
TRANS-ANETHOLE
First draft prepared by Dr F.S.D. Lin,
Division of Toxicological Review and Evaluation,
Center for Food Safety and Applied Nutrition,
US Food and Drug Administration
1. EXPLANATION
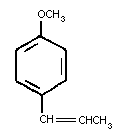
Trans-anethole is a flavoring agent present in the essential
oils of anise, fennel and star anise. Chemically it is an
alkenylbenzene identified as (1-methoxy-4-(1-propenyl) benzene or
para-propenylanisole, with the following chemical structure:
 Trans-anethole was first evaluated by the eleventh meeting of
the Committee when a conditional ADI of 0-1.25 mg per kg of body
weight was allocated (Annex 1, Ref. 14). After re-evaluation at the
twenty-third meeting (Annex 1, reference 50), a temporary ADI of 0-2.5
mg per kg of body weight was allocated pending submission of the
results of an adequate long-term study. After further reviews at the
thirty-first and thirty-third meetings (Annex 1, references 77 and 83,
the Committee extended the temporary ADI but reduced it to 0-1.2 mg
per kg of body weight pending further details of the long-term study
and a review of the detailed study records and of the histological
material.
Since the last evaluation, the new data have been reviewed,
together with new data which has become available in the interim, and
are summarized and discussed in the following monograph. The
previously published monograph has been expanded and is reproduced in
its entirety below.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.2.1 Absorption, distribution, biotransformation and excretion
Trans-anethole is rapidly absorbed and distributed in the rat (Le
Bourhis, 1973; Fritsch et al., 1975) and in the mouse (Le Bourhis,
1968; Strolin-Benedetti & Le Bourhis, 1972; Le Bourhis, 1973). The
same authors reported rapid metabolism in both species and identified
the following urinary metabolites:
% dose in urine
1. p-hydroxypropenylbenzene 32
2. p-hydroxycinnamic acid 15
3. p-methoxybenzoic acid 4
4. p-methoxyhippuric acid 43
5. p-methoxyacetophenone trace
Metabolites (1) and (2) appeared largely as the glucuronide or
sulfate. Virtually identical results were obtained by the i.p. route
(Solheim & Scheline, 1973). Administration to rats of ring or methoxy
side-chain 14C-labelled trans-anethole confirmed that virtually all
ring metabolites were eliminated in urine within 48 hrs with
substantial demethylation and consequent appearance of the methoxy 14C
in expired air, and to a small extent the body and faeces (Strolin-
Benedetti & Le Bourhis, 1972).
Trans-anethole was hydroxylated at the 3'-carbon by hepatic
microsomes from rats and mice (Swanson et al., 1981).
In five human subjects given a 500 mg dose the 24-hour
metabolites were anisic acid (52%) and p-hydroxybenzoic acid (5%).
Trans-anethole was not detectable in the blood (Le Bourhis, 1973).
Metabolism in rabbits appeared to be similar to that in humans
(Axelrod, 1956; Le Bourhis, 1970).
More recently, Sangster et al., have compared the routes of
metabolic degradation of various anisole derivatives, including
trans-anethole, in both rat and mouse, and the route of degradation
was dose dependent (Sangster, 1983; Sangster et al., 1984a,b).
After administration of a single dose of 50 mg trans-[14C methoxy]-
anethole/kg b.w. orally to female rats or i.p. to male mice, the major
routes of excretion were in the urine or as 14CO2 in expired air;
excretion in the faeces or as volatile metabolites (other than CO2)
in expired air was low (<2% of the dose). Eleven urinary metabolites
were identified in the rat and ten in the mouse, arising from side-
chain oxidation and cleavage, O-demethylation and conjugation.
Approximately 4.4% and 10.6% of the dose was excreted as two
diastereoisomers of 1-(4'methoxyphenyl)propan-1,2-diol in mice and
rats respectively; these metabolites had presumably arisen from
epoxidation of the side-chain double bond (Sangster et al., 1984a).
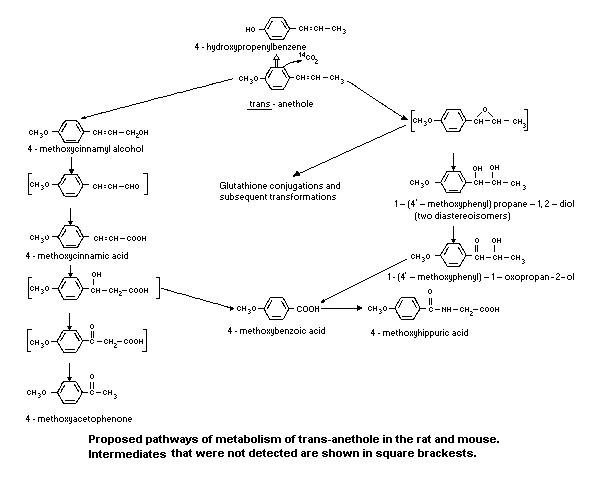
The pathways of metabolism identified are as shown in Figure 1.
Trans-anethole was first evaluated by the eleventh meeting of
the Committee when a conditional ADI of 0-1.25 mg per kg of body
weight was allocated (Annex 1, Ref. 14). After re-evaluation at the
twenty-third meeting (Annex 1, reference 50), a temporary ADI of 0-2.5
mg per kg of body weight was allocated pending submission of the
results of an adequate long-term study. After further reviews at the
thirty-first and thirty-third meetings (Annex 1, references 77 and 83,
the Committee extended the temporary ADI but reduced it to 0-1.2 mg
per kg of body weight pending further details of the long-term study
and a review of the detailed study records and of the histological
material.
Since the last evaluation, the new data have been reviewed,
together with new data which has become available in the interim, and
are summarized and discussed in the following monograph. The
previously published monograph has been expanded and is reproduced in
its entirety below.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.2.1 Absorption, distribution, biotransformation and excretion
Trans-anethole is rapidly absorbed and distributed in the rat (Le
Bourhis, 1973; Fritsch et al., 1975) and in the mouse (Le Bourhis,
1968; Strolin-Benedetti & Le Bourhis, 1972; Le Bourhis, 1973). The
same authors reported rapid metabolism in both species and identified
the following urinary metabolites:
% dose in urine
1. p-hydroxypropenylbenzene 32
2. p-hydroxycinnamic acid 15
3. p-methoxybenzoic acid 4
4. p-methoxyhippuric acid 43
5. p-methoxyacetophenone trace
Metabolites (1) and (2) appeared largely as the glucuronide or
sulfate. Virtually identical results were obtained by the i.p. route
(Solheim & Scheline, 1973). Administration to rats of ring or methoxy
side-chain 14C-labelled trans-anethole confirmed that virtually all
ring metabolites were eliminated in urine within 48 hrs with
substantial demethylation and consequent appearance of the methoxy 14C
in expired air, and to a small extent the body and faeces (Strolin-
Benedetti & Le Bourhis, 1972).
Trans-anethole was hydroxylated at the 3'-carbon by hepatic
microsomes from rats and mice (Swanson et al., 1981).
In five human subjects given a 500 mg dose the 24-hour
metabolites were anisic acid (52%) and p-hydroxybenzoic acid (5%).
Trans-anethole was not detectable in the blood (Le Bourhis, 1973).
Metabolism in rabbits appeared to be similar to that in humans
(Axelrod, 1956; Le Bourhis, 1970).
More recently, Sangster et al., have compared the routes of
metabolic degradation of various anisole derivatives, including
trans-anethole, in both rat and mouse, and the route of degradation
was dose dependent (Sangster, 1983; Sangster et al., 1984a,b).
After administration of a single dose of 50 mg trans-[14C methoxy]-
anethole/kg b.w. orally to female rats or i.p. to male mice, the major
routes of excretion were in the urine or as 14CO2 in expired air;
excretion in the faeces or as volatile metabolites (other than CO2)
in expired air was low (<2% of the dose). Eleven urinary metabolites
were identified in the rat and ten in the mouse, arising from side-
chain oxidation and cleavage, O-demethylation and conjugation.
Approximately 4.4% and 10.6% of the dose was excreted as two
diastereoisomers of 1-(4'methoxyphenyl)propan-1,2-diol in mice and
rats respectively; these metabolites had presumably arisen from
epoxidation of the side-chain double bond (Sangster et al., 1984a).
The pathways of metabolism identified are as shown in Figure 1.
 In studies of the dose dependence of trans-anethole metabolism,
single doses of 0.05, 5, 50, or 1500 mg/kg b.w. were administered to
female rats by gavage; similar doses were given to male mice
intraperitoneally with an additional dose level of 250 mg/kg b.w. also
being included. Dose and species-dependent differences in metabolism
were observed. The major route of metabolism was via O-
demethylation in both species and the significance of this route
decreased with increase in dose from 56 and 72% of the 0.05 mg/kg dose
to 32 and 35% of the highest dose in rats and mice respectively. With
regard to side-chain oxidation, the rat favoured the epoxidation route
and elimination of the diol-isomers rose from 2% to 15% of the dose
over the range of doses studied. In the mouse, w-oxidation was
favoured and the diols were formed in smaller amounts of from 1% to
4.5% over the same dose range (Sangster et al., 1984b). It should
be noted that the inter-species differences were compounded by the sex
difference between the species and by the different route of
administration.
Studies of the metabolism of several structurally-related food
flavours, including trans-anethole, were carried out in human
volunteers. Two male subjects received oral doses of 1 mg
14C[methoxy]- trans-anethole and the urine was collected over a 48
hour period subsequently; aliquots of expired air also were collected
at 30 minute intervals for 8 hours after dosing. Approximately 65% of
the activity was excreted in the 48 hour urine, mainly in the first 8
hours, while an estimated 20% of the dose was excreted as 14CO2 in
exhaled air over the first 8 hours. Most of the urinary activity was
in the form of 4-methoxyhippuric acid (ca 55% of the dose) and 4-
methoxybenzoic acid (ca 3.5% of the dose); approximately 3% of the
dose was excreted as the two diastereoisomers of 1-(4'-
methoxyphenyl)propane-1,2-diol (Sangster et al., 1987).
In a study of the dose-dependence of the metabolism of trans-
anethole in human volunteers, 5 male subjects received oral doses of
1, 50 or 250 mg 14C-[methoxy]- trans-anethole on three separate
occasions in milk. 14CO2 was estimated in expired air at intervals
up to 24 hours and radioactivity was measured in urine at intervals up
to 48 hours. Over the range of doses studied, dose had no systematic
effect on the rate or route of excretion, the major routes being urine
(54-69% of the dose) and expired air (13-17% of the dose). The
principal urinary metabolite (>90% of urinary 14C) was
methoxyhippuric acid with much smaller amounts of 4-methoxy-benzoic
acid and up to three other metabolites; the pattern of urinary
metabolites was unaffected by dose size (Caldwell & Sutton, 1988).
Studies of DNA binding of several alkenylbenzenes, including
trans-anethole, were carried out following i.p. administration to
neonatal and adult mice. Very low levels of adduct formation were
detected only when the dose (300 mg/kg i.p.) approached the LD50 (400
mg/kg b.w.).
Groups of 8 male and 8 female CD Sprague-Dawley rats were
administered (methoxy-14C)- trans-anethole at gavage doses of 100,
250, 500 and 1000 mg/kg (16 uCi/kg). Radioactivities appearing in the
urine, faeces and exhaled CO2 were determined at 24 hour intervals
for up to 72 hours, but only the summarized urine and CO2 data were
provided in the report. The urinary metabolites of trans-anethole
in these animals are said to be still under investigation. Based on
the available disposition data, the authors have concluded the
following:
(1) Excretion of 14C in the urine and expired air was delayed
as the dose was increased, with a progressively smaller
fraction of the total radioactivity excreted during 0-24
hours but a greater fraction in 24-48 hours.
(2) As the dose was increased, the excretion route was shifted
from the expired air to the urine.
(3) Females consistently excreted less of the given doses in 0-
24 hours than males, "with recovery of 14C in the urine and
as CO2 being approximately equally affected" (FEMA, 1989).
The Committee noted that the above data were not analyzed
statistically for intergroup differences. Also noted is
that with the possible exception of the dose-dependent delay
of its excretion, the other dose-dependent effects on the
disposition of trans-anethole, as concluded by the
authors, were not very apparent. Furthermore, the author's
conclusion that there is a sex-dependent difference in the
elimination of trans-anethole from the test animals can
not be conclusively supported by the data provided.
2.2 Toxicological Studies
2.2.1 Acute toxicity
Species Route LD50 Reference
(mg/kg/bw)
Mouse per os 1820-5000 Levenstein,
1960, Jenner,
1964, Boissier
et al., 1967
Mouse i.p. 650 Caujolle &
Meynier, 1958
1410 Boissier et al.,
1967
Rat per os 2090-3208 Taylor et al.,
1964, Shelanski,
1958, Boissier
et al., 1967
Rat i.p. 900 Boissier et al.,
1967
2670 Caujolle &
Meynier, 1958
Guinea-pig per os 2160 Jenner et al.,
1964
2.2.2 Short-term studies
2.2.2.1 Rat
Rats (number not given) receiving anethole in the diet at 0.25%
for one year showed no adverse effects while those receiving 1.0% for
15 weeks showed slight hydropic alterations of hepatic cells (Taylor,
et al., 1964).
Groups of five male and five female rats maintained on diets
containing trans-anethole at 0, 0.1, 0.3, 1.0 or 3.0% for 90 days
showed no effects at 0.1% but dose-related hepatic cell oedema,
degeneration and regeneration at 0.3% and higher. In a parallel
experiment with the cis-isomer, similar changes were noted at 0.03%
and higher (Shelanski, 1958).
Groups of 10 adult male rabbits and adult male and female rats
(number per group not specified) were given orally once per week in
their drinking water a quantity of trans-anethole corresponding to
7 daily doses of 11.4 mg/kg/day over a period of 90 days. Another
group of rats (21 days old), 10 rats per group were treated with
anethole identically but only for a period of 21 days. Neither
measurements of growth, electroencephalograms, electrocardiograms nor
gross and histopathological examinations revealed any evidence of
adverse toxic effects in any group treated either for 21 or 90 days
(Vignoli et al., 1965).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rat
Groups of 25 male and female rats maintained at 0.2, 0.5, 1.0 or
2.0% trans-anethole in the diet for 12-22 months showed no effects
at any level in clinical chemistry, haematology, histopathology nor
mortality. Slower weight gain and decreased fat storage were noted
only at the 1.0 and 2.0% levels. In a paired feeding study, trans-
anethole reduced the rate of weight gain (Le Bourhis, 1973).
Trans-anethole was administered to Sprague-Dawley rats in the
diet at concentrations of 0, 0.25, 0.5 or 1% for 117 weeks. The group
sizes were 52 males and 52 females in two separate control groups and
in the intermediate and high dose groups, and 78 males and 78 females
in the low dose group. A supplementary group of 26 males and 26
females received the trans-anethole at a level of 1% in the diet for
the first 54 weeks of the study, then 10 animals of each sex were
placed on the control diet until week 121; the remaining animals of
each sex continued to receive the diet containing 1% trans-anethole.
Food intake was recorded daily during weeks 1-32 and for one day every
4 weeks thereafter; body weight gain was monitored at weekly intervals
up to week 26 and then at monthly intervals. The animals were
inspected clinically at frequent intervals during the course of the
study and the animals were palpated weekly from week 27 and the
appearance of palpable masses recorded. Moribund animals were killed
in the course of the study; these animals and those sacrificed at
termination were subjected to detailed gross and histopathological
examination (40 tissues). Also at termination, haematological
examinations were performed, including RBC, haemoglobin, MCV,
haematocrit, MCHC, leucocyte count and differential blood count.
There was a retardation in body weight gain in all treated
animals during the first six months of treatment after which the
weight gain of the 0.25% and 0.5% groups was similar to controls,
although the weight deficit was not recovered; in the high dose group
the weight gain remained less than controls. The deficit of weight
gain was associated with reduced food intake due to unpalatability of
the diet and animals removed from the 1% diet after 54 weeks rapidly
gained weight. There were no treatment-related effects on mortality
except for a significantly decreased mortality in the high-dose group
males. Haematological examination did not reveal any treatment-
related differences but comparison of organ weights revealed an
increase in relative liver weight of all treatment groups of females
(P<0.001 in the highest dose group). Treatment-related
histopathological changes were reported in the liver in the form of
hepatocytic vacuolation (males, 1% dose group), sinusoidal dilatation
(males and females in the 1% group, females in the 0.5% group),
focal/nodular hyperplasia (males and females in the 1% group, males in
the 0.5% group) and hepatocellular hypertrophy in females of the 0.5%
and 1% groups.
There was a reported statistically significant increase in the
incidence of benign hepatocellular adenomas and hepatocellular
carcinomas in females in the 1% dose group, but it was pointed out
that this was within the range of spontaneous incidence of similar
lesions in historical controls for the strain of rat used compiled
between 1977 and 1985 (Truhaut et al., 1988). It was considered
that the trend of increase in incidence of benign and malignant liver
cell tumours represented a secondary response to a non-specific effect
rather than a direct genotoxic effect.
