
FURAZOLIDONE
First draft prepared by
Mrs J.E.M van Koten-Vermeulen
Mrs M.F.A. Wouters
Dr F.X.R. van Leeuwen
Laboratory for Toxicology
National Institute of Public Health and Environmental Protection
Bilthoven, The Netherlands
1. EXPLANATION
Furazolidone is a nitrofuran derivative used both
therapeutically and prophylactically as an antimicrobial agent in
poulty, pigs, rabbits and fish.
Furazolidone had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
Two male rats were fed a single oral dose of 330 mg
14C-furazolidone/kg feed. One rat died within 24 h. About 95% of
the administered radioactivity was excreted in 48 h, the major part
in the urine. Most of the residual radioactivity was seen in the
liver, followed by kidney, testes and lungs. The lowest amount was
found in the spleen (Ray, 1959).
A comparison was made between rats dosed with
14C-furazolidone labelled at the aldehyde carbon on the nitrofuran
ring and rats dosed with 14C-furazolidone labelled at the
methylene carbons of the oxazolidine ring. Two rats treated with
methylene-labelled 14C-furazolidone were pretreated with 330 mg
furazolidone/kg feed for a period of 19 days. After 48 h rats
treated with aldehyde-labelled furazolidone excreted the major part
(45.9%) via the urine, followed by the faeces (38.2%) and 2.1% was
exhaled as 14CO2. The major route of excreted methylene-labelled
furazolidone was via urine (71.6%), followed by faeces (34.5%) and
expired air (3.6%). After prefeeding more radioactivity was excreted
in the urine and less in the faeces. After treatment with aldehyde
labelled 14C-furazolidone most radioactivity was found in kidneys
and liver, followed by heart, muscle and testes. After treatment
with methylene-labelled 14C-furazolidone most radioactivity was
found in the liver and kidneys, followed by heart, blood, testes,
muscle and fat. There was no difference between treated and
pretreated rats (Bowman, 1961a).
Rats received a diet containing methylene-labelled
14C-furazolidone for 10 days, after pretreatement with unlabelled
furazolidone for 19 days. Excretion in urine and faeces was 62.2 and
25% of the applied radioactivity, respectively. 14C-Labelled
residue concentration in various tissues was liver>kidney>heart>
blood>muscle>testes>fat. A gradual increase in residues occurred
over the 10-day feeding period, with apparent plateau concentrations
in heart and fat tissues (14.8 and 6.55 mg/kg, respectively) being
reached after 8 days. Elimination was biphasic with a fast and slow
component. The half-lives found in tissues were for the liver 0.9
and 4.8 days, for kidney 0.7 and 7.5 days, for heart 1.1 and 14.1
days, for blood 0.8 and 14.8 days, for muscle 1.0 and 29.0 days and
for testes 0.7 and 8.2 days (Bowman, 1961b).
Four cannulated bile duct rats were administered by intubation
16.5 mg 14C-furazolidone(methylene- and formyl-labelled)/kg bw in
PEG-200. Rats were sacrificed after 72 h. Excretion was 46.6% of the
absorbed dose in urine and 36.5% in bile. The residual radioactivity
in tissues was 3.7%. Four rats were administered 30 mg
14C-furazolidone/kg feed. Rats were sacrificed after 72 h.
Excretion was 51.5% in urine, 34.6% in bile and 4.3% was found in
tissues (Hawkins, 1992).
Four rats per group were administered 14C-furazolidone
(methyl and formyl labelled) in pelleted control lyophilized liver
or muscle (300 mg/kg). The consumed liver was equivalent to a dose
level of approximately 14 mg/kg bw (range of 6 to 18 mg/kg bw) and
the muscle consumed was equivalent to a dose of 13 mg/kg bw. In rats
fed with liver 73% of the dose was absorbed; 18% of the dose was
excreted via the bile, 51% in urine, and 4% was found in tissues. In
rats treated with muscle the urine contained 55% of the dose, bile
35% and tissues 5% (Hawkins et al., 1992).
2.1.1.2 Chickens
Formyl-labelled 14C-furazolidone was administered to chickens
at a rate of 220 mg/kg feed for 4 days following a 21-day feeding
period with 220 mg unlabelled furazolidone/kg feed. Maximum levels
of radioactivity present in liver and kidney (15.6 and 11.7
mg-equivalents/kg, respectively) were about 5 to 8 times the
radioactivity present in fat and muscle. Radioactivity in these
tissues was still present (0.31-0.49 mg-equivalents/kg) after 16
days withdrawal. Depletion of radioactivity appeared to be a
biphasic process, comprised of a fast component with a half-life of
1.5 days and a slower component with a half-life of about 4 days.
Chickens administered formyl 14C-furazolidone for 6, 8, 16 or
31 days and sacrificed after a 4-day withdrawal period showed
plateau levels in tissues after 16 days. After 31 days feeding and 4
days withdrawal liver contained about 7 mg-equivalents/kg (Buzard
et al., 1960).
White leghorn chicks fed furazolidone at a level of 220 mg/kg
feed for three weeks were consecutively treated with
14C-furazolidone (methylene-labelled) for 4 days. Depletion was
biphasic with a rapidly excreted component (half-life of about 0.4
days) and a slower component (half-life of about 4 days). At 11 days
withdrawal radioactivity found in liver, kidney, muscle and fat was
0.867, 0.576, 0.466 and 1.46 mg-equivalents/kg, respectively. In a
32 day-feeding study with 220 mg 14C-furazolidone
(methylene-labelled) tissue saturation was attained within 16 days
(Buzard et al., 1961).
Chickens were administered 220 mg formyl-labelled
14C-furazolidone/kg in the diet for 10 days. 14C-Content was
measured in daily urine, expired air and faeces samples. The
chickens were sacrificed on day 11. Recovery in urine and faeces was
92% of the total dose and 0.6% in expired air. Total recovery in
tissue was 2.8% of the administered 14C, with major amounts in
liver, kidney, crop and its contents (Heotis et al., 1963).
2.1.1.3 Pigs
A Yorkshire barrow maintained on feed containing 330 mg/kg
furazolidone for 3 weeks was orally administered 1.25 mg
14C-furazolidone (formyl-labelled)/kg bw. Urine and faeces were
collected and the pig was sacrificed 48 h after dosing. The total
14C-recovery in urine and faeces was 90%. Most radioactivity in
tissues was found in kidney, liver and thyroid followed by bile,
blood, muscle and fat. A large number of radiolabelled urinary
metabolites were found but not identified (Tennent & Ray, 1971).
One female pig was treated with formyl-labelled
14C-furazolidone and another one was treated with
methylene-labelled 14C-furazolidone at a rate of 5 mg/kg bw/day
for 5 days. A total of 42% and 47%, respectively, of applied
radioactivity was excreted via the urine. Radioactivity in edible
tissues was highest in liver (5.15 and 7.80 mg-equivalent/kg,
respectively) and lowest in muscle (1.00 and 1.05 mg-equivalent/kg,
respectively). In urine at least 15 metabolites were found, less
than 5% comprising the parent compound. None of the metabolites
accounted for more than 15% of total radioactivity (Craine, 1977).
Four male pigs were treated with 14C-furazolidone (labelled
in both the formyl and the methylene position) in their feed for 5
consecutive days at a rate of 5 mg/kg bw/day. Pigs were sacrificed 5
or 14 days after the last dose. Total excretion in urine was 51-56%
of the administered dose. After 14 days only 0.3% of the
radioactivity was detected. At least 11 metabolites were found in
urine but no parent compound was found. Measurable levels of
radioactivity were present in tissues of all four pigs, with highest
levels in liver and kidney. After 14 days withdrawal the liver and
kidney contained 1 mg-equivalent/kg (Craine, 1978).
Piglets were orally dosed with 12 mg/kg bw 14C-furazolidone
(methylene-labelled) for 10 days. Peak blood and plasma levels of
the parent compound reached within 30 min were 835 and 955 ng/ml,
respectively, followed by a decrease which resulted in no detectable
levels 3-4 h after the last administration. The half-life in blood
and plasma was about 60 minutes. One day after withdrawal all
radioactivity was associated with plasma proteins; 61% and 18% of
the total dosed radioactivity was excreted via the urine and faeces,
respectively. Radioactivity in tissues analyzed 2 h after the last
administration was largest in liver, kidney, fat and muscle. After a
withdrawal period of 14 days detectable amounts were still present.
From the residual radioactivity 2 h after sacrifice 9%, 14% and 35%
could not be extracted from kidney, liver and muscle, respectively;
after 14 days withdrawal these amounts were 31%, 27% and 56%,
respectively (Vroomen et al., 1986).
2.1.2 Biotransformation
2.1.2.1 In vitro
Metabolism of furazolidone (N-(5-nitro-2-furfuryliden)-3-amino-
2-oxazolidone) was investigated by using milk xanthine oxidase and
rat liver 9000 g supernatant. One of the major metabolites of the
incubation mixture was identified as 2,3-dihydro-3-cyanomethyl-
2-hydroxy-5-nitro-1alpha,2-di(2-oxo-oxazolidin-3-yl)iminomethyl-furo
[2,3-ß]furan. In addition N-(5-amino-2-furfuryliden)-3-amino-
2-oxazolidone was identified as a minor metabolite (Tatsumi et al.,
1981).
A fast conversion of 14C-furazolidone (methylene-labelled)
was observed in liver microsomes of 3MC-induced rats incubated under
anaerobic and aerobic conditions. The 2 major metabolites formed
were 3-(4-cyano-2-oxobutylid-ene-amino)-2-oxazolidone and
2,3-dihydro-3-cyanomethyl-2-hydroxy-5-nitro-1ý,2-di(2-oxo-
oxazolidin-3-yl)iminomethyl-furo[2,3-ß]furan, both accounting for
about 16.5% of the total extractable radioactivity (Vroomen 1987).
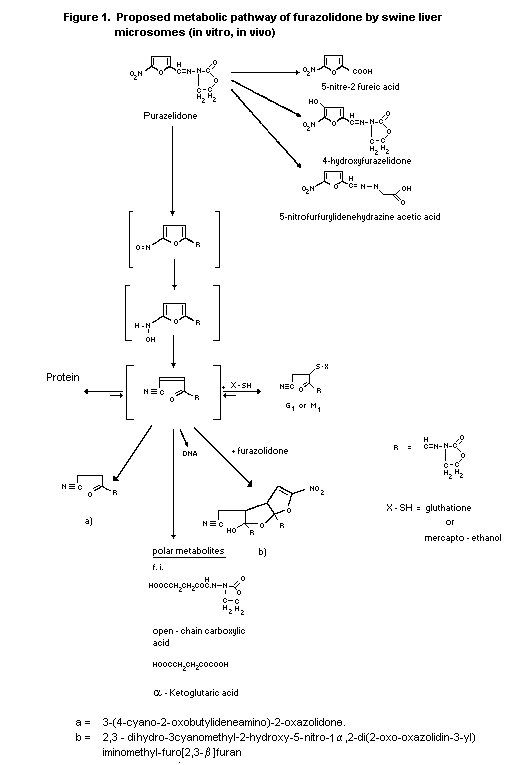
Metabolism was studied with 14C-furazolidone
(methylene-labelled) in swine liver microsomes under aerobic and
anaerobic conditions. 3-(4-cyano-2-oxobutyli-deneamino)-
2-oxazolidone and 2,3-dihydro-3-cyanomethyl-2-hydroxyl-5-nitro-
1alpha, 2-di(2-oxo-oxazolidin-3-yl)iminomethyl-furo[2,3-ß]furan were
the major ethylacetate extractable metabolites, formed via the
open-chain acrylonitril-derivative of furazolidone. Another
metabolite formed by microsomes in the presence of mercaptoethanol
was identified as a mercaptoethanol conjugate
M1-(3-(4-cyano-3-ß-hydroxyethyl-mercapto-2-oxobutylidene
amino)-2-oxazolidone). In the presence of GSH, another metabolite
was formed, shown to be a GSH-conjugate of furazolidone (Figure 1)
(Vroomen et al., 1987b, 1988)
Furazolidone was rapidly transformed by porcine hepatocyte
cultures, resulting partly in the formation of
3-(4-cyano-2-oxobutylidene amino)-2-oxazo-lidone which amounted to
15% of total metabolites (Hoogenboom et al., 1991).
 2.1.2.2 In vivo
[(5-Nitrofurfurylidene)hydrazino]acetic acid has been isolated
from urine of rats treated with 20 mg furazolidone/kg bw (Morrison,
1976).
A small part of the radioactivity in rat urine after treatment
with 14C-furazolidone was identified as
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone and
N-(5-acetamido-2-furfurylidene)-3-amino-2-oxazolidone (Tatsumi &
Takahashi, 1982).
Male Wistar rats were given orally 100 mg 14C-furazolidone
(formyl-labelled)/kg bw. Urine was collected and analyzed for
possible metabolites. N-(4-carboxy-2-oxobutylideneamino)-
2-oxazolidone, alpha-ketoglutaric acid,
3-(4-cyano-2-oxobutylidenamino)-2-oxazolidone and
N-(5-acetoamido-2-furfurylidene)-3-amino-2-oxazolidone were
identified (Tatsumi et al., 1984; White 1989).
Colostomized chickens received a single oral dose of 30 mg
furazolidone/kg bw. An average of 7.5% of the dose was excreted in
the urine in 12 h. Four urinary metabolites containing a furan ring,
of which only one was a nitrofuran, were detected. Only traces of
unchanged furazolidone were found (Craine & Ray, 1972).
The metabolites of aldehyde-labelled 14C-furazolidone in pig
urine were investigated. More than thirty radio-labelled metabolites
were found. Three primary metabolites of furazolidone were
identified as 5-nitro-2-furoic acid, an orange-coloured "415"
chromogen and a yellow "415" chromogen, with half-lives of 30, 22
and 19 minutes, respectively. Some metabolites are naturally
occurring chemicals and include a yellow urinary pigment, carbonates
and a number of ninhydrin positive materials which lose
radioactivity when treated with ninhydrin indicating that these
materials are amino acids or conjugates of amino acids (Ray, 1962).
The orange "415" metabolite contains an intact
5-nitro-2-furfural moiety and is the most abundant single
furazolidone-related metabolite found in pig urine (Ray & Hayes,
1963).
Analysis of urine from a pig treated with 14C-furazolidone,
also labelled with 15N in the 5-nitro position, showed 14
metabolites. Eleven of them degraded to two or three degradation
products, which could not be identified. The presence of 15N was
detected in one minor metabolite. The authors concluded that during
the metabolism of furazolidone the nitro group was removed
extensively from the molecule (Kouba, 1979).
The open-chain cyano-derivative of furazolidone,
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone was a minor
metabolite in plasma and tissues of swine with a plasma half-life of
4 h (Figure 1) (Vroomen et al., 1987a).
Four human subjects were given a single dose of 400 mg
furazolidone/day administered in a tablet or a capsule. Using an
HPLC method, 24 h urine samples collected after administration
contained 0.003% to 0.16% unchanged furazolidone. By using a
specific complexation method (detection limit 2 µg/ml) no
furazolidone was detected (White, 1989).
Furazolidone was given to 10 human adults as 2 daily doses of
200 mg each for 21 days. Plasma levels analysed by HPLC (detection
limit 0.002 µg/ml) ranged from trace quantities to 0.489 µg/ml
(White, 1989).
2.1.3 Special studies on bound residues
Muscle tissue was prepared from a piglet receiving
14C-furazolidone for 10 days and sacrificed 2 h after the last
administration. Groups of 2 female Wistar WU rats were fed diets
containing non-extracted muscle tissue (group 1), muscle tissue
obtained after 4 extractions with water (group 2) (no extractions
with organic solvents were carried out) or the water extracts
obtained from muscle tissue after four extraction steps with water
for 4 days (group 3). Amounts of radioactivity in groups 1, 2, and
3, were, respectively, 26, 28 and 38% in urine, 23, 38 and 24%, in
faeces, 5.0, 7.4 and 4.8% in tissues, and 4.2, 5.3 and 3.1% in the
remaining carcass. Total recovery was 62%, 81% and 71% for groups 1,
2 and 3, respectively. The amount of non-extractable fractions
obtained from rat liver and muscle tissues were, respectively, 34
and 27% for group 1, 29% and 39% for group 2 and 32% and 21% for
group 3 (Vroomen, et al., 1990).
From a metabolism study with pig hepatocytes it was shown that
at least 70% of the bound residues still contain the
3-amino-2-oxazolidone side chain of furazolidone releasable upon
mild acid treatment. At least 25% of the bound residues in vivo
were shown to contain a releasable side-chain (day 0 animal)
(Hoogenboom 1991b).
The bioavailability of total and non-extractable
14C-furazolidone-derived radioactivity found in swine tissues at 0
and 45-day withdrawal has been investigated in bile duct cannulated
male rats. The pigs had been treated with 300 mg 14C-furazolidone
(both methylene- and formyl-labelled)/kg feed for 14 days. Zero-day
withdrawal liver was extracted with methanol:water, methanol, ether
and ethylacetate. A total of 44% of the radioactivity was extracted.
Zero-day withdrawal muscle, 45-day withdrawal liver and 45-day
withdrawal muscle were prepared in a similar way with different
extraction methods but no methanol:water extraction was carried out,
in order to prevent solubilization of small protein molecules which
may contain bound radioactivity. From 0-day withdrawal muscle 22% of
the radioactivity was extracted. From 45-day withdrawal liver and
muscle 8.3% and 14% of the radioactivity was extracted,
respectively.
Pelleted freeze dried extracted and non-extracted 0 and 45 day
withdrawal liver and muscle were dosed for 24 h to groups of 4
Sprague-Dawley rats. The rats were pretreated with control liver or
muscle for 24 h. Bile, urine and faeces were collected for up to 72
h. After 72 h the rats were sacrificed and the liver and
gastrointestinal tract were analyzed. The excretion pattern for the
differently-treated rats was similar. Rats treated with 0 and 45 day
withdrawal liver and muscle containing total residue excreted 10-27%
in the urine and 1.5-2.7% in bile, while 7.4-16% was found in
tissues. Rats treated with non-extractable tissue excreted 17-21% in
urine and 1.1-3.0% in bile; 4.8-15% was found in tissues (Hawkins
et al., 1992).
2.1.4 Effects on enzymes and other biochemical parameters
The effect of oral administration of 500 mg furazolidone/kg bw
on the activity of monoamine oxidase (MAO) was studied in rats.
Twenty-four hours after treatment MAO activity in liver and brain
was inhibited by 95%. Enzyme inhibition was detectable in liver 6 h
and in brain 12 h after administration, while maximal inhibition was
observed between 24 and 48 h. MAO activity returned to control
values in liver and brain within 10 days and 2-3 weeks,
respectively. The levels of noradrenaline and serotonin in brain
increased by 60-70%, dopamine in brain by 20% and noradrenaline in
heart by 60% when measured 1 to 3 days after application. In
contrast, adrenomedullary catecholamines were reduced by 30% one and
3 days after administration. Repeated application of furazolidone
elicited a cumulative effect: 15 mg/kg bw on 10 consecutive days was
as effective as a single dose of 90-120 mg furazolidone/kg bw.
Following application of furazolidone (125-500 mg/kg bw) the
sympathomimetic actions of tyramine given intravenously and
intraduodenally were potentiated up to 100-fold. According to the
authors the inhibition of MAO is probably due to a metabolite of
furazolidone that contains a free hydrazine group (Palm et al.,
1967).
After single oral doses of 2-100 mg furazolidone/kg bw a
decrease in rat liver-mitochondrial MAO was observed in about 4 h.
Maximal inhibition occurred in 16-24 h. Enzyme activity returned to
normal after 21 days. Furazolidone in a saturated solution did not
affect rat liver MAO in vitro (Stern et al., 1967).
In chickens fed 400 mg furazolidone/kg feed for 10 days MAO
activity in the brain, heart and alimentary tract was inhibited. No
MAO inhibition in the liver was found. Treatment increased the
amount of 5-hydroxy-tryptamine (5-HT) in the brain and potentiated
the vasodepressor action of tyramine. The amounts of adrenaline,
noradrenaline and the vasodepressor action in the brain were
unaffected by treatment (Ali & Bartlet, 1982).
In ducklings administered 400 mg furazolidone/kg feed for 10
days or 200 mg/kg bw by crop tube MAO activity in the liver was
inhibited. No inhibition was found in other organs (Ali & Bartlet,
1982).
Significant diamine oxidase (DAO) inhibition was observed in
various tissues (plasma, duodenal mucosa, liver, heart and brain) of
rabbits given orally 50 mg furazolidone/kg bw for 5 consecutive
days. Recovery was complete in all organs 14 days after the
withdrawal of furazolidone (Ali, 1983).
MAO inhibition was measured in primary cultures of pig
hepatocytes incubated with furazolidone. A dose-related inhibition
was observed; the effect was completely reversible upon withdrawal
of furazolidone. The proposed but not proven metabolites
ß-hydroxye-thylhydrazine and 3-amino-2-oxazolidone incubated with
the same cells caused an irreversible inhibition of MAO activity
(Hoogenboom, 1991c).
Six hundred mg furazolidone/kg administered in the diet to male
Wistar rats for 7 days did not affect cytochrome P-450
concentrations, but increased absolute cytochrome b 5 levels.
The activity of NADPH-cytochrome c reductase and aminopyrine
N-demethylase was decreased, but that of aniline hydroxylase was
increased (Fukuhara & Takabatake, 1977).
Thirty mg furazolidone/kg bw administered orally to large male
white turkeys produced significant increases in hypothalamic
concentrations of noradrenaline, adrenaline and dopa. One week after
withdrawal the amine concentrations returned to normal (Ali et al.,
1988).
In an in vitro experiment 14C-furazolidone was covalently
bound to rat liver microsomal protein; the binding could be
inhibited by addition of gluthathione or mercaptoethanol.
Furazolidone did not interact with added calf thymus DNA in the
presence of microsomes (Vroomen, 1987).
In an in vitro study using swine liver microsomes it was
shown that the mercaptoethanol conjugate (M1) and the glutathione
conjugate (G1) can bind covalently to microsomal protein.
According to the author this reaction is reversible (Vroomen et
al., 1988). In the case of pig hepatoccytes, a decrease in
GSH-levels did not result in an increased formation of bound
residues. Furthermore, no proof was obtained for the reversibility
of bound residues upon incubation with MSH (Hoogenboom, Personal
communication, 1992).
Furazolidone binds to DNA and possibly renders steric hindrance
to DNA replication, which leads to the inhibition of biosynthesis of
DNA in Vibrio cholerae cells (Chatterjee et al., 1975).
DNA-binding of 14C-furazolidone in piglet tissue varied from
87-382 pmol-equivalents of furazolidone per mg DNA (Vroomen et al.,
1986).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies on furazolidone are
summarized in Table 1.
Table 1. Acute toxicity of furazolidone
Species Sex Route Purity LD50 Reference
(mg/kg bw)
Mouse M&F oral 100% 1110 Mitchell et al., 1990b
Rat M&F oral 100% 1508 Mitchell et al., 1990a
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Male Wistar rats (6/group) were fed diets containing 0, 10, 100
or 200 mg furazolidone (purity not given)/kg in the diet for 13
months (equivalent to 0, 0.5, 5 or 10 mg/kg bw/day). Mortality rates
were 1/6, 2/6, 1/6 and 2/6 for the control, low-, mid- and high-dose
groups, respectively. No effects were observed on food consumption
and body weight. No changes were observed in the number of
erythrocytes and the number of leucocytes in blood samples of 2
rats/group taken at the end of the experiment. A slight increase in
relative liver and spleen weights were observed at the highest dose.
At histopathology a slight hyperthrophy of the liver cells was
observed at all dose levels. No effects were observed on kidney,
spleen, heart and testis (Aiso et al., 1962).
The effects of feeding various nitrofurans was studied.
Thirty-five female Holtzman weanling rats were administered 1000 mg
furazolidone (purity not given)/kg feed for 45 weeks (equivalent to
50 mg/kg bw/day). A matched control group was used. The rats were
maintained on a control diet for an additional 8 weeks. Observations
included clinical signs, feed consumption, feed efficiency and body
weight. Ten rats with palpable tumours were necropsied at week 42
and the remaining rats at termination of the experiment. At
termination mortality was significantly increased as compared to the
control group. Feed consumption, feed efficiency and body-weight
gain were significantly decreased in treated rats. Treated rats had
significantly more palpable mammary tumours from week 35 onwards
than the control rats. This was a poorly-reported study and no
detailed histopathology is available (Siedler & Searfoss, 1966).
In another experiment group of rats (Carworth, 20/sex/group)
received diets with various nitrofurans for 45 weeks followed by a
7-week recovery period. An untreated control group was used.
Furazolidone was fed at a rate of 1000 mg/kg feed (equivalent to 50
mg/kg bw/day). Observations included body weight, feed consumption,
feed efficiency and pathology. After five weeks on treatment and at
termination both male and female rats showed a significant decrease
in body-weight gain, feed consumption and feed efficiency. A
significantly increased incidence of palpable mammary tumours was
observed in female rats. At microscopy most tumours were found to be
adenomas, fibromas, and fibroadenomas with or without cyst
formation. Two of the 72 tumours observed were adenocarcinomas. This
was a poorly-reported study (Siedler & Searfoss, 1967).
2.2.2.2 Dogs
In a very limited study groups of 2 male and 2 female beagle
dogs were given micronized furazolidone by gavage at doses of 5, 11
or 23 mg/kg bw/day for 90 days. Another group of 1 male and 1 female
beagle dogs received 23 mg/kg bw/day furazolidone (as crystals) in
gelatin capsules for 88 days. Some neurological signs and testicular
degeneration in the 11 and 23 mg/kg bw/day dose groups were seen.
Most dogs suffered from pneumonia (Borgmann et al., 1964).
Groups of beagle dogs were orally administered furazolidone
(purity not given) in gelatin capsules. Four males and 4 females
received 7.5 mg/kg bw/day for 6 months. Two males were given 25
mg/kg bw/day for 6 months and 6 male and 4 female dogs received this
dose for periods varying from 28-118 days during 6 months of the
study. The highest-dose group consisted of 3 males and 2 females
given 50 mg/kg bw/day for periods varying from 16-37 days during 6
months of the study. The number of dogs used for controls were 12
(for 126 days) and 10 (for 6 months). No effects were observed on
clinical signs, haematology, blood biochemistry, urinalysis, organ
weight and macroscopy. Body weight was decreased in the mid- and
high-dose groups. In all treated dogs neurological symptoms and
histopathological changes in the region of the basal ganglia,
decreased sperm and tubular testicular degeneration were reported.
The authors concluded that the neurological effects as well as the
testicular changes were reversible. This was a poorly-reported study
that was not performed according to current standards (Paul, 1955).
Three groups of 2 male and 2 female beagle dogs were fed diets
containing 0, 30 or 100 mg furazolidone/kg for 2 years (equivalent
to 0.8 or 2.5 mg/kg bw/day). No effects were observed on body
weight, haematology, blood biochemistry, urinalysis, organ weight
and histopathology. Only an abstract of this limited study was
available (Huffman, 1965b).
Groups of beagle dogs (ARC, 4/sex/group) were orally
administered 0, 1, 5 or 15 mg/kg bw/day furazolidone (purity not
given) for 2 years. Additionally, 2 male dogs per group were
included to assess effects on semen quality. Two groups of 5 male
and 5 female beagle dogs, raised at different kennels (ARC + HRA)
were included to further assess cataractogenic properties of
furazolidone at the 15 mg/kg bw/day dose level. Observations
included clinical signs, body weight, ophthalmoscopy, neurological
examinations, recording of ECGs, haematology, clinical chemistry and
urinalysis, macroscopy and histopathology.
Because neurological symptoms were observed in 5/8 high-dose
dogs and 2 high-dose dogs from the semen evaluation study, these
dogs were discontinued from the study. Cataracts were observed in
3/5 male and 5/5 female ARC dogs and in 2/5 male and 1/5 female HRA
dogs. Decreased sperm motility and abnormal sperm were observed in
the mid- and high-dose groups. Increased relative kidney weight was
observed in high-dose females and decreased testes weight in mid-
and high-dose males. Only abstract was available (King et al.,
1971a).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of Swiss MBR/ICR mice (50/sex/group) were fed diets
containing 0, 75, 150 or 300 mg/kg furazolidone (purity not given)
for 13 months (equal to average daily doses of 12, 24 or
47 mg/kg bw). All mice were maintained on normal diet for 10
additional months. During the treatment period as well as during the
post-treatment period, no substance-related effects were observed on
feed consumption and body weight. Survival was decreased in mid- and
high-dose females at the end of the treatment period and in
high-dose males and mid- and high-dose females at 23 months. At
histopathology the incidence of bronchial adenocarcinomas was
significanly increased in the mid- and high-dose groups in both
sexes (incidences for males: 13/49, 19/48, 26/50 and 37/50 and for
females: 15/50, 18/50, 20/47 and 30/48 at the control, low-, mid-
and high-doses, respectively). The incidence of lymphosarcomas was
significantly increased in mid- and high-dose males (incidences:
1/49, 7/48, 10/50 and 10/50 for the control, low-, mid- and
high-doses, respectively) (Halliday et al., 1974b).
2.2.3.2 Rats
Groups of Sprague-Dawley rats (35/sex/group) were fed diets
containing furazolidone for 2 years. The actual average consumption
over 2 years was 0, 0.7, 4 or 10 mg/kg bw/day for males and 0, 0.8,
4.3 or 14 mg/kg bw/day for females. Additional groups (5/sex) were
maintained and killed after 1 year. No effects were observed on
clinical signs, body weight, feed consumption, blood biochemistry,
urinalysis and sternal bone marrow sections. Survival tended to
decrease with dose in females (26, 22, 23 and 15 surviving after two
years for control, low-, mid- and high-dose groups, respectively).
At termination mid- and high-dose female rats showed dose-related
decreased erythrocyte, haemoglobin and haematocrit counts and an
increased neutrophil count. Females from the highest-dose group
showed an increased neutrophil/lymphocyte ratio. Relative liver
weight was increased in high-dose males. At histopathology male rats
from the highest dose group showed an increase in parathyroid
hyperplasia without 'renal ricketts'. In male and female rats an
increase in adrenal cortical hyperplasia was observed at the highest
dose. High-dose females showed an increase in thyroid atrophy.
Females exhibited increased mammary tumour incidences as shown in
Table 2. The authors concluded that the mean onset time of mammary
neoplasms was approximately 2 months earlier in the mid- and
high-dose females than in the other groups (King et al., 1972a;
Halliday et al., 1973a).
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 250, 500 or 1000 mg/kg furazolidone (purity not given)
for 20 months (equivalent to 12.5, 25 or 50 mg/kg bw/day). The
surviving rats were maintained on control diets for at least 4
months or until 90% of the rats had died. Observations included
clinical signs, mortality, body weight and feed consumption.
Haematological and clinical chemistry examinations were performed
after 1 year and at the end of the treatment period. Extensive
histopathological examinations were performed on all moribund and
sacrificed rats. At the mid- (males only) and high-doses the
mortality rate was increased; 90% mortality in the highest dose was
observed after 24 months. At the end of the treatment period
body-weight gain was significantly decreased at 500 and 1000 mg/kg
Table 2. Mammary tumour incidences in female Sprague-Dawley rats
control low mid high
No. of animals examined 34 35 33 35
Multiple mammary tumours 3 6 11 19
Malignant mammary tumours 1 3 3 5
Mammary fibroadenomas 0 6 2 10
Mammary adenocarcinomas 1 2 2 3
Mammary carcinosarcomas 0 1 2 1
feed. At 1000 mg/kg feed haemoglobin, haematocrit (also at 500 mg/kg
feed) and the number of erythrocytes (also in high-dose females)
were significantly decreased in male rats. An increase in 'non-renal
ricketts' was observed in high- dose male rats. The incidence of
testicular atrophy was increased in mid- and high-dose males and the
incidence of adrenal cortical hyperplasia, lipolysis, congestion and
haemorrhage was increased in high-dose males only. Tumour incidences
are given in Table 3. In high-dose females a significant increase in
the incidence of mammary gland adenocarcinomas was observed. In
addition, an increased incidence of sebaceous gland adenomas and
thyroid adenomas was observed in both sexes at the mid- and
high-dose and of basal-cell epithelioma and carcinomas in males of
the high-dose group. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1974a).
Sprague-Dawley rats (50/sex) were fed diets containing 0, 250,
500 or 1000 mg furazolidone/kg for 20 months (equivalent to 12.5, 25
or 50 mg/kg bw/day). The study was carried out following the same
protocol as described above for Fischer 344 rats. Mortality for
control, low-, mid- and high-dose groups was 4/50, 11/50, 17/50 and
30/50 for males and 9/50, 12/50, 8/50 and 29/50 for females,
respectively. High-dose animals were sacrificed at day 666, compared
to day 895 for controls. Body-weight gain was significantly
decreased in mid- and high-dose males and high-dose females. At the
end of the treatment period the number of erythrocytes was decreased
in females at 500 and 1000 mg/kg feed. High-dose males showed an
increased neutrophil/lymphocyte ratio and a decreased lymphocyte
count. Histopathology showed an increased incidence of hepatic
necrosis in all treated rats, especially high-dose females.
Testicular atrophy was seen in mid- and high-dose males. A
dose-related increase in adrenal cortical hyperplasia was observed
in females at all dose levels. Tumour incidences are given in Table
4. In the high-dose group, significantly increased incidences were
reported for mammary adenocarcinomas in females and for neural
astrocytomas in males. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1973b).
Table 3. Tumour incidences in Fischer 344 rats
males females
contr. low mid high contr. low mid high
No. of animals examined 49 50 50 49 49 50 50 50
Dermal fibromas 2 6 1 10 0 3 7 3
Sebaceous adenomas 0 0 8 11 1 2 6 10
Sebaceous adenocarcinomas 0 1 1 1 0 1 0 1
Basal cell epitheliomas 0 2 4 8 0 0 0 0
Basal cell carcinomas 0 0 0 2 0 0 0 0
Mammary neoplasms 1 1 2 0 11 29 40 30
Mammary adenocarcinomas 0 0 0 0 0 0 0 6
Lymphoreticular neoplasms 11 1 6 2 11 7 7 6
Pituitary adenomas 4 2 4 1 24 13 23 3
Thyroid adenomas 1 2 12 19 0 1 12 7
Testicular interstitial 42 44 31 0
cell tumours
Table 4. Tumour incidences in Sprague-Dawley rats
males females
contr. low mid high contr. low mid high
No. of animals examined 50 49 50 49 49 50 50 50
Dermal fibroma 2 6 5 7 3 1 2 7
Sebaceous adenoma 1 0 0 6 0 0 0 0
Sebaceous adenocarcinomas 0 0 0 1 0 0 0 0
Mammary neoplasm 2 4 5 1 29 41 45 40
Mammary adenocarcinoma 0 0 1 0 1 0 3 8
Mammary carcinosarcoma 0 0 0 0 0 1 0 0
Lymphoreticular neoplasms 7 6 5 7 6 1 4 0
Pituitary adenomas 10 9 6 3 29 17 17 1
Thyroid adenomas 0 1 1 5 0 0 1 1
Neural astrocytomas 0 0 2 5 1 0 1 0
2.2.4 Reproduction studies
2.2.4.1 Rats
A special study was performed to evaluate the effects on the
reproductive system of male rats fed furazolidone in the diet. Five
male rats (strain not specified)/group received diets containing 330
or 660 mg furazolidone/kg for 14 weeks, 3 male rats/group were given
330 or 660 for 12 weeks (equivalent to 16 or 33 mg/kg bw/day) with a
recovery period of 2 weeks. The control group consisted of 4 rats.
Observations included testes and epididymis weight and macroscopy
and histopathology of the reproductive tract. No dose-related
effects were observed in rats from the low-dose group. Testes weight
was markedly decreased in high-dose rats. This effect was more
pronounced in rats fed furazolidone for 14 weeks than in rats dosed
12 weeks with 2 weeks recovery. Histopathology of the testes of
high-dose rats revealed oedema of the interstitium, and atrophy and
degeneration of the sperm-producing tubules. Occasionally stasis of
previously produced sperm in the epididymus was seen and some
epididymi were devoid of sperm. The effects were still present after
the recovery period (Larson, 1963a).
Because there was some evidence that the testicular damage
observed after feeding of 660 mg furazolidone/kg in the diet was not
completely reversible gross and microscopic examinations were
performed on male rats 5 days and 6 weeks after withdrawal from a
diet containing 660 mg furazolidone/kg. Another 2 males were
retained for 14 weeks, the additional 8 weeks being required to
allow non-treated females to reach breeding age. Each treated male
(and one control male) cohabited with 2 females each. Testes weight
was decreased in 4/6 rats and in 3/6 rats atrophic seminiferous
tubules and abundant intertubular transudate were seen. In 2 of
these male rats the epididimi had sperm stasis. All prostates were
normal. Rats used in the breeding trials showed normal libido and
fecundated females but the litters that were produced had slightly
fewer young than those from the litters sired by the control male
(Larson 1963b).
In a three-generation reproduction study groups of rats were
fed diets containing 0, 30 or 100 mg furazolidone/kg. The F0
generation was maintained on the furazolidone diet and sacrificed
after 2 years. No effects were found on haematology, body weight and
histopathology after 2 years. Decrease in testes weight was
dose-related. Reproductive performance was not affected. Only a
summary of this study was available (Huffman, 1965a).
In a three-generation reproduction study groups of
Sprague-Dawley rats (20/sex) were used. Female rats only were
administered 500 mg furazolidone/kg feed. Because of growth
depression the dose level was lowered to 400 mg/kg on day 16 and to
250 mg/kg on day 37. Three matings per generation were performed.
The pups from the first litter were sacrificed 21 days after birth.
Pups from the second litter were used for the second generation and
pups from the third litter were used for teratological examination.
No treatment-related effects were found on reproduction parameters
resulting in a NOEL equivalent to 12.5 mg/kg bw/day (Borgmann &
Prytherch, 1964b; Borgman & Prytherch, 1966).
