
TRANQUILLIZERS
The tranquillizing agents considered by the Committee have
several points in common. All are old compounds in their class, for
all of them the data available to the Committee were inadequate in
certain respects, all are often used shortly before slaughter in
pigs, and all leave residues in edible tissues. Furthermore,
administration by injection is known to create a local area of high
concentration of the drug which, in part, is likely to be present at
slaughter and, if in edible tissue, is a potential hazard to the
consumer.
The Committee therefore advises against the use of these drugs
for any purpose in the immediate pre-slaughter period, especially
when given by injection into the tissues.
AZAPERONE
First Draft prepared by
Dr. G. Roberts, Environmental Health Branch
Department of Community Services and Health
Canberra, Australia
1. EXPLANATION
Azaperone is a butyrophenone neuroleptic tranquillizer for use
in pigs. The therapeutic dose range is 0.4 to 2.0 mg/kg given
intramuscularly. This is the first occasion on which azaperone has
been evaluated by the Joint FAO/WHO Expert Committee on Food
Additives.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Rats were given an oral dose of 1 mg/kg tritiated azaperone in
aqueous 0.01 M tartaric acid. Over a 4-day period the amount of
radioactivity recovered was 16% in urine and 81% in faeces, most of
which was collected within the first 24 hours. At the end of the
4-day period, less than 1% of the dose was found in organs and
tissues, with highest levels in the liver, kidney and heart
(Heykants, 1973).
After a subcutaneous injection of 1 mg/kg azaperone, rats
excreted 20-25% in urine, most within 24 hours, and 60-80% in faeces
mostly within 48 hours. At the end of 4 days, radioactivity could
not be detected in tissues (Heykants et al., 1971b).
In subcutaneously dosed rats, peak levels of total
radioactivity and unchanged azaperone were detected in blood, liver
and brain within 30 min. Thereafter, elimination of azaperone from
brain and blood was rapid (down to 1% of peak after 8 hours) but
slower in liver (down to 25% of peak after 8 hours). Total
radioactivity diminished more slowly indicating slower elimination
of metabolites (Heykants et al., 1971a).
The fate of a subcutaneous injection of azaperone was similar
in pregnant rats. Peak levels in placenta and fetus occurred after
60 min followed by rapid elimination. In tissues, the proportion of
radioactivity as unchanged drug decreased quickly indicating rapid
degradation of azaperone and slower elimination of metabolites
(Heykants, 1974).
Azaperol, a metabolite of azaperone, was given by intravenous
injection to rats. Measurement of liver, kidney and brain tissue
levels revealed elimination half-lives of 45 min, 15 min and 15 min
respectively. Some 6% of the dose was converted to azaperone (Rauws
et al., 1976).
2.1.2 Biotransformation
After an oral dose in rats, only 1.5% of urinary radioactivity
and 34% of faecal radioactivity were in the form of unchanged drug.
By comparison in subcutaneously injected rats, less unchanged
azaperone (12%) was found in faeces (Heykants, 1973).
The biotransformation of azaperone was rapid in subcutaneously
dosed rats and was thought to occur mainly in the liver. As early as
15 min post treatment, 75% of radioactivity in the liver was in the
form of metabolites (Heykants et al., 1971a).
The excreta from subcutaneously dosed rats were analyzed for
degradation products. The main metabolites arose from oxidative
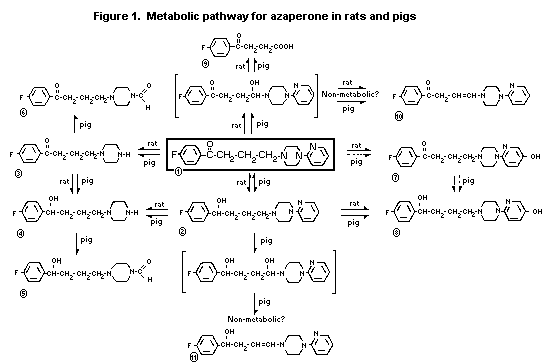
removal of the pyridyl group (3 in Fig. 1) and acetylation of the
resulting free piperazine. The former, which was almost exclusively
in faeces, the latter was in both urine and faeces and totalled
about 50% of radioactivity. Another three metabolites, amounting to
15% of the dose and representing oxidative N-dealkylation, were
found in both urine and faeces (Heykants et al., 1971b).
Using rat liver fractions in vitro, azaperone was shown to be
metabolized to a greater extent by the 16000 x g supernatant than
the microsomal fraction. The main metabolic pathways were reduction
of the butanone (2 in Fig. 1), hydroxylation of the pyridine group
(7 in Fig. 1), oxidative N-dearylation (3 in Fig. 1) and oxidative
N-dealkylation (Meuldermans et al., 1973).
Azaperone was incubated with the 16000 x g supernatant from rat
liver for 1 hour at 37°C. About 10% was unmetabolised, 22% was as
azaperol (2 in Fig 1), 15% was due to hydroxylation of the pyridine
group (7 in Fig 1) with lesser amounts of metabolites 3, 4, 8, 9,
and 10 in Fig 1 (Meuldermans et al., 1975).
2.1.3 Effects on hepatic enzymes
Mice were injected subcutaneously with 4 mg/kg azaperone. At
the end of 7 days, the protein content of liver or microsomes was
unaffected. Cytochrome P-450 concentration was increased but
NADPH-cytochrome C-reductase activity was reduced (Pekkanen and
Salminen, 1973).
 2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Results of acute studies with azaperone
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral 385 Niemegeers et
M s.c. 179 al., (1974)
M i.v. 38-42
Rat M oral 245
M s.c. 450
M i.v. 28
Guinea M oral 202
pig
Dogs N.S. oral > 20
N.S. s.c. > 20
The major toxic signs in rodents were ptosis, sedation, tremors
and occasionally clonic seizures. Ptosis and sedation were observed
in dogs with vomiting after oral dosing.
In mice, the i.v. LD50 values for metabolites 2 and 8 were 56
and 150 mg/kg respectively, higher than for azaperone (Niemegeers,
1975).
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 10 male and 10 female Wistar rats were given
subcutaneous injections of 0, 2.5, 10, and 40 mg/kg b.w./day
azaperone for 13 weeks. The vehicle used was 0.9% NaCl while the
purity of azaperone was not stated.
Treated rats in all groups were sedated for 2 hours following
dosing and at 40 mg/kg b.w./day passive behaviour was exhibited
throughout the study. There were no deaths due to treatment. In
males only, body weight gain was non-significantly reduced at 2.5
and 10 mg/kg b.w./day and markedly reduced at 40 mg/kg b.w./day.
Haematology, blood chemistry and urinalysis were examined at
the end of the study. The only apparent effects were a shift in the
differential white cell count from lymphocytes to neutrophils in
males at 10 mg/kg b.w./day and males and females at 40 mg/kg
b.w./day and a slight increase in serum alkaline phosphatase in
males at 10 and 40 mg/kg b.w./day.
At necropsy, spleens "looked degenerated" at 40 mg/kg b.w./day.
Thymus weights were decreased in males at 10 mg/kg b.w./day and
males and females at 40 mg/kg b.w./day. In females, liver weights
were increased and ovaries showed reduced numbers of corpora lutea
and increased glandular tissue at 40 mg/kg b.w./day (Marsboom et
al., 1967).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity 98-102%) for
15 weeks. There were no signs of toxicity during the study. Food
consumption and body weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood chemistry and urinalysis were
examined at the end of the study. The only notable findings were
decreased serum cholesterol in males and females, increased
urobilinogen in males and increased urinary creatinine in females,
all at 1600 ppm.
Gross pathology was unremarkable. Brain weights were heavier at
1600 ppm. Slight bile duct proliferation was seen in livers of 400
and 1600 ppm males. In females, ovaries showed "active large corpora
lutea" and there were "reduced eosinophils" in the uterine wall,
"mucified aspect" of vaginal mucosa, more developed alveolar tissue
in the mammary gland and "stimulation of erythrosinophils" in the
pituitary. The effects in females were expressed at 1600 ppm and to
a lesser extent at 400 ppm. The NOEL was 100 ppm, equal to 10 mg/kg
b.w./day (Marsboom et al., 1969).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 6 and 12 months. Based on food intake the average doses were 8,
31, and 130 mg/kg b.w./day for 6 months and 8, 30, and 127 mg/kg
b.w./day for 12 months.
Dose-related sedation was observed in all drug-treated groups
during the entire experimental period. Survival was unaffected. Food
consumption and body weight gains were depressed at 1600 ppm while
in the 400 ppm group weight gain was affected in the 6-month study
only.
Ophthalmoscopy, haematology, blood chemistry and urinalysis
were examined at the end of 6 and 12 months. Serum cholesterol was
decreased in the 1600 ppm group at 6 months but not at later times.
Serum bilirubin, BUN and urinary urobilinogen were higher in females
of the 1600 ppm group at 6 and 12 months.
At autopsy, gross pathology was unaffected. Brain weight was
increased at 1600 ppm after both 6 and 12 months of dosing. Septal
cell proliferation in the lung was marked at 6 and 12 months in the
1600 ppm group and led to lipoid pneumonia.
The females of the 1600 ppm group exhibited a "prolonged
diestrus in the uterus" (atrophic at 12 months) accompanied by
"reduced activity in the ovaries" (low numbers of active corpora
lutea and abundant interstitial glandular tissue), "mucification and
thin layered epithelium" with no cornification in the vagina and
"more extensive chromophobe tissue" in the pituitary. These findings
in reproductive tissues were more marked at 12 months. Apart from
the pharmacological effect of sedation, the NOEL was 8 mg/kg
b.w./day (Marsboom et al., 1976a).
2.2.2.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, or 20 mg/kg b.w./day of azaperone (purity 99.7%) for 13 weeks.
Dosing was carried out during 6 days per week in capsules.
Dogs of the 20 mg/kg b.w./day group exhibited a sedative effect
for 3 to 4 hours post-dosing in addition to decreased general
activity, ptosis and catatonia. Emesis and salivation were seen
occasionally at 5 mg/kg b.w./day and frequently at 20 mg/kg
b.w./day. Physical examinations revealed one female in each drug
treatment group with transient swelling of mammary glands.
Body weight was not clearly affected by dosing. Ophthalmoscopy,
ECG, blood pressure, haematology, blood biochemistry and urinalysis
were examined pre-treatment and monthly during the study. None of
these parameters was altered.
At terminal necropsy, liver weights tended to increased values
at 5 and 20 mg/kg b.w./day, but a dose-relationship was not evident.
Gross and histopathology were unaffected. The NOEL was 1.25 mg/kg
b.w./day (Marsboom et al., 1973).
2.2.3 Long term/carcinogenicity studies
2.2.3.1 Rats
Concomitant with the 6 and 12 month rat studies (see Section
2.2.2.1), groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 18 months. Based on food intake the average doses were 7, 29,
and 115 mg/kg b.w./day for 18 months.
As in the shorter-term studies, sedation was noted at all doses
while food intake and weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood biochemistry and urinalysis were
examined at the end of the study. Serum bilirubin and BUN and
urinary urobilinogen were increased in females of the 1600 ppm
group.
At necropsy, brain weight was increased in 1600 ppm rats. Gross
pathology was unremarkable. Septal cell proliferation in the lung
leading to lipoid pneumonia was marked at 1600 ppm. Effects seen in
the pituitary, ovary, uterus, and vagina at 6 and 12 months were not
evident in this 18-month experiment. Tumours were not increased.
Pharmacological effects were noted at all doses, the NOEL for
toxicological effects was 29 mg/kg b.w./day (Marsboom et al.,
1976a).
2.2.3.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, and 20 mg/kg b.w./day of azaperone (purity not stated) for 24
months. Animals were dosed 6 days/week in capsules.
One male dog given 20 mg/kg b.w./day azaperone died in the 64th
week. Signs of intoxication included sedation, back arching,
protrusion of the tongue, head shaking, muscle tremors, apnea,
lacrimation, increased salivation, and emesis. These effects were
seen in most dogs at 20 mg/kg b.w./day and some dogs at 5 mg/kg
b.w./day with sporadic emesis and salivation at 1.25 mg/kg b.w./day.
Body weight gain was unaffected.
Ophthalmoscopy, ECG, BP, haematology, blood biochemistry and
urinalysis were examined pre-dosing and every 3 months during the
study. None of these parameters was altered.
At autopsy, there was increased bile on the duodenal mucosa in
the 5 and 20 mg/kg b.w./day groups. The weights of adrenal glands
and the liver were increased at 20 mg/kg b.w./day. The following
microscopic changes were noted mainly in females of the 1.25 and
5 mg/kg b.w./day groups with virtually no effects at 20 mg/kg
b.w./day: "more marked or protracted metoestral period" -- active
corpora lutea in 2 low-dose (LD) and 1 mid-dose (MD), "fatty
superficial epithelium" in uterus of 2 LD and 2 MD, "more resting
aspect" of genital tract with "thin layered vaginal epithelium" in
all LD and 2 MD, atrophy of uterine wall in 1 high-dose (HD),
stimulation of mammary gland in LD and MD and "stimulation of
erythrosinophilic tissue" in the pituitary in 2 LD. A NOEL was not
identified (Marsboom et al., 1976b).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation study was carried out in Wistar rats. The
initial generation (F0) was allowed to deliver and suckle pups.
The second generation (F1) was mated within treatment groups
avoiding brother-sister matings, and was allowed to deliver and
suckle pups. The third generation (F2) was mated as in the F1
but the females were killed on gestation day 22.
Azaperone (purity not stated) was administered in the diet at
concentrations of 0, 25, 100, and 400 ppm food. The dietary levels
resulted in doses of approximately 2.5, 10, and 40 mg/kg b.w./day.
Only adult females were treated, on gestation days 6 to 15 in each
generation. Group sizes were 20 F0 females, 29-33 F1 females,
and 40-52 F2 females.
There were no mortalities among treated dams in any generation
but other toxic signs were not recorded. Maternal weight gain and
pregnancy rate were similar between groups.
Litter size, pup weight and post-natal weight gain were
unaffected in F1 and F2 offspring. Pup survival was reduced at
40 mg/kg b.w./day during the F2 lactation period only.
Uterine examination of F2 dams revealed no effects on
implantation, resorption or fetal weight. In the 40 mg/kg b.w./day
group, there were 2 F3 fetuses without metacarpal bones of the
foreleg and 1 without metatarsal bones of the hind leg (unilateral).
The NOEL was 10 mg/kg b.w./day (Marsboom, 1974a).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 29 pregnant CRL:COBS-CD-1 mice were given gavage
doses of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone
(purity not stated). Treatment was on gestation days 6 to 15 and
females were killed on gestation day 18. The vehicle contained
tartaric acid, sodium bisulfite, methyl and propyl paraben.
A number of animals died in the vehicle and drug-treated
groups, death was attributed to dosing difficulties as a result of
conditioned aversion to the vehicle. Decreased activity, ptosis,
impaired righting reflex and catalepsy were seen 1 to 3 hours
post-dosing with 10 and 40 mg/kg b.w./day azaperone.
Maternal weight gain was reduced in the vehicle control group
and further reduced at 10 and 40 mg/kg b.w./day. This effect on body
weight may be a reflection of lower litter sizes which were due to
both reduced numbers of corpora lutea and slightly increased
resorptions. Implantation rate, fetal weight and survival were not
significantly affected.
