
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
CARVONE AND STRUCTURALLY RELATED SUBSTANCES
First draft prepared by
Dr P.J. Abbott
Australia New Zealand Food Authority, Canberra, Australia
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of the Procedure for the Safety Evaluation
of Flavouring Agents
Consideration of combined intakes from use as
flavouring agents
Conclusions
Relevant background information
Biological data
Absorption and metabolism
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity and
carcinogenicity
Genotoxicity
Other relevant studies
References
1. EVALUATION
1.1 Introduction
The Committee evaluated the safety of carvone and eight related
substances using the Procedure for the Safety Evaluation of Flavouring
Agents (Figure 1, p. 222, and Annex 1, reference 131). The substances
in this group are terpenoid ketones, secondary alcohols, and related
esters containing a 2-menthyl carbon skeleton (Table 1).
The Committee previously evaluated carvone on several occasions.
A conditional ADI of 0-1.25 mg/kg bw for the (+) and (-) isomers was
established at the eleventh meeting (Annex 1, reference 14). A
temporary ADI of 0-1 mg/kg bw was established for (+)- and (-)-carvone
at the twenty-third meeting (Annex 1, reference 50), which was
extended at the twenty-fifth, twenty-seventh, thirtieth, and
thirty-third meetings (Annex 1, references 55, 62, 73, and 83). At its
thirty-seventh meeting, the Committee determined that the (+) and (-)
enantiomers should be evaluated separately. Owing to lack of data on
(-)-carvone per se, the temporary ADI for (-)-carvone was not
extended. In its review of (+)-carvone, the Committee considered a
long-term study of toxicity and carcinogenicity in mice, short-term
studies of toxicity in mice and rats, and tests for mutagenicity
in vitro. On the basis of a NOEL of 93 mg/kg bw per day in a
three-month study of toxicity in rats, the Committee established an
ADI for (+)-carvone of 0-1 mg/kg bw per day.
Table 1. Summary of the safety evaluation of carvone and structurally related substances used as flavouring agents
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
para-Menthan-2-one 375 499-70-7 0.01/1 II Yes No - - No safety
concern
 para-Menthan-2-ol 376 499-69-4 0.01/7 I Yes No - - No safety
concern
para-Menthan-2-ol 376 499-69-4 0.01/7 I Yes No - - No safety
concern
 Dihydrocarvone 377 7764-50-3 0.02/180 II Yes No - - No safety
concern
Dihydrocarvone 377 7764-50-3 0.02/180 II Yes No - - No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Dihydrocarveol 378 619-01-2 3/320 I Yes No - - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Dihydrocarveol 378 619-01-2 3/320 I Yes No - - No safety
concern
 Dihydrocarvyl 379 20777-49-5 15/0.1 I Yes No - - No safety
acetate concern
Dihydrocarvyl 379 20777-49-5 15/0.1 I Yes No - - No safety
acetate concern
 Carvoneb 380 2244-16-8(+) 2800/9900 II Yes Yes No Yes No safety
6485-40-1(-) concern
Carvoneb 380 2244-16-8(+) 2800/9900 II Yes Yes No Yes No safety
6485-40-1(-) concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Carveol 381 99-48-9 15/140 I Yes No - - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Carveol 381 99-48-9 15/140 I Yes No - - No safety
concern
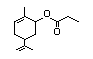
 Carvyl acetate 382 97-42-7 6/36 I Yes No - - No safety
concern
Carvyl acetate 382 97-42-7 6/36 I Yes No - - No safety
concern
 Carvyl propionate 383 97-45-0 N/D/0.04 I Yes No - - No safety
concern
Carvyl propionate 383 97-45-0 N/D/0.04 I Yes No - - No safety
concern
 a Threshold of concern is 1800 µg/day for class I and 540 µg/day for class II.
b The ADI of 0-1 mg/kg bw previously established for (+) carvone at the thirty-seventh meeting was maintained.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from surveys
in Europe (International Organization of the Flavor Industry, 1995)
and the United States (US National Academy of Sciences, 1987) (see
Table 2). The estimated total daily per capita intake of carvone
(No. 380, stereochemistry unspecified) and related substances from use
as flavouring agents is 2.8 mg/person in Europe and 10 mg/person in
the United States. Carvone accounts for approximately 99% of the total
annual per capita intake of this group of substances when used as
flavouring agents in Europe and 96% in the United States.
Seven of the substances in this group have been reported to occur
naturally in foods, including fruits, spices, and berries. (-)-Carvone
(55-75%) has been reported in the oils of Mentha (spearmint).
(+)-Carvone (20-75%) has been reported in Carum (caraway) and
Anethum (dill) (Maarse et al., 1994). Quantitative data on the
natural occurrence and consumption ratios have been reported for five
of these substances (Nos 377, 379, 380, 381, and 382), which indicate
that they are consumed predominantly in traditional foods (i.e.
consumption ratio > 1) (Stofberg & Kirschner, 1985; Stofberg &
Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are terpenoid ketones, secondary
alcohols, and related esters containing a 2-menthyl carbon skeleton.
The group consists of three ketones (Nos 375, 377, and 380), three
secondary alcohols (Nos 376, 378, and 381), and three esters (Nos 379,
382, and 383). Their structures are shown in Table 1.
Terpenoid esters
Each of the tthree esters would be expected to be hydrolysed to
its corresponding alcohol and carboxylic acid by carboxylesterases,
which predominate in hepatocytes. Esters of carveol (Nos 382 and 383)
and dihydrocarveol (No. 379) would be expected to be hydrolysed to
yield carveol (No. 381) and dihydrocarveol (No. 378), respectively,
and the corresponding saturated aliphatic carboxylic acids.
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
* reduction of the ketone, followed by conjugation of the resulting
alcohol with glucuronic acid;
* side-chain oxidation yielding polar metabolites, which may be
conjugated and excreted;
Table 2. Most recent annual usage of carvone and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
p-Menthan-2-one (375)
Europe 0.1 0.01 0.0002
United States 3 1 0.01
p-Menthan-2-ol (376)
Europe 0.1 0.01 0.0002
United States 37 7 0.1
Dihydrocarvone (377)
Europe 0.1 0.02 0.0003
United States 920 180 3
Dihydrocarveol (378)
Europe 3 1 0.01
United States 320 61 1
Dihydrocarvyl acetate (379)
Europe 80 15 0.3
United States 0.3 0.1 0.001
Carvone (380)
Europe 15 000 2800 46
United States 52 000 9900 170
Carveol (381)
Europe 78 15 0.2
United States 740 140 2
Carvyl acetate (382)
Europe 33 6 0.1
United States 190 36 1
Carvyl propionate (383)
Europe - - -
United States 0.2 0.04 0.001
a National Academy of Sciences (1987); International Organization of the
Flavouring Industry (1995)
* glutathione conjugation of ketones, followed by excretion;
* hydrogenation of the endocyclic double bond of carveol; and
* excretion of unchanged parent compound.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), six members of this
group are in class I, namely, para-menthan-2-ol (No. 376),
dihydrocarveol (No. 378), dihydrocarvyl acetate (No. 379),
carveol (No. 381), carvyl acetate (No. 382), and carvyl
propionate (No. 383), while three members are in class II
because they contain an alpha,ß-unsaturated ketone, namely,
para-menthan-2-one (No. 375), para-menth-8-en-2-one (No.
377), and carvone (No. 380).
Step 2. Sufficient data were available on carvone to define the
major pathways of metabolism. This compound contains all of
the key structural elements and potential sites of
metabolism of all other members in the group. Better data
were available on the metabolism of isophorone
(3,4,5-trimethyl-cyclohex-2-en-1-one), which is not a member
of this group of flavouring agents but which shows close
structural similarities to carvone and uses the same routes
of metabolism (reduction of the carbonyl group, conjugation
of the resulting alcohol, and side-chain oxidation). It is
predicted that carvone and other alpha,ß-unsaturated ketones
in the group would undergo glutathione conjugation, but this
would be expected to be a minor route because of the low
reactivity in vitro, a conclusion supported by the
toxicological data.
For the three terpenoid esters in class I, the most
likely route of metabolism is hydrolysis to carveol or
dihydrocarveol. For the three terpenoid alcohols, carveol,
dihydrocarveol, and para-menthan-2-ol, which are also in
class I, the available data indicate that the most likely
route of metabolism is conjugation with glucuronic acid,
followed by excretion; however, other routes of metabolism
such as side-chain oxidation followed by conjugation and
excretion may also occur. In each case, metabolism yields
innocuous metabolites, and the evaluation should proceed via
the 'A' side of the scheme.
For the three terpenoid ketones in class II, the
available data indicate that the most likely route of
metabolism is reduction of the ketone to the corresponding
alcohol, followed by conjugation with glutathione and
excretion. Other routes of metabolism may occur, such as
side-chain oxidation followed by conjugation and direct
conjugation of the ketone with glutathione. In all cases,
metabolism yields innocuous metabolites, and the evaluation
should proceed via the 'A' side of the scheme.
Step A3. The intakes of the six terpenoid esters and alcohols in
class I are below the threshold of concern for this class
(1800 µg/person per day), and they would not be expected to
be of safety concern. The intake of two of the three
terpenoid ketones in class II, para-menthan-2-one and
para-menth-8-en-2-one, is below the threshold of concern
for this class (540 µg/person per day), and these substances
would not be expected to be of safety concern. The intakes
of carvone in both Europe (2800 µg/person per day;
International Organization of the Flavor Industry, 1994) and
the United States (9900 µg/person per day; US Academy of
Sciences, 1987) are above the threshold of concern. This
substance therefore proceeds to step A4.
Step A4. Carvone is not endogenous in humans. Its safety evaluation
therefore proceeds to step A5.
Step A5. A NOEL for (+)-carvone of 93 mg/kg bw per day (three-month
study in rats) was identified by the Committee at its
thirty-seventh meeting (Annex 1, reference 94). If it is
assumed that all of the carvone consumed was (+)-carvone, a
margin of safety of > 500 exists between this NOEL and the
per capita daily intake for the (+) isomer of carvone.
Therefore, this substance would not be expected to be of
safety concern. The (-) isomer of carvone would be expected
to share a common metabolic pathway with (+)-carvone. A NOEL
of 125 mg/kg bw per day for carvone (isomer unspecified) was
identified at the eleventh meeting (Annex 1, reference 14),
and the only material in commerce at that time was
(-)-carvone. This NOEL is considered to apply to (-)-carvone
and, if it is assumed that all of the carvone consumed was
(-)-carvone, a margin of safety of > 750 exists between
this NOEL and the per capita daily intake for the (-)
isomer of carvone. Therefore, this substance would not be
expected to be of safety concern.
Table 1 summarizes the evaluations of the nine substances in this
group.
1.5 Consideration of combined intakes from use as flavouring agents
All nine substances are expected to be efficiently metabolized to
innocuous substances. In the unlikely event that carvone, which
accounts for > 95% of the total estimated dietary intake of the
group, was consumed concomitantly with the eight related substances,
the estimated combined intake would exceed the human intake threshold
for structural classes I and II, but, in the opinion of the Committee,
this would not give rise to perturbations outside the physiological
range.
1.6 Conclusions
The results of the evaluations of carvone and related substances
indicate that these substances would not present safety concerns at
the current estimated intake. In using the procedure, the Committee
noted that all of the available data on toxicity are consistent with
the results of the safety evaluation. The ADI established previously
for (+)-carvone was maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption and metabolism
Terpenoid esters
The three esters would be expected to be hydrolysed to their
corresponding alcohol and carboxylic acid by carboxylesterases, which
predominate in hepatocytes (Heymann, 1980). Esters of carveol (Nos 382
and 383) and dihydrocarveol (No. 379) would be expected to be
hydrolysed to yield carveol and dihydrocarveol, respectively, and the
corresponding saturated aliphatic carboxylic acids. Evidence that this
metabolic route is used for these esters comes from studies of related
compounds, namely, the (-)-menthol esters, (-)-menthol ethylene glycol
carbonate and (-)-menthol propylene glycol carbonate, which were
completely hydrolysed after incubation with a rat liver homogenate
(Anon., 1994). Similarly, more than 80% of radiolabelled cyclandelate,
a structurally related ester, was hydrolysed after 20 min of
incubation with rat hepatic microsomes (White et al., 1990), and
rabbits given bornyl acetate orally excreted borneol as the glucuronic
acid conjugate (Williams, 1959).
