
OLAQUINDOX
1. EXPLANATION
Olaquindox (I) is a quinoxaline 1,4-dioxide structurally related
to carbadox, quindoxin, and cyadox. The chemical structure is shown
in Figure 1.
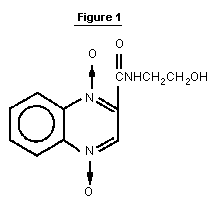 The compound is used as a growth promoter in pigs and is supplied
as a 10% premix in feed for admixture in the final feed at rates of
50-100 ppm for starter rations and 25-50 ppm in grower/fattener feeds
(Windholz et al., 1983; Anon,1989; Kulczyk et al., 1979; Baars
et al., 1988). It has not previously been evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. Biological Data
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion.
2.1.1.1 Rats
Olaquindox is well absorbed when given orally to rats.
Approximately 85% of the radioactivity from a dose of 10 mg/kg b.w. 3-
14C-olaquindox was excreted in the urine. Most radioactivity was
found in the urine within 3 hours of administration. The remainder
was eliminated in the faeces. Less than 1% was recovered in expired
air as carbon dioxide. Experiments with 3-14C-olaquindox
intraduodenally administered to rats with bile duct fistulas suggested
that around 18% of the dose was excreted in the bile. Similar
findings were made after intravenous dosing. Distribution occurred in
a generalized manner throughout the body after oral dosing and most of
the radioactivity had disappeared by 24 hours. Autoradiography
revealed the highest amount in the rat kidney at 4 hours, indicative
of the extent of urinary excretion already noted. Slightly elevated
concentrations were also observed in liver, testes, adrenals and hair
follicles. When dogs were given 10 mg/kg 3-14C-olaquindox, a similar
metabolic profile was noted to that seen in the rat (Duhm et al.,
1970).
2.1.1.2 Pigs
Olaquindox was rapidly absorbed when given orally to pigs. Over
90% of an oral dose of 2 mg/kg b.w. was eliminated in the urine within
24 hours, which is indicative of rapid and extensive absorption. The
remainder was excreted in the faeces. Maximum plasma levels were
attained within 1-2 hours of dosing (1-2 ppm). This was followed by
a rapid decline in plasma levels reaching around 0.03 ppm by 24 hours
and 0.005-0.01 ppm by 48 hours. Radioactivity was present in all
tissues when examined 2 days after dosing, but the levels were
extremely low. In the kidney and liver, levels of 110 and 52 ppb were
found, while levels in muscle were only 9 ppb. After 8 days, levels
in liver and kidney had fallen to 27 and 12 ppb, respectively, while
those in muscle were in the range of 2.5 ppb. By 28 days after dosing
only low levels were found in kidney and muscle (0.9 and 0.5-0.8 ppb,
respectively) with slightly higher concentrations in the liver (2 ppb)
(Duhm et al., 1973).
When pigs were dosed at levels in the range of those recommended
in use (up to 100 ppm in the diet) for up to 20 weeks, relatively high
levels were found in the kidney (around 2000 ppb) with relatively
moderate levels in the liver (300 ppb) when the animals were killed
six hours after drug withdrawal. When killed 2 days after withdrawal,
levels had fallen to below the limits of detection (50 ppb) in liver,
kidney and muscle. Pigs given diets containing olaquindox at levels
in excess of those recommended (160 or 250 ppm) for up to 4 weeks also
had high initial levels in kidney, liver and muscle but these had
fallen to below the limits of detection by day 2 after withdrawal
(Medenwald, 1974; Medenwald & Gericke, 1974; Bories & Bourdon., 1977).
Similar findings were made in other studies where pigs were given
diets containing 100 or 150 ppm olaquindox for 12-30 weeks (Takase &
Komachi, 1975; 1976).
After pigs were given diets containing up to 45 ppm olaquindox
for the duration of the fattening period, the highest levels were
found in the liver (0.14 ppm) and kidney (0.28 ppm) 6 hours after
withdrawal. By 24 hours the levels were below the limit of detection
(0.1 ppm) (Leibetseder, 1980). Similar results were noted when pigs
were given diets containing 10 ppm olaquindox (Anderson & Szabo,
1982).
2.1.2 Biotransformation
The biotransformation of olaquindox has been investigated only in
the pig. The majority of an oral dose of olaquindox (70%) was
excreted in the urine unchanged. The major metabolites appeared to be
the reduced compounds, the 1- or 4-mono-N-oxides (16%). Three other
compounds thought to be carboxylic acid derivatives made up the
remainder (Duhm et al., 1973). Later work led to the elucidation of
the structures of these metabolites in the pig. Again the major
urinary component after oral dosing was olaquindox with about 7%
present as the 4-mono-N-oxide. Omega oxidation produced the
2-carboxymethylaminocarbonyl compound and its 4-mono-N-oxide
derivative (6%). Some of the corresponding 1-mono-N-oxide moiety of
the 2-carboxymethylaminocarbonyl was also noted (1%). The remaining
metabolite was the di-desoxy derivative of
2-carboxymethylaminocarbonyl compound, 2-carboxymethylaminocarbonyl-3-
methyl quinoxaline (>1%) (Maul et al., 1979a and b).
2.1.3 Effects on enzymes and other biochemical parameters
Olaquindox has been shown to cause a decline in plasma
aldosterone levels in the pig accompanied at higher dietary levels by
hyponatraemia and hyperkalaemia. These findings are considered in
more depth in the section on short-term toxicity studies (van der
Molen et al, 1989; Baars et al, 1988).
2.2 Toxicological Studies
2.2.1 Acute Toxicity
Acute toxicity studies are summarized in Table 1.
In a series of experiments, groups of 10 male mice were given
oral doses of 2500-5000 mg/kg b.w. olaquindox as an aqueous suspension
in 2% carboxymethylcellulose. Vehicle controls or undosed groups did
not appear to have been used. Only 1/10 mice died at the lowest dose
used while 100% lethality was noted at the highest dose. Signs of
toxicity included decreased activity, lowering of the eyelids, and
irregular breathing. Animals died 2-14 days after olaquindox
administration. Discolored livers and yellowish-green intestinal
contents were noted on gross examination. Similar findings were made
when groups of male rats were given olaquindox in a similar manner at
doses of 1400-2000 mg/kg b.w. (Tettenborn, 1969).
Table 1: Acute toxicity of olaquindox
Species/strain Sex Route LD50 Reference
(mg/kg b.w.)
Mouse/CF1 M oral 3316 Tettenborn, 1969
M s.c. 2237 Tettenborn, 1969
Rat/Wistar M oral 1704 Tettenborn, 1969
M s.c. 1275 Tettenborn, 1969
M+F inhalation >1751 mg/m3* Thyssen, 1982
F oral 1657 Steinhoff, 1973
Rabbit/cross M+F oral 1000-2000 Tettenborn, 1969
M+F s.c. 1000-2500 Tettenborn, 1969
Cat/cross M+F oral 1000 Tettenborn, 1969
M+F s.c. 500 Tettenborn, 1969
Dog/beagle M+F oral emetic** Tettenborn, 1969
M+F s.c. *** Tettenborn, 1969
* 4 Hour LC50 value.
** Lethal doses could not be achieved due to emesis.
*** 250 mg/kg produced local reaction; temporary inappetence, higher
doses were not tried
Groups of two rabbits, each presumably 1 male and 1 female, were
dosed orally with olaquindox in 2% methylcarboxycellulose. No deaths
occurred at the lowest dose of 500 mg/kg b.w. while in those given
1000 or 2000 mg/kg b.w., 1/2 animals died. All the animals given 4000
mg/kg b.w. died. Similar observations were made in groups of two cats
given 500, 1000 or 2000 mg/kg b.w., with both animals given the
highest dose dying. Emesis was the main toxicological sign noted.
Dogs given oral doses of up to 100 mg/kg b.w. olaquindox showed no
signs of toxicity but those given 250-2000 vomited; no lethalities
occurred. Vehicle controls or undosed animals did not appear to have
been used in these studies (Tettenborn, 1969).
When given by the subcutaneous route as a suspension in 2%
carboxmethylcellulose, the acute toxicity of olaquindox was more
marked (Table 1). Doses of 500 to 2500 mg/kg b.w. and above proved
lethal to mice, rats, rabbits and cats. For unspecified technical
reasons, dogs were given only 250 mg/kg b.w. olaquindox, which
produced a local reaction and temporary inappetence a week later
(Tettenborn, 1969).
2.2.2 Short-term studies
2.2.2.1. Mice
Groups of 20 male and 20 female BOM-NMRI mice were fed diets
containing 0, 300, 600, 1200, 2400 and 4800 ppm olaquindox,
approximately equivalent to 0, 45, 90, 180, 360 and 720 mg/kg
b.w./day, for 90 days, as a dose-finding exercise for a
carcinogenicity study (see Section 2.2.3).
Signs of toxicity were non-specific and included shaggy fur,
dyspnoea and reduced motility. A marked reduction in body weight
occurred at the highest dietary level in both sexes, and in males
given 1200 and 2400 ppm. During the study 1/20 females died at the
600 ppm level as did 18/20 and 5/20 males and females, respectively,
at the 1200 ppm level. All the mice given the two highest doses died.
No deaths occurred in other groups. At necropsy, haemorrhagic lungs
were the main findings. Microscopic examination was not conducted
(Steinhoff & Gunselmann, 1982).
2.2.2.2 Rats
Groups of 10 male and 10 female Wistar rats were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in 2% aqueous
carboxymethylcellulose by stomach tube, for 5 days per week for 13
weeks.
After 6-8 weeks, signs of toxicity included reddening of the ears
and plantar surfaces; weakness and emaciation occurred in animals
given the highest dose. These animals also developed moist, blood
encrusted nostrils. In the eighth week of the study, fatalities began
to occur, consequently the animals were killed. No clinical signs of
toxicity or compound-related deaths occurred in the other groups.
There were no adverse haematological effects in any groups at 4 weeks
including the high dose group, nor at 12 weeks in remaining animals.
Clinical chemistry was normal at 4 weeks in all dose groups and in
animals given 0, 20 or 60 mg/kg b.w./day olaquindox at 12 weeks.
However, at 8 weeks in the high dose animals (prior to death), blood
sugar was significantly reduced, while serum aspartate
aminotransferase was elevated. Urinalysis was normal in all groups at
4 weeks and in all but the high dose group (not available for
examination due to deaths) at 12 weeks.
Absolute organ weights at 90 days indicated a significant
splenomegaly and increases in testicular and ovarian weights in
animals given 60 mg/kg b.w./day olaquindox. These findings were also
reflected in relative organ weights except for splenic weights in
females. There was a significant decrease in relative adrenal weights
in females given 60 mg/kg b.w./day olaquindox.
Gross examination revealed reddening of the pyloric area of the
stomach in the high dose animals and pale and atrophied adrenals. All
the females given 60 mg/kg b.w./day and 5/10 of those given 20 mg/kg
b.w./day had enlarged, reddened ovaries with numerous pin-point
darkened nodules (corpora lutea).
Microscopic examination revealed adrenal atrophy in the high and
mid dose groups, with degenerative changes in the cortical areas. Some
of the female high dose animals had thyroid atrophy. Female rats
given the mid and low dose had no ovarian atrophy but moderate
atrophic changes were noted in the ovaries of 4/5 high dose females
(Hoffmann 1969; Urwin & Mawdesly-Thomas, 1969).
The experiment was later repeated using lower doses: 0, 1, 5 and
20 mg/kg b.w./day. All other factors were identical to the original
study. No clinical signs were observed during the study and there
were no effects on body weights. There were no haematological or
clinical chemistry abnormalities; urinalyses were normal.
At necropsy, increases in adrenal weights in males given the two
highest daily doses were noted. Increases in ovarian weights occurred
in females given the two highest doses. Histopathologic examination
revealed no changes in any organs in any of the treatment groups. The
no-effect level in these studies therefore was 1 mg/kg b.w./day
(Hoffmann, 1972; Urwin & Spicer, 1971).
In a 90 day dietary study, olaquindox was given to groups of 20
male and 20 female Norway rats at levels of 0, 50, 150 and 300 ppm,
approximately equivalent to oral doses of 0, 5, 15 and 30 mg/kg
b.w./day. Haematological and clinical chemistry investigations were
conducted at days 0, 35, and 63 and at the end of the study.
No signs of toxicity were noted during the study and there were
no effects on haematology and clinical chemistry. Gross and
microscopic examination revealed no compound-related changes
(Nastuneak et al, 1986).
In an inhalation study, groups of 10 male and 10 female Wistar
rats were exposed to 0, 10, 207 and 541 mg/m3 olaquindox dust, 6
hours/day, 5 days per week for 3 weeks under dynamic exposure
conditions. Based upon number and particle mass median diameter,
between 30-80% of the particles were inhalable.
No adverse effects occurred in the rats exposed to the lowest
concentration of olaquindox nor in the males of the intermediate
concentration group. Females in this group showed non-specific
effects ("sluggishness") for the first 5 days of exposure but after
this they appeared normal. Effects of a similar nature and duration
occurred in the high concentration males but in females, signs of
respiratory distress were noted from the 11th day of exposure in
addition to the non-specific effects seen in other exposed groups.
No deaths occurred and only minor effects on body weights were
observed. There were no effects on haematology nor on clinical
chemistry. Urinalyses were normal. No gross abnormalities were
evident at autopsy and relative and absolute organ weights were in the
normal range. There were no adverse histopathological findings. The
non-specific signs and particularly the respiratory effects may have
been due to the physical effects of the olaquindox particles (Thyssen,
1983).
2.2.2.3 Rabbits
Olaquindox in Lutrol was applied to the shaven intact dorsal
surface of 3 groups of 6 New Zealand white rabbits (3 male and 3
female) at doses of 0, 50 or 250 mg/kg b.w./day for 6 hours/day, 5
days per week for 3 weeks, without an occlusive dressing. In an
identical manner, olaquindox was applied to the abraded skin of 3
groups of 3 male and 3 female rabbits.
No signs of toxicity were observed in treated animals and skin
reactions due to olaquindox were not seen in the abraded or intact
skin groups. No mortalities occurred. Clinical chemistry and
urinalyses at the end of the study were comparable to control values.
There were no effects on body weights. At necropsy, no abnormalities
were noted and no histopathological changes attributable to olaquindox
treatment were found (Heimann & Schilde, 1982).
2.2.2.4 Dogs
Groups of 2 male and 2 female beagle dogs were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in gelatin capsules for
90 days.
Vomiting occurred during the first week in dogs given the highest
dose. Salivation was noted and food intake declined. The animals
became emaciated. Dogs given the intermediate dose showed inappetence
and salivation occurred. No effects were noted in low dose animals.
All the high dose animals died within 20 days of the start of the
study. One dog given the intermediate dose died on day 40 while the
remainder were killed in extremis after 46 or 56 doses. No animals
given the low dose died.
There were no notable changes in haematology in treated dogs.
Clinical chemistry revealed increases in blood urea in all 4 dogs
given the high dose. Intermittent elevations of blood urea were noted
in the other dosed groups. No abnormalities in urinalyses occurred.
Gross examination of high dose animals suggested an irritant
effect on the gastrointestinal tract, with congestion in the lungs.
The livers were discolored. No abnormalities were noted in the low
dose animals. Histopathologic examination revealed live cell
enlargement and fatty degeneration in dogs given 60 or 180 mg/kg
b.w./day olaquindox, with fatty degeneration of the cells of the
kidney tubules. No histopathologic changes were found in low dose
dogs and the no-effect level in this study appeared to be 20 mg/kg
b.w./day (Lorke & Tettenborn, 1969; Mawdesley-Thomas & Urwin, 1969).
2.2.2.5 Pigs
Groups of 5 castrated male and female German landrace pigs
weighing 9-10 kg each were fed diets containing 0, 100, 160 and 250
ppm olaquindox for 20 weeks.
At the highest dietary level, 5 pigs died and weight gain was
significantly reduced. Animals given 100 and 160 ppm in the diet had
higher rates of weight gain than controls. No haematological effects
occurred. Plasma creatinine and urea were elevated in the
intermediate and high dietary level groups. Hyperkalaemia and
hyponatraemia were noted in pigs given 250 ppm dietary olaquindox.
Urinalyses were normal.
High dose pigs showed a grey-brown discoloration of the renal
cortex but there were no effects on relative organ weights. Tubular
dilatation and flattening of the tubular epithelium occurred in
intermediate and high dietary level pig kidneys and the adrenals of
these animals displayed enlarged cortical epithelial cells. The no-
effect level in this study was 100 ppm olaquindox in the diet (Gericke
& Dycka, 1974; Hoffman et al., 1974).
Groups of 7 hybrid piglets (4 weeks old, at least 3 females per
group and castrated males) were fed diets containing 0, 25, 50, 100
and 200 (2 groups) ppm olaquindox for 6 weeks.
After 2 weeks, dry faeces were produced by piglets given 100 or
200 ppm olaquindox. The drinking of urine from the floor of pens or
directly from urinating pen-mates was noted in pigs given 50 ppm
olaquindox. A decrease in abdominal volume occurred in piglets given
100 or 200 ppm olaquindox after 5 weeks and in the 25 ppm group in
week 6, but not in animals give 50 ppm. Significant rises in serum
albumin values occurred in piglets given 100 or 200 ppm olaquindox
from week 2 onwards, and marked rises in serum urea values occurred in
the 200 ppm group from week 4 and in the 100 ppm group from week 5.
Gross and microscopic pathology were not conducted (Nabuurs et al,
1989).
Groups of 6 female and 6 castrated male hybrid piglets were fed
diets containing 0, 25, 50, 100 or 200 ppm olaquindox for 6 weeks, in
a study of the effects of treatment on plasma aldosterone, sodium and
potassium levels. There was a gradual decline in plasma aldosterone
which was significant in all but the 25 ppm group by week 5. After 6
weeks the decline was significant in all dosed groups except for that
given 100 ppm where a small rise was noted. Hyponatraemia occurred in
the 25 and 200 ppm groups after weeks 0-2 and a continuous decline in
the 200 ppm group occurred after week 3. In the groups given 25 and
100 ppm olaquindox the levels decreased continuously from 2-3 weeks.
Animals in the 50 ppm group were unaffected. Although elevated
potassium levels occurred in the 50 and 100 ppm groups, only piglets
given 200 ppm were considered to be hypokalaemic (van der Molen
et al, 1989; Baars et al, 1988).
Similar effects have been noted in pigs given other quinoxaline
N-oxide drugs, namely cyadox and carbadox (van der Molen et al,
1985). The latter drug caused these effects after accidental
overdosage of pigs (Power et al, 1989). The toxicity appears to be
due to specific effects on the aldosterone-releasing zona glomerulosa
of the adrenals (van der Molen et al, 1986).
2.2.2.6 Rhesus monkeys
Olaquindox in gelatin capsules was given orally to two groups of
3 male and 3 female rhesus monkeys at doses of 0 and 20 mg/kg b.w./day
and to two groups of 3 males and 5 females at doses of 5 and 40 mg/kg
b.w./day, 7 days a week for 19 weeks. Surviving high dose females
were entered onto a 17 week recovery period.
Animals given the highest dose showed a general loss of condition
and loss of body weight. Suppression of weight gain occurred at the
intermediate dose while growth promotion occurred at the low dose.
Suppression of appetite was evident from week 12 in high dose monkeys.
Vaginal cytology indicated a suppression of ovulation in the high
dose females and in 1/3 animals given the intermediate dose. There was
some evidence of recovery after dosing ceased in the high dose
females. Deaths occurred in 2/3 males and 1/5 females form the high
dose groups during the dosing period and 2 females died during the
first two weeks of the recovery period.
Electrocardiography and ophthalmoscopy were normal in all
animals. Urinalyses and haematology were essentially normal at 5
weeks but serum aspartate aminotransferase values in high dose males
were elevated. At 8 weeks plasma biochemistry was normal but packed
cell volume and red cell counts were lowered in high dose animals.
Urinary glucose was found in 3 high dose males. After 15 weeks of
dosing packed cell volume and haemoglobin showed slight reductions in
high dose males while plasma glucose levels were reduced but not
significantly.
In 7/8 high dose monkeys, urine was positive for glucose and for
total reducing substances. One urine showed a lower pH and one was
positive for ketones. When examined at 15 weeks, red blood cell
values were reduced in high dose monkeys. Plasma glucose values were
reduced in high dose animals and protein and glucose were present in
the urines; pH of urine was lowered in high dose animals. At the end
of the 19 week dosing period no haematological examinations were
performed. Plasma glucose levels were reduced in high dose animals
while plasma urea values were increased. Hypokalaemia was noted in
high and intermediate dose animals. Glucose and total reducing
substances were increased in high dose monkeys while pH was lowered.
Macroscopic examination revealed pallor of the kidney in high
dose animals. Suppression of ovulation occurred in high dose females,
and abdominal abscesses in high dose males.
Histopathological examination revealed fatty changes in the
centrilobular areas of the liver in all high dose animals and
deposition of fat in the kidney tubules in all monkeys from this
group. Brown pigmentation of the zona reticularis of the adrenals
occurred in high dose monkeys. Immature testes were noted in males
given 20 and 40 mg/kg b.w./day olaquindox while inactivity of the
ovaries in high dose females and in 1/3 intermediate dose females was
observed. The no effect level in this oral study in rhesus monkey
was 5 mg/kg b.w./day (Heywood et al, 1972).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 20 male and 20 female NMRI mice were given nominal
doses of 0, 15 or 75 mg/kg b.w./day olaquindox in the drinking water.
Low dose mice were given a total of 6.6 g/kg b.w. for 635 days and
high dose mice were given a total of 32.1 g/kg b.w. for 634 days. The
study was terminated when all the mice had died. Animals were not
dosed over holidays and no mention was made of week-ends.
At termination, no excess tumour incidence was noted.
Lymphadenosis was reported in 1/40 control mice while 2/40 high dose
animals had tumours (thymoma and malignant thymus cell tumour) as did
2/40 low dose mice (pulmonary carcinoma and bronchial carcinoma.
Only small numbers of mice were used in this study and survival
was poor, although it was better in the high dose group than in
controls. The mean survival had a high standard deviation (mean
survival 340 ± 187, 338 ± 224 and 403 ± 194 days for mice given 0, 15
or 75 mg/kg b.w./day, respectively).
Moreover, even when survival was adjusted for unexplained deaths
which occurred in the first 82 days in controls, the treatment time
was still less than is currently viewed as normal for a mouse
carcinogenicity study. The short life-span on test may be due to the
fact that the animals were already around 50 days old when the study
commenced (Schmael, 1973).
In a later study, groups of 75 male and 75 female NMRI mice were
given diets containing 0, 40, 120 or 360 ppm olaquindox, equivalent to
0, 6, 18 or 57 mg/kg b.w./day, for life (until death or sacrifice in
a moribund state).
The only effects on body weights were in males and females given
the highest dietary level: male weights fell slightly below control
values from day 50 and female weights declined after day 200.
Haematological examination at weeks 4, 13, 26, 52 and 78 revealed no
abnormalities and survival times were unaffected by olaquindox intake;
remaining males and females died around day 890 (approximately 29
months).
At necropsy there were no differences in liver, kidney, spleen,
heart, testes, or brain weights, and no increases in non-neoplastic
findings were found in treated mice. No increased tumour incidence
was found in animals given 40 or 120 ppm dietary olaquindox but at 360
ppm there was an increase in the total number of tumours and in the
number of animals with benign tumours. These were due to increases in
the incidence of pulmonary adenoma and adrenal cortical adenoma in
males and in pulmonary adenoma and ovarian granulosa cell tumours in
females (Table 2). There were no increases in the incidence of any
malignant tumour types (Steinhoff & Gunselman, 1982).
Table 2: Tumor incidence in mice given olaquindox in the diet
0 ppm 40 ppm 120 ppm 360 ppm
Males
pulmonary adenoma 11 (15%) 17 (23%) 14 (19%) 27 (36%)
adrenal cortical 5 (7%) 3 (4%) 6 (8%) 13 (17%)
adenoma
Females
pulmonary adenoma 8 (11%) (7%) 7 (9%) 11 (15%)
ovarian granulosa 10 (13%) 16 (21%) 15 (20%) 20 (27%)
cell tumor
2.2.3.2 Rats
Groups of 20 male and 20 female Wistar rats were given
olaquindox, once per week for up to 560 days. The total dose was 4.7
g/kg b.w. with individual doses being in the range of 50-150 mg/kg
b.w. Each dose was given by gavage as a suspension in physiological
saline. Controls were given physiological saline only, but this was
administered by intraperitoneal injection and not by gavage as they
simultaneously acted as controls for other studies.
Survival in treated animals was better than controls (875 ± 105
days for male and 818 ± 167 days for female rats given olaquindox
compared with 797 ± 215 and 779 ± 187 days, respectively, for male and
female controls).
The incidence of tumours was not presented in a detailed tabular
form but was given graphically and in separate tables which did not
allow a full comparison. However, there was no elevated incidence of
any tumour type in treated animals when compared with controls and the
number of tumour-bearing animals was similar in both the treated and
control groups (Steinhoff, 1973).
Groups of 80 BR 46 rats were given drinking water containing
olaquindox at levels to ensure an intake of 15 or 75 mg/kg b.w./day.
A group of 49 rats given water only served as controls. Male and
female rats were used in the study but the sex ratio was not
specified. The treated water was supplied 5 days a week until the
animals died.
Survival of animals treated with olaquindox was better than
controls (704 ± 161 days and 655 ± 229 days in low and high dose rats,
respectively, compared with 554 ± 248 days in control rats). The
carcinogenicity data was presented in an unclear manner and separate
data for male and female animals were not given. The only tumour
which showed an increased incidence was mammary fibroadenoma (1/40,
2.5%; 3/46, 6.5% and 7/46, 15% in controls and in low and high dose
rats, respectively). However, the absence of data on male and female
incidence of this tumour renders the values uninterpretable (Schmael,
1973).
As part of a chronic toxicity/carcinogenicity study with
in utero exposure, groups of 75 males and 75 females were given
diets containing 0, 40, 120 or 360 ppm olaquindox. These doses were
approximately equivalent to oral doses of 0, 3, 10 and 30 mg/kg
b.w./day, for one week prior to mating and during a 3 week 1:1 mating
period. After mating the males were removed and the females given the
diets containing olaquindox until the young were 4 weeks old. At this
time the young from each treatment group were divided into groups of
25 males and 25 females and given the same diets as initially given to
their parents. The study was continued until the young had been
treated for 2 years. Clinical chemistry, haematology, and urinalyses
were conducted on groups of 5 males and 5 females at 4, 14, 26, 54 and
102 weeks after commencement of treatment of the F1 generation.
No overt signs of toxicity were noted in treated animals but
after day 400 of treatment, male and female animals given the highest
dietary level showed a marked and statistically significant reduction
in body weight when compared with control values.
Clinical chemistry suggested an elevated creatinine level in the
blood of rats given 360 ppm dietary olaquindox but all the values were
within the normal range. The albumen content of the urine was
generally lower in treated animals than in controls.
At gross and microscopic examination, there were no increases in
the incidences of non-neoplastic diseases and no increased incidence
of any tumour types. This study employed too few animals to allow an
assessment of carcinogenic potential (Steinhoff, 1977).
Olaquindox was tested in a carcinogenicity study using a similar
protocol on groups of 50 male and 50 female rats remaining in the F1
generation from the chronic toxicity carcinogenicity test described
above. The groups were given diets containing 0, 40, 120 and 360 ppm
olaquindox, approximately equivalent to oral doses of 0, 3, 10, and 30
mg/kg b.w./day. No clinical chemistry or haematological tests were
conducted, however, and the study was terminated when the duration was
approximately 3 years (1065 days for males and 1120 days for females),
at which time 20% of the controls (males and females separately)
remained alive.
No signs of toxicity were noted in treated animals except for
reductions in body weights in rats given 360 ppm after day 500.
Reductions occurred despite the fact that in terms of feed consumption
in g/kg b.w./day these animals had consumed more.
At termination there was a significant decrease in survival in
animals given the highest dietary level (98% mortality in males and
females) and in females given 40 ppm olaquindox (92% mortality)
compared with controls (80% mortality). Mortality in the other
dietary groups was only slightly more than in controls (82-86%).
At necropsy there were no differences between treated animals and
controls for the number of animals of each sex with total tumours,
primary tumours, malignant and benign tumours, malignant tumours with
metastases and total benign tumours. Similarly, for particular tumour
sites (benign and malignant tumours plus metastases) there were no
excess incidences except for slight increases in the incidence of
adrenal, reticuloendothelial and seminal vesicle neoplasms, but these
were due, except for the adrenal tumours, to metastases or
infiltrations from other organs. Moreover, the adrenal tumours were
not increased with respect to any particular histological type and
again were often of metastatic origin (Steinhoff & Boehme, 1978).
There were some anomalies in the reporting of this study which
appear to be due to arithmetical errors. The summary table of tumour
incidence cites a benign tumour in female controls which is not listed
in the detailed histopathology tables. The summary data also claims
4 malignant tumours in males given the highest dietary level whereas
the histopathology table lists 9. However, these differences do not
affect the outcome of the study or its assessment.
2.4 Reproduction Studies
2.4.1 Mice
Groups of 20 pregnant NMRI mice were given oral doses of 0, 20,
60, or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. On day 18 of
gestation the fetuses were delivered by Caesarean section and these
were weighed and examined for gross malformations and by alizarin
staining.
None of the pregnant animals died during the test but animals
given the highest dose showed reduction in body weight or rate of
weight gain. The numbers of implantations, live fetuses and
resorptions were similar in all dosed groups. At the highest dose,
fetal weights were significantly lower than in controls. The
incidence of malformations in all treated groups was similar to those
seen in controls (Lorke, 1971a).
2.4.2 Rats
Groups of 20 female pregnant FB 30 rats were given oral doses of
0, 20, 60 or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. Fetuses were
delivered by Caesarean section on day 20 of gestation and these were
examined in the same manner as for the mouse study described above.
The pregnant rats given the highest daily dose showed reductions
in body weights or rate of weight gain compared with control values.
These animals also showed a higher incidence of resorptions and lower
numbers of live fetuses. Fetal weights were lower in the high dose
animals. These indices were similar to controls and to rats given 20
or 60 mg/kg b.w./day olaquindox.
The incidence of malformations in fetuses from dams given 20 or
60 mg/kg b.w./day olaquindox was similar to controls but at the
highest dose level there was an elevated incidence of malformed
fetuses, with 5 malformations reported at a dose level of 180 mg/kg
b.w./day.
In this study, therefore, there was a teratogenic effect at the
highest dose level given to pregnant rats (180 mg/kg b.w./day) on days
6-15 of gestation. The no-effect level in this study was 60 mg/kg
b.w./day (Lorke, 1971b).
A 3-generation study was conducted in groups of 10 male and 20
female FB30 rats using 0, 20, 100 and 500 ppm olaquindox in the diet,
equivalent to oral doses of 0, 1, 5 and 25 mg/kg b.w./day. The F0
animals were given the diets containing olaquindox throughout the
study including during the mating periods. Animals were mated after
dosing for 70 days. Animals which died during the study were
necropsied, while young animals were examined macroscopically after
birth and subsequently observed during the rearing period for evidence
of abnormalities. The F3b animals were sacrificed at 3 weeks of age
and subjected to macroscopic and microscopic examination.
Treatment had no effect on body weights except that body weight
of F0 generation females given the highest dietary level of
olaquindox were slightly higher than weights of controls.
The fertility rate was also lowered in F0 animals in the first
and second matings when given the highest level, but there were no
effects on litter size nor on rearing rates. F1a and F1b animals did
not differ from controls in terms of birth weights except for a
slight, non-statistical increase in those derived from F0 dams given
the highest dietary level. After the second F0 mating the average
number of young in the F1b generation was similar in all treatment
groups, but at 5 days there was a significant reduction for dams given
500 ppm olaquindox (6/litter) compared with values in other groups and
controls (10-12/litter). Birth weights of the F1b generation were
unaffected.
When the F1b generation was mated, the gestation rates in animals
derived from animals initially given the highest level of olaquindox
were reduced (80-84%) compared with values for other groups (90-100%).
The average numbers of young per litter were also reduced at the high
dietary level in both the F2a and F2b generations, both at birth and
5 days after birth (8/litter compared with 11/litter in controls).
The F2 birth weights were unaffected and there was some improvement
in the rearing rates up to 4 weeks.
In the F3 generation, fertility was again affected in animals
originally derived from the high dietary level rates, with gestation
rates of 70-84% compared with 90-100% in other groups. The average
number of young per litter was also reduced at the high dietary level
(5-7/litter) compared with other groups (8.5-10.8/litter). There were
no effects on rearing rates nor F3 birth weights. No malformations
were noted during the course of the study and no abnormalities were
found on gross or histopathological examination of three week old F3b
animals (Loeser, 1974).
In a fertility test in Wistar rats, olaquindox was administered
orally by gavage to groups of 10 male rats at doses of 4 and 10 mg/kg
b.w./kg which were mated with groups of 20 untreated females. Groups
of 10 untreated males were mated with groups of 20 females given
gavage doses of 4 and 10 mg/kg b.w./day. Males were dosed for 8 weeks
prior to mating while females were dosed for 3 weeks prior to mating.
Males and females were mated in a 1:2 ratio. Groups of untreated
males and females were mated as controls.
