
CARBADOX
1. EXPLANATION
Carbadox is a growth-promoting and antibacterial drug added to
swine feed at the rate of 50 ppm. In most areas of the world it is
used in animals up to 4 months of age with a 4 week withdrawal period
prior to slaughter for human consumption. Carbadox has not been
evaluated previously by the Joint FAO/WHO Expert Committee on Food
Additives.
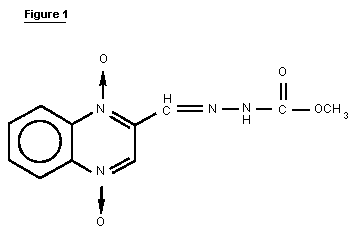
The structure of carbadox is shown in Figure 1.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The elimination of carbadox was studied in rats, swine and
monkeys. Pigs received 3.5 mg/kg of 14C-carbadox after several of
weeks of receiving feed containing 50 g/ton of unlabelled carbadox,
while rats and monkeys received a single dose of 5 mg/kg 14C-carbadox.
Urine and faeces were collected and assayed for radioactivity. The
urinary metabolites were evaluated qualitatively using TLC. Nearly
all (13/15) of the metabolites present in swine urine are found in rat
and monkey urine. One of the other two was a glycine conjugate of
quinoxaline-2-carboxylic acid. All species excreted more than 50% of
the dose in urine (swine: 74%, monkey: 61%, rat: 54%) during the 72
hour collection period. The following activities were reported for
faeces during the 72 hour period: swine, 17%, monkey, 8-10%, and rat,
29%. Total excretion appeared to be in the 70-90% range over the 72
hour period. The author concluded that the distribution of
radioactivity was similar in the three species (von Wittenau, 1969).
2.1.2 Biotransformation
Seven-week-old swine received feed containing 50 g/ton of
unlabelled carbadox for several weeks, then received a single oral
dose of either 3.5 mg/kg or 0.8 mg/kg of 14C-carbadox labelled in the
phenyl ring. Peak radioactivity was observed in plasma approximately
3 hours after dosing. The following were identified in plasma at 5-8
hours post dose: carbadox (13%), desoxycarbadox (9-19%),
carbadoxaldehyde (13%), and quinoxaline-2-carboxylic acid (19%) (all
expressed in terms of total plasma radioactivity). The presence of
carbadoxaldehyde in stomach contents was confirmed. Carbadox was
rapidly eliminated. Approximately 2/3 of the dose was eliminated in
the urine and the remainder in the faeces (total of approximately 90%)
within 48-72 hours. Radioactivity equivalent to approximately 0.1 ppm
carbadox was found to be retained in the liver at 14 days post dose.
Attempts to identify this residual radioactivity were not successful.
The only metabolite identified in liver after 24 hours was the major
urine-eliminated metabolite, quinoxaline-2-carboxylic acid (Figdor
et al., 1969).
In a second study seven-week-old pigs received unlabelled
carbadox in feed at the rate of 50 g/ton for several weeks followed by
a single oral dose of 14C-carbadox labelled in the carbonyl position
via stomach tube. The following evaluations were made: expired air
was evaluated for 14CO2, labelled material in liver and urine was
evaluated to determine if it was methyl carbazate-related, and plasma
and urine were evaluated for free hydrazine. Maximum plasma
concentrations of radiolabelled material occurred at approximately 3
hours. While early plasma concentrations were similar to those found
for ring-labelled carbadox, concentrations at 24 hours remained
somewhat higher. Approximately 50% of the radiolabelled material in
the plasma at 3 hours was identified as carbadox, while methyl
carbazate was estimated to be 30%. The major route of radiolabel
excretion was urinary. However, less than half the total amount
recovered with ring-labelled carbadox was recovered from carbonyl-
labelled carbadox (37% vs 88%). The reason for this is the apparently
high conversion of the radiolabel to CO2 (verified in the rat as up
to 36%). Radiolabelled material equivalent to 0.1 - 0.34 ppm carbadox
was present in liver at 5 days. The authors concluded that some of
this material was incorporated CO2 (potentially 25%). The pig
receiving 7 mg/kg was found to have eliminated 7% of the dose as free
hydrazine in the urine at 24 hours, while pigs receiving lesser doses
were not found to have any identifiable hydrazine in their urine
(Figdor, 1969).
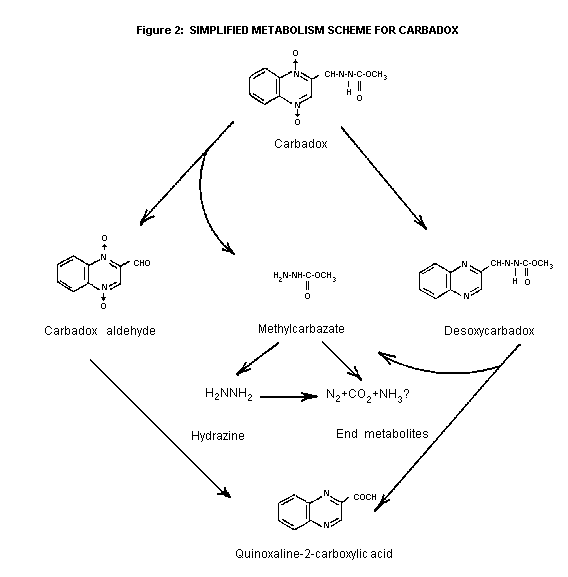
The reported studies support the metabolism scheme illustrated in
Figure 2.
Extensive studies have been performed not only on carbadox, but
also on its metabolites. Studies are summarized separately on each
substance in this monograph.
2.1.3 Effects on enzymes and other biochemical parameters
The persistence of carbadox-induced adrenal lesions was studied
in swine. Groups of 13 animals each received 0, 25, 50, 100, or 200
ppm carbadox in feed for 10 weeks. Five and 11 weeks after withdrawal
2 pigs from each group were necropsied and adrenals evaluated
histologically. At five weeks the pigs in the 25 and 50 ppm groups
showed recovery of adrenal lesions. At 11 weeks pigs in the 100 and
200 ppm groups had not completely recovered. Plasma aldosterone
levels were monitored at 1, 2, 4, 6, and 8 weeks post treatment. At
one week aldosterone levels were depressed in the 100 and 200 ppm
groups vs controls. All other aldosterone measurements of treated
groups were similar to control values (van der Molen et al.,
1989a).
The effect of carbadox on the renin-angiotensin system was
examined in swine. Five groups of 13 five-week-old weaned pigs
received feed containing 0, 50, 100, 150, and 200 ppm carbadox to
provide target doses of 0, 2, 4, 6, and 8 mg/kg body weight for 10
weeks. By nine weeks the plasma renin levels of all treated groups
were significantly higher than controls. At 5 and 10 weeks, renal
immunoreactive renin from two to three pigs was evaluated. All pigs
demonstrated an increase in immunoreactive renin. It was concluded
that carbadox induces activation of the renin-angiotensin system,
secondary to suppression of mineralocorticoid secretion (van der Molen
et al., 1989b).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The elimination of carbadox was studied in rats, swine and
monkeys. Pigs received 3.5 mg/kg of 14C-carbadox after several of
weeks of receiving feed containing 50 g/ton of unlabelled carbadox,
while rats and monkeys received a single dose of 5 mg/kg 14C-carbadox.
Urine and faeces were collected and assayed for radioactivity. The
urinary metabolites were evaluated qualitatively using TLC. Nearly
all (13/15) of the metabolites present in swine urine are found in rat
and monkey urine. One of the other two was a glycine conjugate of
quinoxaline-2-carboxylic acid. All species excreted more than 50% of
the dose in urine (swine: 74%, monkey: 61%, rat: 54%) during the 72
hour collection period. The following activities were reported for
faeces during the 72 hour period: swine, 17%, monkey, 8-10%, and rat,
29%. Total excretion appeared to be in the 70-90% range over the 72
hour period. The author concluded that the distribution of
radioactivity was similar in the three species (von Wittenau, 1969).
2.1.2 Biotransformation
Seven-week-old swine received feed containing 50 g/ton of
unlabelled carbadox for several weeks, then received a single oral
dose of either 3.5 mg/kg or 0.8 mg/kg of 14C-carbadox labelled in the
phenyl ring. Peak radioactivity was observed in plasma approximately
3 hours after dosing. The following were identified in plasma at 5-8
hours post dose: carbadox (13%), desoxycarbadox (9-19%),
carbadoxaldehyde (13%), and quinoxaline-2-carboxylic acid (19%) (all
expressed in terms of total plasma radioactivity). The presence of
carbadoxaldehyde in stomach contents was confirmed. Carbadox was
rapidly eliminated. Approximately 2/3 of the dose was eliminated in
the urine and the remainder in the faeces (total of approximately 90%)
within 48-72 hours. Radioactivity equivalent to approximately 0.1 ppm
carbadox was found to be retained in the liver at 14 days post dose.
Attempts to identify this residual radioactivity were not successful.
The only metabolite identified in liver after 24 hours was the major
urine-eliminated metabolite, quinoxaline-2-carboxylic acid (Figdor
et al., 1969).
In a second study seven-week-old pigs received unlabelled
carbadox in feed at the rate of 50 g/ton for several weeks followed by
a single oral dose of 14C-carbadox labelled in the carbonyl position
via stomach tube. The following evaluations were made: expired air
was evaluated for 14CO2, labelled material in liver and urine was
evaluated to determine if it was methyl carbazate-related, and plasma
and urine were evaluated for free hydrazine. Maximum plasma
concentrations of radiolabelled material occurred at approximately 3
hours. While early plasma concentrations were similar to those found
for ring-labelled carbadox, concentrations at 24 hours remained
somewhat higher. Approximately 50% of the radiolabelled material in
the plasma at 3 hours was identified as carbadox, while methyl
carbazate was estimated to be 30%. The major route of radiolabel
excretion was urinary. However, less than half the total amount
recovered with ring-labelled carbadox was recovered from carbonyl-
labelled carbadox (37% vs 88%). The reason for this is the apparently
high conversion of the radiolabel to CO2 (verified in the rat as up
to 36%). Radiolabelled material equivalent to 0.1 - 0.34 ppm carbadox
was present in liver at 5 days. The authors concluded that some of
this material was incorporated CO2 (potentially 25%). The pig
receiving 7 mg/kg was found to have eliminated 7% of the dose as free
hydrazine in the urine at 24 hours, while pigs receiving lesser doses
were not found to have any identifiable hydrazine in their urine
(Figdor, 1969).
The reported studies support the metabolism scheme illustrated in
Figure 2.
Extensive studies have been performed not only on carbadox, but
also on its metabolites. Studies are summarized separately on each
substance in this monograph.
2.1.3 Effects on enzymes and other biochemical parameters
The persistence of carbadox-induced adrenal lesions was studied
in swine. Groups of 13 animals each received 0, 25, 50, 100, or 200
ppm carbadox in feed for 10 weeks. Five and 11 weeks after withdrawal
2 pigs from each group were necropsied and adrenals evaluated
histologically. At five weeks the pigs in the 25 and 50 ppm groups
showed recovery of adrenal lesions. At 11 weeks pigs in the 100 and
200 ppm groups had not completely recovered. Plasma aldosterone
levels were monitored at 1, 2, 4, 6, and 8 weeks post treatment. At
one week aldosterone levels were depressed in the 100 and 200 ppm
groups vs controls. All other aldosterone measurements of treated
groups were similar to control values (van der Molen et al.,
1989a).
The effect of carbadox on the renin-angiotensin system was
examined in swine. Five groups of 13 five-week-old weaned pigs
received feed containing 0, 50, 100, 150, and 200 ppm carbadox to
provide target doses of 0, 2, 4, 6, and 8 mg/kg body weight for 10
weeks. By nine weeks the plasma renin levels of all treated groups
were significantly higher than controls. At 5 and 10 weeks, renal
immunoreactive renin from two to three pigs was evaluated. All pigs
demonstrated an increase in immunoreactive renin. It was concluded
that carbadox induces activation of the renin-angiotensin system,
secondary to suppression of mineralocorticoid secretion (van der Molen
et al., 1989b).
 The in vitro effect of carbadox on adrenal aldosterone production
was evaluated in porcine adrenal slices obtained from 3-5 week old
pigs. The adrenal slices were maintained in Krebs solution and
exposed to 1-40 µg carbadox/ml. Aldosterone was estimated using RIA
at hourly intervals. A dose-dependent decrease in aldosterone
production occurred with a reduction of 25-35% at 40 µg/ml
(Spierenburg et al; 1988).
2.2. Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with carbadox are shown in
Table 1.
Table 1: Carbadox acute toxicity data
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 2810 Pfizer, undated a
F oral >2810
i.p. 1050
Rat M oral 850
i.p. 810
A study was done to examine the emetic effect of carbadox on
monkeys and dogs. Three animals/group received a single dose of
carbadox orally via capsule. Doses were 25, 10, 5, and 1 mg/kg
b.w./day. In the monkey 1/3 exhibited emesis in the 2 higher dose
groups, while in dogs all dose groups had emesis.
2.2.2 Short-term studies
2.2.2.1 Rats
Twenty Charles River C-D weanling rats were divided and dosed for
30 days as follows: 2-3 males and females at 50 mg/kg b.w./day and
100 mg/kg b.w./day, and 10 males as untreated controls. Carbadox was
administered in the diet. Weight and food consumption were observed
weekly and drug levels adjusted as necessary. CBC at 30 days, urinary
electrolytes at 1, 2, 3, 14, and 30 days, cholesterol at 30 days and
urinalysis at 14 and 30 days were evaluated. Rats received a gross
necropsy at the end of the study and tissues were evaluated
microscopically. A dose-dependent decrease in weight gain and food-
consumption were noted. The authors concluded that all other
parameters were normal (Pfizer, 1963).
2.2.2.2 Dogs
The short-term toxicity of carbadox was studied by dosing dogs 6
days/week for three weeks. Beagle dogs (1/sex/dose) were initially
dosed with 25 or 50 mg/kg b.w./day carbadox via oral capsule. These
doses were later reduced to 10 and then 15 mg/kg b.w./day due to
emesis. A variety of parameters were evaluated including clinical
chemistry, gross necropsy, and microscopic tissue examination at the
termination of the study. The dogs lost weight and had elevated
SGPT's. It was concluded that carbadox is emetic and possibly
hepatotoxic in the dog (Stebbins, 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
The chronic toxicity and potential tumorigenicity of carbadox
were studied in rats. One hundred twenty Charles River C-D rats were
divided into 6 groups (10/sex/dose) and received carbadox in the diet
at rates providing doses of 100, 50, 25, 10, 5, and 0 mg carbadox/kg
b.w./day for 26 months. Feed concentration was adjusted weekly.
Clinical signs, body weights, and food consumption were recorded
weekly. Haematology and urinalysis were evaluated in 5 rats/sex/dose
at 3, 6, 12, 18, and 25 months. Rats were sacrificed at 14 and 112
weeks and received gross necropsies. Tissues were examined
histopathologically.
Survival was reduced for animals in the high-dose groups. The
following observations were reported for animals up to and including
the interim sacrifice:
100 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption, reduced haemoglobin, RBC's and neutropenia.
Microscopic changes reported include pulmonary hemorrhage and edema,
adrenalcortical hemorrhage and degeneration, splenic haemosiderin, and
thymic atrophy. No animals survived beyond the 14 week sacrifice.
50 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption. Microscopic changes reported include pulmonary
hemorrhage and edema, adrenalcortical hemorrhage, necrosis, and
degeneration, splenic haemosiderin and renocorticomedullary fatty
metamorphosis. No animals survived beyond the 14 week sacrifice.
25 mg/kg b.w./day (3 rats/sex necropsied) - Reduced weight gain,
slight adrenal cortical atrophy, degeneration/necrosis, and renal
tubular fatty change.
5 and 10 mg/kg b.w./day (3 rats/sex necropsied) - No clinical, gross
or microscopic changes reported at 3 months.
The following observations were reported for rats from 3 months
up to and including the 26 month sacrifice:
25 mg/kg b.w./day - One female died at 51 weeks with no drug-related
changes noted. All 13 remaining rats were sacrificed by 73 weeks due
to palpable abdominal masses. At necropsy all rats had multiple
hepatic nodules. Ten of 13 rats were diagnosed microscopically to
have benign nodular hyperplasia, while the remaining 3 rats were
determined to have malignant transformation based on metastatic foci
observed in other organs (lung, kidney and lymph nodes).
10 mg/kg b.w./day - One rat died after 67 weeks with
reticuloendothelial neoplasia, a common tumour of aged rats. The
remaining 13 rats were sacrificed between the 64th and 112th weeks.
Eleven of these were observed to have hepatic benign nodular
hyperplasia.
5 mg/kg b.w./day - One male died at 20 weeks due to pulmonary
abscesses. The remaining 13 died or were sacrificed between the 61st
and 112th weeks. Five or these were observed to have hepatic benign
nodular hyperplasia.
Controls One male was sacrificed at 33 weeks with a forestomach
papilloma and pulmonary atelectasis and a second male died at 93 weeks
with myocarditis, nephrosclerosis and peribronchitis. The remaining
animals were sacrificed between weeks 80 and 112 of the study. No
animals in the control group were observed to have hepatic nodular
hyperplasia. The authors suggested that the observed nodular
hyperplasia in treated groups was associated with hepatic injury and
concluded that the rats failed to tolerate carbadox at any of the
tested dose levels. A treatment-related increase in total tumours was
also reported for carbadox-treated rats. The tumour incidence was
lower in the high-dose group, however mortality occurred earlier in
this group than in other treatment groups (Stebbins & Coleman, 1967).
A follow-up study was performed to determine a chronic carbadox
level tolerated by rats. Sixty male Charles River rats (124-173 gms)
and 60 female rats (107-152 gms) of the same strain were divided into
3 groups (20/sex/dose) and received 2.5, 1.0, or 0 mg/kg b.w./day of
carbadox in the diet. Signs, weights and food consumption were
recorded weekly and drug levels were adjusted accordingly.
Haematology, complete urinalysis, and ophthalmoscopic examination were
done at 3, 6, 12, 18, and 24 months on 5 rats/sex/dose. An interim
sacrifice of 5 rats/sex/dose was conducted at 54 weeks with the
remaining rats sacrificed at 106 weeks. Each rat received a gross
necropsy and microscopic examination of standard tissues including:
brain, spinal cord, eye, sub-maxillary gland, thyroid gland, thymus,
heart, aorta, lung, sternum, liver, spleen, pancreas, kidney, adrenal,
stomach, mesenteric lymph node, reproductive tract, urinary bladder,
femoral nerve, femoral bone marrow, and mammary gland with skin.
All parameters evaluated, including histopathologic examination,
were within normal limits at 54 weeks. Survival at 2 years was as
follows: control - 38%, 1.0 mg/kg b.w./day - 45%, 2.5 mg/kg b.w./day
- 43%. At the 2.5 mg/kg b.w./day level 7/27 rats displayed hepatic
benign nodular hyperplasia and 7/27 showed peliosis hepatis. At the
1.0 mg/kg b.w./day level 1/29 rats was found to have hepatic benign
nodular hyperplasia and 3/29 displayed peliosis hepatis. In the
control group 3/29 rats had benign nodular hyperplasia and 2/29 had
peliosis hepatis. Additionally, an increase in total mammary tumours
was reported in the 2.5 mg/kg b.w./day dose group. The authors
concluded that the 1.0 mg/kg b.w./day dose level was tolerated by the
rats for 2 years with no adverse effects, based on the similar
incidence of both hepatic lesions and tumours in other organs in
treatment and control groups (Sigler, 1969a).
Three groups of 28 rats (14/sex) were treated with a target dose
of 25 mg/kg b.w./day of carbadox, or one of two lots of desoxycarbadox
for 11 months to compare the oncogenic activity of carbadox and
desoxycarbadox. An equal sized group served as untreated controls.
The test material was given in the feed for 10 months. Two to four
rats were sacrificed at 30, 60, 90, 191, and 309 days. Animals were
observed daily for toxicity and mortality. Weights and food
consumption were recorded weekly until the 38th week and then every
other week. Rats received a gross necropsy at sacrifice and liver and
adrenals were evaluated histopathologically. A moderate decrease in
weight gain occurred in both sexes receiving carbadox. No other
significant clinical observations were made. At gross necropsy all
groups treated for 10 months showed evidence of hepatic changes
including necrosis and nodule formation. Changes in the carbadox-
treated group were less severe than in the desoxycarbadox-treated
group. Histopathologically all desoxycarbadox-treated rats showed
evidence of hepatocellular carcinoma, while 2/18 rats receiving
carbadox showed evidence of hepatocellular carcinoma. There was a
significant increase in adrenal cortical hemorrhage in both carbadox
and desoxycarbadox-treated groups (King, 1976).
The tumorigenicity of carbadox administered in various dosing
regimens was studied more recently in rats. A total of 119 Wistar
rats of each sex were divided into groups of various size (2 - 18
rats) following parturition and treated with various regimens of
carbadox i.p. (prior to weaning for 8 to 20 days) and/or in the feed
at 300 ppm until study termination at 1 year. Rats were examined and
weighed routinely during the study. Tumours were evaluated grossly
and histopathologically at the end of the study. Weight gains were
reduced. Animals receiving 300 ppm carbadox in feed all developed
hepatic tumours. Rats which received carbadox i.p. developed a higher
incidence of hepatic tumour than untreated controls. The largest
number of spontaneous and rare tumours occurred in groups receiving
carbadox via both routes (Sykora & Vortel, 1986).
2.2.3.2 Monkeys
A study was performed to assess the long-term toxicity of
carbadox in primates. Twenty-eight monkeys were divided into 4 groups
of 7 animals (3 or 4/sex) and were dosed with carbadox in gelatin
capsules 5 days/week. The doses were 20 (5 mg/kg QID), 10 (5 mg/kg
BID) or 5 mg/kg b.w./day and controls. A variety of parameters were
evaluated at 1, 3, 6, 12, and 24 months including haemoglobin,
haematocrit, RBC's, WBC's, clotting time, prothrombin time, complete
urinalysis, blood glucose, BUN, alkaline phosphatase, SGOT and SGPT.
Ophthalmoscopic examinations were performed at the same intervals. At
3 months and 2 years animals were sacrificed and necropsied. The diet
contained 0.2% isoniazid as prophylaxis for tuberculosis.
Elevated transaminase levels were detected at the 3 and 6 month
evaluations in both treated and control animals. The authors
concluded that monkeys tolerated 20 mg/kg/day for two years with no
adverse effect (Coleman, 1967).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation reproduction study with 2 litters per generation
was conducted using feed levels adjusted to provide target doses of
2.5, 1.0 and 10 mg/kg b.w./day. Feed levels were identical to those
used for a concurrent 2 year chronic study. Sixty female and 30 male
Charles River C-D rats were divided into groups of 20 females and 10
males per level for the 1st generation. Two females were placed with
each male for mating. First litter offspring were sacrificed
following a gross examination on lactation day 21. Following a 10 day
rest period the females were rebred to different males. Twenty females
and 10 males from the 2nd litter of each dose level were selected to
become the 2nd generation. These rats were mated at 100 days of age.
Dose levels were maintained and the 2nd and 3rd generation were
treated from weaning throughout the breeding period. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 4 days, number weaned,
litter weight at 24 hours, litter weight at weaning, sex at weaning,
viability index, lactation index, growth index, live birth index, and
gross abnormalities. In addition 10 weanlings/dose level from the 2nd
litter of the 3rd generation received an ophthalmoscopic examination,
gross necropsy, and a standard array of tissues were evaluated
microscopically. Although occasional differences from control group
parameters were noted, these were sporadic and not thought to be
treatment related. A reduced pregnancy rate was reported for all
groups of the third generation, with only the first litter of the
1.0 mg/kg b.w./day treatment group above 50%. Additionally a high
incidence of cannibalism (30 - 44%) occurred in treated groups of the
3rd generation as well as in the second litter of the untreated control
(19%). This cannibalism produced a non dose-related reduction in
lactation index in the treated groups of the 3rd generation
(59 - 79% in treated group litters vs 93 - 98% in controls). No
effects were reported in the first two generations. The authors
concluded that carbadox had no effect on fertility, lactation or
the neonate at any dose level (Sigler, 1969b).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with carbadox are shown in
Table 2.
2.2.6 Special studies on relay toxicity
2.2.6.1 Rats
A study was done to assess the possible risk of toxicity of pork
from pigs fed carbadox. To accomplish the study pigs weighing 60-80
kg were fed carbadox at the rate of 0, 20, and 200 ppm in their feed
for 30 days at which time they were slaughtered. Meat from these pigs
was divided into pieces of approximately 200 grams and frozen at -18
C.
Weanling Wistar rats were divided into groups of 12/sex/dose
(except the high dose which had 24 females). Rats received a dietary
supplement of 10% pork liver from swine fed the carbadox regimens
outlined above. This dietary regimen was continued for 23 - 25
months. All rats received gross necropsies and the following organs
were evaluated microscopically: liver, kidney, adrenal, testes, ovary,
and thyroid. Weights and mortality were recorded. There was no
effect on mortality. No treatment-related histologic changes were
reported (Ferrando et al., 1975).
2.2.6.2 Dogs
In another study 12 beagle dogs weighing about 4.5 kg were fed
for one month with 100 g of control pork as part of a diversified
diet. The dogs were then distributed into 3 treatment groups and
received pork from hogs treated with the above regimens of carbadox at
the rate of 100 gm/day for the first 5 months and then 200 gms for the
remainder of the treatment period (85 months). The dogs were weighed
periodically. Every fourth month haematology, clinical chemistry, and
urinalysis parameters were evaluated.
All dogs were in good health at sacrifice. The dogs received a
gross necropsy at study termination and a standard set of tissues were
evaluated histopathologically. No treatment-related effects were
reported in this study. The authors concluded that there is no danger
to the public health when carbadox is used in pig feed as a growth
promoter at 50 ppm (Ferrando, et al.,1978).
Table 2: Results of genotoxicity assays on carbadox
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 0.0004 - 0.125 Positive Pfizer, undated b
TA1535 (mg/plate)
TA1536, TA1537, Equivocal
TA1538, C340
Ames test TA100 3.1 - 61.7 Positive Beutin et al,
TA98 (+/- S9) (nM) 1981
Ames test TA100 2.5 - 15 µg/plate Positive Yoshimura et al,
TA98 (+/- S9) 1981
Ames test TA100, TA98 .1 umole/plate Positive Negishi, et al,
1980
Ames test TA100 0.001-.02 Positive Voogd et al.,
1980
Mutagenicity Ames E. coli WP2hcr Positive Ohta et al, 1980
test TA100+/-S9 5 - 50 µg/plate Positive
TA98 Positive
TA1538 Negative
TA1537 Negative
TA1535 Negative
Host mediated Assay S. typhimurium 0,1,2, 2.5, 5, 10, Negative Pfizer, undated b
TA1534, TA1952 0,1,2.5 5,7.5, Positive
10,12.5, 20 mg/kg
Repair test B. subtilis (rec) 1 - 100 µg/disc Positive Yoshimura et al.,
S. typhimurium (urv) 1981
Fluctuation test K. pneumoniae .00002 - Positive Voogd et al, 1980
E. coli K12 .005 mmoles/l
Table 2 (contd)
Test System Test Object Concentration Results Reference
Mutagenicity test S. cerevisiae D4 .02% Positive Voogd et al, 1980
Micronucleus test rat bone marrow 5 - 240 mg/kg i.p. Positive Cihak and Srb,
1983
Repair test B. subtilis M45 .1 - 100 Positive Ohta et al,
umole/disk 1980
Chromosomal damage mouse bone marrow 200 mg/kg acute, Positive Pfizer, undated b
10 mg/kg day seven
days
Chromosomal damage in vitro human 0 - 250 µg/ml Positive Pfizer, undated b
lymphocytes
Dominant lethal CD-1 mice 10,50,150,30 mg/kg Negative Pfizer, undated b
assay
QUINOXALINE-2-CARBOXYLIC ACID
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with
quinoxaline-2-carboxylic acid are given in Table 3.
Table 3: Acute toxicity of quinoxaline-2-carboxylic acid
LD50
Species Sex Route (mg/kg) Reference
Rats M Oral 4450 Briggs, 1971
Mice M Oral 1360
2.2.3 Long-term/carcinogenicity Studies
2.2.3.1 Mouse
Groups of 50 Charles River CD mice/sex/dose (100 in controls)
received quinoxaline-2-carboxylic acid in feed for 19 months in a
study of its chronic oral toxicity and tumorigenic potential. Fresh
diets were prepared weekly. Feed and water intake were monitored
weekly for the initial three months and monthly thereafter. Feed
levels of quinoxaline-2-carboxylic acid were adjusted at these
intervals to provide target doses of 0, 25, 50, and 100 mg/kg
b.w./day. Haematology and clinical chemistry were evaluated once,
prior to sacrifice. Necropsy was performed on all animals. Organ
weights were obtained for brain, heart, kidneys, liver, testes, and
ovaries. Histopathologic examination was performed on a standard
array of tissues.
No treatment-related effects were noted in any parameter examined
during the study. Cumulative mortalities were 20% and 15% for control
females and males, respectively, while treatment group cumulative
mortalities ranged from 10% to 20%. Incidence of all tumours was 33%
in female and 46% in male control groups, while the incidences in
treatment groups ranged from 32% to 42%. The authors concluded that
oral administration of quinoxaline-2-carboxylic acid to mice for 19
months produced no evidence of toxicity (Faccini et al., 1979).
2.2.3.2 Rats
Nine male and 9 female Charles River C-D rats were divided into
groups of 3/sex/dose level. Rats received quinoxaline-2-carboxylic
acid in the feed for 2 years. Weight and food consumption were
recorded weekly through week 30 and monthly thereafter. Drug levels
were adjusted to provide target doses of 100, 50 or 0 mg/kg b.w./day.
Rats received clinical examinations weekly and ophthalmoscopic
examinations on days 0, 30, 92, 186, 365, 554, and 733. Haematology
and urinalysis parameters were evaluated on days 31, 93, 182, 274,
370, 461, 547, 644, and 730. Terminal sacrifice was performed on day
735 of the study. All rats received gross necropsies and standard
tissues were evaluated microscopically from each rat. No treatment-
related changes were reported. The authors concluded that
quinoxaline-2-carboxylic acid was tolerated at up to 100 mg/kg
b.w./day when given to rats via feed (Coleman, 1971).