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
(i) reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid;
(ii) side-chain oxidation, yielding polar metabolites which may
be conjugated and excreted;
(iii) glutathione conjugation of ketones, followed by excretion;
(iv) hydrogenation of the endocyclic double bond of carveol; and
(v) excretion of unchanged carvone.
Evidence for each of these pathway is given below:
(i) Reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid
There is considerable evidence that ketones are reduced to the
corresponding secondary alcohol and conjugated mainly with glucuronic
acid (Fischer & Bielig, 1940). In rodents, but probably not in humans,
the conjugate is excreted primarily into the bile, where it may be
hydrolysed to yield the free alcohol (Matthews, 1994). The alcohol may
then enter enterohepatic circulation and be excreted by the kidney
(Hamalainen, 1912; Tamura et al., 1962). If a double bond is present
in the molecule, the metabolite may be hydrogenated to the dihydro
derivative (Fischer & Bielig, 1940; Madyastha & Raj, 1993).
Ketones are reduced primarily by cytosolic carbonyl reductase,
and the reaction is stereoselective to yield a mixture of
diastereomeric alcohols (Leibman & Ortiz, 1973). In experiments in
rabbits, carvone was reduced to carveol, which was converted to the
glucuronic acid conjugate and excreted in urine (Fischer & Bielig,
1940). The glucuronic acid conjugate of dihydrocarveol has been
detected in the urine of rabbits (Hamalainen, 1912).
Groups of five rats (species and sex not specified) received
carvone at 5, 25, 50, 100, or 1000 mg/kg bw in olive oil daily by
gavage for 10 days. Daily urinary elimination of glucuronic acid,
ortho-glucuronide and ascorbic acid increased at daily doses of 100
mg/kg bw (Tamura et al., 1962).
(ii) Side-chain oxidation yielding polar metabolites which may
be conjugated and excreted
Alicyclic ketones containing an alkyl or alkenyl side-chain may
undergo oxidation of the side-chain to form polar metabolites, which
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Ishida et al., 1989; Williams,
1959).
A racemic mixture of carvone was reported to undergo side-chain
oxidation in rabbits. Each of six male rabbits was given approximately
2 g (±)-carvone orally in water. Urine collected over three days was
separated into neutral, acidic, and phenolic fractions. After
hydrolysis of the glucuronic acid and sulfate conjugates,
chromatographic analysis of the neutral fraction revealed the presence
of (+)-9-hydroxycarvone (10%) (Williams, 1959; Ishida et al., 1989).
Oxidation of the side-chain has been observed for other acyclic
(Nishizawa et al., 1987) and alicyclic ketones (Williams, 1940; Nelson
et al., 1992), with cytochrome P450 acting as a catalyst in vitro
(Madyastha & Raj, 1990); however, no effects were observed on the
activity of either cytochrome P450 or cytochrome b5 in male albino
rats given (-)-carvone at 600 mg/kg bw per day orally for three days
(Moorthy et al., 1989).
(iii) Glutathione conjugation of ketones followed by excretion
In a study to examine the ability of carvone and carvyl
derivatives to induce enzymes for the detoxification of carcinogens
(Wattenberg et al., 1989; Lam & Zheng, 1991, 1992), glutathione
S-transferase activity increased and glutathione content decreased
in various tissues of groups of A/J mice given repeated oral doses of
carvone and 10 carvyl derivatives. Groups of four female A/J mice were
given three oral doses, each containing 20 mg of a carvyl derivative
in cottonseed oil, by gavage over two days. The test substances
included alpha,ß-unsaturated ketones, (+)-carvone,
8,9-dihydro-carvone, 9-hydroxycarvone, 9-acetoxycarvone, the
unconjugated ketones 2,3-dihydrocarvone and carvomenthone, and five
carvyl alcohol derivatives. Cytosolic glutathione S-transferase
activity and acid-soluble sulfhydryl levels were measured in mouse
liver, forestomach, lung, and small and large bowel mucosa. Treatment
with carvone and, to a lesser degree, other alpha,ß-unsaturated
ketones increased the activity of glutathione S-transferase in all
tissues by two to four times over that in controls and in animals
treated with other carvyl derivatives. Carvone intake was associated
with a decrease in glutathione content in the liver, lung, and
large-bowel mucosa (Zheng et al., 1992). Carvone rapidly conjugated
with glutathione in the absence of glutathione S-transferase
(Portoghese et al., 1989).
(iv) Hydrogenation of the endocyclic double bond of carveol
Carvone is also metabolized in rabbits by hydrogenation of the
endocyclic double bond to yield 8-menthen-2-ol (dihydrocarveol), which
is excreted unchanged (Fischer & Bielig, 1940).
(v) Excretion of unchanged carvone
Carvone has been detected unchanged in the urine of humans,
presumably arising from its dietary intake (Zlatkis et al., 1973).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of the acute toxicity of carvone and
related substances are shown in Table 3.
2.1.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of all short-term and long-term studies of carvone
are shown in Table 4. Details of the studies that were critical to the
safety evaluation of carvone and related substances are given below.
Table 3. Acute toxicity of carvone and related substances tested by gavage
Substance No. Species Sex LD50 Reference
(mg/kg bw)
Dihydrocarvone 377 Rat NR > 5000 Moreno (1977)
Dihydrocarveol 378 Rat NR > 5000 Moreno (1977)
Dihydrocarvyl acetate 379 Rat NR > 5000 Moreno (1980)
Carvone 380 Rat M/F 1640 Jenner et al. (1964)
Carvone 380 Rat NR 3710 Levenstein (1976)
Carvone 380 Guinea-pig M/F 766 Jenner et al. (1964)
Carveol 381 Rat NR 3000 Keating (1972)
Carvyl acetate 382 Rat NR > 5000 Levenstein (1976)
Carvyl propionate 383 Rat NR > 5000 Levenstein (1976)
NR, not reported; M/F, male and female
Table 4. Short-term and long-term studies of the toxicity of carvone
Species Sex No. groups/ Route Duration NOEL Reference
no. per group (mg/kg bw
per day)
Carvone
Rats M/F 3/10 Oral 16 weeks ND Hagan et al.
to 1 year (1967)
(-)-Carvone
Mice M/F 5/10 Gavage 16 days 328 US National
Toxicology
Program (1990)
Mice M/F 3/40 Gavage 13 weeks 375 US National
2/20 Toxicology
Program (1990)
Rats 5/5 Gavage 16 days 150 US National
Toxicology
Program (1990)
Rats 5/10 Gavage 13 weeks 93 US National
Toxicology
Program (1990)
ND, not determined; M/F, male and female
Carvone
Carvone (unspecified stereochemistry) was administered to four
male Wistar rats for 14 days at a dietary level of 0 or 1% (equivalent
to 500 mg/kg bw per day). Significant increases in serum cholesterol
and triacylglycerol concentrations were reported in rats given carvone
when compared with the controls. Significant decreases in food
consumption and body weights were also reported in treated animals
(Imaizumi et al., 1985).
In a study to examine the toxicity of a large number of
flavouring agents, groups of five male and five female Osborne-Mendel
weanling rats were fed a diet containing carvone at a concentration of
1000 mg/kg (equivalent to 50 mg/kg bw per day) for 27-28 weeks, 2500
mg/kg (equivalent to125 mg/kg bw per day) for one year, or 10 000
mg/kg (equivalent to 750 mg/kg bw per day) for 16 weeks. Although the
stereochemistry of the test material was unspecified, a survey of
industrial producers of carvone who actively marketed carvone during
the period of the study (1960-70) indicated that the material in
commerce in the United States at that time was the (-) isomer (Bauer,
1991; Flynn, 1991; Wrigley Co., 1991). Body weights and food intake
were measured weekly and haematological examinations performed at 3,
6, 12, and 22 months. Although no effects were noted, only a very
limited report of the results of this study was provided. Depressed
body-weight gain and testicular atrophy at a dose of 750 mg/kg bw per
day were the only reported effects. The NOEL was 125 mg/kg bw per day
(Hagan et al., 1967).
(-)-Carvone
Studies on this enantiomer were evaluated at the thirty-seventh
meeting (Annex 1. reference 94)
2.1.2.3 Genotoxicity
The results of studies of the genotoxicity of these substances
are shown in Table 5.
2.1.2.4 Other relevant studies
In a study to examine the anti-carcinogenic properties of
carvone, groups of 15 female A/J mice were given 0.2 mmol (30 mg)
(+)-carvone by intubation I h before administration of
N-nitrosodiethylamine at a dose of 20 mg/kg bw once a week for eight
weeks by intubation. The animals were necropsied 26 weeks after the
initial dose of nitrosamine. (+)-Carvone inhibited forestomach tumour
formation, with a > 63% reduction in the mean number of papillomas
per mouse when compared with the controls. The number of pulmonary
adenomas in mice given (+)-carvone was significantly less (34%) than
in control animals (Wattenberg et al., 1989).
Table 5. Results of assays for the genotoxicity of carvone and related substances
Substance No. End-point Test object Dose Result Reference
Carvone 380 Gene mutation S. typhimurium TA1535, 3 µmol/plate Negative Florin et al. (1980)
TA1537, TA98, TA100
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative National Toxicology
TA98, TA100, TA1537 Program (1990)
Carvone 380 rec assay Bacillus subtilis H17 (rec+) 0.6 ml/disc Negative Matsui et al. (1989)
and M45 (rec-)
(-)-Carvone 380 Sister chromatid Chinese hamster ovary cells 502 µg/ml Equivocal National Toxicology
Program (1990)
exchange
(-)-Carvone 380 Chromosomal Chinese hamster ovary cells 400 µg/ml Equivocal National Toxicology
aberration Program (1990)
Carveol 381 Gene mutation S. typhimurium TA1535, 560 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
Carvyl acetate 382 Gene mutation S. typhimurium TA1535, 333 mg/plate Negativea Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
a With and without metabolic activation
4. REFERENCES
Anon. (1994) In vitro hydrolysis test on menthyl glycolcarbonate.
Unpublished report to the Food and Extract Manufacturers' Association
of the United States. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Bauer, K. (1991) Unpublished report to FEMA regarding the use of
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard -- A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Fischer, F.G., & Bielig, H.J. (1940) Biochemical hydrogenations VII.
Hydrogenation of unsaturated compounds in the animal body.
Hoppe-Seyler's Z. Physiol.Chem., 266, 73-98.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Flynn, C. (1991) Unpublished report to FEMA in relation to
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. Food Cosmet.
Toxicol., 5,141-157.
Hamalainen, J. (1912) Concerning the behavior of alicyclic compounds
with glucuronic acid in organisms. Skand. Arch. Physiol., 27,
141-226.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminalehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Keating, J. (1972) Acute oral toxicity on 1-carveol. Unpublished
report from the Research Institute for Fragrance Materials. Submitted
to WHO by the Food and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Lam, L. & Zheng, B. (1991) Effects of essential oils on glutathione
S-transferase activity in mice. J. Agric. Food. Chem., 39, 660-662.
Lam, L. & Zheng, B. (1992) Inhibitory effects of 2-n-heptylfuran and
2-n-butyl thiophene on benzopyrene induced lung and forestomach
tumorigenesis in A/J mice. Nutr. Cancer, 17, 19-26.
Leibman, K.C. & Ortiz, E. (1973) Mammalian metabolism of terpenoids.
I. Reduction and hydroxylation of camphor and related compounds.
Drug Metab. Disposition, 1, 543-551.
Levenstein, I. (1976) Unpublished report from Leberco Laboratories.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Communications, 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone--A hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Connecticut, Appleton & Lange.
Matsui, S., Yamamoto, R., & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in clorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
Moorthy, B., Madyastha, P. & Madyastha, M. (1989) Heptotoxicity of
pulegone in rats: Its effects on microsomal enzymes in vivo.
Toxicoogy., 55, 327-337.
Moreno, O.M. (1977) Acute oral toxicity of dihydrocarveol and
p-menth-8-en-2-one. Unpublished report from the Research Institute for
Fragrance Materials. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Moreno, O.M. (1980) Acute oral toxicity of dihydrocarvyl acetate.
Unpublished report from the Research Institute for Fragrance
Materials. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II Results from the
testing of 270 chemicals. Environ. Mutag., 8 , 1-119.
