
TIABENDAZOLE (THIABENDAZOLE)
First draft prepared by
Dr G. Roberts
Environmental Health Branch
Department of Health, Housing and Community Services
Canberra, Australia
1. EXPLANATION
Tiabendazole (also known as thiabendazole) had not been
previously reviewed by the Committee, but was evaluated at the 1970
and 1977 Joint FAO/WHO Meetings on Pesticide Residues (FAO/WHO,
1971, 1978). At the 1977 Joint Meeting, an ADI of 0-0.3 mg/kg bw was
established. Tiabendazole is a benzimidazole compound used both as a
broad-spectrum anthelminthic in various animal species and for the
control of parasitic infestations in humans.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution, biotransformation and excretion
2.1.1.1 Mice
Pregnant Jcl:ICR mice were administered a single gavage dose of
1000 mg/kg bw of 14C-tiabendazole on gestation day 9. When an
olive oil vehicle was used, peak plasma radioactivity occurred
within 30 min and was 5-fold higher than the peak level with an
aqueous gum arabic vehicle, achieved 6 h after dosing. After 12 h,
plasma levels were similar with both vehicles and by 72 h they were
negligible. The time course for levels of radioactivity in the
conceptus was similar to that in plasma. Over a 3-day period,
excretion was 74 and 60% in urine and 23 and 18% in faeces for the
olive oil and aqueous gum arabic vehicles, respectively (Yoneyama
et al., 1984).
Pregnant Jcl:ICR mice received single gavage doses of 1300
mg/kg bw of 14C-tiabendazole on gestation day 11. Use of an olive
oil vehicle resulted in 7-fold higher peak blood levels than with
gum arabic. The initial rate of urinary excretion was higher with
the olive oil vehicle, otherwise elimination kinetics were similar
for both vehicles. Over 7 days, 60% was recovered in urine and 37%
in faeces with each vehicle.
Chromatographic analysis of urine revealed 12 to 15% as parent
drug, 22 to 24% as 5-hydroxy tiabendazole, 28 to 29% as 5-hydroxy
tiabendazole glucuronide and 30 to 31% as 5-hydroxy tiabendazole
sulfate. Additionally, a small amount of N-methyl tiabendazole was
identified (Tsuchiya et al., 1987).
Pregnant Crj:CD-1(ICR) mice were given a single oral dose of
1000 mg/kg bw of 14C-tiabendazole in olive oil on gestation day
10. HPLC analysis of 24 h urine revealed the major metabolites as
unchanged tiabendazole, 5-hydroxy tiabendazole and the glucuronide
and sulfate conjugates of the latter. An additional 2 minor
metabolites, eluting between tiabendazole and the 5-hydroxy
metabolite, were detected but the amounts were too low to enable
identification (Fujitani et al., 1991).
2.1.1.2 Rats
Following a dose of 100 mg/kg bw, tiabendazole labelled with
14C in the benzene ring was rapidly absorbed from the
gastrointestinal tract of rats, with a maximum blood concentration
two to three hours after treatment. Radio-activity gradually
disappeared from the blood, and approximately 92% of a dose of 25
mg/kg bw and 80% of a 100 mg/kg bw dose was excreted in the urine
and faeces within three days. Most of the drug and its metabolites
were excreted within the first 24 h. Of the metabolites, 50%
appeared as the glucuronide of 5-hydroxy tiabendazole and 40% as the
sulfate ester of the same aglycone. Traces of unchanged tiabendazole
and 5-hydroxy tiabendazole were also evident (FAO/WHO, 1971).
Male F344/DuCrj rats were given a single oral dose of 800 mg/kg
bw tiabendazole. In addition to the major urinary metabolites
described above, the same 2 minor degradation products as were found
in mouse urine were also detected. Using mass spectrometry, the new
metabolites were identified as 2-acetyl benzimidazole and
4-hydroxytiabendazole (Fujitani et al., 1991).
2.1.1.3 Dogs
Dogs given a single oral dose of 50 mg/kg bw of 14C-labelled
tiabendazole were found to have maximum plasma levels within two
hours. Excretion was essentially complete in eight days, with
approximately 35% of the dose appearing in the urine and 47%
appearing in the faeces (FAO/WHO, 1971).
2.1.1.4 Humans
Sixteen male subjects were administered tiabendazole at dosages
of 1 to 2 g per person in the form of tablets, wafers, capsules or
suspension. The lower dose was rapidly absorbed and peak plasma
concentrations were observed about one hour after treatment. Plasma
drug levels declined rapidly thereafter and reached essentially zero
values between 24 and 48 h. Tiabendazole and its metabolic products
were excreted rapidly in the urine (81-91%) and faeces (2-7%) within
48 h. Approximately half of the material in the urine was associated
with compounds identified as the glucuronide and sulfate esters of
5-hydroxy tiabendazole. In plasma, both unchanged tiabendazole and
free 5-hydroxy tiabendazole were also found. At the higher dosage
level, maximum drug levels in plasma appeared three hours following
dosing. These studies utilized 14C-labelled and unlabelled
tiabendazole (FAO/WHO, 1971; Robinson, 1965).
Based on the metabolism studies, the pathway for tiabendazole
degradation in mammals may be represented as in Figure 1.
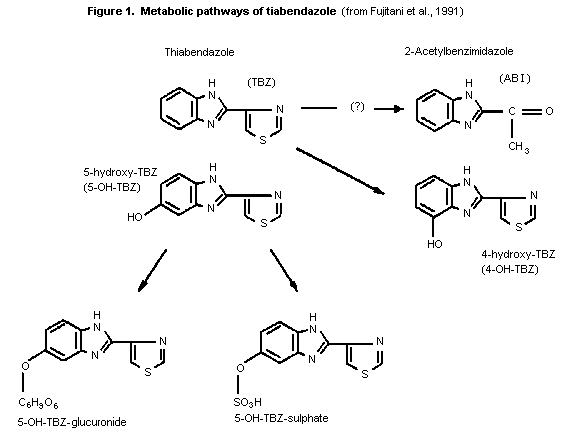 2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with tiabendazole are
summarized in Table 1.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 2400-3810 FAO/WHO, 1971
M&F ip 430 FAO/WHO,1971
M&F iv 150 FAO/WHO, 1971
Rat M&F oral 3330-3600 FAO/WHO, 1971
M&F ip 1850 FAO/WHO, 1971
M&F iv 130 FAO/WHO, 1971
M&F inhalation >397 mg/m3 Gurman et al., 1981
Rabbit M&F oral 3850 FAO/WHO, 1971
M&F dermal >2000 Blaszcak & Auletta,
1987
Sheep ? drench 2000 FAO/WHO, 1971
Goat ? drench >2000 FAO/WHO, 1971
Toxic signs following administration of large doses of
tiabendazole by oral or intraperitoneal routes were generally
similar and consisted of lethargy and stupor. The intravenous
administration of a large dose of tiabendazole hydrochloride
produced narcosis. Death appeared to be due to respiratory failure.
Rabbits that survived large oral doses initially lost weight but
recovered after several days.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In an investigation of renal function, Crj:CD-1 (ICR) mice were
given gavage doses of 0, 250 or 500 mg/kg bw/day tiabendazole
(purity 98.5%) in olive oil. Groups of 6 to 8 males and females were
killed after dosing for 1, 3, 5 or 7 days. There was no effect on
body weight. Water intake was unaffected but urine volume was
increased in all treated groups, significantly so in males given 500
mg/kg bw/day.
There were no meaningful effects on serum creatinine or urea
nitrogen, urinary glucose or protein or the presence of occult blood
in urine. Electrophoresis however, revealed relatively high
molecular weight protein in treated mice, while low molecular weight
protein was found in all groups. There was a progressive
dose-related increase in renal lesions including tubular dilatation
and degenerative desquamation, cell infiltration, fibrosis and
regeneration of tubular epithelium. Electron microscopy showed
evidence of glomerular damage such as flattening of the foot
processes of podocytes and oedematous changes in the mesangium in
treated mice (Tada et al., 1989).
In a 6-week pilot study, groups of 10 male and 10 female
Charles River CD-1 (HaM/ICR) mice were given 0, 50, 150, 300, 600 or
900 mg/kg bw/day tiabendazole (purity unknown) via the diet. There
were no clinical signs or mortality. Food intake and weight gain
were depressed in males at 600 and 900 mg/kg bw/day. Other
parameters were not monitored (Bokelman & Zwickey, 1977).
2.2.2.2 Rats
Rats (Sprague-Dawley Holtzman, 10 males and 10 females per
group) were administered tiabendazole by gavage at dosage levels of
0, 100, 400, 800, 1200 or 1600 mg/kg bw/day. During the 30 day
experimental period, rats in the 800 mg/kg bw/day group decreased
their food intake and gradually lost weight. They were slightly less
active, had ruffled fur and became slightly flaccid; three males and
three females died during the course of the experiment. All of the
rats in the 1200 and 1600 mg/kg bw/day groups died during the 30-day
test period. Rats dosed at 100 and 400 mg/kg bw/day appeared normal
throughout the test period, although a slight depression in
body-weight gain was noted in males at 100 mg/kg bw/day and in
females at 400 mg/kg bw/day. Higher doses produced greater effects
on body-weight gain. Haematologic studies on rats receiving 800
mg/kg bw/day showed a mild neutrophilia with concurrent lymphopenia.
There was also a suggestion of a decrease in red blood cell elements
at 400 mg/kg bw/day and above. Blood biochemistry showed no
meaningful changes.
No gross anatomical changes were noted in rats dosed with 100
mg/kg bw/day, whereas male and female rats treated with 400 mg/kg
bw/day showed thymic involution. A slight enlargement of the liver
was also noted in females at 400 mg/kg bw/day. Animals surviving 800
mg/kg bw/day showed gross signs indicating starvation; normal body
fat depots and subcutaneous fat appeared to be depleted. The liver
and adrenals were slightly enlarged in males and females and the
thymus was involuted. Microscopic pathology showed bone marrow
hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964).
Groups of 12 male and 12 female Albino Crj:CD (SD) rats were
administered gavage doses of 0, 50, 100, 200 or 800 mg/kg bw/day
thiabendazole (purity 100%), suspended in 0.5% methylcellulose, for
28 to 31 days. Severe toxicity was observed at 800 mg/kg bw/day in
the form of decreased activity, sedation, ataxia, recumbency,
flaccidity, loss of righting reflex, piloerection, emaciation, rough
hair coat, alopecia and a high level of mortality. Food intake and
body-weight gain were depressed at 200 and 800 mg/kg bw/day.
Laboratory analyses were carried out in week 4. Serum albumin,
total protein and cholesterol were increased and erythrocytes,
haemoglobin and haematocrit were decreased at 200 and 800 mg/kg
bw/day with reticulocytosis and leucocytopenia at the higher level.
Urinalysis was unchanged. Autopsy revealed changes at all dose
levels. There was haemosiderosis and increased extramedullary
haematopoiesis in the spleen at all doses with increased spleen
weight at 200 mg/kg bw/day and above. Increased thyroid weight and
follicular cell hyperplasia and decreased thymus weight were seen at
50 mg/kg bw/day and above. Lymphoid depletion was observed in the
thymus at 100 mg/kg bw/day and above, in bone marrow at 200 mg/kg
bw/day and above and in spleen and lymph nodes at 800 mg/kg bw/day.
Liver weight and centrilobular hepatocytic hypertrophy were
increased at 100 mg/kg bw/day and above (Usui & Sakaguchi, 1989).
Groups of 20 male and 20 female Sprague-Dawley Crl:CD (SD) BR
rats were given gavage doses of 0, 25, 100 or 400 mg/kg bw/day
tiabendazole (purity not stated), suspended in 0.5% methyl
cellulose, for 13 weeks. Alopecia was noted in some rats of each
treatment group. There was no mortality. Body-weight gain was
reduced at 100 and 400 mg/kg bw/day with lower food intake in 400
mg/kg bw/day males. Ophthalmology at the end of study was
unremarkable.
Clinical laboratory parameters were studied in weeks 6 and 13.
Decreased erythrocytes, haemoglobin and haematocrit and increased
cholesterol were induced at 100 and 400 mg/kg bw/day. Urinary levels
of bilirubin, urobilinogen and nitrite were increased at 400 mg/kg
bw/day. At 100 and 400 mg/kg bw/day, increased liver weight and
centrilobular hypertrophy, enlarged thyroids with follicular cell
hyperplasia and splenic congestion and pigmentation were observed.
In the kidney, renal calculus and transitional epithelial
hyperplasia were evident at 100 and 400 mg/kg bw/day with tubular
degeneration at the highest level. The stomach exhibited acanthosis
and/or degeneration of non-glandular mucosa at 100 and 400 mg/kg
bw/day and cytoplasmic rarefaction and necrosis of glandular mucosa
at 400 mg/kg bw/day. The NOEL was 25 mg/kg bw/day (Kangas et al.,
1989).
Groups of 10 male and 10 female Sprague-Dawley Crl:CD BR rats
were fed diets containing tiabendazole (purity 99.4%) for 13 weeks.
Based on food intake the calculated doses were 0, 9, 37, 150 or 302
mg/kg bw/day. There was no effect on survival or on the eyes at the
end of study. Alopecia was increased at 150 and 302 mg/kg bw/day.
Doses of 37 mg/kg bw/day and above were associated with decreased
body-weight gain and food intake in males; females were similarly
affected at 150 mg/kg bw/day and above.
Clinical laboratory analyses were undertaken in weeks 6 and 13.
At doses of 150 and 302 mg/kg bw/day serum cholesterol was
increased, glucose, erythrocytes, haemoglobin and haematocrit were
depressed and there were increased numbers of abnormal erythrocytes.
Urinalysis was unaffected. Liver weight increases and centrilobular
hypertrophy, increased thyroid weight and follicular cell
hypertrophy and bone marrow erythroid hyperplasia were observed at
37 mg/kg bw/day and above. At 150 and 302 mg/kg bw/day there was
spleen pigmentation and thymus atrophy with skeletal muscle atrophy
in females given 302 mg/kg bw/day. The NOEL was 9 mg/kg bw/day
(Myers & Lankas, 1990).
Rats (Sprague-Dawley Holtzman, 30 males and 30 females per
group) were administered tiabendazole, suspended in 1% methocel, by
gavage for 180 days, at doses of 0, 12.5, 25, 50, 100, 200 or 400
mg/kg bw/day. All of the animals survived throughout the duration of
the experiment. At 400 mg/kg bw/day, a depression in body weight was
observed in both sexes. At 200 mg/kg bw/day, the male rats showed a
slight depression in weight gain. No clinical signs of toxicity were
observed. Haematological studies at 400 mg/kg bw/day indicated a
slight suggestion of a fall of the red blood cell elements,
neutrophilia and lymphopenia which were not evident at levels below
this dose. Blood biochemistry studies and urinalysis were normal at
all dosage levels with a slight polyuria in both male and female
rats at 400 mg/kg bw/day, which was apparently the result of a
slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and
females at 400 mg/kg bw/day and in females at 200 mg/kg bw/day.
There was an increase in liver size at doses of 100 mg/kg bw/day and
above in males and at 50 mg/kg bw/day and above in females. Males
dosed with 200 and 400 mg/kg bw/day and females at 400 mg/kg bw/day
showed an apparent increase in kidney weight. Microscopic
examination of animals at 100 mg/kg bw/day showed a small incidence
of haemosiderosis of the thymus. At 200 and 400 mg/kg bw/day, these
examinations showed considerably more haemosiderosis of the thymus
and colloid depletion in the thyroid. The NOEL was 25 mg/kg bw/day
(FAO/WHO, 1971; Robinson, 1964).
2.2.2.3 Rabbits
Groups of 5 male and 5 female New Zeeland white rabbits
received dermal doses of tiabendazole (purity 98.9%) moistened in
0.9% saline. The drug was applied to the shaved trunk of animals at
doses of 0, 50, 200 or 1000 mg/kg bw, 6 h/day for 3 weeks. Oral
ingestion was prevented by use of a collar. Clinical laboratory
parameters and pathology were investigated at the end of the study.
There was no evidence of local or systemic toxicity (Cavagnaro &
Yamamoto, 1989).
2.2.2.4 Dogs
Groups of 4 male and 4 female beagle dogs were given capsules
of tiabendazole (purity 99.4%) at doses of 0, 35, 75 or 150 mg/kg
bw/day for 14 weeks. Emesis was increased at 75 and 150 mg/kg bw/day
with salivation at 150 mg/kg bw/day. There were no deaths. During
the first 2 weeks of treatment, food consumption and body-weight
gain were decreased in all treated groups. Subsequently, food was
made available overnight rather than for 1 h per day which led to
comparable food intake and weight gain in all groups.
Ophthalmology and ECG examinations were normal throughout the
study. Red blood cells, haemoglobin and haematocrit were depressed
at 150 mg/kg bw/day, more so at weeks 4 and 8 than at week 12. Blood
chemistry and urinalysis were unchanged at any time. Relative but
not absolute liver weights were slightly increased in all treated
groups but with no dose-relationship. At 75 and 150 mg/kg bw/day,
gallbladder cytoplasmic vacuolation was increased in incidence and
severity. The NOEL was 35 mg/kg bw/day (Batham et al., 1989).
Groups comprising two male and two female beagle dogs were
orally administered tiabendazole for periods of over two years at
doses of 0, 20, 100 or 200 mg/kg bw/day. No clinical signs or
morbidity were observed, food and water consumption were normal and
blood and urine chemistry were normal. A slight retardation in
body-weight gain was observed at 200 mg/kg bw/day, which was
accompanied by a slight reduction in total erythrocyte count. At
this level, changes in haemoglobin concentration and haematocrit
were also observed.
No gross pathological observations were noted at the conclusion
of this test and organ weights appeared normal. A significant
haemosiderosis was present in dogs at 100 and 200 mg/kg bw/day in
the spleen, liver, lymph nodes and bone marrow. This was not
associated with an increase in serum haemoglobin. The NOEL was 20
mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964)
Groups of beagle dogs (three males and three females) were
orally administered tiabendazole in capsules for two years at doses
of 0, 20, 50 or 125 mg/kg bw/day. At 125 mg/kg bw/day, two of six
dogs died. One of these dogs had marked liver cirrhosis, seminal
tubular degeneration, bone marrow atrophy and degenerative renal
changes. Slight to moderate reduction in haemoglobin and packed cell
volume occurred. Elevated blood urea nitrogen, serum alkaline
phosphatase and serum GOT and chronic inflammatory liver changes
were evident. Urinary albumin was seen more frequently than in
controls. Inspissated bile entwined in the gall bladder villi was
also observed in one dog. One dog of this group was sacrificed and
was found to have pulmonary arteritis of parasitic origin.
At 50 mg/kg bw/day, growth of male dogs was slightly depressed.
All six dogs given tiabendazole at 50 mg/kg bw/day survived the
study and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa
in one. Five of six dogs given tiabendazole at 20 mg/kg bw/day
survived the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes
in all treated dogs. The NOEL was 20 mg/kg bw/day (FAO/WHO, 1971;
Woodard, 1964).
2.2.2.5 Chickens
Groups of 0.5 week-old male white leghorn chicks (10 to 20 per
group) were fed tiabendazole in the diet at dosage levels ranging
from 1 to 10 000 ppm for a period of 2 1/2 weeks. This level
corresponded to a mean daily intake of tiabendazole ranging from 0.1
to 1200 mg/kg bw. A gradual decrease in growth appeared to occur
with concentrations of 100 ppm tiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg bw/day. Gross pathological
examination of the chicks at a dietary level of 4000 ppm
tiabendazole showed them to be normal except for a smaller size
(FAO/WHO, 1971).
2.2.2.6 Sheep
Thirty weanling wethers were employed in a 16-week study at
doses of 0, 10, 50, 100, 200, 400 or 800 mg/kg bw/day. Tiabendazole
was administered in gelatin capsules. Two of the four sheep dosed at
100 mg/kg bw/day did not survive the test period. However, because
of infection, tiabendazole may not have been directly related to the
cause of their death. All the animals treated at 200 mg/kg bw/day
and above died before the end of the test period. No significant
effects on blood biochemistry or urinalysis were observed. Doses of
50 mg/kg bw/day and above affected body-weight gain after
approximately 120 days on the test. Doses of 10 mg/kg bw/day had no
effect. Food consumption was unaffected at 10 mg/kg bw/day but a
minor decrease was noted at 50 and 100 mg/kg bw/day and a
significant decrease at 200 mg/kg bw/day and above. No gross
pathological changes were observed at doses below 200 mg/kg bw/day.
Starvation and loss of body weight complicated the interpretation of
data concerning organ weights at 200 mg/kg bw/day and above. At 200
to 800 mg/kg bw/day, a moderate hypoplasia of the bone marrow with
replacement by adipose tissue was observed. Loss of colloid in
thyroid was evident at 800 mg/kg bw/day, as well as lymphoid atrophy
at the higher dose levels (FAO/WHO, 1971).
Five groups of two ewe lambs (10 to 12 weeks old) and eight
wether lambs (10 to 19 weeks old) were fed diets containing 0, 100,
320, 1000 or 3200 ppm tiabendazole for periods up to 50 weeks. Sheep
10 weeks of age tolerated 1000 ppm tiabendazole in the diet for the
duration of the experiment. This dose corresponded to 30 to 50 mg/kg
bw/day. The sheep tolerated 3200 ppm in their diet for the first 14
weeks but thereafter lost weight in the final stages of the
experiment (FAO/WHO, 1971).
2.2.2.7 Pigs
Five groups of four barrows each were given a diet containing
0, 320, 1000, 3200 or 10 000 ppm tiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects
for the 14-week period. This concentration corresponded to an intake
of 15 mg/kg bw/day. The concentration of 10 000 ppm tiabendazole in
the diet caused reduced weight gain and reduced food consumption
with no mortality. Gross pathologogical examination revealed no
abnormal conditions (FAO/WHO, 1971).
2.2.2.8 Cattle
Five female holstein calves (seven months old) were fed a diet
containing 0, 320, 1000, 3200 or 10 000 ppm of tiabendazole for a
14-week test period. Calves tolerated 3200 ppm tiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg bw. At 10 000 ppm tiabendazole, the calves grew normally
for the first two weeks, but in the following 12 weeks the weight
gain was about half that observed for the controls. Gross
examination at the time of autopsy revealed no pathological
condition and histological examination of several tissues showed no
changes resulting from the incorporation of tiabendazole in the diet
(FAO/WHO,1971).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female Charles River CD-1 (HaM/ICR)
mice were fed diets containing tiabendazole (purity 99.3%) for up to
2 years. The study was commenced using dietary concentrations of 0
(three groups), 220, 660 or 2000 ppm in males and 0 (three groups),
660, 2000 or 5330 ppm in females. From the seventh week the
concentration of tiabendazole in the feed of the low-dose males and
females was reduced to 60 ppm in an effort to establish a NOEL. The
intake of tiabendazole was 5.6-8.3, 63-121 or 184-372 mg/kg bw/day
in males and 5.7-9.9, 209-368 or 534-1005 mg/kg bw/day in females.
Mortality was increased in groups given the intermediate and
high-doses. At 2000 ppm in males and females and 5330 ppm in
females, myocardial thrombosis was the primary cause of death. The
reason for decreased survival in 660 ppm males was not apparent.
When survival reached 20%, the remaining animals of the group were
sacrificed. Thus 5330 ppm females were killed in week 81, 2000 ppm
males were killed in week 85, 660 ppm males and 2000 ppm females
were killed in week 93, 60 ppm males (originally 220 ppm) were
killed in week 101 and 60 ppm females (originally 660 ppm) were
killed in week 105. A proportion of each control group was killed at
each of these sacrifice times.
Body-weight gain was depressed in 2000 ppm males and females
and 5330 ppm females. Males given 660 ppm had slightly lower weight
gain in the early and latter parts of the study. At the lowest dose,
slightly lower weight gain was noted early in the study, but
following dosage reduction, no further effect was observed. Food
consumption showed no meaningful alterations. Ophthalmoscopy
conducted at termination was unremarkable. Laboratory parameters
were not studied.
At autopsy liver weight was significantly increased in 2000 ppm
males and females and 5330 ppm females. Kidney weight was
significantly decreased in 5330 ppm females with slight decreases in
2000 ppm males and females and 660 ppm males. There were no
pathological lesions associated with the organ weight changes. A
high incidence of atrial thrombosis was seen in 2000 ppm males and
females and 5330 ppm females and was judged to be a major cause of
intercurrent mortality. Tumour incidences were unrelated to
treatment. The NOEL was 6 mg/kg bw/day (Bagdon et al., 1980).
2.2.3.2 Rats
Groups of Charles River rats (35 of each sex) were fed dietary
levels adjusted to provide 0, 10, 40 or 160 mg/kg bw/day of
tiabendazole for up to two years. At the 160 mg/kg bw/day level, a
reduction of weight gain of about 25%, with concomitant reduction in
food consumption and slightly reduced haemoglobin and haematocrit
values were the only changes attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidence or location of neoplasms and histopathological examination
of the tissues.
No effect on food consumption or haemograms were seen at either
10 or 40 mg/kg bw/day. Very slight depression in growth rate was
observed in male rats at 40 mg/kg bw/day but not at 10 mg/kg bw/day.
At 10 mg/kg bw/day, the mean absolute thyroid weights of the male
rats were heavier than the mean thyroid weight of the controls. This
effect was not observed in the rats given 40 or 160 mg/kg bw/day.
The NOEL was 10 mg/kg bw/day (FAO/WHO, 1971; Woodard, 1964).
In a carcinogenicity study, groups of 30 male and 30 female
F344/DuCrj rats were fed diets containing 0, 500, 1000, 2000 or 4000
ppm tiabendazole (purity 98.5%) for 2 years. Based on food
consumption the drug intake was calculated as 21, 43, 90 or 207
mg/kg bw/day in males and 26, 53, 112 or 237 mg/kg bw/day in
females.
There were no signs of toxicity or effects on survival. Food
intake was lower at 4000 ppm whereas body weight gain was reduced at
1000 ppm and above, leading to small and poorly developed animals at
2000 and 4000 ppm. Clinical laboratory parameters were not examined.
At the end of 2 years, all animals were necropsied. Liver
microgranulomas and aggregations of foamy cells in the lung were
increased at 1000 ppm and above. Renal epithelial hyperplasia of
papilla and pelvis were increased at 2000 and 4000 ppm. Preputial or
clitoral gland adenoma incidences were higher than control values at
4000 ppm. The increase was statistically significant (p <0.05) only
for adenomas in the preputial gland. There were no adverse effects
at 500 ppm, equal to a NOEL of 20 mg/kg bw/day (Fujii et al.,
1991).
2.2.4 Reproduction studies
2.2.4.1 Mice
A five-generation reproduction study utilized 25 male and 25
female mice given diets containing tiabendazole at concentrations of
0, 200, 1000 or 5000 ppm. When the mice attained the age of eight
weeks, they were mated and continued on the same diet. The young
from these matings, when they were weaned, were maintained on the
test diet and mated when they reached the age of eight weeks. This
procedure continued for five complete reproductive cycles. No
effects were noted at 200 ppm. At 1000 ppm, a slight decrease in the
weights of weanlings was observed in all five generations. At 5000
ppm, the number of mice born and weaned per litter was reduced and a
marked reduction in the average weanling weight of the young was
observed. The NOEL was 200 ppm, equivalent to 30 mg/kg bw/day
(FAO/WHO, 1971).
