
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
HEXAMETHLENETETRAMINE
CHEMICAL NAMES Hexamethylenetetramine; methenamine; methamin;
hexamethyleneamine
EMPIRICAL FORMULA C6H12N4
STRUCTURAL FORMULA
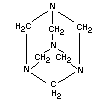 MOLECULAR WEIGHT 140.2
DESCRIPTION White odourless crystals, which sublime at
about 263°C. 1 g yields 1.2 of formaldehyde on
hydrolysis. One part is soluble in 1.5 ml of
water or 12.5 ml of alcohol.
USE As a preservative for fish, meat and pickles.
Biological Data
Biochemical aspects
Formaldehyde and formic acid occur in fresh unpreserved cod-fish
muscle.1,2
Formaldehyde and formic acid are excreted in the urine of dogs and
cats fed a meal of fish not treated with preservatives, the source of
these substances being trimethylamineoxide occurring naturally in fish
muscle.3,4,5 The concentration of free formaldehyde in the urine of
these dogs and cats given unpreserved fish was 4 to 5 times higher
than that found in human subjects who had consumed fish preserved with
hexamethylenetetramine. Humans fed almost exclusively on unpreserved
fish did not show an increase in the excretion of formaldehyde or
formic acid.3
Under acid conditions, or in the presence of proteins,
hexamethylenetetramine decomposes gradually yielding ammonia and
formaldehyde.6
Hexamethylenetetramine liberates free formaldehyde in the stomach.7
Intravenous infusion in dog of formaldehyde, 35 mg/kg as a 0.6%
solution during 8 to 10 minutes, was carried out. Two minutes after
completion of the infusion them was no increase in formaldehyde but a
high increase in the formic acid level in the blood. The formic acid
level decreased by 50% in 1 hour, by 75% after 2 hours, and was back
to normal after 4 hours. In vitro human blood oxidizes 30%
formaldehyde (0.7 mg to 4 ml blood) to formic acid within 4 hours.
Addition of methylene blue (0.05% to 0.2%) results in a complete
oxidation of the formaldehyde.8
Experiments with 14C-labelled formaldehyde given by stomach tube to
rats and mice showed that 5 minutes after application the
radioactivity was distributed over the total body. After 12 hours,
approximately 40% had been expired as 14CO2, 10% had been excreted in
the urine, and 1% in the faeces. The homogenized whole animals
contained 20% of the radioactivity after 24 hours and 10% after 96
hours.9
Further work on the metabolism of hexamethylenetetramine and
formaldehyde is in progress.10,11,12
Special studies
Both hexamethylenetetramine13 and formaldehyde14 have been shown to
act as mutagens in Drosophila.
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat intravenous 9200 15
Short-term studies
Rat. Two groups of 5 male and 5 female rats received respectively
0.4 and 0.2 hexamethylenetetramine per day parenterally or by stomach
tube for 90 days. Groups of 15 male and 15 female rats were also
given 0.4 g hexamethylenetetramine by stomach tube for 333 days.
During the latter experiment 9 males and 3 females died compared with
1 male and 2 females in the control group. In both experiments no
macroscopic changes in organs were observed in the animals receiving
hexamethylenetetramine, nor did the body-weight of the test animals
vary from that of the controls. However, a citrus yellow colour of
the coat was noted.16
Cat. (Work In progress) Since October 1963, 0.124%
hexamethylenetetramine and 0.0375% formaldehyde have been fed in the
diet to groups of 5 and 4 cats respectively.17
Dog. (Work in progress) Since January 1963, 0.125%
hexamethylenetetramine and 0.375% formaldehyde have each been fed to
groups of 6 dogs. In November 1963, 5 litters born after the
commencement of the feeding trials were also included in the
experiment.17
Long-term studies
Mouse. (Work in progress) Since January 1963, 2 groups of 30 males
and 30 females of a special strain of mice with a high incidence of
spontaneous tumours (about 80% after 1 year) have been fed 1%
hexamethylenetetramine and 0.15% formaldehyde respectively.17
Rat. (work in progress) Since April 1963, rats have been fed 0.5%
and 1% hexamethylenetetramine and 0.05% and 0.15% formaldehyde.17
(Work in progress) A long-term, seven-generation feeding study on
rats was commenced in May 1963. 5 mg/kg and 50 mg/kg
hexamethylenetetramine are given in the drinking-water.18
(Work in progress) In March 1964, a three-generation chronic toxicity
test was begun, feeding rats 0.04%, 0.08% and 0.16%
hexamethylenetetramine in the diet.19
Repeated subcutaneous injections of 35-40% solutions of
hexamethylenetetramine produced local sarcomas in 8 out of 14 rats.20
(Work In progress) Two groups of rats (15 male and 15 female) are
being given twice weekly subcutaneous injections with 1 ml of a 40%
solution of hexamethylenetetramine and 0.1% formaldehyde respectively.
