
ALLYL ESTERS
(ALLYL HEXANOATE, ALLYL HEPTANOATE, ALLYL ISOVALERATE)
First draft prepared by Dr R. Walker,
Professor of Food Science, Department of Biochemistry,
University of Surrey, England
1. EXPLANATION
Allyl hexanoate (allyl caproate, 2-propenyl hexanoate) is an
artificial flavour; it has also been reported to occur naturally in
pineapples. A temporary specification for this substance was issued
by the twenty-fourth meeting of the Joint FAO/WHO Expert Committee
on Food Additives (Annex 1, reference 53) but it has not previously
been evaluated by the Committee for an ADI. Allyl heptanoate and
allyl isovalerate have not been identified in nature; these
flavours have not previously been considered by the Committee.
In view of the similarities in metabolism between these
compounds and the fact that they all give rise to allyl alcohol on
hydrolysis, they are evaluated together in the following monograph
for a Group ADI.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Biotransformation
Allyl hexanoate was hydrolysed slowly by artificial gastric
juice in vitro (t´ 1120 min) but more rapidly by simulated
pancreatic juice (t´ 1.98 min). This compound was also hydrolysed
very rapidly by rat small intestinal mucosa preparations in vitro
(t´9.6 x 10-2 sec) and by liver homogenates (t´ 3.96 sec)
(Longland et al., 1977). Similarly, allyl hexanoate was reported
to be completely hydrolysed within 2 hours by pancreatin when
incubated at a concentration of 60 µl/l in the incubation mixture
(Grundschober, 1977).
Hydrolysis of allyl isovalerate by liver homogenates in vitro
proceeds at a slower rate than allyl esters of straight chain acids
(Butterworth et al., 1975; Drake, 1975) and the hepatotoxicity of
a number of allyl esters was correlated to the rate of hydrolysis to
allyl alcohol (see also Short-term Studies).
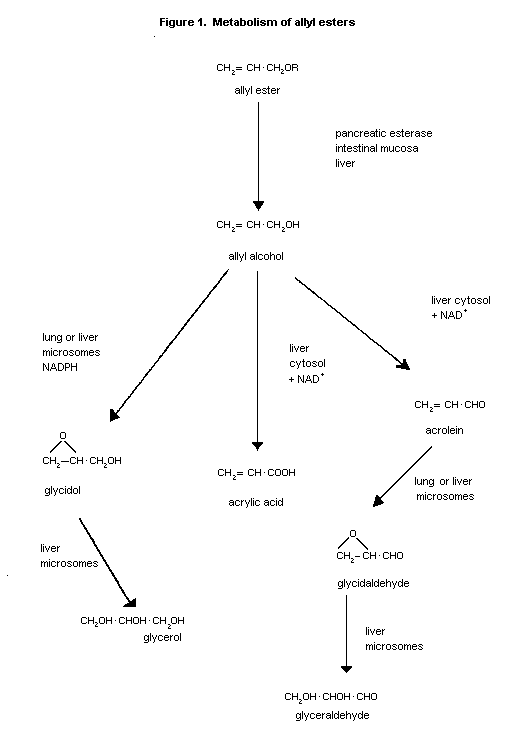
Following hydrolysis, the allyl alcohol liberated is
metabolized via two alternative oxidative pathways leading to the
formation of acrolein or the epoxide, glycidol, as shown in Figure 1
(Patel et al., 1980). The epoxide may then be converted to
glycerol by epoxide hydrolase.
The conversion of allyl alcohol to acrolein is mediated by
alcohol dehydrogenase (ADH), a step which is blocked in the ADH-
deficient rat lung (Patel et al., 1980), in genetically-deficient
deermouse liver (Belinsky, 1985) or by ADH inhibitors (Reid, 1972;
Serafini-Cessi, 1972; Diluzio & Hoffman, 1973; Jaeschke et al.,
1987; Pentilla et al., 1987). The acrolein may then be further
oxidized to acrylic acid by NAD- or NADP-dependent enzymes in the
liver cytosol or microsomes (Jaeschke et al., 1987) or to
glycidaldehyde by a microsomal enzyme with subsequent conversion to
glyceraldehyde by epoxide hydrolase (Patel et al., 1980).
Alternatively, acrolein may react directly both enzymically and
non-enzymically to form stable adducts with glutathione or other low
molecular weight thiol compounds (Ohno et al., 1985). Both
glycidol and glycidaldehyde are substrates for lung and liver
cytosolic glutathione-S-transferases (Patel et al., 1980).
When rats were dosed with allyl esters of weak acids, 3-
hydroxypropylmercapturic acid was detected as a glutathione-derived
metabolite in the urine and bile (Clapp et al., 1969; Kaye &
Young, 1970; Kaye & Young, 1972; Kaye, 1973). The same metabolite
was identified after dosing with allyl alcohol or acrolein and, by
comparison of the percentage conversion after administration of
these compounds or allyl esters of weak acids, it may be concluded
that the esters were completely hydrolysed to allyl alcohol and that
most of the alcohol was converted to acrolein (Kaye, 1973).
 2.2 Toxicological studies
2.2.1 Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Allyl hexanoate
Rat both oral 218 (186-255) Jenner et al.,
1964
Taylor et al.,
1964
both oral 327 (277-386) Meisel, 1982
male oral 393
female oral 276 (215-352)
Guinea both oral 280 (246-319) Jenner et al.,
pig 1964
Rabbit ? dermal 300 (200-600) Shelanski &
Moldovan,
1971
? dermal 820 (700-940) Moreno, 1974
Allyl heptanoate
Mouse both oral 630 (514-772) Jenner et al.,
1964
Rat both oral 500 (392-638) Jenner et al.,
1964
Rabbit ? dermal 810 (440-1180) Moreno, 1974a
Guinea both oral 444 (363-541) Jenner et al.,
pig 1964
Allyl isovalerate
Mouse both oral >500 NTP, 1983
Rat both oral >250 <500 NTP, 1983
? oral 230 (216-290) Moreno, 1977
Rabbit ? dermal 560 (290-1060) Moreno, 1977
2.2.2 Short-term studies
2.2.2.1 Mouse - allyl isovalerate
Groups of 5 male and 5 female B6C3F1 mice were given allyl
isovalerate by gavage in corn oil for 14 consecutive days at daily
doses of 0, 31, 62, 125, 250 or 500 mg/kg b.w. All animals of both
sexes that received 500 mg/kg b.w. died within 48 h of commencement
of the study; all other animals survived to termination.
Inactivity and piloerection were seen in animals receiving 250 and
500 mg/kg b.w. and male mice receiving 250 mg/kg gained no weight;
body weights in other dose groups were comparable to controls at
termination of the study (NTP, 1983).
Groups of 10 male and 10 female B6C3F1 mice, 6 weeks of age at
commencement of the study were dosed with allyl isovalerate by
gavage in corn oil at doses of 0, 15, 31, 62, 125 or 250 mg/kg b.w.
(five day/week) for 13 weeks. At the end of the study, autopsies
were performed on all survivors. Animals from control and highest
dose groups were subjected to detailed histological examination.
In addition the liver was examined histologically for the 62 and 125
mg/kg b.w. groups and in the latter group stomachs were also
examined microscopically. Five out of 10 males and six of 10
females in the highest dose groups died, all but one of the deaths
(a female) being compound related; deaths in other groups were
caused by gavage error. Compound related effects noted at necropsy
or histologically were limited to the 125 and 250 mg/kg b.w. dose
groups and included "thickening" of the urinary bladder wall and
gastric mucosa and small intestine (both groups) ulcerative
inflammation of the stomach, coagulative necrosis of the liver and
cytoplasmic vacuolation of the liver (top dose group only). No
compound related effects were seen in the liver, stomach or bladder
of mice from other groups (NTP, 1983).
2.2.2.2 Rat - allyl heptanoate
Allyl heptanoate was fed to weanling Osborne-Mendel rats of
both sexes for 18 weeks at dietary levels of 0, 1000, 2500 and
10,000 mg/kg diet. There was a dose-related growth depression which
was severe and associated with a poor food efficiency at the highest
dose level only. Gross liver enlargement was observed at all dose
levels; in addition, at the highest dose level kidneys were enlarged
in both sexes and hearts were enlarged in males only. Males in the
2500 and 10 000 mg/kg diet groups were reported to have enlarged
testes (it is not clear from the report whether this "enlargement"
relates to absolute or relative organ weights; if the latter, the
increased relative organ weights may relate to the growth depression
rather than to organ specific effects). Microscopic changes
reported included hydropic degeneration in the periportal areas of
the liver ranging from moderate at the highest dose level to lesser
degrees at lower dose levels. The extent of bile duct proliferation
correlated with the degree of hydropic degeneration and hepatocyte
enlargement also was seen in some groups (Hagan et al., 1965).
Groups of weanling Charles River albino rats of both sexes were
given allyl heptanoate in the diet daily at doses of 0, 49.6, 157
and 496 mg/kg b.w./day for 13 weeks. Weight gain and food intake
were recorded weekly, and urinalysis, haematological and clinical
chemical examinations were carried out at weeks 6 and 12. At
termination, autopsies were performed on all animals and detailed
histological examinations were carried out on all rats from the top
dose group and on half of the control animals. In the rest of the
controls and the other two dose groups, histological examination was
limited to liver and kidney, and to tissues showing gross
abnormalities at autopsy. There was a reduced food intake in the
treated groups, which was statistically significant in the high and
mid-dose groups, and an associated deficit in body weight gain. At
week 6 there was a small but significant depression in leucocyte
count in males of the top dose group only; no such effect was seen
in females and other haematological parameters were normal in
animals of both sexes. At week 12, no significant haematological
differences were seen other than a small non-dose-related decrease
in leucocyte count in the males of the 49.6 mg/kg dose group.
Clinical chemical examinations at weeks 6 and 12 revealed a decrease
in some parameters such as blood glucose, total serum protein and
albumin which appeared to be due to the reduced food intake and not
to specific toxic effects. Urine composition was unaffected by
treatment. At necropsy no gross nor microscopic lesions related to
treatment were observed and elevated relative organ weights in high-
dose males were related to reduced food intake and body weight gain.
It was concluded that daily treatment with allyl heptanoate in this
study did not result in any signs of toxicity; the reasons for the
reduced food intake could not be determined but might have been due
to unpalatability of diet (Damske et al., 1980).
Allyl hexanoate
Groups of 10 male and 10 female Osborne-Mendel rats were given
allyl hexanoate by gavage in corn oil at dose levels of 0, 15, 65
and 100 mg/kg b.w. daily for 18 weeks. Weight gain, food intake and
general condition were recorded weekly. At termination, the
animals were exsanguinated and haematological and gross pathological
examinations were performed. Detailed histological examinations
were performed only on eight rats from the control and high dose
groups; based on observations in the high dose group, livers from
eight animals in the 15 and 65 mg/kg b.w. dose groups were also
examined microscopically. In the high dose group, gross appearance
of the liver was described as nodular and wrinkled with granular or
rough surface. Microscopically the high dose group showed slight to
moderate bile duct proliferation, some "lobular architectural
disarrangement", slight fibrosis and pigment deposition in
macrophages; necrotic foci were seen in 2 of 8 animals examined. In
the 65 mg/kg b.w. dose group, very slight bile-duct proliferation
was observed in 2 of eight animals studied. The livers of the 15
mg/kg b.w. dose group were unaffected by treatment (Hagan et al.,
1967).
In a companion study to the foregoing, 5 rats of each sex
received allyl hexanoate in the diet at a concentration of 1000
mg/kg diet (equivalent to 50mg/kg b.w.) for 28 weeks. No adverse
effects were observed (Hagan et al., 1967).
Groups of 15 male and 15 female Wistar rats were given daily
oral doses of 0, 35 or 100 mg allyl hexanoate/kg b.w. as a solution
in corn oil; a further group of 10 animals of each sex were
similarly dosed with 12 mg/kg b.w./day for 13 weeks. Food and water
intake and body weights were recorded weekly, urinalysis was
performed during week 2, week 5 or 6, and in the final week of
treatment, and renal function tests were also carried out. At
termination, autopsies were performed and organ weights recorded,
and detailed histopathological examinations were conducted.
No differences were noted between treated and control animals
in body weight, water intake, haematological parameters, serum
chemistry, urine composition or in renal concentration tests. There
were slight increases in food intake in the highest dose group.
Liver weights were increased in the 35 and 100 mg/kg b.w. dose
groups and all treated animals showed evidence of periportal
vacuolation which was dose related in incidence and severity and
which in the 100 mg/kg b.w. group was accompanied by enlarged
hepatocytes, focal periportal necrosis and bile-duct proliferation.
Weights of spleen, kidneys, stomach and small intestine were
increased in both sexes in the highest dose group and small
intestine weight was also increased in females of the 35 mg/kg b.w.
group. It was not possible to determine a no observed adverse
effect level for allyl hexanoate in this study (Clode et al.,
1978).
When allyl hexanoate was administered to rats by gavage at a
daily dose level of 15 mg/kg b.w. for 18 weeks there were no
observed adverse effects (this study was reported in summary only)
(Bär & Griepentrog, 1967).
Allyl isovalerate
In similar studies to those described for mice, groups of 5
male and 5 female Fischer F344/N rats were given allyl isovalerate
by gavage for 14 days and groups of 10 animals of each sex were
dosed (5 day/week) for 13 weeks at levels of 0, 31, 62, 125, 250 and
500 mg/kg bw. In the fourteen days study, all rats given 500 mg/kg
b.w. and two rats of each sex given 250 mg/kg b.w. died. At
termination, mean body weights in the 250 mg/kg b.w. group were
depressed relative to weights in the control group by 23% and 13% in
males and females, respectively. Inactivity, laboured breathing,
diarrhoea and piloerection were observed in both sexes in the two
highest dose groups and in necropsy gross dark red areas were seen
in the stomachs of three animals of each sex at the top dose level.
In the thirteen week study, all ten males and 4/10 females that
received 250 mg/kg b.w. died and body weight gain was significantly
depressed in males of the 125 mg/kg b.w. group and females of the
250 mg/kg group. Dose-related effects seen at necropsy were
thickening of the intestinal wall and redness of the mucosal
surfaces of the intestines and urinary bladder. Enlargement of
internal lymph nodes and adrenals was reported but was unaccompanied
by histological lesions. Histopathological examination revealed
multifocal coagulative necrosis, cholangiofibrosis, and bile duct
hyperplasia at the 125 and 250 mg/kg b.w. dose levels. The effects
were dose related in incidence and no such lesions were observed in
the 31 and 62 mg/kg b.w. dose groups (NTP, 1983).
Combinations of esters
Groups of 10 male rats were given by gavage 21 consecutive
daily doses of allyl alcohol and a series of allyl esters (acetate,
propionate, hexanoate, isobutyrate, isovalerate and 2-
ethylhexanoate) at equimolecular doses corresponding to 5, 25 or 60
mg/kg b.w. of allyl alcohol. After 21 days the animals were
sacrificed and the livers examined histologically. The severity of
the liver lesions was classified according to the scheme: periportal
cell enlargement, followed by necrosis and subsequent fibrosis with
bile duct hyperplasia. The severity of the hepatic lesions from the
straight chain esters was similar to that produced by the
corresponding dose of allyl alcohol and more marked than that
produced by the esters of the branched chain acids. The differences
were attributed to differences in the rate of hydrolysis since the
straight chain esters were hydrolysed in vitro approximately 100
times faster than the branched chain esters (Butterworth et al.,
1975).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mouse - allyl isovalerate
Groups of 50 male and 50 female B6C3F1 mice, initially 50 days
old, received allyl isovalerate at doses of 0, 31 or 62 mg/kg b.w.
by gavage in corn oil (10 ml/kg b.w.) five times per week for 103
weeks. Survivors were killed at 112-114 weeks of age. No
significant differences were observed in survival rates in males;
reduced mean body weight gain and significantly lower survival rate
in the lower dose group females was attributed to a high incidence
of a genital tract infection.
An increase in the incidence of epithelial hyperplasia and
squamous cell papillomas was observed in the non-glandular
forestomach in male mice; the observed incidences for hyperplasia
were 1/50, 1/50 and 7/48, and for papillomas 0/50, 1/50 and 3/48 in
the control, low and high dose groups respectively. In females, the
corresponding incidences for forestomach epithelial hyperplasia were
0/50, 2/50 and 3/50, and for squamous cell papillomas were 1/50,
0/50 and 2/50 respectively. The incidence of lymphomas was slightly
elevated in males (5/50, 6/50 and 8/50 in the respective dose
groups) but the increase was not significant by the trend test nor
by the incidental tumour test; in females the corresponding
incidences were 11/50, 11/50 and 18/50 which gave a dose-response
trend, the high dose tumour incidence being significantly higher
(P<0.05) than controls. Significant reductions in tumour incidence
were observed in treated male mice in respect of hepatocellular
carcinomas (18/50, 6/50, 9/50), alveolar/bronchiolar adenomas or
carcinomas (13/50, 6/50 5/49) and for thyroid follicular cell
adenomas (5/47, 0/46, 1/49). No treatment-related non-neoplastic
lesions were observed in mice of either sex (NTP, 1983).
2.2.3.2 Rat - allyl hexanoate
A group of 5 male and 5 female Osborne-Mendel rats were given
allyl hexanoate in the diet at a concentration of 2500 mg/kg
(equivalent to 125 mg/kg b.w.) for 1 year. Body weight and food
intake were recorded weekly and haematological examinations were
carried out at 3, 6, and 12 months. At termination, detailed
histological examinations were performed. No adverse effects were
reported, in contrast with the short-term (18 week) study where
minimal effects (very slight bile duct proliferation) were reported
at a dose level of 65 mg/kg b.w. (Hagan et al., 1967).
When allyl hexanoate was fed to rats at a dietary concentration
of 0.5% for 1.5 years, 2/25 animals developed multiple bile duct
adenomas and proliferative changes of the small bile ducts. An
additional animal was reported to have adenomas (location not
specified) after 8.5 months. The authors concluded that the small
number of animals and tumour incidence were insufficient to allow a
firm conclusion to be reached on the significance (Bär &
Griepentrog, 1967) (This report was in summary only).
Allyl isovalerate
Groups of 50 male and 50 female Fischer 344/N rats, initially
46 days old, were given allyl isovalerate at doses of 0, 31 or 62
mg/kg b.w. by gavage in corn oil (5 ml/kg b.w.) five times per week
for 103 weeks. Survivors were killed at 112-114 weeks of age when
for males the numbers of survivors were 34 controls, 30 low dose and
28 high dose; the corresponding numbers of female survivors were
38, 36 and 29 respectively. There was a dose related increase in
mononuclear-cell leukaemia, the incidences observed were 1/50, 4/50
and 7/50 in males of the control, low- and high dose groups; in
females the corresponding incidences were 4/50, 6/50 and 9/49. In
both sexes there was a significant dose-response trend (p<0.05),
while the incidence in high-dose males was significantly higher than
controls (p<0.05). Increased frequencies of non-neoplastic lesions
(cholangiofibrosis, nodular regeneration, cirrhosis, focal
periportal necrosis, fatty changes and cytoplasmic vacuolation) were
observed in the livers of animals of both sexes in the high dose
group but there was no increase in liver neoplasms (NTP, 1983).
The authors of the NTP report concluded that allyl isovalerate
was carcinogenic, causing increased incidence of haematopoietic
system neoplasms (mononuclear cell leukaemias in male rats and
lymphoma in female mice). In reviewing this and other relevant
biological data, the International Agency for Research on Cancer
concluded that there was limited evidence for the carcinogenicity of
allyl isovalerate to experimental animals (IARC, 1985).
2.2.3.3 Dog - allyl heptanoate
Four groups of 3 male and 3 female beagle dogs were given daily
doses of allyl heptanoate of 0, 5, 25 and 75 mg/kg b.w. by capsule
for up to 18 months. All the dogs in the top dose group died within
3 - 7 months; dogs in the two lower treatment groups were reported
as surviving after 18 months. Administration of 75 mg/kg b.w.
caused depressed growth and macroscopic changes in the appearance of
the liver and haemorrhagic changes in the gastric mucosa. Less
consistent changes were reported in the form of cysts in the urinary
bladder and congestion in the lungs, digestive tract, kidneys,
spleen and lymph nodes. Microscopically the livers showed a slight
to moderate periportal fibrosis associated with slight to moderate
proliferation of the bile duct epithelium. Slight fatty changes
were also observed. The stomachs showed diffuse haemorrhage and
necrosis of the mucosae with instances of focal sub-mucosal
haemorrhage (Hagan et al., 1965).
2.2.4 Special studies on skin irritation
During an acute dermal toxicity study on allyl heptanoate in
rabbits, skin irritancy was evaluated on day 1. At dermal doses of
313-1250 mg/kg slight to moderate redness and oedema were reported
(5000 mg/kg was a lethal dose) (Moreno, 1974). Similar results were
obtained with allyl heptanoate (Moreno, 1974a). Allyl isovalerate
applied undiluted to intact or abraded rabbit skin under occlusion
for 24h was moderately irritating (Moreno, 1977).
In a preliminary to a maximization test, 48 hour closed patch
tests were carried out on the forearms of 5 volunteers with allyl
hexanoate and four subjects displayed grade 1 irritation (Kligman,
1971). Conversely, in a later study in which 5 volunteers were
subjected to patch tests, no signs of irritation were observed with
allyl hexanoate nor with allyl heptanoate (Kligman, 1975; 1975a).