2.2.4.2 Rats
A three-generation, two-litter reproduction study (10 male and
10 female FDRL albino rats per group) at dosages of 0, 20, 40 or 80
mg/kg bw/day showed no adverse effects on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight and decreased
food consumption in the male rats at all dosage levels in the F0-
and F2-generations. Slight decreases in final body weights and
food consumption at the 80 mg/kg bw/day dose level in the F1- and
F2-generation females were also observed (FAO/WHO, 1971; Vogin,
1968)
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
A series of developmental studies were undertaken in Jcl:ICR
mice. Pregnant females were given gavage doses of tiabendazole
(purity 98.5%) in olive oil at various times during the gestation
period. Females were killed on gestation day 18.
Exp. 1: Groups of 39 mice were dosed with 0, 700, 1300 or 2400
mg/kg bw/day on gestation days 7-15. Maternal weight gain was
depressed in a dose-related manner with mortality at 1300 mg/kg
bw/day (5 out of 39) and 2400 mg/kg bw/day (24 out of 39). The
resorption rate was increased at 1300 and 2400 mg/kg bw/day. At all
treatment levels, there were reduced fetal weights and increased
malformations such as cleft palate, fusion of vertebral arches and
vertebral bodies.
Exp. 2: Groups of 7-12 mice were given a dose of 2400 mg/kg bw
on a single day of gestation between days 6 and 15. Mortality was 8
to 55% in treated groups but there were no data on body weight. In
each group, resorptions were increased and fetal body weights were
decreased. Fetal malformations detected were: microcephaly and
exencephaly (following treatment on gestation days 6 to 8); short or
absent tail and anal atresia (day 9); open eyelids (days 7, 8, 10,
13, and 14); reduction deformity of limbs and cleft palate (days 9
to 12); fusion of vertebral arches and bodies (days 7 to 10, and 13)
and fusion of ribs (days 7 to 9). The fetal abnormalities were
judged to be most prominent on gestation day 9. As the types of
malformation were diverse and primarily related to the timing of
treatment, it was suggested that tiabendazole was a non-specific
teratogen.
Exp. 3: Groups of 21 to 31 mice were given a single dose of 30
to 2400 mg/kg bw on gestation day 9. Mortality was induced at 1670
mg/kg bw and above and maternal weight gain was reduced at 1160
mg/kg bw and above. The resorption rate was increased at 1670 mg/kg
bw and above while fetal weight was lower at 60 mg/kg bw and above.
The limb reduction deformity was seen regularly at doses of 480
mg/kg bw and above; the threshold was calculated as being 360 mg/kg
bw. Fusion of vertebral arches, vertebral bodies and ribs was
increased at 240 mg/kg bw and above. Other malformations were not
consistently increased. There were no treatment-related effects at
30 mg/kg bw/day (Ogata et al., 1984).
Follow-up developmental studies were carried out in groups of
16 to 20 pregnant female Jcl:ICR mice. Tiabendazole (purity 98.5%)
was given by gavage in olive oil at doses of 0, 250, 500 or 1000
mg/kg bw/day on gestation day 9. Females were killed on gestation
day 18. Treatment at the two highest doses produced similar effects
to the previous mouse studies.
Pretreatment with SKF-525A (inhibitor of cytochrome P-450)
enhanced the rate of resorptions and the incidence of fetal
malformations. Sodium phenobarbital pretreatment, on the other hand,
resulted in decreases in the resorption rate and reduced fetal
abnormalities to control levels. Pretreatment with glutathione,
cysteine or diethyl maleate (a glutathione-depleting substance) had
no effect on resorptions. However, the incidence of fetal
malformations was increased with glutathione or cysteine and reduced
to control levels by diethyl maleate. Graphs of tissue
concentrations of tiabendazole against time showed the AUC for both
maternal blood and fetal tissue was increased by glutathione and
reduced by diethyl maleate. The overall conclusion was that the
developmental effects appear to be due to unchanged tiabendazole and
not to its degradation products (Ogata et al., 1987; 1989).
2.2.5.2 Rats
Two groups of rats (20 male and 20 female) were placed on a
diet containing 0 or 500 ppm tiabendazole. There were no
abnormalities among the young from either mating attributable to
tiabendazole at 500 ppm. It was concluded that these data presented
no evidence of teratogenecity (FAO/WHO, 1971).
In a preliminary teratology study, Charles River SD rats (8
pregnant females per group) were given gavage doses of 0 or 80 mg/kg
bw/day tiabendazole (purity unknown) on gestation days 8 to 15. Dams
were killed on gestation day 21. There were no effects on resorption
rate or on fetal survival, growth or development (Delatour et al.,
1974).
Groups of 20 pregnant female Wistar rats were administered
gavage doses of 0, 125, 250 or 500 mg/kg bw/day of a commercial
formulation containing 45% tiabendazole and 55% unknown ingredients.
Dosing was on gestation days 6-15 and dams were killed on gestation
day 22. No adverse effects were apparent in females and no
meaningful effects were reported on the resorption rate, fetal
viability, growth or development (Khera et al., 1979).
In a range-finding study, Sprague-Dawley Crl:CD(SD)BR rats were
given gavage doses of 0, 50, 100, 200 or 400 mg/kg bw/day
tiabendazole (purity 99.4%). Groups of 10 pregnant females were
treated on gestation days 6 to 17 and were killed on gestation day
20.
There were no deaths or abortions but all 200 and 400 mg/kg
bw/day females were killed due to severe body-weight effects.
Maternal weight gain was also depressed at 50 and 100 mg/kg bw/day.
Other toxic signs were lethargy and ptosis at 100 mg/kg bw/day and
above and reduced faeces at 200 mg/kg bw/day and above. Increased
protein, albumin and cholesterol were observed at the higher doses
with decreased triglycerides at all dose levels. Resorptions and
fetal morphology were unaffected but fetal weight was reduced at all
treatment levels (Lankas & Wise, 1990a).
Groups of 25 pregnant female Sprague-Dawley Crl:CD(SD)BR rats
were given gavage doses of 0, 10, 40 or 80 mg/kg bw/day tiabendazole
(purity 98.9%) suspended in 0.5% methyl cellulose. Drug
administration was on gestation days 6 to 17 and the dams were
killed on gestation day 20.
At 80 mg/kg bw/day, rats showed ptosis and a few animals
regurgitated a portion of the dose. Food intake and body-weight gain
were depressed at 40 and 80 mg/kg bw/day. There was no mortality or
abortions nor were there increases in the resorption rate or in dead
fetuses. Fetal abnormalities were not produced, but statistically
significantly decreased fetal weight was noted at 40 and 80 mg/kg
bw/day. The NOEL was 10 mg/kg bw/day (Lankas & Wise, 1990b).
2.2.5.3 Rabbits
Groups of 10 to 32 pregnant female New Zeeland white rabbits
were dosed by gavage with 0, 100, 200, 400 or 800 mg/kg bw/day
tiabendazole (purity unknown) suspended in 1% carboxymethyl
cellulose. Treatment was on gestation days 8 to 16 and does were
killed on gestation day 29 or 30.
At 200 mg/kg bw/day and above, does lost weight and the
resorption rate was increased (not dose-related). Fetal weight was
decreased at 400 and 800 mg/kg bw/day but fetal morphology was
unaltered. The NOEL was 100 mg/kg bw/day (Peck, 1966a).
In a range-finding study New Zeeland white rabbits were given
gavage doses of 0, 100, 200, 400 or 800 mg/kg bw/day tiabendazole
(purity 98.9%). Groups of 8 pregnant females were dosed on gestation
days 6 to 18 and does were killed on gestation day 29.
At 800 mg/kg bw/day, abnormal faeces were seen and weight loss
necessitated early sacrifice of this group. Food intake and weight
gain were lower at 200 and 400 mg/kg bw/day. The implantation rate
was lower at 400 mg/kg bw/day but resorptions were not increased.
External fetal examinations were unremarkable (Christian et al.,
1989).
Groups of 18 pregnant female New Zeeland white rabbits were
administered 0, 24, 120 or 600 mg/kg bw/day tiabendazole (purity
98.9%) suspended in 0.5% methyl cellulose. The gavage doses were
given on gestation days 6 to 18 and females were killed on gestation
day 29.
At 600 mg/kg bw/day one doe died, four aborted, abnormal faeces
were increased, food consumption was lower and body weight was lost.
Weight gain was slightly depressed at 120 mg/kg bw/day. There were
increased resorptions at 120 and 600 mg/kg bw/day, including total
resorption of conceptuses in 4 females at 120 mg/kg bw/day. Domed
head, hydrocephaly and enlarged fontanelles were seen in one fetus
at 120 mg/kg bw/day and two fetuses at 600 mg/kg bw/day. The NOEL
was 24 mg/kg bw/day (Hoberman & Lankas, 1989).
Groups of 18 pregnant female New Zeeland white rabbits were
given gavage doses of 0, 50, 150 or 600 mg/kg bw/day tiabendazole
(purity 98.6%) suspended in 0.5% methylcellulose. Treatment was on
gestation days 6 to 18 and dams were killed on gestation day 28.
There were no deaths or abortions related to treatment. Food
intake and body weight gain were reduced at 600 mg/kg bw/day during
the dosing period only. Resorptions were increased slightly but
significantly at 600 mg/kg bw/day. At 600 mg/kg bw/day, there were
increases in the incidences of variation in lung lobation and
incompletely ossified metacarpals. The NOEL was 150 mg/kg bw/day
(Lankas & Wise, 1991).
2.2.5.4 Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg bw
tiabendazole at 2.5 to 8 weeks prior to lambing without any effect
on the birth rate, growth or viability of the lambs. This dose had
no effect on the ewes throughout the test interval or on viability
of the lambs to six weeks of age. Miscellaneous tests using 39 ewes
produced 29 weanling lambs after treatment with tiabendazole at 400
mg/kg bw, whereas, from a control group of 22 ewes, 13 lambs were
weaned. Ewes tolerated a single dose of 18 g tiabendazole per animal
(equal to 235 to 335 mg/kg bw), three to ten days after lambing,
without any effect on the weight or food intake of the ewes or
survival and growth of the lambs up to the weaning time of six weeks
(FAO/WHO, 1971).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with tiabendazole are
summarized in Table 2.
Table 2. Results of genotoxicity studies on tiabendazole1
Test system Test object Treatment Results Reference
Reverse S.typhimurium 10-5000 negative MacGregor, 1976
mutation TA98, TA100, µg/plate
TA1535, TA1537 (+&-S9)2
Reverse S.typhimurium 100-2500 negative Shirasu et al.,
mutation TA1535, TA1537, µg/plate (-S9) 1976a.
TA1538, TA98, 10-1000
TA100, E.coli µg/plate (+S9)2
WP2 hcr-
Reverse S.typhimurium 10-2000 negative Peck, 1977
mutation TA98, TA100, µg/plate
TA1538 (+&-S9)2
TA100, TA1538 10-2000 negative
µg/plate (+S9)3
TA98 10-500 negative
µg/plate (+S9)3
1000-2000 positive
µg/plate (+S9)3
1000-2000 negative
µg/plate (+S9)3
pure tiabendazole
20-1000 positive
µg/plate (+S9)3
concentrated
impurities
Host ICR mice, 300-1000 negative Shirasu et al.,
mediated S.typhimurium mg/kg bw/day for 1976a
G46. 5 days, po
Rec. assay B.subtilis 2-1000 negative Shirasu et al.,
H17, M45 µg/disc (-S9) 1976a
DNA Rat 60-262 negative Lankas, 1989
damage hepatocytes µg/ml
Anaphase- CHO cells 0.06-0.12 negative Mudry de
telophase µg/ml (-S9) Pargament
test 0.24-0.6 positive et al., 1987
µg/ml (-S9)
Table 2. cont'd
Test system Test object Treatment Results Reference
Sister Mouse bone 50-200 negative Mudry de
chromatid marrow mg/kg bw, ip Pargament
exchange et al., 1987
Chromosome Human 2-50 negative Shirasu et al.,
aberration fibroblasts µg/ml (-S9) 1976c
Chromosome Rat bone 100-1000 negative Shirasu et al.,
aberration marrow mg/kg bw, po 1976d
30-300 negative
mg/kg bw/day
for 5 days, po
Micronucleus Mouse bone 125-500 negative Peck, 1977.
test marrow mg/kg bw, po
Micronucleus Mouse bone 50-200 positive Mudry de
test marrow mg/kg bw, ip Pargament
et al., 1987
Dominant Mice 200-600 negative Shirasu et al.,
lethal mg/kg bw/day 1976b,
test for 5 days, po
Dominant Mice 125-500 negative Peck, 1977.
lethal mg/kg bw/day
test for 5 days, po
1 Appropriate positive controls were used.
2 Liver microsomes obtained from Aroclor 1254 pretreated rats.
3 Liver microsomes obtained from phenobarbital pretreated rats.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Male albino guinea-pigs received 10 intracutaneous injections
of a 0.1% suspension of tiabendazole in 0.9% saline. Two weeks after
the induction period an intracutaneous challenge dose failed to
exacerbate reactions at the injection site (Peck, 1966b).
2.2.7.2 Rabbits
There was no evidence of a significant degree of irritation
when 0.5 g tiabendazole was applied in cold cream at concentrations
of 10 and 50% (w/w) for 24 h to intact rabbit skin (FAO/WHO, 1971).
No evidence of irritation was noted other than a very slight
erythema observed for approximately one hour after application of 1
ml of tiabendazole as a 12% suspension in sodium carboxymethyl
cellulose or 10 mg of the dry powder to the conjunctival sac of two
rabbits (FAO/WHO, 1971).
Male and female New Zeeland white rabbits received 100 mg of
tiabendazole powder instilled into the conjunctival sac of the eye.
At 15 min, there was slight conjunctival injection with a clear
discharge which returned to normal within 24 h (Merck, Sharp &
Dohme, undated).
2.2.8 Special studies on pharmacology
Oral doses of tiabendazole (4000 mg/kg bw) produced no striking
acute pharmacological effects on blood pressure or respiration in
either cats or dogs. Similarly, there were no alterations in the
electrocardiogram (FAO/WHO, 1971).
2.3 Observations in humans
A double blind study was carried out with 100 male volunteers,
half of them receiving 250 mg tiabendazole/day for 24 weeks and half
of them a placebo. Each subject was interviewed weekly and any
potential side effect was recorded. General physical examination and
laboratory examinations (haematology, cholesterol, glucose, urea,
alkaline phosphatase, thymol turbidity, bilirubin in serum and
urinalysis) were carried out before the test and after 4, 12 and 24
weeks. PBI in serum and ECGs were carried out only at the beginning
and after 24 weeks. Thirty-six subjects on tiabendazole and 41 on
placebo completed the study. As tiabendazole has a possible
influence on the thyroid the values for PBI were evaluated
carefully. No indication, however, was found of a decrease or
increase in PBI values due to tiabendazole. Under the conditions of
the study, tiabendazole was well tolerated at a dosage of 250 mg/day
during 24 weeks. No effect on any of the parameters studied could be
clearly ascribed to the drug. The NOEL was 3-4 mg/kg bw/day
(Colmore, 1965; FAO/WHO, 1978).
Twenty-three patients with trichinosis received 50 mg/kg bw/day
up to 3 g daily tiabendoazole for 10 days (total dose 25 to 30 g
tiabendazole), which was administered twice a day (after breakfast
and after evening meal). Symptoms of muscle pain and fever subsided
1 to 3 days after commencing tiabendazole. One woman withdrew due to
drug-induced rash, fever and proteinuria. Side-effects occurred in
14/23 treated patients - the most common effects being nausea,
retching and vomiting in 11/23 subjects, aversion to tablets (3/23),
exanthema (3/23), impotence (2/23), diarrhoea (1/23), liver damage
(1/23), fever (1/23) and dizziness (1/23) (Hennekeuser et al.,
1969).
3. COMMENTS
Information from a range of studies on thiabendazole was
available for assessment, including data on kinetics and metabolism,
acute toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity and observations in humans.
Toxicokinetic studies revealed rapid absorption after oral
dosing in mice, rats, dogs, and humans, peak plasma levels occurring
within 3 h of drug administration. The drug was excreted in the
urine and faeces. Elimination of tiabendazole and its degradation
products appeared more rapid in humans than in mice, rats, and dogs,
but metabolism was similar in mice, rats, and humans. The main
urinary metabolites were the glucuronide and sulfate conjugates of
5-hydroxytiabendazole; there were also small amounts of unconjugated
tiabendazole and the 5-hydroxy derivative. At very high doses, other
minor metabolites were detected in rat and mouse urine.
Single oral doses of tiabendazole were slightly toxic
(LD50 > 2000 mg/kg bw) in mice, rats, rabbits, sheep and goats.
Where deaths occurred, they appeared to be due to respiratory
failure.
Following repeated oral administration of tiabendiazole to
mice, rats, dogs, chickens, sheep, pigs, and cattle, the most common
finding was a reduction in food consumption and in body-weight gain.
The effects on weight gain appeared to be greater than could be
accounted for by the depressed food intake. Anaemia was seen in rats
and dogs, and to some extent may have been related to poor
nutrition. However, the presence of increased haemosiderosis in
lymphoid tissues and reticulocytosis in some studies was suggestive
of red cell destruction. The haematological changes occurred at dose
levels of 100 mg/kg bw/day and above for 3 months in rats and 150
mg/kg bw/day and above for 3 months in dogs.
The major target organs for toxicity were the hepatic system,
thyroid gland, and lymphoid organs. Liver hypertrophy was seen in a
3-month study in rats at doses of 37 mg/kg bw/day and above, and in
a 2-year study in dogs minor hepatobiliary changes were observed at
50 mg/kg bw/day and above. Thyroid follicular cell hyperplasia and
colloid depletion were noted in a 3-month study in rats at 37 mg/kg
bw/day and above and in a 16-week study in sheep at 800 mg/kg
bw/day. The thymus, spleen, lymph nodes, and/or bone marrow
exhibited lymphoid depletion or atrophy in a 28-day study in rats at
100 mg/kg bw/day and above and in a 16-week study in sheep at 800
mg/kg bw/day. Effects on blood cells, the liver, and lymphoid organs
are commonly observed with benzimidazoles.
Less common observations included both renal tubular
degeneration and hyperplasia in one 7-day study in mice at 250 mg/kg
bw/day and above and in one 3-month study in rats at 100 mg/kg
bw/day and above. Gastric changes, including mucosal degeneration,
cytoplasmic rarefaction, and/or necrosis, were produced in a 3-month
study in rats at 100 mg/kg bw/day and above. Atrial thrombosis was
present at high incidence in a two-year study in mice at doses of
200 mg/kg bw/day and above that resulted in the death of the
affected animals.
Tumour incidences were not increased in a two-year
carcinogenicity study in mice. The NOEL was 6 mg/kg bw/day, and
there was increased mortality at the next highest level, 60 mg/kg
bw/day.
The incidence of preputial or clitoral gland adenomas was
increased at doses above 200 mg/kg bw/day in one of two 2-year
studies in rats. Statistical significance was achieved only in
males; the animals also displayed poor development due to severe
effects on weight gain which suggests that the maximum tolerated
dose was exceeded. The relevance of these tumours to low-dose
exposure of humans to tiabendiazole is questionable. The NOEL was 10
mg/kg bw/day based on depressed weight gain at the next highest
level, 40 mg/kg bw/day.
Genotoxicity tests were generally negative. In one of three
Salmonella typhimurium reverse-mutation assays, positive results
were obtained in strain TA98 only. Further investigation showed that
an impurity in some batches was responsible for the mutagenic
activity. The manufacturer indicated that no positive results had
been obtained in tests on several hundred further batches. In one
laboratory, micronuclei were induced in mouse bone marrow and
abnormal anaphase-telophase figures were increased in cultured
Chinese hamster ovary cells. The effects were seen only at
relatively high levels and may be indicative of the tubulin-binding
activity characteristic of benzimidazoles (Annex 1, reference 97,
section 3.2.4). A range of other assays for mutation, DNA damage,
and cytogenetic activity were clearly negative.
A multigeneration reproduction study in rats showed no adverse
effects on reproduction parameters up to the highest dose tested, 80
mg/kg bw/day. In mice, litter size and survival during the lactation
period were reduced at 750 mg/kg bw/day, but reproduction was
unaffected at lower doses in a five-generation study. The NOEL was
30 mg/kg bw/day.
Developmental toxicity was examined in mice, rats, rabbits, and
sheep. In a series of studies in mice, embryotoxicity was observed
at 1300 mg/kg bw/day and above, a dose that also resulted in the
death of 5 of 39 females. Fetal malformations were produced at 240
mg/kg bw/day
and above, the main abnormalities being a reduction deformity of the
limbs, cleft palate, and fusion of vertebral arches and vertebral
bodies. Unchanged tiabendazole was implicated as the probable
embryotoxic and teratogenic agent.
The only finding in several rat developmental studies was a
reduction in fetal weight at 40 mg/kg bw/day and above. The NOEL was
10 mg/kg bw/day.
In rabbits, abortions occurred at 600 mg/kg bw/day in one study
on developmental toxicity, but not in the prior range-finding study
at higher doses or in a repeat experiment in which similar treatment
levels were used. Embryotoxicity and low incidences of domed head,
hydrocephaly, and enlarged fontanelles were seen at 120 mg/kg bw/day
and above in one study, but not in the repeat study at similar dose
levels. All effects in rabbits were seen only at maternally toxic
dose levels. The NOEL was 24 mg/kg bw/day.
No adverse reproductive effects were observed in pregnant ewes
given doses of 400 mg/kg bw/day.
In a 24-week study in humans, male volunteers received 250 mg
of tiabendazole/day; there were no treatment-related effects on a
range of physical and biochemical parameters. The NOEL was 3-4 mg/kg
bw/day. In another study, patients with trichinosis were given up to
3 g of tiabendazole daily for 10 days. Side-effects occurred in 14
of 23 patients, the most common being nausea, retching, and
vomiting.
4. EVALUATION
The Committee noted the availability of extensive data in
support of the safety evaluation of tiabendazole and the
manufacturer indicated that further studies had been completed or
were under way. At the 1977 joint FAO/WHO meeting on pesticide
residues, the study in human volunteers was used to establish an ADI
of 0-0.3 mg/kg bw. The Committee considered that the investigations
undertaken as part of this human study were limited in nature, and
that in view of the availability of a number of recent well
conducted animal studies, the NOEL should be derived from the animal
data.
The overall NOEL was 10 mg/kg bw/day based on reduced weight
gain in a 2-year dietary study in rats and decreased fetal weight in
a developmental study in rats. The Committee decided not to use the
NOEL of 6 mg/kg bw/day from the 2-year study in mice as the next
administered dose was considerably higher at 60 mg/kg bw/day.
Using a safety factor of 100, the Committee established an ADI
of 0-100 µg/kg bw for tiabendazole. The Committee noted that the ADI
provided a safety margin corresponding to a factor of over 1000 with
respect to the dose required for fetal malformations in mice.
The Committee asked to see the results from recently completed
and ongoing toxicological studies in order to update the database on
tiabendazole.
5. REFERENCES
BAGDON, W.J., BOKELMAN, D.L. & ZWICKEY, R.E. (1980). Lifetime
carcinogenic study in mice. Unpublished Report No. TT77-014-0 from
Merck, Sharp & Dohme Research Laboratories. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BATHAM, P., REID, S. & OSBORNE, B.E. (1989). A 14-week oral toxicity
study in the beagle dog. Unpublished Report No. 84021 (TT 89-9010)
from Bio-Research Laboratories, Canada. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BLASZCAK, D.L. & AULETTA, C.S. (1987). Acute dermal toxicity study
in rabbits. Unpublished Report No. 4004-86 from Bio/dynamics Inc.,
USA. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
BOKELMAN, D.L. & ZWICKEY, R.E. (1977). Tiabendazole, six-week pilot
study in mice. Unpublished Report No. TT77-004-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CAVAGNORO, J. & YAMAMOTO, K.S. (1989). 23 day dermal toxicity study
in rabbits. Unpublished Report No. HLA 284-161 (TT89-9011) from
Hazleton Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CHRISTIAN, M.S., LANKAS, G.R. & HOBERMAN, A.M. (1989). Oral
range-finding study in pregnant rabbits. Unpublished Report No.
013-029P (TT89-9006) from Argus Research Laboratories, USA.
Submitted to WHO by MSD Research Laboratory, Lauterbach, Germany.
COLMORE, J.P. (1965). Chronic toxicity study of tiabendazole in
volunteers. Unpublished report from University of Oklahoma Medical
Centre, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
DELATOUR, P., LORQUE, G., LAPRAS, M. & DESCHANEL, J.P. (1974).
Embryotoxic properties in the rat, and residues in cattle and sheep,
of three anthelmintics, derivatives of benzimidazole. Bull. Soc.
Sci. Vet. et Med., 76: 147-154.
FAO/WHO (1971). 1970 evaluations of some pesticide residues in food.
AGP: 1970/M/12/1; WHO/Food Add/71.42, Rome.
FAO/WHO (1978). Pesticide residues in food: 1977 evaluations. FAO
Plant Production and Protection Paper, 10 Sup. Rome.
FUJII, T., MIKURIYA, H. & SASAKI, M (1991). Chronic oral toxicity
and carcinogenicity study of tiabendazole in rats. Fd. Chem.
Toxicol., 29: 771-775.
FUJITANI, T., YONEYAMA, M., OGATA, A., UETA, T., MORI, K. &
ICHIKAWA, H. (1991). New metabolites of tiabendazole and the
metabolism of tiabendazole by mouse embryo in vivo and in vitro.
Fd. Chem. Toxicol., 29: 265-274.
GURMAN, J.L., HARDY, R.J., VOELKER, R.W. & DAVIDSON, J.L. (1981).
Acute inhalation toxicity study in rats. Unpublished Report No. HLA
284-129 from Hazleton Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
HENNEKEUSER, H.H., PABST, K., POEPLAU, W. & GEROK, W. (1969).
Tiabendazole for the treatment of trichinosis in humans. Texas Rep.
Biol. Med., 27 (Suppl. 2): 581-596.
HOBERMAN, A.M. & LANKAS, G.R. (1989). Oral developmental toxicity
study in rabbits. Unpublished Report No. 013-029 (TT89-9005) from
Argus Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
KANGAS, L., FARRELL, D. & OSBORNE, B.E. (1989). A 14-week oral
toxicity study in the albino rat. Unpublished Report No. 84114
(TT89-9014) from Bio-Research Laboratories, Canada. Submitted to WHO
by MSD Research Laboratory, Lauterbach, Germany.
KHERA, K.S., WHALEN, C., TRIVETT, G. & ANGERS, G. (1979).
Teratologic assessment of maleic hydrazide and daminozide, and
formulations of ethoxyquin, tiabendazole and naled in rats.
