
ENROFLOXACIN
First draft prepared by
Dr Louis T. Mulligan
Center for Veterinary Medicine
Food and Drug Administration, Rockville, Maryland, USA
1. EXPLANATION
Enrofloxacin is a quinolone carboxylic acid derivative with
antimicrobial action. Enrofloxacin is most effective against gram-
negative bacteria and is indicated for infections of the respiratory,
gastrointestinal and urinary tracts in cattle, pigs and poultry.
Enrofloxacin is bactericidal through inhibition of DNA-gyrase.
Enrofloxacin has not been previously evaluated by the Joint FAO/WHO
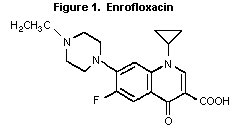
Expert Committee on Food Additives. The structure of enrofloxacin is
shown in Figure 1.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The following information was provided in a summary report of
metabolism and residue data on enrofloxacin. The full study reports
were not provided.
2.1.1.1 Rats
Rats (number, species, strain not specified) were treated orally
or intravenously with a single dose (5 mg/kg bw) of radiolabelled
enrofloxacin. The bioavailability was calculated to be approximately
75%. Enrofloxacin was rapidly absorbed and distributed to all
tissues. Highest concentrations were found in the liver and kidney,
followed by muscle. Fat did not contain appreciable amounts of
radioactivity. Elimination was rapid via urine and faeces. Most of
the radioactivity was excreted during the first 24 hours after
administration. About 40% of the radioactivity was excreted in bile.
The radioactivity in urine was attributed primarily to the unchanged
parent drug, its de-ethylated metabolite, ciprofloxacin, and the
glucuronide of enrofloxacin. Bile contained mainly the unchanged
parent molecule (Altreuther & Klostermann, 1994).
2.1.1.2 Cattle, pigs, chickens, and turkeys
Trials in all species showed that enrofloxacin is absorbed
readily and distributes to all organs and tissues following oral or
parenteral administration. In pigs, when the same dose was
administered by the oral or i.m. route, similar rates of absorption
and subsequent concentrations of drug in tissues were observed.
Following parenteral administration, maximum serum concentrations in
pigs and calves were reached within 1 to 2 hours. The highest drug
concentration initially occurred in the liver in all species.
Following a one-day withdrawal in poultry, highest drug concentrations
were found in the skin. In all species studied, elimination was
primarily via the urine and bile/faeces (Altreuther & Klostermann,
1994).
2.1.2 Biotransformation
2.1.2.1 Rats, cattle, pigs, and poultry
Rats (number, species, strain not specified) were treated orally
with 165 mg/kg bw of radiolabelled enrofloxacin for 3 consecutive
days, then with 50 mg/kg bw on the fourth day. Major urinary
metabolites were enrofloxacin (30-36%), ciprofloxacin (26- 31%), and
enrofloxacin glucuronide (19-29%). Four other components were
identified, each less than 2% of the total residue (Altreuther &
Klostermann, 1994).
In food animal species, ciprofloxacin was the main metabolite
recovered. The liver is considered the primary site of enrofloxacin
metabolism in these species, forming ciprofloxacin through oxidative
dealkylation. Additional metabolites occurred but comprised less than
10% of the total residue (Altreuther & Klostermann, 1994).
2.1.3 Effects on enzymes and other biochemical parameters
The bactericidal in activity of quinolones, including
enrofloxacin, is attributed to their ability to inhibit DNA gyrase
(Mandell & Sande, 1990).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of enrofloxacin is summarized in Table 1.
Table 1. Acute toxicity studies on enrofloxacin
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse male p.o. > 5000 Schmidt, 1985
female 4336
male i.v. 225 Schmidt, 1985
female 220
Rat male p.o. > 5000 Schmidt, 1985
female
Rabbit male p.o. 500-800 Schmidt, 1985
female
male topical > 2000 Eigenberg,1987
female
Dog male p.o. n/a1 Schmidt, 1985
female
1 Not determined due to vomiting of the drug.
Animals were observed daily for 14 days post-exposure in these
studies. Clinical signs associated with enrofloxacin toxicosis
included lethargy, trembling, tonic convulsions, dyspnea, prone and
lateral decubitus, and ataxia. Clinically affected animals that did
not die recovered within 15 minutes to 7 days post-exposure. Gross
post-mortem lesions reported included pulmonary congestion and
haemorrhage.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of Charles River rats (15/sex/group) were administered
diets containing 0, 500, 2000, or 7500 mg enrofloxacin/kg feed (equal
to 36, 150, or 577 mg/kg bw/day for males and 45, 182, or 690 mg/kg
bw/day for females, respectively) for 90 days. No overt effects on
appearance and behaviour were noted at any dose level. Body-weight
gain was reduced at the highest dose in both males (20%) and females
(23%), while the actual diet consumption for males and females
remained constant.
Serum chemistry findings included a significant dose-related
decrease in total plasma protein in the mid- and high-dose males and
females at 6 and 13 weeks. Total bilirubin and alanine aminotrans-
ferase were decreased in the males at 6 weeks but were normal at 13
weeks. Aspartate aminotransferase was decreased in the high-dose
males at 6 and 13 weeks. Globulins were decreased in the high-dose
males at 6 weeks and 13 weeks and high-dose females at 13 weeks. A
corresponding increase in the A/G ratio was seen in the high-dose
males at 6 and 13 weeks.
Haematology findings were unremarkable.
Urinalyses showed a significant decrease in urine sodium output
for males in the mid- and high-dose groups after 6 weeks and for
females in the mid- and high-dose groups at 13 weeks.
Treatment-related gross findings included swollen ears (2 males
and 4 females in the mid-dose group, 4 males and 5 females in the
high-dose group, and 1 female in the control group) and caecal
distension (1 male in the mid-dose group and 3 males and 5 females in
the high-dose group).
Mean and relative prostate weights were statistically
significantly reduced in the mid- and high-dose groups. Mean heart
weights were statistically significantly reduced in high-dose males
and in mid- and high-dose females. Mean liver weights were reduced in
both male and female high-dose animals. The reduction was
statistically significant in females.
Histological findings included auricular chondropathy which was
present in all groups except low-dose males and appeared to be dose-
related (1 control, 1 low dose, 6 mid dose, and 10 high dose). In the
testes of 12/15 rats from the high-dose group, dark, round or oval
cells were distributed among the spermatozoa in the ducts of the
epididymides. These cells were occasionally observed in seminiferous
tubules of 5/15 high-dose rats. These findings were considered
treatment-related.
Based on decreased body-weight gain and microscopic changes
observed in the ducts of the epididymides and testes in the high-dose
group, and gross and microscopic changes indicating auricular
chondropathy in the mid- and high-dose groups, the NOEL was 500 mg/kg
feed, equal to 36 mg/kg bw/day (Kowalski et al., 1985).
A special study was performed in male rats to further assess the
testicular toxicity seen in previous studies. A 13-week recovery
period was included in the study. Thirty male Crl:CD Charles River
rats per group were dosed with 0, 125, 500, or 7500 mg enrofloxacin/kg
feed (equal to 10, 38, or 615 mg/kg bw/day) for 90 days. Fifteen
animals/group were killed on day 91 and the remaining 15 on day 181.
An apparent dose-related decrease in epididymal weights was observed
in all dose groups at 91 and 181 days. The difference achieved
statistical significance (compared to controls) in the mid- and high-
dose groups at both time points. Relative mean testes weight was
significantly decreased in the high-dose group after 91 days.
Bilateral testicular atrophy was seen in two high-dose rats at 181
days. Abnormal spermatozoa were seen at day 91 in the mid- and high-
dose groups but not after the recovery period. The NOEL for this
parameter was 125 mg/kg feed, equal to 10 mg/kg bw/day (Bare et al.,
1988).
2.2.2.2 Dogs
Four groups (4/sex/group) of beagle dogs (3-4 months old, 2.6 to
4.8 kg bw) were administered enrofloxacin in the diet at 0, 100, 320
or 2500 mg/kg feed (equal to 3, 9.6, or 75 mg/kg bw/day, respectively)
for 91 days.
No dogs died during the study. Sporadic incidences of mild
diarrhoea and vomiting were reported early in the study. Vomiting
occurred 5, 6, and nearly 24 hours after ingestion of the test
article. Severe hyperextension (upon manipulation) of carpal joints,
hypoactivity, and abnormal gate or posture, were observed in all
animals in the high-dose group during weeks 1 and 2. Radiographic
examination (8 dogs total) revealed several abnormalities including
valgus deformity at the carpus, asymmetry of the distal ulnar physis,
remodelling of the radial carpal bone, radius curvus, and increased
width of the radial humeral joint. The consulting radiologist
concluded that only the radial carpal remodelling appeared to be dose-
related, and neither bone growth nor density were significantly
affected.
Serum chemistry and haematologic findings were unremarkable.
However, two types of unidentified crystals in urine samples from dogs
in the 2500 mg/kg feed group were considered treatment-related.
Gross postmortem findings included an increase in mean absolute
and relative testicular weights for treated animals when compared to
control animals. However, a clear dose-response relationship was not
established and none of the differences were statistically significant
when compared to controls. In 8/8 high-dose animals and 2/8 mid-dose
animals joint lesions were observed grossly as superficial (0.2 to 0.4
cm in diameter) erosions with dulling of the surfaces on the femoral
head of the hip joint and/or the femoral condyles of the knee joint.
Histologically, joint lesions were characterized by splitting of the
articular cartilage with disorganization of chondrocytes and formation
of "brood capsules" of cartilage cells. Necrosis and disintegration
of hyaline cartilage at the area of splitting was also noted.
There was marked variation in the microscopic appearance of
testes from animal to animal with respect to the stage of maturity,
diameter of the lumen of seminiferous tubules, and vacuolar change in
cells lining the tubules. However, it was not clear whether these
testicular changes were treatment-related because there was no dose-
response relationship in incidence or severity. These variations in
testicular morphology could represent normal variation in the maturing
process of this organ in this species. Due to the uncertainty of the
significance of the testicular findings in the low-dose males in this
study, the Committee concluded that a no-effect level was not observed
(Porter et al., 1987).
In another 90-day dietary study in beagle dogs, 4 groups
(4/sex/group) of adult dogs (12-13 months old weighing 6.4 to 12.5 kg)
received enrofloxacin at 0, 320, 800 or 2000 mg/kg diet (equal to 9.3,
22, or 53 mg/kg bw/day for males, and 8.9, 23, or 51 mg/kg bw/day for
females, respectively). No joint or testicular lesions were reported
in this study (Porter et al., 1985).
A special limited 90-day dietary study in males was performed.
Five groups (4 dogs/group) of 3-month old beagle dogs (weighing 4.1 to
5.7 kg) received enrofloxacin at 0, 10, 20, 40, or 3200 mg/kg diet
(equal to 0, 0.3, 0.6, 1.2, or 92 mg/kg bw/day, respectively). At
termination of the study (day 92) testicular weights were determined
and testes and epididymides collected for histopathological
assessment. No other tissues were collected.
Histologically, the tissues examined contained wide variations in
morphology attributable to differences in sexual maturation. Sections
of epididymides examined were considered normal. Bilateral testicular
degeneration characterized by multinucleated giant cells and cells
with large cytoplasmic and nuclear volumes, sometimes in mitosis, were
reported in the seminiferous tubules of one dog at the high dose.
Seminiferous tubules lined with single layers of spermatogonial cells
and forming open lumens were present in 2/4 dogs in the 10 mg/kg feed
group and 3/4 dogs in each of the 20, 40, and 3200 mg/kg feed groups.
This change was also reported in one control animal. Spermatocytic
giant cells were reported in 0, 1, 2, 2 and 3 dogs from the 0, 10, 20,
40 and 3,200 mg/kg feed groups, respectively.
The author attributed the seminiferous tubule changes to normal
variation in sexual maturity because similar morphology was reported
in the testes of one control dog. The author used this finding to
support a NOEL of 100 mg/kg feed in the 90-day study reported above
(Porter et al., 1987). The Committee concluded that a NOEL for
testicular effects in this special, limited 90-day study could not be
determined due to the inability to determine whether variations in
testicular morphology were normal or due to treatment with the test
article (Porter et al., 1988a).
Three-month old male beagle dogs (4/group) were administered
enrofloxacin at 0, 10, or 40 mg/kg diet (equal to 0.3 or 1.2 mg/kg
bw/day) for 90 days, then maintained on control diets for an
additional 91 days before being killed. The protocol was intended to
evaluate the potential for enrofloxacin testicular toxicity to appear
after animals reach sexual maturity, following exposure at an immature
age (delayed testicular toxicity).
No signs of clinical toxicity were observed nor was body-weight
gain or food consumption affected. Gross and microscopic examination
of testes and epididymides showed no changes indicative of toxicity.
Testes and epididymides appeared mature in all treated animals and
contained normal, mature spermatozoa. The NOEL for delayed testicular
toxicity was 40 mg/kg diet, equal to 1.2 mg/kg bw/day, the highest
dose tested (Porter et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
B6C3F1 mice (60/sex/group) received diets containing 0, 1000,
3300, or 10 000 mg enrofloxacin/kg feed (equal to 323, 1100, or 3520
mg/kg bw/day for males and 373, 1210, or 3700 mg/kg bw/day for
females, respectively) for 24 months. An interim sacrifice
(10/sex/group) was performed after one year. An additional dose group
(10 mice/sex) was administered 20 000 mg enrofloxacin/kg feed (equal
to 8030 mg/kg bw/day for males and 8010 mg/kg bw/day for females,
respectively) and scheduled for interim sacrifice after one year.
During the treatment phase of the study, general appearance and
physical examination findings were within normal limits in all treated
groups. Treatment-related ophthalmological findings included a marked
increase (relative to control animals) in the incidence of focal
lenticular opacity in males and females at 10 000 mg/kg feed.
Histopathological examination of eyes revealed no treatment-related
effects. However, it was noted that focal lenticular changes are only
verifiable to a limited extent with usual histolopathological methods.
Clinical chemical and haematological evaluations were performed
on 10 animals/sex/dose after 12 and 24 months on test. Alkaline
phosphatase levels were significantly lower in females at 3300 mg/kg
feed at 12 months, and at 10 000 mg/kg feed at study termination.
Concurrent with these findings, alanine aminotransferase and aspartate
transaminase values were within normal limits. Total protein was
decreased in males and females at all treatment levels as a result of
decreased globulin fractions.
Haematological analyses revealed a significant decrease in white
blood cell counts in males at 10 000 mg/kg feed at 12 months and at
3300 mg/kg feed at study termination. In females, a significant
decrease in white blood cell counts was noted at 10 000 mg/kg feed at
study termination. At 10 000 mg/kg feed, decreases were seen in
haematocrit, MCV, and MCH values in both sexes, and in the haemoglobin
value in males, at study termination.
Gross necropsy findings included a dose-related incidence of
caecal dilation in males at 1000, 3300, and 10 000 mg/kg feed and in
females at 3300 and 10 000 mg/kg feed. Histopathological examinations
showed neither an increase in the incidence of, nor a decrease in the
time of appearance to tumours in the dosed groups compared to the
controls. Bile duct hyperplasia was seen at 3300 mg/kg feed in males
and 10 000 mg/kg feed in both sexes. Focal papillary mucosal
hyperplasia of the gall bladder was also present in males and females
in these same dose groups. There was no evidence of carcinogenicity
in this study. The NOEL was 1000 mg/kg feed, equal to 323 mg/kg
bw/day (Bomhard et al., 1991a).
2.2.3.2 Rats
A combined long-term toxicity (52 weeks) and carcinogenicity (104
weeks) study of enrofloxacin was conducted in Wistar rats (BOR:WISW).
In the chronic study, 10 rats/sex/group received diets containing 0,
770, 2000, 6000, or 10 000 mg enrofloxacin/kg feed (equal to 0, 41,
103, 338, or 856 mg/kg bw/day for males and 0, 58, 146, 466, or 1000
mg/kg bw/day for females, respectively). In the carcinogenicity
study, 50 rats/sex/group received diets containing 0, 770, 2000, or
6000 mg enrofloxacin/kg feed (equal to 0, 41, 103, or 338 mg/kg bw/day
for males and 0, 58, 146, or 466 mg/kg bw/day for females).
Mortality rates, daily observations, and ophthalmologic findings
in treated animals were comparable to controls. Body-weight gain was
decreased in males in the 6000 mg/kg feed group and in males and
females at 10 000 mg/kg feed. Feed and water intakes were increased
in males and females at 10 000 mg/kg feed. Water intake was also
elevated at 6000 mg/kg feed.
Serum chemistry evaluations were performed after 6, 12, 18, and
24 months. Notable findings included a significant decrease in total
protein in males in all dose groups at all sampling periods. Total
protein was also significantly decreased in females in the high-dose
group both at 6 months and at the end of 2 years, and at 2000 and 10
000 mg/kg feed after 1 year. The albumin value was increased in
males at 10 000 mg/kg feed after 1 year, at 6000 mg/kg feed after 18
months, and in the two highest-dose groups (2000 and 6000 mg/kg feed)
at the end of 2 years. Bilirubin was significantly decreased in
females in all dose groups at the 6-month sampling time only.
Protein electrophoresis performed after 12, 18, and 24 months
showed a decrease in gamma-globulin in males and females in all dose
groups. The author noted that this finding is not unexpected in
animals receiving antimicrobials, as they lower the background
incidence of common pathogens, resulting in a decrease in antigenic
stimulation.
Haematology evaluations were performed after 6, 12, 18, and 24
months. At doses of 2000 mg/kg feed and above, there were
statistically significant and sometimes dose-related decreases in RBC
counts in males and haemoglobin and haematocrit values in both sexes
at various sampling times. However, the values for these parameters
in both sexes in all dose groups were comparable to controls at the
terminal sampling period. After 6 months leucocyte counts in both
sexes were decreased compared to controls at all dose levels. In most
instances, these differences were statistically significant. A trend
toward decreased leucocyte counts in the treatment groups was also
discernible in the subsequent investigations. The author noted that
due to the pharmaco-logical action of antimicrobial compounds,
decreased leucocyte counts are a common finding.
On gross postmortem examination, a decrease in testicular size
(small with altered texture) was noted at 2000 mg/kg feed and above.
Histologically, marked testicular atrophy, decreased spermatogenesis,
and a significant increase in the amount of mineralized concretion in
the testes were noted in these same dose groups. Normal age-related
degenerative changes occurred with greater frequency in treated
animals than in controls. Caecal dilation was reported in both sexes
of rats in the high-dose group and in the males at 2000 mg/kg feed.
Organ weights recorded at both the interim and terminal kill
revealed a decrease in absolute and relative liver weights in males at
2000 mg/kg feed and above. The same changes were seen in females in
these groups at the interim necropsy but not at study termination. At
the interim and terminal kills, absolute weights of the brain, testes
and adrenals were significantly decreased in the 6000 mg/kg feed
males. However, the relative weights of these organs were comparable
to controls.
Histopathological evaluation revealed a dose-related increase in
incidence of hepatic cysts at 24 months, bile duct hyperplasia with
sclerosis at 12 and 24 months, and cystic bile duct hyperplasia at 24
months in both sexes and in all treatment groups. Spinal cord
demyelination/degeneration was reported in all treatment groups.
Because of the high incidence of this finding in controls and without
further definition of lesions into degeneration versus demyelination,
the biological significance of this finding is uncertain. There was
also a marked increase in degenerative changes in the sciatic nerve.
A marked increase in the number of sarcolemmal nuclei in skeletal
muscle cells of animals in the 6000 mg/kg feed group was noted. This
finding is generally associated with degenerative changes in muscle
fibres.
A marked increase in the incidence of cardiomyopathy was
observed in males at 6000 mg/kg feed and in females in all dose
groups. Incidences at 0, 770, 2000, and 6000 mg/kg feed were 10/48,
8/50, 13/50, and 24/50 in males and 8/50, 16/50, 17/50, and 25/50 in
females, respectively. Endocardial neoplasms and Schwann cell-like
hyperplasia were seen in the hearts of some of the animals. These
findings were evaluated by a Pathology Working Group which concluded
that these lesions were not related to treatment with the test
article. Under the conditions of this study there was no evidence of
a carcinogenic effect of the test substance. A NOEL could not be
determined because of the occurrence of bile duct changes and
cardiomyopathy in the low-dose group (Bomhard et al., 1991b; Hall,
1992).
A limited long-term study was performed with Wistar (Bor:WISW)
rats (60/sex/group). Diets containing 0, 100, or 500 mg
enrofloxacin/kg feed (equal to 0, 5.3, or 26 mg/kg bw/day for males
and 0, 7.2, or 36 mg/kg bw/day for females, respectively) were
administered for 24 months. An interim kill of 10 rats/sex/group was
performed at 12 months.
Morbidity, moribundity, and mortality in treated groups were
comparable to controls. General appearance, clinical observations,
and the results of physical and ophthalmoscopic examinations were all
comparable with normal animals of this species and age. Exposure to
enrofloxacin produced no biologically significant alterations in food
or water intake, body weight, clinical chemical parameters or
haematological parameters.
At interim sacrifice, no abnormalities were noted in males or
females on postmortem examination. At terminal sacrifice, an
increased number of males had swollen livers (3/50 [controls], 10/50,
[100 mg/kg feed], and 8/50 [500 mg/kg feed]). However, liver weights
and histopathological findings were within normal limits.
No treatment-related abnormalities were noted in the clinical
chemistry parameters that were evaluated. However, as in the long-
term study summarized above at higher dose levels, the gamma globulin
value in treated animals was consistently lower than in controls.
On histological evaluation, sclerotic bile duct hyperplasia was
more common in both males and females treated with enrofloxacin than
in control animals. The incidence was statistically significantly
increased in the 100 and 500 mg/kg feed groups after 52 weeks of
treatment, and in males and females at 500 mg/kg feed after 104 weeks
of treatment. The total number of females affected was markedly less
than the number of males. Although the incidence of bile duct
hyperplasia at 104 weeks at 100 mg/kg feed was not statistically
significantly different from the control values, the significant
findings in males in both dose groups at 52 weeks suggests that
administration of enrofloxacin accelerates the development of bile
duct sclerosis in male rats.
No evidence of carcinogenicity was reported in this study.
However, the heart and liver were the only two tissues examined
histologically. A NOEL could not be determined in this study (Leser,
1992).
A consulting pathologist conducted a second histopathologic
evaluation of liver slides of male rats from both the interim and
terminal kills in the above study. The consultant's conclusions were
as follows: "There was an increase over controls in the incidence of
bile duct hyperplasia in both dose groups at the interim kill,
although this was probably a spurious finding. At the terminal kill,
an increased incidence occurred only at the 500 ppm level. No clear
effect on lesion severity was noted in any group. The 100 ppm dose
(5.3 mg/kg bw/day) was a no-effect level in this study." (Squire,
1992).
A second limited long-term study was performed on Wistar
(Bor:WISW) rats (60/sex/group), administered enrofloxacin in the diet
at 0, 10, or 50 mg/kg feed (equal to 0, 0.6, or 2.9 mg/kg bw/day for
males and 0, 0.7, or 3.5 mg/kg bw/day for females, respectively) for
24 months. An interim kill of 10 animals/sex/group was performed at
12 months. The purpose of this study was to establish a NOEL for the
treatment-related bile duct hyperplasia observed in previous chronic
studies.
No treatment-related alterations were seen in physical
examinations, ophthalmologic evaluations, feed or water consumption,
body-weight gain, clinical chemistries, urinanalysis, or haematology
at the termination of the study. Postmortem examinations, including
gross observations, organ weights and histopathological examinations
were conducted at 12 and 24 months. Portions of the right and left
anterior liver lobes were collected and prepared for histological
evaluation Only one section per lobe was examined from these hepatic
samples. No treatment-related effects were reported in any of the
parameters monitored during the study. The incidence and severity of
bile duct hyperplasia in the exposed female and male rats were
comparable with the controls. The NOEL was 50 ppm (equal to 2.9 mg/kg
bw/day) (Leser, 1993).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl: CD(SD)Br rats at doses of 0, 500, 2000, or 7500 mg/kg feed. F0
males received the diet for approximately 70 days and the F0 females
for approximately 2 weeks prior to mating. Reproductive performance
of F0 and F1 generations was evaluated, as well as pup parameters.
A gross necropsy was performed on every animal and histopathologic
evaluation was conducted on reproductive organs.
Administration of the test article caused decreased body weights
at 7500 mg/kg feed (equal to 630 mg/kg bw/day) in the F0 and F1
generations. This effect was not accompanied by a reduction in food
consumption. Reproductive performance of the same animals was
significantly affected by the treatment at the high dose, which
included increased gestation length, reductions in the number of
litters, total pups, litter size and number of implants in the F0 and
F1 generations. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations,
which were considered the primary cause for the adverse effects on
fertility. The NOEL was 2000 mg/kg feed, equal to 165 mg/kg bw/day
(Clemens et al., 1986).