In the light of new criteria (Maronpot et al., 1986) for the
diagnosis of proliferative hepatic lesions in rats, the findings of
the above study were reviewed by independent pathologists (Newberne
et al., 1987; Moch, 1990). The results of these reviews are
tabulated below and, in the case of hepatocellular adenomas and
carcinomas, compared with the original diagnosis:
Table 2: Hepatic Lesions
Dose Groups FCA FNH HA HCA HCA
+
HA
Pathologists Male Rats
0% Moch 33/104 2/104 1/104 4/104 5/104
(32%) (2%) (1%) (4%) (5%)
Truhaut - 3/104 3/104 2/104 5/104
(3%) (3%) (2%) (5%)
Newberne 48/104 2/104 1/104 3/104 4/104
(46%) (2%) (1%) (3%) (4%)
0.25%
Moch 32/78 6/78 1/78 3/78 4/78
(41%) (8%) (1%) (4%) (5%)
Truhaut - 3/78 3/78 1/78 4/78
(4%) (4%) (1%) (5%)
Newberne 34/78 7/78 1/78 3/78 4/78
(44%) (9%) (1%) (4%) (5%)
0.5%
Moch 27/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
Truhaut - 8/52 0/52 3/52 3/52
(15%) (6%) (6%)
Newborne 28/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
1.0%
Moch 25/52 14/52 3/52 1/52 4/52
(48%) (27%) (6%) (2%) (8%)
Truhaut - 14/52 4/52 1/52 5/52
(27%) (8%) (2%) (10%)
Newberne 27/52 13/52 3/52 1/52 4/52
(52%) (25%) (6%) (2%) (8%)
Female Rats
0%
Moch 47/104 4/104 4/104 0/104 4/104
(45%) (4%) (4%) (4%)
Truhaut - 11/104 2/104 1/104 3/104
(11%) (2%) (1%) (4%)
Newberne 62/104 6/104 4/104 0/104 4/104
(60%) (6%) (4%) (4%)
Table 2 (contd)
Dose Groups FCA FNH HA HCA HCA
+
HA
0.25%
Moch 42/78 8/78 1/78 0/78 1/78
(54%) (10%) (1%) (1%)
Truhaut - 2/78 2/78 0/78 2/78
(3%) (3%) (3%)
Newberne 53/78 10/78 1/78 0/78 1/78
(68%) (13%) (1%) (1%)
0.5%
Moch 27/52 7/52 0/52 0/52 0/52
(52%) (14%)
Truhaut - 6/52 0/52 0/52 0/52
(12%)
Newberne 32/52 10/52 0/52 0/52 0/52
(62%) (19%)
1%
Moch 16/52 18/52 4/52 6/52 10/52
(31%) (35%) (8%) (12%) (19%)
Truhaut - 15/52 6/52 6/52 12/52
(29%) (12%) (12%) (23%)
Newberne 21/52 15/52 4/52 6/52 10/52
(40%) (29%) (8%) (12%) (19%)
* Hepatic Lesions: FCA = Focus of Cellular Alteration;
FNH = Focus of Nodular Hyperplasia
HA = Hepatocellular Adenoma
HCA = Hepatocellular Carcinoma
As shown above, the findings of the independent pathologists are
in good agreement, all showing a clear increase in high-dose (1%)
female rats with hepatocellular adenoma and/or carcinoma. A similar
increase was not observed in male rats at any dose levels. The
differences observed in the incidence of rats with altered cellular
foci between the original study pathologist and the others, were
primarily due to the fact that the original study pathologist had
diagnosed any vacuolation or clearing of hepatocytes observed as
hepatocellular vacuolation rather than as foci of cellular alteration
(Moch, 1990).
An independent audit of this rat study report showed no
significant discrepancies (Munro & Brillinger, 1989a,b).
2.2.3.2 Mouse
In in the first eight weeks of a 24-week screening test, groups
of 20 female A/He mice received a total dose of 2, 4 or 12.0 g
anethole/kg b.w. in 24 3x/week i.p. injections. The higher dose had
previously been calculated to be the maximum tolerated dose. There
was no increase in the incidence of tumours of the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands.
Survival was reduced to approximately 70% (Stoner et al., 1973). It
should be noted that safrole, which has been shown to be carcinogenic
in other studies, was negative using this protocol, thus there is some
doubt about its validity.
A series of experiments was performed to investigate the
carcinogenic potential of trans-anethole in mice. Groups of male
(56-76 per group) and female (55-61 per group) 4-day old mice were
exposed to trans-anethole by gavage at dose levels of 370 or 740
mg/kg b.w. twice weekly for 10 weeks, then sacrificed at 11-14 months.
In a second experiment, 53 male mice received trans-anethole by
intraperitoneal injection on days 1, 8, 15 and 22 after birth. The
total dose of anethole that each animal received during 4 treatments
was 703 or 1399 mg. Animals from the lower dose group were sacrificed
at 13-18 months and those from the 1390 mg group at 12 months. In a
third experiment, a group of female mice, approximately 8 weeks old,
average body weight 21 g, were fed anethole in the diet at a dose
level of 690 mg/kg for a period of 12 months. After 12 months the
animals received basal diet only and were sacrificed at 18 months.
The fourth experimental group of 17 female mice (8 weeks old) was
given an injection of anethole twice weekly for 12 weeks at a dose
level of 148 mg/kg b.w. The mice were killed 8 months after the first
injection. All animals from each experiment were necropsied and the
various tissues examined histologically. Trans-anethole produced no
different results from controls (Miller et al., 1983).
2.2.4 Special studies on mutagenicity
Two strains of Salmonella typhimurium (TA100 and TA98), with
and without microsomal activation, were used in a plate test to study
the effect of trans-anethole (isolated natural compound), anise oil
(90% trans-anethole) and fennel oil (70% trans-anethole). Each
test material was assayed over the dose range from zero (control) to
the level of cell toxicity. All test materials increased mutagenic
activities with TA100 tester strain with implementation of microsomal
activation (S13) system. Peak mutagenic activity, 4 and 4.4 times
that of the background rate, occurred with 2 mg anise oil/plate and
2.5 mg fennel oil/plate respectively. Isolated natural trans-
anethole was also mutagenic (the dose level and rate of mutagenicity
were not stated). The compound was considered not to be mutagenic
unless it was capable of inducing a mutation (reversion) rate at least
3 times that of the incident background (Marcus & Liechtenstein,
1982).
The mutagenic activities of anethole and its metabolite 3'-
hydroxyanethole were studied using three tester strains of Salmonella
typhimurium (TA1535, TA100 and TA98). Addition of an NADPH-
generating system and liver microsomes and cytosol (S13 fraction) from
Aroclor-treated rats (6.8 mg liver protein/plate) to the incubation
mixture of TA100 tester strain increased mutagenic activities.
Approximately 45 revertants were obtained per µmole anethole. Under
the same conditions, 3'-hydroxyanethole showed no significant
mutagenic activity with less than 7 µmoles/plate. Above this
concentration the S13-mediated mutagenicity increased linearly with
increased doses up to 15 µmoles/plate (about 1000 revertants with 15
µmoles/plate) (Swanson et al., 1979).
Five strains of Salmonella typhimurium (TA1535, TA100, TA1537,
TA1538, TA98), with and without metabolic activation (S9 mix), were
used to study potential mutagenic effects of trans-anethole. The
lowest overtly toxic concentration for trans-anethole was 1
mg/plate. No mutagenic activity was observed at concentrations of up
to 50 µg trans-anethole/plate with or without metabolic activation.
However, the addition of 3'-phosphoadenosine-5'phosphosulphate (PAPS)
to the microsomal assay markedly increased the mutagenicity of trans-
anethole in TA1535 tester strain. The mutation rate observed was
approximately 4, 5, 10, 11, 9 and 3 times that of the background rate
at trans-anethole concentrations of 0.05, 0.20, 1.0, 5.0, 15.0, and
50.0 ug/plate respectively (To et al., 1982).
A further study indicated that trans-anethole showed mutagenic
activity in the Ames S. typhimurium assay but was inactive in a B.
subtilis Rec assay and was negative in an E. coli uvr A reversion
test (Sekizawa & Shibamoto, 1982).
Trans-anethole was inactive in a mouse micronucleus assay 24,
48 and 72 hr after administration by gavage of the second of two daily
doses of 2 ml/kg b.w. to groups of 10 adult males. At this dose level
there was significant mortality (3-4 animals per group) (Siou et al.,
1984). Negative results were also obtained in a mouse micronucleus
assay after i.p. administration to groups of 5 male and 5 female mice
in two doses of 0.25 or 0.5 g/kg b.w., 30 and 6 hours before sacrifice
(Marzin, 1979).
Genotoxicity of trans-anethole was tested, along with some
structurally related compounds (safrole, isosafrole, eugenol,
estragole, allylbenzene, methyleugenol, and p-propylanisole), for its
capability of inducing unscheduled DNA synthesis (UDS) in freshly
isolated rat hepatocytes in primary culture. Hepatocytes were
isolated from male Fischer rats following liver perfusion. After
overnight incubation of the hepatocytes in the presence of the test
chemical, the UDS or DNA repair was measured by determining the amount
of 3H-thymidine incorporated into hepatocyte nuclear DNA during the
repair process. Under the test conditions, trans-anethole did not
induce UDS over the concentration range of 10-6 and 10-2 M, although
cytotoxicity, as measured by LDH leakage, was observed at
concentrations of 10-3 M and above. Of the remaining chemicals
tested, allybenzene, p-propylanisole, isosafrole and eugenol were also
negative in the UDS assay, whereas safrole, methyleugenol and
estragole as well as the positive control, 2-acetylaminofluorene, all
induced UDS in a dose-related manner.
Based on the study results, the authors concluded: "there was an
excellent correlation between UDS induction and known rodent hepato-
carcinogenicity, with safrole, estragole and methyleugenol all
inducing UDS. Anethole, isosafrole, eugenol and allylbenzene, for
which evidence of carcinogenicity is equivocal or negative, did not
induce UDS" (Howes et al., 1990).
In another test, trans-anethole was tested for its ability to
induce UDS in cultured primary hepatocytes isolated from Sprague-
Dawley derived CD rats of both sexes. At concentrations up to 10-2,
the compound did not induce UDS, as measured by 3H-thymidine
incorporation into nuclear DNA. The positive control, 2-
acetylaminofluorene, gave positive response. At concentrations of
10-3 and above, trans-anethole was cytotoxic and the UDS response
was below the control values. The cytotoxicity of trans-anethole
was consistent with the increased leakage of cellular LDH into the
culture medium.
To investigate the possible involvement of a reactive epoxide
intermediate in the observed cytotoxicity, the effect of trans-
anethole on intracellular glutathione levels was studied. The results
showed that anethole caused a dose-related depletion of glutathione in
freshly isolated hepatocytes in suspension. However, when hepatocytes
were pretreated with the non-cytotoxic glutathione depleting agent
dimethyl maleate, there was no enhancement of anethole cytotoxicity,
suggesting that the putative epoxide intermediate may not be of any
toxicological significance (Caldwell & Marshall, 1990).
2.2.5 Special studies on pharmacological effects
The pharmacologic effects of trans-anethole most often noted
are reduction in motor activity, lowering of body temperature and
hypnotic, analgesic and anticonvulsant effects. By either the oral or
i.p. route, administration of more than 10% of the LD50 by that route
appears necessary for significant effects (Boissier et al., 1961;
Seto, 1969; Gruebner, 1972; Le Bourhis & Soene, 1973).
3. COMMENTS
The Committee considered the results of three independent reviews
of the liver histology in the long-term study in rats. It concluded
that there is a clear increase in the incidences of hepatocellular
adenomas and of hepatocellular carcinomas in female rats at 10 mg/kg
in the diet but not at lower doses. In male animals a slight increase
in the incidence of hepatocellular adenomas but not carcinomas was
seen at 10 mg/kg in the diet only. There is also evidence of an
increase in the incidence of non-neoplastic proliferative lesions in
the liver at all dose levels in both sexes.
A review of the report of the long-term rat study showed no
significant discrepancies. The Committee also noted that differences
in survival between treated and control animals could not account for
the increased incidence of liver tumours seen in the study.
The results of two new studies on genotoxicity (unscheduled DNA
synthesis) were negative.
The Committee concluded that insufficient data were available to
permit a final evaluation of the significance of the malignant liver
tumours observed in female rats with respect to the use of trans-
anethole as a food additive. Further metabolic and especially
pharmacokinetic studies in mice, rats, and humans are required for
evaluation in 1992. In addition, a long-term dietary study in mice
may be needed, although the design of such a study will depend upon
the results of the metabolic and pharmacokinetic studies. In view of
the positive results that were obtained in in vitro bacterial gene
mutation tests, the Committee also concluded that chromosome
aberration studies and in vitro tests for gene mutation in mammalian
cells were desirable.
4. EVALUATION
The temporary ADI was extended until 1992, but reduced to 0-0.6
mg per kg of body weight on the basis of the minimal effect level of
2.5 mg/kg in the diet (equivalent to a dose of 125 mg per kg of body
weight per day) for non-neoplastic proliferative changes in the liver
of rats, adjusted using a safety factor of 200.
The need for a reproduction/teratogenicity study will be
considered by the Committee when further relevant data from the above
studies have been reviewed. The Committee also reiterated its
recommendation that an epidemiological study of the effects of
consuming high dietary levels of trans-anethole would be desirable.
5. REFERENCES
AXELROD, J. (1956). Enzymic cleavage of aromatic ethers. Biochem.
J., 63, 634-639.
BOISSIER, J.R., SIMON, P. & LE BOURHIS, B. (1967). Experimental
psychotropic effect of isomeric cis- and trans-anetholes.
Therapie, 22, 309-323.
CALDWELL, J. SUTTON, J.D. (1988). Influence of dose size upon the
disposition of trans-[methoxy-14C]-anethole in human volunteers.
Fd. Chem. Toxicol., (In press).
CALDWELL, J. & MARSHALL, A.D. (1990). The cytotoxicity and
genotoxicity of trans-anethole in isolated rat hepatocytes.
Progress Report submitted to WHO by Anethole Task Force, FEMA,
Washington, D.C. U.S.A., March, 1990.
CAUJOLLE, F. & MEYNIER, D. (1958). Toxicity of tarragon oil and the
anetholes ( cis- and trans-), Acad. Sci. (Paris), 246, 1465-1468.
FEMA (1989). Metabolic and mechanistic studies intended to show that
the low incidence of hepatocellular carcinoma induced in rats by
trans-anethole does not indicate a hazard to humans. Anethole
Progress Report submitted to WHO 11/13/89.
FRITSCH, P., DE SAINT-BLANUAT, G. & DERACHE, R. (1975).
Gastrointestinal absorption, in the rat, of anisole, trans-anethole,
butylhydroxyanisole and safrole. Fd. Cosmet. Toxicol., 13, 359-364.
GRUEBNER, I., KLINGER, W. & ANERMANN, H. (1972). Various substances
and substance classes with inducer properties. II. Arch. Int.
Pharmacodyn. Ther., 196, 288-297.
HOWES, A.J., CHAN, V.S.W. & CALDWELL, J. (1990). Structure-
specificity of the genotoxicity of some naturally occurring
alkenylbenzenes determined by the unscheduled DNA synthesis assay in
rat hepatocytes. Prepublication submitted to Food Chem. Toxicol.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I.
Acute and oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
LE BOURHIS, B. (1968). The metabolism of trans-anethole. Ann.
Biol. Clin., 26, 711-715.
LE BOURHIS, B. (1970). Identification of a few metabolites of trans-
anethole in man, the rabbit and the rat. Ann. Pharm. Fr., 28, 355-
361.
LE BOURHIS, B. (1973). Biological properties of trans-anethole. An
attempt to determine an acceptable daily dosage. Parfums Cosmet.
Savons. Fr., 3, 450-456.
LE BOURHIS, B. & SOENE, A.M. (1973). Studies on the psychotropic
action of some aromatic compounds used in food. Fd. Cosmet.
Toxicol., 11, 1-9.
LEVENSTEIN, I. (1960). Unpublished report of Leberco Laboratories,
Submitted to WHO.
MARCUS, C. & LIECHTENSTEIN, E.P. (1982). Interactions of naturally
occurring food plant components with insecticides and pentobarbital in
rats and mice. J. Agric. Food Chem., 30, 563-568.
MARONPOT, R.R., MONTGOMERY, C.A., BOORMAN, G.A. & MCCONNELL, E.
(1986). National Toxicology Program nomenclature for
hepatoproliferative lesions of rats. Toxicol. Pathol., 14, 263-273.
MARZIN, D. (1979). Recherche d'une action mutagene par le test du
micronucleus chez la souris. Unpublished report of DREBS. Submitted
to WHO by Pernod Ricard.
MILLER, E.C., SWANSON, A.B., PHILLIPS, D.H., FLETCHER, T.L., LIEM, A.
& MILLER, J.A. (1983). Structure-activity studies of the
carcinogenicities in the mouse and rat of some naturally occurring and
synthetic alkenylbenzene derivatives related to safrole and estragole.
Cancer Res., 43, 1124-1134.
MOCH, R.W. (1990). Trans-anethole. Histopathologic review of
microslides containing liver proliferative lesions from a long-term
study in Sprague-Dawley rats. Pathology Report submitted to WHO
April, 1990.
MUNRO, I.C. & BRILLINGER, R. (1989a). Preliminary audit of the
procedures and findings of a 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
MUNRO, I.C. & BRILLINGER, R. (1989b). Addendum to the preliminary and
detailed audit reports of the 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
NEWBERNE, P.M., BROWN, W.R. & CARLTON, W.W. (1987). Histopathologic
evaluation of proliferative lesions in livers of rats fed trans-
anethole for 117-121 weeks (Long-term feeding study in rats, study no.
