
DEXAMETHASONE
First draft prepared by
Dr F.X.R. van Leeuwen
Toxicology Advisory Centre
National Institute of Public Health and Environmental Protection
Bilthoven, Netherlands
1. EXPLANATION
Dexamethasone is a potent synthetic analogue of hydro-cortisone
that has a long history of use in veterinary medicine for the
treatment of a range of metabolic diseases and inflammatory
disorders in companion and farm animals. Animal diseases in which
dexamethasone is an effective treatment include inflammation,
acetonaemia, non-specific skin disease, shock and stress. Its use in
animals is primarily therapeutic. It is also used in human medicine
for the treatment of a wide range of diseases. This wide range of
therapeutic use reflects the broad spectrum of pharmacological
actions of the corticosteroid hormones. The corticosteroids have
effects on several important biochemical pathways and cellular
transport mechanisms including, cellular sodium transport, glycogen
synthesis and antiinflammatory responses.
Dexamethasone had not been previously evaluated by the Joint
FAO/WHO Expert Committee on Food Additives.
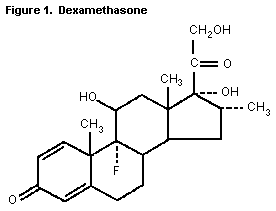
The structure of dexamethasone is shown in Figure 1.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Male Wistar albino rats were administered 0.23 µmol [1,2-3H]
dexamethasone/kg bw, i.p. Urine and faeces were collected up to 4
days after treatment. Within 96 hours 74% of the dose was excreted,
30% in the urine and 44% in the faeces (Rice et al., 1974).
Crl:SD(CD)BR rats were administered a single i.m. dose of 9 µg,
[1,2,4-3H]-dexamethasone/kg bw. Radioactivity was measured up to
96 hours after administration in plasma (pre- and post-freeze
dried), urine, faeces and expired air. Tritium exchange was measured
in stored urine. Highest plasma levels were observed 6 hours after
dosing (3.7 µg equivalents/g), declining rapidly thereafter to 0.15
µg equivalents/g. Within 24 hours 41% of the radioactivity was
excreted in the urine. After 96 hours a mean of 44% of the
radio-activity was excreted. Tritium exchange was observed both in
plasma and urine. Following freeze-drying, the mean loss of
radioactivity 96 hours after dosing was 87% and 37% in plasma and
urine, respectively (Stewart et al., 1992).
Dogs (mixed-breed) were administered dexamethasone alcohol or
dexamethasone 21-isonicotinate as a solution i.v. or i.m. (1 mg/kg
bw), or dexamethasone 21-isonicotinate as a suspension i.m. (0.1 or
1 mg/kg bw). Plasma concentrations were determined with HPLC up to
120 hours after treatment. The elimination half-life after i.v.
administration was 120-140 minutes for both formulations. Following
i.m. administration, absorption was rapid with peak plasma
concentrations at 30-40 minutes for both solutions. Bio-availability
after i.m. administration was 100% for dexamethasone alcohol but 40%
for dexamethasone 21-isonicotinate. After i.m. administration of
dexamethasone 21-isonicotinate as a suspension, dexamethasone was
not detected in plasma, suggesting a long absorption phase (Toutain
et al., 1983).
2.1.2 Biotransformation
2.1.2.1 In vitro
The half-life of dexamethasone 21-isonicotinate was determined
in human, rat, and rabbit sera. In rat and rabbit sera 90 and 99% of
the ester was hydrolyzed after 10 minutes, respectively. The
half-life of in human serum was about 90-100 minutes (Weisenberger,
1972).
Dexamethasone trimethylacetate was rapidly hydrolyzed to
dexamethasone in bovine and equine whole blood; half-lives ranged
from 10-30 minutes for both species (Houghton et al., 1989).
Dexamethasone dimethylbutyrate was hydrolyzed quickly in bovine
plasma, with a half-life of about 1 hour (Coert et al., 1988).
2.1.2.2 Rats
In the urine of rats administered 0.23 µmol/kg bw [1,2-3H]-
dexamethasone i.p., 10% of the administered radioactivity was
associated with one polar metabolite of dexamethasone, likely to be
6-hydroxy-dexamethasone (Rice et al., 1974).
Male Wistar albino rats were administered [3H]-dexamethasone
orally at a dose of 1.14 nmol/kg bw. Thirty-one percent of the
administered radioactivity was excreted in the urine within 4 days
(most of it within the first 24 hours) as unconjugated metabolites.
Unchanged dexamethasone accounted for 14%, 6-hydroxydexamethasone
for 7.4%, and 20-dihydrodexamethasone for 1.1% of the urine
radioactivity. Twenty-five percent of the administered dose was
eliminated in the faeces. Another group of rats was pretreated with
phenytoin 16 hours before dexamethasone treatment. The pretreatment
reduced the rate of total radioactivity excreted in the urine within
24 hours, but the total urinary and faecal excretion after 96 hours
was not significantly reduced (English et al., 1975).
2.1.2.3 Pigs
Four hours after the s.c. administration to pigs of
[1,2-3H]-dexamethasone-21-trimethylacetate, less than 1% of total
plasma radioactivity was extractable as unchanged
[3H]-dexamethasone-21-acetate. The plasma concentration of
dexamethasone was highest (about 3 ng/ml) at 4 hours, declining
rapidly to about 0.5 ng/ml at 24 hours, and slowly thereafter.
Measurable amounts of dexamethasone (>0.2 ng/ml) were still present
at day 5 (Horner, 1989).
2.1.2.4 Humans
No parent compound could be detected in urine of patients after
oral administration of a small dose of dexamethasone (<4 mg/day)
for a few weeks. However, 60% was recovered as
6-ß-hydroxy-dexamethasone and 5-10% as
6-ß-hydroxy-20-dihydrodexamethasone. After the administration of
about 15 mg dexamethasone/day metabolism occurred by an additional
route involving epoxidation and subsequent hydrolysis, resulting in
glycol formation in ring A (Seutter, 1975).
2.1.3 Special studies on macromolecular binding
The binding of dexamethasone to proteins in rat, dog, cow, and
human plasma has been studied in vitro by an equilibrium dialysis
technique. Approximately 85, 73, 74, and 77% was bound in rat, dog,
cow, and human plasma, respectively. Dexamethasone was mainly bound
to the albumin fraction of human plasma (Peets et al., 1969).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of an acute toxicity study is summarized in Table
1.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 15 rats were given daily s.c. injections of 0.5 ml
vehicle or 50 µg/kg bw dexamethasone for 6 weeks. Body- weight gain
was significantly decreased in treated rats. Adrenal weights were
also decreased. Post-mortem examination revealed no pathological
organ changes. Only a summary of this study was available
(Ueberberg, 1964).
In 3 experiments, groups of 15 rats/sex were given 0.125 mg/kg
bw (6 days/week), 0.25 mg/kg bw (5 days/week), or 0.4 mg/kg bw (5
days/week) dexamethasone in tablets for 181-185 days. The control
groups (10 rats/sex/group) received placebo tablets. All mid- and
high-dose animals received 20 mg tetracycline*HCl once a week
beginning on the 39th experimental day.
Dose-related deaths occurred as follows: 4/30 at the low-dose,
14/30 at the mid-dose and 26/30 at the top-dose. Post mortem
examination in all cases showed severe infections. In all dose
groups, body-weight gain was decreased, relative kidney weight was
increased and relative adrenal and thymus weights were decreased. In
the bone marrow the number of neutrophilic forms of leucocytes was
increased and the number of eosinophils was decreased in all
experimental groups as compared to controls. Detailed
histopathological findings of the mid- and high-dose groups were not
presented (Intervet, undated I).
Groups of 20 male and 20 female Wistar rats were administered
s.c. 0, 40, or 79 µg/kg bw/day dexamethasone for 13 weeks. An
additional group of 5 male and 5 female rats was given 79 µg/kg
bw/day dexamethasone s.c. for 13 weeks, then kept for a 7-week
recovery period. Haematological and biochemical examinations were
performed on 5 rats/sex after 10 weeks.
Table 1. Acute toxicity of dexamethasone
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M i.p. 577 Engelhardt, 1963
In all treated males, ALAT activity and total cholesterol
concentrations were increased. Lipid levels were only incidentally
raised. Plasma corticosteroid levels and hepatic glycogen were
decreased in a dose-related manner. In treated males, adrenal
glycogen levels were increased and in males as well as in females
dose-related reductions in adrenal corticosteroids were observed.
Post mortem examination found adrenal and thymus glands that were
markedly smaller with reduced weights when compared to controls. In
some animals no thymus tissue could be found. Compared to the
controls, body weights and most organ weights of treated rats were
lower. Microscopic examination revealed marked changes in the thymus
and the adrenal glands. The adrenal cortex was narrowed due to loss
of the regular structuring of the cells or cell columns, in addition
to a reduction in lipids. The thymus from the treated rats showed
atrophy of the medullary and cortical tissues. After the recovery
period no significant changes compared to the controls were observed
(Bauer et al., 1969a; results of male rats partly reported in
Segro, 1970).
2.2.2.2 Dogs
In a limited study, groups of 4 female beagle dogs were orally
administered a 2 or 8 mg dexamethasone tablet/day (6 days/week) for
26 weeks. No control group was used. Instead, values from previous
studies were used for comparison. One low-dose dog died (not related
to treatment) during the study and 3 high-dose dogs died, of which 2
were due to retro-oesophageal abcesses or gastric ulcers. At
post-mortem examination all remaining dogs were found to have
infections. Alopecia was seen in 1 dog in each dose group. Atrophy
of the lymphatic organs was seen in all dogs, adrenal weight was
decreased, and in the high-dose group the thymus had almost
disappeared (Intervet, undated II).
Groups of mongrel dogs (3 male and 2 female dogs/group) were
orally administered placebo tablets or 125 µg/kg bw/day
dexamethasone 7 days/week for 6 weeks. No treatment-related effects
were observed on clinical signs, body-weight, liver and renal
function tests, urinalysis, or post mortem examination. After 6
weeks blood glucose values were increased in treated animals.
Relative adrenal weights were decreased. In the adrenals, the zona
fasciculata was narrowed in treated dogs. Lipid observed in the
adrenal cortical zones of the controls was not present in treated
dogs. Total 17-ketosteroid excretion in the urine was higher in all
treated groups (Ueberberg, 1963).
Groups of 3 male and 3 female beagle dogs were administered
daily i.m. doses of 0, 40, or 79 µg/kg bw dexamethasone for 13
weeks. Additionally, 2 groups of 3 male and 3 female dogs were given
daily i.m. doses of 79 µg/kg bw dexamethasone for 13 weeks, then
kept for a 4-week recovery period. One female dog from the recovery
group died 14 days after cessation of dosing. No effects were
observed on food consumption or haematological parameters. Decreased
body-weight gain was observed in all treated dogs. ALAT activity was
increased in 1 out of 3 female dogs in both the 40 µg/kg bw/day and
79 µg/kg bw/day groups. Activity returned to normal after the
recovery period. Total lipid levels in serum were increased in all
treated dogs without any dose-dependence. By the end of the recovery
period total lipid levels had decreased, but were still elevated
relative to control levels in all treated dogs. Plasma corticoid
levels were decreased in dogs receiving 79 µg/kg bw/day
dexamethasone but returned to normal by the end of the recovery
period. Increased triglyceride levels in the adrenals and increased
liver glycogen were observed in all treated dogs (without
dose-relationship). The increased adrenal triglyceride levels was
still apparent after the recovery period. Liver weights were
increased in a dose-related manner and honey comb distention was
seen in some hepatic cells. These effects reversed after treatment
was ceased. In all treated dogs, the adrenal weights were lower than
in the controls. Histological examination revealed diminution of the
fascicular and reticular zones of the adrenal cortex without a clear
demarcation between the different zones. After the recovery period
the adrenal weights returned to normal. No thymus or only remnants
of the thymus were found in some treated dogs. When the thymus was
found in recovery animals, it did not differ histologically from the
controls (Bauer et al., 1969b).
2.2.3 Long-term toxicity/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
In a special study 0.15 mg dexamethasone/day (equal to 6 mg/kg
bw/day) was administered s.c. to pregnant A/J mice on days 11-14
post-conception. The mice were killed on day 18. The incidence of
cleft palate was 93% (Walker, 1971).
2.2.5.2 Rats
Groups of 20 pregnant SPF-FW 49 Biberach rats were administered
dexamathasone s.c. at dose levels of 0, 20, 40 or 79 µg/kg bw/day on
days 6-15 of gestation. Females were killed on gestation day 21. One
female died intercurrently, the cause of death could not be
resolved. All treated females failed to gain weight and had lower
food consumption during treatment, but they gained weight after
cessation of treatment. Total food consumption, however, remained
lower in all treated animals compared to controls. The mean number
of implantations was higher in all treated groups than in the
control group. Resorption rates were higher in a dose-related manner
and the number of live offspring was lower in the two highest dose
groups. Litter weight was decreased in a dose-related manner. Both
the variation and malformation rates were increased, but with no
apparent dose-relationship. Retarded ossification of the sternebrae
and hydronephrosis occurred most frequently (Lehmann, 1969a).
Daily doses of 0.05, 0.2, or 0.8 mg dexamethasone/day (equal to
250, 1000 or 4000 µg/kg bw/day) were administered s.c. to pregnant
Holtzmann rats on days 12-15 post-conception. The dams were killed
at day 19. A high frequency of cleft palate (53%) was observed in
litters of rats in the highest dose group. No cleft palates were
observed at lower doses (Walker, 1971).
Groups of 20 pregnant rats (Morini Wistar) were given daily
s.c. injections of 0, 40, or 79 µg dexamethasone/kg bw/day on days
6-15 of gestation. Maternal body-weight gain and food consumption
were decreased in all treated dams. Compared to the controls, the
resorption rate was increased and fetal weights were decreased.
Hydronephrosis was seen in 4 fetuses, 2 at 40 and 2 at 79 µg/kg
bw/day (Segro, 1970).
In a poorly reported study, groups of 10 pregnant rats (SPF,
strain SD-JCL) were given daily s.c. doses of 0, 20, 40, or 80 µg
dexamethasone/kg bw/day on days 6-15 of gestation. Mortality and
body weights were recorded. The dams were killed on day 21 of
pregnancy and the fetuses were delivered by caesarean section.
Maternal body weight was decreased in treated animals. In all
treated rats pre- and post-implantation losses were increased. No
effects were observed on fetal weight. Cleft palate was seen in one
fetus of the control group. One fetus in the 20 µg/kg bw/day dose
group had thoracoschisis. The occurrence of 14th rib was observed in
the control group (25%) as well as in all dose groups. There was no
marked difference relative to controls, but a slight dose-related
effect was observed (incidences ranged from 20 to 28.4% in the
treated groups). Deformity of the sternum was observed in 1/85 rats
in the 20 µg/kg bw/day dose group (Umemura et al., 1972).
In a pilot study groups of Virgin Lati:Han Wistar pregnant rats
(10/group) were orally administered (by gavage) doses of 0, 10, 50,
250, or 1250 µg dexamethasone/kg bw/day on days 7 to 16 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 21 and fetuses were delivered
by caesarean section. Observations included the number of corpora
lutea, implantations, fetus and placenta weight, and sex of viable
fetuses. All fetuses were examined for skeletal and visceral
abnormalities.
Maternal body-weight gain was decreased at doses of 50 µg/kg
bw/day and above and thymus involution was observed at the same dose
levels. In the highest dose group, post-implantation mortality was
increased. Most fetuses that died within the last 24 hours were
malformed. Fetal weight was decreased at doses of 250 and 1250 µg/kg
bw/day. In the 2 highest dose groups retrognathia and cleft palate
of variable severity were observed. Hydrops fetalis and umbilical
hernia was found only at 1250 µg/kg bw/day. Thymus hypoplasia was
observed at 50, 250, and 1250 µg/kg bw/day (4, 2, and 59%,
respectively). The NOEL in this study was 10 µg/kg bw/day (Druga,
1993a).
In the main Segment II study, groups of Virgan Lati:Han Wistar
rats were orally administered (by gavage) 0, 20, 200, or 1000 µg
dexamethasone in methylcellulose/kg bw/day on days 6 to 15 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 20. The visera of the dams
were examined grossly and the thymus was removed and weighed.
Observations included the number of corpora lutea, implantations,
length of umbilical cord, fetus and placenta weight, and sex of
viable fetuses. All fetuses received external, skeletal, and
visceral examinations.