2.2.4.2 Chickens
Groups of New Hampshire chickens were fed diets containing 55,
110 or 220 mg furazolidone/kg feed for 4 to 16 weeks. No effects
were observed on body weight and egg production, hatchability of
eggs and shell quality. Thyroid and adrenal weight were both reduced
(Francis & Shaffner, 1956).
2.2.4.3 Pigs
In a limited study a group of purebred Hampshire and Duroc pigs
(9 sows and 12 gilts) were fed a diet containing 300 mg/kg
furazolidone for two weeks at breeding and 150 mg/kg for three weeks
at farrowing. Another group was maintained as an untreated control
group. There was no difference in the number of pigs born, number of
pigs weaned or weight gain of the pigs. However, pup weight at
weaning was slightly higher in the treated group (Hughes & McMinn,
1963).
2.2.4.4. Goats
Male Nubian goats received furazolidone suspended in distilled
water at a dose of 10 or 40 mg/kg bw. The effect on semen morphology
and biochemistry was studied. At both doses ejaculate volume, the
number of motile spermatozoa per ejaculate and the number of live
spermatozoa per ejaculate and semen fructose concentration were
significantly decreased (Mustafa et al., 1987).
2.2.5 Special studies on embryotoxicity and/or teratogenicity
2.2.5.1 Mice
Groups of Albino C strain mice were administered furazolidone
at doses up to 2 g/kg during pregnancy varying from day 1-11.
Abortions or fetal deaths occurred in all mice treated with doses
> 1 g/kg when treatment started before day 8 of pregnancy.
However when treatment started at day 10 abortions or fetal death
occurred only in 2/9 mice. Litterweight was dose-relatedly decreased
but no congenital abnormalities were observed (Jackson & Robson,
1957).
2.2.5.2 Rats
Groups of 3 Donryu male rats were fed 0 or 100 mg
furazolidone/kg bw for 7 days, and on day 8 the rats were
sacrificed. Relative testes weights were slightly decreased. The
number of mature spermatozoa was decreased and degenerative changes
in the seminiferous tubules (sloughing of spermatocytes and
multi-nucleated cells) were observed (Miyaji et al., 1964).
2.2.5.3 Rabbits
Groups of two rabbits were given an intra-amniotic injection of
1, 2 or 2.5 mg furazolidone on day 14 or 15 of pregnancy. A
laparatomy was performed 5 days after the injection to determine the
effect on pregnancy. Pregnancy interruption was observed at 2.0 and
2.5 mg furazolidone (Jackson & Robson, 1957).
Groups of 10 pregnant New Zeeland white rabbits were orally
administered 30 mg furazolidone (purity not given)/kg bw/day on days
7-15 of pregnancy. On day 29 of pregnancy the dams were sacrificed
and the fetuses were delivered by caesarean section. Feed
consumption and body-weight gain were significantly decreased. No
embryotoxicity or teratogenicity was observed (Borgmann & Prytherch,
1964a).
2.2.5.4 Chickens
Fifty percent mortality was observed in 10-day old chicken
embryos after treatment with 15 mg furazolidone in the inner shell
membrane, 4.2 mg in the allantoic cavity or after treatment with 0.7
mg in the yolk sac (Gentry, 1957).
2.2.5.2 Special studies on genotoxicity
The results of in vitro and in vivo genotoxicity studies
and of genotoxicity studies on metabolites of furazolidone and bound
residues are summarized in Tables 5, 6, and 7, respectively.
Table 5. Results of in vitro genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Ames testa S. typhimurium 0.01-1.0 µg/pl ? positiveb Jagannath et al.,
TA1538, TA98 (1.0 µg/pl slightly 1981g
and TA100 toxic)
TA1535, TA1537 negativeb
Ames testa S. typhimurium* 0.1, 0.5, 1.0 >99% positive Ni, et al., 1987
TA98, TA98NR or 2.5 µg/pl
TA98/1,8-DNP6 2.5 µg/pl toxic
Ames testa S. typhimurium 0.01-0.3 µg/pl 99% positiveb Crebelli et al.,
TA100 >0.3 µg/pl toxic 1982; Carere et
al., 1982
Spot test Escherichia coli 50 µg/plc positive McCalla &
WP2uvrA Voutsinos, 1974
Reverse Euglenia gracilis 25, 50 and 100 ? positive Ebringer et al.,
mutation assay µg/mlc 1976
Forward Vibrio cholerae up to 12 µg/mlc ? positive Chatterjee et al.,
mutation assay OGAWA 154 1983
Prophage Escherichia coli ? ? positive Chatterjee et al.,
induction assay K12 GY5027 1983
Gene conversion S. cerevisiae D4 no details ? positive Voogd et al.,
assay 1982 as cited in:
VanMiert et al.,
1984
Gene mutation Chinese hamster up to 125 µg/ml >99% positive Gao et al., 1989
assaya ovary cells >100 µg toxic
Table 5 cont.
Test system Test object Concentration Purity Results Reference
HGPRT test Mouse L5178Y no details ? positive Voogd et al.,
lymphoma cells 1982
SOS function and Escherichia coli 0.8 and 8.0 µMc ? positive Bryant &
mutation assay WP2 McCalla, 1980
SOS function Escherichia coli 0.1, 0.2, 0.5 ? positive Ohta et al., 1984
assay K12 µg/mlc
Sex-linked D. melanogaster 0.5 mM in DMSOc ? positive Blijleven et al.,
recessive 1977
lethal test
Sex-linked D. melanogaster 0.18, 0.44 or ? positive Kramers, 1982
recessive 0.5 mM in DMSOc
lethal test
Chromosome human 10, 33 and 100 99.8% negative Scheres, 1991b
aberration assay lymphocytes µg/ml in DMSOd
33, 100, 180 positivef
and 333 µg/ml in
DMSOe (333 µg/ml
slightly toxic)
Chromosome human 0.2, 2.0 or ? positive Cohen & Sagi,
aberration assay lymphocytes 20.0 µg/mld 1979
20.0 µg/ml toxic
Chromosome human 0.5-100 µMc ? negative Tonomura &
aberration assay lymphocytes Sasaki, 1973
Table 5 cont.
Test system Test object Concentration Purity Results Reference
Chromosome bovine lymphocytes 0.05-500 mg/lg ? positive Queinnec et al.,
aberration assay porcine lymphocytes 0.05-500 mg/lg positive 1975; Babile et
al., 1978
SCE test human 0.2, 2.0 and ? positive Cohen & Sagi
lymphocytes 20 mg/l in DMSO 1979
20 mg/l toxic
Rec assay Escherichia coli ?? ? positive Chatterjee et al.,
K12 rec- and rec+ 1983
DNA repair Escherichia coli 1.0 to 10 µM ? positive Lu et al., 1979
assay WP2 uvrA >5µM toxic
UDS assay human 5-100 µMc ? negative Tonomura &
lymphocytes Sasaki, 1973
UDS assay Fischer 344 0.5-1000 nM/ml ? positive Probst et al.,
rat hepatocytes in DMSOb,c 1981
* nitroreductase-deficient Salmonella typhimurium tester strains.
a both with and without rat liver S9 fraction.
b positive controls yielded positive results.
c no data about toxicity.
d without metabolic activation.
e with metabolic activation.
f the authors concluded that the results were negative.
g no toxicity observed.
Table 6: Results of in vivo genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Micronucleus Swiss CD-1 mice 300 mg/kg bw 99.8% negative Enninga &
test ip in Weterings 1990
methyl-cellulose
(toxic)
Micronucleus Swiss Webster 100 and 500 mg/kg ? equivocalb Paik, 1985
test mice bw orala
a no toxicity observed
b slight induction, not dose-related
2.2.7 Special studies on endocrine toxicity
In a special study various groups of ovariectomized
Sprague-Dawley, Fischer 344 or Long Evans rats and golden hamsters
received single oral doses varying from 50-500 mg furazolidone/kg
bw. After a 2-week recovery period positive dose-related lordosis
(becoming sexually perceptive) behaviour was observed only in
Sprague-Dawley rats which is, according to the authors, indicative
of an elevated concentration of progesterone in the circulation
(King et al., 1970a).
Groups of immature superovulated Sprague-Dawley rats were
orally dosed with 100 or 500 mg furazolidone/kg bw. In all groups
ovulation was initiated about 24 h earlier than the control group.
The same shift in ovulation time was observed in a positive control
group treated with progesterone (King et al., 1970a).
Feeding of 1000 mg furazolidone/kg feed for 30, 60 or 90 days
produced a marked inhibition of the conversion of progesterone into
corticosterone (11-hydroxylation) in adrenals of Sprague-Dawley
females. A considerably lesser effect was found in Fischer 344
females. This effect could be overcome by addition of NADPH,
indicating an inhibitory effect of furazolidone on the NADPH
generating system (King et al., 1971b).
Table 7. Results of genotoxicity assays on metabolites of furazolidone and
bound residues
Test system Test object Concentration Substance Results Reference
In vitro
Ames testa S. typhimurium 100-5000 µg/plb,c Md negative Scheres, 1991a
TA100, TA98
Ames testa S. typhimurium urine from rats - positivee Crebelli et al.,
TA100 treated with 10, 1982; Carere et
50, 100 mg/kg bw al., 1982
Ames testa S. typhimurium up to 5 µg/pl M1f negativee,g Jagannath &
TA1535,TA1537 Brusick, 1981a; 1981e
Craine, 1981
TA1538, TA98, M2h negativee Jagannath &
TA100 Brusick, 1981b; 1981f
Craine, 1981
M3i negativee Jagannath &
Brusick 1981c; 1981d
Craine, 1981
Ames test a S. typhimurium 0.1 mg/pl M4j negative Vroomen et al.,
TA100, TA98 in DMSO 1987a
Ames test S. typhimurium upto 5 µg/pl GSFk negative Hoogenboom,
TA100 in DMSO MSFl negative 1991a
Reverse Mycobacterium 1000 µg/ml 5-NFAm positive Ebringer et al.,
mutation assay phlei 1976
Euglena gracilis 2.5 and 7.5 5-NFAm positive Ebringer et al.,
µg/ml 1976
DNA repair test rat hepatocytes 10-3M, 10-5Mn Mo negative Mori et al.,
mouse hepatocytes 1988
Table 7 (continued)
a both with and without rat liver S9 fraction
b positive controls yielded positive results
c no toxicity observed
d 3-amino-oxazolidinon-2
e negative control yielded negative results
f whole liver powder from pigs treated with 5 mg furazolidone/kg bw for 5 days
g substances from untreated pigs yielded negative results
h urine isolates from pigs treated with 5 mg furazolidone/kg bw for 5 days
i soluble liver isolate from pigs treated with 5 mg furazolidone/kg bw for 5 days
j 3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone.
k glutathione conjugate of cyano metabolite
l mercaptoethanol conjugate of cyano metabolite
m 5-nitro-2-furaldehyde
n no data about toxicity
o 2-hydroxyethylhydrazine.
Oral administration of 100 or 500 mg furazolidone/kg bw to
mature female rats produced a significant decrease in serum
prolactin during the morning proestrus, but not at afternoon
proestrus. Administration of 500 mg/kg bw during the morning estrus
resulted in a slight decrease in serum prolactin levels (Morrison
et al., 1973).
In a limited experiment the effect of furazolidone treatment on
the tumourigenicity of estrone was studied. Furazolidone treatment
(1000 mg/kg feed to female Sprague-Dawley and Fischer 344 rats) plus
estrone resulted in the development of more mammary masses than with
estrone alone. However, the administration of estrone did not result
in an increased incidence of mammary masses (Morrison et al.,
1972).
Furazolidone (500 or 1000 mg/kg feed) was fed to
adrenalectomized and sham-operated male rats for 30 days, after
which the adrenals and tests were examined. Adrenalectomy did not
alter the testicular atrophy produced by furazolidone. At 1000 mg/kg
feed tubular degeneration in three out of six animals was seen
(Morrison et al., 1974a).
A diet containing 500, 750 or 1000 mg/furazolidone/kg
administered to female rats for 30 days resulted in a significant
increase in serum testosterone at the highest dose (Morrison et
al., 1974b).
In a pilot study rats were administered a single oral dose of
250 or 500 mg/kg bw furazolidone in 0.5% aqueous CMC. At 30 min, 1,
2, 4, 8 and 24 h after administration blood samples were taken for
RIA of prolactin and corticosterone. Furazolidone increased in a
dose-related manner, the corticosterone concentration up to 2 h
after administration, with a return to vehicle control values by 24
h. No effect on plasma prolactin time was observed (Meites, 1984).
Male mature chickens were fed a diet containing 400 or 800 mg
furazolidone/kg feed for 10 days. Testes weight was decreased in
both groups. Treatment with 800 mg/kg produced a significant
reduction in the concentration of testosterone in plasma and testes
and some reduction in ascorbic acid, protein and cholesterol
concentration in the testes. Bolus administration of doses of 40 or
80 mg/kg bw for five days caused the same effect. The size of the
testes, wattles and combs were significantly reduced. Monoamine
oxidase activity in the testes was significantly reduced by
furazolidone. In all the treated birds testicular concentrations of
5-hydroxytryptamine were significantly raised, except in those fed
400 mg furazolidone/kg for 10 days (Ali et al., 1984).
Groups of large white female turkeys were orally dosed with 0,
7.5, 15 or 30 mg furazolidone/kg bw for 7 days in gelatin capsules.
The number of eggs produced by each bird was recorded daily 2 weeks
before, during and 4 weeks after the end of treatment. The
concentrations of luteinizing hormone (LH) and prolactin (PRL) were
analyzed by RIA in plasma samples taken on day -7, 0, +7 and +14. At
the end of the treatment period a dose-related decrease was observed
in egg production and LH concentration (significantly in the mid-
and the high-dose group). No effects were observed on PRL
concentration. After withdrawal only the egg production started to
recover but was still below normal 4 weeks after cessation of
treatment (Ali et al., 1987a; 1988).
When turkeys given 15 or 30 mg furazolidone/kg bw were injected
intramuscularly with 5 µg/kg luteinizing hormone-releasing hormone
(LHRH), no effect on LHRH-induced LH release was observed (Ali et
al., 1987a; 1988).
Groups of male turkeys were orally treated with 0, 1, 2.5, 5 or
20 mg furazolidone/kg bw/day for 14 days. Plasma was analyzed for
LH, testosterone (T) and PRL before, during and after treatment. At
the highest dose the concentrations of LH and T were significantly
decreased, and a tendency towards a decrease was observed at 5 mg/kg
bw/day. At histopathology a decrease in spermatocyte production as
well as corrugation of sperm cell nuclear envelopes and distension
of endoplastic reticulum of elongated spermatides were observed in
the 20 mg/kg bw/day group (Ali et al., 1987b).
Furazolidone administered as a bolus dose (5-500 mg/kg bw) to
male T-line chickens produced a decrease in the amount of
corticosterone in plasma (Bartlet et al., 1990).
In another experiment male T-line and J-line chickens were
given a bolus dose of furazolidone (200 mg/kg bw) which resulted in
a decrease in aspartate transaminase activity in the adrenal gland
and a general disorganization in the structure of the adrenal
cortical cells, leading to atrophy of the adrenal cortical glands
(Bartlet & Khan, 1990).
A concentration of 1 µM furazolidone inhibited significantly
the release of aldosterone from porcine adrenal cells in vitro.
Almost complete inhibition was observed at 100 µM (van den Dungen
et al., 1991).
2.2.8 Special studies on skin and eye irritation
A dose of 500 mg furazolidone (purity 100%) moistened with 0.5
ml of 0.5% saline caused slight erythema in 4/6, 3/6 and 1/6 rabbits
after 0.5, 24, and 48 h respectively, when applied under
semi-occlusive conditions to the clipped intact skin of 3 male and 3
female New Zeeland white rabbits (Mitchell et al., 1990c).
Nine New Zeeland white rabbits received an installation of 30
mg furazolidone (purity 100%) into the conjunctival sac of the right
eye, and 3/9 eyes were washed 20 seconds after treatment. After 24 h
slight conjuctival irritation was observed in both washed and
unwashed eyes. Corneal ulcerations were observed in 2/6 unwashed
eyes only. All eyes were normal at day 7 (Mitchell et al., 1990d).
2.3 Observations in humans
Furazolidone administered to patients with essential
hypertension (no details provided) produced marked supersensitivity
to tyramine and amphetamine, inhibition of intestinal MAO and
increased urinary excretion of tryptamine. Continued administration
produced a cumulative inhibition of MAO (Pettinger, et al., 1968)
Adverse reactions (gastrointestinal 8%, neurological 1.34%,
systemic, 0.56% and dermatological reactions 0.54%) to furazolidone
treatment were observed in 864/10443 patients (8.3%) treated with a
therapeutic dose of < 5 to 7 mg/kg bw/day (Altamirano & Bondani,
1989).
A 43-year old man with a contact allergy to furazolidone was
patch tested to nitrofurans with completely negative results. The
man had a positive patch test reaction to 2% furazolidone both in
PEG-400 and alcohol. Control tests were negative in 25 dermatitis
patients (DeGroot & Conemans, 1990).
3. COMMENTS
The Committee considered data from pharmacodynamic,
pharmacokinetic, metabolism, acute and short-term toxicity,
carcinogenicity, genotoxicity, reproductive, and teratogenicity
studies as well as special studies on endocrine function and some
clinical studies in humans.
The distribution, excretion, and biotransformation of
radiolabelled furazolidone were studied in rats, chickens, pigs, and
humans. After oral administration, furazolidone was rapidly absorbed
and the radioactivity was widely distributed, the highest levels
being found in liver, kidney, fat, and muscle. It was rapidly
metabolized and excreted predominantly in urine. In chicken and
human urine, only trace amounts of unchanged furazolidone could be
detected, and of the large number of metabolites found only some
were identified. In rat and pig urine, the common metabolite
appeared to be the open chain cyanometabolite
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone. The Committee noted
that quantitative information on metabolites was lacking. In pigs, a
substantial portion of the metabolites was bound to macromolecules,
and it appeared that approximately 15-40% of this bound fraction was
bioavailable. However, the Committee questioned whether valid
extraction procedures had been used to isolate these bound
metabolites.
In acute oral toxicity studies in mice and rats furazolidone
was slightly toxic; the LD50 values were of the order of 1100 and
1500 mg/kg bw, respectively.
No NOEL could be established from short-term studies performed
with rats and dogs. Rats receiving furazolidone at doses in the
range 0.5-50 mg/kg bw/day showed hypertrophy of liver cells.
Palpable mammary tumours and a decrease in body weight gain were
observed at 50 mg/kg bw/day. In dogs, dose levels of 5-25 mg/kg
bw/day led to neurological symptoms and histological changes in the
basal ganglia, together with testicular degeneration. It was noted
that the available information was deficient by current standards
and poorly reported.
Two three-generation reproduction studies were performed in
rats. In one study rats were exposed to furazolidone at
concentrations up to 100 mg/kg in feed. In the other study only
female rats were treated with diets containing 500 mg/kg, but this
concentration was gradually reduced to 250 mg/kg in order to avoid
the observed growth depression. No effects on reproductive
performance were observed in either study. The NOEL was equivalent
to 12.5 mg/kg bw/day.
In a special study designed to evaluate the effects on the male
reproductive system, rats exposed to a dietary furazolidone
concentration equivalent to 33 mg/kg bw/day exhibited testicular
degeneration. At 16 mg/kg bw/day no effects were observed.
Neither embryotoxicity nor teratogenicity was observed in
rabbits after oral administration of furazolidone at a dose of 30
mg/kg bw/day.
A carcinogenicity study was conducted in Swiss MBR/ICR mice,
which received a diet containing concentrations of furazolidone
equal to average daily doses of 12, 24, or 47 mg/kg bw/day for 13
months, followed by a control diet for 10 months. In the mid- and
high-dose groups, a significant increase in the incidence of
bronchial adenocarcinomas was observed in both sexes, and the
incidence of lymphosarcomas was significantly increased in male
mice.
In two long-term toxicity/carcinogenicity studies, furazolidone
was administered in the diet to Fischer 344 and Sprague-Dawley rats
at concentrations equivalent to daily doses of 12.5, 25, or 50 mg/kg
bw/day for 20 months. In Fischer 344 rats, a significant increase in
the incidence of mammary gland adenocarcinomas was observed in
females in the high-dose group. In addition, an increase in the
incidence of sebaceous gland adenomas and thyroid adenomas was
observed in both sexes at 25 and 50 mg/kg bw/day and of basal cell
epithelioma and carcinoma in males of the high-dose group. In the
high-dose group of Sprague-Dawley rats, significantly increased
incidences were reported for mammary adenocarcinomas in females and
for neural astrocytomas in males. In both strains of rat, female
animals showed a significant increase in the incidence of mammary
neoplasms (benign and malignant combined) at all dose levels, but
without a dose-response relationship.
Furazolidone has been tested in a wide variety of genotoxicity
studies. Positive findings were recorded in bacterial assays with
and without metabolic activation, in the sex-linked recessive lethal
test in Drosophila melanogaster, in a gene mutation assay with
mammalian cells in vitro, in a sister chromatid exchange test, and
in two DNA-repair tests. Positive as well as negative results were
obtained in chromosome aberration assays with mammalian cells in
vitro, and in tests for unscheduled DNA synthesis. One mouse
micronucleus test was negative, while another gave equivocal
results.
The majority of in vitro genotoxicity tests with postulated
metabolites gave negative results, however, nitrofuraldehyde and
urine from furazolidone-treated rats gave positive results. It was
concluded that furazolidone was genotoxic in vitro.
Several studies were performed on the endocrine effects of
furazolidone. Furazolidone inhibited the conversion of progesterone
into corticosterone in adrenal cells both in vivo and in vitro.
It has been hypothesized that disturbances of steroidogenesis
constituted the underlying mechanism for the increased incidence of
tumours caused by furazolidone. The Committee noted that it was
unlikely that the such a mechanism could account for the increase in
neural astrocytomas and uncommon skin tumours in rats. With respect
to the occurrence of mammary tumours, no information was available
on the effect of furazolidone on plasma progesterone concentrations
and no consistent effects on plasma prolactin concentrations were
observed. The Committee therefore concluded that no support had been
provided for the hypothesized mechanism.
Furazolidone caused reversible inhibition of monoamine oxidase
(MAO) activity in pig hepatocytes in vitro and in liver and brain
tissue of rats following in vivo administration. Irreversible MAO
inhibition both in vitro and in vivo was observed for the
postulated metabolites amino-oxazolidone and hydroxyethyl-hydrazine.
4. EVALUATION
On the basis of the positive effects of furazolidone in
genotoxicity tests in vitro and the increased incidence of
malignant tumours in mice and rats, the Committee concluded that
furazolidone was a genotoxic carcinogen. Since the drug is rapidly
and extensively metabolized, the Committee also considered
information on metabolites of furazolidone. Although a large number
of postulated metabolites produced negative results in genotoxicity
tests, it was noted that only a few of these had been either
identified or quantified in rats and pigs. Furthermore, the
Committee concluded that insufficient data were available on the
nature and toxic potential of compounds released from the bound
residues.
Because of the genotoxic and carcinogenic nature of
furazolidone and the above-mentioned deficiencies with respect to
the data on the metabolites, the Committee was unable to establish
an ADI.
Before considering the compound again, the Committee would wish
to have detailed information on the nature, quantity and toxicity of
the metabolites of furazolidone, including the bound residues.
5. REFERENCES
AISO, K., KANISAWA, M., YAMAOKA, H., TATSUMI, K. & AIKAWA, N.
(1962). Systematic studies on the toxicity of nitrofuran derivatives
(3): Macroscopical and histological examination of rats fed on diet
with furazolidone and 5-(5-nitro-2-vinylfuran)-1, 3,
4-oxadiazoline-2-one for one year. Chiba Daigaku Fuhai Kenkyusho
Hokoku, 15: 21-33. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H. & BARTLET, A.L. (1982). Inhibition of monoamine oxidase in
chicken and ducklings by a microbial metabolite of furazolidone.
Quarterly J. Exp. Physiol., 67(1): 69-79.
ALI, B.H. (1983). The anti-diamine oxidase effect of furazolidone in
rabbits. Comp. Biochem. Physiol., 74C(1): 109-110.
ALI, B.H., HOMEIDA, A.M. & KNIFTON, A. (1984). The effect of
furazolidone on fertility of male chickens. Comp. Biochem.
Physiol., 78C(1): 43-47.
ALI, B.H., SILSBY, J.L. & EL HALAWANI, M.E. (1987a). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
British Poultry Science, 28: 613-621.
ALI, B.H., SILSBY, J.L., LOSETH, K.J., CRABO, B. & EL HALAWANI, M.E.
(1987b). Some effects of furazolidone on the testes and plasma
testosterone, luteinising hormone and prolactin concentrations in
mature male turkeys d.d. 17 June 1987. University of Minnesota. St.
Paul, MN 55108, USA. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL), levels in young turkeys. Gen. Pharmacol., 19(1):
91-95.
ALTAMIRANO, A. & BONDANI, A. (1989). Adverse reactions to
furazolidone and other drugs. Scand. J. Gastroenterol.,
24(suppl.169): 70-80.
BABILE, R., QUEINNEC, G., BERLAND, H.M. & DARRE, R. (1978).
Structure chromosomique des lymphocytes du porc et additifs
alimentaires. C. Soc. Biol., 172:, 546-553.
BARTLET, A.L & KHAN, F.H. (1990). Effects of nitrofurans on adrenal
cortical tissue in chickens. J. Vet. Pharmacol. Therap., 13:
206-216.
BARTLET, A.L., HARVEY, S. & KLANDORF, H. (1990). Contrasting effects
of nitrofurans on plasma corticosterone in chickens following
administration as a bolus or diet additive. J. Vet. Pharmacol.
Therap., 13:, 261-269.
BLIJLEVEN, W.G.H., KORTSELIUS, M.J.H. & KRAMERS, P.G.N. (1977).
Mutagenicity testing of H-193, AF-2 and furazolidone in Drosophila
melanogaster. Mutat. Res. 56: 95-100.
BORGMANN, A.R., MURTI, G.S., COOLEY, R. & LEVIN, R. (1964).
Ninety-day toxicity studies of experimental formulations containing
furoxone (R) (NF-180) in dogs. (Project no. 475.09.02) Unpublished
final report d.d. 29 June 1964, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964a). The effect of furoxone (R)
on fetal growth and development in the rabbit. Unpublished special
report project number no. 458.9 d.d. 28 August 1964 from Pathology
Section, Scientific Department Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964b). The effect of furoxone on
reproduction in rats. Unpublished special report project number no.
460.9 d.d. 3 September 1964 from Pathology Section, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1966). The effect of furoxone on
reproduction in rats. Second and third generation. Unpublished
special report project number no. 460.09 d.d. March, 10, 1966 from
Pathology Section, Scientific Department, The Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BOWMAN, J.S. (1961a). A balance study of furazolidone C14
(methylene and aldehyde-labeled) in male rats. Unpublished report
H-NSC
no. 20-0040-32 from Hazleton, Nuclear Science Corporation,
California. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
BOWMAN, J.S. (1961b). A plateau-elimination study of
furazolidone-C14 (methylene labeled) in male rats. Unpublished
report H-NSC no. 20-0039-32 from Hazleton, Nuclear Science
Corporation, California. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran induced mutagenesis
and error prone repair in Escherichia coli. Chem. Biol. Interact.,
31: 151-166.
BUZARD, J.A., HEOTIS, J.P. & WILLIAMS, C.W. (1960). Chick
distribution studies with C14-(formyl) NF-180. Unpublished interim
report problem 360.3 d.d. 9 December 1960 .Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
BUZARD, J.A., HEOTIS, J.P. & Williams, C.W. (1961). Chick
distribution studies with C14-(methylene) NF-180. Unpublished
interim report problem 360.3 d.d. 15 May 1961. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
CARERE, A., CONTI, L., CREBELLI, R. & MACRI, A. (1982). Quantitative
data on the urinary recovery of mutagenicity in furazolidone-treated
rats. Mutat. Res., 97(6), 461-462.
CHATTERJEE, S.N., MAITI, M. & GHOSH, S. (1975). Interaction of
furazolidone with DNA. Biochem. et Biophysica Acta, 402: 161-165.
CHATTERJEE, S.N., BANERJEE, S.K., PAL, A.K. & BASAK. J. (1983). DNA
damage, prophage induction and mutation by furazolidone. Chem.
Biol. Interact., 45: 315-326.
COHEN, M.M. & SAGI, M. (1979). The effect of nitrofurans on mitosis,
chromosome breakage and sister-chromatid exchange in human
peripheral lymphocytes. Mutat. Res., 59(1): 139-142.
CRAINE, E.M. & RAY, W.H. (1972). Metabolites of furazolidone in
urine of chickens. J. Pharmacol. Science, 61(9): 1495-1497.
CRAINE, E.M. (1977). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 77:10
d.d. 28 December 1977 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1978). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 78:12
d.d. 21 April 1978 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1981). Preparation of isolates of furazolidone
metabolites. Unpublished research report, project no. 80191 d.d. 2
March 1981 from Wil Research Laboratories Inc., Research Division,
Cincinnnati, Ohio. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
CREBELLI, R., CARERE, A., FALCONE, E. & MACRI, A. (1982). A study on
the urinary and fecal excretion of furazolidone in rats by means of
mutagenicity assays. Ecotoxicol. Environ. Saf. 6(5): 448-456.
DeGROOT, A.C. & CONEMANS, J.M.H. (1990). Contact allergy to
furazolidone. Contact Dermatitis, 22:, 202-205.
EBRINGER, L., JURASEK, A., KONICEK, J., KONICKOVA, M., LAHITOVA, N.
& TRIBACUK, S. (1976). Mutagenic action of nitrofurans on Euglena
gracilis and Mycobacterium phlei. Antimicrobial Agents and
Chemotherapy, 9(4): 682-689.
ENNINGA, I.C. & WETERINGS, P.J.J.M. (1990). Micronucleus test in
bone marrow cells of the mouse with furazolidone. Unpublished report
(RCC notox project 026122) d.d. April 1990 from RCC/NOTOX, 5231
DD's-Hertogenbosch, the Netherlands. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
FRANCIS, D.W. & SHAFFNER, C.S. (1956). An investigation of the
morphological changes in young chickens and the reproductive
performance of adult chickens fed furazolidone or nitrofurazone.
Poultry Sci., 35: 1371-1381.
FUKUHARA, M. & TAKABATAKE, E. (1977). The effects of nitrofuran
derivatives on hepatic microsomal mixed-function oxidase activity in
rats. Toxicol. Appl. Pharmacol., 42: 571-581.
GAO, N., NI, Y.C., THORNTON-MANNING, J.R., FU, P.P. & HEFLICH, R.H.
(1989). Mutagenicity of nitrofurantoin and furazolidone in Chinese
hamster ovary cell strains. Mutat. Res., 225: 181-187.
GENTRY, R.F. (1957). The toxicity of certain antibiotics and
furazolidone for chicken embryos. Avian Diseases, 2: 77-82.
HALLIDAY, R.P., SUTTON, M.L., SIGLER, F.W. & LEVIN, R. A. (1973a).
Chronic toxicopathological safety study (two years) of NF-180 in
rats. Unpublished final report project no. 475.09-C d.d. 9 November
1973 from Pathological and Toxicology Section, Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1973b). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished interim report # 2 project no. 475.09D d.d. 9
November 1973 part I: Sprague-Dawley evaluation from Pathological
and Toxicology Section, Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974a). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished final report project no. 475.09D d.d. 31 January
part II: Fischer 344 evaluation from Pathological and Toxicology
Section, Norwich Pharmacal Company, Norwich, New York. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974b). Tumorgenesis
evaluation (twenty-three months) of furazolidone (NF-180) in mice,
Unpublished final report project no. 475.09E d.d. 31 January 1974
from Pathological and Toxicology Section, Research and Development
Department, Norwich Pharmacal Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HAWKINS, D.R., ELSOM, L.F., GIRKIN, R. & CHENG, C.F. (1992). The
bioavailability in rats of tissue residues from swine administered
14C-furazolidone for 14 days and subjected to 0-day, 21-day and
45-day withdrawal periods. Unpublished draft report HRC/SMI
125/911478 from Huntingdon Research Centre Ltd., P.O.Box 2,
Huntingdon, Cambridgeshire, England. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
HEOTIS, J.P., HERRET, R.J. & WILLIAMS, C.W. (1963). Drug metabolism
studies. Incorporation of C14 from formyl-C14-NF-180 into
natural products. Unpublished special report part I: problem 370.3
d.d. 20 September 1963 from Biological Research Division, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
HOOGENBOOM, L.A.P. (1991a). The use of pig hepatocytes to study the
role of glutathione on the biotransformation and toxicity of
furazolidone. In: The uses of pig hepatocytes for biotransformation
and toxicity studies Thesis, Agricultural University of Wageningen,
The Netherlands. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991b). The use of pig hepatocytes for
biotransformation studies of veterinary drugs. In: The use of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991c). The use of pig hepatocytes to study the
inhibition of monoamine oxidase by furazolidone. In: The uses of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P., Van KAMMEN, M., BERGHMANS, M.C.J. & KUIPER, H.A.
(1991). Detection of protein bound metabolites of furazolidone.
Proceedings of the Euroresidue Conference in Noordwijkerhout, The
Netherlands. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965a). Two-year chronic toxicity study and a
three-generation reproductive study in rats. Unpublished report
no. 65:15/H-1R from Hess & Clark, Research Department, Ashland,
Ohio. Summary submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965b). Two-year chronic toxicity test with
furazolidone in dogs. Unpublished report no. 65:16/H-1D) d.d. 30
July 1965 from Hess & Clark, Research Department, Ashland, Ohio.
Abstract submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HUGHES, D.L. & McMINN, C.S. (1963). The effect of furazolidone on
reproduction and enteric infections of swine. Unpublished final
report (Ex 62-12-18a2) Norwich Pharmacal Company, Norwich, USA.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
JACKSON, D. & ROBSON, J.M. (1957). The action of furazolidone on
pregnancy. J. Endocrin., 15: 355-359.
JAGANNATH, D.R. & BRUSICK, D.J. (1981a). Mutagenicity evaluation of
WIL-80191-10-4 dry liver, control in the Ames Salmonella/microsome
plate test. Unpublished final report d.d. March 1981 LBI project
from Litton Bionetics, Inc. Kensington, Maryland 207953. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981b). Mutagenicity evaluation of
WIL-80191-11-2 urine isolate; control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981c). Mutagenicity evaluation of
WIL-80191-10-1 liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981d). Mutagenicity evaluation of
WIL-80191-10-2 dry liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981e). Mutagenicity evaluation of
WIL-80191-10-3 liver isolate control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981f). Mutagenicity evaluation of
WIL-80191-11-1 urine isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981g). Mutagenicity evaluation of
Furazolidone WIL-80191-11-3 in the Ames Salmonella/microsome plate
test. Unpublished final report LBI project from Litton Bionetics,
Inc. Kensington, Maryland 207953. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CURRIE, G.N. HARRINGTON, C. & WELCH, C.A. (1970a).
Effects of nitrofurans on steroidogenesis and mammary hyperplasia
(NF-180 Rx 30309) Unpublished annual report project no. 569.09
d.d.16 June 1970 from Research and Development Department, The
Norwich Pharmacal Comp., Norwich, New York. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CHRISTENSEN, E.F., McLAUGHLIN, J.H., LEVIN, R.A. &
SIGLER, F.W. (1971a). Chronic (2 year) toxicopathologic study of
NF-180 in dogs. Unpublished final report project no. 475.09B d.d. 12
November 1971 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Abstract submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
KING, C.D., CURRIE, G.N., HARRINGTON, C. & WELCH, C. (1971b).
Nitrofurans and steroidogenesis. Unpublished interim report no. 5
project no. 596.09 d.d. 21 December 1971 from Pathological and
Toxicology Section, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L. & LEVIN, R.A. (1972a). Chronic
toxicopathological safety study (two years) of NF-180 in rats.
Unpublished status report no.1 project no. 475.09c d.d. 18 October
1972 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L., WONG, L.C.K. & LAUGHLIN, P.J. (1972b).
Tumorgenesis evaluation (two years) of NF-180 in Spraque-Dawley and
Fischer 344 rats. Unpublished status report no.1 project no. 475.09D
d.d. 18 0ctober 1972 from Pathological and Toxicology Section,
Research and Development Department, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
KOUBA, R.F. (1979). Analysis of components from urine of pigs
treated with furazolidone-14C-15N or furazolidone-14C.
Unpublished report no.: RFK 79:3 from Hess & Clark, Ashland.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
KRAMERS, P.G. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
LARSON, E.J. (1963a). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12a. Addendum to pathology
research report H-12. Unpublished report d.d. 14 November 1963 from
Research Department Hess & Clark, Ashland Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LARSON, E.J. (1963b). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12 d.d. 18 March 1963 from
Research Department Hess & Clark, Ashland, Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli. Mutation and induction and repair of daughter-strand
gaps in DNA. Mutat. Res., 67: 13-144.
McCALLA, D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res. 16(1): 3-16.
MEITES, J. DENINE, E.P. & OBROSKY, K.W. (1984). Determination of
blood levels of prolactin and corticosterone following oral
administration of furazolidone (NF-180) or EU-557 to male
Sprague-Dawley rats: a pilot study. Unpublished final report project
no. 475.59.59 d.d. 30 March 1984 from Norwich Eaton Pharmaceuticals,
Inc., Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990a).
Acute oral toxicity study of furazolidone in rats. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5884-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990b).
Acute oral toxicity study of furazolidone in mice. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5885-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B. & WESSEL, J.L. (1990c).
Primary dermal irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/Dynamics Inc. project
no. 5886-90, East Millstone, New Jersey. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B., ODDE, E.Y. & WESSEL, J.L.
(1990d). Primary irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/dynamics Inc. project
no. 5887-90, East Millstone, New Jersey. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
MIYAJI, T, MIYAMOTO, M & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testis caused by nitrofuran
compounds. Acta Path. Jap., 14(3): 261-273.