Nelson, S.D., McClanahan, R.H., Thomassen, D., Gordon, W.P. & Knebel,
N. (1992) Investigations of mechanisms of reactive metabolite
formation from (R)-(+)-pulegone. Xenobiotica, 22, 1157-1164.
Nishizawa, Y., Abe, S., Yamada, K., Nakamura, T., Yamatsu, I. &
Kinoshita, K. (1987) Identification of urinary and microsomal
metabolites of geranylgeranylacetone in rats. Xenobiotica, 17,
575-584.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Folia
Pharmacol. Jpn., 58, 323-336.
US National Academy of Sciences (1989) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1989) Toxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) and Toxicology
Studies in F344/N Rats (Gavage Studies) (NTP TR, Draft; NIH No.
90-2836), Department of Health and Human Services, Research Triangle
Park, North Caroline, United States.
US National Toxicology Program (1990) oxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) (NTP TR 381),
Department of Health and Human Services, Research Triangle Park, North
Caroline, United States.
Wattenberg, L.W., Sparnins, V.L. & Barany, G. (1989) Inhibition of
N-nitrosodiethylamine carcinogenesis in mice by naturally occurring
organosulfur compounds and monoterpenes. Cancer Res., 49, 2689-2692.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Williams, R.T. (1959) Detoxication mechanisms. In: The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Wrigley Co. (1991) Unpublished report to the Food and Extract
Manufacturers' Association of the United States. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
Zlatkis, A., LIchtenstein, H.A., Tishbee, A. Bertsch, W. & Shunbo, F.
(1973) Concentration and analysis of volatile urinary metabolites.
J. Chromatogr. Sci., 11, 299-302.
a Threshold of concern is 1800 µg/day for class I and 540 µg/day for class II.
b The ADI of 0-1 mg/kg bw previously established for (+) carvone at the thirty-seventh meeting was maintained.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from surveys
in Europe (International Organization of the Flavor Industry, 1995)
and the United States (US National Academy of Sciences, 1987) (see
Table 2). The estimated total daily per capita intake of carvone
(No. 380, stereochemistry unspecified) and related substances from use
as flavouring agents is 2.8 mg/person in Europe and 10 mg/person in
the United States. Carvone accounts for approximately 99% of the total
annual per capita intake of this group of substances when used as
flavouring agents in Europe and 96% in the United States.
Seven of the substances in this group have been reported to occur
naturally in foods, including fruits, spices, and berries. (-)-Carvone
(55-75%) has been reported in the oils of Mentha (spearmint).
(+)-Carvone (20-75%) has been reported in Carum (caraway) and
Anethum (dill) (Maarse et al., 1994). Quantitative data on the
natural occurrence and consumption ratios have been reported for five
of these substances (Nos 377, 379, 380, 381, and 382), which indicate
that they are consumed predominantly in traditional foods (i.e.
consumption ratio > 1) (Stofberg & Kirschner, 1985; Stofberg &
Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are terpenoid ketones, secondary
alcohols, and related esters containing a 2-menthyl carbon skeleton.
The group consists of three ketones (Nos 375, 377, and 380), three
secondary alcohols (Nos 376, 378, and 381), and three esters (Nos 379,
382, and 383). Their structures are shown in Table 1.
Terpenoid esters
Each of the tthree esters would be expected to be hydrolysed to
its corresponding alcohol and carboxylic acid by carboxylesterases,
which predominate in hepatocytes. Esters of carveol (Nos 382 and 383)
and dihydrocarveol (No. 379) would be expected to be hydrolysed to
yield carveol (No. 381) and dihydrocarveol (No. 378), respectively,
and the corresponding saturated aliphatic carboxylic acids.
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
* reduction of the ketone, followed by conjugation of the resulting
alcohol with glucuronic acid;
* side-chain oxidation yielding polar metabolites, which may be
conjugated and excreted;
Table 2. Most recent annual usage of carvone and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
p-Menthan-2-one (375)
Europe 0.1 0.01 0.0002
United States 3 1 0.01
p-Menthan-2-ol (376)
Europe 0.1 0.01 0.0002
United States 37 7 0.1
Dihydrocarvone (377)
Europe 0.1 0.02 0.0003
United States 920 180 3
Dihydrocarveol (378)
Europe 3 1 0.01
United States 320 61 1
Dihydrocarvyl acetate (379)
Europe 80 15 0.3
United States 0.3 0.1 0.001
Carvone (380)
Europe 15 000 2800 46
United States 52 000 9900 170
Carveol (381)
Europe 78 15 0.2
United States 740 140 2
Carvyl acetate (382)
Europe 33 6 0.1
United States 190 36 1
Carvyl propionate (383)
Europe - - -
United States 0.2 0.04 0.001
a National Academy of Sciences (1987); International Organization of the
Flavouring Industry (1995)
* glutathione conjugation of ketones, followed by excretion;
* hydrogenation of the endocyclic double bond of carveol; and
* excretion of unchanged parent compound.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), six members of this
group are in class I, namely, para-menthan-2-ol (No. 376),
dihydrocarveol (No. 378), dihydrocarvyl acetate (No. 379),
carveol (No. 381), carvyl acetate (No. 382), and carvyl
propionate (No. 383), while three members are in class II
because they contain an alpha,ß-unsaturated ketone, namely,
para-menthan-2-one (No. 375), para-menth-8-en-2-one (No.
377), and carvone (No. 380).
Step 2. Sufficient data were available on carvone to define the
major pathways of metabolism. This compound contains all of
the key structural elements and potential sites of
metabolism of all other members in the group. Better data
were available on the metabolism of isophorone
(3,4,5-trimethyl-cyclohex-2-en-1-one), which is not a member
of this group of flavouring agents but which shows close
structural similarities to carvone and uses the same routes
of metabolism (reduction of the carbonyl group, conjugation
of the resulting alcohol, and side-chain oxidation). It is
predicted that carvone and other alpha,ß-unsaturated ketones
in the group would undergo glutathione conjugation, but this
would be expected to be a minor route because of the low
reactivity in vitro, a conclusion supported by the
toxicological data.
For the three terpenoid esters in class I, the most
likely route of metabolism is hydrolysis to carveol or
dihydrocarveol. For the three terpenoid alcohols, carveol,
dihydrocarveol, and para-menthan-2-ol, which are also in
class I, the available data indicate that the most likely
route of metabolism is conjugation with glucuronic acid,
followed by excretion; however, other routes of metabolism
such as side-chain oxidation followed by conjugation and
excretion may also occur. In each case, metabolism yields
innocuous metabolites, and the evaluation should proceed via
the 'A' side of the scheme.
For the three terpenoid ketones in class II, the
available data indicate that the most likely route of
metabolism is reduction of the ketone to the corresponding
alcohol, followed by conjugation with glutathione and
excretion. Other routes of metabolism may occur, such as
side-chain oxidation followed by conjugation and direct
conjugation of the ketone with glutathione. In all cases,
metabolism yields innocuous metabolites, and the evaluation
should proceed via the 'A' side of the scheme.
Step A3. The intakes of the six terpenoid esters and alcohols in
class I are below the threshold of concern for this class
(1800 µg/person per day), and they would not be expected to
be of safety concern. The intake of two of the three
terpenoid ketones in class II, para-menthan-2-one and
para-menth-8-en-2-one, is below the threshold of concern
for this class (540 µg/person per day), and these substances
would not be expected to be of safety concern. The intakes
of carvone in both Europe (2800 µg/person per day;
International Organization of the Flavor Industry, 1994) and
the United States (9900 µg/person per day; US Academy of
Sciences, 1987) are above the threshold of concern. This
substance therefore proceeds to step A4.
Step A4. Carvone is not endogenous in humans. Its safety evaluation
therefore proceeds to step A5.
Step A5. A NOEL for (+)-carvone of 93 mg/kg bw per day (three-month
study in rats) was identified by the Committee at its
thirty-seventh meeting (Annex 1, reference 94). If it is
assumed that all of the carvone consumed was (+)-carvone, a
margin of safety of > 500 exists between this NOEL and the
per capita daily intake for the (+) isomer of carvone.
Therefore, this substance would not be expected to be of
safety concern. The (-) isomer of carvone would be expected
to share a common metabolic pathway with (+)-carvone. A NOEL
of 125 mg/kg bw per day for carvone (isomer unspecified) was
identified at the eleventh meeting (Annex 1, reference 14),
and the only material in commerce at that time was
(-)-carvone. This NOEL is considered to apply to (-)-carvone
and, if it is assumed that all of the carvone consumed was
(-)-carvone, a margin of safety of > 750 exists between
this NOEL and the per capita daily intake for the (-)
isomer of carvone. Therefore, this substance would not be
expected to be of safety concern.
Table 1 summarizes the evaluations of the nine substances in this
group.
1.5 Consideration of combined intakes from use as flavouring agents
All nine substances are expected to be efficiently metabolized to
innocuous substances. In the unlikely event that carvone, which
accounts for > 95% of the total estimated dietary intake of the
group, was consumed concomitantly with the eight related substances,
the estimated combined intake would exceed the human intake threshold
for structural classes I and II, but, in the opinion of the Committee,
this would not give rise to perturbations outside the physiological
range.
1.6 Conclusions
The results of the evaluations of carvone and related substances
indicate that these substances would not present safety concerns at
the current estimated intake. In using the procedure, the Committee
noted that all of the available data on toxicity are consistent with
the results of the safety evaluation. The ADI established previously
for (+)-carvone was maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption and metabolism
Terpenoid esters
The three esters would be expected to be hydrolysed to their
corresponding alcohol and carboxylic acid by carboxylesterases, which
predominate in hepatocytes (Heymann, 1980). Esters of carveol (Nos 382
and 383) and dihydrocarveol (No. 379) would be expected to be
hydrolysed to yield carveol and dihydrocarveol, respectively, and the
corresponding saturated aliphatic carboxylic acids. Evidence that this
metabolic route is used for these esters comes from studies of related
compounds, namely, the (-)-menthol esters, (-)-menthol ethylene glycol
carbonate and (-)-menthol propylene glycol carbonate, which were
completely hydrolysed after incubation with a rat liver homogenate
(Anon., 1994). Similarly, more than 80% of radiolabelled cyclandelate,
a structurally related ester, was hydrolysed after 20 min of
incubation with rat hepatic microsomes (White et al., 1990), and
rabbits given bornyl acetate orally excreted borneol as the glucuronic
acid conjugate (Williams, 1959).
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
(i) reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid;
(ii) side-chain oxidation, yielding polar metabolites which may
be conjugated and excreted;
(iii) glutathione conjugation of ketones, followed by excretion;
(iv) hydrogenation of the endocyclic double bond of carveol; and
(v) excretion of unchanged carvone.
Evidence for each of these pathway is given below:
(i) Reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid
There is considerable evidence that ketones are reduced to the
corresponding secondary alcohol and conjugated mainly with glucuronic
acid (Fischer & Bielig, 1940). In rodents, but probably not in humans,
the conjugate is excreted primarily into the bile, where it may be
hydrolysed to yield the free alcohol (Matthews, 1994). The alcohol may
then enter enterohepatic circulation and be excreted by the kidney
(Hamalainen, 1912; Tamura et al., 1962). If a double bond is present
in the molecule, the metabolite may be hydrogenated to the dihydro
derivative (Fischer & Bielig, 1940; Madyastha & Raj, 1993).
Ketones are reduced primarily by cytosolic carbonyl reductase,
and the reaction is stereoselective to yield a mixture of
diastereomeric alcohols (Leibman & Ortiz, 1973). In experiments in
rabbits, carvone was reduced to carveol, which was converted to the
glucuronic acid conjugate and excreted in urine (Fischer & Bielig,
1940). The glucuronic acid conjugate of dihydrocarveol has been
detected in the urine of rabbits (Hamalainen, 1912).
Groups of five rats (species and sex not specified) received
carvone at 5, 25, 50, 100, or 1000 mg/kg bw in olive oil daily by
gavage for 10 days. Daily urinary elimination of glucuronic acid,
ortho-glucuronide and ascorbic acid increased at daily doses of 100
mg/kg bw (Tamura et al., 1962).
(ii) Side-chain oxidation yielding polar metabolites which may
be conjugated and excreted
Alicyclic ketones containing an alkyl or alkenyl side-chain may
undergo oxidation of the side-chain to form polar metabolites, which
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Ishida et al., 1989; Williams,
1959).