The ratio of male to female fetuses was reduced in the vehicle
and 40 mg/kg b.w./day groups. Fetal examination revealed a slight
delay in ossification of tarsals and phalanges of fore and hind paws
at 40 mg/kg b.w./day. Gross and visceral abnormalities were not
induced. The NOEL was 2.5 mg/kg b.w./day (Mosher et al., 1973).
2.2.5.2 Rats
Groups of pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 22.
Maternal survival and weight gain were unaffected. The number
of implantations and live and dead fetuses was similar in all
groups. Fetal weight and gross, visceral, and skeletal examinations
were unremarkable. There was no effect at the highest dose of
40 mg/kg b.w./day (Marsboom, 1972a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 15 and
females were killed on gestation day 22.
There was no mortality among the dams but weight gain was lower
in the 10 and 40 mg/kg b.w./day groups. The number of implantations
was similar in all groups. Relative to the number of implantations,
the number of resorptions was slightly increased at 40 mg/kg
b.w./day, resulting in slightly smaller litter size. Fetal weight
was reduced at 40 mg/kg b.w./day and the only fetal abnormality was
scoliosis in one 40 mg/kg b.w./day fetus (Marsboom, 1973a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 1 to 21 and
females were killed on gestation day 22.
There was no maternal mortality. Weight gain in the dams was
reduced at 10 and 40 mg/kg b.w./day. The number of implantations was
lower at 10 and 40 mg/kg b.w./day and resorptions were increased at
40 mg/kg b.w./day. Fetal weight was depressed at 40 mg/kg b.w./day
but there was no increase in fetal abnormalities (Marsboom, 1967).
Groups of 25 pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was from gestation day 16 to post-partum
day 21 and dams were allowed to deliver naturally and nurse young
throughout lactation.
There were no maternal deaths and the duration of gestation and
parturition were unaffected. Body weight gain of dams in the 2.5 and
40 mg/kg b.w./day groups was reduced. Litter size, birth weight, and
post-natal weight gain were unremarkable. The survival of pups
through the lactation period was compromised at 40 mg/kg b.w./day.
There were no fetal abnormalities. The NOEL was 10 mg/kg b.w./day
(Marsboom, 1973b).
2.2.5.3 Golden hamsters
Groups of 26 pregnant golden hamsters were given gavage doses
of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity
not stated). Treatment was on gestation days 6 to 10 and females
were killed on gestation day 15. The vehicle contained tartaric
acid, sodium bisulfite, methyl and propyl paraben.
There was no maternal mortality. Toxic signs were ptosis from
2.5 mg/kg b.w./day, decreased motor activity from 10 mg/kg b.w./day,
catalepsy and impaired righting reflex at 40 mg/kg b.w./day during
the 2 hours following dosing.
Body weight gain in dams was depressed at 40 mg/kg b.w./day,
and was associated with lower fetal weight at this dose. The number
of implantations, resorptions and litter size was similar between
groups. Fetal examination revealed only a slight delay in
ossification of metatarsals at 40 mg/kg b.w./day. Discounting
pharmacological effects, the NOEL was 10 mg/kg b.w./day (Mosher et
al., 1974).
2.2.5.4 Rabbits
Groups of 15 pregnant NZ White rabbits were given gavage doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 18 and
females were killed on gestation day 28.
There were no maternal deaths and body weight gain was
depressed at 10 and 40 mg/kg b.w./day. Resorptions or fetal death
were not induced but litter size was slightly lower in the 40 mg/kg
b.w./day group, reflecting lower numbers of implantations. There
were no fetal abnormalities related to treatment. The NOEL was
2.5 mg/kg b.w./day (Marsboom, 1972b).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on azaperone1
Test system Test object Concentration Results Reference
Ames test2 S.typhimurium
TA 1538 > 750 µg/plate positive3 Preiss et al.,
TA 1537 (+ S9 mix) 1982, 1983
TA 98
TA 1535 2500 µg/plate negative Scheutwinkel-
TA 100 (+ S9 mix) Reich, et al.,
1982
TA 1535 2500 µg/plate
TA 1537 (- S9 mix)
TA 98
TA 1538
TA 100
Ames test2 S.typhimurium
TA 1530 2000 µg/plate4 negative Poncelet et al.,
TA 1535 (+ & - S9 1982; Duvergervan
TA 1537 mix) Bogaert et
TA 1538 al., 1987
TA 989
TA 100
Micro Rats 20-160 mg/kg negative Vanparys &
nucleus test orally Marsboom, 1982
Dominant Mice 10-160 mg/kg negative Marsboom,
lethal test orally 1974b
1. Appropriate positive controls were used.
2. Both with and without rat liver S9 fraction.
3. There was no dose relationship.
4. Higher concentrations were bacteriotoxic.
Table 3. Results of Ames tests on azaperone metabolites1,2
(Scheutwinkel-Reich et al., 1982)
Compound Strain Concentration Results
alpha-(4-fluorophenyl)-4- TA 98 > 500 µg/plate (+ S9) positive2
(2-pyridinyl)-piperazine
butanol (Azaperol)
TA 1538 1500 µg/plate (+ S9) positive
TA 1535 2500 µg/plate negative
TA 1537 (+ S9)
TA 100
TA 98 2500 µg/plate negative
TA 1538 (- S9)
TA 1535
TA 1537
TA 100
4-(4-acetyl)-1-piperazinyl- TA 1538 5000 µg/plate positive
41-fluoro-butyrophenone (+ S9)
TA 98 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
TA 98 5000 µg/plate negative
TA 1538 (+ S9)
TA 1535
TA 1537
TA 100
ß-(p-fluorobenzoyl)- TA 98 2500 µg/plate positive2
propanoic acid (+ S9)
TA 1538 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
p-fluorobenzoyl TA 98 5000 µg/plate negative
acetic acid TA 1538 (+ and - S9)
TA 1535
TA 1537
TA 100
1. Appropriate positive controls were used throughout
2. Response not dose-related
2.2.7 Special studies on pharmacology
Table 4. Results of studies with azaperone in pharmacological tests ( Niemegeers
et al, 1974).
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
amphetamine antagonism 2.5 - -
apomorphine antagonism 0.34/9.15 - 0.98
norepineprine antagonism 0.33 - -
tryptamine antagonism 5.9 - -
jumping box test 0.7 - 5(3.95 OR)
W-test
- body weight 1.75 - -
- food intake 2.5 - -
- faecal output 4.0 - -
behavioural observations
- catalepsy 8.0 - -
- ptosis 1.5 - -
open field test
- ambulation 6.7 - -
- rearing 4.1 - -
- defecation 6.9 - -
traumatic shock test 0.021 - -
thermoregulation 3.27 - -
- 37 °C > 320 -
- 30 °C
Table 4. cont'd
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
tail withdrawal test > 40 - -
inhibition of food intake > 10 - -
hot plate test - 7.0 -
inhibition of righting reflex - > 40 -
pentobarbital potentiation - 0.4 -
rotating rod test - 1.64 -
fighting test - 0.74 -
1. lowest effective dose, the NOEL was 0.01 mg/kg sc.
2.3 Observations in humans
A group of 20 male psychotics were studied; 10 remained on their
previous medication, 10 had their medication replaced with azaperone.
Doses were commenced at 0.5 mg three times daily (t.i.d.) increasing
to 20 mg t.i.d over a 17-day period. The maximum dose was then
administered for 2 months.
Clinical observation revealed no symptoms up to 2 mg t.i.d. of
azaperone. At higher doses (from 2.5 mg t.i.d.), sedation was observed
in a dose-related manner and at 20 mg t.i.d. patients started to
complain of dizziness. Haematology and blood chemistry parameters,
examined prior to and at the end of 2´ months azaperone treatment, were
within the normal range (Reyntjens, 1972).
3. COMMENTS
A range of studies on azaperone was submitted for assessment
including data on kinetics and metabolism, acute toxicity,
short-term and long-term toxicity, developmental toxicity, and
genotoxicity. Most of the studies were carried out in the 1970s and
the standard of testing and reporting varied widely.
The kinetic studies with azaperone were insufficient to
determine the extent of absorption from the gastrointestinal tract.
However, by comparison with the excretion pattern after parenteral
dosing, it was estimated that absorption after oral dosing was
probably high. Distribution within the body in rats was extensive
and excretion was primarily in the faeces (81%), with lesser amounts
in urine (16%). Azaperone is extensively and rapidly metabolized.
Two metabolites were found in the pig but not in the rat. However,
these compounds are devoid of significant pharmacological activity,
and therefore do not affect the suitability of the rat as a model
for toxicological testing.
Azaperone was moderately toxic in acute toxicity studies in
mice, rats, guinea pigs, and dogs. Most signs of intoxication
reflected exaggerated pharmacological activity of azaperone in the
central nervous system. A battery of pharmacological studies
indicated that azaperone possesses potent anti-alpha-adrenergic
activity, but these data were inadequate for use in determining a
NOEL as the drug was almost always given by the subcutaneous route.
Short- and long-term toxicity studies were carried out in rats
and dogs. Dose-related sedation was the major effect in both species
and was observed at all treatment levels. Minor hepatotoxicity was
observed at doses at and above 30 mg/kg b.w./day in rats and 5 mg/kg
b.w./day in dogs. In rats only, brain weight was consistently
increased at 30 mg/kg b.w./day, but in the absence of any
pathological change this observation could not be explained.
There were pathological changes in the pituitary and sex
organs, particularly in rats; these were typical of neuroleptic
agents. It has been postulated that the primary effect is
pharmacological and is caused by the blocking of dopamine receptors
in the hypothalamus or pituitary, resulting in increased prolactin
and decreased gonadotrophin secretion. While this could account for
the observed slight stimulation of the pituitary and mammary glands
and the quiescence of the female reproductive tract, direct evidence
for this mechanism was lacking. The effects on the reproductive
organs were slight, in line with the relatively weak anti-dopamine
activity of azaperone. In dogs, such effects were observed only
after dosing for 24 months with 1.25 and 5 mg/kg b.w./day but not at
20 mg/kg b.w./day. In rats, effects were noted after 3, 6, and 12
months but not after 18 months, which suggests the possibility of
adaptation. When pharmacological effects were excluded, the NOELs
were 1.25 mg/kg b.w./day in dogs and 8 mg/kg b.w./day in rats.
The Committee noted that the carcinogenic potential of
azaperone had not been adequately investigated. The only study in
which lifetime exposure was approached was an 18 month study in
rats. However, the duration of dosing was too short and the small
group size (only ten rats of each sex) was inadequate to determine
treatment-related tumour incidences satisfactorily.
Frame shift mutations in Salmonella typhimurium strains were
seen for azaperone and three metabolites in a series of studies
carried out by one group of investigators. However, reversion rates
were only 2-3 times those in controls; there was no dose-response
relationship, and high doses in the presence of rat liver microsomes
were required. This weak response was not reproduced by a second
group of investigators using the same bacterial strains.
Genotoxicity was absent in the micronucleus and dominant lethal
tests in vivo, suggesting that azaperone has low potential for
genetic damage.
In a three-generation study in rats, survival of the pups was
reduced during lactation in one generation at the highest dose of
40 mg/kg b.w./day. There were no adverse effects on other
reproduction parameters. It was recognized, however, that an
unconventional methodology was used in this study in that males were
left untreated and females were dosed on gestation days 6-15 only.
The study was considered to be inadequate to enable the potential
for effects on reproduction and fertility to be fully assessed.
Embryotoxicity and teratogenicity were examined in mice, rats,
golden hamsters, and rabbits. Fetal abnormalities were not observed
in any species. Administration of azaperone during the gestation
period resulted in embryotoxicity in mice at or above 10 mg/kg
b.w./day and in rats at 40 mg/kg b.w./day. Maternal toxicity and
fetotoxicity, in the form of delayed ossification of metatarsals and
metacarpals in mice and golden hamsters and reduced fetal weight in
rats, were noted at 40 mg/kg b.w./day. In a perinatal and postnatal
study in rats, the survival of pups during the lactation period was
reduced at 40 mg/kg b.w./day.
Human psychotic patients treated with up to 2 mg of azaperone
three times a day (about 0.1 mg/kg b.w./day) showed no clinical
effects. At doses of 2.5 mg given three times daily (about
0.125 mg/kg b.w./day) and above there was dose-related sedation, and
at 20 mg three times daily (about 1 mg/kg b.w./day), dizziness.
Haematological and blood chemistry parameters were not affected at
any dose.
There were no effects apart from sedation at 1.25 mg/kg
b.w./day in a 24-month dog study and at 8 mg/kg b.w./day in an
18-month rat study. There was no NOEL for pharmacological activity
in the animals used in the toxicological studies. However, the study
in human subjects provided additional information. The NOEL for
sedation was 2 mg given three times a day. Since the human subjects
were given azaperone in divided doses and it is unclear whether the
doses were additive over the course of the day, the NOEL for
sedation in humans was taken to be about 0.03 mg/kg b.w.
4. EVALUATION
In view of the absence of adequate carcinogenicity and
reproduction studies and the weak mutagenicity findings in bacteria,
the Committee could not establish an ADI. The Committee was aware of
data on the tumorigenic potential of other butyrophenone neuroleptic
agents, but considered that the structural differences between them
were sufficient to preclude the use of this information to support a
temporary ADI for azaperone.
5. REFERENCES
DUVERGER-VAN BOGAERT, M., VANPARYS, PH., DE MEESTER, C. & MARSBOOM,
R. (1987) Mutagenicity evaluation of azaperone in the
Salmonella/microsome test. Drug Chem. Toxicol., 10: 329-338.
HEYKANTS, J. (1973) The excretion and metabolism of azaperone in the
Wistar rat after oral and subcutaneous administration. Unpublished
Research Report No. V1266 from Janssen. Submitted to WHO by Janssen.
HEYKANTS, J. (1974) The transplacental passage of azaperone and its
metabolites in the rat. Unpublished Research Report No. V1552 from
Janssen. Submitted to WHO by Janssen.
HEYKANTS, J., LEWI, P. & JANSSEN, P.A.J. (1971a) On the distribution
and metabolism of azaperone (R1929) in the rat and pig. Part II.
Arzneim.-Forsch., 21: 1263-1269.
HEYKANTS, J., PARDOEL, L. & JANSSEN, P.A.J (1971b) On the
distribution and metabolism of azaperone (R1929) in the rat and pig.
Part I. Arzneim.-Forsch., 29: 982-984.
MARSBOOM, R. (1967) Potential of R1929 for embryotoxicity and
teratogenic effects in rats receiving R1929 subcutaneously.
Unpublished Research Report No. V967 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R. (1972a) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rats. Unpublished Research Report No. V1250
from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1972b) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rabbits. Unpublished Research Report No.
V1249 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973a) Potential of subcutaneous R1929 for
embryotoxicity and teratogenic effects in rats. Unpublished Research
Report No. V1248 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973b) Effects of R1929 in rats after oral
administration during the peri- and post-natal period. Unpublished
Research Report No. V1225 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974a) Potential of oral R1929 for embryotoxicity and
teratogenicity in rats: 3-generation study. Unpublished Research
Report No. V1707 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974b) Dominant lethal test. Unpublished Research
Report No. V1614 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & PARDOEL, L. (1973)
Untitled. Unpublished Research Report No. V1323 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1967)
Subcutaneous safety evaluation of R1929 in rats (13 weeks).
