
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
PROPYL GALLATE
CHEMICAL NAMES Propyl gallate;
n-propyl ester of 3,4,5-trihydroxybenzoic
acid
EMPIRICAL FORMULA C10H12O5
STRUCTURAL FORMULA
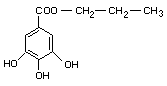 MOLECULAR WEIGHT 212.21
DEFINITION Propyl gallate contains not less then 99% of
C10H12O5 after drying at 110°C for 4 hours.
DESCRIPTION A white to creamy-white, crystalline, odourless
solid, with a slightly bitter taste. 1 g is
soluble in 300 ml of water or in 3.5 ml of
ethanol; freely soluble in fat.
USE As an antioxidant; more effective combined with
citric acid as synergist.
OCTYL GALLATE
CHEMICAL NAME Octyl gallate;
n-octyl ester of 3,4,5-trihydroxybenzoic acid
EMPIRICAL FORMULA C15H22O5
STRUCTURAL FORMULA
MOLECULAR WEIGHT 212.21
DEFINITION Propyl gallate contains not less then 99% of
C10H12O5 after drying at 110°C for 4 hours.
DESCRIPTION A white to creamy-white, crystalline, odourless
solid, with a slightly bitter taste. 1 g is
soluble in 300 ml of water or in 3.5 ml of
ethanol; freely soluble in fat.
USE As an antioxidant; more effective combined with
citric acid as synergist.
OCTYL GALLATE
CHEMICAL NAME Octyl gallate;
n-octyl ester of 3,4,5-trihydroxybenzoic acid
EMPIRICAL FORMULA C15H22O5
STRUCTURAL FORMULA
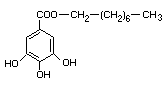 MOLECULAR WEIGHT 282.34
DEFINITION Octyl gallate contains not less than 98.5% of
C15H22O5 after drying at 60°C for 4 hours.
DESCRIPTION A white to creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 2.5 ml of ethanol. It is insoluble
in water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
DODECYL GALLATE
CHEMICAL NAMES Dodecyl gallate;
n-dodecyl (or lauryl) ester of
3,4,5-trihydroxybenzoic acid
SYNONYM Lauryl gallate
EMPIRICAL FORMULA C19H30O5
STRUCTURAL FORMULA
MOLECULAR WEIGHT 282.34
DEFINITION Octyl gallate contains not less than 98.5% of
C15H22O5 after drying at 60°C for 4 hours.
DESCRIPTION A white to creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 2.5 ml of ethanol. It is insoluble
in water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
DODECYL GALLATE
CHEMICAL NAMES Dodecyl gallate;
n-dodecyl (or lauryl) ester of
3,4,5-trihydroxybenzoic acid
SYNONYM Lauryl gallate
EMPIRICAL FORMULA C19H30O5
STRUCTURAL FORMULA
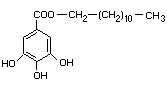 MOLECULAR WEIGHT 338.45
DEFINITION Dodecyl gallate contains not less than 98.5% of
C19H30O5 after drying at 60°C for 4 hours.
DESCRIPTION A white or creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 3.5 ml of ethanol. Insoluble in
water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
Biological Data
Biochemical aspects
The available evidence indicates that the esters are hydrolysed in the
body. Most of the gallic acid is converted into 4-O-methyl gallic
acid. Free gallic acid or a conjugated derivative of 4-O-methyl
gallic acid is excreted in the urine. Conjugation of the 4-O-methyl
gallic acid with glucuronic acid was demonstrated.1
Rats fed a low-methionine, low-choline diet containing 1% gallic acid
developed fatty livers which could be prevented by added choline or
methionine.2
The detailed metabolic pathways for propyl gallate have bean
described.3 There is no evidence to suggest that other esters of
gallic acid differ greatly in their metabolism from the pattern
described for propyl gallate.
Acute Toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Propyl gallate Rat oral 3800 4
Rat oral 3600 5
Rat intraperitoneal 380 4
Mouse oral 2000-3500 5
Octyl gallate Rat oral 4700 6
Rat intraperitoneal 60-80 7
Dodecyl gallate Rat oral 6500 6
Bat intraperitoneal 100-120 7
Short-term studies
Rat. Levels of propyl gallate of 1.2% and 2.3% in the diet caused
interference with weight gain, the bitter taste of the gallate
apparently making the diet unpalatable. The higher dose level caused
some deaths (about 40%) during the first month; the survivors
continued to eat the diet for 10-16 months and showed retarded growth,
but no pathological lesions. The animals that died exhibited renal
damage.4
Weanling rats were given diets containing 2.5% and 5% dodecyl gallate.