Olaquindox administration had no effect on body weights, on
oestrus cycles, or on copulation and conception rates. A significant
reduction in the average number of implantations was noted in the
group in which females given the lower dose of 4 mg/kg b.w./day
olaquindox were mated with untreated males. Pre-implantation losses
were significantly increased in both the groups where females were
treated with olaquindox, and post-implantation losses were increased
in females given the 10 mg/kg b.w./day dose. There were no effects in
the groups where males treated with olaquindox were mated with
untreated females (Gandalovicova & Sykora, 1986).
2.2.5 Special studies on pharmacological properties
Olaquindox has been tested in a number of pharmacological
screening tests in rats and mice including those for anticonvulsive
effects, inhibition of defensive reaction, motor coordination,
analgesia, antihypertensive effects, gastric secretion, bile
secretion, diuresis, blood sugar and blood lipids and thrombocyte
aggregation (bovine plasma). No pharmacological activity was detected
(Kaller, 1970).
2.2.6 Special studies on irritancy and hypersensitivity
Olaquindox as a micronized powder was applied without a vehicle
to the shaved intact and abraded dorsal skin of 6 New Zealand white
rabbits using a 24-hour occlusive dressing. The skin was assessed on
removal of the dressing, after 48 and 72 hours, and at 7 days. Slight
erythema of intact and abraded skin was observed at 24 hours, but not
at the other time points. No oedema was noted. Results indicated a
mild irritant effect (Murman, 1979).
Olaquindox was tested for its ability to induce eye irritation by
application of 15 mg of the micronized substance to the conjunctiva of
the right eyes of 6 New Zealand white rabbits, and to the left
conjunctival sacs of a further 6 rabbits. The material was washed out
of the left eyes with physiological saline after 1 minute. Reactions
were monitored 24, 48, and 72 hours after application and after 7
days.
A slight reddening of the conjunctiva was noted in 4/6 eyes where
olaquindox had been directly applied. Slight chemosis was noted in
2/6 eyes. A slight chemosis was noted in 1/6 rabbit's eyes where
olaquindox has been applied to the conjunctival sac and then washed
out. All reactions subsided within 48 hours. The result suggest that
olaquindox has a slight irritant effect but the mechanical effects of
the dust cannot be ruled out (Murman, 1979).
Olaquindox was tested in the guinea-pig for its ability to induce
hypersensitivity. Olaquindox dissolved in dimethyl sulfoxide or as a
suspension in phosphate buffered saline was administered
intracutaneously into the neck region of groups of 10 Pirbright albino
guinea-pigs on days 1, 3, 6, 9, and 13 of a sensitization schedule.
Four days after the last injection, a suspension of olaquindox in 1:1
acetone/almond oil was applied to the depilated flank and massaged
gently into the skin. To allow for any possible effects of light,
groups of guinea-pigs were treated in a similar manner but were kept
in darkened cages. No indications of sensitization were noted in this
study when assessed by gross examination of the skin for reactions or
when examined histologically (Schlumberger, 1975). (This test is not
widely used and it is unlikely to be as sensitive as those using an
adjuvant (e.g. Freunds') such as the Magnusson-Kligman or Beuhler
models).
2.2.7 Special studies on genotoxicity
Olaquindox has been tested in a wide range of genotoxicity tests
and these are summarized in Table 3. It has produced positive results
in a number of studies designed to test for reverse mutations in
bacteria, including the Ames test with Salmonella typhimurium
strains (Beutin et al, 1981; Yoshimura et al, 1981; Voogd
et al, 1980; Nunoshiba & Nishioka, 1989). A positive result has
been noted in a forward mutation assay with Escherichia coli
(Nunoshiba & Nishioka, 1989).
In vitro study with cultured human lymphocytes and a number of
in vivo assays with mouse bone marrow or Chinese hamster
spermatogonia as the target tissues have demonstrated the clastogenic
activity of olaquindox (Tamura, 1977; Cihak & Vontorkova, 1983; Sram
et al, 1986a; Pokorna, 1986; Herbold, 1983a). Similarly, olaquindox
has produced positive results in several micronucleus tests in the
mouse following oral or inhalation exposure (Herbold, 1983b; Herbold
& Thyssen, 1982; Cihak & Vontorkova, 1985), and in the rat after
intraperitoneal injection (Cihak et al, 1983). However, a dermal
study with a 24 hour exposure was negative (Herbold, 1982a),
reflecting the poor dermal absorption of the substance.
Olaquindox has been tested in two dominant lethal assays in the
male mouse but a weak positive result was observed in only one of
these when a high dose (1 g/kg b.w) was employed (Machemer, 1977a;
Herbold, 1982b). Positive lethal mutations also occurred when female
mice were treated orally with olaquindox despite the use in one study
of doses lower than that which effected a positive result in the male
mouse (200 and 500 mg/kg b.w.) (Machemer, 1977b; Sram et al, 1986b
& c). However a toxic effect in female mice could not be excluded.
Positive results have been obtained in a sister chromatid
exchange test using Chinese hamster V79 cells indicating that
olaquindox may induce DNA damage (Scheutwinkel-Reich & von der Hude,
1984). Positive results in bacterial assays including the SOS
chromotest confirm this possibility (Suter et al, 1978; Beutin
et al, 1981; Yoshimura et al, 1981; Nunoshiba & Nishioka, 1989;
von der Hude et al, 1988). However, there is no evidence that
olaquindox covalently binds to DNA in the rat in vivo (Minini
et al, 1983).
The mutagenicity of a number of olaquindox metabolites has also
been investigated. The omega oxidation product, its 1 and 4
monodesoxy derivatives and its didesoxy derivative have been
investigated in the Ames test using S. typhimurium strains TA 98,
100, 1535 and 1537 with and without rat liver S9 metabolic activation.
All the tests gave negative results (Herbold, 1978; 1979a,b).
Table 3: Results of genotoxicity studies with olaquindox
Test System Test Object Concentration Results Reference
Ames test1 S. typhimurium 3.8-0.5 nmoles/plate Positive Beutin et al., 1981
TA98, 100
Ames test2,4 S. typhimurium 1.9-57 nmoles/plate Positive Beutin et al., 1981
TA100
Ames test1 S. typhimurium 1.25-15µg/plate Positive Yoshimura et al.,
TA98, 100 1981
Ames test2 S.typhimurium 0.01-0.1 mmole/1 Positive Voogd et al., 1980
Ames test1 S. typhimurium 0-50 µg/plate Positive Nunoshiba & Nishioka,
TA98,100 1989
Fluctuation1 test K. pneumoniae 2x10-4-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Fluctuation2 test K. pneumoniae 2x10-5-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Forward1 mutation E. coli 0-20 µg/plate Positive Nunoshiba & Nishioka,
Wp\P2uvrA/pKM101 1989
In vitro Cultured human 3-300 µg/ml Positive Tamura, 1977
cytogenetics lymphocytes
In vivo Mouse bone marrow 20, 500 or 800 mg/kg Negative Sutou, 1977
cytogenetics b.w. oral
In vivo Mouse bone marrow 200-800 mg/ml b.w. oral Negative Cihak & Vontorkova, 1983
cytogenetics
In vivo Mouse bone marrow 20-500 mg/kg b.w. diet Positive Sram, et al., 1986a
cytogenetics 4 and 12 weeks
Table 3 (contd)
Test System Test Object Concentration Results Reference
In vivo Chinese hamster 20 mg/kg b.w. oral, x5 Positive Pokorna, 1986
cytogenetics marrow
In vivo Chinese hamster 2x30-2x1000 mg/kg b.w. Positive Herbold, 1983a
cytogenetics spermatogonia oral
Micronucleus Mouse bone marrow 500 mg/kg b.w. oral, Positive Herbold, 1983b
sampled at 24, 48 or
72 hours; 10-300 mg/kg
b.w. oral, sampled at
24 hours
Micronuceus test Chinese hamster 20 mg/kg b.w. 4.2 and Positive Pokorna, 1986
bone marrow 100 mg/kg b.w. oral,
once
Micronucleus Mouse bone marrow 6.7 mg/m3 and 161 mg/m3 Positive Herbold, & Thyssen, 1982
for 6 hours/day, 2 days,
inhalation
Micronucleus Mouse bone marrow 2034 mg/kg b.w. 30 hours Negative Herbold, 1982a
dermal exposure
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Dominant lethal Mouse (male) 2x1000 mg/kg b.w. week Positive Machemer, 1977a
oral
Table 3 (contd)
Test System Test Object Concentration Results Reference
Dominant lethal Mouse (male) 40, 120 and 360 ppm in Negative Herbold, 1982b
diet for 35 days
equivalent to 6, 18, and
54 mg/kg b.w.
Dominant lethal Mouse (male) 100, 300 and 500 mg/kg b.w. Negative Sram, et al., 1986b
for 4 weeks, 20, 40, 100,
200, and 500 mg/kg b.w.
for 12 weeks, diet
Dominant3 lethal Mouse (female) 30, 100, 300 or 1000 mg/kg b.w. Positive Machemer, 1977b
oral once
Dominant lethal Mouse (female) 20, 40, 100, 200 and 500 mg/kg Positive Sram, et al., 1986c
b.w. diet, for 4 weeks
SOS2 Chromotest E. coli, GE94 0-10 µg/plate Positive Nunoshiba & Nishioka, 1989
(DNA damage)
SOS2 Chromotest E. coli, PQ37 0.001-0.1 mM Positive von der Hude, et al.,
(DNA damage)
DNA damage E. coli, K12 Not applicable Positive Suter, et al., 1978
DNA damage S. typhimurium 100 µg/disc uvr B and recA Positive Beutin, et al., 1981
DNA damage S. typhimurium 1-100 µg/disc Positive Yoshimura, et al., 1981
Mitotic gene2 S. cerevisiae D4 0.05% w/v Positive Voogd, et al., 1980
conversion
Table 3 (contd)
Test System Test Object Concentration Results Reference
DNA binding Rat 500 mg/kg b.w. oral Negative Minini, et al., 1983
in vivo
Sister chromatid Chinese hamster 0-200 µg/ml V79 cells Positive Scheutwinkel-Reich & von
exchange der Hude, 1984
1 With and without rat liver S-9 fraction.
2 In the absence of S-9 fraction.
3 Positive only at the 1000mg/kg b.w. dose.
4 Positive in aerobic and anaerobic conditions.
The results obtained with olaquindox are similar to those noted
with several other quinoxaline di-N-oxides including quindoxin and
carbadox (Suter et al, 1978; English & Dunegan, 1970; Voogd
et al, 1980; Negishi et al, 1980; Beutin et al, 1981 (see also
carbadox)). The mechanisms of action are unknown although neither
olaquindox nor quindoxin binds to DNA (Minini et al, 1983, Suter
et al, 1978).
Electron spin resonance techniques have demonstrated the
generation of free radicals during the reduction of quindoxin while a
related compound 2,3-dihydroxymethyl quinoxaline-1,4-di-N-oxide has
been shown to inhibit DNA synthesis in E. coli (Suter et al,
1978). However, at present the roles of free radicals and inhibition
of DNA synthesis in the mutagenicity of quinoxaline-1,4-dioxides are
unknown.
These studies indicate that olaquindox is genotoxic in a variety
of test systems. It induces mutations in bacterial systems and it
causes chromosome and DNA damage in in vitro and in in vivo
systems. Data available from dominant lethal assays and from an
in vivo cytogenetic study with Chinese hamster spermatogonia suggest
that olaquindox may have the potential to exert its mutagenic effects
on germ line cells.
2.3 Observations in humans
One of the major routes of exposure to olaquindox is likely to be
occupational during the preparation of feed and the feeding of the
final feed to pigs. In an experimental study, no olaquindox was
detected in the workplace air (a stall building housing pigs) during
trough filling operations with a feed containing 50 ppm olaquindox
(Thyssen et al, 1982). Olaquindox was only detected at low levels
in the atmosphere during the preparation of 0.1% feed premix and the
50 ppm final feed starting with a 10% premix of a proprietary product.
Levels in the atmosphere were estimated to be from below 0.4 to below
0.1 µg/m3 air (Inkmann-Koch, 1985a). Analysis of the urine from a
single worker engaged in similar preparation work and in the feeding
of pigs revealed no olaquindox (limit of detection 40 ppb) (Inkmann-
Koch, 1985b).
When applied to the skin of two volunteers as a paste in water
containing 2 g of olaquindox (around 30 mg/kg b.w.) using a 6 hour
occlusive dressing, no olaquindox was detected in the 48 hour urine
using an analytical method with a limit of detection of 0.12 µg/ml
(Beermann, 1982).
There has been one report of allergic contact dermatitis and one
of photocontact dermatitis following occupational exposure to
olaquindox (Bedello et al, 1985; Francalanci et al, 1986). Both
occurred in pig workers exposed to the substance in the animal feed.
There are no reports of systemic toxicity in humans following exposure
to olaquindox.
3. COMMENTS
The toxicological data considered by the Committee included the
results of acute and subchronic studies, together with the results of
studies on mutagenicity, carcinogenicity, and effects on reproduction
and development.
Olaquindox is almost completely absorbed from the
gastrointestinal tract in rats, dogs, and pigs. In studies using
radiolabelled olaquindox, the radioactivity was shown to be widely
distributed in the tissues, with residues in the liver being the most
persistent. The compound was primarily eliminated in the urine, with
lesser amounts being excreted in the faeces and expired air of the
animals. In the pig, elimination was virtually complete by 48 hours
after a single intragastric dose. Any remaining radioactivity was
then eliminated with a half-life of 5-9 days, far in excess of that of
olaquindox (about 3-5 hours). The parent compound accounted for 70%
of the radioactivity in the urine, up to 24 hours after
administration. There were approximately 16 metabolites detected in
the urine; six major metabolites have been fully characterized.
In acute and short-term toxicity studies in rats and mice, rats
were about twice as sensitive as mice. In a 90-day study in rats,
effects were observed in the testes, ovaries, thyroid, and adrenal
cortex at a dose of 5 mg/kg b.w./day and above. These lesions were
described histopathologically as atrophy; and in the adrenal glands
the effect was greatest in the zona glomerulosa. No treatment-related
abnormalities of the pituitary gland were reported. A 90-day study in
beagle dogs and a 19-week study in rhesus monkeys also produced
evidence of toxic effects on the endocrine glands, liver, and kidney.
Of five female monkeys dosed at 40 mg/kg b.w./day, one died during
treatment and two died during the planned 17 week recovery period. The
two survivors failed to re-establish normal ovarian cycles during the
recovery period.
In a 20-week study, groups of five male and five female pigs were
fed up to 250 mg of olaquindox per kg of diet. The plasma
concentrations of urea and creatinine were elevated at 160 and 250 mg
diet, which suggested an effect on the kidney. All of the pigs in the
highest-dose group that survived the study period continued to show
evidence of this effect during a 16 day-recovery period. Plasma
sodium, potassium and chloride concentrations were altered during the
study, but did not return to normal during the recovery period.
Histopathological changes were found at necropsy in kidney and adrenal
tissues from both groups. The manufacturers reported a no-observed-
effect level of 100 mg/kg in feed for this study.
However, in other studies in piglets in which olaquindox was fed
at 25, 50, 100 or 200 mg/kg diet for 6 weeks, a dose-dependent fall in
plasma aldosterone concentration, together with hyponatraemia,
hypochloraemia, and hyperkalaemia occurred in all groups by the end of
the study. Hydropic degeneration of adrenal cortex cells was
recorded. There were no effects on weight gain or clinical signs.
Developmental studies in which the compound was administered
orally were conducted in mice and rats. In mice, fetal weights were
reduced at 180 mg/kg b.w./day, but malformations were absent. In
rats, malformations occurred at 180 mg/kg b.w./day, and reductions in
maternal weight gain, litter size, and fetal weight were also observed
at this dose.
A three-generation reproduction study in which olaquindox was
administered in the diet was conducted in rats. No malformations were
observed, the only findings being reductions in fertility rate and
litter size in the second and third generations at the highest dose of
25 mg/kg b.w./day.
The genotoxicity of olaquindox was investigated in a range of
in vitro and in vivo studies. Positive findings were reported in
assays for point mutation and for DNA damage in bacteria, sister-
chromatid exchange in Chinese hamster V-79 cells, chromosome damage in
human lymphocytes in vitro, mammalian bone-marrow cells in vivo,
and in several micronucleus tests. Two dominant lethal assays in male
mice were negative, but a third gave a weak positive response. Two
dominant lethal assays in female mice gave positive results, but this
may have been partly due to the toxicity of the drug. A weak positive
result was obtained in an in vivo cytogenicity assay using Chinese
hamster spermatogonia. Olaquindox did not bind to rat DNA in vivo.
The Committee considered data from six long-term studies in
rodents, but because of poor survival and deficiencies in experimental
design and reporting, only two carcinogenicity studies, in mice and in
rats, were evaluated.
In the study in mice, doses of olaquindox up to an equivalent of
54 mg/kg b.w./day were administered in the diet for life (up to 635
days). An increase in the incidence of benign adrenal cortical
adenomas and benign proliferative lesions (nodular hyperplasia and
adenoma) in the lung was observed in male mice at the highest dose
level. There was no effect on the incidence of malignant tumours.
In the rat carcinogenicity study, which included fetal exposure
to the drug in utero, olaquindox was administered in the diet at
levels up to an equivalent of 30 mg/kg b.w./day until 80% of the male
and female controls had died (about three years). Survival was
decreased at 30 mg/kg b.w./day in both sexes, but there was no
increase in the incidence of benign or malignant tumours.
4. EVALUATION
The Committee considered olaquindox to be a genotoxic agent.
There was some evidence to suggest that olaquindox was a germ-line
mutagen, but more extensive testing in appropriate mammalian studies
will be required to resolve this issue. In the carcinogenicity
studies only the mouse showed an increase in the incidence of tumours,
and these were benign. Because of doubts over the mechanism of this
effect and the results of the genotoxicity studies, the Committee was
unable to establish an ADI for olaquindox. However, the Committee
concluded that residues resulting from the use of olaquindox in food-
producing animals under conditions of good practice in the use of
veterinary drugs were temporarily acceptable.
The results of studies on the nature and availability of residues
of olaquindox and the results of studies designed to provide an
indication of the toxic potential of these residues are required by
1993.
Depending upon the results of these studies, the following
additional information may be needed:
a) Data to assess the genotoxic potential of olaquindox on
germ-line cells, which would, at a minimum, necessitate a
repeat of the Chinese Hamster spermatogonia study.
b) Studies designed to assess the effects of olaquindox on
adrenal function (including sensitive parameters such as
plasma adrenal hormones and electrolyte levels), sperm
morphology, and fertility in rats, so that a NOEL can be
determined for each of these indicators.
c) Information on the binding of olaquindox or its metabolites
to structural proteins such as tubulin, or to enzymes or
proteins involved in DNA synthesis or repair. (Such binding
may help explain why, despite its obvious genotoxic
potential, olaquindox does not bind to DNA and has given
equivocal results in carcinogenicity studies).
5. REFERENCES
ANDERSON, B., & SZABO, A. (1982). Determination of olaquindox in
swine tissues after short withholding periods. Report No. 08-07-
1981/24-03-1982. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
ANON. (1989). Compendium of Data Sheets for Veterinary Products
1989-90. National Office of Animal Health, Datapharm Publications
Ltd, London.
BAARS, A.J., VAN DER MOLEN, E.J., SPIERENBURG, T.J., DE GRAAF, G.J.,
NABUURS, J.J.A., & JAGER, L.P. (1988). Comparative toxicity of three
quinoxaline-di-N-dioxide feed additives in young pigs. Arch.
Toxicol., Suppl. 12, 405-409.
BEDELLO, P.G., GOITRE, M., CANE, D., & RONCAROLO, G. (1985). Allergic
contact dermatitis to Bayo-N-Ox-1. Contact Derm., 12, 284.
BEERMAN, D. (1982). Cutaneous absorption of olaquinodox in human
volunteers. Doc. No 82/10744. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
BEUTIN, L., PRELLER E., & KOWALSKI, B. (1981). Mutagenicity of
olaquindox, 3its metabolites, and two substituted quinoxaline-di-N-
oxides. Antimicrob. Agents Chemother., 20, 336-343.
BORIES, G., & BOURDON, D. (1977). Residue analysis in pigs. Doc. No.
77/8813. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
CIHAK, R., SRB, V., & VONTOKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. I. Micronucleus
test in rats. Mutat. Res., 116, 129-135.
CIHAK, R., & VONTORKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. II. Metaphase
analysis in mice. Mutat. Res., 117, 311-316.
CIHAK, R., & VONTORKOVA, M. (1985). Cytogentic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. III.
Transplacental micronucleus test in mice. Mutat. Res., 144, 81-84.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A.,
(1970). Resorption, distribution, substance changes, and elimination.
Short test in the rat and in the dog. Report No. 1939. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A., &
SCHEER, M., (1973). Metabolism and residue studies wth 14C-labelled
Bay VA 9391 in swine. Report No. 4151. Unpublished report by Bayer
AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
ENGLISH, A.R., & DUNEGAN, C.M. (1970). Quinoxaline-1, 4-di-N-oxides.
I. Inhibition of deoxyribonucleic acid synthesis in Escherichia
coli by 2, 3-dihydroxy-methyl-quinoxaline-1, 4-di-N-oxide. Proc.
Soc. Exp. Biol. Med., 133, 398-400.
FRANCALANCI, S., GOAL, M., GIORGINI, S., MUCCINELLI, A., & SERTOLI, A.
(1986). Occupational dermatitis from olaquinodox. Contact Derm.,
15, 112-114.
GANDALOVICOVA, D., & SYKORA, I. (1986). Cyadox olaquindox - a
fertility test on rats. Biol. Chem. Vet., (Prague), 22, 47-51.
GERICKE, H., & DYCKA, J. (1974). Olaquindox/Bayo-N-ox.
Investigations into the safety of use of the feed additive. Report
No. 4752. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HEIMANN, K.G. & SCHILDE, B., (1982). BAY VA 9391. Study of subacute
dermal toxicity in rabbits. Report No. 11267(E). Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1978). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No 2. 7824. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979a). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 8091/8092/8099. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979b). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 7824. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982a). Micronucleus test on the mouse to check for
mutagenic effect after cutaneous application. Report No. 10602.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982b). Dominant lethal test on male mice to check for
a mutagenic effect after 35 days of administration in the feed.
Report No 10621. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HERBOLD, B. (1983a). Cytogenetic study of the spermatogonia of the
Chinese hamster in vivo to test for mutagenic activity. Report No.
12179. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1983b). Micronucleus test for a mutagenic effect on on
the mouse. Report No. 11424(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
HERBOLD, B., & THYSSEN, J. (1982). Micronucleus test on the mouse to
test for mutagenic activity after inhalation of dynamically produced
dust. Report No. 10833. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HEYWOOD, R., SORTWELL, R.J., NEWMAN, A.J. & STREET, A.E. (1972).
Report No. 4751/72/186. FB a 9391. Oral Toxicity study in rhesus
monkeys. (Repeated dosage for nineteen weeks and recovery period).
Unpublished report from Huntingdon Research Centre, Huntingdon, UK.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1969). BAY VA 9391. Subchronic toxicity in rats
following oral administration. (Trial over 13 weeks). Report No.
1693. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1972). BAY VA 9391. Subchronic toxicity in rats with
oral administration. (13-week test with low doses). Report No. 3241.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K., LUCKHAUS, G. & DYCKA, J. (1974). BAY VA 931.
Toxicological feeding test in feeder swine. Part 3. Results of the
toxicological, pathologic-anatomic and histopathological studies.
Report No. 4754. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
INKMANN-KOCH, A. (1985a). Determination of the concentration of
olaquindox in the air during the preparation of compound (meal) swine
feed and exposure of workers. Doc. No. 85/11885. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
INKMANN-KOCH, A. (1985b). Determination of olaquindox in urine
samples of a worker follwing the preparation of swine feed from Bayo-
N-Ox. Doc. No. 85/11887. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
KALLER, H. (1970). BAY VA 9391. Pharmacological screening. Report
No. 1809. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
KULCZYK, S.R., HERRERA, R.E., MULKEY, N.S., BREAULT, G.O., WARGO,
J.P., & WAGGONER, T.B. (1979). Determination of physical constants of
BAY VA 9391. Report No. 79/9803. Unpublished report from Analytical
Development Corporation, USA. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
LEIBETSEDER, J. (1980). To determine olaquindox residues in swine.
Doc. No. 80/10022. Unpublished report from the Institute of Nutrition
of the Veterinary Medical University, Vienna, Austria. Submitted to
WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LOESER, E. (1974). BAY Va 9391. Three-generation test in rats.
Report 4748. Unpublished report from Bayer AG, Wuppertal-Elberfeld,
FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE, D. & TETTENBORN, D. (1969). FBa 9391. Subchronic toxicity of
dogs during oral administration. (13 Week test). Report No. 1536.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE D. (1971a). FBa 9391. Examination for embryotoxic effects in
mice. Report No. 2692. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
LORKE, D. (1971b). FBa 9391. Examination for embryotoxic effects in
rats. Report No. 2693. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
MACHEMER, L. (1977a). To evaluate mutagenic potential of BAY VA 9391
in mice utilizing the dominant lethal test. Report No. 6738.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
Machemer (1977b). To investigate BAY VA 9391 for mutagenic effects
using the dominant lethal test. Report No. 6770. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
MAUL, W., SENG, F. & WENDISCH, D. (1979a). Investigations into the
biotransformation of olaquinox in swine. Report No. 8114.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAUL, W, SENG, F. & WENDISCH, D. (1979b). Investigations into the
biotransformation of olaquinox in swine. Report No. 8725.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAWDESLEY-THOMAS, L.E. & URWIN C. (1969). Pathology report of FB a
9391. Sub-chronic toxicity for dogs with oral administration. Report
No. 2921/69/347. Unpublished report from Huntingdon Research Centre,
Huntingdon, UK. Submitted to WHO Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. (1974). Method for the determination of residues of Bay
VA 9391 and its reduction products in animal tissues. Report No.
4751. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. & GERICKE, H. (1974). Toxicological Feeding Test with
Feeder Pig. Part 2. Results of Residue Analysis. Report No. 4753.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MININI, U., LUTZ, W.K., & SCHLATTER, CH. (1983). Lack of in vivo
binding to DNA of olaquindox. Report No. R2570 (P). Unpublished
report from the Institute of Toxicology, Swiss Federal Institute of
Technology and University of Zurich, Schwerzenbach, Switzerland.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MURMAN, P.(1979). BAY VA 9391 (Olaquindox). Testing of primary
dermal and mucosal tolerability in the rabbit. Report No. 8359.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
NABUURS, M.J.A., VAN DER MOLEN, E.J., DE GRAAF, G.J., & JAGER, L.P.
(1989). Clinical Signs and Performance of Pigs Treated with Different
Levels of Carbadox, Cyadox and Olaquindox. J. Vet. Med., 36A,
209-217.
NASTUNEAK, J., KOLOUCH, F., VANOVA, L., DVORAK, M. & SEVICK, B.
(1986). Three month toxicity to laboratory Norway rats: Cyadox
compared with olaquindox. Biol. Chem.Vet. (Prague), 22, 367-380.
NEGISHI, T., TANAKA, K. & HAYATSU, H. (1980). Mutagenicity of
carbadox and several quinoxaline 1,4-dioxide derivatives. Chem.
Pharm. Bull., 28, 1347-1349.
NUNOSHIBA, T., & NISHIOKA, H. (1989). Genotoxicity of quinoxaline
1,4-dioxide derivatives in Escherichia coli and Salmonella
typhimurium. Mutat. Res., 217, 203-209.
POKORNA, D. (1986). Cytogentic analysis of bone marrow cells in
Chinese hamster after the administration of cyadox and olaquindox.
Biol. Chem. Vet. (Prague)., 22, 23-28.
POWER, S.B., DONNELLY, W.J.C., MCLAUGHLIN, J.G., WALSH, M.C. & DROMY,
M.F. (1989). Accidental carbadox overdosage in pigs in an Irish
weaner-producing herd. Vet. Rec., 124, 367-370.
SCHEUTWINKEL-REICH, M. & VON DER HUDE, W. (1984). Sister-chromatid
exchange in Chinese hamster V79 cells exposed to quindoxin, carbadox
and olaquindox. Mutat. Res., 139, 199-202.
SCHLUMBERGER, H.D. (1975). BAY VA 9391: Exclusion of immunogenic
activity. Report No. 5194. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
SCHMAEL, D. (1973). Carcinogenicity test with BAY VA 9391 with oral
application to rats and mice. Report N. 38399. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986a).
Chromosomal aberration in the bone marrow of mice after long-continued
administration cyadox and olaquindox. Biol. Chem. Vet. (Prague), 22,
17-22.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986b). Effect
of long-term oral administration of cyadox and olaquindox on the
frequency of dominant lethal mutations and sperm abnormalities in
mice. Biol. Chem. Vet. (Prague), 22, 37-45.
SRAM, R.J. FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986c).
Dominant lethal mutation in female mice following the oral application
of cyadox and olaquindox. Bio. Chem. Vet. (Prague), 22, 29-35.
STEINHOFF, D. (1973). Carcinogenicity study with Bay VA 9391 after
oral administration to rats. Report No. 4048. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
STEINHOFF, D. (1977). BAY VA 9391. Study as to chronic toxic effect
in rats after oral application. (2-Year trial with additional
transplacental treatment). Report No. 6556. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
STEINHOFF, D., & BOEHME, K. (1978). BAY VA 9391. Carcinogenesis
tests with oral administration to rats. (With transplacental
pretreatment). Report N. 7387. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
STEINHOFF, D., & GUNSELMANN, W. (1982). BAY VA 9391. Carcinogenicity
experiment with oral administration to mice. Report No. 72388
(revised), 100700 (revised), 12251 (revised). Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
SUTER, W., ROSSELET, A., & KNUSEL, F. (1978). Mode of action of
quindoxin and substituted quinoxaline-di-N-oxides on Escherichia
coli. Antimicrob. Agents Chemother., 13, 770-783.
SUTOU, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No 78/9327. Unpublished report from Medical Biology
Laboratory, Life Science Department, Nomura Resarch Station, Japan.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
TAKASE, I., & KOMACHI, S. (1975). Determination of BAY VA 9391
residues in animal tissues. Doc. No. 76/8507. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer Ag, Wuppertal-Elberfeld, FRG.
TAKASE, I & KOMACHI, S. (1976). Residues of BAY VA 9391 (olaquindox)
in tissues and organs of pigs. Doc No. 76/8508. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer AG, Wuppertal-Elberfeld, FRG.
TAMURA, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No. 78/9326. Unpublished report from Keio University,
School of Medicine, Tokyo, Japan. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
TETTENBORN, D. (1969). FBa 9391. Acute toxicity for mouse, rat,
rabbit, cat, and dog with oral and subcutaneous administration.
Report No. 1176. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J. (1982). Olaquindox. Acute inhalation toxicity of the
dust. Report No. 10755. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J., BEERMANN, D., & DORN, H. (1982). Exposure studies at the
place of work on administration of Bayo-N-Ox-Ferkelaufzuchtfutter
(Special Piglet Feed) F2 in pellet or meal form. Report 10731.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
THYSSEN J. (1983). Olaquindox BAY VA 9391. Subacute inhalation study
in rats. Report N. 13032(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
URWIN, C., & MAWDESLEY-THOMAS, L.E. (1969). Pathology report of Bay
a 9391. Subchronic toxicity for rats by oral administration. Report
No. 2989/69/415. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
URWIN, C., & SPICER, E.J.F., (1971). Pathology report of Bay a 9391.
Subchronic toxicity study for rats by oral administration. Report No.
4389/71/545. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
VAN DER MOLEN, E.J., NABUURS, M.J.A., & JAGER, L.P. (1985).
Pathological and clinical changes related to toxicity of carbadox in
weaned pigs. Zbl. Vet. Med., 32, 540-550.
VAN DER MOLEN, E.J., DE GRAAF, G.J., SPIERENBURG, TH. J., NABUURS,
M.J.A., BAARS, A.J. & JAGER, L.P., (1986). Hypoaldosteronism in
piglets induced by carbadox. Experientia., 42, 1247-1249.
VAN DER MOLEN, E.J., BAARS, A.J., DE GRAAF, G.J. & JAGER, L.P. (1989).
Comparative study of the effect of carbadox, olaquindox and cyadox on
aldosterone, sodium and potassium plasma levels in weaned pigs. Res.
Vet. Sci., 47, 11-16.
VON DER HUDE. W., BEHM, C., GURTLER, R., & BASLER, A. (1988).
Evaluation of the SOS chromotest. Mutat. Res., 203, 81-94.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1980). The
mutagenic action of quindoxin, carbadox, olaquindox and some other
N-oxides on bacteria and yeast. Mutat. Res., 78, 233-242.
WINDHOLZ, M., BUDAVARIA, S., BLUMETTI, R.,F., & OTTERBEIN, R.R.S (Eds)
(1983). The Merck Index, 10th Edition, Merck & Co., Rahway, New
Jersey.
YOSHIMURA, H., NAKAMURA, M., & KOEDA, T. (1981). Mutagenicities of
carbadox and olaquindox - growth promoters for pigs. Mutat. Res.,
90, 49-55.
The compound is used as a growth promoter in pigs and is supplied
as a 10% premix in feed for admixture in the final feed at rates of
50-100 ppm for starter rations and 25-50 ppm in grower/fattener feeds
(Windholz et al., 1983; Anon,1989; Kulczyk et al., 1979; Baars
et al., 1988). It has not previously been evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. Biological Data
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion.
