
POLYVINYLPOLYPYRROLIDONE, INSOLUBLE (PVPP)
Explanation
This compound has not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
Introduction
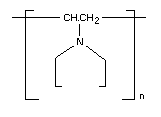
Insoluble polyvinylpolypyrrolidone (PVPP); Crosspovidone;
1-vinyl-2-pyrrolidone crosslinked insoluble polymer is a crosslinked
homopolymer of purified vinyl pyrrolidone.
 BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male Sprague-Dawley rats weighing between 200 and 300 g were
dosed orally (gastric gavage) with 2.25 mg of 14C-PVPP suspended in
0.1 ml of distilled water. At 6, 12, 24 and 48 hours after dosing
urine and faeces were collected. The rats were then sacrificed and the
major organs (spleen, kidneys, liver, lungs, thymus and adrenal
glands) and blood removed. The 14C content of the urine, faeces,
blood and organs was determined. Less than 1% of the administered 14C
dose was detected in the major organs, blood and urine. Sixty-three to
74% of the 14C was recovered in the faeces during the 48-hour period,
with most of the 14C being recovered 12 hours after dosing (Digenis,
1979).
Six Sprague-Dawley (I.F.F. A CREDD) rats (three male and three
female), weighing 180-210 g, received a single oral dose of 14C-PVPP
suspension by gastric intubation. Each dose contained approximately
250 mg PVPP. The PVPP used had been treated by dialysis and
ultrafiltration to remove polymers of M.W. of less than 10 000. Urine
and faeces were collected at 12-hour time intervals for a period of
five days after dosing and 14C excretion determined. The animals were
then sacrificed and the radioactivity remaining in the carcass,
gastrointestinal tract and lungs, determined. About 0.128% of the
administered 14C was excreted in the urine, with most of the 14C
being excreted in the first 24 hours. Eighty to 99% of the 14C was
recovered in the faeces in the period 12-24 hours. Less than 0.1% of
the administered 14C was recovered in the carcass, with most being in
the gastrointestinal tract. There was no evidence of preferential
binding of the 14C to any organ or tissue (Istin, 1979).
In another study, a group of four rats (male) weighing 250-300 g
had their bile ducts catheterized. After resting one day, the rats
received a single dose of 14C-PVPP (250 mg) suspension by gavage.
Bile was collected at 1.5-hour intervals, urine and faeces at 24-hour
intervals. At the termination of the study, the animals were
sacrificed and the 14C in the carcass measured. Approximately
0.00816% of the administered 14C was recovered in the bile. Urine,
faecal and carcass distribution of 14C was similar to that in the
previous study (Istin, 1979).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups each of 26 pregnant rats (SPF rats) were dosed by gavage
with a suspension of PVPP (Kollidon CE 5050) in carboxymethyl
cellulose in doses of 1000 or 3000 mg/kg. Other groups served as
vehicle control or received no treatment. Dosing was from day 6 to day
15 of gestation. The test animals were sacrificed on day 20 of
gestation and the foetuses examined. Two-thirds of the foetuses of
each litter were examined for skeletal changes and the remaining
one-third for visceral changes. Weight gain was similar for all
groups, with the exception of the high-dose group, that showed a
slight decrease in weight gain during the period of administration of
the test compound. However, at termination of the study the weight
gain was comparable in all groups. No significant clinical symptoms
were observed in the test animals. There were no maternal deaths and
no compound-related changes were observed at autopsy. The conception
rate and percentage of live and dead implantations were similar for
test and control animals. The weight of the foetuses of test animals
was slightly lower than those of controls, but no changes were
observed in the foetal length. There were no compound-related skeletal
or visceral abnormalities (Anon., 1977a).
An additional two groups each of 12 pregnant rats (SPF rats) were
dosed by gavage with a suspension of PVPP (Kollidon CD 5050) in
carboxymethyl cellulose in doses of 1000 or 3000 mg/kg bw. Dosing was
from day 15 to day 21 post-parturition. The rats in these groups were
allowed to litter spontaneously and rear their progeny to day 21
post-parturition. There were no significant differences in weight gain
for test and control animals. None of the test animals died. The time
of delivery and average litter size were similar for test and control
animals. At termination of the study, none of the mothers showed any
macroscopic, compound-related effects. The pups of the treated animals
showed similar mortality, body weight increases and behaviour (as
measured by swimming test on day 14) as controls. At autopsy there
were no compound-related skeletal or visceral abnormalities. However,
the heart weight of both male and female pups in the 3000 ppm (0.3%)
group was significantly lower than the control group, as was the
spleen weight of the male group. The kidneys of males and livers of
females also showed some changes from controls (Anon., 1977b).
Short-term studies
Rat
Groups each of 40 rats (equally divided by sex) were maintained
on diets containing 0, 10 000, 25 000, 50 000 or 100 000 ppm (0, 1,
2.5, 5 or 10%) of Kollidon CE 5050 (PVPP) for four weeks, and then for
two weeks on diets free of PVPP. Serum clinical chemistry, haematology
and urinalysis were carried out on days 7, 28 and 42 of the study. At
termination of the study, the animals were autopsied; absolute and
relative weight was determined for the liver, heart, kidney and
spleen. A histological examination was made of the principal organs
and tissues. There was a slight decrease in body weight at the highest
doses fed. Clinical chemistry and haematology for control and test
animals were similar. Urinalysis showed a dose-dependent increase in
urinary casts in the urine sediment of female rats during the first
four weeks of treatment, but this effect was no longer observed when
the rats were returned to diets free of PVPP. No other compound-
related effects were observed. PVPP was not deposited in the mucosa of
the small intestine or the mesenteric lymph nodes (Anon., 1977a). In
another study, groups each of 40 FDRL/Wistar rats equally divided by
sex were fed diets containing 0, 2 or 10% PVPP for 90 days. One of the
control groups was utilized as a pair feeding control with the high-
level groups. Haematological, clinical biochemistry and urinalysis
studies were carried out on day 90 prior to termination of the study.