Similarly, allyl isovalerate was without irritant effect in a closed
patch study on the backs of 28 subjects (Epstein, 1976).
2.2.5 Special studies on contact sensitization
In a maximization test on 25 healthy volunteers, allyl
hexanoate was reported to produce 13 cases of sensitization and was
considered a moderate sensitizer (Kligman, 1975); this contrasts
with an earlier maximization test on a similar number of volunteers
in which no cases of sensitization were detected (Kligman 1971). No
sensitization was observed with allyl heptanoate using a similar
protocol (Kligman, 1975a) nor with allyl isovalerate in 28
volunteers (Epstein, 1976).
2.2.6 Special studies on the haematopoietic and immunologic systems
Following the observation, in carcinogenicity studies on allyl
isovalerate, of marginal increases in mononuclear cell leukaemia in
rats and of malignant lymphoma in mice (NTP, 1983) and in view of
the fact that isovaleric acidaemia has been associated with
pancytopenia in humans, the effects of allyl isovalerate on the
haematopoietic and immune systems of female B6C3F1 mice and Fischer
344/N rats of both sexes were investigated in a short-term (14 day)
study. The animals were dosed by gavage for 5 days per week for 2
weeks with allyl isovalerate in corn oil at dose levels of 0, 31, 62
or 125 mg/kg b.w. (rats and mice) or 250 mg/kg b.w. (rats only).
Haematological, immunological and histological studies were
performed 48-72 h after the final treatment. In addition, bone
marrow slides from female mice from the NTP 13-week study (see
short-term studies) were also examined. The body weights of rats of
both sexes were reduced at the 250mg/kg b.w. dose level and of males
at the 125 mg/kg b.w. level. No changes in the body weights of the
female mice were observed but there was a 20% increase in mean
spleen weight and the splenic follicles were large with prominent
germinal centres. No treatment-related effects were seen in
haematological parameters nor in bone-marrow cellularity in mice or
rats. However, there were significant decreases in pluripotent
haematopoietic stem cells (CFU-S) in the spleen and in granulocyte-
macrophage progenitors (CFU-GM) in the bone marrow of treated mice.
In vitro enzyme assays of these cells showed that haematopoietic
suppression was correlated with a depression of hexose monophosphate
shunt metabolism but that enzymes of the Embden-Meyerhof and
tricarboxylic acid pathways were unaffected. Examination of host
resistance in mice following challenge with Plasmodium yoelii or
Listeria monocytogenes showed no significant differences between
control and treated animals, nor were there other effects on the
immune system. The authors concluded that the myelotoxic effects
were minimal and of a degree that did not alter host resistance
(Hong et al., 1988).
Minimal to moderate hypocellularity of the bone marrow was
observed in the 125 mg/kg b.w. group of mice, both sexes, from the
13-week NTP study and was most striking for megakaryocytes. The
degree of hypocellularity was never severe.
2.2.7 Special studies on genotoxicity
The genotoxicity of allyl hexanoate and allyl isovalerate are
shown on the next page.
2.2.8 Special studies on metabolites
2.2.8.1 Biochemical aspects
For pathways of metabolism of allyl alcohol see Biochemical
Aspects section of allyl esters.
Test system Test object Concentration Result Reference
ALLYL HEXANOATE
Ames test1 S. typhimurium 0-3.5mg/plate - Wild et al., 1983
TA98, TA100,
TA1535, TA1537,
TA1538
Ames test1 S. typhimurium 10.5 µg/plate - Oda et al., 1978
TA98, TA100
Rec assay B. subtilis 0-18 µg/disc + Oda et al., 1978
H17 vs M45
Rec assay B. subtilis 0-20µg/disc - Yoo, 1986
H17 vs M45
Basic test Drosophila 0.5mM in feed - Wild et al., 1983
(sex linked melanogaster
recessive)
Micronucleus Mouse 2 x 156 mg/kg - Wild et al., 1983
test bw i.p.
ALLYL ISOVALERATE
Ames test1,2 S. typhimurium 0 - 1000 µg/ - NTP, 1983;
TA98, TA100 plate Mortelmans et al.,
TA1535, TA1537 1986
Basic test Drosophila 1200 - 2000 - Woodruff et al.,
melanogaster mg/l in feed 1985
4500mg/l injected
injected
(contd)
Test system Test object Concentration Result Reference
ALLYL HEPTANOATE
No mutagenicity data were available for allyl heptanoate
1 Both with and without rat liver S9 fraction
2 Using a preincubation protocol
2.2.8.2 Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse male oral 96(84-110) Dunlap et al., 1958
i.p. 60
Rat ? oral 64(56-74) Smyth et al., 1951
Rat both oral 70(63-79) Taylor et al., 1964
Rat male oral 105(79-140)* Dunlap et al., 1958
99(75-130)**
i.p. 42(32-55)
Rabbit male oral 71(42-125) Dunlap et al., 1958
percut. 89(40-250)
* Rats 111-143 g b.w.
** Rats 170-252 g b.w.
2.2.8.3 Short-term studies
Following acute or short-term exposure to allyl alcohol, the
main target organ is the liver in which typical periportal changes
are observed, ranging from fatty changes to cell necrosis (Piazza,
1915; Dunlap & Hine, 1955; Dunlap et al., 1958; Torkelson et
al., 1959; Rees & Tarlow, 1967; Serafini-Cessi, 1972). The
kidney may also be affected, changes reported include necrosis of
the epithelium of the convoluted tubules and proliferation of
interstitial tissue.
Rat
Groups of 10 rats (strain and sex not specified) received allyl
alcohol in the drinking water at doses of 1.3-1.97 mg/kg b.w. The
top dose level was associated with reduced appetite and increased
mortality. The highest no effect level was 4 mg/kg b.w. The
corresponding no effect level for acrolein was 0.17 mg/kg b.w.
(Smyth et al., 1951).
Groups of six male and six female rats received allyl alcohol
in drinking water at concentrations of 0, 1, 5, 50, 100, 250, 500 or
1000 mg/L for 13 weeks, corresponding to daily intakes of 0.13,
0.62, 5.9, 11.6, 25.5, 41.0 or 72 mg/kg b.w. for males and 0.17,
0.94, 7.34, 13.2, 34.0, 43.7 or 67.4 mg/kg b.w. for females in the
respective dose groups. At termination, histological examination
(12 organs) was performed on half of the animals in each group.
Few gross abnormalities were seen at autopsy; peritoneal fat was
decreased in the 500 mg/L group and absent at 1000 mg/L. The no
effect level reported from this study was approx. 12 mg/kg b.w./day
while at an average 29 mg/kg b.w./day the only noticeable effect was
an increased liver weight in males and kidney weight in females
(Dunlap et al., 1958).
Male Wistar albino rats were given daily intragastric doses of
allyl alcohol of 0 or 30 mg/kg b.w. in corn oil for periods of 1, 10
or 28 days. The administration of a single dose produced marked
periportal necrosis and associated losses of alcohol dehydrogenase
and succinate dehydrogenase activities; hepatic cytochrome P450
concentrations and benzo[a]pyrene hydroxylase activities were
reduced to about 60% of control values. Conversely, further daily
dosing for 10 or 28 days led to a recovery both of histological
appearance and of enzyme activities. It was concluded that
metabolism of allyl alcohol becomes modified by repeated treatment
(Lake et al., 1975). Similarly, no hepatic injury was observed
following 28 daily oral doses of 25 mg/kg b.w., although direct
infusion of acrolein caused typical necrotic changes (Butterworth
et al., 1978).
Groups of 15 male and 15 female Wistar rats were given allyl
alcohol in drinking water at concentrations of 0, 50, 100, 200 or
800 mg/l for 15 weeks. No treatment-related effects were observed
in results of haematological examinations or analysis of serum.
There was a dose-related decrease in fluid intake at all treatment
levels in both sexes while growth and food consumption were reduced
in both sexes at 800 mg/l and males given 200 or 800 mg/L produced
less urine than controls in a period following water deprivation or
water loading. Increased relative weights of liver, spleen and
kidney were observed at both sexes at the top dose level; relative
kidney weights were also higher in the 200 mg/l group and in females
given 100 mg/l. No effects due to treatment were seen at autopsy
or histologically. The no observed adverse effect level was 50
mg/l, equal to 4.8-6.2 mg allyl alcohol/kg b.w./day (Carpanini et
al., 1978).
Although the effects of allyl alcohol are almost exclusively
observed in the liver, changes in the pancreas described as
acidophilia, vacuolation and necrosis of pancreatic acinar cells
were reported following oral administration of a single dose of 50
mg/kg b.w. (Nizze et al., 1979).
2.2.8.4 Special studies on genotoxicity
The genotoxicity of allyl alcohol, acrolein and glycidol are
shown in Table 3.
Test system Test object Compound & Result Reference
concentration
Ames test1 S. typhimurium Allyl alcohol Positive2 Lutz et al., 1982
(liquid TA100 Acrolein Positive
suspension) Glycidol Weak
positive2
Ames test1 S. typhimurium Allyl alcohol Negative Principe et al.,
TA98, TA100, 0.025-0.1 1981
TA1535, TA1537, µl/plate
TA1538
Forward S. coelicolor Allyl alcohol Negative Principe et al.,
mutation 2-100µl/plate 1981
8-azaguanine Aspergillus Allyl alcohol Negative Principe et al.,
resistance nidulans 10-40 µl/plate 1981
(point
mutation)
Ames test1 S. typhimurium Allyl alcohol Negative Bignami et al.,
TA98, TA100, Acrolein Positive 1977
TA1535, TA1538 (TA1538,
TA98)
Ames test1 S. typhimurium Acrolein Negative Sasaki & Endo,
TA98, TA100 TA98, TA100 1978
1 With and without rat liver S9-fraction
2 Lower in the presence of S9-fraction
2.2.8.5 Special studies on mechanisms of liver injury by metabolites
The extent of damage to the liver by allyl alcohol was
increased by the aldehyde dehydrogenase inhibitors disulfiram or
cyanamide (Rikans, 1987; Jaeschke et al., 1987) or by
phenobarbital induction, and was moderated by the alcohol
dehydrogenase inhibitor, pyrazole (Diluzio & Hoffman, 1973). It was
concluded that the toxicity is due to the formation of acrolein from
allyl alcohol (Reid, 1972; Serafini-Cessi, 1972; Patel et al.,
1980; Rikans, 1987). In agreement with this conclusion, co-
administration of ethanol (3 g/kg b.w.) to rats inhibited the rate
of allyl alcohol oxidation by more than 90% and the histological
changes were completely prevented, despite glutathione levels being
depressed (Penttila et al., 1987). As indicated by the results
with disulfiram and cyanamide, oxidation of acrolein by aldehyde
dehydrogenase is an important detoxication step for allyl alcohol-
derived acrolein (Rikans, 1987; Jaeschke et al., 1987).
In mice, allyl alcohol at a dose of 1 mmole/kg b.w. almost
totally depleted hepatic glutathione with subsequent massive lipid
peroxidation while enhanced glutathione levels protected against
hepatotoxicity of allyl alcohol (Jaeschke et al., 1987). In
vitro, allyl alcohol, acrolein and glycidol react with glutathione
by a non-enzymic mechanism (Dore & Montaldo, 1984).
The severity of liver damage 24h after i.p. administration of
allyl alcohol (0.036 µl/kg b.w.) was evaluated in male rats at 4-5,
14-15 or 24-25 months of age. Allyl hepatotoxicity increased as a
function of age but hepatic glutathione levels were unaffected
indicating that the age-related susceptibility was not due to
diminished availability of glutathione (Rikans & Kosanke, 1984).
The location of allyl alcohol or acrolein-induced hepatic
injury is usually periportal but centrilobular necrosis was induced
by using retrograde infusion (Belinsky et al., 1983) and
metabolism of allyl alcohol by alcohol dehydrogenase occurred at
similar rates in both periportal and centrilobular regions. It was
suggested that periportal necrosis seen after oral dosing is due to
greater sensitivity of the mitochondrial respiratory chain to the
toxic effects of acrolein in periportal cells.
2.2.8.6 In vitro studies on metabolite damage to kidney cells
In freshly isolated renal epithelial cells from rats, allyl
alcohol toxicity as assessed by glutathione depletion and loss of
cell viability was more severe in cells from female animals. This
correlated with higher alcohol dehydrogenase activity (Ohno et al.,
1985).
3. COMMENTS
In evaluating these flavours, the Committee noted that they are
rapidly hydrolysed to allyl alcohol and the corresponding acids by
intestinal mucosal, pancreatic and hepatic esterases. The results
of studies on the toxicity of the three esters indicated that the
hepatotoxicity observed at high doses was due to the allyl alcohol
and its metabolites. Accordingly the Committee considered
supplementary toxicological data on allyl alcohol and concluded that
the three esters should be evaluated for a group ADI on the basis of
the allyl alcohol moiety. In its evaluation, the Committee also
took account of the principles relating to food flavours outlined in
Principles for the safety assessment of food additives and
contaminants in food (Annex I, ref. 76).
The hepatotoxicity of the esters and of allyl alcohol was less
marked in repeated-dose short-term studies than in single-dose acute
studies, although the mechanism of the acquired tolerance has not
been fully elucidated. Mutagenicity studies on allyl hexanoate and
allyl isovalerate yielded negative results, while most tests on
allyl alcohol were negative.
The Committee reviewed two long-term carcinogenicity studies in
rats and mice in which allyl isovalerate was administered by gavage
in corn oil at both the maximum tolerated dose and 50% of this dose.
Epithelial hyperplasia and squamous-cell papillomas of the
forestomach were observed in mice, but not rats, at the highest
dose. There was no evidence of hepatic tumours in mice (the liver
being the target organ for short-term toxicity). The Committee
concluded that these results were not relevant to the low-dose,
dietary exposure to allyl isovalerate as a food flavour but were
probably due to the effects of the large bolus doses that were used.
The Committee also noted the small increase in the incidence of
leukaemia reported in the treated rats; however, the incidence was
within the historical control range and no increase in the incidence
of hepatic tumours occurred in rats. Since levels of dietary
exposure to allyl isovalerate in food are much lower than the doses
used in these studies, the Committee concluded that an ADI could be
set.
The evaluation was based on the no-observed-effect-level in
short-term studies on allyl alcohol, with particular reference to
hepatotoxicity; this provides a more conservative estimate than one
based on the no-observed-effect level for the esters. The Committee
noted that a number of other food flavours in use which are allyl
esters and should be considered for inclusion in the group ADI on
the basis of their hydrolysis to allyl alcohol. In addition, in
view of evidence that allyl esters of such fatty acids as acetate,
propionate, isobutyrate, and 2-ethylhexanoate are also rapidly
hydrolysed, the Committee considered that their consumption should
be taken into account since they could contribute to the total
dietary load of allyl alcohol.
4. EVALUATION
The Committee allocated an ADI of 0-0.05 mg/kg b.w. as allyl
alcohol equivalent for allyl heptanoate, allyl hexanoate, and allyl
isovalerate, which corresponds to 0-0.15 mg/kg b.w. allyl
heptanoate, 0-0.13 mg/kg b.w. allyl hexanoate, 0-0.12 mg/kg b.w.
allyl isovalerate, or combinations of these pro rata.
5. REFERENCES
BAR, von F. & GRIEPENTROG, F. (1967) Die situation in der
gesundheitlichen Beurteilung der Aromatisierungsmittel für
Lebensmittel. Medizin und Ernahrung, 8, 244-251.
BELINSKY, S.A., MATSUMURA, T., KAUFFMAN, F.C. & THURMAN, R.G. (1984)
Rates of allyl alcohol metabolism in periportal and pericentral
regions of the liver lobule. Mol. Pharmacol., 25, 158-164.
BELINSKY, S.A., BRADFORD, B.U., FORMAN, D.T., GLASSMAN, E.B.,
FELDER, M.R. & THURMAN, R.G. (1985) Hepatotoxicity due to allyl
alcohol in deermice depends on alcohol dehydrogenase. Hepatology,
5, 1179-1182.
BUTTERWORTH, K.R., CARPANINI, F.M.B., DUNNINGTON, R., GRASSO, P. &
PELLING, R. (1978) The production of periportal necrosis by allyl
alcohol in the rat. Proc. Brit. Pharm. Soc., 57, 353P-354P.
BUTTERWORTH, K.R., CARPANINI, F.M.B., GAUNT, I.F., GRASSO, P. &
LLOYD, A.G. (1975) A new approach to the evaluation of the safety
of flavouring esters. Brit. J. Pharm., 54, 268P.
CARPANINI, F.M.B., GAUNT, I.F., HARDY, J., GANGOLLI, S.D.,
BUTTERWORTH, K.R. & LLOYD, A.G. (1978) Short-term toxicity of
allyl alcohol in rats. Toxicology, 9, 29-45.
CLAPP, J.J., KAYE, C.M. & YOUNG, L. (1969) Observations on the
metabolism of allyl compounds in the rat. Biochem. J., 114, 6P.
CLODE, S.A., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P. & GANGOLLI,
S.D. (1978) Short-term toxicity study of allyl caproate in rats.
Fd. Cosmet. Toxicol., 16, 197-201.
DAMSKE, D.R., MECLER, F.J., BELILES, R.P. & WEIR, R.J. (1980) 90-
day toxicity study in rats: allyl heptanoate. Unpublished report
of Litton Bionetics Inc., LBI project No. 21130-01 & -04. Submitted
to WHO by FEMA.
DILUZIO, N.R. & HOFFMAN, E.O. (1973). Protective influence of
pyrazole on allyl formate induced injury. Gastroenterol., 64,
158.
DORE, M. & MONTALDO, C. (1984) Studi sulla coniugazione in virto
dell'alcool allilico e dei suoi metaboliti con il glutatione
ridotto. Boll. Soc. It. Biol. Sper., 60, 1497-1501.
DUNLAP, M.K. & HINE, C.H. (1955) Toxicity of allyl alcohol. Fed.
Proc., 14, 335.
DUNLAP, M.K., KODAMA, J.K., WELLINGTON, J.S., ANDERSON, H.H. & HINE,
C.H. (1958) The toxicity of allyl alcohol I. Acute and chronic
toxicity. Arch. Ind. Hlth., 18, 303-311.
EPSTEIN, W.L. (1976) Unpublished report to RIFM dated 20th December
1976. Submitted to WHO by FEMA.
GRUNDSCHOBER, F. (1977) Toxicological assessment of flavouring
esters. Toxicology, 8, 387-390.
HAGAN, E.C., JENNER, P.M., JONES, W.I., FITZHUGH, O.G., LONG, E.L.,
BROUWER, J.G. & WEBB, W.K. (1965) Toxic properties of compounds
related to safrole. Toxicol. appl. Pharmacol., 7, 18-24.
HAGAN, E.C., HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES,
W.I., TAYLOR, J.M., LONG, E.L., NELSON, A.A. & BROUWER, J.B. (1967)
Food flavourings and compounds of related structure. II. Subacute
and chronic toxicity. Fd. Cosmet. Toxicol., 5, 141-157.
HONG, H.L., HUFF, J.E., LUSTER, M.I., MARONPOT, R.T., DIETER, M.P.,
HAYES, H.T. & BOORMAN, G.A. (1988) The effects of allyl
isovalerate on the haematopoietic and immunologic systems in
rodents. Fund. Appl. Toxicol., 10, 655-663.
IARC (1985) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans. Vol.36, Allyl compounds, aldehydes,
epoxides and peroxides. International Agency for Research on Cancer;
Lyon, pp. 69-74.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987), The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36, 51-57.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L. & FITZHUGH, O.G.
(1964) Food flavourings and compounds of related structure I. Acute
oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
KAYE, C.M. & YOUNG, L. (1970) Mercapturic acid formation from allyl
compounds in the rat. Biochem. J., 119, 53P.
KAYE, C.M. & YOUNG, L. (1972) The synthesis of mercapturic acids
from allyl compounds in the rat. Biochem. J., 127, 87P.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134, 1093-1101.
KLIGMAN, A.M. (1971) Unpublished report dated 27th September, 1971
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975a) Unpublished report dated 14th February, 1975
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975b) Unpublished report dated 10th June, 1975 to
RIFM. Submitted to WHO by FEMA.
LAKE,, B.G., GANGOLLI, S.D., WRIGHT, M.G., GRASSO, P., CARPANINI,
F.M.B. & BUTTERWORTH, K.R. (1975) The effect of repeated
administration on allyl alcohol-induced hepatotoxicity in the rat.
Biochem. Soc. Trans., 6, 145--146.
LUTZ, D., EDER, E., NEUDECKER, T. & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha, ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93, 305-315.
LONGLAND, R.C., SHILLING, W.H. & GANGOLLI, S.D. (1977) The
hydrolysis of flavouring esters by artificial gastrointestinal
juices and rat tissue preparations. Toxicology, 8, 197-204.
MEISEL, M.L. (1982) Caproate allyl, Ro 81-3538/000: An acute oral
toxicity study (LD50) in the rat. Unpublished report No. 100-
161/106 prepared by Hazleton Laboratories Deutschland GmbH.
Submitted to WHO by FEMA.
MORENO, O.M. (1974a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-676. Submitted to WHO
by FEMA.
MORENO, O.M. (1974b) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-677. Submitted to WHO
by FEMA.
MORENO, O.M. (1977a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 76-1446. Submitted to
WHO by FEMA.