An additional study was conducted to determine whether
quinoxaline-2-carboxylic acid has tumorigenic potential. To
accomplish the study quinoxaline-2-carboxylic acid was administered in
the diet of Charles River Sprague-Dawley rats (20/sex/dose level) for
two years to provide target doses of 0, 10, 25, and 50 mg/kg b.w./day.
Weight and food consumption were monitored weekly and drug
concentration adjusted accordingly. Rats were examined for clinical
signs weekly. Ophthalmologic examinations were performed on all rats
prior to treatment and at 6, 12, 20 and 24 months. Clinical
chemistry, haematology and urine parameters were evaluated in 5 rats
at 6 and 12 months, 2-4 rats at 18 months, and all rats at
termination. At 12 months 5 rats/sex/dose and at 24 months all
remaining rats were sacrificed and received a gross necropsy and
tissues were obtained for microscopic evaluation. Organ weights were
determined for heart, liver, lungs, kidneys, gonads, adrenal, spleen,
and brain. A standard array of tissues was examined
histopathologically.
No treatment-related effects were noted on any of the parameters
evaluated. Cumulative incidences of tumours (based on summing males
and females with 40 rats/dose) occurring in treatment groups for
selected organs of interest are summarized in Table 4. It was
concluded that quinoxaline-2-carboxylic acid in doses of 10, 25, and
50 mg/kg b.w./day over a 2 year period to rats does not produce any
toxicity or elevated tumor incidence (Pfizer, 1971).
Table 4: Cumulative incidence of tumours (males and females combined)
after treatment of rats with quinoxaline-2-carboxylic acid
Treatment Group Mammary gland Pituitary Pancreas Liver
Control 28% 30% 3% 5%
10 mg/kg b.w./day 18 23 10 5
25 mg/kg b.w./day 13 28 3 5
50 mg/kg b.w./day 33 28 3 3
A follow-up chronic study was performed using rats obtained from
the 3-generation study F1 litter. An additional high dose level, 100
mg/kg b.w./day, was added for this study. To accomplish the study
Charles River C-D rats were divided into groups of 50/sex/dose and
received 100, 50, 25, or 0 mg/kg b.w./day of quinoxaline-2-carboxylic
acid in the diet for 29 months. In addition 10 rats/sex in the
control and 100 mg/kg b.w./day groups were added for clinical
chemistry studies at 12 months. Weights were monitored weekly. Food
consumption was recorded weekly during the first 5 months, twice
during the 6th month, and monthly thereafter, and treatment levels
were adjusted accordingly. Clinical examinations were performed
daily. Clinical chemistry parameters were evaluated at 6, 12, and 29
months. Haematology was evaluated at the conclusion of the study.
All rats received a gross necropsy. Liver and kidneys were weighed
from rats sacrificed at 12 months and heart, liver, kidneys, brain,
and testes were weighed during the gross necropsies at 29 months.
Tissues from many organs showing macroscopic abnormality plus a
standard array of grossly normal tissues were evaluated
microscopically.
The general health of the rats was unaffected by treatment. An
outbreak of sialoacryoadenitis occurred between ages 89 and 97 days.
Slightly reduced weight gain (3-5%) was noted in the high-dose groups
in both sexes. The cumulative mortality for controls was 56% in males
and 70% in females, while treatment group cumulative mortality ranged
from 46% to 74%. There was no difference in the incidence of
nonneoplastic lesions among the control and treatment groups, nor did
treatment shorten the lifespan of treatment groups. Treatment of rats
for 2 years did not produce an increase in the incidence of animals
with malignant or benign tumours compared to the control group. The
authors concluded that the NOAEL was 100 mg/kg b.w./day (King, 1979a).
2.2.4 Reproduction studies
2.2.4.1 Rats
The potential reproductive toxicity of quinoxaline-2-carboxylic
acid was studied in rats. The study was performed in conjunction with
a 2-year chronic study. Treatment route was via feed. The study
utilized 2 litters per generation for 3 generations with twenty
rats/sex/dose level using target doses of 25, 50, and 100 mg/kg
b.w./day. The F0 rats were bred following approximately 60 days of
treatment with quinoxaline-2-carboxylic acid. Fifty rats/sex/dose
were selected for the concurrent chronic study from the initial F1
litter. Following a 7 day rest period the females were rebred to
different males to produce the second litter. The subsequent first
litters of each generation were sacrificed at weaning while the second
litters were used to constitute the next generation. These first
litters were reduced to 10 pups/litter (5/sex if possible) for
rearing, then sacrificed, and necropsied at weaning. The 2nd litters
were used to produce the next generation. Subsequent generations of
rats were mated at 100 days of age. Dose levels were maintained and
test animals were treated from weaning throughout the breeding period.
Rats were inspected daily. Food consumption and weights were
determined weekly except for mating periods. Adults of all
generations were sacrificed after weaning of the 2nd litter and
received a gross necropsy with liver weights determined. Blood levels
were determined in F0 rats (5/sex/level) just prior to sacrifice.
Pups were examined daily until weaning and weighed on days 1, 4, and
21 after birth. Gross necropsies were carried out on all pups that
died or were stillborn and on 5 weanlings/sex/dose. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 1, 4, and 21 days, number
weaned, sex ratios, mean pup weights, lactation index, and gross
abnormalities. No treatment-related alterations were observed in any
parameter monitored during the study (King, 1979b).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rabbits
The potential embryotoxicity or teratogenicity of quinoxaline-2-
carboxylic acid was also studied in the New Zealand White Rabbit.
Rabbits were randomly assigned to 4 treatment groups of 19 - 20
animals receiving 0, 25, 50, or 100 mg/kg b.w. quinoxaline-2-
carboxylic acid via gavage on gestation days 7-18. Animals were
observed daily for toxicity and weighed on gestation days 0, 3, 7, 9,
12, 15, 18, 21, 24, and 28. Rabbits were sacrificed on day 28 and the
number of corpora lutea and number and position of live and dead
implants in the uteri were recorded. All fetuses were examined for
external malformations and weighed. Half the fetuses were cleared,
stained with alizarin red and examined for skeletal abnormalities.
The remaining half were fixed in Bouin Allen fluid and examined by
serial sectioning. No treatment related maternal toxicity,
embryotoxicity or malformations were observed (Holmes, 1976a).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with quinoxaline-2-carboxylic
acid are given in table 5.
Table 5: Quinoxaline-2-carboxylic acid genetic toxicology
Test System Test Object Concentration Results Reference
Ames test S. typhimurium .008, .032, Negative Pfizer,
TA1535 .125, .5, 1975
TA1537 2 mg/plate
TA1538
TA 1535+S9 .16, .63, Negative Pfizer,
2.5 mg/plate 1975
Chromosomal Human 50, 100 Negative Pfizer,
aberrations lymphocytes µg/ml 1975
in vitro
DESOXYCARBADOX
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
A long term study was performed to determine the tumorigenic
potential of desoxycarbadox, a carbadox metabolite. Four hundred
Charles River C-D rats (5 weeks of age) were divided into groups of
50/sex/dose level. Rats were distributed randomly according to weight
levels. Desoxycarbadox was administered at target doses of 0, 5, 10,
and 25 mg/kg b.w./day continuously in the diet. Weights were recorded
weekly. Food consumption was monitored weekly the first 3 months and
monthly thereafter. Treatment levels were adjusted accordingly.
Although treatment was planned for 2 years, the test material was
withdrawn from all rats in the 25 mg/kg b.w./day group and 50% of the
rats in each sex in the other 2 treatment groups on day 350, due to
high morbidity and mortality. Administration of desoxycarbadox was
stopped completely on day 416. The study was terminated after 447
days.
Clinical examinations were performed twice weekly. A number of
treatment-related signs were observed. These included: tumours in
the mammary region of both sexes, small cutaneous nodules, and
enlargement of the liver with nodules preceded by weight loss and
polyphagia. There was also a dose-related decrease in weight gain and
a dose-related decrease in survival rate.
Clinical chemistry parameters were evaluated. Desoxycarbadox
administration resulted in dose-related increases in plasma enzyme
activities, urea, and bilirubin. The abnormalities were consistent
with hepatic disorders. The high degree of variability from animal to
animal and lack of reversibility following drug withdrawal indicates
a correlation with tumour progression. A dose-related hypoglycaemia
was noted in both sexes. Haematologic parameters were evaluated in
ten rats/sex/dose at day 413 and the remainder at 447 days. The two
primary changes in haematology in the treated rats were a moderate
hypochromic microcytic anaemia in the 25 mg/kg b.w./day group, a
slight anaemia in the 10 mg/kg b.w./day group, and a neutrophilia in
the 25 mg/kg b.w./day group.
All rats received gross necropsies. Selected organs were
weighed. Histopathologic examination was performed on all grossly
abnormal tissue and routinely on a standard array of tissues. There
was a dose-related increase in pigmentation of renal tubules and
nephrosis. The major pathologic findings related to increased tumour
incidences are summarized in Table 6. The authors concluded that
desoxycarbadox was a potent hepatocarcinogen in the rat and that there
were dose-related increases in other tumours (Reinert, 1976).
Table 6: Incidence of tumours after administration of desoxycarbadox
to rats
Treatment Hepatic Subcutaneous Haemanigiomas Mammary
Group tumours fibromas tumours
Males and females combined Females only
Control 0% 0% 0% 12%
5 mg/kg 75 2 0 24%
b.w./day
10 mg/kg 95 10 5 28
b.w./day
25 mg/kg 100 18 47 27
b.w./day
2.2.5.2 Special Studies on Genotoxicity
The results of genotoxicity studies with desoxycarbadox are
summarized in Table 7.
Table 7: Desoxycarbadox genetic toxicology
Test System Test Object Concentration Result Reference
Ames test TA1535 .0008, .0032, Negative Pfizer, 1975
TA1537 .0125, 0.05, .125,
TA1538 .5, 2, 20 mg/plate
TA1535+S9 .0125, .05, .2, 1, Negative Pfizer, 1975
5 ml/plate
Ames test TA1535 .032, .25, .4, .5 Negative Pfizer, 1975
TA1537 ml/plate plasma;
TA1538 .032, .25. .5 ml/plate
urine1
Host mediated TA1950 500 mg/kg acute Negative Pfizer, 1975
assay
Chromosomal damage Human lymphocytes 200 µg/ml Negative Pfizer, 1975
Rat bone marrow 200 mg/kg acute, Negative Pfizer, 1975
or 100 mg/kg 5 days
10 mg or 25 mg per kg Positive Pfizer, 1975
9 months in feed
Host mediated TA1950 400 or 500 mg/kg Negative Pfizer, 1975
assay in mice
Host mediated TA1950 300 mg/kg with 100 mg/ Negative Pfizer, 1975
assay in rats kg/day previous 31 days
TA1535 200 mg/kg Negative
Table 7 (contd)
Test System Test Object Concentration Result Reference
Yeast assay S. cerevisiae D3 10 mg/ml Negative Holmes, 1976b
& D4
Host mediated TA1950 400 mg/kg +DMNA Negative Holmes, 1976b
assay (mouse)
Ames test TA1537 .01 - 10 mg/plate Negative Holmes, 1976b
TA100
TA98
Ames test TA1535+/- TA100 .005 - 2 mg/plate Positive Holmes, 1976b
S9(rat)
Ames test TA1537+S9 .005 - 2 mg/plate Negative Holmes, 1976b
TA98
Ames test TA1535+S9 from .005 - 2 mg/plate Holmes, 1976b
rat
mouse Positive
hamster Positive
dog Negative
monkey Negative
Negative
Ames test TA1535 Quantitative plate Negative Holmes, 1976b
assay on plasma, liver
& urine at selected
intervals from chronic
study rats fed carbadox
or desoxy-carbadox
Cell transformation BALB/C Swiss 3T3 .1-10 µg/ml Positive Holmes, 1976b
test
1 Urine and plasma collected from mice or rats administered 400 mg/kg desoxycarbadox administered
in 3 hourly doses or 100 mg/kg/day for 5 days.
METHYL CARBAZATE
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
Methyl carbazate is a metabolite of carbadox. The chronic oral
toxicity of methyl carbazate was studied in the rat. Wistar rats were
divided into groups of 12/sex/dose and received methyl carbazate at
target doses of 1 and 10 mg/kg b.w./day in the feed for 21 months.
The colony served as controls. Food consumption was determined every
2 days and weights every 2-3 weeks. Necropsy was performed on all
animals. Organ weights were determined for liver, kidney, adrenals,
testes, and thyroid. Histology was performed on liver, kidneys,
adrenals, thyroid, testes, ovaries, and tissues containing tumours
observed grossly. Three males and 3 females in the high dose group
died before termination. No low dose rats died prior to termination.
No histopathologic evidence of toxicity or evidence of an elevation in
tumours was reported. The authors concluded that administration of
methyl carbazate to rats at dose levels of 1 and 10 mg/kg b.w./day in
the diet produced no evidence of toxicity or carcinogenic potential
(Rutty, 1972).
In a second study weanling Wistar rats were divided into groups
of 24/sex and treated with target doses of 0, 2.5, 5, and 10 mg/kg
b.w./day methyl carbazate in the feed for 710 days. Weights were
determined weekly and food consumption weekly the first 3 months and
monthly thereafter. Drug concentrations were adjusted accordingly.
Clinical examinations were conducted weekly. Blood samples for
clinical chemistry were obtained at terminal sacrifice and from
moribund animals. Haematology was also conducted on these samples and
at 12 months on 6 rats. Rats were sacrificed and necropsied at 710
days. An interim sacrifice was also performed at 12 months. All
moribund and dead rats also received a gross necropsy. Histopathology
was performed on all gross lesions and the following organs: liver,
pituitary, thyroid, parathyroid, adrenal, lung, kidney, urinary
bladder, ovary, and testis.
There were no treatment-related effects noted in any parameters
evaluated in the study. There was histologic evidence of widespread
chronic respiratory disease in all groups. It was concluded that
methyl carbazate had no adverse effect when given to rats in the diet
for 2 years (Ferrando, 1980).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with methyl carbazate are
summarized in Table 8.
Table 8: Genetic toxicity studies with methyl carbazate
Test System Test Object Concentration Results Reference
Ames test TA1535 .0005, .002, Negative Pfizer, 1975
TA1537 .008, .032,
TA1538 .125, 0.5, 2, 5
mg/plate
TA1535+S9 .0125, .05, .2 Negative Pfizer, 1975
1, 5, 10
mg/plate
Host- TA1950 50, 100, 300 Equivocal Pfizer, 1975
mediated mg/kg
assay
Chromosomal Human 50, 100 Negative Pfizer, 1975
damage lymphocytes µg/ml
in vitro
Ames test TA1535+S9 0.5 - 10 Negative Holmes, 1976b
mg/plate
HYDRAZINE
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism, and excretion
Hydrazine toxicokinetic parameters have been determined in the
rabbit. Rabbits received a bolus of 12 mg/kg b.w. Parameters were
derived using a one-compartment model, and spectrophotometric serum
analysis for hydrazine, with 5 rabbits/group. The serum half-life was
2.3 hours and the volume of distribution was 0.63 liters/kg (Keller
et al., 1981).
The metabolism, distribution, and elimination of hydrazine in
rats was studied using 15N-labelled hydrazine or a spectrophotometric
method. About 15% of the 15N label was found as N2. About 20% and
30% of the infused hydrazine was recovered in the urine as acetylated
metabolite and hydrazine, respectively. The serum half-lives were
determined, using a two-compartment model, to be 0.74 hours for the
majority of the hydrazine elimination, and 26.9 hours for a much
slower, smaller elimination component (Dost et al., 1981).
2.1.3 Effects on enzymes and other biochemical parameters
Hydrazine-induced short-term biochemical parameter changes were
investigated following oral administration of hydrazine in Sprague-
Dawley rats. Groups of 5 rats were given doses of hydrazine (0-200
mg/kg b.w.). Some rats received pyruvate azine, phenobarbital,
piperonyl butoxide, or diethylmaleate to more clearly elucidate the
hepatic effects of hydrazine. Hydrazine caused a dose-related
increase in liver triglycerides and liver weight and a decrease in
hepatic glutathione. The threshold dose for toxicity was about 10
mg/kg b.w. While liver weight and glutathione alterations were
evident within 30 minutes, triglyceride alteration was not evident for
4 hours. Pretreatment with phenobarbital decreased toxicity, while
pretreatment with piperonyl butoxide increased toxicity suggesting
hepatotoxicity is due to the parent - not a metabolite. Depletion of
hepatic glutathione was without effect on hepatic toxicity, and
studies with pyruvate azine - a possible oxidative metabolite - also
supported this conclusion (Timbrell, et al., 1982).
Hydrazine-induced short-term histologic and ultrastructural
changes were investigated following oral administration of hydrazine
to Sprague-Dawley rats. Groups of 5 rats were given doses of
hydrazine (0-200 mg/kg b.w.). Some rats received pyruvate azine,
phenobarbital, piperonyl butoxide, or diethylmaleate to more clearly
elucidate the hepatic effects of hydrazine. A dose of 20 mg/kg caused
accumulation of lipid, swelling of mitochondria, and the appearance of
microbodies in periportal and midzonal hepatocytes. Accumulation of
lipid droplets and mitochondrial swelling were detected by electron
microscopy 30 minutes after dosing. Piperonyl butoxide or
phenobarbital treatment increased or decreased fatty liver
respectively, while pyruvate azine was much less effective than
hydrazine in producing this effect (Scales & Timbrell, 1982).
2.2 Toxicological studies
2.2.3 Long-term carcinogenicity studies
2.2.3.1 Mice
Oral administration of hydrazine sulfate to Balb/c female mice at
1.13 mg/day for 46 weeks produced a 100% incidence of lung tumours
(Biancifiori & Ribacchi, 1962).
In another study hydrazine was administrated via gavage to 25
female Swiss mice 5 days/week for 40 weeks at the rate of 0.25 mg/day.
Eighty-five mice served as controls. This dose regimen produced a 46%
incidence of lung tumours in treated mice vs 10% in controls (Roe et
al., 1967).
A study with CBA/Cb/Se mice was performed using hydrazine sulfate
given for 36 weeks by gavage. Twenty-one 8- week-old rats/sex
received 1.13 mg/day. Treated rats had an incidence of 76% and 90%
lung tumours in males and females, respectively, while controls had
3%. Hepatomas were found in 62% of the males and 71% of the females
treated with hydrazine, while 11% of control males and 4% control
females had hepatomas (Severi & Biancifiori 1968).
In another study in mice, 8-week-old CBA mice were divided into
groups of 40-59 mice/sex. Hydrazine sulfate was given by gavage daily
for 150 days at rates of 45, 22, 11, 5.6 and 0 mg/kg b.w./day. Mice
were examined at natural death or following sacrifice when moribund.
Control male mice had a hepatoma incidence of 10% while females had a
3.4% rate. Treated males had hepatoma rates of 60, 48, 28, and 3.8%
at 45, 22, 11, and 5.6 mg/kg b.w./day, respectively. Treated females
had 62.5, 66.6, 8 and 0% hepatomas, respectively. The author reported
many lung tumours in treated groups but did not provide the incidence
rates (Biancifiori, 1970).
2.2.3.2 Hamsters
Eight-week old golden hamsters were divided into 3 groups of 23,
35 and 56 animals which were administered 60 doses of 3.0 mg hydrazine
sulfate via intubation over a 15 week period, 2.8 mg 100 times during
a 20 week period or no hydrazine, respectively. Hepatic lesions
reported include: Fibrosis, reticuloendothelial cell proliferation,
and bile duct proliferation. Hepatic lesions were found in 60-80% of
treated hamsters but in none of the controls. No hepatic tumours were
reported (Biancifiori, 1970).
Negative tumorigenicity results were also obtained in a follow up
chronic study with golden hamsters given 2.3 mg/day over a lifetime.
In this study 50 hamsters/sex received hydrazine in drinking water
from the age of 9 weeks for a lifetime (Toth, 1972).
2.2.3.3 Rats
A chronic oral study was performed in conjunction with the above
mouse study using 8 week old Cb/Se rats. Aqueous hydrazine sulfate
was administered daily by stomach tube at doses of 18 mg/rat to 14
males and 12 mg/rat to 18 females for 68 weeks. Lung tumours were
found in 21 and 27% of dosed male and female rats, respectively.
Control groups of 28 males and 22 females had no lung tumours.
Hepatic tumours were found in 30% of males, while no hepatic tumours
were found in females or controls (Severi & Biancifiori, 1968).
2.2.7.5 Special studies on genotoxicity
The results of genotoxicity studies with hydrazine are summarized
in Table 9.
Table 9: Genetic toxicity studies with hydrazine
Test System Test Object Concentration Results Reference
Bacterial E. coli WP2 0.5 - 2.0 µmole Positive von Wright
uvr A trp & Tikkanen,
1980
Mouse L5178Y 0.1 - 5 mmole Positive Rogers &
lymphoma Back, 1981
cells
Ames test S. typhimurium Positive Ames, 1971
2.3. Observations in humans
No information available
3. COMMENTS
The Committee considered data from short-term, long-term and
"relay" toxicity studies, and from studies on carcinogenicity,
mutagenicity, and reproduction with carbadox, together with data from
mutagenicity and long-term toxicity studies of its metabolites.
The metabolism of carbadox has been studied in rats, monkeys and
pigs using [14C]carbadox, labelled in either the phenyl ring or the
carbonyl group of the side-chain. The metabolism of carbadox was
characterized by the rapid reduction of the N-oxide groups, the
cleavage of the methylcarbazate side-chain, and the liberation of
respired CO2. The primary metabolite in the urine was quinoxaline-2-
carboxylic acid, which was also excreted in conjugated form. The
detectable residues in tissues, up to 24 hours after drug withdrawal,
were carbadox, desoxycarbadox, quinoxaline-1,4-di-N-oxide-2-
carboxaldehyde and quinoxaline-2-carboxylic acid. Hydrazine was a
minor metabolite but would be expected to be present only for a short
time before undergoing further metabolism.
The concentration of carbadox and desoxycarbadox in the tissues
declined rapidly (half-life about 6 hours), and, after 3 days, was
less than 5 µg/kg. In a study in pigs, in which carbonyl-labelled
[14C]carbadox was administered by gavage, the concentration of total
residues in liver at 5 days withdrawal time corresponded to 0.12 mg/kg
methyl carbazate equivalent. However, some of this radioactivity was
due to the presence of amino acids, which had been labelled by
incorporation of 14CO2 when ring-labelled [14C]carbadox was
administered to pigs, extremely low levels of unidentified metabolites
remained in the liver at withdrawal periods longer than 7 days. These
residues were partially released and converted to quinoxaline-2-
carboxylic acid by alkaline digestion of the liver.
The Committee also reviewed recent reports of the effect of
carbadox on adrenal function in pigs, although the information did not
effect the outcome of the evaluation.
Several long-term feeding studies in rats were evaluated which
demonstrated dose-related increases in the incidence of benign and
malignant liver tumours at doses of carbadox above 1.0 mg per kg of
body weight per day. Doses above 25 mg per kg of body weight per day
produced pronounced toxic effects which precluded chronic treatment.
Data from a variety of mammalian and non-mammalian genotoxicity
studies were also reviewed. Positive findings were reported in 14 of
the 15 tests carried out. The Committee concluded that carbadox
appears to be both genotoxic and carcinogenic.
The Committee evaluated a "relay" toxicity study in which pigs
were fed carbadox at up to 200 mg/kg in the diet for 30 days and
killed at zero withdrawal time, their livers were fed to rats as a 10%
dietary component for 2 years. Although this study was conducted
satisfactorily and no treatment-related effects were reported, the
Committee did not use it for assessing the carcinogenicity of carbadox
residues because of the number of animals used and because only small
quantities of residues can be consumed by rats in this type of study.
In a long-term study in rats, desoxycarbadox was reported to
produce an increase in the incidence of tumours. All rats that
received 25 mg per kg of body weight desoxycarbadox daily for 10
months in the diet developed hepatic tumours. The incidence of
tumours was increased in all treated groups, at doses of 5-25 mg per
kg of body weight per day. While the most pronounced change occurred
in the liver, tumour incidence was also elevated at other sites,
including the skin and mammary glands.
The mutagenic potential of desoxycarbadox was investigated in a
range of studies. The Committee noted that, while desoxycarbadox
produced negative results in most mutagenicity test systems, positive
findings were recorded in the cell transformation test and in the Ames
test using liver microsomes from rats treated with polychlorinated
biphenyls. The Committee noted that the tumorigenic potential of
desoxycarbadox was apparently greater than that of the parent
compound, and that desoxycarbadox would therefore probably make a
significant contribution to the tumorigenic activity of carbadox in
rats. However, the Committee also noted that the metabolism data for
carbadox supported the conclusion that desoxycarbadox was a relatively
short-lived intermediate between carbadox and quinoxaline-2-carboxylic
acid.
The Committee also considered information on the carbadox side-
chain metabolite, methyl carbazate. This information consisted of
results from two long-term studies in rats and a number of
mutagenicity assays. In the more informative long-term study (in
which more animals per dose and concurrent controls were used), rats
received methyl carbazate in the diet for 710 days. There was no
increase in the incidence of tumours and no other treatment-related
effects were observed at 10 mg per kg of body weight per day, which
was the highest dose tested. The lack of effect limited the
usefulness of this study. The no-observed-effect level for the study
was 10 mg per kg of body weight per day. The useful mutagenicity
studies were reported to be negative.
The results of studies on the pharmacokinetics, mutagenicity, and
carcinogenicity of hydrazine were also considered by the Committee.
Pharmacokinetic data from rats and rabbits as well as hydrazine's
known chemical reactivity suggest that it should be rapidly
eliminated. The structure of carbadox and its known metabolic
pathways indicate that hydrazine is probably a further metabolite of
methyl carbazate. Hydrazine showed mutagenic and carcinogenic
potential.
In evaluating the potential toxicity of residues of carbadox, the
Committee also considered toxicity data on the carbadox metabolite
quinoxaline-2-carboxylic acid to be useful. In a carcinogenicity
study in mice in which quinoxaline-2-carboxylic acid was administered
in the diet for 19 months, no treatment related increases in tumour
incidence or any other effects on the animals were reported. The no-
observed-effect level was 100 mg per kg of body weight per day.
Several chronic toxicity and carcinogenicity studies in which
quinoxaline-2-carboxylic acid was administered for 24 months were
conducted in rats. There were no effects on the incidence of tumours,
the only findings being a slight reduction in weight gain at 100 mg
per kg of body weight per day. The no-observed-effect level was 50 mg
per kg of body weight per day.
In a three-generation reproduction study in rats, quinoxaline-2-
carboxylic acid was given in the diet at levels of up to 100 mg per kg
of body weight per day. No treatment-related effects were observed.
Developmental studies were conducted in rats and rabbits in which the
compound was administered by gavage at levels of up to 100 mg per kg
of body weight per day. There was no evidence of maternal toxicity,
embryotoxicity, or teratogenicity in either species. The no-observed-
effect levels in these studies were 100 mg per kg of body weight per
day.
4. EVALUATION
With current analytical procedures, quinoxaline-2-carboxylic acid
is the only carbadox metabolite that can be identified in liver from
pigs treated according to good practice in the use of veterinary
drugs. However, uncertainty remains because of the presence of
unidentified residues in liver. Because of the genotoxic and
carcinogenic nature of carbadox and some of its metabolites, the
Committee was not able to establish an ADI.
On the basis of data from studies on the toxicity of quinoxaline-
2-carboxylic acid, and on the metabolism and depletion of carbadox and
the nature of the compounds released from the bound residues, the
Committee concluded that residues resulting from the use of carbadox
in pigs were acceptable, provided that the recommended MRLs are not
exceeded.
5. REFERENCES
AMES, B. (1971). Chemical Mutagens, Vol. 1, Hollaender, A. (ed),
Plenum Press, New York. p. 267.
BEUTIN, L., PRELLER, E., & KOWALSKI, B. (1981). Mutagenicity of
Quindoxin, Its Metabolites, and Two Substituted Quninoxaline-Di-N-
Oxides. Antibacterial Agents and Chemotherapy, 20, 336-343.
BIANCIFIORI, C., & RIBACCHI, R., (1962). Pulmonary Tumour in Mice
Induced by Oral Isoniazid and its Metabolites. Nature, 194, 488-
489.
BIANCIFIORI, C. (1970). Hepatomas in CBA/Cb/Se Mice and Liver Lesions
in Golden Hamsters Induced by Hydrazine Sulfate. J. Nat. Cancer
Inst, 44, 943-953.
BRIGGS, G.B. (1971). Safety Evaluation of CP-16505, Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
CIHAK, R., & SRB, V. (1983). Cytogenetic Effects of Quinoxaline-1,4-
dioxide-type Growth-promoting Agents. Mutat. Res., 116, 129-135.
COLEMAN, G.L., (1967). Two year Chronic Study of GS-6244 (carbadox)
in Monkeys. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
COLEMAN, G.L., (1971). Quinoxaline-2-Carboxylic Acid, First Two Year
Study in Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
DOST, F.N., BRODERICK, D.J., KRIVAK, B.N., & REED, D.J., (1981).
Metabolism of Hydrazine. AFAMRL-TR-81-26. Technical Report. Air
Force Aerospace Medical Research Laboratory, Wright-Patterson AFB, OH.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
FACCINI, J.M., PERRAUD, J., NACHBAUR, J., & MONRO, A.M., (1979). 19-
Month toxicity Study in Mice. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1975).
Application of the Toxicity by Relay Method for the Assessment of
Safety to Human Consumers of Carbadox, a Growth-Promoting Additive, to
the Feed of Pigs Slaughtered for Human Consumption. Toxicology, 3,
369-398.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1978). Human
Consumer Safety in the Use of Carbadox, a Pig Feed Additive, as
Estimated by Seven Years of Relay Toxicity in Beagles. Toxicology,
11, 167-183.
