
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
SATURATED ALIPHATIC ACYCLIC SECONDARY ALCOHOLS, KETONES, AND RELATED
SATURATED AND UNSATURATED ESTERS
First draft prepared by Dr Jœrn Gry
Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration,
Ministry of Food, Agriculture and Fisheries,
Sœborg, Denmark
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of Procedure for the Safety Evaluation
of Flavouring Agents
Consideration of combined intakes from use as
flavouring agents
Conclusions
Relevant background information
Explanation
Intake
Biological data
Absorption, metabolism, and elimination
Ester hydrolysis
gamma-Diketone formation
Excretion
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Genotoxicity
Other relevant studies
References
1. EVALUATION
1.1 Introduction
The Committee evaluated 39 saturated aliphatic acyclic secondary
alcohols, ketones and related saturated and unsaturated esters
(Table 1) using the Procedure for the Safety Evaluation of Flavouring
Agents (Figure 1, p. 222, and Annex 1, reference 131). The Committee
had evaluated five members of this group previously. Acetone was
evaluated as an extraction solvent at the fourteenth meeting (Annex 1,
reference 22), when the Committee considered that, with good
manufacturing practice, the residues in food would be toxicologically
insignificant. The evaluation was tentative owing to lack of relevant
data. Isopropyl alcohol was evaluated at the fourteenth and
twenty-fifth meetings (Annex 1, references 22 and 56); an ADI was not
allocated because of lack of data. Isopropyl acetate was evaluated at
the twenty-third meeting (Annex 1, reference 50), when, because of a
lack of data on hydrolysis and other toxicological end-points, the
Committee was unable to establish an ADI. Isopropyl myristate,
evaluated at the twenty-third meeting (Annex 1, reference 50), was not
allocated an ADI owing to lack of data. 2-Butanone (ethyl methyl
ketone) was evaluated at the twenty-third and twenty-fifth meetings
(Annex 1, references 50 and 56), when the Committee concluded that the
data available were not sufficient for evaluation of the substance,
and no ADI was allocated.
1.2 Estimated daily per capita intake
The total annual volume of the 39 saturated aliphatic acyclic
secondary alcohols, ketones, and related esters in this group is
approximately 750 tonnes in Europe (International Organization of the
Flavor Industry, 1995) and 240 tonnes in the United States (National
Academy of Sciences, 1989). In Europe, 92% of the total annual volume
is accounted for by isopropyl alcohol alone. In the United States, 98%
of the total annual volume of compounds in this group is accounted for
by acetone and isopropyl alcohol. On the basis of the reported annual
volumes, the estimated daily per capita intake of acetone from use
as a flavouring agent is approximately 7.1 mg/day in Europe and 35
mg/day in the United States. Similarly, the total estimated daily
per capita intake of isopropyl alcohol and its nine esters in this
group from use as flavouring agents is about 99 mg/day in Europe and
10 mg/day in the United States.
Saturated aliphatic acyclic secondary alcohols, ketones, and
related esters are the main flavouring components of alcoholic
beverages and a wide variety of fruits (Maarse et al., 1994).
Quantitative data on natural occurrence have been reported for 22 of
the 39 substances in this group. In the United States, intake of 20 of
these 22 substances from natural sources exceeds intake from their use
as flavouring agents; two of the substances, acetone and 3-octanol,
have greater use as flavouring agents (Stofberg & Kirschner, 1985;
Stofberg & Grunschober, 1987).
1.3 Absorption, metabolism, and elimination
In general, saturated aliphatic acyclic secondary alcohols, and
ketones are absorbed through the gastrointestinal tract and rapidly
eliminated from the blood. Peak blood levels are normally obtained
within 1-2 h after dosing. Related esters are anticipated to be
hydrolysed to their component secondary alcohols and aliphatic,
saturated, and unsaturated carboxylic acids, which are also readily
absorbed. Isopropyl alcohol and low-molecular-mass ketones such as
acetone and 2-buta-none are endogenous in humans as components of
fatty acid and carbohydrate metabolism and have been detected in the
blood (Krasavage et al., 1982; Morgott, 1993; Lington & Bevan, 1994).
Isopropyl esters are believed to be hydrolysed to isopropyl
alcohol and their respective saturated aliphatic carboxylic acids.
Hydrolysis is catalysed by classes of enzymes recognized as
carboxylesterases. In mammals, these enzymes occur in most body
tissues but predominate in hepatocytes (Heymann, 1980; Anders, 1989).
Aliphatic ketones are metabolized primarily via reduction to
the corresponding secondary alcohol (Leibman, 1971; Felsted & Bachur,
1980; Bosron & Li, 1980). Secondary alcohols are metabolized by
conjugation with glucuronic acid followed by excretion primarily in
the urine (Neubreuer, 1901). Short-chain aliphatic ketones may also be
metabolized via omega- and/or (omega-1)-oxidation, and/or they may
be excreted unchanged in expired air (Haggard et al., 1945; Scopinaro
et al., 1947; Saito, 1975; Brown et al., 1987; Schwartz, 1989).
omega Oxidation and/or (omega-1)-oxidation become competing pathways
for longer-chain aliphatic ketones at high concentrations (Dietz et
al., 1981; Topping et al., 1994).
An intoxication pathway (i.e. formation of a neurotoxic
gamma-diketone) has been identified for aliphatic ketones that meet
special structural requirements (Krasavage et al., 1980; Topping et
al., 1994). 3-Heptanone is the only substance in this group that may
be metabolized to a gamma-diketone; however, the threshold for
activation of this pathway occurs at near-lethal doses (O'Donoghue et
al., 1984), which are significantly greater than the levels at which
3-heptanone is used as a flavouring substance.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
The stepwise evaluations of the 39 saturated aliphatic acyclic
secondary alcohols, ketones, and related esters used as flavouring
agents are summarized in Table 1.
Step 1. Twenty-eight of the 39 saturated aliphatic acyclic secondary
alcohols, ketones, and related esters are classified in
structural class I. The remaining 11 substances, acyclic
aliphatic 2-alkanones (Nos 283, 288, 292, 296, 298, 299, and
301), 3-alkanones (Nos 285, 290, and 294), and 4-alkanone
(No. 302) with four or more carbons on either side of the
keto group, are in structural class II (Cramer et al.,
1978).
Step 2. The available data indicate that 26 of the 28 secondary
alcohols and ketones would be predicted to be metabolized to
or are innocuous products per se. The 11 esters in this
group are anticipated to be hydrolysed to their component
secondary alcohols (isopropyl alcohol or 3-octanol) and
carboxylic acids (aliphatic saturated acids or the
unsaturated tiglic acid). These hydrolysis products are
either endogenous or can be predicted to be oxidized to
innocuous substances. At current levels of per capita
intake, 37 of the 39 flavouring agents in this group would
not be expected to saturate the metabolic pathways.
Therefore, 37 of the 39 substances in this group were
considered to be metabolized to innocuous products. The
remaining two substances, 3-heptanone (No. 285) and
3-heptanol (No. 286), may undergo oxidation to neurotoxic
gamma-diketones, and these two substances therefore proceed
to step B3.
Table 1. Summary of results of safety evaluations of saturated aliphatic acyclic secondary alcohols, ketones, and related esters
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Acetone 139 7100/36 000 I Yes Yes Yes No safety
concern
CAS No. 67-64-1
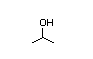
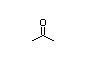 Isopropyl 277 99 000/9 900 I Yes Yes Yes No safety
alcohol concern
CAS No. 67-63-0
Isopropyl 277 99 000/9 900 I Yes Yes Yes No safety
alcohol concern
CAS No. 67-63-0
 2-Butanone 278 110/36 I Yes No No safety
concern
CAS No. 78-93-3
2-Butanone 278 110/36 I Yes No No safety
concern
CAS No. 78-93-3
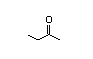 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
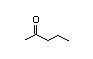
2-Pentanone 279 140/42 I Yes No No safety
concern
CAS No. 107-87-9
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Pentanone 279 140/42 I Yes No No safety
concern
CAS No. 107-87-9
 2-Pentanol 280 6/0.04 I Yes No No safety
concern
CAS No. 6032-29-7
2-Pentanol 280 6/0.04 I Yes No No safety
concern
CAS No. 6032-29-7
 3-Hexanone 281 0.4/1 I Yes No No safety
concern
CAS No. 589-38-8
3-Hexanone 281 0.4/1 I Yes No No safety
concern
CAS No. 589-38-8
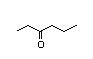 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
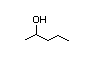
3-Hexanol 282 13/11 I Yes No No safety
concern
CAS No. 623-37-0
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Hexanol 282 13/11 I Yes No No safety
concern
CAS No. 623-37-0
 2-Heptanone 283 110/48 II Yes No No safety
concern
CAS No. 110-43-0
2-Heptanone 283 110/48 II Yes No No safety
concern
CAS No. 110-43-0
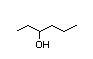
 2-Heptanol 284 8/1 I Yes No No safety
concern
CAS No. 543-49-7
2-Heptanol 284 8/1 I Yes No No safety
concern
CAS No. 543-49-7
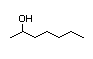 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
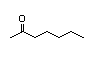
3-Heptanone 285 4/10 II No No Yes No safety
concern
CAS No. 106-35-4
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Heptanone 285 4/10 II No No Yes No safety
concern
CAS No. 106-35-4
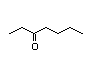 3-Heptanol 286 0.2/0.6 I No No Yes No safety
concern
CAS No. 589-82-2
3-Heptanol 286 0.2/0.6 I No No Yes No safety
concern
CAS No. 589-82-2
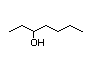 4-Heptanone 287 2/2 I Yes No No safety
concern
CAS No. 123-19-3
4-Heptanone 287 2/2 I Yes No No safety
concern
CAS No. 123-19-3
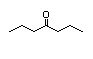 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Octanone 288 110/67 II Yes No No safety
concern
CAS No. 111-13-7
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Octanone 288 110/67 II Yes No No safety
concern
CAS No. 111-13-7
 2-Octanol 289 13/4 I Yes No No safety
concern
CAS No. 123-96-6
2-Octanol 289 13/4 I Yes No No safety
concern
CAS No. 123-96-6
 3-Octanone 290 3/2 II Yes No No safety
concern
CAS No. 106-68-3
3-Octanone 290 3/2 II Yes No No safety
concern
CAS No. 106-68-3
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Octanol 291 6/320 I Yes No No safety
concern
CAS No. 589-98-0
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Octanol 291 6/320 I Yes No No safety
concern
CAS No. 589-98-0
 2-Nonanone 292 380/27 II Yes No No safety
concern
CAS No. 821-55-6
2-Nonanone 292 380/27 II Yes No No safety
concern
CAS No. 821-55-6
 2-Nonanol 293 1/1 I Yes No No safety
concern
CAS No. 628-99-9
2-Nonanol 293 1/1 I Yes No No safety
concern
CAS No. 628-99-9
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Nonanone 294 0.1/0.1 II Yes No No safety
concern
CAS No. 925-78-0
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Nonanone 294 0.1/0.1 II Yes No No safety
concern
CAS No. 925-78-0
 3-Decanol 295 N/D/16 I Yes No No safety
concern
CAS No. 1565-81-7
3-Decanol 295 N/D/16 I Yes No No safety
concern
CAS No. 1565-81-7
 2-Undecanone 296 380/21 II Yes No No safety
concern
CAS No. 112-12-9
2-Undecanone 296 380/21 II Yes No No safety
concern
CAS No. 112-12-9
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Undecanol 297 0.2/0.04 I Yes No No safety
concern
CAS No. 1653-30-1
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Undecanol 297 0.2/0.04 I Yes No No safety
concern
CAS No. 1653-30-1
 2-Tridecanone 298 73/30 II Yes No No safety
concern
CAS No. 593-08-8
2-Tridecanone 298 73/30 II Yes No No safety
concern
CAS No. 593-08-8
 2-Pentadecanone 299 21/430 II Yes No No safety
concern
CAS No. 2345-28-0
2-Pentadecanone 299 21/430 II Yes No No safety
concern
CAS No. 2345-28-0
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Methyl-2- 300 1/0.2 I Yes No No safety
butanol concern
CAS No. 589-75-4
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Methyl-2- 300 1/0.2 I Yes No No safety
butanol concern
CAS No. 589-75-4
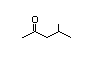
 4-Methyl-2- 301 7/2 II Yes No No safety
concern
CAS No. 108-10-1
4-Methyl-2- 301 7/2 II Yes No No safety
concern
CAS No. 108-10-1
 2,6-Dimethyl-4- 302 0.2/0.06 II Yes No No safety
heptanone concern
CAS No. 108-83-8
2,6-Dimethyl-4- 302 0.2/0.06 II Yes No No safety
heptanone concern
CAS No. 108-83-8
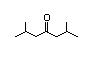 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
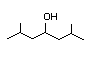
2,6-Dimethyl-4- 303 N/D/5 I Yes No No safety
heptanol concern
CAS No. 108-82-7
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2,6-Dimethyl-4- 303 N/D/5 I Yes No No safety
heptanol concern
CAS No. 108-82-7
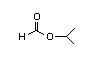
 Isopropyl 304 0.5/0.02 I Yes No No safety
formate concern
CAS No. 625-55-8
Isopropyl 304 0.5/0.02 I Yes No No safety
formate concern
CAS No. 625-55-8
 Isopropyl 305 41/9 I Yes No No safety
acetate concern
CAS No. 108-21-4
Isopropyl 305 41/9 I Yes No No safety
acetate concern
CAS No. 108-21-4
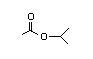 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isoproyl 306 0.01/0.02 I Yes No No safety
propionate concern
CAS No. 637-78-5
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isoproyl 306 0.01/0.02 I Yes No No safety
propionate concern
CAS No. 637-78-5
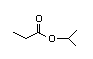 Isopropyl 307 7/0.08 I Yes No No safety
butyrate concern
CAS No. 638-11-9
Isopropyl 307 7/0.08 I Yes No No safety
butyrate concern
CAS No. 638-11-9
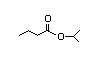 Isopropyl 308 4/0.02 I Yes No No safety
hexanoate concern
CAS No. 2311-46-8
Isopropyl 308 4/0.02 I Yes No No safety
hexanoate concern
CAS No. 2311-46-8
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 309 0.6/0.06 I Yes No No safety
isobutyrate concern
CAS No. 617-50-5
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 309 0.6/0.06 I Yes No No safety
isobutyrate concern
CAS No. 617-50-5
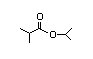 IIsopropyl 310 0.3/0.2 I Yes No No safety
isovalerate concern
CAS No. 32665-23-9
IIsopropyl 310 0.3/0.2 I Yes No No safety
isovalerate concern
CAS No. 32665-23-9
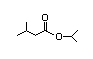 sopropyl 311 23/0.02 I Yes No No safety
myristate concern
CAS No. 110-27-0
sopropyl 311 23/0.02 I Yes No No safety
myristate concern
CAS No. 110-27-0
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 312 0.01/0.1 I Yes No No safety
tiglate concern
CAS No. 6284-46-4
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 312 0.01/0.1 I Yes No No safety
tiglate concern
CAS No. 6284-46-4
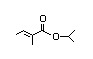 3-Octyl acetate 313 0.7/30 I Yes No No safety
concern
CAS No. 4864-61-3
3-Octyl acetate 313 0.7/30 I Yes No No safety
concern
CAS No. 4864-61-3
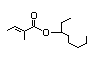
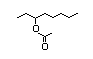 1-Ethylhexyl 448 0.01/29 I Yes No No safety
tiglate concern
(3-Octyl tiglate)
CAS No. 94133-92-3
1-Ethylhexyl 448 0.01/29 I Yes No No safety
tiglate concern
(3-Octyl tiglate)
CAS No. 94133-92-3
 The thresholds for human intake are 1800 µg/day for Class I and 540 µg/day for Class II.
N/D, No intake data reported
Step A3. The daily per capita intakes of 25 of the 27 class-I
substances at this step in Europe and the United States are
below the human intake threshold for class I compounds (1800
µg/person per day), indicating that they pose no safety
concern when used at current levels of estimated intake as
flavouring agents. The intakes of acetone (mg/person per
day) are 7.1 in Europe and 35 in the United States, and the
intakes of isopropyl alcohol are 99 in Europe and 10 in the
United States; these are greater than the human intake
threshold of 1800 µg/person per day (National Academy of
Sciences, 1989; International Organization of the Flavor
Industry, 1995). The intakes of 10 class II substances at
this step in Europe and in the United States are below the
human intake threshold for that class (540 µg/person per
day), indicating that they pose no safety concern at current
levels of estimated intake as flavouring agents.
The total intake of isopropyl alcohol and its nine
esters from use as flavouring agents is also greater than
the human intake threshold for compounds in class I (1800
µg/person per day).
The intake of three additional esters of isopropyl
alcohol (isopropyl benzoate, isopropyl cinnamate, and
isopropyl phenyl-acetate), which were not evaluated as part
of this group, would add about 15 µg/day in Europe and 5
µg/day in the United States to the total intake of isopropyl
alcohol. This potential additional intake is minor and would
not alter the safety evaluation.
Step A4. This step needs to be considered only for acetone and
isopropyl alcohol, the two flavouring agents in this group
for which estimated intake exceeds the class I threshold of
1800 µg/person per day. They are both endogenous, as
components of fatty acid and carbohydrate metabolism, and
have been detected in the blood.
Step B3. The current per capita intake of 3-heptanone (No. 285),
class II, and 3- heptanol (No. 286), class I, do not exceed
the human intake thresholds, 540 and 1800 µg/day,
respectively. Accordingly the evaluation proceeds to step
B4.
Step B4. On the basis of a limited study of the neurotoxic effects of
orally administered 3-heptanone (No. 285) to male rats, the
NOEL was 1000 mg/kg bw per day (O'Donoghue et al., 1984).
This NOEL provides a large safety margin (> 1 000 000 for
3-heptanone and even higher for 3-heptanol) in relation to
current levels of estimated intake of 3-heptanone and
3-heptanol (No. 286) (which might be metabolized to
3-heptanone).
On the basis of the results of the safety evaluation sequence, 28
of the saturated aliphatic acyclic secondary alcohols and ketones and
11 of the related esters of saturated and unsaturated carboxylic acids
evaluated do not pose a safety concern when used at current levels of
intake as flavouring agents.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 39 saturated aliphatic acyclic
secondary alcohols, ketones, and related esters of saturated and
unsaturated carboxylic acids were consumed concomitantly on a daily
basis, the estimated combined intake would exceed the human thresholds
for classes I and II. All of the 39 substances except two (3-heptanone
and 3-heptanol) are expected to be metabolized via well-known
biochemical pathways to innocuous metabolic and/or endogenous
substances; in the opinion of the Committee, the endogenous levels of
these metabolites would not give rise to perturbations outside the
physiological range. Accordingly, even a combined theoretical intake
would be of no safety concern. The combined intake of the two
substances that could potentially form gamma-diketones would also be
of no safety concern.
1.6 Conclusions
The Committee concluded that the substances in this group would
not present safety concerns at the current levels of intake.
Data on toxicity were required only for 3-heptanone (No. 285) and
3-heptanol (No. 286) when applying the procedure to this group of
flavouring agents. The Committee noted that these data and others are
consistent with the results of the safety evaluation using the
procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Twenty-eight saturated aliphatic acyclic ketones and secondary
alcohols with chain lengths from C3 to C15 (Nos 139 and 277-303) and
11 related saturated and unsaturated esters (Nos 304-313 and 448) are
included in this group of flavouring agents (see Table 1). The group
comprises 16 saturated aliphatic acyclic ketones (10 2-alkanones, four
3-alkanones, and two 4-alkanones), 12 saturated aliphatic acyclic
secondary alcohols (seven 2-alkanols, four 3-alkanols, and one
4-alkanol), nine esters of isopropyl alcohol, and two esters of
3-octanol in which the component carboxylic acids are six saturated
and one unsaturated carboxylic acid (tiglic acid).
The substances in this group are structurally related because
they are saturated, aliphatic acyclic ketones, secondary alcohols, or
esters formed from secondary alcohols and aliphatic, saturated or
unsaturated carboxylic acids.
2.2 Intake
The total annual production and the estimated current per
capita intake in Europe and the United States of the 39 saturated
aliphatic acyclic secondary alcohols, ketones, and related esters are
shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
Generally, saturated aliphatic acyclic secondary alcohols and
ketones are absorbed through the gastrointestinal tract and rapidly
eliminated from the blood. The related esters in this group of
flavouring agents are anticipated to be hydrolysed to their component
secondary alcohols and aliphatic saturated and unsaturated carboxylic
acids, which are also readily absorbed, as considered above in section
1.3 and 'General aspects of metabolism', p. 223.
In addition to oxidation and reduction pathways,
low-molecular-mass ketones (carbon chain length, < C-5) may be
excreted unchanged in expired air (Brown et al., 1987). In mammals,
oral doses of volatile ketones or their corresponding alcohols are
eliminated principally as the ketone in expired air; lesser amounts
are excreted in the urine (Haggard et al., 1945; Scopinaro et al.,
1947; Saito, 1975). In rats, 2-pentanone in expired air was the major
metabolite after administration of 2-penta-nol by intraperitoneal
injection; lesser amounts of 2-pentanol were exhaled, and both
metabolites were detected in urine (Haggard et al., 1945). Some of the
flavouring agents in this group may be oxidized to form neurotoxic
gamma-diketones (see below).
2.3.1.1 Ester hydrolysis
Generally, isopropyl esters are anticipated to be hydrolysed to
isopropyl alcohol and their component saturated or unsaturated
aliphatic carboxylic acids (see section 1.3 and 'General aspects of
metabolism', p. 223). Isopropyl tiglate (No. 312) and the two 3-octyl
esters (Nos 313 and 448) may be hydrolysed at a lower rate in the
gastrointestinal tract, but esters that reach the general circulation
intact will be hydrolysed by tissue esterases to the component
carboxylic acid and alcohol.
In general, the ketones and secondary alcohols in this group of
flavouring agents are metabolized by reversible reduction/oxidation of
the ketone/alcohol group and/or side-chain oxidation and/or
conjugation of the alcohol groups, primarily with glucuronic acid (see
'General aspects of metabolism', p. 222).
Table 2. Most recent annual usage of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters as flavouring substances in Europe and United States
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Acetone (139)
Europe 50 000 7 100 120 NA
United States 190 000 36 000 590 NA
Isopropyl alcohol (277)
Europe 690 000 99 000 1 600 NA
United States 52 000 9 900 170 NA
2-Butanone (278)
Europe 790 110 2 NA
United States 190 36 0.6 NA
2-Pentanone (279)
Europe 1 000 140 2 NA
United States 220 42 0.7 NA
2-Pentanol (280)
Europe 44 6 0.1 NA
United States 0.2 0.04 0.0 NA
3-Hexanone (281)
Europe 3 0.4 0.01 NA
United States 5 1 0.02 NA
3-Hexanol (282)
Europe 94 13 0.22 NA
United States 60 11 0.19 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Heptanone (283)
Europe 790 110 2 NA
United States 250 48 0.11 NA
2-Heptanol (284)
Europe 56 8 0.13 NA
United States 5 1 0.02 NA
3-Heptanone (285)
Europe 27 4 0.06 NA
United States 50 10 0.16 NA
3-Heptanol (286)
Europe 1 0.02 0.0 NA
United States 3 0.6 0.01 NA
4-Heptanone (287)
Europe 16 2 0.04 NA
United States 13 2 0.04 NA
2-Octanone (288)
Europe 760 110 2 NA
United States 350 67 1 NA
2-Octanol (289)
Europe 94 13 0.22 NA
United States 19 4 0.06 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octanone (290)
Europe 23 3 0.05 NA
United States 12 2 0.04 NA
3-Octanol (291)
Europe 39 6 0.09 NA
United States 1 700 320 5 NA
2-Nonanone (292)
Europe 2 600 380 6 NA
United States 140 27 0.44 NA
2-Nonanol (293)
Europe 5 1 0.01 NA
United States 3 1 0.01 NA
3-Nonanone (294)
Europe 1 0.1 0.0 NA
United States 0.5 0.1 0.0 NA
3-Decanol (295)
Europe NR N/D 0 NA
United States 82 16 0.26 NA
2-Undecanone (296)
Europe 2 700 380 6 NA
United States 110 21 0.35 NA
2-Undecanol (297)
Europe 2 0.2 0.0 NA
United States 0.2 0.04 0.0 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Tridecanone (298)
Europe 510 73 1 NA
United States 160 30 0.51 NA
2-Pentadecanone (299)
Europe 150 21 0.3 NA
United States 2 300 430 7 NA
3-Methyl-2-butanol (300)
Europe 4 1 0.01 NA
United States 0.9 0.2 0.0 NA
4-Methyl-2-pentanone (301)
Europe 50 7 0.12 NA
United States 8 2 0.03 NA
2,6-Dimethyl-4-heptanone (302)
Europe 1.5 0.2 0.0 NA
United States 0.3 0.06 0.0 NA
2,6-Dimethyl-4-heptanol (303)
Europe NR N/D 0 NA
United States 25 5 0.08 NA
Isopropyl formate (304)
Europe 3.7 0.5 0.01 0.01
United States 0.1 0.02 0.0 0.00
Isopropyl acetate (305)
Europe 290 41 0.69 0.45
United States 46 9 0.15 0.06
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Isopropyl propionate (306)
Europe 0.1 0.01 0.01 0.00
United States 0.1 0.02 0.0 0.00
Isopropyl butyrate (307)
Europe 49 7 0.12 0.05
United States 0.4 0.08 0.0 0.00
Isopropyl hexanoate (308)
Europe 26 4 0.06 0.03
United States 0.1 0.02 0.0 0.00
Isopropyl isobutyrate (309)
Europe 4 0.6 0.01 0.01
United States 0.3 0.06 0.0 0.00
Isopropyl isovalerate (310)
Europe 2 0.3 0.01 0.00
United States 0.9 0.2 0.0 0.00
Isopropyl myristate (311)
Europe 160 23 0.38 0.10
United States 0.1 0.02 0.0 0.00
Isopropyl tiglate (312)
Europe 0.1 0.01 0.0 0.00
United States 0.5 0.1 0.0 0.00
3-Octyl acetate (313)
Europe 5 0.7 0.01 0.00
United States 160 30 0.51 0.20
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octyl tiglate (448)
Europe 0.1 0.01 0.0 0.00
United States 150 29 0.48 0.30
TOTAL
Europe 750 000 NA NA NA
United States 240 000 NA NA NA
TOTAL Isopropyl alcohol
Europe NA 99 000 1 600
United States NA 10 000 170
NA, not applicable; NR, not reported
a Europe, International Organization of the Flavor Industry (1995); United States,
National Academy of Sciences (1989)
b Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where
population (10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the United
States; 0.6 represents the assumption that only 60% of the flavour volume was
reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
c Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily
per capita intake ester
2.3.1.2 gamma-Diketone formation
Oxidation, primarily (omega-1)-oxidation, of certain aliphatic
ketones may yield gamma-diketones (e.g. 2-hexanone and 3-heptanone may
yield 2,5-hexanedione and 2,5-heptanedione, respectively), which, when
administered at high concentrations to experimental animals, may
result in peripheral neuropathy, commonly recognized as 'giant' axonal
neuropathy (Krasavage et al., 1980; Topping et al., 1994). The
structural features of diketone metabolites that induce peripheral
neuropathy have been shown to include gamma-diketone spacing.
gamma-Diketones with terminal methyl substituents (e.g.
2,5-hexanedione) and/or methyl substituents at the 3 and 4 positions
have the most pronounced effects. The severity of the peripheral
neuropathy depends on the size and position of alkyl substituents on
the gamma-diketone. For example, the strongest neurotoxic effects have
been observed with 3,4-dimethyl-substituted 2,5-hexanedione (not a
flavouring substance). When the chain length is increased (i.e. C-7
and greater), the neurotoxic response is generally significantly
reduced (Topping et al., 1994).
