
TYLOSIN
First Draft prepared by
Dr.F.X.R. van Leeuwen
Toxicology Advisory Centre, National Institute of
Public Health and Environmental Protection
Bilthoven, The Netherlands
1. EXPLANATION
Tylosin is a macrolide antibiotic produced by a strain of
Streptomyces fradiae. The compound is active against most
gram-positive bacteria, mycoplasma and certain gram-negative
bacteria. The antibiotic is used in animal feed and veterinary
medicine. The chemical structure of tylosin, and of certain of its
metabolites, is shown at Figure I.
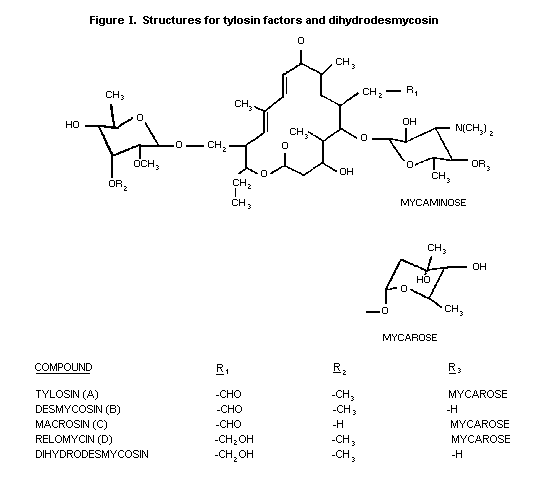 Tylosin was evaluated at the 12th meeting of the Joint FAO/WHO
Expert Committee on Food Additives in 1968 (Annex 1, reference 17).
No ADI was established. It was concluded that tylosin used in animal
feed or in veterinary medicine should not give rise to detectable
residues in edible products of animal origin; when using the
recommended methods of analysis it will be possible to ensure that
residues in meat for human consumption not exceed 0.2 ppm. Since
that time additional data have become available which are summarized
and discussed in this monograph addendum (Annex 1, reference 17).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Fasted rats received a single oral dose of 50 mg/kg b.w.
tylosin base or tylosin tartrate. Tylosin activity was assayed in
serum after 15 and 30 minutes and 1, 2, 4, 5, 7, and 24 hours after
treatment. Peak serum levels < 1.0 mg/l were seen after 1-2
hours. Within 7 hours serum levels decreased to less than the limit
of detection (i.e. 0.10 mg/l, microbiological assay). Four rats were
given i.p. 100 mg/kg b.w. tylosin base. Bile samples were collected
for 2 hours. The bile/serum concentration ratio ranged from 143-266
(Anderson et al., 1966).
Rabbits received i.m. 10 mg/kg b.w. tylosin base as a 5%
aqueous solution acidified with hydrochloric acid to pH 5.5. Serum
levels were determined after 1.5, 4, 7, and 24 hours. Peak serum
levels ranging from 0.57 to 0.88 mg/l were observed after 1.5 hours.
A similar study was carried out using tylosin tartrate in aqueous
(25 mg/kg b.w.) as well as in PEG-200 (10 mg/kg b.w.) solutions.
Peak serum levels at 1.0 hour were 4.7-7.2 and 0.96-1.25,
respectively. Within 24 hours serum levels were below the limits of
detection, which were 0.05 mg/l for tylosin hydrochloride and
0.10 mg/l for tylosin tartrate.
Two dogs given 25 mg/kg b.w. tylosin base orally in capsules
excreted 2% of the dose in the urine within 5 hours. Serum
concentrations were very low, < 0.05 and 3.3 mg/l at 2.5 hours
(microbiological assay). In another study groups of 8 dogs received
orally by capsule 1, 10, or 100 mg/kg b.w./day for 8 days. Blood
levels determined 2 hours after the last dose ranged from
< 0.15 mg/ml to 9.5 mg/ml mostly ranging with the dose. Two dogs
received 25 or 100 mg/kg b.w. tylosin base orally by capsule daily
during 29 days. Serum levels were determined 0, 1, 2, 3, 4, 5, 6,
and 7 hours after the 1st, 15th, and 29th dose. At 25 mg/kg b.w/day
peak serum levels (1.4-2.7 mg/l) were seen 2 hours after dosing and
at 100 mg/kg b.w./day the highest levels (2.7-4.6 mg/l) were seen
2-5 hours after dosing. No accumulation was observed. One dog given
i.v. 10 mg/kg b.w. tylosin base (dissolved in a minimal amount of
hydrochloric acid) excreted a total of 25.2% of the activity in the
urine. During 5 hours after dosing 13.7% of the dose was recovered
from bile. The bile/serum concentration ratio varied from 1230 to
3780. Four dogs were administered 10 mg/kg b.w tylosin base i.v. (as
an aqueous solution acidified with dilute hydrochloric acid). Blood
t´ was calculated as 48 min. Urinary recovery was 18.8% of the
dose during 6 hours after dosing (15.7% within 2 hours). Serum
levels of tylosin in 4 dogs receiving 25 mg tylosin base
intraduodenally at 0.25, 0.5, 1, 2, 3, 4, and 5 hours averaged 0.78,
1.98, 1.77, 1.94, 0.56, 0.29, and 0.13 mg/l, respectively. Urinary
recovery was 7.2% of the dose in 5 hours (Anderson, et al., 1966).
Thirty mg/kg b.w. tylosin tartrate was administered orally by
gavage or intravenously to groups of pigs (5/group, 30-days old). At
38 days of age the treatments were crossed over. Tylosin activity
was measured in blood samples taken at 10 intervals up to 24 hours
after treatment. After oral administration tylosin activity was
present in plasma 10 minutes after dosing with a peak concentration
of 2.4 mg/l at about 1.5 hours. By comparing the areas under the
curve of the tylosin concentration in blood following the 2 routes
of administration a biological availability of 22.5% was determined
(Shionogo & Co. Ltd., 1981).
Tylosin at 110 mg/kg b.w. (as the granulated phosphate) was
orally administered to 3 male and 3 female pigs. Tylosin activity
was assayed in blood samples taken up to 24 hours after dosing.
Serum activity peaked 1 hour after dosing (average 17.81 mg/l); 24
hours after dosing tylosin was not detectable (< 0.1 mg/l) (Van
Duyn & Kline, undated).
Six pigs (weight 56 kg) were orally given 50 mg/kg b.w. tylosin
phosphate in water. Blood and tissue samples were taken at intervals
up to 24 hours. Tylosin levels were detected in serum from 10
minutes to 8 hours after dosing and peaked at 1 hour (8.53 mg/l).
Tylosin was widely distributed in the body with tissue
concentrations in liver and kidney peaking at 1 hour. No activity
was found in the brain or spinal cord. Highest activity was found in
bile and urine (Nakamura et al., 1969).
Young calves (weight range 44.4-59.0 kg) were injected with
10 mg/kg Tylan 200 either subcutaneously or intramuscularly. The
rate of tylosin absorption, time to peak concentration and decline
of serum concentrations were very similar via both routes of
administration (Thomson, undated a).
Groups of calves (average weight 60 kg) received 10 mg
tylosin/kg b.w. subcutaneously or intramuscularly {as formulations
of tylosin tartrate in water, tylosin tartrate in propylene glycol
and water and tylosin base in propylene glycol and water (Tylan 200
injection)}. Another group of calves received the same dose (as
Tylan 200 injection) intravenously. Tylosin activity in serum was
measured in blood samples taken at various intervals after
treatment. Absorption of the formulations containing tylosin
tartrate was faster after subcutaneous and intramuscular
administration than absorption of the formulation containing tylosin
base (Thomson, undated b).
Holstein calves (1-3 weeks, 38-56 kg) were given 1 or 1 to 5
daily intramuscular injections of 17.6 mg tylosin/kg b.w. (as Tylan
200). In all experiments tylosin levels in serum peaked 2 hours
after administration (average 2.0 mg/l) decreasing to about 0.1 mg/l
at 36 hours. Peak lung tissue levels were observed at 6 to 24 hours
post injection varying from 12.6 to 15.7 mg/l. Lung tissue levels
were greater than serum levels and still detectable (2.2 mg/l) at 48
hours after administration (microbiological assay) (Van Duyn &
Folkerts, 1979; Van Duyn & Johnson (undated a); Van Duyn & Handy
(undated)).
A serum half-life of 1.62 hours was established in cows given a
single i.v. injection of 12.5 mg tylosin/kg b.w. (as Tylan 200). The
apparent specific volume of distribution was 1.10 l/kg indicating no
specific accumulation (Gingerich et al., 1977).
Neonatal holstein calves with a natural occuring pneumonia were
treated intramuscularly with 17.6 mg tylosin/kg b.w. (as Tylan 200)
daily for 3 consecutive days. Healthy calves were subjected to the
same treatment. Six hours following the last dose all the calves
were sacrificed and tylosin activity was measured in the lungs.
Tylosin distributed equally into both normal and pneumonic lung
tissue (Thomson, undated a).
Groups of neonatal healthy calves were fed a milk replacer
containing 1.0 g tylosin (as tylosin tartrate) for 4, 7, and 10
days. Serum samples were taken 4 hours after each dose; the animals
were killed after the final sample had been taken and lung tissues
were analyzed. Mean serum and lung tissue levels were 0.41, 0.37,
and 0.42 mg/l, and 1.76, 3.16, and 3.17 mg/l for the 4, 7, and
10-day treatment groups, respectively. Lung/serum tylosin ratios
were 7.24, 9.36, and 14.01, respectively (Buck et al., undated).
Groups of 4 calves (weight 250 kg) received intramuscular
injections with 10 mg tylosin/kg b.w. (as Tylan 200) for 5 days. The
calves were killed 2, 4, 6, 12, or 72 hours after the last
injection. Tylosin activity was measured in serum and lungs. Tylosin
activity in serum was highest at 4 hours after treatment (1.3 mg/l)
and was no longer detectable after 72 hours using a microbiological
assay whose detection limit was 0.05 mg/l. The mean tylosin activity
in the lungs was 5.9, 5.0, 6.6, 4.4, and 0.6 mg/l at 2, 4, 6, 12,
and 72 hours after treatment, respectively (Lilly, undated a).
Four broiler chickens (weight 720 g) were given a single dose
of 50 mg tylosin/bird (as tylosin tartrate) by stomach intubation.
Tylosin activity was detected in serum after 0.5 hours and peak
concentrations of 0.6-4.0 mg/l were found after 2 hours, declining
to negligible after 24 hours. Repeated oral doses of 50 mg tylosin
to chickens (weight 2 kg, dosed at 1, 2, and 3 hours) caused serum
peak levels at 4 hours (about 0.28 mg/l) declining to negligible at
24 hours (Lilly, undated b, undated c).
Groups of 6 chickens (5-7 weeks old, surgically prepared)
received orally 25, 100 or 250 mg tylosin/kg bw (as tartrate). Urine
and faeces were collected during 72 hours. Peak tylosin levels in
urine (< 100 mg/l at the 25 mg/kg dose and > 1400 mg/l at the
250 mg/kg dose) occurred 2-4 hours after dosing and declined rapidly
thereafter. Peak levels in faeces occurred at 8 hours and varied
from 300 to 2000 µg/g with the dose (Lilly, undated d)
2.1.2 Biotransformation
Four male rats, preconditioned on unlabelled tylosin (10 mg/kg
b.w.) for 3 days, received daily during 5 days by gavage 2 ml of a
solution containing 14C-labelled (in lactone ring) tylosin base.
The rats were killed 4 hours after the last dose; 99% of the
radioactivity was excreted in the faeces and 1% in the urine. The
greatest part of the excreted residues was found to be tylosin
factor A, tylosin factor D and dihydrodesmycosin. Less than
0.25 mg/kg total 14C-residue was found in liver and kidney (Sieck
et al., 1978b).
A male pig, preconditioned on feed containing 110 mg/kg
unlabelled tylosin for 2 weeks, received for 3 days feed with
110 mg/kg of feed tylosin base 14C-labelled in the lactone ring.
Four hours after the last dose the pig was killed. The radioactivity
was excreted 99% in the faeces and 1% in the urine. The majority of
the excreted residues (15% of the radioactivity in faeces was not
extractable) was found to be tylosin factor D (33%), tylosin factor
A (6%) and dihydrodesmycosin. At least 10 minor metabolites (< 5%
of activity) were present in the excreta. In liver and kidneys
< 0.25 mg/kg tylosin was found. At least 4 different metabolites
(of which one was detected as dihydrodesmycosin) were detected in
liver and kidneys (Sieck et al., 1978b).
Three pigs received twice daily for 4 days a ration containing
110 mg tylosin base/kg of feed 14C-labelled in the lactone ring. A
control pig received the basal diet throughout the experiment. Pigs
were sacrificed within 4 hours after the last dose. Total
14C-residues in liver and kidney were < 0.28 mg/kg and in
muscle and < 0.04 mg/kg in fat. Investigations by TLC of liver
residues revealed 5 or 6 metabolites. Two of the metabolites could
be identified as tylosin factor A and dihydrodesmycosin, each
representing about 5% of the total extractable liver residue. Mass
spectrometer analysis of the faeces identified 3 metabolites as
tylosin factor A, tylosin factor D and dihydrodesmycosin (Sieck
et al., 1978a; Sieck et al., 1980).
2.2. Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of tylosin formulations and of tylosin are
given in Tables 1 and 2, respectively.
Table 1. The acute toxicity of tylosin formulations
Species Sex Route LD50 LC50 Reference
(mg/kg b.w) (mg/l)
rat M&F oral > 5001 Gries et al.,
1985a
M&F oral > 0.52 > 1.054 Gries et al.,
1 hr > 0.65 1985b
M&F inhal Gries et al.
M&F inhal 1985c
rabbit M&F dermal > 20001 Gries et al.,
1985a
M&F dermal > 20003 Gries et al.,
1985c
M&F dermal > 2.02 Gries et al.,
1985b
1. administered as granulated tylosin concentrate, a formulation
containing 26.7% of tylosin base activity as the phosphate salt
2. ml/kg b.w. administered as undiluted tylan 200 injection
3. administered as tylan soluble, a dry granular formulation, also
known as tylosin tartrate
4. liquid droplet aerosol of tylan 200/injection formulation at
1.05 mg/litre for 1 hour
5. solid particulate aerosol of tylan soluble at 0.6 mg/litre.
Table 2. The acute toxicity of tylosin
Species Sex Route LD50 Reference
(mg/kg b.w.)
mouse F oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 492.51
- i.p. 594.12
- s.c. 13545
- s.c. 784.11
- s.c. > 25002 Gries et al.,
- i.v. 385.71 1983
- i.v. 581.73
- i.v. 588.84
- i.v. 588.95
F i.v. approx 3216
rat M oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 10011
- i.p. > 25005
- i.v. 6955 Gries et al.,
- s.c. 40831 1985c
- s.c. > 30005
M&F oral > 5005
dog M&F oral > 8002 Anderson & Worth,
undated
1. administered as tylosin phosphate
2. administered as tylosin base
3. administered as tylosin hydrochloride
4. administered as tylosin lactate.
5. administered as tylosin tartrate.
6. administered as 20 mg tylosin/ml of acidified sterile water for
injection, USP (2.0%)
After oral administration no deaths were recorded at the
highest dose used; dogs vomited at 800 mg/kg b.w. but not at
400 mg/kg b.w.; at both these doses the dogs salivated and
defecated. Intravenous and intraperitoneal administration caused
depression, prostration, convulsions and death or recovery within 24
hours.
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of rats (Harlan strain, 5 females/group) received daily
s.c. injections of 10, 20, 50 or 100 mg/kg b.w. tylosin base as a
suspension in 5% acacia gum for 1 month. No effects were seen on
food intake, body weight gain, adrenal weight and terminal blood
cell counts. Macroscopy and microscopy did not show abnormalities
(Anderson et al., 1966; study R2-58). Remark: Summary only.
Groups of rats (Harlan strain, 6/sex/group) received daily s.c.
injections of 100, 250, 500, or 1000 mg/kg b.w. tylosin tartrate or
2.5 ml/kg b.w. saline for 1 month. At doses > 250 mg/kg b.w.
diarrhoea was seen during the first week, regressing to soft stools
(occasionally seen at 100 mg/kg b.w. too) during the remainder of
the study. At doses > 250 mg/kg b.w. scarring and scabbing at the
injection site was seen (occasionally at 100 mg/kg b.w.). No effects
were observed on growth, haematology, organ weight, macroscopy and
microscopy (Anderson et al., 1966; study R19-59). Remark: Summary
only.
Groups of rats (Harlan Wistar, 15/sex/group, F1a offspring
from parents fed the same amount of tylosin base for about 10 weeks
prior to mating and during gestation and lactation) were fed diets
containing 0, 0.1, 0.5 or 1.0% tylosin base for 1 year. No
treatment-related effects were observed on mortality, growth, food
consumption, ophthalmoscopy, organ weights, macroscopy and
histopathology. Treated rats appeared moderately hyperirritable and
hyperactive after 7 to 12 months of treatment. An increase in
lymphocytes and a corresponding reduction in neutrophils were
observed in both sexes (significantly in females) at 0.5 and 1.0%. A
trend towards a slightly more alkaline urine occurred in females at
0.5% and 1.0%. The authors concluded that the NOEL was 1.0% tylosin
base, equivalent to 500 mg/kg b.w. However, the Committee concluded
that the NOEL in this study was 0.1% tylosin base, equivalent to
50 mg/kg b.w. (Broddle et al., 1978a).
2.2.2.2 Dogs
Groups of 8 dogs (2/sex mongrel dogs and 2/sex beagle dogs)
received orally by gelatin capsule during 2 years 0, 1, 10 or 100 mg
tylosin base/kg b.w./day. Groups of mongrel dogs (2/sex/group) given
200 or 400 mg tylosin base/kg b.w./day in capsules for 2 years or
longer were subsequently added. Salivation, vomiting and diarrhoea
were observed at 200 and 400 mg/kg b.w./day (at 100 mg/kg b.w./day 1
dog vomited once). Haematology, urinalysis and relative organ
weights did not reveal abnormalities. No changes were observed in
faecal microbiological flora. Liver and kidney function tests
revealed two dogs at 100 mg/kg b.w./day and 1 dog at 400 mg/kg
b.w./day with a transient increased BSP retention time. Macroscopy
and microscopy did not show compound-related changes except for mild
pyelonephritis seen in 1/4 dogs at 200 mg/kg b.w./day and bilateral
nephrosis, mild chronic pyelonephritis and mild cystitis seen in 1/4
dogs at 400 mg/kg b.w./day. Terminal bone-marrow counts were normal
(only measured in normal study). At dose levels > 10 mg/kg
b.w./day serum tylosin levels in blood could be detected. The NOEL
in this study was 100 mg/kg b.w./day (Anderson et al., 1966; study
D4-59). Remark: The limited details provided and the poor reporting
of the study made proper interpretation difficult.
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
In a limited study rats (3/sex/group) were fed diets containing
0, 0.1, 0.3, or 1.0% tylosin base for 17 months. In the 0.3% group 1
female died due to malnutrition. No effects on growth or on terminal
haematological parameters were seen. Relative organ weights revealed
changes in weights of ovaries and uteri due to thickening in uteri
and a decrease in size of the ovaries in 1/3, 3/3, 2/3, and 2/3
female rats at the 0, 0.1, 0.3, and 1.0% levels, respectively.
Macroscopy and microscopic examination revealed squamous metaplasia
of the uterine glands in 2 female rats at the highest dose (Anderson
et al., 1966; Study R9-58). Remark: Incomplete report.
Groups of about 25 male and female Harlan rats (total 213)
received 0, 0.001, 0.01 or 0.1 % tylosin base in their diet for 2
years. Survival was better in tylosin treated groups than in control
rats (54% and 30% respectively). No effects were seen on growth,
haematology, or relative organ weights. Macroscopy and microscopy
revealed an increased number of animals with fatty changes in livers
and kidney at all dose groups and a slight increased incidence of
bile duct proliferation at the 0.1 and 0.01% levels, but neither was
dose-related (Anderson et al., 1966; Study R10-58). Remark:
Incomplete reports of growth and haematology; limited
histopathology.
In another 2-year study groups of Harlan rats (30/sex/group)
were fed diets containg 0, 0.01, or 1.0% tylosin base. Survival was
better in rats fed tylosin than in control rats (57% and 29%
survival in high dose and control rats, respectively). No effects
were seen on growth, haematology, urinalysis, organ weights,
macroscopy and microscopy. A dose-unrelated increase of fatty
changes in liver and kidney was observed. (Anderson et al., 1966;
R3-59). Remark: Incomplete reports of growth, haematology and
urinalysis.
In a very limited study groups of 10 male and 10 female rats
were fed diets containing 0, 2, 5, 10, or 20% tylosin base for up to
2 years. No effects on food consumption, growth and haematology were
observed on rats at 2% and 5%. At the 10% level, growth and food
consumption were slightly reduced. At the highest dose food
consumption and growth were markedly reduced followed by death
(Anderson et al., 1966; R6-60). Remark: Incomplete reports.
Two replicate 2-year studies were carried out with Wistar rats
(40/sex/group in each study derived from F1a offspring from
parents fed diets containing tylosin from 10 weeks prior to mating
up to the time of weaning). The control groups (60/sex/group in each
replicate study) were derived from parents fed untreated diet.