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl:CD (SD)Br rats at doses of 0, 125, 300, or 2000 mg/kg feed
(equivalent to 0, 6.2, 15, or 100 mg/kg bw/day). This study was
intended to evaluate the effect(s) of enrofloxacin on gonadal function
and mating behaviour in treated males, reproductive parameters in
females, and growth and development in the F1 and F2 offspring. F0
males and females received the test compound for 70 and 14 days,
respectively, before mating. A gross necropsy was performed on every
animal and histopathologic evaluation was conducted on testes and
epididymides from each F0 and F1 male.
Administration of the test article caused a reduction in mean
epididymal weights at the highest dose, and alterations in sperm
morphology at 300 and 2000 mg/kg feed. No effects on fertility or
reproductive performance were noted. The NOEL was 125 mg/kg feed,
equivalent to 6.2 mg/kg bw/day (Kowalski et al., 1989).
A specialized male fertility study was performed with
enrofloxacin to determine the timing of the onset of spermatic
dysmorphogenesis and to ascertain whether functional fertility was
restored during a 90-day recovery phase. Diets containing 0 or 7500
mg/kg feed (equivalent to 375 mg/kg bw/day) were administered to
sexually mature Crl:CD BR male rats (60/group) for 90 days. Interim
kills were performed at 3, 6, 9 and 13 weeks, with 15 males from each
group retained to mate with untreated females to assess male
fertility. The first mating was performed after 11 weeks on trial.
From day 91, all males were fed control diet and matings were again
performed on weeks 3, 7, and 11 of recovery. Females were killed on
day 20 of gestation.
Enrofloxacin administered at this dose caused significant
decreases in male fertility, food consumption and body-weight gains.
Testes weights were increased at all interim kills, but were less
than control values at the end of the study. Treatment-related
spermatic dysmorphogenic changes were seen by week 3 on trial. These
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Varying grades of seminiferous
tubular atrophy and other degenerative alterations were observed in
40% of these animals. Complete infertility was diagnosed in the
remaining males (Clemens et al., 1987).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
A teratogenicity (Segment II) study in the rat was performed in
which enrofloxacin as a 5% suspension with carboxymethylcellulose and
polysorbate 80 was administered by gavage to groups of 28 inseminated
COBS CD female rats at 0, 50, 210, or 875 mg/kg bw/day. Dosing was
performed on days 6-15 of gestation. On day 20, all dams were killed
and maternal and fetal parameters were evaluated.
Administration of the test article was maternally toxic at 210
and 875 mg/kg bw/day. Food consumption was higher in high-dose
animals, but there was a significant reduction in mean maternal weight
gain in these animals. In the high-dose litters, fetal weights and
litter size were significantly decreased and fetal resorption and
post-implantation losses were increased. Fetal weights were
significantly decreased in the mid-dose group. Skeletal examination
of the mid- and high-dose groups showed delayed skeletal ossification.
Neither embryotoxicity nor fetotoxicity was seen. No teratogenic
effects were observed. The NOEL was 50 mg/kg bw/day (Clemens &
Hartnagel, 1985).
2.2.5.2 Rabbits
An embryotoxicity (including teratogenicity) study was performed
with enrofloxacin administered by gavage to groups of 16 mated female
chinchilla rabbits at doses of 0, 1, 5, or 25 mg/kg bw/day admixed in
distilled water with 0.5% Tylose. Dosing was performed on days 6-18
of gestation. On day 28, all dams were killed. Because no maternal
effects were seen at doses up to 25 mg/kg bw/day, a supplementary
group of 16 females (with corresponding control group) was added and
administered enrofloxacin at 75 mg/kg bw/day.
Administration of the test article caused decreased food
consumption and lower body weights at 75 mg/kg bw/day. Increased
post-implantation losses were also noted in this group. All other
dose groups showed only incidental findings. The NOEL was 25 mg/kg
bw/day (Becker et al., 1987).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with enrofloxacin are
summarized in Table 2.
2.2.7 Special studies on the human gastrointestinal flora
No in vivo studies were available. In vitro studies of
Escherichia coli (24 strains), Staphylococcus aureus (24
strains) and Enterococcus spp. (24 strains) of human origin showed
the following MIC values for enrofloxacin (E) and ciprofloxacin (C).
Only summary tables were given.
The results of these studies are summarized in Table 3 (Scheer,
1993).
Data on in vitro susceptibility to ciprofloxacin for several
strains of bacteria obtained from "clinical isolates" or "bite wounds"
from humans were also available (Goldstein & Citron, 1988; Wise
et al., 1988).
The results of these studies are summarized in Table 4.
2.3 Observations in humans
Enrofloxacin has been developed specifically for use in
veterinary medicine; therefore, no human data were available.
Ciprofloxacin, the major metabolite of enrofloxacin, is a human drug
and has been used extensively. Clinical data in humans has produced
no evidence of nephrotoxicity or significant drug-related ocular
changes. Since the drug is not recommended for children or pregnant
women, little evidence has been collected to assess the occurrence of
articular or developmental changes in young patients (Schluter, 1987).
Table 2. Genotoxicity assays with enrofloxacin
Test system Test object Concentration Results Reference
In vitro
Unscheduled Rat primary 1-200 mg/ml Negative Cifone & Myhr, 1985
DNA synthesis hepatocytes
Bacterial reverse S. typhimurium 0.004 & 40 Inappropriate Herbold, 1985a
mutation1 TA98, TA100, ng/plate2 & deficient3
TA1535,TA15
TA1537
Mammalian cell CHO/HGPRT 0.25-1.25 Equivocal Den Boer & Hoorn,
forward mg/ml (-S9)4 1987
mutation1 0.375 - 1.25
mg/ml (+S9)5
Mammalian cell CHO/WBI 25-250 mg/ml Positive Taalman & Hoom,
chromosomal (-S9)6 1988
aberration1 0.1-1 mg/ml
(+S9)7
In vivo
Bone marrow Bor:NMRI mice 1-2 g/kg bw Negative Herbold, 1985b
micronucleus
Bone marrow CD rat 0.04-1 g/kg Negative Bootman et al. ,
chromosomal bw8 1987
aberration
Sister chromatid Chinese 2-8 g/kg bw Negative Herbold, 1988
exchange hamster bone
marrow
Table 2 (continued)
1 Both with and without rat liver S9 fraction
2 Positive controls: 2-Aminoanthracene, endoxan (TA100, TA1535),
and trypaflavine (TA98, TA1537).
3 Inappropriate due to substantial bacteriotoxicity; deficient due
to failed positive controls.
4 5-Bromodeoxyuridine as positive control.
5 3-Methylcholanthrene as positive control.
6 Mitomycin C as positive control.
7 Cyclophosphamide as positive control.
8 Low dose previous LD50 studies showed an oral dose tolerance of
>5 g/kg bw but in the present study, 6/30 animals administered 1 g/kg bw
had to be killed in extremis shortly after being dosed.
Table 3. Summary of MIC-values for bacterial species of human origin
Bacteria MIC range MIC50 MIC90
µg/ml µg/ml µg/ml
E C E C E C
E. coli 0.015-0.03 0.015-0.03 0.015 0.015 0.03 0.015
Staphylococcus 0.06-128 0.125-64 0.125 0.25 4.0 32.0
aureus
Enterococcus 0.25-1.0 0.25-2.0 0.5 1.0 1.0 2.0
spp.
Table 4. In vitro susceptibility of bacteria to ciprofloxacin
Bacteria Number of strains MIC range
µg/ml
Bacteroides fragilis 1 15 4-16
Bacteroides spp. 2 17 0.06-32
Clostridium spp. 1 17 0.012-8
E. coli 1 66 0.015-1
Enterobacter spp. 1 51 0.015-2
Enterococci 1 10 1-2
Flavobacterium 2 18 0.5-1
Fusobacterium spp. 2 18 0.25-32
Peptostreptococcus spp. 1 10 0.25-2
Peptostreptococcus spp. 2 16 0.03-8
1 Clinical isolate.
2 Isolated from bite wounds.
3. COMMENTS
The toxicological data available to the Committee were derived
from acute, short-term, long-term, and carcinogenicity studies;
reproductive and developmental studies; mutagenicity studies;
metabolism and residue studies; antimicrobial activity and
observations in humans following treatment with ciprofloxacin, the
major metabolite of enrofloxacin.
In rats orally dosed with 5 mg/kg bw of radiolabelled
enrofloxacin, the bioavailability of the dose was 75%. Enrofloxacin
was rapidly absorbed and distributed to all tissues, with the highest
concentration in the liver and kidney. Enrofloxacin was rapidly
excreted via urine and bile. Radioactivity in the urine was mainly
unchanged parent drug and the de-ethylated compound, ciprofloxacin.
Bile contained primarily unchanged enrofloxacin. Similar results were
obtained with all species of animals tested.
Enrofloxacin is a relatively stable molecule. Metabolism takes
place mainly in the liver, with the main metabolite in all species
being ciprofloxacin, probably formed by an oxidative dealkylation. In
rats four other components were identified, each comprising less than
2% of the total residue.
Single oral or topical doses of enrofloxacin were slightly toxic
to experimental animals (LD50 = 500-5000 mg/kg bw). In mice, single
intravenous doses were moderately toxic (LD50 = approximately 220
mg/kg bw).
Following 90-day oral administration of enrofloxacin to rats,
chronic degenerative changes in the auricular cartilage were seen at
doses equal to 150 and 577 mg/kg bw/day. Treatment-related
morphological changes in the spermatozoa and testicular tubular
atrophy were seen at 577 mg/kg bw/day. These effects were not seen at
a dose equal to 36 mg/kg bw/day.
A special study was performed to further assess the morphological
changes in the male reproductive tract seen in rats in previous
studies. Male rats were administered enrofloxacin in the feed at
doses equal to 10, 38 or 615 mg/kg bw/day followed by a 90-day
recovery period. Statistically significant decreases in epididymal
weights were seen at 91 days in the 38 and 615 mg/kg bw/day groups.
At 615 mg/kg bw/day, the testis/body-weight ratio was significantly
increased, while the epididymides/body-weight ratio was significantly
decreased. Bilateral testicular atrophy was seen in two rats at 615
mg/kg bw/day after the 90-day recovery period. Abnormal spermatozoa
were seen at 615 mg/kg bw/day at days 14 and 91, but not at the end of
the 90-day recovery period. The NOEL for testicular toxicity in this
study was 10 mg/kg bw/day.
To assess effects on testicular toxicity and articular cartilage
in young dogs, a 90-day oral study in three- and four-month old dogs
was performed. Enrofloxacin was administered at dose levels
equivalent to 0, 3, 9.6 or 75 mg/kg bw/day. All treated animals
except those dosed at 3 mg/kg bw/day showed degeneration of the
articular cartilage. The testes/body-weight ratio for all treated
animals was consistently higher than for control animals, but the
difference was not statistically significant. In 4 high-dose and 1
low-dose animals, marked dilatation of the seminiferous tubules, and
focal areas of single layer spermatogonial cells or vacuolated
epithelium in the tubules were considered beyond normal limits. It
could not be determined whether testicular changes observed in the
low-dose group were treatment-related or due to the fact that the
animals were not fully mature. Therefore, a NOEL could not be
determined. The testicular effects seen in this study were not seen
in 12-13-month old dogs treated orally with enrofloxacin up to 52
mg/kg bw/day for 90 days.
A 90-day study was performed in male dogs dosed with enrofloxacin
at dose levels equal to 0.3, 0.6, 1.2, or 92 mg/kg bw/day to determine
a NOEL for the testicular effects of enrofloxacin observed in the
earlier study with 3- to 4-month old dogs. At termination of the
study, testicular weights were determined and testes and epididymides
collected for histopathological assessment. No other tissues were
collected. Testicular weights and testes/body-weight ratios were
elevated over controls at 0.3, 0.6, and 1.2 mg/kg bw/day. In
addition, lameness was seen at 92 mg/kg bw/day following 2-3 days of
enrofloxacin administ-ration. This effect was not seen at 1.2 mg/kg
bw/day. One dog in the 92 mg/kg bw/day group also had bilateral
testicular degenerative changes. The NOEL was 1.2 mg/kg bw/day in
this study.
A 90-day oral study in 3-month old dogs with a 90-day recovery
period was conducted with 0, 0.3, or 1.2 mg/kg bw/day of enrofloxacin
to assure the continued absence of testicular effects after the
animals reached sexual maturity. After the recovery period, gross and
microscopic examination of testes and epididymides showed no changes
indicative of toxicity. Testes and epididymides appeared mature and
contained normal mature spermatozoa. There was no evidence of delayed
testicular toxicity in this study at levels up to and including 1.2
mg/kg bw/day.
Enrofloxacin was not carcinogenic in mice orally dosed at levels
equal to 323, 1100, or 3520 mg/kg bw/day in a 2-year long-term
toxicity/carcinogenicity study. Bile duct hyperplasia was seen in
males in the mid-dose group and at the high dose in both sexes. The
NOEL was 323 mg/kg bw/day.
In a long-term toxicity/carcinogenicity oral study in rats at
doses equal to 41, 103, 338 or 856 mg/kg bw/day, the Committee
concluded that enrofloxacin was not carcinogenic in this species.
However, a NOEL could not be determined for the bile duct hyperplasia
seen in this study. The NOEL for bile duct hyperplasia in further
limited chronic studies with enrofloxacin in rats was 2.9 mg/kg
bw/day.
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of rats
up to a dose equivalent to 630 mg/kg bw/day. Increased gestation
length, reductions in the number of litters, total pups, litter size
and number of implants for the F0 and F1 generations were seen at
630 mg/kg bw/day. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations and
were considered the primary cause for the adverse effects on
fertility. The NOEL for reproductive performance was 165 mg/kg
bw/day.
An additional two-generation reproduction study in rats was
performed with enrofloxacin administered in the feed to groups of rats
at 0, 125, 300, or 2000 mg/kg feed to determine whether the test
compound affected gonadal function and mating behaviour in treated
males or reproductive parameters in females, as well as, to monitor
growth and development in the F1 and F2 offspring. Administration
of the test article caused a reduction in mean epididymal weights at
the highest dose and alterations in sperm morphology at 15 and 100 mg/kg
bw/day. No effects on fertility or reproductive performance were
noted. The NOEL was 125 mg/kg feed, equal to 10 mg/kg bw/day.
A special fertility study was performed in sexually mature male
rats fed enrofloxacin in the diet at doses equivalent to 0 or 375
mg/kg bw/day for 90 days. The purpose of the study was to determine
the timing of the onset of spermatic dysmorphogenesis and to ascertain
whether functional fertility was restored during a 90-day recovery
phase. Administration of the test article caused a significant
decrease in male fertility, food consumption and body-weight gain.
Testes weights were increased at all interim kills, but were lower
than in the control animals at the end of the study. Treatment-
related spermatic dysmorphogenic changes were seen by week 3 and these
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Forty percent of these animals
demonstrated varying grades of seminiferous tubular atrophy and other
degenerative alterations. The remaining males were infertile.
A teratogenicity study was performed in which enrofloxacin was
administered to rats by gavage at 0, 50, 210, or 875 mg/kg bw/day.
Dosing was performed between days 6-15 of gestation. Maternal
toxicity was observed at 210 and 875 mg/kg bw/day. Food consumption
was higher in high-dose animals, but there was a significant reduction
in mean maternal weight gain in these animals. Fetal weights and
litter sizes were significantly decreased and fetal resorption and
post-implantation losses were increased in high-dose litters. Fetal
weights were significantly decreased in the mid-dose group. Skeletal
examination of the mid- and high-dose fetuses revealed delayed
skeletal ossification, which correlated with the decreased fetal
weights. No teratogenic effects were observed in this study. The
NOEL was 50 mg/kg bw/day.
In in vitro mutagenicity testing, enrofloxacin gave equivocal
results in a test for forward mutations in mammalian cells and
positive results in chromosomal aberration tests. Negative results
were obtained in in vivo bone marrow micronucleus, sister chromatid
exchange and bone marrow chromosomal aberration tests. The Committee
concluded that enrofloxacin is not genotoxic.
The potential for adverse effects on the human gastrointestinal
flora was considered from summary data. In vitro MIC data for
ciprofloxacin covering representatives of the human gut flora were
submitted for assessment. Escherichia coli was found to be the
most sensitive bacterium having an MIC50 value of 0.015 µg/ml.
In calculating the ADI this figure was used in the formula
developed at the thirty-eighth meeting of the Committee (Annex 1,
reference 97).
Concentration without
effect on human gut x Daily faecal bolus (g)
Upper limit of flora (µg/ml)a
temporary ADI =
(µg/kg bw) Fraction of
oral dose x Safety factorc x Weight of human (60 kg)
bioavailableb
(0.015 x 20) x 150
=
0.12 x 10 x 60
= 0.6 µg/kg bw
a Since the MIC value was determined using only 105 bacteria/ml a
factor of 20 was used to account for the higher inoculum density
representative of the human intestine. For example,
Enterobacteriaceae and Pseudomonas aeruginosa MIC values
increased 8 to 32-fold when the inoculum size was increased to
107 bacteria/ml.
b The fraction of the dose available to the intestinal microflora
was derived from studies of ciprofloxacin in humans. In human
volunteers given 2 x 50 mg ciprofloxacin the faecal concentration
was found to be 80 mg/kg. In 150 g faeces, this corresponds to
12% of the administered
c A safety factor of 10 was used to account for the possible
variation in absorption between ciprofloxacin and enrofloxacin, an
uncertainty in the biliary excretion of enrofloxacin, and the
variability between individuals.
4. EVALUATION
In view of the absence of information on the effects of
enrofloxacin on microorganisms obtained from the human intestine, the
Committee established a temporary ADI of 0-0.6 µg/kg bw based on the
results of the limited summary data of microbiology testing of
enrofloxacin/ciprofloxacin.
The temporary ADI of 0.6 µg/kg bw calculated from the in vitro
antimicrobial activity data represents an adequate margin of safety
when compared to the NOEL derived from the toxicology endpoint of 1.2
mg/kg bw/day for testicular toxicity.
5. REFERENCES
ALTREUTHER, P. & KLOSTERMANN, L. (1994). Unpublished summary of the
Enrofloxacin metabolism and residue information submitted to the FAO
experts. Submitted to WHO by Bayer AG, Leverkusen, Germany.
BARE, J.J., PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988). A
subchronic (13-week) feeding study followed by a 13-week drug
withdrawal period in male rats with BAY Vp 2674. Unpublished Report
No. MTD0074 from the Toxicology Department, Miles Inc., Elkhart, IN,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73812).
BECKER, H., VOGEL, W., & TERRIER, Ch. (1987). Embryotoxicity
(including teratogenicity) Study with BAY Vp 2674 in the Rabbit.
Unpublished Report No. R4249 (Project No. 058555) from the Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73705).
BOOTMAN, J., HODSON-WALKER, G., & DANCE, C. (1987). BAY Vp 2674;
Investigation of effects on bone marrow chromosomes of the rat after
acute oral administration. Unpublished LSR report No. 87/BAG039/312
(Report No. R4189; Study No. T5023055) from Life Science Research
Ltd., Suffolk, England. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 74166).
BOMHARD, E., RÜHL-FEHLERT, C., & KALINER, G. (1991a). BAY Vp 2674.
Study of chronic toxicity and carcinogenicity in mice (administration
in feed over 24 months). Unpublished Report No. 20263 (Study No.
T8023436) from the Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74229).
BOMHARD, E. & SCHLADT, L. (1991B). BAY Vp 2674. Study of chronic
toxicity and carcinogenicity in rats after administration in feed over
2 years. Unpublished Report No. 20306 (Study No. T8023436) from the
Department of Toxicology, Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany (report No. 74230).
DEN BOER, W.C. & HOORN, A.J.W. (1987). Mutagenicity evaluation of
BAY Vp 2674 in the CHO/HGPRT forward mutation assay: Revised final
report. Unpublished genetics assays Nos. E-9457 & E-9598 (Report No.
R 4071) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73582).
CIFONE, M.A., & MYHR, B.C. (1985). Evaluation of BAY Vp 2674 in the
rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished
Report No. 3168E (Genetic Assay No. 7550; LBI project No. 20991) from
Litton Bionetics, Inc., Kensington, MD. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73055).
CLEMENS, G.R. & HARTNAGEL, R.E. (1985). A teratology (Segment II)
study in the rat with BAY Vp 2674. Unpublished Report No. 53 from the
Toxicology Department, Central Research Services, Miles Laboratories
Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 73159).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1986). A two-generation
reproduction study in rats with BAY Vp 2674. Unpublished Report No.
4 from Toxicology Department, Central Research Services, Miles
Laboratories Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73314).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1987). A specialized
male fertility study with BAY Vp 2674 in the rat. Unpublished Report
No. MTD0030 (Mobay Report No. 73800) from the Toxicology Department,
Central Research Services, Miles Laboratories Inc., Elkhart, IN, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany (report No. 73800).
EIGENBERG, D.A. (1987). Acute dermal toxicity of BAY Vp2674 in albino
rabbits. Unpublished toxicological report No. 926 (Study No. 86-023-
04) from Health, Environment & Safety, Corporate Toxicology
Department, Mobay Corporation, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73606).
GOLDSTEIN, E.J.C., & CITRON, D.M. (1988). Comparative activities of
cefuroxime, amoxicillin-clavulanic acid, ciprofloxacin, enoxacin, and
ofloxacin against aerobic and anaerobic bacteria isolated from bite
wounds. Antimicrob. Agents Chemoth., 32:1143-1148.
HALL, W.D. (CHAIR) (1992). Pathology working group on a 2-year
chronic feeding study with a l-year interim kill in rats on the
compound BAY Vp 2674. Unpublished Pathology Working Group report
(Bayer ID 014379) prepared for the Center for Veterinary Medicine,
U.S. Food & Drug Administration, Department of Health & Human
Services, Rockville, MD, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
HERBOLD, B. (1985a). BAY Vp 2674; Salmonella /microsome test to
evaluate point mutagenic effects. Unpublished Report No. 13523E
(Study No. T9017669) from the Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73213).
HERBOLD, B. (1985b). BAY Vp 2674; Micronucleus test on the mouse to
evaluate for mutagenic effects. Unpublished Report No. 14102E (Study
Nos. T6019042 & T8019675) from Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74164).
HERBOLD, B.A. (1988). BAY Vp 2674; Sister chromatid exchange in the
bone marrow of the Chinese hamster in in vivo as a test for DNA-
modifying effects. Unpublished Report No. 16783 (Study Nos. T5024676
& T8027324) from Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74178).
KOWALSKI, R.L., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985).
Safety evaluation of BAY Vp 2674: Subchronic (13 week) feeding study
in the rat. Unpublished Report No. 63 (No. 73194) from Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73194).
KOWALSKI, R.L., CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1989).
A two-generation reproduction study in rats with BAY Vp 2674.
Unpublished Report No. MTD0093 (Report No. R4705) Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73892).
LESER, K.H. (1992). BAY Vp 2674. Chronic toxicity study in rats after
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 21668A (Study No. T7032787) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74387).
LESER, K.H. (1993). BAY Vp 2674. Chronic toxicity study in rats with
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 22659 (Study No. T6039950) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74472).
MANDELL, G.L., & SANDE, M.A. (1990). Antimicrobial Agents in Goodman
and Gilman's the Pharmacological Basis of Therapeutics, 8th edition,
Gilman, A.G., Rall, T.W., Nies, S.A., and Taylor, P. (eds.). Pergamon
Press, Inc., Maxwell House, Fairview Park, Elmsford, New York 10523,
p. 1058.
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985). Safety
evaluation of BAY Vp 2674: Subchronic (13 week) feeding study in the
dog. Unpublished Report No. 43 (Mobay Report No. 73146) from the
Toxicology Department, Central Research Services, Miles Laboratories,
Inc., Elkhart, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73146).
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1987). Safety
evaluation of BAY Vp 2674: Repeat of a subchronic (13-week) feeding
study in the dog. Unpublished Report No. MTD0031 (BayVet report No.
73146) from the Toxicology Department, Central Research Services,
Miles Laboratories, Inc., Elkhart, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73775).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988a). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs.
Unpublished Report No. MTD0068 from the Toxicology Department, Central
Research Services, Miles Inc., Elkhart, IN, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73788).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988b). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs
followed by a 13-week withdrawal period. Unpublished report Miles
Inc., Elkhart, Report No. MTD0069. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73789).
SCHLUTER, G. (1987). Ciprofloxacin: review of potential toxicologic
effects. Am. J. Med, 82 (suppl 4A): 91-93.
SCHMIDT, M. (1985). Acute toxicity of BAY Vp 2674 in the rat, mouse,
rabbit and dog. Unpublished Report No. 13223(E) from Institute of
Toxicology, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany (report No. 73075).
SQUIRE, R.A. (1992). Evaluation of bile duct hyperplasia in male rat
livers. Unpublished Report No. 74388 (Study No. T7032787; Pathology
No. 3122) from Toxicology, Research & Development Dept., Miles Inc.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74388).
TAALMAN, R.D.F.M. & HOORN, A.J.W. (1988). Clastogenic evaluation of
BAY Vp 2674 in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished HBC study No. E-9804-0-437 (Report No. R 4474; Study No.
T1027516) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74165).