3190 TCR) Unpublished report. Submitted to WHO by Pernod Ricard.
NEWBERNE, P.M., CARLTON, W.W. & BROWN, W.R. (1989). Histopathological
evaluation of proliferative lesions in rats fed trans-anethole in
chronic studies. Fd. Chem. Toxic., 27, 21-26.
SANGSTER, S.A. (1983). The metabolism and disposition of trans-
anethole and p-propenylanisole in rodent species and man. Ph.D.
Thesis, University of London.
SANGSTER, S.A., CALDWELL, J., SMITH, R.L. & FARMER, P.B. (1984a).
Metabolism of anethole I. Pathways of metabolism in the rat and mouse.
Fd. Chem. Toxicol., 22, 695-706.
SANGSTER, S.A., CALDWELL, J. & SMITH, R.L. (1984b). Metabolism of
anethole II. Influence of dose size on the route of metabolism of
trans-anethole in the rat and mouse. Fd. Chem. Toxicol., 22,
707-713.
SANGSTER, S.A., CALDWELL, J., HUTT, A.J. ANTHONY, A. & SMITH, R.L.
(1987). The metabolic disposition of [methoxy-14C]-labelled trans-
anethole, estragole and p-propylanisole in human volunteers.
Xenobiotica, 17, 1223-1232.
SEKIZAWA, J. & SHIBAMOTO, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
SETO, T.A. (1969). Effects of alkylmethoxybenzene and alkyl-
methylenedioxybenzene essential oils on pentobarbital and ethanol
sleeping time. Arch. Int. Pharmacodyn. Ther., 180, 232-240.
SHELANSKI, M.V. (1958). Unpublished report of Industrial Biology
Research and Testing Laboratories. Submitted to WHO.
SIOU, G., LEROND-CONAN, L., EL HAITEM, M. & LACRAMPE, M. (1984).
Recherche d'une eventuelle potentialite genotoxique du p-methoxy-
propenylbenzene par la technique du micronucleus chez la souris.
Unpublished report of C.E.R.T.I. Submitted to WHO by Pernod Ricard.
SOLHEIM, E. & SCHELIEN, R.R. (1973). Metabolism of alkenebenzene
derivatives in the rat I. rho-methoxylallyl benzene (estragole) and
rho-methoxypropenylbenzene (anethole). Xenobiotica, 3, 493-510.
STONER, G.D., SHIMKIN, M.B., KNIAZEIF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumor
response in strain A mice. Cancer Res., 33, 3069-3085.
STROLIN-BENEDETTI, M. & LE BOURHIS, B. (1972). Body distribution and
excretion of trans-anethole-14C. C.R. Acad. Sci. Ser. D., 274,
2378-2381.
SWANSON, A.B., CHAMBLISS, D.D., BLOMQUIST, J.C., MILLER, E.C., &
MILLER, J.A. (1979). The mutagenicities of safrole, estragole,
eugenol, trans-anethole and some of their known or possible
metabolites for Salmonella typhimurium mutants. Mutat. Res., 60,
143-153.
SWANSON, A.B., MILLER, E.C. & MILLER, J.A. (1981). The side chain
epoxidation and hydroxylation of the hepatocarcinogens safrole and
estragole and some related compounds by rat and mouse liver
microsomes. Bichim. Biophys. Acta., 673, 504-516.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964). A comparison of the
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 2, 378-387.
TO, L.P., HUNT, T.P. & ANDERSEN, M.E. (1982). Mutagenicity of trans-
anethole, estragole and safrole in the Ames Salmonella typhimurium
assay. Bull. Environ. Contam. Toxicol., 28, 647-654.
TRUHAUT, R., NEWMAN, J., LE BOURNIS, B., GLOMOT, R. & ATTIA, M.
(1988). A long term feeding study of the toxicity and carcinogenicity
of trans-anethole in rats. Fd. Chem. Toxicol., (in press).
TRUHAUT, R., LE BOURHIS, B., ATTIA, M. GLOMOT, R., NEWMAN, J. &
CALDWELL, J. (1989). Chronic toxicity/carcinogenicity study of
trans-anethole in rats. Fd. Chem. Toxic., 27, 11-20.
VIGNOLI, L., MOREL, M.C. & SIMON, O. (1965). Toxicite chronique du
trans-anethole et des liqueurs anisees a base d'anethole et de
regise. Rev. Path. Comp., 2, 281-287.
In studies of the dose dependence of trans-anethole metabolism,
single doses of 0.05, 5, 50, or 1500 mg/kg b.w. were administered to
female rats by gavage; similar doses were given to male mice
intraperitoneally with an additional dose level of 250 mg/kg b.w. also
being included. Dose and species-dependent differences in metabolism
were observed. The major route of metabolism was via O-
demethylation in both species and the significance of this route
decreased with increase in dose from 56 and 72% of the 0.05 mg/kg dose
to 32 and 35% of the highest dose in rats and mice respectively. With
regard to side-chain oxidation, the rat favoured the epoxidation route
and elimination of the diol-isomers rose from 2% to 15% of the dose
over the range of doses studied. In the mouse, w-oxidation was
favoured and the diols were formed in smaller amounts of from 1% to
4.5% over the same dose range (Sangster et al., 1984b). It should
be noted that the inter-species differences were compounded by the sex
difference between the species and by the different route of
administration.
Studies of the metabolism of several structurally-related food
flavours, including trans-anethole, were carried out in human
volunteers. Two male subjects received oral doses of 1 mg
14C[methoxy]- trans-anethole and the urine was collected over a 48
hour period subsequently; aliquots of expired air also were collected
at 30 minute intervals for 8 hours after dosing. Approximately 65% of
the activity was excreted in the 48 hour urine, mainly in the first 8
hours, while an estimated 20% of the dose was excreted as 14CO2 in
exhaled air over the first 8 hours. Most of the urinary activity was
in the form of 4-methoxyhippuric acid (ca 55% of the dose) and 4-
methoxybenzoic acid (ca 3.5% of the dose); approximately 3% of the
dose was excreted as the two diastereoisomers of 1-(4'-
methoxyphenyl)propane-1,2-diol (Sangster et al., 1987).
In a study of the dose-dependence of the metabolism of trans-
anethole in human volunteers, 5 male subjects received oral doses of
1, 50 or 250 mg 14C-[methoxy]- trans-anethole on three separate
occasions in milk. 14CO2 was estimated in expired air at intervals
up to 24 hours and radioactivity was measured in urine at intervals up
to 48 hours. Over the range of doses studied, dose had no systematic
effect on the rate or route of excretion, the major routes being urine
(54-69% of the dose) and expired air (13-17% of the dose). The
principal urinary metabolite (>90% of urinary 14C) was
methoxyhippuric acid with much smaller amounts of 4-methoxy-benzoic
acid and up to three other metabolites; the pattern of urinary
metabolites was unaffected by dose size (Caldwell & Sutton, 1988).
Studies of DNA binding of several alkenylbenzenes, including
trans-anethole, were carried out following i.p. administration to
neonatal and adult mice. Very low levels of adduct formation were
detected only when the dose (300 mg/kg i.p.) approached the LD50 (400
mg/kg b.w.).
Groups of 8 male and 8 female CD Sprague-Dawley rats were
administered (methoxy-14C)- trans-anethole at gavage doses of 100,
250, 500 and 1000 mg/kg (16 uCi/kg). Radioactivities appearing in the
urine, faeces and exhaled CO2 were determined at 24 hour intervals
for up to 72 hours, but only the summarized urine and CO2 data were
provided in the report. The urinary metabolites of trans-anethole
in these animals are said to be still under investigation. Based on
the available disposition data, the authors have concluded the
following:
(1) Excretion of 14C in the urine and expired air was delayed
as the dose was increased, with a progressively smaller
fraction of the total radioactivity excreted during 0-24
hours but a greater fraction in 24-48 hours.
(2) As the dose was increased, the excretion route was shifted
from the expired air to the urine.
(3) Females consistently excreted less of the given doses in 0-
24 hours than males, "with recovery of 14C in the urine and
as CO2 being approximately equally affected" (FEMA, 1989).
The Committee noted that the above data were not analyzed
statistically for intergroup differences. Also noted is
that with the possible exception of the dose-dependent delay
of its excretion, the other dose-dependent effects on the
disposition of trans-anethole, as concluded by the
authors, were not very apparent. Furthermore, the author's
conclusion that there is a sex-dependent difference in the
elimination of trans-anethole from the test animals can
not be conclusively supported by the data provided.
2.2 Toxicological Studies
2.2.1 Acute toxicity
Species Route LD50 Reference
(mg/kg/bw)
Mouse per os 1820-5000 Levenstein,
1960, Jenner,
1964, Boissier
et al., 1967
Mouse i.p. 650 Caujolle &
Meynier, 1958
1410 Boissier et al.,
1967
Rat per os 2090-3208 Taylor et al.,
1964, Shelanski,
1958, Boissier
et al., 1967
Rat i.p. 900 Boissier et al.,
1967
2670 Caujolle &
Meynier, 1958
Guinea-pig per os 2160 Jenner et al.,
1964
2.2.2 Short-term studies
2.2.2.1 Rat
Rats (number not given) receiving anethole in the diet at 0.25%
for one year showed no adverse effects while those receiving 1.0% for
15 weeks showed slight hydropic alterations of hepatic cells (Taylor,
et al., 1964).
Groups of five male and five female rats maintained on diets
containing trans-anethole at 0, 0.1, 0.3, 1.0 or 3.0% for 90 days
showed no effects at 0.1% but dose-related hepatic cell oedema,
degeneration and regeneration at 0.3% and higher. In a parallel
experiment with the cis-isomer, similar changes were noted at 0.03%
and higher (Shelanski, 1958).
Groups of 10 adult male rabbits and adult male and female rats
(number per group not specified) were given orally once per week in
their drinking water a quantity of trans-anethole corresponding to
7 daily doses of 11.4 mg/kg/day over a period of 90 days. Another
group of rats (21 days old), 10 rats per group were treated with
anethole identically but only for a period of 21 days. Neither
measurements of growth, electroencephalograms, electrocardiograms nor
gross and histopathological examinations revealed any evidence of
adverse toxic effects in any group treated either for 21 or 90 days
(Vignoli et al., 1965).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rat
Groups of 25 male and female rats maintained at 0.2, 0.5, 1.0 or
2.0% trans-anethole in the diet for 12-22 months showed no effects
at any level in clinical chemistry, haematology, histopathology nor
mortality. Slower weight gain and decreased fat storage were noted
only at the 1.0 and 2.0% levels. In a paired feeding study, trans-
anethole reduced the rate of weight gain (Le Bourhis, 1973).
Trans-anethole was administered to Sprague-Dawley rats in the
diet at concentrations of 0, 0.25, 0.5 or 1% for 117 weeks. The group
sizes were 52 males and 52 females in two separate control groups and
in the intermediate and high dose groups, and 78 males and 78 females
in the low dose group. A supplementary group of 26 males and 26
females received the trans-anethole at a level of 1% in the diet for
the first 54 weeks of the study, then 10 animals of each sex were
placed on the control diet until week 121; the remaining animals of
each sex continued to receive the diet containing 1% trans-anethole.
Food intake was recorded daily during weeks 1-32 and for one day every
4 weeks thereafter; body weight gain was monitored at weekly intervals
up to week 26 and then at monthly intervals. The animals were
inspected clinically at frequent intervals during the course of the
study and the animals were palpated weekly from week 27 and the
appearance of palpable masses recorded. Moribund animals were killed
in the course of the study; these animals and those sacrificed at
termination were subjected to detailed gross and histopathological
examination (40 tissues). Also at termination, haematological
examinations were performed, including RBC, haemoglobin, MCV,
haematocrit, MCHC, leucocyte count and differential blood count.
There was a retardation in body weight gain in all treated
animals during the first six months of treatment after which the
weight gain of the 0.25% and 0.5% groups was similar to controls,
although the weight deficit was not recovered; in the high dose group
the weight gain remained less than controls. The deficit of weight
gain was associated with reduced food intake due to unpalatability of
the diet and animals removed from the 1% diet after 54 weeks rapidly
gained weight. There were no treatment-related effects on mortality
except for a significantly decreased mortality in the high-dose group
males. Haematological examination did not reveal any treatment-
related differences but comparison of organ weights revealed an
increase in relative liver weight of all treatment groups of females
(P<0.001 in the highest dose group). Treatment-related
histopathological changes were reported in the liver in the form of
hepatocytic vacuolation (males, 1% dose group), sinusoidal dilatation
(males and females in the 1% group, females in the 0.5% group),
focal/nodular hyperplasia (males and females in the 1% group, males in
the 0.5% group) and hepatocellular hypertrophy in females of the 0.5%
and 1% groups.
There was a reported statistically significant increase in the
incidence of benign hepatocellular adenomas and hepatocellular
carcinomas in females in the 1% dose group, but it was pointed out
that this was within the range of spontaneous incidence of similar
lesions in historical controls for the strain of rat used compiled
between 1977 and 1985 (Truhaut et al., 1988). It was considered
that the trend of increase in incidence of benign and malignant liver
cell tumours represented a secondary response to a non-specific effect
rather than a direct genotoxic effect.
In the light of new criteria (Maronpot et al., 1986) for the
diagnosis of proliferative hepatic lesions in rats, the findings of
the above study were reviewed by independent pathologists (Newberne
et al., 1987; Moch, 1990). The results of these reviews are
tabulated below and, in the case of hepatocellular adenomas and
carcinomas, compared with the original diagnosis:
Table 2: Hepatic Lesions
Dose Groups FCA FNH HA HCA HCA
+
HA
Pathologists Male Rats
0% Moch 33/104 2/104 1/104 4/104 5/104
(32%) (2%) (1%) (4%) (5%)
Truhaut - 3/104 3/104 2/104 5/104
(3%) (3%) (2%) (5%)
Newberne 48/104 2/104 1/104 3/104 4/104
(46%) (2%) (1%) (3%) (4%)
0.25%
Moch 32/78 6/78 1/78 3/78 4/78
(41%) (8%) (1%) (4%) (5%)
Truhaut - 3/78 3/78 1/78 4/78
(4%) (4%) (1%) (5%)
Newberne 34/78 7/78 1/78 3/78 4/78
(44%) (9%) (1%) (4%) (5%)
0.5%
Moch 27/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
Truhaut - 8/52 0/52 3/52 3/52
(15%) (6%) (6%)
Newborne 28/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
1.0%
Moch 25/52 14/52 3/52 1/52 4/52
(48%) (27%) (6%) (2%) (8%)
Truhaut - 14/52 4/52 1/52 5/52
(27%) (8%) (2%) (10%)
Newberne 27/52 13/52 3/52 1/52 4/52
(52%) (25%) (6%) (2%) (8%)
Female Rats
0%
Moch 47/104 4/104 4/104 0/104 4/104
(45%) (4%) (4%) (4%)
Truhaut - 11/104 2/104 1/104 3/104
(11%) (2%) (1%) (4%)
Newberne 62/104 6/104 4/104 0/104 4/104
(60%) (6%) (4%) (4%)
Table 2 (contd)
Dose Groups FCA FNH HA HCA HCA
+
HA
0.25%
Moch 42/78 8/78 1/78 0/78 1/78
(54%) (10%) (1%) (1%)
Truhaut - 2/78 2/78 0/78 2/78
(3%) (3%) (3%)
Newberne 53/78 10/78 1/78 0/78 1/78
(68%) (13%) (1%) (1%)
0.5%
Moch 27/52 7/52 0/52 0/52 0/52
(52%) (14%)
Truhaut - 6/52 0/52 0/52 0/52
(12%)
Newberne 32/52 10/52 0/52 0/52 0/52
(62%) (19%)
1%
Moch 16/52 18/52 4/52 6/52 10/52
(31%) (35%) (8%) (12%) (19%)
Truhaut - 15/52 6/52 6/52 12/52
(29%) (12%) (12%) (23%)
Newberne 21/52 15/52 4/52 6/52 10/52
(40%) (29%) (8%) (12%) (19%)
* Hepatic Lesions: FCA = Focus of Cellular Alteration;
FNH = Focus of Nodular Hyperplasia
HA = Hepatocellular Adenoma
HCA = Hepatocellular Carcinoma
As shown above, the findings of the independent pathologists are
in good agreement, all showing a clear increase in high-dose (1%)
female rats with hepatocellular adenoma and/or carcinoma. A similar
increase was not observed in male rats at any dose levels. The
differences observed in the incidence of rats with altered cellular
foci between the original study pathologist and the others, were
primarily due to the fact that the original study pathologist had
diagnosed any vacuolation or clearing of hepatocytes observed as
hepatocellular vacuolation rather than as foci of cellular alteration
(Moch, 1990).
An independent audit of this rat study report showed no
significant discrepancies (Munro & Brillinger, 1989a,b).
2.2.3.2 Mouse
In in the first eight weeks of a 24-week screening test, groups
of 20 female A/He mice received a total dose of 2, 4 or 12.0 g
anethole/kg b.w. in 24 3x/week i.p. injections. The higher dose had
previously been calculated to be the maximum tolerated dose. There
was no increase in the incidence of tumours of the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands.
Survival was reduced to approximately 70% (Stoner et al., 1973). It
should be noted that safrole, which has been shown to be carcinogenic
in other studies, was negative using this protocol, thus there is some
doubt about its validity.