FERRANDO, R., (1980). Two Year Chronic Toxicity Study in the Rat with
Methyl Carbazate. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
FIGDOR, S.K., (1969). Carbadox in Pigs Radiotracer Metabolism C-14,
Carbonyl. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
FIGDOR, S.K., MCILHENNY, H.M., & VON WITTENAU, M.S., (1969).
Metabolism of Carbadox Using Carbadox Labelled with C14 in the Phenyl
Ring. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
HOLMES, C.L., (1976a). Action of CP-16,505 on Pregnancy and Fetal
Development in Rabbits. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
HOLMES, C.L., (1976b). Addendum Genetic Toxicology Report CP-17,056
(Desoxycarbadox) Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KELLER, W.C., MURPHY, J.P., ANDERSEN, M.E. & BACK, K.C., (1981).
Percutaneous Toxicokinetics of Hydrazine and H70 in the Rabbit.
AFAMRL-TR-81-13. Technical Report. Air Force Aerospace Medical
Research Laboratory, Wright-Patterson AFB, OH. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
KING, T.O., (1976). Target Organ Toxicity of GS-6244 (carbadox) and
CP-17,056 (desoxycarbadox) with Chronic Administration in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
KING, T.O., (1979a). Chronic Study in Rats with Quinoxaline-2-
Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KING, T.O., (1979b). Three Generation Study in Rats with Quinoxaline-
2-Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
NEGISHI, T., TANAKA, K., AND HYATSU, H., (1980). Mutagenicity of
Carbadox and Several Quinoxaline 1,4-Dioxide Derivatives. Chem.
Pharm. Bull., 28, 126-128.
OHTA, T., MORIYA, M., KANEDA, K., WATANABE, T., MIYAZAWA, A., &
SUSIGYAMA, F., (1980). Mutagenicity screening of Feed Additives in
the Microbial System. Mutat. Res., 77, 21-30.
PFIZER (undated a). Acute Toxicity of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (undated b). Genetic Toxicology of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (1963). A thirty-day Subacute Toxicity Study of GS-6244 in
Albino Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
PFIZER (1971). Two Year Study with CP-16,505 (Quinoxaline-2-
Carboxylic Acid) in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
PFIZER (1975). Genetic Toxicology Report CP-17,056 (Desoxycarbadox)
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
REINERT, H. (1976). Desoxycarbadox: Two Year Toxicity Study in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
ROE, F.J., GRANT, G.A., & MILLICAN, D.M., (1967). Carcinogenicity of
Hydrazine and 1,1-Dimethylhydrazine for Mouse Lung. Nature, 216,
375-376.
ROGERS, A.M., & BACK, K.C., (1981). Comparative Mutagenicity of
Hydrazine and 3 Methylated Derivatives in L5178Y Mouse Lymphoma Cells.
Mutat. Res., 89, 321-328.
RUTTY, D.A., (1972). Twenty-one Month Chronic Toxicity Study of
Methyl Carbazate in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
SCALES, M.D., & TIMBRELL, J.A., (1982). Studies on Hydrazine
Hepatotoxicity. 1. Pathological Findings. J. Toxicol and Environ.
Health, 10, 941-953.
SEVERI, L., & BIANCIFIORI, C. (1968). Hepatic Carcinogenesis in
CBA/Cb/Se Mice and Cb/Se Rats by Isonicotinic Acid Hydrazine and
Hydrazine Sulfate. J. Nat. Cancer Inst., 41, 332-349.
SIGLER, F.W., (1969a). A Two-year Chronic Toxicity Study of GS-6244
in Rats. Dose Levels: 2.5, 1.0 or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SIGLER, F.W., (1969b). A Three Generation Reproductive Study of GS-
6244 in Rats. Dose Levels: 2.5, 1.0, or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SPIERENBURG, T.J., BAARS, A.J., DE GRAAF, G.J., & JAGER, L.P., (1988).
Carbadox-induced Inhibition of Aldosterone Production in Porcine
Adrenals in vitro. Toxicology In Vitro, 2, 141-143.
STEBBINS, R.B., (1964). A 3-Week Subacute Toxicity Study of GS-6244
in Rats - Dose Levels of 100, 50, 10, 5, or 0 mg/kg. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
STEBBINS, R.B., & COLEMAN, G.L., (1967). A Twenty-six Month Chronic
Toxicity Study of GS-6244 in Rats - Dose Levels of 100, 50 10, 5, or
0 mg/kg. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
SYKORA, I., & VORTEL, V., (1986). Post-natal Study of the
Carcinogenicity of Cyadox and Carbadox. Biol Vet Praha., 22, 53-62.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J., (1982). Studies on
Hydrazine Hepatotoxicity. 2. Biochemical Findings. J. Toxicol and
Environ. Health, 10, 955-968.
TOTH, B., (1972). Tumorigenesis Studies with 1,2-Dimethylhydrazine
dihydrochloride, Hydrazine Sulfate, and Isonicotinic Acid in Golden
Hamsters. Cancer Res, 32, 804-807.
VAN DER MOLEN, E.J., DE GRAAF, G.J., & BAARS, A.J., (1989a).
Persistence of Carbadox-induced Adrenal Lesions in Pigs Following Drug
Withdrawal and Recovery of Aldosterone Plasma Concentration.
J. Comp. Path., 100, 295-304.
VAN DER MOLEN, E.J., VAN LIESHOUT, J.H., NABUURS, M.J., DERKX, F.H.,
LAMERS, A.P., & MICHELAKIS, A.M., (1989b). Changes in Plasma Renin
Activity and Renal Immunohistochemically Demonstrated Renin in
Carbadox Treated Pigs. Res. Vet. Sci., 46, 401-405.
VON WITTENAU, M.S., (1969). Comparative Metabolism Studies in Swine,
Monkeys, and Rats of Carbadox. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J., (1980). The
Mutagenic Action of Quindoxin, Carbadox, Olaquindox, and Some other N-
oxides on Baceteria and Yeast. Mutat. Res., 78, 233-242.
VON WRIGHT, A. & TIKKANEN, L., (1980). The Comparative Mutagenicities
of Hydrazine and its Mono- and Di- methyl Derivatives in Bacterial
Test Systems. Mutat. Res., 78, 17-23.
YOSHIMURA, H., NAKAMURA, M., KOEDA, T., & YOSHIKAWA, K., (1981).
Mutagenicities of Carbadox and Olaquindox - Growth Promoters for Pigs.
Mutat. Res., 90, 49-55.
The in vitro effect of carbadox on adrenal aldosterone production
was evaluated in porcine adrenal slices obtained from 3-5 week old
pigs. The adrenal slices were maintained in Krebs solution and
exposed to 1-40 µg carbadox/ml. Aldosterone was estimated using RIA
at hourly intervals. A dose-dependent decrease in aldosterone
production occurred with a reduction of 25-35% at 40 µg/ml
(Spierenburg et al; 1988).
2.2. Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with carbadox are shown in
Table 1.
Table 1: Carbadox acute toxicity data
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 2810 Pfizer, undated a
F oral >2810
i.p. 1050
Rat M oral 850
i.p. 810
A study was done to examine the emetic effect of carbadox on
monkeys and dogs. Three animals/group received a single dose of
carbadox orally via capsule. Doses were 25, 10, 5, and 1 mg/kg
b.w./day. In the monkey 1/3 exhibited emesis in the 2 higher dose
groups, while in dogs all dose groups had emesis.
2.2.2 Short-term studies
2.2.2.1 Rats
Twenty Charles River C-D weanling rats were divided and dosed for
30 days as follows: 2-3 males and females at 50 mg/kg b.w./day and
100 mg/kg b.w./day, and 10 males as untreated controls. Carbadox was
administered in the diet. Weight and food consumption were observed
weekly and drug levels adjusted as necessary. CBC at 30 days, urinary
electrolytes at 1, 2, 3, 14, and 30 days, cholesterol at 30 days and
urinalysis at 14 and 30 days were evaluated. Rats received a gross
necropsy at the end of the study and tissues were evaluated
microscopically. A dose-dependent decrease in weight gain and food-
consumption were noted. The authors concluded that all other
parameters were normal (Pfizer, 1963).
2.2.2.2 Dogs
The short-term toxicity of carbadox was studied by dosing dogs 6
days/week for three weeks. Beagle dogs (1/sex/dose) were initially
dosed with 25 or 50 mg/kg b.w./day carbadox via oral capsule. These
doses were later reduced to 10 and then 15 mg/kg b.w./day due to
emesis. A variety of parameters were evaluated including clinical
chemistry, gross necropsy, and microscopic tissue examination at the
termination of the study. The dogs lost weight and had elevated
SGPT's. It was concluded that carbadox is emetic and possibly
hepatotoxic in the dog (Stebbins, 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
The chronic toxicity and potential tumorigenicity of carbadox
were studied in rats. One hundred twenty Charles River C-D rats were
divided into 6 groups (10/sex/dose) and received carbadox in the diet
at rates providing doses of 100, 50, 25, 10, 5, and 0 mg carbadox/kg
b.w./day for 26 months. Feed concentration was adjusted weekly.
Clinical signs, body weights, and food consumption were recorded
weekly. Haematology and urinalysis were evaluated in 5 rats/sex/dose
at 3, 6, 12, 18, and 25 months. Rats were sacrificed at 14 and 112
weeks and received gross necropsies. Tissues were examined
histopathologically.
Survival was reduced for animals in the high-dose groups. The
following observations were reported for animals up to and including
the interim sacrifice:
100 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption, reduced haemoglobin, RBC's and neutropenia.
Microscopic changes reported include pulmonary hemorrhage and edema,
adrenalcortical hemorrhage and degeneration, splenic haemosiderin, and
thymic atrophy. No animals survived beyond the 14 week sacrifice.
50 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption. Microscopic changes reported include pulmonary
hemorrhage and edema, adrenalcortical hemorrhage, necrosis, and
degeneration, splenic haemosiderin and renocorticomedullary fatty
metamorphosis. No animals survived beyond the 14 week sacrifice.
25 mg/kg b.w./day (3 rats/sex necropsied) - Reduced weight gain,
slight adrenal cortical atrophy, degeneration/necrosis, and renal
tubular fatty change.
5 and 10 mg/kg b.w./day (3 rats/sex necropsied) - No clinical, gross
or microscopic changes reported at 3 months.
The following observations were reported for rats from 3 months
up to and including the 26 month sacrifice:
25 mg/kg b.w./day - One female died at 51 weeks with no drug-related
changes noted. All 13 remaining rats were sacrificed by 73 weeks due
to palpable abdominal masses. At necropsy all rats had multiple
hepatic nodules. Ten of 13 rats were diagnosed microscopically to
have benign nodular hyperplasia, while the remaining 3 rats were
determined to have malignant transformation based on metastatic foci
observed in other organs (lung, kidney and lymph nodes).
10 mg/kg b.w./day - One rat died after 67 weeks with
reticuloendothelial neoplasia, a common tumour of aged rats. The
remaining 13 rats were sacrificed between the 64th and 112th weeks.
Eleven of these were observed to have hepatic benign nodular
hyperplasia.
5 mg/kg b.w./day - One male died at 20 weeks due to pulmonary
abscesses. The remaining 13 died or were sacrificed between the 61st
and 112th weeks. Five or these were observed to have hepatic benign
nodular hyperplasia.
Controls One male was sacrificed at 33 weeks with a forestomach
papilloma and pulmonary atelectasis and a second male died at 93 weeks
with myocarditis, nephrosclerosis and peribronchitis. The remaining
animals were sacrificed between weeks 80 and 112 of the study. No
animals in the control group were observed to have hepatic nodular
hyperplasia. The authors suggested that the observed nodular
hyperplasia in treated groups was associated with hepatic injury and
concluded that the rats failed to tolerate carbadox at any of the
tested dose levels. A treatment-related increase in total tumours was
also reported for carbadox-treated rats. The tumour incidence was
lower in the high-dose group, however mortality occurred earlier in
this group than in other treatment groups (Stebbins & Coleman, 1967).
A follow-up study was performed to determine a chronic carbadox
level tolerated by rats. Sixty male Charles River rats (124-173 gms)
and 60 female rats (107-152 gms) of the same strain were divided into
3 groups (20/sex/dose) and received 2.5, 1.0, or 0 mg/kg b.w./day of
carbadox in the diet. Signs, weights and food consumption were
recorded weekly and drug levels were adjusted accordingly.
Haematology, complete urinalysis, and ophthalmoscopic examination were
done at 3, 6, 12, 18, and 24 months on 5 rats/sex/dose. An interim
sacrifice of 5 rats/sex/dose was conducted at 54 weeks with the
remaining rats sacrificed at 106 weeks. Each rat received a gross
necropsy and microscopic examination of standard tissues including:
brain, spinal cord, eye, sub-maxillary gland, thyroid gland, thymus,
heart, aorta, lung, sternum, liver, spleen, pancreas, kidney, adrenal,
stomach, mesenteric lymph node, reproductive tract, urinary bladder,
femoral nerve, femoral bone marrow, and mammary gland with skin.
All parameters evaluated, including histopathologic examination,
were within normal limits at 54 weeks. Survival at 2 years was as
follows: control - 38%, 1.0 mg/kg b.w./day - 45%, 2.5 mg/kg b.w./day
- 43%. At the 2.5 mg/kg b.w./day level 7/27 rats displayed hepatic
benign nodular hyperplasia and 7/27 showed peliosis hepatis. At the
1.0 mg/kg b.w./day level 1/29 rats was found to have hepatic benign
nodular hyperplasia and 3/29 displayed peliosis hepatis. In the
control group 3/29 rats had benign nodular hyperplasia and 2/29 had
peliosis hepatis. Additionally, an increase in total mammary tumours
was reported in the 2.5 mg/kg b.w./day dose group. The authors
concluded that the 1.0 mg/kg b.w./day dose level was tolerated by the
rats for 2 years with no adverse effects, based on the similar
incidence of both hepatic lesions and tumours in other organs in
treatment and control groups (Sigler, 1969a).
Three groups of 28 rats (14/sex) were treated with a target dose
of 25 mg/kg b.w./day of carbadox, or one of two lots of desoxycarbadox
for 11 months to compare the oncogenic activity of carbadox and
desoxycarbadox. An equal sized group served as untreated controls.
The test material was given in the feed for 10 months. Two to four
rats were sacrificed at 30, 60, 90, 191, and 309 days. Animals were
observed daily for toxicity and mortality. Weights and food
consumption were recorded weekly until the 38th week and then every
other week. Rats received a gross necropsy at sacrifice and liver and
adrenals were evaluated histopathologically. A moderate decrease in
weight gain occurred in both sexes receiving carbadox. No other
significant clinical observations were made. At gross necropsy all
groups treated for 10 months showed evidence of hepatic changes
including necrosis and nodule formation. Changes in the carbadox-
treated group were less severe than in the desoxycarbadox-treated
group. Histopathologically all desoxycarbadox-treated rats showed
evidence of hepatocellular carcinoma, while 2/18 rats receiving
carbadox showed evidence of hepatocellular carcinoma. There was a
significant increase in adrenal cortical hemorrhage in both carbadox
and desoxycarbadox-treated groups (King, 1976).
The tumorigenicity of carbadox administered in various dosing
regimens was studied more recently in rats. A total of 119 Wistar
rats of each sex were divided into groups of various size (2 - 18
rats) following parturition and treated with various regimens of
carbadox i.p. (prior to weaning for 8 to 20 days) and/or in the feed
at 300 ppm until study termination at 1 year. Rats were examined and
weighed routinely during the study. Tumours were evaluated grossly
and histopathologically at the end of the study. Weight gains were
reduced. Animals receiving 300 ppm carbadox in feed all developed
hepatic tumours. Rats which received carbadox i.p. developed a higher
incidence of hepatic tumour than untreated controls. The largest
number of spontaneous and rare tumours occurred in groups receiving
carbadox via both routes (Sykora & Vortel, 1986).
2.2.3.2 Monkeys
A study was performed to assess the long-term toxicity of
carbadox in primates. Twenty-eight monkeys were divided into 4 groups
of 7 animals (3 or 4/sex) and were dosed with carbadox in gelatin
capsules 5 days/week. The doses were 20 (5 mg/kg QID), 10 (5 mg/kg
BID) or 5 mg/kg b.w./day and controls. A variety of parameters were
evaluated at 1, 3, 6, 12, and 24 months including haemoglobin,
haematocrit, RBC's, WBC's, clotting time, prothrombin time, complete
urinalysis, blood glucose, BUN, alkaline phosphatase, SGOT and SGPT.
Ophthalmoscopic examinations were performed at the same intervals. At
3 months and 2 years animals were sacrificed and necropsied. The diet
contained 0.2% isoniazid as prophylaxis for tuberculosis.
Elevated transaminase levels were detected at the 3 and 6 month
evaluations in both treated and control animals. The authors
concluded that monkeys tolerated 20 mg/kg/day for two years with no
adverse effect (Coleman, 1967).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation reproduction study with 2 litters per generation
was conducted using feed levels adjusted to provide target doses of
2.5, 1.0 and 10 mg/kg b.w./day. Feed levels were identical to those
used for a concurrent 2 year chronic study. Sixty female and 30 male
Charles River C-D rats were divided into groups of 20 females and 10
males per level for the 1st generation. Two females were placed with
each male for mating. First litter offspring were sacrificed
following a gross examination on lactation day 21. Following a 10 day
rest period the females were rebred to different males. Twenty females
and 10 males from the 2nd litter of each dose level were selected to
become the 2nd generation. These rats were mated at 100 days of age.
Dose levels were maintained and the 2nd and 3rd generation were
treated from weaning throughout the breeding period. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 4 days, number weaned,
litter weight at 24 hours, litter weight at weaning, sex at weaning,
viability index, lactation index, growth index, live birth index, and
gross abnormalities. In addition 10 weanlings/dose level from the 2nd
litter of the 3rd generation received an ophthalmoscopic examination,
gross necropsy, and a standard array of tissues were evaluated
microscopically. Although occasional differences from control group
parameters were noted, these were sporadic and not thought to be
treatment related. A reduced pregnancy rate was reported for all
groups of the third generation, with only the first litter of the
1.0 mg/kg b.w./day treatment group above 50%. Additionally a high
incidence of cannibalism (30 - 44%) occurred in treated groups of the
3rd generation as well as in the second litter of the untreated control
(19%). This cannibalism produced a non dose-related reduction in
lactation index in the treated groups of the 3rd generation
(59 - 79% in treated group litters vs 93 - 98% in controls). No
effects were reported in the first two generations. The authors
concluded that carbadox had no effect on fertility, lactation or
the neonate at any dose level (Sigler, 1969b).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with carbadox are shown in
Table 2.
2.2.6 Special studies on relay toxicity
2.2.6.1 Rats
A study was done to assess the possible risk of toxicity of pork
from pigs fed carbadox. To accomplish the study pigs weighing 60-80
kg were fed carbadox at the rate of 0, 20, and 200 ppm in their feed
for 30 days at which time they were slaughtered. Meat from these pigs
was divided into pieces of approximately 200 grams and frozen at -18
C.
Weanling Wistar rats were divided into groups of 12/sex/dose
(except the high dose which had 24 females). Rats received a dietary
supplement of 10% pork liver from swine fed the carbadox regimens
outlined above. This dietary regimen was continued for 23 - 25
months. All rats received gross necropsies and the following organs
were evaluated microscopically: liver, kidney, adrenal, testes, ovary,
and thyroid. Weights and mortality were recorded. There was no
effect on mortality. No treatment-related histologic changes were
reported (Ferrando et al., 1975).
2.2.6.2 Dogs
In another study 12 beagle dogs weighing about 4.5 kg were fed
for one month with 100 g of control pork as part of a diversified
diet. The dogs were then distributed into 3 treatment groups and
received pork from hogs treated with the above regimens of carbadox at
the rate of 100 gm/day for the first 5 months and then 200 gms for the
remainder of the treatment period (85 months). The dogs were weighed
periodically. Every fourth month haematology, clinical chemistry, and
urinalysis parameters were evaluated.
All dogs were in good health at sacrifice. The dogs received a
gross necropsy at study termination and a standard set of tissues were
evaluated histopathologically. No treatment-related effects were
reported in this study. The authors concluded that there is no danger
to the public health when carbadox is used in pig feed as a growth
promoter at 50 ppm (Ferrando, et al.,1978).
Table 2: Results of genotoxicity assays on carbadox
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 0.0004 - 0.125 Positive Pfizer, undated b
TA1535 (mg/plate)
TA1536, TA1537, Equivocal
TA1538, C340
Ames test TA100 3.1 - 61.7 Positive Beutin et al,
TA98 (+/- S9) (nM) 1981
Ames test TA100 2.5 - 15 µg/plate Positive Yoshimura et al,
TA98 (+/- S9) 1981
Ames test TA100, TA98 .1 umole/plate Positive Negishi, et al,
1980
Ames test TA100 0.001-.02 Positive Voogd et al.,
1980
Mutagenicity Ames E. coli WP2hcr Positive Ohta et al, 1980
test TA100+/-S9 5 - 50 µg/plate Positive
TA98 Positive
TA1538 Negative
TA1537 Negative
TA1535 Negative
Host mediated Assay S. typhimurium 0,1,2, 2.5, 5, 10, Negative Pfizer, undated b
TA1534, TA1952 0,1,2.5 5,7.5, Positive
10,12.5, 20 mg/kg
Repair test B. subtilis (rec) 1 - 100 µg/disc Positive Yoshimura et al.,
S. typhimurium (urv) 1981
Fluctuation test K. pneumoniae .00002 - Positive Voogd et al, 1980
E. coli K12 .005 mmoles/l
Table 2 (contd)
Test System Test Object Concentration Results Reference
Mutagenicity test S. cerevisiae D4 .02% Positive Voogd et al, 1980
Micronucleus test rat bone marrow 5 - 240 mg/kg i.p. Positive Cihak and Srb,
1983
Repair test B. subtilis M45 .1 - 100 Positive Ohta et al,
umole/disk 1980
Chromosomal damage mouse bone marrow 200 mg/kg acute, Positive Pfizer, undated b
10 mg/kg day seven
days
Chromosomal damage in vitro human 0 - 250 µg/ml Positive Pfizer, undated b
lymphocytes
Dominant lethal CD-1 mice 10,50,150,30 mg/kg Negative Pfizer, undated b
assay
QUINOXALINE-2-CARBOXYLIC ACID
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with
quinoxaline-2-carboxylic acid are given in Table 3.
Table 3: Acute toxicity of quinoxaline-2-carboxylic acid
LD50
Species Sex Route (mg/kg) Reference
Rats M Oral 4450 Briggs, 1971
Mice M Oral 1360
2.2.3 Long-term/carcinogenicity Studies
2.2.3.1 Mouse
Groups of 50 Charles River CD mice/sex/dose (100 in controls)
received quinoxaline-2-carboxylic acid in feed for 19 months in a
study of its chronic oral toxicity and tumorigenic potential. Fresh
diets were prepared weekly. Feed and water intake were monitored
weekly for the initial three months and monthly thereafter. Feed
levels of quinoxaline-2-carboxylic acid were adjusted at these
intervals to provide target doses of 0, 25, 50, and 100 mg/kg
b.w./day. Haematology and clinical chemistry were evaluated once,
prior to sacrifice. Necropsy was performed on all animals. Organ
weights were obtained for brain, heart, kidneys, liver, testes, and
ovaries. Histopathologic examination was performed on a standard
array of tissues.
No treatment-related effects were noted in any parameter examined
during the study. Cumulative mortalities were 20% and 15% for control
females and males, respectively, while treatment group cumulative
mortalities ranged from 10% to 20%. Incidence of all tumours was 33%
in female and 46% in male control groups, while the incidences in
treatment groups ranged from 32% to 42%. The authors concluded that
oral administration of quinoxaline-2-carboxylic acid to mice for 19
months produced no evidence of toxicity (Faccini et al., 1979).
2.2.3.2 Rats
Nine male and 9 female Charles River C-D rats were divided into
groups of 3/sex/dose level. Rats received quinoxaline-2-carboxylic
acid in the feed for 2 years. Weight and food consumption were
recorded weekly through week 30 and monthly thereafter. Drug levels
were adjusted to provide target doses of 100, 50 or 0 mg/kg b.w./day.
Rats received clinical examinations weekly and ophthalmoscopic
examinations on days 0, 30, 92, 186, 365, 554, and 733. Haematology
and urinalysis parameters were evaluated on days 31, 93, 182, 274,
370, 461, 547, 644, and 730. Terminal sacrifice was performed on day
735 of the study. All rats received gross necropsies and standard
tissues were evaluated microscopically from each rat. No treatment-
related changes were reported. The authors concluded that
quinoxaline-2-carboxylic acid was tolerated at up to 100 mg/kg
b.w./day when given to rats via feed (Coleman, 1971).
An additional study was conducted to determine whether
quinoxaline-2-carboxylic acid has tumorigenic potential. To
accomplish the study quinoxaline-2-carboxylic acid was administered in
the diet of Charles River Sprague-Dawley rats (20/sex/dose level) for
two years to provide target doses of 0, 10, 25, and 50 mg/kg b.w./day.
Weight and food consumption were monitored weekly and drug
concentration adjusted accordingly. Rats were examined for clinical
signs weekly. Ophthalmologic examinations were performed on all rats
prior to treatment and at 6, 12, 20 and 24 months. Clinical
chemistry, haematology and urine parameters were evaluated in 5 rats
at 6 and 12 months, 2-4 rats at 18 months, and all rats at
termination. At 12 months 5 rats/sex/dose and at 24 months all
remaining rats were sacrificed and received a gross necropsy and
tissues were obtained for microscopic evaluation. Organ weights were
determined for heart, liver, lungs, kidneys, gonads, adrenal, spleen,
and brain. A standard array of tissues was examined
histopathologically.
No treatment-related effects were noted on any of the parameters
evaluated. Cumulative incidences of tumours (based on summing males
and females with 40 rats/dose) occurring in treatment groups for
selected organs of interest are summarized in Table 4. It was
concluded that quinoxaline-2-carboxylic acid in doses of 10, 25, and
50 mg/kg b.w./day over a 2 year period to rats does not produce any
toxicity or elevated tumor incidence (Pfizer, 1971).
Table 4: Cumulative incidence of tumours (males and females combined)
after treatment of rats with quinoxaline-2-carboxylic acid
Treatment Group Mammary gland Pituitary Pancreas Liver
Control 28% 30% 3% 5%
10 mg/kg b.w./day 18 23 10 5
25 mg/kg b.w./day 13 28 3 5
50 mg/kg b.w./day 33 28 3 3
A follow-up chronic study was performed using rats obtained from
the 3-generation study F1 litter. An additional high dose level, 100
mg/kg b.w./day, was added for this study. To accomplish the study
Charles River C-D rats were divided into groups of 50/sex/dose and
received 100, 50, 25, or 0 mg/kg b.w./day of quinoxaline-2-carboxylic
acid in the diet for 29 months. In addition 10 rats/sex in the
control and 100 mg/kg b.w./day groups were added for clinical
chemistry studies at 12 months. Weights were monitored weekly. Food
consumption was recorded weekly during the first 5 months, twice
during the 6th month, and monthly thereafter, and treatment levels
were adjusted accordingly. Clinical examinations were performed
daily. Clinical chemistry parameters were evaluated at 6, 12, and 29
months. Haematology was evaluated at the conclusion of the study.
All rats received a gross necropsy. Liver and kidneys were weighed
from rats sacrificed at 12 months and heart, liver, kidneys, brain,
and testes were weighed during the gross necropsies at 29 months.
Tissues from many organs showing macroscopic abnormality plus a
standard array of grossly normal tissues were evaluated
microscopically.
The general health of the rats was unaffected by treatment. An
outbreak of sialoacryoadenitis occurred between ages 89 and 97 days.
Slightly reduced weight gain (3-5%) was noted in the high-dose groups
in both sexes. The cumulative mortality for controls was 56% in males
and 70% in females, while treatment group cumulative mortality ranged
from 46% to 74%. There was no difference in the incidence of
nonneoplastic lesions among the control and treatment groups, nor did
treatment shorten the lifespan of treatment groups. Treatment of rats
for 2 years did not produce an increase in the incidence of animals
with malignant or benign tumours compared to the control group. The
authors concluded that the NOAEL was 100 mg/kg b.w./day (King, 1979a).
2.2.4 Reproduction studies
2.2.4.1 Rats
The potential reproductive toxicity of quinoxaline-2-carboxylic
acid was studied in rats. The study was performed in conjunction with
a 2-year chronic study. Treatment route was via feed. The study
utilized 2 litters per generation for 3 generations with twenty
rats/sex/dose level using target doses of 25, 50, and 100 mg/kg
b.w./day. The F0 rats were bred following approximately 60 days of
treatment with quinoxaline-2-carboxylic acid. Fifty rats/sex/dose
were selected for the concurrent chronic study from the initial F1
litter. Following a 7 day rest period the females were rebred to
different males to produce the second litter. The subsequent first
litters of each generation were sacrificed at weaning while the second
litters were used to constitute the next generation. These first
litters were reduced to 10 pups/litter (5/sex if possible) for
rearing, then sacrificed, and necropsied at weaning. The 2nd litters
were used to produce the next generation. Subsequent generations of
rats were mated at 100 days of age. Dose levels were maintained and
test animals were treated from weaning throughout the breeding period.
Rats were inspected daily. Food consumption and weights were
determined weekly except for mating periods. Adults of all
generations were sacrificed after weaning of the 2nd litter and
received a gross necropsy with liver weights determined. Blood levels
were determined in F0 rats (5/sex/level) just prior to sacrifice.
Pups were examined daily until weaning and weighed on days 1, 4, and
21 after birth. Gross necropsies were carried out on all pups that
died or were stillborn and on 5 weanlings/sex/dose. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 1, 4, and 21 days, number
weaned, sex ratios, mean pup weights, lactation index, and gross
abnormalities. No treatment-related alterations were observed in any
parameter monitored during the study (King, 1979b).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rabbits
The potential embryotoxicity or teratogenicity of quinoxaline-2-
carboxylic acid was also studied in the New Zealand White Rabbit.