WISE, R., ANDREWS, J.M., ASHBY, J.P. & MATTHEWS, R.S. (1988).
In vitro Activity of lomefloxacin, a new quinolone antimicrobial
agent, in comparison with those of other agents. Antimicrob. Agents
and Chemoth., 32: 617-622.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The following information was provided in a summary report of
metabolism and residue data on enrofloxacin. The full study reports
were not provided.
2.1.1.1 Rats
Rats (number, species, strain not specified) were treated orally
or intravenously with a single dose (5 mg/kg bw) of radiolabelled
enrofloxacin. The bioavailability was calculated to be approximately
75%. Enrofloxacin was rapidly absorbed and distributed to all
tissues. Highest concentrations were found in the liver and kidney,
followed by muscle. Fat did not contain appreciable amounts of
radioactivity. Elimination was rapid via urine and faeces. Most of
the radioactivity was excreted during the first 24 hours after
administration. About 40% of the radioactivity was excreted in bile.
The radioactivity in urine was attributed primarily to the unchanged
parent drug, its de-ethylated metabolite, ciprofloxacin, and the
glucuronide of enrofloxacin. Bile contained mainly the unchanged
parent molecule (Altreuther & Klostermann, 1994).
2.1.1.2 Cattle, pigs, chickens, and turkeys
Trials in all species showed that enrofloxacin is absorbed
readily and distributes to all organs and tissues following oral or
parenteral administration. In pigs, when the same dose was
administered by the oral or i.m. route, similar rates of absorption
and subsequent concentrations of drug in tissues were observed.
Following parenteral administration, maximum serum concentrations in
pigs and calves were reached within 1 to 2 hours. The highest drug
concentration initially occurred in the liver in all species.
Following a one-day withdrawal in poultry, highest drug concentrations
were found in the skin. In all species studied, elimination was
primarily via the urine and bile/faeces (Altreuther & Klostermann,
1994).
2.1.2 Biotransformation
2.1.2.1 Rats, cattle, pigs, and poultry
Rats (number, species, strain not specified) were treated orally
with 165 mg/kg bw of radiolabelled enrofloxacin for 3 consecutive
days, then with 50 mg/kg bw on the fourth day. Major urinary
metabolites were enrofloxacin (30-36%), ciprofloxacin (26- 31%), and
enrofloxacin glucuronide (19-29%). Four other components were
identified, each less than 2% of the total residue (Altreuther &
Klostermann, 1994).
In food animal species, ciprofloxacin was the main metabolite
recovered. The liver is considered the primary site of enrofloxacin
metabolism in these species, forming ciprofloxacin through oxidative
dealkylation. Additional metabolites occurred but comprised less than
10% of the total residue (Altreuther & Klostermann, 1994).
2.1.3 Effects on enzymes and other biochemical parameters
The bactericidal in activity of quinolones, including
enrofloxacin, is attributed to their ability to inhibit DNA gyrase
(Mandell & Sande, 1990).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of enrofloxacin is summarized in Table 1.
Table 1. Acute toxicity studies on enrofloxacin
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse male p.o. > 5000 Schmidt, 1985
female 4336
male i.v. 225 Schmidt, 1985
female 220
Rat male p.o. > 5000 Schmidt, 1985
female
Rabbit male p.o. 500-800 Schmidt, 1985
female
male topical > 2000 Eigenberg,1987
female
Dog male p.o. n/a1 Schmidt, 1985
female
1 Not determined due to vomiting of the drug.
Animals were observed daily for 14 days post-exposure in these
studies. Clinical signs associated with enrofloxacin toxicosis
included lethargy, trembling, tonic convulsions, dyspnea, prone and
lateral decubitus, and ataxia. Clinically affected animals that did
not die recovered within 15 minutes to 7 days post-exposure. Gross
post-mortem lesions reported included pulmonary congestion and
haemorrhage.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of Charles River rats (15/sex/group) were administered
diets containing 0, 500, 2000, or 7500 mg enrofloxacin/kg feed (equal
to 36, 150, or 577 mg/kg bw/day for males and 45, 182, or 690 mg/kg
bw/day for females, respectively) for 90 days. No overt effects on
appearance and behaviour were noted at any dose level. Body-weight
gain was reduced at the highest dose in both males (20%) and females
(23%), while the actual diet consumption for males and females
remained constant.
Serum chemistry findings included a significant dose-related
decrease in total plasma protein in the mid- and high-dose males and
females at 6 and 13 weeks. Total bilirubin and alanine aminotrans-
ferase were decreased in the males at 6 weeks but were normal at 13
weeks. Aspartate aminotransferase was decreased in the high-dose
males at 6 and 13 weeks. Globulins were decreased in the high-dose
males at 6 weeks and 13 weeks and high-dose females at 13 weeks. A
corresponding increase in the A/G ratio was seen in the high-dose
males at 6 and 13 weeks.
Haematology findings were unremarkable.
Urinalyses showed a significant decrease in urine sodium output
for males in the mid- and high-dose groups after 6 weeks and for
females in the mid- and high-dose groups at 13 weeks.
Treatment-related gross findings included swollen ears (2 males
and 4 females in the mid-dose group, 4 males and 5 females in the
high-dose group, and 1 female in the control group) and caecal
distension (1 male in the mid-dose group and 3 males and 5 females in
the high-dose group).
Mean and relative prostate weights were statistically
significantly reduced in the mid- and high-dose groups. Mean heart
weights were statistically significantly reduced in high-dose males
and in mid- and high-dose females. Mean liver weights were reduced in
both male and female high-dose animals. The reduction was
statistically significant in females.
Histological findings included auricular chondropathy which was
present in all groups except low-dose males and appeared to be dose-
related (1 control, 1 low dose, 6 mid dose, and 10 high dose). In the
testes of 12/15 rats from the high-dose group, dark, round or oval
cells were distributed among the spermatozoa in the ducts of the
epididymides. These cells were occasionally observed in seminiferous
tubules of 5/15 high-dose rats. These findings were considered
treatment-related.
Based on decreased body-weight gain and microscopic changes
observed in the ducts of the epididymides and testes in the high-dose
group, and gross and microscopic changes indicating auricular
chondropathy in the mid- and high-dose groups, the NOEL was 500 mg/kg
feed, equal to 36 mg/kg bw/day (Kowalski et al., 1985).
A special study was performed in male rats to further assess the
testicular toxicity seen in previous studies. A 13-week recovery
period was included in the study. Thirty male Crl:CD Charles River
rats per group were dosed with 0, 125, 500, or 7500 mg enrofloxacin/kg
feed (equal to 10, 38, or 615 mg/kg bw/day) for 90 days. Fifteen
animals/group were killed on day 91 and the remaining 15 on day 181.
An apparent dose-related decrease in epididymal weights was observed
in all dose groups at 91 and 181 days. The difference achieved
statistical significance (compared to controls) in the mid- and high-
dose groups at both time points. Relative mean testes weight was
significantly decreased in the high-dose group after 91 days.
Bilateral testicular atrophy was seen in two high-dose rats at 181
days. Abnormal spermatozoa were seen at day 91 in the mid- and high-
dose groups but not after the recovery period. The NOEL for this
parameter was 125 mg/kg feed, equal to 10 mg/kg bw/day (Bare et al.,
1988).
2.2.2.2 Dogs
Four groups (4/sex/group) of beagle dogs (3-4 months old, 2.6 to
4.8 kg bw) were administered enrofloxacin in the diet at 0, 100, 320
or 2500 mg/kg feed (equal to 3, 9.6, or 75 mg/kg bw/day, respectively)
for 91 days.
No dogs died during the study. Sporadic incidences of mild
diarrhoea and vomiting were reported early in the study. Vomiting
occurred 5, 6, and nearly 24 hours after ingestion of the test
article. Severe hyperextension (upon manipulation) of carpal joints,
hypoactivity, and abnormal gate or posture, were observed in all
animals in the high-dose group during weeks 1 and 2. Radiographic
examination (8 dogs total) revealed several abnormalities including
valgus deformity at the carpus, asymmetry of the distal ulnar physis,
remodelling of the radial carpal bone, radius curvus, and increased
width of the radial humeral joint. The consulting radiologist
concluded that only the radial carpal remodelling appeared to be dose-
related, and neither bone growth nor density were significantly
affected.
Serum chemistry and haematologic findings were unremarkable.
However, two types of unidentified crystals in urine samples from dogs
in the 2500 mg/kg feed group were considered treatment-related.
Gross postmortem findings included an increase in mean absolute
and relative testicular weights for treated animals when compared to
control animals. However, a clear dose-response relationship was not
established and none of the differences were statistically significant
when compared to controls. In 8/8 high-dose animals and 2/8 mid-dose
animals joint lesions were observed grossly as superficial (0.2 to 0.4
cm in diameter) erosions with dulling of the surfaces on the femoral
head of the hip joint and/or the femoral condyles of the knee joint.
Histologically, joint lesions were characterized by splitting of the
articular cartilage with disorganization of chondrocytes and formation
of "brood capsules" of cartilage cells. Necrosis and disintegration
of hyaline cartilage at the area of splitting was also noted.
There was marked variation in the microscopic appearance of
testes from animal to animal with respect to the stage of maturity,
diameter of the lumen of seminiferous tubules, and vacuolar change in
cells lining the tubules. However, it was not clear whether these
testicular changes were treatment-related because there was no dose-
response relationship in incidence or severity. These variations in
testicular morphology could represent normal variation in the maturing
process of this organ in this species. Due to the uncertainty of the
significance of the testicular findings in the low-dose males in this
study, the Committee concluded that a no-effect level was not observed
(Porter et al., 1987).
In another 90-day dietary study in beagle dogs, 4 groups
(4/sex/group) of adult dogs (12-13 months old weighing 6.4 to 12.5 kg)
received enrofloxacin at 0, 320, 800 or 2000 mg/kg diet (equal to 9.3,
22, or 53 mg/kg bw/day for males, and 8.9, 23, or 51 mg/kg bw/day for
females, respectively). No joint or testicular lesions were reported
in this study (Porter et al., 1985).
A special limited 90-day dietary study in males was performed.
Five groups (4 dogs/group) of 3-month old beagle dogs (weighing 4.1 to
5.7 kg) received enrofloxacin at 0, 10, 20, 40, or 3200 mg/kg diet
(equal to 0, 0.3, 0.6, 1.2, or 92 mg/kg bw/day, respectively). At
termination of the study (day 92) testicular weights were determined
and testes and epididymides collected for histopathological
assessment. No other tissues were collected.
Histologically, the tissues examined contained wide variations in
morphology attributable to differences in sexual maturation. Sections
of epididymides examined were considered normal. Bilateral testicular
degeneration characterized by multinucleated giant cells and cells
with large cytoplasmic and nuclear volumes, sometimes in mitosis, were
reported in the seminiferous tubules of one dog at the high dose.
Seminiferous tubules lined with single layers of spermatogonial cells
and forming open lumens were present in 2/4 dogs in the 10 mg/kg feed
group and 3/4 dogs in each of the 20, 40, and 3200 mg/kg feed groups.
This change was also reported in one control animal. Spermatocytic
giant cells were reported in 0, 1, 2, 2 and 3 dogs from the 0, 10, 20,
40 and 3,200 mg/kg feed groups, respectively.
The author attributed the seminiferous tubule changes to normal
variation in sexual maturity because similar morphology was reported
in the testes of one control dog. The author used this finding to
support a NOEL of 100 mg/kg feed in the 90-day study reported above
(Porter et al., 1987). The Committee concluded that a NOEL for
testicular effects in this special, limited 90-day study could not be
determined due to the inability to determine whether variations in
testicular morphology were normal or due to treatment with the test
article (Porter et al., 1988a).
Three-month old male beagle dogs (4/group) were administered
enrofloxacin at 0, 10, or 40 mg/kg diet (equal to 0.3 or 1.2 mg/kg
bw/day) for 90 days, then maintained on control diets for an
additional 91 days before being killed. The protocol was intended to
evaluate the potential for enrofloxacin testicular toxicity to appear
after animals reach sexual maturity, following exposure at an immature
age (delayed testicular toxicity).
No signs of clinical toxicity were observed nor was body-weight
gain or food consumption affected. Gross and microscopic examination
of testes and epididymides showed no changes indicative of toxicity.
Testes and epididymides appeared mature in all treated animals and
contained normal, mature spermatozoa. The NOEL for delayed testicular
toxicity was 40 mg/kg diet, equal to 1.2 mg/kg bw/day, the highest
dose tested (Porter et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
B6C3F1 mice (60/sex/group) received diets containing 0, 1000,
3300, or 10 000 mg enrofloxacin/kg feed (equal to 323, 1100, or 3520
mg/kg bw/day for males and 373, 1210, or 3700 mg/kg bw/day for
females, respectively) for 24 months. An interim sacrifice
(10/sex/group) was performed after one year. An additional dose group
(10 mice/sex) was administered 20 000 mg enrofloxacin/kg feed (equal
to 8030 mg/kg bw/day for males and 8010 mg/kg bw/day for females,
respectively) and scheduled for interim sacrifice after one year.
During the treatment phase of the study, general appearance and
physical examination findings were within normal limits in all treated
groups. Treatment-related ophthalmological findings included a marked
increase (relative to control animals) in the incidence of focal
lenticular opacity in males and females at 10 000 mg/kg feed.
Histopathological examination of eyes revealed no treatment-related
effects. However, it was noted that focal lenticular changes are only
verifiable to a limited extent with usual histolopathological methods.
Clinical chemical and haematological evaluations were performed
on 10 animals/sex/dose after 12 and 24 months on test. Alkaline
phosphatase levels were significantly lower in females at 3300 mg/kg
feed at 12 months, and at 10 000 mg/kg feed at study termination.
Concurrent with these findings, alanine aminotransferase and aspartate
transaminase values were within normal limits. Total protein was
decreased in males and females at all treatment levels as a result of
decreased globulin fractions.
Haematological analyses revealed a significant decrease in white
blood cell counts in males at 10 000 mg/kg feed at 12 months and at
3300 mg/kg feed at study termination. In females, a significant
decrease in white blood cell counts was noted at 10 000 mg/kg feed at
study termination. At 10 000 mg/kg feed, decreases were seen in
haematocrit, MCV, and MCH values in both sexes, and in the haemoglobin
value in males, at study termination.
Gross necropsy findings included a dose-related incidence of
caecal dilation in males at 1000, 3300, and 10 000 mg/kg feed and in
females at 3300 and 10 000 mg/kg feed. Histopathological examinations
showed neither an increase in the incidence of, nor a decrease in the
time of appearance to tumours in the dosed groups compared to the
controls. Bile duct hyperplasia was seen at 3300 mg/kg feed in males
and 10 000 mg/kg feed in both sexes. Focal papillary mucosal
hyperplasia of the gall bladder was also present in males and females
in these same dose groups. There was no evidence of carcinogenicity
in this study. The NOEL was 1000 mg/kg feed, equal to 323 mg/kg
bw/day (Bomhard et al., 1991a).
2.2.3.2 Rats
A combined long-term toxicity (52 weeks) and carcinogenicity (104
weeks) study of enrofloxacin was conducted in Wistar rats (BOR:WISW).
In the chronic study, 10 rats/sex/group received diets containing 0,
770, 2000, 6000, or 10 000 mg enrofloxacin/kg feed (equal to 0, 41,
103, 338, or 856 mg/kg bw/day for males and 0, 58, 146, 466, or 1000
mg/kg bw/day for females, respectively). In the carcinogenicity
study, 50 rats/sex/group received diets containing 0, 770, 2000, or
6000 mg enrofloxacin/kg feed (equal to 0, 41, 103, or 338 mg/kg bw/day
for males and 0, 58, 146, or 466 mg/kg bw/day for females).
Mortality rates, daily observations, and ophthalmologic findings
in treated animals were comparable to controls. Body-weight gain was
decreased in males in the 6000 mg/kg feed group and in males and
females at 10 000 mg/kg feed. Feed and water intakes were increased
in males and females at 10 000 mg/kg feed. Water intake was also
elevated at 6000 mg/kg feed.
Serum chemistry evaluations were performed after 6, 12, 18, and
24 months. Notable findings included a significant decrease in total
protein in males in all dose groups at all sampling periods. Total
protein was also significantly decreased in females in the high-dose
group both at 6 months and at the end of 2 years, and at 2000 and 10
000 mg/kg feed after 1 year. The albumin value was increased in
males at 10 000 mg/kg feed after 1 year, at 6000 mg/kg feed after 18
months, and in the two highest-dose groups (2000 and 6000 mg/kg feed)
at the end of 2 years. Bilirubin was significantly decreased in
females in all dose groups at the 6-month sampling time only.
Protein electrophoresis performed after 12, 18, and 24 months
showed a decrease in gamma-globulin in males and females in all dose
groups. The author noted that this finding is not unexpected in
animals receiving antimicrobials, as they lower the background
incidence of common pathogens, resulting in a decrease in antigenic
stimulation.
Haematology evaluations were performed after 6, 12, 18, and 24
months. At doses of 2000 mg/kg feed and above, there were
statistically significant and sometimes dose-related decreases in RBC
counts in males and haemoglobin and haematocrit values in both sexes
at various sampling times. However, the values for these parameters
in both sexes in all dose groups were comparable to controls at the
terminal sampling period. After 6 months leucocyte counts in both
sexes were decreased compared to controls at all dose levels. In most
instances, these differences were statistically significant. A trend
toward decreased leucocyte counts in the treatment groups was also
discernible in the subsequent investigations. The author noted that
due to the pharmaco-logical action of antimicrobial compounds,
decreased leucocyte counts are a common finding.
On gross postmortem examination, a decrease in testicular size
(small with altered texture) was noted at 2000 mg/kg feed and above.
Histologically, marked testicular atrophy, decreased spermatogenesis,
and a significant increase in the amount of mineralized concretion in
the testes were noted in these same dose groups. Normal age-related
degenerative changes occurred with greater frequency in treated
animals than in controls. Caecal dilation was reported in both sexes
of rats in the high-dose group and in the males at 2000 mg/kg feed.
Organ weights recorded at both the interim and terminal kill
revealed a decrease in absolute and relative liver weights in males at
2000 mg/kg feed and above. The same changes were seen in females in
these groups at the interim necropsy but not at study termination. At
the interim and terminal kills, absolute weights of the brain, testes
and adrenals were significantly decreased in the 6000 mg/kg feed
males. However, the relative weights of these organs were comparable
to controls.
Histopathological evaluation revealed a dose-related increase in
incidence of hepatic cysts at 24 months, bile duct hyperplasia with
sclerosis at 12 and 24 months, and cystic bile duct hyperplasia at 24
months in both sexes and in all treatment groups. Spinal cord
demyelination/degeneration was reported in all treatment groups.
Because of the high incidence of this finding in controls and without
further definition of lesions into degeneration versus demyelination,
the biological significance of this finding is uncertain. There was
also a marked increase in degenerative changes in the sciatic nerve.
A marked increase in the number of sarcolemmal nuclei in skeletal
muscle cells of animals in the 6000 mg/kg feed group was noted. This
finding is generally associated with degenerative changes in muscle
fibres.
A marked increase in the incidence of cardiomyopathy was
observed in males at 6000 mg/kg feed and in females in all dose
groups. Incidences at 0, 770, 2000, and 6000 mg/kg feed were 10/48,
8/50, 13/50, and 24/50 in males and 8/50, 16/50, 17/50, and 25/50 in
females, respectively. Endocardial neoplasms and Schwann cell-like
hyperplasia were seen in the hearts of some of the animals. These
findings were evaluated by a Pathology Working Group which concluded
that these lesions were not related to treatment with the test
article. Under the conditions of this study there was no evidence of
a carcinogenic effect of the test substance. A NOEL could not be
determined because of the occurrence of bile duct changes and
cardiomyopathy in the low-dose group (Bomhard et al., 1991b; Hall,
1992).
A limited long-term study was performed with Wistar (Bor:WISW)
rats (60/sex/group). Diets containing 0, 100, or 500 mg
enrofloxacin/kg feed (equal to 0, 5.3, or 26 mg/kg bw/day for males
and 0, 7.2, or 36 mg/kg bw/day for females, respectively) were
administered for 24 months. An interim kill of 10 rats/sex/group was
performed at 12 months.
Morbidity, moribundity, and mortality in treated groups were
comparable to controls. General appearance, clinical observations,
and the results of physical and ophthalmoscopic examinations were all
comparable with normal animals of this species and age. Exposure to
enrofloxacin produced no biologically significant alterations in food
or water intake, body weight, clinical chemical parameters or
haematological parameters.
At interim sacrifice, no abnormalities were noted in males or
females on postmortem examination. At terminal sacrifice, an
increased number of males had swollen livers (3/50 [controls], 10/50,
[100 mg/kg feed], and 8/50 [500 mg/kg feed]). However, liver weights
and histopathological findings were within normal limits.
No treatment-related abnormalities were noted in the clinical
chemistry parameters that were evaluated. However, as in the long-
term study summarized above at higher dose levels, the gamma globulin
value in treated animals was consistently lower than in controls.
On histological evaluation, sclerotic bile duct hyperplasia was
more common in both males and females treated with enrofloxacin than
in control animals. The incidence was statistically significantly
increased in the 100 and 500 mg/kg feed groups after 52 weeks of
treatment, and in males and females at 500 mg/kg feed after 104 weeks
of treatment. The total number of females affected was markedly less
than the number of males. Although the incidence of bile duct
hyperplasia at 104 weeks at 100 mg/kg feed was not statistically
significantly different from the control values, the significant
findings in males in both dose groups at 52 weeks suggests that
administration of enrofloxacin accelerates the development of bile
duct sclerosis in male rats.
No evidence of carcinogenicity was reported in this study.
However, the heart and liver were the only two tissues examined
histologically. A NOEL could not be determined in this study (Leser,
1992).
A consulting pathologist conducted a second histopathologic
evaluation of liver slides of male rats from both the interim and
terminal kills in the above study. The consultant's conclusions were
as follows: "There was an increase over controls in the incidence of
bile duct hyperplasia in both dose groups at the interim kill,
although this was probably a spurious finding. At the terminal kill,
an increased incidence occurred only at the 500 ppm level. No clear
effect on lesion severity was noted in any group. The 100 ppm dose
(5.3 mg/kg bw/day) was a no-effect level in this study." (Squire,
1992).
A second limited long-term study was performed on Wistar
(Bor:WISW) rats (60/sex/group), administered enrofloxacin in the diet
at 0, 10, or 50 mg/kg feed (equal to 0, 0.6, or 2.9 mg/kg bw/day for
males and 0, 0.7, or 3.5 mg/kg bw/day for females, respectively) for
24 months. An interim kill of 10 animals/sex/group was performed at
12 months. The purpose of this study was to establish a NOEL for the
treatment-related bile duct hyperplasia observed in previous chronic
studies.
No treatment-related alterations were seen in physical
examinations, ophthalmologic evaluations, feed or water consumption,
body-weight gain, clinical chemistries, urinanalysis, or haematology
at the termination of the study. Postmortem examinations, including
gross observations, organ weights and histopathological examinations
were conducted at 12 and 24 months. Portions of the right and left
anterior liver lobes were collected and prepared for histological
evaluation Only one section per lobe was examined from these hepatic
samples. No treatment-related effects were reported in any of the
parameters monitored during the study. The incidence and severity of
bile duct hyperplasia in the exposed female and male rats were
comparable with the controls. The NOEL was 50 ppm (equal to 2.9 mg/kg
bw/day) (Leser, 1993).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl: CD(SD)Br rats at doses of 0, 500, 2000, or 7500 mg/kg feed. F0
males received the diet for approximately 70 days and the F0 females
for approximately 2 weeks prior to mating. Reproductive performance
of F0 and F1 generations was evaluated, as well as pup parameters.
A gross necropsy was performed on every animal and histopathologic
evaluation was conducted on reproductive organs.
Administration of the test article caused decreased body weights
at 7500 mg/kg feed (equal to 630 mg/kg bw/day) in the F0 and F1
generations. This effect was not accompanied by a reduction in food
consumption. Reproductive performance of the same animals was
significantly affected by the treatment at the high dose, which
included increased gestation length, reductions in the number of
litters, total pups, litter size and number of implants in the F0 and
F1 generations. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations,
which were considered the primary cause for the adverse effects on
fertility. The NOEL was 2000 mg/kg feed, equal to 165 mg/kg bw/day
(Clemens et al., 1986).
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl:CD (SD)Br rats at doses of 0, 125, 300, or 2000 mg/kg feed
(equivalent to 0, 6.2, 15, or 100 mg/kg bw/day). This study was
intended to evaluate the effect(s) of enrofloxacin on gonadal function
and mating behaviour in treated males, reproductive parameters in
females, and growth and development in the F1 and F2 offspring. F0
males and females received the test compound for 70 and 14 days,
respectively, before mating. A gross necropsy was performed on every
animal and histopathologic evaluation was conducted on testes and
epididymides from each F0 and F1 male.