At termination of the study, the animals were autopsied and absolute
and relative organ/body weights determined for brain, heart,
pituitary, thyroid, liver, kidney, spleen, adrenals and gonads. A
histological examination was made of the principal organs and tissues
from the high-dose and control animals. The behaviour and appearance
of the rats were normal during the study. Food consumption, feed
efficiency and body weight gain were similar for test and control
animals. No compound-related effects were observed in the clinical
studies, or at autopsy or following histological examination of the
tissues (Anon., 1976).
Dog
Groups each of six beagle dogs (equally divided by sex) were
dosed daily by stomach tube with an aqueous suspension of PVPP
equivalent to 1000, 2000 or 5050 mg/kg bw for a period of four weeks.
Control animals received either water or a suspension of cellulose
(400 mg/kg bw) in water. Haematology, clinical chemistry and
urinalysis were carried out prior to administration of the test
compound and at the end of the test period. Electrocardiography
examinations were made before and two hours post-administration of the
test compound. Ophthalmic and auditory tests as well as a dental
inspection were carried out during the course of the study. At the end
of the test period, the dogs were sacrificed and a complete autopsy
was performed. Absolute and relative weights were determined for the
internal organs, and a complete histological examination made of
organs and tissues. In addition, the liver, kidneys and mesenteric
lymph nodes were evaluated for possible PVPP storage. During the
course of the study, there were no compound-related effects on food
and water intake and body weight. Haematology, clinical chemistry and
urinalysis were similar for test and control animals and within normal
limits. No compound-related histopathology was observed. There was no
accumulation of PVPP in the liver, kidneys and mesenteric lymph nodes
(Leuschner et al., 1975).
In another study, groups each of eight beagle dogs (equally
divided by sex) were dosed daily by stomach tube with an aqueous
suspension of PVPP equivalent to 300, 1200 or 4888 mg/kg bw for a
period of 26 weeks. Control animals received either water or 4800 mg
cellulose (type 402-26)/kg bw. Because dogs at the high-dose level
showed increasing emesis during weeks 7-12, the test substance was
given in divided doses. No intolerance was observed at the lower dose
levels. Haematology, clinical chemistry and urinalysis were carried
out on animals prior to administration of the test compounds, and in
weeks 6, 18 and 26 of the test. Electrocardiography, ophthalmic and
auditory tests were also carried out. In addition, an examination of
the circulatory function was made after 26 weeks' treatment. At the
end of the test period, the dogs were sacrificed and a complete
autopsy carried out. Absolute and relative weights were determined for
the internal organs, and a complete histological examination made of
organs and tissues. Special staining techniques were used to
investigate possible PVPP deposition in the liver, kidneys and
mesenteric lymph nodes.
During the course of the study, there were no compound-related
effects on behaviour, food and water intake, and growth. None of the
parameters studied showed any compound-related effects nor was there
any evidence of tissue storage of PVPP (Leuschner et al., 1977).
Comments
Studies with 14C-labelled PVPP in the rat indicate an almost
complete lack of absorption of the orally administered material. The
small amount of 14C detected in the tissues and urine is most likely
due to the presence of low M.W. polymers in the PVPP rather than any
absorption of PVPP per se. Studies in the rat failed to demonstrate
any biliary excretion of the test compound. In addition, there does
not appear to be any accumulation of the PVPP in the gastrointestinal
tract. Short-term feeding studies in the rat and dog, with levels as
high as 10% of the diet in the rat and 2% of the diet in the dog, did
not demonstrate any adverse effects or tissue deposition of PVPP. Any
toxicological questions relating to the safety of the small amount of
absorbed PVPP are adequately answered with previous studies with PVPP
(WHO, 1980). These include lifetime feeding studies in the rat,
teratogenicity studies as well as mutagenicity and transformation
tests in vitro. In addition, exposure to low M.W. weight compounds
that might be present in the PVPP is expected to be infinitesimally
small.
EVALUATION
Estimate of acceptable daily intake for man
ADI not specified.*
* The statement "ADI not specified" means that, on the basis of the
available data (chemical, biochemical, toxicological, and other),
the total daily intake of the substance, arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background in food, does not, in the opinion of the
Committee, represent a hazard to health. For this reason, and for
reasons stated in the individual evaluations, the establishment
of a numerical figure for an acceptable daily intake (ADI) is not
deemed necessary.
REFERENCES
Anon. (1976) Ninety-day feeding study with polyvinylpyrrolidone in
rats. Unpublished report from B.A.S.F., submitted to the World
Health Organization by GAF Corporation, New Jersey, USA
Anon. (1977a) Bericht uber die Prufung der Toxizitat von Kollidon
CE-5050 im 4-Wochen-Futterungsversuch an der Ratte. Unpublished
report from B.A.S.F., submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Anon. (1977b) Prenatal, perinatal, and post natal toxicity of kollidon
CE-5050 in rats. Unpublished study from B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
Digenis, George A. (1979) Oral absorption study of PVPP. Preliminary
report, submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Istin, M. (1979) Study of the urinary, biliary, and fecal execution of
14C by rats treated with labeled polyvinylpyrrolidone by gastric
intubation. (Document prepared by the Laboratory for the Study of
Drug Metabolism, Department of Biology, Atomic Energy Commission,
France, for B.A.S.F.), submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Leuschner, F. et al. (1975) Oral toxicity of kollidon CE-5050 in the
beagle dog. Repeated dosage over four weeks. Unpublished report
for B.A.S.F., submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Leuschner, F. et al. (1977) Toxicity study of kollidon CE-5050 (Batch
XXIV) administered by stomach tube to beagle dogs. Repeated
dosage over six months. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, to B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
World Health Organization (1980) Toxicological evaluation of certain
food additives, WHO Food Additives Series, No. 15
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male Sprague-Dawley rats weighing between 200 and 300 g were
dosed orally (gastric gavage) with 2.25 mg of 14C-PVPP suspended in
0.1 ml of distilled water. At 6, 12, 24 and 48 hours after dosing
urine and faeces were collected. The rats were then sacrificed and the
major organs (spleen, kidneys, liver, lungs, thymus and adrenal
glands) and blood removed. The 14C content of the urine, faeces,
blood and organs was determined. Less than 1% of the administered 14C
dose was detected in the major organs, blood and urine. Sixty-three to
74% of the 14C was recovered in the faeces during the 48-hour period,
with most of the 14C being recovered 12 hours after dosing (Digenis,
1979).