Rabbits were randomly assigned to 4 treatment groups of 19 - 20
animals receiving 0, 25, 50, or 100 mg/kg b.w. quinoxaline-2-
carboxylic acid via gavage on gestation days 7-18. Animals were
observed daily for toxicity and weighed on gestation days 0, 3, 7, 9,
12, 15, 18, 21, 24, and 28. Rabbits were sacrificed on day 28 and the
number of corpora lutea and number and position of live and dead
implants in the uteri were recorded. All fetuses were examined for
external malformations and weighed. Half the fetuses were cleared,
stained with alizarin red and examined for skeletal abnormalities.
The remaining half were fixed in Bouin Allen fluid and examined by
serial sectioning. No treatment related maternal toxicity,
embryotoxicity or malformations were observed (Holmes, 1976a).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with quinoxaline-2-carboxylic
acid are given in table 5.
Table 5: Quinoxaline-2-carboxylic acid genetic toxicology
Test System Test Object Concentration Results Reference
Ames test S. typhimurium .008, .032, Negative Pfizer,
TA1535 .125, .5, 1975
TA1537 2 mg/plate
TA1538
TA 1535+S9 .16, .63, Negative Pfizer,
2.5 mg/plate 1975
Chromosomal Human 50, 100 Negative Pfizer,
aberrations lymphocytes µg/ml 1975
in vitro
DESOXYCARBADOX
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
A long term study was performed to determine the tumorigenic
potential of desoxycarbadox, a carbadox metabolite. Four hundred
Charles River C-D rats (5 weeks of age) were divided into groups of
50/sex/dose level. Rats were distributed randomly according to weight
levels. Desoxycarbadox was administered at target doses of 0, 5, 10,
and 25 mg/kg b.w./day continuously in the diet. Weights were recorded
weekly. Food consumption was monitored weekly the first 3 months and
monthly thereafter. Treatment levels were adjusted accordingly.
Although treatment was planned for 2 years, the test material was
withdrawn from all rats in the 25 mg/kg b.w./day group and 50% of the
rats in each sex in the other 2 treatment groups on day 350, due to
high morbidity and mortality. Administration of desoxycarbadox was
stopped completely on day 416. The study was terminated after 447
days.
Clinical examinations were performed twice weekly. A number of
treatment-related signs were observed. These included: tumours in
the mammary region of both sexes, small cutaneous nodules, and
enlargement of the liver with nodules preceded by weight loss and
polyphagia. There was also a dose-related decrease in weight gain and
a dose-related decrease in survival rate.
Clinical chemistry parameters were evaluated. Desoxycarbadox
administration resulted in dose-related increases in plasma enzyme
activities, urea, and bilirubin. The abnormalities were consistent
with hepatic disorders. The high degree of variability from animal to
animal and lack of reversibility following drug withdrawal indicates
a correlation with tumour progression. A dose-related hypoglycaemia
was noted in both sexes. Haematologic parameters were evaluated in
ten rats/sex/dose at day 413 and the remainder at 447 days. The two
primary changes in haematology in the treated rats were a moderate
hypochromic microcytic anaemia in the 25 mg/kg b.w./day group, a
slight anaemia in the 10 mg/kg b.w./day group, and a neutrophilia in
the 25 mg/kg b.w./day group.
All rats received gross necropsies. Selected organs were
weighed. Histopathologic examination was performed on all grossly
abnormal tissue and routinely on a standard array of tissues. There
was a dose-related increase in pigmentation of renal tubules and
nephrosis. The major pathologic findings related to increased tumour
incidences are summarized in Table 6. The authors concluded that
desoxycarbadox was a potent hepatocarcinogen in the rat and that there
were dose-related increases in other tumours (Reinert, 1976).
Table 6: Incidence of tumours after administration of desoxycarbadox
to rats
Treatment Hepatic Subcutaneous Haemanigiomas Mammary
Group tumours fibromas tumours
Males and females combined Females only
Control 0% 0% 0% 12%
5 mg/kg 75 2 0 24%
b.w./day
10 mg/kg 95 10 5 28
b.w./day
25 mg/kg 100 18 47 27
b.w./day
2.2.5.2 Special Studies on Genotoxicity
The results of genotoxicity studies with desoxycarbadox are
summarized in Table 7.
Table 7: Desoxycarbadox genetic toxicology
Test System Test Object Concentration Result Reference
Ames test TA1535 .0008, .0032, Negative Pfizer, 1975
TA1537 .0125, 0.05, .125,
TA1538 .5, 2, 20 mg/plate
TA1535+S9 .0125, .05, .2, 1, Negative Pfizer, 1975
5 ml/plate
Ames test TA1535 .032, .25, .4, .5 Negative Pfizer, 1975
TA1537 ml/plate plasma;
TA1538 .032, .25. .5 ml/plate
urine1
Host mediated TA1950 500 mg/kg acute Negative Pfizer, 1975
assay
Chromosomal damage Human lymphocytes 200 µg/ml Negative Pfizer, 1975
Rat bone marrow 200 mg/kg acute, Negative Pfizer, 1975
or 100 mg/kg 5 days
10 mg or 25 mg per kg Positive Pfizer, 1975
9 months in feed
Host mediated TA1950 400 or 500 mg/kg Negative Pfizer, 1975
assay in mice
Host mediated TA1950 300 mg/kg with 100 mg/ Negative Pfizer, 1975
assay in rats kg/day previous 31 days
TA1535 200 mg/kg Negative
Table 7 (contd)
Test System Test Object Concentration Result Reference
Yeast assay S. cerevisiae D3 10 mg/ml Negative Holmes, 1976b
& D4
Host mediated TA1950 400 mg/kg +DMNA Negative Holmes, 1976b
assay (mouse)
Ames test TA1537 .01 - 10 mg/plate Negative Holmes, 1976b
TA100
TA98
Ames test TA1535+/- TA100 .005 - 2 mg/plate Positive Holmes, 1976b
S9(rat)
Ames test TA1537+S9 .005 - 2 mg/plate Negative Holmes, 1976b
TA98
Ames test TA1535+S9 from .005 - 2 mg/plate Holmes, 1976b
rat
mouse Positive
hamster Positive
dog Negative
monkey Negative
Negative
Ames test TA1535 Quantitative plate Negative Holmes, 1976b
assay on plasma, liver
& urine at selected
intervals from chronic
study rats fed carbadox
or desoxy-carbadox
Cell transformation BALB/C Swiss 3T3 .1-10 µg/ml Positive Holmes, 1976b
test
1 Urine and plasma collected from mice or rats administered 400 mg/kg desoxycarbadox administered
in 3 hourly doses or 100 mg/kg/day for 5 days.
METHYL CARBAZATE
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
Methyl carbazate is a metabolite of carbadox. The chronic oral
toxicity of methyl carbazate was studied in the rat. Wistar rats were
divided into groups of 12/sex/dose and received methyl carbazate at
target doses of 1 and 10 mg/kg b.w./day in the feed for 21 months.
The colony served as controls. Food consumption was determined every
2 days and weights every 2-3 weeks. Necropsy was performed on all
animals. Organ weights were determined for liver, kidney, adrenals,
testes, and thyroid. Histology was performed on liver, kidneys,
adrenals, thyroid, testes, ovaries, and tissues containing tumours
observed grossly. Three males and 3 females in the high dose group
died before termination. No low dose rats died prior to termination.
No histopathologic evidence of toxicity or evidence of an elevation in
tumours was reported. The authors concluded that administration of
methyl carbazate to rats at dose levels of 1 and 10 mg/kg b.w./day in
the diet produced no evidence of toxicity or carcinogenic potential
(Rutty, 1972).
In a second study weanling Wistar rats were divided into groups
of 24/sex and treated with target doses of 0, 2.5, 5, and 10 mg/kg
b.w./day methyl carbazate in the feed for 710 days. Weights were
determined weekly and food consumption weekly the first 3 months and
monthly thereafter. Drug concentrations were adjusted accordingly.
Clinical examinations were conducted weekly. Blood samples for
clinical chemistry were obtained at terminal sacrifice and from
moribund animals. Haematology was also conducted on these samples and
at 12 months on 6 rats. Rats were sacrificed and necropsied at 710
days. An interim sacrifice was also performed at 12 months. All
moribund and dead rats also received a gross necropsy. Histopathology
was performed on all gross lesions and the following organs: liver,
pituitary, thyroid, parathyroid, adrenal, lung, kidney, urinary
bladder, ovary, and testis.
There were no treatment-related effects noted in any parameters
evaluated in the study. There was histologic evidence of widespread
chronic respiratory disease in all groups. It was concluded that
methyl carbazate had no adverse effect when given to rats in the diet
for 2 years (Ferrando, 1980).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with methyl carbazate are
summarized in Table 8.
Table 8: Genetic toxicity studies with methyl carbazate
Test System Test Object Concentration Results Reference
Ames test TA1535 .0005, .002, Negative Pfizer, 1975
TA1537 .008, .032,
TA1538 .125, 0.5, 2, 5
mg/plate
TA1535+S9 .0125, .05, .2 Negative Pfizer, 1975
1, 5, 10
mg/plate
Host- TA1950 50, 100, 300 Equivocal Pfizer, 1975
mediated mg/kg
assay
Chromosomal Human 50, 100 Negative Pfizer, 1975
damage lymphocytes µg/ml
in vitro
Ames test TA1535+S9 0.5 - 10 Negative Holmes, 1976b
mg/plate
HYDRAZINE
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism, and excretion
Hydrazine toxicokinetic parameters have been determined in the
rabbit. Rabbits received a bolus of 12 mg/kg b.w. Parameters were
derived using a one-compartment model, and spectrophotometric serum
analysis for hydrazine, with 5 rabbits/group. The serum half-life was
2.3 hours and the volume of distribution was 0.63 liters/kg (Keller
et al., 1981).
The metabolism, distribution, and elimination of hydrazine in
rats was studied using 15N-labelled hydrazine or a spectrophotometric
method. About 15% of the 15N label was found as N2. About 20% and
30% of the infused hydrazine was recovered in the urine as acetylated
metabolite and hydrazine, respectively. The serum half-lives were
determined, using a two-compartment model, to be 0.74 hours for the
majority of the hydrazine elimination, and 26.9 hours for a much
slower, smaller elimination component (Dost et al., 1981).
2.1.3 Effects on enzymes and other biochemical parameters
Hydrazine-induced short-term biochemical parameter changes were
investigated following oral administration of hydrazine in Sprague-
Dawley rats. Groups of 5 rats were given doses of hydrazine (0-200
mg/kg b.w.). Some rats received pyruvate azine, phenobarbital,
piperonyl butoxide, or diethylmaleate to more clearly elucidate the
hepatic effects of hydrazine. Hydrazine caused a dose-related
increase in liver triglycerides and liver weight and a decrease in
hepatic glutathione. The threshold dose for toxicity was about 10
mg/kg b.w. While liver weight and glutathione alterations were
evident within 30 minutes, triglyceride alteration was not evident for
4 hours. Pretreatment with phenobarbital decreased toxicity, while
pretreatment with piperonyl butoxide increased toxicity suggesting
hepatotoxicity is due to the parent - not a metabolite. Depletion of
hepatic glutathione was without effect on hepatic toxicity, and
studies with pyruvate azine - a possible oxidative metabolite - also
supported this conclusion (Timbrell, et al., 1982).
Hydrazine-induced short-term histologic and ultrastructural
changes were investigated following oral administration of hydrazine
to Sprague-Dawley rats. Groups of 5 rats were given doses of
hydrazine (0-200 mg/kg b.w.). Some rats received pyruvate azine,
phenobarbital, piperonyl butoxide, or diethylmaleate to more clearly
elucidate the hepatic effects of hydrazine. A dose of 20 mg/kg caused
accumulation of lipid, swelling of mitochondria, and the appearance of
microbodies in periportal and midzonal hepatocytes. Accumulation of
lipid droplets and mitochondrial swelling were detected by electron
microscopy 30 minutes after dosing. Piperonyl butoxide or
phenobarbital treatment increased or decreased fatty liver
respectively, while pyruvate azine was much less effective than
hydrazine in producing this effect (Scales & Timbrell, 1982).
2.2 Toxicological studies
2.2.3 Long-term carcinogenicity studies
2.2.3.1 Mice
Oral administration of hydrazine sulfate to Balb/c female mice at
1.13 mg/day for 46 weeks produced a 100% incidence of lung tumours
(Biancifiori & Ribacchi, 1962).
In another study hydrazine was administrated via gavage to 25
female Swiss mice 5 days/week for 40 weeks at the rate of 0.25 mg/day.
Eighty-five mice served as controls. This dose regimen produced a 46%
incidence of lung tumours in treated mice vs 10% in controls (Roe et
al., 1967).
A study with CBA/Cb/Se mice was performed using hydrazine sulfate
given for 36 weeks by gavage. Twenty-one 8- week-old rats/sex
received 1.13 mg/day. Treated rats had an incidence of 76% and 90%
lung tumours in males and females, respectively, while controls had
3%. Hepatomas were found in 62% of the males and 71% of the females
treated with hydrazine, while 11% of control males and 4% control
females had hepatomas (Severi & Biancifiori 1968).
In another study in mice, 8-week-old CBA mice were divided into
groups of 40-59 mice/sex. Hydrazine sulfate was given by gavage daily
for 150 days at rates of 45, 22, 11, 5.6 and 0 mg/kg b.w./day. Mice
were examined at natural death or following sacrifice when moribund.
Control male mice had a hepatoma incidence of 10% while females had a
3.4% rate. Treated males had hepatoma rates of 60, 48, 28, and 3.8%
at 45, 22, 11, and 5.6 mg/kg b.w./day, respectively. Treated females
had 62.5, 66.6, 8 and 0% hepatomas, respectively. The author reported
many lung tumours in treated groups but did not provide the incidence
rates (Biancifiori, 1970).
2.2.3.2 Hamsters
Eight-week old golden hamsters were divided into 3 groups of 23,
35 and 56 animals which were administered 60 doses of 3.0 mg hydrazine
sulfate via intubation over a 15 week period, 2.8 mg 100 times during
a 20 week period or no hydrazine, respectively. Hepatic lesions
reported include: Fibrosis, reticuloendothelial cell proliferation,
and bile duct proliferation. Hepatic lesions were found in 60-80% of
treated hamsters but in none of the controls. No hepatic tumours were
reported (Biancifiori, 1970).
Negative tumorigenicity results were also obtained in a follow up
chronic study with golden hamsters given 2.3 mg/day over a lifetime.
In this study 50 hamsters/sex received hydrazine in drinking water
from the age of 9 weeks for a lifetime (Toth, 1972).
2.2.3.3 Rats
A chronic oral study was performed in conjunction with the above
mouse study using 8 week old Cb/Se rats. Aqueous hydrazine sulfate
was administered daily by stomach tube at doses of 18 mg/rat to 14
males and 12 mg/rat to 18 females for 68 weeks. Lung tumours were
found in 21 and 27% of dosed male and female rats, respectively.
Control groups of 28 males and 22 females had no lung tumours.
Hepatic tumours were found in 30% of males, while no hepatic tumours
were found in females or controls (Severi & Biancifiori, 1968).
2.2.7.5 Special studies on genotoxicity
The results of genotoxicity studies with hydrazine are summarized
in Table 9.
Table 9: Genetic toxicity studies with hydrazine
Test System Test Object Concentration Results Reference
Bacterial E. coli WP2 0.5 - 2.0 µmole Positive von Wright
uvr A trp & Tikkanen,
1980
Mouse L5178Y 0.1 - 5 mmole Positive Rogers &
lymphoma Back, 1981
cells
Ames test S. typhimurium Positive Ames, 1971
2.3. Observations in humans
No information available
3. COMMENTS
The Committee considered data from short-term, long-term and
"relay" toxicity studies, and from studies on carcinogenicity,
mutagenicity, and reproduction with carbadox, together with data from
mutagenicity and long-term toxicity studies of its metabolites.
The metabolism of carbadox has been studied in rats, monkeys and
pigs using [14C]carbadox, labelled in either the phenyl ring or the
carbonyl group of the side-chain. The metabolism of carbadox was
characterized by the rapid reduction of the N-oxide groups, the
cleavage of the methylcarbazate side-chain, and the liberation of
respired CO2. The primary metabolite in the urine was quinoxaline-2-
carboxylic acid, which was also excreted in conjugated form. The
detectable residues in tissues, up to 24 hours after drug withdrawal,
were carbadox, desoxycarbadox, quinoxaline-1,4-di-N-oxide-2-
carboxaldehyde and quinoxaline-2-carboxylic acid. Hydrazine was a
minor metabolite but would be expected to be present only for a short
time before undergoing further metabolism.
The concentration of carbadox and desoxycarbadox in the tissues
declined rapidly (half-life about 6 hours), and, after 3 days, was
less than 5 µg/kg. In a study in pigs, in which carbonyl-labelled
[14C]carbadox was administered by gavage, the concentration of total
residues in liver at 5 days withdrawal time corresponded to 0.12 mg/kg
methyl carbazate equivalent. However, some of this radioactivity was
due to the presence of amino acids, which had been labelled by
incorporation of 14CO2 when ring-labelled [14C]carbadox was
administered to pigs, extremely low levels of unidentified metabolites
remained in the liver at withdrawal periods longer than 7 days. These
residues were partially released and converted to quinoxaline-2-
carboxylic acid by alkaline digestion of the liver.
The Committee also reviewed recent reports of the effect of
carbadox on adrenal function in pigs, although the information did not
effect the outcome of the evaluation.
Several long-term feeding studies in rats were evaluated which
demonstrated dose-related increases in the incidence of benign and
malignant liver tumours at doses of carbadox above 1.0 mg per kg of
body weight per day. Doses above 25 mg per kg of body weight per day
produced pronounced toxic effects which precluded chronic treatment.
Data from a variety of mammalian and non-mammalian genotoxicity
studies were also reviewed. Positive findings were reported in 14 of
the 15 tests carried out. The Committee concluded that carbadox
appears to be both genotoxic and carcinogenic.
The Committee evaluated a "relay" toxicity study in which pigs
were fed carbadox at up to 200 mg/kg in the diet for 30 days and
killed at zero withdrawal time, their livers were fed to rats as a 10%
dietary component for 2 years. Although this study was conducted
satisfactorily and no treatment-related effects were reported, the
Committee did not use it for assessing the carcinogenicity of carbadox
residues because of the number of animals used and because only small
quantities of residues can be consumed by rats in this type of study.
In a long-term study in rats, desoxycarbadox was reported to
produce an increase in the incidence of tumours. All rats that
received 25 mg per kg of body weight desoxycarbadox daily for 10
months in the diet developed hepatic tumours. The incidence of
tumours was increased in all treated groups, at doses of 5-25 mg per
kg of body weight per day. While the most pronounced change occurred
in the liver, tumour incidence was also elevated at other sites,
including the skin and mammary glands.
The mutagenic potential of desoxycarbadox was investigated in a
range of studies. The Committee noted that, while desoxycarbadox
produced negative results in most mutagenicity test systems, positive
findings were recorded in the cell transformation test and in the Ames
test using liver microsomes from rats treated with polychlorinated
biphenyls. The Committee noted that the tumorigenic potential of
desoxycarbadox was apparently greater than that of the parent
compound, and that desoxycarbadox would therefore probably make a
significant contribution to the tumorigenic activity of carbadox in
rats. However, the Committee also noted that the metabolism data for
carbadox supported the conclusion that desoxycarbadox was a relatively
short-lived intermediate between carbadox and quinoxaline-2-carboxylic
acid.
The Committee also considered information on the carbadox side-
chain metabolite, methyl carbazate. This information consisted of
results from two long-term studies in rats and a number of
mutagenicity assays. In the more informative long-term study (in
which more animals per dose and concurrent controls were used), rats
received methyl carbazate in the diet for 710 days. There was no
increase in the incidence of tumours and no other treatment-related
effects were observed at 10 mg per kg of body weight per day, which
was the highest dose tested. The lack of effect limited the
usefulness of this study. The no-observed-effect level for the study
was 10 mg per kg of body weight per day. The useful mutagenicity
studies were reported to be negative.
The results of studies on the pharmacokinetics, mutagenicity, and
carcinogenicity of hydrazine were also considered by the Committee.
Pharmacokinetic data from rats and rabbits as well as hydrazine's
known chemical reactivity suggest that it should be rapidly
eliminated. The structure of carbadox and its known metabolic
pathways indicate that hydrazine is probably a further metabolite of
methyl carbazate. Hydrazine showed mutagenic and carcinogenic
potential.
In evaluating the potential toxicity of residues of carbadox, the
Committee also considered toxicity data on the carbadox metabolite
quinoxaline-2-carboxylic acid to be useful. In a carcinogenicity
study in mice in which quinoxaline-2-carboxylic acid was administered
in the diet for 19 months, no treatment related increases in tumour
incidence or any other effects on the animals were reported. The no-
observed-effect level was 100 mg per kg of body weight per day.
Several chronic toxicity and carcinogenicity studies in which
quinoxaline-2-carboxylic acid was administered for 24 months were
conducted in rats. There were no effects on the incidence of tumours,
the only findings being a slight reduction in weight gain at 100 mg
per kg of body weight per day. The no-observed-effect level was 50 mg
per kg of body weight per day.
In a three-generation reproduction study in rats, quinoxaline-2-
carboxylic acid was given in the diet at levels of up to 100 mg per kg
of body weight per day. No treatment-related effects were observed.
Developmental studies were conducted in rats and rabbits in which the
compound was administered by gavage at levels of up to 100 mg per kg
of body weight per day. There was no evidence of maternal toxicity,
embryotoxicity, or teratogenicity in either species. The no-observed-
effect levels in these studies were 100 mg per kg of body weight per
day.
4. EVALUATION
With current analytical procedures, quinoxaline-2-carboxylic acid
is the only carbadox metabolite that can be identified in liver from
pigs treated according to good practice in the use of veterinary
drugs. However, uncertainty remains because of the presence of
unidentified residues in liver. Because of the genotoxic and
carcinogenic nature of carbadox and some of its metabolites, the
Committee was not able to establish an ADI.
On the basis of data from studies on the toxicity of quinoxaline-
2-carboxylic acid, and on the metabolism and depletion of carbadox and
the nature of the compounds released from the bound residues, the
Committee concluded that residues resulting from the use of carbadox
in pigs were acceptable, provided that the recommended MRLs are not
exceeded.
5. REFERENCES
AMES, B. (1971). Chemical Mutagens, Vol. 1, Hollaender, A. (ed),
Plenum Press, New York. p. 267.
BEUTIN, L., PRELLER, E., & KOWALSKI, B. (1981). Mutagenicity of
Quindoxin, Its Metabolites, and Two Substituted Quninoxaline-Di-N-
Oxides. Antibacterial Agents and Chemotherapy, 20, 336-343.
BIANCIFIORI, C., & RIBACCHI, R., (1962). Pulmonary Tumour in Mice
Induced by Oral Isoniazid and its Metabolites. Nature, 194, 488-
489.
BIANCIFIORI, C. (1970). Hepatomas in CBA/Cb/Se Mice and Liver Lesions
in Golden Hamsters Induced by Hydrazine Sulfate. J. Nat. Cancer
Inst, 44, 943-953.
BRIGGS, G.B. (1971). Safety Evaluation of CP-16505, Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
CIHAK, R., & SRB, V. (1983). Cytogenetic Effects of Quinoxaline-1,4-
dioxide-type Growth-promoting Agents. Mutat. Res., 116, 129-135.
COLEMAN, G.L., (1967). Two year Chronic Study of GS-6244 (carbadox)
in Monkeys. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
COLEMAN, G.L., (1971). Quinoxaline-2-Carboxylic Acid, First Two Year
Study in Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
DOST, F.N., BRODERICK, D.J., KRIVAK, B.N., & REED, D.J., (1981).
Metabolism of Hydrazine. AFAMRL-TR-81-26. Technical Report. Air
Force Aerospace Medical Research Laboratory, Wright-Patterson AFB, OH.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
FACCINI, J.M., PERRAUD, J., NACHBAUR, J., & MONRO, A.M., (1979). 19-
Month toxicity Study in Mice. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1975).
Application of the Toxicity by Relay Method for the Assessment of
Safety to Human Consumers of Carbadox, a Growth-Promoting Additive, to
the Feed of Pigs Slaughtered for Human Consumption. Toxicology, 3,
369-398.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1978). Human
Consumer Safety in the Use of Carbadox, a Pig Feed Additive, as
Estimated by Seven Years of Relay Toxicity in Beagles. Toxicology,
11, 167-183.
FERRANDO, R., (1980). Two Year Chronic Toxicity Study in the Rat with
Methyl Carbazate. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
FIGDOR, S.K., (1969). Carbadox in Pigs Radiotracer Metabolism C-14,
Carbonyl. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
FIGDOR, S.K., MCILHENNY, H.M., & VON WITTENAU, M.S., (1969).
Metabolism of Carbadox Using Carbadox Labelled with C14 in the Phenyl
Ring. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
HOLMES, C.L., (1976a). Action of CP-16,505 on Pregnancy and Fetal
Development in Rabbits. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
HOLMES, C.L., (1976b). Addendum Genetic Toxicology Report CP-17,056
(Desoxycarbadox) Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KELLER, W.C., MURPHY, J.P., ANDERSEN, M.E. & BACK, K.C., (1981).
Percutaneous Toxicokinetics of Hydrazine and H70 in the Rabbit.
AFAMRL-TR-81-13. Technical Report. Air Force Aerospace Medical
Research Laboratory, Wright-Patterson AFB, OH. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
KING, T.O., (1976). Target Organ Toxicity of GS-6244 (carbadox) and
CP-17,056 (desoxycarbadox) with Chronic Administration in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
KING, T.O., (1979a). Chronic Study in Rats with Quinoxaline-2-
Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KING, T.O., (1979b). Three Generation Study in Rats with Quinoxaline-
2-Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
NEGISHI, T., TANAKA, K., AND HYATSU, H., (1980). Mutagenicity of
Carbadox and Several Quinoxaline 1,4-Dioxide Derivatives. Chem.
Pharm. Bull., 28, 126-128.
OHTA, T., MORIYA, M., KANEDA, K., WATANABE, T., MIYAZAWA, A., &
SUSIGYAMA, F., (1980). Mutagenicity screening of Feed Additives in
the Microbial System. Mutat. Res., 77, 21-30.
PFIZER (undated a). Acute Toxicity of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (undated b). Genetic Toxicology of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (1963). A thirty-day Subacute Toxicity Study of GS-6244 in
Albino Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
PFIZER (1971). Two Year Study with CP-16,505 (Quinoxaline-2-
Carboxylic Acid) in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
PFIZER (1975). Genetic Toxicology Report CP-17,056 (Desoxycarbadox)
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
REINERT, H. (1976). Desoxycarbadox: Two Year Toxicity Study in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
ROE, F.J., GRANT, G.A., & MILLICAN, D.M., (1967). Carcinogenicity of
Hydrazine and 1,1-Dimethylhydrazine for Mouse Lung. Nature, 216,
375-376.
ROGERS, A.M., & BACK, K.C., (1981). Comparative Mutagenicity of
Hydrazine and 3 Methylated Derivatives in L5178Y Mouse Lymphoma Cells.
Mutat. Res., 89, 321-328.
RUTTY, D.A., (1972). Twenty-one Month Chronic Toxicity Study of
Methyl Carbazate in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
SCALES, M.D., & TIMBRELL, J.A., (1982). Studies on Hydrazine
Hepatotoxicity. 1. Pathological Findings. J. Toxicol and Environ.
Health, 10, 941-953.
SEVERI, L., & BIANCIFIORI, C. (1968). Hepatic Carcinogenesis in
CBA/Cb/Se Mice and Cb/Se Rats by Isonicotinic Acid Hydrazine and
Hydrazine Sulfate. J. Nat. Cancer Inst., 41, 332-349.
SIGLER, F.W., (1969a). A Two-year Chronic Toxicity Study of GS-6244
in Rats. Dose Levels: 2.5, 1.0 or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SIGLER, F.W., (1969b). A Three Generation Reproductive Study of GS-
6244 in Rats. Dose Levels: 2.5, 1.0, or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SPIERENBURG, T.J., BAARS, A.J., DE GRAAF, G.J., & JAGER, L.P., (1988).
Carbadox-induced Inhibition of Aldosterone Production in Porcine
Adrenals in vitro. Toxicology In Vitro, 2, 141-143.
STEBBINS, R.B., (1964). A 3-Week Subacute Toxicity Study of GS-6244
in Rats - Dose Levels of 100, 50, 10, 5, or 0 mg/kg. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
STEBBINS, R.B., & COLEMAN, G.L., (1967). A Twenty-six Month Chronic
Toxicity Study of GS-6244 in Rats - Dose Levels of 100, 50 10, 5, or
0 mg/kg. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
SYKORA, I., & VORTEL, V., (1986). Post-natal Study of the
Carcinogenicity of Cyadox and Carbadox. Biol Vet Praha., 22, 53-62.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J., (1982). Studies on
Hydrazine Hepatotoxicity. 2. Biochemical Findings. J. Toxicol and
Environ. Health, 10, 955-968.
TOTH, B., (1972). Tumorigenesis Studies with 1,2-Dimethylhydrazine
dihydrochloride, Hydrazine Sulfate, and Isonicotinic Acid in Golden
Hamsters. Cancer Res, 32, 804-807.
VAN DER MOLEN, E.J., DE GRAAF, G.J., & BAARS, A.J., (1989a).
Persistence of Carbadox-induced Adrenal Lesions in Pigs Following Drug
Withdrawal and Recovery of Aldosterone Plasma Concentration.
J. Comp. Path., 100, 295-304.
VAN DER MOLEN, E.J., VAN LIESHOUT, J.H., NABUURS, M.J., DERKX, F.H.,
LAMERS, A.P., & MICHELAKIS, A.M., (1989b). Changes in Plasma Renin
Activity and Renal Immunohistochemically Demonstrated Renin in
Carbadox Treated Pigs. Res. Vet. Sci., 46, 401-405.
VON WITTENAU, M.S., (1969). Comparative Metabolism Studies in Swine,
Monkeys, and Rats of Carbadox. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J., (1980). The
Mutagenic Action of Quindoxin, Carbadox, Olaquindox, and Some other N-
oxides on Baceteria and Yeast. Mutat. Res., 78, 233-242.