Treated groups were fed diets containing 0.1, 0.5, or 1.0% tylosin
base. Observations included clinical signs, mortality, food
consumption, food efficiency, body weight, terminal haematology and
biochemistry, urinalysis, organ weights, macroscopy, and
histopathology. There were trends towards improved survival in
males, although overall survival was low (20% and 28% for the
control and treated groups, respectively), and increased food
consumption and body weight gain in both males and females in all
treated groups. At histopathology an increased incidence of
pituitary adenomas in males (but not in females) was observed. The
combined incidence of pituitary adenomas in males for both
replicates was 6/120 (5%) in the control group, 9/80 (11%) in the
low dose (0.1% tylosin) group, 18/80 (22.5%) in the mid dose (0.5%)
group, and 20/80 (25%) in the high dose (1.0%) group. The authors
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. The incidence of malignant tumours was unaffected in males or
in females (Gries, 1980)
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 7-8 male and 14-17 female ICR mice were fed diets
containing 0, 0.1, or 1.0% tylosin (composition unknown) for two
succesive generations with 2 litters/generation. Some of the mice
were maintained on an ordinary diet, but all the mice received the
experimental diet prior to delivery of the offspring. No
treatment-related effects were observed on reproductive performance
(sexual maturation, number of pups or weaning of pups) (Tsubura
et al., undated). Remark: Only a few summary tables were available.
2.2.4.2 Rats
A 3-generation reproduction study was performed with 2 groups
of 5 male and 10 female Harlan rats/group receiving 0 or 1.0%
tylosin base in their diet. Fertility, viability, gestation and
lactation indices in the F0 generation rats did not reveal any
abnormality. Growth was equal in all generations for both control
and treated rats. In each succeeding generation the capability for
reproduction and perpetuation was unaffected (Anderson et al., 1966;
R3-59). Remark: Incomplete report.
In a special study weanling Wistar rats (25/sex/group, 6-7
weeks old) were fed diets containing 0.1, 0.5, or 1.0% tylosin base
for 10 weeks prior to mating and thereafter for about 6 months
total. A control group consisted of 35 rats/sex. Only one litter was
bred. No effects were observed on parental body weight, food
consumption, reproductive indices (male or female fertility,
gestation length, number of live fetuses, mean litter weight or
offspring survival) or biochemistry. High dose male rats revealed
significantly decreased white blood cells at termination. Sera
collected from parental rats (approx. 150 days on experimental
diets) did not contain detectable levels of tylosin (< 0.1 mg/l).
Offspring were physically normal and were assigned to the one year
toxicity study with tylosin (see Section 2.2.2.1) (Broddle, et al.,
1978b). Remark: Only summarized data given on reproductive indices.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1. Mice
Groups of 10 pregnant mice (2 strains, CBA and A/Jax) received
orally 100, 500, or 1000 mg/kg b.w./day tylosin (composition not
given) in 0.1 ml water from days 7-12 of gestation. Two control
groups of 3 and 5 mice received saline or remained untreated,
respectively. Females were killed on day 18 of pregnancy. No effects
were observed on number of corpora lutea, number of implantations,
number of early and late deaths, number of embryos alive, or fetal
development (Tsuchikawa & Akabori, undated).
Four groups of mice given 0 or 500 mg/kg b.w. and a further 2
pregnant females (A/Jax x male CBA) per group, receiving orally 0 or
1000 mg tylosin/kg b.w./day during days 7-12 of gestation, were
allowed to deliver and rear their young for 4 weeks. No effects were
observed in growth, survival, or genital system of all mice born
determined up to 9 weeks. At 8 weeks after birth rearing ability,
hearing ability and kinetic functions were not effected. At the 9th
week the mice were killed and visceral and skeletal examinations
were performed. No abnormalities were seen (Tsuchikawa & Akabori,
undated).
2.2.5.2 Rats
Groups of 15 Wistar rats were fed diets containing 0.1, 1.0, or
10.0% tylosin (composition not given; equal to 60.5, 725, or
4800 mg/kg b.w./day, respectively) in their diet during days 1-20 of
gestation. A control group consisted of 10 rats. Females were killed
on day 20 of gestation. Observations included number of resorptions
and live and dead fetuses, sex ratio, fetal weight and external,
visceral and skeletal abnormalities. Fetus weight at the highest
dose was slightly decreased (Terashima, undated).
In another study 3 groups of 15 Wistar rats received 0, 1.0, or
10.0% tylosin (composition not given, equal to 0, 725, or 4800 mg/kg
b.w.) during days 1-20 of gestation. Normal delivery was allowed.
Number of fetuses, sex ratio, external abnormalities and growth
during the weaning period (3 weeks) were determined. Motor functions
and senses were examined. The weanlings were killed and visceral and
skeletal examinations were performed. A slightly reduced growth was
observed in weanlings at the highest dose (Terashima, undated).
2.2.6 Special studies on genotoxicity
Tylosin was tested for genotoxicity in an in vitro
chromosomal assay with Chinese hamster ovary cells, a mouse lymphoma
assay and an in vivo assay for cytogenetic damage. The results are
summarized in Table 3.
2.2.7 Special studies on microbiological activity
The anti-microbial activity of tylosin has been described in
the published literature. Tylosin is markedly active in vitro
against gram-positive bacteria, certain gram-negative bacteria and
mycobacteria; it is inactive against Enterobacteriaceae (McGuire
et al., 1961).
Table 3: Results of genotoxicity assays on Tylosin
Test system Test Concentration Purity Results Reference
object of substance
tested
Chromosome Chinese 500-1000 99.3 - Gries,
aberration hamster µg/ml2, 1990a
assay1 ovary 250-750
cells µg/ml3, both
in DMSO
Lymphoma Mouse 10-1000 µg/ml2 99.3 +5 Gries,
assay L5178Y 10-1000 µg/ml3 1990b
TK+/- (1000 µg/ml
cells toxic) both in
DMSO
Micronucleus ICR 2 daily doses 966 - Gries,
mice of 1250, 2500 1990c
or 5000 mg/kg4
1. Mitomycin C and cyclophosphamide, used as positive controls, yielded
positive results
2. without metabolic activation
3. with metabolic activation
4. the positive control cyclophosphamide yielded positive results.
5. positive at cytotoxic dose
6. administered as tylosin base
In recent studies the minimal inhibitory concentration (MIC) of
tylosin has been determined for bacterial pathogens isolated from
target animal species of European and North American origin since
1984. Additional information on sensitivity of bacterial isolates to
tylosin was obtained from literature published since 1980. Tylosin
was active against most Gram-positive bacteria and mycoplasmas
tested in vitro. Activity against Gram-negative bacteria was
generally lower. Tylosin was also found to be active against
isolates of Chlamydia psittaci. MIC values for streptocci,
enterococci and staphylococci are given in Table 4 (Herd, 1990).
Table 4. MIC1 values for tylosin against some Gram-positive bacteria
Organism MIC range Total No.
(mg/1) of isolates
Streptococcus pyogenes 0-1 - 0.2 5
Streptococcus pneumoniae 0.2 - 0.4 4
Streptococcus dysgalactiae 0.06 - 128 50
Streptococcus agalactiae 0.125 - 0.5 51
Streptococcus suis 0.125 - > 128 42
Streptococcus uberis 0.125 - > 128 53
Enterococcus faecalis 0.25 - > 128 31
Staphylococcus aureus2 0.125 - > 128 98
Staphylococcus Spp.3 0.78 - 1.0 7
1. Minimum inhibitory concentration
2. Coagulase - positive
3. Coagulase - negative
2.2.8 Special studies on neurotoxicity
Three cats received daily for 90 days subcutaneously 200 mg/kg
b.w. tylosin tartrate as a 20% solution divided in 2 doses during
the first 37 days and thereafter as a single dose (with addition of
sodium citrate for buffering). An untreated control group consisted
of 3 cats. Slight reduction (25-35%) was observed in the
post-rotatory nystagmus response, but the auditory response appeared
normal. All cats landed on their four feet when dropped from 1
metre. No ataxia was seen (Anderson, 1966; C2-59)
2.2.9 Special studies on skin and eye irritation
Granulated tylosin concentrate (2 g/kg b.w.) was not irritating
when applied to the rabbit skin but when the formulation (52 mg
(0.1 ml)) was instilled to the rabbit eye corneal dullness, slight
corneal opacity, slight to marked irititis and moderate
conjunctivitis were observed. The effects resolved within 14 days of
treatment (Gries et al., 1985a).
The formulation Tylan 200 injection (2 ml/kg b.w.) caused very
slight erythema to the rabbit skin and the formulation (0.1 ml)
caused slight conjunctival hyperemia to the rabbit eye both within
24 hours after application. No skin and eye irritation were observed
after 48 hours (Gries, et al., 1985b).
The formulation Tylan soluble (2 g/kg b.w.) caused very slight
erythema and slight desquamation to the rabbit skin. Slight to
moderate corneal opacity, marked iritis and moderate conjunctivitis
were observed following the instillation of 58 mg of the formulation
into the rabbit eye; these effects had cleared within a week (Gries
et al., 1985c).
2.2.10 Special studies on skin sensitization
Tylosin tartrate was used as a positive control in a
sensitization test by the method of Landsteiner and Jacobs conducted
for tilmicosin. Guinea-pigs (12) were administered 10 intracutaneous
injections of 50 mg/ml tylosin tartrate; a challenge with a further
dose was given after 14 days. At challenge a mild positive response
was observed (Jordan et al., 1989).
2.3. Observations in humans
Trials in human subjects have been conducted to investigate
antibiotic-resistant bacteria. In one trial 12 or 11 human
volunteers were given 20 mg tylosin per day or a placebo,
respectively, for up to 6 months. There was no significant change in
the total number of tylosin-resistant staphylococci and lactobacilli
in weekly faecal samples or in coliform or yeast forms whereas a
significant increase was observed in total number of resistant
streptococci. In another trial no tylosin resistant organisms were
detected in specimens (including faeces samples) from hospitalized
patients who had never had any exposure to the nonmedical antibiotic
tylosin. In both studies no regular pattern was observed in
cross-resistance to related antibiotics (Malin & Silliker, 1966)
In a Japanese trial 2 human volunteers were given 2 or 5 mg
tylosin (as 1 mg tablets; composition not given) per day for 3
months. Faeces were inspected for E. coli and Enterococci and
Staphylococci at intervals of 1 to 2 weeks from 2 months prior to
administration and 3 months from the start of administration. No
tendency towards increased resistance was noted (Kuwabara, undated).
Tylosin resistance was examined in human cultures of
Staphylococcus aureus, Streptococcus pyogenes and Campylobacter
spp. Of 3812 human cultures isolated between 1985 and 1987 only
1.0% were resistant to tylosin. There was no evidence for a
significant animal source of these resistant cultures (Lacey, 1988).
3. COMMENTS
The Committee considered toxicological data on tylosin,
including the results of studies on biochemical aspects,
mutagenicity, and microbiological activity. The Committee noted that
most of the toxicity studies had been carried out about 20 years
ago, had not been conducted according to current protocols, and were
poorly reported.
Following administration of tylosin by various routes, peak
serum levels in rats, dogs, pigs, and cattle were observed within
1-2 hours, and then declined rapidly. In pigs, about 22% was
bioavailable after oral administration. Excretion of tylosin was
rapid and largely in the bile.
After oral administration of radiolabelled tylosin to rats and
pigs 99% of the radioactivity was excreted via the faeces. Major
products identified in faeces were tylosin (factor A), macrosin
(factor C), relomycin (factor D), and dihydrodesmycosin. In pig
liver and kidney, only very small amounts of tylosin and
dihydrodesmycosin could be found.
Several short- and long-term studies in rats and dogs were
performed. In a 1-year study in rats, tylosin base was administered
in the diet at concentrations up to 10 g/kg of feed. A NOEL of
1 g/kg of feed, equivalent to 50 mg/kg b.w./day, was established,
based on haematological and urinary pH changes. In a study in dogs,
tylosin base was administered orally at dose levels up to 400 mg/kg
b.w./day for 2 years. At the two highest dose levels, salivation,
vomiting, and diarrhoea as well as mild pyelonephritis were
observed. The NOEL was 100 mg/kg b.w./day.
In two replicate, but not independent, carcinogenicity studies
in rats, tylosin base was administered in the diet for 2 years at
levels up to 10 g/kg of feed. Food consumption and body-weight gain
were increased in both males and females in all treated groups. In
male rats, a dose-related increase in pituitary adenomas was
observed, from 5% in the control to 25% in the highest-dose group.
There was no such increase in female rats. The authors of the report
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. However, this hypothesis was neither tested experimentally nor
verifiable in detail by the Committee because individual body
weights at about 12 months of age were not available.
No effects on reproduction performance were observed in a
two-generation study in mice and in one- and three-generation
studies in rats. No malformations were observed in mice or rats, but
the Committee noted that these studies were poorly reported.
Tylosin was not mutagenic in an in vitro test for chromosomal
aberrations and in an in vivo micronucleus test. In a mouse
lymphoma assay, no activity was found with metabolic activation;
however a weak, but significant activity was observed in the absence
of such activation.
In studies in human volunteers, there was no evidence of the
emergence of cross-resistance to therapeutically important
antibiotics, but volunteers given oral doses of 20 mg of tylosin
daily for 6 months showed an increase in the number of resistant
streptococci. The Committee concluded that additional studies
showing no microbiological effects in two individuals at doses up to
5 mg/person/day were inadequate to establish a NOEL. In addition, no
suitable in vitro data were available to establish a NOEL with
respect to the microbiological risk for humans.
4. EVALUATION
Because of the deficiencies in the toxicological and
microbiological data, the Committee was not able to establish an
ADI.
5. REFERENCES
ANDERSON, R.C. (undated). Tylosin absorption and excretion studies.
Study numbered 893//FAANIM/AM/14-26. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C., HARRIS, P.N., LEE, C.C., MAZE, N., SMALL, R.M. &
WORTH, H.M. (1966) The toxicology and pharmacology of tylosin, an
antibiotic, and some salts of tylosin. Unpublished Report Ref.
X/E/4. dated 19 December 1966 from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C. & WORTH, H.M. (undated) The acute toxicity of tylosin
phosphate. Unpublished report from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO under
893/TACUTE/AM//1-26 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., GRIES,
C.L., GIBSON, W.R. & MORTON, D.M. (1978a) Chronic toxicity of
tylosin fed to rats for one year. Unpublished Report from study
R-307 from Toxicology Division, Lilly Research Laboratories.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., KITCHEN,
D.N., GIBSON, W.R. & MORTON, D.M. (1978b) A study of a parental
population of rats bred to produce offspring assigned to one- and
two-year dietary studies of tylosin. Unpublished Report Study No.
R-1176 dated October 1978 from Toxicology Division, Lilly Research
Laboratories. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
BUCK, A.M., COOPER, T.R. & THOMSON, T.D. (undated) Serum and lung
tylosin activity in neonatal calves treated with 1.0 gram tylosin
tartrate B.I.D. in milk replacer. Unpublished Report T1Y768501 from
Lilly Research Centre Laboratories, Animal Health Applications
Research, Greenfield, Indiana. Submitted to WHO under
893/SP/FBLDLV/AM/10/10-15 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
GINGERICH, D.A., BAGGOT, J.D. & KOWALSKI, J.J. (1977) Tylosin
antimicrobial activity and pharmacokinetics in cows. Can. Vet.
Jour., 18(4), 96-100.
GRIES, C.L. (1980) The toxicological evaluation of tylosin (compound
27892) given to Wistar rats in the diet for two years. Unpublished
report from studies R-287 and R-297 from Toxicology Division, Lilly
Research Laboratories. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
GRIES, C.L., McKINLEY, E.R. & QUARLES, J.P. (1983) Acute comparative
intravenous toxicity testing of tylosin, desmycosin and macrocin in
the ICR mouse. Unpublished report dated August 1983 with studies
M-V-46-83, M-V-45-83, and M-V-44-83 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985a) The acute oral,
dermal and ocular toxicity of granulated tylosin concentrate.
Unpublished report dated April, 1985 with studies R-O-365-79,
R-366-79, B-D-109-79, and B-E-94-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985b) The acute oral,
dermal, ocular and inhalation toxicity of tylan 200 injection.
Unpublished report dated September 1985 with studies R-O-344-79,
R-O-343-79, B-D-103-79, B-E-87-79, and R-H-39-79 from Toxicology
Division, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
GRIES, C.L., NEGILSKI, D.S. & DOWNS, O.S. (1985c) The acute oral,
dermal, ocular and inhalation toxicity of tylan soluble. Unpublished
report dated September 1985 with studies R-O-367-79, R-O-368-70,
B-D-94-79, B-E-90-79, and R-H-40-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L. (1990a) The effect of tylosin on the in vitro
induction of chromosome aberrations in chinese hamster ovary cells.
Unpublished report with studies 891109CTX3279, 891129CAB3279, and
891220CAB3279 from Lilly Research Laboratories, Toxicology Division,
Greenfield, Indiana.
GRIES, C.L. (1990b) The effect of tylosin on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished report with studies 891011MLT3279,
891017MLA3279, and 891114MLA3279 from Lilly Research Laboratories,
Toxicology Division, Greenfield, Indiana.
GRIES, C.L. (1990c) The effect of tylosin (compound 027892) on the
in vivo induction of micronuclei in bone marrow of ICR mice.
Unpublished report, study 891212MNT3279 from Lilly Research
Laboratories, Toxicology Division, Greenfield, Indiana.
HERD, R.M. (1990) The antimicrobial activity of tylosin in vitro
1980-1989. Unpublished Report dated 27-06-90 from Lilly Research
Centre Ltd. Submitted to WHO by Lilly, Windlesham, Surrey U.K.
JORDAN, W.H., GARDNER, J.B. & WEAVER, D.E. (1989) An intracutaneous
sensitization stidy in albino guinea pigs with tilmicosin.
Unpublished toxicology report No. 32 from Toxicology divison, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
KUWABARA, S. (undated) A study on the effect of continuous and
minute amount of tylosin on human intestinal flora. Medical
Department Toho University. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
LACEY, R.W. (1988) Rarity of tylosin resistance in human pathogenic
bacteria. Report from Department of Microbiology, University of
Leeds, Leeds, England. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
LILLY (undated a) The determination of tylosin levels in lung and
serum following administration of tylan 200 by intramuscular
injection to weaned calves. Unpublished Report T1Z769001.
Supplementary information submitted to WHO under
893/IJB200/FDISTR/AM/26-35 by Lilly Research Centre, Windlesham,
Surrey, England.
LILLY (undated b) Tylosin absorption studies in chickens.
Unpublished Report VPR-17-418 submitted under 893//FAANIM/AM/1-9 to
WHO by Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated c) Tylosin blood titres in chickens. Unpublished
Report VPR-21-418 submitted to WHO under 893//FBLDLV/AM//1-3 by
Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated d) Tylosin excretion studies in chickens. Unpublished
Report VPR-67-418 submitted to WHO under 893//FEXCRE/AM//6-13 WHO by
Lilly Research Centre, Windlesham, Surrey, England.
MALIN, B. & SILLIKER, J.H. (1966) Low level tylosin and the
emergence of antibiotic-resistant bacteria in humans. Antimicrobial
agents and chemotherapy, 1966. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
McGUIRE, J.M., BONIECE, W.S., HIGGENS, C.E., HOEHN, M.M., STARK,
W.M., WESTHEAD, J. & WOLFE, R.N. (1961) Tylosin, a new antibiotic: I
Microbiological studies. Antibiotics & Chemotherapy, XI (5), 1961,
320-327.
NAKAMURA, H. ET AL. (1969) Studies on distribution of antibiotics
inblood and tissue. XIX. Blood level and tissue concentration of
tylosin in pigs after oral administration of tylosin phosphate.
Proc. Jap. Soc. Vet. Science, 67th meeting, No. 128.
SHIONOGO & Co., Ltd. (1981) Blood levels of tylosin tartrate
following oral administration and intravenous injection in pigs.
Report No. 3174674755 from Department of Animal Science Development,
Shionogo & Co., Ltd. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978a) 14C tylosin tissue residue study in swine.
Unpublished Report dated November 1978 from Agricultural
Biochemistry, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978b) Metabolism of tylosin in swine and rat. Unpublished
Report dated November 1978 from Agricultural Biochemistry, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
SIECK, R.F. ET AL. (1980) 14C tylosin residue study in swine.
Experiment ABC-0016 dated March 1980 from Agricultural Biochemistry,
Lilly Research Laboratories, Greenfield, Indiana. Submitted to WHO
by Lilly Research Centre Ltd.. Windlesham, Surrey, England.
TERASHIMA, H. (undated) The effect of tylosin on a fetus and a
suckling-young of Wistar strain rat. Department of Pathology,
Medical School of Osaka City University. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
THOMSON, T.D. (undated a) Relative bioavailability of TYLAN 200
injection administered subcutaneously and intramuscularly in young
calves. Unpublished Report T1Z768106 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana,. Submitted
to WHO under 893/IJB200/FAANIM/AM/1-6 by Lilly Research Centre,
Windlesham, Surrey, England.
THOMSON, T.D. (undated b). The relative absorption rates of tylosin
base and tylosin tartrate from aqueous and polypropylene glycol
vehicles. Unpublished Report T1Z768107 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana. Submitted to
WHO under 893/IJB200/FAANIM/AM/6-19 by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
THOMSON, T.D. (undated c). Tylosin distribution in pneumonic calf
lung. Unpublished Report T1Z768203 from Lilly Research Laboratories,
Animal Science Division, Greenfield, Indiana. Submitted to WHO under
893/IJB200/FDISTR/AM/1-6 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
TSUBURA, Y., TOYOSHIMA, K., SANO, S., NISHII, Y. & TANI, M.
(undated) Effect of Tylosin on mouse breeding. Report from Second
Department of Pathology Nara Medical College. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
TSUCHIKAWA, K. & AKABORI, A. (undated) On the teratogenicity of
tylosin. Report from National Institute for Genetics, Shionogi and
Co. Submitted to WHO by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
VAN DUYN, R.L. & HANDY, P.R. (undated) Tylosin serum and lung tissue
levels following five daily intramuscular injections. Unpublished
Report 766-G125-86 from Lilly Research Laboratories, Animal Science
Division, Greenfield, Indiana. Submitted to WHO under
893/IJB/FBLDLV/AM/37-43 by Lilly Research Centre, Windlesham,
Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated a) Tylosin serum and lung
tissue levels following a single intramuscular injection with
tylosin in calves. Unpublished Report 766-G125-73 from Lilly
Research Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/24-30 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated b) Tylosin levels in lung
tissue and serum from calves injected once intramuscularly with
tylosin base. Unpublished Report VPR-352-766 from Lilly Research
Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/31-26 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & VOLKERTS, T.M. (1979) Concentrations of tylosin in
blood and lung tissue from calves given single and repeated daily
intramuscular doses. Vet. Med., March 1979, 375/377.
