
CLOSANTEL
1. EXPLANATION
Closantel is a broad-spectrum antiparasitic agent used against
several species and developmental stages of trematodes, nematodes and
arthropods. The anti-trematode activity of closantel is mainly used
against liver fluke. The anti-nematode and anti-arthropod activity is
especially used against those species which feed on blood or plasma.
The drug is widely used in sheep and cattle and can be used
either parenterally (s.c. or i.m.) or orally for both prophylactic and
therapeutic purposes and is available as drench, bolus and injectable
formulations. Closantel has also been combined with mebendazole and
several other benzimidazoles in drench formulations for sheep and with
levamisole in a bolus for cattle (Marsboom et al., 1989).
Closantel has not been evaluated previously by the Joint FAO/WHO
Expert Committee on Food Additives.
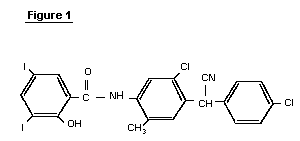
Closantel is a salicylanilide derivative with the structure shown
in Figure 1.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Sheep
Eight sheep, four of each sex, were used in this study. Two male
and two female animals were treated with a single intramuscular dose
of 5 mg/kg body weight of closantel. The remaining group of four
animals was treated with a single oral dose of closantel at 10 mg/kg
body weight. The solution used was the same (5% in propyleneglycol and
water).
Blood samples were taken from one male and one female of each
group 4, 8, 24, 48 and 96 hours after drug administration and then
twice weekly up to the time of sacrifice (2, 4, 6, and 8 weeks after
administration). Concentrations of unchanged closantel were
determined by gas-liquid chromatography.
Maximum plasma levels for both routes of administration were
reached 8 to 24 hours after dosing, ranging from 51 to 68 µg/ml after
intramuscular injection (5 mg/kg bw) and from 48 to 62 µg/ml after
oral administration (10 mg/kg b.w.). The drug was eliminated from the
plasma with a half-life of about 15 days, irrespective of the route of
administration. Up to 60% of the intramuscular dose was present in
the plasma up to about 4 days after injection, whereas only 25% to 30%
of the oral dose had reached the systemic circulation in the same time
interval.
The levels of closantel in tissues were 7 to 21 times lower than
the corresponding plasma concentrations. Highest tissue
concentrations were found in the lung and the kidney. Tissue levels
in liver, muscle and fat were somewhat lower (Michiels et al.,
1977a).
Two groups of five Texel sheep received orally or intramuscularly
10 and 5 mg/kg body weight, respectively, of 14C-labelled closantel
(specific activity 23.5 µCi/mg - radiochemical purity of 97.0%). The
solution was a 5% solution (propyleneglycol-water) with a specific
activity of 4.2 µCi/ml.
Blood samples were taken from one animal per group 4, 8, 24, 48,
96 and 168 hours after drug administration and then weekly up to the
time of sacrifice (14, 21, 35, 42 and 56 days) after treatment. Urine
and faeces were collected daily from dosing up to the time of
sacrifice. Samples of liver, lung, hind-leg muscle, mesenteric fat,
intact kidneys and heart were taken from each animal at sacrifice.
Peak plasma concentrations of radioactivity, occurring at 8-24
hours after drug administration, were 47.0 ± 11 and 47.9 ± 4.4 µg
equiv./ml for the oral and intramuscular route respectively. Plasma
radioactivity was almost exclusively due to the unmetabolized drug.
Closantel was eliminated from plasma with a half-life of 26.7 and 22.7
days after oral and intramuscular administration respectively,
indicating that the systemic availability of orally administered
closantel was half that of an intramuscular dose.
The average blood to plasma level ratio for radioactivity was
0.65, indicative of negligible binding to blood cells, whereas the
fraction bound to plasma proteins was very high (>99%). These data
were similar to those obtained for blood or plasma spiked with 14C-
closantel.
In the same study, within 8 weeks after oral or intramuscular
dosing, about 80% of the administrated radioactivity was excreted in
the faeces, and only 0.5% in the urine. The larger faecal excretion
of the radioactivity during the first two days after oral dosing
(43.3% in contrast with 10.4% after the intramuscular dose) reflected
the smaller systemic availability. Unchanged closantel accounted for
80 to 90% of the faecal radioactivity. As investigated by radio-HPLC,
the main metabolite in the faeces was monoiodoclosantel the 3-
iodoisomer and the 5-iodoisomer).
Residual radioactivity in all tissues but liver was exclusively
due to closantel and tissue levels were similar after the oral and the
intramuscular dose. Highest levels were found in the lung and kidney:
3.3-3.8 µg/g at 14-21 days after dosing (with 5 mg/kg b.w. i.m. or 10
mg/kg b.w. p.o.). Corresponding levels in heart were 1.7-2.8 µg/g, in
muscle 0.41-0.75 and in fat 0.08-0.42 µg/g. Radioactivity levels in
the liver were comparable with heart levels; in liver the contribution
of unmetabolized closantel was on average 71 and 61% after oral and
intramuscular administration respectively. Monoiodoclosantel was the
main metabolite accounting for 30-40% in liver. Plasma levels were
higher than corresponding tissue levels: 6 to 7 times for lung and
kidney, 9 to 15 times for heart and liver, about 30 times for muscle
and some 100 times for fat. Since these plasma to tissue level ratios
were independent of the time interval after dosing and of the route of
administration, the elimination of closantel from plasma presents an
excellent reflection of the depletion from tissues (Meuldermans
et al., 1982).
2.1.1.2 Cattle
Three dairy cows were treated with a single intramuscular dose of
closantel at 5 mg/kg body weight given as 5% injectable solution.
Blood and milk samples were taken before administration and at the
following intervals : 4 and 8 hours, and 1, 2, 3, 4, 5, 6, 7, 14, 21,
28 and 35 days.
Maximum levels of unaltered drug in plasma were reached 2 to 4
days after administration amounting to about 45 µg/ml. This peak
level corresponds to nearly 45% of the administered dose, assuming
that the whole plasma volume accounts for 5% of the cows' body weight.
Maximal concentrations in milk, amounting to about 1 µg/ml were
found about 4 days after intramuscular injection. Assuming a daily
production of 20 liters approximately 1% of the administered dose was
excreted with the milk per day.
From 4 days after injection, elimination of closantel from both
plasma and milk occurred with the same half-life of about 12 days,
indicating an equilibrium between both body fluids. Concentrations of
closantel in the milk, however, were nearly 45 times lower than the
corresponding plasma levels. No major variations between the 3 cows
could be observed either for plasma or for milk (Michiels et al.,
1977b).
2.1.2. Biotransformation
2.1.2.1 Rats
Five male Wistar rats (about 242g) were dosed orally with 14C-
closantel at 10 mg/kg b.w. The drug, labeled in the 14C-carbonyl
carbon, had a radiochemical purity of approx. 97%. Urine and faeces
were collected at 24-hr periods up to 10 days post dosing. Plasma was
collected at sacrifice after the 10-day collection period. Samples
were analyzed for total radioactivity by combustion and counting or by
direct counting. Unchanged drug and metabolites were determined using
HPLC and co-chromatography with reference standards.
Radioactivity was found to be excreted primarily through the
faeces. After 10 days, faecal excretion amounted to 88.4% of the
dose. Over that same time period, only 0.4% of the dose was in the
urine. Approximately half the dose was excreted within 2 days after
dosing.
An examination of the faeces for metabolites evidenced unchanged
closantel (90% of the radioactivity in the faeces at 0-24 hr
collection period, 76% at 192-240 hr) and monoidoclosantel (3.4% of
the sample radioactivity at the first collection, 19% at the last).
The monoiodoclosantel was stated to be predominantly the 3-iodo
isomer. Also present in faeces were deiodinated closantel (trace
amounts) and an unidentified metabolite (approx. 3-6% of the faecal
radioactivity).
In the urine, closantel and a metabolite that co-eluted with
monoiodosalicylic acid were observed. This latter compound would
result from reductive deiodination and amide hydrolysis of closantel.
No sulfate or glucuronide conjugates were detected.
Total residues of closantel in plasma amounted to 3.54 ppm 10
days after dosing. Even after that 10-day period, closantel was 93.4%
of the total radioactivity. Monoiodoclosantel was 4.7% of the plasma
radioactivity.
These data demonstrate the similarity of the metabolism of
closantel in rats and sheep. The scheme shown in Figure 2 would,
therefore, apply to rats, with the possibility of amide hydrolysis
occurring after the initial deiodination step (Mannens, et al.,
1989).
2.1.2.2 Sheep
Following oral (10 mg/kg b.w.) or intramuscular (5 mg/kg b.w.)
administration of 14C-closantel to sheep, HPLC analysis of urine and
faecal extracts indicated that about 90% of the excreted radioactivity
was due to unmetabolized closantel. With radio-HPLC, apart from the
main peak of closantel, two additional radioactivity peaks were
detected in the faecal extracts which, according to HPLC
cochromatography with authentic reference compounds, corresponded to
two monoiodoclosantel isomers. Quantification of the peaks showed
that 3-monoiodoclosantel was present in a relatively larger amount
than the 5-isomer (see Figure 2). In urine, the monoiodoclosantel
isomers were of relatively minor importance, whereas other
metabolites, not corresponding to available reference compounds, were
observed. Since the total urinary excretion of radioactivity amounted
to only 0.5% of the administered dose, no attempts were made to
elucidate these structures. Neither deiodinated closantel nor 3,5-
diiodosalicylic acid could be detected in the excreta (Michiels et
al., 1987).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Sheep
Eight sheep, four of each sex, were used in this study. Two male
and two female animals were treated with a single intramuscular dose
of 5 mg/kg body weight of closantel. The remaining group of four
animals was treated with a single oral dose of closantel at 10 mg/kg
body weight. The solution used was the same (5% in propyleneglycol and
water).
Blood samples were taken from one male and one female of each
group 4, 8, 24, 48 and 96 hours after drug administration and then
twice weekly up to the time of sacrifice (2, 4, 6, and 8 weeks after
administration). Concentrations of unchanged closantel were
determined by gas-liquid chromatography.
Maximum plasma levels for both routes of administration were
reached 8 to 24 hours after dosing, ranging from 51 to 68 µg/ml after
intramuscular injection (5 mg/kg bw) and from 48 to 62 µg/ml after
oral administration (10 mg/kg b.w.). The drug was eliminated from the
plasma with a half-life of about 15 days, irrespective of the route of
administration. Up to 60% of the intramuscular dose was present in
the plasma up to about 4 days after injection, whereas only 25% to 30%
of the oral dose had reached the systemic circulation in the same time
interval.
The levels of closantel in tissues were 7 to 21 times lower than
the corresponding plasma concentrations. Highest tissue
concentrations were found in the lung and the kidney. Tissue levels
in liver, muscle and fat were somewhat lower (Michiels et al.,
1977a).
Two groups of five Texel sheep received orally or intramuscularly
10 and 5 mg/kg body weight, respectively, of 14C-labelled closantel
(specific activity 23.5 µCi/mg - radiochemical purity of 97.0%). The
solution was a 5% solution (propyleneglycol-water) with a specific
activity of 4.2 µCi/ml.
Blood samples were taken from one animal per group 4, 8, 24, 48,
96 and 168 hours after drug administration and then weekly up to the
time of sacrifice (14, 21, 35, 42 and 56 days) after treatment. Urine
and faeces were collected daily from dosing up to the time of
sacrifice. Samples of liver, lung, hind-leg muscle, mesenteric fat,
intact kidneys and heart were taken from each animal at sacrifice.
Peak plasma concentrations of radioactivity, occurring at 8-24
hours after drug administration, were 47.0 ± 11 and 47.9 ± 4.4 µg
equiv./ml for the oral and intramuscular route respectively. Plasma
radioactivity was almost exclusively due to the unmetabolized drug.
Closantel was eliminated from plasma with a half-life of 26.7 and 22.7
days after oral and intramuscular administration respectively,
indicating that the systemic availability of orally administered
closantel was half that of an intramuscular dose.
The average blood to plasma level ratio for radioactivity was
0.65, indicative of negligible binding to blood cells, whereas the
fraction bound to plasma proteins was very high (>99%). These data
were similar to those obtained for blood or plasma spiked with 14C-
closantel.
In the same study, within 8 weeks after oral or intramuscular
dosing, about 80% of the administrated radioactivity was excreted in
the faeces, and only 0.5% in the urine. The larger faecal excretion
of the radioactivity during the first two days after oral dosing
(43.3% in contrast with 10.4% after the intramuscular dose) reflected
the smaller systemic availability. Unchanged closantel accounted for
80 to 90% of the faecal radioactivity. As investigated by radio-HPLC,
the main metabolite in the faeces was monoiodoclosantel the 3-
iodoisomer and the 5-iodoisomer).
Residual radioactivity in all tissues but liver was exclusively
due to closantel and tissue levels were similar after the oral and the
intramuscular dose. Highest levels were found in the lung and kidney:
3.3-3.8 µg/g at 14-21 days after dosing (with 5 mg/kg b.w. i.m. or 10
mg/kg b.w. p.o.). Corresponding levels in heart were 1.7-2.8 µg/g, in
muscle 0.41-0.75 and in fat 0.08-0.42 µg/g. Radioactivity levels in
the liver were comparable with heart levels; in liver the contribution
of unmetabolized closantel was on average 71 and 61% after oral and
intramuscular administration respectively. Monoiodoclosantel was the
main metabolite accounting for 30-40% in liver. Plasma levels were
higher than corresponding tissue levels: 6 to 7 times for lung and
kidney, 9 to 15 times for heart and liver, about 30 times for muscle
and some 100 times for fat. Since these plasma to tissue level ratios
were independent of the time interval after dosing and of the route of
administration, the elimination of closantel from plasma presents an
excellent reflection of the depletion from tissues (Meuldermans
et al., 1982).
2.1.1.2 Cattle
Three dairy cows were treated with a single intramuscular dose of
closantel at 5 mg/kg body weight given as 5% injectable solution.
Blood and milk samples were taken before administration and at the
following intervals : 4 and 8 hours, and 1, 2, 3, 4, 5, 6, 7, 14, 21,
28 and 35 days.
Maximum levels of unaltered drug in plasma were reached 2 to 4
days after administration amounting to about 45 µg/ml. This peak
level corresponds to nearly 45% of the administered dose, assuming
that the whole plasma volume accounts for 5% of the cows' body weight.
Maximal concentrations in milk, amounting to about 1 µg/ml were
found about 4 days after intramuscular injection. Assuming a daily
production of 20 liters approximately 1% of the administered dose was
excreted with the milk per day.
From 4 days after injection, elimination of closantel from both
plasma and milk occurred with the same half-life of about 12 days,
indicating an equilibrium between both body fluids. Concentrations of
closantel in the milk, however, were nearly 45 times lower than the
corresponding plasma levels. No major variations between the 3 cows
could be observed either for plasma or for milk (Michiels et al.,
1977b).
2.1.2. Biotransformation
2.1.2.1 Rats
Five male Wistar rats (about 242g) were dosed orally with 14C-
closantel at 10 mg/kg b.w. The drug, labeled in the 14C-carbonyl
carbon, had a radiochemical purity of approx. 97%. Urine and faeces
were collected at 24-hr periods up to 10 days post dosing. Plasma was
collected at sacrifice after the 10-day collection period. Samples
were analyzed for total radioactivity by combustion and counting or by
direct counting. Unchanged drug and metabolites were determined using
HPLC and co-chromatography with reference standards.
Radioactivity was found to be excreted primarily through the
faeces. After 10 days, faecal excretion amounted to 88.4% of the
dose. Over that same time period, only 0.4% of the dose was in the
urine. Approximately half the dose was excreted within 2 days after
dosing.
An examination of the faeces for metabolites evidenced unchanged
closantel (90% of the radioactivity in the faeces at 0-24 hr
collection period, 76% at 192-240 hr) and monoidoclosantel (3.4% of
the sample radioactivity at the first collection, 19% at the last).
The monoiodoclosantel was stated to be predominantly the 3-iodo
isomer. Also present in faeces were deiodinated closantel (trace
amounts) and an unidentified metabolite (approx. 3-6% of the faecal
radioactivity).
In the urine, closantel and a metabolite that co-eluted with
monoiodosalicylic acid were observed. This latter compound would
result from reductive deiodination and amide hydrolysis of closantel.
No sulfate or glucuronide conjugates were detected.
Total residues of closantel in plasma amounted to 3.54 ppm 10
days after dosing. Even after that 10-day period, closantel was 93.4%
of the total radioactivity. Monoiodoclosantel was 4.7% of the plasma
radioactivity.
These data demonstrate the similarity of the metabolism of
closantel in rats and sheep. The scheme shown in Figure 2 would,
therefore, apply to rats, with the possibility of amide hydrolysis
occurring after the initial deiodination step (Mannens, et al.,
1989).
2.1.2.2 Sheep
Following oral (10 mg/kg b.w.) or intramuscular (5 mg/kg b.w.)
administration of 14C-closantel to sheep, HPLC analysis of urine and
faecal extracts indicated that about 90% of the excreted radioactivity
was due to unmetabolized closantel. With radio-HPLC, apart from the
main peak of closantel, two additional radioactivity peaks were
detected in the faecal extracts which, according to HPLC
cochromatography with authentic reference compounds, corresponded to
two monoiodoclosantel isomers. Quantification of the peaks showed
that 3-monoiodoclosantel was present in a relatively larger amount
than the 5-isomer (see Figure 2). In urine, the monoiodoclosantel
isomers were of relatively minor importance, whereas other
metabolites, not corresponding to available reference compounds, were
observed. Since the total urinary excretion of radioactivity amounted
to only 0.5% of the administered dose, no attempts were made to
elucidate these structures. Neither deiodinated closantel nor 3,5-
diiodosalicylic acid could be detected in the excreta (Michiels et
al., 1987).
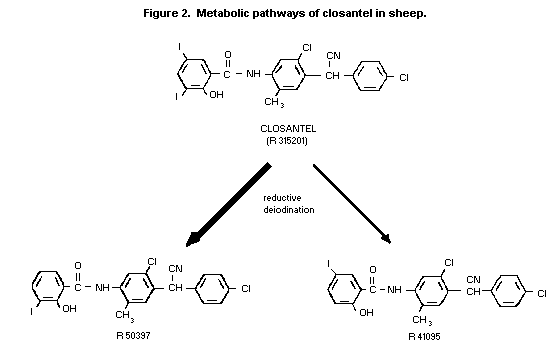 The position of the 14C label is indicated by the asterisk in the
structure of closantel.
2.1.3 Effects on enzymes and other biochemical parameters
The mode of action of salicylanilides has been reviewed in two
articles. Closantel is described as a hydrogen ionophore resulting in
the uncoupling of electron transport-associated phosphorylation (Behm
et al., 1985; Prichard, 1987).
Morphological and biochemical studies supporting this mechanism
of action are available. Ultrastructural changes were observed in
F. hepatica after treatment of artificially-infected sheep with 5
mg/kg body weight of closantel adminstered i.m. An untreated animal
served as the control. Each of the treated sheep was sacrificed at
4, 8, 12 and 24 hours after injection and flukes were collected in the
liver. Changes in morphology of F. hepatica's absorptive tissues
(intestine, gastrodermis, tegument and parenchymal cells) were noted.
Most common changes concerned mitochondria (Verheyen et al.,
1980).
In vitro and ex vivo studies conducted with F. hepatica
(rats, sheep) showed: closantel inhibits the phosphorylation from
electron transport in rat liver mitochondria in vitro (Van den
Bossche et al., 1979).
In vitro and ex vivo, studies conducted with F. hepatica
(rats) showed that closantel disturbs phosphorylation in liver fluke
mitochondria with no effect in rat liver mitochondria in vivo
(Van den Bossche et al., 1980).
In vitro studies conducted with F. hepatica (rats) showed
that closantel is an uncoupler of oxidative phosphorylation from
electron transport in rat liver and an inhibitor of mitochondrial
phosphorylation in F. hepatica. (Van den Bossche & Verhoeven,
1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity resulting from single dosing of closantel
solution in several animal species is summarized in Table 1.
In rats and mice the gross effects observed in the lethal dose
range were hypotonia, ataxia, diarrhoea and dyspnoea. In sheep and
cattle clinical signs of toxicity were anorrhexia, labored breathing,
recumbency, general weakness and decreased vision, or blindness,
appearing approximately one week after dosing. At the lethal dose,
anorrhexia, hypotonia, and quadriplegia preceded death.