MORI, H., SUGIE, S., YOSHIMI, N., IWATA, H., NISHIKAWA, A.,
MATSUKUBO, K., SHIMIZU, H. & HIRONO, I. (1988). Genotoxicity of a
variety of hydrazine derivatives in the hepatocyte primary culture
DNA repair test using rat and mouse hepatocytes. Jpn. J. Cancer
Res. (Gann), 29: 2145-2156.
MORRISON, J.L., CURRIE, G.N., HARRINGTON, C. & WELCH, C.A. (1972).
The effects of estrone and NF-180 on mammary tumor formation.
Unpublished special report project no. 596.09 d.d. 12 October 1972
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1973). Effects of
NF-180 (acute) on serum prolactin levels. Unpublished final report
project no. 596.03 d.d. 24 October 1973 from Biochemistry Section,
The Norwich Pharmacol Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974a). Effects of
adrenalectomy on NF-180 induced testicular atrophy. Unpublished
final report project no. 475.03-F d.d. 24 May 1974 from Biochemistry
Section, The Norwich Pharmacol Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974b). Effects of
NF-180 treatment on plasma steroids in the female Sprague-Dawley
rat. Unpublished final report project no. 475.03-31 d.d. 18
September 1974 from Biochemistry Section, The Norwich Pharmacol
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MORRISON, J.L. (1976). Identification of NF-682 as a metabolite of
NF-180 in rats. Unpublished report d.d. 2 July 1976 attachment 5
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MUSTAFA, A. I., ALI, B.H. & HASSAN, T. (1987). Semen characteristics
in furazolidone-treated goats. Reprod. Nutr. Develop., 27(1A):
89-94.
NI, Y.-C., HEFLICH, R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98nr
and TA98/1,8-DNP6. Mutat. Res., 192(1): 15-22.
OHTA, T., NAKAMURA, N., MORIYA, M., SHIRASU, Y. & KADA, T. (1984).
The SOS-function-inducing activity of chemical mutagens in
Escherichia coli. Mutat. Res., 131: 101-109.
PAIK, S.G. (1985). Micronucleus induction in mouse bone marrow cells
of some nitrofuran, 5-nitroimidazole and nitrothiazole derivatives
used as trichomonacides in Korea. Environ. Mutag. Carcinogens,
5(2): 61-72.
PALM, D., MAGNUS, U., GROBECKER, H. & JONSSON, J. (1967). Hemmung
der monoami-noxydase durch bakteriostatisch wirksame
nitrofuran-derivative. Naunyn-Schmiedebergs Arch. Pharmak., 256:
281-300.
PAUL, H. (1955). Oral chronic toxicity study on Nf-180 in dogs
(study) Unpublished report d.d. 16 June 1955 from Eaton
Laboratories, Norwich, New York. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
PETTINGER, W.A., SOYANGCO, F.G. & OATES, J.A. (1968). Inhibition of
monoamine oxidase in man by furazolidone. Clinical Pharmacol.
Therapeut., 9(4): 442-447.
PROBST, G.S., McMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K. &
NEAL, S.B. (1981). Chemically-induced unscheduled DNA synthesis in
primary rat hepatocytes cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3: 11-32.
QUEINNEC, G., BABILE, R., DARRE, R., BERLAND, H.M. & ESPINASSE, J.
(1975). Induction of abnormalities in chromosomes (of cattle and
swine) by furazolidone or chloramphicol. Veterinary Bul., 46: 330.
RAY, W.H. (1959). Preliminary metabolism study of position
C14-labelled furazolidone in the white rat. Unpublished report no.
59-3 d.d. 18-09070 from Hess & Clark, Ashland, Ohio. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. (1962). An investigation of the metabolites of
furazolidone in swine urine. Unpublished research report no. 62:3
d.d. 29 May 1962 from Hess & Clark, Ashland, Ohio. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. & HAYES, M. (1963). Evidence that the orange '415'
metabolite of furazolidone in swine is a nitrofuran. Unpublished
research report 63:2 d.d. 25 January 1963 from Hess & Clark,
Ashland, Ohio. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
SCHERES, H.M.E. (1991a). Evaluation of the mutagenic activity of
3-amino-oxazolidinon-2 in the Ames Salmonella/microsome test.
Unpublished report project nr. d.d.15 January 1991 from RCC Notox,
5231 DD's Hertogenbosch. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
SCHERES, H.M.E. (1991b). Evaluation of the ability of furazolidone
to induce chromosome aberrations in cultured peripheral human
lymphocytes. Unpublished report project no. 038385 d.d. 9 January
1991 from Notox RCC, 5231 DD's Hertogenbosch. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
13 October 1966 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
4 January 1976 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
STERN, I.J. HOOLIFIELD, R.D., WILK, S. & BUZARD, J.A. (1967). The
anti-monoamine oxidase effects of furazolidone. J. Pharmacol. Exp.
Therapeut., 156(3): 492-499.
TATSUMI, K. & TAKAHASHI, Y. (1982). Metabolic fate of furazolidone
in rats. Chem. Pharm. Bull. Tokyo, 30(9), 3435-3438.
TATSUMI, K. YAMADA, H., YOSHIMURA, H. & KAWAZOE, Y (1981).
Metabolism of furazolidone by milk xanthine oxidase and rat liver
9000 g supernatant: Formation of a unique nitrofuran metabolite and
an aminofuran derivative. Arch. Biochem. Biophysics, 208(1):
167-174.
TATSUMI, K., NAKABEPPU, H., TAKAHASHI, Y., & KITAMURA, S. (1984).
Metabolism in vivo of furazolidone: evidence for formation of an
open-chain carboxylic acid and aketoglutaric acid from the
nitrofuran in rats. Arch. Biochem. Biophysics, 234(1): 112-116.
TENNENT, D.M. & RAY, W.H. (1971). Metabolism of furazolidone in
swine (355994). Proc. Soc. Exp. Biol. Med., 138(3): 808-810.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan. J. Genetics, 48(4): 291-294.
Van Den DUNGEN, H.M., JAGER, L.P., LUYCKX, N.B. L., De GRAAF, G.J. &
BAARS, A.J. (1991). Adrenal toxicity of carbadox and furazolidone
in vitro. Act. Vet. Scand., 87: 338-340.
VOOGD, C.E., KRAMERS, P.G.N., KNAAP, A.G.A.C., OUD, J.H. & Van WENT,
G.F. (1982). Mutagenicity of feed additives. Postersession RIV,
Bilthoven. as cited in Van MIERT, A.S.J.P.A.M., VROOMEN, L.H.M. &
JAGER, L.P. (1984) Farmacologie en toxicologie van furazolidon en
carbadox: een literatuuronderzoek. Tijdschr. Diergeneeskd.
109(22): 928-932.
VROOMEN, L.H., BERGHMANS, M.C., Van LEEUWEN, P., Van Der STRUIJS,
T.D.B.M, De VRIES, P.H.U. & KUIPER, H.A. (1986). Kinetics of
14C-furazolidone in piglets upon oral administration during 10
days and its interaction with tissue macromolecules. Food Add.
Contamin., 3: 331-346.
VROOMEN, L.H. (1987). Quantitative studies of the metabolism of
furazolidone by rat liver microsomes. Toxicol. in vitro, 1(2):
97-104.
VROOMEN, L.H., BERGHMANS, M.C., HEKMAN, P., HOOGENBOOM, L.A.P. &
KUIPER, H.A. (1987a). The elimination of furazolidone and its
open-chain cyano-derivative from adult swine. Xenobiotica, 17:
1427-1435.
VROOMEN, L.H., GROTEN, J.P., Van MUISWINKEL, K., Van VELDHUIZEN,
P.J. & Van BLADEREN, P.J. (1987b). Identification of a reactive
intermediate of furazolidone formed by swine liver microsomes.
Chem. Biol. Interact., 64(1-2): 167-180.
VROOMEN,L.H., BERGHMANS, M.C., GROTEN, J.P., KOEMAN, J.H. & Van
BLADEREN, P.J. (1988). Reversible interaction of a reactive
intermediate derived from furazolidone with glutathione and protein.
Toxicol. Appl. Pharmacol., 95: 53-60.
VROOMEN, L.H., BERGHMANS, M.C., Van BLADEREN, P.J., GROTEN, J.P.,
WISSINK, C.J. & KUIPER, H.A. (1990). In vivo and in vitro
metabolic studies of furazolidone: A risk evaluation. Drug. Metab.
Rev., 22(6-8): 663-676.
WHITE, A.H. (1989). Absorption, distribution, metabolism and
excretion of furazolidone. Scand. J. Gastroenterol.,
24(suppl. 169): 4-10.
2.1.2.2 In vivo
[(5-Nitrofurfurylidene)hydrazino]acetic acid has been isolated
from urine of rats treated with 20 mg furazolidone/kg bw (Morrison,
1976).
A small part of the radioactivity in rat urine after treatment
with 14C-furazolidone was identified as
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone and
N-(5-acetamido-2-furfurylidene)-3-amino-2-oxazolidone (Tatsumi &
Takahashi, 1982).
Male Wistar rats were given orally 100 mg 14C-furazolidone
(formyl-labelled)/kg bw. Urine was collected and analyzed for
possible metabolites. N-(4-carboxy-2-oxobutylideneamino)-
2-oxazolidone, alpha-ketoglutaric acid,
3-(4-cyano-2-oxobutylidenamino)-2-oxazolidone and
N-(5-acetoamido-2-furfurylidene)-3-amino-2-oxazolidone were
identified (Tatsumi et al., 1984; White 1989).
Colostomized chickens received a single oral dose of 30 mg
furazolidone/kg bw. An average of 7.5% of the dose was excreted in
the urine in 12 h. Four urinary metabolites containing a furan ring,
of which only one was a nitrofuran, were detected. Only traces of
unchanged furazolidone were found (Craine & Ray, 1972).
The metabolites of aldehyde-labelled 14C-furazolidone in pig
urine were investigated. More than thirty radio-labelled metabolites
were found. Three primary metabolites of furazolidone were
identified as 5-nitro-2-furoic acid, an orange-coloured "415"
chromogen and a yellow "415" chromogen, with half-lives of 30, 22
and 19 minutes, respectively. Some metabolites are naturally
occurring chemicals and include a yellow urinary pigment, carbonates
and a number of ninhydrin positive materials which lose
radioactivity when treated with ninhydrin indicating that these
materials are amino acids or conjugates of amino acids (Ray, 1962).
The orange "415" metabolite contains an intact
5-nitro-2-furfural moiety and is the most abundant single
furazolidone-related metabolite found in pig urine (Ray & Hayes,
1963).
Analysis of urine from a pig treated with 14C-furazolidone,
also labelled with 15N in the 5-nitro position, showed 14
metabolites. Eleven of them degraded to two or three degradation
products, which could not be identified. The presence of 15N was
detected in one minor metabolite. The authors concluded that during
the metabolism of furazolidone the nitro group was removed
extensively from the molecule (Kouba, 1979).
The open-chain cyano-derivative of furazolidone,
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone was a minor
metabolite in plasma and tissues of swine with a plasma half-life of
4 h (Figure 1) (Vroomen et al., 1987a).
Four human subjects were given a single dose of 400 mg
furazolidone/day administered in a tablet or a capsule. Using an
HPLC method, 24 h urine samples collected after administration
contained 0.003% to 0.16% unchanged furazolidone. By using a
specific complexation method (detection limit 2 µg/ml) no
furazolidone was detected (White, 1989).
Furazolidone was given to 10 human adults as 2 daily doses of
200 mg each for 21 days. Plasma levels analysed by HPLC (detection
limit 0.002 µg/ml) ranged from trace quantities to 0.489 µg/ml
(White, 1989).
2.1.3 Special studies on bound residues
Muscle tissue was prepared from a piglet receiving
14C-furazolidone for 10 days and sacrificed 2 h after the last
administration. Groups of 2 female Wistar WU rats were fed diets
containing non-extracted muscle tissue (group 1), muscle tissue
obtained after 4 extractions with water (group 2) (no extractions
with organic solvents were carried out) or the water extracts
obtained from muscle tissue after four extraction steps with water
for 4 days (group 3). Amounts of radioactivity in groups 1, 2, and
3, were, respectively, 26, 28 and 38% in urine, 23, 38 and 24%, in
faeces, 5.0, 7.4 and 4.8% in tissues, and 4.2, 5.3 and 3.1% in the
remaining carcass. Total recovery was 62%, 81% and 71% for groups 1,
2 and 3, respectively. The amount of non-extractable fractions
obtained from rat liver and muscle tissues were, respectively, 34
and 27% for group 1, 29% and 39% for group 2 and 32% and 21% for
group 3 (Vroomen, et al., 1990).
From a metabolism study with pig hepatocytes it was shown that
at least 70% of the bound residues still contain the
3-amino-2-oxazolidone side chain of furazolidone releasable upon
mild acid treatment. At least 25% of the bound residues in vivo
were shown to contain a releasable side-chain (day 0 animal)
(Hoogenboom 1991b).
The bioavailability of total and non-extractable
14C-furazolidone-derived radioactivity found in swine tissues at 0
and 45-day withdrawal has been investigated in bile duct cannulated
male rats. The pigs had been treated with 300 mg 14C-furazolidone
(both methylene- and formyl-labelled)/kg feed for 14 days. Zero-day
withdrawal liver was extracted with methanol:water, methanol, ether
and ethylacetate. A total of 44% of the radioactivity was extracted.
Zero-day withdrawal muscle, 45-day withdrawal liver and 45-day
withdrawal muscle were prepared in a similar way with different
extraction methods but no methanol:water extraction was carried out,
in order to prevent solubilization of small protein molecules which
may contain bound radioactivity. From 0-day withdrawal muscle 22% of
the radioactivity was extracted. From 45-day withdrawal liver and
muscle 8.3% and 14% of the radioactivity was extracted,
respectively.
Pelleted freeze dried extracted and non-extracted 0 and 45 day
withdrawal liver and muscle were dosed for 24 h to groups of 4
Sprague-Dawley rats. The rats were pretreated with control liver or
muscle for 24 h. Bile, urine and faeces were collected for up to 72
h. After 72 h the rats were sacrificed and the liver and
gastrointestinal tract were analyzed. The excretion pattern for the
differently-treated rats was similar. Rats treated with 0 and 45 day
withdrawal liver and muscle containing total residue excreted 10-27%
in the urine and 1.5-2.7% in bile, while 7.4-16% was found in
tissues. Rats treated with non-extractable tissue excreted 17-21% in
urine and 1.1-3.0% in bile; 4.8-15% was found in tissues (Hawkins
et al., 1992).
2.1.4 Effects on enzymes and other biochemical parameters
The effect of oral administration of 500 mg furazolidone/kg bw
on the activity of monoamine oxidase (MAO) was studied in rats.
Twenty-four hours after treatment MAO activity in liver and brain
was inhibited by 95%. Enzyme inhibition was detectable in liver 6 h
and in brain 12 h after administration, while maximal inhibition was
observed between 24 and 48 h. MAO activity returned to control
values in liver and brain within 10 days and 2-3 weeks,
respectively. The levels of noradrenaline and serotonin in brain
increased by 60-70%, dopamine in brain by 20% and noradrenaline in
heart by 60% when measured 1 to 3 days after application. In
contrast, adrenomedullary catecholamines were reduced by 30% one and
3 days after administration. Repeated application of furazolidone
elicited a cumulative effect: 15 mg/kg bw on 10 consecutive days was
as effective as a single dose of 90-120 mg furazolidone/kg bw.
Following application of furazolidone (125-500 mg/kg bw) the
sympathomimetic actions of tyramine given intravenously and
intraduodenally were potentiated up to 100-fold. According to the
authors the inhibition of MAO is probably due to a metabolite of
furazolidone that contains a free hydrazine group (Palm et al.,
1967).
After single oral doses of 2-100 mg furazolidone/kg bw a
decrease in rat liver-mitochondrial MAO was observed in about 4 h.
Maximal inhibition occurred in 16-24 h. Enzyme activity returned to
normal after 21 days. Furazolidone in a saturated solution did not
affect rat liver MAO in vitro (Stern et al., 1967).
In chickens fed 400 mg furazolidone/kg feed for 10 days MAO
activity in the brain, heart and alimentary tract was inhibited. No
MAO inhibition in the liver was found. Treatment increased the
amount of 5-hydroxy-tryptamine (5-HT) in the brain and potentiated
the vasodepressor action of tyramine. The amounts of adrenaline,
noradrenaline and the vasodepressor action in the brain were
unaffected by treatment (Ali & Bartlet, 1982).
In ducklings administered 400 mg furazolidone/kg feed for 10
days or 200 mg/kg bw by crop tube MAO activity in the liver was
inhibited. No inhibition was found in other organs (Ali & Bartlet,
1982).
Significant diamine oxidase (DAO) inhibition was observed in
various tissues (plasma, duodenal mucosa, liver, heart and brain) of
rabbits given orally 50 mg furazolidone/kg bw for 5 consecutive
days. Recovery was complete in all organs 14 days after the
withdrawal of furazolidone (Ali, 1983).
MAO inhibition was measured in primary cultures of pig
hepatocytes incubated with furazolidone. A dose-related inhibition
was observed; the effect was completely reversible upon withdrawal
of furazolidone. The proposed but not proven metabolites
ß-hydroxye-thylhydrazine and 3-amino-2-oxazolidone incubated with
the same cells caused an irreversible inhibition of MAO activity
(Hoogenboom, 1991c).
Six hundred mg furazolidone/kg administered in the diet to male
Wistar rats for 7 days did not affect cytochrome P-450
concentrations, but increased absolute cytochrome b 5 levels.
The activity of NADPH-cytochrome c reductase and aminopyrine
N-demethylase was decreased, but that of aniline hydroxylase was
increased (Fukuhara & Takabatake, 1977).
Thirty mg furazolidone/kg bw administered orally to large male
white turkeys produced significant increases in hypothalamic
concentrations of noradrenaline, adrenaline and dopa. One week after
withdrawal the amine concentrations returned to normal (Ali et al.,
1988).
In an in vitro experiment 14C-furazolidone was covalently
bound to rat liver microsomal protein; the binding could be
inhibited by addition of gluthathione or mercaptoethanol.
Furazolidone did not interact with added calf thymus DNA in the
presence of microsomes (Vroomen, 1987).
In an in vitro study using swine liver microsomes it was
shown that the mercaptoethanol conjugate (M1) and the glutathione
conjugate (G1) can bind covalently to microsomal protein.
According to the author this reaction is reversible (Vroomen et
al., 1988). In the case of pig hepatoccytes, a decrease in
GSH-levels did not result in an increased formation of bound
residues. Furthermore, no proof was obtained for the reversibility
of bound residues upon incubation with MSH (Hoogenboom, Personal
communication, 1992).
Furazolidone binds to DNA and possibly renders steric hindrance
to DNA replication, which leads to the inhibition of biosynthesis of
DNA in Vibrio cholerae cells (Chatterjee et al., 1975).
DNA-binding of 14C-furazolidone in piglet tissue varied from
87-382 pmol-equivalents of furazolidone per mg DNA (Vroomen et al.,
1986).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies on furazolidone are
summarized in Table 1.
Table 1. Acute toxicity of furazolidone
Species Sex Route Purity LD50 Reference
(mg/kg bw)
Mouse M&F oral 100% 1110 Mitchell et al., 1990b
Rat M&F oral 100% 1508 Mitchell et al., 1990a
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Male Wistar rats (6/group) were fed diets containing 0, 10, 100
or 200 mg furazolidone (purity not given)/kg in the diet for 13
months (equivalent to 0, 0.5, 5 or 10 mg/kg bw/day). Mortality rates
were 1/6, 2/6, 1/6 and 2/6 for the control, low-, mid- and high-dose
groups, respectively. No effects were observed on food consumption
and body weight. No changes were observed in the number of
erythrocytes and the number of leucocytes in blood samples of 2
rats/group taken at the end of the experiment. A slight increase in
relative liver and spleen weights were observed at the highest dose.
At histopathology a slight hyperthrophy of the liver cells was
observed at all dose levels. No effects were observed on kidney,
spleen, heart and testis (Aiso et al., 1962).
The effects of feeding various nitrofurans was studied.
Thirty-five female Holtzman weanling rats were administered 1000 mg
furazolidone (purity not given)/kg feed for 45 weeks (equivalent to
50 mg/kg bw/day). A matched control group was used. The rats were
maintained on a control diet for an additional 8 weeks. Observations
included clinical signs, feed consumption, feed efficiency and body
weight. Ten rats with palpable tumours were necropsied at week 42
and the remaining rats at termination of the experiment. At
termination mortality was significantly increased as compared to the
control group. Feed consumption, feed efficiency and body-weight
gain were significantly decreased in treated rats. Treated rats had
significantly more palpable mammary tumours from week 35 onwards
than the control rats. This was a poorly-reported study and no
detailed histopathology is available (Siedler & Searfoss, 1966).
In another experiment group of rats (Carworth, 20/sex/group)
received diets with various nitrofurans for 45 weeks followed by a
7-week recovery period. An untreated control group was used.
Furazolidone was fed at a rate of 1000 mg/kg feed (equivalent to 50
mg/kg bw/day). Observations included body weight, feed consumption,
feed efficiency and pathology. After five weeks on treatment and at
termination both male and female rats showed a significant decrease
in body-weight gain, feed consumption and feed efficiency. A
significantly increased incidence of palpable mammary tumours was
observed in female rats. At microscopy most tumours were found to be
adenomas, fibromas, and fibroadenomas with or without cyst
formation. Two of the 72 tumours observed were adenocarcinomas. This
was a poorly-reported study (Siedler & Searfoss, 1967).
2.2.2.2 Dogs
In a very limited study groups of 2 male and 2 female beagle
dogs were given micronized furazolidone by gavage at doses of 5, 11
or 23 mg/kg bw/day for 90 days. Another group of 1 male and 1 female
beagle dogs received 23 mg/kg bw/day furazolidone (as crystals) in
gelatin capsules for 88 days. Some neurological signs and testicular
degeneration in the 11 and 23 mg/kg bw/day dose groups were seen.
Most dogs suffered from pneumonia (Borgmann et al., 1964).
Groups of beagle dogs were orally administered furazolidone
(purity not given) in gelatin capsules. Four males and 4 females
received 7.5 mg/kg bw/day for 6 months. Two males were given 25
mg/kg bw/day for 6 months and 6 male and 4 female dogs received this
dose for periods varying from 28-118 days during 6 months of the
study. The highest-dose group consisted of 3 males and 2 females
given 50 mg/kg bw/day for periods varying from 16-37 days during 6
months of the study. The number of dogs used for controls were 12
(for 126 days) and 10 (for 6 months). No effects were observed on
clinical signs, haematology, blood biochemistry, urinalysis, organ
weight and macroscopy. Body weight was decreased in the mid- and
high-dose groups. In all treated dogs neurological symptoms and
histopathological changes in the region of the basal ganglia,
decreased sperm and tubular testicular degeneration were reported.
The authors concluded that the neurological effects as well as the
testicular changes were reversible. This was a poorly-reported study
that was not performed according to current standards (Paul, 1955).
Three groups of 2 male and 2 female beagle dogs were fed diets
containing 0, 30 or 100 mg furazolidone/kg for 2 years (equivalent
to 0.8 or 2.5 mg/kg bw/day). No effects were observed on body
weight, haematology, blood biochemistry, urinalysis, organ weight
and histopathology. Only an abstract of this limited study was
available (Huffman, 1965b).
Groups of beagle dogs (ARC, 4/sex/group) were orally
administered 0, 1, 5 or 15 mg/kg bw/day furazolidone (purity not
given) for 2 years. Additionally, 2 male dogs per group were
included to assess effects on semen quality. Two groups of 5 male
and 5 female beagle dogs, raised at different kennels (ARC + HRA)
were included to further assess cataractogenic properties of
furazolidone at the 15 mg/kg bw/day dose level. Observations
included clinical signs, body weight, ophthalmoscopy, neurological
examinations, recording of ECGs, haematology, clinical chemistry and
urinalysis, macroscopy and histopathology.
Because neurological symptoms were observed in 5/8 high-dose
dogs and 2 high-dose dogs from the semen evaluation study, these
dogs were discontinued from the study. Cataracts were observed in
3/5 male and 5/5 female ARC dogs and in 2/5 male and 1/5 female HRA
dogs. Decreased sperm motility and abnormal sperm were observed in
the mid- and high-dose groups. Increased relative kidney weight was
observed in high-dose females and decreased testes weight in mid-
and high-dose males. Only abstract was available (King et al.,
1971a).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of Swiss MBR/ICR mice (50/sex/group) were fed diets
containing 0, 75, 150 or 300 mg/kg furazolidone (purity not given)
for 13 months (equal to average daily doses of 12, 24 or
47 mg/kg bw). All mice were maintained on normal diet for 10
additional months. During the treatment period as well as during the
post-treatment period, no substance-related effects were observed on
feed consumption and body weight. Survival was decreased in mid- and
high-dose females at the end of the treatment period and in
high-dose males and mid- and high-dose females at 23 months. At
histopathology the incidence of bronchial adenocarcinomas was
significanly increased in the mid- and high-dose groups in both
sexes (incidences for males: 13/49, 19/48, 26/50 and 37/50 and for
females: 15/50, 18/50, 20/47 and 30/48 at the control, low-, mid-
and high-doses, respectively). The incidence of lymphosarcomas was
significantly increased in mid- and high-dose males (incidences:
1/49, 7/48, 10/50 and 10/50 for the control, low-, mid- and
high-doses, respectively) (Halliday et al., 1974b).
2.2.3.2 Rats
Groups of Sprague-Dawley rats (35/sex/group) were fed diets
containing furazolidone for 2 years. The actual average consumption
over 2 years was 0, 0.7, 4 or 10 mg/kg bw/day for males and 0, 0.8,
4.3 or 14 mg/kg bw/day for females. Additional groups (5/sex) were
maintained and killed after 1 year. No effects were observed on
clinical signs, body weight, feed consumption, blood biochemistry,
urinalysis and sternal bone marrow sections. Survival tended to
decrease with dose in females (26, 22, 23 and 15 surviving after two
years for control, low-, mid- and high-dose groups, respectively).
At termination mid- and high-dose female rats showed dose-related
decreased erythrocyte, haemoglobin and haematocrit counts and an
increased neutrophil count. Females from the highest-dose group
showed an increased neutrophil/lymphocyte ratio. Relative liver
weight was increased in high-dose males. At histopathology male rats
from the highest dose group showed an increase in parathyroid
hyperplasia without 'renal ricketts'. In male and female rats an
increase in adrenal cortical hyperplasia was observed at the highest
dose. High-dose females showed an increase in thyroid atrophy.
Females exhibited increased mammary tumour incidences as shown in
Table 2. The authors concluded that the mean onset time of mammary
neoplasms was approximately 2 months earlier in the mid- and
high-dose females than in the other groups (King et al., 1972a;
Halliday et al., 1973a).
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 250, 500 or 1000 mg/kg furazolidone (purity not given)
for 20 months (equivalent to 12.5, 25 or 50 mg/kg bw/day). The
surviving rats were maintained on control diets for at least 4
months or until 90% of the rats had died. Observations included
clinical signs, mortality, body weight and feed consumption.
Haematological and clinical chemistry examinations were performed
after 1 year and at the end of the treatment period. Extensive
histopathological examinations were performed on all moribund and
sacrificed rats. At the mid- (males only) and high-doses the
mortality rate was increased; 90% mortality in the highest dose was
observed after 24 months. At the end of the treatment period
body-weight gain was significantly decreased at 500 and 1000 mg/kg
Table 2. Mammary tumour incidences in female Sprague-Dawley rats
control low mid high
No. of animals examined 34 35 33 35
Multiple mammary tumours 3 6 11 19
Malignant mammary tumours 1 3 3 5
Mammary fibroadenomas 0 6 2 10
Mammary adenocarcinomas 1 2 2 3
Mammary carcinosarcomas 0 1 2 1
feed. At 1000 mg/kg feed haemoglobin, haematocrit (also at 500 mg/kg
feed) and the number of erythrocytes (also in high-dose females)
were significantly decreased in male rats. An increase in 'non-renal
ricketts' was observed in high- dose male rats. The incidence of
testicular atrophy was increased in mid- and high-dose males and the
incidence of adrenal cortical hyperplasia, lipolysis, congestion and
haemorrhage was increased in high-dose males only. Tumour incidences
are given in Table 3. In high-dose females a significant increase in
the incidence of mammary gland adenocarcinomas was observed. In
addition, an increased incidence of sebaceous gland adenomas and
thyroid adenomas was observed in both sexes at the mid- and
high-dose and of basal-cell epithelioma and carcinomas in males of
the high-dose group. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1974a).
Sprague-Dawley rats (50/sex) were fed diets containing 0, 250,
500 or 1000 mg furazolidone/kg for 20 months (equivalent to 12.5, 25
or 50 mg/kg bw/day). The study was carried out following the same
protocol as described above for Fischer 344 rats. Mortality for
control, low-, mid- and high-dose groups was 4/50, 11/50, 17/50 and
30/50 for males and 9/50, 12/50, 8/50 and 29/50 for females,
respectively. High-dose animals were sacrificed at day 666, compared
to day 895 for controls. Body-weight gain was significantly
decreased in mid- and high-dose males and high-dose females. At the
end of the treatment period the number of erythrocytes was decreased
in females at 500 and 1000 mg/kg feed. High-dose males showed an
increased neutrophil/lymphocyte ratio and a decreased lymphocyte
count. Histopathology showed an increased incidence of hepatic
necrosis in all treated rats, especially high-dose females.
Testicular atrophy was seen in mid- and high-dose males. A
dose-related increase in adrenal cortical hyperplasia was observed
in females at all dose levels. Tumour incidences are given in Table
4. In the high-dose group, significantly increased incidences were
reported for mammary adenocarcinomas in females and for neural
astrocytomas in males. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1973b).
Table 3. Tumour incidences in Fischer 344 rats
males females
contr. low mid high contr. low mid high
No. of animals examined 49 50 50 49 49 50 50 50
Dermal fibromas 2 6 1 10 0 3 7 3
Sebaceous adenomas 0 0 8 11 1 2 6 10
Sebaceous adenocarcinomas 0 1 1 1 0 1 0 1
Basal cell epitheliomas 0 2 4 8 0 0 0 0
Basal cell carcinomas 0 0 0 2 0 0 0 0
Mammary neoplasms 1 1 2 0 11 29 40 30
Mammary adenocarcinomas 0 0 0 0 0 0 0 6
Lymphoreticular neoplasms 11 1 6 2 11 7 7 6
Pituitary adenomas 4 2 4 1 24 13 23 3
Thyroid adenomas 1 2 12 19 0 1 12 7
Testicular interstitial 42 44 31 0
cell tumours
Table 4. Tumour incidences in Sprague-Dawley rats
males females
contr. low mid high contr. low mid high
No. of animals examined 50 49 50 49 49 50 50 50
Dermal fibroma 2 6 5 7 3 1 2 7
Sebaceous adenoma 1 0 0 6 0 0 0 0
Sebaceous adenocarcinomas 0 0 0 1 0 0 0 0
Mammary neoplasm 2 4 5 1 29 41 45 40
Mammary adenocarcinoma 0 0 1 0 1 0 3 8
Mammary carcinosarcoma 0 0 0 0 0 1 0 0
Lymphoreticular neoplasms 7 6 5 7 6 1 4 0
Pituitary adenomas 10 9 6 3 29 17 17 1
Thyroid adenomas 0 1 1 5 0 0 1 1
Neural astrocytomas 0 0 2 5 1 0 1 0
2.2.4 Reproduction studies
2.2.4.1 Rats
A special study was performed to evaluate the effects on the
reproductive system of male rats fed furazolidone in the diet. Five
male rats (strain not specified)/group received diets containing 330
or 660 mg furazolidone/kg for 14 weeks, 3 male rats/group were given
330 or 660 for 12 weeks (equivalent to 16 or 33 mg/kg bw/day) with a
recovery period of 2 weeks. The control group consisted of 4 rats.
Observations included testes and epididymis weight and macroscopy
and histopathology of the reproductive tract. No dose-related
effects were observed in rats from the low-dose group. Testes weight
was markedly decreased in high-dose rats. This effect was more
pronounced in rats fed furazolidone for 14 weeks than in rats dosed
12 weeks with 2 weeks recovery. Histopathology of the testes of
high-dose rats revealed oedema of the interstitium, and atrophy and
degeneration of the sperm-producing tubules. Occasionally stasis of
previously produced sperm in the epididymus was seen and some
epididymi were devoid of sperm. The effects were still present after
the recovery period (Larson, 1963a).
Because there was some evidence that the testicular damage
observed after feeding of 660 mg furazolidone/kg in the diet was not
completely reversible gross and microscopic examinations were
performed on male rats 5 days and 6 weeks after withdrawal from a
diet containing 660 mg furazolidone/kg. Another 2 males were
retained for 14 weeks, the additional 8 weeks being required to
allow non-treated females to reach breeding age. Each treated male
(and one control male) cohabited with 2 females each. Testes weight
was decreased in 4/6 rats and in 3/6 rats atrophic seminiferous
tubules and abundant intertubular transudate were seen. In 2 of
these male rats the epididimi had sperm stasis. All prostates were
normal. Rats used in the breeding trials showed normal libido and
fecundated females but the litters that were produced had slightly
fewer young than those from the litters sired by the control male
(Larson 1963b).
In a three-generation reproduction study groups of rats were
fed diets containing 0, 30 or 100 mg furazolidone/kg. The F0
generation was maintained on the furazolidone diet and sacrificed
after 2 years. No effects were found on haematology, body weight and
histopathology after 2 years. Decrease in testes weight was
dose-related. Reproductive performance was not affected. Only a
summary of this study was available (Huffman, 1965a).
In a three-generation reproduction study groups of
Sprague-Dawley rats (20/sex) were used. Female rats only were
administered 500 mg furazolidone/kg feed. Because of growth
depression the dose level was lowered to 400 mg/kg on day 16 and to
250 mg/kg on day 37. Three matings per generation were performed.
The pups from the first litter were sacrificed 21 days after birth.
Pups from the second litter were used for the second generation and
pups from the third litter were used for teratological examination.
No treatment-related effects were found on reproduction parameters
resulting in a NOEL equivalent to 12.5 mg/kg bw/day (Borgmann &
Prytherch, 1964b; Borgman & Prytherch, 1966).
2.2.4.2 Chickens
Groups of New Hampshire chickens were fed diets containing 55,
110 or 220 mg furazolidone/kg feed for 4 to 16 weeks. No effects
were observed on body weight and egg production, hatchability of
eggs and shell quality. Thyroid and adrenal weight were both reduced
(Francis & Shaffner, 1956).
2.2.4.3 Pigs
In a limited study a group of purebred Hampshire and Duroc pigs
(9 sows and 12 gilts) were fed a diet containing 300 mg/kg
furazolidone for two weeks at breeding and 150 mg/kg for three weeks
at farrowing. Another group was maintained as an untreated control
group. There was no difference in the number of pigs born, number of
pigs weaned or weight gain of the pigs. However, pup weight at
weaning was slightly higher in the treated group (Hughes & McMinn,
1963).
2.2.4.4. Goats
Male Nubian goats received furazolidone suspended in distilled
water at a dose of 10 or 40 mg/kg bw. The effect on semen morphology
and biochemistry was studied. At both doses ejaculate volume, the
number of motile spermatozoa per ejaculate and the number of live
spermatozoa per ejaculate and semen fructose concentration were
significantly decreased (Mustafa et al., 1987).
2.2.5 Special studies on embryotoxicity and/or teratogenicity
2.2.5.1 Mice
Groups of Albino C strain mice were administered furazolidone
at doses up to 2 g/kg during pregnancy varying from day 1-11.
Abortions or fetal deaths occurred in all mice treated with doses
> 1 g/kg when treatment started before day 8 of pregnancy.
However when treatment started at day 10 abortions or fetal death
occurred only in 2/9 mice. Litterweight was dose-relatedly decreased
but no congenital abnormalities were observed (Jackson & Robson,
1957).
2.2.5.2 Rats
Groups of 3 Donryu male rats were fed 0 or 100 mg
furazolidone/kg bw for 7 days, and on day 8 the rats were
sacrificed. Relative testes weights were slightly decreased. The
number of mature spermatozoa was decreased and degenerative changes
in the seminiferous tubules (sloughing of spermatocytes and
multi-nucleated cells) were observed (Miyaji et al., 1964).
2.2.5.3 Rabbits
Groups of two rabbits were given an intra-amniotic injection of
1, 2 or 2.5 mg furazolidone on day 14 or 15 of pregnancy. A
laparatomy was performed 5 days after the injection to determine the
effect on pregnancy. Pregnancy interruption was observed at 2.0 and
2.5 mg furazolidone (Jackson & Robson, 1957).
Groups of 10 pregnant New Zeeland white rabbits were orally
administered 30 mg furazolidone (purity not given)/kg bw/day on days
7-15 of pregnancy. On day 29 of pregnancy the dams were sacrificed
and the fetuses were delivered by caesarean section. Feed
consumption and body-weight gain were significantly decreased. No
embryotoxicity or teratogenicity was observed (Borgmann & Prytherch,
1964a).
2.2.5.4 Chickens
Fifty percent mortality was observed in 10-day old chicken
embryos after treatment with 15 mg furazolidone in the inner shell
membrane, 4.2 mg in the allantoic cavity or after treatment with 0.7
mg in the yolk sac (Gentry, 1957).
2.2.5.2 Special studies on genotoxicity
The results of in vitro and in vivo genotoxicity studies
and of genotoxicity studies on metabolites of furazolidone and bound
residues are summarized in Tables 5, 6, and 7, respectively.