Six Sprague-Dawley (I.F.F. A CREDD) rats (three male and three
female), weighing 180-210 g, received a single oral dose of 14C-PVPP
suspension by gastric intubation. Each dose contained approximately
250 mg PVPP. The PVPP used had been treated by dialysis and
ultrafiltration to remove polymers of M.W. of less than 10 000. Urine
and faeces were collected at 12-hour time intervals for a period of
five days after dosing and 14C excretion determined. The animals were
then sacrificed and the radioactivity remaining in the carcass,
gastrointestinal tract and lungs, determined. About 0.128% of the
administered 14C was excreted in the urine, with most of the 14C
being excreted in the first 24 hours. Eighty to 99% of the 14C was
recovered in the faeces in the period 12-24 hours. Less than 0.1% of
the administered 14C was recovered in the carcass, with most being in
the gastrointestinal tract. There was no evidence of preferential
binding of the 14C to any organ or tissue (Istin, 1979).
In another study, a group of four rats (male) weighing 250-300 g
had their bile ducts catheterized. After resting one day, the rats
received a single dose of 14C-PVPP (250 mg) suspension by gavage.
Bile was collected at 1.5-hour intervals, urine and faeces at 24-hour
intervals. At the termination of the study, the animals were
sacrificed and the 14C in the carcass measured. Approximately
0.00816% of the administered 14C was recovered in the bile. Urine,
faecal and carcass distribution of 14C was similar to that in the
previous study (Istin, 1979).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups each of 26 pregnant rats (SPF rats) were dosed by gavage
with a suspension of PVPP (Kollidon CE 5050) in carboxymethyl
cellulose in doses of 1000 or 3000 mg/kg. Other groups served as
vehicle control or received no treatment. Dosing was from day 6 to day
15 of gestation. The test animals were sacrificed on day 20 of
gestation and the foetuses examined. Two-thirds of the foetuses of
each litter were examined for skeletal changes and the remaining
one-third for visceral changes. Weight gain was similar for all
groups, with the exception of the high-dose group, that showed a
slight decrease in weight gain during the period of administration of
the test compound. However, at termination of the study the weight
gain was comparable in all groups. No significant clinical symptoms
were observed in the test animals. There were no maternal deaths and
no compound-related changes were observed at autopsy. The conception
rate and percentage of live and dead implantations were similar for
test and control animals. The weight of the foetuses of test animals
was slightly lower than those of controls, but no changes were
observed in the foetal length. There were no compound-related skeletal
or visceral abnormalities (Anon., 1977a).
An additional two groups each of 12 pregnant rats (SPF rats) were
dosed by gavage with a suspension of PVPP (Kollidon CD 5050) in
carboxymethyl cellulose in doses of 1000 or 3000 mg/kg bw. Dosing was
from day 15 to day 21 post-parturition. The rats in these groups were
allowed to litter spontaneously and rear their progeny to day 21
post-parturition. There were no significant differences in weight gain
for test and control animals. None of the test animals died. The time
of delivery and average litter size were similar for test and control
animals. At termination of the study, none of the mothers showed any
macroscopic, compound-related effects. The pups of the treated animals
showed similar mortality, body weight increases and behaviour (as
measured by swimming test on day 14) as controls. At autopsy there
were no compound-related skeletal or visceral abnormalities. However,
the heart weight of both male and female pups in the 3000 ppm (0.3%)
group was significantly lower than the control group, as was the
spleen weight of the male group. The kidneys of males and livers of
females also showed some changes from controls (Anon., 1977b).
Short-term studies
Rat
Groups each of 40 rats (equally divided by sex) were maintained
on diets containing 0, 10 000, 25 000, 50 000 or 100 000 ppm (0, 1,
2.5, 5 or 10%) of Kollidon CE 5050 (PVPP) for four weeks, and then for
two weeks on diets free of PVPP. Serum clinical chemistry, haematology
and urinalysis were carried out on days 7, 28 and 42 of the study. At
termination of the study, the animals were autopsied; absolute and
relative weight was determined for the liver, heart, kidney and
spleen. A histological examination was made of the principal organs
and tissues. There was a slight decrease in body weight at the highest
doses fed. Clinical chemistry and haematology for control and test
animals were similar. Urinalysis showed a dose-dependent increase in
urinary casts in the urine sediment of female rats during the first
four weeks of treatment, but this effect was no longer observed when
the rats were returned to diets free of PVPP. No other compound-
related effects were observed. PVPP was not deposited in the mucosa of
the small intestine or the mesenteric lymph nodes (Anon., 1977a). In
another study, groups each of 40 FDRL/Wistar rats equally divided by
sex were fed diets containing 0, 2 or 10% PVPP for 90 days. One of the
control groups was utilized as a pair feeding control with the high-
level groups. Haematological, clinical biochemistry and urinalysis
studies were carried out on day 90 prior to termination of the study.