VAN DUYN, R.L. & KLINE, R.M. (undated) Tylosin minigranule oral
blood levels in swine. Pharmacological experiment VPR-275-766
submitted to WHO under 893/G/FBLDLV/AM/1-2 by Lilly Research Centre,
Windlesham, Surrey, England.
Tylosin was evaluated at the 12th meeting of the Joint FAO/WHO
Expert Committee on Food Additives in 1968 (Annex 1, reference 17).
No ADI was established. It was concluded that tylosin used in animal
feed or in veterinary medicine should not give rise to detectable
residues in edible products of animal origin; when using the
recommended methods of analysis it will be possible to ensure that
residues in meat for human consumption not exceed 0.2 ppm. Since
that time additional data have become available which are summarized
and discussed in this monograph addendum (Annex 1, reference 17).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Fasted rats received a single oral dose of 50 mg/kg b.w.
tylosin base or tylosin tartrate. Tylosin activity was assayed in
serum after 15 and 30 minutes and 1, 2, 4, 5, 7, and 24 hours after
treatment. Peak serum levels < 1.0 mg/l were seen after 1-2
hours. Within 7 hours serum levels decreased to less than the limit
of detection (i.e. 0.10 mg/l, microbiological assay). Four rats were
given i.p. 100 mg/kg b.w. tylosin base. Bile samples were collected
for 2 hours. The bile/serum concentration ratio ranged from 143-266
(Anderson et al., 1966).
Rabbits received i.m. 10 mg/kg b.w. tylosin base as a 5%
aqueous solution acidified with hydrochloric acid to pH 5.5. Serum
levels were determined after 1.5, 4, 7, and 24 hours. Peak serum
levels ranging from 0.57 to 0.88 mg/l were observed after 1.5 hours.
A similar study was carried out using tylosin tartrate in aqueous
(25 mg/kg b.w.) as well as in PEG-200 (10 mg/kg b.w.) solutions.
Peak serum levels at 1.0 hour were 4.7-7.2 and 0.96-1.25,
respectively. Within 24 hours serum levels were below the limits of
detection, which were 0.05 mg/l for tylosin hydrochloride and
0.10 mg/l for tylosin tartrate.
Two dogs given 25 mg/kg b.w. tylosin base orally in capsules
excreted 2% of the dose in the urine within 5 hours. Serum
concentrations were very low, < 0.05 and 3.3 mg/l at 2.5 hours
(microbiological assay). In another study groups of 8 dogs received
orally by capsule 1, 10, or 100 mg/kg b.w./day for 8 days. Blood
levels determined 2 hours after the last dose ranged from
< 0.15 mg/ml to 9.5 mg/ml mostly ranging with the dose. Two dogs
received 25 or 100 mg/kg b.w. tylosin base orally by capsule daily
during 29 days. Serum levels were determined 0, 1, 2, 3, 4, 5, 6,
and 7 hours after the 1st, 15th, and 29th dose. At 25 mg/kg b.w/day
peak serum levels (1.4-2.7 mg/l) were seen 2 hours after dosing and
at 100 mg/kg b.w./day the highest levels (2.7-4.6 mg/l) were seen
2-5 hours after dosing. No accumulation was observed. One dog given
i.v. 10 mg/kg b.w. tylosin base (dissolved in a minimal amount of
hydrochloric acid) excreted a total of 25.2% of the activity in the
urine. During 5 hours after dosing 13.7% of the dose was recovered
from bile. The bile/serum concentration ratio varied from 1230 to
3780. Four dogs were administered 10 mg/kg b.w tylosin base i.v. (as
an aqueous solution acidified with dilute hydrochloric acid). Blood
t´ was calculated as 48 min. Urinary recovery was 18.8% of the
dose during 6 hours after dosing (15.7% within 2 hours). Serum
levels of tylosin in 4 dogs receiving 25 mg tylosin base
intraduodenally at 0.25, 0.5, 1, 2, 3, 4, and 5 hours averaged 0.78,
1.98, 1.77, 1.94, 0.56, 0.29, and 0.13 mg/l, respectively. Urinary
recovery was 7.2% of the dose in 5 hours (Anderson, et al., 1966).
Thirty mg/kg b.w. tylosin tartrate was administered orally by
gavage or intravenously to groups of pigs (5/group, 30-days old). At
38 days of age the treatments were crossed over. Tylosin activity
was measured in blood samples taken at 10 intervals up to 24 hours
after treatment. After oral administration tylosin activity was
present in plasma 10 minutes after dosing with a peak concentration
of 2.4 mg/l at about 1.5 hours. By comparing the areas under the
curve of the tylosin concentration in blood following the 2 routes
of administration a biological availability of 22.5% was determined
(Shionogo & Co. Ltd., 1981).
Tylosin at 110 mg/kg b.w. (as the granulated phosphate) was
orally administered to 3 male and 3 female pigs. Tylosin activity
was assayed in blood samples taken up to 24 hours after dosing.
Serum activity peaked 1 hour after dosing (average 17.81 mg/l); 24
hours after dosing tylosin was not detectable (< 0.1 mg/l) (Van
Duyn & Kline, undated).
Six pigs (weight 56 kg) were orally given 50 mg/kg b.w. tylosin
phosphate in water. Blood and tissue samples were taken at intervals
up to 24 hours. Tylosin levels were detected in serum from 10
minutes to 8 hours after dosing and peaked at 1 hour (8.53 mg/l).
Tylosin was widely distributed in the body with tissue
concentrations in liver and kidney peaking at 1 hour. No activity
was found in the brain or spinal cord. Highest activity was found in
bile and urine (Nakamura et al., 1969).
Young calves (weight range 44.4-59.0 kg) were injected with
10 mg/kg Tylan 200 either subcutaneously or intramuscularly. The
rate of tylosin absorption, time to peak concentration and decline
of serum concentrations were very similar via both routes of
administration (Thomson, undated a).
Groups of calves (average weight 60 kg) received 10 mg
tylosin/kg b.w. subcutaneously or intramuscularly {as formulations
of tylosin tartrate in water, tylosin tartrate in propylene glycol
and water and tylosin base in propylene glycol and water (Tylan 200
injection)}. Another group of calves received the same dose (as
Tylan 200 injection) intravenously. Tylosin activity in serum was
measured in blood samples taken at various intervals after
treatment. Absorption of the formulations containing tylosin
tartrate was faster after subcutaneous and intramuscular
administration than absorption of the formulation containing tylosin
base (Thomson, undated b).
Holstein calves (1-3 weeks, 38-56 kg) were given 1 or 1 to 5
daily intramuscular injections of 17.6 mg tylosin/kg b.w. (as Tylan
200). In all experiments tylosin levels in serum peaked 2 hours
after administration (average 2.0 mg/l) decreasing to about 0.1 mg/l
at 36 hours. Peak lung tissue levels were observed at 6 to 24 hours
post injection varying from 12.6 to 15.7 mg/l. Lung tissue levels
were greater than serum levels and still detectable (2.2 mg/l) at 48
hours after administration (microbiological assay) (Van Duyn &
Folkerts, 1979; Van Duyn & Johnson (undated a); Van Duyn & Handy
(undated)).
A serum half-life of 1.62 hours was established in cows given a
single i.v. injection of 12.5 mg tylosin/kg b.w. (as Tylan 200). The
apparent specific volume of distribution was 1.10 l/kg indicating no
specific accumulation (Gingerich et al., 1977).
Neonatal holstein calves with a natural occuring pneumonia were
treated intramuscularly with 17.6 mg tylosin/kg b.w. (as Tylan 200)
daily for 3 consecutive days. Healthy calves were subjected to the
same treatment. Six hours following the last dose all the calves
were sacrificed and tylosin activity was measured in the lungs.
Tylosin distributed equally into both normal and pneumonic lung
tissue (Thomson, undated a).
Groups of neonatal healthy calves were fed a milk replacer
containing 1.0 g tylosin (as tylosin tartrate) for 4, 7, and 10
days. Serum samples were taken 4 hours after each dose; the animals
were killed after the final sample had been taken and lung tissues
were analyzed. Mean serum and lung tissue levels were 0.41, 0.37,
and 0.42 mg/l, and 1.76, 3.16, and 3.17 mg/l for the 4, 7, and
10-day treatment groups, respectively. Lung/serum tylosin ratios
were 7.24, 9.36, and 14.01, respectively (Buck et al., undated).
Groups of 4 calves (weight 250 kg) received intramuscular
injections with 10 mg tylosin/kg b.w. (as Tylan 200) for 5 days. The
calves were killed 2, 4, 6, 12, or 72 hours after the last
injection. Tylosin activity was measured in serum and lungs. Tylosin
activity in serum was highest at 4 hours after treatment (1.3 mg/l)
and was no longer detectable after 72 hours using a microbiological
assay whose detection limit was 0.05 mg/l. The mean tylosin activity
in the lungs was 5.9, 5.0, 6.6, 4.4, and 0.6 mg/l at 2, 4, 6, 12,
and 72 hours after treatment, respectively (Lilly, undated a).
Four broiler chickens (weight 720 g) were given a single dose
of 50 mg tylosin/bird (as tylosin tartrate) by stomach intubation.
Tylosin activity was detected in serum after 0.5 hours and peak
concentrations of 0.6-4.0 mg/l were found after 2 hours, declining
to negligible after 24 hours. Repeated oral doses of 50 mg tylosin
to chickens (weight 2 kg, dosed at 1, 2, and 3 hours) caused serum
peak levels at 4 hours (about 0.28 mg/l) declining to negligible at
24 hours (Lilly, undated b, undated c).
Groups of 6 chickens (5-7 weeks old, surgically prepared)
received orally 25, 100 or 250 mg tylosin/kg bw (as tartrate). Urine
and faeces were collected during 72 hours. Peak tylosin levels in
urine (< 100 mg/l at the 25 mg/kg dose and > 1400 mg/l at the
250 mg/kg dose) occurred 2-4 hours after dosing and declined rapidly
thereafter. Peak levels in faeces occurred at 8 hours and varied
from 300 to 2000 µg/g with the dose (Lilly, undated d)
2.1.2 Biotransformation
Four male rats, preconditioned on unlabelled tylosin (10 mg/kg
b.w.) for 3 days, received daily during 5 days by gavage 2 ml of a
solution containing 14C-labelled (in lactone ring) tylosin base.
The rats were killed 4 hours after the last dose; 99% of the
radioactivity was excreted in the faeces and 1% in the urine. The
greatest part of the excreted residues was found to be tylosin
factor A, tylosin factor D and dihydrodesmycosin. Less than
0.25 mg/kg total 14C-residue was found in liver and kidney (Sieck
et al., 1978b).
A male pig, preconditioned on feed containing 110 mg/kg
unlabelled tylosin for 2 weeks, received for 3 days feed with
110 mg/kg of feed tylosin base 14C-labelled in the lactone ring.
Four hours after the last dose the pig was killed. The radioactivity
was excreted 99% in the faeces and 1% in the urine. The majority of
the excreted residues (15% of the radioactivity in faeces was not
extractable) was found to be tylosin factor D (33%), tylosin factor
A (6%) and dihydrodesmycosin. At least 10 minor metabolites (< 5%
of activity) were present in the excreta. In liver and kidneys
< 0.25 mg/kg tylosin was found. At least 4 different metabolites
(of which one was detected as dihydrodesmycosin) were detected in
liver and kidneys (Sieck et al., 1978b).
Three pigs received twice daily for 4 days a ration containing
110 mg tylosin base/kg of feed 14C-labelled in the lactone ring. A
control pig received the basal diet throughout the experiment. Pigs
were sacrificed within 4 hours after the last dose. Total
14C-residues in liver and kidney were < 0.28 mg/kg and in
muscle and < 0.04 mg/kg in fat. Investigations by TLC of liver
residues revealed 5 or 6 metabolites. Two of the metabolites could
be identified as tylosin factor A and dihydrodesmycosin, each
representing about 5% of the total extractable liver residue. Mass
spectrometer analysis of the faeces identified 3 metabolites as
tylosin factor A, tylosin factor D and dihydrodesmycosin (Sieck
et al., 1978a; Sieck et al., 1980).
2.2. Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of tylosin formulations and of tylosin are
given in Tables 1 and 2, respectively.
Table 1. The acute toxicity of tylosin formulations
Species Sex Route LD50 LC50 Reference
(mg/kg b.w) (mg/l)
rat M&F oral > 5001 Gries et al.,
1985a
M&F oral > 0.52 > 1.054 Gries et al.,
1 hr > 0.65 1985b
M&F inhal Gries et al.
M&F inhal 1985c
rabbit M&F dermal > 20001 Gries et al.,
1985a
M&F dermal > 20003 Gries et al.,
1985c
M&F dermal > 2.02 Gries et al.,
1985b
1. administered as granulated tylosin concentrate, a formulation
containing 26.7% of tylosin base activity as the phosphate salt
2. ml/kg b.w. administered as undiluted tylan 200 injection
3. administered as tylan soluble, a dry granular formulation, also
known as tylosin tartrate
4. liquid droplet aerosol of tylan 200/injection formulation at
1.05 mg/litre for 1 hour
5. solid particulate aerosol of tylan soluble at 0.6 mg/litre.
Table 2. The acute toxicity of tylosin
Species Sex Route LD50 Reference
(mg/kg b.w.)
mouse F oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 492.51
- i.p. 594.12
- s.c. 13545
- s.c. 784.11
- s.c. > 25002 Gries et al.,
- i.v. 385.71 1983
- i.v. 581.73
- i.v. 588.84
- i.v. 588.95
F i.v. approx 3216
rat M oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 10011
- i.p. > 25005
- i.v. 6955 Gries et al.,
- s.c. 40831 1985c
- s.c. > 30005
M&F oral > 5005
dog M&F oral > 8002 Anderson & Worth,
undated
1. administered as tylosin phosphate
2. administered as tylosin base
3. administered as tylosin hydrochloride
4. administered as tylosin lactate.
5. administered as tylosin tartrate.
6. administered as 20 mg tylosin/ml of acidified sterile water for
injection, USP (2.0%)
After oral administration no deaths were recorded at the
highest dose used; dogs vomited at 800 mg/kg b.w. but not at
400 mg/kg b.w.; at both these doses the dogs salivated and
defecated. Intravenous and intraperitoneal administration caused
depression, prostration, convulsions and death or recovery within 24
hours.
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of rats (Harlan strain, 5 females/group) received daily
s.c. injections of 10, 20, 50 or 100 mg/kg b.w. tylosin base as a
suspension in 5% acacia gum for 1 month. No effects were seen on
food intake, body weight gain, adrenal weight and terminal blood
cell counts. Macroscopy and microscopy did not show abnormalities
(Anderson et al., 1966; study R2-58). Remark: Summary only.
Groups of rats (Harlan strain, 6/sex/group) received daily s.c.
injections of 100, 250, 500, or 1000 mg/kg b.w. tylosin tartrate or
2.5 ml/kg b.w. saline for 1 month. At doses > 250 mg/kg b.w.
diarrhoea was seen during the first week, regressing to soft stools
(occasionally seen at 100 mg/kg b.w. too) during the remainder of
the study. At doses > 250 mg/kg b.w. scarring and scabbing at the
injection site was seen (occasionally at 100 mg/kg b.w.). No effects
were observed on growth, haematology, organ weight, macroscopy and
microscopy (Anderson et al., 1966; study R19-59). Remark: Summary
only.
Groups of rats (Harlan Wistar, 15/sex/group, F1a offspring
from parents fed the same amount of tylosin base for about 10 weeks
prior to mating and during gestation and lactation) were fed diets
containing 0, 0.1, 0.5 or 1.0% tylosin base for 1 year. No
treatment-related effects were observed on mortality, growth, food
consumption, ophthalmoscopy, organ weights, macroscopy and
histopathology. Treated rats appeared moderately hyperirritable and
hyperactive after 7 to 12 months of treatment. An increase in
lymphocytes and a corresponding reduction in neutrophils were
observed in both sexes (significantly in females) at 0.5 and 1.0%. A
trend towards a slightly more alkaline urine occurred in females at
0.5% and 1.0%. The authors concluded that the NOEL was 1.0% tylosin
base, equivalent to 500 mg/kg b.w. However, the Committee concluded
that the NOEL in this study was 0.1% tylosin base, equivalent to
50 mg/kg b.w. (Broddle et al., 1978a).
2.2.2.2 Dogs
Groups of 8 dogs (2/sex mongrel dogs and 2/sex beagle dogs)
received orally by gelatin capsule during 2 years 0, 1, 10 or 100 mg
tylosin base/kg b.w./day. Groups of mongrel dogs (2/sex/group) given
200 or 400 mg tylosin base/kg b.w./day in capsules for 2 years or
longer were subsequently added. Salivation, vomiting and diarrhoea
were observed at 200 and 400 mg/kg b.w./day (at 100 mg/kg b.w./day 1
dog vomited once). Haematology, urinalysis and relative organ
weights did not reveal abnormalities. No changes were observed in
faecal microbiological flora. Liver and kidney function tests
revealed two dogs at 100 mg/kg b.w./day and 1 dog at 400 mg/kg
b.w./day with a transient increased BSP retention time. Macroscopy
and microscopy did not show compound-related changes except for mild
pyelonephritis seen in 1/4 dogs at 200 mg/kg b.w./day and bilateral
nephrosis, mild chronic pyelonephritis and mild cystitis seen in 1/4
dogs at 400 mg/kg b.w./day. Terminal bone-marrow counts were normal
(only measured in normal study). At dose levels > 10 mg/kg
b.w./day serum tylosin levels in blood could be detected. The NOEL
in this study was 100 mg/kg b.w./day (Anderson et al., 1966; study
D4-59). Remark: The limited details provided and the poor reporting
of the study made proper interpretation difficult.
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
In a limited study rats (3/sex/group) were fed diets containing
0, 0.1, 0.3, or 1.0% tylosin base for 17 months. In the 0.3% group 1
female died due to malnutrition. No effects on growth or on terminal
haematological parameters were seen. Relative organ weights revealed
changes in weights of ovaries and uteri due to thickening in uteri
and a decrease in size of the ovaries in 1/3, 3/3, 2/3, and 2/3
female rats at the 0, 0.1, 0.3, and 1.0% levels, respectively.
Macroscopy and microscopic examination revealed squamous metaplasia
of the uterine glands in 2 female rats at the highest dose (Anderson
et al., 1966; Study R9-58). Remark: Incomplete report.
Groups of about 25 male and female Harlan rats (total 213)
received 0, 0.001, 0.01 or 0.1 % tylosin base in their diet for 2
years. Survival was better in tylosin treated groups than in control
rats (54% and 30% respectively). No effects were seen on growth,
haematology, or relative organ weights. Macroscopy and microscopy
revealed an increased number of animals with fatty changes in livers
and kidney at all dose groups and a slight increased incidence of
bile duct proliferation at the 0.1 and 0.01% levels, but neither was
dose-related (Anderson et al., 1966; Study R10-58). Remark:
Incomplete reports of growth and haematology; limited
histopathology.
In another 2-year study groups of Harlan rats (30/sex/group)
were fed diets containg 0, 0.01, or 1.0% tylosin base. Survival was
better in rats fed tylosin than in control rats (57% and 29%
survival in high dose and control rats, respectively). No effects
were seen on growth, haematology, urinalysis, organ weights,
macroscopy and microscopy. A dose-unrelated increase of fatty
changes in liver and kidney was observed. (Anderson et al., 1966;
R3-59). Remark: Incomplete reports of growth, haematology and
urinalysis.
In a very limited study groups of 10 male and 10 female rats
were fed diets containing 0, 2, 5, 10, or 20% tylosin base for up to
2 years. No effects on food consumption, growth and haematology were
observed on rats at 2% and 5%. At the 10% level, growth and food
consumption were slightly reduced. At the highest dose food
consumption and growth were markedly reduced followed by death
(Anderson et al., 1966; R6-60). Remark: Incomplete reports.
Two replicate 2-year studies were carried out with Wistar rats
(40/sex/group in each study derived from F1a offspring from
parents fed diets containing tylosin from 10 weeks prior to mating
up to the time of weaning). The control groups (60/sex/group in each
replicate study) were derived from parents fed untreated diet.
Treated groups were fed diets containing 0.1, 0.5, or 1.0% tylosin
base. Observations included clinical signs, mortality, food
consumption, food efficiency, body weight, terminal haematology and
biochemistry, urinalysis, organ weights, macroscopy, and
histopathology. There were trends towards improved survival in
males, although overall survival was low (20% and 28% for the
control and treated groups, respectively), and increased food
consumption and body weight gain in both males and females in all
treated groups. At histopathology an increased incidence of
pituitary adenomas in males (but not in females) was observed. The
combined incidence of pituitary adenomas in males for both
replicates was 6/120 (5%) in the control group, 9/80 (11%) in the
low dose (0.1% tylosin) group, 18/80 (22.5%) in the mid dose (0.5%)
group, and 20/80 (25%) in the high dose (1.0%) group. The authors
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. The incidence of malignant tumours was unaffected in males or
in females (Gries, 1980)
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 7-8 male and 14-17 female ICR mice were fed diets
containing 0, 0.1, or 1.0% tylosin (composition unknown) for two
succesive generations with 2 litters/generation. Some of the mice
were maintained on an ordinary diet, but all the mice received the
experimental diet prior to delivery of the offspring. No
treatment-related effects were observed on reproductive performance
(sexual maturation, number of pups or weaning of pups) (Tsubura
et al., undated). Remark: Only a few summary tables were available.
