
NITROFURAL (NITROFURAZONE)
First draft prepared by
Dr K.N. Woodward
Veterinary Medicine Directorate
Ministry of Agriculture, Fisheries and Food
Weybridge, Surrey, United Kingdom
1. EXPLANATION
Nitrofural also known as nitrofurazone, is a broad-spectrum
bactericidal drug. It also possesses some antiprotozoal activity. It
is used both therapeutically and prophylactically in a number of
food-producing species including pigs, sheep, goats, cattle,
chickens and turkeys (Orphahell, 1991). The chemical structure is
shown below in Figure 1.
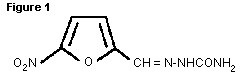 Nitrofural had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
Nitrofural appeared to be well absorbed in the rat after oral
administration of 100 mg/kg bw. At 4 h after administration plasma
levels had reached 4.5 mg/l. No other time points were investigated.
Around 5% and 0.5% of the dose were excreted in the 24 h urine and
faeces, respectively. As acknowledged by the authors, the values are
likely to be underestimates as nitrofurazone was shown in this study
to be rapidly metabolized after incubation with rat liver slices.
There was no evidence of deposition in any organ (Paul et al.,
1960).
2.1.1.2 Cattle
A single oral dose of 14 mg/kg bw nitrofural administered to
five pre-ruminant calves resulted in maximum plasma concentrations
of 3.5 mg/litre at 3 h after administration. The final elimination
half-life was 5 h and around 2% of the administered dose was
recovered in the urine (Nouws et al., 1987).
2.1.2 Biotransformation
As described in paragraph 2.1.1.1, nitrofural is rapidly
metabolized by rat liver slices. Nitrofurans, including nitrofural,
undergo metabolic reduction at the nitro group to generate reactive
species which can covalently bind to cellular macromolecules
(Polnaszek et al., 1984; Kutcher & McCalla, 1984; McCalla 1979;
McCalla et al., 1975). Data obtained from studies with isolated
perfused rat liver suggested that conjugation to glutathione
followed by biliary excretion was important; only 0.27% of the
administered perfusate was excreted unchanged (Sorrentino et al.,
1987). Conjugation with amino acids such as methionine may also
occur (Hoener, 1988).
Ring opening of nitrofurans is known to occur (Swaminathan &
Lower, 1978) but there are insufficient data to demonstrate this
with nitrofurazone.
2.1.3 Effects on enzymes and other biochemical parameters
In chickens nitrofural increased gamma-aminobutyric acid and
glutamate concentrations in the brain at oral doses of 25 and 50
mg/kg bw for 5 days (Ali, 1988). Nitrofural, given orally at doses
of 10 and 20 mg/kg bw for 7 days, induced thiamine deficiency in
chickens (Ali 1983).
Nitrofural was found to inhibit the uptake of oxygen by various
rat tissues (Paul et al., 1952). Nitrofural inhibited respiration
in isolated mouse liver mitochondria suggesting that these may be
potential sites for toxicity (Lim et al., 1986).
Nitrofural and other nitrofurans are relatively potent
inhibitors of MAO in mammalian and avian species (Hoogenboom, 1991).
As with other nitrofurans, nitrofural can affect the endocrine
system resulting in alterations of hormone levels. In laying
turkeys, nitrofural decreased luteinizing hormone and prolactin in
plasma (Ali et al., 1987). Similar effects were noted in young
male turkeys (Ali et al., 1988).
Nitrofural has been shown to inhibit utilization of
progesterone as a steroid percursor by the adrenal in rats. In this
study it was shown that nitrofurans, including nitrofural, block
11-hydroxylation of deoxycorti-costerone to corticosterone.
Sprague-Dawley rats (1 year old) given nitrofurans for 325 days
underwent constant oestrous. The authors concluded that nitrofurans
were antagonists of normal adrenal function and believed that this
could lead to endocrine stress (King & Currie, 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The LD50 values for nitrofural are shown in Table 1. Signs of
toxicity included roughened coats, hyperexcitability, tremors,
reduced activity, weakness, convulsion, and respiratory collapse
(Krantz & Evans 1945; Anderson, 1983).
Table 1. Acute toxicity of nitrofural
Species/strain Sex Route LD50 Reference
(mg/kg bw)
Rat (unspecified) M oral 590 Krantz & Evans,
1945
Rat (Donryu) M&F oral 590 Miyaji, 1971
Rat (Wistar) M oral 800 Anderson, 1983
Mouse ? oral 380 Krantz & Evans,
(unspecified) 1945
Mouse (ICR Jcl) M&F oral 590 Miyaji, 1971
Mouse ? i.p. 300 Smith et al.,
(unspecified) 1963
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 5 male and 5 female B6C3F1 mice were given
diets containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 94.5, 188, 375, 750 or 1500 mg/kg bw/day for 14
days.
All mice given the three highest dietary levels died during the
study, as did 3/5 given 188 mg/kg bw/day. Animals in all the groups
except females given 94.5 mg/kg bw/day lost weight and all dosed
animals displayed rough coats and convulsive seizures (National
Toxicology Program (NTP), 1988).
Groups of 10 male and 10 female B6C3F1 mice were given
diets containing 0, 70, 150, 310, 620 or 1250 ppm nitrofural,
equivalent to 0, 10, 23, 47, 93 or 188 mg/kg bw/day for 13 weeks.
High mortality was noted at the highest dietary level (6/10
males and 9/10 females). Mortality was relatively high at the
penultimate dietary level (3/10 males and 5/10 females). Elevated
relative liver weights were noted in mice given the two highest
dietary levels. Animals given the two highest dietary levels also
displayed hyperexcitability and convulsive seizures, and males in
these groups had a very high incidence (80-90%) of testicular
hypoplasia (NTP, 1988).
2.2.2.2 Rats
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 63, 125, 250, 500 or 1000 mg/kg bw/day, for 14
days.
All rats given 500 or 1000 mg/kg bw/day died. Feed consumption
was decreased at all levels in excess of the lowest level. Signs of
toxicity included lethargy and roughened coats. Seizures occurred in
animals given 250 mg/kg bw/day and above. Histopathologic
examination revealed aspermatogenesis in dosed males (NTP, 1988).
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 150, 310, 620, 1200 or 2500 ppm nitrofural, equivalent
to 0, 15, 31, 62, 120 or 250 mg/kg bw/day, for 13 weeks.
All rats survived until the end of the study but final body
weights of animals given 62, 120 or 250 mg/kg bw/day were 11%, 32%
and 55% lower, respectively, than control values. Relative liver
weights for treated rats were significantly higher than control
animals. Atrophy constricture of the hind-quarters occurred in males
and females given the highest dietary level.
Moderate to severe degeneration of the seminiferous epithelium
of the testes was noted in males given the 4 highest doses. The
no-effect-level was 15 mg/kg bw/day. Males and females given the two
highest dietary levels had osteoporosis in the metaphyseal region.
(NTP, 1988).
In a study designed to investigate effects on the testes,
45-day-old male Donryu rats were given a diet containing 0.2%
nitrofural (equal to 300 mg/kg bw/day) for 9 days. Three to 6 rats
given nitrofural were sacrificed every day after feeding began.
A decrease in testis weight occurred by day 5 but
histologically, a decrease in spermatozoa and spermatids occurred at
day 1, with disappearance by days 3 and 4. Here, the stratified
appearance of the normal testis had disappeared and only basal
spermatogonia and Sertoli cells remained. Slight oedema and
exudation occurred in interstitial tissue but Leydig cells appeared
normal (Miyaji et al., 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female B6C3F1 mice were given
diets containing 0, 150 or 310 ppm nitrofural equal to 0, 14 or 29
mg/kg bw/day for 2 years.
There were no effects on body weights and survival was only
slightly affected at the highest dose in males and females (27/50
and 35/50 for males and females, respectively) compared with
controls (39/50 for each sex).
Females given nitrofural were found to have increased
incidences of ovarian atrophy (44/50 and 38/50 in low- and high-dose
groups respectively) compared with controls (7/47) and tubular cell
hyperplasia (23/50 and 21/50 in low- and high-dose groups) when
compared with controls (1/47). However, there were no effects on the
testes in males.
There were increased incidences of ovarian granulosa cell
tumours in low- (4/50) and high-dose (9/50) females when compared
with control values (1/47). Similarly, there were increased
incidences of benign mixed tumours of the ovary in low- (17/50) and
high-dose (20/50) females compared with controls (0/47). There were
no elevated incidences of any tumour type in male mice (NTP, 1988;
Kari et al., 1989).
In a study of transplacental carcinogenesis, a group of 20
pregnant ICR/Jcl mice was given 3 subcutaneous injections at
75 mg/kg bw nitrofural on days 13, 15 and 17 of gestation. The
incidence of tumours in offspring was not increased when compared
with untreated controls when they were examined 32 weeks after
birth.
In the same study, newborn mice (61) were given a subcutaneous
injection at 75 mg/kg bw 12 h after birth and on days 7, 14 and 21.
The offspring were examined at 32 weeks after birth when an
increased incidence of pulmonary papillary adenomas was noted
(Nomura et al., 1984).
2.2.3.2 Rats
A group of 20 female Holtzman rats was fed a diet containing
1000 ppm nitrofural (150 mg/kg bw/day) for 36 weeks. A similar group
of 30 females was fed the same level for 44.5 weeks. Animals were
killed 15-19 weeks after dosing was completed. At termination, there
was an elevated incidence of mammary tumours in rats fed
nitrofural-containing diets when compared with controls (Morris
et al., 1969; Morris, 1965).
Groups of 35 female Holtzman rats were fed diets containing
0, 500 or 1000 ppm nitrofural (0, 75 or 150 mg/kg bw/day) for 45
weeks, and then a basal diet for a further 8 weeks. Rats consuming
nitrofural at 75 mg/kg bw/day nitrofural had no increased tumour
incidence but in those given 150 mg/kg bw/day the numbers of
tumour-bearing rats were increased. At week 53, the numbers with
detectable mammary tumours were significantly increased in both the
75 (10/35) and 150 (12/35) mg/kg bw/day groups, when compared with
untreated controls (2/35). None of the reported tumours were
malignant (Siedler & Searfoss, 1966).
Groups of 20 male and 20 female CFE rats were fed diets
containing nitrofural to give a daily intake of 50-55 mg/kg bw/day
for 45 weeks. At the end of this period the animals were returned to
the basal diets for a further 7 weeks. There was no increased
incidence of any tumours in the male rats. A significant increase in
the incidence of benign mammary tumours was noted at week 52 (12/20)
compared with untreated controls (0/20) (Siedler & Searfoss, 1967).
Groups of 50 male and 50 female F344/N rats were fed diets
containing 0, 310 or 620 ppm nitrofural equal to 0, 11 or 24 mg/kg
bw/day for 2 years. There were reductions in the rate of weight gain
and final body weights were reduced in males and females given
dietary nitrofural. Mortality was increased in males given the high
dietary level (30/50; 60%) compared with controls (17/50; 34%).
The major non-neoplastic finding was degeneration of joint
articular cartilages in males and females in several areas of the
body, notably, the vertebral and knee joints. A no-effect-level was
not identifiable. Testicular degeneration occurred in 49/50 low
dietary level and 47/50 high dietary level males compared with 12/50
in controls.
There was an increased incidence of mammary fibroadenomas in
both low and high dietary level females (36/50; 72% in both groups)
when compared with controls (8/49; 16%) but the incidence of
adenocarcinomas was not elevated. In males, the incidence of
mesotheliomas in the tunica vaginalis was increased but not in a
dose-related manner (0/50, 7/50 and 2/50 in the control, low and
high dietary level groups). There was also an increased incidence of
sebaceous adenoma of the skin of male rats but only at the high
dietary level (0/50, 0/50 or 5/50 for controls, low and high dietary
levels, respectively). A slightly increased incidence of carcinoma
of the preputial gland was seen in males (1/50, 8/50 and 5/50 in the
control, low and high dietary groups, respectively). The incidence
of testicular tumours was reduced in dosed males (NTP, 1988; Kari
et al., 1989).
It was concluded by the authors that the data from male rats
provided only equivocal evidence of carcinogenicity. The increases
in incidences of the various tumours were small and not
dose-related.
The induction of ovarian tumours noted in mice treated with
chemicals is relatively rare. Such an effect had been noted with
only 8 chemicals from the NTP studies among which were nitrofural
itself, 1,3-butadiene, 4-vinylcyclohexene, nitrofurantoin, benzene
and delta-9-tetrahydrocannabinol. The tumours were mainly in mice
but rats too were also affected (Maronpot, 1987).
Nitrofural is unusual in that of 143 chemicals which had been
tested by the NTP by 1989, only 5, including nitrofural, produced
only benign neoplastic lesions (Huff et al., 1989). Many other
nitrofurans tested have been shown to be carcinogenic in rodents and
many of these have produced malignant tumours (Cohen & Bryan, 1973;
Cohen, 1978; Cohen et al., 1973).
Nitrofural and other nitrofurans inhibited the
hepatocarcinogenesis by 4-(dimethylamino) azobenzene in rats (Akao
et al., 1971). It also inhibited the growth of fibrosarcomas in
mice and demonstrated antineoplastic properties against seminomas
and carcinoma of the prostate in humans (Friedgood & Green, 1950;
Friedgood & Ripstein, 1951).
The International Agency for Research on Cancer (IARC) has
concluded that there was limited evidence for the carcinogenicity of
nitrofurazone in experimental animals and inadequate evidence for
its carcinogenicity in humans. In its overall evaluation, IARC
placed nitrofural in Group 3 (not classifiable as to its
carcinogenicity) (IARC, 1990).
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on teratogenicity
2.2.5.1 Mice
A group of 16 pregnant ICR/Jcl mice was given subcutaneous
injections at 100 mg/kg bw nitrofural on days 9, 10 and 11 of
gestation. A group of 6 pregnant mice was given a single
subcutaneous injection at 300 mg/kg bw nitrofural on day 10 of
gestation. Controls were given water only on days 9, 10 and 11 of
gestation.
The 100 mg/kg bw regime did not produce an elevated incidence
of malformations in 185 fetuses examined. However, the 300 mg/kg bw
dose on day 10 of gestation led to an overall incidence of
malformations of 21% compared with 0.3% in controls. These were
mainly tail anomalies, oligodactyly, and leg defects (unspecified)
(Nomura et al., 1984).
In a prescreening developmental test, female CD-1 mice were
given nitrofural (100 mg/kg bw/day) by gavage on days 6-13 of
gestation. Nitrofural caused a decrease in the numbers of viable
litters (28/35 compared with 45/45 in controls) and slight
reductions in birth weights. By the authors' criteria, these effects
constituted positive results (Hardin et al., 1987).
In a preliminary teratogenicity study, groups of 6-7 CD-1 mice
were given diets containing 0, 100, 500, 1000, 2000 or 3000 ppm
nitrofural, equivalent to 0, 15, 75, 150, 300 or 450 mg/kg bw/day,
on days 6-17 of gestation.
Dams given the two highest dietary levels died during the
study. There were no differences in food consumption in the other
groups and no effects on weight gain. There were no differences in
implantation site and resorption parameters except in those given 75
mg/kg bw/day where an increased incidence of resorptions was noted
(Price et al., 1985).
In a second preliminary teratology study, groups of 12 pregnant
CD-1 mice were given diets containing 0, 75, 250, 500 or 750 ppm
nitrofural equivalent to 0, 11, 38, 75 or 112 mg/kg bw/day, on days
6-17 of gestation. Approximately 8% mortality occurred at the
highest dietary level and hyperactivity, tremor, paralysis and
convulsions were seen at the two highest levels. There were
dose-related decreases in food consumption and body weights. Gravid
uterine weights decreased with increasing nitrofural intake. There
were no effects on implants/dam but the numbers of resorptions per
litter increased with dose. There were no increases in the
incidences of malformations (Price et al., 1985).
In the main teratogenicity study, groups of 26 pregnant CD-1
mice were given diets containing 0, 38, 75, 250 or 500 ppm
nitrofural, equivalent to 0, 6, 11, 38 or 75 mg/kg bw/day on days
6-15 of gestation. There were no maternal deaths and no effects on
food and water consumption. Gravid uterine weights were unaffected
by compound intake and there were no effects on reproductive
parameters except for an increased incidence of late fetal deaths at
marginally maternally toxic doses. The NOEL was 11 mg/kg bw/day
(Price et al., 1985).
2.2.5.2 Rabbits
Groups of 22-27 New Zeeland white rabbits were given gavage
doses of 0, 5, 10, 15 or 20 mg/kg bw/day nitrofural in corn oil on
days 6-19 of gestation.
At sacrifice, the uterine contents of 121 pregnant animals were
examined. There was no evidence of any teratogenic effect nor
materno- or embryo/feto-toxicity at doses of 5-15 mg/kg bw/day. At
20 mg/kg bw/day, two dams died, there was reduced weight gain,
relative and absolute maternal liver weights were increased and an
increased incidence of fetal malformations/litter was observed,
which was suggestive of fetotoxicity. Hence, effects on the
developing fetus occurred only at maternally toxic levels
(Price et al., 1987). The NOEL was 15 mg/kg bw/day.
2.2.6 Special studies on genotoxicity
Nitrofural has been tested in a range of studies (Table 2). In
general, in vitro studies of forward mutation in bacteria and
mammalian cells, sister chromatid exchange, DNA damage and tests for
clastogenicity have proved positive while in vivo studies,
including those for cytogenetic effects and the micronucleus test,
have proved negative.
In studies where the metabolic activation was incorporated into
the agar layer, the mutagenic activities of a number of nitrofurans
(and nitroimidazoles) were reduced, suggesting that the metabolites
of reductive metabolism may be less mutagenic than those formed
after oxidative metabolism (Skeggs et al., 1984). Metabolic
reduction is believed to be the major route of biotransformation of
nitrofurans and nitroimidazoles and the metabolites so formed may
account for the radiosensitizing properties of these agents (Olive,
1979, 1980, 1978b).
The results obtained with nitrofural are similar to those noted
with a wide range of nitrofurans, and in particular, the propensity
of these compounds for activity in in vitro systems is evident
(Klemencic & Wang, 1978). The mechanism of mutagenicity is unclear,
as is the route of activation, although nitroreduction appears to be
an early step (McCalla, 1981, 1983; Matsuoka et al., 1979; McCalla
et al., 1970, 1971). Nitrofural and other nitrofurans are
cytotoxic to hepatocytes in vitro (Hoogenboom et al., 1991), but
as the tumours caused by nitrofural are largely in endocrine organs,
this is unlikely to be relevant to carcinogenesis by this substance.
2.3 Observations in humans
Nitrofural is a skin sensitizer in humans and there have been
reports of contact dermatitis following exposure to the drug (Bajaj
& Gupta, 1986; Laubstein and Niedergesass, 1970; Lo et al., 1990).
When used as an experimental antineoplastic agent in humans
with prostatic or testicular tumours, testicular degeneration
occurred similar to that noted in experimental animals (Friedgood &
Ripstein, 1951). The doses used were unclear in the original text.
In the Collaborative Perinatal Project conducted in the USA by
the National Institute of Neurological and Communicative Diseases
and Stroke in 1958-1965, only 0.5% (234/50282) of mother-child pairs
had been exposed to nitrofural during months 1-4 of gestation. There
was no increased risk of malformations in this group (Heinonem
et al., 1977).
Table 2. Results of genotoxicity assays on nitrofurazone
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0-75 µg/ml Positive Baars et al.,
TA1538 1980
Ames test1 S. typhimurium 0-5 µg/plate Positive Goodman et al.,
TA98, TA100 1977
Ames test1 S. typhimurium 0-100 µg/plate Positive NTP, 1988
TA100, TA1535,
TA1537, TA98
Ames test1 S. typhimurium 1-20 µg/plate Positive Ni et al., 1987
TA98, 98NR, 98/
1.8-DNP6
Ames test1 S. typhimurium 0.1-10 µg/plate Positive Yahagi et al.,
TA1535, TA100 1976
Ames test1 S. typhimurium 0-250 nmole/plate Positive Gajewska et al.,
TA97, TA102 1990
Ames test1 S. typhimurium 0.1-100 µg/plate Positive Chandler &
TA98 Parenti, 1982
Forward mutation S. typhimurium 0-0.5 µg/ml Positive Karube et al.,
TA100 electrode 1982
Forward mutation1 E. coli 0-75 µg/ml Positive Baars et al.,
343/113/(R-9 1980
Table 2. cont'd
Test system Test object Concentration Results Reference
Forward mutation1 E. coli WP2 0-240 µM Weak Bryant & McCalla,
Positive 1980
Forward mutation1 E. coli K12 0-10 mm Positive Chessin et al.,
1978
Forward mutation1 E. coli W2 10-300 µM Positive Lu et al., 1979
WP2 uvrA 0-250 µM Positive
Forward mutation2 E. coli K12 not specified Positive Maier et al.,
343/13 1979
Forward mutation1 E. coli WP2 30-120 µM Positive McCalla &
uvrA- Voutsinos, 1974
Forward mutation1 E. coli B and S unspecified Positive Zampieri &
strains Greenberg, 1974
Fluctuation test1 S. typhimurium 0-1 ng/ml Positive Green et al., 1977
TA1535, TA100
Positive
E. coli WP2 uvrA 0-100 ng/ml
Forward mutation1 A. nidulans 0-1000 µg/plate Negative Bignami et al.,
1982
Forward mutation1 N. crassa 0-200 µg/ml Positive Ong, 1977
74-OR60-29A
SOS Chromotest E. coli PQ37 0.01-01 ng/sample Positive Gajewska et al.,
1990
Table 2. cont'd
Test system Test object Concentration Results Reference
DNA damage Mouse L cells 500 µM Positive Olive & McCalla,
1977
DNA damage Mouse L929 cells )0-500 µM Positive Olive & McCalla,
Hamster BHK21 ) 1975
cells )
Human KB cells )
DNA damage Swiss mice 0.1% in diet Positive Olive, 1978a
in vitro (150 mg/kg bw/day)
Unscheduled DNA Rat thymocytes unspecified Positive Tempel, 1980
synthesis
Unscheduled DNA Rat and mouse 1.1 x 10-3-1.1 mg/ml Negative Mori et al., 1987
synthesis hepatocytes
Sister chromatid Chinese hamster 0-15 µg/ml Positive NTP, 1988
exchange ovary cells
Forward mutation1 Mouse lymphoma 0-300 µg/ml Positive NTP, 1988
L5178Y cells 0-400 µg/ml
Forward mutation1 Chinese hamster 100 µg/ml Positive Olive, 1981
V79 cells
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Phillips, 1983
ovary - HGPRT
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Anderson &
ovary - HGPRT Phillips, 1985
Table 2. cont'd
Test system Test object Concentration Results Reference
Sex-linked D. melanogaster 5 mM Negative Kramers, 1982
recessive lethal
mutation
In vitro Chinese hamster 0-200 µg/ml Positive Anderson &
cytogenetics bone marrow Phillips, 1985
In vitro Chinese hamster 0-600 µg/ml Positive NTP, 1988
cytogenetics ovary
In vitro Chinese hamster 0.1 mg/ml Positive3 Matsuoaka et al.,
cytogenetics cells (male 1979
In vitro Human lymphocytes not specified Positive Tonomura &
cytogenetics Sasaki, 1978
In vivo Chinese hamster 0-400 mg/kg bw Negative Anderson &
cytogenetics bone marrow Phillips, 1985
In vivo Wistar rat bone 0-400 mg/kg bw or Negative Anderson, 1983
cytogenetics marrow 1-150 mg/kg
bw/day for 5 days
Micronucleus test Sprague-Dawley rat )0-60 mg/kg bw; Negative Goodman et al.,
)half dose 30 h and 1977
Long Evans rat )half dose 6 h,
)prior to sacrifice
1 With and without hepatic metabolic activation.
2 With and without testicular metabolic activation.
3 In presence but not absence of metabolic activation.
3. COMMENTS
The results of acute and short-term toxicity studies,
carcinogenicity studies, and teratogenicity studies were considered
by the Committee, in addition to several genotoxicity studies. Some
limited pharmacokinetic data were also available which demonstrated
good absorption of the drug in rats and cattle, but no data were
available on the extent of biotransformation nor on the identities
of any metabolites formed. A comparison with other nitrofurans
suggests that nitrofural probably undergoes extensive
biotransformation.