MORENO, O.M. (1977b) Unpublished report to RIFM dated 27th
January, 1977. Submitted to WHO by FEMA.
MORTELMANS, K., HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986) Salmonella Mutagenicity tests: II. Results
from the testing of 270 chemicals. Environ. Mutagen., 8, Supp.7,
1-119.
NATIONAL TOXICOLOGY PROGRAM (NTP) (1983) Carcinogenesis studies of
allyl isovalerate. Report No. NTP-TR-253; PB-83-2509.
NIZZE, E., LAPIS, K. & KOVACS, L. (1979) Allyl alcohol-induced
changes in the rat exocrine pancreas. Digestion, 19, 359-369.
ODA, Y., HAMONO, Y., INOUE, K., YAMAMOTO, H., NIIHARA, T. & KUNITA,
N. (1978) Mutagenicity of food flavours in bacteria (1st report).
Shouhin Eisei Hen, 9, 177-181.
OHNO, Y., JONES, T.W. & ORMSTAD, K. (1985) Allyl alcohol toxicity
in isolated renal epithelial cells: protective effects of low
molecular weight thiols. Chem. biol. Interactions, 52, 289-299.
PATEL, J.M., WOOD, J.C. & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparation. Drug Metab. Disposition, 8, 305-308.
PENTTILÄ, K.E., MÄKINEN, J. & LINDROS, K.O. (1987) Allyl alcohol
liver injury: suppression by ethanol and relation to transient
glutathione depletion. Pharmacol. Toxicol., 60, 340-344.
PIAZZA, J.G. (1915) Zur kehntnis der Wirkung der Allylverbindungen
Z. Exp. Path. Ther., 17, 318.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE, E.,
FABRIXI, M., CONTI, G. & COMBA, P. (1981) Mutagenicity of
compounds of industrial and agricultural relevance in Salmonella,
Streptomyces and Aspergillus. J. Sci. Fd. Agric. 32, 826-832.
REES, K.R. & TARLOW, M.J. (1967) The hepatotoxic action of allyl
formate. Biochem. J., 104, 757-761.
REID, W.D. (1972) Mechanism of allyl alcohol-induced hepatic
necrosis. Experientia, 28, 1058-1061.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver
aldehyde dehydrogenases: Relation to allyl alcohol hepatotoxicity.
Drug Metab. Disp., 15, 356-362.
RIKANS, L.E. & KOSANKE, S.D. (1984) Effect of aging on liver
glutathione levels and hepatocellular injury from carbon
tetrachloride, allyl alcohol or galactosamine. Drug and Chemical
Toxicology, 7, 595-604
SERAFINI-CESSI, F. (1972) Conversion of allyl alcohol into
acrolein by rat liver. Biochem. J., 128, 1103-1107.
SHELANSKI, M.V. & MOLDOVAN, M. (1971) Unpublished report of Food
and Drug Research Laboratories Inc. to RIFM. Submitted to WHO by
FEMA.
SMYTH, H.F., CARPENTER, C.P. & WEIL, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-
122.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964) A comparison of
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 6, 378-387.
TORKELSON, T.R., WOLF, M.A., OYEN, F. & ROWE, V.K. (1959) Vapor
toxicity of allyl alcohol as determined on laboratory animals. J.
Am. Ind. Hyg. Assoc., 20, 224-229.
WILD, D., KING, M.T., GOCKE, E. & ECKHARDT, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, Basc and micronucleus tests. Fd. Chem.
Toxicol., 21, 707-719.
YOO, Y.S. (1986) Mutagenic and antimutagenic activities of
flavouring agents used in foodstuffs. J. Osaka City Med. Center,
34, 267-288.222.
2.2 Toxicological studies
2.2.1 Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Allyl hexanoate
Rat both oral 218 (186-255) Jenner et al.,
1964
Taylor et al.,
1964
both oral 327 (277-386) Meisel, 1982
male oral 393
female oral 276 (215-352)
Guinea both oral 280 (246-319) Jenner et al.,
pig 1964
Rabbit ? dermal 300 (200-600) Shelanski &
Moldovan,
1971
? dermal 820 (700-940) Moreno, 1974
Allyl heptanoate
Mouse both oral 630 (514-772) Jenner et al.,
1964
Rat both oral 500 (392-638) Jenner et al.,
1964
Rabbit ? dermal 810 (440-1180) Moreno, 1974a
Guinea both oral 444 (363-541) Jenner et al.,
pig 1964
Allyl isovalerate
Mouse both oral >500 NTP, 1983
Rat both oral >250 <500 NTP, 1983
? oral 230 (216-290) Moreno, 1977
Rabbit ? dermal 560 (290-1060) Moreno, 1977
2.2.2 Short-term studies
2.2.2.1 Mouse - allyl isovalerate
Groups of 5 male and 5 female B6C3F1 mice were given allyl
isovalerate by gavage in corn oil for 14 consecutive days at daily
doses of 0, 31, 62, 125, 250 or 500 mg/kg b.w. All animals of both
sexes that received 500 mg/kg b.w. died within 48 h of commencement
of the study; all other animals survived to termination.
Inactivity and piloerection were seen in animals receiving 250 and
500 mg/kg b.w. and male mice receiving 250 mg/kg gained no weight;
body weights in other dose groups were comparable to controls at
termination of the study (NTP, 1983).
Groups of 10 male and 10 female B6C3F1 mice, 6 weeks of age at
commencement of the study were dosed with allyl isovalerate by
gavage in corn oil at doses of 0, 15, 31, 62, 125 or 250 mg/kg b.w.
(five day/week) for 13 weeks. At the end of the study, autopsies
were performed on all survivors. Animals from control and highest
dose groups were subjected to detailed histological examination.
In addition the liver was examined histologically for the 62 and 125
mg/kg b.w. groups and in the latter group stomachs were also
examined microscopically. Five out of 10 males and six of 10
females in the highest dose groups died, all but one of the deaths
(a female) being compound related; deaths in other groups were
caused by gavage error. Compound related effects noted at necropsy
or histologically were limited to the 125 and 250 mg/kg b.w. dose
groups and included "thickening" of the urinary bladder wall and
gastric mucosa and small intestine (both groups) ulcerative
inflammation of the stomach, coagulative necrosis of the liver and
cytoplasmic vacuolation of the liver (top dose group only). No
compound related effects were seen in the liver, stomach or bladder
of mice from other groups (NTP, 1983).
2.2.2.2 Rat - allyl heptanoate
Allyl heptanoate was fed to weanling Osborne-Mendel rats of
both sexes for 18 weeks at dietary levels of 0, 1000, 2500 and
10,000 mg/kg diet. There was a dose-related growth depression which
was severe and associated with a poor food efficiency at the highest
dose level only. Gross liver enlargement was observed at all dose
levels; in addition, at the highest dose level kidneys were enlarged
in both sexes and hearts were enlarged in males only. Males in the
2500 and 10 000 mg/kg diet groups were reported to have enlarged
testes (it is not clear from the report whether this "enlargement"
relates to absolute or relative organ weights; if the latter, the
increased relative organ weights may relate to the growth depression
rather than to organ specific effects). Microscopic changes
reported included hydropic degeneration in the periportal areas of
the liver ranging from moderate at the highest dose level to lesser
degrees at lower dose levels. The extent of bile duct proliferation
correlated with the degree of hydropic degeneration and hepatocyte
enlargement also was seen in some groups (Hagan et al., 1965).
Groups of weanling Charles River albino rats of both sexes were
given allyl heptanoate in the diet daily at doses of 0, 49.6, 157
and 496 mg/kg b.w./day for 13 weeks. Weight gain and food intake
were recorded weekly, and urinalysis, haematological and clinical
chemical examinations were carried out at weeks 6 and 12. At
termination, autopsies were performed on all animals and detailed
histological examinations were carried out on all rats from the top
dose group and on half of the control animals. In the rest of the
controls and the other two dose groups, histological examination was
limited to liver and kidney, and to tissues showing gross
abnormalities at autopsy. There was a reduced food intake in the
treated groups, which was statistically significant in the high and
mid-dose groups, and an associated deficit in body weight gain. At
week 6 there was a small but significant depression in leucocyte
count in males of the top dose group only; no such effect was seen
in females and other haematological parameters were normal in
animals of both sexes. At week 12, no significant haematological
differences were seen other than a small non-dose-related decrease
in leucocyte count in the males of the 49.6 mg/kg dose group.
Clinical chemical examinations at weeks 6 and 12 revealed a decrease
in some parameters such as blood glucose, total serum protein and
albumin which appeared to be due to the reduced food intake and not
to specific toxic effects. Urine composition was unaffected by
treatment. At necropsy no gross nor microscopic lesions related to
treatment were observed and elevated relative organ weights in high-
dose males were related to reduced food intake and body weight gain.
It was concluded that daily treatment with allyl heptanoate in this
study did not result in any signs of toxicity; the reasons for the
reduced food intake could not be determined but might have been due
to unpalatability of diet (Damske et al., 1980).
Allyl hexanoate
Groups of 10 male and 10 female Osborne-Mendel rats were given
allyl hexanoate by gavage in corn oil at dose levels of 0, 15, 65
and 100 mg/kg b.w. daily for 18 weeks. Weight gain, food intake and
general condition were recorded weekly. At termination, the
animals were exsanguinated and haematological and gross pathological
examinations were performed. Detailed histological examinations
were performed only on eight rats from the control and high dose
groups; based on observations in the high dose group, livers from
eight animals in the 15 and 65 mg/kg b.w. dose groups were also
examined microscopically. In the high dose group, gross appearance
of the liver was described as nodular and wrinkled with granular or
rough surface. Microscopically the high dose group showed slight to
moderate bile duct proliferation, some "lobular architectural
disarrangement", slight fibrosis and pigment deposition in
macrophages; necrotic foci were seen in 2 of 8 animals examined. In
the 65 mg/kg b.w. dose group, very slight bile-duct proliferation
was observed in 2 of eight animals studied. The livers of the 15
mg/kg b.w. dose group were unaffected by treatment (Hagan et al.,
1967).
In a companion study to the foregoing, 5 rats of each sex
received allyl hexanoate in the diet at a concentration of 1000
mg/kg diet (equivalent to 50mg/kg b.w.) for 28 weeks. No adverse
effects were observed (Hagan et al., 1967).
Groups of 15 male and 15 female Wistar rats were given daily
oral doses of 0, 35 or 100 mg allyl hexanoate/kg b.w. as a solution
in corn oil; a further group of 10 animals of each sex were
similarly dosed with 12 mg/kg b.w./day for 13 weeks. Food and water
intake and body weights were recorded weekly, urinalysis was
performed during week 2, week 5 or 6, and in the final week of
treatment, and renal function tests were also carried out. At
termination, autopsies were performed and organ weights recorded,
and detailed histopathological examinations were conducted.
No differences were noted between treated and control animals
in body weight, water intake, haematological parameters, serum
chemistry, urine composition or in renal concentration tests. There
were slight increases in food intake in the highest dose group.
Liver weights were increased in the 35 and 100 mg/kg b.w. dose
groups and all treated animals showed evidence of periportal
vacuolation which was dose related in incidence and severity and
which in the 100 mg/kg b.w. group was accompanied by enlarged
hepatocytes, focal periportal necrosis and bile-duct proliferation.
Weights of spleen, kidneys, stomach and small intestine were
increased in both sexes in the highest dose group and small
intestine weight was also increased in females of the 35 mg/kg b.w.
group. It was not possible to determine a no observed adverse
effect level for allyl hexanoate in this study (Clode et al.,
1978).
When allyl hexanoate was administered to rats by gavage at a
daily dose level of 15 mg/kg b.w. for 18 weeks there were no
observed adverse effects (this study was reported in summary only)
(Bär & Griepentrog, 1967).
Allyl isovalerate
In similar studies to those described for mice, groups of 5
male and 5 female Fischer F344/N rats were given allyl isovalerate
by gavage for 14 days and groups of 10 animals of each sex were
dosed (5 day/week) for 13 weeks at levels of 0, 31, 62, 125, 250 and
500 mg/kg bw. In the fourteen days study, all rats given 500 mg/kg
b.w. and two rats of each sex given 250 mg/kg b.w. died. At
termination, mean body weights in the 250 mg/kg b.w. group were
depressed relative to weights in the control group by 23% and 13% in
males and females, respectively. Inactivity, laboured breathing,
diarrhoea and piloerection were observed in both sexes in the two
highest dose groups and in necropsy gross dark red areas were seen
in the stomachs of three animals of each sex at the top dose level.
In the thirteen week study, all ten males and 4/10 females that
received 250 mg/kg b.w. died and body weight gain was significantly
depressed in males of the 125 mg/kg b.w. group and females of the
250 mg/kg group. Dose-related effects seen at necropsy were
thickening of the intestinal wall and redness of the mucosal
surfaces of the intestines and urinary bladder. Enlargement of
internal lymph nodes and adrenals was reported but was unaccompanied
by histological lesions. Histopathological examination revealed
multifocal coagulative necrosis, cholangiofibrosis, and bile duct
hyperplasia at the 125 and 250 mg/kg b.w. dose levels. The effects
were dose related in incidence and no such lesions were observed in
the 31 and 62 mg/kg b.w. dose groups (NTP, 1983).
Combinations of esters
Groups of 10 male rats were given by gavage 21 consecutive
daily doses of allyl alcohol and a series of allyl esters (acetate,
propionate, hexanoate, isobutyrate, isovalerate and 2-
ethylhexanoate) at equimolecular doses corresponding to 5, 25 or 60
mg/kg b.w. of allyl alcohol. After 21 days the animals were
sacrificed and the livers examined histologically. The severity of
the liver lesions was classified according to the scheme: periportal
cell enlargement, followed by necrosis and subsequent fibrosis with
bile duct hyperplasia. The severity of the hepatic lesions from the
straight chain esters was similar to that produced by the
corresponding dose of allyl alcohol and more marked than that
produced by the esters of the branched chain acids. The differences
were attributed to differences in the rate of hydrolysis since the
straight chain esters were hydrolysed in vitro approximately 100
times faster than the branched chain esters (Butterworth et al.,
1975).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mouse - allyl isovalerate
Groups of 50 male and 50 female B6C3F1 mice, initially 50 days
old, received allyl isovalerate at doses of 0, 31 or 62 mg/kg b.w.
by gavage in corn oil (10 ml/kg b.w.) five times per week for 103
weeks. Survivors were killed at 112-114 weeks of age. No
significant differences were observed in survival rates in males;
reduced mean body weight gain and significantly lower survival rate
in the lower dose group females was attributed to a high incidence
of a genital tract infection.
An increase in the incidence of epithelial hyperplasia and
squamous cell papillomas was observed in the non-glandular
forestomach in male mice; the observed incidences for hyperplasia
were 1/50, 1/50 and 7/48, and for papillomas 0/50, 1/50 and 3/48 in
the control, low and high dose groups respectively. In females, the
corresponding incidences for forestomach epithelial hyperplasia were
0/50, 2/50 and 3/50, and for squamous cell papillomas were 1/50,
0/50 and 2/50 respectively. The incidence of lymphomas was slightly
elevated in males (5/50, 6/50 and 8/50 in the respective dose
groups) but the increase was not significant by the trend test nor
by the incidental tumour test; in females the corresponding
incidences were 11/50, 11/50 and 18/50 which gave a dose-response
trend, the high dose tumour incidence being significantly higher
(P<0.05) than controls. Significant reductions in tumour incidence
were observed in treated male mice in respect of hepatocellular
carcinomas (18/50, 6/50, 9/50), alveolar/bronchiolar adenomas or
carcinomas (13/50, 6/50 5/49) and for thyroid follicular cell
adenomas (5/47, 0/46, 1/49). No treatment-related non-neoplastic
lesions were observed in mice of either sex (NTP, 1983).
2.2.3.2 Rat - allyl hexanoate
A group of 5 male and 5 female Osborne-Mendel rats were given
allyl hexanoate in the diet at a concentration of 2500 mg/kg
(equivalent to 125 mg/kg b.w.) for 1 year. Body weight and food
intake were recorded weekly and haematological examinations were
carried out at 3, 6, and 12 months. At termination, detailed
histological examinations were performed. No adverse effects were
reported, in contrast with the short-term (18 week) study where
minimal effects (very slight bile duct proliferation) were reported
at a dose level of 65 mg/kg b.w. (Hagan et al., 1967).
When allyl hexanoate was fed to rats at a dietary concentration
of 0.5% for 1.5 years, 2/25 animals developed multiple bile duct
adenomas and proliferative changes of the small bile ducts. An
additional animal was reported to have adenomas (location not
specified) after 8.5 months. The authors concluded that the small
number of animals and tumour incidence were insufficient to allow a
firm conclusion to be reached on the significance (Bär &
Griepentrog, 1967) (This report was in summary only).
Allyl isovalerate
Groups of 50 male and 50 female Fischer 344/N rats, initially
46 days old, were given allyl isovalerate at doses of 0, 31 or 62
mg/kg b.w. by gavage in corn oil (5 ml/kg b.w.) five times per week
for 103 weeks. Survivors were killed at 112-114 weeks of age when
for males the numbers of survivors were 34 controls, 30 low dose and
28 high dose; the corresponding numbers of female survivors were
38, 36 and 29 respectively. There was a dose related increase in
mononuclear-cell leukaemia, the incidences observed were 1/50, 4/50
and 7/50 in males of the control, low- and high dose groups; in
females the corresponding incidences were 4/50, 6/50 and 9/49. In
both sexes there was a significant dose-response trend (p<0.05),
while the incidence in high-dose males was significantly higher than
controls (p<0.05). Increased frequencies of non-neoplastic lesions
(cholangiofibrosis, nodular regeneration, cirrhosis, focal
periportal necrosis, fatty changes and cytoplasmic vacuolation) were
observed in the livers of animals of both sexes in the high dose
group but there was no increase in liver neoplasms (NTP, 1983).
The authors of the NTP report concluded that allyl isovalerate
was carcinogenic, causing increased incidence of haematopoietic
system neoplasms (mononuclear cell leukaemias in male rats and
lymphoma in female mice). In reviewing this and other relevant
biological data, the International Agency for Research on Cancer
concluded that there was limited evidence for the carcinogenicity of
allyl isovalerate to experimental animals (IARC, 1985).
2.2.3.3 Dog - allyl heptanoate
Four groups of 3 male and 3 female beagle dogs were given daily
doses of allyl heptanoate of 0, 5, 25 and 75 mg/kg b.w. by capsule
for up to 18 months. All the dogs in the top dose group died within
3 - 7 months; dogs in the two lower treatment groups were reported
as surviving after 18 months. Administration of 75 mg/kg b.w.
caused depressed growth and macroscopic changes in the appearance of
the liver and haemorrhagic changes in the gastric mucosa. Less
consistent changes were reported in the form of cysts in the urinary
bladder and congestion in the lungs, digestive tract, kidneys,
spleen and lymph nodes. Microscopically the livers showed a slight
to moderate periportal fibrosis associated with slight to moderate
proliferation of the bile duct epithelium. Slight fatty changes
were also observed. The stomachs showed diffuse haemorrhage and
necrosis of the mucosae with instances of focal sub-mucosal
haemorrhage (Hagan et al., 1965).
2.2.4 Special studies on skin irritation
During an acute dermal toxicity study on allyl heptanoate in
rabbits, skin irritancy was evaluated on day 1. At dermal doses of
313-1250 mg/kg slight to moderate redness and oedema were reported
(5000 mg/kg was a lethal dose) (Moreno, 1974). Similar results were
obtained with allyl heptanoate (Moreno, 1974a). Allyl isovalerate
applied undiluted to intact or abraded rabbit skin under occlusion
for 24h was moderately irritating (Moreno, 1977).
In a preliminary to a maximization test, 48 hour closed patch
tests were carried out on the forearms of 5 volunteers with allyl
hexanoate and four subjects displayed grade 1 irritation (Kligman,
1971). Conversely, in a later study in which 5 volunteers were
subjected to patch tests, no signs of irritation were observed with
allyl hexanoate nor with allyl heptanoate (Kligman, 1975; 1975a).
Similarly, allyl isovalerate was without irritant effect in a closed
patch study on the backs of 28 subjects (Epstein, 1976).
2.2.5 Special studies on contact sensitization
In a maximization test on 25 healthy volunteers, allyl
hexanoate was reported to produce 13 cases of sensitization and was
considered a moderate sensitizer (Kligman, 1975); this contrasts
with an earlier maximization test on a similar number of volunteers
in which no cases of sensitization were detected (Kligman 1971). No
sensitization was observed with allyl heptanoate using a similar
protocol (Kligman, 1975a) nor with allyl isovalerate in 28
volunteers (Epstein, 1976).
2.2.6 Special studies on the haematopoietic and immunologic systems
Following the observation, in carcinogenicity studies on allyl
isovalerate, of marginal increases in mononuclear cell leukaemia in
rats and of malignant lymphoma in mice (NTP, 1983) and in view of
the fact that isovaleric acidaemia has been associated with
pancytopenia in humans, the effects of allyl isovalerate on the
haematopoietic and immune systems of female B6C3F1 mice and Fischer
344/N rats of both sexes were investigated in a short-term (14 day)
study. The animals were dosed by gavage for 5 days per week for 2
weeks with allyl isovalerate in corn oil at dose levels of 0, 31, 62
or 125 mg/kg b.w. (rats and mice) or 250 mg/kg b.w. (rats only).