Table 1: Acute toxicity data
Species Sex Route LD50 Reference
(mg/kg b.w.)
M Oral 331
Mouse F 453 Niemegeers, 1976
M i.m. 56.8
F 256.8
M Oral 342
Rat F 262 Niemegeers, 1976
M i.m. 325.9
F 28.4
Sheep Oral > 40 Marsboom, 1976a
i.m. > 40
Cattle Oral > 40 Marsboom, 1976a
i.m. > 20
2.2.2 Short-term studies
2.2.2.1 Rats
Four groups of Wistar rats (10 males and 10 females/group) were
fed diets containing closantel at 0, 25, 100 and 400 mg/kg diet
(equivalent to 0, 2.5, 10 and 40 mg/kg b.w./day) for 13 weeks. All
animals were observed daily for mortality and physical signs. Body
weight and food consumption were recorded weekly. Clinical
haematology, clinical biochemistry and urine analysis were performed
at time of sacrifice. Prior to sacrifice, a complete physical
examination and slit-lamp examination of the eyes were performed on
all rats. At sacrifice, detailed gross and histopathological
examinations (34 tissues) were also performed and organs were weighed.
Mortality, clinical behaviour, or body weight were not adversely
influenced by treatment. Food consumption was slightly decreased in
females (low and high dosage groups). Erythrocyte counts were
slightly decreased in the high-dose male and female groups.
Lymphocyte counts were slightly increased in males of the same group.
However, glucose was slightly increased in males at the high dose and
in females at the middle and high doses. Total bilirubin was also
increased in males at the high dose. Organ weights were generally
comparable between the controls and dosed rats, but decreased heart
weight in males and increased relative weight of the lung in females
were noted in the three dosed groups. The recorded values fell
within the range of historical control values however. An increased
absolute and relative weight of the gonads in males in the high dosage
group was noted.
A cystic distention of the epididymis was observed in one low
dose, two medium dose and seven high dose males. The effect was
considered to be drug and dose related. In one out of ten rats dosed
at 10 mg/kg b.w./day and eight out of ten rats dosed at 40 mg/kg
b.w./day spermatic granulomas with round cell infiltration, oedema and
fibrosis were found. Histological examination also revealed some
centrilobular fatty deposition in the livers of males dosed at 40
mg/kg b.w./day, but not in females. A slight deposition of the same
type was noted in two male rats dosed at 10 mg/kg b.w./day and in one
rat dosed at 2.5 mg/kg b.w./day.
Male gonads, and secondarily the liver, appear as target organs
for toxicity. The Committee concluded that the NOEL in this study was
2.5 mg/kg b.w./day (Marsboom et al., 1977a).
2.2.2.2 Dogs
Four groups of beagle dogs (3 males and 3 females/group) received
closantel powder in gelatin capsules at rates of 0, 2.5, 10 and 40
mg/kg b.w. each day for 3 months. All animals were observed daily for
mortality and physical signs. Before the study and at termination,
ophthalmoscopic and slit-lamp examinations of the eyes were performed.
Food consumption could not be recorded accurately because of wastage.
Body weights were determined weekly. Heart rate, ECG and blood
pressure values were recorded monthly. Clinical haematology, clinical
biochemistry and urine analysis were performed on all dogs 2 weeks
before the experiment began and at weeks 0, 2, 4, 8 and 13. After
sacrifice detailed gross and histopathological examinations (39
tissues) were also performed and selected organs were weighed (13
organs).
Mortality, clinical behaviour, body weight, haematology,
ophthalmoscopy, heart rate, ECG and blood pressure values were not
affected by treatment. All haematological values were within the
range of historical values. However, an increase of coagulation time
was noted at week 13 in the high dosage group. A slight increase of
total bilirubin was noted at week 13 in the medium and high dosage
groups; and a slight increase of SGOT at weeks 8 and 13 for the high
dosage group was observed. Urine analysis was unchanged. Organ
weights were not affected by treatment. Gross pathology showed no
difference between controls and treated animals. Histological
examinations were unremarkable except for a very slight increase of
fatty deposition in the liver of males and females in the high dose
group.
The liver appeared to be the target organ for toxicity in this
study. The NOEL was 2.5 mg/kg b.w./day, based on hepatotoxicity
observed at higher doses (Marsboom & Herin, 1978).
2.2.2.3 Sheep
Five groups of Suffolk sheep (3 males and 3 females/group)
received a total of ten doses of a solution of closantel (0, 10, and
40 mg/kg b.w. p.o. or 5 and 20 mg/kg b.w. i.m. given at four weekly
intervals. All animals were observed daily for mortality and physical
signs. Ophthalmoscopic and slit-lamp examinations of the eyes were
performed, once before the start of the study and again after the last
administration of the test substance. Body weight was determined
before and every four weeks during the study. Body temperature was
recorded before the study, daily during the first month, and
periodically thereafter. Clinical haematology and clinical
biochemistry were conducted prior to dosing and every 4 weeks during
the study. After sacrifice, gross pathology and histopathology
(liver, heart muscle, pancreas, kidneys, epididymis, ovaries, adrenals
and thyroids) were performed. The animals were sacrificed on day 1,
and weeks 4, 8, 12 and 16 after the last dosing. This was done in
order to study reversibility of possible lesions and to determine the
residual levels of closantel in various organs.
Four animals died from kidney disease. Closantel did not produce
mortality. Transient salivation and diarrhoea were observed in
animals dosed at 40 mg/kg b.w. p.o. route. A slight decrease of body
weight gain was observed in animals dosed at 20 mg/kg b.w. i.m. No
adverse effects on haematological parameters were noted. The few
slight variations observed in clinical chemistry were not dose-
related. Gross pathology showed reactions at the injection site in
two animals which had received 10 i.m. doses at 20 mg/kg b.w.
Histological examinations confirmed muscle irritation at the injection
site (5 and 20 mg/kg b.w.). Irregular degeneration of the germinal
epithelium was observed at 20 mg/kg b.w. i.m. (2 animals) and at 40
mg/kg b.w. p.o. (2 animals) (Marsboom et al., 1977b).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of albino Swiss mice (50 males and 50 females/group)
were fed diets containing 0, 25, 100 and 400 mg/kg diet, equivalent to
approximately 0, 5, 20 and 80 mg/kg b.w./day of closantel for 18
months. All animals were observed daily for mortality and physical
signs. Full necropsy was performed on all animals which died or were
sacrificed during the course of the study, and at the termination of
the study on all surviving animals.
Histopathological examination was performed systematically on
lungs, liver, pancreas, kidneys, spleen, lymph nodes, testes, ovaries,
mammary glands, adrenals, epididymis and hypophysis.
No significant effects on overall survival rate or on the time at
which mortalities occurred were noted in the various dosage groups of
either males or females. No dose related effects on health, behaviour
and appearance or gross pathology or the incidence or types of tumours
in albino Swiss mice were observed (Verstraeten et al. 1981a).
2.2.3.2 Rats
Four groups of Wistar rats (50 males and 50 females/group) were
fed diets containing 0, 25, 100 and 400 mg/kg, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day, closantel during 24 months. All animals
were observed daily for mortality and physical signs. Gross
examination was conducted on all animals which died or were sacrificed
during the course of the study, and at the termination of the study on
all surviving animals.
Gross lesion examination was performed on lungs, liver, pancreas,
kidneys, spleen, lymph nodes, testes, epididymis, ovaries, mammary
glands, adrenals and thyroids in animals killed during the study or at
the termination.
At the end of the study, cumulative mortalities in different
groups (0, 2.5, 10, and 40 mg/kg b.w./day) were 46/50, 43/50, 38/50,
49/50 for males and 32/50, 37/50, 36/50, 42/50 for females,
respectively. This rather high mortality could be related to the
typical short life-expectancy of the Wistar rat strain, bred in the
Janssen Laboratories used in this study. Closantel did not have a
very consistent effect on mortality. In females, a slight increase of
mortality was noted in the high dosage groups, whereas in males the
final mortality was not influenced by the treatment. The overall
mortality in rats was not affected at doses of 25 and 10 mg/kg b.w. in
either males or females. At 40 mg/kg b.w./day a slight increase of
mortality was noted in females.
The observed necropsy findings were comparable between groups
except for an increased incidence of spermatic granulomas in the 10
and 40 mg/kg b.w./day dosed males.
The over-all tumour rates were 40, 46, 58, 42% in males and 84,
75, 64, 47% in females in the control, low, mid and high dose groups
respectively. In females, there was a dose-dependent decrease of
total tumour incidence in the medium and high dosage groups. The
rather high number of adenomas seen in the 10 mg/kg b.w./day dosage
group is likely to be related to the longer survival of this dosage
group.
There was a significantly increased number of tumours of the
haematopoietic system in the mid-dose male rats. This increase did
not appear to be caused by the administration of closantel because:
- the increase was seen only at this level.
- the number of tumours of the haematopoietic system in the
control male rats, namely 3 tumours in 48 examined animals,
was unusually low when compared with the historical control
data from 9 other carcinogenicity studies done at the
Janssen facilities using the same Wistar rat in identical
experimental conditions.
Based upon comparison with the historical control data the
incidences of tumours of the haematopoietic system seen in the mid-and
low-dose males in this study were within the normal range of the
Wistar rat strain, whereas those of the control and the high-dose
males were significantly lower than normal. In the females, the
distribution of the different tumour types among the various groups
was very similar.
The incidence of histologically confirmed spermatic granulomas
seen in the epididymis of male rats is summarized in Table 2.
Table 2: Incidence of spermatic granulomas
Dosage group Incidence p-value
(mg/kg food) n/N
Control 0/48 -
25 0/49 -
100 7/50 <0.05
400 30/50 <0.001
An increase in the vacuolization of the optic nerve was observed
in the high-dose males and females.
The NOEL in this study was 2.5 mg/kg b.w./day (Verstraeten et
al., 1981b).
2.2.4 Reproduction study
2.2.4.1 Rats
Closantel was administered orally by gavage to male and female
Wistar rats once monthly during 3 successive generations each
producing 2 litters. The doses were 0, 2.5, 10 and 40 mg/kg b.w./mo
administered to 20 males and females/group.
Studied parameters in adults were: physical signs, body weight
gain and food consumption of dams during pregnancy, mortality and
gross pathology of males and females, and occurrence of mating,
pregnancy rates, and duration of gestation. Studied parameters in
pups were: litter size; weight at birth, weight and survival rate at
3 weeks of age, and abnormalities. The testes and epididymis were
examined histologically.
In adults, no adverse effects on body weight were observed
except at 40 mg/kg b.w./mo, where the marginal decrease of weight gain
in the 2nd litter of the 2nd and 3rd generation was associated with a
decrease in litter size. Food consumption was not affected.
Mortalities of males and females were comparable between groups. A
tendency to decreased pregnancy rate was noted at 40 mg/kg b.w./mo.
Occurrence of mating and duration of gestation were comparable between
groups.
In pups, no adverse effects on litter size could be detected,
except, at 40 mg/kg b.w./mo., a slight decrease in the number of
implants in the 2nd generation (2nd litter) and the 3rd generation
(1st and 2nd litters). No embryotoxicity effects were noted. Weight
at birth and at 3 weeks of age were comparable between groups.
Survival rate at 3 weeks of age was considered to be normal in all
groups.
Histopathological findings of spermatic granulomas (1/56 animals
at 10 mg/kg b.w./mo and 14/46 at 40 mg/kg b.w./mo) confirmed
previously reported findings in the rat.
The NOEL for closantel in this study was 2.5 mg/kg b.w./month
(Dirkx & Marsboom, 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg of closantel in the diet, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day during the period of organogenesis (day 6 to
day 15). The females were sacrificed on the 22nd day post mating.
Body weight, food consumption and mortalities of dams were measured.
Implantation number, pregnancies and pups, litter size and weight at
birth, resorption number, live and dead fetuses, number and
distribution of live, dead and resorbed embryos, and abnormalities
were measured.
There were no differences between groups. Teratogenic effects
were not observed (Marsboom, 1975).
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg closantel, equivalent to 0, 2.5, 10 and 40 mg/kg
b.w./day, from day 16 of pregnancy and during the 3 week lactation
period. Body weight, food consumption, mortality, pregnancy rate, and
the gestation period of dams were measured. Cannibalism, litter size,
weight at birth and at 2 and 3 weeks after birth, number of live and
stillborn fetuses, survival of pups at 4 days and 2 and 3 weeks after
birth and abnormalities in pups were quantified.
There were no differences between groups and no effects related
to treatment were observed (Marsboom, 1979).
2.2.5.2 Rabbits
Female New Zealand White rabbits (19 in the control group and 20
in each experimental group) received closantel (0, 10 and 40 mg/kg
b.w./day) orally by gavage from day 6 through day 18 of pregnancy, the
period of organogenesis. Females were sacrificed on the 28th day of
gestation. They were necropsied and checked for gross abnormalities.
The examined parameters were (for dams): pregnancy, body weight and
mortality; (for pups): resorption numbers, live and dead fetuses,
litter size, weight at delivery, survival rates after 24 hours, and
abnormalities.
One control female died on day 2 of the experiment from
pneumonia. No differences between groups were observed for body
weight, pregnancy and embryotoxic effects.
In the control group, two neonates from one female had bifurcated
ribs; in the low dosage group, 3 neonates from 3 different females
had either a deformed left fore-leg, a bifurcated rib or a waved rib;
in the high dosage group, 2 neonates from 2 different females had
either deformed thoracic bones or acrania.
Teratogenic or embryotoxic effects were not observed in this
study (Marsboom, 1976b).
2.2.5.3 Sheep
Ewes (274), separated into 10 treatment groups, received
closantel orally at 0, 20, or 40 mg/kg, either on day 11, 17 or 23
after mating. No influence of treatment was noted on the ovarian
cycle or on subsequent ovulation, copulation and conception. No
embryotoxic or teratogenic effects were reported in the clinical trial
(Chevis, 1977).
2.2.6 Special studies on fertility
2.2.6.1 Rats
The study was conducted using 20 males and 20 females/group.
Dose levels were 0, 25, 100 and 400 mg/kg food. Males were treated
for a minimum of 60 days prior to mating with non-dosed females.
Females were treated for at least 14 days prior to mating with non-
dosed males and then throughout gestation. Parameters studied were:
body weight, food consumption, pregnancy outcome, mortality in
parenteral and filial animals, number of implantations, litter size
and weight, the number and distribution of live, dead and resorbed
fetuses, and foetal abnormalities.
Body weight gain was comparable in all parental groups, except
for a lower weight gain in non-dosed females mated with dosed males of
the 400 mg/kg food group; food consumption was comparable in all
groups; no differences in pregnancy outcome were noted except a
reduced pregnancy rate in the high dosage group of non-dosed females
mated with dosed males; there were no mortalities.
No embryotoxic effects were noted except for a decrease in the
number of implants and litter size in the group of non-dosed females
mated with high-dosed males. No abnormalities were related to the
administration of closantel.
Fertility was not affected by the administration of closantel
except for a decreased pregnancy rate in non-dosed females mated with
high-dosed males (Marsboom, 1978).
2.2.6.2 Other species
Semen of bulls treated either once at approximately 2 mg/kg b.w.
or three times at 5 mg/kg b.w. intramuscularly at 8 weekly intervals
was normal (Debruyne, 1978); (Retief, undated a).
Semen of rams treated three times, by the oral route at 20 mg/kg
b.w. at 8 weekly intervals, was normal. (Retief, undated b).
In rams treated (15 mg/kg b.w. by the oral route) 3 or 5 times at
3 or 4 weekly intervals, the semen was normal, and the testes and
epididymis presented a normal structure (Johns, 1981).
2.2.7 Special studies on genotoxicity
Several tests were conducted to assess the genotoxicity of
closantel, they are summarized in Table 3.
Table 3: Results of genotoxicity assays on closantel
Test System Test object Concentration Results Reference
Cytotoxicity - L 5178 Y mouse 0.3-500 µg/ml Cytotoxic effects Enninger, 1989
lymphoma cells (1) in all 3 test
- V 79 Chinese systems. Reduced
hamster cells in presence of S9
- Chinese hamster mix
ovary (CHO) cells
Ames test (1) S. typhimurium 10-2000 µg/plate (2) Negative Poncelet, 1981
TA 1530, TA 1535,
TA 1538, TA 98,
TA 100
Ames test (1) S. typhimurium 1-1000 µg/plate (2) Negative Vanparays &
TA 1535, TA 1538, TA Marsboom, 1987
97, TA 98,
TA 100
In vivo: Drosophila melanogaster 10-50 ppm (3) Negative Vanparays &
sex linked recessive Marsboom, 1981
lethal test
In vivo: Male mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978a
oral treatment
(4)
In vivo: Female mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978b
oral treatment
(4)
DNA repair assay Cultured rat 0.3-100 µg/ml Negative Weterings, 1985
hepatocytes (5)
Table 3 (continued)
1 Both with and without rat liver S9 fraction
2 Sodium azide, nitrofluorene and 2-aminoanthracene were used as positive controls
3 Procarbazine was used as positive control
4 Cyclophosphamide used as positive control
5 7,12-Dimethyl benzanthracene was used positive control
2.3 Observations in humans
A single oral dose of closantel of 2.5 mg/kg b.w. in 5 patients
and a single s.c. injection of 2.5 mg/kg b.w. in one patient were
administered against F. hepatica. All orally treated patients
complained of the bitter taste of the drug, and one presented with
nausea and vomiting. In the last case, the dose was repeated adding
sugar and was then well tolerated. After the s.c. injection,
tachycardia, sweating, a metallic taste in the mouth, induction of
micturition and defecation, reddening of the skin, nervousness,
stress, excitation and a sensation of anguish occurred.
Haematology tests (haematocrit, WBC count and differential
prothrombin-time), clinical biochemistry tests (ALP, SGOT, SGPT,
cholesterol, and glucose), and urinalysis remained unchanged (2
examinations were carried out: one before, and the other seven days
after treatment) (Bernardiner, 1979).
Patients (14) were treated with a single oral dose of 5 mg/kg
b.w. of closantel. The treatment was well tolerated but poor efficacy
against Ancylostoma duodenale was obtained (Borda, 1980).
Patients (13) were treated with a single dose of closantel (6 at
5 mg/kg b.w; 3 at 7.5 mg/kg b.w.; 4 at 10 mg/kg b.w.). In all
treatment groups, side effects such as diarrhoea, drowsiness and
blurred vision were observed (Saowakontha, undated).
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of short-term studies, and of
studies of carcinogenicity, genotoxicity and effects on reproduction
and development.
In studies in rats and sheep, closantel was shown to be strongly
bound to the plasma proteins. The compound was poorly metabolized,
mainly to 3- and 5- monoiodoclosantel. About 90% of the administered
dose was excreted in the faeces, and about 90% was unchanged.
In a study in rats in which closantel was administered in the
diet at levels up to the equivalent of 40 mg per kg of body weight per
day for 13 weeks, spermatic granulomas in the epididymis and hepatic
fatty changes in the liver were observed at the highest dose level.
The NOEL was 2.5 mg per kg of body weight per day.
Dogs were dosed for 3 months by oral administration of the
compound in gelatin capsules at levels up to 40 mg per kg of body
weight per day. There were fatty changes in the liver at the highest
dose level. The NOEL for this study was 2.5 mg per kg of body weight
per day.
A carcinogenicity study was conducted in mice, which received
closantel in the diet at levels up to 80 mg per kg of body weight per
day for 18 months. There were no dose-related effects on the total
numbers of tumours nor on any individual type of tumour. No other
effects were observed in this study.
In a carcinogenicity study in rats in which closantel was
administered in the diet at levels up to 40 mg per kg of body weight
per day for 24 months, there was no effect on the overall incidence of
tumours. However, by comparison with concurrent controls, there was
a statistically significant increase in the incidence of
haematopoietic tumours in male rats at 10 mg per kg of body weight per
day. This incidence was nevertheless within the historical control
range, while the incidences found in the control and high-dose groups
were significantly lower than in the historical controls. Spermatic
granulomas were observed. The NOEL for this study was 2.5 mg per kg
of body weight per day.
Adverse effects on reproduction were probably associated with
toxicity to the male reproductive organs, and were seen only at doses
of 2.5 mg/kg body weight per day and above. In a fertility study in
rats, in which closantel was administered in the diet, there was a
reduced pregnancy rate in untreated females paired with males
receiving the highest dose of 40 mg per kg of body weight per day. A
three-generation reproduction study was conducted in male and female
rats, in which the compound was administered once monthly by gavage.