All animals fed the smaller quantity were dead within 10 days, and all
animals fed the larger quantity died within 7 days.8
Rats fed for 70 days on a diet containing 7% fat and 0.2% dodecyl
gallate showed no effect on body-weight.9
Weanling rats were fed diets which contained 20% lard and 0%, 0.1%,
0.2%, O.3%, 0.4% and 0.5% propyl gallate for 6 weeks. There was no
effect on body-weight, liver weight, liver weight to body-weight
ratio, left adrenal weight, total liver lipid, composition of liver
polyunsaturated fatty acids, liver cholesterol, adrenal cholesterol or
serum sodium.10
Propyl gallate was added to the dietary fat of weanling rats at levels
of 0.02% in the fat for 13 weeks. The fat content of the diet
accounted for 30% of its calorific value. There was a very slight
inhibition of growth. The same rats were then placed on a partial
starvation diet and kept until they died. The survival time of the
animals which had received the propyl gallate was considerably reduced
and the reduction in their total body protein was greater.11
Guinea-pig. Propyl gallate fed to guinea-pigs in groups of 20 at a
level of O.O117% in the diet for 14 months caused no detectable ill
effects.4
Dog. A level of 0.0117% of propyl gallate in the diet was well
tolerated by a group of 7 dogs over a period of 14 months.4
Pig. Diets containing 0.2% of propyl, octyl or dodecyl gallate were
fed to pigs without demonstrable ill effect; no anaemia was
observed.12
Long-term studies
Rat and mouse. A level of 5% propyl gallate in the diet in a 2-year
chronic toxicity test on rats and mice gave rise to patchy hyperplasia
in the proventiculus. At a level of 1% no difference was noted
between test and control animals.5
Rats in groups of 10 males and 10 females were fed for 2 years on
diets containing 0%, 0.00117%, 0.0117%, 0.117%, 1.17% and 2.34% of
propyl gallate. The groups receiving 1.17% and 2.34% of propyl
gallate showed stunted growth and evidence of renal damage. In the
other groups, there was no detectable effect on haemoglobin,
erythrocyte or leucocyte levels in the blood, or on the histological
appearance of the organs examined.4
Propyl, octyl and dodecyl gallate were fed to rats at concentrations
of 0.035%, 0.2% and 0.5% in the diet. Growth was affected only at the
0.5% level of dodecyl gallate; there was significant retardation,
particularly in the second generation. At this level of dodecyl
gallate, some litters were lost in the second generation because they
were not sufficiently fed by the mothers. A slight hypochromic
anaemia was noticed in the groups on diets containing 0.2% octyl and
dodecyl gallate. No abnormalities were observed in the organs or
tissues of the rats at autopsy.12
Young rats in groups of 12 males and 12 females were fed diets
containing 7% fat and 0.2% octyl or dodecyl gallate. There was no
significant difference between test and control animals over 3
generations.6
Comment on experimental studies reported
The acute and short-term toxicity studies reported are fairly
extensive and cover a wide range of animal species. The results of
the experiments involving partial starvation are of great interest and
indicate a higher toxicity of propyl gallate than the other studies.
Evaluation of these experiments is difficult, however, in the absence
of comparable studies with other food additives. Several long-term
studies have been made and the levels of feeding varied widely. There
are some discrepancies in the results reported. However, the
long-term studies on rats provide a basis for evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
With one exception, in long-term studies in rats gallates caused no
demonstrable ill effects when fed at a level of 0.2%; in one
investigation, however, this level resulted in hypochromic anaemia.
It seems likely that this may have been due to interference with iron
absorption, but the cause was not established. Haematological effects
were carefully examined in a number of other investigations and no
abnormalities were seen. It seem probable, therefore, that this
effect was related to the particular circumstances of one study and
can be properly disregarded in arriving at the level that causes no
significant effects in the rat. The level that causes no significant
toxicological effect in the rat is 100 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-0.5
Comment
Attention should be given to the amounts of other phenolic
antioxidants in the diet.
