
TRICLABENDAZOLE
First draft prepared by
Dr G. Roberts
Environmental Health Branch
Department of Health, Housing and Community Services
Canberra, Australia
1. EXPLANATION
Triclabendazole is a benzimidazole anthelminthic agent. It is
used for the control of liver fluke infestation in sheep, goats and
cattle. Triclabendazole had not been previously evaluated by the
Joint FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution, biotransformation and excretion
2.1.1.1 Rats
Following single oral doses of 0.5 or 25 mg/kg bw of
14C-labelled triclabendazole to rats, excretion was rapid with
approximately 93% of the dose eliminated in 48 h. At the end of 144
h, total recovery amounted to 98% of which 88 to 95% was in faeces,
4 to 10% in urine, less than 0.05% in expired air and up to 1% in
tissues. There were no differences related to sex or dosage. In a
bile cannulated rat given 5 mg/kg bw by gavage, biliary excretion in
49 h was 34% of the administered dose (Muecke, 1981; Hambock, 1983).
Degradation products were examined using chromatographic
analysis of excreta from the previous rat study. Faecal metabolites
were: unchanged drug (6-9% of dose), triclabendazole sulfoxide
(20-27%), triclabendazole sulfone (3%), 2-benzimidazolone (8-10%),
4-hydroxy triclabendazole plus minor unknowns (11-12%), unknown
substances (9-13%) and non-extractable radio-label (16-32%). The
metabolites in urine were generally more polar in nature;
2-benzimidazolone was the only identified metabolite. It was
reported that qualitatively similar metabolites were seen in sheep
and goats (Hambock, 1983).
2.1.1.2 Rabbits
Female rabbits were given single oral doses of 3 or 26 mg/kg bw
of 14C-labelled triclabendazole. Peak plasma radioactivity was
reached in 8 h and levels declined rapidly thereafter. A comparison
of the kinetics following oral and intravenous doses indicated
extensive absorption from the gastrointestinal tract. Unchanged drug
was not detected in plasma after the low dose and only small amounts
were seen after the higher dose. Triclabendazole sulfoxide was the
main plasma metabolite during the first 24 h, with the sulfone at
similar or slightly higher levels thereafter. Other metabolites
(which were not identified) began appearing between 24 and 48 h.
Within 7 days, 80 to 90% of the radioactivity was eliminated of
which 66 to 76% was in faeces and 7 to 22% in urine. The greater
urinary excretion was noted at the higher dose. The sulfoxide and
sulfone as well as other metabolites were detected in urine, but
unchanged triclabendazole was not found (Wiegand et al., 1991a,b).
2.1.1.3 Dogs
Dogs received single oral doses of 0.5, 5 or 40 mg/kg bw of
14C-triclabendazole. Peak plasma radioactivity was achieved at 8 h
with the lower doses and 24 h at the highest dose, which was
maintained for 2 to 3 days. By comparison with the kinetics after an
intravenous dose, approximately 40% of the oral dose was absorbed.
Unchanged drug was essentially non-detectable in plasma. During the
first 8 to 12 h, the sulfoxide was present at a greater
concentration than the sulfone but at later times the sulfone was
the major metabolite and significant levels were found even after 7
days. Only 1% of the dose was excreted in the urine (Schutz et al.,
1991).
2.1.1.4 Humans
Three patients infected with Fasciola hepatica were given
single oral doses of 10 mg/kg bw triclabendazole in the form of
Fasinex tablets. In fasted subjects, peak plasma levels occurred at
2 h. HPLC analysis showed that parent drug was present at low levels
with high levels of the sulfoxide metabolite and intermediate levels
of the sulfone. Unchanged triclabendazole was not detected after 8
h. Low levels of sulfoxide and sulfone were still present at 24 h,
the final analysis point. In one patient, administration 1 h after a
meal resulted in plasma levels approximately 3-fold higher than in
fasted subjects, suggesting enhanced absorption under these
conditions (Poltera et al., 1989).
Based on studies in experimental animals, the proposed pathway
of metabolism of triclabendazole is outlined in Figure 1.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies on triclabendazole are
summarized in Table 1.
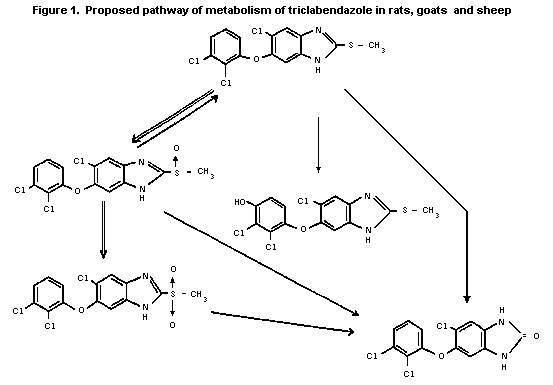 Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M+F oral >8000 Bathe & Sachsse (1979a)
Rat M+F oral >8000 Bathe & Sachsse (1979b)
M+F dermal >4000 Bathe & Sachsse (1979c)
M+F inhalation >500 mg/m3 Ullman & Sachsse (1979b)
M+F ip 1666 Bathe & Sachsse (1979d)
Rabbit M+F oral 206 Ullman & Sachsse (1979a)
The major toxic signs were sedation, dyspnea, exophthalmos,
ruffled fur and curved body position after oral, dermal or
inhalation exposure. Ataxia was also noted following intraperitoneal
dosing.
The oral LD50 in male and female rats of the sulfoxide and
sulfone metabolites was greater than 5000 mg/kg bw. Toxic signs were
similar to those induced by triclabendazole (Sarasin, 1982a,b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Charles River CD rats were fed
diets containing 0, 10, 100 or 1000 ppm triclabendazole (purity 97%)
for 13 weeks. The calculated intake of the test material was 0.7,
6.6 and 68.5 mg/kg bw/day in males and 0.8, 7.9 and 87.3 mg/kg
bw/day in females.
There was no effect on survival and there were no overt
clinical signs. Food and water intake were reduced in 1000 ppm males
while body-weight gain was depressed in 100 ppm males and 1000 ppm
males and females. Ophthalmological and hearing tests carried out
before treatment and at weeks 6 and 13 revealed no treatment-related
effects.
Haematology, blood chemistry and urinalysis were performed
before treatment and at weeks 5 and 12. Numbers of erythrocytes,
haemoglobin and haematocrit were reduced in 1000 ppm males and
females; lymphocytes and therefore total white blood cells were
reduced in 1000 ppm females. Serum alkaline phosphatase was
increased in 1000 ppm groups. Cholesterol, albumin and total protein
were increased at 1000 ppm, particularly in females. Urine volume
was reduced in 1000 ppm males.
All animals were autopsied. Organ weights were unaffected.
Gross examination showed increases in pale kidneys and livers at 100
and 1000 ppm and in congested lungs at 1000 ppm, but pathology was
unremarkable. The NOEL was 10 ppm, equal to 0.7 mg/kg bw/day (Hunter
et al., 1982).
2.2.2.2 Dogs
Groups of 6 male and 6 female beagle dogs were fed diets
containing 0, 10, 100, or 1000 ppm triclabendazole (purity 97.6%)
for 13 weeks. The calculated intake of the test material was 0.35,
3.4 or 37 mg/kg bw/day in males and 0.35, 3.5 or 39 mg/kg bw/day in
females.
There were no overt clinical signs of toxicity. Food intake was
unaffected but body-weight gain was markedly depressed in 1000 ppm
dogs. Ophthalmology was tested before treatment and at weeks 6 and
13 with no evidence of treatment-related effects. ECG's revealed
prolongation of the QT interval and QTc value in 1000 ppm males and
females at weeks 5 and 9 but not at week 13.
Haematology and blood chemistry were examined before treatment
and at weeks 5, 9 and 13. Erythrocytes, haemoglobin and haematocrit
were markedly reduced at 1000 ppm throughout the study, with
evidence of reticulocytosis at week 9 only. Alkaline phosphatase was
increased in 100 and 1000 ppm groups; serum GPT and cholesterol were
increased in 1000 ppm dogs. Urinalysis, performed before treatment
and at weeks 6 and 13, was unaffected.
A full necropsy was undertaken on all animals;
treatment-related effects were seen at the 1000 ppm dose only. Liver
weight was increased and showed centrilobular hepatocellular pigment
granules accompanied by cytoplasmic basophilia, glycogen depletion
and foci of pigmented macrophages. Ovaries and testes were immature
with lower organ weights - females failing to reach oestrous and
males showing incomplete spermatogenesis. The NOEL was 10 ppm, equal
to 0.35 mg/kg bw/day (Taupin, 1981).
2.2.3 Long-term/carcinogenicity studies.
2.2.3.1 Mice
Groups of 80 male and 80 female albino Tif:MAGf mice were fed
diets containing 0, 3, 15, 60 or 300 ppm triclabendazole (purity
99.5%) for 2 years. Within each group, animals were designated for
interim sacrifice at 1 year (10 M and 10 F), for use in clinical
laboratory measurements (10 M and 10 F) and a further 10 M and 10 F
for examination of haematological parameters. The calculated intake
of the test material was 0.29, 1.44, 5.7 or 29.6 mg/kg bw/day in
males and 0.27, 1.39, 5.35 or 28.7 mg/kg bw/day in females.
Treated mice showed no overt signs of toxicity and mortality
was comparable to controls. There were no meaningful effects on food
and water intake while body-weight gain was slightly elevated in all
treated male groups, particularly during the first year. However,
the effect was small (10% or less), not dose-related and was not
observed in females.
Ophthalmology, haematology, blood chemistry and urinalysis were
performed at 6 month intervals. Serum levels of alkaline
phosphatase, GPT and GOT were increased in the 300 ppm groups during
the first year and in all treated groups in the second year. Effects
in the second year were not dose-related and significance was
achieved only occasionally.
All animals were subjected to a full necropsy. There were no
findings attributable to treatment at the interim sacrifice. At the
end of the study, absolute and relative liver weights were increased
at 15 ppm and above. Hepatomas were increased in all treated female
groups, but significance was not reached at the 99% level. The
incidence of carcinomas and time to appearance of tumours were
unaffected (Table 2).
Table 2. Hepatic tumour incidence in female mice
Triclabendazole (ppm) 0 3 15 60 300
Females in study 80 80 80 80 80
Benign hepatoma 7 16 15 9 20
Hepatocelular carcinoma 1 2 2 2 3
Hepatoblastoma 0 0 0 1 0
Total hepatic tumours 8 18 17 12 23
The NOEL was 3 ppm, equal to 0.27 mg/kg bw/day (Basler
et al., 1988a).
2.2.3.2 Rats
Groups of 60 male and 60 female Charles River CD rats were fed
diets containing 0, 3, 15, 30 or 100 ppm triclabendazole (purity
99.5%) for 2 years. Additional groups were designated for interim
sacrifice at 1 year (10 M and 10 F) and for haematology,
biochemistry and urinalysis investigations (20 M and 20 F). The
calculated intake of the test material was 0.1, 0.6, 1.2 or 4.0
mg/kg bw/day for males and 0.2, 0.7, 1.5 or 5.2 mg/kg bw/day for
females.
There was no effect on survival and there were no overt toxic
signs. Food and water intake were unaffected but body-weight gain
was depressed at 100 ppm, significant only in females. Ophthalmology
and hearing tests, measured every six months, were also unaffected.
Clinical laboratory parameters were examined pre-test and at
weeks 13, 26, 51, 78 and 104. Lymphocyte counts were reduced at
termination in 30 and 100 ppm groups but the values were reported to
be within normal variation. Slight decreases in plasma chloride and
BUN and increases in calcium and protein in 100 ppm males and slight
increases in protein and decreases in BUN in all treated female
groups were judged to be of no toxicological significance as the
variations were small and not consistent throughout the study and
were reported to be within normal variation. There were no
alterations in urinalysis.
A full necropsy was performed on all rats. Kidney weights were
lower in 100 ppm males at the interim kill but not at the terminal
sacrifice. At pathology, pancreatic islet cell adenomas were
increased in 15 and 100 ppm males. The relationship to treatment was
questionable as statistical significance was not achieved, there was
no dose relationship and greater numbers of carcinomas were seen in
control males (Table 3).
Table 3. Pancreatic islet cell tumours in male rats (70/group)
Dose (ppm) 0 3 15 30 100
Adenomas 3 4 12 4 11
Carcinomas 6 4 4 2 4
Total 9 8 16 6 15
The NOEL was 30 ppm, equal to 1.2 mg/kg bw/day (Charnley
et al., 1986).
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 20 male and 20 female Tif:RAIf rats were fed diets
containing 0, 3, 15 or 75 ppm triclabendazole (purity 97.6%). Dosing
of the initial generation (P) commenced 62 days prior to mating and
was continued throughout the second generation (F1) until post
partum day 35 of the third generation (F2) pups. The calculated
average intake of test compound was 0.2, 1.1 or 5.5 mg/kg bw/day in
males and 0.3, 1.4 or 7.4 mg/kg bw/day in females.
Observations of P and F1 generation adults revealed no overt
signs of toxicity, no effects on body-weight gain and no influence
on reproductive parameters.
Examination of pups, including a range of early developmental
and behavioural tests, showed no meaningful changes in the F1
offspring. In the F2 pups, mortality was increased in 15 and 75
ppm groups during lactation. The increase was significant when
compared to concurrent controls but not when compared to the F1
groups. Body weights at weaning were lower at 15 and 75 ppm but
significance was not achieved. The NOEL was 75 ppm, equal to 5.5
mg/kg bw/day (Fritz et al., 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity.
2.2.5.1 Rats
Groups of 25 pregnant Tif:RAIf rats were given gavage doses of
0, 10, 30 or 100 mg/kg bw/day of triclabendazole (purity 97.6%) as a
suspension in 0.5% (w/v) carboxymethyl cellulose. Treatment was on
gestation days 6 to 15 and females were killed on gestation day 21.
There was no mortality or clinical signs related to treatment.
Body-weight gain and food consumption were markedly depressed at 100
mg/kg bw/day with slight reduction in weight gain at 10 and 30 mg/kg
bw/day. Gross pathology of dams was unremarkable.
The implantation rate was similar in each group and treatment
did not influence the number of resorptions or fetal deaths. Live
fetus weight was reduced at 100 mg/kg bw/day but no external,
visceral or skeletal malformations were induced. The NOEL was 30
mg/kg bw/day (Giese et al., 1981a; Giese, 1987).
Groups of 20 pregnant Sprague-Dawley rats were given gavage
doses of 0, 10, 25, 50, 100 or 200 mg/kg bw/kg of triclabendazole
(purity 100%) as a suspension in water. Treatment was on gestation
days 8 to 15 and dams were killed on gestation day 21.
Maternal weight gain was depressed at 100 and 200 mg/kg bw/day
with no signs of toxicity at lower levels. Uterine and fetal
examinations revealed no effects on embryo or fetal survival or on
fetal malformations. The only fetal effect was reduced body weight
at 100 and 200 mg/kg bw/day. The NOEL was 50 mg/kg bw/day
(Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 pregnant chinchilla rabbits were given gavage
doses of 0, 3, 10 or 20 mg/kg bw/day of triclabendazole (purity
unknown) as a suspension in 0.5% (w/v) carboxymethyl cellulose.
Treatment was on gestation days 6 to 18 with does killed on
gestation day 28.
Three low-dose females died and diarrhoea was noted in a few
animals from each treated group. Maternal body-weight gain during
the study was similar in all groups but when "corrected" by
subtracting the weight of uterus and contents, there was a slight
non-dose related decrease in the treated groups.
The numbers of implantations, resorptions and fetal deaths were
similar in each group and fetal body weight was unaffected. Fetal
examinations showed unossified phalanges of fore and hind limbs at
10 and 20 mg/kg bw/day. One 20 mg/kg bw/day fetus exhibited
omphalocele which is rare in this strain of rabbit. The NOEL was 3
mg/kg bw/day (Giese et al., 1981b)
2.2.5.3 Sheep
Merino ewes were drenched with a single dose of 0, 5 or 10
mg/kg bw triclabendazole in the third trimester. There were no
effects on lambing or on the morphology of offspring (Strong & Ryan,
1981).
In a series of experiments, merino ewes were drenched with
doses of 0 or 30 mg/kg bw/day triclabendazole before conception and
during the first trimester of pregnancy as follows:
A. 3 treatments during the first 24 days of gestation.
B. 4 treatments during the first 43 days of gestation.
C. 2 treatments 2 to 3 weeks before conception plus
2 treatments during the first 25 days of gestation.
D. 3 treatments 2 to 4 weeks before conception plus
1 treatment 7 to 9 days after conception.
E. 3 treatments 2 to 4 weeks before conception.
None of the treatment regimes caused clinical signs of toxicity
in the ewes, nor were there effects on lambing or development of
lambs (Strong, 1981).
Groups of 27 to 28 pregnant Merino ewes were drenched with a
single dose of 0 or 50 mg/kg bw of triclabendazole. Drug
administration was on days 12, 17, 21, 24 or 28 days after mating.
There were no effects on lambing performance or development of lambs
(Strong & Steiger, 1983).