There was a decrease in the pregnancy rate and number of implants per
animal at the highest dose level of 40 mg per kg of body weight.
Spermatic granulomas were seen at 10 and 40 mg per kg of body weight
but not at 2.5 mg per kg of body weight.
In teratogenicity studies in rabbits and rats, no teratogenic or
toxic effects were evident at doses up to 40 mg per kg of body weight
per day. The rabbits received closantel by gavage on days 6 to 18 of
pregnancy, while the rats, were given the compound in the diet on days
6 to 15 of pregnancy.
Closantel gave negative results in a range of in vitro and
in vivo mutagenicity studies. No tests for clastogenicity were
performed, but the Committee noted that carcinogenicity studies had
been carried out in two species.
Limited clinical reports were available from 33 patients who were
treated with a single oral or parenteral dose of closantel at 2.5 to
10 mg per kg of body weight. There were no adverse effects.
4. EVALUATION
An ADI of 0-0.03 mg per kg of body weight was established for
closantel based on the NOEL of 2.5 mg per kg of body weight per day in
rats and a safety factor of 100.
5. REFERENCES
BEHM, C.A. & BRYANT, C. (1985). The mode of action of some modern
anthelmintics. In Anderson, N. & Waller, J.P. (Eds). Resistance in
Nematodes to anthelmintic drugs. Commonwealth Scientific & Industrial
Research Organization. Australia, pp. 57-67.
BERNARDINER, E. (1979). Preliminary results of the study with
Closantel in fluke disease. Unpublished data. N 1807 from Johnson-
Johnson. Buenos-Aires, Argentina. Submitted to WHO by Janssen
Pharmaceutica.
BORDA, E. (1980). Clinical efficacy and safety of Closantel (R 31520)
on pregnancy in sheep. Unpublished Report N 22122 from Corrientes,
Argentina. Submitted to WHO by Janssen Pharmaceutica.
CHEVIS, R. (1977). Investigation of the effect of Closantel (R 31522)
from on pregnancy in sheep. Unpublished trial Report V 2744, from
ETHNOR (PTY) LTD. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
DEBRUYNE, R. (1978). Fertility control in male cattle after
administration of Closantel (R 31520). Unpublished Report V 2859,
from Artificial Insemination Center. Oostmalle, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
DIRKX, P. & MARSBOOM, R. (1984). Oral three-generation study in
Wistar rats. Unpublished Report V 5183 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
ENNINGER, I.C. (1989). Evaluation of the cytotoxic activity of
Closantel R 31520 in L 5178 Y mouse lymphoma cells, in V 79 Chinese
hamster cells and in Chinese hamster ovary (CHO) cells. Unpublished
Report V 6999 from RCC NOTOX 2BV. S'-Hertogenbosch, the Netherlands.
Submitted to WHO by Janssen Pharmaceutica.
JOHNS, D. (1981). To determine the effect of Closantel on the
fertility of rams after five consecutive oral treatments at twice the
recommended rate. Unpublished Report V 4362, from Smith-Kline Animal
Health. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
KANE, H.J., BEHM, C.A. & BRYANT, C. (1980). Metabolic studies on the
new fasciolicidal drug, Closantel. Mol. and Biochem. Parasitol., 1,
347-355.
MANNENS, G., MOSTAMANS, E., VERBOVEN, P., HENDRICKX, J., HURKMANS, R.,
VAN LEEMPUT, L., MEULDERMANS, W. AND HEYKANTS, J. (1989). The
excretion and metabolism of 14-C-closantel in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished report V 7201. Submitted
to FAO by Janssen Pharmaceutica.
MARSBOOM, R. (1975). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished Report V 2403- from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1976a). Acute toxicity studies in sheep and cattle
single dosing: oral/intramuscular. Unpublished report preclinical
research Report V 2402 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in New Zeeland White rabbits (SEGMENT II). Unpublished Report V 2404
from Jansen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium, Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977a). Oral
toxicity study in Wistar rats (repeated dosages for 13 weeks).
Unpublished Report V 2700 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium, Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R., HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977b).
Oral and intramuscular toxicity study in Suffolk Sheep (10 doses per
animal one every fourth week). Unpublished Report V 2701 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1978). Oral male and female fertility study in Wistar
rats. (Segment I). Unpublished Report V 2860 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. & HERIN, V. (1978). Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished Report V 2985 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1979). Oral Embryotoxicity and teratogenicity study in
Wistar rats (peri. and postnatal study) (Segment III). Unpublished
Report V 3847 from Janssen Research Products Information Service,
Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, R. LAMPO, A., VAN CAUTEREN, H., VANPARYS, P.H., MAES, L. &
HEYKANTS, J. (1989). Review report on the toxicological,
pharmacodynamic and pharmacokinetic documentation related to
Closantel. Unpublished executive review report from Janssen Research
Foundation Products Information Service, Janssen Pharmaceutica,
Beerse, Belgium. Submitted to WHO by Jansen Pharmaceutica.
MEULDERMANS, W., MICHIELS, M., WOESTENBORGHS, R., VAN-HOUDT, J.,
LORREYNE, W., HENDRICKX, J., HEYKANTS, J. & DESPLENTER, L. (1982).
Absorption, tissue distribution, excretion and metabolism of
Closantel-14C after intramuscular and oral administration in sheep.
Unpublished preclinical research report V 4523 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Jansen Pharmaceutica.
MICHIELS, M., WOESTENBORGHS, R., HEYKANTS, J. & MARSBOOM, R. (1977a).
On the absorption and distribution of Closantel (R 31520) in sheep
after oral and intramuscular administration. Unpublished preclinical
research report V 2709 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MICHIELS, M., HENDRICKX, J., HEYKANTS, J., MARSBOOM, R. (1977b).
Plasma and milk concentrations of Closantel in cattle after a single
intramuscular administration. Unpublished preclinical research report
V 2706 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MICHIELS, M., MEULDERMANS, W. & HEYKANTS, J. (1987). The metabolism
and fat of Closantel (Flukiver) in sheep and cattle. Drug. Met.
Review, 18, (2 & 3), 235-251.
NIEMEGEERS, C.J.E. (1976). Acute intramuscular and oral toxicity of
R 31520 in mice and rats. Unpublished preclinical research report V
2396 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Jansssen
Pharmaceutica.
PONCELET, F. (1981). In vitro mutagenicity study of Closantel R
31520, lot B 4901. Unpublished Report V 3944 from Laboratoires de
Toxicologie, UCL. Woluwe, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
PRICHARD, R.K. (1987). The pharmacology of anthelmintics in
livestock. Int. J. Parasitol. 17, 473-482.
RETIEF, G. (undated a). To determine the effect of Closantel
administered s.c. at 5 mg/kg livemass after 3 treatments spaced at 8
weekly intervals on the semen quality of bulls. Unpublished Report V
3782 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
RETIEF, G. (undated b). To determine the effect of Closantel on the
fertility of rams after three consecutive oral treatments, 8 weeks
apart at double the recommended dosage rate. Unpublished Report V
3783 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
SOAWAKONTHA, S. (undated). Unpublished report from Thailand.
Submitted to WHO by Janssen Pharmaceutica.
VAN DEN BOSSCHE, H., VERHOEVEN, H., VANPARIJS. O., LAWERS, H. &
THIENPONT, H. (1979). Closantel a new antiparasitic hydrogen
ionophore. Arch. Int. Physiol. Biochimie., 87, 851-852.
VAN DEN BOSSCHE, H., VERHOEVEN, H. & LAWERS, H. (1980). Uncoupling of
liver mitochondria associated with fascioliasis in rats.
Normalization by Closantel. In: Van den Bossche (Ed). The host
invader interplay, Elsevier, Amsterdam, pp. 699-704.
VAN DEN BOSSCHE, H. & VERHOEVEN, H. (1983). Effects of albumin on the
Closantel induced alterations of mitochondrial processes. Unpublished
preclinical research report V 4546 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978a). Closantel R 31520. Dominant
lethal test in male mice. Unpublished report V 3144 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978b). Closantel R 31520. Dominant
lethal test in female mice. Unpublished report V 2998 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1981). Closantel R 31520. Sex linked
recessive lethal test on Drosophila Melanogaster. Unpublished report
V 4058 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1987). Closantel R 31520. Ames
reverse mutation test. Unpublished report V 6517 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VERHEYEN, A., VANPARYS, O., LAWERS, H. & THIENPONT, D. (1980). The
influence of Closantel administration to sheep on the ultrastructure
of the adult liver fluke F. hepatica L. In: van den Bossche (Ed).,
The host invader interplay, Elsevier, Amsterdam, pp. 705-708.
VERSTRAETEN, A., VANDENBERGHE, J. & MARSBOOM, R. (1981a). Oral
carcinogenicity study in Albino Swiss mice (repeated dosage for 18
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
VERSTRAETEN, A., VANDENBERGHE, J., HERIN, V. & MARSBOOM, R. (1981b).
Oral carcinogenicity study in Wistar rats (repeated dosage for 24
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
WETERINGS, P.J.J.M. (1985). Evaluation of the DNA repair inducing
ability of R 31520 in a primary culture of rat hepatocytes.
Unpublished Report V 5740 from NOTOX v.o.f, S'-Hertogenbosch, The
Netherlands. Submitted to WHO by Janssen Pharmaceutica.
The position of the 14C label is indicated by the asterisk in the
structure of closantel.
2.1.3 Effects on enzymes and other biochemical parameters
The mode of action of salicylanilides has been reviewed in two
articles. Closantel is described as a hydrogen ionophore resulting in
the uncoupling of electron transport-associated phosphorylation (Behm
et al., 1985; Prichard, 1987).
Morphological and biochemical studies supporting this mechanism
of action are available. Ultrastructural changes were observed in
F. hepatica after treatment of artificially-infected sheep with 5
mg/kg body weight of closantel adminstered i.m. An untreated animal
served as the control. Each of the treated sheep was sacrificed at
4, 8, 12 and 24 hours after injection and flukes were collected in the
liver. Changes in morphology of F. hepatica's absorptive tissues
(intestine, gastrodermis, tegument and parenchymal cells) were noted.
Most common changes concerned mitochondria (Verheyen et al.,
1980).
In vitro and ex vivo studies conducted with F. hepatica
(rats, sheep) showed: closantel inhibits the phosphorylation from
electron transport in rat liver mitochondria in vitro (Van den
Bossche et al., 1979).
In vitro and ex vivo, studies conducted with F. hepatica
(rats) showed that closantel disturbs phosphorylation in liver fluke
mitochondria with no effect in rat liver mitochondria in vivo
(Van den Bossche et al., 1980).
In vitro studies conducted with F. hepatica (rats) showed
that closantel is an uncoupler of oxidative phosphorylation from
electron transport in rat liver and an inhibitor of mitochondrial
phosphorylation in F. hepatica. (Van den Bossche & Verhoeven,
1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity resulting from single dosing of closantel
solution in several animal species is summarized in Table 1.
In rats and mice the gross effects observed in the lethal dose
range were hypotonia, ataxia, diarrhoea and dyspnoea. In sheep and
cattle clinical signs of toxicity were anorrhexia, labored breathing,
recumbency, general weakness and decreased vision, or blindness,
appearing approximately one week after dosing. At the lethal dose,
anorrhexia, hypotonia, and quadriplegia preceded death.
Table 1: Acute toxicity data
Species Sex Route LD50 Reference
(mg/kg b.w.)
M Oral 331
Mouse F 453 Niemegeers, 1976
M i.m. 56.8
F 256.8
M Oral 342
Rat F 262 Niemegeers, 1976
M i.m. 325.9
F 28.4
Sheep Oral > 40 Marsboom, 1976a
i.m. > 40
Cattle Oral > 40 Marsboom, 1976a
i.m. > 20
2.2.2 Short-term studies
2.2.2.1 Rats
Four groups of Wistar rats (10 males and 10 females/group) were
fed diets containing closantel at 0, 25, 100 and 400 mg/kg diet
(equivalent to 0, 2.5, 10 and 40 mg/kg b.w./day) for 13 weeks. All
animals were observed daily for mortality and physical signs. Body
weight and food consumption were recorded weekly. Clinical
haematology, clinical biochemistry and urine analysis were performed
at time of sacrifice. Prior to sacrifice, a complete physical
examination and slit-lamp examination of the eyes were performed on
all rats. At sacrifice, detailed gross and histopathological
examinations (34 tissues) were also performed and organs were weighed.
Mortality, clinical behaviour, or body weight were not adversely
influenced by treatment. Food consumption was slightly decreased in
females (low and high dosage groups). Erythrocyte counts were
slightly decreased in the high-dose male and female groups.
Lymphocyte counts were slightly increased in males of the same group.
However, glucose was slightly increased in males at the high dose and
in females at the middle and high doses. Total bilirubin was also
increased in males at the high dose. Organ weights were generally
comparable between the controls and dosed rats, but decreased heart
weight in males and increased relative weight of the lung in females
were noted in the three dosed groups. The recorded values fell
within the range of historical control values however. An increased
absolute and relative weight of the gonads in males in the high dosage
group was noted.
A cystic distention of the epididymis was observed in one low
dose, two medium dose and seven high dose males. The effect was
considered to be drug and dose related. In one out of ten rats dosed
at 10 mg/kg b.w./day and eight out of ten rats dosed at 40 mg/kg
b.w./day spermatic granulomas with round cell infiltration, oedema and
fibrosis were found. Histological examination also revealed some
centrilobular fatty deposition in the livers of males dosed at 40
mg/kg b.w./day, but not in females. A slight deposition of the same
type was noted in two male rats dosed at 10 mg/kg b.w./day and in one
rat dosed at 2.5 mg/kg b.w./day.
Male gonads, and secondarily the liver, appear as target organs
for toxicity. The Committee concluded that the NOEL in this study was
2.5 mg/kg b.w./day (Marsboom et al., 1977a).
2.2.2.2 Dogs
Four groups of beagle dogs (3 males and 3 females/group) received
closantel powder in gelatin capsules at rates of 0, 2.5, 10 and 40
mg/kg b.w. each day for 3 months. All animals were observed daily for
mortality and physical signs. Before the study and at termination,
ophthalmoscopic and slit-lamp examinations of the eyes were performed.
Food consumption could not be recorded accurately because of wastage.
Body weights were determined weekly. Heart rate, ECG and blood
pressure values were recorded monthly. Clinical haematology, clinical
biochemistry and urine analysis were performed on all dogs 2 weeks
before the experiment began and at weeks 0, 2, 4, 8 and 13. After
sacrifice detailed gross and histopathological examinations (39
tissues) were also performed and selected organs were weighed (13
organs).
Mortality, clinical behaviour, body weight, haematology,
ophthalmoscopy, heart rate, ECG and blood pressure values were not
affected by treatment. All haematological values were within the
range of historical values. However, an increase of coagulation time
was noted at week 13 in the high dosage group. A slight increase of
total bilirubin was noted at week 13 in the medium and high dosage
groups; and a slight increase of SGOT at weeks 8 and 13 for the high
dosage group was observed. Urine analysis was unchanged. Organ
weights were not affected by treatment. Gross pathology showed no
difference between controls and treated animals. Histological
examinations were unremarkable except for a very slight increase of
fatty deposition in the liver of males and females in the high dose
group.
The liver appeared to be the target organ for toxicity in this
study. The NOEL was 2.5 mg/kg b.w./day, based on hepatotoxicity
observed at higher doses (Marsboom & Herin, 1978).
2.2.2.3 Sheep
Five groups of Suffolk sheep (3 males and 3 females/group)
received a total of ten doses of a solution of closantel (0, 10, and
40 mg/kg b.w. p.o. or 5 and 20 mg/kg b.w. i.m. given at four weekly
intervals. All animals were observed daily for mortality and physical
signs. Ophthalmoscopic and slit-lamp examinations of the eyes were
performed, once before the start of the study and again after the last
administration of the test substance. Body weight was determined
before and every four weeks during the study. Body temperature was
recorded before the study, daily during the first month, and
periodically thereafter. Clinical haematology and clinical
biochemistry were conducted prior to dosing and every 4 weeks during
the study. After sacrifice, gross pathology and histopathology
(liver, heart muscle, pancreas, kidneys, epididymis, ovaries, adrenals
and thyroids) were performed. The animals were sacrificed on day 1,
and weeks 4, 8, 12 and 16 after the last dosing. This was done in
order to study reversibility of possible lesions and to determine the
residual levels of closantel in various organs.
Four animals died from kidney disease. Closantel did not produce
mortality. Transient salivation and diarrhoea were observed in
animals dosed at 40 mg/kg b.w. p.o. route. A slight decrease of body
weight gain was observed in animals dosed at 20 mg/kg b.w. i.m. No
adverse effects on haematological parameters were noted. The few
slight variations observed in clinical chemistry were not dose-
related. Gross pathology showed reactions at the injection site in
two animals which had received 10 i.m. doses at 20 mg/kg b.w.
Histological examinations confirmed muscle irritation at the injection
site (5 and 20 mg/kg b.w.). Irregular degeneration of the germinal
epithelium was observed at 20 mg/kg b.w. i.m. (2 animals) and at 40
mg/kg b.w. p.o. (2 animals) (Marsboom et al., 1977b).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of albino Swiss mice (50 males and 50 females/group)
were fed diets containing 0, 25, 100 and 400 mg/kg diet, equivalent to
approximately 0, 5, 20 and 80 mg/kg b.w./day of closantel for 18
months. All animals were observed daily for mortality and physical
signs. Full necropsy was performed on all animals which died or were
sacrificed during the course of the study, and at the termination of
the study on all surviving animals.
Histopathological examination was performed systematically on
lungs, liver, pancreas, kidneys, spleen, lymph nodes, testes, ovaries,
mammary glands, adrenals, epididymis and hypophysis.
No significant effects on overall survival rate or on the time at
which mortalities occurred were noted in the various dosage groups of
either males or females. No dose related effects on health, behaviour
and appearance or gross pathology or the incidence or types of tumours
in albino Swiss mice were observed (Verstraeten et al. 1981a).
2.2.3.2 Rats
Four groups of Wistar rats (50 males and 50 females/group) were
fed diets containing 0, 25, 100 and 400 mg/kg, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day, closantel during 24 months. All animals
were observed daily for mortality and physical signs. Gross
examination was conducted on all animals which died or were sacrificed
during the course of the study, and at the termination of the study on
all surviving animals.
Gross lesion examination was performed on lungs, liver, pancreas,
kidneys, spleen, lymph nodes, testes, epididymis, ovaries, mammary
glands, adrenals and thyroids in animals killed during the study or at
the termination.
At the end of the study, cumulative mortalities in different
groups (0, 2.5, 10, and 40 mg/kg b.w./day) were 46/50, 43/50, 38/50,
49/50 for males and 32/50, 37/50, 36/50, 42/50 for females,
respectively. This rather high mortality could be related to the
typical short life-expectancy of the Wistar rat strain, bred in the
Janssen Laboratories used in this study. Closantel did not have a
very consistent effect on mortality. In females, a slight increase of
mortality was noted in the high dosage groups, whereas in males the
final mortality was not influenced by the treatment. The overall
mortality in rats was not affected at doses of 25 and 10 mg/kg b.w. in
either males or females. At 40 mg/kg b.w./day a slight increase of
mortality was noted in females.
The observed necropsy findings were comparable between groups
except for an increased incidence of spermatic granulomas in the 10
and 40 mg/kg b.w./day dosed males.
The over-all tumour rates were 40, 46, 58, 42% in males and 84,
75, 64, 47% in females in the control, low, mid and high dose groups
respectively. In females, there was a dose-dependent decrease of
total tumour incidence in the medium and high dosage groups. The
rather high number of adenomas seen in the 10 mg/kg b.w./day dosage
group is likely to be related to the longer survival of this dosage
group.
There was a significantly increased number of tumours of the
haematopoietic system in the mid-dose male rats. This increase did
not appear to be caused by the administration of closantel because:
- the increase was seen only at this level.
- the number of tumours of the haematopoietic system in the
control male rats, namely 3 tumours in 48 examined animals,
was unusually low when compared with the historical control
data from 9 other carcinogenicity studies done at the
Janssen facilities using the same Wistar rat in identical
experimental conditions.
Based upon comparison with the historical control data the
incidences of tumours of the haematopoietic system seen in the mid-and
low-dose males in this study were within the normal range of the
Wistar rat strain, whereas those of the control and the high-dose
males were significantly lower than normal. In the females, the
distribution of the different tumour types among the various groups
was very similar.