Administration of the test article caused a reduction in mean
epididymal weights at the highest dose, and alterations in sperm
morphology at 300 and 2000 mg/kg feed. No effects on fertility or
reproductive performance were noted. The NOEL was 125 mg/kg feed,
equivalent to 6.2 mg/kg bw/day (Kowalski et al., 1989).
A specialized male fertility study was performed with
enrofloxacin to determine the timing of the onset of spermatic
dysmorphogenesis and to ascertain whether functional fertility was
restored during a 90-day recovery phase. Diets containing 0 or 7500
mg/kg feed (equivalent to 375 mg/kg bw/day) were administered to
sexually mature Crl:CD BR male rats (60/group) for 90 days. Interim
kills were performed at 3, 6, 9 and 13 weeks, with 15 males from each
group retained to mate with untreated females to assess male
fertility. The first mating was performed after 11 weeks on trial.
From day 91, all males were fed control diet and matings were again
performed on weeks 3, 7, and 11 of recovery. Females were killed on
day 20 of gestation.
Enrofloxacin administered at this dose caused significant
decreases in male fertility, food consumption and body-weight gains.
Testes weights were increased at all interim kills, but were less
than control values at the end of the study. Treatment-related
spermatic dysmorphogenic changes were seen by week 3 on trial. These
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Varying grades of seminiferous
tubular atrophy and other degenerative alterations were observed in
40% of these animals. Complete infertility was diagnosed in the
remaining males (Clemens et al., 1987).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
A teratogenicity (Segment II) study in the rat was performed in
which enrofloxacin as a 5% suspension with carboxymethylcellulose and
polysorbate 80 was administered by gavage to groups of 28 inseminated
COBS CD female rats at 0, 50, 210, or 875 mg/kg bw/day. Dosing was
performed on days 6-15 of gestation. On day 20, all dams were killed
and maternal and fetal parameters were evaluated.
Administration of the test article was maternally toxic at 210
and 875 mg/kg bw/day. Food consumption was higher in high-dose
animals, but there was a significant reduction in mean maternal weight
gain in these animals. In the high-dose litters, fetal weights and
litter size were significantly decreased and fetal resorption and
post-implantation losses were increased. Fetal weights were
significantly decreased in the mid-dose group. Skeletal examination
of the mid- and high-dose groups showed delayed skeletal ossification.
Neither embryotoxicity nor fetotoxicity was seen. No teratogenic
effects were observed. The NOEL was 50 mg/kg bw/day (Clemens &
Hartnagel, 1985).
2.2.5.2 Rabbits
An embryotoxicity (including teratogenicity) study was performed
with enrofloxacin administered by gavage to groups of 16 mated female
chinchilla rabbits at doses of 0, 1, 5, or 25 mg/kg bw/day admixed in
distilled water with 0.5% Tylose. Dosing was performed on days 6-18
of gestation. On day 28, all dams were killed. Because no maternal
effects were seen at doses up to 25 mg/kg bw/day, a supplementary
group of 16 females (with corresponding control group) was added and
administered enrofloxacin at 75 mg/kg bw/day.
Administration of the test article caused decreased food
consumption and lower body weights at 75 mg/kg bw/day. Increased
post-implantation losses were also noted in this group. All other
dose groups showed only incidental findings. The NOEL was 25 mg/kg
bw/day (Becker et al., 1987).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with enrofloxacin are
summarized in Table 2.
2.2.7 Special studies on the human gastrointestinal flora
No in vivo studies were available. In vitro studies of
Escherichia coli (24 strains), Staphylococcus aureus (24
strains) and Enterococcus spp. (24 strains) of human origin showed
the following MIC values for enrofloxacin (E) and ciprofloxacin (C).
Only summary tables were given.
The results of these studies are summarized in Table 3 (Scheer,
1993).
Data on in vitro susceptibility to ciprofloxacin for several
strains of bacteria obtained from "clinical isolates" or "bite wounds"
from humans were also available (Goldstein & Citron, 1988; Wise
et al., 1988).
The results of these studies are summarized in Table 4.
2.3 Observations in humans
Enrofloxacin has been developed specifically for use in
veterinary medicine; therefore, no human data were available.
Ciprofloxacin, the major metabolite of enrofloxacin, is a human drug
and has been used extensively. Clinical data in humans has produced
no evidence of nephrotoxicity or significant drug-related ocular
changes. Since the drug is not recommended for children or pregnant
women, little evidence has been collected to assess the occurrence of
articular or developmental changes in young patients (Schluter, 1987).
Table 2. Genotoxicity assays with enrofloxacin
Test system Test object Concentration Results Reference
In vitro
Unscheduled Rat primary 1-200 mg/ml Negative Cifone & Myhr, 1985
DNA synthesis hepatocytes
Bacterial reverse S. typhimurium 0.004 & 40 Inappropriate Herbold, 1985a
mutation1 TA98, TA100, ng/plate2 & deficient3
TA1535,TA15
TA1537
Mammalian cell CHO/HGPRT 0.25-1.25 Equivocal Den Boer & Hoorn,
forward mg/ml (-S9)4 1987
mutation1 0.375 - 1.25
mg/ml (+S9)5
Mammalian cell CHO/WBI 25-250 mg/ml Positive Taalman & Hoom,
chromosomal (-S9)6 1988
aberration1 0.1-1 mg/ml
(+S9)7
In vivo
Bone marrow Bor:NMRI mice 1-2 g/kg bw Negative Herbold, 1985b
micronucleus
Bone marrow CD rat 0.04-1 g/kg Negative Bootman et al. ,
chromosomal bw8 1987
aberration
Sister chromatid Chinese 2-8 g/kg bw Negative Herbold, 1988
exchange hamster bone
marrow
Table 2 (continued)
1 Both with and without rat liver S9 fraction
2 Positive controls: 2-Aminoanthracene, endoxan (TA100, TA1535),
and trypaflavine (TA98, TA1537).
3 Inappropriate due to substantial bacteriotoxicity; deficient due
to failed positive controls.
4 5-Bromodeoxyuridine as positive control.
5 3-Methylcholanthrene as positive control.
6 Mitomycin C as positive control.
7 Cyclophosphamide as positive control.
8 Low dose previous LD50 studies showed an oral dose tolerance of
>5 g/kg bw but in the present study, 6/30 animals administered 1 g/kg bw
had to be killed in extremis shortly after being dosed.
Table 3. Summary of MIC-values for bacterial species of human origin
Bacteria MIC range MIC50 MIC90
µg/ml µg/ml µg/ml
E C E C E C
E. coli 0.015-0.03 0.015-0.03 0.015 0.015 0.03 0.015
Staphylococcus 0.06-128 0.125-64 0.125 0.25 4.0 32.0
aureus
Enterococcus 0.25-1.0 0.25-2.0 0.5 1.0 1.0 2.0
spp.
Table 4. In vitro susceptibility of bacteria to ciprofloxacin
Bacteria Number of strains MIC range
µg/ml
Bacteroides fragilis 1 15 4-16
Bacteroides spp. 2 17 0.06-32
Clostridium spp. 1 17 0.012-8
E. coli 1 66 0.015-1
Enterobacter spp. 1 51 0.015-2
Enterococci 1 10 1-2
Flavobacterium 2 18 0.5-1
Fusobacterium spp. 2 18 0.25-32
Peptostreptococcus spp. 1 10 0.25-2
Peptostreptococcus spp. 2 16 0.03-8
1 Clinical isolate.
2 Isolated from bite wounds.
3. COMMENTS
The toxicological data available to the Committee were derived
from acute, short-term, long-term, and carcinogenicity studies;
reproductive and developmental studies; mutagenicity studies;
metabolism and residue studies; antimicrobial activity and
observations in humans following treatment with ciprofloxacin, the
major metabolite of enrofloxacin.
In rats orally dosed with 5 mg/kg bw of radiolabelled
enrofloxacin, the bioavailability of the dose was 75%. Enrofloxacin
was rapidly absorbed and distributed to all tissues, with the highest
concentration in the liver and kidney. Enrofloxacin was rapidly
excreted via urine and bile. Radioactivity in the urine was mainly
unchanged parent drug and the de-ethylated compound, ciprofloxacin.
Bile contained primarily unchanged enrofloxacin. Similar results were
obtained with all species of animals tested.
Enrofloxacin is a relatively stable molecule. Metabolism takes
place mainly in the liver, with the main metabolite in all species
being ciprofloxacin, probably formed by an oxidative dealkylation. In
rats four other components were identified, each comprising less than
2% of the total residue.
Single oral or topical doses of enrofloxacin were slightly toxic
to experimental animals (LD50 = 500-5000 mg/kg bw). In mice, single
intravenous doses were moderately toxic (LD50 = approximately 220
mg/kg bw).
Following 90-day oral administration of enrofloxacin to rats,
chronic degenerative changes in the auricular cartilage were seen at
doses equal to 150 and 577 mg/kg bw/day. Treatment-related
morphological changes in the spermatozoa and testicular tubular
atrophy were seen at 577 mg/kg bw/day. These effects were not seen at
a dose equal to 36 mg/kg bw/day.
A special study was performed to further assess the morphological
changes in the male reproductive tract seen in rats in previous
studies. Male rats were administered enrofloxacin in the feed at
doses equal to 10, 38 or 615 mg/kg bw/day followed by a 90-day
recovery period. Statistically significant decreases in epididymal
weights were seen at 91 days in the 38 and 615 mg/kg bw/day groups.
At 615 mg/kg bw/day, the testis/body-weight ratio was significantly
increased, while the epididymides/body-weight ratio was significantly
decreased. Bilateral testicular atrophy was seen in two rats at 615
mg/kg bw/day after the 90-day recovery period. Abnormal spermatozoa
were seen at 615 mg/kg bw/day at days 14 and 91, but not at the end of
the 90-day recovery period. The NOEL for testicular toxicity in this
study was 10 mg/kg bw/day.
To assess effects on testicular toxicity and articular cartilage
in young dogs, a 90-day oral study in three- and four-month old dogs
was performed. Enrofloxacin was administered at dose levels
equivalent to 0, 3, 9.6 or 75 mg/kg bw/day. All treated animals
except those dosed at 3 mg/kg bw/day showed degeneration of the
articular cartilage. The testes/body-weight ratio for all treated
animals was consistently higher than for control animals, but the
difference was not statistically significant. In 4 high-dose and 1
low-dose animals, marked dilatation of the seminiferous tubules, and
focal areas of single layer spermatogonial cells or vacuolated
epithelium in the tubules were considered beyond normal limits. It
could not be determined whether testicular changes observed in the
low-dose group were treatment-related or due to the fact that the
animals were not fully mature. Therefore, a NOEL could not be
determined. The testicular effects seen in this study were not seen
in 12-13-month old dogs treated orally with enrofloxacin up to 52
mg/kg bw/day for 90 days.
A 90-day study was performed in male dogs dosed with enrofloxacin
at dose levels equal to 0.3, 0.6, 1.2, or 92 mg/kg bw/day to determine
a NOEL for the testicular effects of enrofloxacin observed in the
earlier study with 3- to 4-month old dogs. At termination of the
study, testicular weights were determined and testes and epididymides
collected for histopathological assessment. No other tissues were
collected. Testicular weights and testes/body-weight ratios were
elevated over controls at 0.3, 0.6, and 1.2 mg/kg bw/day. In
addition, lameness was seen at 92 mg/kg bw/day following 2-3 days of
enrofloxacin administ-ration. This effect was not seen at 1.2 mg/kg
bw/day. One dog in the 92 mg/kg bw/day group also had bilateral
testicular degenerative changes. The NOEL was 1.2 mg/kg bw/day in
this study.
A 90-day oral study in 3-month old dogs with a 90-day recovery
period was conducted with 0, 0.3, or 1.2 mg/kg bw/day of enrofloxacin
to assure the continued absence of testicular effects after the
animals reached sexual maturity. After the recovery period, gross and
microscopic examination of testes and epididymides showed no changes
indicative of toxicity. Testes and epididymides appeared mature and
contained normal mature spermatozoa. There was no evidence of delayed
testicular toxicity in this study at levels up to and including 1.2
mg/kg bw/day.
Enrofloxacin was not carcinogenic in mice orally dosed at levels
equal to 323, 1100, or 3520 mg/kg bw/day in a 2-year long-term
toxicity/carcinogenicity study. Bile duct hyperplasia was seen in
males in the mid-dose group and at the high dose in both sexes. The
NOEL was 323 mg/kg bw/day.
In a long-term toxicity/carcinogenicity oral study in rats at
doses equal to 41, 103, 338 or 856 mg/kg bw/day, the Committee
concluded that enrofloxacin was not carcinogenic in this species.
However, a NOEL could not be determined for the bile duct hyperplasia
seen in this study. The NOEL for bile duct hyperplasia in further
limited chronic studies with enrofloxacin in rats was 2.9 mg/kg
bw/day.
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of rats
up to a dose equivalent to 630 mg/kg bw/day. Increased gestation
length, reductions in the number of litters, total pups, litter size
and number of implants for the F0 and F1 generations were seen at
630 mg/kg bw/day. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations and
were considered the primary cause for the adverse effects on
fertility. The NOEL for reproductive performance was 165 mg/kg
bw/day.
An additional two-generation reproduction study in rats was
performed with enrofloxacin administered in the feed to groups of rats
at 0, 125, 300, or 2000 mg/kg feed to determine whether the test
compound affected gonadal function and mating behaviour in treated
males or reproductive parameters in females, as well as, to monitor
growth and development in the F1 and F2 offspring. Administration
of the test article caused a reduction in mean epididymal weights at
the highest dose and alterations in sperm morphology at 15 and 100 mg/kg
bw/day. No effects on fertility or reproductive performance were
noted. The NOEL was 125 mg/kg feed, equal to 10 mg/kg bw/day.
A special fertility study was performed in sexually mature male
rats fed enrofloxacin in the diet at doses equivalent to 0 or 375
mg/kg bw/day for 90 days. The purpose of the study was to determine
the timing of the onset of spermatic dysmorphogenesis and to ascertain
whether functional fertility was restored during a 90-day recovery
phase. Administration of the test article caused a significant
decrease in male fertility, food consumption and body-weight gain.
Testes weights were increased at all interim kills, but were lower
than in the control animals at the end of the study. Treatment-
related spermatic dysmorphogenic changes were seen by week 3 and these
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Forty percent of these animals
demonstrated varying grades of seminiferous tubular atrophy and other
degenerative alterations. The remaining males were infertile.
A teratogenicity study was performed in which enrofloxacin was
administered to rats by gavage at 0, 50, 210, or 875 mg/kg bw/day.
Dosing was performed between days 6-15 of gestation. Maternal
toxicity was observed at 210 and 875 mg/kg bw/day. Food consumption
was higher in high-dose animals, but there was a significant reduction
in mean maternal weight gain in these animals. Fetal weights and
litter sizes were significantly decreased and fetal resorption and
post-implantation losses were increased in high-dose litters. Fetal
weights were significantly decreased in the mid-dose group. Skeletal
examination of the mid- and high-dose fetuses revealed delayed
skeletal ossification, which correlated with the decreased fetal
weights. No teratogenic effects were observed in this study. The
NOEL was 50 mg/kg bw/day.
In in vitro mutagenicity testing, enrofloxacin gave equivocal
results in a test for forward mutations in mammalian cells and
positive results in chromosomal aberration tests. Negative results
were obtained in in vivo bone marrow micronucleus, sister chromatid
exchange and bone marrow chromosomal aberration tests. The Committee
concluded that enrofloxacin is not genotoxic.
The potential for adverse effects on the human gastrointestinal
flora was considered from summary data. In vitro MIC data for
ciprofloxacin covering representatives of the human gut flora were
submitted for assessment. Escherichia coli was found to be the
most sensitive bacterium having an MIC50 value of 0.015 µg/ml.
In calculating the ADI this figure was used in the formula
developed at the thirty-eighth meeting of the Committee (Annex 1,
reference 97).
Concentration without
effect on human gut x Daily faecal bolus (g)
Upper limit of flora (µg/ml)a
temporary ADI =
(µg/kg bw) Fraction of
oral dose x Safety factorc x Weight of human (60 kg)
bioavailableb
(0.015 x 20) x 150
=
0.12 x 10 x 60
= 0.6 µg/kg bw
a Since the MIC value was determined using only 105 bacteria/ml a
factor of 20 was used to account for the higher inoculum density
representative of the human intestine. For example,
Enterobacteriaceae and Pseudomonas aeruginosa MIC values
increased 8 to 32-fold when the inoculum size was increased to
107 bacteria/ml.
b The fraction of the dose available to the intestinal microflora
was derived from studies of ciprofloxacin in humans. In human
volunteers given 2 x 50 mg ciprofloxacin the faecal concentration
was found to be 80 mg/kg. In 150 g faeces, this corresponds to
12% of the administered
c A safety factor of 10 was used to account for the possible
variation in absorption between ciprofloxacin and enrofloxacin, an
uncertainty in the biliary excretion of enrofloxacin, and the
variability between individuals.
4. EVALUATION
In view of the absence of information on the effects of
enrofloxacin on microorganisms obtained from the human intestine, the
Committee established a temporary ADI of 0-0.6 µg/kg bw based on the
results of the limited summary data of microbiology testing of
enrofloxacin/ciprofloxacin.
The temporary ADI of 0.6 µg/kg bw calculated from the in vitro
antimicrobial activity data represents an adequate margin of safety
when compared to the NOEL derived from the toxicology endpoint of 1.2
mg/kg bw/day for testicular toxicity.
5. REFERENCES
ALTREUTHER, P. & KLOSTERMANN, L. (1994). Unpublished summary of the
Enrofloxacin metabolism and residue information submitted to the FAO
experts. Submitted to WHO by Bayer AG, Leverkusen, Germany.
BARE, J.J., PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988). A
subchronic (13-week) feeding study followed by a 13-week drug
withdrawal period in male rats with BAY Vp 2674. Unpublished Report
No. MTD0074 from the Toxicology Department, Miles Inc., Elkhart, IN,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73812).
BECKER, H., VOGEL, W., & TERRIER, Ch. (1987). Embryotoxicity
(including teratogenicity) Study with BAY Vp 2674 in the Rabbit.
Unpublished Report No. R4249 (Project No. 058555) from the Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73705).
BOOTMAN, J., HODSON-WALKER, G., & DANCE, C. (1987). BAY Vp 2674;
Investigation of effects on bone marrow chromosomes of the rat after
acute oral administration. Unpublished LSR report No. 87/BAG039/312
(Report No. R4189; Study No. T5023055) from Life Science Research
Ltd., Suffolk, England. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 74166).
BOMHARD, E., RÜHL-FEHLERT, C., & KALINER, G. (1991a). BAY Vp 2674.
Study of chronic toxicity and carcinogenicity in mice (administration
in feed over 24 months). Unpublished Report No. 20263 (Study No.
T8023436) from the Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74229).
BOMHARD, E. & SCHLADT, L. (1991B). BAY Vp 2674. Study of chronic
toxicity and carcinogenicity in rats after administration in feed over
2 years. Unpublished Report No. 20306 (Study No. T8023436) from the
Department of Toxicology, Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany (report No. 74230).
DEN BOER, W.C. & HOORN, A.J.W. (1987). Mutagenicity evaluation of
BAY Vp 2674 in the CHO/HGPRT forward mutation assay: Revised final
report. Unpublished genetics assays Nos. E-9457 & E-9598 (Report No.
R 4071) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73582).
CIFONE, M.A., & MYHR, B.C. (1985). Evaluation of BAY Vp 2674 in the
rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished
Report No. 3168E (Genetic Assay No. 7550; LBI project No. 20991) from
Litton Bionetics, Inc., Kensington, MD. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73055).
CLEMENS, G.R. & HARTNAGEL, R.E. (1985). A teratology (Segment II)
study in the rat with BAY Vp 2674. Unpublished Report No. 53 from the
Toxicology Department, Central Research Services, Miles Laboratories
Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 73159).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1986). A two-generation
reproduction study in rats with BAY Vp 2674. Unpublished Report No.
4 from Toxicology Department, Central Research Services, Miles
Laboratories Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73314).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1987). A specialized
male fertility study with BAY Vp 2674 in the rat. Unpublished Report
No. MTD0030 (Mobay Report No. 73800) from the Toxicology Department,
Central Research Services, Miles Laboratories Inc., Elkhart, IN, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany (report No. 73800).
EIGENBERG, D.A. (1987). Acute dermal toxicity of BAY Vp2674 in albino
rabbits. Unpublished toxicological report No. 926 (Study No. 86-023-
04) from Health, Environment & Safety, Corporate Toxicology
Department, Mobay Corporation, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73606).
GOLDSTEIN, E.J.C., & CITRON, D.M. (1988). Comparative activities of
cefuroxime, amoxicillin-clavulanic acid, ciprofloxacin, enoxacin, and
ofloxacin against aerobic and anaerobic bacteria isolated from bite
wounds. Antimicrob. Agents Chemoth., 32:1143-1148.
HALL, W.D. (CHAIR) (1992). Pathology working group on a 2-year
chronic feeding study with a l-year interim kill in rats on the
compound BAY Vp 2674. Unpublished Pathology Working Group report
(Bayer ID 014379) prepared for the Center for Veterinary Medicine,
U.S. Food & Drug Administration, Department of Health & Human
Services, Rockville, MD, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
HERBOLD, B. (1985a). BAY Vp 2674; Salmonella /microsome test to
evaluate point mutagenic effects. Unpublished Report No. 13523E
(Study No. T9017669) from the Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73213).
HERBOLD, B. (1985b). BAY Vp 2674; Micronucleus test on the mouse to
evaluate for mutagenic effects. Unpublished Report No. 14102E (Study
Nos. T6019042 & T8019675) from Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74164).
HERBOLD, B.A. (1988). BAY Vp 2674; Sister chromatid exchange in the
bone marrow of the Chinese hamster in in vivo as a test for DNA-
modifying effects. Unpublished Report No. 16783 (Study Nos. T5024676
& T8027324) from Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74178).
KOWALSKI, R.L., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985).
Safety evaluation of BAY Vp 2674: Subchronic (13 week) feeding study
in the rat. Unpublished Report No. 63 (No. 73194) from Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73194).
KOWALSKI, R.L., CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1989).
A two-generation reproduction study in rats with BAY Vp 2674.
Unpublished Report No. MTD0093 (Report No. R4705) Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73892).
LESER, K.H. (1992). BAY Vp 2674. Chronic toxicity study in rats after
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 21668A (Study No. T7032787) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74387).
LESER, K.H. (1993). BAY Vp 2674. Chronic toxicity study in rats with
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 22659 (Study No. T6039950) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74472).
MANDELL, G.L., & SANDE, M.A. (1990). Antimicrobial Agents in Goodman
and Gilman's the Pharmacological Basis of Therapeutics, 8th edition,
Gilman, A.G., Rall, T.W., Nies, S.A., and Taylor, P. (eds.). Pergamon
Press, Inc., Maxwell House, Fairview Park, Elmsford, New York 10523,
p. 1058.
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985). Safety
evaluation of BAY Vp 2674: Subchronic (13 week) feeding study in the
dog. Unpublished Report No. 43 (Mobay Report No. 73146) from the
Toxicology Department, Central Research Services, Miles Laboratories,
Inc., Elkhart, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73146).
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1987). Safety
evaluation of BAY Vp 2674: Repeat of a subchronic (13-week) feeding
study in the dog. Unpublished Report No. MTD0031 (BayVet report No.
73146) from the Toxicology Department, Central Research Services,
Miles Laboratories, Inc., Elkhart, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73775).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988a). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs.
Unpublished Report No. MTD0068 from the Toxicology Department, Central
Research Services, Miles Inc., Elkhart, IN, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73788).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988b). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs
followed by a 13-week withdrawal period. Unpublished report Miles
Inc., Elkhart, Report No. MTD0069. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73789).
SCHLUTER, G. (1987). Ciprofloxacin: review of potential toxicologic
effects. Am. J. Med, 82 (suppl 4A): 91-93.
SCHMIDT, M. (1985). Acute toxicity of BAY Vp 2674 in the rat, mouse,
rabbit and dog. Unpublished Report No. 13223(E) from Institute of
Toxicology, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany (report No. 73075).
SQUIRE, R.A. (1992). Evaluation of bile duct hyperplasia in male rat
livers. Unpublished Report No. 74388 (Study No. T7032787; Pathology
No. 3122) from Toxicology, Research & Development Dept., Miles Inc.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74388).
TAALMAN, R.D.F.M. & HOORN, A.J.W. (1988). Clastogenic evaluation of
BAY Vp 2674 in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished HBC study No. E-9804-0-437 (Report No. R 4474; Study No.
T1027516) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74165).
WISE, R., ANDREWS, J.M., ASHBY, J.P. & MATTHEWS, R.S. (1988).
In vitro Activity of lomefloxacin, a new quinolone antimicrobial
agent, in comparison with those of other agents. Antimicrob. Agents
and Chemoth., 32: 617-622.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The following information was provided in a summary report of
metabolism and residue data on enrofloxacin. The full study reports
were not provided.
