the substrate for
aldehyde dehydroge-nase-mediated oxidation may be the thiohemiacetal
(Brabec, 1993).
Branched-chain aliphatic primary alcohols and aldehydes are
substrates for alcohol dehydrogenase and aldehyde dehydrogenase,
although the rates of oxidation are lower than those for linear
primary alcohols and aldehydes (Blair & Bodley, 1969; Hedlund &
Kiessling, 1969; Albro, 1975; Saito, 1975; Ambroziak & Pietruszko,
1991). Unsaturated, linear and branched-chain primary alcohols are
better substrates than the saturated analogues, but the reverse is
true of aldehydes (Pietruszko et al., 1973). Citral, a mixture of two
unsaturated branched-chain aldehydes (neral and geranial), was not
oxidized to the corresponding acid by aldehyde dehydrogenase in rat
liver preparations in vitro but was reduced by alcohol dehydrogenase
in the presence of NADH (Boyer & Petersen, 1990); oxidation is a major
metabolic pathway in vivo, resulting in acid metabolites.
(b) Secondary and tertiary alcohols
Secondary alcohol groups, such as those present in carveol and
ß-ionol, undergo reversible oxidation to the corresponding ketone (see
'ketone reduction', below). The alcohol is the more important form
in vivo because of its removal from the equilibrium by conjugation
with glucuronic acid. Tertiary alcohol groups, such as those present
in linalool and terpineol, do not undergo oxidative metabolism, and
tertiary alcohols are eliminated by conjugation.
(iii) Conjugation of alcohols
Conjugation represents the major pathway of metabolism for both
secondary and tertiary alcohols (Kamil et al., 1953). Extensive
conjugation with glucuronic acid is followed by excretion primarily in
the urine or bile, depending on the relative molecular mass and the
animal species. Glucuronic acid conjugation is a major phase-2
metabolic reaction, and both the liver and intestinal wall have a high
capacity for glucuronidation of a wide range of substrates. Sulfate
conjugation of alcohols occurs in many tissues, especially the liver,
and results in the formation of highly polar, water-soluble excretory
products. The urine is the main route of elimination of sulfate
conjugates of alcohols resulting from the metabolism of the flavouring
agents evaluated at the present meeting.
Glucuronic acid conjugation and excretion represent the primary
route of elimination of linalool, and conjugation with glucuronic acid
is the major pathway of metabolism of menthol. In rodents, the
glucuronic acid conjugates of linalool (Parke et al., 1974) and
menthol (Yamaguchi et al., 1994) are excreted primarily via the bile
into the intestine, where they may be hydrolysed to yield the free
alcohol, which undergoes reabsorption and subsequent oxidative
metabolism. The metabolites of menthol are eliminated in the urine and
faeces either unchanged or conjugated with glucuronic acid (Yamaguchi
et al., 1994). Bicyclic tertiary alcohols are relatively stable
in vivo, and in rabbits thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo[2.2.1]heptan-2-ol) are extensively conjugated
with glucuronic acid (Williams, 1938).
(iv) Reduction of ketones
Aliphatic ketones are metabolized primarily via reduction to
the corresponding secondary alcohol (Leibman, 1971; Felsted & Bachur,
1980; Bosron & Li, 1980). Reduction of aliphatic ketones is mediated
by alcohol dehydrogenases and NADH/NADPH-dependent cytosolic carbonyl
reductases (Bosron & Li, 1980). The reaction catalysed by carbonyl
reductase is stereoselective and favours formation of the S enantiomer
of the alcohol. The reaction is reversible under physiological
conditions, and oxidation of the secondary alcohol may lead to the
formation of the corresponding ketone in vivo (Felsted & Bachur,
1980). Ketones have been shown to undergo reduction to alcohols in
human hepatic microsomes (Leibman, 1971). Three reductases have been
purified from human liver cytosol: two aldehyde reductases, which can
reduce aliphatic aldehydes, alicyclic ketones, and alpha-diketones,
and a carbonyl reductase which is active with a broad range of
aldehyde and ketone substrates (Nakayama et al., 1985). Carbonyl
reductase activity in human liver shows limited interindividual
variability, and the enzyme reduces both 4-nitrobenzaldehyde and
4-nitroacetophenone (Iwata et al., 1993).
Alicyclic monoketones, such as cyclohexanone, are reduced to the
corresponding alcohols or undergo alpha-hydroxylation and reduction to
yield diols, which are excreted as the glucuronic acid conjugates.