The incidence of histologically confirmed spermatic granulomas
seen in the epididymis of male rats is summarized in Table 2.
Table 2: Incidence of spermatic granulomas
Dosage group Incidence p-value
(mg/kg food) n/N
Control 0/48 -
25 0/49 -
100 7/50 <0.05
400 30/50 <0.001
An increase in the vacuolization of the optic nerve was observed
in the high-dose males and females.
The NOEL in this study was 2.5 mg/kg b.w./day (Verstraeten et
al., 1981b).
2.2.4 Reproduction study
2.2.4.1 Rats
Closantel was administered orally by gavage to male and female
Wistar rats once monthly during 3 successive generations each
producing 2 litters. The doses were 0, 2.5, 10 and 40 mg/kg b.w./mo
administered to 20 males and females/group.
Studied parameters in adults were: physical signs, body weight
gain and food consumption of dams during pregnancy, mortality and
gross pathology of males and females, and occurrence of mating,
pregnancy rates, and duration of gestation. Studied parameters in
pups were: litter size; weight at birth, weight and survival rate at
3 weeks of age, and abnormalities. The testes and epididymis were
examined histologically.
In adults, no adverse effects on body weight were observed
except at 40 mg/kg b.w./mo, where the marginal decrease of weight gain
in the 2nd litter of the 2nd and 3rd generation was associated with a
decrease in litter size. Food consumption was not affected.
Mortalities of males and females were comparable between groups. A
tendency to decreased pregnancy rate was noted at 40 mg/kg b.w./mo.
Occurrence of mating and duration of gestation were comparable between
groups.
In pups, no adverse effects on litter size could be detected,
except, at 40 mg/kg b.w./mo., a slight decrease in the number of
implants in the 2nd generation (2nd litter) and the 3rd generation
(1st and 2nd litters). No embryotoxicity effects were noted. Weight
at birth and at 3 weeks of age were comparable between groups.
Survival rate at 3 weeks of age was considered to be normal in all
groups.
Histopathological findings of spermatic granulomas (1/56 animals
at 10 mg/kg b.w./mo and 14/46 at 40 mg/kg b.w./mo) confirmed
previously reported findings in the rat.
The NOEL for closantel in this study was 2.5 mg/kg b.w./month
(Dirkx & Marsboom, 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg of closantel in the diet, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day during the period of organogenesis (day 6 to
day 15). The females were sacrificed on the 22nd day post mating.
Body weight, food consumption and mortalities of dams were measured.
Implantation number, pregnancies and pups, litter size and weight at
birth, resorption number, live and dead fetuses, number and
distribution of live, dead and resorbed embryos, and abnormalities
were measured.
There were no differences between groups. Teratogenic effects
were not observed (Marsboom, 1975).
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg closantel, equivalent to 0, 2.5, 10 and 40 mg/kg
b.w./day, from day 16 of pregnancy and during the 3 week lactation
period. Body weight, food consumption, mortality, pregnancy rate, and
the gestation period of dams were measured. Cannibalism, litter size,
weight at birth and at 2 and 3 weeks after birth, number of live and
stillborn fetuses, survival of pups at 4 days and 2 and 3 weeks after
birth and abnormalities in pups were quantified.
There were no differences between groups and no effects related
to treatment were observed (Marsboom, 1979).
2.2.5.2 Rabbits
Female New Zealand White rabbits (19 in the control group and 20
in each experimental group) received closantel (0, 10 and 40 mg/kg
b.w./day) orally by gavage from day 6 through day 18 of pregnancy, the
period of organogenesis. Females were sacrificed on the 28th day of
gestation. They were necropsied and checked for gross abnormalities.
The examined parameters were (for dams): pregnancy, body weight and
mortality; (for pups): resorption numbers, live and dead fetuses,
litter size, weight at delivery, survival rates after 24 hours, and
abnormalities.
One control female died on day 2 of the experiment from
pneumonia. No differences between groups were observed for body
weight, pregnancy and embryotoxic effects.
In the control group, two neonates from one female had bifurcated
ribs; in the low dosage group, 3 neonates from 3 different females
had either a deformed left fore-leg, a bifurcated rib or a waved rib;
in the high dosage group, 2 neonates from 2 different females had
either deformed thoracic bones or acrania.
Teratogenic or embryotoxic effects were not observed in this
study (Marsboom, 1976b).
2.2.5.3 Sheep
Ewes (274), separated into 10 treatment groups, received
closantel orally at 0, 20, or 40 mg/kg, either on day 11, 17 or 23
after mating. No influence of treatment was noted on the ovarian
cycle or on subsequent ovulation, copulation and conception. No
embryotoxic or teratogenic effects were reported in the clinical trial
(Chevis, 1977).
2.2.6 Special studies on fertility
2.2.6.1 Rats
The study was conducted using 20 males and 20 females/group.
Dose levels were 0, 25, 100 and 400 mg/kg food. Males were treated
for a minimum of 60 days prior to mating with non-dosed females.
Females were treated for at least 14 days prior to mating with non-
dosed males and then throughout gestation. Parameters studied were:
body weight, food consumption, pregnancy outcome, mortality in
parenteral and filial animals, number of implantations, litter size
and weight, the number and distribution of live, dead and resorbed
fetuses, and foetal abnormalities.
Body weight gain was comparable in all parental groups, except
for a lower weight gain in non-dosed females mated with dosed males of
the 400 mg/kg food group; food consumption was comparable in all
groups; no differences in pregnancy outcome were noted except a
reduced pregnancy rate in the high dosage group of non-dosed females
mated with dosed males; there were no mortalities.
No embryotoxic effects were noted except for a decrease in the
number of implants and litter size in the group of non-dosed females
mated with high-dosed males. No abnormalities were related to the
administration of closantel.
Fertility was not affected by the administration of closantel
except for a decreased pregnancy rate in non-dosed females mated with
high-dosed males (Marsboom, 1978).
2.2.6.2 Other species
Semen of bulls treated either once at approximately 2 mg/kg b.w.
or three times at 5 mg/kg b.w. intramuscularly at 8 weekly intervals
was normal (Debruyne, 1978); (Retief, undated a).
Semen of rams treated three times, by the oral route at 20 mg/kg
b.w. at 8 weekly intervals, was normal. (Retief, undated b).
In rams treated (15 mg/kg b.w. by the oral route) 3 or 5 times at
3 or 4 weekly intervals, the semen was normal, and the testes and
epididymis presented a normal structure (Johns, 1981).
2.2.7 Special studies on genotoxicity
Several tests were conducted to assess the genotoxicity of
closantel, they are summarized in Table 3.
Table 3: Results of genotoxicity assays on closantel
Test System Test object Concentration Results Reference
Cytotoxicity - L 5178 Y mouse 0.3-500 µg/ml Cytotoxic effects Enninger, 1989
lymphoma cells (1) in all 3 test
- V 79 Chinese systems. Reduced
hamster cells in presence of S9
- Chinese hamster mix
ovary (CHO) cells
Ames test (1) S. typhimurium 10-2000 µg/plate (2) Negative Poncelet, 1981
TA 1530, TA 1535,
TA 1538, TA 98,
TA 100
Ames test (1) S. typhimurium 1-1000 µg/plate (2) Negative Vanparays &
TA 1535, TA 1538, TA Marsboom, 1987
97, TA 98,
TA 100
In vivo: Drosophila melanogaster 10-50 ppm (3) Negative Vanparays &
sex linked recessive Marsboom, 1981
lethal test
In vivo: Male mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978a
oral treatment
(4)
In vivo: Female mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978b
oral treatment
(4)
DNA repair assay Cultured rat 0.3-100 µg/ml Negative Weterings, 1985
hepatocytes (5)
Table 3 (continued)
1 Both with and without rat liver S9 fraction
2 Sodium azide, nitrofluorene and 2-aminoanthracene were used as positive controls
3 Procarbazine was used as positive control
4 Cyclophosphamide used as positive control
5 7,12-Dimethyl benzanthracene was used positive control
2.3 Observations in humans
A single oral dose of closantel of 2.5 mg/kg b.w. in 5 patients
and a single s.c. injection of 2.5 mg/kg b.w. in one patient were
administered against F. hepatica. All orally treated patients
complained of the bitter taste of the drug, and one presented with
nausea and vomiting. In the last case, the dose was repeated adding
sugar and was then well tolerated. After the s.c. injection,
tachycardia, sweating, a metallic taste in the mouth, induction of
micturition and defecation, reddening of the skin, nervousness,
stress, excitation and a sensation of anguish occurred.
Haematology tests (haematocrit, WBC count and differential
prothrombin-time), clinical biochemistry tests (ALP, SGOT, SGPT,
cholesterol, and glucose), and urinalysis remained unchanged (2
examinations were carried out: one before, and the other seven days
after treatment) (Bernardiner, 1979).
Patients (14) were treated with a single oral dose of 5 mg/kg
b.w. of closantel. The treatment was well tolerated but poor efficacy
against Ancylostoma duodenale was obtained (Borda, 1980).
Patients (13) were treated with a single dose of closantel (6 at
5 mg/kg b.w; 3 at 7.5 mg/kg b.w.; 4 at 10 mg/kg b.w.). In all
treatment groups, side effects such as diarrhoea, drowsiness and
blurred vision were observed (Saowakontha, undated).
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of short-term studies, and of
studies of carcinogenicity, genotoxicity and effects on reproduction
and development.
In studies in rats and sheep, closantel was shown to be strongly
bound to the plasma proteins. The compound was poorly metabolized,
mainly to 3- and 5- monoiodoclosantel. About 90% of the administered
dose was excreted in the faeces, and about 90% was unchanged.
In a study in rats in which closantel was administered in the
diet at levels up to the equivalent of 40 mg per kg of body weight per
day for 13 weeks, spermatic granulomas in the epididymis and hepatic
fatty changes in the liver were observed at the highest dose level.
The NOEL was 2.5 mg per kg of body weight per day.
Dogs were dosed for 3 months by oral administration of the
compound in gelatin capsules at levels up to 40 mg per kg of body
weight per day. There were fatty changes in the liver at the highest
dose level. The NOEL for this study was 2.5 mg per kg of body weight
per day.
A carcinogenicity study was conducted in mice, which received
closantel in the diet at levels up to 80 mg per kg of body weight per
day for 18 months. There were no dose-related effects on the total
numbers of tumours nor on any individual type of tumour. No other
effects were observed in this study.
In a carcinogenicity study in rats in which closantel was
administered in the diet at levels up to 40 mg per kg of body weight
per day for 24 months, there was no effect on the overall incidence of
tumours. However, by comparison with concurrent controls, there was
a statistically significant increase in the incidence of
haematopoietic tumours in male rats at 10 mg per kg of body weight per
day. This incidence was nevertheless within the historical control
range, while the incidences found in the control and high-dose groups
were significantly lower than in the historical controls. Spermatic
granulomas were observed. The NOEL for this study was 2.5 mg per kg
of body weight per day.
Adverse effects on reproduction were probably associated with
toxicity to the male reproductive organs, and were seen only at doses
of 2.5 mg/kg body weight per day and above. In a fertility study in
rats, in which closantel was administered in the diet, there was a
reduced pregnancy rate in untreated females paired with males
receiving the highest dose of 40 mg per kg of body weight per day. A
three-generation reproduction study was conducted in male and female
rats, in which the compound was administered once monthly by gavage.
There was a decrease in the pregnancy rate and number of implants per
animal at the highest dose level of 40 mg per kg of body weight.
Spermatic granulomas were seen at 10 and 40 mg per kg of body weight
but not at 2.5 mg per kg of body weight.
In teratogenicity studies in rabbits and rats, no teratogenic or
toxic effects were evident at doses up to 40 mg per kg of body weight
per day. The rabbits received closantel by gavage on days 6 to 18 of
pregnancy, while the rats, were given the compound in the diet on days
6 to 15 of pregnancy.
Closantel gave negative results in a range of in vitro and
in vivo mutagenicity studies. No tests for clastogenicity were
performed, but the Committee noted that carcinogenicity studies had
been carried out in two species.
Limited clinical reports were available from 33 patients who were
treated with a single oral or parenteral dose of closantel at 2.5 to
10 mg per kg of body weight. There were no adverse effects.
4. EVALUATION
An ADI of 0-0.03 mg per kg of body weight was established for
closantel based on the NOEL of 2.5 mg per kg of body weight per day in
rats and a safety factor of 100.
5. REFERENCES
BEHM, C.A. & BRYANT, C. (1985). The mode of action of some modern
anthelmintics. In Anderson, N. & Waller, J.P. (Eds). Resistance in
Nematodes to anthelmintic drugs. Commonwealth Scientific & Industrial
Research Organization. Australia, pp. 57-67.
BERNARDINER, E. (1979). Preliminary results of the study with
Closantel in fluke disease. Unpublished data. N 1807 from Johnson-
Johnson. Buenos-Aires, Argentina. Submitted to WHO by Janssen
Pharmaceutica.
BORDA, E. (1980). Clinical efficacy and safety of Closantel (R 31520)
on pregnancy in sheep. Unpublished Report N 22122 from Corrientes,
Argentina. Submitted to WHO by Janssen Pharmaceutica.
CHEVIS, R. (1977). Investigation of the effect of Closantel (R 31522)
from on pregnancy in sheep. Unpublished trial Report V 2744, from
ETHNOR (PTY) LTD. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
DEBRUYNE, R. (1978). Fertility control in male cattle after
administration of Closantel (R 31520). Unpublished Report V 2859,
from Artificial Insemination Center. Oostmalle, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
DIRKX, P. & MARSBOOM, R. (1984). Oral three-generation study in
Wistar rats. Unpublished Report V 5183 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
ENNINGER, I.C. (1989). Evaluation of the cytotoxic activity of
Closantel R 31520 in L 5178 Y mouse lymphoma cells, in V 79 Chinese
hamster cells and in Chinese hamster ovary (CHO) cells. Unpublished
Report V 6999 from RCC NOTOX 2BV. S'-Hertogenbosch, the Netherlands.
Submitted to WHO by Janssen Pharmaceutica.
JOHNS, D. (1981). To determine the effect of Closantel on the
fertility of rams after five consecutive oral treatments at twice the
recommended rate. Unpublished Report V 4362, from Smith-Kline Animal
Health. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
KANE, H.J., BEHM, C.A. & BRYANT, C. (1980). Metabolic studies on the
new fasciolicidal drug, Closantel. Mol. and Biochem. Parasitol., 1,
347-355.
MANNENS, G., MOSTAMANS, E., VERBOVEN, P., HENDRICKX, J., HURKMANS, R.,
VAN LEEMPUT, L., MEULDERMANS, W. AND HEYKANTS, J. (1989). The
excretion and metabolism of 14-C-closantel in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished report V 7201. Submitted
to FAO by Janssen Pharmaceutica.
MARSBOOM, R. (1975). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished Report V 2403- from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1976a). Acute toxicity studies in sheep and cattle
single dosing: oral/intramuscular. Unpublished report preclinical
research Report V 2402 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in New Zeeland White rabbits (SEGMENT II). Unpublished Report V 2404
from Jansen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium, Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977a). Oral
toxicity study in Wistar rats (repeated dosages for 13 weeks).
Unpublished Report V 2700 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium, Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R., HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977b).
Oral and intramuscular toxicity study in Suffolk Sheep (10 doses per
animal one every fourth week). Unpublished Report V 2701 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1978). Oral male and female fertility study in Wistar
rats. (Segment I). Unpublished Report V 2860 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. & HERIN, V. (1978). Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished Report V 2985 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1979). Oral Embryotoxicity and teratogenicity study in
Wistar rats (peri. and postnatal study) (Segment III). Unpublished
Report V 3847 from Janssen Research Products Information Service,
Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, R. LAMPO, A., VAN CAUTEREN, H., VANPARYS, P.H., MAES, L. &
HEYKANTS, J. (1989). Review report on the toxicological,
pharmacodynamic and pharmacokinetic documentation related to
Closantel. Unpublished executive review report from Janssen Research
Foundation Products Information Service, Janssen Pharmaceutica,
Beerse, Belgium. Submitted to WHO by Jansen Pharmaceutica.
MEULDERMANS, W., MICHIELS, M., WOESTENBORGHS, R., VAN-HOUDT, J.,
LORREYNE, W., HENDRICKX, J., HEYKANTS, J. & DESPLENTER, L. (1982).
Absorption, tissue distribution, excretion and metabolism of
Closantel-14C after intramuscular and oral administration in sheep.
Unpublished preclinical research report V 4523 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Jansen Pharmaceutica.
MICHIELS, M., WOESTENBORGHS, R., HEYKANTS, J. & MARSBOOM, R. (1977a).
On the absorption and distribution of Closantel (R 31520) in sheep
after oral and intramuscular administration. Unpublished preclinical
research report V 2709 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MICHIELS, M., HENDRICKX, J., HEYKANTS, J., MARSBOOM, R. (1977b).
Plasma and milk concentrations of Closantel in cattle after a single
intramuscular administration. Unpublished preclinical research report
V 2706 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MICHIELS, M., MEULDERMANS, W. & HEYKANTS, J. (1987). The metabolism
and fat of Closantel (Flukiver) in sheep and cattle. Drug. Met.
Review, 18, (2 & 3), 235-251.
NIEMEGEERS, C.J.E. (1976). Acute intramuscular and oral toxicity of
R 31520 in mice and rats. Unpublished preclinical research report V
2396 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Jansssen
Pharmaceutica.
PONCELET, F. (1981). In vitro mutagenicity study of Closantel R
31520, lot B 4901. Unpublished Report V 3944 from Laboratoires de
Toxicologie, UCL. Woluwe, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
PRICHARD, R.K. (1987). The pharmacology of anthelmintics in
livestock. Int. J. Parasitol. 17, 473-482.
RETIEF, G. (undated a). To determine the effect of Closantel
administered s.c. at 5 mg/kg livemass after 3 treatments spaced at 8
weekly intervals on the semen quality of bulls. Unpublished Report V
3782 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
RETIEF, G. (undated b). To determine the effect of Closantel on the
fertility of rams after three consecutive oral treatments, 8 weeks
apart at double the recommended dosage rate. Unpublished Report V
3783 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
SOAWAKONTHA, S. (undated). Unpublished report from Thailand.
Submitted to WHO by Janssen Pharmaceutica.
VAN DEN BOSSCHE, H., VERHOEVEN, H., VANPARIJS. O., LAWERS, H. &
THIENPONT, H. (1979). Closantel a new antiparasitic hydrogen
ionophore. Arch. Int. Physiol. Biochimie., 87, 851-852.
VAN DEN BOSSCHE, H., VERHOEVEN, H. & LAWERS, H. (1980). Uncoupling of
liver mitochondria associated with fascioliasis in rats.
Normalization by Closantel. In: Van den Bossche (Ed). The host
invader interplay, Elsevier, Amsterdam, pp. 699-704.
VAN DEN BOSSCHE, H. & VERHOEVEN, H. (1983). Effects of albumin on the
Closantel induced alterations of mitochondrial processes. Unpublished
preclinical research report V 4546 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978a). Closantel R 31520. Dominant
lethal test in male mice. Unpublished report V 3144 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978b). Closantel R 31520. Dominant
lethal test in female mice. Unpublished report V 2998 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1981). Closantel R 31520. Sex linked
recessive lethal test on Drosophila Melanogaster. Unpublished report
V 4058 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1987). Closantel R 31520. Ames
reverse mutation test. Unpublished report V 6517 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VERHEYEN, A., VANPARYS, O., LAWERS, H. & THIENPONT, D. (1980). The
influence of Closantel administration to sheep on the ultrastructure
of the adult liver fluke F. hepatica L. In: van den Bossche (Ed).,
The host invader interplay, Elsevier, Amsterdam, pp. 705-708.
VERSTRAETEN, A., VANDENBERGHE, J. & MARSBOOM, R. (1981a). Oral
carcinogenicity study in Albino Swiss mice (repeated dosage for 18
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
VERSTRAETEN, A., VANDENBERGHE, J., HERIN, V. & MARSBOOM, R. (1981b).
Oral carcinogenicity study in Wistar rats (repeated dosage for 24
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
WETERINGS, P.J.J.M. (1985). Evaluation of the DNA repair inducing
ability of R 31520 in a primary culture of rat hepatocytes.