3-Heptanone and 3-heptanol (Nos 285 and 286) are the only
compounds in this group of flavouring substances that may be
metabolized to yield a neurotoxic gamma-diketone (Topping et al.,
1994); however, the longer chain length of 3-heptanone reduces its
neurotoxic effect , which is observed only at high doses.
In a poorly described study of the neurotoxic effects of some
aliphatic ketones, 3-heptanone administered to female Wistar rats in
the drinking-water for 120 days did not produce significant
neurological alterations (Homan & Maronpot, 1978).
Groups of five male Crl rats were exposed to atmospheres
containing 700 mg/kg 3-heptanone for two 20- and two 16-h periods per
week, for a total of 88 exposures over 164 days (approximately
24 weeks). After the fourth, thirtieth, and eighty-fifth exposures,
serum was analysed for 2,5-heptanedione. The maximum mean serum
concentrations reached 10 µg/ml after four exposures but decreased to
6-7 µg/ml after 30 and 85 exposures. No neurotoxicity was observed
(Katz et al., 1980).
Groups of two Crl rats were given 250, 500, 1000, 2000, or
4000 mg/kg bw 3-heptanone by gavage on five days per week for
14 weeks. No peripheral neuropathy was observed at doses
< 1000 mg/kg bw. At 2000 mg/kg bw, approaching the LD50 in rats
(2760 mg/kg bw), 3-heptanone induced peripheral neuropathy (O'Donoghue
et al., 1984).
2.3.1.3 Excretion
In studies limited to the identification of urinary glucuronide
metabolites, a series of aliphatic secondary alcohols was administered
by stomach tube to rabbits, and the urinary excretion of glucuronic
acid conjugates was determined after 24 h. The compounds, doses, and
average urinary output of glucuronide are listed in Table 3. The
results show that secondary alcohols, either administered directly or
formed via ketone reduction as demonstrated in one case with
2-heptanone (41 % urinary glucuronide), are largely excreted as
glucuronic acid conjugates (Kamil et al., 1953).
Table 3. Urinary excretion of glucuronides after
administration of aliphatic secondary alcohols to
rabbits by gavage
Substance No. Dose Urinary
(mg/kg bw)a glucuronide (%)
2-Propanol 277 1002 10.2
2-Butanol - 618 14.4
2-Pentanol 280 735 44.8
2-Heptanol 284 965 54.6
3-Heptanol 286 965 61.9
4-Heptanol - 965 67.4
2-Octanol 289 1081 15.5
From Kamil et al. (1953)
a Calculated from dose of 25 mmol/3-kg rabbit (for
2-propanol, 50 mmol/3 kg)
Generally, secondary alcohols (and ketones, by reduction to
secondary alcohols) are excreted after their conjugation with
glucuronic acid, primarily in the urine, or in the bile, depending on
the relative molecular mass and the animal species (see 'General
aspects of metabolism', p. 222). After hydrolysis of the esters in
this group, see above, the component secondary alcohols are
metabolized and excreted, and the component acids are metabolized in
the fatty acid ß-oxidation and other well-known metabolic pathways.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 25 of the 39 substances
in this group and range from 400 to 9800 mg/kg bw. The vast majority
of the values are > 1000 mg/kg bw, demonstrating that the acute
toxicity of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters given orally is extremely low (see e.g. Tanii et
al., 1986).
2.3.2.2 Short-term studies of toxicity
The results of short-term studies with six saturated aliphatic
acyclic secondary alcohols and ketones are summarized in Table 4 and
described below, together with some further studies. There were no
adequate studies available on the related esters.
Acetone
In a 14-day study, groups of five male and five female Fischer
344/N rats were given drinking-water ad libitum containing acetone
at concentrations of 0, 5000, 10 000, 20 000, 50 000, or 100 000 mg/kg
(equivalent to 0, 500, 1000, 2000, 5000, and 10 000 mg/kg bw per day).
Water consumption was depressed in females at 2000 mg/kg bw per day
and in all animals at 5000 or 10 000 mg/kg bw per day, but this was
not accompanied by signs of dehydration. The body weights of males at
5000 or 10 000 mg/kg bw per day and females at 10 000 mg/kg bw per day
were depressed. Statistically significant increases in relative kidney
and liver weights were observed n both male and female rats at a dose
of 2000 or 5000 mg/kg bw per day. In addition, statistically
significant increases in relative testis weight were observed at 5000
mg/kg bw per day but not at 10 000 mg/kg bw per day. No
histopathological abnormalities were observed in rats at any dose
tested (Dietz, 1991).
Groups of five male and five female B6C3F1 mice were exposed to
acetone in drinking-water ad libitum at concentrations of 0, 5000,
10 000, 20 000, 50 000, or 100 000 mg/kg (equivalent to 0, 1250, 2500,
5000, 12 500, and 25 000 mg/kg bw per day) for 14 days. Water
consumption was depressed at 12 500 or 25 000 mg/kg bw per day as
compared with controls. Growth retardation was seen in males and
females at 25 000 mg/kg bw per day but was more pronounced in males.
Statistically significant increases in relative kidney weights were
seen in both males and females at 12 500 or 25 000 mg/kg bw per day,
and statistically significant changes in relative liver weights were
observed in males at 1250, 2500, 5000, 12 500, or 25 000 mg/kg bw per
day and in females at 5000, 12 500, or 25 000 mg/kg bw per day.
Minimal to mild centrilobular hepatocellular hypertrophy was observed
at doses of 5000, 12 500, and 25 000 mg/kg bw per day in males and 12
500 and 25 000 mg/kg bw per day in females. Moderate centrilobular
hepatocellular hypertrophy was observed in males at 25 000 mg/kg bw
per day (Dietz, 1991).
In a 13-week study, groups of 10 male and 10 female rats were
given drinking-water ad libitum containing acetone at concentrations
of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg (equivalent to 0,
250, 500, 1000, 2000, and 5000 mg/kg bw per day). Water consumption
was depressed in males at 5000 mg/kg bw per day and in females at 2000
or 5000 mg/kg bw per day, but this was not accompanied by signs of
dehydration. Body-weight gain was significantly decreased in males and
females at 5000 mg/kg bw per day. Relative kidney weights were
significantly increased in both male and female rats exposed to
acetone at 5000 mg/kg bw per day and in females at 2000 mg/kg bw per
day. Increased incidences of neuropathy were seen in all males,
including the controls, but at greatest incidence and severity at the
two highest doses. Relative liver weights were significantly increased
in males and females at 2000 or 5000 mg/kg bw per day. No significant
differences in relative organ weight were observed at doses below 2000
mg/kg bw per day. At 5000 mg/kg bw per day, the relative testis
weights were significantly increased. The authors noted that such
organ weight changes are difficult to interpret because of the effect
of high doses of acetone on body weight; they therefore may not be
biologically significant. Furthermore, testicular toxicants typically
decrease testis weights. Decreased sperm motility, caudal weight, and
epididymal weight and an increased incidence of abnormal sperm were
also seen in males at 5000 mg/kg bw per day. The NOEL was 1000 mg/kg
bw per day (Dietz, 1991).
In a 13-week study, groups of 10 female and 10 male B6C3F1 mice
were given drinking-water containing acetone. Females received acetone
at concentrations of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg,
equivalent to 0, 625, 1250, 2500, 5000, or 12 500 mg/kg bw per day.
Males received acetone at a concentration of 0, 1250, 2500, 5000,
10 000, or 20 000 mg/kg, equivalent to 0, 313, 625, 1250, 2500, and
5000 mg/kg bw per day. The fluid intake of all females was depressed
as compared with controls. Absolute and relative liver weights and
absolute and relative spleen weights were significantly increased in
female mice at 12 500 mg/kg bw per day. Centrilobular hepatocellular
hypertrophy of minimal severity was seen in two female mice receiving
12 500 mg/kg bw per day, but no compound-related lesions were found in
male mice. The NOEL was 2500 mg/kg bw per day (Dietz, 1991).
Acetone was administered to groups of 30 male and 30 female
Sprague-Dawley rats by gavage for 90 days at doses of 100, 500, or
2500 mg/kg bw per day. Female rats at the two highest doses had
increased absolute kidney weights, and relative weight increases were
seen in the kidney, liver, and brain of both males and females at the
highest dose. Red blood cell parameters were significantly increased
in males at 45 days and animals of each sex at 90 days, at the highest
dose. Histopathological examination showed a significant increase in
tubular degeneration and hyalin droplet accumulation in males at
500 mg/kg bw per day and in animals of each sex at 2500 mg/kg bw per
day. Histolopathological examination of the liver and kidneys revealed
no treatment-dependent abnormalities. The NOEL was 100 mg/kg bw per
day (Sonawane et al., 1986).
Isopropyl alcohol
The potential toxicity of isopropyl alcohol was investigated in a
12-week study with 110 three-month-old male Wistar rats given 0, 1, 2,
3, or 5% in their drinking-water for the duration of the study. The
highest concentration was lowered to 4% during week 2 because of
unpalatability. The average daily intakes were reported to be 0, 870,
1300, 1700, and 2500 mg/kg bw. Body weights were statistically
significantly decreased in rats at 1700 or 2500 mg/kg bw per day.
Statistically significant, dose-related changes in relative liver
weights were observed at the three highest doses; significant
increases in testis weights were observed at 2500 mg/kg bw per day;
significant increases in relative kidney weights were seen at the
three highest doses; and statistically significant increases in
relative adrenal weights were observed at the two highest doses.
Increased formation of hyaline casts and increased hyaline droplet
content were observed in the proximal tubules of the kidneys in a
dose-dependent fashion. The NOEL was 870 mg/kg bw per day (Pilegaard &
Ladefoged, 1993).
Isopropyl alcohol was ingested by groups of eight adult men at
doses of 0, 2.6, or 6.4 mg/kg bw per day for six weeks. No
significant changes were observed in the chemical or cellular
composition of blood or urine, in the ability of the liver to excrete
sulfobromophthalein, in the optical properties of the eye, or in the
general well-being of the volunteers (Wills et al., 1969).
2-Heptanone
Groups of 15 male and 15 female CFE rats were given 2-heptanone
by gavage for 13 weeks at doses of 0, 20, 100, or 500 mg/kg bw per
day. In addition, groups of five male and five female rats received
doses of 0, 100, or 500 mg/kg bw per day for two and six weeks,
respectively. At week 13, the absolute and relative liver weights were
significantly increased in animals of each sex at 500 mg/kg bw per
day, and the absolute and relative kidney weights were significantly
increased in male rats at this dose; however, histological examination
showed no treatment-related damage to the liver or kidney. Significant
increases in the excretion of cells in the urine and enlarged kidneys
were observed in animals at 100 and 500 mg/kg bw per day. As no
abnormal function was detected in concentration tests, and there was
no histopathological damage in the kidneys, the significance of the
excretion of cells in the urine is unclear. The NOEL was 20 mg/kg bw
per day (Gaunt et al., 1972).
In a study of the role of the chain length of aliphatic ketones
in the causation of central and peripheral distal axonopathy, five
Sprague-Dawley rats of unspecified sex were given 0.5% 2-heptanone
(500 mg/kg bw per day) in their drinking-water for 12 weeks. The
animals were observed for hindlimb weakness, and pathological
examinations were conducted on tissues removed from the nervous
system. No giant axonal swelling or secondary myelin changes were
observed in treated animals (Spencer et al., 1978).
3-Heptanone
3-Heptanone, the only ketone in the group that is able to form a
gamma-diketone by (omega-1)-oxidation, was also the only ketone in
this group that caused axonal neuropathy in test animals. In a 14-week
study of the neurotoxic effects of oral exposure to 3-heptanone, 44
male rats were dosed by gavage on five days a week for the duration of
the study with 250, 500, 1000, 2000, or 4000 mg/kg bw per day of
3-heptanone alone; 3-heptanone followed by 1500 mg/kg bw per day
methyl ethyl ketone; 750 mg/kg bw per day methyl ethyl ketone; or 1500
mg/kg bw per day 5-methyl-2-octanone. Animals in many treated groups
developed central nervous system depression or narcosis, and those
given 2000 or 4000 mg/kg bw per day 3-heptanone in combination with
methyl ethyl ketone or 5-methyl-2-octanone died after one or two
doses. Histopathological analysis revealed central-peripheral distal
axonopathy characterized by 'giant' axonal swelling and
neurofilamentous hyperplasia in rats at 2000 mg/kg bw per day
3-heptanone. It was concluded that 3-heptanone has low neurotoxic
potential, as oral doses of < 1000 mg/kg bw per day did not induce
axonal neuropathy or the associated clinical signs. The NOEL was 1000
mg/kg bw per day (O'Donoghue et al., 1984).
4-Heptanone
4-Heptanone was administered to eight male Charles River rats by
gavage for 90 days at a dose of 2000 mg/kg bw per day, determined in a
previous study of acute toxicity to be the maximum tolerated dose
(Krasavage & O'Donoghue, 1979). After one week, however, four of the
rats had died, and the dose was reduced to 1000 mg/kg bw per day. The
mean relative liver weight was statistically significantly greater
than that of controls. Statistically significant increases in the
relative weights of the kidneys, adrenal glands, and testes were also
observed. Histopathological examination revealed hepatocyte
hypertrophy, adrenal gland congestion, and macroscopically enlarged
livers (O'Donoghue & Krasavage, 1980).
2,6-Dimethyl-4-heptanone
2,6-Dimethyl-4-heptanone (purity, 67%) was administered to eight
male Charles River rats by gavage for 90 days at 0 or 2000 mg/kg bw
per day, which was determined in a previous study to be the maximum
tolerated dose (Krasavage & O'Donoghue, 1979). The absolute and
relative liver weights, the relative kidney weights, and the absolute
and relative adrenal gland weights were statistically significantly
greater than those of controls; the absolute (but not the relative)
weights of the brain and heart were significantly depressed.
Histopathological examination revealed compound-related changes in the
stomach, liver, and kidneys. The stomachs of all animals showed
hyperkeratosis with or without pseudoepitheliomatous hyperplasia
associated with irritation due to direct contact with the solvent. In
the liver, minor or moderate hepatocyte hypertrophy was observed. In
the kidney, hyalin droplet formation was present in the proximal
tubular epithelium, with sporadic occurrence of regenerating tubular
epithelium and tubular dilation with casts (O'Donoghue & Krasavage,
1980).
2.3.2.3 Genotoxicity
Seven representative aliphatic acyclic secondary alcohols,
ketones, and related esters in this group have been tested for
genotoxicity. The results are summarized in Table 5 and described
below.
Table 4. Short-term studies of the toxicity of saturated aliphatic acyclic secondary alcohols and ketones
Substance No. Species Sex Test Route Time NOEL Reference
groupsa (mg/kg bw
per day)
Acetone 139 Rat Male/female 5/20 Drinking-water 91 days 1000 Dietz (1991)
Acetone 139 Mouse Male/female 5/20 Drinking-water 91 days 2500 Dietz (1991)
Acetone 139 Rat Male/female 3/60 Gavage 90 days 100 Sonawaneet al. (1986)
Isopropyl alcohol 277 Human Male 2/8 Oral 6 weeks 6.4b Wills et al. (1969)
Isopropyl alcohol 277 Rat Male 4/22 Drinking-water 90 days 870 Pilegaard &
Ladefoged (1993)
2-Heptanone 283 Rat Male/female 3/30 Gavage 91 days 20 Gaunt et al. (1972)
3-Heptanone 285 Rat Male 5/9 Gavage 14 weeks 1000 O'Donoghue et al.
(1984)
4-Heptanone 287 Rat Male 1/8 Gavage 90 days < 1000c O'Donoghue &
Krasavage (1980)
2,6-Dimethyl-4-heptanone 302 Rat Male 1/8 Gavage 90 days < 2000c O'Donohgue &
Krasavage (1980)
a No. of test groups/No. of animals per group
b This study was performed at either a single dose or multiple doses that induced no adverse effects. Therefore, this dose is not a true NOEL
but is the highest dose tested that produced no adverse effects. The actual NOEL would be higher.
c No NOEL established
Table 5. Results of assays for the genotoxicity of saturated aliphatic secondary alcohols, ketones, and related esters
Compound No. End-point Test object Concentration Result Reference
Acetone 139 rec Gene mutation B. subtilis NR Negativea Kawachi et al. (1980)
rec Gene mutation B. subtilis NR Negative Ishizaki et al.
(1979)
Reverse mutation S. typhimurium TA100 0.1-1000 µg/plate Negative Rapson et al.
(1980)
Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutation S. typhimurium TA98, NR Negativea Kawachi et al. (1980)
TA100
Reverse mutationb S. typhimurium TA98, 30 µl/plate Negativec Yamaguchi (1985)
TA100 (24 mg/plate)
Reverse mutation S. typhimurium TA97, < 10 000 µg/plate Negativea McCann et al. (1975)
TA98, TA100, TA1535,
TA1537
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA1535,
TA1537
Reverse mutation S. typhimurium TA100 500 µg/plate Negativea Yamaguchi (1982)
Sister chromatid exchange Human embryo fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Hamster lung fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Chinese hamster ovary < 10 µg/ml Negative Sasaki et al. (1980)
cells
Sister chromatid exchange Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Sister chromatid exchange Diploid human fibroblasts 5 µg/ml Negative Sasaki et al. (1980)
Sister chromatid exchange Human lymphocytes 6.8 mmol/plate Negative Norppa et al. (1983)
(395 µg/ml)
Chromosomal aberration Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Chromosomal aberration Hamster lung fibroblasts NR Positivec Kawachi et al. (1980)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
Isopropyl alcohol 277 Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538, E. coli WP2uvrA
Reverse mutationb S. typhimurium TA97, < 10 mg/plated Negativea Zeiger et al. (1992)
TA98, TA102, TA104,
TA1535, TA100, TA1537
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Chemical Manufacturers'
cells, hprt locus Association (1990)
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Kapp et al. (1993)
cells, hprt locus
2-Butanone 278 Reverse mutation S. typhimurium TA98, 10 000 µg/plate Negativea Douglas et al. (1980)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA102, 1 mg/plate Negative Marnett et al.(1985)
TA104
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA98, 0.05-32 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.04-26 µg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA104,
TA1535, TA1537
Reverse mutation S. typhimurium TA102 5000 µg/plate Negativec Müller et al. (1993)
Reverse mutation S. typhimurium TA98, 4000 µg/plate Negativee Brooks et al. (1988)
TA100, TA1535, TA1537,
TA1538; E. coli WP2uvrA
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.67-12 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.54-10 mg/ml)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
2-Butanone (contd) 278 Unscheduled DNA Human lymphocytes 0.01 mol/L Negativea Perocco et al. (1983)
synthesis (0.72 mg/ml)
Unscheduled DNA Rat hepatocytes 0.1-5 µl/ml Negative O'Donoghue et al. (1988)
synthesis (7.2-360 mg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary cells 1000 µg/ml Negativea Brooks et al. (1988)
4-Methyl-2-pentanone 301 Reverse mutation S. typhimurium TA98, 0.04-4 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.03-3 mg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, TA98, < 6667 µg/plate Negativea Zeiger et al. (1992)
TA100, TA1535
Reverse mutation E. coli WP2uvrA 8000 µg/plate Negativec Brooks et al. (1988)
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.32-4.2 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.26-3.4 mg/ml)
Unscheduled DNA Rat hepatocytes 0.01-1 µl/ml Negative O'Donoghue et al. (1988)
synthesis (8-80 µg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary 1000 µg/ml Negativea Brooks et al. (1988)
cells
2,6-Dimethyl- 302 Reverse mutationb S. typhimurium TA98, 1-333 µg/plate Negativea Mortelmans et al. (1986)
4-heptanone TA100, TA1535, TA1537
Isopropyl acetate 305 Reverse mutationb S. typhimurium TA97, TA98, < 10 mg/plate Negativea Zeiger et al. (1992)
TA100, TA1537, TA1538
Isopropyl myristate 311 Reverse mutation S. typhimurium TA98, TA100, 50 µg/plate Negativea Blevins & Taylor
(spot test) TA1535, TA1537, TA1538 (1982)
a With and without metabolic activation
b Modified (pre-incubation) protocol
c Without metabolic activation
d Maximum non-toxic dose
e With metabolic activation
In vitro
No evidence of mutagenicity was reported for eight aliphatic
acyclic secondary alcohols, ketones, and related esters in multiple
assays in the standard Ames or preincubation protocol with
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA104,
TA1535, TA1537, and TA1538 or Escherichia coli strain WP2 uvrA,
with or without metabolic activation (McCann et al., 1975; Douglas et
al., 1980; Florin et al., 1980; Kawachi et al., 1980; Rapson et al.,
1980; Blevins & Taylor, 1982; Yamaguchi, 1982; Marnett et al., 1985;
Shimizu et al., 1985; Yamaguchi, 1985; Mortelmans et al., 1986; Brooks
et al., 1988; O'Donoghue et al., 1988; Zeiger et al., 1992; Müller et
al., 1993). The concentrations tested ranged from 0.1 to 10 000
µg/plate and often approached cytotoxic levels. The results of other
bacterial assays, such as the rec assay in Bacillus subtilis and
the E. coli reversion assay also indicated that saturated aliphatic
secondary alcohols are not mutagenic (Ishizaki et al., 1979; Kawachi
et al., 1980). In the yeast Saccharomyces cerevisiae, 2-butanone and
methyl isobutylketone did not induce gene conversion at concentrations
up to 5 mg/ml either with or without metabolic activation (Brooks
et al., 1988).
In the L5178Y/ tk+/- mouse lymphoma assay, 2-butanone was not
mutagenic when tested with and without metabolic activation at 11
concentrations ranging from 0.67 µl/ml (non-toxic) to 12 µl/ml (100%
toxic) (O'Donoghue et al., 1988). In the same study,
4-methyl-2-pentanone was not mutagenic at 10 concentrations ranging
from 0.6 µl/ml (non-toxic) to 3.7 µl/ml (100% toxic) with and without
metabolic activation, even though equivocal, non-dose-related
responses were observed at high concentrations without activation.
Isopropyl alcohol was tested at eight concentrations ranging between
0.5 and 5 mg/ml with and without metabolic activation in an assay for
forward mutation at the hypoxanthine-guanine phosphoribosyl
transferase (hprt) locus in Chinese hamster ovary cells (Chemical
Manufacturers' Association, 1990). Statistically significantly
increased mutant frequencies were seen in two of 34 treated cultures,
but these were not dose-related and not reproducible in subsequent
trials (Kapp et al., 1993).
In standard assays for cytogeneticity, acetone did not increase
the frequency of sister chromatid exchange in Chinese hamster ovary
cells (Loveday et al., 1990), hamster lung fibroblasts, or Don-6
cells, (Kawachi et al., 1980; Sasaki et al., 1980) and did not
increase the frequency of sister chromatid exchange in human
lymphocytes (Norppa et al., 1983) with and without metabolic
activation at concentrations up to 5000 µg/ml (Loveday et al., 1990).
Acetone did not induce chromosomal aberrations in Chinese hamster
ovary cells at concentrations up to a maximum of 5000 µg/ml (Loveday
et al., 1990). In a test conducted at an unspecified concentration,
acetone induced chromosomal aberrations in hamster lung fibroblasts
(Kawachi et al., 1980), whereas 2-butanone and methyl isobutylketone
gave negative results in assays in rat hepatocytes and Chinese hamster
ovary cells when tested at concentrations up to 1 mg/ml (Brooks et
al., 1988).
2-Butanone did not induce unscheduled DNA synthesis in human
lymphocytes treated with concentrations up to 0.01 mol/L, with and
without metabolic activation (Perocco et al., 1983). 2-Butanone and
4-methyl-2-pentanone did not induce unscheduled DNA synthesis in rat
hepatocytes when tested at concentrations up to 5 and 1 µl/ml,
respectively (O'Donoghue et al., 1988).
In vivo
Acetone, isopropyl alcohol, 2-butanone, and methyl-2-pentanone
did not induce micronucleus formation at doses of 400-2500 mg/kg bw
(Basler, 1986; O'Donoghue et al., 1988; Kapp et al., 1993), which
approximated the LD20 (O'Donoghue et al., 1988) and the LD50 (Basler,
1986) of the tested compounds.
2.3.2.4 Other relevant studies
Isopropyl alcohol
The effects of long-term consumption of isopropyl alcohol on
gonad function and embryonic and perinatal development were studied
during an investigation to establish the maximum permissible
concentrations of isopropyl alcohol in water bodies. The compound was
administered for 20 days to 869 inbred white rats and for 45 days to
488 rats which had progeny at doses of 1008 and 252 mg/kg bw per day,
respectively; for three months at a dose of 1800 mg/kg bw per day; and
for six months at doses of 18, 1.8, or 0.18 mg/kg bw per day. The
embryos were examined macroanatomically on day 21 of gestation to
determine embryotoxicity, and the offspring of treated male and female
rats mated with treated or untreated controls were observed for
developmental abnormalities.
Dams given 252 or 1008 mg/kg bw per day isopropyl alcohol before
the onset of pregnancy had a significantly reduced number of embryos,
but a similar effect was not seen in those given 1800 mg/kg bw per
day. Total embryonic mortality was significantly increased only in
dams given 1008 mg/kg bw per day; however, no essential differences in
embryonic development were noted. The reproductive function of females
receiving isopropyl alcohol for six months was adversely affected. The
percentage mortality of the progeny of treated male and female
pairings and treated male and control female pairings reached a
significant level only in the groups receiving 18 mg/kg bw per day for
six months. The weights of the progeny of treated males and females
were also significantly reduced. The authors concluded that long-term
intake of isopropyl alcohol can affect generative function, however
only at concentrations that far exceed the current maximum allowable
concentration of isopropyl alcohol (0.25 mg/litre) in water supplies
(Antonova & Salmina, 1978).
Groups of 25 timed-pregnant Sprague-Dawley rats were given 0,
400, 800, or 1200 mg/kg bw per day isopropyl alcohol by gavage on days
6-15 of gestation. Deaths were observed in the groups at 800 mg/kg bw
(4%) and 1200 mg/kg bw (8%) per day. Reduced maternal gestational
weight gain on days 0-20 associated with significantly reduced gravid
uterine weights were noted at 800 and 1200 mg/kg bw per day. There
were no adverse maternal or developmental effects at 400 mg/kg bw per
day and no teratogenic effects at any dose (Tyl et al., 1994).
Groups of 15 artificially inseminated New Zealand white rabbits
were given isopropyl alcohol at 0, 120, 240, or 480 mg/kg bw per day
by gavage on days 6-18 of gestation. Four does at 480 mg/kg bw per day
died, and severe clinical signs of toxicity and statistically
significantly reduced food consumption were observed. No
treatment-related maternal or developmental effects were observed at
120 or 240 mg/kg bw per day, and no teratogenicity was seen at any
dose (Tyl et al., 1994).
Rats were exposed to isopropyl alcohol in the drinking-water for
three generations, with average intakes of 1470, 1380, and 1290 mg/kg
bw per day for the first, second, and third generations, respectively.
The growth of the first generation was retarded initially but had
returned to normal by the 13th week. There were no other effects on
growth and no effects on reproduction (Lehman et al., 1945).
Methyl ethyl ketone
Groups of 10 virgin and 30 plug-positive female Swiss CD-1 mice
were exposed to 0, 400, 1000, or 3000 mg/kg methyl ethyl ketone
vapours for 10 consecutive days. The pregnant mice appeared to be
relatively insensitive, but the offspring of mice at the highest dose
showed significant signs of toxicity. No maternal or developmental
toxicity was observed at doses < 1000 mg/kg vapour (Mast et al.,
1989).
4. REFERENCES
Antonova, V.I. & Salmina, Z.A. (1978) [Maximum permissible
concentration of isopropyl alcohol in water bodies with regard to its
actions on gonads and progeny.] Gig. Sanit., 1, 8-11 (in Russian).
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Intermediary Xenobiotic Metabolism in Animals,
pp. 81-97.
Basler, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat. Res., 174, 11-13.
Blevins, R.D. & Taylor, D.E. (1982) Mutagenicity screening of
twenty-five cosmetic ingredients with the Salmonella/microsome test.
