
ANTIPROTOZOAL AGENT
DICLAZURIL
First draft prepared by
Dr L.T. Mulligan
Division of Toxicology and Environmental Sciences
Center for Veterinary Medicine
Food and Drug Administration
Rockville, MD, USA
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Long-term toxicity/carcinogenicity studies
Reproductive toxicity studies
Special studies on teratogenicity/embryotoxicity
Special studies on genotoxicity
Observations in humans
Microbiological properties
Pharmacological effects
Comments
Evaluation
References
1. EXPLANATION
Diclazuril is an anticoccidial drug used in major poultry
species, such as broiler chickens, replacement pullets and turkeys, as
well as in rabbits, lambs and minor edible bird species. It has not
previously been reviewed by the Committee.
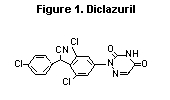
The chemical name of the compound is: 2,6-dichloro-a-
(4-chlorophenyl)-4-(4,5-dihydro-3,5-dioxo-1,2,4-triazin-2(3H)-yl)
benzeneacetonitrile. The structure is shown in Figure 1.
Figure 1. Diclazuril
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Rats
Male Wistar rats received 10 mg 14C-diclazuril/kg bw in an
aqueous suspension. On day 1, 90% of the dosed radioactivity was
excreted in the faeces. After 4 days, 92% was excreted in the faeces
and 0.04% in the urine. Unchanged diclazuril (UD) accounted for most
of the total radioactivity in the faeces, with two metabolites
accounting for less than 0.5% (Mannens et al., 1992).
In another study, concentrations of total radioactivity and UD
were measured in blood, plasma and tissues for up to 96 h after oral
administration of 10 mg 14C-diclazuril/kg bw in an aqueous
suspension to male Wistar rats. The absorption of diclazuril was
limited. Most of the dose was recovered from the GI contents. Maximum
plasma concentrations of total radioactivity and UD were approximately
1 µg/ml and occurred after 8 h. On day 1, total radioactivity in
plasma was almost exclusively UD, but later the UD/total radioactivity
ratio gradually declined. The blood-to-plasma ratio of total
radioactivity was about 0.7, indicating a limited distribution to
blood cells. Distribution to the systemic tissues was rapid but
limited. The concentration of total radioactivity in the liver was
about 50% of the plasma level and kidney, lung and heart levels were
about 20-30%, while muscle and brain concentrations were 5-7% of the
plasma levels. UD depleted from tissues with a half-life of 1.5 days.
The half-life of total radioactivity was 53.0 h (Van Beijsterveldt
et al., 1992).
2.1.1.2 Rabbits
Absorption, distribution, metabolism and excretion was studied in
2 separate studies, with male rabbits receiving a single oral dose of
14C-diclazuril at 1 mg/kg bw. The excretion of radioactivity was
fairly rapid since within 48 h, 70% was recovered in the faeces and 3%
in the urine. After 10 days, more than 98% was excreted. Gall bladder
contained radioactivity, but accounting at most for 0.02% of the dose,
implying that biliary excretion was a minor elimination pathway.
Various metabolites were identified in urine. Two main metabolites
were a glucuronide and a sulfate conjugate. In faeces, UD represented
the main part of the radioactivity, accounting for approximately 66%
of the dose. Some minor metabolites were observed. None of the urinary
or faecal metabolites accounted for more than 2% of the dose. UD
represented almost all the plasma radioactivity at least up to 120 h
after dosing. The elimination from plasma proceeded with an apparent
half-life of 2-2.5 days. The distribution to tissues was limited and
the elimination half-lives were similar to that from plasma, except
for the liver with an elimination half-life of 3 days. The easily
extractable liver residue indicated freedom from concern over bound
residues (Meuldermans et al., 1988a; Michiels et al., 1988).
2.1.1.3 Chickens
In a single-dose study, 14C-diclazuril was administered to
broiler chickens in a lactose mixture at a dose of 1 mg/kg bw. Maximum
radioactivity concentrations of 1.5-2.0 µg-eq./ml were attained in
plasma 6 h after administration. The elimination subsequently
proceeded with a half-life of approximately 50 h.
There was a rapid equilibrium between plasma and tissue levels.
The tissue concentrations were 2 to 10 times lower than the
corresponding plasma concentrations. The liver and the kidneys showed
the highest concentrations at all time points. The half-life in
tissues was approximately 50 h, similar to that found in plasma.
Parent diclazuril accounted for more than 90% in the liver at 24-h
liver, and no metabolites were detectable (<4%). About half of the
dose was excreted within 24 h, almost exclusively as UD. After 10
days, the cumulative excretion was over 95%. A degradation product,
coded DM5, accounted for 5.3% of the dose in the 0-96 h excreta. Other
metabolites accounted for <2% each. DM5 was not definitively
identified. It was demonstrated to be a derivative of
4-amino-2,6-dichloro-a-(4-chlorophenyl) benzeneacetonitrile, formed by
cleavage and subsequent degradation of the triazine-dione ring
(Meuldermans et al., 1988b).
2.1.1.4 Turkeys
In a single-dose study, 14C-diclazuril was administered in a
lactose mixture at a dose of 1 mg/kg bw. Maximum radioactivity
concentrations of 1.78 ± 0.19 µg-eq./ml were attained in plasma 6 h
after administration. The subsequent elimination proceeded with a
half-life of approximately 38 h. The blood-to-plasma ratio was on
average 0.66 throughout the 10-day observation period, implying a
minor distribution to the blood cells. There was a rapid but limited
distribution between plasma and tissues. The tissue concentrations
were slightly to markedly lower than the corresponding plasma
concentrations. The depletion rate was similar in all tissues with a
half-life in the range of 34-46 h. UD accounted for 98% of the sample
radioactivity for the livers sampled after 6 h, and 85% for the livers
sampled after 48 and 72 h. No metabolites accounted for more than 10%
of the radioactivity in liver. At 24 h after dosing, 55% of the dose
was excreted. The cumulative excretion was 88% after 5 days and 94.8 ±
0.8% after 10 days. At least 8 metabolites were identified in excreta.
Parent diclazuril, however, accounted for the major part of the
excreted radioactivity, representing 55.8% of the dose. The
triazine-dione ring cleavage product previously seen in broilers
accounted for 6.3% of the dose in the 0-96 h excreta. An unidentified
metabolite accounted for about 2.4% of the radioactivity. All other
metabolites accounted for < 2% each (Meuldermans et al., 1990).
2.1.1.5 Sheep
Poor absorption of diclazuril was observed in sheep after oral
administration of 1 mg/kg bw. Maximum concentrations in plasma of
0.012 to 0.016 µg/ml were reached at 24-48 h after dosing and were
lower than the quantification limit (< 0.01 µg/ml) at all other
sampling times (Monbaliu et al., 1993).
In summary, in all species investigated, there were little
indications of metabolism of diclazuril. As a result,
diclazuril-derived substances were eliminated mainly as unchanged
parent substance in the excreta of bird species, and in the faeces of
rats and rabbits. Urine of the latter species was only a very minor
excretion route and some metabolized substances could be observed. In
edible tissues, the residues consisted almost entirely of unchanged
diclazuril.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with diclazuril are
summarized in Table 1.
Only in mice and rats dosed i.p. at 5000 mg/kg bw was mortality
observed. Clinical effects in mice and rats were non-specific and were
mainly on the CNS. In dogs, vomiting and defecation were seen after
treatment. Autopsy did not reveal drug-related macroscopic changes in
any study.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 3-month dose-range-finding study, SPF Albino Swiss mice
(10/sex/group) were dosed orally at 200, 400, 800 or 1600 mg/kg of
feed, equivalent to 30, 60, 120 or 240 mg/kg bw/day. Increased
centrilobular swelling of the liver was observed in male mice at the
three highest doses (3/10, 4/10, 9/10, 10/10, and 10/10 at 0, 30, 60,
120 and 240 mg/kg bw/day, respectively), and at 240 mg/kg bw/day in
females. At 240 mg/kg bw/day, decreased body-weight gain, increased
liver weight and paleness of the liver were observed in males. The
NOEL in this study was 30 mg/kg bw/day (Verstraeten et al., 1990).
Table 1. Acute toxicity studies on diclazuril
Species Route Sex LD50 Reference
(mg/kg bw)
Mouse oral M & F >5000 Niemegeers, 1986a
sc M & F >5000 Niemegeers, 1987a
ip M & F >5000 Niemegeers, 1987b
Rat oral M & F >5000 Niemegeers, 1986b
sc M & F >5000 Niemegeers, 1987c
ip M 5000 Niemegeers, 1987d
F >5000 Niemegeers, 1987d
Rabbit dermal M & F >4000 Teuns & Marsboom
1988a,b
Dog oral M & F >5000 Niemegeers, 1987e
In a 3-month toxicity study, SPF Albino Swiss mice (20/sex/group)
were dosed with diclazuril at 1000, 2000 or 3000 mg/kg of feed (equal
to 290, 500 or 850 mg/kg bw/day in males and 290, 610 or 920 mg/kg
bw/day in females). No mortality or adverse gross clinical effects
were noted. At all dose levels, the males showed decreased serum total
bilirubin and other altered serum variables, increased liver weight
and centrilobular hepatocellular swelling. Cytoplasmic vacuolation was
seen in 1 male at 500 mg/kg bw/day, and in 3 males at 850 mg/kg
bw/day. A NOEL was not identified in this study. It was noted that
feed wasting had probably caused overestimation of test article intake
and that the calculated dosage was about twice that obtainable from
dietary intake conversion tables (Verstraeten et al., 1992a).
2.2.2.2 Rats
In two dietary studies, Wistar rats (20/sex/group) were dosed
with diclazuril for 3 months at 50, 200 or 800 mg/kg of feed (equal to
4, 17 or 69 mg/kg bw/day in males and 6, 21 or 89 mg/kg bw/day in
females), and at levels of 1000, 2000 or 3000 mg/kg (equal to 71, 140
or 210 mg/kg bw/day in males and 82, 160 or 240 mg/kg bw/day in
females).
In the first study, male rats dosed at 17 and 69 mg/kg bw/day
showed centrilobular swelling with an increased presence of lipid
vacuoles in the centrilobular hepatocytes. Increased absolute and
relative liver weights were seen in both males and females at the
highest dose levels. The NOELs for these liver effects were 4 and
21 mg/kg bw/day for males and females, respectively (Verstraeten
et al., 1986a).
In the second study, swelling of the centrilobular hepatocytes
was seen in both male and female groups at all doses. Hepatocytic
cytoplasmic vacuolization was noted in males at doses of 140 and
210 mg/kg bw/day. No mortalities occurred in either sex at any dose
level in this study, nor were any relevant changes revealed by
clinical chemistry, haematology or urinalysis. Relative liver weights
were, however, increased in males and females in the highest dose
group. A NOEL was not identified in this study (Verstraeten et al.,
1992b).
In a 12-month study in Wistar rats (20/sex/group) with
diclazuril, doses were 16, 63, 250 and 1000 mg/kg of feed (equal to 1,
4, 18 or 74 mg/kg bw/day in males and 2, 6, 23 or 88 mg/kg bw/day in
females). No drug-related changes were observed at doses up to 6 mg/kg
bw/day for females and 18 mg/kg bw/day for males. Dosing at 23 and
88 mg/kg bw/day in females resulted in the presence of histiocytic
aggregates in the mesenteric lymph node. At 74 and 88 mg/kg bw/day in
males and females, respectively, histological examination showed
centrilobular swelling in the hepatocytes (males only), histiocytic
aggregates in the mesenteric lymph node and an increased incidence of
foamy cells in the lungs. There was no effect on clinical signs, food
consumption, body-weight gain, mortality, haematology, serum analysis
or urinalysis. The NOELs for the liver effects observed in this study
were 6 mg/kg bw/day for females and 18 mg/kg bw//day for males
(Verstraeten et al., 1988a).
2.2.2.3 Dogs
Diclazuril in gelatin capsules was administered to beagle dogs
(4/sex/group) at doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two
additional male and female animals in the control and high-dose groups
were included for a 1-month withdrawal study. At 80 mg/kg bw/day in
both males and females, a fine granular, yellow to brown pigment was
seen in the cytoplasm of the hepatocytes. In males at this dose level,
a significant increase in serum BUN values was also seen. Both liver
pathology and BUN values were normal in the high-dose animals
following the 1-month recovery period (Verstaeten et al., 1986b).
A 12-month toxicity study in beagle dogs of the same design and
dose levels confirmed the findings of the earlier study. The liver
changes were comparable in nature and intensity in both studies. The
NOEL was 20 mg/kg bw/day (Verstraeten et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 25-month combined long-term toxicity/carcinogenicity study,
Swiss mice (50/sex/group) were administered doses of diclazuril of 16,
63, 250 or 1000 mg/kg of feed (equal to 3, 11, 47 or 190 mg/kg bw/day
in males and 4, 14, 53 or 220 mg/kg bw/day in females).
Males at 185 mg/kg bw/day showed reduced body-weight gain and
food consumption and an increased incidence of cachexia. Increased
incidences of hepatic changes, mainly characterized by parenchymal
centrilobular swelling, and fatty overload characterized by the
presence of lipid vacuoles in the centrilobular hepatocytes and
swollen sinusoidal cells, were seen in males at 11, 47 and 190 mg/kg
bw/day, and in females at 220 mg/kg bw/day. The NOEL in this study was
16 mg/kg of feed, equal to 3 mg/kg bw/day (Verstraeten et al., 1989a).
2.2.3.2 Rats
Diclazuril was given in the diet for 28 months to Wistar rats
(50/sex/group) at dose levels of 16, 63, 250 or 1000 mg/kg of feed,
equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20, or
80 mg/kg bw/day in females. At 250 and 1000 mg/kg of feed,
diclazuril-related changes were the increased presence of
histiocytosis (males at 61 mg/kg bw/day, females at 20 and 80 mg/kg
bw/day) and of pigmented macrophages (females at 80 mg/kg bw/day) in
the mesenteric lymph nodes. The lung histiocytosis was observed and
illustrated by an increased presence of foamy cells. The Committee
regarded the highest dose used in this study to be sufficient to
adequately assess carcinogenicity. Taking this together with the
absence of a biologically significant increase in the occurrence of
tumours of any type at any location, the Committee concluded that
diclazuril was not carcinogenic in this study. The NOEL was 63 mg/kg
of feed, equal to 4 mg/kg bw/day (Verstraeten et al., 1989b).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A 2-generation study was performed in Wistar rats, with each
generation producing 2 litters. Doses were 50, 200 or 800 mg/kg of
feed, equivalent to 5, 20 or 80 mg/kg bw/day, given continuously in
the diet. The copulation and fertility indices and the duration of
gestation were comparable between controls and dosed animals.
Teratogenicity was not observed in any litter. In the first
generation, the only adverse effect observed was a lowered birth
weight in pups at 80 mg/kg bw/day. In the second generation,
body-weight gain during pregnancy was slightly lower at 80 mg/kg
bw/day, and food consumption during pregnancy and lactation was
decreased at 20 and 80 mg/kg bw/day, indicating maternal toxicity at
these doses. Birth weight at 80 mg/kg bw/day and the weight of pups at
weaning and survival rate at 20 and 80 mg/kg bw/day were decreased.
The NOEL in this study was 50 mg/kg of feed, equivalent to 5 mg/kg
bw/day (Dirkx et al., 1988a).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
In two studies in Wistar rats, diclazuril was given in the diet
at 12.5-1600 mg/kg, equivalent to 1-160 mg/kg bw/day, from days 6 to
16 of pregnancy. Dosing at 1 and 5 mg/kg bw/day did not adversely
influence dams or their progeny. At 20-160 mg/kg bw/day, slight
maternal toxicity was observed, characterized by a lower body-weight
gain. Litter weights were also decreased in these dosage groups.
Teratogenicity was not evident at any dose level. The NOEL in these
studies was 5 mg/kg bw/day (Dirkx et al., 1987a,b).
2.2.5.2 Rabbits
Oral dosing of New Zealand white rabbits with diclazuril by
gavage from days 6 to 18 of pregnancy, at doses of 5, 20 or 80 mg/kg
bw/day did not result in any adverse effect on the dams or their
litter. No teratogenicity was observed (Dirkx et al., 1988b)
In a repeat study, doses were 40, 80 or 160 mg/kg bw/day. There
was no evidence in these studies that the rabbits were exposed to
sufficient diclazuril to evaluate the teratogenicity of the drug
(Gillardin et al., 1989).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with diclazuril are
summarized in Table 2.
2.3 Observations in humans
Humans have had limited exposure to diclazuril. Since it is
active against Eimeria species, which is the cause of coccidiosis in
animals, it has been administered to AIDS patients with Isospora
belli infection. Eight patients with I. belli diarrhea were
treated with 200 mg diclazuril for 7 days. Clinical improvement was
seen and the diarrhea disappeared. No side effects of the drug was
cited (Kayembe et al., 1989).
Table 2. Results of genotoxicity studies on Diclazuril
Test System Test Object Dose Results Reference
DNA Repair Test Induction of UDS in primary 0.3-30 µg/ml Negative Weterings, 1985
rat hepatocytes
SOS Chromotest Induction of galactosidase 1-1000 ng/well Negative ±S9 Vanparijs, 1985
synthesis in genetically
engineered E. coli K-12
Ames Test Histidine reversion in S. 10-500 µg/plate Negative de Meester &
(2 test reports) typhimurium; tryptophan Leonard, 1985;
reversion in E. coli de Meester &
Leonard, 1986
Mouse Lymphoma Mammalian cell gene mutation; 5-100 µg/ml Negative ±S9 Young, 1989
TK+/- Assay thymidine kinase locus;
trifluorothymidine resistance
Drosophila SLRL Sex-linked recessive lethality 500, 2000 ppm Negative Vanparijs, 1986a
Test
Human Lymphocyte Structural chromosome 25-300 µg Negative Leonard, 1988
Chromosome aberrations
Aberrations
Table 2. Results of genotoxicity studies on Diclazuril (cont'd)
Test System Test Object Dose Results Reference
Micronucleus Test Induction of micronuclei in 80-5120 mg/kg Negative Vanparijs, 1986c;
(2 test reports) mouse bone marrow Vanparijs, 1991
Mouse Dominant Induction of dominant lethal 40-160 mg/kg Inadequate1 Vanparijs, 1986b
Lethal Test mutations in male mouse germ
cells
1 No mortality or clinical signs of toxicity and no evidence of target tissue exposure to the test article were
reported. The doses were inadequate in this test.