Unpublished Report V 5740 from NOTOX v.o.f, S'-Hertogenbosch, The
Netherlands. Submitted to WHO by Janssen Pharmaceutica.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Sheep
Eight sheep, four of each sex, were used in this study. Two male
and two female animals were treated with a single intramuscular dose
of 5 mg/kg body weight of closantel. The remaining group of four
animals was treated with a single oral dose of closantel at 10 mg/kg
body weight. The solution used was the same (5% in propyleneglycol and
water).
Blood samples were taken from one male and one female of each
group 4, 8, 24, 48 and 96 hours after drug administration and then
twice weekly up to the time of sacrifice (2, 4, 6, and 8 weeks after
administration). Concentrations of unchanged closantel were
determined by gas-liquid chromatography.
Maximum plasma levels for both routes of administration were
reached 8 to 24 hours after dosing, ranging from 51 to 68 µg/ml after
intramuscular injection (5 mg/kg bw) and from 48 to 62 µg/ml after
oral administration (10 mg/kg b.w.). The drug was eliminated from the
plasma with a half-life of about 15 days, irrespective of the route of
administration. Up to 60% of the intramuscular dose was present in
the plasma up to about 4 days after injection, whereas only 25% to 30%
of the oral dose had reached the systemic circulation in the same time
interval.
The levels of closantel in tissues were 7 to 21 times lower than
the corresponding plasma concentrations. Highest tissue
concentrations were found in the lung and the kidney. Tissue levels
in liver, muscle and fat were somewhat lower (Michiels et al.,
1977a).
Two groups of five Texel sheep received orally or intramuscularly
10 and 5 mg/kg body weight, respectively, of 14C-labelled closantel
(specific activity 23.5 µCi/mg - radiochemical purity of 97.0%). The
solution was a 5% solution (propyleneglycol-water) with a specific
activity of 4.2 µCi/ml.
Blood samples were taken from one animal per group 4, 8, 24, 48,
96 and 168 hours after drug administration and then weekly up to the
time of sacrifice (14, 21, 35, 42 and 56 days) after treatment. Urine
and faeces were collected daily from dosing up to the time of
sacrifice. Samples of liver, lung, hind-leg muscle, mesenteric fat,
intact kidneys and heart were taken from each animal at sacrifice.
Peak plasma concentrations of radioactivity, occurring at 8-24
hours after drug administration, were 47.0 ± 11 and 47.9 ± 4.4 µg
equiv./ml for the oral and intramuscular route respectively. Plasma
radioactivity was almost exclusively due to the unmetabolized drug.
Closantel was eliminated from plasma with a half-life of 26.7 and 22.7
days after oral and intramuscular administration respectively,
indicating that the systemic availability of orally administered
closantel was half that of an intramuscular dose.
The average blood to plasma level ratio for radioactivity was
0.65, indicative of negligible binding to blood cells, whereas the
fraction bound to plasma proteins was very high (>99%). These data
were similar to those obtained for blood or plasma spiked with 14C-
closantel.
In the same study, within 8 weeks after oral or intramuscular
dosing, about 80% of the administrated radioactivity was excreted in
the faeces, and only 0.5% in the urine. The larger faecal excretion
of the radioactivity during the first two days after oral dosing
(43.3% in contrast with 10.4% after the intramuscular dose) reflected
the smaller systemic availability. Unchanged closantel accounted for
80 to 90% of the faecal radioactivity. As investigated by radio-HPLC,
the main metabolite in the faeces was monoiodoclosantel the 3-
iodoisomer and the 5-iodoisomer).
Residual radioactivity in all tissues but liver was exclusively
due to closantel and tissue levels were similar after the oral and the
intramuscular dose. Highest levels were found in the lung and kidney:
3.3-3.8 µg/g at 14-21 days after dosing (with 5 mg/kg b.w. i.m. or 10
mg/kg b.w. p.o.). Corresponding levels in heart were 1.7-2.8 µg/g, in
muscle 0.41-0.75 and in fat 0.08-0.42 µg/g. Radioactivity levels in
the liver were comparable with heart levels; in liver the contribution
of unmetabolized closantel was on average 71 and 61% after oral and
intramuscular administration respectively. Monoiodoclosantel was the
main metabolite accounting for 30-40% in liver. Plasma levels were
higher than corresponding tissue levels: 6 to 7 times for lung and
kidney, 9 to 15 times for heart and liver, about 30 times for muscle
and some 100 times for fat. Since these plasma to tissue level ratios
were independent of the time interval after dosing and of the route of
administration, the elimination of closantel from plasma presents an
excellent reflection of the depletion from tissues (Meuldermans
et al., 1982).
2.1.1.2 Cattle
Three dairy cows were treated with a single intramuscular dose of
closantel at 5 mg/kg body weight given as 5% injectable solution.
Blood and milk samples were taken before administration and at the
following intervals : 4 and 8 hours, and 1, 2, 3, 4, 5, 6, 7, 14, 21,
28 and 35 days.
Maximum levels of unaltered drug in plasma were reached 2 to 4
days after administration amounting to about 45 µg/ml. This peak
level corresponds to nearly 45% of the administered dose, assuming
that the whole plasma volume accounts for 5% of the cows' body weight.
Maximal concentrations in milk, amounting to about 1 µg/ml were
found about 4 days after intramuscular injection. Assuming a daily
production of 20 liters approximately 1% of the administered dose was
excreted with the milk per day.
From 4 days after injection, elimination of closantel from both
plasma and milk occurred with the same half-life of about 12 days,
indicating an equilibrium between both body fluids. Concentrations of
closantel in the milk, however, were nearly 45 times lower than the
corresponding plasma levels. No major variations between the 3 cows
could be observed either for plasma or for milk (Michiels et al.,
1977b).
2.1.2. Biotransformation
2.1.2.1 Rats
Five male Wistar rats (about 242g) were dosed orally with 14C-
closantel at 10 mg/kg b.w. The drug, labeled in the 14C-carbonyl
carbon, had a radiochemical purity of approx. 97%. Urine and faeces
were collected at 24-hr periods up to 10 days post dosing. Plasma was
collected at sacrifice after the 10-day collection period. Samples
were analyzed for total radioactivity by combustion and counting or by
direct counting. Unchanged drug and metabolites were determined using
HPLC and co-chromatography with reference standards.
Radioactivity was found to be excreted primarily through the
faeces. After 10 days, faecal excretion amounted to 88.4% of the
dose. Over that same time period, only 0.4% of the dose was in the
urine. Approximately half the dose was excreted within 2 days after
dosing.
An examination of the faeces for metabolites evidenced unchanged
closantel (90% of the radioactivity in the faeces at 0-24 hr
collection period, 76% at 192-240 hr) and monoidoclosantel (3.4% of
the sample radioactivity at the first collection, 19% at the last).
The monoiodoclosantel was stated to be predominantly the 3-iodo
isomer. Also present in faeces were deiodinated closantel (trace
amounts) and an unidentified metabolite (approx. 3-6% of the faecal
radioactivity).
In the urine, closantel and a metabolite that co-eluted with
monoiodosalicylic acid were observed. This latter compound would
result from reductive deiodination and amide hydrolysis of closantel.
No sulfate or glucuronide conjugates were detected.
Total residues of closantel in plasma amounted to 3.54 ppm 10
days after dosing. Even after that 10-day period, closantel was 93.4%
of the total radioactivity. Monoiodoclosantel was 4.7% of the plasma
radioactivity.
These data demonstrate the similarity of the metabolism of
closantel in rats and sheep. The scheme shown in Figure 2 would,
therefore, apply to rats, with the possibility of amide hydrolysis
occurring after the initial deiodination step (Mannens, et al.,
1989).
2.1.2.2 Sheep
Following oral (10 mg/kg b.w.) or intramuscular (5 mg/kg b.w.)
administration of 14C-closantel to sheep, HPLC analysis of urine and
faecal extracts indicated that about 90% of the excreted radioactivity
was due to unmetabolized closantel. With radio-HPLC, apart from the
main peak of closantel, two additional radioactivity peaks were
detected in the faecal extracts which, according to HPLC
cochromatography with authentic reference compounds, corresponded to
two monoiodoclosantel isomers. Quantification of the peaks showed
that 3-monoiodoclosantel was present in a relatively larger amount
than the 5-isomer (see Figure 2). In urine, the monoiodoclosantel
isomers were of relatively minor importance, whereas other
metabolites, not corresponding to available reference compounds, were
observed. Since the total urinary excretion of radioactivity amounted
to only 0.5% of the administered dose, no attempts were made to
elucidate these structures. Neither deiodinated closantel nor 3,5-
diiodosalicylic acid could be detected in the excreta (Michiels et
al., 1987).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Sheep
Eight sheep, four of each sex, were used in this study. Two male
and two female animals were treated with a single intramuscular dose
of 5 mg/kg body weight of closantel. The remaining group of four
animals was treated with a single oral dose of closantel at 10 mg/kg
body weight. The solution used was the same (5% in propyleneglycol and
water).
Blood samples were taken from one male and one female of each
group 4, 8, 24, 48 and 96 hours after drug administration and then
twice weekly up to the time of sacrifice (2, 4, 6, and 8 weeks after
administration). Concentrations of unchanged closantel were
determined by gas-liquid chromatography.
Maximum plasma levels for both routes of administration were
reached 8 to 24 hours after dosing, ranging from 51 to 68 µg/ml after
intramuscular injection (5 mg/kg bw) and from 48 to 62 µg/ml after
oral administration (10 mg/kg b.w.). The drug was eliminated from the
plasma with a half-life of about 15 days, irrespective of the route of
administration. Up to 60% of the intramuscular dose was present in
the plasma up to about 4 days after injection, whereas only 25% to 30%
of the oral dose had reached the systemic circulation in the same time
interval.
The levels of closantel in tissues were 7 to 21 times lower than
the corresponding plasma concentrations. Highest tissue
concentrations were found in the lung and the kidney. Tissue levels
in liver, muscle and fat were somewhat lower (Michiels et al.,
1977a).
Two groups of five Texel sheep received orally or intramuscularly
10 and 5 mg/kg body weight, respectively, of 14C-labelled closantel
(specific activity 23.5 µCi/mg - radiochemical purity of 97.0%). The
solution was a 5% solution (propyleneglycol-water) with a specific
activity of 4.2 µCi/ml.
Blood samples were taken from one animal per group 4, 8, 24, 48,
96 and 168 hours after drug administration and then weekly up to the
time of sacrifice (14, 21, 35, 42 and 56 days) after treatment. Urine
and faeces were collected daily from dosing up to the time of
sacrifice. Samples of liver, lung, hind-leg muscle, mesenteric fat,
intact kidneys and heart were taken from each animal at sacrifice.
Peak plasma concentrations of radioactivity, occurring at 8-24
hours after drug administration, were 47.0 ± 11 and 47.9 ± 4.4 µg
equiv./ml for the oral and intramuscular route respectively. Plasma
radioactivity was almost exclusively due to the unmetabolized drug.
Closantel was eliminated from plasma with a half-life of 26.7 and 22.7
days after oral and intramuscular administration respectively,
indicating that the systemic availability of orally administered
closantel was half that of an intramuscular dose.
The average blood to plasma level ratio for radioactivity was
0.65, indicative of negligible binding to blood cells, whereas the
fraction bound to plasma proteins was very high (>99%). These data
were similar to those obtained for blood or plasma spiked with 14C-
closantel.
In the same study, within 8 weeks after oral or intramuscular
dosing, about 80% of the administrated radioactivity was excreted in
the faeces, and only 0.5% in the urine. The larger faecal excretion
of the radioactivity during the first two days after oral dosing
(43.3% in contrast with 10.4% after the intramuscular dose) reflected
the smaller systemic availability. Unchanged closantel accounted for
80 to 90% of the faecal radioactivity. As investigated by radio-HPLC,
the main metabolite in the faeces was monoiodoclosantel the 3-
iodoisomer and the 5-iodoisomer).
Residual radioactivity in all tissues but liver was exclusively
due to closantel and tissue levels were similar after the oral and the
intramuscular dose. Highest levels were found in the lung and kidney:
3.3-3.8 µg/g at 14-21 days after dosing (with 5 mg/kg b.w. i.m. or 10
mg/kg b.w. p.o.). Corresponding levels in heart were 1.7-2.8 µg/g, in
muscle 0.41-0.75 and in fat 0.08-0.42 µg/g. Radioactivity levels in
the liver were comparable with heart levels; in liver the contribution
of unmetabolized closantel was on average 71 and 61% after oral and
intramuscular administration respectively. Monoiodoclosantel was the
main metabolite accounting for 30-40% in liver. Plasma levels were
higher than corresponding tissue levels: 6 to 7 times for lung and
kidney, 9 to 15 times for heart and liver, about 30 times for muscle
and some 100 times for fat. Since these plasma to tissue level ratios
were independent of the time interval after dosing and of the route of
administration, the elimination of closantel from plasma presents an
excellent reflection of the depletion from tissues (Meuldermans
et al., 1982).
2.1.1.2 Cattle
Three dairy cows were treated with a single intramuscular dose of
closantel at 5 mg/kg body weight given as 5% injectable solution.
Blood and milk samples were taken before administration and at the
following intervals : 4 and 8 hours, and 1, 2, 3, 4, 5, 6, 7, 14, 21,
28 and 35 days.
Maximum levels of unaltered drug in plasma were reached 2 to 4
days after administration amounting to about 45 µg/ml. This peak
level corresponds to nearly 45% of the administered dose, assuming
that the whole plasma volume accounts for 5% of the cows' body weight.
Maximal concentrations in milk, amounting to about 1 µg/ml were
found about 4 days after intramuscular injection. Assuming a daily
production of 20 liters approximately 1% of the administered dose was
excreted with the milk per day.
From 4 days after injection, elimination of closantel from both
plasma and milk occurred with the same half-life of about 12 days,
indicating an equilibrium between both body fluids. Concentrations of
closantel in the milk, however, were nearly 45 times lower than the
corresponding plasma levels. No major variations between the 3 cows
could be observed either for plasma or for milk (Michiels et al.,
1977b).
2.1.2. Biotransformation
2.1.2.1 Rats
Five male Wistar rats (about 242g) were dosed orally with 14C-
closantel at 10 mg/kg b.w. The drug, labeled in the 14C-carbonyl
carbon, had a radiochemical purity of approx. 97%. Urine and faeces
were collected at 24-hr periods up to 10 days post dosing. Plasma was
collected at sacrifice after the 10-day collection period. Samples
were analyzed for total radioactivity by combustion and counting or by
direct counting. Unchanged drug and metabolites were determined using
HPLC and co-chromatography with reference standards.
Radioactivity was found to be excreted primarily through the
faeces. After 10 days, faecal excretion amounted to 88.4% of the
dose. Over that same time period, only 0.4% of the dose was in the
urine. Approximately half the dose was excreted within 2 days after
dosing.
An examination of the faeces for metabolites evidenced unchanged
closantel (90% of the radioactivity in the faeces at 0-24 hr
collection period, 76% at 192-240 hr) and monoidoclosantel (3.4% of
the sample radioactivity at the first collection, 19% at the last).
The monoiodoclosantel was stated to be predominantly the 3-iodo
isomer. Also present in faeces were deiodinated closantel (trace
amounts) and an unidentified metabolite (approx. 3-6% of the faecal
radioactivity).
In the urine, closantel and a metabolite that co-eluted with
monoiodosalicylic acid were observed. This latter compound would
result from reductive deiodination and amide hydrolysis of closantel.
No sulfate or glucuronide conjugates were detected.
Total residues of closantel in plasma amounted to 3.54 ppm 10
days after dosing. Even after that 10-day period, closantel was 93.4%
of the total radioactivity. Monoiodoclosantel was 4.7% of the plasma
radioactivity.
These data demonstrate the similarity of the metabolism of
closantel in rats and sheep. The scheme shown in Figure 2 would,
therefore, apply to rats, with the possibility of amide hydrolysis
occurring after the initial deiodination step (Mannens, et al.,
1989).
2.1.2.2 Sheep
Following oral (10 mg/kg b.w.) or intramuscular (5 mg/kg b.w.)
administration of 14C-closantel to sheep, HPLC analysis of urine and
faecal extracts indicated that about 90% of the excreted radioactivity
was due to unmetabolized closantel. With radio-HPLC, apart from the
main peak of closantel, two additional radioactivity peaks were
detected in the faecal extracts which, according to HPLC
cochromatography with authentic reference compounds, corresponded to
two monoiodoclosantel isomers. Quantification of the peaks showed
that 3-monoiodoclosantel was present in a relatively larger amount
than the 5-isomer (see Figure 2). In urine, the monoiodoclosantel
isomers were of relatively minor importance, whereas other
metabolites, not corresponding to available reference compounds, were
observed. Since the total urinary excretion of radioactivity amounted
to only 0.5% of the administered dose, no attempts were made to
elucidate these structures. Neither deiodinated closantel nor 3,5-
diiodosalicylic acid could be detected in the excreta (Michiels et
al., 1987).
 The position of the 14C label is indicated by the asterisk in the
structure of closantel.
2.1.3 Effects on enzymes and other biochemical parameters
The mode of action of salicylanilides has been reviewed in two
articles. Closantel is described as a hydrogen ionophore resulting in
the uncoupling of electron transport-associated phosphorylation (Behm
et al., 1985; Prichard, 1987).
Morphological and biochemical studies supporting this mechanism
of action are available. Ultrastructural changes were observed in
F. hepatica after treatment of artificially-infected sheep with 5
mg/kg body weight of closantel adminstered i.m. An untreated animal
served as the control. Each of the treated sheep was sacrificed at
4, 8, 12 and 24 hours after injection and flukes were collected in the
liver. Changes in morphology of F. hepatica's absorptive tissues
(intestine, gastrodermis, tegument and parenchymal cells) were noted.
Most common changes concerned mitochondria (Verheyen et al.,
1980).
In vitro and ex vivo studies conducted with F. hepatica
(rats, sheep) showed: closantel inhibits the phosphorylation from
electron transport in rat liver mitochondria in vitro (Van den
Bossche et al., 1979).
In vitro and ex vivo, studies conducted with F. hepatica
(rats) showed that closantel disturbs phosphorylation in liver fluke
mitochondria with no effect in rat liver mitochondria in vivo
(Van den Bossche et al., 1980).
In vitro studies conducted with F. hepatica (rats) showed
that closantel is an uncoupler of oxidative phosphorylation from
electron transport in rat liver and an inhibitor of mitochondrial
phosphorylation in F. hepatica. (Van den Bossche & Verhoeven,
1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity resulting from single dosing of closantel
solution in several animal species is summarized in Table 1.
In rats and mice the gross effects observed in the lethal dose
range were hypotonia, ataxia, diarrhoea and dyspnoea. In sheep and
cattle clinical signs of toxicity were anorrhexia, labored breathing,
recumbency, general weakness and decreased vision, or blindness,
appearing approximately one week after dosing. At the lethal dose,
anorrhexia, hypotonia, and quadriplegia preceded death.
Table 1: Acute toxicity data
Species Sex Route LD50 Reference
(mg/kg b.w.)
M Oral 331
Mouse F 453 Niemegeers, 1976
M i.m. 56.8
F 256.8
M Oral 342
Rat F 262 Niemegeers, 1976
M i.m. 325.9
F 28.4
Sheep Oral > 40 Marsboom, 1976a
i.m. > 40
Cattle Oral > 40 Marsboom, 1976a
i.m. > 20
2.2.2 Short-term studies
2.2.2.1 Rats
Four groups of Wistar rats (10 males and 10 females/group) were
fed diets containing closantel at 0, 25, 100 and 400 mg/kg diet
(equivalent to 0, 2.5, 10 and 40 mg/kg b.w./day) for 13 weeks. All
animals were observed daily for mortality and physical signs. Body
weight and food consumption were recorded weekly. Clinical
haematology, clinical biochemistry and urine analysis were performed
at time of sacrifice. Prior to sacrifice, a complete physical
examination and slit-lamp examination of the eyes were performed on
all rats. At sacrifice, detailed gross and histopathological
examinations (34 tissues) were also performed and organs were weighed.
Mortality, clinical behaviour, or body weight were not adversely
influenced by treatment. Food consumption was slightly decreased in
females (low and high dosage groups). Erythrocyte counts were
slightly decreased in the high-dose male and female groups.
Lymphocyte counts were slightly increased in males of the same group.