A series of experiments was performed to investigate the
carcinogenic potential of trans-anethole in mice. Groups of male
(56-76 per group) and female (55-61 per group) 4-day old mice were
exposed to trans-anethole by gavage at dose levels of 370 or 740
mg/kg b.w. twice weekly for 10 weeks, then sacrificed at 11-14 months.
In a second experiment, 53 male mice received trans-anethole by
intraperitoneal injection on days 1, 8, 15 and 22 after birth. The
total dose of anethole that each animal received during 4 treatments
was 703 or 1399 mg. Animals from the lower dose group were sacrificed
at 13-18 months and those from the 1390 mg group at 12 months. In a
third experiment, a group of female mice, approximately 8 weeks old,
average body weight 21 g, were fed anethole in the diet at a dose
level of 690 mg/kg for a period of 12 months. After 12 months the
animals received basal diet only and were sacrificed at 18 months.
The fourth experimental group of 17 female mice (8 weeks old) was
given an injection of anethole twice weekly for 12 weeks at a dose
level of 148 mg/kg b.w. The mice were killed 8 months after the first
injection. All animals from each experiment were necropsied and the
various tissues examined histologically. Trans-anethole produced no
different results from controls (Miller et al., 1983).
2.2.4 Special studies on mutagenicity
Two strains of Salmonella typhimurium (TA100 and TA98), with
and without microsomal activation, were used in a plate test to study
the effect of trans-anethole (isolated natural compound), anise oil
(90% trans-anethole) and fennel oil (70% trans-anethole). Each
test material was assayed over the dose range from zero (control) to
the level of cell toxicity. All test materials increased mutagenic
activities with TA100 tester strain with implementation of microsomal
activation (S13) system. Peak mutagenic activity, 4 and 4.4 times
that of the background rate, occurred with 2 mg anise oil/plate and
2.5 mg fennel oil/plate respectively. Isolated natural trans-
anethole was also mutagenic (the dose level and rate of mutagenicity
were not stated). The compound was considered not to be mutagenic
unless it was capable of inducing a mutation (reversion) rate at least
3 times that of the incident background (Marcus & Liechtenstein,
1982).
The mutagenic activities of anethole and its metabolite 3'-
hydroxyanethole were studied using three tester strains of Salmonella
typhimurium (TA1535, TA100 and TA98). Addition of an NADPH-
generating system and liver microsomes and cytosol (S13 fraction) from
Aroclor-treated rats (6.8 mg liver protein/plate) to the incubation
mixture of TA100 tester strain increased mutagenic activities.
Approximately 45 revertants were obtained per µmole anethole. Under
the same conditions, 3'-hydroxyanethole showed no significant
mutagenic activity with less than 7 µmoles/plate. Above this
concentration the S13-mediated mutagenicity increased linearly with
increased doses up to 15 µmoles/plate (about 1000 revertants with 15
µmoles/plate) (Swanson et al., 1979).
Five strains of Salmonella typhimurium (TA1535, TA100, TA1537,
TA1538, TA98), with and without metabolic activation (S9 mix), were
used to study potential mutagenic effects of trans-anethole. The
lowest overtly toxic concentration for trans-anethole was 1
mg/plate. No mutagenic activity was observed at concentrations of up
to 50 µg trans-anethole/plate with or without metabolic activation.
However, the addition of 3'-phosphoadenosine-5'phosphosulphate (PAPS)
to the microsomal assay markedly increased the mutagenicity of trans-
anethole in TA1535 tester strain. The mutation rate observed was
approximately 4, 5, 10, 11, 9 and 3 times that of the background rate
at trans-anethole concentrations of 0.05, 0.20, 1.0, 5.0, 15.0, and
50.0 ug/plate respectively (To et al., 1982).
A further study indicated that trans-anethole showed mutagenic
activity in the Ames S. typhimurium assay but was inactive in a B.
subtilis Rec assay and was negative in an E. coli uvr A reversion
test (Sekizawa & Shibamoto, 1982).
Trans-anethole was inactive in a mouse micronucleus assay 24,
48 and 72 hr after administration by gavage of the second of two daily
doses of 2 ml/kg b.w. to groups of 10 adult males. At this dose level
there was significant mortality (3-4 animals per group) (Siou et al.,
1984). Negative results were also obtained in a mouse micronucleus
assay after i.p. administration to groups of 5 male and 5 female mice
in two doses of 0.25 or 0.5 g/kg b.w., 30 and 6 hours before sacrifice
(Marzin, 1979).
Genotoxicity of trans-anethole was tested, along with some
structurally related compounds (safrole, isosafrole, eugenol,
estragole, allylbenzene, methyleugenol, and p-propylanisole), for its
capability of inducing unscheduled DNA synthesis (UDS) in freshly
isolated rat hepatocytes in primary culture. Hepatocytes were
isolated from male Fischer rats following liver perfusion. After
overnight incubation of the hepatocytes in the presence of the test
chemical, the UDS or DNA repair was measured by determining the amount
of 3H-thymidine incorporated into hepatocyte nuclear DNA during the
repair process. Under the test conditions, trans-anethole did not
induce UDS over the concentration range of 10-6 and 10-2 M, although
cytotoxicity, as measured by LDH leakage, was observed at
concentrations of 10-3 M and above. Of the remaining chemicals
tested, allybenzene, p-propylanisole, isosafrole and eugenol were also
negative in the UDS assay, whereas safrole, methyleugenol and
estragole as well as the positive control, 2-acetylaminofluorene, all
induced UDS in a dose-related manner.
Based on the study results, the authors concluded: "there was an
excellent correlation between UDS induction and known rodent hepato-
carcinogenicity, with safrole, estragole and methyleugenol all
inducing UDS. Anethole, isosafrole, eugenol and allylbenzene, for
which evidence of carcinogenicity is equivocal or negative, did not
induce UDS" (Howes et al., 1990).
In another test, trans-anethole was tested for its ability to
induce UDS in cultured primary hepatocytes isolated from Sprague-
Dawley derived CD rats of both sexes. At concentrations up to 10-2,
the compound did not induce UDS, as measured by 3H-thymidine
incorporation into nuclear DNA. The positive control, 2-
acetylaminofluorene, gave positive response. At concentrations of
10-3 and above, trans-anethole was cytotoxic and the UDS response
was below the control values. The cytotoxicity of trans-anethole
was consistent with the increased leakage of cellular LDH into the
culture medium.
To investigate the possible involvement of a reactive epoxide
intermediate in the observed cytotoxicity, the effect of trans-
anethole on intracellular glutathione levels was studied. The results
showed that anethole caused a dose-related depletion of glutathione in
freshly isolated hepatocytes in suspension. However, when hepatocytes
were pretreated with the non-cytotoxic glutathione depleting agent
dimethyl maleate, there was no enhancement of anethole cytotoxicity,
suggesting that the putative epoxide intermediate may not be of any
toxicological significance (Caldwell & Marshall, 1990).
2.2.5 Special studies on pharmacological effects
The pharmacologic effects of trans-anethole most often noted
are reduction in motor activity, lowering of body temperature and
hypnotic, analgesic and anticonvulsant effects. By either the oral or
i.p. route, administration of more than 10% of the LD50 by that route
appears necessary for significant effects (Boissier et al., 1961;
Seto, 1969; Gruebner, 1972; Le Bourhis & Soene, 1973).
3. COMMENTS
The Committee considered the results of three independent reviews
of the liver histology in the long-term study in rats. It concluded
that there is a clear increase in the incidences of hepatocellular
adenomas and of hepatocellular carcinomas in female rats at 10 mg/kg
in the diet but not at lower doses. In male animals a slight increase
in the incidence of hepatocellular adenomas but not carcinomas was
seen at 10 mg/kg in the diet only. There is also evidence of an
increase in the incidence of non-neoplastic proliferative lesions in
the liver at all dose levels in both sexes.
A review of the report of the long-term rat study showed no
significant discrepancies. The Committee also noted that differences
in survival between treated and control animals could not account for
the increased incidence of liver tumours seen in the study.
The results of two new studies on genotoxicity (unscheduled DNA
synthesis) were negative.
The Committee concluded that insufficient data were available to
permit a final evaluation of the significance of the malignant liver
tumours observed in female rats with respect to the use of trans-
anethole as a food additive. Further metabolic and especially
pharmacokinetic studies in mice, rats, and humans are required for
evaluation in 1992. In addition, a long-term dietary study in mice
may be needed, although the design of such a study will depend upon
the results of the metabolic and pharmacokinetic studies. In view of
the positive results that were obtained in in vitro bacterial gene
mutation tests, the Committee also concluded that chromosome
aberration studies and in vitro tests for gene mutation in mammalian
cells were desirable.
4. EVALUATION
The temporary ADI was extended until 1992, but reduced to 0-0.6
mg per kg of body weight on the basis of the minimal effect level of
2.5 mg/kg in the diet (equivalent to a dose of 125 mg per kg of body
weight per day) for non-neoplastic proliferative changes in the liver
of rats, adjusted using a safety factor of 200.
The need for a reproduction/teratogenicity study will be
considered by the Committee when further relevant data from the above
studies have been reviewed. The Committee also reiterated its
recommendation that an epidemiological study of the effects of
consuming high dietary levels of trans-anethole would be desirable.
5. REFERENCES
AXELROD, J. (1956). Enzymic cleavage of aromatic ethers. Biochem.
J., 63, 634-639.
BOISSIER, J.R., SIMON, P. & LE BOURHIS, B. (1967). Experimental
psychotropic effect of isomeric cis- and trans-anetholes.
Therapie, 22, 309-323.
CALDWELL, J. SUTTON, J.D. (1988). Influence of dose size upon the
disposition of trans-[methoxy-14C]-anethole in human volunteers.
Fd. Chem. Toxicol., (In press).
CALDWELL, J. & MARSHALL, A.D. (1990). The cytotoxicity and
genotoxicity of trans-anethole in isolated rat hepatocytes.
Progress Report submitted to WHO by Anethole Task Force, FEMA,
Washington, D.C. U.S.A., March, 1990.
CAUJOLLE, F. & MEYNIER, D. (1958). Toxicity of tarragon oil and the
anetholes ( cis- and trans-), Acad. Sci. (Paris), 246, 1465-1468.
FEMA (1989). Metabolic and mechanistic studies intended to show that
the low incidence of hepatocellular carcinoma induced in rats by
trans-anethole does not indicate a hazard to humans. Anethole
Progress Report submitted to WHO 11/13/89.
FRITSCH, P., DE SAINT-BLANUAT, G. & DERACHE, R. (1975).
Gastrointestinal absorption, in the rat, of anisole, trans-anethole,
butylhydroxyanisole and safrole. Fd. Cosmet. Toxicol., 13, 359-364.
GRUEBNER, I., KLINGER, W. & ANERMANN, H. (1972). Various substances
and substance classes with inducer properties. II. Arch. Int.
Pharmacodyn. Ther., 196, 288-297.
HOWES, A.J., CHAN, V.S.W. & CALDWELL, J. (1990). Structure-
specificity of the genotoxicity of some naturally occurring
alkenylbenzenes determined by the unscheduled DNA synthesis assay in
rat hepatocytes. Prepublication submitted to Food Chem. Toxicol.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I.
Acute and oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
LE BOURHIS, B. (1968). The metabolism of trans-anethole. Ann.
Biol. Clin., 26, 711-715.
LE BOURHIS, B. (1970). Identification of a few metabolites of trans-
anethole in man, the rabbit and the rat. Ann. Pharm. Fr., 28, 355-
361.
LE BOURHIS, B. (1973). Biological properties of trans-anethole. An
attempt to determine an acceptable daily dosage. Parfums Cosmet.
Savons. Fr., 3, 450-456.
LE BOURHIS, B. & SOENE, A.M. (1973). Studies on the psychotropic
action of some aromatic compounds used in food. Fd. Cosmet.
Toxicol., 11, 1-9.
LEVENSTEIN, I. (1960). Unpublished report of Leberco Laboratories,
Submitted to WHO.
MARCUS, C. & LIECHTENSTEIN, E.P. (1982). Interactions of naturally
occurring food plant components with insecticides and pentobarbital in
rats and mice. J. Agric. Food Chem., 30, 563-568.
MARONPOT, R.R., MONTGOMERY, C.A., BOORMAN, G.A. & MCCONNELL, E.
(1986). National Toxicology Program nomenclature for
hepatoproliferative lesions of rats. Toxicol. Pathol., 14, 263-273.
MARZIN, D. (1979). Recherche d'une action mutagene par le test du
micronucleus chez la souris. Unpublished report of DREBS. Submitted
to WHO by Pernod Ricard.
MILLER, E.C., SWANSON, A.B., PHILLIPS, D.H., FLETCHER, T.L., LIEM, A.
& MILLER, J.A. (1983). Structure-activity studies of the
carcinogenicities in the mouse and rat of some naturally occurring and
synthetic alkenylbenzene derivatives related to safrole and estragole.
Cancer Res., 43, 1124-1134.
MOCH, R.W. (1990). Trans-anethole. Histopathologic review of
microslides containing liver proliferative lesions from a long-term
study in Sprague-Dawley rats. Pathology Report submitted to WHO
April, 1990.
MUNRO, I.C. & BRILLINGER, R. (1989a). Preliminary audit of the
procedures and findings of a 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
MUNRO, I.C. & BRILLINGER, R. (1989b). Addendum to the preliminary and
detailed audit reports of the 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
NEWBERNE, P.M., BROWN, W.R. & CARLTON, W.W. (1987). Histopathologic
evaluation of proliferative lesions in livers of rats fed trans-
anethole for 117-121 weeks (Long-term feeding study in rats, study no.
3190 TCR) Unpublished report. Submitted to WHO by Pernod Ricard.
NEWBERNE, P.M., CARLTON, W.W. & BROWN, W.R. (1989). Histopathological
evaluation of proliferative lesions in rats fed trans-anethole in
chronic studies. Fd. Chem. Toxic., 27, 21-26.
SANGSTER, S.A. (1983). The metabolism and disposition of trans-
anethole and p-propenylanisole in rodent species and man. Ph.D.
Thesis, University of London.
SANGSTER, S.A., CALDWELL, J., SMITH, R.L. & FARMER, P.B. (1984a).
Metabolism of anethole I. Pathways of metabolism in the rat and mouse.
Fd. Chem. Toxicol., 22, 695-706.
SANGSTER, S.A., CALDWELL, J. & SMITH, R.L. (1984b). Metabolism of
anethole II. Influence of dose size on the route of metabolism of
trans-anethole in the rat and mouse. Fd. Chem. Toxicol., 22,
707-713.
SANGSTER, S.A., CALDWELL, J., HUTT, A.J. ANTHONY, A. & SMITH, R.L.
(1987). The metabolic disposition of [methoxy-14C]-labelled trans-
anethole, estragole and p-propylanisole in human volunteers.
Xenobiotica, 17, 1223-1232.
SEKIZAWA, J. & SHIBAMOTO, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
SETO, T.A. (1969). Effects of alkylmethoxybenzene and alkyl-
methylenedioxybenzene essential oils on pentobarbital and ethanol
sleeping time. Arch. Int. Pharmacodyn. Ther., 180, 232-240.
SHELANSKI, M.V. (1958). Unpublished report of Industrial Biology
Research and Testing Laboratories. Submitted to WHO.
SIOU, G., LEROND-CONAN, L., EL HAITEM, M. & LACRAMPE, M. (1984).
Recherche d'une eventuelle potentialite genotoxique du p-methoxy-
propenylbenzene par la technique du micronucleus chez la souris.
Unpublished report of C.E.R.T.I. Submitted to WHO by Pernod Ricard.
SOLHEIM, E. & SCHELIEN, R.R. (1973). Metabolism of alkenebenzene
derivatives in the rat I. rho-methoxylallyl benzene (estragole) and
rho-methoxypropenylbenzene (anethole). Xenobiotica, 3, 493-510.
STONER, G.D., SHIMKIN, M.B., KNIAZEIF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumor
response in strain A mice. Cancer Res., 33, 3069-3085.
STROLIN-BENEDETTI, M. & LE BOURHIS, B. (1972). Body distribution and
excretion of trans-anethole-14C. C.R. Acad. Sci. Ser. D., 274,
2378-2381.
SWANSON, A.B., CHAMBLISS, D.D., BLOMQUIST, J.C., MILLER, E.C., &
MILLER, J.A. (1979). The mutagenicities of safrole, estragole,
eugenol, trans-anethole and some of their known or possible
metabolites for Salmonella typhimurium mutants. Mutat. Res., 60,
143-153.
SWANSON, A.B., MILLER, E.C. & MILLER, J.A. (1981). The side chain
epoxidation and hydroxylation of the hepatocarcinogens safrole and
estragole and some related compounds by rat and mouse liver
microsomes. Bichim. Biophys. Acta., 673, 504-516.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964). A comparison of the
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 2, 378-387.
TO, L.P., HUNT, T.P. & ANDERSEN, M.E. (1982). Mutagenicity of trans-
anethole, estragole and safrole in the Ames Salmonella typhimurium
assay. Bull. Environ. Contam. Toxicol., 28, 647-654.
TRUHAUT, R., NEWMAN, J., LE BOURNIS, B., GLOMOT, R. & ATTIA, M.
(1988). A long term feeding study of the toxicity and carcinogenicity
of trans-anethole in rats. Fd. Chem. Toxicol., (in press).
TRUHAUT, R., LE BOURHIS, B., ATTIA, M. GLOMOT, R., NEWMAN, J. &
CALDWELL, J. (1989). Chronic toxicity/carcinogenicity study of
trans-anethole in rats. Fd. Chem. Toxic., 27, 11-20.