In acute oral toxicity studies, nitrofural was slightly toxic
to mice (LD50 = 300-600 mg/kg bw) and rats (LD50 = 600-800 mg/kg
bw).
Testicular hypoplasia was noted in rats and mice given dietary
nitrofural for 13 weeks. The NOEL for this effect was 15 mg/kg
bw/day in a 13-week study in rats.
Nitrofural was tested for its ability to induce forward
mutations in bacteria, yeast, and mammalian cells in vitro. In the
majority of these studies it produced positive results. Positive
results were also noted in tests for DNA damage in bacteria and in
mammalian cells in vitro, in in vitro studies for clastogenic
effects in Chinese hamster ovary or bone marrow cells, and in human
lymphocytes. However, in vivo studies of clastogenic potential in
Chinese hamster or rat bone marrow were negative. The results
suggest that nitrofural is genotoxic in vitro but not in vivo.
Carcinogenicity studies with nitrofural were conducted in rats
and mice. In a 2-year dietary study in B6C3F1 mice, the
animals were given nitrofural equal to daily oral doses of 14 or
29 mg/kg bw/day. In female mice, nitrofural induced a high incidence
of ovarian atrophy and of ovarian tubular-cell hyperplasia in both
dose groups when compared with controls. There were also increased
incidences of ovarian granulosa-cell and benign mixed tumours in
low-and high-dose groups. There were no elevated incidences of any
tumour type in male mice.
In a 2-year carcinogenicity study in rats, testicular
degeneration was one of the major non-neoplastic findings. There
were also elevated incidences of degeneration of the joint articular
cartilages at several sites in both males and females. No-effect
levels could not be identified for these findings. There was an
increased incidence of mammary fibroadenomas in females given the
low and high doses of 11 and 24 mg/kg bw/day.
Teratogenicity studies were conducted in mice and rabbits. In
mice, the rate of late fetal death was increased in treated animals
at maternally toxic doses; the NOEL was 11 mg/kg bw/day. Pregnant
rabbits were given gavage doses of up to 20 mg/kg bw/day. At the
highest dose, there was an elevated incidence of minor fetal
malformations per litter but this dose produced maternal toxicity.
The NOEL was 15 mg/kg bw/day. The data suggest that nitrofural is
not a direct teratogen but produces fetotoxic effects at maternally
toxic doses in mice and rabbits.
Although nitrofural was tumorigenic in rats and mice, the
tumours it produced were benign and were restricted to endocrine
organs and the mammary gland. Moreover, the mutagenicity studies
suggest that nitrofural is genotoxic in vitro but not in vivo.
Taken together, the data suggest that nitrofural is a secondary
carcinogen producing its effects in endocrine-responsive organs by a
mechanism that remains to be elucidated. In rats, nitrofural has
been shown to disrupt the utilization of progesterone and blocks the
synthesis of cortisone from deoxycortisone. Effects on
steroidogenesis may therefore be involved in the process of tumour
formation. The closely related compound nitrofurantoin also produced
benign ovarian tumours in the same strain of mouse, which were
thought to be secondary to drug-induced ovarian atrophy. This type
of effect cannot be excluded in the biogenesis of the ovarian
tumours produced by nitrofural. However, the Committee recognized
that no-effect levels for these effects could not be identified. The
Committee noted that nitrofural had been reviewed by the
International Agency for Research on Cancer, which concluded that
there was limited evidence for the carcinogenicity of nitrofural in
animals but inadequate evidence for humans.
4. EVALUATION
The Committee concluded that it could not establish an ADI for
nitrofural because no-effect levels had not been established for the
tumorigenic effects. It noted that, although a no-effect level
(15 mg/kg bw/day) had been established in rats for testicular
hypoplasia in a 13-week study, a slightly lower dose (11 mg/kg
bw/day) resulted in a high incidence of testicular degeneration in
the 2-year carcinogenicity study. There was no NOEL for this effect
in the 2-year study and, moreover, no study on reproductive
performance was available. The degenerative changes in the joints of
rats were seen in both males and females but a no-effect level could
not be established.
Before reviewing nitrofural again the Committee would wish to
see:
1. Further data from long-term studies in rats which would
allow the identification of no-effect levels for the
effects on joint articular cartilages and for testicular
degeneration.
2. Data to support the view that tumour formation in rodents
following nitrofural administration has an endocrine
origin. If a mechanism were demonstrated, no-effect levels
for suitable end-points would need to be identified.
3. Additional data on the identity, quantity and biological
characteristics of nitrofural metabolites.
5. REFERENCES
AKAO, M., KURODA, K., KANISAWA, M. & MIYAKI, K, (1971). Influence of
some nitrofurans on carcinogenesis in rats fed 4-(dimethylamino)
azobenzene. Gann, 62: 479-484.
ALI, B.H. (1983). The effect of nitrofurazone on the thiamine status
of chickens. Comp. Biochem. Physiol., C.76: 131-134.
ALI, B.H., SILSBY, J. & EL HALAWANI, M.E. (1987). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
Brit. Poultry Sci., 28: 613-621.
ALI, B.H. (1988). Effect of furazolidone and nitrofurazone on brain
gamma-aminobutyric acid and glutamate concentrations in chickens.
Clin. Exp. Pharmacol. Physiol., 15: 959-963.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL) levels in young turkeys. Gen. Pharmacol., 19:
91-95.
ANDERSON, D. (1983) Cytogenetic analysis in rat bone marrow with
nitrofurazone. Project No. 466.59.63. Unpublished report for Norwich
Eaton Pharmaceuticals, Inc. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
ANDERSON, D. & PHILLIPS, B.J. (1985). Nitrofurazone - genotoxicity
studies in mammalian cells in vitro and in vivo. Food Chem.
Toxicol., 23: 1091-1098.
BAARS, A.J., BLIJLEVEN, W.G.H., MOHN, G.R., NATARAJAN, A.T. &
BREIMER, D.D. (1980). Preliminary studies on the ability of
Drosophila microsomal preparations to activate mutagens and
carcinogens. Mutat. Res., 72: 257-264.
BAJAJ A.K. & GUPTA, S.C. (1986). Contact hypersensitivity to topical
antibacterial agents. Int. J. Dermatol., 25: 103-105.
BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L., CREBELLI, R. &
FABRIZI, M. (1982). Evaluation of 2 different genetic markers for
the detection of frameshift and missense mutagens in A. nidulans.
Mutat. Res., 97: 293-302.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran-induced mutagenesis
and error prone repair in Escherichia coli. Chem-Biol. Interact.,
31: 151-166.
CHANDLER, A.D. & PARENTI, R.R. (1982). Isolation and
characterisation of a nitrofurazone resistant strain of Salmonella
TA98. Environ. Mutagen., 4: 319-320.
CHESSIN, H., McLAUGHLIN, T., MROCZKOWSKI, Z., RUPP, W.D. & LOW, K.B.
(1978). Radiosensitization, mutagenicity, and toxicity of
Escherichia coli by several nitrofurans and nitroimidazoles.
Radiat. Res., 75: 424-431.
COHEN S.M. & BRYAN, G.T. (1973). Carcinogenesis caused by nitrofuran
derivatives. Pharmacology and the Future of Man. Proc. Vth Int.
Congr. Pharmacol., San Francisco, Karger, Basel, 161-170.
COHEN, S.M., ETURK, F., VON ESCH, A.M., CROVETTI, A.J. & BRYAN, G.T.
(1973). Carcinogenicity of 5-nitrofurans, 5-nitromidazoles,
4-nitrobenzenes and related compounds. J. Natl. Cancer Inst., 51:
403-417.
COHEN, S.M. (1978). Toxicity and carcinogenicity of nitrofurans.
Carcinogenesis, 4: 171-231.
FRIEDGOOD, C.E. & GREEN, M.N. (1950). The effect of nitrofurazone on
the growth of fibrosarcoma in mice. Cancer Res., 10: 613-615.
FRIEDGOOD, C.E. & RIPSTEIN, C.B. (1951). The effect of nitrofurazone
on tumours of the human testis and prostate gland. Surg. Forum,
329-333.
GAJEWSKA, J., SZCZYPKA, M., TUDEK, B. & SZYMCZYK, T. (1990). Studies
on the effect of ascorbic acid and selenium on the genotoxicity of
nitrofurans : nitrofurazone and furazolidone. Mutat. Res., 232:
191-197.
GOODMAN, D., AUFRERE, M., VORE, M. & MEYERS, F. (1977). Metabolism
of nitrofurazone. 61st Annual Meeting of the Federation of American
Societies for Experimental Biology, Chicago, Illinois.
GREEN, M.H.L., ROGERS, A.M., MURIEL, W.J., WARD, A.C. & McCALLA,
D.R. (1977). Use of a simplified fluctuation test to detect and
characterize mutagenesis by nitrofurans. Mutat. Res., 44: 139-143.
HARDIN, B.D., SCHULER, R.L., BURG, J.R., BOOTH, G.M., HAZELDEN,
K.P., MacKENZIE, K.M., PICCIRILLO, V.J., & SMITH, K.N. (1987).
Evaluation of 60 chemicals in a preliminary developmental toxicity
test. Terat. Carcinogen. Mutagen., 7: 29-48.
HEINONEN, O.P., SLONE, D. & SHAPIRO, S. (1977). (Eds). Antimicrobial
and antiparasitic agents. In: Birth Defects and Drugs in Pregnancy,
Publishing Sciences Group, Inc, Littleton, MA, 296-313.
HOENER, B-A. (1988). Nitrofurazone : Kinetics and oxidative stress
in the singlepass isolated perfused rat liver. Biochem. Pharmacol.,
37: 1629-1636.
HOOGENBOOM, L.A., OORSPRONG, M.B.M., VAN VLIET, T. & KUIPER, H.A.
(1991). The use of pig hepatocytes for cytotoxicity studies of
veterinary drugs: A comparative study with furazolidone and other
nitrofurans. Toxicol. in Vitro, 5: 31-38.
HOOGENBOOM, L.A. (1991). The use of pig hepatocytes for
biotransformation and toxicity studies. Thesis,
Landbouwuniversiteit, Wageningen, Netherlands.
HUFF, J.E., EUSTIS, S.L. & HASEMAN, J.K. (1989). Occurrence and
relevance of chemically-induced benign neoplasms in long-term
carcinogenicity studies. Cancer Metastasis Rev., 8: 1-22.
IARC (1990). Monographs on the evaluation of carcinogenic risks to
humans. Pharmaceutical Drugs, IARC, Lyon, 50: 195-209.
KARI, F.W., HUFF, J.E., LEININGER, J., HASEMAN, J.K. & EUSTIS, S.L.
(1989). Toxicity and carcinogenicity of nitrofurazone in F344/N rats
and B6C3F1 mice. Food Chem. Toxicol., 27: 129-137.
KARUBE, I., NAKAHARA, T., MATSUNAGA, T. & SUZUKI, S. (1982).
Salmonella electrode for screening mutagens. Anal. Chem., 54:
1725-1727.
KING, C.D. & CURRIE, G.N. (1972). Effects of nitrofurans on
steroidogenesis and mammary hyperplasia. Unpublished report. Norwich
Pharmacal Company, Project No. 596.09. Submitted to WHO by Orphahell
B.V., Midrecht, Netherlands.
KLEMENCIC, J.M. & WANG, C.Y. (1978). Mutagenicity of nitrofurans.
In: Bryan, G.T. ed. Carcinogenesis, 4: 99-130, New York, Raven
Press.
KRAMERS, P.G.N. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
KRANTZ, J.C. & EVANS, W.E. (1945). A contribution to the
pharmacology of 5-nitro-2-furaldehyde semicarbazone. J. Pharmacol.
Exp. Therap., 85: 324-331.
KUTCHER, W.W. & McCALLA, D.R. (1984). Aerobic reduction of
5-nitro-2-furaldehyde semicarbazone by rat liver xanthine
dehydrogenase. Biochem. Pharmacol., 33: 799-805.
LAUBSTEIN V.H. & NIEDEGESASS, G. (1970). Untersuchungen uber
gruppensensibilisierungen bei nitrofuranderivaten. Derm. Mschr.,
156: 1-8.
LIM, L.O., BORTELL, R. & NEIMS, A.H. (1986). Nitrofurantoin
inhibition of mouse liver mitochondrial respiration involving
NAD-linked substrates. Toxicol. Appl. Pharmacol., 84: 493-499.
LO, J.S., TAYLOR, J.S. & ORIBA, H. (1990). Occupational allergic
contact dermatitis to airborne nitrofurazone. Dermatologic Clinics,
8: 165-168.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli (mutation and induction and repair of daughter-strand
gaps in DNA). Mutat. Res., 67: 133-144.
MAIER, P., PHILPOT, R.M., MOHN, G.R. & MALLING, H.V. (1979).
Influence of subcellular fractions of mammalian testes on the
mutagenic activity of nitrofurans toward Escherichia coli. Mutat.
Res., 63: 233-243.
MARONPOT, R.R. (1987). Ovarian toxicity and carcinogenicity in eight
recent National Toxicology Program studies. Environ. Health
Perspect., 73: 125-130.
MATSUOKA, A., HAYASHI, M. & ISHIDATE, M.J, (1979). Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutat. Res., 66: 277-290.
McCALLA, D.R. (1979). Nitrofurans : antibiotics. In: Hahn F(Ed),
Mechanism of Action of Antibacterial Agents (Vol IV). Berlin,
Springer Verlag, 176-213.
McCALLA, D.R., OLIVE P.L., TU, Y. & FAN, M. (1975). Nitrofurazone -
reducing enzymes in E. coli and their role in drug activation
in vivo. Canad. J Microbiol., 21: 1484-1491.
McCALLA D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res., 26: 3-16.
McCALLA, D.R. (1981). Metabolic activation of nitroheterocyclic
compounds in bacteria and mammalian cells. In: Short-Term Tests for
Chemical Carcinogens, Springer, New York, 36-47.
McCALLA, D.R. (1983). Mutagenicity of nitrofuran derivatives:
Review. Environ. Mutagen., 5: 745-765.
McCALLA, D.R., REUVERS, A. & KAISER, C (1971). "Activation" of
nitrofurans in animal tissues. Biochem. Pharmacol., 20: 3532-3537.
McCALLA, D.R., REUVERS, A. & KAISER, C. (1970). Mode of action of
nitrofurazone. J. Bacteriol., 104: 1126-1134.
MIYAJI, T., MIYAMOTO, M, & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testes caused by nitrofuran
compounds. Acta Path. Jap., 14: 261-273.
MIYAJI, T. (1971). Acute and chronic toxicity of furylfuramide in
rats and mice. Tohoku J. Exp. Med., 103: 331-369.
MORI, H., SUGIE, S., YOSHIMI, M., KINOUCHI, T. & OHNISHI, Y. (1987).
Genotoxicity of a variety of nitroarenes and other nitro compounds
in DNA-repair tests with rat and mouse hepatocytes. Mutat. Res.,
190: 159-167.
MORRIS, J.E. (1965). The carcinogenic activity in the rat of some
derivatives of 5-nitrofurn. PhD Thesis, University of Wisconsin,
Madison, USA.
MORRIS, J.E., PRICE, J.M., LALICH, J.J. & STEIN, R.J. (1969). The
carcinogenic activity of some 5-nitrofuran derivatives in the rat.
Cancer Res., 29: 2145-2156.
NI, Y.C., HEFLICH R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98NR
and TA98/1,8-DNP-6. Mutat. Res., 192: 15-22.
NOMURA, T., KIMURA, S., KANZAKI, T., TANAKA, H., SHIBAK, K.,
NAKAJIMA, H., ISA, Y., KUROKAWA, N., HATANAKA, T., KINUTA, M.,
MASADA, K. & SAKAMOTO, Y. (1984). Induction of tumours and
malformations in mice after prenatal treatment with some antibiotic
drugs. Med. J. Osaka Univ., 35: 13-17.
NOUWS, J.F.M., VREE, T.B., AERTS, M.M.L., DEGEN, M. & DRIESSENS, F.
(1987). Some pharmacokinetic data about furaltadone and
nitrofurazone administered orally to preruminant calves. Vet. Q.,
9: 208-214.
NTP (1988). Toxicology and carcinogenesis studies of nitrofurazone
(CAS No. 59-87-0) in F344/N rats and B6C3F1 mice. Technical
Report Series No. 337. US Department of Health and Human Services,
Public Health Service/National Institutes of Health National
Toxicology Program.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1986). Nitrofuran mutagenicity:
Induction of frameshift mutations. Mutat. Res., 175: 149-152.
OLIVE, P.L. & McCALLA, D.R. (1975). Damage to mammalian cell DNA by
nitrofurans. Cancer Res., 35: 781-784.
OLIVE, P.L. & McCALLA, D.R. (1977). Cytotoxicity and DNA damage to
mammalian cells by nitrofurans. Chem.-Biol. Interact., 16:
223-233.
OLIVE, P.L. (1978a) Nitrofurazone-induced DNA damage to tissues of
mice. Chem.-Biol. Interact., 20: 323-331.
OLIVE, P.L. (1978b). Macromolecular, antineoplastic, and
radiosensitization effects of nitrofurans. Carcinogenesis, 4:
131-169.
OLIVE, P.L. (1979). Influence of cellular environment on toxicity of
nitroheterocycles. Chem.-Biol. Interact., 27: 281-290.
OLIVE, P.L. (1980). Mechanisms of the in vitro toxicity of
nitroheterocycles, including flagyl and misonidazole. Section of
Radiobiology, The Johns Hopkins Oncology Center, Baltimore, MD,
Radiation Sensitizers: Their Use in the Clinical Management of
Cancer, 5: 39-45.
OLIVE, P.L. (1981). Correlation between the half-wave reduction
potentials of nitroheterocycles and their mutagenicity in Chinese
hamster V79 spheroids. Mutat. Res., 82: 137-145.
ONG T-M. (1977). Mutagenic activities of nitrofurans in Neurospora
crassa. Mutat. Res., 56: 13-20.
ORPHAHELL, B.V. (1991). Furazolidone-nitrofurazone. Summary volume
1. Unpublished report. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
PAUL, M.F., PAUL, H.E., BENDER, R.C., KOPKO, F., HARRINGTON, C.M.,
ELLS, V.R. & BUZARD, J.A. (1960). Studies on the distribution and
excretion of certain nitrofurans. Antibiotics and
Chemotherap., 10: 287-302.
PAUL, H.E., PAUL, M.F., KOPKO, F. & MAIN, R.J. (1952). Effect of
furacin (5-nitro-2-furaldehyde semicarbazone) on the in vitro
metabolism of mammalian tissues. Proc. Soc. Exp. Biol. Med., 79:
555-558.
PHILLIPS, B.J. (1983). Study of mutation at HGPRT locus in cultured
Chinese hamster ovary cells with nitrofurans. Unpublished report
(466.59.62), Norwich Eaton Pharmaceuticals, Inc. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
POLNASZEK, C.F., PETERSON, F.J., HOLTZMAN, J.L. & MASON, R.P.
(1984). No detectable reaction of the anion radical metabolite of
nitrofurans with reduced glutathione or macromolecules. Chem.-Biol.
Interact., 51: 263-271.
PRICE, C.J., GEORGE, J.D. MARR, M.C. (1985). Teratologic evaluation
of nitrofurazone (CAS No 59-87-0) administered to CD-1 mice on
gestational days 6 though 15. National Toxicology Program,
NTP-85-335, PB86-145844/GAR.
PRICE, C.J., GEORGE, J.D. & MARR, M.C. (1987). Teratologic
evaluation of nitrofurazone (CAS No 59-87-0) administered to New
Zealand white rabbits on gestational days 6 through 19. National
Toxicology Program, NTP-87-200, PB88-130984/GAR.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No. 440.07)
(Special Report), Norwich Pharmacal Company. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No.440.07)
(Addendum to Special Report), Norwich Pharmacal Company. Submitted
to WHO by Orphahell B.V., Mijdrecht, Netherlands.
SKEGGS, H.R., BERGLUND, R.M., Van Den HEUVEL W.J.A., MROZIK, H.,
WISLOCKI, P.G. & WOLF, F.J. (1984). Effect of liver enzymes on the
mutagenicity of nitroheterocyclic compounds : activation of 3a, 4,
5, 6, 7, 7a - hexahydro-3-(1-methyl-5-nitro-1H-imidazole-2-yl)-
1,2-benzisoxazole and deactivation of nitrofurans and
nitroimidazoles in the Ames test. Mutat. Res., 136: 1-8.
SMITH, C.G., GRADY, J.E. & NORTHAN, J.I. (1963). Relationship
between cytotoxicity in vitro and whole animal toxicity. Cancer
Chemotherapy Reports, 20: 9-12.
SORRENTINO, D., BODE, W. & HOENER, B-A. (1987). Nitrofurazone
disposition by perfused rat liver. Effect of dose size and
glutathione depletion. Biochem Pharmacol., 36: 915-918.
STITZEL, K.A., McCONNELL, R.F. & DIERCKMANN, T.A. (1989). Effects of
nirofurantoin on the primary and secondary reproductive organs of
female 2B6C3F1 mice. Toxicol. Pathol., 17: 774-781.
SWAMINATHAN, S. & LOWER, G.M. (1978). Biotransformation and
excretion of nitrofurans. Carcinogenesis, 4: 59-97.
TEMPEL, K. (1980). Unscheduled DNA synthesis of rat thymocytes under
the influence of radioprotective and radiosensitizing agents.
Naunyn-Schmiedebergs Arch. Pharmacol., 311: R7.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan J. Genetics, 48: 292-294.
YAHAGI, T., MATSUSHIMA, T., NAGAO, M., SEINO, Y., SUGIMURA, T. &
BRYAN, G.T. (1976). Mutagenicities of nitrofuran derivatives on a
bacterial tester strain with a R factor plasmid. Mutat. Res., 40:
9-14.
ZAMPIERI, A. & GREENBERG, J. (1964). Nitrofurazone as a mutagen in
Escherichia coli. Biochem. Biophys. Res Commun., 14: 172-176.