At termination of the study, the animals were autopsied and absolute
and relative organ/body weights determined for brain, heart,
pituitary, thyroid, liver, kidney, spleen, adrenals and gonads. A
histological examination was made of the principal organs and tissues
from the high-dose and control animals. The behaviour and appearance
of the rats were normal during the study. Food consumption, feed
efficiency and body weight gain were similar for test and control
animals. No compound-related effects were observed in the clinical
studies, or at autopsy or following histological examination of the
tissues (Anon., 1976).
Dog
Groups each of six beagle dogs (equally divided by sex) were
dosed daily by stomach tube with an aqueous suspension of PVPP
equivalent to 1000, 2000 or 5050 mg/kg bw for a period of four weeks.
Control animals received either water or a suspension of cellulose
(400 mg/kg bw) in water. Haematology, clinical chemistry and
urinalysis were carried out prior to administration of the test
compound and at the end of the test period. Electrocardiography
examinations were made before and two hours post-administration of the
test compound. Ophthalmic and auditory tests as well as a dental
inspection were carried out during the course of the study. At the end
of the test period, the dogs were sacrificed and a complete autopsy
was performed. Absolute and relative weights were determined for the
internal organs, and a complete histological examination made of
organs and tissues. In addition, the liver, kidneys and mesenteric
lymph nodes were evaluated for possible PVPP storage. During the
course of the study, there were no compound-related effects on food
and water intake and body weight. Haematology, clinical chemistry and
urinalysis were similar for test and control animals and within normal
limits. No compound-related histopathology was observed. There was no
accumulation of PVPP in the liver, kidneys and mesenteric lymph nodes
(Leuschner et al., 1975).
In another study, groups each of eight beagle dogs (equally
divided by sex) were dosed daily by stomach tube with an aqueous
suspension of PVPP equivalent to 300, 1200 or 4888 mg/kg bw for a
period of 26 weeks. Control animals received either water or 4800 mg
cellulose (type 402-26)/kg bw. Because dogs at the high-dose level
showed increasing emesis during weeks 7-12, the test substance was
given in divided doses. No intolerance was observed at the lower dose
levels. Haematology, clinical chemistry and urinalysis were carried
out on animals prior to administration of the test compounds, and in
weeks 6, 18 and 26 of the test. Electrocardiography, ophthalmic and
auditory tests were also carried out. In addition, an examination of
the circulatory function was made after 26 weeks' treatment. At the
end of the test period, the dogs were sacrificed and a complete
autopsy carried out. Absolute and relative weights were determined for
the internal organs, and a complete histological examination made of
organs and tissues. Special staining techniques were used to
investigate possible PVPP deposition in the liver, kidneys and
mesenteric lymph nodes.
During the course of the study, there were no compound-related
effects on behaviour, food and water intake, and growth. None of the
parameters studied showed any compound-related effects nor was there
any evidence of tissue storage of PVPP (Leuschner et al., 1977).
Comments
Studies with 14C-labelled PVPP in the rat indicate an almost
complete lack of absorption of the orally administered material. The
small amount of 14C detected in the tissues and urine is most likely
due to the presence of low M.W. polymers in the PVPP rather than any
absorption of PVPP per se. Studies in the rat failed to demonstrate
any biliary excretion of the test compound. In addition, there does
not appear to be any accumulation of the PVPP in the gastrointestinal
tract. Short-term feeding studies in the rat and dog, with levels as
high as 10% of the diet in the rat and 2% of the diet in the dog, did
not demonstrate any adverse effects or tissue deposition of PVPP. Any
toxicological questions relating to the safety of the small amount of
absorbed PVPP are adequately answered with previous studies with PVPP
(WHO, 1980). These include lifetime feeding studies in the rat,
teratogenicity studies as well as mutagenicity and transformation
tests in vitro. In addition, exposure to low M.W. weight compounds
that might be present in the PVPP is expected to be infinitesimally
small.
EVALUATION
Estimate of acceptable daily intake for man
ADI not specified.*
* The statement "ADI not specified" means that, on the basis of the
available data (chemical, biochemical, toxicological, and other),
the total daily intake of the substance, arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background in food, does not, in the opinion of the
Committee, represent a hazard to health. For this reason, and for
reasons stated in the individual evaluations, the establishment
of a numerical figure for an acceptable daily intake (ADI) is not
deemed necessary.
REFERENCES
Anon. (1976) Ninety-day feeding study with polyvinylpyrrolidone in
rats. Unpublished report from B.A.S.F., submitted to the World
Health Organization by GAF Corporation, New Jersey, USA
Anon. (1977a) Bericht uber die Prufung der Toxizitat von Kollidon
CE-5050 im 4-Wochen-Futterungsversuch an der Ratte. Unpublished
report from B.A.S.F., submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Anon. (1977b) Prenatal, perinatal, and post natal toxicity of kollidon
CE-5050 in rats. Unpublished study from B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
Digenis, George A. (1979) Oral absorption study of PVPP. Preliminary
report, submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Istin, M. (1979) Study of the urinary, biliary, and fecal execution of
14C by rats treated with labeled polyvinylpyrrolidone by gastric
intubation. (Document prepared by the Laboratory for the Study of
Drug Metabolism, Department of Biology, Atomic Energy Commission,
France, for B.A.S.F.), submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Leuschner, F. et al. (1975) Oral toxicity of kollidon CE-5050 in the
beagle dog. Repeated dosage over four weeks. Unpublished report
for B.A.S.F., submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Leuschner, F. et al. (1977) Toxicity study of kollidon CE-5050 (Batch
XXIV) administered by stomach tube to beagle dogs. Repeated
dosage over six months. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, to B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
World Health Organization (1980) Toxicological evaluation of certain
food additives, WHO Food Additives Series, No. 15
 BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male Sprague-Dawley rats weighing between 200 and 300 g were
dosed orally (gastric gavage) with 2.25 mg of 14C-PVPP suspended in
0.1 ml of distilled water. At 6, 12, 24 and 48 hours after dosing
urine and faeces were collected. The rats were then sacrificed and the
major organs (spleen, kidneys, liver, lungs, thymus and adrenal
glands) and blood removed. The 14C content of the urine, faeces,
blood and organs was determined. Less than 1% of the administered 14C
dose was detected in the major organs, blood and urine. Sixty-three to
74% of the 14C was recovered in the faeces during the 48-hour period,
with most of the 14C being recovered 12 hours after dosing (Digenis,
1979).