A racemic mixture of carvone was reported to undergo side-chain
oxidation in rabbits. Each of six male rabbits was given approximately
2 g (±)-carvone orally in water. Urine collected over three days was
separated into neutral, acidic, and phenolic fractions. After
hydrolysis of the glucuronic acid and sulfate conjugates,
chromatographic analysis of the neutral fraction revealed the presence
of (+)-9-hydroxycarvone (10%) (Williams, 1959; Ishida et al., 1989).
Oxidation of the side-chain has been observed for other acyclic
(Nishizawa et al., 1987) and alicyclic ketones (Williams, 1940; Nelson
et al., 1992), with cytochrome P450 acting as a catalyst in vitro
(Madyastha & Raj, 1990); however, no effects were observed on the
activity of either cytochrome P450 or cytochrome b5 in male albino
rats given (-)-carvone at 600 mg/kg bw per day orally for three days
(Moorthy et al., 1989).
(iii) Glutathione conjugation of ketones followed by excretion
In a study to examine the ability of carvone and carvyl
derivatives to induce enzymes for the detoxification of carcinogens
(Wattenberg et al., 1989; Lam & Zheng, 1991, 1992), glutathione
S-transferase activity increased and glutathione content decreased
in various tissues of groups of A/J mice given repeated oral doses of
carvone and 10 carvyl derivatives. Groups of four female A/J mice were
given three oral doses, each containing 20 mg of a carvyl derivative
in cottonseed oil, by gavage over two days. The test substances
included alpha,ß-unsaturated ketones, (+)-carvone,
8,9-dihydro-carvone, 9-hydroxycarvone, 9-acetoxycarvone, the
unconjugated ketones 2,3-dihydrocarvone and carvomenthone, and five
carvyl alcohol derivatives. Cytosolic glutathione S-transferase
activity and acid-soluble sulfhydryl levels were measured in mouse
liver, forestomach, lung, and small and large bowel mucosa. Treatment
with carvone and, to a lesser degree, other alpha,ß-unsaturated
ketones increased the activity of glutathione S-transferase in all
tissues by two to four times over that in controls and in animals
treated with other carvyl derivatives. Carvone intake was associated
with a decrease in glutathione content in the liver, lung, and
large-bowel mucosa (Zheng et al., 1992). Carvone rapidly conjugated
with glutathione in the absence of glutathione S-transferase
(Portoghese et al., 1989).
(iv) Hydrogenation of the endocyclic double bond of carveol
Carvone is also metabolized in rabbits by hydrogenation of the
endocyclic double bond to yield 8-menthen-2-ol (dihydrocarveol), which
is excreted unchanged (Fischer & Bielig, 1940).
(v) Excretion of unchanged carvone
Carvone has been detected unchanged in the urine of humans,
presumably arising from its dietary intake (Zlatkis et al., 1973).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of the acute toxicity of carvone and
related substances are shown in Table 3.
2.1.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of all short-term and long-term studies of carvone
are shown in Table 4. Details of the studies that were critical to the
safety evaluation of carvone and related substances are given below.
Table 3. Acute toxicity of carvone and related substances tested by gavage
Substance No. Species Sex LD50 Reference
(mg/kg bw)
Dihydrocarvone 377 Rat NR > 5000 Moreno (1977)
Dihydrocarveol 378 Rat NR > 5000 Moreno (1977)
Dihydrocarvyl acetate 379 Rat NR > 5000 Moreno (1980)
Carvone 380 Rat M/F 1640 Jenner et al. (1964)
Carvone 380 Rat NR 3710 Levenstein (1976)
Carvone 380 Guinea-pig M/F 766 Jenner et al. (1964)
Carveol 381 Rat NR 3000 Keating (1972)
Carvyl acetate 382 Rat NR > 5000 Levenstein (1976)
Carvyl propionate 383 Rat NR > 5000 Levenstein (1976)
NR, not reported; M/F, male and female
Table 4. Short-term and long-term studies of the toxicity of carvone
Species Sex No. groups/ Route Duration NOEL Reference
no. per group (mg/kg bw
per day)
Carvone
Rats M/F 3/10 Oral 16 weeks ND Hagan et al.
to 1 year (1967)
(-)-Carvone
Mice M/F 5/10 Gavage 16 days 328 US National
Toxicology
Program (1990)
Mice M/F 3/40 Gavage 13 weeks 375 US National
2/20 Toxicology
Program (1990)
Rats 5/5 Gavage 16 days 150 US National
Toxicology
Program (1990)
Rats 5/10 Gavage 13 weeks 93 US National
Toxicology
Program (1990)
ND, not determined; M/F, male and female
Carvone
Carvone (unspecified stereochemistry) was administered to four
male Wistar rats for 14 days at a dietary level of 0 or 1% (equivalent
to 500 mg/kg bw per day). Significant increases in serum cholesterol
and triacylglycerol concentrations were reported in rats given carvone
when compared with the controls. Significant decreases in food
consumption and body weights were also reported in treated animals
(Imaizumi et al., 1985).
In a study to examine the toxicity of a large number of
flavouring agents, groups of five male and five female Osborne-Mendel
weanling rats were fed a diet containing carvone at a concentration of
1000 mg/kg (equivalent to 50 mg/kg bw per day) for 27-28 weeks, 2500
mg/kg (equivalent to125 mg/kg bw per day) for one year, or 10 000
mg/kg (equivalent to 750 mg/kg bw per day) for 16 weeks. Although the
stereochemistry of the test material was unspecified, a survey of
industrial producers of carvone who actively marketed carvone during
the period of the study (1960-70) indicated that the material in
commerce in the United States at that time was the (-) isomer (Bauer,
1991; Flynn, 1991; Wrigley Co., 1991). Body weights and food intake
were measured weekly and haematological examinations performed at 3,
6, 12, and 22 months. Although no effects were noted, only a very
limited report of the results of this study was provided. Depressed
body-weight gain and testicular atrophy at a dose of 750 mg/kg bw per
day were the only reported effects. The NOEL was 125 mg/kg bw per day
(Hagan et al., 1967).
(-)-Carvone
Studies on this enantiomer were evaluated at the thirty-seventh
meeting (Annex 1. reference 94)
2.1.2.3 Genotoxicity
The results of studies of the genotoxicity of these substances
are shown in Table 5.
2.1.2.4 Other relevant studies
In a study to examine the anti-carcinogenic properties of
carvone, groups of 15 female A/J mice were given 0.2 mmol (30 mg)
(+)-carvone by intubation I h before administration of
N-nitrosodiethylamine at a dose of 20 mg/kg bw once a week for eight
weeks by intubation. The animals were necropsied 26 weeks after the
initial dose of nitrosamine. (+)-Carvone inhibited forestomach tumour
formation, with a > 63% reduction in the mean number of papillomas
per mouse when compared with the controls. The number of pulmonary
adenomas in mice given (+)-carvone was significantly less (34%) than
in control animals (Wattenberg et al., 1989).
Table 5. Results of assays for the genotoxicity of carvone and related substances
Substance No. End-point Test object Dose Result Reference
Carvone 380 Gene mutation S. typhimurium TA1535, 3 µmol/plate Negative Florin et al. (1980)
TA1537, TA98, TA100
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative National Toxicology
TA98, TA100, TA1537 Program (1990)
Carvone 380 rec assay Bacillus subtilis H17 (rec+) 0.6 ml/disc Negative Matsui et al. (1989)
and M45 (rec-)
(-)-Carvone 380 Sister chromatid Chinese hamster ovary cells 502 µg/ml Equivocal National Toxicology
Program (1990)
exchange
(-)-Carvone 380 Chromosomal Chinese hamster ovary cells 400 µg/ml Equivocal National Toxicology
aberration Program (1990)
Carveol 381 Gene mutation S. typhimurium TA1535, 560 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
Carvyl acetate 382 Gene mutation S. typhimurium TA1535, 333 mg/plate Negativea Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
a With and without metabolic activation
4. REFERENCES
Anon. (1994) In vitro hydrolysis test on menthyl glycolcarbonate.
Unpublished report to the Food and Extract Manufacturers' Association
of the United States. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Bauer, K. (1991) Unpublished report to FEMA regarding the use of
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard -- A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Fischer, F.G., & Bielig, H.J. (1940) Biochemical hydrogenations VII.
Hydrogenation of unsaturated compounds in the animal body.
Hoppe-Seyler's Z. Physiol.Chem., 266, 73-98.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Flynn, C. (1991) Unpublished report to FEMA in relation to
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. Food Cosmet.
Toxicol., 5,141-157.
Hamalainen, J. (1912) Concerning the behavior of alicyclic compounds
with glucuronic acid in organisms. Skand. Arch. Physiol., 27,
141-226.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminalehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Keating, J. (1972) Acute oral toxicity on 1-carveol. Unpublished
report from the Research Institute for Fragrance Materials. Submitted
to WHO by the Food and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Lam, L. & Zheng, B. (1991) Effects of essential oils on glutathione
S-transferase activity in mice. J. Agric. Food. Chem., 39, 660-662.
Lam, L. & Zheng, B. (1992) Inhibitory effects of 2-n-heptylfuran and
2-n-butyl thiophene on benzopyrene induced lung and forestomach
tumorigenesis in A/J mice. Nutr. Cancer, 17, 19-26.
Leibman, K.C. & Ortiz, E. (1973) Mammalian metabolism of terpenoids.
I. Reduction and hydroxylation of camphor and related compounds.
Drug Metab. Disposition, 1, 543-551.
Levenstein, I. (1976) Unpublished report from Leberco Laboratories.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Communications, 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone--A hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Connecticut, Appleton & Lange.
Matsui, S., Yamamoto, R., & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in clorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
Moorthy, B., Madyastha, P. & Madyastha, M. (1989) Heptotoxicity of
pulegone in rats: Its effects on microsomal enzymes in vivo.
Toxicoogy., 55, 327-337.
Moreno, O.M. (1977) Acute oral toxicity of dihydrocarveol and
p-menth-8-en-2-one. Unpublished report from the Research Institute for
Fragrance Materials. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Moreno, O.M. (1980) Acute oral toxicity of dihydrocarvyl acetate.
Unpublished report from the Research Institute for Fragrance
Materials. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II Results from the
testing of 270 chemicals. Environ. Mutag., 8 , 1-119.
Nelson, S.D., McClanahan, R.H., Thomassen, D., Gordon, W.P. & Knebel,
N. (1992) Investigations of mechanisms of reactive metabolite
formation from (R)-(+)-pulegone. Xenobiotica, 22, 1157-1164.
Nishizawa, Y., Abe, S., Yamada, K., Nakamura, T., Yamatsu, I. &
Kinoshita, K. (1987) Identification of urinary and microsomal
metabolites of geranylgeranylacetone in rats. Xenobiotica, 17,
575-584.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Folia
Pharmacol. Jpn., 58, 323-336.
US National Academy of Sciences (1989) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1989) Toxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) and Toxicology
Studies in F344/N Rats (Gavage Studies) (NTP TR, Draft; NIH No.
90-2836), Department of Health and Human Services, Research Triangle
Park, North Caroline, United States.
US National Toxicology Program (1990) oxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) (NTP TR 381),
Department of Health and Human Services, Research Triangle Park, North
Caroline, United States.
Wattenberg, L.W., Sparnins, V.L. & Barany, G. (1989) Inhibition of
N-nitrosodiethylamine carcinogenesis in mice by naturally occurring
organosulfur compounds and monoterpenes. Cancer Res., 49, 2689-2692.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Williams, R.T. (1959) Detoxication mechanisms. In: The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Wrigley Co. (1991) Unpublished report to the Food and Extract
Manufacturers' Association of the United States. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
Zlatkis, A., LIchtenstein, H.A., Tishbee, A. Bertsch, W. & Shunbo, F.
(1973) Concentration and analysis of volatile urinary metabolites.
