
ANTHELMINTHIC AGENTS
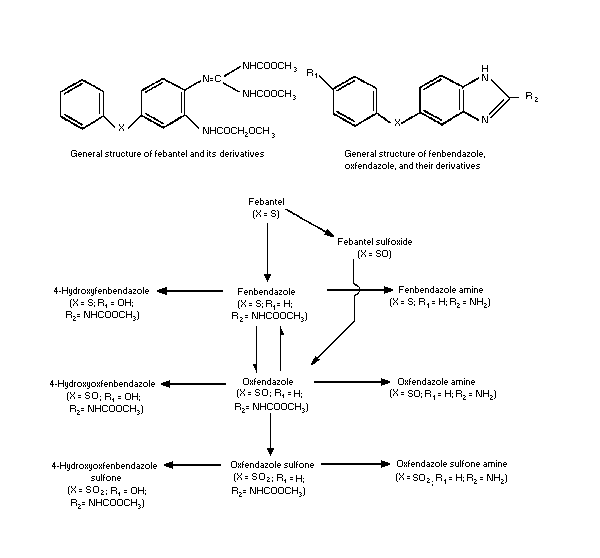
The Commitee considered the three anthelminthic agents,
fenbendazole, oxfendazole and febantel. Of these, the first two are
benzimadozoles that are metabolically inerconvertible in vivo.
Febantel is a prodrug that can be converted in vivo by cyclization
to fenbendazole or following oxidation at the sulfur atom and
subsequent cyclization to oxfendazole( see figure below).
The Committee has written separate monographs for each of these
agents. These monographs are followed by a summary which discusses
them together.
 FEBANTEL
First Draft Prepared by
Dr. K.N. Woodward
Veterinary Medicines Directorate
Weybridge, Surrey, England
1. EXPLANATION
Febantel (N-{-[2,3-bis-(methoxycarbonyl)-guanido]-5-
(phenylthio)-phenyl}-2-methoxyacetamide) is an anthelmintic agent
active against a range of gastrointestinal parasites in animals. It
is a prodrug and in vivo undergoes hydrolytic removal of a
methoxyacetyl group and cyclisation to yield the benzimidazole,
fenbendazole (Wollweber et al., 1978; Delatour & Parish, 1986). It
has not previously been evaluated by the Joint FAO/WHO Expert
Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
Following intraduodenal dosing of rats with 14C-labelled
febantel, around 25-30% of the dose was excreted in the urine,
suggesting moderate absorption after oral administration. After
intravenous and intraduodenal dosing around 70% of the dose was
excreted in the bile indicating that initial absorption after oral
administration may be higher. Around 20% of the oral dose of
febantel was detected in the urine of sheep over a 4-day period
after dosing (Anon. 1987). In the cow, metabolites of febantel were
excreted in the milk after oral doses (Delatour et al., 1983a). The
liver and to a lesser extent the kidney were target tissues for
febantel-related metabolites in the rat, sheep and cattle (Bayer,
1987).
2.1.2 Biotransformation
In vivo, febantel undergoes cyclization to fenbendazole which
is interconvertible with oxfendazole. In addition, oxidation at the
sulfur atom in febantel yields the sulfoxide which can undergo
hydrolytic cleavage and cyclisation to produce oxfendazole. A total
of 9 metabolites has been identified in the rat. Sheep were given
25 mg/kg b.w. and the urine collected. A similar metabolic pattern
was noted in the two species, and oxfendazole and fenbendazole were
identified along with the other metabolites including the p-hydroxy
derivative of fenbendazole (Anon. 1987; Delatour et al., 1983b;
Delatour et al., 1982a). Similar findings were made in cattle (Anon.
1987). Fenbendazole and oxfendazole were identified in cow's milk
after oral dosing (Delatour et al., 1983a). It has been shown that
liver microsomes from various species can convert febantel to its
sulfoxide; sheep microsomes displayed the highest activity in this
respect. Similarly, microsomes from a number of species converted
fenbendazole to oxfendazole. The experiments together suggest that
the liver is responsible for the oxidative metabolism of both
febantel and fenbendazole (Montesissa et al., 1989).
A general metabolism scheme showing the metabolism of all 3
benzimidazole compounds considered by the Committee may be found on
page 1(note: to check).
2.2 Toxicological studies
2.2.1 Acute toxicity
Febantel was of low toxicity in the mouse, rat, and dog after
oral administration. It was somewhat more toxic to the rabbit by
this route (Table 1). It was of low acute toxicity to the rat
following inhalation exposure, and to the rabbit after dermal
exposure.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral > 10000 Muermann, 1975
Mouse M s.c. > 10000
Rat M oral 10605
Rat M&F inh. > 20001 Nelson et al., 1977
Rabbit F oral ca. 1250 Muermann, 1975
Rabbit M&F dermal > 20002 Nelson & Burke, 1977
Dog M&F oral > 10000 Muermann, 1975
1. 1 h exposure, µg/1
2. 24 h occlusive dressing
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 15 male and 15 female Wistar rats were given 0, 20,
50, and 125 mg/kg b.w./day febantel, 7 days per week by stomach
tube, for 3 months. Body weights and food consumption were measured
once a week, and clinical laboratory studies were conducted after 1
and 3 months.
No clinical signs were noted during the study and behaviour and
feed consumption were unaffected by febantel administration. Body
weights in all the groups were comparable with control values except
for males given 20 mg/kg b.w./day, which showed a higher rate of
weight gain. No compound-related deaths occurred during the study.
Haematology was normal at 1 and 3 months, as was clinical
biochemistry. There were no adverse findings following urinalyses.
At necropsy, no gross effects were noted. Relative and absolute
liver weights were increased in females given the highest dose;
relative liver weights were increased in corresponding males.
High-dose females had reduced thymus weights when compared with
controls. The only drug-related finding on histopathological
examination was fatty infiltration of the liver in several high-dose
rats. The no-effect level in this study was 50 mg/kg b.w./day
(Bomhard and Luckhaus, 1976).
2.2.2.2 Dogs
Groups of 3 male and 3 female pure-bred beagles were given
doses of 0, 20, 60, and 180 mg/kg b.w./day febantel in a gelatin
capsule 7 days per week for 13 weeks. Body weights were measured
weekly and body temperatures before the start of the study and at
weeks 3, 6, and 13. Neurological, haematological and ophthalmoscopic
examinations and electrocardiographic (ECG) investigations were
conducted at weeks 3, 6, and 13. Urinalyses were conducted at the
start of the study and at weeks 3, 6, and 13.
No signs of toxicity occurred in dogs given 20 and 60 mg/kg
b.w./day. Food consumption was reduced in some dogs given 180 mg/kg
b.w./day, and occasional diarrhoea and ataxia occurred. One animal
given this dose was killed on day 26 in a moribund state. This
animal was emaciated and had bleeding gums. It became ataxic and was
therefore sacrificed. Animals given 20 and 60 mg/kg b.w./day had
rates of body weight gain comparable with controls but in those
given the high dose, weight gain was depressed during the first 3
weeks. Temperatures in all dogs were comparable with those in
controls and no adverse effects were noted on ophthalmoscopic and
neurological examinations. ECGs were normal and no occult blood was
found on faecal examinations.
Haematologic examination showed no compound-related effects on
red blood cells in dogs given 20 mg/kg b.w./day but those given the
higher doses showed reductions in haematocrit, haemoglobin and
erythrocyte counts. Thrombocyte counts were unaffected. Leukopenia
was seen in 3/6, 1/6, and 6/6 dogs given 20, 60, and 180 mg/kg
b.w./day febantel. Differential blood counts revealed
granulocytopenia. There were no effects on clinical chemistry or
urinalysis values.
At necropsy, there were no apparent gross effects on dogs given
20 or 60 mg/kg b.w./day febantel but dogs given 180 mg/kg b.w./day
had red markings in the mucosa of the stomach and small intestine
with reddening of the mesenteric lymph nodes. There was atrophy of
the white splenic pulp in one animal. Testicular weights were
reduced at all 3 dose levels.
Histopathological examination revealed atrophy of the splenic
follicles in dogs given the highest dose and in one animal given
60 mg/kg b.w./day. Testicular hypoplasia was evident in all treated
groups but this was more pronounced in high-dose animals. No
compound-related changes occurred in the epithelia of the stomach,
oesophagus, or small intestine; there were no effects on mitoses in
the crypts (Machemer & Luckhaus, 1976).
As an extension of the study, groups of 3 male and 3 female
beagle dogs were given doses of 0, 5, and 10 mg/kg b.w./day febantel
in capsules. There were no signs of toxicity noted and haematology
was unaffected by compound administration. There was no excess
incidence of splenic or testicular effects in the treated dogs. The
no-effect level in this study was 10 mg/kg b.w./day (Machemer &
Luckhaus, 1976).
In a 52-week study, groups of 4 male and 4 female beagle dogs
were given diets containing 0, 40, 200, and 1000 ppm febantel
(equivalent to 0, 0.1, 5, and 25 mg/kg b.w./day). In this study,
body weights were recorded weekly, reflexes (corneal, pupillary,
patellar, extensor and reflexor) before the start of the study and
at weeks 4, 7, 13, 25, 39, and 52, and ophthalmoscopy at weeks 4, 7,
13, 25, 39, and 52. Haematology, biochemistry and urinalysis were
also conducted at these time points. Body temperature and pulse
rates were also measured during the study.
Animals given up to 200 ppm dietary febantel exhibited no signs
of toxicity, and there were no effects on body temperature or pulse
rate in any of the treated groups. Animals given the highest dietary
level showed a much reduced feed intake (approximately 75% of
control values). Body weights of males were significantly reduced
and female dogs showed a reduction in the rate of weight gain. One
dog in the high dietary level group showed a marked reduction in
body weight (3.5 kg at week 39) and was found to have opacification
of the lenses.
Three of the 8 dogs given the highest dietary level died during
the course of the study (one accidentally); there were no deaths in
any of the other groups. There were no haematological effects in the
animals given up to 200 ppm febantel. However, in high-level
animals, particularly in the three which died, there were reductions
in haematocrit, haemoglobin and erythrocyte values. The thrombocyte
count was markedly lowered in one of these animals. There was also a
marked reduction in leucocyte counts at the 1000 ppm level. This was
characterized by a significant reduction in granulocytes.
Biochemical studies revealed significant increases in the
activities of hepatic enzymes indicative of some degree of liver
damage, at the highest dietary level. There were no effects on
urinalyses.
At necropsy, there were significant reductions in testicular
and prostate weights in high-level males. Histological examination
revealed drastic reductions in leukopoiesis and erythropoiesis in
the marrow of high-level animals. Lymphofollicular atrophy of the
lymph nodes (including the spleen) occurred in these dogs.
Myocardial necrosis occurred in one high dietary level dog and this
was considered by the authors to be secondary to hypoxemia due to
the effects on blood elements. Centrilobular and panlobular
degeneration of the liver were noted in dogs given 1000 ppm.
Testicular and prostatic hypoplasia were evident in high-level
males. The no-effect level in this 52-week study in the dog was
200 ppm in the diet, equivalent to 5 mg/kg b.w./day (Hoffmann &
Nash, 1983).
2.2.3 Long term/carcinogencity studies
2.2.3.1 Mice
In a combined chronic toxicity and carcinogenicity study,
groups of 60 male and 60 female NMRI mice were given diets
containing 0, 50, 200, and 800 ppm febantel, equal to 0, 10, 42, and
170 mg/kg b.w./day for males and 0, 15, 58, and 250 mg/kg b.w./day
for females, for up to 21 months. From these groups 10 male and 10
female mice were sacrificed after 1 year.
There were no effects on mortality attributable to febantel
intake, nor were there any effects on appearance or behaviour. Feed
and water intake values in treated mice were comparable with
controls. Body weights of treated males were similar to controls but
in females body weights of mice given the highest dietary level were
slightly but significantly lower than control values in the first 11
months of the study. After 11 months, the body weights of the
high-level animals became comparable with those of controls.
Haematological studies revealed no effects in mice given up to
200 ppm febantel in the diet. However, males given the highest
dietary level had significant increases in the platelet count and
significant decreases in mean corpuscular haemoglobin contents.
Females were found to have lower haematocrit values and, at the end
of the study, reductions in erythrocyte counts. Blood biochemistry
was investigated at 12 and 21 months. The activity of serum
aspartate aminotransferase was significantly decreased in all the
treated males and decreased in females given the low and high levels
of dietary febantel; the intermediate-level mice however had
increased activity of this enzyme. The significance of this is
unknown although the authors of the report believed it to be
toxicologically irrelevant. Alkaline phosphatase, serum alanine
aminotransferase, creatine kinase, total protein, bilirubin, urea,
cholesterol and glucose were unaffected. Serum creatinine was
lowered in all treated mice.
There were no gross abnormalities which could be attributed to
compound intake in mice which died during the study, in those killed
in a moribund condition, in the groups killed at 12 months nor in
those sacrificed at termination.
The only effects on organ weights were those noted in the liver
in animals given the highest dietary level. There were significant
increases in absolute and relative liver weights at 12 and 21 months
in these animals.
At histopathological examination, the only adverse finding was
an increased fat accumulation in the livers of high-level female
animals. There was no increase in the incidence of any benign or
malignant tumour type (Bomhard & Kaliner, 1985).
2.2.3.2 Rats
In a combined chronic toxicity and carcinogenicity study,
groups of 100 male and 100 female Wistar rats were fed diets
containing 0, 20, 100, and 500 ppm febantel, equal to 0, 2, 8, and
40 mg/kg b.w./day, for an eleven-week period prior to mating, during
a 20-day mating period, and during the subsequent lactation period
(up to 33 days). A total of 60 male and 60 female pups from each
dose group was then given the same dietary level as the respective
parental animals for up to 30 months. Groups of 10 male and 10
female rats from each group were sacrificed after 65 weeks.
There were no effects on appearance, behaviour, mortality, or
food and water intake, and no effects on body weight at levels of up
to 100 ppm febantel. However, the highest dietary level depressed
body weights in parental animals and in their young. Litter size was
reduced significantly at birth, 7 days, and at week 3 in animals
given the highest level. Levels of up to and including 100 ppm had
no effects on pup weight but pups in the 500 ppm level group had
significant reductions in weight when compared with controls at
birth, 7 days and week 3. Body weights of these animals remained
reduced throughout the course of the study. There were no effects on
mortality in any of the treated groups.
No haematological abnormalities were noted at week 27, 53, 106,
or 132 in animals given up to 100 ppm febantel. In animals given the
highest level, mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentrations were reduced, sometimes significantly, at
all 4 examination points, in male and female rats. There were no
cytological differences, however, between control and treated
animals.
Clinical chemistry revealed significant increases in alkaline
phosphatase activities in males and females given the highest level
of febantel.
Gross examination of rats which died during the study, in those
subject to interim sacrifice at week 65 and in animals killed at
termination revealed no abnormalities. There were significant
increases in liver weights, both absolute and relative, at
termination in males and females given the highest dietary level,
and in relative liver weights at week 65 in males and females.
Histopathological examination of rats sacrificed at week 65
revealed fatty vacuolation of the liver in animals given the highest
dietary level as the only non-neoplastic finding. Similar
observations were made at terminal sacrifice. There was no increased
incidence of any tumour type in any of the treatment groups.
However, only animals given the highest level and controls were
subject to full histopathology; rats given the other doses had only
the lungs, liver and kidneys examined histologically. The NOEL for
non-neoplastic effects was 100 ppm, equal to 8 mg/kg b.w./day
(Bomhard & Mager, 1987).
2.2.4 Reproduction Studies
2.2.4.1 Rats
A two-generation study was conducted in accordance with OECD
Guidelines (Guideline 416). Groups of 25 male and 25 female rats
were given dietary febantel at levels of 0, 20, 100, and 500 ppm,
equivalent to 0, 2, 10, and 50 mg/kg b.w./day. The F0 generation
animals were fed the diets for 100 days prior to mating. Rats were
fed febantel-containing diets throughout the study period, including
the period of gestation and rearing of the young.
The F0 animals were mated to produce F1a and F1b
generations. The F1b rats were mated to produce F2a and F2b
generations. All the F0 and F1b females were necropsied 1-3
weeks after weaning. All the F1b young not required for mating and
all the F2b young were also necropsied when approximately 4 weeks
old.
Treatment of the F0 animals had no effects on appearance,
behaviour or coat quality at any of the dietary levels used. Females
in all treatment groups gained weight at the same rate as controls,
as did males given up to 100 ppm. However, high-level F0 males
were lighter at all times than corresponding controls. There were no
effects on food intake. In the F1a and F1b generations there
were no effects on food intake. In the F1a and F1b generations
there were no effects on insemination index, fertility index,
gestation index, gestation period or on the numbers of successfully
mated females. The male:female ratio was unaffected, and the total
number of young at each dietary level was similar. Litter size was
slightly reduced in both F1 generations at the highest dietary
level. The viability of young at the highest level was also reduced
in both F1 generations. When pup weights were measured at birth
and four weeks after birth, these were lower in young from the
highest dose level and also in the 100 ppm group 4 weeks after
birth. There was no excess incidence of malformations in any of the
treated groups when compared with controls.