VON WRIGHT, A. & TIKKANEN, L., (1980). The Comparative Mutagenicities
of Hydrazine and its Mono- and Di- methyl Derivatives in Bacterial
Test Systems. Mutat. Res., 78, 17-23.
YOSHIMURA, H., NAKAMURA, M., KOEDA, T., & YOSHIKAWA, K., (1981).
Mutagenicities of Carbadox and Olaquindox - Growth Promoters for Pigs.
Mutat. Res., 90, 49-55.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The elimination of carbadox was studied in rats, swine and
monkeys. Pigs received 3.5 mg/kg of 14C-carbadox after several of
weeks of receiving feed containing 50 g/ton of unlabelled carbadox,
while rats and monkeys received a single dose of 5 mg/kg 14C-carbadox.
Urine and faeces were collected and assayed for radioactivity. The
urinary metabolites were evaluated qualitatively using TLC. Nearly
all (13/15) of the metabolites present in swine urine are found in rat
and monkey urine. One of the other two was a glycine conjugate of
quinoxaline-2-carboxylic acid. All species excreted more than 50% of
the dose in urine (swine: 74%, monkey: 61%, rat: 54%) during the 72
hour collection period. The following activities were reported for
faeces during the 72 hour period: swine, 17%, monkey, 8-10%, and rat,
29%. Total excretion appeared to be in the 70-90% range over the 72
hour period. The author concluded that the distribution of
radioactivity was similar in the three species (von Wittenau, 1969).
2.1.2 Biotransformation
Seven-week-old swine received feed containing 50 g/ton of
unlabelled carbadox for several weeks, then received a single oral
dose of either 3.5 mg/kg or 0.8 mg/kg of 14C-carbadox labelled in the
phenyl ring. Peak radioactivity was observed in plasma approximately
3 hours after dosing. The following were identified in plasma at 5-8
hours post dose: carbadox (13%), desoxycarbadox (9-19%),
carbadoxaldehyde (13%), and quinoxaline-2-carboxylic acid (19%) (all
expressed in terms of total plasma radioactivity). The presence of
carbadoxaldehyde in stomach contents was confirmed. Carbadox was
rapidly eliminated. Approximately 2/3 of the dose was eliminated in
the urine and the remainder in the faeces (total of approximately 90%)
within 48-72 hours. Radioactivity equivalent to approximately 0.1 ppm
carbadox was found to be retained in the liver at 14 days post dose.
Attempts to identify this residual radioactivity were not successful.
The only metabolite identified in liver after 24 hours was the major
urine-eliminated metabolite, quinoxaline-2-carboxylic acid (Figdor
et al., 1969).
In a second study seven-week-old pigs received unlabelled
carbadox in feed at the rate of 50 g/ton for several weeks followed by
a single oral dose of 14C-carbadox labelled in the carbonyl position
via stomach tube. The following evaluations were made: expired air
was evaluated for 14CO2, labelled material in liver and urine was
evaluated to determine if it was methyl carbazate-related, and plasma
and urine were evaluated for free hydrazine. Maximum plasma
concentrations of radiolabelled material occurred at approximately 3
hours. While early plasma concentrations were similar to those found
for ring-labelled carbadox, concentrations at 24 hours remained
somewhat higher. Approximately 50% of the radiolabelled material in
the plasma at 3 hours was identified as carbadox, while methyl
carbazate was estimated to be 30%. The major route of radiolabel
excretion was urinary. However, less than half the total amount
recovered with ring-labelled carbadox was recovered from carbonyl-
labelled carbadox (37% vs 88%). The reason for this is the apparently
high conversion of the radiolabel to CO2 (verified in the rat as up
to 36%). Radiolabelled material equivalent to 0.1 - 0.34 ppm carbadox
was present in liver at 5 days. The authors concluded that some of
this material was incorporated CO2 (potentially 25%). The pig
receiving 7 mg/kg was found to have eliminated 7% of the dose as free
hydrazine in the urine at 24 hours, while pigs receiving lesser doses
were not found to have any identifiable hydrazine in their urine
(Figdor, 1969).
The reported studies support the metabolism scheme illustrated in
Figure 2.
Extensive studies have been performed not only on carbadox, but
also on its metabolites. Studies are summarized separately on each
substance in this monograph.
2.1.3 Effects on enzymes and other biochemical parameters
The persistence of carbadox-induced adrenal lesions was studied
in swine. Groups of 13 animals each received 0, 25, 50, 100, or 200
ppm carbadox in feed for 10 weeks. Five and 11 weeks after withdrawal
2 pigs from each group were necropsied and adrenals evaluated
histologically. At five weeks the pigs in the 25 and 50 ppm groups
showed recovery of adrenal lesions. At 11 weeks pigs in the 100 and
200 ppm groups had not completely recovered. Plasma aldosterone
levels were monitored at 1, 2, 4, 6, and 8 weeks post treatment. At
one week aldosterone levels were depressed in the 100 and 200 ppm
groups vs controls. All other aldosterone measurements of treated
groups were similar to control values (van der Molen et al.,
1989a).
The effect of carbadox on the renin-angiotensin system was
examined in swine. Five groups of 13 five-week-old weaned pigs
received feed containing 0, 50, 100, 150, and 200 ppm carbadox to
provide target doses of 0, 2, 4, 6, and 8 mg/kg body weight for 10
weeks. By nine weeks the plasma renin levels of all treated groups
were significantly higher than controls. At 5 and 10 weeks, renal
immunoreactive renin from two to three pigs was evaluated. All pigs
demonstrated an increase in immunoreactive renin. It was concluded
that carbadox induces activation of the renin-angiotensin system,
secondary to suppression of mineralocorticoid secretion (van der Molen
et al., 1989b).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The elimination of carbadox was studied in rats, swine and
monkeys. Pigs received 3.5 mg/kg of 14C-carbadox after several of
weeks of receiving feed containing 50 g/ton of unlabelled carbadox,
while rats and monkeys received a single dose of 5 mg/kg 14C-carbadox.
Urine and faeces were collected and assayed for radioactivity. The
urinary metabolites were evaluated qualitatively using TLC. Nearly
all (13/15) of the metabolites present in swine urine are found in rat
and monkey urine. One of the other two was a glycine conjugate of
quinoxaline-2-carboxylic acid. All species excreted more than 50% of
the dose in urine (swine: 74%, monkey: 61%, rat: 54%) during the 72
hour collection period. The following activities were reported for
faeces during the 72 hour period: swine, 17%, monkey, 8-10%, and rat,
29%. Total excretion appeared to be in the 70-90% range over the 72
hour period. The author concluded that the distribution of
radioactivity was similar in the three species (von Wittenau, 1969).
2.1.2 Biotransformation
Seven-week-old swine received feed containing 50 g/ton of
unlabelled carbadox for several weeks, then received a single oral
dose of either 3.5 mg/kg or 0.8 mg/kg of 14C-carbadox labelled in the
phenyl ring. Peak radioactivity was observed in plasma approximately
3 hours after dosing. The following were identified in plasma at 5-8
hours post dose: carbadox (13%), desoxycarbadox (9-19%),
carbadoxaldehyde (13%), and quinoxaline-2-carboxylic acid (19%) (all
expressed in terms of total plasma radioactivity). The presence of
carbadoxaldehyde in stomach contents was confirmed. Carbadox was
rapidly eliminated. Approximately 2/3 of the dose was eliminated in
the urine and the remainder in the faeces (total of approximately 90%)
within 48-72 hours. Radioactivity equivalent to approximately 0.1 ppm
carbadox was found to be retained in the liver at 14 days post dose.
Attempts to identify this residual radioactivity were not successful.
The only metabolite identified in liver after 24 hours was the major
urine-eliminated metabolite, quinoxaline-2-carboxylic acid (Figdor
et al., 1969).
In a second study seven-week-old pigs received unlabelled
carbadox in feed at the rate of 50 g/ton for several weeks followed by
a single oral dose of 14C-carbadox labelled in the carbonyl position
via stomach tube. The following evaluations were made: expired air
was evaluated for 14CO2, labelled material in liver and urine was
evaluated to determine if it was methyl carbazate-related, and plasma
and urine were evaluated for free hydrazine. Maximum plasma
concentrations of radiolabelled material occurred at approximately 3
hours. While early plasma concentrations were similar to those found
for ring-labelled carbadox, concentrations at 24 hours remained
somewhat higher. Approximately 50% of the radiolabelled material in
the plasma at 3 hours was identified as carbadox, while methyl
carbazate was estimated to be 30%. The major route of radiolabel
excretion was urinary. However, less than half the total amount
recovered with ring-labelled carbadox was recovered from carbonyl-
labelled carbadox (37% vs 88%). The reason for this is the apparently
high conversion of the radiolabel to CO2 (verified in the rat as up
to 36%). Radiolabelled material equivalent to 0.1 - 0.34 ppm carbadox
was present in liver at 5 days. The authors concluded that some of
this material was incorporated CO2 (potentially 25%). The pig
receiving 7 mg/kg was found to have eliminated 7% of the dose as free
hydrazine in the urine at 24 hours, while pigs receiving lesser doses
were not found to have any identifiable hydrazine in their urine
(Figdor, 1969).
The reported studies support the metabolism scheme illustrated in
Figure 2.
Extensive studies have been performed not only on carbadox, but
also on its metabolites. Studies are summarized separately on each
substance in this monograph.
2.1.3 Effects on enzymes and other biochemical parameters
The persistence of carbadox-induced adrenal lesions was studied
in swine. Groups of 13 animals each received 0, 25, 50, 100, or 200
ppm carbadox in feed for 10 weeks. Five and 11 weeks after withdrawal
2 pigs from each group were necropsied and adrenals evaluated
histologically. At five weeks the pigs in the 25 and 50 ppm groups
showed recovery of adrenal lesions. At 11 weeks pigs in the 100 and
200 ppm groups had not completely recovered. Plasma aldosterone
levels were monitored at 1, 2, 4, 6, and 8 weeks post treatment. At
one week aldosterone levels were depressed in the 100 and 200 ppm
groups vs controls. All other aldosterone measurements of treated
groups were similar to control values (van der Molen et al.,
1989a).
The effect of carbadox on the renin-angiotensin system was
examined in swine. Five groups of 13 five-week-old weaned pigs
received feed containing 0, 50, 100, 150, and 200 ppm carbadox to
provide target doses of 0, 2, 4, 6, and 8 mg/kg body weight for 10
weeks. By nine weeks the plasma renin levels of all treated groups
were significantly higher than controls. At 5 and 10 weeks, renal
immunoreactive renin from two to three pigs was evaluated. All pigs
demonstrated an increase in immunoreactive renin. It was concluded
that carbadox induces activation of the renin-angiotensin system,
secondary to suppression of mineralocorticoid secretion (van der Molen
et al., 1989b).
 The in vitro effect of carbadox on adrenal aldosterone production
was evaluated in porcine adrenal slices obtained from 3-5 week old
pigs. The adrenal slices were maintained in Krebs solution and
exposed to 1-40 µg carbadox/ml. Aldosterone was estimated using RIA
at hourly intervals. A dose-dependent decrease in aldosterone
production occurred with a reduction of 25-35% at 40 µg/ml
(Spierenburg et al; 1988).
2.2. Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with carbadox are shown in
Table 1.
Table 1: Carbadox acute toxicity data
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 2810 Pfizer, undated a
F oral >2810
i.p. 1050
Rat M oral 850
i.p. 810
A study was done to examine the emetic effect of carbadox on
monkeys and dogs. Three animals/group received a single dose of
carbadox orally via capsule. Doses were 25, 10, 5, and 1 mg/kg
b.w./day. In the monkey 1/3 exhibited emesis in the 2 higher dose
groups, while in dogs all dose groups had emesis.
2.2.2 Short-term studies
2.2.2.1 Rats
Twenty Charles River C-D weanling rats were divided and dosed for
30 days as follows: 2-3 males and females at 50 mg/kg b.w./day and
100 mg/kg b.w./day, and 10 males as untreated controls. Carbadox was
administered in the diet. Weight and food consumption were observed
weekly and drug levels adjusted as necessary. CBC at 30 days, urinary
electrolytes at 1, 2, 3, 14, and 30 days, cholesterol at 30 days and
urinalysis at 14 and 30 days were evaluated. Rats received a gross
necropsy at the end of the study and tissues were evaluated
microscopically. A dose-dependent decrease in weight gain and food-
consumption were noted. The authors concluded that all other
parameters were normal (Pfizer, 1963).
2.2.2.2 Dogs
The short-term toxicity of carbadox was studied by dosing dogs 6
days/week for three weeks. Beagle dogs (1/sex/dose) were initially
dosed with 25 or 50 mg/kg b.w./day carbadox via oral capsule. These
doses were later reduced to 10 and then 15 mg/kg b.w./day due to
emesis. A variety of parameters were evaluated including clinical
chemistry, gross necropsy, and microscopic tissue examination at the
termination of the study. The dogs lost weight and had elevated
SGPT's. It was concluded that carbadox is emetic and possibly
hepatotoxic in the dog (Stebbins, 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
The chronic toxicity and potential tumorigenicity of carbadox
were studied in rats. One hundred twenty Charles River C-D rats were
divided into 6 groups (10/sex/dose) and received carbadox in the diet
at rates providing doses of 100, 50, 25, 10, 5, and 0 mg carbadox/kg
b.w./day for 26 months. Feed concentration was adjusted weekly.
Clinical signs, body weights, and food consumption were recorded
weekly. Haematology and urinalysis were evaluated in 5 rats/sex/dose
at 3, 6, 12, 18, and 25 months. Rats were sacrificed at 14 and 112
weeks and received gross necropsies. Tissues were examined
histopathologically.
Survival was reduced for animals in the high-dose groups. The
following observations were reported for animals up to and including
the interim sacrifice:
100 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption, reduced haemoglobin, RBC's and neutropenia.
Microscopic changes reported include pulmonary hemorrhage and edema,
adrenalcortical hemorrhage and degeneration, splenic haemosiderin, and
thymic atrophy. No animals survived beyond the 14 week sacrifice.
50 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption. Microscopic changes reported include pulmonary
hemorrhage and edema, adrenalcortical hemorrhage, necrosis, and
degeneration, splenic haemosiderin and renocorticomedullary fatty
metamorphosis. No animals survived beyond the 14 week sacrifice.
25 mg/kg b.w./day (3 rats/sex necropsied) - Reduced weight gain,
slight adrenal cortical atrophy, degeneration/necrosis, and renal
tubular fatty change.
5 and 10 mg/kg b.w./day (3 rats/sex necropsied) - No clinical, gross
or microscopic changes reported at 3 months.
The following observations were reported for rats from 3 months
up to and including the 26 month sacrifice:
25 mg/kg b.w./day - One female died at 51 weeks with no drug-related
changes noted. All 13 remaining rats were sacrificed by 73 weeks due
to palpable abdominal masses. At necropsy all rats had multiple
hepatic nodules. Ten of 13 rats were diagnosed microscopically to
have benign nodular hyperplasia, while the remaining 3 rats were
determined to have malignant transformation based on metastatic foci
observed in other organs (lung, kidney and lymph nodes).
10 mg/kg b.w./day - One rat died after 67 weeks with
reticuloendothelial neoplasia, a common tumour of aged rats. The
remaining 13 rats were sacrificed between the 64th and 112th weeks.
Eleven of these were observed to have hepatic benign nodular
hyperplasia.
5 mg/kg b.w./day - One male died at 20 weeks due to pulmonary
abscesses. The remaining 13 died or were sacrificed between the 61st
and 112th weeks. Five or these were observed to have hepatic benign
nodular hyperplasia.
Controls One male was sacrificed at 33 weeks with a forestomach
papilloma and pulmonary atelectasis and a second male died at 93 weeks
with myocarditis, nephrosclerosis and peribronchitis. The remaining
animals were sacrificed between weeks 80 and 112 of the study. No
animals in the control group were observed to have hepatic nodular
hyperplasia. The authors suggested that the observed nodular
hyperplasia in treated groups was associated with hepatic injury and
concluded that the rats failed to tolerate carbadox at any of the
tested dose levels. A treatment-related increase in total tumours was
also reported for carbadox-treated rats. The tumour incidence was
lower in the high-dose group, however mortality occurred earlier in
this group than in other treatment groups (Stebbins & Coleman, 1967).
A follow-up study was performed to determine a chronic carbadox
level tolerated by rats. Sixty male Charles River rats (124-173 gms)
and 60 female rats (107-152 gms) of the same strain were divided into
3 groups (20/sex/dose) and received 2.5, 1.0, or 0 mg/kg b.w./day of
carbadox in the diet. Signs, weights and food consumption were
recorded weekly and drug levels were adjusted accordingly.
Haematology, complete urinalysis, and ophthalmoscopic examination were
done at 3, 6, 12, 18, and 24 months on 5 rats/sex/dose. An interim
sacrifice of 5 rats/sex/dose was conducted at 54 weeks with the
remaining rats sacrificed at 106 weeks. Each rat received a gross
necropsy and microscopic examination of standard tissues including:
brain, spinal cord, eye, sub-maxillary gland, thyroid gland, thymus,
heart, aorta, lung, sternum, liver, spleen, pancreas, kidney, adrenal,
stomach, mesenteric lymph node, reproductive tract, urinary bladder,
femoral nerve, femoral bone marrow, and mammary gland with skin.
All parameters evaluated, including histopathologic examination,
were within normal limits at 54 weeks. Survival at 2 years was as
follows: control - 38%, 1.0 mg/kg b.w./day - 45%, 2.5 mg/kg b.w./day
- 43%. At the 2.5 mg/kg b.w./day level 7/27 rats displayed hepatic
benign nodular hyperplasia and 7/27 showed peliosis hepatis. At the
1.0 mg/kg b.w./day level 1/29 rats was found to have hepatic benign
nodular hyperplasia and 3/29 displayed peliosis hepatis. In the
control group 3/29 rats had benign nodular hyperplasia and 2/29 had
peliosis hepatis. Additionally, an increase in total mammary tumours
was reported in the 2.5 mg/kg b.w./day dose group. The authors
concluded that the 1.0 mg/kg b.w./day dose level was tolerated by the
rats for 2 years with no adverse effects, based on the similar
incidence of both hepatic lesions and tumours in other organs in
treatment and control groups (Sigler, 1969a).
Three groups of 28 rats (14/sex) were treated with a target dose
of 25 mg/kg b.w./day of carbadox, or one of two lots of desoxycarbadox
for 11 months to compare the oncogenic activity of carbadox and
desoxycarbadox. An equal sized group served as untreated controls.
The test material was given in the feed for 10 months. Two to four
rats were sacrificed at 30, 60, 90, 191, and 309 days. Animals were
observed daily for toxicity and mortality. Weights and food
consumption were recorded weekly until the 38th week and then every
other week. Rats received a gross necropsy at sacrifice and liver and
adrenals were evaluated histopathologically. A moderate decrease in
weight gain occurred in both sexes receiving carbadox. No other
significant clinical observations were made. At gross necropsy all
groups treated for 10 months showed evidence of hepatic changes
including necrosis and nodule formation. Changes in the carbadox-
treated group were less severe than in the desoxycarbadox-treated
group. Histopathologically all desoxycarbadox-treated rats showed
evidence of hepatocellular carcinoma, while 2/18 rats receiving
carbadox showed evidence of hepatocellular carcinoma. There was a
significant increase in adrenal cortical hemorrhage in both carbadox
and desoxycarbadox-treated groups (King, 1976).
The tumorigenicity of carbadox administered in various dosing
regimens was studied more recently in rats. A total of 119 Wistar
rats of each sex were divided into groups of various size (2 - 18
rats) following parturition and treated with various regimens of
carbadox i.p. (prior to weaning for 8 to 20 days) and/or in the feed
at 300 ppm until study termination at 1 year. Rats were examined and
weighed routinely during the study. Tumours were evaluated grossly
and histopathologically at the end of the study. Weight gains were
reduced. Animals receiving 300 ppm carbadox in feed all developed
hepatic tumours. Rats which received carbadox i.p. developed a higher
incidence of hepatic tumour than untreated controls. The largest
number of spontaneous and rare tumours occurred in groups receiving
carbadox via both routes (Sykora & Vortel, 1986).
2.2.3.2 Monkeys
A study was performed to assess the long-term toxicity of
carbadox in primates. Twenty-eight monkeys were divided into 4 groups
of 7 animals (3 or 4/sex) and were dosed with carbadox in gelatin
capsules 5 days/week. The doses were 20 (5 mg/kg QID), 10 (5 mg/kg
BID) or 5 mg/kg b.w./day and controls. A variety of parameters were
evaluated at 1, 3, 6, 12, and 24 months including haemoglobin,
haematocrit, RBC's, WBC's, clotting time, prothrombin time, complete
urinalysis, blood glucose, BUN, alkaline phosphatase, SGOT and SGPT.
Ophthalmoscopic examinations were performed at the same intervals. At
3 months and 2 years animals were sacrificed and necropsied. The diet
contained 0.2% isoniazid as prophylaxis for tuberculosis.
Elevated transaminase levels were detected at the 3 and 6 month
evaluations in both treated and control animals. The authors
concluded that monkeys tolerated 20 mg/kg/day for two years with no
adverse effect (Coleman, 1967).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation reproduction study with 2 litters per generation
was conducted using feed levels adjusted to provide target doses of
2.5, 1.0 and 10 mg/kg b.w./day. Feed levels were identical to those
used for a concurrent 2 year chronic study. Sixty female and 30 male
Charles River C-D rats were divided into groups of 20 females and 10
males per level for the 1st generation. Two females were placed with
each male for mating. First litter offspring were sacrificed
following a gross examination on lactation day 21. Following a 10 day
rest period the females were rebred to different males. Twenty females
and 10 males from the 2nd litter of each dose level were selected to
become the 2nd generation. These rats were mated at 100 days of age.
Dose levels were maintained and the 2nd and 3rd generation were
treated from weaning throughout the breeding period. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 4 days, number weaned,
litter weight at 24 hours, litter weight at weaning, sex at weaning,
viability index, lactation index, growth index, live birth index, and
gross abnormalities. In addition 10 weanlings/dose level from the 2nd
litter of the 3rd generation received an ophthalmoscopic examination,
gross necropsy, and a standard array of tissues were evaluated
microscopically. Although occasional differences from control group
parameters were noted, these were sporadic and not thought to be
treatment related. A reduced pregnancy rate was reported for all
groups of the third generation, with only the first litter of the
1.0 mg/kg b.w./day treatment group above 50%. Additionally a high
incidence of cannibalism (30 - 44%) occurred in treated groups of the
3rd generation as well as in the second litter of the untreated control
(19%). This cannibalism produced a non dose-related reduction in
lactation index in the treated groups of the 3rd generation
(59 - 79% in treated group litters vs 93 - 98% in controls). No
effects were reported in the first two generations. The authors
concluded that carbadox had no effect on fertility, lactation or
the neonate at any dose level (Sigler, 1969b).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with carbadox are shown in
Table 2.
2.2.6 Special studies on relay toxicity
2.2.6.1 Rats
A study was done to assess the possible risk of toxicity of pork
from pigs fed carbadox. To accomplish the study pigs weighing 60-80
kg were fed carbadox at the rate of 0, 20, and 200 ppm in their feed
for 30 days at which time they were slaughtered. Meat from these pigs
was divided into pieces of approximately 200 grams and frozen at -18
C.
Weanling Wistar rats were divided into groups of 12/sex/dose
(except the high dose which had 24 females). Rats received a dietary
supplement of 10% pork liver from swine fed the carbadox regimens
outlined above. This dietary regimen was continued for 23 - 25
months. All rats received gross necropsies and the following organs
were evaluated microscopically: liver, kidney, adrenal, testes, ovary,
and thyroid. Weights and mortality were recorded. There was no
effect on mortality. No treatment-related histologic changes were
reported (Ferrando et al., 1975).
2.2.6.2 Dogs
In another study 12 beagle dogs weighing about 4.5 kg were fed
for one month with 100 g of control pork as part of a diversified
diet. The dogs were then distributed into 3 treatment groups and
received pork from hogs treated with the above regimens of carbadox at
the rate of 100 gm/day for the first 5 months and then 200 gms for the
remainder of the treatment period (85 months). The dogs were weighed
periodically. Every fourth month haematology, clinical chemistry, and
urinalysis parameters were evaluated.
All dogs were in good health at sacrifice. The dogs received a
gross necropsy at study termination and a standard set of tissues were
evaluated histopathologically. No treatment-related effects were
reported in this study. The authors concluded that there is no danger
to the public health when carbadox is used in pig feed as a growth
promoter at 50 ppm (Ferrando, et al.,1978).
Table 2: Results of genotoxicity assays on carbadox
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 0.0004 - 0.125 Positive Pfizer, undated b
TA1535 (mg/plate)
TA1536, TA1537, Equivocal
TA1538, C340
Ames test TA100 3.1 - 61.7 Positive Beutin et al,
TA98 (+/- S9) (nM) 1981
Ames test TA100 2.5 - 15 µg/plate Positive Yoshimura et al,
TA98 (+/- S9) 1981
Ames test TA100, TA98 .1 umole/plate Positive Negishi, et al,
1980
Ames test TA100 0.001-.02 Positive Voogd et al.,
1980
Mutagenicity Ames E. coli WP2hcr Positive Ohta et al, 1980
test TA100+/-S9 5 - 50 µg/plate Positive
TA98 Positive
TA1538 Negative
TA1537 Negative
TA1535 Negative
Host mediated Assay S. typhimurium 0,1,2, 2.5, 5, 10, Negative Pfizer, undated b
TA1534, TA1952 0,1,2.5 5,7.5, Positive
10,12.5, 20 mg/kg
Repair test B. subtilis (rec) 1 - 100 µg/disc Positive Yoshimura et al.,
S. typhimurium (urv) 1981
Fluctuation test K. pneumoniae .00002 - Positive Voogd et al, 1980
E. coli K12 .005 mmoles/l
Table 2 (contd)
Test System Test Object Concentration Results Reference
Mutagenicity test S. cerevisiae D4 .02% Positive Voogd et al, 1980
Micronucleus test rat bone marrow 5 - 240 mg/kg i.p. Positive Cihak and Srb,
1983
Repair test B. subtilis M45 .1 - 100 Positive Ohta et al,
umole/disk 1980
Chromosomal damage mouse bone marrow 200 mg/kg acute, Positive Pfizer, undated b
10 mg/kg day seven
days
Chromosomal damage in vitro human 0 - 250 µg/ml Positive Pfizer, undated b
lymphocytes
Dominant lethal CD-1 mice 10,50,150,30 mg/kg Negative Pfizer, undated b
assay
QUINOXALINE-2-CARBOXYLIC ACID
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with
quinoxaline-2-carboxylic acid are given in Table 3.
Table 3: Acute toxicity of quinoxaline-2-carboxylic acid
LD50
Species Sex Route (mg/kg) Reference
Rats M Oral 4450 Briggs, 1971
Mice M Oral 1360
2.2.3 Long-term/carcinogenicity Studies
2.2.3.1 Mouse
Groups of 50 Charles River CD mice/sex/dose (100 in controls)
received quinoxaline-2-carboxylic acid in feed for 19 months in a
study of its chronic oral toxicity and tumorigenic potential. Fresh
diets were prepared weekly. Feed and water intake were monitored
weekly for the initial three months and monthly thereafter. Feed
levels of quinoxaline-2-carboxylic acid were adjusted at these
intervals to provide target doses of 0, 25, 50, and 100 mg/kg
b.w./day. Haematology and clinical chemistry were evaluated once,
prior to sacrifice. Necropsy was performed on all animals. Organ
weights were obtained for brain, heart, kidneys, liver, testes, and
ovaries. Histopathologic examination was performed on a standard
array of tissues.
No treatment-related effects were noted in any parameter examined
during the study. Cumulative mortalities were 20% and 15% for control
females and males, respectively, while treatment group cumulative
mortalities ranged from 10% to 20%. Incidence of all tumours was 33%
in female and 46% in male control groups, while the incidences in
treatment groups ranged from 32% to 42%. The authors concluded that
oral administration of quinoxaline-2-carboxylic acid to mice for 19
months produced no evidence of toxicity (Faccini et al., 1979).
2.2.3.2 Rats
Nine male and 9 female Charles River C-D rats were divided into
groups of 3/sex/dose level. Rats received quinoxaline-2-carboxylic
acid in the feed for 2 years. Weight and food consumption were
recorded weekly through week 30 and monthly thereafter. Drug levels
were adjusted to provide target doses of 100, 50 or 0 mg/kg b.w./day.
Rats received clinical examinations weekly and ophthalmoscopic
examinations on days 0, 30, 92, 186, 365, 554, and 733. Haematology
and urinalysis parameters were evaluated on days 31, 93, 182, 274,
370, 461, 547, 644, and 730. Terminal sacrifice was performed on day
735 of the study. All rats received gross necropsies and standard
tissues were evaluated microscopically from each rat. No treatment-
related changes were reported. The authors concluded that
quinoxaline-2-carboxylic acid was tolerated at up to 100 mg/kg
b.w./day when given to rats via feed (Coleman, 1971).
An additional study was conducted to determine whether
quinoxaline-2-carboxylic acid has tumorigenic potential. To
accomplish the study quinoxaline-2-carboxylic acid was administered in
the diet of Charles River Sprague-Dawley rats (20/sex/dose level) for
two years to provide target doses of 0, 10, 25, and 50 mg/kg b.w./day.
Weight and food consumption were monitored weekly and drug
concentration adjusted accordingly. Rats were examined for clinical
signs weekly. Ophthalmologic examinations were performed on all rats
prior to treatment and at 6, 12, 20 and 24 months. Clinical
chemistry, haematology and urine parameters were evaluated in 5 rats
at 6 and 12 months, 2-4 rats at 18 months, and all rats at
termination. At 12 months 5 rats/sex/dose and at 24 months all
remaining rats were sacrificed and received a gross necropsy and
tissues were obtained for microscopic evaluation. Organ weights were
determined for heart, liver, lungs, kidneys, gonads, adrenal, spleen,
and brain. A standard array of tissues was examined
histopathologically.