Diclazuril at a similar dose was administered for crypto-
sporidiosis in a single AIDS patient. A complete eradication of
cryptosporidial oocysts from the faeces was seen following treatment.
No cutaneous reactions or modification of biochemical parameter were
documented. There were no comments on additional effects following
drug treatment (Menichetti et al., 1991).
2.4 Microbiological properties
The in vitro antimicrobial activity of diclazuril was
investigated in 11 pathogenic and saprogenic fungi, 6 zoo-pathogenic
and 5 plant-pathogenic bacteria, including both Gram-positive and
Gram-negative organisms. It was found that diclazuril possesses
negligible antifungal and no antibacterial activity at 100 µg/ml
(Van Cutsem et al., 1985).
In another experiment, the antimicrobial activity of diclazuril
was evaluated in 2 strains of Bacillus subtilis and in 1 strain of
Sarcina lutea, which are commonly used for testing residues of
antibiotics in meat or other food products. No antibacterial activity
was found (Van Cutsem et al., 1986).
2.5 Pharmacological effects
In a general screen for drug activity, diclazuril was dosed to
rats by the i.p. route at 40 mg/kg bw. Diclazuril was devoid of
neuroleptic, sedating, analgesic, hypnotic, cholimimetic, constipating
or anticonvulsant properties (Lampo et al., 1995).
3. COMMENTS
The Committee considered data from studies on acute, short-term,
and long-term toxicity/carcinogenicity, reproductive and developmental
toxicity, metabolism, pharmacokinetics and antimicrobial effects.
Pharmacokinetic studies in rats given radiolabelled drug
suggested that the absorption of diclazuril from the gut was limited,
as 90% of the radioactive dose was excreted in the faeces within 24 h.
After 4 days, 92% of the dose had been excreted in the faeces and
0.04% in the urine. Unchanged diclazuril accounted for most of the
total radioactivity in the faeces, with two metabolites accounting for
less than 0.5%. Distribution into the tissues was rapid but limited.
The concentration of total radioactivity in the liver was about half
that in the plasma; the corresponding figures for kidney, lung and
heart were in the range 20-30%. With time, metabolites gradually
accounted for a higher proportion of the tissue radioactivity.
Single oral doses of diclazuril of up to 5 g/kg bw caused no
mortality in experimental animals. Clinical effects in mice and rats
were non-specific and mainly of CNS origin. In dogs, vomiting and
defaecation were seen after treatment.
In a 3-month dose-ranging study in mice, mortality was not seen
with oral doses up to 1600 mg/kg in the diet, equivalent to 240 mg/kg
bw/day. At this dose, changes indicative of mild liver damage were
reported, including increases in relative liver weight, swelling of
the centrilobular hepatocytes and vacuolization of hepatocytes in
males; females showed swelling of the centrilobular hepatocytes only.
The NOEL was 30 mg/kg bw/day.
In a 3-month toxicity study in mice in which the highest dose was
3000 mg/kg in the diet, equal to 850 mg/kg bw/day in males and
920 mg/kg bw/day in females, neither mortality nor adverse clinical
effects were noted. No mortality occurred in treated mice, but at all
dose levels (290-850 mg/kg bw/day) males showed decreased serum levels
of total bilirubin. This and other changes revealed by haematological
and biochemical changes in the blood, although occasionally
statistically significant, were either marginal with respect to
historical controls or not dose-related. Liver weights were increased
at all doses in males and at the highest dose in females. Swelling of
the centrilobular hepatocytes was observed in males dosed at 500 and
850 mg/kg bw/day. A NOEL was not identified in this study. It was
noted that wastage of feed had probably caused overestimation of
dietary intake of the drug and that the calculated dosage was about
twice that derived from dietary intake conversion tables.
Rats were given diclazuril at doses of 50-800 mg/kg in the diet,
equal to 4-69 mg/kg bw/day in males and 6-89 mg/kg bw/day in females
for 3 months. Male rats dosed at 17 and 69 mg/kg bw/day showed
swelling of the centrilobular hepatocytes with an increase in lipid
vacuolization. Absolute and relative liver weights were increased in
both males and females at the highest dose. These changes fell within
the range of historical controls. The NOELs in this study were 4 and
21 mg/kg bw/day for males and females, respectively.
In a second 3-month study, in which rats were dosed at 1000-3000
mg/kg in the diet, equal to 71-210 mg/kg bw/day in males and 82-240
mg/kg bw/day in females, swelling of centrilobular hepatocytes
was seen in both sexes at all doses. Vacuolization of the cytoplasm of
hepatocytes was noted in males dosed at 140 mg/kg bw/day and above. No
deaths occurred in either sex at any dose level in this study, nor was
any relevant change revealed by clinical chemical analysis,
haematology or urinalysis. Relative liver weights were, however,
increased in males and females in the highest-dose group. A NOEL was
not identified in this study.
In a 12-month study in rats given diclazuril at doses of
16-1000 mg/kg in the diet, equal to 1-74 mg/kg bw/day in males and
2-88 mg/kg bw/day in females, no drug-related changes were observed at
doses at or below 6 and 18 mg/kg bw/day for females and males,
respectively. Histological examination showed histocytic aggregates in
the mesenteric lymph node in males at 74 mg/kg bw/day and at both 23
and 88 mg/kg bw/day in females. Swelling of centrilobular hepatocytes
was seen in males dosed at 74 mg/kg bw/day, while females dosed at
88 mg/kg bw/day had increased clusters of foamy cells in the lungs.
The NOEL in this study was 6 mg/kg bw/day.
Diclazuril capsules were administered to 4 dogs of each sex at
doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two additional male
and female animals were included in the control and highest-dose
groups in a 1-month withdrawal study. At 80 mg/kg bw/day in both males
and females, a free granular, yellow brown pigment was present in the
cytoplasm of hepatocytes. In males at this dose level, a significant
increase in serum urea (expressed as nitrogen) was also seen. Both
changes were reversible, as shown by the 4 animals of the recovery
group. A 12-month toxicity study in dogs of the same design and dose
levels as the 3-month study but lacking a recovery group, confirmed
the findings of the earlier study. The liver changes were comparable
in nature and intensity in both studies. The NOEL in these studies was
20 mg/kg bw/day.
A 25-month combined long-term toxicity/carcinogenicity study was
performed in mice. This study included periodic haematological
examinations, analysis of serum samples at the end of the study and
extensive histopathological examinations. Diclazuril was administered
at dose levels of 16, 63, 250 or 1000 mg/kg in the diet, equal to 3,
11, 47 or 190 mg/kg bw/day in males and 4, 14, 53 or 220 mg/kg bw/day
in females. While there was evidence of inhibition of the rate of
body-weight gain in male mice at the highest-dose, histopathological
examination revealed only minor hepatic morphological changes of the
type also found in the 3-month studies in mice. In view of the results
of the two 3-month studies in mice of the same strain, the Committee
considered that the toxic potential of doses exceeding those used in
the carcinogenicity study had been evaluated. Analysis of serum
samples collected at the end of these two 3-month studies provided
evidence of the existence of a threshold of between 1000 and
2000 mg/kg in the diet, above which the mouse is unable to absorb
ingested diclazuril. This threshold is close to the highest dose used
in the long-term toxicity/carcinogenicity study. In the light of this
finding, together with the absence of any biologically significant
increase in the incidence of tumours, the Committee concluded that
diclazuril had been adequately tested and was not carcinogenic in
mice. The NOEL in this study was 3 mg/kg bw/day.
A 28-month combined long-term toxicity/carcinogenicity study was
performed in rats given 16, 63, 250 or 1000 mg/kg diclazuril in the
feed (equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20 or
80 mg/kg bw/day in females). This study included periodic
haematological examinations, analysis of serum samples at the end of
the study and extensive histopathological examinations. As in the
study with mice, no effects of obvious toxicological significance were
noted, but reactive histiocytosis of the mesenteric lymph nodes was
observed in female rats at 20 and 80 mg/kg bw/day and in males at
61 mg/kg bw/day. However, as with the studies in mice, serum samples
collected at the end of the two 3-month studies allowed an exploration
of the relationship between the oral intake of diclazuril and the
resultant concentration in serum. A threshold existed for the
absorption of diclazuril in the region of 2000 mg/kg in the feed, i.e.
about two times higher than the highest dose used in the long-term
toxicity/carcinogenicity study. In the light of this finding together
with the absence of any biologically significant increase in the
incidence of tumours, the Committee concluded that diclazuril had been
adequately tested and was not carcinogenic in the rat. The NOEL in
this study was 4 mg/kg bw/day.
A two-generation reproductive toxicity study was performed in
rats in which each generation produced two litters. The doses were
equivalent to 5, 20 or 80 mg/kg bw/day. In the first generation, the
only adverse effect observed was a reduction in birth weight of pups
at 80 mg/kg bw/day. In the second generation, body-weight gain of dams
during pregnancy was slightly lower at 80 mg/kg bw/day, and food
consumption during pregnancy and lactation was decreased at 20 and
80 mg/kg bw/day, indicating maternal toxicity at these doses. As in
the first generation, birth weights were lower at 80 mg/kg bw/day, and
the survival rates and weight of pups at weaning were also decreased
at 20 and 80 mg/kg bw/day. The NOEL in this study was 5 mg/kg bw/day.
In two teratogenicity studies in rats, diclazuril was
administered in the feed at doses equivalent to 1-160 mg/kg bw/day
from days 6 to 16 of pregnancy. Dosing equivalent to 1 and 5 mg/kg
bw/day did not result in any adverse effects on the dams or their
progeny. At a dose equivalent to 20 mg/kg bw/day, however, slight
maternal toxicity was observed, characterized by a reduction in
body-weight gain. Litter weights were decreased in groups receiving
20-160 mg/kg bw/day. At none of the doses was teratogenicity evident.
The NOEL in these studies was 5 mg/kg bw/day.
In two studies in rabbits, administration of diclazuril by gavage
at doses of 5-160 mg/kg bw/day from days 6 to 18 of pregnancy did not
result in any adverse effects on the dams or their offspring. From
these findings and because studies of the enteric absorption of
diclazuril in the rabbit were lacking, the Committee concluded that
there was no evidence that exposure to diclazuril was sufficient to
enable the teratogenicity of the drug to be evaluated. This conclusion
was supported by the occurrence of adverse effects in rabbits exposed
to Eimeria spp. following administration of the drug in the feed at
a dose equivalent to 13 mg/kg bw/day for 5 weeks. Furthermore, the
Committee noted that materno- and fetotoxicity had been observed in
rabbits dosed with a diclazuril analogue at 80 mg/kg bw/day.
Diclazuril was studied in a range of in vivo and in vitro
assays with a variety of genetic end-points. Negative findings in all
these assays enabled the Committee to conclude that diclazuril was not
genotoxic.
4. EVALUATION
The Committee established a temporary ADI of 0-20 µg/kg bw for
diclazuril, based on the NOEL of 3 mg/kg bw/day in the 2-year
toxicity/carcinogenicity study in mice and a safety factor of 200. The
ADI was rounded to one significant figure, consistent with accepted
rounding procedures (Annex 1, reference 91, section 2.7).
The results of a teratogenicity study in rabbits supported by
evidence that the doses administered were sufficiently high for the
teratogenic potential of diclazuril to be adequately explored are
required for evaluation in 1998.
5. REFERENCES
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987a).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1504. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987b).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1651. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1988a). Diclazuril-
oral 2-generation reproduction study with two litters per generation
in Wistar rats. Unpublished nonclinical laboratory study Exp. No. 1663.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren H., and Marsboom, R. (1988b). Diclazuril -
oral embryotoxicity and teratogenicity study in New Zealand White
rabbits. Unpublished nonclinical laboratory study Exp. No. 1821.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Gillardin, J.M., Van Cauteren H., Sanz, G., and Marsboom, R. (1989).
Embryotoxicity and teratogenicity study in New Zealand white rabbits.
Unpublished nonclinical laboratory study Exp. No. 2212. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Kayembe K., Desmet P., Henry, M.C., and Stoffels, P. (1989).
Diclazuril for Isospora belli infection in AIDS [Letter] Lancet;
1:1397-1398.
Lampo, A., Vanparys, P.H., Vandenberghe, J., Coussement, W.,
Teuns, G., Maes, L., and Van Cauteren, H. (1995). R 64433 - Review
report on the pharmacodynamic and toxicological documentation on
diclazuril. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Leonard, A., et al.. (1988). The in vivo chromosome aberration assay
on human lymphocytes. Unpublished nonclinical laboratory report
SCK 86/02D/R 64433, Mol, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Mannens, G., Van Leemput, L., and Heykants, J. (1992). A study on the
excretion and metabolism of 14C-Diclazuril in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished non-clinical
pharmacokinetics report on R 64433 FK 1217. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
de Meester, C. and Leonard A. (1985). R 64433-Ames reversion mutation
test with Salmonella typhimurium and Escherichia coli. Unpublished
nonclinical laboratory study. Exp. No. 19, UCL, Laboratory of Terato-
genesis and Mutagenesis. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
de Meester, C. and Leonard A. (1986). R 64433-Ames reversion mutation
test with S. typhimurium and E. coli. Unpublished nonclinical
laboratory study Exp. No 22, UCL, Laboratory of Teratogenesis and
Mutagenesis, Brussels, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Menichetti, F., Moretti, M.V., Marroni, M., Papili, R., and
DiCandilo, F. (1991). Diclazuril for cryptosporidiosis in AIDS
[Letter]. Am. J. Med.; 90(2): 271-2.
Meuldermans, W., Hendrickx, J., Mostmans, E., Verboven, P.,
Borgmans, C., Michiels, M., and Heykants, J. (1988a). The excretion
and metabolism of Diclazuil after a single oral dose in rabbits.
Unpublished preclinical research report. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Michiels, M., Hendrickx, J., Geerts, R.,
Hurkmans, R., Mostmans, E., Lauwers, W., and Heykant J. (1988b)
14C-Diclazuril (14C-R64433) in the broiler chicken: Total
radioactivity depletion and metabolism after single oral
administration at 1 mg/kg. Unpublished preclinical research report.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Van Beijsterveldt, L., Hendrickx, J., Geerts, R.,
Mostmans, E., Borgmans, C., Verboven, P., Woestenborghs, R., Van
Leemput, L., and Heykant, J. (1990). 14C-Diclazuril (14C-R64433)
in the turkey: Absorption, metabolism, excretion and tissue depletion
after a single oral dose of 1 mg/kg. Unpublished preclinical research
report on R64433. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Michiels, M., Hendriks, R., Geerts, R., Meuldermans, W., and
Heykant, J. (1988). Absorption and tissue distribution of
14C-Diclazuril (14C-R64433) in the rabbit after a single oral dose
of 1 mg/kg. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Monbaliu, J., Van Leemput, L., and Heykants, J. (1993). Plasma levels
of Diclazuril in sheep after oral administration of a 0.25% drench
formulation at 1 mg Diclazuril per kg. Unpublished non-clinical
pharmacokinetics report on R64433 FK-992. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986a). The acute oral toxicity of R 64433 in
mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986b). The acute oral toxicity of R 64433 in
rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987a). The acute subcutaneous toxicity of R 64433
in mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987b). The acute intraperitoneal toxicity of
R 64433 in mice. Unpublished preclinical research report. Submitted to
WHO (JECFA) by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987c) The acute subcutaneous toxicity of R 64433
in rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987d) The acute intraperitoneal toxicity of
R 64433 in rats. Unpublished preclinical research report. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987e). The acute oral toxicity of R 64433 in
Beagle dogs. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988a). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 1917.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988b). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 2035.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Beijsterveldt, L., Van Leemput, L., and Heykants, J. (1992).
Absorption and tissue distribution of 14C-Diclazuril in the male
Wistar rat after single oral administration at 10 mg/kg in aqueous
suspension. Unpublished non-clinical pharmacokinetics report on R64433
FK-1213. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven, F., and Zaman, R. (1985). The
antimicrobial activity of R 64433. Unpublished nonclinical laboratory
study. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven F., and Zaman, R. (1986). The antimicrobial
activity of R 64433: additional data. Unpublished nonclinical
laboratory study. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1985). R 64433 - SOS chromosoomtest
in Escherichia coli. Unpublished nonclinical laboratory study
No. 1600. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986a). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished nonclinical laboratory
study No. 1501 + 1657. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986b). R 64433 - Dominant lethal
test in male mice. Unpublished nonclinical laboratory study
Exp. No 1609. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986c). R 64433 - Micronucleus test
in mice. Unpublished nonclinical laboratory study Exp. No 1744.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1991). Micronucleus test in mice.
Unpublished nonclinical laboratory study, Exp. No. 2506. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1986a). Subchronic toxicity study in Wistar rats (repeated dosage for
3 months). Unpublished nonclinical laboratory study Exp. No. 1502.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Van Cauteren, H., and Marsboom, R.
(1986b). Subchronic toxicity study in Beagle dogs (repeated dosage for
3 months + 1 month recovery). Unpublished nonclinical laboratory study
Exp. No. 1503. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1988a). Oral longterm toxicity study in Wistar rats (repeated dosage
for 12 months). Unpublished nonclinical laboratory study Exp.
No. 1755. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Teuns, G., Coussement, W., Van Cauteren, H., and
Marsboom, R. (1988b). Oral longterm toxicity study in Beagle dogs
(repeated dosage for 12 months). Unpublished nonclinical laboratory
study Exp. No. 1698. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauterern, H., and Marsboom, R. (1989a).
R 64433 - Oral carcinogencity study in Swiss mice (repeated dosage for
18 up to 24 months). Unpublished nonclinical laboratory study Exp.
No. 1649. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauteren, H., and Marsboom, R. (1989b).
R 64433 - Oral carcinogenicity study in Wistar rats (repeated dosage
for 24 up to 30 months). Unpublished nonclinical laboratory study Exp.
No. 1650. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1990). Subchronic oral feeding toxicity study (pilot) in SPF Albino
Swiss mice. Unpublished nonclinical laboratory study Exp. No. 1665.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A. Coussement, W., and
Van Cauteren, H. (1992a). Three-month oral toxicity study in SPF
Albino Swiss mice. Unpublished nonclinical laboratory study Exp.
No. 2614. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A., Coussement, W., and
Van Cauteren, H. (1992b). Three-month oral toxicity in SPF Wistar
rats. Unpublished nonclinical laboratory study Exp. No. 2613.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Weterings, PJJM., et al. (1985). Evaluation of the DNA repair
inducing ability of R 64433 in a primary culture of rat hepatocytes.