Unpublished Research Report No. V974 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1969)
Untitled. Unpublished Research Report No. V968 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976a)
Oral toxicity study in Wistar rats. Unpublished Research Report No.
V2490 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976b)
Oral toxicity study in Beagle dogs. Unpublished Research Report No.
N2491 from Janssen. Submitted to WHO by Janssen.
MEULDERMANS, W., HEYKANTS, J. & LAUWERS, W. (1973) An in vitro
study of the metabolism of azaperone (Stresnil R). Unpublished
Research Report No. V1505A from Janssen. Submitted to WHO by
Janssen.
MEULDERMANS, W.E.G., LAUWERS, W.F.J., KNAEPS, A.G., MICHIELS, L.J.M.
& HEYKANTS, J.J.P. (1975) In vitro metabolism of azaperone by rats
and pig liver fractions. Unpublished Research Report No. V1983 from
Janssen. Submitted to WHO by Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1973) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in
CRL:COBS-CD-1(ICR)BR outbred albino mice. Unpublished Research
Report No. 402 from McNeil Laboratories. Submitted to WHO by
Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1974) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in golden
hamsters. Unpublished Research Report No. 422 from McNeil
Laboratories. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E. (1975) The acute intravenous toxicity of R1929,
R2138 and R34189 in mice. Unpublished Research Report No. V2156 from
Janssen. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E., VAN NUETEN, J.M. & JANSSEN, P.A.J. (1974)
Azaperone, a sedative neuroleptic of the butyrophenone series with
pronounced anti-aggressive and anti-shock activity in animals.
Arzneim.-Forsch., 24: 1798-1806.
PEKKANEN, T.J. & SALMINEN, K. (1973) Azaperone and the hepatic
microsomes: effects on cytochrome P-450 concentration and on
NADPH-cytochrome c-reductase activity. Acta. Pharmacol. Toxicol.,
32: 285-288.
PONCELOT, F., DE MEESTER, C. & CRUTZEN-FAYT, C. (1982) In vitro
mutagenicity of R1929 Lot C2001. Unpublished Research Report No.
V4549 from Laboratoire de Toxicologie et Bromatologie, Brussels.
Submitted to WHO by Janssen.
PREISS, A.M., SCHEUTWINKEL-REICH, M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation, with the Salmonella/microsome
test, of psychopharmaceuticals used in meat production. Mutation
Res., 104: 333-337.
PREISS, A., SCHEUTWINKEL-REICH, M. & STAN, H-J. (1983) Mutagenitat
einiger in der Tiermast verwendeter Tranquilizer im
Salmonella/Mikrosomen-Test. Fleischwirtsch., 63: 243-244.
RAUWS, A.G., OLLING, M., FREUDENTHAL, J. & TEN HAM, M. (1976)
Azaperol, a new metabolite of the veterinary butyrophenone
tranquilizer azaperone. Toxicol. appl. Pharmacol., 35: 333-339.
REYNTJENS, A. (1972) Safety evaluation of azaperone treatment.
Unpublished Research Report No. V1164 from Janssen. Submitted to WHO
by Janssen.
SCHEUTWINKEL-REICH, M., PREISS, A.M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation of the butyrophenone tranquilizer
azaperone and its main metabolites with the Salmonella/microsome
test. Mutation Res., 104: 339-344.
VANPARYS, P.H. & MARSBOOM, R. (1982) Micronucleus test in rats.
Unpublished Research Report No. V4377 from Janssen. Submitted to WHO
by Janssen.
2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Results of acute studies with azaperone
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral 385 Niemegeers et
M s.c. 179 al., (1974)
M i.v. 38-42
Rat M oral 245
M s.c. 450
M i.v. 28
Guinea M oral 202
pig
Dogs N.S. oral > 20
N.S. s.c. > 20
The major toxic signs in rodents were ptosis, sedation, tremors
and occasionally clonic seizures. Ptosis and sedation were observed
in dogs with vomiting after oral dosing.
In mice, the i.v. LD50 values for metabolites 2 and 8 were 56
and 150 mg/kg respectively, higher than for azaperone (Niemegeers,
1975).
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 10 male and 10 female Wistar rats were given
subcutaneous injections of 0, 2.5, 10, and 40 mg/kg b.w./day
azaperone for 13 weeks. The vehicle used was 0.9% NaCl while the
purity of azaperone was not stated.
Treated rats in all groups were sedated for 2 hours following
dosing and at 40 mg/kg b.w./day passive behaviour was exhibited
throughout the study. There were no deaths due to treatment. In
males only, body weight gain was non-significantly reduced at 2.5
and 10 mg/kg b.w./day and markedly reduced at 40 mg/kg b.w./day.
Haematology, blood chemistry and urinalysis were examined at
the end of the study. The only apparent effects were a shift in the
differential white cell count from lymphocytes to neutrophils in
males at 10 mg/kg b.w./day and males and females at 40 mg/kg
b.w./day and a slight increase in serum alkaline phosphatase in
males at 10 and 40 mg/kg b.w./day.
At necropsy, spleens "looked degenerated" at 40 mg/kg b.w./day.
Thymus weights were decreased in males at 10 mg/kg b.w./day and
males and females at 40 mg/kg b.w./day. In females, liver weights
were increased and ovaries showed reduced numbers of corpora lutea
and increased glandular tissue at 40 mg/kg b.w./day (Marsboom et
al., 1967).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity 98-102%) for
15 weeks. There were no signs of toxicity during the study. Food
consumption and body weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood chemistry and urinalysis were
examined at the end of the study. The only notable findings were
decreased serum cholesterol in males and females, increased
urobilinogen in males and increased urinary creatinine in females,
all at 1600 ppm.
Gross pathology was unremarkable. Brain weights were heavier at
1600 ppm. Slight bile duct proliferation was seen in livers of 400
and 1600 ppm males. In females, ovaries showed "active large corpora
lutea" and there were "reduced eosinophils" in the uterine wall,
"mucified aspect" of vaginal mucosa, more developed alveolar tissue
in the mammary gland and "stimulation of erythrosinophils" in the
pituitary. The effects in females were expressed at 1600 ppm and to
a lesser extent at 400 ppm. The NOEL was 100 ppm, equal to 10 mg/kg
b.w./day (Marsboom et al., 1969).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 6 and 12 months. Based on food intake the average doses were 8,
31, and 130 mg/kg b.w./day for 6 months and 8, 30, and 127 mg/kg
b.w./day for 12 months.
Dose-related sedation was observed in all drug-treated groups
during the entire experimental period. Survival was unaffected. Food
consumption and body weight gains were depressed at 1600 ppm while
in the 400 ppm group weight gain was affected in the 6-month study
only.
Ophthalmoscopy, haematology, blood chemistry and urinalysis
were examined at the end of 6 and 12 months. Serum cholesterol was
decreased in the 1600 ppm group at 6 months but not at later times.
Serum bilirubin, BUN and urinary urobilinogen were higher in females
of the 1600 ppm group at 6 and 12 months.
At autopsy, gross pathology was unaffected. Brain weight was
increased at 1600 ppm after both 6 and 12 months of dosing. Septal
cell proliferation in the lung was marked at 6 and 12 months in the
1600 ppm group and led to lipoid pneumonia.
The females of the 1600 ppm group exhibited a "prolonged
diestrus in the uterus" (atrophic at 12 months) accompanied by
"reduced activity in the ovaries" (low numbers of active corpora
lutea and abundant interstitial glandular tissue), "mucification and
thin layered epithelium" with no cornification in the vagina and
"more extensive chromophobe tissue" in the pituitary. These findings
in reproductive tissues were more marked at 12 months. Apart from
the pharmacological effect of sedation, the NOEL was 8 mg/kg
b.w./day (Marsboom et al., 1976a).
2.2.2.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, or 20 mg/kg b.w./day of azaperone (purity 99.7%) for 13 weeks.
Dosing was carried out during 6 days per week in capsules.
Dogs of the 20 mg/kg b.w./day group exhibited a sedative effect
for 3 to 4 hours post-dosing in addition to decreased general
activity, ptosis and catatonia. Emesis and salivation were seen
occasionally at 5 mg/kg b.w./day and frequently at 20 mg/kg
b.w./day. Physical examinations revealed one female in each drug
treatment group with transient swelling of mammary glands.
Body weight was not clearly affected by dosing. Ophthalmoscopy,
ECG, blood pressure, haematology, blood biochemistry and urinalysis
were examined pre-treatment and monthly during the study. None of
these parameters was altered.
At terminal necropsy, liver weights tended to increased values
at 5 and 20 mg/kg b.w./day, but a dose-relationship was not evident.
Gross and histopathology were unaffected. The NOEL was 1.25 mg/kg
b.w./day (Marsboom et al., 1973).
2.2.3 Long term/carcinogenicity studies
2.2.3.1 Rats
Concomitant with the 6 and 12 month rat studies (see Section
2.2.2.1), groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 18 months. Based on food intake the average doses were 7, 29,
and 115 mg/kg b.w./day for 18 months.
As in the shorter-term studies, sedation was noted at all doses
while food intake and weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood biochemistry and urinalysis were
examined at the end of the study. Serum bilirubin and BUN and
urinary urobilinogen were increased in females of the 1600 ppm
group.
At necropsy, brain weight was increased in 1600 ppm rats. Gross
pathology was unremarkable. Septal cell proliferation in the lung
leading to lipoid pneumonia was marked at 1600 ppm. Effects seen in
the pituitary, ovary, uterus, and vagina at 6 and 12 months were not
evident in this 18-month experiment. Tumours were not increased.
Pharmacological effects were noted at all doses, the NOEL for
toxicological effects was 29 mg/kg b.w./day (Marsboom et al.,
1976a).
2.2.3.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, and 20 mg/kg b.w./day of azaperone (purity not stated) for 24
months. Animals were dosed 6 days/week in capsules.
One male dog given 20 mg/kg b.w./day azaperone died in the 64th
week. Signs of intoxication included sedation, back arching,
protrusion of the tongue, head shaking, muscle tremors, apnea,
lacrimation, increased salivation, and emesis. These effects were
seen in most dogs at 20 mg/kg b.w./day and some dogs at 5 mg/kg
b.w./day with sporadic emesis and salivation at 1.25 mg/kg b.w./day.
Body weight gain was unaffected.
Ophthalmoscopy, ECG, BP, haematology, blood biochemistry and
urinalysis were examined pre-dosing and every 3 months during the
study. None of these parameters was altered.
At autopsy, there was increased bile on the duodenal mucosa in
the 5 and 20 mg/kg b.w./day groups. The weights of adrenal glands
and the liver were increased at 20 mg/kg b.w./day. The following
microscopic changes were noted mainly in females of the 1.25 and
5 mg/kg b.w./day groups with virtually no effects at 20 mg/kg
b.w./day: "more marked or protracted metoestral period" -- active
corpora lutea in 2 low-dose (LD) and 1 mid-dose (MD), "fatty
superficial epithelium" in uterus of 2 LD and 2 MD, "more resting
aspect" of genital tract with "thin layered vaginal epithelium" in
all LD and 2 MD, atrophy of uterine wall in 1 high-dose (HD),
stimulation of mammary gland in LD and MD and "stimulation of
erythrosinophilic tissue" in the pituitary in 2 LD. A NOEL was not
identified (Marsboom et al., 1976b).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation study was carried out in Wistar rats. The
initial generation (F0) was allowed to deliver and suckle pups.
The second generation (F1) was mated within treatment groups
avoiding brother-sister matings, and was allowed to deliver and
suckle pups. The third generation (F2) was mated as in the F1
but the females were killed on gestation day 22.
Azaperone (purity not stated) was administered in the diet at
concentrations of 0, 25, 100, and 400 ppm food. The dietary levels
resulted in doses of approximately 2.5, 10, and 40 mg/kg b.w./day.
Only adult females were treated, on gestation days 6 to 15 in each
generation. Group sizes were 20 F0 females, 29-33 F1 females,
and 40-52 F2 females.
There were no mortalities among treated dams in any generation
but other toxic signs were not recorded. Maternal weight gain and
pregnancy rate were similar between groups.
Litter size, pup weight and post-natal weight gain were
unaffected in F1 and F2 offspring. Pup survival was reduced at
40 mg/kg b.w./day during the F2 lactation period only.
Uterine examination of F2 dams revealed no effects on
implantation, resorption or fetal weight. In the 40 mg/kg b.w./day
group, there were 2 F3 fetuses without metacarpal bones of the
foreleg and 1 without metatarsal bones of the hind leg (unilateral).
The NOEL was 10 mg/kg b.w./day (Marsboom, 1974a).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 29 pregnant CRL:COBS-CD-1 mice were given gavage
doses of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone
(purity not stated). Treatment was on gestation days 6 to 15 and
females were killed on gestation day 18. The vehicle contained
tartaric acid, sodium bisulfite, methyl and propyl paraben.
A number of animals died in the vehicle and drug-treated
groups, death was attributed to dosing difficulties as a result of
conditioned aversion to the vehicle. Decreased activity, ptosis,
impaired righting reflex and catalepsy were seen 1 to 3 hours
post-dosing with 10 and 40 mg/kg b.w./day azaperone.
Maternal weight gain was reduced in the vehicle control group
and further reduced at 10 and 40 mg/kg b.w./day. This effect on body
weight may be a reflection of lower litter sizes which were due to
both reduced numbers of corpora lutea and slightly increased
resorptions. Implantation rate, fetal weight and survival were not
significantly affected.
The ratio of male to female fetuses was reduced in the vehicle
and 40 mg/kg b.w./day groups. Fetal examination revealed a slight
delay in ossification of tarsals and phalanges of fore and hind paws
at 40 mg/kg b.w./day. Gross and visceral abnormalities were not
induced. The NOEL was 2.5 mg/kg b.w./day (Mosher et al., 1973).
2.2.5.2 Rats
Groups of pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 22.
Maternal survival and weight gain were unaffected. The number
of implantations and live and dead fetuses was similar in all
groups. Fetal weight and gross, visceral, and skeletal examinations
were unremarkable. There was no effect at the highest dose of
40 mg/kg b.w./day (Marsboom, 1972a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 15 and
females were killed on gestation day 22.
There was no mortality among the dams but weight gain was lower
in the 10 and 40 mg/kg b.w./day groups. The number of implantations
was similar in all groups. Relative to the number of implantations,
the number of resorptions was slightly increased at 40 mg/kg
b.w./day, resulting in slightly smaller litter size. Fetal weight
was reduced at 40 mg/kg b.w./day and the only fetal abnormality was
scoliosis in one 40 mg/kg b.w./day fetus (Marsboom, 1973a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 1 to 21 and
females were killed on gestation day 22.
There was no maternal mortality. Weight gain in the dams was
reduced at 10 and 40 mg/kg b.w./day. The number of implantations was
lower at 10 and 40 mg/kg b.w./day and resorptions were increased at
40 mg/kg b.w./day. Fetal weight was depressed at 40 mg/kg b.w./day
but there was no increase in fetal abnormalities (Marsboom, 1967).
Groups of 25 pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was from gestation day 16 to post-partum
day 21 and dams were allowed to deliver naturally and nurse young
throughout lactation.
