
CHLORTETRACYCLINE AND TETRACYCLINE
First draft prepared by
M.F.A. Wouters
J.E.M. van Koten-Vermeulen
F.X.R. van Leeuwen
Toxicology Advisory Centre
National Institute of Public Health and
Environmental Protection
Bilthoven, Netherlands
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Interactions with bones and teeth
Observations in humans
Biotransformation
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Long-term toxicity/carcinogenicity studies
Reproductive toxicity studies
Special studies on embryotoxicity/teratogenicity
Special studies on genotoxicity
Special studies on microbiological effects
Special studies on eye irritation and sensitization
Other studies
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
Chlortetracycline (CTC) and tetracycline (TC) are broad-spectrum
antimicrobial drugs with a long history of use in humans and animals.
TC is used primarily for the short-term oral treatment of clinical
diseases, while CTC is usually administered in the feed or water for
prophylactic purposes.
The tetracyclines (TCs) as a group were previously evaluated at
the twelfth meeting of the Committee (Annex 1, reference 17), when a
temporary ADI of 0-0.15 mg/kg bw was established. Oxytetracycline
(OTC) was re-evaluated at the thirty-sixth meeting of the Committee
(Annex 1, reference 91), when an ADI of 0-3 µg/kg bw was established,
based on effects on the human gut flora. Additional data have become
available on CTC and TC since the twelfth meeting, which were
evaluated at the present meeting.
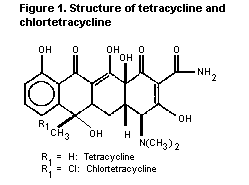
CTC and TC are closely related in structure (see Figure 1). The
toxicological profiles and spectra of antimicrobial and biological
activity are similar and therefore the Committee considered data on
the two compounds together in evaluating their toxicological
potential.
Figure 1. Structure of tetracycline and chlortetracycline
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Tetracycline (TC)
Rats
Fasted white rats were given a single oral dose of TC
hydrochloride by gavage corresponding to 75 mg/kg bw TC base. The rats
were sacrificed 1, 2, 3, 4 or 6 h after treatment and plasma and
tissue levels recorded.
Peak plasma concentrations of 3.6 mg/l were observed 2 h after
treatment, declining to 0.5 mg/l after 6 h. Peak tissue levels were
found 2 h after treatment with highest levels in the liver and kidney
(Berté & Vandoni, 1962).
To investigate the importance of the biliary route of excretion,
4 rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw tritium- labelled TC. Radioactivity was measured in
urine, bile and GI tract 24 h following treatment.
In non-ligated rats, 85-92% of the total radioactivity was
recovered of which 67-72% was excreted in urine and 18-20% in faeces.
About 70-85% of the total radioactivity was recovered in the rats with
ligated bile ducts, 68% and 88% being recovered in the urine of each
rat, and 30% and 9% in the bile. Only very small amounts (on average
2.5%) were recovered in the GI tract. When the same dose of TC was
administered to rats with ligated ureters, no increase in the
excretion of TC in the faeces was observed (Wulf & Eisner, 1961).
Male Sprague-Dawley rats were injected with 10 mg/kg bw 3H-7-TC
hydrochloride (98% pure) in the femoral vein over 5 min. The
intestinal absorption of TC hydrochloride excreted in bile was
evaluated using in situ intestinal preparations. About 73% of TC
excreted in the bile was reabsorbed in the intestinal lumen,
indicating enterohepatic circulation (Adir, 1975).
Single i.v. doses of 15 or 4 mg/kg bw tritium-labelled TC
hydrochloride were administered to 2 rats and 1 dog, respectively.
Within 72 h, 69.2% and 19.5% of the radioactivity was recovered in the
urine and faeces of rats, respectively. Within 168 h, 71% of the
radioactive dose was recovered in the urine and 9% in the faeces of
the dog (Eisner & Wulf, 1962).
Dogs
Tritium-labeled TC hydrochloride was administered i.v. to 2
beagle dogs at 10 mg TC/kg bw to study the distribution of TC
throughout the body. The animals were sacrificed after 4 h and the
various organs and tissues examined for radioactivity. The organs
containing the highest radioactivity were liver and kidney, containing
on average 15 and 45 mg/kg, respectively. A large proportion of the
recovered TC activity was found in the urine, intestinal contents and
bile. No radioactivity was measured in subcutaneous fat (Kelly, 1964).
Beagle dogs given single oral doses of 25 mg TC/kg bw showed peak
TC levels averaging 3 µg/ml 2 h after dosing, declining to an average
of 0.27 µg/ml by 24 h post-dosing. Excretion in the urine accounted
for 10% of the administered dose within 72 h after dosing.
When dogs were given a single i.v. dose of 10 mg TC/kg bw,
average serum TC levels of 10.6 µg/ml were found at 24 h, and
0.14 µg/ml at 48 h after dosing. Excretion in the urine was 58% of the
administered dose by 72 h after dosing (microbiological assay;
sensitivity 0.05-0.1 µg/ml) (Kanegis, 1958).
Pigs
The bioavailability of TC hydrochloride administered orally to
fasted gilts was calculated from the area under the plasma
concentration in time curve (AUC) and estimated to be 23%. After i.v.
administration the disposition kinetics of TC in plasma were best
described by a tri-exponential equation. The drug had a rapid
distribution phase followed by a relatively slow elimination phase,
with half-life of 16 h (Kniffen et al, 1989).
2.1.1.2 Chlortetracycline (CTC)
Rats
14C-Labelled CTC was orally administered to rats at a dose of
60 mg/kg bw. Radioactivity was measured in urine and faeces after
24, 48 and 72 h. Radioactivity was found primarily in the faeces
(92% within 72 h, the major part being excreted within 24 h). About 5%
of the radioactivity was recovered in urine.
When 30 mg/kg bw 14C-CTC was administered i.p., 33% of the
radioactivity was excreted in the urine within the first 24 h, and 5%
in the faeces. Between 24 and 72 h, 7% was excreted in the urine and
40% in the faeces.
To investigate the importance of the biliary route of excretion,
rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw 14C-CTC. Radioactivity was measured in urine, bile
and GI tract, 24 h following treatment.
In non-ligated rats, 75-79% of the total radioactivity was
recovered of which 35-37% was excreted in urine and 44-38% in faeces.
In the rats with ligated bile ducts, about 47-63% of the total
radioactivity was recovered, with 66% and 43% being recovered in the
urine of each rat, and 22% and 51% in the bile. Only very small
amounts (on average 5%) were recovered in the GI tract (Wulf &
Eisner, 1961).
Groups of 6 rats given single oral doses of 75 mg CTC/kg bw had
plasma levels of 2.1 mg/l at 1 h after administration, declining to
0.8 mg/l after 6 h. The rats were sacrificed at 1, 2, 3, 4 or 6 h
after treatment. Tissue levels were highest in liver and kidneys at
all observations. Maximum concentration was found after 2 h in the
liver, and after 1 h in the kidney (Berte & Vandoni, 1962).
Oral doses ranging from 6 to 800 mg CTC/kg bw administered to
female rats and male guinea-pigs gave no proportional increases in
serum CTC concentrations. When guinea-pigs received the same doses
each day for 9 days, higher serum levels were found than with single
doses. Serum CTC levels were increased by the simultaneous
administration of various adjuvants, such as citric acid. This effect
(observed in doses of CTC up to 200 mg/kg bw) was apparent within 1 h
of administration and lasted at least 8 h (Eisner et al., 1953)
Rabbits
Ten adult white Californian rabbits (males and females) received
a single oral dose of 20 mg/kg bw of technical CTC or CTC-chloride.
Serum CTC levels averaged 2.3 mg/l 3 h after dosing. The concentration
of CTC declined to a mean value of 0.09 mg/l by 12 h, and to 0.08 mg/l
by 24 h after dosing. The highest concentrations were found in the
liver (1.53 mg/kg) 24-h post-dosing, followed by kidney, lung and
heart. No measurable levels were found in muscle (detection limit:
37.5 µg/kg) (Neuschl, 1991)
Dogs
Four beagle dogs were given a single oral dose of 25 mg
CTC/kg bw. Peak serum CTC levels ranged from 0.40-1.9 mg/l 2 h after
dosing, declining to an average of 0.21 mg/l by 24 h. When the dogs
were given an i.v. injection of 10 mg CTC/kg bw, serum levels averaged
6.6 mg/l 1 h after administration, declining to 2.4 µg/ml at 8 h,
0.29 µg/ml at 24 h and 0.06 µg/ml at 48 h (Kanegis, 1958).
Two female beagle dogs received a single i.v. injection of 10 mg
14C-CTC/kg bw. The dogs were sacrificed 4 h after dosing. The organ
containing the highest radioactivity was the liver (30 mg/kg),
followed by kidney (25 mg/kg), ileum (15 mg/kg), jejunum (12 mg/kg)
and heart (10 mg/kg). A large proportion of the recovered
radioactivity was found in urine, intestinal contents and bile. With
the exception of subcutaneous fat, radioactivity was found throughout
all tissues and fluids examined (Kelly, 1964).
2.1.2 Interactions with bones and teeth
Tissues from mice, rats, guinea-pigs, rabbits and dogs
parenterally treated with TCs (TC, CTC or OTC, doses varying from
0.1 - 50 mg/kg bw) were examined in U.V. light. In all tissues with
the exception of the brain, a brilliant yellow-gold fluorescence
was apparent within 30 min, irrespective of the dose. The induced
fluorescence disappeared from all tissues (except bone) within 6 h
after a single parenteral injection. However, bone fluorescence
persisted throughout a 10-week observation period after dosing
(Milch et al., 1967).
Single oral doses of 250 mg/kg bw tritium-labelled TC or single
oral dose of 250 mg 14C-CTC/kg bw were administered to male rats
(Sherman strain). Radioactive residues in the femur of the rats
treated with TC were 9.6, 1.9 and 0.4 mg/kg of bone measured after
4 h, 24 h, and 4 weeks, respectively. The radioactivity averaged
12 mg/kg of bone 4 h after CTC treatment, and 2.3 mg/kg after 4 weeks.
After a lifetime diet containing 0.5 to 1000 mg CTC/kg of feed,
the maximum radioactivity in femur was 570 mg/kg. After i.p.
injections with 10-150 mg TC/kg bw, the concentrations found in the
femur were directly related to the administered dose and were much
higher than the concentrations measured after oral treatment with
250 mg TC/kg bw (Buyske et al., 1960).
2.1.3 Observations in humans
In humans, about 30% of an oral therapeutic dose of CTC was
absorbed on an empty stomach. For TC and OTC, the absorption was
60-80% (Sande & Mandell, 1990).
Oral doses of 250 to 500 mg every 6 h produced plasma
concentrations of TC ranging from 1 to 5 mg/l. Intravenous injections
of 250-500 mg produced plasma concentrations of about 15-20 mg/l at
0.5 h, falling to 4-10 mg/l at 1 to 2 h. At 12 h, 1-3 mg/l was still
present. In circulation, TCs are bound to plasma proteins in varying
degrees (24-65% for TC, and about 47% for CTC). TCs appeared in the
milk of nursing mothers where concentrations were be 60% or more of
those in the plasma. TCs diffused across the placenta and appeared in
the fetal circulation at concentrations of about 25 to 75% of those in
the maternal blood. The plasma half-lives of CTC and TC were reported
to be about 8-10 h and 5.5 h, respectively (Martindale, 1989).
Absorption of TCs was impaired by milk products, sodium
bicarbonate, aluminium hydroxide and iron preparations due to
chelation and increase in gastric pH (Sande & Mandell, 1990).
2.1.4 Biotransformation
2.1.4.1 Tetracycline
The metabolism of 14C-TC was examined in rats following single
i.p. or oral administration of 60 mg/kg bw, and in dogs after single
oral administration of 25 mg/kg bw tritium-labelled TC. Approximately
90% of the administered radioactivity in the rat was eliminated either
by urinary or faecal route. A significant portion of the remaining
activity was bound as chelated TC in the skeleton of the animal. With
the exception of this chelate, TC was chemically unaltered by the rat.
Urine of dogs contained only unchanged TC (Kelly & Buyske, 1960).
2.1.4.2 Chlortetracycline
Male Wistar rats (n=6) and male beagle dogs (n=2) were
administered 14C-labelled CTC via different routes (oral:
60 mg/kg bw; i.p.: 30 mg/kg bw; i.v.: 15-60 mg/kg bw). The
recovery of antimicrobial activity was significantly lower than the
recovery of radioactivity by all routes of administration and
excretion. The major 'presumed' metabolite was identified as
4-epichlortetracycline, which accounted for 23-35% of the
radioactivity in the urine of rats, and 31-60% in dogs. It was
essentially inactive in microbiological assays. It was not clear
whether this represented a true metabolite or was a degradation
product due to alkaline treatment. The presence of small amounts
(5-10%) of isochlortetracycline in the urine and faeces of some
animals was also demonstrated (Wulf & Eisner, 1961; Eisner & Wulf,
1963).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with TC and CTC after oral,
i.v. or s.c. administration are summarized in Tables 1 and 2,
respectively.
Dogs developed transient hyperpnea, weakness and anorexia
following an i.v. dose of 50 and 100 mg CTC/kg bw, and a dose of
150 mg/kg bw caused respiratory distress, general paresis, somnolence
and death within a few hours (Hines, 1956).
Table 1. Acute toxicity of tetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. oral TC n.s. 2550 Tubaro et al., 1964
mouse n.s. oral TC n.s. >3000 Cunningham et al., 1954
mouse (42d) n.s. oral TC n.s. 808 Goldenthal, 1971
(3d) 300
mouse n.s. i.v. TC n.s. 160 Tubaro et al., 1964
mouse n.s i.v. TC n.s. 157 Maffii & Mainardi, 1955
mouse n.s. i.v. TC n.s. 170 Cunningham et al., 1954
mouse n.s. i.p. TC n.s. 340 Tubaro et al., 1964
mouse n.s. i.p. TC n.s. 330 Cunningham et al., 1954
rat M oral TC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral TC n.s. >3000 Cunningham et al., 1954
rat (49(d) n.s. oral TC n.s. 807 Goldenthal, 1971
(3d) 360
Table 1. Acute toxicity of tetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat (adult) n.s. oral TC HCl n.s. 6443 Goldenthal, 1971
(<2d) 3827
rat n.s. i.v. TC n.s. 220 Cunningham et al., 1954
rat n.s. i.v. TC n.s. 128 Maffii & Mainardi, 1955
rat n.s. i.v. TC HCl n.s. 375 Diermeier, 1959
rat n.s. i.p. TC n.s. 320 Cunningham et al., 1954
n.s. not specified
d age of animal in days
2.2.2 Short-term toxicity studies
2.2.2.1 Tetracycline
Mice
Groups of male mice (10/sex/group) were given by gavage 0, 20 or
100 mg/kg bw TC hydrochloride once daily, 5 days/week for 6 weeks as a
2% starch suspension containing 4-6% test substance. At termination of
the study a complete blood count was performed in the high-dose group
and no significant haematological alterations were observed.
Accelerated growth rates were observed in dosed mice when compared to
the control mice (Cunningham et al., 1954).
In a range-finding study, groups of B6C3F1 mice (6-7 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 470, 950, 1800, 3700
or 7500 mg/kg bw/day, respectively). No mortalities occurred. Final
mean body weight was slightly decreased in males (16%) and females
(6%) at the 5% level. The TC concentration in bone increased with
increasing dose of TC hydrochloride (NTP, 1989).
Rats
In a range-finding study, groups of F344/N rats (7-8 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 155, 315, 625, 1250 or
2500 mg/kg bw/day, respectively). No mortalities occurred. Cytoplasmic
vacuolization was observed in the livers of male rats at the two
highest dose groups. Bone marrow atrophy was seen in both female and
male rats at 1250 and 2500 mg/kg bw/day. The concentration of TC in
bone increased with increasing dose of TC hydrochloride (NTP, 1989)
Dogs
Mongrel dogs (8-4/group) were given a capsule of 0 or
250 mg/kg bw TC once daily, 6 days/week for 98 days. No mortality
occurred. No effects were seen on peripheral blood and no pathological
changes were observed (Nelson & Radomski, 1954).
Groups of 4 mongrel dogs were orally administered 10 or
100 mg/kg bw TC twice daily, 5 days/week for 3 months. No effects were
observed on body weight, liver function (bromosulfophthalein
clearance), kidney function (phenolsulfophtalein clearance),
blood-clotting time, non-protein nitrogen levels, blood sugar values
or complete blood counts (Cunningham et al., 1954).
Table 2. Acute toxicity of chlortetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse M & F oral CTC HCl n.s. >3000 Cunningham, 1954
mouse F oral CTC HCl n.s. 2150 Tubaro & Banci, 1964
mouse M & F oral CTC HCl n.s. 3350 (M) Bacharach, 1959
4200 (F)
mouse n.s. oral duomycin n.s. >3000 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 155 Cunningham, 1954
mouse F i.v. CTC HCl n.s. 93 Tubaro & Banci, 1964
mouse n.s. i.v. duomycin n.s. 102 Harned, 1948
mouse n.s. i.v. duomycin n.s. 134 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 102 (M) Bacharach, 1959
108 (F)
mouse F i.v. CTC bisulfate n.s. 113 Levinskas 1963
mouse n.s. s.c. duomycin n.s. >600 Harned, 1948
mouse M & F s.c. CTC HCl n.s. 5500 (M) Bacharach, 1959
8250 (F)
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. i.p. CTC HCl n.s. 192 Cunningham, 1954
mouse M & F i.p. CTC HCl n.s. 128 (M) Bacharach, 1959
168 (F)
mouse F i.p. CTC HCl n.s. 214 Tubaro & Banci, 1964
rat M & F oral CTC HCl n.s. >3000 Cunningham, 1954
rat F oral CTC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral CTC-HCl n.s. 55001 Goldenthal, 1971
103002
rat F oral calcium CTC n.s. >10000 Levinskas, 1963
rat n.s. oral Aureo Biomass n.s. >5000 Lowe & Fisher, 1993
(summary only)
rat n.s. oral duomycin n.s. >3000 Harned, 1948
rat M & F i.v. CTC HCl n.s. 160 Cunningham, 1954
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat M i.v. CTC HCl n.s. 167 Diermeier, 1959
rat n.s. i.v. duomycin n.s. 118 Harned, 1948
rat n.s. i.p. CTC HCl n.s. 335 Cunningham, 1954
guinea pig n.s. i.v. duomycin n.s. 100 Harned, 1948
guinea pig n.s. s.c. duomycin n.s. >300 Harned, 1948
n.s. not specified
1 < 2 days old
2 adult
Groups of male dogs (4 mongrel dogs and 4 beagle dogs) were fed
diets containing 0.1, 0.3 or 1% TC hydrochloride for 24 months
(equivalent to 25, 75 or 250 mg/kg bw/day, respectively). An interim
sacrifice of 1 beagle and 1 mongrel dog of each group was performed at
12 months for microscopic examination. No effects were observed on
clinical signs, mortality, body weight, food consumption, haematology,
ALP, bromosulfophtalein clearance, urea nitrogen determinations,
testes and epididymides weight, macroscopy, concentration and motility
of the spermatozoa, or appearance of semen. The bony structures of all
dosed dogs showed a yellowish coloration. The intensity of the colour
was related to the dose fed. A brownish-black pigmentation with a
dose-related intensity was observed in the thyroid glands of the dogs
of all groups. Microscopic examination of the thyroids revealed
intracytoplasmic granules in the follicular epithelial cells of most
of the treated dogs. This effect might be caused by deposits of TC or
its metabolites. Histopathological changes were not observed
(Deichmann et al., 1964).
2.2.2.2 Chlortetracycline
Mice
Groups of 10 male mice were given 0, 20 or 100 mg CTC/kg bw/day,
once daily by gavage, 5 days/week for 6 weeks. At 100 mg/kg bw/day,
complete blood count indicated that there were no significant
haematological alterations. Beneficial effects such as increased
growth were observed at 20 and 100 mg/kg bw/day (Cunningham, 1954).
Two groups of 20 mice received 20 or 100 mg/kg bw/day of CTC for
3 months (route not specified). Final body weight of both groups
exceeded those of the controls by 15%. No evidence of toxicity was
seen and no difference in blood counts was found at the end of
experiment (Hines, 1956).
Groups of 20 male mice (strain not specified) received orally
0, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw, twice daily). There were no
significant effects on mortality, clinical signs, body-weight gain,
haematology, macroscopy or microscopy. The NOEL in this study was
200 mg/kg bw/day (Harned et al., 1948).
Rats
Sherman rats (6/sex/group) received diets containing 0, 2.0 or
5.0% CTC for 28 days (equivalent to 2000 or 5000 mg/kg bw/day).
Body-weight gain was significantly decreased at 5000 mg/kg bw/day. No
other examinations were performed (Halliday & Fanelli, 1960).
Two groups of 20 rats each were given oral doses of 150 or
300 mg/kg bw/day CTC for 3 months. In both groups, body weight of the
animals was higher than the controls. No difference in blood counts
was found at the end of the experiment (Hines, 1956).
Groups of 20 male rats (strain not specified) received by gavage
0, 10, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw twice daily). No treatment-related
effects were seen on mortality, clinical signs, body weight,
haemoglobin, blood pressure, macroscopy or microscopy. The NOEL in
this study was 200 mg/kg bw/day (Harned et al., 1948).
Dogs
Oral administration of 100 mg CTC/kg bw/day to dogs (number and
strain not specified) for 17 days, followed by 100 mg/kg bw twice
daily for 14 days, produced no unfavourable effects on general
appearance, growth rate, haemoglobin, complete blood count, gross or
microscopic examinations (Hines, 1956).
Dogs (4/group) received 10, 50 or 100 mg of CTC/kg bw/day for 14
weeks (route not specified). At the end of this period, liver and
kidney function tests, blood sugar values, blood coagulation time,
blood non-protein nitrogen and complete blood counts did not differ
significantly from pre-test values (Hines, 1956)
Ten dogs (strain not specified) were given CTC twice daily
(morning and afternoon) by capsule at a total dose of 100 mg/kg
bw/day, 5 days/week for 9 to 15 weeks. CTC treatment had no effect on
general appearance, body weight, haematology, clotting time, liver and
kidney function (Bromosulfophtalein and phenolsulfophtalein
clearance), urinalysis, gross or microscopic examinations
(Harned, 1948).
Ten mongrel dogs (8-19 kg; 2-5 years old) received capsules
containing 250 mg CTC/kg bw/day, 6 days/week, for 98 days. Another 4
dogs received the same dose intermittently, 2 weeks on the drug and 3
weeks off, for 121 days in total. Five dogs of the continuously
treated group died. These animals had progressive weight loss, apathy
and anorexia. Haemoglobin, RBC, granulocyte and total leucocyte counts
were slightly depressed. The pathologic changes observed included
emaciation, fatty liver, excess fat in the kidney, depletion of the
bone marrow and atrophic changes in the spleen, lymph nodes and
skeletal muscle (Nelson & Radomski, 1954).
Beagle and basenji dogs (2/sex/group; 9-12 months old) received
daily a single oral dose of 10 or 100 mg CTC-HCl/kg bw in capsules for
54 weeks; a third group consisting of 1 female beagle dog, 2 male and
1 female basenji dogs were given 50 mg/kg bw/day. No control group was
used. Observations included haematology, clinical chemistry,
urinalysis, faecal smears, macroscopy, organ weight, microscopy, and
terminal tissue concentration.
Treatment-related GI disorders (vomiting, diarrhea, appetite loss
and swelling of the anal glands) were observed, and particularly in
the first half of the experiment in all groups, most often in basenji
dogs. The average blood concentration 4 h after administration of the
dose (calculated for each group from 12-14 determinations carried out
over a period of 1 year) were 0.34 µg/ml, 0.58 µg/ml and 0.94 µg/ml at
10, 50 and 100 mg/kg bw/day, respectively. Thyroid weight was high in
one male at 100 mg/kg bw/day, but the shape and appearance of the
gland were normal. Slight yellow discolouration in the bones was
observed at necropsy in the high-dose dogs, and 2 dogs at each dose
level showed evidence of chronic gastritis. Measurable tissue
concentrations (no details given) were found in bone > kidney >
liver but none in brain, heart or spleen. The NOEL in this study was
100 mg/kg bw/day (Dessau & Sisson, 1957).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Tetracycline
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets for 103
weeks containing 0, 1.25 or 2.5% TC-HCl (purity 91%), equal to 1500 or
3000 mg/kg bw/day for males, and 1500 or 3500 mg/kg bw/day for
females. Observations included clinical signs, body weight, feed
consumption, macroscopy, and histopathology.
Survival was increased for male mice (31/50, 43/50, 43/50); no
differences were observed in females (37/50, 35/50, 38/50). Mean body
weights in both dose groups were slightly lower for both sexes
compared to control mice. Dosing had no effects on feed consumption.
The tumour incidence was not significantly increased in either sex. On
the contrary, dosed female mice did not develop hepatocellular
adenomas or carcinomas (combined incidence: 10/49 for controls; 0/48
at 1.25%; 0/50 at 2.5%) (NTP, 1989; Dietz et al., 1991).
Rats
Groups of weanling male rats (Osborne-Mendel) were fed diets
containing 0 (180 rats), 100 (100 rats), 1000 (130 rats) or 3000
(100 rats) mg TC-HCl/kg of feed, equivalent to 5, 50 or 150 mg/kg
bw/day for 2 years. Ten Rats/group were killed at frequent intervals.
Sacrificed and moribund rats were macroscopically and histologically
examined. No effects were seen on clinical signs, body weight, food
consumption or haematological examinations. Beneficial effects were
observed during the first 18-19 months, all dosed rats appearing more
vigorous and gaining weight more rapidly than the control rats.
Mortality rates were lower in the treated rats than in the control
rats. At histopathology, yellow discoloration of long bones and
calvarium was observed at the highest dose. Tumour incidence was not
enhanced (Deichmann et al., 1964).
Groups of F344/N rats (50/sex/group) were fed diets containing
0, 1.25% or 2.5% mg TC-HCl (purity 91%), equal to 440 or 910 mg/kg
bw/day for males, and 510 or 1060 mg/kg bw/day for females for
103 weeks. Observations included clinical signs, body weight, feed
consumption, macroscopy and histopathology.
The survival of female rats of both the low- and high-dose groups
was significantly greater than that of the controls (control 27/50,
low-dose 39/50, high-dose 38/50). No compound-related effects on body
weight or feed consumption were observed. A dose-related increased
incidence of basophilic cytoplasmic changes and clear cell changes
were observed in the livers of male rats. The incidence and severity
of nephropathy were reduced in dosed male rats (control 48/50,
severity 2.8; mid-dose 35/50, severity 1.6; high-dose 36/50, severity
1.5). The tumour incidence was not enhanced (NTP, 1989; Dietz et al.,
1991).
2.2.3.2 Chlortetracycline
Rats
Forty rats (sex or strain not specified) were maintained for 52
weeks on a diet containing 1% CTC, equivalent to 1000 mg/kg bw/day.
There was no significant difference in body weight and no evidence of
toxicity was seen.
In a parallel experiment another group of 40 rats (sex or strain
not specified) received 5% CTC, equivalent to 5000 mg/kg bw/day. Ten
animals died within the first 10 weeks. The remaining 30 rats survived
for the 52-week test period and had a 33% lower weight than the
controls, and an apparent yellow staining of all tissues (Hines, 1956).
Groups of Sherman rats (20/sex/group) were fed diets containing
CTC at concentrations of 0, 1, 5, 20, 100, 500, 2000, 10000 or
50000 mg/kg of feed, equal to 0, 0.07, 0.35, 1.3, 7, 34, 130, 700 or
5200 mg/kg bw/day, respectively. Observations were made on mortality,
clinical signs, body-weight gain, food consumption, haematology,
macroscopy, organ weight, and microscopy.
During the first 12 weeks of the study, 8 male rats in the
highest dose group died compared to none in the other groups. At
termination, the total mortality for males was not different from
controls. Both males and females in the 50000 mg/kg group showed signs
of GI irritation which included abdominal distension, nose and mouth
encrustation and salivation. Body-weight gain was reduced in both
sexes at 50000 mg/kg. WBC was reduced in both sexes at 50000 mg/kg.
Microscopic changes at 50000 mg/kg included accumulation of yellow
pigment in splenic lymph follicles for both sexes, testicular atrophy
occasionally with degenerative changes in seminiferous tubulus, fatty
infiltration of the liver in males and infiltration of monocytes
sometimes coupled with interstitial fibrosis in the lungs in both
sexes. Fluorometric determinations showed the presence of significant
amounts of CTC at dose levels of 500 mg/kg of feed and higher. No
blood or urine biochemistry was carried out. The tumour incidence was
not enhanced. The NOEL in this study was 10000 mg/kg diet, equal to
700 mg/kg bw/day (Dessau & Sullivan, 1958; Dessau & Sullivan, 1961).
2.2.4 Reproductive toxicity studies
2.2.4.1 Chlortetracycline
Mice
Mice maintained through 4 reproductive cycles on diets containing
175 mg CTC/kg of feed (equivalent to 25 mg/kg bw/day) did not differ
significantly from parallel control groups in the number of young born
per litter, the number of surviving pups or the average body weight at
the end of 4 weeks (Hines, 1956).
Rats
Rats maintained through 2 reproductive cycles on a diet
containing 45 mg CTC/kg of feed (equivalent to 2 mg/kg bw/day) did not
differ significantly from parallel control groups in the number of
young per litter, the number of surviving pups or the average body
weight at the end of 4 weeks (Hines, 1956).
A 2-generation reproductive toxicity study was carried out with 2
groups of 5 male and 15 female rats (Sherman strain, approximately
21-day old). One group received the control diet and the other
received the same diet containing 1% CTC, equivalent to 500 mg/kg
bw/day. When the rats were approximately 100-day old, the animals were
mated to produce the first generation. All litters were reduced to 8-9
rats. Pups were weaned to the diet given to their parents. Second
generation breeding was carried out with 21 female and 7 male rats in
the 1% group and a few more rats per group in the control group.
Observations were made for clinical signs, body weight, food
consumption, fertility, reproductive performance and pup growth. Male
body weights were slightly lower than controls for both parental
generations. No further toxicity, nor effects on male or female
reproduction were observed (Hallesy & Hine, 1964).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Tetracycline
Groups of 16 pregnant Wistar rats were given 0 or 150-200 mg TC
chlorhydrate/rat/day from days 1 to 18 of gestation (Group I) and
another group received the same treatment from days 1 to 28 after
delivery (Group II). At day 20 of gestation, 3 females of group I and
3 control females were sacrificed and the uterine contents examined.
The remaining rats were allowed to deliver their litters and the
offspring were killed and developmental effects investigated. No
effects were observed on gestation duration, body size or weight, and
no malformations were observed. Pups of group II exhibited a 15%
reduction in the length of the legs in comparison with the controls at
28 days of age. Examination by UV light of the pups skeleton revealed
typical TC-associated fluorescence, indicating that TC had been
absorbed in the bones (Hurley & Tuchmann-Duplessis, 1963).
Sprague-Dawley male and female rats were given 500 mg/kg TC-HCl
in the feed, equivalent to 25 mg/kg bw/day, starting 3 days prior to
mating. Groups of 15-20 pregnant rats continued to receive the
treatment throughout gestation. Some of the pregnant rats (number not
given) were sacrificed on day 21 of gestation and the contents of the
uteri were examined. The remainder of the dams were allowed to deliver
their litters. No significant effects were observed on resorptions,
pregnancy rate, offspring mortality, litter size or weight. At
external skeletal and visceral examinations, the only treatment-
related effects observed were in animals delivered by section and
included an increase in the occurrence of hydroureter (14%;
control:0.8%) and fragmented lumbar (3%; control:0%). In animals
delivered naturally, the incidence for hydroureter was 15% and 12% for
control and treated rats, respectively (McColl et al., 1965).
Pregnant Wistar rats (number not given) were orally dosed from
days 1 to 21 of gestation with TC or TC-HCl at doses of 54, 270 or
540 mg/kg bw/day, and 40, 200 or 400 mg/kg bw/day, respectively. All
rats were sacrificed on day 21, and fetuses were removed and examined
for skeletal anomalies. A reduction of ossification in the anterior
extremities, but not in the posterior ones was observed, more
frequently in the fetuses of the highest dose groups (Szumigowska-
Szrajber & Jeske, 1970).