Unpublished nonclinical report No. 0114/ER 154, Notox Pathobiology
Research, The Netherlands. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Young, R.R. (1989). Mutagenticity test on in mouse lymphoma forward
mutation assay. Unpublished nonclinical report 10779-0-431, Hazleton
Laboratories America, Inc., USA. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Rats
Male Wistar rats received 10 mg 14C-diclazuril/kg bw in an
aqueous suspension. On day 1, 90% of the dosed radioactivity was
excreted in the faeces. After 4 days, 92% was excreted in the faeces
and 0.04% in the urine. Unchanged diclazuril (UD) accounted for most
of the total radioactivity in the faeces, with two metabolites
accounting for less than 0.5% (Mannens et al., 1992).
In another study, concentrations of total radioactivity and UD
were measured in blood, plasma and tissues for up to 96 h after oral
administration of 10 mg 14C-diclazuril/kg bw in an aqueous
suspension to male Wistar rats. The absorption of diclazuril was
limited. Most of the dose was recovered from the GI contents. Maximum
plasma concentrations of total radioactivity and UD were approximately
1 µg/ml and occurred after 8 h. On day 1, total radioactivity in
plasma was almost exclusively UD, but later the UD/total radioactivity
ratio gradually declined. The blood-to-plasma ratio of total
radioactivity was about 0.7, indicating a limited distribution to
blood cells. Distribution to the systemic tissues was rapid but
limited. The concentration of total radioactivity in the liver was
about 50% of the plasma level and kidney, lung and heart levels were
about 20-30%, while muscle and brain concentrations were 5-7% of the
plasma levels. UD depleted from tissues with a half-life of 1.5 days.
The half-life of total radioactivity was 53.0 h (Van Beijsterveldt
et al., 1992).
2.1.1.2 Rabbits
Absorption, distribution, metabolism and excretion was studied in
2 separate studies, with male rabbits receiving a single oral dose of
14C-diclazuril at 1 mg/kg bw. The excretion of radioactivity was
fairly rapid since within 48 h, 70% was recovered in the faeces and 3%
in the urine. After 10 days, more than 98% was excreted. Gall bladder
contained radioactivity, but accounting at most for 0.02% of the dose,
implying that biliary excretion was a minor elimination pathway.
Various metabolites were identified in urine. Two main metabolites
were a glucuronide and a sulfate conjugate. In faeces, UD represented
the main part of the radioactivity, accounting for approximately 66%
of the dose. Some minor metabolites were observed. None of the urinary
or faecal metabolites accounted for more than 2% of the dose. UD
represented almost all the plasma radioactivity at least up to 120 h
after dosing. The elimination from plasma proceeded with an apparent
half-life of 2-2.5 days. The distribution to tissues was limited and
the elimination half-lives were similar to that from plasma, except
for the liver with an elimination half-life of 3 days. The easily
extractable liver residue indicated freedom from concern over bound
residues (Meuldermans et al., 1988a; Michiels et al., 1988).
2.1.1.3 Chickens
In a single-dose study, 14C-diclazuril was administered to
broiler chickens in a lactose mixture at a dose of 1 mg/kg bw. Maximum
radioactivity concentrations of 1.5-2.0 µg-eq./ml were attained in
plasma 6 h after administration. The elimination subsequently
proceeded with a half-life of approximately 50 h.
There was a rapid equilibrium between plasma and tissue levels.
The tissue concentrations were 2 to 10 times lower than the
corresponding plasma concentrations. The liver and the kidneys showed
the highest concentrations at all time points. The half-life in
tissues was approximately 50 h, similar to that found in plasma.
Parent diclazuril accounted for more than 90% in the liver at 24-h
liver, and no metabolites were detectable (<4%). About half of the
dose was excreted within 24 h, almost exclusively as UD. After 10
days, the cumulative excretion was over 95%. A degradation product,
coded DM5, accounted for 5.3% of the dose in the 0-96 h excreta. Other
metabolites accounted for <2% each. DM5 was not definitively
identified. It was demonstrated to be a derivative of
4-amino-2,6-dichloro-a-(4-chlorophenyl) benzeneacetonitrile, formed by
cleavage and subsequent degradation of the triazine-dione ring
(Meuldermans et al., 1988b).
2.1.1.4 Turkeys
In a single-dose study, 14C-diclazuril was administered in a
lactose mixture at a dose of 1 mg/kg bw. Maximum radioactivity
concentrations of 1.78 ± 0.19 µg-eq./ml were attained in plasma 6 h
after administration. The subsequent elimination proceeded with a
half-life of approximately 38 h. The blood-to-plasma ratio was on
average 0.66 throughout the 10-day observation period, implying a
minor distribution to the blood cells. There was a rapid but limited
distribution between plasma and tissues. The tissue concentrations
were slightly to markedly lower than the corresponding plasma
concentrations. The depletion rate was similar in all tissues with a
half-life in the range of 34-46 h. UD accounted for 98% of the sample
radioactivity for the livers sampled after 6 h, and 85% for the livers
sampled after 48 and 72 h. No metabolites accounted for more than 10%
of the radioactivity in liver. At 24 h after dosing, 55% of the dose
was excreted. The cumulative excretion was 88% after 5 days and 94.8 ±
0.8% after 10 days. At least 8 metabolites were identified in excreta.
Parent diclazuril, however, accounted for the major part of the
excreted radioactivity, representing 55.8% of the dose. The
triazine-dione ring cleavage product previously seen in broilers
accounted for 6.3% of the dose in the 0-96 h excreta. An unidentified
metabolite accounted for about 2.4% of the radioactivity. All other
metabolites accounted for < 2% each (Meuldermans et al., 1990).
2.1.1.5 Sheep
Poor absorption of diclazuril was observed in sheep after oral
administration of 1 mg/kg bw. Maximum concentrations in plasma of
0.012 to 0.016 µg/ml were reached at 24-48 h after dosing and were
lower than the quantification limit (< 0.01 µg/ml) at all other
sampling times (Monbaliu et al., 1993).
In summary, in all species investigated, there were little
indications of metabolism of diclazuril. As a result,
diclazuril-derived substances were eliminated mainly as unchanged
parent substance in the excreta of bird species, and in the faeces of
rats and rabbits. Urine of the latter species was only a very minor
excretion route and some metabolized substances could be observed. In
edible tissues, the residues consisted almost entirely of unchanged
diclazuril.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with diclazuril are
summarized in Table 1.
Only in mice and rats dosed i.p. at 5000 mg/kg bw was mortality
observed. Clinical effects in mice and rats were non-specific and were
mainly on the CNS. In dogs, vomiting and defecation were seen after
treatment. Autopsy did not reveal drug-related macroscopic changes in
any study.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 3-month dose-range-finding study, SPF Albino Swiss mice
(10/sex/group) were dosed orally at 200, 400, 800 or 1600 mg/kg of
feed, equivalent to 30, 60, 120 or 240 mg/kg bw/day. Increased
centrilobular swelling of the liver was observed in male mice at the
three highest doses (3/10, 4/10, 9/10, 10/10, and 10/10 at 0, 30, 60,
120 and 240 mg/kg bw/day, respectively), and at 240 mg/kg bw/day in
females. At 240 mg/kg bw/day, decreased body-weight gain, increased
liver weight and paleness of the liver were observed in males. The
NOEL in this study was 30 mg/kg bw/day (Verstraeten et al., 1990).
Table 1. Acute toxicity studies on diclazuril
Species Route Sex LD50 Reference
(mg/kg bw)
Mouse oral M & F >5000 Niemegeers, 1986a
sc M & F >5000 Niemegeers, 1987a
ip M & F >5000 Niemegeers, 1987b
Rat oral M & F >5000 Niemegeers, 1986b
sc M & F >5000 Niemegeers, 1987c
ip M 5000 Niemegeers, 1987d
F >5000 Niemegeers, 1987d
Rabbit dermal M & F >4000 Teuns & Marsboom
1988a,b
Dog oral M & F >5000 Niemegeers, 1987e
In a 3-month toxicity study, SPF Albino Swiss mice (20/sex/group)
were dosed with diclazuril at 1000, 2000 or 3000 mg/kg of feed (equal
to 290, 500 or 850 mg/kg bw/day in males and 290, 610 or 920 mg/kg
bw/day in females). No mortality or adverse gross clinical effects
were noted. At all dose levels, the males showed decreased serum total
bilirubin and other altered serum variables, increased liver weight
and centrilobular hepatocellular swelling. Cytoplasmic vacuolation was
seen in 1 male at 500 mg/kg bw/day, and in 3 males at 850 mg/kg
bw/day. A NOEL was not identified in this study. It was noted that
feed wasting had probably caused overestimation of test article intake
and that the calculated dosage was about twice that obtainable from
dietary intake conversion tables (Verstraeten et al., 1992a).
2.2.2.2 Rats
In two dietary studies, Wistar rats (20/sex/group) were dosed
with diclazuril for 3 months at 50, 200 or 800 mg/kg of feed (equal to
4, 17 or 69 mg/kg bw/day in males and 6, 21 or 89 mg/kg bw/day in
females), and at levels of 1000, 2000 or 3000 mg/kg (equal to 71, 140
or 210 mg/kg bw/day in males and 82, 160 or 240 mg/kg bw/day in
females).
In the first study, male rats dosed at 17 and 69 mg/kg bw/day
showed centrilobular swelling with an increased presence of lipid
vacuoles in the centrilobular hepatocytes. Increased absolute and
relative liver weights were seen in both males and females at the
highest dose levels. The NOELs for these liver effects were 4 and
21 mg/kg bw/day for males and females, respectively (Verstraeten
et al., 1986a).
In the second study, swelling of the centrilobular hepatocytes
was seen in both male and female groups at all doses. Hepatocytic
cytoplasmic vacuolization was noted in males at doses of 140 and
210 mg/kg bw/day. No mortalities occurred in either sex at any dose
level in this study, nor were any relevant changes revealed by
clinical chemistry, haematology or urinalysis. Relative liver weights
were, however, increased in males and females in the highest dose
group. A NOEL was not identified in this study (Verstraeten et al.,
1992b).
In a 12-month study in Wistar rats (20/sex/group) with
diclazuril, doses were 16, 63, 250 and 1000 mg/kg of feed (equal to 1,
4, 18 or 74 mg/kg bw/day in males and 2, 6, 23 or 88 mg/kg bw/day in
females). No drug-related changes were observed at doses up to 6 mg/kg
bw/day for females and 18 mg/kg bw/day for males. Dosing at 23 and
88 mg/kg bw/day in females resulted in the presence of histiocytic
aggregates in the mesenteric lymph node. At 74 and 88 mg/kg bw/day in
males and females, respectively, histological examination showed
centrilobular swelling in the hepatocytes (males only), histiocytic
aggregates in the mesenteric lymph node and an increased incidence of
foamy cells in the lungs. There was no effect on clinical signs, food
consumption, body-weight gain, mortality, haematology, serum analysis
or urinalysis. The NOELs for the liver effects observed in this study
were 6 mg/kg bw/day for females and 18 mg/kg bw//day for males
(Verstraeten et al., 1988a).
2.2.2.3 Dogs
Diclazuril in gelatin capsules was administered to beagle dogs
(4/sex/group) at doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two
additional male and female animals in the control and high-dose groups
were included for a 1-month withdrawal study. At 80 mg/kg bw/day in
both males and females, a fine granular, yellow to brown pigment was
seen in the cytoplasm of the hepatocytes. In males at this dose level,
a significant increase in serum BUN values was also seen. Both liver
pathology and BUN values were normal in the high-dose animals
following the 1-month recovery period (Verstaeten et al., 1986b).
A 12-month toxicity study in beagle dogs of the same design and
dose levels confirmed the findings of the earlier study. The liver
changes were comparable in nature and intensity in both studies. The
NOEL was 20 mg/kg bw/day (Verstraeten et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 25-month combined long-term toxicity/carcinogenicity study,
Swiss mice (50/sex/group) were administered doses of diclazuril of 16,
63, 250 or 1000 mg/kg of feed (equal to 3, 11, 47 or 190 mg/kg bw/day
in males and 4, 14, 53 or 220 mg/kg bw/day in females).
Males at 185 mg/kg bw/day showed reduced body-weight gain and
food consumption and an increased incidence of cachexia. Increased
incidences of hepatic changes, mainly characterized by parenchymal
centrilobular swelling, and fatty overload characterized by the
presence of lipid vacuoles in the centrilobular hepatocytes and
swollen sinusoidal cells, were seen in males at 11, 47 and 190 mg/kg
bw/day, and in females at 220 mg/kg bw/day. The NOEL in this study was
16 mg/kg of feed, equal to 3 mg/kg bw/day (Verstraeten et al., 1989a).
2.2.3.2 Rats
Diclazuril was given in the diet for 28 months to Wistar rats
(50/sex/group) at dose levels of 16, 63, 250 or 1000 mg/kg of feed,
equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20, or
80 mg/kg bw/day in females. At 250 and 1000 mg/kg of feed,
diclazuril-related changes were the increased presence of
histiocytosis (males at 61 mg/kg bw/day, females at 20 and 80 mg/kg
bw/day) and of pigmented macrophages (females at 80 mg/kg bw/day) in
the mesenteric lymph nodes. The lung histiocytosis was observed and
illustrated by an increased presence of foamy cells. The Committee
regarded the highest dose used in this study to be sufficient to
adequately assess carcinogenicity. Taking this together with the
absence of a biologically significant increase in the occurrence of
tumours of any type at any location, the Committee concluded that
diclazuril was not carcinogenic in this study. The NOEL was 63 mg/kg
of feed, equal to 4 mg/kg bw/day (Verstraeten et al., 1989b).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A 2-generation study was performed in Wistar rats, with each
generation producing 2 litters. Doses were 50, 200 or 800 mg/kg of
feed, equivalent to 5, 20 or 80 mg/kg bw/day, given continuously in
the diet. The copulation and fertility indices and the duration of
gestation were comparable between controls and dosed animals.
Teratogenicity was not observed in any litter. In the first
generation, the only adverse effect observed was a lowered birth
weight in pups at 80 mg/kg bw/day. In the second generation,
body-weight gain during pregnancy was slightly lower at 80 mg/kg
bw/day, and food consumption during pregnancy and lactation was
decreased at 20 and 80 mg/kg bw/day, indicating maternal toxicity at
these doses. Birth weight at 80 mg/kg bw/day and the weight of pups at
weaning and survival rate at 20 and 80 mg/kg bw/day were decreased.
The NOEL in this study was 50 mg/kg of feed, equivalent to 5 mg/kg
bw/day (Dirkx et al., 1988a).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
In two studies in Wistar rats, diclazuril was given in the diet
at 12.5-1600 mg/kg, equivalent to 1-160 mg/kg bw/day, from days 6 to
16 of pregnancy. Dosing at 1 and 5 mg/kg bw/day did not adversely
influence dams or their progeny. At 20-160 mg/kg bw/day, slight
maternal toxicity was observed, characterized by a lower body-weight
gain. Litter weights were also decreased in these dosage groups.
Teratogenicity was not evident at any dose level. The NOEL in these
studies was 5 mg/kg bw/day (Dirkx et al., 1987a,b).
2.2.5.2 Rabbits
Oral dosing of New Zealand white rabbits with diclazuril by
gavage from days 6 to 18 of pregnancy, at doses of 5, 20 or 80 mg/kg
bw/day did not result in any adverse effect on the dams or their
litter. No teratogenicity was observed (Dirkx et al., 1988b)
In a repeat study, doses were 40, 80 or 160 mg/kg bw/day. There
was no evidence in these studies that the rabbits were exposed to
sufficient diclazuril to evaluate the teratogenicity of the drug
(Gillardin et al., 1989).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with diclazuril are
summarized in Table 2.
2.3 Observations in humans
Humans have had limited exposure to diclazuril. Since it is
active against Eimeria species, which is the cause of coccidiosis in
animals, it has been administered to AIDS patients with Isospora
belli infection. Eight patients with I. belli diarrhea were
treated with 200 mg diclazuril for 7 days. Clinical improvement was
seen and the diarrhea disappeared. No side effects of the drug was
cited (Kayembe et al., 1989).
Table 2. Results of genotoxicity studies on Diclazuril
Test System Test Object Dose Results Reference
DNA Repair Test Induction of UDS in primary 0.3-30 µg/ml Negative Weterings, 1985
rat hepatocytes
SOS Chromotest Induction of galactosidase 1-1000 ng/well Negative ±S9 Vanparijs, 1985
synthesis in genetically
engineered E. coli K-12
Ames Test Histidine reversion in S. 10-500 µg/plate Negative de Meester &
(2 test reports) typhimurium; tryptophan Leonard, 1985;
reversion in E. coli de Meester &
Leonard, 1986
Mouse Lymphoma Mammalian cell gene mutation; 5-100 µg/ml Negative ±S9 Young, 1989
TK+/- Assay thymidine kinase locus;
trifluorothymidine resistance
Drosophila SLRL Sex-linked recessive lethality 500, 2000 ppm Negative Vanparijs, 1986a
Test
Human Lymphocyte Structural chromosome 25-300 µg Negative Leonard, 1988
Chromosome aberrations
Aberrations
Table 2. Results of genotoxicity studies on Diclazuril (cont'd)
Test System Test Object Dose Results Reference
Micronucleus Test Induction of micronuclei in 80-5120 mg/kg Negative Vanparijs, 1986c;
(2 test reports) mouse bone marrow Vanparijs, 1991
Mouse Dominant Induction of dominant lethal 40-160 mg/kg Inadequate1 Vanparijs, 1986b
Lethal Test mutations in male mouse germ
cells
1 No mortality or clinical signs of toxicity and no evidence of target tissue exposure to the test article were
reported. The doses were inadequate in this test.
Diclazuril at a similar dose was administered for crypto-
sporidiosis in a single AIDS patient. A complete eradication of
cryptosporidial oocysts from the faeces was seen following treatment.
No cutaneous reactions or modification of biochemical parameter were
documented. There were no comments on additional effects following
drug treatment (Menichetti et al., 1991).
2.4 Microbiological properties
The in vitro antimicrobial activity of diclazuril was
investigated in 11 pathogenic and saprogenic fungi, 6 zoo-pathogenic
and 5 plant-pathogenic bacteria, including both Gram-positive and
Gram-negative organisms. It was found that diclazuril possesses
negligible antifungal and no antibacterial activity at 100 µg/ml
(Van Cutsem et al., 1985).