VIGNOLI, L., MOREL, M.C. & SIMON, O. (1965). Toxicite chronique du
trans-anethole et des liqueurs anisees a base d'anethole et de
regise. Rev. Path. Comp., 2, 281-287.
 Trans-anethole was first evaluated by the eleventh meeting of
the Committee when a conditional ADI of 0-1.25 mg per kg of body
weight was allocated (Annex 1, Ref. 14). After re-evaluation at the
twenty-third meeting (Annex 1, reference 50), a temporary ADI of 0-2.5
mg per kg of body weight was allocated pending submission of the
results of an adequate long-term study. After further reviews at the
thirty-first and thirty-third meetings (Annex 1, references 77 and 83,
the Committee extended the temporary ADI but reduced it to 0-1.2 mg
per kg of body weight pending further details of the long-term study
and a review of the detailed study records and of the histological
material.
Since the last evaluation, the new data have been reviewed,
together with new data which has become available in the interim, and
are summarized and discussed in the following monograph. The
previously published monograph has been expanded and is reproduced in
its entirety below.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.2.1 Absorption, distribution, biotransformation and excretion
Trans-anethole is rapidly absorbed and distributed in the rat (Le
Bourhis, 1973; Fritsch et al., 1975) and in the mouse (Le Bourhis,
1968; Strolin-Benedetti & Le Bourhis, 1972; Le Bourhis, 1973). The
same authors reported rapid metabolism in both species and identified
the following urinary metabolites:
% dose in urine
1. p-hydroxypropenylbenzene 32
2. p-hydroxycinnamic acid 15
3. p-methoxybenzoic acid 4
4. p-methoxyhippuric acid 43
5. p-methoxyacetophenone trace
Metabolites (1) and (2) appeared largely as the glucuronide or
sulfate. Virtually identical results were obtained by the i.p. route
(Solheim & Scheline, 1973). Administration to rats of ring or methoxy
side-chain 14C-labelled trans-anethole confirmed that virtually all
ring metabolites were eliminated in urine within 48 hrs with
substantial demethylation and consequent appearance of the methoxy 14C
in expired air, and to a small extent the body and faeces (Strolin-
Benedetti & Le Bourhis, 1972).
Trans-anethole was hydroxylated at the 3'-carbon by hepatic
microsomes from rats and mice (Swanson et al., 1981).
In five human subjects given a 500 mg dose the 24-hour
metabolites were anisic acid (52%) and p-hydroxybenzoic acid (5%).
Trans-anethole was not detectable in the blood (Le Bourhis, 1973).
Metabolism in rabbits appeared to be similar to that in humans
(Axelrod, 1956; Le Bourhis, 1970).
More recently, Sangster et al., have compared the routes of
metabolic degradation of various anisole derivatives, including
trans-anethole, in both rat and mouse, and the route of degradation
was dose dependent (Sangster, 1983; Sangster et al., 1984a,b).
After administration of a single dose of 50 mg trans-[14C methoxy]-
anethole/kg b.w. orally to female rats or i.p. to male mice, the major
routes of excretion were in the urine or as 14CO2 in expired air;
excretion in the faeces or as volatile metabolites (other than CO2)
in expired air was low (<2% of the dose). Eleven urinary metabolites
were identified in the rat and ten in the mouse, arising from side-
chain oxidation and cleavage, O-demethylation and conjugation.
Approximately 4.4% and 10.6% of the dose was excreted as two
diastereoisomers of 1-(4'methoxyphenyl)propan-1,2-diol in mice and
rats respectively; these metabolites had presumably arisen from
epoxidation of the side-chain double bond (Sangster et al., 1984a).
The pathways of metabolism identified are as shown in Figure 1.
Trans-anethole was first evaluated by the eleventh meeting of
the Committee when a conditional ADI of 0-1.25 mg per kg of body
weight was allocated (Annex 1, Ref. 14). After re-evaluation at the
twenty-third meeting (Annex 1, reference 50), a temporary ADI of 0-2.5
mg per kg of body weight was allocated pending submission of the
results of an adequate long-term study. After further reviews at the
thirty-first and thirty-third meetings (Annex 1, references 77 and 83,
the Committee extended the temporary ADI but reduced it to 0-1.2 mg
per kg of body weight pending further details of the long-term study
and a review of the detailed study records and of the histological
material.
Since the last evaluation, the new data have been reviewed,
together with new data which has become available in the interim, and
are summarized and discussed in the following monograph. The
previously published monograph has been expanded and is reproduced in
its entirety below.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.2.1 Absorption, distribution, biotransformation and excretion
Trans-anethole is rapidly absorbed and distributed in the rat (Le
Bourhis, 1973; Fritsch et al., 1975) and in the mouse (Le Bourhis,
1968; Strolin-Benedetti & Le Bourhis, 1972; Le Bourhis, 1973). The
same authors reported rapid metabolism in both species and identified
the following urinary metabolites:
% dose in urine
1. p-hydroxypropenylbenzene 32
2. p-hydroxycinnamic acid 15
3. p-methoxybenzoic acid 4
4. p-methoxyhippuric acid 43
5. p-methoxyacetophenone trace
Metabolites (1) and (2) appeared largely as the glucuronide or
sulfate. Virtually identical results were obtained by the i.p. route
(Solheim & Scheline, 1973). Administration to rats of ring or methoxy
side-chain 14C-labelled trans-anethole confirmed that virtually all
ring metabolites were eliminated in urine within 48 hrs with
substantial demethylation and consequent appearance of the methoxy 14C
in expired air, and to a small extent the body and faeces (Strolin-
Benedetti & Le Bourhis, 1972).
Trans-anethole was hydroxylated at the 3'-carbon by hepatic
microsomes from rats and mice (Swanson et al., 1981).
In five human subjects given a 500 mg dose the 24-hour
metabolites were anisic acid (52%) and p-hydroxybenzoic acid (5%).
Trans-anethole was not detectable in the blood (Le Bourhis, 1973).
Metabolism in rabbits appeared to be similar to that in humans
(Axelrod, 1956; Le Bourhis, 1970).
More recently, Sangster et al., have compared the routes of
metabolic degradation of various anisole derivatives, including
trans-anethole, in both rat and mouse, and the route of degradation
was dose dependent (Sangster, 1983; Sangster et al., 1984a,b).
After administration of a single dose of 50 mg trans-[14C methoxy]-
anethole/kg b.w. orally to female rats or i.p. to male mice, the major
routes of excretion were in the urine or as 14CO2 in expired air;
excretion in the faeces or as volatile metabolites (other than CO2)
in expired air was low (<2% of the dose). Eleven urinary metabolites
were identified in the rat and ten in the mouse, arising from side-
chain oxidation and cleavage, O-demethylation and conjugation.
Approximately 4.4% and 10.6% of the dose was excreted as two
diastereoisomers of 1-(4'methoxyphenyl)propan-1,2-diol in mice and
rats respectively; these metabolites had presumably arisen from
epoxidation of the side-chain double bond (Sangster et al., 1984a).
The pathways of metabolism identified are as shown in Figure 1.
 In studies of the dose dependence of trans-anethole metabolism,
single doses of 0.05, 5, 50, or 1500 mg/kg b.w. were administered to
female rats by gavage; similar doses were given to male mice
intraperitoneally with an additional dose level of 250 mg/kg b.w. also
being included. Dose and species-dependent differences in metabolism
were observed. The major route of metabolism was via O-
demethylation in both species and the significance of this route
decreased with increase in dose from 56 and 72% of the 0.05 mg/kg dose
to 32 and 35% of the highest dose in rats and mice respectively. With
regard to side-chain oxidation, the rat favoured the epoxidation route
and elimination of the diol-isomers rose from 2% to 15% of the dose
over the range of doses studied. In the mouse, w-oxidation was
favoured and the diols were formed in smaller amounts of from 1% to
4.5% over the same dose range (Sangster et al., 1984b). It should
be noted that the inter-species differences were compounded by the sex
difference between the species and by the different route of
administration.
Studies of the metabolism of several structurally-related food
flavours, including trans-anethole, were carried out in human
volunteers. Two male subjects received oral doses of 1 mg
14C[methoxy]- trans-anethole and the urine was collected over a 48
hour period subsequently; aliquots of expired air also were collected
at 30 minute intervals for 8 hours after dosing. Approximately 65% of
the activity was excreted in the 48 hour urine, mainly in the first 8
hours, while an estimated 20% of the dose was excreted as 14CO2 in
exhaled air over the first 8 hours. Most of the urinary activity was
in the form of 4-methoxyhippuric acid (ca 55% of the dose) and 4-
methoxybenzoic acid (ca 3.5% of the dose); approximately 3% of the
dose was excreted as the two diastereoisomers of 1-(4'-
methoxyphenyl)propane-1,2-diol (Sangster et al., 1987).
In a study of the dose-dependence of the metabolism of trans-
anethole in human volunteers, 5 male subjects received oral doses of
1, 50 or 250 mg 14C-[methoxy]- trans-anethole on three separate
occasions in milk. 14CO2 was estimated in expired air at intervals
up to 24 hours and radioactivity was measured in urine at intervals up
to 48 hours. Over the range of doses studied, dose had no systematic
effect on the rate or route of excretion, the major routes being urine
(54-69% of the dose) and expired air (13-17% of the dose). The
principal urinary metabolite (>90% of urinary 14C) was
methoxyhippuric acid with much smaller amounts of 4-methoxy-benzoic
acid and up to three other metabolites; the pattern of urinary
metabolites was unaffected by dose size (Caldwell & Sutton, 1988).
Studies of DNA binding of several alkenylbenzenes, including
trans-anethole, were carried out following i.p. administration to
neonatal and adult mice. Very low levels of adduct formation were
detected only when the dose (300 mg/kg i.p.) approached the LD50 (400
mg/kg b.w.).
Groups of 8 male and 8 female CD Sprague-Dawley rats were
administered (methoxy-14C)- trans-anethole at gavage doses of 100,
250, 500 and 1000 mg/kg (16 uCi/kg). Radioactivities appearing in the
urine, faeces and exhaled CO2 were determined at 24 hour intervals
for up to 72 hours, but only the summarized urine and CO2 data were
provided in the report. The urinary metabolites of trans-anethole
in these animals are said to be still under investigation. Based on
the available disposition data, the authors have concluded the
following:
(1) Excretion of 14C in the urine and expired air was delayed
as the dose was increased, with a progressively smaller
fraction of the total radioactivity excreted during 0-24
hours but a greater fraction in 24-48 hours.
(2) As the dose was increased, the excretion route was shifted
from the expired air to the urine.
(3) Females consistently excreted less of the given doses in 0-
24 hours than males, "with recovery of 14C in the urine and
as CO2 being approximately equally affected" (FEMA, 1989).
The Committee noted that the above data were not analyzed
statistically for intergroup differences. Also noted is
that with the possible exception of the dose-dependent delay
of its excretion, the other dose-dependent effects on the
disposition of trans-anethole, as concluded by the
authors, were not very apparent. Furthermore, the author's
conclusion that there is a sex-dependent difference in the
elimination of trans-anethole from the test animals can
not be conclusively supported by the data provided.
2.2 Toxicological Studies
2.2.1 Acute toxicity
Species Route LD50 Reference
(mg/kg/bw)
Mouse per os 1820-5000 Levenstein,
1960, Jenner,
1964, Boissier
et al., 1967
Mouse i.p. 650 Caujolle &
Meynier, 1958
1410 Boissier et al.,
1967
Rat per os 2090-3208 Taylor et al.,
1964, Shelanski,
1958, Boissier
et al., 1967
Rat i.p. 900 Boissier et al.,
1967
2670 Caujolle &
Meynier, 1958
Guinea-pig per os 2160 Jenner et al.,
1964
2.2.2 Short-term studies
2.2.2.1 Rat
Rats (number not given) receiving anethole in the diet at 0.25%
for one year showed no adverse effects while those receiving 1.0% for
15 weeks showed slight hydropic alterations of hepatic cells (Taylor,
et al., 1964).
Groups of five male and five female rats maintained on diets
containing trans-anethole at 0, 0.1, 0.3, 1.0 or 3.0% for 90 days
showed no effects at 0.1% but dose-related hepatic cell oedema,
degeneration and regeneration at 0.3% and higher. In a parallel
experiment with the cis-isomer, similar changes were noted at 0.03%
and higher (Shelanski, 1958).
Groups of 10 adult male rabbits and adult male and female rats
(number per group not specified) were given orally once per week in
their drinking water a quantity of trans-anethole corresponding to
7 daily doses of 11.4 mg/kg/day over a period of 90 days. Another
group of rats (21 days old), 10 rats per group were treated with
anethole identically but only for a period of 21 days. Neither
measurements of growth, electroencephalograms, electrocardiograms nor
gross and histopathological examinations revealed any evidence of
adverse toxic effects in any group treated either for 21 or 90 days
(Vignoli et al., 1965).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rat
Groups of 25 male and female rats maintained at 0.2, 0.5, 1.0 or
2.0% trans-anethole in the diet for 12-22 months showed no effects
at any level in clinical chemistry, haematology, histopathology nor
mortality. Slower weight gain and decreased fat storage were noted
only at the 1.0 and 2.0% levels. In a paired feeding study, trans-
anethole reduced the rate of weight gain (Le Bourhis, 1973).
Trans-anethole was administered to Sprague-Dawley rats in the
diet at concentrations of 0, 0.25, 0.5 or 1% for 117 weeks. The group
sizes were 52 males and 52 females in two separate control groups and
in the intermediate and high dose groups, and 78 males and 78 females
in the low dose group. A supplementary group of 26 males and 26
females received the trans-anethole at a level of 1% in the diet for
the first 54 weeks of the study, then 10 animals of each sex were
placed on the control diet until week 121; the remaining animals of
each sex continued to receive the diet containing 1% trans-anethole.
Food intake was recorded daily during weeks 1-32 and for one day every
4 weeks thereafter; body weight gain was monitored at weekly intervals
up to week 26 and then at monthly intervals. The animals were
inspected clinically at frequent intervals during the course of the
study and the animals were palpated weekly from week 27 and the
appearance of palpable masses recorded. Moribund animals were killed
in the course of the study; these animals and those sacrificed at
termination were subjected to detailed gross and histopathological
examination (40 tissues). Also at termination, haematological
examinations were performed, including RBC, haemoglobin, MCV,
haematocrit, MCHC, leucocyte count and differential blood count.
There was a retardation in body weight gain in all treated
animals during the first six months of treatment after which the
weight gain of the 0.25% and 0.5% groups was similar to controls,
although the weight deficit was not recovered; in the high dose group
the weight gain remained less than controls. The deficit of weight
gain was associated with reduced food intake due to unpalatability of
the diet and animals removed from the 1% diet after 54 weeks rapidly
gained weight. There were no treatment-related effects on mortality
except for a significantly decreased mortality in the high-dose group
males. Haematological examination did not reveal any treatment-
related differences but comparison of organ weights revealed an
increase in relative liver weight of all treatment groups of females
(P<0.001 in the highest dose group). Treatment-related
histopathological changes were reported in the liver in the form of
hepatocytic vacuolation (males, 1% dose group), sinusoidal dilatation
(males and females in the 1% group, females in the 0.5% group),
focal/nodular hyperplasia (males and females in the 1% group, males in
the 0.5% group) and hepatocellular hypertrophy in females of the 0.5%
and 1% groups.
There was a reported statistically significant increase in the
incidence of benign hepatocellular adenomas and hepatocellular
carcinomas in females in the 1% dose group, but it was pointed out
that this was within the range of spontaneous incidence of similar
lesions in historical controls for the strain of rat used compiled
between 1977 and 1985 (Truhaut et al., 1988). It was considered
that the trend of increase in incidence of benign and malignant liver
cell tumours represented a secondary response to a non-specific effect
rather than a direct genotoxic effect.