There were no maternal deaths and the duration of gestation and
parturition were unaffected. Body weight gain of dams in the 2.5 and
40 mg/kg b.w./day groups was reduced. Litter size, birth weight, and
post-natal weight gain were unremarkable. The survival of pups
through the lactation period was compromised at 40 mg/kg b.w./day.
There were no fetal abnormalities. The NOEL was 10 mg/kg b.w./day
(Marsboom, 1973b).
2.2.5.3 Golden hamsters
Groups of 26 pregnant golden hamsters were given gavage doses
of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity
not stated). Treatment was on gestation days 6 to 10 and females
were killed on gestation day 15. The vehicle contained tartaric
acid, sodium bisulfite, methyl and propyl paraben.
There was no maternal mortality. Toxic signs were ptosis from
2.5 mg/kg b.w./day, decreased motor activity from 10 mg/kg b.w./day,
catalepsy and impaired righting reflex at 40 mg/kg b.w./day during
the 2 hours following dosing.
Body weight gain in dams was depressed at 40 mg/kg b.w./day,
and was associated with lower fetal weight at this dose. The number
of implantations, resorptions and litter size was similar between
groups. Fetal examination revealed only a slight delay in
ossification of metatarsals at 40 mg/kg b.w./day. Discounting
pharmacological effects, the NOEL was 10 mg/kg b.w./day (Mosher et
al., 1974).
2.2.5.4 Rabbits
Groups of 15 pregnant NZ White rabbits were given gavage doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 18 and
females were killed on gestation day 28.
There were no maternal deaths and body weight gain was
depressed at 10 and 40 mg/kg b.w./day. Resorptions or fetal death
were not induced but litter size was slightly lower in the 40 mg/kg
b.w./day group, reflecting lower numbers of implantations. There
were no fetal abnormalities related to treatment. The NOEL was
2.5 mg/kg b.w./day (Marsboom, 1972b).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on azaperone1
Test system Test object Concentration Results Reference
Ames test2 S.typhimurium
TA 1538 > 750 µg/plate positive3 Preiss et al.,
TA 1537 (+ S9 mix) 1982, 1983
TA 98
TA 1535 2500 µg/plate negative Scheutwinkel-
TA 100 (+ S9 mix) Reich, et al.,
1982
TA 1535 2500 µg/plate
TA 1537 (- S9 mix)
TA 98
TA 1538
TA 100
Ames test2 S.typhimurium
TA 1530 2000 µg/plate4 negative Poncelet et al.,
TA 1535 (+ & - S9 1982; Duvergervan
TA 1537 mix) Bogaert et
TA 1538 al., 1987
TA 989
TA 100
Micro Rats 20-160 mg/kg negative Vanparys &
nucleus test orally Marsboom, 1982
Dominant Mice 10-160 mg/kg negative Marsboom,
lethal test orally 1974b
1. Appropriate positive controls were used.
2. Both with and without rat liver S9 fraction.
3. There was no dose relationship.
4. Higher concentrations were bacteriotoxic.
Table 3. Results of Ames tests on azaperone metabolites1,2
(Scheutwinkel-Reich et al., 1982)
Compound Strain Concentration Results
alpha-(4-fluorophenyl)-4- TA 98 > 500 µg/plate (+ S9) positive2
(2-pyridinyl)-piperazine
butanol (Azaperol)
TA 1538 1500 µg/plate (+ S9) positive
TA 1535 2500 µg/plate negative
TA 1537 (+ S9)
TA 100
TA 98 2500 µg/plate negative
TA 1538 (- S9)
TA 1535
TA 1537
TA 100
4-(4-acetyl)-1-piperazinyl- TA 1538 5000 µg/plate positive
41-fluoro-butyrophenone (+ S9)
TA 98 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
TA 98 5000 µg/plate negative
TA 1538 (+ S9)
TA 1535
TA 1537
TA 100
ß-(p-fluorobenzoyl)- TA 98 2500 µg/plate positive2
propanoic acid (+ S9)
TA 1538 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
p-fluorobenzoyl TA 98 5000 µg/plate negative
acetic acid TA 1538 (+ and - S9)
TA 1535
TA 1537
TA 100
1. Appropriate positive controls were used throughout
2. Response not dose-related
2.2.7 Special studies on pharmacology
Table 4. Results of studies with azaperone in pharmacological tests ( Niemegeers
et al, 1974).
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
amphetamine antagonism 2.5 - -
apomorphine antagonism 0.34/9.15 - 0.98
norepineprine antagonism 0.33 - -
tryptamine antagonism 5.9 - -
jumping box test 0.7 - 5(3.95 OR)
W-test
- body weight 1.75 - -
- food intake 2.5 - -
- faecal output 4.0 - -
behavioural observations
- catalepsy 8.0 - -
- ptosis 1.5 - -
open field test
- ambulation 6.7 - -
- rearing 4.1 - -
- defecation 6.9 - -
traumatic shock test 0.021 - -
thermoregulation 3.27 - -
- 37 °C > 320 -
- 30 °C
Table 4. cont'd
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
tail withdrawal test > 40 - -
inhibition of food intake > 10 - -
hot plate test - 7.0 -
inhibition of righting reflex - > 40 -
pentobarbital potentiation - 0.4 -
rotating rod test - 1.64 -
fighting test - 0.74 -
1. lowest effective dose, the NOEL was 0.01 mg/kg sc.
2.3 Observations in humans
A group of 20 male psychotics were studied; 10 remained on their
previous medication, 10 had their medication replaced with azaperone.
Doses were commenced at 0.5 mg three times daily (t.i.d.) increasing
to 20 mg t.i.d over a 17-day period. The maximum dose was then
administered for 2 months.
Clinical observation revealed no symptoms up to 2 mg t.i.d. of
azaperone. At higher doses (from 2.5 mg t.i.d.), sedation was observed
in a dose-related manner and at 20 mg t.i.d. patients started to
complain of dizziness. Haematology and blood chemistry parameters,
examined prior to and at the end of 2´ months azaperone treatment, were
within the normal range (Reyntjens, 1972).
3. COMMENTS
A range of studies on azaperone was submitted for assessment
including data on kinetics and metabolism, acute toxicity,
short-term and long-term toxicity, developmental toxicity, and
genotoxicity. Most of the studies were carried out in the 1970s and
the standard of testing and reporting varied widely.
The kinetic studies with azaperone were insufficient to
determine the extent of absorption from the gastrointestinal tract.
However, by comparison with the excretion pattern after parenteral
dosing, it was estimated that absorption after oral dosing was
probably high. Distribution within the body in rats was extensive
and excretion was primarily in the faeces (81%), with lesser amounts
in urine (16%). Azaperone is extensively and rapidly metabolized.
Two metabolites were found in the pig but not in the rat. However,
these compounds are devoid of significant pharmacological activity,
and therefore do not affect the suitability of the rat as a model
for toxicological testing.
Azaperone was moderately toxic in acute toxicity studies in
mice, rats, guinea pigs, and dogs. Most signs of intoxication
reflected exaggerated pharmacological activity of azaperone in the
central nervous system. A battery of pharmacological studies
indicated that azaperone possesses potent anti-alpha-adrenergic
activity, but these data were inadequate for use in determining a
NOEL as the drug was almost always given by the subcutaneous route.
Short- and long-term toxicity studies were carried out in rats
and dogs. Dose-related sedation was the major effect in both species
and was observed at all treatment levels. Minor hepatotoxicity was
observed at doses at and above 30 mg/kg b.w./day in rats and 5 mg/kg
b.w./day in dogs. In rats only, brain weight was consistently
increased at 30 mg/kg b.w./day, but in the absence of any
pathological change this observation could not be explained.
There were pathological changes in the pituitary and sex
organs, particularly in rats; these were typical of neuroleptic
agents. It has been postulated that the primary effect is
pharmacological and is caused by the blocking of dopamine receptors
in the hypothalamus or pituitary, resulting in increased prolactin
and decreased gonadotrophin secretion. While this could account for
the observed slight stimulation of the pituitary and mammary glands
and the quiescence of the female reproductive tract, direct evidence
for this mechanism was lacking. The effects on the reproductive
organs were slight, in line with the relatively weak anti-dopamine
activity of azaperone. In dogs, such effects were observed only
after dosing for 24 months with 1.25 and 5 mg/kg b.w./day but not at
20 mg/kg b.w./day. In rats, effects were noted after 3, 6, and 12
months but not after 18 months, which suggests the possibility of
adaptation. When pharmacological effects were excluded, the NOELs
were 1.25 mg/kg b.w./day in dogs and 8 mg/kg b.w./day in rats.
The Committee noted that the carcinogenic potential of
azaperone had not been adequately investigated. The only study in
which lifetime exposure was approached was an 18 month study in
rats. However, the duration of dosing was too short and the small
group size (only ten rats of each sex) was inadequate to determine
treatment-related tumour incidences satisfactorily.
Frame shift mutations in Salmonella typhimurium strains were
seen for azaperone and three metabolites in a series of studies
carried out by one group of investigators. However, reversion rates
were only 2-3 times those in controls; there was no dose-response
relationship, and high doses in the presence of rat liver microsomes
were required. This weak response was not reproduced by a second
group of investigators using the same bacterial strains.
Genotoxicity was absent in the micronucleus and dominant lethal
tests in vivo, suggesting that azaperone has low potential for
genetic damage.
In a three-generation study in rats, survival of the pups was
reduced during lactation in one generation at the highest dose of
40 mg/kg b.w./day. There were no adverse effects on other
reproduction parameters. It was recognized, however, that an
unconventional methodology was used in this study in that males were
left untreated and females were dosed on gestation days 6-15 only.
The study was considered to be inadequate to enable the potential
for effects on reproduction and fertility to be fully assessed.
Embryotoxicity and teratogenicity were examined in mice, rats,
golden hamsters, and rabbits. Fetal abnormalities were not observed
in any species. Administration of azaperone during the gestation
period resulted in embryotoxicity in mice at or above 10 mg/kg
b.w./day and in rats at 40 mg/kg b.w./day. Maternal toxicity and
fetotoxicity, in the form of delayed ossification of metatarsals and
metacarpals in mice and golden hamsters and reduced fetal weight in
rats, were noted at 40 mg/kg b.w./day. In a perinatal and postnatal
study in rats, the survival of pups during the lactation period was
reduced at 40 mg/kg b.w./day.
Human psychotic patients treated with up to 2 mg of azaperone
three times a day (about 0.1 mg/kg b.w./day) showed no clinical
effects. At doses of 2.5 mg given three times daily (about
0.125 mg/kg b.w./day) and above there was dose-related sedation, and
at 20 mg three times daily (about 1 mg/kg b.w./day), dizziness.
Haematological and blood chemistry parameters were not affected at
any dose.
There were no effects apart from sedation at 1.25 mg/kg
b.w./day in a 24-month dog study and at 8 mg/kg b.w./day in an
18-month rat study. There was no NOEL for pharmacological activity
in the animals used in the toxicological studies. However, the study
in human subjects provided additional information. The NOEL for
sedation was 2 mg given three times a day. Since the human subjects
were given azaperone in divided doses and it is unclear whether the
doses were additive over the course of the day, the NOEL for
sedation in humans was taken to be about 0.03 mg/kg b.w.
4. EVALUATION
In view of the absence of adequate carcinogenicity and
reproduction studies and the weak mutagenicity findings in bacteria,
the Committee could not establish an ADI. The Committee was aware of
data on the tumorigenic potential of other butyrophenone neuroleptic
agents, but considered that the structural differences between them
were sufficient to preclude the use of this information to support a
temporary ADI for azaperone.
5. REFERENCES
DUVERGER-VAN BOGAERT, M., VANPARYS, PH., DE MEESTER, C. & MARSBOOM,
R. (1987) Mutagenicity evaluation of azaperone in the
Salmonella/microsome test. Drug Chem. Toxicol., 10: 329-338.
HEYKANTS, J. (1973) The excretion and metabolism of azaperone in the
Wistar rat after oral and subcutaneous administration. Unpublished
Research Report No. V1266 from Janssen. Submitted to WHO by Janssen.
HEYKANTS, J. (1974) The transplacental passage of azaperone and its
metabolites in the rat. Unpublished Research Report No. V1552 from
Janssen. Submitted to WHO by Janssen.
HEYKANTS, J., LEWI, P. & JANSSEN, P.A.J. (1971a) On the distribution
and metabolism of azaperone (R1929) in the rat and pig. Part II.
Arzneim.-Forsch., 21: 1263-1269.
HEYKANTS, J., PARDOEL, L. & JANSSEN, P.A.J (1971b) On the
distribution and metabolism of azaperone (R1929) in the rat and pig.
Part I. Arzneim.-Forsch., 29: 982-984.
MARSBOOM, R. (1967) Potential of R1929 for embryotoxicity and
teratogenic effects in rats receiving R1929 subcutaneously.
Unpublished Research Report No. V967 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R. (1972a) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rats. Unpublished Research Report No. V1250
from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1972b) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rabbits. Unpublished Research Report No.
V1249 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973a) Potential of subcutaneous R1929 for
embryotoxicity and teratogenic effects in rats. Unpublished Research
Report No. V1248 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973b) Effects of R1929 in rats after oral
administration during the peri- and post-natal period. Unpublished
Research Report No. V1225 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974a) Potential of oral R1929 for embryotoxicity and
teratogenicity in rats: 3-generation study. Unpublished Research
Report No. V1707 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974b) Dominant lethal test. Unpublished Research
Report No. V1614 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & PARDOEL, L. (1973)
Untitled. Unpublished Research Report No. V1323 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1967)
Subcutaneous safety evaluation of R1929 in rats (13 weeks).
Unpublished Research Report No. V974 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1969)
Untitled. Unpublished Research Report No. V968 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976a)
Oral toxicity study in Wistar rats. Unpublished Research Report No.
V2490 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976b)
Oral toxicity study in Beagle dogs. Unpublished Research Report No.
N2491 from Janssen. Submitted to WHO by Janssen.
MEULDERMANS, W., HEYKANTS, J. & LAUWERS, W. (1973) An in vitro
study of the metabolism of azaperone (Stresnil R). Unpublished
Research Report No. V1505A from Janssen. Submitted to WHO by
Janssen.
MEULDERMANS, W.E.G., LAUWERS, W.F.J., KNAEPS, A.G., MICHIELS, L.J.M.
& HEYKANTS, J.J.P. (1975) In vitro metabolism of azaperone by rats
and pig liver fractions. Unpublished Research Report No. V1983 from
Janssen. Submitted to WHO by Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1973) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in
CRL:COBS-CD-1(ICR)BR outbred albino mice. Unpublished Research
Report No. 402 from McNeil Laboratories. Submitted to WHO by
Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1974) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in golden
hamsters. Unpublished Research Report No. 422 from McNeil
Laboratories. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E. (1975) The acute intravenous toxicity of R1929,
R2138 and R34189 in mice. Unpublished Research Report No. V2156 from
Janssen. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E., VAN NUETEN, J.M. & JANSSEN, P.A.J. (1974)
Azaperone, a sedative neuroleptic of the butyrophenone series with
pronounced anti-aggressive and anti-shock activity in animals.
Arzneim.-Forsch., 24: 1798-1806.
