
TRENBOLONE ACETATE
EXPLANATION
Trenbolone acetate was considered at the twenty-sixth meeting of
the Joint FAO/WHO Expert Committee on Food Additives (Annex 1,
reference 59), but it could not be evaluated at that time because the
necessary documentation on residue levels, good animal husbandry in
relation to the use of the agent, and details of methods of analysis
were not available.
At the twenty-seventh meeting (Annex 1, reference 62) the
Committee provisionally accepted the use of trenbolone acetate as an
anabolic agent for the production of meat for human consumption in
accordance with good animal husbandry practice, and requested the
submission of the results of a study known to be in progress to
establish a no-hormonal-effect level in non-human primates.
This monograph contains the data previously considered by the
Committee, as well as data that have been submitted recently.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and metabolism
Rats
Male Sprague-Dawley (bile duct cannulated) rats received single
i.v. doses of 28 mg/kg b.w. 3H-labelled trenbolone acetate (TBA).
Eighty-four percent of the administered radioactivity was excreted via
the bile in 24 hours after dosing (6% "free", 37% as the glucuronide,
and 37% as the sulfate). 3-Ketotrienic structures accounted for 66% of
biliary radioactivity; 17-alpha-hydroxytrenbolone (alpha-TBOH) was not
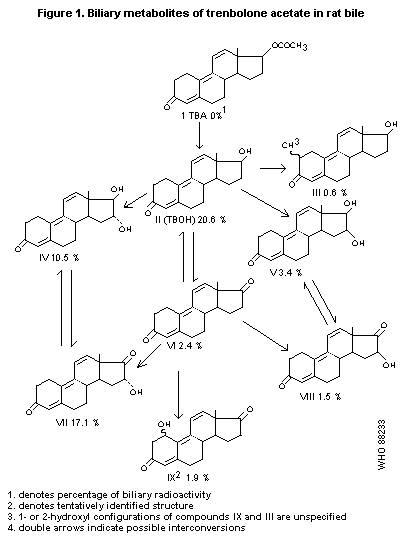
detected in the bile. The identified 3-ketotrienic metabolites are
presented in Figure 1 (Pottier et al., 1978).
Cattle
Two male calves, each given s.c. implantations with 140 mg TBA at
the base of the right ear, showed a high urinary elimination rate of
trenbolone (TBOH) (detected fluorometrically). Within 3 hours after
application relatively high concentrations were measured (50-80 ng/mg
creatinine); the maximum TBOH level was reached after 10 hours (about
120 ng/mg creatinine) followed by a sudden drop within two days.
Additional implantation of estradiol-17ß reduced TBOH excretion very
slightly (Bouffault, 1977).
Groups of 3 - 4 bull calves were given s.c. implantations of
20 mg 3H-estradiol-17ß or 20 mg 3H-estradiol-17ß + 140 mg TBOH.
TBOH caused a marked delay in estradiol excretion. In calves receiving
estradiol only, the maximum plasma estradiol-17ß level was 3 nmole/l,
and 95% of the applied radioactivity was excreted in the urine and
faeces within 20 days; after more than 31 days radioactivity was no
longer detectable in the urine or faeces. Calves treated with TBOH
showed a maximum plasma estradiol-17ß level of 0.33 nmole/l and
excretion of radioactivity was observed up to 107 days after
administration; at that time faecal and urinary radioactivity levels
were still 1.4 - 3 nCi/g (Riis & Suresh, 1976).
Twelve calves weighing 150 - 200 kg each received s.c. implants
in the ear containing 200 mg 3H-TBA. Half of the animals were
sacrificed at 15 days, the other half at 30 days after implantation.
Blood samples were taken at intervals between dosing and sacrifice. At
sacrifice, the liver, kidneys, and samples of muscle, fat, and bile
were taken for analysis. Concentrations of radioactivity in the plasma
were fairly constant during the experimental period, with mean levels
of 4 to 5 ng equivalents/ml. Tissue concentrations of radioactivity
were either similar at 15 and 30 days or were higher at 30 days.
 Highest concentrations were found in the liver (42 and 49 ng
equivalents/g at 15 and 30 days, respectively). Lower concentrations
were found in the kidneys (15 - 20 ng equivalents/g) and muscle and
fat (2 - 3 ng equivalents/g). High concentrations of radioactivity in
the bile (1073 and 736 ng equivalents/ml at 15 and 30 days,
respectively) indicate its importance in excretion of this compound.
Comparison of total and non-volatile radioactivity showed that only a
small amount of tritiated water was produced. About 10% of the
radioactivity in the liver samples was extracted by diethyl ether or
ethyl acetate, and this proportion increased to about 20 - 30%
following incubation with ß-glucuronidase, indicating the presence of
a glucuronide(s) (Hawkins et al., 1984).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. One heifer was killed 60 days after implantation;
the implant was removed from the other heifer after 60 days and the
animal was killed 16 days later. The H content in the liver, kidneys,
muscle, and fat varied from 0.5 to 25 ppb. Of these residues, 1 - 5%
was TBA, TBOH, and trenbolone glucuronide; up to 5% was found in other
organic-soluble material. Of the remaining radioactivity, about 50%
was water soluble, and the insoluble residue could be made water
soluble by treatment with the proteolytic enzymes pepsin and trypsin
(Ryan & Hoffman, 1978).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. After 60 days the implants, which still contained
31% of the initial radioactivity, were removed. One heifer was killed
immediately, the other was maintained for 16 days after implant
removal and then killed. Ethyl acetate-extractable radioactivity in
blood plasma could largely be ascribed to TBOH; in most cases no TBA
was found in plasma. Plasma concentrations during days 1 - 55 after
dosing were 5 - 13 ppb; after 58 days a large increase in both total
and nonvolatile radioactivity was observed (17 - 20 ppb). The
half-lives for plasma disappearance of total and non-volatile
radioactivity were 32 and 29 days, respectively, during the
implantation period and 18 and 14 days, respectively, during the
withdrawal period. Plasma ethyl acetate-extractable radioactivity
amounted to 10 - 74% of the total radioactivity during days 1 - 55
after implantation, and this declined to 5% at 16 days after implant
removal. In the 16 days from implant removal to sacrifice,
radioactivity decreased by 58% in muscle, 75% in liver, 77% in
kidneys, and 74% in fat (Chasseaud et al., 1976).
Heifers (aged 15 months, number not given) were given daily oral
doses of 0.4 or 8 mg TBA per animal for 9 weeks. After 1 and 2 weeks
TBA was detected in the urine. Two weeks after drug withdrawal the
compound was detected in some urine samples, whereas after 3 weeks no
TBA was detected (Stephany et al., 1976).
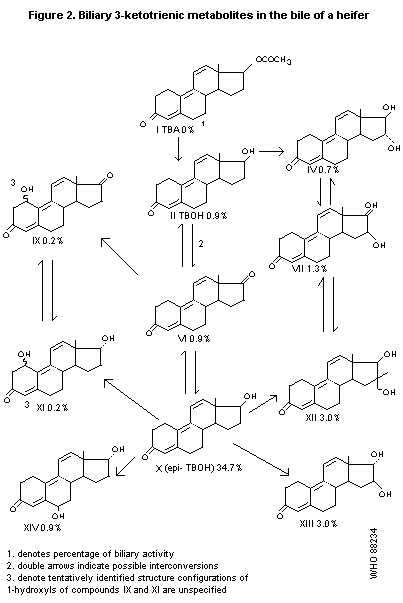
A 14-month-old heifer, after i.v. administration of 10 mg/kg b.w.
TBA, excreted 80% of the administered radioactivity in the bile during
the first 24 hours; 3.5% was in the free form, 30% was excreted as
glucuronides, and 30% as sulfates. Metabolites with the 3-ketotrienic
structure that were identified in the bile are presented in Figure 2.
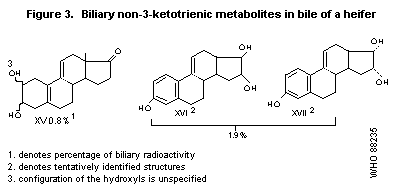
Three compounds that had lost their ketotrienic structure were also
isolated; these metabolites are presented in Figure 3. Less than 1% of
the administered radioactivity was isolated as tritiated water
(Pottier et al., 1978).
Specimens of muscle from the back and rear leg and specimens from
the liver were taken from two heifers that had been implanted two
months earlier with 300 mg 3H-TBA. In addition, bile was collected
by catheterization of one heifer on days preceding slaughter. The
radioactivity content of muscle, independent of its location, was
one-tenth the level in liver, whereas radioactivity levels in the bile
were 15 times higher than in liver tissue, alpha-TBOH and ß-TBOH
concentrations were determined by reverse isotopic dilution. On
average, the concentration of ß-TBOH was 0.05 to 0.1 ppb in various
tissues, whereas that of alpha-TBOH, which was only 0.005 ppb in the
muscle, reached 0.88 ppb in the liver. Following enzymolysis, ß-TBOH
was not detected in the bile, which contained, by contrast, nearly
200 ppb alpha-TBOH. Thus, alpha-TBOH represented 10% of total TBOH in
muscle, 90 - 95% in the liver, and more than 99% of the total in bile
(Pottier, 1979).
3H-TBA was implanted in the ears of two heifers (300 mg;
388 mCi) and the distribution of the radioactivity in liver and muscle
tissue was determined, applying rigorously standardized organic or
aqueous extraction procedures, either directly or following enzymatic
hydrolysis and proteolytic procedures. These steps yielded almost 100%
recovery of the radioactivity and indicate that only 5 to 15% of the
total residues were extractable with organic solvents. The remaining
radioactivity was either soluble in aqueous media or remained bound to
tissue structures. In another experiment, liver tissue from a calf
treated with 3500 mg TBA 68 days prior to slaughter was examined by
applying radioimmunoassay techniques to determine TBA/TBOH ratios.
Trienic-steroid type residues were obtained only from fractions
containing residues extractable with organic solvents (Hoffman et al.,
1984).
Two barren cows, after i.v. administration of 10 mg 3H-TBA per
animal, displayed very rapid hydrolysis of 3H-TBA in the blood
plasma; after 0.1 hour, only 2% of the radioactivity was recovered as
TBA, whereas 70% was recovered as TBOH. After 2 hours, radioactivity
could no longer be extracted, and in the extracted fraction polar
components predominated. From 3 - 8 hours TBOH disappeared from the
blood (half-life, 1.5 hours) (Pottier et al., 1975).
Highest concentrations were found in the liver (42 and 49 ng
equivalents/g at 15 and 30 days, respectively). Lower concentrations
were found in the kidneys (15 - 20 ng equivalents/g) and muscle and
fat (2 - 3 ng equivalents/g). High concentrations of radioactivity in
the bile (1073 and 736 ng equivalents/ml at 15 and 30 days,
respectively) indicate its importance in excretion of this compound.
Comparison of total and non-volatile radioactivity showed that only a
small amount of tritiated water was produced. About 10% of the
radioactivity in the liver samples was extracted by diethyl ether or
ethyl acetate, and this proportion increased to about 20 - 30%
following incubation with ß-glucuronidase, indicating the presence of
a glucuronide(s) (Hawkins et al., 1984).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. One heifer was killed 60 days after implantation;
the implant was removed from the other heifer after 60 days and the
animal was killed 16 days later. The H content in the liver, kidneys,
muscle, and fat varied from 0.5 to 25 ppb. Of these residues, 1 - 5%
was TBA, TBOH, and trenbolone glucuronide; up to 5% was found in other
organic-soluble material. Of the remaining radioactivity, about 50%
was water soluble, and the insoluble residue could be made water
soluble by treatment with the proteolytic enzymes pepsin and trypsin
(Ryan & Hoffman, 1978).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. After 60 days the implants, which still contained
31% of the initial radioactivity, were removed. One heifer was killed
immediately, the other was maintained for 16 days after implant
removal and then killed. Ethyl acetate-extractable radioactivity in
blood plasma could largely be ascribed to TBOH; in most cases no TBA
was found in plasma. Plasma concentrations during days 1 - 55 after
dosing were 5 - 13 ppb; after 58 days a large increase in both total
and nonvolatile radioactivity was observed (17 - 20 ppb). The
half-lives for plasma disappearance of total and non-volatile
radioactivity were 32 and 29 days, respectively, during the
implantation period and 18 and 14 days, respectively, during the
withdrawal period. Plasma ethyl acetate-extractable radioactivity
amounted to 10 - 74% of the total radioactivity during days 1 - 55
after implantation, and this declined to 5% at 16 days after implant
removal. In the 16 days from implant removal to sacrifice,
radioactivity decreased by 58% in muscle, 75% in liver, 77% in
kidneys, and 74% in fat (Chasseaud et al., 1976).
Heifers (aged 15 months, number not given) were given daily oral
doses of 0.4 or 8 mg TBA per animal for 9 weeks. After 1 and 2 weeks
TBA was detected in the urine. Two weeks after drug withdrawal the
compound was detected in some urine samples, whereas after 3 weeks no
TBA was detected (Stephany et al., 1976).
A 14-month-old heifer, after i.v. administration of 10 mg/kg b.w.
TBA, excreted 80% of the administered radioactivity in the bile during
the first 24 hours; 3.5% was in the free form, 30% was excreted as
glucuronides, and 30% as sulfates. Metabolites with the 3-ketotrienic
structure that were identified in the bile are presented in Figure 2.
Three compounds that had lost their ketotrienic structure were also
isolated; these metabolites are presented in Figure 3. Less than 1% of
the administered radioactivity was isolated as tritiated water
(Pottier et al., 1978).
Specimens of muscle from the back and rear leg and specimens from
the liver were taken from two heifers that had been implanted two
months earlier with 300 mg 3H-TBA. In addition, bile was collected
by catheterization of one heifer on days preceding slaughter. The
radioactivity content of muscle, independent of its location, was
one-tenth the level in liver, whereas radioactivity levels in the bile
were 15 times higher than in liver tissue, alpha-TBOH and ß-TBOH
concentrations were determined by reverse isotopic dilution. On
average, the concentration of ß-TBOH was 0.05 to 0.1 ppb in various
tissues, whereas that of alpha-TBOH, which was only 0.005 ppb in the
muscle, reached 0.88 ppb in the liver. Following enzymolysis, ß-TBOH
was not detected in the bile, which contained, by contrast, nearly
200 ppb alpha-TBOH. Thus, alpha-TBOH represented 10% of total TBOH in
muscle, 90 - 95% in the liver, and more than 99% of the total in bile
(Pottier, 1979).
3H-TBA was implanted in the ears of two heifers (300 mg;
388 mCi) and the distribution of the radioactivity in liver and muscle
tissue was determined, applying rigorously standardized organic or
aqueous extraction procedures, either directly or following enzymatic
hydrolysis and proteolytic procedures. These steps yielded almost 100%
recovery of the radioactivity and indicate that only 5 to 15% of the
total residues were extractable with organic solvents. The remaining
radioactivity was either soluble in aqueous media or remained bound to
tissue structures. In another experiment, liver tissue from a calf
treated with 3500 mg TBA 68 days prior to slaughter was examined by
applying radioimmunoassay techniques to determine TBA/TBOH ratios.
Trienic-steroid type residues were obtained only from fractions
containing residues extractable with organic solvents (Hoffman et al.,
1984).
Two barren cows, after i.v. administration of 10 mg 3H-TBA per
animal, displayed very rapid hydrolysis of 3H-TBA in the blood
plasma; after 0.1 hour, only 2% of the radioactivity was recovered as
TBA, whereas 70% was recovered as TBOH. After 2 hours, radioactivity
could no longer be extracted, and in the extracted fraction polar
components predominated. From 3 - 8 hours TBOH disappeared from the
blood (half-life, 1.5 hours) (Pottier et al., 1975).
 In two barren cows after s.c. implantation of 300 mg 3H-TBA per
animal at the base of the ear, slow resorption from the implant
occurred; the half-life of disappearance from the implant was 68 - 84
days. About 33% of the radioactivity was extracted in the blood plasma
over the 3-month period after implantation, 70% of which was accounted
for by TBOH. The main routes of excretion were via the bile and urine.
Tissue levels after 3 months were about 1 ppb, except in the liver
(6.5 ppb) and kidneys (4.5 ppb). Twenty-five percent of the tissue
radioactivity was extractable, 40% of which was TBOH. In the liver and
kidneys, however, only 10% was extractable, while in perirenal fat up
to 88% of the radioactivity was extractable. In perirenal fat 50% of
the radioactivity was TBA. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity (Pottier et al., 1973;
Pottier et al., 1975).
Slow resorption from s.c. implants of 300 mg 3H-TBA occurred in
2 lactating cows. The half-life for disappearance from the implant was
approximately 60 days. About 17% of the radioactivity present in the
blood plasma over the period of 5 months after implantation was
extractable. Less than 1% of the radioactivity was excreted in milk.
Ten percent of the milk radioactivity was extractable and 25% of this
extractable radioactivity was TBOH. Tissue levels after 5 months were
about 1 ppb, except in the liver (3.4 ppb) and kidneys (2.7 ppb).
About 25% of the tissue radioactivity was extractable, except in the
liver and kidneys (both 10%); about 40% of this extractable
radioactivity was TBOH. In contrast, 88% of total radioactivity in
perirenal fat was extractable, of which 50% was TBA. Unchanged TBA was
found in no other tissues. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity after 5 months (Pottier
et al., 1973; Pottier et al., 1975).
Two steers were given by single s.c. implantations 300 mg
3H-TBA in combination with 40 mg estradiol; the implants were
removed 60 days later, at which time 28% of the radioactivity remained
in them. Ethyl acetate-extractable radioactivity in blood plasma was
primarily ascribed to TBOH; in most cases no TBA was found in the
plasma. One animal was killed immediately after removal of the
implant. Plasma concentrations in this animal declined with half-lives
of 26 days for both total and non-volatile radioactivity; ethyl
acetate-extractable radioactivity in the plasma of this animal ranged
between 3 - 5% of the total radioactivity. In the other animal, which
was killed 16 days after removal of the implant, plasma concentrations
declined during days 1 - 60, with half-lives of 50 and 55 days for
total and non-volatile radioactivity, respectively. In the 16 days
from implant removal to sacrifice, radioactivity decreased by 46% in
muscle, 2% in liver and kidneys, and 29% in fat (Chasseaud et al.,
1976).
Relay bioavailability
Groups of 3 rats were fed freeze-dried or ethyl acetate-extracted
liver, kidney, or muscle obtained from two heifers killed 60 days
after s.c. implantation with 300 mg 3H-TBA. 3H-TBA levels in the
heifers averaged 30 ng equivalents/g in the liver, 24 ng equivalents/g
in the kidneys, and 3.2 ng equivalents/g in muscle. Radioactivity
excretion during the 3 days after feeding these tissues to rats is
presented in Table 1 (Hawkins et al., 1979).
Groups of 3 bile duct-cannulated rats that had been fasted for 24
hours were fed during 1 hour freeze dried liver, kidney, or muscle
from the two heifers described in the previous paragraph.
Radioactivity disposition during 48 hours after feeding of these
tissues is presented in Table 2 (Hawkins et al., 1979).
Table 1. Excretion of radioactivity by rats after being fed tissues
from heifers implanted with 3H-TBA
Excretion in percent of
administered radioactivity
Treatment Tissue Urine Faeces Total
Freeze-dried tissue Liver 3 81 84
Kidney 2 93 94
Muscle 6 85 91
Extracted tissue Liver 5 78 83
Kidney 2 103 105
Muscle 2 73 75
Table 2. Excretion of radioactivity by bile duct-cannulated rats
after feeding of tissue from heifers implanted with 3H-TBA
Excretion in percent of administered radioactivity
Tissue Bile Urine Faeces GI tract + contents Total
Liver 7 5 59 2 74
Kidney 3 1 31 60 95
Muscle 3 2 56 not detected 61
Effects on protein binding
The affinity of alpha-TBOH and ß-TBOH for corticosteroid binding
globulin, measured in vitro using the human plasma of elderly women,
was very low, less than 0.1% bound, compared with 10% for testos-
terone. The affinity of alpha-TBOH and ß-TBOH for testosterone and
estradiol binding globulin was 1% of that measured for testosterone.
When alpha-3H-TBOH was incubated in vitro with female human plasma,
it readily bound to the albumin fraction; only 4% was present
as free TBOH. The total blood clearance of ß-TBOH was twice that of
testosterone (Philibert & Moguilewsky, 1983).
Effects on estradiol-17ß excretion and nitrogen retention
Cattle
Plasma residues of estradiol-17ß in cattle were affected by the
presence of TBA in the s.c. implant. Plasma levels of estradiol-17ß
remained greater than 0.05 ppb for nine weeks in steers after
treatment with 200 mg TBA in combination with 40 mg estradiol-17ß,
whereas the residual levels decreased below 0.05 ppb within 5 weeks
after implantation of 40 mg estradiol-17ß alone (Heitzman & Hardwood,
1977).
Implantation of 40 mg TBA in the dewlap of Friesian bulls
(11 - 16 weeks of age) did not affect nitrogen retention. Implantation
of 140 mg TBA in combination with 20 mg estradiol-17ß at the same
site, however, resulted in a 47% decrease in nitrogen retention
(van der Wal, 1975).
Pigs
Pigs (males, females, and castrated males) were given s.c.
implantations with either 20 mg estradiol-17ß or 20 mg estradiol-17ß
in combination with 140 mg TBA. At 5 weeks after implantation, steroid
estrogens were hardly detectable in the faeces, and serum values for
estradiol-17ß were very low in both groups. Urine estradiol-17ß levels
were 6 - 82 µg/l in the estradiol-17ß group and 16 - 135 µg/l in the
combination group (Kroes et al., 1976a).
Toxicological studies
Special studies on carcinogenicity potential
Rats
Male Wistar rats (number not specified) were injected i.p.
with 15 µg/kg b.w. 3H-estradiol-17ß (53.6 Ci/mmole), 19 µg/kg
b.w. 3H-testosterone (54.0 Ci/mmole), 17 µg/kg b.w. 3H-TBA,
(57.0 Ci/mmole), or 30 µg/kg b.w. 3H-zeranol (50.0 Ci/mmole), all in
95% ethanol solution. The animals were sacrificed 16 hours after
injection and the Covalent Binding Indices (CBI, Lutz, 1979) of the
chemicals to DNA in the liver were quantitated. The CBIs were 11.4,
4.80, 5.62, and 1.65 for estradiol-17ß, testosterone, TBA, and
zeranol, respectively (weak carcinogens have a CBI approx. or equal
10, Lutz, 1979). The positive control, N-hydroxy-acetylaminofluorene,
had a CBI value of 262 (Barraud et al., 1983).
The CBI of TBA as a function of time was measured by
administering 0.83 mCi (22 - 40 µg/kg b.w.) 3H-TBA i.p. to 8 male
rats. The animals were killed at 4, 8, 12, 20, 24, 36, 48, and 96
hours. The highest CBI, 7.82 was obtained after 24 hours; after 96
hours the CBI was 1.11 (Barraud et al., 1983).
Treatment of rodents with initiators of liver cancer can give
rise to phenotypically altered cells which, under suitable conditions,
will develop into foci of potentially pre-neoplastic cells. These foci
may either regress or develop into malignant nodules, but because they
only take a few weeks to become apparent, induction of such foci
represents a useful short-term indication of tumour-initiating
capacity.
alpha-TBOH or ß-TBOH (2.5, 5, or 10 mg/kg b.w.), ethinyl
estradiol (0.05 mg/kg b.w.), testosterone (10 mg/kg b.w.),
nitrosomorpholine (25 mg/kg b.w.), or diethylnitrosamine (200 mg/kg
b.w.) were administered by i.p. injection approximately 18 hours after
partial hepatectomy to Fisher 344 CDF rats (5 males and 5 females per
group). Two groups presented only with vehicle and one untreated group
of 5 males and 5 females each were used as controls. The animals were
allowed to recover for a further 13 days after treatment with the test
agent. The animals then were supplied with tap water and powdered diet
containing 0.02% 2-acetylaminofluorene, except that the diet supplied
to animals in one of the vehicle control groups contained no
acetylaminofluorene. Seven days after commencing the new dietary
regime the animals were treated with carbon tetrachloride at 2 ml per
kg b.w. by intragastric gavage (animals in the vehicle control group
not given acetylaminofluorene were not treated with carbon
tetrachloride). Seven days later the animals were killed by cervical
dislocation and the livers were removed for microscopic examination.
Most of the animals showed moderate lethargy and other clinical
signs for two or three days following the operative procedure, but no
compound-related adverse signs were evident. No significant
treatment-related effects on body or liver weight were reported. Only
animals treated with nitrosomorpholine or diethylnitrosamine showed
significant increases in liver foci compared with the vehicle control
or untreated groups.
None of the steroids examined in this study (including alpha-TBOH
and ß-TBOH) showed any evidence of inducing pre-neoplastic liver foci
at the dose levels tested. The authors concluded that none of these
steroids showed any evidence of being a liver rumour initiator in this
assay (Allen & Proudlock, 1987).
Special study on immunoresponse
Cattle
Antibody production in male and female calves (about 25 animals
per group) was investigated after s.c. implantation of placebo
(lactose), 20 mg estradiol-17ß, 140 mg TBA, or 140 mg TBA + 20 mg
estradiol-17ß. A slight, non-significant immunodepressive effect was
seen in male calves treated with either estradiol-17ß or TBA alone. In
the males treated with the combination, this effect was significant.
In female calves the immunoresponse was unaffected (Gropp et al.,
1975).
Special studies on mutagenicity
The results of mutagenicity assays on TBOH and TBA are summarized
in Table 3.
Special studies on no-hormonal effect levels
Pigs
Groups of 3 - 7 (11 in the control group) mature male large white
hybrid pigs, 8 - 10 months of age, were administered orally 0.1, 1,
10, 16, 24, or 36 µg ß-TBOH/kg b.w./day or 0.1, 10, 100, 160, 240, or
360 µg alpha-TBOH/kg b.w./day in gelatine capsules with the feed for
14 consecutive days after castration. They were maintained for 14 more
days, then killed and examined post mortem. Serial blood samples
were collected prior to castration (day 0) and on days 7, 14, 21, and
28; LH was determined on days 0 and 14 in the animals treated with
alpha-TBOH and on days 0, 14, and 28 in the animals treated with
ß-TBOH. After sacrifice, the pituitary, prostate, and seminal vesicles
were examined by gross pathology and histopathology. Based on changes
in plasma luteinizing hormone (LH) concentrations, distinct hormonal
effects occurred at 160 µg alpha-TBOH/kg b.w./day and at 16 µg
ß-TBOH/kg b.w./day. Thus, the no-observed-effect levels in this study
were 10 µg ß-TBOH/kg b.w./day and 100 µg alpha-TBOH/kg b.w./day. The
morphological examinations performed in this study are of limited
value, since a 14-day period elapsed between final treatment and
slaughter, allowing regression of likely effects. Thus, the
observation of treatment-related androgenic effects in the 16, 28, and
36 µg ß-TBOH/kg b.w./day group points toward the high hormonal
activity of this compound (Roberts & Cameron, 1985).
Four groups of 4 male and 4 female large white hybrid pigs were
given orally TBA incorporated into the diet for 14 consecutive weeks
at 0, 0.1, 2, or 20 ppm (equal to 0, 2.0-3.1, 40-62, or 400-620 µg/kg
b.w./day). Blood samples were obtained for steroid hormone assays
before treatment commenced and after 6 and 12 weeks of treatment.
Table 3. Results of mutagenicity assays on TBOH and TBA
Concentration
Test Test of substance
system object tested Results Reference
Ames S. typhimurium 10-10000 µg/ Negative Hossat et al., 1978
test1 TA98, TA100, plate TBA or
TA1535, TA1537 7:1 TBA:
TA1538 estradiol-17ß2
Ames S. typhimurium 1000, 2000, Negative Ingerowski et al., 1981
test1 TA98, TA100, 3000 µg/plate at 1000
TA1535, TA1537 TBOH µg/plate;
TA1538 equivocal at
cytotoxic
concentrations
Ames S.typhimurium 0.5 - 500 µg/ Negative Richold et al., 1982a
test1 TA98, TA100, plate alpha-TBOH;
TA1535, TA1537 15 - 1500 µg/
TA1538 plate ß-TBOH
Ames S. typhimurium 0.06 - 2 µg/ Negative Schiffman et al., 1985
test1 TA98, TA100 plate TBOH
Clastogenic Human lymphocytes 6, 30, or 60 Negative Richold et al., 1982b
potential in vitro µg/ml alpha-TBOH
or ß-TBOH2,3
Cell Mouse lymphoma 15 - 45 µg/ml Equivocal6 Richold et al.,
mutation L5178Y cells alpha-TBOH; 15 - 1982c, 1983
assay4 65 µ/ml ß-
TBOH5
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Cell Syrian hamster 5, 10, 15 µg/ Equivocal7 Schiffman et al., 1985
transformation embryo fibroblasts ml TBOH
assay
Cell Mouse C3H1OT1/2 2 - 25 µg/ml Equivocal Henderson et al., 1987a
transformation cells ß-TBOH (- act.)
assay1 (-S-9 mix)5
5 - 20 µg/ml Positive
ß-TBOH (+ act.)
(+S-9 mix)8
Forward Chinese hamster 25 - 100 µg/ml Negative Edgar et al., 1985
mutation ovary cells ß-TBOH
assay1 (HGPRT locus) (-S-9 mix)9
25 - 150 µg/ml
ß-TBOH
(+S-9 mix)5
Forward Chinese hamster 25 - 500 µg/ml Negative Henderson et al.,
mutation ovary cells ß-TBOH5 1986a
assay1 (HGPRT locus)
Forward Chinese hamster 3 - 75 µg/ml Negative Henderson et al.,
mutation V79 cells ß-TBOH 1987b
assay1 (HGPRT locus) (-S-9 mix)9
12 - 125 µg/ml
ß-TBOH
(+S-9 mix)
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Micronuclei Chinese hamster 1 - 10 µg/ml Equivocal Henderson et al.,
induction1 ovary cells ß-TBOH (- act.) 1986b
(-S-9 mix)10
6 - 60 µg/ml Negative
ß-TBOH (+ act.)
(+S-9 mix)10
Chromosome Chinese hamster 1 - 10 µg/ml Negative Allen et al., 1985
aberration ovary cells ß-TBOH
assay1 (-S-9 mix)3
6 - 60 µg/ml
ß-TBOH
(+S-9 mix)11
Unscheduled HeLa cells and 2.5 - 15 µg/ml Negative Schiffman et al.,
DNA Syrian hamster 1985
synthesis embryo cells
DNA Cultured human 1 - 512 µg/ml Negative13 Allen & Proudlock,
repair epithelioid alpha-TBOH or 1983
assay1 cells ß-TBOH12
In vivo Rat bone marrow 100 mg/kg b.w. Negative Richold & Richardson,
cytogenetics alpha-TBOH or 1982
assay ß-TBOH once;
25 or 50 mg
alpha-TBOH or
ß-TBOH 4 times3
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
In vivo Erythrocytes 100 mg/kg b.w. Negative Allen et al., 1980
micronucleus ß-TBOH3
test
1 Both with and without rat liver S-9 fraction.
2 Dimethylsulfoxide was used as the solvent.
3 Mytomycin C was used as a positive control.
4 Only with rat liver S-9 fraction.
5 20-Methylcholanthrene was used as a positive control.
6 alpha-TBOH at > 22 µg/ml and ß-TBOH at > 15 µg/ml were toxic to the
cells. Both substances induced 2-fold increases in mutation frequency,
but with alpha-TBOH this occurred only at highly toxic concentrations.
7 There was an inverse dose relationship, with the largest number of
transformations occurring at the lowest dose.
8 2-Acetamidofluorene was used as a positive control.
9 Ethylmethanesulfonate was used as a positive control.
10 Colchicine was used as a positive control.
11 Cyclophosphamide was used as a positive control.
12 4-Nitroguinoline-1-oxide (-S-9 mix) and 2-aminoanthracene (+S-9 mix)
were used as positive controls.
13 Increases in nuclear grain count were observed in a limited number of
cultures in one experiment only.
A detailed post-mortem examination was carried out on all pigs
at termination, and tissues were retained for histological
examination. All animals remained generally in good health throughout
the study, and no abnormal clinical signs were noted that could be
associated with experimental treatment. No significant effects of
treatment on body weight, food consumption, or ophthalmoscopy were
noted. There was a marked, dose-related reduction in serum
testosterone levels in male pigs, mean values in the highest-dose
group at week 6 and in pigs fed 2 and 20 ppm TBA at week 12 being
significantly lower than control values (p < 0.01). Testosterone
levels in female pigs remained low at all 3 assay points.
Serum progesterone levels in females were variable. This is not
unexpected, since both progesterone and estradiol show a marked cycle
variation and the assay samples were obtained at arbitrary time points
without regard to the stage of the estrus cycle of individual pigs.
However, mean values at week 12 did indicate a dose-related reduction
in serum progesterone levels in female pigs, and on statistical
examination there was found to be a significant trend with dose
(p < 0.05). Progesterone values for male pigs were less variable and
lower on average than for females and no consistent treatment-related
differences were noted.
Levels of estradiol-17ß in female pigs were generally low,
although there was evidence of some increase in estrogenic secretion
at week 12 in pigs from all groups; no appreciable differences between
groups were observed. Estradiol levels in males were in general higher
than in females; a dose-related reduction in estradiol mean values was
noted at weeks 6 and 12. The results of the hormone assays point to a
no-hormonal-effect level below 2 µg/kg b.w./day.
Treatment-related reductions in mean weights of testes, ovaries,
and uteri were noted. Apparently, the most sensitive organs are the
testes and ovaries. At the lowest dose, no effects on any tissue
weights were observed. The main findings associated with treatment at
the 2 higher levels were atrophy of testicular interstitial cells,
suppression of cyclic ovarian activity, the consequent absence of
glandular development of the uterine endometrium, and lack of alveolar
development and secretion in the mammary glands (highest group only).
Treatment-related changes were not seen in the gonads, uteri, or
mammary glands in any of the animals examined in the lowest-dose group
(Roberts & Cameron, 1985).
Four groups of 5 male and 5 female pigs were given orally TBA as
solutions in corn oil in gelatine capsules with the food for 14
consecutive weeks at 0, 5.0, 7.5, or 10.0 µg/kg b.w./day. Venous blood
samples were taken before treatment began and at weekly intervals
throughout the test period. Plasma radioimmunoassays for testosterone,
estradiol-17ß, and progesterone were carried out. Pigs were sacrificed
after 14 weeks and examined post-mortem. Tissues processed for
histological examination included the testes, ovaries, seminal
vesicles, uterus, and mammary gland. In males, comparison with the
control group showed some differences in testosterone levels at weeks
1 and 5, which reached statistical significance in the two highest
dose groups. However, these differences were not sustained and were in
the opposite direction (i.e. increases compared with the control) to
the dose-related changes (i.e. suppression) noted in the study
described in the previous paragraphs. Testosterone levels in females
generally remained low in all groups.
No significant group differences were found in estradiol levels
in males. In females, estradiol levels generally remained low in all
groups. Mean progesterone levels in males were statistically
significantly lower than in controls between weeks 7 and 10 in the
highest-dose group and at week 10 in the group administered 7.5 µg
TBA/kg b.w./day. However, the mean pre-dose levels were also lower in
pigs in these two groups compared with the controls and analysis of
mean levels over weeks 6 to 14 showed no significant effects. Thus,
these differences were not considered by the authors of the report to
represent a real treatment effect. In female pigs, mean progesterone
values were variable due to cyclic changes, but no consistent group
differences were apparent. Examination of the time of onset of cycling
and the peak level of progesterone recorded did not show significant
effects. No significant differences in organ weights were noted in any
groups. No treatment-related changes in the morphology of the organs
examined were noted in any groups (Roberts & Cameron, 1985).
Monkeys
The antigonadotropic activity of ß-TBOH was tested in acutely
castrated male rhesus macaque monkeys aged 8 - 17 years. A seminal
vesicle biopsy was obtained at the end of the 30-day treatment period
to examine possible changes in androgenic activity induced by TBOH.
ß-TBOH was given orally at 0, 1, 20, or 400 µg/day (three control
animals and two animals per treatment group). From 17 days after
castration until the end of the experiment, the lowest-dose group was
given 1600 µg/day ß-TBOH. Administration of the compound did not
suppress the post-castration elevation in either LH or follicle
stimulating hormone (FSH) secretion, which occurs after removal of the
testes. Although TBOH and testosterone showed no antigonadotropic
activity in this model system, the monkeys receiving 400 and
1600 µg/day TBOH maintained partial or complete seminal vesicle
morphology consonant with an androgenic effect. The expected reduction
in the serum levels of testosterone and estradiol occurred after
castration, and TBOH treatment did not alter the serum concentrations
of these hormones or the typical diurnal pattern of activity within
the hypothalmic-pituitary-adrenal axis. The authors concluded that the
no-hormonal-effect level in this study was 20 µg/day, equivalent to
2 µg/kg b.w./day (Hess, 1983).
TBA was administered in Sustagen/Jello/bran diet cubes for three
cycles, or a maximum of 122 days, to three groups of six mature female
rhesus macaque monkeys weighing 6 kg each at 60, 240, or 960 µg/day.
Blood samples were obtained on a daily basis from all animals during a
pretreatment menstrual cycle, at 3-day intervals during the first two
treatment menstrual cycles, and daily during the last treatment
menstrual cycle. Serum concentrations of estradiol, progesterone, LH,
and FSH were determined by radioimunoassay. Treatment with 960 µg/day
TBA resulted in maximum average serum levels of 2.3 ng/ml of ß-TBOH,
and this dose may have inhibited gonadotropin secretion and ovarian
function in 3 of 16 reproductive cycles.
It was concluded that 960 µg TBA/day had an inhibitory effect on
the pituitary gonadal axis. The anovulatory stage was reached rather
suddenly and from the data presented no conclusions with respect to
changes in the endogenous hormone concentrations, which might signal
this effect, can be drawn. In the 240 µg/day group one animal
exhibited anovulation, which may have been related to treatment. No
effects were observed at 60 µg/day, equivalent to 10 µg/kg b.w./day
(Hess, 1984).
Special studies on relay toxicity
Rats
Female veal calves were given s.c. implantations of 0, 140, or
3500 mg/animal TBA and killed after 10 weeks. A homogenate of veal
meat and veal organs (tongue, heart, lungs, spleen, liver (partial),
and one kidney) from these calves was mixed with the diet of rats in a
two-generation reproduction study. The total length of the experiment
was 114 weeks. The F1a generation was used to study teratogenic
effects. In the group of rats receiving a diet containing 230 ppb TBA,
a slight growth depression was seen. No effects were seen on other
parameters, which included mortality, feed consumption, growth,
fertility, reproduction (copulation, conception rate, duration of
gestation, mean litter weight, litter size, fetal body weight,
mortality rate, and fetal body weight after 3 weeks), haematology,
biochemistry, organ weight, and gross- and histopathology
(Gropp et al., 1978).
Special studies on reproduction
Rats
Groups of 40 male and 80 female rats weighing 133 - 143 g each
were given diets containing 0, 0.5, 1, 4, or 16 ppm TBA from week 9
before mating until day 21 post-partum. An additional group was
handled similarly, in which females were fed 50 ppm TBA from day 1 of
pregnancy until day 21 post-partum. At day 21 post-partum all parent
animals were killed without further examination. There were no major
differences between nominal and detected dietary levels of TBA at the
beginning and end of the study. In females in the 4 and 16 ppm groups,
growth was increased throughout the study (10 - 20%); in the 50 ppm
group growth was increased after mating (10%). A dose-related decrease
in pregnancy rate was seen in the 1, 4, 16, and 50 ppm groups (maximum
-30%). At day 4 post-partum each litter was reduced in size to 4
females and 4 males; up to that time pup mortality was increased in
all dosed groups compared with controls. Litter size and litter weight
on days 0, 4, 12, and 21 post-partum were decreased in the 4 and 16
ppm groups (maximum about -10%) and in the 50 ppm group (about -25%).
Mean pup weights were decreased only in the 50 ppm group from day 4
post-partum onward (maximum -15%) (Hunter et al., 1982).
Groups of 12 male and 12 female rats were fed diets containing
0, 25, 50, or 100 ppm TBA for 63 days and then mated. In these groups,
12/12, 10/12, 4/12, and 1/12 females, respectively, were pregnant
after mating (Ross, 1980).
In a multi-generation reproduction study in CRL:COBS CD(SD)BR
Charles River rats, dietary concentrations of 0, 0.5, 3, or 18 ppm TBA
were fed to F0-generation male rats for nine weeks and female rats
for two weeks prior to mating, then through to termination. Two groups
of F1 generation rats were selected, reared, and mated. One group
was treated continuously at the same dietary concentrations as rats in
the F0 generation ("treated" group) and one was removed from
exposure to TBA at 3 weeks of age and maintained without treatment
throughout ("untreated" group).
Treatment with TBA at 18 ppm was associated with the following
effects: a) generally higher body weights affecting both F0-and
F1-generation treated males and females and females of the untreated
F1 generation; b) depression in mean body weight gain during
gestation affecting both matings of the F0 generation; c) signs of
virilization, namely coarseness of the coat and discoloration of the
skin in F0-generation animals and treated F1-generation females;
d) clitoral prominence in treated F1-generation females and to a
lesser extent in untreated F1-generation females. Similar effects
were observed in F2-generation females from treated F1 parents,
but not in offspring of the untreated F1 generation; e) the presence
of occlusive strands in the vagina and/or precocious/incomplete
vaginal opening affecting treated F1 pups and F2 pups from the
treated F1 generation; f) a delay in the occurrence of testicular
descent affecting F2 pups from the treated F1 generation; g) a
marked reduction in pregnancy rate affecting the second mating of the
F0 generation and the treated F1 generation; h) an increase in
pre-coital time for the second mating of the F0 generation and for
the treated F1 generation; i) a marginal extension of the duration
of gestation affecting the F0 generation and the treated F1
generation; j) a marked increase in the incidence of extended
parturition and total litter loss in the treated F1 generation and a
significant increase in the percentage of males per litter; k) lower
litter size and litter weight, either at birth or at 20-day sacrifice,
after both matings of the F0-generation and of the treated F1
generation; 1) increased post-implantation/pre-birth losses in the
F0 and treated F1 generations; m) at terminal autopsy, findings
additional to those previously described, namely an increase in the
incidence of depression in the forestomach epithelium affecting F0-
and F1-generation males; n) significant reductions in seminal
vesicle/prostate weights in F0- and F1-generation males and
increases in mean ovary weights among F0- and F1-generation
treated females; o) significant decreases in weights of seminal
vesicles/prostate, testes, and epididymes in F1-and F2-generation
male pups at six weeks of age. F1- and F2-generation female pups
showed reduction of adrenal weight at six weeks of age; p) a
significant reduction in anogenital distance among male fetuses and a
marginal increase in the incidence of skeletalvariants after the
second mating of rats of the F0-generation (teratology phase).