Further Work Considered Desirable
1. long-term studies in other species than the rat, with special
attention to possible interference with iron metabolism and
haematopoiesis.
2. Metabolic studies in human subjects.
3. Further studies on the effects of stress on the toxicity of this
and other food additives.
References
1. Booth, A. N., Masri, M. S., Robbins, D. J., Emerson, O. H., Jones,
F. T. & DeEds, F. (1959) J. biol. Chem., 234, 3014
2. Booth, A. N., Robbins, D. J. & DeEds, F. (1961) J. Nutr., 75, 104
3. Dacre, J. O. (1960) J. N.Z. Inst. Chem., 24, 161
4. Orten, J. M., Kuyper, A. C. & Smith, A. H. (1948) Food Techn., 2,
308
5. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodward, G. (1951)
Advanc. Food Res., 3, 197
6. Sluis. K. J. H. van (1951) Food Manuf., 26, 99
7. Esch, G. J. van, Genderen, H. van (1954) Netherlands Institute of
Public Health, Report No. 481
8. Allen, C. S. & DeEds, F. D. (1951) J. Amer. Oil Chem. Soc., 28, 304
9. Tollenaar, F. D. (1954) Fette u. Seifen, 56, 41
10. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
11.Buckman, N. D. (1962) Vop. Pitan., 21, 68
12. Esch, G. J. van (1955) Voeding, 16, 683
MOLECULAR WEIGHT 338.45
DEFINITION Dodecyl gallate contains not less than 98.5% of
C19H30O5 after drying at 60°C for 4 hours.
DESCRIPTION A white or creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 3.5 ml of ethanol. Insoluble in
water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
Biological Data
Biochemical aspects
The available evidence indicates that the esters are hydrolysed in the
body. Most of the gallic acid is converted into 4-O-methyl gallic
acid. Free gallic acid or a conjugated derivative of 4-O-methyl
gallic acid is excreted in the urine. Conjugation of the 4-O-methyl
gallic acid with glucuronic acid was demonstrated.1
Rats fed a low-methionine, low-choline diet containing 1% gallic acid
developed fatty livers which could be prevented by added choline or
methionine.2
The detailed metabolic pathways for propyl gallate have bean
described.3 There is no evidence to suggest that other esters of
gallic acid differ greatly in their metabolism from the pattern
described for propyl gallate.
Acute Toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Propyl gallate Rat oral 3800 4
Rat oral 3600 5
Rat intraperitoneal 380 4
Mouse oral 2000-3500 5
Octyl gallate Rat oral 4700 6
Rat intraperitoneal 60-80 7
Dodecyl gallate Rat oral 6500 6
Bat intraperitoneal 100-120 7
Short-term studies
Rat. Levels of propyl gallate of 1.2% and 2.3% in the diet caused
interference with weight gain, the bitter taste of the gallate
apparently making the diet unpalatable. The higher dose level caused
some deaths (about 40%) during the first month; the survivors
continued to eat the diet for 10-16 months and showed retarded growth,
but no pathological lesions. The animals that died exhibited renal
damage.4
Weanling rats were given diets containing 2.5% and 5% dodecyl gallate.