Nitrofural had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
Nitrofural appeared to be well absorbed in the rat after oral
administration of 100 mg/kg bw. At 4 h after administration plasma
levels had reached 4.5 mg/l. No other time points were investigated.
Around 5% and 0.5% of the dose were excreted in the 24 h urine and
faeces, respectively. As acknowledged by the authors, the values are
likely to be underestimates as nitrofurazone was shown in this study
to be rapidly metabolized after incubation with rat liver slices.
There was no evidence of deposition in any organ (Paul et al.,
1960).
2.1.1.2 Cattle
A single oral dose of 14 mg/kg bw nitrofural administered to
five pre-ruminant calves resulted in maximum plasma concentrations
of 3.5 mg/litre at 3 h after administration. The final elimination
half-life was 5 h and around 2% of the administered dose was
recovered in the urine (Nouws et al., 1987).
2.1.2 Biotransformation
As described in paragraph 2.1.1.1, nitrofural is rapidly
metabolized by rat liver slices. Nitrofurans, including nitrofural,
undergo metabolic reduction at the nitro group to generate reactive
species which can covalently bind to cellular macromolecules
(Polnaszek et al., 1984; Kutcher & McCalla, 1984; McCalla 1979;
McCalla et al., 1975). Data obtained from studies with isolated
perfused rat liver suggested that conjugation to glutathione
followed by biliary excretion was important; only 0.27% of the
administered perfusate was excreted unchanged (Sorrentino et al.,
1987). Conjugation with amino acids such as methionine may also
occur (Hoener, 1988).
Ring opening of nitrofurans is known to occur (Swaminathan &
Lower, 1978) but there are insufficient data to demonstrate this
with nitrofurazone.
2.1.3 Effects on enzymes and other biochemical parameters
In chickens nitrofural increased gamma-aminobutyric acid and
glutamate concentrations in the brain at oral doses of 25 and 50
mg/kg bw for 5 days (Ali, 1988). Nitrofural, given orally at doses
of 10 and 20 mg/kg bw for 7 days, induced thiamine deficiency in
chickens (Ali 1983).
Nitrofural was found to inhibit the uptake of oxygen by various
rat tissues (Paul et al., 1952). Nitrofural inhibited respiration
in isolated mouse liver mitochondria suggesting that these may be
potential sites for toxicity (Lim et al., 1986).
Nitrofural and other nitrofurans are relatively potent
inhibitors of MAO in mammalian and avian species (Hoogenboom, 1991).
As with other nitrofurans, nitrofural can affect the endocrine
system resulting in alterations of hormone levels. In laying
turkeys, nitrofural decreased luteinizing hormone and prolactin in
plasma (Ali et al., 1987). Similar effects were noted in young
male turkeys (Ali et al., 1988).
Nitrofural has been shown to inhibit utilization of
progesterone as a steroid percursor by the adrenal in rats. In this
study it was shown that nitrofurans, including nitrofural, block
11-hydroxylation of deoxycorti-costerone to corticosterone.
Sprague-Dawley rats (1 year old) given nitrofurans for 325 days
underwent constant oestrous. The authors concluded that nitrofurans
were antagonists of normal adrenal function and believed that this
could lead to endocrine stress (King & Currie, 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The LD50 values for nitrofural are shown in Table 1. Signs of
toxicity included roughened coats, hyperexcitability, tremors,
reduced activity, weakness, convulsion, and respiratory collapse
(Krantz & Evans 1945; Anderson, 1983).
Table 1. Acute toxicity of nitrofural
Species/strain Sex Route LD50 Reference
(mg/kg bw)
Rat (unspecified) M oral 590 Krantz & Evans,
1945
Rat (Donryu) M&F oral 590 Miyaji, 1971
Rat (Wistar) M oral 800 Anderson, 1983
Mouse ? oral 380 Krantz & Evans,
(unspecified) 1945
Mouse (ICR Jcl) M&F oral 590 Miyaji, 1971
Mouse ? i.p. 300 Smith et al.,
(unspecified) 1963
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 5 male and 5 female B6C3F1 mice were given
diets containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 94.5, 188, 375, 750 or 1500 mg/kg bw/day for 14
days.
All mice given the three highest dietary levels died during the
study, as did 3/5 given 188 mg/kg bw/day. Animals in all the groups
except females given 94.5 mg/kg bw/day lost weight and all dosed
animals displayed rough coats and convulsive seizures (National
Toxicology Program (NTP), 1988).
Groups of 10 male and 10 female B6C3F1 mice were given
diets containing 0, 70, 150, 310, 620 or 1250 ppm nitrofural,
equivalent to 0, 10, 23, 47, 93 or 188 mg/kg bw/day for 13 weeks.
High mortality was noted at the highest dietary level (6/10
males and 9/10 females). Mortality was relatively high at the
penultimate dietary level (3/10 males and 5/10 females). Elevated
relative liver weights were noted in mice given the two highest
dietary levels. Animals given the two highest dietary levels also
displayed hyperexcitability and convulsive seizures, and males in
these groups had a very high incidence (80-90%) of testicular
hypoplasia (NTP, 1988).
2.2.2.2 Rats
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 63, 125, 250, 500 or 1000 mg/kg bw/day, for 14
days.
All rats given 500 or 1000 mg/kg bw/day died. Feed consumption
was decreased at all levels in excess of the lowest level. Signs of
toxicity included lethargy and roughened coats. Seizures occurred in
animals given 250 mg/kg bw/day and above. Histopathologic
examination revealed aspermatogenesis in dosed males (NTP, 1988).
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 150, 310, 620, 1200 or 2500 ppm nitrofural, equivalent
to 0, 15, 31, 62, 120 or 250 mg/kg bw/day, for 13 weeks.
All rats survived until the end of the study but final body
weights of animals given 62, 120 or 250 mg/kg bw/day were 11%, 32%
and 55% lower, respectively, than control values. Relative liver
weights for treated rats were significantly higher than control
animals. Atrophy constricture of the hind-quarters occurred in males
and females given the highest dietary level.
Moderate to severe degeneration of the seminiferous epithelium
of the testes was noted in males given the 4 highest doses. The
no-effect-level was 15 mg/kg bw/day. Males and females given the two
highest dietary levels had osteoporosis in the metaphyseal region.
(NTP, 1988).
In a study designed to investigate effects on the testes,
45-day-old male Donryu rats were given a diet containing 0.2%
nitrofural (equal to 300 mg/kg bw/day) for 9 days. Three to 6 rats
given nitrofural were sacrificed every day after feeding began.
A decrease in testis weight occurred by day 5 but
histologically, a decrease in spermatozoa and spermatids occurred at
day 1, with disappearance by days 3 and 4. Here, the stratified
appearance of the normal testis had disappeared and only basal
spermatogonia and Sertoli cells remained. Slight oedema and
exudation occurred in interstitial tissue but Leydig cells appeared
normal (Miyaji et al., 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female B6C3F1 mice were given
diets containing 0, 150 or 310 ppm nitrofural equal to 0, 14 or 29
mg/kg bw/day for 2 years.
There were no effects on body weights and survival was only
slightly affected at the highest dose in males and females (27/50
and 35/50 for males and females, respectively) compared with
controls (39/50 for each sex).
Females given nitrofural were found to have increased
incidences of ovarian atrophy (44/50 and 38/50 in low- and high-dose
groups respectively) compared with controls (7/47) and tubular cell
hyperplasia (23/50 and 21/50 in low- and high-dose groups) when
compared with controls (1/47). However, there were no effects on the
testes in males.
There were increased incidences of ovarian granulosa cell
tumours in low- (4/50) and high-dose (9/50) females when compared
with control values (1/47). Similarly, there were increased
incidences of benign mixed tumours of the ovary in low- (17/50) and
high-dose (20/50) females compared with controls (0/47). There were
no elevated incidences of any tumour type in male mice (NTP, 1988;
Kari et al., 1989).
In a study of transplacental carcinogenesis, a group of 20
pregnant ICR/Jcl mice was given 3 subcutaneous injections at
75 mg/kg bw nitrofural on days 13, 15 and 17 of gestation. The
incidence of tumours in offspring was not increased when compared
with untreated controls when they were examined 32 weeks after
birth.
In the same study, newborn mice (61) were given a subcutaneous
injection at 75 mg/kg bw 12 h after birth and on days 7, 14 and 21.
The offspring were examined at 32 weeks after birth when an
increased incidence of pulmonary papillary adenomas was noted
(Nomura et al., 1984).
2.2.3.2 Rats
A group of 20 female Holtzman rats was fed a diet containing
1000 ppm nitrofural (150 mg/kg bw/day) for 36 weeks. A similar group
of 30 females was fed the same level for 44.5 weeks. Animals were
killed 15-19 weeks after dosing was completed. At termination, there
was an elevated incidence of mammary tumours in rats fed
nitrofural-containing diets when compared with controls (Morris
et al., 1969; Morris, 1965).
Groups of 35 female Holtzman rats were fed diets containing
0, 500 or 1000 ppm nitrofural (0, 75 or 150 mg/kg bw/day) for 45
weeks, and then a basal diet for a further 8 weeks. Rats consuming
nitrofural at 75 mg/kg bw/day nitrofural had no increased tumour
incidence but in those given 150 mg/kg bw/day the numbers of
tumour-bearing rats were increased. At week 53, the numbers with
detectable mammary tumours were significantly increased in both the
75 (10/35) and 150 (12/35) mg/kg bw/day groups, when compared with
untreated controls (2/35). None of the reported tumours were
malignant (Siedler & Searfoss, 1966).
Groups of 20 male and 20 female CFE rats were fed diets
containing nitrofural to give a daily intake of 50-55 mg/kg bw/day
for 45 weeks. At the end of this period the animals were returned to
the basal diets for a further 7 weeks. There was no increased
incidence of any tumours in the male rats. A significant increase in
the incidence of benign mammary tumours was noted at week 52 (12/20)
compared with untreated controls (0/20) (Siedler & Searfoss, 1967).
Groups of 50 male and 50 female F344/N rats were fed diets
containing 0, 310 or 620 ppm nitrofural equal to 0, 11 or 24 mg/kg
bw/day for 2 years. There were reductions in the rate of weight gain
and final body weights were reduced in males and females given
dietary nitrofural. Mortality was increased in males given the high
dietary level (30/50; 60%) compared with controls (17/50; 34%).
The major non-neoplastic finding was degeneration of joint
articular cartilages in males and females in several areas of the
body, notably, the vertebral and knee joints. A no-effect-level was
not identifiable. Testicular degeneration occurred in 49/50 low
dietary level and 47/50 high dietary level males compared with 12/50
in controls.
There was an increased incidence of mammary fibroadenomas in
both low and high dietary level females (36/50; 72% in both groups)
when compared with controls (8/49; 16%) but the incidence of
adenocarcinomas was not elevated. In males, the incidence of
mesotheliomas in the tunica vaginalis was increased but not in a
dose-related manner (0/50, 7/50 and 2/50 in the control, low and
high dietary level groups). There was also an increased incidence of
sebaceous adenoma of the skin of male rats but only at the high
dietary level (0/50, 0/50 or 5/50 for controls, low and high dietary
levels, respectively). A slightly increased incidence of carcinoma
of the preputial gland was seen in males (1/50, 8/50 and 5/50 in the
control, low and high dietary groups, respectively). The incidence
of testicular tumours was reduced in dosed males (NTP, 1988; Kari
et al., 1989).
It was concluded by the authors that the data from male rats
provided only equivocal evidence of carcinogenicity. The increases
in incidences of the various tumours were small and not
dose-related.
The induction of ovarian tumours noted in mice treated with
chemicals is relatively rare. Such an effect had been noted with
only 8 chemicals from the NTP studies among which were nitrofural
itself, 1,3-butadiene, 4-vinylcyclohexene, nitrofurantoin, benzene
and delta-9-tetrahydrocannabinol. The tumours were mainly in mice
but rats too were also affected (Maronpot, 1987).
Nitrofural is unusual in that of 143 chemicals which had been
tested by the NTP by 1989, only 5, including nitrofural, produced
only benign neoplastic lesions (Huff et al., 1989). Many other
nitrofurans tested have been shown to be carcinogenic in rodents and
many of these have produced malignant tumours (Cohen & Bryan, 1973;
Cohen, 1978; Cohen et al., 1973).
Nitrofural and other nitrofurans inhibited the
hepatocarcinogenesis by 4-(dimethylamino) azobenzene in rats (Akao
et al., 1971). It also inhibited the growth of fibrosarcomas in
mice and demonstrated antineoplastic properties against seminomas
and carcinoma of the prostate in humans (Friedgood & Green, 1950;
Friedgood & Ripstein, 1951).
The International Agency for Research on Cancer (IARC) has
concluded that there was limited evidence for the carcinogenicity of
nitrofurazone in experimental animals and inadequate evidence for
its carcinogenicity in humans. In its overall evaluation, IARC
placed nitrofural in Group 3 (not classifiable as to its
carcinogenicity) (IARC, 1990).
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on teratogenicity
2.2.5.1 Mice
A group of 16 pregnant ICR/Jcl mice was given subcutaneous
injections at 100 mg/kg bw nitrofural on days 9, 10 and 11 of
gestation. A group of 6 pregnant mice was given a single
subcutaneous injection at 300 mg/kg bw nitrofural on day 10 of
gestation. Controls were given water only on days 9, 10 and 11 of
gestation.
The 100 mg/kg bw regime did not produce an elevated incidence
of malformations in 185 fetuses examined. However, the 300 mg/kg bw
dose on day 10 of gestation led to an overall incidence of
malformations of 21% compared with 0.3% in controls. These were
mainly tail anomalies, oligodactyly, and leg defects (unspecified)
(Nomura et al., 1984).
In a prescreening developmental test, female CD-1 mice were
given nitrofural (100 mg/kg bw/day) by gavage on days 6-13 of
gestation. Nitrofural caused a decrease in the numbers of viable
litters (28/35 compared with 45/45 in controls) and slight
reductions in birth weights. By the authors' criteria, these effects
constituted positive results (Hardin et al., 1987).
In a preliminary teratogenicity study, groups of 6-7 CD-1 mice
were given diets containing 0, 100, 500, 1000, 2000 or 3000 ppm
nitrofural, equivalent to 0, 15, 75, 150, 300 or 450 mg/kg bw/day,
on days 6-17 of gestation.
Dams given the two highest dietary levels died during the
study. There were no differences in food consumption in the other
groups and no effects on weight gain. There were no differences in
implantation site and resorption parameters except in those given 75
mg/kg bw/day where an increased incidence of resorptions was noted
(Price et al., 1985).
In a second preliminary teratology study, groups of 12 pregnant
CD-1 mice were given diets containing 0, 75, 250, 500 or 750 ppm
nitrofural equivalent to 0, 11, 38, 75 or 112 mg/kg bw/day, on days
6-17 of gestation. Approximately 8% mortality occurred at the
highest dietary level and hyperactivity, tremor, paralysis and
convulsions were seen at the two highest levels. There were
dose-related decreases in food consumption and body weights. Gravid
uterine weights decreased with increasing nitrofural intake. There
were no effects on implants/dam but the numbers of resorptions per
litter increased with dose. There were no increases in the
incidences of malformations (Price et al., 1985).
In the main teratogenicity study, groups of 26 pregnant CD-1
mice were given diets containing 0, 38, 75, 250 or 500 ppm
nitrofural, equivalent to 0, 6, 11, 38 or 75 mg/kg bw/day on days
6-15 of gestation. There were no maternal deaths and no effects on
food and water consumption. Gravid uterine weights were unaffected
by compound intake and there were no effects on reproductive
parameters except for an increased incidence of late fetal deaths at
marginally maternally toxic doses. The NOEL was 11 mg/kg bw/day
(Price et al., 1985).
2.2.5.2 Rabbits
Groups of 22-27 New Zeeland white rabbits were given gavage
doses of 0, 5, 10, 15 or 20 mg/kg bw/day nitrofural in corn oil on
days 6-19 of gestation.
At sacrifice, the uterine contents of 121 pregnant animals were
examined. There was no evidence of any teratogenic effect nor
materno- or embryo/feto-toxicity at doses of 5-15 mg/kg bw/day. At
20 mg/kg bw/day, two dams died, there was reduced weight gain,
relative and absolute maternal liver weights were increased and an
increased incidence of fetal malformations/litter was observed,
which was suggestive of fetotoxicity. Hence, effects on the
developing fetus occurred only at maternally toxic levels
(Price et al., 1987). The NOEL was 15 mg/kg bw/day.
2.2.6 Special studies on genotoxicity
Nitrofural has been tested in a range of studies (Table 2). In
general, in vitro studies of forward mutation in bacteria and
mammalian cells, sister chromatid exchange, DNA damage and tests for
clastogenicity have proved positive while in vivo studies,
including those for cytogenetic effects and the micronucleus test,
have proved negative.
In studies where the metabolic activation was incorporated into
the agar layer, the mutagenic activities of a number of nitrofurans
(and nitroimidazoles) were reduced, suggesting that the metabolites
of reductive metabolism may be less mutagenic than those formed
after oxidative metabolism (Skeggs et al., 1984). Metabolic
reduction is believed to be the major route of biotransformation of
nitrofurans and nitroimidazoles and the metabolites so formed may
account for the radiosensitizing properties of these agents (Olive,
1979, 1980, 1978b).
The results obtained with nitrofural are similar to those noted
with a wide range of nitrofurans, and in particular, the propensity
of these compounds for activity in in vitro systems is evident
(Klemencic & Wang, 1978). The mechanism of mutagenicity is unclear,
as is the route of activation, although nitroreduction appears to be
an early step (McCalla, 1981, 1983; Matsuoka et al., 1979; McCalla
et al., 1970, 1971). Nitrofural and other nitrofurans are
cytotoxic to hepatocytes in vitro (Hoogenboom et al., 1991), but
as the tumours caused by nitrofural are largely in endocrine organs,
this is unlikely to be relevant to carcinogenesis by this substance.
2.3 Observations in humans
Nitrofural is a skin sensitizer in humans and there have been
reports of contact dermatitis following exposure to the drug (Bajaj
& Gupta, 1986; Laubstein and Niedergesass, 1970; Lo et al., 1990).
When used as an experimental antineoplastic agent in humans
with prostatic or testicular tumours, testicular degeneration
occurred similar to that noted in experimental animals (Friedgood &
Ripstein, 1951). The doses used were unclear in the original text.
In the Collaborative Perinatal Project conducted in the USA by
the National Institute of Neurological and Communicative Diseases
and Stroke in 1958-1965, only 0.5% (234/50282) of mother-child pairs
had been exposed to nitrofural during months 1-4 of gestation. There
was no increased risk of malformations in this group (Heinonem
et al., 1977).
Table 2. Results of genotoxicity assays on nitrofurazone
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0-75 µg/ml Positive Baars et al.,
TA1538 1980
Ames test1 S. typhimurium 0-5 µg/plate Positive Goodman et al.,
TA98, TA100 1977
Ames test1 S. typhimurium 0-100 µg/plate Positive NTP, 1988
TA100, TA1535,
TA1537, TA98
Ames test1 S. typhimurium 1-20 µg/plate Positive Ni et al., 1987
TA98, 98NR, 98/
1.8-DNP6
Ames test1 S. typhimurium 0.1-10 µg/plate Positive Yahagi et al.,
TA1535, TA100 1976
Ames test1 S. typhimurium 0-250 nmole/plate Positive Gajewska et al.,
TA97, TA102 1990
Ames test1 S. typhimurium 0.1-100 µg/plate Positive Chandler &
TA98 Parenti, 1982
Forward mutation S. typhimurium 0-0.5 µg/ml Positive Karube et al.,
TA100 electrode 1982
Forward mutation1 E. coli 0-75 µg/ml Positive Baars et al.,
343/113/(R-9 1980
Table 2. cont'd
Test system Test object Concentration Results Reference
Forward mutation1 E. coli WP2 0-240 µM Weak Bryant & McCalla,
Positive 1980
Forward mutation1 E. coli K12 0-10 mm Positive Chessin et al.,
1978
Forward mutation1 E. coli W2 10-300 µM Positive Lu et al., 1979
WP2 uvrA 0-250 µM Positive
Forward mutation2 E. coli K12 not specified Positive Maier et al.,
343/13 1979
Forward mutation1 E. coli WP2 30-120 µM Positive McCalla &
uvrA- Voutsinos, 1974
Forward mutation1 E. coli B and S unspecified Positive Zampieri &
strains Greenberg, 1974
Fluctuation test1 S. typhimurium 0-1 ng/ml Positive Green et al., 1977
TA1535, TA100
Positive
E. coli WP2 uvrA 0-100 ng/ml
Forward mutation1 A. nidulans 0-1000 µg/plate Negative Bignami et al.,
1982
Forward mutation1 N. crassa 0-200 µg/ml Positive Ong, 1977
74-OR60-29A
SOS Chromotest E. coli PQ37 0.01-01 ng/sample Positive Gajewska et al.,
1990
Table 2. cont'd
Test system Test object Concentration Results Reference
DNA damage Mouse L cells 500 µM Positive Olive & McCalla,
1977
DNA damage Mouse L929 cells )0-500 µM Positive Olive & McCalla,
Hamster BHK21 ) 1975
cells )
Human KB cells )
DNA damage Swiss mice 0.1% in diet Positive Olive, 1978a
in vitro (150 mg/kg bw/day)
Unscheduled DNA Rat thymocytes unspecified Positive Tempel, 1980
synthesis
Unscheduled DNA Rat and mouse 1.1 x 10-3-1.1 mg/ml Negative Mori et al., 1987
synthesis hepatocytes
Sister chromatid Chinese hamster 0-15 µg/ml Positive NTP, 1988
exchange ovary cells
Forward mutation1 Mouse lymphoma 0-300 µg/ml Positive NTP, 1988
L5178Y cells 0-400 µg/ml
Forward mutation1 Chinese hamster 100 µg/ml Positive Olive, 1981
V79 cells
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Phillips, 1983
ovary - HGPRT
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Anderson &
ovary - HGPRT Phillips, 1985
Table 2. cont'd
Test system Test object Concentration Results Reference
Sex-linked D. melanogaster 5 mM Negative Kramers, 1982
recessive lethal
mutation
In vitro Chinese hamster 0-200 µg/ml Positive Anderson &
cytogenetics bone marrow Phillips, 1985
In vitro Chinese hamster 0-600 µg/ml Positive NTP, 1988
cytogenetics ovary
In vitro Chinese hamster 0.1 mg/ml Positive3 Matsuoaka et al.,
cytogenetics cells (male 1979
In vitro Human lymphocytes not specified Positive Tonomura &
cytogenetics Sasaki, 1978
In vivo Chinese hamster 0-400 mg/kg bw Negative Anderson &
cytogenetics bone marrow Phillips, 1985
In vivo Wistar rat bone 0-400 mg/kg bw or Negative Anderson, 1983
cytogenetics marrow 1-150 mg/kg
bw/day for 5 days
Micronucleus test Sprague-Dawley rat )0-60 mg/kg bw; Negative Goodman et al.,
)half dose 30 h and 1977
Long Evans rat )half dose 6 h,
)prior to sacrifice
1 With and without hepatic metabolic activation.
2 With and without testicular metabolic activation.
3 In presence but not absence of metabolic activation.
3. COMMENTS
The results of acute and short-term toxicity studies,
carcinogenicity studies, and teratogenicity studies were considered
by the Committee, in addition to several genotoxicity studies. Some
limited pharmacokinetic data were also available which demonstrated
good absorption of the drug in rats and cattle, but no data were
available on the extent of biotransformation nor on the identities
of any metabolites formed. A comparison with other nitrofurans
suggests that nitrofural probably undergoes extensive
biotransformation.