Haematological, immunological and histological studies were
performed 48-72 h after the final treatment. In addition, bone
marrow slides from female mice from the NTP 13-week study (see
short-term studies) were also examined. The body weights of rats of
both sexes were reduced at the 250mg/kg b.w. dose level and of males
at the 125 mg/kg b.w. level. No changes in the body weights of the
female mice were observed but there was a 20% increase in mean
spleen weight and the splenic follicles were large with prominent
germinal centres. No treatment-related effects were seen in
haematological parameters nor in bone-marrow cellularity in mice or
rats. However, there were significant decreases in pluripotent
haematopoietic stem cells (CFU-S) in the spleen and in granulocyte-
macrophage progenitors (CFU-GM) in the bone marrow of treated mice.
In vitro enzyme assays of these cells showed that haematopoietic
suppression was correlated with a depression of hexose monophosphate
shunt metabolism but that enzymes of the Embden-Meyerhof and
tricarboxylic acid pathways were unaffected. Examination of host
resistance in mice following challenge with Plasmodium yoelii or
Listeria monocytogenes showed no significant differences between
control and treated animals, nor were there other effects on the
immune system. The authors concluded that the myelotoxic effects
were minimal and of a degree that did not alter host resistance
(Hong et al., 1988).
Minimal to moderate hypocellularity of the bone marrow was
observed in the 125 mg/kg b.w. group of mice, both sexes, from the
13-week NTP study and was most striking for megakaryocytes. The
degree of hypocellularity was never severe.
2.2.7 Special studies on genotoxicity
The genotoxicity of allyl hexanoate and allyl isovalerate are
shown on the next page.
2.2.8 Special studies on metabolites
2.2.8.1 Biochemical aspects
For pathways of metabolism of allyl alcohol see Biochemical
Aspects section of allyl esters.
Test system Test object Concentration Result Reference
ALLYL HEXANOATE
Ames test1 S. typhimurium 0-3.5mg/plate - Wild et al., 1983
TA98, TA100,
TA1535, TA1537,
TA1538
Ames test1 S. typhimurium 10.5 µg/plate - Oda et al., 1978
TA98, TA100
Rec assay B. subtilis 0-18 µg/disc + Oda et al., 1978
H17 vs M45
Rec assay B. subtilis 0-20µg/disc - Yoo, 1986
H17 vs M45
Basic test Drosophila 0.5mM in feed - Wild et al., 1983
(sex linked melanogaster
recessive)
Micronucleus Mouse 2 x 156 mg/kg - Wild et al., 1983
test bw i.p.
ALLYL ISOVALERATE
Ames test1,2 S. typhimurium 0 - 1000 µg/ - NTP, 1983;
TA98, TA100 plate Mortelmans et al.,
TA1535, TA1537 1986
Basic test Drosophila 1200 - 2000 - Woodruff et al.,
melanogaster mg/l in feed 1985
4500mg/l injected
injected
(contd)
Test system Test object Concentration Result Reference
ALLYL HEPTANOATE
No mutagenicity data were available for allyl heptanoate
1 Both with and without rat liver S9 fraction
2 Using a preincubation protocol
2.2.8.2 Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse male oral 96(84-110) Dunlap et al., 1958
i.p. 60
Rat ? oral 64(56-74) Smyth et al., 1951
Rat both oral 70(63-79) Taylor et al., 1964
Rat male oral 105(79-140)* Dunlap et al., 1958
99(75-130)**
i.p. 42(32-55)
Rabbit male oral 71(42-125) Dunlap et al., 1958
percut. 89(40-250)
* Rats 111-143 g b.w.
** Rats 170-252 g b.w.
2.2.8.3 Short-term studies
Following acute or short-term exposure to allyl alcohol, the
main target organ is the liver in which typical periportal changes
are observed, ranging from fatty changes to cell necrosis (Piazza,
1915; Dunlap & Hine, 1955; Dunlap et al., 1958; Torkelson et
al., 1959; Rees & Tarlow, 1967; Serafini-Cessi, 1972). The
kidney may also be affected, changes reported include necrosis of
the epithelium of the convoluted tubules and proliferation of
interstitial tissue.
Rat
Groups of 10 rats (strain and sex not specified) received allyl
alcohol in the drinking water at doses of 1.3-1.97 mg/kg b.w. The
top dose level was associated with reduced appetite and increased
mortality. The highest no effect level was 4 mg/kg b.w. The
corresponding no effect level for acrolein was 0.17 mg/kg b.w.
(Smyth et al., 1951).
Groups of six male and six female rats received allyl alcohol
in drinking water at concentrations of 0, 1, 5, 50, 100, 250, 500 or
1000 mg/L for 13 weeks, corresponding to daily intakes of 0.13,
0.62, 5.9, 11.6, 25.5, 41.0 or 72 mg/kg b.w. for males and 0.17,
0.94, 7.34, 13.2, 34.0, 43.7 or 67.4 mg/kg b.w. for females in the
respective dose groups. At termination, histological examination
(12 organs) was performed on half of the animals in each group.
Few gross abnormalities were seen at autopsy; peritoneal fat was
decreased in the 500 mg/L group and absent at 1000 mg/L. The no
effect level reported from this study was approx. 12 mg/kg b.w./day
while at an average 29 mg/kg b.w./day the only noticeable effect was
an increased liver weight in males and kidney weight in females
(Dunlap et al., 1958).
Male Wistar albino rats were given daily intragastric doses of
allyl alcohol of 0 or 30 mg/kg b.w. in corn oil for periods of 1, 10
or 28 days. The administration of a single dose produced marked
periportal necrosis and associated losses of alcohol dehydrogenase
and succinate dehydrogenase activities; hepatic cytochrome P450
concentrations and benzo[a]pyrene hydroxylase activities were
reduced to about 60% of control values. Conversely, further daily
dosing for 10 or 28 days led to a recovery both of histological
appearance and of enzyme activities. It was concluded that
metabolism of allyl alcohol becomes modified by repeated treatment
(Lake et al., 1975). Similarly, no hepatic injury was observed
following 28 daily oral doses of 25 mg/kg b.w., although direct
infusion of acrolein caused typical necrotic changes (Butterworth
et al., 1978).
Groups of 15 male and 15 female Wistar rats were given allyl
alcohol in drinking water at concentrations of 0, 50, 100, 200 or
800 mg/l for 15 weeks. No treatment-related effects were observed
in results of haematological examinations or analysis of serum.
There was a dose-related decrease in fluid intake at all treatment
levels in both sexes while growth and food consumption were reduced
in both sexes at 800 mg/l and males given 200 or 800 mg/L produced
less urine than controls in a period following water deprivation or
water loading. Increased relative weights of liver, spleen and
kidney were observed at both sexes at the top dose level; relative
kidney weights were also higher in the 200 mg/l group and in females
given 100 mg/l. No effects due to treatment were seen at autopsy
or histologically. The no observed adverse effect level was 50
mg/l, equal to 4.8-6.2 mg allyl alcohol/kg b.w./day (Carpanini et
al., 1978).
Although the effects of allyl alcohol are almost exclusively
observed in the liver, changes in the pancreas described as
acidophilia, vacuolation and necrosis of pancreatic acinar cells
were reported following oral administration of a single dose of 50
mg/kg b.w. (Nizze et al., 1979).
2.2.8.4 Special studies on genotoxicity
The genotoxicity of allyl alcohol, acrolein and glycidol are
shown in Table 3.
Test system Test object Compound & Result Reference
concentration
Ames test1 S. typhimurium Allyl alcohol Positive2 Lutz et al., 1982
(liquid TA100 Acrolein Positive
suspension) Glycidol Weak
positive2
Ames test1 S. typhimurium Allyl alcohol Negative Principe et al.,
TA98, TA100, 0.025-0.1 1981
TA1535, TA1537, µl/plate
TA1538
Forward S. coelicolor Allyl alcohol Negative Principe et al.,
mutation 2-100µl/plate 1981
8-azaguanine Aspergillus Allyl alcohol Negative Principe et al.,
resistance nidulans 10-40 µl/plate 1981
(point
mutation)
Ames test1 S. typhimurium Allyl alcohol Negative Bignami et al.,
TA98, TA100, Acrolein Positive 1977
TA1535, TA1538 (TA1538,
TA98)
Ames test1 S. typhimurium Acrolein Negative Sasaki & Endo,
TA98, TA100 TA98, TA100 1978
1 With and without rat liver S9-fraction
2 Lower in the presence of S9-fraction
2.2.8.5 Special studies on mechanisms of liver injury by metabolites
The extent of damage to the liver by allyl alcohol was
increased by the aldehyde dehydrogenase inhibitors disulfiram or
cyanamide (Rikans, 1987; Jaeschke et al., 1987) or by
phenobarbital induction, and was moderated by the alcohol
dehydrogenase inhibitor, pyrazole (Diluzio & Hoffman, 1973). It was
concluded that the toxicity is due to the formation of acrolein from
allyl alcohol (Reid, 1972; Serafini-Cessi, 1972; Patel et al.,
1980; Rikans, 1987). In agreement with this conclusion, co-
administration of ethanol (3 g/kg b.w.) to rats inhibited the rate
of allyl alcohol oxidation by more than 90% and the histological
changes were completely prevented, despite glutathione levels being
depressed (Penttila et al., 1987). As indicated by the results
with disulfiram and cyanamide, oxidation of acrolein by aldehyde
dehydrogenase is an important detoxication step for allyl alcohol-
derived acrolein (Rikans, 1987; Jaeschke et al., 1987).
In mice, allyl alcohol at a dose of 1 mmole/kg b.w. almost
totally depleted hepatic glutathione with subsequent massive lipid
peroxidation while enhanced glutathione levels protected against
hepatotoxicity of allyl alcohol (Jaeschke et al., 1987). In
vitro, allyl alcohol, acrolein and glycidol react with glutathione
by a non-enzymic mechanism (Dore & Montaldo, 1984).
The severity of liver damage 24h after i.p. administration of
allyl alcohol (0.036 µl/kg b.w.) was evaluated in male rats at 4-5,
14-15 or 24-25 months of age. Allyl hepatotoxicity increased as a
function of age but hepatic glutathione levels were unaffected
indicating that the age-related susceptibility was not due to
diminished availability of glutathione (Rikans & Kosanke, 1984).
The location of allyl alcohol or acrolein-induced hepatic
injury is usually periportal but centrilobular necrosis was induced
by using retrograde infusion (Belinsky et al., 1983) and
metabolism of allyl alcohol by alcohol dehydrogenase occurred at
similar rates in both periportal and centrilobular regions. It was
suggested that periportal necrosis seen after oral dosing is due to
greater sensitivity of the mitochondrial respiratory chain to the
toxic effects of acrolein in periportal cells.
2.2.8.6 In vitro studies on metabolite damage to kidney cells
In freshly isolated renal epithelial cells from rats, allyl
alcohol toxicity as assessed by glutathione depletion and loss of
cell viability was more severe in cells from female animals. This
correlated with higher alcohol dehydrogenase activity (Ohno et al.,
1985).
3. COMMENTS
In evaluating these flavours, the Committee noted that they are
rapidly hydrolysed to allyl alcohol and the corresponding acids by
intestinal mucosal, pancreatic and hepatic esterases. The results
of studies on the toxicity of the three esters indicated that the
hepatotoxicity observed at high doses was due to the allyl alcohol
and its metabolites. Accordingly the Committee considered
supplementary toxicological data on allyl alcohol and concluded that
the three esters should be evaluated for a group ADI on the basis of
the allyl alcohol moiety. In its evaluation, the Committee also
took account of the principles relating to food flavours outlined in
Principles for the safety assessment of food additives and
contaminants in food (Annex I, ref. 76).
The hepatotoxicity of the esters and of allyl alcohol was less
marked in repeated-dose short-term studies than in single-dose acute
studies, although the mechanism of the acquired tolerance has not
been fully elucidated. Mutagenicity studies on allyl hexanoate and
allyl isovalerate yielded negative results, while most tests on
allyl alcohol were negative.
The Committee reviewed two long-term carcinogenicity studies in
rats and mice in which allyl isovalerate was administered by gavage
in corn oil at both the maximum tolerated dose and 50% of this dose.
Epithelial hyperplasia and squamous-cell papillomas of the
forestomach were observed in mice, but not rats, at the highest
dose. There was no evidence of hepatic tumours in mice (the liver
being the target organ for short-term toxicity). The Committee
concluded that these results were not relevant to the low-dose,
dietary exposure to allyl isovalerate as a food flavour but were
probably due to the effects of the large bolus doses that were used.
The Committee also noted the small increase in the incidence of
leukaemia reported in the treated rats; however, the incidence was
within the historical control range and no increase in the incidence
of hepatic tumours occurred in rats. Since levels of dietary
exposure to allyl isovalerate in food are much lower than the doses
used in these studies, the Committee concluded that an ADI could be
set.
The evaluation was based on the no-observed-effect-level in
short-term studies on allyl alcohol, with particular reference to
hepatotoxicity; this provides a more conservative estimate than one
based on the no-observed-effect level for the esters. The Committee
noted that a number of other food flavours in use which are allyl
esters and should be considered for inclusion in the group ADI on
the basis of their hydrolysis to allyl alcohol. In addition, in
view of evidence that allyl esters of such fatty acids as acetate,
propionate, isobutyrate, and 2-ethylhexanoate are also rapidly
hydrolysed, the Committee considered that their consumption should
be taken into account since they could contribute to the total
dietary load of allyl alcohol.
4. EVALUATION
The Committee allocated an ADI of 0-0.05 mg/kg b.w. as allyl
alcohol equivalent for allyl heptanoate, allyl hexanoate, and allyl
isovalerate, which corresponds to 0-0.15 mg/kg b.w. allyl
heptanoate, 0-0.13 mg/kg b.w. allyl hexanoate, 0-0.12 mg/kg b.w.
allyl isovalerate, or combinations of these pro rata.
5. REFERENCES
BAR, von F. & GRIEPENTROG, F. (1967) Die situation in der
gesundheitlichen Beurteilung der Aromatisierungsmittel für
Lebensmittel. Medizin und Ernahrung, 8, 244-251.
BELINSKY, S.A., MATSUMURA, T., KAUFFMAN, F.C. & THURMAN, R.G. (1984)
Rates of allyl alcohol metabolism in periportal and pericentral
regions of the liver lobule. Mol. Pharmacol., 25, 158-164.
BELINSKY, S.A., BRADFORD, B.U., FORMAN, D.T., GLASSMAN, E.B.,
FELDER, M.R. & THURMAN, R.G. (1985) Hepatotoxicity due to allyl
alcohol in deermice depends on alcohol dehydrogenase. Hepatology,
5, 1179-1182.
BUTTERWORTH, K.R., CARPANINI, F.M.B., DUNNINGTON, R., GRASSO, P. &
PELLING, R. (1978) The production of periportal necrosis by allyl
alcohol in the rat. Proc. Brit. Pharm. Soc., 57, 353P-354P.
BUTTERWORTH, K.R., CARPANINI, F.M.B., GAUNT, I.F., GRASSO, P. &
LLOYD, A.G. (1975) A new approach to the evaluation of the safety
of flavouring esters. Brit. J. Pharm., 54, 268P.
CARPANINI, F.M.B., GAUNT, I.F., HARDY, J., GANGOLLI, S.D.,
BUTTERWORTH, K.R. & LLOYD, A.G. (1978) Short-term toxicity of
allyl alcohol in rats. Toxicology, 9, 29-45.
CLAPP, J.J., KAYE, C.M. & YOUNG, L. (1969) Observations on the
metabolism of allyl compounds in the rat. Biochem. J., 114, 6P.
CLODE, S.A., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P. & GANGOLLI,
S.D. (1978) Short-term toxicity study of allyl caproate in rats.
Fd. Cosmet. Toxicol., 16, 197-201.
DAMSKE, D.R., MECLER, F.J., BELILES, R.P. & WEIR, R.J. (1980) 90-
day toxicity study in rats: allyl heptanoate. Unpublished report
of Litton Bionetics Inc., LBI project No. 21130-01 & -04. Submitted
to WHO by FEMA.
DILUZIO, N.R. & HOFFMAN, E.O. (1973). Protective influence of
pyrazole on allyl formate induced injury. Gastroenterol., 64,
158.
DORE, M. & MONTALDO, C. (1984) Studi sulla coniugazione in virto
dell'alcool allilico e dei suoi metaboliti con il glutatione
ridotto. Boll. Soc. It. Biol. Sper., 60, 1497-1501.
DUNLAP, M.K. & HINE, C.H. (1955) Toxicity of allyl alcohol. Fed.
Proc., 14, 335.
DUNLAP, M.K., KODAMA, J.K., WELLINGTON, J.S., ANDERSON, H.H. & HINE,
C.H. (1958) The toxicity of allyl alcohol I. Acute and chronic
toxicity. Arch. Ind. Hlth., 18, 303-311.
EPSTEIN, W.L. (1976) Unpublished report to RIFM dated 20th December
1976. Submitted to WHO by FEMA.
GRUNDSCHOBER, F. (1977) Toxicological assessment of flavouring
esters. Toxicology, 8, 387-390.
HAGAN, E.C., JENNER, P.M., JONES, W.I., FITZHUGH, O.G., LONG, E.L.,
BROUWER, J.G. & WEBB, W.K. (1965) Toxic properties of compounds
related to safrole. Toxicol. appl. Pharmacol., 7, 18-24.
HAGAN, E.C., HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES,
W.I., TAYLOR, J.M., LONG, E.L., NELSON, A.A. & BROUWER, J.B. (1967)
Food flavourings and compounds of related structure. II. Subacute
and chronic toxicity. Fd. Cosmet. Toxicol., 5, 141-157.
HONG, H.L., HUFF, J.E., LUSTER, M.I., MARONPOT, R.T., DIETER, M.P.,
HAYES, H.T. & BOORMAN, G.A. (1988) The effects of allyl
isovalerate on the haematopoietic and immunologic systems in
rodents. Fund. Appl. Toxicol., 10, 655-663.
IARC (1985) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans. Vol.36, Allyl compounds, aldehydes,
epoxides and peroxides. International Agency for Research on Cancer;
Lyon, pp. 69-74.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987), The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36, 51-57.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L. & FITZHUGH, O.G.
(1964) Food flavourings and compounds of related structure I. Acute
oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
KAYE, C.M. & YOUNG, L. (1970) Mercapturic acid formation from allyl
compounds in the rat. Biochem. J., 119, 53P.
KAYE, C.M. & YOUNG, L. (1972) The synthesis of mercapturic acids
from allyl compounds in the rat. Biochem. J., 127, 87P.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134, 1093-1101.
KLIGMAN, A.M. (1971) Unpublished report dated 27th September, 1971
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975a) Unpublished report dated 14th February, 1975
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975b) Unpublished report dated 10th June, 1975 to
RIFM. Submitted to WHO by FEMA.
LAKE,, B.G., GANGOLLI, S.D., WRIGHT, M.G., GRASSO, P., CARPANINI,
F.M.B. & BUTTERWORTH, K.R. (1975) The effect of repeated
administration on allyl alcohol-induced hepatotoxicity in the rat.
Biochem. Soc. Trans., 6, 145--146.
LUTZ, D., EDER, E., NEUDECKER, T. & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha, ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93, 305-315.
LONGLAND, R.C., SHILLING, W.H. & GANGOLLI, S.D. (1977) The
hydrolysis of flavouring esters by artificial gastrointestinal
juices and rat tissue preparations. Toxicology, 8, 197-204.
MEISEL, M.L. (1982) Caproate allyl, Ro 81-3538/000: An acute oral
toxicity study (LD50) in the rat. Unpublished report No. 100-
161/106 prepared by Hazleton Laboratories Deutschland GmbH.
Submitted to WHO by FEMA.
MORENO, O.M. (1974a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-676. Submitted to WHO
by FEMA.
MORENO, O.M. (1974b) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-677. Submitted to WHO
by FEMA.
MORENO, O.M. (1977a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 76-1446. Submitted to
WHO by FEMA.
MORENO, O.M. (1977b) Unpublished report to RIFM dated 27th
January, 1977. Submitted to WHO by FEMA.
MORTELMANS, K., HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986) Salmonella Mutagenicity tests: II. Results
from the testing of 270 chemicals. Environ. Mutagen., 8, Supp.7,
1-119.
NATIONAL TOXICOLOGY PROGRAM (NTP) (1983) Carcinogenesis studies of
allyl isovalerate. Report No. NTP-TR-253; PB-83-2509.
NIZZE, E., LAPIS, K. & KOVACS, L. (1979) Allyl alcohol-induced
changes in the rat exocrine pancreas. Digestion, 19, 359-369.
ODA, Y., HAMONO, Y., INOUE, K., YAMAMOTO, H., NIIHARA, T. & KUNITA,
N. (1978) Mutagenicity of food flavours in bacteria (1st report).
Shouhin Eisei Hen, 9, 177-181.
OHNO, Y., JONES, T.W. & ORMSTAD, K. (1985) Allyl alcohol toxicity
in isolated renal epithelial cells: protective effects of low
molecular weight thiols. Chem. biol. Interactions, 52, 289-299.
PATEL, J.M., WOOD, J.C. & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparation. Drug Metab. Disposition, 8, 305-308.