All animals fed the smaller quantity were dead within 10 days, and all
animals fed the larger quantity died within 7 days.8
Rats fed for 70 days on a diet containing 7% fat and 0.2% dodecyl
gallate showed no effect on body-weight.9
Weanling rats were fed diets which contained 20% lard and 0%, 0.1%,
0.2%, O.3%, 0.4% and 0.5% propyl gallate for 6 weeks. There was no
effect on body-weight, liver weight, liver weight to body-weight
ratio, left adrenal weight, total liver lipid, composition of liver
polyunsaturated fatty acids, liver cholesterol, adrenal cholesterol or
serum sodium.10
Propyl gallate was added to the dietary fat of weanling rats at levels
of 0.02% in the fat for 13 weeks. The fat content of the diet
accounted for 30% of its calorific value. There was a very slight
inhibition of growth. The same rats were then placed on a partial
starvation diet and kept until they died. The survival time of the
animals which had received the propyl gallate was considerably reduced
and the reduction in their total body protein was greater.11
Guinea-pig. Propyl gallate fed to guinea-pigs in groups of 20 at a
level of O.O117% in the diet for 14 months caused no detectable ill
effects.4
Dog. A level of 0.0117% of propyl gallate in the diet was well
tolerated by a group of 7 dogs over a period of 14 months.4
Pig. Diets containing 0.2% of propyl, octyl or dodecyl gallate were
fed to pigs without demonstrable ill effect; no anaemia was
observed.12
Long-term studies
Rat and mouse. A level of 5% propyl gallate in the diet in a 2-year
chronic toxicity test on rats and mice gave rise to patchy hyperplasia
in the proventiculus. At a level of 1% no difference was noted
between test and control animals.5
Rats in groups of 10 males and 10 females were fed for 2 years on
diets containing 0%, 0.00117%, 0.0117%, 0.117%, 1.17% and 2.34% of
propyl gallate. The groups receiving 1.17% and 2.34% of propyl
gallate showed stunted growth and evidence of renal damage. In the
other groups, there was no detectable effect on haemoglobin,
erythrocyte or leucocyte levels in the blood, or on the histological
appearance of the organs examined.4
Propyl, octyl and dodecyl gallate were fed to rats at concentrations
of 0.035%, 0.2% and 0.5% in the diet. Growth was affected only at the
0.5% level of dodecyl gallate; there was significant retardation,
particularly in the second generation. At this level of dodecyl
gallate, some litters were lost in the second generation because they
were not sufficiently fed by the mothers. A slight hypochromic
anaemia was noticed in the groups on diets containing 0.2% octyl and
dodecyl gallate. No abnormalities were observed in the organs or
tissues of the rats at autopsy.12
Young rats in groups of 12 males and 12 females were fed diets
containing 7% fat and 0.2% octyl or dodecyl gallate. There was no
significant difference between test and control animals over 3
generations.6
Comment on experimental studies reported
The acute and short-term toxicity studies reported are fairly
extensive and cover a wide range of animal species. The results of
the experiments involving partial starvation are of great interest and
indicate a higher toxicity of propyl gallate than the other studies.
Evaluation of these experiments is difficult, however, in the absence
of comparable studies with other food additives. Several long-term
studies have been made and the levels of feeding varied widely. There
are some discrepancies in the results reported. However, the
long-term studies on rats provide a basis for evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
With one exception, in long-term studies in rats gallates caused no
demonstrable ill effects when fed at a level of 0.2%; in one
investigation, however, this level resulted in hypochromic anaemia.
It seems likely that this may have been due to interference with iron
absorption, but the cause was not established. Haematological effects
were carefully examined in a number of other investigations and no
abnormalities were seen. It seem probable, therefore, that this
effect was related to the particular circumstances of one study and
can be properly disregarded in arriving at the level that causes no
significant effects in the rat. The level that causes no significant
toxicological effect in the rat is 100 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-0.5
Comment
Attention should be given to the amounts of other phenolic
antioxidants in the diet.
Further Work Considered Desirable
1. long-term studies in other species than the rat, with special
attention to possible interference with iron metabolism and
haematopoiesis.
2. Metabolic studies in human subjects.
3. Further studies on the effects of stress on the toxicity of this
and other food additives.
References
1. Booth, A. N., Masri, M. S., Robbins, D. J., Emerson, O. H., Jones,
F. T. & DeEds, F. (1959) J. biol. Chem., 234, 3014
2. Booth, A. N., Robbins, D. J. & DeEds, F. (1961) J. Nutr., 75, 104
3. Dacre, J. O. (1960) J. N.Z. Inst. Chem., 24, 161
4. Orten, J. M., Kuyper, A. C. & Smith, A. H. (1948) Food Techn., 2,
308
5. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodward, G. (1951)
Advanc. Food Res., 3, 197
6. Sluis. K. J. H. van (1951) Food Manuf., 26, 99
7. Esch, G. J. van, Genderen, H. van (1954) Netherlands Institute of
Public Health, Report No. 481
8. Allen, C. S. & DeEds, F. D. (1951) J. Amer. Oil Chem. Soc., 28, 304
9. Tollenaar, F. D. (1954) Fette u. Seifen, 56, 41
10. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
11.Buckman, N. D. (1962) Vop. Pitan., 21, 68
12. Esch, G. J. van (1955) Voeding, 16, 683
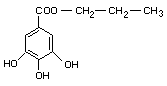 MOLECULAR WEIGHT 212.21
DEFINITION Propyl gallate contains not less then 99% of
C10H12O5 after drying at 110°C for 4 hours.