J. Environ. Sci. Health, B14: 563-577.
LANKAS, G.R. (1989). In vitro alkaline elution/rat hepatocyte
assay. Unpublished report nos. TT88-8850 and TT89-8312 from Merck,
Sharp & Dohme Research Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990a). Oral range-finding study in
pregnant rats. Unpublished Report No. TT90-713-1 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990b). Oral development toxicity study
in rats. Unpublished Report No. TT90-713-0 from Merck, Sharp & Dohme
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1991). Oral developmental toxicity study
in rabbits. Unpublished Report No. TT90-734-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
MacGREGOR, J. (1976). Mutagenicity tests of tiabendazole in the Ames
Salmonella typhimurium reverse mutation test. Unpublished report
from Western Regional Research Centre, California, USA. Submitted to
WHO by MSD Research Laboratory, Lauterbach, Germany.
MERCK, SHARP & DOHME (undated). Primary eye irritation in rabbits.
Unpublished Report No. TT81-2693 from Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
MUDRY DE PARGAMENT, M.D., LABAL DE VINUESA, M. & LARRIPA, I. (1987).
Mutagenic bioassay of certain pharmacological drugs. I Tiabendazole
(TBZ). Mutation Res., 188: 1-6.
MYERS, B.A. & LANKAS, G.R. (1990). A 14-week dietary toxicity study
in rats. Unpublished Report No. HLA 284-169 (TT 90-9002) from
Hazleton Laboratories, USA and Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
OGATA, A., ANDO, H., KUBO, Y. & HIRAGA, K. (1984). Teratogenicity of
tiabendazole in ICR mice. Fd. Chem. Toxicol., 22: 509-520.
OGATA, A., YONEYAMA, M., SASAKI, M., SUZUKI, K. & IMAMICHI, T.
(1987). Effects of pretreatment with SKF-525A or sodium
phenobarbital on tiabendazole-induced teratogenicity in ICR mice.
Fd. Chem. Toxicol., 25: 119-124.
OGATA, A., FUJITANI, T., YONEYAMA, M., SASAKI, M. & SUZUKI, K.
(1989). Glutathione and cysteine enhance and diethylmaleate reduces
tiabendazole teratogenicity in mice. Fd. Chem. Toxicol., 27:
117-123.
PECK, H.M. (1966a). Reproduction study in the rabbit. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
PECK, H.M. (1966b). Cutaneous sensitisation in the guinea pig.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
PECK, H.M. (1977). Mutagenic evaluation. Unpublished report from
Merck, Sharp & Dohme. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
ROBINSON, H.J. (1964). Tiabendazole preclinical evaluation.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
ROBINSON, H.J. (1965). Tiabendazole safety evaluation. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
SHIRASU, Y., MORIYA, M. & KATO, K. (1976a). Mutagenicity testing on
tiabendazole (TBZ) in microbial systems. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TERAMOTO, S. & KANEDA, M. (1976b). Dominant lethal
studies with tiabendazole in mice. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976c).
Cytogenetic studies with tiabendazole in cultured human fibroblasts.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976d).
Cytogenetic studies with tiabendazole in rat bone marrow cells.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
TADA, Y., YONEYAMA, M., KABASHIMA, J., FUJITANI, T. & NAKANO, M.
(1989). Effects of tiabendazole on the kidneys of ICR mice.
Fd. Chem. Toxicol., 27: 307-315.
TSUCHIYA, T., TANAKA, A., FUKUOKA, M., SATO, M. & YAMAHA, T. (1987).
Metabolism of tiabendazole and teratogenic potential of its
metabolites in pregnant mice. Chem. Pharm. Bull., 35: 2985-2993.
USUI, T. & SAKAGUCHI, T. (1989). 5 week oral toxicity study in rats.
Unpublished report nos. TT86-9819 and TT87-9809 from Banyu
Pharmaceutical Development Research Laboratories, Japan. Submitted
to WHO by MSD Research Laboratory, Lauterbach, Germany.
VOGIN, E.E. (1968). Multigeneration reproduction and lactation
studies with tiabendazole. Unpublished report from Food and Drug
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
WOODARD, G. (1964). Safety evaluation by oral administration to dogs
and rats for 104 weeks. Unpublished report from Woodard Research
Corporation, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
YONEYAMA, M., OGATA, A., FUJII, T. & HIRAGA, K. (1984). The
maternal-foetal distribution of tiabendazole administered in two
different vehicles to pregnant mice. Fd. Chem. Toxicol., 22:
731-735.
2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with tiabendazole are
summarized in Table 1.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 2400-3810 FAO/WHO, 1971
M&F ip 430 FAO/WHO,1971
M&F iv 150 FAO/WHO, 1971
Rat M&F oral 3330-3600 FAO/WHO, 1971
M&F ip 1850 FAO/WHO, 1971
M&F iv 130 FAO/WHO, 1971
M&F inhalation >397 mg/m3 Gurman et al., 1981
Rabbit M&F oral 3850 FAO/WHO, 1971
M&F dermal >2000 Blaszcak & Auletta,
1987
Sheep ? drench 2000 FAO/WHO, 1971
Goat ? drench >2000 FAO/WHO, 1971
Toxic signs following administration of large doses of
tiabendazole by oral or intraperitoneal routes were generally
similar and consisted of lethargy and stupor. The intravenous
administration of a large dose of tiabendazole hydrochloride
produced narcosis. Death appeared to be due to respiratory failure.
Rabbits that survived large oral doses initially lost weight but
recovered after several days.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In an investigation of renal function, Crj:CD-1 (ICR) mice were
given gavage doses of 0, 250 or 500 mg/kg bw/day tiabendazole
(purity 98.5%) in olive oil. Groups of 6 to 8 males and females were
killed after dosing for 1, 3, 5 or 7 days. There was no effect on
body weight. Water intake was unaffected but urine volume was
increased in all treated groups, significantly so in males given 500
mg/kg bw/day.
There were no meaningful effects on serum creatinine or urea
nitrogen, urinary glucose or protein or the presence of occult blood
in urine. Electrophoresis however, revealed relatively high
molecular weight protein in treated mice, while low molecular weight
protein was found in all groups. There was a progressive
dose-related increase in renal lesions including tubular dilatation
and degenerative desquamation, cell infiltration, fibrosis and
regeneration of tubular epithelium. Electron microscopy showed
evidence of glomerular damage such as flattening of the foot
processes of podocytes and oedematous changes in the mesangium in
treated mice (Tada et al., 1989).
In a 6-week pilot study, groups of 10 male and 10 female
Charles River CD-1 (HaM/ICR) mice were given 0, 50, 150, 300, 600 or
900 mg/kg bw/day tiabendazole (purity unknown) via the diet. There
were no clinical signs or mortality. Food intake and weight gain
were depressed in males at 600 and 900 mg/kg bw/day. Other
parameters were not monitored (Bokelman & Zwickey, 1977).
2.2.2.2 Rats
Rats (Sprague-Dawley Holtzman, 10 males and 10 females per
group) were administered tiabendazole by gavage at dosage levels of
0, 100, 400, 800, 1200 or 1600 mg/kg bw/day. During the 30 day
experimental period, rats in the 800 mg/kg bw/day group decreased
their food intake and gradually lost weight. They were slightly less
active, had ruffled fur and became slightly flaccid; three males and
three females died during the course of the experiment. All of the
rats in the 1200 and 1600 mg/kg bw/day groups died during the 30-day
test period. Rats dosed at 100 and 400 mg/kg bw/day appeared normal
throughout the test period, although a slight depression in
body-weight gain was noted in males at 100 mg/kg bw/day and in
females at 400 mg/kg bw/day. Higher doses produced greater effects
on body-weight gain. Haematologic studies on rats receiving 800
mg/kg bw/day showed a mild neutrophilia with concurrent lymphopenia.
There was also a suggestion of a decrease in red blood cell elements
at 400 mg/kg bw/day and above. Blood biochemistry showed no
meaningful changes.
No gross anatomical changes were noted in rats dosed with 100
mg/kg bw/day, whereas male and female rats treated with 400 mg/kg
bw/day showed thymic involution. A slight enlargement of the liver
was also noted in females at 400 mg/kg bw/day. Animals surviving 800
mg/kg bw/day showed gross signs indicating starvation; normal body
fat depots and subcutaneous fat appeared to be depleted. The liver
and adrenals were slightly enlarged in males and females and the
thymus was involuted. Microscopic pathology showed bone marrow
hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964).
Groups of 12 male and 12 female Albino Crj:CD (SD) rats were
administered gavage doses of 0, 50, 100, 200 or 800 mg/kg bw/day
thiabendazole (purity 100%), suspended in 0.5% methylcellulose, for
28 to 31 days. Severe toxicity was observed at 800 mg/kg bw/day in
the form of decreased activity, sedation, ataxia, recumbency,
flaccidity, loss of righting reflex, piloerection, emaciation, rough
hair coat, alopecia and a high level of mortality. Food intake and
body-weight gain were depressed at 200 and 800 mg/kg bw/day.
Laboratory analyses were carried out in week 4. Serum albumin,
total protein and cholesterol were increased and erythrocytes,
haemoglobin and haematocrit were decreased at 200 and 800 mg/kg
bw/day with reticulocytosis and leucocytopenia at the higher level.
Urinalysis was unchanged. Autopsy revealed changes at all dose
levels. There was haemosiderosis and increased extramedullary
haematopoiesis in the spleen at all doses with increased spleen
weight at 200 mg/kg bw/day and above. Increased thyroid weight and
follicular cell hyperplasia and decreased thymus weight were seen at
50 mg/kg bw/day and above. Lymphoid depletion was observed in the
thymus at 100 mg/kg bw/day and above, in bone marrow at 200 mg/kg
bw/day and above and in spleen and lymph nodes at 800 mg/kg bw/day.
Liver weight and centrilobular hepatocytic hypertrophy were
increased at 100 mg/kg bw/day and above (Usui & Sakaguchi, 1989).
Groups of 20 male and 20 female Sprague-Dawley Crl:CD (SD) BR
rats were given gavage doses of 0, 25, 100 or 400 mg/kg bw/day
tiabendazole (purity not stated), suspended in 0.5% methyl
cellulose, for 13 weeks. Alopecia was noted in some rats of each
treatment group. There was no mortality. Body-weight gain was
reduced at 100 and 400 mg/kg bw/day with lower food intake in 400
mg/kg bw/day males. Ophthalmology at the end of study was
unremarkable.
Clinical laboratory parameters were studied in weeks 6 and 13.
Decreased erythrocytes, haemoglobin and haematocrit and increased
cholesterol were induced at 100 and 400 mg/kg bw/day. Urinary levels
of bilirubin, urobilinogen and nitrite were increased at 400 mg/kg
bw/day. At 100 and 400 mg/kg bw/day, increased liver weight and
centrilobular hypertrophy, enlarged thyroids with follicular cell
hyperplasia and splenic congestion and pigmentation were observed.
In the kidney, renal calculus and transitional epithelial
hyperplasia were evident at 100 and 400 mg/kg bw/day with tubular
degeneration at the highest level. The stomach exhibited acanthosis
and/or degeneration of non-glandular mucosa at 100 and 400 mg/kg
bw/day and cytoplasmic rarefaction and necrosis of glandular mucosa
at 400 mg/kg bw/day. The NOEL was 25 mg/kg bw/day (Kangas et al.,
1989).
Groups of 10 male and 10 female Sprague-Dawley Crl:CD BR rats
were fed diets containing tiabendazole (purity 99.4%) for 13 weeks.
Based on food intake the calculated doses were 0, 9, 37, 150 or 302
mg/kg bw/day. There was no effect on survival or on the eyes at the
end of study. Alopecia was increased at 150 and 302 mg/kg bw/day.
Doses of 37 mg/kg bw/day and above were associated with decreased
body-weight gain and food intake in males; females were similarly
affected at 150 mg/kg bw/day and above.
Clinical laboratory analyses were undertaken in weeks 6 and 13.
At doses of 150 and 302 mg/kg bw/day serum cholesterol was
increased, glucose, erythrocytes, haemoglobin and haematocrit were
depressed and there were increased numbers of abnormal erythrocytes.
Urinalysis was unaffected. Liver weight increases and centrilobular
hypertrophy, increased thyroid weight and follicular cell
hypertrophy and bone marrow erythroid hyperplasia were observed at
37 mg/kg bw/day and above. At 150 and 302 mg/kg bw/day there was
spleen pigmentation and thymus atrophy with skeletal muscle atrophy
in females given 302 mg/kg bw/day. The NOEL was 9 mg/kg bw/day
(Myers & Lankas, 1990).
Rats (Sprague-Dawley Holtzman, 30 males and 30 females per
group) were administered tiabendazole, suspended in 1% methocel, by
gavage for 180 days, at doses of 0, 12.5, 25, 50, 100, 200 or 400
mg/kg bw/day. All of the animals survived throughout the duration of
the experiment. At 400 mg/kg bw/day, a depression in body weight was
observed in both sexes. At 200 mg/kg bw/day, the male rats showed a
slight depression in weight gain. No clinical signs of toxicity were
observed. Haematological studies at 400 mg/kg bw/day indicated a
slight suggestion of a fall of the red blood cell elements,
neutrophilia and lymphopenia which were not evident at levels below
this dose. Blood biochemistry studies and urinalysis were normal at
all dosage levels with a slight polyuria in both male and female
rats at 400 mg/kg bw/day, which was apparently the result of a
slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and
females at 400 mg/kg bw/day and in females at 200 mg/kg bw/day.
There was an increase in liver size at doses of 100 mg/kg bw/day and
above in males and at 50 mg/kg bw/day and above in females. Males
dosed with 200 and 400 mg/kg bw/day and females at 400 mg/kg bw/day
showed an apparent increase in kidney weight. Microscopic
examination of animals at 100 mg/kg bw/day showed a small incidence
of haemosiderosis of the thymus. At 200 and 400 mg/kg bw/day, these
examinations showed considerably more haemosiderosis of the thymus
and colloid depletion in the thyroid. The NOEL was 25 mg/kg bw/day
(FAO/WHO, 1971; Robinson, 1964).
2.2.2.3 Rabbits
Groups of 5 male and 5 female New Zeeland white rabbits
received dermal doses of tiabendazole (purity 98.9%) moistened in
0.9% saline. The drug was applied to the shaved trunk of animals at
doses of 0, 50, 200 or 1000 mg/kg bw, 6 h/day for 3 weeks. Oral
ingestion was prevented by use of a collar. Clinical laboratory
parameters and pathology were investigated at the end of the study.
There was no evidence of local or systemic toxicity (Cavagnaro &
Yamamoto, 1989).
2.2.2.4 Dogs
Groups of 4 male and 4 female beagle dogs were given capsules
of tiabendazole (purity 99.4%) at doses of 0, 35, 75 or 150 mg/kg
bw/day for 14 weeks. Emesis was increased at 75 and 150 mg/kg bw/day
with salivation at 150 mg/kg bw/day. There were no deaths. During
the first 2 weeks of treatment, food consumption and body-weight
gain were decreased in all treated groups. Subsequently, food was
made available overnight rather than for 1 h per day which led to
comparable food intake and weight gain in all groups.
Ophthalmology and ECG examinations were normal throughout the
study. Red blood cells, haemoglobin and haematocrit were depressed
at 150 mg/kg bw/day, more so at weeks 4 and 8 than at week 12. Blood
chemistry and urinalysis were unchanged at any time. Relative but
not absolute liver weights were slightly increased in all treated
groups but with no dose-relationship. At 75 and 150 mg/kg bw/day,
gallbladder cytoplasmic vacuolation was increased in incidence and
severity. The NOEL was 35 mg/kg bw/day (Batham et al., 1989).
Groups comprising two male and two female beagle dogs were
orally administered tiabendazole for periods of over two years at
doses of 0, 20, 100 or 200 mg/kg bw/day. No clinical signs or
morbidity were observed, food and water consumption were normal and
blood and urine chemistry were normal. A slight retardation in
body-weight gain was observed at 200 mg/kg bw/day, which was
accompanied by a slight reduction in total erythrocyte count. At
this level, changes in haemoglobin concentration and haematocrit
were also observed.
No gross pathological observations were noted at the conclusion
of this test and organ weights appeared normal. A significant
haemosiderosis was present in dogs at 100 and 200 mg/kg bw/day in
the spleen, liver, lymph nodes and bone marrow. This was not
associated with an increase in serum haemoglobin. The NOEL was 20
mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964)
Groups of beagle dogs (three males and three females) were
orally administered tiabendazole in capsules for two years at doses
of 0, 20, 50 or 125 mg/kg bw/day. At 125 mg/kg bw/day, two of six
dogs died. One of these dogs had marked liver cirrhosis, seminal
tubular degeneration, bone marrow atrophy and degenerative renal
changes. Slight to moderate reduction in haemoglobin and packed cell
volume occurred. Elevated blood urea nitrogen, serum alkaline
phosphatase and serum GOT and chronic inflammatory liver changes
were evident. Urinary albumin was seen more frequently than in
controls. Inspissated bile entwined in the gall bladder villi was
also observed in one dog. One dog of this group was sacrificed and
was found to have pulmonary arteritis of parasitic origin.
At 50 mg/kg bw/day, growth of male dogs was slightly depressed.
All six dogs given tiabendazole at 50 mg/kg bw/day survived the
study and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa
in one. Five of six dogs given tiabendazole at 20 mg/kg bw/day
survived the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes
in all treated dogs. The NOEL was 20 mg/kg bw/day (FAO/WHO, 1971;
Woodard, 1964).
2.2.2.5 Chickens
Groups of 0.5 week-old male white leghorn chicks (10 to 20 per
group) were fed tiabendazole in the diet at dosage levels ranging
from 1 to 10 000 ppm for a period of 2 1/2 weeks. This level
corresponded to a mean daily intake of tiabendazole ranging from 0.1
to 1200 mg/kg bw. A gradual decrease in growth appeared to occur
with concentrations of 100 ppm tiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg bw/day. Gross pathological
examination of the chicks at a dietary level of 4000 ppm
tiabendazole showed them to be normal except for a smaller size
(FAO/WHO, 1971).
2.2.2.6 Sheep
Thirty weanling wethers were employed in a 16-week study at
doses of 0, 10, 50, 100, 200, 400 or 800 mg/kg bw/day. Tiabendazole
was administered in gelatin capsules. Two of the four sheep dosed at
100 mg/kg bw/day did not survive the test period. However, because
of infection, tiabendazole may not have been directly related to the
cause of their death. All the animals treated at 200 mg/kg bw/day
and above died before the end of the test period. No significant
effects on blood biochemistry or urinalysis were observed. Doses of
50 mg/kg bw/day and above affected body-weight gain after
approximately 120 days on the test. Doses of 10 mg/kg bw/day had no
effect. Food consumption was unaffected at 10 mg/kg bw/day but a
minor decrease was noted at 50 and 100 mg/kg bw/day and a
significant decrease at 200 mg/kg bw/day and above. No gross
pathological changes were observed at doses below 200 mg/kg bw/day.
Starvation and loss of body weight complicated the interpretation of
data concerning organ weights at 200 mg/kg bw/day and above. At 200
to 800 mg/kg bw/day, a moderate hypoplasia of the bone marrow with
replacement by adipose tissue was observed. Loss of colloid in
thyroid was evident at 800 mg/kg bw/day, as well as lymphoid atrophy
at the higher dose levels (FAO/WHO, 1971).
Five groups of two ewe lambs (10 to 12 weeks old) and eight
wether lambs (10 to 19 weeks old) were fed diets containing 0, 100,
320, 1000 or 3200 ppm tiabendazole for periods up to 50 weeks. Sheep
10 weeks of age tolerated 1000 ppm tiabendazole in the diet for the
duration of the experiment. This dose corresponded to 30 to 50 mg/kg
bw/day. The sheep tolerated 3200 ppm in their diet for the first 14
weeks but thereafter lost weight in the final stages of the
experiment (FAO/WHO, 1971).
2.2.2.7 Pigs
Five groups of four barrows each were given a diet containing
0, 320, 1000, 3200 or 10 000 ppm tiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects
for the 14-week period. This concentration corresponded to an intake
of 15 mg/kg bw/day. The concentration of 10 000 ppm tiabendazole in
the diet caused reduced weight gain and reduced food consumption
with no mortality. Gross pathologogical examination revealed no
abnormal conditions (FAO/WHO, 1971).
2.2.2.8 Cattle
Five female holstein calves (seven months old) were fed a diet
containing 0, 320, 1000, 3200 or 10 000 ppm of tiabendazole for a
14-week test period. Calves tolerated 3200 ppm tiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg bw. At 10 000 ppm tiabendazole, the calves grew normally
for the first two weeks, but in the following 12 weeks the weight
gain was about half that observed for the controls. Gross
examination at the time of autopsy revealed no pathological
condition and histological examination of several tissues showed no
changes resulting from the incorporation of tiabendazole in the diet
(FAO/WHO,1971).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female Charles River CD-1 (HaM/ICR)
mice were fed diets containing tiabendazole (purity 99.3%) for up to
2 years. The study was commenced using dietary concentrations of 0
(three groups), 220, 660 or 2000 ppm in males and 0 (three groups),
660, 2000 or 5330 ppm in females. From the seventh week the
concentration of tiabendazole in the feed of the low-dose males and
females was reduced to 60 ppm in an effort to establish a NOEL. The
intake of tiabendazole was 5.6-8.3, 63-121 or 184-372 mg/kg bw/day
in males and 5.7-9.9, 209-368 or 534-1005 mg/kg bw/day in females.
Mortality was increased in groups given the intermediate and
high-doses. At 2000 ppm in males and females and 5330 ppm in
females, myocardial thrombosis was the primary cause of death. The
reason for decreased survival in 660 ppm males was not apparent.
When survival reached 20%, the remaining animals of the group were
sacrificed. Thus 5330 ppm females were killed in week 81, 2000 ppm
males were killed in week 85, 660 ppm males and 2000 ppm females
were killed in week 93, 60 ppm males (originally 220 ppm) were
killed in week 101 and 60 ppm females (originally 660 ppm) were
killed in week 105. A proportion of each control group was killed at
each of these sacrifice times.
Body-weight gain was depressed in 2000 ppm males and females
and 5330 ppm females. Males given 660 ppm had slightly lower weight
gain in the early and latter parts of the study. At the lowest dose,
slightly lower weight gain was noted early in the study, but
following dosage reduction, no further effect was observed. Food
consumption showed no meaningful alterations. Ophthalmoscopy
conducted at termination was unremarkable. Laboratory parameters
were not studied.
At autopsy liver weight was significantly increased in 2000 ppm
males and females and 5330 ppm females. Kidney weight was
significantly decreased in 5330 ppm females with slight decreases in
2000 ppm males and females and 660 ppm males. There were no
pathological lesions associated with the organ weight changes. A
high incidence of atrial thrombosis was seen in 2000 ppm males and
females and 5330 ppm females and was judged to be a major cause of
intercurrent mortality. Tumour incidences were unrelated to
treatment. The NOEL was 6 mg/kg bw/day (Bagdon et al., 1980).
2.2.3.2 Rats
Groups of Charles River rats (35 of each sex) were fed dietary
levels adjusted to provide 0, 10, 40 or 160 mg/kg bw/day of
tiabendazole for up to two years. At the 160 mg/kg bw/day level, a
reduction of weight gain of about 25%, with concomitant reduction in
food consumption and slightly reduced haemoglobin and haematocrit
values were the only changes attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidence or location of neoplasms and histopathological examination
of the tissues.
No effect on food consumption or haemograms were seen at either
10 or 40 mg/kg bw/day. Very slight depression in growth rate was
observed in male rats at 40 mg/kg bw/day but not at 10 mg/kg bw/day.
At 10 mg/kg bw/day, the mean absolute thyroid weights of the male
rats were heavier than the mean thyroid weight of the controls. This
effect was not observed in the rats given 40 or 160 mg/kg bw/day.
The NOEL was 10 mg/kg bw/day (FAO/WHO, 1971; Woodard, 1964).
In a carcinogenicity study, groups of 30 male and 30 female
F344/DuCrj rats were fed diets containing 0, 500, 1000, 2000 or 4000
ppm tiabendazole (purity 98.5%) for 2 years. Based on food
consumption the drug intake was calculated as 21, 43, 90 or 207
mg/kg bw/day in males and 26, 53, 112 or 237 mg/kg bw/day in
females.
There were no signs of toxicity or effects on survival. Food
intake was lower at 4000 ppm whereas body weight gain was reduced at
1000 ppm and above, leading to small and poorly developed animals at
2000 and 4000 ppm. Clinical laboratory parameters were not examined.
At the end of 2 years, all animals were necropsied. Liver
microgranulomas and aggregations of foamy cells in the lung were
increased at 1000 ppm and above. Renal epithelial hyperplasia of
papilla and pelvis were increased at 2000 and 4000 ppm. Preputial or
clitoral gland adenoma incidences were higher than control values at
4000 ppm. The increase was statistically significant (p <0.05) only
for adenomas in the preputial gland. There were no adverse effects
at 500 ppm, equal to a NOEL of 20 mg/kg bw/day (Fujii et al.,
1991).
2.2.4 Reproduction studies
2.2.4.1 Mice
A five-generation reproduction study utilized 25 male and 25
female mice given diets containing tiabendazole at concentrations of
0, 200, 1000 or 5000 ppm. When the mice attained the age of eight
weeks, they were mated and continued on the same diet. The young
from these matings, when they were weaned, were maintained on the
test diet and mated when they reached the age of eight weeks. This
procedure continued for five complete reproductive cycles. No
effects were noted at 200 ppm. At 1000 ppm, a slight decrease in the
weights of weanlings was observed in all five generations. At 5000
ppm, the number of mice born and weaned per litter was reduced and a
marked reduction in the average weanling weight of the young was
observed. The NOEL was 200 ppm, equivalent to 30 mg/kg bw/day
(FAO/WHO, 1971).
2.2.4.2 Rats
A three-generation, two-litter reproduction study (10 male and
10 female FDRL albino rats per group) at dosages of 0, 20, 40 or 80
mg/kg bw/day showed no adverse effects on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight and decreased
food consumption in the male rats at all dosage levels in the F0-
and F2-generations. Slight decreases in final body weights and
food consumption at the 80 mg/kg bw/day dose level in the F1- and
F2-generation females were also observed (FAO/WHO, 1971; Vogin,
1968)
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
A series of developmental studies were undertaken in Jcl:ICR
mice. Pregnant females were given gavage doses of tiabendazole
(purity 98.5%) in olive oil at various times during the gestation
period. Females were killed on gestation day 18.
Exp. 1: Groups of 39 mice were dosed with 0, 700, 1300 or 2400
mg/kg bw/day on gestation days 7-15. Maternal weight gain was
depressed in a dose-related manner with mortality at 1300 mg/kg
bw/day (5 out of 39) and 2400 mg/kg bw/day (24 out of 39). The
resorption rate was increased at 1300 and 2400 mg/kg bw/day. At all
treatment levels, there were reduced fetal weights and increased
malformations such as cleft palate, fusion of vertebral arches and
vertebral bodies.