Maternal body weight and body-weight gain and food consumption
were decreased at 200 and 1000 µg/kg bw/day. Thymus involution was
observed at the same dose levels. Post-implantation mortality was
increased at the highest dose. Fetal weight was decreased at 200 and
1000 µg/kg bw/day. Umbilical cord length was reduced at 200 and 1000
µg/kg bw/day and the length, thicknesss, and index of the femur were
markedly lower at 1000 µg/kg bw/day. High-dose fetuses showed an
increased incidence of malformations, including hydrops fetalis,
retrognathia, cleft palate, umbilical hernia of variable severity,
split sternum, malformed vertebrae, malformed upper limb bones, and
micromelia. Thymus hypoplasia was observed at 20, 200, and 1000
µg/kg bw/day (4, 2, and 16%, respectively). In the high-dose group,
dystrophy of gonads was also observed. A no-effect level was not
observed in this study (Druga, 1993b).
2.2.5.3 Rabbits
Dexamethasone, as one glucocorticoid in a series of 6, was
administered i.m. at dose levels ranging from 0.1 to 4 mg/day (equal
to 25 to 1000 µg/kg bw/day) to pregnant rabbits on days 13.5-16.5
post-conception. Litter resorptions were observed at 750 and 1000 µg
dexamethasone/kg bw/day. Cleft palate was observed at >62 µg/kg
bw/day. No effects on resorption or cleft palate were observed at 25
µg/kg bw/day (Walker, 1967).
In a comparative study, groups of 15 pregnant rabbits ("SPF
Himalyan"/Biberach) were administered s.c. 0, 20, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. The dams were
killed on day 29 of pregnancy. During dosing maternal body weight
remained stationary or was reduced, particularly in the second half
of the dosing period. A dose-related increase in resorption rate and
number of runts was observed. A dose-related decrease in fetal
weight was also observed. The incidence of flexure of the forefeet
and of malformations (palatoschisis, gastroschisis, exencephaly,
encephalocele and menigocele, anotia, and ectrodactyly) was
increased in a dose-related manner in all treatment groups.
Malformations of the extremities such as haemibrachia, hypoplasia of
tibia and fibula, and acheiria were observed (Lehmann, 1969b).
In a similar study, groups of 15 pregnant New Zealand white
rabbits were given daily s.c. injections of 0, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. Compared to the
control group all treated dams had reduced body weight and their
resorption rates were increased. No treatment-related effect on
gross malformations was observed (Segro, 1970).
2.2.6 Special studies on endocrine toxicity
Groups of Cpb:WU rats (10/sex/group) were orally administered
(by gavage) doses of 0, 0.3, 1, 3, 10, 30, or 100 µg dexamethasone/
kg bw/day for 90 days. Observations included clinical signs, body
weight, water consumption, haematology, IgG/IgM determinations,
gross post mortem examination, adrenal and thymus weight,
histopathology, ACTH stimulation test (4 rats/sex/group), and
corticosteroid determinations.
Significant decreases in body-weight gain (more pronounced in
males than in females) were observed at doses of >10 µg/kg
bw/day. In males of the same groups sluggishness and erected fur
were observed, which were considered to be treatment-related. WBC
and differential WBC counts were significantly decreased in rats at
>10 µg/kg bw/day. WBC counts were also significantly reduced in
females at 3 µg/kg bw/day. IgG and IgM levels were significantly
decreased at 100 µg/kg bw/day. Decreases in adrenal and thymus
weights were observed and accompanied by histopathological changes,
including atrophy and structural disorganization, at >10 µg/kg
bw/day. In the same dose groups dose-related decreases in
corticosterone levels with or without ACTH stimulation were
observed. The Committee concluded that the NOEL in this study was at
1 µg/kg bw/day (De Jong & Coert, 1987).
Rats (6/group) were administered a dose of 0, 0.5, 1, 1.5, 2,
or 4 µg dexamethasone/kg bw/day by gavage for 1 and 7 days. Five
hours after the last application the rats were killed and blood and
liver samples were taken. Tyrosine amino transferase (TAT) activity
in the supernatant of liver homogenates was measured and serum
corticosterone concentrations were determined. TAT activity was
increased in a dose-dependent manner at 2 and 4 µg/kg bw/day and a
significant decrease in serum corticosterone was seen at 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day (Kietzman, 1991;
Bette & Kietzmann, 1991).
2.2.7 Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
2.2.8 Special studies on immune response
The influence of dexamethasone-21-isonicotinate on the course
of experimental bacterial infections of mice and their therapy, on
the phagocytotic activity of the reticulo-endothelial system and the
serum protein picture was investigated. No clear effect on
antibiotic therapy was observed. Dexamethasone-21-isonicotinate
decreased the activity of Celasin C and penicillin G-procain against
streptococcal infections and improved the activity of both
substances against staphylococcal infections. No quantitative effect
was observed upon serum albumin, alpha1, alpha2, ß1, ß2 or
gamma-globulins of mice both 48 hours after a single s.c. treatment
or 24 hours after 5 days of treatment (once a day s.c.) with 75,
150, or 300 µg dexamethasone 21-isonicotinate/kg bw (Goeth &
Lechner, 1978).
2.3 Observations in humans
The corticosteroid suppressive effect of dexamethasone is well
known and used for the definitive diagnosis of Cushing syndrome in
human patients. People with Cushing syndrome suffer from a chronic
over-production of cortisol resulting from an over-production of
ACTH. In the "low- and high-dose dexamethasone suppression test" the
subjects receive 0.5 or 2 mg dexamethasone orally every 6 hours for
Table 2: Results of genotoxicity assays on dexamethasone and
dexamethasone-21-isonicotinate
Test system Test object Concentration Purity Results Reference
In vitro
Ames testa S.typhimurium 10-1000 99% negativec Baumeister,
TA98, 100, µg/pl 1988a
1535, 1537
E. coli , WP2
Fluctuation Mouse 12.5-400 99.4% negativee Clements,
assayb,d lymphoma µg/ml 1992
L5178Y cells
In vivo
Micronucleus NMRI mice i.v. 5 mg/kgf 97.5% negativeg Baumeister,
testsa 1988b
a Test substance: dexamethasone 21-isonicotinate.
b With and without metabolic activation.
c Appropriate positive controls were used.
d Test substance: dexamethasone.
e 4-Nitroquinoline and benzo(a)pyrene were used as positive controls.
f The initial dosage (107.5 mg/kg) had to be reduced due to acute
toxicity (tonic convulsions, deaths) of the excipient 1,
2-propylenglycol.
g Cyclophosphamide was used as positive control.
2 consecutive days. In normal healthy persons cortisol production,
determined as 17-hydroxycorticosteroid or 17-ketosteroid excretion
in urine, is suppressed, whereas in patients with Cushing syndrome
cortisol production is not suppressed. No significant clinical side
effects of this dexamethasone suppression test were reported (Crapo,
1979).
3. COMMENTS
Information from studies on dexamethasone, including data on
kinetics, metabolism, acute and short-term toxicity, developmental
toxicity, and genotoxicity, was available for assessment.
Toxicokinetic studies revealed rapid absorption after i.m.
administration to dogs and rats with peak plasma levels found after
30 minutes and 6 hours, respectively. Dexamethasone is rapidly
excreted in urine and faeces. Dexamethasone esters are rapidly
hydrolyzed in serum. Biotransformation in rats and humans is
comparable and involves mainly hydroxylation to 6-hydroxy- and
20-dihydro-dexamethasone. However, there was additional evidence
that at high (therapeutic) doses in people, dexamethasone is
metabolized by an additional route involving epoxidation.
Following repeated oral administration of dexamethasone to rats
and dogs in short-term toxicity studies the main target organs were
the thymus and the adrenal gland. Corticosteroid concentrations in
plasma and hepatic glycogen were reduced, whereas serum lipid levels
were increased. In rats dosed orally with 0.3, 1, 3, 10, 30, or 100
µg dexamethasone/kg bw/day for 90 days, thymus involution and
morphological changes in the adrenal gland and a decrease in
corticosterone and white blood cell counts were observed in male and
female rats at doses above 10 µg/kg bw/day. Due to the decrease in
white blood cell counts in female rats at 3 µg/kg bw/day this dose
was considered to be a marginal effect level. In a study with rats
orally dosed with 0.5, 1, 1.5, 2, or 4 µg/kg bw/day dexamethasone
for 7 days the corticosterone concentration was reduced in the
highest-dose group and the activity of tyrosine aminotransferase in
the liver was increased in a dose-related manner at 2 and 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day.
No reproduction studies with dexamethasone were available but
an increase in pre- and post-implantation loss and a reduction in
fetal weight were observed in teratogenicity studies in mice, rats,
and rabbits receiving dexamethasone by injection. In these studies
malformations such as hydrops fetalis, cleft palate, exencephaly,
and encephalocele were observed at maternally toxic dose levels.
In oral teratogenicity studies with rats using dose levels
ranging from 10 to 1250/kg bw/day, maternal toxicity was found at 50
µg/kg bw/day and above. At doses at and above 1000 µg/kg bw/day,
dexamethasone caused structural malformations (hydrops fetalis,
cleft palate). Thymus involution and a decrease in body weight were
observed in fetuses, resulting in an overall NOEL for embryotoxicity
in rats of 10 µg/kg bw/day.
Long-term toxicity/carcinogenicity data were not available.
4. EVALUATION
Based on its long history of use in human medicine and because
dexamethasone was negative in in vitro gene mutation assays with
bacteria and mammalian cells and in an in vivo micronucleus test
with mice, the Committee was not concerned about the carcinogenic
potential of dexamethasone.
Using a safety factor of 100 the Committee established an ADI
of 0-0.015 µg/kg bw/day for dexamethasone based on a NOEL of 1.5
µg/kg bw/day for the induction of tyrosine aminotransferase activity
in rat liver. Due to the careful selection of the dose levels in
this study the Committee did not round this figure.
5. REFERENCES
BAUER, O., LEHMANN, & UEBERBERG, H. (1969a) Rat study to screen
pyridine-4-carboxylic acid-(dexamethasone-21')-ester (HE 111) for
subacute toxicity in comparison with dexamethasone. Unpublished
report d.d. 12 June 1969 from Dr. K. Thomas GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUER, O., PAPPRITZ, & UEBERBERG, H. (1969b) Investigation into the
subacute toxicity of pyridine-4-carboxylic acid (dexamethasibe-21')
ester (HE 111) in comparison with dexamethasone in dogs. Unpublished
report No. AX-U-30 d.d.21-4-1969 from Dr. Karl Thomas, GmbH,
Biberach, Germany. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988a) Mutagenicity study with HE III XX (Voren) in
the S. typhimurium and E. coli/mammalian microsome assay (Ames
Test). Unpublished report no. Gen Tox. 39/87 d.d. 5 February 1988
from Dr. K. Thomas, GmbH, Dept. of Experimental Pathology and
Toxicology, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988b) Mutagenicity study with HE III XX (Voren) in
the bone marrow micronucleus assay. Unpublished report no. Gen Tox.
40/87 d.d. 8 February 1988 from Dr. K. Thomas, GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BETTE, P. & KIETZMANN, M. (1991) Effect of dexamethasone on tyrosine
amino-transferase activity in rat liver - a sensitive test to define
its hormonal no-effect-level. Acta Vet. Scand., 87: 200-202.
CRAPO, L. (1979) Cushings syndrome: A review of diagnostic tests.
Metabolism , 28: 955-977.
CLEMENTS, J. (1992) Study to determine the ability of dexamethasone
to induce mutations at the thymidine kinase (tk) locus in mouse
lymphoma L5178Y cells using a fluctuation assay. Unpublished report
no. 2TKREBSG.001 from Hazleton Microtest Limited, York, England.
Submitted to WHO by Intervet International B.V., Boxmeer, The
Netherlands.
COERT, A., HOEIJMAKERS, M., VAN RENS, P. (1988) The in vitro
hydrolysis of dexamethasone dimethylbutyrate in cows plasma.
Unpublished subject report no. 10 d.d. 3-2-1988 from Intervet
Boxmeer. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
De JONG, H. & COERT, A. (1987) The determination of the hormonal
no-effect-level of dexamethasone in rats after 90 days of oral
dosing (NEL study). Unpublished report d.d. 28-8-1987 from Intervet
Boxmeer. Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
DRUGA. A. (1993a) Pilot teratology study (Segment II) of
dexamethasone in rats. Unpublished report no. 9218 by Institute for
Drug Research, Budapest, Hungary. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim.
DRUGA, A. (1993b) Teratology study (segment II) of dexamethsone in
rats. Unpublished report study nr. 9219 by Institute for Drug
Research, Budapest, Hungary. Submitted to WHO by Intervet
International B.V., Boxmeer, The Netherlands and Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGELHARDT, G. (1963). Pharmacological exposé on substance HE III.
Unpublished report no.: AX-U-26 d.d. 17 January 1963 from Dr. K.
Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGLISH, J., CHAKRABORTY, J. & MARKS, V. (1975) The metabolism of
dexamethasone in the rat - effect of phenytoin. J. Steroid
Biochem., 6: 65-68.
GOETH, H. & LECHNER, V. (1978) Investigation on the influence of
Voren on the course of experimental infections and their treatment
with antibiotics and sulphonamides as well as on the phagocytotic
activity of the reticulo-endothelial system (RES) and the serum
protein picture in mice. Berl. Munch. Wschr., 91: 87-93.
HORNER, M.W. (1989) Pharmacokinetics of dexamethasone in pig plasma
following subcutaneous injection of Opticortenol S. Unpublished
report d.d. November 1989, Ciba Geigy Project no. 4. from
Horseracing Forensic Labortory, Newmarket. Submitted to WHO by Ciba
Geigy, Division Agro, Basle, Switzerland.
HOUGHTON, E., GRAINGER, L. & TEALE, P. (1989) The in vitro
hydrolysis of dexamethasone trimethylacetate in whole blood from the
horse and cow. Unpublished report from Horseracing Forensic
Laboratory, Newmarket, July 1989. Submitted to WHO by Ciba Geigy,
Division Agro, Basle, Switzerland.
INTERVET, (undated I) Chronic oral toxicity study with dexamethasone
in rats. Unpublished and unsigned report from Intervet d.d. mid
1960's? Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
INTERVET, (undated II) Chronic oral toxicity study with
dexamethasone in dogs. Unpublished and unsigned report from Intervet
d.d. mid-1960's? Submitted to WHO by Intervet International B.V.,
Boxmeer. The Netherlands.
KIETZMAN, M. (1991) Report of a study in rats to investigate the
effect of dexamethasone on tyrosine aminotransferase activity in the
liver and on serum corticosterone levels. Veterinary College,
Hannover (27-1-91). Unpublished report submitted to WHO by
Boehringer Ibgelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969a) Reproduction study comparing the compound HEIII
with dexamethasone in gestating rats. Unpublished report no. AUXI
0031 from Dr Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969b) Reproduction study on the substance HEIII versus
dexamethasone in pregnant rabbits. Unpublished report d.d.
24-11-1969 no. U69-0018 from Dr Karl Thomas, GmbH, Biberach,
Germany. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
PEETS, E.A., STAM, M. & SYMCHOWICZ, C. (1969) Plasma binding of
betamethasone-3H, dexamethasone-3H, and cortisol-14C - a
comparative study. Biochem. Pharmacol., 18: 1655-1663.
RICE, M.J. TREDGER, J.M., CHAKRABORTY, J. & PARKE, D.V. (1974) The
metabolism of dexamethasone in the rat. Biochem. Soc. Transact.,
53rd Meeting, Bristol, 2: 107-109.
SEGRO, G. (1970) Expertise on HE111 in rats and rabbits. Report d.d
1-4-1970 from University of Sienna, Italy. Unpublished report no.
AX-U43 from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
SEUTTER, E. (1975) Metabolism of systematically given
corticosteroids. Dermatologica , 151: 129-134.
STEWART, F.P., KÖRÖSI, S.A. & HOPKINS, R. (1992)
[1,2,4-3H]-dexamethasone: tritium exchange following intramuscular
administration to the rat. Unpublished report 6938-806/2 d.d.January
1992 from Hazleton UK Limited, Harrogate, England. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
TOUTAIN, P.L., ALVINERIE, M. & RUCKEBUSCH, Y. (1983)
Pharmacokinetics of dexamethasone and its effect on adrenal gland
function in the dog. Am. J. Vet. Res., 44: 212-217.
UEBERBERG, H. (1963) Comparative experimental trials in dogs with
pyridine- 4-carboxylic acid (dexamethasone-21')ester (HEIII) and
dexamethasone. Unpublished report no. AX-U-3 d.d.20-12-1963 from Dr
Karl Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UEBERBERG, H. (1964) Comparative investigations in rats with
substance HEIII and dexamethasone. Unpublished report d.d. 23-3-1964
from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UMEMURA, M., KAST, A. & IIDA, H. (1972) Teratologica; testing of
substances dexamethasone and HEIII in rats. Unpublished report d.d.