The development of rat embryonic tissue, transplanted in athymic
(nude) mice was studied for several weeks. Limb buds of fetal rats
were cut into small pieces and transplanted into the subcutaneous
tissue of the back of female nude mice. On the 7th, 9th and 11th days
after grafting, the host mice were treated intraperitoneally with 1500
or 3000 mg/kg TC suspended in 0.55% CMC. Control mice were treated
with the vehicle only. On day 20, the grafted tissue was examined
macroscopically and histologically. No treatment-related effects were
observed on the growth of the grafted limbs or on the differentiation
of the grafts (Shiota et al., 1990).
Groups of 5 female rabbits (strain unspecified) and groups of 5
female Wistar rats were injected i.v. from days 10 to 20 of pregnancy
with 10 mg/kg bw/day of TC or CTC. Rabbit and rat neonates did not
show significant variations in weight between the groups or any
malformation (examinations in UV-light), however, the study was very
limited (Tubaro, 1964).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with TC and CTC are given
in Tables 3 and 4, respectively.
2.2.7 Special studies on microbiological effects
The TCs are primarily bacteriostatic antibiotics inhibiting
protein synthesis in susceptible microorganisms. Following transport
through the cytoplasmic membrane, TCs bind, predominantly reversibly
to the 30 S sub-unit of bacterial ribosomes, thereby preventing access
of aminoacyl tRNA to the acceptor site on the mRNA-ribosome complex
(Sande & Mandell, 1990; Pratt & Fekety, 1986).
The TCs possess a wide range of antimicrobial activity against
Gram-positive and Gram-negative bacteria. They are also active against
some microorganisms that are resistant to agents that exert their
effects on the bacterial cell wall such as Rickettsiae, Mycoplasma,
Chlamydia, some atypical mycobacteria and amebae. They have little
activity against fungi (Sande & Mandell, 1985).
Up to 50% of the antimicrobial activity of CTC can be inhibited
by the presence of magnesium sulfate. The relationship between CTC
inhibition and the magnesium sulfate concentration (50-200 mM) is
linear (Chiang, 1982)
Table 3. Results of genotoxicity studies on tetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S.typhimurium 0-10 µg/plate negative1 NTP, 1989
TA100, TA98 toxic > 3 µg/plate
TA1535, TA1537
Mouse lymphoma L5178Y/TK+/- -S9:25-300 µg/ml negative1 NTP, 1989
assay cells toxic > 200 µg/ml
+S92:10-120 µg/ml negative1
+S93:20-120 µg/ml weakly
positive1
Sister chromatid Chinese hamster -S9:5-49.9 µg/ml negative1 NTP, 1989
exchange assay ovary cells toxic >40.2 µg/ml
+S9:302-600 µg/ml negative1
toxic 600 µg/ml
Chromosome Human 10 µg/ml equivocal Meisner et al.,
aberration assay lymphocytes 1977
Chromosome Chinese hamster -S9:39.9-400 µg/ml negative1 NTP, 1989
aberration assay ovary cells toxic 400 µg/ml
+S9:1000-2750 µg/ml negative1
Table 3. Results of genotoxicity studies on tetracycline (cont'd)
Test system Test object Concentration Result Reference
Gene mutation FM3A cells from 10-100 µg/ml positive4 Tsutsui, et al.,
C3H mice 1976
In vivo
Chromosome Allium cepa 12-20 µg/ml5 positive Mann, 1978
aberration assay
SLRL assay Drosophila injection: NTP, 1989
melanogaster 5000-5300 ppm negative
feeding:
9005 ppm negative
1 with and without metabolic activation
2 S9 prepared from the liver of Aroclor 1254-induced F344 rats
3 S9 prepared from liver of non-induced F344 rats
4 induction of an 8-azaguanine-resistant mutation chromosomal damage
inhibition of protein and DNA synthesis
5 TC pure was used as test substance
Therapy with TCs is associated with a high rate of development of
supra-infections with bacteria, yeast or fungi not sensitive to these
agents, particularly in patients with diabetes and other conditions
that reduce the natural host defense mechanisms
(Pratt & Fekety, 1986).
2.2.7.1 In vitro studies
MIC values for TC for different bacterial strains of animal
origin are given in Table 5.
MICs for TC, CTC and OTC, ranging from 0.1- > 100 µg/ml, have
been reported for a number of clinical isolates, including
Staphylococcus spp., Proteus spp. and E. coli. The data indicate
that the antimicrobial potency of the three TCs for various
micro-organisms is quite similar (Walter & Heilmeyer, 1975).
The antimicrobial potency of CTC in relation to that of OTC was
established in 34 isolates of 3 different animal pathogens (P.
hemolytica, P. multocida and B. bronchiseptica). The geometric mean
for the MICs was 0.32 µg/ml for CTC and 0.52 µg/ml for OTC. For
E. coli (only one isolate), the MIC was 4.8 µg/ml for CTC, and
2.8 µg/ml for OTC (Rogalski, 1985; Gustafson, 1995).
The MIC observed for S. aureus in vitro was 0.19 µg/ml for CTC,
0.21 µg/ml for TC, and 0.55 µg/ml for OTC (Fourtillan & Lefebvre,
1985).
In in vitro studies with E. coli of animal and human origin,
MIC50 values of 2 µg/ml for CTC and 4 µg/ml for OTC were found. MIC
values for E. faecalis and E. faecium also indicated a 2-fold
higher potency for CTC than for OTC (Devriese, 1994).
The antimicrobial activity of TC was compared with that of OTC in
a range of bacterial species (10 strains/species) isolated from faeces
of healthy humans. The concentrations of TC and OTC in the MIC
determinations ranged from 0.06 to 32 µg/ml. The bacterial density was
107 colony forming units/ml. The results are presented in Table 6.
The sensitivity of the strains of most bacterial species was similar
for TC and OTC, whereas TC was active at lower concentrations for
Bifidobacterium spp., Eubacterium spp. (p<0.05), and Fusobacterium spp.
(p<0.1), and at higher concentrations for Spectrococcus spp. (p<0.05).
The geometric mean MIC, taking into consideration all tested strains
(using a value of 32 µg/ml for resistant strains) was 3.2 µg/ml for
TC and 3.8 µg/ml for OTC (Richez, 1994).
Table 4. Results of genotoxicity studies on chlortetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S. typhimurium 0.1-15 µg/plate; negative1,2 Mulligan, 1989
TA100,TA98,TA1535 conc > 1.0 µg
TA 1337, TA1538 were toxic
E. coli WP-2uvrA-
Amest test S. typhimurium up to 10 µg/plate; negative1 Sharma et al., 19893
TA100, TA98, toxicity was
TA1535, TA1537 seen
E. coli WP-2uvrA-
HGPRT assay CHO cells +act: 20-100 µg/ml negative Harbell, 1988
-act: 25-125 µg/ml
toxicity was
observed at the
highest conc.
UDS rat hepatocytes 25-75 µg/ml; negative Thilagar, 1988
toxicity was seen
at the highest dose
Table 4. Results of genotoxicity studies on chlortetracycline (cont'd)
Test system Test object Concentration Result Reference
In vivo
chromosome rat orally 500, 2500, negative Putman, 1988
aberrations 5000 mg/kg bw4
chromosome Vicia sativa 1% solution negative Sharma &
aberrations Lens esculenta " equivocal Bhattacharyya, 1967
1 with and without metabolic activation
2 no positive controls were used; background was not given
3 abstract only
4 no effects on mitotic index
Table 5. Summary of MIC values for different bacterial species for
tetracycline (Pratt & Fakety, 1986)
Species MIC (µg/ml)1
Escherichia coli 12.5 (3.1-500)
Proteus mirabilis >100 (50->100)
Enterobacter 25 (6.3-50)
1 Values given are the mean (range in parenthesis)
2.2.7.2 In vivo studies
The in vivo activity of S. aureus in mice was comparable for
the three different TCs; ED50 values were 7.6, 7.2 and 5.8 mg/kg bw,
for CTC, TC and OTC, respectively (Fourtillan & Lefebvre, 1985)
2.2.8 Special studies on eye irritation and sensitization
CTC produced local irritation when injected i.p., i.m. or s.c. or
when administered directly to the rabbit eye. Eye irritation from 1%
solutions was mild and completely reversible within 48 h (Harned et
al., 1948).
2.2.9 Other studies
2.2.9.1 Cardiovascular effects
Six anaesthetized male rabbits were given i.v. injections of 1, 2
or 5 mg TC-HCl/kg bw dissolved in 0.5 ml saline. The injections were
performed in 3, 10, 20 or 60 seconds. A dose- and injection
time-dependent slowing of the heart rate (from 270-300 (normal) to 100
beats/min or less) was observed 1-3 min after the injection. No effect
on arterial blood pressure was found.
Respiration was depressed which led at high doses to respiratory
arrest or to a slow, shallow respiration for 1-2 min (Gyrd-Hansen,
1980).
Table 6. MIC values (µg/ml) for tetracycline and oxytetracycline
against bacterial species obtained from human isolates.
TETRACYCLINE OXYTETRACYCLINE
Bacterial species MIC50 geometric MIC50 geometric
mean mean
Escherichia coli > 32 > 32 > 32 > 32
Bifidobacterium spp. 16 8.6 > 32 > 17.1
Bacteroides fragilis 4 2.5 4 2.5
Eubacterium spp. 2* 1.6 4 2.6
Clostridium spp. 0.062 0.2 0.25 0.2
Streptococcus spp. 16 > 19.7 8 > 13.9
Fusobacterium spp. 0.125 0.1 0.25 0.2
Lactobacillus spp. 2* 1.9 2 2.3
Proteus spp. > 32 > 32 > 32 > 32
Peptostreptococcus 2 3.2 4 4.0
spp.
* MIC50 = MIC90
2.2.9.2 Hepatotoxicity
TC-HCl (>0.25 mM) inhibited reversibly the ß-oxidation of
fatty acids as well as the tricarboxylic acid cycle activity of mouse
and human liver mitochondria in vitro. After i.p. administration of
1 mmol/kg bw to mice following an oral administration of a tracer dose
of [U-14C]palmitic acid, an increase in the levels of hepatic
triglycerides was observed and liver histology showed microvesicular
steatosis (Freneaux et al., 1988).
The effects of TC on the synthesis and secretion of triglycerides
and proteins were studied in isolated rat hepatocytes. TC produced a
concentration-dependent inhibition of 14C-triglyceride secretion
without affecting triglyceride synthesis and protein secretion
(Deboyser et al., 1989).
2.2.9.3 Nephrotoxicity
Groups of Wistar albino rats were given single i.v. injections of
150 mg TC/kg bw into the tail vein. Different experiments failed to
demonstrate any significant ischaemia in the kidneys (Tapp & Lowe,
1966)
2.3 Observations in humans
2.3.1 Effects after medical treatment
In human therapy, the usual oral dose for TC-HCl is 250 or 500 mg
every 6 h for adults as specified in the US National Formulary and
British Pharmacopoeia. CTC is approved as capsules (50, 100 and
250 mg) and by injection (100, 200 and 500 mg).
A variety of adverse effects in humans have been reported from
the use of TCs. The TCs are irritating substances. When given i.v.
they can cause thrombophlebitis. Intramuscular administration is
painful and not recommended. Given orally, TCs can cause epigastric
burning, abdominal discomfort, nausea and vomiting (Schindel, 1965;
NTP, 1989; Sande & Mandell, 1990).
Bone and teeth discoloration are known to occur in humans under
clinical treatment with high levels of TC drugs. Children receiving
long- or short-term therapy with a TC may develop a yellow or brownish
discoloration of the enamel. The duration of therapy appears to be
less important than the total quantity of antibiotic administered. The
risk of this effect is highest when the TC is given to neonates and
babies prior to the first dentition. However, pigmentation of the
permanent dentition may develop if the drug is given between the ages
of 2 months and 8 years, when the teeth are being calcified. An early
characteristic of this effect is a yellow fluorescence of the dental
pigment.
Treatment of pregnant patients with TCs may produce
discolouration of the teeth in their children, but exposure also
occurs during breast feeding. (Schindel, 1965; Piper et al., 1988;
Friedman, et al., 1990; Sande & Mandell, 1990).
TCs are deposited in the skeleton during gestation and throughout
childhood. A 40% reduction in bone growth, as determined by
measurements of fibulas, was demonstrated in premature infants treated
with these agents. This effect is readily reversible if the period of
exposure to the drug is short (Sande & Mandell, 1990; Pratt & Fekety,
1986).
No toxic effects were reported from treatment of premature
infants, children, adults or elderly with CTC. Effects after short-
and long-term exposure included increased body-weight gain, and
prophylactic activity against acute and chronic infections and
diarrhea (Hines, 1956).
All the TCs (dose levels not specified) can cause phototoxicity,
manifested by mild to severe skin reactions when the skin is exposed
to direct sunlight (Sande & Mandell, 1990; Schindel, 1965).
Liver effects, characterized by fatty accumulation have been
observed, predominantly following repeated parenteral administration
of TCs (dose levels were not specified). TCs can aggravate uremia in
patients with impaired renal function. For unknown reasons, pregnant
woman appear to be more susceptible to the development of hepatic
damage (Pratt & Fekety, 1986; Sande & Mandell, 1990; Friedman,
et al., 1990).
TCs rarely cause allergic reactions. Hypersensitivity to TCs have
been described as allergic manifestations of mucosal tissues. Central
nervous system side effects and effects on blood cells were rarely
described. Long-term therapy (dose levels not specified) with TCs may
produce changes in the peripheral blood. Leucocytosis, atypical
lymphocytes, toxic granulation of granulocytes, and thrombocytopenic
purpura have been observed.
The TCs may cause increased intracranial pressure and tense
bulging of the fontanels (pseudotumor cerebri) in young infants, when
given in therapeutic doses. Young adults complain of headache, nausea,
vomiting and diplopia.
TC is given orally in low doses (usually 100 mg/person/day) for
the treatment of chronic severe acne vulgaris. A common complication
of treatment with TCs is minor GI irritation (Sande & Mandell, 1990;
Pratt & Fekety, 1986).
3. COMMENTS
Toxicological data
The Committee considered data from studies on pharmacokinetics,
biotransformation, acute and short-term toxicity, reproductive
toxicity, teratogenicity, long-term toxicity/carcinogenicity,
genotoxicity, antimicrobial effects and observations in humans.
Aspects of the comparative toxicity of these compounds with OTC were
also considered.
Pharmacokinetic studies showed that CTC and TC were rapidly
absorbed following oral administration. Peak plasma concentrations
were higher in rats receiving TC than in those receiving the same dose
of CTC. In humans receiving oral therapeutic doses, 30% of the dose of
CTC was absorbed, as compared with 60-80% of the dose of TC. TC is
excreted mainly in urine, whereas CTC is excreted in urine and faeces
in comparable amounts. After absorption following administration by
various routes, CTC and TC are widely distributed in the body, the
highest levels are present in the liver and kidney. Detectable levels
of TCs in bone persist for more than 28 weeks after dosing. The plasma
half-lives of CTC and TC in humans are about 5 and 8 h, respectively.
TCs have been found in the breast milk of women receiving therapeutic
doses and can cross the placenta.
Orally administered CTC and TC have low acute toxicity in rats
and mice, LD50s ranging from 2150 to >5000 mg/kg bw.
In a 13-week range-finding study with mice in which TC was
incorporated in the diet at concentrations equivalent to 470, 950,
1800, 3700 or 7500 mg/kg bw/day, the only sign of toxicity observed
was a decrease in body weight in the highest-dosed group. In a 13-week
range-finding study in which rats were dosed at 155, 315, 625, 1250 or
2500 mg TC/kg bw/day, vacuolization of liver cells and bone marrow
atrophy were observed at 1250 and 2500 mg/kg bw/day. No toxicity was
observed in dogs given TC orally at dose levels of up to
250 mg/kg bw/day for 24 months.
CTC did not cause any toxic effects in short-term toxicity
studies with rats or mice at doses of up to 200 mg/kg bw/day. In dogs
given CTC orally at a dose of 250 mg/kg bw/day for 98 days, increased
incidences of mortality, fatty liver and bone marrow atrophy were
observed. In another experiment in dogs which received CTC orally at
doses of up to 100 mg/kg bw/day for 54 weeks, no effects were
observed, except for signs of GI disorders; these were considered of
minor importance because the increase in body-weight gain was normal.
In carcinogenicity studies with mice and rats, TC was administered
in the diet at doses of up to 3500 and 1060 mg/kg bw/day, respectively.
Survival rates were increased in male mice, while body weights were
slightly lower than controls for both sexes. Survival rates were also
increased in female rats, but male rats showed a dose-related increase
in basophilic cytoplasmic changes and clear cell changes in the liver.
There were no increases in tumour incidence in mice or rats.
In a chronic toxicity/carcinogenicity study, CTC was administered
in the diet of rats at doses varying from 0.07 to 5200 mg/kg bw/day.
In the highest dose group, GI irritation, decreased body-weight gain
and a decreased number of white blood cells were observed in both
sexes, while testicular atrophy was observed in males. Microscopic
changes in the highest-dose group included infiltration of monocytes
in the lungs of males and females, and fatty changes in the liver in
males. Tumour incidence was not increased. The NOEL was 700 mg/kg
bw/day. The Committee concluded that there was no evidence that CTC or
TC is carcinogenic.
In a two-generation reproductive toxicity study in which CTC was
administered to rats in the diet at a dose equivalent to 500 mg/kg
bw/day, no effects on reproductive performance were observed.
Reproductive toxicity data were not available on TC. In a number of
teratogenicity studies with rats, TC and TC hydrochloride caused a
reduction in ossification at the highest oral dose tested, 540 or
400 mg/kg bw/day, respectively. No irreversible structural changes
were found on skeletal or visceral examination. In studies with a
limited number (5 per group) of rats and rabbits, no evidence of
teratogenicity was found at intravenous doses of 10 mg of CTC or TC/kg
bw/day, administered on days 10-20 of pregnancy. The Committee
concluded that CTC and TC did not cause significant toxic effects on
either reproduction or development.
The genotoxicity of CTC and TC was investigated in a wide range
of in vitro and in vivo test systems. CTC gave negative results in
all tests. TC was negative in the majority of in vitro and in vivo
assays, but induced gene mutations in one in vitro test in mammalian
cells and chromosomal aberrations in plants. The Committee considered
these effects to be due to the inhibiting effect of TCs on protein
synthesis, based on their binding to ribosomes, and concluded that CTC
and TC do not pose a genotoxic hazard.
Microbiological data
In vitro studies of the antimicrobial effect of TCs on
pathogenic microorganisms of animal origin indicate that the
activities of CTC and OTC are not significantly different. MIC values
(geometric mean) for CTC and OTC were 0.32 and 0.52 µg/ml,
respectively.
In a limited number of microorganisms representative of human gut
flora, the antimicrobial activity of CTC was of the same order as that
of OTC.
The antimicrobial activity of TC was compared with that of OTC
using a wide range of bacterial species isolated from the faeces of
healthy human volunteers. The sensitivity of most species was similar
for TC and OTC except for Bifidobacterium, Fusobacterium and
Eubacterium species which were slightly more sensitive to TC, and
Streptococcus spp. which were slightly more sensitive to OTC. The
geometric mean MICs for all tested strains were 3.2 µg/ml for TC and
3.8 µg/ml for OTC.
4. EVALUATION
After reviewing the available toxicological and antimicrobial
data, the Committee concluded that the antimicrobial data provided the
most appropriate end-point for the evaluation of CTC and TC.
In view of the similarity of the antimicrobial activity of TC,
CTC and OTC, the Committee established a group ADI of 0-3 µg/kg bw for
the three drugs separately or in combination. This ADI was established
for OTC at the thirty-sixth meeting of the Committee (Annex 1,
reference 91), and is based on the NOEL of 2 mg/person/day for the
effects of OTC on the gut flora in human volunteers and a safety
factor of 10.
The Committee noted that this ADI provides an adequate margin of
safety when compared with the lowest NOEL for toxicological effects of
100 mg/kg bw/day for CTC in dogs.
5. REFERENCES
Adir, J. (1975) Enterohepatic circulation of tetracycline in rats.
J. Pharm. Sci, 64(11) 1847-1850.
Bacharach, A.L., Clark, B.J., McCulloch, m. & Tomich, E.G. (1959)
Comparative toxicity studies on ten antibiotics in current use.
J. Pharm. Toxicol. 11: 737-741.
Berté, F. & Vandoni, G. (1962) On the intestinal absorption and
organotropism of some tertacyclines. Chemotherapia 5, 219-230.
Buyske, D.A., Eisner, H.J. & Kelly, R.G. (1960) Concentration and
persistence of tetracycline and chlortetracycline in bone.
J. Pharm. Exper. Therap. 130, 150-156.
Chiang, T (1982) Inhibition of chlortetracycline activity by magnesium
ions. J. Assoc. Off. Anal. Chem., 65 (5), 1044-1047.
Cunningham, R.W., Hines, L.R., Stokey, E.H., Vessey, R.E. & Yuda, N.N.
(1954) Pharmacology of tetracycline. Antibiotics Annual, 63-69.
Deboyser, D., Goethals, F., Krack, G. & Roberfroid, M. (1989)
Investigation into the mechnism of tetracycline-induced steatosis:
study in isolated hepatocytes. Tox.appl.pharmacol., 97, 473-479.
Deichmann, W.B., Bernal, E., Anderson, W.A.D., Keplinger, M.,
Landeen, K., Mcdonald, W., Mahon, R. & Stebbins, R. (1964) The chronic
oral toxicity of oxytetracycline HCl and tetracycline HCl in the rat,
dog and pig. Ind.Med.Surg., 787-806.
Dessau, F.I. & Sisson, G., (1957) Aureomycinr life studies. Studies
of chlortetracycline hydrochloride (food grade in dogs). Project
number 77601. Unpublished report of American Cynamid Company.
P.R. 3, 440-493. Research Division. Pearl River Laboratories.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Dessau, F.I. & Sullivan, W.J., (1958) Aureomycinr life studies.
Studies of chronic toxicity of CL 13,555: Chlortetracycline
hydrochloride (food grade) in rats. Project number 77601 American
Cyanamid Company, Experimental Pathology Research Division, Pearl
River Laboratories. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Dessau, F.I. & Sullivan, W.J. (1961) A two year study of the toxicity
of chlotetracycline hydrochloride in rats. Toxicol and Appl.
Pharmacol., 3, 654-677.
Devriese, L.A. (1994) Comparative minimal inhibitory concentration
determinations with chlortetracycline and oxytetracycline on gut
bacteria of animal and human origin. Faculty of Veterinary Medicine.
University of Gent, Belgium. Submitted to WHO by American Cyanamid
Company, Princeton, USA.
Diermeier, H.F. (1959) Acute oral and intravenous toxicity of rats of
a A-VIII hydrochloride (CL 22,416), chlortetracycline hydrocholoride
(CL 13,555), and tetracycline hydrochloride (CL13,554). P.R. 5,
899-907. Unpublished report of American Cyanamid Company, Lederle
laboratories, Pearl River, New York. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Dietz, D.D., Abdo, K.M., Haseman, J.K., Eustis, S.L. & Huff, J.E.
(1991) Comparative toxicity and carcinogenicity studies of
tetracycline and oxytetracycline in rats and mice. Fund. Appl.
Toxicol. 17 (2), 335-346.
Eisner, H.J. et al., (1953) The enhancement of serum levels of
Aureomycin experimental animals. J. Pharmacol. Exp. Ther.
108, 442-449.
Eisner, H.J. & Wulf, R.J. (1962) The metabolic fate of
chlortetracycline and some comparisons with other tetracyclines.
J. pharmacol, exp. therap., 142, 122-11.
Fourtillan & Lefebvre (1985) cited in Rogalski, W. (1985) Chemical
modifications of the tetracyclines. In: The tetracyclines. Eds.
Hlavka and Boothe. Springer Verlag, Berlin.
Freneaux, E., Labbe, G., Letteron, P. The Le Dinh, T.L., Degott, C.
Geneve, J. Larrey, D. & Pessayre, D. (1988) Inhibition of the
mitochondrial oxidation of fatty acids by tetracycline in mice and in
man: possible role in microvesicular steatosis induced by this
antibiotic. Hepatology, 8(5), 1056-1062.
Friedman, J.M., Little, B.B., Brent, R.L., Cordero, J.F., Hanson,
J.W., & Shepard, T.H., (1990) Potential human teratogenicity of
frequently prescribed drugs. Obstetics and Gynecol, 75, 594-599.
Goldenthal, E.I. (1971) A compilation of LD50 values in newborn and
adult animals. Tox. and Appl. Pharm, 18, 185-207.
Gustafson, R.H. (1995) MIC tests of veterinary clinical isolates,
tetracycline. Memo to D.L. Ingle, d.d. january 11, 1995. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Gyrd-Hansen, N (1980) The effect of tetracyclines on the rabbit heart.
Zbl.vet.med. A, 27, 228-237.
Halliday, S.L. & Fanelli, G. (1960) Antibacterial tetracycline:
relative toxicity of CL 13,555, chlortetracycline hydrochloride and
CL 30,782, sodium benzoate orally in rats. Project number 14797.
P.R. 6, 1428-1439. Unpublished report of American Cyanamid Company,
Lederle Laboratories, Pearl River, New York, Submitted to WHO by
American Cyanamid Company, Princeton, USA.
Harned, B.K. Cunningham, R.W. Clark, M.C., Cosgrove, P., Hine, C.H.,
Mccauley, W.J., Stokey, E., Vessey R.E., Yuda, N.N. & Subbarow, Y.
(1948) The pharmacology of Duomycin. Ann. NYAS 51, 182-210.ALLESY,
D.W. & HINE, C.H., (1964) The effect of chlortetracycline
hydrochloride on the fertility and growth of rats.
Tox. and Appl. Pharm. 6, 9-14.
Harbell, J.W. (1988) CHO/HGPRT mutation assay. Study number
T8133.332021. Unpublished report of Microbilogical Associates, 1988.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Hines, L.R. (1956) An apprasal of the effects of long-term
chlortetracycline administration. Antibiotics. chemother.,
6 (11), 623-641.
Hurley, L.S. & Tuchmann-Duplessis, H (1963) Influence de la
tetracycline sur le developpement pré- et post-natal du rat.
Compt. rend. acad. sci., 257, 302-304.
Kanegis, L. (1958) The comarative pharmacology of tetracyclines:
initial studies on serum levels and urinary excretion of antibiotic
A-VIII following single oral and intravenous doses in the dog. Report
P.R. 4:884-921. American Cyanamid Company (Pearl River). Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Kelly, R.G. (1964) Antibacterial tetracyclines. In tissue distribution
of tetracycline and chlortetracycline. American Cyanamid Company
Report P.R. 9:485-492 (Pearl River). Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Kelly, R.G. & Buyske, D.A. (1960) Metabolism of tetracycline in the
rat and the dog. J. Pharmacol. Exp. Therap., 130, 144-149. Submitted
to the WHO by American Cyanamid Company, Princeton, USA.
Kniffen, T.S., Bane, D.P., Hall, W.F., Koritz, G.D. & Bevill, R.F.
(1989) Bioavailability, pharmacokinetics, and plasma concentration of
tetracycline hydrochloride fed to swine. Am. J. Vet. Res.
50, 518-521.
Levinskas, C.J. (1963) Auromycinr and formulation: Acute oral and
intravenous toxicity. Central Mediacal Department Report
No. 6305, 53-5,. Submitted to the WHO by American Cyanamid Company,
Princeton, USA.
Lowe C.A. & Fisher J. (1993) Rat oral LD50 study with Aureo Biomass.
Report number A93-61. Data from American Cyanamid Company, Agricultural
Research Division. Submitted to WHO by American Cyanamid Company,
Princeton, USA. (summary only)
Maffii, G. & Mainardi, L. (1955) Studio Farmacologico su un nuovo
antibiotico: la tetraciclina. Il Farmaco Ed. Sci 10 (4), 197-210.
Mann, S.K. (1978) Interaction of tetracycline (TC) with chromosomes in
Allium cepa Environm. exp. botany. 18, 201-205.
Martindale (1989) The Extra pharmacopoeia. 29th edition. Eds. J.E.F.
Reynolds. London, The Pharmaceutical Press.
Mccoll, J.D. Globus, M. & Robinson, S (1965) Effect of some therapeutic
agents on the developing rat fetus. Tox. appl. pharmacol. 7,
409-417.
Meisner, L.F., Chuprevich, T.W. & Inhorn, S.L. (1977) Mechanisms of
chromatid breakage in human lymphocyte cultures. Acta. cytol.
21(4), 555-558.
Milch, R.A., Rall, D.P. & Tobie, J.E. (1967) Bone localization of the
tetracyclines. J. Nat. Canc. Inst. 19, 87-91.
Mulligan, E. (1989) Microbial mutagenicity test report for screening
study MMR89-026. American Cyanamid Company, Report number MMR89-026,
20 April 1989. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Nelson, A.A. & Radomski, J.L. (1954) Comparative pathological study in
dogs of feeding of six broad-spectrum antibiotics. Antibiotics and
Chemotherapy IV, (11), 1174-1180.
Neuschl, J. (1991) Comparison of some pharmacokinetic parameters of
tetracyclines which are most frequently used in veterinary medicine.
Arch. Exp. Veterinarmed. 45, 105-112.
NTP (1989) NTP technical report on the toxicology and carcinogenesis
studies of tetracycline hydrochloride (Cas no. 64-75-5) in F344/N rats
and B6C3F1 mice (feed studies) NTP toxicology program P.O. Box 12233.
NTP TR 344 NIH Publication No. 89-2600. U.S. Department of health and
human services.
Piper, J.M., Baum, C., Kennedy, D.L. & Price, P. (1988) Maternal use
of prescribed drugs associated with recognized fetal adverse drug
reactions. Am. J. Obstet. and Gynecol. 159 (5) 1173-1177.
Pratt, W.B. & Fekety, R. (1986) The antimicrobial drugs. Oxford
University Press, New York, pp 205-228.
Putman, D.L. (1988) Acute in vivo cytogenetics assay in rats. Final
report. Report number T8133.105003. Unpublished report of
Microbiological Associates, 11-7-1988. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Richez, P. (1994) Antibacterial activity of tetracycline and
oxytetracycline against human gut flora. Study No. SVL 029.
Unpublished report of Datavet Veterinary Drug Consultants,
May 31 1994. Submitted to WHO by Intervet International,
Boxmeer, Netherlands.
Rogalski, W. (1985) Chemical modifications of the tetracyclines.
Chapter 5, pp 233-. In: The tetracyclines. Eds. Hlavka and Boothe.
Springer Verlag, Berlin.
Sande, M.A. & Mandell, G.L. (1990) Antimicrobial agents:
tetracyclines, chloramphenicol, erythromycin and miscellaneous
antibacterial agents. In: Goodman and Gilman's The pharmacological
basis of therapeutics, 8th edition. Gilman, A.G., Rall, T.W., Nies,
A.S. and Taylor, P. eds. Pergamon Press, New York, (1990),
pp 1117-1145.
Schindel, L.E. (1965) Clinical side-effects of the tetracyclines.
Antibiotica et Chemotherapia Advances 13, pp. 300-316.
Sharma, A.K. & Bhattacharyya, G.N. (1967) A study on the respomse of
chromosomes to antibiotic treatment. Acta Biol. Acad. Sc. Hung.
18 (1), 67-75.
Sharma, R.K., Traul, K.A., Caterson, C.R., Harbell, J., Thilagar, A. &
Putman, D. (1989) Genotoxicity evaluation of chlortetracycline.
Environ. Mol. Mutagen 14 (suppl. 15), 183. (summary only).
Shiota, K., Uwabe, C. Yamamoto, M. & Arishima, K. (1990) Teratogenic
drugs inhibit the differentiation of fetal rat limb buds grafted in
athymic (nude) mice. Reprod. toxicol. 4, 95-103.
Szumigowska-Szrajber, G. & Jeske J. (1970) Effect of tetracycline
base, tetracycline hydrochloride and oxytetracycline base on
developmental changes in metatarsal bones of extremities of rat
fetuses. Acta polon. pharm. 27 (I), 85-90.
Tapp, E. & Lowe, M.B. (1966) Tetracycline toxicity. Experientia
22, 530-531.
Thilagar, A. (1988) Test for chemical induction of unscheduled DNA
synthesis in rat primary hepatocyte cultures by autoradiography. Test
report 0082-5100. Unpublished report of Sitek Research Laboratories,
22-7-1988. Submitted to WHO by American Cyanamid Company, Princeton,
USA.
Tsutsui, T., Umeda, M., Sou, M. & Maizumi, H. (1976) Effect of
tetracycline on cultured mouse cells. Mutat. res. 40, 261-268.