J. Chromatogr. Sci., 11, 299-302.
 para-Menthan-2-ol 376 499-69-4 0.01/7 I Yes No - - No safety
concern
para-Menthan-2-ol 376 499-69-4 0.01/7 I Yes No - - No safety
concern
 Dihydrocarvone 377 7764-50-3 0.02/180 II Yes No - - No safety
concern
Dihydrocarvone 377 7764-50-3 0.02/180 II Yes No - - No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Dihydrocarveol 378 619-01-2 3/320 I Yes No - - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Dihydrocarveol 378 619-01-2 3/320 I Yes No - - No safety
concern
 Dihydrocarvyl 379 20777-49-5 15/0.1 I Yes No - - No safety
acetate concern
Dihydrocarvyl 379 20777-49-5 15/0.1 I Yes No - - No safety
acetate concern
 Carvoneb 380 2244-16-8(+) 2800/9900 II Yes Yes No Yes No safety
6485-40-1(-) concern
Carvoneb 380 2244-16-8(+) 2800/9900 II Yes Yes No Yes No safety
6485-40-1(-) concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Carveol 381 99-48-9 15/140 I Yes No - - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step 1 Step 2 Step A3 Step A4 Step A5 Conclusion
capita intake, Structural Metabolized Intake exceed Endogenous? Adequate based on
Europe/USA class to innocuous threshold of NOEL for current
(µg/day) products? concern?a substance or levels of
related intake
substance?
Carveol 381 99-48-9 15/140 I Yes No - - No safety
concern
 Carvyl acetate 382 97-42-7 6/36 I Yes No - - No safety
concern
Carvyl acetate 382 97-42-7 6/36 I Yes No - - No safety
concern
 Carvyl propionate 383 97-45-0 N/D/0.04 I Yes No - - No safety
concern
Carvyl propionate 383 97-45-0 N/D/0.04 I Yes No - - No safety
concern
 a Threshold of concern is 1800 µg/day for class I and 540 µg/day for class II.
b The ADI of 0-1 mg/kg bw previously established for (+) carvone at the thirty-seventh meeting was maintained.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from surveys
in Europe (International Organization of the Flavor Industry, 1995)
and the United States (US National Academy of Sciences, 1987) (see
Table 2). The estimated total daily per capita intake of carvone
(No. 380, stereochemistry unspecified) and related substances from use
as flavouring agents is 2.8 mg/person in Europe and 10 mg/person in
the United States. Carvone accounts for approximately 99% of the total
annual per capita intake of this group of substances when used as
flavouring agents in Europe and 96% in the United States.
Seven of the substances in this group have been reported to occur
naturally in foods, including fruits, spices, and berries. (-)-Carvone
(55-75%) has been reported in the oils of Mentha (spearmint).
(+)-Carvone (20-75%) has been reported in Carum (caraway) and
Anethum (dill) (Maarse et al., 1994). Quantitative data on the
natural occurrence and consumption ratios have been reported for five
of these substances (Nos 377, 379, 380, 381, and 382), which indicate
that they are consumed predominantly in traditional foods (i.e.
consumption ratio > 1) (Stofberg & Kirschner, 1985; Stofberg &
Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are terpenoid ketones, secondary
alcohols, and related esters containing a 2-menthyl carbon skeleton.
The group consists of three ketones (Nos 375, 377, and 380), three
secondary alcohols (Nos 376, 378, and 381), and three esters (Nos 379,
382, and 383). Their structures are shown in Table 1.
Terpenoid esters
Each of the tthree esters would be expected to be hydrolysed to
its corresponding alcohol and carboxylic acid by carboxylesterases,
which predominate in hepatocytes. Esters of carveol (Nos 382 and 383)
and dihydrocarveol (No. 379) would be expected to be hydrolysed to
yield carveol (No. 381) and dihydrocarveol (No. 378), respectively,
and the corresponding saturated aliphatic carboxylic acids.
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
* reduction of the ketone, followed by conjugation of the resulting
alcohol with glucuronic acid;
* side-chain oxidation yielding polar metabolites, which may be
conjugated and excreted;
Table 2. Most recent annual usage of carvone and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
p-Menthan-2-one (375)
Europe 0.1 0.01 0.0002
United States 3 1 0.01
p-Menthan-2-ol (376)
Europe 0.1 0.01 0.0002
United States 37 7 0.1
Dihydrocarvone (377)
Europe 0.1 0.02 0.0003
United States 920 180 3
Dihydrocarveol (378)
Europe 3 1 0.01
United States 320 61 1
Dihydrocarvyl acetate (379)
Europe 80 15 0.3
United States 0.3 0.1 0.001
Carvone (380)
Europe 15 000 2800 46
United States 52 000 9900 170
Carveol (381)
Europe 78 15 0.2
United States 740 140 2
Carvyl acetate (382)
Europe 33 6 0.1
United States 190 36 1
Carvyl propionate (383)
Europe - - -
United States 0.2 0.04 0.001
a National Academy of Sciences (1987); International Organization of the
Flavouring Industry (1995)
* glutathione conjugation of ketones, followed by excretion;
* hydrogenation of the endocyclic double bond of carveol; and
* excretion of unchanged parent compound.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), six members of this
group are in class I, namely, para-menthan-2-ol (No. 376),
dihydrocarveol (No. 378), dihydrocarvyl acetate (No. 379),
carveol (No. 381), carvyl acetate (No. 382), and carvyl
propionate (No. 383), while three members are in class II
because they contain an alpha,ß-unsaturated ketone, namely,
para-menthan-2-one (No. 375), para-menth-8-en-2-one (No.
377), and carvone (No. 380).
Step 2. Sufficient data were available on carvone to define the
major pathways of metabolism. This compound contains all of
the key structural elements and potential sites of
metabolism of all other members in the group. Better data
were available on the metabolism of isophorone
(3,4,5-trimethyl-cyclohex-2-en-1-one), which is not a member
of this group of flavouring agents but which shows close
structural similarities to carvone and uses the same routes
of metabolism (reduction of the carbonyl group, conjugation
of the resulting alcohol, and side-chain oxidation). It is
predicted that carvone and other alpha,ß-unsaturated ketones
in the group would undergo glutathione conjugation, but this
would be expected to be a minor route because of the low
reactivity in vitro, a conclusion supported by the
toxicological data.
For the three terpenoid esters in class I, the most
likely route of metabolism is hydrolysis to carveol or
dihydrocarveol. For the three terpenoid alcohols, carveol,
dihydrocarveol, and para-menthan-2-ol, which are also in
class I, the available data indicate that the most likely
route of metabolism is conjugation with glucuronic acid,
followed by excretion; however, other routes of metabolism
such as side-chain oxidation followed by conjugation and
excretion may also occur. In each case, metabolism yields
innocuous metabolites, and the evaluation should proceed via
the 'A' side of the scheme.
For the three terpenoid ketones in class II, the
available data indicate that the most likely route of
metabolism is reduction of the ketone to the corresponding
alcohol, followed by conjugation with glutathione and
excretion. Other routes of metabolism may occur, such as
side-chain oxidation followed by conjugation and direct
conjugation of the ketone with glutathione. In all cases,
metabolism yields innocuous metabolites, and the evaluation
should proceed via the 'A' side of the scheme.
Step A3. The intakes of the six terpenoid esters and alcohols in
class I are below the threshold of concern for this class
(1800 µg/person per day), and they would not be expected to
be of safety concern. The intake of two of the three
terpenoid ketones in class II, para-menthan-2-one and
para-menth-8-en-2-one, is below the threshold of concern
for this class (540 µg/person per day), and these substances
would not be expected to be of safety concern. The intakes
of carvone in both Europe (2800 µg/person per day;
International Organization of the Flavor Industry, 1994) and
the United States (9900 µg/person per day; US Academy of
Sciences, 1987) are above the threshold of concern. This
substance therefore proceeds to step A4.
Step A4. Carvone is not endogenous in humans. Its safety evaluation
therefore proceeds to step A5.
Step A5. A NOEL for (+)-carvone of 93 mg/kg bw per day (three-month
study in rats) was identified by the Committee at its
thirty-seventh meeting (Annex 1, reference 94). If it is
assumed that all of the carvone consumed was (+)-carvone, a
margin of safety of > 500 exists between this NOEL and the
per capita daily intake for the (+) isomer of carvone.
Therefore, this substance would not be expected to be of
safety concern. The (-) isomer of carvone would be expected
to share a common metabolic pathway with (+)-carvone. A NOEL
of 125 mg/kg bw per day for carvone (isomer unspecified) was
identified at the eleventh meeting (Annex 1, reference 14),
and the only material in commerce at that time was
(-)-carvone. This NOEL is considered to apply to (-)-carvone
and, if it is assumed that all of the carvone consumed was
(-)-carvone, a margin of safety of > 750 exists between
this NOEL and the per capita daily intake for the (-)
isomer of carvone. Therefore, this substance would not be
expected to be of safety concern.
Table 1 summarizes the evaluations of the nine substances in this
group.
1.5 Consideration of combined intakes from use as flavouring agents
All nine substances are expected to be efficiently metabolized to
innocuous substances. In the unlikely event that carvone, which
accounts for > 95% of the total estimated dietary intake of the
group, was consumed concomitantly with the eight related substances,
the estimated combined intake would exceed the human intake threshold
for structural classes I and II, but, in the opinion of the Committee,
this would not give rise to perturbations outside the physiological
range.
1.6 Conclusions
The results of the evaluations of carvone and related substances
indicate that these substances would not present safety concerns at
the current estimated intake. In using the procedure, the Committee
noted that all of the available data on toxicity are consistent with
the results of the safety evaluation. The ADI established previously
for (+)-carvone was maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption and metabolism
Terpenoid esters
The three esters would be expected to be hydrolysed to their
corresponding alcohol and carboxylic acid by carboxylesterases, which
predominate in hepatocytes (Heymann, 1980). Esters of carveol (Nos 382
and 383) and dihydrocarveol (No. 379) would be expected to be
hydrolysed to yield carveol and dihydrocarveol, respectively, and the
corresponding saturated aliphatic carboxylic acids. Evidence that this
metabolic route is used for these esters comes from studies of related
compounds, namely, the (-)-menthol esters, (-)-menthol ethylene glycol
carbonate and (-)-menthol propylene glycol carbonate, which were
completely hydrolysed after incubation with a rat liver homogenate
(Anon., 1994). Similarly, more than 80% of radiolabelled cyclandelate,
a structurally related ester, was hydrolysed after 20 min of
incubation with rat hepatic microsomes (White et al., 1990), and
rabbits given bornyl acetate orally excreted borneol as the glucuronic
acid conjugate (Williams, 1959).
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
(i) reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid;
(ii) side-chain oxidation, yielding polar metabolites which may
be conjugated and excreted;
(iii) glutathione conjugation of ketones, followed by excretion;
(iv) hydrogenation of the endocyclic double bond of carveol; and
(v) excretion of unchanged carvone.
Evidence for each of these pathway is given below:
(i) Reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid
There is considerable evidence that ketones are reduced to the
corresponding secondary alcohol and conjugated mainly with glucuronic
acid (Fischer & Bielig, 1940). In rodents, but probably not in humans,
the conjugate is excreted primarily into the bile, where it may be
hydrolysed to yield the free alcohol (Matthews, 1994). The alcohol may
then enter enterohepatic circulation and be excreted by the kidney
(Hamalainen, 1912; Tamura et al., 1962). If a double bond is present
in the molecule, the metabolite may be hydrogenated to the dihydro
derivative (Fischer & Bielig, 1940; Madyastha & Raj, 1993).
Ketones are reduced primarily by cytosolic carbonyl reductase,
and the reaction is stereoselective to yield a mixture of
diastereomeric alcohols (Leibman & Ortiz, 1973). In experiments in
rabbits, carvone was reduced to carveol, which was converted to the
glucuronic acid conjugate and excreted in urine (Fischer & Bielig,
1940). The glucuronic acid conjugate of dihydrocarveol has been
detected in the urine of rabbits (Hamalainen, 1912).
Groups of five rats (species and sex not specified) received
carvone at 5, 25, 50, 100, or 1000 mg/kg bw in olive oil daily by
gavage for 10 days. Daily urinary elimination of glucuronic acid,
ortho-glucuronide and ascorbic acid increased at daily doses of 100
mg/kg bw (Tamura et al., 1962).
(ii) Side-chain oxidation yielding polar metabolites which may
be conjugated and excreted
Alicyclic ketones containing an alkyl or alkenyl side-chain may
undergo oxidation of the side-chain to form polar metabolites, which
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Ishida et al., 1989; Williams,
1959).