PEKKANEN, T.J. & SALMINEN, K. (1973) Azaperone and the hepatic
microsomes: effects on cytochrome P-450 concentration and on
NADPH-cytochrome c-reductase activity. Acta. Pharmacol. Toxicol.,
32: 285-288.
PONCELOT, F., DE MEESTER, C. & CRUTZEN-FAYT, C. (1982) In vitro
mutagenicity of R1929 Lot C2001. Unpublished Research Report No.
V4549 from Laboratoire de Toxicologie et Bromatologie, Brussels.
Submitted to WHO by Janssen.
PREISS, A.M., SCHEUTWINKEL-REICH, M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation, with the Salmonella/microsome
test, of psychopharmaceuticals used in meat production. Mutation
Res., 104: 333-337.
PREISS, A., SCHEUTWINKEL-REICH, M. & STAN, H-J. (1983) Mutagenitat
einiger in der Tiermast verwendeter Tranquilizer im
Salmonella/Mikrosomen-Test. Fleischwirtsch., 63: 243-244.
RAUWS, A.G., OLLING, M., FREUDENTHAL, J. & TEN HAM, M. (1976)
Azaperol, a new metabolite of the veterinary butyrophenone
tranquilizer azaperone. Toxicol. appl. Pharmacol., 35: 333-339.
REYNTJENS, A. (1972) Safety evaluation of azaperone treatment.
Unpublished Research Report No. V1164 from Janssen. Submitted to WHO
by Janssen.
SCHEUTWINKEL-REICH, M., PREISS, A.M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation of the butyrophenone tranquilizer
azaperone and its main metabolites with the Salmonella/microsome
test. Mutation Res., 104: 339-344.
VANPARYS, P.H. & MARSBOOM, R. (1982) Micronucleus test in rats.
Unpublished Research Report No. V4377 from Janssen. Submitted to WHO
by Janssen.
 2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Results of acute studies with azaperone
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral 385 Niemegeers et
M s.c. 179 al., (1974)
M i.v. 38-42
Rat M oral 245
M s.c. 450
M i.v. 28
Guinea M oral 202
pig
Dogs N.S. oral > 20
N.S. s.c. > 20
The major toxic signs in rodents were ptosis, sedation, tremors
and occasionally clonic seizures. Ptosis and sedation were observed
in dogs with vomiting after oral dosing.
In mice, the i.v. LD50 values for metabolites 2 and 8 were 56
and 150 mg/kg respectively, higher than for azaperone (Niemegeers,
1975).
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 10 male and 10 female Wistar rats were given
subcutaneous injections of 0, 2.5, 10, and 40 mg/kg b.w./day
azaperone for 13 weeks. The vehicle used was 0.9% NaCl while the
purity of azaperone was not stated.
Treated rats in all groups were sedated for 2 hours following
dosing and at 40 mg/kg b.w./day passive behaviour was exhibited
throughout the study. There were no deaths due to treatment. In
males only, body weight gain was non-significantly reduced at 2.5
and 10 mg/kg b.w./day and markedly reduced at 40 mg/kg b.w./day.
Haematology, blood chemistry and urinalysis were examined at
the end of the study. The only apparent effects were a shift in the
differential white cell count from lymphocytes to neutrophils in
males at 10 mg/kg b.w./day and males and females at 40 mg/kg
b.w./day and a slight increase in serum alkaline phosphatase in
males at 10 and 40 mg/kg b.w./day.
At necropsy, spleens "looked degenerated" at 40 mg/kg b.w./day.
Thymus weights were decreased in males at 10 mg/kg b.w./day and
males and females at 40 mg/kg b.w./day. In females, liver weights
were increased and ovaries showed reduced numbers of corpora lutea
and increased glandular tissue at 40 mg/kg b.w./day (Marsboom et
al., 1967).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity 98-102%) for
15 weeks. There were no signs of toxicity during the study. Food
consumption and body weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood chemistry and urinalysis were
examined at the end of the study. The only notable findings were
decreased serum cholesterol in males and females, increased
urobilinogen in males and increased urinary creatinine in females,
all at 1600 ppm.
Gross pathology was unremarkable. Brain weights were heavier at
1600 ppm. Slight bile duct proliferation was seen in livers of 400
and 1600 ppm males. In females, ovaries showed "active large corpora
lutea" and there were "reduced eosinophils" in the uterine wall,
"mucified aspect" of vaginal mucosa, more developed alveolar tissue
in the mammary gland and "stimulation of erythrosinophils" in the
pituitary. The effects in females were expressed at 1600 ppm and to
a lesser extent at 400 ppm. The NOEL was 100 ppm, equal to 10 mg/kg
b.w./day (Marsboom et al., 1969).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 6 and 12 months. Based on food intake the average doses were 8,
31, and 130 mg/kg b.w./day for 6 months and 8, 30, and 127 mg/kg
b.w./day for 12 months.
Dose-related sedation was observed in all drug-treated groups
during the entire experimental period. Survival was unaffected. Food
consumption and body weight gains were depressed at 1600 ppm while
in the 400 ppm group weight gain was affected in the 6-month study
only.
Ophthalmoscopy, haematology, blood chemistry and urinalysis
were examined at the end of 6 and 12 months. Serum cholesterol was
decreased in the 1600 ppm group at 6 months but not at later times.
Serum bilirubin, BUN and urinary urobilinogen were higher in females
of the 1600 ppm group at 6 and 12 months.
At autopsy, gross pathology was unaffected. Brain weight was
increased at 1600 ppm after both 6 and 12 months of dosing. Septal
cell proliferation in the lung was marked at 6 and 12 months in the
1600 ppm group and led to lipoid pneumonia.
The females of the 1600 ppm group exhibited a "prolonged
diestrus in the uterus" (atrophic at 12 months) accompanied by
"reduced activity in the ovaries" (low numbers of active corpora
lutea and abundant interstitial glandular tissue), "mucification and
thin layered epithelium" with no cornification in the vagina and
"more extensive chromophobe tissue" in the pituitary. These findings
in reproductive tissues were more marked at 12 months. Apart from
the pharmacological effect of sedation, the NOEL was 8 mg/kg
b.w./day (Marsboom et al., 1976a).
2.2.2.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, or 20 mg/kg b.w./day of azaperone (purity 99.7%) for 13 weeks.
Dosing was carried out during 6 days per week in capsules.
Dogs of the 20 mg/kg b.w./day group exhibited a sedative effect
for 3 to 4 hours post-dosing in addition to decreased general
activity, ptosis and catatonia. Emesis and salivation were seen
occasionally at 5 mg/kg b.w./day and frequently at 20 mg/kg
b.w./day. Physical examinations revealed one female in each drug
treatment group with transient swelling of mammary glands.
Body weight was not clearly affected by dosing. Ophthalmoscopy,
ECG, blood pressure, haematology, blood biochemistry and urinalysis
were examined pre-treatment and monthly during the study. None of
these parameters was altered.
At terminal necropsy, liver weights tended to increased values
at 5 and 20 mg/kg b.w./day, but a dose-relationship was not evident.
Gross and histopathology were unaffected. The NOEL was 1.25 mg/kg
b.w./day (Marsboom et al., 1973).
2.2.3 Long term/carcinogenicity studies
2.2.3.1 Rats
Concomitant with the 6 and 12 month rat studies (see Section
2.2.2.1), groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 18 months. Based on food intake the average doses were 7, 29,
and 115 mg/kg b.w./day for 18 months.
As in the shorter-term studies, sedation was noted at all doses
while food intake and weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood biochemistry and urinalysis were
examined at the end of the study. Serum bilirubin and BUN and
urinary urobilinogen were increased in females of the 1600 ppm
group.
At necropsy, brain weight was increased in 1600 ppm rats. Gross
pathology was unremarkable. Septal cell proliferation in the lung
leading to lipoid pneumonia was marked at 1600 ppm. Effects seen in
the pituitary, ovary, uterus, and vagina at 6 and 12 months were not
evident in this 18-month experiment. Tumours were not increased.
Pharmacological effects were noted at all doses, the NOEL for
toxicological effects was 29 mg/kg b.w./day (Marsboom et al.,
1976a).
2.2.3.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, and 20 mg/kg b.w./day of azaperone (purity not stated) for 24
months. Animals were dosed 6 days/week in capsules.
One male dog given 20 mg/kg b.w./day azaperone died in the 64th
week. Signs of intoxication included sedation, back arching,
protrusion of the tongue, head shaking, muscle tremors, apnea,
lacrimation, increased salivation, and emesis. These effects were
seen in most dogs at 20 mg/kg b.w./day and some dogs at 5 mg/kg
b.w./day with sporadic emesis and salivation at 1.25 mg/kg b.w./day.
Body weight gain was unaffected.
Ophthalmoscopy, ECG, BP, haematology, blood biochemistry and
urinalysis were examined pre-dosing and every 3 months during the
study. None of these parameters was altered.
At autopsy, there was increased bile on the duodenal mucosa in
the 5 and 20 mg/kg b.w./day groups. The weights of adrenal glands
and the liver were increased at 20 mg/kg b.w./day. The following
microscopic changes were noted mainly in females of the 1.25 and
5 mg/kg b.w./day groups with virtually no effects at 20 mg/kg
b.w./day: "more marked or protracted metoestral period" -- active
corpora lutea in 2 low-dose (LD) and 1 mid-dose (MD), "fatty
superficial epithelium" in uterus of 2 LD and 2 MD, "more resting
aspect" of genital tract with "thin layered vaginal epithelium" in
all LD and 2 MD, atrophy of uterine wall in 1 high-dose (HD),
stimulation of mammary gland in LD and MD and "stimulation of
erythrosinophilic tissue" in the pituitary in 2 LD. A NOEL was not
identified (Marsboom et al., 1976b).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation study was carried out in Wistar rats. The
initial generation (F0) was allowed to deliver and suckle pups.
The second generation (F1) was mated within treatment groups
avoiding brother-sister matings, and was allowed to deliver and
suckle pups. The third generation (F2) was mated as in the F1
but the females were killed on gestation day 22.
Azaperone (purity not stated) was administered in the diet at
concentrations of 0, 25, 100, and 400 ppm food. The dietary levels
resulted in doses of approximately 2.5, 10, and 40 mg/kg b.w./day.
Only adult females were treated, on gestation days 6 to 15 in each
generation. Group sizes were 20 F0 females, 29-33 F1 females,
and 40-52 F2 females.
There were no mortalities among treated dams in any generation
but other toxic signs were not recorded. Maternal weight gain and
pregnancy rate were similar between groups.
Litter size, pup weight and post-natal weight gain were
unaffected in F1 and F2 offspring. Pup survival was reduced at
40 mg/kg b.w./day during the F2 lactation period only.
Uterine examination of F2 dams revealed no effects on
implantation, resorption or fetal weight. In the 40 mg/kg b.w./day
group, there were 2 F3 fetuses without metacarpal bones of the
foreleg and 1 without metatarsal bones of the hind leg (unilateral).
The NOEL was 10 mg/kg b.w./day (Marsboom, 1974a).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 29 pregnant CRL:COBS-CD-1 mice were given gavage
doses of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone
(purity not stated). Treatment was on gestation days 6 to 15 and
females were killed on gestation day 18. The vehicle contained
tartaric acid, sodium bisulfite, methyl and propyl paraben.
A number of animals died in the vehicle and drug-treated
groups, death was attributed to dosing difficulties as a result of
conditioned aversion to the vehicle. Decreased activity, ptosis,
impaired righting reflex and catalepsy were seen 1 to 3 hours
post-dosing with 10 and 40 mg/kg b.w./day azaperone.
Maternal weight gain was reduced in the vehicle control group
and further reduced at 10 and 40 mg/kg b.w./day. This effect on body
weight may be a reflection of lower litter sizes which were due to
both reduced numbers of corpora lutea and slightly increased
resorptions. Implantation rate, fetal weight and survival were not
significantly affected.
The ratio of male to female fetuses was reduced in the vehicle
and 40 mg/kg b.w./day groups. Fetal examination revealed a slight
delay in ossification of tarsals and phalanges of fore and hind paws
at 40 mg/kg b.w./day. Gross and visceral abnormalities were not
induced. The NOEL was 2.5 mg/kg b.w./day (Mosher et al., 1973).
2.2.5.2 Rats
Groups of pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 22.
Maternal survival and weight gain were unaffected. The number
of implantations and live and dead fetuses was similar in all
groups. Fetal weight and gross, visceral, and skeletal examinations
were unremarkable. There was no effect at the highest dose of
40 mg/kg b.w./day (Marsboom, 1972a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 15 and
females were killed on gestation day 22.
There was no mortality among the dams but weight gain was lower
in the 10 and 40 mg/kg b.w./day groups. The number of implantations
was similar in all groups. Relative to the number of implantations,
the number of resorptions was slightly increased at 40 mg/kg
b.w./day, resulting in slightly smaller litter size. Fetal weight
was reduced at 40 mg/kg b.w./day and the only fetal abnormality was
scoliosis in one 40 mg/kg b.w./day fetus (Marsboom, 1973a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 1 to 21 and
females were killed on gestation day 22.
There was no maternal mortality. Weight gain in the dams was
reduced at 10 and 40 mg/kg b.w./day. The number of implantations was
lower at 10 and 40 mg/kg b.w./day and resorptions were increased at
40 mg/kg b.w./day. Fetal weight was depressed at 40 mg/kg b.w./day
but there was no increase in fetal abnormalities (Marsboom, 1967).
Groups of 25 pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was from gestation day 16 to post-partum
day 21 and dams were allowed to deliver naturally and nurse young
throughout lactation.
There were no maternal deaths and the duration of gestation and
parturition were unaffected. Body weight gain of dams in the 2.5 and
40 mg/kg b.w./day groups was reduced. Litter size, birth weight, and
post-natal weight gain were unremarkable. The survival of pups
through the lactation period was compromised at 40 mg/kg b.w./day.
There were no fetal abnormalities. The NOEL was 10 mg/kg b.w./day
(Marsboom, 1973b).
2.2.5.3 Golden hamsters
Groups of 26 pregnant golden hamsters were given gavage doses
of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity
not stated). Treatment was on gestation days 6 to 10 and females
were killed on gestation day 15. The vehicle contained tartaric
acid, sodium bisulfite, methyl and propyl paraben.
There was no maternal mortality. Toxic signs were ptosis from
2.5 mg/kg b.w./day, decreased motor activity from 10 mg/kg b.w./day,
catalepsy and impaired righting reflex at 40 mg/kg b.w./day during
the 2 hours following dosing.
Body weight gain in dams was depressed at 40 mg/kg b.w./day,
and was associated with lower fetal weight at this dose. The number
of implantations, resorptions and litter size was similar between
groups. Fetal examination revealed only a slight delay in
ossification of metatarsals at 40 mg/kg b.w./day. Discounting
pharmacological effects, the NOEL was 10 mg/kg b.w./day (Mosher et
al., 1974).
2.2.5.4 Rabbits
Groups of 15 pregnant NZ White rabbits were given gavage doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 18 and
females were killed on gestation day 28.