No treatment-related effects were noted on any of the parameters
evaluated. Cumulative incidences of tumours (based on summing males
and females with 40 rats/dose) occurring in treatment groups for
selected organs of interest are summarized in Table 4. It was
concluded that quinoxaline-2-carboxylic acid in doses of 10, 25, and
50 mg/kg b.w./day over a 2 year period to rats does not produce any
toxicity or elevated tumor incidence (Pfizer, 1971).
Table 4: Cumulative incidence of tumours (males and females combined)
after treatment of rats with quinoxaline-2-carboxylic acid
Treatment Group Mammary gland Pituitary Pancreas Liver
Control 28% 30% 3% 5%
10 mg/kg b.w./day 18 23 10 5
25 mg/kg b.w./day 13 28 3 5
50 mg/kg b.w./day 33 28 3 3
A follow-up chronic study was performed using rats obtained from
the 3-generation study F1 litter. An additional high dose level, 100
mg/kg b.w./day, was added for this study. To accomplish the study
Charles River C-D rats were divided into groups of 50/sex/dose and
received 100, 50, 25, or 0 mg/kg b.w./day of quinoxaline-2-carboxylic
acid in the diet for 29 months. In addition 10 rats/sex in the
control and 100 mg/kg b.w./day groups were added for clinical
chemistry studies at 12 months. Weights were monitored weekly. Food
consumption was recorded weekly during the first 5 months, twice
during the 6th month, and monthly thereafter, and treatment levels
were adjusted accordingly. Clinical examinations were performed
daily. Clinical chemistry parameters were evaluated at 6, 12, and 29
months. Haematology was evaluated at the conclusion of the study.
All rats received a gross necropsy. Liver and kidneys were weighed
from rats sacrificed at 12 months and heart, liver, kidneys, brain,
and testes were weighed during the gross necropsies at 29 months.
Tissues from many organs showing macroscopic abnormality plus a
standard array of grossly normal tissues were evaluated
microscopically.
The general health of the rats was unaffected by treatment. An
outbreak of sialoacryoadenitis occurred between ages 89 and 97 days.
Slightly reduced weight gain (3-5%) was noted in the high-dose groups
in both sexes. The cumulative mortality for controls was 56% in males
and 70% in females, while treatment group cumulative mortality ranged
from 46% to 74%. There was no difference in the incidence of
nonneoplastic lesions among the control and treatment groups, nor did
treatment shorten the lifespan of treatment groups. Treatment of rats
for 2 years did not produce an increase in the incidence of animals
with malignant or benign tumours compared to the control group. The
authors concluded that the NOAEL was 100 mg/kg b.w./day (King, 1979a).
2.2.4 Reproduction studies
2.2.4.1 Rats
The potential reproductive toxicity of quinoxaline-2-carboxylic
acid was studied in rats. The study was performed in conjunction with
a 2-year chronic study. Treatment route was via feed. The study
utilized 2 litters per generation for 3 generations with twenty
rats/sex/dose level using target doses of 25, 50, and 100 mg/kg
b.w./day. The F0 rats were bred following approximately 60 days of
treatment with quinoxaline-2-carboxylic acid. Fifty rats/sex/dose
were selected for the concurrent chronic study from the initial F1
litter. Following a 7 day rest period the females were rebred to
different males to produce the second litter. The subsequent first
litters of each generation were sacrificed at weaning while the second
litters were used to constitute the next generation. These first
litters were reduced to 10 pups/litter (5/sex if possible) for
rearing, then sacrificed, and necropsied at weaning. The 2nd litters
were used to produce the next generation. Subsequent generations of
rats were mated at 100 days of age. Dose levels were maintained and
test animals were treated from weaning throughout the breeding period.
Rats were inspected daily. Food consumption and weights were
determined weekly except for mating periods. Adults of all
generations were sacrificed after weaning of the 2nd litter and
received a gross necropsy with liver weights determined. Blood levels
were determined in F0 rats (5/sex/level) just prior to sacrifice.
Pups were examined daily until weaning and weighed on days 1, 4, and
21 after birth. Gross necropsies were carried out on all pups that
died or were stillborn and on 5 weanlings/sex/dose. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 1, 4, and 21 days, number
weaned, sex ratios, mean pup weights, lactation index, and gross
abnormalities. No treatment-related alterations were observed in any
parameter monitored during the study (King, 1979b).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rabbits
The potential embryotoxicity or teratogenicity of quinoxaline-2-
carboxylic acid was also studied in the New Zealand White Rabbit.
Rabbits were randomly assigned to 4 treatment groups of 19 - 20
animals receiving 0, 25, 50, or 100 mg/kg b.w. quinoxaline-2-
carboxylic acid via gavage on gestation days 7-18. Animals were
observed daily for toxicity and weighed on gestation days 0, 3, 7, 9,
12, 15, 18, 21, 24, and 28. Rabbits were sacrificed on day 28 and the
number of corpora lutea and number and position of live and dead
implants in the uteri were recorded. All fetuses were examined for
external malformations and weighed. Half the fetuses were cleared,
stained with alizarin red and examined for skeletal abnormalities.
The remaining half were fixed in Bouin Allen fluid and examined by
serial sectioning. No treatment related maternal toxicity,
embryotoxicity or malformations were observed (Holmes, 1976a).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with quinoxaline-2-carboxylic
acid are given in table 5.
Table 5: Quinoxaline-2-carboxylic acid genetic toxicology
Test System Test Object Concentration Results Reference
Ames test S. typhimurium .008, .032, Negative Pfizer,
TA1535 .125, .5, 1975
TA1537 2 mg/plate
TA1538
TA 1535+S9 .16, .63, Negative Pfizer,
2.5 mg/plate 1975
Chromosomal Human 50, 100 Negative Pfizer,
aberrations lymphocytes µg/ml 1975
in vitro
DESOXYCARBADOX
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
A long term study was performed to determine the tumorigenic
potential of desoxycarbadox, a carbadox metabolite. Four hundred
Charles River C-D rats (5 weeks of age) were divided into groups of
50/sex/dose level. Rats were distributed randomly according to weight
levels. Desoxycarbadox was administered at target doses of 0, 5, 10,
and 25 mg/kg b.w./day continuously in the diet. Weights were recorded
weekly. Food consumption was monitored weekly the first 3 months and
monthly thereafter. Treatment levels were adjusted accordingly.
Although treatment was planned for 2 years, the test material was
withdrawn from all rats in the 25 mg/kg b.w./day group and 50% of the
rats in each sex in the other 2 treatment groups on day 350, due to
high morbidity and mortality. Administration of desoxycarbadox was
stopped completely on day 416. The study was terminated after 447
days.
Clinical examinations were performed twice weekly. A number of
treatment-related signs were observed. These included: tumours in
the mammary region of both sexes, small cutaneous nodules, and
enlargement of the liver with nodules preceded by weight loss and
polyphagia. There was also a dose-related decrease in weight gain and
a dose-related decrease in survival rate.
Clinical chemistry parameters were evaluated. Desoxycarbadox
administration resulted in dose-related increases in plasma enzyme
activities, urea, and bilirubin. The abnormalities were consistent
with hepatic disorders. The high degree of variability from animal to
animal and lack of reversibility following drug withdrawal indicates
a correlation with tumour progression. A dose-related hypoglycaemia
was noted in both sexes. Haematologic parameters were evaluated in
ten rats/sex/dose at day 413 and the remainder at 447 days. The two
primary changes in haematology in the treated rats were a moderate
hypochromic microcytic anaemia in the 25 mg/kg b.w./day group, a
slight anaemia in the 10 mg/kg b.w./day group, and a neutrophilia in
the 25 mg/kg b.w./day group.
All rats received gross necropsies. Selected organs were
weighed. Histopathologic examination was performed on all grossly
abnormal tissue and routinely on a standard array of tissues. There
was a dose-related increase in pigmentation of renal tubules and
nephrosis. The major pathologic findings related to increased tumour
incidences are summarized in Table 6. The authors concluded that
desoxycarbadox was a potent hepatocarcinogen in the rat and that there
were dose-related increases in other tumours (Reinert, 1976).
Table 6: Incidence of tumours after administration of desoxycarbadox
to rats
Treatment Hepatic Subcutaneous Haemanigiomas Mammary
Group tumours fibromas tumours
Males and females combined Females only
Control 0% 0% 0% 12%
5 mg/kg 75 2 0 24%
b.w./day
10 mg/kg 95 10 5 28
b.w./day
25 mg/kg 100 18 47 27
b.w./day
2.2.5.2 Special Studies on Genotoxicity
The results of genotoxicity studies with desoxycarbadox are
summarized in Table 7.
Table 7: Desoxycarbadox genetic toxicology
Test System Test Object Concentration Result Reference
Ames test TA1535 .0008, .0032, Negative Pfizer, 1975
TA1537 .0125, 0.05, .125,
TA1538 .5, 2, 20 mg/plate
TA1535+S9 .0125, .05, .2, 1, Negative Pfizer, 1975
5 ml/plate
Ames test TA1535 .032, .25, .4, .5 Negative Pfizer, 1975
TA1537 ml/plate plasma;
TA1538 .032, .25. .5 ml/plate
urine1
Host mediated TA1950 500 mg/kg acute Negative Pfizer, 1975
assay
Chromosomal damage Human lymphocytes 200 µg/ml Negative Pfizer, 1975
Rat bone marrow 200 mg/kg acute, Negative Pfizer, 1975
or 100 mg/kg 5 days
10 mg or 25 mg per kg Positive Pfizer, 1975
9 months in feed
Host mediated TA1950 400 or 500 mg/kg Negative Pfizer, 1975
assay in mice
Host mediated TA1950 300 mg/kg with 100 mg/ Negative Pfizer, 1975
assay in rats kg/day previous 31 days
TA1535 200 mg/kg Negative
Table 7 (contd)
Test System Test Object Concentration Result Reference
Yeast assay S. cerevisiae D3 10 mg/ml Negative Holmes, 1976b
& D4
Host mediated TA1950 400 mg/kg +DMNA Negative Holmes, 1976b
assay (mouse)
Ames test TA1537 .01 - 10 mg/plate Negative Holmes, 1976b
TA100
TA98
Ames test TA1535+/- TA100 .005 - 2 mg/plate Positive Holmes, 1976b
S9(rat)
Ames test TA1537+S9 .005 - 2 mg/plate Negative Holmes, 1976b
TA98
Ames test TA1535+S9 from .005 - 2 mg/plate Holmes, 1976b
rat
mouse Positive
hamster Positive
dog Negative
monkey Negative
Negative
Ames test TA1535 Quantitative plate Negative Holmes, 1976b
assay on plasma, liver
& urine at selected
intervals from chronic
study rats fed carbadox
or desoxy-carbadox
Cell transformation BALB/C Swiss 3T3 .1-10 µg/ml Positive Holmes, 1976b
test
1 Urine and plasma collected from mice or rats administered 400 mg/kg desoxycarbadox administered
in 3 hourly doses or 100 mg/kg/day for 5 days.
METHYL CARBAZATE
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
Methyl carbazate is a metabolite of carbadox. The chronic oral
toxicity of methyl carbazate was studied in the rat. Wistar rats were
divided into groups of 12/sex/dose and received methyl carbazate at
target doses of 1 and 10 mg/kg b.w./day in the feed for 21 months.
The colony served as controls. Food consumption was determined every
2 days and weights every 2-3 weeks. Necropsy was performed on all
animals. Organ weights were determined for liver, kidney, adrenals,
testes, and thyroid. Histology was performed on liver, kidneys,
adrenals, thyroid, testes, ovaries, and tissues containing tumours
observed grossly. Three males and 3 females in the high dose group
died before termination. No low dose rats died prior to termination.
No histopathologic evidence of toxicity or evidence of an elevation in
tumours was reported. The authors concluded that administration of
methyl carbazate to rats at dose levels of 1 and 10 mg/kg b.w./day in
the diet produced no evidence of toxicity or carcinogenic potential
(Rutty, 1972).
In a second study weanling Wistar rats were divided into groups
of 24/sex and treated with target doses of 0, 2.5, 5, and 10 mg/kg
b.w./day methyl carbazate in the feed for 710 days. Weights were
determined weekly and food consumption weekly the first 3 months and
monthly thereafter. Drug concentrations were adjusted accordingly.
Clinical examinations were conducted weekly. Blood samples for
clinical chemistry were obtained at terminal sacrifice and from
moribund animals. Haematology was also conducted on these samples and
at 12 months on 6 rats. Rats were sacrificed and necropsied at 710
days. An interim sacrifice was also performed at 12 months. All
moribund and dead rats also received a gross necropsy. Histopathology
was performed on all gross lesions and the following organs: liver,
pituitary, thyroid, parathyroid, adrenal, lung, kidney, urinary
bladder, ovary, and testis.
There were no treatment-related effects noted in any parameters
evaluated in the study. There was histologic evidence of widespread
chronic respiratory disease in all groups. It was concluded that
methyl carbazate had no adverse effect when given to rats in the diet
for 2 years (Ferrando, 1980).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with methyl carbazate are
summarized in Table 8.
Table 8: Genetic toxicity studies with methyl carbazate
Test System Test Object Concentration Results Reference
Ames test TA1535 .0005, .002, Negative Pfizer, 1975
TA1537 .008, .032,
TA1538 .125, 0.5, 2, 5
mg/plate
TA1535+S9 .0125, .05, .2 Negative Pfizer, 1975
1, 5, 10
mg/plate
Host- TA1950 50, 100, 300 Equivocal Pfizer, 1975
mediated mg/kg
assay
Chromosomal Human 50, 100 Negative Pfizer, 1975
damage lymphocytes µg/ml
in vitro
Ames test TA1535+S9 0.5 - 10 Negative Holmes, 1976b
mg/plate
HYDRAZINE
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism, and excretion
Hydrazine toxicokinetic parameters have been determined in the
rabbit. Rabbits received a bolus of 12 mg/kg b.w. Parameters were
derived using a one-compartment model, and spectrophotometric serum
analysis for hydrazine, with 5 rabbits/group. The serum half-life was
2.3 hours and the volume of distribution was 0.63 liters/kg (Keller
et al., 1981).
The metabolism, distribution, and elimination of hydrazine in
rats was studied using 15N-labelled hydrazine or a spectrophotometric
method. About 15% of the 15N label was found as N2. About 20% and
30% of the infused hydrazine was recovered in the urine as acetylated
metabolite and hydrazine, respectively. The serum half-lives were
determined, using a two-compartment model, to be 0.74 hours for the
majority of the hydrazine elimination, and 26.9 hours for a much
slower, smaller elimination component (Dost et al., 1981).
2.1.3 Effects on enzymes and other biochemical parameters
Hydrazine-induced short-term biochemical parameter changes were
investigated following oral administration of hydrazine in Sprague-
Dawley rats. Groups of 5 rats were given doses of hydrazine (0-200
mg/kg b.w.). Some rats received pyruvate azine, phenobarbital,
piperonyl butoxide, or diethylmaleate to more clearly elucidate the
hepatic effects of hydrazine. Hydrazine caused a dose-related
increase in liver triglycerides and liver weight and a decrease in
hepatic glutathione. The threshold dose for toxicity was about 10
mg/kg b.w. While liver weight and glutathione alterations were
evident within 30 minutes, triglyceride alteration was not evident for
4 hours. Pretreatment with phenobarbital decreased toxicity, while
pretreatment with piperonyl butoxide increased toxicity suggesting
hepatotoxicity is due to the parent - not a metabolite. Depletion of
hepatic glutathione was without effect on hepatic toxicity, and
studies with pyruvate azine - a possible oxidative metabolite - also
supported this conclusion (Timbrell, et al., 1982).
Hydrazine-induced short-term histologic and ultrastructural
changes were investigated following oral administration of hydrazine
to Sprague-Dawley rats. Groups of 5 rats were given doses of
hydrazine (0-200 mg/kg b.w.). Some rats received pyruvate azine,
phenobarbital, piperonyl butoxide, or diethylmaleate to more clearly
elucidate the hepatic effects of hydrazine. A dose of 20 mg/kg caused
accumulation of lipid, swelling of mitochondria, and the appearance of
microbodies in periportal and midzonal hepatocytes. Accumulation of
lipid droplets and mitochondrial swelling were detected by electron
microscopy 30 minutes after dosing. Piperonyl butoxide or
phenobarbital treatment increased or decreased fatty liver
respectively, while pyruvate azine was much less effective than
hydrazine in producing this effect (Scales & Timbrell, 1982).
2.2 Toxicological studies
2.2.3 Long-term carcinogenicity studies
2.2.3.1 Mice
Oral administration of hydrazine sulfate to Balb/c female mice at
1.13 mg/day for 46 weeks produced a 100% incidence of lung tumours
(Biancifiori & Ribacchi, 1962).
In another study hydrazine was administrated via gavage to 25
female Swiss mice 5 days/week for 40 weeks at the rate of 0.25 mg/day.
Eighty-five mice served as controls. This dose regimen produced a 46%
incidence of lung tumours in treated mice vs 10% in controls (Roe et
al., 1967).
A study with CBA/Cb/Se mice was performed using hydrazine sulfate
given for 36 weeks by gavage. Twenty-one 8- week-old rats/sex
received 1.13 mg/day. Treated rats had an incidence of 76% and 90%
lung tumours in males and females, respectively, while controls had
3%. Hepatomas were found in 62% of the males and 71% of the females
treated with hydrazine, while 11% of control males and 4% control
females had hepatomas (Severi & Biancifiori 1968).
In another study in mice, 8-week-old CBA mice were divided into
groups of 40-59 mice/sex. Hydrazine sulfate was given by gavage daily
for 150 days at rates of 45, 22, 11, 5.6 and 0 mg/kg b.w./day. Mice
were examined at natural death or following sacrifice when moribund.
Control male mice had a hepatoma incidence of 10% while females had a
3.4% rate. Treated males had hepatoma rates of 60, 48, 28, and 3.8%
at 45, 22, 11, and 5.6 mg/kg b.w./day, respectively. Treated females
had 62.5, 66.6, 8 and 0% hepatomas, respectively. The author reported
many lung tumours in treated groups but did not provide the incidence
rates (Biancifiori, 1970).
2.2.3.2 Hamsters
Eight-week old golden hamsters were divided into 3 groups of 23,
35 and 56 animals which were administered 60 doses of 3.0 mg hydrazine
sulfate via intubation over a 15 week period, 2.8 mg 100 times during
a 20 week period or no hydrazine, respectively. Hepatic lesions
reported include: Fibrosis, reticuloendothelial cell proliferation,
and bile duct proliferation. Hepatic lesions were found in 60-80% of
treated hamsters but in none of the controls. No hepatic tumours were
reported (Biancifiori, 1970).
Negative tumorigenicity results were also obtained in a follow up
chronic study with golden hamsters given 2.3 mg/day over a lifetime.
In this study 50 hamsters/sex received hydrazine in drinking water
from the age of 9 weeks for a lifetime (Toth, 1972).
2.2.3.3 Rats
A chronic oral study was performed in conjunction with the above
mouse study using 8 week old Cb/Se rats. Aqueous hydrazine sulfate
was administered daily by stomach tube at doses of 18 mg/rat to 14
males and 12 mg/rat to 18 females for 68 weeks. Lung tumours were
found in 21 and 27% of dosed male and female rats, respectively.
Control groups of 28 males and 22 females had no lung tumours.
Hepatic tumours were found in 30% of males, while no hepatic tumours
were found in females or controls (Severi & Biancifiori, 1968).
2.2.7.5 Special studies on genotoxicity
The results of genotoxicity studies with hydrazine are summarized
in Table 9.
Table 9: Genetic toxicity studies with hydrazine
Test System Test Object Concentration Results Reference
Bacterial E. coli WP2 0.5 - 2.0 µmole Positive von Wright
uvr A trp & Tikkanen,
1980
Mouse L5178Y 0.1 - 5 mmole Positive Rogers &
lymphoma Back, 1981
cells
Ames test S. typhimurium Positive Ames, 1971
2.3. Observations in humans
No information available
3. COMMENTS
The Committee considered data from short-term, long-term and
"relay" toxicity studies, and from studies on carcinogenicity,
mutagenicity, and reproduction with carbadox, together with data from
mutagenicity and long-term toxicity studies of its metabolites.
The metabolism of carbadox has been studied in rats, monkeys and
pigs using [14C]carbadox, labelled in either the phenyl ring or the
carbonyl group of the side-chain. The metabolism of carbadox was
characterized by the rapid reduction of the N-oxide groups, the
cleavage of the methylcarbazate side-chain, and the liberation of
respired CO2. The primary metabolite in the urine was quinoxaline-2-
carboxylic acid, which was also excreted in conjugated form. The
detectable residues in tissues, up to 24 hours after drug withdrawal,
were carbadox, desoxycarbadox, quinoxaline-1,4-di-N-oxide-2-
carboxaldehyde and quinoxaline-2-carboxylic acid. Hydrazine was a
minor metabolite but would be expected to be present only for a short
time before undergoing further metabolism.
The concentration of carbadox and desoxycarbadox in the tissues
declined rapidly (half-life about 6 hours), and, after 3 days, was
less than 5 µg/kg. In a study in pigs, in which carbonyl-labelled
[14C]carbadox was administered by gavage, the concentration of total
residues in liver at 5 days withdrawal time corresponded to 0.12 mg/kg
methyl carbazate equivalent. However, some of this radioactivity was
due to the presence of amino acids, which had been labelled by
incorporation of 14CO2 when ring-labelled [14C]carbadox was
administered to pigs, extremely low levels of unidentified metabolites
remained in the liver at withdrawal periods longer than 7 days. These
residues were partially released and converted to quinoxaline-2-
carboxylic acid by alkaline digestion of the liver.
The Committee also reviewed recent reports of the effect of
carbadox on adrenal function in pigs, although the information did not
effect the outcome of the evaluation.
Several long-term feeding studies in rats were evaluated which
demonstrated dose-related increases in the incidence of benign and
malignant liver tumours at doses of carbadox above 1.0 mg per kg of
body weight per day. Doses above 25 mg per kg of body weight per day
produced pronounced toxic effects which precluded chronic treatment.
Data from a variety of mammalian and non-mammalian genotoxicity
studies were also reviewed. Positive findings were reported in 14 of
the 15 tests carried out. The Committee concluded that carbadox
appears to be both genotoxic and carcinogenic.
The Committee evaluated a "relay" toxicity study in which pigs
were fed carbadox at up to 200 mg/kg in the diet for 30 days and
killed at zero withdrawal time, their livers were fed to rats as a 10%
dietary component for 2 years. Although this study was conducted
satisfactorily and no treatment-related effects were reported, the
Committee did not use it for assessing the carcinogenicity of carbadox
residues because of the number of animals used and because only small
quantities of residues can be consumed by rats in this type of study.
In a long-term study in rats, desoxycarbadox was reported to
produce an increase in the incidence of tumours. All rats that
received 25 mg per kg of body weight desoxycarbadox daily for 10
months in the diet developed hepatic tumours. The incidence of
tumours was increased in all treated groups, at doses of 5-25 mg per
kg of body weight per day. While the most pronounced change occurred
in the liver, tumour incidence was also elevated at other sites,
including the skin and mammary glands.
The mutagenic potential of desoxycarbadox was investigated in a
range of studies. The Committee noted that, while desoxycarbadox
produced negative results in most mutagenicity test systems, positive
findings were recorded in the cell transformation test and in the Ames
test using liver microsomes from rats treated with polychlorinated
biphenyls. The Committee noted that the tumorigenic potential of
desoxycarbadox was apparently greater than that of the parent
compound, and that desoxycarbadox would therefore probably make a
significant contribution to the tumorigenic activity of carbadox in
rats. However, the Committee also noted that the metabolism data for
carbadox supported the conclusion that desoxycarbadox was a relatively
short-lived intermediate between carbadox and quinoxaline-2-carboxylic
acid.
The Committee also considered information on the carbadox side-
chain metabolite, methyl carbazate. This information consisted of
results from two long-term studies in rats and a number of
mutagenicity assays. In the more informative long-term study (in
which more animals per dose and concurrent controls were used), rats
received methyl carbazate in the diet for 710 days. There was no
increase in the incidence of tumours and no other treatment-related
effects were observed at 10 mg per kg of body weight per day, which
was the highest dose tested. The lack of effect limited the
usefulness of this study. The no-observed-effect level for the study
was 10 mg per kg of body weight per day. The useful mutagenicity
studies were reported to be negative.
The results of studies on the pharmacokinetics, mutagenicity, and
carcinogenicity of hydrazine were also considered by the Committee.
Pharmacokinetic data from rats and rabbits as well as hydrazine's
known chemical reactivity suggest that it should be rapidly
eliminated. The structure of carbadox and its known metabolic
pathways indicate that hydrazine is probably a further metabolite of
methyl carbazate. Hydrazine showed mutagenic and carcinogenic
potential.
In evaluating the potential toxicity of residues of carbadox, the
Committee also considered toxicity data on the carbadox metabolite
quinoxaline-2-carboxylic acid to be useful. In a carcinogenicity
study in mice in which quinoxaline-2-carboxylic acid was administered
in the diet for 19 months, no treatment related increases in tumour
incidence or any other effects on the animals were reported. The no-
observed-effect level was 100 mg per kg of body weight per day.
Several chronic toxicity and carcinogenicity studies in which
quinoxaline-2-carboxylic acid was administered for 24 months were
conducted in rats. There were no effects on the incidence of tumours,
the only findings being a slight reduction in weight gain at 100 mg
per kg of body weight per day. The no-observed-effect level was 50 mg
per kg of body weight per day.
In a three-generation reproduction study in rats, quinoxaline-2-
carboxylic acid was given in the diet at levels of up to 100 mg per kg
of body weight per day. No treatment-related effects were observed.
Developmental studies were conducted in rats and rabbits in which the
compound was administered by gavage at levels of up to 100 mg per kg
of body weight per day. There was no evidence of maternal toxicity,
embryotoxicity, or teratogenicity in either species. The no-observed-
effect levels in these studies were 100 mg per kg of body weight per
day.
4. EVALUATION
With current analytical procedures, quinoxaline-2-carboxylic acid
is the only carbadox metabolite that can be identified in liver from
pigs treated according to good practice in the use of veterinary
drugs. However, uncertainty remains because of the presence of
unidentified residues in liver. Because of the genotoxic and
carcinogenic nature of carbadox and some of its metabolites, the
Committee was not able to establish an ADI.
On the basis of data from studies on the toxicity of quinoxaline-
2-carboxylic acid, and on the metabolism and depletion of carbadox and
the nature of the compounds released from the bound residues, the
Committee concluded that residues resulting from the use of carbadox
in pigs were acceptable, provided that the recommended MRLs are not
exceeded.
5. REFERENCES
AMES, B. (1971). Chemical Mutagens, Vol. 1, Hollaender, A. (ed),
Plenum Press, New York. p. 267.
BEUTIN, L., PRELLER, E., & KOWALSKI, B. (1981). Mutagenicity of
Quindoxin, Its Metabolites, and Two Substituted Quninoxaline-Di-N-
Oxides. Antibacterial Agents and Chemotherapy, 20, 336-343.
BIANCIFIORI, C., & RIBACCHI, R., (1962). Pulmonary Tumour in Mice
Induced by Oral Isoniazid and its Metabolites. Nature, 194, 488-
489.
BIANCIFIORI, C. (1970). Hepatomas in CBA/Cb/Se Mice and Liver Lesions
in Golden Hamsters Induced by Hydrazine Sulfate. J. Nat. Cancer
Inst, 44, 943-953.
BRIGGS, G.B. (1971). Safety Evaluation of CP-16505, Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
CIHAK, R., & SRB, V. (1983). Cytogenetic Effects of Quinoxaline-1,4-
dioxide-type Growth-promoting Agents. Mutat. Res., 116, 129-135.
COLEMAN, G.L., (1967). Two year Chronic Study of GS-6244 (carbadox)
in Monkeys. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
COLEMAN, G.L., (1971). Quinoxaline-2-Carboxylic Acid, First Two Year
Study in Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
DOST, F.N., BRODERICK, D.J., KRIVAK, B.N., & REED, D.J., (1981).
Metabolism of Hydrazine. AFAMRL-TR-81-26. Technical Report. Air
Force Aerospace Medical Research Laboratory, Wright-Patterson AFB, OH.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
FACCINI, J.M., PERRAUD, J., NACHBAUR, J., & MONRO, A.M., (1979). 19-
Month toxicity Study in Mice. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1975).
Application of the Toxicity by Relay Method for the Assessment of
Safety to Human Consumers of Carbadox, a Growth-Promoting Additive, to
the Feed of Pigs Slaughtered for Human Consumption. Toxicology, 3,
369-398.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1978). Human
Consumer Safety in the Use of Carbadox, a Pig Feed Additive, as
Estimated by Seven Years of Relay Toxicity in Beagles. Toxicology,
11, 167-183.
FERRANDO, R., (1980). Two Year Chronic Toxicity Study in the Rat with
Methyl Carbazate. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
FIGDOR, S.K., (1969). Carbadox in Pigs Radiotracer Metabolism C-14,
Carbonyl. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
FIGDOR, S.K., MCILHENNY, H.M., & VON WITTENAU, M.S., (1969).
Metabolism of Carbadox Using Carbadox Labelled with C14 in the Phenyl
Ring. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
HOLMES, C.L., (1976a). Action of CP-16,505 on Pregnancy and Fetal
Development in Rabbits. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
HOLMES, C.L., (1976b). Addendum Genetic Toxicology Report CP-17,056
(Desoxycarbadox) Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KELLER, W.C., MURPHY, J.P., ANDERSEN, M.E. & BACK, K.C., (1981).
Percutaneous Toxicokinetics of Hydrazine and H70 in the Rabbit.
AFAMRL-TR-81-13. Technical Report. Air Force Aerospace Medical
Research Laboratory, Wright-Patterson AFB, OH. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
KING, T.O., (1976). Target Organ Toxicity of GS-6244 (carbadox) and
CP-17,056 (desoxycarbadox) with Chronic Administration in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
KING, T.O., (1979a). Chronic Study in Rats with Quinoxaline-2-
Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KING, T.O., (1979b). Three Generation Study in Rats with Quinoxaline-
2-Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
NEGISHI, T., TANAKA, K., AND HYATSU, H., (1980). Mutagenicity of
Carbadox and Several Quinoxaline 1,4-Dioxide Derivatives. Chem.