Tubaro, E. (1964) Possible relationship between tetracycline stability
and effect on foetal skeleton. Brit. J. Pharmacol. Chemotherap.
23, 445-448.
Tubaro, E. & Banci, F. (1964) The pharmacology of chlormethylene-
cycline, a new chlortetracycline. Arzneimittel-Forschung, 14,
95-100.
Tubaro, E., Barletta, M. & Banci, F. (1964) Some pharmacological
aspects of a new water-soluble tetracycline. J.pharm. pharmacol.
16, 33-37.
Walter, A.M. & Heilmeyer, L. (1975) Antibiotika-Fibel. Antibiotika und
Chemotherapeutila Therapie mikrobieller infektionen, pp.323-324.
Georg Thieme Verlag Stuttgart.
Wulf, R. & Eisner, H. (1961) Antibacterial tetracyclines: Metabolism
of chlortetracycline in the rat and in the dog. American Cyanamid
Company Report C.P. 1, 626-683. Experiment P-70-21-FT. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Tetracycline (TC)
Rats
Fasted white rats were given a single oral dose of TC
hydrochloride by gavage corresponding to 75 mg/kg bw TC base. The rats
were sacrificed 1, 2, 3, 4 or 6 h after treatment and plasma and
tissue levels recorded.
Peak plasma concentrations of 3.6 mg/l were observed 2 h after
treatment, declining to 0.5 mg/l after 6 h. Peak tissue levels were
found 2 h after treatment with highest levels in the liver and kidney
(Berté & Vandoni, 1962).
To investigate the importance of the biliary route of excretion,
4 rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw tritium- labelled TC. Radioactivity was measured in
urine, bile and GI tract 24 h following treatment.
In non-ligated rats, 85-92% of the total radioactivity was
recovered of which 67-72% was excreted in urine and 18-20% in faeces.
About 70-85% of the total radioactivity was recovered in the rats with
ligated bile ducts, 68% and 88% being recovered in the urine of each
rat, and 30% and 9% in the bile. Only very small amounts (on average
2.5%) were recovered in the GI tract. When the same dose of TC was
administered to rats with ligated ureters, no increase in the
excretion of TC in the faeces was observed (Wulf & Eisner, 1961).
Male Sprague-Dawley rats were injected with 10 mg/kg bw 3H-7-TC
hydrochloride (98% pure) in the femoral vein over 5 min. The
intestinal absorption of TC hydrochloride excreted in bile was
evaluated using in situ intestinal preparations. About 73% of TC
excreted in the bile was reabsorbed in the intestinal lumen,
indicating enterohepatic circulation (Adir, 1975).
Single i.v. doses of 15 or 4 mg/kg bw tritium-labelled TC
hydrochloride were administered to 2 rats and 1 dog, respectively.
Within 72 h, 69.2% and 19.5% of the radioactivity was recovered in the
urine and faeces of rats, respectively. Within 168 h, 71% of the
radioactive dose was recovered in the urine and 9% in the faeces of
the dog (Eisner & Wulf, 1962).
Dogs
Tritium-labeled TC hydrochloride was administered i.v. to 2
beagle dogs at 10 mg TC/kg bw to study the distribution of TC
throughout the body. The animals were sacrificed after 4 h and the
various organs and tissues examined for radioactivity. The organs
containing the highest radioactivity were liver and kidney, containing
on average 15 and 45 mg/kg, respectively. A large proportion of the
recovered TC activity was found in the urine, intestinal contents and
bile. No radioactivity was measured in subcutaneous fat (Kelly, 1964).
Beagle dogs given single oral doses of 25 mg TC/kg bw showed peak
TC levels averaging 3 µg/ml 2 h after dosing, declining to an average
of 0.27 µg/ml by 24 h post-dosing. Excretion in the urine accounted
for 10% of the administered dose within 72 h after dosing.
When dogs were given a single i.v. dose of 10 mg TC/kg bw,
average serum TC levels of 10.6 µg/ml were found at 24 h, and
0.14 µg/ml at 48 h after dosing. Excretion in the urine was 58% of the
administered dose by 72 h after dosing (microbiological assay;
sensitivity 0.05-0.1 µg/ml) (Kanegis, 1958).
Pigs
The bioavailability of TC hydrochloride administered orally to
fasted gilts was calculated from the area under the plasma
concentration in time curve (AUC) and estimated to be 23%. After i.v.
administration the disposition kinetics of TC in plasma were best
described by a tri-exponential equation. The drug had a rapid
distribution phase followed by a relatively slow elimination phase,
with half-life of 16 h (Kniffen et al, 1989).
2.1.1.2 Chlortetracycline (CTC)
Rats
14C-Labelled CTC was orally administered to rats at a dose of
60 mg/kg bw. Radioactivity was measured in urine and faeces after
24, 48 and 72 h. Radioactivity was found primarily in the faeces
(92% within 72 h, the major part being excreted within 24 h). About 5%
of the radioactivity was recovered in urine.
When 30 mg/kg bw 14C-CTC was administered i.p., 33% of the
radioactivity was excreted in the urine within the first 24 h, and 5%
in the faeces. Between 24 and 72 h, 7% was excreted in the urine and
40% in the faeces.
To investigate the importance of the biliary route of excretion,
rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw 14C-CTC. Radioactivity was measured in urine, bile
and GI tract, 24 h following treatment.
In non-ligated rats, 75-79% of the total radioactivity was
recovered of which 35-37% was excreted in urine and 44-38% in faeces.
In the rats with ligated bile ducts, about 47-63% of the total
radioactivity was recovered, with 66% and 43% being recovered in the
urine of each rat, and 22% and 51% in the bile. Only very small
amounts (on average 5%) were recovered in the GI tract (Wulf &
Eisner, 1961).
Groups of 6 rats given single oral doses of 75 mg CTC/kg bw had
plasma levels of 2.1 mg/l at 1 h after administration, declining to
0.8 mg/l after 6 h. The rats were sacrificed at 1, 2, 3, 4 or 6 h
after treatment. Tissue levels were highest in liver and kidneys at
all observations. Maximum concentration was found after 2 h in the
liver, and after 1 h in the kidney (Berte & Vandoni, 1962).
Oral doses ranging from 6 to 800 mg CTC/kg bw administered to
female rats and male guinea-pigs gave no proportional increases in
serum CTC concentrations. When guinea-pigs received the same doses
each day for 9 days, higher serum levels were found than with single
doses. Serum CTC levels were increased by the simultaneous
administration of various adjuvants, such as citric acid. This effect
(observed in doses of CTC up to 200 mg/kg bw) was apparent within 1 h
of administration and lasted at least 8 h (Eisner et al., 1953)
Rabbits
Ten adult white Californian rabbits (males and females) received
a single oral dose of 20 mg/kg bw of technical CTC or CTC-chloride.
Serum CTC levels averaged 2.3 mg/l 3 h after dosing. The concentration
of CTC declined to a mean value of 0.09 mg/l by 12 h, and to 0.08 mg/l
by 24 h after dosing. The highest concentrations were found in the
liver (1.53 mg/kg) 24-h post-dosing, followed by kidney, lung and
heart. No measurable levels were found in muscle (detection limit:
37.5 µg/kg) (Neuschl, 1991)
Dogs
Four beagle dogs were given a single oral dose of 25 mg
CTC/kg bw. Peak serum CTC levels ranged from 0.40-1.9 mg/l 2 h after
dosing, declining to an average of 0.21 mg/l by 24 h. When the dogs
were given an i.v. injection of 10 mg CTC/kg bw, serum levels averaged
6.6 mg/l 1 h after administration, declining to 2.4 µg/ml at 8 h,
0.29 µg/ml at 24 h and 0.06 µg/ml at 48 h (Kanegis, 1958).
Two female beagle dogs received a single i.v. injection of 10 mg
14C-CTC/kg bw. The dogs were sacrificed 4 h after dosing. The organ
containing the highest radioactivity was the liver (30 mg/kg),
followed by kidney (25 mg/kg), ileum (15 mg/kg), jejunum (12 mg/kg)
and heart (10 mg/kg). A large proportion of the recovered
radioactivity was found in urine, intestinal contents and bile. With
the exception of subcutaneous fat, radioactivity was found throughout
all tissues and fluids examined (Kelly, 1964).
2.1.2 Interactions with bones and teeth
Tissues from mice, rats, guinea-pigs, rabbits and dogs
parenterally treated with TCs (TC, CTC or OTC, doses varying from
0.1 - 50 mg/kg bw) were examined in U.V. light. In all tissues with
the exception of the brain, a brilliant yellow-gold fluorescence
was apparent within 30 min, irrespective of the dose. The induced
fluorescence disappeared from all tissues (except bone) within 6 h
after a single parenteral injection. However, bone fluorescence
persisted throughout a 10-week observation period after dosing
(Milch et al., 1967).
Single oral doses of 250 mg/kg bw tritium-labelled TC or single
oral dose of 250 mg 14C-CTC/kg bw were administered to male rats
(Sherman strain). Radioactive residues in the femur of the rats
treated with TC were 9.6, 1.9 and 0.4 mg/kg of bone measured after
4 h, 24 h, and 4 weeks, respectively. The radioactivity averaged
12 mg/kg of bone 4 h after CTC treatment, and 2.3 mg/kg after 4 weeks.
After a lifetime diet containing 0.5 to 1000 mg CTC/kg of feed,
the maximum radioactivity in femur was 570 mg/kg. After i.p.
injections with 10-150 mg TC/kg bw, the concentrations found in the
femur were directly related to the administered dose and were much
higher than the concentrations measured after oral treatment with
250 mg TC/kg bw (Buyske et al., 1960).
2.1.3 Observations in humans
In humans, about 30% of an oral therapeutic dose of CTC was
absorbed on an empty stomach. For TC and OTC, the absorption was
60-80% (Sande & Mandell, 1990).
Oral doses of 250 to 500 mg every 6 h produced plasma
concentrations of TC ranging from 1 to 5 mg/l. Intravenous injections
of 250-500 mg produced plasma concentrations of about 15-20 mg/l at
0.5 h, falling to 4-10 mg/l at 1 to 2 h. At 12 h, 1-3 mg/l was still
present. In circulation, TCs are bound to plasma proteins in varying
degrees (24-65% for TC, and about 47% for CTC). TCs appeared in the
milk of nursing mothers where concentrations were be 60% or more of
those in the plasma. TCs diffused across the placenta and appeared in
the fetal circulation at concentrations of about 25 to 75% of those in
the maternal blood. The plasma half-lives of CTC and TC were reported
to be about 8-10 h and 5.5 h, respectively (Martindale, 1989).
Absorption of TCs was impaired by milk products, sodium
bicarbonate, aluminium hydroxide and iron preparations due to
chelation and increase in gastric pH (Sande & Mandell, 1990).
2.1.4 Biotransformation
2.1.4.1 Tetracycline
The metabolism of 14C-TC was examined in rats following single
i.p. or oral administration of 60 mg/kg bw, and in dogs after single
oral administration of 25 mg/kg bw tritium-labelled TC. Approximately
90% of the administered radioactivity in the rat was eliminated either
by urinary or faecal route. A significant portion of the remaining
activity was bound as chelated TC in the skeleton of the animal. With
the exception of this chelate, TC was chemically unaltered by the rat.
Urine of dogs contained only unchanged TC (Kelly & Buyske, 1960).
2.1.4.2 Chlortetracycline
Male Wistar rats (n=6) and male beagle dogs (n=2) were
administered 14C-labelled CTC via different routes (oral:
60 mg/kg bw; i.p.: 30 mg/kg bw; i.v.: 15-60 mg/kg bw). The
recovery of antimicrobial activity was significantly lower than the
recovery of radioactivity by all routes of administration and
excretion. The major 'presumed' metabolite was identified as
4-epichlortetracycline, which accounted for 23-35% of the
radioactivity in the urine of rats, and 31-60% in dogs. It was
essentially inactive in microbiological assays. It was not clear
whether this represented a true metabolite or was a degradation
product due to alkaline treatment. The presence of small amounts
(5-10%) of isochlortetracycline in the urine and faeces of some
animals was also demonstrated (Wulf & Eisner, 1961; Eisner & Wulf,
1963).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with TC and CTC after oral,
i.v. or s.c. administration are summarized in Tables 1 and 2,
respectively.
Dogs developed transient hyperpnea, weakness and anorexia
following an i.v. dose of 50 and 100 mg CTC/kg bw, and a dose of
150 mg/kg bw caused respiratory distress, general paresis, somnolence
and death within a few hours (Hines, 1956).
Table 1. Acute toxicity of tetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. oral TC n.s. 2550 Tubaro et al., 1964
mouse n.s. oral TC n.s. >3000 Cunningham et al., 1954
mouse (42d) n.s. oral TC n.s. 808 Goldenthal, 1971
(3d) 300
mouse n.s. i.v. TC n.s. 160 Tubaro et al., 1964
mouse n.s i.v. TC n.s. 157 Maffii & Mainardi, 1955
mouse n.s. i.v. TC n.s. 170 Cunningham et al., 1954
mouse n.s. i.p. TC n.s. 340 Tubaro et al., 1964
mouse n.s. i.p. TC n.s. 330 Cunningham et al., 1954
rat M oral TC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral TC n.s. >3000 Cunningham et al., 1954
rat (49(d) n.s. oral TC n.s. 807 Goldenthal, 1971
(3d) 360
Table 1. Acute toxicity of tetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat (adult) n.s. oral TC HCl n.s. 6443 Goldenthal, 1971
(<2d) 3827
rat n.s. i.v. TC n.s. 220 Cunningham et al., 1954
rat n.s. i.v. TC n.s. 128 Maffii & Mainardi, 1955
rat n.s. i.v. TC HCl n.s. 375 Diermeier, 1959
rat n.s. i.p. TC n.s. 320 Cunningham et al., 1954
n.s. not specified
d age of animal in days
2.2.2 Short-term toxicity studies
2.2.2.1 Tetracycline
Mice
Groups of male mice (10/sex/group) were given by gavage 0, 20 or
100 mg/kg bw TC hydrochloride once daily, 5 days/week for 6 weeks as a
2% starch suspension containing 4-6% test substance. At termination of
the study a complete blood count was performed in the high-dose group
and no significant haematological alterations were observed.
Accelerated growth rates were observed in dosed mice when compared to
the control mice (Cunningham et al., 1954).
In a range-finding study, groups of B6C3F1 mice (6-7 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 470, 950, 1800, 3700
or 7500 mg/kg bw/day, respectively). No mortalities occurred. Final
mean body weight was slightly decreased in males (16%) and females
(6%) at the 5% level. The TC concentration in bone increased with
increasing dose of TC hydrochloride (NTP, 1989).
Rats
In a range-finding study, groups of F344/N rats (7-8 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 155, 315, 625, 1250 or
2500 mg/kg bw/day, respectively). No mortalities occurred. Cytoplasmic
vacuolization was observed in the livers of male rats at the two
highest dose groups. Bone marrow atrophy was seen in both female and
male rats at 1250 and 2500 mg/kg bw/day. The concentration of TC in
bone increased with increasing dose of TC hydrochloride (NTP, 1989)
Dogs
Mongrel dogs (8-4/group) were given a capsule of 0 or
250 mg/kg bw TC once daily, 6 days/week for 98 days. No mortality
occurred. No effects were seen on peripheral blood and no pathological
changes were observed (Nelson & Radomski, 1954).
Groups of 4 mongrel dogs were orally administered 10 or
100 mg/kg bw TC twice daily, 5 days/week for 3 months. No effects were
observed on body weight, liver function (bromosulfophthalein
clearance), kidney function (phenolsulfophtalein clearance),
blood-clotting time, non-protein nitrogen levels, blood sugar values
or complete blood counts (Cunningham et al., 1954).
Table 2. Acute toxicity of chlortetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse M & F oral CTC HCl n.s. >3000 Cunningham, 1954
mouse F oral CTC HCl n.s. 2150 Tubaro & Banci, 1964
mouse M & F oral CTC HCl n.s. 3350 (M) Bacharach, 1959
4200 (F)
mouse n.s. oral duomycin n.s. >3000 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 155 Cunningham, 1954
mouse F i.v. CTC HCl n.s. 93 Tubaro & Banci, 1964
mouse n.s. i.v. duomycin n.s. 102 Harned, 1948
mouse n.s. i.v. duomycin n.s. 134 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 102 (M) Bacharach, 1959
108 (F)
mouse F i.v. CTC bisulfate n.s. 113 Levinskas 1963
mouse n.s. s.c. duomycin n.s. >600 Harned, 1948
mouse M & F s.c. CTC HCl n.s. 5500 (M) Bacharach, 1959
8250 (F)
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. i.p. CTC HCl n.s. 192 Cunningham, 1954
mouse M & F i.p. CTC HCl n.s. 128 (M) Bacharach, 1959
168 (F)
mouse F i.p. CTC HCl n.s. 214 Tubaro & Banci, 1964
rat M & F oral CTC HCl n.s. >3000 Cunningham, 1954
rat F oral CTC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral CTC-HCl n.s. 55001 Goldenthal, 1971
103002
rat F oral calcium CTC n.s. >10000 Levinskas, 1963
rat n.s. oral Aureo Biomass n.s. >5000 Lowe & Fisher, 1993
(summary only)
rat n.s. oral duomycin n.s. >3000 Harned, 1948
rat M & F i.v. CTC HCl n.s. 160 Cunningham, 1954
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat M i.v. CTC HCl n.s. 167 Diermeier, 1959
rat n.s. i.v. duomycin n.s. 118 Harned, 1948
rat n.s. i.p. CTC HCl n.s. 335 Cunningham, 1954
guinea pig n.s. i.v. duomycin n.s. 100 Harned, 1948
guinea pig n.s. s.c. duomycin n.s. >300 Harned, 1948
n.s. not specified
1 < 2 days old
2 adult
Groups of male dogs (4 mongrel dogs and 4 beagle dogs) were fed
diets containing 0.1, 0.3 or 1% TC hydrochloride for 24 months
(equivalent to 25, 75 or 250 mg/kg bw/day, respectively). An interim
sacrifice of 1 beagle and 1 mongrel dog of each group was performed at
12 months for microscopic examination. No effects were observed on
clinical signs, mortality, body weight, food consumption, haematology,
ALP, bromosulfophtalein clearance, urea nitrogen determinations,
testes and epididymides weight, macroscopy, concentration and motility
of the spermatozoa, or appearance of semen. The bony structures of all
dosed dogs showed a yellowish coloration. The intensity of the colour
was related to the dose fed. A brownish-black pigmentation with a
dose-related intensity was observed in the thyroid glands of the dogs
of all groups. Microscopic examination of the thyroids revealed
intracytoplasmic granules in the follicular epithelial cells of most
of the treated dogs. This effect might be caused by deposits of TC or
its metabolites. Histopathological changes were not observed
(Deichmann et al., 1964).
2.2.2.2 Chlortetracycline
Mice
Groups of 10 male mice were given 0, 20 or 100 mg CTC/kg bw/day,
once daily by gavage, 5 days/week for 6 weeks. At 100 mg/kg bw/day,
complete blood count indicated that there were no significant
haematological alterations. Beneficial effects such as increased
growth were observed at 20 and 100 mg/kg bw/day (Cunningham, 1954).
Two groups of 20 mice received 20 or 100 mg/kg bw/day of CTC for
3 months (route not specified). Final body weight of both groups
exceeded those of the controls by 15%. No evidence of toxicity was
seen and no difference in blood counts was found at the end of
experiment (Hines, 1956).
Groups of 20 male mice (strain not specified) received orally
0, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw, twice daily). There were no
significant effects on mortality, clinical signs, body-weight gain,
haematology, macroscopy or microscopy. The NOEL in this study was
200 mg/kg bw/day (Harned et al., 1948).
Rats
Sherman rats (6/sex/group) received diets containing 0, 2.0 or
5.0% CTC for 28 days (equivalent to 2000 or 5000 mg/kg bw/day).
Body-weight gain was significantly decreased at 5000 mg/kg bw/day. No
other examinations were performed (Halliday & Fanelli, 1960).
Two groups of 20 rats each were given oral doses of 150 or
300 mg/kg bw/day CTC for 3 months. In both groups, body weight of the
animals was higher than the controls. No difference in blood counts
was found at the end of the experiment (Hines, 1956).
Groups of 20 male rats (strain not specified) received by gavage
0, 10, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw twice daily). No treatment-related
effects were seen on mortality, clinical signs, body weight,
haemoglobin, blood pressure, macroscopy or microscopy. The NOEL in
this study was 200 mg/kg bw/day (Harned et al., 1948).
Dogs
Oral administration of 100 mg CTC/kg bw/day to dogs (number and
strain not specified) for 17 days, followed by 100 mg/kg bw twice
daily for 14 days, produced no unfavourable effects on general
appearance, growth rate, haemoglobin, complete blood count, gross or
microscopic examinations (Hines, 1956).
Dogs (4/group) received 10, 50 or 100 mg of CTC/kg bw/day for 14
weeks (route not specified). At the end of this period, liver and
kidney function tests, blood sugar values, blood coagulation time,
blood non-protein nitrogen and complete blood counts did not differ
significantly from pre-test values (Hines, 1956)
Ten dogs (strain not specified) were given CTC twice daily
(morning and afternoon) by capsule at a total dose of 100 mg/kg
bw/day, 5 days/week for 9 to 15 weeks. CTC treatment had no effect on
general appearance, body weight, haematology, clotting time, liver and
kidney function (Bromosulfophtalein and phenolsulfophtalein
clearance), urinalysis, gross or microscopic examinations
(Harned, 1948).
Ten mongrel dogs (8-19 kg; 2-5 years old) received capsules
containing 250 mg CTC/kg bw/day, 6 days/week, for 98 days. Another 4
dogs received the same dose intermittently, 2 weeks on the drug and 3
weeks off, for 121 days in total. Five dogs of the continuously
treated group died. These animals had progressive weight loss, apathy
and anorexia. Haemoglobin, RBC, granulocyte and total leucocyte counts
were slightly depressed. The pathologic changes observed included
emaciation, fatty liver, excess fat in the kidney, depletion of the
bone marrow and atrophic changes in the spleen, lymph nodes and
skeletal muscle (Nelson & Radomski, 1954).
Beagle and basenji dogs (2/sex/group; 9-12 months old) received
daily a single oral dose of 10 or 100 mg CTC-HCl/kg bw in capsules for
54 weeks; a third group consisting of 1 female beagle dog, 2 male and
1 female basenji dogs were given 50 mg/kg bw/day. No control group was
used. Observations included haematology, clinical chemistry,
urinalysis, faecal smears, macroscopy, organ weight, microscopy, and
terminal tissue concentration.
Treatment-related GI disorders (vomiting, diarrhea, appetite loss
and swelling of the anal glands) were observed, and particularly in
the first half of the experiment in all groups, most often in basenji
dogs. The average blood concentration 4 h after administration of the
dose (calculated for each group from 12-14 determinations carried out
over a period of 1 year) were 0.34 µg/ml, 0.58 µg/ml and 0.94 µg/ml at
10, 50 and 100 mg/kg bw/day, respectively. Thyroid weight was high in
one male at 100 mg/kg bw/day, but the shape and appearance of the
gland were normal. Slight yellow discolouration in the bones was
observed at necropsy in the high-dose dogs, and 2 dogs at each dose
level showed evidence of chronic gastritis. Measurable tissue
concentrations (no details given) were found in bone > kidney >
liver but none in brain, heart or spleen. The NOEL in this study was
100 mg/kg bw/day (Dessau & Sisson, 1957).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Tetracycline
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets for 103
weeks containing 0, 1.25 or 2.5% TC-HCl (purity 91%), equal to 1500 or
3000 mg/kg bw/day for males, and 1500 or 3500 mg/kg bw/day for
females. Observations included clinical signs, body weight, feed
consumption, macroscopy, and histopathology.
Survival was increased for male mice (31/50, 43/50, 43/50); no
differences were observed in females (37/50, 35/50, 38/50). Mean body
weights in both dose groups were slightly lower for both sexes
compared to control mice. Dosing had no effects on feed consumption.
The tumour incidence was not significantly increased in either sex. On
the contrary, dosed female mice did not develop hepatocellular
adenomas or carcinomas (combined incidence: 10/49 for controls; 0/48
at 1.25%; 0/50 at 2.5%) (NTP, 1989; Dietz et al., 1991).
Rats
Groups of weanling male rats (Osborne-Mendel) were fed diets
containing 0 (180 rats), 100 (100 rats), 1000 (130 rats) or 3000
(100 rats) mg TC-HCl/kg of feed, equivalent to 5, 50 or 150 mg/kg
bw/day for 2 years. Ten Rats/group were killed at frequent intervals.
Sacrificed and moribund rats were macroscopically and histologically
examined. No effects were seen on clinical signs, body weight, food
consumption or haematological examinations. Beneficial effects were
observed during the first 18-19 months, all dosed rats appearing more
vigorous and gaining weight more rapidly than the control rats.
Mortality rates were lower in the treated rats than in the control
rats. At histopathology, yellow discoloration of long bones and
calvarium was observed at the highest dose. Tumour incidence was not
enhanced (Deichmann et al., 1964).
Groups of F344/N rats (50/sex/group) were fed diets containing
0, 1.25% or 2.5% mg TC-HCl (purity 91%), equal to 440 or 910 mg/kg
bw/day for males, and 510 or 1060 mg/kg bw/day for females for
103 weeks. Observations included clinical signs, body weight, feed
consumption, macroscopy and histopathology.
The survival of female rats of both the low- and high-dose groups
was significantly greater than that of the controls (control 27/50,
low-dose 39/50, high-dose 38/50). No compound-related effects on body
weight or feed consumption were observed. A dose-related increased
incidence of basophilic cytoplasmic changes and clear cell changes
were observed in the livers of male rats. The incidence and severity
of nephropathy were reduced in dosed male rats (control 48/50,
severity 2.8; mid-dose 35/50, severity 1.6; high-dose 36/50, severity
1.5). The tumour incidence was not enhanced (NTP, 1989; Dietz et al.,
1991).
2.2.3.2 Chlortetracycline
Rats
Forty rats (sex or strain not specified) were maintained for 52
weeks on a diet containing 1% CTC, equivalent to 1000 mg/kg bw/day.
There was no significant difference in body weight and no evidence of
toxicity was seen.
In a parallel experiment another group of 40 rats (sex or strain
not specified) received 5% CTC, equivalent to 5000 mg/kg bw/day. Ten
animals died within the first 10 weeks. The remaining 30 rats survived
for the 52-week test period and had a 33% lower weight than the
controls, and an apparent yellow staining of all tissues (Hines, 1956).
Groups of Sherman rats (20/sex/group) were fed diets containing
CTC at concentrations of 0, 1, 5, 20, 100, 500, 2000, 10000 or
50000 mg/kg of feed, equal to 0, 0.07, 0.35, 1.3, 7, 34, 130, 700 or
5200 mg/kg bw/day, respectively. Observations were made on mortality,
clinical signs, body-weight gain, food consumption, haematology,
macroscopy, organ weight, and microscopy.
During the first 12 weeks of the study, 8 male rats in the
highest dose group died compared to none in the other groups. At
termination, the total mortality for males was not different from
controls. Both males and females in the 50000 mg/kg group showed signs
of GI irritation which included abdominal distension, nose and mouth
encrustation and salivation. Body-weight gain was reduced in both
sexes at 50000 mg/kg. WBC was reduced in both sexes at 50000 mg/kg.
Microscopic changes at 50000 mg/kg included accumulation of yellow
pigment in splenic lymph follicles for both sexes, testicular atrophy
occasionally with degenerative changes in seminiferous tubulus, fatty
infiltration of the liver in males and infiltration of monocytes
sometimes coupled with interstitial fibrosis in the lungs in both
sexes. Fluorometric determinations showed the presence of significant
amounts of CTC at dose levels of 500 mg/kg of feed and higher. No
blood or urine biochemistry was carried out. The tumour incidence was
not enhanced. The NOEL in this study was 10000 mg/kg diet, equal to
700 mg/kg bw/day (Dessau & Sullivan, 1958; Dessau & Sullivan, 1961).
2.2.4 Reproductive toxicity studies
2.2.4.1 Chlortetracycline
Mice
Mice maintained through 4 reproductive cycles on diets containing
175 mg CTC/kg of feed (equivalent to 25 mg/kg bw/day) did not differ
significantly from parallel control groups in the number of young born
per litter, the number of surviving pups or the average body weight at
the end of 4 weeks (Hines, 1956).
Rats
Rats maintained through 2 reproductive cycles on a diet
containing 45 mg CTC/kg of feed (equivalent to 2 mg/kg bw/day) did not
differ significantly from parallel control groups in the number of
young per litter, the number of surviving pups or the average body
weight at the end of 4 weeks (Hines, 1956).
A 2-generation reproductive toxicity study was carried out with 2
groups of 5 male and 15 female rats (Sherman strain, approximately
21-day old). One group received the control diet and the other
received the same diet containing 1% CTC, equivalent to 500 mg/kg
bw/day. When the rats were approximately 100-day old, the animals were
mated to produce the first generation. All litters were reduced to 8-9
rats. Pups were weaned to the diet given to their parents. Second
generation breeding was carried out with 21 female and 7 male rats in
the 1% group and a few more rats per group in the control group.
Observations were made for clinical signs, body weight, food
consumption, fertility, reproductive performance and pup growth. Male
body weights were slightly lower than controls for both parental
generations. No further toxicity, nor effects on male or female
reproduction were observed (Hallesy & Hine, 1964).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Tetracycline
Groups of 16 pregnant Wistar rats were given 0 or 150-200 mg TC
chlorhydrate/rat/day from days 1 to 18 of gestation (Group I) and
another group received the same treatment from days 1 to 28 after
delivery (Group II). At day 20 of gestation, 3 females of group I and
3 control females were sacrificed and the uterine contents examined.
The remaining rats were allowed to deliver their litters and the
offspring were killed and developmental effects investigated. No
effects were observed on gestation duration, body size or weight, and
no malformations were observed. Pups of group II exhibited a 15%
reduction in the length of the legs in comparison with the controls at
28 days of age. Examination by UV light of the pups skeleton revealed
typical TC-associated fluorescence, indicating that TC had been
absorbed in the bones (Hurley & Tuchmann-Duplessis, 1963).
Sprague-Dawley male and female rats were given 500 mg/kg TC-HCl
in the feed, equivalent to 25 mg/kg bw/day, starting 3 days prior to
mating. Groups of 15-20 pregnant rats continued to receive the
treatment throughout gestation. Some of the pregnant rats (number not
given) were sacrificed on day 21 of gestation and the contents of the
uteri were examined. The remainder of the dams were allowed to deliver
their litters. No significant effects were observed on resorptions,
pregnancy rate, offspring mortality, litter size or weight. At
external skeletal and visceral examinations, the only treatment-
related effects observed were in animals delivered by section and
included an increase in the occurrence of hydroureter (14%;
control:0.8%) and fragmented lumbar (3%; control:0%). In animals
delivered naturally, the incidence for hydroureter was 15% and 12% for
control and treated rats, respectively (McColl et al., 1965).
Pregnant Wistar rats (number not given) were orally dosed from
days 1 to 21 of gestation with TC or TC-HCl at doses of 54, 270 or
540 mg/kg bw/day, and 40, 200 or 400 mg/kg bw/day, respectively. All
rats were sacrificed on day 21, and fetuses were removed and examined
for skeletal anomalies. A reduction of ossification in the anterior
extremities, but not in the posterior ones was observed, more
frequently in the fetuses of the highest dose groups (Szumigowska-
Szrajber & Jeske, 1970).
The development of rat embryonic tissue, transplanted in athymic
(nude) mice was studied for several weeks. Limb buds of fetal rats
were cut into small pieces and transplanted into the subcutaneous
tissue of the back of female nude mice. On the 7th, 9th and 11th days
after grafting, the host mice were treated intraperitoneally with 1500
or 3000 mg/kg TC suspended in 0.55% CMC. Control mice were treated
with the vehicle only. On day 20, the grafted tissue was examined
macroscopically and histologically. No treatment-related effects were
observed on the growth of the grafted limbs or on the differentiation
of the grafts (Shiota et al., 1990).
Groups of 5 female rabbits (strain unspecified) and groups of 5
female Wistar rats were injected i.v. from days 10 to 20 of pregnancy
with 10 mg/kg bw/day of TC or CTC. Rabbit and rat neonates did not
show significant variations in weight between the groups or any
malformation (examinations in UV-light), however, the study was very
limited (Tubaro, 1964).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with TC and CTC are given
in Tables 3 and 4, respectively.