DESCRIPTION A white to creamy-white, crystalline, odourless
solid, with a slightly bitter taste. 1 g is
soluble in 300 ml of water or in 3.5 ml of
ethanol; freely soluble in fat.
USE As an antioxidant; more effective combined with
citric acid as synergist.
OCTYL GALLATE
CHEMICAL NAME Octyl gallate;
n-octyl ester of 3,4,5-trihydroxybenzoic acid
EMPIRICAL FORMULA C15H22O5
STRUCTURAL FORMULA
MOLECULAR WEIGHT 212.21
DEFINITION Propyl gallate contains not less then 99% of
C10H12O5 after drying at 110°C for 4 hours.
DESCRIPTION A white to creamy-white, crystalline, odourless
solid, with a slightly bitter taste. 1 g is
soluble in 300 ml of water or in 3.5 ml of
ethanol; freely soluble in fat.
USE As an antioxidant; more effective combined with
citric acid as synergist.
OCTYL GALLATE
CHEMICAL NAME Octyl gallate;
n-octyl ester of 3,4,5-trihydroxybenzoic acid
EMPIRICAL FORMULA C15H22O5
STRUCTURAL FORMULA
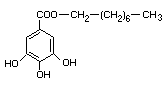 MOLECULAR WEIGHT 282.34
DEFINITION Octyl gallate contains not less than 98.5% of
C15H22O5 after drying at 60°C for 4 hours.
DESCRIPTION A white to creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 2.5 ml of ethanol. It is insoluble
in water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
DODECYL GALLATE
CHEMICAL NAMES Dodecyl gallate;
n-dodecyl (or lauryl) ester of
3,4,5-trihydroxybenzoic acid
SYNONYM Lauryl gallate
EMPIRICAL FORMULA C19H30O5
STRUCTURAL FORMULA
MOLECULAR WEIGHT 282.34
DEFINITION Octyl gallate contains not less than 98.5% of
C15H22O5 after drying at 60°C for 4 hours.
DESCRIPTION A white to creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 2.5 ml of ethanol. It is insoluble
in water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
DODECYL GALLATE
CHEMICAL NAMES Dodecyl gallate;
n-dodecyl (or lauryl) ester of
3,4,5-trihydroxybenzoic acid
SYNONYM Lauryl gallate
EMPIRICAL FORMULA C19H30O5
STRUCTURAL FORMULA
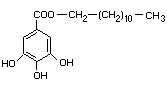 MOLECULAR WEIGHT 338.45
DEFINITION Dodecyl gallate contains not less than 98.5% of
C19H30O5 after drying at 60°C for 4 hours.
DESCRIPTION A white or creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 3.5 ml of ethanol. Insoluble in
water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
Biological Data
Biochemical aspects
The available evidence indicates that the esters are hydrolysed in the
body. Most of the gallic acid is converted into 4-O-methyl gallic
acid. Free gallic acid or a conjugated derivative of 4-O-methyl
gallic acid is excreted in the urine. Conjugation of the 4-O-methyl
gallic acid with glucuronic acid was demonstrated.1
Rats fed a low-methionine, low-choline diet containing 1% gallic acid
developed fatty livers which could be prevented by added choline or
methionine.2
The detailed metabolic pathways for propyl gallate have bean
described.3 There is no evidence to suggest that other esters of
gallic acid differ greatly in their metabolism from the pattern
described for propyl gallate.
Acute Toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Propyl gallate Rat oral 3800 4
Rat oral 3600 5
Rat intraperitoneal 380 4
Mouse oral 2000-3500 5
Octyl gallate Rat oral 4700 6
Rat intraperitoneal 60-80 7
Dodecyl gallate Rat oral 6500 6
Bat intraperitoneal 100-120 7
Short-term studies
Rat. Levels of propyl gallate of 1.2% and 2.3% in the diet caused
interference with weight gain, the bitter taste of the gallate
apparently making the diet unpalatable. The higher dose level caused
some deaths (about 40%) during the first month; the survivors
continued to eat the diet for 10-16 months and showed retarded growth,
but no pathological lesions. The animals that died exhibited renal
damage.4
Weanling rats were given diets containing 2.5% and 5% dodecyl gallate.