J. Environ. Sci. Health, A17, 217-239.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Brooks T.M., Meyer, A.L. & Hutson, D.H. (1988) The genetic toxicology
of some hydrocarbon and oxygenated solvents. Mutagenesis, 3,
227-232.
Brown, W.D., Setzer, J.V., Dick, R.B., Phipps, F.C. & Lowry, L.K.
(1987) Body burden profiles of single and mixed solvent exposures.
J. Occup. Med. , 29, 877-883.
Chemical Manufacturers' Association (1990) Unpublished submission to
the United States Environmental Protection Agency.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dietz D. (1991) Toxicity studies of acetone in F344/N rats and B6C3F1
mice (drinking water studies), National Toxicology Program (NIH
Publication No. 91-3122), Bethesda, Maryland, United States.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Douglas, G.R., Nestmann, E.R., Betts, J.L., Mueller, J.C., Lee, E.G.-
H., Stich, H.F., San, R.H.C., Brouzes, R.J.P., Chmelauskas, A.L.,
Paavila, H.D. & Walden, C.C. (1980) Mutagenic activity in pulp mill
effluents. In: Jolley, R.L. et al., eds, Water Chlorination:
Environmental Impact and Health Effects, Ann Arbor, Michigan, Ann
Arbor Science Publishers, Vol. 3.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, New York, Academic
Press, Vol. I, pp. 281-293.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18, 219-232.
Gaunt, I.F., Carpanini, F.M.B., Wright, M.G., Grasso, P. & Gangolli,
S.D. (1972) Short-term toxicity of methyl amyl ketone in rats. Food
Cosmetic Toxicol., 10, 625-636.
Haggard, H.W., Miller, D.P. & Greenberg, L.A. (1945) The amyl alcohols
and their ketones: Their metabolic fates and comparative toxicities.
J. Ind. Hyg. Toxicol., 27, 1-14.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, Vol. I, pp. 291-323.
Homan, E.R. & Maronpot, R.R. (1978) Neurotoxic evaluation of some
aliphatic ketones (Abstract). Toxicol. Appl. Pharmacol., 45, 312.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Ishizaki, M., Oyamada, N., Ueno, S., Katsumura, K. & Hosogai, Y.
(1979) Mutagenicity of degradation and reaction products of butyl
hydroxy anisol with sodium nitrite or potassium nitrate by irradiation
of ultra violet ray. J. Food Hyg. Soc. Jpn, 20, 143-146.
Kamil, I.A., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; The glucuronic acid conjugation of acyclic
aliphatic alcohols . J. Biochem., 53, 129-136.
Kapp, R.W., Marino, D.J., Gardiner, T.H., Maston, L.W., McKee, R.H.,
Tyler, T.R., Ivett, J.L. & Young, R.R. (1993) In vitro and in vivo
assays of isopropanol for mutagenicity. Environ. Mol. Mutag., 22,
93-100.
Katz, G.V., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J. (1980)
Comparative neurotoxicity and metabolism of ethyl n-butyl ketone and
methyl n-butyl ketone in rats. Toxicol. Appl. Pharmacol., 52,
153-158.
Kawachi, T., Komatsu, T., Kada, T., Ishidate, M., Masamichi, S.,
Sugiyama, T. & Tazima, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In:
Williams, G.M. et al., eds, The Predictive Value of Short-term
Screening Tests in Carcinogenicity Evaluation, Amsterdam,
Elsevier/North-Holland Biomedical Press.
Krasavage, W.J. & O'Donoghue, J.L. (1979) Repeated oral administration
of five ketones and n-heptane to rats. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Krasavage, W.J., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J.
(1980) The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 53, 433-441.
Krasavage, W.J., O'Donoghue, J.L. & DiVincenzo, G.D. (1982) Ketones.
In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 3rd Ed., New York, John Wiley & Sons, Vol. IIC, pp.
4720-4727.
Lehman, A.J., Schwerma, H. & Rickards, E. (1945) Isopropyl alcohol:
Acquired tolerance in dogs, rate of disappearance from the blood
stream in various species and effects on successive generation of
rats. J. Pharmacol. Exp. Therap., 85, 61-69.
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, pp. 2585-2760.
Loveday, K.S., Anderson, B.E., Resnick, M.A. & Zeiger, E. (1990)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. V: Results with 46 chemicals. Environ.
Mol. Mutag., 16, 272-303.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mast, T.J., Dill, J.A., Evanoff, J.J., Rommereim, R.L., Weigel, R.J. &
Westerberg, R.B. (1989) Inhalation developmental toxicology studies:
Teratology study of methyl ethyl ketone in mice. National Toxicology
Program (No. NIH-Y01-ES-70153), Bethesda, Maryland, United States.
McCann, J., Choi, E., Yamasaki, E. & Ames, B.N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay of 300
chemicals. Proc. Natl Acad. Sci. USA, 72, 5135-5139.
Morgott, D.A. (1993) Acetone. In: Clayton, G.D. & Clayton, F.E., eds,
Patty's Industrial Hygiene and Toxicology, 4th Ed., New York, John
Wiley & Sons, pp. 149-281.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Müller, W., Engelhart, G., Herbold, B., Jäckh, R. & Jung, R. (1993)
Evaluation of mutagenicity testing with Salmonella typhimurium TA102
in three different laboratories. Environ. Health Perspectives,
Suppl. 101, 33-36.
National Academy of Sciences (1989) 1989 Poundage and Technical
Effects. Update of Substances Added to Food, Washington DC.
Neubreuer, O. (1901) Concerning the pairing of glucuronic acid with
materials of the aliphatic series. Arch. Exp. Pathol. Pharmacol.,
46, 133-154.
Norppa, H., Vainio, H. & Sorsa, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res. 43, 3579-3582.
O'Donohogue, J.L. & Krasavage, W.J. (1980) 90-day repeated oral
administration of five ketones and n-heptane to rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
O'Donoghue, J.L., Krasavage, W.J., DiVincenzo, G.D. & Katz, G.V.
(1984) Further studies on ketone neurotoxicity and interactions.
Toxicol. Appl. Pharmacol., 72, 201-209.
O'Donoghue, J.L., Haworth, S.R., Curren, R.D., Kirby, P.E., Lawlor,
T., Moran, E.J., Phillips, R.D., Putnam, D.L., Rogers-Back, A.M.,
Slesinski, R.S. & Thilagar, A. (1988) Mutagenicity studies on ketone
solvents: Methyl ethyl ketone, methyl isobutyl ketone, and isophorone.
Mutat. Res., 206, 149-161.
Perocco, P., Bolognesi, S. & Alberghini, W. (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol. Lett., 16, 69-75.
Pilegaard, K. & Ladefoged, O. (1993) Toxic effects in rats of twelve
weeks' dosing of 2-propanol, and neurotoxicity measured by
densitometric measurements of glial fibrillary acidic protein in the
dorsal hippocampus. In Vivo, 7, 325-330.
Rapson, W.H., Nazar, M.A. & Butzky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ.
Contam. Toxicol., 24, 590-596.
Saito, M. (1975) Studies on the metabolism of lower alcohols
(Abstract). Nichidai Igaku Zasshi, 34, 569-585.
Sasaki, M., Sugimura, K., Yoshida, M.A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster cells.
Kromosomo II, 20, 574-584.
Schwartz, L. (1989) [On the oxidation of acetones and homologous
ketones from fatty acids.] Arch. Exp. Pathol. Pharmacol., 40, 168
(in German).
Scopinaro, D., Ghiani, P. & De Cecco, A. (1947) [Ketolytic fate of
acetone. II. Acetone metabolism in normal subjects.] Policlinico
(Rome) Sez, Med., 54, 70 (in Italian).
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Sonawane, B., de Rosa, C., Rubenstein, R., Mayhew, D., Becker, S.V. &
Dietz, D. (1986) Estimation of reference dose (RfD) for oral exposure
to acetone (Abstract). J. Am. Coll. Toxicol.., 5, 605.
Spencer, P.S., Bischoff, M.C. & Schaumburg, H.H. (1978) On the
specific molecular configuration of neurotoxic aliphatic hexacarbon
compounds causing central-peripheral distal axonopathy. Toxicol.
Appl. Pharmacol., 44, 17-28.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tanii, H., Tsuji, H. & Hashimoto, K. (1986) Structure-toxicity
relationship of monoketones. Toxicol. Lett., 30, 13-17.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Tyl, R.W., Masten, L.W., Marr, M.C., Myers, C.B., Slauter, R.W.,
Gardiner, T.H., Strother, D.E., McKee, R.H. & Tyler, T.R. (1994)
Developmental toxicity evaluation of isopropanol by gavage in rats and
rabbits. Fundam. Appl. Toxicol., 22, 139-151.
Wills, J.H., Jameson, E.M. & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15, 560-565.
Yamaguchi, T. (1982) Mutagenicity of trioses and methyl glyoxal on
Salmonella typhimurium. Agric. Biol. Chem. 46, 849-851.
Yamaguchi, T. (1985) Stimulating effects of organic solvents on the
mutagenicities of sugar-degradation compounds. Agric. Biol. Chem.
49, 3363-3368.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
The thresholds for human intake are 1800 µg/day for Class I and 540 µg/day for Class II.
N/D, No intake data reported
Step A3. The daily per capita intakes of 25 of the 27 class-I
substances at this step in Europe and the United States are
below the human intake threshold for class I compounds (1800
µg/person per day), indicating that they pose no safety
concern when used at current levels of estimated intake as
flavouring agents. The intakes of acetone (mg/person per
day) are 7.1 in Europe and 35 in the United States, and the
intakes of isopropyl alcohol are 99 in Europe and 10 in the
United States; these are greater than the human intake
threshold of 1800 µg/person per day (National Academy of
Sciences, 1989; International Organization of the Flavor
Industry, 1995). The intakes of 10 class II substances at
this step in Europe and in the United States are below the
human intake threshold for that class (540 µg/person per
day), indicating that they pose no safety concern at current
levels of estimated intake as flavouring agents.
The total intake of isopropyl alcohol and its nine
esters from use as flavouring agents is also greater than
the human intake threshold for compounds in class I (1800
µg/person per day).
The intake of three additional esters of isopropyl
alcohol (isopropyl benzoate, isopropyl cinnamate, and
isopropyl phenyl-acetate), which were not evaluated as part
of this group, would add about 15 µg/day in Europe and 5
µg/day in the United States to the total intake of isopropyl
alcohol. This potential additional intake is minor and would
not alter the safety evaluation.
Step A4. This step needs to be considered only for acetone and
isopropyl alcohol, the two flavouring agents in this group
for which estimated intake exceeds the class I threshold of
1800 µg/person per day. They are both endogenous, as
components of fatty acid and carbohydrate metabolism, and
have been detected in the blood.
Step B3. The current per capita intake of 3-heptanone (No. 285),
class II, and 3- heptanol (No. 286), class I, do not exceed
the human intake thresholds, 540 and 1800 µg/day,
respectively. Accordingly the evaluation proceeds to step
B4.
Step B4. On the basis of a limited study of the neurotoxic effects of
orally administered 3-heptanone (No. 285) to male rats, the
NOEL was 1000 mg/kg bw per day (O'Donoghue et al., 1984).
This NOEL provides a large safety margin (> 1 000 000 for
3-heptanone and even higher for 3-heptanol) in relation to
current levels of estimated intake of 3-heptanone and
3-heptanol (No. 286) (which might be metabolized to
3-heptanone).
On the basis of the results of the safety evaluation sequence, 28
of the saturated aliphatic acyclic secondary alcohols and ketones and
11 of the related esters of saturated and unsaturated carboxylic acids
evaluated do not pose a safety concern when used at current levels of
intake as flavouring agents.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 39 saturated aliphatic acyclic
secondary alcohols, ketones, and related esters of saturated and
unsaturated carboxylic acids were consumed concomitantly on a daily
basis, the estimated combined intake would exceed the human thresholds
for classes I and II. All of the 39 substances except two (3-heptanone
and 3-heptanol) are expected to be metabolized via well-known
biochemical pathways to innocuous metabolic and/or endogenous
substances; in the opinion of the Committee, the endogenous levels of
these metabolites would not give rise to perturbations outside the
physiological range. Accordingly, even a combined theoretical intake
would be of no safety concern. The combined intake of the two
substances that could potentially form gamma-diketones would also be
of no safety concern.
1.6 Conclusions
The Committee concluded that the substances in this group would
not present safety concerns at the current levels of intake.
Data on toxicity were required only for 3-heptanone (No. 285) and
3-heptanol (No. 286) when applying the procedure to this group of
flavouring agents. The Committee noted that these data and others are
consistent with the results of the safety evaluation using the
procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Twenty-eight saturated aliphatic acyclic ketones and secondary
alcohols with chain lengths from C3 to C15 (Nos 139 and 277-303) and
11 related saturated and unsaturated esters (Nos 304-313 and 448) are
included in this group of flavouring agents (see Table 1). The group
comprises 16 saturated aliphatic acyclic ketones (10 2-alkanones, four
3-alkanones, and two 4-alkanones), 12 saturated aliphatic acyclic
secondary alcohols (seven 2-alkanols, four 3-alkanols, and one
4-alkanol), nine esters of isopropyl alcohol, and two esters of
3-octanol in which the component carboxylic acids are six saturated
and one unsaturated carboxylic acid (tiglic acid).
The substances in this group are structurally related because
they are saturated, aliphatic acyclic ketones, secondary alcohols, or
esters formed from secondary alcohols and aliphatic, saturated or
unsaturated carboxylic acids.
2.2 Intake
The total annual production and the estimated current per
capita intake in Europe and the United States of the 39 saturated
aliphatic acyclic secondary alcohols, ketones, and related esters are
shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
Generally, saturated aliphatic acyclic secondary alcohols and
ketones are absorbed through the gastrointestinal tract and rapidly
eliminated from the blood. The related esters in this group of
flavouring agents are anticipated to be hydrolysed to their component
secondary alcohols and aliphatic saturated and unsaturated carboxylic
acids, which are also readily absorbed, as considered above in section
1.3 and 'General aspects of metabolism', p. 223.
In addition to oxidation and reduction pathways,
low-molecular-mass ketones (carbon chain length, < C-5) may be
excreted unchanged in expired air (Brown et al., 1987). In mammals,
oral doses of volatile ketones or their corresponding alcohols are
eliminated principally as the ketone in expired air; lesser amounts
are excreted in the urine (Haggard et al., 1945; Scopinaro et al.,
1947; Saito, 1975). In rats, 2-pentanone in expired air was the major
metabolite after administration of 2-penta-nol by intraperitoneal
injection; lesser amounts of 2-pentanol were exhaled, and both
metabolites were detected in urine (Haggard et al., 1945). Some of the
flavouring agents in this group may be oxidized to form neurotoxic
gamma-diketones (see below).
2.3.1.1 Ester hydrolysis
Generally, isopropyl esters are anticipated to be hydrolysed to
isopropyl alcohol and their component saturated or unsaturated
aliphatic carboxylic acids (see section 1.3 and 'General aspects of
metabolism', p. 223). Isopropyl tiglate (No. 312) and the two 3-octyl
esters (Nos 313 and 448) may be hydrolysed at a lower rate in the
gastrointestinal tract, but esters that reach the general circulation
intact will be hydrolysed by tissue esterases to the component
carboxylic acid and alcohol.
In general, the ketones and secondary alcohols in this group of
flavouring agents are metabolized by reversible reduction/oxidation of
the ketone/alcohol group and/or side-chain oxidation and/or
conjugation of the alcohol groups, primarily with glucuronic acid (see
'General aspects of metabolism', p. 222).
Table 2. Most recent annual usage of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters as flavouring substances in Europe and United States
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Acetone (139)
Europe 50 000 7 100 120 NA
United States 190 000 36 000 590 NA
Isopropyl alcohol (277)
Europe 690 000 99 000 1 600 NA
United States 52 000 9 900 170 NA
2-Butanone (278)
Europe 790 110 2 NA
United States 190 36 0.6 NA
2-Pentanone (279)
Europe 1 000 140 2 NA
United States 220 42 0.7 NA
2-Pentanol (280)
Europe 44 6 0.1 NA
United States 0.2 0.04 0.0 NA
3-Hexanone (281)
Europe 3 0.4 0.01 NA
United States 5 1 0.02 NA
3-Hexanol (282)
Europe 94 13 0.22 NA
United States 60 11 0.19 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Heptanone (283)
Europe 790 110 2 NA
United States 250 48 0.11 NA
2-Heptanol (284)
Europe 56 8 0.13 NA
United States 5 1 0.02 NA
3-Heptanone (285)
Europe 27 4 0.06 NA
United States 50 10 0.16 NA
3-Heptanol (286)
Europe 1 0.02 0.0 NA
United States 3 0.6 0.01 NA
4-Heptanone (287)
Europe 16 2 0.04 NA
United States 13 2 0.04 NA
2-Octanone (288)
Europe 760 110 2 NA
United States 350 67 1 NA
2-Octanol (289)
Europe 94 13 0.22 NA
United States 19 4 0.06 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octanone (290)
Europe 23 3 0.05 NA
United States 12 2 0.04 NA
3-Octanol (291)
Europe 39 6 0.09 NA
United States 1 700 320 5 NA
2-Nonanone (292)
Europe 2 600 380 6 NA
United States 140 27 0.44 NA
2-Nonanol (293)
Europe 5 1 0.01 NA
United States 3 1 0.01 NA
3-Nonanone (294)
Europe 1 0.1 0.0 NA
United States 0.5 0.1 0.0 NA
3-Decanol (295)
Europe NR N/D 0 NA
United States 82 16 0.26 NA
2-Undecanone (296)
Europe 2 700 380 6 NA
United States 110 21 0.35 NA
2-Undecanol (297)
Europe 2 0.2 0.0 NA
United States 0.2 0.04 0.0 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Tridecanone (298)
Europe 510 73 1 NA
United States 160 30 0.51 NA
2-Pentadecanone (299)
Europe 150 21 0.3 NA
United States 2 300 430 7 NA
3-Methyl-2-butanol (300)
Europe 4 1 0.01 NA
United States 0.9 0.2 0.0 NA
4-Methyl-2-pentanone (301)
Europe 50 7 0.12 NA
United States 8 2 0.03 NA
2,6-Dimethyl-4-heptanone (302)
Europe 1.5 0.2 0.0 NA
United States 0.3 0.06 0.0 NA
2,6-Dimethyl-4-heptanol (303)
Europe NR N/D 0 NA
United States 25 5 0.08 NA
Isopropyl formate (304)
Europe 3.7 0.5 0.01 0.01
United States 0.1 0.02 0.0 0.00
Isopropyl acetate (305)
Europe 290 41 0.69 0.45
United States 46 9 0.15 0.06
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Isopropyl propionate (306)
Europe 0.1 0.01 0.01 0.00
United States 0.1 0.02 0.0 0.00
Isopropyl butyrate (307)
Europe 49 7 0.12 0.05
United States 0.4 0.08 0.0 0.00
Isopropyl hexanoate (308)
Europe 26 4 0.06 0.03
United States 0.1 0.02 0.0 0.00
Isopropyl isobutyrate (309)
Europe 4 0.6 0.01 0.01
United States 0.3 0.06 0.0 0.00
Isopropyl isovalerate (310)
Europe 2 0.3 0.01 0.00
United States 0.9 0.2 0.0 0.00
Isopropyl myristate (311)
Europe 160 23 0.38 0.10
United States 0.1 0.02 0.0 0.00
Isopropyl tiglate (312)
Europe 0.1 0.01 0.0 0.00
United States 0.5 0.1 0.0 0.00
3-Octyl acetate (313)
Europe 5 0.7 0.01 0.00
United States 160 30 0.51 0.20
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octyl tiglate (448)
Europe 0.1 0.01 0.0 0.00
United States 150 29 0.48 0.30
TOTAL
Europe 750 000 NA NA NA
United States 240 000 NA NA NA
TOTAL Isopropyl alcohol
Europe NA 99 000 1 600
United States NA 10 000 170
NA, not applicable; NR, not reported
a Europe, International Organization of the Flavor Industry (1995); United States,
National Academy of Sciences (1989)
b Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where
population (10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the United
States; 0.6 represents the assumption that only 60% of the flavour volume was
reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
c Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily
per capita intake ester
2.3.1.2 gamma-Diketone formation
Oxidation, primarily (omega-1)-oxidation, of certain aliphatic
ketones may yield gamma-diketones (e.g. 2-hexanone and 3-heptanone may
yield 2,5-hexanedione and 2,5-heptanedione, respectively), which, when
administered at high concentrations to experimental animals, may
result in peripheral neuropathy, commonly recognized as 'giant' axonal
neuropathy (Krasavage et al., 1980; Topping et al., 1994). The
structural features of diketone metabolites that induce peripheral
neuropathy have been shown to include gamma-diketone spacing.
gamma-Diketones with terminal methyl substituents (e.g.
2,5-hexanedione) and/or methyl substituents at the 3 and 4 positions
have the most pronounced effects. The severity of the peripheral
neuropathy depends on the size and position of alkyl substituents on
the gamma-diketone. For example, the strongest neurotoxic effects have
been observed with 3,4-dimethyl-substituted 2,5-hexanedione (not a
flavouring substance). When the chain length is increased (i.e. C-7
and greater), the neurotoxic response is generally significantly
reduced (Topping et al., 1994).
3-Heptanone and 3-heptanol (Nos 285 and 286) are the only
compounds in this group of flavouring substances that may be
metabolized to yield a neurotoxic gamma-diketone (Topping et al.,
1994); however, the longer chain length of 3-heptanone reduces its
neurotoxic effect , which is observed only at high doses.
In a poorly described study of the neurotoxic effects of some
aliphatic ketones, 3-heptanone administered to female Wistar rats in
the drinking-water for 120 days did not produce significant
neurological alterations (Homan & Maronpot, 1978).
Groups of five male Crl rats were exposed to atmospheres
containing 700 mg/kg 3-heptanone for two 20- and two 16-h periods per
week, for a total of 88 exposures over 164 days (approximately
24 weeks). After the fourth, thirtieth, and eighty-fifth exposures,
serum was analysed for 2,5-heptanedione. The maximum mean serum
concentrations reached 10 µg/ml after four exposures but decreased to
6-7 µg/ml after 30 and 85 exposures. No neurotoxicity was observed
(Katz et al., 1980).
Groups of two Crl rats were given 250, 500, 1000, 2000, or
4000 mg/kg bw 3-heptanone by gavage on five days per week for
14 weeks. No peripheral neuropathy was observed at doses
< 1000 mg/kg bw. At 2000 mg/kg bw, approaching the LD50 in rats
(2760 mg/kg bw), 3-heptanone induced peripheral neuropathy (O'Donoghue
et al., 1984).
2.3.1.3 Excretion
In studies limited to the identification of urinary glucuronide
metabolites, a series of aliphatic secondary alcohols was administered
by stomach tube to rabbits, and the urinary excretion of glucuronic
acid conjugates was determined after 24 h. The compounds, doses, and
average urinary output of glucuronide are listed in Table 3. The
results show that secondary alcohols, either administered directly or
formed via ketone reduction as demonstrated in one case with
2-heptanone (41 % urinary glucuronide), are largely excreted as
glucuronic acid conjugates (Kamil et al., 1953).
Table 3. Urinary excretion of glucuronides after
administration of aliphatic secondary alcohols to
rabbits by gavage
Substance No. Dose Urinary
(mg/kg bw)a glucuronide (%)
2-Propanol 277 1002 10.2
2-Butanol - 618 14.4
2-Pentanol 280 735 44.8
2-Heptanol 284 965 54.6
3-Heptanol 286 965 61.9
4-Heptanol - 965 67.4
2-Octanol 289 1081 15.5
From Kamil et al. (1953)
a Calculated from dose of 25 mmol/3-kg rabbit (for
2-propanol, 50 mmol/3 kg)
Generally, secondary alcohols (and ketones, by reduction to
secondary alcohols) are excreted after their conjugation with
glucuronic acid, primarily in the urine, or in the bile, depending on
the relative molecular mass and the animal species (see 'General
aspects of metabolism', p. 222). After hydrolysis of the esters in
this group, see above, the component secondary alcohols are
metabolized and excreted, and the component acids are metabolized in
the fatty acid ß-oxidation and other well-known metabolic pathways.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 25 of the 39 substances
in this group and range from 400 to 9800 mg/kg bw. The vast majority
of the values are > 1000 mg/kg bw, demonstrating that the acute
toxicity of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters given orally is extremely low (see e.g. Tanii et
al., 1986).
2.3.2.2 Short-term studies of toxicity
The results of short-term studies with six saturated aliphatic
acyclic secondary alcohols and ketones are summarized in Table 4 and
described below, together with some further studies. There were no
adequate studies available on the related esters.
Acetone
In a 14-day study, groups of five male and five female Fischer
344/N rats were given drinking-water ad libitum containing acetone
at concentrations of 0, 5000, 10 000, 20 000, 50 000, or 100 000 mg/kg
(equivalent to 0, 500, 1000, 2000, 5000, and 10 000 mg/kg bw per day).
Water consumption was depressed in females at 2000 mg/kg bw per day
and in all animals at 5000 or 10 000 mg/kg bw per day, but this was
not accompanied by signs of dehydration. The body weights of males at
5000 or 10 000 mg/kg bw per day and females at 10 000 mg/kg bw per day
were depressed. Statistically significant increases in relative kidney
and liver weights were observed n both male and female rats at a dose
of 2000 or 5000 mg/kg bw per day. In addition, statistically
significant increases in relative testis weight were observed at 5000
mg/kg bw per day but not at 10 000 mg/kg bw per day. No
histopathological abnormalities were observed in rats at any dose
tested (Dietz, 1991).
Groups of five male and five female B6C3F1 mice were exposed to
acetone in drinking-water ad libitum at concentrations of 0, 5000,
10 000, 20 000, 50 000, or 100 000 mg/kg (equivalent to 0, 1250, 2500,
5000, 12 500, and 25 000 mg/kg bw per day) for 14 days. Water
consumption was depressed at 12 500 or 25 000 mg/kg bw per day as
compared with controls. Growth retardation was seen in males and
females at 25 000 mg/kg bw per day but was more pronounced in males.
Statistically significant increases in relative kidney weights were
seen in both males and females at 12 500 or 25 000 mg/kg bw per day,
and statistically significant changes in relative liver weights were
observed in males at 1250, 2500, 5000, 12 500, or 25 000 mg/kg bw per
day and in females at 5000, 12 500, or 25 000 mg/kg bw per day.
Minimal to mild centrilobular hepatocellular hypertrophy was observed
at doses of 5000, 12 500, and 25 000 mg/kg bw per day in males and 12
500 and 25 000 mg/kg bw per day in females. Moderate centrilobular
hepatocellular hypertrophy was observed in males at 25 000 mg/kg bw
per day (Dietz, 1991).
In a 13-week study, groups of 10 male and 10 female rats were
given drinking-water ad libitum containing acetone at concentrations
of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg (equivalent to 0,
250, 500, 1000, 2000, and 5000 mg/kg bw per day). Water consumption
was depressed in males at 5000 mg/kg bw per day and in females at 2000
or 5000 mg/kg bw per day, but this was not accompanied by signs of
dehydration. Body-weight gain was significantly decreased in males and
females at 5000 mg/kg bw per day. Relative kidney weights were
significantly increased in both male and female rats exposed to
acetone at 5000 mg/kg bw per day and in females at 2000 mg/kg bw per
day. Increased incidences of neuropathy were seen in all males,
including the controls, but at greatest incidence and severity at the
two highest doses. Relative liver weights were significantly increased
in males and females at 2000 or 5000 mg/kg bw per day. No significant
differences in relative organ weight were observed at doses below 2000
mg/kg bw per day. At 5000 mg/kg bw per day, the relative testis
weights were significantly increased. The authors noted that such
organ weight changes are difficult to interpret because of the effect
of high doses of acetone on body weight; they therefore may not be
biologically significant. Furthermore, testicular toxicants typically
decrease testis weights. Decreased sperm motility, caudal weight, and
epididymal weight and an increased incidence of abnormal sperm were
also seen in males at 5000 mg/kg bw per day. The NOEL was 1000 mg/kg
bw per day (Dietz, 1991).