Exp. 2: Groups of 7-12 mice were given a dose of 2400 mg/kg bw
on a single day of gestation between days 6 and 15. Mortality was 8
to 55% in treated groups but there were no data on body weight. In
each group, resorptions were increased and fetal body weights were
decreased. Fetal malformations detected were: microcephaly and
exencephaly (following treatment on gestation days 6 to 8); short or
absent tail and anal atresia (day 9); open eyelids (days 7, 8, 10,
13, and 14); reduction deformity of limbs and cleft palate (days 9
to 12); fusion of vertebral arches and bodies (days 7 to 10, and 13)
and fusion of ribs (days 7 to 9). The fetal abnormalities were
judged to be most prominent on gestation day 9. As the types of
malformation were diverse and primarily related to the timing of
treatment, it was suggested that tiabendazole was a non-specific
teratogen.
Exp. 3: Groups of 21 to 31 mice were given a single dose of 30
to 2400 mg/kg bw on gestation day 9. Mortality was induced at 1670
mg/kg bw and above and maternal weight gain was reduced at 1160
mg/kg bw and above. The resorption rate was increased at 1670 mg/kg
bw and above while fetal weight was lower at 60 mg/kg bw and above.
The limb reduction deformity was seen regularly at doses of 480
mg/kg bw and above; the threshold was calculated as being 360 mg/kg
bw. Fusion of vertebral arches, vertebral bodies and ribs was
increased at 240 mg/kg bw and above. Other malformations were not
consistently increased. There were no treatment-related effects at
30 mg/kg bw/day (Ogata et al., 1984).
Follow-up developmental studies were carried out in groups of
16 to 20 pregnant female Jcl:ICR mice. Tiabendazole (purity 98.5%)
was given by gavage in olive oil at doses of 0, 250, 500 or 1000
mg/kg bw/day on gestation day 9. Females were killed on gestation
day 18. Treatment at the two highest doses produced similar effects
to the previous mouse studies.
Pretreatment with SKF-525A (inhibitor of cytochrome P-450)
enhanced the rate of resorptions and the incidence of fetal
malformations. Sodium phenobarbital pretreatment, on the other hand,
resulted in decreases in the resorption rate and reduced fetal
abnormalities to control levels. Pretreatment with glutathione,
cysteine or diethyl maleate (a glutathione-depleting substance) had
no effect on resorptions. However, the incidence of fetal
malformations was increased with glutathione or cysteine and reduced
to control levels by diethyl maleate. Graphs of tissue
concentrations of tiabendazole against time showed the AUC for both
maternal blood and fetal tissue was increased by glutathione and
reduced by diethyl maleate. The overall conclusion was that the
developmental effects appear to be due to unchanged tiabendazole and
not to its degradation products (Ogata et al., 1987; 1989).
2.2.5.2 Rats
Two groups of rats (20 male and 20 female) were placed on a
diet containing 0 or 500 ppm tiabendazole. There were no
abnormalities among the young from either mating attributable to
tiabendazole at 500 ppm. It was concluded that these data presented
no evidence of teratogenecity (FAO/WHO, 1971).
In a preliminary teratology study, Charles River SD rats (8
pregnant females per group) were given gavage doses of 0 or 80 mg/kg
bw/day tiabendazole (purity unknown) on gestation days 8 to 15. Dams
were killed on gestation day 21. There were no effects on resorption
rate or on fetal survival, growth or development (Delatour et al.,
1974).
Groups of 20 pregnant female Wistar rats were administered
gavage doses of 0, 125, 250 or 500 mg/kg bw/day of a commercial
formulation containing 45% tiabendazole and 55% unknown ingredients.
Dosing was on gestation days 6-15 and dams were killed on gestation
day 22. No adverse effects were apparent in females and no
meaningful effects were reported on the resorption rate, fetal
viability, growth or development (Khera et al., 1979).
In a range-finding study, Sprague-Dawley Crl:CD(SD)BR rats were
given gavage doses of 0, 50, 100, 200 or 400 mg/kg bw/day
tiabendazole (purity 99.4%). Groups of 10 pregnant females were
treated on gestation days 6 to 17 and were killed on gestation day
20.
There were no deaths or abortions but all 200 and 400 mg/kg
bw/day females were killed due to severe body-weight effects.
Maternal weight gain was also depressed at 50 and 100 mg/kg bw/day.
Other toxic signs were lethargy and ptosis at 100 mg/kg bw/day and
above and reduced faeces at 200 mg/kg bw/day and above. Increased
protein, albumin and cholesterol were observed at the higher doses
with decreased triglycerides at all dose levels. Resorptions and
fetal morphology were unaffected but fetal weight was reduced at all
treatment levels (Lankas & Wise, 1990a).
Groups of 25 pregnant female Sprague-Dawley Crl:CD(SD)BR rats
were given gavage doses of 0, 10, 40 or 80 mg/kg bw/day tiabendazole
(purity 98.9%) suspended in 0.5% methyl cellulose. Drug
administration was on gestation days 6 to 17 and the dams were
killed on gestation day 20.
At 80 mg/kg bw/day, rats showed ptosis and a few animals
regurgitated a portion of the dose. Food intake and body-weight gain
were depressed at 40 and 80 mg/kg bw/day. There was no mortality or
abortions nor were there increases in the resorption rate or in dead
fetuses. Fetal abnormalities were not produced, but statistically
significantly decreased fetal weight was noted at 40 and 80 mg/kg
bw/day. The NOEL was 10 mg/kg bw/day (Lankas & Wise, 1990b).
2.2.5.3 Rabbits
Groups of 10 to 32 pregnant female New Zeeland white rabbits
were dosed by gavage with 0, 100, 200, 400 or 800 mg/kg bw/day
tiabendazole (purity unknown) suspended in 1% carboxymethyl
cellulose. Treatment was on gestation days 8 to 16 and does were
killed on gestation day 29 or 30.
At 200 mg/kg bw/day and above, does lost weight and the
resorption rate was increased (not dose-related). Fetal weight was
decreased at 400 and 800 mg/kg bw/day but fetal morphology was
unaltered. The NOEL was 100 mg/kg bw/day (Peck, 1966a).
In a range-finding study New Zeeland white rabbits were given
gavage doses of 0, 100, 200, 400 or 800 mg/kg bw/day tiabendazole
(purity 98.9%). Groups of 8 pregnant females were dosed on gestation
days 6 to 18 and does were killed on gestation day 29.
At 800 mg/kg bw/day, abnormal faeces were seen and weight loss
necessitated early sacrifice of this group. Food intake and weight
gain were lower at 200 and 400 mg/kg bw/day. The implantation rate
was lower at 400 mg/kg bw/day but resorptions were not increased.
External fetal examinations were unremarkable (Christian et al.,
1989).
Groups of 18 pregnant female New Zeeland white rabbits were
administered 0, 24, 120 or 600 mg/kg bw/day tiabendazole (purity
98.9%) suspended in 0.5% methyl cellulose. The gavage doses were
given on gestation days 6 to 18 and females were killed on gestation
day 29.
At 600 mg/kg bw/day one doe died, four aborted, abnormal faeces
were increased, food consumption was lower and body weight was lost.
Weight gain was slightly depressed at 120 mg/kg bw/day. There were
increased resorptions at 120 and 600 mg/kg bw/day, including total
resorption of conceptuses in 4 females at 120 mg/kg bw/day. Domed
head, hydrocephaly and enlarged fontanelles were seen in one fetus
at 120 mg/kg bw/day and two fetuses at 600 mg/kg bw/day. The NOEL
was 24 mg/kg bw/day (Hoberman & Lankas, 1989).
Groups of 18 pregnant female New Zeeland white rabbits were
given gavage doses of 0, 50, 150 or 600 mg/kg bw/day tiabendazole
(purity 98.6%) suspended in 0.5% methylcellulose. Treatment was on
gestation days 6 to 18 and dams were killed on gestation day 28.
There were no deaths or abortions related to treatment. Food
intake and body weight gain were reduced at 600 mg/kg bw/day during
the dosing period only. Resorptions were increased slightly but
significantly at 600 mg/kg bw/day. At 600 mg/kg bw/day, there were
increases in the incidences of variation in lung lobation and
incompletely ossified metacarpals. The NOEL was 150 mg/kg bw/day
(Lankas & Wise, 1991).
2.2.5.4 Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg bw
tiabendazole at 2.5 to 8 weeks prior to lambing without any effect
on the birth rate, growth or viability of the lambs. This dose had
no effect on the ewes throughout the test interval or on viability
of the lambs to six weeks of age. Miscellaneous tests using 39 ewes
produced 29 weanling lambs after treatment with tiabendazole at 400
mg/kg bw, whereas, from a control group of 22 ewes, 13 lambs were
weaned. Ewes tolerated a single dose of 18 g tiabendazole per animal
(equal to 235 to 335 mg/kg bw), three to ten days after lambing,
without any effect on the weight or food intake of the ewes or
survival and growth of the lambs up to the weaning time of six weeks
(FAO/WHO, 1971).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with tiabendazole are
summarized in Table 2.
Table 2. Results of genotoxicity studies on tiabendazole1
Test system Test object Treatment Results Reference
Reverse S.typhimurium 10-5000 negative MacGregor, 1976
mutation TA98, TA100, µg/plate
TA1535, TA1537 (+&-S9)2
Reverse S.typhimurium 100-2500 negative Shirasu et al.,
mutation TA1535, TA1537, µg/plate (-S9) 1976a.
TA1538, TA98, 10-1000
TA100, E.coli µg/plate (+S9)2
WP2 hcr-
Reverse S.typhimurium 10-2000 negative Peck, 1977
mutation TA98, TA100, µg/plate
TA1538 (+&-S9)2
TA100, TA1538 10-2000 negative
µg/plate (+S9)3
TA98 10-500 negative
µg/plate (+S9)3
1000-2000 positive
µg/plate (+S9)3
1000-2000 negative
µg/plate (+S9)3
pure tiabendazole
20-1000 positive
µg/plate (+S9)3
concentrated
impurities
Host ICR mice, 300-1000 negative Shirasu et al.,
mediated S.typhimurium mg/kg bw/day for 1976a
G46. 5 days, po
Rec. assay B.subtilis 2-1000 negative Shirasu et al.,
H17, M45 µg/disc (-S9) 1976a
DNA Rat 60-262 negative Lankas, 1989
damage hepatocytes µg/ml
Anaphase- CHO cells 0.06-0.12 negative Mudry de
telophase µg/ml (-S9) Pargament
test 0.24-0.6 positive et al., 1987
µg/ml (-S9)
Table 2. cont'd
Test system Test object Treatment Results Reference
Sister Mouse bone 50-200 negative Mudry de
chromatid marrow mg/kg bw, ip Pargament
exchange et al., 1987
Chromosome Human 2-50 negative Shirasu et al.,
aberration fibroblasts µg/ml (-S9) 1976c
Chromosome Rat bone 100-1000 negative Shirasu et al.,
aberration marrow mg/kg bw, po 1976d
30-300 negative
mg/kg bw/day
for 5 days, po
Micronucleus Mouse bone 125-500 negative Peck, 1977.
test marrow mg/kg bw, po
Micronucleus Mouse bone 50-200 positive Mudry de
test marrow mg/kg bw, ip Pargament
et al., 1987
Dominant Mice 200-600 negative Shirasu et al.,
lethal mg/kg bw/day 1976b,
test for 5 days, po
Dominant Mice 125-500 negative Peck, 1977.
lethal mg/kg bw/day
test for 5 days, po
1 Appropriate positive controls were used.
2 Liver microsomes obtained from Aroclor 1254 pretreated rats.
3 Liver microsomes obtained from phenobarbital pretreated rats.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Male albino guinea-pigs received 10 intracutaneous injections
of a 0.1% suspension of tiabendazole in 0.9% saline. Two weeks after
the induction period an intracutaneous challenge dose failed to
exacerbate reactions at the injection site (Peck, 1966b).
2.2.7.2 Rabbits
There was no evidence of a significant degree of irritation
when 0.5 g tiabendazole was applied in cold cream at concentrations
of 10 and 50% (w/w) for 24 h to intact rabbit skin (FAO/WHO, 1971).
No evidence of irritation was noted other than a very slight
erythema observed for approximately one hour after application of 1
ml of tiabendazole as a 12% suspension in sodium carboxymethyl
cellulose or 10 mg of the dry powder to the conjunctival sac of two
rabbits (FAO/WHO, 1971).
Male and female New Zeeland white rabbits received 100 mg of
tiabendazole powder instilled into the conjunctival sac of the eye.
At 15 min, there was slight conjunctival injection with a clear
discharge which returned to normal within 24 h (Merck, Sharp &
Dohme, undated).
2.2.8 Special studies on pharmacology
Oral doses of tiabendazole (4000 mg/kg bw) produced no striking
acute pharmacological effects on blood pressure or respiration in
either cats or dogs. Similarly, there were no alterations in the
electrocardiogram (FAO/WHO, 1971).
2.3 Observations in humans
A double blind study was carried out with 100 male volunteers,
half of them receiving 250 mg tiabendazole/day for 24 weeks and half
of them a placebo. Each subject was interviewed weekly and any
potential side effect was recorded. General physical examination and
laboratory examinations (haematology, cholesterol, glucose, urea,
alkaline phosphatase, thymol turbidity, bilirubin in serum and
urinalysis) were carried out before the test and after 4, 12 and 24
weeks. PBI in serum and ECGs were carried out only at the beginning
and after 24 weeks. Thirty-six subjects on tiabendazole and 41 on
placebo completed the study. As tiabendazole has a possible
influence on the thyroid the values for PBI were evaluated
carefully. No indication, however, was found of a decrease or
increase in PBI values due to tiabendazole. Under the conditions of
the study, tiabendazole was well tolerated at a dosage of 250 mg/day
during 24 weeks. No effect on any of the parameters studied could be
clearly ascribed to the drug. The NOEL was 3-4 mg/kg bw/day
(Colmore, 1965; FAO/WHO, 1978).
Twenty-three patients with trichinosis received 50 mg/kg bw/day
up to 3 g daily tiabendoazole for 10 days (total dose 25 to 30 g
tiabendazole), which was administered twice a day (after breakfast
and after evening meal). Symptoms of muscle pain and fever subsided
1 to 3 days after commencing tiabendazole. One woman withdrew due to
drug-induced rash, fever and proteinuria. Side-effects occurred in
14/23 treated patients - the most common effects being nausea,
retching and vomiting in 11/23 subjects, aversion to tablets (3/23),
exanthema (3/23), impotence (2/23), diarrhoea (1/23), liver damage
(1/23), fever (1/23) and dizziness (1/23) (Hennekeuser et al.,
1969).
3. COMMENTS
Information from a range of studies on thiabendazole was
available for assessment, including data on kinetics and metabolism,
acute toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity and observations in humans.
Toxicokinetic studies revealed rapid absorption after oral
dosing in mice, rats, dogs, and humans, peak plasma levels occurring
within 3 h of drug administration. The drug was excreted in the
urine and faeces. Elimination of tiabendazole and its degradation
products appeared more rapid in humans than in mice, rats, and dogs,
but metabolism was similar in mice, rats, and humans. The main
urinary metabolites were the glucuronide and sulfate conjugates of
5-hydroxytiabendazole; there were also small amounts of unconjugated
tiabendazole and the 5-hydroxy derivative. At very high doses, other
minor metabolites were detected in rat and mouse urine.
Single oral doses of tiabendazole were slightly toxic
(LD50 > 2000 mg/kg bw) in mice, rats, rabbits, sheep and goats.
Where deaths occurred, they appeared to be due to respiratory
failure.
Following repeated oral administration of tiabendiazole to
mice, rats, dogs, chickens, sheep, pigs, and cattle, the most common
finding was a reduction in food consumption and in body-weight gain.
The effects on weight gain appeared to be greater than could be
accounted for by the depressed food intake. Anaemia was seen in rats
and dogs, and to some extent may have been related to poor
nutrition. However, the presence of increased haemosiderosis in
lymphoid tissues and reticulocytosis in some studies was suggestive
of red cell destruction. The haematological changes occurred at dose
levels of 100 mg/kg bw/day and above for 3 months in rats and 150
mg/kg bw/day and above for 3 months in dogs.
The major target organs for toxicity were the hepatic system,
thyroid gland, and lymphoid organs. Liver hypertrophy was seen in a
3-month study in rats at doses of 37 mg/kg bw/day and above, and in
a 2-year study in dogs minor hepatobiliary changes were observed at
50 mg/kg bw/day and above. Thyroid follicular cell hyperplasia and
colloid depletion were noted in a 3-month study in rats at 37 mg/kg
bw/day and above and in a 16-week study in sheep at 800 mg/kg
bw/day. The thymus, spleen, lymph nodes, and/or bone marrow
exhibited lymphoid depletion or atrophy in a 28-day study in rats at
100 mg/kg bw/day and above and in a 16-week study in sheep at 800
mg/kg bw/day. Effects on blood cells, the liver, and lymphoid organs
are commonly observed with benzimidazoles.
Less common observations included both renal tubular
degeneration and hyperplasia in one 7-day study in mice at 250 mg/kg
bw/day and above and in one 3-month study in rats at 100 mg/kg
bw/day and above. Gastric changes, including mucosal degeneration,
cytoplasmic rarefaction, and/or necrosis, were produced in a 3-month
study in rats at 100 mg/kg bw/day and above. Atrial thrombosis was
present at high incidence in a two-year study in mice at doses of
200 mg/kg bw/day and above that resulted in the death of the
affected animals.
Tumour incidences were not increased in a two-year
carcinogenicity study in mice. The NOEL was 6 mg/kg bw/day, and
there was increased mortality at the next highest level, 60 mg/kg
bw/day.
The incidence of preputial or clitoral gland adenomas was
increased at doses above 200 mg/kg bw/day in one of two 2-year
studies in rats. Statistical significance was achieved only in
males; the animals also displayed poor development due to severe
effects on weight gain which suggests that the maximum tolerated
dose was exceeded. The relevance of these tumours to low-dose
exposure of humans to tiabendiazole is questionable. The NOEL was 10
mg/kg bw/day based on depressed weight gain at the next highest
level, 40 mg/kg bw/day.
Genotoxicity tests were generally negative. In one of three
Salmonella typhimurium reverse-mutation assays, positive results
were obtained in strain TA98 only. Further investigation showed that
an impurity in some batches was responsible for the mutagenic
activity. The manufacturer indicated that no positive results had
been obtained in tests on several hundred further batches. In one
laboratory, micronuclei were induced in mouse bone marrow and
abnormal anaphase-telophase figures were increased in cultured
Chinese hamster ovary cells. The effects were seen only at
relatively high levels and may be indicative of the tubulin-binding
activity characteristic of benzimidazoles (Annex 1, reference 97,
section 3.2.4). A range of other assays for mutation, DNA damage,
and cytogenetic activity were clearly negative.
A multigeneration reproduction study in rats showed no adverse
effects on reproduction parameters up to the highest dose tested, 80
mg/kg bw/day. In mice, litter size and survival during the lactation
period were reduced at 750 mg/kg bw/day, but reproduction was
unaffected at lower doses in a five-generation study. The NOEL was
30 mg/kg bw/day.
Developmental toxicity was examined in mice, rats, rabbits, and
sheep. In a series of studies in mice, embryotoxicity was observed
at 1300 mg/kg bw/day and above, a dose that also resulted in the
death of 5 of 39 females. Fetal malformations were produced at 240
mg/kg bw/day
and above, the main abnormalities being a reduction deformity of the
limbs, cleft palate, and fusion of vertebral arches and vertebral
bodies. Unchanged tiabendazole was implicated as the probable
embryotoxic and teratogenic agent.
The only finding in several rat developmental studies was a
reduction in fetal weight at 40 mg/kg bw/day and above. The NOEL was
10 mg/kg bw/day.
In rabbits, abortions occurred at 600 mg/kg bw/day in one study
on developmental toxicity, but not in the prior range-finding study
at higher doses or in a repeat experiment in which similar treatment
levels were used. Embryotoxicity and low incidences of domed head,
hydrocephaly, and enlarged fontanelles were seen at 120 mg/kg bw/day
and above in one study, but not in the repeat study at similar dose
levels. All effects in rabbits were seen only at maternally toxic
dose levels. The NOEL was 24 mg/kg bw/day.
No adverse reproductive effects were observed in pregnant ewes
given doses of 400 mg/kg bw/day.
In a 24-week study in humans, male volunteers received 250 mg
of tiabendazole/day; there were no treatment-related effects on a
range of physical and biochemical parameters. The NOEL was 3-4 mg/kg
bw/day. In another study, patients with trichinosis were given up to
3 g of tiabendazole daily for 10 days. Side-effects occurred in 14
of 23 patients, the most common being nausea, retching, and
vomiting.
4. EVALUATION
The Committee noted the availability of extensive data in
support of the safety evaluation of tiabendazole and the
manufacturer indicated that further studies had been completed or
were under way. At the 1977 joint FAO/WHO meeting on pesticide
residues, the study in human volunteers was used to establish an ADI
of 0-0.3 mg/kg bw. The Committee considered that the investigations
undertaken as part of this human study were limited in nature, and
that in view of the availability of a number of recent well
conducted animal studies, the NOEL should be derived from the animal
data.
The overall NOEL was 10 mg/kg bw/day based on reduced weight
gain in a 2-year dietary study in rats and decreased fetal weight in
a developmental study in rats. The Committee decided not to use the
NOEL of 6 mg/kg bw/day from the 2-year study in mice as the next
administered dose was considerably higher at 60 mg/kg bw/day.
Using a safety factor of 100, the Committee established an ADI
of 0-100 µg/kg bw for tiabendazole. The Committee noted that the ADI
provided a safety margin corresponding to a factor of over 1000 with
respect to the dose required for fetal malformations in mice.
The Committee asked to see the results from recently completed
and ongoing toxicological studies in order to update the database on
tiabendazole.
5. REFERENCES
BAGDON, W.J., BOKELMAN, D.L. & ZWICKEY, R.E. (1980). Lifetime
carcinogenic study in mice. Unpublished Report No. TT77-014-0 from
Merck, Sharp & Dohme Research Laboratories. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BATHAM, P., REID, S. & OSBORNE, B.E. (1989). A 14-week oral toxicity
study in the beagle dog. Unpublished Report No. 84021 (TT 89-9010)
from Bio-Research Laboratories, Canada. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BLASZCAK, D.L. & AULETTA, C.S. (1987). Acute dermal toxicity study
in rabbits. Unpublished Report No. 4004-86 from Bio/dynamics Inc.,
USA. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
BOKELMAN, D.L. & ZWICKEY, R.E. (1977). Tiabendazole, six-week pilot
study in mice. Unpublished Report No. TT77-004-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CAVAGNORO, J. & YAMAMOTO, K.S. (1989). 23 day dermal toxicity study
in rabbits. Unpublished Report No. HLA 284-161 (TT89-9011) from
Hazleton Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CHRISTIAN, M.S., LANKAS, G.R. & HOBERMAN, A.M. (1989). Oral
range-finding study in pregnant rabbits. Unpublished Report No.
013-029P (TT89-9006) from Argus Research Laboratories, USA.
Submitted to WHO by MSD Research Laboratory, Lauterbach, Germany.
COLMORE, J.P. (1965). Chronic toxicity study of tiabendazole in
volunteers. Unpublished report from University of Oklahoma Medical
Centre, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
DELATOUR, P., LORQUE, G., LAPRAS, M. & DESCHANEL, J.P. (1974).
Embryotoxic properties in the rat, and residues in cattle and sheep,
of three anthelmintics, derivatives of benzimidazole. Bull. Soc.
Sci. Vet. et Med., 76: 147-154.
FAO/WHO (1971). 1970 evaluations of some pesticide residues in food.
AGP: 1970/M/12/1; WHO/Food Add/71.42, Rome.
FAO/WHO (1978). Pesticide residues in food: 1977 evaluations. FAO
Plant Production and Protection Paper, 10 Sup. Rome.
FUJII, T., MIKURIYA, H. & SASAKI, M (1991). Chronic oral toxicity
and carcinogenicity study of tiabendazole in rats. Fd. Chem.
Toxicol., 29: 771-775.
FUJITANI, T., YONEYAMA, M., OGATA, A., UETA, T., MORI, K. &
ICHIKAWA, H. (1991). New metabolites of tiabendazole and the
metabolism of tiabendazole by mouse embryo in vivo and in vitro.
Fd. Chem. Toxicol., 29: 265-274.
GURMAN, J.L., HARDY, R.J., VOELKER, R.W. & DAVIDSON, J.L. (1981).
Acute inhalation toxicity study in rats. Unpublished Report No. HLA
284-129 from Hazleton Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
HENNEKEUSER, H.H., PABST, K., POEPLAU, W. & GEROK, W. (1969).
Tiabendazole for the treatment of trichinosis in humans. Texas Rep.
Biol. Med., 27 (Suppl. 2): 581-596.
HOBERMAN, A.M. & LANKAS, G.R. (1989). Oral developmental toxicity
study in rabbits. Unpublished Report No. 013-029 (TT89-9005) from
Argus Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
KANGAS, L., FARRELL, D. & OSBORNE, B.E. (1989). A 14-week oral
toxicity study in the albino rat. Unpublished Report No. 84114
(TT89-9014) from Bio-Research Laboratories, Canada. Submitted to WHO
by MSD Research Laboratory, Lauterbach, Germany.
KHERA, K.S., WHALEN, C., TRIVETT, G. & ANGERS, G. (1979).
Teratologic assessment of maleic hydrazide and daminozide, and
formulations of ethoxyquin, tiabendazole and naled in rats.
J. Environ. Sci. Health, B14: 563-577.
LANKAS, G.R. (1989). In vitro alkaline elution/rat hepatocyte
assay. Unpublished report nos. TT88-8850 and TT89-8312 from Merck,
Sharp & Dohme Research Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990a). Oral range-finding study in
pregnant rats. Unpublished Report No. TT90-713-1 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990b). Oral development toxicity study
in rats. Unpublished Report No. TT90-713-0 from Merck, Sharp & Dohme
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1991). Oral developmental toxicity study
in rabbits. Unpublished Report No. TT90-734-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
MacGREGOR, J. (1976). Mutagenicity tests of tiabendazole in the Ames
Salmonella typhimurium reverse mutation test. Unpublished report
from Western Regional Research Centre, California, USA. Submitted to
WHO by MSD Research Laboratory, Lauterbach, Germany.
MERCK, SHARP & DOHME (undated). Primary eye irritation in rabbits.
Unpublished Report No. TT81-2693 from Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
MUDRY DE PARGAMENT, M.D., LABAL DE VINUESA, M. & LARRIPA, I. (1987).
Mutagenic bioassay of certain pharmacological drugs. I Tiabendazole
(TBZ). Mutation Res., 188: 1-6.
MYERS, B.A. & LANKAS, G.R. (1990). A 14-week dietary toxicity study
in rats. Unpublished Report No. HLA 284-169 (TT 90-9002) from
Hazleton Laboratories, USA and Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
OGATA, A., ANDO, H., KUBO, Y. & HIRAGA, K. (1984). Teratogenicity of
tiabendazole in ICR mice. Fd. Chem. Toxicol., 22: 509-520.
OGATA, A., YONEYAMA, M., SASAKI, M., SUZUKI, K. & IMAMICHI, T.
(1987). Effects of pretreatment with SKF-525A or sodium
phenobarbital on tiabendazole-induced teratogenicity in ICR mice.