In the F1b generation, the only effects noted were reductions
in body weights in males given the highest level of febantel in the
diet, and reductions in body weights in females given 100 and 500
ppm. There were no effects on feed consumption. The insemination
index, fertility index, gestation index, gestation period and the
numbers of successfully mated females were unaffected by compound
treatment. The numbers of young at birth were slightly but
significantly reduced in the F2a and F2b generations after 100
and 500 ppm but the male:female ratios were unaffected at any of the
dietary levels. The number of young surviving lactation was slightly
reduced at 500 ppm in the F2b generation.
Mean body weights were significantly reduced in both F2
generations at the highest dietary level. The incidence of
malformations was not increased at any treatment level.
Necropsy of F0 animals revealed pale livers at 100 and 500
ppm. All F1b and F2b animals sacrificed at 4 weeks of age were
necropsied. Discoloured livers were noted at 100 and 500 ppm. At
histopathological examination, hepatocellular hypertrophy was noted
in the livers of F0 rats given 100 and 500 ppm febantel. Fatty
degeneration of the liver occurred in F1b rats at 500 ppm.
Glycogen deposition was seen in the livers of F2b animals given
100 and 500 ppm. There were no effects on organ weights of F1b
animals (liver, kidney, testes and ovaries) given up to 100 ppm
febantel but animals given 500 ppm had decreased absolute kidney
weights and increased relative liver weights.
This study therefore revealed no teratogenic effects at levels
of up to 500 ppm dietary febantel. However, parental animals given
the highest dose displayed lowered body weights. Litter size and
viability of young were reduced at the highest level in the F1
generation. Similar effects were noted in the F2 generation at the
high dietary level. Mean body weights were significantly reduced at
the highest level. The no-effect level for reproductive performance
was 100 ppm, but taking into account hepatic hypertrophy and fat and
glycogen deposition, the no-effect level in this study was 20 ppm,
equivalent to 2 mg/kg b.w./day (Eiben, 1985).
2.2.5. Special studies on genotoxocity
Febantel was not mutagenic in the Ames test; it did not induce
DNA repair in a bacterial test and was negative in an in vivo
cytogenetics assay. It did not increase the incidence of
micronucleated polychromatic erythrocytes in the mouse (Table 2).
However, it did give positive results in the mouse dominant lethal
test.
Two metabolites of febantel, fenbendazole (see also
corresponding monograph) and
2-amino-5-phenylsulfinyl-2-benzimidazole (the 2-amino derivative of
fenbendazole) were also tested for genotoxic potential. The results
are given in Table 3. Oxfendazole has been tested and is shown at
2.2.5 in the oxfendazole monograph in this volume. Both compounds
were negative in the Ames test and in tests for DNA repair in
primary rat hepatocytes. However, fenbendazole produced a positive
response in the presence, but not in the absence, of rat liver S9.
Benzimidazoles are a group of compounds which bind to tubulin
and may possibly disrupt mitosis. Benzimidazole itself is positive
in the Ames test (Davidse, 1977; Seiler, 1978). However, febantel as
a pro-benzimidazole is unlikely to be typical of the group except
under circumstances where conversion to fenbendazole, oxfendazole
and their metabolites is possible (see corresponding monographs).
Table 2. Results of genotoxicity assays on febantel
Test system Test object Concentration Results Reference
In vitro
Ames test1 S.typhimurium 0-5000 Negative Hoorn et al.,
TA1535, µg/plate 1980
TA1537,
TA1538, TA98,
TA100
Ames test1 S.typhimurium 0-5000 Negative Mourot, 1990
TA97a, TA98, µg/plate
TA100, TA102
DNA E.coli 0-5000 Negative Hoorn et al.,
repair1 pol A+, pol A- µg/plate 1980
In vivo
Cytogenetics Chinese 1000 mg/kg Negative Machemer,
assay hamster b.w. twice at 1975a
(marrow and 24 h interval2
spermatogonia)
Micro-nucleus Mouse (NMRI) 500 and 1000 Negative Machemer,
test mg/kg b.w. 1975b
twice at 24 h
intervals3
Dominant Mouse (NMRI) 500 and 2000 Positive4 Machemer &
lethal mg/kg Dycka, 1976
b.w./day twice
at 24 h
intervals
1. Both with and without rat liver S-9 fraction.
2. Animals killed and marrow harvested 6 and 48 h after second dose.
3. Animals killed 6 h after second dose.
4. No effects with lower dose but higher dose significantly reduced
fertility at 2, 4, 5 and 6 weeks of mating.
Table 3. Results of genotoxicity studies on fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole
Test Test Object Concentration Results Reference
System
Fenbendazole
Ames test1 S.typhimurium 10000 µg/plate Negative Rabenold &
TA1535, Brusick,
TA1537 1982a
Forward Mouse up to 62.5 Weakly Cifone &
mutation1 lymphoma µg/ml Positive2 Myrh, 1983a
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Rabenold &
hepatocytes Bruskick,
1982b
2-Amino-5-phenylsulfinyl-2-benzimidazole
Ames test1 S.typhimurium 1-10000 Negative Rabenold &
TA1535, µg/plate Brusick,
TA1537, 1982b
TA1538,
TA98, TA100
Forward Mouse up to 300 Positive2 Cifone &
mutation1 lymphoma µg/ml Myhr, 1983b
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Myhr &
hepatocytes Brusick,
1982b
1. Both with and without rat liver S-9.
2. Positive in the presence, but not absence of metabolic activation.
2.2.6 Special studies on teratogenicity
2.2.6.1 Rats
Groups of 20-24 pregnant FB30 (Long Evans) rats were given
febantel as an oral suspension in 0.5% aqueous tylose solution, by
stomach tube at 0, 10, 30, and 100 mg/kg b.w./day on days 6-15 of
gestation. Animals were killed on day 20 of gestation and the
uterine contents examined.
No signs of overt toxicity occurred at any dose level, but
animals given the highest dose gained less weight than those in
other groups including the control group. The number of fertilized
animals was similar in each group, but the number of pregnant
animals in the high dose group at termination was much lower (33%)
than in the other groups (all 100%), indicating severe
embryotoxicity. The numbers of implantations, fetuses and
resorptions was similar for animals given up to 30 mg/kg b.w./day
and controls. However, in those given 100 mg/kg b.w./day, the number
of implantation sites was similar to control values, but the number
of resorptions was markedly increased. Fetal and placental weights
were also significantly decreased at the high dose level.
No teratogenic effects were noted at doses of up to 30 mg/kg
b.w./day. At 100 mg/kg b.w./day, retardation of bone development and
teratogenic effects including anophthalmia, dysplastic
microphthalmia and multiple deformities of the ribs and spine were
noted in 4/25 fetuses examined. Gross examination of one maternal
animal that died during treatment at termination revealed severe
damage to the gastrointestinal tract at 100 mg/kg b.w./day
(Machemer, 1975c).
A study in the rat (unspecified strain) showed febantel to be
teratogenic when given at 46, 67, and 89 mg/kg b.w./day on days 8-15
of gestation. Its sulfoxide was also teratogenic in the rat at 46
and 93 mg/kg b.w./day. The no-effect level for febantel was 22 mg/kg
b.w./day (Delatour et al., 1982a).
Studies with febantel and its metabolites fenbendazole and
oxfendazole suggested that the active metabolite responsible for
embryotoxic effects of febantel was oxfendazole; when febantel was
given to rats simultaneously with SKF 525A, an inhibitor of
microsomal oxidation, the embryotoxicity was abolished (Delatour
et al., 1982a; Delatour et al., 1982b; Delatour, 1983; see also
accompanying monographs on fenbendazole and oxfendazole).
These studies show that febantel is embryotoxic and teratogenic
in the rat; the lowest no-effect level noted was 22 mg/kg b.w./day.
2.3 Observations in humans
No data were available.
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of acute and short-term studies
and of studies of carcinogenicity, genotoxicity, reproduction, and
development.
The main route of metabolism in rats, sheep, and cattle appears
to be cyclization to yield fenbendazole. Oxidation at the sulfur
atom can also occur to yield the sulfoxide which then undergoes
cyclization to give oxfendazole. Both fenbendazole and oxfendazole
can then undergo further metabolism.
In a study in rats in which febantel was given by gavage at
doses of up to 125 mg/kg b.w./day for 7 days, the only drug-related
effect was fatty infiltration of the liver. The NOEL was 50 mg/kg
b.w./day. Dogs were given febantel daily in gelatin capsules for 13
weeks at doses of up to 180 mg/kg b.w./day. Dogs given 60 and
180 mg/kg b.w./day showed testicular hypoplasia; and reductions in
haematocrit, haemoglobin, and erythrocyte counts. Leukopenia was
noted in all treated groups although the effect was not
dose-related. Agranulocytosis was noted in the high dose dogs, and
one high dose animal out of six also had splenic atrophy. In an
extension of the study using doses of up to 10 mg/kg b.w./day, no
haematological, splenic, or testicular effects were seen. The NOEL
was 10 mg/kg b.w./day. In a 52-week study in dogs given febantel at
levels of up to 1 g/kg in the diet (equivalent to 25 mg/kg
b.w./day), reductions in haemotocrit, haemoglobin, and erythrocyte
counts occurred at the highest dietary level. Testicular and
lymphofollicular (including splenic) atrophy also occurred at this
dietary level. The NOEL was 200 mg/kg in the diet, equivalent to
5 mg/kg b.w./day.
A carcinogenicity study was conducted in mice, which received
febantel in the diet at levels of up to 170 mg/kg b.w./day for males
and 250 mg/kg b.w./day for females for up to 21 months. There were
no changes in tumour incidence as compared with control values.
In a combined long-term toxicity and carcinogenicity study,
rats were given febantel in the diet at levels of up to 40 mg/kg
b.w./day during an 11-week period prior to mating, during a 20 day
mating period, and during lactation. A total of 60 male and 60
female pups from each dose group was then given the same dietary
level as the parents for up to 30 months. Females given the highest
dietary level showed reductions in mean corpuscular haemoglobin and
increases in absolute and relative liver weights. These animals had
fatty vacuolation of the liver at histopathological examination both
at week 65 after weaning and at the end of the study. There was no
increase in tumour incidence, but the Committee recognized that the
doses used may not have been sufficiently high for the carcinogenic
potential of this compound to have been adequately explored. The
NOEL for non-neoplastic effects was 8 mg/kg b.w./day.
In a two-generation study in which rats were given febantel in
the diet at up to 50 mg/kg b.w./day, litter size and viability of
the young were reduced at the highest dietary level in both
generations. The NOEL for reproductive performance was 10 mg/kg
b.w./day; however, if hepatic hypertrophy and glycogen deposition
were taken into account, the overall NOEL was 2 mg/kg b.w./day.
In a teratogenicity study in rats, severe maternal toxicity and
embryotoxicity occurred at the highest dose level. The NOEL was
22 mg/kg b.w./day. Teratogenicity was not seen in the absence of
maternal toxicity and embryotoxicity.
The genotoxic potential of febantel was investigated in a
number of test systems. Negative results were reported in two Ames
tests, in a test for DNA repair in bacteria, in an in vivo
cytogenetic test in the hamster, and in the mouse micronucleus test.
However, a positive result was found in the dominant lethal test in
the mouse. Two metabolites of febantel, fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole (the amino-derivative of
oxfendazole), also gave negative results in the Ames test and in a
test for DNA repair in primary rat hepatocytes, but both gave
positive results in the mouse lymphoma tk-locus genotoxicity assay
in the presence of metabolic activation, though not in its absence.
No studies of febantel in humans were available to the
Committee.
4. EVALUATION
The Committee noted that no carcinogenic effects were seen in
rats and mice; the significance of the positive results found with
the compound and its metabolites in a small number of tests for
genotoxic potential was therefore unknown. The major effects were
those noted in the 13- and 52-week studies in dogs, in the
teratogenicity studies in rats, where severe maternal toxicity and
embryotoxicity were noted, and in the two-generation study in rats,
which was the most sensitive study and gave a NOEL 2 mg/kg b.w./day.
The Committee established a temporary ADI of 0-10 µg/kg b.w.
for febantel using a safety factor of 200. The ADI was made
temporary because of the inadequate dosing in the rat
carcinogenicity study.
Even though a temporary ADI was established, it was not used
for recommending MRLs. Before the toxicological issues relating to
this compound can be resolved, additional information on its
genotoxic and carcinogenic potential will have to be provided
(see Summary section on the benzimadozoles).
5. REFERENCES
BAYER (1987) Residue studies synopsis. Febantel 45.5% oral paste.
Unpublished report from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & LUCKHAUS, G. (1976) BAY 5757. Toxicological studies in
rats. Studies with oesophageal probe application over 3 months.
Unpublished report No. 5823 from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
BOMHARD, E. & KALINER, G. (1985) BAY Vh 5757. Chronic toxicity and
carcinogenicity studies in mice. 21-Month feeding study. Unpublished
report No. 13961 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & NAGER, H. (19875) BAY Vh 5757. Combined chronic
toxicity and carcinogenicity study on Wistar rats. Unpublished
report No. 15844 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983a) Mutagenicity evaluation of BAY L
5156 in the mouse lymphoma forward mutation assay. Unpublished
Project No. 20989 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983b) Mutagenicity assay of BAY K 7648
in the mouse lymphoma forward mutation assay. Unpublished Project
No. 20989 from Litton Bionetics Inc., Kensington, MD, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DAVIDSE, L.C. (1977) Mode of action, selectivity and mutagenicity of
benzimidazole compounds. Neth. J. Plant Pathol., 83 (Suppl.1):
135-144.
DELATOUR, P. (1982) Some aspects of the teratogenicity of veterinary
drugs. Vet. Res. Commun., 7: 125-131.
DELATOUR, P., DANDON, M., GARNIER, F. & BENOIT, E. (1982a)
Metabolism-embryotoxicity relationship of febantel in the rat and
sheep. Ann. Rech. Vet., 13: 163-170.
DELATOUR, P., YOSHIMURA, H., GARNIER, F. & BENOIT, E. (1982b)
Embryotoxicity compared with metabolites of oxfendazole. Rec. Med.
Vet., 158: 369-373.
DELATOUR, P., GARNIER, F. & BENOIT, E. (1983a) Kinetics of four
metabolites of febantel in cow's milk. Vet. Res. Commun., 6:
37-42.
DELATOUR, P., TIBERGHIEN, M.P. & BESSE, S. (1983b) An HPLC procedure
for the quantification of five metabolites of febantel in sheep
serum. J. Vet. Pharmacol. Therap. 6: 233-235.
DELATOUR, P. & PARISH, R. (1986) Benzimidazole anthelmintics and
related compounds: Toxicity and evaluation of residues. In: Rico AG
(Ed). Drug Residues in Animals, London Academic Press, 175-204.
EIBEN, R. (1985) BAY Vh 5757. 2-Generation study in rats.
Unpublished report No. 13967 from Bayer AG. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
HOFFMAN, K. & NASH, G. (1983) BAY Vh 5757. Chronic oral toxicity in
dogs (12-month feeding test). Unpublished report No. 11593 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
HOORN, A.J.W., JAGANNATH, D.R. & BRUSICK. D.J. (1980) BAY Vh 5757.
Febantel. Mutagenicity evaluation HE100, PI.571201, 100% in the Ames
Salmonella/microsome plate test and DNA repair test. Unpublished
Project No. 20998 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975a) BAY Vh 5757. Orientational cytological studies
in bone marrow and spermatogonia of Chinese hamsters to test for
mutagenic effects. Unpublished report No. 5460 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975b) BAY Vh 5757. Micronucleus test in mice for
evaluation of mutagenic effects. Unpublished report No. 5729 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975c) BAY Vh 5757. Studies for embryotoxic and
teratogenic effects in rats after oral application. Unpublished
report No. 5644 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & LUCKHAUS, G. (1976) BAY Vh 5757. Sub-chronic toxicity
test on dogs with oral application (13 week experiment). Unpublished
report No. 6422 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & DYCKA, J. (1976) BAY Vh 5757. Dominant lethal test on
male mice to test for mutagenic effects. Unpublished report No. 6031
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MONTESISSA, C., TRACCIARI, J.M., FADINI, L. & BERETTA, C. (1989)
Comparative microsomal oxidation of febantel and its metabolite
fenbendazole in various animal species. Xenobiotica, 19: 97-100.
MOUROT, D. (1990) Febantel. Ames Test. Unpublished report from
Centre National d'Etudes Vétérinaires et Alimentaires. Submitted to
WHO by Centre National d'Etudes Vétérinaires et Alimentaires,
Fougčres, France.