2.1.1.1 Rats
Rats (number, species, strain not specified) were treated orally
or intravenously with a single dose (5 mg/kg bw) of radiolabelled
enrofloxacin. The bioavailability was calculated to be approximately
75%. Enrofloxacin was rapidly absorbed and distributed to all
tissues. Highest concentrations were found in the liver and kidney,
followed by muscle. Fat did not contain appreciable amounts of
radioactivity. Elimination was rapid via urine and faeces. Most of
the radioactivity was excreted during the first 24 hours after
administration. About 40% of the radioactivity was excreted in bile.
The radioactivity in urine was attributed primarily to the unchanged
parent drug, its de-ethylated metabolite, ciprofloxacin, and the
glucuronide of enrofloxacin. Bile contained mainly the unchanged
parent molecule (Altreuther & Klostermann, 1994).
2.1.1.2 Cattle, pigs, chickens, and turkeys
Trials in all species showed that enrofloxacin is absorbed
readily and distributes to all organs and tissues following oral or
parenteral administration. In pigs, when the same dose was
administered by the oral or i.m. route, similar rates of absorption
and subsequent concentrations of drug in tissues were observed.
Following parenteral administration, maximum serum concentrations in
pigs and calves were reached within 1 to 2 hours. The highest drug
concentration initially occurred in the liver in all species.
Following a one-day withdrawal in poultry, highest drug concentrations
were found in the skin. In all species studied, elimination was
primarily via the urine and bile/faeces (Altreuther & Klostermann,
1994).
2.1.2 Biotransformation
2.1.2.1 Rats, cattle, pigs, and poultry
Rats (number, species, strain not specified) were treated orally
with 165 mg/kg bw of radiolabelled enrofloxacin for 3 consecutive
days, then with 50 mg/kg bw on the fourth day. Major urinary
metabolites were enrofloxacin (30-36%), ciprofloxacin (26- 31%), and
enrofloxacin glucuronide (19-29%). Four other components were
identified, each less than 2% of the total residue (Altreuther &
Klostermann, 1994).
In food animal species, ciprofloxacin was the main metabolite
recovered. The liver is considered the primary site of enrofloxacin
metabolism in these species, forming ciprofloxacin through oxidative
dealkylation. Additional metabolites occurred but comprised less than
10% of the total residue (Altreuther & Klostermann, 1994).
2.1.3 Effects on enzymes and other biochemical parameters
The bactericidal in activity of quinolones, including
enrofloxacin, is attributed to their ability to inhibit DNA gyrase
(Mandell & Sande, 1990).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of enrofloxacin is summarized in Table 1.
Table 1. Acute toxicity studies on enrofloxacin
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse male p.o. > 5000 Schmidt, 1985
female 4336
male i.v. 225 Schmidt, 1985
female 220
Rat male p.o. > 5000 Schmidt, 1985
female
Rabbit male p.o. 500-800 Schmidt, 1985
female
male topical > 2000 Eigenberg,1987
female
Dog male p.o. n/a1 Schmidt, 1985
female
1 Not determined due to vomiting of the drug.
Animals were observed daily for 14 days post-exposure in these
studies. Clinical signs associated with enrofloxacin toxicosis
included lethargy, trembling, tonic convulsions, dyspnea, prone and
lateral decubitus, and ataxia. Clinically affected animals that did
not die recovered within 15 minutes to 7 days post-exposure. Gross
post-mortem lesions reported included pulmonary congestion and
haemorrhage.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of Charles River rats (15/sex/group) were administered
diets containing 0, 500, 2000, or 7500 mg enrofloxacin/kg feed (equal
to 36, 150, or 577 mg/kg bw/day for males and 45, 182, or 690 mg/kg
bw/day for females, respectively) for 90 days. No overt effects on
appearance and behaviour were noted at any dose level. Body-weight
gain was reduced at the highest dose in both males (20%) and females
(23%), while the actual diet consumption for males and females
remained constant.
Serum chemistry findings included a significant dose-related
decrease in total plasma protein in the mid- and high-dose males and
females at 6 and 13 weeks. Total bilirubin and alanine aminotrans-
ferase were decreased in the males at 6 weeks but were normal at 13
weeks. Aspartate aminotransferase was decreased in the high-dose
males at 6 and 13 weeks. Globulins were decreased in the high-dose
males at 6 weeks and 13 weeks and high-dose females at 13 weeks. A
corresponding increase in the A/G ratio was seen in the high-dose
males at 6 and 13 weeks.
Haematology findings were unremarkable.
Urinalyses showed a significant decrease in urine sodium output
for males in the mid- and high-dose groups after 6 weeks and for
females in the mid- and high-dose groups at 13 weeks.
Treatment-related gross findings included swollen ears (2 males
and 4 females in the mid-dose group, 4 males and 5 females in the
high-dose group, and 1 female in the control group) and caecal
distension (1 male in the mid-dose group and 3 males and 5 females in
the high-dose group).
Mean and relative prostate weights were statistically
significantly reduced in the mid- and high-dose groups. Mean heart
weights were statistically significantly reduced in high-dose males
and in mid- and high-dose females. Mean liver weights were reduced in
both male and female high-dose animals. The reduction was
statistically significant in females.
Histological findings included auricular chondropathy which was
present in all groups except low-dose males and appeared to be dose-
related (1 control, 1 low dose, 6 mid dose, and 10 high dose). In the
testes of 12/15 rats from the high-dose group, dark, round or oval
cells were distributed among the spermatozoa in the ducts of the
epididymides. These cells were occasionally observed in seminiferous
tubules of 5/15 high-dose rats. These findings were considered
treatment-related.
Based on decreased body-weight gain and microscopic changes
observed in the ducts of the epididymides and testes in the high-dose
group, and gross and microscopic changes indicating auricular
chondropathy in the mid- and high-dose groups, the NOEL was 500 mg/kg
feed, equal to 36 mg/kg bw/day (Kowalski et al., 1985).
A special study was performed in male rats to further assess the
testicular toxicity seen in previous studies. A 13-week recovery
period was included in the study. Thirty male Crl:CD Charles River
rats per group were dosed with 0, 125, 500, or 7500 mg enrofloxacin/kg
feed (equal to 10, 38, or 615 mg/kg bw/day) for 90 days. Fifteen
animals/group were killed on day 91 and the remaining 15 on day 181.
An apparent dose-related decrease in epididymal weights was observed
in all dose groups at 91 and 181 days. The difference achieved
statistical significance (compared to controls) in the mid- and high-
dose groups at both time points. Relative mean testes weight was
significantly decreased in the high-dose group after 91 days.
Bilateral testicular atrophy was seen in two high-dose rats at 181
days. Abnormal spermatozoa were seen at day 91 in the mid- and high-
dose groups but not after the recovery period. The NOEL for this
parameter was 125 mg/kg feed, equal to 10 mg/kg bw/day (Bare et al.,
1988).
2.2.2.2 Dogs
Four groups (4/sex/group) of beagle dogs (3-4 months old, 2.6 to
4.8 kg bw) were administered enrofloxacin in the diet at 0, 100, 320
or 2500 mg/kg feed (equal to 3, 9.6, or 75 mg/kg bw/day, respectively)
for 91 days.
No dogs died during the study. Sporadic incidences of mild
diarrhoea and vomiting were reported early in the study. Vomiting
occurred 5, 6, and nearly 24 hours after ingestion of the test
article. Severe hyperextension (upon manipulation) of carpal joints,
hypoactivity, and abnormal gate or posture, were observed in all
animals in the high-dose group during weeks 1 and 2. Radiographic
examination (8 dogs total) revealed several abnormalities including
valgus deformity at the carpus, asymmetry of the distal ulnar physis,
remodelling of the radial carpal bone, radius curvus, and increased
width of the radial humeral joint. The consulting radiologist
concluded that only the radial carpal remodelling appeared to be dose-
related, and neither bone growth nor density were significantly
affected.
Serum chemistry and haematologic findings were unremarkable.
However, two types of unidentified crystals in urine samples from dogs
in the 2500 mg/kg feed group were considered treatment-related.
Gross postmortem findings included an increase in mean absolute
and relative testicular weights for treated animals when compared to
control animals. However, a clear dose-response relationship was not
established and none of the differences were statistically significant
when compared to controls. In 8/8 high-dose animals and 2/8 mid-dose
animals joint lesions were observed grossly as superficial (0.2 to 0.4
cm in diameter) erosions with dulling of the surfaces on the femoral
head of the hip joint and/or the femoral condyles of the knee joint.
Histologically, joint lesions were characterized by splitting of the
articular cartilage with disorganization of chondrocytes and formation
of "brood capsules" of cartilage cells. Necrosis and disintegration
of hyaline cartilage at the area of splitting was also noted.
There was marked variation in the microscopic appearance of
testes from animal to animal with respect to the stage of maturity,
diameter of the lumen of seminiferous tubules, and vacuolar change in
cells lining the tubules. However, it was not clear whether these
testicular changes were treatment-related because there was no dose-
response relationship in incidence or severity. These variations in
testicular morphology could represent normal variation in the maturing
process of this organ in this species. Due to the uncertainty of the
significance of the testicular findings in the low-dose males in this
study, the Committee concluded that a no-effect level was not observed
(Porter et al., 1987).
In another 90-day dietary study in beagle dogs, 4 groups
(4/sex/group) of adult dogs (12-13 months old weighing 6.4 to 12.5 kg)
received enrofloxacin at 0, 320, 800 or 2000 mg/kg diet (equal to 9.3,
22, or 53 mg/kg bw/day for males, and 8.9, 23, or 51 mg/kg bw/day for
females, respectively). No joint or testicular lesions were reported
in this study (Porter et al., 1985).
A special limited 90-day dietary study in males was performed.
Five groups (4 dogs/group) of 3-month old beagle dogs (weighing 4.1 to
5.7 kg) received enrofloxacin at 0, 10, 20, 40, or 3200 mg/kg diet
(equal to 0, 0.3, 0.6, 1.2, or 92 mg/kg bw/day, respectively). At
termination of the study (day 92) testicular weights were determined
and testes and epididymides collected for histopathological
assessment. No other tissues were collected.
Histologically, the tissues examined contained wide variations in
morphology attributable to differences in sexual maturation. Sections
of epididymides examined were considered normal. Bilateral testicular
degeneration characterized by multinucleated giant cells and cells
with large cytoplasmic and nuclear volumes, sometimes in mitosis, were
reported in the seminiferous tubules of one dog at the high dose.
Seminiferous tubules lined with single layers of spermatogonial cells
and forming open lumens were present in 2/4 dogs in the 10 mg/kg feed
group and 3/4 dogs in each of the 20, 40, and 3200 mg/kg feed groups.
This change was also reported in one control animal. Spermatocytic
giant cells were reported in 0, 1, 2, 2 and 3 dogs from the 0, 10, 20,
40 and 3,200 mg/kg feed groups, respectively.
The author attributed the seminiferous tubule changes to normal
variation in sexual maturity because similar morphology was reported
in the testes of one control dog. The author used this finding to
support a NOEL of 100 mg/kg feed in the 90-day study reported above
(Porter et al., 1987). The Committee concluded that a NOEL for
testicular effects in this special, limited 90-day study could not be
determined due to the inability to determine whether variations in
testicular morphology were normal or due to treatment with the test
article (Porter et al., 1988a).
Three-month old male beagle dogs (4/group) were administered
enrofloxacin at 0, 10, or 40 mg/kg diet (equal to 0.3 or 1.2 mg/kg
bw/day) for 90 days, then maintained on control diets for an
additional 91 days before being killed. The protocol was intended to
evaluate the potential for enrofloxacin testicular toxicity to appear
after animals reach sexual maturity, following exposure at an immature
age (delayed testicular toxicity).
No signs of clinical toxicity were observed nor was body-weight
gain or food consumption affected. Gross and microscopic examination
of testes and epididymides showed no changes indicative of toxicity.
Testes and epididymides appeared mature in all treated animals and
contained normal, mature spermatozoa. The NOEL for delayed testicular
toxicity was 40 mg/kg diet, equal to 1.2 mg/kg bw/day, the highest
dose tested (Porter et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
B6C3F1 mice (60/sex/group) received diets containing 0, 1000,
3300, or 10 000 mg enrofloxacin/kg feed (equal to 323, 1100, or 3520
mg/kg bw/day for males and 373, 1210, or 3700 mg/kg bw/day for
females, respectively) for 24 months. An interim sacrifice
(10/sex/group) was performed after one year. An additional dose group
(10 mice/sex) was administered 20 000 mg enrofloxacin/kg feed (equal
to 8030 mg/kg bw/day for males and 8010 mg/kg bw/day for females,
respectively) and scheduled for interim sacrifice after one year.
During the treatment phase of the study, general appearance and
physical examination findings were within normal limits in all treated
groups. Treatment-related ophthalmological findings included a marked
increase (relative to control animals) in the incidence of focal
lenticular opacity in males and females at 10 000 mg/kg feed.
Histopathological examination of eyes revealed no treatment-related
effects. However, it was noted that focal lenticular changes are only
verifiable to a limited extent with usual histolopathological methods.
Clinical chemical and haematological evaluations were performed
on 10 animals/sex/dose after 12 and 24 months on test. Alkaline
phosphatase levels were significantly lower in females at 3300 mg/kg
feed at 12 months, and at 10 000 mg/kg feed at study termination.
Concurrent with these findings, alanine aminotransferase and aspartate
transaminase values were within normal limits. Total protein was
decreased in males and females at all treatment levels as a result of
decreased globulin fractions.
Haematological analyses revealed a significant decrease in white
blood cell counts in males at 10 000 mg/kg feed at 12 months and at
3300 mg/kg feed at study termination. In females, a significant
decrease in white blood cell counts was noted at 10 000 mg/kg feed at
study termination. At 10 000 mg/kg feed, decreases were seen in
haematocrit, MCV, and MCH values in both sexes, and in the haemoglobin
value in males, at study termination.
Gross necropsy findings included a dose-related incidence of
caecal dilation in males at 1000, 3300, and 10 000 mg/kg feed and in
females at 3300 and 10 000 mg/kg feed. Histopathological examinations
showed neither an increase in the incidence of, nor a decrease in the
time of appearance to tumours in the dosed groups compared to the
controls. Bile duct hyperplasia was seen at 3300 mg/kg feed in males
and 10 000 mg/kg feed in both sexes. Focal papillary mucosal
hyperplasia of the gall bladder was also present in males and females
in these same dose groups. There was no evidence of carcinogenicity
in this study. The NOEL was 1000 mg/kg feed, equal to 323 mg/kg
bw/day (Bomhard et al., 1991a).
2.2.3.2 Rats
A combined long-term toxicity (52 weeks) and carcinogenicity (104
weeks) study of enrofloxacin was conducted in Wistar rats (BOR:WISW).
In the chronic study, 10 rats/sex/group received diets containing 0,
770, 2000, 6000, or 10 000 mg enrofloxacin/kg feed (equal to 0, 41,
103, 338, or 856 mg/kg bw/day for males and 0, 58, 146, 466, or 1000
mg/kg bw/day for females, respectively). In the carcinogenicity
study, 50 rats/sex/group received diets containing 0, 770, 2000, or
6000 mg enrofloxacin/kg feed (equal to 0, 41, 103, or 338 mg/kg bw/day
for males and 0, 58, 146, or 466 mg/kg bw/day for females).
Mortality rates, daily observations, and ophthalmologic findings
in treated animals were comparable to controls. Body-weight gain was
decreased in males in the 6000 mg/kg feed group and in males and
females at 10 000 mg/kg feed. Feed and water intakes were increased
in males and females at 10 000 mg/kg feed. Water intake was also
elevated at 6000 mg/kg feed.
Serum chemistry evaluations were performed after 6, 12, 18, and
24 months. Notable findings included a significant decrease in total
protein in males in all dose groups at all sampling periods. Total
protein was also significantly decreased in females in the high-dose
group both at 6 months and at the end of 2 years, and at 2000 and 10
000 mg/kg feed after 1 year. The albumin value was increased in
males at 10 000 mg/kg feed after 1 year, at 6000 mg/kg feed after 18
months, and in the two highest-dose groups (2000 and 6000 mg/kg feed)
at the end of 2 years. Bilirubin was significantly decreased in
females in all dose groups at the 6-month sampling time only.
Protein electrophoresis performed after 12, 18, and 24 months
showed a decrease in gamma-globulin in males and females in all dose
groups. The author noted that this finding is not unexpected in
animals receiving antimicrobials, as they lower the background
incidence of common pathogens, resulting in a decrease in antigenic
stimulation.
Haematology evaluations were performed after 6, 12, 18, and 24
months. At doses of 2000 mg/kg feed and above, there were
statistically significant and sometimes dose-related decreases in RBC
counts in males and haemoglobin and haematocrit values in both sexes
at various sampling times. However, the values for these parameters
in both sexes in all dose groups were comparable to controls at the
terminal sampling period. After 6 months leucocyte counts in both
sexes were decreased compared to controls at all dose levels. In most
instances, these differences were statistically significant. A trend
toward decreased leucocyte counts in the treatment groups was also
discernible in the subsequent investigations. The author noted that
due to the pharmaco-logical action of antimicrobial compounds,
decreased leucocyte counts are a common finding.
On gross postmortem examination, a decrease in testicular size
(small with altered texture) was noted at 2000 mg/kg feed and above.
Histologically, marked testicular atrophy, decreased spermatogenesis,
and a significant increase in the amount of mineralized concretion in
the testes were noted in these same dose groups. Normal age-related
degenerative changes occurred with greater frequency in treated
animals than in controls. Caecal dilation was reported in both sexes
of rats in the high-dose group and in the males at 2000 mg/kg feed.
Organ weights recorded at both the interim and terminal kill
revealed a decrease in absolute and relative liver weights in males at
2000 mg/kg feed and above. The same changes were seen in females in
these groups at the interim necropsy but not at study termination. At
the interim and terminal kills, absolute weights of the brain, testes
and adrenals were significantly decreased in the 6000 mg/kg feed
males. However, the relative weights of these organs were comparable
to controls.
Histopathological evaluation revealed a dose-related increase in
incidence of hepatic cysts at 24 months, bile duct hyperplasia with
sclerosis at 12 and 24 months, and cystic bile duct hyperplasia at 24
months in both sexes and in all treatment groups. Spinal cord
demyelination/degeneration was reported in all treatment groups.
Because of the high incidence of this finding in controls and without
further definition of lesions into degeneration versus demyelination,
the biological significance of this finding is uncertain. There was
also a marked increase in degenerative changes in the sciatic nerve.
A marked increase in the number of sarcolemmal nuclei in skeletal
muscle cells of animals in the 6000 mg/kg feed group was noted. This
finding is generally associated with degenerative changes in muscle
fibres.
A marked increase in the incidence of cardiomyopathy was
observed in males at 6000 mg/kg feed and in females in all dose
groups. Incidences at 0, 770, 2000, and 6000 mg/kg feed were 10/48,
8/50, 13/50, and 24/50 in males and 8/50, 16/50, 17/50, and 25/50 in
females, respectively. Endocardial neoplasms and Schwann cell-like
hyperplasia were seen in the hearts of some of the animals. These
findings were evaluated by a Pathology Working Group which concluded
that these lesions were not related to treatment with the test
article. Under the conditions of this study there was no evidence of
a carcinogenic effect of the test substance. A NOEL could not be
determined because of the occurrence of bile duct changes and
cardiomyopathy in the low-dose group (Bomhard et al., 1991b; Hall,
1992).
A limited long-term study was performed with Wistar (Bor:WISW)
rats (60/sex/group). Diets containing 0, 100, or 500 mg
enrofloxacin/kg feed (equal to 0, 5.3, or 26 mg/kg bw/day for males
and 0, 7.2, or 36 mg/kg bw/day for females, respectively) were
administered for 24 months. An interim kill of 10 rats/sex/group was
performed at 12 months.
Morbidity, moribundity, and mortality in treated groups were
comparable to controls. General appearance, clinical observations,
and the results of physical and ophthalmoscopic examinations were all
comparable with normal animals of this species and age. Exposure to
enrofloxacin produced no biologically significant alterations in food
or water intake, body weight, clinical chemical parameters or
haematological parameters.
At interim sacrifice, no abnormalities were noted in males or
females on postmortem examination. At terminal sacrifice, an
increased number of males had swollen livers (3/50 [controls], 10/50,
[100 mg/kg feed], and 8/50 [500 mg/kg feed]). However, liver weights
and histopathological findings were within normal limits.
No treatment-related abnormalities were noted in the clinical
chemistry parameters that were evaluated. However, as in the long-
term study summarized above at higher dose levels, the gamma globulin
value in treated animals was consistently lower than in controls.
On histological evaluation, sclerotic bile duct hyperplasia was
more common in both males and females treated with enrofloxacin than
in control animals. The incidence was statistically significantly
increased in the 100 and 500 mg/kg feed groups after 52 weeks of
treatment, and in males and females at 500 mg/kg feed after 104 weeks
of treatment. The total number of females affected was markedly less
than the number of males. Although the incidence of bile duct
hyperplasia at 104 weeks at 100 mg/kg feed was not statistically
significantly different from the control values, the significant
findings in males in both dose groups at 52 weeks suggests that
administration of enrofloxacin accelerates the development of bile
duct sclerosis in male rats.
No evidence of carcinogenicity was reported in this study.
However, the heart and liver were the only two tissues examined
histologically. A NOEL could not be determined in this study (Leser,
1992).
A consulting pathologist conducted a second histopathologic
evaluation of liver slides of male rats from both the interim and
terminal kills in the above study. The consultant's conclusions were
as follows: "There was an increase over controls in the incidence of
bile duct hyperplasia in both dose groups at the interim kill,
although this was probably a spurious finding. At the terminal kill,
an increased incidence occurred only at the 500 ppm level. No clear
effect on lesion severity was noted in any group. The 100 ppm dose
(5.3 mg/kg bw/day) was a no-effect level in this study." (Squire,
1992).
A second limited long-term study was performed on Wistar
(Bor:WISW) rats (60/sex/group), administered enrofloxacin in the diet
at 0, 10, or 50 mg/kg feed (equal to 0, 0.6, or 2.9 mg/kg bw/day for
males and 0, 0.7, or 3.5 mg/kg bw/day for females, respectively) for
24 months. An interim kill of 10 animals/sex/group was performed at
12 months. The purpose of this study was to establish a NOEL for the
treatment-related bile duct hyperplasia observed in previous chronic
studies.
No treatment-related alterations were seen in physical
examinations, ophthalmologic evaluations, feed or water consumption,
body-weight gain, clinical chemistries, urinanalysis, or haematology
at the termination of the study. Postmortem examinations, including
gross observations, organ weights and histopathological examinations
were conducted at 12 and 24 months. Portions of the right and left
anterior liver lobes were collected and prepared for histological
evaluation Only one section per lobe was examined from these hepatic
samples. No treatment-related effects were reported in any of the
parameters monitored during the study. The incidence and severity of
bile duct hyperplasia in the exposed female and male rats were
comparable with the controls. The NOEL was 50 ppm (equal to 2.9 mg/kg
bw/day) (Leser, 1993).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl: CD(SD)Br rats at doses of 0, 500, 2000, or 7500 mg/kg feed. F0
males received the diet for approximately 70 days and the F0 females
for approximately 2 weeks prior to mating. Reproductive performance
of F0 and F1 generations was evaluated, as well as pup parameters.
A gross necropsy was performed on every animal and histopathologic
evaluation was conducted on reproductive organs.
Administration of the test article caused decreased body weights
at 7500 mg/kg feed (equal to 630 mg/kg bw/day) in the F0 and F1
generations. This effect was not accompanied by a reduction in food
consumption. Reproductive performance of the same animals was
significantly affected by the treatment at the high dose, which
included increased gestation length, reductions in the number of
litters, total pups, litter size and number of implants in the F0 and
F1 generations. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations,
which were considered the primary cause for the adverse effects on
fertility. The NOEL was 2000 mg/kg feed, equal to 165 mg/kg bw/day
(Clemens et al., 1986).
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl:CD (SD)Br rats at doses of 0, 125, 300, or 2000 mg/kg feed
(equivalent to 0, 6.2, 15, or 100 mg/kg bw/day). This study was
intended to evaluate the effect(s) of enrofloxacin on gonadal function
and mating behaviour in treated males, reproductive parameters in
females, and growth and development in the F1 and F2 offspring. F0
males and females received the test compound for 70 and 14 days,
respectively, before mating. A gross necropsy was performed on every
animal and histopathologic evaluation was conducted on testes and
epididymides from each F0 and F1 male.
Administration of the test article caused a reduction in mean
epididymal weights at the highest dose, and alterations in sperm
morphology at 300 and 2000 mg/kg feed. No effects on fertility or
reproductive performance were noted. The NOEL was 125 mg/kg feed,
equivalent to 6.2 mg/kg bw/day (Kowalski et al., 1989).
A specialized male fertility study was performed with
enrofloxacin to determine the timing of the onset of spermatic
dysmorphogenesis and to ascertain whether functional fertility was
restored during a 90-day recovery phase. Diets containing 0 or 7500
mg/kg feed (equivalent to 375 mg/kg bw/day) were administered to
sexually mature Crl:CD BR male rats (60/group) for 90 days. Interim
kills were performed at 3, 6, 9 and 13 weeks, with 15 males from each
group retained to mate with untreated females to assess male
fertility. The first mating was performed after 11 weeks on trial.