In a 13-week study, groups of 10 female and 10 male B6C3F1 mice
were given drinking-water containing acetone. Females received acetone
at concentrations of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg,
equivalent to 0, 625, 1250, 2500, 5000, or 12 500 mg/kg bw per day.
Males received acetone at a concentration of 0, 1250, 2500, 5000,
10 000, or 20 000 mg/kg, equivalent to 0, 313, 625, 1250, 2500, and
5000 mg/kg bw per day. The fluid intake of all females was depressed
as compared with controls. Absolute and relative liver weights and
absolute and relative spleen weights were significantly increased in
female mice at 12 500 mg/kg bw per day. Centrilobular hepatocellular
hypertrophy of minimal severity was seen in two female mice receiving
12 500 mg/kg bw per day, but no compound-related lesions were found in
male mice. The NOEL was 2500 mg/kg bw per day (Dietz, 1991).
Acetone was administered to groups of 30 male and 30 female
Sprague-Dawley rats by gavage for 90 days at doses of 100, 500, or
2500 mg/kg bw per day. Female rats at the two highest doses had
increased absolute kidney weights, and relative weight increases were
seen in the kidney, liver, and brain of both males and females at the
highest dose. Red blood cell parameters were significantly increased
in males at 45 days and animals of each sex at 90 days, at the highest
dose. Histopathological examination showed a significant increase in
tubular degeneration and hyalin droplet accumulation in males at
500 mg/kg bw per day and in animals of each sex at 2500 mg/kg bw per
day. Histolopathological examination of the liver and kidneys revealed
no treatment-dependent abnormalities. The NOEL was 100 mg/kg bw per
day (Sonawane et al., 1986).
Isopropyl alcohol
The potential toxicity of isopropyl alcohol was investigated in a
12-week study with 110 three-month-old male Wistar rats given 0, 1, 2,
3, or 5% in their drinking-water for the duration of the study. The
highest concentration was lowered to 4% during week 2 because of
unpalatability. The average daily intakes were reported to be 0, 870,
1300, 1700, and 2500 mg/kg bw. Body weights were statistically
significantly decreased in rats at 1700 or 2500 mg/kg bw per day.
Statistically significant, dose-related changes in relative liver
weights were observed at the three highest doses; significant
increases in testis weights were observed at 2500 mg/kg bw per day;
significant increases in relative kidney weights were seen at the
three highest doses; and statistically significant increases in
relative adrenal weights were observed at the two highest doses.
Increased formation of hyaline casts and increased hyaline droplet
content were observed in the proximal tubules of the kidneys in a
dose-dependent fashion. The NOEL was 870 mg/kg bw per day (Pilegaard &
Ladefoged, 1993).
Isopropyl alcohol was ingested by groups of eight adult men at
doses of 0, 2.6, or 6.4 mg/kg bw per day for six weeks. No
significant changes were observed in the chemical or cellular
composition of blood or urine, in the ability of the liver to excrete
sulfobromophthalein, in the optical properties of the eye, or in the
general well-being of the volunteers (Wills et al., 1969).
2-Heptanone
Groups of 15 male and 15 female CFE rats were given 2-heptanone
by gavage for 13 weeks at doses of 0, 20, 100, or 500 mg/kg bw per
day. In addition, groups of five male and five female rats received
doses of 0, 100, or 500 mg/kg bw per day for two and six weeks,
respectively. At week 13, the absolute and relative liver weights were
significantly increased in animals of each sex at 500 mg/kg bw per
day, and the absolute and relative kidney weights were significantly
increased in male rats at this dose; however, histological examination
showed no treatment-related damage to the liver or kidney. Significant
increases in the excretion of cells in the urine and enlarged kidneys
were observed in animals at 100 and 500 mg/kg bw per day. As no
abnormal function was detected in concentration tests, and there was
no histopathological damage in the kidneys, the significance of the
excretion of cells in the urine is unclear. The NOEL was 20 mg/kg bw
per day (Gaunt et al., 1972).
In a study of the role of the chain length of aliphatic ketones
in the causation of central and peripheral distal axonopathy, five
Sprague-Dawley rats of unspecified sex were given 0.5% 2-heptanone
(500 mg/kg bw per day) in their drinking-water for 12 weeks. The
animals were observed for hindlimb weakness, and pathological
examinations were conducted on tissues removed from the nervous
system. No giant axonal swelling or secondary myelin changes were
observed in treated animals (Spencer et al., 1978).
3-Heptanone
3-Heptanone, the only ketone in the group that is able to form a
gamma-diketone by (omega-1)-oxidation, was also the only ketone in
this group that caused axonal neuropathy in test animals. In a 14-week
study of the neurotoxic effects of oral exposure to 3-heptanone, 44
male rats were dosed by gavage on five days a week for the duration of
the study with 250, 500, 1000, 2000, or 4000 mg/kg bw per day of
3-heptanone alone; 3-heptanone followed by 1500 mg/kg bw per day
methyl ethyl ketone; 750 mg/kg bw per day methyl ethyl ketone; or 1500
mg/kg bw per day 5-methyl-2-octanone. Animals in many treated groups
developed central nervous system depression or narcosis, and those
given 2000 or 4000 mg/kg bw per day 3-heptanone in combination with
methyl ethyl ketone or 5-methyl-2-octanone died after one or two
doses. Histopathological analysis revealed central-peripheral distal
axonopathy characterized by 'giant' axonal swelling and
neurofilamentous hyperplasia in rats at 2000 mg/kg bw per day
3-heptanone. It was concluded that 3-heptanone has low neurotoxic
potential, as oral doses of < 1000 mg/kg bw per day did not induce
axonal neuropathy or the associated clinical signs. The NOEL was 1000
mg/kg bw per day (O'Donoghue et al., 1984).
4-Heptanone
4-Heptanone was administered to eight male Charles River rats by
gavage for 90 days at a dose of 2000 mg/kg bw per day, determined in a
previous study of acute toxicity to be the maximum tolerated dose
(Krasavage & O'Donoghue, 1979). After one week, however, four of the
rats had died, and the dose was reduced to 1000 mg/kg bw per day. The
mean relative liver weight was statistically significantly greater
than that of controls. Statistically significant increases in the
relative weights of the kidneys, adrenal glands, and testes were also
observed. Histopathological examination revealed hepatocyte
hypertrophy, adrenal gland congestion, and macroscopically enlarged
livers (O'Donoghue & Krasavage, 1980).
2,6-Dimethyl-4-heptanone
2,6-Dimethyl-4-heptanone (purity, 67%) was administered to eight
male Charles River rats by gavage for 90 days at 0 or 2000 mg/kg bw
per day, which was determined in a previous study to be the maximum
tolerated dose (Krasavage & O'Donoghue, 1979). The absolute and
relative liver weights, the relative kidney weights, and the absolute
and relative adrenal gland weights were statistically significantly
greater than those of controls; the absolute (but not the relative)
weights of the brain and heart were significantly depressed.
Histopathological examination revealed compound-related changes in the
stomach, liver, and kidneys. The stomachs of all animals showed
hyperkeratosis with or without pseudoepitheliomatous hyperplasia
associated with irritation due to direct contact with the solvent. In
the liver, minor or moderate hepatocyte hypertrophy was observed. In
the kidney, hyalin droplet formation was present in the proximal
tubular epithelium, with sporadic occurrence of regenerating tubular
epithelium and tubular dilation with casts (O'Donoghue & Krasavage,
1980).
2.3.2.3 Genotoxicity
Seven representative aliphatic acyclic secondary alcohols,
ketones, and related esters in this group have been tested for
genotoxicity. The results are summarized in Table 5 and described
below.
Table 4. Short-term studies of the toxicity of saturated aliphatic acyclic secondary alcohols and ketones
Substance No. Species Sex Test Route Time NOEL Reference
groupsa (mg/kg bw
per day)
Acetone 139 Rat Male/female 5/20 Drinking-water 91 days 1000 Dietz (1991)
Acetone 139 Mouse Male/female 5/20 Drinking-water 91 days 2500 Dietz (1991)
Acetone 139 Rat Male/female 3/60 Gavage 90 days 100 Sonawaneet al. (1986)
Isopropyl alcohol 277 Human Male 2/8 Oral 6 weeks 6.4b Wills et al. (1969)
Isopropyl alcohol 277 Rat Male 4/22 Drinking-water 90 days 870 Pilegaard &
Ladefoged (1993)
2-Heptanone 283 Rat Male/female 3/30 Gavage 91 days 20 Gaunt et al. (1972)
3-Heptanone 285 Rat Male 5/9 Gavage 14 weeks 1000 O'Donoghue et al.
(1984)
4-Heptanone 287 Rat Male 1/8 Gavage 90 days < 1000c O'Donoghue &
Krasavage (1980)
2,6-Dimethyl-4-heptanone 302 Rat Male 1/8 Gavage 90 days < 2000c O'Donohgue &
Krasavage (1980)
a No. of test groups/No. of animals per group
b This study was performed at either a single dose or multiple doses that induced no adverse effects. Therefore, this dose is not a true NOEL
but is the highest dose tested that produced no adverse effects. The actual NOEL would be higher.
c No NOEL established
Table 5. Results of assays for the genotoxicity of saturated aliphatic secondary alcohols, ketones, and related esters
Compound No. End-point Test object Concentration Result Reference
Acetone 139 rec Gene mutation B. subtilis NR Negativea Kawachi et al. (1980)
rec Gene mutation B. subtilis NR Negative Ishizaki et al.
(1979)
Reverse mutation S. typhimurium TA100 0.1-1000 µg/plate Negative Rapson et al.
(1980)
Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutation S. typhimurium TA98, NR Negativea Kawachi et al. (1980)
TA100
Reverse mutationb S. typhimurium TA98, 30 µl/plate Negativec Yamaguchi (1985)
TA100 (24 mg/plate)
Reverse mutation S. typhimurium TA97, < 10 000 µg/plate Negativea McCann et al. (1975)
TA98, TA100, TA1535,
TA1537
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA1535,
TA1537
Reverse mutation S. typhimurium TA100 500 µg/plate Negativea Yamaguchi (1982)
Sister chromatid exchange Human embryo fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Hamster lung fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Chinese hamster ovary < 10 µg/ml Negative Sasaki et al. (1980)
cells
Sister chromatid exchange Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Sister chromatid exchange Diploid human fibroblasts 5 µg/ml Negative Sasaki et al. (1980)
Sister chromatid exchange Human lymphocytes 6.8 mmol/plate Negative Norppa et al. (1983)
(395 µg/ml)
Chromosomal aberration Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Chromosomal aberration Hamster lung fibroblasts NR Positivec Kawachi et al. (1980)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
Isopropyl alcohol 277 Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538, E. coli WP2uvrA
Reverse mutationb S. typhimurium TA97, < 10 mg/plated Negativea Zeiger et al. (1992)
TA98, TA102, TA104,
TA1535, TA100, TA1537
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Chemical Manufacturers'
cells, hprt locus Association (1990)
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Kapp et al. (1993)
cells, hprt locus
2-Butanone 278 Reverse mutation S. typhimurium TA98, 10 000 µg/plate Negativea Douglas et al. (1980)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA102, 1 mg/plate Negative Marnett et al.(1985)
TA104
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA98, 0.05-32 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.04-26 µg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA104,
TA1535, TA1537
Reverse mutation S. typhimurium TA102 5000 µg/plate Negativec Müller et al. (1993)
Reverse mutation S. typhimurium TA98, 4000 µg/plate Negativee Brooks et al. (1988)
TA100, TA1535, TA1537,
TA1538; E. coli WP2uvrA
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.67-12 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.54-10 mg/ml)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
2-Butanone (contd) 278 Unscheduled DNA Human lymphocytes 0.01 mol/L Negativea Perocco et al. (1983)
synthesis (0.72 mg/ml)
Unscheduled DNA Rat hepatocytes 0.1-5 µl/ml Negative O'Donoghue et al. (1988)
synthesis (7.2-360 mg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary cells 1000 µg/ml Negativea Brooks et al. (1988)
4-Methyl-2-pentanone 301 Reverse mutation S. typhimurium TA98, 0.04-4 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.03-3 mg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, TA98, < 6667 µg/plate Negativea Zeiger et al. (1992)
TA100, TA1535
Reverse mutation E. coli WP2uvrA 8000 µg/plate Negativec Brooks et al. (1988)
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.32-4.2 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.26-3.4 mg/ml)
Unscheduled DNA Rat hepatocytes 0.01-1 µl/ml Negative O'Donoghue et al. (1988)
synthesis (8-80 µg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary 1000 µg/ml Negativea Brooks et al. (1988)
cells
2,6-Dimethyl- 302 Reverse mutationb S. typhimurium TA98, 1-333 µg/plate Negativea Mortelmans et al. (1986)
4-heptanone TA100, TA1535, TA1537
Isopropyl acetate 305 Reverse mutationb S. typhimurium TA97, TA98, < 10 mg/plate Negativea Zeiger et al. (1992)
TA100, TA1537, TA1538
Isopropyl myristate 311 Reverse mutation S. typhimurium TA98, TA100, 50 µg/plate Negativea Blevins & Taylor
(spot test) TA1535, TA1537, TA1538 (1982)
a With and without metabolic activation
b Modified (pre-incubation) protocol
c Without metabolic activation
d Maximum non-toxic dose
e With metabolic activation
In vitro
No evidence of mutagenicity was reported for eight aliphatic
acyclic secondary alcohols, ketones, and related esters in multiple
assays in the standard Ames or preincubation protocol with
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA104,
TA1535, TA1537, and TA1538 or Escherichia coli strain WP2 uvrA,
with or without metabolic activation (McCann et al., 1975; Douglas et
al., 1980; Florin et al., 1980; Kawachi et al., 1980; Rapson et al.,
1980; Blevins & Taylor, 1982; Yamaguchi, 1982; Marnett et al., 1985;
Shimizu et al., 1985; Yamaguchi, 1985; Mortelmans et al., 1986; Brooks
et al., 1988; O'Donoghue et al., 1988; Zeiger et al., 1992; Müller et
al., 1993). The concentrations tested ranged from 0.1 to 10 000
µg/plate and often approached cytotoxic levels. The results of other
bacterial assays, such as the rec assay in Bacillus subtilis and
the E. coli reversion assay also indicated that saturated aliphatic
secondary alcohols are not mutagenic (Ishizaki et al., 1979; Kawachi
et al., 1980). In the yeast Saccharomyces cerevisiae, 2-butanone and
methyl isobutylketone did not induce gene conversion at concentrations
up to 5 mg/ml either with or without metabolic activation (Brooks
et al., 1988).
In the L5178Y/ tk+/- mouse lymphoma assay, 2-butanone was not
mutagenic when tested with and without metabolic activation at 11
concentrations ranging from 0.67 µl/ml (non-toxic) to 12 µl/ml (100%
toxic) (O'Donoghue et al., 1988). In the same study,
4-methyl-2-pentanone was not mutagenic at 10 concentrations ranging
from 0.6 µl/ml (non-toxic) to 3.7 µl/ml (100% toxic) with and without
metabolic activation, even though equivocal, non-dose-related
responses were observed at high concentrations without activation.
Isopropyl alcohol was tested at eight concentrations ranging between
0.5 and 5 mg/ml with and without metabolic activation in an assay for
forward mutation at the hypoxanthine-guanine phosphoribosyl
transferase (hprt) locus in Chinese hamster ovary cells (Chemical
Manufacturers' Association, 1990). Statistically significantly
increased mutant frequencies were seen in two of 34 treated cultures,
but these were not dose-related and not reproducible in subsequent
trials (Kapp et al., 1993).
In standard assays for cytogeneticity, acetone did not increase
the frequency of sister chromatid exchange in Chinese hamster ovary
cells (Loveday et al., 1990), hamster lung fibroblasts, or Don-6
cells, (Kawachi et al., 1980; Sasaki et al., 1980) and did not
increase the frequency of sister chromatid exchange in human
lymphocytes (Norppa et al., 1983) with and without metabolic
activation at concentrations up to 5000 µg/ml (Loveday et al., 1990).
Acetone did not induce chromosomal aberrations in Chinese hamster
ovary cells at concentrations up to a maximum of 5000 µg/ml (Loveday
et al., 1990). In a test conducted at an unspecified concentration,
acetone induced chromosomal aberrations in hamster lung fibroblasts
(Kawachi et al., 1980), whereas 2-butanone and methyl isobutylketone
gave negative results in assays in rat hepatocytes and Chinese hamster
ovary cells when tested at concentrations up to 1 mg/ml (Brooks et
al., 1988).
2-Butanone did not induce unscheduled DNA synthesis in human
lymphocytes treated with concentrations up to 0.01 mol/L, with and
without metabolic activation (Perocco et al., 1983). 2-Butanone and
4-methyl-2-pentanone did not induce unscheduled DNA synthesis in rat
hepatocytes when tested at concentrations up to 5 and 1 µl/ml,
respectively (O'Donoghue et al., 1988).
In vivo
Acetone, isopropyl alcohol, 2-butanone, and methyl-2-pentanone
did not induce micronucleus formation at doses of 400-2500 mg/kg bw
(Basler, 1986; O'Donoghue et al., 1988; Kapp et al., 1993), which
approximated the LD20 (O'Donoghue et al., 1988) and the LD50 (Basler,
1986) of the tested compounds.
2.3.2.4 Other relevant studies
Isopropyl alcohol
The effects of long-term consumption of isopropyl alcohol on
gonad function and embryonic and perinatal development were studied
during an investigation to establish the maximum permissible
concentrations of isopropyl alcohol in water bodies. The compound was
administered for 20 days to 869 inbred white rats and for 45 days to
488 rats which had progeny at doses of 1008 and 252 mg/kg bw per day,
respectively; for three months at a dose of 1800 mg/kg bw per day; and
for six months at doses of 18, 1.8, or 0.18 mg/kg bw per day. The
embryos were examined macroanatomically on day 21 of gestation to
determine embryotoxicity, and the offspring of treated male and female
rats mated with treated or untreated controls were observed for
developmental abnormalities.
Dams given 252 or 1008 mg/kg bw per day isopropyl alcohol before
the onset of pregnancy had a significantly reduced number of embryos,
but a similar effect was not seen in those given 1800 mg/kg bw per
day. Total embryonic mortality was significantly increased only in
dams given 1008 mg/kg bw per day; however, no essential differences in
embryonic development were noted. The reproductive function of females
receiving isopropyl alcohol for six months was adversely affected. The
percentage mortality of the progeny of treated male and female
pairings and treated male and control female pairings reached a
significant level only in the groups receiving 18 mg/kg bw per day for
six months. The weights of the progeny of treated males and females
were also significantly reduced. The authors concluded that long-term
intake of isopropyl alcohol can affect generative function, however
only at concentrations that far exceed the current maximum allowable
concentration of isopropyl alcohol (0.25 mg/litre) in water supplies
(Antonova & Salmina, 1978).
Groups of 25 timed-pregnant Sprague-Dawley rats were given 0,
400, 800, or 1200 mg/kg bw per day isopropyl alcohol by gavage on days
6-15 of gestation. Deaths were observed in the groups at 800 mg/kg bw
(4%) and 1200 mg/kg bw (8%) per day. Reduced maternal gestational
weight gain on days 0-20 associated with significantly reduced gravid
uterine weights were noted at 800 and 1200 mg/kg bw per day. There
were no adverse maternal or developmental effects at 400 mg/kg bw per
day and no teratogenic effects at any dose (Tyl et al., 1994).
Groups of 15 artificially inseminated New Zealand white rabbits
were given isopropyl alcohol at 0, 120, 240, or 480 mg/kg bw per day
by gavage on days 6-18 of gestation. Four does at 480 mg/kg bw per day
died, and severe clinical signs of toxicity and statistically
significantly reduced food consumption were observed. No
treatment-related maternal or developmental effects were observed at
120 or 240 mg/kg bw per day, and no teratogenicity was seen at any
dose (Tyl et al., 1994).
Rats were exposed to isopropyl alcohol in the drinking-water for
three generations, with average intakes of 1470, 1380, and 1290 mg/kg
bw per day for the first, second, and third generations, respectively.
The growth of the first generation was retarded initially but had
returned to normal by the 13th week. There were no other effects on
growth and no effects on reproduction (Lehman et al., 1945).
Methyl ethyl ketone
Groups of 10 virgin and 30 plug-positive female Swiss CD-1 mice
were exposed to 0, 400, 1000, or 3000 mg/kg methyl ethyl ketone
vapours for 10 consecutive days. The pregnant mice appeared to be
relatively insensitive, but the offspring of mice at the highest dose
showed significant signs of toxicity. No maternal or developmental
toxicity was observed at doses < 1000 mg/kg vapour (Mast et al.,
1989).
4. REFERENCES
Antonova, V.I. & Salmina, Z.A. (1978) [Maximum permissible
concentration of isopropyl alcohol in water bodies with regard to its
actions on gonads and progeny.] Gig. Sanit., 1, 8-11 (in Russian).
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Intermediary Xenobiotic Metabolism in Animals,
pp. 81-97.
Basler, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat. Res., 174, 11-13.
Blevins, R.D. & Taylor, D.E. (1982) Mutagenicity screening of
twenty-five cosmetic ingredients with the Salmonella/microsome test.
J. Environ. Sci. Health, A17, 217-239.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Brooks T.M., Meyer, A.L. & Hutson, D.H. (1988) The genetic toxicology
of some hydrocarbon and oxygenated solvents. Mutagenesis, 3,
227-232.
Brown, W.D., Setzer, J.V., Dick, R.B., Phipps, F.C. & Lowry, L.K.
(1987) Body burden profiles of single and mixed solvent exposures.
J. Occup. Med. , 29, 877-883.
Chemical Manufacturers' Association (1990) Unpublished submission to
the United States Environmental Protection Agency.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dietz D. (1991) Toxicity studies of acetone in F344/N rats and B6C3F1
mice (drinking water studies), National Toxicology Program (NIH
Publication No. 91-3122), Bethesda, Maryland, United States.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Douglas, G.R., Nestmann, E.R., Betts, J.L., Mueller, J.C., Lee, E.G.-
H., Stich, H.F., San, R.H.C., Brouzes, R.J.P., Chmelauskas, A.L.,
Paavila, H.D. & Walden, C.C. (1980) Mutagenic activity in pulp mill
effluents. In: Jolley, R.L. et al., eds, Water Chlorination:
Environmental Impact and Health Effects, Ann Arbor, Michigan, Ann
Arbor Science Publishers, Vol. 3.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, New York, Academic
Press, Vol. I, pp. 281-293.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18, 219-232.
Gaunt, I.F., Carpanini, F.M.B., Wright, M.G., Grasso, P. & Gangolli,
S.D. (1972) Short-term toxicity of methyl amyl ketone in rats. Food
Cosmetic Toxicol., 10, 625-636.
Haggard, H.W., Miller, D.P. & Greenberg, L.A. (1945) The amyl alcohols
and their ketones: Their metabolic fates and comparative toxicities.
J. Ind. Hyg. Toxicol., 27, 1-14.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, Vol. I, pp. 291-323.
Homan, E.R. & Maronpot, R.R. (1978) Neurotoxic evaluation of some
aliphatic ketones (Abstract). Toxicol. Appl. Pharmacol., 45, 312.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Ishizaki, M., Oyamada, N., Ueno, S., Katsumura, K. & Hosogai, Y.
(1979) Mutagenicity of degradation and reaction products of butyl
hydroxy anisol with sodium nitrite or potassium nitrate by irradiation
of ultra violet ray. J. Food Hyg. Soc. Jpn, 20, 143-146.
Kamil, I.A., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; The glucuronic acid conjugation of acyclic
aliphatic alcohols . J. Biochem., 53, 129-136.
Kapp, R.W., Marino, D.J., Gardiner, T.H., Maston, L.W., McKee, R.H.,
Tyler, T.R., Ivett, J.L. & Young, R.R. (1993) In vitro and in vivo
assays of isopropanol for mutagenicity. Environ. Mol. Mutag., 22,
93-100.
Katz, G.V., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J. (1980)
Comparative neurotoxicity and metabolism of ethyl n-butyl ketone and
methyl n-butyl ketone in rats. Toxicol. Appl. Pharmacol., 52,
153-158.
Kawachi, T., Komatsu, T., Kada, T., Ishidate, M., Masamichi, S.,
Sugiyama, T. & Tazima, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In:
Williams, G.M. et al., eds, The Predictive Value of Short-term
Screening Tests in Carcinogenicity Evaluation, Amsterdam,
Elsevier/North-Holland Biomedical Press.
Krasavage, W.J. & O'Donoghue, J.L. (1979) Repeated oral administration
of five ketones and n-heptane to rats. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Krasavage, W.J., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J.
(1980) The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 53, 433-441.
Krasavage, W.J., O'Donoghue, J.L. & DiVincenzo, G.D. (1982) Ketones.
In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 3rd Ed., New York, John Wiley & Sons, Vol. IIC, pp.
4720-4727.
Lehman, A.J., Schwerma, H. & Rickards, E. (1945) Isopropyl alcohol:
Acquired tolerance in dogs, rate of disappearance from the blood
stream in various species and effects on successive generation of
rats. J. Pharmacol. Exp. Therap., 85, 61-69.
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, pp. 2585-2760.
Loveday, K.S., Anderson, B.E., Resnick, M.A. & Zeiger, E. (1990)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. V: Results with 46 chemicals. Environ.
Mol. Mutag., 16, 272-303.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mast, T.J., Dill, J.A., Evanoff, J.J., Rommereim, R.L., Weigel, R.J. &
Westerberg, R.B. (1989) Inhalation developmental toxicology studies:
Teratology study of methyl ethyl ketone in mice. National Toxicology
Program (No. NIH-Y01-ES-70153), Bethesda, Maryland, United States.
McCann, J., Choi, E., Yamasaki, E. & Ames, B.N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay of 300
chemicals. Proc. Natl Acad. Sci. USA, 72, 5135-5139.
Morgott, D.A. (1993) Acetone. In: Clayton, G.D. & Clayton, F.E., eds,
Patty's Industrial Hygiene and Toxicology, 4th Ed., New York, John
Wiley & Sons, pp. 149-281.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Müller, W., Engelhart, G., Herbold, B., Jäckh, R. & Jung, R. (1993)
Evaluation of mutagenicity testing with Salmonella typhimurium TA102
in three different laboratories. Environ. Health Perspectives,
Suppl. 101, 33-36.
National Academy of Sciences (1989) 1989 Poundage and Technical
Effects. Update of Substances Added to Food, Washington DC.
Neubreuer, O. (1901) Concerning the pairing of glucuronic acid with
materials of the aliphatic series. Arch. Exp. Pathol. Pharmacol.,
46, 133-154.
Norppa, H., Vainio, H. & Sorsa, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res. 43, 3579-3582.
O'Donohogue, J.L. & Krasavage, W.J. (1980) 90-day repeated oral
administration of five ketones and n-heptane to rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
O'Donoghue, J.L., Krasavage, W.J., DiVincenzo, G.D. & Katz, G.V.
(1984) Further studies on ketone neurotoxicity and interactions.
Toxicol. Appl. Pharmacol., 72, 201-209.
O'Donoghue, J.L., Haworth, S.R., Curren, R.D., Kirby, P.E., Lawlor,
T., Moran, E.J., Phillips, R.D., Putnam, D.L., Rogers-Back, A.M.,
Slesinski, R.S. & Thilagar, A. (1988) Mutagenicity studies on ketone
solvents: Methyl ethyl ketone, methyl isobutyl ketone, and isophorone.
Mutat. Res., 206, 149-161.
Perocco, P., Bolognesi, S. & Alberghini, W. (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol. Lett., 16, 69-75.
Pilegaard, K. & Ladefoged, O. (1993) Toxic effects in rats of twelve
weeks' dosing of 2-propanol, and neurotoxicity measured by
densitometric measurements of glial fibrillary acidic protein in the
dorsal hippocampus. In Vivo, 7, 325-330.