In the light of new criteria (Maronpot et al., 1986) for the
diagnosis of proliferative hepatic lesions in rats, the findings of
the above study were reviewed by independent pathologists (Newberne
et al., 1987; Moch, 1990). The results of these reviews are
tabulated below and, in the case of hepatocellular adenomas and
carcinomas, compared with the original diagnosis:
Table 2: Hepatic Lesions
Dose Groups FCA FNH HA HCA HCA
+
HA
Pathologists Male Rats
0% Moch 33/104 2/104 1/104 4/104 5/104
(32%) (2%) (1%) (4%) (5%)
Truhaut - 3/104 3/104 2/104 5/104
(3%) (3%) (2%) (5%)
Newberne 48/104 2/104 1/104 3/104 4/104
(46%) (2%) (1%) (3%) (4%)
0.25%
Moch 32/78 6/78 1/78 3/78 4/78
(41%) (8%) (1%) (4%) (5%)
Truhaut - 3/78 3/78 1/78 4/78
(4%) (4%) (1%) (5%)
Newberne 34/78 7/78 1/78 3/78 4/78
(44%) (9%) (1%) (4%) (5%)
0.5%
Moch 27/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
Truhaut - 8/52 0/52 3/52 3/52
(15%) (6%) (6%)
Newborne 28/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
1.0%
Moch 25/52 14/52 3/52 1/52 4/52
(48%) (27%) (6%) (2%) (8%)
Truhaut - 14/52 4/52 1/52 5/52
(27%) (8%) (2%) (10%)
Newberne 27/52 13/52 3/52 1/52 4/52
(52%) (25%) (6%) (2%) (8%)
Female Rats
0%
Moch 47/104 4/104 4/104 0/104 4/104
(45%) (4%) (4%) (4%)
Truhaut - 11/104 2/104 1/104 3/104
(11%) (2%) (1%) (4%)
Newberne 62/104 6/104 4/104 0/104 4/104
(60%) (6%) (4%) (4%)
Table 2 (contd)
Dose Groups FCA FNH HA HCA HCA
+
HA
0.25%
Moch 42/78 8/78 1/78 0/78 1/78
(54%) (10%) (1%) (1%)
Truhaut - 2/78 2/78 0/78 2/78
(3%) (3%) (3%)
Newberne 53/78 10/78 1/78 0/78 1/78
(68%) (13%) (1%) (1%)
0.5%
Moch 27/52 7/52 0/52 0/52 0/52
(52%) (14%)
Truhaut - 6/52 0/52 0/52 0/52
(12%)
Newberne 32/52 10/52 0/52 0/52 0/52
(62%) (19%)
1%
Moch 16/52 18/52 4/52 6/52 10/52
(31%) (35%) (8%) (12%) (19%)
Truhaut - 15/52 6/52 6/52 12/52
(29%) (12%) (12%) (23%)
Newberne 21/52 15/52 4/52 6/52 10/52
(40%) (29%) (8%) (12%) (19%)
* Hepatic Lesions: FCA = Focus of Cellular Alteration;
FNH = Focus of Nodular Hyperplasia
HA = Hepatocellular Adenoma
HCA = Hepatocellular Carcinoma
As shown above, the findings of the independent pathologists are
in good agreement, all showing a clear increase in high-dose (1%)
female rats with hepatocellular adenoma and/or carcinoma. A similar
increase was not observed in male rats at any dose levels. The
differences observed in the incidence of rats with altered cellular
foci between the original study pathologist and the others, were
primarily due to the fact that the original study pathologist had
diagnosed any vacuolation or clearing of hepatocytes observed as
hepatocellular vacuolation rather than as foci of cellular alteration
(Moch, 1990).
An independent audit of this rat study report showed no
significant discrepancies (Munro & Brillinger, 1989a,b).
2.2.3.2 Mouse
In in the first eight weeks of a 24-week screening test, groups
of 20 female A/He mice received a total dose of 2, 4 or 12.0 g
anethole/kg b.w. in 24 3x/week i.p. injections. The higher dose had
previously been calculated to be the maximum tolerated dose. There
was no increase in the incidence of tumours of the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands.
Survival was reduced to approximately 70% (Stoner et al., 1973). It
should be noted that safrole, which has been shown to be carcinogenic
in other studies, was negative using this protocol, thus there is some
doubt about its validity.
A series of experiments was performed to investigate the
carcinogenic potential of trans-anethole in mice. Groups of male
(56-76 per group) and female (55-61 per group) 4-day old mice were
exposed to trans-anethole by gavage at dose levels of 370 or 740
mg/kg b.w. twice weekly for 10 weeks, then sacrificed at 11-14 months.
In a second experiment, 53 male mice received trans-anethole by
intraperitoneal injection on days 1, 8, 15 and 22 after birth. The
total dose of anethole that each animal received during 4 treatments
was 703 or 1399 mg. Animals from the lower dose group were sacrificed
at 13-18 months and those from the 1390 mg group at 12 months. In a
third experiment, a group of female mice, approximately 8 weeks old,
average body weight 21 g, were fed anethole in the diet at a dose
level of 690 mg/kg for a period of 12 months. After 12 months the
animals received basal diet only and were sacrificed at 18 months.
The fourth experimental group of 17 female mice (8 weeks old) was
given an injection of anethole twice weekly for 12 weeks at a dose
level of 148 mg/kg b.w. The mice were killed 8 months after the first
injection. All animals from each experiment were necropsied and the
various tissues examined histologically. Trans-anethole produced no
different results from controls (Miller et al., 1983).
2.2.4 Special studies on mutagenicity
Two strains of Salmonella typhimurium (TA100 and TA98), with
and without microsomal activation, were used in a plate test to study
the effect of trans-anethole (isolated natural compound), anise oil
(90% trans-anethole) and fennel oil (70% trans-anethole). Each
test material was assayed over the dose range from zero (control) to
the level of cell toxicity. All test materials increased mutagenic
activities with TA100 tester strain with implementation of microsomal
activation (S13) system. Peak mutagenic activity, 4 and 4.4 times
that of the background rate, occurred with 2 mg anise oil/plate and
2.5 mg fennel oil/plate respectively. Isolated natural trans-
anethole was also mutagenic (the dose level and rate of mutagenicity
were not stated). The compound was considered not to be mutagenic
unless it was capable of inducing a mutation (reversion) rate at least
3 times that of the incident background (Marcus & Liechtenstein,
1982).
The mutagenic activities of anethole and its metabolite 3'-
hydroxyanethole were studied using three tester strains of Salmonella
typhimurium (TA1535, TA100 and TA98). Addition of an NADPH-
generating system and liver microsomes and cytosol (S13 fraction) from
Aroclor-treated rats (6.8 mg liver protein/plate) to the incubation
mixture of TA100 tester strain increased mutagenic activities.
Approximately 45 revertants were obtained per µmole anethole. Under
the same conditions, 3'-hydroxyanethole showed no significant
mutagenic activity with less than 7 µmoles/plate. Above this
concentration the S13-mediated mutagenicity increased linearly with
increased doses up to 15 µmoles/plate (about 1000 revertants with 15
µmoles/plate) (Swanson et al., 1979).
Five strains of Salmonella typhimurium (TA1535, TA100, TA1537,
TA1538, TA98), with and without metabolic activation (S9 mix), were
used to study potential mutagenic effects of trans-anethole. The
lowest overtly toxic concentration for trans-anethole was 1
mg/plate. No mutagenic activity was observed at concentrations of up
to 50 µg trans-anethole/plate with or without metabolic activation.
However, the addition of 3'-phosphoadenosine-5'phosphosulphate (PAPS)
to the microsomal assay markedly increased the mutagenicity of trans-
anethole in TA1535 tester strain. The mutation rate observed was
approximately 4, 5, 10, 11, 9 and 3 times that of the background rate
at trans-anethole concentrations of 0.05, 0.20, 1.0, 5.0, 15.0, and
50.0 ug/plate respectively (To et al., 1982).
A further study indicated that trans-anethole showed mutagenic
activity in the Ames S. typhimurium assay but was inactive in a B.
subtilis Rec assay and was negative in an E. coli uvr A reversion
test (Sekizawa & Shibamoto, 1982).
Trans-anethole was inactive in a mouse micronucleus assay 24,
48 and 72 hr after administration by gavage of the second of two daily
doses of 2 ml/kg b.w. to groups of 10 adult males. At this dose level
there was significant mortality (3-4 animals per group) (Siou et al.,
1984). Negative results were also obtained in a mouse micronucleus
assay after i.p. administration to groups of 5 male and 5 female mice
in two doses of 0.25 or 0.5 g/kg b.w., 30 and 6 hours before sacrifice
(Marzin, 1979).
Genotoxicity of trans-anethole was tested, along with some
structurally related compounds (safrole, isosafrole, eugenol,
estragole, allylbenzene, methyleugenol, and p-propylanisole), for its
capability of inducing unscheduled DNA synthesis (UDS) in freshly
isolated rat hepatocytes in primary culture. Hepatocytes were
isolated from male Fischer rats following liver perfusion. After
overnight incubation of the hepatocytes in the presence of the test
chemical, the UDS or DNA repair was measured by determining the amount
of 3H-thymidine incorporated into hepatocyte nuclear DNA during the
repair process. Under the test conditions, trans-anethole did not
induce UDS over the concentration range of 10-6 and 10-2 M, although
cytotoxicity, as measured by LDH leakage, was observed at
concentrations of 10-3 M and above. Of the remaining chemicals
tested, allybenzene, p-propylanisole, isosafrole and eugenol were also
negative in the UDS assay, whereas safrole, methyleugenol and
estragole as well as the positive control, 2-acetylaminofluorene, all
induced UDS in a dose-related manner.
Based on the study results, the authors concluded: "there was an
excellent correlation between UDS induction and known rodent hepato-
carcinogenicity, with safrole, estragole and methyleugenol all
inducing UDS. Anethole, isosafrole, eugenol and allylbenzene, for
which evidence of carcinogenicity is equivocal or negative, did not
induce UDS" (Howes et al., 1990).
In another test, trans-anethole was tested for its ability to
induce UDS in cultured primary hepatocytes isolated from Sprague-
Dawley derived CD rats of both sexes. At concentrations up to 10-2,
the compound did not induce UDS, as measured by 3H-thymidine
incorporation into nuclear DNA. The positive control, 2-
acetylaminofluorene, gave positive response. At concentrations of
10-3 and above, trans-anethole was cytotoxic and the UDS response
was below the control values. The cytotoxicity of trans-anethole
was consistent with the increased leakage of cellular LDH into the
culture medium.
To investigate the possible involvement of a reactive epoxide
intermediate in the observed cytotoxicity, the effect of trans-
anethole on intracellular glutathione levels was studied. The results
showed that anethole caused a dose-related depletion of glutathione in
freshly isolated hepatocytes in suspension. However, when hepatocytes
were pretreated with the non-cytotoxic glutathione depleting agent
dimethyl maleate, there was no enhancement of anethole cytotoxicity,
suggesting that the putative epoxide intermediate may not be of any
toxicological significance (Caldwell & Marshall, 1990).
2.2.5 Special studies on pharmacological effects
The pharmacologic effects of trans-anethole most often noted
are reduction in motor activity, lowering of body temperature and
hypnotic, analgesic and anticonvulsant effects. By either the oral or
i.p. route, administration of more than 10% of the LD50 by that route
appears necessary for significant effects (Boissier et al., 1961;
Seto, 1969; Gruebner, 1972; Le Bourhis & Soene, 1973).
3. COMMENTS
The Committee considered the results of three independent reviews
of the liver histology in the long-term study in rats. It concluded
that there is a clear increase in the incidences of hepatocellular
adenomas and of hepatocellular carcinomas in female rats at 10 mg/kg
in the diet but not at lower doses. In male animals a slight increase
in the incidence of hepatocellular adenomas but not carcinomas was
seen at 10 mg/kg in the diet only. There is also evidence of an
increase in the incidence of non-neoplastic proliferative lesions in
the liver at all dose levels in both sexes.
A review of the report of the long-term rat study showed no
significant discrepancies. The Committee also noted that differences
in survival between treated and control animals could not account for
the increased incidence of liver tumours seen in the study.
The results of two new studies on genotoxicity (unscheduled DNA
synthesis) were negative.
The Committee concluded that insufficient data were available to
permit a final evaluation of the significance of the malignant liver
tumours observed in female rats with respect to the use of trans-
anethole as a food additive. Further metabolic and especially
pharmacokinetic studies in mice, rats, and humans are required for
evaluation in 1992. In addition, a long-term dietary study in mice
may be needed, although the design of such a study will depend upon
the results of the metabolic and pharmacokinetic studies. In view of
the positive results that were obtained in in vitro bacterial gene
mutation tests, the Committee also concluded that chromosome
aberration studies and in vitro tests for gene mutation in mammalian
cells were desirable.
4. EVALUATION
The temporary ADI was extended until 1992, but reduced to 0-0.6
mg per kg of body weight on the basis of the minimal effect level of
2.5 mg/kg in the diet (equivalent to a dose of 125 mg per kg of body
weight per day) for non-neoplastic proliferative changes in the liver
of rats, adjusted using a safety factor of 200.
The need for a reproduction/teratogenicity study will be
considered by the Committee when further relevant data from the above
studies have been reviewed. The Committee also reiterated its
recommendation that an epidemiological study of the effects of
consuming high dietary levels of trans-anethole would be desirable.
5. REFERENCES
AXELROD, J. (1956). Enzymic cleavage of aromatic ethers. Biochem.
J., 63, 634-639.
BOISSIER, J.R., SIMON, P. & LE BOURHIS, B. (1967). Experimental
psychotropic effect of isomeric cis- and trans-anetholes.
Therapie, 22, 309-323.
CALDWELL, J. SUTTON, J.D. (1988). Influence of dose size upon the
disposition of trans-[methoxy-14C]-anethole in human volunteers.
Fd. Chem. Toxicol., (In press).
CALDWELL, J. & MARSHALL, A.D. (1990). The cytotoxicity and
genotoxicity of trans-anethole in isolated rat hepatocytes.
Progress Report submitted to WHO by Anethole Task Force, FEMA,
Washington, D.C. U.S.A., March, 1990.
CAUJOLLE, F. & MEYNIER, D. (1958). Toxicity of tarragon oil and the
anetholes ( cis- and trans-), Acad. Sci. (Paris), 246, 1465-1468.
FEMA (1989). Metabolic and mechanistic studies intended to show that
the low incidence of hepatocellular carcinoma induced in rats by
trans-anethole does not indicate a hazard to humans. Anethole
Progress Report submitted to WHO 11/13/89.
FRITSCH, P., DE SAINT-BLANUAT, G. & DERACHE, R. (1975).
Gastrointestinal absorption, in the rat, of anisole, trans-anethole,
butylhydroxyanisole and safrole. Fd. Cosmet. Toxicol., 13, 359-364.
GRUEBNER, I., KLINGER, W. & ANERMANN, H. (1972). Various substances
and substance classes with inducer properties. II. Arch. Int.
Pharmacodyn. Ther., 196, 288-297.
HOWES, A.J., CHAN, V.S.W. & CALDWELL, J. (1990). Structure-
specificity of the genotoxicity of some naturally occurring
alkenylbenzenes determined by the unscheduled DNA synthesis assay in
rat hepatocytes. Prepublication submitted to Food Chem. Toxicol.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I.
Acute and oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
LE BOURHIS, B. (1968). The metabolism of trans-anethole. Ann.
Biol. Clin., 26, 711-715.
LE BOURHIS, B. (1970). Identification of a few metabolites of trans-
anethole in man, the rabbit and the rat. Ann. Pharm. Fr., 28, 355-
361.
LE BOURHIS, B. (1973). Biological properties of trans-anethole. An
attempt to determine an acceptable daily dosage. Parfums Cosmet.
Savons. Fr., 3, 450-456.
LE BOURHIS, B. & SOENE, A.M. (1973). Studies on the psychotropic
action of some aromatic compounds used in food. Fd. Cosmet.
Toxicol., 11, 1-9.
LEVENSTEIN, I. (1960). Unpublished report of Leberco Laboratories,
Submitted to WHO.
MARCUS, C. & LIECHTENSTEIN, E.P. (1982). Interactions of naturally
occurring food plant components with insecticides and pentobarbital in
rats and mice. J. Agric. Food Chem., 30, 563-568.
MARONPOT, R.R., MONTGOMERY, C.A., BOORMAN, G.A. & MCCONNELL, E.
(1986). National Toxicology Program nomenclature for
hepatoproliferative lesions of rats. Toxicol. Pathol., 14, 263-273.
MARZIN, D. (1979). Recherche d'une action mutagene par le test du
micronucleus chez la souris. Unpublished report of DREBS. Submitted
to WHO by Pernod Ricard.
MILLER, E.C., SWANSON, A.B., PHILLIPS, D.H., FLETCHER, T.L., LIEM, A.
& MILLER, J.A. (1983). Structure-activity studies of the
carcinogenicities in the mouse and rat of some naturally occurring and
synthetic alkenylbenzene derivatives related to safrole and estragole.
Cancer Res., 43, 1124-1134.
MOCH, R.W. (1990). Trans-anethole. Histopathologic review of
microslides containing liver proliferative lesions from a long-term
study in Sprague-Dawley rats. Pathology Report submitted to WHO
April, 1990.
MUNRO, I.C. & BRILLINGER, R. (1989a). Preliminary audit of the
procedures and findings of a 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
MUNRO, I.C. & BRILLINGER, R. (1989b). Addendum to the preliminary and
detailed audit reports of the 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
NEWBERNE, P.M., BROWN, W.R. & CARLTON, W.W. (1987). Histopathologic
evaluation of proliferative lesions in livers of rats fed trans-
anethole for 117-121 weeks (Long-term feeding study in rats, study no.
3190 TCR) Unpublished report. Submitted to WHO by Pernod Ricard.
NEWBERNE, P.M., CARLTON, W.W. & BROWN, W.R. (1989). Histopathological
evaluation of proliferative lesions in rats fed trans-anethole in
chronic studies. Fd. Chem. Toxic., 27, 21-26.
SANGSTER, S.A. (1983). The metabolism and disposition of trans-
anethole and p-propenylanisole in rodent species and man. Ph.D.
Thesis, University of London.
SANGSTER, S.A., CALDWELL, J., SMITH, R.L. & FARMER, P.B. (1984a).
Metabolism of anethole I. Pathways of metabolism in the rat and mouse.
Fd. Chem. Toxicol., 22, 695-706.
SANGSTER, S.A., CALDWELL, J. & SMITH, R.L. (1984b). Metabolism of
anethole II. Influence of dose size on the route of metabolism of
trans-anethole in the rat and mouse. Fd. Chem. Toxicol., 22,
707-713.
SANGSTER, S.A., CALDWELL, J., HUTT, A.J. ANTHONY, A. & SMITH, R.L.