In acute oral toxicity studies, nitrofural was slightly toxic
to mice (LD50 = 300-600 mg/kg bw) and rats (LD50 = 600-800 mg/kg
bw).
Testicular hypoplasia was noted in rats and mice given dietary
nitrofural for 13 weeks. The NOEL for this effect was 15 mg/kg
bw/day in a 13-week study in rats.
Nitrofural was tested for its ability to induce forward
mutations in bacteria, yeast, and mammalian cells in vitro. In the
majority of these studies it produced positive results. Positive
results were also noted in tests for DNA damage in bacteria and in
mammalian cells in vitro, in in vitro studies for clastogenic
effects in Chinese hamster ovary or bone marrow cells, and in human
lymphocytes. However, in vivo studies of clastogenic potential in
Chinese hamster or rat bone marrow were negative. The results
suggest that nitrofural is genotoxic in vitro but not in vivo.
Carcinogenicity studies with nitrofural were conducted in rats
and mice. In a 2-year dietary study in B6C3F1 mice, the
animals were given nitrofural equal to daily oral doses of 14 or
29 mg/kg bw/day. In female mice, nitrofural induced a high incidence
of ovarian atrophy and of ovarian tubular-cell hyperplasia in both
dose groups when compared with controls. There were also increased
incidences of ovarian granulosa-cell and benign mixed tumours in
low-and high-dose groups. There were no elevated incidences of any
tumour type in male mice.
In a 2-year carcinogenicity study in rats, testicular
degeneration was one of the major non-neoplastic findings. There
were also elevated incidences of degeneration of the joint articular
cartilages at several sites in both males and females. No-effect
levels could not be identified for these findings. There was an
increased incidence of mammary fibroadenomas in females given the
low and high doses of 11 and 24 mg/kg bw/day.
Teratogenicity studies were conducted in mice and rabbits. In
mice, the rate of late fetal death was increased in treated animals
at maternally toxic doses; the NOEL was 11 mg/kg bw/day. Pregnant
rabbits were given gavage doses of up to 20 mg/kg bw/day. At the
highest dose, there was an elevated incidence of minor fetal
malformations per litter but this dose produced maternal toxicity.
The NOEL was 15 mg/kg bw/day. The data suggest that nitrofural is
not a direct teratogen but produces fetotoxic effects at maternally
toxic doses in mice and rabbits.
Although nitrofural was tumorigenic in rats and mice, the
tumours it produced were benign and were restricted to endocrine
organs and the mammary gland. Moreover, the mutagenicity studies
suggest that nitrofural is genotoxic in vitro but not in vivo.
Taken together, the data suggest that nitrofural is a secondary
carcinogen producing its effects in endocrine-responsive organs by a
mechanism that remains to be elucidated. In rats, nitrofural has
been shown to disrupt the utilization of progesterone and blocks the
synthesis of cortisone from deoxycortisone. Effects on
steroidogenesis may therefore be involved in the process of tumour
formation. The closely related compound nitrofurantoin also produced
benign ovarian tumours in the same strain of mouse, which were
thought to be secondary to drug-induced ovarian atrophy. This type
of effect cannot be excluded in the biogenesis of the ovarian
tumours produced by nitrofural. However, the Committee recognized
that no-effect levels for these effects could not be identified. The
Committee noted that nitrofural had been reviewed by the
International Agency for Research on Cancer, which concluded that
there was limited evidence for the carcinogenicity of nitrofural in
animals but inadequate evidence for humans.
4. EVALUATION
The Committee concluded that it could not establish an ADI for
nitrofural because no-effect levels had not been established for the
tumorigenic effects. It noted that, although a no-effect level
(15 mg/kg bw/day) had been established in rats for testicular
hypoplasia in a 13-week study, a slightly lower dose (11 mg/kg
bw/day) resulted in a high incidence of testicular degeneration in
the 2-year carcinogenicity study. There was no NOEL for this effect
in the 2-year study and, moreover, no study on reproductive
performance was available. The degenerative changes in the joints of
rats were seen in both males and females but a no-effect level could
not be established.
Before reviewing nitrofural again the Committee would wish to
see:
1. Further data from long-term studies in rats which would
allow the identification of no-effect levels for the
effects on joint articular cartilages and for testicular
degeneration.
2. Data to support the view that tumour formation in rodents
following nitrofural administration has an endocrine
origin. If a mechanism were demonstrated, no-effect levels
for suitable end-points would need to be identified.
3. Additional data on the identity, quantity and biological
characteristics of nitrofural metabolites.
5. REFERENCES
AKAO, M., KURODA, K., KANISAWA, M. & MIYAKI, K, (1971). Influence of
some nitrofurans on carcinogenesis in rats fed 4-(dimethylamino)
azobenzene. Gann, 62: 479-484.
ALI, B.H. (1983). The effect of nitrofurazone on the thiamine status
of chickens. Comp. Biochem. Physiol., C.76: 131-134.
ALI, B.H., SILSBY, J. & EL HALAWANI, M.E. (1987). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
Brit. Poultry Sci., 28: 613-621.
ALI, B.H. (1988). Effect of furazolidone and nitrofurazone on brain
gamma-aminobutyric acid and glutamate concentrations in chickens.
Clin. Exp. Pharmacol. Physiol., 15: 959-963.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL) levels in young turkeys. Gen. Pharmacol., 19:
91-95.
ANDERSON, D. (1983) Cytogenetic analysis in rat bone marrow with
nitrofurazone. Project No. 466.59.63. Unpublished report for Norwich
Eaton Pharmaceuticals, Inc. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
ANDERSON, D. & PHILLIPS, B.J. (1985). Nitrofurazone - genotoxicity
studies in mammalian cells in vitro and in vivo. Food Chem.
Toxicol., 23: 1091-1098.
BAARS, A.J., BLIJLEVEN, W.G.H., MOHN, G.R., NATARAJAN, A.T. &
BREIMER, D.D. (1980). Preliminary studies on the ability of
Drosophila microsomal preparations to activate mutagens and
carcinogens. Mutat. Res., 72: 257-264.
BAJAJ A.K. & GUPTA, S.C. (1986). Contact hypersensitivity to topical
antibacterial agents. Int. J. Dermatol., 25: 103-105.
BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L., CREBELLI, R. &
FABRIZI, M. (1982). Evaluation of 2 different genetic markers for
the detection of frameshift and missense mutagens in A. nidulans.
Mutat. Res., 97: 293-302.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran-induced mutagenesis
and error prone repair in Escherichia coli. Chem-Biol. Interact.,
31: 151-166.
CHANDLER, A.D. & PARENTI, R.R. (1982). Isolation and
characterisation of a nitrofurazone resistant strain of Salmonella
TA98. Environ. Mutagen., 4: 319-320.
CHESSIN, H., McLAUGHLIN, T., MROCZKOWSKI, Z., RUPP, W.D. & LOW, K.B.
(1978). Radiosensitization, mutagenicity, and toxicity of
Escherichia coli by several nitrofurans and nitroimidazoles.
Radiat. Res., 75: 424-431.
COHEN S.M. & BRYAN, G.T. (1973). Carcinogenesis caused by nitrofuran
derivatives. Pharmacology and the Future of Man. Proc. Vth Int.
Congr. Pharmacol., San Francisco, Karger, Basel, 161-170.
COHEN, S.M., ETURK, F., VON ESCH, A.M., CROVETTI, A.J. & BRYAN, G.T.
(1973). Carcinogenicity of 5-nitrofurans, 5-nitromidazoles,
4-nitrobenzenes and related compounds. J. Natl. Cancer Inst., 51:
403-417.
COHEN, S.M. (1978). Toxicity and carcinogenicity of nitrofurans.
Carcinogenesis, 4: 171-231.
FRIEDGOOD, C.E. & GREEN, M.N. (1950). The effect of nitrofurazone on
the growth of fibrosarcoma in mice. Cancer Res., 10: 613-615.
FRIEDGOOD, C.E. & RIPSTEIN, C.B. (1951). The effect of nitrofurazone
on tumours of the human testis and prostate gland. Surg. Forum,
329-333.
GAJEWSKA, J., SZCZYPKA, M., TUDEK, B. & SZYMCZYK, T. (1990). Studies
on the effect of ascorbic acid and selenium on the genotoxicity of
nitrofurans : nitrofurazone and furazolidone. Mutat. Res., 232:
191-197.
GOODMAN, D., AUFRERE, M., VORE, M. & MEYERS, F. (1977). Metabolism
of nitrofurazone. 61st Annual Meeting of the Federation of American
Societies for Experimental Biology, Chicago, Illinois.
GREEN, M.H.L., ROGERS, A.M., MURIEL, W.J., WARD, A.C. & McCALLA,
D.R. (1977). Use of a simplified fluctuation test to detect and
characterize mutagenesis by nitrofurans. Mutat. Res., 44: 139-143.
HARDIN, B.D., SCHULER, R.L., BURG, J.R., BOOTH, G.M., HAZELDEN,
K.P., MacKENZIE, K.M., PICCIRILLO, V.J., & SMITH, K.N. (1987).
Evaluation of 60 chemicals in a preliminary developmental toxicity
test. Terat. Carcinogen. Mutagen., 7: 29-48.
HEINONEN, O.P., SLONE, D. & SHAPIRO, S. (1977). (Eds). Antimicrobial
and antiparasitic agents. In: Birth Defects and Drugs in Pregnancy,
Publishing Sciences Group, Inc, Littleton, MA, 296-313.
HOENER, B-A. (1988). Nitrofurazone : Kinetics and oxidative stress
in the singlepass isolated perfused rat liver. Biochem. Pharmacol.,
37: 1629-1636.
HOOGENBOOM, L.A., OORSPRONG, M.B.M., VAN VLIET, T. & KUIPER, H.A.
(1991). The use of pig hepatocytes for cytotoxicity studies of
veterinary drugs: A comparative study with furazolidone and other
nitrofurans. Toxicol. in Vitro, 5: 31-38.
HOOGENBOOM, L.A. (1991). The use of pig hepatocytes for
biotransformation and toxicity studies. Thesis,
Landbouwuniversiteit, Wageningen, Netherlands.
HUFF, J.E., EUSTIS, S.L. & HASEMAN, J.K. (1989). Occurrence and
relevance of chemically-induced benign neoplasms in long-term
carcinogenicity studies. Cancer Metastasis Rev., 8: 1-22.
IARC (1990). Monographs on the evaluation of carcinogenic risks to
humans. Pharmaceutical Drugs, IARC, Lyon, 50: 195-209.
KARI, F.W., HUFF, J.E., LEININGER, J., HASEMAN, J.K. & EUSTIS, S.L.
(1989). Toxicity and carcinogenicity of nitrofurazone in F344/N rats
and B6C3F1 mice. Food Chem. Toxicol., 27: 129-137.
KARUBE, I., NAKAHARA, T., MATSUNAGA, T. & SUZUKI, S. (1982).
Salmonella electrode for screening mutagens. Anal. Chem., 54:
1725-1727.
KING, C.D. & CURRIE, G.N. (1972). Effects of nitrofurans on
steroidogenesis and mammary hyperplasia. Unpublished report. Norwich
Pharmacal Company, Project No. 596.09. Submitted to WHO by Orphahell
B.V., Midrecht, Netherlands.
KLEMENCIC, J.M. & WANG, C.Y. (1978). Mutagenicity of nitrofurans.
In: Bryan, G.T. ed. Carcinogenesis, 4: 99-130, New York, Raven
Press.
KRAMERS, P.G.N. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
KRANTZ, J.C. & EVANS, W.E. (1945). A contribution to the
pharmacology of 5-nitro-2-furaldehyde semicarbazone. J. Pharmacol.
Exp. Therap., 85: 324-331.
KUTCHER, W.W. & McCALLA, D.R. (1984). Aerobic reduction of
5-nitro-2-furaldehyde semicarbazone by rat liver xanthine
dehydrogenase. Biochem. Pharmacol., 33: 799-805.
LAUBSTEIN V.H. & NIEDEGESASS, G. (1970). Untersuchungen uber
gruppensensibilisierungen bei nitrofuranderivaten. Derm. Mschr.,
156: 1-8.
LIM, L.O., BORTELL, R. & NEIMS, A.H. (1986). Nitrofurantoin
inhibition of mouse liver mitochondrial respiration involving
NAD-linked substrates. Toxicol. Appl. Pharmacol., 84: 493-499.
LO, J.S., TAYLOR, J.S. & ORIBA, H. (1990). Occupational allergic
contact dermatitis to airborne nitrofurazone. Dermatologic Clinics,
8: 165-168.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli (mutation and induction and repair of daughter-strand
gaps in DNA). Mutat. Res., 67: 133-144.
MAIER, P., PHILPOT, R.M., MOHN, G.R. & MALLING, H.V. (1979).
Influence of subcellular fractions of mammalian testes on the
mutagenic activity of nitrofurans toward Escherichia coli. Mutat.
Res., 63: 233-243.
MARONPOT, R.R. (1987). Ovarian toxicity and carcinogenicity in eight
recent National Toxicology Program studies. Environ. Health
Perspect., 73: 125-130.
MATSUOKA, A., HAYASHI, M. & ISHIDATE, M.J, (1979). Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutat. Res., 66: 277-290.
McCALLA, D.R. (1979). Nitrofurans : antibiotics. In: Hahn F(Ed),
Mechanism of Action of Antibacterial Agents (Vol IV). Berlin,
Springer Verlag, 176-213.
McCALLA, D.R., OLIVE P.L., TU, Y. & FAN, M. (1975). Nitrofurazone -
reducing enzymes in E. coli and their role in drug activation
in vivo. Canad. J Microbiol., 21: 1484-1491.
McCALLA D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res., 26: 3-16.
McCALLA, D.R. (1981). Metabolic activation of nitroheterocyclic
compounds in bacteria and mammalian cells. In: Short-Term Tests for
Chemical Carcinogens, Springer, New York, 36-47.
McCALLA, D.R. (1983). Mutagenicity of nitrofuran derivatives:
Review. Environ. Mutagen., 5: 745-765.
McCALLA, D.R., REUVERS, A. & KAISER, C (1971). "Activation" of
nitrofurans in animal tissues. Biochem. Pharmacol., 20: 3532-3537.
McCALLA, D.R., REUVERS, A. & KAISER, C. (1970). Mode of action of
nitrofurazone. J. Bacteriol., 104: 1126-1134.
MIYAJI, T., MIYAMOTO, M, & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testes caused by nitrofuran
compounds. Acta Path. Jap., 14: 261-273.
MIYAJI, T. (1971). Acute and chronic toxicity of furylfuramide in
rats and mice. Tohoku J. Exp. Med., 103: 331-369.
MORI, H., SUGIE, S., YOSHIMI, M., KINOUCHI, T. & OHNISHI, Y. (1987).
Genotoxicity of a variety of nitroarenes and other nitro compounds
in DNA-repair tests with rat and mouse hepatocytes. Mutat. Res.,
190: 159-167.
MORRIS, J.E. (1965). The carcinogenic activity in the rat of some
derivatives of 5-nitrofurn. PhD Thesis, University of Wisconsin,
Madison, USA.
MORRIS, J.E., PRICE, J.M., LALICH, J.J. & STEIN, R.J. (1969). The
carcinogenic activity of some 5-nitrofuran derivatives in the rat.
Cancer Res., 29: 2145-2156.
NI, Y.C., HEFLICH R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98NR
and TA98/1,8-DNP-6. Mutat. Res., 192: 15-22.
NOMURA, T., KIMURA, S., KANZAKI, T., TANAKA, H., SHIBAK, K.,
NAKAJIMA, H., ISA, Y., KUROKAWA, N., HATANAKA, T., KINUTA, M.,
MASADA, K. & SAKAMOTO, Y. (1984). Induction of tumours and
malformations in mice after prenatal treatment with some antibiotic
drugs. Med. J. Osaka Univ., 35: 13-17.
NOUWS, J.F.M., VREE, T.B., AERTS, M.M.L., DEGEN, M. & DRIESSENS, F.
(1987). Some pharmacokinetic data about furaltadone and
nitrofurazone administered orally to preruminant calves. Vet. Q.,
9: 208-214.
NTP (1988). Toxicology and carcinogenesis studies of nitrofurazone
(CAS No. 59-87-0) in F344/N rats and B6C3F1 mice. Technical
Report Series No. 337. US Department of Health and Human Services,
Public Health Service/National Institutes of Health National
Toxicology Program.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1986). Nitrofuran mutagenicity:
Induction of frameshift mutations. Mutat. Res., 175: 149-152.
OLIVE, P.L. & McCALLA, D.R. (1975). Damage to mammalian cell DNA by
nitrofurans. Cancer Res., 35: 781-784.
OLIVE, P.L. & McCALLA, D.R. (1977). Cytotoxicity and DNA damage to
mammalian cells by nitrofurans. Chem.-Biol. Interact., 16:
223-233.
OLIVE, P.L. (1978a) Nitrofurazone-induced DNA damage to tissues of
mice. Chem.-Biol. Interact., 20: 323-331.
OLIVE, P.L. (1978b). Macromolecular, antineoplastic, and
radiosensitization effects of nitrofurans. Carcinogenesis, 4:
131-169.
OLIVE, P.L. (1979). Influence of cellular environment on toxicity of
nitroheterocycles. Chem.-Biol. Interact., 27: 281-290.
OLIVE, P.L. (1980). Mechanisms of the in vitro toxicity of
nitroheterocycles, including flagyl and misonidazole. Section of
Radiobiology, The Johns Hopkins Oncology Center, Baltimore, MD,
Radiation Sensitizers: Their Use in the Clinical Management of
Cancer, 5: 39-45.
OLIVE, P.L. (1981). Correlation between the half-wave reduction
potentials of nitroheterocycles and their mutagenicity in Chinese
hamster V79 spheroids. Mutat. Res., 82: 137-145.
ONG T-M. (1977). Mutagenic activities of nitrofurans in Neurospora
crassa. Mutat. Res., 56: 13-20.
ORPHAHELL, B.V. (1991). Furazolidone-nitrofurazone. Summary volume
1. Unpublished report. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
PAUL, M.F., PAUL, H.E., BENDER, R.C., KOPKO, F., HARRINGTON, C.M.,
ELLS, V.R. & BUZARD, J.A. (1960). Studies on the distribution and
excretion of certain nitrofurans. Antibiotics and
Chemotherap., 10: 287-302.
PAUL, H.E., PAUL, M.F., KOPKO, F. & MAIN, R.J. (1952). Effect of
furacin (5-nitro-2-furaldehyde semicarbazone) on the in vitro
metabolism of mammalian tissues. Proc. Soc. Exp. Biol. Med., 79:
555-558.
PHILLIPS, B.J. (1983). Study of mutation at HGPRT locus in cultured
Chinese hamster ovary cells with nitrofurans. Unpublished report
(466.59.62), Norwich Eaton Pharmaceuticals, Inc. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
POLNASZEK, C.F., PETERSON, F.J., HOLTZMAN, J.L. & MASON, R.P.
(1984). No detectable reaction of the anion radical metabolite of
nitrofurans with reduced glutathione or macromolecules. Chem.-Biol.
Interact., 51: 263-271.
PRICE, C.J., GEORGE, J.D. MARR, M.C. (1985). Teratologic evaluation
of nitrofurazone (CAS No 59-87-0) administered to CD-1 mice on
gestational days 6 though 15. National Toxicology Program,
NTP-85-335, PB86-145844/GAR.
PRICE, C.J., GEORGE, J.D. & MARR, M.C. (1987). Teratologic
evaluation of nitrofurazone (CAS No 59-87-0) administered to New
Zealand white rabbits on gestational days 6 through 19. National
Toxicology Program, NTP-87-200, PB88-130984/GAR.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No. 440.07)
(Special Report), Norwich Pharmacal Company. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No.440.07)
(Addendum to Special Report), Norwich Pharmacal Company. Submitted
to WHO by Orphahell B.V., Mijdrecht, Netherlands.
SKEGGS, H.R., BERGLUND, R.M., Van Den HEUVEL W.J.A., MROZIK, H.,
WISLOCKI, P.G. & WOLF, F.J. (1984). Effect of liver enzymes on the
mutagenicity of nitroheterocyclic compounds : activation of 3a, 4,
5, 6, 7, 7a - hexahydro-3-(1-methyl-5-nitro-1H-imidazole-2-yl)-
1,2-benzisoxazole and deactivation of nitrofurans and
nitroimidazoles in the Ames test. Mutat. Res., 136: 1-8.
SMITH, C.G., GRADY, J.E. & NORTHAN, J.I. (1963). Relationship
between cytotoxicity in vitro and whole animal toxicity. Cancer
Chemotherapy Reports, 20: 9-12.
SORRENTINO, D., BODE, W. & HOENER, B-A. (1987). Nitrofurazone
disposition by perfused rat liver. Effect of dose size and
glutathione depletion. Biochem Pharmacol., 36: 915-918.
STITZEL, K.A., McCONNELL, R.F. & DIERCKMANN, T.A. (1989). Effects of
nirofurantoin on the primary and secondary reproductive organs of
female 2B6C3F1 mice. Toxicol. Pathol., 17: 774-781.
SWAMINATHAN, S. & LOWER, G.M. (1978). Biotransformation and
excretion of nitrofurans. Carcinogenesis, 4: 59-97.
TEMPEL, K. (1980). Unscheduled DNA synthesis of rat thymocytes under
the influence of radioprotective and radiosensitizing agents.
Naunyn-Schmiedebergs Arch. Pharmacol., 311: R7.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan J. Genetics, 48: 292-294.
YAHAGI, T., MATSUSHIMA, T., NAGAO, M., SEINO, Y., SUGIMURA, T. &
BRYAN, G.T. (1976). Mutagenicities of nitrofuran derivatives on a
bacterial tester strain with a R factor plasmid. Mutat. Res., 40:
9-14.
ZAMPIERI, A. & GREENBERG, J. (1964). Nitrofurazone as a mutagen in
Escherichia coli. Biochem. Biophys. Res Commun., 14: 172-176.
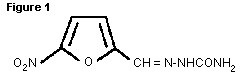 Nitrofural had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
Nitrofural appeared to be well absorbed in the rat after oral
administration of 100 mg/kg bw. At 4 h after administration plasma
levels had reached 4.5 mg/l. No other time points were investigated.
Around 5% and 0.5% of the dose were excreted in the 24 h urine and
faeces, respectively. As acknowledged by the authors, the values are
likely to be underestimates as nitrofurazone was shown in this study
to be rapidly metabolized after incubation with rat liver slices.
There was no evidence of deposition in any organ (Paul et al.,
1960).