2.2.7 Special studies on microbiological effects
The TCs are primarily bacteriostatic antibiotics inhibiting
protein synthesis in susceptible microorganisms. Following transport
through the cytoplasmic membrane, TCs bind, predominantly reversibly
to the 30 S sub-unit of bacterial ribosomes, thereby preventing access
of aminoacyl tRNA to the acceptor site on the mRNA-ribosome complex
(Sande & Mandell, 1990; Pratt & Fekety, 1986).
The TCs possess a wide range of antimicrobial activity against
Gram-positive and Gram-negative bacteria. They are also active against
some microorganisms that are resistant to agents that exert their
effects on the bacterial cell wall such as Rickettsiae, Mycoplasma,
Chlamydia, some atypical mycobacteria and amebae. They have little
activity against fungi (Sande & Mandell, 1985).
Up to 50% of the antimicrobial activity of CTC can be inhibited
by the presence of magnesium sulfate. The relationship between CTC
inhibition and the magnesium sulfate concentration (50-200 mM) is
linear (Chiang, 1982)
Table 3. Results of genotoxicity studies on tetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S.typhimurium 0-10 µg/plate negative1 NTP, 1989
TA100, TA98 toxic > 3 µg/plate
TA1535, TA1537
Mouse lymphoma L5178Y/TK+/- -S9:25-300 µg/ml negative1 NTP, 1989
assay cells toxic > 200 µg/ml
+S92:10-120 µg/ml negative1
+S93:20-120 µg/ml weakly
positive1
Sister chromatid Chinese hamster -S9:5-49.9 µg/ml negative1 NTP, 1989
exchange assay ovary cells toxic >40.2 µg/ml
+S9:302-600 µg/ml negative1
toxic 600 µg/ml
Chromosome Human 10 µg/ml equivocal Meisner et al.,
aberration assay lymphocytes 1977
Chromosome Chinese hamster -S9:39.9-400 µg/ml negative1 NTP, 1989
aberration assay ovary cells toxic 400 µg/ml
+S9:1000-2750 µg/ml negative1
Table 3. Results of genotoxicity studies on tetracycline (cont'd)
Test system Test object Concentration Result Reference
Gene mutation FM3A cells from 10-100 µg/ml positive4 Tsutsui, et al.,
C3H mice 1976
In vivo
Chromosome Allium cepa 12-20 µg/ml5 positive Mann, 1978
aberration assay
SLRL assay Drosophila injection: NTP, 1989
melanogaster 5000-5300 ppm negative
feeding:
9005 ppm negative
1 with and without metabolic activation
2 S9 prepared from the liver of Aroclor 1254-induced F344 rats
3 S9 prepared from liver of non-induced F344 rats
4 induction of an 8-azaguanine-resistant mutation chromosomal damage
inhibition of protein and DNA synthesis
5 TC pure was used as test substance
Therapy with TCs is associated with a high rate of development of
supra-infections with bacteria, yeast or fungi not sensitive to these
agents, particularly in patients with diabetes and other conditions
that reduce the natural host defense mechanisms
(Pratt & Fekety, 1986).
2.2.7.1 In vitro studies
MIC values for TC for different bacterial strains of animal
origin are given in Table 5.
MICs for TC, CTC and OTC, ranging from 0.1- > 100 µg/ml, have
been reported for a number of clinical isolates, including
Staphylococcus spp., Proteus spp. and E. coli. The data indicate
that the antimicrobial potency of the three TCs for various
micro-organisms is quite similar (Walter & Heilmeyer, 1975).
The antimicrobial potency of CTC in relation to that of OTC was
established in 34 isolates of 3 different animal pathogens (P.
hemolytica, P. multocida and B. bronchiseptica). The geometric mean
for the MICs was 0.32 µg/ml for CTC and 0.52 µg/ml for OTC. For
E. coli (only one isolate), the MIC was 4.8 µg/ml for CTC, and
2.8 µg/ml for OTC (Rogalski, 1985; Gustafson, 1995).
The MIC observed for S. aureus in vitro was 0.19 µg/ml for CTC,
0.21 µg/ml for TC, and 0.55 µg/ml for OTC (Fourtillan & Lefebvre,
1985).
In in vitro studies with E. coli of animal and human origin,
MIC50 values of 2 µg/ml for CTC and 4 µg/ml for OTC were found. MIC
values for E. faecalis and E. faecium also indicated a 2-fold
higher potency for CTC than for OTC (Devriese, 1994).
The antimicrobial activity of TC was compared with that of OTC in
a range of bacterial species (10 strains/species) isolated from faeces
of healthy humans. The concentrations of TC and OTC in the MIC
determinations ranged from 0.06 to 32 µg/ml. The bacterial density was
107 colony forming units/ml. The results are presented in Table 6.
The sensitivity of the strains of most bacterial species was similar
for TC and OTC, whereas TC was active at lower concentrations for
Bifidobacterium spp., Eubacterium spp. (p<0.05), and Fusobacterium spp.
(p<0.1), and at higher concentrations for Spectrococcus spp. (p<0.05).
The geometric mean MIC, taking into consideration all tested strains
(using a value of 32 µg/ml for resistant strains) was 3.2 µg/ml for
TC and 3.8 µg/ml for OTC (Richez, 1994).
Table 4. Results of genotoxicity studies on chlortetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S. typhimurium 0.1-15 µg/plate; negative1,2 Mulligan, 1989
TA100,TA98,TA1535 conc > 1.0 µg
TA 1337, TA1538 were toxic
E. coli WP-2uvrA-
Amest test S. typhimurium up to 10 µg/plate; negative1 Sharma et al., 19893
TA100, TA98, toxicity was
TA1535, TA1537 seen
E. coli WP-2uvrA-
HGPRT assay CHO cells +act: 20-100 µg/ml negative Harbell, 1988
-act: 25-125 µg/ml
toxicity was
observed at the
highest conc.
UDS rat hepatocytes 25-75 µg/ml; negative Thilagar, 1988
toxicity was seen
at the highest dose
Table 4. Results of genotoxicity studies on chlortetracycline (cont'd)
Test system Test object Concentration Result Reference
In vivo
chromosome rat orally 500, 2500, negative Putman, 1988
aberrations 5000 mg/kg bw4
chromosome Vicia sativa 1% solution negative Sharma &
aberrations Lens esculenta " equivocal Bhattacharyya, 1967
1 with and without metabolic activation
2 no positive controls were used; background was not given
3 abstract only
4 no effects on mitotic index
Table 5. Summary of MIC values for different bacterial species for
tetracycline (Pratt & Fakety, 1986)
Species MIC (µg/ml)1
Escherichia coli 12.5 (3.1-500)
Proteus mirabilis >100 (50->100)
Enterobacter 25 (6.3-50)
1 Values given are the mean (range in parenthesis)
2.2.7.2 In vivo studies
The in vivo activity of S. aureus in mice was comparable for
the three different TCs; ED50 values were 7.6, 7.2 and 5.8 mg/kg bw,
for CTC, TC and OTC, respectively (Fourtillan & Lefebvre, 1985)
2.2.8 Special studies on eye irritation and sensitization
CTC produced local irritation when injected i.p., i.m. or s.c. or
when administered directly to the rabbit eye. Eye irritation from 1%
solutions was mild and completely reversible within 48 h (Harned et
al., 1948).
2.2.9 Other studies
2.2.9.1 Cardiovascular effects
Six anaesthetized male rabbits were given i.v. injections of 1, 2
or 5 mg TC-HCl/kg bw dissolved in 0.5 ml saline. The injections were
performed in 3, 10, 20 or 60 seconds. A dose- and injection
time-dependent slowing of the heart rate (from 270-300 (normal) to 100
beats/min or less) was observed 1-3 min after the injection. No effect
on arterial blood pressure was found.
Respiration was depressed which led at high doses to respiratory
arrest or to a slow, shallow respiration for 1-2 min (Gyrd-Hansen,
1980).
Table 6. MIC values (µg/ml) for tetracycline and oxytetracycline
against bacterial species obtained from human isolates.
TETRACYCLINE OXYTETRACYCLINE
Bacterial species MIC50 geometric MIC50 geometric
mean mean
Escherichia coli > 32 > 32 > 32 > 32
Bifidobacterium spp. 16 8.6 > 32 > 17.1
Bacteroides fragilis 4 2.5 4 2.5
Eubacterium spp. 2* 1.6 4 2.6
Clostridium spp. 0.062 0.2 0.25 0.2
Streptococcus spp. 16 > 19.7 8 > 13.9
Fusobacterium spp. 0.125 0.1 0.25 0.2
Lactobacillus spp. 2* 1.9 2 2.3
Proteus spp. > 32 > 32 > 32 > 32
Peptostreptococcus 2 3.2 4 4.0
spp.
* MIC50 = MIC90
2.2.9.2 Hepatotoxicity
TC-HCl (>0.25 mM) inhibited reversibly the ß-oxidation of
fatty acids as well as the tricarboxylic acid cycle activity of mouse
and human liver mitochondria in vitro. After i.p. administration of
1 mmol/kg bw to mice following an oral administration of a tracer dose
of [U-14C]palmitic acid, an increase in the levels of hepatic
triglycerides was observed and liver histology showed microvesicular
steatosis (Freneaux et al., 1988).
The effects of TC on the synthesis and secretion of triglycerides
and proteins were studied in isolated rat hepatocytes. TC produced a
concentration-dependent inhibition of 14C-triglyceride secretion
without affecting triglyceride synthesis and protein secretion
(Deboyser et al., 1989).
2.2.9.3 Nephrotoxicity
Groups of Wistar albino rats were given single i.v. injections of
150 mg TC/kg bw into the tail vein. Different experiments failed to
demonstrate any significant ischaemia in the kidneys (Tapp & Lowe,
1966)
2.3 Observations in humans
2.3.1 Effects after medical treatment
In human therapy, the usual oral dose for TC-HCl is 250 or 500 mg
every 6 h for adults as specified in the US National Formulary and
British Pharmacopoeia. CTC is approved as capsules (50, 100 and
250 mg) and by injection (100, 200 and 500 mg).
A variety of adverse effects in humans have been reported from
the use of TCs. The TCs are irritating substances. When given i.v.
they can cause thrombophlebitis. Intramuscular administration is
painful and not recommended. Given orally, TCs can cause epigastric
burning, abdominal discomfort, nausea and vomiting (Schindel, 1965;
NTP, 1989; Sande & Mandell, 1990).
Bone and teeth discoloration are known to occur in humans under
clinical treatment with high levels of TC drugs. Children receiving
long- or short-term therapy with a TC may develop a yellow or brownish
discoloration of the enamel. The duration of therapy appears to be
less important than the total quantity of antibiotic administered. The
risk of this effect is highest when the TC is given to neonates and
babies prior to the first dentition. However, pigmentation of the
permanent dentition may develop if the drug is given between the ages
of 2 months and 8 years, when the teeth are being calcified. An early
characteristic of this effect is a yellow fluorescence of the dental
pigment.
Treatment of pregnant patients with TCs may produce
discolouration of the teeth in their children, but exposure also
occurs during breast feeding. (Schindel, 1965; Piper et al., 1988;
Friedman, et al., 1990; Sande & Mandell, 1990).
TCs are deposited in the skeleton during gestation and throughout
childhood. A 40% reduction in bone growth, as determined by
measurements of fibulas, was demonstrated in premature infants treated
with these agents. This effect is readily reversible if the period of
exposure to the drug is short (Sande & Mandell, 1990; Pratt & Fekety,
1986).
No toxic effects were reported from treatment of premature
infants, children, adults or elderly with CTC. Effects after short-
and long-term exposure included increased body-weight gain, and
prophylactic activity against acute and chronic infections and
diarrhea (Hines, 1956).
All the TCs (dose levels not specified) can cause phototoxicity,
manifested by mild to severe skin reactions when the skin is exposed
to direct sunlight (Sande & Mandell, 1990; Schindel, 1965).
Liver effects, characterized by fatty accumulation have been
observed, predominantly following repeated parenteral administration
of TCs (dose levels were not specified). TCs can aggravate uremia in
patients with impaired renal function. For unknown reasons, pregnant
woman appear to be more susceptible to the development of hepatic
damage (Pratt & Fekety, 1986; Sande & Mandell, 1990; Friedman,
et al., 1990).
TCs rarely cause allergic reactions. Hypersensitivity to TCs have
been described as allergic manifestations of mucosal tissues. Central
nervous system side effects and effects on blood cells were rarely
described. Long-term therapy (dose levels not specified) with TCs may
produce changes in the peripheral blood. Leucocytosis, atypical
lymphocytes, toxic granulation of granulocytes, and thrombocytopenic
purpura have been observed.
The TCs may cause increased intracranial pressure and tense
bulging of the fontanels (pseudotumor cerebri) in young infants, when
given in therapeutic doses. Young adults complain of headache, nausea,
vomiting and diplopia.
TC is given orally in low doses (usually 100 mg/person/day) for
the treatment of chronic severe acne vulgaris. A common complication
of treatment with TCs is minor GI irritation (Sande & Mandell, 1990;
Pratt & Fekety, 1986).
3. COMMENTS
Toxicological data
The Committee considered data from studies on pharmacokinetics,
biotransformation, acute and short-term toxicity, reproductive
toxicity, teratogenicity, long-term toxicity/carcinogenicity,
genotoxicity, antimicrobial effects and observations in humans.
Aspects of the comparative toxicity of these compounds with OTC were
also considered.
Pharmacokinetic studies showed that CTC and TC were rapidly
absorbed following oral administration. Peak plasma concentrations
were higher in rats receiving TC than in those receiving the same dose
of CTC. In humans receiving oral therapeutic doses, 30% of the dose of
CTC was absorbed, as compared with 60-80% of the dose of TC. TC is
excreted mainly in urine, whereas CTC is excreted in urine and faeces
in comparable amounts. After absorption following administration by
various routes, CTC and TC are widely distributed in the body, the
highest levels are present in the liver and kidney. Detectable levels
of TCs in bone persist for more than 28 weeks after dosing. The plasma
half-lives of CTC and TC in humans are about 5 and 8 h, respectively.
TCs have been found in the breast milk of women receiving therapeutic
doses and can cross the placenta.
Orally administered CTC and TC have low acute toxicity in rats
and mice, LD50s ranging from 2150 to >5000 mg/kg bw.
In a 13-week range-finding study with mice in which TC was
incorporated in the diet at concentrations equivalent to 470, 950,
1800, 3700 or 7500 mg/kg bw/day, the only sign of toxicity observed
was a decrease in body weight in the highest-dosed group. In a 13-week
range-finding study in which rats were dosed at 155, 315, 625, 1250 or
2500 mg TC/kg bw/day, vacuolization of liver cells and bone marrow
atrophy were observed at 1250 and 2500 mg/kg bw/day. No toxicity was
observed in dogs given TC orally at dose levels of up to
250 mg/kg bw/day for 24 months.
CTC did not cause any toxic effects in short-term toxicity
studies with rats or mice at doses of up to 200 mg/kg bw/day. In dogs
given CTC orally at a dose of 250 mg/kg bw/day for 98 days, increased
incidences of mortality, fatty liver and bone marrow atrophy were
observed. In another experiment in dogs which received CTC orally at
doses of up to 100 mg/kg bw/day for 54 weeks, no effects were
observed, except for signs of GI disorders; these were considered of
minor importance because the increase in body-weight gain was normal.
In carcinogenicity studies with mice and rats, TC was administered
in the diet at doses of up to 3500 and 1060 mg/kg bw/day, respectively.
Survival rates were increased in male mice, while body weights were
slightly lower than controls for both sexes. Survival rates were also
increased in female rats, but male rats showed a dose-related increase
in basophilic cytoplasmic changes and clear cell changes in the liver.
There were no increases in tumour incidence in mice or rats.
In a chronic toxicity/carcinogenicity study, CTC was administered
in the diet of rats at doses varying from 0.07 to 5200 mg/kg bw/day.
In the highest dose group, GI irritation, decreased body-weight gain
and a decreased number of white blood cells were observed in both
sexes, while testicular atrophy was observed in males. Microscopic
changes in the highest-dose group included infiltration of monocytes
in the lungs of males and females, and fatty changes in the liver in
males. Tumour incidence was not increased. The NOEL was 700 mg/kg
bw/day. The Committee concluded that there was no evidence that CTC or
TC is carcinogenic.
In a two-generation reproductive toxicity study in which CTC was
administered to rats in the diet at a dose equivalent to 500 mg/kg
bw/day, no effects on reproductive performance were observed.
Reproductive toxicity data were not available on TC. In a number of
teratogenicity studies with rats, TC and TC hydrochloride caused a
reduction in ossification at the highest oral dose tested, 540 or
400 mg/kg bw/day, respectively. No irreversible structural changes
were found on skeletal or visceral examination. In studies with a
limited number (5 per group) of rats and rabbits, no evidence of
teratogenicity was found at intravenous doses of 10 mg of CTC or TC/kg
bw/day, administered on days 10-20 of pregnancy. The Committee
concluded that CTC and TC did not cause significant toxic effects on
either reproduction or development.
The genotoxicity of CTC and TC was investigated in a wide range
of in vitro and in vivo test systems. CTC gave negative results in
all tests. TC was negative in the majority of in vitro and in vivo
assays, but induced gene mutations in one in vitro test in mammalian
cells and chromosomal aberrations in plants. The Committee considered
these effects to be due to the inhibiting effect of TCs on protein
synthesis, based on their binding to ribosomes, and concluded that CTC
and TC do not pose a genotoxic hazard.
Microbiological data
In vitro studies of the antimicrobial effect of TCs on
pathogenic microorganisms of animal origin indicate that the
activities of CTC and OTC are not significantly different. MIC values
(geometric mean) for CTC and OTC were 0.32 and 0.52 µg/ml,
respectively.
In a limited number of microorganisms representative of human gut
flora, the antimicrobial activity of CTC was of the same order as that
of OTC.
The antimicrobial activity of TC was compared with that of OTC
using a wide range of bacterial species isolated from the faeces of
healthy human volunteers. The sensitivity of most species was similar
for TC and OTC except for Bifidobacterium, Fusobacterium and
Eubacterium species which were slightly more sensitive to TC, and
Streptococcus spp. which were slightly more sensitive to OTC. The
geometric mean MICs for all tested strains were 3.2 µg/ml for TC and
3.8 µg/ml for OTC.
4. EVALUATION
After reviewing the available toxicological and antimicrobial
data, the Committee concluded that the antimicrobial data provided the
most appropriate end-point for the evaluation of CTC and TC.
In view of the similarity of the antimicrobial activity of TC,
CTC and OTC, the Committee established a group ADI of 0-3 µg/kg bw for
the three drugs separately or in combination. This ADI was established
for OTC at the thirty-sixth meeting of the Committee (Annex 1,
reference 91), and is based on the NOEL of 2 mg/person/day for the
effects of OTC on the gut flora in human volunteers and a safety
factor of 10.
The Committee noted that this ADI provides an adequate margin of
safety when compared with the lowest NOEL for toxicological effects of
100 mg/kg bw/day for CTC in dogs.
5. REFERENCES
Adir, J. (1975) Enterohepatic circulation of tetracycline in rats.
J. Pharm. Sci, 64(11) 1847-1850.
Bacharach, A.L., Clark, B.J., McCulloch, m. & Tomich, E.G. (1959)
Comparative toxicity studies on ten antibiotics in current use.
J. Pharm. Toxicol. 11: 737-741.
Berté, F. & Vandoni, G. (1962) On the intestinal absorption and
organotropism of some tertacyclines. Chemotherapia 5, 219-230.
Buyske, D.A., Eisner, H.J. & Kelly, R.G. (1960) Concentration and
persistence of tetracycline and chlortetracycline in bone.
J. Pharm. Exper. Therap. 130, 150-156.
Chiang, T (1982) Inhibition of chlortetracycline activity by magnesium
ions. J. Assoc. Off. Anal. Chem., 65 (5), 1044-1047.
Cunningham, R.W., Hines, L.R., Stokey, E.H., Vessey, R.E. & Yuda, N.N.
(1954) Pharmacology of tetracycline. Antibiotics Annual, 63-69.
Deboyser, D., Goethals, F., Krack, G. & Roberfroid, M. (1989)
Investigation into the mechnism of tetracycline-induced steatosis:
study in isolated hepatocytes. Tox.appl.pharmacol., 97, 473-479.
Deichmann, W.B., Bernal, E., Anderson, W.A.D., Keplinger, M.,
Landeen, K., Mcdonald, W., Mahon, R. & Stebbins, R. (1964) The chronic
oral toxicity of oxytetracycline HCl and tetracycline HCl in the rat,
dog and pig. Ind.Med.Surg., 787-806.
Dessau, F.I. & Sisson, G., (1957) Aureomycinr life studies. Studies
of chlortetracycline hydrochloride (food grade in dogs). Project
number 77601. Unpublished report of American Cynamid Company.
P.R. 3, 440-493. Research Division. Pearl River Laboratories.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Dessau, F.I. & Sullivan, W.J., (1958) Aureomycinr life studies.
Studies of chronic toxicity of CL 13,555: Chlortetracycline
hydrochloride (food grade) in rats. Project number 77601 American
Cyanamid Company, Experimental Pathology Research Division, Pearl
River Laboratories. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Dessau, F.I. & Sullivan, W.J. (1961) A two year study of the toxicity
of chlotetracycline hydrochloride in rats. Toxicol and Appl.
Pharmacol., 3, 654-677.
Devriese, L.A. (1994) Comparative minimal inhibitory concentration
determinations with chlortetracycline and oxytetracycline on gut
bacteria of animal and human origin. Faculty of Veterinary Medicine.
University of Gent, Belgium. Submitted to WHO by American Cyanamid
Company, Princeton, USA.
Diermeier, H.F. (1959) Acute oral and intravenous toxicity of rats of
a A-VIII hydrochloride (CL 22,416), chlortetracycline hydrocholoride
(CL 13,555), and tetracycline hydrochloride (CL13,554). P.R. 5,
899-907. Unpublished report of American Cyanamid Company, Lederle
laboratories, Pearl River, New York. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Dietz, D.D., Abdo, K.M., Haseman, J.K., Eustis, S.L. & Huff, J.E.
(1991) Comparative toxicity and carcinogenicity studies of
tetracycline and oxytetracycline in rats and mice. Fund. Appl.
Toxicol. 17 (2), 335-346.
Eisner, H.J. et al., (1953) The enhancement of serum levels of
Aureomycin experimental animals. J. Pharmacol. Exp. Ther.
108, 442-449.
Eisner, H.J. & Wulf, R.J. (1962) The metabolic fate of
chlortetracycline and some comparisons with other tetracyclines.
J. pharmacol, exp. therap., 142, 122-11.
Fourtillan & Lefebvre (1985) cited in Rogalski, W. (1985) Chemical
modifications of the tetracyclines. In: The tetracyclines. Eds.
Hlavka and Boothe. Springer Verlag, Berlin.
Freneaux, E., Labbe, G., Letteron, P. The Le Dinh, T.L., Degott, C.
Geneve, J. Larrey, D. & Pessayre, D. (1988) Inhibition of the
mitochondrial oxidation of fatty acids by tetracycline in mice and in
man: possible role in microvesicular steatosis induced by this
antibiotic. Hepatology, 8(5), 1056-1062.
Friedman, J.M., Little, B.B., Brent, R.L., Cordero, J.F., Hanson,
J.W., & Shepard, T.H., (1990) Potential human teratogenicity of
frequently prescribed drugs. Obstetics and Gynecol, 75, 594-599.
Goldenthal, E.I. (1971) A compilation of LD50 values in newborn and
adult animals. Tox. and Appl. Pharm, 18, 185-207.
Gustafson, R.H. (1995) MIC tests of veterinary clinical isolates,
tetracycline. Memo to D.L. Ingle, d.d. january 11, 1995. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Gyrd-Hansen, N (1980) The effect of tetracyclines on the rabbit heart.
Zbl.vet.med. A, 27, 228-237.
Halliday, S.L. & Fanelli, G. (1960) Antibacterial tetracycline:
relative toxicity of CL 13,555, chlortetracycline hydrochloride and
CL 30,782, sodium benzoate orally in rats. Project number 14797.
P.R. 6, 1428-1439. Unpublished report of American Cyanamid Company,
Lederle Laboratories, Pearl River, New York, Submitted to WHO by
American Cyanamid Company, Princeton, USA.
Harned, B.K. Cunningham, R.W. Clark, M.C., Cosgrove, P., Hine, C.H.,
Mccauley, W.J., Stokey, E., Vessey R.E., Yuda, N.N. & Subbarow, Y.
(1948) The pharmacology of Duomycin. Ann. NYAS 51, 182-210.ALLESY,
D.W. & HINE, C.H., (1964) The effect of chlortetracycline
hydrochloride on the fertility and growth of rats.
Tox. and Appl. Pharm. 6, 9-14.
Harbell, J.W. (1988) CHO/HGPRT mutation assay. Study number
T8133.332021. Unpublished report of Microbilogical Associates, 1988.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Hines, L.R. (1956) An apprasal of the effects of long-term
chlortetracycline administration. Antibiotics. chemother.,
6 (11), 623-641.
Hurley, L.S. & Tuchmann-Duplessis, H (1963) Influence de la
tetracycline sur le developpement pré- et post-natal du rat.
Compt. rend. acad. sci., 257, 302-304.
Kanegis, L. (1958) The comarative pharmacology of tetracyclines:
initial studies on serum levels and urinary excretion of antibiotic
A-VIII following single oral and intravenous doses in the dog. Report
P.R. 4:884-921. American Cyanamid Company (Pearl River). Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Kelly, R.G. (1964) Antibacterial tetracyclines. In tissue distribution
of tetracycline and chlortetracycline. American Cyanamid Company
Report P.R. 9:485-492 (Pearl River). Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Kelly, R.G. & Buyske, D.A. (1960) Metabolism of tetracycline in the
rat and the dog. J. Pharmacol. Exp. Therap., 130, 144-149. Submitted
to the WHO by American Cyanamid Company, Princeton, USA.
Kniffen, T.S., Bane, D.P., Hall, W.F., Koritz, G.D. & Bevill, R.F.
(1989) Bioavailability, pharmacokinetics, and plasma concentration of
tetracycline hydrochloride fed to swine. Am. J. Vet. Res.
50, 518-521.
Levinskas, C.J. (1963) Auromycinr and formulation: Acute oral and
intravenous toxicity. Central Mediacal Department Report
No. 6305, 53-5,. Submitted to the WHO by American Cyanamid Company,
Princeton, USA.
Lowe C.A. & Fisher J. (1993) Rat oral LD50 study with Aureo Biomass.
Report number A93-61. Data from American Cyanamid Company, Agricultural
Research Division. Submitted to WHO by American Cyanamid Company,
Princeton, USA. (summary only)
Maffii, G. & Mainardi, L. (1955) Studio Farmacologico su un nuovo
antibiotico: la tetraciclina. Il Farmaco Ed. Sci 10 (4), 197-210.
Mann, S.K. (1978) Interaction of tetracycline (TC) with chromosomes in
Allium cepa Environm. exp. botany. 18, 201-205.
Martindale (1989) The Extra pharmacopoeia. 29th edition. Eds. J.E.F.
Reynolds. London, The Pharmaceutical Press.
Mccoll, J.D. Globus, M. & Robinson, S (1965) Effect of some therapeutic
agents on the developing rat fetus. Tox. appl. pharmacol. 7,
409-417.
Meisner, L.F., Chuprevich, T.W. & Inhorn, S.L. (1977) Mechanisms of
chromatid breakage in human lymphocyte cultures. Acta. cytol.
21(4), 555-558.
Milch, R.A., Rall, D.P. & Tobie, J.E. (1967) Bone localization of the
tetracyclines. J. Nat. Canc. Inst. 19, 87-91.
Mulligan, E. (1989) Microbial mutagenicity test report for screening
study MMR89-026. American Cyanamid Company, Report number MMR89-026,
20 April 1989. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Nelson, A.A. & Radomski, J.L. (1954) Comparative pathological study in
dogs of feeding of six broad-spectrum antibiotics. Antibiotics and
Chemotherapy IV, (11), 1174-1180.
Neuschl, J. (1991) Comparison of some pharmacokinetic parameters of
tetracyclines which are most frequently used in veterinary medicine.
Arch. Exp. Veterinarmed. 45, 105-112.
NTP (1989) NTP technical report on the toxicology and carcinogenesis
studies of tetracycline hydrochloride (Cas no. 64-75-5) in F344/N rats
and B6C3F1 mice (feed studies) NTP toxicology program P.O. Box 12233.
NTP TR 344 NIH Publication No. 89-2600. U.S. Department of health and
human services.
Piper, J.M., Baum, C., Kennedy, D.L. & Price, P. (1988) Maternal use
of prescribed drugs associated with recognized fetal adverse drug
reactions. Am. J. Obstet. and Gynecol. 159 (5) 1173-1177.
Pratt, W.B. & Fekety, R. (1986) The antimicrobial drugs. Oxford
University Press, New York, pp 205-228.
Putman, D.L. (1988) Acute in vivo cytogenetics assay in rats. Final
report. Report number T8133.105003. Unpublished report of
Microbiological Associates, 11-7-1988. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Richez, P. (1994) Antibacterial activity of tetracycline and
oxytetracycline against human gut flora. Study No. SVL 029.
Unpublished report of Datavet Veterinary Drug Consultants,
May 31 1994. Submitted to WHO by Intervet International,
Boxmeer, Netherlands.
Rogalski, W. (1985) Chemical modifications of the tetracyclines.
Chapter 5, pp 233-. In: The tetracyclines. Eds. Hlavka and Boothe.
Springer Verlag, Berlin.
Sande, M.A. & Mandell, G.L. (1990) Antimicrobial agents:
tetracyclines, chloramphenicol, erythromycin and miscellaneous
antibacterial agents. In: Goodman and Gilman's The pharmacological
basis of therapeutics, 8th edition. Gilman, A.G., Rall, T.W., Nies,
A.S. and Taylor, P. eds. Pergamon Press, New York, (1990),
pp 1117-1145.
Schindel, L.E. (1965) Clinical side-effects of the tetracyclines.
Antibiotica et Chemotherapia Advances 13, pp. 300-316.
Sharma, A.K. & Bhattacharyya, G.N. (1967) A study on the respomse of
chromosomes to antibiotic treatment. Acta Biol. Acad. Sc. Hung.
18 (1), 67-75.
Sharma, R.K., Traul, K.A., Caterson, C.R., Harbell, J., Thilagar, A. &
Putman, D. (1989) Genotoxicity evaluation of chlortetracycline.
Environ. Mol. Mutagen 14 (suppl. 15), 183. (summary only).
Shiota, K., Uwabe, C. Yamamoto, M. & Arishima, K. (1990) Teratogenic
drugs inhibit the differentiation of fetal rat limb buds grafted in
athymic (nude) mice. Reprod. toxicol. 4, 95-103.
Szumigowska-Szrajber, G. & Jeske J. (1970) Effect of tetracycline
base, tetracycline hydrochloride and oxytetracycline base on
developmental changes in metatarsal bones of extremities of rat
fetuses. Acta polon. pharm. 27 (I), 85-90.
Tapp, E. & Lowe, M.B. (1966) Tetracycline toxicity. Experientia
22, 530-531.
Thilagar, A. (1988) Test for chemical induction of unscheduled DNA
synthesis in rat primary hepatocyte cultures by autoradiography. Test
report 0082-5100. Unpublished report of Sitek Research Laboratories,
22-7-1988. Submitted to WHO by American Cyanamid Company, Princeton,
USA.
Tsutsui, T., Umeda, M., Sou, M. & Maizumi, H. (1976) Effect of
tetracycline on cultured mouse cells. Mutat. res. 40, 261-268.
Tubaro, E. (1964) Possible relationship between tetracycline stability
and effect on foetal skeleton. Brit. J. Pharmacol. Chemotherap.
23, 445-448.
Tubaro, E. & Banci, F. (1964) The pharmacology of chlormethylene-
cycline, a new chlortetracycline. Arzneimittel-Forschung, 14,
95-100.
Tubaro, E., Barletta, M. & Banci, F. (1964) Some pharmacological
aspects of a new water-soluble tetracycline. J.pharm. pharmacol.
16, 33-37.
Walter, A.M. & Heilmeyer, L. (1975) Antibiotika-Fibel. Antibiotika und
Chemotherapeutila Therapie mikrobieller infektionen, pp.323-324.