(1987). The metabolic disposition of [methoxy-14C]-labelled trans-
anethole, estragole and p-propylanisole in human volunteers.
Xenobiotica, 17, 1223-1232.
SEKIZAWA, J. & SHIBAMOTO, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
SETO, T.A. (1969). Effects of alkylmethoxybenzene and alkyl-
methylenedioxybenzene essential oils on pentobarbital and ethanol
sleeping time. Arch. Int. Pharmacodyn. Ther., 180, 232-240.
SHELANSKI, M.V. (1958). Unpublished report of Industrial Biology
Research and Testing Laboratories. Submitted to WHO.
SIOU, G., LEROND-CONAN, L., EL HAITEM, M. & LACRAMPE, M. (1984).
Recherche d'une eventuelle potentialite genotoxique du p-methoxy-
propenylbenzene par la technique du micronucleus chez la souris.
Unpublished report of C.E.R.T.I. Submitted to WHO by Pernod Ricard.
SOLHEIM, E. & SCHELIEN, R.R. (1973). Metabolism of alkenebenzene
derivatives in the rat I. rho-methoxylallyl benzene (estragole) and
rho-methoxypropenylbenzene (anethole). Xenobiotica, 3, 493-510.
STONER, G.D., SHIMKIN, M.B., KNIAZEIF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumor
response in strain A mice. Cancer Res., 33, 3069-3085.
STROLIN-BENEDETTI, M. & LE BOURHIS, B. (1972). Body distribution and
excretion of trans-anethole-14C. C.R. Acad. Sci. Ser. D., 274,
2378-2381.
SWANSON, A.B., CHAMBLISS, D.D., BLOMQUIST, J.C., MILLER, E.C., &
MILLER, J.A. (1979). The mutagenicities of safrole, estragole,
eugenol, trans-anethole and some of their known or possible
metabolites for Salmonella typhimurium mutants. Mutat. Res., 60,
143-153.
SWANSON, A.B., MILLER, E.C. & MILLER, J.A. (1981). The side chain
epoxidation and hydroxylation of the hepatocarcinogens safrole and
estragole and some related compounds by rat and mouse liver
microsomes. Bichim. Biophys. Acta., 673, 504-516.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964). A comparison of the
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 2, 378-387.
TO, L.P., HUNT, T.P. & ANDERSEN, M.E. (1982). Mutagenicity of trans-
anethole, estragole and safrole in the Ames Salmonella typhimurium
assay. Bull. Environ. Contam. Toxicol., 28, 647-654.
TRUHAUT, R., NEWMAN, J., LE BOURNIS, B., GLOMOT, R. & ATTIA, M.
(1988). A long term feeding study of the toxicity and carcinogenicity
of trans-anethole in rats. Fd. Chem. Toxicol., (in press).
TRUHAUT, R., LE BOURHIS, B., ATTIA, M. GLOMOT, R., NEWMAN, J. &
CALDWELL, J. (1989). Chronic toxicity/carcinogenicity study of
trans-anethole in rats. Fd. Chem. Toxic., 27, 11-20.
VIGNOLI, L., MOREL, M.C. & SIMON, O. (1965). Toxicite chronique du
trans-anethole et des liqueurs anisees a base d'anethole et de
regise. Rev. Path. Comp., 2, 281-287.
In studies of the dose dependence of trans-anethole metabolism,
single doses of 0.05, 5, 50, or 1500 mg/kg b.w. were administered to
female rats by gavage; similar doses were given to male mice
intraperitoneally with an additional dose level of 250 mg/kg b.w. also
being included. Dose and species-dependent differences in metabolism
were observed. The major route of metabolism was via O-
demethylation in both species and the significance of this route
decreased with increase in dose from 56 and 72% of the 0.05 mg/kg dose
to 32 and 35% of the highest dose in rats and mice respectively. With
regard to side-chain oxidation, the rat favoured the epoxidation route
and elimination of the diol-isomers rose from 2% to 15% of the dose
over the range of doses studied. In the mouse, w-oxidation was
favoured and the diols were formed in smaller amounts of from 1% to
4.5% over the same dose range (Sangster et al., 1984b). It should
be noted that the inter-species differences were compounded by the sex
difference between the species and by the different route of
administration.
Studies of the metabolism of several structurally-related food
flavours, including trans-anethole, were carried out in human
volunteers. Two male subjects received oral doses of 1 mg
14C[methoxy]- trans-anethole and the urine was collected over a 48
hour period subsequently; aliquots of expired air also were collected
at 30 minute intervals for 8 hours after dosing. Approximately 65% of
the activity was excreted in the 48 hour urine, mainly in the first 8
hours, while an estimated 20% of the dose was excreted as 14CO2 in
exhaled air over the first 8 hours. Most of the urinary activity was
in the form of 4-methoxyhippuric acid (ca 55% of the dose) and 4-
methoxybenzoic acid (ca 3.5% of the dose); approximately 3% of the
dose was excreted as the two diastereoisomers of 1-(4'-
methoxyphenyl)propane-1,2-diol (Sangster et al., 1987).
In a study of the dose-dependence of the metabolism of trans-
anethole in human volunteers, 5 male subjects received oral doses of
1, 50 or 250 mg 14C-[methoxy]- trans-anethole on three separate
occasions in milk. 14CO2 was estimated in expired air at intervals
up to 24 hours and radioactivity was measured in urine at intervals up
to 48 hours. Over the range of doses studied, dose had no systematic
effect on the rate or route of excretion, the major routes being urine
(54-69% of the dose) and expired air (13-17% of the dose). The
principal urinary metabolite (>90% of urinary 14C) was
methoxyhippuric acid with much smaller amounts of 4-methoxy-benzoic
acid and up to three other metabolites; the pattern of urinary
metabolites was unaffected by dose size (Caldwell & Sutton, 1988).
Studies of DNA binding of several alkenylbenzenes, including
trans-anethole, were carried out following i.p. administration to
neonatal and adult mice. Very low levels of adduct formation were
detected only when the dose (300 mg/kg i.p.) approached the LD50 (400
mg/kg b.w.).
Groups of 8 male and 8 female CD Sprague-Dawley rats were
administered (methoxy-14C)- trans-anethole at gavage doses of 100,
250, 500 and 1000 mg/kg (16 uCi/kg). Radioactivities appearing in the
urine, faeces and exhaled CO2 were determined at 24 hour intervals
for up to 72 hours, but only the summarized urine and CO2 data were
provided in the report. The urinary metabolites of trans-anethole
in these animals are said to be still under investigation. Based on
the available disposition data, the authors have concluded the
following:
(1) Excretion of 14C in the urine and expired air was delayed
as the dose was increased, with a progressively smaller
fraction of the total radioactivity excreted during 0-24
hours but a greater fraction in 24-48 hours.
(2) As the dose was increased, the excretion route was shifted
from the expired air to the urine.
(3) Females consistently excreted less of the given doses in 0-
24 hours than males, "with recovery of 14C in the urine and
as CO2 being approximately equally affected" (FEMA, 1989).
The Committee noted that the above data were not analyzed
statistically for intergroup differences. Also noted is
that with the possible exception of the dose-dependent delay
of its excretion, the other dose-dependent effects on the
disposition of trans-anethole, as concluded by the
authors, were not very apparent. Furthermore, the author's
conclusion that there is a sex-dependent difference in the
elimination of trans-anethole from the test animals can
not be conclusively supported by the data provided.
2.2 Toxicological Studies
2.2.1 Acute toxicity
Species Route LD50 Reference
(mg/kg/bw)
Mouse per os 1820-5000 Levenstein,
1960, Jenner,
1964, Boissier
et al., 1967
Mouse i.p. 650 Caujolle &
Meynier, 1958
1410 Boissier et al.,
1967
Rat per os 2090-3208 Taylor et al.,
1964, Shelanski,
1958, Boissier
et al., 1967
Rat i.p. 900 Boissier et al.,
1967
2670 Caujolle &
Meynier, 1958
Guinea-pig per os 2160 Jenner et al.,
1964
2.2.2 Short-term studies
2.2.2.1 Rat
Rats (number not given) receiving anethole in the diet at 0.25%
for one year showed no adverse effects while those receiving 1.0% for
15 weeks showed slight hydropic alterations of hepatic cells (Taylor,
et al., 1964).
Groups of five male and five female rats maintained on diets
containing trans-anethole at 0, 0.1, 0.3, 1.0 or 3.0% for 90 days
showed no effects at 0.1% but dose-related hepatic cell oedema,
degeneration and regeneration at 0.3% and higher. In a parallel
experiment with the cis-isomer, similar changes were noted at 0.03%
and higher (Shelanski, 1958).
Groups of 10 adult male rabbits and adult male and female rats
(number per group not specified) were given orally once per week in
their drinking water a quantity of trans-anethole corresponding to
7 daily doses of 11.4 mg/kg/day over a period of 90 days. Another
group of rats (21 days old), 10 rats per group were treated with
anethole identically but only for a period of 21 days. Neither
measurements of growth, electroencephalograms, electrocardiograms nor
gross and histopathological examinations revealed any evidence of
adverse toxic effects in any group treated either for 21 or 90 days
(Vignoli et al., 1965).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rat
Groups of 25 male and female rats maintained at 0.2, 0.5, 1.0 or
2.0% trans-anethole in the diet for 12-22 months showed no effects
at any level in clinical chemistry, haematology, histopathology nor
mortality. Slower weight gain and decreased fat storage were noted
only at the 1.0 and 2.0% levels. In a paired feeding study, trans-
anethole reduced the rate of weight gain (Le Bourhis, 1973).
Trans-anethole was administered to Sprague-Dawley rats in the
diet at concentrations of 0, 0.25, 0.5 or 1% for 117 weeks. The group
sizes were 52 males and 52 females in two separate control groups and
in the intermediate and high dose groups, and 78 males and 78 females
in the low dose group. A supplementary group of 26 males and 26
females received the trans-anethole at a level of 1% in the diet for
the first 54 weeks of the study, then 10 animals of each sex were
placed on the control diet until week 121; the remaining animals of
each sex continued to receive the diet containing 1% trans-anethole.
Food intake was recorded daily during weeks 1-32 and for one day every
4 weeks thereafter; body weight gain was monitored at weekly intervals
up to week 26 and then at monthly intervals. The animals were
inspected clinically at frequent intervals during the course of the
study and the animals were palpated weekly from week 27 and the
appearance of palpable masses recorded. Moribund animals were killed
in the course of the study; these animals and those sacrificed at
termination were subjected to detailed gross and histopathological
examination (40 tissues). Also at termination, haematological
examinations were performed, including RBC, haemoglobin, MCV,
haematocrit, MCHC, leucocyte count and differential blood count.
There was a retardation in body weight gain in all treated
animals during the first six months of treatment after which the
weight gain of the 0.25% and 0.5% groups was similar to controls,
although the weight deficit was not recovered; in the high dose group
the weight gain remained less than controls. The deficit of weight
gain was associated with reduced food intake due to unpalatability of
the diet and animals removed from the 1% diet after 54 weeks rapidly
gained weight. There were no treatment-related effects on mortality
except for a significantly decreased mortality in the high-dose group
males. Haematological examination did not reveal any treatment-
related differences but comparison of organ weights revealed an
increase in relative liver weight of all treatment groups of females
(P<0.001 in the highest dose group). Treatment-related
histopathological changes were reported in the liver in the form of
hepatocytic vacuolation (males, 1% dose group), sinusoidal dilatation
(males and females in the 1% group, females in the 0.5% group),
focal/nodular hyperplasia (males and females in the 1% group, males in
the 0.5% group) and hepatocellular hypertrophy in females of the 0.5%
and 1% groups.
There was a reported statistically significant increase in the
incidence of benign hepatocellular adenomas and hepatocellular
carcinomas in females in the 1% dose group, but it was pointed out
that this was within the range of spontaneous incidence of similar
lesions in historical controls for the strain of rat used compiled
between 1977 and 1985 (Truhaut et al., 1988). It was considered
that the trend of increase in incidence of benign and malignant liver
cell tumours represented a secondary response to a non-specific effect
rather than a direct genotoxic effect.
In the light of new criteria (Maronpot et al., 1986) for the
diagnosis of proliferative hepatic lesions in rats, the findings of
the above study were reviewed by independent pathologists (Newberne
et al., 1987; Moch, 1990). The results of these reviews are
tabulated below and, in the case of hepatocellular adenomas and
carcinomas, compared with the original diagnosis:
Table 2: Hepatic Lesions
Dose Groups FCA FNH HA HCA HCA
+
HA
Pathologists Male Rats
0% Moch 33/104 2/104 1/104 4/104 5/104
(32%) (2%) (1%) (4%) (5%)
Truhaut - 3/104 3/104 2/104 5/104
(3%) (3%) (2%) (5%)
Newberne 48/104 2/104 1/104 3/104 4/104
(46%) (2%) (1%) (3%) (4%)
0.25%
Moch 32/78 6/78 1/78 3/78 4/78
(41%) (8%) (1%) (4%) (5%)
Truhaut - 3/78 3/78 1/78 4/78
(4%) (4%) (1%) (5%)
Newberne 34/78 7/78 1/78 3/78 4/78
(44%) (9%) (1%) (4%) (5%)
0.5%
Moch 27/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
Truhaut - 8/52 0/52 3/52 3/52
(15%) (6%) (6%)
Newborne 28/52 6/52 1/52 3/52 4/52
(52%) (12%) (2%) (6%) (8%)
1.0%
Moch 25/52 14/52 3/52 1/52 4/52
(48%) (27%) (6%) (2%) (8%)
Truhaut - 14/52 4/52 1/52 5/52
(27%) (8%) (2%) (10%)
Newberne 27/52 13/52 3/52 1/52 4/52
(52%) (25%) (6%) (2%) (8%)
Female Rats
0%
Moch 47/104 4/104 4/104 0/104 4/104
(45%) (4%) (4%) (4%)
Truhaut - 11/104 2/104 1/104 3/104
(11%) (2%) (1%) (4%)
Newberne 62/104 6/104 4/104 0/104 4/104
(60%) (6%) (4%) (4%)
Table 2 (contd)
Dose Groups FCA FNH HA HCA HCA
+
HA
0.25%
Moch 42/78 8/78 1/78 0/78 1/78
(54%) (10%) (1%) (1%)
Truhaut - 2/78 2/78 0/78 2/78
(3%) (3%) (3%)
Newberne 53/78 10/78 1/78 0/78 1/78
(68%) (13%) (1%) (1%)
0.5%
Moch 27/52 7/52 0/52 0/52 0/52
(52%) (14%)
Truhaut - 6/52 0/52 0/52 0/52
(12%)
Newberne 32/52 10/52 0/52 0/52 0/52
(62%) (19%)
1%
Moch 16/52 18/52 4/52 6/52 10/52
(31%) (35%) (8%) (12%) (19%)
Truhaut - 15/52 6/52 6/52 12/52
(29%) (12%) (12%) (23%)
Newberne 21/52 15/52 4/52 6/52 10/52
(40%) (29%) (8%) (12%) (19%)
* Hepatic Lesions: FCA = Focus of Cellular Alteration;
FNH = Focus of Nodular Hyperplasia
HA = Hepatocellular Adenoma
HCA = Hepatocellular Carcinoma
As shown above, the findings of the independent pathologists are
in good agreement, all showing a clear increase in high-dose (1%)
female rats with hepatocellular adenoma and/or carcinoma. A similar
increase was not observed in male rats at any dose levels. The
differences observed in the incidence of rats with altered cellular
foci between the original study pathologist and the others, were
primarily due to the fact that the original study pathologist had
diagnosed any vacuolation or clearing of hepatocytes observed as
hepatocellular vacuolation rather than as foci of cellular alteration
(Moch, 1990).
An independent audit of this rat study report showed no
significant discrepancies (Munro & Brillinger, 1989a,b).
2.2.3.2 Mouse
In in the first eight weeks of a 24-week screening test, groups
of 20 female A/He mice received a total dose of 2, 4 or 12.0 g
anethole/kg b.w. in 24 3x/week i.p. injections. The higher dose had
previously been calculated to be the maximum tolerated dose. There
was no increase in the incidence of tumours of the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands.
Survival was reduced to approximately 70% (Stoner et al., 1973). It
should be noted that safrole, which has been shown to be carcinogenic
in other studies, was negative using this protocol, thus there is some
doubt about its validity.
A series of experiments was performed to investigate the
carcinogenic potential of trans-anethole in mice. Groups of male
(56-76 per group) and female (55-61 per group) 4-day old mice were
exposed to trans-anethole by gavage at dose levels of 370 or 740
mg/kg b.w. twice weekly for 10 weeks, then sacrificed at 11-14 months.
In a second experiment, 53 male mice received trans-anethole by
intraperitoneal injection on days 1, 8, 15 and 22 after birth. The
total dose of anethole that each animal received during 4 treatments
was 703 or 1399 mg. Animals from the lower dose group were sacrificed
at 13-18 months and those from the 1390 mg group at 12 months. In a
third experiment, a group of female mice, approximately 8 weeks old,
average body weight 21 g, were fed anethole in the diet at a dose
level of 690 mg/kg for a period of 12 months. After 12 months the
animals received basal diet only and were sacrificed at 18 months.
The fourth experimental group of 17 female mice (8 weeks old) was
given an injection of anethole twice weekly for 12 weeks at a dose
level of 148 mg/kg b.w. The mice were killed 8 months after the first
injection. All animals from each experiment were necropsied and the
various tissues examined histologically. Trans-anethole produced no
different results from controls (Miller et al., 1983).