Fd. Chem. Toxicol., 25: 119-124.
OGATA, A., FUJITANI, T., YONEYAMA, M., SASAKI, M. & SUZUKI, K.
(1989). Glutathione and cysteine enhance and diethylmaleate reduces
tiabendazole teratogenicity in mice. Fd. Chem. Toxicol., 27:
117-123.
PECK, H.M. (1966a). Reproduction study in the rabbit. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
PECK, H.M. (1966b). Cutaneous sensitisation in the guinea pig.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
PECK, H.M. (1977). Mutagenic evaluation. Unpublished report from
Merck, Sharp & Dohme. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
ROBINSON, H.J. (1964). Tiabendazole preclinical evaluation.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
ROBINSON, H.J. (1965). Tiabendazole safety evaluation. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
SHIRASU, Y., MORIYA, M. & KATO, K. (1976a). Mutagenicity testing on
tiabendazole (TBZ) in microbial systems. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TERAMOTO, S. & KANEDA, M. (1976b). Dominant lethal
studies with tiabendazole in mice. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976c).
Cytogenetic studies with tiabendazole in cultured human fibroblasts.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976d).
Cytogenetic studies with tiabendazole in rat bone marrow cells.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
TADA, Y., YONEYAMA, M., KABASHIMA, J., FUJITANI, T. & NAKANO, M.
(1989). Effects of tiabendazole on the kidneys of ICR mice.
Fd. Chem. Toxicol., 27: 307-315.
TSUCHIYA, T., TANAKA, A., FUKUOKA, M., SATO, M. & YAMAHA, T. (1987).
Metabolism of tiabendazole and teratogenic potential of its
metabolites in pregnant mice. Chem. Pharm. Bull., 35: 2985-2993.
USUI, T. & SAKAGUCHI, T. (1989). 5 week oral toxicity study in rats.
Unpublished report nos. TT86-9819 and TT87-9809 from Banyu
Pharmaceutical Development Research Laboratories, Japan. Submitted
to WHO by MSD Research Laboratory, Lauterbach, Germany.
VOGIN, E.E. (1968). Multigeneration reproduction and lactation
studies with tiabendazole. Unpublished report from Food and Drug
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
WOODARD, G. (1964). Safety evaluation by oral administration to dogs
and rats for 104 weeks. Unpublished report from Woodard Research
Corporation, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
YONEYAMA, M., OGATA, A., FUJII, T. & HIRAGA, K. (1984). The
maternal-foetal distribution of tiabendazole administered in two
different vehicles to pregnant mice. Fd. Chem. Toxicol., 22:
731-735.
 2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with tiabendazole are
summarized in Table 1.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 2400-3810 FAO/WHO, 1971
M&F ip 430 FAO/WHO,1971
M&F iv 150 FAO/WHO, 1971
Rat M&F oral 3330-3600 FAO/WHO, 1971
M&F ip 1850 FAO/WHO, 1971
M&F iv 130 FAO/WHO, 1971
M&F inhalation >397 mg/m3 Gurman et al., 1981
Rabbit M&F oral 3850 FAO/WHO, 1971
M&F dermal >2000 Blaszcak & Auletta,
1987
Sheep ? drench 2000 FAO/WHO, 1971
Goat ? drench >2000 FAO/WHO, 1971
Toxic signs following administration of large doses of
tiabendazole by oral or intraperitoneal routes were generally
similar and consisted of lethargy and stupor. The intravenous
administration of a large dose of tiabendazole hydrochloride
produced narcosis. Death appeared to be due to respiratory failure.
Rabbits that survived large oral doses initially lost weight but
recovered after several days.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In an investigation of renal function, Crj:CD-1 (ICR) mice were
given gavage doses of 0, 250 or 500 mg/kg bw/day tiabendazole
(purity 98.5%) in olive oil. Groups of 6 to 8 males and females were
killed after dosing for 1, 3, 5 or 7 days. There was no effect on
body weight. Water intake was unaffected but urine volume was
increased in all treated groups, significantly so in males given 500
mg/kg bw/day.
There were no meaningful effects on serum creatinine or urea
nitrogen, urinary glucose or protein or the presence of occult blood
in urine. Electrophoresis however, revealed relatively high
molecular weight protein in treated mice, while low molecular weight
protein was found in all groups. There was a progressive
dose-related increase in renal lesions including tubular dilatation
and degenerative desquamation, cell infiltration, fibrosis and
regeneration of tubular epithelium. Electron microscopy showed
evidence of glomerular damage such as flattening of the foot
processes of podocytes and oedematous changes in the mesangium in
treated mice (Tada et al., 1989).
In a 6-week pilot study, groups of 10 male and 10 female
Charles River CD-1 (HaM/ICR) mice were given 0, 50, 150, 300, 600 or
900 mg/kg bw/day tiabendazole (purity unknown) via the diet. There
were no clinical signs or mortality. Food intake and weight gain
were depressed in males at 600 and 900 mg/kg bw/day. Other
parameters were not monitored (Bokelman & Zwickey, 1977).
2.2.2.2 Rats
Rats (Sprague-Dawley Holtzman, 10 males and 10 females per
group) were administered tiabendazole by gavage at dosage levels of
0, 100, 400, 800, 1200 or 1600 mg/kg bw/day. During the 30 day
experimental period, rats in the 800 mg/kg bw/day group decreased
their food intake and gradually lost weight. They were slightly less
active, had ruffled fur and became slightly flaccid; three males and
three females died during the course of the experiment. All of the
rats in the 1200 and 1600 mg/kg bw/day groups died during the 30-day
test period. Rats dosed at 100 and 400 mg/kg bw/day appeared normal
throughout the test period, although a slight depression in
body-weight gain was noted in males at 100 mg/kg bw/day and in
females at 400 mg/kg bw/day. Higher doses produced greater effects
on body-weight gain. Haematologic studies on rats receiving 800
mg/kg bw/day showed a mild neutrophilia with concurrent lymphopenia.
There was also a suggestion of a decrease in red blood cell elements
at 400 mg/kg bw/day and above. Blood biochemistry showed no
meaningful changes.
No gross anatomical changes were noted in rats dosed with 100
mg/kg bw/day, whereas male and female rats treated with 400 mg/kg
bw/day showed thymic involution. A slight enlargement of the liver
was also noted in females at 400 mg/kg bw/day. Animals surviving 800
mg/kg bw/day showed gross signs indicating starvation; normal body
fat depots and subcutaneous fat appeared to be depleted. The liver
and adrenals were slightly enlarged in males and females and the
thymus was involuted. Microscopic pathology showed bone marrow
hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964).
Groups of 12 male and 12 female Albino Crj:CD (SD) rats were
administered gavage doses of 0, 50, 100, 200 or 800 mg/kg bw/day
thiabendazole (purity 100%), suspended in 0.5% methylcellulose, for
28 to 31 days. Severe toxicity was observed at 800 mg/kg bw/day in
the form of decreased activity, sedation, ataxia, recumbency,
flaccidity, loss of righting reflex, piloerection, emaciation, rough
hair coat, alopecia and a high level of mortality. Food intake and
body-weight gain were depressed at 200 and 800 mg/kg bw/day.
Laboratory analyses were carried out in week 4. Serum albumin,
total protein and cholesterol were increased and erythrocytes,
haemoglobin and haematocrit were decreased at 200 and 800 mg/kg
bw/day with reticulocytosis and leucocytopenia at the higher level.
Urinalysis was unchanged. Autopsy revealed changes at all dose
levels. There was haemosiderosis and increased extramedullary
haematopoiesis in the spleen at all doses with increased spleen
weight at 200 mg/kg bw/day and above. Increased thyroid weight and
follicular cell hyperplasia and decreased thymus weight were seen at
50 mg/kg bw/day and above. Lymphoid depletion was observed in the
thymus at 100 mg/kg bw/day and above, in bone marrow at 200 mg/kg
bw/day and above and in spleen and lymph nodes at 800 mg/kg bw/day.
Liver weight and centrilobular hepatocytic hypertrophy were
increased at 100 mg/kg bw/day and above (Usui & Sakaguchi, 1989).
Groups of 20 male and 20 female Sprague-Dawley Crl:CD (SD) BR
rats were given gavage doses of 0, 25, 100 or 400 mg/kg bw/day
tiabendazole (purity not stated), suspended in 0.5% methyl
cellulose, for 13 weeks. Alopecia was noted in some rats of each
treatment group. There was no mortality. Body-weight gain was
reduced at 100 and 400 mg/kg bw/day with lower food intake in 400
mg/kg bw/day males. Ophthalmology at the end of study was
unremarkable.
Clinical laboratory parameters were studied in weeks 6 and 13.
Decreased erythrocytes, haemoglobin and haematocrit and increased
cholesterol were induced at 100 and 400 mg/kg bw/day. Urinary levels
of bilirubin, urobilinogen and nitrite were increased at 400 mg/kg
bw/day. At 100 and 400 mg/kg bw/day, increased liver weight and
centrilobular hypertrophy, enlarged thyroids with follicular cell
hyperplasia and splenic congestion and pigmentation were observed.
In the kidney, renal calculus and transitional epithelial
hyperplasia were evident at 100 and 400 mg/kg bw/day with tubular
degeneration at the highest level. The stomach exhibited acanthosis
and/or degeneration of non-glandular mucosa at 100 and 400 mg/kg
bw/day and cytoplasmic rarefaction and necrosis of glandular mucosa
at 400 mg/kg bw/day. The NOEL was 25 mg/kg bw/day (Kangas et al.,
1989).
Groups of 10 male and 10 female Sprague-Dawley Crl:CD BR rats
were fed diets containing tiabendazole (purity 99.4%) for 13 weeks.
Based on food intake the calculated doses were 0, 9, 37, 150 or 302
mg/kg bw/day. There was no effect on survival or on the eyes at the
end of study. Alopecia was increased at 150 and 302 mg/kg bw/day.
Doses of 37 mg/kg bw/day and above were associated with decreased
body-weight gain and food intake in males; females were similarly
affected at 150 mg/kg bw/day and above.
Clinical laboratory analyses were undertaken in weeks 6 and 13.
At doses of 150 and 302 mg/kg bw/day serum cholesterol was
increased, glucose, erythrocytes, haemoglobin and haematocrit were
depressed and there were increased numbers of abnormal erythrocytes.
Urinalysis was unaffected. Liver weight increases and centrilobular
hypertrophy, increased thyroid weight and follicular cell
hypertrophy and bone marrow erythroid hyperplasia were observed at
37 mg/kg bw/day and above. At 150 and 302 mg/kg bw/day there was
spleen pigmentation and thymus atrophy with skeletal muscle atrophy
in females given 302 mg/kg bw/day. The NOEL was 9 mg/kg bw/day
(Myers & Lankas, 1990).
Rats (Sprague-Dawley Holtzman, 30 males and 30 females per
group) were administered tiabendazole, suspended in 1% methocel, by
gavage for 180 days, at doses of 0, 12.5, 25, 50, 100, 200 or 400
mg/kg bw/day. All of the animals survived throughout the duration of
the experiment. At 400 mg/kg bw/day, a depression in body weight was
observed in both sexes. At 200 mg/kg bw/day, the male rats showed a
slight depression in weight gain. No clinical signs of toxicity were
observed. Haematological studies at 400 mg/kg bw/day indicated a
slight suggestion of a fall of the red blood cell elements,
neutrophilia and lymphopenia which were not evident at levels below
this dose. Blood biochemistry studies and urinalysis were normal at
all dosage levels with a slight polyuria in both male and female
rats at 400 mg/kg bw/day, which was apparently the result of a
slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and
females at 400 mg/kg bw/day and in females at 200 mg/kg bw/day.
There was an increase in liver size at doses of 100 mg/kg bw/day and
above in males and at 50 mg/kg bw/day and above in females. Males
dosed with 200 and 400 mg/kg bw/day and females at 400 mg/kg bw/day
showed an apparent increase in kidney weight. Microscopic
examination of animals at 100 mg/kg bw/day showed a small incidence
of haemosiderosis of the thymus. At 200 and 400 mg/kg bw/day, these
examinations showed considerably more haemosiderosis of the thymus
and colloid depletion in the thyroid. The NOEL was 25 mg/kg bw/day
(FAO/WHO, 1971; Robinson, 1964).
2.2.2.3 Rabbits
Groups of 5 male and 5 female New Zeeland white rabbits
received dermal doses of tiabendazole (purity 98.9%) moistened in
0.9% saline. The drug was applied to the shaved trunk of animals at
doses of 0, 50, 200 or 1000 mg/kg bw, 6 h/day for 3 weeks. Oral
ingestion was prevented by use of a collar. Clinical laboratory
parameters and pathology were investigated at the end of the study.
There was no evidence of local or systemic toxicity (Cavagnaro &
Yamamoto, 1989).
2.2.2.4 Dogs
Groups of 4 male and 4 female beagle dogs were given capsules
of tiabendazole (purity 99.4%) at doses of 0, 35, 75 or 150 mg/kg
bw/day for 14 weeks. Emesis was increased at 75 and 150 mg/kg bw/day
with salivation at 150 mg/kg bw/day. There were no deaths. During
the first 2 weeks of treatment, food consumption and body-weight
gain were decreased in all treated groups. Subsequently, food was
made available overnight rather than for 1 h per day which led to
comparable food intake and weight gain in all groups.
Ophthalmology and ECG examinations were normal throughout the
study. Red blood cells, haemoglobin and haematocrit were depressed
at 150 mg/kg bw/day, more so at weeks 4 and 8 than at week 12. Blood
chemistry and urinalysis were unchanged at any time. Relative but
not absolute liver weights were slightly increased in all treated
groups but with no dose-relationship. At 75 and 150 mg/kg bw/day,
gallbladder cytoplasmic vacuolation was increased in incidence and
severity. The NOEL was 35 mg/kg bw/day (Batham et al., 1989).
Groups comprising two male and two female beagle dogs were
orally administered tiabendazole for periods of over two years at
doses of 0, 20, 100 or 200 mg/kg bw/day. No clinical signs or
morbidity were observed, food and water consumption were normal and
blood and urine chemistry were normal. A slight retardation in
body-weight gain was observed at 200 mg/kg bw/day, which was
accompanied by a slight reduction in total erythrocyte count. At
this level, changes in haemoglobin concentration and haematocrit
were also observed.
No gross pathological observations were noted at the conclusion
of this test and organ weights appeared normal. A significant
haemosiderosis was present in dogs at 100 and 200 mg/kg bw/day in
the spleen, liver, lymph nodes and bone marrow. This was not
associated with an increase in serum haemoglobin. The NOEL was 20
mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964)
Groups of beagle dogs (three males and three females) were
orally administered tiabendazole in capsules for two years at doses
of 0, 20, 50 or 125 mg/kg bw/day. At 125 mg/kg bw/day, two of six
dogs died. One of these dogs had marked liver cirrhosis, seminal
tubular degeneration, bone marrow atrophy and degenerative renal
changes. Slight to moderate reduction in haemoglobin and packed cell
volume occurred. Elevated blood urea nitrogen, serum alkaline
phosphatase and serum GOT and chronic inflammatory liver changes
were evident. Urinary albumin was seen more frequently than in
controls. Inspissated bile entwined in the gall bladder villi was
also observed in one dog. One dog of this group was sacrificed and
was found to have pulmonary arteritis of parasitic origin.
At 50 mg/kg bw/day, growth of male dogs was slightly depressed.
All six dogs given tiabendazole at 50 mg/kg bw/day survived the
study and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa
in one. Five of six dogs given tiabendazole at 20 mg/kg bw/day
survived the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes
in all treated dogs. The NOEL was 20 mg/kg bw/day (FAO/WHO, 1971;
Woodard, 1964).
2.2.2.5 Chickens
Groups of 0.5 week-old male white leghorn chicks (10 to 20 per
group) were fed tiabendazole in the diet at dosage levels ranging
from 1 to 10 000 ppm for a period of 2 1/2 weeks. This level
corresponded to a mean daily intake of tiabendazole ranging from 0.1
to 1200 mg/kg bw. A gradual decrease in growth appeared to occur
with concentrations of 100 ppm tiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg bw/day. Gross pathological
examination of the chicks at a dietary level of 4000 ppm
tiabendazole showed them to be normal except for a smaller size
(FAO/WHO, 1971).
2.2.2.6 Sheep
Thirty weanling wethers were employed in a 16-week study at
doses of 0, 10, 50, 100, 200, 400 or 800 mg/kg bw/day. Tiabendazole
was administered in gelatin capsules. Two of the four sheep dosed at
100 mg/kg bw/day did not survive the test period. However, because
of infection, tiabendazole may not have been directly related to the
cause of their death. All the animals treated at 200 mg/kg bw/day
and above died before the end of the test period. No significant
effects on blood biochemistry or urinalysis were observed. Doses of
50 mg/kg bw/day and above affected body-weight gain after
approximately 120 days on the test. Doses of 10 mg/kg bw/day had no
effect. Food consumption was unaffected at 10 mg/kg bw/day but a
minor decrease was noted at 50 and 100 mg/kg bw/day and a
significant decrease at 200 mg/kg bw/day and above. No gross
pathological changes were observed at doses below 200 mg/kg bw/day.
Starvation and loss of body weight complicated the interpretation of
data concerning organ weights at 200 mg/kg bw/day and above. At 200
to 800 mg/kg bw/day, a moderate hypoplasia of the bone marrow with
replacement by adipose tissue was observed. Loss of colloid in
thyroid was evident at 800 mg/kg bw/day, as well as lymphoid atrophy
at the higher dose levels (FAO/WHO, 1971).
Five groups of two ewe lambs (10 to 12 weeks old) and eight
wether lambs (10 to 19 weeks old) were fed diets containing 0, 100,
320, 1000 or 3200 ppm tiabendazole for periods up to 50 weeks. Sheep
10 weeks of age tolerated 1000 ppm tiabendazole in the diet for the
duration of the experiment. This dose corresponded to 30 to 50 mg/kg
bw/day. The sheep tolerated 3200 ppm in their diet for the first 14
weeks but thereafter lost weight in the final stages of the
experiment (FAO/WHO, 1971).
2.2.2.7 Pigs
Five groups of four barrows each were given a diet containing
0, 320, 1000, 3200 or 10 000 ppm tiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects
for the 14-week period. This concentration corresponded to an intake
of 15 mg/kg bw/day. The concentration of 10 000 ppm tiabendazole in
the diet caused reduced weight gain and reduced food consumption
with no mortality. Gross pathologogical examination revealed no
abnormal conditions (FAO/WHO, 1971).
2.2.2.8 Cattle
Five female holstein calves (seven months old) were fed a diet
containing 0, 320, 1000, 3200 or 10 000 ppm of tiabendazole for a
14-week test period. Calves tolerated 3200 ppm tiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg bw. At 10 000 ppm tiabendazole, the calves grew normally
for the first two weeks, but in the following 12 weeks the weight
gain was about half that observed for the controls. Gross
examination at the time of autopsy revealed no pathological
condition and histological examination of several tissues showed no
changes resulting from the incorporation of tiabendazole in the diet
(FAO/WHO,1971).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female Charles River CD-1 (HaM/ICR)
mice were fed diets containing tiabendazole (purity 99.3%) for up to
2 years. The study was commenced using dietary concentrations of 0
(three groups), 220, 660 or 2000 ppm in males and 0 (three groups),
660, 2000 or 5330 ppm in females. From the seventh week the
concentration of tiabendazole in the feed of the low-dose males and
females was reduced to 60 ppm in an effort to establish a NOEL. The
intake of tiabendazole was 5.6-8.3, 63-121 or 184-372 mg/kg bw/day
in males and 5.7-9.9, 209-368 or 534-1005 mg/kg bw/day in females.
Mortality was increased in groups given the intermediate and
high-doses. At 2000 ppm in males and females and 5330 ppm in
females, myocardial thrombosis was the primary cause of death. The
reason for decreased survival in 660 ppm males was not apparent.
When survival reached 20%, the remaining animals of the group were
sacrificed. Thus 5330 ppm females were killed in week 81, 2000 ppm
males were killed in week 85, 660 ppm males and 2000 ppm females
were killed in week 93, 60 ppm males (originally 220 ppm) were
killed in week 101 and 60 ppm females (originally 660 ppm) were
killed in week 105. A proportion of each control group was killed at
each of these sacrifice times.
Body-weight gain was depressed in 2000 ppm males and females
and 5330 ppm females. Males given 660 ppm had slightly lower weight
gain in the early and latter parts of the study. At the lowest dose,
slightly lower weight gain was noted early in the study, but
following dosage reduction, no further effect was observed. Food
consumption showed no meaningful alterations. Ophthalmoscopy
conducted at termination was unremarkable. Laboratory parameters
were not studied.
At autopsy liver weight was significantly increased in 2000 ppm
males and females and 5330 ppm females. Kidney weight was
significantly decreased in 5330 ppm females with slight decreases in
2000 ppm males and females and 660 ppm males. There were no
pathological lesions associated with the organ weight changes. A
high incidence of atrial thrombosis was seen in 2000 ppm males and
females and 5330 ppm females and was judged to be a major cause of
intercurrent mortality. Tumour incidences were unrelated to
treatment. The NOEL was 6 mg/kg bw/day (Bagdon et al., 1980).
2.2.3.2 Rats
Groups of Charles River rats (35 of each sex) were fed dietary
levels adjusted to provide 0, 10, 40 or 160 mg/kg bw/day of
tiabendazole for up to two years. At the 160 mg/kg bw/day level, a
reduction of weight gain of about 25%, with concomitant reduction in
food consumption and slightly reduced haemoglobin and haematocrit
values were the only changes attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidence or location of neoplasms and histopathological examination
of the tissues.
No effect on food consumption or haemograms were seen at either
10 or 40 mg/kg bw/day. Very slight depression in growth rate was
observed in male rats at 40 mg/kg bw/day but not at 10 mg/kg bw/day.
At 10 mg/kg bw/day, the mean absolute thyroid weights of the male
rats were heavier than the mean thyroid weight of the controls. This
effect was not observed in the rats given 40 or 160 mg/kg bw/day.
The NOEL was 10 mg/kg bw/day (FAO/WHO, 1971; Woodard, 1964).
In a carcinogenicity study, groups of 30 male and 30 female
F344/DuCrj rats were fed diets containing 0, 500, 1000, 2000 or 4000
ppm tiabendazole (purity 98.5%) for 2 years. Based on food
consumption the drug intake was calculated as 21, 43, 90 or 207
mg/kg bw/day in males and 26, 53, 112 or 237 mg/kg bw/day in
females.
There were no signs of toxicity or effects on survival. Food
intake was lower at 4000 ppm whereas body weight gain was reduced at
1000 ppm and above, leading to small and poorly developed animals at
2000 and 4000 ppm. Clinical laboratory parameters were not examined.
At the end of 2 years, all animals were necropsied. Liver
microgranulomas and aggregations of foamy cells in the lung were
increased at 1000 ppm and above. Renal epithelial hyperplasia of
papilla and pelvis were increased at 2000 and 4000 ppm. Preputial or
clitoral gland adenoma incidences were higher than control values at
4000 ppm. The increase was statistically significant (p <0.05) only
for adenomas in the preputial gland. There were no adverse effects
at 500 ppm, equal to a NOEL of 20 mg/kg bw/day (Fujii et al.,
1991).
2.2.4 Reproduction studies
2.2.4.1 Mice
A five-generation reproduction study utilized 25 male and 25
female mice given diets containing tiabendazole at concentrations of
0, 200, 1000 or 5000 ppm. When the mice attained the age of eight
weeks, they were mated and continued on the same diet. The young
from these matings, when they were weaned, were maintained on the
test diet and mated when they reached the age of eight weeks. This
procedure continued for five complete reproductive cycles. No
effects were noted at 200 ppm. At 1000 ppm, a slight decrease in the
weights of weanlings was observed in all five generations. At 5000
ppm, the number of mice born and weaned per litter was reduced and a
marked reduction in the average weanling weight of the young was
observed. The NOEL was 200 ppm, equivalent to 30 mg/kg bw/day
(FAO/WHO, 1971).
2.2.4.2 Rats
A three-generation, two-litter reproduction study (10 male and
10 female FDRL albino rats per group) at dosages of 0, 20, 40 or 80
mg/kg bw/day showed no adverse effects on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight and decreased
food consumption in the male rats at all dosage levels in the F0-
and F2-generations. Slight decreases in final body weights and
food consumption at the 80 mg/kg bw/day dose level in the F1- and
F2-generation females were also observed (FAO/WHO, 1971; Vogin,
1968)
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
A series of developmental studies were undertaken in Jcl:ICR
mice. Pregnant females were given gavage doses of tiabendazole
(purity 98.5%) in olive oil at various times during the gestation
period. Females were killed on gestation day 18.
Exp. 1: Groups of 39 mice were dosed with 0, 700, 1300 or 2400
mg/kg bw/day on gestation days 7-15. Maternal weight gain was
depressed in a dose-related manner with mortality at 1300 mg/kg
bw/day (5 out of 39) and 2400 mg/kg bw/day (24 out of 39). The
resorption rate was increased at 1300 and 2400 mg/kg bw/day. At all
treatment levels, there were reduced fetal weights and increased
malformations such as cleft palate, fusion of vertebral arches and
vertebral bodies.
Exp. 2: Groups of 7-12 mice were given a dose of 2400 mg/kg bw
on a single day of gestation between days 6 and 15. Mortality was 8
to 55% in treated groups but there were no data on body weight. In
each group, resorptions were increased and fetal body weights were
decreased. Fetal malformations detected were: microcephaly and
exencephaly (following treatment on gestation days 6 to 8); short or
absent tail and anal atresia (day 9); open eyelids (days 7, 8, 10,
13, and 14); reduction deformity of limbs and cleft palate (days 9
to 12); fusion of vertebral arches and bodies (days 7 to 10, and 13)
and fusion of ribs (days 7 to 9). The fetal abnormalities were
judged to be most prominent on gestation day 9. As the types of
malformation were diverse and primarily related to the timing of
treatment, it was suggested that tiabendazole was a non-specific
teratogen.
Exp. 3: Groups of 21 to 31 mice were given a single dose of 30
to 2400 mg/kg bw on gestation day 9. Mortality was induced at 1670
mg/kg bw and above and maternal weight gain was reduced at 1160
mg/kg bw and above. The resorption rate was increased at 1670 mg/kg
bw and above while fetal weight was lower at 60 mg/kg bw and above.
The limb reduction deformity was seen regularly at doses of 480
mg/kg bw and above; the threshold was calculated as being 360 mg/kg
bw. Fusion of vertebral arches, vertebral bodies and ribs was
increased at 240 mg/kg bw and above. Other malformations were not
consistently increased. There were no treatment-related effects at
30 mg/kg bw/day (Ogata et al., 1984).
Follow-up developmental studies were carried out in groups of
16 to 20 pregnant female Jcl:ICR mice. Tiabendazole (purity 98.5%)
was given by gavage in olive oil at doses of 0, 250, 500 or 1000
mg/kg bw/day on gestation day 9. Females were killed on gestation
day 18. Treatment at the two highest doses produced similar effects
to the previous mouse studies.