MUERMANN, P. (1975) BAY Vh 5757. Acute toxicity in mice, rats,
rabbits and dogs. Unpublished report No. 5378 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY h 5156 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY k 7648 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
NELSON, D.L. & BURKE, M.A. (1977) Acute dermal toxicity of BAY Vh
5757 - Drug substance. Unpublished report from Chemagro Agricultural
Division, Mobay Chemical Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
NELSON, D.L., ANDERSON, R.H. & HOSS, H.E. (1977) The acute
inhalation toxicity of BAY Vh 5757 technical to rats. Unpublished
report from Chemagro Agricultural Division, Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982a) Mutagenicity evaluation of BAY
L 5156 Batch 810030 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionetics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982b) Mutagenicity of BAY k 76485156
Batch 810032 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionectics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
SEILER, J.P. (1978) The mutagenic mode of action of benzimidazole.
Experientia, 34: 851-853.
SHARMA, S. & ABUZAR, S. (1983) The benzimidazole anthelmintics -
chemistry and biological activity. Prog. Drug. Res., 27: 85-161.
WOLLWEBER, H., KOLLING, H., WIDDIG, A., THOMAS, H., SCHULZ, H-P. &
MURMANN, P. (1978). Febantel, a new broad spectrum anthelmintic.
Arzneim Forsch., 28: 2193-2195.
FEBANTEL
First Draft Prepared by
Dr. K.N. Woodward
Veterinary Medicines Directorate
Weybridge, Surrey, England
1. EXPLANATION
Febantel (N-{-[2,3-bis-(methoxycarbonyl)-guanido]-5-
(phenylthio)-phenyl}-2-methoxyacetamide) is an anthelmintic agent
active against a range of gastrointestinal parasites in animals. It
is a prodrug and in vivo undergoes hydrolytic removal of a
methoxyacetyl group and cyclisation to yield the benzimidazole,
fenbendazole (Wollweber et al., 1978; Delatour & Parish, 1986). It
has not previously been evaluated by the Joint FAO/WHO Expert
Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
Following intraduodenal dosing of rats with 14C-labelled
febantel, around 25-30% of the dose was excreted in the urine,
suggesting moderate absorption after oral administration. After
intravenous and intraduodenal dosing around 70% of the dose was
excreted in the bile indicating that initial absorption after oral
administration may be higher. Around 20% of the oral dose of
febantel was detected in the urine of sheep over a 4-day period
after dosing (Anon. 1987). In the cow, metabolites of febantel were
excreted in the milk after oral doses (Delatour et al., 1983a). The
liver and to a lesser extent the kidney were target tissues for
febantel-related metabolites in the rat, sheep and cattle (Bayer,
1987).
2.1.2 Biotransformation
In vivo, febantel undergoes cyclization to fenbendazole which
is interconvertible with oxfendazole. In addition, oxidation at the
sulfur atom in febantel yields the sulfoxide which can undergo
hydrolytic cleavage and cyclisation to produce oxfendazole. A total
of 9 metabolites has been identified in the rat. Sheep were given
25 mg/kg b.w. and the urine collected. A similar metabolic pattern
was noted in the two species, and oxfendazole and fenbendazole were
identified along with the other metabolites including the p-hydroxy
derivative of fenbendazole (Anon. 1987; Delatour et al., 1983b;
Delatour et al., 1982a). Similar findings were made in cattle (Anon.
1987). Fenbendazole and oxfendazole were identified in cow's milk
after oral dosing (Delatour et al., 1983a). It has been shown that
liver microsomes from various species can convert febantel to its
sulfoxide; sheep microsomes displayed the highest activity in this
respect. Similarly, microsomes from a number of species converted
fenbendazole to oxfendazole. The experiments together suggest that
the liver is responsible for the oxidative metabolism of both
febantel and fenbendazole (Montesissa et al., 1989).
A general metabolism scheme showing the metabolism of all 3
benzimidazole compounds considered by the Committee may be found on
page 1(note: to check).
2.2 Toxicological studies
2.2.1 Acute toxicity
Febantel was of low toxicity in the mouse, rat, and dog after
oral administration. It was somewhat more toxic to the rabbit by
this route (Table 1). It was of low acute toxicity to the rat
following inhalation exposure, and to the rabbit after dermal
exposure.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral > 10000 Muermann, 1975
Mouse M s.c. > 10000
Rat M oral 10605
Rat M&F inh. > 20001 Nelson et al., 1977
Rabbit F oral ca. 1250 Muermann, 1975
Rabbit M&F dermal > 20002 Nelson & Burke, 1977
Dog M&F oral > 10000 Muermann, 1975
1. 1 h exposure, µg/1
2. 24 h occlusive dressing
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 15 male and 15 female Wistar rats were given 0, 20,
50, and 125 mg/kg b.w./day febantel, 7 days per week by stomach
tube, for 3 months. Body weights and food consumption were measured
once a week, and clinical laboratory studies were conducted after 1
and 3 months.
No clinical signs were noted during the study and behaviour and
feed consumption were unaffected by febantel administration. Body
weights in all the groups were comparable with control values except
for males given 20 mg/kg b.w./day, which showed a higher rate of
weight gain. No compound-related deaths occurred during the study.
Haematology was normal at 1 and 3 months, as was clinical
biochemistry. There were no adverse findings following urinalyses.
At necropsy, no gross effects were noted. Relative and absolute
liver weights were increased in females given the highest dose;
relative liver weights were increased in corresponding males.
High-dose females had reduced thymus weights when compared with
controls. The only drug-related finding on histopathological
examination was fatty infiltration of the liver in several high-dose
rats. The no-effect level in this study was 50 mg/kg b.w./day
(Bomhard and Luckhaus, 1976).
2.2.2.2 Dogs
Groups of 3 male and 3 female pure-bred beagles were given
doses of 0, 20, 60, and 180 mg/kg b.w./day febantel in a gelatin
capsule 7 days per week for 13 weeks. Body weights were measured
weekly and body temperatures before the start of the study and at
weeks 3, 6, and 13. Neurological, haematological and ophthalmoscopic
examinations and electrocardiographic (ECG) investigations were
conducted at weeks 3, 6, and 13. Urinalyses were conducted at the
start of the study and at weeks 3, 6, and 13.
No signs of toxicity occurred in dogs given 20 and 60 mg/kg
b.w./day. Food consumption was reduced in some dogs given 180 mg/kg
b.w./day, and occasional diarrhoea and ataxia occurred. One animal
given this dose was killed on day 26 in a moribund state. This
animal was emaciated and had bleeding gums. It became ataxic and was
therefore sacrificed. Animals given 20 and 60 mg/kg b.w./day had
rates of body weight gain comparable with controls but in those
given the high dose, weight gain was depressed during the first 3
weeks. Temperatures in all dogs were comparable with those in
controls and no adverse effects were noted on ophthalmoscopic and
neurological examinations. ECGs were normal and no occult blood was
found on faecal examinations.
Haematologic examination showed no compound-related effects on
red blood cells in dogs given 20 mg/kg b.w./day but those given the
higher doses showed reductions in haematocrit, haemoglobin and
erythrocyte counts. Thrombocyte counts were unaffected. Leukopenia
was seen in 3/6, 1/6, and 6/6 dogs given 20, 60, and 180 mg/kg
b.w./day febantel. Differential blood counts revealed
granulocytopenia. There were no effects on clinical chemistry or
urinalysis values.
At necropsy, there were no apparent gross effects on dogs given
20 or 60 mg/kg b.w./day febantel but dogs given 180 mg/kg b.w./day
had red markings in the mucosa of the stomach and small intestine
with reddening of the mesenteric lymph nodes. There was atrophy of
the white splenic pulp in one animal. Testicular weights were
reduced at all 3 dose levels.
Histopathological examination revealed atrophy of the splenic
follicles in dogs given the highest dose and in one animal given
60 mg/kg b.w./day. Testicular hypoplasia was evident in all treated
groups but this was more pronounced in high-dose animals. No
compound-related changes occurred in the epithelia of the stomach,
oesophagus, or small intestine; there were no effects on mitoses in
the crypts (Machemer & Luckhaus, 1976).
As an extension of the study, groups of 3 male and 3 female
beagle dogs were given doses of 0, 5, and 10 mg/kg b.w./day febantel
in capsules. There were no signs of toxicity noted and haematology
was unaffected by compound administration. There was no excess
incidence of splenic or testicular effects in the treated dogs. The
no-effect level in this study was 10 mg/kg b.w./day (Machemer &
Luckhaus, 1976).
In a 52-week study, groups of 4 male and 4 female beagle dogs
were given diets containing 0, 40, 200, and 1000 ppm febantel
(equivalent to 0, 0.1, 5, and 25 mg/kg b.w./day). In this study,
body weights were recorded weekly, reflexes (corneal, pupillary,
patellar, extensor and reflexor) before the start of the study and
at weeks 4, 7, 13, 25, 39, and 52, and ophthalmoscopy at weeks 4, 7,
13, 25, 39, and 52. Haematology, biochemistry and urinalysis were
also conducted at these time points. Body temperature and pulse
rates were also measured during the study.
Animals given up to 200 ppm dietary febantel exhibited no signs
of toxicity, and there were no effects on body temperature or pulse
rate in any of the treated groups. Animals given the highest dietary
level showed a much reduced feed intake (approximately 75% of
control values). Body weights of males were significantly reduced
and female dogs showed a reduction in the rate of weight gain. One
dog in the high dietary level group showed a marked reduction in
body weight (3.5 kg at week 39) and was found to have opacification
of the lenses.
Three of the 8 dogs given the highest dietary level died during
the course of the study (one accidentally); there were no deaths in
any of the other groups. There were no haematological effects in the
animals given up to 200 ppm febantel. However, in high-level
animals, particularly in the three which died, there were reductions
in haematocrit, haemoglobin and erythrocyte values. The thrombocyte
count was markedly lowered in one of these animals. There was also a
marked reduction in leucocyte counts at the 1000 ppm level. This was
characterized by a significant reduction in granulocytes.
Biochemical studies revealed significant increases in the
activities of hepatic enzymes indicative of some degree of liver
damage, at the highest dietary level. There were no effects on
urinalyses.
At necropsy, there were significant reductions in testicular
and prostate weights in high-level males. Histological examination
revealed drastic reductions in leukopoiesis and erythropoiesis in
the marrow of high-level animals. Lymphofollicular atrophy of the
lymph nodes (including the spleen) occurred in these dogs.
Myocardial necrosis occurred in one high dietary level dog and this
was considered by the authors to be secondary to hypoxemia due to
the effects on blood elements. Centrilobular and panlobular
degeneration of the liver were noted in dogs given 1000 ppm.
Testicular and prostatic hypoplasia were evident in high-level
males. The no-effect level in this 52-week study in the dog was
200 ppm in the diet, equivalent to 5 mg/kg b.w./day (Hoffmann &
Nash, 1983).
2.2.3 Long term/carcinogencity studies
2.2.3.1 Mice
In a combined chronic toxicity and carcinogenicity study,
groups of 60 male and 60 female NMRI mice were given diets
containing 0, 50, 200, and 800 ppm febantel, equal to 0, 10, 42, and
170 mg/kg b.w./day for males and 0, 15, 58, and 250 mg/kg b.w./day
for females, for up to 21 months. From these groups 10 male and 10
female mice were sacrificed after 1 year.
There were no effects on mortality attributable to febantel
intake, nor were there any effects on appearance or behaviour. Feed
and water intake values in treated mice were comparable with
controls. Body weights of treated males were similar to controls but
in females body weights of mice given the highest dietary level were
slightly but significantly lower than control values in the first 11
months of the study. After 11 months, the body weights of the
high-level animals became comparable with those of controls.
Haematological studies revealed no effects in mice given up to
200 ppm febantel in the diet. However, males given the highest
dietary level had significant increases in the platelet count and
significant decreases in mean corpuscular haemoglobin contents.
Females were found to have lower haematocrit values and, at the end
of the study, reductions in erythrocyte counts. Blood biochemistry
was investigated at 12 and 21 months. The activity of serum
aspartate aminotransferase was significantly decreased in all the
treated males and decreased in females given the low and high levels
of dietary febantel; the intermediate-level mice however had
increased activity of this enzyme. The significance of this is
unknown although the authors of the report believed it to be
toxicologically irrelevant. Alkaline phosphatase, serum alanine
aminotransferase, creatine kinase, total protein, bilirubin, urea,
cholesterol and glucose were unaffected. Serum creatinine was
lowered in all treated mice.
There were no gross abnormalities which could be attributed to
compound intake in mice which died during the study, in those killed
in a moribund condition, in the groups killed at 12 months nor in
those sacrificed at termination.
The only effects on organ weights were those noted in the liver
in animals given the highest dietary level. There were significant
increases in absolute and relative liver weights at 12 and 21 months
in these animals.
At histopathological examination, the only adverse finding was
an increased fat accumulation in the livers of high-level female
animals. There was no increase in the incidence of any benign or
malignant tumour type (Bomhard & Kaliner, 1985).
2.2.3.2 Rats
In a combined chronic toxicity and carcinogenicity study,
groups of 100 male and 100 female Wistar rats were fed diets
containing 0, 20, 100, and 500 ppm febantel, equal to 0, 2, 8, and
40 mg/kg b.w./day, for an eleven-week period prior to mating, during
a 20-day mating period, and during the subsequent lactation period
(up to 33 days). A total of 60 male and 60 female pups from each
dose group was then given the same dietary level as the respective
parental animals for up to 30 months. Groups of 10 male and 10
female rats from each group were sacrificed after 65 weeks.
There were no effects on appearance, behaviour, mortality, or
food and water intake, and no effects on body weight at levels of up
to 100 ppm febantel. However, the highest dietary level depressed
body weights in parental animals and in their young. Litter size was
reduced significantly at birth, 7 days, and at week 3 in animals
given the highest level. Levels of up to and including 100 ppm had
no effects on pup weight but pups in the 500 ppm level group had
significant reductions in weight when compared with controls at
birth, 7 days and week 3. Body weights of these animals remained
reduced throughout the course of the study. There were no effects on
mortality in any of the treated groups.
No haematological abnormalities were noted at week 27, 53, 106,
or 132 in animals given up to 100 ppm febantel. In animals given the
highest level, mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentrations were reduced, sometimes significantly, at
all 4 examination points, in male and female rats. There were no
cytological differences, however, between control and treated
animals.
Clinical chemistry revealed significant increases in alkaline
phosphatase activities in males and females given the highest level
of febantel.
Gross examination of rats which died during the study, in those
subject to interim sacrifice at week 65 and in animals killed at
termination revealed no abnormalities. There were significant
increases in liver weights, both absolute and relative, at
termination in males and females given the highest dietary level,
and in relative liver weights at week 65 in males and females.
Histopathological examination of rats sacrificed at week 65
revealed fatty vacuolation of the liver in animals given the highest
dietary level as the only non-neoplastic finding. Similar
observations were made at terminal sacrifice. There was no increased
incidence of any tumour type in any of the treatment groups.
However, only animals given the highest level and controls were
subject to full histopathology; rats given the other doses had only
the lungs, liver and kidneys examined histologically. The NOEL for
non-neoplastic effects was 100 ppm, equal to 8 mg/kg b.w./day
(Bomhard & Mager, 1987).
2.2.4 Reproduction Studies
2.2.4.1 Rats
A two-generation study was conducted in accordance with OECD
Guidelines (Guideline 416). Groups of 25 male and 25 female rats
were given dietary febantel at levels of 0, 20, 100, and 500 ppm,
equivalent to 0, 2, 10, and 50 mg/kg b.w./day. The F0 generation
animals were fed the diets for 100 days prior to mating. Rats were
fed febantel-containing diets throughout the study period, including
the period of gestation and rearing of the young.
The F0 animals were mated to produce F1a and F1b
generations. The F1b rats were mated to produce F2a and F2b
generations. All the F0 and F1b females were necropsied 1-3
weeks after weaning. All the F1b young not required for mating and
all the F2b young were also necropsied when approximately 4 weeks
old.
Treatment of the F0 animals had no effects on appearance,
behaviour or coat quality at any of the dietary levels used. Females
in all treatment groups gained weight at the same rate as controls,
as did males given up to 100 ppm. However, high-level F0 males
were lighter at all times than corresponding controls. There were no
effects on food intake. In the F1a and F1b generations there
were no effects on food intake. In the F1a and F1b generations
there were no effects on insemination index, fertility index,
gestation index, gestation period or on the numbers of successfully
mated females. The male:female ratio was unaffected, and the total
number of young at each dietary level was similar. Litter size was
slightly reduced in both F1 generations at the highest dietary
level. The viability of young at the highest level was also reduced
in both F1 generations. When pup weights were measured at birth
and four weeks after birth, these were lower in young from the
highest dose level and also in the 100 ppm group 4 weeks after
birth. There was no excess incidence of malformations in any of the
treated groups when compared with controls.
In the F1b generation, the only effects noted were reductions
in body weights in males given the highest level of febantel in the
diet, and reductions in body weights in females given 100 and 500
ppm. There were no effects on feed consumption. The insemination
index, fertility index, gestation index, gestation period and the
numbers of successfully mated females were unaffected by compound
treatment. The numbers of young at birth were slightly but
significantly reduced in the F2a and F2b generations after 100
and 500 ppm but the male:female ratios were unaffected at any of the
dietary levels. The number of young surviving lactation was slightly
reduced at 500 ppm in the F2b generation.