Unsaturated ketones, such as menthone, dihydrocarvone, and
carvone, undergo extensive reduction of the ketone function to yield
the corresponding secondary alcohols (pulegone, dihydrocarveol, and
carveol), which are conjugated mainly with glucuronic acid and then
excreted in the urine (Hamalainen, 1912; Duterte-Catella et al., 1978;
Madyastha & Raj, 1993) or bile. In rodents, but probably not in
humans, the conjugate is excreted primarily into the bile and may then
be hydrolysed to yield the free alcohol (Matthews, 1994), which may
undergo enterohepatic recirculation and finally be excreted by the
kidney (Tamura et al., 1962). Conjugates with relative molecular
masses below about 500, such as the glucuronides of menthan-2-ol,
dihydrocarveol, and carveol, would be eliminated by humans in the
urine; the absence of significant biliary excretion would reduce the
extent of secondary oxidative metabolism.
In general, alicyclic alpha-diketones are metabolized via the
reduction pathway. Methyl-substituted diketones may be reduced to the
corresponding hydroxyketones and diols, which are excreted in the
urine as glucuronic acid conjugates. alpha-Hydroxyketones and their
diol metabolites may be excreted as glucuronic acid conjugates. This
pathway is favoured at high concentrations in vivo, especially for
longer-chain-length ketones. If the carbonyl function is located
elsewhere on the chain, detoxification occurs predominantly by
reduction.
(v) Reduction of double bonds
Reduction of endocyclic and exocyclic double bonds occurs in some
of the flavouring agents evaluated at the present meeting. For
example, the endocyclic double bond of carvone is reduced to form
dihydrocarvone and dihydrocarveol, and the exocyclic double bond in
ß-ionone is reduced to give dihydro-ß-ionol (Ide & Toki, 1970). The
enzymes involved in these reactions have not been characterized; the
intestinal microflora may be involved, either before absorption or
after biliary excretion.
(vi) Oxidation of side-chains
The position and size of substituents in carboxylic acids
influences the route of metabolism. As chain length and lipophilicity
increase, omega-oxidation competes favourably with ß-oxidative
cleavage.
(a) ß -Oxidation
Linear and branched-chain saturated carboxylic acids are
substrates for ß cleavage to yield acetyl coenzyme A (acetyl CoA) and
a new CoA thioester of the carboxylic acid that has been reduced by
two carbon atoms. This process continues, to give complete oxidation
or until a branch point is reached. Acids with an even number of
carbon atoms continue to be cleaved to acetyl CoA, while acids with an
odd number of carbon atoms yield acetyl CoA and propionyl CoA. Acetyl
CoA enters the citric acid cycle directly, whereas propionyl CoA is
transformed into succinyl CoA which then enters the citric acid cycle.
Acids with a methyl substituent located at an even-numbered carbon
(e.g. 2-methylpentanoic acid and 4-methyldecanoic acid) are
metabolized to carbon dioxide via ß-oxidative cleavage in the fatty
acid pathway. If the methyl group is located at the 3 position,
ß-oxidation is inhibited and omega-oxidation predominates, leading
primarily to polar diacid metabolites that are excreted in the urine.
Ethyl or propyl substituents located at the alpha or ß position in
relation to the carboxylic acid group inhibit metabolism to carbon
dioxide (Deuel, 1957), in which case there is direct conjugation of
the acid with glucuronic acid or omega-oxidation followed by
conjugation.
Linear unsaturated carboxylic acids participate in normal fatty
acid metabolism. In this pathway, the saturated fatty acid is
condensed with CoA, which then undergoes catalytic dehydrogenation
mediated by acyl CoA dehydrogenase (Voet & Voet, 1990). The resulting
trans-2,3-unsaturated ester (trans-Delta2-enoyl CoA) is converted
to the 3-keto-thioester, which undergoes ß cleavage to yield an acetyl
CoA fragment and a new thioester reduced by two carbons. Cleavage of
acetyl CoA units continues along the carbon chain until the position
of unsaturation is reached. If unsaturation begins at an even-numbered
carbon, acetyl CoA fragmentation yields a Delta2-enoyl CoA product
which is a substrate for further fatty acid oxidation. If unsaturation
begins at an odd-numbered carbon, acetyl CoA fragmentation will
eventually yield Delta3-enoyl CoA, which cannot enter the fatty acid
cycle until it is isomerized to the trans-Delta2-enoyl CoA by enoyl
CoA isomerase. If the stereochemistry of the double bond is cis, it
is isomerized to the trans double bond by the action of
3-hydroxyacyl CoA epimerase before entering the fatty acid oxidation
pathway. Short-chain acids containing terminal unsaturation may be
metabolized via desaturation to yield a substrate which may
participate in the fatty acid pathway. For example, 4-pentenoic acid
is converted into the CoA thioester; this is dehydrogenated to yield
trans-2,4-pentadienoyl CoA, which undergoes NADPH-dependent
enzyme-catalysed reduction of the Delta2-alkene to
trans-2-pentenoic acid, which then participates in normal fatty acid
oxidation (Schulz, 1983). A second, more minor pathway involves
ß-oxidation to yield 3-keto-4-pentenoyl CoA.