2.1.1.1 Rats
Olaquindox is well absorbed when given orally to rats.
Approximately 85% of the radioactivity from a dose of 10 mg/kg b.w. 3-
14C-olaquindox was excreted in the urine. Most radioactivity was
found in the urine within 3 hours of administration. The remainder
was eliminated in the faeces. Less than 1% was recovered in expired
air as carbon dioxide. Experiments with 3-14C-olaquindox
intraduodenally administered to rats with bile duct fistulas suggested
that around 18% of the dose was excreted in the bile. Similar
findings were made after intravenous dosing. Distribution occurred in
a generalized manner throughout the body after oral dosing and most of
the radioactivity had disappeared by 24 hours. Autoradiography
revealed the highest amount in the rat kidney at 4 hours, indicative
of the extent of urinary excretion already noted. Slightly elevated
concentrations were also observed in liver, testes, adrenals and hair
follicles. When dogs were given 10 mg/kg 3-14C-olaquindox, a similar
metabolic profile was noted to that seen in the rat (Duhm et al.,
1970).
2.1.1.2 Pigs
Olaquindox was rapidly absorbed when given orally to pigs. Over
90% of an oral dose of 2 mg/kg b.w. was eliminated in the urine within
24 hours, which is indicative of rapid and extensive absorption. The
remainder was excreted in the faeces. Maximum plasma levels were
attained within 1-2 hours of dosing (1-2 ppm). This was followed by
a rapid decline in plasma levels reaching around 0.03 ppm by 24 hours
and 0.005-0.01 ppm by 48 hours. Radioactivity was present in all
tissues when examined 2 days after dosing, but the levels were
extremely low. In the kidney and liver, levels of 110 and 52 ppb were
found, while levels in muscle were only 9 ppb. After 8 days, levels
in liver and kidney had fallen to 27 and 12 ppb, respectively, while
those in muscle were in the range of 2.5 ppb. By 28 days after dosing
only low levels were found in kidney and muscle (0.9 and 0.5-0.8 ppb,
respectively) with slightly higher concentrations in the liver (2 ppb)
(Duhm et al., 1973).
When pigs were dosed at levels in the range of those recommended
in use (up to 100 ppm in the diet) for up to 20 weeks, relatively high
levels were found in the kidney (around 2000 ppb) with relatively
moderate levels in the liver (300 ppb) when the animals were killed
six hours after drug withdrawal. When killed 2 days after withdrawal,
levels had fallen to below the limits of detection (50 ppb) in liver,
kidney and muscle. Pigs given diets containing olaquindox at levels
in excess of those recommended (160 or 250 ppm) for up to 4 weeks also
had high initial levels in kidney, liver and muscle but these had
fallen to below the limits of detection by day 2 after withdrawal
(Medenwald, 1974; Medenwald & Gericke, 1974; Bories & Bourdon., 1977).
Similar findings were made in other studies where pigs were given
diets containing 100 or 150 ppm olaquindox for 12-30 weeks (Takase &
Komachi, 1975; 1976).
After pigs were given diets containing up to 45 ppm olaquindox
for the duration of the fattening period, the highest levels were
found in the liver (0.14 ppm) and kidney (0.28 ppm) 6 hours after
withdrawal. By 24 hours the levels were below the limit of detection
(0.1 ppm) (Leibetseder, 1980). Similar results were noted when pigs
were given diets containing 10 ppm olaquindox (Anderson & Szabo,
1982).
2.1.2 Biotransformation
The biotransformation of olaquindox has been investigated only in
the pig. The majority of an oral dose of olaquindox (70%) was
excreted in the urine unchanged. The major metabolites appeared to be
the reduced compounds, the 1- or 4-mono-N-oxides (16%). Three other
compounds thought to be carboxylic acid derivatives made up the
remainder (Duhm et al., 1973). Later work led to the elucidation of
the structures of these metabolites in the pig. Again the major
urinary component after oral dosing was olaquindox with about 7%
present as the 4-mono-N-oxide. Omega oxidation produced the
2-carboxymethylaminocarbonyl compound and its 4-mono-N-oxide
derivative (6%). Some of the corresponding 1-mono-N-oxide moiety of
the 2-carboxymethylaminocarbonyl was also noted (1%). The remaining
metabolite was the di-desoxy derivative of
2-carboxymethylaminocarbonyl compound, 2-carboxymethylaminocarbonyl-3-
methyl quinoxaline (>1%) (Maul et al., 1979a and b).
2.1.3 Effects on enzymes and other biochemical parameters
Olaquindox has been shown to cause a decline in plasma
aldosterone levels in the pig accompanied at higher dietary levels by
hyponatraemia and hyperkalaemia. These findings are considered in
more depth in the section on short-term toxicity studies (van der
Molen et al, 1989; Baars et al, 1988).
2.2 Toxicological Studies
2.2.1 Acute Toxicity
Acute toxicity studies are summarized in Table 1.
In a series of experiments, groups of 10 male mice were given
oral doses of 2500-5000 mg/kg b.w. olaquindox as an aqueous suspension
in 2% carboxymethylcellulose. Vehicle controls or undosed groups did
not appear to have been used. Only 1/10 mice died at the lowest dose
used while 100% lethality was noted at the highest dose. Signs of
toxicity included decreased activity, lowering of the eyelids, and
irregular breathing. Animals died 2-14 days after olaquindox
administration. Discolored livers and yellowish-green intestinal
contents were noted on gross examination. Similar findings were made
when groups of male rats were given olaquindox in a similar manner at
doses of 1400-2000 mg/kg b.w. (Tettenborn, 1969).
Table 1: Acute toxicity of olaquindox
Species/strain Sex Route LD50 Reference
(mg/kg b.w.)
Mouse/CF1 M oral 3316 Tettenborn, 1969
M s.c. 2237 Tettenborn, 1969
Rat/Wistar M oral 1704 Tettenborn, 1969
M s.c. 1275 Tettenborn, 1969
M+F inhalation >1751 mg/m3* Thyssen, 1982
F oral 1657 Steinhoff, 1973
Rabbit/cross M+F oral 1000-2000 Tettenborn, 1969
M+F s.c. 1000-2500 Tettenborn, 1969
Cat/cross M+F oral 1000 Tettenborn, 1969
M+F s.c. 500 Tettenborn, 1969
Dog/beagle M+F oral emetic** Tettenborn, 1969
M+F s.c. *** Tettenborn, 1969
* 4 Hour LC50 value.
** Lethal doses could not be achieved due to emesis.
*** 250 mg/kg produced local reaction; temporary inappetence, higher
doses were not tried
Groups of two rabbits, each presumably 1 male and 1 female, were
dosed orally with olaquindox in 2% methylcarboxycellulose. No deaths
occurred at the lowest dose of 500 mg/kg b.w. while in those given
1000 or 2000 mg/kg b.w., 1/2 animals died. All the animals given 4000
mg/kg b.w. died. Similar observations were made in groups of two cats
given 500, 1000 or 2000 mg/kg b.w., with both animals given the
highest dose dying. Emesis was the main toxicological sign noted.
Dogs given oral doses of up to 100 mg/kg b.w. olaquindox showed no
signs of toxicity but those given 250-2000 vomited; no lethalities
occurred. Vehicle controls or undosed animals did not appear to have
been used in these studies (Tettenborn, 1969).
When given by the subcutaneous route as a suspension in 2%
carboxmethylcellulose, the acute toxicity of olaquindox was more
marked (Table 1). Doses of 500 to 2500 mg/kg b.w. and above proved
lethal to mice, rats, rabbits and cats. For unspecified technical
reasons, dogs were given only 250 mg/kg b.w. olaquindox, which
produced a local reaction and temporary inappetence a week later
(Tettenborn, 1969).
2.2.2 Short-term studies
2.2.2.1. Mice
Groups of 20 male and 20 female BOM-NMRI mice were fed diets
containing 0, 300, 600, 1200, 2400 and 4800 ppm olaquindox,
approximately equivalent to 0, 45, 90, 180, 360 and 720 mg/kg
b.w./day, for 90 days, as a dose-finding exercise for a
carcinogenicity study (see Section 2.2.3).
Signs of toxicity were non-specific and included shaggy fur,
dyspnoea and reduced motility. A marked reduction in body weight
occurred at the highest dietary level in both sexes, and in males
given 1200 and 2400 ppm. During the study 1/20 females died at the
600 ppm level as did 18/20 and 5/20 males and females, respectively,
at the 1200 ppm level. All the mice given the two highest doses died.
No deaths occurred in other groups. At necropsy, haemorrhagic lungs
were the main findings. Microscopic examination was not conducted
(Steinhoff & Gunselmann, 1982).
2.2.2.2 Rats
Groups of 10 male and 10 female Wistar rats were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in 2% aqueous
carboxymethylcellulose by stomach tube, for 5 days per week for 13
weeks.
After 6-8 weeks, signs of toxicity included reddening of the ears
and plantar surfaces; weakness and emaciation occurred in animals
given the highest dose. These animals also developed moist, blood
encrusted nostrils. In the eighth week of the study, fatalities began
to occur, consequently the animals were killed. No clinical signs of
toxicity or compound-related deaths occurred in the other groups.
There were no adverse haematological effects in any groups at 4 weeks
including the high dose group, nor at 12 weeks in remaining animals.
Clinical chemistry was normal at 4 weeks in all dose groups and in
animals given 0, 20 or 60 mg/kg b.w./day olaquindox at 12 weeks.
However, at 8 weeks in the high dose animals (prior to death), blood
sugar was significantly reduced, while serum aspartate
aminotransferase was elevated. Urinalysis was normal in all groups at
4 weeks and in all but the high dose group (not available for
examination due to deaths) at 12 weeks.
Absolute organ weights at 90 days indicated a significant
splenomegaly and increases in testicular and ovarian weights in
animals given 60 mg/kg b.w./day olaquindox. These findings were also
reflected in relative organ weights except for splenic weights in
females. There was a significant decrease in relative adrenal weights
in females given 60 mg/kg b.w./day olaquindox.
Gross examination revealed reddening of the pyloric area of the
stomach in the high dose animals and pale and atrophied adrenals. All
the females given 60 mg/kg b.w./day and 5/10 of those given 20 mg/kg
b.w./day had enlarged, reddened ovaries with numerous pin-point
darkened nodules (corpora lutea).
Microscopic examination revealed adrenal atrophy in the high and
mid dose groups, with degenerative changes in the cortical areas. Some
of the female high dose animals had thyroid atrophy. Female rats
given the mid and low dose had no ovarian atrophy but moderate
atrophic changes were noted in the ovaries of 4/5 high dose females
(Hoffmann 1969; Urwin & Mawdesly-Thomas, 1969).
The experiment was later repeated using lower doses: 0, 1, 5 and
20 mg/kg b.w./day. All other factors were identical to the original
study. No clinical signs were observed during the study and there
were no effects on body weights. There were no haematological or
clinical chemistry abnormalities; urinalyses were normal.
At necropsy, increases in adrenal weights in males given the two
highest daily doses were noted. Increases in ovarian weights occurred
in females given the two highest doses. Histopathologic examination
revealed no changes in any organs in any of the treatment groups. The
no-effect level in these studies therefore was 1 mg/kg b.w./day
(Hoffmann, 1972; Urwin & Spicer, 1971).
In a 90 day dietary study, olaquindox was given to groups of 20
male and 20 female Norway rats at levels of 0, 50, 150 and 300 ppm,
approximately equivalent to oral doses of 0, 5, 15 and 30 mg/kg
b.w./day. Haematological and clinical chemistry investigations were
conducted at days 0, 35, and 63 and at the end of the study.
No signs of toxicity were noted during the study and there were
no effects on haematology and clinical chemistry. Gross and
microscopic examination revealed no compound-related changes
(Nastuneak et al, 1986).
In an inhalation study, groups of 10 male and 10 female Wistar
rats were exposed to 0, 10, 207 and 541 mg/m3 olaquindox dust, 6
hours/day, 5 days per week for 3 weeks under dynamic exposure
conditions. Based upon number and particle mass median diameter,
between 30-80% of the particles were inhalable.
No adverse effects occurred in the rats exposed to the lowest
concentration of olaquindox nor in the males of the intermediate
concentration group. Females in this group showed non-specific
effects ("sluggishness") for the first 5 days of exposure but after
this they appeared normal. Effects of a similar nature and duration
occurred in the high concentration males but in females, signs of
respiratory distress were noted from the 11th day of exposure in
addition to the non-specific effects seen in other exposed groups.
No deaths occurred and only minor effects on body weights were
observed. There were no effects on haematology nor on clinical
chemistry. Urinalyses were normal. No gross abnormalities were
evident at autopsy and relative and absolute organ weights were in the
normal range. There were no adverse histopathological findings. The
non-specific signs and particularly the respiratory effects may have
been due to the physical effects of the olaquindox particles (Thyssen,
1983).
2.2.2.3 Rabbits
Olaquindox in Lutrol was applied to the shaven intact dorsal
surface of 3 groups of 6 New Zealand white rabbits (3 male and 3
female) at doses of 0, 50 or 250 mg/kg b.w./day for 6 hours/day, 5
days per week for 3 weeks, without an occlusive dressing. In an
identical manner, olaquindox was applied to the abraded skin of 3
groups of 3 male and 3 female rabbits.
No signs of toxicity were observed in treated animals and skin
reactions due to olaquindox were not seen in the abraded or intact
skin groups. No mortalities occurred. Clinical chemistry and
urinalyses at the end of the study were comparable to control values.
There were no effects on body weights. At necropsy, no abnormalities
were noted and no histopathological changes attributable to olaquindox
treatment were found (Heimann & Schilde, 1982).
2.2.2.4 Dogs
Groups of 2 male and 2 female beagle dogs were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in gelatin capsules for
90 days.
Vomiting occurred during the first week in dogs given the highest
dose. Salivation was noted and food intake declined. The animals
became emaciated. Dogs given the intermediate dose showed inappetence
and salivation occurred. No effects were noted in low dose animals.
All the high dose animals died within 20 days of the start of the
study. One dog given the intermediate dose died on day 40 while the
remainder were killed in extremis after 46 or 56 doses. No animals
given the low dose died.
There were no notable changes in haematology in treated dogs.
Clinical chemistry revealed increases in blood urea in all 4 dogs
given the high dose. Intermittent elevations of blood urea were noted
in the other dosed groups. No abnormalities in urinalyses occurred.
Gross examination of high dose animals suggested an irritant
effect on the gastrointestinal tract, with congestion in the lungs.
The livers were discolored. No abnormalities were noted in the low
dose animals. Histopathologic examination revealed live cell
enlargement and fatty degeneration in dogs given 60 or 180 mg/kg
b.w./day olaquindox, with fatty degeneration of the cells of the
kidney tubules. No histopathologic changes were found in low dose
dogs and the no-effect level in this study appeared to be 20 mg/kg
b.w./day (Lorke & Tettenborn, 1969; Mawdesley-Thomas & Urwin, 1969).
2.2.2.5 Pigs
Groups of 5 castrated male and female German landrace pigs
weighing 9-10 kg each were fed diets containing 0, 100, 160 and 250
ppm olaquindox for 20 weeks.
At the highest dietary level, 5 pigs died and weight gain was
significantly reduced. Animals given 100 and 160 ppm in the diet had
higher rates of weight gain than controls. No haematological effects
occurred. Plasma creatinine and urea were elevated in the
intermediate and high dietary level groups. Hyperkalaemia and
hyponatraemia were noted in pigs given 250 ppm dietary olaquindox.
Urinalyses were normal.
High dose pigs showed a grey-brown discoloration of the renal
cortex but there were no effects on relative organ weights. Tubular
dilatation and flattening of the tubular epithelium occurred in
intermediate and high dietary level pig kidneys and the adrenals of
these animals displayed enlarged cortical epithelial cells. The no-
effect level in this study was 100 ppm olaquindox in the diet (Gericke
& Dycka, 1974; Hoffman et al., 1974).
Groups of 7 hybrid piglets (4 weeks old, at least 3 females per
group and castrated males) were fed diets containing 0, 25, 50, 100
and 200 (2 groups) ppm olaquindox for 6 weeks.
After 2 weeks, dry faeces were produced by piglets given 100 or
200 ppm olaquindox. The drinking of urine from the floor of pens or
directly from urinating pen-mates was noted in pigs given 50 ppm
olaquindox. A decrease in abdominal volume occurred in piglets given
100 or 200 ppm olaquindox after 5 weeks and in the 25 ppm group in
week 6, but not in animals give 50 ppm. Significant rises in serum
albumin values occurred in piglets given 100 or 200 ppm olaquindox
from week 2 onwards, and marked rises in serum urea values occurred in
the 200 ppm group from week 4 and in the 100 ppm group from week 5.
Gross and microscopic pathology were not conducted (Nabuurs et al,
1989).
Groups of 6 female and 6 castrated male hybrid piglets were fed
diets containing 0, 25, 50, 100 or 200 ppm olaquindox for 6 weeks, in
a study of the effects of treatment on plasma aldosterone, sodium and
potassium levels. There was a gradual decline in plasma aldosterone
which was significant in all but the 25 ppm group by week 5. After 6
weeks the decline was significant in all dosed groups except for that
given 100 ppm where a small rise was noted. Hyponatraemia occurred in
the 25 and 200 ppm groups after weeks 0-2 and a continuous decline in
the 200 ppm group occurred after week 3. In the groups given 25 and
100 ppm olaquindox the levels decreased continuously from 2-3 weeks.
Animals in the 50 ppm group were unaffected. Although elevated
potassium levels occurred in the 50 and 100 ppm groups, only piglets
given 200 ppm were considered to be hypokalaemic (van der Molen
et al, 1989; Baars et al, 1988).
Similar effects have been noted in pigs given other quinoxaline
N-oxide drugs, namely cyadox and carbadox (van der Molen et al,
1985). The latter drug caused these effects after accidental
overdosage of pigs (Power et al, 1989). The toxicity appears to be
due to specific effects on the aldosterone-releasing zona glomerulosa
of the adrenals (van der Molen et al, 1986).
2.2.2.6 Rhesus monkeys
Olaquindox in gelatin capsules was given orally to two groups of
3 male and 3 female rhesus monkeys at doses of 0 and 20 mg/kg b.w./day
and to two groups of 3 males and 5 females at doses of 5 and 40 mg/kg
b.w./day, 7 days a week for 19 weeks. Surviving high dose females
were entered onto a 17 week recovery period.
Animals given the highest dose showed a general loss of condition
and loss of body weight. Suppression of weight gain occurred at the
intermediate dose while growth promotion occurred at the low dose.
Suppression of appetite was evident from week 12 in high dose monkeys.
Vaginal cytology indicated a suppression of ovulation in the high
dose females and in 1/3 animals given the intermediate dose. There was
some evidence of recovery after dosing ceased in the high dose
females. Deaths occurred in 2/3 males and 1/5 females form the high
dose groups during the dosing period and 2 females died during the
first two weeks of the recovery period.
Electrocardiography and ophthalmoscopy were normal in all
animals. Urinalyses and haematology were essentially normal at 5
weeks but serum aspartate aminotransferase values in high dose males
were elevated. At 8 weeks plasma biochemistry was normal but packed
cell volume and red cell counts were lowered in high dose animals.
Urinary glucose was found in 3 high dose males. After 15 weeks of
dosing packed cell volume and haemoglobin showed slight reductions in
high dose males while plasma glucose levels were reduced but not
significantly.
In 7/8 high dose monkeys, urine was positive for glucose and for
total reducing substances. One urine showed a lower pH and one was
positive for ketones. When examined at 15 weeks, red blood cell
values were reduced in high dose monkeys. Plasma glucose values were
reduced in high dose animals and protein and glucose were present in
the urines; pH of urine was lowered in high dose animals. At the end
of the 19 week dosing period no haematological examinations were
performed. Plasma glucose levels were reduced in high dose animals
while plasma urea values were increased. Hypokalaemia was noted in
high and intermediate dose animals. Glucose and total reducing
substances were increased in high dose monkeys while pH was lowered.
Macroscopic examination revealed pallor of the kidney in high
dose animals. Suppression of ovulation occurred in high dose females,
and abdominal abscesses in high dose males.
Histopathological examination revealed fatty changes in the
centrilobular areas of the liver in all high dose animals and
deposition of fat in the kidney tubules in all monkeys from this
group. Brown pigmentation of the zona reticularis of the adrenals
occurred in high dose monkeys. Immature testes were noted in males
given 20 and 40 mg/kg b.w./day olaquindox while inactivity of the
ovaries in high dose females and in 1/3 intermediate dose females was
observed. The no effect level in this oral study in rhesus monkey
was 5 mg/kg b.w./day (Heywood et al, 1972).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 20 male and 20 female NMRI mice were given nominal
doses of 0, 15 or 75 mg/kg b.w./day olaquindox in the drinking water.
Low dose mice were given a total of 6.6 g/kg b.w. for 635 days and
high dose mice were given a total of 32.1 g/kg b.w. for 634 days. The
study was terminated when all the mice had died. Animals were not
dosed over holidays and no mention was made of week-ends.
At termination, no excess tumour incidence was noted.
Lymphadenosis was reported in 1/40 control mice while 2/40 high dose
animals had tumours (thymoma and malignant thymus cell tumour) as did
2/40 low dose mice (pulmonary carcinoma and bronchial carcinoma.
Only small numbers of mice were used in this study and survival
was poor, although it was better in the high dose group than in
controls. The mean survival had a high standard deviation (mean
survival 340 ± 187, 338 ± 224 and 403 ± 194 days for mice given 0, 15
or 75 mg/kg b.w./day, respectively).
Moreover, even when survival was adjusted for unexplained deaths
which occurred in the first 82 days in controls, the treatment time
was still less than is currently viewed as normal for a mouse
carcinogenicity study. The short life-span on test may be due to the
fact that the animals were already around 50 days old when the study
commenced (Schmael, 1973).
In a later study, groups of 75 male and 75 female NMRI mice were
given diets containing 0, 40, 120 or 360 ppm olaquindox, equivalent to
0, 6, 18 or 57 mg/kg b.w./day, for life (until death or sacrifice in
a moribund state).
The only effects on body weights were in males and females given
the highest dietary level: male weights fell slightly below control
values from day 50 and female weights declined after day 200.
Haematological examination at weeks 4, 13, 26, 52 and 78 revealed no
abnormalities and survival times were unaffected by olaquindox intake;
remaining males and females died around day 890 (approximately 29
months).
At necropsy there were no differences in liver, kidney, spleen,
heart, testes, or brain weights, and no increases in non-neoplastic
findings were found in treated mice. No increased tumour incidence
was found in animals given 40 or 120 ppm dietary olaquindox but at 360
ppm there was an increase in the total number of tumours and in the
number of animals with benign tumours. These were due to increases in
the incidence of pulmonary adenoma and adrenal cortical adenoma in
males and in pulmonary adenoma and ovarian granulosa cell tumours in
females (Table 2). There were no increases in the incidence of any
malignant tumour types (Steinhoff & Gunselman, 1982).
Table 2: Tumor incidence in mice given olaquindox in the diet
0 ppm 40 ppm 120 ppm 360 ppm
Males
pulmonary adenoma 11 (15%) 17 (23%) 14 (19%) 27 (36%)
adrenal cortical 5 (7%) 3 (4%) 6 (8%) 13 (17%)
adenoma
Females
pulmonary adenoma 8 (11%) (7%) 7 (9%) 11 (15%)
ovarian granulosa 10 (13%) 16 (21%) 15 (20%) 20 (27%)
cell tumor
2.2.3.2 Rats
Groups of 20 male and 20 female Wistar rats were given
olaquindox, once per week for up to 560 days. The total dose was 4.7
g/kg b.w. with individual doses being in the range of 50-150 mg/kg
b.w. Each dose was given by gavage as a suspension in physiological
saline. Controls were given physiological saline only, but this was
administered by intraperitoneal injection and not by gavage as they
simultaneously acted as controls for other studies.
Survival in treated animals was better than controls (875 ± 105
days for male and 818 ± 167 days for female rats given olaquindox
compared with 797 ± 215 and 779 ± 187 days, respectively, for male and
female controls).
The incidence of tumours was not presented in a detailed tabular
form but was given graphically and in separate tables which did not
allow a full comparison. However, there was no elevated incidence of
any tumour type in treated animals when compared with controls and the
number of tumour-bearing animals was similar in both the treated and
control groups (Steinhoff, 1973).
Groups of 80 BR 46 rats were given drinking water containing
olaquindox at levels to ensure an intake of 15 or 75 mg/kg b.w./day.
A group of 49 rats given water only served as controls. Male and
female rats were used in the study but the sex ratio was not
specified. The treated water was supplied 5 days a week until the
animals died.
Survival of animals treated with olaquindox was better than
controls (704 ± 161 days and 655 ± 229 days in low and high dose rats,
respectively, compared with 554 ± 248 days in control rats). The
carcinogenicity data was presented in an unclear manner and separate
data for male and female animals were not given. The only tumour
which showed an increased incidence was mammary fibroadenoma (1/40,
2.5%; 3/46, 6.5% and 7/46, 15% in controls and in low and high dose
rats, respectively). However, the absence of data on male and female
incidence of this tumour renders the values uninterpretable (Schmael,
1973).
As part of a chronic toxicity/carcinogenicity study with
in utero exposure, groups of 75 males and 75 females were given
diets containing 0, 40, 120 or 360 ppm olaquindox. These doses were
approximately equivalent to oral doses of 0, 3, 10 and 30 mg/kg
b.w./day, for one week prior to mating and during a 3 week 1:1 mating
period. After mating the males were removed and the females given the
diets containing olaquindox until the young were 4 weeks old. At this
time the young from each treatment group were divided into groups of
25 males and 25 females and given the same diets as initially given to
their parents. The study was continued until the young had been
treated for 2 years. Clinical chemistry, haematology, and urinalyses
were conducted on groups of 5 males and 5 females at 4, 14, 26, 54 and
102 weeks after commencement of treatment of the F1 generation.
No overt signs of toxicity were noted in treated animals but
after day 400 of treatment, male and female animals given the highest
dietary level showed a marked and statistically significant reduction
in body weight when compared with control values.
Clinical chemistry suggested an elevated creatinine level in the
blood of rats given 360 ppm dietary olaquindox but all the values were
within the normal range. The albumen content of the urine was
generally lower in treated animals than in controls.
At gross and microscopic examination, there were no increases in
the incidences of non-neoplastic diseases and no increased incidence
of any tumour types. This study employed too few animals to allow an
assessment of carcinogenic potential (Steinhoff, 1977).
Olaquindox was tested in a carcinogenicity study using a similar
protocol on groups of 50 male and 50 female rats remaining in the F1
generation from the chronic toxicity carcinogenicity test described
above. The groups were given diets containing 0, 40, 120 and 360 ppm
olaquindox, approximately equivalent to oral doses of 0, 3, 10, and 30
mg/kg b.w./day. No clinical chemistry or haematological tests were
conducted, however, and the study was terminated when the duration was
approximately 3 years (1065 days for males and 1120 days for females),
at which time 20% of the controls (males and females separately)
remained alive.
No signs of toxicity were noted in treated animals except for
reductions in body weights in rats given 360 ppm after day 500.
Reductions occurred despite the fact that in terms of feed consumption
in g/kg b.w./day these animals had consumed more.
At termination there was a significant decrease in survival in
animals given the highest dietary level (98% mortality in males and
females) and in females given 40 ppm olaquindox (92% mortality)
compared with controls (80% mortality). Mortality in the other
dietary groups was only slightly more than in controls (82-86%).
At necropsy there were no differences between treated animals and
controls for the number of animals of each sex with total tumours,
primary tumours, malignant and benign tumours, malignant tumours with
metastases and total benign tumours. Similarly, for particular tumour
sites (benign and malignant tumours plus metastases) there were no
excess incidences except for slight increases in the incidence of
adrenal, reticuloendothelial and seminal vesicle neoplasms, but these
were due, except for the adrenal tumours, to metastases or
infiltrations from other organs. Moreover, the adrenal tumours were
not increased with respect to any particular histological type and
again were often of metastatic origin (Steinhoff & Boehme, 1978).
There were some anomalies in the reporting of this study which
appear to be due to arithmetical errors. The summary table of tumour
incidence cites a benign tumour in female controls which is not listed
in the detailed histopathology tables. The summary data also claims
4 malignant tumours in males given the highest dietary level whereas
the histopathology table lists 9. However, these differences do not
affect the outcome of the study or its assessment.
2.4 Reproduction Studies
2.4.1 Mice
Groups of 20 pregnant NMRI mice were given oral doses of 0, 20,
60, or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. On day 18 of
gestation the fetuses were delivered by Caesarean section and these
were weighed and examined for gross malformations and by alizarin
staining.
None of the pregnant animals died during the test but animals
given the highest dose showed reduction in body weight or rate of
weight gain. The numbers of implantations, live fetuses and
resorptions were similar in all dosed groups. At the highest dose,
fetal weights were significantly lower than in controls. The
incidence of malformations in all treated groups was similar to those
seen in controls (Lorke, 1971a).
2.4.2 Rats
Groups of 20 female pregnant FB 30 rats were given oral doses of
0, 20, 60 or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. Fetuses were
delivered by Caesarean section on day 20 of gestation and these were
examined in the same manner as for the mouse study described above.
The pregnant rats given the highest daily dose showed reductions
in body weights or rate of weight gain compared with control values.
These animals also showed a higher incidence of resorptions and lower
numbers of live fetuses. Fetal weights were lower in the high dose
animals. These indices were similar to controls and to rats given 20
or 60 mg/kg b.w./day olaquindox.
The incidence of malformations in fetuses from dams given 20 or
60 mg/kg b.w./day olaquindox was similar to controls but at the
highest dose level there was an elevated incidence of malformed
fetuses, with 5 malformations reported at a dose level of 180 mg/kg
b.w./day.
In this study, therefore, there was a teratogenic effect at the
highest dose level given to pregnant rats (180 mg/kg b.w./day) on days
6-15 of gestation. The no-effect level in this study was 60 mg/kg
b.w./day (Lorke, 1971b).
A 3-generation study was conducted in groups of 10 male and 20
female FB30 rats using 0, 20, 100 and 500 ppm olaquindox in the diet,
equivalent to oral doses of 0, 1, 5 and 25 mg/kg b.w./day. The F0
animals were given the diets containing olaquindox throughout the
study including during the mating periods. Animals were mated after
dosing for 70 days. Animals which died during the study were
necropsied, while young animals were examined macroscopically after
birth and subsequently observed during the rearing period for evidence
of abnormalities. The F3b animals were sacrificed at 3 weeks of age
and subjected to macroscopic and microscopic examination.
Treatment had no effect on body weights except that body weight
of F0 generation females given the highest dietary level of
olaquindox were slightly higher than weights of controls.
The fertility rate was also lowered in F0 animals in the first
and second matings when given the highest level, but there were no
effects on litter size nor on rearing rates. F1a and F1b animals did
not differ from controls in terms of birth weights except for a
slight, non-statistical increase in those derived from F0 dams given
the highest dietary level. After the second F0 mating the average
number of young in the F1b generation was similar in all treatment
groups, but at 5 days there was a significant reduction for dams given
500 ppm olaquindox (6/litter) compared with values in other groups and
controls (10-12/litter). Birth weights of the F1b generation were
unaffected.
When the F1b generation was mated, the gestation rates in animals
derived from animals initially given the highest level of olaquindox
were reduced (80-84%) compared with values for other groups (90-100%).
The average numbers of young per litter were also reduced at the high
dietary level in both the F2a and F2b generations, both at birth and
5 days after birth (8/litter compared with 11/litter in controls).
The F2 birth weights were unaffected and there was some improvement
in the rearing rates up to 4 weeks.
In the F3 generation, fertility was again affected in animals
originally derived from the high dietary level rates, with gestation
rates of 70-84% compared with 90-100% in other groups. The average
number of young per litter was also reduced at the high dietary level
(5-7/litter) compared with other groups (8.5-10.8/litter). There were
no effects on rearing rates nor F3 birth weights. No malformations
were noted during the course of the study and no abnormalities were
found on gross or histopathological examination of three week old F3b
animals (Loeser, 1974).
In a fertility test in Wistar rats, olaquindox was administered
orally by gavage to groups of 10 male rats at doses of 4 and 10 mg/kg
b.w./kg which were mated with groups of 20 untreated females. Groups
of 10 untreated males were mated with groups of 20 females given
gavage doses of 4 and 10 mg/kg b.w./day. Males were dosed for 8 weeks
prior to mating while females were dosed for 3 weeks prior to mating.
Males and females were mated in a 1:2 ratio. Groups of untreated
males and females were mated as controls.
Olaquindox administration had no effect on body weights, on
oestrus cycles, or on copulation and conception rates. A significant
reduction in the average number of implantations was noted in the
group in which females given the lower dose of 4 mg/kg b.w./day
olaquindox were mated with untreated males. Pre-implantation losses
were significantly increased in both the groups where females were
treated with olaquindox, and post-implantation losses were increased
in females given the 10 mg/kg b.w./day dose. There were no effects in
the groups where males treated with olaquindox were mated with
untreated females (Gandalovicova & Sykora, 1986).