From day 91, all males were fed control diet and matings were again
performed on weeks 3, 7, and 11 of recovery. Females were killed on
day 20 of gestation.
Enrofloxacin administered at this dose caused significant
decreases in male fertility, food consumption and body-weight gains.
Testes weights were increased at all interim kills, but were less
than control values at the end of the study. Treatment-related
spermatic dysmorphogenic changes were seen by week 3 on trial. These
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Varying grades of seminiferous
tubular atrophy and other degenerative alterations were observed in
40% of these animals. Complete infertility was diagnosed in the
remaining males (Clemens et al., 1987).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
A teratogenicity (Segment II) study in the rat was performed in
which enrofloxacin as a 5% suspension with carboxymethylcellulose and
polysorbate 80 was administered by gavage to groups of 28 inseminated
COBS CD female rats at 0, 50, 210, or 875 mg/kg bw/day. Dosing was
performed on days 6-15 of gestation. On day 20, all dams were killed
and maternal and fetal parameters were evaluated.
Administration of the test article was maternally toxic at 210
and 875 mg/kg bw/day. Food consumption was higher in high-dose
animals, but there was a significant reduction in mean maternal weight
gain in these animals. In the high-dose litters, fetal weights and
litter size were significantly decreased and fetal resorption and
post-implantation losses were increased. Fetal weights were
significantly decreased in the mid-dose group. Skeletal examination
of the mid- and high-dose groups showed delayed skeletal ossification.
Neither embryotoxicity nor fetotoxicity was seen. No teratogenic
effects were observed. The NOEL was 50 mg/kg bw/day (Clemens &
Hartnagel, 1985).
2.2.5.2 Rabbits
An embryotoxicity (including teratogenicity) study was performed
with enrofloxacin administered by gavage to groups of 16 mated female
chinchilla rabbits at doses of 0, 1, 5, or 25 mg/kg bw/day admixed in
distilled water with 0.5% Tylose. Dosing was performed on days 6-18
of gestation. On day 28, all dams were killed. Because no maternal
effects were seen at doses up to 25 mg/kg bw/day, a supplementary
group of 16 females (with corresponding control group) was added and
administered enrofloxacin at 75 mg/kg bw/day.
Administration of the test article caused decreased food
consumption and lower body weights at 75 mg/kg bw/day. Increased
post-implantation losses were also noted in this group. All other
dose groups showed only incidental findings. The NOEL was 25 mg/kg
bw/day (Becker et al., 1987).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with enrofloxacin are
summarized in Table 2.
2.2.7 Special studies on the human gastrointestinal flora
No in vivo studies were available. In vitro studies of
Escherichia coli (24 strains), Staphylococcus aureus (24
strains) and Enterococcus spp. (24 strains) of human origin showed
the following MIC values for enrofloxacin (E) and ciprofloxacin (C).
Only summary tables were given.
The results of these studies are summarized in Table 3 (Scheer,
1993).
Data on in vitro susceptibility to ciprofloxacin for several
strains of bacteria obtained from "clinical isolates" or "bite wounds"
from humans were also available (Goldstein & Citron, 1988; Wise
et al., 1988).
The results of these studies are summarized in Table 4.
2.3 Observations in humans
Enrofloxacin has been developed specifically for use in
veterinary medicine; therefore, no human data were available.
Ciprofloxacin, the major metabolite of enrofloxacin, is a human drug
and has been used extensively. Clinical data in humans has produced
no evidence of nephrotoxicity or significant drug-related ocular
changes. Since the drug is not recommended for children or pregnant
women, little evidence has been collected to assess the occurrence of
articular or developmental changes in young patients (Schluter, 1987).
Table 2. Genotoxicity assays with enrofloxacin
Test system Test object Concentration Results Reference
In vitro
Unscheduled Rat primary 1-200 mg/ml Negative Cifone & Myhr, 1985
DNA synthesis hepatocytes
Bacterial reverse S. typhimurium 0.004 & 40 Inappropriate Herbold, 1985a
mutation1 TA98, TA100, ng/plate2 & deficient3
TA1535,TA15
TA1537
Mammalian cell CHO/HGPRT 0.25-1.25 Equivocal Den Boer & Hoorn,
forward mg/ml (-S9)4 1987
mutation1 0.375 - 1.25
mg/ml (+S9)5
Mammalian cell CHO/WBI 25-250 mg/ml Positive Taalman & Hoom,
chromosomal (-S9)6 1988
aberration1 0.1-1 mg/ml
(+S9)7
In vivo
Bone marrow Bor:NMRI mice 1-2 g/kg bw Negative Herbold, 1985b
micronucleus
Bone marrow CD rat 0.04-1 g/kg Negative Bootman et al. ,
chromosomal bw8 1987
aberration
Sister chromatid Chinese 2-8 g/kg bw Negative Herbold, 1988
exchange hamster bone
marrow
Table 2 (continued)
1 Both with and without rat liver S9 fraction
2 Positive controls: 2-Aminoanthracene, endoxan (TA100, TA1535),
and trypaflavine (TA98, TA1537).
3 Inappropriate due to substantial bacteriotoxicity; deficient due
to failed positive controls.
4 5-Bromodeoxyuridine as positive control.
5 3-Methylcholanthrene as positive control.
6 Mitomycin C as positive control.
7 Cyclophosphamide as positive control.
8 Low dose previous LD50 studies showed an oral dose tolerance of
>5 g/kg bw but in the present study, 6/30 animals administered 1 g/kg bw
had to be killed in extremis shortly after being dosed.
Table 3. Summary of MIC-values for bacterial species of human origin
Bacteria MIC range MIC50 MIC90
µg/ml µg/ml µg/ml
E C E C E C
E. coli 0.015-0.03 0.015-0.03 0.015 0.015 0.03 0.015
Staphylococcus 0.06-128 0.125-64 0.125 0.25 4.0 32.0
aureus
Enterococcus 0.25-1.0 0.25-2.0 0.5 1.0 1.0 2.0
spp.
Table 4. In vitro susceptibility of bacteria to ciprofloxacin
Bacteria Number of strains MIC range
µg/ml
Bacteroides fragilis 1 15 4-16
Bacteroides spp. 2 17 0.06-32
Clostridium spp. 1 17 0.012-8
E. coli 1 66 0.015-1
Enterobacter spp. 1 51 0.015-2
Enterococci 1 10 1-2
Flavobacterium 2 18 0.5-1
Fusobacterium spp. 2 18 0.25-32
Peptostreptococcus spp. 1 10 0.25-2
Peptostreptococcus spp. 2 16 0.03-8
1 Clinical isolate.
2 Isolated from bite wounds.
3. COMMENTS
The toxicological data available to the Committee were derived
from acute, short-term, long-term, and carcinogenicity studies;
reproductive and developmental studies; mutagenicity studies;
metabolism and residue studies; antimicrobial activity and
observations in humans following treatment with ciprofloxacin, the
major metabolite of enrofloxacin.
In rats orally dosed with 5 mg/kg bw of radiolabelled
enrofloxacin, the bioavailability of the dose was 75%. Enrofloxacin
was rapidly absorbed and distributed to all tissues, with the highest
concentration in the liver and kidney. Enrofloxacin was rapidly
excreted via urine and bile. Radioactivity in the urine was mainly
unchanged parent drug and the de-ethylated compound, ciprofloxacin.
Bile contained primarily unchanged enrofloxacin. Similar results were
obtained with all species of animals tested.
Enrofloxacin is a relatively stable molecule. Metabolism takes
place mainly in the liver, with the main metabolite in all species
being ciprofloxacin, probably formed by an oxidative dealkylation. In
rats four other components were identified, each comprising less than
2% of the total residue.
Single oral or topical doses of enrofloxacin were slightly toxic
to experimental animals (LD50 = 500-5000 mg/kg bw). In mice, single
intravenous doses were moderately toxic (LD50 = approximately 220
mg/kg bw).
Following 90-day oral administration of enrofloxacin to rats,
chronic degenerative changes in the auricular cartilage were seen at
doses equal to 150 and 577 mg/kg bw/day. Treatment-related
morphological changes in the spermatozoa and testicular tubular
atrophy were seen at 577 mg/kg bw/day. These effects were not seen at
a dose equal to 36 mg/kg bw/day.
A special study was performed to further assess the morphological
changes in the male reproductive tract seen in rats in previous
studies. Male rats were administered enrofloxacin in the feed at
doses equal to 10, 38 or 615 mg/kg bw/day followed by a 90-day
recovery period. Statistically significant decreases in epididymal
weights were seen at 91 days in the 38 and 615 mg/kg bw/day groups.
At 615 mg/kg bw/day, the testis/body-weight ratio was significantly
increased, while the epididymides/body-weight ratio was significantly
decreased. Bilateral testicular atrophy was seen in two rats at 615
mg/kg bw/day after the 90-day recovery period. Abnormal spermatozoa
were seen at 615 mg/kg bw/day at days 14 and 91, but not at the end of
the 90-day recovery period. The NOEL for testicular toxicity in this
study was 10 mg/kg bw/day.
To assess effects on testicular toxicity and articular cartilage
in young dogs, a 90-day oral study in three- and four-month old dogs
was performed. Enrofloxacin was administered at dose levels
equivalent to 0, 3, 9.6 or 75 mg/kg bw/day. All treated animals
except those dosed at 3 mg/kg bw/day showed degeneration of the
articular cartilage. The testes/body-weight ratio for all treated
animals was consistently higher than for control animals, but the
difference was not statistically significant. In 4 high-dose and 1
low-dose animals, marked dilatation of the seminiferous tubules, and
focal areas of single layer spermatogonial cells or vacuolated
epithelium in the tubules were considered beyond normal limits. It
could not be determined whether testicular changes observed in the
low-dose group were treatment-related or due to the fact that the
animals were not fully mature. Therefore, a NOEL could not be
determined. The testicular effects seen in this study were not seen
in 12-13-month old dogs treated orally with enrofloxacin up to 52
mg/kg bw/day for 90 days.
A 90-day study was performed in male dogs dosed with enrofloxacin
at dose levels equal to 0.3, 0.6, 1.2, or 92 mg/kg bw/day to determine
a NOEL for the testicular effects of enrofloxacin observed in the
earlier study with 3- to 4-month old dogs. At termination of the
study, testicular weights were determined and testes and epididymides
collected for histopathological assessment. No other tissues were
collected. Testicular weights and testes/body-weight ratios were
elevated over controls at 0.3, 0.6, and 1.2 mg/kg bw/day. In
addition, lameness was seen at 92 mg/kg bw/day following 2-3 days of
enrofloxacin administ-ration. This effect was not seen at 1.2 mg/kg
bw/day. One dog in the 92 mg/kg bw/day group also had bilateral
testicular degenerative changes. The NOEL was 1.2 mg/kg bw/day in
this study.
A 90-day oral study in 3-month old dogs with a 90-day recovery
period was conducted with 0, 0.3, or 1.2 mg/kg bw/day of enrofloxacin
to assure the continued absence of testicular effects after the
animals reached sexual maturity. After the recovery period, gross and
microscopic examination of testes and epididymides showed no changes
indicative of toxicity. Testes and epididymides appeared mature and
contained normal mature spermatozoa. There was no evidence of delayed
testicular toxicity in this study at levels up to and including 1.2
mg/kg bw/day.
Enrofloxacin was not carcinogenic in mice orally dosed at levels
equal to 323, 1100, or 3520 mg/kg bw/day in a 2-year long-term
toxicity/carcinogenicity study. Bile duct hyperplasia was seen in
males in the mid-dose group and at the high dose in both sexes. The
NOEL was 323 mg/kg bw/day.
In a long-term toxicity/carcinogenicity oral study in rats at
doses equal to 41, 103, 338 or 856 mg/kg bw/day, the Committee
concluded that enrofloxacin was not carcinogenic in this species.
However, a NOEL could not be determined for the bile duct hyperplasia
seen in this study. The NOEL for bile duct hyperplasia in further
limited chronic studies with enrofloxacin in rats was 2.9 mg/kg
bw/day.
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of rats
up to a dose equivalent to 630 mg/kg bw/day. Increased gestation
length, reductions in the number of litters, total pups, litter size
and number of implants for the F0 and F1 generations were seen at
630 mg/kg bw/day. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations and
were considered the primary cause for the adverse effects on
fertility. The NOEL for reproductive performance was 165 mg/kg
bw/day.
An additional two-generation reproduction study in rats was
performed with enrofloxacin administered in the feed to groups of rats
at 0, 125, 300, or 2000 mg/kg feed to determine whether the test
compound affected gonadal function and mating behaviour in treated
males or reproductive parameters in females, as well as, to monitor
growth and development in the F1 and F2 offspring. Administration
of the test article caused a reduction in mean epididymal weights at
the highest dose and alterations in sperm morphology at 15 and 100 mg/kg
bw/day. No effects on fertility or reproductive performance were
noted. The NOEL was 125 mg/kg feed, equal to 10 mg/kg bw/day.
A special fertility study was performed in sexually mature male
rats fed enrofloxacin in the diet at doses equivalent to 0 or 375
mg/kg bw/day for 90 days. The purpose of the study was to determine
the timing of the onset of spermatic dysmorphogenesis and to ascertain
whether functional fertility was restored during a 90-day recovery
phase. Administration of the test article caused a significant
decrease in male fertility, food consumption and body-weight gain.
Testes weights were increased at all interim kills, but were lower
than in the control animals at the end of the study. Treatment-
related spermatic dysmorphogenic changes were seen by week 3 and these
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Forty percent of these animals
demonstrated varying grades of seminiferous tubular atrophy and other
degenerative alterations. The remaining males were infertile.
A teratogenicity study was performed in which enrofloxacin was
administered to rats by gavage at 0, 50, 210, or 875 mg/kg bw/day.
Dosing was performed between days 6-15 of gestation. Maternal
toxicity was observed at 210 and 875 mg/kg bw/day. Food consumption
was higher in high-dose animals, but there was a significant reduction
in mean maternal weight gain in these animals. Fetal weights and
litter sizes were significantly decreased and fetal resorption and
post-implantation losses were increased in high-dose litters. Fetal
weights were significantly decreased in the mid-dose group. Skeletal
examination of the mid- and high-dose fetuses revealed delayed
skeletal ossification, which correlated with the decreased fetal
weights. No teratogenic effects were observed in this study. The
NOEL was 50 mg/kg bw/day.
In in vitro mutagenicity testing, enrofloxacin gave equivocal
results in a test for forward mutations in mammalian cells and
positive results in chromosomal aberration tests. Negative results
were obtained in in vivo bone marrow micronucleus, sister chromatid
exchange and bone marrow chromosomal aberration tests. The Committee
concluded that enrofloxacin is not genotoxic.
The potential for adverse effects on the human gastrointestinal
flora was considered from summary data. In vitro MIC data for
ciprofloxacin covering representatives of the human gut flora were
submitted for assessment. Escherichia coli was found to be the
most sensitive bacterium having an MIC50 value of 0.015 µg/ml.
In calculating the ADI this figure was used in the formula
developed at the thirty-eighth meeting of the Committee (Annex 1,
reference 97).
Concentration without
effect on human gut x Daily faecal bolus (g)
Upper limit of flora (µg/ml)a
temporary ADI =
(µg/kg bw) Fraction of
oral dose x Safety factorc x Weight of human (60 kg)
bioavailableb
(0.015 x 20) x 150
=
0.12 x 10 x 60
= 0.6 µg/kg bw
a Since the MIC value was determined using only 105 bacteria/ml a
factor of 20 was used to account for the higher inoculum density
representative of the human intestine. For example,
Enterobacteriaceae and Pseudomonas aeruginosa MIC values
increased 8 to 32-fold when the inoculum size was increased to
107 bacteria/ml.
b The fraction of the dose available to the intestinal microflora
was derived from studies of ciprofloxacin in humans. In human
volunteers given 2 x 50 mg ciprofloxacin the faecal concentration
was found to be 80 mg/kg. In 150 g faeces, this corresponds to
12% of the administered
c A safety factor of 10 was used to account for the possible
variation in absorption between ciprofloxacin and enrofloxacin, an
uncertainty in the biliary excretion of enrofloxacin, and the
variability between individuals.
4. EVALUATION
In view of the absence of information on the effects of
enrofloxacin on microorganisms obtained from the human intestine, the
Committee established a temporary ADI of 0-0.6 µg/kg bw based on the
results of the limited summary data of microbiology testing of
enrofloxacin/ciprofloxacin.
The temporary ADI of 0.6 µg/kg bw calculated from the in vitro
antimicrobial activity data represents an adequate margin of safety
when compared to the NOEL derived from the toxicology endpoint of 1.2
mg/kg bw/day for testicular toxicity.
5. REFERENCES
ALTREUTHER, P. & KLOSTERMANN, L. (1994). Unpublished summary of the
Enrofloxacin metabolism and residue information submitted to the FAO
experts. Submitted to WHO by Bayer AG, Leverkusen, Germany.
BARE, J.J., PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988). A
subchronic (13-week) feeding study followed by a 13-week drug
withdrawal period in male rats with BAY Vp 2674. Unpublished Report
No. MTD0074 from the Toxicology Department, Miles Inc., Elkhart, IN,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73812).
BECKER, H., VOGEL, W., & TERRIER, Ch. (1987). Embryotoxicity
(including teratogenicity) Study with BAY Vp 2674 in the Rabbit.
Unpublished Report No. R4249 (Project No. 058555) from the Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73705).
BOOTMAN, J., HODSON-WALKER, G., & DANCE, C. (1987). BAY Vp 2674;
Investigation of effects on bone marrow chromosomes of the rat after
acute oral administration. Unpublished LSR report No. 87/BAG039/312
(Report No. R4189; Study No. T5023055) from Life Science Research
Ltd., Suffolk, England. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 74166).
BOMHARD, E., RÜHL-FEHLERT, C., & KALINER, G. (1991a). BAY Vp 2674.
Study of chronic toxicity and carcinogenicity in mice (administration
in feed over 24 months). Unpublished Report No. 20263 (Study No.
T8023436) from the Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74229).
BOMHARD, E. & SCHLADT, L. (1991B). BAY Vp 2674. Study of chronic
toxicity and carcinogenicity in rats after administration in feed over
2 years. Unpublished Report No. 20306 (Study No. T8023436) from the
Department of Toxicology, Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany (report No. 74230).
DEN BOER, W.C. & HOORN, A.J.W. (1987). Mutagenicity evaluation of
BAY Vp 2674 in the CHO/HGPRT forward mutation assay: Revised final
report. Unpublished genetics assays Nos. E-9457 & E-9598 (Report No.
R 4071) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73582).
CIFONE, M.A., & MYHR, B.C. (1985). Evaluation of BAY Vp 2674 in the
rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished
Report No. 3168E (Genetic Assay No. 7550; LBI project No. 20991) from
Litton Bionetics, Inc., Kensington, MD. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73055).
CLEMENS, G.R. & HARTNAGEL, R.E. (1985). A teratology (Segment II)
study in the rat with BAY Vp 2674. Unpublished Report No. 53 from the
Toxicology Department, Central Research Services, Miles Laboratories
Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 73159).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1986). A two-generation
reproduction study in rats with BAY Vp 2674. Unpublished Report No.
4 from Toxicology Department, Central Research Services, Miles
Laboratories Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73314).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1987). A specialized
male fertility study with BAY Vp 2674 in the rat. Unpublished Report
No. MTD0030 (Mobay Report No. 73800) from the Toxicology Department,
Central Research Services, Miles Laboratories Inc., Elkhart, IN, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany (report No. 73800).
EIGENBERG, D.A. (1987). Acute dermal toxicity of BAY Vp2674 in albino
rabbits. Unpublished toxicological report No. 926 (Study No. 86-023-
04) from Health, Environment & Safety, Corporate Toxicology
Department, Mobay Corporation, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73606).
GOLDSTEIN, E.J.C., & CITRON, D.M. (1988). Comparative activities of
cefuroxime, amoxicillin-clavulanic acid, ciprofloxacin, enoxacin, and
ofloxacin against aerobic and anaerobic bacteria isolated from bite
wounds. Antimicrob. Agents Chemoth., 32:1143-1148.
HALL, W.D. (CHAIR) (1992). Pathology working group on a 2-year
chronic feeding study with a l-year interim kill in rats on the
compound BAY Vp 2674. Unpublished Pathology Working Group report
(Bayer ID 014379) prepared for the Center for Veterinary Medicine,
U.S. Food & Drug Administration, Department of Health & Human
Services, Rockville, MD, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
HERBOLD, B. (1985a). BAY Vp 2674; Salmonella /microsome test to
evaluate point mutagenic effects. Unpublished Report No. 13523E
(Study No. T9017669) from the Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73213).
HERBOLD, B. (1985b). BAY Vp 2674; Micronucleus test on the mouse to
evaluate for mutagenic effects. Unpublished Report No. 14102E (Study
Nos. T6019042 & T8019675) from Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74164).
HERBOLD, B.A. (1988). BAY Vp 2674; Sister chromatid exchange in the
bone marrow of the Chinese hamster in in vivo as a test for DNA-
modifying effects. Unpublished Report No. 16783 (Study Nos. T5024676
& T8027324) from Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74178).
KOWALSKI, R.L., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985).
Safety evaluation of BAY Vp 2674: Subchronic (13 week) feeding study
in the rat. Unpublished Report No. 63 (No. 73194) from Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73194).
KOWALSKI, R.L., CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1989).
A two-generation reproduction study in rats with BAY Vp 2674.
Unpublished Report No. MTD0093 (Report No. R4705) Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73892).
LESER, K.H. (1992). BAY Vp 2674. Chronic toxicity study in rats after
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 21668A (Study No. T7032787) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74387).
LESER, K.H. (1993). BAY Vp 2674. Chronic toxicity study in rats with
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 22659 (Study No. T6039950) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74472).
MANDELL, G.L., & SANDE, M.A. (1990). Antimicrobial Agents in Goodman
and Gilman's the Pharmacological Basis of Therapeutics, 8th edition,
Gilman, A.G., Rall, T.W., Nies, S.A., and Taylor, P. (eds.). Pergamon
Press, Inc., Maxwell House, Fairview Park, Elmsford, New York 10523,
p. 1058.
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985). Safety
evaluation of BAY Vp 2674: Subchronic (13 week) feeding study in the
dog. Unpublished Report No. 43 (Mobay Report No. 73146) from the
Toxicology Department, Central Research Services, Miles Laboratories,
Inc., Elkhart, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73146).
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1987). Safety
evaluation of BAY Vp 2674: Repeat of a subchronic (13-week) feeding
study in the dog. Unpublished Report No. MTD0031 (BayVet report No.
73146) from the Toxicology Department, Central Research Services,
Miles Laboratories, Inc., Elkhart, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73775).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988a). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs.
Unpublished Report No. MTD0068 from the Toxicology Department, Central
Research Services, Miles Inc., Elkhart, IN, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73788).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988b). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs
followed by a 13-week withdrawal period. Unpublished report Miles
Inc., Elkhart, Report No. MTD0069. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73789).
SCHLUTER, G. (1987). Ciprofloxacin: review of potential toxicologic
effects. Am. J. Med, 82 (suppl 4A): 91-93.
SCHMIDT, M. (1985). Acute toxicity of BAY Vp 2674 in the rat, mouse,
rabbit and dog. Unpublished Report No. 13223(E) from Institute of
Toxicology, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany (report No. 73075).
SQUIRE, R.A. (1992). Evaluation of bile duct hyperplasia in male rat
livers. Unpublished Report No. 74388 (Study No. T7032787; Pathology
No. 3122) from Toxicology, Research & Development Dept., Miles Inc.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74388).
TAALMAN, R.D.F.M. & HOORN, A.J.W. (1988). Clastogenic evaluation of
BAY Vp 2674 in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished HBC study No. E-9804-0-437 (Report No. R 4474; Study No.
T1027516) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74165).
WISE, R., ANDREWS, J.M., ASHBY, J.P. & MATTHEWS, R.S. (1988).
In vitro Activity of lomefloxacin, a new quinolone antimicrobial
agent, in comparison with those of other agents. Antimicrob. Agents
and Chemoth., 32: 617-622.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
The following information was provided in a summary report of
metabolism and residue data on enrofloxacin. The full study reports
were not provided.
2.1.1.1 Rats
Rats (number, species, strain not specified) were treated orally
or intravenously with a single dose (5 mg/kg bw) of radiolabelled
enrofloxacin. The bioavailability was calculated to be approximately
75%. Enrofloxacin was rapidly absorbed and distributed to all
tissues. Highest concentrations were found in the liver and kidney,
followed by muscle. Fat did not contain appreciable amounts of
radioactivity. Elimination was rapid via urine and faeces. Most of
the radioactivity was excreted during the first 24 hours after
administration. About 40% of the radioactivity was excreted in bile.
The radioactivity in urine was attributed primarily to the unchanged
parent drug, its de-ethylated metabolite, ciprofloxacin, and the
glucuronide of enrofloxacin. Bile contained mainly the unchanged
parent molecule (Altreuther & Klostermann, 1994).