Mean body weights were significantly reduced in both F2
generations at the highest dietary level. The incidence of
malformations was not increased at any treatment level.
Necropsy of F0 animals revealed pale livers at 100 and 500
ppm. All F1b and F2b animals sacrificed at 4 weeks of age were
necropsied. Discoloured livers were noted at 100 and 500 ppm. At
histopathological examination, hepatocellular hypertrophy was noted
in the livers of F0 rats given 100 and 500 ppm febantel. Fatty
degeneration of the liver occurred in F1b rats at 500 ppm.
Glycogen deposition was seen in the livers of F2b animals given
100 and 500 ppm. There were no effects on organ weights of F1b
animals (liver, kidney, testes and ovaries) given up to 100 ppm
febantel but animals given 500 ppm had decreased absolute kidney
weights and increased relative liver weights.
This study therefore revealed no teratogenic effects at levels
of up to 500 ppm dietary febantel. However, parental animals given
the highest dose displayed lowered body weights. Litter size and
viability of young were reduced at the highest level in the F1
generation. Similar effects were noted in the F2 generation at the
high dietary level. Mean body weights were significantly reduced at
the highest level. The no-effect level for reproductive performance
was 100 ppm, but taking into account hepatic hypertrophy and fat and
glycogen deposition, the no-effect level in this study was 20 ppm,
equivalent to 2 mg/kg b.w./day (Eiben, 1985).
2.2.5. Special studies on genotoxocity
Febantel was not mutagenic in the Ames test; it did not induce
DNA repair in a bacterial test and was negative in an in vivo
cytogenetics assay. It did not increase the incidence of
micronucleated polychromatic erythrocytes in the mouse (Table 2).
However, it did give positive results in the mouse dominant lethal
test.
Two metabolites of febantel, fenbendazole (see also
corresponding monograph) and
2-amino-5-phenylsulfinyl-2-benzimidazole (the 2-amino derivative of
fenbendazole) were also tested for genotoxic potential. The results
are given in Table 3. Oxfendazole has been tested and is shown at
2.2.5 in the oxfendazole monograph in this volume. Both compounds
were negative in the Ames test and in tests for DNA repair in
primary rat hepatocytes. However, fenbendazole produced a positive
response in the presence, but not in the absence, of rat liver S9.
Benzimidazoles are a group of compounds which bind to tubulin
and may possibly disrupt mitosis. Benzimidazole itself is positive
in the Ames test (Davidse, 1977; Seiler, 1978). However, febantel as
a pro-benzimidazole is unlikely to be typical of the group except
under circumstances where conversion to fenbendazole, oxfendazole
and their metabolites is possible (see corresponding monographs).
Table 2. Results of genotoxicity assays on febantel
Test system Test object Concentration Results Reference
In vitro
Ames test1 S.typhimurium 0-5000 Negative Hoorn et al.,
TA1535, µg/plate 1980
TA1537,
TA1538, TA98,
TA100
Ames test1 S.typhimurium 0-5000 Negative Mourot, 1990
TA97a, TA98, µg/plate
TA100, TA102
DNA E.coli 0-5000 Negative Hoorn et al.,
repair1 pol A+, pol A- µg/plate 1980
In vivo
Cytogenetics Chinese 1000 mg/kg Negative Machemer,
assay hamster b.w. twice at 1975a
(marrow and 24 h interval2
spermatogonia)
Micro-nucleus Mouse (NMRI) 500 and 1000 Negative Machemer,
test mg/kg b.w. 1975b
twice at 24 h
intervals3
Dominant Mouse (NMRI) 500 and 2000 Positive4 Machemer &
lethal mg/kg Dycka, 1976
b.w./day twice
at 24 h
intervals
1. Both with and without rat liver S-9 fraction.
2. Animals killed and marrow harvested 6 and 48 h after second dose.
3. Animals killed 6 h after second dose.
4. No effects with lower dose but higher dose significantly reduced
fertility at 2, 4, 5 and 6 weeks of mating.
Table 3. Results of genotoxicity studies on fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole
Test Test Object Concentration Results Reference
System
Fenbendazole
Ames test1 S.typhimurium 10000 µg/plate Negative Rabenold &
TA1535, Brusick,
TA1537 1982a
Forward Mouse up to 62.5 Weakly Cifone &
mutation1 lymphoma µg/ml Positive2 Myrh, 1983a
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Rabenold &
hepatocytes Bruskick,
1982b
2-Amino-5-phenylsulfinyl-2-benzimidazole
Ames test1 S.typhimurium 1-10000 Negative Rabenold &
TA1535, µg/plate Brusick,
TA1537, 1982b
TA1538,
TA98, TA100
Forward Mouse up to 300 Positive2 Cifone &
mutation1 lymphoma µg/ml Myhr, 1983b
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Myhr &
hepatocytes Brusick,
1982b
1. Both with and without rat liver S-9.
2. Positive in the presence, but not absence of metabolic activation.
2.2.6 Special studies on teratogenicity
2.2.6.1 Rats
Groups of 20-24 pregnant FB30 (Long Evans) rats were given
febantel as an oral suspension in 0.5% aqueous tylose solution, by
stomach tube at 0, 10, 30, and 100 mg/kg b.w./day on days 6-15 of
gestation. Animals were killed on day 20 of gestation and the
uterine contents examined.
No signs of overt toxicity occurred at any dose level, but
animals given the highest dose gained less weight than those in
other groups including the control group. The number of fertilized
animals was similar in each group, but the number of pregnant
animals in the high dose group at termination was much lower (33%)
than in the other groups (all 100%), indicating severe
embryotoxicity. The numbers of implantations, fetuses and
resorptions was similar for animals given up to 30 mg/kg b.w./day
and controls. However, in those given 100 mg/kg b.w./day, the number
of implantation sites was similar to control values, but the number
of resorptions was markedly increased. Fetal and placental weights
were also significantly decreased at the high dose level.
No teratogenic effects were noted at doses of up to 30 mg/kg
b.w./day. At 100 mg/kg b.w./day, retardation of bone development and
teratogenic effects including anophthalmia, dysplastic
microphthalmia and multiple deformities of the ribs and spine were
noted in 4/25 fetuses examined. Gross examination of one maternal
animal that died during treatment at termination revealed severe
damage to the gastrointestinal tract at 100 mg/kg b.w./day
(Machemer, 1975c).
A study in the rat (unspecified strain) showed febantel to be
teratogenic when given at 46, 67, and 89 mg/kg b.w./day on days 8-15
of gestation. Its sulfoxide was also teratogenic in the rat at 46
and 93 mg/kg b.w./day. The no-effect level for febantel was 22 mg/kg
b.w./day (Delatour et al., 1982a).
Studies with febantel and its metabolites fenbendazole and
oxfendazole suggested that the active metabolite responsible for
embryotoxic effects of febantel was oxfendazole; when febantel was
given to rats simultaneously with SKF 525A, an inhibitor of
microsomal oxidation, the embryotoxicity was abolished (Delatour
et al., 1982a; Delatour et al., 1982b; Delatour, 1983; see also
accompanying monographs on fenbendazole and oxfendazole).
These studies show that febantel is embryotoxic and teratogenic
in the rat; the lowest no-effect level noted was 22 mg/kg b.w./day.
2.3 Observations in humans
No data were available.
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of acute and short-term studies
and of studies of carcinogenicity, genotoxicity, reproduction, and
development.
The main route of metabolism in rats, sheep, and cattle appears
to be cyclization to yield fenbendazole. Oxidation at the sulfur
atom can also occur to yield the sulfoxide which then undergoes
cyclization to give oxfendazole. Both fenbendazole and oxfendazole
can then undergo further metabolism.
In a study in rats in which febantel was given by gavage at
doses of up to 125 mg/kg b.w./day for 7 days, the only drug-related
effect was fatty infiltration of the liver. The NOEL was 50 mg/kg
b.w./day. Dogs were given febantel daily in gelatin capsules for 13
weeks at doses of up to 180 mg/kg b.w./day. Dogs given 60 and
180 mg/kg b.w./day showed testicular hypoplasia; and reductions in
haematocrit, haemoglobin, and erythrocyte counts. Leukopenia was
noted in all treated groups although the effect was not
dose-related. Agranulocytosis was noted in the high dose dogs, and
one high dose animal out of six also had splenic atrophy. In an
extension of the study using doses of up to 10 mg/kg b.w./day, no
haematological, splenic, or testicular effects were seen. The NOEL
was 10 mg/kg b.w./day. In a 52-week study in dogs given febantel at
levels of up to 1 g/kg in the diet (equivalent to 25 mg/kg
b.w./day), reductions in haemotocrit, haemoglobin, and erythrocyte
counts occurred at the highest dietary level. Testicular and
lymphofollicular (including splenic) atrophy also occurred at this
dietary level. The NOEL was 200 mg/kg in the diet, equivalent to
5 mg/kg b.w./day.
A carcinogenicity study was conducted in mice, which received
febantel in the diet at levels of up to 170 mg/kg b.w./day for males
and 250 mg/kg b.w./day for females for up to 21 months. There were
no changes in tumour incidence as compared with control values.
In a combined long-term toxicity and carcinogenicity study,
rats were given febantel in the diet at levels of up to 40 mg/kg
b.w./day during an 11-week period prior to mating, during a 20 day
mating period, and during lactation. A total of 60 male and 60
female pups from each dose group was then given the same dietary
level as the parents for up to 30 months. Females given the highest
dietary level showed reductions in mean corpuscular haemoglobin and
increases in absolute and relative liver weights. These animals had
fatty vacuolation of the liver at histopathological examination both
at week 65 after weaning and at the end of the study. There was no
increase in tumour incidence, but the Committee recognized that the
doses used may not have been sufficiently high for the carcinogenic
potential of this compound to have been adequately explored. The
NOEL for non-neoplastic effects was 8 mg/kg b.w./day.
In a two-generation study in which rats were given febantel in
the diet at up to 50 mg/kg b.w./day, litter size and viability of
the young were reduced at the highest dietary level in both
generations. The NOEL for reproductive performance was 10 mg/kg
b.w./day; however, if hepatic hypertrophy and glycogen deposition
were taken into account, the overall NOEL was 2 mg/kg b.w./day.
In a teratogenicity study in rats, severe maternal toxicity and
embryotoxicity occurred at the highest dose level. The NOEL was
22 mg/kg b.w./day. Teratogenicity was not seen in the absence of
maternal toxicity and embryotoxicity.
The genotoxic potential of febantel was investigated in a
number of test systems. Negative results were reported in two Ames
tests, in a test for DNA repair in bacteria, in an in vivo
cytogenetic test in the hamster, and in the mouse micronucleus test.
However, a positive result was found in the dominant lethal test in
the mouse. Two metabolites of febantel, fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole (the amino-derivative of
oxfendazole), also gave negative results in the Ames test and in a
test for DNA repair in primary rat hepatocytes, but both gave
positive results in the mouse lymphoma tk-locus genotoxicity assay
in the presence of metabolic activation, though not in its absence.
No studies of febantel in humans were available to the
Committee.
4. EVALUATION
The Committee noted that no carcinogenic effects were seen in
rats and mice; the significance of the positive results found with
the compound and its metabolites in a small number of tests for
genotoxic potential was therefore unknown. The major effects were
those noted in the 13- and 52-week studies in dogs, in the
teratogenicity studies in rats, where severe maternal toxicity and
embryotoxicity were noted, and in the two-generation study in rats,
which was the most sensitive study and gave a NOEL 2 mg/kg b.w./day.
The Committee established a temporary ADI of 0-10 µg/kg b.w.
for febantel using a safety factor of 200. The ADI was made
temporary because of the inadequate dosing in the rat
carcinogenicity study.
Even though a temporary ADI was established, it was not used
for recommending MRLs. Before the toxicological issues relating to
this compound can be resolved, additional information on its
genotoxic and carcinogenic potential will have to be provided
(see Summary section on the benzimadozoles).
5. REFERENCES
BAYER (1987) Residue studies synopsis. Febantel 45.5% oral paste.
Unpublished report from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & LUCKHAUS, G. (1976) BAY 5757. Toxicological studies in
rats. Studies with oesophageal probe application over 3 months.
Unpublished report No. 5823 from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
BOMHARD, E. & KALINER, G. (1985) BAY Vh 5757. Chronic toxicity and
carcinogenicity studies in mice. 21-Month feeding study. Unpublished
report No. 13961 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & NAGER, H. (19875) BAY Vh 5757. Combined chronic
toxicity and carcinogenicity study on Wistar rats. Unpublished
report No. 15844 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983a) Mutagenicity evaluation of BAY L
5156 in the mouse lymphoma forward mutation assay. Unpublished
Project No. 20989 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983b) Mutagenicity assay of BAY K 7648
in the mouse lymphoma forward mutation assay. Unpublished Project
No. 20989 from Litton Bionetics Inc., Kensington, MD, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DAVIDSE, L.C. (1977) Mode of action, selectivity and mutagenicity of
benzimidazole compounds. Neth. J. Plant Pathol., 83 (Suppl.1):
135-144.
DELATOUR, P. (1982) Some aspects of the teratogenicity of veterinary
drugs. Vet. Res. Commun., 7: 125-131.
DELATOUR, P., DANDON, M., GARNIER, F. & BENOIT, E. (1982a)
Metabolism-embryotoxicity relationship of febantel in the rat and
sheep. Ann. Rech. Vet., 13: 163-170.
DELATOUR, P., YOSHIMURA, H., GARNIER, F. & BENOIT, E. (1982b)
Embryotoxicity compared with metabolites of oxfendazole. Rec. Med.
Vet., 158: 369-373.
DELATOUR, P., GARNIER, F. & BENOIT, E. (1983a) Kinetics of four
metabolites of febantel in cow's milk. Vet. Res. Commun., 6:
37-42.
DELATOUR, P., TIBERGHIEN, M.P. & BESSE, S. (1983b) An HPLC procedure
for the quantification of five metabolites of febantel in sheep
serum. J. Vet. Pharmacol. Therap. 6: 233-235.
DELATOUR, P. & PARISH, R. (1986) Benzimidazole anthelmintics and
related compounds: Toxicity and evaluation of residues. In: Rico AG
(Ed). Drug Residues in Animals, London Academic Press, 175-204.
EIBEN, R. (1985) BAY Vh 5757. 2-Generation study in rats.
Unpublished report No. 13967 from Bayer AG. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
HOFFMAN, K. & NASH, G. (1983) BAY Vh 5757. Chronic oral toxicity in
dogs (12-month feeding test). Unpublished report No. 11593 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
HOORN, A.J.W., JAGANNATH, D.R. & BRUSICK. D.J. (1980) BAY Vh 5757.
Febantel. Mutagenicity evaluation HE100, PI.571201, 100% in the Ames
Salmonella/microsome plate test and DNA repair test. Unpublished
Project No. 20998 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975a) BAY Vh 5757. Orientational cytological studies
in bone marrow and spermatogonia of Chinese hamsters to test for
mutagenic effects. Unpublished report No. 5460 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975b) BAY Vh 5757. Micronucleus test in mice for
evaluation of mutagenic effects. Unpublished report No. 5729 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975c) BAY Vh 5757. Studies for embryotoxic and
teratogenic effects in rats after oral application. Unpublished
report No. 5644 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & LUCKHAUS, G. (1976) BAY Vh 5757. Sub-chronic toxicity
test on dogs with oral application (13 week experiment). Unpublished
report No. 6422 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & DYCKA, J. (1976) BAY Vh 5757. Dominant lethal test on
male mice to test for mutagenic effects. Unpublished report No. 6031
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MONTESISSA, C., TRACCIARI, J.M., FADINI, L. & BERETTA, C. (1989)
Comparative microsomal oxidation of febantel and its metabolite
fenbendazole in various animal species. Xenobiotica, 19: 97-100.
MOUROT, D. (1990) Febantel. Ames Test. Unpublished report from
Centre National d'Etudes Vétérinaires et Alimentaires. Submitted to
WHO by Centre National d'Etudes Vétérinaires et Alimentaires,
Fougčres, France.
MUERMANN, P. (1975) BAY Vh 5757. Acute toxicity in mice, rats,
rabbits and dogs. Unpublished report No. 5378 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY h 5156 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY k 7648 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
NELSON, D.L. & BURKE, M.A. (1977) Acute dermal toxicity of BAY Vh
5757 - Drug substance. Unpublished report from Chemagro Agricultural
Division, Mobay Chemical Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
NELSON, D.L., ANDERSON, R.H. & HOSS, H.E. (1977) The acute
inhalation toxicity of BAY Vh 5757 technical to rats. Unpublished
report from Chemagro Agricultural Division, Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982a) Mutagenicity evaluation of BAY
L 5156 Batch 810030 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionetics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982b) Mutagenicity of BAY k 76485156
Batch 810032 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionectics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
SEILER, J.P. (1978) The mutagenic mode of action of benzimidazole.
Experientia, 34: 851-853.