Table 5. Results of in vitro genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Ames testa S. typhimurium 0.01-1.0 µg/pl ? positiveb Jagannath et al.,
TA1538, TA98 (1.0 µg/pl slightly 1981g
and TA100 toxic)
TA1535, TA1537 negativeb
Ames testa S. typhimurium* 0.1, 0.5, 1.0 >99% positive Ni, et al., 1987
TA98, TA98NR or 2.5 µg/pl
TA98/1,8-DNP6 2.5 µg/pl toxic
Ames testa S. typhimurium 0.01-0.3 µg/pl 99% positiveb Crebelli et al.,
TA100 >0.3 µg/pl toxic 1982; Carere et
al., 1982
Spot test Escherichia coli 50 µg/plc positive McCalla &
WP2uvrA Voutsinos, 1974
Reverse Euglenia gracilis 25, 50 and 100 ? positive Ebringer et al.,
mutation assay µg/mlc 1976
Forward Vibrio cholerae up to 12 µg/mlc ? positive Chatterjee et al.,
mutation assay OGAWA 154 1983
Prophage Escherichia coli ? ? positive Chatterjee et al.,
induction assay K12 GY5027 1983
Gene conversion S. cerevisiae D4 no details ? positive Voogd et al.,
assay 1982 as cited in:
VanMiert et al.,
1984
Gene mutation Chinese hamster up to 125 µg/ml >99% positive Gao et al., 1989
assaya ovary cells >100 µg toxic
Table 5 cont.
Test system Test object Concentration Purity Results Reference
HGPRT test Mouse L5178Y no details ? positive Voogd et al.,
lymphoma cells 1982
SOS function and Escherichia coli 0.8 and 8.0 µMc ? positive Bryant &
mutation assay WP2 McCalla, 1980
SOS function Escherichia coli 0.1, 0.2, 0.5 ? positive Ohta et al., 1984
assay K12 µg/mlc
Sex-linked D. melanogaster 0.5 mM in DMSOc ? positive Blijleven et al.,
recessive 1977
lethal test
Sex-linked D. melanogaster 0.18, 0.44 or ? positive Kramers, 1982
recessive 0.5 mM in DMSOc
lethal test
Chromosome human 10, 33 and 100 99.8% negative Scheres, 1991b
aberration assay lymphocytes µg/ml in DMSOd
33, 100, 180 positivef
and 333 µg/ml in
DMSOe (333 µg/ml
slightly toxic)
Chromosome human 0.2, 2.0 or ? positive Cohen & Sagi,
aberration assay lymphocytes 20.0 µg/mld 1979
20.0 µg/ml toxic
Chromosome human 0.5-100 µMc ? negative Tonomura &
aberration assay lymphocytes Sasaki, 1973
Table 5 cont.
Test system Test object Concentration Purity Results Reference
Chromosome bovine lymphocytes 0.05-500 mg/lg ? positive Queinnec et al.,
aberration assay porcine lymphocytes 0.05-500 mg/lg positive 1975; Babile et
al., 1978
SCE test human 0.2, 2.0 and ? positive Cohen & Sagi
lymphocytes 20 mg/l in DMSO 1979
20 mg/l toxic
Rec assay Escherichia coli ?? ? positive Chatterjee et al.,
K12 rec- and rec+ 1983
DNA repair Escherichia coli 1.0 to 10 µM ? positive Lu et al., 1979
assay WP2 uvrA >5µM toxic
UDS assay human 5-100 µMc ? negative Tonomura &
lymphocytes Sasaki, 1973
UDS assay Fischer 344 0.5-1000 nM/ml ? positive Probst et al.,
rat hepatocytes in DMSOb,c 1981
* nitroreductase-deficient Salmonella typhimurium tester strains.
a both with and without rat liver S9 fraction.
b positive controls yielded positive results.
c no data about toxicity.
d without metabolic activation.
e with metabolic activation.
f the authors concluded that the results were negative.
g no toxicity observed.
Table 6: Results of in vivo genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Micronucleus Swiss CD-1 mice 300 mg/kg bw 99.8% negative Enninga &
test ip in Weterings 1990
methyl-cellulose
(toxic)
Micronucleus Swiss Webster 100 and 500 mg/kg ? equivocalb Paik, 1985
test mice bw orala
a no toxicity observed
b slight induction, not dose-related
2.2.7 Special studies on endocrine toxicity
In a special study various groups of ovariectomized
Sprague-Dawley, Fischer 344 or Long Evans rats and golden hamsters
received single oral doses varying from 50-500 mg furazolidone/kg
bw. After a 2-week recovery period positive dose-related lordosis
(becoming sexually perceptive) behaviour was observed only in
Sprague-Dawley rats which is, according to the authors, indicative
of an elevated concentration of progesterone in the circulation
(King et al., 1970a).
Groups of immature superovulated Sprague-Dawley rats were
orally dosed with 100 or 500 mg furazolidone/kg bw. In all groups
ovulation was initiated about 24 h earlier than the control group.
The same shift in ovulation time was observed in a positive control
group treated with progesterone (King et al., 1970a).
Feeding of 1000 mg furazolidone/kg feed for 30, 60 or 90 days
produced a marked inhibition of the conversion of progesterone into
corticosterone (11-hydroxylation) in adrenals of Sprague-Dawley
females. A considerably lesser effect was found in Fischer 344
females. This effect could be overcome by addition of NADPH,
indicating an inhibitory effect of furazolidone on the NADPH
generating system (King et al., 1971b).
Table 7. Results of genotoxicity assays on metabolites of furazolidone and
bound residues
Test system Test object Concentration Substance Results Reference
In vitro
Ames testa S. typhimurium 100-5000 µg/plb,c Md negative Scheres, 1991a
TA100, TA98
Ames testa S. typhimurium urine from rats - positivee Crebelli et al.,
TA100 treated with 10, 1982; Carere et
50, 100 mg/kg bw al., 1982
Ames testa S. typhimurium up to 5 µg/pl M1f negativee,g Jagannath &
TA1535,TA1537 Brusick, 1981a; 1981e
Craine, 1981
TA1538, TA98, M2h negativee Jagannath &
TA100 Brusick, 1981b; 1981f
Craine, 1981
M3i negativee Jagannath &
Brusick 1981c; 1981d
Craine, 1981
Ames test a S. typhimurium 0.1 mg/pl M4j negative Vroomen et al.,
TA100, TA98 in DMSO 1987a
Ames test S. typhimurium upto 5 µg/pl GSFk negative Hoogenboom,
TA100 in DMSO MSFl negative 1991a
Reverse Mycobacterium 1000 µg/ml 5-NFAm positive Ebringer et al.,
mutation assay phlei 1976
Euglena gracilis 2.5 and 7.5 5-NFAm positive Ebringer et al.,
µg/ml 1976
DNA repair test rat hepatocytes 10-3M, 10-5Mn Mo negative Mori et al.,
mouse hepatocytes 1988
Table 7 (continued)
a both with and without rat liver S9 fraction
b positive controls yielded positive results
c no toxicity observed
d 3-amino-oxazolidinon-2
e negative control yielded negative results
f whole liver powder from pigs treated with 5 mg furazolidone/kg bw for 5 days
g substances from untreated pigs yielded negative results
h urine isolates from pigs treated with 5 mg furazolidone/kg bw for 5 days
i soluble liver isolate from pigs treated with 5 mg furazolidone/kg bw for 5 days
j 3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone.
k glutathione conjugate of cyano metabolite
l mercaptoethanol conjugate of cyano metabolite
m 5-nitro-2-furaldehyde
n no data about toxicity
o 2-hydroxyethylhydrazine.
Oral administration of 100 or 500 mg furazolidone/kg bw to
mature female rats produced a significant decrease in serum
prolactin during the morning proestrus, but not at afternoon
proestrus. Administration of 500 mg/kg bw during the morning estrus
resulted in a slight decrease in serum prolactin levels (Morrison
et al., 1973).
In a limited experiment the effect of furazolidone treatment on
the tumourigenicity of estrone was studied. Furazolidone treatment
(1000 mg/kg feed to female Sprague-Dawley and Fischer 344 rats) plus
estrone resulted in the development of more mammary masses than with
estrone alone. However, the administration of estrone did not result
in an increased incidence of mammary masses (Morrison et al.,
1972).
Furazolidone (500 or 1000 mg/kg feed) was fed to
adrenalectomized and sham-operated male rats for 30 days, after
which the adrenals and tests were examined. Adrenalectomy did not
alter the testicular atrophy produced by furazolidone. At 1000 mg/kg
feed tubular degeneration in three out of six animals was seen
(Morrison et al., 1974a).
A diet containing 500, 750 or 1000 mg/furazolidone/kg
administered to female rats for 30 days resulted in a significant
increase in serum testosterone at the highest dose (Morrison et
al., 1974b).
In a pilot study rats were administered a single oral dose of
250 or 500 mg/kg bw furazolidone in 0.5% aqueous CMC. At 30 min, 1,
2, 4, 8 and 24 h after administration blood samples were taken for
RIA of prolactin and corticosterone. Furazolidone increased in a
dose-related manner, the corticosterone concentration up to 2 h
after administration, with a return to vehicle control values by 24
h. No effect on plasma prolactin time was observed (Meites, 1984).
Male mature chickens were fed a diet containing 400 or 800 mg
furazolidone/kg feed for 10 days. Testes weight was decreased in
both groups. Treatment with 800 mg/kg produced a significant
reduction in the concentration of testosterone in plasma and testes
and some reduction in ascorbic acid, protein and cholesterol
concentration in the testes. Bolus administration of doses of 40 or
80 mg/kg bw for five days caused the same effect. The size of the
testes, wattles and combs were significantly reduced. Monoamine
oxidase activity in the testes was significantly reduced by
furazolidone. In all the treated birds testicular concentrations of
5-hydroxytryptamine were significantly raised, except in those fed
400 mg furazolidone/kg for 10 days (Ali et al., 1984).
Groups of large white female turkeys were orally dosed with 0,
7.5, 15 or 30 mg furazolidone/kg bw for 7 days in gelatin capsules.
The number of eggs produced by each bird was recorded daily 2 weeks
before, during and 4 weeks after the end of treatment. The
concentrations of luteinizing hormone (LH) and prolactin (PRL) were
analyzed by RIA in plasma samples taken on day -7, 0, +7 and +14. At
the end of the treatment period a dose-related decrease was observed
in egg production and LH concentration (significantly in the mid-
and the high-dose group). No effects were observed on PRL
concentration. After withdrawal only the egg production started to
recover but was still below normal 4 weeks after cessation of
treatment (Ali et al., 1987a; 1988).
When turkeys given 15 or 30 mg furazolidone/kg bw were injected
intramuscularly with 5 µg/kg luteinizing hormone-releasing hormone
(LHRH), no effect on LHRH-induced LH release was observed (Ali et
al., 1987a; 1988).
Groups of male turkeys were orally treated with 0, 1, 2.5, 5 or
20 mg furazolidone/kg bw/day for 14 days. Plasma was analyzed for
LH, testosterone (T) and PRL before, during and after treatment. At
the highest dose the concentrations of LH and T were significantly
decreased, and a tendency towards a decrease was observed at 5 mg/kg
bw/day. At histopathology a decrease in spermatocyte production as
well as corrugation of sperm cell nuclear envelopes and distension
of endoplastic reticulum of elongated spermatides were observed in
the 20 mg/kg bw/day group (Ali et al., 1987b).
Furazolidone administered as a bolus dose (5-500 mg/kg bw) to
male T-line chickens produced a decrease in the amount of
corticosterone in plasma (Bartlet et al., 1990).
In another experiment male T-line and J-line chickens were
given a bolus dose of furazolidone (200 mg/kg bw) which resulted in
a decrease in aspartate transaminase activity in the adrenal gland
and a general disorganization in the structure of the adrenal
cortical cells, leading to atrophy of the adrenal cortical glands
(Bartlet & Khan, 1990).
A concentration of 1 µM furazolidone inhibited significantly
the release of aldosterone from porcine adrenal cells in vitro.
Almost complete inhibition was observed at 100 µM (van den Dungen
et al., 1991).
2.2.8 Special studies on skin and eye irritation
A dose of 500 mg furazolidone (purity 100%) moistened with 0.5
ml of 0.5% saline caused slight erythema in 4/6, 3/6 and 1/6 rabbits
after 0.5, 24, and 48 h respectively, when applied under
semi-occlusive conditions to the clipped intact skin of 3 male and 3
female New Zeeland white rabbits (Mitchell et al., 1990c).
Nine New Zeeland white rabbits received an installation of 30
mg furazolidone (purity 100%) into the conjunctival sac of the right
eye, and 3/9 eyes were washed 20 seconds after treatment. After 24 h
slight conjuctival irritation was observed in both washed and
unwashed eyes. Corneal ulcerations were observed in 2/6 unwashed
eyes only. All eyes were normal at day 7 (Mitchell et al., 1990d).
2.3 Observations in humans
Furazolidone administered to patients with essential
hypertension (no details provided) produced marked supersensitivity
to tyramine and amphetamine, inhibition of intestinal MAO and
increased urinary excretion of tryptamine. Continued administration
produced a cumulative inhibition of MAO (Pettinger, et al., 1968)
Adverse reactions (gastrointestinal 8%, neurological 1.34%,
systemic, 0.56% and dermatological reactions 0.54%) to furazolidone
treatment were observed in 864/10443 patients (8.3%) treated with a
therapeutic dose of < 5 to 7 mg/kg bw/day (Altamirano & Bondani,
1989).
A 43-year old man with a contact allergy to furazolidone was
patch tested to nitrofurans with completely negative results. The
man had a positive patch test reaction to 2% furazolidone both in
PEG-400 and alcohol. Control tests were negative in 25 dermatitis
patients (DeGroot & Conemans, 1990).
3. COMMENTS
The Committee considered data from pharmacodynamic,
pharmacokinetic, metabolism, acute and short-term toxicity,
carcinogenicity, genotoxicity, reproductive, and teratogenicity
studies as well as special studies on endocrine function and some
clinical studies in humans.
The distribution, excretion, and biotransformation of
radiolabelled furazolidone were studied in rats, chickens, pigs, and
humans. After oral administration, furazolidone was rapidly absorbed
and the radioactivity was widely distributed, the highest levels
being found in liver, kidney, fat, and muscle. It was rapidly
metabolized and excreted predominantly in urine. In chicken and
human urine, only trace amounts of unchanged furazolidone could be
detected, and of the large number of metabolites found only some
were identified. In rat and pig urine, the common metabolite
appeared to be the open chain cyanometabolite
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone. The Committee noted
that quantitative information on metabolites was lacking. In pigs, a
substantial portion of the metabolites was bound to macromolecules,
and it appeared that approximately 15-40% of this bound fraction was
bioavailable. However, the Committee questioned whether valid
extraction procedures had been used to isolate these bound
metabolites.
In acute oral toxicity studies in mice and rats furazolidone
was slightly toxic; the LD50 values were of the order of 1100 and
1500 mg/kg bw, respectively.
No NOEL could be established from short-term studies performed
with rats and dogs. Rats receiving furazolidone at doses in the
range 0.5-50 mg/kg bw/day showed hypertrophy of liver cells.
Palpable mammary tumours and a decrease in body weight gain were
observed at 50 mg/kg bw/day. In dogs, dose levels of 5-25 mg/kg
bw/day led to neurological symptoms and histological changes in the
basal ganglia, together with testicular degeneration. It was noted
that the available information was deficient by current standards
and poorly reported.
Two three-generation reproduction studies were performed in
rats. In one study rats were exposed to furazolidone at
concentrations up to 100 mg/kg in feed. In the other study only
female rats were treated with diets containing 500 mg/kg, but this
concentration was gradually reduced to 250 mg/kg in order to avoid
the observed growth depression. No effects on reproductive
performance were observed in either study. The NOEL was equivalent
to 12.5 mg/kg bw/day.
In a special study designed to evaluate the effects on the male
reproductive system, rats exposed to a dietary furazolidone
concentration equivalent to 33 mg/kg bw/day exhibited testicular
degeneration. At 16 mg/kg bw/day no effects were observed.
Neither embryotoxicity nor teratogenicity was observed in
rabbits after oral administration of furazolidone at a dose of 30
mg/kg bw/day.
A carcinogenicity study was conducted in Swiss MBR/ICR mice,
which received a diet containing concentrations of furazolidone
equal to average daily doses of 12, 24, or 47 mg/kg bw/day for 13
months, followed by a control diet for 10 months. In the mid- and
high-dose groups, a significant increase in the incidence of
bronchial adenocarcinomas was observed in both sexes, and the
incidence of lymphosarcomas was significantly increased in male
mice.
In two long-term toxicity/carcinogenicity studies, furazolidone
was administered in the diet to Fischer 344 and Sprague-Dawley rats
at concentrations equivalent to daily doses of 12.5, 25, or 50 mg/kg
bw/day for 20 months. In Fischer 344 rats, a significant increase in
the incidence of mammary gland adenocarcinomas was observed in
females in the high-dose group. In addition, an increase in the
incidence of sebaceous gland adenomas and thyroid adenomas was
observed in both sexes at 25 and 50 mg/kg bw/day and of basal cell
epithelioma and carcinoma in males of the high-dose group. In the
high-dose group of Sprague-Dawley rats, significantly increased
incidences were reported for mammary adenocarcinomas in females and
for neural astrocytomas in males. In both strains of rat, female
animals showed a significant increase in the incidence of mammary
neoplasms (benign and malignant combined) at all dose levels, but
without a dose-response relationship.
Furazolidone has been tested in a wide variety of genotoxicity
studies. Positive findings were recorded in bacterial assays with
and without metabolic activation, in the sex-linked recessive lethal
test in Drosophila melanogaster, in a gene mutation assay with
mammalian cells in vitro, in a sister chromatid exchange test, and
in two DNA-repair tests. Positive as well as negative results were
obtained in chromosome aberration assays with mammalian cells in
vitro, and in tests for unscheduled DNA synthesis. One mouse
micronucleus test was negative, while another gave equivocal
results.
The majority of in vitro genotoxicity tests with postulated
metabolites gave negative results, however, nitrofuraldehyde and
urine from furazolidone-treated rats gave positive results. It was
concluded that furazolidone was genotoxic in vitro.
Several studies were performed on the endocrine effects of
furazolidone. Furazolidone inhibited the conversion of progesterone
into corticosterone in adrenal cells both in vivo and in vitro.
It has been hypothesized that disturbances of steroidogenesis
constituted the underlying mechanism for the increased incidence of
tumours caused by furazolidone. The Committee noted that it was
unlikely that the such a mechanism could account for the increase in
neural astrocytomas and uncommon skin tumours in rats. With respect
to the occurrence of mammary tumours, no information was available
on the effect of furazolidone on plasma progesterone concentrations
and no consistent effects on plasma prolactin concentrations were
observed. The Committee therefore concluded that no support had been
provided for the hypothesized mechanism.
Furazolidone caused reversible inhibition of monoamine oxidase
(MAO) activity in pig hepatocytes in vitro and in liver and brain
tissue of rats following in vivo administration. Irreversible MAO
inhibition both in vitro and in vivo was observed for the
postulated metabolites amino-oxazolidone and hydroxyethyl-hydrazine.
4. EVALUATION
On the basis of the positive effects of furazolidone in
genotoxicity tests in vitro and the increased incidence of
malignant tumours in mice and rats, the Committee concluded that
furazolidone was a genotoxic carcinogen. Since the drug is rapidly
and extensively metabolized, the Committee also considered
information on metabolites of furazolidone. Although a large number
of postulated metabolites produced negative results in genotoxicity
tests, it was noted that only a few of these had been either
identified or quantified in rats and pigs. Furthermore, the
Committee concluded that insufficient data were available on the
nature and toxic potential of compounds released from the bound
residues.
Because of the genotoxic and carcinogenic nature of
furazolidone and the above-mentioned deficiencies with respect to
the data on the metabolites, the Committee was unable to establish
an ADI.
Before considering the compound again, the Committee would wish
to have detailed information on the nature, quantity and toxicity of
the metabolites of furazolidone, including the bound residues.
5. REFERENCES
AISO, K., KANISAWA, M., YAMAOKA, H., TATSUMI, K. & AIKAWA, N.
(1962). Systematic studies on the toxicity of nitrofuran derivatives
(3): Macroscopical and histological examination of rats fed on diet
with furazolidone and 5-(5-nitro-2-vinylfuran)-1, 3,
4-oxadiazoline-2-one for one year. Chiba Daigaku Fuhai Kenkyusho
Hokoku, 15: 21-33. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H. & BARTLET, A.L. (1982). Inhibition of monoamine oxidase in
chicken and ducklings by a microbial metabolite of furazolidone.
Quarterly J. Exp. Physiol., 67(1): 69-79.
ALI, B.H. (1983). The anti-diamine oxidase effect of furazolidone in
rabbits. Comp. Biochem. Physiol., 74C(1): 109-110.
ALI, B.H., HOMEIDA, A.M. & KNIFTON, A. (1984). The effect of
furazolidone on fertility of male chickens. Comp. Biochem.
Physiol., 78C(1): 43-47.
ALI, B.H., SILSBY, J.L. & EL HALAWANI, M.E. (1987a). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
British Poultry Science, 28: 613-621.
ALI, B.H., SILSBY, J.L., LOSETH, K.J., CRABO, B. & EL HALAWANI, M.E.
(1987b). Some effects of furazolidone on the testes and plasma
testosterone, luteinising hormone and prolactin concentrations in
mature male turkeys d.d. 17 June 1987. University of Minnesota. St.
Paul, MN 55108, USA. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL), levels in young turkeys. Gen. Pharmacol., 19(1):
91-95.
ALTAMIRANO, A. & BONDANI, A. (1989). Adverse reactions to
furazolidone and other drugs. Scand. J. Gastroenterol.,
24(suppl.169): 70-80.
BABILE, R., QUEINNEC, G., BERLAND, H.M. & DARRE, R. (1978).
Structure chromosomique des lymphocytes du porc et additifs
alimentaires. C. Soc. Biol., 172:, 546-553.
BARTLET, A.L & KHAN, F.H. (1990). Effects of nitrofurans on adrenal
cortical tissue in chickens. J. Vet. Pharmacol. Therap., 13:
206-216.
BARTLET, A.L., HARVEY, S. & KLANDORF, H. (1990). Contrasting effects
of nitrofurans on plasma corticosterone in chickens following
administration as a bolus or diet additive. J. Vet. Pharmacol.
Therap., 13:, 261-269.
BLIJLEVEN, W.G.H., KORTSELIUS, M.J.H. & KRAMERS, P.G.N. (1977).
Mutagenicity testing of H-193, AF-2 and furazolidone in Drosophila
melanogaster. Mutat. Res. 56: 95-100.
BORGMANN, A.R., MURTI, G.S., COOLEY, R. & LEVIN, R. (1964).
Ninety-day toxicity studies of experimental formulations containing
furoxone (R) (NF-180) in dogs. (Project no. 475.09.02) Unpublished
final report d.d. 29 June 1964, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964a). The effect of furoxone (R)
on fetal growth and development in the rabbit. Unpublished special
report project number no. 458.9 d.d. 28 August 1964 from Pathology
Section, Scientific Department Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964b). The effect of furoxone on
reproduction in rats. Unpublished special report project number no.
460.9 d.d. 3 September 1964 from Pathology Section, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1966). The effect of furoxone on
reproduction in rats. Second and third generation. Unpublished
special report project number no. 460.09 d.d. March, 10, 1966 from
Pathology Section, Scientific Department, The Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BOWMAN, J.S. (1961a). A balance study of furazolidone C14
(methylene and aldehyde-labeled) in male rats. Unpublished report
H-NSC
no. 20-0040-32 from Hazleton, Nuclear Science Corporation,
California. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
BOWMAN, J.S. (1961b). A plateau-elimination study of
furazolidone-C14 (methylene labeled) in male rats. Unpublished
report H-NSC no. 20-0039-32 from Hazleton, Nuclear Science
Corporation, California. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran induced mutagenesis
and error prone repair in Escherichia coli. Chem. Biol. Interact.,
31: 151-166.
BUZARD, J.A., HEOTIS, J.P. & WILLIAMS, C.W. (1960). Chick
distribution studies with C14-(formyl) NF-180. Unpublished interim
report problem 360.3 d.d. 9 December 1960 .Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
BUZARD, J.A., HEOTIS, J.P. & Williams, C.W. (1961). Chick
distribution studies with C14-(methylene) NF-180. Unpublished
interim report problem 360.3 d.d. 15 May 1961. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
CARERE, A., CONTI, L., CREBELLI, R. & MACRI, A. (1982). Quantitative
data on the urinary recovery of mutagenicity in furazolidone-treated
rats. Mutat. Res., 97(6), 461-462.
CHATTERJEE, S.N., MAITI, M. & GHOSH, S. (1975). Interaction of
furazolidone with DNA. Biochem. et Biophysica Acta, 402: 161-165.
CHATTERJEE, S.N., BANERJEE, S.K., PAL, A.K. & BASAK. J. (1983). DNA
damage, prophage induction and mutation by furazolidone. Chem.
Biol. Interact., 45: 315-326.
COHEN, M.M. & SAGI, M. (1979). The effect of nitrofurans on mitosis,
chromosome breakage and sister-chromatid exchange in human
peripheral lymphocytes. Mutat. Res., 59(1): 139-142.
CRAINE, E.M. & RAY, W.H. (1972). Metabolites of furazolidone in
urine of chickens. J. Pharmacol. Science, 61(9): 1495-1497.
CRAINE, E.M. (1977). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 77:10
d.d. 28 December 1977 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1978). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 78:12
d.d. 21 April 1978 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1981). Preparation of isolates of furazolidone
metabolites. Unpublished research report, project no. 80191 d.d. 2
March 1981 from Wil Research Laboratories Inc., Research Division,
Cincinnnati, Ohio. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
CREBELLI, R., CARERE, A., FALCONE, E. & MACRI, A. (1982). A study on
the urinary and fecal excretion of furazolidone in rats by means of
mutagenicity assays. Ecotoxicol. Environ. Saf. 6(5): 448-456.
DeGROOT, A.C. & CONEMANS, J.M.H. (1990). Contact allergy to
furazolidone. Contact Dermatitis, 22:, 202-205.
EBRINGER, L., JURASEK, A., KONICEK, J., KONICKOVA, M., LAHITOVA, N.
& TRIBACUK, S. (1976). Mutagenic action of nitrofurans on Euglena
gracilis and Mycobacterium phlei. Antimicrobial Agents and
Chemotherapy, 9(4): 682-689.
ENNINGA, I.C. & WETERINGS, P.J.J.M. (1990). Micronucleus test in
bone marrow cells of the mouse with furazolidone. Unpublished report
(RCC notox project 026122) d.d. April 1990 from RCC/NOTOX, 5231
DD's-Hertogenbosch, the Netherlands. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
FRANCIS, D.W. & SHAFFNER, C.S. (1956). An investigation of the
morphological changes in young chickens and the reproductive
performance of adult chickens fed furazolidone or nitrofurazone.
Poultry Sci., 35: 1371-1381.
FUKUHARA, M. & TAKABATAKE, E. (1977). The effects of nitrofuran
derivatives on hepatic microsomal mixed-function oxidase activity in
rats. Toxicol. Appl. Pharmacol., 42: 571-581.
GAO, N., NI, Y.C., THORNTON-MANNING, J.R., FU, P.P. & HEFLICH, R.H.
(1989). Mutagenicity of nitrofurantoin and furazolidone in Chinese
hamster ovary cell strains. Mutat. Res., 225: 181-187.
GENTRY, R.F. (1957). The toxicity of certain antibiotics and
furazolidone for chicken embryos. Avian Diseases, 2: 77-82.
HALLIDAY, R.P., SUTTON, M.L., SIGLER, F.W. & LEVIN, R. A. (1973a).
Chronic toxicopathological safety study (two years) of NF-180 in
rats. Unpublished final report project no. 475.09-C d.d. 9 November
1973 from Pathological and Toxicology Section, Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1973b). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished interim report # 2 project no. 475.09D d.d. 9
November 1973 part I: Sprague-Dawley evaluation from Pathological
and Toxicology Section, Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974a). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished final report project no. 475.09D d.d. 31 January
part II: Fischer 344 evaluation from Pathological and Toxicology
Section, Norwich Pharmacal Company, Norwich, New York. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974b). Tumorgenesis
evaluation (twenty-three months) of furazolidone (NF-180) in mice,
Unpublished final report project no. 475.09E d.d. 31 January 1974
from Pathological and Toxicology Section, Research and Development
Department, Norwich Pharmacal Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HAWKINS, D.R., ELSOM, L.F., GIRKIN, R. & CHENG, C.F. (1992). The
bioavailability in rats of tissue residues from swine administered
14C-furazolidone for 14 days and subjected to 0-day, 21-day and
45-day withdrawal periods. Unpublished draft report HRC/SMI
125/911478 from Huntingdon Research Centre Ltd., P.O.Box 2,
Huntingdon, Cambridgeshire, England. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
HEOTIS, J.P., HERRET, R.J. & WILLIAMS, C.W. (1963). Drug metabolism
studies. Incorporation of C14 from formyl-C14-NF-180 into
natural products. Unpublished special report part I: problem 370.3
d.d. 20 September 1963 from Biological Research Division, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
HOOGENBOOM, L.A.P. (1991a). The use of pig hepatocytes to study the
role of glutathione on the biotransformation and toxicity of
furazolidone. In: The uses of pig hepatocytes for biotransformation
and toxicity studies Thesis, Agricultural University of Wageningen,
The Netherlands. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991b). The use of pig hepatocytes for
biotransformation studies of veterinary drugs. In: The use of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991c). The use of pig hepatocytes to study the
inhibition of monoamine oxidase by furazolidone. In: The uses of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P., Van KAMMEN, M., BERGHMANS, M.C.J. & KUIPER, H.A.
(1991). Detection of protein bound metabolites of furazolidone.
Proceedings of the Euroresidue Conference in Noordwijkerhout, The
Netherlands. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965a). Two-year chronic toxicity study and a
three-generation reproductive study in rats. Unpublished report
no. 65:15/H-1R from Hess & Clark, Research Department, Ashland,
Ohio. Summary submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965b). Two-year chronic toxicity test with
furazolidone in dogs. Unpublished report no. 65:16/H-1D) d.d. 30
July 1965 from Hess & Clark, Research Department, Ashland, Ohio.
Abstract submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HUGHES, D.L. & McMINN, C.S. (1963). The effect of furazolidone on
reproduction and enteric infections of swine. Unpublished final
report (Ex 62-12-18a2) Norwich Pharmacal Company, Norwich, USA.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
JACKSON, D. & ROBSON, J.M. (1957). The action of furazolidone on
pregnancy. J. Endocrin., 15: 355-359.
JAGANNATH, D.R. & BRUSICK, D.J. (1981a). Mutagenicity evaluation of
WIL-80191-10-4 dry liver, control in the Ames Salmonella/microsome
plate test. Unpublished final report d.d. March 1981 LBI project
from Litton Bionetics, Inc. Kensington, Maryland 207953. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981b). Mutagenicity evaluation of
WIL-80191-11-2 urine isolate; control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981c). Mutagenicity evaluation of
WIL-80191-10-1 liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981d). Mutagenicity evaluation of
WIL-80191-10-2 dry liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981e). Mutagenicity evaluation of
WIL-80191-10-3 liver isolate control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981f). Mutagenicity evaluation of
WIL-80191-11-1 urine isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981g). Mutagenicity evaluation of
Furazolidone WIL-80191-11-3 in the Ames Salmonella/microsome plate
test. Unpublished final report LBI project from Litton Bionetics,
Inc. Kensington, Maryland 207953. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CURRIE, G.N. HARRINGTON, C. & WELCH, C.A. (1970a).
Effects of nitrofurans on steroidogenesis and mammary hyperplasia
(NF-180 Rx 30309) Unpublished annual report project no. 569.09
d.d.16 June 1970 from Research and Development Department, The
Norwich Pharmacal Comp., Norwich, New York. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CHRISTENSEN, E.F., McLAUGHLIN, J.H., LEVIN, R.A. &
SIGLER, F.W. (1971a). Chronic (2 year) toxicopathologic study of
NF-180 in dogs. Unpublished final report project no. 475.09B d.d. 12
November 1971 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Abstract submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
KING, C.D., CURRIE, G.N., HARRINGTON, C. & WELCH, C. (1971b).
Nitrofurans and steroidogenesis. Unpublished interim report no. 5
project no. 596.09 d.d. 21 December 1971 from Pathological and
Toxicology Section, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L. & LEVIN, R.A. (1972a). Chronic
toxicopathological safety study (two years) of NF-180 in rats.
Unpublished status report no.1 project no. 475.09c d.d. 18 October
1972 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L., WONG, L.C.K. & LAUGHLIN, P.J. (1972b).
Tumorgenesis evaluation (two years) of NF-180 in Spraque-Dawley and
Fischer 344 rats. Unpublished status report no.1 project no. 475.09D
d.d. 18 0ctober 1972 from Pathological and Toxicology Section,
Research and Development Department, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
KOUBA, R.F. (1979). Analysis of components from urine of pigs
treated with furazolidone-14C-15N or furazolidone-14C.
Unpublished report no.: RFK 79:3 from Hess & Clark, Ashland.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
KRAMERS, P.G. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
LARSON, E.J. (1963a). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12a. Addendum to pathology
research report H-12. Unpublished report d.d. 14 November 1963 from
Research Department Hess & Clark, Ashland Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LARSON, E.J. (1963b). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12 d.d. 18 March 1963 from
Research Department Hess & Clark, Ashland, Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli. Mutation and induction and repair of daughter-strand
gaps in DNA. Mutat. Res., 67: 13-144.
McCALLA, D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res. 16(1): 3-16.
MEITES, J. DENINE, E.P. & OBROSKY, K.W. (1984). Determination of
blood levels of prolactin and corticosterone following oral
administration of furazolidone (NF-180) or EU-557 to male
Sprague-Dawley rats: a pilot study. Unpublished final report project
no. 475.59.59 d.d. 30 March 1984 from Norwich Eaton Pharmaceuticals,
Inc., Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990a).
Acute oral toxicity study of furazolidone in rats. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5884-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990b).
Acute oral toxicity study of furazolidone in mice. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5885-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B. & WESSEL, J.L. (1990c).
Primary dermal irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/Dynamics Inc. project
no. 5886-90, East Millstone, New Jersey. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B., ODDE, E.Y. & WESSEL, J.L.
(1990d). Primary irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/dynamics Inc. project
no. 5887-90, East Millstone, New Jersey. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
MIYAJI, T, MIYAMOTO, M & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testis caused by nitrofuran
compounds. Acta Path. Jap., 14(3): 261-273.
MORI, H., SUGIE, S., YOSHIMI, N., IWATA, H., NISHIKAWA, A.,
MATSUKUBO, K., SHIMIZU, H. & HIRONO, I. (1988). Genotoxicity of a
variety of hydrazine derivatives in the hepatocyte primary culture
DNA repair test using rat and mouse hepatocytes. Jpn. J. Cancer
Res. (Gann), 29: 2145-2156.
MORRISON, J.L., CURRIE, G.N., HARRINGTON, C. & WELCH, C.A. (1972).
The effects of estrone and NF-180 on mammary tumor formation.
Unpublished special report project no. 596.09 d.d. 12 October 1972
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1973). Effects of
NF-180 (acute) on serum prolactin levels. Unpublished final report
project no. 596.03 d.d. 24 October 1973 from Biochemistry Section,
The Norwich Pharmacol Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974a). Effects of
adrenalectomy on NF-180 induced testicular atrophy. Unpublished
final report project no. 475.03-F d.d. 24 May 1974 from Biochemistry
Section, The Norwich Pharmacol Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974b). Effects of
NF-180 treatment on plasma steroids in the female Sprague-Dawley
rat. Unpublished final report project no. 475.03-31 d.d. 18
September 1974 from Biochemistry Section, The Norwich Pharmacol
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MORRISON, J.L. (1976). Identification of NF-682 as a metabolite of
NF-180 in rats. Unpublished report d.d. 2 July 1976 attachment 5
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MUSTAFA, A. I., ALI, B.H. & HASSAN, T. (1987). Semen characteristics
in furazolidone-treated goats. Reprod. Nutr. Develop., 27(1A):
89-94.
NI, Y.-C., HEFLICH, R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98nr
and TA98/1,8-DNP6. Mutat. Res., 192(1): 15-22.
OHTA, T., NAKAMURA, N., MORIYA, M., SHIRASU, Y. & KADA, T. (1984).
The SOS-function-inducing activity of chemical mutagens in
Escherichia coli. Mutat. Res., 131: 101-109.
PAIK, S.G. (1985). Micronucleus induction in mouse bone marrow cells
of some nitrofuran, 5-nitroimidazole and nitrothiazole derivatives
used as trichomonacides in Korea. Environ. Mutag. Carcinogens,
5(2): 61-72.
PALM, D., MAGNUS, U., GROBECKER, H. & JONSSON, J. (1967). Hemmung
der monoami-noxydase durch bakteriostatisch wirksame
nitrofuran-derivative. Naunyn-Schmiedebergs Arch. Pharmak., 256:
281-300.
PAUL, H. (1955). Oral chronic toxicity study on Nf-180 in dogs
(study) Unpublished report d.d. 16 June 1955 from Eaton
Laboratories, Norwich, New York. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
PETTINGER, W.A., SOYANGCO, F.G. & OATES, J.A. (1968). Inhibition of
monoamine oxidase in man by furazolidone. Clinical Pharmacol.
Therapeut., 9(4): 442-447.
PROBST, G.S., McMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K. &
NEAL, S.B. (1981). Chemically-induced unscheduled DNA synthesis in
primary rat hepatocytes cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3: 11-32.
QUEINNEC, G., BABILE, R., DARRE, R., BERLAND, H.M. & ESPINASSE, J.
(1975). Induction of abnormalities in chromosomes (of cattle and
swine) by furazolidone or chloramphicol. Veterinary Bul., 46: 330.
RAY, W.H. (1959). Preliminary metabolism study of position
C14-labelled furazolidone in the white rat. Unpublished report no.
59-3 d.d. 18-09070 from Hess & Clark, Ashland, Ohio. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. (1962). An investigation of the metabolites of
furazolidone in swine urine. Unpublished research report no. 62:3
d.d. 29 May 1962 from Hess & Clark, Ashland, Ohio. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. & HAYES, M. (1963). Evidence that the orange '415'
metabolite of furazolidone in swine is a nitrofuran. Unpublished
research report 63:2 d.d. 25 January 1963 from Hess & Clark,
Ashland, Ohio. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
SCHERES, H.M.E. (1991a). Evaluation of the mutagenic activity of
3-amino-oxazolidinon-2 in the Ames Salmonella/microsome test.