2.2.4.2 Rats
A 3-generation reproduction study was performed with 2 groups
of 5 male and 10 female Harlan rats/group receiving 0 or 1.0%
tylosin base in their diet. Fertility, viability, gestation and
lactation indices in the F0 generation rats did not reveal any
abnormality. Growth was equal in all generations for both control
and treated rats. In each succeeding generation the capability for
reproduction and perpetuation was unaffected (Anderson et al., 1966;
R3-59). Remark: Incomplete report.
In a special study weanling Wistar rats (25/sex/group, 6-7
weeks old) were fed diets containing 0.1, 0.5, or 1.0% tylosin base
for 10 weeks prior to mating and thereafter for about 6 months
total. A control group consisted of 35 rats/sex. Only one litter was
bred. No effects were observed on parental body weight, food
consumption, reproductive indices (male or female fertility,
gestation length, number of live fetuses, mean litter weight or
offspring survival) or biochemistry. High dose male rats revealed
significantly decreased white blood cells at termination. Sera
collected from parental rats (approx. 150 days on experimental
diets) did not contain detectable levels of tylosin (< 0.1 mg/l).
Offspring were physically normal and were assigned to the one year
toxicity study with tylosin (see Section 2.2.2.1) (Broddle, et al.,
1978b). Remark: Only summarized data given on reproductive indices.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1. Mice
Groups of 10 pregnant mice (2 strains, CBA and A/Jax) received
orally 100, 500, or 1000 mg/kg b.w./day tylosin (composition not
given) in 0.1 ml water from days 7-12 of gestation. Two control
groups of 3 and 5 mice received saline or remained untreated,
respectively. Females were killed on day 18 of pregnancy. No effects
were observed on number of corpora lutea, number of implantations,
number of early and late deaths, number of embryos alive, or fetal
development (Tsuchikawa & Akabori, undated).
Four groups of mice given 0 or 500 mg/kg b.w. and a further 2
pregnant females (A/Jax x male CBA) per group, receiving orally 0 or
1000 mg tylosin/kg b.w./day during days 7-12 of gestation, were
allowed to deliver and rear their young for 4 weeks. No effects were
observed in growth, survival, or genital system of all mice born
determined up to 9 weeks. At 8 weeks after birth rearing ability,
hearing ability and kinetic functions were not effected. At the 9th
week the mice were killed and visceral and skeletal examinations
were performed. No abnormalities were seen (Tsuchikawa & Akabori,
undated).
2.2.5.2 Rats
Groups of 15 Wistar rats were fed diets containing 0.1, 1.0, or
10.0% tylosin (composition not given; equal to 60.5, 725, or
4800 mg/kg b.w./day, respectively) in their diet during days 1-20 of
gestation. A control group consisted of 10 rats. Females were killed
on day 20 of gestation. Observations included number of resorptions
and live and dead fetuses, sex ratio, fetal weight and external,
visceral and skeletal abnormalities. Fetus weight at the highest
dose was slightly decreased (Terashima, undated).
In another study 3 groups of 15 Wistar rats received 0, 1.0, or
10.0% tylosin (composition not given, equal to 0, 725, or 4800 mg/kg
b.w.) during days 1-20 of gestation. Normal delivery was allowed.
Number of fetuses, sex ratio, external abnormalities and growth
during the weaning period (3 weeks) were determined. Motor functions
and senses were examined. The weanlings were killed and visceral and
skeletal examinations were performed. A slightly reduced growth was
observed in weanlings at the highest dose (Terashima, undated).
2.2.6 Special studies on genotoxicity
Tylosin was tested for genotoxicity in an in vitro
chromosomal assay with Chinese hamster ovary cells, a mouse lymphoma
assay and an in vivo assay for cytogenetic damage. The results are
summarized in Table 3.
2.2.7 Special studies on microbiological activity
The anti-microbial activity of tylosin has been described in
the published literature. Tylosin is markedly active in vitro
against gram-positive bacteria, certain gram-negative bacteria and
mycobacteria; it is inactive against Enterobacteriaceae (McGuire
et al., 1961).
Table 3: Results of genotoxicity assays on Tylosin
Test system Test Concentration Purity Results Reference
object of substance
tested
Chromosome Chinese 500-1000 99.3 - Gries,
aberration hamster µg/ml2, 1990a
assay1 ovary 250-750
cells µg/ml3, both
in DMSO
Lymphoma Mouse 10-1000 µg/ml2 99.3 +5 Gries,
assay L5178Y 10-1000 µg/ml3 1990b
TK+/- (1000 µg/ml
cells toxic) both in
DMSO
Micronucleus ICR 2 daily doses 966 - Gries,
mice of 1250, 2500 1990c
or 5000 mg/kg4
1. Mitomycin C and cyclophosphamide, used as positive controls, yielded
positive results
2. without metabolic activation
3. with metabolic activation
4. the positive control cyclophosphamide yielded positive results.
5. positive at cytotoxic dose
6. administered as tylosin base
In recent studies the minimal inhibitory concentration (MIC) of
tylosin has been determined for bacterial pathogens isolated from
target animal species of European and North American origin since
1984. Additional information on sensitivity of bacterial isolates to
tylosin was obtained from literature published since 1980. Tylosin
was active against most Gram-positive bacteria and mycoplasmas
tested in vitro. Activity against Gram-negative bacteria was
generally lower. Tylosin was also found to be active against
isolates of Chlamydia psittaci. MIC values for streptocci,
enterococci and staphylococci are given in Table 4 (Herd, 1990).
Table 4. MIC1 values for tylosin against some Gram-positive bacteria
Organism MIC range Total No.
(mg/1) of isolates
Streptococcus pyogenes 0-1 - 0.2 5
Streptococcus pneumoniae 0.2 - 0.4 4
Streptococcus dysgalactiae 0.06 - 128 50
Streptococcus agalactiae 0.125 - 0.5 51
Streptococcus suis 0.125 - > 128 42
Streptococcus uberis 0.125 - > 128 53
Enterococcus faecalis 0.25 - > 128 31
Staphylococcus aureus2 0.125 - > 128 98
Staphylococcus Spp.3 0.78 - 1.0 7
1. Minimum inhibitory concentration
2. Coagulase - positive
3. Coagulase - negative
2.2.8 Special studies on neurotoxicity
Three cats received daily for 90 days subcutaneously 200 mg/kg
b.w. tylosin tartrate as a 20% solution divided in 2 doses during
the first 37 days and thereafter as a single dose (with addition of
sodium citrate for buffering). An untreated control group consisted
of 3 cats. Slight reduction (25-35%) was observed in the
post-rotatory nystagmus response, but the auditory response appeared
normal. All cats landed on their four feet when dropped from 1
metre. No ataxia was seen (Anderson, 1966; C2-59)
2.2.9 Special studies on skin and eye irritation
Granulated tylosin concentrate (2 g/kg b.w.) was not irritating
when applied to the rabbit skin but when the formulation (52 mg
(0.1 ml)) was instilled to the rabbit eye corneal dullness, slight
corneal opacity, slight to marked irititis and moderate
conjunctivitis were observed. The effects resolved within 14 days of
treatment (Gries et al., 1985a).
The formulation Tylan 200 injection (2 ml/kg b.w.) caused very
slight erythema to the rabbit skin and the formulation (0.1 ml)
caused slight conjunctival hyperemia to the rabbit eye both within
24 hours after application. No skin and eye irritation were observed
after 48 hours (Gries, et al., 1985b).
The formulation Tylan soluble (2 g/kg b.w.) caused very slight
erythema and slight desquamation to the rabbit skin. Slight to
moderate corneal opacity, marked iritis and moderate conjunctivitis
were observed following the instillation of 58 mg of the formulation
into the rabbit eye; these effects had cleared within a week (Gries
et al., 1985c).
2.2.10 Special studies on skin sensitization
Tylosin tartrate was used as a positive control in a
sensitization test by the method of Landsteiner and Jacobs conducted
for tilmicosin. Guinea-pigs (12) were administered 10 intracutaneous
injections of 50 mg/ml tylosin tartrate; a challenge with a further
dose was given after 14 days. At challenge a mild positive response
was observed (Jordan et al., 1989).
2.3. Observations in humans
Trials in human subjects have been conducted to investigate
antibiotic-resistant bacteria. In one trial 12 or 11 human
volunteers were given 20 mg tylosin per day or a placebo,
respectively, for up to 6 months. There was no significant change in
the total number of tylosin-resistant staphylococci and lactobacilli
in weekly faecal samples or in coliform or yeast forms whereas a
significant increase was observed in total number of resistant
streptococci. In another trial no tylosin resistant organisms were
detected in specimens (including faeces samples) from hospitalized
patients who had never had any exposure to the nonmedical antibiotic
tylosin. In both studies no regular pattern was observed in
cross-resistance to related antibiotics (Malin & Silliker, 1966)
In a Japanese trial 2 human volunteers were given 2 or 5 mg
tylosin (as 1 mg tablets; composition not given) per day for 3
months. Faeces were inspected for E. coli and Enterococci and
Staphylococci at intervals of 1 to 2 weeks from 2 months prior to
administration and 3 months from the start of administration. No
tendency towards increased resistance was noted (Kuwabara, undated).
Tylosin resistance was examined in human cultures of
Staphylococcus aureus, Streptococcus pyogenes and Campylobacter
spp. Of 3812 human cultures isolated between 1985 and 1987 only
1.0% were resistant to tylosin. There was no evidence for a
significant animal source of these resistant cultures (Lacey, 1988).
3. COMMENTS
The Committee considered toxicological data on tylosin,
including the results of studies on biochemical aspects,
mutagenicity, and microbiological activity. The Committee noted that
most of the toxicity studies had been carried out about 20 years
ago, had not been conducted according to current protocols, and were
poorly reported.
Following administration of tylosin by various routes, peak
serum levels in rats, dogs, pigs, and cattle were observed within
1-2 hours, and then declined rapidly. In pigs, about 22% was
bioavailable after oral administration. Excretion of tylosin was
rapid and largely in the bile.
After oral administration of radiolabelled tylosin to rats and
pigs 99% of the radioactivity was excreted via the faeces. Major
products identified in faeces were tylosin (factor A), macrosin
(factor C), relomycin (factor D), and dihydrodesmycosin. In pig
liver and kidney, only very small amounts of tylosin and
dihydrodesmycosin could be found.
Several short- and long-term studies in rats and dogs were
performed. In a 1-year study in rats, tylosin base was administered
in the diet at concentrations up to 10 g/kg of feed. A NOEL of
1 g/kg of feed, equivalent to 50 mg/kg b.w./day, was established,
based on haematological and urinary pH changes. In a study in dogs,
tylosin base was administered orally at dose levels up to 400 mg/kg
b.w./day for 2 years. At the two highest dose levels, salivation,
vomiting, and diarrhoea as well as mild pyelonephritis were
observed. The NOEL was 100 mg/kg b.w./day.
In two replicate, but not independent, carcinogenicity studies
in rats, tylosin base was administered in the diet for 2 years at
levels up to 10 g/kg of feed. Food consumption and body-weight gain
were increased in both males and females in all treated groups. In
male rats, a dose-related increase in pituitary adenomas was
observed, from 5% in the control to 25% in the highest-dose group.
There was no such increase in female rats. The authors of the report
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. However, this hypothesis was neither tested experimentally nor
verifiable in detail by the Committee because individual body
weights at about 12 months of age were not available.
No effects on reproduction performance were observed in a
two-generation study in mice and in one- and three-generation
studies in rats. No malformations were observed in mice or rats, but
the Committee noted that these studies were poorly reported.
Tylosin was not mutagenic in an in vitro test for chromosomal
aberrations and in an in vivo micronucleus test. In a mouse
lymphoma assay, no activity was found with metabolic activation;
however a weak, but significant activity was observed in the absence
of such activation.
In studies in human volunteers, there was no evidence of the
emergence of cross-resistance to therapeutically important
antibiotics, but volunteers given oral doses of 20 mg of tylosin
daily for 6 months showed an increase in the number of resistant
streptococci. The Committee concluded that additional studies
showing no microbiological effects in two individuals at doses up to
5 mg/person/day were inadequate to establish a NOEL. In addition, no
suitable in vitro data were available to establish a NOEL with
respect to the microbiological risk for humans.
4. EVALUATION
Because of the deficiencies in the toxicological and
microbiological data, the Committee was not able to establish an
ADI.
5. REFERENCES
ANDERSON, R.C. (undated). Tylosin absorption and excretion studies.
Study numbered 893//FAANIM/AM/14-26. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C., HARRIS, P.N., LEE, C.C., MAZE, N., SMALL, R.M. &
WORTH, H.M. (1966) The toxicology and pharmacology of tylosin, an
antibiotic, and some salts of tylosin. Unpublished Report Ref.
X/E/4. dated 19 December 1966 from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C. & WORTH, H.M. (undated) The acute toxicity of tylosin
phosphate. Unpublished report from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO under
893/TACUTE/AM//1-26 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., GRIES,
C.L., GIBSON, W.R. & MORTON, D.M. (1978a) Chronic toxicity of
tylosin fed to rats for one year. Unpublished Report from study
R-307 from Toxicology Division, Lilly Research Laboratories.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., KITCHEN,
D.N., GIBSON, W.R. & MORTON, D.M. (1978b) A study of a parental
population of rats bred to produce offspring assigned to one- and
two-year dietary studies of tylosin. Unpublished Report Study No.
R-1176 dated October 1978 from Toxicology Division, Lilly Research
Laboratories. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
BUCK, A.M., COOPER, T.R. & THOMSON, T.D. (undated) Serum and lung
tylosin activity in neonatal calves treated with 1.0 gram tylosin
tartrate B.I.D. in milk replacer. Unpublished Report T1Y768501 from
Lilly Research Centre Laboratories, Animal Health Applications
Research, Greenfield, Indiana. Submitted to WHO under
893/SP/FBLDLV/AM/10/10-15 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
GINGERICH, D.A., BAGGOT, J.D. & KOWALSKI, J.J. (1977) Tylosin
antimicrobial activity and pharmacokinetics in cows. Can. Vet.
Jour., 18(4), 96-100.
GRIES, C.L. (1980) The toxicological evaluation of tylosin (compound
27892) given to Wistar rats in the diet for two years. Unpublished
report from studies R-287 and R-297 from Toxicology Division, Lilly
Research Laboratories. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
GRIES, C.L., McKINLEY, E.R. & QUARLES, J.P. (1983) Acute comparative
intravenous toxicity testing of tylosin, desmycosin and macrocin in
the ICR mouse. Unpublished report dated August 1983 with studies
M-V-46-83, M-V-45-83, and M-V-44-83 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985a) The acute oral,
dermal and ocular toxicity of granulated tylosin concentrate.
Unpublished report dated April, 1985 with studies R-O-365-79,
R-366-79, B-D-109-79, and B-E-94-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985b) The acute oral,
dermal, ocular and inhalation toxicity of tylan 200 injection.
Unpublished report dated September 1985 with studies R-O-344-79,
R-O-343-79, B-D-103-79, B-E-87-79, and R-H-39-79 from Toxicology
Division, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
GRIES, C.L., NEGILSKI, D.S. & DOWNS, O.S. (1985c) The acute oral,
dermal, ocular and inhalation toxicity of tylan soluble. Unpublished
report dated September 1985 with studies R-O-367-79, R-O-368-70,
B-D-94-79, B-E-90-79, and R-H-40-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L. (1990a) The effect of tylosin on the in vitro
induction of chromosome aberrations in chinese hamster ovary cells.
Unpublished report with studies 891109CTX3279, 891129CAB3279, and
891220CAB3279 from Lilly Research Laboratories, Toxicology Division,
Greenfield, Indiana.
GRIES, C.L. (1990b) The effect of tylosin on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished report with studies 891011MLT3279,
891017MLA3279, and 891114MLA3279 from Lilly Research Laboratories,
Toxicology Division, Greenfield, Indiana.
GRIES, C.L. (1990c) The effect of tylosin (compound 027892) on the
in vivo induction of micronuclei in bone marrow of ICR mice.
Unpublished report, study 891212MNT3279 from Lilly Research
Laboratories, Toxicology Division, Greenfield, Indiana.
HERD, R.M. (1990) The antimicrobial activity of tylosin in vitro
1980-1989. Unpublished Report dated 27-06-90 from Lilly Research
Centre Ltd. Submitted to WHO by Lilly, Windlesham, Surrey U.K.
JORDAN, W.H., GARDNER, J.B. & WEAVER, D.E. (1989) An intracutaneous
sensitization stidy in albino guinea pigs with tilmicosin.
Unpublished toxicology report No. 32 from Toxicology divison, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
KUWABARA, S. (undated) A study on the effect of continuous and
minute amount of tylosin on human intestinal flora. Medical
Department Toho University. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
LACEY, R.W. (1988) Rarity of tylosin resistance in human pathogenic
bacteria. Report from Department of Microbiology, University of
Leeds, Leeds, England. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
LILLY (undated a) The determination of tylosin levels in lung and
serum following administration of tylan 200 by intramuscular
injection to weaned calves. Unpublished Report T1Z769001.
Supplementary information submitted to WHO under
893/IJB200/FDISTR/AM/26-35 by Lilly Research Centre, Windlesham,
Surrey, England.
LILLY (undated b) Tylosin absorption studies in chickens.
Unpublished Report VPR-17-418 submitted under 893//FAANIM/AM/1-9 to
WHO by Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated c) Tylosin blood titres in chickens. Unpublished
Report VPR-21-418 submitted to WHO under 893//FBLDLV/AM//1-3 by
Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated d) Tylosin excretion studies in chickens. Unpublished
Report VPR-67-418 submitted to WHO under 893//FEXCRE/AM//6-13 WHO by
Lilly Research Centre, Windlesham, Surrey, England.
MALIN, B. & SILLIKER, J.H. (1966) Low level tylosin and the
emergence of antibiotic-resistant bacteria in humans. Antimicrobial
agents and chemotherapy, 1966. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
McGUIRE, J.M., BONIECE, W.S., HIGGENS, C.E., HOEHN, M.M., STARK,
W.M., WESTHEAD, J. & WOLFE, R.N. (1961) Tylosin, a new antibiotic: I
Microbiological studies. Antibiotics & Chemotherapy, XI (5), 1961,
320-327.
NAKAMURA, H. ET AL. (1969) Studies on distribution of antibiotics
inblood and tissue. XIX. Blood level and tissue concentration of
tylosin in pigs after oral administration of tylosin phosphate.
Proc. Jap. Soc. Vet. Science, 67th meeting, No. 128.
SHIONOGO & Co., Ltd. (1981) Blood levels of tylosin tartrate
following oral administration and intravenous injection in pigs.
Report No. 3174674755 from Department of Animal Science Development,
Shionogo & Co., Ltd. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978a) 14C tylosin tissue residue study in swine.
Unpublished Report dated November 1978 from Agricultural
Biochemistry, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978b) Metabolism of tylosin in swine and rat. Unpublished
Report dated November 1978 from Agricultural Biochemistry, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
SIECK, R.F. ET AL. (1980) 14C tylosin residue study in swine.
Experiment ABC-0016 dated March 1980 from Agricultural Biochemistry,
Lilly Research Laboratories, Greenfield, Indiana. Submitted to WHO
by Lilly Research Centre Ltd.. Windlesham, Surrey, England.
TERASHIMA, H. (undated) The effect of tylosin on a fetus and a
suckling-young of Wistar strain rat. Department of Pathology,
Medical School of Osaka City University. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
THOMSON, T.D. (undated a) Relative bioavailability of TYLAN 200
injection administered subcutaneously and intramuscularly in young
calves. Unpublished Report T1Z768106 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana,. Submitted
to WHO under 893/IJB200/FAANIM/AM/1-6 by Lilly Research Centre,
Windlesham, Surrey, England.
THOMSON, T.D. (undated b). The relative absorption rates of tylosin
base and tylosin tartrate from aqueous and polypropylene glycol
vehicles. Unpublished Report T1Z768107 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana. Submitted to
WHO under 893/IJB200/FAANIM/AM/6-19 by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
THOMSON, T.D. (undated c). Tylosin distribution in pneumonic calf
lung. Unpublished Report T1Z768203 from Lilly Research Laboratories,
Animal Science Division, Greenfield, Indiana. Submitted to WHO under
893/IJB200/FDISTR/AM/1-6 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
TSUBURA, Y., TOYOSHIMA, K., SANO, S., NISHII, Y. & TANI, M.
(undated) Effect of Tylosin on mouse breeding. Report from Second
Department of Pathology Nara Medical College. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
TSUCHIKAWA, K. & AKABORI, A. (undated) On the teratogenicity of
tylosin. Report from National Institute for Genetics, Shionogi and
Co. Submitted to WHO by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
VAN DUYN, R.L. & HANDY, P.R. (undated) Tylosin serum and lung tissue
levels following five daily intramuscular injections. Unpublished
Report 766-G125-86 from Lilly Research Laboratories, Animal Science
Division, Greenfield, Indiana. Submitted to WHO under
893/IJB/FBLDLV/AM/37-43 by Lilly Research Centre, Windlesham,
Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated a) Tylosin serum and lung
tissue levels following a single intramuscular injection with
tylosin in calves. Unpublished Report 766-G125-73 from Lilly
Research Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/24-30 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated b) Tylosin levels in lung
tissue and serum from calves injected once intramuscularly with
tylosin base. Unpublished Report VPR-352-766 from Lilly Research
Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/31-26 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & VOLKERTS, T.M. (1979) Concentrations of tylosin in
blood and lung tissue from calves given single and repeated daily
intramuscular doses. Vet. Med., March 1979, 375/377.
VAN DUYN, R.L. & KLINE, R.M. (undated) Tylosin minigranule oral
blood levels in swine. Pharmacological experiment VPR-275-766
submitted to WHO under 893/G/FBLDLV/AM/1-2 by Lilly Research Centre,
Windlesham, Surrey, England.
 Tylosin was evaluated at the 12th meeting of the Joint FAO/WHO
Expert Committee on Food Additives in 1968 (Annex 1, reference 17).