PENTTILÄ, K.E., MÄKINEN, J. & LINDROS, K.O. (1987) Allyl alcohol
liver injury: suppression by ethanol and relation to transient
glutathione depletion. Pharmacol. Toxicol., 60, 340-344.
PIAZZA, J.G. (1915) Zur kehntnis der Wirkung der Allylverbindungen
Z. Exp. Path. Ther., 17, 318.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE, E.,
FABRIXI, M., CONTI, G. & COMBA, P. (1981) Mutagenicity of
compounds of industrial and agricultural relevance in Salmonella,
Streptomyces and Aspergillus. J. Sci. Fd. Agric. 32, 826-832.
REES, K.R. & TARLOW, M.J. (1967) The hepatotoxic action of allyl
formate. Biochem. J., 104, 757-761.
REID, W.D. (1972) Mechanism of allyl alcohol-induced hepatic
necrosis. Experientia, 28, 1058-1061.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver
aldehyde dehydrogenases: Relation to allyl alcohol hepatotoxicity.
Drug Metab. Disp., 15, 356-362.
RIKANS, L.E. & KOSANKE, S.D. (1984) Effect of aging on liver
glutathione levels and hepatocellular injury from carbon
tetrachloride, allyl alcohol or galactosamine. Drug and Chemical
Toxicology, 7, 595-604
SERAFINI-CESSI, F. (1972) Conversion of allyl alcohol into
acrolein by rat liver. Biochem. J., 128, 1103-1107.
SHELANSKI, M.V. & MOLDOVAN, M. (1971) Unpublished report of Food
and Drug Research Laboratories Inc. to RIFM. Submitted to WHO by
FEMA.
SMYTH, H.F., CARPENTER, C.P. & WEIL, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-
122.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964) A comparison of
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 6, 378-387.
TORKELSON, T.R., WOLF, M.A., OYEN, F. & ROWE, V.K. (1959) Vapor
toxicity of allyl alcohol as determined on laboratory animals. J.
Am. Ind. Hyg. Assoc., 20, 224-229.
WILD, D., KING, M.T., GOCKE, E. & ECKHARDT, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, Basc and micronucleus tests. Fd. Chem.
Toxicol., 21, 707-719.
YOO, Y.S. (1986) Mutagenic and antimutagenic activities of
flavouring agents used in foodstuffs. J. Osaka City Med. Center,
34, 267-288.222.
 2.2 Toxicological studies
2.2.1 Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Allyl hexanoate
Rat both oral 218 (186-255) Jenner et al.,
1964
Taylor et al.,
1964
both oral 327 (277-386) Meisel, 1982
male oral 393
female oral 276 (215-352)
Guinea both oral 280 (246-319) Jenner et al.,
pig 1964
Rabbit ? dermal 300 (200-600) Shelanski &
Moldovan,
1971
? dermal 820 (700-940) Moreno, 1974
Allyl heptanoate
Mouse both oral 630 (514-772) Jenner et al.,
1964
Rat both oral 500 (392-638) Jenner et al.,
1964
Rabbit ? dermal 810 (440-1180) Moreno, 1974a
Guinea both oral 444 (363-541) Jenner et al.,
pig 1964
Allyl isovalerate
Mouse both oral >500 NTP, 1983
Rat both oral >250 <500 NTP, 1983
? oral 230 (216-290) Moreno, 1977
Rabbit ? dermal 560 (290-1060) Moreno, 1977
2.2.2 Short-term studies
2.2.2.1 Mouse - allyl isovalerate
Groups of 5 male and 5 female B6C3F1 mice were given allyl
isovalerate by gavage in corn oil for 14 consecutive days at daily
doses of 0, 31, 62, 125, 250 or 500 mg/kg b.w. All animals of both
sexes that received 500 mg/kg b.w. died within 48 h of commencement
of the study; all other animals survived to termination.
Inactivity and piloerection were seen in animals receiving 250 and
500 mg/kg b.w. and male mice receiving 250 mg/kg gained no weight;
body weights in other dose groups were comparable to controls at
termination of the study (NTP, 1983).
Groups of 10 male and 10 female B6C3F1 mice, 6 weeks of age at
commencement of the study were dosed with allyl isovalerate by
gavage in corn oil at doses of 0, 15, 31, 62, 125 or 250 mg/kg b.w.
(five day/week) for 13 weeks. At the end of the study, autopsies
were performed on all survivors. Animals from control and highest
dose groups were subjected to detailed histological examination.
In addition the liver was examined histologically for the 62 and 125
mg/kg b.w. groups and in the latter group stomachs were also
examined microscopically. Five out of 10 males and six of 10
females in the highest dose groups died, all but one of the deaths
(a female) being compound related; deaths in other groups were
caused by gavage error. Compound related effects noted at necropsy
or histologically were limited to the 125 and 250 mg/kg b.w. dose
groups and included "thickening" of the urinary bladder wall and
gastric mucosa and small intestine (both groups) ulcerative
inflammation of the stomach, coagulative necrosis of the liver and
cytoplasmic vacuolation of the liver (top dose group only). No
compound related effects were seen in the liver, stomach or bladder
of mice from other groups (NTP, 1983).
2.2.2.2 Rat - allyl heptanoate
Allyl heptanoate was fed to weanling Osborne-Mendel rats of
both sexes for 18 weeks at dietary levels of 0, 1000, 2500 and
10,000 mg/kg diet. There was a dose-related growth depression which
was severe and associated with a poor food efficiency at the highest
dose level only. Gross liver enlargement was observed at all dose
levels; in addition, at the highest dose level kidneys were enlarged
in both sexes and hearts were enlarged in males only. Males in the
2500 and 10 000 mg/kg diet groups were reported to have enlarged
testes (it is not clear from the report whether this "enlargement"
relates to absolute or relative organ weights; if the latter, the
increased relative organ weights may relate to the growth depression
rather than to organ specific effects). Microscopic changes
reported included hydropic degeneration in the periportal areas of
the liver ranging from moderate at the highest dose level to lesser
degrees at lower dose levels. The extent of bile duct proliferation
correlated with the degree of hydropic degeneration and hepatocyte
enlargement also was seen in some groups (Hagan et al., 1965).
Groups of weanling Charles River albino rats of both sexes were
given allyl heptanoate in the diet daily at doses of 0, 49.6, 157
and 496 mg/kg b.w./day for 13 weeks. Weight gain and food intake
were recorded weekly, and urinalysis, haematological and clinical
chemical examinations were carried out at weeks 6 and 12. At
termination, autopsies were performed on all animals and detailed
histological examinations were carried out on all rats from the top
dose group and on half of the control animals. In the rest of the
controls and the other two dose groups, histological examination was
limited to liver and kidney, and to tissues showing gross
abnormalities at autopsy. There was a reduced food intake in the
treated groups, which was statistically significant in the high and
mid-dose groups, and an associated deficit in body weight gain. At
week 6 there was a small but significant depression in leucocyte
count in males of the top dose group only; no such effect was seen
in females and other haematological parameters were normal in
animals of both sexes. At week 12, no significant haematological
differences were seen other than a small non-dose-related decrease
in leucocyte count in the males of the 49.6 mg/kg dose group.
Clinical chemical examinations at weeks 6 and 12 revealed a decrease
in some parameters such as blood glucose, total serum protein and
albumin which appeared to be due to the reduced food intake and not
to specific toxic effects. Urine composition was unaffected by
treatment. At necropsy no gross nor microscopic lesions related to
treatment were observed and elevated relative organ weights in high-
dose males were related to reduced food intake and body weight gain.
It was concluded that daily treatment with allyl heptanoate in this
study did not result in any signs of toxicity; the reasons for the
reduced food intake could not be determined but might have been due
to unpalatability of diet (Damske et al., 1980).
Allyl hexanoate
Groups of 10 male and 10 female Osborne-Mendel rats were given
allyl hexanoate by gavage in corn oil at dose levels of 0, 15, 65
and 100 mg/kg b.w. daily for 18 weeks. Weight gain, food intake and
general condition were recorded weekly. At termination, the
animals were exsanguinated and haematological and gross pathological
examinations were performed. Detailed histological examinations
were performed only on eight rats from the control and high dose
groups; based on observations in the high dose group, livers from
eight animals in the 15 and 65 mg/kg b.w. dose groups were also
examined microscopically. In the high dose group, gross appearance
of the liver was described as nodular and wrinkled with granular or
rough surface. Microscopically the high dose group showed slight to
moderate bile duct proliferation, some "lobular architectural
disarrangement", slight fibrosis and pigment deposition in
macrophages; necrotic foci were seen in 2 of 8 animals examined. In
the 65 mg/kg b.w. dose group, very slight bile-duct proliferation
was observed in 2 of eight animals studied. The livers of the 15
mg/kg b.w. dose group were unaffected by treatment (Hagan et al.,
1967).
In a companion study to the foregoing, 5 rats of each sex
received allyl hexanoate in the diet at a concentration of 1000
mg/kg diet (equivalent to 50mg/kg b.w.) for 28 weeks. No adverse
effects were observed (Hagan et al., 1967).
Groups of 15 male and 15 female Wistar rats were given daily
oral doses of 0, 35 or 100 mg allyl hexanoate/kg b.w. as a solution
in corn oil; a further group of 10 animals of each sex were
similarly dosed with 12 mg/kg b.w./day for 13 weeks. Food and water
intake and body weights were recorded weekly, urinalysis was
performed during week 2, week 5 or 6, and in the final week of
treatment, and renal function tests were also carried out. At
termination, autopsies were performed and organ weights recorded,
and detailed histopathological examinations were conducted.
No differences were noted between treated and control animals
in body weight, water intake, haematological parameters, serum
chemistry, urine composition or in renal concentration tests. There
were slight increases in food intake in the highest dose group.
Liver weights were increased in the 35 and 100 mg/kg b.w. dose
groups and all treated animals showed evidence of periportal
vacuolation which was dose related in incidence and severity and
which in the 100 mg/kg b.w. group was accompanied by enlarged
hepatocytes, focal periportal necrosis and bile-duct proliferation.
Weights of spleen, kidneys, stomach and small intestine were
increased in both sexes in the highest dose group and small
intestine weight was also increased in females of the 35 mg/kg b.w.
group. It was not possible to determine a no observed adverse
effect level for allyl hexanoate in this study (Clode et al.,
1978).
When allyl hexanoate was administered to rats by gavage at a
daily dose level of 15 mg/kg b.w. for 18 weeks there were no
observed adverse effects (this study was reported in summary only)
(Bär & Griepentrog, 1967).
Allyl isovalerate
In similar studies to those described for mice, groups of 5
male and 5 female Fischer F344/N rats were given allyl isovalerate
by gavage for 14 days and groups of 10 animals of each sex were
dosed (5 day/week) for 13 weeks at levels of 0, 31, 62, 125, 250 and
500 mg/kg bw. In the fourteen days study, all rats given 500 mg/kg
b.w. and two rats of each sex given 250 mg/kg b.w. died. At
termination, mean body weights in the 250 mg/kg b.w. group were
depressed relative to weights in the control group by 23% and 13% in
males and females, respectively. Inactivity, laboured breathing,
diarrhoea and piloerection were observed in both sexes in the two
highest dose groups and in necropsy gross dark red areas were seen
in the stomachs of three animals of each sex at the top dose level.
In the thirteen week study, all ten males and 4/10 females that
received 250 mg/kg b.w. died and body weight gain was significantly
depressed in males of the 125 mg/kg b.w. group and females of the
250 mg/kg group. Dose-related effects seen at necropsy were
thickening of the intestinal wall and redness of the mucosal
surfaces of the intestines and urinary bladder. Enlargement of
internal lymph nodes and adrenals was reported but was unaccompanied
by histological lesions. Histopathological examination revealed
multifocal coagulative necrosis, cholangiofibrosis, and bile duct
hyperplasia at the 125 and 250 mg/kg b.w. dose levels. The effects
were dose related in incidence and no such lesions were observed in
the 31 and 62 mg/kg b.w. dose groups (NTP, 1983).
Combinations of esters
Groups of 10 male rats were given by gavage 21 consecutive
daily doses of allyl alcohol and a series of allyl esters (acetate,
propionate, hexanoate, isobutyrate, isovalerate and 2-
ethylhexanoate) at equimolecular doses corresponding to 5, 25 or 60
mg/kg b.w. of allyl alcohol. After 21 days the animals were
sacrificed and the livers examined histologically. The severity of
the liver lesions was classified according to the scheme: periportal
cell enlargement, followed by necrosis and subsequent fibrosis with
bile duct hyperplasia. The severity of the hepatic lesions from the
straight chain esters was similar to that produced by the
corresponding dose of allyl alcohol and more marked than that
produced by the esters of the branched chain acids. The differences
were attributed to differences in the rate of hydrolysis since the
straight chain esters were hydrolysed in vitro approximately 100
times faster than the branched chain esters (Butterworth et al.,
1975).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mouse - allyl isovalerate
Groups of 50 male and 50 female B6C3F1 mice, initially 50 days
old, received allyl isovalerate at doses of 0, 31 or 62 mg/kg b.w.
by gavage in corn oil (10 ml/kg b.w.) five times per week for 103
weeks. Survivors were killed at 112-114 weeks of age. No
significant differences were observed in survival rates in males;
reduced mean body weight gain and significantly lower survival rate
in the lower dose group females was attributed to a high incidence
of a genital tract infection.
An increase in the incidence of epithelial hyperplasia and
squamous cell papillomas was observed in the non-glandular
forestomach in male mice; the observed incidences for hyperplasia
were 1/50, 1/50 and 7/48, and for papillomas 0/50, 1/50 and 3/48 in
the control, low and high dose groups respectively. In females, the
corresponding incidences for forestomach epithelial hyperplasia were
0/50, 2/50 and 3/50, and for squamous cell papillomas were 1/50,
0/50 and 2/50 respectively. The incidence of lymphomas was slightly
elevated in males (5/50, 6/50 and 8/50 in the respective dose
groups) but the increase was not significant by the trend test nor
by the incidental tumour test; in females the corresponding
incidences were 11/50, 11/50 and 18/50 which gave a dose-response
trend, the high dose tumour incidence being significantly higher
(P<0.05) than controls. Significant reductions in tumour incidence
were observed in treated male mice in respect of hepatocellular
carcinomas (18/50, 6/50, 9/50), alveolar/bronchiolar adenomas or
carcinomas (13/50, 6/50 5/49) and for thyroid follicular cell
adenomas (5/47, 0/46, 1/49). No treatment-related non-neoplastic
lesions were observed in mice of either sex (NTP, 1983).
2.2.3.2 Rat - allyl hexanoate
A group of 5 male and 5 female Osborne-Mendel rats were given
allyl hexanoate in the diet at a concentration of 2500 mg/kg
(equivalent to 125 mg/kg b.w.) for 1 year. Body weight and food
intake were recorded weekly and haematological examinations were
carried out at 3, 6, and 12 months. At termination, detailed
histological examinations were performed. No adverse effects were
reported, in contrast with the short-term (18 week) study where
minimal effects (very slight bile duct proliferation) were reported
at a dose level of 65 mg/kg b.w. (Hagan et al., 1967).
When allyl hexanoate was fed to rats at a dietary concentration
of 0.5% for 1.5 years, 2/25 animals developed multiple bile duct
adenomas and proliferative changes of the small bile ducts. An
additional animal was reported to have adenomas (location not
specified) after 8.5 months. The authors concluded that the small
number of animals and tumour incidence were insufficient to allow a
firm conclusion to be reached on the significance (Bär &
Griepentrog, 1967) (This report was in summary only).
Allyl isovalerate
Groups of 50 male and 50 female Fischer 344/N rats, initially
46 days old, were given allyl isovalerate at doses of 0, 31 or 62
mg/kg b.w. by gavage in corn oil (5 ml/kg b.w.) five times per week
for 103 weeks. Survivors were killed at 112-114 weeks of age when
for males the numbers of survivors were 34 controls, 30 low dose and
28 high dose; the corresponding numbers of female survivors were
38, 36 and 29 respectively. There was a dose related increase in
mononuclear-cell leukaemia, the incidences observed were 1/50, 4/50
and 7/50 in males of the control, low- and high dose groups; in
females the corresponding incidences were 4/50, 6/50 and 9/49. In
both sexes there was a significant dose-response trend (p<0.05),
while the incidence in high-dose males was significantly higher than
controls (p<0.05). Increased frequencies of non-neoplastic lesions
(cholangiofibrosis, nodular regeneration, cirrhosis, focal
periportal necrosis, fatty changes and cytoplasmic vacuolation) were
observed in the livers of animals of both sexes in the high dose
group but there was no increase in liver neoplasms (NTP, 1983).
The authors of the NTP report concluded that allyl isovalerate
was carcinogenic, causing increased incidence of haematopoietic
system neoplasms (mononuclear cell leukaemias in male rats and
lymphoma in female mice). In reviewing this and other relevant
biological data, the International Agency for Research on Cancer
concluded that there was limited evidence for the carcinogenicity of
allyl isovalerate to experimental animals (IARC, 1985).
2.2.3.3 Dog - allyl heptanoate
Four groups of 3 male and 3 female beagle dogs were given daily
doses of allyl heptanoate of 0, 5, 25 and 75 mg/kg b.w. by capsule
for up to 18 months. All the dogs in the top dose group died within
3 - 7 months; dogs in the two lower treatment groups were reported
as surviving after 18 months. Administration of 75 mg/kg b.w.
caused depressed growth and macroscopic changes in the appearance of
the liver and haemorrhagic changes in the gastric mucosa. Less
consistent changes were reported in the form of cysts in the urinary
bladder and congestion in the lungs, digestive tract, kidneys,
spleen and lymph nodes. Microscopically the livers showed a slight
to moderate periportal fibrosis associated with slight to moderate
proliferation of the bile duct epithelium. Slight fatty changes
were also observed. The stomachs showed diffuse haemorrhage and
necrosis of the mucosae with instances of focal sub-mucosal
haemorrhage (Hagan et al., 1965).
2.2.4 Special studies on skin irritation
During an acute dermal toxicity study on allyl heptanoate in
rabbits, skin irritancy was evaluated on day 1. At dermal doses of
313-1250 mg/kg slight to moderate redness and oedema were reported
(5000 mg/kg was a lethal dose) (Moreno, 1974). Similar results were
obtained with allyl heptanoate (Moreno, 1974a). Allyl isovalerate
applied undiluted to intact or abraded rabbit skin under occlusion
for 24h was moderately irritating (Moreno, 1977).
In a preliminary to a maximization test, 48 hour closed patch
tests were carried out on the forearms of 5 volunteers with allyl
hexanoate and four subjects displayed grade 1 irritation (Kligman,
1971). Conversely, in a later study in which 5 volunteers were
subjected to patch tests, no signs of irritation were observed with
allyl hexanoate nor with allyl heptanoate (Kligman, 1975; 1975a).
Similarly, allyl isovalerate was without irritant effect in a closed
patch study on the backs of 28 subjects (Epstein, 1976).
2.2.5 Special studies on contact sensitization
In a maximization test on 25 healthy volunteers, allyl
hexanoate was reported to produce 13 cases of sensitization and was
considered a moderate sensitizer (Kligman, 1975); this contrasts
with an earlier maximization test on a similar number of volunteers
in which no cases of sensitization were detected (Kligman 1971). No
sensitization was observed with allyl heptanoate using a similar
protocol (Kligman, 1975a) nor with allyl isovalerate in 28
volunteers (Epstein, 1976).
2.2.6 Special studies on the haematopoietic and immunologic systems
Following the observation, in carcinogenicity studies on allyl
isovalerate, of marginal increases in mononuclear cell leukaemia in
rats and of malignant lymphoma in mice (NTP, 1983) and in view of
the fact that isovaleric acidaemia has been associated with
pancytopenia in humans, the effects of allyl isovalerate on the
haematopoietic and immune systems of female B6C3F1 mice and Fischer
344/N rats of both sexes were investigated in a short-term (14 day)
study. The animals were dosed by gavage for 5 days per week for 2
weeks with allyl isovalerate in corn oil at dose levels of 0, 31, 62
or 125 mg/kg b.w. (rats and mice) or 250 mg/kg b.w. (rats only).
Haematological, immunological and histological studies were
performed 48-72 h after the final treatment. In addition, bone
marrow slides from female mice from the NTP 13-week study (see
short-term studies) were also examined. The body weights of rats of
both sexes were reduced at the 250mg/kg b.w. dose level and of males
at the 125 mg/kg b.w. level. No changes in the body weights of the
female mice were observed but there was a 20% increase in mean
spleen weight and the splenic follicles were large with prominent
germinal centres. No treatment-related effects were seen in
haematological parameters nor in bone-marrow cellularity in mice or
rats. However, there were significant decreases in pluripotent
haematopoietic stem cells (CFU-S) in the spleen and in granulocyte-
macrophage progenitors (CFU-GM) in the bone marrow of treated mice.
In vitro enzyme assays of these cells showed that haematopoietic
suppression was correlated with a depression of hexose monophosphate
shunt metabolism but that enzymes of the Embden-Meyerhof and
tricarboxylic acid pathways were unaffected. Examination of host
resistance in mice following challenge with Plasmodium yoelii or
Listeria monocytogenes showed no significant differences between
control and treated animals, nor were there other effects on the
immune system. The authors concluded that the myelotoxic effects
were minimal and of a degree that did not alter host resistance
(Hong et al., 1988).