Unpublished report project nr. d.d.15 January 1991 from RCC Notox,
5231 DD's Hertogenbosch. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
SCHERES, H.M.E. (1991b). Evaluation of the ability of furazolidone
to induce chromosome aberrations in cultured peripheral human
lymphocytes. Unpublished report project no. 038385 d.d. 9 January
1991 from Notox RCC, 5231 DD's Hertogenbosch. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
13 October 1966 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
4 January 1976 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
STERN, I.J. HOOLIFIELD, R.D., WILK, S. & BUZARD, J.A. (1967). The
anti-monoamine oxidase effects of furazolidone. J. Pharmacol. Exp.
Therapeut., 156(3): 492-499.
TATSUMI, K. & TAKAHASHI, Y. (1982). Metabolic fate of furazolidone
in rats. Chem. Pharm. Bull. Tokyo, 30(9), 3435-3438.
TATSUMI, K. YAMADA, H., YOSHIMURA, H. & KAWAZOE, Y (1981).
Metabolism of furazolidone by milk xanthine oxidase and rat liver
9000 g supernatant: Formation of a unique nitrofuran metabolite and
an aminofuran derivative. Arch. Biochem. Biophysics, 208(1):
167-174.
TATSUMI, K., NAKABEPPU, H., TAKAHASHI, Y., & KITAMURA, S. (1984).
Metabolism in vivo of furazolidone: evidence for formation of an
open-chain carboxylic acid and aketoglutaric acid from the
nitrofuran in rats. Arch. Biochem. Biophysics, 234(1): 112-116.
TENNENT, D.M. & RAY, W.H. (1971). Metabolism of furazolidone in
swine (355994). Proc. Soc. Exp. Biol. Med., 138(3): 808-810.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan. J. Genetics, 48(4): 291-294.
Van Den DUNGEN, H.M., JAGER, L.P., LUYCKX, N.B. L., De GRAAF, G.J. &
BAARS, A.J. (1991). Adrenal toxicity of carbadox and furazolidone
in vitro. Act. Vet. Scand., 87: 338-340.
VOOGD, C.E., KRAMERS, P.G.N., KNAAP, A.G.A.C., OUD, J.H. & Van WENT,
G.F. (1982). Mutagenicity of feed additives. Postersession RIV,
Bilthoven. as cited in Van MIERT, A.S.J.P.A.M., VROOMEN, L.H.M. &
JAGER, L.P. (1984) Farmacologie en toxicologie van furazolidon en
carbadox: een literatuuronderzoek. Tijdschr. Diergeneeskd.
109(22): 928-932.
VROOMEN, L.H., BERGHMANS, M.C., Van LEEUWEN, P., Van Der STRUIJS,
T.D.B.M, De VRIES, P.H.U. & KUIPER, H.A. (1986). Kinetics of
14C-furazolidone in piglets upon oral administration during 10
days and its interaction with tissue macromolecules. Food Add.
Contamin., 3: 331-346.
VROOMEN, L.H. (1987). Quantitative studies of the metabolism of
furazolidone by rat liver microsomes. Toxicol. in vitro, 1(2):
97-104.
VROOMEN, L.H., BERGHMANS, M.C., HEKMAN, P., HOOGENBOOM, L.A.P. &
KUIPER, H.A. (1987a). The elimination of furazolidone and its
open-chain cyano-derivative from adult swine. Xenobiotica, 17:
1427-1435.
VROOMEN, L.H., GROTEN, J.P., Van MUISWINKEL, K., Van VELDHUIZEN,
P.J. & Van BLADEREN, P.J. (1987b). Identification of a reactive
intermediate of furazolidone formed by swine liver microsomes.
Chem. Biol. Interact., 64(1-2): 167-180.
VROOMEN,L.H., BERGHMANS, M.C., GROTEN, J.P., KOEMAN, J.H. & Van
BLADEREN, P.J. (1988). Reversible interaction of a reactive
intermediate derived from furazolidone with glutathione and protein.
Toxicol. Appl. Pharmacol., 95: 53-60.
VROOMEN, L.H., BERGHMANS, M.C., Van BLADEREN, P.J., GROTEN, J.P.,
WISSINK, C.J. & KUIPER, H.A. (1990). In vivo and in vitro
metabolic studies of furazolidone: A risk evaluation. Drug. Metab.
Rev., 22(6-8): 663-676.
WHITE, A.H. (1989). Absorption, distribution, metabolism and
excretion of furazolidone. Scand. J. Gastroenterol.,
24(suppl. 169): 4-10.
 2.1.2.2 In vivo
[(5-Nitrofurfurylidene)hydrazino]acetic acid has been isolated
from urine of rats treated with 20 mg furazolidone/kg bw (Morrison,
1976).
A small part of the radioactivity in rat urine after treatment
with 14C-furazolidone was identified as
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone and
N-(5-acetamido-2-furfurylidene)-3-amino-2-oxazolidone (Tatsumi &
Takahashi, 1982).
Male Wistar rats were given orally 100 mg 14C-furazolidone
(formyl-labelled)/kg bw. Urine was collected and analyzed for
possible metabolites. N-(4-carboxy-2-oxobutylideneamino)-
2-oxazolidone, alpha-ketoglutaric acid,
3-(4-cyano-2-oxobutylidenamino)-2-oxazolidone and
N-(5-acetoamido-2-furfurylidene)-3-amino-2-oxazolidone were
identified (Tatsumi et al., 1984; White 1989).
Colostomized chickens received a single oral dose of 30 mg
furazolidone/kg bw. An average of 7.5% of the dose was excreted in
the urine in 12 h. Four urinary metabolites containing a furan ring,
of which only one was a nitrofuran, were detected. Only traces of
unchanged furazolidone were found (Craine & Ray, 1972).
The metabolites of aldehyde-labelled 14C-furazolidone in pig
urine were investigated. More than thirty radio-labelled metabolites
were found. Three primary metabolites of furazolidone were
identified as 5-nitro-2-furoic acid, an orange-coloured "415"
chromogen and a yellow "415" chromogen, with half-lives of 30, 22
and 19 minutes, respectively. Some metabolites are naturally
occurring chemicals and include a yellow urinary pigment, carbonates
and a number of ninhydrin positive materials which lose
radioactivity when treated with ninhydrin indicating that these
materials are amino acids or conjugates of amino acids (Ray, 1962).
The orange "415" metabolite contains an intact
5-nitro-2-furfural moiety and is the most abundant single
furazolidone-related metabolite found in pig urine (Ray & Hayes,
1963).
Analysis of urine from a pig treated with 14C-furazolidone,
also labelled with 15N in the 5-nitro position, showed 14
metabolites. Eleven of them degraded to two or three degradation
products, which could not be identified. The presence of 15N was
detected in one minor metabolite. The authors concluded that during
the metabolism of furazolidone the nitro group was removed
extensively from the molecule (Kouba, 1979).
The open-chain cyano-derivative of furazolidone,
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone was a minor
metabolite in plasma and tissues of swine with a plasma half-life of
4 h (Figure 1) (Vroomen et al., 1987a).
Four human subjects were given a single dose of 400 mg
furazolidone/day administered in a tablet or a capsule. Using an
HPLC method, 24 h urine samples collected after administration
contained 0.003% to 0.16% unchanged furazolidone. By using a
specific complexation method (detection limit 2 µg/ml) no
furazolidone was detected (White, 1989).
Furazolidone was given to 10 human adults as 2 daily doses of
200 mg each for 21 days. Plasma levels analysed by HPLC (detection
limit 0.002 µg/ml) ranged from trace quantities to 0.489 µg/ml
(White, 1989).
2.1.3 Special studies on bound residues
Muscle tissue was prepared from a piglet receiving
14C-furazolidone for 10 days and sacrificed 2 h after the last
administration. Groups of 2 female Wistar WU rats were fed diets
containing non-extracted muscle tissue (group 1), muscle tissue
obtained after 4 extractions with water (group 2) (no extractions
with organic solvents were carried out) or the water extracts
obtained from muscle tissue after four extraction steps with water
for 4 days (group 3). Amounts of radioactivity in groups 1, 2, and
3, were, respectively, 26, 28 and 38% in urine, 23, 38 and 24%, in
faeces, 5.0, 7.4 and 4.8% in tissues, and 4.2, 5.3 and 3.1% in the
remaining carcass. Total recovery was 62%, 81% and 71% for groups 1,
2 and 3, respectively. The amount of non-extractable fractions
obtained from rat liver and muscle tissues were, respectively, 34
and 27% for group 1, 29% and 39% for group 2 and 32% and 21% for
group 3 (Vroomen, et al., 1990).
From a metabolism study with pig hepatocytes it was shown that
at least 70% of the bound residues still contain the
3-amino-2-oxazolidone side chain of furazolidone releasable upon
mild acid treatment. At least 25% of the bound residues in vivo
were shown to contain a releasable side-chain (day 0 animal)
(Hoogenboom 1991b).
The bioavailability of total and non-extractable
14C-furazolidone-derived radioactivity found in swine tissues at 0
and 45-day withdrawal has been investigated in bile duct cannulated
male rats. The pigs had been treated with 300 mg 14C-furazolidone
(both methylene- and formyl-labelled)/kg feed for 14 days. Zero-day
withdrawal liver was extracted with methanol:water, methanol, ether
and ethylacetate. A total of 44% of the radioactivity was extracted.
Zero-day withdrawal muscle, 45-day withdrawal liver and 45-day
withdrawal muscle were prepared in a similar way with different
extraction methods but no methanol:water extraction was carried out,
in order to prevent solubilization of small protein molecules which
may contain bound radioactivity. From 0-day withdrawal muscle 22% of
the radioactivity was extracted. From 45-day withdrawal liver and
muscle 8.3% and 14% of the radioactivity was extracted,
respectively.
Pelleted freeze dried extracted and non-extracted 0 and 45 day
withdrawal liver and muscle were dosed for 24 h to groups of 4
Sprague-Dawley rats. The rats were pretreated with control liver or
muscle for 24 h. Bile, urine and faeces were collected for up to 72
h. After 72 h the rats were sacrificed and the liver and
gastrointestinal tract were analyzed. The excretion pattern for the
differently-treated rats was similar. Rats treated with 0 and 45 day
withdrawal liver and muscle containing total residue excreted 10-27%
in the urine and 1.5-2.7% in bile, while 7.4-16% was found in
tissues. Rats treated with non-extractable tissue excreted 17-21% in
urine and 1.1-3.0% in bile; 4.8-15% was found in tissues (Hawkins
et al., 1992).
2.1.4 Effects on enzymes and other biochemical parameters
The effect of oral administration of 500 mg furazolidone/kg bw
on the activity of monoamine oxidase (MAO) was studied in rats.
Twenty-four hours after treatment MAO activity in liver and brain
was inhibited by 95%. Enzyme inhibition was detectable in liver 6 h
and in brain 12 h after administration, while maximal inhibition was
observed between 24 and 48 h. MAO activity returned to control
values in liver and brain within 10 days and 2-3 weeks,
respectively. The levels of noradrenaline and serotonin in brain
increased by 60-70%, dopamine in brain by 20% and noradrenaline in
heart by 60% when measured 1 to 3 days after application. In
contrast, adrenomedullary catecholamines were reduced by 30% one and
3 days after administration. Repeated application of furazolidone
elicited a cumulative effect: 15 mg/kg bw on 10 consecutive days was
as effective as a single dose of 90-120 mg furazolidone/kg bw.
Following application of furazolidone (125-500 mg/kg bw) the
sympathomimetic actions of tyramine given intravenously and
intraduodenally were potentiated up to 100-fold. According to the
authors the inhibition of MAO is probably due to a metabolite of
furazolidone that contains a free hydrazine group (Palm et al.,
1967).
After single oral doses of 2-100 mg furazolidone/kg bw a
decrease in rat liver-mitochondrial MAO was observed in about 4 h.
Maximal inhibition occurred in 16-24 h. Enzyme activity returned to
normal after 21 days. Furazolidone in a saturated solution did not
affect rat liver MAO in vitro (Stern et al., 1967).
In chickens fed 400 mg furazolidone/kg feed for 10 days MAO
activity in the brain, heart and alimentary tract was inhibited. No
MAO inhibition in the liver was found. Treatment increased the
amount of 5-hydroxy-tryptamine (5-HT) in the brain and potentiated
the vasodepressor action of tyramine. The amounts of adrenaline,
noradrenaline and the vasodepressor action in the brain were
unaffected by treatment (Ali & Bartlet, 1982).
In ducklings administered 400 mg furazolidone/kg feed for 10
days or 200 mg/kg bw by crop tube MAO activity in the liver was
inhibited. No inhibition was found in other organs (Ali & Bartlet,
1982).
Significant diamine oxidase (DAO) inhibition was observed in
various tissues (plasma, duodenal mucosa, liver, heart and brain) of
rabbits given orally 50 mg furazolidone/kg bw for 5 consecutive
days. Recovery was complete in all organs 14 days after the
withdrawal of furazolidone (Ali, 1983).
MAO inhibition was measured in primary cultures of pig
hepatocytes incubated with furazolidone. A dose-related inhibition
was observed; the effect was completely reversible upon withdrawal
of furazolidone. The proposed but not proven metabolites
ß-hydroxye-thylhydrazine and 3-amino-2-oxazolidone incubated with
the same cells caused an irreversible inhibition of MAO activity
(Hoogenboom, 1991c).
Six hundred mg furazolidone/kg administered in the diet to male
Wistar rats for 7 days did not affect cytochrome P-450
concentrations, but increased absolute cytochrome b 5 levels.
The activity of NADPH-cytochrome c reductase and aminopyrine
N-demethylase was decreased, but that of aniline hydroxylase was
increased (Fukuhara & Takabatake, 1977).
Thirty mg furazolidone/kg bw administered orally to large male
white turkeys produced significant increases in hypothalamic
concentrations of noradrenaline, adrenaline and dopa. One week after
withdrawal the amine concentrations returned to normal (Ali et al.,
1988).
In an in vitro experiment 14C-furazolidone was covalently
bound to rat liver microsomal protein; the binding could be
inhibited by addition of gluthathione or mercaptoethanol.
Furazolidone did not interact with added calf thymus DNA in the
presence of microsomes (Vroomen, 1987).
In an in vitro study using swine liver microsomes it was
shown that the mercaptoethanol conjugate (M1) and the glutathione
conjugate (G1) can bind covalently to microsomal protein.
According to the author this reaction is reversible (Vroomen et
al., 1988). In the case of pig hepatoccytes, a decrease in
GSH-levels did not result in an increased formation of bound
residues. Furthermore, no proof was obtained for the reversibility
of bound residues upon incubation with MSH (Hoogenboom, Personal
communication, 1992).
Furazolidone binds to DNA and possibly renders steric hindrance
to DNA replication, which leads to the inhibition of biosynthesis of
DNA in Vibrio cholerae cells (Chatterjee et al., 1975).
DNA-binding of 14C-furazolidone in piglet tissue varied from
87-382 pmol-equivalents of furazolidone per mg DNA (Vroomen et al.,
1986).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies on furazolidone are
summarized in Table 1.
Table 1. Acute toxicity of furazolidone
Species Sex Route Purity LD50 Reference
(mg/kg bw)
Mouse M&F oral 100% 1110 Mitchell et al., 1990b
Rat M&F oral 100% 1508 Mitchell et al., 1990a
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Male Wistar rats (6/group) were fed diets containing 0, 10, 100
or 200 mg furazolidone (purity not given)/kg in the diet for 13
months (equivalent to 0, 0.5, 5 or 10 mg/kg bw/day). Mortality rates
were 1/6, 2/6, 1/6 and 2/6 for the control, low-, mid- and high-dose
groups, respectively. No effects were observed on food consumption
and body weight. No changes were observed in the number of
erythrocytes and the number of leucocytes in blood samples of 2
rats/group taken at the end of the experiment. A slight increase in
relative liver and spleen weights were observed at the highest dose.
At histopathology a slight hyperthrophy of the liver cells was
observed at all dose levels. No effects were observed on kidney,
spleen, heart and testis (Aiso et al., 1962).
The effects of feeding various nitrofurans was studied.
Thirty-five female Holtzman weanling rats were administered 1000 mg
furazolidone (purity not given)/kg feed for 45 weeks (equivalent to
50 mg/kg bw/day). A matched control group was used. The rats were
maintained on a control diet for an additional 8 weeks. Observations
included clinical signs, feed consumption, feed efficiency and body
weight. Ten rats with palpable tumours were necropsied at week 42
and the remaining rats at termination of the experiment. At
termination mortality was significantly increased as compared to the
control group. Feed consumption, feed efficiency and body-weight
gain were significantly decreased in treated rats. Treated rats had
significantly more palpable mammary tumours from week 35 onwards
than the control rats. This was a poorly-reported study and no
detailed histopathology is available (Siedler & Searfoss, 1966).
In another experiment group of rats (Carworth, 20/sex/group)
received diets with various nitrofurans for 45 weeks followed by a
7-week recovery period. An untreated control group was used.
Furazolidone was fed at a rate of 1000 mg/kg feed (equivalent to 50
mg/kg bw/day). Observations included body weight, feed consumption,
feed efficiency and pathology. After five weeks on treatment and at
termination both male and female rats showed a significant decrease
in body-weight gain, feed consumption and feed efficiency. A
significantly increased incidence of palpable mammary tumours was
observed in female rats. At microscopy most tumours were found to be
adenomas, fibromas, and fibroadenomas with or without cyst
formation. Two of the 72 tumours observed were adenocarcinomas. This
was a poorly-reported study (Siedler & Searfoss, 1967).
2.2.2.2 Dogs
In a very limited study groups of 2 male and 2 female beagle
dogs were given micronized furazolidone by gavage at doses of 5, 11
or 23 mg/kg bw/day for 90 days. Another group of 1 male and 1 female
beagle dogs received 23 mg/kg bw/day furazolidone (as crystals) in
gelatin capsules for 88 days. Some neurological signs and testicular
degeneration in the 11 and 23 mg/kg bw/day dose groups were seen.
Most dogs suffered from pneumonia (Borgmann et al., 1964).
Groups of beagle dogs were orally administered furazolidone
(purity not given) in gelatin capsules. Four males and 4 females
received 7.5 mg/kg bw/day for 6 months. Two males were given 25
mg/kg bw/day for 6 months and 6 male and 4 female dogs received this
dose for periods varying from 28-118 days during 6 months of the
study. The highest-dose group consisted of 3 males and 2 females
given 50 mg/kg bw/day for periods varying from 16-37 days during 6
months of the study. The number of dogs used for controls were 12
(for 126 days) and 10 (for 6 months). No effects were observed on
clinical signs, haematology, blood biochemistry, urinalysis, organ
weight and macroscopy. Body weight was decreased in the mid- and
high-dose groups. In all treated dogs neurological symptoms and
histopathological changes in the region of the basal ganglia,
decreased sperm and tubular testicular degeneration were reported.
The authors concluded that the neurological effects as well as the
testicular changes were reversible. This was a poorly-reported study
that was not performed according to current standards (Paul, 1955).
Three groups of 2 male and 2 female beagle dogs were fed diets
containing 0, 30 or 100 mg furazolidone/kg for 2 years (equivalent
to 0.8 or 2.5 mg/kg bw/day). No effects were observed on body
weight, haematology, blood biochemistry, urinalysis, organ weight
and histopathology. Only an abstract of this limited study was
available (Huffman, 1965b).
Groups of beagle dogs (ARC, 4/sex/group) were orally
administered 0, 1, 5 or 15 mg/kg bw/day furazolidone (purity not
given) for 2 years. Additionally, 2 male dogs per group were
included to assess effects on semen quality. Two groups of 5 male
and 5 female beagle dogs, raised at different kennels (ARC + HRA)
were included to further assess cataractogenic properties of
furazolidone at the 15 mg/kg bw/day dose level. Observations
included clinical signs, body weight, ophthalmoscopy, neurological
examinations, recording of ECGs, haematology, clinical chemistry and
urinalysis, macroscopy and histopathology.
Because neurological symptoms were observed in 5/8 high-dose
dogs and 2 high-dose dogs from the semen evaluation study, these
dogs were discontinued from the study. Cataracts were observed in
3/5 male and 5/5 female ARC dogs and in 2/5 male and 1/5 female HRA
dogs. Decreased sperm motility and abnormal sperm were observed in
the mid- and high-dose groups. Increased relative kidney weight was
observed in high-dose females and decreased testes weight in mid-
and high-dose males. Only abstract was available (King et al.,
1971a).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of Swiss MBR/ICR mice (50/sex/group) were fed diets
containing 0, 75, 150 or 300 mg/kg furazolidone (purity not given)
for 13 months (equal to average daily doses of 12, 24 or
47 mg/kg bw). All mice were maintained on normal diet for 10
additional months. During the treatment period as well as during the
post-treatment period, no substance-related effects were observed on
feed consumption and body weight. Survival was decreased in mid- and
high-dose females at the end of the treatment period and in
high-dose males and mid- and high-dose females at 23 months. At
histopathology the incidence of bronchial adenocarcinomas was
significanly increased in the mid- and high-dose groups in both
sexes (incidences for males: 13/49, 19/48, 26/50 and 37/50 and for
females: 15/50, 18/50, 20/47 and 30/48 at the control, low-, mid-
and high-doses, respectively). The incidence of lymphosarcomas was
significantly increased in mid- and high-dose males (incidences:
1/49, 7/48, 10/50 and 10/50 for the control, low-, mid- and
high-doses, respectively) (Halliday et al., 1974b).
2.2.3.2 Rats
Groups of Sprague-Dawley rats (35/sex/group) were fed diets
containing furazolidone for 2 years. The actual average consumption
over 2 years was 0, 0.7, 4 or 10 mg/kg bw/day for males and 0, 0.8,
4.3 or 14 mg/kg bw/day for females. Additional groups (5/sex) were
maintained and killed after 1 year. No effects were observed on
clinical signs, body weight, feed consumption, blood biochemistry,
urinalysis and sternal bone marrow sections. Survival tended to
decrease with dose in females (26, 22, 23 and 15 surviving after two
years for control, low-, mid- and high-dose groups, respectively).
At termination mid- and high-dose female rats showed dose-related
decreased erythrocyte, haemoglobin and haematocrit counts and an
increased neutrophil count. Females from the highest-dose group
showed an increased neutrophil/lymphocyte ratio. Relative liver
weight was increased in high-dose males. At histopathology male rats
from the highest dose group showed an increase in parathyroid
hyperplasia without 'renal ricketts'. In male and female rats an
increase in adrenal cortical hyperplasia was observed at the highest
dose. High-dose females showed an increase in thyroid atrophy.
Females exhibited increased mammary tumour incidences as shown in
Table 2. The authors concluded that the mean onset time of mammary
neoplasms was approximately 2 months earlier in the mid- and
high-dose females than in the other groups (King et al., 1972a;
Halliday et al., 1973a).
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 250, 500 or 1000 mg/kg furazolidone (purity not given)
for 20 months (equivalent to 12.5, 25 or 50 mg/kg bw/day). The
surviving rats were maintained on control diets for at least 4
months or until 90% of the rats had died. Observations included
clinical signs, mortality, body weight and feed consumption.
Haematological and clinical chemistry examinations were performed
after 1 year and at the end of the treatment period. Extensive
histopathological examinations were performed on all moribund and
sacrificed rats. At the mid- (males only) and high-doses the
mortality rate was increased; 90% mortality in the highest dose was
observed after 24 months. At the end of the treatment period
body-weight gain was significantly decreased at 500 and 1000 mg/kg
Table 2. Mammary tumour incidences in female Sprague-Dawley rats
control low mid high
No. of animals examined 34 35 33 35
Multiple mammary tumours 3 6 11 19
Malignant mammary tumours 1 3 3 5
Mammary fibroadenomas 0 6 2 10
Mammary adenocarcinomas 1 2 2 3
Mammary carcinosarcomas 0 1 2 1
feed. At 1000 mg/kg feed haemoglobin, haematocrit (also at 500 mg/kg
feed) and the number of erythrocytes (also in high-dose females)
were significantly decreased in male rats. An increase in 'non-renal
ricketts' was observed in high- dose male rats. The incidence of
testicular atrophy was increased in mid- and high-dose males and the
incidence of adrenal cortical hyperplasia, lipolysis, congestion and
haemorrhage was increased in high-dose males only. Tumour incidences
are given in Table 3. In high-dose females a significant increase in
the incidence of mammary gland adenocarcinomas was observed. In
addition, an increased incidence of sebaceous gland adenomas and
thyroid adenomas was observed in both sexes at the mid- and
high-dose and of basal-cell epithelioma and carcinomas in males of
the high-dose group. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1974a).
Sprague-Dawley rats (50/sex) were fed diets containing 0, 250,
500 or 1000 mg furazolidone/kg for 20 months (equivalent to 12.5, 25
or 50 mg/kg bw/day). The study was carried out following the same
protocol as described above for Fischer 344 rats. Mortality for
control, low-, mid- and high-dose groups was 4/50, 11/50, 17/50 and
30/50 for males and 9/50, 12/50, 8/50 and 29/50 for females,
respectively. High-dose animals were sacrificed at day 666, compared
to day 895 for controls. Body-weight gain was significantly
decreased in mid- and high-dose males and high-dose females. At the
end of the treatment period the number of erythrocytes was decreased
in females at 500 and 1000 mg/kg feed. High-dose males showed an
increased neutrophil/lymphocyte ratio and a decreased lymphocyte
count. Histopathology showed an increased incidence of hepatic
necrosis in all treated rats, especially high-dose females.
Testicular atrophy was seen in mid- and high-dose males. A
dose-related increase in adrenal cortical hyperplasia was observed
in females at all dose levels. Tumour incidences are given in Table
4. In the high-dose group, significantly increased incidences were
reported for mammary adenocarcinomas in females and for neural
astrocytomas in males. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1973b).
Table 3. Tumour incidences in Fischer 344 rats
males females
contr. low mid high contr. low mid high
No. of animals examined 49 50 50 49 49 50 50 50
Dermal fibromas 2 6 1 10 0 3 7 3
Sebaceous adenomas 0 0 8 11 1 2 6 10
Sebaceous adenocarcinomas 0 1 1 1 0 1 0 1
Basal cell epitheliomas 0 2 4 8 0 0 0 0
Basal cell carcinomas 0 0 0 2 0 0 0 0
Mammary neoplasms 1 1 2 0 11 29 40 30
Mammary adenocarcinomas 0 0 0 0 0 0 0 6
Lymphoreticular neoplasms 11 1 6 2 11 7 7 6
Pituitary adenomas 4 2 4 1 24 13 23 3
Thyroid adenomas 1 2 12 19 0 1 12 7
Testicular interstitial 42 44 31 0
cell tumours
Table 4. Tumour incidences in Sprague-Dawley rats
males females
contr. low mid high contr. low mid high
No. of animals examined 50 49 50 49 49 50 50 50
Dermal fibroma 2 6 5 7 3 1 2 7
Sebaceous adenoma 1 0 0 6 0 0 0 0
Sebaceous adenocarcinomas 0 0 0 1 0 0 0 0
Mammary neoplasm 2 4 5 1 29 41 45 40
Mammary adenocarcinoma 0 0 1 0 1 0 3 8
Mammary carcinosarcoma 0 0 0 0 0 1 0 0
Lymphoreticular neoplasms 7 6 5 7 6 1 4 0
Pituitary adenomas 10 9 6 3 29 17 17 1
Thyroid adenomas 0 1 1 5 0 0 1 1
Neural astrocytomas 0 0 2 5 1 0 1 0
2.2.4 Reproduction studies
2.2.4.1 Rats
A special study was performed to evaluate the effects on the
reproductive system of male rats fed furazolidone in the diet. Five
male rats (strain not specified)/group received diets containing 330
or 660 mg furazolidone/kg for 14 weeks, 3 male rats/group were given
330 or 660 for 12 weeks (equivalent to 16 or 33 mg/kg bw/day) with a
recovery period of 2 weeks. The control group consisted of 4 rats.
Observations included testes and epididymis weight and macroscopy
and histopathology of the reproductive tract. No dose-related
effects were observed in rats from the low-dose group. Testes weight
was markedly decreased in high-dose rats. This effect was more
pronounced in rats fed furazolidone for 14 weeks than in rats dosed
12 weeks with 2 weeks recovery. Histopathology of the testes of
high-dose rats revealed oedema of the interstitium, and atrophy and
degeneration of the sperm-producing tubules. Occasionally stasis of
previously produced sperm in the epididymus was seen and some
epididymi were devoid of sperm. The effects were still present after
the recovery period (Larson, 1963a).
Because there was some evidence that the testicular damage
observed after feeding of 660 mg furazolidone/kg in the diet was not
completely reversible gross and microscopic examinations were
performed on male rats 5 days and 6 weeks after withdrawal from a
diet containing 660 mg furazolidone/kg. Another 2 males were
retained for 14 weeks, the additional 8 weeks being required to
allow non-treated females to reach breeding age. Each treated male
(and one control male) cohabited with 2 females each. Testes weight
was decreased in 4/6 rats and in 3/6 rats atrophic seminiferous
tubules and abundant intertubular transudate were seen. In 2 of
these male rats the epididimi had sperm stasis. All prostates were
normal. Rats used in the breeding trials showed normal libido and
fecundated females but the litters that were produced had slightly
fewer young than those from the litters sired by the control male
(Larson 1963b).
In a three-generation reproduction study groups of rats were
fed diets containing 0, 30 or 100 mg furazolidone/kg. The F0
generation was maintained on the furazolidone diet and sacrificed
after 2 years. No effects were found on haematology, body weight and
histopathology after 2 years. Decrease in testes weight was
dose-related. Reproductive performance was not affected. Only a
summary of this study was available (Huffman, 1965a).
In a three-generation reproduction study groups of
Sprague-Dawley rats (20/sex) were used. Female rats only were
administered 500 mg furazolidone/kg feed. Because of growth
depression the dose level was lowered to 400 mg/kg on day 16 and to
250 mg/kg on day 37. Three matings per generation were performed.
The pups from the first litter were sacrificed 21 days after birth.
Pups from the second litter were used for the second generation and
pups from the third litter were used for teratological examination.
No treatment-related effects were found on reproduction parameters
resulting in a NOEL equivalent to 12.5 mg/kg bw/day (Borgmann &
Prytherch, 1964b; Borgman & Prytherch, 1966).
2.2.4.2 Chickens
Groups of New Hampshire chickens were fed diets containing 55,
110 or 220 mg furazolidone/kg feed for 4 to 16 weeks. No effects
were observed on body weight and egg production, hatchability of
eggs and shell quality. Thyroid and adrenal weight were both reduced
(Francis & Shaffner, 1956).
2.2.4.3 Pigs
In a limited study a group of purebred Hampshire and Duroc pigs
(9 sows and 12 gilts) were fed a diet containing 300 mg/kg
furazolidone for two weeks at breeding and 150 mg/kg for three weeks
at farrowing. Another group was maintained as an untreated control
group. There was no difference in the number of pigs born, number of
pigs weaned or weight gain of the pigs. However, pup weight at
weaning was slightly higher in the treated group (Hughes & McMinn,
1963).
2.2.4.4. Goats
Male Nubian goats received furazolidone suspended in distilled
water at a dose of 10 or 40 mg/kg bw. The effect on semen morphology
and biochemistry was studied. At both doses ejaculate volume, the
number of motile spermatozoa per ejaculate and the number of live
spermatozoa per ejaculate and semen fructose concentration were
significantly decreased (Mustafa et al., 1987).
2.2.5 Special studies on embryotoxicity and/or teratogenicity
2.2.5.1 Mice
Groups of Albino C strain mice were administered furazolidone
at doses up to 2 g/kg during pregnancy varying from day 1-11.
Abortions or fetal deaths occurred in all mice treated with doses
> 1 g/kg when treatment started before day 8 of pregnancy.
However when treatment started at day 10 abortions or fetal death
occurred only in 2/9 mice. Litterweight was dose-relatedly decreased
but no congenital abnormalities were observed (Jackson & Robson,
1957).
2.2.5.2 Rats
Groups of 3 Donryu male rats were fed 0 or 100 mg
furazolidone/kg bw for 7 days, and on day 8 the rats were
sacrificed. Relative testes weights were slightly decreased. The
number of mature spermatozoa was decreased and degenerative changes
in the seminiferous tubules (sloughing of spermatocytes and
multi-nucleated cells) were observed (Miyaji et al., 1964).
2.2.5.3 Rabbits
Groups of two rabbits were given an intra-amniotic injection of
1, 2 or 2.5 mg furazolidone on day 14 or 15 of pregnancy. A
laparatomy was performed 5 days after the injection to determine the
effect on pregnancy. Pregnancy interruption was observed at 2.0 and
2.5 mg furazolidone (Jackson & Robson, 1957).
Groups of 10 pregnant New Zeeland white rabbits were orally
administered 30 mg furazolidone (purity not given)/kg bw/day on days
7-15 of pregnancy. On day 29 of pregnancy the dams were sacrificed
and the fetuses were delivered by caesarean section. Feed
consumption and body-weight gain were significantly decreased. No
embryotoxicity or teratogenicity was observed (Borgmann & Prytherch,
1964a).
2.2.5.4 Chickens
Fifty percent mortality was observed in 10-day old chicken
embryos after treatment with 15 mg furazolidone in the inner shell
membrane, 4.2 mg in the allantoic cavity or after treatment with 0.7
mg in the yolk sac (Gentry, 1957).
2.2.5.2 Special studies on genotoxicity
The results of in vitro and in vivo genotoxicity studies
and of genotoxicity studies on metabolites of furazolidone and bound
residues are summarized in Tables 5, 6, and 7, respectively.
Table 5. Results of in vitro genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Ames testa S. typhimurium 0.01-1.0 µg/pl ? positiveb Jagannath et al.,
TA1538, TA98 (1.0 µg/pl slightly 1981g
and TA100 toxic)
TA1535, TA1537 negativeb
Ames testa S. typhimurium* 0.1, 0.5, 1.0 >99% positive Ni, et al., 1987
TA98, TA98NR or 2.5 µg/pl
TA98/1,8-DNP6 2.5 µg/pl toxic
Ames testa S. typhimurium 0.01-0.3 µg/pl 99% positiveb Crebelli et al.,
TA100 >0.3 µg/pl toxic 1982; Carere et
al., 1982
Spot test Escherichia coli 50 µg/plc positive McCalla &
WP2uvrA Voutsinos, 1974
Reverse Euglenia gracilis 25, 50 and 100 ? positive Ebringer et al.,
mutation assay µg/mlc 1976
Forward Vibrio cholerae up to 12 µg/mlc ? positive Chatterjee et al.,
mutation assay OGAWA 154 1983
Prophage Escherichia coli ? ? positive Chatterjee et al.,
induction assay K12 GY5027 1983
Gene conversion S. cerevisiae D4 no details ? positive Voogd et al.,
assay 1982 as cited in:
VanMiert et al.,
1984
Gene mutation Chinese hamster up to 125 µg/ml >99% positive Gao et al., 1989
assaya ovary cells >100 µg toxic
Table 5 cont.
Test system Test object Concentration Purity Results Reference
HGPRT test Mouse L5178Y no details ? positive Voogd et al.,
lymphoma cells 1982
SOS function and Escherichia coli 0.8 and 8.0 µMc ? positive Bryant &
mutation assay WP2 McCalla, 1980
SOS function Escherichia coli 0.1, 0.2, 0.5 ? positive Ohta et al., 1984
assay K12 µg/mlc
Sex-linked D. melanogaster 0.5 mM in DMSOc ? positive Blijleven et al.,
recessive 1977
lethal test
Sex-linked D. melanogaster 0.18, 0.44 or ? positive Kramers, 1982
recessive 0.5 mM in DMSOc
lethal test
Chromosome human 10, 33 and 100 99.8% negative Scheres, 1991b
aberration assay lymphocytes µg/ml in DMSOd
33, 100, 180 positivef
and 333 µg/ml in
DMSOe (333 µg/ml
slightly toxic)
Chromosome human 0.2, 2.0 or ? positive Cohen & Sagi,
aberration assay lymphocytes 20.0 µg/mld 1979
20.0 µg/ml toxic
Chromosome human 0.5-100 µMc ? negative Tonomura &
aberration assay lymphocytes Sasaki, 1973
Table 5 cont.
Test system Test object Concentration Purity Results Reference
Chromosome bovine lymphocytes 0.05-500 mg/lg ? positive Queinnec et al.,
aberration assay porcine lymphocytes 0.05-500 mg/lg positive 1975; Babile et
al., 1978
SCE test human 0.2, 2.0 and ? positive Cohen & Sagi
lymphocytes 20 mg/l in DMSO 1979
20 mg/l toxic
Rec assay Escherichia coli ?? ? positive Chatterjee et al.,
K12 rec- and rec+ 1983
DNA repair Escherichia coli 1.0 to 10 µM ? positive Lu et al., 1979
assay WP2 uvrA >5µM toxic
UDS assay human 5-100 µMc ? negative Tonomura &
lymphocytes Sasaki, 1973
UDS assay Fischer 344 0.5-1000 nM/ml ? positive Probst et al.,
rat hepatocytes in DMSOb,c 1981
* nitroreductase-deficient Salmonella typhimurium tester strains.
a both with and without rat liver S9 fraction.
b positive controls yielded positive results.
c no data about toxicity.
d without metabolic activation.
e with metabolic activation.
f the authors concluded that the results were negative.
g no toxicity observed.
Table 6: Results of in vivo genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Micronucleus Swiss CD-1 mice 300 mg/kg bw 99.8% negative Enninga &
test ip in Weterings 1990
methyl-cellulose
(toxic)
Micronucleus Swiss Webster 100 and 500 mg/kg ? equivocalb Paik, 1985
test mice bw orala
a no toxicity observed
b slight induction, not dose-related
2.2.7 Special studies on endocrine toxicity
In a special study various groups of ovariectomized
Sprague-Dawley, Fischer 344 or Long Evans rats and golden hamsters
received single oral doses varying from 50-500 mg furazolidone/kg
bw. After a 2-week recovery period positive dose-related lordosis
(becoming sexually perceptive) behaviour was observed only in
Sprague-Dawley rats which is, according to the authors, indicative
of an elevated concentration of progesterone in the circulation
(King et al., 1970a).