Six Sprague-Dawley (I.F.F. A CREDD) rats (three male and three
female), weighing 180-210 g, received a single oral dose of 14C-PVPP
suspension by gastric intubation. Each dose contained approximately
250 mg PVPP. The PVPP used had been treated by dialysis and
ultrafiltration to remove polymers of M.W. of less than 10 000. Urine
and faeces were collected at 12-hour time intervals for a period of
five days after dosing and 14C excretion determined. The animals were
then sacrificed and the radioactivity remaining in the carcass,
gastrointestinal tract and lungs, determined. About 0.128% of the
administered 14C was excreted in the urine, with most of the 14C
being excreted in the first 24 hours. Eighty to 99% of the 14C was
recovered in the faeces in the period 12-24 hours. Less than 0.1% of
the administered 14C was recovered in the carcass, with most being in
the gastrointestinal tract. There was no evidence of preferential
binding of the 14C to any organ or tissue (Istin, 1979).
In another study, a group of four rats (male) weighing 250-300 g
had their bile ducts catheterized. After resting one day, the rats
received a single dose of 14C-PVPP (250 mg) suspension by gavage.
Bile was collected at 1.5-hour intervals, urine and faeces at 24-hour
intervals. At the termination of the study, the animals were
sacrificed and the 14C in the carcass measured. Approximately
0.00816% of the administered 14C was recovered in the bile. Urine,
faecal and carcass distribution of 14C was similar to that in the
previous study (Istin, 1979).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups each of 26 pregnant rats (SPF rats) were dosed by gavage
with a suspension of PVPP (Kollidon CE 5050) in carboxymethyl
cellulose in doses of 1000 or 3000 mg/kg. Other groups served as
vehicle control or received no treatment. Dosing was from day 6 to day
15 of gestation. The test animals were sacrificed on day 20 of
gestation and the foetuses examined. Two-thirds of the foetuses of
each litter were examined for skeletal changes and the remaining
one-third for visceral changes. Weight gain was similar for all
groups, with the exception of the high-dose group, that showed a
slight decrease in weight gain during the period of administration of
the test compound. However, at termination of the study the weight
gain was comparable in all groups. No significant clinical symptoms
were observed in the test animals. There were no maternal deaths and
no compound-related changes were observed at autopsy. The conception
rate and percentage of live and dead implantations were similar for
test and control animals. The weight of the foetuses of test animals
was slightly lower than those of controls, but no changes were
observed in the foetal length. There were no compound-related skeletal
or visceral abnormalities (Anon., 1977a).
An additional two groups each of 12 pregnant rats (SPF rats) were
dosed by gavage with a suspension of PVPP (Kollidon CD 5050) in
carboxymethyl cellulose in doses of 1000 or 3000 mg/kg bw. Dosing was
from day 15 to day 21 post-parturition. The rats in these groups were
allowed to litter spontaneously and rear their progeny to day 21
post-parturition. There were no significant differences in weight gain
for test and control animals. None of the test animals died. The time
of delivery and average litter size were similar for test and control
animals. At termination of the study, none of the mothers showed any
macroscopic, compound-related effects. The pups of the treated animals
showed similar mortality, body weight increases and behaviour (as
measured by swimming test on day 14) as controls. At autopsy there
were no compound-related skeletal or visceral abnormalities. However,
the heart weight of both male and female pups in the 3000 ppm (0.3%)
group was significantly lower than the control group, as was the
spleen weight of the male group. The kidneys of males and livers of
females also showed some changes from controls (Anon., 1977b).
Short-term studies
Rat
Groups each of 40 rats (equally divided by sex) were maintained
on diets containing 0, 10 000, 25 000, 50 000 or 100 000 ppm (0, 1,
2.5, 5 or 10%) of Kollidon CE 5050 (PVPP) for four weeks, and then for
two weeks on diets free of PVPP. Serum clinical chemistry, haematology
and urinalysis were carried out on days 7, 28 and 42 of the study. At
termination of the study, the animals were autopsied; absolute and
relative weight was determined for the liver, heart, kidney and
spleen. A histological examination was made of the principal organs
and tissues. There was a slight decrease in body weight at the highest
doses fed. Clinical chemistry and haematology for control and test
animals were similar. Urinalysis showed a dose-dependent increase in
urinary casts in the urine sediment of female rats during the first
four weeks of treatment, but this effect was no longer observed when
the rats were returned to diets free of PVPP. No other compound-
related effects were observed. PVPP was not deposited in the mucosa of
the small intestine or the mesenteric lymph nodes (Anon., 1977a). In
another study, groups each of 40 FDRL/Wistar rats equally divided by
sex were fed diets containing 0, 2 or 10% PVPP for 90 days. One of the
control groups was utilized as a pair feeding control with the high-
level groups. Haematological, clinical biochemistry and urinalysis
studies were carried out on day 90 prior to termination of the study.