2.2.5.4 Cattle
Hereford cows were drenched with a single dose of 24 mg/kg bw
triclabendazole during the first month of pregnancy. Calving and
uterine development of offspring were unaffected (Bowen & Ryan,
1985a).
Hereford or black poll cows were drenched with a single dose of
0 or 24 mg/kg bw/day triclabendazole during the second, third,
fourth and sixth to seventh month of pregnancy. There were no
difficulties at calving or abnormalities in the calves (Bowen &
Ryan, 1985b).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies on triclabendazole are
summarized in Table 4.
Table 4. Results of genotoxicity studies on triclabendazole1
Test system Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.5-1250 µg/plate Negative Ogorek & Arni,
TA98, TA100, 1987a
TA1535, TA1537,
TA1538
Forward mutation2 Chinese hamster 0.025-0.5 µg/ml (-S9) Negative Dollenmeier &
V79 cells 3.5-70 µg/ml (+S9) Puri, 1988
Unscheduled Primary rat 0.3-40 µg/ml Negative Hertner & Puri,
DNA-synthesis hepatocytes 1988
Unscheduled Human fibroblasts 0.4-60 µg/ml Negative Meyer & Puri,
DNA-synthesis 1988
Nucleus anomaly Chinese hamster 172-688 mg/kg bw Negative Hool et al.,
bone marrow by gavage 1982
Sister chromatid Chinese hamster 173-692 mg/kg bw by Negative Hool & Muller,
exchange bone marrow gavage 1982
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Groups of 10 male and 10 female pirbright guinea-pigs were used
in an optimization test. The induction phase was carried out by
administering 10 intracutaneous injections of a 0.1% (w/v)
suspension in propylene glycol/saline. Epidermal challenge produced
no reaction but an intradermal challenge resulted in a positive
sensitization reaction (Ullmann & Sachsse, 1979e)
2.2.7.2 Rabbits
Groups of 3 male and 3 female New Zeeland white rabbits were
used in a patch test. Triclabendazole was suspended in propylene
glycol/saline and applied for 24 h. The test material was judged to
be a slight skin irritant (Ullmann & Sachsse, 1979c)
Groups of 3 male and 6 female New Zeeland white rabbits
received 0.1 g of triclabendazole instilled into the conjunctival
sac of the eye. Subsequent examination failed to reveal any eye
irritation (Ullmann & Sachsse, 1979d).
2.2.8 Special studies on triclabendazole and fenbendazole in
combination
A series of studies was conducted using test material
containing 50% triclabendazole and 50% fenbendazole that are briefly
reviewed below.
Pregnant rats were treated with gavage doses of 0, 10, 40 or
120 mg/kg bw/day test material during gestation days 6 to 15. At 120
mg/kg bw/day, dams had a reduced food consumption and there were
increases in partial abortion of conceptuses and in resorptions.
Fetotoxicity at the same level included decreased fetal weight,
reduced ossification, kinked tail, meningo-encephalocele and
hydrocephaly. Delayed ossification was present in the 40 mg/kg
bw/day fetuses while there were no adverse findings in the 10 mg/kg
bw/day group (Giese et al., 1985).
In two developmental studies in sheep, pregnant ewes received
either two doses of 0 or 50 mg/kg bw or a single dose of 0 or 150
mg/kg bw, during the first month of pregnancy. There were no effects
on lambing performance and the only adverse effect on offspring was
grossly misshapen kidneys, with disorganized internal structure, in
3 of 25 lambs at the higher-dose level (Bowen & Heckenberg, 1985;
Richards et al., 1985).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Saccharomyces cerevisiae D7 were
negative, both in the presence and absence of rat liver microsomes
(Deparade & Arni, 1983; Hool & Arni, 1985). Chromosome aberrations
in cultured human lymphocytes were increased at 22 and 44 µg/ml
(dose-related), but not at 11 µg/ml, only in the presence of a rat
liver S9 fraction (Strasser & Arni, 1985a). The bone marrow of
Chinese hamsters revealed an increase in nucleus anomalies (single
Jolly bodies only) following oral doses of 1000, 2000 or 4000 mg/kg
bw. While the increases were statistically significant they were
only slight and non dose-related (Strasser & Muller, 1984).
Chromosome aberrations were not induced in mouse primary and
secondary spermatocytes or spermatogonia after oral doses of up to
2500 mg/kg bw (Strasser & Arni, 1985b; Strasser et al., 1985).
2.2.9 Special studies on triclabendazole and levamisole in
combination
A series of studies was conducted using test material
containing 61.5% triclabendazole and 38.5% levamisole that are
briefly reviewed below.
Acute studies in male and female rats revealed LD50 values of
1996 mg/kg bw by the oral route and >2000 mg/kg bw by the dermal
route (Hartmann & Schoch, 1987a,b). In rabbits, slight and
reversible eye irritation was observed while the test material was
judged to be non-irritant to skin (Schoch, 1987a,b).
Rats were administered 0, 20, 200 or 2000 ppm of the test
material in the feed for 3 months. Body-weight gain was depressed at
200 ppm and above and anaemia was apparent at 2000 ppm. Increased
serum levels of liver enzymes and cholesterol and decreased levels
of triglycerides were suggestive of hepatocellular damage at 2000
ppm and, to a lesser extent, at 200 ppm. Necropsy revealed the
following slight changes at 2000 ppm: chronic lesion of renal
tubules, spermatic granulomas in the epididymis, fatty change in the
adrenal cortex, thymic atrophy and skeletal muscle atrophy. There
were no effects at 20 ppm, equal to 1.4 mg/kg bw/day (Basler
et al., 1988b).
Dogs were fed diets containing 0, 20, 60, 240 or 1000 ppm for 3
months. At 1000 ppm, body weight gain and food intake were reduced
and half the animals died. The administration of this dose was
discontinued after 45 days. Anaemia, increased alkaline phosphatase
and cholesterol were evident at 240 ppm and above. Increases were
observed in liver weight at 240 ppm and spleen weight at 60 and 240
ppm. There was increased haematopoiesis of the sternum at 60 ppm and
above. Survivors in the 1000 ppm group, which were not dosed for the
previous 45 days, showed at least partial recovery of toxic effects.
There were no effects at 20 ppm, equal to 0.75 mg/kg bw/day (Monnot,
1988).
Pregnant rats were treated with gavage doses of 0, 10, 80 or
160 mg/kg bw/day during gestation days 6 to 15. Ruffled fur was seen
at 160 mg/kg bw/day and increased wet bedding was noted at 80 mg/kg
bw/day and above, suggesting polyuria. Maternal body-weight gain and
food intake were depressed at 80 and 160 mg/kg bw/day. The only
fetal effects, seen at 80 and 160 mg/kg bw/day, were decreased fetal
body weight, increased bipartite sternebrae and reduced
ossification. There were no adverse effects at 10 mg/kg bw/day
(Thouin et al., 1988).
Pregnant rabbits received gavage doses of 0, 1.5, 30 or 50
mg/kg bw/day on gestation days 7 to 19. In the 50 mg/kg bw/day
group, there was reduced body weight gain, one abortion and total
resorption of conceptuses in one rabbit. The resorption rate was
slightly increased at 50 mg/kg bw/day but significance was not
achieved. There were no increased developmental effects in any
group. No adverse effect was noted up to 30 mg/kg bw/day (Khalil
et al., 1989).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Chinese hamster V79 cells gave negative
results both in the presence and absence of rat liver microsomes
(Ogorek & Arni, 1987b; Dollenmeier & Puri, 1987). Using isolated rat
hepatocytes and human fibroblasts there was no evidence of
unscheduled DNA synthesis in exposed cells (Hertner & Puri, 1987;
Meyer & Puri, 1987). Micronuclei were not induced in bone marrow of
mice dosed orally with the test substance (Strasser et al., 1988).
3. COMMENTS
A range of studies on triclabendazole was submitted for
assessment, including data on kinetics and metabolism, acute
toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity.
Approximately 40-50% was absorbed following the administration
of single oral doses of radiolabelled triclabendazole to rats and
dogs. Oral absorption was considerably higher in rabbits but could
not be quantified in a human study. Peak plasma levels of
radioactivity were generally achieved within 8 h; elimination
(primarily in the faeces) was rapid in rats, rabbits, and humans. In
dogs, peak plasma levels of radioactivity were maintained for 2-3
days and significant levels persisted for more than 7 days.
Triclabendazole was rapidly and extensively degraded in rabbits,
dogs, and humans, the sulfoxide and sulfone being the main
metabolites found in plasma. In addition to these oxidation
products, rat excreta contained 4-hydroxytriclabendazole and
2-benzimidazolone.
Single oral doses of triclabendazole were slightly toxic (LD50
> 8000 mg/kg bw) in mice and rats and moderately toxic (LD50 =
206 mg/kg bw) in rabbits, whose greater sensitivity was probably due
to a greater bioavailability of oral doses.
Findings after short-term oral dosing in rats and dogs were
primarily non-specific. In 3-month studies, decreased weight gain
was observed at 7 mg/kg bw/day and above in rats and at 37 mg/kg
bw/day in dogs; anaemia was seen at 68 mg/kg bw/day and 37 mg/kg
bw/day, respectively. Evidence of hepatotoxicity, for example
increases in plasma levels of liver enzymes and cholesterol and in
liver weight, was seen at approximately 70 mg/kg bw/day in rats and
37 mg/kg bw/day in dogs. Minor non-neoplastic liver lesions were
observed in a 3-month study in dogs at 37 mg/kg bw/day. The NOELs
were 0.7 mg/kg bw/day in rats and 0.35 mg/kg bw/day in dogs.
Hepatomas were seen in female mice given up to 29 mg/kg bw/day
for 2 years. The incidences of adenomas were higher than in controls
at all dose levels, but a dose-response relationship was not found.
Significance at the 99% level, which is widely used to assess the
significance of tumours that occur at a high background rate, was
not reached for this common benign mouse tumour. In addition, there
was no significant increase in the incidence of hepatocellular
carcinomas at any dose level. The NOEL was 0.27 mg/kg bw/day based
on increased liver weight at the next highest dose, 1.4 mg/kg
bw/day. Tumours were not induced in a 2-year dietary study in rats,
the NOEL being 1.2 mg/kg bw/day based on reduced weight gain at 4
mg/kg bw/day. Several different in vitro and in vivo
genotoxicity studies were clearly negative. These results suggest
that triclabendazole has no carcinogenic potential.
In a two-generation reproduction study in rats, postnatal
survival and growth were reduced during the lactation period of the
second generation at 1 mg/kg bw/day and above. However, the
incidence of these effects was not dose-related and was similar to
that in the first-generation offspring. In addition, statistical
significance was not achieved, and it was concluded that the
findings were not related to treatment. The NOEL was 5.5 mg/kg
bw/day.
Developmental studies in rats and rabbits provided no evidence
of embryotoxicity. Fetotoxicity, in the form of reduced fetal weight
in rats and decreased ossification in rabbits, was observed at 100
and 10 mg/kg bw/day, respectively. One rabbit fetus at 20 mg/kg
bw/day exhibited omphalocele, a rare abnormality in the strain used.
All the above effects were present only at maternally toxic dose
levels. Drug treatment during pregnancy in sheep and cattle did not
affect growth and development of the conceptus. The NOELs were 50
mg/kg bw/day in rats and 3 mg/kg bw/day in rabbits.
Special studies with triclabendazole in combination with either
fenbendazole (developmental and genotoxicity studies) or levamisole
(acute, subchronic, developmental, and genotoxicity studies) did not
reveal any synergism between the drugs.
4. EVALUATION
The database in support of the safety evaluation of
triclabendazole is substantial. The lowest NOEL was 0.27mg/kg
bw/day, based on increased liver weight in the long-term study in
mice. Using a safety factor of 100, the Committee established an ADI
for triclabendazole of 0-3 µg/kg bw.
5. REFERENCES
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, G., MALINOWSKI, W.,
CHRISTEN, P., FORMICA, G. & GFELLER, W. (1988a). Lifetime
carcinogenicity and chronic toxicity study in mice. Unpublished
Report No. 811708 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, A.L., MALINOWSKI,
W. & GFELLER, W. (1988b), 3-month oral toxicity study in rats
(administration in feed). Unpublished Report No. 861575 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979a). Report on acute oral LD50 in the
mouse of technical CGA 89'317. Unpublished Report No. 791073 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979b). Report on acute oral LD50 in the
rat of CGA 89'317. Unpublished Report No. 790038 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BATHE, R. & SACHSSE, K. (1979c). Report on acute dermal LD5O in
the rat of technical CGA 89'317. Unpublished Report No. 791076 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979d). Report on acute intraperitoneal
LD50 in the rat of CGA 89'317. Unpublished Report No. 790039 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BOWEN, F.L. & HECKENBERG, K. (1985). Reproduction study in sheep:
triclabendazole plus fenbendazole. Unpublished Report No. 85/12/1060
from CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985a). A field trial to assess the safety
of triclabendazole for cows during their first month of pregnancy.
Unpublished Report No. 85/11/1054 from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985b). Field trials to assess the safety
of triclabendazole for cows during the second to sixth month of
pregnancy. Unpublished Report No. 85/11/1055 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
CHARNLEY, B.N., HEYWOOD, R., STREET, A.E., IMM, S.A.,
GIBSON, W.A., GOPINATH, C., ALMOND, R.H. & DAWE, I.S. (1986).
Potential tumorigenic and toxic effects in prolonged dietary
administration to rats. Unpublished Report No. CBG 318/85595
(811709) from Huntingdon Research Centre, UK. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
DEPARADE, E. & ARNI, P. (1983). Salmonella/mammalian-microsome
mutagenicity test. Unpublished Report No. 831517 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1987). Point mutation test with Chinese
hamster cells V79. Unpublished Report No. 871011 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1988). Point mutation test with Chinese
hamster cells V79 with CGA 89'317 tech. Unpublished Report No.
871170 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
FRITZ, H., SUTER, H.P., ZAK, F., NAYLOR, D.C. & HESS, R. (1984).
Report on CGA 89'317: 2-Generation toxicity study in rats.
Unpublished Report No. 811540 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K. (1987). Amendment No. 1 to Final Report on CGA 89'317
tech., Teratology study (Seg. II) in rats. Unpublished Report
amendment No. 802099-1 from CIBA-GEIGY, Switzerland. Submitted to
WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & GFELLER, W. (1985). Report on CGA 89317
Tech and CGA 50560 Tech (1:1). Teratology study in rats. Unpublished
Report No. 831213 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981a). Report on CGA 89'317
tech. Teratology study (Seg. II) in rats. Unpublished Report No.
802099 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981b). Report on CGA 89'317
tech. Teratology study in rabbits. Unpublished Report No. 802090
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
HAMBOCK, H. (1983). The metabolic fate of CGA 89317 in sheep, rat
and the lactating goat. Unpublished Report No. 41/83 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987a). Acute oral toxicity in the rat.
Unpublished Report No. 861571 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987b). Acute dermal toxicity in the
rat. Unpublished Report No. 861574 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1987). Autoradiographic DNA-repair test on
rat hepatocytes. Unpublished Report No. 861580 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1988). Autoradiographic DNA repair test on
rat hepatocytes (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871173 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
HOOL, G. & ARNI, P. (1985). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro. Unpublished Report No.
840945 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G., LANGAUER, M. & MULLER, D. (1982). Nucleus anomaly test in
somatic interphase nuclei, CGA 89'317, Chinese hamster (test for
mutagenic effects on bone marrow cells). Unpublished Report No.
801392 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G. & MULLER, D. (1982). Sister chromatid exchange study, CGA
89'317, Chinese hamster (Test for mutagenic effect on bone marrow
cells). Unpublished Report No. 820209 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HUNTER, B., BERRYMAN, E.L., HEYWOOD, R., PRENTICE, D.E., STREET,
A.E., GIBSON, W.A., SINGH, H. & OFFER, J.M. (1982). CGA 89,317, a
13-week toxicity study in rats by incorporation in the diet.
Unpublished Report No. CBG/252/81151 (791817) from Huntingdon
Research Centre, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
KHALIL, S., THONIN, M.H., WEBER H., GRUENDER, G. & BASLER, W.
(1989). Developmental toxicity (teratogenicity study). Unpublished
Report No. 881433 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1987). Autoradiographic DNA-repair test on
human fibroblasts. Unpublished Report No. 861581 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1988). Autoradiographic DNA repair test on
human fibroblasts (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871172 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MONNOT, G. (1988). 13-week dietary administration toxicity study in
the beagle dog. Unpublished Report No. 380/520 (861576) from
Hazleton, France. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
MUECKE, W. (1981). Distribution, degradation and excretion of CGA
89317 in the rat. Unpublished Report No. 27/81 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987a). Salmonella/mammalian microsome
mutagenicity test (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871171 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987b). Salmonella/mammalian microsome
mutagenicity test. Unpublished Report No. 861582 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
POLTERA, A.A., ROUAN, M.C., LECAILLON, J.B. & DUBOIS, J.P. (1989).
Kinetics in humans. Unpublished Report No. CRB R37/1989 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
RICHARDS, R.J., BOUVARD, H.R. & STRITTMATTER, J. (1985). Effect on
reproduction in sheep of triclabendazole/fenbendazole treatments
given during the first month of pregnancy. Unpublished Report No.