2.1.1.2 Cattle
A single oral dose of 14 mg/kg bw nitrofural administered to
five pre-ruminant calves resulted in maximum plasma concentrations
of 3.5 mg/litre at 3 h after administration. The final elimination
half-life was 5 h and around 2% of the administered dose was
recovered in the urine (Nouws et al., 1987).
2.1.2 Biotransformation
As described in paragraph 2.1.1.1, nitrofural is rapidly
metabolized by rat liver slices. Nitrofurans, including nitrofural,
undergo metabolic reduction at the nitro group to generate reactive
species which can covalently bind to cellular macromolecules
(Polnaszek et al., 1984; Kutcher & McCalla, 1984; McCalla 1979;
McCalla et al., 1975). Data obtained from studies with isolated
perfused rat liver suggested that conjugation to glutathione
followed by biliary excretion was important; only 0.27% of the
administered perfusate was excreted unchanged (Sorrentino et al.,
1987). Conjugation with amino acids such as methionine may also
occur (Hoener, 1988).
Ring opening of nitrofurans is known to occur (Swaminathan &
Lower, 1978) but there are insufficient data to demonstrate this
with nitrofurazone.
2.1.3 Effects on enzymes and other biochemical parameters
In chickens nitrofural increased gamma-aminobutyric acid and
glutamate concentrations in the brain at oral doses of 25 and 50
mg/kg bw for 5 days (Ali, 1988). Nitrofural, given orally at doses
of 10 and 20 mg/kg bw for 7 days, induced thiamine deficiency in
chickens (Ali 1983).
Nitrofural was found to inhibit the uptake of oxygen by various
rat tissues (Paul et al., 1952). Nitrofural inhibited respiration
in isolated mouse liver mitochondria suggesting that these may be
potential sites for toxicity (Lim et al., 1986).
Nitrofural and other nitrofurans are relatively potent
inhibitors of MAO in mammalian and avian species (Hoogenboom, 1991).
As with other nitrofurans, nitrofural can affect the endocrine
system resulting in alterations of hormone levels. In laying
turkeys, nitrofural decreased luteinizing hormone and prolactin in
plasma (Ali et al., 1987). Similar effects were noted in young
male turkeys (Ali et al., 1988).
Nitrofural has been shown to inhibit utilization of
progesterone as a steroid percursor by the adrenal in rats. In this
study it was shown that nitrofurans, including nitrofural, block
11-hydroxylation of deoxycorti-costerone to corticosterone.
Sprague-Dawley rats (1 year old) given nitrofurans for 325 days
underwent constant oestrous. The authors concluded that nitrofurans
were antagonists of normal adrenal function and believed that this
could lead to endocrine stress (King & Currie, 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The LD50 values for nitrofural are shown in Table 1. Signs of
toxicity included roughened coats, hyperexcitability, tremors,
reduced activity, weakness, convulsion, and respiratory collapse
(Krantz & Evans 1945; Anderson, 1983).
Table 1. Acute toxicity of nitrofural
Species/strain Sex Route LD50 Reference
(mg/kg bw)
Rat (unspecified) M oral 590 Krantz & Evans,
1945
Rat (Donryu) M&F oral 590 Miyaji, 1971
Rat (Wistar) M oral 800 Anderson, 1983
Mouse ? oral 380 Krantz & Evans,
(unspecified) 1945
Mouse (ICR Jcl) M&F oral 590 Miyaji, 1971
Mouse ? i.p. 300 Smith et al.,
(unspecified) 1963
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 5 male and 5 female B6C3F1 mice were given
diets containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 94.5, 188, 375, 750 or 1500 mg/kg bw/day for 14
days.
All mice given the three highest dietary levels died during the
study, as did 3/5 given 188 mg/kg bw/day. Animals in all the groups
except females given 94.5 mg/kg bw/day lost weight and all dosed
animals displayed rough coats and convulsive seizures (National
Toxicology Program (NTP), 1988).
Groups of 10 male and 10 female B6C3F1 mice were given
diets containing 0, 70, 150, 310, 620 or 1250 ppm nitrofural,
equivalent to 0, 10, 23, 47, 93 or 188 mg/kg bw/day for 13 weeks.
High mortality was noted at the highest dietary level (6/10
males and 9/10 females). Mortality was relatively high at the
penultimate dietary level (3/10 males and 5/10 females). Elevated
relative liver weights were noted in mice given the two highest
dietary levels. Animals given the two highest dietary levels also
displayed hyperexcitability and convulsive seizures, and males in
these groups had a very high incidence (80-90%) of testicular
hypoplasia (NTP, 1988).
2.2.2.2 Rats
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 63, 125, 250, 500 or 1000 mg/kg bw/day, for 14
days.
All rats given 500 or 1000 mg/kg bw/day died. Feed consumption
was decreased at all levels in excess of the lowest level. Signs of
toxicity included lethargy and roughened coats. Seizures occurred in
animals given 250 mg/kg bw/day and above. Histopathologic
examination revealed aspermatogenesis in dosed males (NTP, 1988).
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 150, 310, 620, 1200 or 2500 ppm nitrofural, equivalent
to 0, 15, 31, 62, 120 or 250 mg/kg bw/day, for 13 weeks.
All rats survived until the end of the study but final body
weights of animals given 62, 120 or 250 mg/kg bw/day were 11%, 32%
and 55% lower, respectively, than control values. Relative liver
weights for treated rats were significantly higher than control
animals. Atrophy constricture of the hind-quarters occurred in males
and females given the highest dietary level.
Moderate to severe degeneration of the seminiferous epithelium
of the testes was noted in males given the 4 highest doses. The
no-effect-level was 15 mg/kg bw/day. Males and females given the two
highest dietary levels had osteoporosis in the metaphyseal region.
(NTP, 1988).
In a study designed to investigate effects on the testes,
45-day-old male Donryu rats were given a diet containing 0.2%
nitrofural (equal to 300 mg/kg bw/day) for 9 days. Three to 6 rats
given nitrofural were sacrificed every day after feeding began.
A decrease in testis weight occurred by day 5 but
histologically, a decrease in spermatozoa and spermatids occurred at
day 1, with disappearance by days 3 and 4. Here, the stratified
appearance of the normal testis had disappeared and only basal
spermatogonia and Sertoli cells remained. Slight oedema and
exudation occurred in interstitial tissue but Leydig cells appeared
normal (Miyaji et al., 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female B6C3F1 mice were given
diets containing 0, 150 or 310 ppm nitrofural equal to 0, 14 or 29
mg/kg bw/day for 2 years.
There were no effects on body weights and survival was only
slightly affected at the highest dose in males and females (27/50
and 35/50 for males and females, respectively) compared with
controls (39/50 for each sex).
Females given nitrofural were found to have increased
incidences of ovarian atrophy (44/50 and 38/50 in low- and high-dose
groups respectively) compared with controls (7/47) and tubular cell
hyperplasia (23/50 and 21/50 in low- and high-dose groups) when
compared with controls (1/47). However, there were no effects on the
testes in males.
There were increased incidences of ovarian granulosa cell
tumours in low- (4/50) and high-dose (9/50) females when compared
with control values (1/47). Similarly, there were increased
incidences of benign mixed tumours of the ovary in low- (17/50) and
high-dose (20/50) females compared with controls (0/47). There were
no elevated incidences of any tumour type in male mice (NTP, 1988;
Kari et al., 1989).
In a study of transplacental carcinogenesis, a group of 20
pregnant ICR/Jcl mice was given 3 subcutaneous injections at
75 mg/kg bw nitrofural on days 13, 15 and 17 of gestation. The
incidence of tumours in offspring was not increased when compared
with untreated controls when they were examined 32 weeks after
birth.
In the same study, newborn mice (61) were given a subcutaneous
injection at 75 mg/kg bw 12 h after birth and on days 7, 14 and 21.
The offspring were examined at 32 weeks after birth when an
increased incidence of pulmonary papillary adenomas was noted
(Nomura et al., 1984).
2.2.3.2 Rats
A group of 20 female Holtzman rats was fed a diet containing
1000 ppm nitrofural (150 mg/kg bw/day) for 36 weeks. A similar group
of 30 females was fed the same level for 44.5 weeks. Animals were
killed 15-19 weeks after dosing was completed. At termination, there
was an elevated incidence of mammary tumours in rats fed
nitrofural-containing diets when compared with controls (Morris
et al., 1969; Morris, 1965).
Groups of 35 female Holtzman rats were fed diets containing
0, 500 or 1000 ppm nitrofural (0, 75 or 150 mg/kg bw/day) for 45
weeks, and then a basal diet for a further 8 weeks. Rats consuming
nitrofural at 75 mg/kg bw/day nitrofural had no increased tumour
incidence but in those given 150 mg/kg bw/day the numbers of
tumour-bearing rats were increased. At week 53, the numbers with
detectable mammary tumours were significantly increased in both the
75 (10/35) and 150 (12/35) mg/kg bw/day groups, when compared with
untreated controls (2/35). None of the reported tumours were
malignant (Siedler & Searfoss, 1966).
Groups of 20 male and 20 female CFE rats were fed diets
containing nitrofural to give a daily intake of 50-55 mg/kg bw/day
for 45 weeks. At the end of this period the animals were returned to
the basal diets for a further 7 weeks. There was no increased
incidence of any tumours in the male rats. A significant increase in
the incidence of benign mammary tumours was noted at week 52 (12/20)
compared with untreated controls (0/20) (Siedler & Searfoss, 1967).
Groups of 50 male and 50 female F344/N rats were fed diets
containing 0, 310 or 620 ppm nitrofural equal to 0, 11 or 24 mg/kg
bw/day for 2 years. There were reductions in the rate of weight gain
and final body weights were reduced in males and females given
dietary nitrofural. Mortality was increased in males given the high
dietary level (30/50; 60%) compared with controls (17/50; 34%).
The major non-neoplastic finding was degeneration of joint
articular cartilages in males and females in several areas of the
body, notably, the vertebral and knee joints. A no-effect-level was
not identifiable. Testicular degeneration occurred in 49/50 low
dietary level and 47/50 high dietary level males compared with 12/50
in controls.
There was an increased incidence of mammary fibroadenomas in
both low and high dietary level females (36/50; 72% in both groups)
when compared with controls (8/49; 16%) but the incidence of
adenocarcinomas was not elevated. In males, the incidence of
mesotheliomas in the tunica vaginalis was increased but not in a
dose-related manner (0/50, 7/50 and 2/50 in the control, low and
high dietary level groups). There was also an increased incidence of
sebaceous adenoma of the skin of male rats but only at the high
dietary level (0/50, 0/50 or 5/50 for controls, low and high dietary
levels, respectively). A slightly increased incidence of carcinoma
of the preputial gland was seen in males (1/50, 8/50 and 5/50 in the
control, low and high dietary groups, respectively). The incidence
of testicular tumours was reduced in dosed males (NTP, 1988; Kari
et al., 1989).
It was concluded by the authors that the data from male rats
provided only equivocal evidence of carcinogenicity. The increases
in incidences of the various tumours were small and not
dose-related.
The induction of ovarian tumours noted in mice treated with
chemicals is relatively rare. Such an effect had been noted with
only 8 chemicals from the NTP studies among which were nitrofural
itself, 1,3-butadiene, 4-vinylcyclohexene, nitrofurantoin, benzene
and delta-9-tetrahydrocannabinol. The tumours were mainly in mice
but rats too were also affected (Maronpot, 1987).
Nitrofural is unusual in that of 143 chemicals which had been
tested by the NTP by 1989, only 5, including nitrofural, produced
only benign neoplastic lesions (Huff et al., 1989). Many other
nitrofurans tested have been shown to be carcinogenic in rodents and
many of these have produced malignant tumours (Cohen & Bryan, 1973;
Cohen, 1978; Cohen et al., 1973).
Nitrofural and other nitrofurans inhibited the
hepatocarcinogenesis by 4-(dimethylamino) azobenzene in rats (Akao
et al., 1971). It also inhibited the growth of fibrosarcomas in
mice and demonstrated antineoplastic properties against seminomas
and carcinoma of the prostate in humans (Friedgood & Green, 1950;
Friedgood & Ripstein, 1951).
The International Agency for Research on Cancer (IARC) has
concluded that there was limited evidence for the carcinogenicity of
nitrofurazone in experimental animals and inadequate evidence for
its carcinogenicity in humans. In its overall evaluation, IARC
placed nitrofural in Group 3 (not classifiable as to its
carcinogenicity) (IARC, 1990).
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on teratogenicity
2.2.5.1 Mice
A group of 16 pregnant ICR/Jcl mice was given subcutaneous
injections at 100 mg/kg bw nitrofural on days 9, 10 and 11 of
gestation. A group of 6 pregnant mice was given a single
subcutaneous injection at 300 mg/kg bw nitrofural on day 10 of
gestation. Controls were given water only on days 9, 10 and 11 of
gestation.
The 100 mg/kg bw regime did not produce an elevated incidence
of malformations in 185 fetuses examined. However, the 300 mg/kg bw
dose on day 10 of gestation led to an overall incidence of
malformations of 21% compared with 0.3% in controls. These were
mainly tail anomalies, oligodactyly, and leg defects (unspecified)
(Nomura et al., 1984).
In a prescreening developmental test, female CD-1 mice were
given nitrofural (100 mg/kg bw/day) by gavage on days 6-13 of
gestation. Nitrofural caused a decrease in the numbers of viable
litters (28/35 compared with 45/45 in controls) and slight
reductions in birth weights. By the authors' criteria, these effects
constituted positive results (Hardin et al., 1987).
In a preliminary teratogenicity study, groups of 6-7 CD-1 mice
were given diets containing 0, 100, 500, 1000, 2000 or 3000 ppm
nitrofural, equivalent to 0, 15, 75, 150, 300 or 450 mg/kg bw/day,
on days 6-17 of gestation.
Dams given the two highest dietary levels died during the
study. There were no differences in food consumption in the other
groups and no effects on weight gain. There were no differences in
implantation site and resorption parameters except in those given 75
mg/kg bw/day where an increased incidence of resorptions was noted
(Price et al., 1985).
In a second preliminary teratology study, groups of 12 pregnant
CD-1 mice were given diets containing 0, 75, 250, 500 or 750 ppm
nitrofural equivalent to 0, 11, 38, 75 or 112 mg/kg bw/day, on days
6-17 of gestation. Approximately 8% mortality occurred at the
highest dietary level and hyperactivity, tremor, paralysis and
convulsions were seen at the two highest levels. There were
dose-related decreases in food consumption and body weights. Gravid
uterine weights decreased with increasing nitrofural intake. There
were no effects on implants/dam but the numbers of resorptions per
litter increased with dose. There were no increases in the
incidences of malformations (Price et al., 1985).
In the main teratogenicity study, groups of 26 pregnant CD-1
mice were given diets containing 0, 38, 75, 250 or 500 ppm
nitrofural, equivalent to 0, 6, 11, 38 or 75 mg/kg bw/day on days
6-15 of gestation. There were no maternal deaths and no effects on
food and water consumption. Gravid uterine weights were unaffected
by compound intake and there were no effects on reproductive
parameters except for an increased incidence of late fetal deaths at
marginally maternally toxic doses. The NOEL was 11 mg/kg bw/day
(Price et al., 1985).
2.2.5.2 Rabbits
Groups of 22-27 New Zeeland white rabbits were given gavage
doses of 0, 5, 10, 15 or 20 mg/kg bw/day nitrofural in corn oil on
days 6-19 of gestation.
At sacrifice, the uterine contents of 121 pregnant animals were
examined. There was no evidence of any teratogenic effect nor
materno- or embryo/feto-toxicity at doses of 5-15 mg/kg bw/day. At
20 mg/kg bw/day, two dams died, there was reduced weight gain,
relative and absolute maternal liver weights were increased and an
increased incidence of fetal malformations/litter was observed,
which was suggestive of fetotoxicity. Hence, effects on the
developing fetus occurred only at maternally toxic levels
(Price et al., 1987). The NOEL was 15 mg/kg bw/day.
2.2.6 Special studies on genotoxicity
Nitrofural has been tested in a range of studies (Table 2). In
general, in vitro studies of forward mutation in bacteria and
mammalian cells, sister chromatid exchange, DNA damage and tests for
clastogenicity have proved positive while in vivo studies,
including those for cytogenetic effects and the micronucleus test,
have proved negative.
In studies where the metabolic activation was incorporated into
the agar layer, the mutagenic activities of a number of nitrofurans
(and nitroimidazoles) were reduced, suggesting that the metabolites
of reductive metabolism may be less mutagenic than those formed
after oxidative metabolism (Skeggs et al., 1984). Metabolic
reduction is believed to be the major route of biotransformation of
nitrofurans and nitroimidazoles and the metabolites so formed may
account for the radiosensitizing properties of these agents (Olive,
1979, 1980, 1978b).
The results obtained with nitrofural are similar to those noted
with a wide range of nitrofurans, and in particular, the propensity
of these compounds for activity in in vitro systems is evident
(Klemencic & Wang, 1978). The mechanism of mutagenicity is unclear,
as is the route of activation, although nitroreduction appears to be
an early step (McCalla, 1981, 1983; Matsuoka et al., 1979; McCalla
et al., 1970, 1971). Nitrofural and other nitrofurans are
cytotoxic to hepatocytes in vitro (Hoogenboom et al., 1991), but
as the tumours caused by nitrofural are largely in endocrine organs,
this is unlikely to be relevant to carcinogenesis by this substance.
2.3 Observations in humans
Nitrofural is a skin sensitizer in humans and there have been
reports of contact dermatitis following exposure to the drug (Bajaj
& Gupta, 1986; Laubstein and Niedergesass, 1970; Lo et al., 1990).
When used as an experimental antineoplastic agent in humans
with prostatic or testicular tumours, testicular degeneration
occurred similar to that noted in experimental animals (Friedgood &
Ripstein, 1951). The doses used were unclear in the original text.
In the Collaborative Perinatal Project conducted in the USA by
the National Institute of Neurological and Communicative Diseases
and Stroke in 1958-1965, only 0.5% (234/50282) of mother-child pairs
had been exposed to nitrofural during months 1-4 of gestation. There
was no increased risk of malformations in this group (Heinonem
et al., 1977).
Table 2. Results of genotoxicity assays on nitrofurazone
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0-75 µg/ml Positive Baars et al.,
TA1538 1980
Ames test1 S. typhimurium 0-5 µg/plate Positive Goodman et al.,
TA98, TA100 1977
Ames test1 S. typhimurium 0-100 µg/plate Positive NTP, 1988
TA100, TA1535,
TA1537, TA98
Ames test1 S. typhimurium 1-20 µg/plate Positive Ni et al., 1987
TA98, 98NR, 98/
1.8-DNP6
Ames test1 S. typhimurium 0.1-10 µg/plate Positive Yahagi et al.,
TA1535, TA100 1976
Ames test1 S. typhimurium 0-250 nmole/plate Positive Gajewska et al.,
TA97, TA102 1990
Ames test1 S. typhimurium 0.1-100 µg/plate Positive Chandler &
TA98 Parenti, 1982
Forward mutation S. typhimurium 0-0.5 µg/ml Positive Karube et al.,
TA100 electrode 1982
Forward mutation1 E. coli 0-75 µg/ml Positive Baars et al.,
343/113/(R-9 1980
Table 2. cont'd
Test system Test object Concentration Results Reference
Forward mutation1 E. coli WP2 0-240 µM Weak Bryant & McCalla,
Positive 1980
Forward mutation1 E. coli K12 0-10 mm Positive Chessin et al.,
1978
Forward mutation1 E. coli W2 10-300 µM Positive Lu et al., 1979
WP2 uvrA 0-250 µM Positive
Forward mutation2 E. coli K12 not specified Positive Maier et al.,
343/13 1979
Forward mutation1 E. coli WP2 30-120 µM Positive McCalla &
uvrA- Voutsinos, 1974
Forward mutation1 E. coli B and S unspecified Positive Zampieri &
strains Greenberg, 1974
Fluctuation test1 S. typhimurium 0-1 ng/ml Positive Green et al., 1977
TA1535, TA100
Positive
E. coli WP2 uvrA 0-100 ng/ml
Forward mutation1 A. nidulans 0-1000 µg/plate Negative Bignami et al.,
1982
Forward mutation1 N. crassa 0-200 µg/ml Positive Ong, 1977
74-OR60-29A
SOS Chromotest E. coli PQ37 0.01-01 ng/sample Positive Gajewska et al.,
1990
Table 2. cont'd
Test system Test object Concentration Results Reference
DNA damage Mouse L cells 500 µM Positive Olive & McCalla,
1977
DNA damage Mouse L929 cells )0-500 µM Positive Olive & McCalla,
Hamster BHK21 ) 1975
cells )
Human KB cells )
DNA damage Swiss mice 0.1% in diet Positive Olive, 1978a
in vitro (150 mg/kg bw/day)
Unscheduled DNA Rat thymocytes unspecified Positive Tempel, 1980
synthesis
Unscheduled DNA Rat and mouse 1.1 x 10-3-1.1 mg/ml Negative Mori et al., 1987
synthesis hepatocytes
Sister chromatid Chinese hamster 0-15 µg/ml Positive NTP, 1988
exchange ovary cells
Forward mutation1 Mouse lymphoma 0-300 µg/ml Positive NTP, 1988
L5178Y cells 0-400 µg/ml
Forward mutation1 Chinese hamster 100 µg/ml Positive Olive, 1981
V79 cells
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Phillips, 1983
ovary - HGPRT
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Anderson &
ovary - HGPRT Phillips, 1985
Table 2. cont'd
Test system Test object Concentration Results Reference
Sex-linked D. melanogaster 5 mM Negative Kramers, 1982
recessive lethal
mutation
In vitro Chinese hamster 0-200 µg/ml Positive Anderson &
cytogenetics bone marrow Phillips, 1985
In vitro Chinese hamster 0-600 µg/ml Positive NTP, 1988
cytogenetics ovary
In vitro Chinese hamster 0.1 mg/ml Positive3 Matsuoaka et al.,
cytogenetics cells (male 1979
In vitro Human lymphocytes not specified Positive Tonomura &
cytogenetics Sasaki, 1978
In vivo Chinese hamster 0-400 mg/kg bw Negative Anderson &
cytogenetics bone marrow Phillips, 1985
In vivo Wistar rat bone 0-400 mg/kg bw or Negative Anderson, 1983
cytogenetics marrow 1-150 mg/kg
bw/day for 5 days
Micronucleus test Sprague-Dawley rat )0-60 mg/kg bw; Negative Goodman et al.,
)half dose 30 h and 1977
Long Evans rat )half dose 6 h,
)prior to sacrifice
1 With and without hepatic metabolic activation.
2 With and without testicular metabolic activation.
3 In presence but not absence of metabolic activation.
3. COMMENTS
The results of acute and short-term toxicity studies,
carcinogenicity studies, and teratogenicity studies were considered
by the Committee, in addition to several genotoxicity studies. Some
limited pharmacokinetic data were also available which demonstrated
good absorption of the drug in rats and cattle, but no data were
available on the extent of biotransformation nor on the identities
of any metabolites formed. A comparison with other nitrofurans
suggests that nitrofural probably undergoes extensive
biotransformation.
In acute oral toxicity studies, nitrofural was slightly toxic
to mice (LD50 = 300-600 mg/kg bw) and rats (LD50 = 600-800 mg/kg
bw).