SHARMA, S. & ABUZAR, S. (1983) The benzimidazole anthelmintics -
chemistry and biological activity. Prog. Drug. Res., 27: 85-161.
WOLLWEBER, H., KOLLING, H., WIDDIG, A., THOMAS, H., SCHULZ, H-P. &
MURMANN, P. (1978). Febantel, a new broad spectrum anthelmintic.
Arzneim Forsch., 28: 2193-2195.
 FEBANTEL
First Draft Prepared by
Dr. K.N. Woodward
Veterinary Medicines Directorate
Weybridge, Surrey, England
1. EXPLANATION
Febantel (N-{-[2,3-bis-(methoxycarbonyl)-guanido]-5-
(phenylthio)-phenyl}-2-methoxyacetamide) is an anthelmintic agent
active against a range of gastrointestinal parasites in animals. It
is a prodrug and in vivo undergoes hydrolytic removal of a
methoxyacetyl group and cyclisation to yield the benzimidazole,
fenbendazole (Wollweber et al., 1978; Delatour & Parish, 1986). It
has not previously been evaluated by the Joint FAO/WHO Expert
Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
Following intraduodenal dosing of rats with 14C-labelled
febantel, around 25-30% of the dose was excreted in the urine,
suggesting moderate absorption after oral administration. After
intravenous and intraduodenal dosing around 70% of the dose was
excreted in the bile indicating that initial absorption after oral
administration may be higher. Around 20% of the oral dose of
febantel was detected in the urine of sheep over a 4-day period
after dosing (Anon. 1987). In the cow, metabolites of febantel were
excreted in the milk after oral doses (Delatour et al., 1983a). The
liver and to a lesser extent the kidney were target tissues for
febantel-related metabolites in the rat, sheep and cattle (Bayer,
1987).
2.1.2 Biotransformation
In vivo, febantel undergoes cyclization to fenbendazole which
is interconvertible with oxfendazole. In addition, oxidation at the
sulfur atom in febantel yields the sulfoxide which can undergo
hydrolytic cleavage and cyclisation to produce oxfendazole. A total
of 9 metabolites has been identified in the rat. Sheep were given
25 mg/kg b.w. and the urine collected. A similar metabolic pattern
was noted in the two species, and oxfendazole and fenbendazole were
identified along with the other metabolites including the p-hydroxy
derivative of fenbendazole (Anon. 1987; Delatour et al., 1983b;
Delatour et al., 1982a). Similar findings were made in cattle (Anon.
1987). Fenbendazole and oxfendazole were identified in cow's milk
after oral dosing (Delatour et al., 1983a). It has been shown that
liver microsomes from various species can convert febantel to its
sulfoxide; sheep microsomes displayed the highest activity in this
respect. Similarly, microsomes from a number of species converted
fenbendazole to oxfendazole. The experiments together suggest that
the liver is responsible for the oxidative metabolism of both
febantel and fenbendazole (Montesissa et al., 1989).
A general metabolism scheme showing the metabolism of all 3
benzimidazole compounds considered by the Committee may be found on
page 1(note: to check).
2.2 Toxicological studies
2.2.1 Acute toxicity
Febantel was of low toxicity in the mouse, rat, and dog after
oral administration. It was somewhat more toxic to the rabbit by
this route (Table 1). It was of low acute toxicity to the rat
following inhalation exposure, and to the rabbit after dermal
exposure.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral > 10000 Muermann, 1975
Mouse M s.c. > 10000
Rat M oral 10605
Rat M&F inh. > 20001 Nelson et al., 1977
Rabbit F oral ca. 1250 Muermann, 1975
Rabbit M&F dermal > 20002 Nelson & Burke, 1977
Dog M&F oral > 10000 Muermann, 1975
1. 1 h exposure, µg/1
2. 24 h occlusive dressing
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 15 male and 15 female Wistar rats were given 0, 20,
50, and 125 mg/kg b.w./day febantel, 7 days per week by stomach
tube, for 3 months. Body weights and food consumption were measured
once a week, and clinical laboratory studies were conducted after 1
and 3 months.
No clinical signs were noted during the study and behaviour and
feed consumption were unaffected by febantel administration. Body
weights in all the groups were comparable with control values except
for males given 20 mg/kg b.w./day, which showed a higher rate of
weight gain. No compound-related deaths occurred during the study.
Haematology was normal at 1 and 3 months, as was clinical
biochemistry. There were no adverse findings following urinalyses.
At necropsy, no gross effects were noted. Relative and absolute
liver weights were increased in females given the highest dose;
relative liver weights were increased in corresponding males.
High-dose females had reduced thymus weights when compared with
controls. The only drug-related finding on histopathological
examination was fatty infiltration of the liver in several high-dose
rats. The no-effect level in this study was 50 mg/kg b.w./day
(Bomhard and Luckhaus, 1976).
2.2.2.2 Dogs
Groups of 3 male and 3 female pure-bred beagles were given
doses of 0, 20, 60, and 180 mg/kg b.w./day febantel in a gelatin
capsule 7 days per week for 13 weeks. Body weights were measured
weekly and body temperatures before the start of the study and at
weeks 3, 6, and 13. Neurological, haematological and ophthalmoscopic
examinations and electrocardiographic (ECG) investigations were
conducted at weeks 3, 6, and 13. Urinalyses were conducted at the
start of the study and at weeks 3, 6, and 13.
No signs of toxicity occurred in dogs given 20 and 60 mg/kg
b.w./day. Food consumption was reduced in some dogs given 180 mg/kg
b.w./day, and occasional diarrhoea and ataxia occurred. One animal
given this dose was killed on day 26 in a moribund state. This
animal was emaciated and had bleeding gums. It became ataxic and was
therefore sacrificed. Animals given 20 and 60 mg/kg b.w./day had
rates of body weight gain comparable with controls but in those
given the high dose, weight gain was depressed during the first 3
weeks. Temperatures in all dogs were comparable with those in
controls and no adverse effects were noted on ophthalmoscopic and
neurological examinations. ECGs were normal and no occult blood was
found on faecal examinations.
Haematologic examination showed no compound-related effects on
red blood cells in dogs given 20 mg/kg b.w./day but those given the
higher doses showed reductions in haematocrit, haemoglobin and
erythrocyte counts. Thrombocyte counts were unaffected. Leukopenia
was seen in 3/6, 1/6, and 6/6 dogs given 20, 60, and 180 mg/kg
b.w./day febantel. Differential blood counts revealed
granulocytopenia. There were no effects on clinical chemistry or
urinalysis values.
At necropsy, there were no apparent gross effects on dogs given
20 or 60 mg/kg b.w./day febantel but dogs given 180 mg/kg b.w./day
had red markings in the mucosa of the stomach and small intestine
with reddening of the mesenteric lymph nodes. There was atrophy of
the white splenic pulp in one animal. Testicular weights were
reduced at all 3 dose levels.
Histopathological examination revealed atrophy of the splenic
follicles in dogs given the highest dose and in one animal given
60 mg/kg b.w./day. Testicular hypoplasia was evident in all treated
groups but this was more pronounced in high-dose animals. No
compound-related changes occurred in the epithelia of the stomach,
oesophagus, or small intestine; there were no effects on mitoses in
the crypts (Machemer & Luckhaus, 1976).
As an extension of the study, groups of 3 male and 3 female
beagle dogs were given doses of 0, 5, and 10 mg/kg b.w./day febantel
in capsules. There were no signs of toxicity noted and haematology
was unaffected by compound administration. There was no excess
incidence of splenic or testicular effects in the treated dogs. The
no-effect level in this study was 10 mg/kg b.w./day (Machemer &
Luckhaus, 1976).
In a 52-week study, groups of 4 male and 4 female beagle dogs
were given diets containing 0, 40, 200, and 1000 ppm febantel
(equivalent to 0, 0.1, 5, and 25 mg/kg b.w./day). In this study,
body weights were recorded weekly, reflexes (corneal, pupillary,
patellar, extensor and reflexor) before the start of the study and
at weeks 4, 7, 13, 25, 39, and 52, and ophthalmoscopy at weeks 4, 7,
13, 25, 39, and 52. Haematology, biochemistry and urinalysis were
also conducted at these time points. Body temperature and pulse
rates were also measured during the study.
Animals given up to 200 ppm dietary febantel exhibited no signs
of toxicity, and there were no effects on body temperature or pulse
rate in any of the treated groups. Animals given the highest dietary
level showed a much reduced feed intake (approximately 75% of
control values). Body weights of males were significantly reduced
and female dogs showed a reduction in the rate of weight gain. One
dog in the high dietary level group showed a marked reduction in
body weight (3.5 kg at week 39) and was found to have opacification
of the lenses.
Three of the 8 dogs given the highest dietary level died during
the course of the study (one accidentally); there were no deaths in
any of the other groups. There were no haematological effects in the
animals given up to 200 ppm febantel. However, in high-level
animals, particularly in the three which died, there were reductions
in haematocrit, haemoglobin and erythrocyte values. The thrombocyte
count was markedly lowered in one of these animals. There was also a
marked reduction in leucocyte counts at the 1000 ppm level. This was
characterized by a significant reduction in granulocytes.
Biochemical studies revealed significant increases in the
activities of hepatic enzymes indicative of some degree of liver
damage, at the highest dietary level. There were no effects on
urinalyses.
At necropsy, there were significant reductions in testicular
and prostate weights in high-level males. Histological examination
revealed drastic reductions in leukopoiesis and erythropoiesis in
the marrow of high-level animals. Lymphofollicular atrophy of the
lymph nodes (including the spleen) occurred in these dogs.
Myocardial necrosis occurred in one high dietary level dog and this
was considered by the authors to be secondary to hypoxemia due to
the effects on blood elements. Centrilobular and panlobular
degeneration of the liver were noted in dogs given 1000 ppm.
Testicular and prostatic hypoplasia were evident in high-level
males. The no-effect level in this 52-week study in the dog was
200 ppm in the diet, equivalent to 5 mg/kg b.w./day (Hoffmann &
Nash, 1983).
2.2.3 Long term/carcinogencity studies
2.2.3.1 Mice
In a combined chronic toxicity and carcinogenicity study,
groups of 60 male and 60 female NMRI mice were given diets
containing 0, 50, 200, and 800 ppm febantel, equal to 0, 10, 42, and
170 mg/kg b.w./day for males and 0, 15, 58, and 250 mg/kg b.w./day
for females, for up to 21 months. From these groups 10 male and 10
female mice were sacrificed after 1 year.
There were no effects on mortality attributable to febantel
intake, nor were there any effects on appearance or behaviour. Feed
and water intake values in treated mice were comparable with
controls. Body weights of treated males were similar to controls but
in females body weights of mice given the highest dietary level were
slightly but significantly lower than control values in the first 11
months of the study. After 11 months, the body weights of the
high-level animals became comparable with those of controls.
Haematological studies revealed no effects in mice given up to
200 ppm febantel in the diet. However, males given the highest
dietary level had significant increases in the platelet count and
significant decreases in mean corpuscular haemoglobin contents.
Females were found to have lower haematocrit values and, at the end
of the study, reductions in erythrocyte counts. Blood biochemistry
was investigated at 12 and 21 months. The activity of serum
aspartate aminotransferase was significantly decreased in all the
treated males and decreased in females given the low and high levels
of dietary febantel; the intermediate-level mice however had
increased activity of this enzyme. The significance of this is
unknown although the authors of the report believed it to be
toxicologically irrelevant. Alkaline phosphatase, serum alanine
aminotransferase, creatine kinase, total protein, bilirubin, urea,
cholesterol and glucose were unaffected. Serum creatinine was
lowered in all treated mice.
There were no gross abnormalities which could be attributed to
compound intake in mice which died during the study, in those killed
in a moribund condition, in the groups killed at 12 months nor in
those sacrificed at termination.
The only effects on organ weights were those noted in the liver
in animals given the highest dietary level. There were significant
increases in absolute and relative liver weights at 12 and 21 months
in these animals.
At histopathological examination, the only adverse finding was
an increased fat accumulation in the livers of high-level female
animals. There was no increase in the incidence of any benign or
malignant tumour type (Bomhard & Kaliner, 1985).
2.2.3.2 Rats
In a combined chronic toxicity and carcinogenicity study,
groups of 100 male and 100 female Wistar rats were fed diets
containing 0, 20, 100, and 500 ppm febantel, equal to 0, 2, 8, and
40 mg/kg b.w./day, for an eleven-week period prior to mating, during
a 20-day mating period, and during the subsequent lactation period
(up to 33 days). A total of 60 male and 60 female pups from each
dose group was then given the same dietary level as the respective
parental animals for up to 30 months. Groups of 10 male and 10
female rats from each group were sacrificed after 65 weeks.
There were no effects on appearance, behaviour, mortality, or
food and water intake, and no effects on body weight at levels of up
to 100 ppm febantel. However, the highest dietary level depressed
body weights in parental animals and in their young. Litter size was
reduced significantly at birth, 7 days, and at week 3 in animals
given the highest level. Levels of up to and including 100 ppm had
no effects on pup weight but pups in the 500 ppm level group had
significant reductions in weight when compared with controls at
birth, 7 days and week 3. Body weights of these animals remained
reduced throughout the course of the study. There were no effects on
mortality in any of the treated groups.
No haematological abnormalities were noted at week 27, 53, 106,
or 132 in animals given up to 100 ppm febantel. In animals given the
highest level, mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentrations were reduced, sometimes significantly, at
all 4 examination points, in male and female rats. There were no
cytological differences, however, between control and treated
animals.
Clinical chemistry revealed significant increases in alkaline
phosphatase activities in males and females given the highest level
of febantel.
Gross examination of rats which died during the study, in those
subject to interim sacrifice at week 65 and in animals killed at
termination revealed no abnormalities. There were significant
increases in liver weights, both absolute and relative, at
termination in males and females given the highest dietary level,
and in relative liver weights at week 65 in males and females.
Histopathological examination of rats sacrificed at week 65
revealed fatty vacuolation of the liver in animals given the highest
dietary level as the only non-neoplastic finding. Similar
observations were made at terminal sacrifice. There was no increased
incidence of any tumour type in any of the treatment groups.
However, only animals given the highest level and controls were
subject to full histopathology; rats given the other doses had only
the lungs, liver and kidneys examined histologically. The NOEL for
non-neoplastic effects was 100 ppm, equal to 8 mg/kg b.w./day
(Bomhard & Mager, 1987).
2.2.4 Reproduction Studies
2.2.4.1 Rats
A two-generation study was conducted in accordance with OECD
Guidelines (Guideline 416). Groups of 25 male and 25 female rats
were given dietary febantel at levels of 0, 20, 100, and 500 ppm,
equivalent to 0, 2, 10, and 50 mg/kg b.w./day. The F0 generation
animals were fed the diets for 100 days prior to mating. Rats were
fed febantel-containing diets throughout the study period, including
the period of gestation and rearing of the young.
The F0 animals were mated to produce F1a and F1b
generations. The F1b rats were mated to produce F2a and F2b
generations. All the F0 and F1b females were necropsied 1-3
weeks after weaning. All the F1b young not required for mating and
all the F2b young were also necropsied when approximately 4 weeks
old.
Treatment of the F0 animals had no effects on appearance,
behaviour or coat quality at any of the dietary levels used. Females
in all treatment groups gained weight at the same rate as controls,
as did males given up to 100 ppm. However, high-level F0 males
were lighter at all times than corresponding controls. There were no
effects on food intake. In the F1a and F1b generations there
were no effects on food intake. In the F1a and F1b generations
there were no effects on insemination index, fertility index,
gestation index, gestation period or on the numbers of successfully
mated females. The male:female ratio was unaffected, and the total
number of young at each dietary level was similar. Litter size was
slightly reduced in both F1 generations at the highest dietary
level. The viability of young at the highest level was also reduced
in both F1 generations. When pup weights were measured at birth
and four weeks after birth, these were lower in young from the
highest dose level and also in the 100 ppm group 4 weeks after
birth. There was no excess incidence of malformations in any of the
treated groups when compared with controls.
In the F1b generation, the only effects noted were reductions
in body weights in males given the highest level of febantel in the
diet, and reductions in body weights in females given 100 and 500
ppm. There were no effects on feed consumption. The insemination
index, fertility index, gestation index, gestation period and the
numbers of successfully mated females were unaffected by compound
treatment. The numbers of young at birth were slightly but
significantly reduced in the F2a and F2b generations after 100
and 500 ppm but the male:female ratios were unaffected at any of the
dietary levels. The number of young surviving lactation was slightly
reduced at 500 ppm in the F2b generation.
Mean body weights were significantly reduced in both F2
generations at the highest dietary level. The incidence of
malformations was not increased at any treatment level.
Necropsy of F0 animals revealed pale livers at 100 and 500
ppm. All F1b and F2b animals sacrificed at 4 weeks of age were
necropsied. Discoloured livers were noted at 100 and 500 ppm. At
histopathological examination, hepatocellular hypertrophy was noted
in the livers of F0 rats given 100 and 500 ppm febantel. Fatty
degeneration of the liver occurred in F1b rats at 500 ppm.