Groups of immature superovulated Sprague-Dawley rats were
orally dosed with 100 or 500 mg furazolidone/kg bw. In all groups
ovulation was initiated about 24 h earlier than the control group.
The same shift in ovulation time was observed in a positive control
group treated with progesterone (King et al., 1970a).
Feeding of 1000 mg furazolidone/kg feed for 30, 60 or 90 days
produced a marked inhibition of the conversion of progesterone into
corticosterone (11-hydroxylation) in adrenals of Sprague-Dawley
females. A considerably lesser effect was found in Fischer 344
females. This effect could be overcome by addition of NADPH,
indicating an inhibitory effect of furazolidone on the NADPH
generating system (King et al., 1971b).
Table 7. Results of genotoxicity assays on metabolites of furazolidone and
bound residues
Test system Test object Concentration Substance Results Reference
In vitro
Ames testa S. typhimurium 100-5000 µg/plb,c Md negative Scheres, 1991a
TA100, TA98
Ames testa S. typhimurium urine from rats - positivee Crebelli et al.,
TA100 treated with 10, 1982; Carere et
50, 100 mg/kg bw al., 1982
Ames testa S. typhimurium up to 5 µg/pl M1f negativee,g Jagannath &
TA1535,TA1537 Brusick, 1981a; 1981e
Craine, 1981
TA1538, TA98, M2h negativee Jagannath &
TA100 Brusick, 1981b; 1981f
Craine, 1981
M3i negativee Jagannath &
Brusick 1981c; 1981d
Craine, 1981
Ames test a S. typhimurium 0.1 mg/pl M4j negative Vroomen et al.,
TA100, TA98 in DMSO 1987a
Ames test S. typhimurium upto 5 µg/pl GSFk negative Hoogenboom,
TA100 in DMSO MSFl negative 1991a
Reverse Mycobacterium 1000 µg/ml 5-NFAm positive Ebringer et al.,
mutation assay phlei 1976
Euglena gracilis 2.5 and 7.5 5-NFAm positive Ebringer et al.,
µg/ml 1976
DNA repair test rat hepatocytes 10-3M, 10-5Mn Mo negative Mori et al.,
mouse hepatocytes 1988
Table 7 (continued)
a both with and without rat liver S9 fraction
b positive controls yielded positive results
c no toxicity observed
d 3-amino-oxazolidinon-2
e negative control yielded negative results
f whole liver powder from pigs treated with 5 mg furazolidone/kg bw for 5 days
g substances from untreated pigs yielded negative results
h urine isolates from pigs treated with 5 mg furazolidone/kg bw for 5 days
i soluble liver isolate from pigs treated with 5 mg furazolidone/kg bw for 5 days
j 3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone.
k glutathione conjugate of cyano metabolite
l mercaptoethanol conjugate of cyano metabolite
m 5-nitro-2-furaldehyde
n no data about toxicity
o 2-hydroxyethylhydrazine.
Oral administration of 100 or 500 mg furazolidone/kg bw to
mature female rats produced a significant decrease in serum
prolactin during the morning proestrus, but not at afternoon
proestrus. Administration of 500 mg/kg bw during the morning estrus
resulted in a slight decrease in serum prolactin levels (Morrison
et al., 1973).
In a limited experiment the effect of furazolidone treatment on
the tumourigenicity of estrone was studied. Furazolidone treatment
(1000 mg/kg feed to female Sprague-Dawley and Fischer 344 rats) plus
estrone resulted in the development of more mammary masses than with
estrone alone. However, the administration of estrone did not result
in an increased incidence of mammary masses (Morrison et al.,
1972).
Furazolidone (500 or 1000 mg/kg feed) was fed to
adrenalectomized and sham-operated male rats for 30 days, after
which the adrenals and tests were examined. Adrenalectomy did not
alter the testicular atrophy produced by furazolidone. At 1000 mg/kg
feed tubular degeneration in three out of six animals was seen
(Morrison et al., 1974a).
A diet containing 500, 750 or 1000 mg/furazolidone/kg
administered to female rats for 30 days resulted in a significant
increase in serum testosterone at the highest dose (Morrison et
al., 1974b).
In a pilot study rats were administered a single oral dose of
250 or 500 mg/kg bw furazolidone in 0.5% aqueous CMC. At 30 min, 1,
2, 4, 8 and 24 h after administration blood samples were taken for
RIA of prolactin and corticosterone. Furazolidone increased in a
dose-related manner, the corticosterone concentration up to 2 h
after administration, with a return to vehicle control values by 24
h. No effect on plasma prolactin time was observed (Meites, 1984).
Male mature chickens were fed a diet containing 400 or 800 mg
furazolidone/kg feed for 10 days. Testes weight was decreased in
both groups. Treatment with 800 mg/kg produced a significant
reduction in the concentration of testosterone in plasma and testes
and some reduction in ascorbic acid, protein and cholesterol
concentration in the testes. Bolus administration of doses of 40 or
80 mg/kg bw for five days caused the same effect. The size of the
testes, wattles and combs were significantly reduced. Monoamine
oxidase activity in the testes was significantly reduced by
furazolidone. In all the treated birds testicular concentrations of
5-hydroxytryptamine were significantly raised, except in those fed
400 mg furazolidone/kg for 10 days (Ali et al., 1984).
Groups of large white female turkeys were orally dosed with 0,
7.5, 15 or 30 mg furazolidone/kg bw for 7 days in gelatin capsules.
The number of eggs produced by each bird was recorded daily 2 weeks
before, during and 4 weeks after the end of treatment. The
concentrations of luteinizing hormone (LH) and prolactin (PRL) were
analyzed by RIA in plasma samples taken on day -7, 0, +7 and +14. At
the end of the treatment period a dose-related decrease was observed
in egg production and LH concentration (significantly in the mid-
and the high-dose group). No effects were observed on PRL
concentration. After withdrawal only the egg production started to
recover but was still below normal 4 weeks after cessation of
treatment (Ali et al., 1987a; 1988).
When turkeys given 15 or 30 mg furazolidone/kg bw were injected
intramuscularly with 5 µg/kg luteinizing hormone-releasing hormone
(LHRH), no effect on LHRH-induced LH release was observed (Ali et
al., 1987a; 1988).
Groups of male turkeys were orally treated with 0, 1, 2.5, 5 or
20 mg furazolidone/kg bw/day for 14 days. Plasma was analyzed for
LH, testosterone (T) and PRL before, during and after treatment. At
the highest dose the concentrations of LH and T were significantly
decreased, and a tendency towards a decrease was observed at 5 mg/kg
bw/day. At histopathology a decrease in spermatocyte production as
well as corrugation of sperm cell nuclear envelopes and distension
of endoplastic reticulum of elongated spermatides were observed in
the 20 mg/kg bw/day group (Ali et al., 1987b).
Furazolidone administered as a bolus dose (5-500 mg/kg bw) to
male T-line chickens produced a decrease in the amount of
corticosterone in plasma (Bartlet et al., 1990).
In another experiment male T-line and J-line chickens were
given a bolus dose of furazolidone (200 mg/kg bw) which resulted in
a decrease in aspartate transaminase activity in the adrenal gland
and a general disorganization in the structure of the adrenal
cortical cells, leading to atrophy of the adrenal cortical glands
(Bartlet & Khan, 1990).
A concentration of 1 µM furazolidone inhibited significantly
the release of aldosterone from porcine adrenal cells in vitro.
Almost complete inhibition was observed at 100 µM (van den Dungen
et al., 1991).
2.2.8 Special studies on skin and eye irritation
A dose of 500 mg furazolidone (purity 100%) moistened with 0.5
ml of 0.5% saline caused slight erythema in 4/6, 3/6 and 1/6 rabbits
after 0.5, 24, and 48 h respectively, when applied under
semi-occlusive conditions to the clipped intact skin of 3 male and 3
female New Zeeland white rabbits (Mitchell et al., 1990c).
Nine New Zeeland white rabbits received an installation of 30
mg furazolidone (purity 100%) into the conjunctival sac of the right
eye, and 3/9 eyes were washed 20 seconds after treatment. After 24 h
slight conjuctival irritation was observed in both washed and
unwashed eyes. Corneal ulcerations were observed in 2/6 unwashed
eyes only. All eyes were normal at day 7 (Mitchell et al., 1990d).
2.3 Observations in humans
Furazolidone administered to patients with essential
hypertension (no details provided) produced marked supersensitivity
to tyramine and amphetamine, inhibition of intestinal MAO and
increased urinary excretion of tryptamine. Continued administration
produced a cumulative inhibition of MAO (Pettinger, et al., 1968)
Adverse reactions (gastrointestinal 8%, neurological 1.34%,
systemic, 0.56% and dermatological reactions 0.54%) to furazolidone
treatment were observed in 864/10443 patients (8.3%) treated with a
therapeutic dose of < 5 to 7 mg/kg bw/day (Altamirano & Bondani,
1989).
A 43-year old man with a contact allergy to furazolidone was
patch tested to nitrofurans with completely negative results. The
man had a positive patch test reaction to 2% furazolidone both in
PEG-400 and alcohol. Control tests were negative in 25 dermatitis
patients (DeGroot & Conemans, 1990).
3. COMMENTS
The Committee considered data from pharmacodynamic,
pharmacokinetic, metabolism, acute and short-term toxicity,
carcinogenicity, genotoxicity, reproductive, and teratogenicity
studies as well as special studies on endocrine function and some
clinical studies in humans.
The distribution, excretion, and biotransformation of
radiolabelled furazolidone were studied in rats, chickens, pigs, and
humans. After oral administration, furazolidone was rapidly absorbed
and the radioactivity was widely distributed, the highest levels
being found in liver, kidney, fat, and muscle. It was rapidly
metabolized and excreted predominantly in urine. In chicken and
human urine, only trace amounts of unchanged furazolidone could be
detected, and of the large number of metabolites found only some
were identified. In rat and pig urine, the common metabolite
appeared to be the open chain cyanometabolite
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone. The Committee noted
that quantitative information on metabolites was lacking. In pigs, a
substantial portion of the metabolites was bound to macromolecules,
and it appeared that approximately 15-40% of this bound fraction was
bioavailable. However, the Committee questioned whether valid
extraction procedures had been used to isolate these bound
metabolites.
In acute oral toxicity studies in mice and rats furazolidone
was slightly toxic; the LD50 values were of the order of 1100 and
1500 mg/kg bw, respectively.
No NOEL could be established from short-term studies performed
with rats and dogs. Rats receiving furazolidone at doses in the
range 0.5-50 mg/kg bw/day showed hypertrophy of liver cells.
Palpable mammary tumours and a decrease in body weight gain were
observed at 50 mg/kg bw/day. In dogs, dose levels of 5-25 mg/kg
bw/day led to neurological symptoms and histological changes in the
basal ganglia, together with testicular degeneration. It was noted
that the available information was deficient by current standards
and poorly reported.
Two three-generation reproduction studies were performed in
rats. In one study rats were exposed to furazolidone at
concentrations up to 100 mg/kg in feed. In the other study only
female rats were treated with diets containing 500 mg/kg, but this
concentration was gradually reduced to 250 mg/kg in order to avoid
the observed growth depression. No effects on reproductive
performance were observed in either study. The NOEL was equivalent
to 12.5 mg/kg bw/day.
In a special study designed to evaluate the effects on the male
reproductive system, rats exposed to a dietary furazolidone
concentration equivalent to 33 mg/kg bw/day exhibited testicular
degeneration. At 16 mg/kg bw/day no effects were observed.
Neither embryotoxicity nor teratogenicity was observed in
rabbits after oral administration of furazolidone at a dose of 30
mg/kg bw/day.
A carcinogenicity study was conducted in Swiss MBR/ICR mice,
which received a diet containing concentrations of furazolidone
equal to average daily doses of 12, 24, or 47 mg/kg bw/day for 13
months, followed by a control diet for 10 months. In the mid- and
high-dose groups, a significant increase in the incidence of
bronchial adenocarcinomas was observed in both sexes, and the
incidence of lymphosarcomas was significantly increased in male
mice.
In two long-term toxicity/carcinogenicity studies, furazolidone
was administered in the diet to Fischer 344 and Sprague-Dawley rats
at concentrations equivalent to daily doses of 12.5, 25, or 50 mg/kg
bw/day for 20 months. In Fischer 344 rats, a significant increase in
the incidence of mammary gland adenocarcinomas was observed in
females in the high-dose group. In addition, an increase in the
incidence of sebaceous gland adenomas and thyroid adenomas was
observed in both sexes at 25 and 50 mg/kg bw/day and of basal cell
epithelioma and carcinoma in males of the high-dose group. In the
high-dose group of Sprague-Dawley rats, significantly increased
incidences were reported for mammary adenocarcinomas in females and
for neural astrocytomas in males. In both strains of rat, female
animals showed a significant increase in the incidence of mammary
neoplasms (benign and malignant combined) at all dose levels, but
without a dose-response relationship.
Furazolidone has been tested in a wide variety of genotoxicity
studies. Positive findings were recorded in bacterial assays with
and without metabolic activation, in the sex-linked recessive lethal
test in Drosophila melanogaster, in a gene mutation assay with
mammalian cells in vitro, in a sister chromatid exchange test, and
in two DNA-repair tests. Positive as well as negative results were
obtained in chromosome aberration assays with mammalian cells in
vitro, and in tests for unscheduled DNA synthesis. One mouse
micronucleus test was negative, while another gave equivocal
results.
The majority of in vitro genotoxicity tests with postulated
metabolites gave negative results, however, nitrofuraldehyde and
urine from furazolidone-treated rats gave positive results. It was
concluded that furazolidone was genotoxic in vitro.
Several studies were performed on the endocrine effects of
furazolidone. Furazolidone inhibited the conversion of progesterone
into corticosterone in adrenal cells both in vivo and in vitro.
It has been hypothesized that disturbances of steroidogenesis
constituted the underlying mechanism for the increased incidence of
tumours caused by furazolidone. The Committee noted that it was
unlikely that the such a mechanism could account for the increase in
neural astrocytomas and uncommon skin tumours in rats. With respect
to the occurrence of mammary tumours, no information was available
on the effect of furazolidone on plasma progesterone concentrations
and no consistent effects on plasma prolactin concentrations were
observed. The Committee therefore concluded that no support had been
provided for the hypothesized mechanism.
Furazolidone caused reversible inhibition of monoamine oxidase
(MAO) activity in pig hepatocytes in vitro and in liver and brain
tissue of rats following in vivo administration. Irreversible MAO
inhibition both in vitro and in vivo was observed for the
postulated metabolites amino-oxazolidone and hydroxyethyl-hydrazine.
4. EVALUATION
On the basis of the positive effects of furazolidone in
genotoxicity tests in vitro and the increased incidence of
malignant tumours in mice and rats, the Committee concluded that
furazolidone was a genotoxic carcinogen. Since the drug is rapidly
and extensively metabolized, the Committee also considered
information on metabolites of furazolidone. Although a large number
of postulated metabolites produced negative results in genotoxicity
tests, it was noted that only a few of these had been either
identified or quantified in rats and pigs. Furthermore, the
Committee concluded that insufficient data were available on the
nature and toxic potential of compounds released from the bound
residues.
Because of the genotoxic and carcinogenic nature of
furazolidone and the above-mentioned deficiencies with respect to
the data on the metabolites, the Committee was unable to establish
an ADI.
Before considering the compound again, the Committee would wish
to have detailed information on the nature, quantity and toxicity of
the metabolites of furazolidone, including the bound residues.
5. REFERENCES
AISO, K., KANISAWA, M., YAMAOKA, H., TATSUMI, K. & AIKAWA, N.
(1962). Systematic studies on the toxicity of nitrofuran derivatives
(3): Macroscopical and histological examination of rats fed on diet
with furazolidone and 5-(5-nitro-2-vinylfuran)-1, 3,
4-oxadiazoline-2-one for one year. Chiba Daigaku Fuhai Kenkyusho
Hokoku, 15: 21-33. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H. & BARTLET, A.L. (1982). Inhibition of monoamine oxidase in
chicken and ducklings by a microbial metabolite of furazolidone.
Quarterly J. Exp. Physiol., 67(1): 69-79.
ALI, B.H. (1983). The anti-diamine oxidase effect of furazolidone in
rabbits. Comp. Biochem. Physiol., 74C(1): 109-110.
ALI, B.H., HOMEIDA, A.M. & KNIFTON, A. (1984). The effect of
furazolidone on fertility of male chickens. Comp. Biochem.
Physiol., 78C(1): 43-47.
ALI, B.H., SILSBY, J.L. & EL HALAWANI, M.E. (1987a). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
British Poultry Science, 28: 613-621.
ALI, B.H., SILSBY, J.L., LOSETH, K.J., CRABO, B. & EL HALAWANI, M.E.
(1987b). Some effects of furazolidone on the testes and plasma
testosterone, luteinising hormone and prolactin concentrations in
mature male turkeys d.d. 17 June 1987. University of Minnesota. St.
Paul, MN 55108, USA. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL), levels in young turkeys. Gen. Pharmacol., 19(1):
91-95.
ALTAMIRANO, A. & BONDANI, A. (1989). Adverse reactions to
furazolidone and other drugs. Scand. J. Gastroenterol.,
24(suppl.169): 70-80.
BABILE, R., QUEINNEC, G., BERLAND, H.M. & DARRE, R. (1978).
Structure chromosomique des lymphocytes du porc et additifs
alimentaires. C. Soc. Biol., 172:, 546-553.
BARTLET, A.L & KHAN, F.H. (1990). Effects of nitrofurans on adrenal
cortical tissue in chickens. J. Vet. Pharmacol. Therap., 13:
206-216.
BARTLET, A.L., HARVEY, S. & KLANDORF, H. (1990). Contrasting effects
of nitrofurans on plasma corticosterone in chickens following
administration as a bolus or diet additive. J. Vet. Pharmacol.
Therap., 13:, 261-269.
BLIJLEVEN, W.G.H., KORTSELIUS, M.J.H. & KRAMERS, P.G.N. (1977).
Mutagenicity testing of H-193, AF-2 and furazolidone in Drosophila
melanogaster. Mutat. Res. 56: 95-100.
BORGMANN, A.R., MURTI, G.S., COOLEY, R. & LEVIN, R. (1964).
Ninety-day toxicity studies of experimental formulations containing
furoxone (R) (NF-180) in dogs. (Project no. 475.09.02) Unpublished
final report d.d. 29 June 1964, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964a). The effect of furoxone (R)
on fetal growth and development in the rabbit. Unpublished special
report project number no. 458.9 d.d. 28 August 1964 from Pathology
Section, Scientific Department Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964b). The effect of furoxone on
reproduction in rats. Unpublished special report project number no.
460.9 d.d. 3 September 1964 from Pathology Section, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1966). The effect of furoxone on
reproduction in rats. Second and third generation. Unpublished
special report project number no. 460.09 d.d. March, 10, 1966 from
Pathology Section, Scientific Department, The Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BOWMAN, J.S. (1961a). A balance study of furazolidone C14
(methylene and aldehyde-labeled) in male rats. Unpublished report
H-NSC
no. 20-0040-32 from Hazleton, Nuclear Science Corporation,
California. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
BOWMAN, J.S. (1961b). A plateau-elimination study of
furazolidone-C14 (methylene labeled) in male rats. Unpublished
report H-NSC no. 20-0039-32 from Hazleton, Nuclear Science
Corporation, California. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran induced mutagenesis
and error prone repair in Escherichia coli. Chem. Biol. Interact.,
31: 151-166.
BUZARD, J.A., HEOTIS, J.P. & WILLIAMS, C.W. (1960). Chick
distribution studies with C14-(formyl) NF-180. Unpublished interim
report problem 360.3 d.d. 9 December 1960 .Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
BUZARD, J.A., HEOTIS, J.P. & Williams, C.W. (1961). Chick
distribution studies with C14-(methylene) NF-180. Unpublished
interim report problem 360.3 d.d. 15 May 1961. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
CARERE, A., CONTI, L., CREBELLI, R. & MACRI, A. (1982). Quantitative
data on the urinary recovery of mutagenicity in furazolidone-treated
rats. Mutat. Res., 97(6), 461-462.
CHATTERJEE, S.N., MAITI, M. & GHOSH, S. (1975). Interaction of
furazolidone with DNA. Biochem. et Biophysica Acta, 402: 161-165.
CHATTERJEE, S.N., BANERJEE, S.K., PAL, A.K. & BASAK. J. (1983). DNA
damage, prophage induction and mutation by furazolidone. Chem.
Biol. Interact., 45: 315-326.
COHEN, M.M. & SAGI, M. (1979). The effect of nitrofurans on mitosis,
chromosome breakage and sister-chromatid exchange in human
peripheral lymphocytes. Mutat. Res., 59(1): 139-142.
CRAINE, E.M. & RAY, W.H. (1972). Metabolites of furazolidone in
urine of chickens. J. Pharmacol. Science, 61(9): 1495-1497.
CRAINE, E.M. (1977). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 77:10
d.d. 28 December 1977 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1978). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 78:12
d.d. 21 April 1978 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1981). Preparation of isolates of furazolidone
metabolites. Unpublished research report, project no. 80191 d.d. 2
March 1981 from Wil Research Laboratories Inc., Research Division,
Cincinnnati, Ohio. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
CREBELLI, R., CARERE, A., FALCONE, E. & MACRI, A. (1982). A study on
the urinary and fecal excretion of furazolidone in rats by means of
mutagenicity assays. Ecotoxicol. Environ. Saf. 6(5): 448-456.
DeGROOT, A.C. & CONEMANS, J.M.H. (1990). Contact allergy to
furazolidone. Contact Dermatitis, 22:, 202-205.
EBRINGER, L., JURASEK, A., KONICEK, J., KONICKOVA, M., LAHITOVA, N.
& TRIBACUK, S. (1976). Mutagenic action of nitrofurans on Euglena
gracilis and Mycobacterium phlei. Antimicrobial Agents and
Chemotherapy, 9(4): 682-689.
ENNINGA, I.C. & WETERINGS, P.J.J.M. (1990). Micronucleus test in
bone marrow cells of the mouse with furazolidone. Unpublished report
(RCC notox project 026122) d.d. April 1990 from RCC/NOTOX, 5231
DD's-Hertogenbosch, the Netherlands. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
FRANCIS, D.W. & SHAFFNER, C.S. (1956). An investigation of the
morphological changes in young chickens and the reproductive
performance of adult chickens fed furazolidone or nitrofurazone.
Poultry Sci., 35: 1371-1381.
FUKUHARA, M. & TAKABATAKE, E. (1977). The effects of nitrofuran
derivatives on hepatic microsomal mixed-function oxidase activity in
rats. Toxicol. Appl. Pharmacol., 42: 571-581.
GAO, N., NI, Y.C., THORNTON-MANNING, J.R., FU, P.P. & HEFLICH, R.H.
(1989). Mutagenicity of nitrofurantoin and furazolidone in Chinese
hamster ovary cell strains. Mutat. Res., 225: 181-187.
GENTRY, R.F. (1957). The toxicity of certain antibiotics and
furazolidone for chicken embryos. Avian Diseases, 2: 77-82.
HALLIDAY, R.P., SUTTON, M.L., SIGLER, F.W. & LEVIN, R. A. (1973a).
Chronic toxicopathological safety study (two years) of NF-180 in
rats. Unpublished final report project no. 475.09-C d.d. 9 November
1973 from Pathological and Toxicology Section, Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1973b). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished interim report # 2 project no. 475.09D d.d. 9
November 1973 part I: Sprague-Dawley evaluation from Pathological
and Toxicology Section, Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974a). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished final report project no. 475.09D d.d. 31 January
part II: Fischer 344 evaluation from Pathological and Toxicology
Section, Norwich Pharmacal Company, Norwich, New York. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974b). Tumorgenesis
evaluation (twenty-three months) of furazolidone (NF-180) in mice,
Unpublished final report project no. 475.09E d.d. 31 January 1974
from Pathological and Toxicology Section, Research and Development
Department, Norwich Pharmacal Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HAWKINS, D.R., ELSOM, L.F., GIRKIN, R. & CHENG, C.F. (1992). The
bioavailability in rats of tissue residues from swine administered
14C-furazolidone for 14 days and subjected to 0-day, 21-day and
45-day withdrawal periods. Unpublished draft report HRC/SMI
125/911478 from Huntingdon Research Centre Ltd., P.O.Box 2,
Huntingdon, Cambridgeshire, England. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
HEOTIS, J.P., HERRET, R.J. & WILLIAMS, C.W. (1963). Drug metabolism
studies. Incorporation of C14 from formyl-C14-NF-180 into
natural products. Unpublished special report part I: problem 370.3
d.d. 20 September 1963 from Biological Research Division, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
HOOGENBOOM, L.A.P. (1991a). The use of pig hepatocytes to study the
role of glutathione on the biotransformation and toxicity of
furazolidone. In: The uses of pig hepatocytes for biotransformation
and toxicity studies Thesis, Agricultural University of Wageningen,
The Netherlands. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991b). The use of pig hepatocytes for
biotransformation studies of veterinary drugs. In: The use of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991c). The use of pig hepatocytes to study the
inhibition of monoamine oxidase by furazolidone. In: The uses of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P., Van KAMMEN, M., BERGHMANS, M.C.J. & KUIPER, H.A.
(1991). Detection of protein bound metabolites of furazolidone.
Proceedings of the Euroresidue Conference in Noordwijkerhout, The
Netherlands. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965a). Two-year chronic toxicity study and a
three-generation reproductive study in rats. Unpublished report
no. 65:15/H-1R from Hess & Clark, Research Department, Ashland,
Ohio. Summary submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965b). Two-year chronic toxicity test with
furazolidone in dogs. Unpublished report no. 65:16/H-1D) d.d. 30
July 1965 from Hess & Clark, Research Department, Ashland, Ohio.
Abstract submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HUGHES, D.L. & McMINN, C.S. (1963). The effect of furazolidone on
reproduction and enteric infections of swine. Unpublished final
report (Ex 62-12-18a2) Norwich Pharmacal Company, Norwich, USA.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
JACKSON, D. & ROBSON, J.M. (1957). The action of furazolidone on
pregnancy. J. Endocrin., 15: 355-359.
JAGANNATH, D.R. & BRUSICK, D.J. (1981a). Mutagenicity evaluation of
WIL-80191-10-4 dry liver, control in the Ames Salmonella/microsome
plate test. Unpublished final report d.d. March 1981 LBI project
from Litton Bionetics, Inc. Kensington, Maryland 207953. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981b). Mutagenicity evaluation of
WIL-80191-11-2 urine isolate; control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981c). Mutagenicity evaluation of
WIL-80191-10-1 liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981d). Mutagenicity evaluation of
WIL-80191-10-2 dry liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981e). Mutagenicity evaluation of
WIL-80191-10-3 liver isolate control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981f). Mutagenicity evaluation of
WIL-80191-11-1 urine isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981g). Mutagenicity evaluation of
Furazolidone WIL-80191-11-3 in the Ames Salmonella/microsome plate
test. Unpublished final report LBI project from Litton Bionetics,
Inc. Kensington, Maryland 207953. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CURRIE, G.N. HARRINGTON, C. & WELCH, C.A. (1970a).
Effects of nitrofurans on steroidogenesis and mammary hyperplasia
(NF-180 Rx 30309) Unpublished annual report project no. 569.09
d.d.16 June 1970 from Research and Development Department, The
Norwich Pharmacal Comp., Norwich, New York. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CHRISTENSEN, E.F., McLAUGHLIN, J.H., LEVIN, R.A. &
SIGLER, F.W. (1971a). Chronic (2 year) toxicopathologic study of
NF-180 in dogs. Unpublished final report project no. 475.09B d.d. 12
November 1971 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Abstract submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
KING, C.D., CURRIE, G.N., HARRINGTON, C. & WELCH, C. (1971b).
Nitrofurans and steroidogenesis. Unpublished interim report no. 5
project no. 596.09 d.d. 21 December 1971 from Pathological and
Toxicology Section, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L. & LEVIN, R.A. (1972a). Chronic
toxicopathological safety study (two years) of NF-180 in rats.
Unpublished status report no.1 project no. 475.09c d.d. 18 October
1972 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L., WONG, L.C.K. & LAUGHLIN, P.J. (1972b).
Tumorgenesis evaluation (two years) of NF-180 in Spraque-Dawley and
Fischer 344 rats. Unpublished status report no.1 project no. 475.09D
d.d. 18 0ctober 1972 from Pathological and Toxicology Section,
Research and Development Department, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
KOUBA, R.F. (1979). Analysis of components from urine of pigs
treated with furazolidone-14C-15N or furazolidone-14C.
Unpublished report no.: RFK 79:3 from Hess & Clark, Ashland.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
KRAMERS, P.G. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
LARSON, E.J. (1963a). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12a. Addendum to pathology
research report H-12. Unpublished report d.d. 14 November 1963 from
Research Department Hess & Clark, Ashland Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LARSON, E.J. (1963b). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12 d.d. 18 March 1963 from
Research Department Hess & Clark, Ashland, Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli. Mutation and induction and repair of daughter-strand
gaps in DNA. Mutat. Res., 67: 13-144.
McCALLA, D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res. 16(1): 3-16.
MEITES, J. DENINE, E.P. & OBROSKY, K.W. (1984). Determination of
blood levels of prolactin and corticosterone following oral
administration of furazolidone (NF-180) or EU-557 to male
Sprague-Dawley rats: a pilot study. Unpublished final report project
no. 475.59.59 d.d. 30 March 1984 from Norwich Eaton Pharmaceuticals,
Inc., Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990a).
Acute oral toxicity study of furazolidone in rats. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5884-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990b).
Acute oral toxicity study of furazolidone in mice. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5885-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B. & WESSEL, J.L. (1990c).
Primary dermal irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/Dynamics Inc. project
no. 5886-90, East Millstone, New Jersey. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B., ODDE, E.Y. & WESSEL, J.L.
(1990d). Primary irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/dynamics Inc. project
no. 5887-90, East Millstone, New Jersey. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
MIYAJI, T, MIYAMOTO, M & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testis caused by nitrofuran
compounds. Acta Path. Jap., 14(3): 261-273.
MORI, H., SUGIE, S., YOSHIMI, N., IWATA, H., NISHIKAWA, A.,
MATSUKUBO, K., SHIMIZU, H. & HIRONO, I. (1988). Genotoxicity of a
variety of hydrazine derivatives in the hepatocyte primary culture
DNA repair test using rat and mouse hepatocytes. Jpn. J. Cancer
Res. (Gann), 29: 2145-2156.
MORRISON, J.L., CURRIE, G.N., HARRINGTON, C. & WELCH, C.A. (1972).
The effects of estrone and NF-180 on mammary tumor formation.
Unpublished special report project no. 596.09 d.d. 12 October 1972
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1973). Effects of
NF-180 (acute) on serum prolactin levels. Unpublished final report
project no. 596.03 d.d. 24 October 1973 from Biochemistry Section,
The Norwich Pharmacol Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974a). Effects of
adrenalectomy on NF-180 induced testicular atrophy. Unpublished
final report project no. 475.03-F d.d. 24 May 1974 from Biochemistry
Section, The Norwich Pharmacol Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974b). Effects of
NF-180 treatment on plasma steroids in the female Sprague-Dawley
rat. Unpublished final report project no. 475.03-31 d.d. 18
September 1974 from Biochemistry Section, The Norwich Pharmacol
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MORRISON, J.L. (1976). Identification of NF-682 as a metabolite of
NF-180 in rats. Unpublished report d.d. 2 July 1976 attachment 5
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MUSTAFA, A. I., ALI, B.H. & HASSAN, T. (1987). Semen characteristics
in furazolidone-treated goats. Reprod. Nutr. Develop., 27(1A):
89-94.
NI, Y.-C., HEFLICH, R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98nr
and TA98/1,8-DNP6. Mutat. Res., 192(1): 15-22.
OHTA, T., NAKAMURA, N., MORIYA, M., SHIRASU, Y. & KADA, T. (1984).
The SOS-function-inducing activity of chemical mutagens in
Escherichia coli. Mutat. Res., 131: 101-109.
PAIK, S.G. (1985). Micronucleus induction in mouse bone marrow cells
of some nitrofuran, 5-nitroimidazole and nitrothiazole derivatives
used as trichomonacides in Korea. Environ. Mutag. Carcinogens,
5(2): 61-72.
PALM, D., MAGNUS, U., GROBECKER, H. & JONSSON, J. (1967). Hemmung
der monoami-noxydase durch bakteriostatisch wirksame
nitrofuran-derivative. Naunyn-Schmiedebergs Arch. Pharmak., 256:
281-300.
PAUL, H. (1955). Oral chronic toxicity study on Nf-180 in dogs
(study) Unpublished report d.d. 16 June 1955 from Eaton
Laboratories, Norwich, New York. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
PETTINGER, W.A., SOYANGCO, F.G. & OATES, J.A. (1968). Inhibition of
monoamine oxidase in man by furazolidone. Clinical Pharmacol.
Therapeut., 9(4): 442-447.
PROBST, G.S., McMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K. &
NEAL, S.B. (1981). Chemically-induced unscheduled DNA synthesis in
primary rat hepatocytes cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3: 11-32.
QUEINNEC, G., BABILE, R., DARRE, R., BERLAND, H.M. & ESPINASSE, J.
(1975). Induction of abnormalities in chromosomes (of cattle and
swine) by furazolidone or chloramphicol. Veterinary Bul., 46: 330.
RAY, W.H. (1959). Preliminary metabolism study of position
C14-labelled furazolidone in the white rat. Unpublished report no.
59-3 d.d. 18-09070 from Hess & Clark, Ashland, Ohio. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. (1962). An investigation of the metabolites of
furazolidone in swine urine. Unpublished research report no. 62:3
d.d. 29 May 1962 from Hess & Clark, Ashland, Ohio. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. & HAYES, M. (1963). Evidence that the orange '415'
metabolite of furazolidone in swine is a nitrofuran. Unpublished
research report 63:2 d.d. 25 January 1963 from Hess & Clark,
Ashland, Ohio. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
SCHERES, H.M.E. (1991a). Evaluation of the mutagenic activity of
3-amino-oxazolidinon-2 in the Ames Salmonella/microsome test.
Unpublished report project nr. d.d.15 January 1991 from RCC Notox,
5231 DD's Hertogenbosch. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
SCHERES, H.M.E. (1991b). Evaluation of the ability of furazolidone
to induce chromosome aberrations in cultured peripheral human
lymphocytes. Unpublished report project no. 038385 d.d. 9 January
1991 from Notox RCC, 5231 DD's Hertogenbosch. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
13 October 1966 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
4 January 1976 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
STERN, I.J. HOOLIFIELD, R.D., WILK, S. & BUZARD, J.A. (1967). The
anti-monoamine oxidase effects of furazolidone. J. Pharmacol. Exp.
Therapeut., 156(3): 492-499.
TATSUMI, K. & TAKAHASHI, Y. (1982). Metabolic fate of furazolidone
in rats. Chem. Pharm. Bull. Tokyo, 30(9), 3435-3438.
TATSUMI, K. YAMADA, H., YOSHIMURA, H. & KAWAZOE, Y (1981).
Metabolism of furazolidone by milk xanthine oxidase and rat liver
9000 g supernatant: Formation of a unique nitrofuran metabolite and
an aminofuran derivative. Arch. Biochem. Biophysics, 208(1):
167-174.
TATSUMI, K., NAKABEPPU, H., TAKAHASHI, Y., & KITAMURA, S. (1984).
Metabolism in vivo of furazolidone: evidence for formation of an
open-chain carboxylic acid and aketoglutaric acid from the
nitrofuran in rats. Arch. Biochem. Biophysics, 234(1): 112-116.
TENNENT, D.M. & RAY, W.H. (1971). Metabolism of furazolidone in
swine (355994). Proc. Soc. Exp. Biol. Med., 138(3): 808-810.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan. J. Genetics, 48(4): 291-294.
Van Den DUNGEN, H.M., JAGER, L.P., LUYCKX, N.B. L., De GRAAF, G.J. &
BAARS, A.J. (1991). Adrenal toxicity of carbadox and furazolidone
in vitro. Act. Vet. Scand., 87: 338-340.
VOOGD, C.E., KRAMERS, P.G.N., KNAAP, A.G.A.C., OUD, J.H. & Van WENT,
G.F. (1982). Mutagenicity of feed additives. Postersession RIV,
Bilthoven. as cited in Van MIERT, A.S.J.P.A.M., VROOMEN, L.H.M. &
JAGER, L.P. (1984) Farmacologie en toxicologie van furazolidon en
carbadox: een literatuuronderzoek. Tijdschr. Diergeneeskd.
109(22): 928-932.
VROOMEN, L.H., BERGHMANS, M.C., Van LEEUWEN, P., Van Der STRUIJS,
T.D.B.M, De VRIES, P.H.U. & KUIPER, H.A. (1986). Kinetics of
14C-furazolidone in piglets upon oral administration during 10
days and its interaction with tissue macromolecules. Food Add.
Contamin., 3: 331-346.
VROOMEN, L.H. (1987). Quantitative studies of the metabolism of
furazolidone by rat liver microsomes. Toxicol. in vitro, 1(2):
97-104.
VROOMEN, L.H., BERGHMANS, M.C., HEKMAN, P., HOOGENBOOM, L.A.P. &
KUIPER, H.A. (1987a). The elimination of furazolidone and its
open-chain cyano-derivative from adult swine. Xenobiotica, 17:
1427-1435.
VROOMEN, L.H., GROTEN, J.P., Van MUISWINKEL, K., Van VELDHUIZEN,
P.J. & Van BLADEREN, P.J. (1987b). Identification of a reactive
intermediate of furazolidone formed by swine liver microsomes.
Chem. Biol. Interact., 64(1-2): 167-180.