However, glucose was slightly increased in males at the high dose and
in females at the middle and high doses. Total bilirubin was also
increased in males at the high dose. Organ weights were generally
comparable between the controls and dosed rats, but decreased heart
weight in males and increased relative weight of the lung in females
were noted in the three dosed groups. The recorded values fell
within the range of historical control values however. An increased
absolute and relative weight of the gonads in males in the high dosage
group was noted.
A cystic distention of the epididymis was observed in one low
dose, two medium dose and seven high dose males. The effect was
considered to be drug and dose related. In one out of ten rats dosed
at 10 mg/kg b.w./day and eight out of ten rats dosed at 40 mg/kg
b.w./day spermatic granulomas with round cell infiltration, oedema and
fibrosis were found. Histological examination also revealed some
centrilobular fatty deposition in the livers of males dosed at 40
mg/kg b.w./day, but not in females. A slight deposition of the same
type was noted in two male rats dosed at 10 mg/kg b.w./day and in one
rat dosed at 2.5 mg/kg b.w./day.
Male gonads, and secondarily the liver, appear as target organs
for toxicity. The Committee concluded that the NOEL in this study was
2.5 mg/kg b.w./day (Marsboom et al., 1977a).
2.2.2.2 Dogs
Four groups of beagle dogs (3 males and 3 females/group) received
closantel powder in gelatin capsules at rates of 0, 2.5, 10 and 40
mg/kg b.w. each day for 3 months. All animals were observed daily for
mortality and physical signs. Before the study and at termination,
ophthalmoscopic and slit-lamp examinations of the eyes were performed.
Food consumption could not be recorded accurately because of wastage.
Body weights were determined weekly. Heart rate, ECG and blood
pressure values were recorded monthly. Clinical haematology, clinical
biochemistry and urine analysis were performed on all dogs 2 weeks
before the experiment began and at weeks 0, 2, 4, 8 and 13. After
sacrifice detailed gross and histopathological examinations (39
tissues) were also performed and selected organs were weighed (13
organs).
Mortality, clinical behaviour, body weight, haematology,
ophthalmoscopy, heart rate, ECG and blood pressure values were not
affected by treatment. All haematological values were within the
range of historical values. However, an increase of coagulation time
was noted at week 13 in the high dosage group. A slight increase of
total bilirubin was noted at week 13 in the medium and high dosage
groups; and a slight increase of SGOT at weeks 8 and 13 for the high
dosage group was observed. Urine analysis was unchanged. Organ
weights were not affected by treatment. Gross pathology showed no
difference between controls and treated animals. Histological
examinations were unremarkable except for a very slight increase of
fatty deposition in the liver of males and females in the high dose
group.
The liver appeared to be the target organ for toxicity in this
study. The NOEL was 2.5 mg/kg b.w./day, based on hepatotoxicity
observed at higher doses (Marsboom & Herin, 1978).
2.2.2.3 Sheep
Five groups of Suffolk sheep (3 males and 3 females/group)
received a total of ten doses of a solution of closantel (0, 10, and
40 mg/kg b.w. p.o. or 5 and 20 mg/kg b.w. i.m. given at four weekly
intervals. All animals were observed daily for mortality and physical
signs. Ophthalmoscopic and slit-lamp examinations of the eyes were
performed, once before the start of the study and again after the last
administration of the test substance. Body weight was determined
before and every four weeks during the study. Body temperature was
recorded before the study, daily during the first month, and
periodically thereafter. Clinical haematology and clinical
biochemistry were conducted prior to dosing and every 4 weeks during
the study. After sacrifice, gross pathology and histopathology
(liver, heart muscle, pancreas, kidneys, epididymis, ovaries, adrenals
and thyroids) were performed. The animals were sacrificed on day 1,
and weeks 4, 8, 12 and 16 after the last dosing. This was done in
order to study reversibility of possible lesions and to determine the
residual levels of closantel in various organs.
Four animals died from kidney disease. Closantel did not produce
mortality. Transient salivation and diarrhoea were observed in
animals dosed at 40 mg/kg b.w. p.o. route. A slight decrease of body
weight gain was observed in animals dosed at 20 mg/kg b.w. i.m. No
adverse effects on haematological parameters were noted. The few
slight variations observed in clinical chemistry were not dose-
related. Gross pathology showed reactions at the injection site in
two animals which had received 10 i.m. doses at 20 mg/kg b.w.
Histological examinations confirmed muscle irritation at the injection
site (5 and 20 mg/kg b.w.). Irregular degeneration of the germinal
epithelium was observed at 20 mg/kg b.w. i.m. (2 animals) and at 40
mg/kg b.w. p.o. (2 animals) (Marsboom et al., 1977b).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of albino Swiss mice (50 males and 50 females/group)
were fed diets containing 0, 25, 100 and 400 mg/kg diet, equivalent to
approximately 0, 5, 20 and 80 mg/kg b.w./day of closantel for 18
months. All animals were observed daily for mortality and physical
signs. Full necropsy was performed on all animals which died or were
sacrificed during the course of the study, and at the termination of
the study on all surviving animals.
Histopathological examination was performed systematically on
lungs, liver, pancreas, kidneys, spleen, lymph nodes, testes, ovaries,
mammary glands, adrenals, epididymis and hypophysis.
No significant effects on overall survival rate or on the time at
which mortalities occurred were noted in the various dosage groups of
either males or females. No dose related effects on health, behaviour
and appearance or gross pathology or the incidence or types of tumours
in albino Swiss mice were observed (Verstraeten et al. 1981a).
2.2.3.2 Rats
Four groups of Wistar rats (50 males and 50 females/group) were
fed diets containing 0, 25, 100 and 400 mg/kg, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day, closantel during 24 months. All animals
were observed daily for mortality and physical signs. Gross
examination was conducted on all animals which died or were sacrificed
during the course of the study, and at the termination of the study on
all surviving animals.
Gross lesion examination was performed on lungs, liver, pancreas,
kidneys, spleen, lymph nodes, testes, epididymis, ovaries, mammary
glands, adrenals and thyroids in animals killed during the study or at
the termination.
At the end of the study, cumulative mortalities in different
groups (0, 2.5, 10, and 40 mg/kg b.w./day) were 46/50, 43/50, 38/50,
49/50 for males and 32/50, 37/50, 36/50, 42/50 for females,
respectively. This rather high mortality could be related to the
typical short life-expectancy of the Wistar rat strain, bred in the
Janssen Laboratories used in this study. Closantel did not have a
very consistent effect on mortality. In females, a slight increase of
mortality was noted in the high dosage groups, whereas in males the
final mortality was not influenced by the treatment. The overall
mortality in rats was not affected at doses of 25 and 10 mg/kg b.w. in
either males or females. At 40 mg/kg b.w./day a slight increase of
mortality was noted in females.
The observed necropsy findings were comparable between groups
except for an increased incidence of spermatic granulomas in the 10
and 40 mg/kg b.w./day dosed males.
The over-all tumour rates were 40, 46, 58, 42% in males and 84,
75, 64, 47% in females in the control, low, mid and high dose groups
respectively. In females, there was a dose-dependent decrease of
total tumour incidence in the medium and high dosage groups. The
rather high number of adenomas seen in the 10 mg/kg b.w./day dosage
group is likely to be related to the longer survival of this dosage
group.
There was a significantly increased number of tumours of the
haematopoietic system in the mid-dose male rats. This increase did
not appear to be caused by the administration of closantel because:
- the increase was seen only at this level.
- the number of tumours of the haematopoietic system in the
control male rats, namely 3 tumours in 48 examined animals,
was unusually low when compared with the historical control
data from 9 other carcinogenicity studies done at the
Janssen facilities using the same Wistar rat in identical
experimental conditions.
Based upon comparison with the historical control data the
incidences of tumours of the haematopoietic system seen in the mid-and
low-dose males in this study were within the normal range of the
Wistar rat strain, whereas those of the control and the high-dose
males were significantly lower than normal. In the females, the
distribution of the different tumour types among the various groups
was very similar.
The incidence of histologically confirmed spermatic granulomas
seen in the epididymis of male rats is summarized in Table 2.
Table 2: Incidence of spermatic granulomas
Dosage group Incidence p-value
(mg/kg food) n/N
Control 0/48 -
25 0/49 -
100 7/50 <0.05
400 30/50 <0.001
An increase in the vacuolization of the optic nerve was observed
in the high-dose males and females.
The NOEL in this study was 2.5 mg/kg b.w./day (Verstraeten et
al., 1981b).
2.2.4 Reproduction study
2.2.4.1 Rats
Closantel was administered orally by gavage to male and female
Wistar rats once monthly during 3 successive generations each
producing 2 litters. The doses were 0, 2.5, 10 and 40 mg/kg b.w./mo
administered to 20 males and females/group.
Studied parameters in adults were: physical signs, body weight
gain and food consumption of dams during pregnancy, mortality and
gross pathology of males and females, and occurrence of mating,
pregnancy rates, and duration of gestation. Studied parameters in
pups were: litter size; weight at birth, weight and survival rate at
3 weeks of age, and abnormalities. The testes and epididymis were
examined histologically.
In adults, no adverse effects on body weight were observed
except at 40 mg/kg b.w./mo, where the marginal decrease of weight gain
in the 2nd litter of the 2nd and 3rd generation was associated with a
decrease in litter size. Food consumption was not affected.
Mortalities of males and females were comparable between groups. A
tendency to decreased pregnancy rate was noted at 40 mg/kg b.w./mo.
Occurrence of mating and duration of gestation were comparable between
groups.
In pups, no adverse effects on litter size could be detected,
except, at 40 mg/kg b.w./mo., a slight decrease in the number of
implants in the 2nd generation (2nd litter) and the 3rd generation
(1st and 2nd litters). No embryotoxicity effects were noted. Weight
at birth and at 3 weeks of age were comparable between groups.
Survival rate at 3 weeks of age was considered to be normal in all
groups.
Histopathological findings of spermatic granulomas (1/56 animals
at 10 mg/kg b.w./mo and 14/46 at 40 mg/kg b.w./mo) confirmed
previously reported findings in the rat.
The NOEL for closantel in this study was 2.5 mg/kg b.w./month
(Dirkx & Marsboom, 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg of closantel in the diet, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day during the period of organogenesis (day 6 to
day 15). The females were sacrificed on the 22nd day post mating.
Body weight, food consumption and mortalities of dams were measured.
Implantation number, pregnancies and pups, litter size and weight at
birth, resorption number, live and dead fetuses, number and
distribution of live, dead and resorbed embryos, and abnormalities
were measured.
There were no differences between groups. Teratogenic effects
were not observed (Marsboom, 1975).
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg closantel, equivalent to 0, 2.5, 10 and 40 mg/kg
b.w./day, from day 16 of pregnancy and during the 3 week lactation
period. Body weight, food consumption, mortality, pregnancy rate, and
the gestation period of dams were measured. Cannibalism, litter size,
weight at birth and at 2 and 3 weeks after birth, number of live and
stillborn fetuses, survival of pups at 4 days and 2 and 3 weeks after
birth and abnormalities in pups were quantified.
There were no differences between groups and no effects related
to treatment were observed (Marsboom, 1979).
2.2.5.2 Rabbits
Female New Zealand White rabbits (19 in the control group and 20
in each experimental group) received closantel (0, 10 and 40 mg/kg
b.w./day) orally by gavage from day 6 through day 18 of pregnancy, the
period of organogenesis. Females were sacrificed on the 28th day of
gestation. They were necropsied and checked for gross abnormalities.
The examined parameters were (for dams): pregnancy, body weight and
mortality; (for pups): resorption numbers, live and dead fetuses,
litter size, weight at delivery, survival rates after 24 hours, and
abnormalities.
One control female died on day 2 of the experiment from
pneumonia. No differences between groups were observed for body
weight, pregnancy and embryotoxic effects.
In the control group, two neonates from one female had bifurcated
ribs; in the low dosage group, 3 neonates from 3 different females
had either a deformed left fore-leg, a bifurcated rib or a waved rib;
in the high dosage group, 2 neonates from 2 different females had
either deformed thoracic bones or acrania.
Teratogenic or embryotoxic effects were not observed in this
study (Marsboom, 1976b).
2.2.5.3 Sheep
Ewes (274), separated into 10 treatment groups, received
closantel orally at 0, 20, or 40 mg/kg, either on day 11, 17 or 23
after mating. No influence of treatment was noted on the ovarian
cycle or on subsequent ovulation, copulation and conception. No
embryotoxic or teratogenic effects were reported in the clinical trial
(Chevis, 1977).
2.2.6 Special studies on fertility
2.2.6.1 Rats
The study was conducted using 20 males and 20 females/group.
Dose levels were 0, 25, 100 and 400 mg/kg food. Males were treated
for a minimum of 60 days prior to mating with non-dosed females.
Females were treated for at least 14 days prior to mating with non-
dosed males and then throughout gestation. Parameters studied were:
body weight, food consumption, pregnancy outcome, mortality in
parenteral and filial animals, number of implantations, litter size
and weight, the number and distribution of live, dead and resorbed
fetuses, and foetal abnormalities.
Body weight gain was comparable in all parental groups, except
for a lower weight gain in non-dosed females mated with dosed males of
the 400 mg/kg food group; food consumption was comparable in all
groups; no differences in pregnancy outcome were noted except a
reduced pregnancy rate in the high dosage group of non-dosed females
mated with dosed males; there were no mortalities.
No embryotoxic effects were noted except for a decrease in the
number of implants and litter size in the group of non-dosed females
mated with high-dosed males. No abnormalities were related to the
administration of closantel.
Fertility was not affected by the administration of closantel
except for a decreased pregnancy rate in non-dosed females mated with
high-dosed males (Marsboom, 1978).
2.2.6.2 Other species
Semen of bulls treated either once at approximately 2 mg/kg b.w.
or three times at 5 mg/kg b.w. intramuscularly at 8 weekly intervals
was normal (Debruyne, 1978); (Retief, undated a).
Semen of rams treated three times, by the oral route at 20 mg/kg
b.w. at 8 weekly intervals, was normal. (Retief, undated b).
In rams treated (15 mg/kg b.w. by the oral route) 3 or 5 times at
3 or 4 weekly intervals, the semen was normal, and the testes and
epididymis presented a normal structure (Johns, 1981).
2.2.7 Special studies on genotoxicity
Several tests were conducted to assess the genotoxicity of
closantel, they are summarized in Table 3.
Table 3: Results of genotoxicity assays on closantel
Test System Test object Concentration Results Reference
Cytotoxicity - L 5178 Y mouse 0.3-500 µg/ml Cytotoxic effects Enninger, 1989
lymphoma cells (1) in all 3 test
- V 79 Chinese systems. Reduced
hamster cells in presence of S9
- Chinese hamster mix
ovary (CHO) cells
Ames test (1) S. typhimurium 10-2000 µg/plate (2) Negative Poncelet, 1981
TA 1530, TA 1535,
TA 1538, TA 98,
TA 100
Ames test (1) S. typhimurium 1-1000 µg/plate (2) Negative Vanparays &
TA 1535, TA 1538, TA Marsboom, 1987
97, TA 98,
TA 100
In vivo: Drosophila melanogaster 10-50 ppm (3) Negative Vanparays &
sex linked recessive Marsboom, 1981
lethal test
In vivo: Male mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978a
oral treatment
(4)
In vivo: Female mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978b
oral treatment
(4)
DNA repair assay Cultured rat 0.3-100 µg/ml Negative Weterings, 1985
hepatocytes (5)
Table 3 (continued)
1 Both with and without rat liver S9 fraction
2 Sodium azide, nitrofluorene and 2-aminoanthracene were used as positive controls
3 Procarbazine was used as positive control
4 Cyclophosphamide used as positive control
5 7,12-Dimethyl benzanthracene was used positive control
2.3 Observations in humans
A single oral dose of closantel of 2.5 mg/kg b.w. in 5 patients
and a single s.c. injection of 2.5 mg/kg b.w. in one patient were
administered against F. hepatica. All orally treated patients
complained of the bitter taste of the drug, and one presented with
nausea and vomiting. In the last case, the dose was repeated adding
sugar and was then well tolerated. After the s.c. injection,
tachycardia, sweating, a metallic taste in the mouth, induction of
micturition and defecation, reddening of the skin, nervousness,
stress, excitation and a sensation of anguish occurred.
Haematology tests (haematocrit, WBC count and differential
prothrombin-time), clinical biochemistry tests (ALP, SGOT, SGPT,
cholesterol, and glucose), and urinalysis remained unchanged (2
examinations were carried out: one before, and the other seven days
after treatment) (Bernardiner, 1979).
Patients (14) were treated with a single oral dose of 5 mg/kg
b.w. of closantel. The treatment was well tolerated but poor efficacy
against Ancylostoma duodenale was obtained (Borda, 1980).
Patients (13) were treated with a single dose of closantel (6 at
5 mg/kg b.w; 3 at 7.5 mg/kg b.w.; 4 at 10 mg/kg b.w.). In all
treatment groups, side effects such as diarrhoea, drowsiness and
blurred vision were observed (Saowakontha, undated).
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of short-term studies, and of
studies of carcinogenicity, genotoxicity and effects on reproduction
and development.
In studies in rats and sheep, closantel was shown to be strongly
bound to the plasma proteins. The compound was poorly metabolized,
mainly to 3- and 5- monoiodoclosantel. About 90% of the administered
dose was excreted in the faeces, and about 90% was unchanged.
In a study in rats in which closantel was administered in the
diet at levels up to the equivalent of 40 mg per kg of body weight per
day for 13 weeks, spermatic granulomas in the epididymis and hepatic
fatty changes in the liver were observed at the highest dose level.
The NOEL was 2.5 mg per kg of body weight per day.
Dogs were dosed for 3 months by oral administration of the
compound in gelatin capsules at levels up to 40 mg per kg of body
weight per day. There were fatty changes in the liver at the highest
dose level. The NOEL for this study was 2.5 mg per kg of body weight
per day.
A carcinogenicity study was conducted in mice, which received
closantel in the diet at levels up to 80 mg per kg of body weight per
day for 18 months. There were no dose-related effects on the total
numbers of tumours nor on any individual type of tumour. No other
effects were observed in this study.
In a carcinogenicity study in rats in which closantel was
administered in the diet at levels up to 40 mg per kg of body weight
per day for 24 months, there was no effect on the overall incidence of
tumours. However, by comparison with concurrent controls, there was
a statistically significant increase in the incidence of
haematopoietic tumours in male rats at 10 mg per kg of body weight per
day. This incidence was nevertheless within the historical control
range, while the incidences found in the control and high-dose groups
were significantly lower than in the historical controls. Spermatic
granulomas were observed. The NOEL for this study was 2.5 mg per kg
of body weight per day.
Adverse effects on reproduction were probably associated with
toxicity to the male reproductive organs, and were seen only at doses
of 2.5 mg/kg body weight per day and above. In a fertility study in
rats, in which closantel was administered in the diet, there was a
reduced pregnancy rate in untreated females paired with males
receiving the highest dose of 40 mg per kg of body weight per day. A
three-generation reproduction study was conducted in male and female
rats, in which the compound was administered once monthly by gavage.
There was a decrease in the pregnancy rate and number of implants per
animal at the highest dose level of 40 mg per kg of body weight.
Spermatic granulomas were seen at 10 and 40 mg per kg of body weight
but not at 2.5 mg per kg of body weight.
In teratogenicity studies in rabbits and rats, no teratogenic or
toxic effects were evident at doses up to 40 mg per kg of body weight
per day. The rabbits received closantel by gavage on days 6 to 18 of
pregnancy, while the rats, were given the compound in the diet on days
6 to 15 of pregnancy.
Closantel gave negative results in a range of in vitro and
in vivo mutagenicity studies. No tests for clastogenicity were
performed, but the Committee noted that carcinogenicity studies had
been carried out in two species.
Limited clinical reports were available from 33 patients who were
treated with a single oral or parenteral dose of closantel at 2.5 to
10 mg per kg of body weight. There were no adverse effects.
4. EVALUATION
An ADI of 0-0.03 mg per kg of body weight was established for
closantel based on the NOEL of 2.5 mg per kg of body weight per day in
rats and a safety factor of 100.
5. REFERENCES
BEHM, C.A. & BRYANT, C. (1985). The mode of action of some modern
anthelmintics. In Anderson, N. & Waller, J.P. (Eds). Resistance in
Nematodes to anthelmintic drugs. Commonwealth Scientific & Industrial
Research Organization. Australia, pp. 57-67.
BERNARDINER, E. (1979). Preliminary results of the study with
Closantel in fluke disease. Unpublished data. N 1807 from Johnson-
Johnson. Buenos-Aires, Argentina. Submitted to WHO by Janssen
Pharmaceutica.