Testicular hypoplasia was noted in rats and mice given dietary
nitrofural for 13 weeks. The NOEL for this effect was 15 mg/kg
bw/day in a 13-week study in rats.
Nitrofural was tested for its ability to induce forward
mutations in bacteria, yeast, and mammalian cells in vitro. In the
majority of these studies it produced positive results. Positive
results were also noted in tests for DNA damage in bacteria and in
mammalian cells in vitro, in in vitro studies for clastogenic
effects in Chinese hamster ovary or bone marrow cells, and in human
lymphocytes. However, in vivo studies of clastogenic potential in
Chinese hamster or rat bone marrow were negative. The results
suggest that nitrofural is genotoxic in vitro but not in vivo.
Carcinogenicity studies with nitrofural were conducted in rats
and mice. In a 2-year dietary study in B6C3F1 mice, the
animals were given nitrofural equal to daily oral doses of 14 or
29 mg/kg bw/day. In female mice, nitrofural induced a high incidence
of ovarian atrophy and of ovarian tubular-cell hyperplasia in both
dose groups when compared with controls. There were also increased
incidences of ovarian granulosa-cell and benign mixed tumours in
low-and high-dose groups. There were no elevated incidences of any
tumour type in male mice.
In a 2-year carcinogenicity study in rats, testicular
degeneration was one of the major non-neoplastic findings. There
were also elevated incidences of degeneration of the joint articular
cartilages at several sites in both males and females. No-effect
levels could not be identified for these findings. There was an
increased incidence of mammary fibroadenomas in females given the
low and high doses of 11 and 24 mg/kg bw/day.
Teratogenicity studies were conducted in mice and rabbits. In
mice, the rate of late fetal death was increased in treated animals
at maternally toxic doses; the NOEL was 11 mg/kg bw/day. Pregnant
rabbits were given gavage doses of up to 20 mg/kg bw/day. At the
highest dose, there was an elevated incidence of minor fetal
malformations per litter but this dose produced maternal toxicity.
The NOEL was 15 mg/kg bw/day. The data suggest that nitrofural is
not a direct teratogen but produces fetotoxic effects at maternally
toxic doses in mice and rabbits.
Although nitrofural was tumorigenic in rats and mice, the
tumours it produced were benign and were restricted to endocrine
organs and the mammary gland. Moreover, the mutagenicity studies
suggest that nitrofural is genotoxic in vitro but not in vivo.
Taken together, the data suggest that nitrofural is a secondary
carcinogen producing its effects in endocrine-responsive organs by a
mechanism that remains to be elucidated. In rats, nitrofural has
been shown to disrupt the utilization of progesterone and blocks the
synthesis of cortisone from deoxycortisone. Effects on
steroidogenesis may therefore be involved in the process of tumour
formation. The closely related compound nitrofurantoin also produced
benign ovarian tumours in the same strain of mouse, which were
thought to be secondary to drug-induced ovarian atrophy. This type
of effect cannot be excluded in the biogenesis of the ovarian
tumours produced by nitrofural. However, the Committee recognized
that no-effect levels for these effects could not be identified. The
Committee noted that nitrofural had been reviewed by the
International Agency for Research on Cancer, which concluded that
there was limited evidence for the carcinogenicity of nitrofural in
animals but inadequate evidence for humans.
4. EVALUATION
The Committee concluded that it could not establish an ADI for
nitrofural because no-effect levels had not been established for the
tumorigenic effects. It noted that, although a no-effect level
(15 mg/kg bw/day) had been established in rats for testicular
hypoplasia in a 13-week study, a slightly lower dose (11 mg/kg
bw/day) resulted in a high incidence of testicular degeneration in
the 2-year carcinogenicity study. There was no NOEL for this effect
in the 2-year study and, moreover, no study on reproductive
performance was available. The degenerative changes in the joints of
rats were seen in both males and females but a no-effect level could
not be established.
Before reviewing nitrofural again the Committee would wish to
see:
1. Further data from long-term studies in rats which would
allow the identification of no-effect levels for the
effects on joint articular cartilages and for testicular
degeneration.
2. Data to support the view that tumour formation in rodents
following nitrofural administration has an endocrine
origin. If a mechanism were demonstrated, no-effect levels
for suitable end-points would need to be identified.
3. Additional data on the identity, quantity and biological
characteristics of nitrofural metabolites.
5. REFERENCES
AKAO, M., KURODA, K., KANISAWA, M. & MIYAKI, K, (1971). Influence of
some nitrofurans on carcinogenesis in rats fed 4-(dimethylamino)
azobenzene. Gann, 62: 479-484.
ALI, B.H. (1983). The effect of nitrofurazone on the thiamine status
of chickens. Comp. Biochem. Physiol., C.76: 131-134.
ALI, B.H., SILSBY, J. & EL HALAWANI, M.E. (1987). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
Brit. Poultry Sci., 28: 613-621.
ALI, B.H. (1988). Effect of furazolidone and nitrofurazone on brain
gamma-aminobutyric acid and glutamate concentrations in chickens.
Clin. Exp. Pharmacol. Physiol., 15: 959-963.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL) levels in young turkeys. Gen. Pharmacol., 19:
91-95.
ANDERSON, D. (1983) Cytogenetic analysis in rat bone marrow with
nitrofurazone. Project No. 466.59.63. Unpublished report for Norwich
Eaton Pharmaceuticals, Inc. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
ANDERSON, D. & PHILLIPS, B.J. (1985). Nitrofurazone - genotoxicity
studies in mammalian cells in vitro and in vivo. Food Chem.
Toxicol., 23: 1091-1098.
BAARS, A.J., BLIJLEVEN, W.G.H., MOHN, G.R., NATARAJAN, A.T. &
BREIMER, D.D. (1980). Preliminary studies on the ability of
Drosophila microsomal preparations to activate mutagens and
carcinogens. Mutat. Res., 72: 257-264.
BAJAJ A.K. & GUPTA, S.C. (1986). Contact hypersensitivity to topical
antibacterial agents. Int. J. Dermatol., 25: 103-105.
BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L., CREBELLI, R. &
FABRIZI, M. (1982). Evaluation of 2 different genetic markers for
the detection of frameshift and missense mutagens in A. nidulans.
Mutat. Res., 97: 293-302.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran-induced mutagenesis
and error prone repair in Escherichia coli. Chem-Biol. Interact.,
31: 151-166.
CHANDLER, A.D. & PARENTI, R.R. (1982). Isolation and
characterisation of a nitrofurazone resistant strain of Salmonella
TA98. Environ. Mutagen., 4: 319-320.
CHESSIN, H., McLAUGHLIN, T., MROCZKOWSKI, Z., RUPP, W.D. & LOW, K.B.
(1978). Radiosensitization, mutagenicity, and toxicity of
Escherichia coli by several nitrofurans and nitroimidazoles.
Radiat. Res., 75: 424-431.
COHEN S.M. & BRYAN, G.T. (1973). Carcinogenesis caused by nitrofuran
derivatives. Pharmacology and the Future of Man. Proc. Vth Int.
Congr. Pharmacol., San Francisco, Karger, Basel, 161-170.
COHEN, S.M., ETURK, F., VON ESCH, A.M., CROVETTI, A.J. & BRYAN, G.T.
(1973). Carcinogenicity of 5-nitrofurans, 5-nitromidazoles,
4-nitrobenzenes and related compounds. J. Natl. Cancer Inst., 51:
403-417.
COHEN, S.M. (1978). Toxicity and carcinogenicity of nitrofurans.
Carcinogenesis, 4: 171-231.
FRIEDGOOD, C.E. & GREEN, M.N. (1950). The effect of nitrofurazone on
the growth of fibrosarcoma in mice. Cancer Res., 10: 613-615.
FRIEDGOOD, C.E. & RIPSTEIN, C.B. (1951). The effect of nitrofurazone
on tumours of the human testis and prostate gland. Surg. Forum,
329-333.
GAJEWSKA, J., SZCZYPKA, M., TUDEK, B. & SZYMCZYK, T. (1990). Studies
on the effect of ascorbic acid and selenium on the genotoxicity of
nitrofurans : nitrofurazone and furazolidone. Mutat. Res., 232:
191-197.
GOODMAN, D., AUFRERE, M., VORE, M. & MEYERS, F. (1977). Metabolism
of nitrofurazone. 61st Annual Meeting of the Federation of American
Societies for Experimental Biology, Chicago, Illinois.
GREEN, M.H.L., ROGERS, A.M., MURIEL, W.J., WARD, A.C. & McCALLA,
D.R. (1977). Use of a simplified fluctuation test to detect and
characterize mutagenesis by nitrofurans. Mutat. Res., 44: 139-143.
HARDIN, B.D., SCHULER, R.L., BURG, J.R., BOOTH, G.M., HAZELDEN,
K.P., MacKENZIE, K.M., PICCIRILLO, V.J., & SMITH, K.N. (1987).
Evaluation of 60 chemicals in a preliminary developmental toxicity
test. Terat. Carcinogen. Mutagen., 7: 29-48.
HEINONEN, O.P., SLONE, D. & SHAPIRO, S. (1977). (Eds). Antimicrobial
and antiparasitic agents. In: Birth Defects and Drugs in Pregnancy,
Publishing Sciences Group, Inc, Littleton, MA, 296-313.
HOENER, B-A. (1988). Nitrofurazone : Kinetics and oxidative stress
in the singlepass isolated perfused rat liver. Biochem. Pharmacol.,
37: 1629-1636.
HOOGENBOOM, L.A., OORSPRONG, M.B.M., VAN VLIET, T. & KUIPER, H.A.
(1991). The use of pig hepatocytes for cytotoxicity studies of
veterinary drugs: A comparative study with furazolidone and other
nitrofurans. Toxicol. in Vitro, 5: 31-38.
HOOGENBOOM, L.A. (1991). The use of pig hepatocytes for
biotransformation and toxicity studies. Thesis,
Landbouwuniversiteit, Wageningen, Netherlands.
HUFF, J.E., EUSTIS, S.L. & HASEMAN, J.K. (1989). Occurrence and
relevance of chemically-induced benign neoplasms in long-term
carcinogenicity studies. Cancer Metastasis Rev., 8: 1-22.
IARC (1990). Monographs on the evaluation of carcinogenic risks to
humans. Pharmaceutical Drugs, IARC, Lyon, 50: 195-209.
KARI, F.W., HUFF, J.E., LEININGER, J., HASEMAN, J.K. & EUSTIS, S.L.
(1989). Toxicity and carcinogenicity of nitrofurazone in F344/N rats
and B6C3F1 mice. Food Chem. Toxicol., 27: 129-137.
KARUBE, I., NAKAHARA, T., MATSUNAGA, T. & SUZUKI, S. (1982).
Salmonella electrode for screening mutagens. Anal. Chem., 54:
1725-1727.
KING, C.D. & CURRIE, G.N. (1972). Effects of nitrofurans on
steroidogenesis and mammary hyperplasia. Unpublished report. Norwich
Pharmacal Company, Project No. 596.09. Submitted to WHO by Orphahell
B.V., Midrecht, Netherlands.
KLEMENCIC, J.M. & WANG, C.Y. (1978). Mutagenicity of nitrofurans.
In: Bryan, G.T. ed. Carcinogenesis, 4: 99-130, New York, Raven
Press.
KRAMERS, P.G.N. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
KRANTZ, J.C. & EVANS, W.E. (1945). A contribution to the
pharmacology of 5-nitro-2-furaldehyde semicarbazone. J. Pharmacol.
Exp. Therap., 85: 324-331.
KUTCHER, W.W. & McCALLA, D.R. (1984). Aerobic reduction of
5-nitro-2-furaldehyde semicarbazone by rat liver xanthine
dehydrogenase. Biochem. Pharmacol., 33: 799-805.
LAUBSTEIN V.H. & NIEDEGESASS, G. (1970). Untersuchungen uber
gruppensensibilisierungen bei nitrofuranderivaten. Derm. Mschr.,
156: 1-8.
LIM, L.O., BORTELL, R. & NEIMS, A.H. (1986). Nitrofurantoin
inhibition of mouse liver mitochondrial respiration involving
NAD-linked substrates. Toxicol. Appl. Pharmacol., 84: 493-499.
LO, J.S., TAYLOR, J.S. & ORIBA, H. (1990). Occupational allergic
contact dermatitis to airborne nitrofurazone. Dermatologic Clinics,
8: 165-168.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli (mutation and induction and repair of daughter-strand
gaps in DNA). Mutat. Res., 67: 133-144.
MAIER, P., PHILPOT, R.M., MOHN, G.R. & MALLING, H.V. (1979).
Influence of subcellular fractions of mammalian testes on the
mutagenic activity of nitrofurans toward Escherichia coli. Mutat.
Res., 63: 233-243.
MARONPOT, R.R. (1987). Ovarian toxicity and carcinogenicity in eight
recent National Toxicology Program studies. Environ. Health
Perspect., 73: 125-130.
MATSUOKA, A., HAYASHI, M. & ISHIDATE, M.J, (1979). Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutat. Res., 66: 277-290.
McCALLA, D.R. (1979). Nitrofurans : antibiotics. In: Hahn F(Ed),
Mechanism of Action of Antibacterial Agents (Vol IV). Berlin,
Springer Verlag, 176-213.
McCALLA, D.R., OLIVE P.L., TU, Y. & FAN, M. (1975). Nitrofurazone -
reducing enzymes in E. coli and their role in drug activation
in vivo. Canad. J Microbiol., 21: 1484-1491.
McCALLA D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res., 26: 3-16.
McCALLA, D.R. (1981). Metabolic activation of nitroheterocyclic
compounds in bacteria and mammalian cells. In: Short-Term Tests for
Chemical Carcinogens, Springer, New York, 36-47.
McCALLA, D.R. (1983). Mutagenicity of nitrofuran derivatives:
Review. Environ. Mutagen., 5: 745-765.
McCALLA, D.R., REUVERS, A. & KAISER, C (1971). "Activation" of
nitrofurans in animal tissues. Biochem. Pharmacol., 20: 3532-3537.
McCALLA, D.R., REUVERS, A. & KAISER, C. (1970). Mode of action of
nitrofurazone. J. Bacteriol., 104: 1126-1134.
MIYAJI, T., MIYAMOTO, M, & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testes caused by nitrofuran
compounds. Acta Path. Jap., 14: 261-273.
MIYAJI, T. (1971). Acute and chronic toxicity of furylfuramide in
rats and mice. Tohoku J. Exp. Med., 103: 331-369.
MORI, H., SUGIE, S., YOSHIMI, M., KINOUCHI, T. & OHNISHI, Y. (1987).
Genotoxicity of a variety of nitroarenes and other nitro compounds
in DNA-repair tests with rat and mouse hepatocytes. Mutat. Res.,
190: 159-167.
MORRIS, J.E. (1965). The carcinogenic activity in the rat of some
derivatives of 5-nitrofurn. PhD Thesis, University of Wisconsin,
Madison, USA.
MORRIS, J.E., PRICE, J.M., LALICH, J.J. & STEIN, R.J. (1969). The
carcinogenic activity of some 5-nitrofuran derivatives in the rat.
Cancer Res., 29: 2145-2156.
NI, Y.C., HEFLICH R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98NR
and TA98/1,8-DNP-6. Mutat. Res., 192: 15-22.
NOMURA, T., KIMURA, S., KANZAKI, T., TANAKA, H., SHIBAK, K.,
NAKAJIMA, H., ISA, Y., KUROKAWA, N., HATANAKA, T., KINUTA, M.,
MASADA, K. & SAKAMOTO, Y. (1984). Induction of tumours and
malformations in mice after prenatal treatment with some antibiotic
drugs. Med. J. Osaka Univ., 35: 13-17.
NOUWS, J.F.M., VREE, T.B., AERTS, M.M.L., DEGEN, M. & DRIESSENS, F.
(1987). Some pharmacokinetic data about furaltadone and
nitrofurazone administered orally to preruminant calves. Vet. Q.,
9: 208-214.
NTP (1988). Toxicology and carcinogenesis studies of nitrofurazone
(CAS No. 59-87-0) in F344/N rats and B6C3F1 mice. Technical
Report Series No. 337. US Department of Health and Human Services,
Public Health Service/National Institutes of Health National
Toxicology Program.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1986). Nitrofuran mutagenicity:
Induction of frameshift mutations. Mutat. Res., 175: 149-152.
OLIVE, P.L. & McCALLA, D.R. (1975). Damage to mammalian cell DNA by
nitrofurans. Cancer Res., 35: 781-784.
OLIVE, P.L. & McCALLA, D.R. (1977). Cytotoxicity and DNA damage to
mammalian cells by nitrofurans. Chem.-Biol. Interact., 16:
223-233.
OLIVE, P.L. (1978a) Nitrofurazone-induced DNA damage to tissues of
mice. Chem.-Biol. Interact., 20: 323-331.
OLIVE, P.L. (1978b). Macromolecular, antineoplastic, and
radiosensitization effects of nitrofurans. Carcinogenesis, 4:
131-169.
OLIVE, P.L. (1979). Influence of cellular environment on toxicity of
nitroheterocycles. Chem.-Biol. Interact., 27: 281-290.
OLIVE, P.L. (1980). Mechanisms of the in vitro toxicity of
nitroheterocycles, including flagyl and misonidazole. Section of
Radiobiology, The Johns Hopkins Oncology Center, Baltimore, MD,
Radiation Sensitizers: Their Use in the Clinical Management of
Cancer, 5: 39-45.
OLIVE, P.L. (1981). Correlation between the half-wave reduction
potentials of nitroheterocycles and their mutagenicity in Chinese
hamster V79 spheroids. Mutat. Res., 82: 137-145.
ONG T-M. (1977). Mutagenic activities of nitrofurans in Neurospora
crassa. Mutat. Res., 56: 13-20.
ORPHAHELL, B.V. (1991). Furazolidone-nitrofurazone. Summary volume
1. Unpublished report. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
PAUL, M.F., PAUL, H.E., BENDER, R.C., KOPKO, F., HARRINGTON, C.M.,
ELLS, V.R. & BUZARD, J.A. (1960). Studies on the distribution and
excretion of certain nitrofurans. Antibiotics and
Chemotherap., 10: 287-302.
PAUL, H.E., PAUL, M.F., KOPKO, F. & MAIN, R.J. (1952). Effect of
furacin (5-nitro-2-furaldehyde semicarbazone) on the in vitro
metabolism of mammalian tissues. Proc. Soc. Exp. Biol. Med., 79:
555-558.
PHILLIPS, B.J. (1983). Study of mutation at HGPRT locus in cultured
Chinese hamster ovary cells with nitrofurans. Unpublished report
(466.59.62), Norwich Eaton Pharmaceuticals, Inc. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
POLNASZEK, C.F., PETERSON, F.J., HOLTZMAN, J.L. & MASON, R.P.
(1984). No detectable reaction of the anion radical metabolite of
nitrofurans with reduced glutathione or macromolecules. Chem.-Biol.
Interact., 51: 263-271.
PRICE, C.J., GEORGE, J.D. MARR, M.C. (1985). Teratologic evaluation
of nitrofurazone (CAS No 59-87-0) administered to CD-1 mice on
gestational days 6 though 15. National Toxicology Program,
NTP-85-335, PB86-145844/GAR.
PRICE, C.J., GEORGE, J.D. & MARR, M.C. (1987). Teratologic
evaluation of nitrofurazone (CAS No 59-87-0) administered to New
Zealand white rabbits on gestational days 6 through 19. National
Toxicology Program, NTP-87-200, PB88-130984/GAR.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No. 440.07)
(Special Report), Norwich Pharmacal Company. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No.440.07)
(Addendum to Special Report), Norwich Pharmacal Company. Submitted
to WHO by Orphahell B.V., Mijdrecht, Netherlands.
SKEGGS, H.R., BERGLUND, R.M., Van Den HEUVEL W.J.A., MROZIK, H.,
WISLOCKI, P.G. & WOLF, F.J. (1984). Effect of liver enzymes on the
mutagenicity of nitroheterocyclic compounds : activation of 3a, 4,
5, 6, 7, 7a - hexahydro-3-(1-methyl-5-nitro-1H-imidazole-2-yl)-
1,2-benzisoxazole and deactivation of nitrofurans and
nitroimidazoles in the Ames test. Mutat. Res., 136: 1-8.
SMITH, C.G., GRADY, J.E. & NORTHAN, J.I. (1963). Relationship
between cytotoxicity in vitro and whole animal toxicity. Cancer
Chemotherapy Reports, 20: 9-12.
SORRENTINO, D., BODE, W. & HOENER, B-A. (1987). Nitrofurazone
disposition by perfused rat liver. Effect of dose size and
glutathione depletion. Biochem Pharmacol., 36: 915-918.
STITZEL, K.A., McCONNELL, R.F. & DIERCKMANN, T.A. (1989). Effects of
nirofurantoin on the primary and secondary reproductive organs of
female 2B6C3F1 mice. Toxicol. Pathol., 17: 774-781.
SWAMINATHAN, S. & LOWER, G.M. (1978). Biotransformation and
excretion of nitrofurans. Carcinogenesis, 4: 59-97.
TEMPEL, K. (1980). Unscheduled DNA synthesis of rat thymocytes under
the influence of radioprotective and radiosensitizing agents.
Naunyn-Schmiedebergs Arch. Pharmacol., 311: R7.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan J. Genetics, 48: 292-294.
YAHAGI, T., MATSUSHIMA, T., NAGAO, M., SEINO, Y., SUGIMURA, T. &
BRYAN, G.T. (1976). Mutagenicities of nitrofuran derivatives on a
bacterial tester strain with a R factor plasmid. Mutat. Res., 40:
9-14.
ZAMPIERI, A. & GREENBERG, J. (1964). Nitrofurazone as a mutagen in
Escherichia coli. Biochem. Biophys. Res Commun., 14: 172-176.
Nitrofural had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
Nitrofural appeared to be well absorbed in the rat after oral
administration of 100 mg/kg bw. At 4 h after administration plasma
levels had reached 4.5 mg/l. No other time points were investigated.
Around 5% and 0.5% of the dose were excreted in the 24 h urine and
faeces, respectively. As acknowledged by the authors, the values are
likely to be underestimates as nitrofurazone was shown in this study
to be rapidly metabolized after incubation with rat liver slices.
There was no evidence of deposition in any organ (Paul et al.,
1960).
2.1.1.2 Cattle
A single oral dose of 14 mg/kg bw nitrofural administered to
five pre-ruminant calves resulted in maximum plasma concentrations
of 3.5 mg/litre at 3 h after administration. The final elimination
half-life was 5 h and around 2% of the administered dose was
recovered in the urine (Nouws et al., 1987).
2.1.2 Biotransformation
As described in paragraph 2.1.1.1, nitrofural is rapidly
metabolized by rat liver slices. Nitrofurans, including nitrofural,
undergo metabolic reduction at the nitro group to generate reactive
species which can covalently bind to cellular macromolecules
(Polnaszek et al., 1984; Kutcher & McCalla, 1984; McCalla 1979;
McCalla et al., 1975). Data obtained from studies with isolated
perfused rat liver suggested that conjugation to glutathione
followed by biliary excretion was important; only 0.27% of the
administered perfusate was excreted unchanged (Sorrentino et al.,
1987). Conjugation with amino acids such as methionine may also
occur (Hoener, 1988).