2.2.5 Special studies on pharmacological properties
Olaquindox has been tested in a number of pharmacological
screening tests in rats and mice including those for anticonvulsive
effects, inhibition of defensive reaction, motor coordination,
analgesia, antihypertensive effects, gastric secretion, bile
secretion, diuresis, blood sugar and blood lipids and thrombocyte
aggregation (bovine plasma). No pharmacological activity was detected
(Kaller, 1970).
2.2.6 Special studies on irritancy and hypersensitivity
Olaquindox as a micronized powder was applied without a vehicle
to the shaved intact and abraded dorsal skin of 6 New Zealand white
rabbits using a 24-hour occlusive dressing. The skin was assessed on
removal of the dressing, after 48 and 72 hours, and at 7 days. Slight
erythema of intact and abraded skin was observed at 24 hours, but not
at the other time points. No oedema was noted. Results indicated a
mild irritant effect (Murman, 1979).
Olaquindox was tested for its ability to induce eye irritation by
application of 15 mg of the micronized substance to the conjunctiva of
the right eyes of 6 New Zealand white rabbits, and to the left
conjunctival sacs of a further 6 rabbits. The material was washed out
of the left eyes with physiological saline after 1 minute. Reactions
were monitored 24, 48, and 72 hours after application and after 7
days.
A slight reddening of the conjunctiva was noted in 4/6 eyes where
olaquindox had been directly applied. Slight chemosis was noted in
2/6 eyes. A slight chemosis was noted in 1/6 rabbit's eyes where
olaquindox has been applied to the conjunctival sac and then washed
out. All reactions subsided within 48 hours. The result suggest that
olaquindox has a slight irritant effect but the mechanical effects of
the dust cannot be ruled out (Murman, 1979).
Olaquindox was tested in the guinea-pig for its ability to induce
hypersensitivity. Olaquindox dissolved in dimethyl sulfoxide or as a
suspension in phosphate buffered saline was administered
intracutaneously into the neck region of groups of 10 Pirbright albino
guinea-pigs on days 1, 3, 6, 9, and 13 of a sensitization schedule.
Four days after the last injection, a suspension of olaquindox in 1:1
acetone/almond oil was applied to the depilated flank and massaged
gently into the skin. To allow for any possible effects of light,
groups of guinea-pigs were treated in a similar manner but were kept
in darkened cages. No indications of sensitization were noted in this
study when assessed by gross examination of the skin for reactions or
when examined histologically (Schlumberger, 1975). (This test is not
widely used and it is unlikely to be as sensitive as those using an
adjuvant (e.g. Freunds') such as the Magnusson-Kligman or Beuhler
models).
2.2.7 Special studies on genotoxicity
Olaquindox has been tested in a wide range of genotoxicity tests
and these are summarized in Table 3. It has produced positive results
in a number of studies designed to test for reverse mutations in
bacteria, including the Ames test with Salmonella typhimurium
strains (Beutin et al, 1981; Yoshimura et al, 1981; Voogd
et al, 1980; Nunoshiba & Nishioka, 1989). A positive result has
been noted in a forward mutation assay with Escherichia coli
(Nunoshiba & Nishioka, 1989).
In vitro study with cultured human lymphocytes and a number of
in vivo assays with mouse bone marrow or Chinese hamster
spermatogonia as the target tissues have demonstrated the clastogenic
activity of olaquindox (Tamura, 1977; Cihak & Vontorkova, 1983; Sram
et al, 1986a; Pokorna, 1986; Herbold, 1983a). Similarly, olaquindox
has produced positive results in several micronucleus tests in the
mouse following oral or inhalation exposure (Herbold, 1983b; Herbold
& Thyssen, 1982; Cihak & Vontorkova, 1985), and in the rat after
intraperitoneal injection (Cihak et al, 1983). However, a dermal
study with a 24 hour exposure was negative (Herbold, 1982a),
reflecting the poor dermal absorption of the substance.
Olaquindox has been tested in two dominant lethal assays in the
male mouse but a weak positive result was observed in only one of
these when a high dose (1 g/kg b.w) was employed (Machemer, 1977a;
Herbold, 1982b). Positive lethal mutations also occurred when female
mice were treated orally with olaquindox despite the use in one study
of doses lower than that which effected a positive result in the male
mouse (200 and 500 mg/kg b.w.) (Machemer, 1977b; Sram et al, 1986b
& c). However a toxic effect in female mice could not be excluded.
Positive results have been obtained in a sister chromatid
exchange test using Chinese hamster V79 cells indicating that
olaquindox may induce DNA damage (Scheutwinkel-Reich & von der Hude,
1984). Positive results in bacterial assays including the SOS
chromotest confirm this possibility (Suter et al, 1978; Beutin
et al, 1981; Yoshimura et al, 1981; Nunoshiba & Nishioka, 1989;
von der Hude et al, 1988). However, there is no evidence that
olaquindox covalently binds to DNA in the rat in vivo (Minini
et al, 1983).
The mutagenicity of a number of olaquindox metabolites has also
been investigated. The omega oxidation product, its 1 and 4
monodesoxy derivatives and its didesoxy derivative have been
investigated in the Ames test using S. typhimurium strains TA 98,
100, 1535 and 1537 with and without rat liver S9 metabolic activation.
All the tests gave negative results (Herbold, 1978; 1979a,b).
Table 3: Results of genotoxicity studies with olaquindox
Test System Test Object Concentration Results Reference
Ames test1 S. typhimurium 3.8-0.5 nmoles/plate Positive Beutin et al., 1981
TA98, 100
Ames test2,4 S. typhimurium 1.9-57 nmoles/plate Positive Beutin et al., 1981
TA100
Ames test1 S. typhimurium 1.25-15µg/plate Positive Yoshimura et al.,
TA98, 100 1981
Ames test2 S.typhimurium 0.01-0.1 mmole/1 Positive Voogd et al., 1980
Ames test1 S. typhimurium 0-50 µg/plate Positive Nunoshiba & Nishioka,
TA98,100 1989
Fluctuation1 test K. pneumoniae 2x10-4-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Fluctuation2 test K. pneumoniae 2x10-5-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Forward1 mutation E. coli 0-20 µg/plate Positive Nunoshiba & Nishioka,
Wp\P2uvrA/pKM101 1989
In vitro Cultured human 3-300 µg/ml Positive Tamura, 1977
cytogenetics lymphocytes
In vivo Mouse bone marrow 20, 500 or 800 mg/kg Negative Sutou, 1977
cytogenetics b.w. oral
In vivo Mouse bone marrow 200-800 mg/ml b.w. oral Negative Cihak & Vontorkova, 1983
cytogenetics
In vivo Mouse bone marrow 20-500 mg/kg b.w. diet Positive Sram, et al., 1986a
cytogenetics 4 and 12 weeks
Table 3 (contd)
Test System Test Object Concentration Results Reference
In vivo Chinese hamster 20 mg/kg b.w. oral, x5 Positive Pokorna, 1986
cytogenetics marrow
In vivo Chinese hamster 2x30-2x1000 mg/kg b.w. Positive Herbold, 1983a
cytogenetics spermatogonia oral
Micronucleus Mouse bone marrow 500 mg/kg b.w. oral, Positive Herbold, 1983b
sampled at 24, 48 or
72 hours; 10-300 mg/kg
b.w. oral, sampled at
24 hours
Micronuceus test Chinese hamster 20 mg/kg b.w. 4.2 and Positive Pokorna, 1986
bone marrow 100 mg/kg b.w. oral,
once
Micronucleus Mouse bone marrow 6.7 mg/m3 and 161 mg/m3 Positive Herbold, & Thyssen, 1982
for 6 hours/day, 2 days,
inhalation
Micronucleus Mouse bone marrow 2034 mg/kg b.w. 30 hours Negative Herbold, 1982a
dermal exposure
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Dominant lethal Mouse (male) 2x1000 mg/kg b.w. week Positive Machemer, 1977a
oral
Table 3 (contd)
Test System Test Object Concentration Results Reference
Dominant lethal Mouse (male) 40, 120 and 360 ppm in Negative Herbold, 1982b
diet for 35 days
equivalent to 6, 18, and
54 mg/kg b.w.
Dominant lethal Mouse (male) 100, 300 and 500 mg/kg b.w. Negative Sram, et al., 1986b
for 4 weeks, 20, 40, 100,
200, and 500 mg/kg b.w.
for 12 weeks, diet
Dominant3 lethal Mouse (female) 30, 100, 300 or 1000 mg/kg b.w. Positive Machemer, 1977b
oral once
Dominant lethal Mouse (female) 20, 40, 100, 200 and 500 mg/kg Positive Sram, et al., 1986c
b.w. diet, for 4 weeks
SOS2 Chromotest E. coli, GE94 0-10 µg/plate Positive Nunoshiba & Nishioka, 1989
(DNA damage)
SOS2 Chromotest E. coli, PQ37 0.001-0.1 mM Positive von der Hude, et al.,
(DNA damage)
DNA damage E. coli, K12 Not applicable Positive Suter, et al., 1978
DNA damage S. typhimurium 100 µg/disc uvr B and recA Positive Beutin, et al., 1981
DNA damage S. typhimurium 1-100 µg/disc Positive Yoshimura, et al., 1981
Mitotic gene2 S. cerevisiae D4 0.05% w/v Positive Voogd, et al., 1980
conversion
Table 3 (contd)
Test System Test Object Concentration Results Reference
DNA binding Rat 500 mg/kg b.w. oral Negative Minini, et al., 1983
in vivo
Sister chromatid Chinese hamster 0-200 µg/ml V79 cells Positive Scheutwinkel-Reich & von
exchange der Hude, 1984
1 With and without rat liver S-9 fraction.
2 In the absence of S-9 fraction.
3 Positive only at the 1000mg/kg b.w. dose.
4 Positive in aerobic and anaerobic conditions.
The results obtained with olaquindox are similar to those noted
with several other quinoxaline di-N-oxides including quindoxin and
carbadox (Suter et al, 1978; English & Dunegan, 1970; Voogd
et al, 1980; Negishi et al, 1980; Beutin et al, 1981 (see also
carbadox)). The mechanisms of action are unknown although neither
olaquindox nor quindoxin binds to DNA (Minini et al, 1983, Suter
et al, 1978).
Electron spin resonance techniques have demonstrated the
generation of free radicals during the reduction of quindoxin while a
related compound 2,3-dihydroxymethyl quinoxaline-1,4-di-N-oxide has
been shown to inhibit DNA synthesis in E. coli (Suter et al,
1978). However, at present the roles of free radicals and inhibition
of DNA synthesis in the mutagenicity of quinoxaline-1,4-dioxides are
unknown.
These studies indicate that olaquindox is genotoxic in a variety
of test systems. It induces mutations in bacterial systems and it
causes chromosome and DNA damage in in vitro and in in vivo
systems. Data available from dominant lethal assays and from an
in vivo cytogenetic study with Chinese hamster spermatogonia suggest
that olaquindox may have the potential to exert its mutagenic effects
on germ line cells.
2.3 Observations in humans
One of the major routes of exposure to olaquindox is likely to be
occupational during the preparation of feed and the feeding of the
final feed to pigs. In an experimental study, no olaquindox was
detected in the workplace air (a stall building housing pigs) during
trough filling operations with a feed containing 50 ppm olaquindox
(Thyssen et al, 1982). Olaquindox was only detected at low levels
in the atmosphere during the preparation of 0.1% feed premix and the
50 ppm final feed starting with a 10% premix of a proprietary product.
Levels in the atmosphere were estimated to be from below 0.4 to below
0.1 µg/m3 air (Inkmann-Koch, 1985a). Analysis of the urine from a
single worker engaged in similar preparation work and in the feeding
of pigs revealed no olaquindox (limit of detection 40 ppb) (Inkmann-
Koch, 1985b).
When applied to the skin of two volunteers as a paste in water
containing 2 g of olaquindox (around 30 mg/kg b.w.) using a 6 hour
occlusive dressing, no olaquindox was detected in the 48 hour urine
using an analytical method with a limit of detection of 0.12 µg/ml
(Beermann, 1982).
There has been one report of allergic contact dermatitis and one
of photocontact dermatitis following occupational exposure to
olaquindox (Bedello et al, 1985; Francalanci et al, 1986). Both
occurred in pig workers exposed to the substance in the animal feed.
There are no reports of systemic toxicity in humans following exposure
to olaquindox.
3. COMMENTS
The toxicological data considered by the Committee included the
results of acute and subchronic studies, together with the results of
studies on mutagenicity, carcinogenicity, and effects on reproduction
and development.
Olaquindox is almost completely absorbed from the
gastrointestinal tract in rats, dogs, and pigs. In studies using
radiolabelled olaquindox, the radioactivity was shown to be widely
distributed in the tissues, with residues in the liver being the most
persistent. The compound was primarily eliminated in the urine, with
lesser amounts being excreted in the faeces and expired air of the
animals. In the pig, elimination was virtually complete by 48 hours
after a single intragastric dose. Any remaining radioactivity was
then eliminated with a half-life of 5-9 days, far in excess of that of
olaquindox (about 3-5 hours). The parent compound accounted for 70%
of the radioactivity in the urine, up to 24 hours after
administration. There were approximately 16 metabolites detected in
the urine; six major metabolites have been fully characterized.
In acute and short-term toxicity studies in rats and mice, rats
were about twice as sensitive as mice. In a 90-day study in rats,
effects were observed in the testes, ovaries, thyroid, and adrenal
cortex at a dose of 5 mg/kg b.w./day and above. These lesions were
described histopathologically as atrophy; and in the adrenal glands
the effect was greatest in the zona glomerulosa. No treatment-related
abnormalities of the pituitary gland were reported. A 90-day study in
beagle dogs and a 19-week study in rhesus monkeys also produced
evidence of toxic effects on the endocrine glands, liver, and kidney.
Of five female monkeys dosed at 40 mg/kg b.w./day, one died during
treatment and two died during the planned 17 week recovery period. The
two survivors failed to re-establish normal ovarian cycles during the
recovery period.
In a 20-week study, groups of five male and five female pigs were
fed up to 250 mg of olaquindox per kg of diet. The plasma
concentrations of urea and creatinine were elevated at 160 and 250 mg
diet, which suggested an effect on the kidney. All of the pigs in the
highest-dose group that survived the study period continued to show
evidence of this effect during a 16 day-recovery period. Plasma
sodium, potassium and chloride concentrations were altered during the
study, but did not return to normal during the recovery period.
Histopathological changes were found at necropsy in kidney and adrenal
tissues from both groups. The manufacturers reported a no-observed-
effect level of 100 mg/kg in feed for this study.
However, in other studies in piglets in which olaquindox was fed
at 25, 50, 100 or 200 mg/kg diet for 6 weeks, a dose-dependent fall in
plasma aldosterone concentration, together with hyponatraemia,
hypochloraemia, and hyperkalaemia occurred in all groups by the end of
the study. Hydropic degeneration of adrenal cortex cells was
recorded. There were no effects on weight gain or clinical signs.
Developmental studies in which the compound was administered
orally were conducted in mice and rats. In mice, fetal weights were
reduced at 180 mg/kg b.w./day, but malformations were absent. In
rats, malformations occurred at 180 mg/kg b.w./day, and reductions in
maternal weight gain, litter size, and fetal weight were also observed
at this dose.
A three-generation reproduction study in which olaquindox was
administered in the diet was conducted in rats. No malformations were
observed, the only findings being reductions in fertility rate and
litter size in the second and third generations at the highest dose of
25 mg/kg b.w./day.
The genotoxicity of olaquindox was investigated in a range of
in vitro and in vivo studies. Positive findings were reported in
assays for point mutation and for DNA damage in bacteria, sister-
chromatid exchange in Chinese hamster V-79 cells, chromosome damage in
human lymphocytes in vitro, mammalian bone-marrow cells in vivo,
and in several micronucleus tests. Two dominant lethal assays in male
mice were negative, but a third gave a weak positive response. Two
dominant lethal assays in female mice gave positive results, but this
may have been partly due to the toxicity of the drug. A weak positive
result was obtained in an in vivo cytogenicity assay using Chinese
hamster spermatogonia. Olaquindox did not bind to rat DNA in vivo.
The Committee considered data from six long-term studies in
rodents, but because of poor survival and deficiencies in experimental
design and reporting, only two carcinogenicity studies, in mice and in
rats, were evaluated.
In the study in mice, doses of olaquindox up to an equivalent of
54 mg/kg b.w./day were administered in the diet for life (up to 635
days). An increase in the incidence of benign adrenal cortical
adenomas and benign proliferative lesions (nodular hyperplasia and
adenoma) in the lung was observed in male mice at the highest dose
level. There was no effect on the incidence of malignant tumours.
In the rat carcinogenicity study, which included fetal exposure
to the drug in utero, olaquindox was administered in the diet at
levels up to an equivalent of 30 mg/kg b.w./day until 80% of the male
and female controls had died (about three years). Survival was
decreased at 30 mg/kg b.w./day in both sexes, but there was no
increase in the incidence of benign or malignant tumours.
4. EVALUATION
The Committee considered olaquindox to be a genotoxic agent.
There was some evidence to suggest that olaquindox was a germ-line
mutagen, but more extensive testing in appropriate mammalian studies
will be required to resolve this issue. In the carcinogenicity
studies only the mouse showed an increase in the incidence of tumours,
and these were benign. Because of doubts over the mechanism of this
effect and the results of the genotoxicity studies, the Committee was
unable to establish an ADI for olaquindox. However, the Committee
concluded that residues resulting from the use of olaquindox in food-
producing animals under conditions of good practice in the use of
veterinary drugs were temporarily acceptable.
The results of studies on the nature and availability of residues
of olaquindox and the results of studies designed to provide an
indication of the toxic potential of these residues are required by
1993.
Depending upon the results of these studies, the following
additional information may be needed:
a) Data to assess the genotoxic potential of olaquindox on
germ-line cells, which would, at a minimum, necessitate a
repeat of the Chinese Hamster spermatogonia study.
b) Studies designed to assess the effects of olaquindox on
adrenal function (including sensitive parameters such as
plasma adrenal hormones and electrolyte levels), sperm
morphology, and fertility in rats, so that a NOEL can be
determined for each of these indicators.
c) Information on the binding of olaquindox or its metabolites
to structural proteins such as tubulin, or to enzymes or
proteins involved in DNA synthesis or repair. (Such binding
may help explain why, despite its obvious genotoxic
potential, olaquindox does not bind to DNA and has given
equivocal results in carcinogenicity studies).
5. REFERENCES
ANDERSON, B., & SZABO, A. (1982). Determination of olaquindox in
swine tissues after short withholding periods. Report No. 08-07-
1981/24-03-1982. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
ANON. (1989). Compendium of Data Sheets for Veterinary Products
1989-90. National Office of Animal Health, Datapharm Publications
Ltd, London.
BAARS, A.J., VAN DER MOLEN, E.J., SPIERENBURG, T.J., DE GRAAF, G.J.,
NABUURS, J.J.A., & JAGER, L.P. (1988). Comparative toxicity of three
quinoxaline-di-N-dioxide feed additives in young pigs. Arch.
Toxicol., Suppl. 12, 405-409.
BEDELLO, P.G., GOITRE, M., CANE, D., & RONCAROLO, G. (1985). Allergic
contact dermatitis to Bayo-N-Ox-1. Contact Derm., 12, 284.
BEERMAN, D. (1982). Cutaneous absorption of olaquinodox in human
volunteers. Doc. No 82/10744. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
BEUTIN, L., PRELLER E., & KOWALSKI, B. (1981). Mutagenicity of
olaquindox, 3its metabolites, and two substituted quinoxaline-di-N-
oxides. Antimicrob. Agents Chemother., 20, 336-343.
BORIES, G., & BOURDON, D. (1977). Residue analysis in pigs. Doc. No.
77/8813. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
CIHAK, R., SRB, V., & VONTOKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. I. Micronucleus
test in rats. Mutat. Res., 116, 129-135.
CIHAK, R., & VONTORKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. II. Metaphase
analysis in mice. Mutat. Res., 117, 311-316.
CIHAK, R., & VONTORKOVA, M. (1985). Cytogentic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. III.
Transplacental micronucleus test in mice. Mutat. Res., 144, 81-84.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A.,
(1970). Resorption, distribution, substance changes, and elimination.
Short test in the rat and in the dog. Report No. 1939. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A., &
SCHEER, M., (1973). Metabolism and residue studies wth 14C-labelled
Bay VA 9391 in swine. Report No. 4151. Unpublished report by Bayer
AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
ENGLISH, A.R., & DUNEGAN, C.M. (1970). Quinoxaline-1, 4-di-N-oxides.
I. Inhibition of deoxyribonucleic acid synthesis in Escherichia
coli by 2, 3-dihydroxy-methyl-quinoxaline-1, 4-di-N-oxide. Proc.
Soc. Exp. Biol. Med., 133, 398-400.
FRANCALANCI, S., GOAL, M., GIORGINI, S., MUCCINELLI, A., & SERTOLI, A.
(1986). Occupational dermatitis from olaquinodox. Contact Derm.,
15, 112-114.
GANDALOVICOVA, D., & SYKORA, I. (1986). Cyadox olaquindox - a
fertility test on rats. Biol. Chem. Vet., (Prague), 22, 47-51.
GERICKE, H., & DYCKA, J. (1974). Olaquindox/Bayo-N-ox.
Investigations into the safety of use of the feed additive. Report
No. 4752. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HEIMANN, K.G. & SCHILDE, B., (1982). BAY VA 9391. Study of subacute
dermal toxicity in rabbits. Report No. 11267(E). Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1978). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No 2. 7824. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979a). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 8091/8092/8099. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979b). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 7824. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982a). Micronucleus test on the mouse to check for
mutagenic effect after cutaneous application. Report No. 10602.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982b). Dominant lethal test on male mice to check for
a mutagenic effect after 35 days of administration in the feed.
Report No 10621. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HERBOLD, B. (1983a). Cytogenetic study of the spermatogonia of the
Chinese hamster in vivo to test for mutagenic activity. Report No.
12179. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1983b). Micronucleus test for a mutagenic effect on on
the mouse. Report No. 11424(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
HERBOLD, B., & THYSSEN, J. (1982). Micronucleus test on the mouse to
test for mutagenic activity after inhalation of dynamically produced
dust. Report No. 10833. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HEYWOOD, R., SORTWELL, R.J., NEWMAN, A.J. & STREET, A.E. (1972).
Report No. 4751/72/186. FB a 9391. Oral Toxicity study in rhesus
monkeys. (Repeated dosage for nineteen weeks and recovery period).
Unpublished report from Huntingdon Research Centre, Huntingdon, UK.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1969). BAY VA 9391. Subchronic toxicity in rats
following oral administration. (Trial over 13 weeks). Report No.
1693. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1972). BAY VA 9391. Subchronic toxicity in rats with
oral administration. (13-week test with low doses). Report No. 3241.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K., LUCKHAUS, G. & DYCKA, J. (1974). BAY VA 931.
Toxicological feeding test in feeder swine. Part 3. Results of the
toxicological, pathologic-anatomic and histopathological studies.
Report No. 4754. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
INKMANN-KOCH, A. (1985a). Determination of the concentration of
olaquindox in the air during the preparation of compound (meal) swine
feed and exposure of workers. Doc. No. 85/11885. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
INKMANN-KOCH, A. (1985b). Determination of olaquindox in urine
samples of a worker follwing the preparation of swine feed from Bayo-
N-Ox. Doc. No. 85/11887. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
KALLER, H. (1970). BAY VA 9391. Pharmacological screening. Report
No. 1809. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
KULCZYK, S.R., HERRERA, R.E., MULKEY, N.S., BREAULT, G.O., WARGO,
J.P., & WAGGONER, T.B. (1979). Determination of physical constants of
BAY VA 9391. Report No. 79/9803. Unpublished report from Analytical
Development Corporation, USA. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
LEIBETSEDER, J. (1980). To determine olaquindox residues in swine.
Doc. No. 80/10022. Unpublished report from the Institute of Nutrition
of the Veterinary Medical University, Vienna, Austria. Submitted to
WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LOESER, E. (1974). BAY Va 9391. Three-generation test in rats.
Report 4748. Unpublished report from Bayer AG, Wuppertal-Elberfeld,
FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE, D. & TETTENBORN, D. (1969). FBa 9391. Subchronic toxicity of
dogs during oral administration. (13 Week test). Report No. 1536.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE D. (1971a). FBa 9391. Examination for embryotoxic effects in
mice. Report No. 2692. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
LORKE, D. (1971b). FBa 9391. Examination for embryotoxic effects in
rats. Report No. 2693. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
MACHEMER, L. (1977a). To evaluate mutagenic potential of BAY VA 9391
in mice utilizing the dominant lethal test. Report No. 6738.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
Machemer (1977b). To investigate BAY VA 9391 for mutagenic effects
using the dominant lethal test. Report No. 6770. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
MAUL, W., SENG, F. & WENDISCH, D. (1979a). Investigations into the
biotransformation of olaquinox in swine. Report No. 8114.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAUL, W, SENG, F. & WENDISCH, D. (1979b). Investigations into the
biotransformation of olaquinox in swine. Report No. 8725.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAWDESLEY-THOMAS, L.E. & URWIN C. (1969). Pathology report of FB a
9391. Sub-chronic toxicity for dogs with oral administration. Report
No. 2921/69/347. Unpublished report from Huntingdon Research Centre,
Huntingdon, UK. Submitted to WHO Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. (1974). Method for the determination of residues of Bay
VA 9391 and its reduction products in animal tissues. Report No.
4751. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. & GERICKE, H. (1974). Toxicological Feeding Test with
Feeder Pig. Part 2. Results of Residue Analysis. Report No. 4753.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MININI, U., LUTZ, W.K., & SCHLATTER, CH. (1983). Lack of in vivo
binding to DNA of olaquindox. Report No. R2570 (P). Unpublished
report from the Institute of Toxicology, Swiss Federal Institute of
Technology and University of Zurich, Schwerzenbach, Switzerland.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MURMAN, P.(1979). BAY VA 9391 (Olaquindox). Testing of primary
dermal and mucosal tolerability in the rabbit. Report No. 8359.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
NABUURS, M.J.A., VAN DER MOLEN, E.J., DE GRAAF, G.J., & JAGER, L.P.
(1989). Clinical Signs and Performance of Pigs Treated with Different
Levels of Carbadox, Cyadox and Olaquindox. J. Vet. Med., 36A,
209-217.
NASTUNEAK, J., KOLOUCH, F., VANOVA, L., DVORAK, M. & SEVICK, B.
(1986). Three month toxicity to laboratory Norway rats: Cyadox
compared with olaquindox. Biol. Chem.Vet. (Prague), 22, 367-380.
NEGISHI, T., TANAKA, K. & HAYATSU, H. (1980). Mutagenicity of
carbadox and several quinoxaline 1,4-dioxide derivatives. Chem.
Pharm. Bull., 28, 1347-1349.
NUNOSHIBA, T., & NISHIOKA, H. (1989). Genotoxicity of quinoxaline
1,4-dioxide derivatives in Escherichia coli and Salmonella
typhimurium. Mutat. Res., 217, 203-209.
POKORNA, D. (1986). Cytogentic analysis of bone marrow cells in
Chinese hamster after the administration of cyadox and olaquindox.
Biol. Chem. Vet. (Prague)., 22, 23-28.
POWER, S.B., DONNELLY, W.J.C., MCLAUGHLIN, J.G., WALSH, M.C. & DROMY,
M.F. (1989). Accidental carbadox overdosage in pigs in an Irish
weaner-producing herd. Vet. Rec., 124, 367-370.
SCHEUTWINKEL-REICH, M. & VON DER HUDE, W. (1984). Sister-chromatid
exchange in Chinese hamster V79 cells exposed to quindoxin, carbadox
and olaquindox. Mutat. Res., 139, 199-202.
SCHLUMBERGER, H.D. (1975). BAY VA 9391: Exclusion of immunogenic
activity. Report No. 5194. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
SCHMAEL, D. (1973). Carcinogenicity test with BAY VA 9391 with oral
application to rats and mice. Report N. 38399. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986a).
Chromosomal aberration in the bone marrow of mice after long-continued
administration cyadox and olaquindox. Biol. Chem. Vet. (Prague), 22,
17-22.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986b). Effect
of long-term oral administration of cyadox and olaquindox on the
frequency of dominant lethal mutations and sperm abnormalities in
mice. Biol. Chem. Vet. (Prague), 22, 37-45.
SRAM, R.J. FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986c).
Dominant lethal mutation in female mice following the oral application
of cyadox and olaquindox. Bio. Chem. Vet. (Prague), 22, 29-35.
STEINHOFF, D. (1973). Carcinogenicity study with Bay VA 9391 after
oral administration to rats. Report No. 4048. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
STEINHOFF, D. (1977). BAY VA 9391. Study as to chronic toxic effect
in rats after oral application. (2-Year trial with additional
transplacental treatment). Report No. 6556. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
STEINHOFF, D., & BOEHME, K. (1978). BAY VA 9391. Carcinogenesis
tests with oral administration to rats. (With transplacental
pretreatment). Report N. 7387. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
STEINHOFF, D., & GUNSELMANN, W. (1982). BAY VA 9391. Carcinogenicity
experiment with oral administration to mice. Report No. 72388
(revised), 100700 (revised), 12251 (revised). Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
SUTER, W., ROSSELET, A., & KNUSEL, F. (1978). Mode of action of
quindoxin and substituted quinoxaline-di-N-oxides on Escherichia
coli. Antimicrob. Agents Chemother., 13, 770-783.
SUTOU, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No 78/9327. Unpublished report from Medical Biology
Laboratory, Life Science Department, Nomura Resarch Station, Japan.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
TAKASE, I., & KOMACHI, S. (1975). Determination of BAY VA 9391
residues in animal tissues. Doc. No. 76/8507. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer Ag, Wuppertal-Elberfeld, FRG.
TAKASE, I & KOMACHI, S. (1976). Residues of BAY VA 9391 (olaquindox)
in tissues and organs of pigs. Doc No. 76/8508. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer AG, Wuppertal-Elberfeld, FRG.
TAMURA, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No. 78/9326. Unpublished report from Keio University,
School of Medicine, Tokyo, Japan. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
TETTENBORN, D. (1969). FBa 9391. Acute toxicity for mouse, rat,
rabbit, cat, and dog with oral and subcutaneous administration.
Report No. 1176. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J. (1982). Olaquindox. Acute inhalation toxicity of the
dust. Report No. 10755. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J., BEERMANN, D., & DORN, H. (1982). Exposure studies at the
place of work on administration of Bayo-N-Ox-Ferkelaufzuchtfutter
(Special Piglet Feed) F2 in pellet or meal form. Report 10731.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
THYSSEN J. (1983). Olaquindox BAY VA 9391. Subacute inhalation study
in rats. Report N. 13032(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
URWIN, C., & MAWDESLEY-THOMAS, L.E. (1969). Pathology report of Bay
a 9391. Subchronic toxicity for rats by oral administration. Report
No. 2989/69/415. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
URWIN, C., & SPICER, E.J.F., (1971). Pathology report of Bay a 9391.
Subchronic toxicity study for rats by oral administration. Report No.
4389/71/545. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
VAN DER MOLEN, E.J., NABUURS, M.J.A., & JAGER, L.P. (1985).
Pathological and clinical changes related to toxicity of carbadox in
weaned pigs. Zbl. Vet. Med., 32, 540-550.
VAN DER MOLEN, E.J., DE GRAAF, G.J., SPIERENBURG, TH. J., NABUURS,
M.J.A., BAARS, A.J. & JAGER, L.P., (1986). Hypoaldosteronism in
piglets induced by carbadox. Experientia., 42, 1247-1249.
VAN DER MOLEN, E.J., BAARS, A.J., DE GRAAF, G.J. & JAGER, L.P. (1989).
Comparative study of the effect of carbadox, olaquindox and cyadox on
aldosterone, sodium and potassium plasma levels in weaned pigs. Res.
Vet. Sci., 47, 11-16.
VON DER HUDE. W., BEHM, C., GURTLER, R., & BASLER, A. (1988).
Evaluation of the SOS chromotest. Mutat. Res., 203, 81-94.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1980). The
mutagenic action of quindoxin, carbadox, olaquindox and some other
N-oxides on bacteria and yeast. Mutat. Res., 78, 233-242.
WINDHOLZ, M., BUDAVARIA, S., BLUMETTI, R.,F., & OTTERBEIN, R.R.S (Eds)
(1983). The Merck Index, 10th Edition, Merck & Co., Rahway, New
Jersey.
YOSHIMURA, H., NAKAMURA, M., & KOEDA, T. (1981). Mutagenicities of
carbadox and olaquindox - growth promoters for pigs. Mutat. Res.,
90, 49-55.
 The compound is used as a growth promoter in pigs and is supplied
as a 10% premix in feed for admixture in the final feed at rates of
50-100 ppm for starter rations and 25-50 ppm in grower/fattener feeds
(Windholz et al., 1983; Anon,1989; Kulczyk et al., 1979; Baars
et al., 1988). It has not previously been evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. Biological Data
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion.
2.1.1.1 Rats
Olaquindox is well absorbed when given orally to rats.