There were no maternal deaths and body weight gain was
depressed at 10 and 40 mg/kg b.w./day. Resorptions or fetal death
were not induced but litter size was slightly lower in the 40 mg/kg
b.w./day group, reflecting lower numbers of implantations. There
were no fetal abnormalities related to treatment. The NOEL was
2.5 mg/kg b.w./day (Marsboom, 1972b).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on azaperone1
Test system Test object Concentration Results Reference
Ames test2 S.typhimurium
TA 1538 > 750 µg/plate positive3 Preiss et al.,
TA 1537 (+ S9 mix) 1982, 1983
TA 98
TA 1535 2500 µg/plate negative Scheutwinkel-
TA 100 (+ S9 mix) Reich, et al.,
1982
TA 1535 2500 µg/plate
TA 1537 (- S9 mix)
TA 98
TA 1538
TA 100
Ames test2 S.typhimurium
TA 1530 2000 µg/plate4 negative Poncelet et al.,
TA 1535 (+ & - S9 1982; Duvergervan
TA 1537 mix) Bogaert et
TA 1538 al., 1987
TA 989
TA 100
Micro Rats 20-160 mg/kg negative Vanparys &
nucleus test orally Marsboom, 1982
Dominant Mice 10-160 mg/kg negative Marsboom,
lethal test orally 1974b
1. Appropriate positive controls were used.
2. Both with and without rat liver S9 fraction.
3. There was no dose relationship.
4. Higher concentrations were bacteriotoxic.
Table 3. Results of Ames tests on azaperone metabolites1,2
(Scheutwinkel-Reich et al., 1982)
Compound Strain Concentration Results
alpha-(4-fluorophenyl)-4- TA 98 > 500 µg/plate (+ S9) positive2
(2-pyridinyl)-piperazine
butanol (Azaperol)
TA 1538 1500 µg/plate (+ S9) positive
TA 1535 2500 µg/plate negative
TA 1537 (+ S9)
TA 100
TA 98 2500 µg/plate negative
TA 1538 (- S9)
TA 1535
TA 1537
TA 100
4-(4-acetyl)-1-piperazinyl- TA 1538 5000 µg/plate positive
41-fluoro-butyrophenone (+ S9)
TA 98 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
TA 98 5000 µg/plate negative
TA 1538 (+ S9)
TA 1535
TA 1537
TA 100
ß-(p-fluorobenzoyl)- TA 98 2500 µg/plate positive2
propanoic acid (+ S9)
TA 1538 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
p-fluorobenzoyl TA 98 5000 µg/plate negative
acetic acid TA 1538 (+ and - S9)
TA 1535
TA 1537
TA 100
1. Appropriate positive controls were used throughout
2. Response not dose-related
2.2.7 Special studies on pharmacology
Table 4. Results of studies with azaperone in pharmacological tests ( Niemegeers
et al, 1974).
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
amphetamine antagonism 2.5 - -
apomorphine antagonism 0.34/9.15 - 0.98
norepineprine antagonism 0.33 - -
tryptamine antagonism 5.9 - -
jumping box test 0.7 - 5(3.95 OR)
W-test
- body weight 1.75 - -
- food intake 2.5 - -
- faecal output 4.0 - -
behavioural observations
- catalepsy 8.0 - -
- ptosis 1.5 - -
open field test
- ambulation 6.7 - -
- rearing 4.1 - -
- defecation 6.9 - -
traumatic shock test 0.021 - -
thermoregulation 3.27 - -
- 37 °C > 320 -
- 30 °C
Table 4. cont'd
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
tail withdrawal test > 40 - -
inhibition of food intake > 10 - -
hot plate test - 7.0 -
inhibition of righting reflex - > 40 -
pentobarbital potentiation - 0.4 -
rotating rod test - 1.64 -
fighting test - 0.74 -
1. lowest effective dose, the NOEL was 0.01 mg/kg sc.
2.3 Observations in humans
A group of 20 male psychotics were studied; 10 remained on their
previous medication, 10 had their medication replaced with azaperone.
Doses were commenced at 0.5 mg three times daily (t.i.d.) increasing
to 20 mg t.i.d over a 17-day period. The maximum dose was then
administered for 2 months.
Clinical observation revealed no symptoms up to 2 mg t.i.d. of
azaperone. At higher doses (from 2.5 mg t.i.d.), sedation was observed
in a dose-related manner and at 20 mg t.i.d. patients started to
complain of dizziness. Haematology and blood chemistry parameters,
examined prior to and at the end of 2´ months azaperone treatment, were
within the normal range (Reyntjens, 1972).
3. COMMENTS
A range of studies on azaperone was submitted for assessment
including data on kinetics and metabolism, acute toxicity,
short-term and long-term toxicity, developmental toxicity, and
genotoxicity. Most of the studies were carried out in the 1970s and
the standard of testing and reporting varied widely.
The kinetic studies with azaperone were insufficient to
determine the extent of absorption from the gastrointestinal tract.
However, by comparison with the excretion pattern after parenteral
dosing, it was estimated that absorption after oral dosing was
probably high. Distribution within the body in rats was extensive
and excretion was primarily in the faeces (81%), with lesser amounts
in urine (16%). Azaperone is extensively and rapidly metabolized.
Two metabolites were found in the pig but not in the rat. However,
these compounds are devoid of significant pharmacological activity,
and therefore do not affect the suitability of the rat as a model
for toxicological testing.
Azaperone was moderately toxic in acute toxicity studies in
mice, rats, guinea pigs, and dogs. Most signs of intoxication
reflected exaggerated pharmacological activity of azaperone in the
central nervous system. A battery of pharmacological studies
indicated that azaperone possesses potent anti-alpha-adrenergic
activity, but these data were inadequate for use in determining a
NOEL as the drug was almost always given by the subcutaneous route.
Short- and long-term toxicity studies were carried out in rats
and dogs. Dose-related sedation was the major effect in both species
and was observed at all treatment levels. Minor hepatotoxicity was
observed at doses at and above 30 mg/kg b.w./day in rats and 5 mg/kg
b.w./day in dogs. In rats only, brain weight was consistently
increased at 30 mg/kg b.w./day, but in the absence of any
pathological change this observation could not be explained.
There were pathological changes in the pituitary and sex
organs, particularly in rats; these were typical of neuroleptic
agents. It has been postulated that the primary effect is
pharmacological and is caused by the blocking of dopamine receptors
in the hypothalamus or pituitary, resulting in increased prolactin
and decreased gonadotrophin secretion. While this could account for
the observed slight stimulation of the pituitary and mammary glands
and the quiescence of the female reproductive tract, direct evidence
for this mechanism was lacking. The effects on the reproductive
organs were slight, in line with the relatively weak anti-dopamine
activity of azaperone. In dogs, such effects were observed only
after dosing for 24 months with 1.25 and 5 mg/kg b.w./day but not at
20 mg/kg b.w./day. In rats, effects were noted after 3, 6, and 12
months but not after 18 months, which suggests the possibility of
adaptation. When pharmacological effects were excluded, the NOELs
were 1.25 mg/kg b.w./day in dogs and 8 mg/kg b.w./day in rats.
The Committee noted that the carcinogenic potential of
azaperone had not been adequately investigated. The only study in
which lifetime exposure was approached was an 18 month study in
rats. However, the duration of dosing was too short and the small
group size (only ten rats of each sex) was inadequate to determine
treatment-related tumour incidences satisfactorily.
Frame shift mutations in Salmonella typhimurium strains were
seen for azaperone and three metabolites in a series of studies
carried out by one group of investigators. However, reversion rates
were only 2-3 times those in controls; there was no dose-response
relationship, and high doses in the presence of rat liver microsomes
were required. This weak response was not reproduced by a second
group of investigators using the same bacterial strains.
Genotoxicity was absent in the micronucleus and dominant lethal
tests in vivo, suggesting that azaperone has low potential for
genetic damage.
In a three-generation study in rats, survival of the pups was
reduced during lactation in one generation at the highest dose of
40 mg/kg b.w./day. There were no adverse effects on other
reproduction parameters. It was recognized, however, that an
unconventional methodology was used in this study in that males were
left untreated and females were dosed on gestation days 6-15 only.
The study was considered to be inadequate to enable the potential
for effects on reproduction and fertility to be fully assessed.
Embryotoxicity and teratogenicity were examined in mice, rats,
golden hamsters, and rabbits. Fetal abnormalities were not observed
in any species. Administration of azaperone during the gestation
period resulted in embryotoxicity in mice at or above 10 mg/kg
b.w./day and in rats at 40 mg/kg b.w./day. Maternal toxicity and
fetotoxicity, in the form of delayed ossification of metatarsals and
metacarpals in mice and golden hamsters and reduced fetal weight in
rats, were noted at 40 mg/kg b.w./day. In a perinatal and postnatal
study in rats, the survival of pups during the lactation period was
reduced at 40 mg/kg b.w./day.
Human psychotic patients treated with up to 2 mg of azaperone
three times a day (about 0.1 mg/kg b.w./day) showed no clinical
effects. At doses of 2.5 mg given three times daily (about
0.125 mg/kg b.w./day) and above there was dose-related sedation, and
at 20 mg three times daily (about 1 mg/kg b.w./day), dizziness.
Haematological and blood chemistry parameters were not affected at
any dose.
There were no effects apart from sedation at 1.25 mg/kg
b.w./day in a 24-month dog study and at 8 mg/kg b.w./day in an
18-month rat study. There was no NOEL for pharmacological activity
in the animals used in the toxicological studies. However, the study
in human subjects provided additional information. The NOEL for
sedation was 2 mg given three times a day. Since the human subjects
were given azaperone in divided doses and it is unclear whether the
doses were additive over the course of the day, the NOEL for
sedation in humans was taken to be about 0.03 mg/kg b.w.
4. EVALUATION
In view of the absence of adequate carcinogenicity and
reproduction studies and the weak mutagenicity findings in bacteria,
the Committee could not establish an ADI. The Committee was aware of
data on the tumorigenic potential of other butyrophenone neuroleptic
agents, but considered that the structural differences between them
were sufficient to preclude the use of this information to support a
temporary ADI for azaperone.
5. REFERENCES
DUVERGER-VAN BOGAERT, M., VANPARYS, PH., DE MEESTER, C. & MARSBOOM,
R. (1987) Mutagenicity evaluation of azaperone in the
Salmonella/microsome test. Drug Chem. Toxicol., 10: 329-338.
HEYKANTS, J. (1973) The excretion and metabolism of azaperone in the
Wistar rat after oral and subcutaneous administration. Unpublished
Research Report No. V1266 from Janssen. Submitted to WHO by Janssen.
HEYKANTS, J. (1974) The transplacental passage of azaperone and its
metabolites in the rat. Unpublished Research Report No. V1552 from
Janssen. Submitted to WHO by Janssen.
HEYKANTS, J., LEWI, P. & JANSSEN, P.A.J. (1971a) On the distribution
and metabolism of azaperone (R1929) in the rat and pig. Part II.
Arzneim.-Forsch., 21: 1263-1269.
HEYKANTS, J., PARDOEL, L. & JANSSEN, P.A.J (1971b) On the
distribution and metabolism of azaperone (R1929) in the rat and pig.
Part I. Arzneim.-Forsch., 29: 982-984.
MARSBOOM, R. (1967) Potential of R1929 for embryotoxicity and
teratogenic effects in rats receiving R1929 subcutaneously.
Unpublished Research Report No. V967 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R. (1972a) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rats. Unpublished Research Report No. V1250
from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1972b) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rabbits. Unpublished Research Report No.
V1249 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973a) Potential of subcutaneous R1929 for
embryotoxicity and teratogenic effects in rats. Unpublished Research
Report No. V1248 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973b) Effects of R1929 in rats after oral
administration during the peri- and post-natal period. Unpublished
Research Report No. V1225 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974a) Potential of oral R1929 for embryotoxicity and
teratogenicity in rats: 3-generation study. Unpublished Research
Report No. V1707 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974b) Dominant lethal test. Unpublished Research
Report No. V1614 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & PARDOEL, L. (1973)
Untitled. Unpublished Research Report No. V1323 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1967)
Subcutaneous safety evaluation of R1929 in rats (13 weeks).
Unpublished Research Report No. V974 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1969)
Untitled. Unpublished Research Report No. V968 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976a)
Oral toxicity study in Wistar rats. Unpublished Research Report No.
V2490 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976b)
Oral toxicity study in Beagle dogs. Unpublished Research Report No.
N2491 from Janssen. Submitted to WHO by Janssen.
MEULDERMANS, W., HEYKANTS, J. & LAUWERS, W. (1973) An in vitro
study of the metabolism of azaperone (Stresnil R). Unpublished
Research Report No. V1505A from Janssen. Submitted to WHO by
Janssen.
MEULDERMANS, W.E.G., LAUWERS, W.F.J., KNAEPS, A.G., MICHIELS, L.J.M.
& HEYKANTS, J.J.P. (1975) In vitro metabolism of azaperone by rats
and pig liver fractions. Unpublished Research Report No. V1983 from
Janssen. Submitted to WHO by Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1973) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in
CRL:COBS-CD-1(ICR)BR outbred albino mice. Unpublished Research
Report No. 402 from McNeil Laboratories. Submitted to WHO by
Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1974) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in golden
hamsters. Unpublished Research Report No. 422 from McNeil
Laboratories. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E. (1975) The acute intravenous toxicity of R1929,
R2138 and R34189 in mice. Unpublished Research Report No. V2156 from
Janssen. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E., VAN NUETEN, J.M. & JANSSEN, P.A.J. (1974)
Azaperone, a sedative neuroleptic of the butyrophenone series with
pronounced anti-aggressive and anti-shock activity in animals.
Arzneim.-Forsch., 24: 1798-1806.
PEKKANEN, T.J. & SALMINEN, K. (1973) Azaperone and the hepatic
microsomes: effects on cytochrome P-450 concentration and on
NADPH-cytochrome c-reductase activity. Acta. Pharmacol. Toxicol.,
32: 285-288.
PONCELOT, F., DE MEESTER, C. & CRUTZEN-FAYT, C. (1982) In vitro
mutagenicity of R1929 Lot C2001. Unpublished Research Report No.
V4549 from Laboratoire de Toxicologie et Bromatologie, Brussels.
Submitted to WHO by Janssen.
PREISS, A.M., SCHEUTWINKEL-REICH, M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation, with the Salmonella/microsome
test, of psychopharmaceuticals used in meat production. Mutation
Res., 104: 333-337.
PREISS, A., SCHEUTWINKEL-REICH, M. & STAN, H-J. (1983) Mutagenitat
einiger in der Tiermast verwendeter Tranquilizer im
Salmonella/Mikrosomen-Test. Fleischwirtsch., 63: 243-244.
RAUWS, A.G., OLLING, M., FREUDENTHAL, J. & TEN HAM, M. (1976)
Azaperol, a new metabolite of the veterinary butyrophenone
tranquilizer azaperone. Toxicol. appl. Pharmacol., 35: 333-339.
REYNTJENS, A. (1972) Safety evaluation of azaperone treatment.
Unpublished Research Report No. V1164 from Janssen. Submitted to WHO
by Janssen.
SCHEUTWINKEL-REICH, M., PREISS, A.M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation of the butyrophenone tranquilizer
azaperone and its main metabolites with the Salmonella/microsome
test. Mutation Res., 104: 339-344.
VANPARYS, P.H. & MARSBOOM, R. (1982) Micronucleus test in rats.