All animals fed the smaller quantity were dead within 10 days, and all
animals fed the larger quantity died within 7 days.8
Rats fed for 70 days on a diet containing 7% fat and 0.2% dodecyl
gallate showed no effect on body-weight.9
Weanling rats were fed diets which contained 20% lard and 0%, 0.1%,
0.2%, O.3%, 0.4% and 0.5% propyl gallate for 6 weeks. There was no
effect on body-weight, liver weight, liver weight to body-weight
ratio, left adrenal weight, total liver lipid, composition of liver
polyunsaturated fatty acids, liver cholesterol, adrenal cholesterol or
serum sodium.10
Propyl gallate was added to the dietary fat of weanling rats at levels
of 0.02% in the fat for 13 weeks. The fat content of the diet
accounted for 30% of its calorific value. There was a very slight
inhibition of growth. The same rats were then placed on a partial
starvation diet and kept until they died. The survival time of the
animals which had received the propyl gallate was considerably reduced
and the reduction in their total body protein was greater.11
Guinea-pig. Propyl gallate fed to guinea-pigs in groups of 20 at a
level of O.O117% in the diet for 14 months caused no detectable ill
effects.4
Dog. A level of 0.0117% of propyl gallate in the diet was well
tolerated by a group of 7 dogs over a period of 14 months.4
Pig. Diets containing 0.2% of propyl, octyl or dodecyl gallate were
fed to pigs without demonstrable ill effect; no anaemia was
observed.12
Long-term studies
Rat and mouse. A level of 5% propyl gallate in the diet in a 2-year
chronic toxicity test on rats and mice gave rise to patchy hyperplasia
in the proventiculus. At a level of 1% no difference was noted
between test and control animals.5
Rats in groups of 10 males and 10 females were fed for 2 years on
diets containing 0%, 0.00117%, 0.0117%, 0.117%, 1.17% and 2.34% of
propyl gallate. The groups receiving 1.17% and 2.34% of propyl
gallate showed stunted growth and evidence of renal damage. In the
other groups, there was no detectable effect on haemoglobin,
erythrocyte or leucocyte levels in the blood, or on the histological
appearance of the organs examined.4
Propyl, octyl and dodecyl gallate were fed to rats at concentrations
of 0.035%, 0.2% and 0.5% in the diet. Growth was affected only at the
0.5% level of dodecyl gallate; there was significant retardation,
particularly in the second generation. At this level of dodecyl
gallate, some litters were lost in the second generation because they
were not sufficiently fed by the mothers. A slight hypochromic
anaemia was noticed in the groups on diets containing 0.2% octyl and
dodecyl gallate. No abnormalities were observed in the organs or
tissues of the rats at autopsy.12
Young rats in groups of 12 males and 12 females were fed diets
containing 7% fat and 0.2% octyl or dodecyl gallate. There was no
significant difference between test and control animals over 3
generations.6
Comment on experimental studies reported
The acute and short-term toxicity studies reported are fairly
extensive and cover a wide range of animal species. The results of
the experiments involving partial starvation are of great interest and
indicate a higher toxicity of propyl gallate than the other studies.
Evaluation of these experiments is difficult, however, in the absence
of comparable studies with other food additives. Several long-term
studies have been made and the levels of feeding varied widely. There
are some discrepancies in the results reported. However, the
long-term studies on rats provide a basis for evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
With one exception, in long-term studies in rats gallates caused no
demonstrable ill effects when fed at a level of 0.2%; in one
investigation, however, this level resulted in hypochromic anaemia.
It seems likely that this may have been due to interference with iron
absorption, but the cause was not established. Haematological effects
were carefully examined in a number of other investigations and no
abnormalities were seen. It seem probable, therefore, that this
effect was related to the particular circumstances of one study and
can be properly disregarded in arriving at the level that causes no
significant effects in the rat. The level that causes no significant
toxicological effect in the rat is 100 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-0.5
Comment
Attention should be given to the amounts of other phenolic
antioxidants in the diet.
Further Work Considered Desirable
1. long-term studies in other species than the rat, with special
attention to possible interference with iron metabolism and
haematopoiesis.
2. Metabolic studies in human subjects.
3. Further studies on the effects of stress on the toxicity of this
and other food additives.