Approximately 85% of the radioactivity from a dose of 10 mg/kg b.w. 3-
14C-olaquindox was excreted in the urine. Most radioactivity was
found in the urine within 3 hours of administration. The remainder
was eliminated in the faeces. Less than 1% was recovered in expired
air as carbon dioxide. Experiments with 3-14C-olaquindox
intraduodenally administered to rats with bile duct fistulas suggested
that around 18% of the dose was excreted in the bile. Similar
findings were made after intravenous dosing. Distribution occurred in
a generalized manner throughout the body after oral dosing and most of
the radioactivity had disappeared by 24 hours. Autoradiography
revealed the highest amount in the rat kidney at 4 hours, indicative
of the extent of urinary excretion already noted. Slightly elevated
concentrations were also observed in liver, testes, adrenals and hair
follicles. When dogs were given 10 mg/kg 3-14C-olaquindox, a similar
metabolic profile was noted to that seen in the rat (Duhm et al.,
1970).
2.1.1.2 Pigs
Olaquindox was rapidly absorbed when given orally to pigs. Over
90% of an oral dose of 2 mg/kg b.w. was eliminated in the urine within
24 hours, which is indicative of rapid and extensive absorption. The
remainder was excreted in the faeces. Maximum plasma levels were
attained within 1-2 hours of dosing (1-2 ppm). This was followed by
a rapid decline in plasma levels reaching around 0.03 ppm by 24 hours
and 0.005-0.01 ppm by 48 hours. Radioactivity was present in all
tissues when examined 2 days after dosing, but the levels were
extremely low. In the kidney and liver, levels of 110 and 52 ppb were
found, while levels in muscle were only 9 ppb. After 8 days, levels
in liver and kidney had fallen to 27 and 12 ppb, respectively, while
those in muscle were in the range of 2.5 ppb. By 28 days after dosing
only low levels were found in kidney and muscle (0.9 and 0.5-0.8 ppb,
respectively) with slightly higher concentrations in the liver (2 ppb)
(Duhm et al., 1973).
When pigs were dosed at levels in the range of those recommended
in use (up to 100 ppm in the diet) for up to 20 weeks, relatively high
levels were found in the kidney (around 2000 ppb) with relatively
moderate levels in the liver (300 ppb) when the animals were killed
six hours after drug withdrawal. When killed 2 days after withdrawal,
levels had fallen to below the limits of detection (50 ppb) in liver,
kidney and muscle. Pigs given diets containing olaquindox at levels
in excess of those recommended (160 or 250 ppm) for up to 4 weeks also
had high initial levels in kidney, liver and muscle but these had
fallen to below the limits of detection by day 2 after withdrawal
(Medenwald, 1974; Medenwald & Gericke, 1974; Bories & Bourdon., 1977).
Similar findings were made in other studies where pigs were given
diets containing 100 or 150 ppm olaquindox for 12-30 weeks (Takase &
Komachi, 1975; 1976).
After pigs were given diets containing up to 45 ppm olaquindox
for the duration of the fattening period, the highest levels were
found in the liver (0.14 ppm) and kidney (0.28 ppm) 6 hours after
withdrawal. By 24 hours the levels were below the limit of detection
(0.1 ppm) (Leibetseder, 1980). Similar results were noted when pigs
were given diets containing 10 ppm olaquindox (Anderson & Szabo,
1982).
2.1.2 Biotransformation
The biotransformation of olaquindox has been investigated only in
the pig. The majority of an oral dose of olaquindox (70%) was
excreted in the urine unchanged. The major metabolites appeared to be
the reduced compounds, the 1- or 4-mono-N-oxides (16%). Three other
compounds thought to be carboxylic acid derivatives made up the
remainder (Duhm et al., 1973). Later work led to the elucidation of
the structures of these metabolites in the pig. Again the major
urinary component after oral dosing was olaquindox with about 7%
present as the 4-mono-N-oxide. Omega oxidation produced the
2-carboxymethylaminocarbonyl compound and its 4-mono-N-oxide
derivative (6%). Some of the corresponding 1-mono-N-oxide moiety of
the 2-carboxymethylaminocarbonyl was also noted (1%). The remaining
metabolite was the di-desoxy derivative of
2-carboxymethylaminocarbonyl compound, 2-carboxymethylaminocarbonyl-3-
methyl quinoxaline (>1%) (Maul et al., 1979a and b).
2.1.3 Effects on enzymes and other biochemical parameters
Olaquindox has been shown to cause a decline in plasma
aldosterone levels in the pig accompanied at higher dietary levels by
hyponatraemia and hyperkalaemia. These findings are considered in
more depth in the section on short-term toxicity studies (van der
Molen et al, 1989; Baars et al, 1988).
2.2 Toxicological Studies
2.2.1 Acute Toxicity
Acute toxicity studies are summarized in Table 1.
In a series of experiments, groups of 10 male mice were given
oral doses of 2500-5000 mg/kg b.w. olaquindox as an aqueous suspension
in 2% carboxymethylcellulose. Vehicle controls or undosed groups did
not appear to have been used. Only 1/10 mice died at the lowest dose
used while 100% lethality was noted at the highest dose. Signs of
toxicity included decreased activity, lowering of the eyelids, and
irregular breathing. Animals died 2-14 days after olaquindox
administration. Discolored livers and yellowish-green intestinal
contents were noted on gross examination. Similar findings were made
when groups of male rats were given olaquindox in a similar manner at
doses of 1400-2000 mg/kg b.w. (Tettenborn, 1969).
Table 1: Acute toxicity of olaquindox
Species/strain Sex Route LD50 Reference
(mg/kg b.w.)
Mouse/CF1 M oral 3316 Tettenborn, 1969
M s.c. 2237 Tettenborn, 1969
Rat/Wistar M oral 1704 Tettenborn, 1969
M s.c. 1275 Tettenborn, 1969
M+F inhalation >1751 mg/m3* Thyssen, 1982
F oral 1657 Steinhoff, 1973
Rabbit/cross M+F oral 1000-2000 Tettenborn, 1969
M+F s.c. 1000-2500 Tettenborn, 1969
Cat/cross M+F oral 1000 Tettenborn, 1969
M+F s.c. 500 Tettenborn, 1969
Dog/beagle M+F oral emetic** Tettenborn, 1969
M+F s.c. *** Tettenborn, 1969
* 4 Hour LC50 value.
** Lethal doses could not be achieved due to emesis.
*** 250 mg/kg produced local reaction; temporary inappetence, higher
doses were not tried
Groups of two rabbits, each presumably 1 male and 1 female, were
dosed orally with olaquindox in 2% methylcarboxycellulose. No deaths
occurred at the lowest dose of 500 mg/kg b.w. while in those given
1000 or 2000 mg/kg b.w., 1/2 animals died. All the animals given 4000
mg/kg b.w. died. Similar observations were made in groups of two cats
given 500, 1000 or 2000 mg/kg b.w., with both animals given the
highest dose dying. Emesis was the main toxicological sign noted.
Dogs given oral doses of up to 100 mg/kg b.w. olaquindox showed no
signs of toxicity but those given 250-2000 vomited; no lethalities
occurred. Vehicle controls or undosed animals did not appear to have
been used in these studies (Tettenborn, 1969).
When given by the subcutaneous route as a suspension in 2%
carboxmethylcellulose, the acute toxicity of olaquindox was more
marked (Table 1). Doses of 500 to 2500 mg/kg b.w. and above proved
lethal to mice, rats, rabbits and cats. For unspecified technical
reasons, dogs were given only 250 mg/kg b.w. olaquindox, which
produced a local reaction and temporary inappetence a week later
(Tettenborn, 1969).
2.2.2 Short-term studies
2.2.2.1. Mice
Groups of 20 male and 20 female BOM-NMRI mice were fed diets
containing 0, 300, 600, 1200, 2400 and 4800 ppm olaquindox,
approximately equivalent to 0, 45, 90, 180, 360 and 720 mg/kg
b.w./day, for 90 days, as a dose-finding exercise for a
carcinogenicity study (see Section 2.2.3).
Signs of toxicity were non-specific and included shaggy fur,
dyspnoea and reduced motility. A marked reduction in body weight
occurred at the highest dietary level in both sexes, and in males
given 1200 and 2400 ppm. During the study 1/20 females died at the
600 ppm level as did 18/20 and 5/20 males and females, respectively,
at the 1200 ppm level. All the mice given the two highest doses died.
No deaths occurred in other groups. At necropsy, haemorrhagic lungs
were the main findings. Microscopic examination was not conducted
(Steinhoff & Gunselmann, 1982).
2.2.2.2 Rats
Groups of 10 male and 10 female Wistar rats were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in 2% aqueous
carboxymethylcellulose by stomach tube, for 5 days per week for 13
weeks.
After 6-8 weeks, signs of toxicity included reddening of the ears
and plantar surfaces; weakness and emaciation occurred in animals
given the highest dose. These animals also developed moist, blood
encrusted nostrils. In the eighth week of the study, fatalities began
to occur, consequently the animals were killed. No clinical signs of
toxicity or compound-related deaths occurred in the other groups.
There were no adverse haematological effects in any groups at 4 weeks
including the high dose group, nor at 12 weeks in remaining animals.
Clinical chemistry was normal at 4 weeks in all dose groups and in
animals given 0, 20 or 60 mg/kg b.w./day olaquindox at 12 weeks.
However, at 8 weeks in the high dose animals (prior to death), blood
sugar was significantly reduced, while serum aspartate
aminotransferase was elevated. Urinalysis was normal in all groups at
4 weeks and in all but the high dose group (not available for
examination due to deaths) at 12 weeks.
Absolute organ weights at 90 days indicated a significant
splenomegaly and increases in testicular and ovarian weights in
animals given 60 mg/kg b.w./day olaquindox. These findings were also
reflected in relative organ weights except for splenic weights in
females. There was a significant decrease in relative adrenal weights
in females given 60 mg/kg b.w./day olaquindox.
Gross examination revealed reddening of the pyloric area of the
stomach in the high dose animals and pale and atrophied adrenals. All
the females given 60 mg/kg b.w./day and 5/10 of those given 20 mg/kg
b.w./day had enlarged, reddened ovaries with numerous pin-point
darkened nodules (corpora lutea).
Microscopic examination revealed adrenal atrophy in the high and
mid dose groups, with degenerative changes in the cortical areas. Some
of the female high dose animals had thyroid atrophy. Female rats
given the mid and low dose had no ovarian atrophy but moderate
atrophic changes were noted in the ovaries of 4/5 high dose females
(Hoffmann 1969; Urwin & Mawdesly-Thomas, 1969).
The experiment was later repeated using lower doses: 0, 1, 5 and
20 mg/kg b.w./day. All other factors were identical to the original
study. No clinical signs were observed during the study and there
were no effects on body weights. There were no haematological or
clinical chemistry abnormalities; urinalyses were normal.
At necropsy, increases in adrenal weights in males given the two
highest daily doses were noted. Increases in ovarian weights occurred
in females given the two highest doses. Histopathologic examination
revealed no changes in any organs in any of the treatment groups. The
no-effect level in these studies therefore was 1 mg/kg b.w./day
(Hoffmann, 1972; Urwin & Spicer, 1971).
In a 90 day dietary study, olaquindox was given to groups of 20
male and 20 female Norway rats at levels of 0, 50, 150 and 300 ppm,
approximately equivalent to oral doses of 0, 5, 15 and 30 mg/kg
b.w./day. Haematological and clinical chemistry investigations were
conducted at days 0, 35, and 63 and at the end of the study.
No signs of toxicity were noted during the study and there were
no effects on haematology and clinical chemistry. Gross and
microscopic examination revealed no compound-related changes
(Nastuneak et al, 1986).
In an inhalation study, groups of 10 male and 10 female Wistar
rats were exposed to 0, 10, 207 and 541 mg/m3 olaquindox dust, 6
hours/day, 5 days per week for 3 weeks under dynamic exposure
conditions. Based upon number and particle mass median diameter,
between 30-80% of the particles were inhalable.
No adverse effects occurred in the rats exposed to the lowest
concentration of olaquindox nor in the males of the intermediate
concentration group. Females in this group showed non-specific
effects ("sluggishness") for the first 5 days of exposure but after
this they appeared normal. Effects of a similar nature and duration
occurred in the high concentration males but in females, signs of
respiratory distress were noted from the 11th day of exposure in
addition to the non-specific effects seen in other exposed groups.
No deaths occurred and only minor effects on body weights were
observed. There were no effects on haematology nor on clinical
chemistry. Urinalyses were normal. No gross abnormalities were
evident at autopsy and relative and absolute organ weights were in the
normal range. There were no adverse histopathological findings. The
non-specific signs and particularly the respiratory effects may have
been due to the physical effects of the olaquindox particles (Thyssen,
1983).
2.2.2.3 Rabbits
Olaquindox in Lutrol was applied to the shaven intact dorsal
surface of 3 groups of 6 New Zealand white rabbits (3 male and 3
female) at doses of 0, 50 or 250 mg/kg b.w./day for 6 hours/day, 5
days per week for 3 weeks, without an occlusive dressing. In an
identical manner, olaquindox was applied to the abraded skin of 3
groups of 3 male and 3 female rabbits.
No signs of toxicity were observed in treated animals and skin
reactions due to olaquindox were not seen in the abraded or intact
skin groups. No mortalities occurred. Clinical chemistry and
urinalyses at the end of the study were comparable to control values.
There were no effects on body weights. At necropsy, no abnormalities
were noted and no histopathological changes attributable to olaquindox
treatment were found (Heimann & Schilde, 1982).
2.2.2.4 Dogs
Groups of 2 male and 2 female beagle dogs were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in gelatin capsules for
90 days.
Vomiting occurred during the first week in dogs given the highest
dose. Salivation was noted and food intake declined. The animals
became emaciated. Dogs given the intermediate dose showed inappetence
and salivation occurred. No effects were noted in low dose animals.
All the high dose animals died within 20 days of the start of the
study. One dog given the intermediate dose died on day 40 while the
remainder were killed in extremis after 46 or 56 doses. No animals
given the low dose died.
There were no notable changes in haematology in treated dogs.
Clinical chemistry revealed increases in blood urea in all 4 dogs
given the high dose. Intermittent elevations of blood urea were noted
in the other dosed groups. No abnormalities in urinalyses occurred.
Gross examination of high dose animals suggested an irritant
effect on the gastrointestinal tract, with congestion in the lungs.
The livers were discolored. No abnormalities were noted in the low
dose animals. Histopathologic examination revealed live cell
enlargement and fatty degeneration in dogs given 60 or 180 mg/kg
b.w./day olaquindox, with fatty degeneration of the cells of the
kidney tubules. No histopathologic changes were found in low dose
dogs and the no-effect level in this study appeared to be 20 mg/kg
b.w./day (Lorke & Tettenborn, 1969; Mawdesley-Thomas & Urwin, 1969).
2.2.2.5 Pigs
Groups of 5 castrated male and female German landrace pigs
weighing 9-10 kg each were fed diets containing 0, 100, 160 and 250
ppm olaquindox for 20 weeks.
At the highest dietary level, 5 pigs died and weight gain was
significantly reduced. Animals given 100 and 160 ppm in the diet had
higher rates of weight gain than controls. No haematological effects
occurred. Plasma creatinine and urea were elevated in the
intermediate and high dietary level groups. Hyperkalaemia and
hyponatraemia were noted in pigs given 250 ppm dietary olaquindox.
Urinalyses were normal.
High dose pigs showed a grey-brown discoloration of the renal
cortex but there were no effects on relative organ weights. Tubular
dilatation and flattening of the tubular epithelium occurred in
intermediate and high dietary level pig kidneys and the adrenals of
these animals displayed enlarged cortical epithelial cells. The no-
effect level in this study was 100 ppm olaquindox in the diet (Gericke
& Dycka, 1974; Hoffman et al., 1974).
Groups of 7 hybrid piglets (4 weeks old, at least 3 females per
group and castrated males) were fed diets containing 0, 25, 50, 100
and 200 (2 groups) ppm olaquindox for 6 weeks.
After 2 weeks, dry faeces were produced by piglets given 100 or
200 ppm olaquindox. The drinking of urine from the floor of pens or
directly from urinating pen-mates was noted in pigs given 50 ppm
olaquindox. A decrease in abdominal volume occurred in piglets given
100 or 200 ppm olaquindox after 5 weeks and in the 25 ppm group in
week 6, but not in animals give 50 ppm. Significant rises in serum
albumin values occurred in piglets given 100 or 200 ppm olaquindox
from week 2 onwards, and marked rises in serum urea values occurred in
the 200 ppm group from week 4 and in the 100 ppm group from week 5.
Gross and microscopic pathology were not conducted (Nabuurs et al,
1989).
Groups of 6 female and 6 castrated male hybrid piglets were fed
diets containing 0, 25, 50, 100 or 200 ppm olaquindox for 6 weeks, in
a study of the effects of treatment on plasma aldosterone, sodium and
potassium levels. There was a gradual decline in plasma aldosterone
which was significant in all but the 25 ppm group by week 5. After 6
weeks the decline was significant in all dosed groups except for that
given 100 ppm where a small rise was noted. Hyponatraemia occurred in
the 25 and 200 ppm groups after weeks 0-2 and a continuous decline in
the 200 ppm group occurred after week 3. In the groups given 25 and
100 ppm olaquindox the levels decreased continuously from 2-3 weeks.
Animals in the 50 ppm group were unaffected. Although elevated
potassium levels occurred in the 50 and 100 ppm groups, only piglets
given 200 ppm were considered to be hypokalaemic (van der Molen
et al, 1989; Baars et al, 1988).
Similar effects have been noted in pigs given other quinoxaline
N-oxide drugs, namely cyadox and carbadox (van der Molen et al,
1985). The latter drug caused these effects after accidental
overdosage of pigs (Power et al, 1989). The toxicity appears to be
due to specific effects on the aldosterone-releasing zona glomerulosa
of the adrenals (van der Molen et al, 1986).
2.2.2.6 Rhesus monkeys
Olaquindox in gelatin capsules was given orally to two groups of
3 male and 3 female rhesus monkeys at doses of 0 and 20 mg/kg b.w./day
and to two groups of 3 males and 5 females at doses of 5 and 40 mg/kg
b.w./day, 7 days a week for 19 weeks. Surviving high dose females
were entered onto a 17 week recovery period.
Animals given the highest dose showed a general loss of condition
and loss of body weight. Suppression of weight gain occurred at the
intermediate dose while growth promotion occurred at the low dose.
Suppression of appetite was evident from week 12 in high dose monkeys.
Vaginal cytology indicated a suppression of ovulation in the high
dose females and in 1/3 animals given the intermediate dose. There was
some evidence of recovery after dosing ceased in the high dose
females. Deaths occurred in 2/3 males and 1/5 females form the high
dose groups during the dosing period and 2 females died during the
first two weeks of the recovery period.
Electrocardiography and ophthalmoscopy were normal in all
animals. Urinalyses and haematology were essentially normal at 5
weeks but serum aspartate aminotransferase values in high dose males
were elevated. At 8 weeks plasma biochemistry was normal but packed
cell volume and red cell counts were lowered in high dose animals.
Urinary glucose was found in 3 high dose males. After 15 weeks of
dosing packed cell volume and haemoglobin showed slight reductions in
high dose males while plasma glucose levels were reduced but not
significantly.
In 7/8 high dose monkeys, urine was positive for glucose and for
total reducing substances. One urine showed a lower pH and one was
positive for ketones. When examined at 15 weeks, red blood cell
values were reduced in high dose monkeys. Plasma glucose values were
reduced in high dose animals and protein and glucose were present in
the urines; pH of urine was lowered in high dose animals. At the end
of the 19 week dosing period no haematological examinations were
performed. Plasma glucose levels were reduced in high dose animals
while plasma urea values were increased. Hypokalaemia was noted in
high and intermediate dose animals. Glucose and total reducing
substances were increased in high dose monkeys while pH was lowered.
Macroscopic examination revealed pallor of the kidney in high
dose animals. Suppression of ovulation occurred in high dose females,
and abdominal abscesses in high dose males.
Histopathological examination revealed fatty changes in the
centrilobular areas of the liver in all high dose animals and
deposition of fat in the kidney tubules in all monkeys from this
group. Brown pigmentation of the zona reticularis of the adrenals
occurred in high dose monkeys. Immature testes were noted in males
given 20 and 40 mg/kg b.w./day olaquindox while inactivity of the
ovaries in high dose females and in 1/3 intermediate dose females was
observed. The no effect level in this oral study in rhesus monkey
was 5 mg/kg b.w./day (Heywood et al, 1972).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 20 male and 20 female NMRI mice were given nominal
doses of 0, 15 or 75 mg/kg b.w./day olaquindox in the drinking water.
Low dose mice were given a total of 6.6 g/kg b.w. for 635 days and
high dose mice were given a total of 32.1 g/kg b.w. for 634 days. The
study was terminated when all the mice had died. Animals were not
dosed over holidays and no mention was made of week-ends.
At termination, no excess tumour incidence was noted.
Lymphadenosis was reported in 1/40 control mice while 2/40 high dose
animals had tumours (thymoma and malignant thymus cell tumour) as did
2/40 low dose mice (pulmonary carcinoma and bronchial carcinoma.
Only small numbers of mice were used in this study and survival
was poor, although it was better in the high dose group than in
controls. The mean survival had a high standard deviation (mean
survival 340 ± 187, 338 ± 224 and 403 ± 194 days for mice given 0, 15
or 75 mg/kg b.w./day, respectively).
Moreover, even when survival was adjusted for unexplained deaths
which occurred in the first 82 days in controls, the treatment time
was still less than is currently viewed as normal for a mouse
carcinogenicity study. The short life-span on test may be due to the
fact that the animals were already around 50 days old when the study
commenced (Schmael, 1973).
In a later study, groups of 75 male and 75 female NMRI mice were
given diets containing 0, 40, 120 or 360 ppm olaquindox, equivalent to
0, 6, 18 or 57 mg/kg b.w./day, for life (until death or sacrifice in
a moribund state).
The only effects on body weights were in males and females given
the highest dietary level: male weights fell slightly below control
values from day 50 and female weights declined after day 200.
Haematological examination at weeks 4, 13, 26, 52 and 78 revealed no
abnormalities and survival times were unaffected by olaquindox intake;
remaining males and females died around day 890 (approximately 29
months).
At necropsy there were no differences in liver, kidney, spleen,
heart, testes, or brain weights, and no increases in non-neoplastic
findings were found in treated mice. No increased tumour incidence
was found in animals given 40 or 120 ppm dietary olaquindox but at 360
ppm there was an increase in the total number of tumours and in the
number of animals with benign tumours. These were due to increases in
the incidence of pulmonary adenoma and adrenal cortical adenoma in
males and in pulmonary adenoma and ovarian granulosa cell tumours in
females (Table 2). There were no increases in the incidence of any
malignant tumour types (Steinhoff & Gunselman, 1982).
Table 2: Tumor incidence in mice given olaquindox in the diet
0 ppm 40 ppm 120 ppm 360 ppm
Males
pulmonary adenoma 11 (15%) 17 (23%) 14 (19%) 27 (36%)
adrenal cortical 5 (7%) 3 (4%) 6 (8%) 13 (17%)
adenoma
Females
pulmonary adenoma 8 (11%) (7%) 7 (9%) 11 (15%)
ovarian granulosa 10 (13%) 16 (21%) 15 (20%) 20 (27%)
cell tumor
2.2.3.2 Rats
Groups of 20 male and 20 female Wistar rats were given
olaquindox, once per week for up to 560 days. The total dose was 4.7
g/kg b.w. with individual doses being in the range of 50-150 mg/kg
b.w. Each dose was given by gavage as a suspension in physiological
saline. Controls were given physiological saline only, but this was
administered by intraperitoneal injection and not by gavage as they
simultaneously acted as controls for other studies.
Survival in treated animals was better than controls (875 ± 105
days for male and 818 ± 167 days for female rats given olaquindox
compared with 797 ± 215 and 779 ± 187 days, respectively, for male and
female controls).
The incidence of tumours was not presented in a detailed tabular
form but was given graphically and in separate tables which did not
allow a full comparison. However, there was no elevated incidence of
any tumour type in treated animals when compared with controls and the
number of tumour-bearing animals was similar in both the treated and
control groups (Steinhoff, 1973).
Groups of 80 BR 46 rats were given drinking water containing
olaquindox at levels to ensure an intake of 15 or 75 mg/kg b.w./day.
A group of 49 rats given water only served as controls. Male and
female rats were used in the study but the sex ratio was not
specified. The treated water was supplied 5 days a week until the
animals died.
Survival of animals treated with olaquindox was better than
controls (704 ± 161 days and 655 ± 229 days in low and high dose rats,
respectively, compared with 554 ± 248 days in control rats). The
carcinogenicity data was presented in an unclear manner and separate
data for male and female animals were not given. The only tumour
which showed an increased incidence was mammary fibroadenoma (1/40,
2.5%; 3/46, 6.5% and 7/46, 15% in controls and in low and high dose
rats, respectively). However, the absence of data on male and female
incidence of this tumour renders the values uninterpretable (Schmael,
1973).
As part of a chronic toxicity/carcinogenicity study with
in utero exposure, groups of 75 males and 75 females were given
diets containing 0, 40, 120 or 360 ppm olaquindox. These doses were
approximately equivalent to oral doses of 0, 3, 10 and 30 mg/kg
b.w./day, for one week prior to mating and during a 3 week 1:1 mating
period. After mating the males were removed and the females given the
diets containing olaquindox until the young were 4 weeks old. At this
time the young from each treatment group were divided into groups of
25 males and 25 females and given the same diets as initially given to
their parents. The study was continued until the young had been
treated for 2 years. Clinical chemistry, haematology, and urinalyses
were conducted on groups of 5 males and 5 females at 4, 14, 26, 54 and
102 weeks after commencement of treatment of the F1 generation.
No overt signs of toxicity were noted in treated animals but
after day 400 of treatment, male and female animals given the highest
dietary level showed a marked and statistically significant reduction
in body weight when compared with control values.
Clinical chemistry suggested an elevated creatinine level in the
blood of rats given 360 ppm dietary olaquindox but all the values were
within the normal range. The albumen content of the urine was
generally lower in treated animals than in controls.
At gross and microscopic examination, there were no increases in
the incidences of non-neoplastic diseases and no increased incidence
of any tumour types. This study employed too few animals to allow an
assessment of carcinogenic potential (Steinhoff, 1977).
Olaquindox was tested in a carcinogenicity study using a similar
protocol on groups of 50 male and 50 female rats remaining in the F1
generation from the chronic toxicity carcinogenicity test described
above. The groups were given diets containing 0, 40, 120 and 360 ppm
olaquindox, approximately equivalent to oral doses of 0, 3, 10, and 30
mg/kg b.w./day. No clinical chemistry or haematological tests were
conducted, however, and the study was terminated when the duration was
approximately 3 years (1065 days for males and 1120 days for females),
at which time 20% of the controls (males and females separately)
remained alive.
No signs of toxicity were noted in treated animals except for
reductions in body weights in rats given 360 ppm after day 500.
Reductions occurred despite the fact that in terms of feed consumption
in g/kg b.w./day these animals had consumed more.
At termination there was a significant decrease in survival in
animals given the highest dietary level (98% mortality in males and
females) and in females given 40 ppm olaquindox (92% mortality)
compared with controls (80% mortality). Mortality in the other
dietary groups was only slightly more than in controls (82-86%).
At necropsy there were no differences between treated animals and
controls for the number of animals of each sex with total tumours,
primary tumours, malignant and benign tumours, malignant tumours with
metastases and total benign tumours. Similarly, for particular tumour
sites (benign and malignant tumours plus metastases) there were no
excess incidences except for slight increases in the incidence of
adrenal, reticuloendothelial and seminal vesicle neoplasms, but these
were due, except for the adrenal tumours, to metastases or
infiltrations from other organs. Moreover, the adrenal tumours were
not increased with respect to any particular histological type and
again were often of metastatic origin (Steinhoff & Boehme, 1978).
There were some anomalies in the reporting of this study which
appear to be due to arithmetical errors. The summary table of tumour
incidence cites a benign tumour in female controls which is not listed
in the detailed histopathology tables. The summary data also claims
4 malignant tumours in males given the highest dietary level whereas
the histopathology table lists 9. However, these differences do not
affect the outcome of the study or its assessment.
2.4 Reproduction Studies
2.4.1 Mice
Groups of 20 pregnant NMRI mice were given oral doses of 0, 20,
60, or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. On day 18 of
gestation the fetuses were delivered by Caesarean section and these
were weighed and examined for gross malformations and by alizarin
staining.
None of the pregnant animals died during the test but animals
given the highest dose showed reduction in body weight or rate of
weight gain. The numbers of implantations, live fetuses and
resorptions were similar in all dosed groups. At the highest dose,
fetal weights were significantly lower than in controls. The
incidence of malformations in all treated groups was similar to those
seen in controls (Lorke, 1971a).
2.4.2 Rats
Groups of 20 female pregnant FB 30 rats were given oral doses of
0, 20, 60 or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. Fetuses were
delivered by Caesarean section on day 20 of gestation and these were
examined in the same manner as for the mouse study described above.
The pregnant rats given the highest daily dose showed reductions
in body weights or rate of weight gain compared with control values.
These animals also showed a higher incidence of resorptions and lower
numbers of live fetuses. Fetal weights were lower in the high dose
animals. These indices were similar to controls and to rats given 20
or 60 mg/kg b.w./day olaquindox.
The incidence of malformations in fetuses from dams given 20 or
60 mg/kg b.w./day olaquindox was similar to controls but at the
highest dose level there was an elevated incidence of malformed
fetuses, with 5 malformations reported at a dose level of 180 mg/kg
b.w./day.
In this study, therefore, there was a teratogenic effect at the
highest dose level given to pregnant rats (180 mg/kg b.w./day) on days
6-15 of gestation. The no-effect level in this study was 60 mg/kg
b.w./day (Lorke, 1971b).
A 3-generation study was conducted in groups of 10 male and 20
female FB30 rats using 0, 20, 100 and 500 ppm olaquindox in the diet,
equivalent to oral doses of 0, 1, 5 and 25 mg/kg b.w./day. The F0
animals were given the diets containing olaquindox throughout the
study including during the mating periods. Animals were mated after
dosing for 70 days. Animals which died during the study were
necropsied, while young animals were examined macroscopically after
birth and subsequently observed during the rearing period for evidence
of abnormalities. The F3b animals were sacrificed at 3 weeks of age
and subjected to macroscopic and microscopic examination.
Treatment had no effect on body weights except that body weight
of F0 generation females given the highest dietary level of
olaquindox were slightly higher than weights of controls.
The fertility rate was also lowered in F0 animals in the first
and second matings when given the highest level, but there were no
effects on litter size nor on rearing rates. F1a and F1b animals did
not differ from controls in terms of birth weights except for a
slight, non-statistical increase in those derived from F0 dams given
the highest dietary level. After the second F0 mating the average
number of young in the F1b generation was similar in all treatment
groups, but at 5 days there was a significant reduction for dams given
500 ppm olaquindox (6/litter) compared with values in other groups and
controls (10-12/litter). Birth weights of the F1b generation were
unaffected.
When the F1b generation was mated, the gestation rates in animals
derived from animals initially given the highest level of olaquindox
were reduced (80-84%) compared with values for other groups (90-100%).
The average numbers of young per litter were also reduced at the high
dietary level in both the F2a and F2b generations, both at birth and
5 days after birth (8/litter compared with 11/litter in controls).
The F2 birth weights were unaffected and there was some improvement
in the rearing rates up to 4 weeks.
In the F3 generation, fertility was again affected in animals
originally derived from the high dietary level rates, with gestation
rates of 70-84% compared with 90-100% in other groups. The average
number of young per litter was also reduced at the high dietary level
(5-7/litter) compared with other groups (8.5-10.8/litter). There were
no effects on rearing rates nor F3 birth weights. No malformations
were noted during the course of the study and no abnormalities were
found on gross or histopathological examination of three week old F3b
animals (Loeser, 1974).
In a fertility test in Wistar rats, olaquindox was administered
orally by gavage to groups of 10 male rats at doses of 4 and 10 mg/kg
b.w./kg which were mated with groups of 20 untreated females. Groups
of 10 untreated males were mated with groups of 20 females given
gavage doses of 4 and 10 mg/kg b.w./day. Males were dosed for 8 weeks
prior to mating while females were dosed for 3 weeks prior to mating.
Males and females were mated in a 1:2 ratio. Groups of untreated
males and females were mated as controls.
Olaquindox administration had no effect on body weights, on
oestrus cycles, or on copulation and conception rates. A significant
reduction in the average number of implantations was noted in the
group in which females given the lower dose of 4 mg/kg b.w./day
olaquindox were mated with untreated males. Pre-implantation losses
were significantly increased in both the groups where females were
treated with olaquindox, and post-implantation losses were increased
in females given the 10 mg/kg b.w./day dose. There were no effects in
the groups where males treated with olaquindox were mated with
untreated females (Gandalovicova & Sykora, 1986).
2.2.5 Special studies on pharmacological properties
Olaquindox has been tested in a number of pharmacological
screening tests in rats and mice including those for anticonvulsive
effects, inhibition of defensive reaction, motor coordination,
analgesia, antihypertensive effects, gastric secretion, bile
secretion, diuresis, blood sugar and blood lipids and thrombocyte
aggregation (bovine plasma). No pharmacological activity was detected
(Kaller, 1970).
2.2.6 Special studies on irritancy and hypersensitivity
Olaquindox as a micronized powder was applied without a vehicle
to the shaved intact and abraded dorsal skin of 6 New Zealand white
rabbits using a 24-hour occlusive dressing. The skin was assessed on
removal of the dressing, after 48 and 72 hours, and at 7 days. Slight
erythema of intact and abraded skin was observed at 24 hours, but not
at the other time points. No oedema was noted. Results indicated a
mild irritant effect (Murman, 1979).