Pretreatment with SKF-525A (inhibitor of cytochrome P-450)
enhanced the rate of resorptions and the incidence of fetal
malformations. Sodium phenobarbital pretreatment, on the other hand,
resulted in decreases in the resorption rate and reduced fetal
abnormalities to control levels. Pretreatment with glutathione,
cysteine or diethyl maleate (a glutathione-depleting substance) had
no effect on resorptions. However, the incidence of fetal
malformations was increased with glutathione or cysteine and reduced
to control levels by diethyl maleate. Graphs of tissue
concentrations of tiabendazole against time showed the AUC for both
maternal blood and fetal tissue was increased by glutathione and
reduced by diethyl maleate. The overall conclusion was that the
developmental effects appear to be due to unchanged tiabendazole and
not to its degradation products (Ogata et al., 1987; 1989).
2.2.5.2 Rats
Two groups of rats (20 male and 20 female) were placed on a
diet containing 0 or 500 ppm tiabendazole. There were no
abnormalities among the young from either mating attributable to
tiabendazole at 500 ppm. It was concluded that these data presented
no evidence of teratogenecity (FAO/WHO, 1971).
In a preliminary teratology study, Charles River SD rats (8
pregnant females per group) were given gavage doses of 0 or 80 mg/kg
bw/day tiabendazole (purity unknown) on gestation days 8 to 15. Dams
were killed on gestation day 21. There were no effects on resorption
rate or on fetal survival, growth or development (Delatour et al.,
1974).
Groups of 20 pregnant female Wistar rats were administered
gavage doses of 0, 125, 250 or 500 mg/kg bw/day of a commercial
formulation containing 45% tiabendazole and 55% unknown ingredients.
Dosing was on gestation days 6-15 and dams were killed on gestation
day 22. No adverse effects were apparent in females and no
meaningful effects were reported on the resorption rate, fetal
viability, growth or development (Khera et al., 1979).
In a range-finding study, Sprague-Dawley Crl:CD(SD)BR rats were
given gavage doses of 0, 50, 100, 200 or 400 mg/kg bw/day
tiabendazole (purity 99.4%). Groups of 10 pregnant females were
treated on gestation days 6 to 17 and were killed on gestation day
20.
There were no deaths or abortions but all 200 and 400 mg/kg
bw/day females were killed due to severe body-weight effects.
Maternal weight gain was also depressed at 50 and 100 mg/kg bw/day.
Other toxic signs were lethargy and ptosis at 100 mg/kg bw/day and
above and reduced faeces at 200 mg/kg bw/day and above. Increased
protein, albumin and cholesterol were observed at the higher doses
with decreased triglycerides at all dose levels. Resorptions and
fetal morphology were unaffected but fetal weight was reduced at all
treatment levels (Lankas & Wise, 1990a).
Groups of 25 pregnant female Sprague-Dawley Crl:CD(SD)BR rats
were given gavage doses of 0, 10, 40 or 80 mg/kg bw/day tiabendazole
(purity 98.9%) suspended in 0.5% methyl cellulose. Drug
administration was on gestation days 6 to 17 and the dams were
killed on gestation day 20.
At 80 mg/kg bw/day, rats showed ptosis and a few animals
regurgitated a portion of the dose. Food intake and body-weight gain
were depressed at 40 and 80 mg/kg bw/day. There was no mortality or
abortions nor were there increases in the resorption rate or in dead
fetuses. Fetal abnormalities were not produced, but statistically
significantly decreased fetal weight was noted at 40 and 80 mg/kg
bw/day. The NOEL was 10 mg/kg bw/day (Lankas & Wise, 1990b).
2.2.5.3 Rabbits
Groups of 10 to 32 pregnant female New Zeeland white rabbits
were dosed by gavage with 0, 100, 200, 400 or 800 mg/kg bw/day
tiabendazole (purity unknown) suspended in 1% carboxymethyl
cellulose. Treatment was on gestation days 8 to 16 and does were
killed on gestation day 29 or 30.
At 200 mg/kg bw/day and above, does lost weight and the
resorption rate was increased (not dose-related). Fetal weight was
decreased at 400 and 800 mg/kg bw/day but fetal morphology was
unaltered. The NOEL was 100 mg/kg bw/day (Peck, 1966a).
In a range-finding study New Zeeland white rabbits were given
gavage doses of 0, 100, 200, 400 or 800 mg/kg bw/day tiabendazole
(purity 98.9%). Groups of 8 pregnant females were dosed on gestation
days 6 to 18 and does were killed on gestation day 29.
At 800 mg/kg bw/day, abnormal faeces were seen and weight loss
necessitated early sacrifice of this group. Food intake and weight
gain were lower at 200 and 400 mg/kg bw/day. The implantation rate
was lower at 400 mg/kg bw/day but resorptions were not increased.
External fetal examinations were unremarkable (Christian et al.,
1989).
Groups of 18 pregnant female New Zeeland white rabbits were
administered 0, 24, 120 or 600 mg/kg bw/day tiabendazole (purity
98.9%) suspended in 0.5% methyl cellulose. The gavage doses were
given on gestation days 6 to 18 and females were killed on gestation
day 29.
At 600 mg/kg bw/day one doe died, four aborted, abnormal faeces
were increased, food consumption was lower and body weight was lost.
Weight gain was slightly depressed at 120 mg/kg bw/day. There were
increased resorptions at 120 and 600 mg/kg bw/day, including total
resorption of conceptuses in 4 females at 120 mg/kg bw/day. Domed
head, hydrocephaly and enlarged fontanelles were seen in one fetus
at 120 mg/kg bw/day and two fetuses at 600 mg/kg bw/day. The NOEL
was 24 mg/kg bw/day (Hoberman & Lankas, 1989).
Groups of 18 pregnant female New Zeeland white rabbits were
given gavage doses of 0, 50, 150 or 600 mg/kg bw/day tiabendazole
(purity 98.6%) suspended in 0.5% methylcellulose. Treatment was on
gestation days 6 to 18 and dams were killed on gestation day 28.
There were no deaths or abortions related to treatment. Food
intake and body weight gain were reduced at 600 mg/kg bw/day during
the dosing period only. Resorptions were increased slightly but
significantly at 600 mg/kg bw/day. At 600 mg/kg bw/day, there were
increases in the incidences of variation in lung lobation and
incompletely ossified metacarpals. The NOEL was 150 mg/kg bw/day
(Lankas & Wise, 1991).
2.2.5.4 Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg bw
tiabendazole at 2.5 to 8 weeks prior to lambing without any effect
on the birth rate, growth or viability of the lambs. This dose had
no effect on the ewes throughout the test interval or on viability
of the lambs to six weeks of age. Miscellaneous tests using 39 ewes
produced 29 weanling lambs after treatment with tiabendazole at 400
mg/kg bw, whereas, from a control group of 22 ewes, 13 lambs were
weaned. Ewes tolerated a single dose of 18 g tiabendazole per animal
(equal to 235 to 335 mg/kg bw), three to ten days after lambing,
without any effect on the weight or food intake of the ewes or
survival and growth of the lambs up to the weaning time of six weeks
(FAO/WHO, 1971).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with tiabendazole are
summarized in Table 2.
Table 2. Results of genotoxicity studies on tiabendazole1
Test system Test object Treatment Results Reference
Reverse S.typhimurium 10-5000 negative MacGregor, 1976
mutation TA98, TA100, µg/plate
TA1535, TA1537 (+&-S9)2
Reverse S.typhimurium 100-2500 negative Shirasu et al.,
mutation TA1535, TA1537, µg/plate (-S9) 1976a.
TA1538, TA98, 10-1000
TA100, E.coli µg/plate (+S9)2
WP2 hcr-
Reverse S.typhimurium 10-2000 negative Peck, 1977
mutation TA98, TA100, µg/plate
TA1538 (+&-S9)2
TA100, TA1538 10-2000 negative
µg/plate (+S9)3
TA98 10-500 negative
µg/plate (+S9)3
1000-2000 positive
µg/plate (+S9)3
1000-2000 negative
µg/plate (+S9)3
pure tiabendazole
20-1000 positive
µg/plate (+S9)3
concentrated
impurities
Host ICR mice, 300-1000 negative Shirasu et al.,
mediated S.typhimurium mg/kg bw/day for 1976a
G46. 5 days, po
Rec. assay B.subtilis 2-1000 negative Shirasu et al.,
H17, M45 µg/disc (-S9) 1976a
DNA Rat 60-262 negative Lankas, 1989
damage hepatocytes µg/ml
Anaphase- CHO cells 0.06-0.12 negative Mudry de
telophase µg/ml (-S9) Pargament
test 0.24-0.6 positive et al., 1987
µg/ml (-S9)
Table 2. cont'd
Test system Test object Treatment Results Reference
Sister Mouse bone 50-200 negative Mudry de
chromatid marrow mg/kg bw, ip Pargament
exchange et al., 1987
Chromosome Human 2-50 negative Shirasu et al.,
aberration fibroblasts µg/ml (-S9) 1976c
Chromosome Rat bone 100-1000 negative Shirasu et al.,
aberration marrow mg/kg bw, po 1976d
30-300 negative
mg/kg bw/day
for 5 days, po
Micronucleus Mouse bone 125-500 negative Peck, 1977.
test marrow mg/kg bw, po
Micronucleus Mouse bone 50-200 positive Mudry de
test marrow mg/kg bw, ip Pargament
et al., 1987
Dominant Mice 200-600 negative Shirasu et al.,
lethal mg/kg bw/day 1976b,
test for 5 days, po
Dominant Mice 125-500 negative Peck, 1977.
lethal mg/kg bw/day
test for 5 days, po
1 Appropriate positive controls were used.
2 Liver microsomes obtained from Aroclor 1254 pretreated rats.
3 Liver microsomes obtained from phenobarbital pretreated rats.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Male albino guinea-pigs received 10 intracutaneous injections
of a 0.1% suspension of tiabendazole in 0.9% saline. Two weeks after
the induction period an intracutaneous challenge dose failed to
exacerbate reactions at the injection site (Peck, 1966b).
2.2.7.2 Rabbits
There was no evidence of a significant degree of irritation
when 0.5 g tiabendazole was applied in cold cream at concentrations
of 10 and 50% (w/w) for 24 h to intact rabbit skin (FAO/WHO, 1971).
No evidence of irritation was noted other than a very slight
erythema observed for approximately one hour after application of 1
ml of tiabendazole as a 12% suspension in sodium carboxymethyl
cellulose or 10 mg of the dry powder to the conjunctival sac of two
rabbits (FAO/WHO, 1971).
Male and female New Zeeland white rabbits received 100 mg of
tiabendazole powder instilled into the conjunctival sac of the eye.
At 15 min, there was slight conjunctival injection with a clear
discharge which returned to normal within 24 h (Merck, Sharp &
Dohme, undated).
2.2.8 Special studies on pharmacology
Oral doses of tiabendazole (4000 mg/kg bw) produced no striking
acute pharmacological effects on blood pressure or respiration in
either cats or dogs. Similarly, there were no alterations in the
electrocardiogram (FAO/WHO, 1971).
2.3 Observations in humans
A double blind study was carried out with 100 male volunteers,
half of them receiving 250 mg tiabendazole/day for 24 weeks and half
of them a placebo. Each subject was interviewed weekly and any
potential side effect was recorded. General physical examination and
laboratory examinations (haematology, cholesterol, glucose, urea,
alkaline phosphatase, thymol turbidity, bilirubin in serum and
urinalysis) were carried out before the test and after 4, 12 and 24
weeks. PBI in serum and ECGs were carried out only at the beginning
and after 24 weeks. Thirty-six subjects on tiabendazole and 41 on
placebo completed the study. As tiabendazole has a possible
influence on the thyroid the values for PBI were evaluated
carefully. No indication, however, was found of a decrease or
increase in PBI values due to tiabendazole. Under the conditions of
the study, tiabendazole was well tolerated at a dosage of 250 mg/day
during 24 weeks. No effect on any of the parameters studied could be
clearly ascribed to the drug. The NOEL was 3-4 mg/kg bw/day
(Colmore, 1965; FAO/WHO, 1978).
Twenty-three patients with trichinosis received 50 mg/kg bw/day
up to 3 g daily tiabendoazole for 10 days (total dose 25 to 30 g
tiabendazole), which was administered twice a day (after breakfast
and after evening meal). Symptoms of muscle pain and fever subsided
1 to 3 days after commencing tiabendazole. One woman withdrew due to
drug-induced rash, fever and proteinuria. Side-effects occurred in
14/23 treated patients - the most common effects being nausea,
retching and vomiting in 11/23 subjects, aversion to tablets (3/23),
exanthema (3/23), impotence (2/23), diarrhoea (1/23), liver damage
(1/23), fever (1/23) and dizziness (1/23) (Hennekeuser et al.,
1969).
3. COMMENTS
Information from a range of studies on thiabendazole was
available for assessment, including data on kinetics and metabolism,
acute toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity and observations in humans.
Toxicokinetic studies revealed rapid absorption after oral
dosing in mice, rats, dogs, and humans, peak plasma levels occurring
within 3 h of drug administration. The drug was excreted in the
urine and faeces. Elimination of tiabendazole and its degradation
products appeared more rapid in humans than in mice, rats, and dogs,
but metabolism was similar in mice, rats, and humans. The main
urinary metabolites were the glucuronide and sulfate conjugates of
5-hydroxytiabendazole; there were also small amounts of unconjugated
tiabendazole and the 5-hydroxy derivative. At very high doses, other
minor metabolites were detected in rat and mouse urine.
Single oral doses of tiabendazole were slightly toxic
(LD50 > 2000 mg/kg bw) in mice, rats, rabbits, sheep and goats.
Where deaths occurred, they appeared to be due to respiratory
failure.
Following repeated oral administration of tiabendiazole to
mice, rats, dogs, chickens, sheep, pigs, and cattle, the most common
finding was a reduction in food consumption and in body-weight gain.
The effects on weight gain appeared to be greater than could be
accounted for by the depressed food intake. Anaemia was seen in rats
and dogs, and to some extent may have been related to poor
nutrition. However, the presence of increased haemosiderosis in
lymphoid tissues and reticulocytosis in some studies was suggestive
of red cell destruction. The haematological changes occurred at dose
levels of 100 mg/kg bw/day and above for 3 months in rats and 150
mg/kg bw/day and above for 3 months in dogs.
The major target organs for toxicity were the hepatic system,
thyroid gland, and lymphoid organs. Liver hypertrophy was seen in a
3-month study in rats at doses of 37 mg/kg bw/day and above, and in
a 2-year study in dogs minor hepatobiliary changes were observed at
50 mg/kg bw/day and above. Thyroid follicular cell hyperplasia and
colloid depletion were noted in a 3-month study in rats at 37 mg/kg
bw/day and above and in a 16-week study in sheep at 800 mg/kg
bw/day. The thymus, spleen, lymph nodes, and/or bone marrow
exhibited lymphoid depletion or atrophy in a 28-day study in rats at
100 mg/kg bw/day and above and in a 16-week study in sheep at 800
mg/kg bw/day. Effects on blood cells, the liver, and lymphoid organs
are commonly observed with benzimidazoles.
Less common observations included both renal tubular
degeneration and hyperplasia in one 7-day study in mice at 250 mg/kg
bw/day and above and in one 3-month study in rats at 100 mg/kg
bw/day and above. Gastric changes, including mucosal degeneration,
cytoplasmic rarefaction, and/or necrosis, were produced in a 3-month
study in rats at 100 mg/kg bw/day and above. Atrial thrombosis was
present at high incidence in a two-year study in mice at doses of
200 mg/kg bw/day and above that resulted in the death of the
affected animals.
Tumour incidences were not increased in a two-year
carcinogenicity study in mice. The NOEL was 6 mg/kg bw/day, and
there was increased mortality at the next highest level, 60 mg/kg
bw/day.
The incidence of preputial or clitoral gland adenomas was
increased at doses above 200 mg/kg bw/day in one of two 2-year
studies in rats. Statistical significance was achieved only in
males; the animals also displayed poor development due to severe
effects on weight gain which suggests that the maximum tolerated
dose was exceeded. The relevance of these tumours to low-dose
exposure of humans to tiabendiazole is questionable. The NOEL was 10
mg/kg bw/day based on depressed weight gain at the next highest
level, 40 mg/kg bw/day.
Genotoxicity tests were generally negative. In one of three
Salmonella typhimurium reverse-mutation assays, positive results
were obtained in strain TA98 only. Further investigation showed that
an impurity in some batches was responsible for the mutagenic
activity. The manufacturer indicated that no positive results had
been obtained in tests on several hundred further batches. In one
laboratory, micronuclei were induced in mouse bone marrow and
abnormal anaphase-telophase figures were increased in cultured
Chinese hamster ovary cells. The effects were seen only at
relatively high levels and may be indicative of the tubulin-binding
activity characteristic of benzimidazoles (Annex 1, reference 97,
section 3.2.4). A range of other assays for mutation, DNA damage,
and cytogenetic activity were clearly negative.
A multigeneration reproduction study in rats showed no adverse
effects on reproduction parameters up to the highest dose tested, 80
mg/kg bw/day. In mice, litter size and survival during the lactation
period were reduced at 750 mg/kg bw/day, but reproduction was
unaffected at lower doses in a five-generation study. The NOEL was
30 mg/kg bw/day.
Developmental toxicity was examined in mice, rats, rabbits, and
sheep. In a series of studies in mice, embryotoxicity was observed
at 1300 mg/kg bw/day and above, a dose that also resulted in the
death of 5 of 39 females. Fetal malformations were produced at 240
mg/kg bw/day
and above, the main abnormalities being a reduction deformity of the
limbs, cleft palate, and fusion of vertebral arches and vertebral
bodies. Unchanged tiabendazole was implicated as the probable
embryotoxic and teratogenic agent.
The only finding in several rat developmental studies was a
reduction in fetal weight at 40 mg/kg bw/day and above. The NOEL was
10 mg/kg bw/day.
In rabbits, abortions occurred at 600 mg/kg bw/day in one study
on developmental toxicity, but not in the prior range-finding study
at higher doses or in a repeat experiment in which similar treatment
levels were used. Embryotoxicity and low incidences of domed head,
hydrocephaly, and enlarged fontanelles were seen at 120 mg/kg bw/day
and above in one study, but not in the repeat study at similar dose
levels. All effects in rabbits were seen only at maternally toxic
dose levels. The NOEL was 24 mg/kg bw/day.
No adverse reproductive effects were observed in pregnant ewes
given doses of 400 mg/kg bw/day.
In a 24-week study in humans, male volunteers received 250 mg
of tiabendazole/day; there were no treatment-related effects on a
range of physical and biochemical parameters. The NOEL was 3-4 mg/kg
bw/day. In another study, patients with trichinosis were given up to
3 g of tiabendazole daily for 10 days. Side-effects occurred in 14
of 23 patients, the most common being nausea, retching, and
vomiting.
4. EVALUATION
The Committee noted the availability of extensive data in
support of the safety evaluation of tiabendazole and the
manufacturer indicated that further studies had been completed or
were under way. At the 1977 joint FAO/WHO meeting on pesticide
residues, the study in human volunteers was used to establish an ADI
of 0-0.3 mg/kg bw. The Committee considered that the investigations
undertaken as part of this human study were limited in nature, and
that in view of the availability of a number of recent well
conducted animal studies, the NOEL should be derived from the animal
data.
The overall NOEL was 10 mg/kg bw/day based on reduced weight
gain in a 2-year dietary study in rats and decreased fetal weight in
a developmental study in rats. The Committee decided not to use the
NOEL of 6 mg/kg bw/day from the 2-year study in mice as the next
administered dose was considerably higher at 60 mg/kg bw/day.
Using a safety factor of 100, the Committee established an ADI
of 0-100 µg/kg bw for tiabendazole. The Committee noted that the ADI
provided a safety margin corresponding to a factor of over 1000 with
respect to the dose required for fetal malformations in mice.
The Committee asked to see the results from recently completed
and ongoing toxicological studies in order to update the database on
tiabendazole.
5. REFERENCES
BAGDON, W.J., BOKELMAN, D.L. & ZWICKEY, R.E. (1980). Lifetime
carcinogenic study in mice. Unpublished Report No. TT77-014-0 from
Merck, Sharp & Dohme Research Laboratories. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BATHAM, P., REID, S. & OSBORNE, B.E. (1989). A 14-week oral toxicity
study in the beagle dog. Unpublished Report No. 84021 (TT 89-9010)
from Bio-Research Laboratories, Canada. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BLASZCAK, D.L. & AULETTA, C.S. (1987). Acute dermal toxicity study
in rabbits. Unpublished Report No. 4004-86 from Bio/dynamics Inc.,
USA. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
BOKELMAN, D.L. & ZWICKEY, R.E. (1977). Tiabendazole, six-week pilot
study in mice. Unpublished Report No. TT77-004-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CAVAGNORO, J. & YAMAMOTO, K.S. (1989). 23 day dermal toxicity study
in rabbits. Unpublished Report No. HLA 284-161 (TT89-9011) from
Hazleton Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CHRISTIAN, M.S., LANKAS, G.R. & HOBERMAN, A.M. (1989). Oral
range-finding study in pregnant rabbits. Unpublished Report No.
013-029P (TT89-9006) from Argus Research Laboratories, USA.
Submitted to WHO by MSD Research Laboratory, Lauterbach, Germany.
COLMORE, J.P. (1965). Chronic toxicity study of tiabendazole in
volunteers. Unpublished report from University of Oklahoma Medical
Centre, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
DELATOUR, P., LORQUE, G., LAPRAS, M. & DESCHANEL, J.P. (1974).
Embryotoxic properties in the rat, and residues in cattle and sheep,
of three anthelmintics, derivatives of benzimidazole. Bull. Soc.
Sci. Vet. et Med., 76: 147-154.
FAO/WHO (1971). 1970 evaluations of some pesticide residues in food.
AGP: 1970/M/12/1; WHO/Food Add/71.42, Rome.
FAO/WHO (1978). Pesticide residues in food: 1977 evaluations. FAO
Plant Production and Protection Paper, 10 Sup. Rome.
FUJII, T., MIKURIYA, H. & SASAKI, M (1991). Chronic oral toxicity
and carcinogenicity study of tiabendazole in rats. Fd. Chem.
Toxicol., 29: 771-775.
FUJITANI, T., YONEYAMA, M., OGATA, A., UETA, T., MORI, K. &
ICHIKAWA, H. (1991). New metabolites of tiabendazole and the
metabolism of tiabendazole by mouse embryo in vivo and in vitro.
Fd. Chem. Toxicol., 29: 265-274.
GURMAN, J.L., HARDY, R.J., VOELKER, R.W. & DAVIDSON, J.L. (1981).
Acute inhalation toxicity study in rats. Unpublished Report No. HLA
284-129 from Hazleton Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
HENNEKEUSER, H.H., PABST, K., POEPLAU, W. & GEROK, W. (1969).
Tiabendazole for the treatment of trichinosis in humans. Texas Rep.
Biol. Med., 27 (Suppl. 2): 581-596.
HOBERMAN, A.M. & LANKAS, G.R. (1989). Oral developmental toxicity
study in rabbits. Unpublished Report No. 013-029 (TT89-9005) from
Argus Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
KANGAS, L., FARRELL, D. & OSBORNE, B.E. (1989). A 14-week oral
toxicity study in the albino rat. Unpublished Report No. 84114
(TT89-9014) from Bio-Research Laboratories, Canada. Submitted to WHO
by MSD Research Laboratory, Lauterbach, Germany.
KHERA, K.S., WHALEN, C., TRIVETT, G. & ANGERS, G. (1979).
Teratologic assessment of maleic hydrazide and daminozide, and
formulations of ethoxyquin, tiabendazole and naled in rats.
J. Environ. Sci. Health, B14: 563-577.
LANKAS, G.R. (1989). In vitro alkaline elution/rat hepatocyte
assay. Unpublished report nos. TT88-8850 and TT89-8312 from Merck,
Sharp & Dohme Research Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990a). Oral range-finding study in
pregnant rats. Unpublished Report No. TT90-713-1 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990b). Oral development toxicity study
in rats. Unpublished Report No. TT90-713-0 from Merck, Sharp & Dohme
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1991). Oral developmental toxicity study
in rabbits. Unpublished Report No. TT90-734-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
MacGREGOR, J. (1976). Mutagenicity tests of tiabendazole in the Ames
Salmonella typhimurium reverse mutation test. Unpublished report
from Western Regional Research Centre, California, USA. Submitted to
WHO by MSD Research Laboratory, Lauterbach, Germany.
MERCK, SHARP & DOHME (undated). Primary eye irritation in rabbits.
Unpublished Report No. TT81-2693 from Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
MUDRY DE PARGAMENT, M.D., LABAL DE VINUESA, M. & LARRIPA, I. (1987).
Mutagenic bioassay of certain pharmacological drugs. I Tiabendazole
(TBZ). Mutation Res., 188: 1-6.
MYERS, B.A. & LANKAS, G.R. (1990). A 14-week dietary toxicity study
in rats. Unpublished Report No. HLA 284-169 (TT 90-9002) from
Hazleton Laboratories, USA and Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
OGATA, A., ANDO, H., KUBO, Y. & HIRAGA, K. (1984). Teratogenicity of
tiabendazole in ICR mice. Fd. Chem. Toxicol., 22: 509-520.
OGATA, A., YONEYAMA, M., SASAKI, M., SUZUKI, K. & IMAMICHI, T.
(1987). Effects of pretreatment with SKF-525A or sodium
phenobarbital on tiabendazole-induced teratogenicity in ICR mice.
Fd. Chem. Toxicol., 25: 119-124.
OGATA, A., FUJITANI, T., YONEYAMA, M., SASAKI, M. & SUZUKI, K.
(1989). Glutathione and cysteine enhance and diethylmaleate reduces
tiabendazole teratogenicity in mice. Fd. Chem. Toxicol., 27:
117-123.
PECK, H.M. (1966a). Reproduction study in the rabbit. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
PECK, H.M. (1966b). Cutaneous sensitisation in the guinea pig.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
PECK, H.M. (1977). Mutagenic evaluation. Unpublished report from
Merck, Sharp & Dohme. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
ROBINSON, H.J. (1964). Tiabendazole preclinical evaluation.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
ROBINSON, H.J. (1965). Tiabendazole safety evaluation. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
SHIRASU, Y., MORIYA, M. & KATO, K. (1976a). Mutagenicity testing on
tiabendazole (TBZ) in microbial systems. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TERAMOTO, S. & KANEDA, M. (1976b). Dominant lethal
studies with tiabendazole in mice. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976c).
Cytogenetic studies with tiabendazole in cultured human fibroblasts.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976d).
Cytogenetic studies with tiabendazole in rat bone marrow cells.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
TADA, Y., YONEYAMA, M., KABASHIMA, J., FUJITANI, T. & NAKANO, M.
(1989). Effects of tiabendazole on the kidneys of ICR mice.
Fd. Chem. Toxicol., 27: 307-315.
TSUCHIYA, T., TANAKA, A., FUKUOKA, M., SATO, M. & YAMAHA, T. (1987).
Metabolism of tiabendazole and teratogenic potential of its
metabolites in pregnant mice. Chem. Pharm. Bull., 35: 2985-2993.