Georg Thieme Verlag Stuttgart.
Wulf, R. & Eisner, H. (1961) Antibacterial tetracyclines: Metabolism
of chlortetracycline in the rat and in the dog. American Cyanamid
Company Report C.P. 1, 626-683. Experiment P-70-21-FT. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Tetracycline (TC)
Rats
Fasted white rats were given a single oral dose of TC
hydrochloride by gavage corresponding to 75 mg/kg bw TC base. The rats
were sacrificed 1, 2, 3, 4 or 6 h after treatment and plasma and
tissue levels recorded.
Peak plasma concentrations of 3.6 mg/l were observed 2 h after
treatment, declining to 0.5 mg/l after 6 h. Peak tissue levels were
found 2 h after treatment with highest levels in the liver and kidney
(Berté & Vandoni, 1962).
To investigate the importance of the biliary route of excretion,
4 rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw tritium- labelled TC. Radioactivity was measured in
urine, bile and GI tract 24 h following treatment.
In non-ligated rats, 85-92% of the total radioactivity was
recovered of which 67-72% was excreted in urine and 18-20% in faeces.
About 70-85% of the total radioactivity was recovered in the rats with
ligated bile ducts, 68% and 88% being recovered in the urine of each
rat, and 30% and 9% in the bile. Only very small amounts (on average
2.5%) were recovered in the GI tract. When the same dose of TC was
administered to rats with ligated ureters, no increase in the
excretion of TC in the faeces was observed (Wulf & Eisner, 1961).
Male Sprague-Dawley rats were injected with 10 mg/kg bw 3H-7-TC
hydrochloride (98% pure) in the femoral vein over 5 min. The
intestinal absorption of TC hydrochloride excreted in bile was
evaluated using in situ intestinal preparations. About 73% of TC
excreted in the bile was reabsorbed in the intestinal lumen,
indicating enterohepatic circulation (Adir, 1975).
Single i.v. doses of 15 or 4 mg/kg bw tritium-labelled TC
hydrochloride were administered to 2 rats and 1 dog, respectively.
Within 72 h, 69.2% and 19.5% of the radioactivity was recovered in the
urine and faeces of rats, respectively. Within 168 h, 71% of the
radioactive dose was recovered in the urine and 9% in the faeces of
the dog (Eisner & Wulf, 1962).
Dogs
Tritium-labeled TC hydrochloride was administered i.v. to 2
beagle dogs at 10 mg TC/kg bw to study the distribution of TC
throughout the body. The animals were sacrificed after 4 h and the
various organs and tissues examined for radioactivity. The organs
containing the highest radioactivity were liver and kidney, containing
on average 15 and 45 mg/kg, respectively. A large proportion of the
recovered TC activity was found in the urine, intestinal contents and
bile. No radioactivity was measured in subcutaneous fat (Kelly, 1964).
Beagle dogs given single oral doses of 25 mg TC/kg bw showed peak
TC levels averaging 3 µg/ml 2 h after dosing, declining to an average
of 0.27 µg/ml by 24 h post-dosing. Excretion in the urine accounted
for 10% of the administered dose within 72 h after dosing.
When dogs were given a single i.v. dose of 10 mg TC/kg bw,
average serum TC levels of 10.6 µg/ml were found at 24 h, and
0.14 µg/ml at 48 h after dosing. Excretion in the urine was 58% of the
administered dose by 72 h after dosing (microbiological assay;
sensitivity 0.05-0.1 µg/ml) (Kanegis, 1958).
Pigs
The bioavailability of TC hydrochloride administered orally to
fasted gilts was calculated from the area under the plasma
concentration in time curve (AUC) and estimated to be 23%. After i.v.
administration the disposition kinetics of TC in plasma were best
described by a tri-exponential equation. The drug had a rapid
distribution phase followed by a relatively slow elimination phase,
with half-life of 16 h (Kniffen et al, 1989).
2.1.1.2 Chlortetracycline (CTC)
Rats
14C-Labelled CTC was orally administered to rats at a dose of
60 mg/kg bw. Radioactivity was measured in urine and faeces after
24, 48 and 72 h. Radioactivity was found primarily in the faeces
(92% within 72 h, the major part being excreted within 24 h). About 5%
of the radioactivity was recovered in urine.
When 30 mg/kg bw 14C-CTC was administered i.p., 33% of the
radioactivity was excreted in the urine within the first 24 h, and 5%
in the faeces. Between 24 and 72 h, 7% was excreted in the urine and
40% in the faeces.
To investigate the importance of the biliary route of excretion,
rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw 14C-CTC. Radioactivity was measured in urine, bile
and GI tract, 24 h following treatment.
In non-ligated rats, 75-79% of the total radioactivity was
recovered of which 35-37% was excreted in urine and 44-38% in faeces.
In the rats with ligated bile ducts, about 47-63% of the total
radioactivity was recovered, with 66% and 43% being recovered in the
urine of each rat, and 22% and 51% in the bile. Only very small
amounts (on average 5%) were recovered in the GI tract (Wulf &
Eisner, 1961).
Groups of 6 rats given single oral doses of 75 mg CTC/kg bw had
plasma levels of 2.1 mg/l at 1 h after administration, declining to
0.8 mg/l after 6 h. The rats were sacrificed at 1, 2, 3, 4 or 6 h
after treatment. Tissue levels were highest in liver and kidneys at
all observations. Maximum concentration was found after 2 h in the
liver, and after 1 h in the kidney (Berte & Vandoni, 1962).
Oral doses ranging from 6 to 800 mg CTC/kg bw administered to
female rats and male guinea-pigs gave no proportional increases in
serum CTC concentrations. When guinea-pigs received the same doses
each day for 9 days, higher serum levels were found than with single
doses. Serum CTC levels were increased by the simultaneous
administration of various adjuvants, such as citric acid. This effect
(observed in doses of CTC up to 200 mg/kg bw) was apparent within 1 h
of administration and lasted at least 8 h (Eisner et al., 1953)
Rabbits
Ten adult white Californian rabbits (males and females) received
a single oral dose of 20 mg/kg bw of technical CTC or CTC-chloride.
Serum CTC levels averaged 2.3 mg/l 3 h after dosing. The concentration
of CTC declined to a mean value of 0.09 mg/l by 12 h, and to 0.08 mg/l
by 24 h after dosing. The highest concentrations were found in the
liver (1.53 mg/kg) 24-h post-dosing, followed by kidney, lung and
heart. No measurable levels were found in muscle (detection limit:
37.5 µg/kg) (Neuschl, 1991)
Dogs
Four beagle dogs were given a single oral dose of 25 mg
CTC/kg bw. Peak serum CTC levels ranged from 0.40-1.9 mg/l 2 h after
dosing, declining to an average of 0.21 mg/l by 24 h. When the dogs
were given an i.v. injection of 10 mg CTC/kg bw, serum levels averaged
6.6 mg/l 1 h after administration, declining to 2.4 µg/ml at 8 h,
0.29 µg/ml at 24 h and 0.06 µg/ml at 48 h (Kanegis, 1958).
Two female beagle dogs received a single i.v. injection of 10 mg
14C-CTC/kg bw. The dogs were sacrificed 4 h after dosing. The organ
containing the highest radioactivity was the liver (30 mg/kg),
followed by kidney (25 mg/kg), ileum (15 mg/kg), jejunum (12 mg/kg)
and heart (10 mg/kg). A large proportion of the recovered
radioactivity was found in urine, intestinal contents and bile. With
the exception of subcutaneous fat, radioactivity was found throughout
all tissues and fluids examined (Kelly, 1964).
2.1.2 Interactions with bones and teeth
Tissues from mice, rats, guinea-pigs, rabbits and dogs
parenterally treated with TCs (TC, CTC or OTC, doses varying from
0.1 - 50 mg/kg bw) were examined in U.V. light. In all tissues with
the exception of the brain, a brilliant yellow-gold fluorescence
was apparent within 30 min, irrespective of the dose. The induced
fluorescence disappeared from all tissues (except bone) within 6 h
after a single parenteral injection. However, bone fluorescence
persisted throughout a 10-week observation period after dosing
(Milch et al., 1967).
Single oral doses of 250 mg/kg bw tritium-labelled TC or single
oral dose of 250 mg 14C-CTC/kg bw were administered to male rats
(Sherman strain). Radioactive residues in the femur of the rats
treated with TC were 9.6, 1.9 and 0.4 mg/kg of bone measured after
4 h, 24 h, and 4 weeks, respectively. The radioactivity averaged
12 mg/kg of bone 4 h after CTC treatment, and 2.3 mg/kg after 4 weeks.
After a lifetime diet containing 0.5 to 1000 mg CTC/kg of feed,
the maximum radioactivity in femur was 570 mg/kg. After i.p.
injections with 10-150 mg TC/kg bw, the concentrations found in the
femur were directly related to the administered dose and were much
higher than the concentrations measured after oral treatment with
250 mg TC/kg bw (Buyske et al., 1960).
2.1.3 Observations in humans
In humans, about 30% of an oral therapeutic dose of CTC was
absorbed on an empty stomach. For TC and OTC, the absorption was
60-80% (Sande & Mandell, 1990).
Oral doses of 250 to 500 mg every 6 h produced plasma
concentrations of TC ranging from 1 to 5 mg/l. Intravenous injections
of 250-500 mg produced plasma concentrations of about 15-20 mg/l at
0.5 h, falling to 4-10 mg/l at 1 to 2 h. At 12 h, 1-3 mg/l was still
present. In circulation, TCs are bound to plasma proteins in varying
degrees (24-65% for TC, and about 47% for CTC). TCs appeared in the
milk of nursing mothers where concentrations were be 60% or more of
those in the plasma. TCs diffused across the placenta and appeared in
the fetal circulation at concentrations of about 25 to 75% of those in
the maternal blood. The plasma half-lives of CTC and TC were reported
to be about 8-10 h and 5.5 h, respectively (Martindale, 1989).
Absorption of TCs was impaired by milk products, sodium
bicarbonate, aluminium hydroxide and iron preparations due to
chelation and increase in gastric pH (Sande & Mandell, 1990).
2.1.4 Biotransformation
2.1.4.1 Tetracycline
The metabolism of 14C-TC was examined in rats following single
i.p. or oral administration of 60 mg/kg bw, and in dogs after single
oral administration of 25 mg/kg bw tritium-labelled TC. Approximately
90% of the administered radioactivity in the rat was eliminated either
by urinary or faecal route. A significant portion of the remaining
activity was bound as chelated TC in the skeleton of the animal. With
the exception of this chelate, TC was chemically unaltered by the rat.
Urine of dogs contained only unchanged TC (Kelly & Buyske, 1960).
2.1.4.2 Chlortetracycline
Male Wistar rats (n=6) and male beagle dogs (n=2) were
administered 14C-labelled CTC via different routes (oral:
60 mg/kg bw; i.p.: 30 mg/kg bw; i.v.: 15-60 mg/kg bw). The
recovery of antimicrobial activity was significantly lower than the
recovery of radioactivity by all routes of administration and
excretion. The major 'presumed' metabolite was identified as
4-epichlortetracycline, which accounted for 23-35% of the
radioactivity in the urine of rats, and 31-60% in dogs. It was
essentially inactive in microbiological assays. It was not clear
whether this represented a true metabolite or was a degradation
product due to alkaline treatment. The presence of small amounts
(5-10%) of isochlortetracycline in the urine and faeces of some
animals was also demonstrated (Wulf & Eisner, 1961; Eisner & Wulf,
1963).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with TC and CTC after oral,
i.v. or s.c. administration are summarized in Tables 1 and 2,
respectively.
Dogs developed transient hyperpnea, weakness and anorexia
following an i.v. dose of 50 and 100 mg CTC/kg bw, and a dose of
150 mg/kg bw caused respiratory distress, general paresis, somnolence
and death within a few hours (Hines, 1956).
Table 1. Acute toxicity of tetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. oral TC n.s. 2550 Tubaro et al., 1964
mouse n.s. oral TC n.s. >3000 Cunningham et al., 1954
mouse (42d) n.s. oral TC n.s. 808 Goldenthal, 1971
(3d) 300
mouse n.s. i.v. TC n.s. 160 Tubaro et al., 1964
mouse n.s i.v. TC n.s. 157 Maffii & Mainardi, 1955
mouse n.s. i.v. TC n.s. 170 Cunningham et al., 1954
mouse n.s. i.p. TC n.s. 340 Tubaro et al., 1964
mouse n.s. i.p. TC n.s. 330 Cunningham et al., 1954
rat M oral TC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral TC n.s. >3000 Cunningham et al., 1954
rat (49(d) n.s. oral TC n.s. 807 Goldenthal, 1971
(3d) 360
Table 1. Acute toxicity of tetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat (adult) n.s. oral TC HCl n.s. 6443 Goldenthal, 1971
(<2d) 3827
rat n.s. i.v. TC n.s. 220 Cunningham et al., 1954
rat n.s. i.v. TC n.s. 128 Maffii & Mainardi, 1955
rat n.s. i.v. TC HCl n.s. 375 Diermeier, 1959
rat n.s. i.p. TC n.s. 320 Cunningham et al., 1954
n.s. not specified
d age of animal in days
2.2.2 Short-term toxicity studies
2.2.2.1 Tetracycline
Mice
Groups of male mice (10/sex/group) were given by gavage 0, 20 or
100 mg/kg bw TC hydrochloride once daily, 5 days/week for 6 weeks as a
2% starch suspension containing 4-6% test substance. At termination of
the study a complete blood count was performed in the high-dose group
and no significant haematological alterations were observed.
Accelerated growth rates were observed in dosed mice when compared to
the control mice (Cunningham et al., 1954).
In a range-finding study, groups of B6C3F1 mice (6-7 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 470, 950, 1800, 3700
or 7500 mg/kg bw/day, respectively). No mortalities occurred. Final
mean body weight was slightly decreased in males (16%) and females
(6%) at the 5% level. The TC concentration in bone increased with
increasing dose of TC hydrochloride (NTP, 1989).
Rats
In a range-finding study, groups of F344/N rats (7-8 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 155, 315, 625, 1250 or
2500 mg/kg bw/day, respectively). No mortalities occurred. Cytoplasmic
vacuolization was observed in the livers of male rats at the two
highest dose groups. Bone marrow atrophy was seen in both female and
male rats at 1250 and 2500 mg/kg bw/day. The concentration of TC in
bone increased with increasing dose of TC hydrochloride (NTP, 1989)
Dogs
Mongrel dogs (8-4/group) were given a capsule of 0 or
250 mg/kg bw TC once daily, 6 days/week for 98 days. No mortality
occurred. No effects were seen on peripheral blood and no pathological
changes were observed (Nelson & Radomski, 1954).
Groups of 4 mongrel dogs were orally administered 10 or
100 mg/kg bw TC twice daily, 5 days/week for 3 months. No effects were
observed on body weight, liver function (bromosulfophthalein
clearance), kidney function (phenolsulfophtalein clearance),
blood-clotting time, non-protein nitrogen levels, blood sugar values
or complete blood counts (Cunningham et al., 1954).
Table 2. Acute toxicity of chlortetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse M & F oral CTC HCl n.s. >3000 Cunningham, 1954
mouse F oral CTC HCl n.s. 2150 Tubaro & Banci, 1964
mouse M & F oral CTC HCl n.s. 3350 (M) Bacharach, 1959
4200 (F)
mouse n.s. oral duomycin n.s. >3000 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 155 Cunningham, 1954
mouse F i.v. CTC HCl n.s. 93 Tubaro & Banci, 1964
mouse n.s. i.v. duomycin n.s. 102 Harned, 1948
mouse n.s. i.v. duomycin n.s. 134 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 102 (M) Bacharach, 1959
108 (F)
mouse F i.v. CTC bisulfate n.s. 113 Levinskas 1963
mouse n.s. s.c. duomycin n.s. >600 Harned, 1948
mouse M & F s.c. CTC HCl n.s. 5500 (M) Bacharach, 1959
8250 (F)
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. i.p. CTC HCl n.s. 192 Cunningham, 1954
mouse M & F i.p. CTC HCl n.s. 128 (M) Bacharach, 1959
168 (F)
mouse F i.p. CTC HCl n.s. 214 Tubaro & Banci, 1964
rat M & F oral CTC HCl n.s. >3000 Cunningham, 1954
rat F oral CTC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral CTC-HCl n.s. 55001 Goldenthal, 1971
103002
rat F oral calcium CTC n.s. >10000 Levinskas, 1963
rat n.s. oral Aureo Biomass n.s. >5000 Lowe & Fisher, 1993
(summary only)
rat n.s. oral duomycin n.s. >3000 Harned, 1948
rat M & F i.v. CTC HCl n.s. 160 Cunningham, 1954
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat M i.v. CTC HCl n.s. 167 Diermeier, 1959
rat n.s. i.v. duomycin n.s. 118 Harned, 1948
rat n.s. i.p. CTC HCl n.s. 335 Cunningham, 1954
guinea pig n.s. i.v. duomycin n.s. 100 Harned, 1948
guinea pig n.s. s.c. duomycin n.s. >300 Harned, 1948
n.s. not specified
1 < 2 days old
2 adult
Groups of male dogs (4 mongrel dogs and 4 beagle dogs) were fed
diets containing 0.1, 0.3 or 1% TC hydrochloride for 24 months
(equivalent to 25, 75 or 250 mg/kg bw/day, respectively). An interim
sacrifice of 1 beagle and 1 mongrel dog of each group was performed at
12 months for microscopic examination. No effects were observed on
clinical signs, mortality, body weight, food consumption, haematology,
ALP, bromosulfophtalein clearance, urea nitrogen determinations,
testes and epididymides weight, macroscopy, concentration and motility
of the spermatozoa, or appearance of semen. The bony structures of all
dosed dogs showed a yellowish coloration. The intensity of the colour
was related to the dose fed. A brownish-black pigmentation with a
dose-related intensity was observed in the thyroid glands of the dogs
of all groups. Microscopic examination of the thyroids revealed
intracytoplasmic granules in the follicular epithelial cells of most
of the treated dogs. This effect might be caused by deposits of TC or
its metabolites. Histopathological changes were not observed
(Deichmann et al., 1964).
2.2.2.2 Chlortetracycline
Mice
Groups of 10 male mice were given 0, 20 or 100 mg CTC/kg bw/day,
once daily by gavage, 5 days/week for 6 weeks. At 100 mg/kg bw/day,
complete blood count indicated that there were no significant
haematological alterations. Beneficial effects such as increased
growth were observed at 20 and 100 mg/kg bw/day (Cunningham, 1954).
Two groups of 20 mice received 20 or 100 mg/kg bw/day of CTC for
3 months (route not specified). Final body weight of both groups
exceeded those of the controls by 15%. No evidence of toxicity was
seen and no difference in blood counts was found at the end of
experiment (Hines, 1956).
Groups of 20 male mice (strain not specified) received orally
0, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw, twice daily). There were no
significant effects on mortality, clinical signs, body-weight gain,
haematology, macroscopy or microscopy. The NOEL in this study was
200 mg/kg bw/day (Harned et al., 1948).
Rats
Sherman rats (6/sex/group) received diets containing 0, 2.0 or
5.0% CTC for 28 days (equivalent to 2000 or 5000 mg/kg bw/day).
Body-weight gain was significantly decreased at 5000 mg/kg bw/day. No
other examinations were performed (Halliday & Fanelli, 1960).
Two groups of 20 rats each were given oral doses of 150 or
300 mg/kg bw/day CTC for 3 months. In both groups, body weight of the
animals was higher than the controls. No difference in blood counts
was found at the end of the experiment (Hines, 1956).
Groups of 20 male rats (strain not specified) received by gavage
0, 10, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw twice daily). No treatment-related
effects were seen on mortality, clinical signs, body weight,
haemoglobin, blood pressure, macroscopy or microscopy. The NOEL in
this study was 200 mg/kg bw/day (Harned et al., 1948).
Dogs
Oral administration of 100 mg CTC/kg bw/day to dogs (number and
strain not specified) for 17 days, followed by 100 mg/kg bw twice
daily for 14 days, produced no unfavourable effects on general
appearance, growth rate, haemoglobin, complete blood count, gross or
microscopic examinations (Hines, 1956).
Dogs (4/group) received 10, 50 or 100 mg of CTC/kg bw/day for 14
weeks (route not specified). At the end of this period, liver and
kidney function tests, blood sugar values, blood coagulation time,
blood non-protein nitrogen and complete blood counts did not differ
significantly from pre-test values (Hines, 1956)
Ten dogs (strain not specified) were given CTC twice daily
(morning and afternoon) by capsule at a total dose of 100 mg/kg
bw/day, 5 days/week for 9 to 15 weeks. CTC treatment had no effect on
general appearance, body weight, haematology, clotting time, liver and
kidney function (Bromosulfophtalein and phenolsulfophtalein
clearance), urinalysis, gross or microscopic examinations
(Harned, 1948).
Ten mongrel dogs (8-19 kg; 2-5 years old) received capsules
containing 250 mg CTC/kg bw/day, 6 days/week, for 98 days. Another 4
dogs received the same dose intermittently, 2 weeks on the drug and 3
weeks off, for 121 days in total. Five dogs of the continuously
treated group died. These animals had progressive weight loss, apathy
and anorexia. Haemoglobin, RBC, granulocyte and total leucocyte counts
were slightly depressed. The pathologic changes observed included
emaciation, fatty liver, excess fat in the kidney, depletion of the
bone marrow and atrophic changes in the spleen, lymph nodes and
skeletal muscle (Nelson & Radomski, 1954).
Beagle and basenji dogs (2/sex/group; 9-12 months old) received
daily a single oral dose of 10 or 100 mg CTC-HCl/kg bw in capsules for
54 weeks; a third group consisting of 1 female beagle dog, 2 male and
1 female basenji dogs were given 50 mg/kg bw/day. No control group was
used. Observations included haematology, clinical chemistry,
urinalysis, faecal smears, macroscopy, organ weight, microscopy, and
terminal tissue concentration.
Treatment-related GI disorders (vomiting, diarrhea, appetite loss
and swelling of the anal glands) were observed, and particularly in
the first half of the experiment in all groups, most often in basenji
dogs. The average blood concentration 4 h after administration of the
dose (calculated for each group from 12-14 determinations carried out
over a period of 1 year) were 0.34 µg/ml, 0.58 µg/ml and 0.94 µg/ml at
10, 50 and 100 mg/kg bw/day, respectively. Thyroid weight was high in
one male at 100 mg/kg bw/day, but the shape and appearance of the
gland were normal. Slight yellow discolouration in the bones was
observed at necropsy in the high-dose dogs, and 2 dogs at each dose
level showed evidence of chronic gastritis. Measurable tissue
concentrations (no details given) were found in bone > kidney >
liver but none in brain, heart or spleen. The NOEL in this study was
100 mg/kg bw/day (Dessau & Sisson, 1957).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Tetracycline
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets for 103
weeks containing 0, 1.25 or 2.5% TC-HCl (purity 91%), equal to 1500 or
3000 mg/kg bw/day for males, and 1500 or 3500 mg/kg bw/day for
females. Observations included clinical signs, body weight, feed
consumption, macroscopy, and histopathology.
Survival was increased for male mice (31/50, 43/50, 43/50); no
differences were observed in females (37/50, 35/50, 38/50). Mean body
weights in both dose groups were slightly lower for both sexes
compared to control mice. Dosing had no effects on feed consumption.
The tumour incidence was not significantly increased in either sex. On
the contrary, dosed female mice did not develop hepatocellular
adenomas or carcinomas (combined incidence: 10/49 for controls; 0/48
at 1.25%; 0/50 at 2.5%) (NTP, 1989; Dietz et al., 1991).
Rats
Groups of weanling male rats (Osborne-Mendel) were fed diets
containing 0 (180 rats), 100 (100 rats), 1000 (130 rats) or 3000
(100 rats) mg TC-HCl/kg of feed, equivalent to 5, 50 or 150 mg/kg
bw/day for 2 years. Ten Rats/group were killed at frequent intervals.
Sacrificed and moribund rats were macroscopically and histologically
examined. No effects were seen on clinical signs, body weight, food
consumption or haematological examinations. Beneficial effects were
observed during the first 18-19 months, all dosed rats appearing more
vigorous and gaining weight more rapidly than the control rats.
Mortality rates were lower in the treated rats than in the control
rats. At histopathology, yellow discoloration of long bones and
calvarium was observed at the highest dose. Tumour incidence was not
enhanced (Deichmann et al., 1964).
Groups of F344/N rats (50/sex/group) were fed diets containing
0, 1.25% or 2.5% mg TC-HCl (purity 91%), equal to 440 or 910 mg/kg
bw/day for males, and 510 or 1060 mg/kg bw/day for females for
103 weeks. Observations included clinical signs, body weight, feed
consumption, macroscopy and histopathology.
The survival of female rats of both the low- and high-dose groups
was significantly greater than that of the controls (control 27/50,
low-dose 39/50, high-dose 38/50). No compound-related effects on body
weight or feed consumption were observed. A dose-related increased
incidence of basophilic cytoplasmic changes and clear cell changes
were observed in the livers of male rats. The incidence and severity
of nephropathy were reduced in dosed male rats (control 48/50,
severity 2.8; mid-dose 35/50, severity 1.6; high-dose 36/50, severity
1.5). The tumour incidence was not enhanced (NTP, 1989; Dietz et al.,
1991).
2.2.3.2 Chlortetracycline
Rats
Forty rats (sex or strain not specified) were maintained for 52
weeks on a diet containing 1% CTC, equivalent to 1000 mg/kg bw/day.
There was no significant difference in body weight and no evidence of
toxicity was seen.
In a parallel experiment another group of 40 rats (sex or strain
not specified) received 5% CTC, equivalent to 5000 mg/kg bw/day. Ten
animals died within the first 10 weeks. The remaining 30 rats survived
for the 52-week test period and had a 33% lower weight than the
controls, and an apparent yellow staining of all tissues (Hines, 1956).
Groups of Sherman rats (20/sex/group) were fed diets containing
CTC at concentrations of 0, 1, 5, 20, 100, 500, 2000, 10000 or
50000 mg/kg of feed, equal to 0, 0.07, 0.35, 1.3, 7, 34, 130, 700 or
5200 mg/kg bw/day, respectively. Observations were made on mortality,
clinical signs, body-weight gain, food consumption, haematology,
macroscopy, organ weight, and microscopy.
During the first 12 weeks of the study, 8 male rats in the
highest dose group died compared to none in the other groups. At
termination, the total mortality for males was not different from
controls. Both males and females in the 50000 mg/kg group showed signs
of GI irritation which included abdominal distension, nose and mouth
encrustation and salivation. Body-weight gain was reduced in both
sexes at 50000 mg/kg. WBC was reduced in both sexes at 50000 mg/kg.
Microscopic changes at 50000 mg/kg included accumulation of yellow
pigment in splenic lymph follicles for both sexes, testicular atrophy
occasionally with degenerative changes in seminiferous tubulus, fatty
infiltration of the liver in males and infiltration of monocytes
sometimes coupled with interstitial fibrosis in the lungs in both
sexes. Fluorometric determinations showed the presence of significant
amounts of CTC at dose levels of 500 mg/kg of feed and higher. No
blood or urine biochemistry was carried out. The tumour incidence was
not enhanced. The NOEL in this study was 10000 mg/kg diet, equal to
700 mg/kg bw/day (Dessau & Sullivan, 1958; Dessau & Sullivan, 1961).
2.2.4 Reproductive toxicity studies
2.2.4.1 Chlortetracycline
Mice
Mice maintained through 4 reproductive cycles on diets containing
175 mg CTC/kg of feed (equivalent to 25 mg/kg bw/day) did not differ
significantly from parallel control groups in the number of young born
per litter, the number of surviving pups or the average body weight at
the end of 4 weeks (Hines, 1956).
Rats
Rats maintained through 2 reproductive cycles on a diet
containing 45 mg CTC/kg of feed (equivalent to 2 mg/kg bw/day) did not
differ significantly from parallel control groups in the number of
young per litter, the number of surviving pups or the average body
weight at the end of 4 weeks (Hines, 1956).
A 2-generation reproductive toxicity study was carried out with 2
groups of 5 male and 15 female rats (Sherman strain, approximately
21-day old). One group received the control diet and the other
received the same diet containing 1% CTC, equivalent to 500 mg/kg
bw/day. When the rats were approximately 100-day old, the animals were
mated to produce the first generation. All litters were reduced to 8-9
rats. Pups were weaned to the diet given to their parents. Second
generation breeding was carried out with 21 female and 7 male rats in
the 1% group and a few more rats per group in the control group.
Observations were made for clinical signs, body weight, food
consumption, fertility, reproductive performance and pup growth. Male
body weights were slightly lower than controls for both parental
generations. No further toxicity, nor effects on male or female
reproduction were observed (Hallesy & Hine, 1964).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Tetracycline
Groups of 16 pregnant Wistar rats were given 0 or 150-200 mg TC
chlorhydrate/rat/day from days 1 to 18 of gestation (Group I) and
another group received the same treatment from days 1 to 28 after
delivery (Group II). At day 20 of gestation, 3 females of group I and
3 control females were sacrificed and the uterine contents examined.
The remaining rats were allowed to deliver their litters and the
offspring were killed and developmental effects investigated. No
effects were observed on gestation duration, body size or weight, and
no malformations were observed. Pups of group II exhibited a 15%
reduction in the length of the legs in comparison with the controls at
28 days of age. Examination by UV light of the pups skeleton revealed
typical TC-associated fluorescence, indicating that TC had been
absorbed in the bones (Hurley & Tuchmann-Duplessis, 1963).
Sprague-Dawley male and female rats were given 500 mg/kg TC-HCl
in the feed, equivalent to 25 mg/kg bw/day, starting 3 days prior to
mating. Groups of 15-20 pregnant rats continued to receive the
treatment throughout gestation. Some of the pregnant rats (number not
given) were sacrificed on day 21 of gestation and the contents of the
uteri were examined. The remainder of the dams were allowed to deliver
their litters. No significant effects were observed on resorptions,
pregnancy rate, offspring mortality, litter size or weight. At
external skeletal and visceral examinations, the only treatment-
related effects observed were in animals delivered by section and
included an increase in the occurrence of hydroureter (14%;
control:0.8%) and fragmented lumbar (3%; control:0%). In animals
delivered naturally, the incidence for hydroureter was 15% and 12% for
control and treated rats, respectively (McColl et al., 1965).
Pregnant Wistar rats (number not given) were orally dosed from
days 1 to 21 of gestation with TC or TC-HCl at doses of 54, 270 or
540 mg/kg bw/day, and 40, 200 or 400 mg/kg bw/day, respectively. All
rats were sacrificed on day 21, and fetuses were removed and examined
for skeletal anomalies. A reduction of ossification in the anterior
extremities, but not in the posterior ones was observed, more
frequently in the fetuses of the highest dose groups (Szumigowska-
Szrajber & Jeske, 1970).
The development of rat embryonic tissue, transplanted in athymic
(nude) mice was studied for several weeks. Limb buds of fetal rats
were cut into small pieces and transplanted into the subcutaneous
tissue of the back of female nude mice. On the 7th, 9th and 11th days
after grafting, the host mice were treated intraperitoneally with 1500
or 3000 mg/kg TC suspended in 0.55% CMC. Control mice were treated
with the vehicle only. On day 20, the grafted tissue was examined
macroscopically and histologically. No treatment-related effects were
observed on the growth of the grafted limbs or on the differentiation
of the grafts (Shiota et al., 1990).
Groups of 5 female rabbits (strain unspecified) and groups of 5
female Wistar rats were injected i.v. from days 10 to 20 of pregnancy
with 10 mg/kg bw/day of TC or CTC. Rabbit and rat neonates did not
show significant variations in weight between the groups or any
malformation (examinations in UV-light), however, the study was very
limited (Tubaro, 1964).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with TC and CTC are given
in Tables 3 and 4, respectively.
2.2.7 Special studies on microbiological effects
The TCs are primarily bacteriostatic antibiotics inhibiting
protein synthesis in susceptible microorganisms. Following transport
through the cytoplasmic membrane, TCs bind, predominantly reversibly
to the 30 S sub-unit of bacterial ribosomes, thereby preventing access
of aminoacyl tRNA to the acceptor site on the mRNA-ribosome complex
(Sande & Mandell, 1990; Pratt & Fekety, 1986).