3-3-1972 from Boehringer Ingelheim GmbH Pharma Research Japan.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
International Division, Ingelheim, Germany.
WALKER, B.E. (1967) Induction of cleft palate in rabbits by several
glucocorticoids. Proc. Soc. Exp. Biol. Med., 125: 1281-1284.
WALKER, B.E. (1971) Induction of cleft palate in rats with
anti-inflammatory drugs. Teratology , 4: 39-42.
WEISENBERGER, H. (1972) Species differences in the hydrolysis of
dexamethasone 21-isonicotinate by serum esterases. Klin. Wschr.,
50: 665.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Male Wistar albino rats were administered 0.23 µmol [1,2-3H]
dexamethasone/kg bw, i.p. Urine and faeces were collected up to 4
days after treatment. Within 96 hours 74% of the dose was excreted,
30% in the urine and 44% in the faeces (Rice et al., 1974).
Crl:SD(CD)BR rats were administered a single i.m. dose of 9 µg,
[1,2,4-3H]-dexamethasone/kg bw. Radioactivity was measured up to
96 hours after administration in plasma (pre- and post-freeze
dried), urine, faeces and expired air. Tritium exchange was measured
in stored urine. Highest plasma levels were observed 6 hours after
dosing (3.7 µg equivalents/g), declining rapidly thereafter to 0.15
µg equivalents/g. Within 24 hours 41% of the radioactivity was
excreted in the urine. After 96 hours a mean of 44% of the
radio-activity was excreted. Tritium exchange was observed both in
plasma and urine. Following freeze-drying, the mean loss of
radioactivity 96 hours after dosing was 87% and 37% in plasma and
urine, respectively (Stewart et al., 1992).
Dogs (mixed-breed) were administered dexamethasone alcohol or
dexamethasone 21-isonicotinate as a solution i.v. or i.m. (1 mg/kg
bw), or dexamethasone 21-isonicotinate as a suspension i.m. (0.1 or
1 mg/kg bw). Plasma concentrations were determined with HPLC up to
120 hours after treatment. The elimination half-life after i.v.
administration was 120-140 minutes for both formulations. Following
i.m. administration, absorption was rapid with peak plasma
concentrations at 30-40 minutes for both solutions. Bio-availability
after i.m. administration was 100% for dexamethasone alcohol but 40%
for dexamethasone 21-isonicotinate. After i.m. administration of
dexamethasone 21-isonicotinate as a suspension, dexamethasone was
not detected in plasma, suggesting a long absorption phase (Toutain
et al., 1983).
2.1.2 Biotransformation
2.1.2.1 In vitro
The half-life of dexamethasone 21-isonicotinate was determined
in human, rat, and rabbit sera. In rat and rabbit sera 90 and 99% of
the ester was hydrolyzed after 10 minutes, respectively. The
half-life of in human serum was about 90-100 minutes (Weisenberger,
1972).
Dexamethasone trimethylacetate was rapidly hydrolyzed to
dexamethasone in bovine and equine whole blood; half-lives ranged
from 10-30 minutes for both species (Houghton et al., 1989).
Dexamethasone dimethylbutyrate was hydrolyzed quickly in bovine
plasma, with a half-life of about 1 hour (Coert et al., 1988).
2.1.2.2 Rats
In the urine of rats administered 0.23 µmol/kg bw [1,2-3H]-
dexamethasone i.p., 10% of the administered radioactivity was
associated with one polar metabolite of dexamethasone, likely to be
6-hydroxy-dexamethasone (Rice et al., 1974).
Male Wistar albino rats were administered [3H]-dexamethasone
orally at a dose of 1.14 nmol/kg bw. Thirty-one percent of the
administered radioactivity was excreted in the urine within 4 days
(most of it within the first 24 hours) as unconjugated metabolites.
Unchanged dexamethasone accounted for 14%, 6-hydroxydexamethasone
for 7.4%, and 20-dihydrodexamethasone for 1.1% of the urine
radioactivity. Twenty-five percent of the administered dose was
eliminated in the faeces. Another group of rats was pretreated with
phenytoin 16 hours before dexamethasone treatment. The pretreatment
reduced the rate of total radioactivity excreted in the urine within
24 hours, but the total urinary and faecal excretion after 96 hours
was not significantly reduced (English et al., 1975).
2.1.2.3 Pigs
Four hours after the s.c. administration to pigs of
[1,2-3H]-dexamethasone-21-trimethylacetate, less than 1% of total
plasma radioactivity was extractable as unchanged
[3H]-dexamethasone-21-acetate. The plasma concentration of
dexamethasone was highest (about 3 ng/ml) at 4 hours, declining
rapidly to about 0.5 ng/ml at 24 hours, and slowly thereafter.
Measurable amounts of dexamethasone (>0.2 ng/ml) were still present
at day 5 (Horner, 1989).
2.1.2.4 Humans
No parent compound could be detected in urine of patients after
oral administration of a small dose of dexamethasone (<4 mg/day)
for a few weeks. However, 60% was recovered as
6-ß-hydroxy-dexamethasone and 5-10% as
6-ß-hydroxy-20-dihydrodexamethasone. After the administration of
about 15 mg dexamethasone/day metabolism occurred by an additional
route involving epoxidation and subsequent hydrolysis, resulting in
glycol formation in ring A (Seutter, 1975).
2.1.3 Special studies on macromolecular binding
The binding of dexamethasone to proteins in rat, dog, cow, and
human plasma has been studied in vitro by an equilibrium dialysis
technique. Approximately 85, 73, 74, and 77% was bound in rat, dog,
cow, and human plasma, respectively. Dexamethasone was mainly bound
to the albumin fraction of human plasma (Peets et al., 1969).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of an acute toxicity study is summarized in Table
1.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 15 rats were given daily s.c. injections of 0.5 ml
vehicle or 50 µg/kg bw dexamethasone for 6 weeks. Body- weight gain
was significantly decreased in treated rats. Adrenal weights were
also decreased. Post-mortem examination revealed no pathological
organ changes. Only a summary of this study was available
(Ueberberg, 1964).
In 3 experiments, groups of 15 rats/sex were given 0.125 mg/kg
bw (6 days/week), 0.25 mg/kg bw (5 days/week), or 0.4 mg/kg bw (5
days/week) dexamethasone in tablets for 181-185 days. The control
groups (10 rats/sex/group) received placebo tablets. All mid- and
high-dose animals received 20 mg tetracycline*HCl once a week
beginning on the 39th experimental day.
Dose-related deaths occurred as follows: 4/30 at the low-dose,
14/30 at the mid-dose and 26/30 at the top-dose. Post mortem
examination in all cases showed severe infections. In all dose
groups, body-weight gain was decreased, relative kidney weight was
increased and relative adrenal and thymus weights were decreased. In
the bone marrow the number of neutrophilic forms of leucocytes was
increased and the number of eosinophils was decreased in all
experimental groups as compared to controls. Detailed
histopathological findings of the mid- and high-dose groups were not
presented (Intervet, undated I).
Groups of 20 male and 20 female Wistar rats were administered
s.c. 0, 40, or 79 µg/kg bw/day dexamethasone for 13 weeks. An
additional group of 5 male and 5 female rats was given 79 µg/kg
bw/day dexamethasone s.c. for 13 weeks, then kept for a 7-week
recovery period. Haematological and biochemical examinations were
performed on 5 rats/sex after 10 weeks.
Table 1. Acute toxicity of dexamethasone
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M i.p. 577 Engelhardt, 1963
In all treated males, ALAT activity and total cholesterol
concentrations were increased. Lipid levels were only incidentally
raised. Plasma corticosteroid levels and hepatic glycogen were
decreased in a dose-related manner. In treated males, adrenal
glycogen levels were increased and in males as well as in females
dose-related reductions in adrenal corticosteroids were observed.
Post mortem examination found adrenal and thymus glands that were
markedly smaller with reduced weights when compared to controls. In
some animals no thymus tissue could be found. Compared to the
controls, body weights and most organ weights of treated rats were
lower. Microscopic examination revealed marked changes in the thymus
and the adrenal glands. The adrenal cortex was narrowed due to loss
of the regular structuring of the cells or cell columns, in addition
to a reduction in lipids. The thymus from the treated rats showed
atrophy of the medullary and cortical tissues. After the recovery
period no significant changes compared to the controls were observed
(Bauer et al., 1969a; results of male rats partly reported in
Segro, 1970).
2.2.2.2 Dogs
In a limited study, groups of 4 female beagle dogs were orally
administered a 2 or 8 mg dexamethasone tablet/day (6 days/week) for
26 weeks. No control group was used. Instead, values from previous
studies were used for comparison. One low-dose dog died (not related
to treatment) during the study and 3 high-dose dogs died, of which 2
were due to retro-oesophageal abcesses or gastric ulcers. At
post-mortem examination all remaining dogs were found to have
infections. Alopecia was seen in 1 dog in each dose group. Atrophy
of the lymphatic organs was seen in all dogs, adrenal weight was
decreased, and in the high-dose group the thymus had almost
disappeared (Intervet, undated II).
Groups of mongrel dogs (3 male and 2 female dogs/group) were
orally administered placebo tablets or 125 µg/kg bw/day
dexamethasone 7 days/week for 6 weeks. No treatment-related effects
were observed on clinical signs, body-weight, liver and renal
function tests, urinalysis, or post mortem examination. After 6
weeks blood glucose values were increased in treated animals.
Relative adrenal weights were decreased. In the adrenals, the zona
fasciculata was narrowed in treated dogs. Lipid observed in the
adrenal cortical zones of the controls was not present in treated
dogs. Total 17-ketosteroid excretion in the urine was higher in all
treated groups (Ueberberg, 1963).
Groups of 3 male and 3 female beagle dogs were administered
daily i.m. doses of 0, 40, or 79 µg/kg bw dexamethasone for 13
weeks. Additionally, 2 groups of 3 male and 3 female dogs were given
daily i.m. doses of 79 µg/kg bw dexamethasone for 13 weeks, then
kept for a 4-week recovery period. One female dog from the recovery
group died 14 days after cessation of dosing. No effects were
observed on food consumption or haematological parameters. Decreased
body-weight gain was observed in all treated dogs. ALAT activity was
increased in 1 out of 3 female dogs in both the 40 µg/kg bw/day and
79 µg/kg bw/day groups. Activity returned to normal after the
recovery period. Total lipid levels in serum were increased in all
treated dogs without any dose-dependence. By the end of the recovery
period total lipid levels had decreased, but were still elevated
relative to control levels in all treated dogs. Plasma corticoid
levels were decreased in dogs receiving 79 µg/kg bw/day
dexamethasone but returned to normal by the end of the recovery
period. Increased triglyceride levels in the adrenals and increased
liver glycogen were observed in all treated dogs (without
dose-relationship). The increased adrenal triglyceride levels was
still apparent after the recovery period. Liver weights were
increased in a dose-related manner and honey comb distention was
seen in some hepatic cells. These effects reversed after treatment
was ceased. In all treated dogs, the adrenal weights were lower than
in the controls. Histological examination revealed diminution of the
fascicular and reticular zones of the adrenal cortex without a clear
demarcation between the different zones. After the recovery period
the adrenal weights returned to normal. No thymus or only remnants
of the thymus were found in some treated dogs. When the thymus was
found in recovery animals, it did not differ histologically from the
controls (Bauer et al., 1969b).
2.2.3 Long-term toxicity/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
In a special study 0.15 mg dexamethasone/day (equal to 6 mg/kg
bw/day) was administered s.c. to pregnant A/J mice on days 11-14
post-conception. The mice were killed on day 18. The incidence of
cleft palate was 93% (Walker, 1971).
2.2.5.2 Rats
Groups of 20 pregnant SPF-FW 49 Biberach rats were administered
dexamathasone s.c. at dose levels of 0, 20, 40 or 79 µg/kg bw/day on
days 6-15 of gestation. Females were killed on gestation day 21. One
female died intercurrently, the cause of death could not be
resolved. All treated females failed to gain weight and had lower
food consumption during treatment, but they gained weight after
cessation of treatment. Total food consumption, however, remained
lower in all treated animals compared to controls. The mean number
of implantations was higher in all treated groups than in the
control group. Resorption rates were higher in a dose-related manner
and the number of live offspring was lower in the two highest dose
groups. Litter weight was decreased in a dose-related manner. Both
the variation and malformation rates were increased, but with no
apparent dose-relationship. Retarded ossification of the sternebrae
and hydronephrosis occurred most frequently (Lehmann, 1969a).
Daily doses of 0.05, 0.2, or 0.8 mg dexamethasone/day (equal to
250, 1000 or 4000 µg/kg bw/day) were administered s.c. to pregnant
Holtzmann rats on days 12-15 post-conception. The dams were killed
at day 19. A high frequency of cleft palate (53%) was observed in
litters of rats in the highest dose group. No cleft palates were
observed at lower doses (Walker, 1971).
Groups of 20 pregnant rats (Morini Wistar) were given daily
s.c. injections of 0, 40, or 79 µg dexamethasone/kg bw/day on days
6-15 of gestation. Maternal body-weight gain and food consumption
were decreased in all treated dams. Compared to the controls, the
resorption rate was increased and fetal weights were decreased.
Hydronephrosis was seen in 4 fetuses, 2 at 40 and 2 at 79 µg/kg
bw/day (Segro, 1970).
In a poorly reported study, groups of 10 pregnant rats (SPF,
strain SD-JCL) were given daily s.c. doses of 0, 20, 40, or 80 µg
dexamethasone/kg bw/day on days 6-15 of gestation. Mortality and
body weights were recorded. The dams were killed on day 21 of
pregnancy and the fetuses were delivered by caesarean section.
Maternal body weight was decreased in treated animals. In all
treated rats pre- and post-implantation losses were increased. No
effects were observed on fetal weight. Cleft palate was seen in one
fetus of the control group. One fetus in the 20 µg/kg bw/day dose
group had thoracoschisis. The occurrence of 14th rib was observed in
the control group (25%) as well as in all dose groups. There was no
marked difference relative to controls, but a slight dose-related
effect was observed (incidences ranged from 20 to 28.4% in the
treated groups). Deformity of the sternum was observed in 1/85 rats
in the 20 µg/kg bw/day dose group (Umemura et al., 1972).
In a pilot study groups of Virgin Lati:Han Wistar pregnant rats
(10/group) were orally administered (by gavage) doses of 0, 10, 50,
250, or 1250 µg dexamethasone/kg bw/day on days 7 to 16 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 21 and fetuses were delivered
by caesarean section. Observations included the number of corpora
lutea, implantations, fetus and placenta weight, and sex of viable
fetuses. All fetuses were examined for skeletal and visceral
abnormalities.
Maternal body-weight gain was decreased at doses of 50 µg/kg
bw/day and above and thymus involution was observed at the same dose
levels. In the highest dose group, post-implantation mortality was
increased. Most fetuses that died within the last 24 hours were
malformed. Fetal weight was decreased at doses of 250 and 1250 µg/kg
bw/day. In the 2 highest dose groups retrognathia and cleft palate
of variable severity were observed. Hydrops fetalis and umbilical
hernia was found only at 1250 µg/kg bw/day. Thymus hypoplasia was
observed at 50, 250, and 1250 µg/kg bw/day (4, 2, and 59%,
respectively). The NOEL in this study was 10 µg/kg bw/day (Druga,
1993a).
In the main Segment II study, groups of Virgan Lati:Han Wistar
rats were orally administered (by gavage) 0, 20, 200, or 1000 µg
dexamethasone in methylcellulose/kg bw/day on days 6 to 15 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 20. The visera of the dams
were examined grossly and the thymus was removed and weighed.
Observations included the number of corpora lutea, implantations,
length of umbilical cord, fetus and placenta weight, and sex of
viable fetuses. All fetuses received external, skeletal, and
visceral examinations.
Maternal body weight and body-weight gain and food consumption
were decreased at 200 and 1000 µg/kg bw/day. Thymus involution was
observed at the same dose levels. Post-implantation mortality was
increased at the highest dose. Fetal weight was decreased at 200 and
1000 µg/kg bw/day. Umbilical cord length was reduced at 200 and 1000
µg/kg bw/day and the length, thicknesss, and index of the femur were
markedly lower at 1000 µg/kg bw/day. High-dose fetuses showed an
increased incidence of malformations, including hydrops fetalis,
retrognathia, cleft palate, umbilical hernia of variable severity,
split sternum, malformed vertebrae, malformed upper limb bones, and
micromelia. Thymus hypoplasia was observed at 20, 200, and 1000
µg/kg bw/day (4, 2, and 16%, respectively). In the high-dose group,
dystrophy of gonads was also observed. A no-effect level was not
observed in this study (Druga, 1993b).