Glycogen deposition was seen in the livers of F2b animals given
100 and 500 ppm. There were no effects on organ weights of F1b
animals (liver, kidney, testes and ovaries) given up to 100 ppm
febantel but animals given 500 ppm had decreased absolute kidney
weights and increased relative liver weights.
This study therefore revealed no teratogenic effects at levels
of up to 500 ppm dietary febantel. However, parental animals given
the highest dose displayed lowered body weights. Litter size and
viability of young were reduced at the highest level in the F1
generation. Similar effects were noted in the F2 generation at the
high dietary level. Mean body weights were significantly reduced at
the highest level. The no-effect level for reproductive performance
was 100 ppm, but taking into account hepatic hypertrophy and fat and
glycogen deposition, the no-effect level in this study was 20 ppm,
equivalent to 2 mg/kg b.w./day (Eiben, 1985).
2.2.5. Special studies on genotoxocity
Febantel was not mutagenic in the Ames test; it did not induce
DNA repair in a bacterial test and was negative in an in vivo
cytogenetics assay. It did not increase the incidence of
micronucleated polychromatic erythrocytes in the mouse (Table 2).
However, it did give positive results in the mouse dominant lethal
test.
Two metabolites of febantel, fenbendazole (see also
corresponding monograph) and
2-amino-5-phenylsulfinyl-2-benzimidazole (the 2-amino derivative of
fenbendazole) were also tested for genotoxic potential. The results
are given in Table 3. Oxfendazole has been tested and is shown at
2.2.5 in the oxfendazole monograph in this volume. Both compounds
were negative in the Ames test and in tests for DNA repair in
primary rat hepatocytes. However, fenbendazole produced a positive
response in the presence, but not in the absence, of rat liver S9.
Benzimidazoles are a group of compounds which bind to tubulin
and may possibly disrupt mitosis. Benzimidazole itself is positive
in the Ames test (Davidse, 1977; Seiler, 1978). However, febantel as
a pro-benzimidazole is unlikely to be typical of the group except
under circumstances where conversion to fenbendazole, oxfendazole
and their metabolites is possible (see corresponding monographs).
Table 2. Results of genotoxicity assays on febantel
Test system Test object Concentration Results Reference
In vitro
Ames test1 S.typhimurium 0-5000 Negative Hoorn et al.,
TA1535, µg/plate 1980
TA1537,
TA1538, TA98,
TA100
Ames test1 S.typhimurium 0-5000 Negative Mourot, 1990
TA97a, TA98, µg/plate
TA100, TA102
DNA E.coli 0-5000 Negative Hoorn et al.,
repair1 pol A+, pol A- µg/plate 1980
In vivo
Cytogenetics Chinese 1000 mg/kg Negative Machemer,
assay hamster b.w. twice at 1975a
(marrow and 24 h interval2
spermatogonia)
Micro-nucleus Mouse (NMRI) 500 and 1000 Negative Machemer,
test mg/kg b.w. 1975b
twice at 24 h
intervals3
Dominant Mouse (NMRI) 500 and 2000 Positive4 Machemer &
lethal mg/kg Dycka, 1976
b.w./day twice
at 24 h
intervals
1. Both with and without rat liver S-9 fraction.
2. Animals killed and marrow harvested 6 and 48 h after second dose.
3. Animals killed 6 h after second dose.
4. No effects with lower dose but higher dose significantly reduced
fertility at 2, 4, 5 and 6 weeks of mating.
Table 3. Results of genotoxicity studies on fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole
Test Test Object Concentration Results Reference
System
Fenbendazole
Ames test1 S.typhimurium 10000 µg/plate Negative Rabenold &
TA1535, Brusick,
TA1537 1982a
Forward Mouse up to 62.5 Weakly Cifone &
mutation1 lymphoma µg/ml Positive2 Myrh, 1983a
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Rabenold &
hepatocytes Bruskick,
1982b
2-Amino-5-phenylsulfinyl-2-benzimidazole
Ames test1 S.typhimurium 1-10000 Negative Rabenold &
TA1535, µg/plate Brusick,
TA1537, 1982b
TA1538,
TA98, TA100
Forward Mouse up to 300 Positive2 Cifone &
mutation1 lymphoma µg/ml Myhr, 1983b
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Myhr &
hepatocytes Brusick,
1982b
1. Both with and without rat liver S-9.
2. Positive in the presence, but not absence of metabolic activation.
2.2.6 Special studies on teratogenicity
2.2.6.1 Rats
Groups of 20-24 pregnant FB30 (Long Evans) rats were given
febantel as an oral suspension in 0.5% aqueous tylose solution, by
stomach tube at 0, 10, 30, and 100 mg/kg b.w./day on days 6-15 of
gestation. Animals were killed on day 20 of gestation and the
uterine contents examined.
No signs of overt toxicity occurred at any dose level, but
animals given the highest dose gained less weight than those in
other groups including the control group. The number of fertilized
animals was similar in each group, but the number of pregnant
animals in the high dose group at termination was much lower (33%)
than in the other groups (all 100%), indicating severe
embryotoxicity. The numbers of implantations, fetuses and
resorptions was similar for animals given up to 30 mg/kg b.w./day
and controls. However, in those given 100 mg/kg b.w./day, the number
of implantation sites was similar to control values, but the number
of resorptions was markedly increased. Fetal and placental weights
were also significantly decreased at the high dose level.
No teratogenic effects were noted at doses of up to 30 mg/kg
b.w./day. At 100 mg/kg b.w./day, retardation of bone development and
teratogenic effects including anophthalmia, dysplastic
microphthalmia and multiple deformities of the ribs and spine were
noted in 4/25 fetuses examined. Gross examination of one maternal
animal that died during treatment at termination revealed severe
damage to the gastrointestinal tract at 100 mg/kg b.w./day
(Machemer, 1975c).
A study in the rat (unspecified strain) showed febantel to be
teratogenic when given at 46, 67, and 89 mg/kg b.w./day on days 8-15
of gestation. Its sulfoxide was also teratogenic in the rat at 46
and 93 mg/kg b.w./day. The no-effect level for febantel was 22 mg/kg
b.w./day (Delatour et al., 1982a).
Studies with febantel and its metabolites fenbendazole and
oxfendazole suggested that the active metabolite responsible for
embryotoxic effects of febantel was oxfendazole; when febantel was
given to rats simultaneously with SKF 525A, an inhibitor of
microsomal oxidation, the embryotoxicity was abolished (Delatour
et al., 1982a; Delatour et al., 1982b; Delatour, 1983; see also
accompanying monographs on fenbendazole and oxfendazole).
These studies show that febantel is embryotoxic and teratogenic
in the rat; the lowest no-effect level noted was 22 mg/kg b.w./day.
2.3 Observations in humans
No data were available.
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of acute and short-term studies
and of studies of carcinogenicity, genotoxicity, reproduction, and
development.
The main route of metabolism in rats, sheep, and cattle appears
to be cyclization to yield fenbendazole. Oxidation at the sulfur
atom can also occur to yield the sulfoxide which then undergoes
cyclization to give oxfendazole. Both fenbendazole and oxfendazole
can then undergo further metabolism.
In a study in rats in which febantel was given by gavage at
doses of up to 125 mg/kg b.w./day for 7 days, the only drug-related
effect was fatty infiltration of the liver. The NOEL was 50 mg/kg
b.w./day. Dogs were given febantel daily in gelatin capsules for 13
weeks at doses of up to 180 mg/kg b.w./day. Dogs given 60 and
180 mg/kg b.w./day showed testicular hypoplasia; and reductions in
haematocrit, haemoglobin, and erythrocyte counts. Leukopenia was
noted in all treated groups although the effect was not
dose-related. Agranulocytosis was noted in the high dose dogs, and
one high dose animal out of six also had splenic atrophy. In an
extension of the study using doses of up to 10 mg/kg b.w./day, no
haematological, splenic, or testicular effects were seen. The NOEL
was 10 mg/kg b.w./day. In a 52-week study in dogs given febantel at
levels of up to 1 g/kg in the diet (equivalent to 25 mg/kg
b.w./day), reductions in haemotocrit, haemoglobin, and erythrocyte
counts occurred at the highest dietary level. Testicular and
lymphofollicular (including splenic) atrophy also occurred at this
dietary level. The NOEL was 200 mg/kg in the diet, equivalent to
5 mg/kg b.w./day.
A carcinogenicity study was conducted in mice, which received
febantel in the diet at levels of up to 170 mg/kg b.w./day for males
and 250 mg/kg b.w./day for females for up to 21 months. There were
no changes in tumour incidence as compared with control values.
In a combined long-term toxicity and carcinogenicity study,
rats were given febantel in the diet at levels of up to 40 mg/kg
b.w./day during an 11-week period prior to mating, during a 20 day
mating period, and during lactation. A total of 60 male and 60
female pups from each dose group was then given the same dietary
level as the parents for up to 30 months. Females given the highest
dietary level showed reductions in mean corpuscular haemoglobin and
increases in absolute and relative liver weights. These animals had
fatty vacuolation of the liver at histopathological examination both
at week 65 after weaning and at the end of the study. There was no
increase in tumour incidence, but the Committee recognized that the
doses used may not have been sufficiently high for the carcinogenic
potential of this compound to have been adequately explored. The
NOEL for non-neoplastic effects was 8 mg/kg b.w./day.
In a two-generation study in which rats were given febantel in
the diet at up to 50 mg/kg b.w./day, litter size and viability of
the young were reduced at the highest dietary level in both
generations. The NOEL for reproductive performance was 10 mg/kg
b.w./day; however, if hepatic hypertrophy and glycogen deposition
were taken into account, the overall NOEL was 2 mg/kg b.w./day.
In a teratogenicity study in rats, severe maternal toxicity and
embryotoxicity occurred at the highest dose level. The NOEL was
22 mg/kg b.w./day. Teratogenicity was not seen in the absence of
maternal toxicity and embryotoxicity.
The genotoxic potential of febantel was investigated in a
number of test systems. Negative results were reported in two Ames
tests, in a test for DNA repair in bacteria, in an in vivo
cytogenetic test in the hamster, and in the mouse micronucleus test.
However, a positive result was found in the dominant lethal test in
the mouse. Two metabolites of febantel, fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole (the amino-derivative of
oxfendazole), also gave negative results in the Ames test and in a
test for DNA repair in primary rat hepatocytes, but both gave
positive results in the mouse lymphoma tk-locus genotoxicity assay
in the presence of metabolic activation, though not in its absence.
No studies of febantel in humans were available to the
Committee.
4. EVALUATION
The Committee noted that no carcinogenic effects were seen in
rats and mice; the significance of the positive results found with
the compound and its metabolites in a small number of tests for
genotoxic potential was therefore unknown. The major effects were
those noted in the 13- and 52-week studies in dogs, in the
teratogenicity studies in rats, where severe maternal toxicity and
embryotoxicity were noted, and in the two-generation study in rats,
which was the most sensitive study and gave a NOEL 2 mg/kg b.w./day.
The Committee established a temporary ADI of 0-10 µg/kg b.w.
for febantel using a safety factor of 200. The ADI was made
temporary because of the inadequate dosing in the rat
carcinogenicity study.
Even though a temporary ADI was established, it was not used
for recommending MRLs. Before the toxicological issues relating to
this compound can be resolved, additional information on its
genotoxic and carcinogenic potential will have to be provided
(see Summary section on the benzimadozoles).
5. REFERENCES
BAYER (1987) Residue studies synopsis. Febantel 45.5% oral paste.
Unpublished report from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & LUCKHAUS, G. (1976) BAY 5757. Toxicological studies in
rats. Studies with oesophageal probe application over 3 months.
Unpublished report No. 5823 from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
BOMHARD, E. & KALINER, G. (1985) BAY Vh 5757. Chronic toxicity and
carcinogenicity studies in mice. 21-Month feeding study. Unpublished
report No. 13961 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & NAGER, H. (19875) BAY Vh 5757. Combined chronic
toxicity and carcinogenicity study on Wistar rats. Unpublished
report No. 15844 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983a) Mutagenicity evaluation of BAY L
5156 in the mouse lymphoma forward mutation assay. Unpublished
Project No. 20989 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983b) Mutagenicity assay of BAY K 7648
in the mouse lymphoma forward mutation assay. Unpublished Project
No. 20989 from Litton Bionetics Inc., Kensington, MD, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DAVIDSE, L.C. (1977) Mode of action, selectivity and mutagenicity of
benzimidazole compounds. Neth. J. Plant Pathol., 83 (Suppl.1):
135-144.
DELATOUR, P. (1982) Some aspects of the teratogenicity of veterinary
drugs. Vet. Res. Commun., 7: 125-131.
DELATOUR, P., DANDON, M., GARNIER, F. & BENOIT, E. (1982a)
Metabolism-embryotoxicity relationship of febantel in the rat and
sheep. Ann. Rech. Vet., 13: 163-170.
DELATOUR, P., YOSHIMURA, H., GARNIER, F. & BENOIT, E. (1982b)
Embryotoxicity compared with metabolites of oxfendazole. Rec. Med.
Vet., 158: 369-373.
DELATOUR, P., GARNIER, F. & BENOIT, E. (1983a) Kinetics of four
metabolites of febantel in cow's milk. Vet. Res. Commun., 6:
37-42.
DELATOUR, P., TIBERGHIEN, M.P. & BESSE, S. (1983b) An HPLC procedure
for the quantification of five metabolites of febantel in sheep
serum. J. Vet. Pharmacol. Therap. 6: 233-235.
DELATOUR, P. & PARISH, R. (1986) Benzimidazole anthelmintics and
related compounds: Toxicity and evaluation of residues. In: Rico AG
(Ed). Drug Residues in Animals, London Academic Press, 175-204.
EIBEN, R. (1985) BAY Vh 5757. 2-Generation study in rats.
Unpublished report No. 13967 from Bayer AG. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
HOFFMAN, K. & NASH, G. (1983) BAY Vh 5757. Chronic oral toxicity in
dogs (12-month feeding test). Unpublished report No. 11593 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
HOORN, A.J.W., JAGANNATH, D.R. & BRUSICK. D.J. (1980) BAY Vh 5757.
Febantel. Mutagenicity evaluation HE100, PI.571201, 100% in the Ames
Salmonella/microsome plate test and DNA repair test. Unpublished
Project No. 20998 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975a) BAY Vh 5757. Orientational cytological studies
in bone marrow and spermatogonia of Chinese hamsters to test for
mutagenic effects. Unpublished report No. 5460 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975b) BAY Vh 5757. Micronucleus test in mice for
evaluation of mutagenic effects. Unpublished report No. 5729 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975c) BAY Vh 5757. Studies for embryotoxic and
teratogenic effects in rats after oral application. Unpublished
report No. 5644 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & LUCKHAUS, G. (1976) BAY Vh 5757. Sub-chronic toxicity
test on dogs with oral application (13 week experiment). Unpublished
report No. 6422 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & DYCKA, J. (1976) BAY Vh 5757. Dominant lethal test on
male mice to test for mutagenic effects. Unpublished report No. 6031
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MONTESISSA, C., TRACCIARI, J.M., FADINI, L. & BERETTA, C. (1989)
Comparative microsomal oxidation of febantel and its metabolite
fenbendazole in various animal species. Xenobiotica, 19: 97-100.
MOUROT, D. (1990) Febantel. Ames Test. Unpublished report from
Centre National d'Etudes Vétérinaires et Alimentaires. Submitted to
WHO by Centre National d'Etudes Vétérinaires et Alimentaires,
Fougčres, France.
MUERMANN, P. (1975) BAY Vh 5757. Acute toxicity in mice, rats,
rabbits and dogs. Unpublished report No. 5378 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY h 5156 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY k 7648 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
NELSON, D.L. & BURKE, M.A. (1977) Acute dermal toxicity of BAY Vh
5757 - Drug substance. Unpublished report from Chemagro Agricultural
Division, Mobay Chemical Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
NELSON, D.L., ANDERSON, R.H. & HOSS, H.E. (1977) The acute
inhalation toxicity of BAY Vh 5757 technical to rats. Unpublished
report from Chemagro Agricultural Division, Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982a) Mutagenicity evaluation of BAY
L 5156 Batch 810030 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionetics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982b) Mutagenicity of BAY k 76485156
Batch 810032 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionectics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
SEILER, J.P. (1978) The mutagenic mode of action of benzimidazole.
Experientia, 34: 851-853.
SHARMA, S. & ABUZAR, S. (1983) The benzimidazole anthelmintics -
chemistry and biological activity. Prog. Drug. Res., 27: 85-161.
WOLLWEBER, H., KOLLING, H., WIDDIG, A., THOMAS, H., SCHULZ, H-P. &
MURMANN, P. (1978). Febantel, a new broad spectrum anthelmintic.
Arzneim Forsch., 28: 2193-2195.