The TCs possess a wide range of antimicrobial activity against
Gram-positive and Gram-negative bacteria. They are also active against
some microorganisms that are resistant to agents that exert their
effects on the bacterial cell wall such as Rickettsiae, Mycoplasma,
Chlamydia, some atypical mycobacteria and amebae. They have little
activity against fungi (Sande & Mandell, 1985).
Up to 50% of the antimicrobial activity of CTC can be inhibited
by the presence of magnesium sulfate. The relationship between CTC
inhibition and the magnesium sulfate concentration (50-200 mM) is
linear (Chiang, 1982)
Table 3. Results of genotoxicity studies on tetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S.typhimurium 0-10 µg/plate negative1 NTP, 1989
TA100, TA98 toxic > 3 µg/plate
TA1535, TA1537
Mouse lymphoma L5178Y/TK+/- -S9:25-300 µg/ml negative1 NTP, 1989
assay cells toxic > 200 µg/ml
+S92:10-120 µg/ml negative1
+S93:20-120 µg/ml weakly
positive1
Sister chromatid Chinese hamster -S9:5-49.9 µg/ml negative1 NTP, 1989
exchange assay ovary cells toxic >40.2 µg/ml
+S9:302-600 µg/ml negative1
toxic 600 µg/ml
Chromosome Human 10 µg/ml equivocal Meisner et al.,
aberration assay lymphocytes 1977
Chromosome Chinese hamster -S9:39.9-400 µg/ml negative1 NTP, 1989
aberration assay ovary cells toxic 400 µg/ml
+S9:1000-2750 µg/ml negative1
Table 3. Results of genotoxicity studies on tetracycline (cont'd)
Test system Test object Concentration Result Reference
Gene mutation FM3A cells from 10-100 µg/ml positive4 Tsutsui, et al.,
C3H mice 1976
In vivo
Chromosome Allium cepa 12-20 µg/ml5 positive Mann, 1978
aberration assay
SLRL assay Drosophila injection: NTP, 1989
melanogaster 5000-5300 ppm negative
feeding:
9005 ppm negative
1 with and without metabolic activation
2 S9 prepared from the liver of Aroclor 1254-induced F344 rats
3 S9 prepared from liver of non-induced F344 rats
4 induction of an 8-azaguanine-resistant mutation chromosomal damage
inhibition of protein and DNA synthesis
5 TC pure was used as test substance
Therapy with TCs is associated with a high rate of development of
supra-infections with bacteria, yeast or fungi not sensitive to these
agents, particularly in patients with diabetes and other conditions
that reduce the natural host defense mechanisms
(Pratt & Fekety, 1986).
2.2.7.1 In vitro studies
MIC values for TC for different bacterial strains of animal
origin are given in Table 5.
MICs for TC, CTC and OTC, ranging from 0.1- > 100 µg/ml, have
been reported for a number of clinical isolates, including
Staphylococcus spp., Proteus spp. and E. coli. The data indicate
that the antimicrobial potency of the three TCs for various
micro-organisms is quite similar (Walter & Heilmeyer, 1975).
The antimicrobial potency of CTC in relation to that of OTC was
established in 34 isolates of 3 different animal pathogens (P.
hemolytica, P. multocida and B. bronchiseptica). The geometric mean
for the MICs was 0.32 µg/ml for CTC and 0.52 µg/ml for OTC. For
E. coli (only one isolate), the MIC was 4.8 µg/ml for CTC, and
2.8 µg/ml for OTC (Rogalski, 1985; Gustafson, 1995).
The MIC observed for S. aureus in vitro was 0.19 µg/ml for CTC,
0.21 µg/ml for TC, and 0.55 µg/ml for OTC (Fourtillan & Lefebvre,
1985).
In in vitro studies with E. coli of animal and human origin,
MIC50 values of 2 µg/ml for CTC and 4 µg/ml for OTC were found. MIC
values for E. faecalis and E. faecium also indicated a 2-fold
higher potency for CTC than for OTC (Devriese, 1994).
The antimicrobial activity of TC was compared with that of OTC in
a range of bacterial species (10 strains/species) isolated from faeces
of healthy humans. The concentrations of TC and OTC in the MIC
determinations ranged from 0.06 to 32 µg/ml. The bacterial density was
107 colony forming units/ml. The results are presented in Table 6.
The sensitivity of the strains of most bacterial species was similar
for TC and OTC, whereas TC was active at lower concentrations for
Bifidobacterium spp., Eubacterium spp. (p<0.05), and Fusobacterium spp.
(p<0.1), and at higher concentrations for Spectrococcus spp. (p<0.05).
The geometric mean MIC, taking into consideration all tested strains
(using a value of 32 µg/ml for resistant strains) was 3.2 µg/ml for
TC and 3.8 µg/ml for OTC (Richez, 1994).
Table 4. Results of genotoxicity studies on chlortetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S. typhimurium 0.1-15 µg/plate; negative1,2 Mulligan, 1989
TA100,TA98,TA1535 conc > 1.0 µg
TA 1337, TA1538 were toxic
E. coli WP-2uvrA-
Amest test S. typhimurium up to 10 µg/plate; negative1 Sharma et al., 19893
TA100, TA98, toxicity was
TA1535, TA1537 seen
E. coli WP-2uvrA-
HGPRT assay CHO cells +act: 20-100 µg/ml negative Harbell, 1988
-act: 25-125 µg/ml
toxicity was
observed at the
highest conc.
UDS rat hepatocytes 25-75 µg/ml; negative Thilagar, 1988
toxicity was seen
at the highest dose
Table 4. Results of genotoxicity studies on chlortetracycline (cont'd)
Test system Test object Concentration Result Reference
In vivo
chromosome rat orally 500, 2500, negative Putman, 1988
aberrations 5000 mg/kg bw4
chromosome Vicia sativa 1% solution negative Sharma &
aberrations Lens esculenta " equivocal Bhattacharyya, 1967
1 with and without metabolic activation
2 no positive controls were used; background was not given
3 abstract only
4 no effects on mitotic index
Table 5. Summary of MIC values for different bacterial species for
tetracycline (Pratt & Fakety, 1986)
Species MIC (µg/ml)1
Escherichia coli 12.5 (3.1-500)
Proteus mirabilis >100 (50->100)
Enterobacter 25 (6.3-50)
1 Values given are the mean (range in parenthesis)
2.2.7.2 In vivo studies
The in vivo activity of S. aureus in mice was comparable for
the three different TCs; ED50 values were 7.6, 7.2 and 5.8 mg/kg bw,
for CTC, TC and OTC, respectively (Fourtillan & Lefebvre, 1985)
2.2.8 Special studies on eye irritation and sensitization
CTC produced local irritation when injected i.p., i.m. or s.c. or
when administered directly to the rabbit eye. Eye irritation from 1%
solutions was mild and completely reversible within 48 h (Harned et
al., 1948).
2.2.9 Other studies
2.2.9.1 Cardiovascular effects
Six anaesthetized male rabbits were given i.v. injections of 1, 2
or 5 mg TC-HCl/kg bw dissolved in 0.5 ml saline. The injections were
performed in 3, 10, 20 or 60 seconds. A dose- and injection
time-dependent slowing of the heart rate (from 270-300 (normal) to 100
beats/min or less) was observed 1-3 min after the injection. No effect
on arterial blood pressure was found.
Respiration was depressed which led at high doses to respiratory
arrest or to a slow, shallow respiration for 1-2 min (Gyrd-Hansen,
1980).
Table 6. MIC values (µg/ml) for tetracycline and oxytetracycline
against bacterial species obtained from human isolates.
TETRACYCLINE OXYTETRACYCLINE
Bacterial species MIC50 geometric MIC50 geometric
mean mean
Escherichia coli > 32 > 32 > 32 > 32
Bifidobacterium spp. 16 8.6 > 32 > 17.1
Bacteroides fragilis 4 2.5 4 2.5
Eubacterium spp. 2* 1.6 4 2.6
Clostridium spp. 0.062 0.2 0.25 0.2
Streptococcus spp. 16 > 19.7 8 > 13.9
Fusobacterium spp. 0.125 0.1 0.25 0.2
Lactobacillus spp. 2* 1.9 2 2.3
Proteus spp. > 32 > 32 > 32 > 32
Peptostreptococcus 2 3.2 4 4.0
spp.
* MIC50 = MIC90
2.2.9.2 Hepatotoxicity
TC-HCl (>0.25 mM) inhibited reversibly the ß-oxidation of
fatty acids as well as the tricarboxylic acid cycle activity of mouse
and human liver mitochondria in vitro. After i.p. administration of
1 mmol/kg bw to mice following an oral administration of a tracer dose
of [U-14C]palmitic acid, an increase in the levels of hepatic
triglycerides was observed and liver histology showed microvesicular
steatosis (Freneaux et al., 1988).
The effects of TC on the synthesis and secretion of triglycerides
and proteins were studied in isolated rat hepatocytes. TC produced a
concentration-dependent inhibition of 14C-triglyceride secretion
without affecting triglyceride synthesis and protein secretion
(Deboyser et al., 1989).
2.2.9.3 Nephrotoxicity
Groups of Wistar albino rats were given single i.v. injections of
150 mg TC/kg bw into the tail vein. Different experiments failed to
demonstrate any significant ischaemia in the kidneys (Tapp & Lowe,
1966)
2.3 Observations in humans
2.3.1 Effects after medical treatment
In human therapy, the usual oral dose for TC-HCl is 250 or 500 mg
every 6 h for adults as specified in the US National Formulary and
British Pharmacopoeia. CTC is approved as capsules (50, 100 and
250 mg) and by injection (100, 200 and 500 mg).
A variety of adverse effects in humans have been reported from
the use of TCs. The TCs are irritating substances. When given i.v.
they can cause thrombophlebitis. Intramuscular administration is
painful and not recommended. Given orally, TCs can cause epigastric
burning, abdominal discomfort, nausea and vomiting (Schindel, 1965;
NTP, 1989; Sande & Mandell, 1990).
Bone and teeth discoloration are known to occur in humans under
clinical treatment with high levels of TC drugs. Children receiving
long- or short-term therapy with a TC may develop a yellow or brownish
discoloration of the enamel. The duration of therapy appears to be
less important than the total quantity of antibiotic administered. The
risk of this effect is highest when the TC is given to neonates and
babies prior to the first dentition. However, pigmentation of the
permanent dentition may develop if the drug is given between the ages
of 2 months and 8 years, when the teeth are being calcified. An early
characteristic of this effect is a yellow fluorescence of the dental
pigment.
Treatment of pregnant patients with TCs may produce
discolouration of the teeth in their children, but exposure also
occurs during breast feeding. (Schindel, 1965; Piper et al., 1988;
Friedman, et al., 1990; Sande & Mandell, 1990).
TCs are deposited in the skeleton during gestation and throughout
childhood. A 40% reduction in bone growth, as determined by
measurements of fibulas, was demonstrated in premature infants treated
with these agents. This effect is readily reversible if the period of
exposure to the drug is short (Sande & Mandell, 1990; Pratt & Fekety,
1986).
No toxic effects were reported from treatment of premature
infants, children, adults or elderly with CTC. Effects after short-
and long-term exposure included increased body-weight gain, and
prophylactic activity against acute and chronic infections and
diarrhea (Hines, 1956).
All the TCs (dose levels not specified) can cause phototoxicity,
manifested by mild to severe skin reactions when the skin is exposed
to direct sunlight (Sande & Mandell, 1990; Schindel, 1965).
Liver effects, characterized by fatty accumulation have been
observed, predominantly following repeated parenteral administration
of TCs (dose levels were not specified). TCs can aggravate uremia in
patients with impaired renal function. For unknown reasons, pregnant
woman appear to be more susceptible to the development of hepatic
damage (Pratt & Fekety, 1986; Sande & Mandell, 1990; Friedman,
et al., 1990).
TCs rarely cause allergic reactions. Hypersensitivity to TCs have
been described as allergic manifestations of mucosal tissues. Central
nervous system side effects and effects on blood cells were rarely
described. Long-term therapy (dose levels not specified) with TCs may
produce changes in the peripheral blood. Leucocytosis, atypical
lymphocytes, toxic granulation of granulocytes, and thrombocytopenic
purpura have been observed.
The TCs may cause increased intracranial pressure and tense
bulging of the fontanels (pseudotumor cerebri) in young infants, when
given in therapeutic doses. Young adults complain of headache, nausea,
vomiting and diplopia.
TC is given orally in low doses (usually 100 mg/person/day) for
the treatment of chronic severe acne vulgaris. A common complication
of treatment with TCs is minor GI irritation (Sande & Mandell, 1990;
Pratt & Fekety, 1986).
3. COMMENTS
Toxicological data
The Committee considered data from studies on pharmacokinetics,
biotransformation, acute and short-term toxicity, reproductive
toxicity, teratogenicity, long-term toxicity/carcinogenicity,
genotoxicity, antimicrobial effects and observations in humans.
Aspects of the comparative toxicity of these compounds with OTC were
also considered.
Pharmacokinetic studies showed that CTC and TC were rapidly
absorbed following oral administration. Peak plasma concentrations
were higher in rats receiving TC than in those receiving the same dose
of CTC. In humans receiving oral therapeutic doses, 30% of the dose of
CTC was absorbed, as compared with 60-80% of the dose of TC. TC is
excreted mainly in urine, whereas CTC is excreted in urine and faeces
in comparable amounts. After absorption following administration by
various routes, CTC and TC are widely distributed in the body, the
highest levels are present in the liver and kidney. Detectable levels
of TCs in bone persist for more than 28 weeks after dosing. The plasma
half-lives of CTC and TC in humans are about 5 and 8 h, respectively.
TCs have been found in the breast milk of women receiving therapeutic
doses and can cross the placenta.
Orally administered CTC and TC have low acute toxicity in rats
and mice, LD50s ranging from 2150 to >5000 mg/kg bw.
In a 13-week range-finding study with mice in which TC was
incorporated in the diet at concentrations equivalent to 470, 950,
1800, 3700 or 7500 mg/kg bw/day, the only sign of toxicity observed
was a decrease in body weight in the highest-dosed group. In a 13-week
range-finding study in which rats were dosed at 155, 315, 625, 1250 or
2500 mg TC/kg bw/day, vacuolization of liver cells and bone marrow
atrophy were observed at 1250 and 2500 mg/kg bw/day. No toxicity was
observed in dogs given TC orally at dose levels of up to
250 mg/kg bw/day for 24 months.
CTC did not cause any toxic effects in short-term toxicity
studies with rats or mice at doses of up to 200 mg/kg bw/day. In dogs
given CTC orally at a dose of 250 mg/kg bw/day for 98 days, increased
incidences of mortality, fatty liver and bone marrow atrophy were
observed. In another experiment in dogs which received CTC orally at
doses of up to 100 mg/kg bw/day for 54 weeks, no effects were
observed, except for signs of GI disorders; these were considered of
minor importance because the increase in body-weight gain was normal.
In carcinogenicity studies with mice and rats, TC was administered
in the diet at doses of up to 3500 and 1060 mg/kg bw/day, respectively.
Survival rates were increased in male mice, while body weights were
slightly lower than controls for both sexes. Survival rates were also
increased in female rats, but male rats showed a dose-related increase
in basophilic cytoplasmic changes and clear cell changes in the liver.
There were no increases in tumour incidence in mice or rats.
In a chronic toxicity/carcinogenicity study, CTC was administered
in the diet of rats at doses varying from 0.07 to 5200 mg/kg bw/day.
In the highest dose group, GI irritation, decreased body-weight gain
and a decreased number of white blood cells were observed in both
sexes, while testicular atrophy was observed in males. Microscopic
changes in the highest-dose group included infiltration of monocytes
in the lungs of males and females, and fatty changes in the liver in
males. Tumour incidence was not increased. The NOEL was 700 mg/kg
bw/day. The Committee concluded that there was no evidence that CTC or
TC is carcinogenic.
In a two-generation reproductive toxicity study in which CTC was
administered to rats in the diet at a dose equivalent to 500 mg/kg
bw/day, no effects on reproductive performance were observed.
Reproductive toxicity data were not available on TC. In a number of
teratogenicity studies with rats, TC and TC hydrochloride caused a
reduction in ossification at the highest oral dose tested, 540 or
400 mg/kg bw/day, respectively. No irreversible structural changes
were found on skeletal or visceral examination. In studies with a
limited number (5 per group) of rats and rabbits, no evidence of
teratogenicity was found at intravenous doses of 10 mg of CTC or TC/kg
bw/day, administered on days 10-20 of pregnancy. The Committee
concluded that CTC and TC did not cause significant toxic effects on
either reproduction or development.
The genotoxicity of CTC and TC was investigated in a wide range
of in vitro and in vivo test systems. CTC gave negative results in
all tests. TC was negative in the majority of in vitro and in vivo
assays, but induced gene mutations in one in vitro test in mammalian
cells and chromosomal aberrations in plants. The Committee considered
these effects to be due to the inhibiting effect of TCs on protein
synthesis, based on their binding to ribosomes, and concluded that CTC
and TC do not pose a genotoxic hazard.
Microbiological data
In vitro studies of the antimicrobial effect of TCs on
pathogenic microorganisms of animal origin indicate that the
activities of CTC and OTC are not significantly different. MIC values
(geometric mean) for CTC and OTC were 0.32 and 0.52 µg/ml,
respectively.
In a limited number of microorganisms representative of human gut
flora, the antimicrobial activity of CTC was of the same order as that
of OTC.
The antimicrobial activity of TC was compared with that of OTC
using a wide range of bacterial species isolated from the faeces of
healthy human volunteers. The sensitivity of most species was similar
for TC and OTC except for Bifidobacterium, Fusobacterium and
Eubacterium species which were slightly more sensitive to TC, and
Streptococcus spp. which were slightly more sensitive to OTC. The
geometric mean MICs for all tested strains were 3.2 µg/ml for TC and
3.8 µg/ml for OTC.
4. EVALUATION
After reviewing the available toxicological and antimicrobial
data, the Committee concluded that the antimicrobial data provided the
most appropriate end-point for the evaluation of CTC and TC.
In view of the similarity of the antimicrobial activity of TC,
CTC and OTC, the Committee established a group ADI of 0-3 µg/kg bw for
the three drugs separately or in combination. This ADI was established
for OTC at the thirty-sixth meeting of the Committee (Annex 1,
reference 91), and is based on the NOEL of 2 mg/person/day for the
effects of OTC on the gut flora in human volunteers and a safety
factor of 10.
The Committee noted that this ADI provides an adequate margin of
safety when compared with the lowest NOEL for toxicological effects of
100 mg/kg bw/day for CTC in dogs.
5. REFERENCES
Adir, J. (1975) Enterohepatic circulation of tetracycline in rats.
J. Pharm. Sci, 64(11) 1847-1850.
Bacharach, A.L., Clark, B.J., McCulloch, m. & Tomich, E.G. (1959)
Comparative toxicity studies on ten antibiotics in current use.
J. Pharm. Toxicol. 11: 737-741.
Berté, F. & Vandoni, G. (1962) On the intestinal absorption and
organotropism of some tertacyclines. Chemotherapia 5, 219-230.
Buyske, D.A., Eisner, H.J. & Kelly, R.G. (1960) Concentration and
persistence of tetracycline and chlortetracycline in bone.
J. Pharm. Exper. Therap. 130, 150-156.
Chiang, T (1982) Inhibition of chlortetracycline activity by magnesium
ions. J. Assoc. Off. Anal. Chem., 65 (5), 1044-1047.
Cunningham, R.W., Hines, L.R., Stokey, E.H., Vessey, R.E. & Yuda, N.N.
(1954) Pharmacology of tetracycline. Antibiotics Annual, 63-69.
Deboyser, D., Goethals, F., Krack, G. & Roberfroid, M. (1989)
Investigation into the mechnism of tetracycline-induced steatosis:
study in isolated hepatocytes. Tox.appl.pharmacol., 97, 473-479.
Deichmann, W.B., Bernal, E., Anderson, W.A.D., Keplinger, M.,
Landeen, K., Mcdonald, W., Mahon, R. & Stebbins, R. (1964) The chronic
oral toxicity of oxytetracycline HCl and tetracycline HCl in the rat,
dog and pig. Ind.Med.Surg., 787-806.
Dessau, F.I. & Sisson, G., (1957) Aureomycinr life studies. Studies
of chlortetracycline hydrochloride (food grade in dogs). Project
number 77601. Unpublished report of American Cynamid Company.
P.R. 3, 440-493. Research Division. Pearl River Laboratories.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Dessau, F.I. & Sullivan, W.J., (1958) Aureomycinr life studies.
Studies of chronic toxicity of CL 13,555: Chlortetracycline
hydrochloride (food grade) in rats. Project number 77601 American
Cyanamid Company, Experimental Pathology Research Division, Pearl
River Laboratories. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Dessau, F.I. & Sullivan, W.J. (1961) A two year study of the toxicity
of chlotetracycline hydrochloride in rats. Toxicol and Appl.
Pharmacol., 3, 654-677.
Devriese, L.A. (1994) Comparative minimal inhibitory concentration
determinations with chlortetracycline and oxytetracycline on gut
bacteria of animal and human origin. Faculty of Veterinary Medicine.
University of Gent, Belgium. Submitted to WHO by American Cyanamid
Company, Princeton, USA.
Diermeier, H.F. (1959) Acute oral and intravenous toxicity of rats of
a A-VIII hydrochloride (CL 22,416), chlortetracycline hydrocholoride
(CL 13,555), and tetracycline hydrochloride (CL13,554). P.R. 5,
899-907. Unpublished report of American Cyanamid Company, Lederle
laboratories, Pearl River, New York. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Dietz, D.D., Abdo, K.M., Haseman, J.K., Eustis, S.L. & Huff, J.E.
(1991) Comparative toxicity and carcinogenicity studies of
tetracycline and oxytetracycline in rats and mice. Fund. Appl.
Toxicol. 17 (2), 335-346.
Eisner, H.J. et al., (1953) The enhancement of serum levels of
Aureomycin experimental animals. J. Pharmacol. Exp. Ther.
108, 442-449.
Eisner, H.J. & Wulf, R.J. (1962) The metabolic fate of
chlortetracycline and some comparisons with other tetracyclines.
J. pharmacol, exp. therap., 142, 122-11.
Fourtillan & Lefebvre (1985) cited in Rogalski, W. (1985) Chemical
modifications of the tetracyclines. In: The tetracyclines. Eds.
Hlavka and Boothe. Springer Verlag, Berlin.
Freneaux, E., Labbe, G., Letteron, P. The Le Dinh, T.L., Degott, C.
Geneve, J. Larrey, D. & Pessayre, D. (1988) Inhibition of the
mitochondrial oxidation of fatty acids by tetracycline in mice and in
man: possible role in microvesicular steatosis induced by this
antibiotic. Hepatology, 8(5), 1056-1062.
Friedman, J.M., Little, B.B., Brent, R.L., Cordero, J.F., Hanson,
J.W., & Shepard, T.H., (1990) Potential human teratogenicity of
frequently prescribed drugs. Obstetics and Gynecol, 75, 594-599.
Goldenthal, E.I. (1971) A compilation of LD50 values in newborn and
adult animals. Tox. and Appl. Pharm, 18, 185-207.
Gustafson, R.H. (1995) MIC tests of veterinary clinical isolates,
tetracycline. Memo to D.L. Ingle, d.d. january 11, 1995. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Gyrd-Hansen, N (1980) The effect of tetracyclines on the rabbit heart.
Zbl.vet.med. A, 27, 228-237.
Halliday, S.L. & Fanelli, G. (1960) Antibacterial tetracycline:
relative toxicity of CL 13,555, chlortetracycline hydrochloride and
CL 30,782, sodium benzoate orally in rats. Project number 14797.
P.R. 6, 1428-1439. Unpublished report of American Cyanamid Company,
Lederle Laboratories, Pearl River, New York, Submitted to WHO by
American Cyanamid Company, Princeton, USA.
Harned, B.K. Cunningham, R.W. Clark, M.C., Cosgrove, P., Hine, C.H.,
Mccauley, W.J., Stokey, E., Vessey R.E., Yuda, N.N. & Subbarow, Y.
(1948) The pharmacology of Duomycin. Ann. NYAS 51, 182-210.ALLESY,
D.W. & HINE, C.H., (1964) The effect of chlortetracycline
hydrochloride on the fertility and growth of rats.
Tox. and Appl. Pharm. 6, 9-14.
Harbell, J.W. (1988) CHO/HGPRT mutation assay. Study number
T8133.332021. Unpublished report of Microbilogical Associates, 1988.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Hines, L.R. (1956) An apprasal of the effects of long-term
chlortetracycline administration. Antibiotics. chemother.,
6 (11), 623-641.
Hurley, L.S. & Tuchmann-Duplessis, H (1963) Influence de la
tetracycline sur le developpement pré- et post-natal du rat.
Compt. rend. acad. sci., 257, 302-304.
Kanegis, L. (1958) The comarative pharmacology of tetracyclines:
initial studies on serum levels and urinary excretion of antibiotic
A-VIII following single oral and intravenous doses in the dog. Report
P.R. 4:884-921. American Cyanamid Company (Pearl River). Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Kelly, R.G. (1964) Antibacterial tetracyclines. In tissue distribution
of tetracycline and chlortetracycline. American Cyanamid Company
Report P.R. 9:485-492 (Pearl River). Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Kelly, R.G. & Buyske, D.A. (1960) Metabolism of tetracycline in the
rat and the dog. J. Pharmacol. Exp. Therap., 130, 144-149. Submitted
to the WHO by American Cyanamid Company, Princeton, USA.
Kniffen, T.S., Bane, D.P., Hall, W.F., Koritz, G.D. & Bevill, R.F.
(1989) Bioavailability, pharmacokinetics, and plasma concentration of
tetracycline hydrochloride fed to swine. Am. J. Vet. Res.
50, 518-521.
Levinskas, C.J. (1963) Auromycinr and formulation: Acute oral and
intravenous toxicity. Central Mediacal Department Report
No. 6305, 53-5,. Submitted to the WHO by American Cyanamid Company,
Princeton, USA.
Lowe C.A. & Fisher J. (1993) Rat oral LD50 study with Aureo Biomass.
Report number A93-61. Data from American Cyanamid Company, Agricultural
Research Division. Submitted to WHO by American Cyanamid Company,
Princeton, USA. (summary only)
Maffii, G. & Mainardi, L. (1955) Studio Farmacologico su un nuovo
antibiotico: la tetraciclina. Il Farmaco Ed. Sci 10 (4), 197-210.
Mann, S.K. (1978) Interaction of tetracycline (TC) with chromosomes in
Allium cepa Environm. exp. botany. 18, 201-205.
Martindale (1989) The Extra pharmacopoeia. 29th edition. Eds. J.E.F.
Reynolds. London, The Pharmaceutical Press.
Mccoll, J.D. Globus, M. & Robinson, S (1965) Effect of some therapeutic
agents on the developing rat fetus. Tox. appl. pharmacol. 7,
409-417.
Meisner, L.F., Chuprevich, T.W. & Inhorn, S.L. (1977) Mechanisms of
chromatid breakage in human lymphocyte cultures. Acta. cytol.
21(4), 555-558.
Milch, R.A., Rall, D.P. & Tobie, J.E. (1967) Bone localization of the
tetracyclines. J. Nat. Canc. Inst. 19, 87-91.
Mulligan, E. (1989) Microbial mutagenicity test report for screening
study MMR89-026. American Cyanamid Company, Report number MMR89-026,
20 April 1989. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Nelson, A.A. & Radomski, J.L. (1954) Comparative pathological study in
dogs of feeding of six broad-spectrum antibiotics. Antibiotics and
Chemotherapy IV, (11), 1174-1180.
Neuschl, J. (1991) Comparison of some pharmacokinetic parameters of
tetracyclines which are most frequently used in veterinary medicine.
Arch. Exp. Veterinarmed. 45, 105-112.
NTP (1989) NTP technical report on the toxicology and carcinogenesis
studies of tetracycline hydrochloride (Cas no. 64-75-5) in F344/N rats
and B6C3F1 mice (feed studies) NTP toxicology program P.O. Box 12233.
NTP TR 344 NIH Publication No. 89-2600. U.S. Department of health and
human services.
Piper, J.M., Baum, C., Kennedy, D.L. & Price, P. (1988) Maternal use
of prescribed drugs associated with recognized fetal adverse drug
reactions. Am. J. Obstet. and Gynecol. 159 (5) 1173-1177.
Pratt, W.B. & Fekety, R. (1986) The antimicrobial drugs. Oxford
University Press, New York, pp 205-228.
Putman, D.L. (1988) Acute in vivo cytogenetics assay in rats. Final
report. Report number T8133.105003. Unpublished report of
Microbiological Associates, 11-7-1988. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Richez, P. (1994) Antibacterial activity of tetracycline and
oxytetracycline against human gut flora. Study No. SVL 029.
Unpublished report of Datavet Veterinary Drug Consultants,
May 31 1994. Submitted to WHO by Intervet International,
Boxmeer, Netherlands.
Rogalski, W. (1985) Chemical modifications of the tetracyclines.
Chapter 5, pp 233-. In: The tetracyclines. Eds. Hlavka and Boothe.
Springer Verlag, Berlin.
Sande, M.A. & Mandell, G.L. (1990) Antimicrobial agents:
tetracyclines, chloramphenicol, erythromycin and miscellaneous
antibacterial agents. In: Goodman and Gilman's The pharmacological
basis of therapeutics, 8th edition. Gilman, A.G., Rall, T.W., Nies,
A.S. and Taylor, P. eds. Pergamon Press, New York, (1990),
pp 1117-1145.
Schindel, L.E. (1965) Clinical side-effects of the tetracyclines.
Antibiotica et Chemotherapia Advances 13, pp. 300-316.
Sharma, A.K. & Bhattacharyya, G.N. (1967) A study on the respomse of
chromosomes to antibiotic treatment. Acta Biol. Acad. Sc. Hung.
18 (1), 67-75.
Sharma, R.K., Traul, K.A., Caterson, C.R., Harbell, J., Thilagar, A. &
Putman, D. (1989) Genotoxicity evaluation of chlortetracycline.
Environ. Mol. Mutagen 14 (suppl. 15), 183. (summary only).
Shiota, K., Uwabe, C. Yamamoto, M. & Arishima, K. (1990) Teratogenic
drugs inhibit the differentiation of fetal rat limb buds grafted in
athymic (nude) mice. Reprod. toxicol. 4, 95-103.
Szumigowska-Szrajber, G. & Jeske J. (1970) Effect of tetracycline
base, tetracycline hydrochloride and oxytetracycline base on
developmental changes in metatarsal bones of extremities of rat
fetuses. Acta polon. pharm. 27 (I), 85-90.
Tapp, E. & Lowe, M.B. (1966) Tetracycline toxicity. Experientia
22, 530-531.
Thilagar, A. (1988) Test for chemical induction of unscheduled DNA
synthesis in rat primary hepatocyte cultures by autoradiography. Test
report 0082-5100. Unpublished report of Sitek Research Laboratories,
22-7-1988. Submitted to WHO by American Cyanamid Company, Princeton,
USA.
Tsutsui, T., Umeda, M., Sou, M. & Maizumi, H. (1976) Effect of
tetracycline on cultured mouse cells. Mutat. res. 40, 261-268.
Tubaro, E. (1964) Possible relationship between tetracycline stability
and effect on foetal skeleton. Brit. J. Pharmacol. Chemotherap.
23, 445-448.
Tubaro, E. & Banci, F. (1964) The pharmacology of chlormethylene-
cycline, a new chlortetracycline. Arzneimittel-Forschung, 14,
95-100.
Tubaro, E., Barletta, M. & Banci, F. (1964) Some pharmacological
aspects of a new water-soluble tetracycline. J.pharm. pharmacol.
16, 33-37.
Walter, A.M. & Heilmeyer, L. (1975) Antibiotika-Fibel. Antibiotika und
Chemotherapeutila Therapie mikrobieller infektionen, pp.323-324.
Georg Thieme Verlag Stuttgart.
Wulf, R. & Eisner, H. (1961) Antibacterial tetracyclines: Metabolism
of chlortetracycline in the rat and in the dog. American Cyanamid
Company Report C.P. 1, 626-683. Experiment P-70-21-FT. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Tetracycline (TC)
Rats
Fasted white rats were given a single oral dose of TC
hydrochloride by gavage corresponding to 75 mg/kg bw TC base. The rats
were sacrificed 1, 2, 3, 4 or 6 h after treatment and plasma and
tissue levels recorded.
Peak plasma concentrations of 3.6 mg/l were observed 2 h after
treatment, declining to 0.5 mg/l after 6 h. Peak tissue levels were
found 2 h after treatment with highest levels in the liver and kidney
(Berté & Vandoni, 1962).