No ADI was established. It was concluded that tylosin used in animal
feed or in veterinary medicine should not give rise to detectable
residues in edible products of animal origin; when using the
recommended methods of analysis it will be possible to ensure that
residues in meat for human consumption not exceed 0.2 ppm. Since
that time additional data have become available which are summarized
and discussed in this monograph addendum (Annex 1, reference 17).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Fasted rats received a single oral dose of 50 mg/kg b.w.
tylosin base or tylosin tartrate. Tylosin activity was assayed in
serum after 15 and 30 minutes and 1, 2, 4, 5, 7, and 24 hours after
treatment. Peak serum levels < 1.0 mg/l were seen after 1-2
hours. Within 7 hours serum levels decreased to less than the limit
of detection (i.e. 0.10 mg/l, microbiological assay). Four rats were
given i.p. 100 mg/kg b.w. tylosin base. Bile samples were collected
for 2 hours. The bile/serum concentration ratio ranged from 143-266
(Anderson et al., 1966).
Rabbits received i.m. 10 mg/kg b.w. tylosin base as a 5%
aqueous solution acidified with hydrochloric acid to pH 5.5. Serum
levels were determined after 1.5, 4, 7, and 24 hours. Peak serum
levels ranging from 0.57 to 0.88 mg/l were observed after 1.5 hours.
A similar study was carried out using tylosin tartrate in aqueous
(25 mg/kg b.w.) as well as in PEG-200 (10 mg/kg b.w.) solutions.
Peak serum levels at 1.0 hour were 4.7-7.2 and 0.96-1.25,
respectively. Within 24 hours serum levels were below the limits of
detection, which were 0.05 mg/l for tylosin hydrochloride and
0.10 mg/l for tylosin tartrate.
Two dogs given 25 mg/kg b.w. tylosin base orally in capsules
excreted 2% of the dose in the urine within 5 hours. Serum
concentrations were very low, < 0.05 and 3.3 mg/l at 2.5 hours
(microbiological assay). In another study groups of 8 dogs received
orally by capsule 1, 10, or 100 mg/kg b.w./day for 8 days. Blood
levels determined 2 hours after the last dose ranged from
< 0.15 mg/ml to 9.5 mg/ml mostly ranging with the dose. Two dogs
received 25 or 100 mg/kg b.w. tylosin base orally by capsule daily
during 29 days. Serum levels were determined 0, 1, 2, 3, 4, 5, 6,
and 7 hours after the 1st, 15th, and 29th dose. At 25 mg/kg b.w/day
peak serum levels (1.4-2.7 mg/l) were seen 2 hours after dosing and
at 100 mg/kg b.w./day the highest levels (2.7-4.6 mg/l) were seen
2-5 hours after dosing. No accumulation was observed. One dog given
i.v. 10 mg/kg b.w. tylosin base (dissolved in a minimal amount of
hydrochloric acid) excreted a total of 25.2% of the activity in the
urine. During 5 hours after dosing 13.7% of the dose was recovered
from bile. The bile/serum concentration ratio varied from 1230 to
3780. Four dogs were administered 10 mg/kg b.w tylosin base i.v. (as
an aqueous solution acidified with dilute hydrochloric acid). Blood
t´ was calculated as 48 min. Urinary recovery was 18.8% of the
dose during 6 hours after dosing (15.7% within 2 hours). Serum
levels of tylosin in 4 dogs receiving 25 mg tylosin base
intraduodenally at 0.25, 0.5, 1, 2, 3, 4, and 5 hours averaged 0.78,
1.98, 1.77, 1.94, 0.56, 0.29, and 0.13 mg/l, respectively. Urinary
recovery was 7.2% of the dose in 5 hours (Anderson, et al., 1966).
Thirty mg/kg b.w. tylosin tartrate was administered orally by
gavage or intravenously to groups of pigs (5/group, 30-days old). At
38 days of age the treatments were crossed over. Tylosin activity
was measured in blood samples taken at 10 intervals up to 24 hours
after treatment. After oral administration tylosin activity was
present in plasma 10 minutes after dosing with a peak concentration
of 2.4 mg/l at about 1.5 hours. By comparing the areas under the
curve of the tylosin concentration in blood following the 2 routes
of administration a biological availability of 22.5% was determined
(Shionogo & Co. Ltd., 1981).
Tylosin at 110 mg/kg b.w. (as the granulated phosphate) was
orally administered to 3 male and 3 female pigs. Tylosin activity
was assayed in blood samples taken up to 24 hours after dosing.
Serum activity peaked 1 hour after dosing (average 17.81 mg/l); 24
hours after dosing tylosin was not detectable (< 0.1 mg/l) (Van
Duyn & Kline, undated).
Six pigs (weight 56 kg) were orally given 50 mg/kg b.w. tylosin
phosphate in water. Blood and tissue samples were taken at intervals
up to 24 hours. Tylosin levels were detected in serum from 10
minutes to 8 hours after dosing and peaked at 1 hour (8.53 mg/l).
Tylosin was widely distributed in the body with tissue
concentrations in liver and kidney peaking at 1 hour. No activity
was found in the brain or spinal cord. Highest activity was found in
bile and urine (Nakamura et al., 1969).
Young calves (weight range 44.4-59.0 kg) were injected with
10 mg/kg Tylan 200 either subcutaneously or intramuscularly. The
rate of tylosin absorption, time to peak concentration and decline
of serum concentrations were very similar via both routes of
administration (Thomson, undated a).
Groups of calves (average weight 60 kg) received 10 mg
tylosin/kg b.w. subcutaneously or intramuscularly {as formulations
of tylosin tartrate in water, tylosin tartrate in propylene glycol
and water and tylosin base in propylene glycol and water (Tylan 200
injection)}. Another group of calves received the same dose (as
Tylan 200 injection) intravenously. Tylosin activity in serum was
measured in blood samples taken at various intervals after
treatment. Absorption of the formulations containing tylosin
tartrate was faster after subcutaneous and intramuscular
administration than absorption of the formulation containing tylosin
base (Thomson, undated b).
Holstein calves (1-3 weeks, 38-56 kg) were given 1 or 1 to 5
daily intramuscular injections of 17.6 mg tylosin/kg b.w. (as Tylan
200). In all experiments tylosin levels in serum peaked 2 hours
after administration (average 2.0 mg/l) decreasing to about 0.1 mg/l
at 36 hours. Peak lung tissue levels were observed at 6 to 24 hours
post injection varying from 12.6 to 15.7 mg/l. Lung tissue levels
were greater than serum levels and still detectable (2.2 mg/l) at 48
hours after administration (microbiological assay) (Van Duyn &
Folkerts, 1979; Van Duyn & Johnson (undated a); Van Duyn & Handy
(undated)).
A serum half-life of 1.62 hours was established in cows given a
single i.v. injection of 12.5 mg tylosin/kg b.w. (as Tylan 200). The
apparent specific volume of distribution was 1.10 l/kg indicating no
specific accumulation (Gingerich et al., 1977).
Neonatal holstein calves with a natural occuring pneumonia were
treated intramuscularly with 17.6 mg tylosin/kg b.w. (as Tylan 200)
daily for 3 consecutive days. Healthy calves were subjected to the
same treatment. Six hours following the last dose all the calves
were sacrificed and tylosin activity was measured in the lungs.
Tylosin distributed equally into both normal and pneumonic lung
tissue (Thomson, undated a).
Groups of neonatal healthy calves were fed a milk replacer
containing 1.0 g tylosin (as tylosin tartrate) for 4, 7, and 10
days. Serum samples were taken 4 hours after each dose; the animals
were killed after the final sample had been taken and lung tissues
were analyzed. Mean serum and lung tissue levels were 0.41, 0.37,
and 0.42 mg/l, and 1.76, 3.16, and 3.17 mg/l for the 4, 7, and
10-day treatment groups, respectively. Lung/serum tylosin ratios
were 7.24, 9.36, and 14.01, respectively (Buck et al., undated).
Groups of 4 calves (weight 250 kg) received intramuscular
injections with 10 mg tylosin/kg b.w. (as Tylan 200) for 5 days. The
calves were killed 2, 4, 6, 12, or 72 hours after the last
injection. Tylosin activity was measured in serum and lungs. Tylosin
activity in serum was highest at 4 hours after treatment (1.3 mg/l)
and was no longer detectable after 72 hours using a microbiological
assay whose detection limit was 0.05 mg/l. The mean tylosin activity
in the lungs was 5.9, 5.0, 6.6, 4.4, and 0.6 mg/l at 2, 4, 6, 12,
and 72 hours after treatment, respectively (Lilly, undated a).
Four broiler chickens (weight 720 g) were given a single dose
of 50 mg tylosin/bird (as tylosin tartrate) by stomach intubation.
Tylosin activity was detected in serum after 0.5 hours and peak
concentrations of 0.6-4.0 mg/l were found after 2 hours, declining
to negligible after 24 hours. Repeated oral doses of 50 mg tylosin
to chickens (weight 2 kg, dosed at 1, 2, and 3 hours) caused serum
peak levels at 4 hours (about 0.28 mg/l) declining to negligible at
24 hours (Lilly, undated b, undated c).
Groups of 6 chickens (5-7 weeks old, surgically prepared)
received orally 25, 100 or 250 mg tylosin/kg bw (as tartrate). Urine
and faeces were collected during 72 hours. Peak tylosin levels in
urine (< 100 mg/l at the 25 mg/kg dose and > 1400 mg/l at the
250 mg/kg dose) occurred 2-4 hours after dosing and declined rapidly
thereafter. Peak levels in faeces occurred at 8 hours and varied
from 300 to 2000 µg/g with the dose (Lilly, undated d)
2.1.2 Biotransformation
Four male rats, preconditioned on unlabelled tylosin (10 mg/kg
b.w.) for 3 days, received daily during 5 days by gavage 2 ml of a
solution containing 14C-labelled (in lactone ring) tylosin base.
The rats were killed 4 hours after the last dose; 99% of the
radioactivity was excreted in the faeces and 1% in the urine. The
greatest part of the excreted residues was found to be tylosin
factor A, tylosin factor D and dihydrodesmycosin. Less than
0.25 mg/kg total 14C-residue was found in liver and kidney (Sieck
et al., 1978b).
A male pig, preconditioned on feed containing 110 mg/kg
unlabelled tylosin for 2 weeks, received for 3 days feed with
110 mg/kg of feed tylosin base 14C-labelled in the lactone ring.
Four hours after the last dose the pig was killed. The radioactivity
was excreted 99% in the faeces and 1% in the urine. The majority of
the excreted residues (15% of the radioactivity in faeces was not
extractable) was found to be tylosin factor D (33%), tylosin factor
A (6%) and dihydrodesmycosin. At least 10 minor metabolites (< 5%
of activity) were present in the excreta. In liver and kidneys
< 0.25 mg/kg tylosin was found. At least 4 different metabolites
(of which one was detected as dihydrodesmycosin) were detected in
liver and kidneys (Sieck et al., 1978b).
Three pigs received twice daily for 4 days a ration containing
110 mg tylosin base/kg of feed 14C-labelled in the lactone ring. A
control pig received the basal diet throughout the experiment. Pigs
were sacrificed within 4 hours after the last dose. Total
14C-residues in liver and kidney were < 0.28 mg/kg and in
muscle and < 0.04 mg/kg in fat. Investigations by TLC of liver
residues revealed 5 or 6 metabolites. Two of the metabolites could
be identified as tylosin factor A and dihydrodesmycosin, each
representing about 5% of the total extractable liver residue. Mass
spectrometer analysis of the faeces identified 3 metabolites as
tylosin factor A, tylosin factor D and dihydrodesmycosin (Sieck
et al., 1978a; Sieck et al., 1980).
2.2. Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of tylosin formulations and of tylosin are
given in Tables 1 and 2, respectively.
Table 1. The acute toxicity of tylosin formulations
Species Sex Route LD50 LC50 Reference
(mg/kg b.w) (mg/l)
rat M&F oral > 5001 Gries et al.,
1985a
M&F oral > 0.52 > 1.054 Gries et al.,
1 hr > 0.65 1985b
M&F inhal Gries et al.
M&F inhal 1985c
rabbit M&F dermal > 20001 Gries et al.,
1985a
M&F dermal > 20003 Gries et al.,
1985c
M&F dermal > 2.02 Gries et al.,
1985b
1. administered as granulated tylosin concentrate, a formulation
containing 26.7% of tylosin base activity as the phosphate salt
2. ml/kg b.w. administered as undiluted tylan 200 injection
3. administered as tylan soluble, a dry granular formulation, also
known as tylosin tartrate
4. liquid droplet aerosol of tylan 200/injection formulation at
1.05 mg/litre for 1 hour
5. solid particulate aerosol of tylan soluble at 0.6 mg/litre.
Table 2. The acute toxicity of tylosin
Species Sex Route LD50 Reference
(mg/kg b.w.)
mouse F oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 492.51
- i.p. 594.12
- s.c. 13545
- s.c. 784.11
- s.c. > 25002 Gries et al.,
- i.v. 385.71 1983
- i.v. 581.73
- i.v. 588.84
- i.v. 588.95
F i.v. approx 3216
rat M oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 10011
- i.p. > 25005
- i.v. 6955 Gries et al.,
- s.c. 40831 1985c
- s.c. > 30005
M&F oral > 5005
dog M&F oral > 8002 Anderson & Worth,
undated
1. administered as tylosin phosphate
2. administered as tylosin base
3. administered as tylosin hydrochloride
4. administered as tylosin lactate.
5. administered as tylosin tartrate.
6. administered as 20 mg tylosin/ml of acidified sterile water for
injection, USP (2.0%)
After oral administration no deaths were recorded at the
highest dose used; dogs vomited at 800 mg/kg b.w. but not at
400 mg/kg b.w.; at both these doses the dogs salivated and
defecated. Intravenous and intraperitoneal administration caused
depression, prostration, convulsions and death or recovery within 24
hours.
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of rats (Harlan strain, 5 females/group) received daily
s.c. injections of 10, 20, 50 or 100 mg/kg b.w. tylosin base as a
suspension in 5% acacia gum for 1 month. No effects were seen on
food intake, body weight gain, adrenal weight and terminal blood
cell counts. Macroscopy and microscopy did not show abnormalities
(Anderson et al., 1966; study R2-58). Remark: Summary only.
Groups of rats (Harlan strain, 6/sex/group) received daily s.c.
injections of 100, 250, 500, or 1000 mg/kg b.w. tylosin tartrate or
2.5 ml/kg b.w. saline for 1 month. At doses > 250 mg/kg b.w.
diarrhoea was seen during the first week, regressing to soft stools
(occasionally seen at 100 mg/kg b.w. too) during the remainder of
the study. At doses > 250 mg/kg b.w. scarring and scabbing at the
injection site was seen (occasionally at 100 mg/kg b.w.). No effects
were observed on growth, haematology, organ weight, macroscopy and
microscopy (Anderson et al., 1966; study R19-59). Remark: Summary
only.
Groups of rats (Harlan Wistar, 15/sex/group, F1a offspring
from parents fed the same amount of tylosin base for about 10 weeks
prior to mating and during gestation and lactation) were fed diets
containing 0, 0.1, 0.5 or 1.0% tylosin base for 1 year. No
treatment-related effects were observed on mortality, growth, food
consumption, ophthalmoscopy, organ weights, macroscopy and
histopathology. Treated rats appeared moderately hyperirritable and
hyperactive after 7 to 12 months of treatment. An increase in
lymphocytes and a corresponding reduction in neutrophils were
observed in both sexes (significantly in females) at 0.5 and 1.0%. A
trend towards a slightly more alkaline urine occurred in females at
0.5% and 1.0%. The authors concluded that the NOEL was 1.0% tylosin
base, equivalent to 500 mg/kg b.w. However, the Committee concluded
that the NOEL in this study was 0.1% tylosin base, equivalent to
50 mg/kg b.w. (Broddle et al., 1978a).
2.2.2.2 Dogs
Groups of 8 dogs (2/sex mongrel dogs and 2/sex beagle dogs)
received orally by gelatin capsule during 2 years 0, 1, 10 or 100 mg
tylosin base/kg b.w./day. Groups of mongrel dogs (2/sex/group) given
200 or 400 mg tylosin base/kg b.w./day in capsules for 2 years or
longer were subsequently added. Salivation, vomiting and diarrhoea
were observed at 200 and 400 mg/kg b.w./day (at 100 mg/kg b.w./day 1
dog vomited once). Haematology, urinalysis and relative organ
weights did not reveal abnormalities. No changes were observed in
faecal microbiological flora. Liver and kidney function tests
revealed two dogs at 100 mg/kg b.w./day and 1 dog at 400 mg/kg
b.w./day with a transient increased BSP retention time. Macroscopy
and microscopy did not show compound-related changes except for mild
pyelonephritis seen in 1/4 dogs at 200 mg/kg b.w./day and bilateral
nephrosis, mild chronic pyelonephritis and mild cystitis seen in 1/4
dogs at 400 mg/kg b.w./day. Terminal bone-marrow counts were normal
(only measured in normal study). At dose levels > 10 mg/kg
b.w./day serum tylosin levels in blood could be detected. The NOEL
in this study was 100 mg/kg b.w./day (Anderson et al., 1966; study
D4-59). Remark: The limited details provided and the poor reporting
of the study made proper interpretation difficult.
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
In a limited study rats (3/sex/group) were fed diets containing
0, 0.1, 0.3, or 1.0% tylosin base for 17 months. In the 0.3% group 1
female died due to malnutrition. No effects on growth or on terminal
haematological parameters were seen. Relative organ weights revealed
changes in weights of ovaries and uteri due to thickening in uteri
and a decrease in size of the ovaries in 1/3, 3/3, 2/3, and 2/3
female rats at the 0, 0.1, 0.3, and 1.0% levels, respectively.
Macroscopy and microscopic examination revealed squamous metaplasia
of the uterine glands in 2 female rats at the highest dose (Anderson
et al., 1966; Study R9-58). Remark: Incomplete report.
Groups of about 25 male and female Harlan rats (total 213)
received 0, 0.001, 0.01 or 0.1 % tylosin base in their diet for 2
years. Survival was better in tylosin treated groups than in control
rats (54% and 30% respectively). No effects were seen on growth,
haematology, or relative organ weights. Macroscopy and microscopy
revealed an increased number of animals with fatty changes in livers
and kidney at all dose groups and a slight increased incidence of
bile duct proliferation at the 0.1 and 0.01% levels, but neither was
dose-related (Anderson et al., 1966; Study R10-58). Remark:
Incomplete reports of growth and haematology; limited
histopathology.
In another 2-year study groups of Harlan rats (30/sex/group)
were fed diets containg 0, 0.01, or 1.0% tylosin base. Survival was
better in rats fed tylosin than in control rats (57% and 29%
survival in high dose and control rats, respectively). No effects
were seen on growth, haematology, urinalysis, organ weights,
macroscopy and microscopy. A dose-unrelated increase of fatty
changes in liver and kidney was observed. (Anderson et al., 1966;
R3-59). Remark: Incomplete reports of growth, haematology and
urinalysis.
In a very limited study groups of 10 male and 10 female rats
were fed diets containing 0, 2, 5, 10, or 20% tylosin base for up to
2 years. No effects on food consumption, growth and haematology were
observed on rats at 2% and 5%. At the 10% level, growth and food
consumption were slightly reduced. At the highest dose food
consumption and growth were markedly reduced followed by death
(Anderson et al., 1966; R6-60). Remark: Incomplete reports.
Two replicate 2-year studies were carried out with Wistar rats
(40/sex/group in each study derived from F1a offspring from
parents fed diets containing tylosin from 10 weeks prior to mating
up to the time of weaning). The control groups (60/sex/group in each
replicate study) were derived from parents fed untreated diet.
Treated groups were fed diets containing 0.1, 0.5, or 1.0% tylosin
base. Observations included clinical signs, mortality, food
consumption, food efficiency, body weight, terminal haematology and
biochemistry, urinalysis, organ weights, macroscopy, and
histopathology. There were trends towards improved survival in
males, although overall survival was low (20% and 28% for the
control and treated groups, respectively), and increased food
consumption and body weight gain in both males and females in all
treated groups. At histopathology an increased incidence of
pituitary adenomas in males (but not in females) was observed. The
combined incidence of pituitary adenomas in males for both
replicates was 6/120 (5%) in the control group, 9/80 (11%) in the
low dose (0.1% tylosin) group, 18/80 (22.5%) in the mid dose (0.5%)
group, and 20/80 (25%) in the high dose (1.0%) group. The authors
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. The incidence of malignant tumours was unaffected in males or
in females (Gries, 1980)
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 7-8 male and 14-17 female ICR mice were fed diets
containing 0, 0.1, or 1.0% tylosin (composition unknown) for two
succesive generations with 2 litters/generation. Some of the mice
were maintained on an ordinary diet, but all the mice received the
experimental diet prior to delivery of the offspring. No
treatment-related effects were observed on reproductive performance
(sexual maturation, number of pups or weaning of pups) (Tsubura
et al., undated). Remark: Only a few summary tables were available.
2.2.4.2 Rats
A 3-generation reproduction study was performed with 2 groups
of 5 male and 10 female Harlan rats/group receiving 0 or 1.0%
tylosin base in their diet. Fertility, viability, gestation and
lactation indices in the F0 generation rats did not reveal any
abnormality. Growth was equal in all generations for both control
and treated rats. In each succeeding generation the capability for
reproduction and perpetuation was unaffected (Anderson et al., 1966;
R3-59). Remark: Incomplete report.
In a special study weanling Wistar rats (25/sex/group, 6-7
weeks old) were fed diets containing 0.1, 0.5, or 1.0% tylosin base
for 10 weeks prior to mating and thereafter for about 6 months
total. A control group consisted of 35 rats/sex. Only one litter was
bred. No effects were observed on parental body weight, food
consumption, reproductive indices (male or female fertility,
gestation length, number of live fetuses, mean litter weight or
offspring survival) or biochemistry. High dose male rats revealed
significantly decreased white blood cells at termination. Sera
collected from parental rats (approx. 150 days on experimental
diets) did not contain detectable levels of tylosin (< 0.1 mg/l).