Treatment with TBA at 3 ppm was associated with the following
effects: a) retarded body weight gain at the first mating of the
F0-generation; b) coarseness of the coat and discoloration of the
skin affecting one female in the F1-generation; c) a significant
delay in the mean age of vaginal opening in females in the
F1-generation and in F2 offspring from treated F1 parents; d)
the occurrence of incomplete vaginal opening or occlusive strands in
the vagina affecting occasional animals of the F1-generation at 6
week autopsy; e) a slight, but not statistically significant, delay in
testicular descent affecting F2 male offspring from treated F1
parents; f) significantly lower litter size at birth after the first
mating of the F0-generation; g) lower litter weight after the second
mating of the F0-generation; h) marginally reduced litter size in
offspring of the treated F1-generation; i) a significant decrease in
the weight of seminal vesicles/prostate, testes, and epididymes in
F1 and F2 male pups at six weeks of age.
The only apparent effects of treatment at 0.5 ppm were as
follows: a) higher group mean body weights of males from the F0
generation; b) a slight but not statistically significant delay in the
mean age of vaginal opening in F1 pups and F2 pups from the
treated F1 generation (subsequent mating performance and resulting
litter parameters were comparable to those of controls); c) a
statistically significant decrease in seminal vesicles/prostate weight
in treated F1 male pups and in F2 male pups from treated parents
at six weeks of age; d) a significant decrease in weight of epididymes
in F2 males.
The authors concluded that, in terms of reproductive performance
of the two generations examined in this study, as assessed by the
ability of parents to produce and sustain their litters, TBA exerted a
marked effect at 18 ppm and some effect at 3 ppm. At the lowest dose
level examined (0.5 ppm), slight effects were observed, which were
more marked in F2 pups than in F1 pups of a comparable age.
However, the authors concluded that in terms of reproductive
performance TBA exerted no effect at 0.5 ppm. The reproductive
performance for all groups of F1 animals following withdrawal from
treatment showed no marked differences from those of the control group
(James et al., 1985).
Histological examination of the testes, epididymes, seminal
vesicles, and prostate of 6-week-old male F2-generation rats from
treated F1 animals in the study described above revealed no
morphological abnormalities, although lower group mean weights were
recorded in treated groups compared to controls. All rats were
considered normal for that age, when they are not quite fully sexually
mature, with spermiogenesis proceeding to tailed spermatids in the
testes but no spermatoozoa in the epididymes (Offer, 1985).
In a study in rats, dietary concentrations of 0, 0.1, 0.3, 0.5,
3, or 18 ppm TBA were fed to F0 males and females from two weeks
prior to mating until termination of pregnancy. The F1 litters were
reared through the weaning period. On day 22 post-partum the male
offspring were sacrificed, examined macroscopically, and the testes,
seminal vesicles/prostate, and epididymes from each pup were weighed
and preserved. Female offspring were sacrificed on day 24 post-partum
and examined macroscopically.
Treatment with TBA at 18 ppm was associated with the following
effects: a) slightly higher mean body weights of F0 females, but
lower body weight gain during gestation and slightly lower weight gain
of males over the last three weeks of treatment; b) clitoral
prominence at autopsy in 22/29 F0 females and in all F1 female
offspring from approximately 3 weeks of age; c) a statistically
significant extension in duration of gestation; d) total litter loss
in 4/29 F0 females; e) effects on litter parameters, including
reduced litter size, lower litter weight, marginally higher pup
mortality, and higher mean pup weight; f) lower testicular weight and
higher mean weight of seminal vesicles/prostate in F1 males at 22
days of age.
Treatment at 3 ppm was associated with: a) slightly higher mean
body weights of females and slightly lower weights of males in the
three weeks prior to termination; b) a marginal extension in duration
of gestation; c) effects on litter parameters, including reduced
litter size, and marginally higher pup mortality and mean pup weight;
d) lower testicular weight and higher mean seminal vesicle/prostate
weight in F1 males.
The only effects associated with treatment at 0.1, 0.3, and
0.5 ppm TBA were slightly higher mean body weights of females and the
marginal differences observed at 3 and 18 ppm in litter parameters
(James et al., 1986).
Special studies on teratogenicity
Rats
Four groups of 6 pregnant rats each were given by oral gavage
0, 2.5, 5, or 10 mg/kg b.w./day TBA from days 6 - 15 of gestation. The
vehicle was 2.5% ethanol in 1% methylcellulose. Mortality, growth,
number of corpora lutea, number and distribution of live and dead
young, litter weight, mean fetal weight, microscopic fetal
abnormalities, sex ratio, and fetal crown-rump distance were all
unaffected by treatment (James et al., 1982).
Groups of 20 pregnant rats received by oral garage 0, 5, 10, or
20 mg/kg b.w./day TBA from days 6 - 15 of gestation. The vehicle was
2.5% ethanol in 1% methylcellulose. In the 10 and 20 mg/kg b.w./ day
groups, 9/20 and 15/20 dams, respectively, showed hair loss. A
dose-related decrease in growth was seen in all dosed groups (maximum
20%). Pregnancy rate, number of liver and dead young, number of
implantations and corpora lutea, litter weight, mean fetal weight,
incidence of major malformations, incidence of minor visceral
anomalies (Wilson's technique), and fetal crown-rump distance were all
unaffected. The incidences of skeletal variants (number of ribs,
number of normal and variant sternebrae) were unaffected. Mean
anogential distances in male fetuses preserved in Bouin's solution
were slightly shorter than those of controls, with an apparent
slightly decreasing trend with increase in dosage; the difference at
20 mg/kg b.w./day attained borderline statistical significance
(P < 0.05). Male fetuses preserved in alcohol did not show the same
trend in mean values, although the variation in anogenital distance
was greater in pups in the high-dose group than in controls; ranges of
fixation weight and crown-rump distance length were also higher in the
high-dose group than in controls. Mean anogenital distances in females
preserved in Bouin's solution and in alcohol were comparable in all
groups (James et al., 1982).
Acute toxicity
Table 4 summarizes the results of acute toxicity studies on TBA.
Table 4. Acute toxicity of TBA
LD50
Species Sex Route Vehicle (mg/kg b.w.) Reference
Mouse M/F oral 40% ethanol 1500 Audegond et al.,
in corn oil 1981a
M i.p. ethanol + 10% 565 Escuret & Bas, 1978
sesame oil
F i.p. ethanol + 10% 643 Escuret & Bas, 1978
sesame oil
Rat M/F oral 10% ethanol 5000 Audegond et 1981b
in corn oil
M i.p. 10% ethanol 1601 Escuret & Bas, 1978
in corn oil
F i.p. 10% ethanol 1772 Escuret & Bas, 1978
in corn oil
Dog M/F oral via capsules 1000 Audegond et al.,
1981c
Dogs
Anaesthetized dogs were given i.v. 1, 2, 5, or 10 mg/kg b.w. TBA
as a 20 ml/mg solution in 92% acetylmethylamine. Dogs in the 2, 5, and
10 mg/kg b.w. groups showed a dose-related decrease in blood pressure;
at 10 mg/kg b.w., this was accompanied by slight bradycardia. A
decrease in blood pressure in the same groups was seen after injection
with adrenalin and noradrenalin; an increase was seen after
acetylcholine injection. Changes in reactions to histamine were not
noted in any groups (Seeger, 1971a).
Short-term studies
Mice
Groups of 8 male and 8 female mice weighing 19 - 25 g each were
given 0, 25, 50, or 100 ppm TBA in the diet for 8 weeks. Mortality,
appearance, behaviour, body weight, food consumption, and food
conversion were unaffected by treatment. Females in all treatment
groups exhibited significant decreases in absolute and relative liver
weights and significant increases in absolute and relative uterine
weights. Absolute and relative ovarian weights were decreased
significantly in females in the 50 and 100 ppm groups, while absolute
and relative testes weights were decreased significantly in males in
the highest-dose group. No effects on the weights of adrenals,
kidneys, prostate, seminal vesicles, or spleen were noted. Females
showed dose-related suppression of ovarian cyclic activity
characterized histopathologically by the absence of, or few, corpora
lutea in the gonads, a dose-related reduction in the amount of stroma,
and reduction in the number of endometrial glands in the uteri
(Hunter et al., 1976a).
TBA was administered to groups of 8 male and 8 female Swiss
albino CFLP mice for 10 weeks at levels of 0, 1, 2, 5, or 10 ppm of
the diet, equal to mean intakes of 0, 0.12, 0.24, 0.56, or 1.2 mg/kg
b.w./day for males and 0, 0.13, 0.25, 0.66, or 1.4 mg/kg b.w./day for
females. There were no signs of reaction to treatment, including no
treatment-related effects on food intake or body weight gain. No
treatment-related abnormalities were seen; the absolute and relative
weights of all organs examined were considered to be within normal
limits for mice of this strain and age. The only organs examined
histologically were the prostate, seminal vesicles, testes, ovaries,
and uterus from mice in the control and highest-dose group. All
histopathological parameters were within normal limits (Hunter et al.,
1976b).
Rats
Sixteen male rats that had been castrated between 21 and 24 days
of age were given total oral doses of 0, 0.75, 3, 12, or 48 mg TBA
from days 2 - 11 after castration in 10 daily portions. At autopsy,
one day after the last application, dose-related increases in the
absolute weight of the prostate (maximum +440%) and seminal vesicles
(maximum +400%) were seen in all treated groups. In the three
highest-dose groups a dose-related increase in the musculo levator ani
weight (maximum +250%) was noted (Schröder, 1971a).
Groups of 10 male and 10 female CFY rats were fed diets
containing TBA at concentrations of 0, 25, 50, or 100 ppm for 13
weeks. Group mean intakes during the treatment period were 0, 1.8,
3.8, or 7.6 mg/kg b.w./day for males and 0, 2.2, 4.2, or 8.4 mg/kg
b.w./day for females. At all the tested doses, the females exhibited
better efficiency of feed utilization than the males, causing higher
weight gain.
At 25 ppm, lower prostate weight (-36%), which was not associated
with morphological changes, was observed. At 50 ppm, lower prostate
and seminal vesicle weights (-50 and -30%, respectively), which were
not associated with morphological or uterine changes, were observed in
two rats.
At 100 ppm, the following changes were observed: lower neutrophil
and lymphocyte values in males at weeks 12 and 13 (-35%); lower
seminal vesicle weight, which was not associated with morphological
changes (-60%); lower prostate weight (-80%), which was associated
with small alveoli lined by cuboidal epithelium in 5 rats; and uterine
changes characterized by an apparent reduction of the endometrial
stroma with dilated uterine glands and also a corrugated appearance of
the endometrial and glandular epithelium in 6 rats (Hunter et al.,
1976c).
Groups of 10 male and 10 female rats weighing 60 g each were
given 0, 50, 100, 200, or 1000 µg/kg b.w./day TBA orally 6 days per
week for 3 months. The compound was presented as 0.5 ml of an aqueous
solution containing 0.9% NaCl, 0.4% polysorbate 80, 0.5% carboxy-
methylcellulose, and 0.9% benzyl alcohol. Determinations were
carried out in half of the animals at termination only.
Growth of females was slightly increased; in the two highest-dose
groups growth of males was decreased. Haematological parameters were
unaffected. In all dosed groups, SGOT and SGPT were decreased; total
cholesterol was decreased in the 100, 200, and 1000 µg/kg b.w./day
groups. In the highest-dose group a slight decrease in blood glucose
was seen; urea was slightly increased only in the females in this
group. Increases were observed in liver weight in males and females in
the 200 and 1000 µg/kg b.w./day groups, kidney weight in males and
females in the highest-dose group, and spleen weight in females in the
200 and 1000 µg/kg b.w./day groups. In females, ovary weight was
increased in the highest-dose group and uterus weight was decreased in
the 100 and 200 µg/kg b.w./day groups. In males, decreases were seen
in seminal vesicle weight in the 100, 200, and 1000 µg/kg b.w./day
groups and in prostate weight in the 100 and 1000 µg/kg b.w./day
groups. Gross pathological examination revealed atrophy of the
prostate, seminal vesicles, and testes in rats in the highest-dose
group. Histologically, changes in the ovary (cysts and released
follicles in all test groups) and in the uterus ("dentelle uterine" in
the 200 and 1000 µg/kg b.w./day groups) were observed in females. In
males in the highest-dose group a delay in spermatogenesis and aplasia
in the seminal vesicles and prostate were observed (Seeger, 1971a, b).
Female rats fed diets containing 0.01, 0.1, 2.5, 5, 10, 20, 40,
80, or 160 ppm TBA exhibited increases in uterus weight at levels
higher than 40 ppm. Vaginal smears in all groups were negative; some
proliferation of the vaginal mucosa was seen at the highest dose level
(Huis in't Veld et al., 1973).
Female rats weighing 60 - 65 g each were ovariectomized and given
s.c. doses of 0, 0.2, 1.0, or 5.0 mg/day TBA in sesame oil for 4 days.
At termination, on day 5, a dose-related increase in uterus weight was
seen in all dosed groups (maximum +550%). The estrogenic activity of
TBA was less than 0.1% of the estrogenic activity of estradiol-17ß
(Schröder, 1971b).
Male castrated rats weighing 100 g each were given daily s.c.
doses of 4, 20, or 100 µg TBA, 4, 20, or 100 µg ß-TBOH, or 20, 100,
500, or 1000 µg alpha-TBOH for 9 days. Dosing was started 1 day after
castration. One control group was used. At sacrifice, one day after
the last injection, the weights of the prostate, musculo levator ani,
and seminal vesicles were recorded. In animals dosed with both TBA and
ß-TBOH, organ weights were increased in a dose-related manner in all
test groups. In the animals administered alpha-TBOH, organ weights
were increased at the three highest dose levels (Escuret & Bas, 1978).
Male castrated rats weighing 65 - 75 g each were given daily s.c.
doses of 0, 0.02, 0.1, or 0.5 mg TBA as a solution in sesame oil for
10 days after castration. At sacrifice on day 11, dose-related
increases were seen in the weights of muscolo levator ani (maximum
+250%), prostate (maximum +1400%), and seminal vesicles (maximum
+2500%) in all groups. In this experiment TBA showed distinct anabolic
and androgenic activity that was 5 times higher than that of
testosterone and 20 times higher than that of 17-ethynyl-19-nor-
testosterone (Schröder, 1971b).
Groups of 10 male and 10 female rats weighing 123 - 131 g each
were dosed 6 times per week s.c. with 0, 200, 1000, or 5000 µg/kg
b.w./day TBA over a period of 2 months. The compound was applied as a
solution in 1:1 syncortyl and arachis oil.
Growth of females was increased in all groups; by contrast,
growth of males in the highest-dose group was decreased. Haematology
and blood biochemistry determinations, which were performed at
termination in 5 rats/sex/group, revealed slight increases in Hb and
haematocrit values and slight leucocytopenia (due to lymphocytopenia)
in all test groups. Other blood abnormalities that were noted were a
decrease in glucose in females in the 5000 µg/kg b.w./day group (not
determined in other groups), a decrease in BUN in females in the 1000
and 5000 µg/kg b.w./day groups and in males in the 5000 µg/kg b.w./day
group, and a decrease in cholesterol in males and females in the 1000
and 5000 µg/kg b.w./day groups.
In all test groups, absolute and relative kidney weights were
increased and absolute and relative adrenal and thymus weights were
decreased. Ovary weights were decreased in a dose-related manner in
females in all test groups. In females in the 200 and 1000 µg/kg
b.w./day groups uterus weights were decreased. All treated females
showed an increase in absolute liver weight. Males showed a decrease
in testes weight in all treated groups and in seminal vesicle and
prostate weight in the 1000 and 5000 µg/kg b.w./day groups. Besides
these weight changes, atrophy was noted in the thymus, ovaries, and
testes; hypertrophy was seen in the seminal vesicles and prostate
(Sovetal, 1970; Seeger, 1971a).
Rabbits
Groups of 4 - 6 rabbits weighing 2 kg each were administered s.c.
during 4 days 0, 0.05, 0.5, 2, or 5 mg/kg b.w./day TBA. Liver function
was examined (SGOT activity and BSP excretion). At 2 mg/kg b.w./day
SGOT activity was increased slightly, while at 5 mg/kg b.w./day SGOT
activity was increased significantly. BSP excretion was not affected
in any of the test groups (Seeger, 1971a).
Pigs
Male, female, and castrated male pigs were fed diets containing
1-2 ppm TBA, alone or in combination with 2 ppm estradiol-17ß or 2 ppm
ethynylestradiol, for 5 - 8 weeks. At 5 and 6.5 weeks after compound
withdrawal TBOH was not detected in the urine. Urinary excretion of
total steroid estrogen was not increased at 7 weeks after dose
withdrawal (Kroes et al., 1976a).
Groups of 4 male and 4 female domestic pigs (Sus scrofa) were fed
diets containing 0, 0.1, 2, or 20 ppm TBA (equivalent to 0, 4, 80, or
800 µg/kg b.w./day TBA, respectively) for 14 weeks. No treatment-
related effects on mortality, body weight, or ophthalmoscopy were
seen. After 6 and 12 weeks several haematological (i.e., Hb, RBC, PCV,
WBC, diff. WBC, and prothrombin index) and blood biochemical
parameters (i.e., glucose, protein, albumin, albumin/globulin ratio,
SAP, calcium, and creatinine), were unaffected. At 2 and 20 ppm,
dose-related increases were seen in platelets (maximum +70%, not
significant), urea (maximum +3%, significant) and cholesterol (maximum
+100%, significant). SGOT increased in a dose-dependent manner in all
test groups (maximum, +100%, significant). There were dose-related
decreases in blood levels of testosterone and estradiol in males in
all dosed groups (both maximum -95%, significant); progesterone was
markedly decreased (maximum -99%, significant) in females in the two
highest-dose groups. In the same groups, there were dose-related
changes in the absolute and relative weights of the liver (maximum
+30%, significant), uterus (maximum -50%, significant), kidney
(maximum +25%, significant), and testis (maximum -55%, significant).
In the highest-dose group changes were observed in the weights of the
pituitary (-15%, significant) and seminal vesicles (+280%,
significant). There was an increase in thyroid weight at all three
dose levels (maximum +20%, not significant).
Histopathological examination showed the following dose-related
abnormalities in the 2 and 20 ppm groups: in the liver, enlargement of
the hepatocytes with associated ground glass appearance of the
cytoplast; in testes, moderate to complete interstitial cell atrophy
(with normal spermatogenesis within the seminiferous tubules); in
ovaries, evidence of suppressed or abnormal cyclic activity
characterized by the absence of maturing follicles and/or mature or
early regressing corpora lutea; and, in the uteri, absence of
glandular development in the endometrium. This last observation and
the lack of alveolar development and secretion noted in the mammary
glands of the 2 ppm group were probably associated with the anestrous
state suggested by the findings in the ovaries (Ross et al., 1980).
Cattle
Groups of 8 male calves were given s.c. implants of 140 mg TBA +
20 mg estradiol-17ß at 8 or 4 weeks before slaughter. An additional
group was dosed with 200 mg/kg b.w. testosterone + 20 mg/kg b.w.
estradiol-17ß on both occasions. In all treatment groups histological
examination of prostates showed increased secretory activity and
hyperplastic and metaplastic changes (Verbeke et al., 1975).
Six weeks after single s.c. application of 20 mg estradiol-17ß to
male calves, distinct changes in seminal vesicles were found after
histological examination. Treatment of calves with 20 mg estradiol-17ß
combined with 140 mg TBOH, applied s.c., caused very slight changes in
seminal vesicles in 4/11 calves, while in 3/7 animals with unchanged
seminal vesicles, androgenic stimulation was observed (Kroes, 1972).
Male calves that were 11 weeks of age received by s.c.
implantation 20 mg estradiol-17ß alone or 20 mg estradiol-17ß in
combination with 140 mg TBA. Urinary secretion of total steroid
estrogens was high in the animals given estradiol-17ß alone during the
first 12 days after application. After 3 weeks normal values were
reached. In the estradiol-17ß/TBA combination group, gradual and
prolonged excretion of steroid estrogens for up to 42 days after
implantation occurred; after 56 days normal values were reached.
Qualitative determinations of estradiol-17ß and estradiol-17ß in urine
showed that the alpha epimer was present in almost all urine samples;
estradiol-17ß was found in the urine of those calves administered
estradiol-17ß alone. In the combination group, estradiol-17ß was found
in urine at day 21 only. Histological examination of prostates
revealed squamous metaplasia of gland epithelium after both treatments
(Kroes et al., 1976b).
A total of 1480 female calves, aged 7 weeks, were administered by
percutaneous implantation 0, 140, or 3500 mg TBA (groups 1 - 3,
respectively) or 140 mg TBA + 20 mg estradiol-17ß (group 4), 1400 mg
TBA + 200 mg estradiol-17ß (group 5), or 3500 mg TBA + 500 mg
estradiol-17ß (group 6). The calves were slaughtered 10 weeks after
implantation.
Blood parameters (glucose, GOT, GPT, AP, LDH, cholesterol,
bilirubin, Hb, and PCV), urinary density, and pH were unaffected in
all groups. Calcium and phosphorus levels in serum and bone were not
changed; however, magnesium levels in serum and deposition of
magnesium in bone were decreased in groups 3, 5 and 6. In group 3
slight, and in groups 4, 5 and 6 marked, increases in uterus weight
were seen, which were accompanied by proliferation of uterine
glandular cells, while the lumen of the uterus was partially filled
with a watery liquid. In dosed groups ovarian weights were reduced,
accompanied by a diminution in follicular size. These weight changes
were most marked in groups 2, 3 and 6. The diminution of follicular
size, which was accompanied by a decrease in the number of follicles,
was most marked in groups 5 and 6. In all groups, a dose-related
decrease in thymus weight was seen. In group 3 abnormal development of
the clitoris was noted. Histopathological examination showed a
non-dose related proliferation and secretion of glandular tissue in
the mammary glands of animals in groups 4, 5 and 6. Heart, lever,
kidneys, pituitary, pineal gland, adrenal glands, thyroid, and
skeletal muscle did not show abnormalities (Gropp et al., 1975).
Steers and heifers were seven 300 mg TBA by s.c. implantation. An
additional group of steers was given implants containing 140 mg TBA +
20 mg estradiol-17ß. In all groups, plasma urea was decreased during
the 9-week observation period after treatment. Other plasma parameters
(glucose, calcium, phosphorus, magnesium, sodium, potassium, and total
protein) were unaffected. No changes in plasma concentrations of
insulin or growth hormone were seen. A decrease in thyroxine levels in
steers was observed during the 9-week observation period after
treatment; the decrease was most marked in those administered TBA and
estradiol-17ß in combination. A marked reduction, about -50%, in
thymus weight was observed in steers (Heitzman, 1975).
Steers and oxen given, by s.c. implantation, 140 mg TBA alone or
140 mg TBA in combination with 20 mg estradiol-17ß. Control animals
received implantations with the carrier. The combination of drugs
affected urinary excretion of exogenous estradiol-17ß in oxen and
possibly in steers. Histological examination of the prostate revealed
squamous metaplasia in the combination group. In both treated groups,
more active epithelium of the prostate was seen, compared to the
control group (Kroes et al., 1976c).
Long-term studies
Mice
Groups of 64 male and 64 female Swiss albino CFLP mice, weighing
22 - 25 g each, were given diets containing 0, 0.5, 1.0, 10 or 100 ppm
TBA (equal to 0, 0.004, 0.09, 0.86, or 8.6 mg/kg b.w./day TBA for
males, respectively, and 0, 0.005, 0.10, 0.96, or 9.5 mg/kg b.w./day
TBA for females, respectively) for 95 - 104 weeks (the test was ended
when survival was 20% in males or females in the control group). After
13 weeks 12 mice/sex were killed. At that time significant increases
were observed in the absolute and relative weights of the kidneys in
males and females at 100 ppm (20 - 40% increases). Significant
decreases were seen in the weights of the spleen of top-dose females
(-20%) and significant increases were seen at 1.0, 10, and 100 ppm in
males (+25%). Dose-related relative decreases in weights of the uterus
were observed in all dosed females (maximum -25%). At interim
sacrifice, inhibition of ovulation, characterized by a lack of corpora
lutea in all females in the highest-dose group, was observed.
Follicular development proceeded to the mature follicle stage. The
uteri of all females in the 100 ppm group were consistent with the
diestrous stage of the cycle. In the spleens of 6/12 males in the
100 ppm group an increased number of polymor-phonuclear leucocytes in
the red pulp was seen (0/12 in controls); congested sinuses were noted
in 2/12 males of this group (0/12 in controls).
At terminal sacrifice no organ weights were recorded. Terminal
gross- and histopathological examination showed an increase in liver
nodular hyperplasia and dose-related tumours in the male dose groups;
these increases were statistically significant at the two highest
doses. The incidence of liver tumours was also increased in females in
the highest-dose group (8/52 versus 4/51 in controls). There was an
increase in incidence of hepatocyte vacuolation in males in the
100 ppm group. In 100 ppm females, gross pathological examination
showed an increase in the incidence of enlarged and swollen kidneys,
accompanied by a marginal increase in the incidence of nephritis. In
the same group, increases in ovarian cysts (in 10/20 animals versus
1/11 in controls) and enlarged, abscessed, or cystic preputial glands
(in 4/20 females versus 0/11 controls) were seen. The spleens of 4/20
females in the 100 ppm group appeared small (Hunter et al., 1981).
Rats
Six groups of 65 male and 65 female Sprague-Dawley CFY rats
weighing 150 - 200 g each were given diets containing 0, 0.5, 1.0,
4.0, 16, or 50 ppm TBA (equal to 0, 0.02, 0.04, 0.14, 0.56, or
1.80 mg/kg b.w./day TBA for males, respectively, end 0, 0.02, 0.04,
0.16, 0.64, or 1.92 mg/kg b.w./day for females, respectively) for 112
weeks. The parents of the test animals had been dosed at the same
levels from week 9 before mating until day 21 post-partum (except for
the 50 ppm group in which only the dams had been dosed from day 0 of
pregnancy until day 21 post-partum).
The females in the 4.0, 16, and 50 ppm groups showed prominent
pudendum. A dose-related incidence of pendulous anogenital skin was
seen in all female dose groups (maximum incidence, 85%). In males a
dose-related reduction in testes size was seen in all dose groups,
except at 0.5 ppm (maximum incidence, 45%). Growth and food
consumption were decreased throughout the study in males administered
50 ppm TBA (-15 and -10%, respectively). Water consumption was
decreased in the same group up to week 51 (-15%). Urinalysis (9
parameters measured in 5 rats/sex of the 0, 16, and 50 ppm groups on 6
occasions throughout the study) showed no treatment-related changes.
Haematology (10 parameters measured in 10 rats/sex of the 0, 16,
and 50 ppm groups on 6 occasions throughout the study) showed dose-
related slight increases in some parameters in females in the 16 and
50 ppm groups. Male haematology values were generally unaffected by
treatment. Blood biochemistry (13 parameters) showed no compound-
related changes.
At interim sacrifice at week 78, 13 - 14 rats/sex/group were
killed. Macroscopic examination revealed a prominent clitoris in 5/14
females fed 16 ppm TBA and in 13/13 females fed 50 ppm TBA. The males
of the 50 ppm group showed atrophy of the testes, seminal vesicles,
and prostate. Interim organ weighings revealed, in the males fed 16
and 50 ppm TBA, dose-related decreases in absolute testes and prostate
weights. Adrenals and pituitary weights were decreased in both sexes
in the 50 ppm group. Weights of the ovaries were increased in all dose
groups, but without dose relation.
At the end of the experiment, similar changes in the absolute
weights of the testes, prostate, and adrenals were found. In the males
of the highest-dose group, decreases were observed in the weights of
the pituitary (-30%), thyroid (-20%), kidney (-30%), spleen (-30%),
and liver (-20%). The weight of the ovaries was markedly decreased in
the highest-dose females (-60%).
Terminal gross- and histopathological evaluation revealed a
slightly increased incidence of foci or areas of ground-glass
hepatocytes in the livers of the highest-dose females. Urinary bladder
calculi and associated epithelial hyperplasia joined by cystitis and
pyelitis were seen in females in the same dose group. Both the male
and female reproductive tracts were affected. Testicular, prostatic,
and vesicular atrophic changes were seen in males in the 16 and 50 ppm
groups. Absence of corpora lutea, vaginal inflammation and
mucification, uterine endometritis, dilatation, decreased endometrial
thickness, and the development of clitoral bone were seen in the
50 ppm females. Clitoral enlargement was seen in females in the two
highest-dose groups. Neoplastic histopathology showed an increase in
the incidence of pancreatic islet cell tumours in the 50 ppm group
(Hunter et al., 1982).
Observations in human beings
Male and female human volunteers were given i.m. doses of 5 or
10 mg TBA every-other-day during 14 days. In the subjects administered
5 mg TBA, nitrogen balance was disturbed, including retention of
nitrogen. In the 10 mg group, some women showed disturbances of the
menstruation cycle. The same dose caused a slight but significant
reduction of excretion of 17-ketosteroids. No effects on
17-hydroxy-corticosteroid excretion were seen. Blood parameters
(protein, cholesterol, coagulation factors, and prothrombin and
thrombin time) were unaffected by treatment (Krüskemper et al.,
1967).
COMMENTS
TBA is a synthetic steroid with anabolic properties. At the
17-position in the molecule two epimers, alpha and ß, are possible.
The ß-epimer of TBA is the commercial product. It is administered to
cattle either alone or in combination with estradiol-17ß or zeranol as
a subcutaneous implant in the ear to improve body weight, feed
conversion, and nitrogen retention. It is usually implanted 60 - 90
days before the intended date of slaughter.
After administration to cattle, TBA is rapidly hydrolyzed to
TBOH, the major metabolite being alpha-TBOH, occurring in the excreta,
bile, and liver. In muscle most of the TBOH is present as ß-TBOH.
Experiments with implantation of 200 mg of radiolabelled TBA in calves
and heifers showed that maximum levels of residues occurred about 30
days after implantation. The highest mean concentration of residues as
TBOH equivalents was 50 µg/kg in liver, while muscle contained
3 µg/kg.
The results of the studies requested at the twenty-seventh
meeting were submitted for consideration by the present Committee. In
addition, new toxicological data were available on reproduction and
mutagenicity. The Committee also reviewed previously available data
including metabolic, teratogenicity, and carcinogenicity data.
Acute toxicity studies in several species showed TBA to be of low
toxicity, when given orally.
Experiments had been performed in rats to assess the effect of
TBA on pregnancy, on the reproductive function of multiple
generations, and on the development of the offspring to weaning.
Treatment at 0.3 and 0.5 ppm in the diet, equivalent to about
20-30 µg/kg b.w./day, was associated with mean weekly body weights for
female rats that were only slightly higher than those of controls, and
marginal differences in litter parameters, while treatment with TBA at
3.0 and 18.0 ppm in the diet was associated with a hormonal effect. It
was considered that TBA exerted no effect on reproductive performance
in the rat at 0.5 ppm in the diet, equivalent to 30 µg/kg b.w./day. No
teratogenic effect was seen in two feeding studies in rats at very
high doses of TBA. In a comprehensive range of in vivo and in vitro
mutagenicity studies, all tests were negative for TBA, ß-TBOH, and
TBA's major metabolite, alpha-TBOH, with the exception of the mutation
assay in mouse lymphoma cells, which gave equivocal results with
ß-TBOH and alpha-TBOH. The Committee also noted a report of an
equivocal result in a transformation study with ß-TBOH in Syrian
hamster embryo fibroblasts, and took into account the recognized
difficulty in interpreting results from this type of study.
The Committee reaffirmed the opinion expressed at its
twenty-seventh meeting regarding the results of long-term feeding
studies with TBA with rats and mice (Annex 1, reference 62). It
considered that the liver hyperplasia and tumours in mice fed high
doses of TBA (0.9 - 9 mg/kg b.w./day) and the slight increase in the
incidence of islet-cell tumours of the pancreas of rats fed TBA at
1.85 mg/kg b.w./day (the highest dose in the study) arose as a
consequence of the hormonal activity of TBOH.
The Committee therefore concluded that its safety assessment
could be based on establishing the no-hormonal-effect level. It
reviewed a study with castrated male rhesus macaque monkeys
administered ß-TBOH orally, and considered that this model could be
relevant to the human population. The castrated male rhesus monkey is
highly sensitive to compounds with antigonadotropic activity; the
Committee therefore adopted a conservative approach by using this
study as the basis for establishing an ADI for human beings. Despite
the small numbers in each group of monkeys studied, and the advanced
age of the animals used, the Committee set 2 µg/kg b.w./day as a
no-hormonal-effect level, based on assessment of histological changes
in the seminal vesicles. In the intact female rhesus monkey, TBA had a
clear no-hormonal-effect level of 10 µg/kg b.w./day. The Committee
also considered that the pig was a sensitive model for assessing
hormonal effects and noted that, here too, TBA had a no-hormonal-
effect level of 2 µg/kg b.w./day based on assessment of pathological
changes in the testes. Another study in the pig demonstrated that the
hormonal activity of ß-TBOH was about ten times that of alpha-TBOH. No
data on individual animals were available for any of the pig studies.
In the absence of satisfactory toxicological data the Committee
was unable to establish a separate no-effect level for the alpha-TBOH
metabolite. It also noted that this metabolite was not produced in
significant amounts in the rat, which made it inadvisable to
extrapolate from data generated from ß-epimer experiments in that
species.
EVALUATION
Level causing no hormonal effect
Pig: 0.1 ppm in the diet, equal to 2 µg/kg b.w./day.
Monkey: 2 µg/kg b.w./day.
Estimate of temporary acceptable daily intake
0 - 0.01 µg/kg b.w.
Further work or information
Required (by 1990):
( a) the final reports, with supporting data, for the tissue residue
studies in which TBA was administered to heifers and TBA in
combination with estradiol-17ß was administered to steers;
( b) histopathological data on individual animals from the three
hormonal studies in pigs that were reviewed by the Committee;
( c) results from a 90-day study in an appropriate species, with
orally administered alpha-TBOH.
REFERENCES
Allen, J.A. & Proudlock, R.J. (1983). Autoradiographic assessment of
DNA repair in mammalian cells after exposure to 17-alpha-hydroxy-
trenbolone and to 17-ß-hydroxytrenbolone. Unpublished report
No. RSL 619/83731 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Brooker, P.C., & McCaffrey, K.J. (1985). Analysis of
metaphase chromosomes obtained from CHO cells cultured in vitro and
treated with 17-ß-hydroxytrenbolone. Unpublished report No. RSL
676/85631 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Proudlock, R.J., & Pugh, L.C. (1986). Micronucleus test
on 17-ß-hydroxytrenbolone. Unpublished report No. RSL 728/861306 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Allen, J.A. & Proudlock, R.J. (1987). Assessment of 17-alpha- and
17-ß-hydroxytrenbolone in the Solt-Farber system for induction of
liver foci. Unpublished report No. RSL 711/861252 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981a). R 1697 (trenbolone
acetate). Single administration study by oral route in the mouse.
Unpublished report No. 81213-A-JL/36-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981b). R 1697 (trenbolone
acetate). Single administration study by oral route in the rat.
Unpublished report No. 81212-A-JL/37-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, g., & Glomot, R. (1981c). R 1697 (trenbolone
acetate). Single administration study by oral route in the dog.
Unpublished report No. 81216 from Roussel Uclaf Research Centre.
Submitted to WHO by Roussel Uclaf, Paris, France.
Barraud, B., Lugnier, A., & Dirheimer, G. (1983). In vivo covalent
binding to rat liver DNA of trenbolone as compared to 17-ß-estradiol,
testosterone and zeranol. In: Anabolics in animal production,
International Office of Epizootics, Paris, pp. 193-206.
Bouffault, J.C. (1977). Expert statement concerning the excretion of
trenbolone and oestradiol-17-alpha in urine of calves after
subcutaneous implantation of trenbolone acetate and oestradiol 17-ß.
Unpublished report submitted to WHO by Roussel Uclaf, Paris, France.
Chasseaud, L.F., Hawkins, D.R., Elsom, L.F., Parsonage, M.T.,
Ross, D.B., & Roberts, N.L. (1976). Plasma and tissue concentrations
of radioactivity in cattle after implantation of pellets containing
3H-trenboloneacetete, 3H-oestradiol or combinations of radioactive
drugs with the non-radioactive form of the other. Unpublished report
No. SVT/11/76351 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Edgar, D.H., Bosworth, H.J., Ransome, S.J., & Banks, S.J. (1985). An
assessment of the mutagenic potential of 17-ß-hydroxytrenbolone in
mammalian cells in vitro using the Chinese hamster ovary/HGPRT locus
assay. Unpublished report No. RSL 677/85642 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Escuret, P. & Bas M.N. (1978). DL-50 chez le rat et chez le souris.
Unpublished report Recherches Veterinaires R.U.-essai 5.77.71 R.
Submitted to WHO by Roussel Uclaf, Paris, France.
Gropp, J., Herlyn, D., Boehncke, E., Schulz, V., Sanderleben, J.V.,
Hanichen, Th., & Geisel, O. (1975). Physiological data including
evaluation of immunoresponse in relation to anabolic effects on veal
calves. Published in "Anabolic agents in animal production",
FAO/WHO Symposium, pp. 131-141, March 1975.
Gropp, J., Buntenkotter, S., & Kaemmerer, K. (1978). Relay toxicity of
anabolic agents, general considerations and some experimental data.
Published at Relay Toxicity and Bioavailability Symposium,
5 October 1978. Report submitted to WHO by Roussel Uclaf,
Paris, France.
Hawkins, D.R., Weston, K.T., Ray, J., Ross, D.B., & Cameron, D.M.
(1979). The bioavailability of trenboloneacetate residues in the
edible tissues of heifers after oral administration to rats.
Unpublished report No. RSL/328/7959 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hawkins, D.R., Waller, A.R., Moore, D.H., Jordan, M.C., Roberts, N.L.,
& Cameron, D. (1984). Tissue residues of radioactivity at 15 and 30
days after implantation of 3H-trenboloneacetate in calves.
Unpublished report No. HRC/RSL/636 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Heitzman, R.J. (1975). The effectiveness of anabolic agents in
increasing rate of growth in farm animals; report on experiments in
cattle. Published in "Anabolic agents in animal production", FAO/WHO
Symposium, pp. 89-98, March 1975.
Heitzman, R.J. & Hardwood, D.J. (1977). Residue levels of trenbolone
and oestradiol-17-ß in plasma and tissues of steers implanted with
anabolic steroid preparations, Br. Vet. J., 133, 564.
Henderson, L.M., Bosworth, H.J., Ransome, S.J., Banks, S.J., & Ozel,
K.J. (1986a). An assessment of the mutagenic potential of 17-alpha-
hydroxytrenbolone in mammalian cells in vitro using the Chinese
hamster ovary/HPRT locus assay. Unpublished report No. RSL 713/86713
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Henderson, L.M., Brooker, P.C., & Howell, A. (1986b). An assessment of
the mutagenic potential of 17-ß-hydroxytrenbolone in mammalian cells
in vitro using a Chinese hamster ovary micronucleus test.
Unpublished report No. RSL 729/861287 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., & Bosworth, H.J. (1987a).
An assessment of the carcinogenic potential of 17-ß-hydroxytrenbolone
and testosterone in an in vitro cell transformation assay using
mouse C3H10T1/2 cells. Unpublished report No. RSL 691/861257 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., Bosworth, H.J.,
Bottoms, M., & Williams, J. (1987b). An assessment of the mutagenic
potential of 17-ß-hydroxytrenbolone in mammalian cells in vitro
using the Chinese hamster V79/HPRT locus assay. Unpublished report
No. RSL 712/861249 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hess, D.L. (1983). Determination of a hormonal no-effect dose level
for 17-ß-trenbolone Rhesus Macaque. Unpublished report from Oregon
Regional Primate Research Center, Beaverton, OR, USA. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hess, D.L. (1984). Determination of the hormonal no-effect dose level
for trenbolone acetate in the female Rhesus Macaque. Unpublished
report from Oregon Regional Primate Research Center, Beaverton, OR,
USA. Submitted to WHO by Roussel Uclaf, Paris, France.
Hoffman, B., Schopper, D., & Karg, H. (1984). Investigations on the
occurrence of non-extractable residues of trenbolone acetate in cattle
tissues in respect to their bioavailability and immunological
reactivity. Food Additives and Contaminants., 1, p. 253.
Hossack, D.J.N., Richold, M., & Jones, E. (1978). Ames metabolic
activation test to assess the potential mutagenic effect of trenbolone
acetate, 17-ß-oestradiol and 7:1 mixture of trenbolone with
17-ß-oestradiol. Unpublished report No. RSL 351/781136 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Huis in't Veld, L.G., Have, J. ten, & Kok, G.L. (1973). Hormonale
werkingen van trenbolone-acetaat. Unpublished report No. U 146/73 ENDO
from the National Institute of Public Health, The Netherlands.