VROOMEN,L.H., BERGHMANS, M.C., GROTEN, J.P., KOEMAN, J.H. & Van
BLADEREN, P.J. (1988). Reversible interaction of a reactive
intermediate derived from furazolidone with glutathione and protein.
Toxicol. Appl. Pharmacol., 95: 53-60.
VROOMEN, L.H., BERGHMANS, M.C., Van BLADEREN, P.J., GROTEN, J.P.,
WISSINK, C.J. & KUIPER, H.A. (1990). In vivo and in vitro
metabolic studies of furazolidone: A risk evaluation. Drug. Metab.
Rev., 22(6-8): 663-676.
WHITE, A.H. (1989). Absorption, distribution, metabolism and
excretion of furazolidone. Scand. J. Gastroenterol.,
24(suppl. 169): 4-10.
2.1.2.2 In vivo
[(5-Nitrofurfurylidene)hydrazino]acetic acid has been isolated
from urine of rats treated with 20 mg furazolidone/kg bw (Morrison,
1976).
A small part of the radioactivity in rat urine after treatment
with 14C-furazolidone was identified as
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone and
N-(5-acetamido-2-furfurylidene)-3-amino-2-oxazolidone (Tatsumi &
Takahashi, 1982).
Male Wistar rats were given orally 100 mg 14C-furazolidone
(formyl-labelled)/kg bw. Urine was collected and analyzed for
possible metabolites. N-(4-carboxy-2-oxobutylideneamino)-
2-oxazolidone, alpha-ketoglutaric acid,
3-(4-cyano-2-oxobutylidenamino)-2-oxazolidone and
N-(5-acetoamido-2-furfurylidene)-3-amino-2-oxazolidone were
identified (Tatsumi et al., 1984; White 1989).
Colostomized chickens received a single oral dose of 30 mg
furazolidone/kg bw. An average of 7.5% of the dose was excreted in
the urine in 12 h. Four urinary metabolites containing a furan ring,
of which only one was a nitrofuran, were detected. Only traces of
unchanged furazolidone were found (Craine & Ray, 1972).
The metabolites of aldehyde-labelled 14C-furazolidone in pig
urine were investigated. More than thirty radio-labelled metabolites
were found. Three primary metabolites of furazolidone were
identified as 5-nitro-2-furoic acid, an orange-coloured "415"
chromogen and a yellow "415" chromogen, with half-lives of 30, 22
and 19 minutes, respectively. Some metabolites are naturally
occurring chemicals and include a yellow urinary pigment, carbonates
and a number of ninhydrin positive materials which lose
radioactivity when treated with ninhydrin indicating that these
materials are amino acids or conjugates of amino acids (Ray, 1962).
The orange "415" metabolite contains an intact
5-nitro-2-furfural moiety and is the most abundant single
furazolidone-related metabolite found in pig urine (Ray & Hayes,
1963).
Analysis of urine from a pig treated with 14C-furazolidone,
also labelled with 15N in the 5-nitro position, showed 14
metabolites. Eleven of them degraded to two or three degradation
products, which could not be identified. The presence of 15N was
detected in one minor metabolite. The authors concluded that during
the metabolism of furazolidone the nitro group was removed
extensively from the molecule (Kouba, 1979).
The open-chain cyano-derivative of furazolidone,
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone was a minor
metabolite in plasma and tissues of swine with a plasma half-life of
4 h (Figure 1) (Vroomen et al., 1987a).
Four human subjects were given a single dose of 400 mg
furazolidone/day administered in a tablet or a capsule. Using an
HPLC method, 24 h urine samples collected after administration
contained 0.003% to 0.16% unchanged furazolidone. By using a
specific complexation method (detection limit 2 µg/ml) no
furazolidone was detected (White, 1989).
Furazolidone was given to 10 human adults as 2 daily doses of
200 mg each for 21 days. Plasma levels analysed by HPLC (detection
limit 0.002 µg/ml) ranged from trace quantities to 0.489 µg/ml
(White, 1989).
2.1.3 Special studies on bound residues
Muscle tissue was prepared from a piglet receiving
14C-furazolidone for 10 days and sacrificed 2 h after the last
administration. Groups of 2 female Wistar WU rats were fed diets
containing non-extracted muscle tissue (group 1), muscle tissue
obtained after 4 extractions with water (group 2) (no extractions
with organic solvents were carried out) or the water extracts
obtained from muscle tissue after four extraction steps with water
for 4 days (group 3). Amounts of radioactivity in groups 1, 2, and
3, were, respectively, 26, 28 and 38% in urine, 23, 38 and 24%, in
faeces, 5.0, 7.4 and 4.8% in tissues, and 4.2, 5.3 and 3.1% in the
remaining carcass. Total recovery was 62%, 81% and 71% for groups 1,
2 and 3, respectively. The amount of non-extractable fractions
obtained from rat liver and muscle tissues were, respectively, 34
and 27% for group 1, 29% and 39% for group 2 and 32% and 21% for
group 3 (Vroomen, et al., 1990).
From a metabolism study with pig hepatocytes it was shown that
at least 70% of the bound residues still contain the
3-amino-2-oxazolidone side chain of furazolidone releasable upon
mild acid treatment. At least 25% of the bound residues in vivo
were shown to contain a releasable side-chain (day 0 animal)
(Hoogenboom 1991b).
The bioavailability of total and non-extractable
14C-furazolidone-derived radioactivity found in swine tissues at 0
and 45-day withdrawal has been investigated in bile duct cannulated
male rats. The pigs had been treated with 300 mg 14C-furazolidone
(both methylene- and formyl-labelled)/kg feed for 14 days. Zero-day
withdrawal liver was extracted with methanol:water, methanol, ether
and ethylacetate. A total of 44% of the radioactivity was extracted.
Zero-day withdrawal muscle, 45-day withdrawal liver and 45-day
withdrawal muscle were prepared in a similar way with different
extraction methods but no methanol:water extraction was carried out,
in order to prevent solubilization of small protein molecules which
may contain bound radioactivity. From 0-day withdrawal muscle 22% of
the radioactivity was extracted. From 45-day withdrawal liver and
muscle 8.3% and 14% of the radioactivity was extracted,
respectively.
Pelleted freeze dried extracted and non-extracted 0 and 45 day
withdrawal liver and muscle were dosed for 24 h to groups of 4
Sprague-Dawley rats. The rats were pretreated with control liver or
muscle for 24 h. Bile, urine and faeces were collected for up to 72
h. After 72 h the rats were sacrificed and the liver and
gastrointestinal tract were analyzed. The excretion pattern for the
differently-treated rats was similar. Rats treated with 0 and 45 day
withdrawal liver and muscle containing total residue excreted 10-27%
in the urine and 1.5-2.7% in bile, while 7.4-16% was found in
tissues. Rats treated with non-extractable tissue excreted 17-21% in
urine and 1.1-3.0% in bile; 4.8-15% was found in tissues (Hawkins
et al., 1992).
2.1.4 Effects on enzymes and other biochemical parameters
The effect of oral administration of 500 mg furazolidone/kg bw
on the activity of monoamine oxidase (MAO) was studied in rats.
Twenty-four hours after treatment MAO activity in liver and brain
was inhibited by 95%. Enzyme inhibition was detectable in liver 6 h
and in brain 12 h after administration, while maximal inhibition was
observed between 24 and 48 h. MAO activity returned to control
values in liver and brain within 10 days and 2-3 weeks,
respectively. The levels of noradrenaline and serotonin in brain
increased by 60-70%, dopamine in brain by 20% and noradrenaline in
heart by 60% when measured 1 to 3 days after application. In
contrast, adrenomedullary catecholamines were reduced by 30% one and
3 days after administration. Repeated application of furazolidone
elicited a cumulative effect: 15 mg/kg bw on 10 consecutive days was
as effective as a single dose of 90-120 mg furazolidone/kg bw.
Following application of furazolidone (125-500 mg/kg bw) the
sympathomimetic actions of tyramine given intravenously and
intraduodenally were potentiated up to 100-fold. According to the
authors the inhibition of MAO is probably due to a metabolite of
furazolidone that contains a free hydrazine group (Palm et al.,
1967).
After single oral doses of 2-100 mg furazolidone/kg bw a
decrease in rat liver-mitochondrial MAO was observed in about 4 h.
Maximal inhibition occurred in 16-24 h. Enzyme activity returned to
normal after 21 days. Furazolidone in a saturated solution did not
affect rat liver MAO in vitro (Stern et al., 1967).
In chickens fed 400 mg furazolidone/kg feed for 10 days MAO
activity in the brain, heart and alimentary tract was inhibited. No
MAO inhibition in the liver was found. Treatment increased the
amount of 5-hydroxy-tryptamine (5-HT) in the brain and potentiated
the vasodepressor action of tyramine. The amounts of adrenaline,
noradrenaline and the vasodepressor action in the brain were
unaffected by treatment (Ali & Bartlet, 1982).
In ducklings administered 400 mg furazolidone/kg feed for 10
days or 200 mg/kg bw by crop tube MAO activity in the liver was
inhibited. No inhibition was found in other organs (Ali & Bartlet,
1982).
Significant diamine oxidase (DAO) inhibition was observed in
various tissues (plasma, duodenal mucosa, liver, heart and brain) of
rabbits given orally 50 mg furazolidone/kg bw for 5 consecutive
days. Recovery was complete in all organs 14 days after the
withdrawal of furazolidone (Ali, 1983).
MAO inhibition was measured in primary cultures of pig
hepatocytes incubated with furazolidone. A dose-related inhibition
was observed; the effect was completely reversible upon withdrawal
of furazolidone. The proposed but not proven metabolites
ß-hydroxye-thylhydrazine and 3-amino-2-oxazolidone incubated with
the same cells caused an irreversible inhibition of MAO activity
(Hoogenboom, 1991c).
Six hundred mg furazolidone/kg administered in the diet to male
Wistar rats for 7 days did not affect cytochrome P-450
concentrations, but increased absolute cytochrome b 5 levels.
The activity of NADPH-cytochrome c reductase and aminopyrine
N-demethylase was decreased, but that of aniline hydroxylase was
increased (Fukuhara & Takabatake, 1977).
Thirty mg furazolidone/kg bw administered orally to large male
white turkeys produced significant increases in hypothalamic
concentrations of noradrenaline, adrenaline and dopa. One week after
withdrawal the amine concentrations returned to normal (Ali et al.,
1988).
In an in vitro experiment 14C-furazolidone was covalently
bound to rat liver microsomal protein; the binding could be
inhibited by addition of gluthathione or mercaptoethanol.
Furazolidone did not interact with added calf thymus DNA in the
presence of microsomes (Vroomen, 1987).
In an in vitro study using swine liver microsomes it was
shown that the mercaptoethanol conjugate (M1) and the glutathione
conjugate (G1) can bind covalently to microsomal protein.
According to the author this reaction is reversible (Vroomen et
al., 1988). In the case of pig hepatoccytes, a decrease in
GSH-levels did not result in an increased formation of bound
residues. Furthermore, no proof was obtained for the reversibility
of bound residues upon incubation with MSH (Hoogenboom, Personal
communication, 1992).
Furazolidone binds to DNA and possibly renders steric hindrance
to DNA replication, which leads to the inhibition of biosynthesis of
DNA in Vibrio cholerae cells (Chatterjee et al., 1975).
DNA-binding of 14C-furazolidone in piglet tissue varied from
87-382 pmol-equivalents of furazolidone per mg DNA (Vroomen et al.,
1986).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies on furazolidone are
summarized in Table 1.
Table 1. Acute toxicity of furazolidone
Species Sex Route Purity LD50 Reference
(mg/kg bw)
Mouse M&F oral 100% 1110 Mitchell et al., 1990b
Rat M&F oral 100% 1508 Mitchell et al., 1990a
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Male Wistar rats (6/group) were fed diets containing 0, 10, 100
or 200 mg furazolidone (purity not given)/kg in the diet for 13
months (equivalent to 0, 0.5, 5 or 10 mg/kg bw/day). Mortality rates
were 1/6, 2/6, 1/6 and 2/6 for the control, low-, mid- and high-dose
groups, respectively. No effects were observed on food consumption
and body weight. No changes were observed in the number of
erythrocytes and the number of leucocytes in blood samples of 2
rats/group taken at the end of the experiment. A slight increase in
relative liver and spleen weights were observed at the highest dose.
At histopathology a slight hyperthrophy of the liver cells was
observed at all dose levels. No effects were observed on kidney,
spleen, heart and testis (Aiso et al., 1962).
The effects of feeding various nitrofurans was studied.
Thirty-five female Holtzman weanling rats were administered 1000 mg
furazolidone (purity not given)/kg feed for 45 weeks (equivalent to
50 mg/kg bw/day). A matched control group was used. The rats were
maintained on a control diet for an additional 8 weeks. Observations
included clinical signs, feed consumption, feed efficiency and body
weight. Ten rats with palpable tumours were necropsied at week 42
and the remaining rats at termination of the experiment. At
termination mortality was significantly increased as compared to the
control group. Feed consumption, feed efficiency and body-weight
gain were significantly decreased in treated rats. Treated rats had
significantly more palpable mammary tumours from week 35 onwards
than the control rats. This was a poorly-reported study and no
detailed histopathology is available (Siedler & Searfoss, 1966).
In another experiment group of rats (Carworth, 20/sex/group)
received diets with various nitrofurans for 45 weeks followed by a
7-week recovery period. An untreated control group was used.
Furazolidone was fed at a rate of 1000 mg/kg feed (equivalent to 50
mg/kg bw/day). Observations included body weight, feed consumption,
feed efficiency and pathology. After five weeks on treatment and at
termination both male and female rats showed a significant decrease
in body-weight gain, feed consumption and feed efficiency. A
significantly increased incidence of palpable mammary tumours was
observed in female rats. At microscopy most tumours were found to be
adenomas, fibromas, and fibroadenomas with or without cyst
formation. Two of the 72 tumours observed were adenocarcinomas. This
was a poorly-reported study (Siedler & Searfoss, 1967).
2.2.2.2 Dogs
In a very limited study groups of 2 male and 2 female beagle
dogs were given micronized furazolidone by gavage at doses of 5, 11
or 23 mg/kg bw/day for 90 days. Another group of 1 male and 1 female
beagle dogs received 23 mg/kg bw/day furazolidone (as crystals) in
gelatin capsules for 88 days. Some neurological signs and testicular
degeneration in the 11 and 23 mg/kg bw/day dose groups were seen.
Most dogs suffered from pneumonia (Borgmann et al., 1964).
Groups of beagle dogs were orally administered furazolidone
(purity not given) in gelatin capsules. Four males and 4 females
received 7.5 mg/kg bw/day for 6 months. Two males were given 25
mg/kg bw/day for 6 months and 6 male and 4 female dogs received this
dose for periods varying from 28-118 days during 6 months of the
study. The highest-dose group consisted of 3 males and 2 females
given 50 mg/kg bw/day for periods varying from 16-37 days during 6
months of the study. The number of dogs used for controls were 12
(for 126 days) and 10 (for 6 months). No effects were observed on
clinical signs, haematology, blood biochemistry, urinalysis, organ
weight and macroscopy. Body weight was decreased in the mid- and
high-dose groups. In all treated dogs neurological symptoms and
histopathological changes in the region of the basal ganglia,
decreased sperm and tubular testicular degeneration were reported.
The authors concluded that the neurological effects as well as the
testicular changes were reversible. This was a poorly-reported study
that was not performed according to current standards (Paul, 1955).
Three groups of 2 male and 2 female beagle dogs were fed diets
containing 0, 30 or 100 mg furazolidone/kg for 2 years (equivalent
to 0.8 or 2.5 mg/kg bw/day). No effects were observed on body
weight, haematology, blood biochemistry, urinalysis, organ weight
and histopathology. Only an abstract of this limited study was
available (Huffman, 1965b).
Groups of beagle dogs (ARC, 4/sex/group) were orally
administered 0, 1, 5 or 15 mg/kg bw/day furazolidone (purity not
given) for 2 years. Additionally, 2 male dogs per group were
included to assess effects on semen quality. Two groups of 5 male
and 5 female beagle dogs, raised at different kennels (ARC + HRA)
were included to further assess cataractogenic properties of
furazolidone at the 15 mg/kg bw/day dose level. Observations
included clinical signs, body weight, ophthalmoscopy, neurological
examinations, recording of ECGs, haematology, clinical chemistry and
urinalysis, macroscopy and histopathology.
Because neurological symptoms were observed in 5/8 high-dose
dogs and 2 high-dose dogs from the semen evaluation study, these
dogs were discontinued from the study. Cataracts were observed in
3/5 male and 5/5 female ARC dogs and in 2/5 male and 1/5 female HRA
dogs. Decreased sperm motility and abnormal sperm were observed in
the mid- and high-dose groups. Increased relative kidney weight was
observed in high-dose females and decreased testes weight in mid-
and high-dose males. Only abstract was available (King et al.,
1971a).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of Swiss MBR/ICR mice (50/sex/group) were fed diets
containing 0, 75, 150 or 300 mg/kg furazolidone (purity not given)
for 13 months (equal to average daily doses of 12, 24 or
47 mg/kg bw). All mice were maintained on normal diet for 10
additional months. During the treatment period as well as during the
post-treatment period, no substance-related effects were observed on
feed consumption and body weight. Survival was decreased in mid- and
high-dose females at the end of the treatment period and in
high-dose males and mid- and high-dose females at 23 months. At
histopathology the incidence of bronchial adenocarcinomas was
significanly increased in the mid- and high-dose groups in both
sexes (incidences for males: 13/49, 19/48, 26/50 and 37/50 and for
females: 15/50, 18/50, 20/47 and 30/48 at the control, low-, mid-
and high-doses, respectively). The incidence of lymphosarcomas was
significantly increased in mid- and high-dose males (incidences:
1/49, 7/48, 10/50 and 10/50 for the control, low-, mid- and
high-doses, respectively) (Halliday et al., 1974b).
2.2.3.2 Rats
Groups of Sprague-Dawley rats (35/sex/group) were fed diets
containing furazolidone for 2 years. The actual average consumption
over 2 years was 0, 0.7, 4 or 10 mg/kg bw/day for males and 0, 0.8,
4.3 or 14 mg/kg bw/day for females. Additional groups (5/sex) were
maintained and killed after 1 year. No effects were observed on
clinical signs, body weight, feed consumption, blood biochemistry,
urinalysis and sternal bone marrow sections. Survival tended to
decrease with dose in females (26, 22, 23 and 15 surviving after two
years for control, low-, mid- and high-dose groups, respectively).
At termination mid- and high-dose female rats showed dose-related
decreased erythrocyte, haemoglobin and haematocrit counts and an
increased neutrophil count. Females from the highest-dose group
showed an increased neutrophil/lymphocyte ratio. Relative liver
weight was increased in high-dose males. At histopathology male rats
from the highest dose group showed an increase in parathyroid
hyperplasia without 'renal ricketts'. In male and female rats an
increase in adrenal cortical hyperplasia was observed at the highest
dose. High-dose females showed an increase in thyroid atrophy.
Females exhibited increased mammary tumour incidences as shown in
Table 2. The authors concluded that the mean onset time of mammary
neoplasms was approximately 2 months earlier in the mid- and
high-dose females than in the other groups (King et al., 1972a;
Halliday et al., 1973a).
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 250, 500 or 1000 mg/kg furazolidone (purity not given)
for 20 months (equivalent to 12.5, 25 or 50 mg/kg bw/day). The
surviving rats were maintained on control diets for at least 4
months or until 90% of the rats had died. Observations included
clinical signs, mortality, body weight and feed consumption.
Haematological and clinical chemistry examinations were performed
after 1 year and at the end of the treatment period. Extensive
histopathological examinations were performed on all moribund and
sacrificed rats. At the mid- (males only) and high-doses the
mortality rate was increased; 90% mortality in the highest dose was
observed after 24 months. At the end of the treatment period
body-weight gain was significantly decreased at 500 and 1000 mg/kg
Table 2. Mammary tumour incidences in female Sprague-Dawley rats
control low mid high
No. of animals examined 34 35 33 35
Multiple mammary tumours 3 6 11 19
Malignant mammary tumours 1 3 3 5
Mammary fibroadenomas 0 6 2 10
Mammary adenocarcinomas 1 2 2 3
Mammary carcinosarcomas 0 1 2 1
feed. At 1000 mg/kg feed haemoglobin, haematocrit (also at 500 mg/kg
feed) and the number of erythrocytes (also in high-dose females)
were significantly decreased in male rats. An increase in 'non-renal
ricketts' was observed in high- dose male rats. The incidence of
testicular atrophy was increased in mid- and high-dose males and the
incidence of adrenal cortical hyperplasia, lipolysis, congestion and
haemorrhage was increased in high-dose males only. Tumour incidences
are given in Table 3. In high-dose females a significant increase in
the incidence of mammary gland adenocarcinomas was observed. In
addition, an increased incidence of sebaceous gland adenomas and
thyroid adenomas was observed in both sexes at the mid- and
high-dose and of basal-cell epithelioma and carcinomas in males of
the high-dose group. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1974a).
Sprague-Dawley rats (50/sex) were fed diets containing 0, 250,
500 or 1000 mg furazolidone/kg for 20 months (equivalent to 12.5, 25
or 50 mg/kg bw/day). The study was carried out following the same
protocol as described above for Fischer 344 rats. Mortality for
control, low-, mid- and high-dose groups was 4/50, 11/50, 17/50 and
30/50 for males and 9/50, 12/50, 8/50 and 29/50 for females,
respectively. High-dose animals were sacrificed at day 666, compared
to day 895 for controls. Body-weight gain was significantly
decreased in mid- and high-dose males and high-dose females. At the
end of the treatment period the number of erythrocytes was decreased
in females at 500 and 1000 mg/kg feed. High-dose males showed an
increased neutrophil/lymphocyte ratio and a decreased lymphocyte
count. Histopathology showed an increased incidence of hepatic
necrosis in all treated rats, especially high-dose females.
Testicular atrophy was seen in mid- and high-dose males. A
dose-related increase in adrenal cortical hyperplasia was observed
in females at all dose levels. Tumour incidences are given in Table
4. In the high-dose group, significantly increased incidences were
reported for mammary adenocarcinomas in females and for neural
astrocytomas in males. Female rats showed a significant increase in
the incidence of mammary neoplasms (benign and malignant combined)
at all dose levels, but without a dose-response relationship (King
et al., 1972b; Halliday et al., 1973b).
Table 3. Tumour incidences in Fischer 344 rats
males females
contr. low mid high contr. low mid high
No. of animals examined 49 50 50 49 49 50 50 50
Dermal fibromas 2 6 1 10 0 3 7 3
Sebaceous adenomas 0 0 8 11 1 2 6 10
Sebaceous adenocarcinomas 0 1 1 1 0 1 0 1
Basal cell epitheliomas 0 2 4 8 0 0 0 0
Basal cell carcinomas 0 0 0 2 0 0 0 0
Mammary neoplasms 1 1 2 0 11 29 40 30
Mammary adenocarcinomas 0 0 0 0 0 0 0 6
Lymphoreticular neoplasms 11 1 6 2 11 7 7 6
Pituitary adenomas 4 2 4 1 24 13 23 3
Thyroid adenomas 1 2 12 19 0 1 12 7
Testicular interstitial 42 44 31 0
cell tumours
Table 4. Tumour incidences in Sprague-Dawley rats
males females
contr. low mid high contr. low mid high
No. of animals examined 50 49 50 49 49 50 50 50
Dermal fibroma 2 6 5 7 3 1 2 7
Sebaceous adenoma 1 0 0 6 0 0 0 0
Sebaceous adenocarcinomas 0 0 0 1 0 0 0 0
Mammary neoplasm 2 4 5 1 29 41 45 40
Mammary adenocarcinoma 0 0 1 0 1 0 3 8
Mammary carcinosarcoma 0 0 0 0 0 1 0 0
Lymphoreticular neoplasms 7 6 5 7 6 1 4 0
Pituitary adenomas 10 9 6 3 29 17 17 1
Thyroid adenomas 0 1 1 5 0 0 1 1
Neural astrocytomas 0 0 2 5 1 0 1 0
2.2.4 Reproduction studies
2.2.4.1 Rats
A special study was performed to evaluate the effects on the
reproductive system of male rats fed furazolidone in the diet. Five
male rats (strain not specified)/group received diets containing 330
or 660 mg furazolidone/kg for 14 weeks, 3 male rats/group were given
330 or 660 for 12 weeks (equivalent to 16 or 33 mg/kg bw/day) with a
recovery period of 2 weeks. The control group consisted of 4 rats.
Observations included testes and epididymis weight and macroscopy
and histopathology of the reproductive tract. No dose-related
effects were observed in rats from the low-dose group. Testes weight
was markedly decreased in high-dose rats. This effect was more
pronounced in rats fed furazolidone for 14 weeks than in rats dosed
12 weeks with 2 weeks recovery. Histopathology of the testes of
high-dose rats revealed oedema of the interstitium, and atrophy and
degeneration of the sperm-producing tubules. Occasionally stasis of
previously produced sperm in the epididymus was seen and some
epididymi were devoid of sperm. The effects were still present after
the recovery period (Larson, 1963a).
Because there was some evidence that the testicular damage
observed after feeding of 660 mg furazolidone/kg in the diet was not
completely reversible gross and microscopic examinations were
performed on male rats 5 days and 6 weeks after withdrawal from a
diet containing 660 mg furazolidone/kg. Another 2 males were
retained for 14 weeks, the additional 8 weeks being required to
allow non-treated females to reach breeding age. Each treated male
(and one control male) cohabited with 2 females each. Testes weight
was decreased in 4/6 rats and in 3/6 rats atrophic seminiferous
tubules and abundant intertubular transudate were seen. In 2 of
these male rats the epididimi had sperm stasis. All prostates were
normal. Rats used in the breeding trials showed normal libido and
fecundated females but the litters that were produced had slightly
fewer young than those from the litters sired by the control male
(Larson 1963b).
In a three-generation reproduction study groups of rats were
fed diets containing 0, 30 or 100 mg furazolidone/kg. The F0
generation was maintained on the furazolidone diet and sacrificed
after 2 years. No effects were found on haematology, body weight and
histopathology after 2 years. Decrease in testes weight was
dose-related. Reproductive performance was not affected. Only a
summary of this study was available (Huffman, 1965a).
In a three-generation reproduction study groups of
Sprague-Dawley rats (20/sex) were used. Female rats only were
administered 500 mg furazolidone/kg feed. Because of growth
depression the dose level was lowered to 400 mg/kg on day 16 and to
250 mg/kg on day 37. Three matings per generation were performed.
The pups from the first litter were sacrificed 21 days after birth.
Pups from the second litter were used for the second generation and
pups from the third litter were used for teratological examination.
No treatment-related effects were found on reproduction parameters
resulting in a NOEL equivalent to 12.5 mg/kg bw/day (Borgmann &
Prytherch, 1964b; Borgman & Prytherch, 1966).
2.2.4.2 Chickens
Groups of New Hampshire chickens were fed diets containing 55,
110 or 220 mg furazolidone/kg feed for 4 to 16 weeks. No effects
were observed on body weight and egg production, hatchability of
eggs and shell quality. Thyroid and adrenal weight were both reduced
(Francis & Shaffner, 1956).
2.2.4.3 Pigs
In a limited study a group of purebred Hampshire and Duroc pigs
(9 sows and 12 gilts) were fed a diet containing 300 mg/kg
furazolidone for two weeks at breeding and 150 mg/kg for three weeks
at farrowing. Another group was maintained as an untreated control
group. There was no difference in the number of pigs born, number of
pigs weaned or weight gain of the pigs. However, pup weight at
weaning was slightly higher in the treated group (Hughes & McMinn,
1963).
2.2.4.4. Goats
Male Nubian goats received furazolidone suspended in distilled
water at a dose of 10 or 40 mg/kg bw. The effect on semen morphology
and biochemistry was studied. At both doses ejaculate volume, the
number of motile spermatozoa per ejaculate and the number of live
spermatozoa per ejaculate and semen fructose concentration were
significantly decreased (Mustafa et al., 1987).
2.2.5 Special studies on embryotoxicity and/or teratogenicity
2.2.5.1 Mice
Groups of Albino C strain mice were administered furazolidone
at doses up to 2 g/kg during pregnancy varying from day 1-11.
Abortions or fetal deaths occurred in all mice treated with doses
> 1 g/kg when treatment started before day 8 of pregnancy.
However when treatment started at day 10 abortions or fetal death
occurred only in 2/9 mice. Litterweight was dose-relatedly decreased
but no congenital abnormalities were observed (Jackson & Robson,
1957).
2.2.5.2 Rats
Groups of 3 Donryu male rats were fed 0 or 100 mg
furazolidone/kg bw for 7 days, and on day 8 the rats were
sacrificed. Relative testes weights were slightly decreased. The
number of mature spermatozoa was decreased and degenerative changes
in the seminiferous tubules (sloughing of spermatocytes and
multi-nucleated cells) were observed (Miyaji et al., 1964).
2.2.5.3 Rabbits
Groups of two rabbits were given an intra-amniotic injection of
1, 2 or 2.5 mg furazolidone on day 14 or 15 of pregnancy. A
laparatomy was performed 5 days after the injection to determine the
effect on pregnancy. Pregnancy interruption was observed at 2.0 and
2.5 mg furazolidone (Jackson & Robson, 1957).
Groups of 10 pregnant New Zeeland white rabbits were orally
administered 30 mg furazolidone (purity not given)/kg bw/day on days
7-15 of pregnancy. On day 29 of pregnancy the dams were sacrificed
and the fetuses were delivered by caesarean section. Feed
consumption and body-weight gain were significantly decreased. No
embryotoxicity or teratogenicity was observed (Borgmann & Prytherch,
1964a).
2.2.5.4 Chickens
Fifty percent mortality was observed in 10-day old chicken
embryos after treatment with 15 mg furazolidone in the inner shell
membrane, 4.2 mg in the allantoic cavity or after treatment with 0.7
mg in the yolk sac (Gentry, 1957).
2.2.5.2 Special studies on genotoxicity
The results of in vitro and in vivo genotoxicity studies
and of genotoxicity studies on metabolites of furazolidone and bound
residues are summarized in Tables 5, 6, and 7, respectively.
Table 5. Results of in vitro genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Ames testa S. typhimurium 0.01-1.0 µg/pl ? positiveb Jagannath et al.,
TA1538, TA98 (1.0 µg/pl slightly 1981g
and TA100 toxic)
TA1535, TA1537 negativeb
Ames testa S. typhimurium* 0.1, 0.5, 1.0 >99% positive Ni, et al., 1987
TA98, TA98NR or 2.5 µg/pl
TA98/1,8-DNP6 2.5 µg/pl toxic
Ames testa S. typhimurium 0.01-0.3 µg/pl 99% positiveb Crebelli et al.,
TA100 >0.3 µg/pl toxic 1982; Carere et
al., 1982
Spot test Escherichia coli 50 µg/plc positive McCalla &
WP2uvrA Voutsinos, 1974
Reverse Euglenia gracilis 25, 50 and 100 ? positive Ebringer et al.,
mutation assay µg/mlc 1976
Forward Vibrio cholerae up to 12 µg/mlc ? positive Chatterjee et al.,
mutation assay OGAWA 154 1983
Prophage Escherichia coli ? ? positive Chatterjee et al.,
induction assay K12 GY5027 1983
Gene conversion S. cerevisiae D4 no details ? positive Voogd et al.,
assay 1982 as cited in:
VanMiert et al.,
1984
Gene mutation Chinese hamster up to 125 µg/ml >99% positive Gao et al., 1989
assaya ovary cells >100 µg toxic
Table 5 cont.
Test system Test object Concentration Purity Results Reference
HGPRT test Mouse L5178Y no details ? positive Voogd et al.,
lymphoma cells 1982
SOS function and Escherichia coli 0.8 and 8.0 µMc ? positive Bryant &
mutation assay WP2 McCalla, 1980
SOS function Escherichia coli 0.1, 0.2, 0.5 ? positive Ohta et al., 1984
assay K12 µg/mlc
Sex-linked D. melanogaster 0.5 mM in DMSOc ? positive Blijleven et al.,
recessive 1977
lethal test
Sex-linked D. melanogaster 0.18, 0.44 or ? positive Kramers, 1982
recessive 0.5 mM in DMSOc
lethal test
Chromosome human 10, 33 and 100 99.8% negative Scheres, 1991b
aberration assay lymphocytes µg/ml in DMSOd
33, 100, 180 positivef
and 333 µg/ml in
DMSOe (333 µg/ml
slightly toxic)
Chromosome human 0.2, 2.0 or ? positive Cohen & Sagi,
aberration assay lymphocytes 20.0 µg/mld 1979
20.0 µg/ml toxic
Chromosome human 0.5-100 µMc ? negative Tonomura &
aberration assay lymphocytes Sasaki, 1973
Table 5 cont.
Test system Test object Concentration Purity Results Reference
Chromosome bovine lymphocytes 0.05-500 mg/lg ? positive Queinnec et al.,
aberration assay porcine lymphocytes 0.05-500 mg/lg positive 1975; Babile et
al., 1978
SCE test human 0.2, 2.0 and ? positive Cohen & Sagi
lymphocytes 20 mg/l in DMSO 1979
20 mg/l toxic
Rec assay Escherichia coli ?? ? positive Chatterjee et al.,
K12 rec- and rec+ 1983
DNA repair Escherichia coli 1.0 to 10 µM ? positive Lu et al., 1979
assay WP2 uvrA >5µM toxic
UDS assay human 5-100 µMc ? negative Tonomura &
lymphocytes Sasaki, 1973
UDS assay Fischer 344 0.5-1000 nM/ml ? positive Probst et al.,
rat hepatocytes in DMSOb,c 1981
* nitroreductase-deficient Salmonella typhimurium tester strains.
a both with and without rat liver S9 fraction.
b positive controls yielded positive results.
c no data about toxicity.
d without metabolic activation.
e with metabolic activation.
f the authors concluded that the results were negative.
g no toxicity observed.
Table 6: Results of in vivo genotoxicity assays on furazolidone
Test system Test object Concentration Purity Results Reference
Micronucleus Swiss CD-1 mice 300 mg/kg bw 99.8% negative Enninga &
test ip in Weterings 1990
methyl-cellulose
(toxic)
Micronucleus Swiss Webster 100 and 500 mg/kg ? equivocalb Paik, 1985
test mice bw orala
a no toxicity observed
b slight induction, not dose-related
2.2.7 Special studies on endocrine toxicity
In a special study various groups of ovariectomized
Sprague-Dawley, Fischer 344 or Long Evans rats and golden hamsters
received single oral doses varying from 50-500 mg furazolidone/kg
bw. After a 2-week recovery period positive dose-related lordosis
(becoming sexually perceptive) behaviour was observed only in
Sprague-Dawley rats which is, according to the authors, indicative
of an elevated concentration of progesterone in the circulation
(King et al., 1970a).
Groups of immature superovulated Sprague-Dawley rats were
orally dosed with 100 or 500 mg furazolidone/kg bw. In all groups
ovulation was initiated about 24 h earlier than the control group.
The same shift in ovulation time was observed in a positive control
group treated with progesterone (King et al., 1970a).
Feeding of 1000 mg furazolidone/kg feed for 30, 60 or 90 days
produced a marked inhibition of the conversion of progesterone into
corticosterone (11-hydroxylation) in adrenals of Sprague-Dawley
females. A considerably lesser effect was found in Fischer 344
females. This effect could be overcome by addition of NADPH,
indicating an inhibitory effect of furazolidone on the NADPH
generating system (King et al., 1971b).
Table 7. Results of genotoxicity assays on metabolites of furazolidone and
bound residues
Test system Test object Concentration Substance Results Reference
In vitro
Ames testa S. typhimurium 100-5000 µg/plb,c Md negative Scheres, 1991a
TA100, TA98
Ames testa S. typhimurium urine from rats - positivee Crebelli et al.,
TA100 treated with 10, 1982; Carere et
50, 100 mg/kg bw al., 1982
Ames testa S. typhimurium up to 5 µg/pl M1f negativee,g Jagannath &
TA1535,TA1537 Brusick, 1981a; 1981e
Craine, 1981
TA1538, TA98, M2h negativee Jagannath &
TA100 Brusick, 1981b; 1981f
Craine, 1981
M3i negativee Jagannath &
Brusick 1981c; 1981d
Craine, 1981
Ames test a S. typhimurium 0.1 mg/pl M4j negative Vroomen et al.,
TA100, TA98 in DMSO 1987a
Ames test S. typhimurium upto 5 µg/pl GSFk negative Hoogenboom,
TA100 in DMSO MSFl negative 1991a
Reverse Mycobacterium 1000 µg/ml 5-NFAm positive Ebringer et al.,
mutation assay phlei 1976
Euglena gracilis 2.5 and 7.5 5-NFAm positive Ebringer et al.,
µg/ml 1976
DNA repair test rat hepatocytes 10-3M, 10-5Mn Mo negative Mori et al.,
mouse hepatocytes 1988
Table 7 (continued)
a both with and without rat liver S9 fraction
b positive controls yielded positive results
c no toxicity observed
d 3-amino-oxazolidinon-2
e negative control yielded negative results
f whole liver powder from pigs treated with 5 mg furazolidone/kg bw for 5 days
g substances from untreated pigs yielded negative results
h urine isolates from pigs treated with 5 mg furazolidone/kg bw for 5 days
i soluble liver isolate from pigs treated with 5 mg furazolidone/kg bw for 5 days
j 3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone.
k glutathione conjugate of cyano metabolite
l mercaptoethanol conjugate of cyano metabolite
m 5-nitro-2-furaldehyde
n no data about toxicity
o 2-hydroxyethylhydrazine.
Oral administration of 100 or 500 mg furazolidone/kg bw to
mature female rats produced a significant decrease in serum
prolactin during the morning proestrus, but not at afternoon
proestrus. Administration of 500 mg/kg bw during the morning estrus
resulted in a slight decrease in serum prolactin levels (Morrison
et al., 1973).