At termination of the study, the animals were autopsied and absolute
and relative organ/body weights determined for brain, heart,
pituitary, thyroid, liver, kidney, spleen, adrenals and gonads. A
histological examination was made of the principal organs and tissues
from the high-dose and control animals. The behaviour and appearance
of the rats were normal during the study. Food consumption, feed
efficiency and body weight gain were similar for test and control
animals. No compound-related effects were observed in the clinical
studies, or at autopsy or following histological examination of the
tissues (Anon., 1976).
Dog
Groups each of six beagle dogs (equally divided by sex) were
dosed daily by stomach tube with an aqueous suspension of PVPP
equivalent to 1000, 2000 or 5050 mg/kg bw for a period of four weeks.
Control animals received either water or a suspension of cellulose
(400 mg/kg bw) in water. Haematology, clinical chemistry and
urinalysis were carried out prior to administration of the test
compound and at the end of the test period. Electrocardiography
examinations were made before and two hours post-administration of the
test compound. Ophthalmic and auditory tests as well as a dental
inspection were carried out during the course of the study. At the end
of the test period, the dogs were sacrificed and a complete autopsy
was performed. Absolute and relative weights were determined for the
internal organs, and a complete histological examination made of
organs and tissues. In addition, the liver, kidneys and mesenteric
lymph nodes were evaluated for possible PVPP storage. During the
course of the study, there were no compound-related effects on food
and water intake and body weight. Haematology, clinical chemistry and
urinalysis were similar for test and control animals and within normal
limits. No compound-related histopathology was observed. There was no
accumulation of PVPP in the liver, kidneys and mesenteric lymph nodes
(Leuschner et al., 1975).
In another study, groups each of eight beagle dogs (equally
divided by sex) were dosed daily by stomach tube with an aqueous
suspension of PVPP equivalent to 300, 1200 or 4888 mg/kg bw for a
period of 26 weeks. Control animals received either water or 4800 mg
cellulose (type 402-26)/kg bw. Because dogs at the high-dose level
showed increasing emesis during weeks 7-12, the test substance was
given in divided doses. No intolerance was observed at the lower dose
levels. Haematology, clinical chemistry and urinalysis were carried
out on animals prior to administration of the test compounds, and in
weeks 6, 18 and 26 of the test. Electrocardiography, ophthalmic and
auditory tests were also carried out. In addition, an examination of
the circulatory function was made after 26 weeks' treatment. At the
end of the test period, the dogs were sacrificed and a complete
autopsy carried out. Absolute and relative weights were determined for
the internal organs, and a complete histological examination made of
organs and tissues. Special staining techniques were used to
investigate possible PVPP deposition in the liver, kidneys and
mesenteric lymph nodes.
During the course of the study, there were no compound-related
effects on behaviour, food and water intake, and growth. None of the
parameters studied showed any compound-related effects nor was there
any evidence of tissue storage of PVPP (Leuschner et al., 1977).
Comments
Studies with 14C-labelled PVPP in the rat indicate an almost
complete lack of absorption of the orally administered material. The
small amount of 14C detected in the tissues and urine is most likely
due to the presence of low M.W. polymers in the PVPP rather than any
absorption of PVPP per se. Studies in the rat failed to demonstrate
any biliary excretion of the test compound. In addition, there does
not appear to be any accumulation of the PVPP in the gastrointestinal
tract. Short-term feeding studies in the rat and dog, with levels as
high as 10% of the diet in the rat and 2% of the diet in the dog, did
not demonstrate any adverse effects or tissue deposition of PVPP. Any
toxicological questions relating to the safety of the small amount of
absorbed PVPP are adequately answered with previous studies with PVPP
(WHO, 1980). These include lifetime feeding studies in the rat,
teratogenicity studies as well as mutagenicity and transformation
tests in vitro. In addition, exposure to low M.W. weight compounds
that might be present in the PVPP is expected to be infinitesimally
small.
EVALUATION
Estimate of acceptable daily intake for man
ADI not specified.*
* The statement "ADI not specified" means that, on the basis of the
available data (chemical, biochemical, toxicological, and other),
the total daily intake of the substance, arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background in food, does not, in the opinion of the
Committee, represent a hazard to health. For this reason, and for
reasons stated in the individual evaluations, the establishment
of a numerical figure for an acceptable daily intake (ADI) is not
deemed necessary.
REFERENCES
Anon. (1976) Ninety-day feeding study with polyvinylpyrrolidone in
rats. Unpublished report from B.A.S.F., submitted to the World
Health Organization by GAF Corporation, New Jersey, USA
Anon. (1977a) Bericht uber die Prufung der Toxizitat von Kollidon
CE-5050 im 4-Wochen-Futterungsversuch an der Ratte. Unpublished
report from B.A.S.F., submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Anon. (1977b) Prenatal, perinatal, and post natal toxicity of kollidon
CE-5050 in rats. Unpublished study from B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
Digenis, George A. (1979) Oral absorption study of PVPP. Preliminary
report, submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Istin, M. (1979) Study of the urinary, biliary, and fecal execution of
14C by rats treated with labeled polyvinylpyrrolidone by gastric
intubation. (Document prepared by the Laboratory for the Study of
Drug Metabolism, Department of Biology, Atomic Energy Commission,
France, for B.A.S.F.), submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Leuschner, F. et al. (1975) Oral toxicity of kollidon CE-5050 in the
beagle dog. Repeated dosage over four weeks. Unpublished report
for B.A.S.F., submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Leuschner, F. et al. (1977) Toxicity study of kollidon CE-5050 (Batch
XXIV) administered by stomach tube to beagle dogs. Repeated
dosage over six months. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, to B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
World Health Organization (1980) Toxicological evaluation of certain
food additives, WHO Food Additives Series, No. 15
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male Sprague-Dawley rats weighing between 200 and 300 g were
dosed orally (gastric gavage) with 2.25 mg of 14C-PVPP suspended in
0.1 ml of distilled water. At 6, 12, 24 and 48 hours after dosing
urine and faeces were collected. The rats were then sacrificed and the
major organs (spleen, kidneys, liver, lungs, thymus and adrenal
glands) and blood removed. The 14C content of the urine, faeces,
blood and organs was determined. Less than 1% of the administered 14C
dose was detected in the major organs, blood and urine. Sixty-three to
74% of the 14C was recovered in the faeces during the 48-hour period,
with most of the 14C being recovered 12 hours after dosing (Digenis,
1979).