Olaquindox was tested for its ability to induce eye irritation by
application of 15 mg of the micronized substance to the conjunctiva of
the right eyes of 6 New Zealand white rabbits, and to the left
conjunctival sacs of a further 6 rabbits. The material was washed out
of the left eyes with physiological saline after 1 minute. Reactions
were monitored 24, 48, and 72 hours after application and after 7
days.
A slight reddening of the conjunctiva was noted in 4/6 eyes where
olaquindox had been directly applied. Slight chemosis was noted in
2/6 eyes. A slight chemosis was noted in 1/6 rabbit's eyes where
olaquindox has been applied to the conjunctival sac and then washed
out. All reactions subsided within 48 hours. The result suggest that
olaquindox has a slight irritant effect but the mechanical effects of
the dust cannot be ruled out (Murman, 1979).
Olaquindox was tested in the guinea-pig for its ability to induce
hypersensitivity. Olaquindox dissolved in dimethyl sulfoxide or as a
suspension in phosphate buffered saline was administered
intracutaneously into the neck region of groups of 10 Pirbright albino
guinea-pigs on days 1, 3, 6, 9, and 13 of a sensitization schedule.
Four days after the last injection, a suspension of olaquindox in 1:1
acetone/almond oil was applied to the depilated flank and massaged
gently into the skin. To allow for any possible effects of light,
groups of guinea-pigs were treated in a similar manner but were kept
in darkened cages. No indications of sensitization were noted in this
study when assessed by gross examination of the skin for reactions or
when examined histologically (Schlumberger, 1975). (This test is not
widely used and it is unlikely to be as sensitive as those using an
adjuvant (e.g. Freunds') such as the Magnusson-Kligman or Beuhler
models).
2.2.7 Special studies on genotoxicity
Olaquindox has been tested in a wide range of genotoxicity tests
and these are summarized in Table 3. It has produced positive results
in a number of studies designed to test for reverse mutations in
bacteria, including the Ames test with Salmonella typhimurium
strains (Beutin et al, 1981; Yoshimura et al, 1981; Voogd
et al, 1980; Nunoshiba & Nishioka, 1989). A positive result has
been noted in a forward mutation assay with Escherichia coli
(Nunoshiba & Nishioka, 1989).
In vitro study with cultured human lymphocytes and a number of
in vivo assays with mouse bone marrow or Chinese hamster
spermatogonia as the target tissues have demonstrated the clastogenic
activity of olaquindox (Tamura, 1977; Cihak & Vontorkova, 1983; Sram
et al, 1986a; Pokorna, 1986; Herbold, 1983a). Similarly, olaquindox
has produced positive results in several micronucleus tests in the
mouse following oral or inhalation exposure (Herbold, 1983b; Herbold
& Thyssen, 1982; Cihak & Vontorkova, 1985), and in the rat after
intraperitoneal injection (Cihak et al, 1983). However, a dermal
study with a 24 hour exposure was negative (Herbold, 1982a),
reflecting the poor dermal absorption of the substance.
Olaquindox has been tested in two dominant lethal assays in the
male mouse but a weak positive result was observed in only one of
these when a high dose (1 g/kg b.w) was employed (Machemer, 1977a;
Herbold, 1982b). Positive lethal mutations also occurred when female
mice were treated orally with olaquindox despite the use in one study
of doses lower than that which effected a positive result in the male
mouse (200 and 500 mg/kg b.w.) (Machemer, 1977b; Sram et al, 1986b
& c). However a toxic effect in female mice could not be excluded.
Positive results have been obtained in a sister chromatid
exchange test using Chinese hamster V79 cells indicating that
olaquindox may induce DNA damage (Scheutwinkel-Reich & von der Hude,
1984). Positive results in bacterial assays including the SOS
chromotest confirm this possibility (Suter et al, 1978; Beutin
et al, 1981; Yoshimura et al, 1981; Nunoshiba & Nishioka, 1989;
von der Hude et al, 1988). However, there is no evidence that
olaquindox covalently binds to DNA in the rat in vivo (Minini
et al, 1983).
The mutagenicity of a number of olaquindox metabolites has also
been investigated. The omega oxidation product, its 1 and 4
monodesoxy derivatives and its didesoxy derivative have been
investigated in the Ames test using S. typhimurium strains TA 98,
100, 1535 and 1537 with and without rat liver S9 metabolic activation.
All the tests gave negative results (Herbold, 1978; 1979a,b).
Table 3: Results of genotoxicity studies with olaquindox
Test System Test Object Concentration Results Reference
Ames test1 S. typhimurium 3.8-0.5 nmoles/plate Positive Beutin et al., 1981
TA98, 100
Ames test2,4 S. typhimurium 1.9-57 nmoles/plate Positive Beutin et al., 1981
TA100
Ames test1 S. typhimurium 1.25-15µg/plate Positive Yoshimura et al.,
TA98, 100 1981
Ames test2 S.typhimurium 0.01-0.1 mmole/1 Positive Voogd et al., 1980
Ames test1 S. typhimurium 0-50 µg/plate Positive Nunoshiba & Nishioka,
TA98,100 1989
Fluctuation1 test K. pneumoniae 2x10-4-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Fluctuation2 test K. pneumoniae 2x10-5-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Forward1 mutation E. coli 0-20 µg/plate Positive Nunoshiba & Nishioka,
Wp\P2uvrA/pKM101 1989
In vitro Cultured human 3-300 µg/ml Positive Tamura, 1977
cytogenetics lymphocytes
In vivo Mouse bone marrow 20, 500 or 800 mg/kg Negative Sutou, 1977
cytogenetics b.w. oral
In vivo Mouse bone marrow 200-800 mg/ml b.w. oral Negative Cihak & Vontorkova, 1983
cytogenetics
In vivo Mouse bone marrow 20-500 mg/kg b.w. diet Positive Sram, et al., 1986a
cytogenetics 4 and 12 weeks
Table 3 (contd)
Test System Test Object Concentration Results Reference
In vivo Chinese hamster 20 mg/kg b.w. oral, x5 Positive Pokorna, 1986
cytogenetics marrow
In vivo Chinese hamster 2x30-2x1000 mg/kg b.w. Positive Herbold, 1983a
cytogenetics spermatogonia oral
Micronucleus Mouse bone marrow 500 mg/kg b.w. oral, Positive Herbold, 1983b
sampled at 24, 48 or
72 hours; 10-300 mg/kg
b.w. oral, sampled at
24 hours
Micronuceus test Chinese hamster 20 mg/kg b.w. 4.2 and Positive Pokorna, 1986
bone marrow 100 mg/kg b.w. oral,
once
Micronucleus Mouse bone marrow 6.7 mg/m3 and 161 mg/m3 Positive Herbold, & Thyssen, 1982
for 6 hours/day, 2 days,
inhalation
Micronucleus Mouse bone marrow 2034 mg/kg b.w. 30 hours Negative Herbold, 1982a
dermal exposure
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Dominant lethal Mouse (male) 2x1000 mg/kg b.w. week Positive Machemer, 1977a
oral
Table 3 (contd)
Test System Test Object Concentration Results Reference
Dominant lethal Mouse (male) 40, 120 and 360 ppm in Negative Herbold, 1982b
diet for 35 days
equivalent to 6, 18, and
54 mg/kg b.w.
Dominant lethal Mouse (male) 100, 300 and 500 mg/kg b.w. Negative Sram, et al., 1986b
for 4 weeks, 20, 40, 100,
200, and 500 mg/kg b.w.
for 12 weeks, diet
Dominant3 lethal Mouse (female) 30, 100, 300 or 1000 mg/kg b.w. Positive Machemer, 1977b
oral once
Dominant lethal Mouse (female) 20, 40, 100, 200 and 500 mg/kg Positive Sram, et al., 1986c
b.w. diet, for 4 weeks
SOS2 Chromotest E. coli, GE94 0-10 µg/plate Positive Nunoshiba & Nishioka, 1989
(DNA damage)
SOS2 Chromotest E. coli, PQ37 0.001-0.1 mM Positive von der Hude, et al.,
(DNA damage)
DNA damage E. coli, K12 Not applicable Positive Suter, et al., 1978
DNA damage S. typhimurium 100 µg/disc uvr B and recA Positive Beutin, et al., 1981
DNA damage S. typhimurium 1-100 µg/disc Positive Yoshimura, et al., 1981
Mitotic gene2 S. cerevisiae D4 0.05% w/v Positive Voogd, et al., 1980
conversion
Table 3 (contd)
Test System Test Object Concentration Results Reference
DNA binding Rat 500 mg/kg b.w. oral Negative Minini, et al., 1983
in vivo
Sister chromatid Chinese hamster 0-200 µg/ml V79 cells Positive Scheutwinkel-Reich & von
exchange der Hude, 1984
1 With and without rat liver S-9 fraction.
2 In the absence of S-9 fraction.
3 Positive only at the 1000mg/kg b.w. dose.
4 Positive in aerobic and anaerobic conditions.
The results obtained with olaquindox are similar to those noted
with several other quinoxaline di-N-oxides including quindoxin and
carbadox (Suter et al, 1978; English & Dunegan, 1970; Voogd
et al, 1980; Negishi et al, 1980; Beutin et al, 1981 (see also
carbadox)). The mechanisms of action are unknown although neither
olaquindox nor quindoxin binds to DNA (Minini et al, 1983, Suter
et al, 1978).
Electron spin resonance techniques have demonstrated the
generation of free radicals during the reduction of quindoxin while a
related compound 2,3-dihydroxymethyl quinoxaline-1,4-di-N-oxide has
been shown to inhibit DNA synthesis in E. coli (Suter et al,
1978). However, at present the roles of free radicals and inhibition
of DNA synthesis in the mutagenicity of quinoxaline-1,4-dioxides are
unknown.
These studies indicate that olaquindox is genotoxic in a variety
of test systems. It induces mutations in bacterial systems and it
causes chromosome and DNA damage in in vitro and in in vivo
systems. Data available from dominant lethal assays and from an
in vivo cytogenetic study with Chinese hamster spermatogonia suggest
that olaquindox may have the potential to exert its mutagenic effects
on germ line cells.
2.3 Observations in humans
One of the major routes of exposure to olaquindox is likely to be
occupational during the preparation of feed and the feeding of the
final feed to pigs. In an experimental study, no olaquindox was
detected in the workplace air (a stall building housing pigs) during
trough filling operations with a feed containing 50 ppm olaquindox
(Thyssen et al, 1982). Olaquindox was only detected at low levels
in the atmosphere during the preparation of 0.1% feed premix and the
50 ppm final feed starting with a 10% premix of a proprietary product.
Levels in the atmosphere were estimated to be from below 0.4 to below
0.1 µg/m3 air (Inkmann-Koch, 1985a). Analysis of the urine from a
single worker engaged in similar preparation work and in the feeding
of pigs revealed no olaquindox (limit of detection 40 ppb) (Inkmann-
Koch, 1985b).
When applied to the skin of two volunteers as a paste in water
containing 2 g of olaquindox (around 30 mg/kg b.w.) using a 6 hour
occlusive dressing, no olaquindox was detected in the 48 hour urine
using an analytical method with a limit of detection of 0.12 µg/ml
(Beermann, 1982).
There has been one report of allergic contact dermatitis and one
of photocontact dermatitis following occupational exposure to
olaquindox (Bedello et al, 1985; Francalanci et al, 1986). Both
occurred in pig workers exposed to the substance in the animal feed.
There are no reports of systemic toxicity in humans following exposure
to olaquindox.
3. COMMENTS
The toxicological data considered by the Committee included the
results of acute and subchronic studies, together with the results of
studies on mutagenicity, carcinogenicity, and effects on reproduction
and development.
Olaquindox is almost completely absorbed from the
gastrointestinal tract in rats, dogs, and pigs. In studies using
radiolabelled olaquindox, the radioactivity was shown to be widely
distributed in the tissues, with residues in the liver being the most
persistent. The compound was primarily eliminated in the urine, with
lesser amounts being excreted in the faeces and expired air of the
animals. In the pig, elimination was virtually complete by 48 hours
after a single intragastric dose. Any remaining radioactivity was
then eliminated with a half-life of 5-9 days, far in excess of that of
olaquindox (about 3-5 hours). The parent compound accounted for 70%
of the radioactivity in the urine, up to 24 hours after
administration. There were approximately 16 metabolites detected in
the urine; six major metabolites have been fully characterized.
In acute and short-term toxicity studies in rats and mice, rats
were about twice as sensitive as mice. In a 90-day study in rats,
effects were observed in the testes, ovaries, thyroid, and adrenal
cortex at a dose of 5 mg/kg b.w./day and above. These lesions were
described histopathologically as atrophy; and in the adrenal glands
the effect was greatest in the zona glomerulosa. No treatment-related
abnormalities of the pituitary gland were reported. A 90-day study in
beagle dogs and a 19-week study in rhesus monkeys also produced
evidence of toxic effects on the endocrine glands, liver, and kidney.
Of five female monkeys dosed at 40 mg/kg b.w./day, one died during
treatment and two died during the planned 17 week recovery period. The
two survivors failed to re-establish normal ovarian cycles during the
recovery period.
In a 20-week study, groups of five male and five female pigs were
fed up to 250 mg of olaquindox per kg of diet. The plasma
concentrations of urea and creatinine were elevated at 160 and 250 mg
diet, which suggested an effect on the kidney. All of the pigs in the
highest-dose group that survived the study period continued to show
evidence of this effect during a 16 day-recovery period. Plasma
sodium, potassium and chloride concentrations were altered during the
study, but did not return to normal during the recovery period.
Histopathological changes were found at necropsy in kidney and adrenal
tissues from both groups. The manufacturers reported a no-observed-
effect level of 100 mg/kg in feed for this study.
However, in other studies in piglets in which olaquindox was fed
at 25, 50, 100 or 200 mg/kg diet for 6 weeks, a dose-dependent fall in
plasma aldosterone concentration, together with hyponatraemia,
hypochloraemia, and hyperkalaemia occurred in all groups by the end of
the study. Hydropic degeneration of adrenal cortex cells was
recorded. There were no effects on weight gain or clinical signs.
Developmental studies in which the compound was administered
orally were conducted in mice and rats. In mice, fetal weights were
reduced at 180 mg/kg b.w./day, but malformations were absent. In
rats, malformations occurred at 180 mg/kg b.w./day, and reductions in
maternal weight gain, litter size, and fetal weight were also observed
at this dose.
A three-generation reproduction study in which olaquindox was
administered in the diet was conducted in rats. No malformations were
observed, the only findings being reductions in fertility rate and
litter size in the second and third generations at the highest dose of
25 mg/kg b.w./day.
The genotoxicity of olaquindox was investigated in a range of
in vitro and in vivo studies. Positive findings were reported in
assays for point mutation and for DNA damage in bacteria, sister-
chromatid exchange in Chinese hamster V-79 cells, chromosome damage in
human lymphocytes in vitro, mammalian bone-marrow cells in vivo,
and in several micronucleus tests. Two dominant lethal assays in male
mice were negative, but a third gave a weak positive response. Two
dominant lethal assays in female mice gave positive results, but this
may have been partly due to the toxicity of the drug. A weak positive
result was obtained in an in vivo cytogenicity assay using Chinese
hamster spermatogonia. Olaquindox did not bind to rat DNA in vivo.
The Committee considered data from six long-term studies in
rodents, but because of poor survival and deficiencies in experimental
design and reporting, only two carcinogenicity studies, in mice and in
rats, were evaluated.
In the study in mice, doses of olaquindox up to an equivalent of
54 mg/kg b.w./day were administered in the diet for life (up to 635
days). An increase in the incidence of benign adrenal cortical
adenomas and benign proliferative lesions (nodular hyperplasia and
adenoma) in the lung was observed in male mice at the highest dose
level. There was no effect on the incidence of malignant tumours.
In the rat carcinogenicity study, which included fetal exposure
to the drug in utero, olaquindox was administered in the diet at
levels up to an equivalent of 30 mg/kg b.w./day until 80% of the male
and female controls had died (about three years). Survival was
decreased at 30 mg/kg b.w./day in both sexes, but there was no
increase in the incidence of benign or malignant tumours.
4. EVALUATION
The Committee considered olaquindox to be a genotoxic agent.
There was some evidence to suggest that olaquindox was a germ-line
mutagen, but more extensive testing in appropriate mammalian studies
will be required to resolve this issue. In the carcinogenicity
studies only the mouse showed an increase in the incidence of tumours,
and these were benign. Because of doubts over the mechanism of this
effect and the results of the genotoxicity studies, the Committee was
unable to establish an ADI for olaquindox. However, the Committee
concluded that residues resulting from the use of olaquindox in food-
producing animals under conditions of good practice in the use of
veterinary drugs were temporarily acceptable.
The results of studies on the nature and availability of residues
of olaquindox and the results of studies designed to provide an
indication of the toxic potential of these residues are required by
1993.
Depending upon the results of these studies, the following
additional information may be needed:
a) Data to assess the genotoxic potential of olaquindox on
germ-line cells, which would, at a minimum, necessitate a
repeat of the Chinese Hamster spermatogonia study.
b) Studies designed to assess the effects of olaquindox on
adrenal function (including sensitive parameters such as
plasma adrenal hormones and electrolyte levels), sperm
morphology, and fertility in rats, so that a NOEL can be
determined for each of these indicators.
c) Information on the binding of olaquindox or its metabolites
to structural proteins such as tubulin, or to enzymes or
proteins involved in DNA synthesis or repair. (Such binding
may help explain why, despite its obvious genotoxic
potential, olaquindox does not bind to DNA and has given
equivocal results in carcinogenicity studies).
5. REFERENCES
ANDERSON, B., & SZABO, A. (1982). Determination of olaquindox in
swine tissues after short withholding periods. Report No. 08-07-
1981/24-03-1982. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
ANON. (1989). Compendium of Data Sheets for Veterinary Products
1989-90. National Office of Animal Health, Datapharm Publications
Ltd, London.
BAARS, A.J., VAN DER MOLEN, E.J., SPIERENBURG, T.J., DE GRAAF, G.J.,
NABUURS, J.J.A., & JAGER, L.P. (1988). Comparative toxicity of three
quinoxaline-di-N-dioxide feed additives in young pigs. Arch.
Toxicol., Suppl. 12, 405-409.
BEDELLO, P.G., GOITRE, M., CANE, D., & RONCAROLO, G. (1985). Allergic
contact dermatitis to Bayo-N-Ox-1. Contact Derm., 12, 284.
BEERMAN, D. (1982). Cutaneous absorption of olaquinodox in human
volunteers. Doc. No 82/10744. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
BEUTIN, L., PRELLER E., & KOWALSKI, B. (1981). Mutagenicity of
olaquindox, 3its metabolites, and two substituted quinoxaline-di-N-
oxides. Antimicrob. Agents Chemother., 20, 336-343.
BORIES, G., & BOURDON, D. (1977). Residue analysis in pigs. Doc. No.
77/8813. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
CIHAK, R., SRB, V., & VONTOKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. I. Micronucleus
test in rats. Mutat. Res., 116, 129-135.
CIHAK, R., & VONTORKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. II. Metaphase
analysis in mice. Mutat. Res., 117, 311-316.
CIHAK, R., & VONTORKOVA, M. (1985). Cytogentic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. III.
Transplacental micronucleus test in mice. Mutat. Res., 144, 81-84.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A.,
(1970). Resorption, distribution, substance changes, and elimination.
Short test in the rat and in the dog. Report No. 1939. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A., &
SCHEER, M., (1973). Metabolism and residue studies wth 14C-labelled
Bay VA 9391 in swine. Report No. 4151. Unpublished report by Bayer
AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
ENGLISH, A.R., & DUNEGAN, C.M. (1970). Quinoxaline-1, 4-di-N-oxides.
I. Inhibition of deoxyribonucleic acid synthesis in Escherichia
coli by 2, 3-dihydroxy-methyl-quinoxaline-1, 4-di-N-oxide. Proc.
Soc. Exp. Biol. Med., 133, 398-400.
FRANCALANCI, S., GOAL, M., GIORGINI, S., MUCCINELLI, A., & SERTOLI, A.
(1986). Occupational dermatitis from olaquinodox. Contact Derm.,
15, 112-114.
GANDALOVICOVA, D., & SYKORA, I. (1986). Cyadox olaquindox - a
fertility test on rats. Biol. Chem. Vet., (Prague), 22, 47-51.
GERICKE, H., & DYCKA, J. (1974). Olaquindox/Bayo-N-ox.
Investigations into the safety of use of the feed additive. Report
No. 4752. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HEIMANN, K.G. & SCHILDE, B., (1982). BAY VA 9391. Study of subacute
dermal toxicity in rabbits. Report No. 11267(E). Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1978). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No 2. 7824. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979a). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 8091/8092/8099. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979b). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 7824. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982a). Micronucleus test on the mouse to check for
mutagenic effect after cutaneous application. Report No. 10602.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982b). Dominant lethal test on male mice to check for
a mutagenic effect after 35 days of administration in the feed.
Report No 10621. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HERBOLD, B. (1983a). Cytogenetic study of the spermatogonia of the
Chinese hamster in vivo to test for mutagenic activity. Report No.
12179. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1983b). Micronucleus test for a mutagenic effect on on
the mouse. Report No. 11424(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
HERBOLD, B., & THYSSEN, J. (1982). Micronucleus test on the mouse to
test for mutagenic activity after inhalation of dynamically produced
dust. Report No. 10833. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HEYWOOD, R., SORTWELL, R.J., NEWMAN, A.J. & STREET, A.E. (1972).
Report No. 4751/72/186. FB a 9391. Oral Toxicity study in rhesus
monkeys. (Repeated dosage for nineteen weeks and recovery period).
Unpublished report from Huntingdon Research Centre, Huntingdon, UK.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1969). BAY VA 9391. Subchronic toxicity in rats
following oral administration. (Trial over 13 weeks). Report No.
1693. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1972). BAY VA 9391. Subchronic toxicity in rats with
oral administration. (13-week test with low doses). Report No. 3241.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K., LUCKHAUS, G. & DYCKA, J. (1974). BAY VA 931.
Toxicological feeding test in feeder swine. Part 3. Results of the
toxicological, pathologic-anatomic and histopathological studies.
Report No. 4754. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
INKMANN-KOCH, A. (1985a). Determination of the concentration of
olaquindox in the air during the preparation of compound (meal) swine
feed and exposure of workers. Doc. No. 85/11885. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
INKMANN-KOCH, A. (1985b). Determination of olaquindox in urine
samples of a worker follwing the preparation of swine feed from Bayo-
N-Ox. Doc. No. 85/11887. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
KALLER, H. (1970). BAY VA 9391. Pharmacological screening. Report
No. 1809. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
KULCZYK, S.R., HERRERA, R.E., MULKEY, N.S., BREAULT, G.O., WARGO,
J.P., & WAGGONER, T.B. (1979). Determination of physical constants of
BAY VA 9391. Report No. 79/9803. Unpublished report from Analytical
Development Corporation, USA. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
LEIBETSEDER, J. (1980). To determine olaquindox residues in swine.
Doc. No. 80/10022. Unpublished report from the Institute of Nutrition
of the Veterinary Medical University, Vienna, Austria. Submitted to
WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LOESER, E. (1974). BAY Va 9391. Three-generation test in rats.
Report 4748. Unpublished report from Bayer AG, Wuppertal-Elberfeld,
FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE, D. & TETTENBORN, D. (1969). FBa 9391. Subchronic toxicity of
dogs during oral administration. (13 Week test). Report No. 1536.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE D. (1971a). FBa 9391. Examination for embryotoxic effects in
mice. Report No. 2692. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
LORKE, D. (1971b). FBa 9391. Examination for embryotoxic effects in
rats. Report No. 2693. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
MACHEMER, L. (1977a). To evaluate mutagenic potential of BAY VA 9391
in mice utilizing the dominant lethal test. Report No. 6738.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
Machemer (1977b). To investigate BAY VA 9391 for mutagenic effects
using the dominant lethal test. Report No. 6770. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
MAUL, W., SENG, F. & WENDISCH, D. (1979a). Investigations into the
biotransformation of olaquinox in swine. Report No. 8114.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAUL, W, SENG, F. & WENDISCH, D. (1979b). Investigations into the
biotransformation of olaquinox in swine. Report No. 8725.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAWDESLEY-THOMAS, L.E. & URWIN C. (1969). Pathology report of FB a
9391. Sub-chronic toxicity for dogs with oral administration. Report
No. 2921/69/347. Unpublished report from Huntingdon Research Centre,
Huntingdon, UK. Submitted to WHO Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. (1974). Method for the determination of residues of Bay
VA 9391 and its reduction products in animal tissues. Report No.
4751. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. & GERICKE, H. (1974). Toxicological Feeding Test with
Feeder Pig. Part 2. Results of Residue Analysis. Report No. 4753.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MININI, U., LUTZ, W.K., & SCHLATTER, CH. (1983). Lack of in vivo
binding to DNA of olaquindox. Report No. R2570 (P). Unpublished
report from the Institute of Toxicology, Swiss Federal Institute of
Technology and University of Zurich, Schwerzenbach, Switzerland.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MURMAN, P.(1979). BAY VA 9391 (Olaquindox). Testing of primary
dermal and mucosal tolerability in the rabbit. Report No. 8359.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
NABUURS, M.J.A., VAN DER MOLEN, E.J., DE GRAAF, G.J., & JAGER, L.P.
(1989). Clinical Signs and Performance of Pigs Treated with Different
Levels of Carbadox, Cyadox and Olaquindox. J. Vet. Med., 36A,
209-217.
NASTUNEAK, J., KOLOUCH, F., VANOVA, L., DVORAK, M. & SEVICK, B.
(1986). Three month toxicity to laboratory Norway rats: Cyadox
compared with olaquindox. Biol. Chem.Vet. (Prague), 22, 367-380.
NEGISHI, T., TANAKA, K. & HAYATSU, H. (1980). Mutagenicity of
carbadox and several quinoxaline 1,4-dioxide derivatives. Chem.
Pharm. Bull., 28, 1347-1349.
NUNOSHIBA, T., & NISHIOKA, H. (1989). Genotoxicity of quinoxaline
1,4-dioxide derivatives in Escherichia coli and Salmonella
typhimurium. Mutat. Res., 217, 203-209.
POKORNA, D. (1986). Cytogentic analysis of bone marrow cells in
Chinese hamster after the administration of cyadox and olaquindox.
Biol. Chem. Vet. (Prague)., 22, 23-28.
POWER, S.B., DONNELLY, W.J.C., MCLAUGHLIN, J.G., WALSH, M.C. & DROMY,
M.F. (1989). Accidental carbadox overdosage in pigs in an Irish
weaner-producing herd. Vet. Rec., 124, 367-370.
SCHEUTWINKEL-REICH, M. & VON DER HUDE, W. (1984). Sister-chromatid
exchange in Chinese hamster V79 cells exposed to quindoxin, carbadox
and olaquindox. Mutat. Res., 139, 199-202.
SCHLUMBERGER, H.D. (1975). BAY VA 9391: Exclusion of immunogenic
activity. Report No. 5194. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
SCHMAEL, D. (1973). Carcinogenicity test with BAY VA 9391 with oral
application to rats and mice. Report N. 38399. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986a).
Chromosomal aberration in the bone marrow of mice after long-continued
administration cyadox and olaquindox. Biol. Chem. Vet. (Prague), 22,
17-22.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986b). Effect
of long-term oral administration of cyadox and olaquindox on the
frequency of dominant lethal mutations and sperm abnormalities in
mice. Biol. Chem. Vet. (Prague), 22, 37-45.
SRAM, R.J. FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986c).
Dominant lethal mutation in female mice following the oral application
of cyadox and olaquindox. Bio. Chem. Vet. (Prague), 22, 29-35.
STEINHOFF, D. (1973). Carcinogenicity study with Bay VA 9391 after
oral administration to rats. Report No. 4048. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
STEINHOFF, D. (1977). BAY VA 9391. Study as to chronic toxic effect
in rats after oral application. (2-Year trial with additional
transplacental treatment). Report No. 6556. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
STEINHOFF, D., & BOEHME, K. (1978). BAY VA 9391. Carcinogenesis
tests with oral administration to rats. (With transplacental
pretreatment). Report N. 7387. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
STEINHOFF, D., & GUNSELMANN, W. (1982). BAY VA 9391. Carcinogenicity
experiment with oral administration to mice. Report No. 72388
(revised), 100700 (revised), 12251 (revised). Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
SUTER, W., ROSSELET, A., & KNUSEL, F. (1978). Mode of action of
quindoxin and substituted quinoxaline-di-N-oxides on Escherichia
coli. Antimicrob. Agents Chemother., 13, 770-783.
SUTOU, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No 78/9327. Unpublished report from Medical Biology
Laboratory, Life Science Department, Nomura Resarch Station, Japan.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
TAKASE, I., & KOMACHI, S. (1975). Determination of BAY VA 9391
residues in animal tissues. Doc. No. 76/8507. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer Ag, Wuppertal-Elberfeld, FRG.
TAKASE, I & KOMACHI, S. (1976). Residues of BAY VA 9391 (olaquindox)
in tissues and organs of pigs. Doc No. 76/8508. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer AG, Wuppertal-Elberfeld, FRG.
TAMURA, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No. 78/9326. Unpublished report from Keio University,
School of Medicine, Tokyo, Japan. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
TETTENBORN, D. (1969). FBa 9391. Acute toxicity for mouse, rat,
rabbit, cat, and dog with oral and subcutaneous administration.
Report No. 1176. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J. (1982). Olaquindox. Acute inhalation toxicity of the
dust. Report No. 10755. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J., BEERMANN, D., & DORN, H. (1982). Exposure studies at the
place of work on administration of Bayo-N-Ox-Ferkelaufzuchtfutter
(Special Piglet Feed) F2 in pellet or meal form. Report 10731.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
THYSSEN J. (1983). Olaquindox BAY VA 9391. Subacute inhalation study
in rats. Report N. 13032(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
URWIN, C., & MAWDESLEY-THOMAS, L.E. (1969). Pathology report of Bay
a 9391. Subchronic toxicity for rats by oral administration. Report
No. 2989/69/415. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
URWIN, C., & SPICER, E.J.F., (1971). Pathology report of Bay a 9391.
Subchronic toxicity study for rats by oral administration. Report No.
4389/71/545. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
VAN DER MOLEN, E.J., NABUURS, M.J.A., & JAGER, L.P. (1985).
Pathological and clinical changes related to toxicity of carbadox in
weaned pigs. Zbl. Vet. Med., 32, 540-550.
VAN DER MOLEN, E.J., DE GRAAF, G.J., SPIERENBURG, TH. J., NABUURS,
M.J.A., BAARS, A.J. & JAGER, L.P., (1986). Hypoaldosteronism in
piglets induced by carbadox. Experientia., 42, 1247-1249.
VAN DER MOLEN, E.J., BAARS, A.J., DE GRAAF, G.J. & JAGER, L.P. (1989).
Comparative study of the effect of carbadox, olaquindox and cyadox on
aldosterone, sodium and potassium plasma levels in weaned pigs. Res.
Vet. Sci., 47, 11-16.
VON DER HUDE. W., BEHM, C., GURTLER, R., & BASLER, A. (1988).
Evaluation of the SOS chromotest. Mutat. Res., 203, 81-94.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1980). The
mutagenic action of quindoxin, carbadox, olaquindox and some other
N-oxides on bacteria and yeast. Mutat. Res., 78, 233-242.
WINDHOLZ, M., BUDAVARIA, S., BLUMETTI, R.,F., & OTTERBEIN, R.R.S (Eds)
(1983). The Merck Index, 10th Edition, Merck & Co., Rahway, New
Jersey.
YOSHIMURA, H., NAKAMURA, M., & KOEDA, T. (1981). Mutagenicities of
carbadox and olaquindox - growth promoters for pigs. Mutat. Res.,
90, 49-55.
The compound is used as a growth promoter in pigs and is supplied
as a 10% premix in feed for admixture in the final feed at rates of
50-100 ppm for starter rations and 25-50 ppm in grower/fattener feeds
(Windholz et al., 1983; Anon,1989; Kulczyk et al., 1979; Baars
et al., 1988). It has not previously been evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. Biological Data
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion.
2.1.1.1 Rats
Olaquindox is well absorbed when given orally to rats.
Approximately 85% of the radioactivity from a dose of 10 mg/kg b.w. 3-
14C-olaquindox was excreted in the urine. Most radioactivity was
found in the urine within 3 hours of administration. The remainder
was eliminated in the faeces. Less than 1% was recovered in expired
air as carbon dioxide. Experiments with 3-14C-olaquindox
intraduodenally administered to rats with bile duct fistulas suggested
that around 18% of the dose was excreted in the bile. Similar
findings were made after intravenous dosing. Distribution occurred in
a generalized manner throughout the body after oral dosing and most of
the radioactivity had disappeared by 24 hours. Autoradiography
revealed the highest amount in the rat kidney at 4 hours, indicative
of the extent of urinary excretion already noted. Slightly elevated
concentrations were also observed in liver, testes, adrenals and hair
follicles. When dogs were given 10 mg/kg 3-14C-olaquindox, a similar
metabolic profile was noted to that seen in the rat (Duhm et al.,
1970).