Two control groups, each having 7 male and 7 female rats, are being
given respectively NaCl and saccharose solution of equal osmotic
pressure to that of the 40% hexamethylenetetramine solution.
Evaluation
The toxicological data are considered to be insufficient for
evaluation of an acceptable level for use as a food additive in man.
In addition, hexamethylenetetramine produces sarcomas in rats after
subcutaneous injection and has a mutagenic action in insects.
It is concluded that, pending the evaluation of the results of work
now in progress, hexamethylenetetramine should not be used as a food
additive in human foodstuffs.
References
1. Malorny, G. (1964) FAO Symposium on the Significance of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/11
2. Amano, K. & Yamada, K. (1964) FAO Symposium on the Significanae of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/9
3. Malorny, G. & Rietbrock. N. (1962) Naturwissenschaften 49, 520
4. Malorny, G., Rietbrock, N. & Schassan, H. H. (1963) Arch. exp.
Path. Pharmak., 246, 62
5. Schassan, H. H. (1963) Inauguraldissertation, Hamburg
6. Hutschenreuter, H. (1956) Z. Lebensmitt.-Untersuch., 104, 161
7. Malorny. G. & Rietbrock. N. (1963) Vortrag auf der X. Tagung des
Ernährungswissenschaftlichen Beirats der deutschen Fischwirtschaft,
Bremen.
8. Malorny. G., Rietbrock. N. & Schneider, M. (1964) Arch. exp. Path.
Pharmak., 247, 381
9. Buss, J., Kuschinsky, K., Kewitz, H. & Koransky, W. (1964) Arch.
exp. Path. Pharmak., 247, 380
10. Malorny, G., Rietbrock, N. & Schneider, M. (1964) Der
Stoffwechsel des Formaldehyds. 1. Die Oxydation des Formaldehyds im
Blut (In preparation)
11. Malorny, G. & Reitbrock, N. (1964) Pharmakologisch-toxikologische
Wirkungen des Hexamethlentetramins und seines Spaltproduktes
Formaldehyd (In preparation)
12. Malorny, G., Netter, K. J. & Seidel, G. (1964) Untersuchungen
über die Hemmbarkeit enzymatischer Reaktionen durch Formaldehyd und
einige seiner Homologen (In Preparation)
13. Auerbach. C. (1951) Cold Spr. Harb. Symp. quant. Biol., 16, 199
14. Rapoport. I. A. (1946) C.R. Acad. Sci. USSR, 54, 65
15. Langecker. H. (1954) Arch. exp. Path. Pharmak., 221, 166
16. Brendel. R. (1964) Arzneimittel-Forsch., 14, 51
17. Kewitz, H. (1964) Work in progress, Institute for Veterinary
Pharmacology, Free University of Berlin
18. Malorny, G. (1964) Work in progresss, Pharmacological Institute,
University of Hamburg
19. Berglund, F. (1964) Work in progress, National Institute of
Public Health, Stockholm
20. Watangabe, G. & Sugimoto, S. (1955) Gann, 46, 365
MOLECULAR WEIGHT 140.2
DESCRIPTION White odourless crystals, which sublime at
about 263°C. 1 g yields 1.2 of formaldehyde on
hydrolysis. One part is soluble in 1.5 ml of
water or 12.5 ml of alcohol.
USE As a preservative for fish, meat and pickles.