Hunter, B., Macrae, S., Prentice, D.E., & Brooks, P.N. (1976a).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 235/76316
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Macrae, S., Prentice, D.E., & Majedd, S.K. (1976b).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 213/75992
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Bathman, P., Heywood, R., Street, A.E., Prentice, D.E., &
Majeed, S.K. (1976c). Trenboloneacetate oral toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RSL
237/76426 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Graham, C., Gibson, W.A., Read, R., Gregson, R., Heywood
R., Offer, J., Prentice, D., & Woodhouse, R. (1981). Long-term feeding
of trenboloneacetate to mice (final report). Unpublished report volume
I, II and III, No. RSL/267/80122 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Batham, P., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D., Gregson, R., & Offer, J. (1982). Trenboloneacetate
potential tumorigenic and toxic effects in prolonged administration to
rats following "in utero" exposure (final report). Unpublished report
volume I, II, III and IV, No. RSL 295-G/80974 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ingerowski, G.H., Scheutwinkel-Reich, M., & Stan, H.J. (1981).
Mutagenicity studies on veterinary anabolic drugs with the
Salmonella/microsome test. Mutagen. Res., 91, 93-98.
James, P.A., Cox, R., Clark, R., Palmer, A.K., & Offer, J. (1982).
Effect of trenboloneacetate on pregnancy of the rat. Unpublished
report No. RSL 458/81553 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., John, D.M., Gibson, W.A., Offer, J.M., &
Anderson, A. (1985). Effect of trenbolone acetate on reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/841004 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., & Parker, C.A. (1986). Supplementary study of
the effect of trenbolone acetate on pregnancy of the rat and
development of the offspring. Unpublished report No. RSL 695/851572
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Kroes, R. (1972). De invloed van oestradiol en een combinatie van
oestradiol en trienbolone op de vesiculae semenalis van mestkalveren.
Unpublished report No. 162/72 Path. from the National Institute of
Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976a). Onderzoek
naar de invloed van ethynylestradiol, 17-alpha-oestradiol en
trenboloneacetaat alleen of in combinatie op beren, borgen en geiten.
Uitscheiding in urine en faeces en residuen in serum (ILOB proeven
98.06 98.07, 98.08, 98.09, 118.04 en 118.05). Unpublished report
No. 216/76 Path. from the National Institute of Public Health,
The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976b). Onderzoek
naar de invloed van combinatie-preparaten van mannelijke en
vrouwelijke hormonen of hormonaal werkende stoffen of mannelijke
mestkalveren (Een biologisch, chemisch en histologish onderzoek, ILOB
proef 25.14 P 72/82). Unpublished report No. 193/76 Path. from the
National Institute of Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., Both-Miedema, R., & van de Berg, R.
(1976c). Onderzoek naar de invloed van 17-ß-oestradiol, trenbolone-
acetaat en testosteron alleen of in combinatie, op stieren en ossen
(Uitscheiding in urine, aanwezigheid in serum en histologisch
onderzoek prostaat, ILOB-proeven 134-02 a, 134.02 b en 134.03).
Unpublished report No. 218/76 Path. from the National Institute of
Public Health, The Netherlands.
Kruskemper, H.L., Morgner, K.D., & Noell, G. (1967). Klinische
pharmakologie von Trienolonen, eine Gruppe neuartiger anabol wirksamer
Oestran-Derivate, Arzneim. Forsch., 17, 449-454.
Lutz, W.K (1979). In vivo covalent binding of organic chemicals to
DNA as a quantitative indicator in the process of chemical
carcinogenesis. Mutation Res., 65, 289-356.
Offer, J.M. (1985). Effect of trenbolone acetate on the reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/85959 (Addendum to RSL 634/841004) from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Philibert, D. & Moguilewsky, M. (1983). Liaison de la testosterone de
la 17-ß-OH-trenbolone (RU 2341) et de son isomere, la 17-alpha-
OH-trenbolone (RU 27423) au niveau du plasma humain. Unpublished
report No. AN 39 from Roussel Uclaf Research Centre. Submitted to WHO
by Roussel Uclaf, Paris, France.
Pottier, J. Busigny, M., & Gradadam, J.A. (1973). Tissue residues in
cows after implantation of trenbolone acetate. J. Anim. Sci.,
37, 256 (Abstract No. 110F).
Pottier, J., Busigny, M., & Gradaclam, J.A. (1975). Plasma kinetics,
excretion in milk and tissue levels in the cow following implantation
of trenbolone acetate. J. Anim. Sci., 41, 968.
Pottier, J., Cousty, C., Heitzman, R.J., & Reynolds, I.P. (1978).
Metabolites trenbolone acetate R in the bile of the cow and the rat.
Report presented during the 1978 Joint Meeting of the American Dairy
Association and the American Society of Animal Science, Michigan State
University, East Lansing, 9-13 June. Submitted to WHO by Roussel
Uclaf, Paris, France.
Pottier, J. (1979). Trenboloneacetate tissue and biliary
concentrations of the position of 17 epimers of trenbolone in heifers
after 2 months of implantation. Unpublished report No. 799 from
Roussel Uclaf. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. & Richardson, J.C. (1982). Metaphase analysis on
17-ß-hydroxytrenbolone and 17-alpha-hydroxytrenbolone. Unpublished
report No. RSL/512/81962 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M., Jones, E., & Hales, J.F. (1982a). Ames metabolic
activation test to assess the potential mutagenic effect of
17-alpha-hydroxytrenbolone, 17-ß-hydroxytrenbolone and
17-alpha-oestradiol. Unpublished report No. RSL 567/82767 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Richold, M., Richardson, J.C., Proudlock, R.J., & Fleming, P.M.
(1982b). Effect of 17-ß-hydroxytrenbolone and 17-alpha-hydroxy-
trenbolone on cultured human lymphocytes. Unpublished report No. RSL
544/82136 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. Dehnel, J.M., & Ransome, S. (1982c). An assessment of the
mutagenic potential of 4 compounds using an in vitro mammalian cell
test system. Unpublished report No. RSL 513/8286 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Richold, M., Edgar, D.H., Ransome, S.J., & Banks, S.J. (1983). An
assessment of the mutagenic potential of four compounds using an
in vitro mammalian cell test system. Unpublished report from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Riis, P.M. & Suresh, T.P. (1976). The effect of a synthetic steroid
(trenbolone) on the rate of release and excretion of subcutaneously
administered estradiol in calves. Steroids, 27, 5-15.
Roberts, N.L. & Cameron D.M. (1985). Hormonal effects of orally
administered trenbolone. Findings from three studies in the domestic
pig. Unpublished report No. RSL 591/83499 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ross, D.B. (1980). Toxicology and residues of trenboloneacetate.
Published in: Conference on the Use, Residues and Toxicology of Growth
Promoters, Dublin, 9 April 1980, pp. 34-48.
Ross, D.B., Roberts, N.L., Cameron, D.M., Street, A.E., Crook, D.,
Prentice, D.E., & Cherry, C.P. (1980). Oral toxicity of trenbolone
acetate to growing pigs (Sus scrofa)- preliminary 14 weeks feeding
study. Unpublished report No. RSL 357/80152 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ryan, J.J. & Hoffman, B. (1978). Trenbolone acetate: experiences with
residues in cattle tissues. J.A.O.A.C., 61, 1274-1279.
Schiffman, D., Metzler, M., Neudecker, T., & Henschler, D. (1985).
Morphological transformation of Syrian hamster embryo fibroblasts by
the anabolic agent trenbolone. Arch. Toxicol., 58, 59-63.
Schroder, R. (1971a). R 1697: Prüfung der androgenen und
muskelbildenden Wirkung bei oraler Verabreichung. Unpublished report
from Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Schroder, R. (1971b). R 1697: Untersuchung auf androgene und anabole
Wirkung, Untersuchung auf oestrogene Wirkung. Unpublished report from
Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Seeger, K. (1971a). Anabolikum R 1697 (A 50273) as us vet.
Unpublished report from Hoechst A.G. Submitted to WHO by Roussel
Uclaf, Paris, France.
Seeger, K. (1971b). R 1967: Chronische Toxizitat per oral. Unpublished
report from Hoechst A.G. Submitted to WHO by Roussel Uclaf,
Paris, France.
Sovetal (1970). Toxicité cronique par voie sous-cutanée chez le rat
male et chez la femelle. Unpublished report No. R 1697 from Sovetal.
Submitted to WHO by Roussel Uclaf, Paris, France.
Stephany, R.W., Bosch, D., van den Sahertian, E.Th., & Kroes, R
(1976). Onderzoek naar de invloed van orale toediening van
trenboloneacetaat op vaarzen (ILOB-proef 134-01) (Eenchemisch
onderzoek). Unpublished report No. 215/76 Path. From the National
Institute of Public Health, The Netherlands.
Verbeke, R., Debackere M., Hicquet, R., Lauwers, H., Stevens, J.,
Pottier, G., Moer, D., Van Hoof, J., & Vermeersch, G. (1975). Quality
of the meat after the application of anabolic agents in young calves.
Published in: Anabolic Agents in Animal Production, FAO/WHO Symposium,
March 1975, pp. 123-130.
van de Wal, P. (1975). General aspects of the effectiveness of
anabolic agents in increasing protein production in farm animals, in
particular in bull calves. Published in: Anabolic Agents in Animal
Production, FAO/WHO Symposium, March 1975, pp. 60-78.
In two barren cows after s.c. implantation of 300 mg 3H-TBA per
animal at the base of the ear, slow resorption from the implant
occurred; the half-life of disappearance from the implant was 68 - 84
days. About 33% of the radioactivity was extracted in the blood plasma
over the 3-month period after implantation, 70% of which was accounted
for by TBOH. The main routes of excretion were via the bile and urine.
Tissue levels after 3 months were about 1 ppb, except in the liver
(6.5 ppb) and kidneys (4.5 ppb). Twenty-five percent of the tissue
radioactivity was extractable, 40% of which was TBOH. In the liver and
kidneys, however, only 10% was extractable, while in perirenal fat up
to 88% of the radioactivity was extractable. In perirenal fat 50% of
the radioactivity was TBA. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity (Pottier et al., 1973;
Pottier et al., 1975).
Slow resorption from s.c. implants of 300 mg 3H-TBA occurred in
2 lactating cows. The half-life for disappearance from the implant was
approximately 60 days. About 17% of the radioactivity present in the
blood plasma over the period of 5 months after implantation was
extractable. Less than 1% of the radioactivity was excreted in milk.
Ten percent of the milk radioactivity was extractable and 25% of this
extractable radioactivity was TBOH. Tissue levels after 5 months were
about 1 ppb, except in the liver (3.4 ppb) and kidneys (2.7 ppb).
About 25% of the tissue radioactivity was extractable, except in the
liver and kidneys (both 10%); about 40% of this extractable
radioactivity was TBOH. In contrast, 88% of total radioactivity in
perirenal fat was extractable, of which 50% was TBA. Unchanged TBA was
found in no other tissues. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity after 5 months (Pottier
et al., 1973; Pottier et al., 1975).
Two steers were given by single s.c. implantations 300 mg
3H-TBA in combination with 40 mg estradiol; the implants were
removed 60 days later, at which time 28% of the radioactivity remained
in them. Ethyl acetate-extractable radioactivity in blood plasma was
primarily ascribed to TBOH; in most cases no TBA was found in the
plasma. One animal was killed immediately after removal of the
implant. Plasma concentrations in this animal declined with half-lives
of 26 days for both total and non-volatile radioactivity; ethyl
acetate-extractable radioactivity in the plasma of this animal ranged
between 3 - 5% of the total radioactivity. In the other animal, which
was killed 16 days after removal of the implant, plasma concentrations
declined during days 1 - 60, with half-lives of 50 and 55 days for
total and non-volatile radioactivity, respectively. In the 16 days
from implant removal to sacrifice, radioactivity decreased by 46% in
muscle, 2% in liver and kidneys, and 29% in fat (Chasseaud et al.,
1976).
Relay bioavailability
Groups of 3 rats were fed freeze-dried or ethyl acetate-extracted
liver, kidney, or muscle obtained from two heifers killed 60 days
after s.c. implantation with 300 mg 3H-TBA. 3H-TBA levels in the
heifers averaged 30 ng equivalents/g in the liver, 24 ng equivalents/g
in the kidneys, and 3.2 ng equivalents/g in muscle. Radioactivity
excretion during the 3 days after feeding these tissues to rats is
presented in Table 1 (Hawkins et al., 1979).
Groups of 3 bile duct-cannulated rats that had been fasted for 24
hours were fed during 1 hour freeze dried liver, kidney, or muscle
from the two heifers described in the previous paragraph.
Radioactivity disposition during 48 hours after feeding of these
tissues is presented in Table 2 (Hawkins et al., 1979).
Table 1. Excretion of radioactivity by rats after being fed tissues
from heifers implanted with 3H-TBA
Excretion in percent of
administered radioactivity
Treatment Tissue Urine Faeces Total
Freeze-dried tissue Liver 3 81 84
Kidney 2 93 94
Muscle 6 85 91
Extracted tissue Liver 5 78 83
Kidney 2 103 105
Muscle 2 73 75
Table 2. Excretion of radioactivity by bile duct-cannulated rats
after feeding of tissue from heifers implanted with 3H-TBA
Excretion in percent of administered radioactivity
Tissue Bile Urine Faeces GI tract + contents Total
Liver 7 5 59 2 74
Kidney 3 1 31 60 95
Muscle 3 2 56 not detected 61
Effects on protein binding
The affinity of alpha-TBOH and ß-TBOH for corticosteroid binding
globulin, measured in vitro using the human plasma of elderly women,
was very low, less than 0.1% bound, compared with 10% for testos-
terone. The affinity of alpha-TBOH and ß-TBOH for testosterone and
estradiol binding globulin was 1% of that measured for testosterone.
When alpha-3H-TBOH was incubated in vitro with female human plasma,
it readily bound to the albumin fraction; only 4% was present
as free TBOH. The total blood clearance of ß-TBOH was twice that of
testosterone (Philibert & Moguilewsky, 1983).
Effects on estradiol-17ß excretion and nitrogen retention
Cattle
Plasma residues of estradiol-17ß in cattle were affected by the
presence of TBA in the s.c. implant. Plasma levels of estradiol-17ß
remained greater than 0.05 ppb for nine weeks in steers after
treatment with 200 mg TBA in combination with 40 mg estradiol-17ß,
whereas the residual levels decreased below 0.05 ppb within 5 weeks
after implantation of 40 mg estradiol-17ß alone (Heitzman & Hardwood,
1977).
Implantation of 40 mg TBA in the dewlap of Friesian bulls
(11 - 16 weeks of age) did not affect nitrogen retention. Implantation
of 140 mg TBA in combination with 20 mg estradiol-17ß at the same
site, however, resulted in a 47% decrease in nitrogen retention
(van der Wal, 1975).
Pigs
Pigs (males, females, and castrated males) were given s.c.
implantations with either 20 mg estradiol-17ß or 20 mg estradiol-17ß
in combination with 140 mg TBA. At 5 weeks after implantation, steroid
estrogens were hardly detectable in the faeces, and serum values for
estradiol-17ß were very low in both groups. Urine estradiol-17ß levels
were 6 - 82 µg/l in the estradiol-17ß group and 16 - 135 µg/l in the
combination group (Kroes et al., 1976a).
Toxicological studies
Special studies on carcinogenicity potential
Rats
Male Wistar rats (number not specified) were injected i.p.
with 15 µg/kg b.w. 3H-estradiol-17ß (53.6 Ci/mmole), 19 µg/kg
b.w. 3H-testosterone (54.0 Ci/mmole), 17 µg/kg b.w. 3H-TBA,
(57.0 Ci/mmole), or 30 µg/kg b.w. 3H-zeranol (50.0 Ci/mmole), all in
95% ethanol solution. The animals were sacrificed 16 hours after
injection and the Covalent Binding Indices (CBI, Lutz, 1979) of the
chemicals to DNA in the liver were quantitated. The CBIs were 11.4,
4.80, 5.62, and 1.65 for estradiol-17ß, testosterone, TBA, and
zeranol, respectively (weak carcinogens have a CBI approx. or equal
10, Lutz, 1979). The positive control, N-hydroxy-acetylaminofluorene,
had a CBI value of 262 (Barraud et al., 1983).
The CBI of TBA as a function of time was measured by
administering 0.83 mCi (22 - 40 µg/kg b.w.) 3H-TBA i.p. to 8 male
rats. The animals were killed at 4, 8, 12, 20, 24, 36, 48, and 96
hours. The highest CBI, 7.82 was obtained after 24 hours; after 96
hours the CBI was 1.11 (Barraud et al., 1983).
Treatment of rodents with initiators of liver cancer can give
rise to phenotypically altered cells which, under suitable conditions,
will develop into foci of potentially pre-neoplastic cells. These foci
may either regress or develop into malignant nodules, but because they
only take a few weeks to become apparent, induction of such foci
represents a useful short-term indication of tumour-initiating
capacity.
alpha-TBOH or ß-TBOH (2.5, 5, or 10 mg/kg b.w.), ethinyl
estradiol (0.05 mg/kg b.w.), testosterone (10 mg/kg b.w.),
nitrosomorpholine (25 mg/kg b.w.), or diethylnitrosamine (200 mg/kg
b.w.) were administered by i.p. injection approximately 18 hours after
partial hepatectomy to Fisher 344 CDF rats (5 males and 5 females per
group). Two groups presented only with vehicle and one untreated group
of 5 males and 5 females each were used as controls. The animals were
allowed to recover for a further 13 days after treatment with the test
agent. The animals then were supplied with tap water and powdered diet
containing 0.02% 2-acetylaminofluorene, except that the diet supplied
to animals in one of the vehicle control groups contained no
acetylaminofluorene. Seven days after commencing the new dietary
regime the animals were treated with carbon tetrachloride at 2 ml per
kg b.w. by intragastric gavage (animals in the vehicle control group
not given acetylaminofluorene were not treated with carbon
tetrachloride). Seven days later the animals were killed by cervical
dislocation and the livers were removed for microscopic examination.
Most of the animals showed moderate lethargy and other clinical
signs for two or three days following the operative procedure, but no
compound-related adverse signs were evident. No significant
treatment-related effects on body or liver weight were reported. Only
animals treated with nitrosomorpholine or diethylnitrosamine showed
significant increases in liver foci compared with the vehicle control
or untreated groups.
None of the steroids examined in this study (including alpha-TBOH
and ß-TBOH) showed any evidence of inducing pre-neoplastic liver foci
at the dose levels tested. The authors concluded that none of these
steroids showed any evidence of being a liver rumour initiator in this
assay (Allen & Proudlock, 1987).
Special study on immunoresponse
Cattle
Antibody production in male and female calves (about 25 animals
per group) was investigated after s.c. implantation of placebo
(lactose), 20 mg estradiol-17ß, 140 mg TBA, or 140 mg TBA + 20 mg
estradiol-17ß. A slight, non-significant immunodepressive effect was
seen in male calves treated with either estradiol-17ß or TBA alone. In
the males treated with the combination, this effect was significant.
In female calves the immunoresponse was unaffected (Gropp et al.,
1975).
Special studies on mutagenicity
The results of mutagenicity assays on TBOH and TBA are summarized
in Table 3.
Special studies on no-hormonal effect levels
Pigs
Groups of 3 - 7 (11 in the control group) mature male large white
hybrid pigs, 8 - 10 months of age, were administered orally 0.1, 1,
10, 16, 24, or 36 µg ß-TBOH/kg b.w./day or 0.1, 10, 100, 160, 240, or
360 µg alpha-TBOH/kg b.w./day in gelatine capsules with the feed for
14 consecutive days after castration. They were maintained for 14 more
days, then killed and examined post mortem. Serial blood samples
were collected prior to castration (day 0) and on days 7, 14, 21, and
28; LH was determined on days 0 and 14 in the animals treated with
alpha-TBOH and on days 0, 14, and 28 in the animals treated with
ß-TBOH. After sacrifice, the pituitary, prostate, and seminal vesicles
were examined by gross pathology and histopathology. Based on changes
in plasma luteinizing hormone (LH) concentrations, distinct hormonal
effects occurred at 160 µg alpha-TBOH/kg b.w./day and at 16 µg
ß-TBOH/kg b.w./day. Thus, the no-observed-effect levels in this study
were 10 µg ß-TBOH/kg b.w./day and 100 µg alpha-TBOH/kg b.w./day. The
morphological examinations performed in this study are of limited
value, since a 14-day period elapsed between final treatment and
slaughter, allowing regression of likely effects. Thus, the
observation of treatment-related androgenic effects in the 16, 28, and
36 µg ß-TBOH/kg b.w./day group points toward the high hormonal
activity of this compound (Roberts & Cameron, 1985).
Four groups of 4 male and 4 female large white hybrid pigs were
given orally TBA incorporated into the diet for 14 consecutive weeks
at 0, 0.1, 2, or 20 ppm (equal to 0, 2.0-3.1, 40-62, or 400-620 µg/kg
b.w./day). Blood samples were obtained for steroid hormone assays
before treatment commenced and after 6 and 12 weeks of treatment.
Table 3. Results of mutagenicity assays on TBOH and TBA
Concentration
Test Test of substance
system object tested Results Reference
Ames S. typhimurium 10-10000 µg/ Negative Hossat et al., 1978
test1 TA98, TA100, plate TBA or
TA1535, TA1537 7:1 TBA:
TA1538 estradiol-17ß2
Ames S. typhimurium 1000, 2000, Negative Ingerowski et al., 1981
test1 TA98, TA100, 3000 µg/plate at 1000
TA1535, TA1537 TBOH µg/plate;
TA1538 equivocal at
cytotoxic
concentrations
Ames S.typhimurium 0.5 - 500 µg/ Negative Richold et al., 1982a
test1 TA98, TA100, plate alpha-TBOH;
TA1535, TA1537 15 - 1500 µg/
TA1538 plate ß-TBOH
Ames S. typhimurium 0.06 - 2 µg/ Negative Schiffman et al., 1985
test1 TA98, TA100 plate TBOH
Clastogenic Human lymphocytes 6, 30, or 60 Negative Richold et al., 1982b
potential in vitro µg/ml alpha-TBOH
or ß-TBOH2,3
Cell Mouse lymphoma 15 - 45 µg/ml Equivocal6 Richold et al.,
mutation L5178Y cells alpha-TBOH; 15 - 1982c, 1983
assay4 65 µ/ml ß-
TBOH5
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Cell Syrian hamster 5, 10, 15 µg/ Equivocal7 Schiffman et al., 1985
transformation embryo fibroblasts ml TBOH
assay
Cell Mouse C3H1OT1/2 2 - 25 µg/ml Equivocal Henderson et al., 1987a
transformation cells ß-TBOH (- act.)
assay1 (-S-9 mix)5
5 - 20 µg/ml Positive
ß-TBOH (+ act.)
(+S-9 mix)8
Forward Chinese hamster 25 - 100 µg/ml Negative Edgar et al., 1985
mutation ovary cells ß-TBOH
assay1 (HGPRT locus) (-S-9 mix)9
25 - 150 µg/ml
ß-TBOH
(+S-9 mix)5
Forward Chinese hamster 25 - 500 µg/ml Negative Henderson et al.,
mutation ovary cells ß-TBOH5 1986a
assay1 (HGPRT locus)
Forward Chinese hamster 3 - 75 µg/ml Negative Henderson et al.,
mutation V79 cells ß-TBOH 1987b
assay1 (HGPRT locus) (-S-9 mix)9
12 - 125 µg/ml
ß-TBOH
(+S-9 mix)
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Micronuclei Chinese hamster 1 - 10 µg/ml Equivocal Henderson et al.,
induction1 ovary cells ß-TBOH (- act.) 1986b
(-S-9 mix)10
6 - 60 µg/ml Negative
ß-TBOH (+ act.)
(+S-9 mix)10
Chromosome Chinese hamster 1 - 10 µg/ml Negative Allen et al., 1985
aberration ovary cells ß-TBOH
assay1 (-S-9 mix)3
6 - 60 µg/ml
ß-TBOH
(+S-9 mix)11
Unscheduled HeLa cells and 2.5 - 15 µg/ml Negative Schiffman et al.,
DNA Syrian hamster 1985
synthesis embryo cells
DNA Cultured human 1 - 512 µg/ml Negative13 Allen & Proudlock,
repair epithelioid alpha-TBOH or 1983
assay1 cells ß-TBOH12
In vivo Rat bone marrow 100 mg/kg b.w. Negative Richold & Richardson,
cytogenetics alpha-TBOH or 1982
assay ß-TBOH once;
25 or 50 mg
alpha-TBOH or
ß-TBOH 4 times3
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
In vivo Erythrocytes 100 mg/kg b.w. Negative Allen et al., 1980
micronucleus ß-TBOH3
test
1 Both with and without rat liver S-9 fraction.
2 Dimethylsulfoxide was used as the solvent.
3 Mytomycin C was used as a positive control.
4 Only with rat liver S-9 fraction.
5 20-Methylcholanthrene was used as a positive control.
6 alpha-TBOH at > 22 µg/ml and ß-TBOH at > 15 µg/ml were toxic to the
cells. Both substances induced 2-fold increases in mutation frequency,
but with alpha-TBOH this occurred only at highly toxic concentrations.
7 There was an inverse dose relationship, with the largest number of
transformations occurring at the lowest dose.
8 2-Acetamidofluorene was used as a positive control.
9 Ethylmethanesulfonate was used as a positive control.
10 Colchicine was used as a positive control.
11 Cyclophosphamide was used as a positive control.
12 4-Nitroguinoline-1-oxide (-S-9 mix) and 2-aminoanthracene (+S-9 mix)
were used as positive controls.
13 Increases in nuclear grain count were observed in a limited number of
cultures in one experiment only.
A detailed post-mortem examination was carried out on all pigs
at termination, and tissues were retained for histological
examination. All animals remained generally in good health throughout
the study, and no abnormal clinical signs were noted that could be
associated with experimental treatment. No significant effects of
treatment on body weight, food consumption, or ophthalmoscopy were
noted. There was a marked, dose-related reduction in serum
testosterone levels in male pigs, mean values in the highest-dose
group at week 6 and in pigs fed 2 and 20 ppm TBA at week 12 being
significantly lower than control values (p < 0.01). Testosterone
levels in female pigs remained low at all 3 assay points.
Serum progesterone levels in females were variable. This is not
unexpected, since both progesterone and estradiol show a marked cycle
variation and the assay samples were obtained at arbitrary time points
without regard to the stage of the estrus cycle of individual pigs.
However, mean values at week 12 did indicate a dose-related reduction
in serum progesterone levels in female pigs, and on statistical
examination there was found to be a significant trend with dose
(p < 0.05). Progesterone values for male pigs were less variable and
lower on average than for females and no consistent treatment-related
differences were noted.
Levels of estradiol-17ß in female pigs were generally low,
although there was evidence of some increase in estrogenic secretion
at week 12 in pigs from all groups; no appreciable differences between
groups were observed. Estradiol levels in males were in general higher
than in females; a dose-related reduction in estradiol mean values was
noted at weeks 6 and 12. The results of the hormone assays point to a
no-hormonal-effect level below 2 µg/kg b.w./day.
Treatment-related reductions in mean weights of testes, ovaries,
and uteri were noted. Apparently, the most sensitive organs are the
testes and ovaries. At the lowest dose, no effects on any tissue
weights were observed. The main findings associated with treatment at
the 2 higher levels were atrophy of testicular interstitial cells,
suppression of cyclic ovarian activity, the consequent absence of
glandular development of the uterine endometrium, and lack of alveolar
development and secretion in the mammary glands (highest group only).
Treatment-related changes were not seen in the gonads, uteri, or
mammary glands in any of the animals examined in the lowest-dose group
(Roberts & Cameron, 1985).
Four groups of 5 male and 5 female pigs were given orally TBA as
solutions in corn oil in gelatine capsules with the food for 14
consecutive weeks at 0, 5.0, 7.5, or 10.0 µg/kg b.w./day. Venous blood
samples were taken before treatment began and at weekly intervals
throughout the test period. Plasma radioimmunoassays for testosterone,
estradiol-17ß, and progesterone were carried out. Pigs were sacrificed
after 14 weeks and examined post-mortem. Tissues processed for
histological examination included the testes, ovaries, seminal
vesicles, uterus, and mammary gland. In males, comparison with the
control group showed some differences in testosterone levels at weeks
1 and 5, which reached statistical significance in the two highest
dose groups. However, these differences were not sustained and were in
the opposite direction (i.e. increases compared with the control) to
the dose-related changes (i.e. suppression) noted in the study
described in the previous paragraphs. Testosterone levels in females
generally remained low in all groups.
No significant group differences were found in estradiol levels
in males. In females, estradiol levels generally remained low in all
groups. Mean progesterone levels in males were statistically
significantly lower than in controls between weeks 7 and 10 in the
highest-dose group and at week 10 in the group administered 7.5 µg
TBA/kg b.w./day. However, the mean pre-dose levels were also lower in
pigs in these two groups compared with the controls and analysis of
mean levels over weeks 6 to 14 showed no significant effects. Thus,
these differences were not considered by the authors of the report to
represent a real treatment effect. In female pigs, mean progesterone
values were variable due to cyclic changes, but no consistent group
differences were apparent. Examination of the time of onset of cycling
and the peak level of progesterone recorded did not show significant
effects. No significant differences in organ weights were noted in any
groups. No treatment-related changes in the morphology of the organs
examined were noted in any groups (Roberts & Cameron, 1985).
Monkeys
The antigonadotropic activity of ß-TBOH was tested in acutely
castrated male rhesus macaque monkeys aged 8 - 17 years. A seminal
vesicle biopsy was obtained at the end of the 30-day treatment period
to examine possible changes in androgenic activity induced by TBOH.
ß-TBOH was given orally at 0, 1, 20, or 400 µg/day (three control
animals and two animals per treatment group). From 17 days after
castration until the end of the experiment, the lowest-dose group was
given 1600 µg/day ß-TBOH. Administration of the compound did not
suppress the post-castration elevation in either LH or follicle
stimulating hormone (FSH) secretion, which occurs after removal of the
testes. Although TBOH and testosterone showed no antigonadotropic
activity in this model system, the monkeys receiving 400 and
1600 µg/day TBOH maintained partial or complete seminal vesicle
morphology consonant with an androgenic effect. The expected reduction
in the serum levels of testosterone and estradiol occurred after
castration, and TBOH treatment did not alter the serum concentrations
of these hormones or the typical diurnal pattern of activity within
the hypothalmic-pituitary-adrenal axis. The authors concluded that the
no-hormonal-effect level in this study was 20 µg/day, equivalent to
2 µg/kg b.w./day (Hess, 1983).
TBA was administered in Sustagen/Jello/bran diet cubes for three
cycles, or a maximum of 122 days, to three groups of six mature female
rhesus macaque monkeys weighing 6 kg each at 60, 240, or 960 µg/day.
Blood samples were obtained on a daily basis from all animals during a
pretreatment menstrual cycle, at 3-day intervals during the first two
treatment menstrual cycles, and daily during the last treatment
menstrual cycle. Serum concentrations of estradiol, progesterone, LH,
and FSH were determined by radioimunoassay. Treatment with 960 µg/day
TBA resulted in maximum average serum levels of 2.3 ng/ml of ß-TBOH,
and this dose may have inhibited gonadotropin secretion and ovarian
function in 3 of 16 reproductive cycles.
It was concluded that 960 µg TBA/day had an inhibitory effect on
the pituitary gonadal axis. The anovulatory stage was reached rather
suddenly and from the data presented no conclusions with respect to
changes in the endogenous hormone concentrations, which might signal
this effect, can be drawn. In the 240 µg/day group one animal
exhibited anovulation, which may have been related to treatment. No
effects were observed at 60 µg/day, equivalent to 10 µg/kg b.w./day
(Hess, 1984).
Special studies on relay toxicity
Rats
Female veal calves were given s.c. implantations of 0, 140, or
3500 mg/animal TBA and killed after 10 weeks. A homogenate of veal
meat and veal organs (tongue, heart, lungs, spleen, liver (partial),
and one kidney) from these calves was mixed with the diet of rats in a
two-generation reproduction study. The total length of the experiment
was 114 weeks. The F1a generation was used to study teratogenic
effects. In the group of rats receiving a diet containing 230 ppb TBA,
a slight growth depression was seen. No effects were seen on other
parameters, which included mortality, feed consumption, growth,
fertility, reproduction (copulation, conception rate, duration of
gestation, mean litter weight, litter size, fetal body weight,
mortality rate, and fetal body weight after 3 weeks), haematology,
biochemistry, organ weight, and gross- and histopathology
(Gropp et al., 1978).
Special studies on reproduction
Rats
Groups of 40 male and 80 female rats weighing 133 - 143 g each
were given diets containing 0, 0.5, 1, 4, or 16 ppm TBA from week 9
before mating until day 21 post-partum. An additional group was
handled similarly, in which females were fed 50 ppm TBA from day 1 of
pregnancy until day 21 post-partum. At day 21 post-partum all parent
animals were killed without further examination. There were no major
differences between nominal and detected dietary levels of TBA at the
beginning and end of the study. In females in the 4 and 16 ppm groups,
growth was increased throughout the study (10 - 20%); in the 50 ppm
group growth was increased after mating (10%). A dose-related decrease
in pregnancy rate was seen in the 1, 4, 16, and 50 ppm groups (maximum
-30%). At day 4 post-partum each litter was reduced in size to 4
females and 4 males; up to that time pup mortality was increased in
all dosed groups compared with controls. Litter size and litter weight
on days 0, 4, 12, and 21 post-partum were decreased in the 4 and 16
ppm groups (maximum about -10%) and in the 50 ppm group (about -25%).
Mean pup weights were decreased only in the 50 ppm group from day 4
post-partum onward (maximum -15%) (Hunter et al., 1982).
Groups of 12 male and 12 female rats were fed diets containing
0, 25, 50, or 100 ppm TBA for 63 days and then mated. In these groups,
12/12, 10/12, 4/12, and 1/12 females, respectively, were pregnant
after mating (Ross, 1980).
In a multi-generation reproduction study in CRL:COBS CD(SD)BR
Charles River rats, dietary concentrations of 0, 0.5, 3, or 18 ppm TBA
were fed to F0-generation male rats for nine weeks and female rats
for two weeks prior to mating, then through to termination. Two groups
of F1 generation rats were selected, reared, and mated. One group
was treated continuously at the same dietary concentrations as rats in
the F0 generation ("treated" group) and one was removed from
exposure to TBA at 3 weeks of age and maintained without treatment
throughout ("untreated" group).
Treatment with TBA at 18 ppm was associated with the following
effects: a) generally higher body weights affecting both F0-and
F1-generation treated males and females and females of the untreated
F1 generation; b) depression in mean body weight gain during
gestation affecting both matings of the F0 generation; c) signs of
virilization, namely coarseness of the coat and discoloration of the
skin in F0-generation animals and treated F1-generation females;
d) clitoral prominence in treated F1-generation females and to a
lesser extent in untreated F1-generation females. Similar effects
were observed in F2-generation females from treated F1 parents,
but not in offspring of the untreated F1 generation; e) the presence
of occlusive strands in the vagina and/or precocious/incomplete
vaginal opening affecting treated F1 pups and F2 pups from the
treated F1 generation; f) a delay in the occurrence of testicular
descent affecting F2 pups from the treated F1 generation; g) a
marked reduction in pregnancy rate affecting the second mating of the
F0 generation and the treated F1 generation; h) an increase in
pre-coital time for the second mating of the F0 generation and for
the treated F1 generation; i) a marginal extension of the duration
of gestation affecting the F0 generation and the treated F1
generation; j) a marked increase in the incidence of extended
parturition and total litter loss in the treated F1 generation and a
significant increase in the percentage of males per litter; k) lower
litter size and litter weight, either at birth or at 20-day sacrifice,
after both matings of the F0-generation and of the treated F1
generation; 1) increased post-implantation/pre-birth losses in the
F0 and treated F1 generations; m) at terminal autopsy, findings
additional to those previously described, namely an increase in the
incidence of depression in the forestomach epithelium affecting F0-
and F1-generation males; n) significant reductions in seminal
vesicle/prostate weights in F0- and F1-generation males and
increases in mean ovary weights among F0- and F1-generation
treated females; o) significant decreases in weights of seminal
vesicles/prostate, testes, and epididymes in F1-and F2-generation
male pups at six weeks of age. F1- and F2-generation female pups
showed reduction of adrenal weight at six weeks of age; p) a
significant reduction in anogenital distance among male fetuses and a
marginal increase in the incidence of skeletalvariants after the
second mating of rats of the F0-generation (teratology phase).
Treatment with TBA at 3 ppm was associated with the following
effects: a) retarded body weight gain at the first mating of the
F0-generation; b) coarseness of the coat and discoloration of the
skin affecting one female in the F1-generation; c) a significant
delay in the mean age of vaginal opening in females in the
F1-generation and in F2 offspring from treated F1 parents; d)
the occurrence of incomplete vaginal opening or occlusive strands in
the vagina affecting occasional animals of the F1-generation at 6
week autopsy; e) a slight, but not statistically significant, delay in
testicular descent affecting F2 male offspring from treated F1
parents; f) significantly lower litter size at birth after the first
mating of the F0-generation; g) lower litter weight after the second
mating of the F0-generation; h) marginally reduced litter size in
offspring of the treated F1-generation; i) a significant decrease in
the weight of seminal vesicles/prostate, testes, and epididymes in
F1 and F2 male pups at six weeks of age.
The only apparent effects of treatment at 0.5 ppm were as
follows: a) higher group mean body weights of males from the F0
generation; b) a slight but not statistically significant delay in the
mean age of vaginal opening in F1 pups and F2 pups from the
treated F1 generation (subsequent mating performance and resulting
litter parameters were comparable to those of controls); c) a
statistically significant decrease in seminal vesicles/prostate weight
in treated F1 male pups and in F2 male pups from treated parents
at six weeks of age; d) a significant decrease in weight of epididymes
in F2 males.
The authors concluded that, in terms of reproductive performance
of the two generations examined in this study, as assessed by the
ability of parents to produce and sustain their litters, TBA exerted a
marked effect at 18 ppm and some effect at 3 ppm. At the lowest dose
level examined (0.5 ppm), slight effects were observed, which were
more marked in F2 pups than in F1 pups of a comparable age.
However, the authors concluded that in terms of reproductive
performance TBA exerted no effect at 0.5 ppm. The reproductive
performance for all groups of F1 animals following withdrawal from
treatment showed no marked differences from those of the control group
(James et al., 1985).
Histological examination of the testes, epididymes, seminal
vesicles, and prostate of 6-week-old male F2-generation rats from
treated F1 animals in the study described above revealed no
morphological abnormalities, although lower group mean weights were
recorded in treated groups compared to controls. All rats were
considered normal for that age, when they are not quite fully sexually
mature, with spermiogenesis proceeding to tailed spermatids in the
testes but no spermatoozoa in the epididymes (Offer, 1985).
In a study in rats, dietary concentrations of 0, 0.1, 0.3, 0.5,
3, or 18 ppm TBA were fed to F0 males and females from two weeks
prior to mating until termination of pregnancy. The F1 litters were
reared through the weaning period. On day 22 post-partum the male
offspring were sacrificed, examined macroscopically, and the testes,
seminal vesicles/prostate, and epididymes from each pup were weighed
and preserved. Female offspring were sacrificed on day 24 post-partum
and examined macroscopically.
Treatment with TBA at 18 ppm was associated with the following
effects: a) slightly higher mean body weights of F0 females, but
lower body weight gain during gestation and slightly lower weight gain
of males over the last three weeks of treatment; b) clitoral
prominence at autopsy in 22/29 F0 females and in all F1 female
offspring from approximately 3 weeks of age; c) a statistically
significant extension in duration of gestation; d) total litter loss
in 4/29 F0 females; e) effects on litter parameters, including
reduced litter size, lower litter weight, marginally higher pup
mortality, and higher mean pup weight; f) lower testicular weight and
higher mean weight of seminal vesicles/prostate in F1 males at 22
days of age.
Treatment at 3 ppm was associated with: a) slightly higher mean
body weights of females and slightly lower weights of males in the
three weeks prior to termination; b) a marginal extension in duration
of gestation; c) effects on litter parameters, including reduced
litter size, and marginally higher pup mortality and mean pup weight;
d) lower testicular weight and higher mean seminal vesicle/prostate
weight in F1 males.
The only effects associated with treatment at 0.1, 0.3, and
0.5 ppm TBA were slightly higher mean body weights of females and the
marginal differences observed at 3 and 18 ppm in litter parameters
(James et al., 1986).
Special studies on teratogenicity
Rats
Four groups of 6 pregnant rats each were given by oral gavage
0, 2.5, 5, or 10 mg/kg b.w./day TBA from days 6 - 15 of gestation. The
vehicle was 2.5% ethanol in 1% methylcellulose. Mortality, growth,
number of corpora lutea, number and distribution of live and dead
young, litter weight, mean fetal weight, microscopic fetal
abnormalities, sex ratio, and fetal crown-rump distance were all
unaffected by treatment (James et al., 1982).
Groups of 20 pregnant rats received by oral garage 0, 5, 10, or
20 mg/kg b.w./day TBA from days 6 - 15 of gestation. The vehicle was
2.5% ethanol in 1% methylcellulose. In the 10 and 20 mg/kg b.w./ day
groups, 9/20 and 15/20 dams, respectively, showed hair loss. A
dose-related decrease in growth was seen in all dosed groups (maximum
20%). Pregnancy rate, number of liver and dead young, number of
implantations and corpora lutea, litter weight, mean fetal weight,
incidence of major malformations, incidence of minor visceral
anomalies (Wilson's technique), and fetal crown-rump distance were all
unaffected. The incidences of skeletal variants (number of ribs,
number of normal and variant sternebrae) were unaffected. Mean
anogential distances in male fetuses preserved in Bouin's solution
were slightly shorter than those of controls, with an apparent
slightly decreasing trend with increase in dosage; the difference at
20 mg/kg b.w./day attained borderline statistical significance
(P < 0.05). Male fetuses preserved in alcohol did not show the same
trend in mean values, although the variation in anogenital distance
was greater in pups in the high-dose group than in controls; ranges of
fixation weight and crown-rump distance length were also higher in the
high-dose group than in controls. Mean anogenital distances in females
preserved in Bouin's solution and in alcohol were comparable in all
groups (James et al., 1982).
Acute toxicity
Table 4 summarizes the results of acute toxicity studies on TBA.