CRA 154/85T from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982a). Report on acute oral LD50 in the rat of CGA
110'752. Unpublished Report No. 811294 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982b). Report on acute oral LD50 in the rat of CGA
110'753. Unpublished Report No. 811295 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987a). Acute dermal irritation/corrosion study in the
rabbit. Unpublished Report No. 861573 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987b). Acute eye irritation/corrosion study in the
rabbit. Unpublished Report No. 861572 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991). Kinetics in dogs
and rats. Unpublished Report No. CRB R25/1991 from CIBA-GEIGY,
France and Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985a). Chromosome studies on human
lymphocytes in vitro. Unpublished Report No. 840944 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985b). Chromosome studies on male germinal
epithelium of mouse spermatogonia. Unpublished Report No. 840943
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., LANGAUER, M. & ARNI, P. (1988). Micronucleus test
(mouse). Unpublished Report No. 861579 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRASSER, F. & MULLER, D. (1984). Nucleus anomaly test in somatic
interphase nuclei of Chinese hamster. Unpublished Report No. 831518
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., PURI, E. & ARNI, P. (1985). Chromosome studies on the
germinal epithelium of mouse spermatocytes. Unpublished Report No.
840946 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
STRONG, M.B. (1981). Studies on the lambing performance of ewes
following multiple dosing with CGA-89317 during the first trimester
of pregnancy. Unpublished Report No. 81/10/881 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRONG, M.B. & RYAN, K.J. (1981) Preliminary field studies with
CGA-89317 in pregnant ewes. Unpublished Report No. 81/1/845 from
CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRONG, M.B. & STEIGER, R.F. (1983). Studies on the lambing
performance of ewes given a single elevated dose of CGA-89317
(triclabendazole) during the first trimester of pregnancy.
Unpublished Report No. 83/10/967A from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
TAUPIN, P.J.Y. (1981). CGA 89'317, 13 week dietary toxicity study in
the dog. Unpublished Report No. 2588--380/6 from Hazleton
Laboratories Europe, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
THOUIN, M.H., SCHOCH, M., SCHMIDT, B & GFELLER, W. (1988).
Developmental toxicity (teratogenicity) study. Unpublished Report
No. 861577 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979a). Report on acute oral LD50 in
the rabbit of technical CGA 89'317. Unpublished Report No. 791074
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979b). Report on acute aerosol
inhalation toxicity in the rat of technical CGA 89'317. Unpublished
Report No. 791078 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, L. (1979c). Report on skin irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790040 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979d). Report on eye irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790041 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979e). Report on skin sensitizing
(contact allergenic) effect in guinea pigs of technical CGA 89'317.
Unpublished Report No. 791077 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991a).
Kinetics in female rabbits. Unpublished Report No. CRB R22/1991 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H. & PROBST, A. (1991b). Absorption and
disposition studies in female rabbits. Unpublished Report No. DM
12/1991 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
YOSHIMURA, H. (1987). Teratogenic evaluation of triclabendazole in
rats. Toxicology, 43: 283-287.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M+F oral >8000 Bathe & Sachsse (1979a)
Rat M+F oral >8000 Bathe & Sachsse (1979b)
M+F dermal >4000 Bathe & Sachsse (1979c)
M+F inhalation >500 mg/m3 Ullman & Sachsse (1979b)
M+F ip 1666 Bathe & Sachsse (1979d)
Rabbit M+F oral 206 Ullman & Sachsse (1979a)
The major toxic signs were sedation, dyspnea, exophthalmos,
ruffled fur and curved body position after oral, dermal or
inhalation exposure. Ataxia was also noted following intraperitoneal
dosing.
The oral LD50 in male and female rats of the sulfoxide and
sulfone metabolites was greater than 5000 mg/kg bw. Toxic signs were
similar to those induced by triclabendazole (Sarasin, 1982a,b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Charles River CD rats were fed
diets containing 0, 10, 100 or 1000 ppm triclabendazole (purity 97%)
for 13 weeks. The calculated intake of the test material was 0.7,
6.6 and 68.5 mg/kg bw/day in males and 0.8, 7.9 and 87.3 mg/kg
bw/day in females.
There was no effect on survival and there were no overt
clinical signs. Food and water intake were reduced in 1000 ppm males
while body-weight gain was depressed in 100 ppm males and 1000 ppm
males and females. Ophthalmological and hearing tests carried out
before treatment and at weeks 6 and 13 revealed no treatment-related
effects.
Haematology, blood chemistry and urinalysis were performed
before treatment and at weeks 5 and 12. Numbers of erythrocytes,
haemoglobin and haematocrit were reduced in 1000 ppm males and
females; lymphocytes and therefore total white blood cells were
reduced in 1000 ppm females. Serum alkaline phosphatase was
increased in 1000 ppm groups. Cholesterol, albumin and total protein
were increased at 1000 ppm, particularly in females. Urine volume
was reduced in 1000 ppm males.
All animals were autopsied. Organ weights were unaffected.
Gross examination showed increases in pale kidneys and livers at 100
and 1000 ppm and in congested lungs at 1000 ppm, but pathology was
unremarkable. The NOEL was 10 ppm, equal to 0.7 mg/kg bw/day (Hunter
et al., 1982).
2.2.2.2 Dogs
Groups of 6 male and 6 female beagle dogs were fed diets
containing 0, 10, 100, or 1000 ppm triclabendazole (purity 97.6%)
for 13 weeks. The calculated intake of the test material was 0.35,
3.4 or 37 mg/kg bw/day in males and 0.35, 3.5 or 39 mg/kg bw/day in
females.
There were no overt clinical signs of toxicity. Food intake was
unaffected but body-weight gain was markedly depressed in 1000 ppm
dogs. Ophthalmology was tested before treatment and at weeks 6 and
13 with no evidence of treatment-related effects. ECG's revealed
prolongation of the QT interval and QTc value in 1000 ppm males and
females at weeks 5 and 9 but not at week 13.
Haematology and blood chemistry were examined before treatment
and at weeks 5, 9 and 13. Erythrocytes, haemoglobin and haematocrit
were markedly reduced at 1000 ppm throughout the study, with
evidence of reticulocytosis at week 9 only. Alkaline phosphatase was
increased in 100 and 1000 ppm groups; serum GPT and cholesterol were
increased in 1000 ppm dogs. Urinalysis, performed before treatment
and at weeks 6 and 13, was unaffected.
A full necropsy was undertaken on all animals;
treatment-related effects were seen at the 1000 ppm dose only. Liver
weight was increased and showed centrilobular hepatocellular pigment
granules accompanied by cytoplasmic basophilia, glycogen depletion
and foci of pigmented macrophages. Ovaries and testes were immature
with lower organ weights - females failing to reach oestrous and
males showing incomplete spermatogenesis. The NOEL was 10 ppm, equal
to 0.35 mg/kg bw/day (Taupin, 1981).
2.2.3 Long-term/carcinogenicity studies.
2.2.3.1 Mice
Groups of 80 male and 80 female albino Tif:MAGf mice were fed
diets containing 0, 3, 15, 60 or 300 ppm triclabendazole (purity
99.5%) for 2 years. Within each group, animals were designated for
interim sacrifice at 1 year (10 M and 10 F), for use in clinical
laboratory measurements (10 M and 10 F) and a further 10 M and 10 F
for examination of haematological parameters. The calculated intake
of the test material was 0.29, 1.44, 5.7 or 29.6 mg/kg bw/day in
males and 0.27, 1.39, 5.35 or 28.7 mg/kg bw/day in females.
Treated mice showed no overt signs of toxicity and mortality
was comparable to controls. There were no meaningful effects on food
and water intake while body-weight gain was slightly elevated in all
treated male groups, particularly during the first year. However,
the effect was small (10% or less), not dose-related and was not
observed in females.
Ophthalmology, haematology, blood chemistry and urinalysis were
performed at 6 month intervals. Serum levels of alkaline
phosphatase, GPT and GOT were increased in the 300 ppm groups during
the first year and in all treated groups in the second year. Effects
in the second year were not dose-related and significance was
achieved only occasionally.
All animals were subjected to a full necropsy. There were no
findings attributable to treatment at the interim sacrifice. At the
end of the study, absolute and relative liver weights were increased
at 15 ppm and above. Hepatomas were increased in all treated female
groups, but significance was not reached at the 99% level. The
incidence of carcinomas and time to appearance of tumours were
unaffected (Table 2).
Table 2. Hepatic tumour incidence in female mice
Triclabendazole (ppm) 0 3 15 60 300
Females in study 80 80 80 80 80
Benign hepatoma 7 16 15 9 20
Hepatocelular carcinoma 1 2 2 2 3
Hepatoblastoma 0 0 0 1 0
Total hepatic tumours 8 18 17 12 23
The NOEL was 3 ppm, equal to 0.27 mg/kg bw/day (Basler
et al., 1988a).
2.2.3.2 Rats
Groups of 60 male and 60 female Charles River CD rats were fed
diets containing 0, 3, 15, 30 or 100 ppm triclabendazole (purity
99.5%) for 2 years. Additional groups were designated for interim
sacrifice at 1 year (10 M and 10 F) and for haematology,
biochemistry and urinalysis investigations (20 M and 20 F). The
calculated intake of the test material was 0.1, 0.6, 1.2 or 4.0
mg/kg bw/day for males and 0.2, 0.7, 1.5 or 5.2 mg/kg bw/day for
females.
There was no effect on survival and there were no overt toxic
signs. Food and water intake were unaffected but body-weight gain
was depressed at 100 ppm, significant only in females. Ophthalmology
and hearing tests, measured every six months, were also unaffected.
Clinical laboratory parameters were examined pre-test and at
weeks 13, 26, 51, 78 and 104. Lymphocyte counts were reduced at
termination in 30 and 100 ppm groups but the values were reported to
be within normal variation. Slight decreases in plasma chloride and
BUN and increases in calcium and protein in 100 ppm males and slight
increases in protein and decreases in BUN in all treated female
groups were judged to be of no toxicological significance as the
variations were small and not consistent throughout the study and
were reported to be within normal variation. There were no
alterations in urinalysis.
A full necropsy was performed on all rats. Kidney weights were
lower in 100 ppm males at the interim kill but not at the terminal
sacrifice. At pathology, pancreatic islet cell adenomas were
increased in 15 and 100 ppm males. The relationship to treatment was
questionable as statistical significance was not achieved, there was
no dose relationship and greater numbers of carcinomas were seen in
control males (Table 3).
Table 3. Pancreatic islet cell tumours in male rats (70/group)
Dose (ppm) 0 3 15 30 100
Adenomas 3 4 12 4 11
Carcinomas 6 4 4 2 4
Total 9 8 16 6 15
The NOEL was 30 ppm, equal to 1.2 mg/kg bw/day (Charnley
et al., 1986).
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 20 male and 20 female Tif:RAIf rats were fed diets
containing 0, 3, 15 or 75 ppm triclabendazole (purity 97.6%). Dosing
of the initial generation (P) commenced 62 days prior to mating and
was continued throughout the second generation (F1) until post
partum day 35 of the third generation (F2) pups. The calculated
average intake of test compound was 0.2, 1.1 or 5.5 mg/kg bw/day in
males and 0.3, 1.4 or 7.4 mg/kg bw/day in females.
Observations of P and F1 generation adults revealed no overt
signs of toxicity, no effects on body-weight gain and no influence
on reproductive parameters.
Examination of pups, including a range of early developmental
and behavioural tests, showed no meaningful changes in the F1
offspring. In the F2 pups, mortality was increased in 15 and 75
ppm groups during lactation. The increase was significant when
compared to concurrent controls but not when compared to the F1
groups. Body weights at weaning were lower at 15 and 75 ppm but
significance was not achieved. The NOEL was 75 ppm, equal to 5.5
mg/kg bw/day (Fritz et al., 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity.
2.2.5.1 Rats
Groups of 25 pregnant Tif:RAIf rats were given gavage doses of
0, 10, 30 or 100 mg/kg bw/day of triclabendazole (purity 97.6%) as a
suspension in 0.5% (w/v) carboxymethyl cellulose. Treatment was on
gestation days 6 to 15 and females were killed on gestation day 21.
There was no mortality or clinical signs related to treatment.
Body-weight gain and food consumption were markedly depressed at 100
mg/kg bw/day with slight reduction in weight gain at 10 and 30 mg/kg
bw/day. Gross pathology of dams was unremarkable.
The implantation rate was similar in each group and treatment
did not influence the number of resorptions or fetal deaths. Live
fetus weight was reduced at 100 mg/kg bw/day but no external,
visceral or skeletal malformations were induced. The NOEL was 30
mg/kg bw/day (Giese et al., 1981a; Giese, 1987).
Groups of 20 pregnant Sprague-Dawley rats were given gavage
doses of 0, 10, 25, 50, 100 or 200 mg/kg bw/kg of triclabendazole
(purity 100%) as a suspension in water. Treatment was on gestation
days 8 to 15 and dams were killed on gestation day 21.
Maternal weight gain was depressed at 100 and 200 mg/kg bw/day
with no signs of toxicity at lower levels. Uterine and fetal
examinations revealed no effects on embryo or fetal survival or on
fetal malformations. The only fetal effect was reduced body weight
at 100 and 200 mg/kg bw/day. The NOEL was 50 mg/kg bw/day
(Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 pregnant chinchilla rabbits were given gavage
doses of 0, 3, 10 or 20 mg/kg bw/day of triclabendazole (purity
unknown) as a suspension in 0.5% (w/v) carboxymethyl cellulose.
Treatment was on gestation days 6 to 18 with does killed on
gestation day 28.
Three low-dose females died and diarrhoea was noted in a few
animals from each treated group. Maternal body-weight gain during
the study was similar in all groups but when "corrected" by
subtracting the weight of uterus and contents, there was a slight
non-dose related decrease in the treated groups.
The numbers of implantations, resorptions and fetal deaths were
similar in each group and fetal body weight was unaffected. Fetal
examinations showed unossified phalanges of fore and hind limbs at
10 and 20 mg/kg bw/day. One 20 mg/kg bw/day fetus exhibited
omphalocele which is rare in this strain of rabbit. The NOEL was 3
mg/kg bw/day (Giese et al., 1981b)
2.2.5.3 Sheep
Merino ewes were drenched with a single dose of 0, 5 or 10
mg/kg bw triclabendazole in the third trimester. There were no
effects on lambing or on the morphology of offspring (Strong & Ryan,
1981).
In a series of experiments, merino ewes were drenched with
doses of 0 or 30 mg/kg bw/day triclabendazole before conception and
during the first trimester of pregnancy as follows:
A. 3 treatments during the first 24 days of gestation.
B. 4 treatments during the first 43 days of gestation.
C. 2 treatments 2 to 3 weeks before conception plus
2 treatments during the first 25 days of gestation.
D. 3 treatments 2 to 4 weeks before conception plus
1 treatment 7 to 9 days after conception.
E. 3 treatments 2 to 4 weeks before conception.
None of the treatment regimes caused clinical signs of toxicity
in the ewes, nor were there effects on lambing or development of
lambs (Strong, 1981).
Groups of 27 to 28 pregnant Merino ewes were drenched with a
single dose of 0 or 50 mg/kg bw of triclabendazole. Drug
administration was on days 12, 17, 21, 24 or 28 days after mating.
There were no effects on lambing performance or development of lambs
(Strong & Steiger, 1983).
2.2.5.4 Cattle
Hereford cows were drenched with a single dose of 24 mg/kg bw
triclabendazole during the first month of pregnancy. Calving and
uterine development of offspring were unaffected (Bowen & Ryan,
1985a).
Hereford or black poll cows were drenched with a single dose of
0 or 24 mg/kg bw/day triclabendazole during the second, third,
fourth and sixth to seventh month of pregnancy. There were no
difficulties at calving or abnormalities in the calves (Bowen &
Ryan, 1985b).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies on triclabendazole are
summarized in Table 4.
Table 4. Results of genotoxicity studies on triclabendazole1
Test system Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.5-1250 µg/plate Negative Ogorek & Arni,
TA98, TA100, 1987a
TA1535, TA1537,
TA1538
Forward mutation2 Chinese hamster 0.025-0.5 µg/ml (-S9) Negative Dollenmeier &
V79 cells 3.5-70 µg/ml (+S9) Puri, 1988
Unscheduled Primary rat 0.3-40 µg/ml Negative Hertner & Puri,
DNA-synthesis hepatocytes 1988
Unscheduled Human fibroblasts 0.4-60 µg/ml Negative Meyer & Puri,
DNA-synthesis 1988
Nucleus anomaly Chinese hamster 172-688 mg/kg bw Negative Hool et al.,
bone marrow by gavage 1982
Sister chromatid Chinese hamster 173-692 mg/kg bw by Negative Hool & Muller,
exchange bone marrow gavage 1982
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Groups of 10 male and 10 female pirbright guinea-pigs were used
in an optimization test. The induction phase was carried out by
administering 10 intracutaneous injections of a 0.1% (w/v)
suspension in propylene glycol/saline. Epidermal challenge produced
no reaction but an intradermal challenge resulted in a positive
sensitization reaction (Ullmann & Sachsse, 1979e)
2.2.7.2 Rabbits
Groups of 3 male and 3 female New Zeeland white rabbits were
used in a patch test. Triclabendazole was suspended in propylene
glycol/saline and applied for 24 h. The test material was judged to
be a slight skin irritant (Ullmann & Sachsse, 1979c)
Groups of 3 male and 6 female New Zeeland white rabbits
received 0.1 g of triclabendazole instilled into the conjunctival
sac of the eye. Subsequent examination failed to reveal any eye
irritation (Ullmann & Sachsse, 1979d).