References
1. Booth, A. N., Masri, M. S., Robbins, D. J., Emerson, O. H., Jones,
F. T. & DeEds, F. (1959) J. biol. Chem., 234, 3014
2. Booth, A. N., Robbins, D. J. & DeEds, F. (1961) J. Nutr., 75, 104
3. Dacre, J. O. (1960) J. N.Z. Inst. Chem., 24, 161
4. Orten, J. M., Kuyper, A. C. & Smith, A. H. (1948) Food Techn., 2,
308
5. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodward, G. (1951)
Advanc. Food Res., 3, 197
6. Sluis. K. J. H. van (1951) Food Manuf., 26, 99
7. Esch, G. J. van, Genderen, H. van (1954) Netherlands Institute of
Public Health, Report No. 481
8. Allen, C. S. & DeEds, F. D. (1951) J. Amer. Oil Chem. Soc., 28, 304
9. Tollenaar, F. D. (1954) Fette u. Seifen, 56, 41
10. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
11.Buckman, N. D. (1962) Vop. Pitan., 21, 68
12. Esch, G. J. van (1955) Voeding, 16, 683
MOLECULAR WEIGHT 338.45
DEFINITION Dodecyl gallate contains not less than 98.5% of
C19H30O5 after drying at 60°C for 4 hours.
DESCRIPTION A white or creamy-white, odourless solid, which
may have a slightly bitter taste. 1 g is
soluble in 3.5 ml of ethanol. Insoluble in
water but freely soluble in fats.
USE As an antioxidant; more effective combined with
citric acid as synergist.
Biological Data
Biochemical aspects
The available evidence indicates that the esters are hydrolysed in the
body. Most of the gallic acid is converted into 4-O-methyl gallic
acid. Free gallic acid or a conjugated derivative of 4-O-methyl
gallic acid is excreted in the urine. Conjugation of the 4-O-methyl
gallic acid with glucuronic acid was demonstrated.1
Rats fed a low-methionine, low-choline diet containing 1% gallic acid
developed fatty livers which could be prevented by added choline or
methionine.2
The detailed metabolic pathways for propyl gallate have bean
described.3 There is no evidence to suggest that other esters of
gallic acid differ greatly in their metabolism from the pattern
described for propyl gallate.
Acute Toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Propyl gallate Rat oral 3800 4
Rat oral 3600 5
Rat intraperitoneal 380 4
Mouse oral 2000-3500 5
Octyl gallate Rat oral 4700 6
Rat intraperitoneal 60-80 7
Dodecyl gallate Rat oral 6500 6
Bat intraperitoneal 100-120 7
Short-term studies
Rat. Levels of propyl gallate of 1.2% and 2.3% in the diet caused
interference with weight gain, the bitter taste of the gallate
apparently making the diet unpalatable. The higher dose level caused
some deaths (about 40%) during the first month; the survivors
continued to eat the diet for 10-16 months and showed retarded growth,
but no pathological lesions. The animals that died exhibited renal
damage.4
Weanling rats were given diets containing 2.5% and 5% dodecyl gallate.