USUI, T. & SAKAGUCHI, T. (1989). 5 week oral toxicity study in rats.
Unpublished report nos. TT86-9819 and TT87-9809 from Banyu
Pharmaceutical Development Research Laboratories, Japan. Submitted
to WHO by MSD Research Laboratory, Lauterbach, Germany.
VOGIN, E.E. (1968). Multigeneration reproduction and lactation
studies with tiabendazole. Unpublished report from Food and Drug
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
WOODARD, G. (1964). Safety evaluation by oral administration to dogs
and rats for 104 weeks. Unpublished report from Woodard Research
Corporation, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
YONEYAMA, M., OGATA, A., FUJII, T. & HIRAGA, K. (1984). The
maternal-foetal distribution of tiabendazole administered in two
different vehicles to pregnant mice. Fd. Chem. Toxicol., 22:
731-735.
2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with tiabendazole are
summarized in Table 1.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 2400-3810 FAO/WHO, 1971
M&F ip 430 FAO/WHO,1971
M&F iv 150 FAO/WHO, 1971
Rat M&F oral 3330-3600 FAO/WHO, 1971
M&F ip 1850 FAO/WHO, 1971
M&F iv 130 FAO/WHO, 1971
M&F inhalation >397 mg/m3 Gurman et al., 1981
Rabbit M&F oral 3850 FAO/WHO, 1971
M&F dermal >2000 Blaszcak & Auletta,
1987
Sheep ? drench 2000 FAO/WHO, 1971
Goat ? drench >2000 FAO/WHO, 1971
Toxic signs following administration of large doses of
tiabendazole by oral or intraperitoneal routes were generally
similar and consisted of lethargy and stupor. The intravenous
administration of a large dose of tiabendazole hydrochloride
produced narcosis. Death appeared to be due to respiratory failure.
Rabbits that survived large oral doses initially lost weight but
recovered after several days.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In an investigation of renal function, Crj:CD-1 (ICR) mice were
given gavage doses of 0, 250 or 500 mg/kg bw/day tiabendazole
(purity 98.5%) in olive oil. Groups of 6 to 8 males and females were
killed after dosing for 1, 3, 5 or 7 days. There was no effect on
body weight. Water intake was unaffected but urine volume was
increased in all treated groups, significantly so in males given 500
mg/kg bw/day.
There were no meaningful effects on serum creatinine or urea
nitrogen, urinary glucose or protein or the presence of occult blood
in urine. Electrophoresis however, revealed relatively high
molecular weight protein in treated mice, while low molecular weight
protein was found in all groups. There was a progressive
dose-related increase in renal lesions including tubular dilatation
and degenerative desquamation, cell infiltration, fibrosis and
regeneration of tubular epithelium. Electron microscopy showed
evidence of glomerular damage such as flattening of the foot
processes of podocytes and oedematous changes in the mesangium in
treated mice (Tada et al., 1989).
In a 6-week pilot study, groups of 10 male and 10 female
Charles River CD-1 (HaM/ICR) mice were given 0, 50, 150, 300, 600 or
900 mg/kg bw/day tiabendazole (purity unknown) via the diet. There
were no clinical signs or mortality. Food intake and weight gain
were depressed in males at 600 and 900 mg/kg bw/day. Other
parameters were not monitored (Bokelman & Zwickey, 1977).
2.2.2.2 Rats
Rats (Sprague-Dawley Holtzman, 10 males and 10 females per
group) were administered tiabendazole by gavage at dosage levels of
0, 100, 400, 800, 1200 or 1600 mg/kg bw/day. During the 30 day
experimental period, rats in the 800 mg/kg bw/day group decreased
their food intake and gradually lost weight. They were slightly less
active, had ruffled fur and became slightly flaccid; three males and
three females died during the course of the experiment. All of the
rats in the 1200 and 1600 mg/kg bw/day groups died during the 30-day
test period. Rats dosed at 100 and 400 mg/kg bw/day appeared normal
throughout the test period, although a slight depression in
body-weight gain was noted in males at 100 mg/kg bw/day and in
females at 400 mg/kg bw/day. Higher doses produced greater effects
on body-weight gain. Haematologic studies on rats receiving 800
mg/kg bw/day showed a mild neutrophilia with concurrent lymphopenia.
There was also a suggestion of a decrease in red blood cell elements
at 400 mg/kg bw/day and above. Blood biochemistry showed no
meaningful changes.
No gross anatomical changes were noted in rats dosed with 100
mg/kg bw/day, whereas male and female rats treated with 400 mg/kg
bw/day showed thymic involution. A slight enlargement of the liver
was also noted in females at 400 mg/kg bw/day. Animals surviving 800
mg/kg bw/day showed gross signs indicating starvation; normal body
fat depots and subcutaneous fat appeared to be depleted. The liver
and adrenals were slightly enlarged in males and females and the
thymus was involuted. Microscopic pathology showed bone marrow
hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964).
Groups of 12 male and 12 female Albino Crj:CD (SD) rats were
administered gavage doses of 0, 50, 100, 200 or 800 mg/kg bw/day
thiabendazole (purity 100%), suspended in 0.5% methylcellulose, for
28 to 31 days. Severe toxicity was observed at 800 mg/kg bw/day in
the form of decreased activity, sedation, ataxia, recumbency,
flaccidity, loss of righting reflex, piloerection, emaciation, rough
hair coat, alopecia and a high level of mortality. Food intake and
body-weight gain were depressed at 200 and 800 mg/kg bw/day.
Laboratory analyses were carried out in week 4. Serum albumin,
total protein and cholesterol were increased and erythrocytes,
haemoglobin and haematocrit were decreased at 200 and 800 mg/kg
bw/day with reticulocytosis and leucocytopenia at the higher level.
Urinalysis was unchanged. Autopsy revealed changes at all dose
levels. There was haemosiderosis and increased extramedullary
haematopoiesis in the spleen at all doses with increased spleen
weight at 200 mg/kg bw/day and above. Increased thyroid weight and
follicular cell hyperplasia and decreased thymus weight were seen at
50 mg/kg bw/day and above. Lymphoid depletion was observed in the
thymus at 100 mg/kg bw/day and above, in bone marrow at 200 mg/kg
bw/day and above and in spleen and lymph nodes at 800 mg/kg bw/day.
Liver weight and centrilobular hepatocytic hypertrophy were
increased at 100 mg/kg bw/day and above (Usui & Sakaguchi, 1989).
Groups of 20 male and 20 female Sprague-Dawley Crl:CD (SD) BR
rats were given gavage doses of 0, 25, 100 or 400 mg/kg bw/day
tiabendazole (purity not stated), suspended in 0.5% methyl
cellulose, for 13 weeks. Alopecia was noted in some rats of each
treatment group. There was no mortality. Body-weight gain was
reduced at 100 and 400 mg/kg bw/day with lower food intake in 400
mg/kg bw/day males. Ophthalmology at the end of study was
unremarkable.
Clinical laboratory parameters were studied in weeks 6 and 13.
Decreased erythrocytes, haemoglobin and haematocrit and increased
cholesterol were induced at 100 and 400 mg/kg bw/day. Urinary levels
of bilirubin, urobilinogen and nitrite were increased at 400 mg/kg
bw/day. At 100 and 400 mg/kg bw/day, increased liver weight and
centrilobular hypertrophy, enlarged thyroids with follicular cell
hyperplasia and splenic congestion and pigmentation were observed.
In the kidney, renal calculus and transitional epithelial
hyperplasia were evident at 100 and 400 mg/kg bw/day with tubular
degeneration at the highest level. The stomach exhibited acanthosis
and/or degeneration of non-glandular mucosa at 100 and 400 mg/kg
bw/day and cytoplasmic rarefaction and necrosis of glandular mucosa
at 400 mg/kg bw/day. The NOEL was 25 mg/kg bw/day (Kangas et al.,
1989).
Groups of 10 male and 10 female Sprague-Dawley Crl:CD BR rats
were fed diets containing tiabendazole (purity 99.4%) for 13 weeks.
Based on food intake the calculated doses were 0, 9, 37, 150 or 302
mg/kg bw/day. There was no effect on survival or on the eyes at the
end of study. Alopecia was increased at 150 and 302 mg/kg bw/day.
Doses of 37 mg/kg bw/day and above were associated with decreased
body-weight gain and food intake in males; females were similarly
affected at 150 mg/kg bw/day and above.
Clinical laboratory analyses were undertaken in weeks 6 and 13.
At doses of 150 and 302 mg/kg bw/day serum cholesterol was
increased, glucose, erythrocytes, haemoglobin and haematocrit were
depressed and there were increased numbers of abnormal erythrocytes.
Urinalysis was unaffected. Liver weight increases and centrilobular
hypertrophy, increased thyroid weight and follicular cell
hypertrophy and bone marrow erythroid hyperplasia were observed at
37 mg/kg bw/day and above. At 150 and 302 mg/kg bw/day there was
spleen pigmentation and thymus atrophy with skeletal muscle atrophy
in females given 302 mg/kg bw/day. The NOEL was 9 mg/kg bw/day
(Myers & Lankas, 1990).
Rats (Sprague-Dawley Holtzman, 30 males and 30 females per
group) were administered tiabendazole, suspended in 1% methocel, by
gavage for 180 days, at doses of 0, 12.5, 25, 50, 100, 200 or 400
mg/kg bw/day. All of the animals survived throughout the duration of
the experiment. At 400 mg/kg bw/day, a depression in body weight was
observed in both sexes. At 200 mg/kg bw/day, the male rats showed a
slight depression in weight gain. No clinical signs of toxicity were
observed. Haematological studies at 400 mg/kg bw/day indicated a
slight suggestion of a fall of the red blood cell elements,
neutrophilia and lymphopenia which were not evident at levels below
this dose. Blood biochemistry studies and urinalysis were normal at
all dosage levels with a slight polyuria in both male and female
rats at 400 mg/kg bw/day, which was apparently the result of a
slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and
females at 400 mg/kg bw/day and in females at 200 mg/kg bw/day.
There was an increase in liver size at doses of 100 mg/kg bw/day and
above in males and at 50 mg/kg bw/day and above in females. Males
dosed with 200 and 400 mg/kg bw/day and females at 400 mg/kg bw/day
showed an apparent increase in kidney weight. Microscopic
examination of animals at 100 mg/kg bw/day showed a small incidence
of haemosiderosis of the thymus. At 200 and 400 mg/kg bw/day, these
examinations showed considerably more haemosiderosis of the thymus
and colloid depletion in the thyroid. The NOEL was 25 mg/kg bw/day
(FAO/WHO, 1971; Robinson, 1964).
2.2.2.3 Rabbits
Groups of 5 male and 5 female New Zeeland white rabbits
received dermal doses of tiabendazole (purity 98.9%) moistened in
0.9% saline. The drug was applied to the shaved trunk of animals at
doses of 0, 50, 200 or 1000 mg/kg bw, 6 h/day for 3 weeks. Oral
ingestion was prevented by use of a collar. Clinical laboratory
parameters and pathology were investigated at the end of the study.
There was no evidence of local or systemic toxicity (Cavagnaro &
Yamamoto, 1989).
2.2.2.4 Dogs
Groups of 4 male and 4 female beagle dogs were given capsules
of tiabendazole (purity 99.4%) at doses of 0, 35, 75 or 150 mg/kg
bw/day for 14 weeks. Emesis was increased at 75 and 150 mg/kg bw/day
with salivation at 150 mg/kg bw/day. There were no deaths. During
the first 2 weeks of treatment, food consumption and body-weight
gain were decreased in all treated groups. Subsequently, food was
made available overnight rather than for 1 h per day which led to
comparable food intake and weight gain in all groups.
Ophthalmology and ECG examinations were normal throughout the
study. Red blood cells, haemoglobin and haematocrit were depressed
at 150 mg/kg bw/day, more so at weeks 4 and 8 than at week 12. Blood
chemistry and urinalysis were unchanged at any time. Relative but
not absolute liver weights were slightly increased in all treated
groups but with no dose-relationship. At 75 and 150 mg/kg bw/day,
gallbladder cytoplasmic vacuolation was increased in incidence and
severity. The NOEL was 35 mg/kg bw/day (Batham et al., 1989).
Groups comprising two male and two female beagle dogs were
orally administered tiabendazole for periods of over two years at
doses of 0, 20, 100 or 200 mg/kg bw/day. No clinical signs or
morbidity were observed, food and water consumption were normal and
blood and urine chemistry were normal. A slight retardation in
body-weight gain was observed at 200 mg/kg bw/day, which was
accompanied by a slight reduction in total erythrocyte count. At
this level, changes in haemoglobin concentration and haematocrit
were also observed.
No gross pathological observations were noted at the conclusion
of this test and organ weights appeared normal. A significant
haemosiderosis was present in dogs at 100 and 200 mg/kg bw/day in
the spleen, liver, lymph nodes and bone marrow. This was not
associated with an increase in serum haemoglobin. The NOEL was 20
mg/kg bw/day (FAO/WHO, 1971; Robinson, 1964)
Groups of beagle dogs (three males and three females) were
orally administered tiabendazole in capsules for two years at doses
of 0, 20, 50 or 125 mg/kg bw/day. At 125 mg/kg bw/day, two of six
dogs died. One of these dogs had marked liver cirrhosis, seminal
tubular degeneration, bone marrow atrophy and degenerative renal
changes. Slight to moderate reduction in haemoglobin and packed cell
volume occurred. Elevated blood urea nitrogen, serum alkaline
phosphatase and serum GOT and chronic inflammatory liver changes
were evident. Urinary albumin was seen more frequently than in
controls. Inspissated bile entwined in the gall bladder villi was
also observed in one dog. One dog of this group was sacrificed and
was found to have pulmonary arteritis of parasitic origin.
At 50 mg/kg bw/day, growth of male dogs was slightly depressed.
All six dogs given tiabendazole at 50 mg/kg bw/day survived the
study and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa
in one. Five of six dogs given tiabendazole at 20 mg/kg bw/day
survived the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes
in all treated dogs. The NOEL was 20 mg/kg bw/day (FAO/WHO, 1971;
Woodard, 1964).
2.2.2.5 Chickens
Groups of 0.5 week-old male white leghorn chicks (10 to 20 per
group) were fed tiabendazole in the diet at dosage levels ranging
from 1 to 10 000 ppm for a period of 2 1/2 weeks. This level
corresponded to a mean daily intake of tiabendazole ranging from 0.1
to 1200 mg/kg bw. A gradual decrease in growth appeared to occur
with concentrations of 100 ppm tiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg bw/day. Gross pathological
examination of the chicks at a dietary level of 4000 ppm
tiabendazole showed them to be normal except for a smaller size
(FAO/WHO, 1971).
2.2.2.6 Sheep
Thirty weanling wethers were employed in a 16-week study at
doses of 0, 10, 50, 100, 200, 400 or 800 mg/kg bw/day. Tiabendazole
was administered in gelatin capsules. Two of the four sheep dosed at
100 mg/kg bw/day did not survive the test period. However, because
of infection, tiabendazole may not have been directly related to the
cause of their death. All the animals treated at 200 mg/kg bw/day
and above died before the end of the test period. No significant
effects on blood biochemistry or urinalysis were observed. Doses of
50 mg/kg bw/day and above affected body-weight gain after
approximately 120 days on the test. Doses of 10 mg/kg bw/day had no
effect. Food consumption was unaffected at 10 mg/kg bw/day but a
minor decrease was noted at 50 and 100 mg/kg bw/day and a
significant decrease at 200 mg/kg bw/day and above. No gross
pathological changes were observed at doses below 200 mg/kg bw/day.
Starvation and loss of body weight complicated the interpretation of
data concerning organ weights at 200 mg/kg bw/day and above. At 200
to 800 mg/kg bw/day, a moderate hypoplasia of the bone marrow with
replacement by adipose tissue was observed. Loss of colloid in
thyroid was evident at 800 mg/kg bw/day, as well as lymphoid atrophy
at the higher dose levels (FAO/WHO, 1971).
Five groups of two ewe lambs (10 to 12 weeks old) and eight
wether lambs (10 to 19 weeks old) were fed diets containing 0, 100,
320, 1000 or 3200 ppm tiabendazole for periods up to 50 weeks. Sheep
10 weeks of age tolerated 1000 ppm tiabendazole in the diet for the
duration of the experiment. This dose corresponded to 30 to 50 mg/kg
bw/day. The sheep tolerated 3200 ppm in their diet for the first 14
weeks but thereafter lost weight in the final stages of the
experiment (FAO/WHO, 1971).
2.2.2.7 Pigs
Five groups of four barrows each were given a diet containing
0, 320, 1000, 3200 or 10 000 ppm tiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects
for the 14-week period. This concentration corresponded to an intake
of 15 mg/kg bw/day. The concentration of 10 000 ppm tiabendazole in
the diet caused reduced weight gain and reduced food consumption
with no mortality. Gross pathologogical examination revealed no
abnormal conditions (FAO/WHO, 1971).
2.2.2.8 Cattle
Five female holstein calves (seven months old) were fed a diet
containing 0, 320, 1000, 3200 or 10 000 ppm of tiabendazole for a
14-week test period. Calves tolerated 3200 ppm tiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg bw. At 10 000 ppm tiabendazole, the calves grew normally
for the first two weeks, but in the following 12 weeks the weight
gain was about half that observed for the controls. Gross
examination at the time of autopsy revealed no pathological
condition and histological examination of several tissues showed no
changes resulting from the incorporation of tiabendazole in the diet
(FAO/WHO,1971).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female Charles River CD-1 (HaM/ICR)
mice were fed diets containing tiabendazole (purity 99.3%) for up to
2 years. The study was commenced using dietary concentrations of 0
(three groups), 220, 660 or 2000 ppm in males and 0 (three groups),
660, 2000 or 5330 ppm in females. From the seventh week the
concentration of tiabendazole in the feed of the low-dose males and
females was reduced to 60 ppm in an effort to establish a NOEL. The
intake of tiabendazole was 5.6-8.3, 63-121 or 184-372 mg/kg bw/day
in males and 5.7-9.9, 209-368 or 534-1005 mg/kg bw/day in females.
Mortality was increased in groups given the intermediate and
high-doses. At 2000 ppm in males and females and 5330 ppm in
females, myocardial thrombosis was the primary cause of death. The
reason for decreased survival in 660 ppm males was not apparent.
When survival reached 20%, the remaining animals of the group were
sacrificed. Thus 5330 ppm females were killed in week 81, 2000 ppm
males were killed in week 85, 660 ppm males and 2000 ppm females
were killed in week 93, 60 ppm males (originally 220 ppm) were
killed in week 101 and 60 ppm females (originally 660 ppm) were
killed in week 105. A proportion of each control group was killed at
each of these sacrifice times.
Body-weight gain was depressed in 2000 ppm males and females
and 5330 ppm females. Males given 660 ppm had slightly lower weight
gain in the early and latter parts of the study. At the lowest dose,
slightly lower weight gain was noted early in the study, but
following dosage reduction, no further effect was observed. Food
consumption showed no meaningful alterations. Ophthalmoscopy
conducted at termination was unremarkable. Laboratory parameters
were not studied.
At autopsy liver weight was significantly increased in 2000 ppm
males and females and 5330 ppm females. Kidney weight was
significantly decreased in 5330 ppm females with slight decreases in
2000 ppm males and females and 660 ppm males. There were no
pathological lesions associated with the organ weight changes. A
high incidence of atrial thrombosis was seen in 2000 ppm males and
females and 5330 ppm females and was judged to be a major cause of
intercurrent mortality. Tumour incidences were unrelated to
treatment. The NOEL was 6 mg/kg bw/day (Bagdon et al., 1980).
2.2.3.2 Rats
Groups of Charles River rats (35 of each sex) were fed dietary
levels adjusted to provide 0, 10, 40 or 160 mg/kg bw/day of
tiabendazole for up to two years. At the 160 mg/kg bw/day level, a
reduction of weight gain of about 25%, with concomitant reduction in
food consumption and slightly reduced haemoglobin and haematocrit
values were the only changes attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidence or location of neoplasms and histopathological examination
of the tissues.
No effect on food consumption or haemograms were seen at either
10 or 40 mg/kg bw/day. Very slight depression in growth rate was
observed in male rats at 40 mg/kg bw/day but not at 10 mg/kg bw/day.
At 10 mg/kg bw/day, the mean absolute thyroid weights of the male
rats were heavier than the mean thyroid weight of the controls. This
effect was not observed in the rats given 40 or 160 mg/kg bw/day.
The NOEL was 10 mg/kg bw/day (FAO/WHO, 1971; Woodard, 1964).
In a carcinogenicity study, groups of 30 male and 30 female
F344/DuCrj rats were fed diets containing 0, 500, 1000, 2000 or 4000
ppm tiabendazole (purity 98.5%) for 2 years. Based on food
consumption the drug intake was calculated as 21, 43, 90 or 207
mg/kg bw/day in males and 26, 53, 112 or 237 mg/kg bw/day in
females.
There were no signs of toxicity or effects on survival. Food
intake was lower at 4000 ppm whereas body weight gain was reduced at
1000 ppm and above, leading to small and poorly developed animals at
2000 and 4000 ppm. Clinical laboratory parameters were not examined.
At the end of 2 years, all animals were necropsied. Liver
microgranulomas and aggregations of foamy cells in the lung were
increased at 1000 ppm and above. Renal epithelial hyperplasia of
papilla and pelvis were increased at 2000 and 4000 ppm. Preputial or
clitoral gland adenoma incidences were higher than control values at
4000 ppm. The increase was statistically significant (p <0.05) only
for adenomas in the preputial gland. There were no adverse effects
at 500 ppm, equal to a NOEL of 20 mg/kg bw/day (Fujii et al.,
1991).
2.2.4 Reproduction studies
2.2.4.1 Mice
A five-generation reproduction study utilized 25 male and 25
female mice given diets containing tiabendazole at concentrations of
0, 200, 1000 or 5000 ppm. When the mice attained the age of eight
weeks, they were mated and continued on the same diet. The young
from these matings, when they were weaned, were maintained on the
test diet and mated when they reached the age of eight weeks. This
procedure continued for five complete reproductive cycles. No
effects were noted at 200 ppm. At 1000 ppm, a slight decrease in the
weights of weanlings was observed in all five generations. At 5000
ppm, the number of mice born and weaned per litter was reduced and a
marked reduction in the average weanling weight of the young was
observed. The NOEL was 200 ppm, equivalent to 30 mg/kg bw/day
(FAO/WHO, 1971).
2.2.4.2 Rats
A three-generation, two-litter reproduction study (10 male and
10 female FDRL albino rats per group) at dosages of 0, 20, 40 or 80
mg/kg bw/day showed no adverse effects on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight and decreased
food consumption in the male rats at all dosage levels in the F0-
and F2-generations. Slight decreases in final body weights and
food consumption at the 80 mg/kg bw/day dose level in the F1- and
F2-generation females were also observed (FAO/WHO, 1971; Vogin,
1968)
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
A series of developmental studies were undertaken in Jcl:ICR
mice. Pregnant females were given gavage doses of tiabendazole
(purity 98.5%) in olive oil at various times during the gestation
period. Females were killed on gestation day 18.
Exp. 1: Groups of 39 mice were dosed with 0, 700, 1300 or 2400
mg/kg bw/day on gestation days 7-15. Maternal weight gain was
depressed in a dose-related manner with mortality at 1300 mg/kg
bw/day (5 out of 39) and 2400 mg/kg bw/day (24 out of 39). The
resorption rate was increased at 1300 and 2400 mg/kg bw/day. At all
treatment levels, there were reduced fetal weights and increased
malformations such as cleft palate, fusion of vertebral arches and
vertebral bodies.
Exp. 2: Groups of 7-12 mice were given a dose of 2400 mg/kg bw
on a single day of gestation between days 6 and 15. Mortality was 8
to 55% in treated groups but there were no data on body weight. In
each group, resorptions were increased and fetal body weights were
decreased. Fetal malformations detected were: microcephaly and
exencephaly (following treatment on gestation days 6 to 8); short or
absent tail and anal atresia (day 9); open eyelids (days 7, 8, 10,
13, and 14); reduction deformity of limbs and cleft palate (days 9
to 12); fusion of vertebral arches and bodies (days 7 to 10, and 13)
and fusion of ribs (days 7 to 9). The fetal abnormalities were
judged to be most prominent on gestation day 9. As the types of
malformation were diverse and primarily related to the timing of
treatment, it was suggested that tiabendazole was a non-specific
teratogen.
Exp. 3: Groups of 21 to 31 mice were given a single dose of 30
to 2400 mg/kg bw on gestation day 9. Mortality was induced at 1670
mg/kg bw and above and maternal weight gain was reduced at 1160
mg/kg bw and above. The resorption rate was increased at 1670 mg/kg
bw and above while fetal weight was lower at 60 mg/kg bw and above.
The limb reduction deformity was seen regularly at doses of 480
mg/kg bw and above; the threshold was calculated as being 360 mg/kg
bw. Fusion of vertebral arches, vertebral bodies and ribs was
increased at 240 mg/kg bw and above. Other malformations were not
consistently increased. There were no treatment-related effects at
30 mg/kg bw/day (Ogata et al., 1984).
Follow-up developmental studies were carried out in groups of
16 to 20 pregnant female Jcl:ICR mice. Tiabendazole (purity 98.5%)
was given by gavage in olive oil at doses of 0, 250, 500 or 1000
mg/kg bw/day on gestation day 9. Females were killed on gestation
day 18. Treatment at the two highest doses produced similar effects
to the previous mouse studies.
Pretreatment with SKF-525A (inhibitor of cytochrome P-450)
enhanced the rate of resorptions and the incidence of fetal
malformations. Sodium phenobarbital pretreatment, on the other hand,
resulted in decreases in the resorption rate and reduced fetal
abnormalities to control levels. Pretreatment with glutathione,
cysteine or diethyl maleate (a glutathione-depleting substance) had
no effect on resorptions. However, the incidence of fetal
malformations was increased with glutathione or cysteine and reduced
to control levels by diethyl maleate. Graphs of tissue
concentrations of tiabendazole against time showed the AUC for both
maternal blood and fetal tissue was increased by glutathione and
reduced by diethyl maleate. The overall conclusion was that the
developmental effects appear to be due to unchanged tiabendazole and
not to its degradation products (Ogata et al., 1987; 1989).
2.2.5.2 Rats
Two groups of rats (20 male and 20 female) were placed on a
diet containing 0 or 500 ppm tiabendazole. There were no
abnormalities among the young from either mating attributable to
tiabendazole at 500 ppm. It was concluded that these data presented
no evidence of teratogenecity (FAO/WHO, 1971).
In a preliminary teratology study, Charles River SD rats (8
pregnant females per group) were given gavage doses of 0 or 80 mg/kg
bw/day tiabendazole (purity unknown) on gestation days 8 to 15. Dams
were killed on gestation day 21. There were no effects on resorption
rate or on fetal survival, growth or development (Delatour et al.,
1974).
Groups of 20 pregnant female Wistar rats were administered
gavage doses of 0, 125, 250 or 500 mg/kg bw/day of a commercial
formulation containing 45% tiabendazole and 55% unknown ingredients.