Offspring were physically normal and were assigned to the one year
toxicity study with tylosin (see Section 2.2.2.1) (Broddle, et al.,
1978b). Remark: Only summarized data given on reproductive indices.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1. Mice
Groups of 10 pregnant mice (2 strains, CBA and A/Jax) received
orally 100, 500, or 1000 mg/kg b.w./day tylosin (composition not
given) in 0.1 ml water from days 7-12 of gestation. Two control
groups of 3 and 5 mice received saline or remained untreated,
respectively. Females were killed on day 18 of pregnancy. No effects
were observed on number of corpora lutea, number of implantations,
number of early and late deaths, number of embryos alive, or fetal
development (Tsuchikawa & Akabori, undated).
Four groups of mice given 0 or 500 mg/kg b.w. and a further 2
pregnant females (A/Jax x male CBA) per group, receiving orally 0 or
1000 mg tylosin/kg b.w./day during days 7-12 of gestation, were
allowed to deliver and rear their young for 4 weeks. No effects were
observed in growth, survival, or genital system of all mice born
determined up to 9 weeks. At 8 weeks after birth rearing ability,
hearing ability and kinetic functions were not effected. At the 9th
week the mice were killed and visceral and skeletal examinations
were performed. No abnormalities were seen (Tsuchikawa & Akabori,
undated).
2.2.5.2 Rats
Groups of 15 Wistar rats were fed diets containing 0.1, 1.0, or
10.0% tylosin (composition not given; equal to 60.5, 725, or
4800 mg/kg b.w./day, respectively) in their diet during days 1-20 of
gestation. A control group consisted of 10 rats. Females were killed
on day 20 of gestation. Observations included number of resorptions
and live and dead fetuses, sex ratio, fetal weight and external,
visceral and skeletal abnormalities. Fetus weight at the highest
dose was slightly decreased (Terashima, undated).
In another study 3 groups of 15 Wistar rats received 0, 1.0, or
10.0% tylosin (composition not given, equal to 0, 725, or 4800 mg/kg
b.w.) during days 1-20 of gestation. Normal delivery was allowed.
Number of fetuses, sex ratio, external abnormalities and growth
during the weaning period (3 weeks) were determined. Motor functions
and senses were examined. The weanlings were killed and visceral and
skeletal examinations were performed. A slightly reduced growth was
observed in weanlings at the highest dose (Terashima, undated).
2.2.6 Special studies on genotoxicity
Tylosin was tested for genotoxicity in an in vitro
chromosomal assay with Chinese hamster ovary cells, a mouse lymphoma
assay and an in vivo assay for cytogenetic damage. The results are
summarized in Table 3.
2.2.7 Special studies on microbiological activity
The anti-microbial activity of tylosin has been described in
the published literature. Tylosin is markedly active in vitro
against gram-positive bacteria, certain gram-negative bacteria and
mycobacteria; it is inactive against Enterobacteriaceae (McGuire
et al., 1961).
Table 3: Results of genotoxicity assays on Tylosin
Test system Test Concentration Purity Results Reference
object of substance
tested
Chromosome Chinese 500-1000 99.3 - Gries,
aberration hamster µg/ml2, 1990a
assay1 ovary 250-750
cells µg/ml3, both
in DMSO
Lymphoma Mouse 10-1000 µg/ml2 99.3 +5 Gries,
assay L5178Y 10-1000 µg/ml3 1990b
TK+/- (1000 µg/ml
cells toxic) both in
DMSO
Micronucleus ICR 2 daily doses 966 - Gries,
mice of 1250, 2500 1990c
or 5000 mg/kg4
1. Mitomycin C and cyclophosphamide, used as positive controls, yielded
positive results
2. without metabolic activation
3. with metabolic activation
4. the positive control cyclophosphamide yielded positive results.
5. positive at cytotoxic dose
6. administered as tylosin base
In recent studies the minimal inhibitory concentration (MIC) of
tylosin has been determined for bacterial pathogens isolated from
target animal species of European and North American origin since
1984. Additional information on sensitivity of bacterial isolates to
tylosin was obtained from literature published since 1980. Tylosin
was active against most Gram-positive bacteria and mycoplasmas
tested in vitro. Activity against Gram-negative bacteria was
generally lower. Tylosin was also found to be active against
isolates of Chlamydia psittaci. MIC values for streptocci,
enterococci and staphylococci are given in Table 4 (Herd, 1990).
Table 4. MIC1 values for tylosin against some Gram-positive bacteria
Organism MIC range Total No.
(mg/1) of isolates
Streptococcus pyogenes 0-1 - 0.2 5
Streptococcus pneumoniae 0.2 - 0.4 4
Streptococcus dysgalactiae 0.06 - 128 50
Streptococcus agalactiae 0.125 - 0.5 51
Streptococcus suis 0.125 - > 128 42
Streptococcus uberis 0.125 - > 128 53
Enterococcus faecalis 0.25 - > 128 31
Staphylococcus aureus2 0.125 - > 128 98
Staphylococcus Spp.3 0.78 - 1.0 7
1. Minimum inhibitory concentration
2. Coagulase - positive
3. Coagulase - negative
2.2.8 Special studies on neurotoxicity
Three cats received daily for 90 days subcutaneously 200 mg/kg
b.w. tylosin tartrate as a 20% solution divided in 2 doses during
the first 37 days and thereafter as a single dose (with addition of
sodium citrate for buffering). An untreated control group consisted
of 3 cats. Slight reduction (25-35%) was observed in the
post-rotatory nystagmus response, but the auditory response appeared
normal. All cats landed on their four feet when dropped from 1
metre. No ataxia was seen (Anderson, 1966; C2-59)
2.2.9 Special studies on skin and eye irritation
Granulated tylosin concentrate (2 g/kg b.w.) was not irritating
when applied to the rabbit skin but when the formulation (52 mg
(0.1 ml)) was instilled to the rabbit eye corneal dullness, slight
corneal opacity, slight to marked irititis and moderate
conjunctivitis were observed. The effects resolved within 14 days of
treatment (Gries et al., 1985a).
The formulation Tylan 200 injection (2 ml/kg b.w.) caused very
slight erythema to the rabbit skin and the formulation (0.1 ml)
caused slight conjunctival hyperemia to the rabbit eye both within
24 hours after application. No skin and eye irritation were observed
after 48 hours (Gries, et al., 1985b).
The formulation Tylan soluble (2 g/kg b.w.) caused very slight
erythema and slight desquamation to the rabbit skin. Slight to
moderate corneal opacity, marked iritis and moderate conjunctivitis
were observed following the instillation of 58 mg of the formulation
into the rabbit eye; these effects had cleared within a week (Gries
et al., 1985c).
2.2.10 Special studies on skin sensitization
Tylosin tartrate was used as a positive control in a
sensitization test by the method of Landsteiner and Jacobs conducted
for tilmicosin. Guinea-pigs (12) were administered 10 intracutaneous
injections of 50 mg/ml tylosin tartrate; a challenge with a further
dose was given after 14 days. At challenge a mild positive response
was observed (Jordan et al., 1989).
2.3. Observations in humans
Trials in human subjects have been conducted to investigate
antibiotic-resistant bacteria. In one trial 12 or 11 human
volunteers were given 20 mg tylosin per day or a placebo,
respectively, for up to 6 months. There was no significant change in
the total number of tylosin-resistant staphylococci and lactobacilli
in weekly faecal samples or in coliform or yeast forms whereas a
significant increase was observed in total number of resistant
streptococci. In another trial no tylosin resistant organisms were
detected in specimens (including faeces samples) from hospitalized
patients who had never had any exposure to the nonmedical antibiotic
tylosin. In both studies no regular pattern was observed in
cross-resistance to related antibiotics (Malin & Silliker, 1966)
In a Japanese trial 2 human volunteers were given 2 or 5 mg
tylosin (as 1 mg tablets; composition not given) per day for 3
months. Faeces were inspected for E. coli and Enterococci and
Staphylococci at intervals of 1 to 2 weeks from 2 months prior to
administration and 3 months from the start of administration. No
tendency towards increased resistance was noted (Kuwabara, undated).
Tylosin resistance was examined in human cultures of
Staphylococcus aureus, Streptococcus pyogenes and Campylobacter
spp. Of 3812 human cultures isolated between 1985 and 1987 only
1.0% were resistant to tylosin. There was no evidence for a
significant animal source of these resistant cultures (Lacey, 1988).
3. COMMENTS
The Committee considered toxicological data on tylosin,
including the results of studies on biochemical aspects,
mutagenicity, and microbiological activity. The Committee noted that
most of the toxicity studies had been carried out about 20 years
ago, had not been conducted according to current protocols, and were
poorly reported.
Following administration of tylosin by various routes, peak
serum levels in rats, dogs, pigs, and cattle were observed within
1-2 hours, and then declined rapidly. In pigs, about 22% was
bioavailable after oral administration. Excretion of tylosin was
rapid and largely in the bile.
After oral administration of radiolabelled tylosin to rats and
pigs 99% of the radioactivity was excreted via the faeces. Major
products identified in faeces were tylosin (factor A), macrosin
(factor C), relomycin (factor D), and dihydrodesmycosin. In pig
liver and kidney, only very small amounts of tylosin and
dihydrodesmycosin could be found.
Several short- and long-term studies in rats and dogs were
performed. In a 1-year study in rats, tylosin base was administered
in the diet at concentrations up to 10 g/kg of feed. A NOEL of
1 g/kg of feed, equivalent to 50 mg/kg b.w./day, was established,
based on haematological and urinary pH changes. In a study in dogs,
tylosin base was administered orally at dose levels up to 400 mg/kg
b.w./day for 2 years. At the two highest dose levels, salivation,
vomiting, and diarrhoea as well as mild pyelonephritis were
observed. The NOEL was 100 mg/kg b.w./day.
In two replicate, but not independent, carcinogenicity studies
in rats, tylosin base was administered in the diet for 2 years at
levels up to 10 g/kg of feed. Food consumption and body-weight gain
were increased in both males and females in all treated groups. In
male rats, a dose-related increase in pituitary adenomas was
observed, from 5% in the control to 25% in the highest-dose group.
There was no such increase in female rats. The authors of the report
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. However, this hypothesis was neither tested experimentally nor
verifiable in detail by the Committee because individual body
weights at about 12 months of age were not available.
No effects on reproduction performance were observed in a
two-generation study in mice and in one- and three-generation
studies in rats. No malformations were observed in mice or rats, but
the Committee noted that these studies were poorly reported.
Tylosin was not mutagenic in an in vitro test for chromosomal
aberrations and in an in vivo micronucleus test. In a mouse
lymphoma assay, no activity was found with metabolic activation;
however a weak, but significant activity was observed in the absence
of such activation.
In studies in human volunteers, there was no evidence of the
emergence of cross-resistance to therapeutically important
antibiotics, but volunteers given oral doses of 20 mg of tylosin
daily for 6 months showed an increase in the number of resistant
streptococci. The Committee concluded that additional studies
showing no microbiological effects in two individuals at doses up to
5 mg/person/day were inadequate to establish a NOEL. In addition, no
suitable in vitro data were available to establish a NOEL with
respect to the microbiological risk for humans.
4. EVALUATION
Because of the deficiencies in the toxicological and
microbiological data, the Committee was not able to establish an
ADI.
5. REFERENCES
ANDERSON, R.C. (undated). Tylosin absorption and excretion studies.
Study numbered 893//FAANIM/AM/14-26. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C., HARRIS, P.N., LEE, C.C., MAZE, N., SMALL, R.M. &
WORTH, H.M. (1966) The toxicology and pharmacology of tylosin, an
antibiotic, and some salts of tylosin. Unpublished Report Ref.
X/E/4. dated 19 December 1966 from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C. & WORTH, H.M. (undated) The acute toxicity of tylosin
phosphate. Unpublished report from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO under
893/TACUTE/AM//1-26 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., GRIES,
C.L., GIBSON, W.R. & MORTON, D.M. (1978a) Chronic toxicity of
tylosin fed to rats for one year. Unpublished Report from study
R-307 from Toxicology Division, Lilly Research Laboratories.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., KITCHEN,
D.N., GIBSON, W.R. & MORTON, D.M. (1978b) A study of a parental
population of rats bred to produce offspring assigned to one- and
two-year dietary studies of tylosin. Unpublished Report Study No.
R-1176 dated October 1978 from Toxicology Division, Lilly Research
Laboratories. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
BUCK, A.M., COOPER, T.R. & THOMSON, T.D. (undated) Serum and lung
tylosin activity in neonatal calves treated with 1.0 gram tylosin
tartrate B.I.D. in milk replacer. Unpublished Report T1Y768501 from
Lilly Research Centre Laboratories, Animal Health Applications
Research, Greenfield, Indiana. Submitted to WHO under
893/SP/FBLDLV/AM/10/10-15 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
GINGERICH, D.A., BAGGOT, J.D. & KOWALSKI, J.J. (1977) Tylosin
antimicrobial activity and pharmacokinetics in cows. Can. Vet.
Jour., 18(4), 96-100.
GRIES, C.L. (1980) The toxicological evaluation of tylosin (compound
27892) given to Wistar rats in the diet for two years. Unpublished
report from studies R-287 and R-297 from Toxicology Division, Lilly
Research Laboratories. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
GRIES, C.L., McKINLEY, E.R. & QUARLES, J.P. (1983) Acute comparative
intravenous toxicity testing of tylosin, desmycosin and macrocin in
the ICR mouse. Unpublished report dated August 1983 with studies
M-V-46-83, M-V-45-83, and M-V-44-83 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985a) The acute oral,
dermal and ocular toxicity of granulated tylosin concentrate.
Unpublished report dated April, 1985 with studies R-O-365-79,
R-366-79, B-D-109-79, and B-E-94-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985b) The acute oral,
dermal, ocular and inhalation toxicity of tylan 200 injection.
Unpublished report dated September 1985 with studies R-O-344-79,
R-O-343-79, B-D-103-79, B-E-87-79, and R-H-39-79 from Toxicology
Division, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
GRIES, C.L., NEGILSKI, D.S. & DOWNS, O.S. (1985c) The acute oral,
dermal, ocular and inhalation toxicity of tylan soluble. Unpublished
report dated September 1985 with studies R-O-367-79, R-O-368-70,
B-D-94-79, B-E-90-79, and R-H-40-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L. (1990a) The effect of tylosin on the in vitro
induction of chromosome aberrations in chinese hamster ovary cells.
Unpublished report with studies 891109CTX3279, 891129CAB3279, and
891220CAB3279 from Lilly Research Laboratories, Toxicology Division,
Greenfield, Indiana.
GRIES, C.L. (1990b) The effect of tylosin on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished report with studies 891011MLT3279,
891017MLA3279, and 891114MLA3279 from Lilly Research Laboratories,
Toxicology Division, Greenfield, Indiana.
GRIES, C.L. (1990c) The effect of tylosin (compound 027892) on the
in vivo induction of micronuclei in bone marrow of ICR mice.
Unpublished report, study 891212MNT3279 from Lilly Research
Laboratories, Toxicology Division, Greenfield, Indiana.
HERD, R.M. (1990) The antimicrobial activity of tylosin in vitro
1980-1989. Unpublished Report dated 27-06-90 from Lilly Research
Centre Ltd. Submitted to WHO by Lilly, Windlesham, Surrey U.K.
JORDAN, W.H., GARDNER, J.B. & WEAVER, D.E. (1989) An intracutaneous
sensitization stidy in albino guinea pigs with tilmicosin.
Unpublished toxicology report No. 32 from Toxicology divison, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
KUWABARA, S. (undated) A study on the effect of continuous and
minute amount of tylosin on human intestinal flora. Medical
Department Toho University. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
LACEY, R.W. (1988) Rarity of tylosin resistance in human pathogenic
bacteria. Report from Department of Microbiology, University of
Leeds, Leeds, England. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
LILLY (undated a) The determination of tylosin levels in lung and
serum following administration of tylan 200 by intramuscular
injection to weaned calves. Unpublished Report T1Z769001.
Supplementary information submitted to WHO under
893/IJB200/FDISTR/AM/26-35 by Lilly Research Centre, Windlesham,
Surrey, England.
LILLY (undated b) Tylosin absorption studies in chickens.
Unpublished Report VPR-17-418 submitted under 893//FAANIM/AM/1-9 to
WHO by Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated c) Tylosin blood titres in chickens. Unpublished
Report VPR-21-418 submitted to WHO under 893//FBLDLV/AM//1-3 by
Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated d) Tylosin excretion studies in chickens. Unpublished
Report VPR-67-418 submitted to WHO under 893//FEXCRE/AM//6-13 WHO by
Lilly Research Centre, Windlesham, Surrey, England.
MALIN, B. & SILLIKER, J.H. (1966) Low level tylosin and the
emergence of antibiotic-resistant bacteria in humans. Antimicrobial
agents and chemotherapy, 1966. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
McGUIRE, J.M., BONIECE, W.S., HIGGENS, C.E., HOEHN, M.M., STARK,
W.M., WESTHEAD, J. & WOLFE, R.N. (1961) Tylosin, a new antibiotic: I
Microbiological studies. Antibiotics & Chemotherapy, XI (5), 1961,
320-327.
NAKAMURA, H. ET AL. (1969) Studies on distribution of antibiotics
inblood and tissue. XIX. Blood level and tissue concentration of
tylosin in pigs after oral administration of tylosin phosphate.
Proc. Jap. Soc. Vet. Science, 67th meeting, No. 128.
SHIONOGO & Co., Ltd. (1981) Blood levels of tylosin tartrate
following oral administration and intravenous injection in pigs.
Report No. 3174674755 from Department of Animal Science Development,
Shionogo & Co., Ltd. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978a) 14C tylosin tissue residue study in swine.
Unpublished Report dated November 1978 from Agricultural
Biochemistry, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978b) Metabolism of tylosin in swine and rat. Unpublished
Report dated November 1978 from Agricultural Biochemistry, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
SIECK, R.F. ET AL. (1980) 14C tylosin residue study in swine.
Experiment ABC-0016 dated March 1980 from Agricultural Biochemistry,
Lilly Research Laboratories, Greenfield, Indiana. Submitted to WHO
by Lilly Research Centre Ltd.. Windlesham, Surrey, England.
TERASHIMA, H. (undated) The effect of tylosin on a fetus and a
suckling-young of Wistar strain rat. Department of Pathology,
Medical School of Osaka City University. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
THOMSON, T.D. (undated a) Relative bioavailability of TYLAN 200
injection administered subcutaneously and intramuscularly in young
calves. Unpublished Report T1Z768106 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana,. Submitted
to WHO under 893/IJB200/FAANIM/AM/1-6 by Lilly Research Centre,
Windlesham, Surrey, England.
THOMSON, T.D. (undated b). The relative absorption rates of tylosin
base and tylosin tartrate from aqueous and polypropylene glycol
vehicles. Unpublished Report T1Z768107 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana. Submitted to
WHO under 893/IJB200/FAANIM/AM/6-19 by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
THOMSON, T.D. (undated c). Tylosin distribution in pneumonic calf
lung. Unpublished Report T1Z768203 from Lilly Research Laboratories,
Animal Science Division, Greenfield, Indiana. Submitted to WHO under
893/IJB200/FDISTR/AM/1-6 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
TSUBURA, Y., TOYOSHIMA, K., SANO, S., NISHII, Y. & TANI, M.
(undated) Effect of Tylosin on mouse breeding. Report from Second
Department of Pathology Nara Medical College. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
TSUCHIKAWA, K. & AKABORI, A. (undated) On the teratogenicity of
tylosin. Report from National Institute for Genetics, Shionogi and
Co. Submitted to WHO by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
VAN DUYN, R.L. & HANDY, P.R. (undated) Tylosin serum and lung tissue
levels following five daily intramuscular injections. Unpublished
Report 766-G125-86 from Lilly Research Laboratories, Animal Science
Division, Greenfield, Indiana. Submitted to WHO under
893/IJB/FBLDLV/AM/37-43 by Lilly Research Centre, Windlesham,
Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated a) Tylosin serum and lung
tissue levels following a single intramuscular injection with
tylosin in calves. Unpublished Report 766-G125-73 from Lilly
Research Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/24-30 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated b) Tylosin levels in lung
tissue and serum from calves injected once intramuscularly with
tylosin base. Unpublished Report VPR-352-766 from Lilly Research
Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/31-26 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & VOLKERTS, T.M. (1979) Concentrations of tylosin in
blood and lung tissue from calves given single and repeated daily
intramuscular doses. Vet. Med., March 1979, 375/377.
VAN DUYN, R.L. & KLINE, R.M. (undated) Tylosin minigranule oral
blood levels in swine. Pharmacological experiment VPR-275-766
submitted to WHO under 893/G/FBLDLV/AM/1-2 by Lilly Research Centre,
Windlesham, Surrey, England.
Tylosin was evaluated at the 12th meeting of the Joint FAO/WHO
Expert Committee on Food Additives in 1968 (Annex 1, reference 17).
No ADI was established. It was concluded that tylosin used in animal
feed or in veterinary medicine should not give rise to detectable
residues in edible products of animal origin; when using the
recommended methods of analysis it will be possible to ensure that
residues in meat for human consumption not exceed 0.2 ppm. Since
that time additional data have become available which are summarized
and discussed in this monograph addendum (Annex 1, reference 17).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Fasted rats received a single oral dose of 50 mg/kg b.w.
tylosin base or tylosin tartrate. Tylosin activity was assayed in
serum after 15 and 30 minutes and 1, 2, 4, 5, 7, and 24 hours after
treatment. Peak serum levels < 1.0 mg/l were seen after 1-2
hours. Within 7 hours serum levels decreased to less than the limit
of detection (i.e. 0.10 mg/l, microbiological assay). Four rats were
given i.p. 100 mg/kg b.w. tylosin base. Bile samples were collected
for 2 hours. The bile/serum concentration ratio ranged from 143-266
(Anderson et al., 1966).