2.2.8 Special studies on triclabendazole and fenbendazole in
combination
A series of studies was conducted using test material
containing 50% triclabendazole and 50% fenbendazole that are briefly
reviewed below.
Pregnant rats were treated with gavage doses of 0, 10, 40 or
120 mg/kg bw/day test material during gestation days 6 to 15. At 120
mg/kg bw/day, dams had a reduced food consumption and there were
increases in partial abortion of conceptuses and in resorptions.
Fetotoxicity at the same level included decreased fetal weight,
reduced ossification, kinked tail, meningo-encephalocele and
hydrocephaly. Delayed ossification was present in the 40 mg/kg
bw/day fetuses while there were no adverse findings in the 10 mg/kg
bw/day group (Giese et al., 1985).
In two developmental studies in sheep, pregnant ewes received
either two doses of 0 or 50 mg/kg bw or a single dose of 0 or 150
mg/kg bw, during the first month of pregnancy. There were no effects
on lambing performance and the only adverse effect on offspring was
grossly misshapen kidneys, with disorganized internal structure, in
3 of 25 lambs at the higher-dose level (Bowen & Heckenberg, 1985;
Richards et al., 1985).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Saccharomyces cerevisiae D7 were
negative, both in the presence and absence of rat liver microsomes
(Deparade & Arni, 1983; Hool & Arni, 1985). Chromosome aberrations
in cultured human lymphocytes were increased at 22 and 44 µg/ml
(dose-related), but not at 11 µg/ml, only in the presence of a rat
liver S9 fraction (Strasser & Arni, 1985a). The bone marrow of
Chinese hamsters revealed an increase in nucleus anomalies (single
Jolly bodies only) following oral doses of 1000, 2000 or 4000 mg/kg
bw. While the increases were statistically significant they were
only slight and non dose-related (Strasser & Muller, 1984).
Chromosome aberrations were not induced in mouse primary and
secondary spermatocytes or spermatogonia after oral doses of up to
2500 mg/kg bw (Strasser & Arni, 1985b; Strasser et al., 1985).
2.2.9 Special studies on triclabendazole and levamisole in
combination
A series of studies was conducted using test material
containing 61.5% triclabendazole and 38.5% levamisole that are
briefly reviewed below.
Acute studies in male and female rats revealed LD50 values of
1996 mg/kg bw by the oral route and >2000 mg/kg bw by the dermal
route (Hartmann & Schoch, 1987a,b). In rabbits, slight and
reversible eye irritation was observed while the test material was
judged to be non-irritant to skin (Schoch, 1987a,b).
Rats were administered 0, 20, 200 or 2000 ppm of the test
material in the feed for 3 months. Body-weight gain was depressed at
200 ppm and above and anaemia was apparent at 2000 ppm. Increased
serum levels of liver enzymes and cholesterol and decreased levels
of triglycerides were suggestive of hepatocellular damage at 2000
ppm and, to a lesser extent, at 200 ppm. Necropsy revealed the
following slight changes at 2000 ppm: chronic lesion of renal
tubules, spermatic granulomas in the epididymis, fatty change in the
adrenal cortex, thymic atrophy and skeletal muscle atrophy. There
were no effects at 20 ppm, equal to 1.4 mg/kg bw/day (Basler
et al., 1988b).
Dogs were fed diets containing 0, 20, 60, 240 or 1000 ppm for 3
months. At 1000 ppm, body weight gain and food intake were reduced
and half the animals died. The administration of this dose was
discontinued after 45 days. Anaemia, increased alkaline phosphatase
and cholesterol were evident at 240 ppm and above. Increases were
observed in liver weight at 240 ppm and spleen weight at 60 and 240
ppm. There was increased haematopoiesis of the sternum at 60 ppm and
above. Survivors in the 1000 ppm group, which were not dosed for the
previous 45 days, showed at least partial recovery of toxic effects.
There were no effects at 20 ppm, equal to 0.75 mg/kg bw/day (Monnot,
1988).
Pregnant rats were treated with gavage doses of 0, 10, 80 or
160 mg/kg bw/day during gestation days 6 to 15. Ruffled fur was seen
at 160 mg/kg bw/day and increased wet bedding was noted at 80 mg/kg
bw/day and above, suggesting polyuria. Maternal body-weight gain and
food intake were depressed at 80 and 160 mg/kg bw/day. The only
fetal effects, seen at 80 and 160 mg/kg bw/day, were decreased fetal
body weight, increased bipartite sternebrae and reduced
ossification. There were no adverse effects at 10 mg/kg bw/day
(Thouin et al., 1988).
Pregnant rabbits received gavage doses of 0, 1.5, 30 or 50
mg/kg bw/day on gestation days 7 to 19. In the 50 mg/kg bw/day
group, there was reduced body weight gain, one abortion and total
resorption of conceptuses in one rabbit. The resorption rate was
slightly increased at 50 mg/kg bw/day but significance was not
achieved. There were no increased developmental effects in any
group. No adverse effect was noted up to 30 mg/kg bw/day (Khalil
et al., 1989).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Chinese hamster V79 cells gave negative
results both in the presence and absence of rat liver microsomes
(Ogorek & Arni, 1987b; Dollenmeier & Puri, 1987). Using isolated rat
hepatocytes and human fibroblasts there was no evidence of
unscheduled DNA synthesis in exposed cells (Hertner & Puri, 1987;
Meyer & Puri, 1987). Micronuclei were not induced in bone marrow of
mice dosed orally with the test substance (Strasser et al., 1988).
3. COMMENTS
A range of studies on triclabendazole was submitted for
assessment, including data on kinetics and metabolism, acute
toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity.
Approximately 40-50% was absorbed following the administration
of single oral doses of radiolabelled triclabendazole to rats and
dogs. Oral absorption was considerably higher in rabbits but could
not be quantified in a human study. Peak plasma levels of
radioactivity were generally achieved within 8 h; elimination
(primarily in the faeces) was rapid in rats, rabbits, and humans. In
dogs, peak plasma levels of radioactivity were maintained for 2-3
days and significant levels persisted for more than 7 days.
Triclabendazole was rapidly and extensively degraded in rabbits,
dogs, and humans, the sulfoxide and sulfone being the main
metabolites found in plasma. In addition to these oxidation
products, rat excreta contained 4-hydroxytriclabendazole and
2-benzimidazolone.
Single oral doses of triclabendazole were slightly toxic (LD50
> 8000 mg/kg bw) in mice and rats and moderately toxic (LD50 =
206 mg/kg bw) in rabbits, whose greater sensitivity was probably due
to a greater bioavailability of oral doses.
Findings after short-term oral dosing in rats and dogs were
primarily non-specific. In 3-month studies, decreased weight gain
was observed at 7 mg/kg bw/day and above in rats and at 37 mg/kg
bw/day in dogs; anaemia was seen at 68 mg/kg bw/day and 37 mg/kg
bw/day, respectively. Evidence of hepatotoxicity, for example
increases in plasma levels of liver enzymes and cholesterol and in
liver weight, was seen at approximately 70 mg/kg bw/day in rats and
37 mg/kg bw/day in dogs. Minor non-neoplastic liver lesions were
observed in a 3-month study in dogs at 37 mg/kg bw/day. The NOELs
were 0.7 mg/kg bw/day in rats and 0.35 mg/kg bw/day in dogs.
Hepatomas were seen in female mice given up to 29 mg/kg bw/day
for 2 years. The incidences of adenomas were higher than in controls
at all dose levels, but a dose-response relationship was not found.
Significance at the 99% level, which is widely used to assess the
significance of tumours that occur at a high background rate, was
not reached for this common benign mouse tumour. In addition, there
was no significant increase in the incidence of hepatocellular
carcinomas at any dose level. The NOEL was 0.27 mg/kg bw/day based
on increased liver weight at the next highest dose, 1.4 mg/kg
bw/day. Tumours were not induced in a 2-year dietary study in rats,
the NOEL being 1.2 mg/kg bw/day based on reduced weight gain at 4
mg/kg bw/day. Several different in vitro and in vivo
genotoxicity studies were clearly negative. These results suggest
that triclabendazole has no carcinogenic potential.
In a two-generation reproduction study in rats, postnatal
survival and growth were reduced during the lactation period of the
second generation at 1 mg/kg bw/day and above. However, the
incidence of these effects was not dose-related and was similar to
that in the first-generation offspring. In addition, statistical
significance was not achieved, and it was concluded that the
findings were not related to treatment. The NOEL was 5.5 mg/kg
bw/day.
Developmental studies in rats and rabbits provided no evidence
of embryotoxicity. Fetotoxicity, in the form of reduced fetal weight
in rats and decreased ossification in rabbits, was observed at 100
and 10 mg/kg bw/day, respectively. One rabbit fetus at 20 mg/kg
bw/day exhibited omphalocele, a rare abnormality in the strain used.
All the above effects were present only at maternally toxic dose
levels. Drug treatment during pregnancy in sheep and cattle did not
affect growth and development of the conceptus. The NOELs were 50
mg/kg bw/day in rats and 3 mg/kg bw/day in rabbits.
Special studies with triclabendazole in combination with either
fenbendazole (developmental and genotoxicity studies) or levamisole
(acute, subchronic, developmental, and genotoxicity studies) did not
reveal any synergism between the drugs.
4. EVALUATION
The database in support of the safety evaluation of
triclabendazole is substantial. The lowest NOEL was 0.27mg/kg
bw/day, based on increased liver weight in the long-term study in
mice. Using a safety factor of 100, the Committee established an ADI
for triclabendazole of 0-3 µg/kg bw.
5. REFERENCES
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, G., MALINOWSKI, W.,
CHRISTEN, P., FORMICA, G. & GFELLER, W. (1988a). Lifetime
carcinogenicity and chronic toxicity study in mice. Unpublished
Report No. 811708 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, A.L., MALINOWSKI,
W. & GFELLER, W. (1988b), 3-month oral toxicity study in rats
(administration in feed). Unpublished Report No. 861575 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979a). Report on acute oral LD50 in the
mouse of technical CGA 89'317. Unpublished Report No. 791073 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979b). Report on acute oral LD50 in the
rat of CGA 89'317. Unpublished Report No. 790038 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BATHE, R. & SACHSSE, K. (1979c). Report on acute dermal LD5O in
the rat of technical CGA 89'317. Unpublished Report No. 791076 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979d). Report on acute intraperitoneal
LD50 in the rat of CGA 89'317. Unpublished Report No. 790039 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BOWEN, F.L. & HECKENBERG, K. (1985). Reproduction study in sheep:
triclabendazole plus fenbendazole. Unpublished Report No. 85/12/1060
from CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985a). A field trial to assess the safety
of triclabendazole for cows during their first month of pregnancy.
Unpublished Report No. 85/11/1054 from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985b). Field trials to assess the safety
of triclabendazole for cows during the second to sixth month of
pregnancy. Unpublished Report No. 85/11/1055 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
CHARNLEY, B.N., HEYWOOD, R., STREET, A.E., IMM, S.A.,
GIBSON, W.A., GOPINATH, C., ALMOND, R.H. & DAWE, I.S. (1986).
Potential tumorigenic and toxic effects in prolonged dietary
administration to rats. Unpublished Report No. CBG 318/85595
(811709) from Huntingdon Research Centre, UK. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
DEPARADE, E. & ARNI, P. (1983). Salmonella/mammalian-microsome
mutagenicity test. Unpublished Report No. 831517 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1987). Point mutation test with Chinese
hamster cells V79. Unpublished Report No. 871011 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1988). Point mutation test with Chinese
hamster cells V79 with CGA 89'317 tech. Unpublished Report No.
871170 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
FRITZ, H., SUTER, H.P., ZAK, F., NAYLOR, D.C. & HESS, R. (1984).
Report on CGA 89'317: 2-Generation toxicity study in rats.
Unpublished Report No. 811540 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K. (1987). Amendment No. 1 to Final Report on CGA 89'317
tech., Teratology study (Seg. II) in rats. Unpublished Report
amendment No. 802099-1 from CIBA-GEIGY, Switzerland. Submitted to
WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & GFELLER, W. (1985). Report on CGA 89317
Tech and CGA 50560 Tech (1:1). Teratology study in rats. Unpublished
Report No. 831213 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981a). Report on CGA 89'317
tech. Teratology study (Seg. II) in rats. Unpublished Report No.
802099 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981b). Report on CGA 89'317
tech. Teratology study in rabbits. Unpublished Report No. 802090
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
HAMBOCK, H. (1983). The metabolic fate of CGA 89317 in sheep, rat
and the lactating goat. Unpublished Report No. 41/83 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987a). Acute oral toxicity in the rat.
Unpublished Report No. 861571 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987b). Acute dermal toxicity in the
rat. Unpublished Report No. 861574 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1987). Autoradiographic DNA-repair test on
rat hepatocytes. Unpublished Report No. 861580 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1988). Autoradiographic DNA repair test on
rat hepatocytes (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871173 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
HOOL, G. & ARNI, P. (1985). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro. Unpublished Report No.
840945 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G., LANGAUER, M. & MULLER, D. (1982). Nucleus anomaly test in
somatic interphase nuclei, CGA 89'317, Chinese hamster (test for
mutagenic effects on bone marrow cells). Unpublished Report No.
801392 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G. & MULLER, D. (1982). Sister chromatid exchange study, CGA
89'317, Chinese hamster (Test for mutagenic effect on bone marrow
cells). Unpublished Report No. 820209 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HUNTER, B., BERRYMAN, E.L., HEYWOOD, R., PRENTICE, D.E., STREET,
A.E., GIBSON, W.A., SINGH, H. & OFFER, J.M. (1982). CGA 89,317, a
13-week toxicity study in rats by incorporation in the diet.
Unpublished Report No. CBG/252/81151 (791817) from Huntingdon
Research Centre, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
KHALIL, S., THONIN, M.H., WEBER H., GRUENDER, G. & BASLER, W.
(1989). Developmental toxicity (teratogenicity study). Unpublished
Report No. 881433 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1987). Autoradiographic DNA-repair test on
human fibroblasts. Unpublished Report No. 861581 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1988). Autoradiographic DNA repair test on
human fibroblasts (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871172 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MONNOT, G. (1988). 13-week dietary administration toxicity study in
the beagle dog. Unpublished Report No. 380/520 (861576) from
Hazleton, France. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
MUECKE, W. (1981). Distribution, degradation and excretion of CGA
89317 in the rat. Unpublished Report No. 27/81 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987a). Salmonella/mammalian microsome
mutagenicity test (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871171 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987b). Salmonella/mammalian microsome
mutagenicity test. Unpublished Report No. 861582 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
POLTERA, A.A., ROUAN, M.C., LECAILLON, J.B. & DUBOIS, J.P. (1989).
Kinetics in humans. Unpublished Report No. CRB R37/1989 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
RICHARDS, R.J., BOUVARD, H.R. & STRITTMATTER, J. (1985). Effect on
reproduction in sheep of triclabendazole/fenbendazole treatments
given during the first month of pregnancy. Unpublished Report No.
CRA 154/85T from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982a). Report on acute oral LD50 in the rat of CGA
110'752. Unpublished Report No. 811294 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982b). Report on acute oral LD50 in the rat of CGA
110'753. Unpublished Report No. 811295 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987a). Acute dermal irritation/corrosion study in the
rabbit. Unpublished Report No. 861573 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987b). Acute eye irritation/corrosion study in the
rabbit. Unpublished Report No. 861572 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991). Kinetics in dogs
and rats. Unpublished Report No. CRB R25/1991 from CIBA-GEIGY,
France and Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985a). Chromosome studies on human
lymphocytes in vitro. Unpublished Report No. 840944 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985b). Chromosome studies on male germinal
epithelium of mouse spermatogonia. Unpublished Report No. 840943
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., LANGAUER, M. & ARNI, P. (1988). Micronucleus test
(mouse). Unpublished Report No. 861579 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRASSER, F. & MULLER, D. (1984). Nucleus anomaly test in somatic
interphase nuclei of Chinese hamster. Unpublished Report No. 831518
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., PURI, E. & ARNI, P. (1985). Chromosome studies on the
germinal epithelium of mouse spermatocytes. Unpublished Report No.
840946 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
STRONG, M.B. (1981). Studies on the lambing performance of ewes
following multiple dosing with CGA-89317 during the first trimester
of pregnancy. Unpublished Report No. 81/10/881 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRONG, M.B. & RYAN, K.J. (1981) Preliminary field studies with
CGA-89317 in pregnant ewes. Unpublished Report No. 81/1/845 from
CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRONG, M.B. & STEIGER, R.F. (1983). Studies on the lambing
performance of ewes given a single elevated dose of CGA-89317
(triclabendazole) during the first trimester of pregnancy.