Minimal to moderate hypocellularity of the bone marrow was
observed in the 125 mg/kg b.w. group of mice, both sexes, from the
13-week NTP study and was most striking for megakaryocytes. The
degree of hypocellularity was never severe.
2.2.7 Special studies on genotoxicity
The genotoxicity of allyl hexanoate and allyl isovalerate are
shown on the next page.
2.2.8 Special studies on metabolites
2.2.8.1 Biochemical aspects
For pathways of metabolism of allyl alcohol see Biochemical
Aspects section of allyl esters.
Test system Test object Concentration Result Reference
ALLYL HEXANOATE
Ames test1 S. typhimurium 0-3.5mg/plate - Wild et al., 1983
TA98, TA100,
TA1535, TA1537,
TA1538
Ames test1 S. typhimurium 10.5 µg/plate - Oda et al., 1978
TA98, TA100
Rec assay B. subtilis 0-18 µg/disc + Oda et al., 1978
H17 vs M45
Rec assay B. subtilis 0-20µg/disc - Yoo, 1986
H17 vs M45
Basic test Drosophila 0.5mM in feed - Wild et al., 1983
(sex linked melanogaster
recessive)
Micronucleus Mouse 2 x 156 mg/kg - Wild et al., 1983
test bw i.p.
ALLYL ISOVALERATE
Ames test1,2 S. typhimurium 0 - 1000 µg/ - NTP, 1983;
TA98, TA100 plate Mortelmans et al.,
TA1535, TA1537 1986
Basic test Drosophila 1200 - 2000 - Woodruff et al.,
melanogaster mg/l in feed 1985
4500mg/l injected
injected
(contd)
Test system Test object Concentration Result Reference
ALLYL HEPTANOATE
No mutagenicity data were available for allyl heptanoate
1 Both with and without rat liver S9 fraction
2 Using a preincubation protocol
2.2.8.2 Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse male oral 96(84-110) Dunlap et al., 1958
i.p. 60
Rat ? oral 64(56-74) Smyth et al., 1951
Rat both oral 70(63-79) Taylor et al., 1964
Rat male oral 105(79-140)* Dunlap et al., 1958
99(75-130)**
i.p. 42(32-55)
Rabbit male oral 71(42-125) Dunlap et al., 1958
percut. 89(40-250)
* Rats 111-143 g b.w.
** Rats 170-252 g b.w.
2.2.8.3 Short-term studies
Following acute or short-term exposure to allyl alcohol, the
main target organ is the liver in which typical periportal changes
are observed, ranging from fatty changes to cell necrosis (Piazza,
1915; Dunlap & Hine, 1955; Dunlap et al., 1958; Torkelson et
al., 1959; Rees & Tarlow, 1967; Serafini-Cessi, 1972). The
kidney may also be affected, changes reported include necrosis of
the epithelium of the convoluted tubules and proliferation of
interstitial tissue.
Rat
Groups of 10 rats (strain and sex not specified) received allyl
alcohol in the drinking water at doses of 1.3-1.97 mg/kg b.w. The
top dose level was associated with reduced appetite and increased
mortality. The highest no effect level was 4 mg/kg b.w. The
corresponding no effect level for acrolein was 0.17 mg/kg b.w.
(Smyth et al., 1951).
Groups of six male and six female rats received allyl alcohol
in drinking water at concentrations of 0, 1, 5, 50, 100, 250, 500 or
1000 mg/L for 13 weeks, corresponding to daily intakes of 0.13,
0.62, 5.9, 11.6, 25.5, 41.0 or 72 mg/kg b.w. for males and 0.17,
0.94, 7.34, 13.2, 34.0, 43.7 or 67.4 mg/kg b.w. for females in the
respective dose groups. At termination, histological examination
(12 organs) was performed on half of the animals in each group.
Few gross abnormalities were seen at autopsy; peritoneal fat was
decreased in the 500 mg/L group and absent at 1000 mg/L. The no
effect level reported from this study was approx. 12 mg/kg b.w./day
while at an average 29 mg/kg b.w./day the only noticeable effect was
an increased liver weight in males and kidney weight in females
(Dunlap et al., 1958).
Male Wistar albino rats were given daily intragastric doses of
allyl alcohol of 0 or 30 mg/kg b.w. in corn oil for periods of 1, 10
or 28 days. The administration of a single dose produced marked
periportal necrosis and associated losses of alcohol dehydrogenase
and succinate dehydrogenase activities; hepatic cytochrome P450
concentrations and benzo[a]pyrene hydroxylase activities were
reduced to about 60% of control values. Conversely, further daily
dosing for 10 or 28 days led to a recovery both of histological
appearance and of enzyme activities. It was concluded that
metabolism of allyl alcohol becomes modified by repeated treatment
(Lake et al., 1975). Similarly, no hepatic injury was observed
following 28 daily oral doses of 25 mg/kg b.w., although direct
infusion of acrolein caused typical necrotic changes (Butterworth
et al., 1978).
Groups of 15 male and 15 female Wistar rats were given allyl
alcohol in drinking water at concentrations of 0, 50, 100, 200 or
800 mg/l for 15 weeks. No treatment-related effects were observed
in results of haematological examinations or analysis of serum.
There was a dose-related decrease in fluid intake at all treatment
levels in both sexes while growth and food consumption were reduced
in both sexes at 800 mg/l and males given 200 or 800 mg/L produced
less urine than controls in a period following water deprivation or
water loading. Increased relative weights of liver, spleen and
kidney were observed at both sexes at the top dose level; relative
kidney weights were also higher in the 200 mg/l group and in females
given 100 mg/l. No effects due to treatment were seen at autopsy
or histologically. The no observed adverse effect level was 50
mg/l, equal to 4.8-6.2 mg allyl alcohol/kg b.w./day (Carpanini et
al., 1978).
Although the effects of allyl alcohol are almost exclusively
observed in the liver, changes in the pancreas described as
acidophilia, vacuolation and necrosis of pancreatic acinar cells
were reported following oral administration of a single dose of 50
mg/kg b.w. (Nizze et al., 1979).
2.2.8.4 Special studies on genotoxicity
The genotoxicity of allyl alcohol, acrolein and glycidol are
shown in Table 3.
Test system Test object Compound & Result Reference
concentration
Ames test1 S. typhimurium Allyl alcohol Positive2 Lutz et al., 1982
(liquid TA100 Acrolein Positive
suspension) Glycidol Weak
positive2
Ames test1 S. typhimurium Allyl alcohol Negative Principe et al.,
TA98, TA100, 0.025-0.1 1981
TA1535, TA1537, µl/plate
TA1538
Forward S. coelicolor Allyl alcohol Negative Principe et al.,
mutation 2-100µl/plate 1981
8-azaguanine Aspergillus Allyl alcohol Negative Principe et al.,
resistance nidulans 10-40 µl/plate 1981
(point
mutation)
Ames test1 S. typhimurium Allyl alcohol Negative Bignami et al.,
TA98, TA100, Acrolein Positive 1977
TA1535, TA1538 (TA1538,
TA98)
Ames test1 S. typhimurium Acrolein Negative Sasaki & Endo,
TA98, TA100 TA98, TA100 1978
1 With and without rat liver S9-fraction
2 Lower in the presence of S9-fraction
2.2.8.5 Special studies on mechanisms of liver injury by metabolites
The extent of damage to the liver by allyl alcohol was
increased by the aldehyde dehydrogenase inhibitors disulfiram or
cyanamide (Rikans, 1987; Jaeschke et al., 1987) or by
phenobarbital induction, and was moderated by the alcohol
dehydrogenase inhibitor, pyrazole (Diluzio & Hoffman, 1973). It was
concluded that the toxicity is due to the formation of acrolein from
allyl alcohol (Reid, 1972; Serafini-Cessi, 1972; Patel et al.,
1980; Rikans, 1987). In agreement with this conclusion, co-
administration of ethanol (3 g/kg b.w.) to rats inhibited the rate
of allyl alcohol oxidation by more than 90% and the histological
changes were completely prevented, despite glutathione levels being
depressed (Penttila et al., 1987). As indicated by the results
with disulfiram and cyanamide, oxidation of acrolein by aldehyde
dehydrogenase is an important detoxication step for allyl alcohol-
derived acrolein (Rikans, 1987; Jaeschke et al., 1987).
In mice, allyl alcohol at a dose of 1 mmole/kg b.w. almost
totally depleted hepatic glutathione with subsequent massive lipid
peroxidation while enhanced glutathione levels protected against
hepatotoxicity of allyl alcohol (Jaeschke et al., 1987). In
vitro, allyl alcohol, acrolein and glycidol react with glutathione
by a non-enzymic mechanism (Dore & Montaldo, 1984).
The severity of liver damage 24h after i.p. administration of
allyl alcohol (0.036 µl/kg b.w.) was evaluated in male rats at 4-5,
14-15 or 24-25 months of age. Allyl hepatotoxicity increased as a
function of age but hepatic glutathione levels were unaffected
indicating that the age-related susceptibility was not due to
diminished availability of glutathione (Rikans & Kosanke, 1984).
The location of allyl alcohol or acrolein-induced hepatic
injury is usually periportal but centrilobular necrosis was induced
by using retrograde infusion (Belinsky et al., 1983) and
metabolism of allyl alcohol by alcohol dehydrogenase occurred at
similar rates in both periportal and centrilobular regions. It was
suggested that periportal necrosis seen after oral dosing is due to
greater sensitivity of the mitochondrial respiratory chain to the
toxic effects of acrolein in periportal cells.
2.2.8.6 In vitro studies on metabolite damage to kidney cells
In freshly isolated renal epithelial cells from rats, allyl
alcohol toxicity as assessed by glutathione depletion and loss of
cell viability was more severe in cells from female animals. This
correlated with higher alcohol dehydrogenase activity (Ohno et al.,
1985).
3. COMMENTS
In evaluating these flavours, the Committee noted that they are
rapidly hydrolysed to allyl alcohol and the corresponding acids by
intestinal mucosal, pancreatic and hepatic esterases. The results
of studies on the toxicity of the three esters indicated that the
hepatotoxicity observed at high doses was due to the allyl alcohol
and its metabolites. Accordingly the Committee considered
supplementary toxicological data on allyl alcohol and concluded that
the three esters should be evaluated for a group ADI on the basis of
the allyl alcohol moiety. In its evaluation, the Committee also
took account of the principles relating to food flavours outlined in
Principles for the safety assessment of food additives and
contaminants in food (Annex I, ref. 76).
The hepatotoxicity of the esters and of allyl alcohol was less
marked in repeated-dose short-term studies than in single-dose acute
studies, although the mechanism of the acquired tolerance has not
been fully elucidated. Mutagenicity studies on allyl hexanoate and
allyl isovalerate yielded negative results, while most tests on
allyl alcohol were negative.
The Committee reviewed two long-term carcinogenicity studies in
rats and mice in which allyl isovalerate was administered by gavage
in corn oil at both the maximum tolerated dose and 50% of this dose.
Epithelial hyperplasia and squamous-cell papillomas of the
forestomach were observed in mice, but not rats, at the highest
dose. There was no evidence of hepatic tumours in mice (the liver
being the target organ for short-term toxicity). The Committee
concluded that these results were not relevant to the low-dose,
dietary exposure to allyl isovalerate as a food flavour but were
probably due to the effects of the large bolus doses that were used.
The Committee also noted the small increase in the incidence of
leukaemia reported in the treated rats; however, the incidence was
within the historical control range and no increase in the incidence
of hepatic tumours occurred in rats. Since levels of dietary
exposure to allyl isovalerate in food are much lower than the doses
used in these studies, the Committee concluded that an ADI could be
set.
The evaluation was based on the no-observed-effect-level in
short-term studies on allyl alcohol, with particular reference to
hepatotoxicity; this provides a more conservative estimate than one
based on the no-observed-effect level for the esters. The Committee
noted that a number of other food flavours in use which are allyl
esters and should be considered for inclusion in the group ADI on
the basis of their hydrolysis to allyl alcohol. In addition, in
view of evidence that allyl esters of such fatty acids as acetate,
propionate, isobutyrate, and 2-ethylhexanoate are also rapidly
hydrolysed, the Committee considered that their consumption should
be taken into account since they could contribute to the total
dietary load of allyl alcohol.
4. EVALUATION
The Committee allocated an ADI of 0-0.05 mg/kg b.w. as allyl
alcohol equivalent for allyl heptanoate, allyl hexanoate, and allyl
isovalerate, which corresponds to 0-0.15 mg/kg b.w. allyl
heptanoate, 0-0.13 mg/kg b.w. allyl hexanoate, 0-0.12 mg/kg b.w.
allyl isovalerate, or combinations of these pro rata.
5. REFERENCES
BAR, von F. & GRIEPENTROG, F. (1967) Die situation in der
gesundheitlichen Beurteilung der Aromatisierungsmittel für
Lebensmittel. Medizin und Ernahrung, 8, 244-251.
BELINSKY, S.A., MATSUMURA, T., KAUFFMAN, F.C. & THURMAN, R.G. (1984)
Rates of allyl alcohol metabolism in periportal and pericentral
regions of the liver lobule. Mol. Pharmacol., 25, 158-164.
BELINSKY, S.A., BRADFORD, B.U., FORMAN, D.T., GLASSMAN, E.B.,
FELDER, M.R. & THURMAN, R.G. (1985) Hepatotoxicity due to allyl
alcohol in deermice depends on alcohol dehydrogenase. Hepatology,
5, 1179-1182.
BUTTERWORTH, K.R., CARPANINI, F.M.B., DUNNINGTON, R., GRASSO, P. &
PELLING, R. (1978) The production of periportal necrosis by allyl
alcohol in the rat. Proc. Brit. Pharm. Soc., 57, 353P-354P.
BUTTERWORTH, K.R., CARPANINI, F.M.B., GAUNT, I.F., GRASSO, P. &
LLOYD, A.G. (1975) A new approach to the evaluation of the safety
of flavouring esters. Brit. J. Pharm., 54, 268P.
CARPANINI, F.M.B., GAUNT, I.F., HARDY, J., GANGOLLI, S.D.,
BUTTERWORTH, K.R. & LLOYD, A.G. (1978) Short-term toxicity of
allyl alcohol in rats. Toxicology, 9, 29-45.
CLAPP, J.J., KAYE, C.M. & YOUNG, L. (1969) Observations on the
metabolism of allyl compounds in the rat. Biochem. J., 114, 6P.
CLODE, S.A., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P. & GANGOLLI,
S.D. (1978) Short-term toxicity study of allyl caproate in rats.
Fd. Cosmet. Toxicol., 16, 197-201.
DAMSKE, D.R., MECLER, F.J., BELILES, R.P. & WEIR, R.J. (1980) 90-
day toxicity study in rats: allyl heptanoate. Unpublished report
of Litton Bionetics Inc., LBI project No. 21130-01 & -04. Submitted
to WHO by FEMA.
DILUZIO, N.R. & HOFFMAN, E.O. (1973). Protective influence of
pyrazole on allyl formate induced injury. Gastroenterol., 64,
158.
DORE, M. & MONTALDO, C. (1984) Studi sulla coniugazione in virto
dell'alcool allilico e dei suoi metaboliti con il glutatione
ridotto. Boll. Soc. It. Biol. Sper., 60, 1497-1501.
DUNLAP, M.K. & HINE, C.H. (1955) Toxicity of allyl alcohol. Fed.
Proc., 14, 335.
DUNLAP, M.K., KODAMA, J.K., WELLINGTON, J.S., ANDERSON, H.H. & HINE,
C.H. (1958) The toxicity of allyl alcohol I. Acute and chronic
toxicity. Arch. Ind. Hlth., 18, 303-311.
EPSTEIN, W.L. (1976) Unpublished report to RIFM dated 20th December
1976. Submitted to WHO by FEMA.
GRUNDSCHOBER, F. (1977) Toxicological assessment of flavouring
esters. Toxicology, 8, 387-390.
HAGAN, E.C., JENNER, P.M., JONES, W.I., FITZHUGH, O.G., LONG, E.L.,
BROUWER, J.G. & WEBB, W.K. (1965) Toxic properties of compounds
related to safrole. Toxicol. appl. Pharmacol., 7, 18-24.
HAGAN, E.C., HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES,
W.I., TAYLOR, J.M., LONG, E.L., NELSON, A.A. & BROUWER, J.B. (1967)
Food flavourings and compounds of related structure. II. Subacute
and chronic toxicity. Fd. Cosmet. Toxicol., 5, 141-157.
HONG, H.L., HUFF, J.E., LUSTER, M.I., MARONPOT, R.T., DIETER, M.P.,
HAYES, H.T. & BOORMAN, G.A. (1988) The effects of allyl
isovalerate on the haematopoietic and immunologic systems in
rodents. Fund. Appl. Toxicol., 10, 655-663.
IARC (1985) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans. Vol.36, Allyl compounds, aldehydes,
epoxides and peroxides. International Agency for Research on Cancer;
Lyon, pp. 69-74.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987), The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36, 51-57.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L. & FITZHUGH, O.G.
(1964) Food flavourings and compounds of related structure I. Acute
oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
KAYE, C.M. & YOUNG, L. (1970) Mercapturic acid formation from allyl
compounds in the rat. Biochem. J., 119, 53P.
KAYE, C.M. & YOUNG, L. (1972) The synthesis of mercapturic acids
from allyl compounds in the rat. Biochem. J., 127, 87P.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134, 1093-1101.
KLIGMAN, A.M. (1971) Unpublished report dated 27th September, 1971
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975a) Unpublished report dated 14th February, 1975
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975b) Unpublished report dated 10th June, 1975 to
RIFM. Submitted to WHO by FEMA.
LAKE,, B.G., GANGOLLI, S.D., WRIGHT, M.G., GRASSO, P., CARPANINI,
F.M.B. & BUTTERWORTH, K.R. (1975) The effect of repeated
administration on allyl alcohol-induced hepatotoxicity in the rat.
Biochem. Soc. Trans., 6, 145--146.
LUTZ, D., EDER, E., NEUDECKER, T. & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha, ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93, 305-315.
LONGLAND, R.C., SHILLING, W.H. & GANGOLLI, S.D. (1977) The
hydrolysis of flavouring esters by artificial gastrointestinal
juices and rat tissue preparations. Toxicology, 8, 197-204.
MEISEL, M.L. (1982) Caproate allyl, Ro 81-3538/000: An acute oral
toxicity study (LD50) in the rat. Unpublished report No. 100-
161/106 prepared by Hazleton Laboratories Deutschland GmbH.
Submitted to WHO by FEMA.
MORENO, O.M. (1974a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-676. Submitted to WHO
by FEMA.
MORENO, O.M. (1974b) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-677. Submitted to WHO
by FEMA.
MORENO, O.M. (1977a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 76-1446. Submitted to
WHO by FEMA.
MORENO, O.M. (1977b) Unpublished report to RIFM dated 27th
January, 1977. Submitted to WHO by FEMA.
MORTELMANS, K., HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986) Salmonella Mutagenicity tests: II. Results
from the testing of 270 chemicals. Environ. Mutagen., 8, Supp.7,
1-119.
NATIONAL TOXICOLOGY PROGRAM (NTP) (1983) Carcinogenesis studies of
allyl isovalerate. Report No. NTP-TR-253; PB-83-2509.
NIZZE, E., LAPIS, K. & KOVACS, L. (1979) Allyl alcohol-induced
changes in the rat exocrine pancreas. Digestion, 19, 359-369.
ODA, Y., HAMONO, Y., INOUE, K., YAMAMOTO, H., NIIHARA, T. & KUNITA,
N. (1978) Mutagenicity of food flavours in bacteria (1st report).
Shouhin Eisei Hen, 9, 177-181.
OHNO, Y., JONES, T.W. & ORMSTAD, K. (1985) Allyl alcohol toxicity
in isolated renal epithelial cells: protective effects of low
molecular weight thiols. Chem. biol. Interactions, 52, 289-299.
PATEL, J.M., WOOD, J.C. & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparation. Drug Metab. Disposition, 8, 305-308.
PENTTILÄ, K.E., MÄKINEN, J. & LINDROS, K.O. (1987) Allyl alcohol
liver injury: suppression by ethanol and relation to transient
glutathione depletion. Pharmacol. Toxicol., 60, 340-344.
PIAZZA, J.G. (1915) Zur kehntnis der Wirkung der Allylverbindungen
Z. Exp. Path. Ther., 17, 318.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE, E.,
FABRIXI, M., CONTI, G. & COMBA, P. (1981) Mutagenicity of
compounds of industrial and agricultural relevance in Salmonella,
Streptomyces and Aspergillus. J. Sci. Fd. Agric. 32, 826-832.
REES, K.R. & TARLOW, M.J. (1967) The hepatotoxic action of allyl
formate. Biochem. J., 104, 757-761.
REID, W.D. (1972) Mechanism of allyl alcohol-induced hepatic
necrosis. Experientia, 28, 1058-1061.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver
aldehyde dehydrogenases: Relation to allyl alcohol hepatotoxicity.
Drug Metab. Disp., 15, 356-362.
RIKANS, L.E. & KOSANKE, S.D. (1984) Effect of aging on liver
glutathione levels and hepatocellular injury from carbon
tetrachloride, allyl alcohol or galactosamine. Drug and Chemical
Toxicology, 7, 595-604
SERAFINI-CESSI, F. (1972) Conversion of allyl alcohol into
acrolein by rat liver. Biochem. J., 128, 1103-1107.