Table 4. Acute toxicity of TBA
LD50
Species Sex Route Vehicle (mg/kg b.w.) Reference
Mouse M/F oral 40% ethanol 1500 Audegond et al.,
in corn oil 1981a
M i.p. ethanol + 10% 565 Escuret & Bas, 1978
sesame oil
F i.p. ethanol + 10% 643 Escuret & Bas, 1978
sesame oil
Rat M/F oral 10% ethanol 5000 Audegond et 1981b
in corn oil
M i.p. 10% ethanol 1601 Escuret & Bas, 1978
in corn oil
F i.p. 10% ethanol 1772 Escuret & Bas, 1978
in corn oil
Dog M/F oral via capsules 1000 Audegond et al.,
1981c
Dogs
Anaesthetized dogs were given i.v. 1, 2, 5, or 10 mg/kg b.w. TBA
as a 20 ml/mg solution in 92% acetylmethylamine. Dogs in the 2, 5, and
10 mg/kg b.w. groups showed a dose-related decrease in blood pressure;
at 10 mg/kg b.w., this was accompanied by slight bradycardia. A
decrease in blood pressure in the same groups was seen after injection
with adrenalin and noradrenalin; an increase was seen after
acetylcholine injection. Changes in reactions to histamine were not
noted in any groups (Seeger, 1971a).
Short-term studies
Mice
Groups of 8 male and 8 female mice weighing 19 - 25 g each were
given 0, 25, 50, or 100 ppm TBA in the diet for 8 weeks. Mortality,
appearance, behaviour, body weight, food consumption, and food
conversion were unaffected by treatment. Females in all treatment
groups exhibited significant decreases in absolute and relative liver
weights and significant increases in absolute and relative uterine
weights. Absolute and relative ovarian weights were decreased
significantly in females in the 50 and 100 ppm groups, while absolute
and relative testes weights were decreased significantly in males in
the highest-dose group. No effects on the weights of adrenals,
kidneys, prostate, seminal vesicles, or spleen were noted. Females
showed dose-related suppression of ovarian cyclic activity
characterized histopathologically by the absence of, or few, corpora
lutea in the gonads, a dose-related reduction in the amount of stroma,
and reduction in the number of endometrial glands in the uteri
(Hunter et al., 1976a).
TBA was administered to groups of 8 male and 8 female Swiss
albino CFLP mice for 10 weeks at levels of 0, 1, 2, 5, or 10 ppm of
the diet, equal to mean intakes of 0, 0.12, 0.24, 0.56, or 1.2 mg/kg
b.w./day for males and 0, 0.13, 0.25, 0.66, or 1.4 mg/kg b.w./day for
females. There were no signs of reaction to treatment, including no
treatment-related effects on food intake or body weight gain. No
treatment-related abnormalities were seen; the absolute and relative
weights of all organs examined were considered to be within normal
limits for mice of this strain and age. The only organs examined
histologically were the prostate, seminal vesicles, testes, ovaries,
and uterus from mice in the control and highest-dose group. All
histopathological parameters were within normal limits (Hunter et al.,
1976b).
Rats
Sixteen male rats that had been castrated between 21 and 24 days
of age were given total oral doses of 0, 0.75, 3, 12, or 48 mg TBA
from days 2 - 11 after castration in 10 daily portions. At autopsy,
one day after the last application, dose-related increases in the
absolute weight of the prostate (maximum +440%) and seminal vesicles
(maximum +400%) were seen in all treated groups. In the three
highest-dose groups a dose-related increase in the musculo levator ani
weight (maximum +250%) was noted (Schröder, 1971a).
Groups of 10 male and 10 female CFY rats were fed diets
containing TBA at concentrations of 0, 25, 50, or 100 ppm for 13
weeks. Group mean intakes during the treatment period were 0, 1.8,
3.8, or 7.6 mg/kg b.w./day for males and 0, 2.2, 4.2, or 8.4 mg/kg
b.w./day for females. At all the tested doses, the females exhibited
better efficiency of feed utilization than the males, causing higher
weight gain.
At 25 ppm, lower prostate weight (-36%), which was not associated
with morphological changes, was observed. At 50 ppm, lower prostate
and seminal vesicle weights (-50 and -30%, respectively), which were
not associated with morphological or uterine changes, were observed in
two rats.
At 100 ppm, the following changes were observed: lower neutrophil
and lymphocyte values in males at weeks 12 and 13 (-35%); lower
seminal vesicle weight, which was not associated with morphological
changes (-60%); lower prostate weight (-80%), which was associated
with small alveoli lined by cuboidal epithelium in 5 rats; and uterine
changes characterized by an apparent reduction of the endometrial
stroma with dilated uterine glands and also a corrugated appearance of
the endometrial and glandular epithelium in 6 rats (Hunter et al.,
1976c).
Groups of 10 male and 10 female rats weighing 60 g each were
given 0, 50, 100, 200, or 1000 µg/kg b.w./day TBA orally 6 days per
week for 3 months. The compound was presented as 0.5 ml of an aqueous
solution containing 0.9% NaCl, 0.4% polysorbate 80, 0.5% carboxy-
methylcellulose, and 0.9% benzyl alcohol. Determinations were
carried out in half of the animals at termination only.
Growth of females was slightly increased; in the two highest-dose
groups growth of males was decreased. Haematological parameters were
unaffected. In all dosed groups, SGOT and SGPT were decreased; total
cholesterol was decreased in the 100, 200, and 1000 µg/kg b.w./day
groups. In the highest-dose group a slight decrease in blood glucose
was seen; urea was slightly increased only in the females in this
group. Increases were observed in liver weight in males and females in
the 200 and 1000 µg/kg b.w./day groups, kidney weight in males and
females in the highest-dose group, and spleen weight in females in the
200 and 1000 µg/kg b.w./day groups. In females, ovary weight was
increased in the highest-dose group and uterus weight was decreased in
the 100 and 200 µg/kg b.w./day groups. In males, decreases were seen
in seminal vesicle weight in the 100, 200, and 1000 µg/kg b.w./day
groups and in prostate weight in the 100 and 1000 µg/kg b.w./day
groups. Gross pathological examination revealed atrophy of the
prostate, seminal vesicles, and testes in rats in the highest-dose
group. Histologically, changes in the ovary (cysts and released
follicles in all test groups) and in the uterus ("dentelle uterine" in
the 200 and 1000 µg/kg b.w./day groups) were observed in females. In
males in the highest-dose group a delay in spermatogenesis and aplasia
in the seminal vesicles and prostate were observed (Seeger, 1971a, b).
Female rats fed diets containing 0.01, 0.1, 2.5, 5, 10, 20, 40,
80, or 160 ppm TBA exhibited increases in uterus weight at levels
higher than 40 ppm. Vaginal smears in all groups were negative; some
proliferation of the vaginal mucosa was seen at the highest dose level
(Huis in't Veld et al., 1973).
Female rats weighing 60 - 65 g each were ovariectomized and given
s.c. doses of 0, 0.2, 1.0, or 5.0 mg/day TBA in sesame oil for 4 days.
At termination, on day 5, a dose-related increase in uterus weight was
seen in all dosed groups (maximum +550%). The estrogenic activity of
TBA was less than 0.1% of the estrogenic activity of estradiol-17ß
(Schröder, 1971b).
Male castrated rats weighing 100 g each were given daily s.c.
doses of 4, 20, or 100 µg TBA, 4, 20, or 100 µg ß-TBOH, or 20, 100,
500, or 1000 µg alpha-TBOH for 9 days. Dosing was started 1 day after
castration. One control group was used. At sacrifice, one day after
the last injection, the weights of the prostate, musculo levator ani,
and seminal vesicles were recorded. In animals dosed with both TBA and
ß-TBOH, organ weights were increased in a dose-related manner in all
test groups. In the animals administered alpha-TBOH, organ weights
were increased at the three highest dose levels (Escuret & Bas, 1978).
Male castrated rats weighing 65 - 75 g each were given daily s.c.
doses of 0, 0.02, 0.1, or 0.5 mg TBA as a solution in sesame oil for
10 days after castration. At sacrifice on day 11, dose-related
increases were seen in the weights of muscolo levator ani (maximum
+250%), prostate (maximum +1400%), and seminal vesicles (maximum
+2500%) in all groups. In this experiment TBA showed distinct anabolic
and androgenic activity that was 5 times higher than that of
testosterone and 20 times higher than that of 17-ethynyl-19-nor-
testosterone (Schröder, 1971b).
Groups of 10 male and 10 female rats weighing 123 - 131 g each
were dosed 6 times per week s.c. with 0, 200, 1000, or 5000 µg/kg
b.w./day TBA over a period of 2 months. The compound was applied as a
solution in 1:1 syncortyl and arachis oil.
Growth of females was increased in all groups; by contrast,
growth of males in the highest-dose group was decreased. Haematology
and blood biochemistry determinations, which were performed at
termination in 5 rats/sex/group, revealed slight increases in Hb and
haematocrit values and slight leucocytopenia (due to lymphocytopenia)
in all test groups. Other blood abnormalities that were noted were a
decrease in glucose in females in the 5000 µg/kg b.w./day group (not
determined in other groups), a decrease in BUN in females in the 1000
and 5000 µg/kg b.w./day groups and in males in the 5000 µg/kg b.w./day
group, and a decrease in cholesterol in males and females in the 1000
and 5000 µg/kg b.w./day groups.
In all test groups, absolute and relative kidney weights were
increased and absolute and relative adrenal and thymus weights were
decreased. Ovary weights were decreased in a dose-related manner in
females in all test groups. In females in the 200 and 1000 µg/kg
b.w./day groups uterus weights were decreased. All treated females
showed an increase in absolute liver weight. Males showed a decrease
in testes weight in all treated groups and in seminal vesicle and
prostate weight in the 1000 and 5000 µg/kg b.w./day groups. Besides
these weight changes, atrophy was noted in the thymus, ovaries, and
testes; hypertrophy was seen in the seminal vesicles and prostate
(Sovetal, 1970; Seeger, 1971a).
Rabbits
Groups of 4 - 6 rabbits weighing 2 kg each were administered s.c.
during 4 days 0, 0.05, 0.5, 2, or 5 mg/kg b.w./day TBA. Liver function
was examined (SGOT activity and BSP excretion). At 2 mg/kg b.w./day
SGOT activity was increased slightly, while at 5 mg/kg b.w./day SGOT
activity was increased significantly. BSP excretion was not affected
in any of the test groups (Seeger, 1971a).
Pigs
Male, female, and castrated male pigs were fed diets containing
1-2 ppm TBA, alone or in combination with 2 ppm estradiol-17ß or 2 ppm
ethynylestradiol, for 5 - 8 weeks. At 5 and 6.5 weeks after compound
withdrawal TBOH was not detected in the urine. Urinary excretion of
total steroid estrogen was not increased at 7 weeks after dose
withdrawal (Kroes et al., 1976a).
Groups of 4 male and 4 female domestic pigs (Sus scrofa) were fed
diets containing 0, 0.1, 2, or 20 ppm TBA (equivalent to 0, 4, 80, or
800 µg/kg b.w./day TBA, respectively) for 14 weeks. No treatment-
related effects on mortality, body weight, or ophthalmoscopy were
seen. After 6 and 12 weeks several haematological (i.e., Hb, RBC, PCV,
WBC, diff. WBC, and prothrombin index) and blood biochemical
parameters (i.e., glucose, protein, albumin, albumin/globulin ratio,
SAP, calcium, and creatinine), were unaffected. At 2 and 20 ppm,
dose-related increases were seen in platelets (maximum +70%, not
significant), urea (maximum +3%, significant) and cholesterol (maximum
+100%, significant). SGOT increased in a dose-dependent manner in all
test groups (maximum, +100%, significant). There were dose-related
decreases in blood levels of testosterone and estradiol in males in
all dosed groups (both maximum -95%, significant); progesterone was
markedly decreased (maximum -99%, significant) in females in the two
highest-dose groups. In the same groups, there were dose-related
changes in the absolute and relative weights of the liver (maximum
+30%, significant), uterus (maximum -50%, significant), kidney
(maximum +25%, significant), and testis (maximum -55%, significant).
In the highest-dose group changes were observed in the weights of the
pituitary (-15%, significant) and seminal vesicles (+280%,
significant). There was an increase in thyroid weight at all three
dose levels (maximum +20%, not significant).
Histopathological examination showed the following dose-related
abnormalities in the 2 and 20 ppm groups: in the liver, enlargement of
the hepatocytes with associated ground glass appearance of the
cytoplast; in testes, moderate to complete interstitial cell atrophy
(with normal spermatogenesis within the seminiferous tubules); in
ovaries, evidence of suppressed or abnormal cyclic activity
characterized by the absence of maturing follicles and/or mature or
early regressing corpora lutea; and, in the uteri, absence of
glandular development in the endometrium. This last observation and
the lack of alveolar development and secretion noted in the mammary
glands of the 2 ppm group were probably associated with the anestrous
state suggested by the findings in the ovaries (Ross et al., 1980).
Cattle
Groups of 8 male calves were given s.c. implants of 140 mg TBA +
20 mg estradiol-17ß at 8 or 4 weeks before slaughter. An additional
group was dosed with 200 mg/kg b.w. testosterone + 20 mg/kg b.w.
estradiol-17ß on both occasions. In all treatment groups histological
examination of prostates showed increased secretory activity and
hyperplastic and metaplastic changes (Verbeke et al., 1975).
Six weeks after single s.c. application of 20 mg estradiol-17ß to
male calves, distinct changes in seminal vesicles were found after
histological examination. Treatment of calves with 20 mg estradiol-17ß
combined with 140 mg TBOH, applied s.c., caused very slight changes in
seminal vesicles in 4/11 calves, while in 3/7 animals with unchanged
seminal vesicles, androgenic stimulation was observed (Kroes, 1972).
Male calves that were 11 weeks of age received by s.c.
implantation 20 mg estradiol-17ß alone or 20 mg estradiol-17ß in
combination with 140 mg TBA. Urinary secretion of total steroid
estrogens was high in the animals given estradiol-17ß alone during the
first 12 days after application. After 3 weeks normal values were
reached. In the estradiol-17ß/TBA combination group, gradual and
prolonged excretion of steroid estrogens for up to 42 days after
implantation occurred; after 56 days normal values were reached.
Qualitative determinations of estradiol-17ß and estradiol-17ß in urine
showed that the alpha epimer was present in almost all urine samples;
estradiol-17ß was found in the urine of those calves administered
estradiol-17ß alone. In the combination group, estradiol-17ß was found
in urine at day 21 only. Histological examination of prostates
revealed squamous metaplasia of gland epithelium after both treatments
(Kroes et al., 1976b).
A total of 1480 female calves, aged 7 weeks, were administered by
percutaneous implantation 0, 140, or 3500 mg TBA (groups 1 - 3,
respectively) or 140 mg TBA + 20 mg estradiol-17ß (group 4), 1400 mg
TBA + 200 mg estradiol-17ß (group 5), or 3500 mg TBA + 500 mg
estradiol-17ß (group 6). The calves were slaughtered 10 weeks after
implantation.
Blood parameters (glucose, GOT, GPT, AP, LDH, cholesterol,
bilirubin, Hb, and PCV), urinary density, and pH were unaffected in
all groups. Calcium and phosphorus levels in serum and bone were not
changed; however, magnesium levels in serum and deposition of
magnesium in bone were decreased in groups 3, 5 and 6. In group 3
slight, and in groups 4, 5 and 6 marked, increases in uterus weight
were seen, which were accompanied by proliferation of uterine
glandular cells, while the lumen of the uterus was partially filled
with a watery liquid. In dosed groups ovarian weights were reduced,
accompanied by a diminution in follicular size. These weight changes
were most marked in groups 2, 3 and 6. The diminution of follicular
size, which was accompanied by a decrease in the number of follicles,
was most marked in groups 5 and 6. In all groups, a dose-related
decrease in thymus weight was seen. In group 3 abnormal development of
the clitoris was noted. Histopathological examination showed a
non-dose related proliferation and secretion of glandular tissue in
the mammary glands of animals in groups 4, 5 and 6. Heart, lever,
kidneys, pituitary, pineal gland, adrenal glands, thyroid, and
skeletal muscle did not show abnormalities (Gropp et al., 1975).
Steers and heifers were seven 300 mg TBA by s.c. implantation. An
additional group of steers was given implants containing 140 mg TBA +
20 mg estradiol-17ß. In all groups, plasma urea was decreased during
the 9-week observation period after treatment. Other plasma parameters
(glucose, calcium, phosphorus, magnesium, sodium, potassium, and total
protein) were unaffected. No changes in plasma concentrations of
insulin or growth hormone were seen. A decrease in thyroxine levels in
steers was observed during the 9-week observation period after
treatment; the decrease was most marked in those administered TBA and
estradiol-17ß in combination. A marked reduction, about -50%, in
thymus weight was observed in steers (Heitzman, 1975).
Steers and oxen given, by s.c. implantation, 140 mg TBA alone or
140 mg TBA in combination with 20 mg estradiol-17ß. Control animals
received implantations with the carrier. The combination of drugs
affected urinary excretion of exogenous estradiol-17ß in oxen and
possibly in steers. Histological examination of the prostate revealed
squamous metaplasia in the combination group. In both treated groups,
more active epithelium of the prostate was seen, compared to the
control group (Kroes et al., 1976c).
Long-term studies
Mice
Groups of 64 male and 64 female Swiss albino CFLP mice, weighing
22 - 25 g each, were given diets containing 0, 0.5, 1.0, 10 or 100 ppm
TBA (equal to 0, 0.004, 0.09, 0.86, or 8.6 mg/kg b.w./day TBA for
males, respectively, and 0, 0.005, 0.10, 0.96, or 9.5 mg/kg b.w./day
TBA for females, respectively) for 95 - 104 weeks (the test was ended
when survival was 20% in males or females in the control group). After
13 weeks 12 mice/sex were killed. At that time significant increases
were observed in the absolute and relative weights of the kidneys in
males and females at 100 ppm (20 - 40% increases). Significant
decreases were seen in the weights of the spleen of top-dose females
(-20%) and significant increases were seen at 1.0, 10, and 100 ppm in
males (+25%). Dose-related relative decreases in weights of the uterus
were observed in all dosed females (maximum -25%). At interim
sacrifice, inhibition of ovulation, characterized by a lack of corpora
lutea in all females in the highest-dose group, was observed.
Follicular development proceeded to the mature follicle stage. The
uteri of all females in the 100 ppm group were consistent with the
diestrous stage of the cycle. In the spleens of 6/12 males in the
100 ppm group an increased number of polymor-phonuclear leucocytes in
the red pulp was seen (0/12 in controls); congested sinuses were noted
in 2/12 males of this group (0/12 in controls).
At terminal sacrifice no organ weights were recorded. Terminal
gross- and histopathological examination showed an increase in liver
nodular hyperplasia and dose-related tumours in the male dose groups;
these increases were statistically significant at the two highest
doses. The incidence of liver tumours was also increased in females in
the highest-dose group (8/52 versus 4/51 in controls). There was an
increase in incidence of hepatocyte vacuolation in males in the
100 ppm group. In 100 ppm females, gross pathological examination
showed an increase in the incidence of enlarged and swollen kidneys,
accompanied by a marginal increase in the incidence of nephritis. In
the same group, increases in ovarian cysts (in 10/20 animals versus
1/11 in controls) and enlarged, abscessed, or cystic preputial glands
(in 4/20 females versus 0/11 controls) were seen. The spleens of 4/20
females in the 100 ppm group appeared small (Hunter et al., 1981).
Rats
Six groups of 65 male and 65 female Sprague-Dawley CFY rats
weighing 150 - 200 g each were given diets containing 0, 0.5, 1.0,
4.0, 16, or 50 ppm TBA (equal to 0, 0.02, 0.04, 0.14, 0.56, or
1.80 mg/kg b.w./day TBA for males, respectively, end 0, 0.02, 0.04,
0.16, 0.64, or 1.92 mg/kg b.w./day for females, respectively) for 112
weeks. The parents of the test animals had been dosed at the same
levels from week 9 before mating until day 21 post-partum (except for
the 50 ppm group in which only the dams had been dosed from day 0 of
pregnancy until day 21 post-partum).
The females in the 4.0, 16, and 50 ppm groups showed prominent
pudendum. A dose-related incidence of pendulous anogenital skin was
seen in all female dose groups (maximum incidence, 85%). In males a
dose-related reduction in testes size was seen in all dose groups,
except at 0.5 ppm (maximum incidence, 45%). Growth and food
consumption were decreased throughout the study in males administered
50 ppm TBA (-15 and -10%, respectively). Water consumption was
decreased in the same group up to week 51 (-15%). Urinalysis (9
parameters measured in 5 rats/sex of the 0, 16, and 50 ppm groups on 6
occasions throughout the study) showed no treatment-related changes.
Haematology (10 parameters measured in 10 rats/sex of the 0, 16,
and 50 ppm groups on 6 occasions throughout the study) showed dose-
related slight increases in some parameters in females in the 16 and
50 ppm groups. Male haematology values were generally unaffected by
treatment. Blood biochemistry (13 parameters) showed no compound-
related changes.
At interim sacrifice at week 78, 13 - 14 rats/sex/group were
killed. Macroscopic examination revealed a prominent clitoris in 5/14
females fed 16 ppm TBA and in 13/13 females fed 50 ppm TBA. The males
of the 50 ppm group showed atrophy of the testes, seminal vesicles,
and prostate. Interim organ weighings revealed, in the males fed 16
and 50 ppm TBA, dose-related decreases in absolute testes and prostate
weights. Adrenals and pituitary weights were decreased in both sexes
in the 50 ppm group. Weights of the ovaries were increased in all dose
groups, but without dose relation.
At the end of the experiment, similar changes in the absolute
weights of the testes, prostate, and adrenals were found. In the males
of the highest-dose group, decreases were observed in the weights of
the pituitary (-30%), thyroid (-20%), kidney (-30%), spleen (-30%),
and liver (-20%). The weight of the ovaries was markedly decreased in
the highest-dose females (-60%).
Terminal gross- and histopathological evaluation revealed a
slightly increased incidence of foci or areas of ground-glass
hepatocytes in the livers of the highest-dose females. Urinary bladder
calculi and associated epithelial hyperplasia joined by cystitis and
pyelitis were seen in females in the same dose group. Both the male
and female reproductive tracts were affected. Testicular, prostatic,
and vesicular atrophic changes were seen in males in the 16 and 50 ppm
groups. Absence of corpora lutea, vaginal inflammation and
mucification, uterine endometritis, dilatation, decreased endometrial
thickness, and the development of clitoral bone were seen in the
50 ppm females. Clitoral enlargement was seen in females in the two
highest-dose groups. Neoplastic histopathology showed an increase in
the incidence of pancreatic islet cell tumours in the 50 ppm group
(Hunter et al., 1982).
Observations in human beings
Male and female human volunteers were given i.m. doses of 5 or
10 mg TBA every-other-day during 14 days. In the subjects administered
5 mg TBA, nitrogen balance was disturbed, including retention of
nitrogen. In the 10 mg group, some women showed disturbances of the
menstruation cycle. The same dose caused a slight but significant
reduction of excretion of 17-ketosteroids. No effects on
17-hydroxy-corticosteroid excretion were seen. Blood parameters
(protein, cholesterol, coagulation factors, and prothrombin and
thrombin time) were unaffected by treatment (Krüskemper et al.,
1967).
COMMENTS
TBA is a synthetic steroid with anabolic properties. At the
17-position in the molecule two epimers, alpha and ß, are possible.
The ß-epimer of TBA is the commercial product. It is administered to
cattle either alone or in combination with estradiol-17ß or zeranol as
a subcutaneous implant in the ear to improve body weight, feed
conversion, and nitrogen retention. It is usually implanted 60 - 90
days before the intended date of slaughter.
After administration to cattle, TBA is rapidly hydrolyzed to
TBOH, the major metabolite being alpha-TBOH, occurring in the excreta,
bile, and liver. In muscle most of the TBOH is present as ß-TBOH.
Experiments with implantation of 200 mg of radiolabelled TBA in calves
and heifers showed that maximum levels of residues occurred about 30
days after implantation. The highest mean concentration of residues as
TBOH equivalents was 50 µg/kg in liver, while muscle contained
3 µg/kg.
The results of the studies requested at the twenty-seventh
meeting were submitted for consideration by the present Committee. In
addition, new toxicological data were available on reproduction and
mutagenicity. The Committee also reviewed previously available data
including metabolic, teratogenicity, and carcinogenicity data.
Acute toxicity studies in several species showed TBA to be of low
toxicity, when given orally.
Experiments had been performed in rats to assess the effect of
TBA on pregnancy, on the reproductive function of multiple
generations, and on the development of the offspring to weaning.
Treatment at 0.3 and 0.5 ppm in the diet, equivalent to about
20-30 µg/kg b.w./day, was associated with mean weekly body weights for
female rats that were only slightly higher than those of controls, and
marginal differences in litter parameters, while treatment with TBA at
3.0 and 18.0 ppm in the diet was associated with a hormonal effect. It
was considered that TBA exerted no effect on reproductive performance
in the rat at 0.5 ppm in the diet, equivalent to 30 µg/kg b.w./day. No
teratogenic effect was seen in two feeding studies in rats at very
high doses of TBA. In a comprehensive range of in vivo and in vitro
mutagenicity studies, all tests were negative for TBA, ß-TBOH, and
TBA's major metabolite, alpha-TBOH, with the exception of the mutation
assay in mouse lymphoma cells, which gave equivocal results with
ß-TBOH and alpha-TBOH. The Committee also noted a report of an
equivocal result in a transformation study with ß-TBOH in Syrian
hamster embryo fibroblasts, and took into account the recognized
difficulty in interpreting results from this type of study.
The Committee reaffirmed the opinion expressed at its
twenty-seventh meeting regarding the results of long-term feeding
studies with TBA with rats and mice (Annex 1, reference 62). It
considered that the liver hyperplasia and tumours in mice fed high
doses of TBA (0.9 - 9 mg/kg b.w./day) and the slight increase in the
incidence of islet-cell tumours of the pancreas of rats fed TBA at
1.85 mg/kg b.w./day (the highest dose in the study) arose as a
consequence of the hormonal activity of TBOH.
The Committee therefore concluded that its safety assessment
could be based on establishing the no-hormonal-effect level. It
reviewed a study with castrated male rhesus macaque monkeys
administered ß-TBOH orally, and considered that this model could be
relevant to the human population. The castrated male rhesus monkey is
highly sensitive to compounds with antigonadotropic activity; the
Committee therefore adopted a conservative approach by using this
study as the basis for establishing an ADI for human beings. Despite
the small numbers in each group of monkeys studied, and the advanced
age of the animals used, the Committee set 2 µg/kg b.w./day as a
no-hormonal-effect level, based on assessment of histological changes
in the seminal vesicles. In the intact female rhesus monkey, TBA had a
clear no-hormonal-effect level of 10 µg/kg b.w./day. The Committee
also considered that the pig was a sensitive model for assessing
hormonal effects and noted that, here too, TBA had a no-hormonal-
effect level of 2 µg/kg b.w./day based on assessment of pathological
changes in the testes. Another study in the pig demonstrated that the
hormonal activity of ß-TBOH was about ten times that of alpha-TBOH. No
data on individual animals were available for any of the pig studies.
In the absence of satisfactory toxicological data the Committee
was unable to establish a separate no-effect level for the alpha-TBOH
metabolite. It also noted that this metabolite was not produced in
significant amounts in the rat, which made it inadvisable to
extrapolate from data generated from ß-epimer experiments in that
species.
EVALUATION
Level causing no hormonal effect
Pig: 0.1 ppm in the diet, equal to 2 µg/kg b.w./day.
Monkey: 2 µg/kg b.w./day.
Estimate of temporary acceptable daily intake
0 - 0.01 µg/kg b.w.
Further work or information
Required (by 1990):
( a) the final reports, with supporting data, for the tissue residue
studies in which TBA was administered to heifers and TBA in
combination with estradiol-17ß was administered to steers;
( b) histopathological data on individual animals from the three
hormonal studies in pigs that were reviewed by the Committee;
( c) results from a 90-day study in an appropriate species, with
orally administered alpha-TBOH.
REFERENCES
Allen, J.A. & Proudlock, R.J. (1983). Autoradiographic assessment of
DNA repair in mammalian cells after exposure to 17-alpha-hydroxy-
trenbolone and to 17-ß-hydroxytrenbolone. Unpublished report
No. RSL 619/83731 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Brooker, P.C., & McCaffrey, K.J. (1985). Analysis of
metaphase chromosomes obtained from CHO cells cultured in vitro and
treated with 17-ß-hydroxytrenbolone. Unpublished report No. RSL
676/85631 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Proudlock, R.J., & Pugh, L.C. (1986). Micronucleus test
on 17-ß-hydroxytrenbolone. Unpublished report No. RSL 728/861306 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Allen, J.A. & Proudlock, R.J. (1987). Assessment of 17-alpha- and
17-ß-hydroxytrenbolone in the Solt-Farber system for induction of
liver foci. Unpublished report No. RSL 711/861252 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981a). R 1697 (trenbolone
acetate). Single administration study by oral route in the mouse.
Unpublished report No. 81213-A-JL/36-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981b). R 1697 (trenbolone
acetate). Single administration study by oral route in the rat.
Unpublished report No. 81212-A-JL/37-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, g., & Glomot, R. (1981c). R 1697 (trenbolone
acetate). Single administration study by oral route in the dog.
Unpublished report No. 81216 from Roussel Uclaf Research Centre.
Submitted to WHO by Roussel Uclaf, Paris, France.
Barraud, B., Lugnier, A., & Dirheimer, G. (1983). In vivo covalent
binding to rat liver DNA of trenbolone as compared to 17-ß-estradiol,
testosterone and zeranol. In: Anabolics in animal production,
International Office of Epizootics, Paris, pp. 193-206.
Bouffault, J.C. (1977). Expert statement concerning the excretion of
trenbolone and oestradiol-17-alpha in urine of calves after
subcutaneous implantation of trenbolone acetate and oestradiol 17-ß.
Unpublished report submitted to WHO by Roussel Uclaf, Paris, France.
Chasseaud, L.F., Hawkins, D.R., Elsom, L.F., Parsonage, M.T.,
Ross, D.B., & Roberts, N.L. (1976). Plasma and tissue concentrations
of radioactivity in cattle after implantation of pellets containing
3H-trenboloneacetete, 3H-oestradiol or combinations of radioactive
drugs with the non-radioactive form of the other. Unpublished report
No. SVT/11/76351 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Edgar, D.H., Bosworth, H.J., Ransome, S.J., & Banks, S.J. (1985). An
assessment of the mutagenic potential of 17-ß-hydroxytrenbolone in
mammalian cells in vitro using the Chinese hamster ovary/HGPRT locus
assay. Unpublished report No. RSL 677/85642 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Escuret, P. & Bas M.N. (1978). DL-50 chez le rat et chez le souris.
Unpublished report Recherches Veterinaires R.U.-essai 5.77.71 R.
Submitted to WHO by Roussel Uclaf, Paris, France.
Gropp, J., Herlyn, D., Boehncke, E., Schulz, V., Sanderleben, J.V.,
Hanichen, Th., & Geisel, O. (1975). Physiological data including
evaluation of immunoresponse in relation to anabolic effects on veal
calves. Published in "Anabolic agents in animal production",
FAO/WHO Symposium, pp. 131-141, March 1975.
Gropp, J., Buntenkotter, S., & Kaemmerer, K. (1978). Relay toxicity of
anabolic agents, general considerations and some experimental data.
Published at Relay Toxicity and Bioavailability Symposium,
5 October 1978. Report submitted to WHO by Roussel Uclaf,
Paris, France.
Hawkins, D.R., Weston, K.T., Ray, J., Ross, D.B., & Cameron, D.M.
(1979). The bioavailability of trenboloneacetate residues in the
edible tissues of heifers after oral administration to rats.
Unpublished report No. RSL/328/7959 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hawkins, D.R., Waller, A.R., Moore, D.H., Jordan, M.C., Roberts, N.L.,
& Cameron, D. (1984). Tissue residues of radioactivity at 15 and 30
days after implantation of 3H-trenboloneacetate in calves.
Unpublished report No. HRC/RSL/636 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Heitzman, R.J. (1975). The effectiveness of anabolic agents in
increasing rate of growth in farm animals; report on experiments in
cattle. Published in "Anabolic agents in animal production", FAO/WHO
Symposium, pp. 89-98, March 1975.
Heitzman, R.J. & Hardwood, D.J. (1977). Residue levels of trenbolone
and oestradiol-17-ß in plasma and tissues of steers implanted with
anabolic steroid preparations, Br. Vet. J., 133, 564.
Henderson, L.M., Bosworth, H.J., Ransome, S.J., Banks, S.J., & Ozel,
K.J. (1986a). An assessment of the mutagenic potential of 17-alpha-
hydroxytrenbolone in mammalian cells in vitro using the Chinese
hamster ovary/HPRT locus assay. Unpublished report No. RSL 713/86713
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Henderson, L.M., Brooker, P.C., & Howell, A. (1986b). An assessment of
the mutagenic potential of 17-ß-hydroxytrenbolone in mammalian cells
in vitro using a Chinese hamster ovary micronucleus test.
Unpublished report No. RSL 729/861287 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., & Bosworth, H.J. (1987a).
An assessment of the carcinogenic potential of 17-ß-hydroxytrenbolone
and testosterone in an in vitro cell transformation assay using
mouse C3H10T1/2 cells. Unpublished report No. RSL 691/861257 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., Bosworth, H.J.,
Bottoms, M., & Williams, J. (1987b). An assessment of the mutagenic
potential of 17-ß-hydroxytrenbolone in mammalian cells in vitro
using the Chinese hamster V79/HPRT locus assay. Unpublished report
No. RSL 712/861249 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hess, D.L. (1983). Determination of a hormonal no-effect dose level
for 17-ß-trenbolone Rhesus Macaque. Unpublished report from Oregon
Regional Primate Research Center, Beaverton, OR, USA. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hess, D.L. (1984). Determination of the hormonal no-effect dose level
for trenbolone acetate in the female Rhesus Macaque. Unpublished
report from Oregon Regional Primate Research Center, Beaverton, OR,
USA. Submitted to WHO by Roussel Uclaf, Paris, France.
Hoffman, B., Schopper, D., & Karg, H. (1984). Investigations on the
occurrence of non-extractable residues of trenbolone acetate in cattle
tissues in respect to their bioavailability and immunological
reactivity. Food Additives and Contaminants., 1, p. 253.
Hossack, D.J.N., Richold, M., & Jones, E. (1978). Ames metabolic
activation test to assess the potential mutagenic effect of trenbolone
acetate, 17-ß-oestradiol and 7:1 mixture of trenbolone with
17-ß-oestradiol. Unpublished report No. RSL 351/781136 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Huis in't Veld, L.G., Have, J. ten, & Kok, G.L. (1973). Hormonale
werkingen van trenbolone-acetaat. Unpublished report No. U 146/73 ENDO
from the National Institute of Public Health, The Netherlands.
Hunter, B., Macrae, S., Prentice, D.E., & Brooks, P.N. (1976a).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 235/76316
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Macrae, S., Prentice, D.E., & Majedd, S.K. (1976b).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 213/75992
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Bathman, P., Heywood, R., Street, A.E., Prentice, D.E., &
Majeed, S.K. (1976c). Trenboloneacetate oral toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RSL
237/76426 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Graham, C., Gibson, W.A., Read, R., Gregson, R., Heywood
R., Offer, J., Prentice, D., & Woodhouse, R. (1981). Long-term feeding
of trenboloneacetate to mice (final report). Unpublished report volume
I, II and III, No. RSL/267/80122 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Batham, P., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D., Gregson, R., & Offer, J. (1982). Trenboloneacetate
potential tumorigenic and toxic effects in prolonged administration to
rats following "in utero" exposure (final report). Unpublished report
volume I, II, III and IV, No. RSL 295-G/80974 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ingerowski, G.H., Scheutwinkel-Reich, M., & Stan, H.J. (1981).
Mutagenicity studies on veterinary anabolic drugs with the
Salmonella/microsome test. Mutagen. Res., 91, 93-98.
James, P.A., Cox, R., Clark, R., Palmer, A.K., & Offer, J. (1982).
Effect of trenboloneacetate on pregnancy of the rat. Unpublished
report No. RSL 458/81553 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., John, D.M., Gibson, W.A., Offer, J.M., &
Anderson, A. (1985). Effect of trenbolone acetate on reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/841004 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., & Parker, C.A. (1986). Supplementary study of
the effect of trenbolone acetate on pregnancy of the rat and
development of the offspring. Unpublished report No. RSL 695/851572
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Kroes, R. (1972). De invloed van oestradiol en een combinatie van
oestradiol en trienbolone op de vesiculae semenalis van mestkalveren.
Unpublished report No. 162/72 Path. from the National Institute of
Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976a). Onderzoek
naar de invloed van ethynylestradiol, 17-alpha-oestradiol en
trenboloneacetaat alleen of in combinatie op beren, borgen en geiten.
Uitscheiding in urine en faeces en residuen in serum (ILOB proeven
98.06 98.07, 98.08, 98.09, 118.04 en 118.05). Unpublished report
No. 216/76 Path. from the National Institute of Public Health,
The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976b). Onderzoek
naar de invloed van combinatie-preparaten van mannelijke en
vrouwelijke hormonen of hormonaal werkende stoffen of mannelijke
mestkalveren (Een biologisch, chemisch en histologish onderzoek, ILOB
proef 25.14 P 72/82). Unpublished report No. 193/76 Path. from the
National Institute of Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., Both-Miedema, R., & van de Berg, R.
(1976c). Onderzoek naar de invloed van 17-ß-oestradiol, trenbolone-
acetaat en testosteron alleen of in combinatie, op stieren en ossen
(Uitscheiding in urine, aanwezigheid in serum en histologisch
onderzoek prostaat, ILOB-proeven 134-02 a, 134.02 b en 134.03).
Unpublished report No. 218/76 Path. from the National Institute of
Public Health, The Netherlands.
Kruskemper, H.L., Morgner, K.D., & Noell, G. (1967). Klinische
pharmakologie von Trienolonen, eine Gruppe neuartiger anabol wirksamer
Oestran-Derivate, Arzneim. Forsch., 17, 449-454.
Lutz, W.K (1979). In vivo covalent binding of organic chemicals to
DNA as a quantitative indicator in the process of chemical
carcinogenesis. Mutation Res., 65, 289-356.
Offer, J.M. (1985). Effect of trenbolone acetate on the reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/85959 (Addendum to RSL 634/841004) from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Philibert, D. & Moguilewsky, M. (1983). Liaison de la testosterone de
la 17-ß-OH-trenbolone (RU 2341) et de son isomere, la 17-alpha-
OH-trenbolone (RU 27423) au niveau du plasma humain. Unpublished
report No. AN 39 from Roussel Uclaf Research Centre. Submitted to WHO
by Roussel Uclaf, Paris, France.
Pottier, J. Busigny, M., & Gradadam, J.A. (1973). Tissue residues in
cows after implantation of trenbolone acetate. J. Anim. Sci.,
37, 256 (Abstract No. 110F).
Pottier, J., Busigny, M., & Gradaclam, J.A. (1975). Plasma kinetics,
excretion in milk and tissue levels in the cow following implantation
of trenbolone acetate. J. Anim. Sci., 41, 968.
Pottier, J., Cousty, C., Heitzman, R.J., & Reynolds, I.P. (1978).
Metabolites trenbolone acetate R in the bile of the cow and the rat.
Report presented during the 1978 Joint Meeting of the American Dairy
Association and the American Society of Animal Science, Michigan State
University, East Lansing, 9-13 June. Submitted to WHO by Roussel
Uclaf, Paris, France.
Pottier, J. (1979). Trenboloneacetate tissue and biliary
concentrations of the position of 17 epimers of trenbolone in heifers
after 2 months of implantation. Unpublished report No. 799 from
Roussel Uclaf. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. & Richardson, J.C. (1982). Metaphase analysis on
17-ß-hydroxytrenbolone and 17-alpha-hydroxytrenbolone. Unpublished
report No. RSL/512/81962 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M., Jones, E., & Hales, J.F. (1982a). Ames metabolic
activation test to assess the potential mutagenic effect of
17-alpha-hydroxytrenbolone, 17-ß-hydroxytrenbolone and
17-alpha-oestradiol. Unpublished report No. RSL 567/82767 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Richold, M., Richardson, J.C., Proudlock, R.J., & Fleming, P.M.
(1982b). Effect of 17-ß-hydroxytrenbolone and 17-alpha-hydroxy-
trenbolone on cultured human lymphocytes. Unpublished report No. RSL
544/82136 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. Dehnel, J.M., & Ransome, S. (1982c). An assessment of the
mutagenic potential of 4 compounds using an in vitro mammalian cell
test system. Unpublished report No. RSL 513/8286 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Richold, M., Edgar, D.H., Ransome, S.J., & Banks, S.J. (1983). An
assessment of the mutagenic potential of four compounds using an
in vitro mammalian cell test system. Unpublished report from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Riis, P.M. & Suresh, T.P. (1976). The effect of a synthetic steroid
(trenbolone) on the rate of release and excretion of subcutaneously
administered estradiol in calves. Steroids, 27, 5-15.
Roberts, N.L. & Cameron D.M. (1985). Hormonal effects of orally
administered trenbolone. Findings from three studies in the domestic
pig. Unpublished report No. RSL 591/83499 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ross, D.B. (1980). Toxicology and residues of trenboloneacetate.
Published in: Conference on the Use, Residues and Toxicology of Growth
Promoters, Dublin, 9 April 1980, pp. 34-48.
Ross, D.B., Roberts, N.L., Cameron, D.M., Street, A.E., Crook, D.,
Prentice, D.E., & Cherry, C.P. (1980). Oral toxicity of trenbolone
acetate to growing pigs (Sus scrofa)- preliminary 14 weeks feeding
study. Unpublished report No. RSL 357/80152 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ryan, J.J. & Hoffman, B. (1978). Trenbolone acetate: experiences with
residues in cattle tissues. J.A.O.A.C., 61, 1274-1279.
Schiffman, D., Metzler, M., Neudecker, T., & Henschler, D. (1985).
Morphological transformation of Syrian hamster embryo fibroblasts by
the anabolic agent trenbolone. Arch. Toxicol., 58, 59-63.