To investigate the importance of the biliary route of excretion,
4 rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw tritium- labelled TC. Radioactivity was measured in
urine, bile and GI tract 24 h following treatment.
In non-ligated rats, 85-92% of the total radioactivity was
recovered of which 67-72% was excreted in urine and 18-20% in faeces.
About 70-85% of the total radioactivity was recovered in the rats with
ligated bile ducts, 68% and 88% being recovered in the urine of each
rat, and 30% and 9% in the bile. Only very small amounts (on average
2.5%) were recovered in the GI tract. When the same dose of TC was
administered to rats with ligated ureters, no increase in the
excretion of TC in the faeces was observed (Wulf & Eisner, 1961).
Male Sprague-Dawley rats were injected with 10 mg/kg bw 3H-7-TC
hydrochloride (98% pure) in the femoral vein over 5 min. The
intestinal absorption of TC hydrochloride excreted in bile was
evaluated using in situ intestinal preparations. About 73% of TC
excreted in the bile was reabsorbed in the intestinal lumen,
indicating enterohepatic circulation (Adir, 1975).
Single i.v. doses of 15 or 4 mg/kg bw tritium-labelled TC
hydrochloride were administered to 2 rats and 1 dog, respectively.
Within 72 h, 69.2% and 19.5% of the radioactivity was recovered in the
urine and faeces of rats, respectively. Within 168 h, 71% of the
radioactive dose was recovered in the urine and 9% in the faeces of
the dog (Eisner & Wulf, 1962).
Dogs
Tritium-labeled TC hydrochloride was administered i.v. to 2
beagle dogs at 10 mg TC/kg bw to study the distribution of TC
throughout the body. The animals were sacrificed after 4 h and the
various organs and tissues examined for radioactivity. The organs
containing the highest radioactivity were liver and kidney, containing
on average 15 and 45 mg/kg, respectively. A large proportion of the
recovered TC activity was found in the urine, intestinal contents and
bile. No radioactivity was measured in subcutaneous fat (Kelly, 1964).
Beagle dogs given single oral doses of 25 mg TC/kg bw showed peak
TC levels averaging 3 µg/ml 2 h after dosing, declining to an average
of 0.27 µg/ml by 24 h post-dosing. Excretion in the urine accounted
for 10% of the administered dose within 72 h after dosing.
When dogs were given a single i.v. dose of 10 mg TC/kg bw,
average serum TC levels of 10.6 µg/ml were found at 24 h, and
0.14 µg/ml at 48 h after dosing. Excretion in the urine was 58% of the
administered dose by 72 h after dosing (microbiological assay;
sensitivity 0.05-0.1 µg/ml) (Kanegis, 1958).
Pigs
The bioavailability of TC hydrochloride administered orally to
fasted gilts was calculated from the area under the plasma
concentration in time curve (AUC) and estimated to be 23%. After i.v.
administration the disposition kinetics of TC in plasma were best
described by a tri-exponential equation. The drug had a rapid
distribution phase followed by a relatively slow elimination phase,
with half-life of 16 h (Kniffen et al, 1989).
2.1.1.2 Chlortetracycline (CTC)
Rats
14C-Labelled CTC was orally administered to rats at a dose of
60 mg/kg bw. Radioactivity was measured in urine and faeces after
24, 48 and 72 h. Radioactivity was found primarily in the faeces
(92% within 72 h, the major part being excreted within 24 h). About 5%
of the radioactivity was recovered in urine.
When 30 mg/kg bw 14C-CTC was administered i.p., 33% of the
radioactivity was excreted in the urine within the first 24 h, and 5%
in the faeces. Between 24 and 72 h, 7% was excreted in the urine and
40% in the faeces.
To investigate the importance of the biliary route of excretion,
rats (2 with ligated bile ducts) were administered single i.v. doses
of 15 mg/kg bw 14C-CTC. Radioactivity was measured in urine, bile
and GI tract, 24 h following treatment.
In non-ligated rats, 75-79% of the total radioactivity was
recovered of which 35-37% was excreted in urine and 44-38% in faeces.
In the rats with ligated bile ducts, about 47-63% of the total
radioactivity was recovered, with 66% and 43% being recovered in the
urine of each rat, and 22% and 51% in the bile. Only very small
amounts (on average 5%) were recovered in the GI tract (Wulf &
Eisner, 1961).
Groups of 6 rats given single oral doses of 75 mg CTC/kg bw had
plasma levels of 2.1 mg/l at 1 h after administration, declining to
0.8 mg/l after 6 h. The rats were sacrificed at 1, 2, 3, 4 or 6 h
after treatment. Tissue levels were highest in liver and kidneys at
all observations. Maximum concentration was found after 2 h in the
liver, and after 1 h in the kidney (Berte & Vandoni, 1962).
Oral doses ranging from 6 to 800 mg CTC/kg bw administered to
female rats and male guinea-pigs gave no proportional increases in
serum CTC concentrations. When guinea-pigs received the same doses
each day for 9 days, higher serum levels were found than with single
doses. Serum CTC levels were increased by the simultaneous
administration of various adjuvants, such as citric acid. This effect
(observed in doses of CTC up to 200 mg/kg bw) was apparent within 1 h
of administration and lasted at least 8 h (Eisner et al., 1953)
Rabbits
Ten adult white Californian rabbits (males and females) received
a single oral dose of 20 mg/kg bw of technical CTC or CTC-chloride.
Serum CTC levels averaged 2.3 mg/l 3 h after dosing. The concentration
of CTC declined to a mean value of 0.09 mg/l by 12 h, and to 0.08 mg/l
by 24 h after dosing. The highest concentrations were found in the
liver (1.53 mg/kg) 24-h post-dosing, followed by kidney, lung and
heart. No measurable levels were found in muscle (detection limit:
37.5 µg/kg) (Neuschl, 1991)
Dogs
Four beagle dogs were given a single oral dose of 25 mg
CTC/kg bw. Peak serum CTC levels ranged from 0.40-1.9 mg/l 2 h after
dosing, declining to an average of 0.21 mg/l by 24 h. When the dogs
were given an i.v. injection of 10 mg CTC/kg bw, serum levels averaged
6.6 mg/l 1 h after administration, declining to 2.4 µg/ml at 8 h,
0.29 µg/ml at 24 h and 0.06 µg/ml at 48 h (Kanegis, 1958).
Two female beagle dogs received a single i.v. injection of 10 mg
14C-CTC/kg bw. The dogs were sacrificed 4 h after dosing. The organ
containing the highest radioactivity was the liver (30 mg/kg),
followed by kidney (25 mg/kg), ileum (15 mg/kg), jejunum (12 mg/kg)
and heart (10 mg/kg). A large proportion of the recovered
radioactivity was found in urine, intestinal contents and bile. With
the exception of subcutaneous fat, radioactivity was found throughout
all tissues and fluids examined (Kelly, 1964).
2.1.2 Interactions with bones and teeth
Tissues from mice, rats, guinea-pigs, rabbits and dogs
parenterally treated with TCs (TC, CTC or OTC, doses varying from
0.1 - 50 mg/kg bw) were examined in U.V. light. In all tissues with
the exception of the brain, a brilliant yellow-gold fluorescence
was apparent within 30 min, irrespective of the dose. The induced
fluorescence disappeared from all tissues (except bone) within 6 h
after a single parenteral injection. However, bone fluorescence
persisted throughout a 10-week observation period after dosing
(Milch et al., 1967).
Single oral doses of 250 mg/kg bw tritium-labelled TC or single
oral dose of 250 mg 14C-CTC/kg bw were administered to male rats
(Sherman strain). Radioactive residues in the femur of the rats
treated with TC were 9.6, 1.9 and 0.4 mg/kg of bone measured after
4 h, 24 h, and 4 weeks, respectively. The radioactivity averaged
12 mg/kg of bone 4 h after CTC treatment, and 2.3 mg/kg after 4 weeks.
After a lifetime diet containing 0.5 to 1000 mg CTC/kg of feed,
the maximum radioactivity in femur was 570 mg/kg. After i.p.
injections with 10-150 mg TC/kg bw, the concentrations found in the
femur were directly related to the administered dose and were much
higher than the concentrations measured after oral treatment with
250 mg TC/kg bw (Buyske et al., 1960).
2.1.3 Observations in humans
In humans, about 30% of an oral therapeutic dose of CTC was
absorbed on an empty stomach. For TC and OTC, the absorption was
60-80% (Sande & Mandell, 1990).
Oral doses of 250 to 500 mg every 6 h produced plasma
concentrations of TC ranging from 1 to 5 mg/l. Intravenous injections
of 250-500 mg produced plasma concentrations of about 15-20 mg/l at
0.5 h, falling to 4-10 mg/l at 1 to 2 h. At 12 h, 1-3 mg/l was still
present. In circulation, TCs are bound to plasma proteins in varying
degrees (24-65% for TC, and about 47% for CTC). TCs appeared in the
milk of nursing mothers where concentrations were be 60% or more of
those in the plasma. TCs diffused across the placenta and appeared in
the fetal circulation at concentrations of about 25 to 75% of those in
the maternal blood. The plasma half-lives of CTC and TC were reported
to be about 8-10 h and 5.5 h, respectively (Martindale, 1989).
Absorption of TCs was impaired by milk products, sodium
bicarbonate, aluminium hydroxide and iron preparations due to
chelation and increase in gastric pH (Sande & Mandell, 1990).
2.1.4 Biotransformation
2.1.4.1 Tetracycline
The metabolism of 14C-TC was examined in rats following single
i.p. or oral administration of 60 mg/kg bw, and in dogs after single
oral administration of 25 mg/kg bw tritium-labelled TC. Approximately
90% of the administered radioactivity in the rat was eliminated either
by urinary or faecal route. A significant portion of the remaining
activity was bound as chelated TC in the skeleton of the animal. With
the exception of this chelate, TC was chemically unaltered by the rat.
Urine of dogs contained only unchanged TC (Kelly & Buyske, 1960).
2.1.4.2 Chlortetracycline
Male Wistar rats (n=6) and male beagle dogs (n=2) were
administered 14C-labelled CTC via different routes (oral:
60 mg/kg bw; i.p.: 30 mg/kg bw; i.v.: 15-60 mg/kg bw). The
recovery of antimicrobial activity was significantly lower than the
recovery of radioactivity by all routes of administration and
excretion. The major 'presumed' metabolite was identified as
4-epichlortetracycline, which accounted for 23-35% of the
radioactivity in the urine of rats, and 31-60% in dogs. It was
essentially inactive in microbiological assays. It was not clear
whether this represented a true metabolite or was a degradation
product due to alkaline treatment. The presence of small amounts
(5-10%) of isochlortetracycline in the urine and faeces of some
animals was also demonstrated (Wulf & Eisner, 1961; Eisner & Wulf,
1963).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with TC and CTC after oral,
i.v. or s.c. administration are summarized in Tables 1 and 2,
respectively.
Dogs developed transient hyperpnea, weakness and anorexia
following an i.v. dose of 50 and 100 mg CTC/kg bw, and a dose of
150 mg/kg bw caused respiratory distress, general paresis, somnolence
and death within a few hours (Hines, 1956).
Table 1. Acute toxicity of tetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. oral TC n.s. 2550 Tubaro et al., 1964
mouse n.s. oral TC n.s. >3000 Cunningham et al., 1954
mouse (42d) n.s. oral TC n.s. 808 Goldenthal, 1971
(3d) 300
mouse n.s. i.v. TC n.s. 160 Tubaro et al., 1964
mouse n.s i.v. TC n.s. 157 Maffii & Mainardi, 1955
mouse n.s. i.v. TC n.s. 170 Cunningham et al., 1954
mouse n.s. i.p. TC n.s. 340 Tubaro et al., 1964
mouse n.s. i.p. TC n.s. 330 Cunningham et al., 1954
rat M oral TC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral TC n.s. >3000 Cunningham et al., 1954
rat (49(d) n.s. oral TC n.s. 807 Goldenthal, 1971
(3d) 360
Table 1. Acute toxicity of tetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat (adult) n.s. oral TC HCl n.s. 6443 Goldenthal, 1971
(<2d) 3827
rat n.s. i.v. TC n.s. 220 Cunningham et al., 1954
rat n.s. i.v. TC n.s. 128 Maffii & Mainardi, 1955
rat n.s. i.v. TC HCl n.s. 375 Diermeier, 1959
rat n.s. i.p. TC n.s. 320 Cunningham et al., 1954
n.s. not specified
d age of animal in days
2.2.2 Short-term toxicity studies
2.2.2.1 Tetracycline
Mice
Groups of male mice (10/sex/group) were given by gavage 0, 20 or
100 mg/kg bw TC hydrochloride once daily, 5 days/week for 6 weeks as a
2% starch suspension containing 4-6% test substance. At termination of
the study a complete blood count was performed in the high-dose group
and no significant haematological alterations were observed.
Accelerated growth rates were observed in dosed mice when compared to
the control mice (Cunningham et al., 1954).
In a range-finding study, groups of B6C3F1 mice (6-7 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 470, 950, 1800, 3700
or 7500 mg/kg bw/day, respectively). No mortalities occurred. Final
mean body weight was slightly decreased in males (16%) and females
(6%) at the 5% level. The TC concentration in bone increased with
increasing dose of TC hydrochloride (NTP, 1989).
Rats
In a range-finding study, groups of F344/N rats (7-8 week old;
10-15/sex/group) were fed diets containing 0, 0.31, 0.63, 1.25, 2.5 or
5% TC hydrochloride for 13 weeks (equivalent to 155, 315, 625, 1250 or
2500 mg/kg bw/day, respectively). No mortalities occurred. Cytoplasmic
vacuolization was observed in the livers of male rats at the two
highest dose groups. Bone marrow atrophy was seen in both female and
male rats at 1250 and 2500 mg/kg bw/day. The concentration of TC in
bone increased with increasing dose of TC hydrochloride (NTP, 1989)
Dogs
Mongrel dogs (8-4/group) were given a capsule of 0 or
250 mg/kg bw TC once daily, 6 days/week for 98 days. No mortality
occurred. No effects were seen on peripheral blood and no pathological
changes were observed (Nelson & Radomski, 1954).
Groups of 4 mongrel dogs were orally administered 10 or
100 mg/kg bw TC twice daily, 5 days/week for 3 months. No effects were
observed on body weight, liver function (bromosulfophthalein
clearance), kidney function (phenolsulfophtalein clearance),
blood-clotting time, non-protein nitrogen levels, blood sugar values
or complete blood counts (Cunningham et al., 1954).
Table 2. Acute toxicity of chlortetracycline
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse M & F oral CTC HCl n.s. >3000 Cunningham, 1954
mouse F oral CTC HCl n.s. 2150 Tubaro & Banci, 1964
mouse M & F oral CTC HCl n.s. 3350 (M) Bacharach, 1959
4200 (F)
mouse n.s. oral duomycin n.s. >3000 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 155 Cunningham, 1954
mouse F i.v. CTC HCl n.s. 93 Tubaro & Banci, 1964
mouse n.s. i.v. duomycin n.s. 102 Harned, 1948
mouse n.s. i.v. duomycin n.s. 134 Harned, 1948
mouse M & F i.v. CTC HCl n.s. 102 (M) Bacharach, 1959
108 (F)
mouse F i.v. CTC bisulfate n.s. 113 Levinskas 1963
mouse n.s. s.c. duomycin n.s. >600 Harned, 1948
mouse M & F s.c. CTC HCl n.s. 5500 (M) Bacharach, 1959
8250 (F)
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
mouse n.s. i.p. CTC HCl n.s. 192 Cunningham, 1954
mouse M & F i.p. CTC HCl n.s. 128 (M) Bacharach, 1959
168 (F)
mouse F i.p. CTC HCl n.s. 214 Tubaro & Banci, 1964
rat M & F oral CTC HCl n.s. >3000 Cunningham, 1954
rat F oral CTC HCl n.s. >4000 Diermeier, 1959
rat n.s. oral CTC-HCl n.s. 55001 Goldenthal, 1971
103002
rat F oral calcium CTC n.s. >10000 Levinskas, 1963
rat n.s. oral Aureo Biomass n.s. >5000 Lowe & Fisher, 1993
(summary only)
rat n.s. oral duomycin n.s. >3000 Harned, 1948
rat M & F i.v. CTC HCl n.s. 160 Cunningham, 1954
Table 2. Acute toxicity of chlortetracycline (cont'd)
Species Sex Route Substance Purity LD50 Reference
(mg/kg bw)
rat M i.v. CTC HCl n.s. 167 Diermeier, 1959
rat n.s. i.v. duomycin n.s. 118 Harned, 1948
rat n.s. i.p. CTC HCl n.s. 335 Cunningham, 1954
guinea pig n.s. i.v. duomycin n.s. 100 Harned, 1948
guinea pig n.s. s.c. duomycin n.s. >300 Harned, 1948
n.s. not specified
1 < 2 days old
2 adult
Groups of male dogs (4 mongrel dogs and 4 beagle dogs) were fed
diets containing 0.1, 0.3 or 1% TC hydrochloride for 24 months
(equivalent to 25, 75 or 250 mg/kg bw/day, respectively). An interim
sacrifice of 1 beagle and 1 mongrel dog of each group was performed at
12 months for microscopic examination. No effects were observed on
clinical signs, mortality, body weight, food consumption, haematology,
ALP, bromosulfophtalein clearance, urea nitrogen determinations,
testes and epididymides weight, macroscopy, concentration and motility
of the spermatozoa, or appearance of semen. The bony structures of all
dosed dogs showed a yellowish coloration. The intensity of the colour
was related to the dose fed. A brownish-black pigmentation with a
dose-related intensity was observed in the thyroid glands of the dogs
of all groups. Microscopic examination of the thyroids revealed
intracytoplasmic granules in the follicular epithelial cells of most
of the treated dogs. This effect might be caused by deposits of TC or
its metabolites. Histopathological changes were not observed
(Deichmann et al., 1964).
2.2.2.2 Chlortetracycline
Mice
Groups of 10 male mice were given 0, 20 or 100 mg CTC/kg bw/day,
once daily by gavage, 5 days/week for 6 weeks. At 100 mg/kg bw/day,
complete blood count indicated that there were no significant
haematological alterations. Beneficial effects such as increased
growth were observed at 20 and 100 mg/kg bw/day (Cunningham, 1954).
Two groups of 20 mice received 20 or 100 mg/kg bw/day of CTC for
3 months (route not specified). Final body weight of both groups
exceeded those of the controls by 15%. No evidence of toxicity was
seen and no difference in blood counts was found at the end of
experiment (Hines, 1956).
Groups of 20 male mice (strain not specified) received orally
0, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw, twice daily). There were no
significant effects on mortality, clinical signs, body-weight gain,
haematology, macroscopy or microscopy. The NOEL in this study was
200 mg/kg bw/day (Harned et al., 1948).
Rats
Sherman rats (6/sex/group) received diets containing 0, 2.0 or
5.0% CTC for 28 days (equivalent to 2000 or 5000 mg/kg bw/day).
Body-weight gain was significantly decreased at 5000 mg/kg bw/day. No
other examinations were performed (Halliday & Fanelli, 1960).
Two groups of 20 rats each were given oral doses of 150 or
300 mg/kg bw/day CTC for 3 months. In both groups, body weight of the
animals was higher than the controls. No difference in blood counts
was found at the end of the experiment (Hines, 1956).
Groups of 20 male rats (strain not specified) received by gavage
0, 10, 40 or 100 mg CTC/kg bw/day, 5 days/week for 14 weeks. From the
fourth week onwards, the high dose was increased to 200 mg/kg bw/day
(administration of 100 mg/kg bw twice daily). No treatment-related
effects were seen on mortality, clinical signs, body weight,
haemoglobin, blood pressure, macroscopy or microscopy. The NOEL in
this study was 200 mg/kg bw/day (Harned et al., 1948).
Dogs
Oral administration of 100 mg CTC/kg bw/day to dogs (number and
strain not specified) for 17 days, followed by 100 mg/kg bw twice
daily for 14 days, produced no unfavourable effects on general
appearance, growth rate, haemoglobin, complete blood count, gross or
microscopic examinations (Hines, 1956).
Dogs (4/group) received 10, 50 or 100 mg of CTC/kg bw/day for 14
weeks (route not specified). At the end of this period, liver and
kidney function tests, blood sugar values, blood coagulation time,
blood non-protein nitrogen and complete blood counts did not differ
significantly from pre-test values (Hines, 1956)
Ten dogs (strain not specified) were given CTC twice daily
(morning and afternoon) by capsule at a total dose of 100 mg/kg
bw/day, 5 days/week for 9 to 15 weeks. CTC treatment had no effect on
general appearance, body weight, haematology, clotting time, liver and
kidney function (Bromosulfophtalein and phenolsulfophtalein
clearance), urinalysis, gross or microscopic examinations
(Harned, 1948).
Ten mongrel dogs (8-19 kg; 2-5 years old) received capsules
containing 250 mg CTC/kg bw/day, 6 days/week, for 98 days. Another 4
dogs received the same dose intermittently, 2 weeks on the drug and 3
weeks off, for 121 days in total. Five dogs of the continuously
treated group died. These animals had progressive weight loss, apathy
and anorexia. Haemoglobin, RBC, granulocyte and total leucocyte counts
were slightly depressed. The pathologic changes observed included
emaciation, fatty liver, excess fat in the kidney, depletion of the
bone marrow and atrophic changes in the spleen, lymph nodes and
skeletal muscle (Nelson & Radomski, 1954).
Beagle and basenji dogs (2/sex/group; 9-12 months old) received
daily a single oral dose of 10 or 100 mg CTC-HCl/kg bw in capsules for
54 weeks; a third group consisting of 1 female beagle dog, 2 male and
1 female basenji dogs were given 50 mg/kg bw/day. No control group was
used. Observations included haematology, clinical chemistry,
urinalysis, faecal smears, macroscopy, organ weight, microscopy, and
terminal tissue concentration.
Treatment-related GI disorders (vomiting, diarrhea, appetite loss
and swelling of the anal glands) were observed, and particularly in
the first half of the experiment in all groups, most often in basenji
dogs. The average blood concentration 4 h after administration of the
dose (calculated for each group from 12-14 determinations carried out
over a period of 1 year) were 0.34 µg/ml, 0.58 µg/ml and 0.94 µg/ml at
10, 50 and 100 mg/kg bw/day, respectively. Thyroid weight was high in
one male at 100 mg/kg bw/day, but the shape and appearance of the
gland were normal. Slight yellow discolouration in the bones was
observed at necropsy in the high-dose dogs, and 2 dogs at each dose
level showed evidence of chronic gastritis. Measurable tissue
concentrations (no details given) were found in bone > kidney >
liver but none in brain, heart or spleen. The NOEL in this study was
100 mg/kg bw/day (Dessau & Sisson, 1957).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Tetracycline
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets for 103
weeks containing 0, 1.25 or 2.5% TC-HCl (purity 91%), equal to 1500 or
3000 mg/kg bw/day for males, and 1500 or 3500 mg/kg bw/day for
females. Observations included clinical signs, body weight, feed
consumption, macroscopy, and histopathology.
Survival was increased for male mice (31/50, 43/50, 43/50); no
differences were observed in females (37/50, 35/50, 38/50). Mean body
weights in both dose groups were slightly lower for both sexes
compared to control mice. Dosing had no effects on feed consumption.
The tumour incidence was not significantly increased in either sex. On
the contrary, dosed female mice did not develop hepatocellular
adenomas or carcinomas (combined incidence: 10/49 for controls; 0/48
at 1.25%; 0/50 at 2.5%) (NTP, 1989; Dietz et al., 1991).
Rats
Groups of weanling male rats (Osborne-Mendel) were fed diets
containing 0 (180 rats), 100 (100 rats), 1000 (130 rats) or 3000
(100 rats) mg TC-HCl/kg of feed, equivalent to 5, 50 or 150 mg/kg
bw/day for 2 years. Ten Rats/group were killed at frequent intervals.
Sacrificed and moribund rats were macroscopically and histologically
examined. No effects were seen on clinical signs, body weight, food
consumption or haematological examinations. Beneficial effects were
observed during the first 18-19 months, all dosed rats appearing more
vigorous and gaining weight more rapidly than the control rats.
Mortality rates were lower in the treated rats than in the control
rats. At histopathology, yellow discoloration of long bones and
calvarium was observed at the highest dose. Tumour incidence was not
enhanced (Deichmann et al., 1964).
Groups of F344/N rats (50/sex/group) were fed diets containing
0, 1.25% or 2.5% mg TC-HCl (purity 91%), equal to 440 or 910 mg/kg
bw/day for males, and 510 or 1060 mg/kg bw/day for females for
103 weeks. Observations included clinical signs, body weight, feed
consumption, macroscopy and histopathology.
The survival of female rats of both the low- and high-dose groups
was significantly greater than that of the controls (control 27/50,
low-dose 39/50, high-dose 38/50). No compound-related effects on body
weight or feed consumption were observed. A dose-related increased
incidence of basophilic cytoplasmic changes and clear cell changes
were observed in the livers of male rats. The incidence and severity
of nephropathy were reduced in dosed male rats (control 48/50,
severity 2.8; mid-dose 35/50, severity 1.6; high-dose 36/50, severity
1.5). The tumour incidence was not enhanced (NTP, 1989; Dietz et al.,
1991).
2.2.3.2 Chlortetracycline
Rats
Forty rats (sex or strain not specified) were maintained for 52
weeks on a diet containing 1% CTC, equivalent to 1000 mg/kg bw/day.
There was no significant difference in body weight and no evidence of
toxicity was seen.
In a parallel experiment another group of 40 rats (sex or strain
not specified) received 5% CTC, equivalent to 5000 mg/kg bw/day. Ten
animals died within the first 10 weeks. The remaining 30 rats survived
for the 52-week test period and had a 33% lower weight than the
controls, and an apparent yellow staining of all tissues (Hines, 1956).
Groups of Sherman rats (20/sex/group) were fed diets containing
CTC at concentrations of 0, 1, 5, 20, 100, 500, 2000, 10000 or
50000 mg/kg of feed, equal to 0, 0.07, 0.35, 1.3, 7, 34, 130, 700 or
5200 mg/kg bw/day, respectively. Observations were made on mortality,
clinical signs, body-weight gain, food consumption, haematology,
macroscopy, organ weight, and microscopy.
During the first 12 weeks of the study, 8 male rats in the
highest dose group died compared to none in the other groups. At
termination, the total mortality for males was not different from
controls. Both males and females in the 50000 mg/kg group showed signs
of GI irritation which included abdominal distension, nose and mouth
encrustation and salivation. Body-weight gain was reduced in both
sexes at 50000 mg/kg. WBC was reduced in both sexes at 50000 mg/kg.
Microscopic changes at 50000 mg/kg included accumulation of yellow
pigment in splenic lymph follicles for both sexes, testicular atrophy
occasionally with degenerative changes in seminiferous tubulus, fatty
infiltration of the liver in males and infiltration of monocytes
sometimes coupled with interstitial fibrosis in the lungs in both
sexes. Fluorometric determinations showed the presence of significant
amounts of CTC at dose levels of 500 mg/kg of feed and higher. No
blood or urine biochemistry was carried out. The tumour incidence was
not enhanced. The NOEL in this study was 10000 mg/kg diet, equal to
700 mg/kg bw/day (Dessau & Sullivan, 1958; Dessau & Sullivan, 1961).
2.2.4 Reproductive toxicity studies
2.2.4.1 Chlortetracycline
Mice
Mice maintained through 4 reproductive cycles on diets containing
175 mg CTC/kg of feed (equivalent to 25 mg/kg bw/day) did not differ
significantly from parallel control groups in the number of young born
per litter, the number of surviving pups or the average body weight at
the end of 4 weeks (Hines, 1956).
Rats
Rats maintained through 2 reproductive cycles on a diet
containing 45 mg CTC/kg of feed (equivalent to 2 mg/kg bw/day) did not
differ significantly from parallel control groups in the number of
young per litter, the number of surviving pups or the average body
weight at the end of 4 weeks (Hines, 1956).
A 2-generation reproductive toxicity study was carried out with 2
groups of 5 male and 15 female rats (Sherman strain, approximately
21-day old). One group received the control diet and the other
received the same diet containing 1% CTC, equivalent to 500 mg/kg
bw/day. When the rats were approximately 100-day old, the animals were
mated to produce the first generation. All litters were reduced to 8-9
rats. Pups were weaned to the diet given to their parents. Second
generation breeding was carried out with 21 female and 7 male rats in
the 1% group and a few more rats per group in the control group.
Observations were made for clinical signs, body weight, food
consumption, fertility, reproductive performance and pup growth. Male
body weights were slightly lower than controls for both parental
generations. No further toxicity, nor effects on male or female
reproduction were observed (Hallesy & Hine, 1964).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Tetracycline
Groups of 16 pregnant Wistar rats were given 0 or 150-200 mg TC
chlorhydrate/rat/day from days 1 to 18 of gestation (Group I) and
another group received the same treatment from days 1 to 28 after
delivery (Group II). At day 20 of gestation, 3 females of group I and
3 control females were sacrificed and the uterine contents examined.
The remaining rats were allowed to deliver their litters and the
offspring were killed and developmental effects investigated. No
effects were observed on gestation duration, body size or weight, and
no malformations were observed. Pups of group II exhibited a 15%
reduction in the length of the legs in comparison with the controls at
28 days of age. Examination by UV light of the pups skeleton revealed
typical TC-associated fluorescence, indicating that TC had been
absorbed in the bones (Hurley & Tuchmann-Duplessis, 1963).
Sprague-Dawley male and female rats were given 500 mg/kg TC-HCl
in the feed, equivalent to 25 mg/kg bw/day, starting 3 days prior to
mating. Groups of 15-20 pregnant rats continued to receive the
treatment throughout gestation. Some of the pregnant rats (number not
given) were sacrificed on day 21 of gestation and the contents of the
uteri were examined. The remainder of the dams were allowed to deliver
their litters. No significant effects were observed on resorptions,
pregnancy rate, offspring mortality, litter size or weight. At
external skeletal and visceral examinations, the only treatment-
related effects observed were in animals delivered by section and
included an increase in the occurrence of hydroureter (14%;
control:0.8%) and fragmented lumbar (3%; control:0%). In animals
delivered naturally, the incidence for hydroureter was 15% and 12% for
control and treated rats, respectively (McColl et al., 1965).
Pregnant Wistar rats (number not given) were orally dosed from
days 1 to 21 of gestation with TC or TC-HCl at doses of 54, 270 or
540 mg/kg bw/day, and 40, 200 or 400 mg/kg bw/day, respectively. All
rats were sacrificed on day 21, and fetuses were removed and examined
for skeletal anomalies. A reduction of ossification in the anterior
extremities, but not in the posterior ones was observed, more
frequently in the fetuses of the highest dose groups (Szumigowska-
Szrajber & Jeske, 1970).
The development of rat embryonic tissue, transplanted in athymic
(nude) mice was studied for several weeks. Limb buds of fetal rats
were cut into small pieces and transplanted into the subcutaneous
tissue of the back of female nude mice. On the 7th, 9th and 11th days
after grafting, the host mice were treated intraperitoneally with 1500
or 3000 mg/kg TC suspended in 0.55% CMC. Control mice were treated
with the vehicle only. On day 20, the grafted tissue was examined
macroscopically and histologically. No treatment-related effects were
observed on the growth of the grafted limbs or on the differentiation
of the grafts (Shiota et al., 1990).
Groups of 5 female rabbits (strain unspecified) and groups of 5
female Wistar rats were injected i.v. from days 10 to 20 of pregnancy
with 10 mg/kg bw/day of TC or CTC. Rabbit and rat neonates did not
show significant variations in weight between the groups or any
malformation (examinations in UV-light), however, the study was very
limited (Tubaro, 1964).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with TC and CTC are given
in Tables 3 and 4, respectively.
2.2.7 Special studies on microbiological effects
The TCs are primarily bacteriostatic antibiotics inhibiting
protein synthesis in susceptible microorganisms. Following transport
through the cytoplasmic membrane, TCs bind, predominantly reversibly
to the 30 S sub-unit of bacterial ribosomes, thereby preventing access
of aminoacyl tRNA to the acceptor site on the mRNA-ribosome complex
(Sande & Mandell, 1990; Pratt & Fekety, 1986).