2.2.5.3 Rabbits
Dexamethasone, as one glucocorticoid in a series of 6, was
administered i.m. at dose levels ranging from 0.1 to 4 mg/day (equal
to 25 to 1000 µg/kg bw/day) to pregnant rabbits on days 13.5-16.5
post-conception. Litter resorptions were observed at 750 and 1000 µg
dexamethasone/kg bw/day. Cleft palate was observed at >62 µg/kg
bw/day. No effects on resorption or cleft palate were observed at 25
µg/kg bw/day (Walker, 1967).
In a comparative study, groups of 15 pregnant rabbits ("SPF
Himalyan"/Biberach) were administered s.c. 0, 20, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. The dams were
killed on day 29 of pregnancy. During dosing maternal body weight
remained stationary or was reduced, particularly in the second half
of the dosing period. A dose-related increase in resorption rate and
number of runts was observed. A dose-related decrease in fetal
weight was also observed. The incidence of flexure of the forefeet
and of malformations (palatoschisis, gastroschisis, exencephaly,
encephalocele and menigocele, anotia, and ectrodactyly) was
increased in a dose-related manner in all treatment groups.
Malformations of the extremities such as haemibrachia, hypoplasia of
tibia and fibula, and acheiria were observed (Lehmann, 1969b).
In a similar study, groups of 15 pregnant New Zealand white
rabbits were given daily s.c. injections of 0, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. Compared to the
control group all treated dams had reduced body weight and their
resorption rates were increased. No treatment-related effect on
gross malformations was observed (Segro, 1970).
2.2.6 Special studies on endocrine toxicity
Groups of Cpb:WU rats (10/sex/group) were orally administered
(by gavage) doses of 0, 0.3, 1, 3, 10, 30, or 100 µg dexamethasone/
kg bw/day for 90 days. Observations included clinical signs, body
weight, water consumption, haematology, IgG/IgM determinations,
gross post mortem examination, adrenal and thymus weight,
histopathology, ACTH stimulation test (4 rats/sex/group), and
corticosteroid determinations.
Significant decreases in body-weight gain (more pronounced in
males than in females) were observed at doses of >10 µg/kg
bw/day. In males of the same groups sluggishness and erected fur
were observed, which were considered to be treatment-related. WBC
and differential WBC counts were significantly decreased in rats at
>10 µg/kg bw/day. WBC counts were also significantly reduced in
females at 3 µg/kg bw/day. IgG and IgM levels were significantly
decreased at 100 µg/kg bw/day. Decreases in adrenal and thymus
weights were observed and accompanied by histopathological changes,
including atrophy and structural disorganization, at >10 µg/kg
bw/day. In the same dose groups dose-related decreases in
corticosterone levels with or without ACTH stimulation were
observed. The Committee concluded that the NOEL in this study was at
1 µg/kg bw/day (De Jong & Coert, 1987).
Rats (6/group) were administered a dose of 0, 0.5, 1, 1.5, 2,
or 4 µg dexamethasone/kg bw/day by gavage for 1 and 7 days. Five
hours after the last application the rats were killed and blood and
liver samples were taken. Tyrosine amino transferase (TAT) activity
in the supernatant of liver homogenates was measured and serum
corticosterone concentrations were determined. TAT activity was
increased in a dose-dependent manner at 2 and 4 µg/kg bw/day and a
significant decrease in serum corticosterone was seen at 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day (Kietzman, 1991;
Bette & Kietzmann, 1991).
2.2.7 Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
2.2.8 Special studies on immune response
The influence of dexamethasone-21-isonicotinate on the course
of experimental bacterial infections of mice and their therapy, on
the phagocytotic activity of the reticulo-endothelial system and the
serum protein picture was investigated. No clear effect on
antibiotic therapy was observed. Dexamethasone-21-isonicotinate
decreased the activity of Celasin C and penicillin G-procain against
streptococcal infections and improved the activity of both
substances against staphylococcal infections. No quantitative effect
was observed upon serum albumin, alpha1, alpha2, ß1, ß2 or
gamma-globulins of mice both 48 hours after a single s.c. treatment
or 24 hours after 5 days of treatment (once a day s.c.) with 75,
150, or 300 µg dexamethasone 21-isonicotinate/kg bw (Goeth &
Lechner, 1978).
2.3 Observations in humans
The corticosteroid suppressive effect of dexamethasone is well
known and used for the definitive diagnosis of Cushing syndrome in
human patients. People with Cushing syndrome suffer from a chronic
over-production of cortisol resulting from an over-production of
ACTH. In the "low- and high-dose dexamethasone suppression test" the
subjects receive 0.5 or 2 mg dexamethasone orally every 6 hours for
Table 2: Results of genotoxicity assays on dexamethasone and
dexamethasone-21-isonicotinate
Test system Test object Concentration Purity Results Reference
In vitro
Ames testa S.typhimurium 10-1000 99% negativec Baumeister,
TA98, 100, µg/pl 1988a
1535, 1537
E. coli , WP2
Fluctuation Mouse 12.5-400 99.4% negativee Clements,
assayb,d lymphoma µg/ml 1992
L5178Y cells
In vivo
Micronucleus NMRI mice i.v. 5 mg/kgf 97.5% negativeg Baumeister,
testsa 1988b
a Test substance: dexamethasone 21-isonicotinate.
b With and without metabolic activation.
c Appropriate positive controls were used.
d Test substance: dexamethasone.
e 4-Nitroquinoline and benzo(a)pyrene were used as positive controls.
f The initial dosage (107.5 mg/kg) had to be reduced due to acute
toxicity (tonic convulsions, deaths) of the excipient 1,
2-propylenglycol.
g Cyclophosphamide was used as positive control.
2 consecutive days. In normal healthy persons cortisol production,
determined as 17-hydroxycorticosteroid or 17-ketosteroid excretion
in urine, is suppressed, whereas in patients with Cushing syndrome
cortisol production is not suppressed. No significant clinical side
effects of this dexamethasone suppression test were reported (Crapo,
1979).
3. COMMENTS
Information from studies on dexamethasone, including data on
kinetics, metabolism, acute and short-term toxicity, developmental
toxicity, and genotoxicity, was available for assessment.
Toxicokinetic studies revealed rapid absorption after i.m.
administration to dogs and rats with peak plasma levels found after
30 minutes and 6 hours, respectively. Dexamethasone is rapidly
excreted in urine and faeces. Dexamethasone esters are rapidly
hydrolyzed in serum. Biotransformation in rats and humans is
comparable and involves mainly hydroxylation to 6-hydroxy- and
20-dihydro-dexamethasone. However, there was additional evidence
that at high (therapeutic) doses in people, dexamethasone is
metabolized by an additional route involving epoxidation.
Following repeated oral administration of dexamethasone to rats
and dogs in short-term toxicity studies the main target organs were
the thymus and the adrenal gland. Corticosteroid concentrations in
plasma and hepatic glycogen were reduced, whereas serum lipid levels
were increased. In rats dosed orally with 0.3, 1, 3, 10, 30, or 100
µg dexamethasone/kg bw/day for 90 days, thymus involution and
morphological changes in the adrenal gland and a decrease in
corticosterone and white blood cell counts were observed in male and
female rats at doses above 10 µg/kg bw/day. Due to the decrease in
white blood cell counts in female rats at 3 µg/kg bw/day this dose
was considered to be a marginal effect level. In a study with rats
orally dosed with 0.5, 1, 1.5, 2, or 4 µg/kg bw/day dexamethasone
for 7 days the corticosterone concentration was reduced in the
highest-dose group and the activity of tyrosine aminotransferase in
the liver was increased in a dose-related manner at 2 and 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day.
No reproduction studies with dexamethasone were available but
an increase in pre- and post-implantation loss and a reduction in
fetal weight were observed in teratogenicity studies in mice, rats,
and rabbits receiving dexamethasone by injection. In these studies
malformations such as hydrops fetalis, cleft palate, exencephaly,
and encephalocele were observed at maternally toxic dose levels.
In oral teratogenicity studies with rats using dose levels
ranging from 10 to 1250/kg bw/day, maternal toxicity was found at 50
µg/kg bw/day and above. At doses at and above 1000 µg/kg bw/day,
dexamethasone caused structural malformations (hydrops fetalis,
cleft palate). Thymus involution and a decrease in body weight were
observed in fetuses, resulting in an overall NOEL for embryotoxicity
in rats of 10 µg/kg bw/day.
Long-term toxicity/carcinogenicity data were not available.
4. EVALUATION
Based on its long history of use in human medicine and because
dexamethasone was negative in in vitro gene mutation assays with
bacteria and mammalian cells and in an in vivo micronucleus test
with mice, the Committee was not concerned about the carcinogenic
potential of dexamethasone.
Using a safety factor of 100 the Committee established an ADI
of 0-0.015 µg/kg bw/day for dexamethasone based on a NOEL of 1.5
µg/kg bw/day for the induction of tyrosine aminotransferase activity
in rat liver. Due to the careful selection of the dose levels in
this study the Committee did not round this figure.
5. REFERENCES
BAUER, O., LEHMANN, & UEBERBERG, H. (1969a) Rat study to screen
pyridine-4-carboxylic acid-(dexamethasone-21')-ester (HE 111) for
subacute toxicity in comparison with dexamethasone. Unpublished
report d.d. 12 June 1969 from Dr. K. Thomas GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUER, O., PAPPRITZ, & UEBERBERG, H. (1969b) Investigation into the
subacute toxicity of pyridine-4-carboxylic acid (dexamethasibe-21')
ester (HE 111) in comparison with dexamethasone in dogs. Unpublished
report No. AX-U-30 d.d.21-4-1969 from Dr. Karl Thomas, GmbH,
Biberach, Germany. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988a) Mutagenicity study with HE III XX (Voren) in
the S. typhimurium and E. coli/mammalian microsome assay (Ames
Test). Unpublished report no. Gen Tox. 39/87 d.d. 5 February 1988
from Dr. K. Thomas, GmbH, Dept. of Experimental Pathology and
Toxicology, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988b) Mutagenicity study with HE III XX (Voren) in
the bone marrow micronucleus assay. Unpublished report no. Gen Tox.
40/87 d.d. 8 February 1988 from Dr. K. Thomas, GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BETTE, P. & KIETZMANN, M. (1991) Effect of dexamethasone on tyrosine
amino-transferase activity in rat liver - a sensitive test to define
its hormonal no-effect-level. Acta Vet. Scand., 87: 200-202.
CRAPO, L. (1979) Cushings syndrome: A review of diagnostic tests.
Metabolism , 28: 955-977.
CLEMENTS, J. (1992) Study to determine the ability of dexamethasone
to induce mutations at the thymidine kinase (tk) locus in mouse
lymphoma L5178Y cells using a fluctuation assay. Unpublished report
no. 2TKREBSG.001 from Hazleton Microtest Limited, York, England.
Submitted to WHO by Intervet International B.V., Boxmeer, The
Netherlands.
COERT, A., HOEIJMAKERS, M., VAN RENS, P. (1988) The in vitro
hydrolysis of dexamethasone dimethylbutyrate in cows plasma.
Unpublished subject report no. 10 d.d. 3-2-1988 from Intervet
Boxmeer. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
De JONG, H. & COERT, A. (1987) The determination of the hormonal
no-effect-level of dexamethasone in rats after 90 days of oral
dosing (NEL study). Unpublished report d.d. 28-8-1987 from Intervet
Boxmeer. Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
DRUGA. A. (1993a) Pilot teratology study (Segment II) of
dexamethasone in rats. Unpublished report no. 9218 by Institute for
Drug Research, Budapest, Hungary. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim.
DRUGA, A. (1993b) Teratology study (segment II) of dexamethsone in
rats. Unpublished report study nr. 9219 by Institute for Drug
Research, Budapest, Hungary. Submitted to WHO by Intervet
International B.V., Boxmeer, The Netherlands and Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGELHARDT, G. (1963). Pharmacological exposé on substance HE III.
Unpublished report no.: AX-U-26 d.d. 17 January 1963 from Dr. K.
Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGLISH, J., CHAKRABORTY, J. & MARKS, V. (1975) The metabolism of
dexamethasone in the rat - effect of phenytoin. J. Steroid
Biochem., 6: 65-68.
GOETH, H. & LECHNER, V. (1978) Investigation on the influence of
Voren on the course of experimental infections and their treatment
with antibiotics and sulphonamides as well as on the phagocytotic
activity of the reticulo-endothelial system (RES) and the serum
protein picture in mice. Berl. Munch. Wschr., 91: 87-93.
HORNER, M.W. (1989) Pharmacokinetics of dexamethasone in pig plasma
following subcutaneous injection of Opticortenol S. Unpublished
report d.d. November 1989, Ciba Geigy Project no. 4. from
Horseracing Forensic Labortory, Newmarket. Submitted to WHO by Ciba
Geigy, Division Agro, Basle, Switzerland.
HOUGHTON, E., GRAINGER, L. & TEALE, P. (1989) The in vitro
hydrolysis of dexamethasone trimethylacetate in whole blood from the
horse and cow. Unpublished report from Horseracing Forensic
Laboratory, Newmarket, July 1989. Submitted to WHO by Ciba Geigy,
Division Agro, Basle, Switzerland.
INTERVET, (undated I) Chronic oral toxicity study with dexamethasone
in rats. Unpublished and unsigned report from Intervet d.d. mid
1960's? Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
INTERVET, (undated II) Chronic oral toxicity study with
dexamethasone in dogs. Unpublished and unsigned report from Intervet
d.d. mid-1960's? Submitted to WHO by Intervet International B.V.,
Boxmeer. The Netherlands.
KIETZMAN, M. (1991) Report of a study in rats to investigate the
effect of dexamethasone on tyrosine aminotransferase activity in the
liver and on serum corticosterone levels. Veterinary College,
Hannover (27-1-91). Unpublished report submitted to WHO by
Boehringer Ibgelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969a) Reproduction study comparing the compound HEIII
with dexamethasone in gestating rats. Unpublished report no. AUXI
0031 from Dr Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969b) Reproduction study on the substance HEIII versus
dexamethasone in pregnant rabbits. Unpublished report d.d.
24-11-1969 no. U69-0018 from Dr Karl Thomas, GmbH, Biberach,
Germany. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
PEETS, E.A., STAM, M. & SYMCHOWICZ, C. (1969) Plasma binding of
betamethasone-3H, dexamethasone-3H, and cortisol-14C - a
comparative study. Biochem. Pharmacol., 18: 1655-1663.
RICE, M.J. TREDGER, J.M., CHAKRABORTY, J. & PARKE, D.V. (1974) The
metabolism of dexamethasone in the rat. Biochem. Soc. Transact.,
53rd Meeting, Bristol, 2: 107-109.
SEGRO, G. (1970) Expertise on HE111 in rats and rabbits. Report d.d
1-4-1970 from University of Sienna, Italy. Unpublished report no.
AX-U43 from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
SEUTTER, E. (1975) Metabolism of systematically given
corticosteroids. Dermatologica , 151: 129-134.
STEWART, F.P., KÖRÖSI, S.A. & HOPKINS, R. (1992)
[1,2,4-3H]-dexamethasone: tritium exchange following intramuscular
administration to the rat. Unpublished report 6938-806/2 d.d.January
1992 from Hazleton UK Limited, Harrogate, England. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
TOUTAIN, P.L., ALVINERIE, M. & RUCKEBUSCH, Y. (1983)
Pharmacokinetics of dexamethasone and its effect on adrenal gland
function in the dog. Am. J. Vet. Res., 44: 212-217.
UEBERBERG, H. (1963) Comparative experimental trials in dogs with
pyridine- 4-carboxylic acid (dexamethasone-21')ester (HEIII) and
dexamethasone. Unpublished report no. AX-U-3 d.d.20-12-1963 from Dr
Karl Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UEBERBERG, H. (1964) Comparative investigations in rats with
substance HEIII and dexamethasone. Unpublished report d.d. 23-3-1964
from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UMEMURA, M., KAST, A. & IIDA, H. (1972) Teratologica; testing of
substances dexamethasone and HEIII in rats. Unpublished report d.d.
3-3-1972 from Boehringer Ingelheim GmbH Pharma Research Japan.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
International Division, Ingelheim, Germany.
WALKER, B.E. (1967) Induction of cleft palate in rabbits by several
glucocorticoids. Proc. Soc. Exp. Biol. Med., 125: 1281-1284.