(b) omega- and (omega-1)-Oxidation
omega- and (omega-1)-Oxidation reactions are important in the
elimination of compounds with long and/or complex alkyl chains,
because they eventually yield polar acid metabolites that are
eliminated in the urine (Horning et al., 1976; Ventura et al., 1985;
Madyastha & Srivatsan, 1988a). omega-Oxidation results in the
formation of a primary alcohol, which may undergo further oxidation to
the corresponding carboxylic acid, which may then be excreted in the
urine. For example, linalool undergoes cytochrome P450-mediated
oxidation to form 8-hydroxylinalool and 8-carboxylinalool, whereas
geraniol is oxidized to 8-hydroxygeraniol, 8-carboxygeraniol, and the
dibasic acid, Hildebrandt's acid (Chadha & Madyastha, 1984). Citral,
the aldehyde analogue of geraniol, undergoes oxidation of the aldehyde
group in vivo followed by omega-oxidation to yield a mixture of
diacids and hydroxy acids (Diliberto et al., 1990). Menthol undergoes
omega-oxidation of the side-chain substituents to yield various
polyols and hydroxy acids (Madyastha & Srivatsan, 1988b; Yamaguchi et
al., 1994); the (-) isomer undergoes more extensive oxidation than the
(+) isomer (Williams, 1939).
Short-chain ketones (< C4, e.g. butanone) that contain a
carbonyl function at the 2 position, and alpha-diketones, such as
acetoin, may undergo omega-oxidation of the terminal methyl group to
give a diketocarboxylic acid, which would undergo decarboxylation to
yield an alpha-ketocarboxylic acid. As intermediary metabolites,
alpha-ketoacids undergo oxidative decarboxylation to yield carbon
dioxide and a simple aliphatic carboxylic acid, which may be
completely metabolized via the fatty acid pathway and citric acid
cycle. Almost one-half of a dose of radiolabelled acetoin,
administered by intravenous injection to rats, was eliminated as
carbon dioxide in expired air (Gabriel et al., 1972); about 20% was
eliminated as unidentified urinary metabolites. trans-Methyl styryl
ketone undergoes essentially complete metabolism of the methyl group
after oral administration to rats; the glycine conjugates of
phenylacetic and benzoic acid were the major metabolites in the urine,
while the intermediate methyl styryl carbinol was detected in plasma
(Sauer et al., 1997).
omega-Oxidation of ketones yields hydroxyketones which may be
further reduced to diols and excreted in the urine as glucuronic acid
conjugates. Participation in these pathways depends on chain length,
the position of the carbonyl function, and the dose (Dietz et al.,
1981; Topping et al., 1994). Longer-chain aliphatic ketones (i.e.
carbon chain length > C5) are metabolized primarily via
reduction, but omega- and/or (omega-1)-oxidation are competing
pathways at high concentrations. At high doses, aliphatic ketones may
undergo omega- and/or (omega-1)-oxidation to yield ketoacids and
diketones, respectively.
A number of the flavouring agents evaluated at the present
meeting are alicyclic ketones containing an alkyl or alkenyl side
chain; these undergo oxidation of the side chain (probably by
cytochrome P450: Madyastha & Raj, 1990) to form polar metabolites that
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Williams, 1959; Ishida et al.,
1989).
(c) Oxidation of double bonds
Oxidation of double bonds via an epoxide intermediate to form
diols is probably only a minor pathway of metabolism for a few of the
flavouring agents considered at the present meeting. For example,
alpha-terpineol is oxidized by cytochrome P450 in rats, via an epoxide
intermediate, to a 1,2-dihydroxy metabolite (Madyastha & Srivatsan,
1988a); this diol metabolite has been detected in humans after
ingestion of alpha-terpineol (Horning et al., 1976).