SHELANSKI, M.V. & MOLDOVAN, M. (1971) Unpublished report of Food
and Drug Research Laboratories Inc. to RIFM. Submitted to WHO by
FEMA.
SMYTH, H.F., CARPENTER, C.P. & WEIL, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-
122.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964) A comparison of
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 6, 378-387.
TORKELSON, T.R., WOLF, M.A., OYEN, F. & ROWE, V.K. (1959) Vapor
toxicity of allyl alcohol as determined on laboratory animals. J.
Am. Ind. Hyg. Assoc., 20, 224-229.
WILD, D., KING, M.T., GOCKE, E. & ECKHARDT, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, Basc and micronucleus tests. Fd. Chem.
Toxicol., 21, 707-719.
YOO, Y.S. (1986) Mutagenic and antimutagenic activities of
flavouring agents used in foodstuffs. J. Osaka City Med. Center,
34, 267-288.222.
2.2 Toxicological studies
2.2.1 Acute toxicity
Species Sex Route LD50 Reference
mg/kg b.w.
Allyl hexanoate
Rat both oral 218 (186-255) Jenner et al.,
1964
Taylor et al.,
1964
both oral 327 (277-386) Meisel, 1982
male oral 393
female oral 276 (215-352)
Guinea both oral 280 (246-319) Jenner et al.,
pig 1964
Rabbit ? dermal 300 (200-600) Shelanski &
Moldovan,
1971
? dermal 820 (700-940) Moreno, 1974
Allyl heptanoate
Mouse both oral 630 (514-772) Jenner et al.,
1964
Rat both oral 500 (392-638) Jenner et al.,
1964
Rabbit ? dermal 810 (440-1180) Moreno, 1974a
Guinea both oral 444 (363-541) Jenner et al.,
pig 1964
Allyl isovalerate
Mouse both oral >500 NTP, 1983
Rat both oral >250 <500 NTP, 1983
? oral 230 (216-290) Moreno, 1977
Rabbit ? dermal 560 (290-1060) Moreno, 1977
2.2.2 Short-term studies
2.2.2.1 Mouse - allyl isovalerate
Groups of 5 male and 5 female B6C3F1 mice were given allyl
isovalerate by gavage in corn oil for 14 consecutive days at daily
doses of 0, 31, 62, 125, 250 or 500 mg/kg b.w. All animals of both
sexes that received 500 mg/kg b.w. died within 48 h of commencement
of the study; all other animals survived to termination.
Inactivity and piloerection were seen in animals receiving 250 and
500 mg/kg b.w. and male mice receiving 250 mg/kg gained no weight;
body weights in other dose groups were comparable to controls at
termination of the study (NTP, 1983).
Groups of 10 male and 10 female B6C3F1 mice, 6 weeks of age at
commencement of the study were dosed with allyl isovalerate by
gavage in corn oil at doses of 0, 15, 31, 62, 125 or 250 mg/kg b.w.
(five day/week) for 13 weeks. At the end of the study, autopsies
were performed on all survivors. Animals from control and highest
dose groups were subjected to detailed histological examination.
In addition the liver was examined histologically for the 62 and 125
mg/kg b.w. groups and in the latter group stomachs were also
examined microscopically. Five out of 10 males and six of 10
females in the highest dose groups died, all but one of the deaths
(a female) being compound related; deaths in other groups were
caused by gavage error. Compound related effects noted at necropsy
or histologically were limited to the 125 and 250 mg/kg b.w. dose
groups and included "thickening" of the urinary bladder wall and
gastric mucosa and small intestine (both groups) ulcerative
inflammation of the stomach, coagulative necrosis of the liver and
cytoplasmic vacuolation of the liver (top dose group only). No
compound related effects were seen in the liver, stomach or bladder
of mice from other groups (NTP, 1983).
2.2.2.2 Rat - allyl heptanoate
Allyl heptanoate was fed to weanling Osborne-Mendel rats of
both sexes for 18 weeks at dietary levels of 0, 1000, 2500 and
10,000 mg/kg diet. There was a dose-related growth depression which
was severe and associated with a poor food efficiency at the highest
dose level only. Gross liver enlargement was observed at all dose
levels; in addition, at the highest dose level kidneys were enlarged
in both sexes and hearts were enlarged in males only. Males in the
2500 and 10 000 mg/kg diet groups were reported to have enlarged
testes (it is not clear from the report whether this "enlargement"
relates to absolute or relative organ weights; if the latter, the
increased relative organ weights may relate to the growth depression
rather than to organ specific effects). Microscopic changes
reported included hydropic degeneration in the periportal areas of
the liver ranging from moderate at the highest dose level to lesser
degrees at lower dose levels. The extent of bile duct proliferation
correlated with the degree of hydropic degeneration and hepatocyte
enlargement also was seen in some groups (Hagan et al., 1965).
Groups of weanling Charles River albino rats of both sexes were
given allyl heptanoate in the diet daily at doses of 0, 49.6, 157
and 496 mg/kg b.w./day for 13 weeks. Weight gain and food intake
were recorded weekly, and urinalysis, haematological and clinical
chemical examinations were carried out at weeks 6 and 12. At
termination, autopsies were performed on all animals and detailed
histological examinations were carried out on all rats from the top
dose group and on half of the control animals. In the rest of the
controls and the other two dose groups, histological examination was
limited to liver and kidney, and to tissues showing gross
abnormalities at autopsy. There was a reduced food intake in the
treated groups, which was statistically significant in the high and
mid-dose groups, and an associated deficit in body weight gain. At
week 6 there was a small but significant depression in leucocyte
count in males of the top dose group only; no such effect was seen
in females and other haematological parameters were normal in
animals of both sexes. At week 12, no significant haematological
differences were seen other than a small non-dose-related decrease
in leucocyte count in the males of the 49.6 mg/kg dose group.
Clinical chemical examinations at weeks 6 and 12 revealed a decrease
in some parameters such as blood glucose, total serum protein and
albumin which appeared to be due to the reduced food intake and not
to specific toxic effects. Urine composition was unaffected by
treatment. At necropsy no gross nor microscopic lesions related to
treatment were observed and elevated relative organ weights in high-
dose males were related to reduced food intake and body weight gain.
It was concluded that daily treatment with allyl heptanoate in this
study did not result in any signs of toxicity; the reasons for the
reduced food intake could not be determined but might have been due
to unpalatability of diet (Damske et al., 1980).
Allyl hexanoate
Groups of 10 male and 10 female Osborne-Mendel rats were given
allyl hexanoate by gavage in corn oil at dose levels of 0, 15, 65
and 100 mg/kg b.w. daily for 18 weeks. Weight gain, food intake and
general condition were recorded weekly. At termination, the
animals were exsanguinated and haematological and gross pathological
examinations were performed. Detailed histological examinations
were performed only on eight rats from the control and high dose
groups; based on observations in the high dose group, livers from
eight animals in the 15 and 65 mg/kg b.w. dose groups were also
examined microscopically. In the high dose group, gross appearance
of the liver was described as nodular and wrinkled with granular or
rough surface. Microscopically the high dose group showed slight to
moderate bile duct proliferation, some "lobular architectural
disarrangement", slight fibrosis and pigment deposition in
macrophages; necrotic foci were seen in 2 of 8 animals examined. In
the 65 mg/kg b.w. dose group, very slight bile-duct proliferation
was observed in 2 of eight animals studied. The livers of the 15
mg/kg b.w. dose group were unaffected by treatment (Hagan et al.,
1967).
In a companion study to the foregoing, 5 rats of each sex
received allyl hexanoate in the diet at a concentration of 1000
mg/kg diet (equivalent to 50mg/kg b.w.) for 28 weeks. No adverse
effects were observed (Hagan et al., 1967).
Groups of 15 male and 15 female Wistar rats were given daily
oral doses of 0, 35 or 100 mg allyl hexanoate/kg b.w. as a solution
in corn oil; a further group of 10 animals of each sex were
similarly dosed with 12 mg/kg b.w./day for 13 weeks. Food and water
intake and body weights were recorded weekly, urinalysis was
performed during week 2, week 5 or 6, and in the final week of
treatment, and renal function tests were also carried out. At
termination, autopsies were performed and organ weights recorded,
and detailed histopathological examinations were conducted.
No differences were noted between treated and control animals
in body weight, water intake, haematological parameters, serum
chemistry, urine composition or in renal concentration tests. There
were slight increases in food intake in the highest dose group.
Liver weights were increased in the 35 and 100 mg/kg b.w. dose
groups and all treated animals showed evidence of periportal
vacuolation which was dose related in incidence and severity and
which in the 100 mg/kg b.w. group was accompanied by enlarged
hepatocytes, focal periportal necrosis and bile-duct proliferation.
Weights of spleen, kidneys, stomach and small intestine were
increased in both sexes in the highest dose group and small
intestine weight was also increased in females of the 35 mg/kg b.w.
group. It was not possible to determine a no observed adverse
effect level for allyl hexanoate in this study (Clode et al.,
1978).
When allyl hexanoate was administered to rats by gavage at a
daily dose level of 15 mg/kg b.w. for 18 weeks there were no
observed adverse effects (this study was reported in summary only)
(Bär & Griepentrog, 1967).
Allyl isovalerate
In similar studies to those described for mice, groups of 5
male and 5 female Fischer F344/N rats were given allyl isovalerate
by gavage for 14 days and groups of 10 animals of each sex were
dosed (5 day/week) for 13 weeks at levels of 0, 31, 62, 125, 250 and
500 mg/kg bw. In the fourteen days study, all rats given 500 mg/kg
b.w. and two rats of each sex given 250 mg/kg b.w. died. At
termination, mean body weights in the 250 mg/kg b.w. group were
depressed relative to weights in the control group by 23% and 13% in
males and females, respectively. Inactivity, laboured breathing,
diarrhoea and piloerection were observed in both sexes in the two
highest dose groups and in necropsy gross dark red areas were seen
in the stomachs of three animals of each sex at the top dose level.
In the thirteen week study, all ten males and 4/10 females that
received 250 mg/kg b.w. died and body weight gain was significantly
depressed in males of the 125 mg/kg b.w. group and females of the
250 mg/kg group. Dose-related effects seen at necropsy were
thickening of the intestinal wall and redness of the mucosal
surfaces of the intestines and urinary bladder. Enlargement of
internal lymph nodes and adrenals was reported but was unaccompanied
by histological lesions. Histopathological examination revealed
multifocal coagulative necrosis, cholangiofibrosis, and bile duct
hyperplasia at the 125 and 250 mg/kg b.w. dose levels. The effects
were dose related in incidence and no such lesions were observed in
the 31 and 62 mg/kg b.w. dose groups (NTP, 1983).
Combinations of esters
Groups of 10 male rats were given by gavage 21 consecutive
daily doses of allyl alcohol and a series of allyl esters (acetate,
propionate, hexanoate, isobutyrate, isovalerate and 2-
ethylhexanoate) at equimolecular doses corresponding to 5, 25 or 60
mg/kg b.w. of allyl alcohol. After 21 days the animals were
sacrificed and the livers examined histologically. The severity of
the liver lesions was classified according to the scheme: periportal
cell enlargement, followed by necrosis and subsequent fibrosis with
bile duct hyperplasia. The severity of the hepatic lesions from the
straight chain esters was similar to that produced by the
corresponding dose of allyl alcohol and more marked than that
produced by the esters of the branched chain acids. The differences
were attributed to differences in the rate of hydrolysis since the
straight chain esters were hydrolysed in vitro approximately 100
times faster than the branched chain esters (Butterworth et al.,
1975).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mouse - allyl isovalerate
Groups of 50 male and 50 female B6C3F1 mice, initially 50 days
old, received allyl isovalerate at doses of 0, 31 or 62 mg/kg b.w.
by gavage in corn oil (10 ml/kg b.w.) five times per week for 103
weeks. Survivors were killed at 112-114 weeks of age. No
significant differences were observed in survival rates in males;
reduced mean body weight gain and significantly lower survival rate
in the lower dose group females was attributed to a high incidence
of a genital tract infection.
An increase in the incidence of epithelial hyperplasia and
squamous cell papillomas was observed in the non-glandular
forestomach in male mice; the observed incidences for hyperplasia
were 1/50, 1/50 and 7/48, and for papillomas 0/50, 1/50 and 3/48 in
the control, low and high dose groups respectively. In females, the
corresponding incidences for forestomach epithelial hyperplasia were
0/50, 2/50 and 3/50, and for squamous cell papillomas were 1/50,
0/50 and 2/50 respectively. The incidence of lymphomas was slightly
elevated in males (5/50, 6/50 and 8/50 in the respective dose
groups) but the increase was not significant by the trend test nor
by the incidental tumour test; in females the corresponding
incidences were 11/50, 11/50 and 18/50 which gave a dose-response
trend, the high dose tumour incidence being significantly higher
(P<0.05) than controls. Significant reductions in tumour incidence
were observed in treated male mice in respect of hepatocellular
carcinomas (18/50, 6/50, 9/50), alveolar/bronchiolar adenomas or
carcinomas (13/50, 6/50 5/49) and for thyroid follicular cell
adenomas (5/47, 0/46, 1/49). No treatment-related non-neoplastic
lesions were observed in mice of either sex (NTP, 1983).
2.2.3.2 Rat - allyl hexanoate
A group of 5 male and 5 female Osborne-Mendel rats were given
allyl hexanoate in the diet at a concentration of 2500 mg/kg
(equivalent to 125 mg/kg b.w.) for 1 year. Body weight and food
intake were recorded weekly and haematological examinations were
carried out at 3, 6, and 12 months. At termination, detailed
histological examinations were performed. No adverse effects were
reported, in contrast with the short-term (18 week) study where
minimal effects (very slight bile duct proliferation) were reported
at a dose level of 65 mg/kg b.w. (Hagan et al., 1967).
When allyl hexanoate was fed to rats at a dietary concentration
of 0.5% for 1.5 years, 2/25 animals developed multiple bile duct
adenomas and proliferative changes of the small bile ducts. An
additional animal was reported to have adenomas (location not
specified) after 8.5 months. The authors concluded that the small
number of animals and tumour incidence were insufficient to allow a
firm conclusion to be reached on the significance (Bär &
Griepentrog, 1967) (This report was in summary only).
Allyl isovalerate
Groups of 50 male and 50 female Fischer 344/N rats, initially
46 days old, were given allyl isovalerate at doses of 0, 31 or 62
mg/kg b.w. by gavage in corn oil (5 ml/kg b.w.) five times per week
for 103 weeks. Survivors were killed at 112-114 weeks of age when
for males the numbers of survivors were 34 controls, 30 low dose and
28 high dose; the corresponding numbers of female survivors were
38, 36 and 29 respectively. There was a dose related increase in
mononuclear-cell leukaemia, the incidences observed were 1/50, 4/50
and 7/50 in males of the control, low- and high dose groups; in
females the corresponding incidences were 4/50, 6/50 and 9/49. In
both sexes there was a significant dose-response trend (p<0.05),
while the incidence in high-dose males was significantly higher than
controls (p<0.05). Increased frequencies of non-neoplastic lesions
(cholangiofibrosis, nodular regeneration, cirrhosis, focal
periportal necrosis, fatty changes and cytoplasmic vacuolation) were
observed in the livers of animals of both sexes in the high dose
group but there was no increase in liver neoplasms (NTP, 1983).
The authors of the NTP report concluded that allyl isovalerate
was carcinogenic, causing increased incidence of haematopoietic
system neoplasms (mononuclear cell leukaemias in male rats and
lymphoma in female mice). In reviewing this and other relevant
biological data, the International Agency for Research on Cancer
concluded that there was limited evidence for the carcinogenicity of
allyl isovalerate to experimental animals (IARC, 1985).
2.2.3.3 Dog - allyl heptanoate
Four groups of 3 male and 3 female beagle dogs were given daily
doses of allyl heptanoate of 0, 5, 25 and 75 mg/kg b.w. by capsule
for up to 18 months. All the dogs in the top dose group died within
3 - 7 months; dogs in the two lower treatment groups were reported
as surviving after 18 months. Administration of 75 mg/kg b.w.
caused depressed growth and macroscopic changes in the appearance of
the liver and haemorrhagic changes in the gastric mucosa. Less
consistent changes were reported in the form of cysts in the urinary
bladder and congestion in the lungs, digestive tract, kidneys,
spleen and lymph nodes. Microscopically the livers showed a slight
to moderate periportal fibrosis associated with slight to moderate
proliferation of the bile duct epithelium. Slight fatty changes
were also observed. The stomachs showed diffuse haemorrhage and
necrosis of the mucosae with instances of focal sub-mucosal
haemorrhage (Hagan et al., 1965).
2.2.4 Special studies on skin irritation
During an acute dermal toxicity study on allyl heptanoate in
rabbits, skin irritancy was evaluated on day 1. At dermal doses of
313-1250 mg/kg slight to moderate redness and oedema were reported
(5000 mg/kg was a lethal dose) (Moreno, 1974). Similar results were
obtained with allyl heptanoate (Moreno, 1974a). Allyl isovalerate
applied undiluted to intact or abraded rabbit skin under occlusion
for 24h was moderately irritating (Moreno, 1977).
In a preliminary to a maximization test, 48 hour closed patch
tests were carried out on the forearms of 5 volunteers with allyl
hexanoate and four subjects displayed grade 1 irritation (Kligman,
1971). Conversely, in a later study in which 5 volunteers were
subjected to patch tests, no signs of irritation were observed with
allyl hexanoate nor with allyl heptanoate (Kligman, 1975; 1975a).
Similarly, allyl isovalerate was without irritant effect in a closed
patch study on the backs of 28 subjects (Epstein, 1976).
2.2.5 Special studies on contact sensitization
In a maximization test on 25 healthy volunteers, allyl
hexanoate was reported to produce 13 cases of sensitization and was
considered a moderate sensitizer (Kligman, 1975); this contrasts
with an earlier maximization test on a similar number of volunteers
in which no cases of sensitization were detected (Kligman 1971). No
sensitization was observed with allyl heptanoate using a similar
protocol (Kligman, 1975a) nor with allyl isovalerate in 28
volunteers (Epstein, 1976).
2.2.6 Special studies on the haematopoietic and immunologic systems
Following the observation, in carcinogenicity studies on allyl
isovalerate, of marginal increases in mononuclear cell leukaemia in
rats and of malignant lymphoma in mice (NTP, 1983) and in view of
the fact that isovaleric acidaemia has been associated with
pancytopenia in humans, the effects of allyl isovalerate on the
haematopoietic and immune systems of female B6C3F1 mice and Fischer
344/N rats of both sexes were investigated in a short-term (14 day)
study. The animals were dosed by gavage for 5 days per week for 2
weeks with allyl isovalerate in corn oil at dose levels of 0, 31, 62
or 125 mg/kg b.w. (rats and mice) or 250 mg/kg b.w. (rats only).
Haematological, immunological and histological studies were
performed 48-72 h after the final treatment. In addition, bone
marrow slides from female mice from the NTP 13-week study (see
short-term studies) were also examined. The body weights of rats of
both sexes were reduced at the 250mg/kg b.w. dose level and of males
at the 125 mg/kg b.w. level. No changes in the body weights of the
female mice were observed but there was a 20% increase in mean
spleen weight and the splenic follicles were large with prominent
germinal centres. No treatment-related effects were seen in
haematological parameters nor in bone-marrow cellularity in mice or
rats. However, there were significant decreases in pluripotent
haematopoietic stem cells (CFU-S) in the spleen and in granulocyte-
macrophage progenitors (CFU-GM) in the bone marrow of treated mice.
In vitro enzyme assays of these cells showed that haematopoietic
suppression was correlated with a depression of hexose monophosphate
shunt metabolism but that enzymes of the Embden-Meyerhof and
tricarboxylic acid pathways were unaffected. Examination of host
resistance in mice following challenge with Plasmodium yoelii or
Listeria monocytogenes showed no significant differences between
control and treated animals, nor were there other effects on the
immune system. The authors concluded that the myelotoxic effects
were minimal and of a degree that did not alter host resistance
(Hong et al., 1988).
Minimal to moderate hypocellularity of the bone marrow was
observed in the 125 mg/kg b.w. group of mice, both sexes, from the
13-week NTP study and was most striking for megakaryocytes. The
degree of hypocellularity was never severe.
2.2.7 Special studies on genotoxicity
The genotoxicity of allyl hexanoate and allyl isovalerate are
shown on the next page.
2.2.8 Special studies on metabolites
2.2.8.1 Biochemical aspects
For pathways of metabolism of allyl alcohol see Biochemical
Aspects section of allyl esters.
Test system Test object Concentration Result Reference
ALLYL HEXANOATE
Ames test1 S. typhimurium 0-3.5mg/plate - Wild et al., 1983
TA98, TA100,
TA1535, TA1537,
TA1538
Ames test1 S. typhimurium 10.5 µg/plate - Oda et al., 1978
TA98, TA100
Rec assay B. subtilis 0-18 µg/disc + Oda et al., 1978
H17 vs M45
Rec assay B. subtilis 0-20µg/disc - Yoo, 1986
H17 vs M45
Basic test Drosophila 0.5mM in feed - Wild et al., 1983
(sex linked melanogaster
recessive)
Micronucleus Mouse 2 x 156 mg/kg - Wild et al., 1983
test bw i.p.