2.1.1.2 Cattle, pigs, chickens, and turkeys
Trials in all species showed that enrofloxacin is absorbed
readily and distributes to all organs and tissues following oral or
parenteral administration. In pigs, when the same dose was
administered by the oral or i.m. route, similar rates of absorption
and subsequent concentrations of drug in tissues were observed.
Following parenteral administration, maximum serum concentrations in
pigs and calves were reached within 1 to 2 hours. The highest drug
concentration initially occurred in the liver in all species.
Following a one-day withdrawal in poultry, highest drug concentrations
were found in the skin. In all species studied, elimination was
primarily via the urine and bile/faeces (Altreuther & Klostermann,
1994).
2.1.2 Biotransformation
2.1.2.1 Rats, cattle, pigs, and poultry
Rats (number, species, strain not specified) were treated orally
with 165 mg/kg bw of radiolabelled enrofloxacin for 3 consecutive
days, then with 50 mg/kg bw on the fourth day. Major urinary
metabolites were enrofloxacin (30-36%), ciprofloxacin (26- 31%), and
enrofloxacin glucuronide (19-29%). Four other components were
identified, each less than 2% of the total residue (Altreuther &
Klostermann, 1994).
In food animal species, ciprofloxacin was the main metabolite
recovered. The liver is considered the primary site of enrofloxacin
metabolism in these species, forming ciprofloxacin through oxidative
dealkylation. Additional metabolites occurred but comprised less than
10% of the total residue (Altreuther & Klostermann, 1994).
2.1.3 Effects on enzymes and other biochemical parameters
The bactericidal in activity of quinolones, including
enrofloxacin, is attributed to their ability to inhibit DNA gyrase
(Mandell & Sande, 1990).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of enrofloxacin is summarized in Table 1.
Table 1. Acute toxicity studies on enrofloxacin
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse male p.o. > 5000 Schmidt, 1985
female 4336
male i.v. 225 Schmidt, 1985
female 220
Rat male p.o. > 5000 Schmidt, 1985
female
Rabbit male p.o. 500-800 Schmidt, 1985
female
male topical > 2000 Eigenberg,1987
female
Dog male p.o. n/a1 Schmidt, 1985
female
1 Not determined due to vomiting of the drug.
Animals were observed daily for 14 days post-exposure in these
studies. Clinical signs associated with enrofloxacin toxicosis
included lethargy, trembling, tonic convulsions, dyspnea, prone and
lateral decubitus, and ataxia. Clinically affected animals that did
not die recovered within 15 minutes to 7 days post-exposure. Gross
post-mortem lesions reported included pulmonary congestion and
haemorrhage.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of Charles River rats (15/sex/group) were administered
diets containing 0, 500, 2000, or 7500 mg enrofloxacin/kg feed (equal
to 36, 150, or 577 mg/kg bw/day for males and 45, 182, or 690 mg/kg
bw/day for females, respectively) for 90 days. No overt effects on
appearance and behaviour were noted at any dose level. Body-weight
gain was reduced at the highest dose in both males (20%) and females
(23%), while the actual diet consumption for males and females
remained constant.
Serum chemistry findings included a significant dose-related
decrease in total plasma protein in the mid- and high-dose males and
females at 6 and 13 weeks. Total bilirubin and alanine aminotrans-
ferase were decreased in the males at 6 weeks but were normal at 13
weeks. Aspartate aminotransferase was decreased in the high-dose
males at 6 and 13 weeks. Globulins were decreased in the high-dose
males at 6 weeks and 13 weeks and high-dose females at 13 weeks. A
corresponding increase in the A/G ratio was seen in the high-dose
males at 6 and 13 weeks.
Haematology findings were unremarkable.
Urinalyses showed a significant decrease in urine sodium output
for males in the mid- and high-dose groups after 6 weeks and for
females in the mid- and high-dose groups at 13 weeks.
Treatment-related gross findings included swollen ears (2 males
and 4 females in the mid-dose group, 4 males and 5 females in the
high-dose group, and 1 female in the control group) and caecal
distension (1 male in the mid-dose group and 3 males and 5 females in
the high-dose group).
Mean and relative prostate weights were statistically
significantly reduced in the mid- and high-dose groups. Mean heart
weights were statistically significantly reduced in high-dose males
and in mid- and high-dose females. Mean liver weights were reduced in
both male and female high-dose animals. The reduction was
statistically significant in females.
Histological findings included auricular chondropathy which was
present in all groups except low-dose males and appeared to be dose-
related (1 control, 1 low dose, 6 mid dose, and 10 high dose). In the
testes of 12/15 rats from the high-dose group, dark, round or oval
cells were distributed among the spermatozoa in the ducts of the
epididymides. These cells were occasionally observed in seminiferous
tubules of 5/15 high-dose rats. These findings were considered
treatment-related.
Based on decreased body-weight gain and microscopic changes
observed in the ducts of the epididymides and testes in the high-dose
group, and gross and microscopic changes indicating auricular
chondropathy in the mid- and high-dose groups, the NOEL was 500 mg/kg
feed, equal to 36 mg/kg bw/day (Kowalski et al., 1985).
A special study was performed in male rats to further assess the
testicular toxicity seen in previous studies. A 13-week recovery
period was included in the study. Thirty male Crl:CD Charles River
rats per group were dosed with 0, 125, 500, or 7500 mg enrofloxacin/kg
feed (equal to 10, 38, or 615 mg/kg bw/day) for 90 days. Fifteen
animals/group were killed on day 91 and the remaining 15 on day 181.
An apparent dose-related decrease in epididymal weights was observed
in all dose groups at 91 and 181 days. The difference achieved
statistical significance (compared to controls) in the mid- and high-
dose groups at both time points. Relative mean testes weight was
significantly decreased in the high-dose group after 91 days.
Bilateral testicular atrophy was seen in two high-dose rats at 181
days. Abnormal spermatozoa were seen at day 91 in the mid- and high-
dose groups but not after the recovery period. The NOEL for this
parameter was 125 mg/kg feed, equal to 10 mg/kg bw/day (Bare et al.,
1988).
2.2.2.2 Dogs
Four groups (4/sex/group) of beagle dogs (3-4 months old, 2.6 to
4.8 kg bw) were administered enrofloxacin in the diet at 0, 100, 320
or 2500 mg/kg feed (equal to 3, 9.6, or 75 mg/kg bw/day, respectively)
for 91 days.
No dogs died during the study. Sporadic incidences of mild
diarrhoea and vomiting were reported early in the study. Vomiting
occurred 5, 6, and nearly 24 hours after ingestion of the test
article. Severe hyperextension (upon manipulation) of carpal joints,
hypoactivity, and abnormal gate or posture, were observed in all
animals in the high-dose group during weeks 1 and 2. Radiographic
examination (8 dogs total) revealed several abnormalities including
valgus deformity at the carpus, asymmetry of the distal ulnar physis,
remodelling of the radial carpal bone, radius curvus, and increased
width of the radial humeral joint. The consulting radiologist
concluded that only the radial carpal remodelling appeared to be dose-
related, and neither bone growth nor density were significantly
affected.
Serum chemistry and haematologic findings were unremarkable.
However, two types of unidentified crystals in urine samples from dogs
in the 2500 mg/kg feed group were considered treatment-related.
Gross postmortem findings included an increase in mean absolute
and relative testicular weights for treated animals when compared to
control animals. However, a clear dose-response relationship was not
established and none of the differences were statistically significant
when compared to controls. In 8/8 high-dose animals and 2/8 mid-dose
animals joint lesions were observed grossly as superficial (0.2 to 0.4
cm in diameter) erosions with dulling of the surfaces on the femoral
head of the hip joint and/or the femoral condyles of the knee joint.
Histologically, joint lesions were characterized by splitting of the
articular cartilage with disorganization of chondrocytes and formation
of "brood capsules" of cartilage cells. Necrosis and disintegration
of hyaline cartilage at the area of splitting was also noted.
There was marked variation in the microscopic appearance of
testes from animal to animal with respect to the stage of maturity,
diameter of the lumen of seminiferous tubules, and vacuolar change in
cells lining the tubules. However, it was not clear whether these
testicular changes were treatment-related because there was no dose-
response relationship in incidence or severity. These variations in
testicular morphology could represent normal variation in the maturing
process of this organ in this species. Due to the uncertainty of the
significance of the testicular findings in the low-dose males in this
study, the Committee concluded that a no-effect level was not observed
(Porter et al., 1987).
In another 90-day dietary study in beagle dogs, 4 groups
(4/sex/group) of adult dogs (12-13 months old weighing 6.4 to 12.5 kg)
received enrofloxacin at 0, 320, 800 or 2000 mg/kg diet (equal to 9.3,
22, or 53 mg/kg bw/day for males, and 8.9, 23, or 51 mg/kg bw/day for
females, respectively). No joint or testicular lesions were reported
in this study (Porter et al., 1985).
A special limited 90-day dietary study in males was performed.
Five groups (4 dogs/group) of 3-month old beagle dogs (weighing 4.1 to
5.7 kg) received enrofloxacin at 0, 10, 20, 40, or 3200 mg/kg diet
(equal to 0, 0.3, 0.6, 1.2, or 92 mg/kg bw/day, respectively). At
termination of the study (day 92) testicular weights were determined
and testes and epididymides collected for histopathological
assessment. No other tissues were collected.
Histologically, the tissues examined contained wide variations in
morphology attributable to differences in sexual maturation. Sections
of epididymides examined were considered normal. Bilateral testicular
degeneration characterized by multinucleated giant cells and cells
with large cytoplasmic and nuclear volumes, sometimes in mitosis, were
reported in the seminiferous tubules of one dog at the high dose.
Seminiferous tubules lined with single layers of spermatogonial cells
and forming open lumens were present in 2/4 dogs in the 10 mg/kg feed
group and 3/4 dogs in each of the 20, 40, and 3200 mg/kg feed groups.
This change was also reported in one control animal. Spermatocytic
giant cells were reported in 0, 1, 2, 2 and 3 dogs from the 0, 10, 20,
40 and 3,200 mg/kg feed groups, respectively.
The author attributed the seminiferous tubule changes to normal
variation in sexual maturity because similar morphology was reported
in the testes of one control dog. The author used this finding to
support a NOEL of 100 mg/kg feed in the 90-day study reported above
(Porter et al., 1987). The Committee concluded that a NOEL for
testicular effects in this special, limited 90-day study could not be
determined due to the inability to determine whether variations in
testicular morphology were normal or due to treatment with the test
article (Porter et al., 1988a).
Three-month old male beagle dogs (4/group) were administered
enrofloxacin at 0, 10, or 40 mg/kg diet (equal to 0.3 or 1.2 mg/kg
bw/day) for 90 days, then maintained on control diets for an
additional 91 days before being killed. The protocol was intended to
evaluate the potential for enrofloxacin testicular toxicity to appear
after animals reach sexual maturity, following exposure at an immature
age (delayed testicular toxicity).
No signs of clinical toxicity were observed nor was body-weight
gain or food consumption affected. Gross and microscopic examination
of testes and epididymides showed no changes indicative of toxicity.
Testes and epididymides appeared mature in all treated animals and
contained normal, mature spermatozoa. The NOEL for delayed testicular
toxicity was 40 mg/kg diet, equal to 1.2 mg/kg bw/day, the highest
dose tested (Porter et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
B6C3F1 mice (60/sex/group) received diets containing 0, 1000,
3300, or 10 000 mg enrofloxacin/kg feed (equal to 323, 1100, or 3520
mg/kg bw/day for males and 373, 1210, or 3700 mg/kg bw/day for
females, respectively) for 24 months. An interim sacrifice
(10/sex/group) was performed after one year. An additional dose group
(10 mice/sex) was administered 20 000 mg enrofloxacin/kg feed (equal
to 8030 mg/kg bw/day for males and 8010 mg/kg bw/day for females,
respectively) and scheduled for interim sacrifice after one year.
During the treatment phase of the study, general appearance and
physical examination findings were within normal limits in all treated
groups. Treatment-related ophthalmological findings included a marked
increase (relative to control animals) in the incidence of focal
lenticular opacity in males and females at 10 000 mg/kg feed.
Histopathological examination of eyes revealed no treatment-related
effects. However, it was noted that focal lenticular changes are only
verifiable to a limited extent with usual histolopathological methods.
Clinical chemical and haematological evaluations were performed
on 10 animals/sex/dose after 12 and 24 months on test. Alkaline
phosphatase levels were significantly lower in females at 3300 mg/kg
feed at 12 months, and at 10 000 mg/kg feed at study termination.
Concurrent with these findings, alanine aminotransferase and aspartate
transaminase values were within normal limits. Total protein was
decreased in males and females at all treatment levels as a result of
decreased globulin fractions.
Haematological analyses revealed a significant decrease in white
blood cell counts in males at 10 000 mg/kg feed at 12 months and at
3300 mg/kg feed at study termination. In females, a significant
decrease in white blood cell counts was noted at 10 000 mg/kg feed at
study termination. At 10 000 mg/kg feed, decreases were seen in
haematocrit, MCV, and MCH values in both sexes, and in the haemoglobin
value in males, at study termination.
Gross necropsy findings included a dose-related incidence of
caecal dilation in males at 1000, 3300, and 10 000 mg/kg feed and in
females at 3300 and 10 000 mg/kg feed. Histopathological examinations
showed neither an increase in the incidence of, nor a decrease in the
time of appearance to tumours in the dosed groups compared to the
controls. Bile duct hyperplasia was seen at 3300 mg/kg feed in males
and 10 000 mg/kg feed in both sexes. Focal papillary mucosal
hyperplasia of the gall bladder was also present in males and females
in these same dose groups. There was no evidence of carcinogenicity
in this study. The NOEL was 1000 mg/kg feed, equal to 323 mg/kg
bw/day (Bomhard et al., 1991a).
2.2.3.2 Rats
A combined long-term toxicity (52 weeks) and carcinogenicity (104
weeks) study of enrofloxacin was conducted in Wistar rats (BOR:WISW).
In the chronic study, 10 rats/sex/group received diets containing 0,
770, 2000, 6000, or 10 000 mg enrofloxacin/kg feed (equal to 0, 41,
103, 338, or 856 mg/kg bw/day for males and 0, 58, 146, 466, or 1000
mg/kg bw/day for females, respectively). In the carcinogenicity
study, 50 rats/sex/group received diets containing 0, 770, 2000, or
6000 mg enrofloxacin/kg feed (equal to 0, 41, 103, or 338 mg/kg bw/day
for males and 0, 58, 146, or 466 mg/kg bw/day for females).
Mortality rates, daily observations, and ophthalmologic findings
in treated animals were comparable to controls. Body-weight gain was
decreased in males in the 6000 mg/kg feed group and in males and
females at 10 000 mg/kg feed. Feed and water intakes were increased
in males and females at 10 000 mg/kg feed. Water intake was also
elevated at 6000 mg/kg feed.
Serum chemistry evaluations were performed after 6, 12, 18, and
24 months. Notable findings included a significant decrease in total
protein in males in all dose groups at all sampling periods. Total
protein was also significantly decreased in females in the high-dose
group both at 6 months and at the end of 2 years, and at 2000 and 10
000 mg/kg feed after 1 year. The albumin value was increased in
males at 10 000 mg/kg feed after 1 year, at 6000 mg/kg feed after 18
months, and in the two highest-dose groups (2000 and 6000 mg/kg feed)
at the end of 2 years. Bilirubin was significantly decreased in
females in all dose groups at the 6-month sampling time only.
Protein electrophoresis performed after 12, 18, and 24 months
showed a decrease in gamma-globulin in males and females in all dose
groups. The author noted that this finding is not unexpected in
animals receiving antimicrobials, as they lower the background
incidence of common pathogens, resulting in a decrease in antigenic
stimulation.
Haematology evaluations were performed after 6, 12, 18, and 24
months. At doses of 2000 mg/kg feed and above, there were
statistically significant and sometimes dose-related decreases in RBC
counts in males and haemoglobin and haematocrit values in both sexes
at various sampling times. However, the values for these parameters
in both sexes in all dose groups were comparable to controls at the
terminal sampling period. After 6 months leucocyte counts in both
sexes were decreased compared to controls at all dose levels. In most
instances, these differences were statistically significant. A trend
toward decreased leucocyte counts in the treatment groups was also
discernible in the subsequent investigations. The author noted that
due to the pharmaco-logical action of antimicrobial compounds,
decreased leucocyte counts are a common finding.
On gross postmortem examination, a decrease in testicular size
(small with altered texture) was noted at 2000 mg/kg feed and above.
Histologically, marked testicular atrophy, decreased spermatogenesis,
and a significant increase in the amount of mineralized concretion in
the testes were noted in these same dose groups. Normal age-related
degenerative changes occurred with greater frequency in treated
animals than in controls. Caecal dilation was reported in both sexes
of rats in the high-dose group and in the males at 2000 mg/kg feed.
Organ weights recorded at both the interim and terminal kill
revealed a decrease in absolute and relative liver weights in males at
2000 mg/kg feed and above. The same changes were seen in females in
these groups at the interim necropsy but not at study termination. At
the interim and terminal kills, absolute weights of the brain, testes
and adrenals were significantly decreased in the 6000 mg/kg feed
males. However, the relative weights of these organs were comparable
to controls.
Histopathological evaluation revealed a dose-related increase in
incidence of hepatic cysts at 24 months, bile duct hyperplasia with
sclerosis at 12 and 24 months, and cystic bile duct hyperplasia at 24
months in both sexes and in all treatment groups. Spinal cord
demyelination/degeneration was reported in all treatment groups.
Because of the high incidence of this finding in controls and without
further definition of lesions into degeneration versus demyelination,
the biological significance of this finding is uncertain. There was
also a marked increase in degenerative changes in the sciatic nerve.
A marked increase in the number of sarcolemmal nuclei in skeletal
muscle cells of animals in the 6000 mg/kg feed group was noted. This
finding is generally associated with degenerative changes in muscle
fibres.
A marked increase in the incidence of cardiomyopathy was
observed in males at 6000 mg/kg feed and in females in all dose
groups. Incidences at 0, 770, 2000, and 6000 mg/kg feed were 10/48,
8/50, 13/50, and 24/50 in males and 8/50, 16/50, 17/50, and 25/50 in
females, respectively. Endocardial neoplasms and Schwann cell-like
hyperplasia were seen in the hearts of some of the animals. These
findings were evaluated by a Pathology Working Group which concluded
that these lesions were not related to treatment with the test
article. Under the conditions of this study there was no evidence of
a carcinogenic effect of the test substance. A NOEL could not be
determined because of the occurrence of bile duct changes and
cardiomyopathy in the low-dose group (Bomhard et al., 1991b; Hall,
1992).
A limited long-term study was performed with Wistar (Bor:WISW)
rats (60/sex/group). Diets containing 0, 100, or 500 mg
enrofloxacin/kg feed (equal to 0, 5.3, or 26 mg/kg bw/day for males
and 0, 7.2, or 36 mg/kg bw/day for females, respectively) were
administered for 24 months. An interim kill of 10 rats/sex/group was
performed at 12 months.
Morbidity, moribundity, and mortality in treated groups were
comparable to controls. General appearance, clinical observations,
and the results of physical and ophthalmoscopic examinations were all
comparable with normal animals of this species and age. Exposure to
enrofloxacin produced no biologically significant alterations in food
or water intake, body weight, clinical chemical parameters or
haematological parameters.
At interim sacrifice, no abnormalities were noted in males or
females on postmortem examination. At terminal sacrifice, an
increased number of males had swollen livers (3/50 [controls], 10/50,
[100 mg/kg feed], and 8/50 [500 mg/kg feed]). However, liver weights
and histopathological findings were within normal limits.
No treatment-related abnormalities were noted in the clinical
chemistry parameters that were evaluated. However, as in the long-
term study summarized above at higher dose levels, the gamma globulin
value in treated animals was consistently lower than in controls.
On histological evaluation, sclerotic bile duct hyperplasia was
more common in both males and females treated with enrofloxacin than
in control animals. The incidence was statistically significantly
increased in the 100 and 500 mg/kg feed groups after 52 weeks of
treatment, and in males and females at 500 mg/kg feed after 104 weeks
of treatment. The total number of females affected was markedly less
than the number of males. Although the incidence of bile duct
hyperplasia at 104 weeks at 100 mg/kg feed was not statistically
significantly different from the control values, the significant
findings in males in both dose groups at 52 weeks suggests that
administration of enrofloxacin accelerates the development of bile
duct sclerosis in male rats.
No evidence of carcinogenicity was reported in this study.
However, the heart and liver were the only two tissues examined
histologically. A NOEL could not be determined in this study (Leser,
1992).
A consulting pathologist conducted a second histopathologic
evaluation of liver slides of male rats from both the interim and
terminal kills in the above study. The consultant's conclusions were
as follows: "There was an increase over controls in the incidence of
bile duct hyperplasia in both dose groups at the interim kill,
although this was probably a spurious finding. At the terminal kill,
an increased incidence occurred only at the 500 ppm level. No clear
effect on lesion severity was noted in any group. The 100 ppm dose
(5.3 mg/kg bw/day) was a no-effect level in this study." (Squire,
1992).
A second limited long-term study was performed on Wistar
(Bor:WISW) rats (60/sex/group), administered enrofloxacin in the diet
at 0, 10, or 50 mg/kg feed (equal to 0, 0.6, or 2.9 mg/kg bw/day for
males and 0, 0.7, or 3.5 mg/kg bw/day for females, respectively) for
24 months. An interim kill of 10 animals/sex/group was performed at
12 months. The purpose of this study was to establish a NOEL for the
treatment-related bile duct hyperplasia observed in previous chronic
studies.
No treatment-related alterations were seen in physical
examinations, ophthalmologic evaluations, feed or water consumption,
body-weight gain, clinical chemistries, urinanalysis, or haematology
at the termination of the study. Postmortem examinations, including
gross observations, organ weights and histopathological examinations
were conducted at 12 and 24 months. Portions of the right and left
anterior liver lobes were collected and prepared for histological
evaluation Only one section per lobe was examined from these hepatic
samples. No treatment-related effects were reported in any of the
parameters monitored during the study. The incidence and severity of
bile duct hyperplasia in the exposed female and male rats were
comparable with the controls. The NOEL was 50 ppm (equal to 2.9 mg/kg
bw/day) (Leser, 1993).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl: CD(SD)Br rats at doses of 0, 500, 2000, or 7500 mg/kg feed. F0
males received the diet for approximately 70 days and the F0 females
for approximately 2 weeks prior to mating. Reproductive performance
of F0 and F1 generations was evaluated, as well as pup parameters.
A gross necropsy was performed on every animal and histopathologic
evaluation was conducted on reproductive organs.
Administration of the test article caused decreased body weights
at 7500 mg/kg feed (equal to 630 mg/kg bw/day) in the F0 and F1
generations. This effect was not accompanied by a reduction in food
consumption. Reproductive performance of the same animals was
significantly affected by the treatment at the high dose, which
included increased gestation length, reductions in the number of
litters, total pups, litter size and number of implants in the F0 and
F1 generations. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations,
which were considered the primary cause for the adverse effects on
fertility. The NOEL was 2000 mg/kg feed, equal to 165 mg/kg bw/day
(Clemens et al., 1986).
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of 30
Crl:CD (SD)Br rats at doses of 0, 125, 300, or 2000 mg/kg feed
(equivalent to 0, 6.2, 15, or 100 mg/kg bw/day). This study was
intended to evaluate the effect(s) of enrofloxacin on gonadal function
and mating behaviour in treated males, reproductive parameters in
females, and growth and development in the F1 and F2 offspring. F0
males and females received the test compound for 70 and 14 days,
respectively, before mating. A gross necropsy was performed on every
animal and histopathologic evaluation was conducted on testes and
epididymides from each F0 and F1 male.
Administration of the test article caused a reduction in mean
epididymal weights at the highest dose, and alterations in sperm
morphology at 300 and 2000 mg/kg feed. No effects on fertility or
reproductive performance were noted. The NOEL was 125 mg/kg feed,
equivalent to 6.2 mg/kg bw/day (Kowalski et al., 1989).
A specialized male fertility study was performed with
enrofloxacin to determine the timing of the onset of spermatic
dysmorphogenesis and to ascertain whether functional fertility was
restored during a 90-day recovery phase. Diets containing 0 or 7500
mg/kg feed (equivalent to 375 mg/kg bw/day) were administered to
sexually mature Crl:CD BR male rats (60/group) for 90 days. Interim
kills were performed at 3, 6, 9 and 13 weeks, with 15 males from each
group retained to mate with untreated females to assess male
fertility. The first mating was performed after 11 weeks on trial.
From day 91, all males were fed control diet and matings were again
performed on weeks 3, 7, and 11 of recovery. Females were killed on
day 20 of gestation.
Enrofloxacin administered at this dose caused significant
decreases in male fertility, food consumption and body-weight gains.