Unpublished Research Report No. V4377 from Janssen. Submitted to WHO
by Janssen.
2.2 Toxicological studies
2.2.1 Acute studies
Table 1. Results of acute studies with azaperone
Species Sex Route LD50 Reference
(mg/kg b.w.)
Mouse M oral 385 Niemegeers et
M s.c. 179 al., (1974)
M i.v. 38-42
Rat M oral 245
M s.c. 450
M i.v. 28
Guinea M oral 202
pig
Dogs N.S. oral > 20
N.S. s.c. > 20
The major toxic signs in rodents were ptosis, sedation, tremors
and occasionally clonic seizures. Ptosis and sedation were observed
in dogs with vomiting after oral dosing.
In mice, the i.v. LD50 values for metabolites 2 and 8 were 56
and 150 mg/kg respectively, higher than for azaperone (Niemegeers,
1975).
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of 10 male and 10 female Wistar rats were given
subcutaneous injections of 0, 2.5, 10, and 40 mg/kg b.w./day
azaperone for 13 weeks. The vehicle used was 0.9% NaCl while the
purity of azaperone was not stated.
Treated rats in all groups were sedated for 2 hours following
dosing and at 40 mg/kg b.w./day passive behaviour was exhibited
throughout the study. There were no deaths due to treatment. In
males only, body weight gain was non-significantly reduced at 2.5
and 10 mg/kg b.w./day and markedly reduced at 40 mg/kg b.w./day.
Haematology, blood chemistry and urinalysis were examined at
the end of the study. The only apparent effects were a shift in the
differential white cell count from lymphocytes to neutrophils in
males at 10 mg/kg b.w./day and males and females at 40 mg/kg
b.w./day and a slight increase in serum alkaline phosphatase in
males at 10 and 40 mg/kg b.w./day.
At necropsy, spleens "looked degenerated" at 40 mg/kg b.w./day.
Thymus weights were decreased in males at 10 mg/kg b.w./day and
males and females at 40 mg/kg b.w./day. In females, liver weights
were increased and ovaries showed reduced numbers of corpora lutea
and increased glandular tissue at 40 mg/kg b.w./day (Marsboom et
al., 1967).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity 98-102%) for
15 weeks. There were no signs of toxicity during the study. Food
consumption and body weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood chemistry and urinalysis were
examined at the end of the study. The only notable findings were
decreased serum cholesterol in males and females, increased
urobilinogen in males and increased urinary creatinine in females,
all at 1600 ppm.
Gross pathology was unremarkable. Brain weights were heavier at
1600 ppm. Slight bile duct proliferation was seen in livers of 400
and 1600 ppm males. In females, ovaries showed "active large corpora
lutea" and there were "reduced eosinophils" in the uterine wall,
"mucified aspect" of vaginal mucosa, more developed alveolar tissue
in the mammary gland and "stimulation of erythrosinophils" in the
pituitary. The effects in females were expressed at 1600 ppm and to
a lesser extent at 400 ppm. The NOEL was 100 ppm, equal to 10 mg/kg
b.w./day (Marsboom et al., 1969).
Groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 6 and 12 months. Based on food intake the average doses were 8,
31, and 130 mg/kg b.w./day for 6 months and 8, 30, and 127 mg/kg
b.w./day for 12 months.
Dose-related sedation was observed in all drug-treated groups
during the entire experimental period. Survival was unaffected. Food
consumption and body weight gains were depressed at 1600 ppm while
in the 400 ppm group weight gain was affected in the 6-month study
only.
Ophthalmoscopy, haematology, blood chemistry and urinalysis
were examined at the end of 6 and 12 months. Serum cholesterol was
decreased in the 1600 ppm group at 6 months but not at later times.
Serum bilirubin, BUN and urinary urobilinogen were higher in females
of the 1600 ppm group at 6 and 12 months.
At autopsy, gross pathology was unaffected. Brain weight was
increased at 1600 ppm after both 6 and 12 months of dosing. Septal
cell proliferation in the lung was marked at 6 and 12 months in the
1600 ppm group and led to lipoid pneumonia.
The females of the 1600 ppm group exhibited a "prolonged
diestrus in the uterus" (atrophic at 12 months) accompanied by
"reduced activity in the ovaries" (low numbers of active corpora
lutea and abundant interstitial glandular tissue), "mucification and
thin layered epithelium" with no cornification in the vagina and
"more extensive chromophobe tissue" in the pituitary. These findings
in reproductive tissues were more marked at 12 months. Apart from
the pharmacological effect of sedation, the NOEL was 8 mg/kg
b.w./day (Marsboom et al., 1976a).
2.2.2.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, or 20 mg/kg b.w./day of azaperone (purity 99.7%) for 13 weeks.
Dosing was carried out during 6 days per week in capsules.
Dogs of the 20 mg/kg b.w./day group exhibited a sedative effect
for 3 to 4 hours post-dosing in addition to decreased general
activity, ptosis and catatonia. Emesis and salivation were seen
occasionally at 5 mg/kg b.w./day and frequently at 20 mg/kg
b.w./day. Physical examinations revealed one female in each drug
treatment group with transient swelling of mammary glands.
Body weight was not clearly affected by dosing. Ophthalmoscopy,
ECG, blood pressure, haematology, blood biochemistry and urinalysis
were examined pre-treatment and monthly during the study. None of
these parameters was altered.
At terminal necropsy, liver weights tended to increased values
at 5 and 20 mg/kg b.w./day, but a dose-relationship was not evident.
Gross and histopathology were unaffected. The NOEL was 1.25 mg/kg
b.w./day (Marsboom et al., 1973).
2.2.3 Long term/carcinogenicity studies
2.2.3.1 Rats
Concomitant with the 6 and 12 month rat studies (see Section
2.2.2.1), groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 100, 400, or 1600 ppm azaperone (purity not stated)
for 18 months. Based on food intake the average doses were 7, 29,
and 115 mg/kg b.w./day for 18 months.
As in the shorter-term studies, sedation was noted at all doses
while food intake and weight gain were depressed at 1600 ppm.
Ophthalmoscopy, haematology, blood biochemistry and urinalysis were
examined at the end of the study. Serum bilirubin and BUN and
urinary urobilinogen were increased in females of the 1600 ppm
group.
At necropsy, brain weight was increased in 1600 ppm rats. Gross
pathology was unremarkable. Septal cell proliferation in the lung
leading to lipoid pneumonia was marked at 1600 ppm. Effects seen in
the pituitary, ovary, uterus, and vagina at 6 and 12 months were not
evident in this 18-month experiment. Tumours were not increased.
Pharmacological effects were noted at all doses, the NOEL for
toxicological effects was 29 mg/kg b.w./day (Marsboom et al.,
1976a).
2.2.3.2 Dogs
Groups of 3 male and 3 female beagle dogs were given 0, 1.25,
5, and 20 mg/kg b.w./day of azaperone (purity not stated) for 24
months. Animals were dosed 6 days/week in capsules.
One male dog given 20 mg/kg b.w./day azaperone died in the 64th
week. Signs of intoxication included sedation, back arching,
protrusion of the tongue, head shaking, muscle tremors, apnea,
lacrimation, increased salivation, and emesis. These effects were
seen in most dogs at 20 mg/kg b.w./day and some dogs at 5 mg/kg
b.w./day with sporadic emesis and salivation at 1.25 mg/kg b.w./day.
Body weight gain was unaffected.
Ophthalmoscopy, ECG, BP, haematology, blood biochemistry and
urinalysis were examined pre-dosing and every 3 months during the
study. None of these parameters was altered.
At autopsy, there was increased bile on the duodenal mucosa in
the 5 and 20 mg/kg b.w./day groups. The weights of adrenal glands
and the liver were increased at 20 mg/kg b.w./day. The following
microscopic changes were noted mainly in females of the 1.25 and
5 mg/kg b.w./day groups with virtually no effects at 20 mg/kg
b.w./day: "more marked or protracted metoestral period" -- active
corpora lutea in 2 low-dose (LD) and 1 mid-dose (MD), "fatty
superficial epithelium" in uterus of 2 LD and 2 MD, "more resting
aspect" of genital tract with "thin layered vaginal epithelium" in
all LD and 2 MD, atrophy of uterine wall in 1 high-dose (HD),
stimulation of mammary gland in LD and MD and "stimulation of
erythrosinophilic tissue" in the pituitary in 2 LD. A NOEL was not
identified (Marsboom et al., 1976b).
2.2.4 Reproduction studies
2.2.4.1 Rats
A 3-generation study was carried out in Wistar rats. The
initial generation (F0) was allowed to deliver and suckle pups.
The second generation (F1) was mated within treatment groups
avoiding brother-sister matings, and was allowed to deliver and
suckle pups. The third generation (F2) was mated as in the F1
but the females were killed on gestation day 22.
Azaperone (purity not stated) was administered in the diet at
concentrations of 0, 25, 100, and 400 ppm food. The dietary levels
resulted in doses of approximately 2.5, 10, and 40 mg/kg b.w./day.
Only adult females were treated, on gestation days 6 to 15 in each
generation. Group sizes were 20 F0 females, 29-33 F1 females,
and 40-52 F2 females.
There were no mortalities among treated dams in any generation
but other toxic signs were not recorded. Maternal weight gain and
pregnancy rate were similar between groups.
Litter size, pup weight and post-natal weight gain were
unaffected in F1 and F2 offspring. Pup survival was reduced at
40 mg/kg b.w./day during the F2 lactation period only.
Uterine examination of F2 dams revealed no effects on
implantation, resorption or fetal weight. In the 40 mg/kg b.w./day
group, there were 2 F3 fetuses without metacarpal bones of the
foreleg and 1 without metatarsal bones of the hind leg (unilateral).
The NOEL was 10 mg/kg b.w./day (Marsboom, 1974a).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 29 pregnant CRL:COBS-CD-1 mice were given gavage
doses of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone
(purity not stated). Treatment was on gestation days 6 to 15 and
females were killed on gestation day 18. The vehicle contained
tartaric acid, sodium bisulfite, methyl and propyl paraben.
A number of animals died in the vehicle and drug-treated
groups, death was attributed to dosing difficulties as a result of
conditioned aversion to the vehicle. Decreased activity, ptosis,
impaired righting reflex and catalepsy were seen 1 to 3 hours
post-dosing with 10 and 40 mg/kg b.w./day azaperone.
Maternal weight gain was reduced in the vehicle control group
and further reduced at 10 and 40 mg/kg b.w./day. This effect on body
weight may be a reflection of lower litter sizes which were due to
both reduced numbers of corpora lutea and slightly increased
resorptions. Implantation rate, fetal weight and survival were not
significantly affected.
The ratio of male to female fetuses was reduced in the vehicle
and 40 mg/kg b.w./day groups. Fetal examination revealed a slight
delay in ossification of tarsals and phalanges of fore and hind paws
at 40 mg/kg b.w./day. Gross and visceral abnormalities were not
induced. The NOEL was 2.5 mg/kg b.w./day (Mosher et al., 1973).
2.2.5.2 Rats
Groups of pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was on gestation days 6 to 15 and females
were killed on gestation day 22.
Maternal survival and weight gain were unaffected. The number
of implantations and live and dead fetuses was similar in all
groups. Fetal weight and gross, visceral, and skeletal examinations
were unremarkable. There was no effect at the highest dose of
40 mg/kg b.w./day (Marsboom, 1972a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 15 and
females were killed on gestation day 22.
There was no mortality among the dams but weight gain was lower
in the 10 and 40 mg/kg b.w./day groups. The number of implantations
was similar in all groups. Relative to the number of implantations,
the number of resorptions was slightly increased at 40 mg/kg
b.w./day, resulting in slightly smaller litter size. Fetal weight
was reduced at 40 mg/kg b.w./day and the only fetal abnormality was
scoliosis in one 40 mg/kg b.w./day fetus (Marsboom, 1973a).
Groups of 20 pregnant Wistar rats were given subcutaneous doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 1 to 21 and
females were killed on gestation day 22.
There was no maternal mortality. Weight gain in the dams was
reduced at 10 and 40 mg/kg b.w./day. The number of implantations was
lower at 10 and 40 mg/kg b.w./day and resorptions were increased at
40 mg/kg b.w./day. Fetal weight was depressed at 40 mg/kg b.w./day
but there was no increase in fetal abnormalities (Marsboom, 1967).
Groups of 25 pregnant Wistar rats were given gavage doses of 0,
2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated) in an
aqueous vehicle. Treatment was from gestation day 16 to post-partum
day 21 and dams were allowed to deliver naturally and nurse young
throughout lactation.
There were no maternal deaths and the duration of gestation and
parturition were unaffected. Body weight gain of dams in the 2.5 and
40 mg/kg b.w./day groups was reduced. Litter size, birth weight, and
post-natal weight gain were unremarkable. The survival of pups
through the lactation period was compromised at 40 mg/kg b.w./day.
There were no fetal abnormalities. The NOEL was 10 mg/kg b.w./day
(Marsboom, 1973b).
2.2.5.3 Golden hamsters
Groups of 26 pregnant golden hamsters were given gavage doses
of 0, vehicle, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity
not stated). Treatment was on gestation days 6 to 10 and females
were killed on gestation day 15. The vehicle contained tartaric
acid, sodium bisulfite, methyl and propyl paraben.
There was no maternal mortality. Toxic signs were ptosis from
2.5 mg/kg b.w./day, decreased motor activity from 10 mg/kg b.w./day,
catalepsy and impaired righting reflex at 40 mg/kg b.w./day during
the 2 hours following dosing.
Body weight gain in dams was depressed at 40 mg/kg b.w./day,
and was associated with lower fetal weight at this dose. The number
of implantations, resorptions and litter size was similar between
groups. Fetal examination revealed only a slight delay in
ossification of metatarsals at 40 mg/kg b.w./day. Discounting
pharmacological effects, the NOEL was 10 mg/kg b.w./day (Mosher et
al., 1974).
2.2.5.4 Rabbits
Groups of 15 pregnant NZ White rabbits were given gavage doses
of 0, 2.5, 10, or 40 mg/kg b.w./day of azaperone (purity not stated)
in an aqueous vehicle. Treatment was on gestation days 6 to 18 and
females were killed on gestation day 28.