(vii) Oxidation of alicyclics
Cyclohexane and cyclohexene rings, which are present in the
carvone and ionone groups considered at the present meeting, are
rapidly oxidized in vivo by ring hydroxylation, even when other
pathways of elimination are present. For example, the main pathways of
elimination of cyclohexane are exhalation unchanged in expired air and
excretion in urine as glucuronic acid conjugates of cyclohexanol and
cyclohexane-1,2-diol (Elliott et al., 1959). Cyclohexylamine is highly
water-soluble and is excreted in the urine as both the parent compound
and the glucuronides of ring-hydroxylated metabolites, together with
small amounts of cyclohexane-1,2-diol formed via deamination and
ring hydroxylation (Renwick & Williams, 1972). Ring oxidation, which
is catalysed by cytochrome P450 (CYP3A4), is a major pathway of
metabolism for the substituted cyclohexene ring systems that are
present in vitamin A and its metabolites, such as 13- cis- and
all- trans-retinoic acid, as well as the ionone group of flavouring
agents. Oxidation of the substituted cyclohexene rings present in
retinoids and ionones occurs on the carbon adjacent to the double
bond.
(vii) Conjugation with glutathione
The vast majority of the flavouring agents evaluated at the
present meeting are metabolized by the pathways described above.
Possible exceptions are carvone, carveol (and its esters), and some
members of the ionone group in which a ketone group, or a precursor
secondary alcohol group, is located adjacent to a double bond or
conjugated double bonds (alpha,ß-unsaturated ketone). Glutathione
conjugation is an important detoxification pathway, especially for
reactive compounds. The products of conjugation with glutathione are
usually eliminated in the bile as the glutathione conjugates per se
and in the urine as the N-acetylcysteine metabolites produced from
the glutathione conjugates. Glutathione conjugates may also be split
by C-S lyase (ß-lyase) in the kidney and intestinal microflora, to
give a thiol compound; this reaction represents a bioactivation
process for some chemicals. Only limited data were available on the
metabolism of the ionone group of flavouring agents, and glutathione
conjugation products have not been reported as urinary metabolites of
ionones or of carvone. Oral administration of 20 mg carvone, by gavage
in corn oil, to mice daily for three days caused a reduction in the
concentration of glutathione in the liver and an increase in
glutathione transferase activity (Zheng et al., 1992); however, these
effects may not have been due to the alpha,ß-unsaturated ketone
moiety, because the decrease in glutathione was not found with
alpha,ß-unsaturated carvone analogues lacking the exocyclic allyl
group, while the increase in glutathione transferase activity was
detected with compounds such as para-menthan-2-one which contain
neither an alpha,ß-unsaturated ketone moiety nor an exocyclic allyl
group.
The potential for non-enzymatic interaction with glutathione was
shown in vitro (Portoghese et al., 1989). The greatest reactivity
with glutathione was found with the simplest alpha,ß-unsaturated
ketone (butenone), which had a rate of reaction about 1000 times
greater than that of oct-2-ene-4-one. Damascenone, the 1,3-endocyclic
diene analogue of damascone, and carvone, which were evaluated at the
present meeting, showed rates of reaction that were 17 000, 11 000,
and 320 000 times slower than that of butenone, which indicates low
reactivity.
Overall, the flavouring agents with an alpha,ß-unsaturated ketone
moiety that were evaluated at the present meeting have low reactivity,
and conjugation with glutathione would not be a major route of
metabolism. The low predicted reactivity is supported by the
toxicological data for the ionones and carvone.
REFERENCES
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Ambroziak, W. & Pietruszko, R. (1991) Human aldehyde dehydrogenase:
Activity with aldehyde metabolites of monoamines, diamines, and
polyamines. J. Biol. Chem., 20, 13011-13018.
Beedham, C. (1988) Molybdenum hydroxylases. In: Gorrod, J.W.,
Oelschlager, H. & Caldwell, J., eds, Metabolism of Xenobiotics,
London, Taylor & Francis, pp. 51-58.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
Partial purification and properties. Can. J. Biochem., 47, 265-272.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Boyer, C.S. & Petersen, D.R. (1990) The metabolism of
3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and
cytosolic fractions. Drug Metab. Disposition, 16, 81-86.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, Vol. IIA, pp. 283-327.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Deuel, H.J., ed. (1957) The Lipids, Theit Chemistry and
Biochemistry, New York, Wiley Interscience, Vol. III.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1990) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Duterte-Catella, H., Nguyen, P., Dang, Q. & Truhaut, R. (1978)
Metabolic transformations of the trimethyl-3,5,5, cycloxene-2, one-1
(isophorone). Toxicol. Eur. Res., 1, 209-216.
Eckfeldt, J.H. & Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogeanse from horse liver. In: Wood, W.A., ed., Methods in
Enzymology, New York, Academic Press, pp. 474-479.