Ring opening of nitrofurans is known to occur (Swaminathan &
Lower, 1978) but there are insufficient data to demonstrate this
with nitrofurazone.
2.1.3 Effects on enzymes and other biochemical parameters
In chickens nitrofural increased gamma-aminobutyric acid and
glutamate concentrations in the brain at oral doses of 25 and 50
mg/kg bw for 5 days (Ali, 1988). Nitrofural, given orally at doses
of 10 and 20 mg/kg bw for 7 days, induced thiamine deficiency in
chickens (Ali 1983).
Nitrofural was found to inhibit the uptake of oxygen by various
rat tissues (Paul et al., 1952). Nitrofural inhibited respiration
in isolated mouse liver mitochondria suggesting that these may be
potential sites for toxicity (Lim et al., 1986).
Nitrofural and other nitrofurans are relatively potent
inhibitors of MAO in mammalian and avian species (Hoogenboom, 1991).
As with other nitrofurans, nitrofural can affect the endocrine
system resulting in alterations of hormone levels. In laying
turkeys, nitrofural decreased luteinizing hormone and prolactin in
plasma (Ali et al., 1987). Similar effects were noted in young
male turkeys (Ali et al., 1988).
Nitrofural has been shown to inhibit utilization of
progesterone as a steroid percursor by the adrenal in rats. In this
study it was shown that nitrofurans, including nitrofural, block
11-hydroxylation of deoxycorti-costerone to corticosterone.
Sprague-Dawley rats (1 year old) given nitrofurans for 325 days
underwent constant oestrous. The authors concluded that nitrofurans
were antagonists of normal adrenal function and believed that this
could lead to endocrine stress (King & Currie, 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The LD50 values for nitrofural are shown in Table 1. Signs of
toxicity included roughened coats, hyperexcitability, tremors,
reduced activity, weakness, convulsion, and respiratory collapse
(Krantz & Evans 1945; Anderson, 1983).
Table 1. Acute toxicity of nitrofural
Species/strain Sex Route LD50 Reference
(mg/kg bw)
Rat (unspecified) M oral 590 Krantz & Evans,
1945
Rat (Donryu) M&F oral 590 Miyaji, 1971
Rat (Wistar) M oral 800 Anderson, 1983
Mouse ? oral 380 Krantz & Evans,
(unspecified) 1945
Mouse (ICR Jcl) M&F oral 590 Miyaji, 1971
Mouse ? i.p. 300 Smith et al.,
(unspecified) 1963
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 5 male and 5 female B6C3F1 mice were given
diets containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 94.5, 188, 375, 750 or 1500 mg/kg bw/day for 14
days.
All mice given the three highest dietary levels died during the
study, as did 3/5 given 188 mg/kg bw/day. Animals in all the groups
except females given 94.5 mg/kg bw/day lost weight and all dosed
animals displayed rough coats and convulsive seizures (National
Toxicology Program (NTP), 1988).
Groups of 10 male and 10 female B6C3F1 mice were given
diets containing 0, 70, 150, 310, 620 or 1250 ppm nitrofural,
equivalent to 0, 10, 23, 47, 93 or 188 mg/kg bw/day for 13 weeks.
High mortality was noted at the highest dietary level (6/10
males and 9/10 females). Mortality was relatively high at the
penultimate dietary level (3/10 males and 5/10 females). Elevated
relative liver weights were noted in mice given the two highest
dietary levels. Animals given the two highest dietary levels also
displayed hyperexcitability and convulsive seizures, and males in
these groups had a very high incidence (80-90%) of testicular
hypoplasia (NTP, 1988).
2.2.2.2 Rats
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 630, 1250, 2500, 5000 or 10 000 ppm nitrofural,
equivalent to 0, 63, 125, 250, 500 or 1000 mg/kg bw/day, for 14
days.
All rats given 500 or 1000 mg/kg bw/day died. Feed consumption
was decreased at all levels in excess of the lowest level. Signs of
toxicity included lethargy and roughened coats. Seizures occurred in
animals given 250 mg/kg bw/day and above. Histopathologic
examination revealed aspermatogenesis in dosed males (NTP, 1988).
Groups of 10 male and 10 female F344/N rats were given diets
containing 0, 150, 310, 620, 1200 or 2500 ppm nitrofural, equivalent
to 0, 15, 31, 62, 120 or 250 mg/kg bw/day, for 13 weeks.
All rats survived until the end of the study but final body
weights of animals given 62, 120 or 250 mg/kg bw/day were 11%, 32%
and 55% lower, respectively, than control values. Relative liver
weights for treated rats were significantly higher than control
animals. Atrophy constricture of the hind-quarters occurred in males
and females given the highest dietary level.
Moderate to severe degeneration of the seminiferous epithelium
of the testes was noted in males given the 4 highest doses. The
no-effect-level was 15 mg/kg bw/day. Males and females given the two
highest dietary levels had osteoporosis in the metaphyseal region.
(NTP, 1988).
In a study designed to investigate effects on the testes,
45-day-old male Donryu rats were given a diet containing 0.2%
nitrofural (equal to 300 mg/kg bw/day) for 9 days. Three to 6 rats
given nitrofural were sacrificed every day after feeding began.
A decrease in testis weight occurred by day 5 but
histologically, a decrease in spermatozoa and spermatids occurred at
day 1, with disappearance by days 3 and 4. Here, the stratified
appearance of the normal testis had disappeared and only basal
spermatogonia and Sertoli cells remained. Slight oedema and
exudation occurred in interstitial tissue but Leydig cells appeared
normal (Miyaji et al., 1964).
2.2.3 Long-term/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female B6C3F1 mice were given
diets containing 0, 150 or 310 ppm nitrofural equal to 0, 14 or 29
mg/kg bw/day for 2 years.
There were no effects on body weights and survival was only
slightly affected at the highest dose in males and females (27/50
and 35/50 for males and females, respectively) compared with
controls (39/50 for each sex).
Females given nitrofural were found to have increased
incidences of ovarian atrophy (44/50 and 38/50 in low- and high-dose
groups respectively) compared with controls (7/47) and tubular cell
hyperplasia (23/50 and 21/50 in low- and high-dose groups) when
compared with controls (1/47). However, there were no effects on the
testes in males.
There were increased incidences of ovarian granulosa cell
tumours in low- (4/50) and high-dose (9/50) females when compared
with control values (1/47). Similarly, there were increased
incidences of benign mixed tumours of the ovary in low- (17/50) and
high-dose (20/50) females compared with controls (0/47). There were
no elevated incidences of any tumour type in male mice (NTP, 1988;
Kari et al., 1989).
In a study of transplacental carcinogenesis, a group of 20
pregnant ICR/Jcl mice was given 3 subcutaneous injections at
75 mg/kg bw nitrofural on days 13, 15 and 17 of gestation. The
incidence of tumours in offspring was not increased when compared
with untreated controls when they were examined 32 weeks after
birth.
In the same study, newborn mice (61) were given a subcutaneous
injection at 75 mg/kg bw 12 h after birth and on days 7, 14 and 21.
The offspring were examined at 32 weeks after birth when an
increased incidence of pulmonary papillary adenomas was noted
(Nomura et al., 1984).
2.2.3.2 Rats
A group of 20 female Holtzman rats was fed a diet containing
1000 ppm nitrofural (150 mg/kg bw/day) for 36 weeks. A similar group
of 30 females was fed the same level for 44.5 weeks. Animals were
killed 15-19 weeks after dosing was completed. At termination, there
was an elevated incidence of mammary tumours in rats fed
nitrofural-containing diets when compared with controls (Morris
et al., 1969; Morris, 1965).
Groups of 35 female Holtzman rats were fed diets containing
0, 500 or 1000 ppm nitrofural (0, 75 or 150 mg/kg bw/day) for 45
weeks, and then a basal diet for a further 8 weeks. Rats consuming
nitrofural at 75 mg/kg bw/day nitrofural had no increased tumour
incidence but in those given 150 mg/kg bw/day the numbers of
tumour-bearing rats were increased. At week 53, the numbers with
detectable mammary tumours were significantly increased in both the
75 (10/35) and 150 (12/35) mg/kg bw/day groups, when compared with
untreated controls (2/35). None of the reported tumours were
malignant (Siedler & Searfoss, 1966).
Groups of 20 male and 20 female CFE rats were fed diets
containing nitrofural to give a daily intake of 50-55 mg/kg bw/day
for 45 weeks. At the end of this period the animals were returned to
the basal diets for a further 7 weeks. There was no increased
incidence of any tumours in the male rats. A significant increase in
the incidence of benign mammary tumours was noted at week 52 (12/20)
compared with untreated controls (0/20) (Siedler & Searfoss, 1967).
Groups of 50 male and 50 female F344/N rats were fed diets
containing 0, 310 or 620 ppm nitrofural equal to 0, 11 or 24 mg/kg
bw/day for 2 years. There were reductions in the rate of weight gain
and final body weights were reduced in males and females given
dietary nitrofural. Mortality was increased in males given the high
dietary level (30/50; 60%) compared with controls (17/50; 34%).
The major non-neoplastic finding was degeneration of joint
articular cartilages in males and females in several areas of the
body, notably, the vertebral and knee joints. A no-effect-level was
not identifiable. Testicular degeneration occurred in 49/50 low
dietary level and 47/50 high dietary level males compared with 12/50
in controls.
There was an increased incidence of mammary fibroadenomas in
both low and high dietary level females (36/50; 72% in both groups)
when compared with controls (8/49; 16%) but the incidence of
adenocarcinomas was not elevated. In males, the incidence of
mesotheliomas in the tunica vaginalis was increased but not in a
dose-related manner (0/50, 7/50 and 2/50 in the control, low and
high dietary level groups). There was also an increased incidence of
sebaceous adenoma of the skin of male rats but only at the high
dietary level (0/50, 0/50 or 5/50 for controls, low and high dietary
levels, respectively). A slightly increased incidence of carcinoma
of the preputial gland was seen in males (1/50, 8/50 and 5/50 in the
control, low and high dietary groups, respectively). The incidence
of testicular tumours was reduced in dosed males (NTP, 1988; Kari
et al., 1989).
It was concluded by the authors that the data from male rats
provided only equivocal evidence of carcinogenicity. The increases
in incidences of the various tumours were small and not
dose-related.
The induction of ovarian tumours noted in mice treated with
chemicals is relatively rare. Such an effect had been noted with
only 8 chemicals from the NTP studies among which were nitrofural
itself, 1,3-butadiene, 4-vinylcyclohexene, nitrofurantoin, benzene
and delta-9-tetrahydrocannabinol. The tumours were mainly in mice
but rats too were also affected (Maronpot, 1987).
Nitrofural is unusual in that of 143 chemicals which had been
tested by the NTP by 1989, only 5, including nitrofural, produced
only benign neoplastic lesions (Huff et al., 1989). Many other
nitrofurans tested have been shown to be carcinogenic in rodents and
many of these have produced malignant tumours (Cohen & Bryan, 1973;
Cohen, 1978; Cohen et al., 1973).
Nitrofural and other nitrofurans inhibited the
hepatocarcinogenesis by 4-(dimethylamino) azobenzene in rats (Akao
et al., 1971). It also inhibited the growth of fibrosarcomas in
mice and demonstrated antineoplastic properties against seminomas
and carcinoma of the prostate in humans (Friedgood & Green, 1950;
Friedgood & Ripstein, 1951).
The International Agency for Research on Cancer (IARC) has
concluded that there was limited evidence for the carcinogenicity of
nitrofurazone in experimental animals and inadequate evidence for
its carcinogenicity in humans. In its overall evaluation, IARC
placed nitrofural in Group 3 (not classifiable as to its
carcinogenicity) (IARC, 1990).
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on teratogenicity
2.2.5.1 Mice
A group of 16 pregnant ICR/Jcl mice was given subcutaneous
injections at 100 mg/kg bw nitrofural on days 9, 10 and 11 of
gestation. A group of 6 pregnant mice was given a single
subcutaneous injection at 300 mg/kg bw nitrofural on day 10 of
gestation. Controls were given water only on days 9, 10 and 11 of
gestation.
The 100 mg/kg bw regime did not produce an elevated incidence
of malformations in 185 fetuses examined. However, the 300 mg/kg bw
dose on day 10 of gestation led to an overall incidence of
malformations of 21% compared with 0.3% in controls. These were
mainly tail anomalies, oligodactyly, and leg defects (unspecified)
(Nomura et al., 1984).
In a prescreening developmental test, female CD-1 mice were
given nitrofural (100 mg/kg bw/day) by gavage on days 6-13 of
gestation. Nitrofural caused a decrease in the numbers of viable
litters (28/35 compared with 45/45 in controls) and slight
reductions in birth weights. By the authors' criteria, these effects
constituted positive results (Hardin et al., 1987).
In a preliminary teratogenicity study, groups of 6-7 CD-1 mice
were given diets containing 0, 100, 500, 1000, 2000 or 3000 ppm
nitrofural, equivalent to 0, 15, 75, 150, 300 or 450 mg/kg bw/day,
on days 6-17 of gestation.
Dams given the two highest dietary levels died during the
study. There were no differences in food consumption in the other
groups and no effects on weight gain. There were no differences in
implantation site and resorption parameters except in those given 75
mg/kg bw/day where an increased incidence of resorptions was noted
(Price et al., 1985).
In a second preliminary teratology study, groups of 12 pregnant
CD-1 mice were given diets containing 0, 75, 250, 500 or 750 ppm
nitrofural equivalent to 0, 11, 38, 75 or 112 mg/kg bw/day, on days
6-17 of gestation. Approximately 8% mortality occurred at the
highest dietary level and hyperactivity, tremor, paralysis and
convulsions were seen at the two highest levels. There were
dose-related decreases in food consumption and body weights. Gravid
uterine weights decreased with increasing nitrofural intake. There
were no effects on implants/dam but the numbers of resorptions per
litter increased with dose. There were no increases in the
incidences of malformations (Price et al., 1985).
In the main teratogenicity study, groups of 26 pregnant CD-1
mice were given diets containing 0, 38, 75, 250 or 500 ppm
nitrofural, equivalent to 0, 6, 11, 38 or 75 mg/kg bw/day on days
6-15 of gestation. There were no maternal deaths and no effects on
food and water consumption. Gravid uterine weights were unaffected
by compound intake and there were no effects on reproductive
parameters except for an increased incidence of late fetal deaths at
marginally maternally toxic doses. The NOEL was 11 mg/kg bw/day
(Price et al., 1985).
2.2.5.2 Rabbits
Groups of 22-27 New Zeeland white rabbits were given gavage
doses of 0, 5, 10, 15 or 20 mg/kg bw/day nitrofural in corn oil on
days 6-19 of gestation.
At sacrifice, the uterine contents of 121 pregnant animals were
examined. There was no evidence of any teratogenic effect nor
materno- or embryo/feto-toxicity at doses of 5-15 mg/kg bw/day. At
20 mg/kg bw/day, two dams died, there was reduced weight gain,
relative and absolute maternal liver weights were increased and an
increased incidence of fetal malformations/litter was observed,
which was suggestive of fetotoxicity. Hence, effects on the
developing fetus occurred only at maternally toxic levels
(Price et al., 1987). The NOEL was 15 mg/kg bw/day.
2.2.6 Special studies on genotoxicity
Nitrofural has been tested in a range of studies (Table 2). In
general, in vitro studies of forward mutation in bacteria and
mammalian cells, sister chromatid exchange, DNA damage and tests for
clastogenicity have proved positive while in vivo studies,
including those for cytogenetic effects and the micronucleus test,
have proved negative.
In studies where the metabolic activation was incorporated into
the agar layer, the mutagenic activities of a number of nitrofurans
(and nitroimidazoles) were reduced, suggesting that the metabolites
of reductive metabolism may be less mutagenic than those formed
after oxidative metabolism (Skeggs et al., 1984). Metabolic
reduction is believed to be the major route of biotransformation of
nitrofurans and nitroimidazoles and the metabolites so formed may
account for the radiosensitizing properties of these agents (Olive,
1979, 1980, 1978b).
The results obtained with nitrofural are similar to those noted
with a wide range of nitrofurans, and in particular, the propensity
of these compounds for activity in in vitro systems is evident
(Klemencic & Wang, 1978). The mechanism of mutagenicity is unclear,
as is the route of activation, although nitroreduction appears to be
an early step (McCalla, 1981, 1983; Matsuoka et al., 1979; McCalla
et al., 1970, 1971). Nitrofural and other nitrofurans are
cytotoxic to hepatocytes in vitro (Hoogenboom et al., 1991), but
as the tumours caused by nitrofural are largely in endocrine organs,
this is unlikely to be relevant to carcinogenesis by this substance.
2.3 Observations in humans
Nitrofural is a skin sensitizer in humans and there have been
reports of contact dermatitis following exposure to the drug (Bajaj
& Gupta, 1986; Laubstein and Niedergesass, 1970; Lo et al., 1990).
When used as an experimental antineoplastic agent in humans
with prostatic or testicular tumours, testicular degeneration
occurred similar to that noted in experimental animals (Friedgood &
Ripstein, 1951). The doses used were unclear in the original text.
In the Collaborative Perinatal Project conducted in the USA by
the National Institute of Neurological and Communicative Diseases
and Stroke in 1958-1965, only 0.5% (234/50282) of mother-child pairs
had been exposed to nitrofural during months 1-4 of gestation. There
was no increased risk of malformations in this group (Heinonem
et al., 1977).
Table 2. Results of genotoxicity assays on nitrofurazone
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0-75 µg/ml Positive Baars et al.,
TA1538 1980
Ames test1 S. typhimurium 0-5 µg/plate Positive Goodman et al.,
TA98, TA100 1977
Ames test1 S. typhimurium 0-100 µg/plate Positive NTP, 1988
TA100, TA1535,
TA1537, TA98
Ames test1 S. typhimurium 1-20 µg/plate Positive Ni et al., 1987
TA98, 98NR, 98/
1.8-DNP6
Ames test1 S. typhimurium 0.1-10 µg/plate Positive Yahagi et al.,
TA1535, TA100 1976
Ames test1 S. typhimurium 0-250 nmole/plate Positive Gajewska et al.,
TA97, TA102 1990
Ames test1 S. typhimurium 0.1-100 µg/plate Positive Chandler &
TA98 Parenti, 1982
Forward mutation S. typhimurium 0-0.5 µg/ml Positive Karube et al.,
TA100 electrode 1982
Forward mutation1 E. coli 0-75 µg/ml Positive Baars et al.,
343/113/(R-9 1980
Table 2. cont'd
Test system Test object Concentration Results Reference
Forward mutation1 E. coli WP2 0-240 µM Weak Bryant & McCalla,
Positive 1980
Forward mutation1 E. coli K12 0-10 mm Positive Chessin et al.,
1978
Forward mutation1 E. coli W2 10-300 µM Positive Lu et al., 1979
WP2 uvrA 0-250 µM Positive
Forward mutation2 E. coli K12 not specified Positive Maier et al.,
343/13 1979
Forward mutation1 E. coli WP2 30-120 µM Positive McCalla &
uvrA- Voutsinos, 1974
Forward mutation1 E. coli B and S unspecified Positive Zampieri &
strains Greenberg, 1974
Fluctuation test1 S. typhimurium 0-1 ng/ml Positive Green et al., 1977
TA1535, TA100
Positive
E. coli WP2 uvrA 0-100 ng/ml
Forward mutation1 A. nidulans 0-1000 µg/plate Negative Bignami et al.,
1982
Forward mutation1 N. crassa 0-200 µg/ml Positive Ong, 1977
74-OR60-29A
SOS Chromotest E. coli PQ37 0.01-01 ng/sample Positive Gajewska et al.,
1990
Table 2. cont'd
Test system Test object Concentration Results Reference
DNA damage Mouse L cells 500 µM Positive Olive & McCalla,
1977
DNA damage Mouse L929 cells )0-500 µM Positive Olive & McCalla,
Hamster BHK21 ) 1975
cells )
Human KB cells )
DNA damage Swiss mice 0.1% in diet Positive Olive, 1978a
in vitro (150 mg/kg bw/day)
Unscheduled DNA Rat thymocytes unspecified Positive Tempel, 1980
synthesis
Unscheduled DNA Rat and mouse 1.1 x 10-3-1.1 mg/ml Negative Mori et al., 1987
synthesis hepatocytes
Sister chromatid Chinese hamster 0-15 µg/ml Positive NTP, 1988
exchange ovary cells
Forward mutation1 Mouse lymphoma 0-300 µg/ml Positive NTP, 1988
L5178Y cells 0-400 µg/ml
Forward mutation1 Chinese hamster 100 µg/ml Positive Olive, 1981
V79 cells
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Phillips, 1983
ovary - HGPRT
Forward mutation1 Chinese hamster 0-200 µg/ml Negative Anderson &
ovary - HGPRT Phillips, 1985
Table 2. cont'd
Test system Test object Concentration Results Reference
Sex-linked D. melanogaster 5 mM Negative Kramers, 1982
recessive lethal
mutation
In vitro Chinese hamster 0-200 µg/ml Positive Anderson &
cytogenetics bone marrow Phillips, 1985
In vitro Chinese hamster 0-600 µg/ml Positive NTP, 1988
cytogenetics ovary
In vitro Chinese hamster 0.1 mg/ml Positive3 Matsuoaka et al.,
cytogenetics cells (male 1979
In vitro Human lymphocytes not specified Positive Tonomura &
cytogenetics Sasaki, 1978
In vivo Chinese hamster 0-400 mg/kg bw Negative Anderson &
cytogenetics bone marrow Phillips, 1985
In vivo Wistar rat bone 0-400 mg/kg bw or Negative Anderson, 1983
cytogenetics marrow 1-150 mg/kg
bw/day for 5 days
Micronucleus test Sprague-Dawley rat )0-60 mg/kg bw; Negative Goodman et al.,
)half dose 30 h and 1977
Long Evans rat )half dose 6 h,
)prior to sacrifice
1 With and without hepatic metabolic activation.
2 With and without testicular metabolic activation.
3 In presence but not absence of metabolic activation.
3. COMMENTS
The results of acute and short-term toxicity studies,
carcinogenicity studies, and teratogenicity studies were considered
by the Committee, in addition to several genotoxicity studies. Some
limited pharmacokinetic data were also available which demonstrated
good absorption of the drug in rats and cattle, but no data were
available on the extent of biotransformation nor on the identities
of any metabolites formed. A comparison with other nitrofurans
suggests that nitrofural probably undergoes extensive
biotransformation.
In acute oral toxicity studies, nitrofural was slightly toxic
to mice (LD50 = 300-600 mg/kg bw) and rats (LD50 = 600-800 mg/kg
bw).
Testicular hypoplasia was noted in rats and mice given dietary
nitrofural for 13 weeks. The NOEL for this effect was 15 mg/kg
bw/day in a 13-week study in rats.