Six Sprague-Dawley (I.F.F. A CREDD) rats (three male and three
female), weighing 180-210 g, received a single oral dose of 14C-PVPP
suspension by gastric intubation. Each dose contained approximately
250 mg PVPP. The PVPP used had been treated by dialysis and
ultrafiltration to remove polymers of M.W. of less than 10 000. Urine
and faeces were collected at 12-hour time intervals for a period of
five days after dosing and 14C excretion determined. The animals were
then sacrificed and the radioactivity remaining in the carcass,
gastrointestinal tract and lungs, determined. About 0.128% of the
administered 14C was excreted in the urine, with most of the 14C
being excreted in the first 24 hours. Eighty to 99% of the 14C was
recovered in the faeces in the period 12-24 hours. Less than 0.1% of
the administered 14C was recovered in the carcass, with most being in
the gastrointestinal tract. There was no evidence of preferential
binding of the 14C to any organ or tissue (Istin, 1979).
In another study, a group of four rats (male) weighing 250-300 g
had their bile ducts catheterized. After resting one day, the rats
received a single dose of 14C-PVPP (250 mg) suspension by gavage.
Bile was collected at 1.5-hour intervals, urine and faeces at 24-hour
intervals. At the termination of the study, the animals were
sacrificed and the 14C in the carcass measured. Approximately
0.00816% of the administered 14C was recovered in the bile. Urine,
faecal and carcass distribution of 14C was similar to that in the
previous study (Istin, 1979).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups each of 26 pregnant rats (SPF rats) were dosed by gavage
with a suspension of PVPP (Kollidon CE 5050) in carboxymethyl
cellulose in doses of 1000 or 3000 mg/kg. Other groups served as
vehicle control or received no treatment. Dosing was from day 6 to day
15 of gestation. The test animals were sacrificed on day 20 of
gestation and the foetuses examined. Two-thirds of the foetuses of
each litter were examined for skeletal changes and the remaining
one-third for visceral changes. Weight gain was similar for all
groups, with the exception of the high-dose group, that showed a
slight decrease in weight gain during the period of administration of
the test compound. However, at termination of the study the weight
gain was comparable in all groups. No significant clinical symptoms
were observed in the test animals. There were no maternal deaths and
no compound-related changes were observed at autopsy. The conception
rate and percentage of live and dead implantations were similar for
test and control animals. The weight of the foetuses of test animals
was slightly lower than those of controls, but no changes were
observed in the foetal length. There were no compound-related skeletal
or visceral abnormalities (Anon., 1977a).
An additional two groups each of 12 pregnant rats (SPF rats) were
dosed by gavage with a suspension of PVPP (Kollidon CD 5050) in
carboxymethyl cellulose in doses of 1000 or 3000 mg/kg bw. Dosing was
from day 15 to day 21 post-parturition. The rats in these groups were
allowed to litter spontaneously and rear their progeny to day 21
post-parturition. There were no significant differences in weight gain
for test and control animals. None of the test animals died. The time
of delivery and average litter size were similar for test and control
animals. At termination of the study, none of the mothers showed any
macroscopic, compound-related effects. The pups of the treated animals
showed similar mortality, body weight increases and behaviour (as
measured by swimming test on day 14) as controls. At autopsy there
were no compound-related skeletal or visceral abnormalities. However,
the heart weight of both male and female pups in the 3000 ppm (0.3%)
group was significantly lower than the control group, as was the
spleen weight of the male group. The kidneys of males and livers of
females also showed some changes from controls (Anon., 1977b).
Short-term studies
Rat
Groups each of 40 rats (equally divided by sex) were maintained
on diets containing 0, 10 000, 25 000, 50 000 or 100 000 ppm (0, 1,
2.5, 5 or 10%) of Kollidon CE 5050 (PVPP) for four weeks, and then for
two weeks on diets free of PVPP. Serum clinical chemistry, haematology
and urinalysis were carried out on days 7, 28 and 42 of the study. At
termination of the study, the animals were autopsied; absolute and
relative weight was determined for the liver, heart, kidney and
spleen. A histological examination was made of the principal organs
and tissues. There was a slight decrease in body weight at the highest
doses fed. Clinical chemistry and haematology for control and test
animals were similar. Urinalysis showed a dose-dependent increase in
urinary casts in the urine sediment of female rats during the first
four weeks of treatment, but this effect was no longer observed when
the rats were returned to diets free of PVPP. No other compound-
related effects were observed. PVPP was not deposited in the mucosa of
the small intestine or the mesenteric lymph nodes (Anon., 1977a). In
another study, groups each of 40 FDRL/Wistar rats equally divided by
sex were fed diets containing 0, 2 or 10% PVPP for 90 days. One of the
control groups was utilized as a pair feeding control with the high-
level groups. Haematological, clinical biochemistry and urinalysis
studies were carried out on day 90 prior to termination of the study.
At termination of the study, the animals were autopsied and absolute
and relative organ/body weights determined for brain, heart,
pituitary, thyroid, liver, kidney, spleen, adrenals and gonads. A
histological examination was made of the principal organs and tissues
from the high-dose and control animals. The behaviour and appearance
of the rats were normal during the study. Food consumption, feed
efficiency and body weight gain were similar for test and control
animals. No compound-related effects were observed in the clinical
studies, or at autopsy or following histological examination of the
tissues (Anon., 1976).