Unpublished Report No. 83/10/967A from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
TAUPIN, P.J.Y. (1981). CGA 89'317, 13 week dietary toxicity study in
the dog. Unpublished Report No. 2588--380/6 from Hazleton
Laboratories Europe, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
THOUIN, M.H., SCHOCH, M., SCHMIDT, B & GFELLER, W. (1988).
Developmental toxicity (teratogenicity) study. Unpublished Report
No. 861577 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979a). Report on acute oral LD50 in
the rabbit of technical CGA 89'317. Unpublished Report No. 791074
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979b). Report on acute aerosol
inhalation toxicity in the rat of technical CGA 89'317. Unpublished
Report No. 791078 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, L. (1979c). Report on skin irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790040 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979d). Report on eye irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790041 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979e). Report on skin sensitizing
(contact allergenic) effect in guinea pigs of technical CGA 89'317.
Unpublished Report No. 791077 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991a).
Kinetics in female rabbits. Unpublished Report No. CRB R22/1991 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H. & PROBST, A. (1991b). Absorption and
disposition studies in female rabbits. Unpublished Report No. DM
12/1991 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
YOSHIMURA, H. (1987). Teratogenic evaluation of triclabendazole in
rats. Toxicology, 43: 283-287.
 Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M+F oral >8000 Bathe & Sachsse (1979a)
Rat M+F oral >8000 Bathe & Sachsse (1979b)
M+F dermal >4000 Bathe & Sachsse (1979c)
M+F inhalation >500 mg/m3 Ullman & Sachsse (1979b)
M+F ip 1666 Bathe & Sachsse (1979d)
Rabbit M+F oral 206 Ullman & Sachsse (1979a)
The major toxic signs were sedation, dyspnea, exophthalmos,
ruffled fur and curved body position after oral, dermal or
inhalation exposure. Ataxia was also noted following intraperitoneal
dosing.
The oral LD50 in male and female rats of the sulfoxide and
sulfone metabolites was greater than 5000 mg/kg bw. Toxic signs were
similar to those induced by triclabendazole (Sarasin, 1982a,b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Charles River CD rats were fed
diets containing 0, 10, 100 or 1000 ppm triclabendazole (purity 97%)
for 13 weeks. The calculated intake of the test material was 0.7,
6.6 and 68.5 mg/kg bw/day in males and 0.8, 7.9 and 87.3 mg/kg
bw/day in females.
There was no effect on survival and there were no overt
clinical signs. Food and water intake were reduced in 1000 ppm males
while body-weight gain was depressed in 100 ppm males and 1000 ppm
males and females. Ophthalmological and hearing tests carried out
before treatment and at weeks 6 and 13 revealed no treatment-related
effects.
Haematology, blood chemistry and urinalysis were performed
before treatment and at weeks 5 and 12. Numbers of erythrocytes,
haemoglobin and haematocrit were reduced in 1000 ppm males and
females; lymphocytes and therefore total white blood cells were
reduced in 1000 ppm females. Serum alkaline phosphatase was
increased in 1000 ppm groups. Cholesterol, albumin and total protein
were increased at 1000 ppm, particularly in females. Urine volume
was reduced in 1000 ppm males.
All animals were autopsied. Organ weights were unaffected.
Gross examination showed increases in pale kidneys and livers at 100
and 1000 ppm and in congested lungs at 1000 ppm, but pathology was
unremarkable. The NOEL was 10 ppm, equal to 0.7 mg/kg bw/day (Hunter
et al., 1982).
2.2.2.2 Dogs
Groups of 6 male and 6 female beagle dogs were fed diets
containing 0, 10, 100, or 1000 ppm triclabendazole (purity 97.6%)
for 13 weeks. The calculated intake of the test material was 0.35,
3.4 or 37 mg/kg bw/day in males and 0.35, 3.5 or 39 mg/kg bw/day in
females.
There were no overt clinical signs of toxicity. Food intake was
unaffected but body-weight gain was markedly depressed in 1000 ppm
dogs. Ophthalmology was tested before treatment and at weeks 6 and
13 with no evidence of treatment-related effects. ECG's revealed
prolongation of the QT interval and QTc value in 1000 ppm males and
females at weeks 5 and 9 but not at week 13.
Haematology and blood chemistry were examined before treatment
and at weeks 5, 9 and 13. Erythrocytes, haemoglobin and haematocrit
were markedly reduced at 1000 ppm throughout the study, with
evidence of reticulocytosis at week 9 only. Alkaline phosphatase was
increased in 100 and 1000 ppm groups; serum GPT and cholesterol were
increased in 1000 ppm dogs. Urinalysis, performed before treatment
and at weeks 6 and 13, was unaffected.
A full necropsy was undertaken on all animals;
treatment-related effects were seen at the 1000 ppm dose only. Liver
weight was increased and showed centrilobular hepatocellular pigment
granules accompanied by cytoplasmic basophilia, glycogen depletion
and foci of pigmented macrophages. Ovaries and testes were immature
with lower organ weights - females failing to reach oestrous and
males showing incomplete spermatogenesis. The NOEL was 10 ppm, equal
to 0.35 mg/kg bw/day (Taupin, 1981).
2.2.3 Long-term/carcinogenicity studies.
2.2.3.1 Mice
Groups of 80 male and 80 female albino Tif:MAGf mice were fed
diets containing 0, 3, 15, 60 or 300 ppm triclabendazole (purity
99.5%) for 2 years. Within each group, animals were designated for
interim sacrifice at 1 year (10 M and 10 F), for use in clinical
laboratory measurements (10 M and 10 F) and a further 10 M and 10 F
for examination of haematological parameters. The calculated intake
of the test material was 0.29, 1.44, 5.7 or 29.6 mg/kg bw/day in
males and 0.27, 1.39, 5.35 or 28.7 mg/kg bw/day in females.
Treated mice showed no overt signs of toxicity and mortality
was comparable to controls. There were no meaningful effects on food
and water intake while body-weight gain was slightly elevated in all
treated male groups, particularly during the first year. However,
the effect was small (10% or less), not dose-related and was not
observed in females.
Ophthalmology, haematology, blood chemistry and urinalysis were
performed at 6 month intervals. Serum levels of alkaline
phosphatase, GPT and GOT were increased in the 300 ppm groups during
the first year and in all treated groups in the second year. Effects
in the second year were not dose-related and significance was
achieved only occasionally.
All animals were subjected to a full necropsy. There were no
findings attributable to treatment at the interim sacrifice. At the
end of the study, absolute and relative liver weights were increased
at 15 ppm and above. Hepatomas were increased in all treated female
groups, but significance was not reached at the 99% level. The
incidence of carcinomas and time to appearance of tumours were
unaffected (Table 2).
Table 2. Hepatic tumour incidence in female mice
Triclabendazole (ppm) 0 3 15 60 300
Females in study 80 80 80 80 80
Benign hepatoma 7 16 15 9 20
Hepatocelular carcinoma 1 2 2 2 3
Hepatoblastoma 0 0 0 1 0
Total hepatic tumours 8 18 17 12 23
The NOEL was 3 ppm, equal to 0.27 mg/kg bw/day (Basler
et al., 1988a).
2.2.3.2 Rats
Groups of 60 male and 60 female Charles River CD rats were fed
diets containing 0, 3, 15, 30 or 100 ppm triclabendazole (purity
99.5%) for 2 years. Additional groups were designated for interim
sacrifice at 1 year (10 M and 10 F) and for haematology,
biochemistry and urinalysis investigations (20 M and 20 F). The
calculated intake of the test material was 0.1, 0.6, 1.2 or 4.0
mg/kg bw/day for males and 0.2, 0.7, 1.5 or 5.2 mg/kg bw/day for
females.
There was no effect on survival and there were no overt toxic
signs. Food and water intake were unaffected but body-weight gain
was depressed at 100 ppm, significant only in females. Ophthalmology
and hearing tests, measured every six months, were also unaffected.
Clinical laboratory parameters were examined pre-test and at
weeks 13, 26, 51, 78 and 104. Lymphocyte counts were reduced at
termination in 30 and 100 ppm groups but the values were reported to
be within normal variation. Slight decreases in plasma chloride and
BUN and increases in calcium and protein in 100 ppm males and slight
increases in protein and decreases in BUN in all treated female
groups were judged to be of no toxicological significance as the
variations were small and not consistent throughout the study and
were reported to be within normal variation. There were no
alterations in urinalysis.
A full necropsy was performed on all rats. Kidney weights were
lower in 100 ppm males at the interim kill but not at the terminal
sacrifice. At pathology, pancreatic islet cell adenomas were
increased in 15 and 100 ppm males. The relationship to treatment was
questionable as statistical significance was not achieved, there was
no dose relationship and greater numbers of carcinomas were seen in
control males (Table 3).
Table 3. Pancreatic islet cell tumours in male rats (70/group)
Dose (ppm) 0 3 15 30 100
Adenomas 3 4 12 4 11
Carcinomas 6 4 4 2 4
Total 9 8 16 6 15
The NOEL was 30 ppm, equal to 1.2 mg/kg bw/day (Charnley
et al., 1986).
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 20 male and 20 female Tif:RAIf rats were fed diets
containing 0, 3, 15 or 75 ppm triclabendazole (purity 97.6%). Dosing
of the initial generation (P) commenced 62 days prior to mating and
was continued throughout the second generation (F1) until post
partum day 35 of the third generation (F2) pups. The calculated
average intake of test compound was 0.2, 1.1 or 5.5 mg/kg bw/day in
males and 0.3, 1.4 or 7.4 mg/kg bw/day in females.
Observations of P and F1 generation adults revealed no overt
signs of toxicity, no effects on body-weight gain and no influence
on reproductive parameters.
Examination of pups, including a range of early developmental
and behavioural tests, showed no meaningful changes in the F1
offspring. In the F2 pups, mortality was increased in 15 and 75
ppm groups during lactation. The increase was significant when
compared to concurrent controls but not when compared to the F1
groups. Body weights at weaning were lower at 15 and 75 ppm but
significance was not achieved. The NOEL was 75 ppm, equal to 5.5
mg/kg bw/day (Fritz et al., 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity.
2.2.5.1 Rats
Groups of 25 pregnant Tif:RAIf rats were given gavage doses of
0, 10, 30 or 100 mg/kg bw/day of triclabendazole (purity 97.6%) as a
suspension in 0.5% (w/v) carboxymethyl cellulose. Treatment was on
gestation days 6 to 15 and females were killed on gestation day 21.
There was no mortality or clinical signs related to treatment.
Body-weight gain and food consumption were markedly depressed at 100
mg/kg bw/day with slight reduction in weight gain at 10 and 30 mg/kg
bw/day. Gross pathology of dams was unremarkable.
The implantation rate was similar in each group and treatment
did not influence the number of resorptions or fetal deaths. Live
fetus weight was reduced at 100 mg/kg bw/day but no external,
visceral or skeletal malformations were induced. The NOEL was 30
mg/kg bw/day (Giese et al., 1981a; Giese, 1987).
Groups of 20 pregnant Sprague-Dawley rats were given gavage
doses of 0, 10, 25, 50, 100 or 200 mg/kg bw/kg of triclabendazole
(purity 100%) as a suspension in water. Treatment was on gestation
days 8 to 15 and dams were killed on gestation day 21.
Maternal weight gain was depressed at 100 and 200 mg/kg bw/day
with no signs of toxicity at lower levels. Uterine and fetal
examinations revealed no effects on embryo or fetal survival or on
fetal malformations. The only fetal effect was reduced body weight
at 100 and 200 mg/kg bw/day. The NOEL was 50 mg/kg bw/day
(Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 pregnant chinchilla rabbits were given gavage
doses of 0, 3, 10 or 20 mg/kg bw/day of triclabendazole (purity
unknown) as a suspension in 0.5% (w/v) carboxymethyl cellulose.
Treatment was on gestation days 6 to 18 with does killed on
gestation day 28.
Three low-dose females died and diarrhoea was noted in a few
animals from each treated group. Maternal body-weight gain during
the study was similar in all groups but when "corrected" by
subtracting the weight of uterus and contents, there was a slight
non-dose related decrease in the treated groups.
The numbers of implantations, resorptions and fetal deaths were
similar in each group and fetal body weight was unaffected. Fetal
examinations showed unossified phalanges of fore and hind limbs at
10 and 20 mg/kg bw/day. One 20 mg/kg bw/day fetus exhibited
omphalocele which is rare in this strain of rabbit. The NOEL was 3
mg/kg bw/day (Giese et al., 1981b)
2.2.5.3 Sheep
Merino ewes were drenched with a single dose of 0, 5 or 10
mg/kg bw triclabendazole in the third trimester. There were no
effects on lambing or on the morphology of offspring (Strong & Ryan,
1981).
In a series of experiments, merino ewes were drenched with
doses of 0 or 30 mg/kg bw/day triclabendazole before conception and
during the first trimester of pregnancy as follows:
A. 3 treatments during the first 24 days of gestation.
B. 4 treatments during the first 43 days of gestation.
C. 2 treatments 2 to 3 weeks before conception plus
2 treatments during the first 25 days of gestation.
D. 3 treatments 2 to 4 weeks before conception plus
1 treatment 7 to 9 days after conception.
E. 3 treatments 2 to 4 weeks before conception.
None of the treatment regimes caused clinical signs of toxicity
in the ewes, nor were there effects on lambing or development of
lambs (Strong, 1981).
Groups of 27 to 28 pregnant Merino ewes were drenched with a
single dose of 0 or 50 mg/kg bw of triclabendazole. Drug
administration was on days 12, 17, 21, 24 or 28 days after mating.
There were no effects on lambing performance or development of lambs
(Strong & Steiger, 1983).
2.2.5.4 Cattle
Hereford cows were drenched with a single dose of 24 mg/kg bw
triclabendazole during the first month of pregnancy. Calving and
uterine development of offspring were unaffected (Bowen & Ryan,
1985a).
Hereford or black poll cows were drenched with a single dose of
0 or 24 mg/kg bw/day triclabendazole during the second, third,
fourth and sixth to seventh month of pregnancy. There were no
difficulties at calving or abnormalities in the calves (Bowen &
Ryan, 1985b).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies on triclabendazole are
summarized in Table 4.
Table 4. Results of genotoxicity studies on triclabendazole1
Test system Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.5-1250 µg/plate Negative Ogorek & Arni,
TA98, TA100, 1987a
TA1535, TA1537,
TA1538
Forward mutation2 Chinese hamster 0.025-0.5 µg/ml (-S9) Negative Dollenmeier &
V79 cells 3.5-70 µg/ml (+S9) Puri, 1988
Unscheduled Primary rat 0.3-40 µg/ml Negative Hertner & Puri,
DNA-synthesis hepatocytes 1988
Unscheduled Human fibroblasts 0.4-60 µg/ml Negative Meyer & Puri,
DNA-synthesis 1988
Nucleus anomaly Chinese hamster 172-688 mg/kg bw Negative Hool et al.,
bone marrow by gavage 1982
Sister chromatid Chinese hamster 173-692 mg/kg bw by Negative Hool & Muller,
exchange bone marrow gavage 1982
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Groups of 10 male and 10 female pirbright guinea-pigs were used
in an optimization test. The induction phase was carried out by
administering 10 intracutaneous injections of a 0.1% (w/v)
suspension in propylene glycol/saline. Epidermal challenge produced
no reaction but an intradermal challenge resulted in a positive
sensitization reaction (Ullmann & Sachsse, 1979e)
2.2.7.2 Rabbits
Groups of 3 male and 3 female New Zeeland white rabbits were
used in a patch test. Triclabendazole was suspended in propylene
glycol/saline and applied for 24 h. The test material was judged to
be a slight skin irritant (Ullmann & Sachsse, 1979c)
Groups of 3 male and 6 female New Zeeland white rabbits
received 0.1 g of triclabendazole instilled into the conjunctival
sac of the eye. Subsequent examination failed to reveal any eye
irritation (Ullmann & Sachsse, 1979d).
2.2.8 Special studies on triclabendazole and fenbendazole in
combination
A series of studies was conducted using test material
containing 50% triclabendazole and 50% fenbendazole that are briefly
reviewed below.
Pregnant rats were treated with gavage doses of 0, 10, 40 or
120 mg/kg bw/day test material during gestation days 6 to 15. At 120
mg/kg bw/day, dams had a reduced food consumption and there were
increases in partial abortion of conceptuses and in resorptions.
Fetotoxicity at the same level included decreased fetal weight,
reduced ossification, kinked tail, meningo-encephalocele and
hydrocephaly. Delayed ossification was present in the 40 mg/kg
bw/day fetuses while there were no adverse findings in the 10 mg/kg
bw/day group (Giese et al., 1985).