Nitrofural was tested for its ability to induce forward
mutations in bacteria, yeast, and mammalian cells in vitro. In the
majority of these studies it produced positive results. Positive
results were also noted in tests for DNA damage in bacteria and in
mammalian cells in vitro, in in vitro studies for clastogenic
effects in Chinese hamster ovary or bone marrow cells, and in human
lymphocytes. However, in vivo studies of clastogenic potential in
Chinese hamster or rat bone marrow were negative. The results
suggest that nitrofural is genotoxic in vitro but not in vivo.
Carcinogenicity studies with nitrofural were conducted in rats
and mice. In a 2-year dietary study in B6C3F1 mice, the
animals were given nitrofural equal to daily oral doses of 14 or
29 mg/kg bw/day. In female mice, nitrofural induced a high incidence
of ovarian atrophy and of ovarian tubular-cell hyperplasia in both
dose groups when compared with controls. There were also increased
incidences of ovarian granulosa-cell and benign mixed tumours in
low-and high-dose groups. There were no elevated incidences of any
tumour type in male mice.
In a 2-year carcinogenicity study in rats, testicular
degeneration was one of the major non-neoplastic findings. There
were also elevated incidences of degeneration of the joint articular
cartilages at several sites in both males and females. No-effect
levels could not be identified for these findings. There was an
increased incidence of mammary fibroadenomas in females given the
low and high doses of 11 and 24 mg/kg bw/day.
Teratogenicity studies were conducted in mice and rabbits. In
mice, the rate of late fetal death was increased in treated animals
at maternally toxic doses; the NOEL was 11 mg/kg bw/day. Pregnant
rabbits were given gavage doses of up to 20 mg/kg bw/day. At the
highest dose, there was an elevated incidence of minor fetal
malformations per litter but this dose produced maternal toxicity.
The NOEL was 15 mg/kg bw/day. The data suggest that nitrofural is
not a direct teratogen but produces fetotoxic effects at maternally
toxic doses in mice and rabbits.
Although nitrofural was tumorigenic in rats and mice, the
tumours it produced were benign and were restricted to endocrine
organs and the mammary gland. Moreover, the mutagenicity studies
suggest that nitrofural is genotoxic in vitro but not in vivo.
Taken together, the data suggest that nitrofural is a secondary
carcinogen producing its effects in endocrine-responsive organs by a
mechanism that remains to be elucidated. In rats, nitrofural has
been shown to disrupt the utilization of progesterone and blocks the
synthesis of cortisone from deoxycortisone. Effects on
steroidogenesis may therefore be involved in the process of tumour
formation. The closely related compound nitrofurantoin also produced
benign ovarian tumours in the same strain of mouse, which were
thought to be secondary to drug-induced ovarian atrophy. This type
of effect cannot be excluded in the biogenesis of the ovarian
tumours produced by nitrofural. However, the Committee recognized
that no-effect levels for these effects could not be identified. The
Committee noted that nitrofural had been reviewed by the
International Agency for Research on Cancer, which concluded that
there was limited evidence for the carcinogenicity of nitrofural in
animals but inadequate evidence for humans.
4. EVALUATION
The Committee concluded that it could not establish an ADI for
nitrofural because no-effect levels had not been established for the
tumorigenic effects. It noted that, although a no-effect level
(15 mg/kg bw/day) had been established in rats for testicular
hypoplasia in a 13-week study, a slightly lower dose (11 mg/kg
bw/day) resulted in a high incidence of testicular degeneration in
the 2-year carcinogenicity study. There was no NOEL for this effect
in the 2-year study and, moreover, no study on reproductive
performance was available. The degenerative changes in the joints of
rats were seen in both males and females but a no-effect level could
not be established.
Before reviewing nitrofural again the Committee would wish to
see:
1. Further data from long-term studies in rats which would
allow the identification of no-effect levels for the
effects on joint articular cartilages and for testicular
degeneration.
2. Data to support the view that tumour formation in rodents
following nitrofural administration has an endocrine
origin. If a mechanism were demonstrated, no-effect levels
for suitable end-points would need to be identified.
3. Additional data on the identity, quantity and biological
characteristics of nitrofural metabolites.
5. REFERENCES
AKAO, M., KURODA, K., KANISAWA, M. & MIYAKI, K, (1971). Influence of
some nitrofurans on carcinogenesis in rats fed 4-(dimethylamino)
azobenzene. Gann, 62: 479-484.
ALI, B.H. (1983). The effect of nitrofurazone on the thiamine status
of chickens. Comp. Biochem. Physiol., C.76: 131-134.
ALI, B.H., SILSBY, J. & EL HALAWANI, M.E. (1987). Effect of
furazolidone and nitrofurazone on egg production, on plasma
luteinising hormone and on prolactin concentrations in turkeys.
Brit. Poultry Sci., 28: 613-621.
ALI, B.H. (1988). Effect of furazolidone and nitrofurazone on brain
gamma-aminobutyric acid and glutamate concentrations in chickens.
Clin. Exp. Pharmacol. Physiol., 15: 959-963.
ALI, B.H., SILSBY, J.L., EDENS, F. & EL HALAWANI, M.E. (1988).
Effects of furazolidone or nitrofurazone on the concentrations of
hypothalamic amines and plasma luteinizing hormone (LH) and
prolactin (PRL) levels in young turkeys. Gen. Pharmacol., 19:
91-95.
ANDERSON, D. (1983) Cytogenetic analysis in rat bone marrow with
nitrofurazone. Project No. 466.59.63. Unpublished report for Norwich
Eaton Pharmaceuticals, Inc. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
ANDERSON, D. & PHILLIPS, B.J. (1985). Nitrofurazone - genotoxicity
studies in mammalian cells in vitro and in vivo. Food Chem.
Toxicol., 23: 1091-1098.
BAARS, A.J., BLIJLEVEN, W.G.H., MOHN, G.R., NATARAJAN, A.T. &
BREIMER, D.D. (1980). Preliminary studies on the ability of
Drosophila microsomal preparations to activate mutagens and
carcinogens. Mutat. Res., 72: 257-264.
BAJAJ A.K. & GUPTA, S.C. (1986). Contact hypersensitivity to topical
antibacterial agents. Int. J. Dermatol., 25: 103-105.
BIGNAMI, M., CARERE, A., CONTI, G., CONTI, L., CREBELLI, R. &
FABRIZI, M. (1982). Evaluation of 2 different genetic markers for
the detection of frameshift and missense mutagens in A. nidulans.
Mutat. Res., 97: 293-302.
BRYANT, D.W. & McCALLA, D.R. (1980). Nitrofuran-induced mutagenesis
and error prone repair in Escherichia coli. Chem-Biol. Interact.,
31: 151-166.
CHANDLER, A.D. & PARENTI, R.R. (1982). Isolation and
characterisation of a nitrofurazone resistant strain of Salmonella
TA98. Environ. Mutagen., 4: 319-320.
CHESSIN, H., McLAUGHLIN, T., MROCZKOWSKI, Z., RUPP, W.D. & LOW, K.B.
(1978). Radiosensitization, mutagenicity, and toxicity of
Escherichia coli by several nitrofurans and nitroimidazoles.
Radiat. Res., 75: 424-431.
COHEN S.M. & BRYAN, G.T. (1973). Carcinogenesis caused by nitrofuran
derivatives. Pharmacology and the Future of Man. Proc. Vth Int.
Congr. Pharmacol., San Francisco, Karger, Basel, 161-170.
COHEN, S.M., ETURK, F., VON ESCH, A.M., CROVETTI, A.J. & BRYAN, G.T.
(1973). Carcinogenicity of 5-nitrofurans, 5-nitromidazoles,
4-nitrobenzenes and related compounds. J. Natl. Cancer Inst., 51:
403-417.
COHEN, S.M. (1978). Toxicity and carcinogenicity of nitrofurans.
Carcinogenesis, 4: 171-231.
FRIEDGOOD, C.E. & GREEN, M.N. (1950). The effect of nitrofurazone on
the growth of fibrosarcoma in mice. Cancer Res., 10: 613-615.
FRIEDGOOD, C.E. & RIPSTEIN, C.B. (1951). The effect of nitrofurazone
on tumours of the human testis and prostate gland. Surg. Forum,
329-333.
GAJEWSKA, J., SZCZYPKA, M., TUDEK, B. & SZYMCZYK, T. (1990). Studies
on the effect of ascorbic acid and selenium on the genotoxicity of
nitrofurans : nitrofurazone and furazolidone. Mutat. Res., 232:
191-197.
GOODMAN, D., AUFRERE, M., VORE, M. & MEYERS, F. (1977). Metabolism
of nitrofurazone. 61st Annual Meeting of the Federation of American
Societies for Experimental Biology, Chicago, Illinois.
GREEN, M.H.L., ROGERS, A.M., MURIEL, W.J., WARD, A.C. & McCALLA,
D.R. (1977). Use of a simplified fluctuation test to detect and
characterize mutagenesis by nitrofurans. Mutat. Res., 44: 139-143.
HARDIN, B.D., SCHULER, R.L., BURG, J.R., BOOTH, G.M., HAZELDEN,
K.P., MacKENZIE, K.M., PICCIRILLO, V.J., & SMITH, K.N. (1987).
Evaluation of 60 chemicals in a preliminary developmental toxicity
test. Terat. Carcinogen. Mutagen., 7: 29-48.
HEINONEN, O.P., SLONE, D. & SHAPIRO, S. (1977). (Eds). Antimicrobial
and antiparasitic agents. In: Birth Defects and Drugs in Pregnancy,
Publishing Sciences Group, Inc, Littleton, MA, 296-313.
HOENER, B-A. (1988). Nitrofurazone : Kinetics and oxidative stress
in the singlepass isolated perfused rat liver. Biochem. Pharmacol.,
37: 1629-1636.
HOOGENBOOM, L.A., OORSPRONG, M.B.M., VAN VLIET, T. & KUIPER, H.A.
(1991). The use of pig hepatocytes for cytotoxicity studies of
veterinary drugs: A comparative study with furazolidone and other
nitrofurans. Toxicol. in Vitro, 5: 31-38.
HOOGENBOOM, L.A. (1991). The use of pig hepatocytes for
biotransformation and toxicity studies. Thesis,
Landbouwuniversiteit, Wageningen, Netherlands.
HUFF, J.E., EUSTIS, S.L. & HASEMAN, J.K. (1989). Occurrence and
relevance of chemically-induced benign neoplasms in long-term
carcinogenicity studies. Cancer Metastasis Rev., 8: 1-22.
IARC (1990). Monographs on the evaluation of carcinogenic risks to
humans. Pharmaceutical Drugs, IARC, Lyon, 50: 195-209.
KARI, F.W., HUFF, J.E., LEININGER, J., HASEMAN, J.K. & EUSTIS, S.L.
(1989). Toxicity and carcinogenicity of nitrofurazone in F344/N rats
and B6C3F1 mice. Food Chem. Toxicol., 27: 129-137.
KARUBE, I., NAKAHARA, T., MATSUNAGA, T. & SUZUKI, S. (1982).
Salmonella electrode for screening mutagens. Anal. Chem., 54:
1725-1727.
KING, C.D. & CURRIE, G.N. (1972). Effects of nitrofurans on
steroidogenesis and mammary hyperplasia. Unpublished report. Norwich
Pharmacal Company, Project No. 596.09. Submitted to WHO by Orphahell
B.V., Midrecht, Netherlands.
KLEMENCIC, J.M. & WANG, C.Y. (1978). Mutagenicity of nitrofurans.
In: Bryan, G.T. ed. Carcinogenesis, 4: 99-130, New York, Raven
Press.
KRAMERS, P.G.N. (1982). Studies on the induction of sex-linked
recessive lethal mutations in Drosophila melanogaster by
nitroheterocyclic compounds. Mutat. Res., 101: 209-236.
KRANTZ, J.C. & EVANS, W.E. (1945). A contribution to the
pharmacology of 5-nitro-2-furaldehyde semicarbazone. J. Pharmacol.
Exp. Therap., 85: 324-331.
KUTCHER, W.W. & McCALLA, D.R. (1984). Aerobic reduction of
5-nitro-2-furaldehyde semicarbazone by rat liver xanthine
dehydrogenase. Biochem. Pharmacol., 33: 799-805.
LAUBSTEIN V.H. & NIEDEGESASS, G. (1970). Untersuchungen uber
gruppensensibilisierungen bei nitrofuranderivaten. Derm. Mschr.,
156: 1-8.
LIM, L.O., BORTELL, R. & NEIMS, A.H. (1986). Nitrofurantoin
inhibition of mouse liver mitochondrial respiration involving
NAD-linked substrates. Toxicol. Appl. Pharmacol., 84: 493-499.
LO, J.S., TAYLOR, J.S. & ORIBA, H. (1990). Occupational allergic
contact dermatitis to airborne nitrofurazone. Dermatologic Clinics,
8: 165-168.
LU, C., McCALLA, D.R. & BRYANT, D.W. (1979). Action of nitrofurans
on E. coli (mutation and induction and repair of daughter-strand
gaps in DNA). Mutat. Res., 67: 133-144.
MAIER, P., PHILPOT, R.M., MOHN, G.R. & MALLING, H.V. (1979).
Influence of subcellular fractions of mammalian testes on the
mutagenic activity of nitrofurans toward Escherichia coli. Mutat.
Res., 63: 233-243.
MARONPOT, R.R. (1987). Ovarian toxicity and carcinogenicity in eight
recent National Toxicology Program studies. Environ. Health
Perspect., 73: 125-130.
MATSUOKA, A., HAYASHI, M. & ISHIDATE, M.J, (1979). Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutat. Res., 66: 277-290.
McCALLA, D.R. (1979). Nitrofurans : antibiotics. In: Hahn F(Ed),
Mechanism of Action of Antibacterial Agents (Vol IV). Berlin,
Springer Verlag, 176-213.
McCALLA, D.R., OLIVE P.L., TU, Y. & FAN, M. (1975). Nitrofurazone -
reducing enzymes in E. coli and their role in drug activation
in vivo. Canad. J Microbiol., 21: 1484-1491.
McCALLA D.R. & VOUTSINOS, D. (1974). On the mutagenicity of
nitrofurans. Mutat. Res., 26: 3-16.
McCALLA, D.R. (1981). Metabolic activation of nitroheterocyclic
compounds in bacteria and mammalian cells. In: Short-Term Tests for
Chemical Carcinogens, Springer, New York, 36-47.
McCALLA, D.R. (1983). Mutagenicity of nitrofuran derivatives:
Review. Environ. Mutagen., 5: 745-765.
McCALLA, D.R., REUVERS, A. & KAISER, C (1971). "Activation" of
nitrofurans in animal tissues. Biochem. Pharmacol., 20: 3532-3537.
McCALLA, D.R., REUVERS, A. & KAISER, C. (1970). Mode of action of
nitrofurazone. J. Bacteriol., 104: 1126-1134.
MIYAJI, T., MIYAMOTO, M, & UEDA, Y. (1964). Inhibition of
spermatogenesis and atrophy of the testes caused by nitrofuran
compounds. Acta Path. Jap., 14: 261-273.
MIYAJI, T. (1971). Acute and chronic toxicity of furylfuramide in
rats and mice. Tohoku J. Exp. Med., 103: 331-369.
MORI, H., SUGIE, S., YOSHIMI, M., KINOUCHI, T. & OHNISHI, Y. (1987).
Genotoxicity of a variety of nitroarenes and other nitro compounds
in DNA-repair tests with rat and mouse hepatocytes. Mutat. Res.,
190: 159-167.
MORRIS, J.E. (1965). The carcinogenic activity in the rat of some
derivatives of 5-nitrofurn. PhD Thesis, University of Wisconsin,
Madison, USA.
MORRIS, J.E., PRICE, J.M., LALICH, J.J. & STEIN, R.J. (1969). The
carcinogenic activity of some 5-nitrofuran derivatives in the rat.
Cancer Res., 29: 2145-2156.
NI, Y.C., HEFLICH R.H., KADLUBAR, F.F. & FU, P.P. (1987).
Mutagenicity of nitrofurans in Salmonella typhimurium TA98, TA98NR
and TA98/1,8-DNP-6. Mutat. Res., 192: 15-22.
NOMURA, T., KIMURA, S., KANZAKI, T., TANAKA, H., SHIBAK, K.,
NAKAJIMA, H., ISA, Y., KUROKAWA, N., HATANAKA, T., KINUTA, M.,
MASADA, K. & SAKAMOTO, Y. (1984). Induction of tumours and
malformations in mice after prenatal treatment with some antibiotic
drugs. Med. J. Osaka Univ., 35: 13-17.
NOUWS, J.F.M., VREE, T.B., AERTS, M.M.L., DEGEN, M. & DRIESSENS, F.
(1987). Some pharmacokinetic data about furaltadone and
nitrofurazone administered orally to preruminant calves. Vet. Q.,
9: 208-214.
NTP (1988). Toxicology and carcinogenesis studies of nitrofurazone
(CAS No. 59-87-0) in F344/N rats and B6C3F1 mice. Technical
Report Series No. 337. US Department of Health and Human Services,
Public Health Service/National Institutes of Health National
Toxicology Program.
OBASEIKI-EBOR, E.E. & AKERELE, J.O. (1986). Nitrofuran mutagenicity:
Induction of frameshift mutations. Mutat. Res., 175: 149-152.
OLIVE, P.L. & McCALLA, D.R. (1975). Damage to mammalian cell DNA by
nitrofurans. Cancer Res., 35: 781-784.
OLIVE, P.L. & McCALLA, D.R. (1977). Cytotoxicity and DNA damage to
mammalian cells by nitrofurans. Chem.-Biol. Interact., 16:
223-233.
OLIVE, P.L. (1978a) Nitrofurazone-induced DNA damage to tissues of
mice. Chem.-Biol. Interact., 20: 323-331.
OLIVE, P.L. (1978b). Macromolecular, antineoplastic, and
radiosensitization effects of nitrofurans. Carcinogenesis, 4:
131-169.
OLIVE, P.L. (1979). Influence of cellular environment on toxicity of
nitroheterocycles. Chem.-Biol. Interact., 27: 281-290.
OLIVE, P.L. (1980). Mechanisms of the in vitro toxicity of
nitroheterocycles, including flagyl and misonidazole. Section of
Radiobiology, The Johns Hopkins Oncology Center, Baltimore, MD,
Radiation Sensitizers: Their Use in the Clinical Management of
Cancer, 5: 39-45.
OLIVE, P.L. (1981). Correlation between the half-wave reduction
potentials of nitroheterocycles and their mutagenicity in Chinese
hamster V79 spheroids. Mutat. Res., 82: 137-145.
ONG T-M. (1977). Mutagenic activities of nitrofurans in Neurospora
crassa. Mutat. Res., 56: 13-20.
ORPHAHELL, B.V. (1991). Furazolidone-nitrofurazone. Summary volume
1. Unpublished report. Submitted to WHO by Orphahell B.V.,
Mijdrecht, Netherlands.
PAUL, M.F., PAUL, H.E., BENDER, R.C., KOPKO, F., HARRINGTON, C.M.,
ELLS, V.R. & BUZARD, J.A. (1960). Studies on the distribution and
excretion of certain nitrofurans. Antibiotics and
Chemotherap., 10: 287-302.
PAUL, H.E., PAUL, M.F., KOPKO, F. & MAIN, R.J. (1952). Effect of
furacin (5-nitro-2-furaldehyde semicarbazone) on the in vitro
metabolism of mammalian tissues. Proc. Soc. Exp. Biol. Med., 79:
555-558.
PHILLIPS, B.J. (1983). Study of mutation at HGPRT locus in cultured
Chinese hamster ovary cells with nitrofurans. Unpublished report
(466.59.62), Norwich Eaton Pharmaceuticals, Inc. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
POLNASZEK, C.F., PETERSON, F.J., HOLTZMAN, J.L. & MASON, R.P.
(1984). No detectable reaction of the anion radical metabolite of
nitrofurans with reduced glutathione or macromolecules. Chem.-Biol.
Interact., 51: 263-271.
PRICE, C.J., GEORGE, J.D. MARR, M.C. (1985). Teratologic evaluation
of nitrofurazone (CAS No 59-87-0) administered to CD-1 mice on
gestational days 6 though 15. National Toxicology Program,
NTP-85-335, PB86-145844/GAR.
PRICE, C.J., GEORGE, J.D. & MARR, M.C. (1987). Teratologic
evaluation of nitrofurazone (CAS No 59-87-0) administered to New
Zealand white rabbits on gestational days 6 through 19. National
Toxicology Program, NTP-87-200, PB88-130984/GAR.
SIEDLER, A.J. & SEARFOSS, W. (1966). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No. 440.07)
(Special Report), Norwich Pharmacal Company. Submitted to WHO by
Orphahell B.V., Mijdrecht, Netherlands.
SIEDLER, A.J. & SEARFOSS, W. (1967). Effects of long-term feeding of
various nitrofurans to rats. Unpublished report (Problem No.440.07)
(Addendum to Special Report), Norwich Pharmacal Company. Submitted
to WHO by Orphahell B.V., Mijdrecht, Netherlands.
SKEGGS, H.R., BERGLUND, R.M., Van Den HEUVEL W.J.A., MROZIK, H.,
WISLOCKI, P.G. & WOLF, F.J. (1984). Effect of liver enzymes on the
mutagenicity of nitroheterocyclic compounds : activation of 3a, 4,
5, 6, 7, 7a - hexahydro-3-(1-methyl-5-nitro-1H-imidazole-2-yl)-
1,2-benzisoxazole and deactivation of nitrofurans and
nitroimidazoles in the Ames test. Mutat. Res., 136: 1-8.
SMITH, C.G., GRADY, J.E. & NORTHAN, J.I. (1963). Relationship
between cytotoxicity in vitro and whole animal toxicity. Cancer
Chemotherapy Reports, 20: 9-12.
SORRENTINO, D., BODE, W. & HOENER, B-A. (1987). Nitrofurazone
disposition by perfused rat liver. Effect of dose size and
glutathione depletion. Biochem Pharmacol., 36: 915-918.
STITZEL, K.A., McCONNELL, R.F. & DIERCKMANN, T.A. (1989). Effects of
nirofurantoin on the primary and secondary reproductive organs of
female 2B6C3F1 mice. Toxicol. Pathol., 17: 774-781.
SWAMINATHAN, S. & LOWER, G.M. (1978). Biotransformation and
excretion of nitrofurans. Carcinogenesis, 4: 59-97.
TEMPEL, K. (1980). Unscheduled DNA synthesis of rat thymocytes under
the influence of radioprotective and radiosensitizing agents.
Naunyn-Schmiedebergs Arch. Pharmacol., 311: R7.
TONOMURA, A. & SASAKI, M.S. (1973). Chromosome aberrations and DNA
repair synthesis in cultured human cells exposed to nitrofurans.
Japan J. Genetics, 48: 292-294.
YAHAGI, T., MATSUSHIMA, T., NAGAO, M., SEINO, Y., SUGIMURA, T. &
BRYAN, G.T. (1976). Mutagenicities of nitrofuran derivatives on a
bacterial tester strain with a R factor plasmid. Mutat. Res., 40:
9-14.
ZAMPIERI, A. & GREENBERG, J. (1964). Nitrofurazone as a mutagen in
Escherichia coli. Biochem. Biophys. Res Commun., 14: 172-176.