Pharm. Bull., 28, 126-128.
OHTA, T., MORIYA, M., KANEDA, K., WATANABE, T., MIYAZAWA, A., &
SUSIGYAMA, F., (1980). Mutagenicity screening of Feed Additives in
the Microbial System. Mutat. Res., 77, 21-30.
PFIZER (undated a). Acute Toxicity of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (undated b). Genetic Toxicology of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (1963). A thirty-day Subacute Toxicity Study of GS-6244 in
Albino Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
PFIZER (1971). Two Year Study with CP-16,505 (Quinoxaline-2-
Carboxylic Acid) in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
PFIZER (1975). Genetic Toxicology Report CP-17,056 (Desoxycarbadox)
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
REINERT, H. (1976). Desoxycarbadox: Two Year Toxicity Study in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
ROE, F.J., GRANT, G.A., & MILLICAN, D.M., (1967). Carcinogenicity of
Hydrazine and 1,1-Dimethylhydrazine for Mouse Lung. Nature, 216,
375-376.
ROGERS, A.M., & BACK, K.C., (1981). Comparative Mutagenicity of
Hydrazine and 3 Methylated Derivatives in L5178Y Mouse Lymphoma Cells.
Mutat. Res., 89, 321-328.
RUTTY, D.A., (1972). Twenty-one Month Chronic Toxicity Study of
Methyl Carbazate in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
SCALES, M.D., & TIMBRELL, J.A., (1982). Studies on Hydrazine
Hepatotoxicity. 1. Pathological Findings. J. Toxicol and Environ.
Health, 10, 941-953.
SEVERI, L., & BIANCIFIORI, C. (1968). Hepatic Carcinogenesis in
CBA/Cb/Se Mice and Cb/Se Rats by Isonicotinic Acid Hydrazine and
Hydrazine Sulfate. J. Nat. Cancer Inst., 41, 332-349.
SIGLER, F.W., (1969a). A Two-year Chronic Toxicity Study of GS-6244
in Rats. Dose Levels: 2.5, 1.0 or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SIGLER, F.W., (1969b). A Three Generation Reproductive Study of GS-
6244 in Rats. Dose Levels: 2.5, 1.0, or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SPIERENBURG, T.J., BAARS, A.J., DE GRAAF, G.J., & JAGER, L.P., (1988).
Carbadox-induced Inhibition of Aldosterone Production in Porcine
Adrenals in vitro. Toxicology In Vitro, 2, 141-143.
STEBBINS, R.B., (1964). A 3-Week Subacute Toxicity Study of GS-6244
in Rats - Dose Levels of 100, 50, 10, 5, or 0 mg/kg. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
STEBBINS, R.B., & COLEMAN, G.L., (1967). A Twenty-six Month Chronic
Toxicity Study of GS-6244 in Rats - Dose Levels of 100, 50 10, 5, or
0 mg/kg. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
SYKORA, I., & VORTEL, V., (1986). Post-natal Study of the
Carcinogenicity of Cyadox and Carbadox. Biol Vet Praha., 22, 53-62.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J., (1982). Studies on
Hydrazine Hepatotoxicity. 2. Biochemical Findings. J. Toxicol and
Environ. Health, 10, 955-968.
TOTH, B., (1972). Tumorigenesis Studies with 1,2-Dimethylhydrazine
dihydrochloride, Hydrazine Sulfate, and Isonicotinic Acid in Golden
Hamsters. Cancer Res, 32, 804-807.
VAN DER MOLEN, E.J., DE GRAAF, G.J., & BAARS, A.J., (1989a).
Persistence of Carbadox-induced Adrenal Lesions in Pigs Following Drug
Withdrawal and Recovery of Aldosterone Plasma Concentration.
J. Comp. Path., 100, 295-304.
VAN DER MOLEN, E.J., VAN LIESHOUT, J.H., NABUURS, M.J., DERKX, F.H.,
LAMERS, A.P., & MICHELAKIS, A.M., (1989b). Changes in Plasma Renin
Activity and Renal Immunohistochemically Demonstrated Renin in
Carbadox Treated Pigs. Res. Vet. Sci., 46, 401-405.
VON WITTENAU, M.S., (1969). Comparative Metabolism Studies in Swine,
Monkeys, and Rats of Carbadox. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J., (1980). The
Mutagenic Action of Quindoxin, Carbadox, Olaquindox, and Some other N-
oxides on Baceteria and Yeast. Mutat. Res., 78, 233-242.
VON WRIGHT, A. & TIKKANEN, L., (1980). The Comparative Mutagenicities
of Hydrazine and its Mono- and Di- methyl Derivatives in Bacterial
Test Systems. Mutat. Res., 78, 17-23.
YOSHIMURA, H., NAKAMURA, M., KOEDA, T., & YOSHIKAWA, K., (1981).
Mutagenicities of Carbadox and Olaquindox - Growth Promoters for Pigs.
Mutat. Res., 90, 49-55.
The in vitro effect of carbadox on adrenal aldosterone production
was evaluated in porcine adrenal slices obtained from 3-5 week old
pigs. The adrenal slices were maintained in Krebs solution and
exposed to 1-40 µg carbadox/ml. Aldosterone was estimated using RIA
at hourly intervals. A dose-dependent decrease in aldosterone
production occurred with a reduction of 25-35% at 40 µg/ml
(Spierenburg et al; 1988).
2.2. Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with carbadox are shown in
Table 1.
Table 1: Carbadox acute toxicity data
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 2810 Pfizer, undated a
F oral >2810
i.p. 1050
Rat M oral 850
i.p. 810
A study was done to examine the emetic effect of carbadox on
monkeys and dogs. Three animals/group received a single dose of
carbadox orally via capsule. Doses were 25, 10, 5, and 1 mg/kg
b.w./day. In the monkey 1/3 exhibited emesis in the 2 higher dose
groups, while in dogs all dose groups had emesis.
2.2.2 Short-term studies
2.2.2.1 Rats
Twenty Charles River C-D weanling rats were divided and dosed for
30 days as follows: 2-3 males and females at 50 mg/kg b.w./day and
100 mg/kg b.w./day, and 10 males as untreated controls. Carbadox was
administered in the diet. Weight and food consumption were observed
weekly and drug levels adjusted as necessary. CBC at 30 days, urinary
electrolytes at 1, 2, 3, 14, and 30 days, cholesterol at 30 days and
urinalysis at 14 and 30 days were evaluated. Rats received a gross
necropsy at the end of the study and tissues were evaluated
microscopically. A dose-dependent decrease in weight gain and food-
consumption were noted. The authors concluded that all other
parameters were normal (Pfizer, 1963).
2.2.2.2 Dogs
The short-term toxicity of carbadox was studied by dosing dogs 6
days/week for three weeks. Beagle dogs (1/sex/dose) were initially
dosed with 25 or 50 mg/kg b.w./day carbadox via oral capsule. These
doses were later reduced to 10 and then 15 mg/kg b.w./day due to
emesis. A variety of parameters were evaluated including clinical
chemistry, gross necropsy, and microscopic tissue examination at the
termination of the study. The dogs lost weight and had elevated
SGPT's. It was concluded that carbadox is emetic and possibly
hepatotoxic in the dog (Stebbins, 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
The chronic toxicity and potential tumorigenicity of carbadox
were studied in rats. One hundred twenty Charles River C-D rats were
divided into 6 groups (10/sex/dose) and received carbadox in the diet
at rates providing doses of 100, 50, 25, 10, 5, and 0 mg carbadox/kg
b.w./day for 26 months. Feed concentration was adjusted weekly.
Clinical signs, body weights, and food consumption were recorded
weekly. Haematology and urinalysis were evaluated in 5 rats/sex/dose
at 3, 6, 12, 18, and 25 months. Rats were sacrificed at 14 and 112
weeks and received gross necropsies. Tissues were examined
histopathologically.
Survival was reduced for animals in the high-dose groups. The
following observations were reported for animals up to and including
the interim sacrifice:
100 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption, reduced haemoglobin, RBC's and neutropenia.
Microscopic changes reported include pulmonary hemorrhage and edema,
adrenalcortical hemorrhage and degeneration, splenic haemosiderin, and
thymic atrophy. No animals survived beyond the 14 week sacrifice.
50 mg/kg b.w./day (10 rats/sex necropsied) - Decreased weight gain
and food consumption. Microscopic changes reported include pulmonary
hemorrhage and edema, adrenalcortical hemorrhage, necrosis, and
degeneration, splenic haemosiderin and renocorticomedullary fatty
metamorphosis. No animals survived beyond the 14 week sacrifice.
25 mg/kg b.w./day (3 rats/sex necropsied) - Reduced weight gain,
slight adrenal cortical atrophy, degeneration/necrosis, and renal
tubular fatty change.
5 and 10 mg/kg b.w./day (3 rats/sex necropsied) - No clinical, gross
or microscopic changes reported at 3 months.
The following observations were reported for rats from 3 months
up to and including the 26 month sacrifice:
25 mg/kg b.w./day - One female died at 51 weeks with no drug-related
changes noted. All 13 remaining rats were sacrificed by 73 weeks due
to palpable abdominal masses. At necropsy all rats had multiple
hepatic nodules. Ten of 13 rats were diagnosed microscopically to
have benign nodular hyperplasia, while the remaining 3 rats were
determined to have malignant transformation based on metastatic foci
observed in other organs (lung, kidney and lymph nodes).
10 mg/kg b.w./day - One rat died after 67 weeks with
reticuloendothelial neoplasia, a common tumour of aged rats. The
remaining 13 rats were sacrificed between the 64th and 112th weeks.
Eleven of these were observed to have hepatic benign nodular
hyperplasia.
5 mg/kg b.w./day - One male died at 20 weeks due to pulmonary
abscesses. The remaining 13 died or were sacrificed between the 61st
and 112th weeks. Five or these were observed to have hepatic benign
nodular hyperplasia.
Controls One male was sacrificed at 33 weeks with a forestomach
papilloma and pulmonary atelectasis and a second male died at 93 weeks
with myocarditis, nephrosclerosis and peribronchitis. The remaining
animals were sacrificed between weeks 80 and 112 of the study. No
animals in the control group were observed to have hepatic nodular
hyperplasia. The authors suggested that the observed nodular
hyperplasia in treated groups was associated with hepatic injury and
concluded that the rats failed to tolerate carbadox at any of the
tested dose levels. A treatment-related increase in total tumours was
also reported for carbadox-treated rats. The tumour incidence was
lower in the high-dose group, however mortality occurred earlier in
this group than in other treatment groups (Stebbins & Coleman, 1967).
A follow-up study was performed to determine a chronic carbadox
level tolerated by rats. Sixty male Charles River rats (124-173 gms)
and 60 female rats (107-152 gms) of the same strain were divided into
3 groups (20/sex/dose) and received 2.5, 1.0, or 0 mg/kg b.w./day of
carbadox in the diet. Signs, weights and food consumption were
recorded weekly and drug levels were adjusted accordingly.
Haematology, complete urinalysis, and ophthalmoscopic examination were
done at 3, 6, 12, 18, and 24 months on 5 rats/sex/dose. An interim
sacrifice of 5 rats/sex/dose was conducted at 54 weeks with the
remaining rats sacrificed at 106 weeks. Each rat received a gross
necropsy and microscopic examination of standard tissues including:
brain, spinal cord, eye, sub-maxillary gland, thyroid gland, thymus,
heart, aorta, lung, sternum, liver, spleen, pancreas, kidney, adrenal,
stomach, mesenteric lymph node, reproductive tract, urinary bladder,
femoral nerve, femoral bone marrow, and mammary gland with skin.
All parameters evaluated, including histopathologic examination,
were within normal limits at 54 weeks. Survival at 2 years was as
follows: control - 38%, 1.0 mg/kg b.w./day - 45%, 2.5 mg/kg b.w./day
- 43%. At the 2.5 mg/kg b.w./day level 7/27 rats displayed hepatic
benign nodular hyperplasia and 7/27 showed peliosis hepatis. At the
1.0 mg/kg b.w./day level 1/29 rats was found to have hepatic benign
nodular hyperplasia and 3/29 displayed peliosis hepatis. In the
control group 3/29 rats had benign nodular hyperplasia and 2/29 had
peliosis hepatis. Additionally, an increase in total mammary tumours
was reported in the 2.5 mg/kg b.w./day dose group. The authors
concluded that the 1.0 mg/kg b.w./day dose level was tolerated by the
rats for 2 years with no adverse effects, based on the similar
incidence of both hepatic lesions and tumours in other organs in
treatment and control groups (Sigler, 1969a).
Three groups of 28 rats (14/sex) were treated with a target dose
of 25 mg/kg b.w./day of carbadox, or one of two lots of desoxycarbadox
for 11 months to compare the oncogenic activity of carbadox and
desoxycarbadox. An equal sized group served as untreated controls.
The test material was given in the feed for 10 months. Two to four
rats were sacrificed at 30, 60, 90, 191, and 309 days. Animals were
observed daily for toxicity and mortality. Weights and food
consumption were recorded weekly until the 38th week and then every
other week. Rats received a gross necropsy at sacrifice and liver and
adrenals were evaluated histopathologically. A moderate decrease in
weight gain occurred in both sexes receiving carbadox. No other
significant clinical observations were made. At gross necropsy all
groups treated for 10 months showed evidence of hepatic changes
including necrosis and nodule formation. Changes in the carbadox-
treated group were less severe than in the desoxycarbadox-treated
group. Histopathologically all desoxycarbadox-treated rats showed
evidence of hepatocellular carcinoma, while 2/18 rats receiving
carbadox showed evidence of hepatocellular carcinoma. There was a
significant increase in adrenal cortical hemorrhage in both carbadox
and desoxycarbadox-treated groups (King, 1976).
The tumorigenicity of carbadox administered in various dosing
regimens was studied more recently in rats. A total of 119 Wistar
rats of each sex were divided into groups of various size (2 - 18
rats) following parturition and treated with various regimens of
carbadox i.p. (prior to weaning for 8 to 20 days) and/or in the feed
at 300 ppm until study termination at 1 year. Rats were examined and
weighed routinely during the study. Tumours were evaluated grossly
and histopathologically at the end of the study. Weight gains were
reduced. Animals receiving 300 ppm carbadox in feed all developed
hepatic tumours. Rats which received carbadox i.p. developed a higher
incidence of hepatic tumour than untreated controls. The largest
number of spontaneous and rare tumours occurred in groups receiving
carbadox via both routes (Sykora & Vortel, 1986).
2.2.3.2 Monkeys
A study was performed to assess the long-term toxicity of
carbadox in primates. Twenty-eight monkeys were divided into 4 groups
of 7 animals (3 or 4/sex) and were dosed with carbadox in gelatin
capsules 5 days/week. The doses were 20 (5 mg/kg QID), 10 (5 mg/kg
BID) or 5 mg/kg b.w./day and controls. A variety of parameters were
evaluated at 1, 3, 6, 12, and 24 months including haemoglobin,
haematocrit, RBC's, WBC's, clotting time, prothrombin time, complete
urinalysis, blood glucose, BUN, alkaline phosphatase, SGOT and SGPT.
Ophthalmoscopic examinations were performed at the same intervals. At
3 months and 2 years animals were sacrificed and necropsied. The diet
contained 0.2% isoniazid as prophylaxis for tuberculosis.
Elevated transaminase levels were detected at the 3 and 6 month
evaluations in both treated and control animals. The authors
concluded that monkeys tolerated 20 mg/kg/day for two years with no
adverse effect (Coleman, 1967).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation reproduction study with 2 litters per generation
was conducted using feed levels adjusted to provide target doses of
2.5, 1.0 and 10 mg/kg b.w./day. Feed levels were identical to those
used for a concurrent 2 year chronic study. Sixty female and 30 male
Charles River C-D rats were divided into groups of 20 females and 10
males per level for the 1st generation. Two females were placed with
each male for mating. First litter offspring were sacrificed
following a gross examination on lactation day 21. Following a 10 day
rest period the females were rebred to different males. Twenty females
and 10 males from the 2nd litter of each dose level were selected to
become the 2nd generation. These rats were mated at 100 days of age.
Dose levels were maintained and the 2nd and 3rd generation were
treated from weaning throughout the breeding period. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 4 days, number weaned,
litter weight at 24 hours, litter weight at weaning, sex at weaning,
viability index, lactation index, growth index, live birth index, and
gross abnormalities. In addition 10 weanlings/dose level from the 2nd
litter of the 3rd generation received an ophthalmoscopic examination,
gross necropsy, and a standard array of tissues were evaluated
microscopically. Although occasional differences from control group
parameters were noted, these were sporadic and not thought to be
treatment related. A reduced pregnancy rate was reported for all
groups of the third generation, with only the first litter of the
1.0 mg/kg b.w./day treatment group above 50%. Additionally a high
incidence of cannibalism (30 - 44%) occurred in treated groups of the
3rd generation as well as in the second litter of the untreated control
(19%). This cannibalism produced a non dose-related reduction in
lactation index in the treated groups of the 3rd generation
(59 - 79% in treated group litters vs 93 - 98% in controls). No
effects were reported in the first two generations. The authors
concluded that carbadox had no effect on fertility, lactation or
the neonate at any dose level (Sigler, 1969b).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with carbadox are shown in
Table 2.
2.2.6 Special studies on relay toxicity
2.2.6.1 Rats
A study was done to assess the possible risk of toxicity of pork
from pigs fed carbadox. To accomplish the study pigs weighing 60-80
kg were fed carbadox at the rate of 0, 20, and 200 ppm in their feed
for 30 days at which time they were slaughtered. Meat from these pigs
was divided into pieces of approximately 200 grams and frozen at -18
C.
Weanling Wistar rats were divided into groups of 12/sex/dose
(except the high dose which had 24 females). Rats received a dietary
supplement of 10% pork liver from swine fed the carbadox regimens
outlined above. This dietary regimen was continued for 23 - 25
months. All rats received gross necropsies and the following organs
were evaluated microscopically: liver, kidney, adrenal, testes, ovary,
and thyroid. Weights and mortality were recorded. There was no
effect on mortality. No treatment-related histologic changes were
reported (Ferrando et al., 1975).
2.2.6.2 Dogs
In another study 12 beagle dogs weighing about 4.5 kg were fed
for one month with 100 g of control pork as part of a diversified
diet. The dogs were then distributed into 3 treatment groups and
received pork from hogs treated with the above regimens of carbadox at
the rate of 100 gm/day for the first 5 months and then 200 gms for the
remainder of the treatment period (85 months). The dogs were weighed
periodically. Every fourth month haematology, clinical chemistry, and
urinalysis parameters were evaluated.
All dogs were in good health at sacrifice. The dogs received a
gross necropsy at study termination and a standard set of tissues were
evaluated histopathologically. No treatment-related effects were
reported in this study. The authors concluded that there is no danger
to the public health when carbadox is used in pig feed as a growth
promoter at 50 ppm (Ferrando, et al.,1978).
Table 2: Results of genotoxicity assays on carbadox
Test System Test Object Concentration Results Reference
Ames test S. typhimurium 0.0004 - 0.125 Positive Pfizer, undated b
TA1535 (mg/plate)
TA1536, TA1537, Equivocal
TA1538, C340
Ames test TA100 3.1 - 61.7 Positive Beutin et al,
TA98 (+/- S9) (nM) 1981
Ames test TA100 2.5 - 15 µg/plate Positive Yoshimura et al,
TA98 (+/- S9) 1981
Ames test TA100, TA98 .1 umole/plate Positive Negishi, et al,
1980
Ames test TA100 0.001-.02 Positive Voogd et al.,
1980
Mutagenicity Ames E. coli WP2hcr Positive Ohta et al, 1980
test TA100+/-S9 5 - 50 µg/plate Positive
TA98 Positive
TA1538 Negative
TA1537 Negative
TA1535 Negative
Host mediated Assay S. typhimurium 0,1,2, 2.5, 5, 10, Negative Pfizer, undated b
TA1534, TA1952 0,1,2.5 5,7.5, Positive
10,12.5, 20 mg/kg
Repair test B. subtilis (rec) 1 - 100 µg/disc Positive Yoshimura et al.,
S. typhimurium (urv) 1981
Fluctuation test K. pneumoniae .00002 - Positive Voogd et al, 1980
E. coli K12 .005 mmoles/l
Table 2 (contd)
Test System Test Object Concentration Results Reference
Mutagenicity test S. cerevisiae D4 .02% Positive Voogd et al, 1980
Micronucleus test rat bone marrow 5 - 240 mg/kg i.p. Positive Cihak and Srb,
1983
Repair test B. subtilis M45 .1 - 100 Positive Ohta et al,
umole/disk 1980
Chromosomal damage mouse bone marrow 200 mg/kg acute, Positive Pfizer, undated b
10 mg/kg day seven
days
Chromosomal damage in vitro human 0 - 250 µg/ml Positive Pfizer, undated b
lymphocytes
Dominant lethal CD-1 mice 10,50,150,30 mg/kg Negative Pfizer, undated b
assay
QUINOXALINE-2-CARBOXYLIC ACID
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with
quinoxaline-2-carboxylic acid are given in Table 3.
Table 3: Acute toxicity of quinoxaline-2-carboxylic acid
LD50
Species Sex Route (mg/kg) Reference
Rats M Oral 4450 Briggs, 1971
Mice M Oral 1360
2.2.3 Long-term/carcinogenicity Studies
2.2.3.1 Mouse
Groups of 50 Charles River CD mice/sex/dose (100 in controls)
received quinoxaline-2-carboxylic acid in feed for 19 months in a
study of its chronic oral toxicity and tumorigenic potential. Fresh
diets were prepared weekly. Feed and water intake were monitored
weekly for the initial three months and monthly thereafter. Feed
levels of quinoxaline-2-carboxylic acid were adjusted at these
intervals to provide target doses of 0, 25, 50, and 100 mg/kg
b.w./day. Haematology and clinical chemistry were evaluated once,
prior to sacrifice. Necropsy was performed on all animals. Organ
weights were obtained for brain, heart, kidneys, liver, testes, and
ovaries. Histopathologic examination was performed on a standard
array of tissues.
No treatment-related effects were noted in any parameter examined
during the study. Cumulative mortalities were 20% and 15% for control
females and males, respectively, while treatment group cumulative
mortalities ranged from 10% to 20%. Incidence of all tumours was 33%
in female and 46% in male control groups, while the incidences in
treatment groups ranged from 32% to 42%. The authors concluded that
oral administration of quinoxaline-2-carboxylic acid to mice for 19
months produced no evidence of toxicity (Faccini et al., 1979).
2.2.3.2 Rats
Nine male and 9 female Charles River C-D rats were divided into
groups of 3/sex/dose level. Rats received quinoxaline-2-carboxylic
acid in the feed for 2 years. Weight and food consumption were
recorded weekly through week 30 and monthly thereafter. Drug levels
were adjusted to provide target doses of 100, 50 or 0 mg/kg b.w./day.
Rats received clinical examinations weekly and ophthalmoscopic
examinations on days 0, 30, 92, 186, 365, 554, and 733. Haematology
and urinalysis parameters were evaluated on days 31, 93, 182, 274,
370, 461, 547, 644, and 730. Terminal sacrifice was performed on day
735 of the study. All rats received gross necropsies and standard
tissues were evaluated microscopically from each rat. No treatment-
related changes were reported. The authors concluded that
quinoxaline-2-carboxylic acid was tolerated at up to 100 mg/kg
b.w./day when given to rats via feed (Coleman, 1971).
An additional study was conducted to determine whether
quinoxaline-2-carboxylic acid has tumorigenic potential. To
accomplish the study quinoxaline-2-carboxylic acid was administered in
the diet of Charles River Sprague-Dawley rats (20/sex/dose level) for
two years to provide target doses of 0, 10, 25, and 50 mg/kg b.w./day.
Weight and food consumption were monitored weekly and drug
concentration adjusted accordingly. Rats were examined for clinical
signs weekly. Ophthalmologic examinations were performed on all rats
prior to treatment and at 6, 12, 20 and 24 months. Clinical
chemistry, haematology and urine parameters were evaluated in 5 rats
at 6 and 12 months, 2-4 rats at 18 months, and all rats at
termination. At 12 months 5 rats/sex/dose and at 24 months all
remaining rats were sacrificed and received a gross necropsy and
tissues were obtained for microscopic evaluation. Organ weights were
determined for heart, liver, lungs, kidneys, gonads, adrenal, spleen,
and brain. A standard array of tissues was examined
histopathologically.
No treatment-related effects were noted on any of the parameters
evaluated. Cumulative incidences of tumours (based on summing males
and females with 40 rats/dose) occurring in treatment groups for
selected organs of interest are summarized in Table 4. It was
concluded that quinoxaline-2-carboxylic acid in doses of 10, 25, and
50 mg/kg b.w./day over a 2 year period to rats does not produce any
toxicity or elevated tumor incidence (Pfizer, 1971).
Table 4: Cumulative incidence of tumours (males and females combined)
after treatment of rats with quinoxaline-2-carboxylic acid
Treatment Group Mammary gland Pituitary Pancreas Liver
Control 28% 30% 3% 5%
10 mg/kg b.w./day 18 23 10 5
25 mg/kg b.w./day 13 28 3 5
50 mg/kg b.w./day 33 28 3 3
A follow-up chronic study was performed using rats obtained from
the 3-generation study F1 litter. An additional high dose level, 100
mg/kg b.w./day, was added for this study. To accomplish the study
Charles River C-D rats were divided into groups of 50/sex/dose and
received 100, 50, 25, or 0 mg/kg b.w./day of quinoxaline-2-carboxylic
acid in the diet for 29 months. In addition 10 rats/sex in the
control and 100 mg/kg b.w./day groups were added for clinical
chemistry studies at 12 months. Weights were monitored weekly. Food
consumption was recorded weekly during the first 5 months, twice
during the 6th month, and monthly thereafter, and treatment levels
were adjusted accordingly. Clinical examinations were performed
daily. Clinical chemistry parameters were evaluated at 6, 12, and 29
months. Haematology was evaluated at the conclusion of the study.
All rats received a gross necropsy. Liver and kidneys were weighed
from rats sacrificed at 12 months and heart, liver, kidneys, brain,
and testes were weighed during the gross necropsies at 29 months.
Tissues from many organs showing macroscopic abnormality plus a
standard array of grossly normal tissues were evaluated
microscopically.
The general health of the rats was unaffected by treatment. An
outbreak of sialoacryoadenitis occurred between ages 89 and 97 days.
Slightly reduced weight gain (3-5%) was noted in the high-dose groups
in both sexes. The cumulative mortality for controls was 56% in males
and 70% in females, while treatment group cumulative mortality ranged
from 46% to 74%. There was no difference in the incidence of
nonneoplastic lesions among the control and treatment groups, nor did
treatment shorten the lifespan of treatment groups. Treatment of rats
for 2 years did not produce an increase in the incidence of animals
with malignant or benign tumours compared to the control group. The
authors concluded that the NOAEL was 100 mg/kg b.w./day (King, 1979a).
2.2.4 Reproduction studies
2.2.4.1 Rats
The potential reproductive toxicity of quinoxaline-2-carboxylic
acid was studied in rats. The study was performed in conjunction with
a 2-year chronic study. Treatment route was via feed. The study
utilized 2 litters per generation for 3 generations with twenty
rats/sex/dose level using target doses of 25, 50, and 100 mg/kg
b.w./day. The F0 rats were bred following approximately 60 days of
treatment with quinoxaline-2-carboxylic acid. Fifty rats/sex/dose
were selected for the concurrent chronic study from the initial F1
litter. Following a 7 day rest period the females were rebred to
different males to produce the second litter. The subsequent first
litters of each generation were sacrificed at weaning while the second
litters were used to constitute the next generation. These first
litters were reduced to 10 pups/litter (5/sex if possible) for
rearing, then sacrificed, and necropsied at weaning. The 2nd litters
were used to produce the next generation. Subsequent generations of
rats were mated at 100 days of age. Dose levels were maintained and
test animals were treated from weaning throughout the breeding period.
Rats were inspected daily. Food consumption and weights were
determined weekly except for mating periods. Adults of all
generations were sacrificed after weaning of the 2nd litter and
received a gross necropsy with liver weights determined. Blood levels
were determined in F0 rats (5/sex/level) just prior to sacrifice.
Pups were examined daily until weaning and weighed on days 1, 4, and
21 after birth. Gross necropsies were carried out on all pups that
died or were stillborn and on 5 weanlings/sex/dose. Reproductive
parameters evaluated included: fertility, length of gestation, number
of livebirths, stillbirths, number alive at 1, 4, and 21 days, number
weaned, sex ratios, mean pup weights, lactation index, and gross
abnormalities. No treatment-related alterations were observed in any
parameter monitored during the study (King, 1979b).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rabbits
The potential embryotoxicity or teratogenicity of quinoxaline-2-
carboxylic acid was also studied in the New Zealand White Rabbit.
Rabbits were randomly assigned to 4 treatment groups of 19 - 20
animals receiving 0, 25, 50, or 100 mg/kg b.w. quinoxaline-2-
carboxylic acid via gavage on gestation days 7-18. Animals were
observed daily for toxicity and weighed on gestation days 0, 3, 7, 9,
12, 15, 18, 21, 24, and 28. Rabbits were sacrificed on day 28 and the
number of corpora lutea and number and position of live and dead
implants in the uteri were recorded. All fetuses were examined for
external malformations and weighed. Half the fetuses were cleared,
stained with alizarin red and examined for skeletal abnormalities.
The remaining half were fixed in Bouin Allen fluid and examined by
serial sectioning. No treatment related maternal toxicity,
embryotoxicity or malformations were observed (Holmes, 1976a).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with quinoxaline-2-carboxylic
acid are given in table 5.
Table 5: Quinoxaline-2-carboxylic acid genetic toxicology
Test System Test Object Concentration Results Reference
Ames test S. typhimurium .008, .032, Negative Pfizer,
TA1535 .125, .5, 1975
TA1537 2 mg/plate
TA1538
TA 1535+S9 .16, .63, Negative Pfizer,
2.5 mg/plate 1975
Chromosomal Human 50, 100 Negative Pfizer,
aberrations lymphocytes µg/ml 1975
in vitro
DESOXYCARBADOX
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
A long term study was performed to determine the tumorigenic
potential of desoxycarbadox, a carbadox metabolite. Four hundred
Charles River C-D rats (5 weeks of age) were divided into groups of
50/sex/dose level. Rats were distributed randomly according to weight
levels. Desoxycarbadox was administered at target doses of 0, 5, 10,
and 25 mg/kg b.w./day continuously in the diet. Weights were recorded
weekly. Food consumption was monitored weekly the first 3 months and
monthly thereafter. Treatment levels were adjusted accordingly.