Rapson, W.H., Nazar, M.A. & Butzky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ.
Contam. Toxicol., 24, 590-596.
Saito, M. (1975) Studies on the metabolism of lower alcohols
(Abstract). Nichidai Igaku Zasshi, 34, 569-585.
Sasaki, M., Sugimura, K., Yoshida, M.A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster cells.
Kromosomo II, 20, 574-584.
Schwartz, L. (1989) [On the oxidation of acetones and homologous
ketones from fatty acids.] Arch. Exp. Pathol. Pharmacol., 40, 168
(in German).
Scopinaro, D., Ghiani, P. & De Cecco, A. (1947) [Ketolytic fate of
acetone. II. Acetone metabolism in normal subjects.] Policlinico
(Rome) Sez, Med., 54, 70 (in Italian).
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Sonawane, B., de Rosa, C., Rubenstein, R., Mayhew, D., Becker, S.V. &
Dietz, D. (1986) Estimation of reference dose (RfD) for oral exposure
to acetone (Abstract). J. Am. Coll. Toxicol.., 5, 605.
Spencer, P.S., Bischoff, M.C. & Schaumburg, H.H. (1978) On the
specific molecular configuration of neurotoxic aliphatic hexacarbon
compounds causing central-peripheral distal axonopathy. Toxicol.
Appl. Pharmacol., 44, 17-28.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tanii, H., Tsuji, H. & Hashimoto, K. (1986) Structure-toxicity
relationship of monoketones. Toxicol. Lett., 30, 13-17.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Tyl, R.W., Masten, L.W., Marr, M.C., Myers, C.B., Slauter, R.W.,
Gardiner, T.H., Strother, D.E., McKee, R.H. & Tyler, T.R. (1994)
Developmental toxicity evaluation of isopropanol by gavage in rats and
rabbits. Fundam. Appl. Toxicol., 22, 139-151.
Wills, J.H., Jameson, E.M. & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15, 560-565.
Yamaguchi, T. (1982) Mutagenicity of trioses and methyl glyoxal on
Salmonella typhimurium. Agric. Biol. Chem. 46, 849-851.
Yamaguchi, T. (1985) Stimulating effects of organic solvents on the
mutagenicities of sugar-degradation compounds. Agric. Biol. Chem.
49, 3363-3368.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
 Isopropyl 277 99 000/9 900 I Yes Yes Yes No safety
alcohol concern
CAS No. 67-63-0
Isopropyl 277 99 000/9 900 I Yes Yes Yes No safety
alcohol concern
CAS No. 67-63-0
 2-Butanone 278 110/36 I Yes No No safety
concern
CAS No. 78-93-3
2-Butanone 278 110/36 I Yes No No safety
concern
CAS No. 78-93-3
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Pentanone 279 140/42 I Yes No No safety
concern
CAS No. 107-87-9
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Pentanone 279 140/42 I Yes No No safety
concern
CAS No. 107-87-9
 2-Pentanol 280 6/0.04 I Yes No No safety
concern
CAS No. 6032-29-7
2-Pentanol 280 6/0.04 I Yes No No safety
concern
CAS No. 6032-29-7
 3-Hexanone 281 0.4/1 I Yes No No safety
concern
CAS No. 589-38-8
3-Hexanone 281 0.4/1 I Yes No No safety
concern
CAS No. 589-38-8
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Hexanol 282 13/11 I Yes No No safety
concern
CAS No. 623-37-0
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Hexanol 282 13/11 I Yes No No safety
concern
CAS No. 623-37-0
 2-Heptanone 283 110/48 II Yes No No safety
concern
CAS No. 110-43-0
2-Heptanone 283 110/48 II Yes No No safety
concern
CAS No. 110-43-0
 2-Heptanol 284 8/1 I Yes No No safety
concern
CAS No. 543-49-7
2-Heptanol 284 8/1 I Yes No No safety
concern
CAS No. 543-49-7
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Heptanone 285 4/10 II No No Yes No safety
concern
CAS No. 106-35-4
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Heptanone 285 4/10 II No No Yes No safety
concern
CAS No. 106-35-4
 3-Heptanol 286 0.2/0.6 I No No Yes No safety
concern
CAS No. 589-82-2
3-Heptanol 286 0.2/0.6 I No No Yes No safety
concern
CAS No. 589-82-2
 4-Heptanone 287 2/2 I Yes No No safety
concern
CAS No. 123-19-3
4-Heptanone 287 2/2 I Yes No No safety
concern
CAS No. 123-19-3
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Octanone 288 110/67 II Yes No No safety
concern
CAS No. 111-13-7
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Octanone 288 110/67 II Yes No No safety
concern
CAS No. 111-13-7
 2-Octanol 289 13/4 I Yes No No safety
concern
CAS No. 123-96-6
2-Octanol 289 13/4 I Yes No No safety
concern
CAS No. 123-96-6
 3-Octanone 290 3/2 II Yes No No safety
concern
CAS No. 106-68-3
3-Octanone 290 3/2 II Yes No No safety
concern
CAS No. 106-68-3
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Octanol 291 6/320 I Yes No No safety
concern
CAS No. 589-98-0
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Octanol 291 6/320 I Yes No No safety
concern
CAS No. 589-98-0
 2-Nonanone 292 380/27 II Yes No No safety
concern
CAS No. 821-55-6
2-Nonanone 292 380/27 II Yes No No safety
concern
CAS No. 821-55-6
 2-Nonanol 293 1/1 I Yes No No safety
concern
CAS No. 628-99-9
2-Nonanol 293 1/1 I Yes No No safety
concern
CAS No. 628-99-9
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Nonanone 294 0.1/0.1 II Yes No No safety
concern
CAS No. 925-78-0
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Nonanone 294 0.1/0.1 II Yes No No safety
concern
CAS No. 925-78-0
 3-Decanol 295 N/D/16 I Yes No No safety
concern
CAS No. 1565-81-7
3-Decanol 295 N/D/16 I Yes No No safety
concern
CAS No. 1565-81-7
 2-Undecanone 296 380/21 II Yes No No safety
concern
CAS No. 112-12-9
2-Undecanone 296 380/21 II Yes No No safety
concern
CAS No. 112-12-9
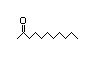 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Undecanol 297 0.2/0.04 I Yes No No safety
concern
CAS No. 1653-30-1
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2-Undecanol 297 0.2/0.04 I Yes No No safety
concern
CAS No. 1653-30-1
 2-Tridecanone 298 73/30 II Yes No No safety
concern
CAS No. 593-08-8
2-Tridecanone 298 73/30 II Yes No No safety
concern
CAS No. 593-08-8
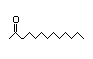 2-Pentadecanone 299 21/430 II Yes No No safety
concern
CAS No. 2345-28-0
2-Pentadecanone 299 21/430 II Yes No No safety
concern
CAS No. 2345-28-0
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Methyl-2- 300 1/0.2 I Yes No No safety
butanol concern
CAS No. 589-75-4
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
3-Methyl-2- 300 1/0.2 I Yes No No safety
butanol concern
CAS No. 589-75-4
 4-Methyl-2- 301 7/2 II Yes No No safety
concern
CAS No. 108-10-1
4-Methyl-2- 301 7/2 II Yes No No safety
concern
CAS No. 108-10-1
 2,6-Dimethyl-4- 302 0.2/0.06 II Yes No No safety
heptanone concern
CAS No. 108-83-8
2,6-Dimethyl-4- 302 0.2/0.06 II Yes No No safety
heptanone concern
CAS No. 108-83-8
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2,6-Dimethyl-4- 303 N/D/5 I Yes No No safety
heptanol concern
CAS No. 108-82-7
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
2,6-Dimethyl-4- 303 N/D/5 I Yes No No safety
heptanol concern
CAS No. 108-82-7
 Isopropyl 304 0.5/0.02 I Yes No No safety
formate concern
CAS No. 625-55-8
Isopropyl 304 0.5/0.02 I Yes No No safety
formate concern
CAS No. 625-55-8
 Isopropyl 305 41/9 I Yes No No safety
acetate concern
CAS No. 108-21-4
Isopropyl 305 41/9 I Yes No No safety
acetate concern
CAS No. 108-21-4
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isoproyl 306 0.01/0.02 I Yes No No safety
propionate concern
CAS No. 637-78-5
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isoproyl 306 0.01/0.02 I Yes No No safety
propionate concern
CAS No. 637-78-5
 Isopropyl 307 7/0.08 I Yes No No safety
butyrate concern
CAS No. 638-11-9
Isopropyl 307 7/0.08 I Yes No No safety
butyrate concern
CAS No. 638-11-9
 Isopropyl 308 4/0.02 I Yes No No safety
hexanoate concern
CAS No. 2311-46-8
Isopropyl 308 4/0.02 I Yes No No safety
hexanoate concern
CAS No. 2311-46-8
 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 309 0.6/0.06 I Yes No No safety
isobutyrate concern
CAS No. 617-50-5
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 309 0.6/0.06 I Yes No No safety
isobutyrate concern
CAS No. 617-50-5
 IIsopropyl 310 0.3/0.2 I Yes No No safety
isovalerate concern
CAS No. 32665-23-9
IIsopropyl 310 0.3/0.2 I Yes No No safety
isovalerate concern
CAS No. 32665-23-9
 sopropyl 311 23/0.02 I Yes No No safety
myristate concern
CAS No. 110-27-0
sopropyl 311 23/0.02 I Yes No No safety
myristate concern
CAS No. 110-27-0
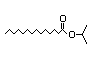 Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 312 0.01/0.1 I Yes No No safety
tiglate concern
CAS No. 6284-46-4
Table 1. (continued)
Substance No. Estimated Step 1 Step 2 Step A3/B3 Step A4 Step B4 Conclusion
per capita Structural Is the Does intake Is the substance Adequate NOEL based on
intake, class substance exceed human or its for the current levels
Europe/USA metabolized intake metabolites substance of intake
(µg/day) to inoccuous threshold? endogenous? or structurally
products? related
substances?
Isopropyl 312 0.01/0.1 I Yes No No safety
tiglate concern
CAS No. 6284-46-4
 3-Octyl acetate 313 0.7/30 I Yes No No safety
concern
CAS No. 4864-61-3
3-Octyl acetate 313 0.7/30 I Yes No No safety
concern
CAS No. 4864-61-3
 1-Ethylhexyl 448 0.01/29 I Yes No No safety
tiglate concern
(3-Octyl tiglate)
CAS No. 94133-92-3
1-Ethylhexyl 448 0.01/29 I Yes No No safety
tiglate concern
(3-Octyl tiglate)
CAS No. 94133-92-3
 The thresholds for human intake are 1800 µg/day for Class I and 540 µg/day for Class II.
N/D, No intake data reported
Step A3. The daily per capita intakes of 25 of the 27 class-I
substances at this step in Europe and the United States are
below the human intake threshold for class I compounds (1800
µg/person per day), indicating that they pose no safety
concern when used at current levels of estimated intake as
flavouring agents. The intakes of acetone (mg/person per
day) are 7.1 in Europe and 35 in the United States, and the
intakes of isopropyl alcohol are 99 in Europe and 10 in the
United States; these are greater than the human intake
threshold of 1800 µg/person per day (National Academy of
Sciences, 1989; International Organization of the Flavor
Industry, 1995). The intakes of 10 class II substances at
this step in Europe and in the United States are below the
human intake threshold for that class (540 µg/person per
day), indicating that they pose no safety concern at current
levels of estimated intake as flavouring agents.
The total intake of isopropyl alcohol and its nine
esters from use as flavouring agents is also greater than
the human intake threshold for compounds in class I (1800
µg/person per day).
The intake of three additional esters of isopropyl
alcohol (isopropyl benzoate, isopropyl cinnamate, and
isopropyl phenyl-acetate), which were not evaluated as part
of this group, would add about 15 µg/day in Europe and 5
µg/day in the United States to the total intake of isopropyl
alcohol. This potential additional intake is minor and would
not alter the safety evaluation.
Step A4. This step needs to be considered only for acetone and
isopropyl alcohol, the two flavouring agents in this group
for which estimated intake exceeds the class I threshold of
1800 µg/person per day. They are both endogenous, as
components of fatty acid and carbohydrate metabolism, and
have been detected in the blood.
Step B3. The current per capita intake of 3-heptanone (No. 285),
class II, and 3- heptanol (No. 286), class I, do not exceed
the human intake thresholds, 540 and 1800 µg/day,
respectively. Accordingly the evaluation proceeds to step
B4.
Step B4. On the basis of a limited study of the neurotoxic effects of
orally administered 3-heptanone (No. 285) to male rats, the
NOEL was 1000 mg/kg bw per day (O'Donoghue et al., 1984).
This NOEL provides a large safety margin (> 1 000 000 for
3-heptanone and even higher for 3-heptanol) in relation to
current levels of estimated intake of 3-heptanone and
3-heptanol (No. 286) (which might be metabolized to
3-heptanone).
On the basis of the results of the safety evaluation sequence, 28
of the saturated aliphatic acyclic secondary alcohols and ketones and
11 of the related esters of saturated and unsaturated carboxylic acids
evaluated do not pose a safety concern when used at current levels of
intake as flavouring agents.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 39 saturated aliphatic acyclic
secondary alcohols, ketones, and related esters of saturated and
unsaturated carboxylic acids were consumed concomitantly on a daily
basis, the estimated combined intake would exceed the human thresholds
for classes I and II. All of the 39 substances except two (3-heptanone
and 3-heptanol) are expected to be metabolized via well-known
biochemical pathways to innocuous metabolic and/or endogenous
substances; in the opinion of the Committee, the endogenous levels of
these metabolites would not give rise to perturbations outside the
physiological range. Accordingly, even a combined theoretical intake
would be of no safety concern. The combined intake of the two
substances that could potentially form gamma-diketones would also be
of no safety concern.
1.6 Conclusions
The Committee concluded that the substances in this group would
not present safety concerns at the current levels of intake.
Data on toxicity were required only for 3-heptanone (No. 285) and
3-heptanol (No. 286) when applying the procedure to this group of
flavouring agents. The Committee noted that these data and others are
consistent with the results of the safety evaluation using the
procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Twenty-eight saturated aliphatic acyclic ketones and secondary
alcohols with chain lengths from C3 to C15 (Nos 139 and 277-303) and
11 related saturated and unsaturated esters (Nos 304-313 and 448) are
included in this group of flavouring agents (see Table 1). The group
comprises 16 saturated aliphatic acyclic ketones (10 2-alkanones, four
3-alkanones, and two 4-alkanones), 12 saturated aliphatic acyclic
secondary alcohols (seven 2-alkanols, four 3-alkanols, and one
4-alkanol), nine esters of isopropyl alcohol, and two esters of
3-octanol in which the component carboxylic acids are six saturated
and one unsaturated carboxylic acid (tiglic acid).
The substances in this group are structurally related because
they are saturated, aliphatic acyclic ketones, secondary alcohols, or
esters formed from secondary alcohols and aliphatic, saturated or
unsaturated carboxylic acids.
2.2 Intake
The total annual production and the estimated current per
capita intake in Europe and the United States of the 39 saturated
aliphatic acyclic secondary alcohols, ketones, and related esters are
shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
Generally, saturated aliphatic acyclic secondary alcohols and
ketones are absorbed through the gastrointestinal tract and rapidly
eliminated from the blood. The related esters in this group of
flavouring agents are anticipated to be hydrolysed to their component
secondary alcohols and aliphatic saturated and unsaturated carboxylic
acids, which are also readily absorbed, as considered above in section
1.3 and 'General aspects of metabolism', p. 223.
In addition to oxidation and reduction pathways,
low-molecular-mass ketones (carbon chain length, < C-5) may be
excreted unchanged in expired air (Brown et al., 1987). In mammals,
oral doses of volatile ketones or their corresponding alcohols are
eliminated principally as the ketone in expired air; lesser amounts
are excreted in the urine (Haggard et al., 1945; Scopinaro et al.,
1947; Saito, 1975). In rats, 2-pentanone in expired air was the major
metabolite after administration of 2-penta-nol by intraperitoneal
injection; lesser amounts of 2-pentanol were exhaled, and both
metabolites were detected in urine (Haggard et al., 1945). Some of the
flavouring agents in this group may be oxidized to form neurotoxic
gamma-diketones (see below).
2.3.1.1 Ester hydrolysis
Generally, isopropyl esters are anticipated to be hydrolysed to
isopropyl alcohol and their component saturated or unsaturated
aliphatic carboxylic acids (see section 1.3 and 'General aspects of
metabolism', p. 223). Isopropyl tiglate (No. 312) and the two 3-octyl
esters (Nos 313 and 448) may be hydrolysed at a lower rate in the
gastrointestinal tract, but esters that reach the general circulation
intact will be hydrolysed by tissue esterases to the component
carboxylic acid and alcohol.
In general, the ketones and secondary alcohols in this group of
flavouring agents are metabolized by reversible reduction/oxidation of
the ketone/alcohol group and/or side-chain oxidation and/or
conjugation of the alcohol groups, primarily with glucuronic acid (see
'General aspects of metabolism', p. 222).
Table 2. Most recent annual usage of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters as flavouring substances in Europe and United States
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Acetone (139)
Europe 50 000 7 100 120 NA
United States 190 000 36 000 590 NA
Isopropyl alcohol (277)
Europe 690 000 99 000 1 600 NA
United States 52 000 9 900 170 NA
2-Butanone (278)
Europe 790 110 2 NA
United States 190 36 0.6 NA
2-Pentanone (279)
Europe 1 000 140 2 NA
United States 220 42 0.7 NA
2-Pentanol (280)
Europe 44 6 0.1 NA
United States 0.2 0.04 0.0 NA
3-Hexanone (281)
Europe 3 0.4 0.01 NA
United States 5 1 0.02 NA
3-Hexanol (282)
Europe 94 13 0.22 NA
United States 60 11 0.19 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Heptanone (283)
Europe 790 110 2 NA
United States 250 48 0.11 NA
2-Heptanol (284)
Europe 56 8 0.13 NA
United States 5 1 0.02 NA
3-Heptanone (285)
Europe 27 4 0.06 NA
United States 50 10 0.16 NA
3-Heptanol (286)
Europe 1 0.02 0.0 NA
United States 3 0.6 0.01 NA
4-Heptanone (287)
Europe 16 2 0.04 NA
United States 13 2 0.04 NA
2-Octanone (288)
Europe 760 110 2 NA
United States 350 67 1 NA
2-Octanol (289)
Europe 94 13 0.22 NA
United States 19 4 0.06 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octanone (290)
Europe 23 3 0.05 NA
United States 12 2 0.04 NA
3-Octanol (291)
Europe 39 6 0.09 NA
United States 1 700 320 5 NA
2-Nonanone (292)
Europe 2 600 380 6 NA
United States 140 27 0.44 NA
2-Nonanol (293)
Europe 5 1 0.01 NA
United States 3 1 0.01 NA
3-Nonanone (294)
Europe 1 0.1 0.0 NA
United States 0.5 0.1 0.0 NA
3-Decanol (295)
Europe NR N/D 0 NA
United States 82 16 0.26 NA
2-Undecanone (296)
Europe 2 700 380 6 NA
United States 110 21 0.35 NA
2-Undecanol (297)
Europe 2 0.2 0.0 NA
United States 0.2 0.04 0.0 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Tridecanone (298)
Europe 510 73 1 NA
United States 160 30 0.51 NA
2-Pentadecanone (299)
Europe 150 21 0.3 NA
United States 2 300 430 7 NA
3-Methyl-2-butanol (300)
Europe 4 1 0.01 NA
United States 0.9 0.2 0.0 NA
4-Methyl-2-pentanone (301)
Europe 50 7 0.12 NA
United States 8 2 0.03 NA
2,6-Dimethyl-4-heptanone (302)
Europe 1.5 0.2 0.0 NA
United States 0.3 0.06 0.0 NA
2,6-Dimethyl-4-heptanol (303)
Europe NR N/D 0 NA
United States 25 5 0.08 NA
Isopropyl formate (304)
Europe 3.7 0.5 0.01 0.01
United States 0.1 0.02 0.0 0.00
Isopropyl acetate (305)
Europe 290 41 0.69 0.45
United States 46 9 0.15 0.06
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Isopropyl propionate (306)
Europe 0.1 0.01 0.01 0.00
United States 0.1 0.02 0.0 0.00
Isopropyl butyrate (307)
Europe 49 7 0.12 0.05
United States 0.4 0.08 0.0 0.00
Isopropyl hexanoate (308)
Europe 26 4 0.06 0.03
United States 0.1 0.02 0.0 0.00
Isopropyl isobutyrate (309)
Europe 4 0.6 0.01 0.01
United States 0.3 0.06 0.0 0.00
Isopropyl isovalerate (310)
Europe 2 0.3 0.01 0.00
United States 0.9 0.2 0.0 0.00
Isopropyl myristate (311)
Europe 160 23 0.38 0.10
United States 0.1 0.02 0.0 0.00
Isopropyl tiglate (312)
Europe 0.1 0.01 0.0 0.00
United States 0.5 0.1 0.0 0.00
3-Octyl acetate (313)
Europe 5 0.7 0.01 0.00
United States 160 30 0.51 0.20
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octyl tiglate (448)
Europe 0.1 0.01 0.0 0.00
United States 150 29 0.48 0.30
TOTAL
Europe 750 000 NA NA NA
United States 240 000 NA NA NA
TOTAL Isopropyl alcohol
Europe NA 99 000 1 600
United States NA 10 000 170
NA, not applicable; NR, not reported
a Europe, International Organization of the Flavor Industry (1995); United States,
National Academy of Sciences (1989)
b Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where
population (10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the United
States; 0.6 represents the assumption that only 60% of the flavour volume was
reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
c Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily
per capita intake ester
2.3.1.2 gamma-Diketone formation
Oxidation, primarily (omega-1)-oxidation, of certain aliphatic
ketones may yield gamma-diketones (e.g. 2-hexanone and 3-heptanone may
yield 2,5-hexanedione and 2,5-heptanedione, respectively), which, when
administered at high concentrations to experimental animals, may
result in peripheral neuropathy, commonly recognized as 'giant' axonal
neuropathy (Krasavage et al., 1980; Topping et al., 1994). The
structural features of diketone metabolites that induce peripheral
neuropathy have been shown to include gamma-diketone spacing.
gamma-Diketones with terminal methyl substituents (e.g.
2,5-hexanedione) and/or methyl substituents at the 3 and 4 positions
have the most pronounced effects. The severity of the peripheral
neuropathy depends on the size and position of alkyl substituents on
the gamma-diketone. For example, the strongest neurotoxic effects have
been observed with 3,4-dimethyl-substituted 2,5-hexanedione (not a
flavouring substance). When the chain length is increased (i.e. C-7
and greater), the neurotoxic response is generally significantly
reduced (Topping et al., 1994).
3-Heptanone and 3-heptanol (Nos 285 and 286) are the only
compounds in this group of flavouring substances that may be
metabolized to yield a neurotoxic gamma-diketone (Topping et al.,
1994); however, the longer chain length of 3-heptanone reduces its
neurotoxic effect , which is observed only at high doses.
In a poorly described study of the neurotoxic effects of some
aliphatic ketones, 3-heptanone administered to female Wistar rats in
the drinking-water for 120 days did not produce significant
neurological alterations (Homan & Maronpot, 1978).
Groups of five male Crl rats were exposed to atmospheres
containing 700 mg/kg 3-heptanone for two 20- and two 16-h periods per
week, for a total of 88 exposures over 164 days (approximately
24 weeks). After the fourth, thirtieth, and eighty-fifth exposures,
serum was analysed for 2,5-heptanedione. The maximum mean serum
concentrations reached 10 µg/ml after four exposures but decreased to
6-7 µg/ml after 30 and 85 exposures. No neurotoxicity was observed
(Katz et al., 1980).
Groups of two Crl rats were given 250, 500, 1000, 2000, or
4000 mg/kg bw 3-heptanone by gavage on five days per week for
14 weeks. No peripheral neuropathy was observed at doses
< 1000 mg/kg bw. At 2000 mg/kg bw, approaching the LD50 in rats
(2760 mg/kg bw), 3-heptanone induced peripheral neuropathy (O'Donoghue
et al., 1984).
2.3.1.3 Excretion
In studies limited to the identification of urinary glucuronide
metabolites, a series of aliphatic secondary alcohols was administered
by stomach tube to rabbits, and the urinary excretion of glucuronic
acid conjugates was determined after 24 h. The compounds, doses, and
average urinary output of glucuronide are listed in Table 3. The
results show that secondary alcohols, either administered directly or
formed via ketone reduction as demonstrated in one case with
2-heptanone (41 % urinary glucuronide), are largely excreted as
glucuronic acid conjugates (Kamil et al., 1953).
Table 3. Urinary excretion of glucuronides after
administration of aliphatic secondary alcohols to
rabbits by gavage
Substance No. Dose Urinary
(mg/kg bw)a glucuronide (%)
2-Propanol 277 1002 10.2
2-Butanol - 618 14.4
2-Pentanol 280 735 44.8
2-Heptanol 284 965 54.6
3-Heptanol 286 965 61.9
4-Heptanol - 965 67.4
2-Octanol 289 1081 15.5
From Kamil et al. (1953)
a Calculated from dose of 25 mmol/3-kg rabbit (for
2-propanol, 50 mmol/3 kg)
Generally, secondary alcohols (and ketones, by reduction to
secondary alcohols) are excreted after their conjugation with
glucuronic acid, primarily in the urine, or in the bile, depending on
the relative molecular mass and the animal species (see 'General
aspects of metabolism', p. 222). After hydrolysis of the esters in
this group, see above, the component secondary alcohols are
metabolized and excreted, and the component acids are metabolized in
the fatty acid ß-oxidation and other well-known metabolic pathways.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 25 of the 39 substances
in this group and range from 400 to 9800 mg/kg bw. The vast majority
of the values are > 1000 mg/kg bw, demonstrating that the acute
toxicity of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters given orally is extremely low (see e.g. Tanii et
al., 1986).
2.3.2.2 Short-term studies of toxicity
The results of short-term studies with six saturated aliphatic
acyclic secondary alcohols and ketones are summarized in Table 4 and
described below, together with some further studies. There were no
adequate studies available on the related esters.
Acetone
In a 14-day study, groups of five male and five female Fischer
344/N rats were given drinking-water ad libitum containing acetone
at concentrations of 0, 5000, 10 000, 20 000, 50 000, or 100 000 mg/kg
(equivalent to 0, 500, 1000, 2000, 5000, and 10 000 mg/kg bw per day).
Water consumption was depressed in females at 2000 mg/kg bw per day
and in all animals at 5000 or 10 000 mg/kg bw per day, but this was
not accompanied by signs of dehydration. The body weights of males at
5000 or 10 000 mg/kg bw per day and females at 10 000 mg/kg bw per day
were depressed. Statistically significant increases in relative kidney
and liver weights were observed n both male and female rats at a dose
of 2000 or 5000 mg/kg bw per day. In addition, statistically
significant increases in relative testis weight were observed at 5000
mg/kg bw per day but not at 10 000 mg/kg bw per day. No
histopathological abnormalities were observed in rats at any dose
tested (Dietz, 1991).
Groups of five male and five female B6C3F1 mice were exposed to
acetone in drinking-water ad libitum at concentrations of 0, 5000,
10 000, 20 000, 50 000, or 100 000 mg/kg (equivalent to 0, 1250, 2500,
5000, 12 500, and 25 000 mg/kg bw per day) for 14 days. Water
consumption was depressed at 12 500 or 25 000 mg/kg bw per day as
compared with controls. Growth retardation was seen in males and
females at 25 000 mg/kg bw per day but was more pronounced in males.
Statistically significant increases in relative kidney weights were
seen in both males and females at 12 500 or 25 000 mg/kg bw per day,
and statistically significant changes in relative liver weights were
observed in males at 1250, 2500, 5000, 12 500, or 25 000 mg/kg bw per
day and in females at 5000, 12 500, or 25 000 mg/kg bw per day.