FEBANTEL
First Draft Prepared by
Dr. K.N. Woodward
Veterinary Medicines Directorate
Weybridge, Surrey, England
1. EXPLANATION
Febantel (N-{-[2,3-bis-(methoxycarbonyl)-guanido]-5-
(phenylthio)-phenyl}-2-methoxyacetamide) is an anthelmintic agent
active against a range of gastrointestinal parasites in animals. It
is a prodrug and in vivo undergoes hydrolytic removal of a
methoxyacetyl group and cyclisation to yield the benzimidazole,
fenbendazole (Wollweber et al., 1978; Delatour & Parish, 1986). It
has not previously been evaluated by the Joint FAO/WHO Expert
Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
Following intraduodenal dosing of rats with 14C-labelled
febantel, around 25-30% of the dose was excreted in the urine,
suggesting moderate absorption after oral administration. After
intravenous and intraduodenal dosing around 70% of the dose was
excreted in the bile indicating that initial absorption after oral
administration may be higher. Around 20% of the oral dose of
febantel was detected in the urine of sheep over a 4-day period
after dosing (Anon. 1987). In the cow, metabolites of febantel were
excreted in the milk after oral doses (Delatour et al., 1983a). The
liver and to a lesser extent the kidney were target tissues for
febantel-related metabolites in the rat, sheep and cattle (Bayer,
1987).
2.1.2 Biotransformation
In vivo, febantel undergoes cyclization to fenbendazole which
is interconvertible with oxfendazole. In addition, oxidation at the
sulfur atom in febantel yields the sulfoxide which can undergo
hydrolytic cleavage and cyclisation to produce oxfendazole. A total
of 9 metabolites has been identified in the rat. Sheep were given
25 mg/kg b.w. and the urine collected. A similar metabolic pattern
was noted in the two species, and oxfendazole and fenbendazole were
identified along with the other metabolites including the p-hydroxy
derivative of fenbendazole (Anon. 1987; Delatour et al., 1983b;
Delatour et al., 1982a). Similar findings were made in cattle (Anon.
1987). Fenbendazole and oxfendazole were identified in cow's milk
after oral dosing (Delatour et al., 1983a). It has been shown that
liver microsomes from various species can convert febantel to its
sulfoxide; sheep microsomes displayed the highest activity in this
respect. Similarly, microsomes from a number of species converted
fenbendazole to oxfendazole. The experiments together suggest that
the liver is responsible for the oxidative metabolism of both
febantel and fenbendazole (Montesissa et al., 1989).
A general metabolism scheme showing the metabolism of all 3
benzimidazole compounds considered by the Committee may be found on
page 1(note: to check).
2.2 Toxicological studies
2.2.1 Acute toxicity
Febantel was of low toxicity in the mouse, rat, and dog after
oral administration. It was somewhat more toxic to the rabbit by
this route (Table 1). It was of low acute toxicity to the rat
following inhalation exposure, and to the rabbit after dermal
exposure.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral > 10000 Muermann, 1975
Mouse M s.c. > 10000
Rat M oral 10605
Rat M&F inh. > 20001 Nelson et al., 1977
Rabbit F oral ca. 1250 Muermann, 1975
Rabbit M&F dermal > 20002 Nelson & Burke, 1977
Dog M&F oral > 10000 Muermann, 1975
1. 1 h exposure, µg/1
2. 24 h occlusive dressing
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 15 male and 15 female Wistar rats were given 0, 20,
50, and 125 mg/kg b.w./day febantel, 7 days per week by stomach
tube, for 3 months. Body weights and food consumption were measured
once a week, and clinical laboratory studies were conducted after 1
and 3 months.
No clinical signs were noted during the study and behaviour and
feed consumption were unaffected by febantel administration. Body
weights in all the groups were comparable with control values except
for males given 20 mg/kg b.w./day, which showed a higher rate of
weight gain. No compound-related deaths occurred during the study.
Haematology was normal at 1 and 3 months, as was clinical
biochemistry. There were no adverse findings following urinalyses.
At necropsy, no gross effects were noted. Relative and absolute
liver weights were increased in females given the highest dose;
relative liver weights were increased in corresponding males.
High-dose females had reduced thymus weights when compared with
controls. The only drug-related finding on histopathological
examination was fatty infiltration of the liver in several high-dose
rats. The no-effect level in this study was 50 mg/kg b.w./day
(Bomhard and Luckhaus, 1976).
2.2.2.2 Dogs
Groups of 3 male and 3 female pure-bred beagles were given
doses of 0, 20, 60, and 180 mg/kg b.w./day febantel in a gelatin
capsule 7 days per week for 13 weeks. Body weights were measured
weekly and body temperatures before the start of the study and at
weeks 3, 6, and 13. Neurological, haematological and ophthalmoscopic
examinations and electrocardiographic (ECG) investigations were
conducted at weeks 3, 6, and 13. Urinalyses were conducted at the
start of the study and at weeks 3, 6, and 13.
No signs of toxicity occurred in dogs given 20 and 60 mg/kg
b.w./day. Food consumption was reduced in some dogs given 180 mg/kg
b.w./day, and occasional diarrhoea and ataxia occurred. One animal
given this dose was killed on day 26 in a moribund state. This
animal was emaciated and had bleeding gums. It became ataxic and was
therefore sacrificed. Animals given 20 and 60 mg/kg b.w./day had
rates of body weight gain comparable with controls but in those
given the high dose, weight gain was depressed during the first 3
weeks. Temperatures in all dogs were comparable with those in
controls and no adverse effects were noted on ophthalmoscopic and
neurological examinations. ECGs were normal and no occult blood was
found on faecal examinations.
Haematologic examination showed no compound-related effects on
red blood cells in dogs given 20 mg/kg b.w./day but those given the
higher doses showed reductions in haematocrit, haemoglobin and
erythrocyte counts. Thrombocyte counts were unaffected. Leukopenia
was seen in 3/6, 1/6, and 6/6 dogs given 20, 60, and 180 mg/kg
b.w./day febantel. Differential blood counts revealed
granulocytopenia. There were no effects on clinical chemistry or
urinalysis values.
At necropsy, there were no apparent gross effects on dogs given
20 or 60 mg/kg b.w./day febantel but dogs given 180 mg/kg b.w./day
had red markings in the mucosa of the stomach and small intestine
with reddening of the mesenteric lymph nodes. There was atrophy of
the white splenic pulp in one animal. Testicular weights were
reduced at all 3 dose levels.
Histopathological examination revealed atrophy of the splenic
follicles in dogs given the highest dose and in one animal given
60 mg/kg b.w./day. Testicular hypoplasia was evident in all treated
groups but this was more pronounced in high-dose animals. No
compound-related changes occurred in the epithelia of the stomach,
oesophagus, or small intestine; there were no effects on mitoses in
the crypts (Machemer & Luckhaus, 1976).
As an extension of the study, groups of 3 male and 3 female
beagle dogs were given doses of 0, 5, and 10 mg/kg b.w./day febantel
in capsules. There were no signs of toxicity noted and haematology
was unaffected by compound administration. There was no excess
incidence of splenic or testicular effects in the treated dogs. The
no-effect level in this study was 10 mg/kg b.w./day (Machemer &
Luckhaus, 1976).
In a 52-week study, groups of 4 male and 4 female beagle dogs
were given diets containing 0, 40, 200, and 1000 ppm febantel
(equivalent to 0, 0.1, 5, and 25 mg/kg b.w./day). In this study,
body weights were recorded weekly, reflexes (corneal, pupillary,
patellar, extensor and reflexor) before the start of the study and
at weeks 4, 7, 13, 25, 39, and 52, and ophthalmoscopy at weeks 4, 7,
13, 25, 39, and 52. Haematology, biochemistry and urinalysis were
also conducted at these time points. Body temperature and pulse
rates were also measured during the study.
Animals given up to 200 ppm dietary febantel exhibited no signs
of toxicity, and there were no effects on body temperature or pulse
rate in any of the treated groups. Animals given the highest dietary
level showed a much reduced feed intake (approximately 75% of
control values). Body weights of males were significantly reduced
and female dogs showed a reduction in the rate of weight gain. One
dog in the high dietary level group showed a marked reduction in
body weight (3.5 kg at week 39) and was found to have opacification
of the lenses.
Three of the 8 dogs given the highest dietary level died during
the course of the study (one accidentally); there were no deaths in
any of the other groups. There were no haematological effects in the
animals given up to 200 ppm febantel. However, in high-level
animals, particularly in the three which died, there were reductions
in haematocrit, haemoglobin and erythrocyte values. The thrombocyte
count was markedly lowered in one of these animals. There was also a
marked reduction in leucocyte counts at the 1000 ppm level. This was
characterized by a significant reduction in granulocytes.
Biochemical studies revealed significant increases in the
activities of hepatic enzymes indicative of some degree of liver
damage, at the highest dietary level. There were no effects on
urinalyses.
At necropsy, there were significant reductions in testicular
and prostate weights in high-level males. Histological examination
revealed drastic reductions in leukopoiesis and erythropoiesis in
the marrow of high-level animals. Lymphofollicular atrophy of the
lymph nodes (including the spleen) occurred in these dogs.
Myocardial necrosis occurred in one high dietary level dog and this
was considered by the authors to be secondary to hypoxemia due to
the effects on blood elements. Centrilobular and panlobular
degeneration of the liver were noted in dogs given 1000 ppm.
Testicular and prostatic hypoplasia were evident in high-level
males. The no-effect level in this 52-week study in the dog was
200 ppm in the diet, equivalent to 5 mg/kg b.w./day (Hoffmann &
Nash, 1983).
2.2.3 Long term/carcinogencity studies
2.2.3.1 Mice
In a combined chronic toxicity and carcinogenicity study,
groups of 60 male and 60 female NMRI mice were given diets
containing 0, 50, 200, and 800 ppm febantel, equal to 0, 10, 42, and
170 mg/kg b.w./day for males and 0, 15, 58, and 250 mg/kg b.w./day
for females, for up to 21 months. From these groups 10 male and 10
female mice were sacrificed after 1 year.
There were no effects on mortality attributable to febantel
intake, nor were there any effects on appearance or behaviour. Feed
and water intake values in treated mice were comparable with
controls. Body weights of treated males were similar to controls but
in females body weights of mice given the highest dietary level were
slightly but significantly lower than control values in the first 11
months of the study. After 11 months, the body weights of the
high-level animals became comparable with those of controls.
Haematological studies revealed no effects in mice given up to
200 ppm febantel in the diet. However, males given the highest
dietary level had significant increases in the platelet count and
significant decreases in mean corpuscular haemoglobin contents.
Females were found to have lower haematocrit values and, at the end
of the study, reductions in erythrocyte counts. Blood biochemistry
was investigated at 12 and 21 months. The activity of serum
aspartate aminotransferase was significantly decreased in all the
treated males and decreased in females given the low and high levels
of dietary febantel; the intermediate-level mice however had
increased activity of this enzyme. The significance of this is
unknown although the authors of the report believed it to be
toxicologically irrelevant. Alkaline phosphatase, serum alanine
aminotransferase, creatine kinase, total protein, bilirubin, urea,
cholesterol and glucose were unaffected. Serum creatinine was
lowered in all treated mice.
There were no gross abnormalities which could be attributed to
compound intake in mice which died during the study, in those killed
in a moribund condition, in the groups killed at 12 months nor in
those sacrificed at termination.
The only effects on organ weights were those noted in the liver
in animals given the highest dietary level. There were significant
increases in absolute and relative liver weights at 12 and 21 months
in these animals.
At histopathological examination, the only adverse finding was
an increased fat accumulation in the livers of high-level female
animals. There was no increase in the incidence of any benign or
malignant tumour type (Bomhard & Kaliner, 1985).
2.2.3.2 Rats
In a combined chronic toxicity and carcinogenicity study,
groups of 100 male and 100 female Wistar rats were fed diets
containing 0, 20, 100, and 500 ppm febantel, equal to 0, 2, 8, and
40 mg/kg b.w./day, for an eleven-week period prior to mating, during
a 20-day mating period, and during the subsequent lactation period
(up to 33 days). A total of 60 male and 60 female pups from each
dose group was then given the same dietary level as the respective
parental animals for up to 30 months. Groups of 10 male and 10
female rats from each group were sacrificed after 65 weeks.
There were no effects on appearance, behaviour, mortality, or
food and water intake, and no effects on body weight at levels of up
to 100 ppm febantel. However, the highest dietary level depressed
body weights in parental animals and in their young. Litter size was
reduced significantly at birth, 7 days, and at week 3 in animals
given the highest level. Levels of up to and including 100 ppm had
no effects on pup weight but pups in the 500 ppm level group had
significant reductions in weight when compared with controls at
birth, 7 days and week 3. Body weights of these animals remained
reduced throughout the course of the study. There were no effects on
mortality in any of the treated groups.
No haematological abnormalities were noted at week 27, 53, 106,
or 132 in animals given up to 100 ppm febantel. In animals given the
highest level, mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentrations were reduced, sometimes significantly, at
all 4 examination points, in male and female rats. There were no
cytological differences, however, between control and treated
animals.
Clinical chemistry revealed significant increases in alkaline
phosphatase activities in males and females given the highest level
of febantel.
Gross examination of rats which died during the study, in those
subject to interim sacrifice at week 65 and in animals killed at
termination revealed no abnormalities. There were significant
increases in liver weights, both absolute and relative, at
termination in males and females given the highest dietary level,
and in relative liver weights at week 65 in males and females.
Histopathological examination of rats sacrificed at week 65
revealed fatty vacuolation of the liver in animals given the highest
dietary level as the only non-neoplastic finding. Similar
observations were made at terminal sacrifice. There was no increased
incidence of any tumour type in any of the treatment groups.
However, only animals given the highest level and controls were
subject to full histopathology; rats given the other doses had only
the lungs, liver and kidneys examined histologically. The NOEL for
non-neoplastic effects was 100 ppm, equal to 8 mg/kg b.w./day
(Bomhard & Mager, 1987).
2.2.4 Reproduction Studies
2.2.4.1 Rats
A two-generation study was conducted in accordance with OECD
Guidelines (Guideline 416). Groups of 25 male and 25 female rats
were given dietary febantel at levels of 0, 20, 100, and 500 ppm,
equivalent to 0, 2, 10, and 50 mg/kg b.w./day. The F0 generation
animals were fed the diets for 100 days prior to mating. Rats were
fed febantel-containing diets throughout the study period, including
the period of gestation and rearing of the young.
The F0 animals were mated to produce F1a and F1b
generations. The F1b rats were mated to produce F2a and F2b
generations. All the F0 and F1b females were necropsied 1-3
weeks after weaning. All the F1b young not required for mating and
all the F2b young were also necropsied when approximately 4 weeks
old.
Treatment of the F0 animals had no effects on appearance,
behaviour or coat quality at any of the dietary levels used. Females
in all treatment groups gained weight at the same rate as controls,
as did males given up to 100 ppm. However, high-level F0 males
were lighter at all times than corresponding controls. There were no
effects on food intake. In the F1a and F1b generations there
were no effects on food intake. In the F1a and F1b generations
there were no effects on insemination index, fertility index,
gestation index, gestation period or on the numbers of successfully
mated females. The male:female ratio was unaffected, and the total
number of young at each dietary level was similar. Litter size was
slightly reduced in both F1 generations at the highest dietary
level. The viability of young at the highest level was also reduced
in both F1 generations. When pup weights were measured at birth
and four weeks after birth, these were lower in young from the
highest dose level and also in the 100 ppm group 4 weeks after
birth. There was no excess incidence of malformations in any of the
treated groups when compared with controls.
In the F1b generation, the only effects noted were reductions
in body weights in males given the highest level of febantel in the
diet, and reductions in body weights in females given 100 and 500
ppm. There were no effects on feed consumption. The insemination
index, fertility index, gestation index, gestation period and the
numbers of successfully mated females were unaffected by compound
treatment. The numbers of young at birth were slightly but
significantly reduced in the F2a and F2b generations after 100
and 500 ppm but the male:female ratios were unaffected at any of the
dietary levels. The number of young surviving lactation was slightly
reduced at 500 ppm in the F2b generation.
Mean body weights were significantly reduced in both F2
generations at the highest dietary level. The incidence of
malformations was not increased at any treatment level.
Necropsy of F0 animals revealed pale livers at 100 and 500
ppm. All F1b and F2b animals sacrificed at 4 weeks of age were
necropsied. Discoloured livers were noted at 100 and 500 ppm. At
histopathological examination, hepatocellular hypertrophy was noted
in the livers of F0 rats given 100 and 500 ppm febantel. Fatty
degeneration of the liver occurred in F1b rats at 500 ppm.
Glycogen deposition was seen in the livers of F2b animals given
100 and 500 ppm. There were no effects on organ weights of F1b
animals (liver, kidney, testes and ovaries) given up to 100 ppm
febantel but animals given 500 ppm had decreased absolute kidney
weights and increased relative liver weights.
This study therefore revealed no teratogenic effects at levels
of up to 500 ppm dietary febantel. However, parental animals given
the highest dose displayed lowered body weights. Litter size and
viability of young were reduced at the highest level in the F1
generation. Similar effects were noted in the F2 generation at the
high dietary level. Mean body weights were significantly reduced at
the highest level. The no-effect level for reproductive performance
was 100 ppm, but taking into account hepatic hypertrophy and fat and
glycogen deposition, the no-effect level in this study was 20 ppm,
equivalent to 2 mg/kg b.w./day (Eiben, 1985).