Although treatment was planned for 2 years, the test material was
withdrawn from all rats in the 25 mg/kg b.w./day group and 50% of the
rats in each sex in the other 2 treatment groups on day 350, due to
high morbidity and mortality. Administration of desoxycarbadox was
stopped completely on day 416. The study was terminated after 447
days.
Clinical examinations were performed twice weekly. A number of
treatment-related signs were observed. These included: tumours in
the mammary region of both sexes, small cutaneous nodules, and
enlargement of the liver with nodules preceded by weight loss and
polyphagia. There was also a dose-related decrease in weight gain and
a dose-related decrease in survival rate.
Clinical chemistry parameters were evaluated. Desoxycarbadox
administration resulted in dose-related increases in plasma enzyme
activities, urea, and bilirubin. The abnormalities were consistent
with hepatic disorders. The high degree of variability from animal to
animal and lack of reversibility following drug withdrawal indicates
a correlation with tumour progression. A dose-related hypoglycaemia
was noted in both sexes. Haematologic parameters were evaluated in
ten rats/sex/dose at day 413 and the remainder at 447 days. The two
primary changes in haematology in the treated rats were a moderate
hypochromic microcytic anaemia in the 25 mg/kg b.w./day group, a
slight anaemia in the 10 mg/kg b.w./day group, and a neutrophilia in
the 25 mg/kg b.w./day group.
All rats received gross necropsies. Selected organs were
weighed. Histopathologic examination was performed on all grossly
abnormal tissue and routinely on a standard array of tissues. There
was a dose-related increase in pigmentation of renal tubules and
nephrosis. The major pathologic findings related to increased tumour
incidences are summarized in Table 6. The authors concluded that
desoxycarbadox was a potent hepatocarcinogen in the rat and that there
were dose-related increases in other tumours (Reinert, 1976).
Table 6: Incidence of tumours after administration of desoxycarbadox
to rats
Treatment Hepatic Subcutaneous Haemanigiomas Mammary
Group tumours fibromas tumours
Males and females combined Females only
Control 0% 0% 0% 12%
5 mg/kg 75 2 0 24%
b.w./day
10 mg/kg 95 10 5 28
b.w./day
25 mg/kg 100 18 47 27
b.w./day
2.2.5.2 Special Studies on Genotoxicity
The results of genotoxicity studies with desoxycarbadox are
summarized in Table 7.
Table 7: Desoxycarbadox genetic toxicology
Test System Test Object Concentration Result Reference
Ames test TA1535 .0008, .0032, Negative Pfizer, 1975
TA1537 .0125, 0.05, .125,
TA1538 .5, 2, 20 mg/plate
TA1535+S9 .0125, .05, .2, 1, Negative Pfizer, 1975
5 ml/plate
Ames test TA1535 .032, .25, .4, .5 Negative Pfizer, 1975
TA1537 ml/plate plasma;
TA1538 .032, .25. .5 ml/plate
urine1
Host mediated TA1950 500 mg/kg acute Negative Pfizer, 1975
assay
Chromosomal damage Human lymphocytes 200 µg/ml Negative Pfizer, 1975
Rat bone marrow 200 mg/kg acute, Negative Pfizer, 1975
or 100 mg/kg 5 days
10 mg or 25 mg per kg Positive Pfizer, 1975
9 months in feed
Host mediated TA1950 400 or 500 mg/kg Negative Pfizer, 1975
assay in mice
Host mediated TA1950 300 mg/kg with 100 mg/ Negative Pfizer, 1975
assay in rats kg/day previous 31 days
TA1535 200 mg/kg Negative
Table 7 (contd)
Test System Test Object Concentration Result Reference
Yeast assay S. cerevisiae D3 10 mg/ml Negative Holmes, 1976b
& D4
Host mediated TA1950 400 mg/kg +DMNA Negative Holmes, 1976b
assay (mouse)
Ames test TA1537 .01 - 10 mg/plate Negative Holmes, 1976b
TA100
TA98
Ames test TA1535+/- TA100 .005 - 2 mg/plate Positive Holmes, 1976b
S9(rat)
Ames test TA1537+S9 .005 - 2 mg/plate Negative Holmes, 1976b
TA98
Ames test TA1535+S9 from .005 - 2 mg/plate Holmes, 1976b
rat
mouse Positive
hamster Positive
dog Negative
monkey Negative
Negative
Ames test TA1535 Quantitative plate Negative Holmes, 1976b
assay on plasma, liver
& urine at selected
intervals from chronic
study rats fed carbadox
or desoxy-carbadox
Cell transformation BALB/C Swiss 3T3 .1-10 µg/ml Positive Holmes, 1976b
test
1 Urine and plasma collected from mice or rats administered 400 mg/kg desoxycarbadox administered
in 3 hourly doses or 100 mg/kg/day for 5 days.
METHYL CARBAZATE
2.2 Toxicological studies
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
Methyl carbazate is a metabolite of carbadox. The chronic oral
toxicity of methyl carbazate was studied in the rat. Wistar rats were
divided into groups of 12/sex/dose and received methyl carbazate at
target doses of 1 and 10 mg/kg b.w./day in the feed for 21 months.
The colony served as controls. Food consumption was determined every
2 days and weights every 2-3 weeks. Necropsy was performed on all
animals. Organ weights were determined for liver, kidney, adrenals,
testes, and thyroid. Histology was performed on liver, kidneys,
adrenals, thyroid, testes, ovaries, and tissues containing tumours
observed grossly. Three males and 3 females in the high dose group
died before termination. No low dose rats died prior to termination.
No histopathologic evidence of toxicity or evidence of an elevation in
tumours was reported. The authors concluded that administration of
methyl carbazate to rats at dose levels of 1 and 10 mg/kg b.w./day in
the diet produced no evidence of toxicity or carcinogenic potential
(Rutty, 1972).
In a second study weanling Wistar rats were divided into groups
of 24/sex and treated with target doses of 0, 2.5, 5, and 10 mg/kg
b.w./day methyl carbazate in the feed for 710 days. Weights were
determined weekly and food consumption weekly the first 3 months and
monthly thereafter. Drug concentrations were adjusted accordingly.
Clinical examinations were conducted weekly. Blood samples for
clinical chemistry were obtained at terminal sacrifice and from
moribund animals. Haematology was also conducted on these samples and
at 12 months on 6 rats. Rats were sacrificed and necropsied at 710
days. An interim sacrifice was also performed at 12 months. All
moribund and dead rats also received a gross necropsy. Histopathology
was performed on all gross lesions and the following organs: liver,
pituitary, thyroid, parathyroid, adrenal, lung, kidney, urinary
bladder, ovary, and testis.
There were no treatment-related effects noted in any parameters
evaluated in the study. There was histologic evidence of widespread
chronic respiratory disease in all groups. It was concluded that
methyl carbazate had no adverse effect when given to rats in the diet
for 2 years (Ferrando, 1980).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with methyl carbazate are
summarized in Table 8.
Table 8: Genetic toxicity studies with methyl carbazate
Test System Test Object Concentration Results Reference
Ames test TA1535 .0005, .002, Negative Pfizer, 1975
TA1537 .008, .032,
TA1538 .125, 0.5, 2, 5
mg/plate
TA1535+S9 .0125, .05, .2 Negative Pfizer, 1975
1, 5, 10
mg/plate
Host- TA1950 50, 100, 300 Equivocal Pfizer, 1975
mediated mg/kg
assay
Chromosomal Human 50, 100 Negative Pfizer, 1975
damage lymphocytes µg/ml
in vitro
Ames test TA1535+S9 0.5 - 10 Negative Holmes, 1976b
mg/plate
HYDRAZINE
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism, and excretion
Hydrazine toxicokinetic parameters have been determined in the
rabbit. Rabbits received a bolus of 12 mg/kg b.w. Parameters were
derived using a one-compartment model, and spectrophotometric serum
analysis for hydrazine, with 5 rabbits/group. The serum half-life was
2.3 hours and the volume of distribution was 0.63 liters/kg (Keller
et al., 1981).
The metabolism, distribution, and elimination of hydrazine in
rats was studied using 15N-labelled hydrazine or a spectrophotometric
method. About 15% of the 15N label was found as N2. About 20% and
30% of the infused hydrazine was recovered in the urine as acetylated
metabolite and hydrazine, respectively. The serum half-lives were
determined, using a two-compartment model, to be 0.74 hours for the
majority of the hydrazine elimination, and 26.9 hours for a much
slower, smaller elimination component (Dost et al., 1981).
2.1.3 Effects on enzymes and other biochemical parameters
Hydrazine-induced short-term biochemical parameter changes were
investigated following oral administration of hydrazine in Sprague-
Dawley rats. Groups of 5 rats were given doses of hydrazine (0-200
mg/kg b.w.). Some rats received pyruvate azine, phenobarbital,
piperonyl butoxide, or diethylmaleate to more clearly elucidate the
hepatic effects of hydrazine. Hydrazine caused a dose-related
increase in liver triglycerides and liver weight and a decrease in
hepatic glutathione. The threshold dose for toxicity was about 10
mg/kg b.w. While liver weight and glutathione alterations were
evident within 30 minutes, triglyceride alteration was not evident for
4 hours. Pretreatment with phenobarbital decreased toxicity, while
pretreatment with piperonyl butoxide increased toxicity suggesting
hepatotoxicity is due to the parent - not a metabolite. Depletion of
hepatic glutathione was without effect on hepatic toxicity, and
studies with pyruvate azine - a possible oxidative metabolite - also
supported this conclusion (Timbrell, et al., 1982).
Hydrazine-induced short-term histologic and ultrastructural
changes were investigated following oral administration of hydrazine
to Sprague-Dawley rats. Groups of 5 rats were given doses of
hydrazine (0-200 mg/kg b.w.). Some rats received pyruvate azine,
phenobarbital, piperonyl butoxide, or diethylmaleate to more clearly
elucidate the hepatic effects of hydrazine. A dose of 20 mg/kg caused
accumulation of lipid, swelling of mitochondria, and the appearance of
microbodies in periportal and midzonal hepatocytes. Accumulation of
lipid droplets and mitochondrial swelling were detected by electron
microscopy 30 minutes after dosing. Piperonyl butoxide or
phenobarbital treatment increased or decreased fatty liver
respectively, while pyruvate azine was much less effective than
hydrazine in producing this effect (Scales & Timbrell, 1982).
2.2 Toxicological studies
2.2.3 Long-term carcinogenicity studies
2.2.3.1 Mice
Oral administration of hydrazine sulfate to Balb/c female mice at
1.13 mg/day for 46 weeks produced a 100% incidence of lung tumours
(Biancifiori & Ribacchi, 1962).
In another study hydrazine was administrated via gavage to 25
female Swiss mice 5 days/week for 40 weeks at the rate of 0.25 mg/day.
Eighty-five mice served as controls. This dose regimen produced a 46%
incidence of lung tumours in treated mice vs 10% in controls (Roe et
al., 1967).
A study with CBA/Cb/Se mice was performed using hydrazine sulfate
given for 36 weeks by gavage. Twenty-one 8- week-old rats/sex
received 1.13 mg/day. Treated rats had an incidence of 76% and 90%
lung tumours in males and females, respectively, while controls had
3%. Hepatomas were found in 62% of the males and 71% of the females
treated with hydrazine, while 11% of control males and 4% control
females had hepatomas (Severi & Biancifiori 1968).
In another study in mice, 8-week-old CBA mice were divided into
groups of 40-59 mice/sex. Hydrazine sulfate was given by gavage daily
for 150 days at rates of 45, 22, 11, 5.6 and 0 mg/kg b.w./day. Mice
were examined at natural death or following sacrifice when moribund.
Control male mice had a hepatoma incidence of 10% while females had a
3.4% rate. Treated males had hepatoma rates of 60, 48, 28, and 3.8%
at 45, 22, 11, and 5.6 mg/kg b.w./day, respectively. Treated females
had 62.5, 66.6, 8 and 0% hepatomas, respectively. The author reported
many lung tumours in treated groups but did not provide the incidence
rates (Biancifiori, 1970).
2.2.3.2 Hamsters
Eight-week old golden hamsters were divided into 3 groups of 23,
35 and 56 animals which were administered 60 doses of 3.0 mg hydrazine
sulfate via intubation over a 15 week period, 2.8 mg 100 times during
a 20 week period or no hydrazine, respectively. Hepatic lesions
reported include: Fibrosis, reticuloendothelial cell proliferation,
and bile duct proliferation. Hepatic lesions were found in 60-80% of
treated hamsters but in none of the controls. No hepatic tumours were
reported (Biancifiori, 1970).
Negative tumorigenicity results were also obtained in a follow up
chronic study with golden hamsters given 2.3 mg/day over a lifetime.
In this study 50 hamsters/sex received hydrazine in drinking water
from the age of 9 weeks for a lifetime (Toth, 1972).
2.2.3.3 Rats
A chronic oral study was performed in conjunction with the above
mouse study using 8 week old Cb/Se rats. Aqueous hydrazine sulfate
was administered daily by stomach tube at doses of 18 mg/rat to 14
males and 12 mg/rat to 18 females for 68 weeks. Lung tumours were
found in 21 and 27% of dosed male and female rats, respectively.
Control groups of 28 males and 22 females had no lung tumours.
Hepatic tumours were found in 30% of males, while no hepatic tumours
were found in females or controls (Severi & Biancifiori, 1968).
2.2.7.5 Special studies on genotoxicity
The results of genotoxicity studies with hydrazine are summarized
in Table 9.
Table 9: Genetic toxicity studies with hydrazine
Test System Test Object Concentration Results Reference
Bacterial E. coli WP2 0.5 - 2.0 µmole Positive von Wright
uvr A trp & Tikkanen,
1980
Mouse L5178Y 0.1 - 5 mmole Positive Rogers &
lymphoma Back, 1981
cells
Ames test S. typhimurium Positive Ames, 1971
2.3. Observations in humans
No information available
3. COMMENTS
The Committee considered data from short-term, long-term and
"relay" toxicity studies, and from studies on carcinogenicity,
mutagenicity, and reproduction with carbadox, together with data from
mutagenicity and long-term toxicity studies of its metabolites.
The metabolism of carbadox has been studied in rats, monkeys and
pigs using [14C]carbadox, labelled in either the phenyl ring or the
carbonyl group of the side-chain. The metabolism of carbadox was
characterized by the rapid reduction of the N-oxide groups, the
cleavage of the methylcarbazate side-chain, and the liberation of
respired CO2. The primary metabolite in the urine was quinoxaline-2-
carboxylic acid, which was also excreted in conjugated form. The
detectable residues in tissues, up to 24 hours after drug withdrawal,
were carbadox, desoxycarbadox, quinoxaline-1,4-di-N-oxide-2-
carboxaldehyde and quinoxaline-2-carboxylic acid. Hydrazine was a
minor metabolite but would be expected to be present only for a short
time before undergoing further metabolism.
The concentration of carbadox and desoxycarbadox in the tissues
declined rapidly (half-life about 6 hours), and, after 3 days, was
less than 5 µg/kg. In a study in pigs, in which carbonyl-labelled
[14C]carbadox was administered by gavage, the concentration of total
residues in liver at 5 days withdrawal time corresponded to 0.12 mg/kg
methyl carbazate equivalent. However, some of this radioactivity was
due to the presence of amino acids, which had been labelled by
incorporation of 14CO2 when ring-labelled [14C]carbadox was
administered to pigs, extremely low levels of unidentified metabolites
remained in the liver at withdrawal periods longer than 7 days. These
residues were partially released and converted to quinoxaline-2-
carboxylic acid by alkaline digestion of the liver.
The Committee also reviewed recent reports of the effect of
carbadox on adrenal function in pigs, although the information did not
effect the outcome of the evaluation.
Several long-term feeding studies in rats were evaluated which
demonstrated dose-related increases in the incidence of benign and
malignant liver tumours at doses of carbadox above 1.0 mg per kg of
body weight per day. Doses above 25 mg per kg of body weight per day
produced pronounced toxic effects which precluded chronic treatment.
Data from a variety of mammalian and non-mammalian genotoxicity
studies were also reviewed. Positive findings were reported in 14 of
the 15 tests carried out. The Committee concluded that carbadox
appears to be both genotoxic and carcinogenic.
The Committee evaluated a "relay" toxicity study in which pigs
were fed carbadox at up to 200 mg/kg in the diet for 30 days and
killed at zero withdrawal time, their livers were fed to rats as a 10%
dietary component for 2 years. Although this study was conducted
satisfactorily and no treatment-related effects were reported, the
Committee did not use it for assessing the carcinogenicity of carbadox
residues because of the number of animals used and because only small
quantities of residues can be consumed by rats in this type of study.
In a long-term study in rats, desoxycarbadox was reported to
produce an increase in the incidence of tumours. All rats that
received 25 mg per kg of body weight desoxycarbadox daily for 10
months in the diet developed hepatic tumours. The incidence of
tumours was increased in all treated groups, at doses of 5-25 mg per
kg of body weight per day. While the most pronounced change occurred
in the liver, tumour incidence was also elevated at other sites,
including the skin and mammary glands.
The mutagenic potential of desoxycarbadox was investigated in a
range of studies. The Committee noted that, while desoxycarbadox
produced negative results in most mutagenicity test systems, positive
findings were recorded in the cell transformation test and in the Ames
test using liver microsomes from rats treated with polychlorinated
biphenyls. The Committee noted that the tumorigenic potential of
desoxycarbadox was apparently greater than that of the parent
compound, and that desoxycarbadox would therefore probably make a
significant contribution to the tumorigenic activity of carbadox in
rats. However, the Committee also noted that the metabolism data for
carbadox supported the conclusion that desoxycarbadox was a relatively
short-lived intermediate between carbadox and quinoxaline-2-carboxylic
acid.
The Committee also considered information on the carbadox side-
chain metabolite, methyl carbazate. This information consisted of
results from two long-term studies in rats and a number of
mutagenicity assays. In the more informative long-term study (in
which more animals per dose and concurrent controls were used), rats
received methyl carbazate in the diet for 710 days. There was no
increase in the incidence of tumours and no other treatment-related
effects were observed at 10 mg per kg of body weight per day, which
was the highest dose tested. The lack of effect limited the
usefulness of this study. The no-observed-effect level for the study
was 10 mg per kg of body weight per day. The useful mutagenicity
studies were reported to be negative.
The results of studies on the pharmacokinetics, mutagenicity, and
carcinogenicity of hydrazine were also considered by the Committee.
Pharmacokinetic data from rats and rabbits as well as hydrazine's
known chemical reactivity suggest that it should be rapidly
eliminated. The structure of carbadox and its known metabolic
pathways indicate that hydrazine is probably a further metabolite of
methyl carbazate. Hydrazine showed mutagenic and carcinogenic
potential.
In evaluating the potential toxicity of residues of carbadox, the
Committee also considered toxicity data on the carbadox metabolite
quinoxaline-2-carboxylic acid to be useful. In a carcinogenicity
study in mice in which quinoxaline-2-carboxylic acid was administered
in the diet for 19 months, no treatment related increases in tumour
incidence or any other effects on the animals were reported. The no-
observed-effect level was 100 mg per kg of body weight per day.
Several chronic toxicity and carcinogenicity studies in which
quinoxaline-2-carboxylic acid was administered for 24 months were
conducted in rats. There were no effects on the incidence of tumours,
the only findings being a slight reduction in weight gain at 100 mg
per kg of body weight per day. The no-observed-effect level was 50 mg
per kg of body weight per day.
In a three-generation reproduction study in rats, quinoxaline-2-
carboxylic acid was given in the diet at levels of up to 100 mg per kg
of body weight per day. No treatment-related effects were observed.
Developmental studies were conducted in rats and rabbits in which the
compound was administered by gavage at levels of up to 100 mg per kg
of body weight per day. There was no evidence of maternal toxicity,
embryotoxicity, or teratogenicity in either species. The no-observed-
effect levels in these studies were 100 mg per kg of body weight per
day.
4. EVALUATION
With current analytical procedures, quinoxaline-2-carboxylic acid
is the only carbadox metabolite that can be identified in liver from
pigs treated according to good practice in the use of veterinary
drugs. However, uncertainty remains because of the presence of
unidentified residues in liver. Because of the genotoxic and
carcinogenic nature of carbadox and some of its metabolites, the
Committee was not able to establish an ADI.
On the basis of data from studies on the toxicity of quinoxaline-
2-carboxylic acid, and on the metabolism and depletion of carbadox and
the nature of the compounds released from the bound residues, the
Committee concluded that residues resulting from the use of carbadox
in pigs were acceptable, provided that the recommended MRLs are not
exceeded.
5. REFERENCES
AMES, B. (1971). Chemical Mutagens, Vol. 1, Hollaender, A. (ed),
Plenum Press, New York. p. 267.
BEUTIN, L., PRELLER, E., & KOWALSKI, B. (1981). Mutagenicity of
Quindoxin, Its Metabolites, and Two Substituted Quninoxaline-Di-N-
Oxides. Antibacterial Agents and Chemotherapy, 20, 336-343.
BIANCIFIORI, C., & RIBACCHI, R., (1962). Pulmonary Tumour in Mice
Induced by Oral Isoniazid and its Metabolites. Nature, 194, 488-
489.
BIANCIFIORI, C. (1970). Hepatomas in CBA/Cb/Se Mice and Liver Lesions
in Golden Hamsters Induced by Hydrazine Sulfate. J. Nat. Cancer
Inst, 44, 943-953.
BRIGGS, G.B. (1971). Safety Evaluation of CP-16505, Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
CIHAK, R., & SRB, V. (1983). Cytogenetic Effects of Quinoxaline-1,4-
dioxide-type Growth-promoting Agents. Mutat. Res., 116, 129-135.
COLEMAN, G.L., (1967). Two year Chronic Study of GS-6244 (carbadox)
in Monkeys. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
COLEMAN, G.L., (1971). Quinoxaline-2-Carboxylic Acid, First Two Year
Study in Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
DOST, F.N., BRODERICK, D.J., KRIVAK, B.N., & REED, D.J., (1981).
Metabolism of Hydrazine. AFAMRL-TR-81-26. Technical Report. Air
Force Aerospace Medical Research Laboratory, Wright-Patterson AFB, OH.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
FACCINI, J.M., PERRAUD, J., NACHBAUR, J., & MONRO, A.M., (1979). 19-
Month toxicity Study in Mice. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1975).
Application of the Toxicity by Relay Method for the Assessment of
Safety to Human Consumers of Carbadox, a Growth-Promoting Additive, to
the Feed of Pigs Slaughtered for Human Consumption. Toxicology, 3,
369-398.
FERRANDO, R., TRUHAUT, R., RAYNAUD, J., & SPANOGHE, J., (1978). Human
Consumer Safety in the Use of Carbadox, a Pig Feed Additive, as
Estimated by Seven Years of Relay Toxicity in Beagles. Toxicology,
11, 167-183.
FERRANDO, R., (1980). Two Year Chronic Toxicity Study in the Rat with
Methyl Carbazate. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
FIGDOR, S.K., (1969). Carbadox in Pigs Radiotracer Metabolism C-14,
Carbonyl. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
FIGDOR, S.K., MCILHENNY, H.M., & VON WITTENAU, M.S., (1969).
Metabolism of Carbadox Using Carbadox Labelled with C14 in the Phenyl
Ring. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
HOLMES, C.L., (1976a). Action of CP-16,505 on Pregnancy and Fetal
Development in Rabbits. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
HOLMES, C.L., (1976b). Addendum Genetic Toxicology Report CP-17,056
(Desoxycarbadox) Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KELLER, W.C., MURPHY, J.P., ANDERSEN, M.E. & BACK, K.C., (1981).
Percutaneous Toxicokinetics of Hydrazine and H70 in the Rabbit.
AFAMRL-TR-81-13. Technical Report. Air Force Aerospace Medical
Research Laboratory, Wright-Patterson AFB, OH. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
KING, T.O., (1976). Target Organ Toxicity of GS-6244 (carbadox) and
CP-17,056 (desoxycarbadox) with Chronic Administration in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
KING, T.O., (1979a). Chronic Study in Rats with Quinoxaline-2-
Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
KING, T.O., (1979b). Three Generation Study in Rats with Quinoxaline-
2-Carboxylic Acid. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
NEGISHI, T., TANAKA, K., AND HYATSU, H., (1980). Mutagenicity of
Carbadox and Several Quinoxaline 1,4-Dioxide Derivatives. Chem.
Pharm. Bull., 28, 126-128.
OHTA, T., MORIYA, M., KANEDA, K., WATANABE, T., MIYAZAWA, A., &
SUSIGYAMA, F., (1980). Mutagenicity screening of Feed Additives in
the Microbial System. Mutat. Res., 77, 21-30.
PFIZER (undated a). Acute Toxicity of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (undated b). Genetic Toxicology of Carbadox. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
PFIZER (1963). A thirty-day Subacute Toxicity Study of GS-6244 in
Albino Rats. Unpublished. Submitted to WHO by Pfizer Central
Research, Groton, CT, USA.
PFIZER (1971). Two Year Study with CP-16,505 (Quinoxaline-2-
Carboxylic Acid) in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
PFIZER (1975). Genetic Toxicology Report CP-17,056 (Desoxycarbadox)
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
REINERT, H. (1976). Desoxycarbadox: Two Year Toxicity Study in Rats.
Unpublished. Submitted to WHO by Pfizer Central Research, Groton, CT,
USA.
ROE, F.J., GRANT, G.A., & MILLICAN, D.M., (1967). Carcinogenicity of
Hydrazine and 1,1-Dimethylhydrazine for Mouse Lung. Nature, 216,
375-376.
ROGERS, A.M., & BACK, K.C., (1981). Comparative Mutagenicity of
Hydrazine and 3 Methylated Derivatives in L5178Y Mouse Lymphoma Cells.
Mutat. Res., 89, 321-328.
RUTTY, D.A., (1972). Twenty-one Month Chronic Toxicity Study of
Methyl Carbazate in Rats. Unpublished. Submitted to WHO by Pfizer
Central Research, Groton, CT, USA.
SCALES, M.D., & TIMBRELL, J.A., (1982). Studies on Hydrazine
Hepatotoxicity. 1. Pathological Findings. J. Toxicol and Environ.
Health, 10, 941-953.
SEVERI, L., & BIANCIFIORI, C. (1968). Hepatic Carcinogenesis in
CBA/Cb/Se Mice and Cb/Se Rats by Isonicotinic Acid Hydrazine and
Hydrazine Sulfate. J. Nat. Cancer Inst., 41, 332-349.
SIGLER, F.W., (1969a). A Two-year Chronic Toxicity Study of GS-6244
in Rats. Dose Levels: 2.5, 1.0 or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SIGLER, F.W., (1969b). A Three Generation Reproductive Study of GS-
6244 in Rats. Dose Levels: 2.5, 1.0, or 0 mg/kg Day. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
SPIERENBURG, T.J., BAARS, A.J., DE GRAAF, G.J., & JAGER, L.P., (1988).
Carbadox-induced Inhibition of Aldosterone Production in Porcine
Adrenals in vitro. Toxicology In Vitro, 2, 141-143.
STEBBINS, R.B., (1964). A 3-Week Subacute Toxicity Study of GS-6244
in Rats - Dose Levels of 100, 50, 10, 5, or 0 mg/kg. Unpublished.
Submitted to WHO by Pfizer Central Research, Groton, CT, USA.
STEBBINS, R.B., & COLEMAN, G.L., (1967). A Twenty-six Month Chronic
Toxicity Study of GS-6244 in Rats - Dose Levels of 100, 50 10, 5, or
0 mg/kg. Unpublished. Submitted to WHO by Pfizer Central Research,
Groton, CT, USA.
SYKORA, I., & VORTEL, V., (1986). Post-natal Study of the
Carcinogenicity of Cyadox and Carbadox. Biol Vet Praha., 22, 53-62.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J., (1982). Studies on
Hydrazine Hepatotoxicity. 2. Biochemical Findings. J. Toxicol and
Environ. Health, 10, 955-968.
TOTH, B., (1972). Tumorigenesis Studies with 1,2-Dimethylhydrazine
dihydrochloride, Hydrazine Sulfate, and Isonicotinic Acid in Golden
Hamsters. Cancer Res, 32, 804-807.
VAN DER MOLEN, E.J., DE GRAAF, G.J., & BAARS, A.J., (1989a).
Persistence of Carbadox-induced Adrenal Lesions in Pigs Following Drug
Withdrawal and Recovery of Aldosterone Plasma Concentration.
J. Comp. Path., 100, 295-304.
VAN DER MOLEN, E.J., VAN LIESHOUT, J.H., NABUURS, M.J., DERKX, F.H.,
LAMERS, A.P., & MICHELAKIS, A.M., (1989b). Changes in Plasma Renin
Activity and Renal Immunohistochemically Demonstrated Renin in
Carbadox Treated Pigs. Res. Vet. Sci., 46, 401-405.
VON WITTENAU, M.S., (1969). Comparative Metabolism Studies in Swine,
Monkeys, and Rats of Carbadox. Unpublished. Submitted to WHO by
Pfizer Central Research, Groton, CT, USA.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J., (1980). The
Mutagenic Action of Quindoxin, Carbadox, Olaquindox, and Some other N-
oxides on Baceteria and Yeast. Mutat. Res., 78, 233-242.
VON WRIGHT, A. & TIKKANEN, L., (1980). The Comparative Mutagenicities
of Hydrazine and its Mono- and Di- methyl Derivatives in Bacterial
Test Systems. Mutat. Res., 78, 17-23.
YOSHIMURA, H., NAKAMURA, M., KOEDA, T., & YOSHIKAWA, K., (1981).
Mutagenicities of Carbadox and Olaquindox - Growth Promoters for Pigs.
Mutat. Res., 90, 49-55.