Schroder, R. (1971a). R 1697: Prüfung der androgenen und
muskelbildenden Wirkung bei oraler Verabreichung. Unpublished report
from Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Schroder, R. (1971b). R 1697: Untersuchung auf androgene und anabole
Wirkung, Untersuchung auf oestrogene Wirkung. Unpublished report from
Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Seeger, K. (1971a). Anabolikum R 1697 (A 50273) as us vet.
Unpublished report from Hoechst A.G. Submitted to WHO by Roussel
Uclaf, Paris, France.
Seeger, K. (1971b). R 1967: Chronische Toxizitat per oral. Unpublished
report from Hoechst A.G. Submitted to WHO by Roussel Uclaf,
Paris, France.
Sovetal (1970). Toxicité cronique par voie sous-cutanée chez le rat
male et chez la femelle. Unpublished report No. R 1697 from Sovetal.
Submitted to WHO by Roussel Uclaf, Paris, France.
Stephany, R.W., Bosch, D., van den Sahertian, E.Th., & Kroes, R
(1976). Onderzoek naar de invloed van orale toediening van
trenboloneacetaat op vaarzen (ILOB-proef 134-01) (Eenchemisch
onderzoek). Unpublished report No. 215/76 Path. From the National
Institute of Public Health, The Netherlands.
Verbeke, R., Debackere M., Hicquet, R., Lauwers, H., Stevens, J.,
Pottier, G., Moer, D., Van Hoof, J., & Vermeersch, G. (1975). Quality
of the meat after the application of anabolic agents in young calves.
Published in: Anabolic Agents in Animal Production, FAO/WHO Symposium,
March 1975, pp. 123-130.
van de Wal, P. (1975). General aspects of the effectiveness of
anabolic agents in increasing protein production in farm animals, in
particular in bull calves. Published in: Anabolic Agents in Animal
Production, FAO/WHO Symposium, March 1975, pp. 60-78.
 Highest concentrations were found in the liver (42 and 49 ng
equivalents/g at 15 and 30 days, respectively). Lower concentrations
were found in the kidneys (15 - 20 ng equivalents/g) and muscle and
fat (2 - 3 ng equivalents/g). High concentrations of radioactivity in
the bile (1073 and 736 ng equivalents/ml at 15 and 30 days,
respectively) indicate its importance in excretion of this compound.
Comparison of total and non-volatile radioactivity showed that only a
small amount of tritiated water was produced. About 10% of the
radioactivity in the liver samples was extracted by diethyl ether or
ethyl acetate, and this proportion increased to about 20 - 30%
following incubation with ß-glucuronidase, indicating the presence of
a glucuronide(s) (Hawkins et al., 1984).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. One heifer was killed 60 days after implantation;
the implant was removed from the other heifer after 60 days and the
animal was killed 16 days later. The H content in the liver, kidneys,
muscle, and fat varied from 0.5 to 25 ppb. Of these residues, 1 - 5%
was TBA, TBOH, and trenbolone glucuronide; up to 5% was found in other
organic-soluble material. Of the remaining radioactivity, about 50%
was water soluble, and the insoluble residue could be made water
soluble by treatment with the proteolytic enzymes pepsin and trypsin
(Ryan & Hoffman, 1978).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. After 60 days the implants, which still contained
31% of the initial radioactivity, were removed. One heifer was killed
immediately, the other was maintained for 16 days after implant
removal and then killed. Ethyl acetate-extractable radioactivity in
blood plasma could largely be ascribed to TBOH; in most cases no TBA
was found in plasma. Plasma concentrations during days 1 - 55 after
dosing were 5 - 13 ppb; after 58 days a large increase in both total
and nonvolatile radioactivity was observed (17 - 20 ppb). The
half-lives for plasma disappearance of total and non-volatile
radioactivity were 32 and 29 days, respectively, during the
implantation period and 18 and 14 days, respectively, during the
withdrawal period. Plasma ethyl acetate-extractable radioactivity
amounted to 10 - 74% of the total radioactivity during days 1 - 55
after implantation, and this declined to 5% at 16 days after implant
removal. In the 16 days from implant removal to sacrifice,
radioactivity decreased by 58% in muscle, 75% in liver, 77% in
kidneys, and 74% in fat (Chasseaud et al., 1976).
Heifers (aged 15 months, number not given) were given daily oral
doses of 0.4 or 8 mg TBA per animal for 9 weeks. After 1 and 2 weeks
TBA was detected in the urine. Two weeks after drug withdrawal the
compound was detected in some urine samples, whereas after 3 weeks no
TBA was detected (Stephany et al., 1976).
A 14-month-old heifer, after i.v. administration of 10 mg/kg b.w.
TBA, excreted 80% of the administered radioactivity in the bile during
the first 24 hours; 3.5% was in the free form, 30% was excreted as
glucuronides, and 30% as sulfates. Metabolites with the 3-ketotrienic
structure that were identified in the bile are presented in Figure 2.
Three compounds that had lost their ketotrienic structure were also
isolated; these metabolites are presented in Figure 3. Less than 1% of
the administered radioactivity was isolated as tritiated water
(Pottier et al., 1978).
Specimens of muscle from the back and rear leg and specimens from
the liver were taken from two heifers that had been implanted two
months earlier with 300 mg 3H-TBA. In addition, bile was collected
by catheterization of one heifer on days preceding slaughter. The
radioactivity content of muscle, independent of its location, was
one-tenth the level in liver, whereas radioactivity levels in the bile
were 15 times higher than in liver tissue, alpha-TBOH and ß-TBOH
concentrations were determined by reverse isotopic dilution. On
average, the concentration of ß-TBOH was 0.05 to 0.1 ppb in various
tissues, whereas that of alpha-TBOH, which was only 0.005 ppb in the
muscle, reached 0.88 ppb in the liver. Following enzymolysis, ß-TBOH
was not detected in the bile, which contained, by contrast, nearly
200 ppb alpha-TBOH. Thus, alpha-TBOH represented 10% of total TBOH in
muscle, 90 - 95% in the liver, and more than 99% of the total in bile
(Pottier, 1979).
3H-TBA was implanted in the ears of two heifers (300 mg;
388 mCi) and the distribution of the radioactivity in liver and muscle
tissue was determined, applying rigorously standardized organic or
aqueous extraction procedures, either directly or following enzymatic
hydrolysis and proteolytic procedures. These steps yielded almost 100%
recovery of the radioactivity and indicate that only 5 to 15% of the
total residues were extractable with organic solvents. The remaining
radioactivity was either soluble in aqueous media or remained bound to
tissue structures. In another experiment, liver tissue from a calf
treated with 3500 mg TBA 68 days prior to slaughter was examined by
applying radioimmunoassay techniques to determine TBA/TBOH ratios.
Trienic-steroid type residues were obtained only from fractions
containing residues extractable with organic solvents (Hoffman et al.,
1984).
Two barren cows, after i.v. administration of 10 mg 3H-TBA per
animal, displayed very rapid hydrolysis of 3H-TBA in the blood
plasma; after 0.1 hour, only 2% of the radioactivity was recovered as
TBA, whereas 70% was recovered as TBOH. After 2 hours, radioactivity
could no longer be extracted, and in the extracted fraction polar
components predominated. From 3 - 8 hours TBOH disappeared from the
blood (half-life, 1.5 hours) (Pottier et al., 1975).
Highest concentrations were found in the liver (42 and 49 ng
equivalents/g at 15 and 30 days, respectively). Lower concentrations
were found in the kidneys (15 - 20 ng equivalents/g) and muscle and
fat (2 - 3 ng equivalents/g). High concentrations of radioactivity in
the bile (1073 and 736 ng equivalents/ml at 15 and 30 days,
respectively) indicate its importance in excretion of this compound.
Comparison of total and non-volatile radioactivity showed that only a
small amount of tritiated water was produced. About 10% of the
radioactivity in the liver samples was extracted by diethyl ether or
ethyl acetate, and this proportion increased to about 20 - 30%
following incubation with ß-glucuronidase, indicating the presence of
a glucuronide(s) (Hawkins et al., 1984).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. One heifer was killed 60 days after implantation;
the implant was removed from the other heifer after 60 days and the
animal was killed 16 days later. The H content in the liver, kidneys,
muscle, and fat varied from 0.5 to 25 ppb. Of these residues, 1 - 5%
was TBA, TBOH, and trenbolone glucuronide; up to 5% was found in other
organic-soluble material. Of the remaining radioactivity, about 50%
was water soluble, and the insoluble residue could be made water
soluble by treatment with the proteolytic enzymes pepsin and trypsin
(Ryan & Hoffman, 1978).
Two heifers were given single s.c. implantations with 300 mg
3H-labelled TBA. After 60 days the implants, which still contained
31% of the initial radioactivity, were removed. One heifer was killed
immediately, the other was maintained for 16 days after implant
removal and then killed. Ethyl acetate-extractable radioactivity in
blood plasma could largely be ascribed to TBOH; in most cases no TBA
was found in plasma. Plasma concentrations during days 1 - 55 after
dosing were 5 - 13 ppb; after 58 days a large increase in both total
and nonvolatile radioactivity was observed (17 - 20 ppb). The
half-lives for plasma disappearance of total and non-volatile
radioactivity were 32 and 29 days, respectively, during the
implantation period and 18 and 14 days, respectively, during the
withdrawal period. Plasma ethyl acetate-extractable radioactivity
amounted to 10 - 74% of the total radioactivity during days 1 - 55
after implantation, and this declined to 5% at 16 days after implant
removal. In the 16 days from implant removal to sacrifice,
radioactivity decreased by 58% in muscle, 75% in liver, 77% in
kidneys, and 74% in fat (Chasseaud et al., 1976).
Heifers (aged 15 months, number not given) were given daily oral
doses of 0.4 or 8 mg TBA per animal for 9 weeks. After 1 and 2 weeks
TBA was detected in the urine. Two weeks after drug withdrawal the
compound was detected in some urine samples, whereas after 3 weeks no
TBA was detected (Stephany et al., 1976).
A 14-month-old heifer, after i.v. administration of 10 mg/kg b.w.
TBA, excreted 80% of the administered radioactivity in the bile during
the first 24 hours; 3.5% was in the free form, 30% was excreted as
glucuronides, and 30% as sulfates. Metabolites with the 3-ketotrienic
structure that were identified in the bile are presented in Figure 2.
Three compounds that had lost their ketotrienic structure were also
isolated; these metabolites are presented in Figure 3. Less than 1% of
the administered radioactivity was isolated as tritiated water
(Pottier et al., 1978).
Specimens of muscle from the back and rear leg and specimens from
the liver were taken from two heifers that had been implanted two
months earlier with 300 mg 3H-TBA. In addition, bile was collected
by catheterization of one heifer on days preceding slaughter. The
radioactivity content of muscle, independent of its location, was
one-tenth the level in liver, whereas radioactivity levels in the bile
were 15 times higher than in liver tissue, alpha-TBOH and ß-TBOH
concentrations were determined by reverse isotopic dilution. On
average, the concentration of ß-TBOH was 0.05 to 0.1 ppb in various
tissues, whereas that of alpha-TBOH, which was only 0.005 ppb in the
muscle, reached 0.88 ppb in the liver. Following enzymolysis, ß-TBOH
was not detected in the bile, which contained, by contrast, nearly
200 ppb alpha-TBOH. Thus, alpha-TBOH represented 10% of total TBOH in
muscle, 90 - 95% in the liver, and more than 99% of the total in bile
(Pottier, 1979).
3H-TBA was implanted in the ears of two heifers (300 mg;
388 mCi) and the distribution of the radioactivity in liver and muscle
tissue was determined, applying rigorously standardized organic or
aqueous extraction procedures, either directly or following enzymatic
hydrolysis and proteolytic procedures. These steps yielded almost 100%
recovery of the radioactivity and indicate that only 5 to 15% of the
total residues were extractable with organic solvents. The remaining
radioactivity was either soluble in aqueous media or remained bound to
tissue structures. In another experiment, liver tissue from a calf
treated with 3500 mg TBA 68 days prior to slaughter was examined by
applying radioimmunoassay techniques to determine TBA/TBOH ratios.
Trienic-steroid type residues were obtained only from fractions
containing residues extractable with organic solvents (Hoffman et al.,
1984).
Two barren cows, after i.v. administration of 10 mg 3H-TBA per
animal, displayed very rapid hydrolysis of 3H-TBA in the blood
plasma; after 0.1 hour, only 2% of the radioactivity was recovered as
TBA, whereas 70% was recovered as TBOH. After 2 hours, radioactivity
could no longer be extracted, and in the extracted fraction polar
components predominated. From 3 - 8 hours TBOH disappeared from the
blood (half-life, 1.5 hours) (Pottier et al., 1975).

 In two barren cows after s.c. implantation of 300 mg 3H-TBA per
animal at the base of the ear, slow resorption from the implant
occurred; the half-life of disappearance from the implant was 68 - 84
days. About 33% of the radioactivity was extracted in the blood plasma
over the 3-month period after implantation, 70% of which was accounted
for by TBOH. The main routes of excretion were via the bile and urine.
Tissue levels after 3 months were about 1 ppb, except in the liver
(6.5 ppb) and kidneys (4.5 ppb). Twenty-five percent of the tissue
radioactivity was extractable, 40% of which was TBOH. In the liver and
kidneys, however, only 10% was extractable, while in perirenal fat up
to 88% of the radioactivity was extractable. In perirenal fat 50% of
the radioactivity was TBA. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity (Pottier et al., 1973;
Pottier et al., 1975).
Slow resorption from s.c. implants of 300 mg 3H-TBA occurred in
2 lactating cows. The half-life for disappearance from the implant was
approximately 60 days. About 17% of the radioactivity present in the
blood plasma over the period of 5 months after implantation was
extractable. Less than 1% of the radioactivity was excreted in milk.
Ten percent of the milk radioactivity was extractable and 25% of this
extractable radioactivity was TBOH. Tissue levels after 5 months were
about 1 ppb, except in the liver (3.4 ppb) and kidneys (2.7 ppb).
About 25% of the tissue radioactivity was extractable, except in the
liver and kidneys (both 10%); about 40% of this extractable
radioactivity was TBOH. In contrast, 88% of total radioactivity in
perirenal fat was extractable, of which 50% was TBA. Unchanged TBA was
found in no other tissues. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity after 5 months (Pottier
et al., 1973; Pottier et al., 1975).
Two steers were given by single s.c. implantations 300 mg
3H-TBA in combination with 40 mg estradiol; the implants were
removed 60 days later, at which time 28% of the radioactivity remained
in them. Ethyl acetate-extractable radioactivity in blood plasma was
primarily ascribed to TBOH; in most cases no TBA was found in the
plasma. One animal was killed immediately after removal of the
implant. Plasma concentrations in this animal declined with half-lives
of 26 days for both total and non-volatile radioactivity; ethyl
acetate-extractable radioactivity in the plasma of this animal ranged
between 3 - 5% of the total radioactivity. In the other animal, which
was killed 16 days after removal of the implant, plasma concentrations
declined during days 1 - 60, with half-lives of 50 and 55 days for
total and non-volatile radioactivity, respectively. In the 16 days
from implant removal to sacrifice, radioactivity decreased by 46% in
muscle, 2% in liver and kidneys, and 29% in fat (Chasseaud et al.,
1976).
Relay bioavailability
Groups of 3 rats were fed freeze-dried or ethyl acetate-extracted
liver, kidney, or muscle obtained from two heifers killed 60 days
after s.c. implantation with 300 mg 3H-TBA. 3H-TBA levels in the
heifers averaged 30 ng equivalents/g in the liver, 24 ng equivalents/g
in the kidneys, and 3.2 ng equivalents/g in muscle. Radioactivity
excretion during the 3 days after feeding these tissues to rats is
presented in Table 1 (Hawkins et al., 1979).
Groups of 3 bile duct-cannulated rats that had been fasted for 24
hours were fed during 1 hour freeze dried liver, kidney, or muscle
from the two heifers described in the previous paragraph.
Radioactivity disposition during 48 hours after feeding of these
tissues is presented in Table 2 (Hawkins et al., 1979).
Table 1. Excretion of radioactivity by rats after being fed tissues
from heifers implanted with 3H-TBA
Excretion in percent of
administered radioactivity
Treatment Tissue Urine Faeces Total
Freeze-dried tissue Liver 3 81 84
Kidney 2 93 94
Muscle 6 85 91
Extracted tissue Liver 5 78 83
Kidney 2 103 105
Muscle 2 73 75
Table 2. Excretion of radioactivity by bile duct-cannulated rats
after feeding of tissue from heifers implanted with 3H-TBA
Excretion in percent of administered radioactivity
Tissue Bile Urine Faeces GI tract + contents Total
Liver 7 5 59 2 74
Kidney 3 1 31 60 95
Muscle 3 2 56 not detected 61
Effects on protein binding
The affinity of alpha-TBOH and ß-TBOH for corticosteroid binding
globulin, measured in vitro using the human plasma of elderly women,
was very low, less than 0.1% bound, compared with 10% for testos-
terone. The affinity of alpha-TBOH and ß-TBOH for testosterone and
estradiol binding globulin was 1% of that measured for testosterone.
When alpha-3H-TBOH was incubated in vitro with female human plasma,
it readily bound to the albumin fraction; only 4% was present
as free TBOH. The total blood clearance of ß-TBOH was twice that of
testosterone (Philibert & Moguilewsky, 1983).
Effects on estradiol-17ß excretion and nitrogen retention
Cattle
Plasma residues of estradiol-17ß in cattle were affected by the
presence of TBA in the s.c. implant. Plasma levels of estradiol-17ß
remained greater than 0.05 ppb for nine weeks in steers after
treatment with 200 mg TBA in combination with 40 mg estradiol-17ß,
whereas the residual levels decreased below 0.05 ppb within 5 weeks
after implantation of 40 mg estradiol-17ß alone (Heitzman & Hardwood,
1977).
Implantation of 40 mg TBA in the dewlap of Friesian bulls
(11 - 16 weeks of age) did not affect nitrogen retention. Implantation
of 140 mg TBA in combination with 20 mg estradiol-17ß at the same
site, however, resulted in a 47% decrease in nitrogen retention
(van der Wal, 1975).
Pigs
Pigs (males, females, and castrated males) were given s.c.
implantations with either 20 mg estradiol-17ß or 20 mg estradiol-17ß
in combination with 140 mg TBA. At 5 weeks after implantation, steroid
estrogens were hardly detectable in the faeces, and serum values for
estradiol-17ß were very low in both groups. Urine estradiol-17ß levels
were 6 - 82 µg/l in the estradiol-17ß group and 16 - 135 µg/l in the
combination group (Kroes et al., 1976a).
Toxicological studies
Special studies on carcinogenicity potential
Rats
Male Wistar rats (number not specified) were injected i.p.
with 15 µg/kg b.w. 3H-estradiol-17ß (53.6 Ci/mmole), 19 µg/kg
b.w. 3H-testosterone (54.0 Ci/mmole), 17 µg/kg b.w. 3H-TBA,
(57.0 Ci/mmole), or 30 µg/kg b.w. 3H-zeranol (50.0 Ci/mmole), all in
95% ethanol solution. The animals were sacrificed 16 hours after
injection and the Covalent Binding Indices (CBI, Lutz, 1979) of the
chemicals to DNA in the liver were quantitated. The CBIs were 11.4,
4.80, 5.62, and 1.65 for estradiol-17ß, testosterone, TBA, and
zeranol, respectively (weak carcinogens have a CBI approx. or equal
10, Lutz, 1979). The positive control, N-hydroxy-acetylaminofluorene,
had a CBI value of 262 (Barraud et al., 1983).
The CBI of TBA as a function of time was measured by
administering 0.83 mCi (22 - 40 µg/kg b.w.) 3H-TBA i.p. to 8 male
rats. The animals were killed at 4, 8, 12, 20, 24, 36, 48, and 96
hours. The highest CBI, 7.82 was obtained after 24 hours; after 96
hours the CBI was 1.11 (Barraud et al., 1983).
Treatment of rodents with initiators of liver cancer can give
rise to phenotypically altered cells which, under suitable conditions,
will develop into foci of potentially pre-neoplastic cells. These foci
may either regress or develop into malignant nodules, but because they
only take a few weeks to become apparent, induction of such foci
represents a useful short-term indication of tumour-initiating
capacity.
alpha-TBOH or ß-TBOH (2.5, 5, or 10 mg/kg b.w.), ethinyl
estradiol (0.05 mg/kg b.w.), testosterone (10 mg/kg b.w.),
nitrosomorpholine (25 mg/kg b.w.), or diethylnitrosamine (200 mg/kg
b.w.) were administered by i.p. injection approximately 18 hours after
partial hepatectomy to Fisher 344 CDF rats (5 males and 5 females per
group). Two groups presented only with vehicle and one untreated group
of 5 males and 5 females each were used as controls. The animals were
allowed to recover for a further 13 days after treatment with the test
agent. The animals then were supplied with tap water and powdered diet
containing 0.02% 2-acetylaminofluorene, except that the diet supplied
to animals in one of the vehicle control groups contained no
acetylaminofluorene. Seven days after commencing the new dietary
regime the animals were treated with carbon tetrachloride at 2 ml per
kg b.w. by intragastric gavage (animals in the vehicle control group
not given acetylaminofluorene were not treated with carbon
tetrachloride). Seven days later the animals were killed by cervical
dislocation and the livers were removed for microscopic examination.
Most of the animals showed moderate lethargy and other clinical
signs for two or three days following the operative procedure, but no
compound-related adverse signs were evident. No significant
treatment-related effects on body or liver weight were reported. Only
animals treated with nitrosomorpholine or diethylnitrosamine showed
significant increases in liver foci compared with the vehicle control
or untreated groups.
None of the steroids examined in this study (including alpha-TBOH
and ß-TBOH) showed any evidence of inducing pre-neoplastic liver foci
at the dose levels tested. The authors concluded that none of these
steroids showed any evidence of being a liver rumour initiator in this
assay (Allen & Proudlock, 1987).
Special study on immunoresponse
Cattle
Antibody production in male and female calves (about 25 animals
per group) was investigated after s.c. implantation of placebo
(lactose), 20 mg estradiol-17ß, 140 mg TBA, or 140 mg TBA + 20 mg
estradiol-17ß. A slight, non-significant immunodepressive effect was
seen in male calves treated with either estradiol-17ß or TBA alone. In
the males treated with the combination, this effect was significant.
In female calves the immunoresponse was unaffected (Gropp et al.,
1975).
Special studies on mutagenicity
The results of mutagenicity assays on TBOH and TBA are summarized
in Table 3.
Special studies on no-hormonal effect levels
Pigs
Groups of 3 - 7 (11 in the control group) mature male large white
hybrid pigs, 8 - 10 months of age, were administered orally 0.1, 1,
10, 16, 24, or 36 µg ß-TBOH/kg b.w./day or 0.1, 10, 100, 160, 240, or
360 µg alpha-TBOH/kg b.w./day in gelatine capsules with the feed for
14 consecutive days after castration. They were maintained for 14 more
days, then killed and examined post mortem. Serial blood samples
were collected prior to castration (day 0) and on days 7, 14, 21, and
28; LH was determined on days 0 and 14 in the animals treated with
alpha-TBOH and on days 0, 14, and 28 in the animals treated with
ß-TBOH. After sacrifice, the pituitary, prostate, and seminal vesicles
were examined by gross pathology and histopathology. Based on changes
in plasma luteinizing hormone (LH) concentrations, distinct hormonal
effects occurred at 160 µg alpha-TBOH/kg b.w./day and at 16 µg
ß-TBOH/kg b.w./day. Thus, the no-observed-effect levels in this study
were 10 µg ß-TBOH/kg b.w./day and 100 µg alpha-TBOH/kg b.w./day. The
morphological examinations performed in this study are of limited
value, since a 14-day period elapsed between final treatment and
slaughter, allowing regression of likely effects. Thus, the
observation of treatment-related androgenic effects in the 16, 28, and
36 µg ß-TBOH/kg b.w./day group points toward the high hormonal
activity of this compound (Roberts & Cameron, 1985).
Four groups of 4 male and 4 female large white hybrid pigs were
given orally TBA incorporated into the diet for 14 consecutive weeks
at 0, 0.1, 2, or 20 ppm (equal to 0, 2.0-3.1, 40-62, or 400-620 µg/kg
b.w./day). Blood samples were obtained for steroid hormone assays
before treatment commenced and after 6 and 12 weeks of treatment.
Table 3. Results of mutagenicity assays on TBOH and TBA
Concentration
Test Test of substance
system object tested Results Reference
Ames S. typhimurium 10-10000 µg/ Negative Hossat et al., 1978
test1 TA98, TA100, plate TBA or
TA1535, TA1537 7:1 TBA:
TA1538 estradiol-17ß2
Ames S. typhimurium 1000, 2000, Negative Ingerowski et al., 1981
test1 TA98, TA100, 3000 µg/plate at 1000
TA1535, TA1537 TBOH µg/plate;
TA1538 equivocal at
cytotoxic
concentrations
Ames S.typhimurium 0.5 - 500 µg/ Negative Richold et al., 1982a
test1 TA98, TA100, plate alpha-TBOH;
TA1535, TA1537 15 - 1500 µg/
TA1538 plate ß-TBOH
Ames S. typhimurium 0.06 - 2 µg/ Negative Schiffman et al., 1985
test1 TA98, TA100 plate TBOH
Clastogenic Human lymphocytes 6, 30, or 60 Negative Richold et al., 1982b
potential in vitro µg/ml alpha-TBOH
or ß-TBOH2,3
Cell Mouse lymphoma 15 - 45 µg/ml Equivocal6 Richold et al.,
mutation L5178Y cells alpha-TBOH; 15 - 1982c, 1983
assay4 65 µ/ml ß-
TBOH5
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Cell Syrian hamster 5, 10, 15 µg/ Equivocal7 Schiffman et al., 1985
transformation embryo fibroblasts ml TBOH
assay
Cell Mouse C3H1OT1/2 2 - 25 µg/ml Equivocal Henderson et al., 1987a
transformation cells ß-TBOH (- act.)
assay1 (-S-9 mix)5
5 - 20 µg/ml Positive
ß-TBOH (+ act.)
(+S-9 mix)8
Forward Chinese hamster 25 - 100 µg/ml Negative Edgar et al., 1985
mutation ovary cells ß-TBOH
assay1 (HGPRT locus) (-S-9 mix)9
25 - 150 µg/ml
ß-TBOH
(+S-9 mix)5
Forward Chinese hamster 25 - 500 µg/ml Negative Henderson et al.,
mutation ovary cells ß-TBOH5 1986a
assay1 (HGPRT locus)
Forward Chinese hamster 3 - 75 µg/ml Negative Henderson et al.,
mutation V79 cells ß-TBOH 1987b
assay1 (HGPRT locus) (-S-9 mix)9
12 - 125 µg/ml
ß-TBOH
(+S-9 mix)
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Micronuclei Chinese hamster 1 - 10 µg/ml Equivocal Henderson et al.,
induction1 ovary cells ß-TBOH (- act.) 1986b
(-S-9 mix)10
6 - 60 µg/ml Negative
ß-TBOH (+ act.)
(+S-9 mix)10
Chromosome Chinese hamster 1 - 10 µg/ml Negative Allen et al., 1985
aberration ovary cells ß-TBOH
assay1 (-S-9 mix)3
6 - 60 µg/ml
ß-TBOH
(+S-9 mix)11
Unscheduled HeLa cells and 2.5 - 15 µg/ml Negative Schiffman et al.,
DNA Syrian hamster 1985
synthesis embryo cells
DNA Cultured human 1 - 512 µg/ml Negative13 Allen & Proudlock,
repair epithelioid alpha-TBOH or 1983
assay1 cells ß-TBOH12
In vivo Rat bone marrow 100 mg/kg b.w. Negative Richold & Richardson,
cytogenetics alpha-TBOH or 1982
assay ß-TBOH once;
25 or 50 mg
alpha-TBOH or
ß-TBOH 4 times3
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
In vivo Erythrocytes 100 mg/kg b.w. Negative Allen et al., 1980
micronucleus ß-TBOH3
test
1 Both with and without rat liver S-9 fraction.
2 Dimethylsulfoxide was used as the solvent.
3 Mytomycin C was used as a positive control.
4 Only with rat liver S-9 fraction.
5 20-Methylcholanthrene was used as a positive control.
6 alpha-TBOH at > 22 µg/ml and ß-TBOH at > 15 µg/ml were toxic to the
cells. Both substances induced 2-fold increases in mutation frequency,
but with alpha-TBOH this occurred only at highly toxic concentrations.
7 There was an inverse dose relationship, with the largest number of
transformations occurring at the lowest dose.
8 2-Acetamidofluorene was used as a positive control.
9 Ethylmethanesulfonate was used as a positive control.
10 Colchicine was used as a positive control.
11 Cyclophosphamide was used as a positive control.
12 4-Nitroguinoline-1-oxide (-S-9 mix) and 2-aminoanthracene (+S-9 mix)
were used as positive controls.
13 Increases in nuclear grain count were observed in a limited number of
cultures in one experiment only.
A detailed post-mortem examination was carried out on all pigs
at termination, and tissues were retained for histological
examination. All animals remained generally in good health throughout
the study, and no abnormal clinical signs were noted that could be
associated with experimental treatment. No significant effects of
treatment on body weight, food consumption, or ophthalmoscopy were
noted. There was a marked, dose-related reduction in serum
testosterone levels in male pigs, mean values in the highest-dose
group at week 6 and in pigs fed 2 and 20 ppm TBA at week 12 being
significantly lower than control values (p < 0.01). Testosterone
levels in female pigs remained low at all 3 assay points.
Serum progesterone levels in females were variable. This is not
unexpected, since both progesterone and estradiol show a marked cycle
variation and the assay samples were obtained at arbitrary time points
without regard to the stage of the estrus cycle of individual pigs.
However, mean values at week 12 did indicate a dose-related reduction
in serum progesterone levels in female pigs, and on statistical
examination there was found to be a significant trend with dose
(p < 0.05). Progesterone values for male pigs were less variable and
lower on average than for females and no consistent treatment-related
differences were noted.
Levels of estradiol-17ß in female pigs were generally low,
although there was evidence of some increase in estrogenic secretion
at week 12 in pigs from all groups; no appreciable differences between
groups were observed. Estradiol levels in males were in general higher
than in females; a dose-related reduction in estradiol mean values was
noted at weeks 6 and 12. The results of the hormone assays point to a
no-hormonal-effect level below 2 µg/kg b.w./day.
Treatment-related reductions in mean weights of testes, ovaries,
and uteri were noted. Apparently, the most sensitive organs are the
testes and ovaries. At the lowest dose, no effects on any tissue
weights were observed. The main findings associated with treatment at
the 2 higher levels were atrophy of testicular interstitial cells,
suppression of cyclic ovarian activity, the consequent absence of
glandular development of the uterine endometrium, and lack of alveolar
development and secretion in the mammary glands (highest group only).
Treatment-related changes were not seen in the gonads, uteri, or
mammary glands in any of the animals examined in the lowest-dose group
(Roberts & Cameron, 1985).
Four groups of 5 male and 5 female pigs were given orally TBA as
solutions in corn oil in gelatine capsules with the food for 14
consecutive weeks at 0, 5.0, 7.5, or 10.0 µg/kg b.w./day. Venous blood
samples were taken before treatment began and at weekly intervals
throughout the test period. Plasma radioimmunoassays for testosterone,
estradiol-17ß, and progesterone were carried out. Pigs were sacrificed
after 14 weeks and examined post-mortem. Tissues processed for
histological examination included the testes, ovaries, seminal
vesicles, uterus, and mammary gland. In males, comparison with the
control group showed some differences in testosterone levels at weeks
1 and 5, which reached statistical significance in the two highest
dose groups. However, these differences were not sustained and were in
the opposite direction (i.e. increases compared with the control) to
the dose-related changes (i.e. suppression) noted in the study
described in the previous paragraphs. Testosterone levels in females
generally remained low in all groups.
No significant group differences were found in estradiol levels
in males. In females, estradiol levels generally remained low in all
groups. Mean progesterone levels in males were statistically
significantly lower than in controls between weeks 7 and 10 in the
highest-dose group and at week 10 in the group administered 7.5 µg
TBA/kg b.w./day. However, the mean pre-dose levels were also lower in
pigs in these two groups compared with the controls and analysis of
mean levels over weeks 6 to 14 showed no significant effects. Thus,
these differences were not considered by the authors of the report to
represent a real treatment effect. In female pigs, mean progesterone
values were variable due to cyclic changes, but no consistent group
differences were apparent. Examination of the time of onset of cycling
and the peak level of progesterone recorded did not show significant
effects. No significant differences in organ weights were noted in any
groups. No treatment-related changes in the morphology of the organs
examined were noted in any groups (Roberts & Cameron, 1985).
Monkeys
The antigonadotropic activity of ß-TBOH was tested in acutely
castrated male rhesus macaque monkeys aged 8 - 17 years. A seminal
vesicle biopsy was obtained at the end of the 30-day treatment period
to examine possible changes in androgenic activity induced by TBOH.
ß-TBOH was given orally at 0, 1, 20, or 400 µg/day (three control
animals and two animals per treatment group). From 17 days after
castration until the end of the experiment, the lowest-dose group was
given 1600 µg/day ß-TBOH. Administration of the compound did not
suppress the post-castration elevation in either LH or follicle
stimulating hormone (FSH) secretion, which occurs after removal of the
testes. Although TBOH and testosterone showed no antigonadotropic
activity in this model system, the monkeys receiving 400 and
1600 µg/day TBOH maintained partial or complete seminal vesicle
morphology consonant with an androgenic effect. The expected reduction
in the serum levels of testosterone and estradiol occurred after
castration, and TBOH treatment did not alter the serum concentrations
of these hormones or the typical diurnal pattern of activity within
the hypothalmic-pituitary-adrenal axis. The authors concluded that the
no-hormonal-effect level in this study was 20 µg/day, equivalent to
2 µg/kg b.w./day (Hess, 1983).
TBA was administered in Sustagen/Jello/bran diet cubes for three
cycles, or a maximum of 122 days, to three groups of six mature female
rhesus macaque monkeys weighing 6 kg each at 60, 240, or 960 µg/day.
Blood samples were obtained on a daily basis from all animals during a
pretreatment menstrual cycle, at 3-day intervals during the first two
treatment menstrual cycles, and daily during the last treatment
menstrual cycle. Serum concentrations of estradiol, progesterone, LH,
and FSH were determined by radioimunoassay. Treatment with 960 µg/day
TBA resulted in maximum average serum levels of 2.3 ng/ml of ß-TBOH,
and this dose may have inhibited gonadotropin secretion and ovarian
function in 3 of 16 reproductive cycles.
It was concluded that 960 µg TBA/day had an inhibitory effect on
the pituitary gonadal axis. The anovulatory stage was reached rather
suddenly and from the data presented no conclusions with respect to
changes in the endogenous hormone concentrations, which might signal
this effect, can be drawn. In the 240 µg/day group one animal
exhibited anovulation, which may have been related to treatment. No
effects were observed at 60 µg/day, equivalent to 10 µg/kg b.w./day
(Hess, 1984).
Special studies on relay toxicity
Rats
Female veal calves were given s.c. implantations of 0, 140, or
3500 mg/animal TBA and killed after 10 weeks. A homogenate of veal
meat and veal organs (tongue, heart, lungs, spleen, liver (partial),
and one kidney) from these calves was mixed with the diet of rats in a
two-generation reproduction study. The total length of the experiment
was 114 weeks. The F1a generation was used to study teratogenic
effects. In the group of rats receiving a diet containing 230 ppb TBA,
a slight growth depression was seen. No effects were seen on other
parameters, which included mortality, feed consumption, growth,
fertility, reproduction (copulation, conception rate, duration of
gestation, mean litter weight, litter size, fetal body weight,
mortality rate, and fetal body weight after 3 weeks), haematology,
biochemistry, organ weight, and gross- and histopathology
(Gropp et al., 1978).
Special studies on reproduction
Rats
Groups of 40 male and 80 female rats weighing 133 - 143 g each
were given diets containing 0, 0.5, 1, 4, or 16 ppm TBA from week 9
before mating until day 21 post-partum. An additional group was
handled similarly, in which females were fed 50 ppm TBA from day 1 of
pregnancy until day 21 post-partum. At day 21 post-partum all parent
animals were killed without further examination. There were no major
differences between nominal and detected dietary levels of TBA at the
beginning and end of the study. In females in the 4 and 16 ppm groups,
growth was increased throughout the study (10 - 20%); in the 50 ppm
group growth was increased after mating (10%). A dose-related decrease
in pregnancy rate was seen in the 1, 4, 16, and 50 ppm groups (maximum
-30%). At day 4 post-partum each litter was reduced in size to 4
females and 4 males; up to that time pup mortality was increased in
all dosed groups compared with controls. Litter size and litter weight
on days 0, 4, 12, and 21 post-partum were decreased in the 4 and 16
ppm groups (maximum about -10%) and in the 50 ppm group (about -25%).
Mean pup weights were decreased only in the 50 ppm group from day 4
post-partum onward (maximum -15%) (Hunter et al., 1982).
Groups of 12 male and 12 female rats were fed diets containing
0, 25, 50, or 100 ppm TBA for 63 days and then mated. In these groups,
12/12, 10/12, 4/12, and 1/12 females, respectively, were pregnant
after mating (Ross, 1980).
In a multi-generation reproduction study in CRL:COBS CD(SD)BR
Charles River rats, dietary concentrations of 0, 0.5, 3, or 18 ppm TBA
were fed to F0-generation male rats for nine weeks and female rats
for two weeks prior to mating, then through to termination. Two groups
of F1 generation rats were selected, reared, and mated. One group
was treated continuously at the same dietary concentrations as rats in
the F0 generation ("treated" group) and one was removed from
exposure to TBA at 3 weeks of age and maintained without treatment
throughout ("untreated" group).
Treatment with TBA at 18 ppm was associated with the following
effects: a) generally higher body weights affecting both F0-and
F1-generation treated males and females and females of the untreated
F1 generation; b) depression in mean body weight gain during
gestation affecting both matings of the F0 generation; c) signs of
virilization, namely coarseness of the coat and discoloration of the
skin in F0-generation animals and treated F1-generation females;
d) clitoral prominence in treated F1-generation females and to a
lesser extent in untreated F1-generation females. Similar effects
were observed in F2-generation females from treated F1 parents,
but not in offspring of the untreated F1 generation; e) the presence
of occlusive strands in the vagina and/or precocious/incomplete
vaginal opening affecting treated F1 pups and F2 pups from the
treated F1 generation; f) a delay in the occurrence of testicular
descent affecting F2 pups from the treated F1 generation; g) a
marked reduction in pregnancy rate affecting the second mating of the
F0 generation and the treated F1 generation; h) an increase in
pre-coital time for the second mating of the F0 generation and for
the treated F1 generation; i) a marginal extension of the duration
of gestation affecting the F0 generation and the treated F1
generation; j) a marked increase in the incidence of extended
parturition and total litter loss in the treated F1 generation and a
significant increase in the percentage of males per litter; k) lower
litter size and litter weight, either at birth or at 20-day sacrifice,
after both matings of the F0-generation and of the treated F1
generation; 1) increased post-implantation/pre-birth losses in the
F0 and treated F1 generations; m) at terminal autopsy, findings
additional to those previously described, namely an increase in the
incidence of depression in the forestomach epithelium affecting F0-
and F1-generation males; n) significant reductions in seminal
vesicle/prostate weights in F0- and F1-generation males and
increases in mean ovary weights among F0- and F1-generation
treated females; o) significant decreases in weights of seminal
vesicles/prostate, testes, and epididymes in F1-and F2-generation
male pups at six weeks of age. F1- and F2-generation female pups
showed reduction of adrenal weight at six weeks of age; p) a
significant reduction in anogenital distance among male fetuses and a
marginal increase in the incidence of skeletalvariants after the
second mating of rats of the F0-generation (teratology phase).
Treatment with TBA at 3 ppm was associated with the following
effects: a) retarded body weight gain at the first mating of the
F0-generation; b) coarseness of the coat and discoloration of the
skin affecting one female in the F1-generation; c) a significant
delay in the mean age of vaginal opening in females in the
F1-generation and in F2 offspring from treated F1 parents; d)
the occurrence of incomplete vaginal opening or occlusive strands in
the vagina affecting occasional animals of the F1-generation at 6
week autopsy; e) a slight, but not statistically significant, delay in
testicular descent affecting F2 male offspring from treated F1
parents; f) significantly lower litter size at birth after the first
mating of the F0-generation; g) lower litter weight after the second
mating of the F0-generation; h) marginally reduced litter size in
offspring of the treated F1-generation; i) a significant decrease in
the weight of seminal vesicles/prostate, testes, and epididymes in
F1 and F2 male pups at six weeks of age.
The only apparent effects of treatment at 0.5 ppm were as
follows: a) higher group mean body weights of males from the F0
generation; b) a slight but not statistically significant delay in the
mean age of vaginal opening in F1 pups and F2 pups from the
treated F1 generation (subsequent mating performance and resulting
litter parameters were comparable to those of controls); c) a
statistically significant decrease in seminal vesicles/prostate weight
in treated F1 male pups and in F2 male pups from treated parents
at six weeks of age; d) a significant decrease in weight of epididymes
in F2 males.
The authors concluded that, in terms of reproductive performance
of the two generations examined in this study, as assessed by the
ability of parents to produce and sustain their litters, TBA exerted a
marked effect at 18 ppm and some effect at 3 ppm. At the lowest dose
level examined (0.5 ppm), slight effects were observed, which were
more marked in F2 pups than in F1 pups of a comparable age.
However, the authors concluded that in terms of reproductive
performance TBA exerted no effect at 0.5 ppm. The reproductive
performance for all groups of F1 animals following withdrawal from
treatment showed no marked differences from those of the control group
(James et al., 1985).
Histological examination of the testes, epididymes, seminal
vesicles, and prostate of 6-week-old male F2-generation rats from
treated F1 animals in the study described above revealed no
morphological abnormalities, although lower group mean weights were
recorded in treated groups compared to controls. All rats were
considered normal for that age, when they are not quite fully sexually
mature, with spermiogenesis proceeding to tailed spermatids in the
testes but no spermatoozoa in the epididymes (Offer, 1985).