WALKER, B.E. (1971) Induction of cleft palate in rats with
anti-inflammatory drugs. Teratology , 4: 39-42.
WEISENBERGER, H. (1972) Species differences in the hydrolysis of
dexamethasone 21-isonicotinate by serum esterases. Klin. Wschr.,
50: 665.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Male Wistar albino rats were administered 0.23 µmol [1,2-3H]
dexamethasone/kg bw, i.p. Urine and faeces were collected up to 4
days after treatment. Within 96 hours 74% of the dose was excreted,
30% in the urine and 44% in the faeces (Rice et al., 1974).
Crl:SD(CD)BR rats were administered a single i.m. dose of 9 µg,
[1,2,4-3H]-dexamethasone/kg bw. Radioactivity was measured up to
96 hours after administration in plasma (pre- and post-freeze
dried), urine, faeces and expired air. Tritium exchange was measured
in stored urine. Highest plasma levels were observed 6 hours after
dosing (3.7 µg equivalents/g), declining rapidly thereafter to 0.15
µg equivalents/g. Within 24 hours 41% of the radioactivity was
excreted in the urine. After 96 hours a mean of 44% of the
radio-activity was excreted. Tritium exchange was observed both in
plasma and urine. Following freeze-drying, the mean loss of
radioactivity 96 hours after dosing was 87% and 37% in plasma and
urine, respectively (Stewart et al., 1992).
Dogs (mixed-breed) were administered dexamethasone alcohol or
dexamethasone 21-isonicotinate as a solution i.v. or i.m. (1 mg/kg
bw), or dexamethasone 21-isonicotinate as a suspension i.m. (0.1 or
1 mg/kg bw). Plasma concentrations were determined with HPLC up to
120 hours after treatment. The elimination half-life after i.v.
administration was 120-140 minutes for both formulations. Following
i.m. administration, absorption was rapid with peak plasma
concentrations at 30-40 minutes for both solutions. Bio-availability
after i.m. administration was 100% for dexamethasone alcohol but 40%
for dexamethasone 21-isonicotinate. After i.m. administration of
dexamethasone 21-isonicotinate as a suspension, dexamethasone was
not detected in plasma, suggesting a long absorption phase (Toutain
et al., 1983).
2.1.2 Biotransformation
2.1.2.1 In vitro
The half-life of dexamethasone 21-isonicotinate was determined
in human, rat, and rabbit sera. In rat and rabbit sera 90 and 99% of
the ester was hydrolyzed after 10 minutes, respectively. The
half-life of in human serum was about 90-100 minutes (Weisenberger,
1972).
Dexamethasone trimethylacetate was rapidly hydrolyzed to
dexamethasone in bovine and equine whole blood; half-lives ranged
from 10-30 minutes for both species (Houghton et al., 1989).
Dexamethasone dimethylbutyrate was hydrolyzed quickly in bovine
plasma, with a half-life of about 1 hour (Coert et al., 1988).
2.1.2.2 Rats
In the urine of rats administered 0.23 µmol/kg bw [1,2-3H]-
dexamethasone i.p., 10% of the administered radioactivity was
associated with one polar metabolite of dexamethasone, likely to be
6-hydroxy-dexamethasone (Rice et al., 1974).
Male Wistar albino rats were administered [3H]-dexamethasone
orally at a dose of 1.14 nmol/kg bw. Thirty-one percent of the
administered radioactivity was excreted in the urine within 4 days
(most of it within the first 24 hours) as unconjugated metabolites.
Unchanged dexamethasone accounted for 14%, 6-hydroxydexamethasone
for 7.4%, and 20-dihydrodexamethasone for 1.1% of the urine
radioactivity. Twenty-five percent of the administered dose was
eliminated in the faeces. Another group of rats was pretreated with
phenytoin 16 hours before dexamethasone treatment. The pretreatment
reduced the rate of total radioactivity excreted in the urine within
24 hours, but the total urinary and faecal excretion after 96 hours
was not significantly reduced (English et al., 1975).
2.1.2.3 Pigs
Four hours after the s.c. administration to pigs of
[1,2-3H]-dexamethasone-21-trimethylacetate, less than 1% of total
plasma radioactivity was extractable as unchanged
[3H]-dexamethasone-21-acetate. The plasma concentration of
dexamethasone was highest (about 3 ng/ml) at 4 hours, declining
rapidly to about 0.5 ng/ml at 24 hours, and slowly thereafter.
Measurable amounts of dexamethasone (>0.2 ng/ml) were still present
at day 5 (Horner, 1989).
2.1.2.4 Humans
No parent compound could be detected in urine of patients after
oral administration of a small dose of dexamethasone (<4 mg/day)
for a few weeks. However, 60% was recovered as
6-ß-hydroxy-dexamethasone and 5-10% as
6-ß-hydroxy-20-dihydrodexamethasone. After the administration of
about 15 mg dexamethasone/day metabolism occurred by an additional
route involving epoxidation and subsequent hydrolysis, resulting in
glycol formation in ring A (Seutter, 1975).
2.1.3 Special studies on macromolecular binding
The binding of dexamethasone to proteins in rat, dog, cow, and
human plasma has been studied in vitro by an equilibrium dialysis
technique. Approximately 85, 73, 74, and 77% was bound in rat, dog,
cow, and human plasma, respectively. Dexamethasone was mainly bound
to the albumin fraction of human plasma (Peets et al., 1969).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of an acute toxicity study is summarized in Table
1.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 15 rats were given daily s.c. injections of 0.5 ml
vehicle or 50 µg/kg bw dexamethasone for 6 weeks. Body- weight gain
was significantly decreased in treated rats. Adrenal weights were
also decreased. Post-mortem examination revealed no pathological
organ changes. Only a summary of this study was available
(Ueberberg, 1964).
In 3 experiments, groups of 15 rats/sex were given 0.125 mg/kg
bw (6 days/week), 0.25 mg/kg bw (5 days/week), or 0.4 mg/kg bw (5
days/week) dexamethasone in tablets for 181-185 days. The control
groups (10 rats/sex/group) received placebo tablets. All mid- and
high-dose animals received 20 mg tetracycline*HCl once a week
beginning on the 39th experimental day.
Dose-related deaths occurred as follows: 4/30 at the low-dose,
14/30 at the mid-dose and 26/30 at the top-dose. Post mortem
examination in all cases showed severe infections. In all dose
groups, body-weight gain was decreased, relative kidney weight was
increased and relative adrenal and thymus weights were decreased. In
the bone marrow the number of neutrophilic forms of leucocytes was
increased and the number of eosinophils was decreased in all
experimental groups as compared to controls. Detailed
histopathological findings of the mid- and high-dose groups were not
presented (Intervet, undated I).
Groups of 20 male and 20 female Wistar rats were administered
s.c. 0, 40, or 79 µg/kg bw/day dexamethasone for 13 weeks. An
additional group of 5 male and 5 female rats was given 79 µg/kg
bw/day dexamethasone s.c. for 13 weeks, then kept for a 7-week
recovery period. Haematological and biochemical examinations were
performed on 5 rats/sex after 10 weeks.
Table 1. Acute toxicity of dexamethasone
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M i.p. 577 Engelhardt, 1963
In all treated males, ALAT activity and total cholesterol
concentrations were increased. Lipid levels were only incidentally
raised. Plasma corticosteroid levels and hepatic glycogen were
decreased in a dose-related manner. In treated males, adrenal
glycogen levels were increased and in males as well as in females
dose-related reductions in adrenal corticosteroids were observed.
Post mortem examination found adrenal and thymus glands that were
markedly smaller with reduced weights when compared to controls. In
some animals no thymus tissue could be found. Compared to the
controls, body weights and most organ weights of treated rats were
lower. Microscopic examination revealed marked changes in the thymus
and the adrenal glands. The adrenal cortex was narrowed due to loss
of the regular structuring of the cells or cell columns, in addition
to a reduction in lipids. The thymus from the treated rats showed
atrophy of the medullary and cortical tissues. After the recovery
period no significant changes compared to the controls were observed
(Bauer et al., 1969a; results of male rats partly reported in
Segro, 1970).
2.2.2.2 Dogs
In a limited study, groups of 4 female beagle dogs were orally
administered a 2 or 8 mg dexamethasone tablet/day (6 days/week) for
26 weeks. No control group was used. Instead, values from previous
studies were used for comparison. One low-dose dog died (not related
to treatment) during the study and 3 high-dose dogs died, of which 2
were due to retro-oesophageal abcesses or gastric ulcers. At
post-mortem examination all remaining dogs were found to have
infections. Alopecia was seen in 1 dog in each dose group. Atrophy
of the lymphatic organs was seen in all dogs, adrenal weight was
decreased, and in the high-dose group the thymus had almost
disappeared (Intervet, undated II).
Groups of mongrel dogs (3 male and 2 female dogs/group) were
orally administered placebo tablets or 125 µg/kg bw/day
dexamethasone 7 days/week for 6 weeks. No treatment-related effects
were observed on clinical signs, body-weight, liver and renal
function tests, urinalysis, or post mortem examination. After 6
weeks blood glucose values were increased in treated animals.
Relative adrenal weights were decreased. In the adrenals, the zona
fasciculata was narrowed in treated dogs. Lipid observed in the
adrenal cortical zones of the controls was not present in treated
dogs. Total 17-ketosteroid excretion in the urine was higher in all
treated groups (Ueberberg, 1963).
Groups of 3 male and 3 female beagle dogs were administered
daily i.m. doses of 0, 40, or 79 µg/kg bw dexamethasone for 13
weeks. Additionally, 2 groups of 3 male and 3 female dogs were given
daily i.m. doses of 79 µg/kg bw dexamethasone for 13 weeks, then
kept for a 4-week recovery period. One female dog from the recovery
group died 14 days after cessation of dosing. No effects were
observed on food consumption or haematological parameters. Decreased
body-weight gain was observed in all treated dogs. ALAT activity was
increased in 1 out of 3 female dogs in both the 40 µg/kg bw/day and
79 µg/kg bw/day groups. Activity returned to normal after the
recovery period. Total lipid levels in serum were increased in all
treated dogs without any dose-dependence. By the end of the recovery
period total lipid levels had decreased, but were still elevated
relative to control levels in all treated dogs. Plasma corticoid
levels were decreased in dogs receiving 79 µg/kg bw/day
dexamethasone but returned to normal by the end of the recovery
period. Increased triglyceride levels in the adrenals and increased
liver glycogen were observed in all treated dogs (without
dose-relationship). The increased adrenal triglyceride levels was
still apparent after the recovery period. Liver weights were
increased in a dose-related manner and honey comb distention was
seen in some hepatic cells. These effects reversed after treatment
was ceased. In all treated dogs, the adrenal weights were lower than
in the controls. Histological examination revealed diminution of the
fascicular and reticular zones of the adrenal cortex without a clear
demarcation between the different zones. After the recovery period
the adrenal weights returned to normal. No thymus or only remnants
of the thymus were found in some treated dogs. When the thymus was
found in recovery animals, it did not differ histologically from the
controls (Bauer et al., 1969b).
2.2.3 Long-term toxicity/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
In a special study 0.15 mg dexamethasone/day (equal to 6 mg/kg
bw/day) was administered s.c. to pregnant A/J mice on days 11-14
post-conception. The mice were killed on day 18. The incidence of
cleft palate was 93% (Walker, 1971).
2.2.5.2 Rats
Groups of 20 pregnant SPF-FW 49 Biberach rats were administered
dexamathasone s.c. at dose levels of 0, 20, 40 or 79 µg/kg bw/day on
days 6-15 of gestation. Females were killed on gestation day 21. One
female died intercurrently, the cause of death could not be
resolved. All treated females failed to gain weight and had lower
food consumption during treatment, but they gained weight after
cessation of treatment. Total food consumption, however, remained
lower in all treated animals compared to controls. The mean number
of implantations was higher in all treated groups than in the
control group. Resorption rates were higher in a dose-related manner
and the number of live offspring was lower in the two highest dose
groups. Litter weight was decreased in a dose-related manner. Both
the variation and malformation rates were increased, but with no
apparent dose-relationship. Retarded ossification of the sternebrae
and hydronephrosis occurred most frequently (Lehmann, 1969a).
Daily doses of 0.05, 0.2, or 0.8 mg dexamethasone/day (equal to
250, 1000 or 4000 µg/kg bw/day) were administered s.c. to pregnant
Holtzmann rats on days 12-15 post-conception. The dams were killed
at day 19. A high frequency of cleft palate (53%) was observed in
litters of rats in the highest dose group. No cleft palates were
observed at lower doses (Walker, 1971).
Groups of 20 pregnant rats (Morini Wistar) were given daily
s.c. injections of 0, 40, or 79 µg dexamethasone/kg bw/day on days
6-15 of gestation. Maternal body-weight gain and food consumption
were decreased in all treated dams. Compared to the controls, the
resorption rate was increased and fetal weights were decreased.
Hydronephrosis was seen in 4 fetuses, 2 at 40 and 2 at 79 µg/kg
bw/day (Segro, 1970).
In a poorly reported study, groups of 10 pregnant rats (SPF,
strain SD-JCL) were given daily s.c. doses of 0, 20, 40, or 80 µg
dexamethasone/kg bw/day on days 6-15 of gestation. Mortality and
body weights were recorded. The dams were killed on day 21 of
pregnancy and the fetuses were delivered by caesarean section.
Maternal body weight was decreased in treated animals. In all
treated rats pre- and post-implantation losses were increased. No
effects were observed on fetal weight. Cleft palate was seen in one
fetus of the control group. One fetus in the 20 µg/kg bw/day dose
group had thoracoschisis. The occurrence of 14th rib was observed in
the control group (25%) as well as in all dose groups. There was no
marked difference relative to controls, but a slight dose-related
effect was observed (incidences ranged from 20 to 28.4% in the
treated groups). Deformity of the sternum was observed in 1/85 rats
in the 20 µg/kg bw/day dose group (Umemura et al., 1972).
In a pilot study groups of Virgin Lati:Han Wistar pregnant rats
(10/group) were orally administered (by gavage) doses of 0, 10, 50,
250, or 1250 µg dexamethasone/kg bw/day on days 7 to 16 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 21 and fetuses were delivered
by caesarean section. Observations included the number of corpora
lutea, implantations, fetus and placenta weight, and sex of viable
fetuses. All fetuses were examined for skeletal and visceral
abnormalities.
Maternal body-weight gain was decreased at doses of 50 µg/kg
bw/day and above and thymus involution was observed at the same dose
levels. In the highest dose group, post-implantation mortality was
increased. Most fetuses that died within the last 24 hours were
malformed. Fetal weight was decreased at doses of 250 and 1250 µg/kg
bw/day. In the 2 highest dose groups retrognathia and cleft palate
of variable severity were observed. Hydrops fetalis and umbilical
hernia was found only at 1250 µg/kg bw/day. Thymus hypoplasia was
observed at 50, 250, and 1250 µg/kg bw/day (4, 2, and 59%,
respectively). The NOEL in this study was 10 µg/kg bw/day (Druga,
1993a).
In the main Segment II study, groups of Virgan Lati:Han Wistar
rats were orally administered (by gavage) 0, 20, 200, or 1000 µg
dexamethasone in methylcellulose/kg bw/day on days 6 to 15 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 20. The visera of the dams
were examined grossly and the thymus was removed and weighed.
Observations included the number of corpora lutea, implantations,
length of umbilical cord, fetus and placenta weight, and sex of
viable fetuses. All fetuses received external, skeletal, and
visceral examinations.
Maternal body weight and body-weight gain and food consumption
were decreased at 200 and 1000 µg/kg bw/day. Thymus involution was
observed at the same dose levels. Post-implantation mortality was
increased at the highest dose. Fetal weight was decreased at 200 and
1000 µg/kg bw/day. Umbilical cord length was reduced at 200 and 1000
µg/kg bw/day and the length, thicknesss, and index of the femur were
markedly lower at 1000 µg/kg bw/day. High-dose fetuses showed an
increased incidence of malformations, including hydrops fetalis,
retrognathia, cleft palate, umbilical hernia of variable severity,
split sternum, malformed vertebrae, malformed upper limb bones, and
micromelia. Thymus hypoplasia was observed at 20, 200, and 1000
µg/kg bw/day (4, 2, and 16%, respectively). In the high-dose group,
dystrophy of gonads was also observed. A no-effect level was not
observed in this study (Druga, 1993b).