2.2.4 Special studies on mutagenicity
Two strains of Salmonella typhimurium (TA100 and TA98), with
and without microsomal activation, were used in a plate test to study
the effect of trans-anethole (isolated natural compound), anise oil
(90% trans-anethole) and fennel oil (70% trans-anethole). Each
test material was assayed over the dose range from zero (control) to
the level of cell toxicity. All test materials increased mutagenic
activities with TA100 tester strain with implementation of microsomal
activation (S13) system. Peak mutagenic activity, 4 and 4.4 times
that of the background rate, occurred with 2 mg anise oil/plate and
2.5 mg fennel oil/plate respectively. Isolated natural trans-
anethole was also mutagenic (the dose level and rate of mutagenicity
were not stated). The compound was considered not to be mutagenic
unless it was capable of inducing a mutation (reversion) rate at least
3 times that of the incident background (Marcus & Liechtenstein,
1982).
The mutagenic activities of anethole and its metabolite 3'-
hydroxyanethole were studied using three tester strains of Salmonella
typhimurium (TA1535, TA100 and TA98). Addition of an NADPH-
generating system and liver microsomes and cytosol (S13 fraction) from
Aroclor-treated rats (6.8 mg liver protein/plate) to the incubation
mixture of TA100 tester strain increased mutagenic activities.
Approximately 45 revertants were obtained per µmole anethole. Under
the same conditions, 3'-hydroxyanethole showed no significant
mutagenic activity with less than 7 µmoles/plate. Above this
concentration the S13-mediated mutagenicity increased linearly with
increased doses up to 15 µmoles/plate (about 1000 revertants with 15
µmoles/plate) (Swanson et al., 1979).
Five strains of Salmonella typhimurium (TA1535, TA100, TA1537,
TA1538, TA98), with and without metabolic activation (S9 mix), were
used to study potential mutagenic effects of trans-anethole. The
lowest overtly toxic concentration for trans-anethole was 1
mg/plate. No mutagenic activity was observed at concentrations of up
to 50 µg trans-anethole/plate with or without metabolic activation.
However, the addition of 3'-phosphoadenosine-5'phosphosulphate (PAPS)
to the microsomal assay markedly increased the mutagenicity of trans-
anethole in TA1535 tester strain. The mutation rate observed was
approximately 4, 5, 10, 11, 9 and 3 times that of the background rate
at trans-anethole concentrations of 0.05, 0.20, 1.0, 5.0, 15.0, and
50.0 ug/plate respectively (To et al., 1982).
A further study indicated that trans-anethole showed mutagenic
activity in the Ames S. typhimurium assay but was inactive in a B.
subtilis Rec assay and was negative in an E. coli uvr A reversion
test (Sekizawa & Shibamoto, 1982).
Trans-anethole was inactive in a mouse micronucleus assay 24,
48 and 72 hr after administration by gavage of the second of two daily
doses of 2 ml/kg b.w. to groups of 10 adult males. At this dose level
there was significant mortality (3-4 animals per group) (Siou et al.,
1984). Negative results were also obtained in a mouse micronucleus
assay after i.p. administration to groups of 5 male and 5 female mice
in two doses of 0.25 or 0.5 g/kg b.w., 30 and 6 hours before sacrifice
(Marzin, 1979).
Genotoxicity of trans-anethole was tested, along with some
structurally related compounds (safrole, isosafrole, eugenol,
estragole, allylbenzene, methyleugenol, and p-propylanisole), for its
capability of inducing unscheduled DNA synthesis (UDS) in freshly
isolated rat hepatocytes in primary culture. Hepatocytes were
isolated from male Fischer rats following liver perfusion. After
overnight incubation of the hepatocytes in the presence of the test
chemical, the UDS or DNA repair was measured by determining the amount
of 3H-thymidine incorporated into hepatocyte nuclear DNA during the
repair process. Under the test conditions, trans-anethole did not
induce UDS over the concentration range of 10-6 and 10-2 M, although
cytotoxicity, as measured by LDH leakage, was observed at
concentrations of 10-3 M and above. Of the remaining chemicals
tested, allybenzene, p-propylanisole, isosafrole and eugenol were also
negative in the UDS assay, whereas safrole, methyleugenol and
estragole as well as the positive control, 2-acetylaminofluorene, all
induced UDS in a dose-related manner.
Based on the study results, the authors concluded: "there was an
excellent correlation between UDS induction and known rodent hepato-
carcinogenicity, with safrole, estragole and methyleugenol all
inducing UDS. Anethole, isosafrole, eugenol and allylbenzene, for
which evidence of carcinogenicity is equivocal or negative, did not
induce UDS" (Howes et al., 1990).
In another test, trans-anethole was tested for its ability to
induce UDS in cultured primary hepatocytes isolated from Sprague-
Dawley derived CD rats of both sexes. At concentrations up to 10-2,
the compound did not induce UDS, as measured by 3H-thymidine
incorporation into nuclear DNA. The positive control, 2-
acetylaminofluorene, gave positive response. At concentrations of
10-3 and above, trans-anethole was cytotoxic and the UDS response
was below the control values. The cytotoxicity of trans-anethole
was consistent with the increased leakage of cellular LDH into the
culture medium.
To investigate the possible involvement of a reactive epoxide
intermediate in the observed cytotoxicity, the effect of trans-
anethole on intracellular glutathione levels was studied. The results
showed that anethole caused a dose-related depletion of glutathione in
freshly isolated hepatocytes in suspension. However, when hepatocytes
were pretreated with the non-cytotoxic glutathione depleting agent
dimethyl maleate, there was no enhancement of anethole cytotoxicity,
suggesting that the putative epoxide intermediate may not be of any
toxicological significance (Caldwell & Marshall, 1990).
2.2.5 Special studies on pharmacological effects
The pharmacologic effects of trans-anethole most often noted
are reduction in motor activity, lowering of body temperature and
hypnotic, analgesic and anticonvulsant effects. By either the oral or
i.p. route, administration of more than 10% of the LD50 by that route
appears necessary for significant effects (Boissier et al., 1961;
Seto, 1969; Gruebner, 1972; Le Bourhis & Soene, 1973).
3. COMMENTS
The Committee considered the results of three independent reviews
of the liver histology in the long-term study in rats. It concluded
that there is a clear increase in the incidences of hepatocellular
adenomas and of hepatocellular carcinomas in female rats at 10 mg/kg
in the diet but not at lower doses. In male animals a slight increase
in the incidence of hepatocellular adenomas but not carcinomas was
seen at 10 mg/kg in the diet only. There is also evidence of an
increase in the incidence of non-neoplastic proliferative lesions in
the liver at all dose levels in both sexes.
A review of the report of the long-term rat study showed no
significant discrepancies. The Committee also noted that differences
in survival between treated and control animals could not account for
the increased incidence of liver tumours seen in the study.
The results of two new studies on genotoxicity (unscheduled DNA
synthesis) were negative.
The Committee concluded that insufficient data were available to
permit a final evaluation of the significance of the malignant liver
tumours observed in female rats with respect to the use of trans-
anethole as a food additive. Further metabolic and especially
pharmacokinetic studies in mice, rats, and humans are required for
evaluation in 1992. In addition, a long-term dietary study in mice
may be needed, although the design of such a study will depend upon
the results of the metabolic and pharmacokinetic studies. In view of
the positive results that were obtained in in vitro bacterial gene
mutation tests, the Committee also concluded that chromosome
aberration studies and in vitro tests for gene mutation in mammalian
cells were desirable.
4. EVALUATION
The temporary ADI was extended until 1992, but reduced to 0-0.6
mg per kg of body weight on the basis of the minimal effect level of
2.5 mg/kg in the diet (equivalent to a dose of 125 mg per kg of body
weight per day) for non-neoplastic proliferative changes in the liver
of rats, adjusted using a safety factor of 200.
The need for a reproduction/teratogenicity study will be
considered by the Committee when further relevant data from the above
studies have been reviewed. The Committee also reiterated its
recommendation that an epidemiological study of the effects of
consuming high dietary levels of trans-anethole would be desirable.
5. REFERENCES
AXELROD, J. (1956). Enzymic cleavage of aromatic ethers. Biochem.
J., 63, 634-639.
BOISSIER, J.R., SIMON, P. & LE BOURHIS, B. (1967). Experimental
psychotropic effect of isomeric cis- and trans-anetholes.
Therapie, 22, 309-323.
CALDWELL, J. SUTTON, J.D. (1988). Influence of dose size upon the
disposition of trans-[methoxy-14C]-anethole in human volunteers.
Fd. Chem. Toxicol., (In press).
CALDWELL, J. & MARSHALL, A.D. (1990). The cytotoxicity and
genotoxicity of trans-anethole in isolated rat hepatocytes.
Progress Report submitted to WHO by Anethole Task Force, FEMA,
Washington, D.C. U.S.A., March, 1990.
CAUJOLLE, F. & MEYNIER, D. (1958). Toxicity of tarragon oil and the
anetholes ( cis- and trans-), Acad. Sci. (Paris), 246, 1465-1468.
FEMA (1989). Metabolic and mechanistic studies intended to show that
the low incidence of hepatocellular carcinoma induced in rats by
trans-anethole does not indicate a hazard to humans. Anethole
Progress Report submitted to WHO 11/13/89.
FRITSCH, P., DE SAINT-BLANUAT, G. & DERACHE, R. (1975).
Gastrointestinal absorption, in the rat, of anisole, trans-anethole,
butylhydroxyanisole and safrole. Fd. Cosmet. Toxicol., 13, 359-364.
GRUEBNER, I., KLINGER, W. & ANERMANN, H. (1972). Various substances
and substance classes with inducer properties. II. Arch. Int.
Pharmacodyn. Ther., 196, 288-297.
HOWES, A.J., CHAN, V.S.W. & CALDWELL, J. (1990). Structure-
specificity of the genotoxicity of some naturally occurring
alkenylbenzenes determined by the unscheduled DNA synthesis assay in
rat hepatocytes. Prepublication submitted to Food Chem. Toxicol.
JENNER, P.M., HAGAN, E.C., TAYLOR, J.M., COOK, E.L., & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I.
Acute and oral toxicity. Fd. Cosmet. Toxicol., 2, 327-343.
LE BOURHIS, B. (1968). The metabolism of trans-anethole. Ann.
Biol. Clin., 26, 711-715.
LE BOURHIS, B. (1970). Identification of a few metabolites of trans-
anethole in man, the rabbit and the rat. Ann. Pharm. Fr., 28, 355-
361.
LE BOURHIS, B. (1973). Biological properties of trans-anethole. An
attempt to determine an acceptable daily dosage. Parfums Cosmet.
Savons. Fr., 3, 450-456.
LE BOURHIS, B. & SOENE, A.M. (1973). Studies on the psychotropic
action of some aromatic compounds used in food. Fd. Cosmet.
Toxicol., 11, 1-9.
LEVENSTEIN, I. (1960). Unpublished report of Leberco Laboratories,
Submitted to WHO.
MARCUS, C. & LIECHTENSTEIN, E.P. (1982). Interactions of naturally
occurring food plant components with insecticides and pentobarbital in
rats and mice. J. Agric. Food Chem., 30, 563-568.
MARONPOT, R.R., MONTGOMERY, C.A., BOORMAN, G.A. & MCCONNELL, E.
(1986). National Toxicology Program nomenclature for
hepatoproliferative lesions of rats. Toxicol. Pathol., 14, 263-273.
MARZIN, D. (1979). Recherche d'une action mutagene par le test du
micronucleus chez la souris. Unpublished report of DREBS. Submitted
to WHO by Pernod Ricard.
MILLER, E.C., SWANSON, A.B., PHILLIPS, D.H., FLETCHER, T.L., LIEM, A.
& MILLER, J.A. (1983). Structure-activity studies of the
carcinogenicities in the mouse and rat of some naturally occurring and
synthetic alkenylbenzene derivatives related to safrole and estragole.
Cancer Res., 43, 1124-1134.
MOCH, R.W. (1990). Trans-anethole. Histopathologic review of
microslides containing liver proliferative lesions from a long-term
study in Sprague-Dawley rats. Pathology Report submitted to WHO
April, 1990.
MUNRO, I.C. & BRILLINGER, R. (1989a). Preliminary audit of the
procedures and findings of a 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
MUNRO, I.C. & BRILLINGER, R. (1989b). Addendum to the preliminary and
detailed audit reports of the 117-week study of trans-anethole in
rats. Unpublished report No. 0388018. Submitted to WHO by CanTox
Inc. Oakville, Ontario, Canada.
NEWBERNE, P.M., BROWN, W.R. & CARLTON, W.W. (1987). Histopathologic
evaluation of proliferative lesions in livers of rats fed trans-
anethole for 117-121 weeks (Long-term feeding study in rats, study no.
3190 TCR) Unpublished report. Submitted to WHO by Pernod Ricard.
NEWBERNE, P.M., CARLTON, W.W. & BROWN, W.R. (1989). Histopathological
evaluation of proliferative lesions in rats fed trans-anethole in
chronic studies. Fd. Chem. Toxic., 27, 21-26.
SANGSTER, S.A. (1983). The metabolism and disposition of trans-
anethole and p-propenylanisole in rodent species and man. Ph.D.
Thesis, University of London.
SANGSTER, S.A., CALDWELL, J., SMITH, R.L. & FARMER, P.B. (1984a).
Metabolism of anethole I. Pathways of metabolism in the rat and mouse.
Fd. Chem. Toxicol., 22, 695-706.
SANGSTER, S.A., CALDWELL, J. & SMITH, R.L. (1984b). Metabolism of
anethole II. Influence of dose size on the route of metabolism of
trans-anethole in the rat and mouse. Fd. Chem. Toxicol., 22,
707-713.
SANGSTER, S.A., CALDWELL, J., HUTT, A.J. ANTHONY, A. & SMITH, R.L.
(1987). The metabolic disposition of [methoxy-14C]-labelled trans-
anethole, estragole and p-propylanisole in human volunteers.
Xenobiotica, 17, 1223-1232.
SEKIZAWA, J. & SHIBAMOTO, T. (1982). Genotoxicity of safrole-related
chemicals in microbial test systems. Mutat. Res., 101, 127-140.
SETO, T.A. (1969). Effects of alkylmethoxybenzene and alkyl-
methylenedioxybenzene essential oils on pentobarbital and ethanol
sleeping time. Arch. Int. Pharmacodyn. Ther., 180, 232-240.
SHELANSKI, M.V. (1958). Unpublished report of Industrial Biology
Research and Testing Laboratories. Submitted to WHO.
SIOU, G., LEROND-CONAN, L., EL HAITEM, M. & LACRAMPE, M. (1984).
Recherche d'une eventuelle potentialite genotoxique du p-methoxy-
propenylbenzene par la technique du micronucleus chez la souris.
Unpublished report of C.E.R.T.I. Submitted to WHO by Pernod Ricard.
SOLHEIM, E. & SCHELIEN, R.R. (1973). Metabolism of alkenebenzene
derivatives in the rat I. rho-methoxylallyl benzene (estragole) and
rho-methoxypropenylbenzene (anethole). Xenobiotica, 3, 493-510.
STONER, G.D., SHIMKIN, M.B., KNIAZEIF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G.B. (1973). Test for carcinogenicity of
food additives and chemotherapeutic agents by the pulmonary tumor
response in strain A mice. Cancer Res., 33, 3069-3085.
STROLIN-BENEDETTI, M. & LE BOURHIS, B. (1972). Body distribution and
excretion of trans-anethole-14C. C.R. Acad. Sci. Ser. D., 274,
2378-2381.
SWANSON, A.B., CHAMBLISS, D.D., BLOMQUIST, J.C., MILLER, E.C., &
MILLER, J.A. (1979). The mutagenicities of safrole, estragole,
eugenol, trans-anethole and some of their known or possible
metabolites for Salmonella typhimurium mutants. Mutat. Res., 60,
143-153.
SWANSON, A.B., MILLER, E.C. & MILLER, J.A. (1981). The side chain
epoxidation and hydroxylation of the hepatocarcinogens safrole and
estragole and some related compounds by rat and mouse liver
microsomes. Bichim. Biophys. Acta., 673, 504-516.
TAYLOR, J.M., JENNER, P.M. & JONES, W.I. (1964). A comparison of the
toxicity of some allyl, propenyl and propyl compounds in the rat.
Toxicol. Appl. Pharmacol., 2, 378-387.
TO, L.P., HUNT, T.P. & ANDERSEN, M.E. (1982). Mutagenicity of trans-
anethole, estragole and safrole in the Ames Salmonella typhimurium
assay. Bull. Environ. Contam. Toxicol., 28, 647-654.
TRUHAUT, R., NEWMAN, J., LE BOURNIS, B., GLOMOT, R. & ATTIA, M.
(1988). A long term feeding study of the toxicity and carcinogenicity
of trans-anethole in rats. Fd. Chem. Toxicol., (in press).
TRUHAUT, R., LE BOURHIS, B., ATTIA, M. GLOMOT, R., NEWMAN, J. &
CALDWELL, J. (1989). Chronic toxicity/carcinogenicity study of
trans-anethole in rats. Fd. Chem. Toxic., 27, 11-20.
VIGNOLI, L., MOREL, M.C. & SIMON, O. (1965). Toxicite chronique du
trans-anethole et des liqueurs anisees a base d'anethole et de
regise. Rev. Path. Comp., 2, 281-287.