Dosing was on gestation days 6-15 and dams were killed on gestation
day 22. No adverse effects were apparent in females and no
meaningful effects were reported on the resorption rate, fetal
viability, growth or development (Khera et al., 1979).
In a range-finding study, Sprague-Dawley Crl:CD(SD)BR rats were
given gavage doses of 0, 50, 100, 200 or 400 mg/kg bw/day
tiabendazole (purity 99.4%). Groups of 10 pregnant females were
treated on gestation days 6 to 17 and were killed on gestation day
20.
There were no deaths or abortions but all 200 and 400 mg/kg
bw/day females were killed due to severe body-weight effects.
Maternal weight gain was also depressed at 50 and 100 mg/kg bw/day.
Other toxic signs were lethargy and ptosis at 100 mg/kg bw/day and
above and reduced faeces at 200 mg/kg bw/day and above. Increased
protein, albumin and cholesterol were observed at the higher doses
with decreased triglycerides at all dose levels. Resorptions and
fetal morphology were unaffected but fetal weight was reduced at all
treatment levels (Lankas & Wise, 1990a).
Groups of 25 pregnant female Sprague-Dawley Crl:CD(SD)BR rats
were given gavage doses of 0, 10, 40 or 80 mg/kg bw/day tiabendazole
(purity 98.9%) suspended in 0.5% methyl cellulose. Drug
administration was on gestation days 6 to 17 and the dams were
killed on gestation day 20.
At 80 mg/kg bw/day, rats showed ptosis and a few animals
regurgitated a portion of the dose. Food intake and body-weight gain
were depressed at 40 and 80 mg/kg bw/day. There was no mortality or
abortions nor were there increases in the resorption rate or in dead
fetuses. Fetal abnormalities were not produced, but statistically
significantly decreased fetal weight was noted at 40 and 80 mg/kg
bw/day. The NOEL was 10 mg/kg bw/day (Lankas & Wise, 1990b).
2.2.5.3 Rabbits
Groups of 10 to 32 pregnant female New Zeeland white rabbits
were dosed by gavage with 0, 100, 200, 400 or 800 mg/kg bw/day
tiabendazole (purity unknown) suspended in 1% carboxymethyl
cellulose. Treatment was on gestation days 8 to 16 and does were
killed on gestation day 29 or 30.
At 200 mg/kg bw/day and above, does lost weight and the
resorption rate was increased (not dose-related). Fetal weight was
decreased at 400 and 800 mg/kg bw/day but fetal morphology was
unaltered. The NOEL was 100 mg/kg bw/day (Peck, 1966a).
In a range-finding study New Zeeland white rabbits were given
gavage doses of 0, 100, 200, 400 or 800 mg/kg bw/day tiabendazole
(purity 98.9%). Groups of 8 pregnant females were dosed on gestation
days 6 to 18 and does were killed on gestation day 29.
At 800 mg/kg bw/day, abnormal faeces were seen and weight loss
necessitated early sacrifice of this group. Food intake and weight
gain were lower at 200 and 400 mg/kg bw/day. The implantation rate
was lower at 400 mg/kg bw/day but resorptions were not increased.
External fetal examinations were unremarkable (Christian et al.,
1989).
Groups of 18 pregnant female New Zeeland white rabbits were
administered 0, 24, 120 or 600 mg/kg bw/day tiabendazole (purity
98.9%) suspended in 0.5% methyl cellulose. The gavage doses were
given on gestation days 6 to 18 and females were killed on gestation
day 29.
At 600 mg/kg bw/day one doe died, four aborted, abnormal faeces
were increased, food consumption was lower and body weight was lost.
Weight gain was slightly depressed at 120 mg/kg bw/day. There were
increased resorptions at 120 and 600 mg/kg bw/day, including total
resorption of conceptuses in 4 females at 120 mg/kg bw/day. Domed
head, hydrocephaly and enlarged fontanelles were seen in one fetus
at 120 mg/kg bw/day and two fetuses at 600 mg/kg bw/day. The NOEL
was 24 mg/kg bw/day (Hoberman & Lankas, 1989).
Groups of 18 pregnant female New Zeeland white rabbits were
given gavage doses of 0, 50, 150 or 600 mg/kg bw/day tiabendazole
(purity 98.6%) suspended in 0.5% methylcellulose. Treatment was on
gestation days 6 to 18 and dams were killed on gestation day 28.
There were no deaths or abortions related to treatment. Food
intake and body weight gain were reduced at 600 mg/kg bw/day during
the dosing period only. Resorptions were increased slightly but
significantly at 600 mg/kg bw/day. At 600 mg/kg bw/day, there were
increases in the incidences of variation in lung lobation and
incompletely ossified metacarpals. The NOEL was 150 mg/kg bw/day
(Lankas & Wise, 1991).
2.2.5.4 Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg bw
tiabendazole at 2.5 to 8 weeks prior to lambing without any effect
on the birth rate, growth or viability of the lambs. This dose had
no effect on the ewes throughout the test interval or on viability
of the lambs to six weeks of age. Miscellaneous tests using 39 ewes
produced 29 weanling lambs after treatment with tiabendazole at 400
mg/kg bw, whereas, from a control group of 22 ewes, 13 lambs were
weaned. Ewes tolerated a single dose of 18 g tiabendazole per animal
(equal to 235 to 335 mg/kg bw), three to ten days after lambing,
without any effect on the weight or food intake of the ewes or
survival and growth of the lambs up to the weaning time of six weeks
(FAO/WHO, 1971).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with tiabendazole are
summarized in Table 2.
Table 2. Results of genotoxicity studies on tiabendazole1
Test system Test object Treatment Results Reference
Reverse S.typhimurium 10-5000 negative MacGregor, 1976
mutation TA98, TA100, µg/plate
TA1535, TA1537 (+&-S9)2
Reverse S.typhimurium 100-2500 negative Shirasu et al.,
mutation TA1535, TA1537, µg/plate (-S9) 1976a.
TA1538, TA98, 10-1000
TA100, E.coli µg/plate (+S9)2
WP2 hcr-
Reverse S.typhimurium 10-2000 negative Peck, 1977
mutation TA98, TA100, µg/plate
TA1538 (+&-S9)2
TA100, TA1538 10-2000 negative
µg/plate (+S9)3
TA98 10-500 negative
µg/plate (+S9)3
1000-2000 positive
µg/plate (+S9)3
1000-2000 negative
µg/plate (+S9)3
pure tiabendazole
20-1000 positive
µg/plate (+S9)3
concentrated
impurities
Host ICR mice, 300-1000 negative Shirasu et al.,
mediated S.typhimurium mg/kg bw/day for 1976a
G46. 5 days, po
Rec. assay B.subtilis 2-1000 negative Shirasu et al.,
H17, M45 µg/disc (-S9) 1976a
DNA Rat 60-262 negative Lankas, 1989
damage hepatocytes µg/ml
Anaphase- CHO cells 0.06-0.12 negative Mudry de
telophase µg/ml (-S9) Pargament
test 0.24-0.6 positive et al., 1987
µg/ml (-S9)
Table 2. cont'd
Test system Test object Treatment Results Reference
Sister Mouse bone 50-200 negative Mudry de
chromatid marrow mg/kg bw, ip Pargament
exchange et al., 1987
Chromosome Human 2-50 negative Shirasu et al.,
aberration fibroblasts µg/ml (-S9) 1976c
Chromosome Rat bone 100-1000 negative Shirasu et al.,
aberration marrow mg/kg bw, po 1976d
30-300 negative
mg/kg bw/day
for 5 days, po
Micronucleus Mouse bone 125-500 negative Peck, 1977.
test marrow mg/kg bw, po
Micronucleus Mouse bone 50-200 positive Mudry de
test marrow mg/kg bw, ip Pargament
et al., 1987
Dominant Mice 200-600 negative Shirasu et al.,
lethal mg/kg bw/day 1976b,
test for 5 days, po
Dominant Mice 125-500 negative Peck, 1977.
lethal mg/kg bw/day
test for 5 days, po
1 Appropriate positive controls were used.
2 Liver microsomes obtained from Aroclor 1254 pretreated rats.
3 Liver microsomes obtained from phenobarbital pretreated rats.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Male albino guinea-pigs received 10 intracutaneous injections
of a 0.1% suspension of tiabendazole in 0.9% saline. Two weeks after
the induction period an intracutaneous challenge dose failed to
exacerbate reactions at the injection site (Peck, 1966b).
2.2.7.2 Rabbits
There was no evidence of a significant degree of irritation
when 0.5 g tiabendazole was applied in cold cream at concentrations
of 10 and 50% (w/w) for 24 h to intact rabbit skin (FAO/WHO, 1971).
No evidence of irritation was noted other than a very slight
erythema observed for approximately one hour after application of 1
ml of tiabendazole as a 12% suspension in sodium carboxymethyl
cellulose or 10 mg of the dry powder to the conjunctival sac of two
rabbits (FAO/WHO, 1971).
Male and female New Zeeland white rabbits received 100 mg of
tiabendazole powder instilled into the conjunctival sac of the eye.
At 15 min, there was slight conjunctival injection with a clear
discharge which returned to normal within 24 h (Merck, Sharp &
Dohme, undated).
2.2.8 Special studies on pharmacology
Oral doses of tiabendazole (4000 mg/kg bw) produced no striking
acute pharmacological effects on blood pressure or respiration in
either cats or dogs. Similarly, there were no alterations in the
electrocardiogram (FAO/WHO, 1971).
2.3 Observations in humans
A double blind study was carried out with 100 male volunteers,
half of them receiving 250 mg tiabendazole/day for 24 weeks and half
of them a placebo. Each subject was interviewed weekly and any
potential side effect was recorded. General physical examination and
laboratory examinations (haematology, cholesterol, glucose, urea,
alkaline phosphatase, thymol turbidity, bilirubin in serum and
urinalysis) were carried out before the test and after 4, 12 and 24
weeks. PBI in serum and ECGs were carried out only at the beginning
and after 24 weeks. Thirty-six subjects on tiabendazole and 41 on
placebo completed the study. As tiabendazole has a possible
influence on the thyroid the values for PBI were evaluated
carefully. No indication, however, was found of a decrease or
increase in PBI values due to tiabendazole. Under the conditions of
the study, tiabendazole was well tolerated at a dosage of 250 mg/day
during 24 weeks. No effect on any of the parameters studied could be
clearly ascribed to the drug. The NOEL was 3-4 mg/kg bw/day
(Colmore, 1965; FAO/WHO, 1978).
Twenty-three patients with trichinosis received 50 mg/kg bw/day
up to 3 g daily tiabendoazole for 10 days (total dose 25 to 30 g
tiabendazole), which was administered twice a day (after breakfast
and after evening meal). Symptoms of muscle pain and fever subsided
1 to 3 days after commencing tiabendazole. One woman withdrew due to
drug-induced rash, fever and proteinuria. Side-effects occurred in
14/23 treated patients - the most common effects being nausea,
retching and vomiting in 11/23 subjects, aversion to tablets (3/23),
exanthema (3/23), impotence (2/23), diarrhoea (1/23), liver damage
(1/23), fever (1/23) and dizziness (1/23) (Hennekeuser et al.,
1969).
3. COMMENTS
Information from a range of studies on thiabendazole was
available for assessment, including data on kinetics and metabolism,
acute toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity and observations in humans.
Toxicokinetic studies revealed rapid absorption after oral
dosing in mice, rats, dogs, and humans, peak plasma levels occurring
within 3 h of drug administration. The drug was excreted in the
urine and faeces. Elimination of tiabendazole and its degradation
products appeared more rapid in humans than in mice, rats, and dogs,
but metabolism was similar in mice, rats, and humans. The main
urinary metabolites were the glucuronide and sulfate conjugates of
5-hydroxytiabendazole; there were also small amounts of unconjugated
tiabendazole and the 5-hydroxy derivative. At very high doses, other
minor metabolites were detected in rat and mouse urine.
Single oral doses of tiabendazole were slightly toxic
(LD50 > 2000 mg/kg bw) in mice, rats, rabbits, sheep and goats.
Where deaths occurred, they appeared to be due to respiratory
failure.
Following repeated oral administration of tiabendiazole to
mice, rats, dogs, chickens, sheep, pigs, and cattle, the most common
finding was a reduction in food consumption and in body-weight gain.
The effects on weight gain appeared to be greater than could be
accounted for by the depressed food intake. Anaemia was seen in rats
and dogs, and to some extent may have been related to poor
nutrition. However, the presence of increased haemosiderosis in
lymphoid tissues and reticulocytosis in some studies was suggestive
of red cell destruction. The haematological changes occurred at dose
levels of 100 mg/kg bw/day and above for 3 months in rats and 150
mg/kg bw/day and above for 3 months in dogs.
The major target organs for toxicity were the hepatic system,
thyroid gland, and lymphoid organs. Liver hypertrophy was seen in a
3-month study in rats at doses of 37 mg/kg bw/day and above, and in
a 2-year study in dogs minor hepatobiliary changes were observed at
50 mg/kg bw/day and above. Thyroid follicular cell hyperplasia and
colloid depletion were noted in a 3-month study in rats at 37 mg/kg
bw/day and above and in a 16-week study in sheep at 800 mg/kg
bw/day. The thymus, spleen, lymph nodes, and/or bone marrow
exhibited lymphoid depletion or atrophy in a 28-day study in rats at
100 mg/kg bw/day and above and in a 16-week study in sheep at 800
mg/kg bw/day. Effects on blood cells, the liver, and lymphoid organs
are commonly observed with benzimidazoles.
Less common observations included both renal tubular
degeneration and hyperplasia in one 7-day study in mice at 250 mg/kg
bw/day and above and in one 3-month study in rats at 100 mg/kg
bw/day and above. Gastric changes, including mucosal degeneration,
cytoplasmic rarefaction, and/or necrosis, were produced in a 3-month
study in rats at 100 mg/kg bw/day and above. Atrial thrombosis was
present at high incidence in a two-year study in mice at doses of
200 mg/kg bw/day and above that resulted in the death of the
affected animals.
Tumour incidences were not increased in a two-year
carcinogenicity study in mice. The NOEL was 6 mg/kg bw/day, and
there was increased mortality at the next highest level, 60 mg/kg
bw/day.
The incidence of preputial or clitoral gland adenomas was
increased at doses above 200 mg/kg bw/day in one of two 2-year
studies in rats. Statistical significance was achieved only in
males; the animals also displayed poor development due to severe
effects on weight gain which suggests that the maximum tolerated
dose was exceeded. The relevance of these tumours to low-dose
exposure of humans to tiabendiazole is questionable. The NOEL was 10
mg/kg bw/day based on depressed weight gain at the next highest
level, 40 mg/kg bw/day.
Genotoxicity tests were generally negative. In one of three
Salmonella typhimurium reverse-mutation assays, positive results
were obtained in strain TA98 only. Further investigation showed that
an impurity in some batches was responsible for the mutagenic
activity. The manufacturer indicated that no positive results had
been obtained in tests on several hundred further batches. In one
laboratory, micronuclei were induced in mouse bone marrow and
abnormal anaphase-telophase figures were increased in cultured
Chinese hamster ovary cells. The effects were seen only at
relatively high levels and may be indicative of the tubulin-binding
activity characteristic of benzimidazoles (Annex 1, reference 97,
section 3.2.4). A range of other assays for mutation, DNA damage,
and cytogenetic activity were clearly negative.
A multigeneration reproduction study in rats showed no adverse
effects on reproduction parameters up to the highest dose tested, 80
mg/kg bw/day. In mice, litter size and survival during the lactation
period were reduced at 750 mg/kg bw/day, but reproduction was
unaffected at lower doses in a five-generation study. The NOEL was
30 mg/kg bw/day.
Developmental toxicity was examined in mice, rats, rabbits, and
sheep. In a series of studies in mice, embryotoxicity was observed
at 1300 mg/kg bw/day and above, a dose that also resulted in the
death of 5 of 39 females. Fetal malformations were produced at 240
mg/kg bw/day
and above, the main abnormalities being a reduction deformity of the
limbs, cleft palate, and fusion of vertebral arches and vertebral
bodies. Unchanged tiabendazole was implicated as the probable
embryotoxic and teratogenic agent.
The only finding in several rat developmental studies was a
reduction in fetal weight at 40 mg/kg bw/day and above. The NOEL was
10 mg/kg bw/day.
In rabbits, abortions occurred at 600 mg/kg bw/day in one study
on developmental toxicity, but not in the prior range-finding study
at higher doses or in a repeat experiment in which similar treatment
levels were used. Embryotoxicity and low incidences of domed head,
hydrocephaly, and enlarged fontanelles were seen at 120 mg/kg bw/day
and above in one study, but not in the repeat study at similar dose
levels. All effects in rabbits were seen only at maternally toxic
dose levels. The NOEL was 24 mg/kg bw/day.
No adverse reproductive effects were observed in pregnant ewes
given doses of 400 mg/kg bw/day.
In a 24-week study in humans, male volunteers received 250 mg
of tiabendazole/day; there were no treatment-related effects on a
range of physical and biochemical parameters. The NOEL was 3-4 mg/kg
bw/day. In another study, patients with trichinosis were given up to
3 g of tiabendazole daily for 10 days. Side-effects occurred in 14
of 23 patients, the most common being nausea, retching, and
vomiting.
4. EVALUATION
The Committee noted the availability of extensive data in
support of the safety evaluation of tiabendazole and the
manufacturer indicated that further studies had been completed or
were under way. At the 1977 joint FAO/WHO meeting on pesticide
residues, the study in human volunteers was used to establish an ADI
of 0-0.3 mg/kg bw. The Committee considered that the investigations
undertaken as part of this human study were limited in nature, and
that in view of the availability of a number of recent well
conducted animal studies, the NOEL should be derived from the animal
data.
The overall NOEL was 10 mg/kg bw/day based on reduced weight
gain in a 2-year dietary study in rats and decreased fetal weight in
a developmental study in rats. The Committee decided not to use the
NOEL of 6 mg/kg bw/day from the 2-year study in mice as the next
administered dose was considerably higher at 60 mg/kg bw/day.
Using a safety factor of 100, the Committee established an ADI
of 0-100 µg/kg bw for tiabendazole. The Committee noted that the ADI
provided a safety margin corresponding to a factor of over 1000 with
respect to the dose required for fetal malformations in mice.
The Committee asked to see the results from recently completed
and ongoing toxicological studies in order to update the database on
tiabendazole.
5. REFERENCES
BAGDON, W.J., BOKELMAN, D.L. & ZWICKEY, R.E. (1980). Lifetime
carcinogenic study in mice. Unpublished Report No. TT77-014-0 from
Merck, Sharp & Dohme Research Laboratories. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BATHAM, P., REID, S. & OSBORNE, B.E. (1989). A 14-week oral toxicity
study in the beagle dog. Unpublished Report No. 84021 (TT 89-9010)
from Bio-Research Laboratories, Canada. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
BLASZCAK, D.L. & AULETTA, C.S. (1987). Acute dermal toxicity study
in rabbits. Unpublished Report No. 4004-86 from Bio/dynamics Inc.,
USA. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
BOKELMAN, D.L. & ZWICKEY, R.E. (1977). Tiabendazole, six-week pilot
study in mice. Unpublished Report No. TT77-004-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CAVAGNORO, J. & YAMAMOTO, K.S. (1989). 23 day dermal toxicity study
in rabbits. Unpublished Report No. HLA 284-161 (TT89-9011) from
Hazleton Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
CHRISTIAN, M.S., LANKAS, G.R. & HOBERMAN, A.M. (1989). Oral
range-finding study in pregnant rabbits. Unpublished Report No.
013-029P (TT89-9006) from Argus Research Laboratories, USA.
Submitted to WHO by MSD Research Laboratory, Lauterbach, Germany.
COLMORE, J.P. (1965). Chronic toxicity study of tiabendazole in
volunteers. Unpublished report from University of Oklahoma Medical
Centre, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
DELATOUR, P., LORQUE, G., LAPRAS, M. & DESCHANEL, J.P. (1974).
Embryotoxic properties in the rat, and residues in cattle and sheep,
of three anthelmintics, derivatives of benzimidazole. Bull. Soc.
Sci. Vet. et Med., 76: 147-154.
FAO/WHO (1971). 1970 evaluations of some pesticide residues in food.
AGP: 1970/M/12/1; WHO/Food Add/71.42, Rome.
FAO/WHO (1978). Pesticide residues in food: 1977 evaluations. FAO
Plant Production and Protection Paper, 10 Sup. Rome.
FUJII, T., MIKURIYA, H. & SASAKI, M (1991). Chronic oral toxicity
and carcinogenicity study of tiabendazole in rats. Fd. Chem.
Toxicol., 29: 771-775.
FUJITANI, T., YONEYAMA, M., OGATA, A., UETA, T., MORI, K. &
ICHIKAWA, H. (1991). New metabolites of tiabendazole and the
metabolism of tiabendazole by mouse embryo in vivo and in vitro.
Fd. Chem. Toxicol., 29: 265-274.
GURMAN, J.L., HARDY, R.J., VOELKER, R.W. & DAVIDSON, J.L. (1981).
Acute inhalation toxicity study in rats. Unpublished Report No. HLA
284-129 from Hazleton Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
HENNEKEUSER, H.H., PABST, K., POEPLAU, W. & GEROK, W. (1969).
Tiabendazole for the treatment of trichinosis in humans. Texas Rep.
Biol. Med., 27 (Suppl. 2): 581-596.
HOBERMAN, A.M. & LANKAS, G.R. (1989). Oral developmental toxicity
study in rabbits. Unpublished Report No. 013-029 (TT89-9005) from
Argus Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
KANGAS, L., FARRELL, D. & OSBORNE, B.E. (1989). A 14-week oral
toxicity study in the albino rat. Unpublished Report No. 84114
(TT89-9014) from Bio-Research Laboratories, Canada. Submitted to WHO
by MSD Research Laboratory, Lauterbach, Germany.
KHERA, K.S., WHALEN, C., TRIVETT, G. & ANGERS, G. (1979).
Teratologic assessment of maleic hydrazide and daminozide, and
formulations of ethoxyquin, tiabendazole and naled in rats.
J. Environ. Sci. Health, B14: 563-577.
LANKAS, G.R. (1989). In vitro alkaline elution/rat hepatocyte
assay. Unpublished report nos. TT88-8850 and TT89-8312 from Merck,
Sharp & Dohme Research Laboratories, USA. Submitted to WHO by MSD
Research Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990a). Oral range-finding study in
pregnant rats. Unpublished Report No. TT90-713-1 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1990b). Oral development toxicity study
in rats. Unpublished Report No. TT90-713-0 from Merck, Sharp & Dohme
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
LANKAS, G.R. & WISE, L.D. (1991). Oral developmental toxicity study
in rabbits. Unpublished Report No. TT90-734-0 from Merck, Sharp &
Dohme Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
MacGREGOR, J. (1976). Mutagenicity tests of tiabendazole in the Ames
Salmonella typhimurium reverse mutation test. Unpublished report
from Western Regional Research Centre, California, USA. Submitted to
WHO by MSD Research Laboratory, Lauterbach, Germany.
MERCK, SHARP & DOHME (undated). Primary eye irritation in rabbits.
Unpublished Report No. TT81-2693 from Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
MUDRY DE PARGAMENT, M.D., LABAL DE VINUESA, M. & LARRIPA, I. (1987).
Mutagenic bioassay of certain pharmacological drugs. I Tiabendazole
(TBZ). Mutation Res., 188: 1-6.
MYERS, B.A. & LANKAS, G.R. (1990). A 14-week dietary toxicity study
in rats. Unpublished Report No. HLA 284-169 (TT 90-9002) from
Hazleton Laboratories, USA and Merck, Sharp & Dohme Research
Laboratories, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
OGATA, A., ANDO, H., KUBO, Y. & HIRAGA, K. (1984). Teratogenicity of
tiabendazole in ICR mice. Fd. Chem. Toxicol., 22: 509-520.
OGATA, A., YONEYAMA, M., SASAKI, M., SUZUKI, K. & IMAMICHI, T.
(1987). Effects of pretreatment with SKF-525A or sodium
phenobarbital on tiabendazole-induced teratogenicity in ICR mice.
Fd. Chem. Toxicol., 25: 119-124.
OGATA, A., FUJITANI, T., YONEYAMA, M., SASAKI, M. & SUZUKI, K.
(1989). Glutathione and cysteine enhance and diethylmaleate reduces
tiabendazole teratogenicity in mice. Fd. Chem. Toxicol., 27:
117-123.
PECK, H.M. (1966a). Reproduction study in the rabbit. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
PECK, H.M. (1966b). Cutaneous sensitisation in the guinea pig.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
PECK, H.M. (1977). Mutagenic evaluation. Unpublished report from
Merck, Sharp & Dohme. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
ROBINSON, H.J. (1964). Tiabendazole preclinical evaluation.
Unpublished report from Merck, Sharp & Dohme. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
ROBINSON, H.J. (1965). Tiabendazole safety evaluation. Unpublished
report from Merck, Sharp & Dohme. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
SHIRASU, Y., MORIYA, M. & KATO, K. (1976a). Mutagenicity testing on
tiabendazole (TBZ) in microbial systems. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TERAMOTO, S. & KANEDA, M. (1976b). Dominant lethal
studies with tiabendazole in mice. Unpublished report from the
Institute of Environmental Toxicology, Japan. Submitted to WHO by
MSD Research Laboratory, Lauterbach, Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976c).
Cytogenetic studies with tiabendazole in cultured human fibroblasts.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
SHIRASU, Y., TEZUKA, H., HEMMI, R. & MURAKAMI, N. (1976d).
Cytogenetic studies with tiabendazole in rat bone marrow cells.
Unpublished report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by MSD Research Laboratory, Lauterbach,
Germany.
TADA, Y., YONEYAMA, M., KABASHIMA, J., FUJITANI, T. & NAKANO, M.
(1989). Effects of tiabendazole on the kidneys of ICR mice.
Fd. Chem. Toxicol., 27: 307-315.
TSUCHIYA, T., TANAKA, A., FUKUOKA, M., SATO, M. & YAMAHA, T. (1987).
Metabolism of tiabendazole and teratogenic potential of its
metabolites in pregnant mice. Chem. Pharm. Bull., 35: 2985-2993.
USUI, T. & SAKAGUCHI, T. (1989). 5 week oral toxicity study in rats.
Unpublished report nos. TT86-9819 and TT87-9809 from Banyu
Pharmaceutical Development Research Laboratories, Japan. Submitted
to WHO by MSD Research Laboratory, Lauterbach, Germany.
VOGIN, E.E. (1968). Multigeneration reproduction and lactation
studies with tiabendazole. Unpublished report from Food and Drug
Research Laboratories, USA. Submitted to WHO by MSD Research
Laboratory, Lauterbach, Germany.
WOODARD, G. (1964). Safety evaluation by oral administration to dogs
and rats for 104 weeks. Unpublished report from Woodard Research
Corporation, USA. Submitted to WHO by MSD Research Laboratory,
Lauterbach, Germany.
YONEYAMA, M., OGATA, A., FUJII, T. & HIRAGA, K. (1984). The
maternal-foetal distribution of tiabendazole administered in two
different vehicles to pregnant mice. Fd. Chem. Toxicol., 22:
731-735.