Eklund, H., Müller-Wille, P., Horjales, E., Futer, O., Holmquist, B.,
Vallee, B.L., Höög, J.-O., Kaiser, R. & Jörnvall, H. (1990) Comparison
of three classes of human liver alcohol dehydrogenase: Emphasis on
different substrate binding pockets. Eur. J. Biochem., 193, 303-310.
Elliott, T.H., Parke, D.V. & Williams, R.T. (1959) Studies in
detoxication: 79. The metabolism of cyclo[14C]hexane and its
derivatives. Biochem. J., 72, 193-200.
Fahelbum, I.M.S. & James, S.P. (1977) The absorption and metabolism of
methyl cinnamate. Toxicology, 7, 123-132.
Fahelbum, I.M.S. & James, S.P. (1979) Absorption, distribution and
metabolism of propyl anthranilate. Toxicology, 12, 75-87.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York,
Academic Press, pp. 281-293.
Gabriel, M.A., Ilbawi, M. & Al-Khalidi, U.A.S. (1972) The oxidation of
acetoin to CO2 in intact animals and in liver mince preparation.
Comp. Biochem. Physiol., 41B, 493-502.
Grundschober, F. (1977) Toxicological assessment of flavouring esters.
Toxicology, 8, 387-390.
Hamalainen, J. (1912) [Concerning the behaviour of alicyclic compounds
with glucuronic acid in organisms.] Skand. Arch. Physiol., 27,
141-226 (in German).
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. 1. The oxidation of some liver aliphatic fusel
alcohols and their effect on the mitochondrial oxidation of various
substrates. Acta Pharmacol. Toxicol., 27, 381-396.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.T. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxidediol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectrscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
Ishida, T., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(±)-7-hydroxycitronellal, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (±)-carvone in rabbits. Xenobiotica, 19,
843-855.
Iwata, N., Inazu, N., Hara, S., Yanase, T., Kano, S., Endo, T.,
Kuriiwa, F., Sato, Y. & Satoh, T. (1993) Interindividual variability
of carbonyl reductase levels in human livers. Biochem. Pharmacol.,
45, 1711-1714.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols: The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochemistry, 53,
129-136.
Klesov, A.A., Lange, L.G., Sytkowski, A.J. & Vallee, B.L. (1977)
[Unusual nature of the substrate specificity of alcohol dehydrogenase
of different origins.] Biorganich. Khim., 3, 1141-1144 (in Russian).
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Commun., 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone -- a hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Madyastha, K.M. & Srivatsan, V. (1988a) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Madyastha, K.M. & Srivatsan, V. (1988b) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Norwalk, Connecticut, Appleton &
Lange, pp. 177-192.
Nakayama, T., Hara, A., Yashiro, K. & Sawada, H. (1985) Reductases for
carbonyl compounds in human liver. Biochem. Pharmacol., 34, 107-117.
Nakayasu, H., Mihara, K. & Sato, R. (1978) Purification and properties
of membrane-bound aldehyde dehydrogenase from rat liver microsomes.
Biochem. Biophys. Res. Commun., 83, 697-703.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Pietruszko, R., Crawford, K. & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenases from human liver,
horse liver and yeast towards saturated and 2-enoic alcohols and
aldehydes. Arch. Biochem. Biophys., 159, 50-60.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Renwick, A.G. & Williams, R.T. (1972) The metabolites of
cyclohexylamine in man and certain animals. Biochem. J., 129,
857-867.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34, 569-585.
Sauer, J.-M., Smith, R.L., Bao, J., Kattnig, M.J., Kuester, R.K.,
McClure, T.D., Mayersohn, M. & Sipes, I.G. (1997) Oral and topical
absorption, disposition kinetics, and the metabolic fate of
trans-methyl styryl ketone in the male Fischer 344 rat. Drug
Metab. Disposition, 25, 732-739.
Savary, P. & Constantin, M.J. (1970) Intestinal hydrolysis and
lymphatic absorption of isopropyl asters of long chain fatty acids in
the rat. Biochem. Biophys. Acta, 218, 195.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. Biochemistry, 22,
1827-1832.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. I. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Fol.
Pharmacol. Jpn., 58, 323-336.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knight, S. &
Knight, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71-79.
Williams, R.T. (1938) Studies in detoxication. II. a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol in the rabbit. b) d-iso-Menthylglucuronide,
a new conjugated glucuronic acid. Biochem. J., 32, 1849-1855.
Williams, R.T. (1939) Studies in detoxication. III. The use of the
glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 33, 1519-1524.
Williams, R.T. (1959) Detoxication Mechanisms: The Metabolism and
Detoxication of Drugs, Toxic Substances and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