In another experiment, the antimicrobial activity of diclazuril
was evaluated in 2 strains of Bacillus subtilis and in 1 strain of
Sarcina lutea, which are commonly used for testing residues of
antibiotics in meat or other food products. No antibacterial activity
was found (Van Cutsem et al., 1986).
2.5 Pharmacological effects
In a general screen for drug activity, diclazuril was dosed to
rats by the i.p. route at 40 mg/kg bw. Diclazuril was devoid of
neuroleptic, sedating, analgesic, hypnotic, cholimimetic, constipating
or anticonvulsant properties (Lampo et al., 1995).
3. COMMENTS
The Committee considered data from studies on acute, short-term,
and long-term toxicity/carcinogenicity, reproductive and developmental
toxicity, metabolism, pharmacokinetics and antimicrobial effects.
Pharmacokinetic studies in rats given radiolabelled drug
suggested that the absorption of diclazuril from the gut was limited,
as 90% of the radioactive dose was excreted in the faeces within 24 h.
After 4 days, 92% of the dose had been excreted in the faeces and
0.04% in the urine. Unchanged diclazuril accounted for most of the
total radioactivity in the faeces, with two metabolites accounting for
less than 0.5%. Distribution into the tissues was rapid but limited.
The concentration of total radioactivity in the liver was about half
that in the plasma; the corresponding figures for kidney, lung and
heart were in the range 20-30%. With time, metabolites gradually
accounted for a higher proportion of the tissue radioactivity.
Single oral doses of diclazuril of up to 5 g/kg bw caused no
mortality in experimental animals. Clinical effects in mice and rats
were non-specific and mainly of CNS origin. In dogs, vomiting and
defaecation were seen after treatment.
In a 3-month dose-ranging study in mice, mortality was not seen
with oral doses up to 1600 mg/kg in the diet, equivalent to 240 mg/kg
bw/day. At this dose, changes indicative of mild liver damage were
reported, including increases in relative liver weight, swelling of
the centrilobular hepatocytes and vacuolization of hepatocytes in
males; females showed swelling of the centrilobular hepatocytes only.
The NOEL was 30 mg/kg bw/day.
In a 3-month toxicity study in mice in which the highest dose was
3000 mg/kg in the diet, equal to 850 mg/kg bw/day in males and
920 mg/kg bw/day in females, neither mortality nor adverse clinical
effects were noted. No mortality occurred in treated mice, but at all
dose levels (290-850 mg/kg bw/day) males showed decreased serum levels
of total bilirubin. This and other changes revealed by haematological
and biochemical changes in the blood, although occasionally
statistically significant, were either marginal with respect to
historical controls or not dose-related. Liver weights were increased
at all doses in males and at the highest dose in females. Swelling of
the centrilobular hepatocytes was observed in males dosed at 500 and
850 mg/kg bw/day. A NOEL was not identified in this study. It was
noted that wastage of feed had probably caused overestimation of
dietary intake of the drug and that the calculated dosage was about
twice that derived from dietary intake conversion tables.
Rats were given diclazuril at doses of 50-800 mg/kg in the diet,
equal to 4-69 mg/kg bw/day in males and 6-89 mg/kg bw/day in females
for 3 months. Male rats dosed at 17 and 69 mg/kg bw/day showed
swelling of the centrilobular hepatocytes with an increase in lipid
vacuolization. Absolute and relative liver weights were increased in
both males and females at the highest dose. These changes fell within
the range of historical controls. The NOELs in this study were 4 and
21 mg/kg bw/day for males and females, respectively.
In a second 3-month study, in which rats were dosed at 1000-3000
mg/kg in the diet, equal to 71-210 mg/kg bw/day in males and 82-240
mg/kg bw/day in females, swelling of centrilobular hepatocytes
was seen in both sexes at all doses. Vacuolization of the cytoplasm of
hepatocytes was noted in males dosed at 140 mg/kg bw/day and above. No
deaths occurred in either sex at any dose level in this study, nor was
any relevant change revealed by clinical chemical analysis,
haematology or urinalysis. Relative liver weights were, however,
increased in males and females in the highest-dose group. A NOEL was
not identified in this study.
In a 12-month study in rats given diclazuril at doses of
16-1000 mg/kg in the diet, equal to 1-74 mg/kg bw/day in males and
2-88 mg/kg bw/day in females, no drug-related changes were observed at
doses at or below 6 and 18 mg/kg bw/day for females and males,
respectively. Histological examination showed histocytic aggregates in
the mesenteric lymph node in males at 74 mg/kg bw/day and at both 23
and 88 mg/kg bw/day in females. Swelling of centrilobular hepatocytes
was seen in males dosed at 74 mg/kg bw/day, while females dosed at
88 mg/kg bw/day had increased clusters of foamy cells in the lungs.
The NOEL in this study was 6 mg/kg bw/day.
Diclazuril capsules were administered to 4 dogs of each sex at
doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two additional male
and female animals were included in the control and highest-dose
groups in a 1-month withdrawal study. At 80 mg/kg bw/day in both males
and females, a free granular, yellow brown pigment was present in the
cytoplasm of hepatocytes. In males at this dose level, a significant
increase in serum urea (expressed as nitrogen) was also seen. Both
changes were reversible, as shown by the 4 animals of the recovery
group. A 12-month toxicity study in dogs of the same design and dose
levels as the 3-month study but lacking a recovery group, confirmed
the findings of the earlier study. The liver changes were comparable
in nature and intensity in both studies. The NOEL in these studies was
20 mg/kg bw/day.
A 25-month combined long-term toxicity/carcinogenicity study was
performed in mice. This study included periodic haematological
examinations, analysis of serum samples at the end of the study and
extensive histopathological examinations. Diclazuril was administered
at dose levels of 16, 63, 250 or 1000 mg/kg in the diet, equal to 3,
11, 47 or 190 mg/kg bw/day in males and 4, 14, 53 or 220 mg/kg bw/day
in females. While there was evidence of inhibition of the rate of
body-weight gain in male mice at the highest-dose, histopathological
examination revealed only minor hepatic morphological changes of the
type also found in the 3-month studies in mice. In view of the results
of the two 3-month studies in mice of the same strain, the Committee
considered that the toxic potential of doses exceeding those used in
the carcinogenicity study had been evaluated. Analysis of serum
samples collected at the end of these two 3-month studies provided
evidence of the existence of a threshold of between 1000 and
2000 mg/kg in the diet, above which the mouse is unable to absorb
ingested diclazuril. This threshold is close to the highest dose used
in the long-term toxicity/carcinogenicity study. In the light of this
finding, together with the absence of any biologically significant
increase in the incidence of tumours, the Committee concluded that
diclazuril had been adequately tested and was not carcinogenic in
mice. The NOEL in this study was 3 mg/kg bw/day.
A 28-month combined long-term toxicity/carcinogenicity study was
performed in rats given 16, 63, 250 or 1000 mg/kg diclazuril in the
feed (equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20 or
80 mg/kg bw/day in females). This study included periodic
haematological examinations, analysis of serum samples at the end of
the study and extensive histopathological examinations. As in the
study with mice, no effects of obvious toxicological significance were
noted, but reactive histiocytosis of the mesenteric lymph nodes was
observed in female rats at 20 and 80 mg/kg bw/day and in males at
61 mg/kg bw/day. However, as with the studies in mice, serum samples
collected at the end of the two 3-month studies allowed an exploration
of the relationship between the oral intake of diclazuril and the
resultant concentration in serum. A threshold existed for the
absorption of diclazuril in the region of 2000 mg/kg in the feed, i.e.
about two times higher than the highest dose used in the long-term
toxicity/carcinogenicity study. In the light of this finding together
with the absence of any biologically significant increase in the
incidence of tumours, the Committee concluded that diclazuril had been
adequately tested and was not carcinogenic in the rat. The NOEL in
this study was 4 mg/kg bw/day.
A two-generation reproductive toxicity study was performed in
rats in which each generation produced two litters. The doses were
equivalent to 5, 20 or 80 mg/kg bw/day. In the first generation, the
only adverse effect observed was a reduction in birth weight of pups
at 80 mg/kg bw/day. In the second generation, body-weight gain of dams
during pregnancy was slightly lower at 80 mg/kg bw/day, and food
consumption during pregnancy and lactation was decreased at 20 and
80 mg/kg bw/day, indicating maternal toxicity at these doses. As in
the first generation, birth weights were lower at 80 mg/kg bw/day, and
the survival rates and weight of pups at weaning were also decreased
at 20 and 80 mg/kg bw/day. The NOEL in this study was 5 mg/kg bw/day.
In two teratogenicity studies in rats, diclazuril was
administered in the feed at doses equivalent to 1-160 mg/kg bw/day
from days 6 to 16 of pregnancy. Dosing equivalent to 1 and 5 mg/kg
bw/day did not result in any adverse effects on the dams or their
progeny. At a dose equivalent to 20 mg/kg bw/day, however, slight
maternal toxicity was observed, characterized by a reduction in
body-weight gain. Litter weights were decreased in groups receiving
20-160 mg/kg bw/day. At none of the doses was teratogenicity evident.
The NOEL in these studies was 5 mg/kg bw/day.
In two studies in rabbits, administration of diclazuril by gavage
at doses of 5-160 mg/kg bw/day from days 6 to 18 of pregnancy did not
result in any adverse effects on the dams or their offspring. From
these findings and because studies of the enteric absorption of
diclazuril in the rabbit were lacking, the Committee concluded that
there was no evidence that exposure to diclazuril was sufficient to
enable the teratogenicity of the drug to be evaluated. This conclusion
was supported by the occurrence of adverse effects in rabbits exposed
to Eimeria spp. following administration of the drug in the feed at
a dose equivalent to 13 mg/kg bw/day for 5 weeks. Furthermore, the
Committee noted that materno- and fetotoxicity had been observed in
rabbits dosed with a diclazuril analogue at 80 mg/kg bw/day.
Diclazuril was studied in a range of in vivo and in vitro
assays with a variety of genetic end-points. Negative findings in all
these assays enabled the Committee to conclude that diclazuril was not
genotoxic.
4. EVALUATION
The Committee established a temporary ADI of 0-20 µg/kg bw for
diclazuril, based on the NOEL of 3 mg/kg bw/day in the 2-year
toxicity/carcinogenicity study in mice and a safety factor of 200. The
ADI was rounded to one significant figure, consistent with accepted
rounding procedures (Annex 1, reference 91, section 2.7).
The results of a teratogenicity study in rabbits supported by
evidence that the doses administered were sufficiently high for the
teratogenic potential of diclazuril to be adequately explored are
required for evaluation in 1998.
5. REFERENCES
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987a).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1504. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987b).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1651. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1988a). Diclazuril-
oral 2-generation reproduction study with two litters per generation
in Wistar rats. Unpublished nonclinical laboratory study Exp. No. 1663.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren H., and Marsboom, R. (1988b). Diclazuril -
oral embryotoxicity and teratogenicity study in New Zealand White
rabbits. Unpublished nonclinical laboratory study Exp. No. 1821.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Gillardin, J.M., Van Cauteren H., Sanz, G., and Marsboom, R. (1989).
Embryotoxicity and teratogenicity study in New Zealand white rabbits.
Unpublished nonclinical laboratory study Exp. No. 2212. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Kayembe K., Desmet P., Henry, M.C., and Stoffels, P. (1989).
Diclazuril for Isospora belli infection in AIDS [Letter] Lancet;
1:1397-1398.
Lampo, A., Vanparys, P.H., Vandenberghe, J., Coussement, W.,
Teuns, G., Maes, L., and Van Cauteren, H. (1995). R 64433 - Review
report on the pharmacodynamic and toxicological documentation on
diclazuril. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Leonard, A., et al.. (1988). The in vivo chromosome aberration assay
on human lymphocytes. Unpublished nonclinical laboratory report
SCK 86/02D/R 64433, Mol, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Mannens, G., Van Leemput, L., and Heykants, J. (1992). A study on the
excretion and metabolism of 14C-Diclazuril in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished non-clinical
pharmacokinetics report on R 64433 FK 1217. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
de Meester, C. and Leonard A. (1985). R 64433-Ames reversion mutation
test with Salmonella typhimurium and Escherichia coli. Unpublished
nonclinical laboratory study. Exp. No. 19, UCL, Laboratory of Terato-
genesis and Mutagenesis. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
de Meester, C. and Leonard A. (1986). R 64433-Ames reversion mutation
test with S. typhimurium and E. coli. Unpublished nonclinical
laboratory study Exp. No 22, UCL, Laboratory of Teratogenesis and
Mutagenesis, Brussels, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Menichetti, F., Moretti, M.V., Marroni, M., Papili, R., and
DiCandilo, F. (1991). Diclazuril for cryptosporidiosis in AIDS
[Letter]. Am. J. Med.; 90(2): 271-2.
Meuldermans, W., Hendrickx, J., Mostmans, E., Verboven, P.,
Borgmans, C., Michiels, M., and Heykants, J. (1988a). The excretion
and metabolism of Diclazuil after a single oral dose in rabbits.
Unpublished preclinical research report. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Michiels, M., Hendrickx, J., Geerts, R.,
Hurkmans, R., Mostmans, E., Lauwers, W., and Heykant J. (1988b)
14C-Diclazuril (14C-R64433) in the broiler chicken: Total
radioactivity depletion and metabolism after single oral
administration at 1 mg/kg. Unpublished preclinical research report.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Van Beijsterveldt, L., Hendrickx, J., Geerts, R.,
Mostmans, E., Borgmans, C., Verboven, P., Woestenborghs, R., Van
Leemput, L., and Heykant, J. (1990). 14C-Diclazuril (14C-R64433)
in the turkey: Absorption, metabolism, excretion and tissue depletion
after a single oral dose of 1 mg/kg. Unpublished preclinical research
report on R64433. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Michiels, M., Hendriks, R., Geerts, R., Meuldermans, W., and
Heykant, J. (1988). Absorption and tissue distribution of
14C-Diclazuril (14C-R64433) in the rabbit after a single oral dose
of 1 mg/kg. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Monbaliu, J., Van Leemput, L., and Heykants, J. (1993). Plasma levels
of Diclazuril in sheep after oral administration of a 0.25% drench
formulation at 1 mg Diclazuril per kg. Unpublished non-clinical
pharmacokinetics report on R64433 FK-992. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986a). The acute oral toxicity of R 64433 in
mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986b). The acute oral toxicity of R 64433 in
rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987a). The acute subcutaneous toxicity of R 64433
in mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987b). The acute intraperitoneal toxicity of
R 64433 in mice. Unpublished preclinical research report. Submitted to
WHO (JECFA) by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987c) The acute subcutaneous toxicity of R 64433
in rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987d) The acute intraperitoneal toxicity of
R 64433 in rats. Unpublished preclinical research report. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987e). The acute oral toxicity of R 64433 in
Beagle dogs. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988a). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 1917.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988b). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 2035.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Beijsterveldt, L., Van Leemput, L., and Heykants, J. (1992).
Absorption and tissue distribution of 14C-Diclazuril in the male
Wistar rat after single oral administration at 10 mg/kg in aqueous
suspension. Unpublished non-clinical pharmacokinetics report on R64433
FK-1213. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven, F., and Zaman, R. (1985). The
antimicrobial activity of R 64433. Unpublished nonclinical laboratory
study. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven F., and Zaman, R. (1986). The antimicrobial
activity of R 64433: additional data. Unpublished nonclinical
laboratory study. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1985). R 64433 - SOS chromosoomtest
in Escherichia coli. Unpublished nonclinical laboratory study
No. 1600. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986a). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished nonclinical laboratory
study No. 1501 + 1657. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986b). R 64433 - Dominant lethal
test in male mice. Unpublished nonclinical laboratory study
Exp. No 1609. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986c). R 64433 - Micronucleus test
in mice. Unpublished nonclinical laboratory study Exp. No 1744.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1991). Micronucleus test in mice.
Unpublished nonclinical laboratory study, Exp. No. 2506. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1986a). Subchronic toxicity study in Wistar rats (repeated dosage for
3 months). Unpublished nonclinical laboratory study Exp. No. 1502.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Van Cauteren, H., and Marsboom, R.
(1986b). Subchronic toxicity study in Beagle dogs (repeated dosage for
3 months + 1 month recovery). Unpublished nonclinical laboratory study
Exp. No. 1503. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1988a). Oral longterm toxicity study in Wistar rats (repeated dosage
for 12 months). Unpublished nonclinical laboratory study Exp.
No. 1755. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Teuns, G., Coussement, W., Van Cauteren, H., and
Marsboom, R. (1988b). Oral longterm toxicity study in Beagle dogs
(repeated dosage for 12 months). Unpublished nonclinical laboratory
study Exp. No. 1698. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauterern, H., and Marsboom, R. (1989a).
R 64433 - Oral carcinogencity study in Swiss mice (repeated dosage for
18 up to 24 months). Unpublished nonclinical laboratory study Exp.
No. 1649. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauteren, H., and Marsboom, R. (1989b).
R 64433 - Oral carcinogenicity study in Wistar rats (repeated dosage
for 24 up to 30 months). Unpublished nonclinical laboratory study Exp.
No. 1650. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1990). Subchronic oral feeding toxicity study (pilot) in SPF Albino
Swiss mice. Unpublished nonclinical laboratory study Exp. No. 1665.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A. Coussement, W., and
Van Cauteren, H. (1992a). Three-month oral toxicity study in SPF
Albino Swiss mice. Unpublished nonclinical laboratory study Exp.
No. 2614. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A., Coussement, W., and
Van Cauteren, H. (1992b). Three-month oral toxicity in SPF Wistar
rats. Unpublished nonclinical laboratory study Exp. No. 2613.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Weterings, PJJM., et al. (1985). Evaluation of the DNA repair
inducing ability of R 64433 in a primary culture of rat hepatocytes.
Unpublished nonclinical report No. 0114/ER 154, Notox Pathobiology
Research, The Netherlands. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Young, R.R. (1989). Mutagenticity test on in mouse lymphoma forward
mutation assay. Unpublished nonclinical report 10779-0-431, Hazleton
Laboratories America, Inc., USA. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Rats
Male Wistar rats received 10 mg 14C-diclazuril/kg bw in an
aqueous suspension. On day 1, 90% of the dosed radioactivity was
excreted in the faeces. After 4 days, 92% was excreted in the faeces
and 0.04% in the urine. Unchanged diclazuril (UD) accounted for most
of the total radioactivity in the faeces, with two metabolites
accounting for less than 0.5% (Mannens et al., 1992).