BORDA, E. (1980). Clinical efficacy and safety of Closantel (R 31520)
on pregnancy in sheep. Unpublished Report N 22122 from Corrientes,
Argentina. Submitted to WHO by Janssen Pharmaceutica.
CHEVIS, R. (1977). Investigation of the effect of Closantel (R 31522)
from on pregnancy in sheep. Unpublished trial Report V 2744, from
ETHNOR (PTY) LTD. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
DEBRUYNE, R. (1978). Fertility control in male cattle after
administration of Closantel (R 31520). Unpublished Report V 2859,
from Artificial Insemination Center. Oostmalle, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
DIRKX, P. & MARSBOOM, R. (1984). Oral three-generation study in
Wistar rats. Unpublished Report V 5183 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
ENNINGER, I.C. (1989). Evaluation of the cytotoxic activity of
Closantel R 31520 in L 5178 Y mouse lymphoma cells, in V 79 Chinese
hamster cells and in Chinese hamster ovary (CHO) cells. Unpublished
Report V 6999 from RCC NOTOX 2BV. S'-Hertogenbosch, the Netherlands.
Submitted to WHO by Janssen Pharmaceutica.
JOHNS, D. (1981). To determine the effect of Closantel on the
fertility of rams after five consecutive oral treatments at twice the
recommended rate. Unpublished Report V 4362, from Smith-Kline Animal
Health. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
KANE, H.J., BEHM, C.A. & BRYANT, C. (1980). Metabolic studies on the
new fasciolicidal drug, Closantel. Mol. and Biochem. Parasitol., 1,
347-355.
MANNENS, G., MOSTAMANS, E., VERBOVEN, P., HENDRICKX, J., HURKMANS, R.,
VAN LEEMPUT, L., MEULDERMANS, W. AND HEYKANTS, J. (1989). The
excretion and metabolism of 14-C-closantel in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished report V 7201. Submitted
to FAO by Janssen Pharmaceutica.
MARSBOOM, R. (1975). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished Report V 2403- from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1976a). Acute toxicity studies in sheep and cattle
single dosing: oral/intramuscular. Unpublished report preclinical
research Report V 2402 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in New Zeeland White rabbits (SEGMENT II). Unpublished Report V 2404
from Jansen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium, Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977a). Oral
toxicity study in Wistar rats (repeated dosages for 13 weeks).
Unpublished Report V 2700 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium, Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R., HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977b).
Oral and intramuscular toxicity study in Suffolk Sheep (10 doses per
animal one every fourth week). Unpublished Report V 2701 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1978). Oral male and female fertility study in Wistar
rats. (Segment I). Unpublished Report V 2860 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. & HERIN, V. (1978). Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished Report V 2985 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1979). Oral Embryotoxicity and teratogenicity study in
Wistar rats (peri. and postnatal study) (Segment III). Unpublished
Report V 3847 from Janssen Research Products Information Service,
Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, R. LAMPO, A., VAN CAUTEREN, H., VANPARYS, P.H., MAES, L. &
HEYKANTS, J. (1989). Review report on the toxicological,
pharmacodynamic and pharmacokinetic documentation related to
Closantel. Unpublished executive review report from Janssen Research
Foundation Products Information Service, Janssen Pharmaceutica,
Beerse, Belgium. Submitted to WHO by Jansen Pharmaceutica.
MEULDERMANS, W., MICHIELS, M., WOESTENBORGHS, R., VAN-HOUDT, J.,
LORREYNE, W., HENDRICKX, J., HEYKANTS, J. & DESPLENTER, L. (1982).
Absorption, tissue distribution, excretion and metabolism of
Closantel-14C after intramuscular and oral administration in sheep.
Unpublished preclinical research report V 4523 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Jansen Pharmaceutica.
MICHIELS, M., WOESTENBORGHS, R., HEYKANTS, J. & MARSBOOM, R. (1977a).
On the absorption and distribution of Closantel (R 31520) in sheep
after oral and intramuscular administration. Unpublished preclinical
research report V 2709 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MICHIELS, M., HENDRICKX, J., HEYKANTS, J., MARSBOOM, R. (1977b).
Plasma and milk concentrations of Closantel in cattle after a single
intramuscular administration. Unpublished preclinical research report
V 2706 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MICHIELS, M., MEULDERMANS, W. & HEYKANTS, J. (1987). The metabolism
and fat of Closantel (Flukiver) in sheep and cattle. Drug. Met.
Review, 18, (2 & 3), 235-251.
NIEMEGEERS, C.J.E. (1976). Acute intramuscular and oral toxicity of
R 31520 in mice and rats. Unpublished preclinical research report V
2396 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Jansssen
Pharmaceutica.
PONCELET, F. (1981). In vitro mutagenicity study of Closantel R
31520, lot B 4901. Unpublished Report V 3944 from Laboratoires de
Toxicologie, UCL. Woluwe, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
PRICHARD, R.K. (1987). The pharmacology of anthelmintics in
livestock. Int. J. Parasitol. 17, 473-482.
RETIEF, G. (undated a). To determine the effect of Closantel
administered s.c. at 5 mg/kg livemass after 3 treatments spaced at 8
weekly intervals on the semen quality of bulls. Unpublished Report V
3782 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
RETIEF, G. (undated b). To determine the effect of Closantel on the
fertility of rams after three consecutive oral treatments, 8 weeks
apart at double the recommended dosage rate. Unpublished Report V
3783 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
SOAWAKONTHA, S. (undated). Unpublished report from Thailand.
Submitted to WHO by Janssen Pharmaceutica.
VAN DEN BOSSCHE, H., VERHOEVEN, H., VANPARIJS. O., LAWERS, H. &
THIENPONT, H. (1979). Closantel a new antiparasitic hydrogen
ionophore. Arch. Int. Physiol. Biochimie., 87, 851-852.
VAN DEN BOSSCHE, H., VERHOEVEN, H. & LAWERS, H. (1980). Uncoupling of
liver mitochondria associated with fascioliasis in rats.
Normalization by Closantel. In: Van den Bossche (Ed). The host
invader interplay, Elsevier, Amsterdam, pp. 699-704.
VAN DEN BOSSCHE, H. & VERHOEVEN, H. (1983). Effects of albumin on the
Closantel induced alterations of mitochondrial processes. Unpublished
preclinical research report V 4546 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978a). Closantel R 31520. Dominant
lethal test in male mice. Unpublished report V 3144 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978b). Closantel R 31520. Dominant
lethal test in female mice. Unpublished report V 2998 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1981). Closantel R 31520. Sex linked
recessive lethal test on Drosophila Melanogaster. Unpublished report
V 4058 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1987). Closantel R 31520. Ames
reverse mutation test. Unpublished report V 6517 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VERHEYEN, A., VANPARYS, O., LAWERS, H. & THIENPONT, D. (1980). The
influence of Closantel administration to sheep on the ultrastructure
of the adult liver fluke F. hepatica L. In: van den Bossche (Ed).,
The host invader interplay, Elsevier, Amsterdam, pp. 705-708.
VERSTRAETEN, A., VANDENBERGHE, J. & MARSBOOM, R. (1981a). Oral
carcinogenicity study in Albino Swiss mice (repeated dosage for 18
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
VERSTRAETEN, A., VANDENBERGHE, J., HERIN, V. & MARSBOOM, R. (1981b).
Oral carcinogenicity study in Wistar rats (repeated dosage for 24
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
WETERINGS, P.J.J.M. (1985). Evaluation of the DNA repair inducing
ability of R 31520 in a primary culture of rat hepatocytes.
Unpublished Report V 5740 from NOTOX v.o.f, S'-Hertogenbosch, The
Netherlands. Submitted to WHO by Janssen Pharmaceutica.
The position of the 14C label is indicated by the asterisk in the
structure of closantel.
2.1.3 Effects on enzymes and other biochemical parameters
The mode of action of salicylanilides has been reviewed in two
articles. Closantel is described as a hydrogen ionophore resulting in
the uncoupling of electron transport-associated phosphorylation (Behm
et al., 1985; Prichard, 1987).
Morphological and biochemical studies supporting this mechanism
of action are available. Ultrastructural changes were observed in
F. hepatica after treatment of artificially-infected sheep with 5
mg/kg body weight of closantel adminstered i.m. An untreated animal
served as the control. Each of the treated sheep was sacrificed at
4, 8, 12 and 24 hours after injection and flukes were collected in the
liver. Changes in morphology of F. hepatica's absorptive tissues
(intestine, gastrodermis, tegument and parenchymal cells) were noted.
Most common changes concerned mitochondria (Verheyen et al.,
1980).
In vitro and ex vivo studies conducted with F. hepatica
(rats, sheep) showed: closantel inhibits the phosphorylation from
electron transport in rat liver mitochondria in vitro (Van den
Bossche et al., 1979).
In vitro and ex vivo, studies conducted with F. hepatica
(rats) showed that closantel disturbs phosphorylation in liver fluke
mitochondria with no effect in rat liver mitochondria in vivo
(Van den Bossche et al., 1980).
In vitro studies conducted with F. hepatica (rats) showed
that closantel is an uncoupler of oxidative phosphorylation from
electron transport in rat liver and an inhibitor of mitochondrial
phosphorylation in F. hepatica. (Van den Bossche & Verhoeven,
1983).
2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity resulting from single dosing of closantel
solution in several animal species is summarized in Table 1.
In rats and mice the gross effects observed in the lethal dose
range were hypotonia, ataxia, diarrhoea and dyspnoea. In sheep and
cattle clinical signs of toxicity were anorrhexia, labored breathing,
recumbency, general weakness and decreased vision, or blindness,
appearing approximately one week after dosing. At the lethal dose,
anorrhexia, hypotonia, and quadriplegia preceded death.
Table 1: Acute toxicity data
Species Sex Route LD50 Reference
(mg/kg b.w.)
M Oral 331
Mouse F 453 Niemegeers, 1976
M i.m. 56.8
F 256.8
M Oral 342
Rat F 262 Niemegeers, 1976
M i.m. 325.9
F 28.4
Sheep Oral > 40 Marsboom, 1976a
i.m. > 40
Cattle Oral > 40 Marsboom, 1976a
i.m. > 20
2.2.2 Short-term studies
2.2.2.1 Rats
Four groups of Wistar rats (10 males and 10 females/group) were
fed diets containing closantel at 0, 25, 100 and 400 mg/kg diet
(equivalent to 0, 2.5, 10 and 40 mg/kg b.w./day) for 13 weeks. All
animals were observed daily for mortality and physical signs. Body
weight and food consumption were recorded weekly. Clinical
haematology, clinical biochemistry and urine analysis were performed
at time of sacrifice. Prior to sacrifice, a complete physical
examination and slit-lamp examination of the eyes were performed on
all rats. At sacrifice, detailed gross and histopathological
examinations (34 tissues) were also performed and organs were weighed.
Mortality, clinical behaviour, or body weight were not adversely
influenced by treatment. Food consumption was slightly decreased in
females (low and high dosage groups). Erythrocyte counts were
slightly decreased in the high-dose male and female groups.
Lymphocyte counts were slightly increased in males of the same group.
However, glucose was slightly increased in males at the high dose and
in females at the middle and high doses. Total bilirubin was also
increased in males at the high dose. Organ weights were generally
comparable between the controls and dosed rats, but decreased heart
weight in males and increased relative weight of the lung in females
were noted in the three dosed groups. The recorded values fell
within the range of historical control values however. An increased
absolute and relative weight of the gonads in males in the high dosage
group was noted.
A cystic distention of the epididymis was observed in one low
dose, two medium dose and seven high dose males. The effect was
considered to be drug and dose related. In one out of ten rats dosed
at 10 mg/kg b.w./day and eight out of ten rats dosed at 40 mg/kg
b.w./day spermatic granulomas with round cell infiltration, oedema and
fibrosis were found. Histological examination also revealed some
centrilobular fatty deposition in the livers of males dosed at 40
mg/kg b.w./day, but not in females. A slight deposition of the same
type was noted in two male rats dosed at 10 mg/kg b.w./day and in one
rat dosed at 2.5 mg/kg b.w./day.
Male gonads, and secondarily the liver, appear as target organs
for toxicity. The Committee concluded that the NOEL in this study was
2.5 mg/kg b.w./day (Marsboom et al., 1977a).
2.2.2.2 Dogs
Four groups of beagle dogs (3 males and 3 females/group) received
closantel powder in gelatin capsules at rates of 0, 2.5, 10 and 40
mg/kg b.w. each day for 3 months. All animals were observed daily for
mortality and physical signs. Before the study and at termination,
ophthalmoscopic and slit-lamp examinations of the eyes were performed.
Food consumption could not be recorded accurately because of wastage.
Body weights were determined weekly. Heart rate, ECG and blood
pressure values were recorded monthly. Clinical haematology, clinical
biochemistry and urine analysis were performed on all dogs 2 weeks
before the experiment began and at weeks 0, 2, 4, 8 and 13. After
sacrifice detailed gross and histopathological examinations (39
tissues) were also performed and selected organs were weighed (13
organs).
Mortality, clinical behaviour, body weight, haematology,
ophthalmoscopy, heart rate, ECG and blood pressure values were not
affected by treatment. All haematological values were within the
range of historical values. However, an increase of coagulation time
was noted at week 13 in the high dosage group. A slight increase of
total bilirubin was noted at week 13 in the medium and high dosage
groups; and a slight increase of SGOT at weeks 8 and 13 for the high
dosage group was observed. Urine analysis was unchanged. Organ
weights were not affected by treatment. Gross pathology showed no
difference between controls and treated animals. Histological
examinations were unremarkable except for a very slight increase of
fatty deposition in the liver of males and females in the high dose
group.
The liver appeared to be the target organ for toxicity in this
study. The NOEL was 2.5 mg/kg b.w./day, based on hepatotoxicity
observed at higher doses (Marsboom & Herin, 1978).
2.2.2.3 Sheep
Five groups of Suffolk sheep (3 males and 3 females/group)
received a total of ten doses of a solution of closantel (0, 10, and
40 mg/kg b.w. p.o. or 5 and 20 mg/kg b.w. i.m. given at four weekly
intervals. All animals were observed daily for mortality and physical
signs. Ophthalmoscopic and slit-lamp examinations of the eyes were
performed, once before the start of the study and again after the last
administration of the test substance. Body weight was determined
before and every four weeks during the study. Body temperature was
recorded before the study, daily during the first month, and
periodically thereafter. Clinical haematology and clinical
biochemistry were conducted prior to dosing and every 4 weeks during
the study. After sacrifice, gross pathology and histopathology
(liver, heart muscle, pancreas, kidneys, epididymis, ovaries, adrenals
and thyroids) were performed. The animals were sacrificed on day 1,
and weeks 4, 8, 12 and 16 after the last dosing. This was done in
order to study reversibility of possible lesions and to determine the
residual levels of closantel in various organs.
Four animals died from kidney disease. Closantel did not produce
mortality. Transient salivation and diarrhoea were observed in
animals dosed at 40 mg/kg b.w. p.o. route. A slight decrease of body
weight gain was observed in animals dosed at 20 mg/kg b.w. i.m. No
adverse effects on haematological parameters were noted. The few
slight variations observed in clinical chemistry were not dose-
related. Gross pathology showed reactions at the injection site in
two animals which had received 10 i.m. doses at 20 mg/kg b.w.
Histological examinations confirmed muscle irritation at the injection
site (5 and 20 mg/kg b.w.). Irregular degeneration of the germinal
epithelium was observed at 20 mg/kg b.w. i.m. (2 animals) and at 40
mg/kg b.w. p.o. (2 animals) (Marsboom et al., 1977b).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Four groups of albino Swiss mice (50 males and 50 females/group)
were fed diets containing 0, 25, 100 and 400 mg/kg diet, equivalent to
approximately 0, 5, 20 and 80 mg/kg b.w./day of closantel for 18
months. All animals were observed daily for mortality and physical
signs. Full necropsy was performed on all animals which died or were
sacrificed during the course of the study, and at the termination of
the study on all surviving animals.
Histopathological examination was performed systematically on
lungs, liver, pancreas, kidneys, spleen, lymph nodes, testes, ovaries,
mammary glands, adrenals, epididymis and hypophysis.
No significant effects on overall survival rate or on the time at
which mortalities occurred were noted in the various dosage groups of
either males or females. No dose related effects on health, behaviour
and appearance or gross pathology or the incidence or types of tumours
in albino Swiss mice were observed (Verstraeten et al. 1981a).
2.2.3.2 Rats
Four groups of Wistar rats (50 males and 50 females/group) were
fed diets containing 0, 25, 100 and 400 mg/kg, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day, closantel during 24 months. All animals
were observed daily for mortality and physical signs. Gross
examination was conducted on all animals which died or were sacrificed
during the course of the study, and at the termination of the study on
all surviving animals.
Gross lesion examination was performed on lungs, liver, pancreas,
kidneys, spleen, lymph nodes, testes, epididymis, ovaries, mammary
glands, adrenals and thyroids in animals killed during the study or at
the termination.
At the end of the study, cumulative mortalities in different
groups (0, 2.5, 10, and 40 mg/kg b.w./day) were 46/50, 43/50, 38/50,
49/50 for males and 32/50, 37/50, 36/50, 42/50 for females,
respectively. This rather high mortality could be related to the
typical short life-expectancy of the Wistar rat strain, bred in the
Janssen Laboratories used in this study. Closantel did not have a
very consistent effect on mortality. In females, a slight increase of
mortality was noted in the high dosage groups, whereas in males the
final mortality was not influenced by the treatment. The overall
mortality in rats was not affected at doses of 25 and 10 mg/kg b.w. in
either males or females. At 40 mg/kg b.w./day a slight increase of
mortality was noted in females.
The observed necropsy findings were comparable between groups
except for an increased incidence of spermatic granulomas in the 10
and 40 mg/kg b.w./day dosed males.
The over-all tumour rates were 40, 46, 58, 42% in males and 84,
75, 64, 47% in females in the control, low, mid and high dose groups
respectively. In females, there was a dose-dependent decrease of
total tumour incidence in the medium and high dosage groups. The
rather high number of adenomas seen in the 10 mg/kg b.w./day dosage
group is likely to be related to the longer survival of this dosage
group.
There was a significantly increased number of tumours of the
haematopoietic system in the mid-dose male rats. This increase did
not appear to be caused by the administration of closantel because:
- the increase was seen only at this level.
- the number of tumours of the haematopoietic system in the
control male rats, namely 3 tumours in 48 examined animals,
was unusually low when compared with the historical control
data from 9 other carcinogenicity studies done at the
Janssen facilities using the same Wistar rat in identical
experimental conditions.
Based upon comparison with the historical control data the
incidences of tumours of the haematopoietic system seen in the mid-and
low-dose males in this study were within the normal range of the
Wistar rat strain, whereas those of the control and the high-dose
males were significantly lower than normal. In the females, the
distribution of the different tumour types among the various groups
was very similar.
The incidence of histologically confirmed spermatic granulomas
seen in the epididymis of male rats is summarized in Table 2.
Table 2: Incidence of spermatic granulomas
Dosage group Incidence p-value
(mg/kg food) n/N
Control 0/48 -
25 0/49 -
100 7/50 <0.05
400 30/50 <0.001
An increase in the vacuolization of the optic nerve was observed
in the high-dose males and females.
The NOEL in this study was 2.5 mg/kg b.w./day (Verstraeten et
al., 1981b).
2.2.4 Reproduction study
2.2.4.1 Rats
Closantel was administered orally by gavage to male and female
Wistar rats once monthly during 3 successive generations each
producing 2 litters. The doses were 0, 2.5, 10 and 40 mg/kg b.w./mo
administered to 20 males and females/group.
Studied parameters in adults were: physical signs, body weight
gain and food consumption of dams during pregnancy, mortality and
gross pathology of males and females, and occurrence of mating,
pregnancy rates, and duration of gestation. Studied parameters in
pups were: litter size; weight at birth, weight and survival rate at
3 weeks of age, and abnormalities. The testes and epididymis were
examined histologically.
In adults, no adverse effects on body weight were observed
except at 40 mg/kg b.w./mo, where the marginal decrease of weight gain
in the 2nd litter of the 2nd and 3rd generation was associated with a
decrease in litter size. Food consumption was not affected.
Mortalities of males and females were comparable between groups. A
tendency to decreased pregnancy rate was noted at 40 mg/kg b.w./mo.