A racemic mixture of carvone was reported to undergo side-chain
oxidation in rabbits. Each of six male rabbits was given approximately
2 g (±)-carvone orally in water. Urine collected over three days was
separated into neutral, acidic, and phenolic fractions. After
hydrolysis of the glucuronic acid and sulfate conjugates,
chromatographic analysis of the neutral fraction revealed the presence
of (+)-9-hydroxycarvone (10%) (Williams, 1959; Ishida et al., 1989).
Oxidation of the side-chain has been observed for other acyclic
(Nishizawa et al., 1987) and alicyclic ketones (Williams, 1940; Nelson
et al., 1992), with cytochrome P450 acting as a catalyst in vitro
(Madyastha & Raj, 1990); however, no effects were observed on the
activity of either cytochrome P450 or cytochrome b5 in male albino
rats given (-)-carvone at 600 mg/kg bw per day orally for three days
(Moorthy et al., 1989).
(iii) Glutathione conjugation of ketones followed by excretion
In a study to examine the ability of carvone and carvyl
derivatives to induce enzymes for the detoxification of carcinogens
(Wattenberg et al., 1989; Lam & Zheng, 1991, 1992), glutathione
S-transferase activity increased and glutathione content decreased
in various tissues of groups of A/J mice given repeated oral doses of
carvone and 10 carvyl derivatives. Groups of four female A/J mice were
given three oral doses, each containing 20 mg of a carvyl derivative
in cottonseed oil, by gavage over two days. The test substances
included alpha,ß-unsaturated ketones, (+)-carvone,
8,9-dihydro-carvone, 9-hydroxycarvone, 9-acetoxycarvone, the
unconjugated ketones 2,3-dihydrocarvone and carvomenthone, and five
carvyl alcohol derivatives. Cytosolic glutathione S-transferase
activity and acid-soluble sulfhydryl levels were measured in mouse
liver, forestomach, lung, and small and large bowel mucosa. Treatment
with carvone and, to a lesser degree, other alpha,ß-unsaturated
ketones increased the activity of glutathione S-transferase in all
tissues by two to four times over that in controls and in animals
treated with other carvyl derivatives. Carvone intake was associated
with a decrease in glutathione content in the liver, lung, and
large-bowel mucosa (Zheng et al., 1992). Carvone rapidly conjugated
with glutathione in the absence of glutathione S-transferase
(Portoghese et al., 1989).
(iv) Hydrogenation of the endocyclic double bond of carveol
Carvone is also metabolized in rabbits by hydrogenation of the
endocyclic double bond to yield 8-menthen-2-ol (dihydrocarveol), which
is excreted unchanged (Fischer & Bielig, 1940).
(v) Excretion of unchanged carvone
Carvone has been detected unchanged in the urine of humans,
presumably arising from its dietary intake (Zlatkis et al., 1973).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of the acute toxicity of carvone and
related substances are shown in Table 3.
2.1.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of all short-term and long-term studies of carvone
are shown in Table 4. Details of the studies that were critical to the
safety evaluation of carvone and related substances are given below.
Table 3. Acute toxicity of carvone and related substances tested by gavage
Substance No. Species Sex LD50 Reference
(mg/kg bw)
Dihydrocarvone 377 Rat NR > 5000 Moreno (1977)
Dihydrocarveol 378 Rat NR > 5000 Moreno (1977)
Dihydrocarvyl acetate 379 Rat NR > 5000 Moreno (1980)
Carvone 380 Rat M/F 1640 Jenner et al. (1964)
Carvone 380 Rat NR 3710 Levenstein (1976)
Carvone 380 Guinea-pig M/F 766 Jenner et al. (1964)
Carveol 381 Rat NR 3000 Keating (1972)
Carvyl acetate 382 Rat NR > 5000 Levenstein (1976)
Carvyl propionate 383 Rat NR > 5000 Levenstein (1976)
NR, not reported; M/F, male and female
Table 4. Short-term and long-term studies of the toxicity of carvone
Species Sex No. groups/ Route Duration NOEL Reference
no. per group (mg/kg bw
per day)
Carvone
Rats M/F 3/10 Oral 16 weeks ND Hagan et al.
to 1 year (1967)
(-)-Carvone
Mice M/F 5/10 Gavage 16 days 328 US National
Toxicology
Program (1990)
Mice M/F 3/40 Gavage 13 weeks 375 US National
2/20 Toxicology
Program (1990)
Rats 5/5 Gavage 16 days 150 US National
Toxicology
Program (1990)
Rats 5/10 Gavage 13 weeks 93 US National
Toxicology
Program (1990)
ND, not determined; M/F, male and female
Carvone
Carvone (unspecified stereochemistry) was administered to four
male Wistar rats for 14 days at a dietary level of 0 or 1% (equivalent
to 500 mg/kg bw per day). Significant increases in serum cholesterol
and triacylglycerol concentrations were reported in rats given carvone
when compared with the controls. Significant decreases in food
consumption and body weights were also reported in treated animals
(Imaizumi et al., 1985).
In a study to examine the toxicity of a large number of
flavouring agents, groups of five male and five female Osborne-Mendel
weanling rats were fed a diet containing carvone at a concentration of
1000 mg/kg (equivalent to 50 mg/kg bw per day) for 27-28 weeks, 2500
mg/kg (equivalent to125 mg/kg bw per day) for one year, or 10 000
mg/kg (equivalent to 750 mg/kg bw per day) for 16 weeks. Although the
stereochemistry of the test material was unspecified, a survey of
industrial producers of carvone who actively marketed carvone during
the period of the study (1960-70) indicated that the material in
commerce in the United States at that time was the (-) isomer (Bauer,
1991; Flynn, 1991; Wrigley Co., 1991). Body weights and food intake
were measured weekly and haematological examinations performed at 3,
6, 12, and 22 months. Although no effects were noted, only a very
limited report of the results of this study was provided. Depressed
body-weight gain and testicular atrophy at a dose of 750 mg/kg bw per
day were the only reported effects. The NOEL was 125 mg/kg bw per day
(Hagan et al., 1967).
(-)-Carvone
Studies on this enantiomer were evaluated at the thirty-seventh
meeting (Annex 1. reference 94)
2.1.2.3 Genotoxicity
The results of studies of the genotoxicity of these substances
are shown in Table 5.
2.1.2.4 Other relevant studies
In a study to examine the anti-carcinogenic properties of
carvone, groups of 15 female A/J mice were given 0.2 mmol (30 mg)
(+)-carvone by intubation I h before administration of
N-nitrosodiethylamine at a dose of 20 mg/kg bw once a week for eight
weeks by intubation. The animals were necropsied 26 weeks after the
initial dose of nitrosamine. (+)-Carvone inhibited forestomach tumour
formation, with a > 63% reduction in the mean number of papillomas
per mouse when compared with the controls. The number of pulmonary
adenomas in mice given (+)-carvone was significantly less (34%) than
in control animals (Wattenberg et al., 1989).
Table 5. Results of assays for the genotoxicity of carvone and related substances
Substance No. End-point Test object Dose Result Reference
Carvone 380 Gene mutation S. typhimurium TA1535, 3 µmol/plate Negative Florin et al. (1980)
TA1537, TA98, TA100
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative National Toxicology
TA98, TA100, TA1537 Program (1990)
Carvone 380 rec assay Bacillus subtilis H17 (rec+) 0.6 ml/disc Negative Matsui et al. (1989)
and M45 (rec-)
(-)-Carvone 380 Sister chromatid Chinese hamster ovary cells 502 µg/ml Equivocal National Toxicology
Program (1990)
exchange
(-)-Carvone 380 Chromosomal Chinese hamster ovary cells 400 µg/ml Equivocal National Toxicology
aberration Program (1990)
Carveol 381 Gene mutation S. typhimurium TA1535, 560 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
Carvyl acetate 382 Gene mutation S. typhimurium TA1535, 333 mg/plate Negativea Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
a With and without metabolic activation
4. REFERENCES
Anon. (1994) In vitro hydrolysis test on menthyl glycolcarbonate.
Unpublished report to the Food and Extract Manufacturers' Association
of the United States. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Bauer, K. (1991) Unpublished report to FEMA regarding the use of
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard -- A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Fischer, F.G., & Bielig, H.J. (1940) Biochemical hydrogenations VII.
Hydrogenation of unsaturated compounds in the animal body.
Hoppe-Seyler's Z. Physiol.Chem., 266, 73-98.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Flynn, C. (1991) Unpublished report to FEMA in relation to
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. Food Cosmet.
Toxicol., 5,141-157.
Hamalainen, J. (1912) Concerning the behavior of alicyclic compounds
with glucuronic acid in organisms. Skand. Arch. Physiol., 27,
141-226.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminalehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Keating, J. (1972) Acute oral toxicity on 1-carveol. Unpublished
report from the Research Institute for Fragrance Materials. Submitted
to WHO by the Food and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Lam, L. & Zheng, B. (1991) Effects of essential oils on glutathione
S-transferase activity in mice. J. Agric. Food. Chem., 39, 660-662.
Lam, L. & Zheng, B. (1992) Inhibitory effects of 2-n-heptylfuran and
2-n-butyl thiophene on benzopyrene induced lung and forestomach
tumorigenesis in A/J mice. Nutr. Cancer, 17, 19-26.
Leibman, K.C. & Ortiz, E. (1973) Mammalian metabolism of terpenoids.
I. Reduction and hydroxylation of camphor and related compounds.
Drug Metab. Disposition, 1, 543-551.
Levenstein, I. (1976) Unpublished report from Leberco Laboratories.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Communications, 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone--A hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Connecticut, Appleton & Lange.
Matsui, S., Yamamoto, R., & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in clorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
Moorthy, B., Madyastha, P. & Madyastha, M. (1989) Heptotoxicity of
pulegone in rats: Its effects on microsomal enzymes in vivo.
Toxicoogy., 55, 327-337.
Moreno, O.M. (1977) Acute oral toxicity of dihydrocarveol and
p-menth-8-en-2-one. Unpublished report from the Research Institute for
Fragrance Materials. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Moreno, O.M. (1980) Acute oral toxicity of dihydrocarvyl acetate.
Unpublished report from the Research Institute for Fragrance
Materials. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II Results from the
testing of 270 chemicals. Environ. Mutag., 8 , 1-119.
Nelson, S.D., McClanahan, R.H., Thomassen, D., Gordon, W.P. & Knebel,
N. (1992) Investigations of mechanisms of reactive metabolite
formation from (R)-(+)-pulegone. Xenobiotica, 22, 1157-1164.
Nishizawa, Y., Abe, S., Yamada, K., Nakamura, T., Yamatsu, I. &
Kinoshita, K. (1987) Identification of urinary and microsomal
metabolites of geranylgeranylacetone in rats. Xenobiotica, 17,
575-584.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Folia
Pharmacol. Jpn., 58, 323-336.
US National Academy of Sciences (1989) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1989) Toxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) and Toxicology
Studies in F344/N Rats (Gavage Studies) (NTP TR, Draft; NIH No.
90-2836), Department of Health and Human Services, Research Triangle
Park, North Caroline, United States.
US National Toxicology Program (1990) oxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) (NTP TR 381),
Department of Health and Human Services, Research Triangle Park, North
Caroline, United States.
Wattenberg, L.W., Sparnins, V.L. & Barany, G. (1989) Inhibition of
N-nitrosodiethylamine carcinogenesis in mice by naturally occurring
organosulfur compounds and monoterpenes. Cancer Res., 49, 2689-2692.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Williams, R.T. (1959) Detoxication mechanisms. In: The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Wrigley Co. (1991) Unpublished report to the Food and Extract
Manufacturers' Association of the United States. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
Zlatkis, A., LIchtenstein, H.A., Tishbee, A. Bertsch, W. & Shunbo, F.
(1973) Concentration and analysis of volatile urinary metabolites.
J. Chromatogr. Sci., 11, 299-302.
a Threshold of concern is 1800 µg/day for class I and 540 µg/day for class II.
b The ADI of 0-1 mg/kg bw previously established for (+) carvone at the thirty-seventh meeting was maintained.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from surveys
in Europe (International Organization of the Flavor Industry, 1995)
and the United States (US National Academy of Sciences, 1987) (see
Table 2). The estimated total daily per capita intake of carvone
(No. 380, stereochemistry unspecified) and related substances from use
as flavouring agents is 2.8 mg/person in Europe and 10 mg/person in
the United States. Carvone accounts for approximately 99% of the total
annual per capita intake of this group of substances when used as
flavouring agents in Europe and 96% in the United States.