2.2.5.3 Rabbits
Dexamethasone, as one glucocorticoid in a series of 6, was
administered i.m. at dose levels ranging from 0.1 to 4 mg/day (equal
to 25 to 1000 µg/kg bw/day) to pregnant rabbits on days 13.5-16.5
post-conception. Litter resorptions were observed at 750 and 1000 µg
dexamethasone/kg bw/day. Cleft palate was observed at >62 µg/kg
bw/day. No effects on resorption or cleft palate were observed at 25
µg/kg bw/day (Walker, 1967).
In a comparative study, groups of 15 pregnant rabbits ("SPF
Himalyan"/Biberach) were administered s.c. 0, 20, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. The dams were
killed on day 29 of pregnancy. During dosing maternal body weight
remained stationary or was reduced, particularly in the second half
of the dosing period. A dose-related increase in resorption rate and
number of runts was observed. A dose-related decrease in fetal
weight was also observed. The incidence of flexure of the forefeet
and of malformations (palatoschisis, gastroschisis, exencephaly,
encephalocele and menigocele, anotia, and ectrodactyly) was
increased in a dose-related manner in all treatment groups.
Malformations of the extremities such as haemibrachia, hypoplasia of
tibia and fibula, and acheiria were observed (Lehmann, 1969b).
In a similar study, groups of 15 pregnant New Zealand white
rabbits were given daily s.c. injections of 0, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. Compared to the
control group all treated dams had reduced body weight and their
resorption rates were increased. No treatment-related effect on
gross malformations was observed (Segro, 1970).
2.2.6 Special studies on endocrine toxicity
Groups of Cpb:WU rats (10/sex/group) were orally administered
(by gavage) doses of 0, 0.3, 1, 3, 10, 30, or 100 µg dexamethasone/
kg bw/day for 90 days. Observations included clinical signs, body
weight, water consumption, haematology, IgG/IgM determinations,
gross post mortem examination, adrenal and thymus weight,
histopathology, ACTH stimulation test (4 rats/sex/group), and
corticosteroid determinations.
Significant decreases in body-weight gain (more pronounced in
males than in females) were observed at doses of >10 µg/kg
bw/day. In males of the same groups sluggishness and erected fur
were observed, which were considered to be treatment-related. WBC
and differential WBC counts were significantly decreased in rats at
>10 µg/kg bw/day. WBC counts were also significantly reduced in
females at 3 µg/kg bw/day. IgG and IgM levels were significantly
decreased at 100 µg/kg bw/day. Decreases in adrenal and thymus
weights were observed and accompanied by histopathological changes,
including atrophy and structural disorganization, at >10 µg/kg
bw/day. In the same dose groups dose-related decreases in
corticosterone levels with or without ACTH stimulation were
observed. The Committee concluded that the NOEL in this study was at
1 µg/kg bw/day (De Jong & Coert, 1987).
Rats (6/group) were administered a dose of 0, 0.5, 1, 1.5, 2,
or 4 µg dexamethasone/kg bw/day by gavage for 1 and 7 days. Five
hours after the last application the rats were killed and blood and
liver samples were taken. Tyrosine amino transferase (TAT) activity
in the supernatant of liver homogenates was measured and serum
corticosterone concentrations were determined. TAT activity was
increased in a dose-dependent manner at 2 and 4 µg/kg bw/day and a
significant decrease in serum corticosterone was seen at 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day (Kietzman, 1991;
Bette & Kietzmann, 1991).
2.2.7 Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
2.2.8 Special studies on immune response
The influence of dexamethasone-21-isonicotinate on the course
of experimental bacterial infections of mice and their therapy, on
the phagocytotic activity of the reticulo-endothelial system and the
serum protein picture was investigated. No clear effect on
antibiotic therapy was observed. Dexamethasone-21-isonicotinate
decreased the activity of Celasin C and penicillin G-procain against
streptococcal infections and improved the activity of both
substances against staphylococcal infections. No quantitative effect
was observed upon serum albumin, alpha1, alpha2, ß1, ß2 or
gamma-globulins of mice both 48 hours after a single s.c. treatment
or 24 hours after 5 days of treatment (once a day s.c.) with 75,
150, or 300 µg dexamethasone 21-isonicotinate/kg bw (Goeth &
Lechner, 1978).
2.3 Observations in humans
The corticosteroid suppressive effect of dexamethasone is well
known and used for the definitive diagnosis of Cushing syndrome in
human patients. People with Cushing syndrome suffer from a chronic
over-production of cortisol resulting from an over-production of
ACTH. In the "low- and high-dose dexamethasone suppression test" the
subjects receive 0.5 or 2 mg dexamethasone orally every 6 hours for
Table 2: Results of genotoxicity assays on dexamethasone and
dexamethasone-21-isonicotinate
Test system Test object Concentration Purity Results Reference
In vitro
Ames testa S.typhimurium 10-1000 99% negativec Baumeister,
TA98, 100, µg/pl 1988a
1535, 1537
E. coli , WP2
Fluctuation Mouse 12.5-400 99.4% negativee Clements,
assayb,d lymphoma µg/ml 1992
L5178Y cells
In vivo
Micronucleus NMRI mice i.v. 5 mg/kgf 97.5% negativeg Baumeister,
testsa 1988b
a Test substance: dexamethasone 21-isonicotinate.
b With and without metabolic activation.
c Appropriate positive controls were used.
d Test substance: dexamethasone.
e 4-Nitroquinoline and benzo(a)pyrene were used as positive controls.
f The initial dosage (107.5 mg/kg) had to be reduced due to acute
toxicity (tonic convulsions, deaths) of the excipient 1,
2-propylenglycol.
g Cyclophosphamide was used as positive control.
2 consecutive days. In normal healthy persons cortisol production,
determined as 17-hydroxycorticosteroid or 17-ketosteroid excretion
in urine, is suppressed, whereas in patients with Cushing syndrome
cortisol production is not suppressed. No significant clinical side
effects of this dexamethasone suppression test were reported (Crapo,
1979).
3. COMMENTS
Information from studies on dexamethasone, including data on
kinetics, metabolism, acute and short-term toxicity, developmental
toxicity, and genotoxicity, was available for assessment.
Toxicokinetic studies revealed rapid absorption after i.m.
administration to dogs and rats with peak plasma levels found after
30 minutes and 6 hours, respectively. Dexamethasone is rapidly
excreted in urine and faeces. Dexamethasone esters are rapidly
hydrolyzed in serum. Biotransformation in rats and humans is
comparable and involves mainly hydroxylation to 6-hydroxy- and
20-dihydro-dexamethasone. However, there was additional evidence
that at high (therapeutic) doses in people, dexamethasone is
metabolized by an additional route involving epoxidation.
Following repeated oral administration of dexamethasone to rats
and dogs in short-term toxicity studies the main target organs were
the thymus and the adrenal gland. Corticosteroid concentrations in
plasma and hepatic glycogen were reduced, whereas serum lipid levels
were increased. In rats dosed orally with 0.3, 1, 3, 10, 30, or 100
µg dexamethasone/kg bw/day for 90 days, thymus involution and
morphological changes in the adrenal gland and a decrease in
corticosterone and white blood cell counts were observed in male and
female rats at doses above 10 µg/kg bw/day. Due to the decrease in
white blood cell counts in female rats at 3 µg/kg bw/day this dose
was considered to be a marginal effect level. In a study with rats
orally dosed with 0.5, 1, 1.5, 2, or 4 µg/kg bw/day dexamethasone
for 7 days the corticosterone concentration was reduced in the
highest-dose group and the activity of tyrosine aminotransferase in
the liver was increased in a dose-related manner at 2 and 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day.
No reproduction studies with dexamethasone were available but
an increase in pre- and post-implantation loss and a reduction in
fetal weight were observed in teratogenicity studies in mice, rats,
and rabbits receiving dexamethasone by injection. In these studies
malformations such as hydrops fetalis, cleft palate, exencephaly,
and encephalocele were observed at maternally toxic dose levels.
In oral teratogenicity studies with rats using dose levels
ranging from 10 to 1250/kg bw/day, maternal toxicity was found at 50
µg/kg bw/day and above. At doses at and above 1000 µg/kg bw/day,
dexamethasone caused structural malformations (hydrops fetalis,
cleft palate). Thymus involution and a decrease in body weight were
observed in fetuses, resulting in an overall NOEL for embryotoxicity
in rats of 10 µg/kg bw/day.
Long-term toxicity/carcinogenicity data were not available.
4. EVALUATION
Based on its long history of use in human medicine and because
dexamethasone was negative in in vitro gene mutation assays with
bacteria and mammalian cells and in an in vivo micronucleus test
with mice, the Committee was not concerned about the carcinogenic
potential of dexamethasone.
Using a safety factor of 100 the Committee established an ADI
of 0-0.015 µg/kg bw/day for dexamethasone based on a NOEL of 1.5
µg/kg bw/day for the induction of tyrosine aminotransferase activity
in rat liver. Due to the careful selection of the dose levels in
this study the Committee did not round this figure.
5. REFERENCES
BAUER, O., LEHMANN, & UEBERBERG, H. (1969a) Rat study to screen
pyridine-4-carboxylic acid-(dexamethasone-21')-ester (HE 111) for
subacute toxicity in comparison with dexamethasone. Unpublished
report d.d. 12 June 1969 from Dr. K. Thomas GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUER, O., PAPPRITZ, & UEBERBERG, H. (1969b) Investigation into the
subacute toxicity of pyridine-4-carboxylic acid (dexamethasibe-21')
ester (HE 111) in comparison with dexamethasone in dogs. Unpublished
report No. AX-U-30 d.d.21-4-1969 from Dr. Karl Thomas, GmbH,
Biberach, Germany. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988a) Mutagenicity study with HE III XX (Voren) in
the S. typhimurium and E. coli/mammalian microsome assay (Ames
Test). Unpublished report no. Gen Tox. 39/87 d.d. 5 February 1988
from Dr. K. Thomas, GmbH, Dept. of Experimental Pathology and
Toxicology, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988b) Mutagenicity study with HE III XX (Voren) in
the bone marrow micronucleus assay. Unpublished report no. Gen Tox.
40/87 d.d. 8 February 1988 from Dr. K. Thomas, GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BETTE, P. & KIETZMANN, M. (1991) Effect of dexamethasone on tyrosine
amino-transferase activity in rat liver - a sensitive test to define
its hormonal no-effect-level. Acta Vet. Scand., 87: 200-202.
CRAPO, L. (1979) Cushings syndrome: A review of diagnostic tests.
Metabolism , 28: 955-977.
CLEMENTS, J. (1992) Study to determine the ability of dexamethasone
to induce mutations at the thymidine kinase (tk) locus in mouse
lymphoma L5178Y cells using a fluctuation assay. Unpublished report
no. 2TKREBSG.001 from Hazleton Microtest Limited, York, England.
Submitted to WHO by Intervet International B.V., Boxmeer, The
Netherlands.
COERT, A., HOEIJMAKERS, M., VAN RENS, P. (1988) The in vitro
hydrolysis of dexamethasone dimethylbutyrate in cows plasma.
Unpublished subject report no. 10 d.d. 3-2-1988 from Intervet
Boxmeer. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
De JONG, H. & COERT, A. (1987) The determination of the hormonal
no-effect-level of dexamethasone in rats after 90 days of oral
dosing (NEL study). Unpublished report d.d. 28-8-1987 from Intervet
Boxmeer. Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
DRUGA. A. (1993a) Pilot teratology study (Segment II) of
dexamethasone in rats. Unpublished report no. 9218 by Institute for
Drug Research, Budapest, Hungary. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim.
DRUGA, A. (1993b) Teratology study (segment II) of dexamethsone in
rats. Unpublished report study nr. 9219 by Institute for Drug
Research, Budapest, Hungary. Submitted to WHO by Intervet
International B.V., Boxmeer, The Netherlands and Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGELHARDT, G. (1963). Pharmacological exposé on substance HE III.
Unpublished report no.: AX-U-26 d.d. 17 January 1963 from Dr. K.
Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGLISH, J., CHAKRABORTY, J. & MARKS, V. (1975) The metabolism of
dexamethasone in the rat - effect of phenytoin. J. Steroid
Biochem., 6: 65-68.
GOETH, H. & LECHNER, V. (1978) Investigation on the influence of
Voren on the course of experimental infections and their treatment
with antibiotics and sulphonamides as well as on the phagocytotic
activity of the reticulo-endothelial system (RES) and the serum
protein picture in mice. Berl. Munch. Wschr., 91: 87-93.
HORNER, M.W. (1989) Pharmacokinetics of dexamethasone in pig plasma
following subcutaneous injection of Opticortenol S. Unpublished
report d.d. November 1989, Ciba Geigy Project no. 4. from
Horseracing Forensic Labortory, Newmarket. Submitted to WHO by Ciba
Geigy, Division Agro, Basle, Switzerland.
HOUGHTON, E., GRAINGER, L. & TEALE, P. (1989) The in vitro
hydrolysis of dexamethasone trimethylacetate in whole blood from the
horse and cow. Unpublished report from Horseracing Forensic
Laboratory, Newmarket, July 1989. Submitted to WHO by Ciba Geigy,
Division Agro, Basle, Switzerland.
INTERVET, (undated I) Chronic oral toxicity study with dexamethasone
in rats. Unpublished and unsigned report from Intervet d.d. mid
1960's? Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
INTERVET, (undated II) Chronic oral toxicity study with
dexamethasone in dogs. Unpublished and unsigned report from Intervet
d.d. mid-1960's? Submitted to WHO by Intervet International B.V.,
Boxmeer. The Netherlands.
KIETZMAN, M. (1991) Report of a study in rats to investigate the
effect of dexamethasone on tyrosine aminotransferase activity in the
liver and on serum corticosterone levels. Veterinary College,
Hannover (27-1-91). Unpublished report submitted to WHO by
Boehringer Ibgelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969a) Reproduction study comparing the compound HEIII
with dexamethasone in gestating rats. Unpublished report no. AUXI
0031 from Dr Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969b) Reproduction study on the substance HEIII versus
dexamethasone in pregnant rabbits. Unpublished report d.d.
24-11-1969 no. U69-0018 from Dr Karl Thomas, GmbH, Biberach,
Germany. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
PEETS, E.A., STAM, M. & SYMCHOWICZ, C. (1969) Plasma binding of
betamethasone-3H, dexamethasone-3H, and cortisol-14C - a
comparative study. Biochem. Pharmacol., 18: 1655-1663.
RICE, M.J. TREDGER, J.M., CHAKRABORTY, J. & PARKE, D.V. (1974) The
metabolism of dexamethasone in the rat. Biochem. Soc. Transact.,
53rd Meeting, Bristol, 2: 107-109.
SEGRO, G. (1970) Expertise on HE111 in rats and rabbits. Report d.d
1-4-1970 from University of Sienna, Italy. Unpublished report no.
AX-U43 from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
SEUTTER, E. (1975) Metabolism of systematically given
corticosteroids. Dermatologica , 151: 129-134.
STEWART, F.P., KÖRÖSI, S.A. & HOPKINS, R. (1992)
[1,2,4-3H]-dexamethasone: tritium exchange following intramuscular
administration to the rat. Unpublished report 6938-806/2 d.d.January
1992 from Hazleton UK Limited, Harrogate, England. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
TOUTAIN, P.L., ALVINERIE, M. & RUCKEBUSCH, Y. (1983)
Pharmacokinetics of dexamethasone and its effect on adrenal gland
function in the dog. Am. J. Vet. Res., 44: 212-217.
UEBERBERG, H. (1963) Comparative experimental trials in dogs with
pyridine- 4-carboxylic acid (dexamethasone-21')ester (HEIII) and
dexamethasone. Unpublished report no. AX-U-3 d.d.20-12-1963 from Dr
Karl Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UEBERBERG, H. (1964) Comparative investigations in rats with
substance HEIII and dexamethasone. Unpublished report d.d. 23-3-1964
from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UMEMURA, M., KAST, A. & IIDA, H. (1972) Teratologica; testing of
substances dexamethasone and HEIII in rats. Unpublished report d.d.
3-3-1972 from Boehringer Ingelheim GmbH Pharma Research Japan.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
International Division, Ingelheim, Germany.
WALKER, B.E. (1967) Induction of cleft palate in rabbits by several
glucocorticoids. Proc. Soc. Exp. Biol. Med., 125: 1281-1284.
WALKER, B.E. (1971) Induction of cleft palate in rats with
anti-inflammatory drugs. Teratology , 4: 39-42.
WEISENBERGER, H. (1972) Species differences in the hydrolysis of
dexamethasone 21-isonicotinate by serum esterases. Klin. Wschr.,
50: 665.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Male Wistar albino rats were administered 0.23 µmol [1,2-3H]
dexamethasone/kg bw, i.p. Urine and faeces were collected up to 4
days after treatment. Within 96 hours 74% of the dose was excreted,
30% in the urine and 44% in the faeces (Rice et al., 1974).