Minimal to mild centrilobular hepatocellular hypertrophy was observed
at doses of 5000, 12 500, and 25 000 mg/kg bw per day in males and 12
500 and 25 000 mg/kg bw per day in females. Moderate centrilobular
hepatocellular hypertrophy was observed in males at 25 000 mg/kg bw
per day (Dietz, 1991).
In a 13-week study, groups of 10 male and 10 female rats were
given drinking-water ad libitum containing acetone at concentrations
of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg (equivalent to 0,
250, 500, 1000, 2000, and 5000 mg/kg bw per day). Water consumption
was depressed in males at 5000 mg/kg bw per day and in females at 2000
or 5000 mg/kg bw per day, but this was not accompanied by signs of
dehydration. Body-weight gain was significantly decreased in males and
females at 5000 mg/kg bw per day. Relative kidney weights were
significantly increased in both male and female rats exposed to
acetone at 5000 mg/kg bw per day and in females at 2000 mg/kg bw per
day. Increased incidences of neuropathy were seen in all males,
including the controls, but at greatest incidence and severity at the
two highest doses. Relative liver weights were significantly increased
in males and females at 2000 or 5000 mg/kg bw per day. No significant
differences in relative organ weight were observed at doses below 2000
mg/kg bw per day. At 5000 mg/kg bw per day, the relative testis
weights were significantly increased. The authors noted that such
organ weight changes are difficult to interpret because of the effect
of high doses of acetone on body weight; they therefore may not be
biologically significant. Furthermore, testicular toxicants typically
decrease testis weights. Decreased sperm motility, caudal weight, and
epididymal weight and an increased incidence of abnormal sperm were
also seen in males at 5000 mg/kg bw per day. The NOEL was 1000 mg/kg
bw per day (Dietz, 1991).
In a 13-week study, groups of 10 female and 10 male B6C3F1 mice
were given drinking-water containing acetone. Females received acetone
at concentrations of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg,
equivalent to 0, 625, 1250, 2500, 5000, or 12 500 mg/kg bw per day.
Males received acetone at a concentration of 0, 1250, 2500, 5000,
10 000, or 20 000 mg/kg, equivalent to 0, 313, 625, 1250, 2500, and
5000 mg/kg bw per day. The fluid intake of all females was depressed
as compared with controls. Absolute and relative liver weights and
absolute and relative spleen weights were significantly increased in
female mice at 12 500 mg/kg bw per day. Centrilobular hepatocellular
hypertrophy of minimal severity was seen in two female mice receiving
12 500 mg/kg bw per day, but no compound-related lesions were found in
male mice. The NOEL was 2500 mg/kg bw per day (Dietz, 1991).
Acetone was administered to groups of 30 male and 30 female
Sprague-Dawley rats by gavage for 90 days at doses of 100, 500, or
2500 mg/kg bw per day. Female rats at the two highest doses had
increased absolute kidney weights, and relative weight increases were
seen in the kidney, liver, and brain of both males and females at the
highest dose. Red blood cell parameters were significantly increased
in males at 45 days and animals of each sex at 90 days, at the highest
dose. Histopathological examination showed a significant increase in
tubular degeneration and hyalin droplet accumulation in males at
500 mg/kg bw per day and in animals of each sex at 2500 mg/kg bw per
day. Histolopathological examination of the liver and kidneys revealed
no treatment-dependent abnormalities. The NOEL was 100 mg/kg bw per
day (Sonawane et al., 1986).
Isopropyl alcohol
The potential toxicity of isopropyl alcohol was investigated in a
12-week study with 110 three-month-old male Wistar rats given 0, 1, 2,
3, or 5% in their drinking-water for the duration of the study. The
highest concentration was lowered to 4% during week 2 because of
unpalatability. The average daily intakes were reported to be 0, 870,
1300, 1700, and 2500 mg/kg bw. Body weights were statistically
significantly decreased in rats at 1700 or 2500 mg/kg bw per day.
Statistically significant, dose-related changes in relative liver
weights were observed at the three highest doses; significant
increases in testis weights were observed at 2500 mg/kg bw per day;
significant increases in relative kidney weights were seen at the
three highest doses; and statistically significant increases in
relative adrenal weights were observed at the two highest doses.
Increased formation of hyaline casts and increased hyaline droplet
content were observed in the proximal tubules of the kidneys in a
dose-dependent fashion. The NOEL was 870 mg/kg bw per day (Pilegaard &
Ladefoged, 1993).
Isopropyl alcohol was ingested by groups of eight adult men at
doses of 0, 2.6, or 6.4 mg/kg bw per day for six weeks. No
significant changes were observed in the chemical or cellular
composition of blood or urine, in the ability of the liver to excrete
sulfobromophthalein, in the optical properties of the eye, or in the
general well-being of the volunteers (Wills et al., 1969).
2-Heptanone
Groups of 15 male and 15 female CFE rats were given 2-heptanone
by gavage for 13 weeks at doses of 0, 20, 100, or 500 mg/kg bw per
day. In addition, groups of five male and five female rats received
doses of 0, 100, or 500 mg/kg bw per day for two and six weeks,
respectively. At week 13, the absolute and relative liver weights were
significantly increased in animals of each sex at 500 mg/kg bw per
day, and the absolute and relative kidney weights were significantly
increased in male rats at this dose; however, histological examination
showed no treatment-related damage to the liver or kidney. Significant
increases in the excretion of cells in the urine and enlarged kidneys
were observed in animals at 100 and 500 mg/kg bw per day. As no
abnormal function was detected in concentration tests, and there was
no histopathological damage in the kidneys, the significance of the
excretion of cells in the urine is unclear. The NOEL was 20 mg/kg bw
per day (Gaunt et al., 1972).
In a study of the role of the chain length of aliphatic ketones
in the causation of central and peripheral distal axonopathy, five
Sprague-Dawley rats of unspecified sex were given 0.5% 2-heptanone
(500 mg/kg bw per day) in their drinking-water for 12 weeks. The
animals were observed for hindlimb weakness, and pathological
examinations were conducted on tissues removed from the nervous
system. No giant axonal swelling or secondary myelin changes were
observed in treated animals (Spencer et al., 1978).
3-Heptanone
3-Heptanone, the only ketone in the group that is able to form a
gamma-diketone by (omega-1)-oxidation, was also the only ketone in
this group that caused axonal neuropathy in test animals. In a 14-week
study of the neurotoxic effects of oral exposure to 3-heptanone, 44
male rats were dosed by gavage on five days a week for the duration of
the study with 250, 500, 1000, 2000, or 4000 mg/kg bw per day of
3-heptanone alone; 3-heptanone followed by 1500 mg/kg bw per day
methyl ethyl ketone; 750 mg/kg bw per day methyl ethyl ketone; or 1500
mg/kg bw per day 5-methyl-2-octanone. Animals in many treated groups
developed central nervous system depression or narcosis, and those
given 2000 or 4000 mg/kg bw per day 3-heptanone in combination with
methyl ethyl ketone or 5-methyl-2-octanone died after one or two
doses. Histopathological analysis revealed central-peripheral distal
axonopathy characterized by 'giant' axonal swelling and
neurofilamentous hyperplasia in rats at 2000 mg/kg bw per day
3-heptanone. It was concluded that 3-heptanone has low neurotoxic
potential, as oral doses of < 1000 mg/kg bw per day did not induce
axonal neuropathy or the associated clinical signs. The NOEL was 1000
mg/kg bw per day (O'Donoghue et al., 1984).
4-Heptanone
4-Heptanone was administered to eight male Charles River rats by
gavage for 90 days at a dose of 2000 mg/kg bw per day, determined in a
previous study of acute toxicity to be the maximum tolerated dose
(Krasavage & O'Donoghue, 1979). After one week, however, four of the
rats had died, and the dose was reduced to 1000 mg/kg bw per day. The
mean relative liver weight was statistically significantly greater
than that of controls. Statistically significant increases in the
relative weights of the kidneys, adrenal glands, and testes were also
observed. Histopathological examination revealed hepatocyte
hypertrophy, adrenal gland congestion, and macroscopically enlarged
livers (O'Donoghue & Krasavage, 1980).
2,6-Dimethyl-4-heptanone
2,6-Dimethyl-4-heptanone (purity, 67%) was administered to eight
male Charles River rats by gavage for 90 days at 0 or 2000 mg/kg bw
per day, which was determined in a previous study to be the maximum
tolerated dose (Krasavage & O'Donoghue, 1979). The absolute and
relative liver weights, the relative kidney weights, and the absolute
and relative adrenal gland weights were statistically significantly
greater than those of controls; the absolute (but not the relative)
weights of the brain and heart were significantly depressed.
Histopathological examination revealed compound-related changes in the
stomach, liver, and kidneys. The stomachs of all animals showed
hyperkeratosis with or without pseudoepitheliomatous hyperplasia
associated with irritation due to direct contact with the solvent. In
the liver, minor or moderate hepatocyte hypertrophy was observed. In
the kidney, hyalin droplet formation was present in the proximal
tubular epithelium, with sporadic occurrence of regenerating tubular
epithelium and tubular dilation with casts (O'Donoghue & Krasavage,
1980).
2.3.2.3 Genotoxicity
Seven representative aliphatic acyclic secondary alcohols,
ketones, and related esters in this group have been tested for
genotoxicity. The results are summarized in Table 5 and described
below.
Table 4. Short-term studies of the toxicity of saturated aliphatic acyclic secondary alcohols and ketones
Substance No. Species Sex Test Route Time NOEL Reference
groupsa (mg/kg bw
per day)
Acetone 139 Rat Male/female 5/20 Drinking-water 91 days 1000 Dietz (1991)
Acetone 139 Mouse Male/female 5/20 Drinking-water 91 days 2500 Dietz (1991)
Acetone 139 Rat Male/female 3/60 Gavage 90 days 100 Sonawaneet al. (1986)
Isopropyl alcohol 277 Human Male 2/8 Oral 6 weeks 6.4b Wills et al. (1969)
Isopropyl alcohol 277 Rat Male 4/22 Drinking-water 90 days 870 Pilegaard &
Ladefoged (1993)
2-Heptanone 283 Rat Male/female 3/30 Gavage 91 days 20 Gaunt et al. (1972)
3-Heptanone 285 Rat Male 5/9 Gavage 14 weeks 1000 O'Donoghue et al.
(1984)
4-Heptanone 287 Rat Male 1/8 Gavage 90 days < 1000c O'Donoghue &
Krasavage (1980)
2,6-Dimethyl-4-heptanone 302 Rat Male 1/8 Gavage 90 days < 2000c O'Donohgue &
Krasavage (1980)
a No. of test groups/No. of animals per group
b This study was performed at either a single dose or multiple doses that induced no adverse effects. Therefore, this dose is not a true NOEL
but is the highest dose tested that produced no adverse effects. The actual NOEL would be higher.
c No NOEL established
Table 5. Results of assays for the genotoxicity of saturated aliphatic secondary alcohols, ketones, and related esters
Compound No. End-point Test object Concentration Result Reference
Acetone 139 rec Gene mutation B. subtilis NR Negativea Kawachi et al. (1980)
rec Gene mutation B. subtilis NR Negative Ishizaki et al.
(1979)
Reverse mutation S. typhimurium TA100 0.1-1000 µg/plate Negative Rapson et al.
(1980)
Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutation S. typhimurium TA98, NR Negativea Kawachi et al. (1980)
TA100
Reverse mutationb S. typhimurium TA98, 30 µl/plate Negativec Yamaguchi (1985)
TA100 (24 mg/plate)
Reverse mutation S. typhimurium TA97, < 10 000 µg/plate Negativea McCann et al. (1975)
TA98, TA100, TA1535,
TA1537
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA1535,
TA1537
Reverse mutation S. typhimurium TA100 500 µg/plate Negativea Yamaguchi (1982)
Sister chromatid exchange Human embryo fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Hamster lung fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Chinese hamster ovary < 10 µg/ml Negative Sasaki et al. (1980)
cells
Sister chromatid exchange Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Sister chromatid exchange Diploid human fibroblasts 5 µg/ml Negative Sasaki et al. (1980)
Sister chromatid exchange Human lymphocytes 6.8 mmol/plate Negative Norppa et al. (1983)
(395 µg/ml)
Chromosomal aberration Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Chromosomal aberration Hamster lung fibroblasts NR Positivec Kawachi et al. (1980)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
Isopropyl alcohol 277 Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538, E. coli WP2uvrA
Reverse mutationb S. typhimurium TA97, < 10 mg/plated Negativea Zeiger et al. (1992)
TA98, TA102, TA104,
TA1535, TA100, TA1537
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Chemical Manufacturers'
cells, hprt locus Association (1990)
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Kapp et al. (1993)
cells, hprt locus
2-Butanone 278 Reverse mutation S. typhimurium TA98, 10 000 µg/plate Negativea Douglas et al. (1980)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA102, 1 mg/plate Negative Marnett et al.(1985)
TA104
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA98, 0.05-32 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.04-26 µg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA104,
TA1535, TA1537
Reverse mutation S. typhimurium TA102 5000 µg/plate Negativec Müller et al. (1993)
Reverse mutation S. typhimurium TA98, 4000 µg/plate Negativee Brooks et al. (1988)
TA100, TA1535, TA1537,
TA1538; E. coli WP2uvrA
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.67-12 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.54-10 mg/ml)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
2-Butanone (contd) 278 Unscheduled DNA Human lymphocytes 0.01 mol/L Negativea Perocco et al. (1983)
synthesis (0.72 mg/ml)
Unscheduled DNA Rat hepatocytes 0.1-5 µl/ml Negative O'Donoghue et al. (1988)
synthesis (7.2-360 mg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary cells 1000 µg/ml Negativea Brooks et al. (1988)
4-Methyl-2-pentanone 301 Reverse mutation S. typhimurium TA98, 0.04-4 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.03-3 mg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, TA98, < 6667 µg/plate Negativea Zeiger et al. (1992)
TA100, TA1535
Reverse mutation E. coli WP2uvrA 8000 µg/plate Negativec Brooks et al. (1988)
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.32-4.2 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.26-3.4 mg/ml)
Unscheduled DNA Rat hepatocytes 0.01-1 µl/ml Negative O'Donoghue et al. (1988)
synthesis (8-80 µg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary 1000 µg/ml Negativea Brooks et al. (1988)
cells
2,6-Dimethyl- 302 Reverse mutationb S. typhimurium TA98, 1-333 µg/plate Negativea Mortelmans et al. (1986)
4-heptanone TA100, TA1535, TA1537
Isopropyl acetate 305 Reverse mutationb S. typhimurium TA97, TA98, < 10 mg/plate Negativea Zeiger et al. (1992)
TA100, TA1537, TA1538
Isopropyl myristate 311 Reverse mutation S. typhimurium TA98, TA100, 50 µg/plate Negativea Blevins & Taylor
(spot test) TA1535, TA1537, TA1538 (1982)
a With and without metabolic activation
b Modified (pre-incubation) protocol
c Without metabolic activation
d Maximum non-toxic dose
e With metabolic activation
In vitro
No evidence of mutagenicity was reported for eight aliphatic
acyclic secondary alcohols, ketones, and related esters in multiple
assays in the standard Ames or preincubation protocol with
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA104,
TA1535, TA1537, and TA1538 or Escherichia coli strain WP2 uvrA,
with or without metabolic activation (McCann et al., 1975; Douglas et
al., 1980; Florin et al., 1980; Kawachi et al., 1980; Rapson et al.,
1980; Blevins & Taylor, 1982; Yamaguchi, 1982; Marnett et al., 1985;
Shimizu et al., 1985; Yamaguchi, 1985; Mortelmans et al., 1986; Brooks
et al., 1988; O'Donoghue et al., 1988; Zeiger et al., 1992; Müller et
al., 1993). The concentrations tested ranged from 0.1 to 10 000
µg/plate and often approached cytotoxic levels. The results of other
bacterial assays, such as the rec assay in Bacillus subtilis and
the E. coli reversion assay also indicated that saturated aliphatic
secondary alcohols are not mutagenic (Ishizaki et al., 1979; Kawachi
et al., 1980). In the yeast Saccharomyces cerevisiae, 2-butanone and
methyl isobutylketone did not induce gene conversion at concentrations
up to 5 mg/ml either with or without metabolic activation (Brooks
et al., 1988).
In the L5178Y/ tk+/- mouse lymphoma assay, 2-butanone was not
mutagenic when tested with and without metabolic activation at 11
concentrations ranging from 0.67 µl/ml (non-toxic) to 12 µl/ml (100%
toxic) (O'Donoghue et al., 1988). In the same study,
4-methyl-2-pentanone was not mutagenic at 10 concentrations ranging
from 0.6 µl/ml (non-toxic) to 3.7 µl/ml (100% toxic) with and without
metabolic activation, even though equivocal, non-dose-related
responses were observed at high concentrations without activation.
Isopropyl alcohol was tested at eight concentrations ranging between
0.5 and 5 mg/ml with and without metabolic activation in an assay for
forward mutation at the hypoxanthine-guanine phosphoribosyl
transferase (hprt) locus in Chinese hamster ovary cells (Chemical
Manufacturers' Association, 1990). Statistically significantly
increased mutant frequencies were seen in two of 34 treated cultures,
but these were not dose-related and not reproducible in subsequent
trials (Kapp et al., 1993).
In standard assays for cytogeneticity, acetone did not increase
the frequency of sister chromatid exchange in Chinese hamster ovary
cells (Loveday et al., 1990), hamster lung fibroblasts, or Don-6
cells, (Kawachi et al., 1980; Sasaki et al., 1980) and did not
increase the frequency of sister chromatid exchange in human
lymphocytes (Norppa et al., 1983) with and without metabolic
activation at concentrations up to 5000 µg/ml (Loveday et al., 1990).
Acetone did not induce chromosomal aberrations in Chinese hamster
ovary cells at concentrations up to a maximum of 5000 µg/ml (Loveday
et al., 1990). In a test conducted at an unspecified concentration,
acetone induced chromosomal aberrations in hamster lung fibroblasts
(Kawachi et al., 1980), whereas 2-butanone and methyl isobutylketone
gave negative results in assays in rat hepatocytes and Chinese hamster
ovary cells when tested at concentrations up to 1 mg/ml (Brooks et
al., 1988).
2-Butanone did not induce unscheduled DNA synthesis in human
lymphocytes treated with concentrations up to 0.01 mol/L, with and
without metabolic activation (Perocco et al., 1983). 2-Butanone and
4-methyl-2-pentanone did not induce unscheduled DNA synthesis in rat
hepatocytes when tested at concentrations up to 5 and 1 µl/ml,
respectively (O'Donoghue et al., 1988).
In vivo
Acetone, isopropyl alcohol, 2-butanone, and methyl-2-pentanone
did not induce micronucleus formation at doses of 400-2500 mg/kg bw
(Basler, 1986; O'Donoghue et al., 1988; Kapp et al., 1993), which
approximated the LD20 (O'Donoghue et al., 1988) and the LD50 (Basler,
1986) of the tested compounds.
2.3.2.4 Other relevant studies
Isopropyl alcohol
The effects of long-term consumption of isopropyl alcohol on
gonad function and embryonic and perinatal development were studied
during an investigation to establish the maximum permissible
concentrations of isopropyl alcohol in water bodies. The compound was
administered for 20 days to 869 inbred white rats and for 45 days to
488 rats which had progeny at doses of 1008 and 252 mg/kg bw per day,
respectively; for three months at a dose of 1800 mg/kg bw per day; and
for six months at doses of 18, 1.8, or 0.18 mg/kg bw per day. The
embryos were examined macroanatomically on day 21 of gestation to
determine embryotoxicity, and the offspring of treated male and female
rats mated with treated or untreated controls were observed for
developmental abnormalities.
Dams given 252 or 1008 mg/kg bw per day isopropyl alcohol before
the onset of pregnancy had a significantly reduced number of embryos,
but a similar effect was not seen in those given 1800 mg/kg bw per
day. Total embryonic mortality was significantly increased only in
dams given 1008 mg/kg bw per day; however, no essential differences in
embryonic development were noted. The reproductive function of females
receiving isopropyl alcohol for six months was adversely affected. The
percentage mortality of the progeny of treated male and female
pairings and treated male and control female pairings reached a
significant level only in the groups receiving 18 mg/kg bw per day for
six months. The weights of the progeny of treated males and females
were also significantly reduced. The authors concluded that long-term
intake of isopropyl alcohol can affect generative function, however
only at concentrations that far exceed the current maximum allowable
concentration of isopropyl alcohol (0.25 mg/litre) in water supplies
(Antonova & Salmina, 1978).
Groups of 25 timed-pregnant Sprague-Dawley rats were given 0,
400, 800, or 1200 mg/kg bw per day isopropyl alcohol by gavage on days
6-15 of gestation. Deaths were observed in the groups at 800 mg/kg bw
(4%) and 1200 mg/kg bw (8%) per day. Reduced maternal gestational
weight gain on days 0-20 associated with significantly reduced gravid
uterine weights were noted at 800 and 1200 mg/kg bw per day. There
were no adverse maternal or developmental effects at 400 mg/kg bw per
day and no teratogenic effects at any dose (Tyl et al., 1994).
Groups of 15 artificially inseminated New Zealand white rabbits
were given isopropyl alcohol at 0, 120, 240, or 480 mg/kg bw per day
by gavage on days 6-18 of gestation. Four does at 480 mg/kg bw per day
died, and severe clinical signs of toxicity and statistically
significantly reduced food consumption were observed. No
treatment-related maternal or developmental effects were observed at
120 or 240 mg/kg bw per day, and no teratogenicity was seen at any
dose (Tyl et al., 1994).
Rats were exposed to isopropyl alcohol in the drinking-water for
three generations, with average intakes of 1470, 1380, and 1290 mg/kg
bw per day for the first, second, and third generations, respectively.
The growth of the first generation was retarded initially but had
returned to normal by the 13th week. There were no other effects on
growth and no effects on reproduction (Lehman et al., 1945).
Methyl ethyl ketone
Groups of 10 virgin and 30 plug-positive female Swiss CD-1 mice
were exposed to 0, 400, 1000, or 3000 mg/kg methyl ethyl ketone
vapours for 10 consecutive days. The pregnant mice appeared to be
relatively insensitive, but the offspring of mice at the highest dose
showed significant signs of toxicity. No maternal or developmental
toxicity was observed at doses < 1000 mg/kg vapour (Mast et al.,
1989).
4. REFERENCES
Antonova, V.I. & Salmina, Z.A. (1978) [Maximum permissible
concentration of isopropyl alcohol in water bodies with regard to its
actions on gonads and progeny.] Gig. Sanit., 1, 8-11 (in Russian).
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Intermediary Xenobiotic Metabolism in Animals,
pp. 81-97.
Basler, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat. Res., 174, 11-13.
Blevins, R.D. & Taylor, D.E. (1982) Mutagenicity screening of
twenty-five cosmetic ingredients with the Salmonella/microsome test.
J. Environ. Sci. Health, A17, 217-239.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Brooks T.M., Meyer, A.L. & Hutson, D.H. (1988) The genetic toxicology
of some hydrocarbon and oxygenated solvents. Mutagenesis, 3,
227-232.
Brown, W.D., Setzer, J.V., Dick, R.B., Phipps, F.C. & Lowry, L.K.
(1987) Body burden profiles of single and mixed solvent exposures.
J. Occup. Med. , 29, 877-883.
Chemical Manufacturers' Association (1990) Unpublished submission to
the United States Environmental Protection Agency.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dietz D. (1991) Toxicity studies of acetone in F344/N rats and B6C3F1
mice (drinking water studies), National Toxicology Program (NIH
Publication No. 91-3122), Bethesda, Maryland, United States.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Douglas, G.R., Nestmann, E.R., Betts, J.L., Mueller, J.C., Lee, E.G.-
H., Stich, H.F., San, R.H.C., Brouzes, R.J.P., Chmelauskas, A.L.,
Paavila, H.D. & Walden, C.C. (1980) Mutagenic activity in pulp mill
effluents. In: Jolley, R.L. et al., eds, Water Chlorination:
Environmental Impact and Health Effects, Ann Arbor, Michigan, Ann
Arbor Science Publishers, Vol. 3.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, New York, Academic
Press, Vol. I, pp. 281-293.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18, 219-232.
Gaunt, I.F., Carpanini, F.M.B., Wright, M.G., Grasso, P. & Gangolli,
S.D. (1972) Short-term toxicity of methyl amyl ketone in rats. Food
Cosmetic Toxicol., 10, 625-636.
Haggard, H.W., Miller, D.P. & Greenberg, L.A. (1945) The amyl alcohols
and their ketones: Their metabolic fates and comparative toxicities.
J. Ind. Hyg. Toxicol., 27, 1-14.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, Vol. I, pp. 291-323.
Homan, E.R. & Maronpot, R.R. (1978) Neurotoxic evaluation of some
aliphatic ketones (Abstract). Toxicol. Appl. Pharmacol., 45, 312.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Ishizaki, M., Oyamada, N., Ueno, S., Katsumura, K. & Hosogai, Y.
(1979) Mutagenicity of degradation and reaction products of butyl
hydroxy anisol with sodium nitrite or potassium nitrate by irradiation
of ultra violet ray. J. Food Hyg. Soc. Jpn, 20, 143-146.
Kamil, I.A., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; The glucuronic acid conjugation of acyclic
aliphatic alcohols . J. Biochem., 53, 129-136.
Kapp, R.W., Marino, D.J., Gardiner, T.H., Maston, L.W., McKee, R.H.,
Tyler, T.R., Ivett, J.L. & Young, R.R. (1993) In vitro and in vivo
assays of isopropanol for mutagenicity. Environ. Mol. Mutag., 22,
93-100.
Katz, G.V., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J. (1980)
Comparative neurotoxicity and metabolism of ethyl n-butyl ketone and
methyl n-butyl ketone in rats. Toxicol. Appl. Pharmacol., 52,
153-158.
Kawachi, T., Komatsu, T., Kada, T., Ishidate, M., Masamichi, S.,
Sugiyama, T. & Tazima, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In:
Williams, G.M. et al., eds, The Predictive Value of Short-term
Screening Tests in Carcinogenicity Evaluation, Amsterdam,
Elsevier/North-Holland Biomedical Press.
Krasavage, W.J. & O'Donoghue, J.L. (1979) Repeated oral administration
of five ketones and n-heptane to rats. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Krasavage, W.J., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J.
(1980) The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 53, 433-441.
Krasavage, W.J., O'Donoghue, J.L. & DiVincenzo, G.D. (1982) Ketones.
In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 3rd Ed., New York, John Wiley & Sons, Vol. IIC, pp.
4720-4727.
Lehman, A.J., Schwerma, H. & Rickards, E. (1945) Isopropyl alcohol:
Acquired tolerance in dogs, rate of disappearance from the blood
stream in various species and effects on successive generation of
rats. J. Pharmacol. Exp. Therap., 85, 61-69.
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, pp. 2585-2760.
Loveday, K.S., Anderson, B.E., Resnick, M.A. & Zeiger, E. (1990)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. V: Results with 46 chemicals. Environ.
Mol. Mutag., 16, 272-303.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mast, T.J., Dill, J.A., Evanoff, J.J., Rommereim, R.L., Weigel, R.J. &
Westerberg, R.B. (1989) Inhalation developmental toxicology studies:
Teratology study of methyl ethyl ketone in mice. National Toxicology
Program (No. NIH-Y01-ES-70153), Bethesda, Maryland, United States.
McCann, J., Choi, E., Yamasaki, E. & Ames, B.N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay of 300
chemicals. Proc. Natl Acad. Sci. USA, 72, 5135-5139.
Morgott, D.A. (1993) Acetone. In: Clayton, G.D. & Clayton, F.E., eds,
Patty's Industrial Hygiene and Toxicology, 4th Ed., New York, John
Wiley & Sons, pp. 149-281.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Müller, W., Engelhart, G., Herbold, B., Jäckh, R. & Jung, R. (1993)
Evaluation of mutagenicity testing with Salmonella typhimurium TA102
in three different laboratories. Environ. Health Perspectives,
Suppl. 101, 33-36.