Seven of the substances in this group have been reported to occur
naturally in foods, including fruits, spices, and berries. (-)-Carvone
(55-75%) has been reported in the oils of Mentha (spearmint).
(+)-Carvone (20-75%) has been reported in Carum (caraway) and
Anethum (dill) (Maarse et al., 1994). Quantitative data on the
natural occurrence and consumption ratios have been reported for five
of these substances (Nos 377, 379, 380, 381, and 382), which indicate
that they are consumed predominantly in traditional foods (i.e.
consumption ratio > 1) (Stofberg & Kirschner, 1985; Stofberg &
Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are terpenoid ketones, secondary
alcohols, and related esters containing a 2-menthyl carbon skeleton.
The group consists of three ketones (Nos 375, 377, and 380), three
secondary alcohols (Nos 376, 378, and 381), and three esters (Nos 379,
382, and 383). Their structures are shown in Table 1.
Terpenoid esters
Each of the tthree esters would be expected to be hydrolysed to
its corresponding alcohol and carboxylic acid by carboxylesterases,
which predominate in hepatocytes. Esters of carveol (Nos 382 and 383)
and dihydrocarveol (No. 379) would be expected to be hydrolysed to
yield carveol (No. 381) and dihydrocarveol (No. 378), respectively,
and the corresponding saturated aliphatic carboxylic acids.
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
* reduction of the ketone, followed by conjugation of the resulting
alcohol with glucuronic acid;
* side-chain oxidation yielding polar metabolites, which may be
conjugated and excreted;
Table 2. Most recent annual usage of carvone and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
p-Menthan-2-one (375)
Europe 0.1 0.01 0.0002
United States 3 1 0.01
p-Menthan-2-ol (376)
Europe 0.1 0.01 0.0002
United States 37 7 0.1
Dihydrocarvone (377)
Europe 0.1 0.02 0.0003
United States 920 180 3
Dihydrocarveol (378)
Europe 3 1 0.01
United States 320 61 1
Dihydrocarvyl acetate (379)
Europe 80 15 0.3
United States 0.3 0.1 0.001
Carvone (380)
Europe 15 000 2800 46
United States 52 000 9900 170
Carveol (381)
Europe 78 15 0.2
United States 740 140 2
Carvyl acetate (382)
Europe 33 6 0.1
United States 190 36 1
Carvyl propionate (383)
Europe - - -
United States 0.2 0.04 0.001
a National Academy of Sciences (1987); International Organization of the
Flavouring Industry (1995)
* glutathione conjugation of ketones, followed by excretion;
* hydrogenation of the endocyclic double bond of carveol; and
* excretion of unchanged parent compound.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), six members of this
group are in class I, namely, para-menthan-2-ol (No. 376),
dihydrocarveol (No. 378), dihydrocarvyl acetate (No. 379),
carveol (No. 381), carvyl acetate (No. 382), and carvyl
propionate (No. 383), while three members are in class II
because they contain an alpha,ß-unsaturated ketone, namely,
para-menthan-2-one (No. 375), para-menth-8-en-2-one (No.
377), and carvone (No. 380).
Step 2. Sufficient data were available on carvone to define the
major pathways of metabolism. This compound contains all of
the key structural elements and potential sites of
metabolism of all other members in the group. Better data
were available on the metabolism of isophorone
(3,4,5-trimethyl-cyclohex-2-en-1-one), which is not a member
of this group of flavouring agents but which shows close
structural similarities to carvone and uses the same routes
of metabolism (reduction of the carbonyl group, conjugation
of the resulting alcohol, and side-chain oxidation). It is
predicted that carvone and other alpha,ß-unsaturated ketones
in the group would undergo glutathione conjugation, but this
would be expected to be a minor route because of the low
reactivity in vitro, a conclusion supported by the
toxicological data.
For the three terpenoid esters in class I, the most
likely route of metabolism is hydrolysis to carveol or
dihydrocarveol. For the three terpenoid alcohols, carveol,
dihydrocarveol, and para-menthan-2-ol, which are also in
class I, the available data indicate that the most likely
route of metabolism is conjugation with glucuronic acid,
followed by excretion; however, other routes of metabolism
such as side-chain oxidation followed by conjugation and
excretion may also occur. In each case, metabolism yields
innocuous metabolites, and the evaluation should proceed via
the 'A' side of the scheme.
For the three terpenoid ketones in class II, the
available data indicate that the most likely route of
metabolism is reduction of the ketone to the corresponding
alcohol, followed by conjugation with glutathione and
excretion. Other routes of metabolism may occur, such as
side-chain oxidation followed by conjugation and direct
conjugation of the ketone with glutathione. In all cases,
metabolism yields innocuous metabolites, and the evaluation
should proceed via the 'A' side of the scheme.
Step A3. The intakes of the six terpenoid esters and alcohols in
class I are below the threshold of concern for this class
(1800 µg/person per day), and they would not be expected to
be of safety concern. The intake of two of the three
terpenoid ketones in class II, para-menthan-2-one and
para-menth-8-en-2-one, is below the threshold of concern
for this class (540 µg/person per day), and these substances
would not be expected to be of safety concern. The intakes
of carvone in both Europe (2800 µg/person per day;
International Organization of the Flavor Industry, 1994) and
the United States (9900 µg/person per day; US Academy of
Sciences, 1987) are above the threshold of concern. This
substance therefore proceeds to step A4.
Step A4. Carvone is not endogenous in humans. Its safety evaluation
therefore proceeds to step A5.
Step A5. A NOEL for (+)-carvone of 93 mg/kg bw per day (three-month
study in rats) was identified by the Committee at its
thirty-seventh meeting (Annex 1, reference 94). If it is
assumed that all of the carvone consumed was (+)-carvone, a
margin of safety of > 500 exists between this NOEL and the
per capita daily intake for the (+) isomer of carvone.
Therefore, this substance would not be expected to be of
safety concern. The (-) isomer of carvone would be expected
to share a common metabolic pathway with (+)-carvone. A NOEL
of 125 mg/kg bw per day for carvone (isomer unspecified) was
identified at the eleventh meeting (Annex 1, reference 14),
and the only material in commerce at that time was
(-)-carvone. This NOEL is considered to apply to (-)-carvone
and, if it is assumed that all of the carvone consumed was
(-)-carvone, a margin of safety of > 750 exists between
this NOEL and the per capita daily intake for the (-)
isomer of carvone. Therefore, this substance would not be
expected to be of safety concern.
Table 1 summarizes the evaluations of the nine substances in this
group.
1.5 Consideration of combined intakes from use as flavouring agents
All nine substances are expected to be efficiently metabolized to
innocuous substances. In the unlikely event that carvone, which
accounts for > 95% of the total estimated dietary intake of the
group, was consumed concomitantly with the eight related substances,
the estimated combined intake would exceed the human intake threshold
for structural classes I and II, but, in the opinion of the Committee,
this would not give rise to perturbations outside the physiological
range.
1.6 Conclusions
The results of the evaluations of carvone and related substances
indicate that these substances would not present safety concerns at
the current estimated intake. In using the procedure, the Committee
noted that all of the available data on toxicity are consistent with
the results of the safety evaluation. The ADI established previously
for (+)-carvone was maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption and metabolism
Terpenoid esters
The three esters would be expected to be hydrolysed to their
corresponding alcohol and carboxylic acid by carboxylesterases, which
predominate in hepatocytes (Heymann, 1980). Esters of carveol (Nos 382
and 383) and dihydrocarveol (No. 379) would be expected to be
hydrolysed to yield carveol and dihydrocarveol, respectively, and the
corresponding saturated aliphatic carboxylic acids. Evidence that this
metabolic route is used for these esters comes from studies of related
compounds, namely, the (-)-menthol esters, (-)-menthol ethylene glycol
carbonate and (-)-menthol propylene glycol carbonate, which were
completely hydrolysed after incubation with a rat liver homogenate
(Anon., 1994). Similarly, more than 80% of radiolabelled cyclandelate,
a structurally related ester, was hydrolysed after 20 min of
incubation with rat hepatic microsomes (White et al., 1990), and
rabbits given bornyl acetate orally excreted borneol as the glucuronic
acid conjugate (Williams, 1959).
Terpenoid alcohols and ketones
The terpenoid alcohols resulting from ester hydrolysis and their
corresponding ketones are metabolized like other alicyclic terpenoid
ketones and secondary alcohols. Five detoxification pathways have been
identified:
(i) reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid;
(ii) side-chain oxidation, yielding polar metabolites which may
be conjugated and excreted;
(iii) glutathione conjugation of ketones, followed by excretion;
(iv) hydrogenation of the endocyclic double bond of carveol; and
(v) excretion of unchanged carvone.
Evidence for each of these pathway is given below:
(i) Reduction of the ketone followed by conjugation of the
resulting alcohol with glucuronic acid
There is considerable evidence that ketones are reduced to the
corresponding secondary alcohol and conjugated mainly with glucuronic
acid (Fischer & Bielig, 1940). In rodents, but probably not in humans,
the conjugate is excreted primarily into the bile, where it may be
hydrolysed to yield the free alcohol (Matthews, 1994). The alcohol may
then enter enterohepatic circulation and be excreted by the kidney
(Hamalainen, 1912; Tamura et al., 1962). If a double bond is present
in the molecule, the metabolite may be hydrogenated to the dihydro
derivative (Fischer & Bielig, 1940; Madyastha & Raj, 1993).
Ketones are reduced primarily by cytosolic carbonyl reductase,
and the reaction is stereoselective to yield a mixture of
diastereomeric alcohols (Leibman & Ortiz, 1973). In experiments in
rabbits, carvone was reduced to carveol, which was converted to the
glucuronic acid conjugate and excreted in urine (Fischer & Bielig,
1940). The glucuronic acid conjugate of dihydrocarveol has been
detected in the urine of rabbits (Hamalainen, 1912).
Groups of five rats (species and sex not specified) received
carvone at 5, 25, 50, 100, or 1000 mg/kg bw in olive oil daily by
gavage for 10 days. Daily urinary elimination of glucuronic acid,
ortho-glucuronide and ascorbic acid increased at daily doses of 100
mg/kg bw (Tamura et al., 1962).
(ii) Side-chain oxidation yielding polar metabolites which may
be conjugated and excreted
Alicyclic ketones containing an alkyl or alkenyl side-chain may
undergo oxidation of the side-chain to form polar metabolites, which
are excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Ishida et al., 1989; Williams,
1959).
A racemic mixture of carvone was reported to undergo side-chain
oxidation in rabbits. Each of six male rabbits was given approximately
2 g (±)-carvone orally in water. Urine collected over three days was
separated into neutral, acidic, and phenolic fractions. After
hydrolysis of the glucuronic acid and sulfate conjugates,
chromatographic analysis of the neutral fraction revealed the presence
of (+)-9-hydroxycarvone (10%) (Williams, 1959; Ishida et al., 1989).
Oxidation of the side-chain has been observed for other acyclic
(Nishizawa et al., 1987) and alicyclic ketones (Williams, 1940; Nelson
et al., 1992), with cytochrome P450 acting as a catalyst in vitro
(Madyastha & Raj, 1990); however, no effects were observed on the
activity of either cytochrome P450 or cytochrome b5 in male albino
rats given (-)-carvone at 600 mg/kg bw per day orally for three days
(Moorthy et al., 1989).
(iii) Glutathione conjugation of ketones followed by excretion
In a study to examine the ability of carvone and carvyl
derivatives to induce enzymes for the detoxification of carcinogens
(Wattenberg et al., 1989; Lam & Zheng, 1991, 1992), glutathione
S-transferase activity increased and glutathione content decreased
in various tissues of groups of A/J mice given repeated oral doses of
carvone and 10 carvyl derivatives. Groups of four female A/J mice were
given three oral doses, each containing 20 mg of a carvyl derivative
in cottonseed oil, by gavage over two days. The test substances
included alpha,ß-unsaturated ketones, (+)-carvone,
8,9-dihydro-carvone, 9-hydroxycarvone, 9-acetoxycarvone, the
unconjugated ketones 2,3-dihydrocarvone and carvomenthone, and five
carvyl alcohol derivatives. Cytosolic glutathione S-transferase
activity and acid-soluble sulfhydryl levels were measured in mouse
liver, forestomach, lung, and small and large bowel mucosa. Treatment
with carvone and, to a lesser degree, other alpha,ß-unsaturated
ketones increased the activity of glutathione S-transferase in all
tissues by two to four times over that in controls and in animals
treated with other carvyl derivatives. Carvone intake was associated
with a decrease in glutathione content in the liver, lung, and
large-bowel mucosa (Zheng et al., 1992). Carvone rapidly conjugated
with glutathione in the absence of glutathione S-transferase
(Portoghese et al., 1989).