In a study in rats, dietary concentrations of 0, 0.1, 0.3, 0.5,
3, or 18 ppm TBA were fed to F0 males and females from two weeks
prior to mating until termination of pregnancy. The F1 litters were
reared through the weaning period. On day 22 post-partum the male
offspring were sacrificed, examined macroscopically, and the testes,
seminal vesicles/prostate, and epididymes from each pup were weighed
and preserved. Female offspring were sacrificed on day 24 post-partum
and examined macroscopically.
Treatment with TBA at 18 ppm was associated with the following
effects: a) slightly higher mean body weights of F0 females, but
lower body weight gain during gestation and slightly lower weight gain
of males over the last three weeks of treatment; b) clitoral
prominence at autopsy in 22/29 F0 females and in all F1 female
offspring from approximately 3 weeks of age; c) a statistically
significant extension in duration of gestation; d) total litter loss
in 4/29 F0 females; e) effects on litter parameters, including
reduced litter size, lower litter weight, marginally higher pup
mortality, and higher mean pup weight; f) lower testicular weight and
higher mean weight of seminal vesicles/prostate in F1 males at 22
days of age.
Treatment at 3 ppm was associated with: a) slightly higher mean
body weights of females and slightly lower weights of males in the
three weeks prior to termination; b) a marginal extension in duration
of gestation; c) effects on litter parameters, including reduced
litter size, and marginally higher pup mortality and mean pup weight;
d) lower testicular weight and higher mean seminal vesicle/prostate
weight in F1 males.
The only effects associated with treatment at 0.1, 0.3, and
0.5 ppm TBA were slightly higher mean body weights of females and the
marginal differences observed at 3 and 18 ppm in litter parameters
(James et al., 1986).
Special studies on teratogenicity
Rats
Four groups of 6 pregnant rats each were given by oral gavage
0, 2.5, 5, or 10 mg/kg b.w./day TBA from days 6 - 15 of gestation. The
vehicle was 2.5% ethanol in 1% methylcellulose. Mortality, growth,
number of corpora lutea, number and distribution of live and dead
young, litter weight, mean fetal weight, microscopic fetal
abnormalities, sex ratio, and fetal crown-rump distance were all
unaffected by treatment (James et al., 1982).
Groups of 20 pregnant rats received by oral garage 0, 5, 10, or
20 mg/kg b.w./day TBA from days 6 - 15 of gestation. The vehicle was
2.5% ethanol in 1% methylcellulose. In the 10 and 20 mg/kg b.w./ day
groups, 9/20 and 15/20 dams, respectively, showed hair loss. A
dose-related decrease in growth was seen in all dosed groups (maximum
20%). Pregnancy rate, number of liver and dead young, number of
implantations and corpora lutea, litter weight, mean fetal weight,
incidence of major malformations, incidence of minor visceral
anomalies (Wilson's technique), and fetal crown-rump distance were all
unaffected. The incidences of skeletal variants (number of ribs,
number of normal and variant sternebrae) were unaffected. Mean
anogential distances in male fetuses preserved in Bouin's solution
were slightly shorter than those of controls, with an apparent
slightly decreasing trend with increase in dosage; the difference at
20 mg/kg b.w./day attained borderline statistical significance
(P < 0.05). Male fetuses preserved in alcohol did not show the same
trend in mean values, although the variation in anogenital distance
was greater in pups in the high-dose group than in controls; ranges of
fixation weight and crown-rump distance length were also higher in the
high-dose group than in controls. Mean anogenital distances in females
preserved in Bouin's solution and in alcohol were comparable in all
groups (James et al., 1982).
Acute toxicity
Table 4 summarizes the results of acute toxicity studies on TBA.
Table 4. Acute toxicity of TBA
LD50
Species Sex Route Vehicle (mg/kg b.w.) Reference
Mouse M/F oral 40% ethanol 1500 Audegond et al.,
in corn oil 1981a
M i.p. ethanol + 10% 565 Escuret & Bas, 1978
sesame oil
F i.p. ethanol + 10% 643 Escuret & Bas, 1978
sesame oil
Rat M/F oral 10% ethanol 5000 Audegond et 1981b
in corn oil
M i.p. 10% ethanol 1601 Escuret & Bas, 1978
in corn oil
F i.p. 10% ethanol 1772 Escuret & Bas, 1978
in corn oil
Dog M/F oral via capsules 1000 Audegond et al.,
1981c
Dogs
Anaesthetized dogs were given i.v. 1, 2, 5, or 10 mg/kg b.w. TBA
as a 20 ml/mg solution in 92% acetylmethylamine. Dogs in the 2, 5, and
10 mg/kg b.w. groups showed a dose-related decrease in blood pressure;
at 10 mg/kg b.w., this was accompanied by slight bradycardia. A
decrease in blood pressure in the same groups was seen after injection
with adrenalin and noradrenalin; an increase was seen after
acetylcholine injection. Changes in reactions to histamine were not
noted in any groups (Seeger, 1971a).
Short-term studies
Mice
Groups of 8 male and 8 female mice weighing 19 - 25 g each were
given 0, 25, 50, or 100 ppm TBA in the diet for 8 weeks. Mortality,
appearance, behaviour, body weight, food consumption, and food
conversion were unaffected by treatment. Females in all treatment
groups exhibited significant decreases in absolute and relative liver
weights and significant increases in absolute and relative uterine
weights. Absolute and relative ovarian weights were decreased
significantly in females in the 50 and 100 ppm groups, while absolute
and relative testes weights were decreased significantly in males in
the highest-dose group. No effects on the weights of adrenals,
kidneys, prostate, seminal vesicles, or spleen were noted. Females
showed dose-related suppression of ovarian cyclic activity
characterized histopathologically by the absence of, or few, corpora
lutea in the gonads, a dose-related reduction in the amount of stroma,
and reduction in the number of endometrial glands in the uteri
(Hunter et al., 1976a).
TBA was administered to groups of 8 male and 8 female Swiss
albino CFLP mice for 10 weeks at levels of 0, 1, 2, 5, or 10 ppm of
the diet, equal to mean intakes of 0, 0.12, 0.24, 0.56, or 1.2 mg/kg
b.w./day for males and 0, 0.13, 0.25, 0.66, or 1.4 mg/kg b.w./day for
females. There were no signs of reaction to treatment, including no
treatment-related effects on food intake or body weight gain. No
treatment-related abnormalities were seen; the absolute and relative
weights of all organs examined were considered to be within normal
limits for mice of this strain and age. The only organs examined
histologically were the prostate, seminal vesicles, testes, ovaries,
and uterus from mice in the control and highest-dose group. All
histopathological parameters were within normal limits (Hunter et al.,
1976b).
Rats
Sixteen male rats that had been castrated between 21 and 24 days
of age were given total oral doses of 0, 0.75, 3, 12, or 48 mg TBA
from days 2 - 11 after castration in 10 daily portions. At autopsy,
one day after the last application, dose-related increases in the
absolute weight of the prostate (maximum +440%) and seminal vesicles
(maximum +400%) were seen in all treated groups. In the three
highest-dose groups a dose-related increase in the musculo levator ani
weight (maximum +250%) was noted (Schröder, 1971a).
Groups of 10 male and 10 female CFY rats were fed diets
containing TBA at concentrations of 0, 25, 50, or 100 ppm for 13
weeks. Group mean intakes during the treatment period were 0, 1.8,
3.8, or 7.6 mg/kg b.w./day for males and 0, 2.2, 4.2, or 8.4 mg/kg
b.w./day for females. At all the tested doses, the females exhibited
better efficiency of feed utilization than the males, causing higher
weight gain.
At 25 ppm, lower prostate weight (-36%), which was not associated
with morphological changes, was observed. At 50 ppm, lower prostate
and seminal vesicle weights (-50 and -30%, respectively), which were
not associated with morphological or uterine changes, were observed in
two rats.
At 100 ppm, the following changes were observed: lower neutrophil
and lymphocyte values in males at weeks 12 and 13 (-35%); lower
seminal vesicle weight, which was not associated with morphological
changes (-60%); lower prostate weight (-80%), which was associated
with small alveoli lined by cuboidal epithelium in 5 rats; and uterine
changes characterized by an apparent reduction of the endometrial
stroma with dilated uterine glands and also a corrugated appearance of
the endometrial and glandular epithelium in 6 rats (Hunter et al.,
1976c).
Groups of 10 male and 10 female rats weighing 60 g each were
given 0, 50, 100, 200, or 1000 µg/kg b.w./day TBA orally 6 days per
week for 3 months. The compound was presented as 0.5 ml of an aqueous
solution containing 0.9% NaCl, 0.4% polysorbate 80, 0.5% carboxy-
methylcellulose, and 0.9% benzyl alcohol. Determinations were
carried out in half of the animals at termination only.
Growth of females was slightly increased; in the two highest-dose
groups growth of males was decreased. Haematological parameters were
unaffected. In all dosed groups, SGOT and SGPT were decreased; total
cholesterol was decreased in the 100, 200, and 1000 µg/kg b.w./day
groups. In the highest-dose group a slight decrease in blood glucose
was seen; urea was slightly increased only in the females in this
group. Increases were observed in liver weight in males and females in
the 200 and 1000 µg/kg b.w./day groups, kidney weight in males and
females in the highest-dose group, and spleen weight in females in the
200 and 1000 µg/kg b.w./day groups. In females, ovary weight was
increased in the highest-dose group and uterus weight was decreased in
the 100 and 200 µg/kg b.w./day groups. In males, decreases were seen
in seminal vesicle weight in the 100, 200, and 1000 µg/kg b.w./day
groups and in prostate weight in the 100 and 1000 µg/kg b.w./day
groups. Gross pathological examination revealed atrophy of the
prostate, seminal vesicles, and testes in rats in the highest-dose
group. Histologically, changes in the ovary (cysts and released
follicles in all test groups) and in the uterus ("dentelle uterine" in
the 200 and 1000 µg/kg b.w./day groups) were observed in females. In
males in the highest-dose group a delay in spermatogenesis and aplasia
in the seminal vesicles and prostate were observed (Seeger, 1971a, b).
Female rats fed diets containing 0.01, 0.1, 2.5, 5, 10, 20, 40,
80, or 160 ppm TBA exhibited increases in uterus weight at levels
higher than 40 ppm. Vaginal smears in all groups were negative; some
proliferation of the vaginal mucosa was seen at the highest dose level
(Huis in't Veld et al., 1973).
Female rats weighing 60 - 65 g each were ovariectomized and given
s.c. doses of 0, 0.2, 1.0, or 5.0 mg/day TBA in sesame oil for 4 days.
At termination, on day 5, a dose-related increase in uterus weight was
seen in all dosed groups (maximum +550%). The estrogenic activity of
TBA was less than 0.1% of the estrogenic activity of estradiol-17ß
(Schröder, 1971b).
Male castrated rats weighing 100 g each were given daily s.c.
doses of 4, 20, or 100 µg TBA, 4, 20, or 100 µg ß-TBOH, or 20, 100,
500, or 1000 µg alpha-TBOH for 9 days. Dosing was started 1 day after
castration. One control group was used. At sacrifice, one day after
the last injection, the weights of the prostate, musculo levator ani,
and seminal vesicles were recorded. In animals dosed with both TBA and
ß-TBOH, organ weights were increased in a dose-related manner in all
test groups. In the animals administered alpha-TBOH, organ weights
were increased at the three highest dose levels (Escuret & Bas, 1978).
Male castrated rats weighing 65 - 75 g each were given daily s.c.
doses of 0, 0.02, 0.1, or 0.5 mg TBA as a solution in sesame oil for
10 days after castration. At sacrifice on day 11, dose-related
increases were seen in the weights of muscolo levator ani (maximum
+250%), prostate (maximum +1400%), and seminal vesicles (maximum
+2500%) in all groups. In this experiment TBA showed distinct anabolic
and androgenic activity that was 5 times higher than that of
testosterone and 20 times higher than that of 17-ethynyl-19-nor-
testosterone (Schröder, 1971b).
Groups of 10 male and 10 female rats weighing 123 - 131 g each
were dosed 6 times per week s.c. with 0, 200, 1000, or 5000 µg/kg
b.w./day TBA over a period of 2 months. The compound was applied as a
solution in 1:1 syncortyl and arachis oil.
Growth of females was increased in all groups; by contrast,
growth of males in the highest-dose group was decreased. Haematology
and blood biochemistry determinations, which were performed at
termination in 5 rats/sex/group, revealed slight increases in Hb and
haematocrit values and slight leucocytopenia (due to lymphocytopenia)
in all test groups. Other blood abnormalities that were noted were a
decrease in glucose in females in the 5000 µg/kg b.w./day group (not
determined in other groups), a decrease in BUN in females in the 1000
and 5000 µg/kg b.w./day groups and in males in the 5000 µg/kg b.w./day
group, and a decrease in cholesterol in males and females in the 1000
and 5000 µg/kg b.w./day groups.
In all test groups, absolute and relative kidney weights were
increased and absolute and relative adrenal and thymus weights were
decreased. Ovary weights were decreased in a dose-related manner in
females in all test groups. In females in the 200 and 1000 µg/kg
b.w./day groups uterus weights were decreased. All treated females
showed an increase in absolute liver weight. Males showed a decrease
in testes weight in all treated groups and in seminal vesicle and
prostate weight in the 1000 and 5000 µg/kg b.w./day groups. Besides
these weight changes, atrophy was noted in the thymus, ovaries, and
testes; hypertrophy was seen in the seminal vesicles and prostate
(Sovetal, 1970; Seeger, 1971a).
Rabbits
Groups of 4 - 6 rabbits weighing 2 kg each were administered s.c.
during 4 days 0, 0.05, 0.5, 2, or 5 mg/kg b.w./day TBA. Liver function
was examined (SGOT activity and BSP excretion). At 2 mg/kg b.w./day
SGOT activity was increased slightly, while at 5 mg/kg b.w./day SGOT
activity was increased significantly. BSP excretion was not affected
in any of the test groups (Seeger, 1971a).
Pigs
Male, female, and castrated male pigs were fed diets containing
1-2 ppm TBA, alone or in combination with 2 ppm estradiol-17ß or 2 ppm
ethynylestradiol, for 5 - 8 weeks. At 5 and 6.5 weeks after compound
withdrawal TBOH was not detected in the urine. Urinary excretion of
total steroid estrogen was not increased at 7 weeks after dose
withdrawal (Kroes et al., 1976a).
Groups of 4 male and 4 female domestic pigs (Sus scrofa) were fed
diets containing 0, 0.1, 2, or 20 ppm TBA (equivalent to 0, 4, 80, or
800 µg/kg b.w./day TBA, respectively) for 14 weeks. No treatment-
related effects on mortality, body weight, or ophthalmoscopy were
seen. After 6 and 12 weeks several haematological (i.e., Hb, RBC, PCV,
WBC, diff. WBC, and prothrombin index) and blood biochemical
parameters (i.e., glucose, protein, albumin, albumin/globulin ratio,
SAP, calcium, and creatinine), were unaffected. At 2 and 20 ppm,
dose-related increases were seen in platelets (maximum +70%, not
significant), urea (maximum +3%, significant) and cholesterol (maximum
+100%, significant). SGOT increased in a dose-dependent manner in all
test groups (maximum, +100%, significant). There were dose-related
decreases in blood levels of testosterone and estradiol in males in
all dosed groups (both maximum -95%, significant); progesterone was
markedly decreased (maximum -99%, significant) in females in the two
highest-dose groups. In the same groups, there were dose-related
changes in the absolute and relative weights of the liver (maximum
+30%, significant), uterus (maximum -50%, significant), kidney
(maximum +25%, significant), and testis (maximum -55%, significant).
In the highest-dose group changes were observed in the weights of the
pituitary (-15%, significant) and seminal vesicles (+280%,
significant). There was an increase in thyroid weight at all three
dose levels (maximum +20%, not significant).
Histopathological examination showed the following dose-related
abnormalities in the 2 and 20 ppm groups: in the liver, enlargement of
the hepatocytes with associated ground glass appearance of the
cytoplast; in testes, moderate to complete interstitial cell atrophy
(with normal spermatogenesis within the seminiferous tubules); in
ovaries, evidence of suppressed or abnormal cyclic activity
characterized by the absence of maturing follicles and/or mature or
early regressing corpora lutea; and, in the uteri, absence of
glandular development in the endometrium. This last observation and
the lack of alveolar development and secretion noted in the mammary
glands of the 2 ppm group were probably associated with the anestrous
state suggested by the findings in the ovaries (Ross et al., 1980).
Cattle
Groups of 8 male calves were given s.c. implants of 140 mg TBA +
20 mg estradiol-17ß at 8 or 4 weeks before slaughter. An additional
group was dosed with 200 mg/kg b.w. testosterone + 20 mg/kg b.w.
estradiol-17ß on both occasions. In all treatment groups histological
examination of prostates showed increased secretory activity and
hyperplastic and metaplastic changes (Verbeke et al., 1975).
Six weeks after single s.c. application of 20 mg estradiol-17ß to
male calves, distinct changes in seminal vesicles were found after
histological examination. Treatment of calves with 20 mg estradiol-17ß
combined with 140 mg TBOH, applied s.c., caused very slight changes in
seminal vesicles in 4/11 calves, while in 3/7 animals with unchanged
seminal vesicles, androgenic stimulation was observed (Kroes, 1972).
Male calves that were 11 weeks of age received by s.c.
implantation 20 mg estradiol-17ß alone or 20 mg estradiol-17ß in
combination with 140 mg TBA. Urinary secretion of total steroid
estrogens was high in the animals given estradiol-17ß alone during the
first 12 days after application. After 3 weeks normal values were
reached. In the estradiol-17ß/TBA combination group, gradual and
prolonged excretion of steroid estrogens for up to 42 days after
implantation occurred; after 56 days normal values were reached.
Qualitative determinations of estradiol-17ß and estradiol-17ß in urine
showed that the alpha epimer was present in almost all urine samples;
estradiol-17ß was found in the urine of those calves administered
estradiol-17ß alone. In the combination group, estradiol-17ß was found
in urine at day 21 only. Histological examination of prostates
revealed squamous metaplasia of gland epithelium after both treatments
(Kroes et al., 1976b).
A total of 1480 female calves, aged 7 weeks, were administered by
percutaneous implantation 0, 140, or 3500 mg TBA (groups 1 - 3,
respectively) or 140 mg TBA + 20 mg estradiol-17ß (group 4), 1400 mg
TBA + 200 mg estradiol-17ß (group 5), or 3500 mg TBA + 500 mg
estradiol-17ß (group 6). The calves were slaughtered 10 weeks after
implantation.
Blood parameters (glucose, GOT, GPT, AP, LDH, cholesterol,
bilirubin, Hb, and PCV), urinary density, and pH were unaffected in
all groups. Calcium and phosphorus levels in serum and bone were not
changed; however, magnesium levels in serum and deposition of
magnesium in bone were decreased in groups 3, 5 and 6. In group 3
slight, and in groups 4, 5 and 6 marked, increases in uterus weight
were seen, which were accompanied by proliferation of uterine
glandular cells, while the lumen of the uterus was partially filled
with a watery liquid. In dosed groups ovarian weights were reduced,
accompanied by a diminution in follicular size. These weight changes
were most marked in groups 2, 3 and 6. The diminution of follicular
size, which was accompanied by a decrease in the number of follicles,
was most marked in groups 5 and 6. In all groups, a dose-related
decrease in thymus weight was seen. In group 3 abnormal development of
the clitoris was noted. Histopathological examination showed a
non-dose related proliferation and secretion of glandular tissue in
the mammary glands of animals in groups 4, 5 and 6. Heart, lever,
kidneys, pituitary, pineal gland, adrenal glands, thyroid, and
skeletal muscle did not show abnormalities (Gropp et al., 1975).
Steers and heifers were seven 300 mg TBA by s.c. implantation. An
additional group of steers was given implants containing 140 mg TBA +
20 mg estradiol-17ß. In all groups, plasma urea was decreased during
the 9-week observation period after treatment. Other plasma parameters
(glucose, calcium, phosphorus, magnesium, sodium, potassium, and total
protein) were unaffected. No changes in plasma concentrations of
insulin or growth hormone were seen. A decrease in thyroxine levels in
steers was observed during the 9-week observation period after
treatment; the decrease was most marked in those administered TBA and
estradiol-17ß in combination. A marked reduction, about -50%, in
thymus weight was observed in steers (Heitzman, 1975).
Steers and oxen given, by s.c. implantation, 140 mg TBA alone or
140 mg TBA in combination with 20 mg estradiol-17ß. Control animals
received implantations with the carrier. The combination of drugs
affected urinary excretion of exogenous estradiol-17ß in oxen and
possibly in steers. Histological examination of the prostate revealed
squamous metaplasia in the combination group. In both treated groups,
more active epithelium of the prostate was seen, compared to the
control group (Kroes et al., 1976c).
Long-term studies
Mice
Groups of 64 male and 64 female Swiss albino CFLP mice, weighing
22 - 25 g each, were given diets containing 0, 0.5, 1.0, 10 or 100 ppm
TBA (equal to 0, 0.004, 0.09, 0.86, or 8.6 mg/kg b.w./day TBA for
males, respectively, and 0, 0.005, 0.10, 0.96, or 9.5 mg/kg b.w./day
TBA for females, respectively) for 95 - 104 weeks (the test was ended
when survival was 20% in males or females in the control group). After
13 weeks 12 mice/sex were killed. At that time significant increases
were observed in the absolute and relative weights of the kidneys in
males and females at 100 ppm (20 - 40% increases). Significant
decreases were seen in the weights of the spleen of top-dose females
(-20%) and significant increases were seen at 1.0, 10, and 100 ppm in
males (+25%). Dose-related relative decreases in weights of the uterus
were observed in all dosed females (maximum -25%). At interim
sacrifice, inhibition of ovulation, characterized by a lack of corpora
lutea in all females in the highest-dose group, was observed.
Follicular development proceeded to the mature follicle stage. The
uteri of all females in the 100 ppm group were consistent with the
diestrous stage of the cycle. In the spleens of 6/12 males in the
100 ppm group an increased number of polymor-phonuclear leucocytes in
the red pulp was seen (0/12 in controls); congested sinuses were noted
in 2/12 males of this group (0/12 in controls).
At terminal sacrifice no organ weights were recorded. Terminal
gross- and histopathological examination showed an increase in liver
nodular hyperplasia and dose-related tumours in the male dose groups;
these increases were statistically significant at the two highest
doses. The incidence of liver tumours was also increased in females in
the highest-dose group (8/52 versus 4/51 in controls). There was an
increase in incidence of hepatocyte vacuolation in males in the
100 ppm group. In 100 ppm females, gross pathological examination
showed an increase in the incidence of enlarged and swollen kidneys,
accompanied by a marginal increase in the incidence of nephritis. In
the same group, increases in ovarian cysts (in 10/20 animals versus
1/11 in controls) and enlarged, abscessed, or cystic preputial glands
(in 4/20 females versus 0/11 controls) were seen. The spleens of 4/20
females in the 100 ppm group appeared small (Hunter et al., 1981).
Rats
Six groups of 65 male and 65 female Sprague-Dawley CFY rats
weighing 150 - 200 g each were given diets containing 0, 0.5, 1.0,
4.0, 16, or 50 ppm TBA (equal to 0, 0.02, 0.04, 0.14, 0.56, or
1.80 mg/kg b.w./day TBA for males, respectively, end 0, 0.02, 0.04,
0.16, 0.64, or 1.92 mg/kg b.w./day for females, respectively) for 112
weeks. The parents of the test animals had been dosed at the same
levels from week 9 before mating until day 21 post-partum (except for
the 50 ppm group in which only the dams had been dosed from day 0 of
pregnancy until day 21 post-partum).
The females in the 4.0, 16, and 50 ppm groups showed prominent
pudendum. A dose-related incidence of pendulous anogenital skin was
seen in all female dose groups (maximum incidence, 85%). In males a
dose-related reduction in testes size was seen in all dose groups,
except at 0.5 ppm (maximum incidence, 45%). Growth and food
consumption were decreased throughout the study in males administered
50 ppm TBA (-15 and -10%, respectively). Water consumption was
decreased in the same group up to week 51 (-15%). Urinalysis (9
parameters measured in 5 rats/sex of the 0, 16, and 50 ppm groups on 6
occasions throughout the study) showed no treatment-related changes.
Haematology (10 parameters measured in 10 rats/sex of the 0, 16,
and 50 ppm groups on 6 occasions throughout the study) showed dose-
related slight increases in some parameters in females in the 16 and
50 ppm groups. Male haematology values were generally unaffected by
treatment. Blood biochemistry (13 parameters) showed no compound-
related changes.
At interim sacrifice at week 78, 13 - 14 rats/sex/group were
killed. Macroscopic examination revealed a prominent clitoris in 5/14
females fed 16 ppm TBA and in 13/13 females fed 50 ppm TBA. The males
of the 50 ppm group showed atrophy of the testes, seminal vesicles,
and prostate. Interim organ weighings revealed, in the males fed 16
and 50 ppm TBA, dose-related decreases in absolute testes and prostate
weights. Adrenals and pituitary weights were decreased in both sexes
in the 50 ppm group. Weights of the ovaries were increased in all dose
groups, but without dose relation.
At the end of the experiment, similar changes in the absolute
weights of the testes, prostate, and adrenals were found. In the males
of the highest-dose group, decreases were observed in the weights of
the pituitary (-30%), thyroid (-20%), kidney (-30%), spleen (-30%),
and liver (-20%). The weight of the ovaries was markedly decreased in
the highest-dose females (-60%).
Terminal gross- and histopathological evaluation revealed a
slightly increased incidence of foci or areas of ground-glass
hepatocytes in the livers of the highest-dose females. Urinary bladder
calculi and associated epithelial hyperplasia joined by cystitis and
pyelitis were seen in females in the same dose group. Both the male
and female reproductive tracts were affected. Testicular, prostatic,
and vesicular atrophic changes were seen in males in the 16 and 50 ppm
groups. Absence of corpora lutea, vaginal inflammation and
mucification, uterine endometritis, dilatation, decreased endometrial
thickness, and the development of clitoral bone were seen in the
50 ppm females. Clitoral enlargement was seen in females in the two
highest-dose groups. Neoplastic histopathology showed an increase in
the incidence of pancreatic islet cell tumours in the 50 ppm group
(Hunter et al., 1982).
Observations in human beings
Male and female human volunteers were given i.m. doses of 5 or
10 mg TBA every-other-day during 14 days. In the subjects administered
5 mg TBA, nitrogen balance was disturbed, including retention of
nitrogen. In the 10 mg group, some women showed disturbances of the
menstruation cycle. The same dose caused a slight but significant
reduction of excretion of 17-ketosteroids. No effects on
17-hydroxy-corticosteroid excretion were seen. Blood parameters
(protein, cholesterol, coagulation factors, and prothrombin and
thrombin time) were unaffected by treatment (Krüskemper et al.,
1967).
COMMENTS
TBA is a synthetic steroid with anabolic properties. At the
17-position in the molecule two epimers, alpha and ß, are possible.
The ß-epimer of TBA is the commercial product. It is administered to
cattle either alone or in combination with estradiol-17ß or zeranol as
a subcutaneous implant in the ear to improve body weight, feed
conversion, and nitrogen retention. It is usually implanted 60 - 90
days before the intended date of slaughter.
After administration to cattle, TBA is rapidly hydrolyzed to
TBOH, the major metabolite being alpha-TBOH, occurring in the excreta,
bile, and liver. In muscle most of the TBOH is present as ß-TBOH.
Experiments with implantation of 200 mg of radiolabelled TBA in calves
and heifers showed that maximum levels of residues occurred about 30
days after implantation. The highest mean concentration of residues as
TBOH equivalents was 50 µg/kg in liver, while muscle contained
3 µg/kg.
The results of the studies requested at the twenty-seventh
meeting were submitted for consideration by the present Committee. In
addition, new toxicological data were available on reproduction and
mutagenicity. The Committee also reviewed previously available data
including metabolic, teratogenicity, and carcinogenicity data.
Acute toxicity studies in several species showed TBA to be of low
toxicity, when given orally.
Experiments had been performed in rats to assess the effect of
TBA on pregnancy, on the reproductive function of multiple
generations, and on the development of the offspring to weaning.
Treatment at 0.3 and 0.5 ppm in the diet, equivalent to about
20-30 µg/kg b.w./day, was associated with mean weekly body weights for
female rats that were only slightly higher than those of controls, and
marginal differences in litter parameters, while treatment with TBA at
3.0 and 18.0 ppm in the diet was associated with a hormonal effect. It
was considered that TBA exerted no effect on reproductive performance
in the rat at 0.5 ppm in the diet, equivalent to 30 µg/kg b.w./day. No
teratogenic effect was seen in two feeding studies in rats at very
high doses of TBA. In a comprehensive range of in vivo and in vitro
mutagenicity studies, all tests were negative for TBA, ß-TBOH, and
TBA's major metabolite, alpha-TBOH, with the exception of the mutation
assay in mouse lymphoma cells, which gave equivocal results with
ß-TBOH and alpha-TBOH. The Committee also noted a report of an
equivocal result in a transformation study with ß-TBOH in Syrian
hamster embryo fibroblasts, and took into account the recognized
difficulty in interpreting results from this type of study.
The Committee reaffirmed the opinion expressed at its
twenty-seventh meeting regarding the results of long-term feeding
studies with TBA with rats and mice (Annex 1, reference 62). It
considered that the liver hyperplasia and tumours in mice fed high
doses of TBA (0.9 - 9 mg/kg b.w./day) and the slight increase in the
incidence of islet-cell tumours of the pancreas of rats fed TBA at
1.85 mg/kg b.w./day (the highest dose in the study) arose as a
consequence of the hormonal activity of TBOH.
The Committee therefore concluded that its safety assessment
could be based on establishing the no-hormonal-effect level. It
reviewed a study with castrated male rhesus macaque monkeys
administered ß-TBOH orally, and considered that this model could be
relevant to the human population. The castrated male rhesus monkey is
highly sensitive to compounds with antigonadotropic activity; the
Committee therefore adopted a conservative approach by using this
study as the basis for establishing an ADI for human beings. Despite
the small numbers in each group of monkeys studied, and the advanced
age of the animals used, the Committee set 2 µg/kg b.w./day as a
no-hormonal-effect level, based on assessment of histological changes
in the seminal vesicles. In the intact female rhesus monkey, TBA had a
clear no-hormonal-effect level of 10 µg/kg b.w./day. The Committee
also considered that the pig was a sensitive model for assessing
hormonal effects and noted that, here too, TBA had a no-hormonal-
effect level of 2 µg/kg b.w./day based on assessment of pathological
changes in the testes. Another study in the pig demonstrated that the
hormonal activity of ß-TBOH was about ten times that of alpha-TBOH. No
data on individual animals were available for any of the pig studies.
In the absence of satisfactory toxicological data the Committee
was unable to establish a separate no-effect level for the alpha-TBOH
metabolite. It also noted that this metabolite was not produced in
significant amounts in the rat, which made it inadvisable to
extrapolate from data generated from ß-epimer experiments in that
species.
EVALUATION
Level causing no hormonal effect
Pig: 0.1 ppm in the diet, equal to 2 µg/kg b.w./day.
Monkey: 2 µg/kg b.w./day.
Estimate of temporary acceptable daily intake
0 - 0.01 µg/kg b.w.
Further work or information
Required (by 1990):
( a) the final reports, with supporting data, for the tissue residue
studies in which TBA was administered to heifers and TBA in
combination with estradiol-17ß was administered to steers;
( b) histopathological data on individual animals from the three
hormonal studies in pigs that were reviewed by the Committee;
( c) results from a 90-day study in an appropriate species, with
orally administered alpha-TBOH.
REFERENCES
Allen, J.A. & Proudlock, R.J. (1983). Autoradiographic assessment of
DNA repair in mammalian cells after exposure to 17-alpha-hydroxy-
trenbolone and to 17-ß-hydroxytrenbolone. Unpublished report
No. RSL 619/83731 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Brooker, P.C., & McCaffrey, K.J. (1985). Analysis of
metaphase chromosomes obtained from CHO cells cultured in vitro and
treated with 17-ß-hydroxytrenbolone. Unpublished report No. RSL
676/85631 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Proudlock, R.J., & Pugh, L.C. (1986). Micronucleus test
on 17-ß-hydroxytrenbolone. Unpublished report No. RSL 728/861306 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Allen, J.A. & Proudlock, R.J. (1987). Assessment of 17-alpha- and
17-ß-hydroxytrenbolone in the Solt-Farber system for induction of
liver foci. Unpublished report No. RSL 711/861252 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981a). R 1697 (trenbolone
acetate). Single administration study by oral route in the mouse.
Unpublished report No. 81213-A-JL/36-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981b). R 1697 (trenbolone
acetate). Single administration study by oral route in the rat.
Unpublished report No. 81212-A-JL/37-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, g., & Glomot, R. (1981c). R 1697 (trenbolone
acetate). Single administration study by oral route in the dog.
Unpublished report No. 81216 from Roussel Uclaf Research Centre.
Submitted to WHO by Roussel Uclaf, Paris, France.
Barraud, B., Lugnier, A., & Dirheimer, G. (1983). In vivo covalent
binding to rat liver DNA of trenbolone as compared to 17-ß-estradiol,
testosterone and zeranol. In: Anabolics in animal production,
International Office of Epizootics, Paris, pp. 193-206.
Bouffault, J.C. (1977). Expert statement concerning the excretion of
trenbolone and oestradiol-17-alpha in urine of calves after
subcutaneous implantation of trenbolone acetate and oestradiol 17-ß.
Unpublished report submitted to WHO by Roussel Uclaf, Paris, France.
Chasseaud, L.F., Hawkins, D.R., Elsom, L.F., Parsonage, M.T.,
Ross, D.B., & Roberts, N.L. (1976). Plasma and tissue concentrations
of radioactivity in cattle after implantation of pellets containing
3H-trenboloneacetete, 3H-oestradiol or combinations of radioactive
drugs with the non-radioactive form of the other. Unpublished report
No. SVT/11/76351 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Edgar, D.H., Bosworth, H.J., Ransome, S.J., & Banks, S.J. (1985). An
assessment of the mutagenic potential of 17-ß-hydroxytrenbolone in
mammalian cells in vitro using the Chinese hamster ovary/HGPRT locus
assay. Unpublished report No. RSL 677/85642 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Escuret, P. & Bas M.N. (1978). DL-50 chez le rat et chez le souris.
Unpublished report Recherches Veterinaires R.U.-essai 5.77.71 R.
Submitted to WHO by Roussel Uclaf, Paris, France.
Gropp, J., Herlyn, D., Boehncke, E., Schulz, V., Sanderleben, J.V.,
Hanichen, Th., & Geisel, O. (1975). Physiological data including
evaluation of immunoresponse in relation to anabolic effects on veal
calves. Published in "Anabolic agents in animal production",
FAO/WHO Symposium, pp. 131-141, March 1975.
Gropp, J., Buntenkotter, S., & Kaemmerer, K. (1978). Relay toxicity of
anabolic agents, general considerations and some experimental data.
Published at Relay Toxicity and Bioavailability Symposium,
5 October 1978. Report submitted to WHO by Roussel Uclaf,
Paris, France.
Hawkins, D.R., Weston, K.T., Ray, J., Ross, D.B., & Cameron, D.M.
(1979). The bioavailability of trenboloneacetate residues in the
edible tissues of heifers after oral administration to rats.
Unpublished report No. RSL/328/7959 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hawkins, D.R., Waller, A.R., Moore, D.H., Jordan, M.C., Roberts, N.L.,
& Cameron, D. (1984). Tissue residues of radioactivity at 15 and 30
days after implantation of 3H-trenboloneacetate in calves.
Unpublished report No. HRC/RSL/636 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Heitzman, R.J. (1975). The effectiveness of anabolic agents in
increasing rate of growth in farm animals; report on experiments in
cattle. Published in "Anabolic agents in animal production", FAO/WHO
Symposium, pp. 89-98, March 1975.
Heitzman, R.J. & Hardwood, D.J. (1977). Residue levels of trenbolone
and oestradiol-17-ß in plasma and tissues of steers implanted with
anabolic steroid preparations, Br. Vet. J., 133, 564.
Henderson, L.M., Bosworth, H.J., Ransome, S.J., Banks, S.J., & Ozel,
K.J. (1986a). An assessment of the mutagenic potential of 17-alpha-
hydroxytrenbolone in mammalian cells in vitro using the Chinese
hamster ovary/HPRT locus assay. Unpublished report No. RSL 713/86713
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Henderson, L.M., Brooker, P.C., & Howell, A. (1986b). An assessment of
the mutagenic potential of 17-ß-hydroxytrenbolone in mammalian cells
in vitro using a Chinese hamster ovary micronucleus test.
Unpublished report No. RSL 729/861287 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., & Bosworth, H.J. (1987a).
An assessment of the carcinogenic potential of 17-ß-hydroxytrenbolone
and testosterone in an in vitro cell transformation assay using
mouse C3H10T1/2 cells. Unpublished report No. RSL 691/861257 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., Bosworth, H.J.,
Bottoms, M., & Williams, J. (1987b). An assessment of the mutagenic
potential of 17-ß-hydroxytrenbolone in mammalian cells in vitro
using the Chinese hamster V79/HPRT locus assay. Unpublished report
No. RSL 712/861249 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hess, D.L. (1983). Determination of a hormonal no-effect dose level
for 17-ß-trenbolone Rhesus Macaque. Unpublished report from Oregon
Regional Primate Research Center, Beaverton, OR, USA. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hess, D.L. (1984). Determination of the hormonal no-effect dose level
for trenbolone acetate in the female Rhesus Macaque. Unpublished
report from Oregon Regional Primate Research Center, Beaverton, OR,
USA. Submitted to WHO by Roussel Uclaf, Paris, France.
Hoffman, B., Schopper, D., & Karg, H. (1984). Investigations on the
occurrence of non-extractable residues of trenbolone acetate in cattle
tissues in respect to their bioavailability and immunological
reactivity. Food Additives and Contaminants., 1, p. 253.
Hossack, D.J.N., Richold, M., & Jones, E. (1978). Ames metabolic
activation test to assess the potential mutagenic effect of trenbolone
acetate, 17-ß-oestradiol and 7:1 mixture of trenbolone with
17-ß-oestradiol. Unpublished report No. RSL 351/781136 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Huis in't Veld, L.G., Have, J. ten, & Kok, G.L. (1973). Hormonale
werkingen van trenbolone-acetaat. Unpublished report No. U 146/73 ENDO
from the National Institute of Public Health, The Netherlands.
Hunter, B., Macrae, S., Prentice, D.E., & Brooks, P.N. (1976a).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 235/76316
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Macrae, S., Prentice, D.E., & Majedd, S.K. (1976b).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 213/75992
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Bathman, P., Heywood, R., Street, A.E., Prentice, D.E., &
Majeed, S.K. (1976c). Trenboloneacetate oral toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RSL
237/76426 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Graham, C., Gibson, W.A., Read, R., Gregson, R., Heywood
R., Offer, J., Prentice, D., & Woodhouse, R. (1981). Long-term feeding
of trenboloneacetate to mice (final report). Unpublished report volume
I, II and III, No. RSL/267/80122 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Batham, P., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D., Gregson, R., & Offer, J. (1982). Trenboloneacetate
potential tumorigenic and toxic effects in prolonged administration to
rats following "in utero" exposure (final report). Unpublished report
volume I, II, III and IV, No. RSL 295-G/80974 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ingerowski, G.H., Scheutwinkel-Reich, M., & Stan, H.J. (1981).
Mutagenicity studies on veterinary anabolic drugs with the
Salmonella/microsome test. Mutagen. Res., 91, 93-98.
James, P.A., Cox, R., Clark, R., Palmer, A.K., & Offer, J. (1982).
Effect of trenboloneacetate on pregnancy of the rat. Unpublished
report No. RSL 458/81553 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., John, D.M., Gibson, W.A., Offer, J.M., &
Anderson, A. (1985). Effect of trenbolone acetate on reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/841004 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., & Parker, C.A. (1986). Supplementary study of
the effect of trenbolone acetate on pregnancy of the rat and
development of the offspring. Unpublished report No. RSL 695/851572
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Kroes, R. (1972). De invloed van oestradiol en een combinatie van
oestradiol en trienbolone op de vesiculae semenalis van mestkalveren.
Unpublished report No. 162/72 Path. from the National Institute of
Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976a). Onderzoek
naar de invloed van ethynylestradiol, 17-alpha-oestradiol en
trenboloneacetaat alleen of in combinatie op beren, borgen en geiten.
Uitscheiding in urine en faeces en residuen in serum (ILOB proeven
98.06 98.07, 98.08, 98.09, 118.04 en 118.05). Unpublished report
No. 216/76 Path. from the National Institute of Public Health,
The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976b). Onderzoek
naar de invloed van combinatie-preparaten van mannelijke en
vrouwelijke hormonen of hormonaal werkende stoffen of mannelijke
mestkalveren (Een biologisch, chemisch en histologish onderzoek, ILOB
proef 25.14 P 72/82). Unpublished report No. 193/76 Path. from the
National Institute of Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., Both-Miedema, R., & van de Berg, R.
(1976c). Onderzoek naar de invloed van 17-ß-oestradiol, trenbolone-
acetaat en testosteron alleen of in combinatie, op stieren en ossen
(Uitscheiding in urine, aanwezigheid in serum en histologisch
onderzoek prostaat, ILOB-proeven 134-02 a, 134.02 b en 134.03).
Unpublished report No. 218/76 Path. from the National Institute of
Public Health, The Netherlands.
Kruskemper, H.L., Morgner, K.D., & Noell, G. (1967). Klinische
pharmakologie von Trienolonen, eine Gruppe neuartiger anabol wirksamer
Oestran-Derivate, Arzneim. Forsch., 17, 449-454.
Lutz, W.K (1979). In vivo covalent binding of organic chemicals to
DNA as a quantitative indicator in the process of chemical
carcinogenesis. Mutation Res., 65, 289-356.