National Academy of Sciences (1989) 1989 Poundage and Technical
Effects. Update of Substances Added to Food, Washington DC.
Neubreuer, O. (1901) Concerning the pairing of glucuronic acid with
materials of the aliphatic series. Arch. Exp. Pathol. Pharmacol.,
46, 133-154.
Norppa, H., Vainio, H. & Sorsa, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res. 43, 3579-3582.
O'Donohogue, J.L. & Krasavage, W.J. (1980) 90-day repeated oral
administration of five ketones and n-heptane to rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
O'Donoghue, J.L., Krasavage, W.J., DiVincenzo, G.D. & Katz, G.V.
(1984) Further studies on ketone neurotoxicity and interactions.
Toxicol. Appl. Pharmacol., 72, 201-209.
O'Donoghue, J.L., Haworth, S.R., Curren, R.D., Kirby, P.E., Lawlor,
T., Moran, E.J., Phillips, R.D., Putnam, D.L., Rogers-Back, A.M.,
Slesinski, R.S. & Thilagar, A. (1988) Mutagenicity studies on ketone
solvents: Methyl ethyl ketone, methyl isobutyl ketone, and isophorone.
Mutat. Res., 206, 149-161.
Perocco, P., Bolognesi, S. & Alberghini, W. (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol. Lett., 16, 69-75.
Pilegaard, K. & Ladefoged, O. (1993) Toxic effects in rats of twelve
weeks' dosing of 2-propanol, and neurotoxicity measured by
densitometric measurements of glial fibrillary acidic protein in the
dorsal hippocampus. In Vivo, 7, 325-330.
Rapson, W.H., Nazar, M.A. & Butzky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ.
Contam. Toxicol., 24, 590-596.
Saito, M. (1975) Studies on the metabolism of lower alcohols
(Abstract). Nichidai Igaku Zasshi, 34, 569-585.
Sasaki, M., Sugimura, K., Yoshida, M.A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster cells.
Kromosomo II, 20, 574-584.
Schwartz, L. (1989) [On the oxidation of acetones and homologous
ketones from fatty acids.] Arch. Exp. Pathol. Pharmacol., 40, 168
(in German).
Scopinaro, D., Ghiani, P. & De Cecco, A. (1947) [Ketolytic fate of
acetone. II. Acetone metabolism in normal subjects.] Policlinico
(Rome) Sez, Med., 54, 70 (in Italian).
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Sonawane, B., de Rosa, C., Rubenstein, R., Mayhew, D., Becker, S.V. &
Dietz, D. (1986) Estimation of reference dose (RfD) for oral exposure
to acetone (Abstract). J. Am. Coll. Toxicol.., 5, 605.
Spencer, P.S., Bischoff, M.C. & Schaumburg, H.H. (1978) On the
specific molecular configuration of neurotoxic aliphatic hexacarbon
compounds causing central-peripheral distal axonopathy. Toxicol.
Appl. Pharmacol., 44, 17-28.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tanii, H., Tsuji, H. & Hashimoto, K. (1986) Structure-toxicity
relationship of monoketones. Toxicol. Lett., 30, 13-17.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Tyl, R.W., Masten, L.W., Marr, M.C., Myers, C.B., Slauter, R.W.,
Gardiner, T.H., Strother, D.E., McKee, R.H. & Tyler, T.R. (1994)
Developmental toxicity evaluation of isopropanol by gavage in rats and
rabbits. Fundam. Appl. Toxicol., 22, 139-151.
Wills, J.H., Jameson, E.M. & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15, 560-565.
Yamaguchi, T. (1982) Mutagenicity of trioses and methyl glyoxal on
Salmonella typhimurium. Agric. Biol. Chem. 46, 849-851.
Yamaguchi, T. (1985) Stimulating effects of organic solvents on the
mutagenicities of sugar-degradation compounds. Agric. Biol. Chem.
49, 3363-3368.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
The thresholds for human intake are 1800 µg/day for Class I and 540 µg/day for Class II.
N/D, No intake data reported
Step A3. The daily per capita intakes of 25 of the 27 class-I
substances at this step in Europe and the United States are
below the human intake threshold for class I compounds (1800
µg/person per day), indicating that they pose no safety
concern when used at current levels of estimated intake as
flavouring agents. The intakes of acetone (mg/person per
day) are 7.1 in Europe and 35 in the United States, and the
intakes of isopropyl alcohol are 99 in Europe and 10 in the
United States; these are greater than the human intake
threshold of 1800 µg/person per day (National Academy of
Sciences, 1989; International Organization of the Flavor
Industry, 1995). The intakes of 10 class II substances at
this step in Europe and in the United States are below the
human intake threshold for that class (540 µg/person per
day), indicating that they pose no safety concern at current
levels of estimated intake as flavouring agents.
The total intake of isopropyl alcohol and its nine
esters from use as flavouring agents is also greater than
the human intake threshold for compounds in class I (1800
µg/person per day).
The intake of three additional esters of isopropyl
alcohol (isopropyl benzoate, isopropyl cinnamate, and
isopropyl phenyl-acetate), which were not evaluated as part
of this group, would add about 15 µg/day in Europe and 5
µg/day in the United States to the total intake of isopropyl
alcohol. This potential additional intake is minor and would
not alter the safety evaluation.
Step A4. This step needs to be considered only for acetone and
isopropyl alcohol, the two flavouring agents in this group
for which estimated intake exceeds the class I threshold of
1800 µg/person per day. They are both endogenous, as
components of fatty acid and carbohydrate metabolism, and
have been detected in the blood.
Step B3. The current per capita intake of 3-heptanone (No. 285),
class II, and 3- heptanol (No. 286), class I, do not exceed
the human intake thresholds, 540 and 1800 µg/day,
respectively. Accordingly the evaluation proceeds to step
B4.
Step B4. On the basis of a limited study of the neurotoxic effects of
orally administered 3-heptanone (No. 285) to male rats, the
NOEL was 1000 mg/kg bw per day (O'Donoghue et al., 1984).
This NOEL provides a large safety margin (> 1 000 000 for
3-heptanone and even higher for 3-heptanol) in relation to
current levels of estimated intake of 3-heptanone and
3-heptanol (No. 286) (which might be metabolized to
3-heptanone).
On the basis of the results of the safety evaluation sequence, 28
of the saturated aliphatic acyclic secondary alcohols and ketones and
11 of the related esters of saturated and unsaturated carboxylic acids
evaluated do not pose a safety concern when used at current levels of
intake as flavouring agents.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 39 saturated aliphatic acyclic
secondary alcohols, ketones, and related esters of saturated and
unsaturated carboxylic acids were consumed concomitantly on a daily
basis, the estimated combined intake would exceed the human thresholds
for classes I and II. All of the 39 substances except two (3-heptanone
and 3-heptanol) are expected to be metabolized via well-known
biochemical pathways to innocuous metabolic and/or endogenous
substances; in the opinion of the Committee, the endogenous levels of
these metabolites would not give rise to perturbations outside the
physiological range. Accordingly, even a combined theoretical intake
would be of no safety concern. The combined intake of the two
substances that could potentially form gamma-diketones would also be
of no safety concern.
1.6 Conclusions
The Committee concluded that the substances in this group would
not present safety concerns at the current levels of intake.
Data on toxicity were required only for 3-heptanone (No. 285) and
3-heptanol (No. 286) when applying the procedure to this group of
flavouring agents. The Committee noted that these data and others are
consistent with the results of the safety evaluation using the
procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Twenty-eight saturated aliphatic acyclic ketones and secondary
alcohols with chain lengths from C3 to C15 (Nos 139 and 277-303) and
11 related saturated and unsaturated esters (Nos 304-313 and 448) are
included in this group of flavouring agents (see Table 1). The group
comprises 16 saturated aliphatic acyclic ketones (10 2-alkanones, four
3-alkanones, and two 4-alkanones), 12 saturated aliphatic acyclic
secondary alcohols (seven 2-alkanols, four 3-alkanols, and one
4-alkanol), nine esters of isopropyl alcohol, and two esters of
3-octanol in which the component carboxylic acids are six saturated
and one unsaturated carboxylic acid (tiglic acid).
The substances in this group are structurally related because
they are saturated, aliphatic acyclic ketones, secondary alcohols, or
esters formed from secondary alcohols and aliphatic, saturated or
unsaturated carboxylic acids.
2.2 Intake
The total annual production and the estimated current per
capita intake in Europe and the United States of the 39 saturated
aliphatic acyclic secondary alcohols, ketones, and related esters are
shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
Generally, saturated aliphatic acyclic secondary alcohols and
ketones are absorbed through the gastrointestinal tract and rapidly
eliminated from the blood. The related esters in this group of
flavouring agents are anticipated to be hydrolysed to their component
secondary alcohols and aliphatic saturated and unsaturated carboxylic
acids, which are also readily absorbed, as considered above in section
1.3 and 'General aspects of metabolism', p. 223.
In addition to oxidation and reduction pathways,
low-molecular-mass ketones (carbon chain length, < C-5) may be
excreted unchanged in expired air (Brown et al., 1987). In mammals,
oral doses of volatile ketones or their corresponding alcohols are
eliminated principally as the ketone in expired air; lesser amounts
are excreted in the urine (Haggard et al., 1945; Scopinaro et al.,
1947; Saito, 1975). In rats, 2-pentanone in expired air was the major
metabolite after administration of 2-penta-nol by intraperitoneal
injection; lesser amounts of 2-pentanol were exhaled, and both
metabolites were detected in urine (Haggard et al., 1945). Some of the
flavouring agents in this group may be oxidized to form neurotoxic
gamma-diketones (see below).
2.3.1.1 Ester hydrolysis
Generally, isopropyl esters are anticipated to be hydrolysed to
isopropyl alcohol and their component saturated or unsaturated
aliphatic carboxylic acids (see section 1.3 and 'General aspects of
metabolism', p. 223). Isopropyl tiglate (No. 312) and the two 3-octyl
esters (Nos 313 and 448) may be hydrolysed at a lower rate in the
gastrointestinal tract, but esters that reach the general circulation
intact will be hydrolysed by tissue esterases to the component
carboxylic acid and alcohol.
In general, the ketones and secondary alcohols in this group of
flavouring agents are metabolized by reversible reduction/oxidation of
the ketone/alcohol group and/or side-chain oxidation and/or
conjugation of the alcohol groups, primarily with glucuronic acid (see
'General aspects of metabolism', p. 222).
Table 2. Most recent annual usage of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters as flavouring substances in Europe and United States
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Acetone (139)
Europe 50 000 7 100 120 NA
United States 190 000 36 000 590 NA
Isopropyl alcohol (277)
Europe 690 000 99 000 1 600 NA
United States 52 000 9 900 170 NA
2-Butanone (278)
Europe 790 110 2 NA
United States 190 36 0.6 NA
2-Pentanone (279)
Europe 1 000 140 2 NA
United States 220 42 0.7 NA
2-Pentanol (280)
Europe 44 6 0.1 NA
United States 0.2 0.04 0.0 NA
3-Hexanone (281)
Europe 3 0.4 0.01 NA
United States 5 1 0.02 NA
3-Hexanol (282)
Europe 94 13 0.22 NA
United States 60 11 0.19 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Heptanone (283)
Europe 790 110 2 NA
United States 250 48 0.11 NA
2-Heptanol (284)
Europe 56 8 0.13 NA
United States 5 1 0.02 NA
3-Heptanone (285)
Europe 27 4 0.06 NA
United States 50 10 0.16 NA
3-Heptanol (286)
Europe 1 0.02 0.0 NA
United States 3 0.6 0.01 NA
4-Heptanone (287)
Europe 16 2 0.04 NA
United States 13 2 0.04 NA
2-Octanone (288)
Europe 760 110 2 NA
United States 350 67 1 NA
2-Octanol (289)
Europe 94 13 0.22 NA
United States 19 4 0.06 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octanone (290)
Europe 23 3 0.05 NA
United States 12 2 0.04 NA
3-Octanol (291)
Europe 39 6 0.09 NA
United States 1 700 320 5 NA
2-Nonanone (292)
Europe 2 600 380 6 NA
United States 140 27 0.44 NA
2-Nonanol (293)
Europe 5 1 0.01 NA
United States 3 1 0.01 NA
3-Nonanone (294)
Europe 1 0.1 0.0 NA
United States 0.5 0.1 0.0 NA
3-Decanol (295)
Europe NR N/D 0 NA
United States 82 16 0.26 NA
2-Undecanone (296)
Europe 2 700 380 6 NA
United States 110 21 0.35 NA
2-Undecanol (297)
Europe 2 0.2 0.0 NA
United States 0.2 0.04 0.0 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
2-Tridecanone (298)
Europe 510 73 1 NA
United States 160 30 0.51 NA
2-Pentadecanone (299)
Europe 150 21 0.3 NA
United States 2 300 430 7 NA
3-Methyl-2-butanol (300)
Europe 4 1 0.01 NA
United States 0.9 0.2 0.0 NA
4-Methyl-2-pentanone (301)
Europe 50 7 0.12 NA
United States 8 2 0.03 NA
2,6-Dimethyl-4-heptanone (302)
Europe 1.5 0.2 0.0 NA
United States 0.3 0.06 0.0 NA
2,6-Dimethyl-4-heptanol (303)
Europe NR N/D 0 NA
United States 25 5 0.08 NA
Isopropyl formate (304)
Europe 3.7 0.5 0.01 0.01
United States 0.1 0.02 0.0 0.00
Isopropyl acetate (305)
Europe 290 41 0.69 0.45
United States 46 9 0.15 0.06
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Isopropyl propionate (306)
Europe 0.1 0.01 0.01 0.00
United States 0.1 0.02 0.0 0.00
Isopropyl butyrate (307)
Europe 49 7 0.12 0.05
United States 0.4 0.08 0.0 0.00
Isopropyl hexanoate (308)
Europe 26 4 0.06 0.03
United States 0.1 0.02 0.0 0.00
Isopropyl isobutyrate (309)
Europe 4 0.6 0.01 0.01
United States 0.3 0.06 0.0 0.00
Isopropyl isovalerate (310)
Europe 2 0.3 0.01 0.00
United States 0.9 0.2 0.0 0.00
Isopropyl myristate (311)
Europe 160 23 0.38 0.10
United States 0.1 0.02 0.0 0.00
Isopropyl tiglate (312)
Europe 0.1 0.01 0.0 0.00
United States 0.5 0.1 0.0 0.00
3-Octyl acetate (313)
Europe 5 0.7 0.01 0.00
United States 160 30 0.51 0.20
Table 2. (continued)
Substance (No.) Most recent Per capita intakeb Per capita intakec,
annual volumea alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
3-Octyl tiglate (448)
Europe 0.1 0.01 0.0 0.00
United States 150 29 0.48 0.30
TOTAL
Europe 750 000 NA NA NA
United States 240 000 NA NA NA
TOTAL Isopropyl alcohol
Europe NA 99 000 1 600
United States NA 10 000 170
NA, not applicable; NR, not reported
a Europe, International Organization of the Flavor Industry (1995); United States,
National Academy of Sciences (1989)
b Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where
population (10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the United
States; 0.6 represents the assumption that only 60% of the flavour volume was
reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
c Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily
per capita intake ester
2.3.1.2 gamma-Diketone formation
Oxidation, primarily (omega-1)-oxidation, of certain aliphatic
ketones may yield gamma-diketones (e.g. 2-hexanone and 3-heptanone may
yield 2,5-hexanedione and 2,5-heptanedione, respectively), which, when
administered at high concentrations to experimental animals, may
result in peripheral neuropathy, commonly recognized as 'giant' axonal
neuropathy (Krasavage et al., 1980; Topping et al., 1994). The
structural features of diketone metabolites that induce peripheral
neuropathy have been shown to include gamma-diketone spacing.
gamma-Diketones with terminal methyl substituents (e.g.
2,5-hexanedione) and/or methyl substituents at the 3 and 4 positions
have the most pronounced effects. The severity of the peripheral
neuropathy depends on the size and position of alkyl substituents on
the gamma-diketone. For example, the strongest neurotoxic effects have
been observed with 3,4-dimethyl-substituted 2,5-hexanedione (not a
flavouring substance). When the chain length is increased (i.e. C-7
and greater), the neurotoxic response is generally significantly
reduced (Topping et al., 1994).
3-Heptanone and 3-heptanol (Nos 285 and 286) are the only
compounds in this group of flavouring substances that may be
metabolized to yield a neurotoxic gamma-diketone (Topping et al.,
1994); however, the longer chain length of 3-heptanone reduces its
neurotoxic effect , which is observed only at high doses.
In a poorly described study of the neurotoxic effects of some
aliphatic ketones, 3-heptanone administered to female Wistar rats in
the drinking-water for 120 days did not produce significant
neurological alterations (Homan & Maronpot, 1978).
Groups of five male Crl rats were exposed to atmospheres
containing 700 mg/kg 3-heptanone for two 20- and two 16-h periods per
week, for a total of 88 exposures over 164 days (approximately
24 weeks). After the fourth, thirtieth, and eighty-fifth exposures,
serum was analysed for 2,5-heptanedione. The maximum mean serum
concentrations reached 10 µg/ml after four exposures but decreased to
6-7 µg/ml after 30 and 85 exposures. No neurotoxicity was observed
(Katz et al., 1980).
Groups of two Crl rats were given 250, 500, 1000, 2000, or
4000 mg/kg bw 3-heptanone by gavage on five days per week for
14 weeks. No peripheral neuropathy was observed at doses
< 1000 mg/kg bw. At 2000 mg/kg bw, approaching the LD50 in rats
(2760 mg/kg bw), 3-heptanone induced peripheral neuropathy (O'Donoghue
et al., 1984).
2.3.1.3 Excretion
In studies limited to the identification of urinary glucuronide
metabolites, a series of aliphatic secondary alcohols was administered
by stomach tube to rabbits, and the urinary excretion of glucuronic
acid conjugates was determined after 24 h. The compounds, doses, and
average urinary output of glucuronide are listed in Table 3. The
results show that secondary alcohols, either administered directly or
formed via ketone reduction as demonstrated in one case with
2-heptanone (41 % urinary glucuronide), are largely excreted as
glucuronic acid conjugates (Kamil et al., 1953).
Table 3. Urinary excretion of glucuronides after
administration of aliphatic secondary alcohols to
rabbits by gavage
Substance No. Dose Urinary
(mg/kg bw)a glucuronide (%)
2-Propanol 277 1002 10.2
2-Butanol - 618 14.4
2-Pentanol 280 735 44.8
2-Heptanol 284 965 54.6
3-Heptanol 286 965 61.9
4-Heptanol - 965 67.4
2-Octanol 289 1081 15.5
From Kamil et al. (1953)
a Calculated from dose of 25 mmol/3-kg rabbit (for
2-propanol, 50 mmol/3 kg)
Generally, secondary alcohols (and ketones, by reduction to
secondary alcohols) are excreted after their conjugation with
glucuronic acid, primarily in the urine, or in the bile, depending on
the relative molecular mass and the animal species (see 'General
aspects of metabolism', p. 222). After hydrolysis of the esters in
this group, see above, the component secondary alcohols are
metabolized and excreted, and the component acids are metabolized in
the fatty acid ß-oxidation and other well-known metabolic pathways.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 25 of the 39 substances
in this group and range from 400 to 9800 mg/kg bw. The vast majority
of the values are > 1000 mg/kg bw, demonstrating that the acute
toxicity of saturated aliphatic acyclic secondary alcohols, ketones,
and related esters given orally is extremely low (see e.g. Tanii et
al., 1986).
2.3.2.2 Short-term studies of toxicity
The results of short-term studies with six saturated aliphatic
acyclic secondary alcohols and ketones are summarized in Table 4 and
described below, together with some further studies. There were no
adequate studies available on the related esters.
Acetone
In a 14-day study, groups of five male and five female Fischer
344/N rats were given drinking-water ad libitum containing acetone
at concentrations of 0, 5000, 10 000, 20 000, 50 000, or 100 000 mg/kg
(equivalent to 0, 500, 1000, 2000, 5000, and 10 000 mg/kg bw per day).
Water consumption was depressed in females at 2000 mg/kg bw per day
and in all animals at 5000 or 10 000 mg/kg bw per day, but this was
not accompanied by signs of dehydration. The body weights of males at
5000 or 10 000 mg/kg bw per day and females at 10 000 mg/kg bw per day
were depressed. Statistically significant increases in relative kidney
and liver weights were observed n both male and female rats at a dose
of 2000 or 5000 mg/kg bw per day. In addition, statistically
significant increases in relative testis weight were observed at 5000
mg/kg bw per day but not at 10 000 mg/kg bw per day. No
histopathological abnormalities were observed in rats at any dose
tested (Dietz, 1991).
Groups of five male and five female B6C3F1 mice were exposed to
acetone in drinking-water ad libitum at concentrations of 0, 5000,
10 000, 20 000, 50 000, or 100 000 mg/kg (equivalent to 0, 1250, 2500,
5000, 12 500, and 25 000 mg/kg bw per day) for 14 days. Water
consumption was depressed at 12 500 or 25 000 mg/kg bw per day as
compared with controls. Growth retardation was seen in males and
females at 25 000 mg/kg bw per day but was more pronounced in males.
Statistically significant increases in relative kidney weights were
seen in both males and females at 12 500 or 25 000 mg/kg bw per day,
and statistically significant changes in relative liver weights were
observed in males at 1250, 2500, 5000, 12 500, or 25 000 mg/kg bw per
day and in females at 5000, 12 500, or 25 000 mg/kg bw per day.
Minimal to mild centrilobular hepatocellular hypertrophy was observed
at doses of 5000, 12 500, and 25 000 mg/kg bw per day in males and 12
500 and 25 000 mg/kg bw per day in females. Moderate centrilobular
hepatocellular hypertrophy was observed in males at 25 000 mg/kg bw
per day (Dietz, 1991).
In a 13-week study, groups of 10 male and 10 female rats were
given drinking-water ad libitum containing acetone at concentrations
of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg (equivalent to 0,
250, 500, 1000, 2000, and 5000 mg/kg bw per day). Water consumption
was depressed in males at 5000 mg/kg bw per day and in females at 2000
or 5000 mg/kg bw per day, but this was not accompanied by signs of
dehydration. Body-weight gain was significantly decreased in males and
females at 5000 mg/kg bw per day. Relative kidney weights were
significantly increased in both male and female rats exposed to
acetone at 5000 mg/kg bw per day and in females at 2000 mg/kg bw per
day. Increased incidences of neuropathy were seen in all males,
including the controls, but at greatest incidence and severity at the
two highest doses. Relative liver weights were significantly increased
in males and females at 2000 or 5000 mg/kg bw per day. No significant
differences in relative organ weight were observed at doses below 2000
mg/kg bw per day. At 5000 mg/kg bw per day, the relative testis
weights were significantly increased. The authors noted that such
organ weight changes are difficult to interpret because of the effect
of high doses of acetone on body weight; they therefore may not be
biologically significant. Furthermore, testicular toxicants typically
decrease testis weights. Decreased sperm motility, caudal weight, and
epididymal weight and an increased incidence of abnormal sperm were
also seen in males at 5000 mg/kg bw per day. The NOEL was 1000 mg/kg
bw per day (Dietz, 1991).
In a 13-week study, groups of 10 female and 10 male B6C3F1 mice
were given drinking-water containing acetone. Females received acetone
at concentrations of 0, 2500, 5000, 10 000, 20 000, or 50 000 mg/kg,
equivalent to 0, 625, 1250, 2500, 5000, or 12 500 mg/kg bw per day.
Males received acetone at a concentration of 0, 1250, 2500, 5000,
10 000, or 20 000 mg/kg, equivalent to 0, 313, 625, 1250, 2500, and
5000 mg/kg bw per day. The fluid intake of all females was depressed
as compared with controls. Absolute and relative liver weights and
absolute and relative spleen weights were significantly increased in
female mice at 12 500 mg/kg bw per day. Centrilobular hepatocellular
hypertrophy of minimal severity was seen in two female mice receiving
12 500 mg/kg bw per day, but no compound-related lesions were found in
male mice. The NOEL was 2500 mg/kg bw per day (Dietz, 1991).
Acetone was administered to groups of 30 male and 30 female
Sprague-Dawley rats by gavage for 90 days at doses of 100, 500, or
2500 mg/kg bw per day. Female rats at the two highest doses had
increased absolute kidney weights, and relative weight increases were
seen in the kidney, liver, and brain of both males and females at the
highest dose. Red blood cell parameters were significantly increased
in males at 45 days and animals of each sex at 90 days, at the highest
dose. Histopathological examination showed a significant increase in
tubular degeneration and hyalin droplet accumulation in males at
500 mg/kg bw per day and in animals of each sex at 2500 mg/kg bw per
day. Histolopathological examination of the liver and kidneys revealed
no treatment-dependent abnormalities. The NOEL was 100 mg/kg bw per
day (Sonawane et al., 1986).
Isopropyl alcohol
The potential toxicity of isopropyl alcohol was investigated in a
12-week study with 110 three-month-old male Wistar rats given 0, 1, 2,
3, or 5% in their drinking-water for the duration of the study. The
highest concentration was lowered to 4% during week 2 because of
unpalatability. The average daily intakes were reported to be 0, 870,
1300, 1700, and 2500 mg/kg bw. Body weights were statistically
significantly decreased in rats at 1700 or 2500 mg/kg bw per day.
Statistically significant, dose-related changes in relative liver
weights were observed at the three highest doses; significant
increases in testis weights were observed at 2500 mg/kg bw per day;
significant increases in relative kidney weights were seen at the
three highest doses; and statistically significant increases in
relative adrenal weights were observed at the two highest doses.
Increased formation of hyaline casts and increased hyaline droplet
content were observed in the proximal tubules of the kidneys in a
dose-dependent fashion. The NOEL was 870 mg/kg bw per day (Pilegaard &
Ladefoged, 1993).
Isopropyl alcohol was ingested by groups of eight adult men at
doses of 0, 2.6, or 6.4 mg/kg bw per day for six weeks. No
significant changes were observed in the chemical or cellular
composition of blood or urine, in the ability of the liver to excrete
sulfobromophthalein, in the optical properties of the eye, or in the
general well-being of the volunteers (Wills et al., 1969).
2-Heptanone
Groups of 15 male and 15 female CFE rats were given 2-heptanone
by gavage for 13 weeks at doses of 0, 20, 100, or 500 mg/kg bw per
day. In addition, groups of five male and five female rats received
doses of 0, 100, or 500 mg/kg bw per day for two and six weeks,
respectively. At week 13, the absolute and relative liver weights were
significantly increased in animals of each sex at 500 mg/kg bw per
day, and the absolute and relative kidney weights were significantly
increased in male rats at this dose; however, histological examination
showed no treatment-related damage to the liver or kidney. Significant
increases in the excretion of cells in the urine and enlarged kidneys
were observed in animals at 100 and 500 mg/kg bw per day. As no
abnormal function was detected in concentration tests, and there was
no histopathological damage in the kidneys, the significance of the
excretion of cells in the urine is unclear. The NOEL was 20 mg/kg bw
per day (Gaunt et al., 1972).
In a study of the role of the chain length of aliphatic ketones
in the causation of central and peripheral distal axonopathy, five
Sprague-Dawley rats of unspecified sex were given 0.5% 2-heptanone
(500 mg/kg bw per day) in their drinking-water for 12 weeks. The
animals were observed for hindlimb weakness, and pathological
examinations were conducted on tissues removed from the nervous
system. No giant axonal swelling or secondary myelin changes were
observed in treated animals (Spencer et al., 1978).