Rabbits received i.m. 10 mg/kg b.w. tylosin base as a 5%
aqueous solution acidified with hydrochloric acid to pH 5.5. Serum
levels were determined after 1.5, 4, 7, and 24 hours. Peak serum
levels ranging from 0.57 to 0.88 mg/l were observed after 1.5 hours.
A similar study was carried out using tylosin tartrate in aqueous
(25 mg/kg b.w.) as well as in PEG-200 (10 mg/kg b.w.) solutions.
Peak serum levels at 1.0 hour were 4.7-7.2 and 0.96-1.25,
respectively. Within 24 hours serum levels were below the limits of
detection, which were 0.05 mg/l for tylosin hydrochloride and
0.10 mg/l for tylosin tartrate.
Two dogs given 25 mg/kg b.w. tylosin base orally in capsules
excreted 2% of the dose in the urine within 5 hours. Serum
concentrations were very low, < 0.05 and 3.3 mg/l at 2.5 hours
(microbiological assay). In another study groups of 8 dogs received
orally by capsule 1, 10, or 100 mg/kg b.w./day for 8 days. Blood
levels determined 2 hours after the last dose ranged from
< 0.15 mg/ml to 9.5 mg/ml mostly ranging with the dose. Two dogs
received 25 or 100 mg/kg b.w. tylosin base orally by capsule daily
during 29 days. Serum levels were determined 0, 1, 2, 3, 4, 5, 6,
and 7 hours after the 1st, 15th, and 29th dose. At 25 mg/kg b.w/day
peak serum levels (1.4-2.7 mg/l) were seen 2 hours after dosing and
at 100 mg/kg b.w./day the highest levels (2.7-4.6 mg/l) were seen
2-5 hours after dosing. No accumulation was observed. One dog given
i.v. 10 mg/kg b.w. tylosin base (dissolved in a minimal amount of
hydrochloric acid) excreted a total of 25.2% of the activity in the
urine. During 5 hours after dosing 13.7% of the dose was recovered
from bile. The bile/serum concentration ratio varied from 1230 to
3780. Four dogs were administered 10 mg/kg b.w tylosin base i.v. (as
an aqueous solution acidified with dilute hydrochloric acid). Blood
t´ was calculated as 48 min. Urinary recovery was 18.8% of the
dose during 6 hours after dosing (15.7% within 2 hours). Serum
levels of tylosin in 4 dogs receiving 25 mg tylosin base
intraduodenally at 0.25, 0.5, 1, 2, 3, 4, and 5 hours averaged 0.78,
1.98, 1.77, 1.94, 0.56, 0.29, and 0.13 mg/l, respectively. Urinary
recovery was 7.2% of the dose in 5 hours (Anderson, et al., 1966).
Thirty mg/kg b.w. tylosin tartrate was administered orally by
gavage or intravenously to groups of pigs (5/group, 30-days old). At
38 days of age the treatments were crossed over. Tylosin activity
was measured in blood samples taken at 10 intervals up to 24 hours
after treatment. After oral administration tylosin activity was
present in plasma 10 minutes after dosing with a peak concentration
of 2.4 mg/l at about 1.5 hours. By comparing the areas under the
curve of the tylosin concentration in blood following the 2 routes
of administration a biological availability of 22.5% was determined
(Shionogo & Co. Ltd., 1981).
Tylosin at 110 mg/kg b.w. (as the granulated phosphate) was
orally administered to 3 male and 3 female pigs. Tylosin activity
was assayed in blood samples taken up to 24 hours after dosing.
Serum activity peaked 1 hour after dosing (average 17.81 mg/l); 24
hours after dosing tylosin was not detectable (< 0.1 mg/l) (Van
Duyn & Kline, undated).
Six pigs (weight 56 kg) were orally given 50 mg/kg b.w. tylosin
phosphate in water. Blood and tissue samples were taken at intervals
up to 24 hours. Tylosin levels were detected in serum from 10
minutes to 8 hours after dosing and peaked at 1 hour (8.53 mg/l).
Tylosin was widely distributed in the body with tissue
concentrations in liver and kidney peaking at 1 hour. No activity
was found in the brain or spinal cord. Highest activity was found in
bile and urine (Nakamura et al., 1969).
Young calves (weight range 44.4-59.0 kg) were injected with
10 mg/kg Tylan 200 either subcutaneously or intramuscularly. The
rate of tylosin absorption, time to peak concentration and decline
of serum concentrations were very similar via both routes of
administration (Thomson, undated a).
Groups of calves (average weight 60 kg) received 10 mg
tylosin/kg b.w. subcutaneously or intramuscularly {as formulations
of tylosin tartrate in water, tylosin tartrate in propylene glycol
and water and tylosin base in propylene glycol and water (Tylan 200
injection)}. Another group of calves received the same dose (as
Tylan 200 injection) intravenously. Tylosin activity in serum was
measured in blood samples taken at various intervals after
treatment. Absorption of the formulations containing tylosin
tartrate was faster after subcutaneous and intramuscular
administration than absorption of the formulation containing tylosin
base (Thomson, undated b).
Holstein calves (1-3 weeks, 38-56 kg) were given 1 or 1 to 5
daily intramuscular injections of 17.6 mg tylosin/kg b.w. (as Tylan
200). In all experiments tylosin levels in serum peaked 2 hours
after administration (average 2.0 mg/l) decreasing to about 0.1 mg/l
at 36 hours. Peak lung tissue levels were observed at 6 to 24 hours
post injection varying from 12.6 to 15.7 mg/l. Lung tissue levels
were greater than serum levels and still detectable (2.2 mg/l) at 48
hours after administration (microbiological assay) (Van Duyn &
Folkerts, 1979; Van Duyn & Johnson (undated a); Van Duyn & Handy
(undated)).
A serum half-life of 1.62 hours was established in cows given a
single i.v. injection of 12.5 mg tylosin/kg b.w. (as Tylan 200). The
apparent specific volume of distribution was 1.10 l/kg indicating no
specific accumulation (Gingerich et al., 1977).
Neonatal holstein calves with a natural occuring pneumonia were
treated intramuscularly with 17.6 mg tylosin/kg b.w. (as Tylan 200)
daily for 3 consecutive days. Healthy calves were subjected to the
same treatment. Six hours following the last dose all the calves
were sacrificed and tylosin activity was measured in the lungs.
Tylosin distributed equally into both normal and pneumonic lung
tissue (Thomson, undated a).
Groups of neonatal healthy calves were fed a milk replacer
containing 1.0 g tylosin (as tylosin tartrate) for 4, 7, and 10
days. Serum samples were taken 4 hours after each dose; the animals
were killed after the final sample had been taken and lung tissues
were analyzed. Mean serum and lung tissue levels were 0.41, 0.37,
and 0.42 mg/l, and 1.76, 3.16, and 3.17 mg/l for the 4, 7, and
10-day treatment groups, respectively. Lung/serum tylosin ratios
were 7.24, 9.36, and 14.01, respectively (Buck et al., undated).
Groups of 4 calves (weight 250 kg) received intramuscular
injections with 10 mg tylosin/kg b.w. (as Tylan 200) for 5 days. The
calves were killed 2, 4, 6, 12, or 72 hours after the last
injection. Tylosin activity was measured in serum and lungs. Tylosin
activity in serum was highest at 4 hours after treatment (1.3 mg/l)
and was no longer detectable after 72 hours using a microbiological
assay whose detection limit was 0.05 mg/l. The mean tylosin activity
in the lungs was 5.9, 5.0, 6.6, 4.4, and 0.6 mg/l at 2, 4, 6, 12,
and 72 hours after treatment, respectively (Lilly, undated a).
Four broiler chickens (weight 720 g) were given a single dose
of 50 mg tylosin/bird (as tylosin tartrate) by stomach intubation.
Tylosin activity was detected in serum after 0.5 hours and peak
concentrations of 0.6-4.0 mg/l were found after 2 hours, declining
to negligible after 24 hours. Repeated oral doses of 50 mg tylosin
to chickens (weight 2 kg, dosed at 1, 2, and 3 hours) caused serum
peak levels at 4 hours (about 0.28 mg/l) declining to negligible at
24 hours (Lilly, undated b, undated c).
Groups of 6 chickens (5-7 weeks old, surgically prepared)
received orally 25, 100 or 250 mg tylosin/kg bw (as tartrate). Urine
and faeces were collected during 72 hours. Peak tylosin levels in
urine (< 100 mg/l at the 25 mg/kg dose and > 1400 mg/l at the
250 mg/kg dose) occurred 2-4 hours after dosing and declined rapidly
thereafter. Peak levels in faeces occurred at 8 hours and varied
from 300 to 2000 µg/g with the dose (Lilly, undated d)
2.1.2 Biotransformation
Four male rats, preconditioned on unlabelled tylosin (10 mg/kg
b.w.) for 3 days, received daily during 5 days by gavage 2 ml of a
solution containing 14C-labelled (in lactone ring) tylosin base.
The rats were killed 4 hours after the last dose; 99% of the
radioactivity was excreted in the faeces and 1% in the urine. The
greatest part of the excreted residues was found to be tylosin
factor A, tylosin factor D and dihydrodesmycosin. Less than
0.25 mg/kg total 14C-residue was found in liver and kidney (Sieck
et al., 1978b).
A male pig, preconditioned on feed containing 110 mg/kg
unlabelled tylosin for 2 weeks, received for 3 days feed with
110 mg/kg of feed tylosin base 14C-labelled in the lactone ring.
Four hours after the last dose the pig was killed. The radioactivity
was excreted 99% in the faeces and 1% in the urine. The majority of
the excreted residues (15% of the radioactivity in faeces was not
extractable) was found to be tylosin factor D (33%), tylosin factor
A (6%) and dihydrodesmycosin. At least 10 minor metabolites (< 5%
of activity) were present in the excreta. In liver and kidneys
< 0.25 mg/kg tylosin was found. At least 4 different metabolites
(of which one was detected as dihydrodesmycosin) were detected in
liver and kidneys (Sieck et al., 1978b).
Three pigs received twice daily for 4 days a ration containing
110 mg tylosin base/kg of feed 14C-labelled in the lactone ring. A
control pig received the basal diet throughout the experiment. Pigs
were sacrificed within 4 hours after the last dose. Total
14C-residues in liver and kidney were < 0.28 mg/kg and in
muscle and < 0.04 mg/kg in fat. Investigations by TLC of liver
residues revealed 5 or 6 metabolites. Two of the metabolites could
be identified as tylosin factor A and dihydrodesmycosin, each
representing about 5% of the total extractable liver residue. Mass
spectrometer analysis of the faeces identified 3 metabolites as
tylosin factor A, tylosin factor D and dihydrodesmycosin (Sieck
et al., 1978a; Sieck et al., 1980).
2.2. Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of tylosin formulations and of tylosin are
given in Tables 1 and 2, respectively.
Table 1. The acute toxicity of tylosin formulations
Species Sex Route LD50 LC50 Reference
(mg/kg b.w) (mg/l)
rat M&F oral > 5001 Gries et al.,
1985a
M&F oral > 0.52 > 1.054 Gries et al.,
1 hr > 0.65 1985b
M&F inhal Gries et al.
M&F inhal 1985c
rabbit M&F dermal > 20001 Gries et al.,
1985a
M&F dermal > 20003 Gries et al.,
1985c
M&F dermal > 2.02 Gries et al.,
1985b
1. administered as granulated tylosin concentrate, a formulation
containing 26.7% of tylosin base activity as the phosphate salt
2. ml/kg b.w. administered as undiluted tylan 200 injection
3. administered as tylan soluble, a dry granular formulation, also
known as tylosin tartrate
4. liquid droplet aerosol of tylan 200/injection formulation at
1.05 mg/litre for 1 hour
5. solid particulate aerosol of tylan soluble at 0.6 mg/litre.
Table 2. The acute toxicity of tylosin
Species Sex Route LD50 Reference
(mg/kg b.w.)
mouse F oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 492.51
- i.p. 594.12
- s.c. 13545
- s.c. 784.11
- s.c. > 25002 Gries et al.,
- i.v. 385.71 1983
- i.v. 581.73
- i.v. 588.84
- i.v. 588.95
F i.v. approx 3216
rat M oral > 62001 Anderson & Worth,
- oral > 50002 undated
- oral > 62005
- i.p. 10011
- i.p. > 25005
- i.v. 6955 Gries et al.,
- s.c. 40831 1985c
- s.c. > 30005
M&F oral > 5005
dog M&F oral > 8002 Anderson & Worth,
undated
1. administered as tylosin phosphate
2. administered as tylosin base
3. administered as tylosin hydrochloride
4. administered as tylosin lactate.
5. administered as tylosin tartrate.
6. administered as 20 mg tylosin/ml of acidified sterile water for
injection, USP (2.0%)
After oral administration no deaths were recorded at the
highest dose used; dogs vomited at 800 mg/kg b.w. but not at
400 mg/kg b.w.; at both these doses the dogs salivated and
defecated. Intravenous and intraperitoneal administration caused
depression, prostration, convulsions and death or recovery within 24
hours.
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of rats (Harlan strain, 5 females/group) received daily
s.c. injections of 10, 20, 50 or 100 mg/kg b.w. tylosin base as a
suspension in 5% acacia gum for 1 month. No effects were seen on
food intake, body weight gain, adrenal weight and terminal blood
cell counts. Macroscopy and microscopy did not show abnormalities
(Anderson et al., 1966; study R2-58). Remark: Summary only.
Groups of rats (Harlan strain, 6/sex/group) received daily s.c.
injections of 100, 250, 500, or 1000 mg/kg b.w. tylosin tartrate or
2.5 ml/kg b.w. saline for 1 month. At doses > 250 mg/kg b.w.
diarrhoea was seen during the first week, regressing to soft stools
(occasionally seen at 100 mg/kg b.w. too) during the remainder of
the study. At doses > 250 mg/kg b.w. scarring and scabbing at the
injection site was seen (occasionally at 100 mg/kg b.w.). No effects
were observed on growth, haematology, organ weight, macroscopy and
microscopy (Anderson et al., 1966; study R19-59). Remark: Summary
only.
Groups of rats (Harlan Wistar, 15/sex/group, F1a offspring
from parents fed the same amount of tylosin base for about 10 weeks
prior to mating and during gestation and lactation) were fed diets
containing 0, 0.1, 0.5 or 1.0% tylosin base for 1 year. No
treatment-related effects were observed on mortality, growth, food
consumption, ophthalmoscopy, organ weights, macroscopy and
histopathology. Treated rats appeared moderately hyperirritable and
hyperactive after 7 to 12 months of treatment. An increase in
lymphocytes and a corresponding reduction in neutrophils were
observed in both sexes (significantly in females) at 0.5 and 1.0%. A
trend towards a slightly more alkaline urine occurred in females at
0.5% and 1.0%. The authors concluded that the NOEL was 1.0% tylosin
base, equivalent to 500 mg/kg b.w. However, the Committee concluded
that the NOEL in this study was 0.1% tylosin base, equivalent to
50 mg/kg b.w. (Broddle et al., 1978a).
2.2.2.2 Dogs
Groups of 8 dogs (2/sex mongrel dogs and 2/sex beagle dogs)
received orally by gelatin capsule during 2 years 0, 1, 10 or 100 mg
tylosin base/kg b.w./day. Groups of mongrel dogs (2/sex/group) given
200 or 400 mg tylosin base/kg b.w./day in capsules for 2 years or
longer were subsequently added. Salivation, vomiting and diarrhoea
were observed at 200 and 400 mg/kg b.w./day (at 100 mg/kg b.w./day 1
dog vomited once). Haematology, urinalysis and relative organ
weights did not reveal abnormalities. No changes were observed in
faecal microbiological flora. Liver and kidney function tests
revealed two dogs at 100 mg/kg b.w./day and 1 dog at 400 mg/kg
b.w./day with a transient increased BSP retention time. Macroscopy
and microscopy did not show compound-related changes except for mild
pyelonephritis seen in 1/4 dogs at 200 mg/kg b.w./day and bilateral
nephrosis, mild chronic pyelonephritis and mild cystitis seen in 1/4
dogs at 400 mg/kg b.w./day. Terminal bone-marrow counts were normal
(only measured in normal study). At dose levels > 10 mg/kg
b.w./day serum tylosin levels in blood could be detected. The NOEL
in this study was 100 mg/kg b.w./day (Anderson et al., 1966; study
D4-59). Remark: The limited details provided and the poor reporting
of the study made proper interpretation difficult.
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Rats
In a limited study rats (3/sex/group) were fed diets containing
0, 0.1, 0.3, or 1.0% tylosin base for 17 months. In the 0.3% group 1
female died due to malnutrition. No effects on growth or on terminal
haematological parameters were seen. Relative organ weights revealed
changes in weights of ovaries and uteri due to thickening in uteri
and a decrease in size of the ovaries in 1/3, 3/3, 2/3, and 2/3
female rats at the 0, 0.1, 0.3, and 1.0% levels, respectively.
Macroscopy and microscopic examination revealed squamous metaplasia
of the uterine glands in 2 female rats at the highest dose (Anderson
et al., 1966; Study R9-58). Remark: Incomplete report.
Groups of about 25 male and female Harlan rats (total 213)
received 0, 0.001, 0.01 or 0.1 % tylosin base in their diet for 2
years. Survival was better in tylosin treated groups than in control
rats (54% and 30% respectively). No effects were seen on growth,
haematology, or relative organ weights. Macroscopy and microscopy
revealed an increased number of animals with fatty changes in livers
and kidney at all dose groups and a slight increased incidence of
bile duct proliferation at the 0.1 and 0.01% levels, but neither was
dose-related (Anderson et al., 1966; Study R10-58). Remark:
Incomplete reports of growth and haematology; limited
histopathology.
In another 2-year study groups of Harlan rats (30/sex/group)
were fed diets containg 0, 0.01, or 1.0% tylosin base. Survival was
better in rats fed tylosin than in control rats (57% and 29%
survival in high dose and control rats, respectively). No effects
were seen on growth, haematology, urinalysis, organ weights,
macroscopy and microscopy. A dose-unrelated increase of fatty
changes in liver and kidney was observed. (Anderson et al., 1966;
R3-59). Remark: Incomplete reports of growth, haematology and
urinalysis.
In a very limited study groups of 10 male and 10 female rats
were fed diets containing 0, 2, 5, 10, or 20% tylosin base for up to
2 years. No effects on food consumption, growth and haematology were
observed on rats at 2% and 5%. At the 10% level, growth and food
consumption were slightly reduced. At the highest dose food
consumption and growth were markedly reduced followed by death
(Anderson et al., 1966; R6-60). Remark: Incomplete reports.
Two replicate 2-year studies were carried out with Wistar rats
(40/sex/group in each study derived from F1a offspring from
parents fed diets containing tylosin from 10 weeks prior to mating
up to the time of weaning). The control groups (60/sex/group in each
replicate study) were derived from parents fed untreated diet.
Treated groups were fed diets containing 0.1, 0.5, or 1.0% tylosin
base. Observations included clinical signs, mortality, food
consumption, food efficiency, body weight, terminal haematology and
biochemistry, urinalysis, organ weights, macroscopy, and
histopathology. There were trends towards improved survival in
males, although overall survival was low (20% and 28% for the
control and treated groups, respectively), and increased food
consumption and body weight gain in both males and females in all
treated groups. At histopathology an increased incidence of
pituitary adenomas in males (but not in females) was observed. The
combined incidence of pituitary adenomas in males for both
replicates was 6/120 (5%) in the control group, 9/80 (11%) in the
low dose (0.1% tylosin) group, 18/80 (22.5%) in the mid dose (0.5%)
group, and 20/80 (25%) in the high dose (1.0%) group. The authors
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. The incidence of malignant tumours was unaffected in males or
in females (Gries, 1980)
2.2.4 Reproduction studies
2.2.4.1 Mice
Groups of 7-8 male and 14-17 female ICR mice were fed diets
containing 0, 0.1, or 1.0% tylosin (composition unknown) for two
succesive generations with 2 litters/generation. Some of the mice
were maintained on an ordinary diet, but all the mice received the
experimental diet prior to delivery of the offspring. No
treatment-related effects were observed on reproductive performance
(sexual maturation, number of pups or weaning of pups) (Tsubura
et al., undated). Remark: Only a few summary tables were available.
2.2.4.2 Rats
A 3-generation reproduction study was performed with 2 groups
of 5 male and 10 female Harlan rats/group receiving 0 or 1.0%
tylosin base in their diet. Fertility, viability, gestation and
lactation indices in the F0 generation rats did not reveal any
abnormality. Growth was equal in all generations for both control
and treated rats. In each succeeding generation the capability for
reproduction and perpetuation was unaffected (Anderson et al., 1966;
R3-59). Remark: Incomplete report.
In a special study weanling Wistar rats (25/sex/group, 6-7
weeks old) were fed diets containing 0.1, 0.5, or 1.0% tylosin base
for 10 weeks prior to mating and thereafter for about 6 months
total. A control group consisted of 35 rats/sex. Only one litter was
bred. No effects were observed on parental body weight, food
consumption, reproductive indices (male or female fertility,
gestation length, number of live fetuses, mean litter weight or
offspring survival) or biochemistry. High dose male rats revealed
significantly decreased white blood cells at termination. Sera
collected from parental rats (approx. 150 days on experimental
diets) did not contain detectable levels of tylosin (< 0.1 mg/l).
Offspring were physically normal and were assigned to the one year
toxicity study with tylosin (see Section 2.2.2.1) (Broddle, et al.,
1978b). Remark: Only summarized data given on reproductive indices.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1. Mice
Groups of 10 pregnant mice (2 strains, CBA and A/Jax) received
orally 100, 500, or 1000 mg/kg b.w./day tylosin (composition not
given) in 0.1 ml water from days 7-12 of gestation. Two control
groups of 3 and 5 mice received saline or remained untreated,
respectively. Females were killed on day 18 of pregnancy. No effects
were observed on number of corpora lutea, number of implantations,
number of early and late deaths, number of embryos alive, or fetal
development (Tsuchikawa & Akabori, undated).