(iv) Hydrogenation of the endocyclic double bond of carveol
Carvone is also metabolized in rabbits by hydrogenation of the
endocyclic double bond to yield 8-menthen-2-ol (dihydrocarveol), which
is excreted unchanged (Fischer & Bielig, 1940).
(v) Excretion of unchanged carvone
Carvone has been detected unchanged in the urine of humans,
presumably arising from its dietary intake (Zlatkis et al., 1973).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of the acute toxicity of carvone and
related substances are shown in Table 3.
2.1.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of all short-term and long-term studies of carvone
are shown in Table 4. Details of the studies that were critical to the
safety evaluation of carvone and related substances are given below.
Table 3. Acute toxicity of carvone and related substances tested by gavage
Substance No. Species Sex LD50 Reference
(mg/kg bw)
Dihydrocarvone 377 Rat NR > 5000 Moreno (1977)
Dihydrocarveol 378 Rat NR > 5000 Moreno (1977)
Dihydrocarvyl acetate 379 Rat NR > 5000 Moreno (1980)
Carvone 380 Rat M/F 1640 Jenner et al. (1964)
Carvone 380 Rat NR 3710 Levenstein (1976)
Carvone 380 Guinea-pig M/F 766 Jenner et al. (1964)
Carveol 381 Rat NR 3000 Keating (1972)
Carvyl acetate 382 Rat NR > 5000 Levenstein (1976)
Carvyl propionate 383 Rat NR > 5000 Levenstein (1976)
NR, not reported; M/F, male and female
Table 4. Short-term and long-term studies of the toxicity of carvone
Species Sex No. groups/ Route Duration NOEL Reference
no. per group (mg/kg bw
per day)
Carvone
Rats M/F 3/10 Oral 16 weeks ND Hagan et al.
to 1 year (1967)
(-)-Carvone
Mice M/F 5/10 Gavage 16 days 328 US National
Toxicology
Program (1990)
Mice M/F 3/40 Gavage 13 weeks 375 US National
2/20 Toxicology
Program (1990)
Rats 5/5 Gavage 16 days 150 US National
Toxicology
Program (1990)
Rats 5/10 Gavage 13 weeks 93 US National
Toxicology
Program (1990)
ND, not determined; M/F, male and female
Carvone
Carvone (unspecified stereochemistry) was administered to four
male Wistar rats for 14 days at a dietary level of 0 or 1% (equivalent
to 500 mg/kg bw per day). Significant increases in serum cholesterol
and triacylglycerol concentrations were reported in rats given carvone
when compared with the controls. Significant decreases in food
consumption and body weights were also reported in treated animals
(Imaizumi et al., 1985).
In a study to examine the toxicity of a large number of
flavouring agents, groups of five male and five female Osborne-Mendel
weanling rats were fed a diet containing carvone at a concentration of
1000 mg/kg (equivalent to 50 mg/kg bw per day) for 27-28 weeks, 2500
mg/kg (equivalent to125 mg/kg bw per day) for one year, or 10 000
mg/kg (equivalent to 750 mg/kg bw per day) for 16 weeks. Although the
stereochemistry of the test material was unspecified, a survey of
industrial producers of carvone who actively marketed carvone during
the period of the study (1960-70) indicated that the material in
commerce in the United States at that time was the (-) isomer (Bauer,
1991; Flynn, 1991; Wrigley Co., 1991). Body weights and food intake
were measured weekly and haematological examinations performed at 3,
6, 12, and 22 months. Although no effects were noted, only a very
limited report of the results of this study was provided. Depressed
body-weight gain and testicular atrophy at a dose of 750 mg/kg bw per
day were the only reported effects. The NOEL was 125 mg/kg bw per day
(Hagan et al., 1967).
(-)-Carvone
Studies on this enantiomer were evaluated at the thirty-seventh
meeting (Annex 1. reference 94)
2.1.2.3 Genotoxicity
The results of studies of the genotoxicity of these substances
are shown in Table 5.
2.1.2.4 Other relevant studies
In a study to examine the anti-carcinogenic properties of
carvone, groups of 15 female A/J mice were given 0.2 mmol (30 mg)
(+)-carvone by intubation I h before administration of
N-nitrosodiethylamine at a dose of 20 mg/kg bw once a week for eight
weeks by intubation. The animals were necropsied 26 weeks after the
initial dose of nitrosamine. (+)-Carvone inhibited forestomach tumour
formation, with a > 63% reduction in the mean number of papillomas
per mouse when compared with the controls. The number of pulmonary
adenomas in mice given (+)-carvone was significantly less (34%) than
in control animals (Wattenberg et al., 1989).
Table 5. Results of assays for the genotoxicity of carvone and related substances
Substance No. End-point Test object Dose Result Reference
Carvone 380 Gene mutation S. typhimurium TA1535, 3 µmol/plate Negative Florin et al. (1980)
TA1537, TA98, TA100
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
(-)-Carvone 380 Gene mutation S. typhimurium TA1535, 333 µg/plate Negative National Toxicology
TA98, TA100, TA1537 Program (1990)
Carvone 380 rec assay Bacillus subtilis H17 (rec+) 0.6 ml/disc Negative Matsui et al. (1989)
and M45 (rec-)
(-)-Carvone 380 Sister chromatid Chinese hamster ovary cells 502 µg/ml Equivocal National Toxicology
Program (1990)
exchange
(-)-Carvone 380 Chromosomal Chinese hamster ovary cells 400 µg/ml Equivocal National Toxicology
aberration Program (1990)
Carveol 381 Gene mutation S. typhimurium TA1535, 560 µg/plate Negative Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
Carvyl acetate 382 Gene mutation S. typhimurium TA1535, 333 mg/plate Negativea Mortelmans et al.
(preincubation) TA98, TA100, TA1537 (1986)
a With and without metabolic activation
4. REFERENCES
Anon. (1994) In vitro hydrolysis test on menthyl glycolcarbonate.
Unpublished report to the Food and Extract Manufacturers' Association
of the United States. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Bauer, K. (1991) Unpublished report to FEMA regarding the use of
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard -- A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Fischer, F.G., & Bielig, H.J. (1940) Biochemical hydrogenations VII.
Hydrogenation of unsaturated compounds in the animal body.
Hoppe-Seyler's Z. Physiol.Chem., 266, 73-98.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Flynn, C. (1991) Unpublished report to FEMA in relation to
l-carvone. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. Food Cosmet.
Toxicol., 5,141-157.
Hamalainen, J. (1912) Concerning the behavior of alicyclic compounds
with glucuronic acid in organisms. Skand. Arch. Physiol., 27,
141-226.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminalehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Keating, J. (1972) Acute oral toxicity on 1-carveol. Unpublished
report from the Research Institute for Fragrance Materials. Submitted
to WHO by the Food and Extract Manufacturers' Association of the
United States, Washington DC, United States.
Lam, L. & Zheng, B. (1991) Effects of essential oils on glutathione
S-transferase activity in mice. J. Agric. Food. Chem., 39, 660-662.
Lam, L. & Zheng, B. (1992) Inhibitory effects of 2-n-heptylfuran and
2-n-butyl thiophene on benzopyrene induced lung and forestomach
tumorigenesis in A/J mice. Nutr. Cancer, 17, 19-26.
Leibman, K.C. & Ortiz, E. (1973) Mammalian metabolism of terpenoids.
I. Reduction and hydroxylation of camphor and related compounds.
Drug Metab. Disposition, 1, 543-551.
Levenstein, I. (1976) Unpublished report from Leberco Laboratories.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Raj, C.P. (1990) Biotransformations of
R-(+)-pulegone and menthofuran in vitro: Chemical basis for
toxicity. Biochem. Biophys. Res. Communications, 3, 1086-1092.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a
monoterpene ketone, R-(+)-pulegone--A hepatotoxin in rat: Isolation
and characterization of new metabolites. Xenobiotica, 23, 509-518.
Matthews, H.B. (1994) Excretion and elimination of toxicants and their
metabolites. In: Hodgson, E. & Levi, P., eds, Introduction to
Biochemical Toxicology, 2nd Ed., Connecticut, Appleton & Lange.
Matsui, S., Yamamoto, R., & Yamada, H. (1989) The Bacillus
subtilis/microsome rec-assay for the detection of DNA damaging
substances which may occur in clorinated and ozonated waters.
Water Sci. Technol., 21, 875-887.
Moorthy, B., Madyastha, P. & Madyastha, M. (1989) Heptotoxicity of
pulegone in rats: Its effects on microsomal enzymes in vivo.
Toxicoogy., 55, 327-337.
Moreno, O.M. (1977) Acute oral toxicity of dihydrocarveol and
p-menth-8-en-2-one. Unpublished report from the Research Institute for
Fragrance Materials. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Moreno, O.M. (1980) Acute oral toxicity of dihydrocarvyl acetate.
Unpublished report from the Research Institute for Fragrance
Materials. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II Results from the
testing of 270 chemicals. Environ. Mutag., 8 , 1-119.
Nelson, S.D., McClanahan, R.H., Thomassen, D., Gordon, W.P. & Knebel,
N. (1992) Investigations of mechanisms of reactive metabolite
formation from (R)-(+)-pulegone. Xenobiotica, 22, 1157-1164.
Nishizawa, Y., Abe, S., Yamada, K., Nakamura, T., Yamatsu, I. &
Kinoshita, K. (1987) Identification of urinary and microsomal
metabolites of geranylgeranylacetone in rats. Xenobiotica, 17,
575-584.
Portoghese, P.S., Kedziora, G.S., Larson, D.L., Bernard, B.K. & Hall,
R.L. (1989) Reactivity of glutathione with alpha,ß-unsaturated ketone
flavoring substances. Food Chem. Toxicol., 27, 773-776.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tamura, S., Tsutsumi, S. & Kizu, K. (1962) Studies on glucuronic acid
metabolism. The influence of borneol, ionone and carvone on the
urinary excretion of glucuronic acid and ascorbic acid. Folia
Pharmacol. Jpn., 58, 323-336.
US National Academy of Sciences (1989) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1989) Toxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) and Toxicology
Studies in F344/N Rats (Gavage Studies) (NTP TR, Draft; NIH No.
90-2836), Department of Health and Human Services, Research Triangle
Park, North Caroline, United States.
US National Toxicology Program (1990) oxicology and Carcinogenesis
Studies of d-Carvone in B6C3F1 Mice (Gavage Studies) (NTP TR 381),
Department of Health and Human Services, Research Triangle Park, North
Caroline, United States.
Wattenberg, L.W., Sparnins, V.L. & Barany, G. (1989) Inhibition of
N-nitrosodiethylamine carcinogenesis in mice by naturally occurring
organosulfur compounds and monoterpenes. Cancer Res., 49, 2689-2692.
White, D.A., Heffron, A., Miciak, B., Middleton, B., Knights, S. &
Knights, D. (1990) Chemical synthesis of dual radiolabelled
cyclandelate and its metabolism in rat hepatocytes and mouse J774
cells. Xenobiotica, 20, 71.
Williams, R.T. (1940) Studies in detoxication. 7. The biological
reduction of l-menthone to d-neomenthol and of d-isomenthone to
d-isomenthol in the rabbit. The conjugation of d-neomenthol with
glucuronic acid. Biochem. J., 34, 690-697.
Williams, R.T. (1959) Detoxication mechanisms. In: The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Wrigley Co. (1991) Unpublished report to the Food and Extract
Manufacturers' Association of the United States. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Zheng, G.-Q., Kenney, P.M. & Lam, K.L.T. (1992) Effects of carvone
compounds on glutathione S-transferase activity in A/J mice.
J. Agric. Food Chem., 40, 751-755.
Zlatkis, A., LIchtenstein, H.A., Tishbee, A. Bertsch, W. & Shunbo, F.
(1973) Concentration and analysis of volatile urinary metabolites.
J. Chromatogr. Sci., 11, 299-302.