The TCs possess a wide range of antimicrobial activity against
Gram-positive and Gram-negative bacteria. They are also active against
some microorganisms that are resistant to agents that exert their
effects on the bacterial cell wall such as Rickettsiae, Mycoplasma,
Chlamydia, some atypical mycobacteria and amebae. They have little
activity against fungi (Sande & Mandell, 1985).
Up to 50% of the antimicrobial activity of CTC can be inhibited
by the presence of magnesium sulfate. The relationship between CTC
inhibition and the magnesium sulfate concentration (50-200 mM) is
linear (Chiang, 1982)
Table 3. Results of genotoxicity studies on tetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S.typhimurium 0-10 µg/plate negative1 NTP, 1989
TA100, TA98 toxic > 3 µg/plate
TA1535, TA1537
Mouse lymphoma L5178Y/TK+/- -S9:25-300 µg/ml negative1 NTP, 1989
assay cells toxic > 200 µg/ml
+S92:10-120 µg/ml negative1
+S93:20-120 µg/ml weakly
positive1
Sister chromatid Chinese hamster -S9:5-49.9 µg/ml negative1 NTP, 1989
exchange assay ovary cells toxic >40.2 µg/ml
+S9:302-600 µg/ml negative1
toxic 600 µg/ml
Chromosome Human 10 µg/ml equivocal Meisner et al.,
aberration assay lymphocytes 1977
Chromosome Chinese hamster -S9:39.9-400 µg/ml negative1 NTP, 1989
aberration assay ovary cells toxic 400 µg/ml
+S9:1000-2750 µg/ml negative1
Table 3. Results of genotoxicity studies on tetracycline (cont'd)
Test system Test object Concentration Result Reference
Gene mutation FM3A cells from 10-100 µg/ml positive4 Tsutsui, et al.,
C3H mice 1976
In vivo
Chromosome Allium cepa 12-20 µg/ml5 positive Mann, 1978
aberration assay
SLRL assay Drosophila injection: NTP, 1989
melanogaster 5000-5300 ppm negative
feeding:
9005 ppm negative
1 with and without metabolic activation
2 S9 prepared from the liver of Aroclor 1254-induced F344 rats
3 S9 prepared from liver of non-induced F344 rats
4 induction of an 8-azaguanine-resistant mutation chromosomal damage
inhibition of protein and DNA synthesis
5 TC pure was used as test substance
Therapy with TCs is associated with a high rate of development of
supra-infections with bacteria, yeast or fungi not sensitive to these
agents, particularly in patients with diabetes and other conditions
that reduce the natural host defense mechanisms
(Pratt & Fekety, 1986).
2.2.7.1 In vitro studies
MIC values for TC for different bacterial strains of animal
origin are given in Table 5.
MICs for TC, CTC and OTC, ranging from 0.1- > 100 µg/ml, have
been reported for a number of clinical isolates, including
Staphylococcus spp., Proteus spp. and E. coli. The data indicate
that the antimicrobial potency of the three TCs for various
micro-organisms is quite similar (Walter & Heilmeyer, 1975).
The antimicrobial potency of CTC in relation to that of OTC was
established in 34 isolates of 3 different animal pathogens (P.
hemolytica, P. multocida and B. bronchiseptica). The geometric mean
for the MICs was 0.32 µg/ml for CTC and 0.52 µg/ml for OTC. For
E. coli (only one isolate), the MIC was 4.8 µg/ml for CTC, and
2.8 µg/ml for OTC (Rogalski, 1985; Gustafson, 1995).
The MIC observed for S. aureus in vitro was 0.19 µg/ml for CTC,
0.21 µg/ml for TC, and 0.55 µg/ml for OTC (Fourtillan & Lefebvre,
1985).
In in vitro studies with E. coli of animal and human origin,
MIC50 values of 2 µg/ml for CTC and 4 µg/ml for OTC were found. MIC
values for E. faecalis and E. faecium also indicated a 2-fold
higher potency for CTC than for OTC (Devriese, 1994).
The antimicrobial activity of TC was compared with that of OTC in
a range of bacterial species (10 strains/species) isolated from faeces
of healthy humans. The concentrations of TC and OTC in the MIC
determinations ranged from 0.06 to 32 µg/ml. The bacterial density was
107 colony forming units/ml. The results are presented in Table 6.
The sensitivity of the strains of most bacterial species was similar
for TC and OTC, whereas TC was active at lower concentrations for
Bifidobacterium spp., Eubacterium spp. (p<0.05), and Fusobacterium spp.
(p<0.1), and at higher concentrations for Spectrococcus spp. (p<0.05).
The geometric mean MIC, taking into consideration all tested strains
(using a value of 32 µg/ml for resistant strains) was 3.2 µg/ml for
TC and 3.8 µg/ml for OTC (Richez, 1994).
Table 4. Results of genotoxicity studies on chlortetracycline
Test system Test object Concentration Result Reference
In vitro
Ames test S. typhimurium 0.1-15 µg/plate; negative1,2 Mulligan, 1989
TA100,TA98,TA1535 conc > 1.0 µg
TA 1337, TA1538 were toxic
E. coli WP-2uvrA-
Amest test S. typhimurium up to 10 µg/plate; negative1 Sharma et al., 19893
TA100, TA98, toxicity was
TA1535, TA1537 seen
E. coli WP-2uvrA-
HGPRT assay CHO cells +act: 20-100 µg/ml negative Harbell, 1988
-act: 25-125 µg/ml
toxicity was
observed at the
highest conc.
UDS rat hepatocytes 25-75 µg/ml; negative Thilagar, 1988
toxicity was seen
at the highest dose
Table 4. Results of genotoxicity studies on chlortetracycline (cont'd)
Test system Test object Concentration Result Reference
In vivo
chromosome rat orally 500, 2500, negative Putman, 1988
aberrations 5000 mg/kg bw4
chromosome Vicia sativa 1% solution negative Sharma &
aberrations Lens esculenta " equivocal Bhattacharyya, 1967
1 with and without metabolic activation
2 no positive controls were used; background was not given
3 abstract only
4 no effects on mitotic index
Table 5. Summary of MIC values for different bacterial species for
tetracycline (Pratt & Fakety, 1986)
Species MIC (µg/ml)1
Escherichia coli 12.5 (3.1-500)
Proteus mirabilis >100 (50->100)
Enterobacter 25 (6.3-50)
1 Values given are the mean (range in parenthesis)
2.2.7.2 In vivo studies
The in vivo activity of S. aureus in mice was comparable for
the three different TCs; ED50 values were 7.6, 7.2 and 5.8 mg/kg bw,
for CTC, TC and OTC, respectively (Fourtillan & Lefebvre, 1985)
2.2.8 Special studies on eye irritation and sensitization
CTC produced local irritation when injected i.p., i.m. or s.c. or
when administered directly to the rabbit eye. Eye irritation from 1%
solutions was mild and completely reversible within 48 h (Harned et
al., 1948).
2.2.9 Other studies
2.2.9.1 Cardiovascular effects
Six anaesthetized male rabbits were given i.v. injections of 1, 2
or 5 mg TC-HCl/kg bw dissolved in 0.5 ml saline. The injections were
performed in 3, 10, 20 or 60 seconds. A dose- and injection
time-dependent slowing of the heart rate (from 270-300 (normal) to 100
beats/min or less) was observed 1-3 min after the injection. No effect
on arterial blood pressure was found.
Respiration was depressed which led at high doses to respiratory
arrest or to a slow, shallow respiration for 1-2 min (Gyrd-Hansen,
1980).
Table 6. MIC values (µg/ml) for tetracycline and oxytetracycline
against bacterial species obtained from human isolates.
TETRACYCLINE OXYTETRACYCLINE
Bacterial species MIC50 geometric MIC50 geometric
mean mean
Escherichia coli > 32 > 32 > 32 > 32
Bifidobacterium spp. 16 8.6 > 32 > 17.1
Bacteroides fragilis 4 2.5 4 2.5
Eubacterium spp. 2* 1.6 4 2.6
Clostridium spp. 0.062 0.2 0.25 0.2
Streptococcus spp. 16 > 19.7 8 > 13.9
Fusobacterium spp. 0.125 0.1 0.25 0.2
Lactobacillus spp. 2* 1.9 2 2.3
Proteus spp. > 32 > 32 > 32 > 32
Peptostreptococcus 2 3.2 4 4.0
spp.
* MIC50 = MIC90
2.2.9.2 Hepatotoxicity
TC-HCl (>0.25 mM) inhibited reversibly the ß-oxidation of
fatty acids as well as the tricarboxylic acid cycle activity of mouse
and human liver mitochondria in vitro. After i.p. administration of
1 mmol/kg bw to mice following an oral administration of a tracer dose
of [U-14C]palmitic acid, an increase in the levels of hepatic
triglycerides was observed and liver histology showed microvesicular
steatosis (Freneaux et al., 1988).
The effects of TC on the synthesis and secretion of triglycerides
and proteins were studied in isolated rat hepatocytes. TC produced a
concentration-dependent inhibition of 14C-triglyceride secretion
without affecting triglyceride synthesis and protein secretion
(Deboyser et al., 1989).
2.2.9.3 Nephrotoxicity
Groups of Wistar albino rats were given single i.v. injections of
150 mg TC/kg bw into the tail vein. Different experiments failed to
demonstrate any significant ischaemia in the kidneys (Tapp & Lowe,
1966)
2.3 Observations in humans
2.3.1 Effects after medical treatment
In human therapy, the usual oral dose for TC-HCl is 250 or 500 mg
every 6 h for adults as specified in the US National Formulary and
British Pharmacopoeia. CTC is approved as capsules (50, 100 and
250 mg) and by injection (100, 200 and 500 mg).
A variety of adverse effects in humans have been reported from
the use of TCs. The TCs are irritating substances. When given i.v.
they can cause thrombophlebitis. Intramuscular administration is
painful and not recommended. Given orally, TCs can cause epigastric
burning, abdominal discomfort, nausea and vomiting (Schindel, 1965;
NTP, 1989; Sande & Mandell, 1990).
Bone and teeth discoloration are known to occur in humans under
clinical treatment with high levels of TC drugs. Children receiving
long- or short-term therapy with a TC may develop a yellow or brownish
discoloration of the enamel. The duration of therapy appears to be
less important than the total quantity of antibiotic administered. The
risk of this effect is highest when the TC is given to neonates and
babies prior to the first dentition. However, pigmentation of the
permanent dentition may develop if the drug is given between the ages
of 2 months and 8 years, when the teeth are being calcified. An early
characteristic of this effect is a yellow fluorescence of the dental
pigment.
Treatment of pregnant patients with TCs may produce
discolouration of the teeth in their children, but exposure also
occurs during breast feeding. (Schindel, 1965; Piper et al., 1988;
Friedman, et al., 1990; Sande & Mandell, 1990).
TCs are deposited in the skeleton during gestation and throughout
childhood. A 40% reduction in bone growth, as determined by
measurements of fibulas, was demonstrated in premature infants treated
with these agents. This effect is readily reversible if the period of
exposure to the drug is short (Sande & Mandell, 1990; Pratt & Fekety,
1986).
No toxic effects were reported from treatment of premature
infants, children, adults or elderly with CTC. Effects after short-
and long-term exposure included increased body-weight gain, and
prophylactic activity against acute and chronic infections and
diarrhea (Hines, 1956).
All the TCs (dose levels not specified) can cause phototoxicity,
manifested by mild to severe skin reactions when the skin is exposed
to direct sunlight (Sande & Mandell, 1990; Schindel, 1965).
Liver effects, characterized by fatty accumulation have been
observed, predominantly following repeated parenteral administration
of TCs (dose levels were not specified). TCs can aggravate uremia in
patients with impaired renal function. For unknown reasons, pregnant
woman appear to be more susceptible to the development of hepatic
damage (Pratt & Fekety, 1986; Sande & Mandell, 1990; Friedman,
et al., 1990).
TCs rarely cause allergic reactions. Hypersensitivity to TCs have
been described as allergic manifestations of mucosal tissues. Central
nervous system side effects and effects on blood cells were rarely
described. Long-term therapy (dose levels not specified) with TCs may
produce changes in the peripheral blood. Leucocytosis, atypical
lymphocytes, toxic granulation of granulocytes, and thrombocytopenic
purpura have been observed.
The TCs may cause increased intracranial pressure and tense
bulging of the fontanels (pseudotumor cerebri) in young infants, when
given in therapeutic doses. Young adults complain of headache, nausea,
vomiting and diplopia.
TC is given orally in low doses (usually 100 mg/person/day) for
the treatment of chronic severe acne vulgaris. A common complication
of treatment with TCs is minor GI irritation (Sande & Mandell, 1990;
Pratt & Fekety, 1986).
3. COMMENTS
Toxicological data
The Committee considered data from studies on pharmacokinetics,
biotransformation, acute and short-term toxicity, reproductive
toxicity, teratogenicity, long-term toxicity/carcinogenicity,
genotoxicity, antimicrobial effects and observations in humans.
Aspects of the comparative toxicity of these compounds with OTC were
also considered.
Pharmacokinetic studies showed that CTC and TC were rapidly
absorbed following oral administration. Peak plasma concentrations
were higher in rats receiving TC than in those receiving the same dose
of CTC. In humans receiving oral therapeutic doses, 30% of the dose of
CTC was absorbed, as compared with 60-80% of the dose of TC. TC is
excreted mainly in urine, whereas CTC is excreted in urine and faeces
in comparable amounts. After absorption following administration by
various routes, CTC and TC are widely distributed in the body, the
highest levels are present in the liver and kidney. Detectable levels
of TCs in bone persist for more than 28 weeks after dosing. The plasma
half-lives of CTC and TC in humans are about 5 and 8 h, respectively.
TCs have been found in the breast milk of women receiving therapeutic
doses and can cross the placenta.
Orally administered CTC and TC have low acute toxicity in rats
and mice, LD50s ranging from 2150 to >5000 mg/kg bw.
In a 13-week range-finding study with mice in which TC was
incorporated in the diet at concentrations equivalent to 470, 950,
1800, 3700 or 7500 mg/kg bw/day, the only sign of toxicity observed
was a decrease in body weight in the highest-dosed group. In a 13-week
range-finding study in which rats were dosed at 155, 315, 625, 1250 or
2500 mg TC/kg bw/day, vacuolization of liver cells and bone marrow
atrophy were observed at 1250 and 2500 mg/kg bw/day. No toxicity was
observed in dogs given TC orally at dose levels of up to
250 mg/kg bw/day for 24 months.
CTC did not cause any toxic effects in short-term toxicity
studies with rats or mice at doses of up to 200 mg/kg bw/day. In dogs
given CTC orally at a dose of 250 mg/kg bw/day for 98 days, increased
incidences of mortality, fatty liver and bone marrow atrophy were
observed. In another experiment in dogs which received CTC orally at
doses of up to 100 mg/kg bw/day for 54 weeks, no effects were
observed, except for signs of GI disorders; these were considered of
minor importance because the increase in body-weight gain was normal.
In carcinogenicity studies with mice and rats, TC was administered
in the diet at doses of up to 3500 and 1060 mg/kg bw/day, respectively.
Survival rates were increased in male mice, while body weights were
slightly lower than controls for both sexes. Survival rates were also
increased in female rats, but male rats showed a dose-related increase
in basophilic cytoplasmic changes and clear cell changes in the liver.
There were no increases in tumour incidence in mice or rats.
In a chronic toxicity/carcinogenicity study, CTC was administered
in the diet of rats at doses varying from 0.07 to 5200 mg/kg bw/day.
In the highest dose group, GI irritation, decreased body-weight gain
and a decreased number of white blood cells were observed in both
sexes, while testicular atrophy was observed in males. Microscopic
changes in the highest-dose group included infiltration of monocytes
in the lungs of males and females, and fatty changes in the liver in
males. Tumour incidence was not increased. The NOEL was 700 mg/kg
bw/day. The Committee concluded that there was no evidence that CTC or
TC is carcinogenic.
In a two-generation reproductive toxicity study in which CTC was
administered to rats in the diet at a dose equivalent to 500 mg/kg
bw/day, no effects on reproductive performance were observed.
Reproductive toxicity data were not available on TC. In a number of
teratogenicity studies with rats, TC and TC hydrochloride caused a
reduction in ossification at the highest oral dose tested, 540 or
400 mg/kg bw/day, respectively. No irreversible structural changes
were found on skeletal or visceral examination. In studies with a
limited number (5 per group) of rats and rabbits, no evidence of
teratogenicity was found at intravenous doses of 10 mg of CTC or TC/kg
bw/day, administered on days 10-20 of pregnancy. The Committee
concluded that CTC and TC did not cause significant toxic effects on
either reproduction or development.
The genotoxicity of CTC and TC was investigated in a wide range
of in vitro and in vivo test systems. CTC gave negative results in
all tests. TC was negative in the majority of in vitro and in vivo
assays, but induced gene mutations in one in vitro test in mammalian
cells and chromosomal aberrations in plants. The Committee considered
these effects to be due to the inhibiting effect of TCs on protein
synthesis, based on their binding to ribosomes, and concluded that CTC
and TC do not pose a genotoxic hazard.
Microbiological data
In vitro studies of the antimicrobial effect of TCs on
pathogenic microorganisms of animal origin indicate that the
activities of CTC and OTC are not significantly different. MIC values
(geometric mean) for CTC and OTC were 0.32 and 0.52 µg/ml,
respectively.
In a limited number of microorganisms representative of human gut
flora, the antimicrobial activity of CTC was of the same order as that
of OTC.
The antimicrobial activity of TC was compared with that of OTC
using a wide range of bacterial species isolated from the faeces of
healthy human volunteers. The sensitivity of most species was similar
for TC and OTC except for Bifidobacterium, Fusobacterium and
Eubacterium species which were slightly more sensitive to TC, and
Streptococcus spp. which were slightly more sensitive to OTC. The
geometric mean MICs for all tested strains were 3.2 µg/ml for TC and
3.8 µg/ml for OTC.
4. EVALUATION
After reviewing the available toxicological and antimicrobial
data, the Committee concluded that the antimicrobial data provided the
most appropriate end-point for the evaluation of CTC and TC.
In view of the similarity of the antimicrobial activity of TC,
CTC and OTC, the Committee established a group ADI of 0-3 µg/kg bw for
the three drugs separately or in combination. This ADI was established
for OTC at the thirty-sixth meeting of the Committee (Annex 1,
reference 91), and is based on the NOEL of 2 mg/person/day for the
effects of OTC on the gut flora in human volunteers and a safety
factor of 10.
The Committee noted that this ADI provides an adequate margin of
safety when compared with the lowest NOEL for toxicological effects of
100 mg/kg bw/day for CTC in dogs.
5. REFERENCES
Adir, J. (1975) Enterohepatic circulation of tetracycline in rats.
J. Pharm. Sci, 64(11) 1847-1850.
Bacharach, A.L., Clark, B.J., McCulloch, m. & Tomich, E.G. (1959)
Comparative toxicity studies on ten antibiotics in current use.
J. Pharm. Toxicol. 11: 737-741.
Berté, F. & Vandoni, G. (1962) On the intestinal absorption and
organotropism of some tertacyclines. Chemotherapia 5, 219-230.
Buyske, D.A., Eisner, H.J. & Kelly, R.G. (1960) Concentration and
persistence of tetracycline and chlortetracycline in bone.
J. Pharm. Exper. Therap. 130, 150-156.
Chiang, T (1982) Inhibition of chlortetracycline activity by magnesium
ions. J. Assoc. Off. Anal. Chem., 65 (5), 1044-1047.
Cunningham, R.W., Hines, L.R., Stokey, E.H., Vessey, R.E. & Yuda, N.N.
(1954) Pharmacology of tetracycline. Antibiotics Annual, 63-69.
Deboyser, D., Goethals, F., Krack, G. & Roberfroid, M. (1989)
Investigation into the mechnism of tetracycline-induced steatosis:
study in isolated hepatocytes. Tox.appl.pharmacol., 97, 473-479.
Deichmann, W.B., Bernal, E., Anderson, W.A.D., Keplinger, M.,
Landeen, K., Mcdonald, W., Mahon, R. & Stebbins, R. (1964) The chronic
oral toxicity of oxytetracycline HCl and tetracycline HCl in the rat,
dog and pig. Ind.Med.Surg., 787-806.
Dessau, F.I. & Sisson, G., (1957) Aureomycinr life studies. Studies
of chlortetracycline hydrochloride (food grade in dogs). Project
number 77601. Unpublished report of American Cynamid Company.
P.R. 3, 440-493. Research Division. Pearl River Laboratories.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Dessau, F.I. & Sullivan, W.J., (1958) Aureomycinr life studies.
Studies of chronic toxicity of CL 13,555: Chlortetracycline
hydrochloride (food grade) in rats. Project number 77601 American
Cyanamid Company, Experimental Pathology Research Division, Pearl
River Laboratories. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Dessau, F.I. & Sullivan, W.J. (1961) A two year study of the toxicity
of chlotetracycline hydrochloride in rats. Toxicol and Appl.
Pharmacol., 3, 654-677.
Devriese, L.A. (1994) Comparative minimal inhibitory concentration
determinations with chlortetracycline and oxytetracycline on gut
bacteria of animal and human origin. Faculty of Veterinary Medicine.
University of Gent, Belgium. Submitted to WHO by American Cyanamid
Company, Princeton, USA.
Diermeier, H.F. (1959) Acute oral and intravenous toxicity of rats of
a A-VIII hydrochloride (CL 22,416), chlortetracycline hydrocholoride
(CL 13,555), and tetracycline hydrochloride (CL13,554). P.R. 5,
899-907. Unpublished report of American Cyanamid Company, Lederle
laboratories, Pearl River, New York. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Dietz, D.D., Abdo, K.M., Haseman, J.K., Eustis, S.L. & Huff, J.E.
(1991) Comparative toxicity and carcinogenicity studies of
tetracycline and oxytetracycline in rats and mice. Fund. Appl.
Toxicol. 17 (2), 335-346.
Eisner, H.J. et al., (1953) The enhancement of serum levels of
Aureomycin experimental animals. J. Pharmacol. Exp. Ther.
108, 442-449.
Eisner, H.J. & Wulf, R.J. (1962) The metabolic fate of
chlortetracycline and some comparisons with other tetracyclines.
J. pharmacol, exp. therap., 142, 122-11.
Fourtillan & Lefebvre (1985) cited in Rogalski, W. (1985) Chemical
modifications of the tetracyclines. In: The tetracyclines. Eds.
Hlavka and Boothe. Springer Verlag, Berlin.
Freneaux, E., Labbe, G., Letteron, P. The Le Dinh, T.L., Degott, C.
Geneve, J. Larrey, D. & Pessayre, D. (1988) Inhibition of the
mitochondrial oxidation of fatty acids by tetracycline in mice and in
man: possible role in microvesicular steatosis induced by this
antibiotic. Hepatology, 8(5), 1056-1062.
Friedman, J.M., Little, B.B., Brent, R.L., Cordero, J.F., Hanson,
J.W., & Shepard, T.H., (1990) Potential human teratogenicity of
frequently prescribed drugs. Obstetics and Gynecol, 75, 594-599.
Goldenthal, E.I. (1971) A compilation of LD50 values in newborn and
adult animals. Tox. and Appl. Pharm, 18, 185-207.
Gustafson, R.H. (1995) MIC tests of veterinary clinical isolates,
tetracycline. Memo to D.L. Ingle, d.d. january 11, 1995. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Gyrd-Hansen, N (1980) The effect of tetracyclines on the rabbit heart.
Zbl.vet.med. A, 27, 228-237.
Halliday, S.L. & Fanelli, G. (1960) Antibacterial tetracycline:
relative toxicity of CL 13,555, chlortetracycline hydrochloride and
CL 30,782, sodium benzoate orally in rats. Project number 14797.
P.R. 6, 1428-1439. Unpublished report of American Cyanamid Company,
Lederle Laboratories, Pearl River, New York, Submitted to WHO by
American Cyanamid Company, Princeton, USA.
Harned, B.K. Cunningham, R.W. Clark, M.C., Cosgrove, P., Hine, C.H.,
Mccauley, W.J., Stokey, E., Vessey R.E., Yuda, N.N. & Subbarow, Y.
(1948) The pharmacology of Duomycin. Ann. NYAS 51, 182-210.ALLESY,
D.W. & HINE, C.H., (1964) The effect of chlortetracycline
hydrochloride on the fertility and growth of rats.
Tox. and Appl. Pharm. 6, 9-14.
Harbell, J.W. (1988) CHO/HGPRT mutation assay. Study number
T8133.332021. Unpublished report of Microbilogical Associates, 1988.
Submitted to WHO by American Cyanamid Company, Princeton, USA.
Hines, L.R. (1956) An apprasal of the effects of long-term
chlortetracycline administration. Antibiotics. chemother.,
6 (11), 623-641.
Hurley, L.S. & Tuchmann-Duplessis, H (1963) Influence de la
tetracycline sur le developpement pré- et post-natal du rat.
Compt. rend. acad. sci., 257, 302-304.
Kanegis, L. (1958) The comarative pharmacology of tetracyclines:
initial studies on serum levels and urinary excretion of antibiotic
A-VIII following single oral and intravenous doses in the dog. Report
P.R. 4:884-921. American Cyanamid Company (Pearl River). Submitted to
WHO by American Cyanamid Company, Princeton, USA.
Kelly, R.G. (1964) Antibacterial tetracyclines. In tissue distribution
of tetracycline and chlortetracycline. American Cyanamid Company
Report P.R. 9:485-492 (Pearl River). Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Kelly, R.G. & Buyske, D.A. (1960) Metabolism of tetracycline in the
rat and the dog. J. Pharmacol. Exp. Therap., 130, 144-149. Submitted
to the WHO by American Cyanamid Company, Princeton, USA.
Kniffen, T.S., Bane, D.P., Hall, W.F., Koritz, G.D. & Bevill, R.F.
(1989) Bioavailability, pharmacokinetics, and plasma concentration of
tetracycline hydrochloride fed to swine. Am. J. Vet. Res.
50, 518-521.
Levinskas, C.J. (1963) Auromycinr and formulation: Acute oral and
intravenous toxicity. Central Mediacal Department Report
No. 6305, 53-5,. Submitted to the WHO by American Cyanamid Company,
Princeton, USA.
Lowe C.A. & Fisher J. (1993) Rat oral LD50 study with Aureo Biomass.
Report number A93-61. Data from American Cyanamid Company, Agricultural
Research Division. Submitted to WHO by American Cyanamid Company,
Princeton, USA. (summary only)
Maffii, G. & Mainardi, L. (1955) Studio Farmacologico su un nuovo
antibiotico: la tetraciclina. Il Farmaco Ed. Sci 10 (4), 197-210.
Mann, S.K. (1978) Interaction of tetracycline (TC) with chromosomes in
Allium cepa Environm. exp. botany. 18, 201-205.
Martindale (1989) The Extra pharmacopoeia. 29th edition. Eds. J.E.F.
Reynolds. London, The Pharmaceutical Press.
Mccoll, J.D. Globus, M. & Robinson, S (1965) Effect of some therapeutic
agents on the developing rat fetus. Tox. appl. pharmacol. 7,
409-417.
Meisner, L.F., Chuprevich, T.W. & Inhorn, S.L. (1977) Mechanisms of
chromatid breakage in human lymphocyte cultures. Acta. cytol.
21(4), 555-558.
Milch, R.A., Rall, D.P. & Tobie, J.E. (1967) Bone localization of the
tetracyclines. J. Nat. Canc. Inst. 19, 87-91.
Mulligan, E. (1989) Microbial mutagenicity test report for screening
study MMR89-026. American Cyanamid Company, Report number MMR89-026,
20 April 1989. Submitted to WHO by American Cyanamid Company,
Princeton, USA.
Nelson, A.A. & Radomski, J.L. (1954) Comparative pathological study in
dogs of feeding of six broad-spectrum antibiotics. Antibiotics and
Chemotherapy IV, (11), 1174-1180.
Neuschl, J. (1991) Comparison of some pharmacokinetic parameters of
tetracyclines which are most frequently used in veterinary medicine.
Arch. Exp. Veterinarmed. 45, 105-112.
NTP (1989) NTP technical report on the toxicology and carcinogenesis
studies of tetracycline hydrochloride (Cas no. 64-75-5) in F344/N rats
and B6C3F1 mice (feed studies) NTP toxicology program P.O. Box 12233.
NTP TR 344 NIH Publication No. 89-2600. U.S. Department of health and
human services.
Piper, J.M., Baum, C., Kennedy, D.L. & Price, P. (1988) Maternal use
of prescribed drugs associated with recognized fetal adverse drug
reactions. Am. J. Obstet. and Gynecol. 159 (5) 1173-1177.
Pratt, W.B. & Fekety, R. (1986) The antimicrobial drugs. Oxford
University Press, New York, pp 205-228.
Putman, D.L. (1988) Acute in vivo cytogenetics assay in rats. Final
report. Report number T8133.105003. Unpublished report of
Microbiological Associates, 11-7-1988. Submitted to WHO by American
Cyanamid Company, Princeton, USA.
Richez, P. (1994) Antibacterial activity of tetracycline and
oxytetracycline against human gut flora. Study No. SVL 029.
Unpublished report of Datavet Veterinary Drug Consultants,
May 31 1994. Submitted to WHO by Intervet International,
Boxmeer, Netherlands.
Rogalski, W. (1985) Chemical modifications of the tetracyclines.
Chapter 5, pp 233-. In: The tetracyclines. Eds. Hlavka and Boothe.
Springer Verlag, Berlin.
Sande, M.A. & Mandell, G.L. (1990) Antimicrobial agents:
tetracyclines, chloramphenicol, erythromycin and miscellaneous
antibacterial agents. In: Goodman and Gilman's The pharmacological
basis of therapeutics, 8th edition. Gilman, A.G., Rall, T.W., Nies,
A.S. and Taylor, P. eds. Pergamon Press, New York, (1990),
pp 1117-1145.
Schindel, L.E. (1965) Clinical side-effects of the tetracyclines.
Antibiotica et Chemotherapia Advances 13, pp. 300-316.
Sharma, A.K. & Bhattacharyya, G.N. (1967) A study on the respomse of
chromosomes to antibiotic treatment. Acta Biol. Acad. Sc. Hung.
18 (1), 67-75.
Sharma, R.K., Traul, K.A., Caterson, C.R., Harbell, J., Thilagar, A. &
Putman, D. (1989) Genotoxicity evaluation of chlortetracycline.
Environ. Mol. Mutagen 14 (suppl. 15), 183. (summary only).
Shiota, K., Uwabe, C. Yamamoto, M. & Arishima, K. (1990) Teratogenic
drugs inhibit the differentiation of fetal rat limb buds grafted in
athymic (nude) mice. Reprod. toxicol. 4, 95-103.
Szumigowska-Szrajber, G. & Jeske J. (1970) Effect of tetracycline
base, tetracycline hydrochloride and oxytetracycline base on
developmental changes in metatarsal bones of extremities of rat
fetuses. Acta polon. pharm. 27 (I), 85-90.
Tapp, E. & Lowe, M.B. (1966) Tetracycline toxicity. Experientia
22, 530-531.
Thilagar, A. (1988) Test for chemical induction of unscheduled DNA
synthesis in rat primary hepatocyte cultures by autoradiography. Test
report 0082-5100. Unpublished report of Sitek Research Laboratories,
22-7-1988. Submitted to WHO by American Cyanamid Company, Princeton,
USA.
Tsutsui, T., Umeda, M., Sou, M. & Maizumi, H. (1976) Effect of
tetracycline on cultured mouse cells. Mutat. res. 40, 261-268.
Tubaro, E. (1964) Possible relationship between tetracycline stability
and effect on foetal skeleton. Brit. J. Pharmacol. Chemotherap.
23, 445-448.
Tubaro, E. & Banci, F. (1964) The pharmacology of chlormethylene-
cycline, a new chlortetracycline. Arzneimittel-Forschung, 14,
95-100.
Tubaro, E., Barletta, M. & Banci, F. (1964) Some pharmacological
aspects of a new water-soluble tetracycline. J.pharm. pharmacol.
16, 33-37.
Walter, A.M. & Heilmeyer, L. (1975) Antibiotika-Fibel. Antibiotika und
Chemotherapeutila Therapie mikrobieller infektionen, pp.323-324.
Georg Thieme Verlag Stuttgart.
Wulf, R. & Eisner, H. (1961) Antibacterial tetracyclines: Metabolism
of chlortetracycline in the rat and in the dog. American Cyanamid
Company Report C.P. 1, 626-683. Experiment P-70-21-FT. Submitted to
WHO by American Cyanamid Company, Princeton, USA.