2.1.1.2 Pigs
Olaquindox was rapidly absorbed when given orally to pigs. Over
90% of an oral dose of 2 mg/kg b.w. was eliminated in the urine within
24 hours, which is indicative of rapid and extensive absorption. The
remainder was excreted in the faeces. Maximum plasma levels were
attained within 1-2 hours of dosing (1-2 ppm). This was followed by
a rapid decline in plasma levels reaching around 0.03 ppm by 24 hours
and 0.005-0.01 ppm by 48 hours. Radioactivity was present in all
tissues when examined 2 days after dosing, but the levels were
extremely low. In the kidney and liver, levels of 110 and 52 ppb were
found, while levels in muscle were only 9 ppb. After 8 days, levels
in liver and kidney had fallen to 27 and 12 ppb, respectively, while
those in muscle were in the range of 2.5 ppb. By 28 days after dosing
only low levels were found in kidney and muscle (0.9 and 0.5-0.8 ppb,
respectively) with slightly higher concentrations in the liver (2 ppb)
(Duhm et al., 1973).
When pigs were dosed at levels in the range of those recommended
in use (up to 100 ppm in the diet) for up to 20 weeks, relatively high
levels were found in the kidney (around 2000 ppb) with relatively
moderate levels in the liver (300 ppb) when the animals were killed
six hours after drug withdrawal. When killed 2 days after withdrawal,
levels had fallen to below the limits of detection (50 ppb) in liver,
kidney and muscle. Pigs given diets containing olaquindox at levels
in excess of those recommended (160 or 250 ppm) for up to 4 weeks also
had high initial levels in kidney, liver and muscle but these had
fallen to below the limits of detection by day 2 after withdrawal
(Medenwald, 1974; Medenwald & Gericke, 1974; Bories & Bourdon., 1977).
Similar findings were made in other studies where pigs were given
diets containing 100 or 150 ppm olaquindox for 12-30 weeks (Takase &
Komachi, 1975; 1976).
After pigs were given diets containing up to 45 ppm olaquindox
for the duration of the fattening period, the highest levels were
found in the liver (0.14 ppm) and kidney (0.28 ppm) 6 hours after
withdrawal. By 24 hours the levels were below the limit of detection
(0.1 ppm) (Leibetseder, 1980). Similar results were noted when pigs
were given diets containing 10 ppm olaquindox (Anderson & Szabo,
1982).
2.1.2 Biotransformation
The biotransformation of olaquindox has been investigated only in
the pig. The majority of an oral dose of olaquindox (70%) was
excreted in the urine unchanged. The major metabolites appeared to be
the reduced compounds, the 1- or 4-mono-N-oxides (16%). Three other
compounds thought to be carboxylic acid derivatives made up the
remainder (Duhm et al., 1973). Later work led to the elucidation of
the structures of these metabolites in the pig. Again the major
urinary component after oral dosing was olaquindox with about 7%
present as the 4-mono-N-oxide. Omega oxidation produced the
2-carboxymethylaminocarbonyl compound and its 4-mono-N-oxide
derivative (6%). Some of the corresponding 1-mono-N-oxide moiety of
the 2-carboxymethylaminocarbonyl was also noted (1%). The remaining
metabolite was the di-desoxy derivative of
2-carboxymethylaminocarbonyl compound, 2-carboxymethylaminocarbonyl-3-
methyl quinoxaline (>1%) (Maul et al., 1979a and b).
2.1.3 Effects on enzymes and other biochemical parameters
Olaquindox has been shown to cause a decline in plasma
aldosterone levels in the pig accompanied at higher dietary levels by
hyponatraemia and hyperkalaemia. These findings are considered in
more depth in the section on short-term toxicity studies (van der
Molen et al, 1989; Baars et al, 1988).
2.2 Toxicological Studies
2.2.1 Acute Toxicity
Acute toxicity studies are summarized in Table 1.
In a series of experiments, groups of 10 male mice were given
oral doses of 2500-5000 mg/kg b.w. olaquindox as an aqueous suspension
in 2% carboxymethylcellulose. Vehicle controls or undosed groups did
not appear to have been used. Only 1/10 mice died at the lowest dose
used while 100% lethality was noted at the highest dose. Signs of
toxicity included decreased activity, lowering of the eyelids, and
irregular breathing. Animals died 2-14 days after olaquindox
administration. Discolored livers and yellowish-green intestinal
contents were noted on gross examination. Similar findings were made
when groups of male rats were given olaquindox in a similar manner at
doses of 1400-2000 mg/kg b.w. (Tettenborn, 1969).
Table 1: Acute toxicity of olaquindox
Species/strain Sex Route LD50 Reference
(mg/kg b.w.)
Mouse/CF1 M oral 3316 Tettenborn, 1969
M s.c. 2237 Tettenborn, 1969
Rat/Wistar M oral 1704 Tettenborn, 1969
M s.c. 1275 Tettenborn, 1969
M+F inhalation >1751 mg/m3* Thyssen, 1982
F oral 1657 Steinhoff, 1973
Rabbit/cross M+F oral 1000-2000 Tettenborn, 1969
M+F s.c. 1000-2500 Tettenborn, 1969
Cat/cross M+F oral 1000 Tettenborn, 1969
M+F s.c. 500 Tettenborn, 1969
Dog/beagle M+F oral emetic** Tettenborn, 1969
M+F s.c. *** Tettenborn, 1969
* 4 Hour LC50 value.
** Lethal doses could not be achieved due to emesis.
*** 250 mg/kg produced local reaction; temporary inappetence, higher
doses were not tried
Groups of two rabbits, each presumably 1 male and 1 female, were
dosed orally with olaquindox in 2% methylcarboxycellulose. No deaths
occurred at the lowest dose of 500 mg/kg b.w. while in those given
1000 or 2000 mg/kg b.w., 1/2 animals died. All the animals given 4000
mg/kg b.w. died. Similar observations were made in groups of two cats
given 500, 1000 or 2000 mg/kg b.w., with both animals given the
highest dose dying. Emesis was the main toxicological sign noted.
Dogs given oral doses of up to 100 mg/kg b.w. olaquindox showed no
signs of toxicity but those given 250-2000 vomited; no lethalities
occurred. Vehicle controls or undosed animals did not appear to have
been used in these studies (Tettenborn, 1969).
When given by the subcutaneous route as a suspension in 2%
carboxmethylcellulose, the acute toxicity of olaquindox was more
marked (Table 1). Doses of 500 to 2500 mg/kg b.w. and above proved
lethal to mice, rats, rabbits and cats. For unspecified technical
reasons, dogs were given only 250 mg/kg b.w. olaquindox, which
produced a local reaction and temporary inappetence a week later
(Tettenborn, 1969).
2.2.2 Short-term studies
2.2.2.1. Mice
Groups of 20 male and 20 female BOM-NMRI mice were fed diets
containing 0, 300, 600, 1200, 2400 and 4800 ppm olaquindox,
approximately equivalent to 0, 45, 90, 180, 360 and 720 mg/kg
b.w./day, for 90 days, as a dose-finding exercise for a
carcinogenicity study (see Section 2.2.3).
Signs of toxicity were non-specific and included shaggy fur,
dyspnoea and reduced motility. A marked reduction in body weight
occurred at the highest dietary level in both sexes, and in males
given 1200 and 2400 ppm. During the study 1/20 females died at the
600 ppm level as did 18/20 and 5/20 males and females, respectively,
at the 1200 ppm level. All the mice given the two highest doses died.
No deaths occurred in other groups. At necropsy, haemorrhagic lungs
were the main findings. Microscopic examination was not conducted
(Steinhoff & Gunselmann, 1982).
2.2.2.2 Rats
Groups of 10 male and 10 female Wistar rats were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in 2% aqueous
carboxymethylcellulose by stomach tube, for 5 days per week for 13
weeks.
After 6-8 weeks, signs of toxicity included reddening of the ears
and plantar surfaces; weakness and emaciation occurred in animals
given the highest dose. These animals also developed moist, blood
encrusted nostrils. In the eighth week of the study, fatalities began
to occur, consequently the animals were killed. No clinical signs of
toxicity or compound-related deaths occurred in the other groups.
There were no adverse haematological effects in any groups at 4 weeks
including the high dose group, nor at 12 weeks in remaining animals.
Clinical chemistry was normal at 4 weeks in all dose groups and in
animals given 0, 20 or 60 mg/kg b.w./day olaquindox at 12 weeks.
However, at 8 weeks in the high dose animals (prior to death), blood
sugar was significantly reduced, while serum aspartate
aminotransferase was elevated. Urinalysis was normal in all groups at
4 weeks and in all but the high dose group (not available for
examination due to deaths) at 12 weeks.
Absolute organ weights at 90 days indicated a significant
splenomegaly and increases in testicular and ovarian weights in
animals given 60 mg/kg b.w./day olaquindox. These findings were also
reflected in relative organ weights except for splenic weights in
females. There was a significant decrease in relative adrenal weights
in females given 60 mg/kg b.w./day olaquindox.
Gross examination revealed reddening of the pyloric area of the
stomach in the high dose animals and pale and atrophied adrenals. All
the females given 60 mg/kg b.w./day and 5/10 of those given 20 mg/kg
b.w./day had enlarged, reddened ovaries with numerous pin-point
darkened nodules (corpora lutea).
Microscopic examination revealed adrenal atrophy in the high and
mid dose groups, with degenerative changes in the cortical areas. Some
of the female high dose animals had thyroid atrophy. Female rats
given the mid and low dose had no ovarian atrophy but moderate
atrophic changes were noted in the ovaries of 4/5 high dose females
(Hoffmann 1969; Urwin & Mawdesly-Thomas, 1969).
The experiment was later repeated using lower doses: 0, 1, 5 and
20 mg/kg b.w./day. All other factors were identical to the original
study. No clinical signs were observed during the study and there
were no effects on body weights. There were no haematological or
clinical chemistry abnormalities; urinalyses were normal.
At necropsy, increases in adrenal weights in males given the two
highest daily doses were noted. Increases in ovarian weights occurred
in females given the two highest doses. Histopathologic examination
revealed no changes in any organs in any of the treatment groups. The
no-effect level in these studies therefore was 1 mg/kg b.w./day
(Hoffmann, 1972; Urwin & Spicer, 1971).
In a 90 day dietary study, olaquindox was given to groups of 20
male and 20 female Norway rats at levels of 0, 50, 150 and 300 ppm,
approximately equivalent to oral doses of 0, 5, 15 and 30 mg/kg
b.w./day. Haematological and clinical chemistry investigations were
conducted at days 0, 35, and 63 and at the end of the study.
No signs of toxicity were noted during the study and there were
no effects on haematology and clinical chemistry. Gross and
microscopic examination revealed no compound-related changes
(Nastuneak et al, 1986).
In an inhalation study, groups of 10 male and 10 female Wistar
rats were exposed to 0, 10, 207 and 541 mg/m3 olaquindox dust, 6
hours/day, 5 days per week for 3 weeks under dynamic exposure
conditions. Based upon number and particle mass median diameter,
between 30-80% of the particles were inhalable.
No adverse effects occurred in the rats exposed to the lowest
concentration of olaquindox nor in the males of the intermediate
concentration group. Females in this group showed non-specific
effects ("sluggishness") for the first 5 days of exposure but after
this they appeared normal. Effects of a similar nature and duration
occurred in the high concentration males but in females, signs of
respiratory distress were noted from the 11th day of exposure in
addition to the non-specific effects seen in other exposed groups.
No deaths occurred and only minor effects on body weights were
observed. There were no effects on haematology nor on clinical
chemistry. Urinalyses were normal. No gross abnormalities were
evident at autopsy and relative and absolute organ weights were in the
normal range. There were no adverse histopathological findings. The
non-specific signs and particularly the respiratory effects may have
been due to the physical effects of the olaquindox particles (Thyssen,
1983).
2.2.2.3 Rabbits
Olaquindox in Lutrol was applied to the shaven intact dorsal
surface of 3 groups of 6 New Zealand white rabbits (3 male and 3
female) at doses of 0, 50 or 250 mg/kg b.w./day for 6 hours/day, 5
days per week for 3 weeks, without an occlusive dressing. In an
identical manner, olaquindox was applied to the abraded skin of 3
groups of 3 male and 3 female rabbits.
No signs of toxicity were observed in treated animals and skin
reactions due to olaquindox were not seen in the abraded or intact
skin groups. No mortalities occurred. Clinical chemistry and
urinalyses at the end of the study were comparable to control values.
There were no effects on body weights. At necropsy, no abnormalities
were noted and no histopathological changes attributable to olaquindox
treatment were found (Heimann & Schilde, 1982).
2.2.2.4 Dogs
Groups of 2 male and 2 female beagle dogs were given oral doses
of 0, 20, 60 or 180 mg/kg b.w./day olaquindox in gelatin capsules for
90 days.
Vomiting occurred during the first week in dogs given the highest
dose. Salivation was noted and food intake declined. The animals
became emaciated. Dogs given the intermediate dose showed inappetence
and salivation occurred. No effects were noted in low dose animals.
All the high dose animals died within 20 days of the start of the
study. One dog given the intermediate dose died on day 40 while the
remainder were killed in extremis after 46 or 56 doses. No animals
given the low dose died.
There were no notable changes in haematology in treated dogs.
Clinical chemistry revealed increases in blood urea in all 4 dogs
given the high dose. Intermittent elevations of blood urea were noted
in the other dosed groups. No abnormalities in urinalyses occurred.
Gross examination of high dose animals suggested an irritant
effect on the gastrointestinal tract, with congestion in the lungs.
The livers were discolored. No abnormalities were noted in the low
dose animals. Histopathologic examination revealed live cell
enlargement and fatty degeneration in dogs given 60 or 180 mg/kg
b.w./day olaquindox, with fatty degeneration of the cells of the
kidney tubules. No histopathologic changes were found in low dose
dogs and the no-effect level in this study appeared to be 20 mg/kg
b.w./day (Lorke & Tettenborn, 1969; Mawdesley-Thomas & Urwin, 1969).
2.2.2.5 Pigs
Groups of 5 castrated male and female German landrace pigs
weighing 9-10 kg each were fed diets containing 0, 100, 160 and 250
ppm olaquindox for 20 weeks.
At the highest dietary level, 5 pigs died and weight gain was
significantly reduced. Animals given 100 and 160 ppm in the diet had
higher rates of weight gain than controls. No haematological effects
occurred. Plasma creatinine and urea were elevated in the
intermediate and high dietary level groups. Hyperkalaemia and
hyponatraemia were noted in pigs given 250 ppm dietary olaquindox.
Urinalyses were normal.
High dose pigs showed a grey-brown discoloration of the renal
cortex but there were no effects on relative organ weights. Tubular
dilatation and flattening of the tubular epithelium occurred in
intermediate and high dietary level pig kidneys and the adrenals of
these animals displayed enlarged cortical epithelial cells. The no-
effect level in this study was 100 ppm olaquindox in the diet (Gericke
& Dycka, 1974; Hoffman et al., 1974).
Groups of 7 hybrid piglets (4 weeks old, at least 3 females per
group and castrated males) were fed diets containing 0, 25, 50, 100
and 200 (2 groups) ppm olaquindox for 6 weeks.
After 2 weeks, dry faeces were produced by piglets given 100 or
200 ppm olaquindox. The drinking of urine from the floor of pens or
directly from urinating pen-mates was noted in pigs given 50 ppm
olaquindox. A decrease in abdominal volume occurred in piglets given
100 or 200 ppm olaquindox after 5 weeks and in the 25 ppm group in
week 6, but not in animals give 50 ppm. Significant rises in serum
albumin values occurred in piglets given 100 or 200 ppm olaquindox
from week 2 onwards, and marked rises in serum urea values occurred in
the 200 ppm group from week 4 and in the 100 ppm group from week 5.
Gross and microscopic pathology were not conducted (Nabuurs et al,
1989).
Groups of 6 female and 6 castrated male hybrid piglets were fed
diets containing 0, 25, 50, 100 or 200 ppm olaquindox for 6 weeks, in
a study of the effects of treatment on plasma aldosterone, sodium and
potassium levels. There was a gradual decline in plasma aldosterone
which was significant in all but the 25 ppm group by week 5. After 6
weeks the decline was significant in all dosed groups except for that
given 100 ppm where a small rise was noted. Hyponatraemia occurred in
the 25 and 200 ppm groups after weeks 0-2 and a continuous decline in
the 200 ppm group occurred after week 3. In the groups given 25 and
100 ppm olaquindox the levels decreased continuously from 2-3 weeks.
Animals in the 50 ppm group were unaffected. Although elevated
potassium levels occurred in the 50 and 100 ppm groups, only piglets
given 200 ppm were considered to be hypokalaemic (van der Molen
et al, 1989; Baars et al, 1988).
Similar effects have been noted in pigs given other quinoxaline
N-oxide drugs, namely cyadox and carbadox (van der Molen et al,
1985). The latter drug caused these effects after accidental
overdosage of pigs (Power et al, 1989). The toxicity appears to be
due to specific effects on the aldosterone-releasing zona glomerulosa
of the adrenals (van der Molen et al, 1986).
2.2.2.6 Rhesus monkeys
Olaquindox in gelatin capsules was given orally to two groups of
3 male and 3 female rhesus monkeys at doses of 0 and 20 mg/kg b.w./day
and to two groups of 3 males and 5 females at doses of 5 and 40 mg/kg
b.w./day, 7 days a week for 19 weeks. Surviving high dose females
were entered onto a 17 week recovery period.
Animals given the highest dose showed a general loss of condition
and loss of body weight. Suppression of weight gain occurred at the
intermediate dose while growth promotion occurred at the low dose.
Suppression of appetite was evident from week 12 in high dose monkeys.
Vaginal cytology indicated a suppression of ovulation in the high
dose females and in 1/3 animals given the intermediate dose. There was
some evidence of recovery after dosing ceased in the high dose
females. Deaths occurred in 2/3 males and 1/5 females form the high
dose groups during the dosing period and 2 females died during the
first two weeks of the recovery period.
Electrocardiography and ophthalmoscopy were normal in all
animals. Urinalyses and haematology were essentially normal at 5
weeks but serum aspartate aminotransferase values in high dose males
were elevated. At 8 weeks plasma biochemistry was normal but packed
cell volume and red cell counts were lowered in high dose animals.
Urinary glucose was found in 3 high dose males. After 15 weeks of
dosing packed cell volume and haemoglobin showed slight reductions in
high dose males while plasma glucose levels were reduced but not
significantly.
In 7/8 high dose monkeys, urine was positive for glucose and for
total reducing substances. One urine showed a lower pH and one was
positive for ketones. When examined at 15 weeks, red blood cell
values were reduced in high dose monkeys. Plasma glucose values were
reduced in high dose animals and protein and glucose were present in
the urines; pH of urine was lowered in high dose animals. At the end
of the 19 week dosing period no haematological examinations were
performed. Plasma glucose levels were reduced in high dose animals
while plasma urea values were increased. Hypokalaemia was noted in
high and intermediate dose animals. Glucose and total reducing
substances were increased in high dose monkeys while pH was lowered.
Macroscopic examination revealed pallor of the kidney in high
dose animals. Suppression of ovulation occurred in high dose females,
and abdominal abscesses in high dose males.
Histopathological examination revealed fatty changes in the
centrilobular areas of the liver in all high dose animals and
deposition of fat in the kidney tubules in all monkeys from this
group. Brown pigmentation of the zona reticularis of the adrenals
occurred in high dose monkeys. Immature testes were noted in males
given 20 and 40 mg/kg b.w./day olaquindox while inactivity of the
ovaries in high dose females and in 1/3 intermediate dose females was
observed. The no effect level in this oral study in rhesus monkey
was 5 mg/kg b.w./day (Heywood et al, 1972).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 20 male and 20 female NMRI mice were given nominal
doses of 0, 15 or 75 mg/kg b.w./day olaquindox in the drinking water.
Low dose mice were given a total of 6.6 g/kg b.w. for 635 days and
high dose mice were given a total of 32.1 g/kg b.w. for 634 days. The
study was terminated when all the mice had died. Animals were not
dosed over holidays and no mention was made of week-ends.
At termination, no excess tumour incidence was noted.
Lymphadenosis was reported in 1/40 control mice while 2/40 high dose
animals had tumours (thymoma and malignant thymus cell tumour) as did
2/40 low dose mice (pulmonary carcinoma and bronchial carcinoma.
Only small numbers of mice were used in this study and survival
was poor, although it was better in the high dose group than in
controls. The mean survival had a high standard deviation (mean
survival 340 ± 187, 338 ± 224 and 403 ± 194 days for mice given 0, 15
or 75 mg/kg b.w./day, respectively).
Moreover, even when survival was adjusted for unexplained deaths
which occurred in the first 82 days in controls, the treatment time
was still less than is currently viewed as normal for a mouse
carcinogenicity study. The short life-span on test may be due to the
fact that the animals were already around 50 days old when the study
commenced (Schmael, 1973).
In a later study, groups of 75 male and 75 female NMRI mice were
given diets containing 0, 40, 120 or 360 ppm olaquindox, equivalent to
0, 6, 18 or 57 mg/kg b.w./day, for life (until death or sacrifice in
a moribund state).
The only effects on body weights were in males and females given
the highest dietary level: male weights fell slightly below control
values from day 50 and female weights declined after day 200.
Haematological examination at weeks 4, 13, 26, 52 and 78 revealed no
abnormalities and survival times were unaffected by olaquindox intake;
remaining males and females died around day 890 (approximately 29
months).
At necropsy there were no differences in liver, kidney, spleen,
heart, testes, or brain weights, and no increases in non-neoplastic
findings were found in treated mice. No increased tumour incidence
was found in animals given 40 or 120 ppm dietary olaquindox but at 360
ppm there was an increase in the total number of tumours and in the
number of animals with benign tumours. These were due to increases in
the incidence of pulmonary adenoma and adrenal cortical adenoma in
males and in pulmonary adenoma and ovarian granulosa cell tumours in
females (Table 2). There were no increases in the incidence of any
malignant tumour types (Steinhoff & Gunselman, 1982).
Table 2: Tumor incidence in mice given olaquindox in the diet
0 ppm 40 ppm 120 ppm 360 ppm
Males
pulmonary adenoma 11 (15%) 17 (23%) 14 (19%) 27 (36%)
adrenal cortical 5 (7%) 3 (4%) 6 (8%) 13 (17%)
adenoma
Females
pulmonary adenoma 8 (11%) (7%) 7 (9%) 11 (15%)
ovarian granulosa 10 (13%) 16 (21%) 15 (20%) 20 (27%)
cell tumor
2.2.3.2 Rats
Groups of 20 male and 20 female Wistar rats were given
olaquindox, once per week for up to 560 days. The total dose was 4.7
g/kg b.w. with individual doses being in the range of 50-150 mg/kg
b.w. Each dose was given by gavage as a suspension in physiological
saline. Controls were given physiological saline only, but this was
administered by intraperitoneal injection and not by gavage as they
simultaneously acted as controls for other studies.
Survival in treated animals was better than controls (875 ± 105
days for male and 818 ± 167 days for female rats given olaquindox
compared with 797 ± 215 and 779 ± 187 days, respectively, for male and
female controls).
The incidence of tumours was not presented in a detailed tabular
form but was given graphically and in separate tables which did not
allow a full comparison. However, there was no elevated incidence of
any tumour type in treated animals when compared with controls and the
number of tumour-bearing animals was similar in both the treated and
control groups (Steinhoff, 1973).
Groups of 80 BR 46 rats were given drinking water containing
olaquindox at levels to ensure an intake of 15 or 75 mg/kg b.w./day.
A group of 49 rats given water only served as controls. Male and
female rats were used in the study but the sex ratio was not
specified. The treated water was supplied 5 days a week until the
animals died.
Survival of animals treated with olaquindox was better than
controls (704 ± 161 days and 655 ± 229 days in low and high dose rats,
respectively, compared with 554 ± 248 days in control rats). The
carcinogenicity data was presented in an unclear manner and separate
data for male and female animals were not given. The only tumour
which showed an increased incidence was mammary fibroadenoma (1/40,
2.5%; 3/46, 6.5% and 7/46, 15% in controls and in low and high dose
rats, respectively). However, the absence of data on male and female
incidence of this tumour renders the values uninterpretable (Schmael,
1973).
As part of a chronic toxicity/carcinogenicity study with
in utero exposure, groups of 75 males and 75 females were given
diets containing 0, 40, 120 or 360 ppm olaquindox. These doses were
approximately equivalent to oral doses of 0, 3, 10 and 30 mg/kg
b.w./day, for one week prior to mating and during a 3 week 1:1 mating
period. After mating the males were removed and the females given the
diets containing olaquindox until the young were 4 weeks old. At this
time the young from each treatment group were divided into groups of
25 males and 25 females and given the same diets as initially given to
their parents. The study was continued until the young had been
treated for 2 years. Clinical chemistry, haematology, and urinalyses
were conducted on groups of 5 males and 5 females at 4, 14, 26, 54 and
102 weeks after commencement of treatment of the F1 generation.
No overt signs of toxicity were noted in treated animals but
after day 400 of treatment, male and female animals given the highest
dietary level showed a marked and statistically significant reduction
in body weight when compared with control values.
Clinical chemistry suggested an elevated creatinine level in the
blood of rats given 360 ppm dietary olaquindox but all the values were
within the normal range. The albumen content of the urine was
generally lower in treated animals than in controls.
At gross and microscopic examination, there were no increases in
the incidences of non-neoplastic diseases and no increased incidence
of any tumour types. This study employed too few animals to allow an
assessment of carcinogenic potential (Steinhoff, 1977).
Olaquindox was tested in a carcinogenicity study using a similar
protocol on groups of 50 male and 50 female rats remaining in the F1
generation from the chronic toxicity carcinogenicity test described
above. The groups were given diets containing 0, 40, 120 and 360 ppm
olaquindox, approximately equivalent to oral doses of 0, 3, 10, and 30
mg/kg b.w./day. No clinical chemistry or haematological tests were
conducted, however, and the study was terminated when the duration was
approximately 3 years (1065 days for males and 1120 days for females),
at which time 20% of the controls (males and females separately)
remained alive.
No signs of toxicity were noted in treated animals except for
reductions in body weights in rats given 360 ppm after day 500.
Reductions occurred despite the fact that in terms of feed consumption
in g/kg b.w./day these animals had consumed more.
At termination there was a significant decrease in survival in
animals given the highest dietary level (98% mortality in males and
females) and in females given 40 ppm olaquindox (92% mortality)
compared with controls (80% mortality). Mortality in the other
dietary groups was only slightly more than in controls (82-86%).
At necropsy there were no differences between treated animals and
controls for the number of animals of each sex with total tumours,
primary tumours, malignant and benign tumours, malignant tumours with
metastases and total benign tumours. Similarly, for particular tumour
sites (benign and malignant tumours plus metastases) there were no
excess incidences except for slight increases in the incidence of
adrenal, reticuloendothelial and seminal vesicle neoplasms, but these
were due, except for the adrenal tumours, to metastases or
infiltrations from other organs. Moreover, the adrenal tumours were
not increased with respect to any particular histological type and
again were often of metastatic origin (Steinhoff & Boehme, 1978).
There were some anomalies in the reporting of this study which
appear to be due to arithmetical errors. The summary table of tumour
incidence cites a benign tumour in female controls which is not listed
in the detailed histopathology tables. The summary data also claims
4 malignant tumours in males given the highest dietary level whereas
the histopathology table lists 9. However, these differences do not
affect the outcome of the study or its assessment.
2.4 Reproduction Studies
2.4.1 Mice
Groups of 20 pregnant NMRI mice were given oral doses of 0, 20,
60, or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. On day 18 of
gestation the fetuses were delivered by Caesarean section and these
were weighed and examined for gross malformations and by alizarin
staining.
None of the pregnant animals died during the test but animals
given the highest dose showed reduction in body weight or rate of
weight gain. The numbers of implantations, live fetuses and
resorptions were similar in all dosed groups. At the highest dose,
fetal weights were significantly lower than in controls. The
incidence of malformations in all treated groups was similar to those
seen in controls (Lorke, 1971a).
2.4.2 Rats
Groups of 20 female pregnant FB 30 rats were given oral doses of
0, 20, 60 or 180 mg/kg b.w./day olaquindox as an aqueous suspension in
tragacanth by gavage from day 6 to day 15 of gestation. Fetuses were
delivered by Caesarean section on day 20 of gestation and these were
examined in the same manner as for the mouse study described above.
The pregnant rats given the highest daily dose showed reductions
in body weights or rate of weight gain compared with control values.
These animals also showed a higher incidence of resorptions and lower
numbers of live fetuses. Fetal weights were lower in the high dose
animals. These indices were similar to controls and to rats given 20
or 60 mg/kg b.w./day olaquindox.
The incidence of malformations in fetuses from dams given 20 or
60 mg/kg b.w./day olaquindox was similar to controls but at the
highest dose level there was an elevated incidence of malformed
fetuses, with 5 malformations reported at a dose level of 180 mg/kg
b.w./day.
In this study, therefore, there was a teratogenic effect at the
highest dose level given to pregnant rats (180 mg/kg b.w./day) on days
6-15 of gestation. The no-effect level in this study was 60 mg/kg
b.w./day (Lorke, 1971b).
A 3-generation study was conducted in groups of 10 male and 20
female FB30 rats using 0, 20, 100 and 500 ppm olaquindox in the diet,
equivalent to oral doses of 0, 1, 5 and 25 mg/kg b.w./day. The F0
animals were given the diets containing olaquindox throughout the
study including during the mating periods. Animals were mated after
dosing for 70 days. Animals which died during the study were
necropsied, while young animals were examined macroscopically after
birth and subsequently observed during the rearing period for evidence
of abnormalities. The F3b animals were sacrificed at 3 weeks of age
and subjected to macroscopic and microscopic examination.
Treatment had no effect on body weights except that body weight
of F0 generation females given the highest dietary level of
olaquindox were slightly higher than weights of controls.
The fertility rate was also lowered in F0 animals in the first
and second matings when given the highest level, but there were no
effects on litter size nor on rearing rates. F1a and F1b animals did
not differ from controls in terms of birth weights except for a
slight, non-statistical increase in those derived from F0 dams given
the highest dietary level. After the second F0 mating the average
number of young in the F1b generation was similar in all treatment
groups, but at 5 days there was a significant reduction for dams given
500 ppm olaquindox (6/litter) compared with values in other groups and
controls (10-12/litter). Birth weights of the F1b generation were
unaffected.
When the F1b generation was mated, the gestation rates in animals
derived from animals initially given the highest level of olaquindox
were reduced (80-84%) compared with values for other groups (90-100%).
The average numbers of young per litter were also reduced at the high
dietary level in both the F2a and F2b generations, both at birth and
5 days after birth (8/litter compared with 11/litter in controls).
The F2 birth weights were unaffected and there was some improvement
in the rearing rates up to 4 weeks.
In the F3 generation, fertility was again affected in animals
originally derived from the high dietary level rates, with gestation
rates of 70-84% compared with 90-100% in other groups. The average
number of young per litter was also reduced at the high dietary level
(5-7/litter) compared with other groups (8.5-10.8/litter). There were
no effects on rearing rates nor F3 birth weights. No malformations
were noted during the course of the study and no abnormalities were
found on gross or histopathological examination of three week old F3b
animals (Loeser, 1974).
In a fertility test in Wistar rats, olaquindox was administered
orally by gavage to groups of 10 male rats at doses of 4 and 10 mg/kg
b.w./kg which were mated with groups of 20 untreated females. Groups
of 10 untreated males were mated with groups of 20 females given
gavage doses of 4 and 10 mg/kg b.w./day. Males were dosed for 8 weeks
prior to mating while females were dosed for 3 weeks prior to mating.
Males and females were mated in a 1:2 ratio. Groups of untreated
males and females were mated as controls.
Olaquindox administration had no effect on body weights, on
oestrus cycles, or on copulation and conception rates. A significant
reduction in the average number of implantations was noted in the
group in which females given the lower dose of 4 mg/kg b.w./day
olaquindox were mated with untreated males. Pre-implantation losses
were significantly increased in both the groups where females were
treated with olaquindox, and post-implantation losses were increased
in females given the 10 mg/kg b.w./day dose. There were no effects in
the groups where males treated with olaquindox were mated with
untreated females (Gandalovicova & Sykora, 1986).
2.2.5 Special studies on pharmacological properties
Olaquindox has been tested in a number of pharmacological
screening tests in rats and mice including those for anticonvulsive
effects, inhibition of defensive reaction, motor coordination,
analgesia, antihypertensive effects, gastric secretion, bile
secretion, diuresis, blood sugar and blood lipids and thrombocyte
aggregation (bovine plasma). No pharmacological activity was detected
(Kaller, 1970).
2.2.6 Special studies on irritancy and hypersensitivity
Olaquindox as a micronized powder was applied without a vehicle
to the shaved intact and abraded dorsal skin of 6 New Zealand white
rabbits using a 24-hour occlusive dressing. The skin was assessed on
removal of the dressing, after 48 and 72 hours, and at 7 days. Slight
erythema of intact and abraded skin was observed at 24 hours, but not
at the other time points. No oedema was noted. Results indicated a
mild irritant effect (Murman, 1979).