Crl:SD(CD)BR rats were administered a single i.m. dose of 9 µg,
[1,2,4-3H]-dexamethasone/kg bw. Radioactivity was measured up to
96 hours after administration in plasma (pre- and post-freeze
dried), urine, faeces and expired air. Tritium exchange was measured
in stored urine. Highest plasma levels were observed 6 hours after
dosing (3.7 µg equivalents/g), declining rapidly thereafter to 0.15
µg equivalents/g. Within 24 hours 41% of the radioactivity was
excreted in the urine. After 96 hours a mean of 44% of the
radio-activity was excreted. Tritium exchange was observed both in
plasma and urine. Following freeze-drying, the mean loss of
radioactivity 96 hours after dosing was 87% and 37% in plasma and
urine, respectively (Stewart et al., 1992).
Dogs (mixed-breed) were administered dexamethasone alcohol or
dexamethasone 21-isonicotinate as a solution i.v. or i.m. (1 mg/kg
bw), or dexamethasone 21-isonicotinate as a suspension i.m. (0.1 or
1 mg/kg bw). Plasma concentrations were determined with HPLC up to
120 hours after treatment. The elimination half-life after i.v.
administration was 120-140 minutes for both formulations. Following
i.m. administration, absorption was rapid with peak plasma
concentrations at 30-40 minutes for both solutions. Bio-availability
after i.m. administration was 100% for dexamethasone alcohol but 40%
for dexamethasone 21-isonicotinate. After i.m. administration of
dexamethasone 21-isonicotinate as a suspension, dexamethasone was
not detected in plasma, suggesting a long absorption phase (Toutain
et al., 1983).
2.1.2 Biotransformation
2.1.2.1 In vitro
The half-life of dexamethasone 21-isonicotinate was determined
in human, rat, and rabbit sera. In rat and rabbit sera 90 and 99% of
the ester was hydrolyzed after 10 minutes, respectively. The
half-life of in human serum was about 90-100 minutes (Weisenberger,
1972).
Dexamethasone trimethylacetate was rapidly hydrolyzed to
dexamethasone in bovine and equine whole blood; half-lives ranged
from 10-30 minutes for both species (Houghton et al., 1989).
Dexamethasone dimethylbutyrate was hydrolyzed quickly in bovine
plasma, with a half-life of about 1 hour (Coert et al., 1988).
2.1.2.2 Rats
In the urine of rats administered 0.23 µmol/kg bw [1,2-3H]-
dexamethasone i.p., 10% of the administered radioactivity was
associated with one polar metabolite of dexamethasone, likely to be
6-hydroxy-dexamethasone (Rice et al., 1974).
Male Wistar albino rats were administered [3H]-dexamethasone
orally at a dose of 1.14 nmol/kg bw. Thirty-one percent of the
administered radioactivity was excreted in the urine within 4 days
(most of it within the first 24 hours) as unconjugated metabolites.
Unchanged dexamethasone accounted for 14%, 6-hydroxydexamethasone
for 7.4%, and 20-dihydrodexamethasone for 1.1% of the urine
radioactivity. Twenty-five percent of the administered dose was
eliminated in the faeces. Another group of rats was pretreated with
phenytoin 16 hours before dexamethasone treatment. The pretreatment
reduced the rate of total radioactivity excreted in the urine within
24 hours, but the total urinary and faecal excretion after 96 hours
was not significantly reduced (English et al., 1975).
2.1.2.3 Pigs
Four hours after the s.c. administration to pigs of
[1,2-3H]-dexamethasone-21-trimethylacetate, less than 1% of total
plasma radioactivity was extractable as unchanged
[3H]-dexamethasone-21-acetate. The plasma concentration of
dexamethasone was highest (about 3 ng/ml) at 4 hours, declining
rapidly to about 0.5 ng/ml at 24 hours, and slowly thereafter.
Measurable amounts of dexamethasone (>0.2 ng/ml) were still present
at day 5 (Horner, 1989).
2.1.2.4 Humans
No parent compound could be detected in urine of patients after
oral administration of a small dose of dexamethasone (<4 mg/day)
for a few weeks. However, 60% was recovered as
6-ß-hydroxy-dexamethasone and 5-10% as
6-ß-hydroxy-20-dihydrodexamethasone. After the administration of
about 15 mg dexamethasone/day metabolism occurred by an additional
route involving epoxidation and subsequent hydrolysis, resulting in
glycol formation in ring A (Seutter, 1975).
2.1.3 Special studies on macromolecular binding
The binding of dexamethasone to proteins in rat, dog, cow, and
human plasma has been studied in vitro by an equilibrium dialysis
technique. Approximately 85, 73, 74, and 77% was bound in rat, dog,
cow, and human plasma, respectively. Dexamethasone was mainly bound
to the albumin fraction of human plasma (Peets et al., 1969).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of an acute toxicity study is summarized in Table
1.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 15 rats were given daily s.c. injections of 0.5 ml
vehicle or 50 µg/kg bw dexamethasone for 6 weeks. Body- weight gain
was significantly decreased in treated rats. Adrenal weights were
also decreased. Post-mortem examination revealed no pathological
organ changes. Only a summary of this study was available
(Ueberberg, 1964).
In 3 experiments, groups of 15 rats/sex were given 0.125 mg/kg
bw (6 days/week), 0.25 mg/kg bw (5 days/week), or 0.4 mg/kg bw (5
days/week) dexamethasone in tablets for 181-185 days. The control
groups (10 rats/sex/group) received placebo tablets. All mid- and
high-dose animals received 20 mg tetracycline*HCl once a week
beginning on the 39th experimental day.
Dose-related deaths occurred as follows: 4/30 at the low-dose,
14/30 at the mid-dose and 26/30 at the top-dose. Post mortem
examination in all cases showed severe infections. In all dose
groups, body-weight gain was decreased, relative kidney weight was
increased and relative adrenal and thymus weights were decreased. In
the bone marrow the number of neutrophilic forms of leucocytes was
increased and the number of eosinophils was decreased in all
experimental groups as compared to controls. Detailed
histopathological findings of the mid- and high-dose groups were not
presented (Intervet, undated I).
Groups of 20 male and 20 female Wistar rats were administered
s.c. 0, 40, or 79 µg/kg bw/day dexamethasone for 13 weeks. An
additional group of 5 male and 5 female rats was given 79 µg/kg
bw/day dexamethasone s.c. for 13 weeks, then kept for a 7-week
recovery period. Haematological and biochemical examinations were
performed on 5 rats/sex after 10 weeks.
Table 1. Acute toxicity of dexamethasone
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M i.p. 577 Engelhardt, 1963
In all treated males, ALAT activity and total cholesterol
concentrations were increased. Lipid levels were only incidentally
raised. Plasma corticosteroid levels and hepatic glycogen were
decreased in a dose-related manner. In treated males, adrenal
glycogen levels were increased and in males as well as in females
dose-related reductions in adrenal corticosteroids were observed.
Post mortem examination found adrenal and thymus glands that were
markedly smaller with reduced weights when compared to controls. In
some animals no thymus tissue could be found. Compared to the
controls, body weights and most organ weights of treated rats were
lower. Microscopic examination revealed marked changes in the thymus
and the adrenal glands. The adrenal cortex was narrowed due to loss
of the regular structuring of the cells or cell columns, in addition
to a reduction in lipids. The thymus from the treated rats showed
atrophy of the medullary and cortical tissues. After the recovery
period no significant changes compared to the controls were observed
(Bauer et al., 1969a; results of male rats partly reported in
Segro, 1970).
2.2.2.2 Dogs
In a limited study, groups of 4 female beagle dogs were orally
administered a 2 or 8 mg dexamethasone tablet/day (6 days/week) for
26 weeks. No control group was used. Instead, values from previous
studies were used for comparison. One low-dose dog died (not related
to treatment) during the study and 3 high-dose dogs died, of which 2
were due to retro-oesophageal abcesses or gastric ulcers. At
post-mortem examination all remaining dogs were found to have
infections. Alopecia was seen in 1 dog in each dose group. Atrophy
of the lymphatic organs was seen in all dogs, adrenal weight was
decreased, and in the high-dose group the thymus had almost
disappeared (Intervet, undated II).
Groups of mongrel dogs (3 male and 2 female dogs/group) were
orally administered placebo tablets or 125 µg/kg bw/day
dexamethasone 7 days/week for 6 weeks. No treatment-related effects
were observed on clinical signs, body-weight, liver and renal
function tests, urinalysis, or post mortem examination. After 6
weeks blood glucose values were increased in treated animals.
Relative adrenal weights were decreased. In the adrenals, the zona
fasciculata was narrowed in treated dogs. Lipid observed in the
adrenal cortical zones of the controls was not present in treated
dogs. Total 17-ketosteroid excretion in the urine was higher in all
treated groups (Ueberberg, 1963).
Groups of 3 male and 3 female beagle dogs were administered
daily i.m. doses of 0, 40, or 79 µg/kg bw dexamethasone for 13
weeks. Additionally, 2 groups of 3 male and 3 female dogs were given
daily i.m. doses of 79 µg/kg bw dexamethasone for 13 weeks, then
kept for a 4-week recovery period. One female dog from the recovery
group died 14 days after cessation of dosing. No effects were
observed on food consumption or haematological parameters. Decreased
body-weight gain was observed in all treated dogs. ALAT activity was
increased in 1 out of 3 female dogs in both the 40 µg/kg bw/day and
79 µg/kg bw/day groups. Activity returned to normal after the
recovery period. Total lipid levels in serum were increased in all
treated dogs without any dose-dependence. By the end of the recovery
period total lipid levels had decreased, but were still elevated
relative to control levels in all treated dogs. Plasma corticoid
levels were decreased in dogs receiving 79 µg/kg bw/day
dexamethasone but returned to normal by the end of the recovery
period. Increased triglyceride levels in the adrenals and increased
liver glycogen were observed in all treated dogs (without
dose-relationship). The increased adrenal triglyceride levels was
still apparent after the recovery period. Liver weights were
increased in a dose-related manner and honey comb distention was
seen in some hepatic cells. These effects reversed after treatment
was ceased. In all treated dogs, the adrenal weights were lower than
in the controls. Histological examination revealed diminution of the
fascicular and reticular zones of the adrenal cortex without a clear
demarcation between the different zones. After the recovery period
the adrenal weights returned to normal. No thymus or only remnants
of the thymus were found in some treated dogs. When the thymus was
found in recovery animals, it did not differ histologically from the
controls (Bauer et al., 1969b).
2.2.3 Long-term toxicity/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available.
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
In a special study 0.15 mg dexamethasone/day (equal to 6 mg/kg
bw/day) was administered s.c. to pregnant A/J mice on days 11-14
post-conception. The mice were killed on day 18. The incidence of
cleft palate was 93% (Walker, 1971).
2.2.5.2 Rats
Groups of 20 pregnant SPF-FW 49 Biberach rats were administered
dexamathasone s.c. at dose levels of 0, 20, 40 or 79 µg/kg bw/day on
days 6-15 of gestation. Females were killed on gestation day 21. One
female died intercurrently, the cause of death could not be
resolved. All treated females failed to gain weight and had lower
food consumption during treatment, but they gained weight after
cessation of treatment. Total food consumption, however, remained
lower in all treated animals compared to controls. The mean number
of implantations was higher in all treated groups than in the
control group. Resorption rates were higher in a dose-related manner
and the number of live offspring was lower in the two highest dose
groups. Litter weight was decreased in a dose-related manner. Both
the variation and malformation rates were increased, but with no
apparent dose-relationship. Retarded ossification of the sternebrae
and hydronephrosis occurred most frequently (Lehmann, 1969a).
Daily doses of 0.05, 0.2, or 0.8 mg dexamethasone/day (equal to
250, 1000 or 4000 µg/kg bw/day) were administered s.c. to pregnant
Holtzmann rats on days 12-15 post-conception. The dams were killed
at day 19. A high frequency of cleft palate (53%) was observed in
litters of rats in the highest dose group. No cleft palates were
observed at lower doses (Walker, 1971).
Groups of 20 pregnant rats (Morini Wistar) were given daily
s.c. injections of 0, 40, or 79 µg dexamethasone/kg bw/day on days
6-15 of gestation. Maternal body-weight gain and food consumption
were decreased in all treated dams. Compared to the controls, the
resorption rate was increased and fetal weights were decreased.
Hydronephrosis was seen in 4 fetuses, 2 at 40 and 2 at 79 µg/kg
bw/day (Segro, 1970).
In a poorly reported study, groups of 10 pregnant rats (SPF,
strain SD-JCL) were given daily s.c. doses of 0, 20, 40, or 80 µg
dexamethasone/kg bw/day on days 6-15 of gestation. Mortality and
body weights were recorded. The dams were killed on day 21 of
pregnancy and the fetuses were delivered by caesarean section.
Maternal body weight was decreased in treated animals. In all
treated rats pre- and post-implantation losses were increased. No
effects were observed on fetal weight. Cleft palate was seen in one
fetus of the control group. One fetus in the 20 µg/kg bw/day dose
group had thoracoschisis. The occurrence of 14th rib was observed in
the control group (25%) as well as in all dose groups. There was no
marked difference relative to controls, but a slight dose-related
effect was observed (incidences ranged from 20 to 28.4% in the
treated groups). Deformity of the sternum was observed in 1/85 rats
in the 20 µg/kg bw/day dose group (Umemura et al., 1972).
In a pilot study groups of Virgin Lati:Han Wistar pregnant rats
(10/group) were orally administered (by gavage) doses of 0, 10, 50,
250, or 1250 µg dexamethasone/kg bw/day on days 7 to 16 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 21 and fetuses were delivered
by caesarean section. Observations included the number of corpora
lutea, implantations, fetus and placenta weight, and sex of viable
fetuses. All fetuses were examined for skeletal and visceral
abnormalities.
Maternal body-weight gain was decreased at doses of 50 µg/kg
bw/day and above and thymus involution was observed at the same dose
levels. In the highest dose group, post-implantation mortality was
increased. Most fetuses that died within the last 24 hours were
malformed. Fetal weight was decreased at doses of 250 and 1250 µg/kg
bw/day. In the 2 highest dose groups retrognathia and cleft palate
of variable severity were observed. Hydrops fetalis and umbilical
hernia was found only at 1250 µg/kg bw/day. Thymus hypoplasia was
observed at 50, 250, and 1250 µg/kg bw/day (4, 2, and 59%,
respectively). The NOEL in this study was 10 µg/kg bw/day (Druga,
1993a).
In the main Segment II study, groups of Virgan Lati:Han Wistar
rats were orally administered (by gavage) 0, 20, 200, or 1000 µg
dexamethasone in methylcellulose/kg bw/day on days 6 to 15 of
gestation. Mortality, body weight, and food consumption were
recorded. All dams were killed on day 20. The visera of the dams
were examined grossly and the thymus was removed and weighed.
Observations included the number of corpora lutea, implantations,
length of umbilical cord, fetus and placenta weight, and sex of
viable fetuses. All fetuses received external, skeletal, and
visceral examinations.
Maternal body weight and body-weight gain and food consumption
were decreased at 200 and 1000 µg/kg bw/day. Thymus involution was
observed at the same dose levels. Post-implantation mortality was
increased at the highest dose. Fetal weight was decreased at 200 and
1000 µg/kg bw/day. Umbilical cord length was reduced at 200 and 1000
µg/kg bw/day and the length, thicknesss, and index of the femur were
markedly lower at 1000 µg/kg bw/day. High-dose fetuses showed an
increased incidence of malformations, including hydrops fetalis,
retrognathia, cleft palate, umbilical hernia of variable severity,
split sternum, malformed vertebrae, malformed upper limb bones, and
micromelia. Thymus hypoplasia was observed at 20, 200, and 1000
µg/kg bw/day (4, 2, and 16%, respectively). In the high-dose group,
dystrophy of gonads was also observed. A no-effect level was not
observed in this study (Druga, 1993b).
2.2.5.3 Rabbits
Dexamethasone, as one glucocorticoid in a series of 6, was
administered i.m. at dose levels ranging from 0.1 to 4 mg/day (equal
to 25 to 1000 µg/kg bw/day) to pregnant rabbits on days 13.5-16.5
post-conception. Litter resorptions were observed at 750 and 1000 µg
dexamethasone/kg bw/day. Cleft palate was observed at >62 µg/kg
bw/day. No effects on resorption or cleft palate were observed at 25
µg/kg bw/day (Walker, 1967).