Offer, J.M. (1985). Effect of trenbolone acetate on the reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/85959 (Addendum to RSL 634/841004) from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Philibert, D. & Moguilewsky, M. (1983). Liaison de la testosterone de
la 17-ß-OH-trenbolone (RU 2341) et de son isomere, la 17-alpha-
OH-trenbolone (RU 27423) au niveau du plasma humain. Unpublished
report No. AN 39 from Roussel Uclaf Research Centre. Submitted to WHO
by Roussel Uclaf, Paris, France.
Pottier, J. Busigny, M., & Gradadam, J.A. (1973). Tissue residues in
cows after implantation of trenbolone acetate. J. Anim. Sci.,
37, 256 (Abstract No. 110F).
Pottier, J., Busigny, M., & Gradaclam, J.A. (1975). Plasma kinetics,
excretion in milk and tissue levels in the cow following implantation
of trenbolone acetate. J. Anim. Sci., 41, 968.
Pottier, J., Cousty, C., Heitzman, R.J., & Reynolds, I.P. (1978).
Metabolites trenbolone acetate R in the bile of the cow and the rat.
Report presented during the 1978 Joint Meeting of the American Dairy
Association and the American Society of Animal Science, Michigan State
University, East Lansing, 9-13 June. Submitted to WHO by Roussel
Uclaf, Paris, France.
Pottier, J. (1979). Trenboloneacetate tissue and biliary
concentrations of the position of 17 epimers of trenbolone in heifers
after 2 months of implantation. Unpublished report No. 799 from
Roussel Uclaf. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. & Richardson, J.C. (1982). Metaphase analysis on
17-ß-hydroxytrenbolone and 17-alpha-hydroxytrenbolone. Unpublished
report No. RSL/512/81962 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M., Jones, E., & Hales, J.F. (1982a). Ames metabolic
activation test to assess the potential mutagenic effect of
17-alpha-hydroxytrenbolone, 17-ß-hydroxytrenbolone and
17-alpha-oestradiol. Unpublished report No. RSL 567/82767 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Richold, M., Richardson, J.C., Proudlock, R.J., & Fleming, P.M.
(1982b). Effect of 17-ß-hydroxytrenbolone and 17-alpha-hydroxy-
trenbolone on cultured human lymphocytes. Unpublished report No. RSL
544/82136 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. Dehnel, J.M., & Ransome, S. (1982c). An assessment of the
mutagenic potential of 4 compounds using an in vitro mammalian cell
test system. Unpublished report No. RSL 513/8286 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Richold, M., Edgar, D.H., Ransome, S.J., & Banks, S.J. (1983). An
assessment of the mutagenic potential of four compounds using an
in vitro mammalian cell test system. Unpublished report from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Riis, P.M. & Suresh, T.P. (1976). The effect of a synthetic steroid
(trenbolone) on the rate of release and excretion of subcutaneously
administered estradiol in calves. Steroids, 27, 5-15.
Roberts, N.L. & Cameron D.M. (1985). Hormonal effects of orally
administered trenbolone. Findings from three studies in the domestic
pig. Unpublished report No. RSL 591/83499 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ross, D.B. (1980). Toxicology and residues of trenboloneacetate.
Published in: Conference on the Use, Residues and Toxicology of Growth
Promoters, Dublin, 9 April 1980, pp. 34-48.
Ross, D.B., Roberts, N.L., Cameron, D.M., Street, A.E., Crook, D.,
Prentice, D.E., & Cherry, C.P. (1980). Oral toxicity of trenbolone
acetate to growing pigs (Sus scrofa)- preliminary 14 weeks feeding
study. Unpublished report No. RSL 357/80152 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ryan, J.J. & Hoffman, B. (1978). Trenbolone acetate: experiences with
residues in cattle tissues. J.A.O.A.C., 61, 1274-1279.
Schiffman, D., Metzler, M., Neudecker, T., & Henschler, D. (1985).
Morphological transformation of Syrian hamster embryo fibroblasts by
the anabolic agent trenbolone. Arch. Toxicol., 58, 59-63.
Schroder, R. (1971a). R 1697: Prüfung der androgenen und
muskelbildenden Wirkung bei oraler Verabreichung. Unpublished report
from Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Schroder, R. (1971b). R 1697: Untersuchung auf androgene und anabole
Wirkung, Untersuchung auf oestrogene Wirkung. Unpublished report from
Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Seeger, K. (1971a). Anabolikum R 1697 (A 50273) as us vet.
Unpublished report from Hoechst A.G. Submitted to WHO by Roussel
Uclaf, Paris, France.
Seeger, K. (1971b). R 1967: Chronische Toxizitat per oral. Unpublished
report from Hoechst A.G. Submitted to WHO by Roussel Uclaf,
Paris, France.
Sovetal (1970). Toxicité cronique par voie sous-cutanée chez le rat
male et chez la femelle. Unpublished report No. R 1697 from Sovetal.
Submitted to WHO by Roussel Uclaf, Paris, France.
Stephany, R.W., Bosch, D., van den Sahertian, E.Th., & Kroes, R
(1976). Onderzoek naar de invloed van orale toediening van
trenboloneacetaat op vaarzen (ILOB-proef 134-01) (Eenchemisch
onderzoek). Unpublished report No. 215/76 Path. From the National
Institute of Public Health, The Netherlands.
Verbeke, R., Debackere M., Hicquet, R., Lauwers, H., Stevens, J.,
Pottier, G., Moer, D., Van Hoof, J., & Vermeersch, G. (1975). Quality
of the meat after the application of anabolic agents in young calves.
Published in: Anabolic Agents in Animal Production, FAO/WHO Symposium,
March 1975, pp. 123-130.
van de Wal, P. (1975). General aspects of the effectiveness of
anabolic agents in increasing protein production in farm animals, in
particular in bull calves. Published in: Anabolic Agents in Animal
Production, FAO/WHO Symposium, March 1975, pp. 60-78.
In two barren cows after s.c. implantation of 300 mg 3H-TBA per
animal at the base of the ear, slow resorption from the implant
occurred; the half-life of disappearance from the implant was 68 - 84
days. About 33% of the radioactivity was extracted in the blood plasma
over the 3-month period after implantation, 70% of which was accounted
for by TBOH. The main routes of excretion were via the bile and urine.
Tissue levels after 3 months were about 1 ppb, except in the liver
(6.5 ppb) and kidneys (4.5 ppb). Twenty-five percent of the tissue
radioactivity was extractable, 40% of which was TBOH. In the liver and
kidneys, however, only 10% was extractable, while in perirenal fat up
to 88% of the radioactivity was extractable. In perirenal fat 50% of
the radioactivity was TBA. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity (Pottier et al., 1973;
Pottier et al., 1975).
Slow resorption from s.c. implants of 300 mg 3H-TBA occurred in
2 lactating cows. The half-life for disappearance from the implant was
approximately 60 days. About 17% of the radioactivity present in the
blood plasma over the period of 5 months after implantation was
extractable. Less than 1% of the radioactivity was excreted in milk.
Ten percent of the milk radioactivity was extractable and 25% of this
extractable radioactivity was TBOH. Tissue levels after 5 months were
about 1 ppb, except in the liver (3.4 ppb) and kidneys (2.7 ppb).
About 25% of the tissue radioactivity was extractable, except in the
liver and kidneys (both 10%); about 40% of this extractable
radioactivity was TBOH. In contrast, 88% of total radioactivity in
perirenal fat was extractable, of which 50% was TBA. Unchanged TBA was
found in no other tissues. Radioactivity levels in the implantation
zone were 8 - 21% of the implanted quantity after 5 months (Pottier
et al., 1973; Pottier et al., 1975).
Two steers were given by single s.c. implantations 300 mg
3H-TBA in combination with 40 mg estradiol; the implants were
removed 60 days later, at which time 28% of the radioactivity remained
in them. Ethyl acetate-extractable radioactivity in blood plasma was
primarily ascribed to TBOH; in most cases no TBA was found in the
plasma. One animal was killed immediately after removal of the
implant. Plasma concentrations in this animal declined with half-lives
of 26 days for both total and non-volatile radioactivity; ethyl
acetate-extractable radioactivity in the plasma of this animal ranged
between 3 - 5% of the total radioactivity. In the other animal, which
was killed 16 days after removal of the implant, plasma concentrations
declined during days 1 - 60, with half-lives of 50 and 55 days for
total and non-volatile radioactivity, respectively. In the 16 days
from implant removal to sacrifice, radioactivity decreased by 46% in
muscle, 2% in liver and kidneys, and 29% in fat (Chasseaud et al.,
1976).
Relay bioavailability
Groups of 3 rats were fed freeze-dried or ethyl acetate-extracted
liver, kidney, or muscle obtained from two heifers killed 60 days
after s.c. implantation with 300 mg 3H-TBA. 3H-TBA levels in the
heifers averaged 30 ng equivalents/g in the liver, 24 ng equivalents/g
in the kidneys, and 3.2 ng equivalents/g in muscle. Radioactivity
excretion during the 3 days after feeding these tissues to rats is
presented in Table 1 (Hawkins et al., 1979).
Groups of 3 bile duct-cannulated rats that had been fasted for 24
hours were fed during 1 hour freeze dried liver, kidney, or muscle
from the two heifers described in the previous paragraph.
Radioactivity disposition during 48 hours after feeding of these
tissues is presented in Table 2 (Hawkins et al., 1979).
Table 1. Excretion of radioactivity by rats after being fed tissues
from heifers implanted with 3H-TBA
Excretion in percent of
administered radioactivity
Treatment Tissue Urine Faeces Total
Freeze-dried tissue Liver 3 81 84
Kidney 2 93 94
Muscle 6 85 91
Extracted tissue Liver 5 78 83
Kidney 2 103 105
Muscle 2 73 75
Table 2. Excretion of radioactivity by bile duct-cannulated rats
after feeding of tissue from heifers implanted with 3H-TBA
Excretion in percent of administered radioactivity
Tissue Bile Urine Faeces GI tract + contents Total
Liver 7 5 59 2 74
Kidney 3 1 31 60 95
Muscle 3 2 56 not detected 61
Effects on protein binding
The affinity of alpha-TBOH and ß-TBOH for corticosteroid binding
globulin, measured in vitro using the human plasma of elderly women,
was very low, less than 0.1% bound, compared with 10% for testos-
terone. The affinity of alpha-TBOH and ß-TBOH for testosterone and
estradiol binding globulin was 1% of that measured for testosterone.
When alpha-3H-TBOH was incubated in vitro with female human plasma,
it readily bound to the albumin fraction; only 4% was present
as free TBOH. The total blood clearance of ß-TBOH was twice that of
testosterone (Philibert & Moguilewsky, 1983).
Effects on estradiol-17ß excretion and nitrogen retention
Cattle
Plasma residues of estradiol-17ß in cattle were affected by the
presence of TBA in the s.c. implant. Plasma levels of estradiol-17ß
remained greater than 0.05 ppb for nine weeks in steers after
treatment with 200 mg TBA in combination with 40 mg estradiol-17ß,
whereas the residual levels decreased below 0.05 ppb within 5 weeks
after implantation of 40 mg estradiol-17ß alone (Heitzman & Hardwood,
1977).
Implantation of 40 mg TBA in the dewlap of Friesian bulls
(11 - 16 weeks of age) did not affect nitrogen retention. Implantation
of 140 mg TBA in combination with 20 mg estradiol-17ß at the same
site, however, resulted in a 47% decrease in nitrogen retention
(van der Wal, 1975).
Pigs
Pigs (males, females, and castrated males) were given s.c.
implantations with either 20 mg estradiol-17ß or 20 mg estradiol-17ß
in combination with 140 mg TBA. At 5 weeks after implantation, steroid
estrogens were hardly detectable in the faeces, and serum values for
estradiol-17ß were very low in both groups. Urine estradiol-17ß levels
were 6 - 82 µg/l in the estradiol-17ß group and 16 - 135 µg/l in the
combination group (Kroes et al., 1976a).
Toxicological studies
Special studies on carcinogenicity potential
Rats
Male Wistar rats (number not specified) were injected i.p.
with 15 µg/kg b.w. 3H-estradiol-17ß (53.6 Ci/mmole), 19 µg/kg
b.w. 3H-testosterone (54.0 Ci/mmole), 17 µg/kg b.w. 3H-TBA,
(57.0 Ci/mmole), or 30 µg/kg b.w. 3H-zeranol (50.0 Ci/mmole), all in
95% ethanol solution. The animals were sacrificed 16 hours after
injection and the Covalent Binding Indices (CBI, Lutz, 1979) of the
chemicals to DNA in the liver were quantitated. The CBIs were 11.4,
4.80, 5.62, and 1.65 for estradiol-17ß, testosterone, TBA, and
zeranol, respectively (weak carcinogens have a CBI approx. or equal
10, Lutz, 1979). The positive control, N-hydroxy-acetylaminofluorene,
had a CBI value of 262 (Barraud et al., 1983).
The CBI of TBA as a function of time was measured by
administering 0.83 mCi (22 - 40 µg/kg b.w.) 3H-TBA i.p. to 8 male
rats. The animals were killed at 4, 8, 12, 20, 24, 36, 48, and 96
hours. The highest CBI, 7.82 was obtained after 24 hours; after 96
hours the CBI was 1.11 (Barraud et al., 1983).
Treatment of rodents with initiators of liver cancer can give
rise to phenotypically altered cells which, under suitable conditions,
will develop into foci of potentially pre-neoplastic cells. These foci
may either regress or develop into malignant nodules, but because they
only take a few weeks to become apparent, induction of such foci
represents a useful short-term indication of tumour-initiating
capacity.
alpha-TBOH or ß-TBOH (2.5, 5, or 10 mg/kg b.w.), ethinyl
estradiol (0.05 mg/kg b.w.), testosterone (10 mg/kg b.w.),
nitrosomorpholine (25 mg/kg b.w.), or diethylnitrosamine (200 mg/kg
b.w.) were administered by i.p. injection approximately 18 hours after
partial hepatectomy to Fisher 344 CDF rats (5 males and 5 females per
group). Two groups presented only with vehicle and one untreated group
of 5 males and 5 females each were used as controls. The animals were
allowed to recover for a further 13 days after treatment with the test
agent. The animals then were supplied with tap water and powdered diet
containing 0.02% 2-acetylaminofluorene, except that the diet supplied
to animals in one of the vehicle control groups contained no
acetylaminofluorene. Seven days after commencing the new dietary
regime the animals were treated with carbon tetrachloride at 2 ml per
kg b.w. by intragastric gavage (animals in the vehicle control group
not given acetylaminofluorene were not treated with carbon
tetrachloride). Seven days later the animals were killed by cervical
dislocation and the livers were removed for microscopic examination.
Most of the animals showed moderate lethargy and other clinical
signs for two or three days following the operative procedure, but no
compound-related adverse signs were evident. No significant
treatment-related effects on body or liver weight were reported. Only
animals treated with nitrosomorpholine or diethylnitrosamine showed
significant increases in liver foci compared with the vehicle control
or untreated groups.
None of the steroids examined in this study (including alpha-TBOH
and ß-TBOH) showed any evidence of inducing pre-neoplastic liver foci
at the dose levels tested. The authors concluded that none of these
steroids showed any evidence of being a liver rumour initiator in this
assay (Allen & Proudlock, 1987).
Special study on immunoresponse
Cattle
Antibody production in male and female calves (about 25 animals
per group) was investigated after s.c. implantation of placebo
(lactose), 20 mg estradiol-17ß, 140 mg TBA, or 140 mg TBA + 20 mg
estradiol-17ß. A slight, non-significant immunodepressive effect was
seen in male calves treated with either estradiol-17ß or TBA alone. In
the males treated with the combination, this effect was significant.
In female calves the immunoresponse was unaffected (Gropp et al.,
1975).
Special studies on mutagenicity
The results of mutagenicity assays on TBOH and TBA are summarized
in Table 3.
Special studies on no-hormonal effect levels
Pigs
Groups of 3 - 7 (11 in the control group) mature male large white
hybrid pigs, 8 - 10 months of age, were administered orally 0.1, 1,
10, 16, 24, or 36 µg ß-TBOH/kg b.w./day or 0.1, 10, 100, 160, 240, or
360 µg alpha-TBOH/kg b.w./day in gelatine capsules with the feed for
14 consecutive days after castration. They were maintained for 14 more
days, then killed and examined post mortem. Serial blood samples
were collected prior to castration (day 0) and on days 7, 14, 21, and
28; LH was determined on days 0 and 14 in the animals treated with
alpha-TBOH and on days 0, 14, and 28 in the animals treated with
ß-TBOH. After sacrifice, the pituitary, prostate, and seminal vesicles
were examined by gross pathology and histopathology. Based on changes
in plasma luteinizing hormone (LH) concentrations, distinct hormonal
effects occurred at 160 µg alpha-TBOH/kg b.w./day and at 16 µg
ß-TBOH/kg b.w./day. Thus, the no-observed-effect levels in this study
were 10 µg ß-TBOH/kg b.w./day and 100 µg alpha-TBOH/kg b.w./day. The
morphological examinations performed in this study are of limited
value, since a 14-day period elapsed between final treatment and
slaughter, allowing regression of likely effects. Thus, the
observation of treatment-related androgenic effects in the 16, 28, and
36 µg ß-TBOH/kg b.w./day group points toward the high hormonal
activity of this compound (Roberts & Cameron, 1985).
Four groups of 4 male and 4 female large white hybrid pigs were
given orally TBA incorporated into the diet for 14 consecutive weeks
at 0, 0.1, 2, or 20 ppm (equal to 0, 2.0-3.1, 40-62, or 400-620 µg/kg
b.w./day). Blood samples were obtained for steroid hormone assays
before treatment commenced and after 6 and 12 weeks of treatment.
Table 3. Results of mutagenicity assays on TBOH and TBA
Concentration
Test Test of substance
system object tested Results Reference
Ames S. typhimurium 10-10000 µg/ Negative Hossat et al., 1978
test1 TA98, TA100, plate TBA or
TA1535, TA1537 7:1 TBA:
TA1538 estradiol-17ß2
Ames S. typhimurium 1000, 2000, Negative Ingerowski et al., 1981
test1 TA98, TA100, 3000 µg/plate at 1000
TA1535, TA1537 TBOH µg/plate;
TA1538 equivocal at
cytotoxic
concentrations
Ames S.typhimurium 0.5 - 500 µg/ Negative Richold et al., 1982a
test1 TA98, TA100, plate alpha-TBOH;
TA1535, TA1537 15 - 1500 µg/
TA1538 plate ß-TBOH
Ames S. typhimurium 0.06 - 2 µg/ Negative Schiffman et al., 1985
test1 TA98, TA100 plate TBOH
Clastogenic Human lymphocytes 6, 30, or 60 Negative Richold et al., 1982b
potential in vitro µg/ml alpha-TBOH
or ß-TBOH2,3
Cell Mouse lymphoma 15 - 45 µg/ml Equivocal6 Richold et al.,
mutation L5178Y cells alpha-TBOH; 15 - 1982c, 1983
assay4 65 µ/ml ß-
TBOH5
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Cell Syrian hamster 5, 10, 15 µg/ Equivocal7 Schiffman et al., 1985
transformation embryo fibroblasts ml TBOH
assay
Cell Mouse C3H1OT1/2 2 - 25 µg/ml Equivocal Henderson et al., 1987a
transformation cells ß-TBOH (- act.)
assay1 (-S-9 mix)5
5 - 20 µg/ml Positive
ß-TBOH (+ act.)
(+S-9 mix)8
Forward Chinese hamster 25 - 100 µg/ml Negative Edgar et al., 1985
mutation ovary cells ß-TBOH
assay1 (HGPRT locus) (-S-9 mix)9
25 - 150 µg/ml
ß-TBOH
(+S-9 mix)5
Forward Chinese hamster 25 - 500 µg/ml Negative Henderson et al.,
mutation ovary cells ß-TBOH5 1986a
assay1 (HGPRT locus)
Forward Chinese hamster 3 - 75 µg/ml Negative Henderson et al.,
mutation V79 cells ß-TBOH 1987b
assay1 (HGPRT locus) (-S-9 mix)9
12 - 125 µg/ml
ß-TBOH
(+S-9 mix)
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
Micronuclei Chinese hamster 1 - 10 µg/ml Equivocal Henderson et al.,
induction1 ovary cells ß-TBOH (- act.) 1986b
(-S-9 mix)10
6 - 60 µg/ml Negative
ß-TBOH (+ act.)
(+S-9 mix)10
Chromosome Chinese hamster 1 - 10 µg/ml Negative Allen et al., 1985
aberration ovary cells ß-TBOH
assay1 (-S-9 mix)3
6 - 60 µg/ml
ß-TBOH
(+S-9 mix)11
Unscheduled HeLa cells and 2.5 - 15 µg/ml Negative Schiffman et al.,
DNA Syrian hamster 1985
synthesis embryo cells
DNA Cultured human 1 - 512 µg/ml Negative13 Allen & Proudlock,
repair epithelioid alpha-TBOH or 1983
assay1 cells ß-TBOH12
In vivo Rat bone marrow 100 mg/kg b.w. Negative Richold & Richardson,
cytogenetics alpha-TBOH or 1982
assay ß-TBOH once;
25 or 50 mg
alpha-TBOH or
ß-TBOH 4 times3
Table 3. Results of mutagenicity assays on TBOH and TBA (cont'd).
Concentration
Test Test of substance
system object tested Results Reference
In vivo Erythrocytes 100 mg/kg b.w. Negative Allen et al., 1980
micronucleus ß-TBOH3
test
1 Both with and without rat liver S-9 fraction.
2 Dimethylsulfoxide was used as the solvent.
3 Mytomycin C was used as a positive control.
4 Only with rat liver S-9 fraction.
5 20-Methylcholanthrene was used as a positive control.
6 alpha-TBOH at > 22 µg/ml and ß-TBOH at > 15 µg/ml were toxic to the
cells. Both substances induced 2-fold increases in mutation frequency,
but with alpha-TBOH this occurred only at highly toxic concentrations.
7 There was an inverse dose relationship, with the largest number of
transformations occurring at the lowest dose.
8 2-Acetamidofluorene was used as a positive control.
9 Ethylmethanesulfonate was used as a positive control.
10 Colchicine was used as a positive control.
11 Cyclophosphamide was used as a positive control.
12 4-Nitroguinoline-1-oxide (-S-9 mix) and 2-aminoanthracene (+S-9 mix)
were used as positive controls.
13 Increases in nuclear grain count were observed in a limited number of
cultures in one experiment only.
A detailed post-mortem examination was carried out on all pigs
at termination, and tissues were retained for histological
examination. All animals remained generally in good health throughout
the study, and no abnormal clinical signs were noted that could be
associated with experimental treatment. No significant effects of
treatment on body weight, food consumption, or ophthalmoscopy were
noted. There was a marked, dose-related reduction in serum
testosterone levels in male pigs, mean values in the highest-dose
group at week 6 and in pigs fed 2 and 20 ppm TBA at week 12 being
significantly lower than control values (p < 0.01). Testosterone
levels in female pigs remained low at all 3 assay points.
Serum progesterone levels in females were variable. This is not
unexpected, since both progesterone and estradiol show a marked cycle
variation and the assay samples were obtained at arbitrary time points
without regard to the stage of the estrus cycle of individual pigs.
However, mean values at week 12 did indicate a dose-related reduction
in serum progesterone levels in female pigs, and on statistical
examination there was found to be a significant trend with dose
(p < 0.05). Progesterone values for male pigs were less variable and
lower on average than for females and no consistent treatment-related
differences were noted.
Levels of estradiol-17ß in female pigs were generally low,
although there was evidence of some increase in estrogenic secretion
at week 12 in pigs from all groups; no appreciable differences between
groups were observed. Estradiol levels in males were in general higher
than in females; a dose-related reduction in estradiol mean values was
noted at weeks 6 and 12. The results of the hormone assays point to a
no-hormonal-effect level below 2 µg/kg b.w./day.
Treatment-related reductions in mean weights of testes, ovaries,
and uteri were noted. Apparently, the most sensitive organs are the
testes and ovaries. At the lowest dose, no effects on any tissue
weights were observed. The main findings associated with treatment at
the 2 higher levels were atrophy of testicular interstitial cells,
suppression of cyclic ovarian activity, the consequent absence of
glandular development of the uterine endometrium, and lack of alveolar
development and secretion in the mammary glands (highest group only).
Treatment-related changes were not seen in the gonads, uteri, or
mammary glands in any of the animals examined in the lowest-dose group
(Roberts & Cameron, 1985).
Four groups of 5 male and 5 female pigs were given orally TBA as
solutions in corn oil in gelatine capsules with the food for 14
consecutive weeks at 0, 5.0, 7.5, or 10.0 µg/kg b.w./day. Venous blood
samples were taken before treatment began and at weekly intervals
throughout the test period. Plasma radioimmunoassays for testosterone,
estradiol-17ß, and progesterone were carried out. Pigs were sacrificed
after 14 weeks and examined post-mortem. Tissues processed for
histological examination included the testes, ovaries, seminal
vesicles, uterus, and mammary gland. In males, comparison with the
control group showed some differences in testosterone levels at weeks
1 and 5, which reached statistical significance in the two highest
dose groups. However, these differences were not sustained and were in
the opposite direction (i.e. increases compared with the control) to
the dose-related changes (i.e. suppression) noted in the study
described in the previous paragraphs. Testosterone levels in females
generally remained low in all groups.
No significant group differences were found in estradiol levels
in males. In females, estradiol levels generally remained low in all
groups. Mean progesterone levels in males were statistically
significantly lower than in controls between weeks 7 and 10 in the
highest-dose group and at week 10 in the group administered 7.5 µg
TBA/kg b.w./day. However, the mean pre-dose levels were also lower in
pigs in these two groups compared with the controls and analysis of
mean levels over weeks 6 to 14 showed no significant effects. Thus,
these differences were not considered by the authors of the report to
represent a real treatment effect. In female pigs, mean progesterone
values were variable due to cyclic changes, but no consistent group
differences were apparent. Examination of the time of onset of cycling
and the peak level of progesterone recorded did not show significant
effects. No significant differences in organ weights were noted in any
groups. No treatment-related changes in the morphology of the organs
examined were noted in any groups (Roberts & Cameron, 1985).
Monkeys
The antigonadotropic activity of ß-TBOH was tested in acutely
castrated male rhesus macaque monkeys aged 8 - 17 years. A seminal
vesicle biopsy was obtained at the end of the 30-day treatment period
to examine possible changes in androgenic activity induced by TBOH.
ß-TBOH was given orally at 0, 1, 20, or 400 µg/day (three control
animals and two animals per treatment group). From 17 days after
castration until the end of the experiment, the lowest-dose group was
given 1600 µg/day ß-TBOH. Administration of the compound did not
suppress the post-castration elevation in either LH or follicle
stimulating hormone (FSH) secretion, which occurs after removal of the
testes. Although TBOH and testosterone showed no antigonadotropic
activity in this model system, the monkeys receiving 400 and
1600 µg/day TBOH maintained partial or complete seminal vesicle
morphology consonant with an androgenic effect. The expected reduction
in the serum levels of testosterone and estradiol occurred after
castration, and TBOH treatment did not alter the serum concentrations
of these hormones or the typical diurnal pattern of activity within
the hypothalmic-pituitary-adrenal axis. The authors concluded that the
no-hormonal-effect level in this study was 20 µg/day, equivalent to
2 µg/kg b.w./day (Hess, 1983).
TBA was administered in Sustagen/Jello/bran diet cubes for three
cycles, or a maximum of 122 days, to three groups of six mature female
rhesus macaque monkeys weighing 6 kg each at 60, 240, or 960 µg/day.
Blood samples were obtained on a daily basis from all animals during a
pretreatment menstrual cycle, at 3-day intervals during the first two
treatment menstrual cycles, and daily during the last treatment
menstrual cycle. Serum concentrations of estradiol, progesterone, LH,
and FSH were determined by radioimunoassay. Treatment with 960 µg/day
TBA resulted in maximum average serum levels of 2.3 ng/ml of ß-TBOH,
and this dose may have inhibited gonadotropin secretion and ovarian
function in 3 of 16 reproductive cycles.
It was concluded that 960 µg TBA/day had an inhibitory effect on
the pituitary gonadal axis. The anovulatory stage was reached rather
suddenly and from the data presented no conclusions with respect to
changes in the endogenous hormone concentrations, which might signal
this effect, can be drawn. In the 240 µg/day group one animal
exhibited anovulation, which may have been related to treatment. No
effects were observed at 60 µg/day, equivalent to 10 µg/kg b.w./day
(Hess, 1984).
Special studies on relay toxicity
Rats
Female veal calves were given s.c. implantations of 0, 140, or
3500 mg/animal TBA and killed after 10 weeks. A homogenate of veal
meat and veal organs (tongue, heart, lungs, spleen, liver (partial),
and one kidney) from these calves was mixed with the diet of rats in a
two-generation reproduction study. The total length of the experiment
was 114 weeks. The F1a generation was used to study teratogenic
effects. In the group of rats receiving a diet containing 230 ppb TBA,
a slight growth depression was seen. No effects were seen on other
parameters, which included mortality, feed consumption, growth,
fertility, reproduction (copulation, conception rate, duration of
gestation, mean litter weight, litter size, fetal body weight,
mortality rate, and fetal body weight after 3 weeks), haematology,
biochemistry, organ weight, and gross- and histopathology
(Gropp et al., 1978).
Special studies on reproduction
Rats
Groups of 40 male and 80 female rats weighing 133 - 143 g each
were given diets containing 0, 0.5, 1, 4, or 16 ppm TBA from week 9
before mating until day 21 post-partum. An additional group was
handled similarly, in which females were fed 50 ppm TBA from day 1 of
pregnancy until day 21 post-partum. At day 21 post-partum all parent
animals were killed without further examination. There were no major
differences between nominal and detected dietary levels of TBA at the
beginning and end of the study. In females in the 4 and 16 ppm groups,
growth was increased throughout the study (10 - 20%); in the 50 ppm
group growth was increased after mating (10%). A dose-related decrease
in pregnancy rate was seen in the 1, 4, 16, and 50 ppm groups (maximum
-30%). At day 4 post-partum each litter was reduced in size to 4
females and 4 males; up to that time pup mortality was increased in
all dosed groups compared with controls. Litter size and litter weight
on days 0, 4, 12, and 21 post-partum were decreased in the 4 and 16
ppm groups (maximum about -10%) and in the 50 ppm group (about -25%).
Mean pup weights were decreased only in the 50 ppm group from day 4
post-partum onward (maximum -15%) (Hunter et al., 1982).
Groups of 12 male and 12 female rats were fed diets containing
0, 25, 50, or 100 ppm TBA for 63 days and then mated. In these groups,
12/12, 10/12, 4/12, and 1/12 females, respectively, were pregnant
after mating (Ross, 1980).
In a multi-generation reproduction study in CRL:COBS CD(SD)BR
Charles River rats, dietary concentrations of 0, 0.5, 3, or 18 ppm TBA
were fed to F0-generation male rats for nine weeks and female rats
for two weeks prior to mating, then through to termination. Two groups
of F1 generation rats were selected, reared, and mated. One group
was treated continuously at the same dietary concentrations as rats in
the F0 generation ("treated" group) and one was removed from
exposure to TBA at 3 weeks of age and maintained without treatment
throughout ("untreated" group).
Treatment with TBA at 18 ppm was associated with the following
effects: a) generally higher body weights affecting both F0-and
F1-generation treated males and females and females of the untreated
F1 generation; b) depression in mean body weight gain during
gestation affecting both matings of the F0 generation; c) signs of
virilization, namely coarseness of the coat and discoloration of the
skin in F0-generation animals and treated F1-generation females;
d) clitoral prominence in treated F1-generation females and to a
lesser extent in untreated F1-generation females. Similar effects
were observed in F2-generation females from treated F1 parents,
but not in offspring of the untreated F1 generation; e) the presence
of occlusive strands in the vagina and/or precocious/incomplete
vaginal opening affecting treated F1 pups and F2 pups from the
treated F1 generation; f) a delay in the occurrence of testicular
descent affecting F2 pups from the treated F1 generation; g) a
marked reduction in pregnancy rate affecting the second mating of the
F0 generation and the treated F1 generation; h) an increase in
pre-coital time for the second mating of the F0 generation and for
the treated F1 generation; i) a marginal extension of the duration
of gestation affecting the F0 generation and the treated F1
generation; j) a marked increase in the incidence of extended
parturition and total litter loss in the treated F1 generation and a
significant increase in the percentage of males per litter; k) lower
litter size and litter weight, either at birth or at 20-day sacrifice,
after both matings of the F0-generation and of the treated F1
generation; 1) increased post-implantation/pre-birth losses in the
F0 and treated F1 generations; m) at terminal autopsy, findings
additional to those previously described, namely an increase in the
incidence of depression in the forestomach epithelium affecting F0-
and F1-generation males; n) significant reductions in seminal
vesicle/prostate weights in F0- and F1-generation males and
increases in mean ovary weights among F0- and F1-generation
treated females; o) significant decreases in weights of seminal
vesicles/prostate, testes, and epididymes in F1-and F2-generation
male pups at six weeks of age. F1- and F2-generation female pups
showed reduction of adrenal weight at six weeks of age; p) a
significant reduction in anogenital distance among male fetuses and a
marginal increase in the incidence of skeletalvariants after the
second mating of rats of the F0-generation (teratology phase).
Treatment with TBA at 3 ppm was associated with the following
effects: a) retarded body weight gain at the first mating of the
F0-generation; b) coarseness of the coat and discoloration of the
skin affecting one female in the F1-generation; c) a significant
delay in the mean age of vaginal opening in females in the
F1-generation and in F2 offspring from treated F1 parents; d)
the occurrence of incomplete vaginal opening or occlusive strands in
the vagina affecting occasional animals of the F1-generation at 6
week autopsy; e) a slight, but not statistically significant, delay in
testicular descent affecting F2 male offspring from treated F1
parents; f) significantly lower litter size at birth after the first
mating of the F0-generation; g) lower litter weight after the second
mating of the F0-generation; h) marginally reduced litter size in
offspring of the treated F1-generation; i) a significant decrease in
the weight of seminal vesicles/prostate, testes, and epididymes in
F1 and F2 male pups at six weeks of age.
The only apparent effects of treatment at 0.5 ppm were as
follows: a) higher group mean body weights of males from the F0
generation; b) a slight but not statistically significant delay in the
mean age of vaginal opening in F1 pups and F2 pups from the
treated F1 generation (subsequent mating performance and resulting
litter parameters were comparable to those of controls); c) a
statistically significant decrease in seminal vesicles/prostate weight
in treated F1 male pups and in F2 male pups from treated parents
at six weeks of age; d) a significant decrease in weight of epididymes
in F2 males.
The authors concluded that, in terms of reproductive performance
of the two generations examined in this study, as assessed by the
ability of parents to produce and sustain their litters, TBA exerted a
marked effect at 18 ppm and some effect at 3 ppm. At the lowest dose
level examined (0.5 ppm), slight effects were observed, which were
more marked in F2 pups than in F1 pups of a comparable age.
However, the authors concluded that in terms of reproductive
performance TBA exerted no effect at 0.5 ppm. The reproductive
performance for all groups of F1 animals following withdrawal from
treatment showed no marked differences from those of the control group
(James et al., 1985).
Histological examination of the testes, epididymes, seminal
vesicles, and prostate of 6-week-old male F2-generation rats from
treated F1 animals in the study described above revealed no
morphological abnormalities, although lower group mean weights were
recorded in treated groups compared to controls. All rats were
considered normal for that age, when they are not quite fully sexually
mature, with spermiogenesis proceeding to tailed spermatids in the
testes but no spermatoozoa in the epididymes (Offer, 1985).
In a study in rats, dietary concentrations of 0, 0.1, 0.3, 0.5,
3, or 18 ppm TBA were fed to F0 males and females from two weeks
prior to mating until termination of pregnancy. The F1 litters were
reared through the weaning period. On day 22 post-partum the male
offspring were sacrificed, examined macroscopically, and the testes,
seminal vesicles/prostate, and epididymes from each pup were weighed
and preserved. Female offspring were sacrificed on day 24 post-partum
and examined macroscopically.
Treatment with TBA at 18 ppm was associated with the following
effects: a) slightly higher mean body weights of F0 females, but
lower body weight gain during gestation and slightly lower weight gain
of males over the last three weeks of treatment; b) clitoral
prominence at autopsy in 22/29 F0 females and in all F1 female
offspring from approximately 3 weeks of age; c) a statistically
significant extension in duration of gestation; d) total litter loss
in 4/29 F0 females; e) effects on litter parameters, including
reduced litter size, lower litter weight, marginally higher pup
mortality, and higher mean pup weight; f) lower testicular weight and
higher mean weight of seminal vesicles/prostate in F1 males at 22
days of age.
Treatment at 3 ppm was associated with: a) slightly higher mean
body weights of females and slightly lower weights of males in the
three weeks prior to termination; b) a marginal extension in duration
of gestation; c) effects on litter parameters, including reduced
litter size, and marginally higher pup mortality and mean pup weight;
d) lower testicular weight and higher mean seminal vesicle/prostate
weight in F1 males.
The only effects associated with treatment at 0.1, 0.3, and
0.5 ppm TBA were slightly higher mean body weights of females and the
marginal differences observed at 3 and 18 ppm in litter parameters
(James et al., 1986).
Special studies on teratogenicity
Rats
Four groups of 6 pregnant rats each were given by oral gavage
0, 2.5, 5, or 10 mg/kg b.w./day TBA from days 6 - 15 of gestation. The
vehicle was 2.5% ethanol in 1% methylcellulose. Mortality, growth,
number of corpora lutea, number and distribution of live and dead
young, litter weight, mean fetal weight, microscopic fetal
abnormalities, sex ratio, and fetal crown-rump distance were all
unaffected by treatment (James et al., 1982).
Groups of 20 pregnant rats received by oral garage 0, 5, 10, or
20 mg/kg b.w./day TBA from days 6 - 15 of gestation. The vehicle was
2.5% ethanol in 1% methylcellulose. In the 10 and 20 mg/kg b.w./ day
groups, 9/20 and 15/20 dams, respectively, showed hair loss. A
dose-related decrease in growth was seen in all dosed groups (maximum
20%). Pregnancy rate, number of liver and dead young, number of
implantations and corpora lutea, litter weight, mean fetal weight,
incidence of major malformations, incidence of minor visceral
anomalies (Wilson's technique), and fetal crown-rump distance were all
unaffected. The incidences of skeletal variants (number of ribs,
number of normal and variant sternebrae) were unaffected. Mean
anogential distances in male fetuses preserved in Bouin's solution
were slightly shorter than those of controls, with an apparent
slightly decreasing trend with increase in dosage; the difference at
20 mg/kg b.w./day attained borderline statistical significance
(P < 0.05). Male fetuses preserved in alcohol did not show the same
trend in mean values, although the variation in anogenital distance
was greater in pups in the high-dose group than in controls; ranges of
fixation weight and crown-rump distance length were also higher in the
high-dose group than in controls. Mean anogenital distances in females
preserved in Bouin's solution and in alcohol were comparable in all
groups (James et al., 1982).
Acute toxicity
Table 4 summarizes the results of acute toxicity studies on TBA.
Table 4. Acute toxicity of TBA
LD50
Species Sex Route Vehicle (mg/kg b.w.) Reference
Mouse M/F oral 40% ethanol 1500 Audegond et al.,
in corn oil 1981a
M i.p. ethanol + 10% 565 Escuret & Bas, 1978
sesame oil
F i.p. ethanol + 10% 643 Escuret & Bas, 1978
sesame oil
Rat M/F oral 10% ethanol 5000 Audegond et 1981b
in corn oil
M i.p. 10% ethanol 1601 Escuret & Bas, 1978
in corn oil
F i.p. 10% ethanol 1772 Escuret & Bas, 1978
in corn oil
Dog M/F oral via capsules 1000 Audegond et al.,
1981c
Dogs
Anaesthetized dogs were given i.v. 1, 2, 5, or 10 mg/kg b.w. TBA
as a 20 ml/mg solution in 92% acetylmethylamine. Dogs in the 2, 5, and
10 mg/kg b.w. groups showed a dose-related decrease in blood pressure;
at 10 mg/kg b.w., this was accompanied by slight bradycardia. A
decrease in blood pressure in the same groups was seen after injection
with adrenalin and noradrenalin; an increase was seen after
acetylcholine injection. Changes in reactions to histamine were not
noted in any groups (Seeger, 1971a).
Short-term studies
Mice
Groups of 8 male and 8 female mice weighing 19 - 25 g each were
given 0, 25, 50, or 100 ppm TBA in the diet for 8 weeks. Mortality,
appearance, behaviour, body weight, food consumption, and food
conversion were unaffected by treatment. Females in all treatment
groups exhibited significant decreases in absolute and relative liver
weights and significant increases in absolute and relative uterine
weights. Absolute and relative ovarian weights were decreased
significantly in females in the 50 and 100 ppm groups, while absolute
and relative testes weights were decreased significantly in males in
the highest-dose group. No effects on the weights of adrenals,
kidneys, prostate, seminal vesicles, or spleen were noted. Females
showed dose-related suppression of ovarian cyclic activity
characterized histopathologically by the absence of, or few, corpora
lutea in the gonads, a dose-related reduction in the amount of stroma,
and reduction in the number of endometrial glands in the uteri
(Hunter et al., 1976a).
TBA was administered to groups of 8 male and 8 female Swiss
albino CFLP mice for 10 weeks at levels of 0, 1, 2, 5, or 10 ppm of
the diet, equal to mean intakes of 0, 0.12, 0.24, 0.56, or 1.2 mg/kg
b.w./day for males and 0, 0.13, 0.25, 0.66, or 1.4 mg/kg b.w./day for
females. There were no signs of reaction to treatment, including no
treatment-related effects on food intake or body weight gain. No
treatment-related abnormalities were seen; the absolute and relative
weights of all organs examined were considered to be within normal
limits for mice of this strain and age. The only organs examined
histologically were the prostate, seminal vesicles, testes, ovaries,
and uterus from mice in the control and highest-dose group. All
histopathological parameters were within normal limits (Hunter et al.,
1976b).