Dog
Groups each of six beagle dogs (equally divided by sex) were
dosed daily by stomach tube with an aqueous suspension of PVPP
equivalent to 1000, 2000 or 5050 mg/kg bw for a period of four weeks.
Control animals received either water or a suspension of cellulose
(400 mg/kg bw) in water. Haematology, clinical chemistry and
urinalysis were carried out prior to administration of the test
compound and at the end of the test period. Electrocardiography
examinations were made before and two hours post-administration of the
test compound. Ophthalmic and auditory tests as well as a dental
inspection were carried out during the course of the study. At the end
of the test period, the dogs were sacrificed and a complete autopsy
was performed. Absolute and relative weights were determined for the
internal organs, and a complete histological examination made of
organs and tissues. In addition, the liver, kidneys and mesenteric
lymph nodes were evaluated for possible PVPP storage. During the
course of the study, there were no compound-related effects on food
and water intake and body weight. Haematology, clinical chemistry and
urinalysis were similar for test and control animals and within normal
limits. No compound-related histopathology was observed. There was no
accumulation of PVPP in the liver, kidneys and mesenteric lymph nodes
(Leuschner et al., 1975).
In another study, groups each of eight beagle dogs (equally
divided by sex) were dosed daily by stomach tube with an aqueous
suspension of PVPP equivalent to 300, 1200 or 4888 mg/kg bw for a
period of 26 weeks. Control animals received either water or 4800 mg
cellulose (type 402-26)/kg bw. Because dogs at the high-dose level
showed increasing emesis during weeks 7-12, the test substance was
given in divided doses. No intolerance was observed at the lower dose
levels. Haematology, clinical chemistry and urinalysis were carried
out on animals prior to administration of the test compounds, and in
weeks 6, 18 and 26 of the test. Electrocardiography, ophthalmic and
auditory tests were also carried out. In addition, an examination of
the circulatory function was made after 26 weeks' treatment. At the
end of the test period, the dogs were sacrificed and a complete
autopsy carried out. Absolute and relative weights were determined for
the internal organs, and a complete histological examination made of
organs and tissues. Special staining techniques were used to
investigate possible PVPP deposition in the liver, kidneys and
mesenteric lymph nodes.
During the course of the study, there were no compound-related
effects on behaviour, food and water intake, and growth. None of the
parameters studied showed any compound-related effects nor was there
any evidence of tissue storage of PVPP (Leuschner et al., 1977).
Comments
Studies with 14C-labelled PVPP in the rat indicate an almost
complete lack of absorption of the orally administered material. The
small amount of 14C detected in the tissues and urine is most likely
due to the presence of low M.W. polymers in the PVPP rather than any
absorption of PVPP per se. Studies in the rat failed to demonstrate
any biliary excretion of the test compound. In addition, there does
not appear to be any accumulation of the PVPP in the gastrointestinal
tract. Short-term feeding studies in the rat and dog, with levels as
high as 10% of the diet in the rat and 2% of the diet in the dog, did
not demonstrate any adverse effects or tissue deposition of PVPP. Any
toxicological questions relating to the safety of the small amount of
absorbed PVPP are adequately answered with previous studies with PVPP
(WHO, 1980). These include lifetime feeding studies in the rat,
teratogenicity studies as well as mutagenicity and transformation
tests in vitro. In addition, exposure to low M.W. weight compounds
that might be present in the PVPP is expected to be infinitesimally
small.
EVALUATION
Estimate of acceptable daily intake for man
ADI not specified.*
* The statement "ADI not specified" means that, on the basis of the
available data (chemical, biochemical, toxicological, and other),
the total daily intake of the substance, arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background in food, does not, in the opinion of the
Committee, represent a hazard to health. For this reason, and for
reasons stated in the individual evaluations, the establishment
of a numerical figure for an acceptable daily intake (ADI) is not
deemed necessary.
REFERENCES
Anon. (1976) Ninety-day feeding study with polyvinylpyrrolidone in
rats. Unpublished report from B.A.S.F., submitted to the World
Health Organization by GAF Corporation, New Jersey, USA
Anon. (1977a) Bericht uber die Prufung der Toxizitat von Kollidon
CE-5050 im 4-Wochen-Futterungsversuch an der Ratte. Unpublished
report from B.A.S.F., submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Anon. (1977b) Prenatal, perinatal, and post natal toxicity of kollidon
CE-5050 in rats. Unpublished study from B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
Digenis, George A. (1979) Oral absorption study of PVPP. Preliminary
report, submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Istin, M. (1979) Study of the urinary, biliary, and fecal execution of
14C by rats treated with labeled polyvinylpyrrolidone by gastric
intubation. (Document prepared by the Laboratory for the Study of
Drug Metabolism, Department of Biology, Atomic Energy Commission,
France, for B.A.S.F.), submitted to the World Health Organization
by GAF Corporation, New Jersey, USA
Leuschner, F. et al. (1975) Oral toxicity of kollidon CE-5050 in the
beagle dog. Repeated dosage over four weeks. Unpublished report
for B.A.S.F., submitted to the World Health Organization by GAF
Corporation, New Jersey, USA
Leuschner, F. et al. (1977) Toxicity study of kollidon CE-5050 (Batch
XXIV) administered by stomach tube to beagle dogs. Repeated
dosage over six months. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, to B.A.S.F., submitted to
the World Health Organization by GAF Corporation, New Jersey, USA
World Health Organization (1980) Toxicological evaluation of certain
food additives, WHO Food Additives Series, No. 15