In two developmental studies in sheep, pregnant ewes received
either two doses of 0 or 50 mg/kg bw or a single dose of 0 or 150
mg/kg bw, during the first month of pregnancy. There were no effects
on lambing performance and the only adverse effect on offspring was
grossly misshapen kidneys, with disorganized internal structure, in
3 of 25 lambs at the higher-dose level (Bowen & Heckenberg, 1985;
Richards et al., 1985).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Saccharomyces cerevisiae D7 were
negative, both in the presence and absence of rat liver microsomes
(Deparade & Arni, 1983; Hool & Arni, 1985). Chromosome aberrations
in cultured human lymphocytes were increased at 22 and 44 µg/ml
(dose-related), but not at 11 µg/ml, only in the presence of a rat
liver S9 fraction (Strasser & Arni, 1985a). The bone marrow of
Chinese hamsters revealed an increase in nucleus anomalies (single
Jolly bodies only) following oral doses of 1000, 2000 or 4000 mg/kg
bw. While the increases were statistically significant they were
only slight and non dose-related (Strasser & Muller, 1984).
Chromosome aberrations were not induced in mouse primary and
secondary spermatocytes or spermatogonia after oral doses of up to
2500 mg/kg bw (Strasser & Arni, 1985b; Strasser et al., 1985).
2.2.9 Special studies on triclabendazole and levamisole in
combination
A series of studies was conducted using test material
containing 61.5% triclabendazole and 38.5% levamisole that are
briefly reviewed below.
Acute studies in male and female rats revealed LD50 values of
1996 mg/kg bw by the oral route and >2000 mg/kg bw by the dermal
route (Hartmann & Schoch, 1987a,b). In rabbits, slight and
reversible eye irritation was observed while the test material was
judged to be non-irritant to skin (Schoch, 1987a,b).
Rats were administered 0, 20, 200 or 2000 ppm of the test
material in the feed for 3 months. Body-weight gain was depressed at
200 ppm and above and anaemia was apparent at 2000 ppm. Increased
serum levels of liver enzymes and cholesterol and decreased levels
of triglycerides were suggestive of hepatocellular damage at 2000
ppm and, to a lesser extent, at 200 ppm. Necropsy revealed the
following slight changes at 2000 ppm: chronic lesion of renal
tubules, spermatic granulomas in the epididymis, fatty change in the
adrenal cortex, thymic atrophy and skeletal muscle atrophy. There
were no effects at 20 ppm, equal to 1.4 mg/kg bw/day (Basler
et al., 1988b).
Dogs were fed diets containing 0, 20, 60, 240 or 1000 ppm for 3
months. At 1000 ppm, body weight gain and food intake were reduced
and half the animals died. The administration of this dose was
discontinued after 45 days. Anaemia, increased alkaline phosphatase
and cholesterol were evident at 240 ppm and above. Increases were
observed in liver weight at 240 ppm and spleen weight at 60 and 240
ppm. There was increased haematopoiesis of the sternum at 60 ppm and
above. Survivors in the 1000 ppm group, which were not dosed for the
previous 45 days, showed at least partial recovery of toxic effects.
There were no effects at 20 ppm, equal to 0.75 mg/kg bw/day (Monnot,
1988).
Pregnant rats were treated with gavage doses of 0, 10, 80 or
160 mg/kg bw/day during gestation days 6 to 15. Ruffled fur was seen
at 160 mg/kg bw/day and increased wet bedding was noted at 80 mg/kg
bw/day and above, suggesting polyuria. Maternal body-weight gain and
food intake were depressed at 80 and 160 mg/kg bw/day. The only
fetal effects, seen at 80 and 160 mg/kg bw/day, were decreased fetal
body weight, increased bipartite sternebrae and reduced
ossification. There were no adverse effects at 10 mg/kg bw/day
(Thouin et al., 1988).
Pregnant rabbits received gavage doses of 0, 1.5, 30 or 50
mg/kg bw/day on gestation days 7 to 19. In the 50 mg/kg bw/day
group, there was reduced body weight gain, one abortion and total
resorption of conceptuses in one rabbit. The resorption rate was
slightly increased at 50 mg/kg bw/day but significance was not
achieved. There were no increased developmental effects in any
group. No adverse effect was noted up to 30 mg/kg bw/day (Khalil
et al., 1989).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Chinese hamster V79 cells gave negative
results both in the presence and absence of rat liver microsomes
(Ogorek & Arni, 1987b; Dollenmeier & Puri, 1987). Using isolated rat
hepatocytes and human fibroblasts there was no evidence of
unscheduled DNA synthesis in exposed cells (Hertner & Puri, 1987;
Meyer & Puri, 1987). Micronuclei were not induced in bone marrow of
mice dosed orally with the test substance (Strasser et al., 1988).
3. COMMENTS
A range of studies on triclabendazole was submitted for
assessment, including data on kinetics and metabolism, acute
toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity.
Approximately 40-50% was absorbed following the administration
of single oral doses of radiolabelled triclabendazole to rats and
dogs. Oral absorption was considerably higher in rabbits but could
not be quantified in a human study. Peak plasma levels of
radioactivity were generally achieved within 8 h; elimination
(primarily in the faeces) was rapid in rats, rabbits, and humans. In
dogs, peak plasma levels of radioactivity were maintained for 2-3
days and significant levels persisted for more than 7 days.
Triclabendazole was rapidly and extensively degraded in rabbits,
dogs, and humans, the sulfoxide and sulfone being the main
metabolites found in plasma. In addition to these oxidation
products, rat excreta contained 4-hydroxytriclabendazole and
2-benzimidazolone.
Single oral doses of triclabendazole were slightly toxic (LD50
> 8000 mg/kg bw) in mice and rats and moderately toxic (LD50 =
206 mg/kg bw) in rabbits, whose greater sensitivity was probably due
to a greater bioavailability of oral doses.
Findings after short-term oral dosing in rats and dogs were
primarily non-specific. In 3-month studies, decreased weight gain
was observed at 7 mg/kg bw/day and above in rats and at 37 mg/kg
bw/day in dogs; anaemia was seen at 68 mg/kg bw/day and 37 mg/kg
bw/day, respectively. Evidence of hepatotoxicity, for example
increases in plasma levels of liver enzymes and cholesterol and in
liver weight, was seen at approximately 70 mg/kg bw/day in rats and
37 mg/kg bw/day in dogs. Minor non-neoplastic liver lesions were
observed in a 3-month study in dogs at 37 mg/kg bw/day. The NOELs
were 0.7 mg/kg bw/day in rats and 0.35 mg/kg bw/day in dogs.
Hepatomas were seen in female mice given up to 29 mg/kg bw/day
for 2 years. The incidences of adenomas were higher than in controls
at all dose levels, but a dose-response relationship was not found.
Significance at the 99% level, which is widely used to assess the
significance of tumours that occur at a high background rate, was
not reached for this common benign mouse tumour. In addition, there
was no significant increase in the incidence of hepatocellular
carcinomas at any dose level. The NOEL was 0.27 mg/kg bw/day based
on increased liver weight at the next highest dose, 1.4 mg/kg
bw/day. Tumours were not induced in a 2-year dietary study in rats,
the NOEL being 1.2 mg/kg bw/day based on reduced weight gain at 4
mg/kg bw/day. Several different in vitro and in vivo
genotoxicity studies were clearly negative. These results suggest
that triclabendazole has no carcinogenic potential.
In a two-generation reproduction study in rats, postnatal
survival and growth were reduced during the lactation period of the
second generation at 1 mg/kg bw/day and above. However, the
incidence of these effects was not dose-related and was similar to
that in the first-generation offspring. In addition, statistical
significance was not achieved, and it was concluded that the
findings were not related to treatment. The NOEL was 5.5 mg/kg
bw/day.
Developmental studies in rats and rabbits provided no evidence
of embryotoxicity. Fetotoxicity, in the form of reduced fetal weight
in rats and decreased ossification in rabbits, was observed at 100
and 10 mg/kg bw/day, respectively. One rabbit fetus at 20 mg/kg
bw/day exhibited omphalocele, a rare abnormality in the strain used.
All the above effects were present only at maternally toxic dose
levels. Drug treatment during pregnancy in sheep and cattle did not
affect growth and development of the conceptus. The NOELs were 50
mg/kg bw/day in rats and 3 mg/kg bw/day in rabbits.
Special studies with triclabendazole in combination with either
fenbendazole (developmental and genotoxicity studies) or levamisole
(acute, subchronic, developmental, and genotoxicity studies) did not
reveal any synergism between the drugs.
4. EVALUATION
The database in support of the safety evaluation of
triclabendazole is substantial. The lowest NOEL was 0.27mg/kg
bw/day, based on increased liver weight in the long-term study in
mice. Using a safety factor of 100, the Committee established an ADI
for triclabendazole of 0-3 µg/kg bw.
5. REFERENCES
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, G., MALINOWSKI, W.,
CHRISTEN, P., FORMICA, G. & GFELLER, W. (1988a). Lifetime
carcinogenicity and chronic toxicity study in mice. Unpublished
Report No. 811708 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, A.L., MALINOWSKI,
W. & GFELLER, W. (1988b), 3-month oral toxicity study in rats
(administration in feed). Unpublished Report No. 861575 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979a). Report on acute oral LD50 in the
mouse of technical CGA 89'317. Unpublished Report No. 791073 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979b). Report on acute oral LD50 in the
rat of CGA 89'317. Unpublished Report No. 790038 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BATHE, R. & SACHSSE, K. (1979c). Report on acute dermal LD5O in
the rat of technical CGA 89'317. Unpublished Report No. 791076 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979d). Report on acute intraperitoneal
LD50 in the rat of CGA 89'317. Unpublished Report No. 790039 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BOWEN, F.L. & HECKENBERG, K. (1985). Reproduction study in sheep:
triclabendazole plus fenbendazole. Unpublished Report No. 85/12/1060
from CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985a). A field trial to assess the safety
of triclabendazole for cows during their first month of pregnancy.
Unpublished Report No. 85/11/1054 from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985b). Field trials to assess the safety
of triclabendazole for cows during the second to sixth month of
pregnancy. Unpublished Report No. 85/11/1055 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
CHARNLEY, B.N., HEYWOOD, R., STREET, A.E., IMM, S.A.,
GIBSON, W.A., GOPINATH, C., ALMOND, R.H. & DAWE, I.S. (1986).
Potential tumorigenic and toxic effects in prolonged dietary
administration to rats. Unpublished Report No. CBG 318/85595
(811709) from Huntingdon Research Centre, UK. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
DEPARADE, E. & ARNI, P. (1983). Salmonella/mammalian-microsome
mutagenicity test. Unpublished Report No. 831517 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1987). Point mutation test with Chinese
hamster cells V79. Unpublished Report No. 871011 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1988). Point mutation test with Chinese
hamster cells V79 with CGA 89'317 tech. Unpublished Report No.
871170 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
FRITZ, H., SUTER, H.P., ZAK, F., NAYLOR, D.C. & HESS, R. (1984).
Report on CGA 89'317: 2-Generation toxicity study in rats.
Unpublished Report No. 811540 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K. (1987). Amendment No. 1 to Final Report on CGA 89'317
tech., Teratology study (Seg. II) in rats. Unpublished Report
amendment No. 802099-1 from CIBA-GEIGY, Switzerland. Submitted to
WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & GFELLER, W. (1985). Report on CGA 89317
Tech and CGA 50560 Tech (1:1). Teratology study in rats. Unpublished
Report No. 831213 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981a). Report on CGA 89'317
tech. Teratology study (Seg. II) in rats. Unpublished Report No.
802099 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981b). Report on CGA 89'317
tech. Teratology study in rabbits. Unpublished Report No. 802090
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
HAMBOCK, H. (1983). The metabolic fate of CGA 89317 in sheep, rat
and the lactating goat. Unpublished Report No. 41/83 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987a). Acute oral toxicity in the rat.
Unpublished Report No. 861571 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987b). Acute dermal toxicity in the
rat. Unpublished Report No. 861574 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1987). Autoradiographic DNA-repair test on
rat hepatocytes. Unpublished Report No. 861580 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1988). Autoradiographic DNA repair test on
rat hepatocytes (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871173 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
HOOL, G. & ARNI, P. (1985). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro. Unpublished Report No.
840945 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G., LANGAUER, M. & MULLER, D. (1982). Nucleus anomaly test in
somatic interphase nuclei, CGA 89'317, Chinese hamster (test for
mutagenic effects on bone marrow cells). Unpublished Report No.
801392 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G. & MULLER, D. (1982). Sister chromatid exchange study, CGA
89'317, Chinese hamster (Test for mutagenic effect on bone marrow
cells). Unpublished Report No. 820209 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HUNTER, B., BERRYMAN, E.L., HEYWOOD, R., PRENTICE, D.E., STREET,
A.E., GIBSON, W.A., SINGH, H. & OFFER, J.M. (1982). CGA 89,317, a
13-week toxicity study in rats by incorporation in the diet.
Unpublished Report No. CBG/252/81151 (791817) from Huntingdon
Research Centre, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
KHALIL, S., THONIN, M.H., WEBER H., GRUENDER, G. & BASLER, W.
(1989). Developmental toxicity (teratogenicity study). Unpublished
Report No. 881433 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1987). Autoradiographic DNA-repair test on
human fibroblasts. Unpublished Report No. 861581 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1988). Autoradiographic DNA repair test on
human fibroblasts (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871172 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MONNOT, G. (1988). 13-week dietary administration toxicity study in
the beagle dog. Unpublished Report No. 380/520 (861576) from
Hazleton, France. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
MUECKE, W. (1981). Distribution, degradation and excretion of CGA
89317 in the rat. Unpublished Report No. 27/81 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987a). Salmonella/mammalian microsome
mutagenicity test (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871171 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987b). Salmonella/mammalian microsome
mutagenicity test. Unpublished Report No. 861582 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
POLTERA, A.A., ROUAN, M.C., LECAILLON, J.B. & DUBOIS, J.P. (1989).
Kinetics in humans. Unpublished Report No. CRB R37/1989 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
RICHARDS, R.J., BOUVARD, H.R. & STRITTMATTER, J. (1985). Effect on
reproduction in sheep of triclabendazole/fenbendazole treatments
given during the first month of pregnancy. Unpublished Report No.
CRA 154/85T from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982a). Report on acute oral LD50 in the rat of CGA
110'752. Unpublished Report No. 811294 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982b). Report on acute oral LD50 in the rat of CGA
110'753. Unpublished Report No. 811295 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987a). Acute dermal irritation/corrosion study in the
rabbit. Unpublished Report No. 861573 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987b). Acute eye irritation/corrosion study in the
rabbit. Unpublished Report No. 861572 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991). Kinetics in dogs
and rats. Unpublished Report No. CRB R25/1991 from CIBA-GEIGY,
France and Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985a). Chromosome studies on human
lymphocytes in vitro. Unpublished Report No. 840944 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985b). Chromosome studies on male germinal
epithelium of mouse spermatogonia. Unpublished Report No. 840943
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., LANGAUER, M. & ARNI, P. (1988). Micronucleus test
(mouse). Unpublished Report No. 861579 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRASSER, F. & MULLER, D. (1984). Nucleus anomaly test in somatic
interphase nuclei of Chinese hamster. Unpublished Report No. 831518
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., PURI, E. & ARNI, P. (1985). Chromosome studies on the
germinal epithelium of mouse spermatocytes. Unpublished Report No.
840946 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
STRONG, M.B. (1981). Studies on the lambing performance of ewes
following multiple dosing with CGA-89317 during the first trimester
of pregnancy. Unpublished Report No. 81/10/881 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRONG, M.B. & RYAN, K.J. (1981) Preliminary field studies with
CGA-89317 in pregnant ewes. Unpublished Report No. 81/1/845 from
CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRONG, M.B. & STEIGER, R.F. (1983). Studies on the lambing
performance of ewes given a single elevated dose of CGA-89317
(triclabendazole) during the first trimester of pregnancy.
Unpublished Report No. 83/10/967A from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
TAUPIN, P.J.Y. (1981). CGA 89'317, 13 week dietary toxicity study in
the dog. Unpublished Report No. 2588--380/6 from Hazleton
Laboratories Europe, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
THOUIN, M.H., SCHOCH, M., SCHMIDT, B & GFELLER, W. (1988).
Developmental toxicity (teratogenicity) study. Unpublished Report
No. 861577 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979a). Report on acute oral LD50 in
the rabbit of technical CGA 89'317. Unpublished Report No. 791074
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979b). Report on acute aerosol
inhalation toxicity in the rat of technical CGA 89'317. Unpublished
Report No. 791078 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, L. (1979c). Report on skin irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790040 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979d). Report on eye irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790041 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979e). Report on skin sensitizing
(contact allergenic) effect in guinea pigs of technical CGA 89'317.
Unpublished Report No. 791077 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991a).
Kinetics in female rabbits. Unpublished Report No. CRB R22/1991 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H. & PROBST, A. (1991b). Absorption and
disposition studies in female rabbits. Unpublished Report No. DM
12/1991 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
YOSHIMURA, H. (1987). Teratogenic evaluation of triclabendazole in
rats. Toxicology, 43: 283-287.
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M+F oral >8000 Bathe & Sachsse (1979a)
Rat M+F oral >8000 Bathe & Sachsse (1979b)
M+F dermal >4000 Bathe & Sachsse (1979c)
M+F inhalation >500 mg/m3 Ullman & Sachsse (1979b)
M+F ip 1666 Bathe & Sachsse (1979d)
Rabbit M+F oral 206 Ullman & Sachsse (1979a)
The major toxic signs were sedation, dyspnea, exophthalmos,
ruffled fur and curved body position after oral, dermal or
inhalation exposure. Ataxia was also noted following intraperitoneal
dosing.