Rats
Sixteen male rats that had been castrated between 21 and 24 days
of age were given total oral doses of 0, 0.75, 3, 12, or 48 mg TBA
from days 2 - 11 after castration in 10 daily portions. At autopsy,
one day after the last application, dose-related increases in the
absolute weight of the prostate (maximum +440%) and seminal vesicles
(maximum +400%) were seen in all treated groups. In the three
highest-dose groups a dose-related increase in the musculo levator ani
weight (maximum +250%) was noted (Schröder, 1971a).
Groups of 10 male and 10 female CFY rats were fed diets
containing TBA at concentrations of 0, 25, 50, or 100 ppm for 13
weeks. Group mean intakes during the treatment period were 0, 1.8,
3.8, or 7.6 mg/kg b.w./day for males and 0, 2.2, 4.2, or 8.4 mg/kg
b.w./day for females. At all the tested doses, the females exhibited
better efficiency of feed utilization than the males, causing higher
weight gain.
At 25 ppm, lower prostate weight (-36%), which was not associated
with morphological changes, was observed. At 50 ppm, lower prostate
and seminal vesicle weights (-50 and -30%, respectively), which were
not associated with morphological or uterine changes, were observed in
two rats.
At 100 ppm, the following changes were observed: lower neutrophil
and lymphocyte values in males at weeks 12 and 13 (-35%); lower
seminal vesicle weight, which was not associated with morphological
changes (-60%); lower prostate weight (-80%), which was associated
with small alveoli lined by cuboidal epithelium in 5 rats; and uterine
changes characterized by an apparent reduction of the endometrial
stroma with dilated uterine glands and also a corrugated appearance of
the endometrial and glandular epithelium in 6 rats (Hunter et al.,
1976c).
Groups of 10 male and 10 female rats weighing 60 g each were
given 0, 50, 100, 200, or 1000 µg/kg b.w./day TBA orally 6 days per
week for 3 months. The compound was presented as 0.5 ml of an aqueous
solution containing 0.9% NaCl, 0.4% polysorbate 80, 0.5% carboxy-
methylcellulose, and 0.9% benzyl alcohol. Determinations were
carried out in half of the animals at termination only.
Growth of females was slightly increased; in the two highest-dose
groups growth of males was decreased. Haematological parameters were
unaffected. In all dosed groups, SGOT and SGPT were decreased; total
cholesterol was decreased in the 100, 200, and 1000 µg/kg b.w./day
groups. In the highest-dose group a slight decrease in blood glucose
was seen; urea was slightly increased only in the females in this
group. Increases were observed in liver weight in males and females in
the 200 and 1000 µg/kg b.w./day groups, kidney weight in males and
females in the highest-dose group, and spleen weight in females in the
200 and 1000 µg/kg b.w./day groups. In females, ovary weight was
increased in the highest-dose group and uterus weight was decreased in
the 100 and 200 µg/kg b.w./day groups. In males, decreases were seen
in seminal vesicle weight in the 100, 200, and 1000 µg/kg b.w./day
groups and in prostate weight in the 100 and 1000 µg/kg b.w./day
groups. Gross pathological examination revealed atrophy of the
prostate, seminal vesicles, and testes in rats in the highest-dose
group. Histologically, changes in the ovary (cysts and released
follicles in all test groups) and in the uterus ("dentelle uterine" in
the 200 and 1000 µg/kg b.w./day groups) were observed in females. In
males in the highest-dose group a delay in spermatogenesis and aplasia
in the seminal vesicles and prostate were observed (Seeger, 1971a, b).
Female rats fed diets containing 0.01, 0.1, 2.5, 5, 10, 20, 40,
80, or 160 ppm TBA exhibited increases in uterus weight at levels
higher than 40 ppm. Vaginal smears in all groups were negative; some
proliferation of the vaginal mucosa was seen at the highest dose level
(Huis in't Veld et al., 1973).
Female rats weighing 60 - 65 g each were ovariectomized and given
s.c. doses of 0, 0.2, 1.0, or 5.0 mg/day TBA in sesame oil for 4 days.
At termination, on day 5, a dose-related increase in uterus weight was
seen in all dosed groups (maximum +550%). The estrogenic activity of
TBA was less than 0.1% of the estrogenic activity of estradiol-17ß
(Schröder, 1971b).
Male castrated rats weighing 100 g each were given daily s.c.
doses of 4, 20, or 100 µg TBA, 4, 20, or 100 µg ß-TBOH, or 20, 100,
500, or 1000 µg alpha-TBOH for 9 days. Dosing was started 1 day after
castration. One control group was used. At sacrifice, one day after
the last injection, the weights of the prostate, musculo levator ani,
and seminal vesicles were recorded. In animals dosed with both TBA and
ß-TBOH, organ weights were increased in a dose-related manner in all
test groups. In the animals administered alpha-TBOH, organ weights
were increased at the three highest dose levels (Escuret & Bas, 1978).
Male castrated rats weighing 65 - 75 g each were given daily s.c.
doses of 0, 0.02, 0.1, or 0.5 mg TBA as a solution in sesame oil for
10 days after castration. At sacrifice on day 11, dose-related
increases were seen in the weights of muscolo levator ani (maximum
+250%), prostate (maximum +1400%), and seminal vesicles (maximum
+2500%) in all groups. In this experiment TBA showed distinct anabolic
and androgenic activity that was 5 times higher than that of
testosterone and 20 times higher than that of 17-ethynyl-19-nor-
testosterone (Schröder, 1971b).
Groups of 10 male and 10 female rats weighing 123 - 131 g each
were dosed 6 times per week s.c. with 0, 200, 1000, or 5000 µg/kg
b.w./day TBA over a period of 2 months. The compound was applied as a
solution in 1:1 syncortyl and arachis oil.
Growth of females was increased in all groups; by contrast,
growth of males in the highest-dose group was decreased. Haematology
and blood biochemistry determinations, which were performed at
termination in 5 rats/sex/group, revealed slight increases in Hb and
haematocrit values and slight leucocytopenia (due to lymphocytopenia)
in all test groups. Other blood abnormalities that were noted were a
decrease in glucose in females in the 5000 µg/kg b.w./day group (not
determined in other groups), a decrease in BUN in females in the 1000
and 5000 µg/kg b.w./day groups and in males in the 5000 µg/kg b.w./day
group, and a decrease in cholesterol in males and females in the 1000
and 5000 µg/kg b.w./day groups.
In all test groups, absolute and relative kidney weights were
increased and absolute and relative adrenal and thymus weights were
decreased. Ovary weights were decreased in a dose-related manner in
females in all test groups. In females in the 200 and 1000 µg/kg
b.w./day groups uterus weights were decreased. All treated females
showed an increase in absolute liver weight. Males showed a decrease
in testes weight in all treated groups and in seminal vesicle and
prostate weight in the 1000 and 5000 µg/kg b.w./day groups. Besides
these weight changes, atrophy was noted in the thymus, ovaries, and
testes; hypertrophy was seen in the seminal vesicles and prostate
(Sovetal, 1970; Seeger, 1971a).
Rabbits
Groups of 4 - 6 rabbits weighing 2 kg each were administered s.c.
during 4 days 0, 0.05, 0.5, 2, or 5 mg/kg b.w./day TBA. Liver function
was examined (SGOT activity and BSP excretion). At 2 mg/kg b.w./day
SGOT activity was increased slightly, while at 5 mg/kg b.w./day SGOT
activity was increased significantly. BSP excretion was not affected
in any of the test groups (Seeger, 1971a).
Pigs
Male, female, and castrated male pigs were fed diets containing
1-2 ppm TBA, alone or in combination with 2 ppm estradiol-17ß or 2 ppm
ethynylestradiol, for 5 - 8 weeks. At 5 and 6.5 weeks after compound
withdrawal TBOH was not detected in the urine. Urinary excretion of
total steroid estrogen was not increased at 7 weeks after dose
withdrawal (Kroes et al., 1976a).
Groups of 4 male and 4 female domestic pigs (Sus scrofa) were fed
diets containing 0, 0.1, 2, or 20 ppm TBA (equivalent to 0, 4, 80, or
800 µg/kg b.w./day TBA, respectively) for 14 weeks. No treatment-
related effects on mortality, body weight, or ophthalmoscopy were
seen. After 6 and 12 weeks several haematological (i.e., Hb, RBC, PCV,
WBC, diff. WBC, and prothrombin index) and blood biochemical
parameters (i.e., glucose, protein, albumin, albumin/globulin ratio,
SAP, calcium, and creatinine), were unaffected. At 2 and 20 ppm,
dose-related increases were seen in platelets (maximum +70%, not
significant), urea (maximum +3%, significant) and cholesterol (maximum
+100%, significant). SGOT increased in a dose-dependent manner in all
test groups (maximum, +100%, significant). There were dose-related
decreases in blood levels of testosterone and estradiol in males in
all dosed groups (both maximum -95%, significant); progesterone was
markedly decreased (maximum -99%, significant) in females in the two
highest-dose groups. In the same groups, there were dose-related
changes in the absolute and relative weights of the liver (maximum
+30%, significant), uterus (maximum -50%, significant), kidney
(maximum +25%, significant), and testis (maximum -55%, significant).
In the highest-dose group changes were observed in the weights of the
pituitary (-15%, significant) and seminal vesicles (+280%,
significant). There was an increase in thyroid weight at all three
dose levels (maximum +20%, not significant).
Histopathological examination showed the following dose-related
abnormalities in the 2 and 20 ppm groups: in the liver, enlargement of
the hepatocytes with associated ground glass appearance of the
cytoplast; in testes, moderate to complete interstitial cell atrophy
(with normal spermatogenesis within the seminiferous tubules); in
ovaries, evidence of suppressed or abnormal cyclic activity
characterized by the absence of maturing follicles and/or mature or
early regressing corpora lutea; and, in the uteri, absence of
glandular development in the endometrium. This last observation and
the lack of alveolar development and secretion noted in the mammary
glands of the 2 ppm group were probably associated with the anestrous
state suggested by the findings in the ovaries (Ross et al., 1980).
Cattle
Groups of 8 male calves were given s.c. implants of 140 mg TBA +
20 mg estradiol-17ß at 8 or 4 weeks before slaughter. An additional
group was dosed with 200 mg/kg b.w. testosterone + 20 mg/kg b.w.
estradiol-17ß on both occasions. In all treatment groups histological
examination of prostates showed increased secretory activity and
hyperplastic and metaplastic changes (Verbeke et al., 1975).
Six weeks after single s.c. application of 20 mg estradiol-17ß to
male calves, distinct changes in seminal vesicles were found after
histological examination. Treatment of calves with 20 mg estradiol-17ß
combined with 140 mg TBOH, applied s.c., caused very slight changes in
seminal vesicles in 4/11 calves, while in 3/7 animals with unchanged
seminal vesicles, androgenic stimulation was observed (Kroes, 1972).
Male calves that were 11 weeks of age received by s.c.
implantation 20 mg estradiol-17ß alone or 20 mg estradiol-17ß in
combination with 140 mg TBA. Urinary secretion of total steroid
estrogens was high in the animals given estradiol-17ß alone during the
first 12 days after application. After 3 weeks normal values were
reached. In the estradiol-17ß/TBA combination group, gradual and
prolonged excretion of steroid estrogens for up to 42 days after
implantation occurred; after 56 days normal values were reached.
Qualitative determinations of estradiol-17ß and estradiol-17ß in urine
showed that the alpha epimer was present in almost all urine samples;
estradiol-17ß was found in the urine of those calves administered
estradiol-17ß alone. In the combination group, estradiol-17ß was found
in urine at day 21 only. Histological examination of prostates
revealed squamous metaplasia of gland epithelium after both treatments
(Kroes et al., 1976b).
A total of 1480 female calves, aged 7 weeks, were administered by
percutaneous implantation 0, 140, or 3500 mg TBA (groups 1 - 3,
respectively) or 140 mg TBA + 20 mg estradiol-17ß (group 4), 1400 mg
TBA + 200 mg estradiol-17ß (group 5), or 3500 mg TBA + 500 mg
estradiol-17ß (group 6). The calves were slaughtered 10 weeks after
implantation.
Blood parameters (glucose, GOT, GPT, AP, LDH, cholesterol,
bilirubin, Hb, and PCV), urinary density, and pH were unaffected in
all groups. Calcium and phosphorus levels in serum and bone were not
changed; however, magnesium levels in serum and deposition of
magnesium in bone were decreased in groups 3, 5 and 6. In group 3
slight, and in groups 4, 5 and 6 marked, increases in uterus weight
were seen, which were accompanied by proliferation of uterine
glandular cells, while the lumen of the uterus was partially filled
with a watery liquid. In dosed groups ovarian weights were reduced,
accompanied by a diminution in follicular size. These weight changes
were most marked in groups 2, 3 and 6. The diminution of follicular
size, which was accompanied by a decrease in the number of follicles,
was most marked in groups 5 and 6. In all groups, a dose-related
decrease in thymus weight was seen. In group 3 abnormal development of
the clitoris was noted. Histopathological examination showed a
non-dose related proliferation and secretion of glandular tissue in
the mammary glands of animals in groups 4, 5 and 6. Heart, lever,
kidneys, pituitary, pineal gland, adrenal glands, thyroid, and
skeletal muscle did not show abnormalities (Gropp et al., 1975).
Steers and heifers were seven 300 mg TBA by s.c. implantation. An
additional group of steers was given implants containing 140 mg TBA +
20 mg estradiol-17ß. In all groups, plasma urea was decreased during
the 9-week observation period after treatment. Other plasma parameters
(glucose, calcium, phosphorus, magnesium, sodium, potassium, and total
protein) were unaffected. No changes in plasma concentrations of
insulin or growth hormone were seen. A decrease in thyroxine levels in
steers was observed during the 9-week observation period after
treatment; the decrease was most marked in those administered TBA and
estradiol-17ß in combination. A marked reduction, about -50%, in
thymus weight was observed in steers (Heitzman, 1975).
Steers and oxen given, by s.c. implantation, 140 mg TBA alone or
140 mg TBA in combination with 20 mg estradiol-17ß. Control animals
received implantations with the carrier. The combination of drugs
affected urinary excretion of exogenous estradiol-17ß in oxen and
possibly in steers. Histological examination of the prostate revealed
squamous metaplasia in the combination group. In both treated groups,
more active epithelium of the prostate was seen, compared to the
control group (Kroes et al., 1976c).
Long-term studies
Mice
Groups of 64 male and 64 female Swiss albino CFLP mice, weighing
22 - 25 g each, were given diets containing 0, 0.5, 1.0, 10 or 100 ppm
TBA (equal to 0, 0.004, 0.09, 0.86, or 8.6 mg/kg b.w./day TBA for
males, respectively, and 0, 0.005, 0.10, 0.96, or 9.5 mg/kg b.w./day
TBA for females, respectively) for 95 - 104 weeks (the test was ended
when survival was 20% in males or females in the control group). After
13 weeks 12 mice/sex were killed. At that time significant increases
were observed in the absolute and relative weights of the kidneys in
males and females at 100 ppm (20 - 40% increases). Significant
decreases were seen in the weights of the spleen of top-dose females
(-20%) and significant increases were seen at 1.0, 10, and 100 ppm in
males (+25%). Dose-related relative decreases in weights of the uterus
were observed in all dosed females (maximum -25%). At interim
sacrifice, inhibition of ovulation, characterized by a lack of corpora
lutea in all females in the highest-dose group, was observed.
Follicular development proceeded to the mature follicle stage. The
uteri of all females in the 100 ppm group were consistent with the
diestrous stage of the cycle. In the spleens of 6/12 males in the
100 ppm group an increased number of polymor-phonuclear leucocytes in
the red pulp was seen (0/12 in controls); congested sinuses were noted
in 2/12 males of this group (0/12 in controls).
At terminal sacrifice no organ weights were recorded. Terminal
gross- and histopathological examination showed an increase in liver
nodular hyperplasia and dose-related tumours in the male dose groups;
these increases were statistically significant at the two highest
doses. The incidence of liver tumours was also increased in females in
the highest-dose group (8/52 versus 4/51 in controls). There was an
increase in incidence of hepatocyte vacuolation in males in the
100 ppm group. In 100 ppm females, gross pathological examination
showed an increase in the incidence of enlarged and swollen kidneys,
accompanied by a marginal increase in the incidence of nephritis. In
the same group, increases in ovarian cysts (in 10/20 animals versus
1/11 in controls) and enlarged, abscessed, or cystic preputial glands
(in 4/20 females versus 0/11 controls) were seen. The spleens of 4/20
females in the 100 ppm group appeared small (Hunter et al., 1981).
Rats
Six groups of 65 male and 65 female Sprague-Dawley CFY rats
weighing 150 - 200 g each were given diets containing 0, 0.5, 1.0,
4.0, 16, or 50 ppm TBA (equal to 0, 0.02, 0.04, 0.14, 0.56, or
1.80 mg/kg b.w./day TBA for males, respectively, end 0, 0.02, 0.04,
0.16, 0.64, or 1.92 mg/kg b.w./day for females, respectively) for 112
weeks. The parents of the test animals had been dosed at the same
levels from week 9 before mating until day 21 post-partum (except for
the 50 ppm group in which only the dams had been dosed from day 0 of
pregnancy until day 21 post-partum).
The females in the 4.0, 16, and 50 ppm groups showed prominent
pudendum. A dose-related incidence of pendulous anogenital skin was
seen in all female dose groups (maximum incidence, 85%). In males a
dose-related reduction in testes size was seen in all dose groups,
except at 0.5 ppm (maximum incidence, 45%). Growth and food
consumption were decreased throughout the study in males administered
50 ppm TBA (-15 and -10%, respectively). Water consumption was
decreased in the same group up to week 51 (-15%). Urinalysis (9
parameters measured in 5 rats/sex of the 0, 16, and 50 ppm groups on 6
occasions throughout the study) showed no treatment-related changes.
Haematology (10 parameters measured in 10 rats/sex of the 0, 16,
and 50 ppm groups on 6 occasions throughout the study) showed dose-
related slight increases in some parameters in females in the 16 and
50 ppm groups. Male haematology values were generally unaffected by
treatment. Blood biochemistry (13 parameters) showed no compound-
related changes.
At interim sacrifice at week 78, 13 - 14 rats/sex/group were
killed. Macroscopic examination revealed a prominent clitoris in 5/14
females fed 16 ppm TBA and in 13/13 females fed 50 ppm TBA. The males
of the 50 ppm group showed atrophy of the testes, seminal vesicles,
and prostate. Interim organ weighings revealed, in the males fed 16
and 50 ppm TBA, dose-related decreases in absolute testes and prostate
weights. Adrenals and pituitary weights were decreased in both sexes
in the 50 ppm group. Weights of the ovaries were increased in all dose
groups, but without dose relation.
At the end of the experiment, similar changes in the absolute
weights of the testes, prostate, and adrenals were found. In the males
of the highest-dose group, decreases were observed in the weights of
the pituitary (-30%), thyroid (-20%), kidney (-30%), spleen (-30%),
and liver (-20%). The weight of the ovaries was markedly decreased in
the highest-dose females (-60%).
Terminal gross- and histopathological evaluation revealed a
slightly increased incidence of foci or areas of ground-glass
hepatocytes in the livers of the highest-dose females. Urinary bladder
calculi and associated epithelial hyperplasia joined by cystitis and
pyelitis were seen in females in the same dose group. Both the male
and female reproductive tracts were affected. Testicular, prostatic,
and vesicular atrophic changes were seen in males in the 16 and 50 ppm
groups. Absence of corpora lutea, vaginal inflammation and
mucification, uterine endometritis, dilatation, decreased endometrial
thickness, and the development of clitoral bone were seen in the
50 ppm females. Clitoral enlargement was seen in females in the two
highest-dose groups. Neoplastic histopathology showed an increase in
the incidence of pancreatic islet cell tumours in the 50 ppm group
(Hunter et al., 1982).
Observations in human beings
Male and female human volunteers were given i.m. doses of 5 or
10 mg TBA every-other-day during 14 days. In the subjects administered
5 mg TBA, nitrogen balance was disturbed, including retention of
nitrogen. In the 10 mg group, some women showed disturbances of the
menstruation cycle. The same dose caused a slight but significant
reduction of excretion of 17-ketosteroids. No effects on
17-hydroxy-corticosteroid excretion were seen. Blood parameters
(protein, cholesterol, coagulation factors, and prothrombin and
thrombin time) were unaffected by treatment (Krüskemper et al.,
1967).
COMMENTS
TBA is a synthetic steroid with anabolic properties. At the
17-position in the molecule two epimers, alpha and ß, are possible.
The ß-epimer of TBA is the commercial product. It is administered to
cattle either alone or in combination with estradiol-17ß or zeranol as
a subcutaneous implant in the ear to improve body weight, feed
conversion, and nitrogen retention. It is usually implanted 60 - 90
days before the intended date of slaughter.
After administration to cattle, TBA is rapidly hydrolyzed to
TBOH, the major metabolite being alpha-TBOH, occurring in the excreta,
bile, and liver. In muscle most of the TBOH is present as ß-TBOH.
Experiments with implantation of 200 mg of radiolabelled TBA in calves
and heifers showed that maximum levels of residues occurred about 30
days after implantation. The highest mean concentration of residues as
TBOH equivalents was 50 µg/kg in liver, while muscle contained
3 µg/kg.
The results of the studies requested at the twenty-seventh
meeting were submitted for consideration by the present Committee. In
addition, new toxicological data were available on reproduction and
mutagenicity. The Committee also reviewed previously available data
including metabolic, teratogenicity, and carcinogenicity data.
Acute toxicity studies in several species showed TBA to be of low
toxicity, when given orally.
Experiments had been performed in rats to assess the effect of
TBA on pregnancy, on the reproductive function of multiple
generations, and on the development of the offspring to weaning.
Treatment at 0.3 and 0.5 ppm in the diet, equivalent to about
20-30 µg/kg b.w./day, was associated with mean weekly body weights for
female rats that were only slightly higher than those of controls, and
marginal differences in litter parameters, while treatment with TBA at
3.0 and 18.0 ppm in the diet was associated with a hormonal effect. It
was considered that TBA exerted no effect on reproductive performance
in the rat at 0.5 ppm in the diet, equivalent to 30 µg/kg b.w./day. No
teratogenic effect was seen in two feeding studies in rats at very
high doses of TBA. In a comprehensive range of in vivo and in vitro
mutagenicity studies, all tests were negative for TBA, ß-TBOH, and
TBA's major metabolite, alpha-TBOH, with the exception of the mutation
assay in mouse lymphoma cells, which gave equivocal results with
ß-TBOH and alpha-TBOH. The Committee also noted a report of an
equivocal result in a transformation study with ß-TBOH in Syrian
hamster embryo fibroblasts, and took into account the recognized
difficulty in interpreting results from this type of study.
The Committee reaffirmed the opinion expressed at its
twenty-seventh meeting regarding the results of long-term feeding
studies with TBA with rats and mice (Annex 1, reference 62). It
considered that the liver hyperplasia and tumours in mice fed high
doses of TBA (0.9 - 9 mg/kg b.w./day) and the slight increase in the
incidence of islet-cell tumours of the pancreas of rats fed TBA at
1.85 mg/kg b.w./day (the highest dose in the study) arose as a
consequence of the hormonal activity of TBOH.
The Committee therefore concluded that its safety assessment
could be based on establishing the no-hormonal-effect level. It
reviewed a study with castrated male rhesus macaque monkeys
administered ß-TBOH orally, and considered that this model could be
relevant to the human population. The castrated male rhesus monkey is
highly sensitive to compounds with antigonadotropic activity; the
Committee therefore adopted a conservative approach by using this
study as the basis for establishing an ADI for human beings. Despite
the small numbers in each group of monkeys studied, and the advanced
age of the animals used, the Committee set 2 µg/kg b.w./day as a
no-hormonal-effect level, based on assessment of histological changes
in the seminal vesicles. In the intact female rhesus monkey, TBA had a
clear no-hormonal-effect level of 10 µg/kg b.w./day. The Committee
also considered that the pig was a sensitive model for assessing
hormonal effects and noted that, here too, TBA had a no-hormonal-
effect level of 2 µg/kg b.w./day based on assessment of pathological
changes in the testes. Another study in the pig demonstrated that the
hormonal activity of ß-TBOH was about ten times that of alpha-TBOH. No
data on individual animals were available for any of the pig studies.
In the absence of satisfactory toxicological data the Committee
was unable to establish a separate no-effect level for the alpha-TBOH
metabolite. It also noted that this metabolite was not produced in
significant amounts in the rat, which made it inadvisable to
extrapolate from data generated from ß-epimer experiments in that
species.
EVALUATION
Level causing no hormonal effect
Pig: 0.1 ppm in the diet, equal to 2 µg/kg b.w./day.
Monkey: 2 µg/kg b.w./day.
Estimate of temporary acceptable daily intake
0 - 0.01 µg/kg b.w.
Further work or information
Required (by 1990):
( a) the final reports, with supporting data, for the tissue residue
studies in which TBA was administered to heifers and TBA in
combination with estradiol-17ß was administered to steers;
( b) histopathological data on individual animals from the three
hormonal studies in pigs that were reviewed by the Committee;
( c) results from a 90-day study in an appropriate species, with
orally administered alpha-TBOH.
REFERENCES
Allen, J.A. & Proudlock, R.J. (1983). Autoradiographic assessment of
DNA repair in mammalian cells after exposure to 17-alpha-hydroxy-
trenbolone and to 17-ß-hydroxytrenbolone. Unpublished report
No. RSL 619/83731 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Brooker, P.C., & McCaffrey, K.J. (1985). Analysis of
metaphase chromosomes obtained from CHO cells cultured in vitro and
treated with 17-ß-hydroxytrenbolone. Unpublished report No. RSL
676/85631 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Allen, J.A., Proudlock, R.J., & Pugh, L.C. (1986). Micronucleus test
on 17-ß-hydroxytrenbolone. Unpublished report No. RSL 728/861306 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Allen, J.A. & Proudlock, R.J. (1987). Assessment of 17-alpha- and
17-ß-hydroxytrenbolone in the Solt-Farber system for induction of
liver foci. Unpublished report No. RSL 711/861252 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981a). R 1697 (trenbolone
acetate). Single administration study by oral route in the mouse.
Unpublished report No. 81213-A-JL/36-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, E., & Glomot, R. (1981b). R 1697 (trenbolone
acetate). Single administration study by oral route in the rat.
Unpublished report No. 81212-A-JL/37-A from Roussel Uclaf Research
Centre. Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L., Collas, g., & Glomot, R. (1981c). R 1697 (trenbolone
acetate). Single administration study by oral route in the dog.
Unpublished report No. 81216 from Roussel Uclaf Research Centre.
Submitted to WHO by Roussel Uclaf, Paris, France.
Barraud, B., Lugnier, A., & Dirheimer, G. (1983). In vivo covalent
binding to rat liver DNA of trenbolone as compared to 17-ß-estradiol,
testosterone and zeranol. In: Anabolics in animal production,
International Office of Epizootics, Paris, pp. 193-206.
Bouffault, J.C. (1977). Expert statement concerning the excretion of
trenbolone and oestradiol-17-alpha in urine of calves after
subcutaneous implantation of trenbolone acetate and oestradiol 17-ß.
Unpublished report submitted to WHO by Roussel Uclaf, Paris, France.
Chasseaud, L.F., Hawkins, D.R., Elsom, L.F., Parsonage, M.T.,
Ross, D.B., & Roberts, N.L. (1976). Plasma and tissue concentrations
of radioactivity in cattle after implantation of pellets containing
3H-trenboloneacetete, 3H-oestradiol or combinations of radioactive
drugs with the non-radioactive form of the other. Unpublished report
No. SVT/11/76351 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Edgar, D.H., Bosworth, H.J., Ransome, S.J., & Banks, S.J. (1985). An
assessment of the mutagenic potential of 17-ß-hydroxytrenbolone in
mammalian cells in vitro using the Chinese hamster ovary/HGPRT locus
assay. Unpublished report No. RSL 677/85642 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Escuret, P. & Bas M.N. (1978). DL-50 chez le rat et chez le souris.
Unpublished report Recherches Veterinaires R.U.-essai 5.77.71 R.
Submitted to WHO by Roussel Uclaf, Paris, France.
Gropp, J., Herlyn, D., Boehncke, E., Schulz, V., Sanderleben, J.V.,
Hanichen, Th., & Geisel, O. (1975). Physiological data including
evaluation of immunoresponse in relation to anabolic effects on veal
calves. Published in "Anabolic agents in animal production",
FAO/WHO Symposium, pp. 131-141, March 1975.
Gropp, J., Buntenkotter, S., & Kaemmerer, K. (1978). Relay toxicity of
anabolic agents, general considerations and some experimental data.
Published at Relay Toxicity and Bioavailability Symposium,
5 October 1978. Report submitted to WHO by Roussel Uclaf,
Paris, France.
Hawkins, D.R., Weston, K.T., Ray, J., Ross, D.B., & Cameron, D.M.
(1979). The bioavailability of trenboloneacetate residues in the
edible tissues of heifers after oral administration to rats.
Unpublished report No. RSL/328/7959 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hawkins, D.R., Waller, A.R., Moore, D.H., Jordan, M.C., Roberts, N.L.,
& Cameron, D. (1984). Tissue residues of radioactivity at 15 and 30
days after implantation of 3H-trenboloneacetate in calves.
Unpublished report No. HRC/RSL/636 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Heitzman, R.J. (1975). The effectiveness of anabolic agents in
increasing rate of growth in farm animals; report on experiments in
cattle. Published in "Anabolic agents in animal production", FAO/WHO
Symposium, pp. 89-98, March 1975.
Heitzman, R.J. & Hardwood, D.J. (1977). Residue levels of trenbolone
and oestradiol-17-ß in plasma and tissues of steers implanted with
anabolic steroid preparations, Br. Vet. J., 133, 564.
Henderson, L.M., Bosworth, H.J., Ransome, S.J., Banks, S.J., & Ozel,
K.J. (1986a). An assessment of the mutagenic potential of 17-alpha-
hydroxytrenbolone in mammalian cells in vitro using the Chinese
hamster ovary/HPRT locus assay. Unpublished report No. RSL 713/86713
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Henderson, L.M., Brooker, P.C., & Howell, A. (1986b). An assessment of
the mutagenic potential of 17-ß-hydroxytrenbolone in mammalian cells
in vitro using a Chinese hamster ovary micronucleus test.
Unpublished report No. RSL 729/861287 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., & Bosworth, H.J. (1987a).
An assessment of the carcinogenic potential of 17-ß-hydroxytrenbolone
and testosterone in an in vitro cell transformation assay using
mouse C3H10T1/2 cells. Unpublished report No. RSL 691/861257 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Henderson, L.M., Ransome, S.J., Banks, S.J., Bosworth, H.J.,
Bottoms, M., & Williams, J. (1987b). An assessment of the mutagenic
potential of 17-ß-hydroxytrenbolone in mammalian cells in vitro
using the Chinese hamster V79/HPRT locus assay. Unpublished report
No. RSL 712/861249 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hess, D.L. (1983). Determination of a hormonal no-effect dose level
for 17-ß-trenbolone Rhesus Macaque. Unpublished report from Oregon
Regional Primate Research Center, Beaverton, OR, USA. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hess, D.L. (1984). Determination of the hormonal no-effect dose level
for trenbolone acetate in the female Rhesus Macaque. Unpublished
report from Oregon Regional Primate Research Center, Beaverton, OR,
USA. Submitted to WHO by Roussel Uclaf, Paris, France.
Hoffman, B., Schopper, D., & Karg, H. (1984). Investigations on the
occurrence of non-extractable residues of trenbolone acetate in cattle
tissues in respect to their bioavailability and immunological
reactivity. Food Additives and Contaminants., 1, p. 253.
Hossack, D.J.N., Richold, M., & Jones, E. (1978). Ames metabolic
activation test to assess the potential mutagenic effect of trenbolone
acetate, 17-ß-oestradiol and 7:1 mixture of trenbolone with
17-ß-oestradiol. Unpublished report No. RSL 351/781136 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Huis in't Veld, L.G., Have, J. ten, & Kok, G.L. (1973). Hormonale
werkingen van trenbolone-acetaat. Unpublished report No. U 146/73 ENDO
from the National Institute of Public Health, The Netherlands.
Hunter, B., Macrae, S., Prentice, D.E., & Brooks, P.N. (1976a).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 235/76316
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Macrae, S., Prentice, D.E., & Majedd, S.K. (1976b).
Preliminary assessment of the toxicity of trenboloneacetate when
incorporated in the diet of mice. Unpublished report No. RSL 213/75992
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Hunter, B., Bathman, P., Heywood, R., Street, A.E., Prentice, D.E., &
Majeed, S.K. (1976c). Trenboloneacetate oral toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RSL
237/76426 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Graham, C., Gibson, W.A., Read, R., Gregson, R., Heywood
R., Offer, J., Prentice, D., & Woodhouse, R. (1981). Long-term feeding
of trenboloneacetate to mice (final report). Unpublished report volume
I, II and III, No. RSL/267/80122 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Roussel Uclaf, Paris, France.
Hunter, B., Batham, P., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D., Gregson, R., & Offer, J. (1982). Trenboloneacetate
potential tumorigenic and toxic effects in prolonged administration to
rats following "in utero" exposure (final report). Unpublished report
volume I, II, III and IV, No. RSL 295-G/80974 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ingerowski, G.H., Scheutwinkel-Reich, M., & Stan, H.J. (1981).
Mutagenicity studies on veterinary anabolic drugs with the
Salmonella/microsome test. Mutagen. Res., 91, 93-98.
James, P.A., Cox, R., Clark, R., Palmer, A.K., & Offer, J. (1982).
Effect of trenboloneacetate on pregnancy of the rat. Unpublished
report No. RSL 458/81553 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., John, D.M., Gibson, W.A., Offer, J.M., &
Anderson, A. (1985). Effect of trenbolone acetate on reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/841004 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
James, P., Smith, J.A., & Parker, C.A. (1986). Supplementary study of
the effect of trenbolone acetate on pregnancy of the rat and
development of the offspring. Unpublished report No. RSL 695/851572
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Roussel Uclaf, Paris, France.
Kroes, R. (1972). De invloed van oestradiol en een combinatie van
oestradiol en trienbolone op de vesiculae semenalis van mestkalveren.
Unpublished report No. 162/72 Path. from the National Institute of
Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976a). Onderzoek
naar de invloed van ethynylestradiol, 17-alpha-oestradiol en
trenboloneacetaat alleen of in combinatie op beren, borgen en geiten.
Uitscheiding in urine en faeces en residuen in serum (ILOB proeven
98.06 98.07, 98.08, 98.09, 118.04 en 118.05). Unpublished report
No. 216/76 Path. from the National Institute of Public Health,
The Netherlands.
Kroes, R., Huis in't Veld, L.G., & Stephany, R.W. (1976b). Onderzoek
naar de invloed van combinatie-preparaten van mannelijke en
vrouwelijke hormonen of hormonaal werkende stoffen of mannelijke
mestkalveren (Een biologisch, chemisch en histologish onderzoek, ILOB
proef 25.14 P 72/82). Unpublished report No. 193/76 Path. from the
National Institute of Public Health, The Netherlands.
Kroes, R., Huis in't Veld, L.G., Both-Miedema, R., & van de Berg, R.
(1976c). Onderzoek naar de invloed van 17-ß-oestradiol, trenbolone-
acetaat en testosteron alleen of in combinatie, op stieren en ossen
(Uitscheiding in urine, aanwezigheid in serum en histologisch
onderzoek prostaat, ILOB-proeven 134-02 a, 134.02 b en 134.03).
Unpublished report No. 218/76 Path. from the National Institute of
Public Health, The Netherlands.
Kruskemper, H.L., Morgner, K.D., & Noell, G. (1967). Klinische
pharmakologie von Trienolonen, eine Gruppe neuartiger anabol wirksamer
Oestran-Derivate, Arzneim. Forsch., 17, 449-454.
Lutz, W.K (1979). In vivo covalent binding of organic chemicals to
DNA as a quantitative indicator in the process of chemical
carcinogenesis. Mutation Res., 65, 289-356.
Offer, J.M. (1985). Effect of trenbolone acetate on the reproductive
function of multiple generations in the rat. Unpublished report
No. RSL 634/85959 (Addendum to RSL 634/841004) from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Philibert, D. & Moguilewsky, M. (1983). Liaison de la testosterone de
la 17-ß-OH-trenbolone (RU 2341) et de son isomere, la 17-alpha-
OH-trenbolone (RU 27423) au niveau du plasma humain. Unpublished
report No. AN 39 from Roussel Uclaf Research Centre. Submitted to WHO
by Roussel Uclaf, Paris, France.
Pottier, J. Busigny, M., & Gradadam, J.A. (1973). Tissue residues in
cows after implantation of trenbolone acetate. J. Anim. Sci.,
37, 256 (Abstract No. 110F).
Pottier, J., Busigny, M., & Gradaclam, J.A. (1975). Plasma kinetics,
excretion in milk and tissue levels in the cow following implantation
of trenbolone acetate. J. Anim. Sci., 41, 968.
Pottier, J., Cousty, C., Heitzman, R.J., & Reynolds, I.P. (1978).
Metabolites trenbolone acetate R in the bile of the cow and the rat.
Report presented during the 1978 Joint Meeting of the American Dairy
Association and the American Society of Animal Science, Michigan State
University, East Lansing, 9-13 June. Submitted to WHO by Roussel
Uclaf, Paris, France.
Pottier, J. (1979). Trenboloneacetate tissue and biliary
concentrations of the position of 17 epimers of trenbolone in heifers
after 2 months of implantation. Unpublished report No. 799 from
Roussel Uclaf. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. & Richardson, J.C. (1982). Metaphase analysis on
17-ß-hydroxytrenbolone and 17-alpha-hydroxytrenbolone. Unpublished
report No. RSL/512/81962 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M., Jones, E., & Hales, J.F. (1982a). Ames metabolic
activation test to assess the potential mutagenic effect of
17-alpha-hydroxytrenbolone, 17-ß-hydroxytrenbolone and
17-alpha-oestradiol. Unpublished report No. RSL 567/82767 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Richold, M., Richardson, J.C., Proudlock, R.J., & Fleming, P.M.
(1982b). Effect of 17-ß-hydroxytrenbolone and 17-alpha-hydroxy-
trenbolone on cultured human lymphocytes. Unpublished report No. RSL
544/82136 from Huntingdon Research Centre, Huntingdon, England.
Submitted to WHO by Roussel Uclaf, Paris, France.
Richold, M. Dehnel, J.M., & Ransome, S. (1982c). An assessment of the
mutagenic potential of 4 compounds using an in vitro mammalian cell
test system. Unpublished report No. RSL 513/8286 from Huntingdon
Research Centre, Huntingdon, England. Submitted to WHO by Roussel
Uclaf, Paris, France.
Richold, M., Edgar, D.H., Ransome, S.J., & Banks, S.J. (1983). An
assessment of the mutagenic potential of four compounds using an
in vitro mammalian cell test system. Unpublished report from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Riis, P.M. & Suresh, T.P. (1976). The effect of a synthetic steroid
(trenbolone) on the rate of release and excretion of subcutaneously
administered estradiol in calves. Steroids, 27, 5-15.
Roberts, N.L. & Cameron D.M. (1985). Hormonal effects of orally
administered trenbolone. Findings from three studies in the domestic
pig. Unpublished report No. RSL 591/83499 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ross, D.B. (1980). Toxicology and residues of trenboloneacetate.
Published in: Conference on the Use, Residues and Toxicology of Growth
Promoters, Dublin, 9 April 1980, pp. 34-48.
Ross, D.B., Roberts, N.L., Cameron, D.M., Street, A.E., Crook, D.,
Prentice, D.E., & Cherry, C.P. (1980). Oral toxicity of trenbolone
acetate to growing pigs (Sus scrofa)- preliminary 14 weeks feeding
study. Unpublished report No. RSL 357/80152 from Huntingdon Research
Centre, Huntingdon, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ryan, J.J. & Hoffman, B. (1978). Trenbolone acetate: experiences with
residues in cattle tissues. J.A.O.A.C., 61, 1274-1279.
Schiffman, D., Metzler, M., Neudecker, T., & Henschler, D. (1985).
Morphological transformation of Syrian hamster embryo fibroblasts by
the anabolic agent trenbolone. Arch. Toxicol., 58, 59-63.
Schroder, R. (1971a). R 1697: Prüfung der androgenen und
muskelbildenden Wirkung bei oraler Verabreichung. Unpublished report
from Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Schroder, R. (1971b). R 1697: Untersuchung auf androgene und anabole
Wirkung, Untersuchung auf oestrogene Wirkung. Unpublished report from
Hoechst A.G. Submitted to WHO by Roussel Uclaf, Paris, France.
Seeger, K. (1971a). Anabolikum R 1697 (A 50273) as us vet.
Unpublished report from Hoechst A.G. Submitted to WHO by Roussel
Uclaf, Paris, France.
Seeger, K. (1971b). R 1967: Chronische Toxizitat per oral. Unpublished
report from Hoechst A.G. Submitted to WHO by Roussel Uclaf,
Paris, France.
Sovetal (1970). Toxicité cronique par voie sous-cutanée chez le rat
male et chez la femelle. Unpublished report No. R 1697 from Sovetal.
Submitted to WHO by Roussel Uclaf, Paris, France.
Stephany, R.W., Bosch, D., van den Sahertian, E.Th., & Kroes, R
(1976). Onderzoek naar de invloed van orale toediening van
trenboloneacetaat op vaarzen (ILOB-proef 134-01) (Eenchemisch
onderzoek). Unpublished report No. 215/76 Path. From the National
Institute of Public Health, The Netherlands.
Verbeke, R., Debackere M., Hicquet, R., Lauwers, H., Stevens, J.,
Pottier, G., Moer, D., Van Hoof, J., & Vermeersch, G. (1975). Quality
of the meat after the application of anabolic agents in young calves.
Published in: Anabolic Agents in Animal Production, FAO/WHO Symposium,
March 1975, pp. 123-130.
van de Wal, P. (1975). General aspects of the effectiveness of
anabolic agents in increasing protein production in farm animals, in
particular in bull calves. Published in: Anabolic Agents in Animal
Production, FAO/WHO Symposium, March 1975, pp. 60-78.