In a comparative study, groups of 15 pregnant rabbits ("SPF
Himalyan"/Biberach) were administered s.c. 0, 20, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. The dams were
killed on day 29 of pregnancy. During dosing maternal body weight
remained stationary or was reduced, particularly in the second half
of the dosing period. A dose-related increase in resorption rate and
number of runts was observed. A dose-related decrease in fetal
weight was also observed. The incidence of flexure of the forefeet
and of malformations (palatoschisis, gastroschisis, exencephaly,
encephalocele and menigocele, anotia, and ectrodactyly) was
increased in a dose-related manner in all treatment groups.
Malformations of the extremities such as haemibrachia, hypoplasia of
tibia and fibula, and acheiria were observed (Lehmann, 1969b).
In a similar study, groups of 15 pregnant New Zealand white
rabbits were given daily s.c. injections of 0, 40, or 79 µg
dexamethasone/kg bw/day on days 6-18 of gestation. Compared to the
control group all treated dams had reduced body weight and their
resorption rates were increased. No treatment-related effect on
gross malformations was observed (Segro, 1970).
2.2.6 Special studies on endocrine toxicity
Groups of Cpb:WU rats (10/sex/group) were orally administered
(by gavage) doses of 0, 0.3, 1, 3, 10, 30, or 100 µg dexamethasone/
kg bw/day for 90 days. Observations included clinical signs, body
weight, water consumption, haematology, IgG/IgM determinations,
gross post mortem examination, adrenal and thymus weight,
histopathology, ACTH stimulation test (4 rats/sex/group), and
corticosteroid determinations.
Significant decreases in body-weight gain (more pronounced in
males than in females) were observed at doses of >10 µg/kg
bw/day. In males of the same groups sluggishness and erected fur
were observed, which were considered to be treatment-related. WBC
and differential WBC counts were significantly decreased in rats at
>10 µg/kg bw/day. WBC counts were also significantly reduced in
females at 3 µg/kg bw/day. IgG and IgM levels were significantly
decreased at 100 µg/kg bw/day. Decreases in adrenal and thymus
weights were observed and accompanied by histopathological changes,
including atrophy and structural disorganization, at >10 µg/kg
bw/day. In the same dose groups dose-related decreases in
corticosterone levels with or without ACTH stimulation were
observed. The Committee concluded that the NOEL in this study was at
1 µg/kg bw/day (De Jong & Coert, 1987).
Rats (6/group) were administered a dose of 0, 0.5, 1, 1.5, 2,
or 4 µg dexamethasone/kg bw/day by gavage for 1 and 7 days. Five
hours after the last application the rats were killed and blood and
liver samples were taken. Tyrosine amino transferase (TAT) activity
in the supernatant of liver homogenates was measured and serum
corticosterone concentrations were determined. TAT activity was
increased in a dose-dependent manner at 2 and 4 µg/kg bw/day and a
significant decrease in serum corticosterone was seen at 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day (Kietzman, 1991;
Bette & Kietzmann, 1991).
2.2.7 Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
2.2.8 Special studies on immune response
The influence of dexamethasone-21-isonicotinate on the course
of experimental bacterial infections of mice and their therapy, on
the phagocytotic activity of the reticulo-endothelial system and the
serum protein picture was investigated. No clear effect on
antibiotic therapy was observed. Dexamethasone-21-isonicotinate
decreased the activity of Celasin C and penicillin G-procain against
streptococcal infections and improved the activity of both
substances against staphylococcal infections. No quantitative effect
was observed upon serum albumin, alpha1, alpha2, ß1, ß2 or
gamma-globulins of mice both 48 hours after a single s.c. treatment
or 24 hours after 5 days of treatment (once a day s.c.) with 75,
150, or 300 µg dexamethasone 21-isonicotinate/kg bw (Goeth &
Lechner, 1978).
2.3 Observations in humans
The corticosteroid suppressive effect of dexamethasone is well
known and used for the definitive diagnosis of Cushing syndrome in
human patients. People with Cushing syndrome suffer from a chronic
over-production of cortisol resulting from an over-production of
ACTH. In the "low- and high-dose dexamethasone suppression test" the
subjects receive 0.5 or 2 mg dexamethasone orally every 6 hours for
Table 2: Results of genotoxicity assays on dexamethasone and
dexamethasone-21-isonicotinate
Test system Test object Concentration Purity Results Reference
In vitro
Ames testa S.typhimurium 10-1000 99% negativec Baumeister,
TA98, 100, µg/pl 1988a
1535, 1537
E. coli , WP2
Fluctuation Mouse 12.5-400 99.4% negativee Clements,
assayb,d lymphoma µg/ml 1992
L5178Y cells
In vivo
Micronucleus NMRI mice i.v. 5 mg/kgf 97.5% negativeg Baumeister,
testsa 1988b
a Test substance: dexamethasone 21-isonicotinate.
b With and without metabolic activation.
c Appropriate positive controls were used.
d Test substance: dexamethasone.
e 4-Nitroquinoline and benzo(a)pyrene were used as positive controls.
f The initial dosage (107.5 mg/kg) had to be reduced due to acute
toxicity (tonic convulsions, deaths) of the excipient 1,
2-propylenglycol.
g Cyclophosphamide was used as positive control.
2 consecutive days. In normal healthy persons cortisol production,
determined as 17-hydroxycorticosteroid or 17-ketosteroid excretion
in urine, is suppressed, whereas in patients with Cushing syndrome
cortisol production is not suppressed. No significant clinical side
effects of this dexamethasone suppression test were reported (Crapo,
1979).
3. COMMENTS
Information from studies on dexamethasone, including data on
kinetics, metabolism, acute and short-term toxicity, developmental
toxicity, and genotoxicity, was available for assessment.
Toxicokinetic studies revealed rapid absorption after i.m.
administration to dogs and rats with peak plasma levels found after
30 minutes and 6 hours, respectively. Dexamethasone is rapidly
excreted in urine and faeces. Dexamethasone esters are rapidly
hydrolyzed in serum. Biotransformation in rats and humans is
comparable and involves mainly hydroxylation to 6-hydroxy- and
20-dihydro-dexamethasone. However, there was additional evidence
that at high (therapeutic) doses in people, dexamethasone is
metabolized by an additional route involving epoxidation.
Following repeated oral administration of dexamethasone to rats
and dogs in short-term toxicity studies the main target organs were
the thymus and the adrenal gland. Corticosteroid concentrations in
plasma and hepatic glycogen were reduced, whereas serum lipid levels
were increased. In rats dosed orally with 0.3, 1, 3, 10, 30, or 100
µg dexamethasone/kg bw/day for 90 days, thymus involution and
morphological changes in the adrenal gland and a decrease in
corticosterone and white blood cell counts were observed in male and
female rats at doses above 10 µg/kg bw/day. Due to the decrease in
white blood cell counts in female rats at 3 µg/kg bw/day this dose
was considered to be a marginal effect level. In a study with rats
orally dosed with 0.5, 1, 1.5, 2, or 4 µg/kg bw/day dexamethasone
for 7 days the corticosterone concentration was reduced in the
highest-dose group and the activity of tyrosine aminotransferase in
the liver was increased in a dose-related manner at 2 and 4 µg/kg
bw/day. The NOEL in this study was 1.5 µg/kg bw/day.
No reproduction studies with dexamethasone were available but
an increase in pre- and post-implantation loss and a reduction in
fetal weight were observed in teratogenicity studies in mice, rats,
and rabbits receiving dexamethasone by injection. In these studies
malformations such as hydrops fetalis, cleft palate, exencephaly,
and encephalocele were observed at maternally toxic dose levels.
In oral teratogenicity studies with rats using dose levels
ranging from 10 to 1250/kg bw/day, maternal toxicity was found at 50
µg/kg bw/day and above. At doses at and above 1000 µg/kg bw/day,
dexamethasone caused structural malformations (hydrops fetalis,
cleft palate). Thymus involution and a decrease in body weight were
observed in fetuses, resulting in an overall NOEL for embryotoxicity
in rats of 10 µg/kg bw/day.
Long-term toxicity/carcinogenicity data were not available.
4. EVALUATION
Based on its long history of use in human medicine and because
dexamethasone was negative in in vitro gene mutation assays with
bacteria and mammalian cells and in an in vivo micronucleus test
with mice, the Committee was not concerned about the carcinogenic
potential of dexamethasone.
Using a safety factor of 100 the Committee established an ADI
of 0-0.015 µg/kg bw/day for dexamethasone based on a NOEL of 1.5
µg/kg bw/day for the induction of tyrosine aminotransferase activity
in rat liver. Due to the careful selection of the dose levels in
this study the Committee did not round this figure.
5. REFERENCES
BAUER, O., LEHMANN, & UEBERBERG, H. (1969a) Rat study to screen
pyridine-4-carboxylic acid-(dexamethasone-21')-ester (HE 111) for
subacute toxicity in comparison with dexamethasone. Unpublished
report d.d. 12 June 1969 from Dr. K. Thomas GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUER, O., PAPPRITZ, & UEBERBERG, H. (1969b) Investigation into the
subacute toxicity of pyridine-4-carboxylic acid (dexamethasibe-21')
ester (HE 111) in comparison with dexamethasone in dogs. Unpublished
report No. AX-U-30 d.d.21-4-1969 from Dr. Karl Thomas, GmbH,
Biberach, Germany. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988a) Mutagenicity study with HE III XX (Voren) in
the S. typhimurium and E. coli/mammalian microsome assay (Ames
Test). Unpublished report no. Gen Tox. 39/87 d.d. 5 February 1988
from Dr. K. Thomas, GmbH, Dept. of Experimental Pathology and
Toxicology, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BAUMEISTER, M. (1988b) Mutagenicity study with HE III XX (Voren) in
the bone marrow micronucleus assay. Unpublished report no. Gen Tox.
40/87 d.d. 8 February 1988 from Dr. K. Thomas, GmbH, Dept. of
Experimental Pathology and Toxicology, Biberach, Germany. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
BETTE, P. & KIETZMANN, M. (1991) Effect of dexamethasone on tyrosine
amino-transferase activity in rat liver - a sensitive test to define
its hormonal no-effect-level. Acta Vet. Scand., 87: 200-202.
CRAPO, L. (1979) Cushings syndrome: A review of diagnostic tests.
Metabolism , 28: 955-977.
CLEMENTS, J. (1992) Study to determine the ability of dexamethasone
to induce mutations at the thymidine kinase (tk) locus in mouse
lymphoma L5178Y cells using a fluctuation assay. Unpublished report
no. 2TKREBSG.001 from Hazleton Microtest Limited, York, England.
Submitted to WHO by Intervet International B.V., Boxmeer, The
Netherlands.
COERT, A., HOEIJMAKERS, M., VAN RENS, P. (1988) The in vitro
hydrolysis of dexamethasone dimethylbutyrate in cows plasma.
Unpublished subject report no. 10 d.d. 3-2-1988 from Intervet
Boxmeer. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
De JONG, H. & COERT, A. (1987) The determination of the hormonal
no-effect-level of dexamethasone in rats after 90 days of oral
dosing (NEL study). Unpublished report d.d. 28-8-1987 from Intervet
Boxmeer. Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
DRUGA. A. (1993a) Pilot teratology study (Segment II) of
dexamethasone in rats. Unpublished report no. 9218 by Institute for
Drug Research, Budapest, Hungary. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim.
DRUGA, A. (1993b) Teratology study (segment II) of dexamethsone in
rats. Unpublished report study nr. 9219 by Institute for Drug
Research, Budapest, Hungary. Submitted to WHO by Intervet
International B.V., Boxmeer, The Netherlands and Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGELHARDT, G. (1963). Pharmacological exposé on substance HE III.
Unpublished report no.: AX-U-26 d.d. 17 January 1963 from Dr. K.
Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
ENGLISH, J., CHAKRABORTY, J. & MARKS, V. (1975) The metabolism of
dexamethasone in the rat - effect of phenytoin. J. Steroid
Biochem., 6: 65-68.
GOETH, H. & LECHNER, V. (1978) Investigation on the influence of
Voren on the course of experimental infections and their treatment
with antibiotics and sulphonamides as well as on the phagocytotic
activity of the reticulo-endothelial system (RES) and the serum
protein picture in mice. Berl. Munch. Wschr., 91: 87-93.
HORNER, M.W. (1989) Pharmacokinetics of dexamethasone in pig plasma
following subcutaneous injection of Opticortenol S. Unpublished
report d.d. November 1989, Ciba Geigy Project no. 4. from
Horseracing Forensic Labortory, Newmarket. Submitted to WHO by Ciba
Geigy, Division Agro, Basle, Switzerland.
HOUGHTON, E., GRAINGER, L. & TEALE, P. (1989) The in vitro
hydrolysis of dexamethasone trimethylacetate in whole blood from the
horse and cow. Unpublished report from Horseracing Forensic
Laboratory, Newmarket, July 1989. Submitted to WHO by Ciba Geigy,
Division Agro, Basle, Switzerland.
INTERVET, (undated I) Chronic oral toxicity study with dexamethasone
in rats. Unpublished and unsigned report from Intervet d.d. mid
1960's? Submitted to WHO by Intervet International B.V., Boxmeer,
The Netherlands.
INTERVET, (undated II) Chronic oral toxicity study with
dexamethasone in dogs. Unpublished and unsigned report from Intervet
d.d. mid-1960's? Submitted to WHO by Intervet International B.V.,
Boxmeer. The Netherlands.
KIETZMAN, M. (1991) Report of a study in rats to investigate the
effect of dexamethasone on tyrosine aminotransferase activity in the
liver and on serum corticosterone levels. Veterinary College,
Hannover (27-1-91). Unpublished report submitted to WHO by
Boehringer Ibgelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969a) Reproduction study comparing the compound HEIII
with dexamethasone in gestating rats. Unpublished report no. AUXI
0031 from Dr Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
LEHMANN, (1969b) Reproduction study on the substance HEIII versus
dexamethasone in pregnant rabbits. Unpublished report d.d.
24-11-1969 no. U69-0018 from Dr Karl Thomas, GmbH, Biberach,
Germany. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim, Germany.
PEETS, E.A., STAM, M. & SYMCHOWICZ, C. (1969) Plasma binding of
betamethasone-3H, dexamethasone-3H, and cortisol-14C - a
comparative study. Biochem. Pharmacol., 18: 1655-1663.
RICE, M.J. TREDGER, J.M., CHAKRABORTY, J. & PARKE, D.V. (1974) The
metabolism of dexamethasone in the rat. Biochem. Soc. Transact.,
53rd Meeting, Bristol, 2: 107-109.
SEGRO, G. (1970) Expertise on HE111 in rats and rabbits. Report d.d
1-4-1970 from University of Sienna, Italy. Unpublished report no.
AX-U43 from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
SEUTTER, E. (1975) Metabolism of systematically given
corticosteroids. Dermatologica , 151: 129-134.
STEWART, F.P., KÖRÖSI, S.A. & HOPKINS, R. (1992)
[1,2,4-3H]-dexamethasone: tritium exchange following intramuscular
administration to the rat. Unpublished report 6938-806/2 d.d.January
1992 from Hazleton UK Limited, Harrogate, England. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
TOUTAIN, P.L., ALVINERIE, M. & RUCKEBUSCH, Y. (1983)
Pharmacokinetics of dexamethasone and its effect on adrenal gland
function in the dog. Am. J. Vet. Res., 44: 212-217.
UEBERBERG, H. (1963) Comparative experimental trials in dogs with
pyridine- 4-carboxylic acid (dexamethasone-21')ester (HEIII) and
dexamethasone. Unpublished report no. AX-U-3 d.d.20-12-1963 from Dr
Karl Thomas, GMBH, Biberach, Germany. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UEBERBERG, H. (1964) Comparative investigations in rats with
substance HEIII and dexamethasone. Unpublished report d.d. 23-3-1964
from Dr. Karl Thomas, GmbH, Biberach, Germany. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
UMEMURA, M., KAST, A. & IIDA, H. (1972) Teratologica; testing of
substances dexamethasone and HEIII in rats. Unpublished report d.d.
3-3-1972 from Boehringer Ingelheim GmbH Pharma Research Japan.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
International Division, Ingelheim, Germany.
WALKER, B.E. (1967) Induction of cleft palate in rabbits by several
glucocorticoids. Proc. Soc. Exp. Biol. Med., 125: 1281-1284.
WALKER, B.E. (1971) Induction of cleft palate in rats with
anti-inflammatory drugs. Teratology , 4: 39-42.
WEISENBERGER, H. (1972) Species differences in the hydrolysis of
dexamethasone 21-isonicotinate by serum esterases. Klin. Wschr.,
50: 665.