In another study, concentrations of total radioactivity and UD
were measured in blood, plasma and tissues for up to 96 h after oral
administration of 10 mg 14C-diclazuril/kg bw in an aqueous
suspension to male Wistar rats. The absorption of diclazuril was
limited. Most of the dose was recovered from the GI contents. Maximum
plasma concentrations of total radioactivity and UD were approximately
1 µg/ml and occurred after 8 h. On day 1, total radioactivity in
plasma was almost exclusively UD, but later the UD/total radioactivity
ratio gradually declined. The blood-to-plasma ratio of total
radioactivity was about 0.7, indicating a limited distribution to
blood cells. Distribution to the systemic tissues was rapid but
limited. The concentration of total radioactivity in the liver was
about 50% of the plasma level and kidney, lung and heart levels were
about 20-30%, while muscle and brain concentrations were 5-7% of the
plasma levels. UD depleted from tissues with a half-life of 1.5 days.
The half-life of total radioactivity was 53.0 h (Van Beijsterveldt
et al., 1992).
2.1.1.2 Rabbits
Absorption, distribution, metabolism and excretion was studied in
2 separate studies, with male rabbits receiving a single oral dose of
14C-diclazuril at 1 mg/kg bw. The excretion of radioactivity was
fairly rapid since within 48 h, 70% was recovered in the faeces and 3%
in the urine. After 10 days, more than 98% was excreted. Gall bladder
contained radioactivity, but accounting at most for 0.02% of the dose,
implying that biliary excretion was a minor elimination pathway.
Various metabolites were identified in urine. Two main metabolites
were a glucuronide and a sulfate conjugate. In faeces, UD represented
the main part of the radioactivity, accounting for approximately 66%
of the dose. Some minor metabolites were observed. None of the urinary
or faecal metabolites accounted for more than 2% of the dose. UD
represented almost all the plasma radioactivity at least up to 120 h
after dosing. The elimination from plasma proceeded with an apparent
half-life of 2-2.5 days. The distribution to tissues was limited and
the elimination half-lives were similar to that from plasma, except
for the liver with an elimination half-life of 3 days. The easily
extractable liver residue indicated freedom from concern over bound
residues (Meuldermans et al., 1988a; Michiels et al., 1988).
2.1.1.3 Chickens
In a single-dose study, 14C-diclazuril was administered to
broiler chickens in a lactose mixture at a dose of 1 mg/kg bw. Maximum
radioactivity concentrations of 1.5-2.0 µg-eq./ml were attained in
plasma 6 h after administration. The elimination subsequently
proceeded with a half-life of approximately 50 h.
There was a rapid equilibrium between plasma and tissue levels.
The tissue concentrations were 2 to 10 times lower than the
corresponding plasma concentrations. The liver and the kidneys showed
the highest concentrations at all time points. The half-life in
tissues was approximately 50 h, similar to that found in plasma.
Parent diclazuril accounted for more than 90% in the liver at 24-h
liver, and no metabolites were detectable (<4%). About half of the
dose was excreted within 24 h, almost exclusively as UD. After 10
days, the cumulative excretion was over 95%. A degradation product,
coded DM5, accounted for 5.3% of the dose in the 0-96 h excreta. Other
metabolites accounted for <2% each. DM5 was not definitively
identified. It was demonstrated to be a derivative of
4-amino-2,6-dichloro-a-(4-chlorophenyl) benzeneacetonitrile, formed by
cleavage and subsequent degradation of the triazine-dione ring
(Meuldermans et al., 1988b).
2.1.1.4 Turkeys
In a single-dose study, 14C-diclazuril was administered in a
lactose mixture at a dose of 1 mg/kg bw. Maximum radioactivity
concentrations of 1.78 ± 0.19 µg-eq./ml were attained in plasma 6 h
after administration. The subsequent elimination proceeded with a
half-life of approximately 38 h. The blood-to-plasma ratio was on
average 0.66 throughout the 10-day observation period, implying a
minor distribution to the blood cells. There was a rapid but limited
distribution between plasma and tissues. The tissue concentrations
were slightly to markedly lower than the corresponding plasma
concentrations. The depletion rate was similar in all tissues with a
half-life in the range of 34-46 h. UD accounted for 98% of the sample
radioactivity for the livers sampled after 6 h, and 85% for the livers
sampled after 48 and 72 h. No metabolites accounted for more than 10%
of the radioactivity in liver. At 24 h after dosing, 55% of the dose
was excreted. The cumulative excretion was 88% after 5 days and 94.8 ±
0.8% after 10 days. At least 8 metabolites were identified in excreta.
Parent diclazuril, however, accounted for the major part of the
excreted radioactivity, representing 55.8% of the dose. The
triazine-dione ring cleavage product previously seen in broilers
accounted for 6.3% of the dose in the 0-96 h excreta. An unidentified
metabolite accounted for about 2.4% of the radioactivity. All other
metabolites accounted for < 2% each (Meuldermans et al., 1990).
2.1.1.5 Sheep
Poor absorption of diclazuril was observed in sheep after oral
administration of 1 mg/kg bw. Maximum concentrations in plasma of
0.012 to 0.016 µg/ml were reached at 24-48 h after dosing and were
lower than the quantification limit (< 0.01 µg/ml) at all other
sampling times (Monbaliu et al., 1993).
In summary, in all species investigated, there were little
indications of metabolism of diclazuril. As a result,
diclazuril-derived substances were eliminated mainly as unchanged
parent substance in the excreta of bird species, and in the faeces of
rats and rabbits. Urine of the latter species was only a very minor
excretion route and some metabolized substances could be observed. In
edible tissues, the residues consisted almost entirely of unchanged
diclazuril.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with diclazuril are
summarized in Table 1.
Only in mice and rats dosed i.p. at 5000 mg/kg bw was mortality
observed. Clinical effects in mice and rats were non-specific and were
mainly on the CNS. In dogs, vomiting and defecation were seen after
treatment. Autopsy did not reveal drug-related macroscopic changes in
any study.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 3-month dose-range-finding study, SPF Albino Swiss mice
(10/sex/group) were dosed orally at 200, 400, 800 or 1600 mg/kg of
feed, equivalent to 30, 60, 120 or 240 mg/kg bw/day. Increased
centrilobular swelling of the liver was observed in male mice at the
three highest doses (3/10, 4/10, 9/10, 10/10, and 10/10 at 0, 30, 60,
120 and 240 mg/kg bw/day, respectively), and at 240 mg/kg bw/day in
females. At 240 mg/kg bw/day, decreased body-weight gain, increased
liver weight and paleness of the liver were observed in males. The
NOEL in this study was 30 mg/kg bw/day (Verstraeten et al., 1990).
Table 1. Acute toxicity studies on diclazuril
Species Route Sex LD50 Reference
(mg/kg bw)
Mouse oral M & F >5000 Niemegeers, 1986a
sc M & F >5000 Niemegeers, 1987a
ip M & F >5000 Niemegeers, 1987b
Rat oral M & F >5000 Niemegeers, 1986b
sc M & F >5000 Niemegeers, 1987c
ip M 5000 Niemegeers, 1987d
F >5000 Niemegeers, 1987d
Rabbit dermal M & F >4000 Teuns & Marsboom
1988a,b
Dog oral M & F >5000 Niemegeers, 1987e
In a 3-month toxicity study, SPF Albino Swiss mice (20/sex/group)
were dosed with diclazuril at 1000, 2000 or 3000 mg/kg of feed (equal
to 290, 500 or 850 mg/kg bw/day in males and 290, 610 or 920 mg/kg
bw/day in females). No mortality or adverse gross clinical effects
were noted. At all dose levels, the males showed decreased serum total
bilirubin and other altered serum variables, increased liver weight
and centrilobular hepatocellular swelling. Cytoplasmic vacuolation was
seen in 1 male at 500 mg/kg bw/day, and in 3 males at 850 mg/kg
bw/day. A NOEL was not identified in this study. It was noted that
feed wasting had probably caused overestimation of test article intake
and that the calculated dosage was about twice that obtainable from
dietary intake conversion tables (Verstraeten et al., 1992a).
2.2.2.2 Rats
In two dietary studies, Wistar rats (20/sex/group) were dosed
with diclazuril for 3 months at 50, 200 or 800 mg/kg of feed (equal to
4, 17 or 69 mg/kg bw/day in males and 6, 21 or 89 mg/kg bw/day in
females), and at levels of 1000, 2000 or 3000 mg/kg (equal to 71, 140
or 210 mg/kg bw/day in males and 82, 160 or 240 mg/kg bw/day in
females).
In the first study, male rats dosed at 17 and 69 mg/kg bw/day
showed centrilobular swelling with an increased presence of lipid
vacuoles in the centrilobular hepatocytes. Increased absolute and
relative liver weights were seen in both males and females at the
highest dose levels. The NOELs for these liver effects were 4 and
21 mg/kg bw/day for males and females, respectively (Verstraeten
et al., 1986a).
In the second study, swelling of the centrilobular hepatocytes
was seen in both male and female groups at all doses. Hepatocytic
cytoplasmic vacuolization was noted in males at doses of 140 and
210 mg/kg bw/day. No mortalities occurred in either sex at any dose
level in this study, nor were any relevant changes revealed by
clinical chemistry, haematology or urinalysis. Relative liver weights
were, however, increased in males and females in the highest dose
group. A NOEL was not identified in this study (Verstraeten et al.,
1992b).
In a 12-month study in Wistar rats (20/sex/group) with
diclazuril, doses were 16, 63, 250 and 1000 mg/kg of feed (equal to 1,
4, 18 or 74 mg/kg bw/day in males and 2, 6, 23 or 88 mg/kg bw/day in
females). No drug-related changes were observed at doses up to 6 mg/kg
bw/day for females and 18 mg/kg bw/day for males. Dosing at 23 and
88 mg/kg bw/day in females resulted in the presence of histiocytic
aggregates in the mesenteric lymph node. At 74 and 88 mg/kg bw/day in
males and females, respectively, histological examination showed
centrilobular swelling in the hepatocytes (males only), histiocytic
aggregates in the mesenteric lymph node and an increased incidence of
foamy cells in the lungs. There was no effect on clinical signs, food
consumption, body-weight gain, mortality, haematology, serum analysis
or urinalysis. The NOELs for the liver effects observed in this study
were 6 mg/kg bw/day for females and 18 mg/kg bw//day for males
(Verstraeten et al., 1988a).
2.2.2.3 Dogs
Diclazuril in gelatin capsules was administered to beagle dogs
(4/sex/group) at doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two
additional male and female animals in the control and high-dose groups
were included for a 1-month withdrawal study. At 80 mg/kg bw/day in
both males and females, a fine granular, yellow to brown pigment was
seen in the cytoplasm of the hepatocytes. In males at this dose level,
a significant increase in serum BUN values was also seen. Both liver
pathology and BUN values were normal in the high-dose animals
following the 1-month recovery period (Verstaeten et al., 1986b).
A 12-month toxicity study in beagle dogs of the same design and
dose levels confirmed the findings of the earlier study. The liver
changes were comparable in nature and intensity in both studies. The
NOEL was 20 mg/kg bw/day (Verstraeten et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 25-month combined long-term toxicity/carcinogenicity study,
Swiss mice (50/sex/group) were administered doses of diclazuril of 16,
63, 250 or 1000 mg/kg of feed (equal to 3, 11, 47 or 190 mg/kg bw/day
in males and 4, 14, 53 or 220 mg/kg bw/day in females).
Males at 185 mg/kg bw/day showed reduced body-weight gain and
food consumption and an increased incidence of cachexia. Increased
incidences of hepatic changes, mainly characterized by parenchymal
centrilobular swelling, and fatty overload characterized by the
presence of lipid vacuoles in the centrilobular hepatocytes and
swollen sinusoidal cells, were seen in males at 11, 47 and 190 mg/kg
bw/day, and in females at 220 mg/kg bw/day. The NOEL in this study was
16 mg/kg of feed, equal to 3 mg/kg bw/day (Verstraeten et al., 1989a).
2.2.3.2 Rats
Diclazuril was given in the diet for 28 months to Wistar rats
(50/sex/group) at dose levels of 16, 63, 250 or 1000 mg/kg of feed,
equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20, or
80 mg/kg bw/day in females. At 250 and 1000 mg/kg of feed,
diclazuril-related changes were the increased presence of
histiocytosis (males at 61 mg/kg bw/day, females at 20 and 80 mg/kg
bw/day) and of pigmented macrophages (females at 80 mg/kg bw/day) in
the mesenteric lymph nodes. The lung histiocytosis was observed and
illustrated by an increased presence of foamy cells. The Committee
regarded the highest dose used in this study to be sufficient to
adequately assess carcinogenicity. Taking this together with the
absence of a biologically significant increase in the occurrence of
tumours of any type at any location, the Committee concluded that
diclazuril was not carcinogenic in this study. The NOEL was 63 mg/kg
of feed, equal to 4 mg/kg bw/day (Verstraeten et al., 1989b).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A 2-generation study was performed in Wistar rats, with each
generation producing 2 litters. Doses were 50, 200 or 800 mg/kg of
feed, equivalent to 5, 20 or 80 mg/kg bw/day, given continuously in
the diet. The copulation and fertility indices and the duration of
gestation were comparable between controls and dosed animals.
Teratogenicity was not observed in any litter. In the first
generation, the only adverse effect observed was a lowered birth
weight in pups at 80 mg/kg bw/day. In the second generation,
body-weight gain during pregnancy was slightly lower at 80 mg/kg
bw/day, and food consumption during pregnancy and lactation was
decreased at 20 and 80 mg/kg bw/day, indicating maternal toxicity at
these doses. Birth weight at 80 mg/kg bw/day and the weight of pups at
weaning and survival rate at 20 and 80 mg/kg bw/day were decreased.
The NOEL in this study was 50 mg/kg of feed, equivalent to 5 mg/kg
bw/day (Dirkx et al., 1988a).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
In two studies in Wistar rats, diclazuril was given in the diet
at 12.5-1600 mg/kg, equivalent to 1-160 mg/kg bw/day, from days 6 to
16 of pregnancy. Dosing at 1 and 5 mg/kg bw/day did not adversely
influence dams or their progeny. At 20-160 mg/kg bw/day, slight
maternal toxicity was observed, characterized by a lower body-weight
gain. Litter weights were also decreased in these dosage groups.
Teratogenicity was not evident at any dose level. The NOEL in these
studies was 5 mg/kg bw/day (Dirkx et al., 1987a,b).
2.2.5.2 Rabbits
Oral dosing of New Zealand white rabbits with diclazuril by
gavage from days 6 to 18 of pregnancy, at doses of 5, 20 or 80 mg/kg
bw/day did not result in any adverse effect on the dams or their
litter. No teratogenicity was observed (Dirkx et al., 1988b)
In a repeat study, doses were 40, 80 or 160 mg/kg bw/day. There
was no evidence in these studies that the rabbits were exposed to
sufficient diclazuril to evaluate the teratogenicity of the drug
(Gillardin et al., 1989).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with diclazuril are
summarized in Table 2.
2.3 Observations in humans
Humans have had limited exposure to diclazuril. Since it is
active against Eimeria species, which is the cause of coccidiosis in
animals, it has been administered to AIDS patients with Isospora
belli infection. Eight patients with I. belli diarrhea were
treated with 200 mg diclazuril for 7 days. Clinical improvement was
seen and the diarrhea disappeared. No side effects of the drug was
cited (Kayembe et al., 1989).
Table 2. Results of genotoxicity studies on Diclazuril
Test System Test Object Dose Results Reference
DNA Repair Test Induction of UDS in primary 0.3-30 µg/ml Negative Weterings, 1985
rat hepatocytes
SOS Chromotest Induction of galactosidase 1-1000 ng/well Negative ±S9 Vanparijs, 1985
synthesis in genetically
engineered E. coli K-12
Ames Test Histidine reversion in S. 10-500 µg/plate Negative de Meester &
(2 test reports) typhimurium; tryptophan Leonard, 1985;
reversion in E. coli de Meester &
Leonard, 1986
Mouse Lymphoma Mammalian cell gene mutation; 5-100 µg/ml Negative ±S9 Young, 1989
TK+/- Assay thymidine kinase locus;
trifluorothymidine resistance
Drosophila SLRL Sex-linked recessive lethality 500, 2000 ppm Negative Vanparijs, 1986a
Test
Human Lymphocyte Structural chromosome 25-300 µg Negative Leonard, 1988
Chromosome aberrations
Aberrations
Table 2. Results of genotoxicity studies on Diclazuril (cont'd)
Test System Test Object Dose Results Reference
Micronucleus Test Induction of micronuclei in 80-5120 mg/kg Negative Vanparijs, 1986c;
(2 test reports) mouse bone marrow Vanparijs, 1991
Mouse Dominant Induction of dominant lethal 40-160 mg/kg Inadequate1 Vanparijs, 1986b
Lethal Test mutations in male mouse germ
cells
1 No mortality or clinical signs of toxicity and no evidence of target tissue exposure to the test article were
reported. The doses were inadequate in this test.
Diclazuril at a similar dose was administered for crypto-
sporidiosis in a single AIDS patient. A complete eradication of
cryptosporidial oocysts from the faeces was seen following treatment.
No cutaneous reactions or modification of biochemical parameter were
documented. There were no comments on additional effects following
drug treatment (Menichetti et al., 1991).
2.4 Microbiological properties
The in vitro antimicrobial activity of diclazuril was
investigated in 11 pathogenic and saprogenic fungi, 6 zoo-pathogenic
and 5 plant-pathogenic bacteria, including both Gram-positive and
Gram-negative organisms. It was found that diclazuril possesses
negligible antifungal and no antibacterial activity at 100 µg/ml
(Van Cutsem et al., 1985).
In another experiment, the antimicrobial activity of diclazuril
was evaluated in 2 strains of Bacillus subtilis and in 1 strain of
Sarcina lutea, which are commonly used for testing residues of
antibiotics in meat or other food products. No antibacterial activity
was found (Van Cutsem et al., 1986).
2.5 Pharmacological effects
In a general screen for drug activity, diclazuril was dosed to
rats by the i.p. route at 40 mg/kg bw. Diclazuril was devoid of
neuroleptic, sedating, analgesic, hypnotic, cholimimetic, constipating
or anticonvulsant properties (Lampo et al., 1995).