ALLYL ISOVALERATE
Ames test1,2 S. typhimurium 0 - 1000 µg/ - NTP, 1983;
TA98, TA100 plate Mortelmans et al.,
TA1535, TA1537 1986
Basic test Drosophila 1200 - 2000 - Woodruff et al.,
melanogaster mg/l in feed 1985
4500mg/l injected
injected
(contd)
Test system Test object Concentration Result Reference
ALLYL HEPTANOATE
No mutagenicity data were available for allyl heptanoate
1 Both with and without rat liver S9 fraction
2 Using a preincubation protocol
2.2.8.2 Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse male oral 96(84-110) Dunlap et al., 1958
i.p. 60
Rat ? oral 64(56-74) Smyth et al., 1951
Rat both oral 70(63-79) Taylor et al., 1964
Rat male oral 105(79-140)* Dunlap et al., 1958
99(75-130)**
i.p. 42(32-55)
Rabbit male oral 71(42-125) Dunlap et al., 1958
percut. 89(40-250)
* Rats 111-143 g b.w.
** Rats 170-252 g b.w.
2.2.8.3 Short-term studies
Following acute or short-term exposure to allyl alcohol, the
main target organ is the liver in which typical periportal changes
are observed, ranging from fatty changes to cell necrosis (Piazza,
1915; Dunlap & Hine, 1955; Dunlap et al., 1958; Torkelson et
al., 1959; Rees & Tarlow, 1967; Serafini-Cessi, 1972). The
kidney may also be affected, changes reported include necrosis of
the epithelium of the convoluted tubules and proliferation of
interstitial tissue.
Rat
Groups of 10 rats (strain and sex not specified) received allyl
alcohol in the drinking water at doses of 1.3-1.97 mg/kg b.w. The
top dose level was associated with reduced appetite and increased
mortality. The highest no effect level was 4 mg/kg b.w. The
corresponding no effect level for acrolein was 0.17 mg/kg b.w.
(Smyth et al., 1951).
Groups of six male and six female rats received allyl alcohol
in drinking water at concentrations of 0, 1, 5, 50, 100, 250, 500 or
1000 mg/L for 13 weeks, corresponding to daily intakes of 0.13,
0.62, 5.9, 11.6, 25.5, 41.0 or 72 mg/kg b.w. for males and 0.17,
0.94, 7.34, 13.2, 34.0, 43.7 or 67.4 mg/kg b.w. for females in the
respective dose groups. At termination, histological examination
(12 organs) was performed on half of the animals in each group.
Few gross abnormalities were seen at autopsy; peritoneal fat was
decreased in the 500 mg/L group and absent at 1000 mg/L. The no
effect level reported from this study was approx. 12 mg/kg b.w./day
while at an average 29 mg/kg b.w./day the only noticeable effect was
an increased liver weight in males and kidney weight in females
(Dunlap et al., 1958).
Male Wistar albino rats were given daily intragastric doses of
allyl alcohol of 0 or 30 mg/kg b.w. in corn oil for periods of 1, 10
or 28 days. The administration of a single dose produced marked
periportal necrosis and associated losses of alcohol dehydrogenase
and succinate dehydrogenase activities; hepatic cytochrome P450
concentrations and benzo[a]pyrene hydroxylase activities were
reduced to about 60% of control values. Conversely, further daily
dosing for 10 or 28 days led to a recovery both of histological
appearance and of enzyme activities. It was concluded that
metabolism of allyl alcohol becomes modified by repeated treatment
(Lake et al., 1975). Similarly, no hepatic injury was observed
following 28 daily oral doses of 25 mg/kg b.w., although direct
infusion of acrolein caused typical necrotic changes (Butterworth
et al., 1978).
Groups of 15 male and 15 female Wistar rats were given allyl
alcohol in drinking water at concentrations of 0, 50, 100, 200 or
800 mg/l for 15 weeks. No treatment-related effects were observed
in results of haematological examinations or analysis of serum.
There was a dose-related decrease in fluid intake at all treatment
levels in both sexes while growth and food consumption were reduced
in both sexes at 800 mg/l and males given 200 or 800 mg/L produced
less urine than controls in a period following water deprivation or
water loading. Increased relative weights of liver, spleen and
kidney were observed at both sexes at the top dose level; relative
kidney weights were also higher in the 200 mg/l group and in females
given 100 mg/l. No effects due to treatment were seen at autopsy
or histologically. The no observed adverse effect level was 50
mg/l, equal to 4.8-6.2 mg allyl alcohol/kg b.w./day (Carpanini et
al., 1978).
Although the effects of allyl alcohol are almost exclusively
observed in the liver, changes in the pancreas described as
acidophilia, vacuolation and necrosis of pancreatic acinar cells
were reported following oral administration of a single dose of 50
mg/kg b.w. (Nizze et al., 1979).
2.2.8.4 Special studies on genotoxicity
The genotoxicity of allyl alcohol, acrolein and glycidol are
shown in Table 3.
Test system Test object Compound & Result Reference
concentration
Ames test1 S. typhimurium Allyl alcohol Positive2 Lutz et al., 1982
(liquid TA100 Acrolein Positive
suspension) Glycidol Weak
positive2
Ames test1 S. typhimurium Allyl alcohol Negative Principe et al.,
TA98, TA100, 0.025-0.1 1981
TA1535, TA1537, µl/plate
TA1538
Forward S. coelicolor Allyl alcohol Negative Principe et al.,
mutation 2-100µl/plate 1981
8-azaguanine Aspergillus Allyl alcohol Negative Principe et al.,
resistance nidulans 10-40 µl/plate 1981
(point
mutation)
Ames test1 S. typhimurium Allyl alcohol Negative Bignami et al.,
TA98, TA100, Acrolein Positive 1977
TA1535, TA1538 (TA1538,
TA98)
Ames test1 S. typhimurium Acrolein Negative Sasaki & Endo,
TA98, TA100 TA98, TA100 1978
1 With and without rat liver S9-fraction
2 Lower in the presence of S9-fraction
2.2.8.5 Special studies on mechanisms of liver injury by metabolites
The extent of damage to the liver by allyl alcohol was
increased by the aldehyde dehydrogenase inhibitors disulfiram or
cyanamide (Rikans, 1987; Jaeschke et al., 1987) or by
phenobarbital induction, and was moderated by the alcohol
dehydrogenase inhibitor, pyrazole (Diluzio & Hoffman, 1973). It was
concluded that the toxicity is due to the formation of acrolein from
allyl alcohol (Reid, 1972; Serafini-Cessi, 1972; Patel et al.,
1980; Rikans, 1987). In agreement with this conclusion, co-
administration of ethanol (3 g/kg b.w.) to rats inhibited the rate
of allyl alcohol oxidation by more than 90% and the histological
changes were completely prevented, despite glutathione levels being
depressed (Penttila et al., 1987). As indicated by the results
with disulfiram and cyanamide, oxidation of acrolein by aldehyde
dehydrogenase is an important detoxication step for allyl alcohol-
derived acrolein (Rikans, 1987; Jaeschke et al., 1987).
In mice, allyl alcohol at a dose of 1 mmole/kg b.w. almost
totally depleted hepatic glutathione with subsequent massive lipid
peroxidation while enhanced glutathione levels protected against
hepatotoxicity of allyl alcohol (Jaeschke et al., 1987). In
vitro, allyl alcohol, acrolein and glycidol react with glutathione
by a non-enzymic mechanism (Dore & Montaldo, 1984).
The severity of liver damage 24h after i.p. administration of
allyl alcohol (0.036 µl/kg b.w.) was evaluated in male rats at 4-5,
14-15 or 24-25 months of age. Allyl hepatotoxicity increased as a
function of age but hepatic glutathione levels were unaffected
indicating that the age-related susceptibility was not due to
diminished availability of glutathione (Rikans & Kosanke, 1984).
The location of allyl alcohol or acrolein-induced hepatic
injury is usually periportal but centrilobular necrosis was induced
by using retrograde infusion (Belinsky et al., 1983) and
metabolism of allyl alcohol by alcohol dehydrogenase occurred at
similar rates in both periportal and centrilobular regions. It was
suggested that periportal necrosis seen after oral dosing is due to
greater sensitivity of the mitochondrial respiratory chain to the
toxic effects of acrolein in periportal cells.
2.2.8.6 In vitro studies on metabolite damage to kidney cells
In freshly isolated renal epithelial cells from rats, allyl
alcohol toxicity as assessed by glutathione depletion and loss of
cell viability was more severe in cells from female animals. This
correlated with higher alcohol dehydrogenase activity (Ohno et al.,
1985).
3. COMMENTS
In evaluating these flavours, the Committee noted that they are
rapidly hydrolysed to allyl alcohol and the corresponding acids by
intestinal mucosal, pancreatic and hepatic esterases. The results
of studies on the toxicity of the three esters indicated that the
hepatotoxicity observed at high doses was due to the allyl alcohol
and its metabolites. Accordingly the Committee considered
supplementary toxicological data on allyl alcohol and concluded that
the three esters should be evaluated for a group ADI on the basis of
the allyl alcohol moiety. In its evaluation, the Committee also
took account of the principles relating to food flavours outlined in
Principles for the safety assessment of food additives and
contaminants in food (Annex I, ref. 76).
The hepatotoxicity of the esters and of allyl alcohol was less
marked in repeated-dose short-term studies than in single-dose acute
studies, although the mechanism of the acquired tolerance has not
been fully elucidated. Mutagenicity studies on allyl hexanoate and
allyl isovalerate yielded negative results, while most tests on
allyl alcohol were negative.
The Committee reviewed two long-term carcinogenicity studies in
rats and mice in which allyl isovalerate was administered by gavage
in corn oil at both the maximum tolerated dose and 50% of this dose.
Epithelial hyperplasia and squamous-cell papillomas of the
forestomach were observed in mice, but not rats, at the highest
dose. There was no evidence of hepatic tumours in mice (the liver
being the target organ for short-term toxicity). The Committee
concluded that these results were not relevant to the low-dose,
dietary exposure to allyl isovalerate as a food flavour but were
probably due to the effects of the large bolus doses that were used.
The Committee also noted the small increase in the incidence of
leukaemia reported in the treated rats; however, the incidence was
within the historical control range and no increase in the incidence
of hepatic tumours occurred in rats. Since levels of dietary
exposure to allyl isovalerate in food are much lower than the doses
used in these studies, the Committee concluded that an ADI could be
set.
The evaluation was based on the no-observed-effect-level in
short-term studies on allyl alcohol, with particular reference to
hepatotoxicity; this provides a more conservative estimate than one
based on the no-observed-effect level for the esters. The Committee
noted that a number of other food flavours in use which are allyl
esters and should be considered for inclusion in the group ADI on
the basis of their hydrolysis to allyl alcohol. In addition, in
view of evidence that allyl esters of such fatty acids as acetate,
propionate, isobutyrate, and 2-ethylhexanoate are also rapidly
hydrolysed, the Committee considered that their consumption should
be taken into account since they could contribute to the total
dietary load of allyl alcohol.
4. EVALUATION
The Committee allocated an ADI of 0-0.05 mg/kg b.w. as allyl
alcohol equivalent for allyl heptanoate, allyl hexanoate, and allyl
isovalerate, which corresponds to 0-0.15 mg/kg b.w. allyl
heptanoate, 0-0.13 mg/kg b.w. allyl hexanoate, 0-0.12 mg/kg b.w.
allyl isovalerate, or combinations of these pro rata.
5. REFERENCES
BAR, von F. & GRIEPENTROG, F. (1967) Die situation in der
gesundheitlichen Beurteilung der Aromatisierungsmittel für
Lebensmittel. Medizin und Ernahrung, 8, 244-251.
BELINSKY, S.A., MATSUMURA, T., KAUFFMAN, F.C. & THURMAN, R.G. (1984)
Rates of allyl alcohol metabolism in periportal and pericentral
regions of the liver lobule. Mol. Pharmacol., 25, 158-164.
BELINSKY, S.A., BRADFORD, B.U., FORMAN, D.T., GLASSMAN, E.B.,
FELDER, M.R. & THURMAN, R.G. (1985) Hepatotoxicity due to allyl
alcohol in deermice depends on alcohol dehydrogenase. Hepatology,
5, 1179-1182.
BUTTERWORTH, K.R., CARPANINI, F.M.B., DUNNINGTON, R., GRASSO, P. &
PELLING, R. (1978) The production of periportal necrosis by allyl
alcohol in the rat. Proc. Brit. Pharm. Soc., 57, 353P-354P.
BUTTERWORTH, K.R., CARPANINI, F.M.B., GAUNT, I.F., GRASSO, P. &
LLOYD, A.G. (1975) A new approach to the evaluation of the safety
of flavouring esters. Brit. J. Pharm., 54, 268P.
CARPANINI, F.M.B., GAUNT, I.F., HARDY, J., GANGOLLI, S.D.,
BUTTERWORTH, K.R. & LLOYD, A.G. (1978) Short-term toxicity of
allyl alcohol in rats. Toxicology, 9, 29-45.
CLAPP, J.J., KAYE, C.M. & YOUNG, L. (1969) Observations on the
metabolism of allyl compounds in the rat. Biochem. J., 114, 6P.
CLODE, S.A., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P. & GANGOLLI,
S.D. (1978) Short-term toxicity study of allyl caproate in rats.
Fd. Cosmet. Toxicol., 16, 197-201.
DAMSKE, D.R., MECLER, F.J., BELILES, R.P. & WEIR, R.J. (1980) 90-
day toxicity study in rats: allyl heptanoate. Unpublished report
of Litton Bionetics Inc., LBI project No. 21130-01 & -04. Submitted
to WHO by FEMA.
DILUZIO, N.R. & HOFFMAN, E.O. (1973). Protective influence of
pyrazole on allyl formate induced injury. Gastroenterol., 64,
158.
DORE, M. & MONTALDO, C. (1984) Studi sulla coniugazione in virto
dell'alcool allilico e dei suoi metaboliti con il glutatione
ridotto. Boll. Soc. It. Biol. Sper., 60, 1497-1501.
DUNLAP, M.K. & HINE, C.H. (1955) Toxicity of allyl alcohol. Fed.
Proc., 14, 335.
DUNLAP, M.K., KODAMA, J.K., WELLINGTON, J.S., ANDERSON, H.H. & HINE,
C.H. (1958) The toxicity of allyl alcohol I. Acute and chronic
toxicity. Arch. Ind. Hlth., 18, 303-311.
EPSTEIN, W.L. (1976) Unpublished report to RIFM dated 20th December
1976. Submitted to WHO by FEMA.
GRUNDSCHOBER, F. (1977) Toxicological assessment of flavouring
esters. Toxicology, 8, 387-390.
HAGAN, E.C., JENNER, P.M., JONES, W.I., FITZHUGH, O.G., LONG, E.L.,
BROUWER, J.G. & WEBB, W.K. (1965) Toxic properties of compounds
related to safrole. Toxicol. appl. Pharmacol., 7, 18-24.
HAGAN, E.C., HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES,
W.I., TAYLOR, J.M., LONG, E.L., NELSON, A.A. & BROUWER, J.B. (1967)
Food flavourings and compounds of related structure. II. Subacute
and chronic toxicity. Fd. Cosmet. Toxicol., 5, 141-157.
HONG, H.L., HUFF, J.E., LUSTER, M.I., MARONPOT, R.T., DIETER, M.P.,
HAYES, H.T. & BOORMAN, G.A. (1988) The effects of allyl
isovalerate on the haematopoietic and immunologic systems in
rodents. Fund. Appl. Toxicol., 10, 655-663.
IARC (1985) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans. Vol.36, Allyl compounds, aldehydes,
epoxides and peroxides. International Agency for Research on Cancer;
Lyon, pp. 69-74.
JAESCHKE, H., KLEINWAECHTER, C. & WENDEL, A. (1987), The role of
acrolein in allyl alcohol-induced lipid peroxidation and liver cell
damage in mice. Biochem. Pharmacol., 36, 51-57.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L. & FITZHUGH, O.G.
(1964) Food flavourings and compounds of related structure I. Acute
oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
KAYE, C.M. & YOUNG, L. (1970) Mercapturic acid formation from allyl
compounds in the rat. Biochem. J., 119, 53P.
KAYE, C.M. & YOUNG, L. (1972) The synthesis of mercapturic acids
from allyl compounds in the rat. Biochem. J., 127, 87P.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134, 1093-1101.
KLIGMAN, A.M. (1971) Unpublished report dated 27th September, 1971
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975a) Unpublished report dated 14th February, 1975
to RIFM. Submitted to WHO by FEMA.
KLIGMAN, A.M. (1975b) Unpublished report dated 10th June, 1975 to
RIFM. Submitted to WHO by FEMA.
LAKE,, B.G., GANGOLLI, S.D., WRIGHT, M.G., GRASSO, P., CARPANINI,
F.M.B. & BUTTERWORTH, K.R. (1975) The effect of repeated
administration on allyl alcohol-induced hepatotoxicity in the rat.
Biochem. Soc. Trans., 6, 145--146.
LUTZ, D., EDER, E., NEUDECKER, T. & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha, ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93, 305-315.
LONGLAND, R.C., SHILLING, W.H. & GANGOLLI, S.D. (1977) The
hydrolysis of flavouring esters by artificial gastrointestinal
juices and rat tissue preparations. Toxicology, 8, 197-204.
MEISEL, M.L. (1982) Caproate allyl, Ro 81-3538/000: An acute oral
toxicity study (LD50) in the rat. Unpublished report No. 100-
161/106 prepared by Hazleton Laboratories Deutschland GmbH.
Submitted to WHO by FEMA.
MORENO, O.M. (1974a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-676. Submitted to WHO
by FEMA.
MORENO, O.M. (1974b) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 74-677. Submitted to WHO
by FEMA.
MORENO, O.M. (1977a) Unpublished report of M.B. Research
Laboratories Inc. to RIFM, Project No. MB 76-1446. Submitted to
WHO by FEMA.
MORENO, O.M. (1977b) Unpublished report to RIFM dated 27th
January, 1977. Submitted to WHO by FEMA.
MORTELMANS, K., HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986) Salmonella Mutagenicity tests: II. Results
from the testing of 270 chemicals. Environ. Mutagen., 8, Supp.7,
1-119.
NATIONAL TOXICOLOGY PROGRAM (NTP) (1983) Carcinogenesis studies of
allyl isovalerate. Report No. NTP-TR-253; PB-83-2509.
NIZZE, E., LAPIS, K. & KOVACS, L. (1979) Allyl alcohol-induced
changes in the rat exocrine pancreas. Digestion, 19, 359-369.
ODA, Y., HAMONO, Y., INOUE, K., YAMAMOTO, H., NIIHARA, T. & KUNITA,
N. (1978) Mutagenicity of food flavours in bacteria (1st report).
Shouhin Eisei Hen, 9, 177-181.
OHNO, Y., JONES, T.W. & ORMSTAD, K. (1985) Allyl alcohol toxicity
in isolated renal epithelial cells: protective effects of low
molecular weight thiols. Chem. biol. Interactions, 52, 289-299.
PATEL, J.M., WOOD, J.C. & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparation. Drug Metab. Disposition, 8, 305-308.
PENTTILÄ, K.E., MÄKINEN, J. & LINDROS, K.O. (1987) Allyl alcohol
liver injury: suppression by ethanol and relation to transient
glutathione depletion. Pharmacol. Toxicol., 60, 340-344.
PIAZZA, J.G. (1915) Zur kehntnis der Wirkung der Allylverbindungen
Z. Exp. Path. Ther., 17, 318.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE, E.,
FABRIXI, M., CONTI, G. & COMBA, P. (1981) Mutagenicity of
compounds of industrial and agricultural relevance in Salmonella,
Streptomyces and Aspergillus. J. Sci. Fd. Agric. 32, 826-832.
REES, K.R. & TARLOW, M.J. (1967) The hepatotoxic action of allyl
formate. Biochem. J., 104, 757-761.
REID, W.D. (1972) Mechanism of allyl alcohol-induced hepatic
necrosis. Experientia, 28, 1058-1061.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver
aldehyde dehydrogenases: Relation to allyl alcohol hepatotoxicity.
Drug Metab. Disp., 15, 356-362.
RIKANS, L.E. & KOSANKE, S.D. (1984) Effect of aging on liver
glutathione levels and hepatocellular injury from carbon
tetrachloride, allyl alcohol or galactosamine. Drug and Chemical
Toxicology, 7, 595-604
SERAFINI-CESSI, F. (1972) Conversion of allyl alcohol into
acrolein by rat liver. Biochem. J., 128, 1103-1107.
SHELANSKI, M.V. & MOLDOVAN, M. (1971) Unpublished report of Food
and Drug Research Laboratories Inc. to RIFM. Submitted to WHO by
FEMA.
SMYTH, H.F., CARPENTER, C.P. & WEIL, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-
122.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964) A comparison of
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 6, 378-387.
TORKELSON, T.R., WOLF, M.A., OYEN, F. & ROWE, V.K. (1959) Vapor
toxicity of allyl alcohol as determined on laboratory animals. J.
Am. Ind. Hyg. Assoc., 20, 224-229.
WILD, D., KING, M.T., GOCKE, E. & ECKHARDT, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, Basc and micronucleus tests. Fd. Chem.
Toxicol., 21, 707-719.
YOO, Y.S. (1986) Mutagenic and antimutagenic activities of
flavouring agents used in foodstuffs. J. Osaka City Med. Center,
34, 267-288.222.