In a limited experiment the effect of furazolidone treatment on
the tumourigenicity of estrone was studied. Furazolidone treatment
(1000 mg/kg feed to female Sprague-Dawley and Fischer 344 rats) plus
estrone resulted in the development of more mammary masses than with
estrone alone. However, the administration of estrone did not result
in an increased incidence of mammary masses (Morrison et al.,
1972).
Furazolidone (500 or 1000 mg/kg feed) was fed to
adrenalectomized and sham-operated male rats for 30 days, after
which the adrenals and tests were examined. Adrenalectomy did not
alter the testicular atrophy produced by furazolidone. At 1000 mg/kg
feed tubular degeneration in three out of six animals was seen
(Morrison et al., 1974a).
A diet containing 500, 750 or 1000 mg/furazolidone/kg
administered to female rats for 30 days resulted in a significant
increase in serum testosterone at the highest dose (Morrison et
al., 1974b).
In a pilot study rats were administered a single oral dose of
250 or 500 mg/kg bw furazolidone in 0.5% aqueous CMC. At 30 min, 1,
2, 4, 8 and 24 h after administration blood samples were taken for
RIA of prolactin and corticosterone. Furazolidone increased in a
dose-related manner, the corticosterone concentration up to 2 h
after administration, with a return to vehicle control values by 24
h. No effect on plasma prolactin time was observed (Meites, 1984).
Male mature chickens were fed a diet containing 400 or 800 mg
furazolidone/kg feed for 10 days. Testes weight was decreased in
both groups. Treatment with 800 mg/kg produced a significant
reduction in the concentration of testosterone in plasma and testes
and some reduction in ascorbic acid, protein and cholesterol
concentration in the testes. Bolus administration of doses of 40 or
80 mg/kg bw for five days caused the same effect. The size of the
testes, wattles and combs were significantly reduced. Monoamine
oxidase activity in the testes was significantly reduced by
furazolidone. In all the treated birds testicular concentrations of
5-hydroxytryptamine were significantly raised, except in those fed
400 mg furazolidone/kg for 10 days (Ali et al., 1984).
Groups of large white female turkeys were orally dosed with 0,
7.5, 15 or 30 mg furazolidone/kg bw for 7 days in gelatin capsules.
The number of eggs produced by each bird was recorded daily 2 weeks
before, during and 4 weeks after the end of treatment. The
concentrations of luteinizing hormone (LH) and prolactin (PRL) were
analyzed by RIA in plasma samples taken on day -7, 0, +7 and +14. At
the end of the treatment period a dose-related decrease was observed
in egg production and LH concentration (significantly in the mid-
and the high-dose group). No effects were observed on PRL
concentration. After withdrawal only the egg production started to
recover but was still below normal 4 weeks after cessation of
treatment (Ali et al., 1987a; 1988).
When turkeys given 15 or 30 mg furazolidone/kg bw were injected
intramuscularly with 5 µg/kg luteinizing hormone-releasing hormone
(LHRH), no effect on LHRH-induced LH release was observed (Ali et
al., 1987a; 1988).
Groups of male turkeys were orally treated with 0, 1, 2.5, 5 or
20 mg furazolidone/kg bw/day for 14 days. Plasma was analyzed for
LH, testosterone (T) and PRL before, during and after treatment. At
the highest dose the concentrations of LH and T were significantly
decreased, and a tendency towards a decrease was observed at 5 mg/kg
bw/day. At histopathology a decrease in spermatocyte production as
well as corrugation of sperm cell nuclear envelopes and distension
of endoplastic reticulum of elongated spermatides were observed in
the 20 mg/kg bw/day group (Ali et al., 1987b).
Furazolidone administered as a bolus dose (5-500 mg/kg bw) to
male T-line chickens produced a decrease in the amount of
corticosterone in plasma (Bartlet et al., 1990).
In another experiment male T-line and J-line chickens were
given a bolus dose of furazolidone (200 mg/kg bw) which resulted in
a decrease in aspartate transaminase activity in the adrenal gland
and a general disorganization in the structure of the adrenal
cortical cells, leading to atrophy of the adrenal cortical glands
(Bartlet & Khan, 1990).
A concentration of 1 µM furazolidone inhibited significantly
the release of aldosterone from porcine adrenal cells in vitro.
Almost complete inhibition was observed at 100 µM (van den Dungen
et al., 1991).
2.2.8 Special studies on skin and eye irritation
A dose of 500 mg furazolidone (purity 100%) moistened with 0.5
ml of 0.5% saline caused slight erythema in 4/6, 3/6 and 1/6 rabbits
after 0.5, 24, and 48 h respectively, when applied under
semi-occlusive conditions to the clipped intact skin of 3 male and 3
female New Zeeland white rabbits (Mitchell et al., 1990c).
Nine New Zeeland white rabbits received an installation of 30
mg furazolidone (purity 100%) into the conjunctival sac of the right
eye, and 3/9 eyes were washed 20 seconds after treatment. After 24 h
slight conjuctival irritation was observed in both washed and
unwashed eyes. Corneal ulcerations were observed in 2/6 unwashed
eyes only. All eyes were normal at day 7 (Mitchell et al., 1990d).
2.3 Observations in humans
Furazolidone administered to patients with essential
hypertension (no details provided) produced marked supersensitivity
to tyramine and amphetamine, inhibition of intestinal MAO and
increased urinary excretion of tryptamine. Continued administration
produced a cumulative inhibition of MAO (Pettinger, et al., 1968)
Adverse reactions (gastrointestinal 8%, neurological 1.34%,
systemic, 0.56% and dermatological reactions 0.54%) to furazolidone
treatment were observed in 864/10443 patients (8.3%) treated with a
therapeutic dose of < 5 to 7 mg/kg bw/day (Altamirano & Bondani,
1989).
A 43-year old man with a contact allergy to furazolidone was
patch tested to nitrofurans with completely negative results. The
man had a positive patch test reaction to 2% furazolidone both in
PEG-400 and alcohol. Control tests were negative in 25 dermatitis
patients (DeGroot & Conemans, 1990).
3. COMMENTS
The Committee considered data from pharmacodynamic,
pharmacokinetic, metabolism, acute and short-term toxicity,
carcinogenicity, genotoxicity, reproductive, and teratogenicity
studies as well as special studies on endocrine function and some
clinical studies in humans.
The distribution, excretion, and biotransformation of
radiolabelled furazolidone were studied in rats, chickens, pigs, and
humans. After oral administration, furazolidone was rapidly absorbed
and the radioactivity was widely distributed, the highest levels
being found in liver, kidney, fat, and muscle. It was rapidly
metabolized and excreted predominantly in urine. In chicken and
human urine, only trace amounts of unchanged furazolidone could be
detected, and of the large number of metabolites found only some
were identified. In rat and pig urine, the common metabolite
appeared to be the open chain cyanometabolite
3-(4-cyano-2-oxobutylideneamino)-2-oxazolidone. The Committee noted
that quantitative information on metabolites was lacking. In pigs, a
substantial portion of the metabolites was bound to macromolecules,
and it appeared that approximately 15-40% of this bound fraction was
bioavailable. However, the Committee questioned whether valid
extraction procedures had been used to isolate these bound
metabolites.
In acute oral toxicity studies in mice and rats furazolidone
was slightly toxic; the LD50 values were of the order of 1100 and
1500 mg/kg bw, respectively.
No NOEL could be established from short-term studies performed
with rats and dogs. Rats receiving furazolidone at doses in the
range 0.5-50 mg/kg bw/day showed hypertrophy of liver cells.
Palpable mammary tumours and a decrease in body weight gain were
observed at 50 mg/kg bw/day. In dogs, dose levels of 5-25 mg/kg
bw/day led to neurological symptoms and histological changes in the
basal ganglia, together with testicular degeneration. It was noted
that the available information was deficient by current standards
and poorly reported.
Two three-generation reproduction studies were performed in
rats. In one study rats were exposed to furazolidone at
concentrations up to 100 mg/kg in feed. In the other study only
female rats were treated with diets containing 500 mg/kg, but this
concentration was gradually reduced to 250 mg/kg in order to avoid
the observed growth depression. No effects on reproductive
performance were observed in either study. The NOEL was equivalent
to 12.5 mg/kg bw/day.
In a special study designed to evaluate the effects on the male
reproductive system, rats exposed to a dietary furazolidone
concentration equivalent to 33 mg/kg bw/day exhibited testicular
degeneration. At 16 mg/kg bw/day no effects were observed.
Neither embryotoxicity nor teratogenicity was observed in
rabbits after oral administration of furazolidone at a dose of 30
mg/kg bw/day.
A carcinogenicity study was conducted in Swiss MBR/ICR mice,
which received a diet containing concentrations of furazolidone
equal to average daily doses of 12, 24, or 47 mg/kg bw/day for 13
months, followed by a control diet for 10 months. In the mid- and
high-dose groups, a significant increase in the incidence of
bronchial adenocarcinomas was observed in both sexes, and the
incidence of lymphosarcomas was significantly increased in male
mice.
In two long-term toxicity/carcinogenicity studies, furazolidone
was administered in the diet to Fischer 344 and Sprague-Dawley rats
at concentrations equivalent to daily doses of 12.5, 25, or 50 mg/kg
bw/day for 20 months. In Fischer 344 rats, a significant increase in
the incidence of mammary gland adenocarcinomas was observed in
females in the high-dose group. In addition, an increase in the
incidence of sebaceous gland adenomas and thyroid adenomas was
observed in both sexes at 25 and 50 mg/kg bw/day and of basal cell
epithelioma and carcinoma in males of the high-dose group. In the
high-dose group of Sprague-Dawley rats, significantly increased
incidences were reported for mammary adenocarcinomas in females and
for neural astrocytomas in males. In both strains of rat, female
animals showed a significant increase in the incidence of mammary
neoplasms (benign and malignant combined) at all dose levels, but
without a dose-response relationship.
Furazolidone has been tested in a wide variety of genotoxicity
studies. Positive findings were recorded in bacterial assays with
and without metabolic activation, in the sex-linked recessive lethal
test in Drosophila melanogaster, in a gene mutation assay with
mammalian cells in vitro, in a sister chromatid exchange test, and
in two DNA-repair tests. Positive as well as negative results were
obtained in chromosome aberration assays with mammalian cells in
vitro, and in tests for unscheduled DNA synthesis. One mouse
micronucleus test was negative, while another gave equivocal
results.
The majority of in vitro genotoxicity tests with postulated
metabolites gave negative results, however, nitrofuraldehyde and
urine from furazolidone-treated rats gave positive results. It was
concluded that furazolidone was genotoxic in vitro.
Several studies were performed on the endocrine effects of
furazolidone. Furazolidone inhibited the conversion of progesterone
into corticosterone in adrenal cells both in vivo and in vitro.
It has been hypothesized that disturbances of steroidogenesis
constituted the underlying mechanism for the increased incidence of
tumours caused by furazolidone. The Committee noted that it was
unlikely that the such a mechanism could account for the increase in
neural astrocytomas and uncommon skin tumours in rats. With respect
to the occurrence of mammary tumours, no information was available
on the effect of furazolidone on plasma progesterone concentrations
and no consistent effects on plasma prolactin concentrations were
observed. The Committee therefore concluded that no support had been
provided for the hypothesized mechanism.
Furazolidone caused reversible inhibition of monoamine oxidase
(MAO) activity in pig hepatocytes in vitro and in liver and brain
tissue of rats following in vivo administration. Irreversible MAO
inhibition both in vitro and in vivo was observed for the
postulated metabolites amino-oxazolidone and hydroxyethyl-hydrazine.
4. EVALUATION
On the basis of the positive effects of furazolidone in
genotoxicity tests in vitro and the increased incidence of
malignant tumours in mice and rats, the Committee concluded that
furazolidone was a genotoxic carcinogen. Since the drug is rapidly
and extensively metabolized, the Committee also considered
information on metabolites of furazolidone. Although a large number
of postulated metabolites produced negative results in genotoxicity
tests, it was noted that only a few of these had been either
identified or quantified in rats and pigs. Furthermore, the
Committee concluded that insufficient data were available on the
nature and toxic potential of compounds released from the bound
residues.
Because of the genotoxic and carcinogenic nature of
furazolidone and the above-mentioned deficiencies with respect to
the data on the metabolites, the Committee was unable to establish
an ADI.
Before considering the compound again, the Committee would wish
to have detailed information on the nature, quantity and toxicity of
the metabolites of furazolidone, including the bound residues.
5. REFERENCES
AISO, K., KANISAWA, M., YAMAOKA, H., TATSUMI, K. & AIKAWA, N.
(1962). Systematic studies on the toxicity of nitrofuran derivatives
(3): Macroscopical and histological examination of rats fed on diet
with furazolidone and 5-(5-nitro-2-vinylfuran)-1, 3,
4-oxadiazoline-2-one for one year. Chiba Daigaku Fuhai Kenkyusho
Hokoku, 15: 21-33. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H. & BARTLET, A.L. (1982). Inhibition of monoamine oxidase in
chicken and ducklings by a microbial metabolite of furazolidone.
Quarterly J. Exp. Physiol., 67(1): 69-79.
ALI, B.H. (1983). The anti-diamine oxidase effect of furazolidone in
rabbits. Comp. Biochem. Physiol., 74C(1): 109-110.
ALI, B.H., HOMEIDA, A.M. & KNIFTON, A. (1984). The effect of
furazolidone on fertility of male chickens. Comp. Biochem.
Physiol., 78C(1): 43-47.
ALI, B.H., SILSBY, J.L. & EL HALAWANI, M.E. (1987a). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
British Poultry Science, 28: 613-621.
ALI, B.H., SILSBY, J.L., LOSETH, K.J., CRABO, B. & EL HALAWANI, M.E.
(1987b). Some effects of furazolidone on the testes and plasma
testosterone, luteinising hormone and prolactin concentrations in
mature male turkeys d.d. 17 June 1987. University of Minnesota. St.
Paul, MN 55108, USA. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL), levels in young turkeys. Gen. Pharmacol., 19(1):
91-95.
ALTAMIRANO, A. & BONDANI, A. (1989). Adverse reactions to
furazolidone and other drugs. Scand. J. Gastroenterol.,
24(suppl.169): 70-80.
BABILE, R., QUEINNEC, G., BERLAND, H.M. & DARRE, R. (1978).
Structure chromosomique des lymphocytes du porc et additifs
alimentaires. C. Soc. Biol., 172:, 546-553.
BARTLET, A.L & KHAN, F.H. (1990). Effects of nitrofurans on adrenal
cortical tissue in chickens. J. Vet. Pharmacol. Therap., 13:
206-216.
BARTLET, A.L., HARVEY, S. & KLANDORF, H. (1990). Contrasting effects
of nitrofurans on plasma corticosterone in chickens following
administration as a bolus or diet additive. J. Vet. Pharmacol.
Therap., 13:, 261-269.
BLIJLEVEN, W.G.H., KORTSELIUS, M.J.H. & KRAMERS, P.G.N. (1977).
Mutagenicity testing of H-193, AF-2 and furazolidone in Drosophila
melanogaster. Mutat. Res. 56: 95-100.
BORGMANN, A.R., MURTI, G.S., COOLEY, R. & LEVIN, R. (1964).
Ninety-day toxicity studies of experimental formulations containing
furoxone (R) (NF-180) in dogs. (Project no. 475.09.02) Unpublished
final report d.d. 29 June 1964, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964a). The effect of furoxone (R)
on fetal growth and development in the rabbit. Unpublished special
report project number no. 458.9 d.d. 28 August 1964 from Pathology
Section, Scientific Department Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1964b). The effect of furoxone on
reproduction in rats. Unpublished special report project number no.
460.9 d.d. 3 September 1964 from Pathology Section, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
BORGMANN, R. & PRYTHERCH, J.P. (1966). The effect of furoxone on
reproduction in rats. Second and third generation. Unpublished
special report project number no. 460.09 d.d. March, 10, 1966 from
Pathology Section, Scientific Department, The Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BOWMAN, J.S. (1961a). A balance study of furazolidone C14
(methylene and aldehyde-labeled) in male rats. Unpublished report
H-NSC
no. 20-0040-32 from Hazleton, Nuclear Science Corporation,
California. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
BOWMAN, J.S. (1961b). A plateau-elimination study of
furazolidone-C14 (methylene labeled) in male rats. Unpublished
report H-NSC no. 20-0039-32 from Hazleton, Nuclear Science
Corporation, California. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran induced mutagenesis
and error prone repair in Escherichia coli. Chem. Biol. Interact.,
31: 151-166.
BUZARD, J.A., HEOTIS, J.P. & WILLIAMS, C.W. (1960). Chick
distribution studies with C14-(formyl) NF-180. Unpublished interim
report problem 360.3 d.d. 9 December 1960 .Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
BUZARD, J.A., HEOTIS, J.P. & Williams, C.W. (1961). Chick
distribution studies with C14-(methylene) NF-180. Unpublished
interim report problem 360.3 d.d. 15 May 1961. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
CARERE, A., CONTI, L., CREBELLI, R. & MACRI, A. (1982). Quantitative
data on the urinary recovery of mutagenicity in furazolidone-treated
rats. Mutat. Res., 97(6), 461-462.
CHATTERJEE, S.N., MAITI, M. & GHOSH, S. (1975). Interaction of
furazolidone with DNA. Biochem. et Biophysica Acta, 402: 161-165.
CHATTERJEE, S.N., BANERJEE, S.K., PAL, A.K. & BASAK. J. (1983). DNA
damage, prophage induction and mutation by furazolidone. Chem.
Biol. Interact., 45: 315-326.
COHEN, M.M. & SAGI, M. (1979). The effect of nitrofurans on mitosis,
chromosome breakage and sister-chromatid exchange in human
peripheral lymphocytes. Mutat. Res., 59(1): 139-142.
CRAINE, E.M. & RAY, W.H. (1972). Metabolites of furazolidone in
urine of chickens. J. Pharmacol. Science, 61(9): 1495-1497.
CRAINE, E.M. (1977). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 77:10
d.d. 28 December 1977 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1978). The disposition of furazolidone-14C to the
urine and tissues of pigs. Unpublished research report no. EMC 78:12
d.d. 21 April 1978 from Hess & Clark Division, Ashland, Ohio.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
CRAINE, E.M. (1981). Preparation of isolates of furazolidone
metabolites. Unpublished research report, project no. 80191 d.d. 2
March 1981 from Wil Research Laboratories Inc., Research Division,
Cincinnnati, Ohio. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
CREBELLI, R., CARERE, A., FALCONE, E. & MACRI, A. (1982). A study on
the urinary and fecal excretion of furazolidone in rats by means of
mutagenicity assays. Ecotoxicol. Environ. Saf. 6(5): 448-456.
DeGROOT, A.C. & CONEMANS, J.M.H. (1990). Contact allergy to
furazolidone. Contact Dermatitis, 22:, 202-205.
EBRINGER, L., JURASEK, A., KONICEK, J., KONICKOVA, M., LAHITOVA, N.
& TRIBACUK, S. (1976). Mutagenic action of nitrofurans on Euglena
gracilis and Mycobacterium phlei. Antimicrobial Agents and
Chemotherapy, 9(4): 682-689.
ENNINGA, I.C. & WETERINGS, P.J.J.M. (1990). Micronucleus test in
bone marrow cells of the mouse with furazolidone. Unpublished report
(RCC notox project 026122) d.d. April 1990 from RCC/NOTOX, 5231
DD's-Hertogenbosch, the Netherlands. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
FRANCIS, D.W. & SHAFFNER, C.S. (1956). An investigation of the
morphological changes in young chickens and the reproductive
performance of adult chickens fed furazolidone or nitrofurazone.
Poultry Sci., 35: 1371-1381.
FUKUHARA, M. & TAKABATAKE, E. (1977). The effects of nitrofuran
derivatives on hepatic microsomal mixed-function oxidase activity in
rats. Toxicol. Appl. Pharmacol., 42: 571-581.
GAO, N., NI, Y.C., THORNTON-MANNING, J.R., FU, P.P. & HEFLICH, R.H.
(1989). Mutagenicity of nitrofurantoin and furazolidone in Chinese
hamster ovary cell strains. Mutat. Res., 225: 181-187.
GENTRY, R.F. (1957). The toxicity of certain antibiotics and
furazolidone for chicken embryos. Avian Diseases, 2: 77-82.
HALLIDAY, R.P., SUTTON, M.L., SIGLER, F.W. & LEVIN, R. A. (1973a).
Chronic toxicopathological safety study (two years) of NF-180 in
rats. Unpublished final report project no. 475.09-C d.d. 9 November
1973 from Pathological and Toxicology Section, Norwich Pharmacal
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1973b). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished interim report # 2 project no. 475.09D d.d. 9
November 1973 part I: Sprague-Dawley evaluation from Pathological
and Toxicology Section, Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974a). Tumorgenesis
evaluation (lifetime) of NF-180 in Sprague-Dawley and Fischer 344
rats. Unpublished final report project no. 475.09D d.d. 31 January
part II: Fischer 344 evaluation from Pathological and Toxicology
Section, Norwich Pharmacal Company, Norwich, New York. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HALLIDAY, R.P., SUTTON, M.L. & SIGLER, F.W. (1974b). Tumorgenesis
evaluation (twenty-three months) of furazolidone (NF-180) in mice,
Unpublished final report project no. 475.09E d.d. 31 January 1974
from Pathological and Toxicology Section, Research and Development
Department, Norwich Pharmacal Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HAWKINS, D.R., ELSOM, L.F., GIRKIN, R. & CHENG, C.F. (1992). The
bioavailability in rats of tissue residues from swine administered
14C-furazolidone for 14 days and subjected to 0-day, 21-day and
45-day withdrawal periods. Unpublished draft report HRC/SMI
125/911478 from Huntingdon Research Centre Ltd., P.O.Box 2,
Huntingdon, Cambridgeshire, England. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
HEOTIS, J.P., HERRET, R.J. & WILLIAMS, C.W. (1963). Drug metabolism
studies. Incorporation of C14 from formyl-C14-NF-180 into
natural products. Unpublished special report part I: problem 370.3
d.d. 20 September 1963 from Biological Research Division, Scientific
Department, The Norwich Pharmacal Company, Norwich, New York.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
HOOGENBOOM, L.A.P. (1991a). The use of pig hepatocytes to study the
role of glutathione on the biotransformation and toxicity of
furazolidone. In: The uses of pig hepatocytes for biotransformation
and toxicity studies Thesis, Agricultural University of Wageningen,
The Netherlands. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991b). The use of pig hepatocytes for
biotransformation studies of veterinary drugs. In: The use of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P. (1991c). The use of pig hepatocytes to study the
inhibition of monoamine oxidase by furazolidone. In: The uses of pig
hepatocytes for biotransformation and toxicity studies Thesis,
Agricultural University of Wageningen, The Netherlands. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
HOOGENBOOM, L.A.P., Van KAMMEN, M., BERGHMANS, M.C.J. & KUIPER, H.A.
(1991). Detection of protein bound metabolites of furazolidone.
Proceedings of the Euroresidue Conference in Noordwijkerhout, The
Netherlands. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965a). Two-year chronic toxicity study and a
three-generation reproductive study in rats. Unpublished report
no. 65:15/H-1R from Hess & Clark, Research Department, Ashland,
Ohio. Summary submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
HUFFMAN, K.W. (1965b). Two-year chronic toxicity test with
furazolidone in dogs. Unpublished report no. 65:16/H-1D) d.d. 30
July 1965 from Hess & Clark, Research Department, Ashland, Ohio.
Abstract submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
HUGHES, D.L. & McMINN, C.S. (1963). The effect of furazolidone on
reproduction and enteric infections of swine. Unpublished final
report (Ex 62-12-18a2) Norwich Pharmacal Company, Norwich, USA.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
JACKSON, D. & ROBSON, J.M. (1957). The action of furazolidone on
pregnancy. J. Endocrin., 15: 355-359.
JAGANNATH, D.R. & BRUSICK, D.J. (1981a). Mutagenicity evaluation of
WIL-80191-10-4 dry liver, control in the Ames Salmonella/microsome
plate test. Unpublished final report d.d. March 1981 LBI project
from Litton Bionetics, Inc. Kensington, Maryland 207953. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981b). Mutagenicity evaluation of
WIL-80191-11-2 urine isolate; control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981c). Mutagenicity evaluation of
WIL-80191-10-1 liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981d). Mutagenicity evaluation of
WIL-80191-10-2 dry liver isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
February 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981e). Mutagenicity evaluation of
WIL-80191-10-3 liver isolate control in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981f). Mutagenicity evaluation of
WIL-80191-11-1 urine isolate treated in the Ames
Salmonella/microsome plate test. Unpublished final report d.d.
March 1981 LBI project from Litton Bionetics, Inc. Kensington,
Maryland 207953. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
JAGANNATH, D.R. & BRUSICK, D.J. (1981g). Mutagenicity evaluation of
Furazolidone WIL-80191-11-3 in the Ames Salmonella/microsome plate
test. Unpublished final report LBI project from Litton Bionetics,
Inc. Kensington, Maryland 207953. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CURRIE, G.N. HARRINGTON, C. & WELCH, C.A. (1970a).
Effects of nitrofurans on steroidogenesis and mammary hyperplasia
(NF-180 Rx 30309) Unpublished annual report project no. 569.09
d.d.16 June 1970 from Research and Development Department, The
Norwich Pharmacal Comp., Norwich, New York. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
KING, C.D., CHRISTENSEN, E.F., McLAUGHLIN, J.H., LEVIN, R.A. &
SIGLER, F.W. (1971a). Chronic (2 year) toxicopathologic study of
NF-180 in dogs. Unpublished final report project no. 475.09B d.d. 12
November 1971 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Abstract submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
KING, C.D., CURRIE, G.N., HARRINGTON, C. & WELCH, C. (1971b).
Nitrofurans and steroidogenesis. Unpublished interim report no. 5
project no. 596.09 d.d. 21 December 1971 from Pathological and
Toxicology Section, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L. & LEVIN, R.A. (1972a). Chronic
toxicopathological safety study (two years) of NF-180 in rats.
Unpublished status report no.1 project no. 475.09c d.d. 18 October
1972 from Pathological and Toxicology Section, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
KING, C.D., SUTTON, M.L., WONG, L.C.K. & LAUGHLIN, P.J. (1972b).
Tumorgenesis evaluation (two years) of NF-180 in Spraque-Dawley and
Fischer 344 rats. Unpublished status report no.1 project no. 475.09D
d.d. 18 0ctober 1972 from Pathological and Toxicology Section,
Research and Development Department, The Norwich Pharmacal Company,
Norwich, New York. Submitted to WHO by SmithKline Beecham Animal
Health, West Chester, PA, USA.
KOUBA, R.F. (1979). Analysis of components from urine of pigs
treated with furazolidone-14C-15N or furazolidone-14C.
Unpublished report no.: RFK 79:3 from Hess & Clark, Ashland.
Submitted to WHO by SmithKline Beecham Animal Health, West Chester,
PA, USA.
KRAMERS, P.G. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
LARSON, E.J. (1963a). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12a. Addendum to pathology
research report H-12. Unpublished report d.d. 14 November 1963 from
Research Department Hess & Clark, Ashland Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LARSON, E.J. (1963b). Evaluation of effects on the reproductive
system of male rats fed NF-180 as 0.033% and 0.066% of the diet.
Unpublished pathology research report H-12 d.d. 18 March 1963 from
Research Department Hess & Clark, Ashland, Ohio. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli. Mutation and induction and repair of daughter-strand
gaps in DNA. Mutat. Res., 67: 13-144.
McCALLA, D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res. 16(1): 3-16.
MEITES, J. DENINE, E.P. & OBROSKY, K.W. (1984). Determination of
blood levels of prolactin and corticosterone following oral
administration of furazolidone (NF-180) or EU-557 to male
Sprague-Dawley rats: a pilot study. Unpublished final report project
no. 475.59.59 d.d. 30 March 1984 from Norwich Eaton Pharmaceuticals,
Inc., Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990a).
Acute oral toxicity study of furazolidone in rats. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5884-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., AREIA, D. & WESSEL, J.L. (1990b).
Acute oral toxicity study of furazolidone in mice. Unpublished
report d.d. 5 July 1991 from Bio/Dynamics Inc. project no. 5885-90,
East Millstone, New Jersey. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B. & WESSEL, J.L. (1990c).
Primary dermal irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/Dynamics Inc. project
no. 5886-90, East Millstone, New Jersey. Submitted to WHO by
Smithkline Beecham Animal Health, West Chester, PA, USA.
MITCHELL, J.M., TRIMMER, J.E., LUKE, B., ODDE, E.Y. & WESSEL, J.L.
(1990d). Primary irritation study of furazolidone in rabbits.
Unpublished report d.d. 5 July 1991 from Bio/dynamics Inc. project
no. 5887-90, East Millstone, New Jersey. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
MIYAJI, T, MIYAMOTO, M & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testis caused by nitrofuran
compounds. Acta Path. Jap., 14(3): 261-273.
MORI, H., SUGIE, S., YOSHIMI, N., IWATA, H., NISHIKAWA, A.,
MATSUKUBO, K., SHIMIZU, H. & HIRONO, I. (1988). Genotoxicity of a
variety of hydrazine derivatives in the hepatocyte primary culture
DNA repair test using rat and mouse hepatocytes. Jpn. J. Cancer
Res. (Gann), 29: 2145-2156.
MORRISON, J.L., CURRIE, G.N., HARRINGTON, C. & WELCH, C.A. (1972).
The effects of estrone and NF-180 on mammary tumor formation.
Unpublished special report project no. 596.09 d.d. 12 October 1972
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1973). Effects of
NF-180 (acute) on serum prolactin levels. Unpublished final report
project no. 596.03 d.d. 24 October 1973 from Biochemistry Section,
The Norwich Pharmacol Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974a). Effects of
adrenalectomy on NF-180 induced testicular atrophy. Unpublished
final report project no. 475.03-F d.d. 24 May 1974 from Biochemistry
Section, The Norwich Pharmacol Company, Norwich, New York. Submitted
to WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
MORRISON, J.L., CURRIE, G.N. & HARRINGTON, C. (1974b). Effects of
NF-180 treatment on plasma steroids in the female Sprague-Dawley
rat. Unpublished final report project no. 475.03-31 d.d. 18
September 1974 from Biochemistry Section, The Norwich Pharmacol
Company, Norwich, New York. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
MORRISON, J.L. (1976). Identification of NF-682 as a metabolite of
NF-180 in rats. Unpublished report d.d. 2 July 1976 attachment 5
from Norwich Pharmacal Company, Norwich, New York. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
MUSTAFA, A. I., ALI, B.H. & HASSAN, T. (1987). Semen characteristics
in furazolidone-treated goats. Reprod. Nutr. Develop., 27(1A):
89-94.
NI, Y.-C., HEFLICH, R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98nr
and TA98/1,8-DNP6. Mutat. Res., 192(1): 15-22.
OHTA, T., NAKAMURA, N., MORIYA, M., SHIRASU, Y. & KADA, T. (1984).
The SOS-function-inducing activity of chemical mutagens in
Escherichia coli. Mutat. Res., 131: 101-109.
PAIK, S.G. (1985). Micronucleus induction in mouse bone marrow cells
of some nitrofuran, 5-nitroimidazole and nitrothiazole derivatives
used as trichomonacides in Korea. Environ. Mutag. Carcinogens,
5(2): 61-72.
PALM, D., MAGNUS, U., GROBECKER, H. & JONSSON, J. (1967). Hemmung
der monoami-noxydase durch bakteriostatisch wirksame
nitrofuran-derivative. Naunyn-Schmiedebergs Arch. Pharmak., 256:
281-300.
PAUL, H. (1955). Oral chronic toxicity study on Nf-180 in dogs
(study) Unpublished report d.d. 16 June 1955 from Eaton
Laboratories, Norwich, New York. Submitted to WHO by SmithKline
Beecham Animal Health, West Chester, PA, USA.
PETTINGER, W.A., SOYANGCO, F.G. & OATES, J.A. (1968). Inhibition of
monoamine oxidase in man by furazolidone. Clinical Pharmacol.
Therapeut., 9(4): 442-447.
PROBST, G.S., McMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K. &
NEAL, S.B. (1981). Chemically-induced unscheduled DNA synthesis in
primary rat hepatocytes cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3: 11-32.
QUEINNEC, G., BABILE, R., DARRE, R., BERLAND, H.M. & ESPINASSE, J.
(1975). Induction of abnormalities in chromosomes (of cattle and
swine) by furazolidone or chloramphicol. Veterinary Bul., 46: 330.
RAY, W.H. (1959). Preliminary metabolism study of position
C14-labelled furazolidone in the white rat. Unpublished report no.
59-3 d.d. 18-09070 from Hess & Clark, Ashland, Ohio. Submitted to
WHO by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. (1962). An investigation of the metabolites of
furazolidone in swine urine. Unpublished research report no. 62:3
d.d. 29 May 1962 from Hess & Clark, Ashland, Ohio. Submitted to WHO
by SmithKline Beecham Animal Health, West Chester, PA, USA.
RAY, W.H. & HAYES, M. (1963). Evidence that the orange '415'
metabolite of furazolidone in swine is a nitrofuran. Unpublished
research report 63:2 d.d. 25 January 1963 from Hess & Clark,
Ashland, Ohio. Submitted to WHO by SmithKline Beecham Animal Health,
West Chester, PA, USA.
SCHERES, H.M.E. (1991a). Evaluation of the mutagenic activity of
3-amino-oxazolidinon-2 in the Ames Salmonella/microsome test.
Unpublished report project nr. d.d.15 January 1991 from RCC Notox,
5231 DD's Hertogenbosch. Submitted to WHO by SmithKline Beecham
Animal Health, West Chester, PA, USA.
SCHERES, H.M.E. (1991b). Evaluation of the ability of furazolidone
to induce chromosome aberrations in cultured peripheral human
lymphocytes. Unpublished report project no. 038385 d.d. 9 January
1991 from Notox RCC, 5231 DD's Hertogenbosch. Submitted to WHO by
SmithKline Beecham Animal Health, West Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
13 October 1966 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report problem 440.07 d.d.
4 January 1976 from Division of Chemotherapy, Research and
Development Department, The Norwich Pharmacal Company, Norwich, New
York. Submitted to WHO by SmithKline Beecham Animal Health, West
Chester, PA, USA.
STERN, I.J. HOOLIFIELD, R.D., WILK, S. & BUZARD, J.A. (1967). The
anti-monoamine oxidase effects of furazolidone. J. Pharmacol. Exp.
Therapeut., 156(3): 492-499.
TATSUMI, K. & TAKAHASHI, Y. (1982). Metabolic fate of furazolidone
in rats. Chem. Pharm. Bull. Tokyo, 30(9), 3435-3438.
TATSUMI, K. YAMADA, H., YOSHIMURA, H. & KAWAZOE, Y (1981).
Metabolism of furazolidone by milk xanthine oxidase and rat liver
9000 g supernatant: Formation of a unique nitrofuran metabolite and
an aminofuran derivative. Arch. Biochem. Biophysics, 208(1):
167-174.
TATSUMI, K., NAKABEPPU, H., TAKAHASHI, Y., & KITAMURA, S. (1984).
Metabolism in vivo of furazolidone: evidence for formation of an
open-chain carboxylic acid and aketoglutaric acid from the
nitrofuran in rats. Arch. Biochem. Biophysics, 234(1): 112-116.
TENNENT, D.M. & RAY, W.H. (1971). Metabolism of furazolidone in
swine (355994). Proc. Soc. Exp. Biol. Med., 138(3): 808-810.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan. J. Genetics, 48(4): 291-294.
Van Den DUNGEN, H.M., JAGER, L.P., LUYCKX, N.B. L., De GRAAF, G.J. &
BAARS, A.J. (1991). Adrenal toxicity of carbadox and furazolidone
in vitro. Act. Vet. Scand., 87: 338-340.
VOOGD, C.E., KRAMERS, P.G.N., KNAAP, A.G.A.C., OUD, J.H. & Van WENT,
G.F. (1982). Mutagenicity of feed additives. Postersession RIV,
Bilthoven. as cited in Van MIERT, A.S.J.P.A.M., VROOMEN, L.H.M. &
JAGER, L.P. (1984) Farmacologie en toxicologie van furazolidon en
carbadox: een literatuuronderzoek. Tijdschr. Diergeneeskd.
109(22): 928-932.
VROOMEN, L.H., BERGHMANS, M.C., Van LEEUWEN, P., Van Der STRUIJS,
T.D.B.M, De VRIES, P.H.U. & KUIPER, H.A. (1986). Kinetics of
14C-furazolidone in piglets upon oral administration during 10
days and its interaction with tissue macromolecules. Food Add.
Contamin., 3: 331-346.
VROOMEN, L.H. (1987). Quantitative studies of the metabolism of
furazolidone by rat liver microsomes. Toxicol. in vitro, 1(2):
97-104.
VROOMEN, L.H., BERGHMANS, M.C., HEKMAN, P., HOOGENBOOM, L.A.P. &
KUIPER, H.A. (1987a). The elimination of furazolidone and its
open-chain cyano-derivative from adult swine. Xenobiotica, 17:
1427-1435.
VROOMEN, L.H., GROTEN, J.P., Van MUISWINKEL, K., Van VELDHUIZEN,
P.J. & Van BLADEREN, P.J. (1987b). Identification of a reactive
intermediate of furazolidone formed by swine liver microsomes.
Chem. Biol. Interact., 64(1-2): 167-180.
VROOMEN,L.H., BERGHMANS, M.C., GROTEN, J.P., KOEMAN, J.H. & Van
BLADEREN, P.J. (1988). Reversible interaction of a reactive
intermediate derived from furazolidone with glutathione and protein.
Toxicol. Appl. Pharmacol., 95: 53-60.
VROOMEN, L.H., BERGHMANS, M.C., Van BLADEREN, P.J., GROTEN, J.P.,
WISSINK, C.J. & KUIPER, H.A. (1990). In vivo and in vitro
metabolic studies of furazolidone: A risk evaluation. Drug. Metab.
Rev., 22(6-8): 663-676.
WHITE, A.H. (1989). Absorption, distribution, metabolism and
excretion of furazolidone. Scand. J. Gastroenterol.,
24(suppl. 169): 4-10.