The oral LD50 in male and female rats of the sulfoxide and
sulfone metabolites was greater than 5000 mg/kg bw. Toxic signs were
similar to those induced by triclabendazole (Sarasin, 1982a,b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Charles River CD rats were fed
diets containing 0, 10, 100 or 1000 ppm triclabendazole (purity 97%)
for 13 weeks. The calculated intake of the test material was 0.7,
6.6 and 68.5 mg/kg bw/day in males and 0.8, 7.9 and 87.3 mg/kg
bw/day in females.
There was no effect on survival and there were no overt
clinical signs. Food and water intake were reduced in 1000 ppm males
while body-weight gain was depressed in 100 ppm males and 1000 ppm
males and females. Ophthalmological and hearing tests carried out
before treatment and at weeks 6 and 13 revealed no treatment-related
effects.
Haematology, blood chemistry and urinalysis were performed
before treatment and at weeks 5 and 12. Numbers of erythrocytes,
haemoglobin and haematocrit were reduced in 1000 ppm males and
females; lymphocytes and therefore total white blood cells were
reduced in 1000 ppm females. Serum alkaline phosphatase was
increased in 1000 ppm groups. Cholesterol, albumin and total protein
were increased at 1000 ppm, particularly in females. Urine volume
was reduced in 1000 ppm males.
All animals were autopsied. Organ weights were unaffected.
Gross examination showed increases in pale kidneys and livers at 100
and 1000 ppm and in congested lungs at 1000 ppm, but pathology was
unremarkable. The NOEL was 10 ppm, equal to 0.7 mg/kg bw/day (Hunter
et al., 1982).
2.2.2.2 Dogs
Groups of 6 male and 6 female beagle dogs were fed diets
containing 0, 10, 100, or 1000 ppm triclabendazole (purity 97.6%)
for 13 weeks. The calculated intake of the test material was 0.35,
3.4 or 37 mg/kg bw/day in males and 0.35, 3.5 or 39 mg/kg bw/day in
females.
There were no overt clinical signs of toxicity. Food intake was
unaffected but body-weight gain was markedly depressed in 1000 ppm
dogs. Ophthalmology was tested before treatment and at weeks 6 and
13 with no evidence of treatment-related effects. ECG's revealed
prolongation of the QT interval and QTc value in 1000 ppm males and
females at weeks 5 and 9 but not at week 13.
Haematology and blood chemistry were examined before treatment
and at weeks 5, 9 and 13. Erythrocytes, haemoglobin and haematocrit
were markedly reduced at 1000 ppm throughout the study, with
evidence of reticulocytosis at week 9 only. Alkaline phosphatase was
increased in 100 and 1000 ppm groups; serum GPT and cholesterol were
increased in 1000 ppm dogs. Urinalysis, performed before treatment
and at weeks 6 and 13, was unaffected.
A full necropsy was undertaken on all animals;
treatment-related effects were seen at the 1000 ppm dose only. Liver
weight was increased and showed centrilobular hepatocellular pigment
granules accompanied by cytoplasmic basophilia, glycogen depletion
and foci of pigmented macrophages. Ovaries and testes were immature
with lower organ weights - females failing to reach oestrous and
males showing incomplete spermatogenesis. The NOEL was 10 ppm, equal
to 0.35 mg/kg bw/day (Taupin, 1981).
2.2.3 Long-term/carcinogenicity studies.
2.2.3.1 Mice
Groups of 80 male and 80 female albino Tif:MAGf mice were fed
diets containing 0, 3, 15, 60 or 300 ppm triclabendazole (purity
99.5%) for 2 years. Within each group, animals were designated for
interim sacrifice at 1 year (10 M and 10 F), for use in clinical
laboratory measurements (10 M and 10 F) and a further 10 M and 10 F
for examination of haematological parameters. The calculated intake
of the test material was 0.29, 1.44, 5.7 or 29.6 mg/kg bw/day in
males and 0.27, 1.39, 5.35 or 28.7 mg/kg bw/day in females.
Treated mice showed no overt signs of toxicity and mortality
was comparable to controls. There were no meaningful effects on food
and water intake while body-weight gain was slightly elevated in all
treated male groups, particularly during the first year. However,
the effect was small (10% or less), not dose-related and was not
observed in females.
Ophthalmology, haematology, blood chemistry and urinalysis were
performed at 6 month intervals. Serum levels of alkaline
phosphatase, GPT and GOT were increased in the 300 ppm groups during
the first year and in all treated groups in the second year. Effects
in the second year were not dose-related and significance was
achieved only occasionally.
All animals were subjected to a full necropsy. There were no
findings attributable to treatment at the interim sacrifice. At the
end of the study, absolute and relative liver weights were increased
at 15 ppm and above. Hepatomas were increased in all treated female
groups, but significance was not reached at the 99% level. The
incidence of carcinomas and time to appearance of tumours were
unaffected (Table 2).
Table 2. Hepatic tumour incidence in female mice
Triclabendazole (ppm) 0 3 15 60 300
Females in study 80 80 80 80 80
Benign hepatoma 7 16 15 9 20
Hepatocelular carcinoma 1 2 2 2 3
Hepatoblastoma 0 0 0 1 0
Total hepatic tumours 8 18 17 12 23
The NOEL was 3 ppm, equal to 0.27 mg/kg bw/day (Basler
et al., 1988a).
2.2.3.2 Rats
Groups of 60 male and 60 female Charles River CD rats were fed
diets containing 0, 3, 15, 30 or 100 ppm triclabendazole (purity
99.5%) for 2 years. Additional groups were designated for interim
sacrifice at 1 year (10 M and 10 F) and for haematology,
biochemistry and urinalysis investigations (20 M and 20 F). The
calculated intake of the test material was 0.1, 0.6, 1.2 or 4.0
mg/kg bw/day for males and 0.2, 0.7, 1.5 or 5.2 mg/kg bw/day for
females.
There was no effect on survival and there were no overt toxic
signs. Food and water intake were unaffected but body-weight gain
was depressed at 100 ppm, significant only in females. Ophthalmology
and hearing tests, measured every six months, were also unaffected.
Clinical laboratory parameters were examined pre-test and at
weeks 13, 26, 51, 78 and 104. Lymphocyte counts were reduced at
termination in 30 and 100 ppm groups but the values were reported to
be within normal variation. Slight decreases in plasma chloride and
BUN and increases in calcium and protein in 100 ppm males and slight
increases in protein and decreases in BUN in all treated female
groups were judged to be of no toxicological significance as the
variations were small and not consistent throughout the study and
were reported to be within normal variation. There were no
alterations in urinalysis.
A full necropsy was performed on all rats. Kidney weights were
lower in 100 ppm males at the interim kill but not at the terminal
sacrifice. At pathology, pancreatic islet cell adenomas were
increased in 15 and 100 ppm males. The relationship to treatment was
questionable as statistical significance was not achieved, there was
no dose relationship and greater numbers of carcinomas were seen in
control males (Table 3).
Table 3. Pancreatic islet cell tumours in male rats (70/group)
Dose (ppm) 0 3 15 30 100
Adenomas 3 4 12 4 11
Carcinomas 6 4 4 2 4
Total 9 8 16 6 15
The NOEL was 30 ppm, equal to 1.2 mg/kg bw/day (Charnley
et al., 1986).
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of 20 male and 20 female Tif:RAIf rats were fed diets
containing 0, 3, 15 or 75 ppm triclabendazole (purity 97.6%). Dosing
of the initial generation (P) commenced 62 days prior to mating and
was continued throughout the second generation (F1) until post
partum day 35 of the third generation (F2) pups. The calculated
average intake of test compound was 0.2, 1.1 or 5.5 mg/kg bw/day in
males and 0.3, 1.4 or 7.4 mg/kg bw/day in females.
Observations of P and F1 generation adults revealed no overt
signs of toxicity, no effects on body-weight gain and no influence
on reproductive parameters.
Examination of pups, including a range of early developmental
and behavioural tests, showed no meaningful changes in the F1
offspring. In the F2 pups, mortality was increased in 15 and 75
ppm groups during lactation. The increase was significant when
compared to concurrent controls but not when compared to the F1
groups. Body weights at weaning were lower at 15 and 75 ppm but
significance was not achieved. The NOEL was 75 ppm, equal to 5.5
mg/kg bw/day (Fritz et al., 1984).
2.2.5 Special studies on embryotoxicity and teratogenicity.
2.2.5.1 Rats
Groups of 25 pregnant Tif:RAIf rats were given gavage doses of
0, 10, 30 or 100 mg/kg bw/day of triclabendazole (purity 97.6%) as a
suspension in 0.5% (w/v) carboxymethyl cellulose. Treatment was on
gestation days 6 to 15 and females were killed on gestation day 21.
There was no mortality or clinical signs related to treatment.
Body-weight gain and food consumption were markedly depressed at 100
mg/kg bw/day with slight reduction in weight gain at 10 and 30 mg/kg
bw/day. Gross pathology of dams was unremarkable.
The implantation rate was similar in each group and treatment
did not influence the number of resorptions or fetal deaths. Live
fetus weight was reduced at 100 mg/kg bw/day but no external,
visceral or skeletal malformations were induced. The NOEL was 30
mg/kg bw/day (Giese et al., 1981a; Giese, 1987).
Groups of 20 pregnant Sprague-Dawley rats were given gavage
doses of 0, 10, 25, 50, 100 or 200 mg/kg bw/kg of triclabendazole
(purity 100%) as a suspension in water. Treatment was on gestation
days 8 to 15 and dams were killed on gestation day 21.
Maternal weight gain was depressed at 100 and 200 mg/kg bw/day
with no signs of toxicity at lower levels. Uterine and fetal
examinations revealed no effects on embryo or fetal survival or on
fetal malformations. The only fetal effect was reduced body weight
at 100 and 200 mg/kg bw/day. The NOEL was 50 mg/kg bw/day
(Yoshimura, 1987).
2.2.5.2 Rabbits
Groups of 20 pregnant chinchilla rabbits were given gavage
doses of 0, 3, 10 or 20 mg/kg bw/day of triclabendazole (purity
unknown) as a suspension in 0.5% (w/v) carboxymethyl cellulose.
Treatment was on gestation days 6 to 18 with does killed on
gestation day 28.
Three low-dose females died and diarrhoea was noted in a few
animals from each treated group. Maternal body-weight gain during
the study was similar in all groups but when "corrected" by
subtracting the weight of uterus and contents, there was a slight
non-dose related decrease in the treated groups.
The numbers of implantations, resorptions and fetal deaths were
similar in each group and fetal body weight was unaffected. Fetal
examinations showed unossified phalanges of fore and hind limbs at
10 and 20 mg/kg bw/day. One 20 mg/kg bw/day fetus exhibited
omphalocele which is rare in this strain of rabbit. The NOEL was 3
mg/kg bw/day (Giese et al., 1981b)
2.2.5.3 Sheep
Merino ewes were drenched with a single dose of 0, 5 or 10
mg/kg bw triclabendazole in the third trimester. There were no
effects on lambing or on the morphology of offspring (Strong & Ryan,
1981).
In a series of experiments, merino ewes were drenched with
doses of 0 or 30 mg/kg bw/day triclabendazole before conception and
during the first trimester of pregnancy as follows:
A. 3 treatments during the first 24 days of gestation.
B. 4 treatments during the first 43 days of gestation.
C. 2 treatments 2 to 3 weeks before conception plus
2 treatments during the first 25 days of gestation.
D. 3 treatments 2 to 4 weeks before conception plus
1 treatment 7 to 9 days after conception.
E. 3 treatments 2 to 4 weeks before conception.
None of the treatment regimes caused clinical signs of toxicity
in the ewes, nor were there effects on lambing or development of
lambs (Strong, 1981).
Groups of 27 to 28 pregnant Merino ewes were drenched with a
single dose of 0 or 50 mg/kg bw of triclabendazole. Drug
administration was on days 12, 17, 21, 24 or 28 days after mating.
There were no effects on lambing performance or development of lambs
(Strong & Steiger, 1983).
2.2.5.4 Cattle
Hereford cows were drenched with a single dose of 24 mg/kg bw
triclabendazole during the first month of pregnancy. Calving and
uterine development of offspring were unaffected (Bowen & Ryan,
1985a).
Hereford or black poll cows were drenched with a single dose of
0 or 24 mg/kg bw/day triclabendazole during the second, third,
fourth and sixth to seventh month of pregnancy. There were no
difficulties at calving or abnormalities in the calves (Bowen &
Ryan, 1985b).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies on triclabendazole are
summarized in Table 4.
Table 4. Results of genotoxicity studies on triclabendazole1
Test system Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.5-1250 µg/plate Negative Ogorek & Arni,
TA98, TA100, 1987a
TA1535, TA1537,
TA1538
Forward mutation2 Chinese hamster 0.025-0.5 µg/ml (-S9) Negative Dollenmeier &
V79 cells 3.5-70 µg/ml (+S9) Puri, 1988
Unscheduled Primary rat 0.3-40 µg/ml Negative Hertner & Puri,
DNA-synthesis hepatocytes 1988
Unscheduled Human fibroblasts 0.4-60 µg/ml Negative Meyer & Puri,
DNA-synthesis 1988
Nucleus anomaly Chinese hamster 172-688 mg/kg bw Negative Hool et al.,
bone marrow by gavage 1982
Sister chromatid Chinese hamster 173-692 mg/kg bw by Negative Hool & Muller,
exchange bone marrow gavage 1982
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
2.2.7 Special studies on irritation and sensitization
2.2.7.1 Guinea-pigs
Groups of 10 male and 10 female pirbright guinea-pigs were used
in an optimization test. The induction phase was carried out by
administering 10 intracutaneous injections of a 0.1% (w/v)
suspension in propylene glycol/saline. Epidermal challenge produced
no reaction but an intradermal challenge resulted in a positive
sensitization reaction (Ullmann & Sachsse, 1979e)
2.2.7.2 Rabbits
Groups of 3 male and 3 female New Zeeland white rabbits were
used in a patch test. Triclabendazole was suspended in propylene
glycol/saline and applied for 24 h. The test material was judged to
be a slight skin irritant (Ullmann & Sachsse, 1979c)
Groups of 3 male and 6 female New Zeeland white rabbits
received 0.1 g of triclabendazole instilled into the conjunctival
sac of the eye. Subsequent examination failed to reveal any eye
irritation (Ullmann & Sachsse, 1979d).
2.2.8 Special studies on triclabendazole and fenbendazole in
combination
A series of studies was conducted using test material
containing 50% triclabendazole and 50% fenbendazole that are briefly
reviewed below.
Pregnant rats were treated with gavage doses of 0, 10, 40 or
120 mg/kg bw/day test material during gestation days 6 to 15. At 120
mg/kg bw/day, dams had a reduced food consumption and there were
increases in partial abortion of conceptuses and in resorptions.
Fetotoxicity at the same level included decreased fetal weight,
reduced ossification, kinked tail, meningo-encephalocele and
hydrocephaly. Delayed ossification was present in the 40 mg/kg
bw/day fetuses while there were no adverse findings in the 10 mg/kg
bw/day group (Giese et al., 1985).
In two developmental studies in sheep, pregnant ewes received
either two doses of 0 or 50 mg/kg bw or a single dose of 0 or 150
mg/kg bw, during the first month of pregnancy. There were no effects
on lambing performance and the only adverse effect on offspring was
grossly misshapen kidneys, with disorganized internal structure, in
3 of 25 lambs at the higher-dose level (Bowen & Heckenberg, 1985;
Richards et al., 1985).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Saccharomyces cerevisiae D7 were
negative, both in the presence and absence of rat liver microsomes
(Deparade & Arni, 1983; Hool & Arni, 1985). Chromosome aberrations
in cultured human lymphocytes were increased at 22 and 44 µg/ml
(dose-related), but not at 11 µg/ml, only in the presence of a rat
liver S9 fraction (Strasser & Arni, 1985a). The bone marrow of
Chinese hamsters revealed an increase in nucleus anomalies (single
Jolly bodies only) following oral doses of 1000, 2000 or 4000 mg/kg
bw. While the increases were statistically significant they were
only slight and non dose-related (Strasser & Muller, 1984).
Chromosome aberrations were not induced in mouse primary and
secondary spermatocytes or spermatogonia after oral doses of up to
2500 mg/kg bw (Strasser & Arni, 1985b; Strasser et al., 1985).
2.2.9 Special studies on triclabendazole and levamisole in
combination
A series of studies was conducted using test material
containing 61.5% triclabendazole and 38.5% levamisole that are
briefly reviewed below.
Acute studies in male and female rats revealed LD50 values of
1996 mg/kg bw by the oral route and >2000 mg/kg bw by the dermal
route (Hartmann & Schoch, 1987a,b). In rabbits, slight and
reversible eye irritation was observed while the test material was
judged to be non-irritant to skin (Schoch, 1987a,b).