3-Heptanone
3-Heptanone, the only ketone in the group that is able to form a
gamma-diketone by (omega-1)-oxidation, was also the only ketone in
this group that caused axonal neuropathy in test animals. In a 14-week
study of the neurotoxic effects of oral exposure to 3-heptanone, 44
male rats were dosed by gavage on five days a week for the duration of
the study with 250, 500, 1000, 2000, or 4000 mg/kg bw per day of
3-heptanone alone; 3-heptanone followed by 1500 mg/kg bw per day
methyl ethyl ketone; 750 mg/kg bw per day methyl ethyl ketone; or 1500
mg/kg bw per day 5-methyl-2-octanone. Animals in many treated groups
developed central nervous system depression or narcosis, and those
given 2000 or 4000 mg/kg bw per day 3-heptanone in combination with
methyl ethyl ketone or 5-methyl-2-octanone died after one or two
doses. Histopathological analysis revealed central-peripheral distal
axonopathy characterized by 'giant' axonal swelling and
neurofilamentous hyperplasia in rats at 2000 mg/kg bw per day
3-heptanone. It was concluded that 3-heptanone has low neurotoxic
potential, as oral doses of < 1000 mg/kg bw per day did not induce
axonal neuropathy or the associated clinical signs. The NOEL was 1000
mg/kg bw per day (O'Donoghue et al., 1984).
4-Heptanone
4-Heptanone was administered to eight male Charles River rats by
gavage for 90 days at a dose of 2000 mg/kg bw per day, determined in a
previous study of acute toxicity to be the maximum tolerated dose
(Krasavage & O'Donoghue, 1979). After one week, however, four of the
rats had died, and the dose was reduced to 1000 mg/kg bw per day. The
mean relative liver weight was statistically significantly greater
than that of controls. Statistically significant increases in the
relative weights of the kidneys, adrenal glands, and testes were also
observed. Histopathological examination revealed hepatocyte
hypertrophy, adrenal gland congestion, and macroscopically enlarged
livers (O'Donoghue & Krasavage, 1980).
2,6-Dimethyl-4-heptanone
2,6-Dimethyl-4-heptanone (purity, 67%) was administered to eight
male Charles River rats by gavage for 90 days at 0 or 2000 mg/kg bw
per day, which was determined in a previous study to be the maximum
tolerated dose (Krasavage & O'Donoghue, 1979). The absolute and
relative liver weights, the relative kidney weights, and the absolute
and relative adrenal gland weights were statistically significantly
greater than those of controls; the absolute (but not the relative)
weights of the brain and heart were significantly depressed.
Histopathological examination revealed compound-related changes in the
stomach, liver, and kidneys. The stomachs of all animals showed
hyperkeratosis with or without pseudoepitheliomatous hyperplasia
associated with irritation due to direct contact with the solvent. In
the liver, minor or moderate hepatocyte hypertrophy was observed. In
the kidney, hyalin droplet formation was present in the proximal
tubular epithelium, with sporadic occurrence of regenerating tubular
epithelium and tubular dilation with casts (O'Donoghue & Krasavage,
1980).
2.3.2.3 Genotoxicity
Seven representative aliphatic acyclic secondary alcohols,
ketones, and related esters in this group have been tested for
genotoxicity. The results are summarized in Table 5 and described
below.
Table 4. Short-term studies of the toxicity of saturated aliphatic acyclic secondary alcohols and ketones
Substance No. Species Sex Test Route Time NOEL Reference
groupsa (mg/kg bw
per day)
Acetone 139 Rat Male/female 5/20 Drinking-water 91 days 1000 Dietz (1991)
Acetone 139 Mouse Male/female 5/20 Drinking-water 91 days 2500 Dietz (1991)
Acetone 139 Rat Male/female 3/60 Gavage 90 days 100 Sonawaneet al. (1986)
Isopropyl alcohol 277 Human Male 2/8 Oral 6 weeks 6.4b Wills et al. (1969)
Isopropyl alcohol 277 Rat Male 4/22 Drinking-water 90 days 870 Pilegaard &
Ladefoged (1993)
2-Heptanone 283 Rat Male/female 3/30 Gavage 91 days 20 Gaunt et al. (1972)
3-Heptanone 285 Rat Male 5/9 Gavage 14 weeks 1000 O'Donoghue et al.
(1984)
4-Heptanone 287 Rat Male 1/8 Gavage 90 days < 1000c O'Donoghue &
Krasavage (1980)
2,6-Dimethyl-4-heptanone 302 Rat Male 1/8 Gavage 90 days < 2000c O'Donohgue &
Krasavage (1980)
a No. of test groups/No. of animals per group
b This study was performed at either a single dose or multiple doses that induced no adverse effects. Therefore, this dose is not a true NOEL
but is the highest dose tested that produced no adverse effects. The actual NOEL would be higher.
c No NOEL established
Table 5. Results of assays for the genotoxicity of saturated aliphatic secondary alcohols, ketones, and related esters
Compound No. End-point Test object Concentration Result Reference
Acetone 139 rec Gene mutation B. subtilis NR Negativea Kawachi et al. (1980)
rec Gene mutation B. subtilis NR Negative Ishizaki et al.
(1979)
Reverse mutation S. typhimurium TA100 0.1-1000 µg/plate Negative Rapson et al.
(1980)
Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutation S. typhimurium TA98, NR Negativea Kawachi et al. (1980)
TA100
Reverse mutationb S. typhimurium TA98, 30 µl/plate Negativec Yamaguchi (1985)
TA100 (24 mg/plate)
Reverse mutation S. typhimurium TA97, < 10 000 µg/plate Negativea McCann et al. (1975)
TA98, TA100, TA1535,
TA1537
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA1535,
TA1537
Reverse mutation S. typhimurium TA100 500 µg/plate Negativea Yamaguchi (1982)
Sister chromatid exchange Human embryo fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Hamster lung fibroblasts NR Negativec Kawachi et al. (1980)
Sister chromatid exchange Chinese hamster ovary < 10 µg/ml Negative Sasaki et al. (1980)
cells
Sister chromatid exchange Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Sister chromatid exchange Diploid human fibroblasts 5 µg/ml Negative Sasaki et al. (1980)
Sister chromatid exchange Human lymphocytes 6.8 mmol/plate Negative Norppa et al. (1983)
(395 µg/ml)
Chromosomal aberration Chinese hamster ovary < 5020 µg/ml Negativea Loveday et al. (1990)
cells
Chromosomal aberration Hamster lung fibroblasts NR Positivec Kawachi et al. (1980)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
Isopropyl alcohol 277 Reverse mutation S. typhimurium TA98, 3 µmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537 (174 µg/plate)
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538, E. coli WP2uvrA
Reverse mutationb S. typhimurium TA97, < 10 mg/plated Negativea Zeiger et al. (1992)
TA98, TA102, TA104,
TA1535, TA100, TA1537
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Chemical Manufacturers'
cells, hprt locus Association (1990)
Forward mutation Chinese hamster ovary 0.5-5 mg/ml Negativea Kapp et al. (1993)
cells, hprt locus
2-Butanone 278 Reverse mutation S. typhimurium TA98, 10 000 µg/plate Negativea Douglas et al. (1980)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA102, 1 mg/plate Negative Marnett et al.(1985)
TA104
Reverse mutationb S. typhimurium TA98, 5-5000 µg/plate Negativea Shimizu et al. (1985)
TA100, TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium TA98, 0.05-32 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.04-26 µg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, < 10 000 µg/plate Negativea Zeiger et al. (1992)
TA98, TA100, TA104,
TA1535, TA1537
Reverse mutation S. typhimurium TA102 5000 µg/plate Negativec Müller et al. (1993)
Reverse mutation S. typhimurium TA98, 4000 µg/plate Negativee Brooks et al. (1988)
TA100, TA1535, TA1537,
TA1538; E. coli WP2uvrA
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.67-12 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.54-10 mg/ml)
Table 5. (continued)
Compound No. End-point Test object Concentration Result Reference
2-Butanone (contd) 278 Unscheduled DNA Human lymphocytes 0.01 mol/L Negativea Perocco et al. (1983)
synthesis (0.72 mg/ml)
Unscheduled DNA Rat hepatocytes 0.1-5 µl/ml Negative O'Donoghue et al. (1988)
synthesis (7.2-360 mg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary cells 1000 µg/ml Negativea Brooks et al. (1988)
4-Methyl-2-pentanone 301 Reverse mutation S. typhimurium TA98, 0.04-4 µl/plate Negativea O'Donoghue et al. (1988)
TA100, TA1535, TA1537, (0.03-3 mg/plate)
TA1538
Reverse mutationb S. typhimurium TA97, TA98, < 6667 µg/plate Negativea Zeiger et al. (1992)
TA100, TA1535
Reverse mutation E. coli WP2uvrA 8000 µg/plate Negativec Brooks et al. (1988)
Gene conversion S. cerevisiae 5 mg/ml Negativea Brooks et al. (1988)
Forward mutation L5178Y/tk+/- mouse 0.32-4.2 µl/ml Negativea O'Donoghue et al. (1988)
lymphoma cells (0.26-3.4 mg/ml)
Unscheduled DNA Rat hepatocytes 0.01-1 µl/ml Negative O'Donoghue et al. (1988)
synthesis (8-80 µg/ml)
Chromosomal aberration Rat hepatocytes 1000 µg/ml Negative Brooks et al. (1988)
Chromosomal aberration Chinese hamster ovary 1000 µg/ml Negativea Brooks et al. (1988)
cells
2,6-Dimethyl- 302 Reverse mutationb S. typhimurium TA98, 1-333 µg/plate Negativea Mortelmans et al. (1986)
4-heptanone TA100, TA1535, TA1537
Isopropyl acetate 305 Reverse mutationb S. typhimurium TA97, TA98, < 10 mg/plate Negativea Zeiger et al. (1992)
TA100, TA1537, TA1538
Isopropyl myristate 311 Reverse mutation S. typhimurium TA98, TA100, 50 µg/plate Negativea Blevins & Taylor
(spot test) TA1535, TA1537, TA1538 (1982)
a With and without metabolic activation
b Modified (pre-incubation) protocol
c Without metabolic activation
d Maximum non-toxic dose
e With metabolic activation
In vitro
No evidence of mutagenicity was reported for eight aliphatic
acyclic secondary alcohols, ketones, and related esters in multiple
assays in the standard Ames or preincubation protocol with
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA104,
TA1535, TA1537, and TA1538 or Escherichia coli strain WP2 uvrA,
with or without metabolic activation (McCann et al., 1975; Douglas et
al., 1980; Florin et al., 1980; Kawachi et al., 1980; Rapson et al.,
1980; Blevins & Taylor, 1982; Yamaguchi, 1982; Marnett et al., 1985;
Shimizu et al., 1985; Yamaguchi, 1985; Mortelmans et al., 1986; Brooks
et al., 1988; O'Donoghue et al., 1988; Zeiger et al., 1992; Müller et
al., 1993). The concentrations tested ranged from 0.1 to 10 000
µg/plate and often approached cytotoxic levels. The results of other
bacterial assays, such as the rec assay in Bacillus subtilis and
the E. coli reversion assay also indicated that saturated aliphatic
secondary alcohols are not mutagenic (Ishizaki et al., 1979; Kawachi
et al., 1980). In the yeast Saccharomyces cerevisiae, 2-butanone and
methyl isobutylketone did not induce gene conversion at concentrations
up to 5 mg/ml either with or without metabolic activation (Brooks
et al., 1988).
In the L5178Y/ tk+/- mouse lymphoma assay, 2-butanone was not
mutagenic when tested with and without metabolic activation at 11
concentrations ranging from 0.67 µl/ml (non-toxic) to 12 µl/ml (100%
toxic) (O'Donoghue et al., 1988). In the same study,
4-methyl-2-pentanone was not mutagenic at 10 concentrations ranging
from 0.6 µl/ml (non-toxic) to 3.7 µl/ml (100% toxic) with and without
metabolic activation, even though equivocal, non-dose-related
responses were observed at high concentrations without activation.
Isopropyl alcohol was tested at eight concentrations ranging between
0.5 and 5 mg/ml with and without metabolic activation in an assay for
forward mutation at the hypoxanthine-guanine phosphoribosyl
transferase (hprt) locus in Chinese hamster ovary cells (Chemical
Manufacturers' Association, 1990). Statistically significantly
increased mutant frequencies were seen in two of 34 treated cultures,
but these were not dose-related and not reproducible in subsequent
trials (Kapp et al., 1993).
In standard assays for cytogeneticity, acetone did not increase
the frequency of sister chromatid exchange in Chinese hamster ovary
cells (Loveday et al., 1990), hamster lung fibroblasts, or Don-6
cells, (Kawachi et al., 1980; Sasaki et al., 1980) and did not
increase the frequency of sister chromatid exchange in human
lymphocytes (Norppa et al., 1983) with and without metabolic
activation at concentrations up to 5000 µg/ml (Loveday et al., 1990).
Acetone did not induce chromosomal aberrations in Chinese hamster
ovary cells at concentrations up to a maximum of 5000 µg/ml (Loveday
et al., 1990). In a test conducted at an unspecified concentration,
acetone induced chromosomal aberrations in hamster lung fibroblasts
(Kawachi et al., 1980), whereas 2-butanone and methyl isobutylketone
gave negative results in assays in rat hepatocytes and Chinese hamster
ovary cells when tested at concentrations up to 1 mg/ml (Brooks et
al., 1988).
2-Butanone did not induce unscheduled DNA synthesis in human
lymphocytes treated with concentrations up to 0.01 mol/L, with and
without metabolic activation (Perocco et al., 1983). 2-Butanone and
4-methyl-2-pentanone did not induce unscheduled DNA synthesis in rat
hepatocytes when tested at concentrations up to 5 and 1 µl/ml,
respectively (O'Donoghue et al., 1988).
In vivo
Acetone, isopropyl alcohol, 2-butanone, and methyl-2-pentanone
did not induce micronucleus formation at doses of 400-2500 mg/kg bw
(Basler, 1986; O'Donoghue et al., 1988; Kapp et al., 1993), which
approximated the LD20 (O'Donoghue et al., 1988) and the LD50 (Basler,
1986) of the tested compounds.
2.3.2.4 Other relevant studies
Isopropyl alcohol
The effects of long-term consumption of isopropyl alcohol on
gonad function and embryonic and perinatal development were studied
during an investigation to establish the maximum permissible
concentrations of isopropyl alcohol in water bodies. The compound was
administered for 20 days to 869 inbred white rats and for 45 days to
488 rats which had progeny at doses of 1008 and 252 mg/kg bw per day,
respectively; for three months at a dose of 1800 mg/kg bw per day; and
for six months at doses of 18, 1.8, or 0.18 mg/kg bw per day. The
embryos were examined macroanatomically on day 21 of gestation to
determine embryotoxicity, and the offspring of treated male and female
rats mated with treated or untreated controls were observed for
developmental abnormalities.
Dams given 252 or 1008 mg/kg bw per day isopropyl alcohol before
the onset of pregnancy had a significantly reduced number of embryos,
but a similar effect was not seen in those given 1800 mg/kg bw per
day. Total embryonic mortality was significantly increased only in
dams given 1008 mg/kg bw per day; however, no essential differences in
embryonic development were noted. The reproductive function of females
receiving isopropyl alcohol for six months was adversely affected. The
percentage mortality of the progeny of treated male and female
pairings and treated male and control female pairings reached a
significant level only in the groups receiving 18 mg/kg bw per day for
six months. The weights of the progeny of treated males and females
were also significantly reduced. The authors concluded that long-term
intake of isopropyl alcohol can affect generative function, however
only at concentrations that far exceed the current maximum allowable
concentration of isopropyl alcohol (0.25 mg/litre) in water supplies
(Antonova & Salmina, 1978).
Groups of 25 timed-pregnant Sprague-Dawley rats were given 0,
400, 800, or 1200 mg/kg bw per day isopropyl alcohol by gavage on days
6-15 of gestation. Deaths were observed in the groups at 800 mg/kg bw
(4%) and 1200 mg/kg bw (8%) per day. Reduced maternal gestational
weight gain on days 0-20 associated with significantly reduced gravid
uterine weights were noted at 800 and 1200 mg/kg bw per day. There
were no adverse maternal or developmental effects at 400 mg/kg bw per
day and no teratogenic effects at any dose (Tyl et al., 1994).
Groups of 15 artificially inseminated New Zealand white rabbits
were given isopropyl alcohol at 0, 120, 240, or 480 mg/kg bw per day
by gavage on days 6-18 of gestation. Four does at 480 mg/kg bw per day
died, and severe clinical signs of toxicity and statistically
significantly reduced food consumption were observed. No
treatment-related maternal or developmental effects were observed at
120 or 240 mg/kg bw per day, and no teratogenicity was seen at any
dose (Tyl et al., 1994).
Rats were exposed to isopropyl alcohol in the drinking-water for
three generations, with average intakes of 1470, 1380, and 1290 mg/kg
bw per day for the first, second, and third generations, respectively.
The growth of the first generation was retarded initially but had
returned to normal by the 13th week. There were no other effects on
growth and no effects on reproduction (Lehman et al., 1945).
Methyl ethyl ketone
Groups of 10 virgin and 30 plug-positive female Swiss CD-1 mice
were exposed to 0, 400, 1000, or 3000 mg/kg methyl ethyl ketone
vapours for 10 consecutive days. The pregnant mice appeared to be
relatively insensitive, but the offspring of mice at the highest dose
showed significant signs of toxicity. No maternal or developmental
toxicity was observed at doses < 1000 mg/kg vapour (Mast et al.,
1989).
4. REFERENCES
Antonova, V.I. & Salmina, Z.A. (1978) [Maximum permissible
concentration of isopropyl alcohol in water bodies with regard to its
actions on gonads and progeny.] Gig. Sanit., 1, 8-11 (in Russian).
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Intermediary Xenobiotic Metabolism in Animals,
pp. 81-97.
Basler, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat. Res., 174, 11-13.
Blevins, R.D. & Taylor, D.E. (1982) Mutagenicity screening of
twenty-five cosmetic ingredients with the Salmonella/microsome test.
J. Environ. Sci. Health, A17, 217-239.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, Vol. 1, New York, Academic
Press, p. 231.
Brooks T.M., Meyer, A.L. & Hutson, D.H. (1988) The genetic toxicology
of some hydrocarbon and oxygenated solvents. Mutagenesis, 3,
227-232.
Brown, W.D., Setzer, J.V., Dick, R.B., Phipps, F.C. & Lowry, L.K.
(1987) Body burden profiles of single and mixed solvent exposures.
J. Occup. Med. , 29, 877-883.
Chemical Manufacturers' Association (1990) Unpublished submission to
the United States Environmental Protection Agency.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dietz D. (1991) Toxicity studies of acetone in F344/N rats and B6C3F1
mice (drinking water studies), National Toxicology Program (NIH
Publication No. 91-3122), Bethesda, Maryland, United States.
Dietz, F.K., Rodriguez-Giaxola, M., Traiger, G.J., Stella, V.J. &
Himmelstein, K.J. (1981) Pharmacokinetics of 2-butanol and its
metabolites in the rat. J. Pharmacokinet. Biopharm., 9, 553-576.
Douglas, G.R., Nestmann, E.R., Betts, J.L., Mueller, J.C., Lee, E.G.-
H., Stich, H.F., San, R.H.C., Brouzes, R.J.P., Chmelauskas, A.L.,
Paavila, H.D. & Walden, C.C. (1980) Mutagenic activity in pulp mill
effluents. In: Jolley, R.L. et al., eds, Water Chlorination:
Environmental Impact and Health Effects, Ann Arbor, Michigan, Ann
Arbor Science Publishers, Vol. 3.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, New York, Academic
Press, Vol. I, pp. 281-293.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 18, 219-232.
Gaunt, I.F., Carpanini, F.M.B., Wright, M.G., Grasso, P. & Gangolli,
S.D. (1972) Short-term toxicity of methyl amyl ketone in rats. Food
Cosmetic Toxicol., 10, 625-636.
Haggard, H.W., Miller, D.P. & Greenberg, L.A. (1945) The amyl alcohols
and their ketones: Their metabolic fates and comparative toxicities.
J. Ind. Hyg. Toxicol., 27, 1-14.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, Vol. I, pp. 291-323.
Homan, E.R. & Maronpot, R.R. (1978) Neurotoxic evaluation of some
aliphatic ketones (Abstract). Toxicol. Appl. Pharmacol., 45, 312.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Ishizaki, M., Oyamada, N., Ueno, S., Katsumura, K. & Hosogai, Y.
(1979) Mutagenicity of degradation and reaction products of butyl
hydroxy anisol with sodium nitrite or potassium nitrate by irradiation
of ultra violet ray. J. Food Hyg. Soc. Jpn, 20, 143-146.
Kamil, I.A., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; The glucuronic acid conjugation of acyclic
aliphatic alcohols . J. Biochem., 53, 129-136.
Kapp, R.W., Marino, D.J., Gardiner, T.H., Maston, L.W., McKee, R.H.,
Tyler, T.R., Ivett, J.L. & Young, R.R. (1993) In vitro and in vivo
assays of isopropanol for mutagenicity. Environ. Mol. Mutag., 22,
93-100.
Katz, G.V., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J. (1980)
Comparative neurotoxicity and metabolism of ethyl n-butyl ketone and
methyl n-butyl ketone in rats. Toxicol. Appl. Pharmacol., 52,
153-158.
Kawachi, T., Komatsu, T., Kada, T., Ishidate, M., Masamichi, S.,
Sugiyama, T. & Tazima, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In:
Williams, G.M. et al., eds, The Predictive Value of Short-term
Screening Tests in Carcinogenicity Evaluation, Amsterdam,
Elsevier/North-Holland Biomedical Press.
Krasavage, W.J. & O'Donoghue, J.L. (1979) Repeated oral administration
of five ketones and n-heptane to rats. Unpublished report. Submitted
to WHO by the Flavor and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Krasavage, W.J., O'Donoghue, J.L., DiVincenzo, G.D. & Terhaar, C.J.
(1980) The relative neurotoxicity of methyl-n-butyl ketone, n-hexane
and their metabolites. Toxicol. Appl. Pharmacol., 53, 433-441.
Krasavage, W.J., O'Donoghue, J.L. & DiVincenzo, G.D. (1982) Ketones.
In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 3rd Ed., New York, John Wiley & Sons, Vol. IIC, pp.
4720-4727.
Lehman, A.J., Schwerma, H. & Rickards, E. (1945) Isopropyl alcohol:
Acquired tolerance in dogs, rate of disappearance from the blood
stream in various species and effects on successive generation of
rats. J. Pharmacol. Exp. Therap., 85, 61-69.
Leibman, K.C. (1971) Reduction of ketones in liver cytosol.
Xenobiotica, 1, 97-104.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E., eds, Patty's Industrial Hygiene and Toxicology, 4th
Ed., New York, John Wiley & Sons, pp. 2585-2760.
Loveday, K.S., Anderson, B.E., Resnick, M.A. & Zeiger, E. (1990)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. V: Results with 46 chemicals. Environ.
Mol. Mutag., 16, 272-303.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Marnett, L.J., Hurd, H.K., Hollstein, M.C., Levin, D.E., Esterbauer,
H. & Ames, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA104. Mutat. Res., 148, 25-34.
Mast, T.J., Dill, J.A., Evanoff, J.J., Rommereim, R.L., Weigel, R.J. &
Westerberg, R.B. (1989) Inhalation developmental toxicology studies:
Teratology study of methyl ethyl ketone in mice. National Toxicology
Program (No. NIH-Y01-ES-70153), Bethesda, Maryland, United States.
McCann, J., Choi, E., Yamasaki, E. & Ames, B.N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay of 300
chemicals. Proc. Natl Acad. Sci. USA, 72, 5135-5139.
Morgott, D.A. (1993) Acetone. In: Clayton, G.D. & Clayton, F.E., eds,
Patty's Industrial Hygiene and Toxicology, 4th Ed., New York, John
Wiley & Sons, pp. 149-281.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Müller, W., Engelhart, G., Herbold, B., Jäckh, R. & Jung, R. (1993)
Evaluation of mutagenicity testing with Salmonella typhimurium TA102
in three different laboratories. Environ. Health Perspectives,
Suppl. 101, 33-36.
National Academy of Sciences (1989) 1989 Poundage and Technical
Effects. Update of Substances Added to Food, Washington DC.
Neubreuer, O. (1901) Concerning the pairing of glucuronic acid with
materials of the aliphatic series. Arch. Exp. Pathol. Pharmacol.,
46, 133-154.
Norppa, H., Vainio, H. & Sorsa, M. (1983) Metabolic activation of
styrene by erythrocytes detected as increased sister chromatid
exchanges in cultured human lymphocytes. Cancer Res. 43, 3579-3582.
O'Donohogue, J.L. & Krasavage, W.J. (1980) 90-day repeated oral
administration of five ketones and n-heptane to rats. Unpublished
report. Submitted to WHO by the Flavor and Extract Manufacturers'
Association of the United States, Washington DC, United States.
O'Donoghue, J.L., Krasavage, W.J., DiVincenzo, G.D. & Katz, G.V.
(1984) Further studies on ketone neurotoxicity and interactions.
Toxicol. Appl. Pharmacol., 72, 201-209.
O'Donoghue, J.L., Haworth, S.R., Curren, R.D., Kirby, P.E., Lawlor,
T., Moran, E.J., Phillips, R.D., Putnam, D.L., Rogers-Back, A.M.,
Slesinski, R.S. & Thilagar, A. (1988) Mutagenicity studies on ketone
solvents: Methyl ethyl ketone, methyl isobutyl ketone, and isophorone.
Mutat. Res., 206, 149-161.
Perocco, P., Bolognesi, S. & Alberghini, W. (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol. Lett., 16, 69-75.
Pilegaard, K. & Ladefoged, O. (1993) Toxic effects in rats of twelve
weeks' dosing of 2-propanol, and neurotoxicity measured by
densitometric measurements of glial fibrillary acidic protein in the
dorsal hippocampus. In Vivo, 7, 325-330.
Rapson, W.H., Nazar, M.A. & Butzky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ.
Contam. Toxicol., 24, 590-596.
Saito, M. (1975) Studies on the metabolism of lower alcohols
(Abstract). Nichidai Igaku Zasshi, 34, 569-585.
Sasaki, M., Sugimura, K., Yoshida, M.A. & Abe, S. (1980) Cytogenetic
effects of 60 chemicals on cultured human and Chinese hamster cells.
Kromosomo II, 20, 574-584.
Schwartz, L. (1989) [On the oxidation of acetones and homologous
ketones from fatty acids.] Arch. Exp. Pathol. Pharmacol., 40, 168
(in German).
Scopinaro, D., Ghiani, P. & De Cecco, A. (1947) [Ketolytic fate of
acetone. II. Acetone metabolism in normal subjects.] Policlinico
(Rome) Sez, Med., 54, 70 (in Italian).
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Sonawane, B., de Rosa, C., Rubenstein, R., Mayhew, D., Becker, S.V. &
Dietz, D. (1986) Estimation of reference dose (RfD) for oral exposure
to acetone (Abstract). J. Am. Coll. Toxicol.., 5, 605.
Spencer, P.S., Bischoff, M.C. & Schaumburg, H.H. (1978) On the
specific molecular configuration of neurotoxic aliphatic hexacarbon
compounds causing central-peripheral distal axonopathy. Toxicol.
Appl. Pharmacol., 44, 17-28.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tanii, H., Tsuji, H. & Hashimoto, K. (1986) Structure-toxicity
relationship of monoketones. Toxicol. Lett., 30, 13-17.
Topping, D.C., Morgott, D.A., David, R.M. & O'Donoghue, J.L. (1994)
Ketones. In: Clayton, G.D. & Clayton, F.E., eds, Patty's Industrial
Hygiene and Toxicology, 4th Ed., New York, John Wiley & Sons, pp.
1739-1878.
Tyl, R.W., Masten, L.W., Marr, M.C., Myers, C.B., Slauter, R.W.,
Gardiner, T.H., Strother, D.E., McKee, R.H. & Tyler, T.R. (1994)
Developmental toxicity evaluation of isopropanol by gavage in rats and
rabbits. Fundam. Appl. Toxicol., 22, 139-151.
Wills, J.H., Jameson, E.M. & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15, 560-565.
Yamaguchi, T. (1982) Mutagenicity of trioses and methyl glyoxal on
Salmonella typhimurium. Agric. Biol. Chem. 46, 849-851.
Yamaguchi, T. (1985) Stimulating effects of organic solvents on the
mutagenicities of sugar-degradation compounds. Agric. Biol. Chem.
49, 3363-3368.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