Four groups of mice given 0 or 500 mg/kg b.w. and a further 2
pregnant females (A/Jax x male CBA) per group, receiving orally 0 or
1000 mg tylosin/kg b.w./day during days 7-12 of gestation, were
allowed to deliver and rear their young for 4 weeks. No effects were
observed in growth, survival, or genital system of all mice born
determined up to 9 weeks. At 8 weeks after birth rearing ability,
hearing ability and kinetic functions were not effected. At the 9th
week the mice were killed and visceral and skeletal examinations
were performed. No abnormalities were seen (Tsuchikawa & Akabori,
undated).
2.2.5.2 Rats
Groups of 15 Wistar rats were fed diets containing 0.1, 1.0, or
10.0% tylosin (composition not given; equal to 60.5, 725, or
4800 mg/kg b.w./day, respectively) in their diet during days 1-20 of
gestation. A control group consisted of 10 rats. Females were killed
on day 20 of gestation. Observations included number of resorptions
and live and dead fetuses, sex ratio, fetal weight and external,
visceral and skeletal abnormalities. Fetus weight at the highest
dose was slightly decreased (Terashima, undated).
In another study 3 groups of 15 Wistar rats received 0, 1.0, or
10.0% tylosin (composition not given, equal to 0, 725, or 4800 mg/kg
b.w.) during days 1-20 of gestation. Normal delivery was allowed.
Number of fetuses, sex ratio, external abnormalities and growth
during the weaning period (3 weeks) were determined. Motor functions
and senses were examined. The weanlings were killed and visceral and
skeletal examinations were performed. A slightly reduced growth was
observed in weanlings at the highest dose (Terashima, undated).
2.2.6 Special studies on genotoxicity
Tylosin was tested for genotoxicity in an in vitro
chromosomal assay with Chinese hamster ovary cells, a mouse lymphoma
assay and an in vivo assay for cytogenetic damage. The results are
summarized in Table 3.
2.2.7 Special studies on microbiological activity
The anti-microbial activity of tylosin has been described in
the published literature. Tylosin is markedly active in vitro
against gram-positive bacteria, certain gram-negative bacteria and
mycobacteria; it is inactive against Enterobacteriaceae (McGuire
et al., 1961).
Table 3: Results of genotoxicity assays on Tylosin
Test system Test Concentration Purity Results Reference
object of substance
tested
Chromosome Chinese 500-1000 99.3 - Gries,
aberration hamster µg/ml2, 1990a
assay1 ovary 250-750
cells µg/ml3, both
in DMSO
Lymphoma Mouse 10-1000 µg/ml2 99.3 +5 Gries,
assay L5178Y 10-1000 µg/ml3 1990b
TK+/- (1000 µg/ml
cells toxic) both in
DMSO
Micronucleus ICR 2 daily doses 966 - Gries,
mice of 1250, 2500 1990c
or 5000 mg/kg4
1. Mitomycin C and cyclophosphamide, used as positive controls, yielded
positive results
2. without metabolic activation
3. with metabolic activation
4. the positive control cyclophosphamide yielded positive results.
5. positive at cytotoxic dose
6. administered as tylosin base
In recent studies the minimal inhibitory concentration (MIC) of
tylosin has been determined for bacterial pathogens isolated from
target animal species of European and North American origin since
1984. Additional information on sensitivity of bacterial isolates to
tylosin was obtained from literature published since 1980. Tylosin
was active against most Gram-positive bacteria and mycoplasmas
tested in vitro. Activity against Gram-negative bacteria was
generally lower. Tylosin was also found to be active against
isolates of Chlamydia psittaci. MIC values for streptocci,
enterococci and staphylococci are given in Table 4 (Herd, 1990).
Table 4. MIC1 values for tylosin against some Gram-positive bacteria
Organism MIC range Total No.
(mg/1) of isolates
Streptococcus pyogenes 0-1 - 0.2 5
Streptococcus pneumoniae 0.2 - 0.4 4
Streptococcus dysgalactiae 0.06 - 128 50
Streptococcus agalactiae 0.125 - 0.5 51
Streptococcus suis 0.125 - > 128 42
Streptococcus uberis 0.125 - > 128 53
Enterococcus faecalis 0.25 - > 128 31
Staphylococcus aureus2 0.125 - > 128 98
Staphylococcus Spp.3 0.78 - 1.0 7
1. Minimum inhibitory concentration
2. Coagulase - positive
3. Coagulase - negative
2.2.8 Special studies on neurotoxicity
Three cats received daily for 90 days subcutaneously 200 mg/kg
b.w. tylosin tartrate as a 20% solution divided in 2 doses during
the first 37 days and thereafter as a single dose (with addition of
sodium citrate for buffering). An untreated control group consisted
of 3 cats. Slight reduction (25-35%) was observed in the
post-rotatory nystagmus response, but the auditory response appeared
normal. All cats landed on their four feet when dropped from 1
metre. No ataxia was seen (Anderson, 1966; C2-59)
2.2.9 Special studies on skin and eye irritation
Granulated tylosin concentrate (2 g/kg b.w.) was not irritating
when applied to the rabbit skin but when the formulation (52 mg
(0.1 ml)) was instilled to the rabbit eye corneal dullness, slight
corneal opacity, slight to marked irititis and moderate
conjunctivitis were observed. The effects resolved within 14 days of
treatment (Gries et al., 1985a).
The formulation Tylan 200 injection (2 ml/kg b.w.) caused very
slight erythema to the rabbit skin and the formulation (0.1 ml)
caused slight conjunctival hyperemia to the rabbit eye both within
24 hours after application. No skin and eye irritation were observed
after 48 hours (Gries, et al., 1985b).
The formulation Tylan soluble (2 g/kg b.w.) caused very slight
erythema and slight desquamation to the rabbit skin. Slight to
moderate corneal opacity, marked iritis and moderate conjunctivitis
were observed following the instillation of 58 mg of the formulation
into the rabbit eye; these effects had cleared within a week (Gries
et al., 1985c).
2.2.10 Special studies on skin sensitization
Tylosin tartrate was used as a positive control in a
sensitization test by the method of Landsteiner and Jacobs conducted
for tilmicosin. Guinea-pigs (12) were administered 10 intracutaneous
injections of 50 mg/ml tylosin tartrate; a challenge with a further
dose was given after 14 days. At challenge a mild positive response
was observed (Jordan et al., 1989).
2.3. Observations in humans
Trials in human subjects have been conducted to investigate
antibiotic-resistant bacteria. In one trial 12 or 11 human
volunteers were given 20 mg tylosin per day or a placebo,
respectively, for up to 6 months. There was no significant change in
the total number of tylosin-resistant staphylococci and lactobacilli
in weekly faecal samples or in coliform or yeast forms whereas a
significant increase was observed in total number of resistant
streptococci. In another trial no tylosin resistant organisms were
detected in specimens (including faeces samples) from hospitalized
patients who had never had any exposure to the nonmedical antibiotic
tylosin. In both studies no regular pattern was observed in
cross-resistance to related antibiotics (Malin & Silliker, 1966)
In a Japanese trial 2 human volunteers were given 2 or 5 mg
tylosin (as 1 mg tablets; composition not given) per day for 3
months. Faeces were inspected for E. coli and Enterococci and
Staphylococci at intervals of 1 to 2 weeks from 2 months prior to
administration and 3 months from the start of administration. No
tendency towards increased resistance was noted (Kuwabara, undated).
Tylosin resistance was examined in human cultures of
Staphylococcus aureus, Streptococcus pyogenes and Campylobacter
spp. Of 3812 human cultures isolated between 1985 and 1987 only
1.0% were resistant to tylosin. There was no evidence for a
significant animal source of these resistant cultures (Lacey, 1988).
3. COMMENTS
The Committee considered toxicological data on tylosin,
including the results of studies on biochemical aspects,
mutagenicity, and microbiological activity. The Committee noted that
most of the toxicity studies had been carried out about 20 years
ago, had not been conducted according to current protocols, and were
poorly reported.
Following administration of tylosin by various routes, peak
serum levels in rats, dogs, pigs, and cattle were observed within
1-2 hours, and then declined rapidly. In pigs, about 22% was
bioavailable after oral administration. Excretion of tylosin was
rapid and largely in the bile.
After oral administration of radiolabelled tylosin to rats and
pigs 99% of the radioactivity was excreted via the faeces. Major
products identified in faeces were tylosin (factor A), macrosin
(factor C), relomycin (factor D), and dihydrodesmycosin. In pig
liver and kidney, only very small amounts of tylosin and
dihydrodesmycosin could be found.
Several short- and long-term studies in rats and dogs were
performed. In a 1-year study in rats, tylosin base was administered
in the diet at concentrations up to 10 g/kg of feed. A NOEL of
1 g/kg of feed, equivalent to 50 mg/kg b.w./day, was established,
based on haematological and urinary pH changes. In a study in dogs,
tylosin base was administered orally at dose levels up to 400 mg/kg
b.w./day for 2 years. At the two highest dose levels, salivation,
vomiting, and diarrhoea as well as mild pyelonephritis were
observed. The NOEL was 100 mg/kg b.w./day.
In two replicate, but not independent, carcinogenicity studies
in rats, tylosin base was administered in the diet for 2 years at
levels up to 10 g/kg of feed. Food consumption and body-weight gain
were increased in both males and females in all treated groups. In
male rats, a dose-related increase in pituitary adenomas was
observed, from 5% in the control to 25% in the highest-dose group.
There was no such increase in female rats. The authors of the report
concluded that the increase in pituitary tumours was an indirect
result of the ability of tylosin to increase survival and weight
gain. However, this hypothesis was neither tested experimentally nor
verifiable in detail by the Committee because individual body
weights at about 12 months of age were not available.
No effects on reproduction performance were observed in a
two-generation study in mice and in one- and three-generation
studies in rats. No malformations were observed in mice or rats, but
the Committee noted that these studies were poorly reported.
Tylosin was not mutagenic in an in vitro test for chromosomal
aberrations and in an in vivo micronucleus test. In a mouse
lymphoma assay, no activity was found with metabolic activation;
however a weak, but significant activity was observed in the absence
of such activation.
In studies in human volunteers, there was no evidence of the
emergence of cross-resistance to therapeutically important
antibiotics, but volunteers given oral doses of 20 mg of tylosin
daily for 6 months showed an increase in the number of resistant
streptococci. The Committee concluded that additional studies
showing no microbiological effects in two individuals at doses up to
5 mg/person/day were inadequate to establish a NOEL. In addition, no
suitable in vitro data were available to establish a NOEL with
respect to the microbiological risk for humans.
4. EVALUATION
Because of the deficiencies in the toxicological and
microbiological data, the Committee was not able to establish an
ADI.
5. REFERENCES
ANDERSON, R.C. (undated). Tylosin absorption and excretion studies.
Study numbered 893//FAANIM/AM/14-26. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C., HARRIS, P.N., LEE, C.C., MAZE, N., SMALL, R.M. &
WORTH, H.M. (1966) The toxicology and pharmacology of tylosin, an
antibiotic, and some salts of tylosin. Unpublished Report Ref.
X/E/4. dated 19 December 1966 from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
ANDERSON, R.C. & WORTH, H.M. (undated) The acute toxicity of tylosin
phosphate. Unpublished report from the Lilly Research Laboratories,
Eli Lilly and Co., Indianapolis, Indiana. Submitted to WHO under
893/TACUTE/AM//1-26 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., GRIES,
C.L., GIBSON, W.R. & MORTON, D.M. (1978a) Chronic toxicity of
tylosin fed to rats for one year. Unpublished Report from study
R-307 from Toxicology Division, Lilly Research Laboratories.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
BRODDLE, W.D., GOSSETT, F.O., ADAMS, E.R., HOFFMAN, D.G., KITCHEN,
D.N., GIBSON, W.R. & MORTON, D.M. (1978b) A study of a parental
population of rats bred to produce offspring assigned to one- and
two-year dietary studies of tylosin. Unpublished Report Study No.
R-1176 dated October 1978 from Toxicology Division, Lilly Research
Laboratories. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
BUCK, A.M., COOPER, T.R. & THOMSON, T.D. (undated) Serum and lung
tylosin activity in neonatal calves treated with 1.0 gram tylosin
tartrate B.I.D. in milk replacer. Unpublished Report T1Y768501 from
Lilly Research Centre Laboratories, Animal Health Applications
Research, Greenfield, Indiana. Submitted to WHO under
893/SP/FBLDLV/AM/10/10-15 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
GINGERICH, D.A., BAGGOT, J.D. & KOWALSKI, J.J. (1977) Tylosin
antimicrobial activity and pharmacokinetics in cows. Can. Vet.
Jour., 18(4), 96-100.
GRIES, C.L. (1980) The toxicological evaluation of tylosin (compound
27892) given to Wistar rats in the diet for two years. Unpublished
report from studies R-287 and R-297 from Toxicology Division, Lilly
Research Laboratories. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
GRIES, C.L., McKINLEY, E.R. & QUARLES, J.P. (1983) Acute comparative
intravenous toxicity testing of tylosin, desmycosin and macrocin in
the ICR mouse. Unpublished report dated August 1983 with studies
M-V-46-83, M-V-45-83, and M-V-44-83 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985a) The acute oral,
dermal and ocular toxicity of granulated tylosin concentrate.
Unpublished report dated April, 1985 with studies R-O-365-79,
R-366-79, B-D-109-79, and B-E-94-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L., DOWNS, O.S. & NEGILSKI, D.S. (1985b) The acute oral,
dermal, ocular and inhalation toxicity of tylan 200 injection.
Unpublished report dated September 1985 with studies R-O-344-79,
R-O-343-79, B-D-103-79, B-E-87-79, and R-H-39-79 from Toxicology
Division, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
GRIES, C.L., NEGILSKI, D.S. & DOWNS, O.S. (1985c) The acute oral,
dermal, ocular and inhalation toxicity of tylan soluble. Unpublished
report dated September 1985 with studies R-O-367-79, R-O-368-70,
B-D-94-79, B-E-90-79, and R-H-40-79 from Toxicology Division, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
GRIES, C.L. (1990a) The effect of tylosin on the in vitro
induction of chromosome aberrations in chinese hamster ovary cells.
Unpublished report with studies 891109CTX3279, 891129CAB3279, and
891220CAB3279 from Lilly Research Laboratories, Toxicology Division,
Greenfield, Indiana.
GRIES, C.L. (1990b) The effect of tylosin on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished report with studies 891011MLT3279,
891017MLA3279, and 891114MLA3279 from Lilly Research Laboratories,
Toxicology Division, Greenfield, Indiana.
GRIES, C.L. (1990c) The effect of tylosin (compound 027892) on the
in vivo induction of micronuclei in bone marrow of ICR mice.
Unpublished report, study 891212MNT3279 from Lilly Research
Laboratories, Toxicology Division, Greenfield, Indiana.
HERD, R.M. (1990) The antimicrobial activity of tylosin in vitro
1980-1989. Unpublished Report dated 27-06-90 from Lilly Research
Centre Ltd. Submitted to WHO by Lilly, Windlesham, Surrey U.K.
JORDAN, W.H., GARDNER, J.B. & WEAVER, D.E. (1989) An intracutaneous
sensitization stidy in albino guinea pigs with tilmicosin.
Unpublished toxicology report No. 32 from Toxicology divison, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
KUWABARA, S. (undated) A study on the effect of continuous and
minute amount of tylosin on human intestinal flora. Medical
Department Toho University. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
LACEY, R.W. (1988) Rarity of tylosin resistance in human pathogenic
bacteria. Report from Department of Microbiology, University of
Leeds, Leeds, England. Submitted to WHO by Lilly Research Centre
Ltd., Windlesham, Surrey, England.
LILLY (undated a) The determination of tylosin levels in lung and
serum following administration of tylan 200 by intramuscular
injection to weaned calves. Unpublished Report T1Z769001.
Supplementary information submitted to WHO under
893/IJB200/FDISTR/AM/26-35 by Lilly Research Centre, Windlesham,
Surrey, England.
LILLY (undated b) Tylosin absorption studies in chickens.
Unpublished Report VPR-17-418 submitted under 893//FAANIM/AM/1-9 to
WHO by Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated c) Tylosin blood titres in chickens. Unpublished
Report VPR-21-418 submitted to WHO under 893//FBLDLV/AM//1-3 by
Lilly Research Centre, Windlesham, Surrey, England.
LILLY (undated d) Tylosin excretion studies in chickens. Unpublished
Report VPR-67-418 submitted to WHO under 893//FEXCRE/AM//6-13 WHO by
Lilly Research Centre, Windlesham, Surrey, England.
MALIN, B. & SILLIKER, J.H. (1966) Low level tylosin and the
emergence of antibiotic-resistant bacteria in humans. Antimicrobial
agents and chemotherapy, 1966. Submitted to WHO by Lilly Research
Centre Ltd., Windlesham, Surrey, England.
McGUIRE, J.M., BONIECE, W.S., HIGGENS, C.E., HOEHN, M.M., STARK,
W.M., WESTHEAD, J. & WOLFE, R.N. (1961) Tylosin, a new antibiotic: I
Microbiological studies. Antibiotics & Chemotherapy, XI (5), 1961,
320-327.
NAKAMURA, H. ET AL. (1969) Studies on distribution of antibiotics
inblood and tissue. XIX. Blood level and tissue concentration of
tylosin in pigs after oral administration of tylosin phosphate.
Proc. Jap. Soc. Vet. Science, 67th meeting, No. 128.
SHIONOGO & Co., Ltd. (1981) Blood levels of tylosin tartrate
following oral administration and intravenous injection in pigs.
Report No. 3174674755 from Department of Animal Science Development,
Shionogo & Co., Ltd. Submitted to WHO by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978a) 14C tylosin tissue residue study in swine.
Unpublished Report dated November 1978 from Agricultural
Biochemistry, Lilly Research Laboratories, Greenfield, Indiana.
Submitted to WHO by Lilly Research Centre Ltd., Windlesham, Surrey,
England.
SIECK, R.F., GRAPER, L.K., GIERA, D. D., HERBERG, R.J. & HAMILL,
R.L. (1978b) Metabolism of tylosin in swine and rat. Unpublished
Report dated November 1978 from Agricultural Biochemistry, Lilly
Research Laboratories, Greenfield, Indiana. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
SIECK, R.F. ET AL. (1980) 14C tylosin residue study in swine.
Experiment ABC-0016 dated March 1980 from Agricultural Biochemistry,
Lilly Research Laboratories, Greenfield, Indiana. Submitted to WHO
by Lilly Research Centre Ltd.. Windlesham, Surrey, England.
TERASHIMA, H. (undated) The effect of tylosin on a fetus and a
suckling-young of Wistar strain rat. Department of Pathology,
Medical School of Osaka City University. Submitted to WHO by Lilly
Research Centre Ltd., Windlesham, Surrey, England.
THOMSON, T.D. (undated a) Relative bioavailability of TYLAN 200
injection administered subcutaneously and intramuscularly in young
calves. Unpublished Report T1Z768106 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana,. Submitted
to WHO under 893/IJB200/FAANIM/AM/1-6 by Lilly Research Centre,
Windlesham, Surrey, England.
THOMSON, T.D. (undated b). The relative absorption rates of tylosin
base and tylosin tartrate from aqueous and polypropylene glycol
vehicles. Unpublished Report T1Z768107 from Lilly Research
Laboratories, Animal Science Div., Greenfield, Indiana. Submitted to
WHO under 893/IJB200/FAANIM/AM/6-19 by Lilly Research Centre Ltd.,
Windlesham, Surrey, England.
THOMSON, T.D. (undated c). Tylosin distribution in pneumonic calf
lung. Unpublished Report T1Z768203 from Lilly Research Laboratories,
Animal Science Division, Greenfield, Indiana. Submitted to WHO under
893/IJB200/FDISTR/AM/1-6 by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
TSUBURA, Y., TOYOSHIMA, K., SANO, S., NISHII, Y. & TANI, M.
(undated) Effect of Tylosin on mouse breeding. Report from Second
Department of Pathology Nara Medical College. Submitted to WHO by
Lilly Research Centre Ltd., Windlesham, Surrey, England.
TSUCHIKAWA, K. & AKABORI, A. (undated) On the teratogenicity of
tylosin. Report from National Institute for Genetics, Shionogi and
Co. Submitted to WHO by Lilly Research Centre Ltd., Windlesham,
Surrey, England.
VAN DUYN, R.L. & HANDY, P.R. (undated) Tylosin serum and lung tissue
levels following five daily intramuscular injections. Unpublished
Report 766-G125-86 from Lilly Research Laboratories, Animal Science
Division, Greenfield, Indiana. Submitted to WHO under
893/IJB/FBLDLV/AM/37-43 by Lilly Research Centre, Windlesham,
Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated a) Tylosin serum and lung
tissue levels following a single intramuscular injection with
tylosin in calves. Unpublished Report 766-G125-73 from Lilly
Research Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/24-30 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & JOHNSON, W.S. (undated b) Tylosin levels in lung
tissue and serum from calves injected once intramuscularly with
tylosin base. Unpublished Report VPR-352-766 from Lilly Research
Laboratories, Animal Science Division, Greenfield, Indiana.
Submitted to WHO under 893/IJB/FBLDLV/AM/31-26 by Lilly Research
Centre, Windlesham, Surrey, England.
VAN DUYN, R.L. & VOLKERTS, T.M. (1979) Concentrations of tylosin in
blood and lung tissue from calves given single and repeated daily
intramuscular doses. Vet. Med., March 1979, 375/377.
VAN DUYN, R.L. & KLINE, R.M. (undated) Tylosin minigranule oral
blood levels in swine. Pharmacological experiment VPR-275-766
submitted to WHO under 893/G/FBLDLV/AM/1-2 by Lilly Research Centre,
Windlesham, Surrey, England.