2.2.5. Special studies on genotoxocity
Febantel was not mutagenic in the Ames test; it did not induce
DNA repair in a bacterial test and was negative in an in vivo
cytogenetics assay. It did not increase the incidence of
micronucleated polychromatic erythrocytes in the mouse (Table 2).
However, it did give positive results in the mouse dominant lethal
test.
Two metabolites of febantel, fenbendazole (see also
corresponding monograph) and
2-amino-5-phenylsulfinyl-2-benzimidazole (the 2-amino derivative of
fenbendazole) were also tested for genotoxic potential. The results
are given in Table 3. Oxfendazole has been tested and is shown at
2.2.5 in the oxfendazole monograph in this volume. Both compounds
were negative in the Ames test and in tests for DNA repair in
primary rat hepatocytes. However, fenbendazole produced a positive
response in the presence, but not in the absence, of rat liver S9.
Benzimidazoles are a group of compounds which bind to tubulin
and may possibly disrupt mitosis. Benzimidazole itself is positive
in the Ames test (Davidse, 1977; Seiler, 1978). However, febantel as
a pro-benzimidazole is unlikely to be typical of the group except
under circumstances where conversion to fenbendazole, oxfendazole
and their metabolites is possible (see corresponding monographs).
Table 2. Results of genotoxicity assays on febantel
Test system Test object Concentration Results Reference
In vitro
Ames test1 S.typhimurium 0-5000 Negative Hoorn et al.,
TA1535, µg/plate 1980
TA1537,
TA1538, TA98,
TA100
Ames test1 S.typhimurium 0-5000 Negative Mourot, 1990
TA97a, TA98, µg/plate
TA100, TA102
DNA E.coli 0-5000 Negative Hoorn et al.,
repair1 pol A+, pol A- µg/plate 1980
In vivo
Cytogenetics Chinese 1000 mg/kg Negative Machemer,
assay hamster b.w. twice at 1975a
(marrow and 24 h interval2
spermatogonia)
Micro-nucleus Mouse (NMRI) 500 and 1000 Negative Machemer,
test mg/kg b.w. 1975b
twice at 24 h
intervals3
Dominant Mouse (NMRI) 500 and 2000 Positive4 Machemer &
lethal mg/kg Dycka, 1976
b.w./day twice
at 24 h
intervals
1. Both with and without rat liver S-9 fraction.
2. Animals killed and marrow harvested 6 and 48 h after second dose.
3. Animals killed 6 h after second dose.
4. No effects with lower dose but higher dose significantly reduced
fertility at 2, 4, 5 and 6 weeks of mating.
Table 3. Results of genotoxicity studies on fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole
Test Test Object Concentration Results Reference
System
Fenbendazole
Ames test1 S.typhimurium 10000 µg/plate Negative Rabenold &
TA1535, Brusick,
TA1537 1982a
Forward Mouse up to 62.5 Weakly Cifone &
mutation1 lymphoma µg/ml Positive2 Myrh, 1983a
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Rabenold &
hepatocytes Bruskick,
1982b
2-Amino-5-phenylsulfinyl-2-benzimidazole
Ames test1 S.typhimurium 1-10000 Negative Rabenold &
TA1535, µg/plate Brusick,
TA1537, 1982b
TA1538,
TA98, TA100
Forward Mouse up to 300 Positive2 Cifone &
mutation1 lymphoma µg/ml Myhr, 1983b
assay
DNA repair1 Primary rat 0.5-100 µg/ml Negative Myhr &
hepatocytes Brusick,
1982b
1. Both with and without rat liver S-9.
2. Positive in the presence, but not absence of metabolic activation.
2.2.6 Special studies on teratogenicity
2.2.6.1 Rats
Groups of 20-24 pregnant FB30 (Long Evans) rats were given
febantel as an oral suspension in 0.5% aqueous tylose solution, by
stomach tube at 0, 10, 30, and 100 mg/kg b.w./day on days 6-15 of
gestation. Animals were killed on day 20 of gestation and the
uterine contents examined.
No signs of overt toxicity occurred at any dose level, but
animals given the highest dose gained less weight than those in
other groups including the control group. The number of fertilized
animals was similar in each group, but the number of pregnant
animals in the high dose group at termination was much lower (33%)
than in the other groups (all 100%), indicating severe
embryotoxicity. The numbers of implantations, fetuses and
resorptions was similar for animals given up to 30 mg/kg b.w./day
and controls. However, in those given 100 mg/kg b.w./day, the number
of implantation sites was similar to control values, but the number
of resorptions was markedly increased. Fetal and placental weights
were also significantly decreased at the high dose level.
No teratogenic effects were noted at doses of up to 30 mg/kg
b.w./day. At 100 mg/kg b.w./day, retardation of bone development and
teratogenic effects including anophthalmia, dysplastic
microphthalmia and multiple deformities of the ribs and spine were
noted in 4/25 fetuses examined. Gross examination of one maternal
animal that died during treatment at termination revealed severe
damage to the gastrointestinal tract at 100 mg/kg b.w./day
(Machemer, 1975c).
A study in the rat (unspecified strain) showed febantel to be
teratogenic when given at 46, 67, and 89 mg/kg b.w./day on days 8-15
of gestation. Its sulfoxide was also teratogenic in the rat at 46
and 93 mg/kg b.w./day. The no-effect level for febantel was 22 mg/kg
b.w./day (Delatour et al., 1982a).
Studies with febantel and its metabolites fenbendazole and
oxfendazole suggested that the active metabolite responsible for
embryotoxic effects of febantel was oxfendazole; when febantel was
given to rats simultaneously with SKF 525A, an inhibitor of
microsomal oxidation, the embryotoxicity was abolished (Delatour
et al., 1982a; Delatour et al., 1982b; Delatour, 1983; see also
accompanying monographs on fenbendazole and oxfendazole).
These studies show that febantel is embryotoxic and teratogenic
in the rat; the lowest no-effect level noted was 22 mg/kg b.w./day.
2.3 Observations in humans
No data were available.
3. COMMENTS
The toxicological data considered by the Committee included the
results of studies on metabolism, of acute and short-term studies
and of studies of carcinogenicity, genotoxicity, reproduction, and
development.
The main route of metabolism in rats, sheep, and cattle appears
to be cyclization to yield fenbendazole. Oxidation at the sulfur
atom can also occur to yield the sulfoxide which then undergoes
cyclization to give oxfendazole. Both fenbendazole and oxfendazole
can then undergo further metabolism.
In a study in rats in which febantel was given by gavage at
doses of up to 125 mg/kg b.w./day for 7 days, the only drug-related
effect was fatty infiltration of the liver. The NOEL was 50 mg/kg
b.w./day. Dogs were given febantel daily in gelatin capsules for 13
weeks at doses of up to 180 mg/kg b.w./day. Dogs given 60 and
180 mg/kg b.w./day showed testicular hypoplasia; and reductions in
haematocrit, haemoglobin, and erythrocyte counts. Leukopenia was
noted in all treated groups although the effect was not
dose-related. Agranulocytosis was noted in the high dose dogs, and
one high dose animal out of six also had splenic atrophy. In an
extension of the study using doses of up to 10 mg/kg b.w./day, no
haematological, splenic, or testicular effects were seen. The NOEL
was 10 mg/kg b.w./day. In a 52-week study in dogs given febantel at
levels of up to 1 g/kg in the diet (equivalent to 25 mg/kg
b.w./day), reductions in haemotocrit, haemoglobin, and erythrocyte
counts occurred at the highest dietary level. Testicular and
lymphofollicular (including splenic) atrophy also occurred at this
dietary level. The NOEL was 200 mg/kg in the diet, equivalent to
5 mg/kg b.w./day.
A carcinogenicity study was conducted in mice, which received
febantel in the diet at levels of up to 170 mg/kg b.w./day for males
and 250 mg/kg b.w./day for females for up to 21 months. There were
no changes in tumour incidence as compared with control values.
In a combined long-term toxicity and carcinogenicity study,
rats were given febantel in the diet at levels of up to 40 mg/kg
b.w./day during an 11-week period prior to mating, during a 20 day
mating period, and during lactation. A total of 60 male and 60
female pups from each dose group was then given the same dietary
level as the parents for up to 30 months. Females given the highest
dietary level showed reductions in mean corpuscular haemoglobin and
increases in absolute and relative liver weights. These animals had
fatty vacuolation of the liver at histopathological examination both
at week 65 after weaning and at the end of the study. There was no
increase in tumour incidence, but the Committee recognized that the
doses used may not have been sufficiently high for the carcinogenic
potential of this compound to have been adequately explored. The
NOEL for non-neoplastic effects was 8 mg/kg b.w./day.
In a two-generation study in which rats were given febantel in
the diet at up to 50 mg/kg b.w./day, litter size and viability of
the young were reduced at the highest dietary level in both
generations. The NOEL for reproductive performance was 10 mg/kg
b.w./day; however, if hepatic hypertrophy and glycogen deposition
were taken into account, the overall NOEL was 2 mg/kg b.w./day.
In a teratogenicity study in rats, severe maternal toxicity and
embryotoxicity occurred at the highest dose level. The NOEL was
22 mg/kg b.w./day. Teratogenicity was not seen in the absence of
maternal toxicity and embryotoxicity.
The genotoxic potential of febantel was investigated in a
number of test systems. Negative results were reported in two Ames
tests, in a test for DNA repair in bacteria, in an in vivo
cytogenetic test in the hamster, and in the mouse micronucleus test.
However, a positive result was found in the dominant lethal test in
the mouse. Two metabolites of febantel, fenbendazole and
2-amino-5-phenylsulfinyl-2-benzimidazole (the amino-derivative of
oxfendazole), also gave negative results in the Ames test and in a
test for DNA repair in primary rat hepatocytes, but both gave
positive results in the mouse lymphoma tk-locus genotoxicity assay
in the presence of metabolic activation, though not in its absence.
No studies of febantel in humans were available to the
Committee.
4. EVALUATION
The Committee noted that no carcinogenic effects were seen in
rats and mice; the significance of the positive results found with
the compound and its metabolites in a small number of tests for
genotoxic potential was therefore unknown. The major effects were
those noted in the 13- and 52-week studies in dogs, in the
teratogenicity studies in rats, where severe maternal toxicity and
embryotoxicity were noted, and in the two-generation study in rats,
which was the most sensitive study and gave a NOEL 2 mg/kg b.w./day.
The Committee established a temporary ADI of 0-10 µg/kg b.w.
for febantel using a safety factor of 200. The ADI was made
temporary because of the inadequate dosing in the rat
carcinogenicity study.
Even though a temporary ADI was established, it was not used
for recommending MRLs. Before the toxicological issues relating to
this compound can be resolved, additional information on its
genotoxic and carcinogenic potential will have to be provided
(see Summary section on the benzimadozoles).
5. REFERENCES
BAYER (1987) Residue studies synopsis. Febantel 45.5% oral paste.
Unpublished report from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & LUCKHAUS, G. (1976) BAY 5757. Toxicological studies in
rats. Studies with oesophageal probe application over 3 months.
Unpublished report No. 5823 from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
BOMHARD, E. & KALINER, G. (1985) BAY Vh 5757. Chronic toxicity and
carcinogenicity studies in mice. 21-Month feeding study. Unpublished
report No. 13961 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
BOMHARD, E. & NAGER, H. (19875) BAY Vh 5757. Combined chronic
toxicity and carcinogenicity study on Wistar rats. Unpublished
report No. 15844 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983a) Mutagenicity evaluation of BAY L
5156 in the mouse lymphoma forward mutation assay. Unpublished
Project No. 20989 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
CIFONE, M.A. & MYRH, B.C. (1983b) Mutagenicity assay of BAY K 7648
in the mouse lymphoma forward mutation assay. Unpublished Project
No. 20989 from Litton Bionetics Inc., Kensington, MD, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
DAVIDSE, L.C. (1977) Mode of action, selectivity and mutagenicity of
benzimidazole compounds. Neth. J. Plant Pathol., 83 (Suppl.1):
135-144.
DELATOUR, P. (1982) Some aspects of the teratogenicity of veterinary
drugs. Vet. Res. Commun., 7: 125-131.
DELATOUR, P., DANDON, M., GARNIER, F. & BENOIT, E. (1982a)
Metabolism-embryotoxicity relationship of febantel in the rat and
sheep. Ann. Rech. Vet., 13: 163-170.
DELATOUR, P., YOSHIMURA, H., GARNIER, F. & BENOIT, E. (1982b)
Embryotoxicity compared with metabolites of oxfendazole. Rec. Med.
Vet., 158: 369-373.
DELATOUR, P., GARNIER, F. & BENOIT, E. (1983a) Kinetics of four
metabolites of febantel in cow's milk. Vet. Res. Commun., 6:
37-42.
DELATOUR, P., TIBERGHIEN, M.P. & BESSE, S. (1983b) An HPLC procedure
for the quantification of five metabolites of febantel in sheep
serum. J. Vet. Pharmacol. Therap. 6: 233-235.
DELATOUR, P. & PARISH, R. (1986) Benzimidazole anthelmintics and
related compounds: Toxicity and evaluation of residues. In: Rico AG
(Ed). Drug Residues in Animals, London Academic Press, 175-204.
EIBEN, R. (1985) BAY Vh 5757. 2-Generation study in rats.
Unpublished report No. 13967 from Bayer AG. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
HOFFMAN, K. & NASH, G. (1983) BAY Vh 5757. Chronic oral toxicity in
dogs (12-month feeding test). Unpublished report No. 11593 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
HOORN, A.J.W., JAGANNATH, D.R. & BRUSICK. D.J. (1980) BAY Vh 5757.
Febantel. Mutagenicity evaluation HE100, PI.571201, 100% in the Ames
Salmonella/microsome plate test and DNA repair test. Unpublished
Project No. 20998 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975a) BAY Vh 5757. Orientational cytological studies
in bone marrow and spermatogonia of Chinese hamsters to test for
mutagenic effects. Unpublished report No. 5460 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975b) BAY Vh 5757. Micronucleus test in mice for
evaluation of mutagenic effects. Unpublished report No. 5729 from
Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MACHEMER, L. (1975c) BAY Vh 5757. Studies for embryotoxic and
teratogenic effects in rats after oral application. Unpublished
report No. 5644 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & LUCKHAUS, G. (1976) BAY Vh 5757. Sub-chronic toxicity
test on dogs with oral application (13 week experiment). Unpublished
report No. 6422 from Bayer AG. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
MACHEMER, L. & DYCKA, J. (1976) BAY Vh 5757. Dominant lethal test on
male mice to test for mutagenic effects. Unpublished report No. 6031
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MONTESISSA, C., TRACCIARI, J.M., FADINI, L. & BERETTA, C. (1989)
Comparative microsomal oxidation of febantel and its metabolite
fenbendazole in various animal species. Xenobiotica, 19: 97-100.
MOUROT, D. (1990) Febantel. Ames Test. Unpublished report from
Centre National d'Etudes Vétérinaires et Alimentaires. Submitted to
WHO by Centre National d'Etudes Vétérinaires et Alimentaires,
Fougčres, France.
MUERMANN, P. (1975) BAY Vh 5757. Acute toxicity in mice, rats,
rabbits and dogs. Unpublished report No. 5378 from Bayer AG.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY h 5156 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
MYHR, B.C. & BRUSICK, D.J. (1982a) Evaluation of BAY k 7648 in the
primary rat hepatocyte unscheduled DNA synthesis assay. Unpublished
project No. 20991 from Litton Bionetics Inc., Kensington, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
NELSON, D.L. & BURKE, M.A. (1977) Acute dermal toxicity of BAY Vh
5757 - Drug substance. Unpublished report from Chemagro Agricultural
Division, Mobay Chemical Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
NELSON, D.L., ANDERSON, R.H. & HOSS, H.E. (1977) The acute
inhalation toxicity of BAY Vh 5757 technical to rats. Unpublished
report from Chemagro Agricultural Division, Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982a) Mutagenicity evaluation of BAY
L 5156 Batch 810030 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionetics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RABENOLD, C. & BRUSICK, D.J. (1982b) Mutagenicity of BAY k 76485156
Batch 810032 in the Ames Salmonella/microsome plate test.
Unpublished project No. 20988 from Litton Bionectics Inc. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
SEILER, J.P. (1978) The mutagenic mode of action of benzimidazole.
Experientia, 34: 851-853.
SHARMA, S. & ABUZAR, S. (1983) The benzimidazole anthelmintics -
chemistry and biological activity. Prog. Drug. Res., 27: 85-161.
WOLLWEBER, H., KOLLING, H., WIDDIG, A., THOMAS, H., SCHULZ, H-P. &
MURMANN, P. (1978). Febantel, a new broad spectrum anthelmintic.
Arzneim Forsch., 28: 2193-2195.