There were no maternal deaths and body weight gain was
depressed at 10 and 40 mg/kg b.w./day. Resorptions or fetal death
were not induced but litter size was slightly lower in the 40 mg/kg
b.w./day group, reflecting lower numbers of implantations. There
were no fetal abnormalities related to treatment. The NOEL was
2.5 mg/kg b.w./day (Marsboom, 1972b).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on azaperone1
Test system Test object Concentration Results Reference
Ames test2 S.typhimurium
TA 1538 > 750 µg/plate positive3 Preiss et al.,
TA 1537 (+ S9 mix) 1982, 1983
TA 98
TA 1535 2500 µg/plate negative Scheutwinkel-
TA 100 (+ S9 mix) Reich, et al.,
1982
TA 1535 2500 µg/plate
TA 1537 (- S9 mix)
TA 98
TA 1538
TA 100
Ames test2 S.typhimurium
TA 1530 2000 µg/plate4 negative Poncelet et al.,
TA 1535 (+ & - S9 1982; Duvergervan
TA 1537 mix) Bogaert et
TA 1538 al., 1987
TA 989
TA 100
Micro Rats 20-160 mg/kg negative Vanparys &
nucleus test orally Marsboom, 1982
Dominant Mice 10-160 mg/kg negative Marsboom,
lethal test orally 1974b
1. Appropriate positive controls were used.
2. Both with and without rat liver S9 fraction.
3. There was no dose relationship.
4. Higher concentrations were bacteriotoxic.
Table 3. Results of Ames tests on azaperone metabolites1,2
(Scheutwinkel-Reich et al., 1982)
Compound Strain Concentration Results
alpha-(4-fluorophenyl)-4- TA 98 > 500 µg/plate (+ S9) positive2
(2-pyridinyl)-piperazine
butanol (Azaperol)
TA 1538 1500 µg/plate (+ S9) positive
TA 1535 2500 µg/plate negative
TA 1537 (+ S9)
TA 100
TA 98 2500 µg/plate negative
TA 1538 (- S9)
TA 1535
TA 1537
TA 100
4-(4-acetyl)-1-piperazinyl- TA 1538 5000 µg/plate positive
41-fluoro-butyrophenone (+ S9)
TA 98 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
TA 98 5000 µg/plate negative
TA 1538 (+ S9)
TA 1535
TA 1537
TA 100
ß-(p-fluorobenzoyl)- TA 98 2500 µg/plate positive2
propanoic acid (+ S9)
TA 1538 5000 µg/plate negative
TA 1535 (- S9)
TA 1537
TA 100
p-fluorobenzoyl TA 98 5000 µg/plate negative
acetic acid TA 1538 (+ and - S9)
TA 1535
TA 1537
TA 100
1. Appropriate positive controls were used throughout
2. Response not dose-related
2.2.7 Special studies on pharmacology
Table 4. Results of studies with azaperone in pharmacological tests ( Niemegeers
et al, 1974).
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
amphetamine antagonism 2.5 - -
apomorphine antagonism 0.34/9.15 - 0.98
norepineprine antagonism 0.33 - -
tryptamine antagonism 5.9 - -
jumping box test 0.7 - 5(3.95 OR)
W-test
- body weight 1.75 - -
- food intake 2.5 - -
- faecal output 4.0 - -
behavioural observations
- catalepsy 8.0 - -
- ptosis 1.5 - -
open field test
- ambulation 6.7 - -
- rearing 4.1 - -
- defecation 6.9 - -
traumatic shock test 0.021 - -
thermoregulation 3.27 - -
- 37 °C > 320 -
- 30 °C
Table 4. cont'd
ED50 value ED50 value ED50 value
(mg/kg SC) (mg/kg SC) (mg/kg SC)
Type of test Rat Mouse Dog
tail withdrawal test > 40 - -
inhibition of food intake > 10 - -
hot plate test - 7.0 -
inhibition of righting reflex - > 40 -
pentobarbital potentiation - 0.4 -
rotating rod test - 1.64 -
fighting test - 0.74 -
1. lowest effective dose, the NOEL was 0.01 mg/kg sc.
2.3 Observations in humans
A group of 20 male psychotics were studied; 10 remained on their
previous medication, 10 had their medication replaced with azaperone.
Doses were commenced at 0.5 mg three times daily (t.i.d.) increasing
to 20 mg t.i.d over a 17-day period. The maximum dose was then
administered for 2 months.
Clinical observation revealed no symptoms up to 2 mg t.i.d. of
azaperone. At higher doses (from 2.5 mg t.i.d.), sedation was observed
in a dose-related manner and at 20 mg t.i.d. patients started to
complain of dizziness. Haematology and blood chemistry parameters,
examined prior to and at the end of 2´ months azaperone treatment, were
within the normal range (Reyntjens, 1972).
3. COMMENTS
A range of studies on azaperone was submitted for assessment
including data on kinetics and metabolism, acute toxicity,
short-term and long-term toxicity, developmental toxicity, and
genotoxicity. Most of the studies were carried out in the 1970s and
the standard of testing and reporting varied widely.
The kinetic studies with azaperone were insufficient to
determine the extent of absorption from the gastrointestinal tract.
However, by comparison with the excretion pattern after parenteral
dosing, it was estimated that absorption after oral dosing was
probably high. Distribution within the body in rats was extensive
and excretion was primarily in the faeces (81%), with lesser amounts
in urine (16%). Azaperone is extensively and rapidly metabolized.
Two metabolites were found in the pig but not in the rat. However,
these compounds are devoid of significant pharmacological activity,
and therefore do not affect the suitability of the rat as a model
for toxicological testing.
Azaperone was moderately toxic in acute toxicity studies in
mice, rats, guinea pigs, and dogs. Most signs of intoxication
reflected exaggerated pharmacological activity of azaperone in the
central nervous system. A battery of pharmacological studies
indicated that azaperone possesses potent anti-alpha-adrenergic
activity, but these data were inadequate for use in determining a
NOEL as the drug was almost always given by the subcutaneous route.
Short- and long-term toxicity studies were carried out in rats
and dogs. Dose-related sedation was the major effect in both species
and was observed at all treatment levels. Minor hepatotoxicity was
observed at doses at and above 30 mg/kg b.w./day in rats and 5 mg/kg
b.w./day in dogs. In rats only, brain weight was consistently
increased at 30 mg/kg b.w./day, but in the absence of any
pathological change this observation could not be explained.
There were pathological changes in the pituitary and sex
organs, particularly in rats; these were typical of neuroleptic
agents. It has been postulated that the primary effect is
pharmacological and is caused by the blocking of dopamine receptors
in the hypothalamus or pituitary, resulting in increased prolactin
and decreased gonadotrophin secretion. While this could account for
the observed slight stimulation of the pituitary and mammary glands
and the quiescence of the female reproductive tract, direct evidence
for this mechanism was lacking. The effects on the reproductive
organs were slight, in line with the relatively weak anti-dopamine
activity of azaperone. In dogs, such effects were observed only
after dosing for 24 months with 1.25 and 5 mg/kg b.w./day but not at
20 mg/kg b.w./day. In rats, effects were noted after 3, 6, and 12
months but not after 18 months, which suggests the possibility of
adaptation. When pharmacological effects were excluded, the NOELs
were 1.25 mg/kg b.w./day in dogs and 8 mg/kg b.w./day in rats.
The Committee noted that the carcinogenic potential of
azaperone had not been adequately investigated. The only study in
which lifetime exposure was approached was an 18 month study in
rats. However, the duration of dosing was too short and the small
group size (only ten rats of each sex) was inadequate to determine
treatment-related tumour incidences satisfactorily.
Frame shift mutations in Salmonella typhimurium strains were
seen for azaperone and three metabolites in a series of studies
carried out by one group of investigators. However, reversion rates
were only 2-3 times those in controls; there was no dose-response
relationship, and high doses in the presence of rat liver microsomes
were required. This weak response was not reproduced by a second
group of investigators using the same bacterial strains.
Genotoxicity was absent in the micronucleus and dominant lethal
tests in vivo, suggesting that azaperone has low potential for
genetic damage.
In a three-generation study in rats, survival of the pups was
reduced during lactation in one generation at the highest dose of
40 mg/kg b.w./day. There were no adverse effects on other
reproduction parameters. It was recognized, however, that an
unconventional methodology was used in this study in that males were
left untreated and females were dosed on gestation days 6-15 only.
The study was considered to be inadequate to enable the potential
for effects on reproduction and fertility to be fully assessed.
Embryotoxicity and teratogenicity were examined in mice, rats,
golden hamsters, and rabbits. Fetal abnormalities were not observed
in any species. Administration of azaperone during the gestation
period resulted in embryotoxicity in mice at or above 10 mg/kg
b.w./day and in rats at 40 mg/kg b.w./day. Maternal toxicity and
fetotoxicity, in the form of delayed ossification of metatarsals and
metacarpals in mice and golden hamsters and reduced fetal weight in
rats, were noted at 40 mg/kg b.w./day. In a perinatal and postnatal
study in rats, the survival of pups during the lactation period was
reduced at 40 mg/kg b.w./day.
Human psychotic patients treated with up to 2 mg of azaperone
three times a day (about 0.1 mg/kg b.w./day) showed no clinical
effects. At doses of 2.5 mg given three times daily (about
0.125 mg/kg b.w./day) and above there was dose-related sedation, and
at 20 mg three times daily (about 1 mg/kg b.w./day), dizziness.
Haematological and blood chemistry parameters were not affected at
any dose.
There were no effects apart from sedation at 1.25 mg/kg
b.w./day in a 24-month dog study and at 8 mg/kg b.w./day in an
18-month rat study. There was no NOEL for pharmacological activity
in the animals used in the toxicological studies. However, the study
in human subjects provided additional information. The NOEL for
sedation was 2 mg given three times a day. Since the human subjects
were given azaperone in divided doses and it is unclear whether the
doses were additive over the course of the day, the NOEL for
sedation in humans was taken to be about 0.03 mg/kg b.w.
4. EVALUATION
In view of the absence of adequate carcinogenicity and
reproduction studies and the weak mutagenicity findings in bacteria,
the Committee could not establish an ADI. The Committee was aware of
data on the tumorigenic potential of other butyrophenone neuroleptic
agents, but considered that the structural differences between them
were sufficient to preclude the use of this information to support a
temporary ADI for azaperone.
5. REFERENCES
DUVERGER-VAN BOGAERT, M., VANPARYS, PH., DE MEESTER, C. & MARSBOOM,
R. (1987) Mutagenicity evaluation of azaperone in the
Salmonella/microsome test. Drug Chem. Toxicol., 10: 329-338.
HEYKANTS, J. (1973) The excretion and metabolism of azaperone in the
Wistar rat after oral and subcutaneous administration. Unpublished
Research Report No. V1266 from Janssen. Submitted to WHO by Janssen.
HEYKANTS, J. (1974) The transplacental passage of azaperone and its
metabolites in the rat. Unpublished Research Report No. V1552 from
Janssen. Submitted to WHO by Janssen.
HEYKANTS, J., LEWI, P. & JANSSEN, P.A.J. (1971a) On the distribution
and metabolism of azaperone (R1929) in the rat and pig. Part II.
Arzneim.-Forsch., 21: 1263-1269.
HEYKANTS, J., PARDOEL, L. & JANSSEN, P.A.J (1971b) On the
distribution and metabolism of azaperone (R1929) in the rat and pig.
Part I. Arzneim.-Forsch., 29: 982-984.
MARSBOOM, R. (1967) Potential of R1929 for embryotoxicity and
teratogenic effects in rats receiving R1929 subcutaneously.
Unpublished Research Report No. V967 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R. (1972a) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rats. Unpublished Research Report No. V1250
from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1972b) Potential of oral R1929 for embryotoxicity and
teratogenic effects in rabbits. Unpublished Research Report No.
V1249 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973a) Potential of subcutaneous R1929 for
embryotoxicity and teratogenic effects in rats. Unpublished Research
Report No. V1248 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1973b) Effects of R1929 in rats after oral
administration during the peri- and post-natal period. Unpublished
Research Report No. V1225 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974a) Potential of oral R1929 for embryotoxicity and
teratogenicity in rats: 3-generation study. Unpublished Research
Report No. V1707 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R. (1974b) Dominant lethal test. Unpublished Research
Report No. V1614 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & PARDOEL, L. (1973)
Untitled. Unpublished Research Report No. V1323 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1967)
Subcutaneous safety evaluation of R1929 in rats (13 weeks).
Unpublished Research Report No. V974 from Janssen. Submitted to WHO
by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1969)
Untitled. Unpublished Research Report No. V968 from Janssen.
Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976a)
Oral toxicity study in Wistar rats. Unpublished Research Report No.
V2490 from Janssen. Submitted to WHO by Janssen.
MARSBOOM, R., VANDESTEENE, R., HERIN, V. & VAN BELLE, H. (1976b)
Oral toxicity study in Beagle dogs. Unpublished Research Report No.
N2491 from Janssen. Submitted to WHO by Janssen.
MEULDERMANS, W., HEYKANTS, J. & LAUWERS, W. (1973) An in vitro
study of the metabolism of azaperone (Stresnil R). Unpublished
Research Report No. V1505A from Janssen. Submitted to WHO by
Janssen.
MEULDERMANS, W.E.G., LAUWERS, W.F.J., KNAEPS, A.G., MICHIELS, L.J.M.
& HEYKANTS, J.J.P. (1975) In vitro metabolism of azaperone by rats
and pig liver fractions. Unpublished Research Report No. V1983 from
Janssen. Submitted to WHO by Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1973) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in
CRL:COBS-CD-1(ICR)BR outbred albino mice. Unpublished Research
Report No. 402 from McNeil Laboratories. Submitted to WHO by
Janssen.
MOSHER, A.H., DANILOVITZ, M.S. & STEELMAN, R.L. (1974) Teratology
study (Segment II) of Stresnil (Azaperone, PM-JR-1929) in golden
hamsters. Unpublished Research Report No. 422 from McNeil
Laboratories. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E. (1975) The acute intravenous toxicity of R1929,
R2138 and R34189 in mice. Unpublished Research Report No. V2156 from
Janssen. Submitted to WHO by Janssen.
NIEMEGEERS, C.J.E., VAN NUETEN, J.M. & JANSSEN, P.A.J. (1974)
Azaperone, a sedative neuroleptic of the butyrophenone series with
pronounced anti-aggressive and anti-shock activity in animals.
Arzneim.-Forsch., 24: 1798-1806.
PEKKANEN, T.J. & SALMINEN, K. (1973) Azaperone and the hepatic
microsomes: effects on cytochrome P-450 concentration and on
NADPH-cytochrome c-reductase activity. Acta. Pharmacol. Toxicol.,
32: 285-288.
PONCELOT, F., DE MEESTER, C. & CRUTZEN-FAYT, C. (1982) In vitro
mutagenicity of R1929 Lot C2001. Unpublished Research Report No.
V4549 from Laboratoire de Toxicologie et Bromatologie, Brussels.
Submitted to WHO by Janssen.
PREISS, A.M., SCHEUTWINKEL-REICH, M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation, with the Salmonella/microsome
test, of psychopharmaceuticals used in meat production. Mutation
Res., 104: 333-337.
PREISS, A., SCHEUTWINKEL-REICH, M. & STAN, H-J. (1983) Mutagenitat
einiger in der Tiermast verwendeter Tranquilizer im
Salmonella/Mikrosomen-Test. Fleischwirtsch., 63: 243-244.
RAUWS, A.G., OLLING, M., FREUDENTHAL, J. & TEN HAM, M. (1976)
Azaperol, a new metabolite of the veterinary butyrophenone
tranquilizer azaperone. Toxicol. appl. Pharmacol., 35: 333-339.
REYNTJENS, A. (1972) Safety evaluation of azaperone treatment.
Unpublished Research Report No. V1164 from Janssen. Submitted to WHO
by Janssen.
SCHEUTWINKEL-REICH, M., PREISS, A.M., FULLE, I., GROHMANN, H.G. &
STAN, H-J. (1982) Investigation of the butyrophenone tranquilizer
azaperone and its main metabolites with the Salmonella/microsome
test. Mutation Res., 104: 339-344.
VANPARYS, P.H. & MARSBOOM, R. (1982) Micronucleus test in rats.
Unpublished Research Report No. V4377 from Janssen. Submitted to WHO
by Janssen.