3. COMMENTS
The Committee considered data from studies on acute, short-term,
and long-term toxicity/carcinogenicity, reproductive and developmental
toxicity, metabolism, pharmacokinetics and antimicrobial effects.
Pharmacokinetic studies in rats given radiolabelled drug
suggested that the absorption of diclazuril from the gut was limited,
as 90% of the radioactive dose was excreted in the faeces within 24 h.
After 4 days, 92% of the dose had been excreted in the faeces and
0.04% in the urine. Unchanged diclazuril accounted for most of the
total radioactivity in the faeces, with two metabolites accounting for
less than 0.5%. Distribution into the tissues was rapid but limited.
The concentration of total radioactivity in the liver was about half
that in the plasma; the corresponding figures for kidney, lung and
heart were in the range 20-30%. With time, metabolites gradually
accounted for a higher proportion of the tissue radioactivity.
Single oral doses of diclazuril of up to 5 g/kg bw caused no
mortality in experimental animals. Clinical effects in mice and rats
were non-specific and mainly of CNS origin. In dogs, vomiting and
defaecation were seen after treatment.
In a 3-month dose-ranging study in mice, mortality was not seen
with oral doses up to 1600 mg/kg in the diet, equivalent to 240 mg/kg
bw/day. At this dose, changes indicative of mild liver damage were
reported, including increases in relative liver weight, swelling of
the centrilobular hepatocytes and vacuolization of hepatocytes in
males; females showed swelling of the centrilobular hepatocytes only.
The NOEL was 30 mg/kg bw/day.
In a 3-month toxicity study in mice in which the highest dose was
3000 mg/kg in the diet, equal to 850 mg/kg bw/day in males and
920 mg/kg bw/day in females, neither mortality nor adverse clinical
effects were noted. No mortality occurred in treated mice, but at all
dose levels (290-850 mg/kg bw/day) males showed decreased serum levels
of total bilirubin. This and other changes revealed by haematological
and biochemical changes in the blood, although occasionally
statistically significant, were either marginal with respect to
historical controls or not dose-related. Liver weights were increased
at all doses in males and at the highest dose in females. Swelling of
the centrilobular hepatocytes was observed in males dosed at 500 and
850 mg/kg bw/day. A NOEL was not identified in this study. It was
noted that wastage of feed had probably caused overestimation of
dietary intake of the drug and that the calculated dosage was about
twice that derived from dietary intake conversion tables.
Rats were given diclazuril at doses of 50-800 mg/kg in the diet,
equal to 4-69 mg/kg bw/day in males and 6-89 mg/kg bw/day in females
for 3 months. Male rats dosed at 17 and 69 mg/kg bw/day showed
swelling of the centrilobular hepatocytes with an increase in lipid
vacuolization. Absolute and relative liver weights were increased in
both males and females at the highest dose. These changes fell within
the range of historical controls. The NOELs in this study were 4 and
21 mg/kg bw/day for males and females, respectively.
In a second 3-month study, in which rats were dosed at 1000-3000
mg/kg in the diet, equal to 71-210 mg/kg bw/day in males and 82-240
mg/kg bw/day in females, swelling of centrilobular hepatocytes
was seen in both sexes at all doses. Vacuolization of the cytoplasm of
hepatocytes was noted in males dosed at 140 mg/kg bw/day and above. No
deaths occurred in either sex at any dose level in this study, nor was
any relevant change revealed by clinical chemical analysis,
haematology or urinalysis. Relative liver weights were, however,
increased in males and females in the highest-dose group. A NOEL was
not identified in this study.
In a 12-month study in rats given diclazuril at doses of
16-1000 mg/kg in the diet, equal to 1-74 mg/kg bw/day in males and
2-88 mg/kg bw/day in females, no drug-related changes were observed at
doses at or below 6 and 18 mg/kg bw/day for females and males,
respectively. Histological examination showed histocytic aggregates in
the mesenteric lymph node in males at 74 mg/kg bw/day and at both 23
and 88 mg/kg bw/day in females. Swelling of centrilobular hepatocytes
was seen in males dosed at 74 mg/kg bw/day, while females dosed at
88 mg/kg bw/day had increased clusters of foamy cells in the lungs.
The NOEL in this study was 6 mg/kg bw/day.
Diclazuril capsules were administered to 4 dogs of each sex at
doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two additional male
and female animals were included in the control and highest-dose
groups in a 1-month withdrawal study. At 80 mg/kg bw/day in both males
and females, a free granular, yellow brown pigment was present in the
cytoplasm of hepatocytes. In males at this dose level, a significant
increase in serum urea (expressed as nitrogen) was also seen. Both
changes were reversible, as shown by the 4 animals of the recovery
group. A 12-month toxicity study in dogs of the same design and dose
levels as the 3-month study but lacking a recovery group, confirmed
the findings of the earlier study. The liver changes were comparable
in nature and intensity in both studies. The NOEL in these studies was
20 mg/kg bw/day.
A 25-month combined long-term toxicity/carcinogenicity study was
performed in mice. This study included periodic haematological
examinations, analysis of serum samples at the end of the study and
extensive histopathological examinations. Diclazuril was administered
at dose levels of 16, 63, 250 or 1000 mg/kg in the diet, equal to 3,
11, 47 or 190 mg/kg bw/day in males and 4, 14, 53 or 220 mg/kg bw/day
in females. While there was evidence of inhibition of the rate of
body-weight gain in male mice at the highest-dose, histopathological
examination revealed only minor hepatic morphological changes of the
type also found in the 3-month studies in mice. In view of the results
of the two 3-month studies in mice of the same strain, the Committee
considered that the toxic potential of doses exceeding those used in
the carcinogenicity study had been evaluated. Analysis of serum
samples collected at the end of these two 3-month studies provided
evidence of the existence of a threshold of between 1000 and
2000 mg/kg in the diet, above which the mouse is unable to absorb
ingested diclazuril. This threshold is close to the highest dose used
in the long-term toxicity/carcinogenicity study. In the light of this
finding, together with the absence of any biologically significant
increase in the incidence of tumours, the Committee concluded that
diclazuril had been adequately tested and was not carcinogenic in
mice. The NOEL in this study was 3 mg/kg bw/day.
A 28-month combined long-term toxicity/carcinogenicity study was
performed in rats given 16, 63, 250 or 1000 mg/kg diclazuril in the
feed (equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20 or
80 mg/kg bw/day in females). This study included periodic
haematological examinations, analysis of serum samples at the end of
the study and extensive histopathological examinations. As in the
study with mice, no effects of obvious toxicological significance were
noted, but reactive histiocytosis of the mesenteric lymph nodes was
observed in female rats at 20 and 80 mg/kg bw/day and in males at
61 mg/kg bw/day. However, as with the studies in mice, serum samples
collected at the end of the two 3-month studies allowed an exploration
of the relationship between the oral intake of diclazuril and the
resultant concentration in serum. A threshold existed for the
absorption of diclazuril in the region of 2000 mg/kg in the feed, i.e.
about two times higher than the highest dose used in the long-term
toxicity/carcinogenicity study. In the light of this finding together
with the absence of any biologically significant increase in the
incidence of tumours, the Committee concluded that diclazuril had been
adequately tested and was not carcinogenic in the rat. The NOEL in
this study was 4 mg/kg bw/day.
A two-generation reproductive toxicity study was performed in
rats in which each generation produced two litters. The doses were
equivalent to 5, 20 or 80 mg/kg bw/day. In the first generation, the
only adverse effect observed was a reduction in birth weight of pups
at 80 mg/kg bw/day. In the second generation, body-weight gain of dams
during pregnancy was slightly lower at 80 mg/kg bw/day, and food
consumption during pregnancy and lactation was decreased at 20 and
80 mg/kg bw/day, indicating maternal toxicity at these doses. As in
the first generation, birth weights were lower at 80 mg/kg bw/day, and
the survival rates and weight of pups at weaning were also decreased
at 20 and 80 mg/kg bw/day. The NOEL in this study was 5 mg/kg bw/day.
In two teratogenicity studies in rats, diclazuril was
administered in the feed at doses equivalent to 1-160 mg/kg bw/day
from days 6 to 16 of pregnancy. Dosing equivalent to 1 and 5 mg/kg
bw/day did not result in any adverse effects on the dams or their
progeny. At a dose equivalent to 20 mg/kg bw/day, however, slight
maternal toxicity was observed, characterized by a reduction in
body-weight gain. Litter weights were decreased in groups receiving
20-160 mg/kg bw/day. At none of the doses was teratogenicity evident.
The NOEL in these studies was 5 mg/kg bw/day.
In two studies in rabbits, administration of diclazuril by gavage
at doses of 5-160 mg/kg bw/day from days 6 to 18 of pregnancy did not
result in any adverse effects on the dams or their offspring. From
these findings and because studies of the enteric absorption of
diclazuril in the rabbit were lacking, the Committee concluded that
there was no evidence that exposure to diclazuril was sufficient to
enable the teratogenicity of the drug to be evaluated. This conclusion
was supported by the occurrence of adverse effects in rabbits exposed
to Eimeria spp. following administration of the drug in the feed at
a dose equivalent to 13 mg/kg bw/day for 5 weeks. Furthermore, the
Committee noted that materno- and fetotoxicity had been observed in
rabbits dosed with a diclazuril analogue at 80 mg/kg bw/day.
Diclazuril was studied in a range of in vivo and in vitro
assays with a variety of genetic end-points. Negative findings in all
these assays enabled the Committee to conclude that diclazuril was not
genotoxic.
4. EVALUATION
The Committee established a temporary ADI of 0-20 µg/kg bw for
diclazuril, based on the NOEL of 3 mg/kg bw/day in the 2-year
toxicity/carcinogenicity study in mice and a safety factor of 200. The
ADI was rounded to one significant figure, consistent with accepted
rounding procedures (Annex 1, reference 91, section 2.7).
The results of a teratogenicity study in rabbits supported by
evidence that the doses administered were sufficiently high for the
teratogenic potential of diclazuril to be adequately explored are
required for evaluation in 1998.
5. REFERENCES
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987a).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1504. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987b).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1651. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1988a). Diclazuril-
oral 2-generation reproduction study with two litters per generation
in Wistar rats. Unpublished nonclinical laboratory study Exp. No. 1663.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren H., and Marsboom, R. (1988b). Diclazuril -
oral embryotoxicity and teratogenicity study in New Zealand White
rabbits. Unpublished nonclinical laboratory study Exp. No. 1821.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Gillardin, J.M., Van Cauteren H., Sanz, G., and Marsboom, R. (1989).
Embryotoxicity and teratogenicity study in New Zealand white rabbits.
Unpublished nonclinical laboratory study Exp. No. 2212. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Kayembe K., Desmet P., Henry, M.C., and Stoffels, P. (1989).
Diclazuril for Isospora belli infection in AIDS [Letter] Lancet;
1:1397-1398.
Lampo, A., Vanparys, P.H., Vandenberghe, J., Coussement, W.,
Teuns, G., Maes, L., and Van Cauteren, H. (1995). R 64433 - Review
report on the pharmacodynamic and toxicological documentation on
diclazuril. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Leonard, A., et al.. (1988). The in vivo chromosome aberration assay
on human lymphocytes. Unpublished nonclinical laboratory report
SCK 86/02D/R 64433, Mol, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Mannens, G., Van Leemput, L., and Heykants, J. (1992). A study on the
excretion and metabolism of 14C-Diclazuril in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished non-clinical
pharmacokinetics report on R 64433 FK 1217. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
de Meester, C. and Leonard A. (1985). R 64433-Ames reversion mutation
test with Salmonella typhimurium and Escherichia coli. Unpublished
nonclinical laboratory study. Exp. No. 19, UCL, Laboratory of Terato-
genesis and Mutagenesis. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
de Meester, C. and Leonard A. (1986). R 64433-Ames reversion mutation
test with S. typhimurium and E. coli. Unpublished nonclinical
laboratory study Exp. No 22, UCL, Laboratory of Teratogenesis and
Mutagenesis, Brussels, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Menichetti, F., Moretti, M.V., Marroni, M., Papili, R., and
DiCandilo, F. (1991). Diclazuril for cryptosporidiosis in AIDS
[Letter]. Am. J. Med.; 90(2): 271-2.
Meuldermans, W., Hendrickx, J., Mostmans, E., Verboven, P.,
Borgmans, C., Michiels, M., and Heykants, J. (1988a). The excretion
and metabolism of Diclazuil after a single oral dose in rabbits.
Unpublished preclinical research report. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Michiels, M., Hendrickx, J., Geerts, R.,
Hurkmans, R., Mostmans, E., Lauwers, W., and Heykant J. (1988b)
14C-Diclazuril (14C-R64433) in the broiler chicken: Total
radioactivity depletion and metabolism after single oral
administration at 1 mg/kg. Unpublished preclinical research report.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Van Beijsterveldt, L., Hendrickx, J., Geerts, R.,
Mostmans, E., Borgmans, C., Verboven, P., Woestenborghs, R., Van
Leemput, L., and Heykant, J. (1990). 14C-Diclazuril (14C-R64433)
in the turkey: Absorption, metabolism, excretion and tissue depletion
after a single oral dose of 1 mg/kg. Unpublished preclinical research
report on R64433. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Michiels, M., Hendriks, R., Geerts, R., Meuldermans, W., and
Heykant, J. (1988). Absorption and tissue distribution of
14C-Diclazuril (14C-R64433) in the rabbit after a single oral dose
of 1 mg/kg. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Monbaliu, J., Van Leemput, L., and Heykants, J. (1993). Plasma levels
of Diclazuril in sheep after oral administration of a 0.25% drench
formulation at 1 mg Diclazuril per kg. Unpublished non-clinical
pharmacokinetics report on R64433 FK-992. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986a). The acute oral toxicity of R 64433 in
mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986b). The acute oral toxicity of R 64433 in
rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987a). The acute subcutaneous toxicity of R 64433
in mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987b). The acute intraperitoneal toxicity of
R 64433 in mice. Unpublished preclinical research report. Submitted to
WHO (JECFA) by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987c) The acute subcutaneous toxicity of R 64433
in rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987d) The acute intraperitoneal toxicity of
R 64433 in rats. Unpublished preclinical research report. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987e). The acute oral toxicity of R 64433 in
Beagle dogs. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988a). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 1917.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988b). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 2035.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Beijsterveldt, L., Van Leemput, L., and Heykants, J. (1992).
Absorption and tissue distribution of 14C-Diclazuril in the male
Wistar rat after single oral administration at 10 mg/kg in aqueous
suspension. Unpublished non-clinical pharmacokinetics report on R64433
FK-1213. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven, F., and Zaman, R. (1985). The
antimicrobial activity of R 64433. Unpublished nonclinical laboratory
study. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven F., and Zaman, R. (1986). The antimicrobial
activity of R 64433: additional data. Unpublished nonclinical
laboratory study. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1985). R 64433 - SOS chromosoomtest
in Escherichia coli. Unpublished nonclinical laboratory study
No. 1600. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986a). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished nonclinical laboratory
study No. 1501 + 1657. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986b). R 64433 - Dominant lethal
test in male mice. Unpublished nonclinical laboratory study
Exp. No 1609. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986c). R 64433 - Micronucleus test
in mice. Unpublished nonclinical laboratory study Exp. No 1744.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1991). Micronucleus test in mice.
Unpublished nonclinical laboratory study, Exp. No. 2506. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1986a). Subchronic toxicity study in Wistar rats (repeated dosage for
3 months). Unpublished nonclinical laboratory study Exp. No. 1502.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Van Cauteren, H., and Marsboom, R.
(1986b). Subchronic toxicity study in Beagle dogs (repeated dosage for
3 months + 1 month recovery). Unpublished nonclinical laboratory study
Exp. No. 1503. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1988a). Oral longterm toxicity study in Wistar rats (repeated dosage
for 12 months). Unpublished nonclinical laboratory study Exp.
No. 1755. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Teuns, G., Coussement, W., Van Cauteren, H., and
Marsboom, R. (1988b). Oral longterm toxicity study in Beagle dogs
(repeated dosage for 12 months). Unpublished nonclinical laboratory
study Exp. No. 1698. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauterern, H., and Marsboom, R. (1989a).
R 64433 - Oral carcinogencity study in Swiss mice (repeated dosage for
18 up to 24 months). Unpublished nonclinical laboratory study Exp.
No. 1649. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauteren, H., and Marsboom, R. (1989b).
R 64433 - Oral carcinogenicity study in Wistar rats (repeated dosage
for 24 up to 30 months). Unpublished nonclinical laboratory study Exp.
No. 1650. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1990). Subchronic oral feeding toxicity study (pilot) in SPF Albino
Swiss mice. Unpublished nonclinical laboratory study Exp. No. 1665.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A. Coussement, W., and
Van Cauteren, H. (1992a). Three-month oral toxicity study in SPF
Albino Swiss mice. Unpublished nonclinical laboratory study Exp.
No. 2614. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A., Coussement, W., and
Van Cauteren, H. (1992b). Three-month oral toxicity in SPF Wistar
rats. Unpublished nonclinical laboratory study Exp. No. 2613.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Weterings, PJJM., et al. (1985). Evaluation of the DNA repair
inducing ability of R 64433 in a primary culture of rat hepatocytes.
Unpublished nonclinical report No. 0114/ER 154, Notox Pathobiology
Research, The Netherlands. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Young, R.R. (1989). Mutagenticity test on in mouse lymphoma forward
mutation assay. Unpublished nonclinical report 10779-0-431, Hazleton
Laboratories America, Inc., USA. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Rats
Male Wistar rats received 10 mg 14C-diclazuril/kg bw in an
aqueous suspension. On day 1, 90% of the dosed radioactivity was
excreted in the faeces. After 4 days, 92% was excreted in the faeces
and 0.04% in the urine. Unchanged diclazuril (UD) accounted for most
of the total radioactivity in the faeces, with two metabolites
accounting for less than 0.5% (Mannens et al., 1992).
In another study, concentrations of total radioactivity and UD
were measured in blood, plasma and tissues for up to 96 h after oral
administration of 10 mg 14C-diclazuril/kg bw in an aqueous
suspension to male Wistar rats. The absorption of diclazuril was
limited. Most of the dose was recovered from the GI contents. Maximum
plasma concentrations of total radioactivity and UD were approximately
1 µg/ml and occurred after 8 h. On day 1, total radioactivity in
plasma was almost exclusively UD, but later the UD/total radioactivity
ratio gradually declined. The blood-to-plasma ratio of total
radioactivity was about 0.7, indicating a limited distribution to
blood cells. Distribution to the systemic tissues was rapid but
limited. The concentration of total radioactivity in the liver was
about 50% of the plasma level and kidney, lung and heart levels were
about 20-30%, while muscle and brain concentrations were 5-7% of the
plasma levels. UD depleted from tissues with a half-life of 1.5 days.