Testes weights were increased at all interim kills, but were less
than control values at the end of the study. Treatment-related
spermatic dysmorphogenic changes were seen by week 3 on trial. These
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Varying grades of seminiferous
tubular atrophy and other degenerative alterations were observed in
40% of these animals. Complete infertility was diagnosed in the
remaining males (Clemens et al., 1987).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
A teratogenicity (Segment II) study in the rat was performed in
which enrofloxacin as a 5% suspension with carboxymethylcellulose and
polysorbate 80 was administered by gavage to groups of 28 inseminated
COBS CD female rats at 0, 50, 210, or 875 mg/kg bw/day. Dosing was
performed on days 6-15 of gestation. On day 20, all dams were killed
and maternal and fetal parameters were evaluated.
Administration of the test article was maternally toxic at 210
and 875 mg/kg bw/day. Food consumption was higher in high-dose
animals, but there was a significant reduction in mean maternal weight
gain in these animals. In the high-dose litters, fetal weights and
litter size were significantly decreased and fetal resorption and
post-implantation losses were increased. Fetal weights were
significantly decreased in the mid-dose group. Skeletal examination
of the mid- and high-dose groups showed delayed skeletal ossification.
Neither embryotoxicity nor fetotoxicity was seen. No teratogenic
effects were observed. The NOEL was 50 mg/kg bw/day (Clemens &
Hartnagel, 1985).
2.2.5.2 Rabbits
An embryotoxicity (including teratogenicity) study was performed
with enrofloxacin administered by gavage to groups of 16 mated female
chinchilla rabbits at doses of 0, 1, 5, or 25 mg/kg bw/day admixed in
distilled water with 0.5% Tylose. Dosing was performed on days 6-18
of gestation. On day 28, all dams were killed. Because no maternal
effects were seen at doses up to 25 mg/kg bw/day, a supplementary
group of 16 females (with corresponding control group) was added and
administered enrofloxacin at 75 mg/kg bw/day.
Administration of the test article caused decreased food
consumption and lower body weights at 75 mg/kg bw/day. Increased
post-implantation losses were also noted in this group. All other
dose groups showed only incidental findings. The NOEL was 25 mg/kg
bw/day (Becker et al., 1987).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with enrofloxacin are
summarized in Table 2.
2.2.7 Special studies on the human gastrointestinal flora
No in vivo studies were available. In vitro studies of
Escherichia coli (24 strains), Staphylococcus aureus (24
strains) and Enterococcus spp. (24 strains) of human origin showed
the following MIC values for enrofloxacin (E) and ciprofloxacin (C).
Only summary tables were given.
The results of these studies are summarized in Table 3 (Scheer,
1993).
Data on in vitro susceptibility to ciprofloxacin for several
strains of bacteria obtained from "clinical isolates" or "bite wounds"
from humans were also available (Goldstein & Citron, 1988; Wise
et al., 1988).
The results of these studies are summarized in Table 4.
2.3 Observations in humans
Enrofloxacin has been developed specifically for use in
veterinary medicine; therefore, no human data were available.
Ciprofloxacin, the major metabolite of enrofloxacin, is a human drug
and has been used extensively. Clinical data in humans has produced
no evidence of nephrotoxicity or significant drug-related ocular
changes. Since the drug is not recommended for children or pregnant
women, little evidence has been collected to assess the occurrence of
articular or developmental changes in young patients (Schluter, 1987).
Table 2. Genotoxicity assays with enrofloxacin
Test system Test object Concentration Results Reference
In vitro
Unscheduled Rat primary 1-200 mg/ml Negative Cifone & Myhr, 1985
DNA synthesis hepatocytes
Bacterial reverse S. typhimurium 0.004 & 40 Inappropriate Herbold, 1985a
mutation1 TA98, TA100, ng/plate2 & deficient3
TA1535,TA15
TA1537
Mammalian cell CHO/HGPRT 0.25-1.25 Equivocal Den Boer & Hoorn,
forward mg/ml (-S9)4 1987
mutation1 0.375 - 1.25
mg/ml (+S9)5
Mammalian cell CHO/WBI 25-250 mg/ml Positive Taalman & Hoom,
chromosomal (-S9)6 1988
aberration1 0.1-1 mg/ml
(+S9)7
In vivo
Bone marrow Bor:NMRI mice 1-2 g/kg bw Negative Herbold, 1985b
micronucleus
Bone marrow CD rat 0.04-1 g/kg Negative Bootman et al. ,
chromosomal bw8 1987
aberration
Sister chromatid Chinese 2-8 g/kg bw Negative Herbold, 1988
exchange hamster bone
marrow
Table 2 (continued)
1 Both with and without rat liver S9 fraction
2 Positive controls: 2-Aminoanthracene, endoxan (TA100, TA1535),
and trypaflavine (TA98, TA1537).
3 Inappropriate due to substantial bacteriotoxicity; deficient due
to failed positive controls.
4 5-Bromodeoxyuridine as positive control.
5 3-Methylcholanthrene as positive control.
6 Mitomycin C as positive control.
7 Cyclophosphamide as positive control.
8 Low dose previous LD50 studies showed an oral dose tolerance of
>5 g/kg bw but in the present study, 6/30 animals administered 1 g/kg bw
had to be killed in extremis shortly after being dosed.
Table 3. Summary of MIC-values for bacterial species of human origin
Bacteria MIC range MIC50 MIC90
µg/ml µg/ml µg/ml
E C E C E C
E. coli 0.015-0.03 0.015-0.03 0.015 0.015 0.03 0.015
Staphylococcus 0.06-128 0.125-64 0.125 0.25 4.0 32.0
aureus
Enterococcus 0.25-1.0 0.25-2.0 0.5 1.0 1.0 2.0
spp.
Table 4. In vitro susceptibility of bacteria to ciprofloxacin
Bacteria Number of strains MIC range
µg/ml
Bacteroides fragilis 1 15 4-16
Bacteroides spp. 2 17 0.06-32
Clostridium spp. 1 17 0.012-8
E. coli 1 66 0.015-1
Enterobacter spp. 1 51 0.015-2
Enterococci 1 10 1-2
Flavobacterium 2 18 0.5-1
Fusobacterium spp. 2 18 0.25-32
Peptostreptococcus spp. 1 10 0.25-2
Peptostreptococcus spp. 2 16 0.03-8
1 Clinical isolate.
2 Isolated from bite wounds.
3. COMMENTS
The toxicological data available to the Committee were derived
from acute, short-term, long-term, and carcinogenicity studies;
reproductive and developmental studies; mutagenicity studies;
metabolism and residue studies; antimicrobial activity and
observations in humans following treatment with ciprofloxacin, the
major metabolite of enrofloxacin.
In rats orally dosed with 5 mg/kg bw of radiolabelled
enrofloxacin, the bioavailability of the dose was 75%. Enrofloxacin
was rapidly absorbed and distributed to all tissues, with the highest
concentration in the liver and kidney. Enrofloxacin was rapidly
excreted via urine and bile. Radioactivity in the urine was mainly
unchanged parent drug and the de-ethylated compound, ciprofloxacin.
Bile contained primarily unchanged enrofloxacin. Similar results were
obtained with all species of animals tested.
Enrofloxacin is a relatively stable molecule. Metabolism takes
place mainly in the liver, with the main metabolite in all species
being ciprofloxacin, probably formed by an oxidative dealkylation. In
rats four other components were identified, each comprising less than
2% of the total residue.
Single oral or topical doses of enrofloxacin were slightly toxic
to experimental animals (LD50 = 500-5000 mg/kg bw). In mice, single
intravenous doses were moderately toxic (LD50 = approximately 220
mg/kg bw).
Following 90-day oral administration of enrofloxacin to rats,
chronic degenerative changes in the auricular cartilage were seen at
doses equal to 150 and 577 mg/kg bw/day. Treatment-related
morphological changes in the spermatozoa and testicular tubular
atrophy were seen at 577 mg/kg bw/day. These effects were not seen at
a dose equal to 36 mg/kg bw/day.
A special study was performed to further assess the morphological
changes in the male reproductive tract seen in rats in previous
studies. Male rats were administered enrofloxacin in the feed at
doses equal to 10, 38 or 615 mg/kg bw/day followed by a 90-day
recovery period. Statistically significant decreases in epididymal
weights were seen at 91 days in the 38 and 615 mg/kg bw/day groups.
At 615 mg/kg bw/day, the testis/body-weight ratio was significantly
increased, while the epididymides/body-weight ratio was significantly
decreased. Bilateral testicular atrophy was seen in two rats at 615
mg/kg bw/day after the 90-day recovery period. Abnormal spermatozoa
were seen at 615 mg/kg bw/day at days 14 and 91, but not at the end of
the 90-day recovery period. The NOEL for testicular toxicity in this
study was 10 mg/kg bw/day.
To assess effects on testicular toxicity and articular cartilage
in young dogs, a 90-day oral study in three- and four-month old dogs
was performed. Enrofloxacin was administered at dose levels
equivalent to 0, 3, 9.6 or 75 mg/kg bw/day. All treated animals
except those dosed at 3 mg/kg bw/day showed degeneration of the
articular cartilage. The testes/body-weight ratio for all treated
animals was consistently higher than for control animals, but the
difference was not statistically significant. In 4 high-dose and 1
low-dose animals, marked dilatation of the seminiferous tubules, and
focal areas of single layer spermatogonial cells or vacuolated
epithelium in the tubules were considered beyond normal limits. It
could not be determined whether testicular changes observed in the
low-dose group were treatment-related or due to the fact that the
animals were not fully mature. Therefore, a NOEL could not be
determined. The testicular effects seen in this study were not seen
in 12-13-month old dogs treated orally with enrofloxacin up to 52
mg/kg bw/day for 90 days.
A 90-day study was performed in male dogs dosed with enrofloxacin
at dose levels equal to 0.3, 0.6, 1.2, or 92 mg/kg bw/day to determine
a NOEL for the testicular effects of enrofloxacin observed in the
earlier study with 3- to 4-month old dogs. At termination of the
study, testicular weights were determined and testes and epididymides
collected for histopathological assessment. No other tissues were
collected. Testicular weights and testes/body-weight ratios were
elevated over controls at 0.3, 0.6, and 1.2 mg/kg bw/day. In
addition, lameness was seen at 92 mg/kg bw/day following 2-3 days of
enrofloxacin administ-ration. This effect was not seen at 1.2 mg/kg
bw/day. One dog in the 92 mg/kg bw/day group also had bilateral
testicular degenerative changes. The NOEL was 1.2 mg/kg bw/day in
this study.
A 90-day oral study in 3-month old dogs with a 90-day recovery
period was conducted with 0, 0.3, or 1.2 mg/kg bw/day of enrofloxacin
to assure the continued absence of testicular effects after the
animals reached sexual maturity. After the recovery period, gross and
microscopic examination of testes and epididymides showed no changes
indicative of toxicity. Testes and epididymides appeared mature and
contained normal mature spermatozoa. There was no evidence of delayed
testicular toxicity in this study at levels up to and including 1.2
mg/kg bw/day.
Enrofloxacin was not carcinogenic in mice orally dosed at levels
equal to 323, 1100, or 3520 mg/kg bw/day in a 2-year long-term
toxicity/carcinogenicity study. Bile duct hyperplasia was seen in
males in the mid-dose group and at the high dose in both sexes. The
NOEL was 323 mg/kg bw/day.
In a long-term toxicity/carcinogenicity oral study in rats at
doses equal to 41, 103, 338 or 856 mg/kg bw/day, the Committee
concluded that enrofloxacin was not carcinogenic in this species.
However, a NOEL could not be determined for the bile duct hyperplasia
seen in this study. The NOEL for bile duct hyperplasia in further
limited chronic studies with enrofloxacin in rats was 2.9 mg/kg
bw/day.
A two-generation reproductive toxicity study in rats was
performed with enrofloxacin administered in the feed to groups of rats
up to a dose equivalent to 630 mg/kg bw/day. Increased gestation
length, reductions in the number of litters, total pups, litter size
and number of implants for the F0 and F1 generations were seen at
630 mg/kg bw/day. Survival and growth rates were significantly
decreased in the progeny of these animals. Spermatic morphologic
alterations were noted in the high-dose males in both generations and
were considered the primary cause for the adverse effects on
fertility. The NOEL for reproductive performance was 165 mg/kg
bw/day.
An additional two-generation reproduction study in rats was
performed with enrofloxacin administered in the feed to groups of rats
at 0, 125, 300, or 2000 mg/kg feed to determine whether the test
compound affected gonadal function and mating behaviour in treated
males or reproductive parameters in females, as well as, to monitor
growth and development in the F1 and F2 offspring. Administration
of the test article caused a reduction in mean epididymal weights at
the highest dose and alterations in sperm morphology at 15 and 100 mg/kg
bw/day. No effects on fertility or reproductive performance were
noted. The NOEL was 125 mg/kg feed, equal to 10 mg/kg bw/day.
A special fertility study was performed in sexually mature male
rats fed enrofloxacin in the diet at doses equivalent to 0 or 375
mg/kg bw/day for 90 days. The purpose of the study was to determine
the timing of the onset of spermatic dysmorphogenesis and to ascertain
whether functional fertility was restored during a 90-day recovery
phase. Administration of the test article caused a significant
decrease in male fertility, food consumption and body-weight gain.
Testes weights were increased at all interim kills, but were lower
than in the control animals at the end of the study. Treatment-
related spermatic dysmorphogenic changes were seen by week 3 and these
changes were partially reversible as functional fertility returned to
13 of 15 previously treated males. Forty percent of these animals
demonstrated varying grades of seminiferous tubular atrophy and other
degenerative alterations. The remaining males were infertile.
A teratogenicity study was performed in which enrofloxacin was
administered to rats by gavage at 0, 50, 210, or 875 mg/kg bw/day.
Dosing was performed between days 6-15 of gestation. Maternal
toxicity was observed at 210 and 875 mg/kg bw/day. Food consumption
was higher in high-dose animals, but there was a significant reduction
in mean maternal weight gain in these animals. Fetal weights and
litter sizes were significantly decreased and fetal resorption and
post-implantation losses were increased in high-dose litters. Fetal
weights were significantly decreased in the mid-dose group. Skeletal
examination of the mid- and high-dose fetuses revealed delayed
skeletal ossification, which correlated with the decreased fetal
weights. No teratogenic effects were observed in this study. The
NOEL was 50 mg/kg bw/day.
In in vitro mutagenicity testing, enrofloxacin gave equivocal
results in a test for forward mutations in mammalian cells and
positive results in chromosomal aberration tests. Negative results
were obtained in in vivo bone marrow micronucleus, sister chromatid
exchange and bone marrow chromosomal aberration tests. The Committee
concluded that enrofloxacin is not genotoxic.
The potential for adverse effects on the human gastrointestinal
flora was considered from summary data. In vitro MIC data for
ciprofloxacin covering representatives of the human gut flora were
submitted for assessment. Escherichia coli was found to be the
most sensitive bacterium having an MIC50 value of 0.015 µg/ml.
In calculating the ADI this figure was used in the formula
developed at the thirty-eighth meeting of the Committee (Annex 1,
reference 97).
Concentration without
effect on human gut x Daily faecal bolus (g)
Upper limit of flora (µg/ml)a
temporary ADI =
(µg/kg bw) Fraction of
oral dose x Safety factorc x Weight of human (60 kg)
bioavailableb
(0.015 x 20) x 150
=
0.12 x 10 x 60
= 0.6 µg/kg bw
a Since the MIC value was determined using only 105 bacteria/ml a
factor of 20 was used to account for the higher inoculum density
representative of the human intestine. For example,
Enterobacteriaceae and Pseudomonas aeruginosa MIC values
increased 8 to 32-fold when the inoculum size was increased to
107 bacteria/ml.
b The fraction of the dose available to the intestinal microflora
was derived from studies of ciprofloxacin in humans. In human
volunteers given 2 x 50 mg ciprofloxacin the faecal concentration
was found to be 80 mg/kg. In 150 g faeces, this corresponds to
12% of the administered
c A safety factor of 10 was used to account for the possible
variation in absorption between ciprofloxacin and enrofloxacin, an
uncertainty in the biliary excretion of enrofloxacin, and the
variability between individuals.
4. EVALUATION
In view of the absence of information on the effects of
enrofloxacin on microorganisms obtained from the human intestine, the
Committee established a temporary ADI of 0-0.6 µg/kg bw based on the
results of the limited summary data of microbiology testing of
enrofloxacin/ciprofloxacin.
The temporary ADI of 0.6 µg/kg bw calculated from the in vitro
antimicrobial activity data represents an adequate margin of safety
when compared to the NOEL derived from the toxicology endpoint of 1.2
mg/kg bw/day for testicular toxicity.
5. REFERENCES
ALTREUTHER, P. & KLOSTERMANN, L. (1994). Unpublished summary of the
Enrofloxacin metabolism and residue information submitted to the FAO
experts. Submitted to WHO by Bayer AG, Leverkusen, Germany.
BARE, J.J., PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988). A
subchronic (13-week) feeding study followed by a 13-week drug
withdrawal period in male rats with BAY Vp 2674. Unpublished Report
No. MTD0074 from the Toxicology Department, Miles Inc., Elkhart, IN,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73812).
BECKER, H., VOGEL, W., & TERRIER, Ch. (1987). Embryotoxicity
(including teratogenicity) Study with BAY Vp 2674 in the Rabbit.
Unpublished Report No. R4249 (Project No. 058555) from the Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73705).
BOOTMAN, J., HODSON-WALKER, G., & DANCE, C. (1987). BAY Vp 2674;
Investigation of effects on bone marrow chromosomes of the rat after
acute oral administration. Unpublished LSR report No. 87/BAG039/312
(Report No. R4189; Study No. T5023055) from Life Science Research
Ltd., Suffolk, England. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 74166).
BOMHARD, E., RÜHL-FEHLERT, C., & KALINER, G. (1991a). BAY Vp 2674.
Study of chronic toxicity and carcinogenicity in mice (administration
in feed over 24 months). Unpublished Report No. 20263 (Study No.
T8023436) from the Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74229).
BOMHARD, E. & SCHLADT, L. (1991B). BAY Vp 2674. Study of chronic
toxicity and carcinogenicity in rats after administration in feed over
2 years. Unpublished Report No. 20306 (Study No. T8023436) from the
Department of Toxicology, Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany (report No. 74230).
DEN BOER, W.C. & HOORN, A.J.W. (1987). Mutagenicity evaluation of
BAY Vp 2674 in the CHO/HGPRT forward mutation assay: Revised final
report. Unpublished genetics assays Nos. E-9457 & E-9598 (Report No.
R 4071) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73582).
CIFONE, M.A., & MYHR, B.C. (1985). Evaluation of BAY Vp 2674 in the
rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished
Report No. 3168E (Genetic Assay No. 7550; LBI project No. 20991) from
Litton Bionetics, Inc., Kensington, MD. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73055).
CLEMENS, G.R. & HARTNAGEL, R.E. (1985). A teratology (Segment II)
study in the rat with BAY Vp 2674. Unpublished Report No. 53 from the
Toxicology Department, Central Research Services, Miles Laboratories
Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany (report No. 73159).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1986). A two-generation
reproduction study in rats with BAY Vp 2674. Unpublished Report No.
4 from Toxicology Department, Central Research Services, Miles
Laboratories Inc., Elkhart, IN, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73314).
CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1987). A specialized
male fertility study with BAY Vp 2674 in the rat. Unpublished Report
No. MTD0030 (Mobay Report No. 73800) from the Toxicology Department,
Central Research Services, Miles Laboratories Inc., Elkhart, IN, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany (report No. 73800).
EIGENBERG, D.A. (1987). Acute dermal toxicity of BAY Vp2674 in albino
rabbits. Unpublished toxicological report No. 926 (Study No. 86-023-
04) from Health, Environment & Safety, Corporate Toxicology
Department, Mobay Corporation, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73606).
GOLDSTEIN, E.J.C., & CITRON, D.M. (1988). Comparative activities of
cefuroxime, amoxicillin-clavulanic acid, ciprofloxacin, enoxacin, and
ofloxacin against aerobic and anaerobic bacteria isolated from bite
wounds. Antimicrob. Agents Chemoth., 32:1143-1148.
HALL, W.D. (CHAIR) (1992). Pathology working group on a 2-year
chronic feeding study with a l-year interim kill in rats on the
compound BAY Vp 2674. Unpublished Pathology Working Group report
(Bayer ID 014379) prepared for the Center for Veterinary Medicine,
U.S. Food & Drug Administration, Department of Health & Human
Services, Rockville, MD, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
HERBOLD, B. (1985a). BAY Vp 2674; Salmonella /microsome test to
evaluate point mutagenic effects. Unpublished Report No. 13523E
(Study No. T9017669) from the Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73213).
HERBOLD, B. (1985b). BAY Vp 2674; Micronucleus test on the mouse to
evaluate for mutagenic effects. Unpublished Report No. 14102E (Study
Nos. T6019042 & T8019675) from Institute of Toxicology, Pharmaceutical
Division, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74164).
HERBOLD, B.A. (1988). BAY Vp 2674; Sister chromatid exchange in the
bone marrow of the Chinese hamster in in vivo as a test for DNA-
modifying effects. Unpublished Report No. 16783 (Study Nos. T5024676
& T8027324) from Department of Toxicology, Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany (report
No. 74178).
KOWALSKI, R.L., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985).
Safety evaluation of BAY Vp 2674: Subchronic (13 week) feeding study
in the rat. Unpublished Report No. 63 (No. 73194) from Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73194).
KOWALSKI, R.L., CLEMENS, G.R., JASTY, V., & HARTNAGEL, R.E. (1989).
A two-generation reproduction study in rats with BAY Vp 2674.
Unpublished Report No. MTD0093 (Report No. R4705) Toxicology
Department, Central Research Services, Miles Laboratories, Elkhart,
IN. Submitted to WHO by Bayer AG, Leverkusen, Germany (report No.
73892).
LESER, K.H. (1992). BAY Vp 2674. Chronic toxicity study in rats after
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 21668A (Study No. T7032787) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74387).
LESER, K.H. (1993). BAY Vp 2674. Chronic toxicity study in rats with
administration in feed over a period of 2 years. (Supplementary study
to determine the dose tolerated without damage). Unpublished Report
No. 22659 (Study No. T6039950) from the Department of Toxicology,
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 74472).
MANDELL, G.L., & SANDE, M.A. (1990). Antimicrobial Agents in Goodman
and Gilman's the Pharmacological Basis of Therapeutics, 8th edition,
Gilman, A.G., Rall, T.W., Nies, S.A., and Taylor, P. (eds.). Pergamon
Press, Inc., Maxwell House, Fairview Park, Elmsford, New York 10523,
p. 1058.
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1985). Safety
evaluation of BAY Vp 2674: Subchronic (13 week) feeding study in the
dog. Unpublished Report No. 43 (Mobay Report No. 73146) from the
Toxicology Department, Central Research Services, Miles Laboratories,
Inc., Elkhart, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 73146).
PORTER, M.C., JASTY, V., BARE, J.J., & HARTNAGEL, R.E. (1987). Safety
evaluation of BAY Vp 2674: Repeat of a subchronic (13-week) feeding
study in the dog. Unpublished Report No. MTD0031 (BayVet report No.
73146) from the Toxicology Department, Central Research Services,
Miles Laboratories, Inc., Elkhart, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73775).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988a). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs.
Unpublished Report No. MTD0068 from the Toxicology Department, Central
Research Services, Miles Inc., Elkhart, IN, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany (report No. 73788).
PORTER, M.C., JASTY, V., & HARTNAGEL, R.E. (1988b). Safety evaluation
of BAY Vp 2674: Subchronic (13-week) feeding study in male dogs
followed by a 13-week withdrawal period. Unpublished report Miles
Inc., Elkhart, Report No. MTD0069. Submitted to WHO by Bayer AG,
Leverkusen, Germany (report No. 73789).
SCHLUTER, G. (1987). Ciprofloxacin: review of potential toxicologic
effects. Am. J. Med, 82 (suppl 4A): 91-93.
SCHMIDT, M. (1985). Acute toxicity of BAY Vp 2674 in the rat, mouse,
rabbit and dog. Unpublished Report No. 13223(E) from Institute of
Toxicology, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany (report No. 73075).
SQUIRE, R.A. (1992). Evaluation of bile duct hyperplasia in male rat
livers. Unpublished Report No. 74388 (Study No. T7032787; Pathology
No. 3122) from Toxicology, Research & Development Dept., Miles Inc.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74388).
TAALMAN, R.D.F.M. & HOORN, A.J.W. (1988). Clastogenic evaluation of
BAY Vp 2674 in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished HBC study No. E-9804-0-437 (Report No. R 4474; Study No.
T1027516) from Hazleton Biotechnologies Laboratory, Veenendaal, The
Netherlands. Submitted to WHO by Bayer AG, Leverkusen, Germany
(report No. 74165).
WISE, R., ANDREWS, J.M., ASHBY, J.P. & MATTHEWS, R.S. (1988).
In vitro Activity of lomefloxacin, a new quinolone antimicrobial
agent, in comparison with those of other agents. Antimicrob. Agents
and Chemoth., 32: 617-622.