Olaquindox was tested for its ability to induce eye irritation by
application of 15 mg of the micronized substance to the conjunctiva of
the right eyes of 6 New Zealand white rabbits, and to the left
conjunctival sacs of a further 6 rabbits. The material was washed out
of the left eyes with physiological saline after 1 minute. Reactions
were monitored 24, 48, and 72 hours after application and after 7
days.
A slight reddening of the conjunctiva was noted in 4/6 eyes where
olaquindox had been directly applied. Slight chemosis was noted in
2/6 eyes. A slight chemosis was noted in 1/6 rabbit's eyes where
olaquindox has been applied to the conjunctival sac and then washed
out. All reactions subsided within 48 hours. The result suggest that
olaquindox has a slight irritant effect but the mechanical effects of
the dust cannot be ruled out (Murman, 1979).
Olaquindox was tested in the guinea-pig for its ability to induce
hypersensitivity. Olaquindox dissolved in dimethyl sulfoxide or as a
suspension in phosphate buffered saline was administered
intracutaneously into the neck region of groups of 10 Pirbright albino
guinea-pigs on days 1, 3, 6, 9, and 13 of a sensitization schedule.
Four days after the last injection, a suspension of olaquindox in 1:1
acetone/almond oil was applied to the depilated flank and massaged
gently into the skin. To allow for any possible effects of light,
groups of guinea-pigs were treated in a similar manner but were kept
in darkened cages. No indications of sensitization were noted in this
study when assessed by gross examination of the skin for reactions or
when examined histologically (Schlumberger, 1975). (This test is not
widely used and it is unlikely to be as sensitive as those using an
adjuvant (e.g. Freunds') such as the Magnusson-Kligman or Beuhler
models).
2.2.7 Special studies on genotoxicity
Olaquindox has been tested in a wide range of genotoxicity tests
and these are summarized in Table 3. It has produced positive results
in a number of studies designed to test for reverse mutations in
bacteria, including the Ames test with Salmonella typhimurium
strains (Beutin et al, 1981; Yoshimura et al, 1981; Voogd
et al, 1980; Nunoshiba & Nishioka, 1989). A positive result has
been noted in a forward mutation assay with Escherichia coli
(Nunoshiba & Nishioka, 1989).
In vitro study with cultured human lymphocytes and a number of
in vivo assays with mouse bone marrow or Chinese hamster
spermatogonia as the target tissues have demonstrated the clastogenic
activity of olaquindox (Tamura, 1977; Cihak & Vontorkova, 1983; Sram
et al, 1986a; Pokorna, 1986; Herbold, 1983a). Similarly, olaquindox
has produced positive results in several micronucleus tests in the
mouse following oral or inhalation exposure (Herbold, 1983b; Herbold
& Thyssen, 1982; Cihak & Vontorkova, 1985), and in the rat after
intraperitoneal injection (Cihak et al, 1983). However, a dermal
study with a 24 hour exposure was negative (Herbold, 1982a),
reflecting the poor dermal absorption of the substance.
Olaquindox has been tested in two dominant lethal assays in the
male mouse but a weak positive result was observed in only one of
these when a high dose (1 g/kg b.w) was employed (Machemer, 1977a;
Herbold, 1982b). Positive lethal mutations also occurred when female
mice were treated orally with olaquindox despite the use in one study
of doses lower than that which effected a positive result in the male
mouse (200 and 500 mg/kg b.w.) (Machemer, 1977b; Sram et al, 1986b
& c). However a toxic effect in female mice could not be excluded.
Positive results have been obtained in a sister chromatid
exchange test using Chinese hamster V79 cells indicating that
olaquindox may induce DNA damage (Scheutwinkel-Reich & von der Hude,
1984). Positive results in bacterial assays including the SOS
chromotest confirm this possibility (Suter et al, 1978; Beutin
et al, 1981; Yoshimura et al, 1981; Nunoshiba & Nishioka, 1989;
von der Hude et al, 1988). However, there is no evidence that
olaquindox covalently binds to DNA in the rat in vivo (Minini
et al, 1983).
The mutagenicity of a number of olaquindox metabolites has also
been investigated. The omega oxidation product, its 1 and 4
monodesoxy derivatives and its didesoxy derivative have been
investigated in the Ames test using S. typhimurium strains TA 98,
100, 1535 and 1537 with and without rat liver S9 metabolic activation.
All the tests gave negative results (Herbold, 1978; 1979a,b).
Table 3: Results of genotoxicity studies with olaquindox
Test System Test Object Concentration Results Reference
Ames test1 S. typhimurium 3.8-0.5 nmoles/plate Positive Beutin et al., 1981
TA98, 100
Ames test2,4 S. typhimurium 1.9-57 nmoles/plate Positive Beutin et al., 1981
TA100
Ames test1 S. typhimurium 1.25-15µg/plate Positive Yoshimura et al.,
TA98, 100 1981
Ames test2 S.typhimurium 0.01-0.1 mmole/1 Positive Voogd et al., 1980
Ames test1 S. typhimurium 0-50 µg/plate Positive Nunoshiba & Nishioka,
TA98,100 1989
Fluctuation1 test K. pneumoniae 2x10-4-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Fluctuation2 test K. pneumoniae 2x10-5-1x10-2 mmole/1 Positive Voogd, et al,. 1980
Forward1 mutation E. coli 0-20 µg/plate Positive Nunoshiba & Nishioka,
Wp\P2uvrA/pKM101 1989
In vitro Cultured human 3-300 µg/ml Positive Tamura, 1977
cytogenetics lymphocytes
In vivo Mouse bone marrow 20, 500 or 800 mg/kg Negative Sutou, 1977
cytogenetics b.w. oral
In vivo Mouse bone marrow 200-800 mg/ml b.w. oral Negative Cihak & Vontorkova, 1983
cytogenetics
In vivo Mouse bone marrow 20-500 mg/kg b.w. diet Positive Sram, et al., 1986a
cytogenetics 4 and 12 weeks
Table 3 (contd)
Test System Test Object Concentration Results Reference
In vivo Chinese hamster 20 mg/kg b.w. oral, x5 Positive Pokorna, 1986
cytogenetics marrow
In vivo Chinese hamster 2x30-2x1000 mg/kg b.w. Positive Herbold, 1983a
cytogenetics spermatogonia oral
Micronucleus Mouse bone marrow 500 mg/kg b.w. oral, Positive Herbold, 1983b
sampled at 24, 48 or
72 hours; 10-300 mg/kg
b.w. oral, sampled at
24 hours
Micronuceus test Chinese hamster 20 mg/kg b.w. 4.2 and Positive Pokorna, 1986
bone marrow 100 mg/kg b.w. oral,
once
Micronucleus Mouse bone marrow 6.7 mg/m3 and 161 mg/m3 Positive Herbold, & Thyssen, 1982
for 6 hours/day, 2 days,
inhalation
Micronucleus Mouse bone marrow 2034 mg/kg b.w. 30 hours Negative Herbold, 1982a
dermal exposure
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Micronucleus Mouse bone marrow 100 mg/kg b.w oral or Positive Cihak & Vontorkova, 1985
intraperitoneal
Dominant lethal Mouse (male) 2x1000 mg/kg b.w. week Positive Machemer, 1977a
oral
Table 3 (contd)
Test System Test Object Concentration Results Reference
Dominant lethal Mouse (male) 40, 120 and 360 ppm in Negative Herbold, 1982b
diet for 35 days
equivalent to 6, 18, and
54 mg/kg b.w.
Dominant lethal Mouse (male) 100, 300 and 500 mg/kg b.w. Negative Sram, et al., 1986b
for 4 weeks, 20, 40, 100,
200, and 500 mg/kg b.w.
for 12 weeks, diet
Dominant3 lethal Mouse (female) 30, 100, 300 or 1000 mg/kg b.w. Positive Machemer, 1977b
oral once
Dominant lethal Mouse (female) 20, 40, 100, 200 and 500 mg/kg Positive Sram, et al., 1986c
b.w. diet, for 4 weeks
SOS2 Chromotest E. coli, GE94 0-10 µg/plate Positive Nunoshiba & Nishioka, 1989
(DNA damage)
SOS2 Chromotest E. coli, PQ37 0.001-0.1 mM Positive von der Hude, et al.,
(DNA damage)
DNA damage E. coli, K12 Not applicable Positive Suter, et al., 1978
DNA damage S. typhimurium 100 µg/disc uvr B and recA Positive Beutin, et al., 1981
DNA damage S. typhimurium 1-100 µg/disc Positive Yoshimura, et al., 1981
Mitotic gene2 S. cerevisiae D4 0.05% w/v Positive Voogd, et al., 1980
conversion
Table 3 (contd)
Test System Test Object Concentration Results Reference
DNA binding Rat 500 mg/kg b.w. oral Negative Minini, et al., 1983
in vivo
Sister chromatid Chinese hamster 0-200 µg/ml V79 cells Positive Scheutwinkel-Reich & von
exchange der Hude, 1984
1 With and without rat liver S-9 fraction.
2 In the absence of S-9 fraction.
3 Positive only at the 1000mg/kg b.w. dose.
4 Positive in aerobic and anaerobic conditions.
The results obtained with olaquindox are similar to those noted
with several other quinoxaline di-N-oxides including quindoxin and
carbadox (Suter et al, 1978; English & Dunegan, 1970; Voogd
et al, 1980; Negishi et al, 1980; Beutin et al, 1981 (see also
carbadox)). The mechanisms of action are unknown although neither
olaquindox nor quindoxin binds to DNA (Minini et al, 1983, Suter
et al, 1978).
Electron spin resonance techniques have demonstrated the
generation of free radicals during the reduction of quindoxin while a
related compound 2,3-dihydroxymethyl quinoxaline-1,4-di-N-oxide has
been shown to inhibit DNA synthesis in E. coli (Suter et al,
1978). However, at present the roles of free radicals and inhibition
of DNA synthesis in the mutagenicity of quinoxaline-1,4-dioxides are
unknown.
These studies indicate that olaquindox is genotoxic in a variety
of test systems. It induces mutations in bacterial systems and it
causes chromosome and DNA damage in in vitro and in in vivo
systems. Data available from dominant lethal assays and from an
in vivo cytogenetic study with Chinese hamster spermatogonia suggest
that olaquindox may have the potential to exert its mutagenic effects
on germ line cells.
2.3 Observations in humans
One of the major routes of exposure to olaquindox is likely to be
occupational during the preparation of feed and the feeding of the
final feed to pigs. In an experimental study, no olaquindox was
detected in the workplace air (a stall building housing pigs) during
trough filling operations with a feed containing 50 ppm olaquindox
(Thyssen et al, 1982). Olaquindox was only detected at low levels
in the atmosphere during the preparation of 0.1% feed premix and the
50 ppm final feed starting with a 10% premix of a proprietary product.
Levels in the atmosphere were estimated to be from below 0.4 to below
0.1 µg/m3 air (Inkmann-Koch, 1985a). Analysis of the urine from a
single worker engaged in similar preparation work and in the feeding
of pigs revealed no olaquindox (limit of detection 40 ppb) (Inkmann-
Koch, 1985b).
When applied to the skin of two volunteers as a paste in water
containing 2 g of olaquindox (around 30 mg/kg b.w.) using a 6 hour
occlusive dressing, no olaquindox was detected in the 48 hour urine
using an analytical method with a limit of detection of 0.12 µg/ml
(Beermann, 1982).
There has been one report of allergic contact dermatitis and one
of photocontact dermatitis following occupational exposure to
olaquindox (Bedello et al, 1985; Francalanci et al, 1986). Both
occurred in pig workers exposed to the substance in the animal feed.
There are no reports of systemic toxicity in humans following exposure
to olaquindox.
3. COMMENTS
The toxicological data considered by the Committee included the
results of acute and subchronic studies, together with the results of
studies on mutagenicity, carcinogenicity, and effects on reproduction
and development.
Olaquindox is almost completely absorbed from the
gastrointestinal tract in rats, dogs, and pigs. In studies using
radiolabelled olaquindox, the radioactivity was shown to be widely
distributed in the tissues, with residues in the liver being the most
persistent. The compound was primarily eliminated in the urine, with
lesser amounts being excreted in the faeces and expired air of the
animals. In the pig, elimination was virtually complete by 48 hours
after a single intragastric dose. Any remaining radioactivity was
then eliminated with a half-life of 5-9 days, far in excess of that of
olaquindox (about 3-5 hours). The parent compound accounted for 70%
of the radioactivity in the urine, up to 24 hours after
administration. There were approximately 16 metabolites detected in
the urine; six major metabolites have been fully characterized.
In acute and short-term toxicity studies in rats and mice, rats
were about twice as sensitive as mice. In a 90-day study in rats,
effects were observed in the testes, ovaries, thyroid, and adrenal
cortex at a dose of 5 mg/kg b.w./day and above. These lesions were
described histopathologically as atrophy; and in the adrenal glands
the effect was greatest in the zona glomerulosa. No treatment-related
abnormalities of the pituitary gland were reported. A 90-day study in
beagle dogs and a 19-week study in rhesus monkeys also produced
evidence of toxic effects on the endocrine glands, liver, and kidney.
Of five female monkeys dosed at 40 mg/kg b.w./day, one died during
treatment and two died during the planned 17 week recovery period. The
two survivors failed to re-establish normal ovarian cycles during the
recovery period.
In a 20-week study, groups of five male and five female pigs were
fed up to 250 mg of olaquindox per kg of diet. The plasma
concentrations of urea and creatinine were elevated at 160 and 250 mg
diet, which suggested an effect on the kidney. All of the pigs in the
highest-dose group that survived the study period continued to show
evidence of this effect during a 16 day-recovery period. Plasma
sodium, potassium and chloride concentrations were altered during the
study, but did not return to normal during the recovery period.
Histopathological changes were found at necropsy in kidney and adrenal
tissues from both groups. The manufacturers reported a no-observed-
effect level of 100 mg/kg in feed for this study.
However, in other studies in piglets in which olaquindox was fed
at 25, 50, 100 or 200 mg/kg diet for 6 weeks, a dose-dependent fall in
plasma aldosterone concentration, together with hyponatraemia,
hypochloraemia, and hyperkalaemia occurred in all groups by the end of
the study. Hydropic degeneration of adrenal cortex cells was
recorded. There were no effects on weight gain or clinical signs.
Developmental studies in which the compound was administered
orally were conducted in mice and rats. In mice, fetal weights were
reduced at 180 mg/kg b.w./day, but malformations were absent. In
rats, malformations occurred at 180 mg/kg b.w./day, and reductions in
maternal weight gain, litter size, and fetal weight were also observed
at this dose.
A three-generation reproduction study in which olaquindox was
administered in the diet was conducted in rats. No malformations were
observed, the only findings being reductions in fertility rate and
litter size in the second and third generations at the highest dose of
25 mg/kg b.w./day.
The genotoxicity of olaquindox was investigated in a range of
in vitro and in vivo studies. Positive findings were reported in
assays for point mutation and for DNA damage in bacteria, sister-
chromatid exchange in Chinese hamster V-79 cells, chromosome damage in
human lymphocytes in vitro, mammalian bone-marrow cells in vivo,
and in several micronucleus tests. Two dominant lethal assays in male
mice were negative, but a third gave a weak positive response. Two
dominant lethal assays in female mice gave positive results, but this
may have been partly due to the toxicity of the drug. A weak positive
result was obtained in an in vivo cytogenicity assay using Chinese
hamster spermatogonia. Olaquindox did not bind to rat DNA in vivo.
The Committee considered data from six long-term studies in
rodents, but because of poor survival and deficiencies in experimental
design and reporting, only two carcinogenicity studies, in mice and in
rats, were evaluated.
In the study in mice, doses of olaquindox up to an equivalent of
54 mg/kg b.w./day were administered in the diet for life (up to 635
days). An increase in the incidence of benign adrenal cortical
adenomas and benign proliferative lesions (nodular hyperplasia and
adenoma) in the lung was observed in male mice at the highest dose
level. There was no effect on the incidence of malignant tumours.
In the rat carcinogenicity study, which included fetal exposure
to the drug in utero, olaquindox was administered in the diet at
levels up to an equivalent of 30 mg/kg b.w./day until 80% of the male
and female controls had died (about three years). Survival was
decreased at 30 mg/kg b.w./day in both sexes, but there was no
increase in the incidence of benign or malignant tumours.
4. EVALUATION
The Committee considered olaquindox to be a genotoxic agent.
There was some evidence to suggest that olaquindox was a germ-line
mutagen, but more extensive testing in appropriate mammalian studies
will be required to resolve this issue. In the carcinogenicity
studies only the mouse showed an increase in the incidence of tumours,
and these were benign. Because of doubts over the mechanism of this
effect and the results of the genotoxicity studies, the Committee was
unable to establish an ADI for olaquindox. However, the Committee
concluded that residues resulting from the use of olaquindox in food-
producing animals under conditions of good practice in the use of
veterinary drugs were temporarily acceptable.
The results of studies on the nature and availability of residues
of olaquindox and the results of studies designed to provide an
indication of the toxic potential of these residues are required by
1993.
Depending upon the results of these studies, the following
additional information may be needed:
a) Data to assess the genotoxic potential of olaquindox on
germ-line cells, which would, at a minimum, necessitate a
repeat of the Chinese Hamster spermatogonia study.
b) Studies designed to assess the effects of olaquindox on
adrenal function (including sensitive parameters such as
plasma adrenal hormones and electrolyte levels), sperm
morphology, and fertility in rats, so that a NOEL can be
determined for each of these indicators.
c) Information on the binding of olaquindox or its metabolites
to structural proteins such as tubulin, or to enzymes or
proteins involved in DNA synthesis or repair. (Such binding
may help explain why, despite its obvious genotoxic
potential, olaquindox does not bind to DNA and has given
equivocal results in carcinogenicity studies).
5. REFERENCES
ANDERSON, B., & SZABO, A. (1982). Determination of olaquindox in
swine tissues after short withholding periods. Report No. 08-07-
1981/24-03-1982. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
ANON. (1989). Compendium of Data Sheets for Veterinary Products
1989-90. National Office of Animal Health, Datapharm Publications
Ltd, London.
BAARS, A.J., VAN DER MOLEN, E.J., SPIERENBURG, T.J., DE GRAAF, G.J.,
NABUURS, J.J.A., & JAGER, L.P. (1988). Comparative toxicity of three
quinoxaline-di-N-dioxide feed additives in young pigs. Arch.
Toxicol., Suppl. 12, 405-409.
BEDELLO, P.G., GOITRE, M., CANE, D., & RONCAROLO, G. (1985). Allergic
contact dermatitis to Bayo-N-Ox-1. Contact Derm., 12, 284.
BEERMAN, D. (1982). Cutaneous absorption of olaquinodox in human
volunteers. Doc. No 82/10744. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
BEUTIN, L., PRELLER E., & KOWALSKI, B. (1981). Mutagenicity of
olaquindox, 3its metabolites, and two substituted quinoxaline-di-N-
oxides. Antimicrob. Agents Chemother., 20, 336-343.
BORIES, G., & BOURDON, D. (1977). Residue analysis in pigs. Doc. No.
77/8813. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
CIHAK, R., SRB, V., & VONTOKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. I. Micronucleus
test in rats. Mutat. Res., 116, 129-135.
CIHAK, R., & VONTORKOVA, M. (1983). Cytogenetic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. II. Metaphase
analysis in mice. Mutat. Res., 117, 311-316.
CIHAK, R., & VONTORKOVA, M. (1985). Cytogentic effects of
quinoxaline-1,4-dioxide-type growth-promoting agents. III.
Transplacental micronucleus test in mice. Mutat. Res., 144, 81-84.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A.,
(1970). Resorption, distribution, substance changes, and elimination.
Short test in the rat and in the dog. Report No. 1939. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
DUHM, B., MAUL, W., MEDENWALD, H., PATZSCHKE, K., & WEGNER, L.A., &
SCHEER, M., (1973). Metabolism and residue studies wth 14C-labelled
Bay VA 9391 in swine. Report No. 4151. Unpublished report by Bayer
AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
ENGLISH, A.R., & DUNEGAN, C.M. (1970). Quinoxaline-1, 4-di-N-oxides.
I. Inhibition of deoxyribonucleic acid synthesis in Escherichia
coli by 2, 3-dihydroxy-methyl-quinoxaline-1, 4-di-N-oxide. Proc.
Soc. Exp. Biol. Med., 133, 398-400.
FRANCALANCI, S., GOAL, M., GIORGINI, S., MUCCINELLI, A., & SERTOLI, A.
(1986). Occupational dermatitis from olaquinodox. Contact Derm.,
15, 112-114.
GANDALOVICOVA, D., & SYKORA, I. (1986). Cyadox olaquindox - a
fertility test on rats. Biol. Chem. Vet., (Prague), 22, 47-51.
GERICKE, H., & DYCKA, J. (1974). Olaquindox/Bayo-N-ox.
Investigations into the safety of use of the feed additive. Report
No. 4752. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HEIMANN, K.G. & SCHILDE, B., (1982). BAY VA 9391. Study of subacute
dermal toxicity in rabbits. Report No. 11267(E). Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1978). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No 2. 7824. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979a). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 8091/8092/8099. Unpublished
report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by
Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1979b). Salmonella/microsome-test in the investigation
of point mutagenic activity. Report No. 7824. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982a). Micronucleus test on the mouse to check for
mutagenic effect after cutaneous application. Report No. 10602.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1982b). Dominant lethal test on male mice to check for
a mutagenic effect after 35 days of administration in the feed.
Report No 10621. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HERBOLD, B. (1983a). Cytogenetic study of the spermatogonia of the
Chinese hamster in vivo to test for mutagenic activity. Report No.
12179. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HERBOLD, B. (1983b). Micronucleus test for a mutagenic effect on on
the mouse. Report No. 11424(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
HERBOLD, B., & THYSSEN, J. (1982). Micronucleus test on the mouse to
test for mutagenic activity after inhalation of dynamically produced
dust. Report No. 10833. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
HEYWOOD, R., SORTWELL, R.J., NEWMAN, A.J. & STREET, A.E. (1972).
Report No. 4751/72/186. FB a 9391. Oral Toxicity study in rhesus
monkeys. (Repeated dosage for nineteen weeks and recovery period).
Unpublished report from Huntingdon Research Centre, Huntingdon, UK.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1969). BAY VA 9391. Subchronic toxicity in rats
following oral administration. (Trial over 13 weeks). Report No.
1693. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K. (1972). BAY VA 9391. Subchronic toxicity in rats with
oral administration. (13-week test with low doses). Report No. 3241.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
HOFFMANN, K., LUCKHAUS, G. & DYCKA, J. (1974). BAY VA 931.
Toxicological feeding test in feeder swine. Part 3. Results of the
toxicological, pathologic-anatomic and histopathological studies.
Report No. 4754. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
INKMANN-KOCH, A. (1985a). Determination of the concentration of
olaquindox in the air during the preparation of compound (meal) swine
feed and exposure of workers. Doc. No. 85/11885. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
INKMANN-KOCH, A. (1985b). Determination of olaquindox in urine
samples of a worker follwing the preparation of swine feed from Bayo-
N-Ox. Doc. No. 85/11887. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
KALLER, H. (1970). BAY VA 9391. Pharmacological screening. Report
No. 1809. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
KULCZYK, S.R., HERRERA, R.E., MULKEY, N.S., BREAULT, G.O., WARGO,
J.P., & WAGGONER, T.B. (1979). Determination of physical constants of
BAY VA 9391. Report No. 79/9803. Unpublished report from Analytical
Development Corporation, USA. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
LEIBETSEDER, J. (1980). To determine olaquindox residues in swine.
Doc. No. 80/10022. Unpublished report from the Institute of Nutrition
of the Veterinary Medical University, Vienna, Austria. Submitted to
WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LOESER, E. (1974). BAY Va 9391. Three-generation test in rats.
Report 4748. Unpublished report from Bayer AG, Wuppertal-Elberfeld,
FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE, D. & TETTENBORN, D. (1969). FBa 9391. Subchronic toxicity of
dogs during oral administration. (13 Week test). Report No. 1536.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
LORKE D. (1971a). FBa 9391. Examination for embryotoxic effects in
mice. Report No. 2692. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
LORKE, D. (1971b). FBa 9391. Examination for embryotoxic effects in
rats. Report No. 2693. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
MACHEMER, L. (1977a). To evaluate mutagenic potential of BAY VA 9391
in mice utilizing the dominant lethal test. Report No. 6738.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
Machemer (1977b). To investigate BAY VA 9391 for mutagenic effects
using the dominant lethal test. Report No. 6770. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
MAUL, W., SENG, F. & WENDISCH, D. (1979a). Investigations into the
biotransformation of olaquinox in swine. Report No. 8114.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAUL, W, SENG, F. & WENDISCH, D. (1979b). Investigations into the
biotransformation of olaquinox in swine. Report No. 8725.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MAWDESLEY-THOMAS, L.E. & URWIN C. (1969). Pathology report of FB a
9391. Sub-chronic toxicity for dogs with oral administration. Report
No. 2921/69/347. Unpublished report from Huntingdon Research Centre,
Huntingdon, UK. Submitted to WHO Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. (1974). Method for the determination of residues of Bay
VA 9391 and its reduction products in animal tissues. Report No.
4751. Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MEDENWALD, H. & GERICKE, H. (1974). Toxicological Feeding Test with
Feeder Pig. Part 2. Results of Residue Analysis. Report No. 4753.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MININI, U., LUTZ, W.K., & SCHLATTER, CH. (1983). Lack of in vivo
binding to DNA of olaquindox. Report No. R2570 (P). Unpublished
report from the Institute of Toxicology, Swiss Federal Institute of
Technology and University of Zurich, Schwerzenbach, Switzerland.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
MURMAN, P.(1979). BAY VA 9391 (Olaquindox). Testing of primary
dermal and mucosal tolerability in the rabbit. Report No. 8359.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
NABUURS, M.J.A., VAN DER MOLEN, E.J., DE GRAAF, G.J., & JAGER, L.P.
(1989). Clinical Signs and Performance of Pigs Treated with Different
Levels of Carbadox, Cyadox and Olaquindox. J. Vet. Med., 36A,
209-217.
NASTUNEAK, J., KOLOUCH, F., VANOVA, L., DVORAK, M. & SEVICK, B.
(1986). Three month toxicity to laboratory Norway rats: Cyadox
compared with olaquindox. Biol. Chem.Vet. (Prague), 22, 367-380.
NEGISHI, T., TANAKA, K. & HAYATSU, H. (1980). Mutagenicity of
carbadox and several quinoxaline 1,4-dioxide derivatives. Chem.
Pharm. Bull., 28, 1347-1349.
NUNOSHIBA, T., & NISHIOKA, H. (1989). Genotoxicity of quinoxaline
1,4-dioxide derivatives in Escherichia coli and Salmonella
typhimurium. Mutat. Res., 217, 203-209.
POKORNA, D. (1986). Cytogentic analysis of bone marrow cells in
Chinese hamster after the administration of cyadox and olaquindox.
Biol. Chem. Vet. (Prague)., 22, 23-28.
POWER, S.B., DONNELLY, W.J.C., MCLAUGHLIN, J.G., WALSH, M.C. & DROMY,
M.F. (1989). Accidental carbadox overdosage in pigs in an Irish
weaner-producing herd. Vet. Rec., 124, 367-370.
SCHEUTWINKEL-REICH, M. & VON DER HUDE, W. (1984). Sister-chromatid
exchange in Chinese hamster V79 cells exposed to quindoxin, carbadox
and olaquindox. Mutat. Res., 139, 199-202.
SCHLUMBERGER, H.D. (1975). BAY VA 9391: Exclusion of immunogenic
activity. Report No. 5194. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
SCHMAEL, D. (1973). Carcinogenicity test with BAY VA 9391 with oral
application to rats and mice. Report N. 38399. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986a).
Chromosomal aberration in the bone marrow of mice after long-continued
administration cyadox and olaquindox. Biol. Chem. Vet. (Prague), 22,
17-22.
SRAM, R.J., FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986b). Effect
of long-term oral administration of cyadox and olaquindox on the
frequency of dominant lethal mutations and sperm abnormalities in
mice. Biol. Chem. Vet. (Prague), 22, 37-45.
SRAM, R.J. FERNANDEZ RODRIQUEZ, S.F., & KOCISOVA, J. (1986c).
Dominant lethal mutation in female mice following the oral application
of cyadox and olaquindox. Bio. Chem. Vet. (Prague), 22, 29-35.
STEINHOFF, D. (1973). Carcinogenicity study with Bay VA 9391 after
oral administration to rats. Report No. 4048. Unpublished report
from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer
AG, Wuppertal-Elberfeld, FRG.
STEINHOFF, D. (1977). BAY VA 9391. Study as to chronic toxic effect
in rats after oral application. (2-Year trial with additional
transplacental treatment). Report No. 6556. Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
STEINHOFF, D., & BOEHME, K. (1978). BAY VA 9391. Carcinogenesis
tests with oral administration to rats. (With transplacental
pretreatment). Report N. 7387. Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
STEINHOFF, D., & GUNSELMANN, W. (1982). BAY VA 9391. Carcinogenicity
experiment with oral administration to mice. Report No. 72388
(revised), 100700 (revised), 12251 (revised). Unpublished report from
Bayer AG, Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
SUTER, W., ROSSELET, A., & KNUSEL, F. (1978). Mode of action of
quindoxin and substituted quinoxaline-di-N-oxides on Escherichia
coli. Antimicrob. Agents Chemother., 13, 770-783.
SUTOU, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No 78/9327. Unpublished report from Medical Biology
Laboratory, Life Science Department, Nomura Resarch Station, Japan.
Submitted to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
TAKASE, I., & KOMACHI, S. (1975). Determination of BAY VA 9391
residues in animal tissues. Doc. No. 76/8507. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer Ag, Wuppertal-Elberfeld, FRG.
TAKASE, I & KOMACHI, S. (1976). Residues of BAY VA 9391 (olaquindox)
in tissues and organs of pigs. Doc No. 76/8508. Unpublished report
from Agricultural Chemicals Institute, Tokyo, Japan. Submitted to WHO
by Bayer AG, Wuppertal-Elberfeld, FRG.
TAMURA, S. (1977). To investigate the mutagenic potential of BAY VA
9391. Doc. No. 78/9326. Unpublished report from Keio University,
School of Medicine, Tokyo, Japan. Submitted to WHO by Bayer AG,
Wuppertal-Elberfeld, FRG.
TETTENBORN, D. (1969). FBa 9391. Acute toxicity for mouse, rat,
rabbit, cat, and dog with oral and subcutaneous administration.
Report No. 1176. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J. (1982). Olaquindox. Acute inhalation toxicity of the
dust. Report No. 10755. Unpublished report from Bayer AG, Wuppertal-
Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
THYSSEN, J., BEERMANN, D., & DORN, H. (1982). Exposure studies at the
place of work on administration of Bayo-N-Ox-Ferkelaufzuchtfutter
(Special Piglet Feed) F2 in pellet or meal form. Report 10731.
Unpublished report from Bayer AG, Wuppertal-Elberfeld, FRG. Submitted
to WHO by Bayer AG, Wuppertal-Elberfeld, FRG.
THYSSEN J. (1983). Olaquindox BAY VA 9391. Subacute inhalation study
in rats. Report N. 13032(E). Unpublished report from Bayer AG,
Wuppertal-Elberfeld, FRG. Submitted to WHO by Bayer AG, Wuppertal-
Elberfeld, FRG.
URWIN, C., & MAWDESLEY-THOMAS, L.E. (1969). Pathology report of Bay
a 9391. Subchronic toxicity for rats by oral administration. Report
No. 2989/69/415. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
URWIN, C., & SPICER, E.J.F., (1971). Pathology report of Bay a 9391.
Subchronic toxicity study for rats by oral administration. Report No.
4389/71/545. Unpublished report from Huntingdon Research Centre.
Huntingdon, UK. Submitted to WHO by Bayer AG, Wuppertal-Elberfeld,
FRG.
VAN DER MOLEN, E.J., NABUURS, M.J.A., & JAGER, L.P. (1985).
Pathological and clinical changes related to toxicity of carbadox in
weaned pigs. Zbl. Vet. Med., 32, 540-550.
VAN DER MOLEN, E.J., DE GRAAF, G.J., SPIERENBURG, TH. J., NABUURS,
M.J.A., BAARS, A.J. & JAGER, L.P., (1986). Hypoaldosteronism in
piglets induced by carbadox. Experientia., 42, 1247-1249.
VAN DER MOLEN, E.J., BAARS, A.J., DE GRAAF, G.J. & JAGER, L.P. (1989).
Comparative study of the effect of carbadox, olaquindox and cyadox on
aldosterone, sodium and potassium plasma levels in weaned pigs. Res.
Vet. Sci., 47, 11-16.
VON DER HUDE. W., BEHM, C., GURTLER, R., & BASLER, A. (1988).
Evaluation of the SOS chromotest. Mutat. Res., 203, 81-94.
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1980). The
mutagenic action of quindoxin, carbadox, olaquindox and some other
N-oxides on bacteria and yeast. Mutat. Res., 78, 233-242.
WINDHOLZ, M., BUDAVARIA, S., BLUMETTI, R.,F., & OTTERBEIN, R.R.S (Eds)
(1983). The Merck Index, 10th Edition, Merck & Co., Rahway, New
Jersey.
YOSHIMURA, H., NAKAMURA, M., & KOEDA, T. (1981). Mutagenicities of
carbadox and olaquindox - growth promoters for pigs. Mutat. Res.,
90, 49-55.