The half-life of total radioactivity was 53.0 h (Van Beijsterveldt
et al., 1992).
2.1.1.2 Rabbits
Absorption, distribution, metabolism and excretion was studied in
2 separate studies, with male rabbits receiving a single oral dose of
14C-diclazuril at 1 mg/kg bw. The excretion of radioactivity was
fairly rapid since within 48 h, 70% was recovered in the faeces and 3%
in the urine. After 10 days, more than 98% was excreted. Gall bladder
contained radioactivity, but accounting at most for 0.02% of the dose,
implying that biliary excretion was a minor elimination pathway.
Various metabolites were identified in urine. Two main metabolites
were a glucuronide and a sulfate conjugate. In faeces, UD represented
the main part of the radioactivity, accounting for approximately 66%
of the dose. Some minor metabolites were observed. None of the urinary
or faecal metabolites accounted for more than 2% of the dose. UD
represented almost all the plasma radioactivity at least up to 120 h
after dosing. The elimination from plasma proceeded with an apparent
half-life of 2-2.5 days. The distribution to tissues was limited and
the elimination half-lives were similar to that from plasma, except
for the liver with an elimination half-life of 3 days. The easily
extractable liver residue indicated freedom from concern over bound
residues (Meuldermans et al., 1988a; Michiels et al., 1988).
2.1.1.3 Chickens
In a single-dose study, 14C-diclazuril was administered to
broiler chickens in a lactose mixture at a dose of 1 mg/kg bw. Maximum
radioactivity concentrations of 1.5-2.0 µg-eq./ml were attained in
plasma 6 h after administration. The elimination subsequently
proceeded with a half-life of approximately 50 h.
There was a rapid equilibrium between plasma and tissue levels.
The tissue concentrations were 2 to 10 times lower than the
corresponding plasma concentrations. The liver and the kidneys showed
the highest concentrations at all time points. The half-life in
tissues was approximately 50 h, similar to that found in plasma.
Parent diclazuril accounted for more than 90% in the liver at 24-h
liver, and no metabolites were detectable (<4%). About half of the
dose was excreted within 24 h, almost exclusively as UD. After 10
days, the cumulative excretion was over 95%. A degradation product,
coded DM5, accounted for 5.3% of the dose in the 0-96 h excreta. Other
metabolites accounted for <2% each. DM5 was not definitively
identified. It was demonstrated to be a derivative of
4-amino-2,6-dichloro-a-(4-chlorophenyl) benzeneacetonitrile, formed by
cleavage and subsequent degradation of the triazine-dione ring
(Meuldermans et al., 1988b).
2.1.1.4 Turkeys
In a single-dose study, 14C-diclazuril was administered in a
lactose mixture at a dose of 1 mg/kg bw. Maximum radioactivity
concentrations of 1.78 ± 0.19 µg-eq./ml were attained in plasma 6 h
after administration. The subsequent elimination proceeded with a
half-life of approximately 38 h. The blood-to-plasma ratio was on
average 0.66 throughout the 10-day observation period, implying a
minor distribution to the blood cells. There was a rapid but limited
distribution between plasma and tissues. The tissue concentrations
were slightly to markedly lower than the corresponding plasma
concentrations. The depletion rate was similar in all tissues with a
half-life in the range of 34-46 h. UD accounted for 98% of the sample
radioactivity for the livers sampled after 6 h, and 85% for the livers
sampled after 48 and 72 h. No metabolites accounted for more than 10%
of the radioactivity in liver. At 24 h after dosing, 55% of the dose
was excreted. The cumulative excretion was 88% after 5 days and 94.8 ±
0.8% after 10 days. At least 8 metabolites were identified in excreta.
Parent diclazuril, however, accounted for the major part of the
excreted radioactivity, representing 55.8% of the dose. The
triazine-dione ring cleavage product previously seen in broilers
accounted for 6.3% of the dose in the 0-96 h excreta. An unidentified
metabolite accounted for about 2.4% of the radioactivity. All other
metabolites accounted for < 2% each (Meuldermans et al., 1990).
2.1.1.5 Sheep
Poor absorption of diclazuril was observed in sheep after oral
administration of 1 mg/kg bw. Maximum concentrations in plasma of
0.012 to 0.016 µg/ml were reached at 24-48 h after dosing and were
lower than the quantification limit (< 0.01 µg/ml) at all other
sampling times (Monbaliu et al., 1993).
In summary, in all species investigated, there were little
indications of metabolism of diclazuril. As a result,
diclazuril-derived substances were eliminated mainly as unchanged
parent substance in the excreta of bird species, and in the faeces of
rats and rabbits. Urine of the latter species was only a very minor
excretion route and some metabolized substances could be observed. In
edible tissues, the residues consisted almost entirely of unchanged
diclazuril.
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with diclazuril are
summarized in Table 1.
Only in mice and rats dosed i.p. at 5000 mg/kg bw was mortality
observed. Clinical effects in mice and rats were non-specific and were
mainly on the CNS. In dogs, vomiting and defecation were seen after
treatment. Autopsy did not reveal drug-related macroscopic changes in
any study.
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
In a 3-month dose-range-finding study, SPF Albino Swiss mice
(10/sex/group) were dosed orally at 200, 400, 800 or 1600 mg/kg of
feed, equivalent to 30, 60, 120 or 240 mg/kg bw/day. Increased
centrilobular swelling of the liver was observed in male mice at the
three highest doses (3/10, 4/10, 9/10, 10/10, and 10/10 at 0, 30, 60,
120 and 240 mg/kg bw/day, respectively), and at 240 mg/kg bw/day in
females. At 240 mg/kg bw/day, decreased body-weight gain, increased
liver weight and paleness of the liver were observed in males. The
NOEL in this study was 30 mg/kg bw/day (Verstraeten et al., 1990).
Table 1. Acute toxicity studies on diclazuril
Species Route Sex LD50 Reference
(mg/kg bw)
Mouse oral M & F >5000 Niemegeers, 1986a
sc M & F >5000 Niemegeers, 1987a
ip M & F >5000 Niemegeers, 1987b
Rat oral M & F >5000 Niemegeers, 1986b
sc M & F >5000 Niemegeers, 1987c
ip M 5000 Niemegeers, 1987d
F >5000 Niemegeers, 1987d
Rabbit dermal M & F >4000 Teuns & Marsboom
1988a,b
Dog oral M & F >5000 Niemegeers, 1987e
In a 3-month toxicity study, SPF Albino Swiss mice (20/sex/group)
were dosed with diclazuril at 1000, 2000 or 3000 mg/kg of feed (equal
to 290, 500 or 850 mg/kg bw/day in males and 290, 610 or 920 mg/kg
bw/day in females). No mortality or adverse gross clinical effects
were noted. At all dose levels, the males showed decreased serum total
bilirubin and other altered serum variables, increased liver weight
and centrilobular hepatocellular swelling. Cytoplasmic vacuolation was
seen in 1 male at 500 mg/kg bw/day, and in 3 males at 850 mg/kg
bw/day. A NOEL was not identified in this study. It was noted that
feed wasting had probably caused overestimation of test article intake
and that the calculated dosage was about twice that obtainable from
dietary intake conversion tables (Verstraeten et al., 1992a).
2.2.2.2 Rats
In two dietary studies, Wistar rats (20/sex/group) were dosed
with diclazuril for 3 months at 50, 200 or 800 mg/kg of feed (equal to
4, 17 or 69 mg/kg bw/day in males and 6, 21 or 89 mg/kg bw/day in
females), and at levels of 1000, 2000 or 3000 mg/kg (equal to 71, 140
or 210 mg/kg bw/day in males and 82, 160 or 240 mg/kg bw/day in
females).
In the first study, male rats dosed at 17 and 69 mg/kg bw/day
showed centrilobular swelling with an increased presence of lipid
vacuoles in the centrilobular hepatocytes. Increased absolute and
relative liver weights were seen in both males and females at the
highest dose levels. The NOELs for these liver effects were 4 and
21 mg/kg bw/day for males and females, respectively (Verstraeten
et al., 1986a).
In the second study, swelling of the centrilobular hepatocytes
was seen in both male and female groups at all doses. Hepatocytic
cytoplasmic vacuolization was noted in males at doses of 140 and
210 mg/kg bw/day. No mortalities occurred in either sex at any dose
level in this study, nor were any relevant changes revealed by
clinical chemistry, haematology or urinalysis. Relative liver weights
were, however, increased in males and females in the highest dose
group. A NOEL was not identified in this study (Verstraeten et al.,
1992b).
In a 12-month study in Wistar rats (20/sex/group) with
diclazuril, doses were 16, 63, 250 and 1000 mg/kg of feed (equal to 1,
4, 18 or 74 mg/kg bw/day in males and 2, 6, 23 or 88 mg/kg bw/day in
females). No drug-related changes were observed at doses up to 6 mg/kg
bw/day for females and 18 mg/kg bw/day for males. Dosing at 23 and
88 mg/kg bw/day in females resulted in the presence of histiocytic
aggregates in the mesenteric lymph node. At 74 and 88 mg/kg bw/day in
males and females, respectively, histological examination showed
centrilobular swelling in the hepatocytes (males only), histiocytic
aggregates in the mesenteric lymph node and an increased incidence of
foamy cells in the lungs. There was no effect on clinical signs, food
consumption, body-weight gain, mortality, haematology, serum analysis
or urinalysis. The NOELs for the liver effects observed in this study
were 6 mg/kg bw/day for females and 18 mg/kg bw//day for males
(Verstraeten et al., 1988a).
2.2.2.3 Dogs
Diclazuril in gelatin capsules was administered to beagle dogs
(4/sex/group) at doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two
additional male and female animals in the control and high-dose groups
were included for a 1-month withdrawal study. At 80 mg/kg bw/day in
both males and females, a fine granular, yellow to brown pigment was
seen in the cytoplasm of the hepatocytes. In males at this dose level,
a significant increase in serum BUN values was also seen. Both liver
pathology and BUN values were normal in the high-dose animals
following the 1-month recovery period (Verstaeten et al., 1986b).
A 12-month toxicity study in beagle dogs of the same design and
dose levels confirmed the findings of the earlier study. The liver
changes were comparable in nature and intensity in both studies. The
NOEL was 20 mg/kg bw/day (Verstraeten et al., 1988b).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
In a 25-month combined long-term toxicity/carcinogenicity study,
Swiss mice (50/sex/group) were administered doses of diclazuril of 16,
63, 250 or 1000 mg/kg of feed (equal to 3, 11, 47 or 190 mg/kg bw/day
in males and 4, 14, 53 or 220 mg/kg bw/day in females).
Males at 185 mg/kg bw/day showed reduced body-weight gain and
food consumption and an increased incidence of cachexia. Increased
incidences of hepatic changes, mainly characterized by parenchymal
centrilobular swelling, and fatty overload characterized by the
presence of lipid vacuoles in the centrilobular hepatocytes and
swollen sinusoidal cells, were seen in males at 11, 47 and 190 mg/kg
bw/day, and in females at 220 mg/kg bw/day. The NOEL in this study was
16 mg/kg of feed, equal to 3 mg/kg bw/day (Verstraeten et al., 1989a).
2.2.3.2 Rats
Diclazuril was given in the diet for 28 months to Wistar rats
(50/sex/group) at dose levels of 16, 63, 250 or 1000 mg/kg of feed,
equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20, or
80 mg/kg bw/day in females. At 250 and 1000 mg/kg of feed,
diclazuril-related changes were the increased presence of
histiocytosis (males at 61 mg/kg bw/day, females at 20 and 80 mg/kg
bw/day) and of pigmented macrophages (females at 80 mg/kg bw/day) in
the mesenteric lymph nodes. The lung histiocytosis was observed and
illustrated by an increased presence of foamy cells. The Committee
regarded the highest dose used in this study to be sufficient to
adequately assess carcinogenicity. Taking this together with the
absence of a biologically significant increase in the occurrence of
tumours of any type at any location, the Committee concluded that
diclazuril was not carcinogenic in this study. The NOEL was 63 mg/kg
of feed, equal to 4 mg/kg bw/day (Verstraeten et al., 1989b).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A 2-generation study was performed in Wistar rats, with each
generation producing 2 litters. Doses were 50, 200 or 800 mg/kg of
feed, equivalent to 5, 20 or 80 mg/kg bw/day, given continuously in
the diet. The copulation and fertility indices and the duration of
gestation were comparable between controls and dosed animals.
Teratogenicity was not observed in any litter. In the first
generation, the only adverse effect observed was a lowered birth
weight in pups at 80 mg/kg bw/day. In the second generation,
body-weight gain during pregnancy was slightly lower at 80 mg/kg
bw/day, and food consumption during pregnancy and lactation was
decreased at 20 and 80 mg/kg bw/day, indicating maternal toxicity at
these doses. Birth weight at 80 mg/kg bw/day and the weight of pups at
weaning and survival rate at 20 and 80 mg/kg bw/day were decreased.
The NOEL in this study was 50 mg/kg of feed, equivalent to 5 mg/kg
bw/day (Dirkx et al., 1988a).
2.2.5 Special studies on teratogenicity/embryotoxicity
2.2.5.1 Rats
In two studies in Wistar rats, diclazuril was given in the diet
at 12.5-1600 mg/kg, equivalent to 1-160 mg/kg bw/day, from days 6 to
16 of pregnancy. Dosing at 1 and 5 mg/kg bw/day did not adversely
influence dams or their progeny. At 20-160 mg/kg bw/day, slight
maternal toxicity was observed, characterized by a lower body-weight
gain. Litter weights were also decreased in these dosage groups.
Teratogenicity was not evident at any dose level. The NOEL in these
studies was 5 mg/kg bw/day (Dirkx et al., 1987a,b).
2.2.5.2 Rabbits
Oral dosing of New Zealand white rabbits with diclazuril by
gavage from days 6 to 18 of pregnancy, at doses of 5, 20 or 80 mg/kg
bw/day did not result in any adverse effect on the dams or their
litter. No teratogenicity was observed (Dirkx et al., 1988b)
In a repeat study, doses were 40, 80 or 160 mg/kg bw/day. There
was no evidence in these studies that the rabbits were exposed to
sufficient diclazuril to evaluate the teratogenicity of the drug
(Gillardin et al., 1989).
2.2.6 Special studies on genotoxicity
The results of the genotoxicity studies with diclazuril are
summarized in Table 2.
2.3 Observations in humans
Humans have had limited exposure to diclazuril. Since it is
active against Eimeria species, which is the cause of coccidiosis in
animals, it has been administered to AIDS patients with Isospora
belli infection. Eight patients with I. belli diarrhea were
treated with 200 mg diclazuril for 7 days. Clinical improvement was
seen and the diarrhea disappeared. No side effects of the drug was
cited (Kayembe et al., 1989).
Table 2. Results of genotoxicity studies on Diclazuril
Test System Test Object Dose Results Reference
DNA Repair Test Induction of UDS in primary 0.3-30 µg/ml Negative Weterings, 1985
rat hepatocytes
SOS Chromotest Induction of galactosidase 1-1000 ng/well Negative ±S9 Vanparijs, 1985
synthesis in genetically
engineered E. coli K-12
Ames Test Histidine reversion in S. 10-500 µg/plate Negative de Meester &
(2 test reports) typhimurium; tryptophan Leonard, 1985;
reversion in E. coli de Meester &
Leonard, 1986
Mouse Lymphoma Mammalian cell gene mutation; 5-100 µg/ml Negative ±S9 Young, 1989
TK+/- Assay thymidine kinase locus;
trifluorothymidine resistance
Drosophila SLRL Sex-linked recessive lethality 500, 2000 ppm Negative Vanparijs, 1986a
Test
Human Lymphocyte Structural chromosome 25-300 µg Negative Leonard, 1988
Chromosome aberrations
Aberrations
Table 2. Results of genotoxicity studies on Diclazuril (cont'd)
Test System Test Object Dose Results Reference
Micronucleus Test Induction of micronuclei in 80-5120 mg/kg Negative Vanparijs, 1986c;
(2 test reports) mouse bone marrow Vanparijs, 1991
Mouse Dominant Induction of dominant lethal 40-160 mg/kg Inadequate1 Vanparijs, 1986b
Lethal Test mutations in male mouse germ
cells
1 No mortality or clinical signs of toxicity and no evidence of target tissue exposure to the test article were
reported. The doses were inadequate in this test.
Diclazuril at a similar dose was administered for crypto-
sporidiosis in a single AIDS patient. A complete eradication of
cryptosporidial oocysts from the faeces was seen following treatment.
No cutaneous reactions or modification of biochemical parameter were
documented. There were no comments on additional effects following
drug treatment (Menichetti et al., 1991).
2.4 Microbiological properties
The in vitro antimicrobial activity of diclazuril was
investigated in 11 pathogenic and saprogenic fungi, 6 zoo-pathogenic
and 5 plant-pathogenic bacteria, including both Gram-positive and
Gram-negative organisms. It was found that diclazuril possesses
negligible antifungal and no antibacterial activity at 100 µg/ml
(Van Cutsem et al., 1985).
In another experiment, the antimicrobial activity of diclazuril
was evaluated in 2 strains of Bacillus subtilis and in 1 strain of
Sarcina lutea, which are commonly used for testing residues of
antibiotics in meat or other food products. No antibacterial activity
was found (Van Cutsem et al., 1986).
2.5 Pharmacological effects
In a general screen for drug activity, diclazuril was dosed to
rats by the i.p. route at 40 mg/kg bw. Diclazuril was devoid of
neuroleptic, sedating, analgesic, hypnotic, cholimimetic, constipating
or anticonvulsant properties (Lampo et al., 1995).
3. COMMENTS
The Committee considered data from studies on acute, short-term,
and long-term toxicity/carcinogenicity, reproductive and developmental
toxicity, metabolism, pharmacokinetics and antimicrobial effects.
Pharmacokinetic studies in rats given radiolabelled drug
suggested that the absorption of diclazuril from the gut was limited,
as 90% of the radioactive dose was excreted in the faeces within 24 h.