All animals fed the smaller quantity were dead within 10 days, and all
animals fed the larger quantity died within 7 days.8
Rats fed for 70 days on a diet containing 7% fat and 0.2% dodecyl
gallate showed no effect on body-weight.9
Weanling rats were fed diets which contained 20% lard and 0%, 0.1%,
0.2%, O.3%, 0.4% and 0.5% propyl gallate for 6 weeks. There was no
effect on body-weight, liver weight, liver weight to body-weight
ratio, left adrenal weight, total liver lipid, composition of liver
polyunsaturated fatty acids, liver cholesterol, adrenal cholesterol or
serum sodium.10
Propyl gallate was added to the dietary fat of weanling rats at levels
of 0.02% in the fat for 13 weeks. The fat content of the diet
accounted for 30% of its calorific value. There was a very slight
inhibition of growth. The same rats were then placed on a partial
starvation diet and kept until they died. The survival time of the
animals which had received the propyl gallate was considerably reduced
and the reduction in their total body protein was greater.11
Guinea-pig. Propyl gallate fed to guinea-pigs in groups of 20 at a
level of O.O117% in the diet for 14 months caused no detectable ill
effects.4
Dog. A level of 0.0117% of propyl gallate in the diet was well
tolerated by a group of 7 dogs over a period of 14 months.4
Pig. Diets containing 0.2% of propyl, octyl or dodecyl gallate were
fed to pigs without demonstrable ill effect; no anaemia was
observed.12
Long-term studies
Rat and mouse. A level of 5% propyl gallate in the diet in a 2-year
chronic toxicity test on rats and mice gave rise to patchy hyperplasia
in the proventiculus. At a level of 1% no difference was noted
between test and control animals.5
Rats in groups of 10 males and 10 females were fed for 2 years on
diets containing 0%, 0.00117%, 0.0117%, 0.117%, 1.17% and 2.34% of
propyl gallate. The groups receiving 1.17% and 2.34% of propyl
gallate showed stunted growth and evidence of renal damage. In the
other groups, there was no detectable effect on haemoglobin,
erythrocyte or leucocyte levels in the blood, or on the histological
appearance of the organs examined.4
Propyl, octyl and dodecyl gallate were fed to rats at concentrations
of 0.035%, 0.2% and 0.5% in the diet. Growth was affected only at the
0.5% level of dodecyl gallate; there was significant retardation,
particularly in the second generation. At this level of dodecyl
gallate, some litters were lost in the second generation because they
were not sufficiently fed by the mothers. A slight hypochromic
anaemia was noticed in the groups on diets containing 0.2% octyl and
dodecyl gallate. No abnormalities were observed in the organs or
tissues of the rats at autopsy.12
Young rats in groups of 12 males and 12 females were fed diets
containing 7% fat and 0.2% octyl or dodecyl gallate. There was no
significant difference between test and control animals over 3
generations.6
Comment on experimental studies reported
The acute and short-term toxicity studies reported are fairly
extensive and cover a wide range of animal species. The results of
the experiments involving partial starvation are of great interest and
indicate a higher toxicity of propyl gallate than the other studies.
Evaluation of these experiments is difficult, however, in the absence
of comparable studies with other food additives. Several long-term
studies have been made and the levels of feeding varied widely. There
are some discrepancies in the results reported. However, the
long-term studies on rats provide a basis for evaluation.
Evaluation
Level causing no significant toxicological effect in the rat
With one exception, in long-term studies in rats gallates caused no
demonstrable ill effects when fed at a level of 0.2%; in one
investigation, however, this level resulted in hypochromic anaemia.
It seems likely that this may have been due to interference with iron
absorption, but the cause was not established. Haematological effects
were carefully examined in a number of other investigations and no
abnormalities were seen. It seem probable, therefore, that this
effect was related to the particular circumstances of one study and
can be properly disregarded in arriving at the level that causes no
significant effects in the rat. The level that causes no significant
toxicological effect in the rat is 100 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-0.5
Comment
Attention should be given to the amounts of other phenolic
antioxidants in the diet.
Further Work Considered Desirable
1. long-term studies in other species than the rat, with special
attention to possible interference with iron metabolism and
haematopoiesis.
2. Metabolic studies in human subjects.
3. Further studies on the effects of stress on the toxicity of this
and other food additives.
References
1. Booth, A. N., Masri, M. S., Robbins, D. J., Emerson, O. H., Jones,
F. T. & DeEds, F. (1959) J. biol. Chem., 234, 3014
2. Booth, A. N., Robbins, D. J. & DeEds, F. (1961) J. Nutr., 75, 104
3. Dacre, J. O. (1960) J. N.Z. Inst. Chem., 24, 161
4. Orten, J. M., Kuyper, A. C. & Smith, A. H. (1948) Food Techn., 2,
308
5. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodward, G. (1951)
Advanc. Food Res., 3, 197
6. Sluis. K. J. H. van (1951) Food Manuf., 26, 99
7. Esch, G. J. van, Genderen, H. van (1954) Netherlands Institute of
Public Health, Report No. 481
8. Allen, C. S. & DeEds, F. D. (1951) J. Amer. Oil Chem. Soc., 28, 304
9. Tollenaar, F. D. (1954) Fette u. Seifen, 56, 41
10. Johnson, A. R. & Hewgill, F. R. (1961) Aust. J. exp. Biol. med.
Sci., 39, 353
11.Buckman, N. D. (1962) Vop. Pitan., 21, 68
12. Esch, G. J. van (1955) Voeding, 16, 683