Rats were administered 0, 20, 200 or 2000 ppm of the test
material in the feed for 3 months. Body-weight gain was depressed at
200 ppm and above and anaemia was apparent at 2000 ppm. Increased
serum levels of liver enzymes and cholesterol and decreased levels
of triglycerides were suggestive of hepatocellular damage at 2000
ppm and, to a lesser extent, at 200 ppm. Necropsy revealed the
following slight changes at 2000 ppm: chronic lesion of renal
tubules, spermatic granulomas in the epididymis, fatty change in the
adrenal cortex, thymic atrophy and skeletal muscle atrophy. There
were no effects at 20 ppm, equal to 1.4 mg/kg bw/day (Basler
et al., 1988b).
Dogs were fed diets containing 0, 20, 60, 240 or 1000 ppm for 3
months. At 1000 ppm, body weight gain and food intake were reduced
and half the animals died. The administration of this dose was
discontinued after 45 days. Anaemia, increased alkaline phosphatase
and cholesterol were evident at 240 ppm and above. Increases were
observed in liver weight at 240 ppm and spleen weight at 60 and 240
ppm. There was increased haematopoiesis of the sternum at 60 ppm and
above. Survivors in the 1000 ppm group, which were not dosed for the
previous 45 days, showed at least partial recovery of toxic effects.
There were no effects at 20 ppm, equal to 0.75 mg/kg bw/day (Monnot,
1988).
Pregnant rats were treated with gavage doses of 0, 10, 80 or
160 mg/kg bw/day during gestation days 6 to 15. Ruffled fur was seen
at 160 mg/kg bw/day and increased wet bedding was noted at 80 mg/kg
bw/day and above, suggesting polyuria. Maternal body-weight gain and
food intake were depressed at 80 and 160 mg/kg bw/day. The only
fetal effects, seen at 80 and 160 mg/kg bw/day, were decreased fetal
body weight, increased bipartite sternebrae and reduced
ossification. There were no adverse effects at 10 mg/kg bw/day
(Thouin et al., 1988).
Pregnant rabbits received gavage doses of 0, 1.5, 30 or 50
mg/kg bw/day on gestation days 7 to 19. In the 50 mg/kg bw/day
group, there was reduced body weight gain, one abortion and total
resorption of conceptuses in one rabbit. The resorption rate was
slightly increased at 50 mg/kg bw/day but significance was not
achieved. There were no increased developmental effects in any
group. No adverse effect was noted up to 30 mg/kg bw/day (Khalil
et al., 1989).
Mutation assays in Salmonella typhimurium strains TA98,
TA100, TA1535 and TA1537 and Chinese hamster V79 cells gave negative
results both in the presence and absence of rat liver microsomes
(Ogorek & Arni, 1987b; Dollenmeier & Puri, 1987). Using isolated rat
hepatocytes and human fibroblasts there was no evidence of
unscheduled DNA synthesis in exposed cells (Hertner & Puri, 1987;
Meyer & Puri, 1987). Micronuclei were not induced in bone marrow of
mice dosed orally with the test substance (Strasser et al., 1988).
3. COMMENTS
A range of studies on triclabendazole was submitted for
assessment, including data on kinetics and metabolism, acute
toxicity, short-term and long-term toxicity, reproductive and
developmental toxicity, and genotoxicity.
Approximately 40-50% was absorbed following the administration
of single oral doses of radiolabelled triclabendazole to rats and
dogs. Oral absorption was considerably higher in rabbits but could
not be quantified in a human study. Peak plasma levels of
radioactivity were generally achieved within 8 h; elimination
(primarily in the faeces) was rapid in rats, rabbits, and humans. In
dogs, peak plasma levels of radioactivity were maintained for 2-3
days and significant levels persisted for more than 7 days.
Triclabendazole was rapidly and extensively degraded in rabbits,
dogs, and humans, the sulfoxide and sulfone being the main
metabolites found in plasma. In addition to these oxidation
products, rat excreta contained 4-hydroxytriclabendazole and
2-benzimidazolone.
Single oral doses of triclabendazole were slightly toxic (LD50
> 8000 mg/kg bw) in mice and rats and moderately toxic (LD50 =
206 mg/kg bw) in rabbits, whose greater sensitivity was probably due
to a greater bioavailability of oral doses.
Findings after short-term oral dosing in rats and dogs were
primarily non-specific. In 3-month studies, decreased weight gain
was observed at 7 mg/kg bw/day and above in rats and at 37 mg/kg
bw/day in dogs; anaemia was seen at 68 mg/kg bw/day and 37 mg/kg
bw/day, respectively. Evidence of hepatotoxicity, for example
increases in plasma levels of liver enzymes and cholesterol and in
liver weight, was seen at approximately 70 mg/kg bw/day in rats and
37 mg/kg bw/day in dogs. Minor non-neoplastic liver lesions were
observed in a 3-month study in dogs at 37 mg/kg bw/day. The NOELs
were 0.7 mg/kg bw/day in rats and 0.35 mg/kg bw/day in dogs.
Hepatomas were seen in female mice given up to 29 mg/kg bw/day
for 2 years. The incidences of adenomas were higher than in controls
at all dose levels, but a dose-response relationship was not found.
Significance at the 99% level, which is widely used to assess the
significance of tumours that occur at a high background rate, was
not reached for this common benign mouse tumour. In addition, there
was no significant increase in the incidence of hepatocellular
carcinomas at any dose level. The NOEL was 0.27 mg/kg bw/day based
on increased liver weight at the next highest dose, 1.4 mg/kg
bw/day. Tumours were not induced in a 2-year dietary study in rats,
the NOEL being 1.2 mg/kg bw/day based on reduced weight gain at 4
mg/kg bw/day. Several different in vitro and in vivo
genotoxicity studies were clearly negative. These results suggest
that triclabendazole has no carcinogenic potential.
In a two-generation reproduction study in rats, postnatal
survival and growth were reduced during the lactation period of the
second generation at 1 mg/kg bw/day and above. However, the
incidence of these effects was not dose-related and was similar to
that in the first-generation offspring. In addition, statistical
significance was not achieved, and it was concluded that the
findings were not related to treatment. The NOEL was 5.5 mg/kg
bw/day.
Developmental studies in rats and rabbits provided no evidence
of embryotoxicity. Fetotoxicity, in the form of reduced fetal weight
in rats and decreased ossification in rabbits, was observed at 100
and 10 mg/kg bw/day, respectively. One rabbit fetus at 20 mg/kg
bw/day exhibited omphalocele, a rare abnormality in the strain used.
All the above effects were present only at maternally toxic dose
levels. Drug treatment during pregnancy in sheep and cattle did not
affect growth and development of the conceptus. The NOELs were 50
mg/kg bw/day in rats and 3 mg/kg bw/day in rabbits.
Special studies with triclabendazole in combination with either
fenbendazole (developmental and genotoxicity studies) or levamisole
(acute, subchronic, developmental, and genotoxicity studies) did not
reveal any synergism between the drugs.
4. EVALUATION
The database in support of the safety evaluation of
triclabendazole is substantial. The lowest NOEL was 0.27mg/kg
bw/day, based on increased liver weight in the long-term study in
mice. Using a safety factor of 100, the Committee established an ADI
for triclabendazole of 0-3 µg/kg bw.
5. REFERENCES
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, G., MALINOWSKI, W.,
CHRISTEN, P., FORMICA, G. & GFELLER, W. (1988a). Lifetime
carcinogenicity and chronic toxicity study in mice. Unpublished
Report No. 811708 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
BASLER, W., GRETENER, P., FROEHLICH, E., KRINKE, A.L., MALINOWSKI,
W. & GFELLER, W. (1988b), 3-month oral toxicity study in rats
(administration in feed). Unpublished Report No. 861575 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979a). Report on acute oral LD50 in the
mouse of technical CGA 89'317. Unpublished Report No. 791073 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979b). Report on acute oral LD50 in the
rat of CGA 89'317. Unpublished Report No. 790038 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BATHE, R. & SACHSSE, K. (1979c). Report on acute dermal LD5O in
the rat of technical CGA 89'317. Unpublished Report No. 791076 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BATHE, R. & SACHSSE, K. (1979d). Report on acute intraperitoneal
LD50 in the rat of CGA 89'317. Unpublished Report No. 790039 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
BOWEN, F.L. & HECKENBERG, K. (1985). Reproduction study in sheep:
triclabendazole plus fenbendazole. Unpublished Report No. 85/12/1060
from CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985a). A field trial to assess the safety
of triclabendazole for cows during their first month of pregnancy.
Unpublished Report No. 85/11/1054 from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
BOWEN, F.L. & RYAN, K.J. (1985b). Field trials to assess the safety
of triclabendazole for cows during the second to sixth month of
pregnancy. Unpublished Report No. 85/11/1055 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
CHARNLEY, B.N., HEYWOOD, R., STREET, A.E., IMM, S.A.,
GIBSON, W.A., GOPINATH, C., ALMOND, R.H. & DAWE, I.S. (1986).
Potential tumorigenic and toxic effects in prolonged dietary
administration to rats. Unpublished Report No. CBG 318/85595
(811709) from Huntingdon Research Centre, UK. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
DEPARADE, E. & ARNI, P. (1983). Salmonella/mammalian-microsome
mutagenicity test. Unpublished Report No. 831517 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1987). Point mutation test with Chinese
hamster cells V79. Unpublished Report No. 871011 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
DOLLENMEIER, P. & PURI, E. (1988). Point mutation test with Chinese
hamster cells V79 with CGA 89'317 tech. Unpublished Report No.
871170 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
FRITZ, H., SUTER, H.P., ZAK, F., NAYLOR, D.C. & HESS, R. (1984).
Report on CGA 89'317: 2-Generation toxicity study in rats.
Unpublished Report No. 811540 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K. (1987). Amendment No. 1 to Final Report on CGA 89'317
tech., Teratology study (Seg. II) in rats. Unpublished Report
amendment No. 802099-1 from CIBA-GEIGY, Switzerland. Submitted to
WHO by CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & GFELLER, W. (1985). Report on CGA 89317
Tech and CGA 50560 Tech (1:1). Teratology study in rats. Unpublished
Report No. 831213 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981a). Report on CGA 89'317
tech. Teratology study (Seg. II) in rats. Unpublished Report No.
802099 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
GIESE, K., SUTER, H.P. & HESS, R. (1981b). Report on CGA 89'317
tech. Teratology study in rabbits. Unpublished Report No. 802090
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
HAMBOCK, H. (1983). The metabolic fate of CGA 89317 in sheep, rat
and the lactating goat. Unpublished Report No. 41/83 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987a). Acute oral toxicity in the rat.
Unpublished Report No. 861571 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HARTMANN, H.R. & SCHOCH, M. (1987b). Acute dermal toxicity in the
rat. Unpublished Report No. 861574 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1987). Autoradiographic DNA-repair test on
rat hepatocytes. Unpublished Report No. 861580 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HERTNER, TH. & PURI, E. (1988). Autoradiographic DNA repair test on
rat hepatocytes (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871173 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
HOOL, G. & ARNI, P. (1985). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro. Unpublished Report No.
840945 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G., LANGAUER, M. & MULLER, D. (1982). Nucleus anomaly test in
somatic interphase nuclei, CGA 89'317, Chinese hamster (test for
mutagenic effects on bone marrow cells). Unpublished Report No.
801392 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
HOOL, G. & MULLER, D. (1982). Sister chromatid exchange study, CGA
89'317, Chinese hamster (Test for mutagenic effect on bone marrow
cells). Unpublished Report No. 820209 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
HUNTER, B., BERRYMAN, E.L., HEYWOOD, R., PRENTICE, D.E., STREET,
A.E., GIBSON, W.A., SINGH, H. & OFFER, J.M. (1982). CGA 89,317, a
13-week toxicity study in rats by incorporation in the diet.
Unpublished Report No. CBG/252/81151 (791817) from Huntingdon
Research Centre, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
KHALIL, S., THONIN, M.H., WEBER H., GRUENDER, G. & BASLER, W.
(1989). Developmental toxicity (teratogenicity study). Unpublished
Report No. 881433 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1987). Autoradiographic DNA-repair test on
human fibroblasts. Unpublished Report No. 861581 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
MEYER, A. & PURI, E. (1988). Autoradiographic DNA repair test on
human fibroblasts (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871172 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
MONNOT, G. (1988). 13-week dietary administration toxicity study in
the beagle dog. Unpublished Report No. 380/520 (861576) from
Hazleton, France. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
MUECKE, W. (1981). Distribution, degradation and excretion of CGA
89317 in the rat. Unpublished Report No. 27/81 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987a). Salmonella/mammalian microsome
mutagenicity test (OECD conform) with CGA 89'317 tech. Unpublished
Report No. 871171 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
OGOREK, B. & ARNI, P. (1987b). Salmonella/mammalian microsome
mutagenicity test. Unpublished Report No. 861582 from CIBA-GEIGY,
Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
POLTERA, A.A., ROUAN, M.C., LECAILLON, J.B. & DUBOIS, J.P. (1989).
Kinetics in humans. Unpublished Report No. CRB R37/1989 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
RICHARDS, R.J., BOUVARD, H.R. & STRITTMATTER, J. (1985). Effect on
reproduction in sheep of triclabendazole/fenbendazole treatments
given during the first month of pregnancy. Unpublished Report No.
CRA 154/85T from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982a). Report on acute oral LD50 in the rat of CGA
110'752. Unpublished Report No. 811294 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SARASIN, G. (1982b). Report on acute oral LD50 in the rat of CGA
110'753. Unpublished Report No. 811295 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987a). Acute dermal irritation/corrosion study in the
rabbit. Unpublished Report No. 861573 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHOCH, M. (1987b). Acute eye irritation/corrosion study in the
rabbit. Unpublished Report No. 861572 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991). Kinetics in dogs
and rats. Unpublished Report No. CRB R25/1991 from CIBA-GEIGY,
France and Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985a). Chromosome studies on human
lymphocytes in vitro. Unpublished Report No. 840944 from
CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRASSER, F. & ARNI, P. (1985b). Chromosome studies on male germinal
epithelium of mouse spermatogonia. Unpublished Report No. 840943
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., LANGAUER, M. & ARNI, P. (1988). Micronucleus test
(mouse). Unpublished Report No. 861579 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRASSER, F. & MULLER, D. (1984). Nucleus anomaly test in somatic
interphase nuclei of Chinese hamster. Unpublished Report No. 831518
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
STRASSER, F., PURI, E. & ARNI, P. (1985). Chromosome studies on the
germinal epithelium of mouse spermatocytes. Unpublished Report No.
840946 from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
STRONG, M.B. (1981). Studies on the lambing performance of ewes
following multiple dosing with CGA-89317 during the first trimester
of pregnancy. Unpublished Report No. 81/10/881 from CIBA-GEIGY,
Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
STRONG, M.B. & RYAN, K.J. (1981) Preliminary field studies with
CGA-89317 in pregnant ewes. Unpublished Report No. 81/1/845 from
CIBA-GEIGY, Australia. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
STRONG, M.B. & STEIGER, R.F. (1983). Studies on the lambing
performance of ewes given a single elevated dose of CGA-89317
(triclabendazole) during the first trimester of pregnancy.
Unpublished Report No. 83/10/967A from CIBA-GEIGY, Australia.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
TAUPIN, P.J.Y. (1981). CGA 89'317, 13 week dietary toxicity study in
the dog. Unpublished Report No. 2588--380/6 from Hazleton
Laboratories Europe, UK. Submitted to WHO by CIBA-GEIGY, AG, Basel,
Switzerland.
THOUIN, M.H., SCHOCH, M., SCHMIDT, B & GFELLER, W. (1988).
Developmental toxicity (teratogenicity) study. Unpublished Report
No. 861577 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979a). Report on acute oral LD50 in
the rabbit of technical CGA 89'317. Unpublished Report No. 791074
from CIBA-GEIGY, Switzerland. Submitted to WHO by CIBA-GEIGY, AG,
Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979b). Report on acute aerosol
inhalation toxicity in the rat of technical CGA 89'317. Unpublished
Report No. 791078 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, L. (1979c). Report on skin irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790040 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979d). Report on eye irritation in the
rabbit after single application of CGA 89'317. Unpublished Report
No. 790041 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
ULLMANN, L. & SACHSSE, K. (1979e). Report on skin sensitizing
(contact allergenic) effect in guinea pigs of technical CGA 89'317.
Unpublished Report No. 791077 from CIBA-GEIGY, Switzerland.
Submitted to WHO by CIBA-GEIGY, AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H., LECAILLON, J.B. & GODBILLON, J. (1991a).
Kinetics in female rabbits. Unpublished Report No. CRB R22/1991 from
CIBA-GEIGY, France and Switzerland. Submitted to WHO by CIBA-GEIGY,
AG, Basel, Switzerland.
WIEGAND, H., SCHUTZ, H. & PROBST, A. (1991b). Absorption and
disposition studies in female rabbits. Unpublished Report No. DM
12/1991 from CIBA-GEIGY, Switzerland. Submitted to WHO by
CIBA-GEIGY, AG, Basel, Switzerland.
YOSHIMURA, H. (1987). Teratogenic evaluation of triclabendazole in
rats. Toxicology, 43: 283-287.