After 4 days, 92% of the dose had been excreted in the faeces and
0.04% in the urine. Unchanged diclazuril accounted for most of the
total radioactivity in the faeces, with two metabolites accounting for
less than 0.5%. Distribution into the tissues was rapid but limited.
The concentration of total radioactivity in the liver was about half
that in the plasma; the corresponding figures for kidney, lung and
heart were in the range 20-30%. With time, metabolites gradually
accounted for a higher proportion of the tissue radioactivity.
Single oral doses of diclazuril of up to 5 g/kg bw caused no
mortality in experimental animals. Clinical effects in mice and rats
were non-specific and mainly of CNS origin. In dogs, vomiting and
defaecation were seen after treatment.
In a 3-month dose-ranging study in mice, mortality was not seen
with oral doses up to 1600 mg/kg in the diet, equivalent to 240 mg/kg
bw/day. At this dose, changes indicative of mild liver damage were
reported, including increases in relative liver weight, swelling of
the centrilobular hepatocytes and vacuolization of hepatocytes in
males; females showed swelling of the centrilobular hepatocytes only.
The NOEL was 30 mg/kg bw/day.
In a 3-month toxicity study in mice in which the highest dose was
3000 mg/kg in the diet, equal to 850 mg/kg bw/day in males and
920 mg/kg bw/day in females, neither mortality nor adverse clinical
effects were noted. No mortality occurred in treated mice, but at all
dose levels (290-850 mg/kg bw/day) males showed decreased serum levels
of total bilirubin. This and other changes revealed by haematological
and biochemical changes in the blood, although occasionally
statistically significant, were either marginal with respect to
historical controls or not dose-related. Liver weights were increased
at all doses in males and at the highest dose in females. Swelling of
the centrilobular hepatocytes was observed in males dosed at 500 and
850 mg/kg bw/day. A NOEL was not identified in this study. It was
noted that wastage of feed had probably caused overestimation of
dietary intake of the drug and that the calculated dosage was about
twice that derived from dietary intake conversion tables.
Rats were given diclazuril at doses of 50-800 mg/kg in the diet,
equal to 4-69 mg/kg bw/day in males and 6-89 mg/kg bw/day in females
for 3 months. Male rats dosed at 17 and 69 mg/kg bw/day showed
swelling of the centrilobular hepatocytes with an increase in lipid
vacuolization. Absolute and relative liver weights were increased in
both males and females at the highest dose. These changes fell within
the range of historical controls. The NOELs in this study were 4 and
21 mg/kg bw/day for males and females, respectively.
In a second 3-month study, in which rats were dosed at 1000-3000
mg/kg in the diet, equal to 71-210 mg/kg bw/day in males and 82-240
mg/kg bw/day in females, swelling of centrilobular hepatocytes
was seen in both sexes at all doses. Vacuolization of the cytoplasm of
hepatocytes was noted in males dosed at 140 mg/kg bw/day and above. No
deaths occurred in either sex at any dose level in this study, nor was
any relevant change revealed by clinical chemical analysis,
haematology or urinalysis. Relative liver weights were, however,
increased in males and females in the highest-dose group. A NOEL was
not identified in this study.
In a 12-month study in rats given diclazuril at doses of
16-1000 mg/kg in the diet, equal to 1-74 mg/kg bw/day in males and
2-88 mg/kg bw/day in females, no drug-related changes were observed at
doses at or below 6 and 18 mg/kg bw/day for females and males,
respectively. Histological examination showed histocytic aggregates in
the mesenteric lymph node in males at 74 mg/kg bw/day and at both 23
and 88 mg/kg bw/day in females. Swelling of centrilobular hepatocytes
was seen in males dosed at 74 mg/kg bw/day, while females dosed at
88 mg/kg bw/day had increased clusters of foamy cells in the lungs.
The NOEL in this study was 6 mg/kg bw/day.
Diclazuril capsules were administered to 4 dogs of each sex at
doses of 5, 20 or 80 mg/kg bw/day for 3 months. Two additional male
and female animals were included in the control and highest-dose
groups in a 1-month withdrawal study. At 80 mg/kg bw/day in both males
and females, a free granular, yellow brown pigment was present in the
cytoplasm of hepatocytes. In males at this dose level, a significant
increase in serum urea (expressed as nitrogen) was also seen. Both
changes were reversible, as shown by the 4 animals of the recovery
group. A 12-month toxicity study in dogs of the same design and dose
levels as the 3-month study but lacking a recovery group, confirmed
the findings of the earlier study. The liver changes were comparable
in nature and intensity in both studies. The NOEL in these studies was
20 mg/kg bw/day.
A 25-month combined long-term toxicity/carcinogenicity study was
performed in mice. This study included periodic haematological
examinations, analysis of serum samples at the end of the study and
extensive histopathological examinations. Diclazuril was administered
at dose levels of 16, 63, 250 or 1000 mg/kg in the diet, equal to 3,
11, 47 or 190 mg/kg bw/day in males and 4, 14, 53 or 220 mg/kg bw/day
in females. While there was evidence of inhibition of the rate of
body-weight gain in male mice at the highest-dose, histopathological
examination revealed only minor hepatic morphological changes of the
type also found in the 3-month studies in mice. In view of the results
of the two 3-month studies in mice of the same strain, the Committee
considered that the toxic potential of doses exceeding those used in
the carcinogenicity study had been evaluated. Analysis of serum
samples collected at the end of these two 3-month studies provided
evidence of the existence of a threshold of between 1000 and
2000 mg/kg in the diet, above which the mouse is unable to absorb
ingested diclazuril. This threshold is close to the highest dose used
in the long-term toxicity/carcinogenicity study. In the light of this
finding, together with the absence of any biologically significant
increase in the incidence of tumours, the Committee concluded that
diclazuril had been adequately tested and was not carcinogenic in
mice. The NOEL in this study was 3 mg/kg bw/day.
A 28-month combined long-term toxicity/carcinogenicity study was
performed in rats given 16, 63, 250 or 1000 mg/kg diclazuril in the
feed (equal to 1, 4, 15 or 61 mg/kg bw/day in males and 1, 5, 20 or
80 mg/kg bw/day in females). This study included periodic
haematological examinations, analysis of serum samples at the end of
the study and extensive histopathological examinations. As in the
study with mice, no effects of obvious toxicological significance were
noted, but reactive histiocytosis of the mesenteric lymph nodes was
observed in female rats at 20 and 80 mg/kg bw/day and in males at
61 mg/kg bw/day. However, as with the studies in mice, serum samples
collected at the end of the two 3-month studies allowed an exploration
of the relationship between the oral intake of diclazuril and the
resultant concentration in serum. A threshold existed for the
absorption of diclazuril in the region of 2000 mg/kg in the feed, i.e.
about two times higher than the highest dose used in the long-term
toxicity/carcinogenicity study. In the light of this finding together
with the absence of any biologically significant increase in the
incidence of tumours, the Committee concluded that diclazuril had been
adequately tested and was not carcinogenic in the rat. The NOEL in
this study was 4 mg/kg bw/day.
A two-generation reproductive toxicity study was performed in
rats in which each generation produced two litters. The doses were
equivalent to 5, 20 or 80 mg/kg bw/day. In the first generation, the
only adverse effect observed was a reduction in birth weight of pups
at 80 mg/kg bw/day. In the second generation, body-weight gain of dams
during pregnancy was slightly lower at 80 mg/kg bw/day, and food
consumption during pregnancy and lactation was decreased at 20 and
80 mg/kg bw/day, indicating maternal toxicity at these doses. As in
the first generation, birth weights were lower at 80 mg/kg bw/day, and
the survival rates and weight of pups at weaning were also decreased
at 20 and 80 mg/kg bw/day. The NOEL in this study was 5 mg/kg bw/day.
In two teratogenicity studies in rats, diclazuril was
administered in the feed at doses equivalent to 1-160 mg/kg bw/day
from days 6 to 16 of pregnancy. Dosing equivalent to 1 and 5 mg/kg
bw/day did not result in any adverse effects on the dams or their
progeny. At a dose equivalent to 20 mg/kg bw/day, however, slight
maternal toxicity was observed, characterized by a reduction in
body-weight gain. Litter weights were decreased in groups receiving
20-160 mg/kg bw/day. At none of the doses was teratogenicity evident.
The NOEL in these studies was 5 mg/kg bw/day.
In two studies in rabbits, administration of diclazuril by gavage
at doses of 5-160 mg/kg bw/day from days 6 to 18 of pregnancy did not
result in any adverse effects on the dams or their offspring. From
these findings and because studies of the enteric absorption of
diclazuril in the rabbit were lacking, the Committee concluded that
there was no evidence that exposure to diclazuril was sufficient to
enable the teratogenicity of the drug to be evaluated. This conclusion
was supported by the occurrence of adverse effects in rabbits exposed
to Eimeria spp. following administration of the drug in the feed at
a dose equivalent to 13 mg/kg bw/day for 5 weeks. Furthermore, the
Committee noted that materno- and fetotoxicity had been observed in
rabbits dosed with a diclazuril analogue at 80 mg/kg bw/day.
Diclazuril was studied in a range of in vivo and in vitro
assays with a variety of genetic end-points. Negative findings in all
these assays enabled the Committee to conclude that diclazuril was not
genotoxic.
4. EVALUATION
The Committee established a temporary ADI of 0-20 µg/kg bw for
diclazuril, based on the NOEL of 3 mg/kg bw/day in the 2-year
toxicity/carcinogenicity study in mice and a safety factor of 200. The
ADI was rounded to one significant figure, consistent with accepted
rounding procedures (Annex 1, reference 91, section 2.7).
The results of a teratogenicity study in rabbits supported by
evidence that the doses administered were sufficiently high for the
teratogenic potential of diclazuril to be adequately explored are
required for evaluation in 1998.
5. REFERENCES
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987a).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1504. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1987b).
R 64433-Embryotoxicity and teratogenicity study in Wistar rats.
Unpublished nonclinical laboratory study Exp. No. 1651. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren, H., and Marsboom, R. (1988a). Diclazuril-
oral 2-generation reproduction study with two litters per generation
in Wistar rats. Unpublished nonclinical laboratory study Exp. No. 1663.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Dirkx, P., Van Cauteren H., and Marsboom, R. (1988b). Diclazuril -
oral embryotoxicity and teratogenicity study in New Zealand White
rabbits. Unpublished nonclinical laboratory study Exp. No. 1821.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Gillardin, J.M., Van Cauteren H., Sanz, G., and Marsboom, R. (1989).
Embryotoxicity and teratogenicity study in New Zealand white rabbits.
Unpublished nonclinical laboratory study Exp. No. 2212. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Kayembe K., Desmet P., Henry, M.C., and Stoffels, P. (1989).
Diclazuril for Isospora belli infection in AIDS [Letter] Lancet;
1:1397-1398.
Lampo, A., Vanparys, P.H., Vandenberghe, J., Coussement, W.,
Teuns, G., Maes, L., and Van Cauteren, H. (1995). R 64433 - Review
report on the pharmacodynamic and toxicological documentation on
diclazuril. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Leonard, A., et al.. (1988). The in vivo chromosome aberration assay
on human lymphocytes. Unpublished nonclinical laboratory report
SCK 86/02D/R 64433, Mol, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Mannens, G., Van Leemput, L., and Heykants, J. (1992). A study on the
excretion and metabolism of 14C-Diclazuril in male Wistar rats after
a single oral dose of 10 mg/kg. Unpublished non-clinical
pharmacokinetics report on R 64433 FK 1217. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
de Meester, C. and Leonard A. (1985). R 64433-Ames reversion mutation
test with Salmonella typhimurium and Escherichia coli. Unpublished
nonclinical laboratory study. Exp. No. 19, UCL, Laboratory of Terato-
genesis and Mutagenesis. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
de Meester, C. and Leonard A. (1986). R 64433-Ames reversion mutation
test with S. typhimurium and E. coli. Unpublished nonclinical
laboratory study Exp. No 22, UCL, Laboratory of Teratogenesis and
Mutagenesis, Brussels, Belgium. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Menichetti, F., Moretti, M.V., Marroni, M., Papili, R., and
DiCandilo, F. (1991). Diclazuril for cryptosporidiosis in AIDS
[Letter]. Am. J. Med.; 90(2): 271-2.
Meuldermans, W., Hendrickx, J., Mostmans, E., Verboven, P.,
Borgmans, C., Michiels, M., and Heykants, J. (1988a). The excretion
and metabolism of Diclazuil after a single oral dose in rabbits.
Unpublished preclinical research report. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Michiels, M., Hendrickx, J., Geerts, R.,
Hurkmans, R., Mostmans, E., Lauwers, W., and Heykant J. (1988b)
14C-Diclazuril (14C-R64433) in the broiler chicken: Total
radioactivity depletion and metabolism after single oral
administration at 1 mg/kg. Unpublished preclinical research report.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Meuldermans, W., Van Beijsterveldt, L., Hendrickx, J., Geerts, R.,
Mostmans, E., Borgmans, C., Verboven, P., Woestenborghs, R., Van
Leemput, L., and Heykant, J. (1990). 14C-Diclazuril (14C-R64433)
in the turkey: Absorption, metabolism, excretion and tissue depletion
after a single oral dose of 1 mg/kg. Unpublished preclinical research
report on R64433. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Michiels, M., Hendriks, R., Geerts, R., Meuldermans, W., and
Heykant, J. (1988). Absorption and tissue distribution of
14C-Diclazuril (14C-R64433) in the rabbit after a single oral dose
of 1 mg/kg. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Monbaliu, J., Van Leemput, L., and Heykants, J. (1993). Plasma levels
of Diclazuril in sheep after oral administration of a 0.25% drench
formulation at 1 mg Diclazuril per kg. Unpublished non-clinical
pharmacokinetics report on R64433 FK-992. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986a). The acute oral toxicity of R 64433 in
mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1986b). The acute oral toxicity of R 64433 in
rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987a). The acute subcutaneous toxicity of R 64433
in mice. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987b). The acute intraperitoneal toxicity of
R 64433 in mice. Unpublished preclinical research report. Submitted to
WHO (JECFA) by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987c) The acute subcutaneous toxicity of R 64433
in rats. Unpublished preclinical research report. Submitted to WHO by
Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987d) The acute intraperitoneal toxicity of
R 64433 in rats. Unpublished preclinical research report. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Niemegeers, C.J.E. (1987e). The acute oral toxicity of R 64433 in
Beagle dogs. Unpublished preclinical research report. Submitted to WHO
by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988a). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 1917.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Teuns, G. and Marsboom, R. (1988b). Acute dermal toxicity study in
rabbits. Unpublished nonclinical laboratory study Exp. No. 2035.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Beijsterveldt, L., Van Leemput, L., and Heykants, J. (1992).
Absorption and tissue distribution of 14C-Diclazuril in the male
Wistar rat after single oral administration at 10 mg/kg in aqueous
suspension. Unpublished non-clinical pharmacokinetics report on R64433
FK-1213. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven, F., and Zaman, R. (1985). The
antimicrobial activity of R 64433. Unpublished nonclinical laboratory
study. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Van Cutsem, J., Van Gerven F., and Zaman, R. (1986). The antimicrobial
activity of R 64433: additional data. Unpublished nonclinical
laboratory study. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1985). R 64433 - SOS chromosoomtest
in Escherichia coli. Unpublished nonclinical laboratory study
No. 1600. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986a). Sex-linked recessive lethal
test in Drosophila melanogaster. Unpublished nonclinical laboratory
study No. 1501 + 1657. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986b). R 64433 - Dominant lethal
test in male mice. Unpublished nonclinical laboratory study
Exp. No 1609. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1986c). R 64433 - Micronucleus test
in mice. Unpublished nonclinical laboratory study Exp. No 1744.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Vanparijs, Ph. and Marsboom, R. (1991). Micronucleus test in mice.
Unpublished nonclinical laboratory study, Exp. No. 2506. Submitted to
WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1986a). Subchronic toxicity study in Wistar rats (repeated dosage for
3 months). Unpublished nonclinical laboratory study Exp. No. 1502.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Van Cauteren, H., and Marsboom, R.
(1986b). Subchronic toxicity study in Beagle dogs (repeated dosage for
3 months + 1 month recovery). Unpublished nonclinical laboratory study
Exp. No. 1503. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1988a). Oral longterm toxicity study in Wistar rats (repeated dosage
for 12 months). Unpublished nonclinical laboratory study Exp.
No. 1755. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Teuns, G., Coussement, W., Van Cauteren, H., and
Marsboom, R. (1988b). Oral longterm toxicity study in Beagle dogs
(repeated dosage for 12 months). Unpublished nonclinical laboratory
study Exp. No. 1698. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauterern, H., and Marsboom, R. (1989a).
R 64433 - Oral carcinogencity study in Swiss mice (repeated dosage for
18 up to 24 months). Unpublished nonclinical laboratory study Exp.
No. 1649. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Gervais, S., Peeters, J., Lenaerts,
P., Van Rompay, J., Van Cauteren, H., and Marsboom, R. (1989b).
R 64433 - Oral carcinogenicity study in Wistar rats (repeated dosage
for 24 up to 30 months). Unpublished nonclinical laboratory study Exp.
No. 1650. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Coussement, W., Van Cauteren, H., and Marsboom, R.
(1990). Subchronic oral feeding toxicity study (pilot) in SPF Albino
Swiss mice. Unpublished nonclinical laboratory study Exp. No. 1665.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A. Coussement, W., and
Van Cauteren, H. (1992a). Three-month oral toxicity study in SPF
Albino Swiss mice. Unpublished nonclinical laboratory study Exp.
No. 2614. Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Verstraeten, A., Vandenberghe, J., Lampo, A., Coussement, W., and
Van Cauteren, H. (1992b). Three-month oral toxicity in SPF Wistar
rats. Unpublished nonclinical laboratory study Exp. No. 2613.
Submitted to WHO by Janssen Pharmaceutica, Beerse, Belgium.
Weterings, PJJM., et al. (1985). Evaluation of the DNA repair
inducing ability of R 64433 in a primary culture of rat hepatocytes.
Unpublished nonclinical report No. 0114/ER 154, Notox Pathobiology
Research, The Netherlands. Submitted to WHO by Janssen Pharmaceutica,
Beerse, Belgium.
Young, R.R. (1989). Mutagenticity test on in mouse lymphoma forward
mutation assay. Unpublished nonclinical report 10779-0-431, Hazleton
Laboratories America, Inc., USA. Submitted to WHO by Janssen
Pharmaceutica, Beerse, Belgium.