Occurrence of mating and duration of gestation were comparable between
groups.
In pups, no adverse effects on litter size could be detected,
except, at 40 mg/kg b.w./mo., a slight decrease in the number of
implants in the 2nd generation (2nd litter) and the 3rd generation
(1st and 2nd litters). No embryotoxicity effects were noted. Weight
at birth and at 3 weeks of age were comparable between groups.
Survival rate at 3 weeks of age was considered to be normal in all
groups.
Histopathological findings of spermatic granulomas (1/56 animals
at 10 mg/kg b.w./mo and 14/46 at 40 mg/kg b.w./mo) confirmed
previously reported findings in the rat.
The NOEL for closantel in this study was 2.5 mg/kg b.w./month
(Dirkx & Marsboom, 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg of closantel in the diet, equivalent to 0, 2.5,
10 and 40 mg/kg b.w./day during the period of organogenesis (day 6 to
day 15). The females were sacrificed on the 22nd day post mating.
Body weight, food consumption and mortalities of dams were measured.
Implantation number, pregnancies and pups, litter size and weight at
birth, resorption number, live and dead fetuses, number and
distribution of live, dead and resorbed embryos, and abnormalities
were measured.
There were no differences between groups. Teratogenic effects
were not observed (Marsboom, 1975).
Four groups of female Wistar rats were fed diets containing 0,
25, 100 and 400 mg/kg closantel, equivalent to 0, 2.5, 10 and 40 mg/kg
b.w./day, from day 16 of pregnancy and during the 3 week lactation
period. Body weight, food consumption, mortality, pregnancy rate, and
the gestation period of dams were measured. Cannibalism, litter size,
weight at birth and at 2 and 3 weeks after birth, number of live and
stillborn fetuses, survival of pups at 4 days and 2 and 3 weeks after
birth and abnormalities in pups were quantified.
There were no differences between groups and no effects related
to treatment were observed (Marsboom, 1979).
2.2.5.2 Rabbits
Female New Zealand White rabbits (19 in the control group and 20
in each experimental group) received closantel (0, 10 and 40 mg/kg
b.w./day) orally by gavage from day 6 through day 18 of pregnancy, the
period of organogenesis. Females were sacrificed on the 28th day of
gestation. They were necropsied and checked for gross abnormalities.
The examined parameters were (for dams): pregnancy, body weight and
mortality; (for pups): resorption numbers, live and dead fetuses,
litter size, weight at delivery, survival rates after 24 hours, and
abnormalities.
One control female died on day 2 of the experiment from
pneumonia. No differences between groups were observed for body
weight, pregnancy and embryotoxic effects.
In the control group, two neonates from one female had bifurcated
ribs; in the low dosage group, 3 neonates from 3 different females
had either a deformed left fore-leg, a bifurcated rib or a waved rib;
in the high dosage group, 2 neonates from 2 different females had
either deformed thoracic bones or acrania.
Teratogenic or embryotoxic effects were not observed in this
study (Marsboom, 1976b).
2.2.5.3 Sheep
Ewes (274), separated into 10 treatment groups, received
closantel orally at 0, 20, or 40 mg/kg, either on day 11, 17 or 23
after mating. No influence of treatment was noted on the ovarian
cycle or on subsequent ovulation, copulation and conception. No
embryotoxic or teratogenic effects were reported in the clinical trial
(Chevis, 1977).
2.2.6 Special studies on fertility
2.2.6.1 Rats
The study was conducted using 20 males and 20 females/group.
Dose levels were 0, 25, 100 and 400 mg/kg food. Males were treated
for a minimum of 60 days prior to mating with non-dosed females.
Females were treated for at least 14 days prior to mating with non-
dosed males and then throughout gestation. Parameters studied were:
body weight, food consumption, pregnancy outcome, mortality in
parenteral and filial animals, number of implantations, litter size
and weight, the number and distribution of live, dead and resorbed
fetuses, and foetal abnormalities.
Body weight gain was comparable in all parental groups, except
for a lower weight gain in non-dosed females mated with dosed males of
the 400 mg/kg food group; food consumption was comparable in all
groups; no differences in pregnancy outcome were noted except a
reduced pregnancy rate in the high dosage group of non-dosed females
mated with dosed males; there were no mortalities.
No embryotoxic effects were noted except for a decrease in the
number of implants and litter size in the group of non-dosed females
mated with high-dosed males. No abnormalities were related to the
administration of closantel.
Fertility was not affected by the administration of closantel
except for a decreased pregnancy rate in non-dosed females mated with
high-dosed males (Marsboom, 1978).
2.2.6.2 Other species
Semen of bulls treated either once at approximately 2 mg/kg b.w.
or three times at 5 mg/kg b.w. intramuscularly at 8 weekly intervals
was normal (Debruyne, 1978); (Retief, undated a).
Semen of rams treated three times, by the oral route at 20 mg/kg
b.w. at 8 weekly intervals, was normal. (Retief, undated b).
In rams treated (15 mg/kg b.w. by the oral route) 3 or 5 times at
3 or 4 weekly intervals, the semen was normal, and the testes and
epididymis presented a normal structure (Johns, 1981).
2.2.7 Special studies on genotoxicity
Several tests were conducted to assess the genotoxicity of
closantel, they are summarized in Table 3.
Table 3: Results of genotoxicity assays on closantel
Test System Test object Concentration Results Reference
Cytotoxicity - L 5178 Y mouse 0.3-500 µg/ml Cytotoxic effects Enninger, 1989
lymphoma cells (1) in all 3 test
- V 79 Chinese systems. Reduced
hamster cells in presence of S9
- Chinese hamster mix
ovary (CHO) cells
Ames test (1) S. typhimurium 10-2000 µg/plate (2) Negative Poncelet, 1981
TA 1530, TA 1535,
TA 1538, TA 98,
TA 100
Ames test (1) S. typhimurium 1-1000 µg/plate (2) Negative Vanparays &
TA 1535, TA 1538, TA Marsboom, 1987
97, TA 98,
TA 100
In vivo: Drosophila melanogaster 10-50 ppm (3) Negative Vanparays &
sex linked recessive Marsboom, 1981
lethal test
In vivo: Male mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978a
oral treatment
(4)
In vivo: Female mice 10, 40, 160 Negative Vanparays &
Dominant lethal test mg/kg single Marsboom, 1978b
oral treatment
(4)
DNA repair assay Cultured rat 0.3-100 µg/ml Negative Weterings, 1985
hepatocytes (5)
Table 3 (continued)
1 Both with and without rat liver S9 fraction
2 Sodium azide, nitrofluorene and 2-aminoanthracene were used as positive controls
3 Procarbazine was used as positive control
4 Cyclophosphamide used as positive control
5 7,12-Dimethyl benzanthracene was used positive control
2.3 Observations in humans
A single oral dose of closantel of 2.5 mg/kg b.w. in 5 patients
and a single s.c. injection of 2.5 mg/kg b.w. in one patient were
administered against F. hepatica. All orally treated patients
complained of the bitter taste of the drug, and one presented with
nausea and vomiting. In the last case, the dose was repeated adding
sugar and was then well tolerated. After the s.c. injection,
tachycardia, sweating, a metallic taste in the mouth, induction of
micturition and defecation, reddening of the skin, nervousness,
stress, excitation and a sensation of anguish occurred.
Haematology tests (haematocrit, WBC count and differential
prothrombin-time), clinical biochemistry tests (ALP, SGOT, SGPT,
cholesterol, and glucose), and urinalysis remained unchanged (2
examinations were carried out: one before, and the other seven days
after treatment) (Bernardiner, 1979).
Patients (14) were treated with a single oral dose of 5 mg/kg
b.w. of closantel. The treatment was well tolerated but poor efficacy
against Ancylostoma duodenale was obtained (Borda, 1980).
Patients (13) were treated with a single dose of closantel (6 at
5 mg/kg b.w; 3 at 7.5 mg/kg b.w.; 4 at 10 mg/kg b.w.). In all
treatment groups, side effects such as diarrhoea, drowsiness and
blurred vision were observed (Saowakontha, undated).
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of short-term studies, and of
studies of carcinogenicity, genotoxicity and effects on reproduction
and development.
In studies in rats and sheep, closantel was shown to be strongly
bound to the plasma proteins. The compound was poorly metabolized,
mainly to 3- and 5- monoiodoclosantel. About 90% of the administered
dose was excreted in the faeces, and about 90% was unchanged.
In a study in rats in which closantel was administered in the
diet at levels up to the equivalent of 40 mg per kg of body weight per
day for 13 weeks, spermatic granulomas in the epididymis and hepatic
fatty changes in the liver were observed at the highest dose level.
The NOEL was 2.5 mg per kg of body weight per day.
Dogs were dosed for 3 months by oral administration of the
compound in gelatin capsules at levels up to 40 mg per kg of body
weight per day. There were fatty changes in the liver at the highest
dose level. The NOEL for this study was 2.5 mg per kg of body weight
per day.
A carcinogenicity study was conducted in mice, which received
closantel in the diet at levels up to 80 mg per kg of body weight per
day for 18 months. There were no dose-related effects on the total
numbers of tumours nor on any individual type of tumour. No other
effects were observed in this study.
In a carcinogenicity study in rats in which closantel was
administered in the diet at levels up to 40 mg per kg of body weight
per day for 24 months, there was no effect on the overall incidence of
tumours. However, by comparison with concurrent controls, there was
a statistically significant increase in the incidence of
haematopoietic tumours in male rats at 10 mg per kg of body weight per
day. This incidence was nevertheless within the historical control
range, while the incidences found in the control and high-dose groups
were significantly lower than in the historical controls. Spermatic
granulomas were observed. The NOEL for this study was 2.5 mg per kg
of body weight per day.
Adverse effects on reproduction were probably associated with
toxicity to the male reproductive organs, and were seen only at doses
of 2.5 mg/kg body weight per day and above. In a fertility study in
rats, in which closantel was administered in the diet, there was a
reduced pregnancy rate in untreated females paired with males
receiving the highest dose of 40 mg per kg of body weight per day. A
three-generation reproduction study was conducted in male and female
rats, in which the compound was administered once monthly by gavage.
There was a decrease in the pregnancy rate and number of implants per
animal at the highest dose level of 40 mg per kg of body weight.
Spermatic granulomas were seen at 10 and 40 mg per kg of body weight
but not at 2.5 mg per kg of body weight.
In teratogenicity studies in rabbits and rats, no teratogenic or
toxic effects were evident at doses up to 40 mg per kg of body weight
per day. The rabbits received closantel by gavage on days 6 to 18 of
pregnancy, while the rats, were given the compound in the diet on days
6 to 15 of pregnancy.
Closantel gave negative results in a range of in vitro and
in vivo mutagenicity studies. No tests for clastogenicity were
performed, but the Committee noted that carcinogenicity studies had
been carried out in two species.
Limited clinical reports were available from 33 patients who were
treated with a single oral or parenteral dose of closantel at 2.5 to
10 mg per kg of body weight. There were no adverse effects.
4. EVALUATION
An ADI of 0-0.03 mg per kg of body weight was established for
closantel based on the NOEL of 2.5 mg per kg of body weight per day in
rats and a safety factor of 100.
5. REFERENCES
BEHM, C.A. & BRYANT, C. (1985). The mode of action of some modern
anthelmintics. In Anderson, N. & Waller, J.P. (Eds). Resistance in
Nematodes to anthelmintic drugs. Commonwealth Scientific & Industrial
Research Organization. Australia, pp. 57-67.
BERNARDINER, E. (1979). Preliminary results of the study with
Closantel in fluke disease. Unpublished data. N 1807 from Johnson-
Johnson. Buenos-Aires, Argentina. Submitted to WHO by Janssen
Pharmaceutica.
BORDA, E. (1980). Clinical efficacy and safety of Closantel (R 31520)
on pregnancy in sheep. Unpublished Report N 22122 from Corrientes,
Argentina. Submitted to WHO by Janssen Pharmaceutica.
CHEVIS, R. (1977). Investigation of the effect of Closantel (R 31522)
from on pregnancy in sheep. Unpublished trial Report V 2744, from
ETHNOR (PTY) LTD. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
DEBRUYNE, R. (1978). Fertility control in male cattle after
administration of Closantel (R 31520). Unpublished Report V 2859,
from Artificial Insemination Center. Oostmalle, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
DIRKX, P. & MARSBOOM, R. (1984). Oral three-generation study in
Wistar rats. Unpublished Report V 5183 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
ENNINGER, I.C. (1989). Evaluation of the cytotoxic activity of
Closantel R 31520 in L 5178 Y mouse lymphoma cells, in V 79 Chinese
hamster cells and in Chinese hamster ovary (CHO) cells. Unpublished
Report V 6999 from RCC NOTOX 2BV. S'-Hertogenbosch, the Netherlands.
Submitted to WHO by Janssen Pharmaceutica.
JOHNS, D. (1981). To determine the effect of Closantel on the
fertility of rams after five consecutive oral treatments at twice the
recommended rate. Unpublished Report V 4362, from Smith-Kline Animal
Health. The Oaks, Australia. Submitted to WHO by Janssen
Pharmaceutica.
KANE, H.J., BEHM, C.A. & BRYANT, C. (1980). Metabolic studies on the
new fasciolicidal drug, Closantel. Mol. and Biochem. Parasitol., 1,
347-355.
MANNENS, G., MOSTAMANS, E., VERBOVEN, P., HENDRICKX, J., HURKMANS, R.,
VAN LEEMPUT, L., MEULDERMANS, W. AND HEYKANTS, J. (1989). The
excretion and metabolism of 14-C-closantel in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished report V 7201. Submitted
to FAO by Janssen Pharmaceutica.
MARSBOOM, R. (1975). Oral embryotoxicity and teratogenicity study in
Wistar rats (Segment II). Unpublished Report V 2403- from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1976a). Acute toxicity studies in sheep and cattle
single dosing: oral/intramuscular. Unpublished report preclinical
research Report V 2402 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R. (1976b). Oral embryotoxicity and teratogenicity study
in New Zeeland White rabbits (SEGMENT II). Unpublished Report V 2404
from Jansen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium, Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977a). Oral
toxicity study in Wistar rats (repeated dosages for 13 weeks).
Unpublished Report V 2700 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium, Submitted to WHO by
Janssen Pharmaceutica.
MARSBOOM, R., HERIN, V., VANDENSTANE, R. & VAN BELLE, H. (1977b).
Oral and intramuscular toxicity study in Suffolk Sheep (10 doses per
animal one every fourth week). Unpublished Report V 2701 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1978). Oral male and female fertility study in Wistar
rats. (Segment I). Unpublished Report V 2860 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. & HERIN, V. (1978). Oral toxicity study in Beagle dogs
(repeated dosage for 3 months). Unpublished Report V 2985 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
MARSBOOM, R. (1979). Oral Embryotoxicity and teratogenicity study in
Wistar rats (peri. and postnatal study) (Segment III). Unpublished
Report V 3847 from Janssen Research Products Information Service,
Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MARSBOOM, R. LAMPO, A., VAN CAUTEREN, H., VANPARYS, P.H., MAES, L. &
HEYKANTS, J. (1989). Review report on the toxicological,
pharmacodynamic and pharmacokinetic documentation related to
Closantel. Unpublished executive review report from Janssen Research
Foundation Products Information Service, Janssen Pharmaceutica,
Beerse, Belgium. Submitted to WHO by Jansen Pharmaceutica.
MEULDERMANS, W., MICHIELS, M., WOESTENBORGHS, R., VAN-HOUDT, J.,
LORREYNE, W., HENDRICKX, J., HEYKANTS, J. & DESPLENTER, L. (1982).
Absorption, tissue distribution, excretion and metabolism of
Closantel-14C after intramuscular and oral administration in sheep.
Unpublished preclinical research report V 4523 from Janssen Research
Products Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Jansen Pharmaceutica.
MICHIELS, M., WOESTENBORGHS, R., HEYKANTS, J. & MARSBOOM, R. (1977a).
On the absorption and distribution of Closantel (R 31520) in sheep
after oral and intramuscular administration. Unpublished preclinical
research report V 2709 from Janssen Research Products Information
Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted to WHO by
Janssen Pharmaceutica.
MICHIELS, M., HENDRICKX, J., HEYKANTS, J., MARSBOOM, R. (1977b).
Plasma and milk concentrations of Closantel in cattle after a single
intramuscular administration. Unpublished preclinical research report
V 2706 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
MICHIELS, M., MEULDERMANS, W. & HEYKANTS, J. (1987). The metabolism
and fat of Closantel (Flukiver) in sheep and cattle. Drug. Met.
Review, 18, (2 & 3), 235-251.
NIEMEGEERS, C.J.E. (1976). Acute intramuscular and oral toxicity of
R 31520 in mice and rats. Unpublished preclinical research report V
2396 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Jansssen
Pharmaceutica.
PONCELET, F. (1981). In vitro mutagenicity study of Closantel R
31520, lot B 4901. Unpublished Report V 3944 from Laboratoires de
Toxicologie, UCL. Woluwe, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
PRICHARD, R.K. (1987). The pharmacology of anthelmintics in
livestock. Int. J. Parasitol. 17, 473-482.
RETIEF, G. (undated a). To determine the effect of Closantel
administered s.c. at 5 mg/kg livemass after 3 treatments spaced at 8
weekly intervals on the semen quality of bulls. Unpublished Report V
3782 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
RETIEF, G. (undated b). To determine the effect of Closantel on the
fertility of rams after three consecutive oral treatments, 8 weeks
apart at double the recommended dosage rate. Unpublished Report V
3783 from Animal Breeding Services (PTY) LTD. Brooklyn, Pretoria.
Submitted to WHO by Janssen Pharmaceutica.
SOAWAKONTHA, S. (undated). Unpublished report from Thailand.
Submitted to WHO by Janssen Pharmaceutica.
VAN DEN BOSSCHE, H., VERHOEVEN, H., VANPARIJS. O., LAWERS, H. &
THIENPONT, H. (1979). Closantel a new antiparasitic hydrogen
ionophore. Arch. Int. Physiol. Biochimie., 87, 851-852.
VAN DEN BOSSCHE, H., VERHOEVEN, H. & LAWERS, H. (1980). Uncoupling of
liver mitochondria associated with fascioliasis in rats.
Normalization by Closantel. In: Van den Bossche (Ed). The host
invader interplay, Elsevier, Amsterdam, pp. 699-704.
VAN DEN BOSSCHE, H. & VERHOEVEN, H. (1983). Effects of albumin on the
Closantel induced alterations of mitochondrial processes. Unpublished
preclinical research report V 4546 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium.
Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978a). Closantel R 31520. Dominant
lethal test in male mice. Unpublished report V 3144 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1978b). Closantel R 31520. Dominant
lethal test in female mice. Unpublished report V 2998 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1981). Closantel R 31520. Sex linked
recessive lethal test on Drosophila Melanogaster. Unpublished report
V 4058 from Janssen Research Products Information Service, Janssen
Pharmaceutica, Beerse, Belgium. Submitted to WHO by Janssen
Pharmaceutica.
VANPARYS, P.H. & MARSBOOM, R. (1987). Closantel R 31520. Ames
reverse mutation test. Unpublished report V 6517 from Janssen
Research Products Information Service, Janssen Pharmaceutica, Beerse,
Belgium. Submitted to WHO by Janssen Pharmaceutica.
VERHEYEN, A., VANPARYS, O., LAWERS, H. & THIENPONT, D. (1980). The
influence of Closantel administration to sheep on the ultrastructure
of the adult liver fluke F. hepatica L. In: van den Bossche (Ed).,
The host invader interplay, Elsevier, Amsterdam, pp. 705-708.
VERSTRAETEN, A., VANDENBERGHE, J. & MARSBOOM, R. (1981a). Oral
carcinogenicity study in Albino Swiss mice (repeated dosage for 18
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
VERSTRAETEN, A., VANDENBERGHE, J., HERIN, V. & MARSBOOM, R. (1981b).
Oral carcinogenicity study in Wistar rats (repeated dosage for 24
months). Unpublished report V 4057 from Janssen Research Products
Information Service, Janssen Pharmaceutica, Beerse, Belgium. Submitted
to WHO by Janssen Pharmaceutica.
WETERINGS, P.J.J.M. (1985). Evaluation of the DNA repair inducing
ability of R 31520 in a primary culture of rat hepatocytes.
Unpublished Report V 5740 from NOTOX v.o.f, S'-Hertogenbosch, The
Netherlands. Submitted to WHO by Janssen Pharmaceutica.