Biological Data
Biochemical aspects
Formaldehyde and formic acid occur in fresh unpreserved cod-fish
muscle.1,2
Formaldehyde and formic acid are excreted in the urine of dogs and
cats fed a meal of fish not treated with preservatives, the source of
these substances being trimethylamineoxide occurring naturally in fish
muscle.3,4,5 The concentration of free formaldehyde in the urine of
these dogs and cats given unpreserved fish was 4 to 5 times higher
than that found in human subjects who had consumed fish preserved with
hexamethylenetetramine. Humans fed almost exclusively on unpreserved
fish did not show an increase in the excretion of formaldehyde or
formic acid.3
Under acid conditions, or in the presence of proteins,
hexamethylenetetramine decomposes gradually yielding ammonia and
formaldehyde.6
Hexamethylenetetramine liberates free formaldehyde in the stomach.7
Intravenous infusion in dog of formaldehyde, 35 mg/kg as a 0.6%
solution during 8 to 10 minutes, was carried out. Two minutes after
completion of the infusion them was no increase in formaldehyde but a
high increase in the formic acid level in the blood. The formic acid
level decreased by 50% in 1 hour, by 75% after 2 hours, and was back
to normal after 4 hours. In vitro human blood oxidizes 30%
formaldehyde (0.7 mg to 4 ml blood) to formic acid within 4 hours.
Addition of methylene blue (0.05% to 0.2%) results in a complete
oxidation of the formaldehyde.8
Experiments with 14C-labelled formaldehyde given by stomach tube to
rats and mice showed that 5 minutes after application the
radioactivity was distributed over the total body. After 12 hours,
approximately 40% had been expired as 14CO2, 10% had been excreted in
the urine, and 1% in the faeces. The homogenized whole animals
contained 20% of the radioactivity after 24 hours and 10% after 96
hours.9
Further work on the metabolism of hexamethylenetetramine and
formaldehyde is in progress.10,11,12
Special studies
Both hexamethylenetetramine13 and formaldehyde14 have been shown to
act as mutagens in Drosophila.
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat intravenous 9200 15
Short-term studies
Rat. Two groups of 5 male and 5 female rats received respectively
0.4 and 0.2 hexamethylenetetramine per day parenterally or by stomach
tube for 90 days. Groups of 15 male and 15 female rats were also
given 0.4 g hexamethylenetetramine by stomach tube for 333 days.
During the latter experiment 9 males and 3 females died compared with
1 male and 2 females in the control group. In both experiments no
macroscopic changes in organs were observed in the animals receiving
hexamethylenetetramine, nor did the body-weight of the test animals
vary from that of the controls. However, a citrus yellow colour of
the coat was noted.16
Cat. (Work In progress) Since October 1963, 0.124%
hexamethylenetetramine and 0.0375% formaldehyde have been fed in the
diet to groups of 5 and 4 cats respectively.17
Dog. (Work in progress) Since January 1963, 0.125%
hexamethylenetetramine and 0.375% formaldehyde have each been fed to
groups of 6 dogs. In November 1963, 5 litters born after the
commencement of the feeding trials were also included in the
experiment.17
Long-term studies
Mouse. (Work in progress) Since January 1963, 2 groups of 30 males
and 30 females of a special strain of mice with a high incidence of
spontaneous tumours (about 80% after 1 year) have been fed 1%
hexamethylenetetramine and 0.15% formaldehyde respectively.17
Rat. (work in progress) Since April 1963, rats have been fed 0.5%
and 1% hexamethylenetetramine and 0.05% and 0.15% formaldehyde.17
(Work in progress) A long-term, seven-generation feeding study on
rats was commenced in May 1963. 5 mg/kg and 50 mg/kg
hexamethylenetetramine are given in the drinking-water.18
(Work in progress) In March 1964, a three-generation chronic toxicity
test was begun, feeding rats 0.04%, 0.08% and 0.16%
hexamethylenetetramine in the diet.19
Repeated subcutaneous injections of 35-40% solutions of
hexamethylenetetramine produced local sarcomas in 8 out of 14 rats.20
(Work In progress) Two groups of rats (15 male and 15 female) are
being given twice weekly subcutaneous injections with 1 ml of a 40%
solution of hexamethylenetetramine and 0.1% formaldehyde respectively.
Two control groups, each having 7 male and 7 female rats, are being
given respectively NaCl and saccharose solution of equal osmotic
pressure to that of the 40% hexamethylenetetramine solution.
Evaluation
The toxicological data are considered to be insufficient for
evaluation of an acceptable level for use as a food additive in man.
In addition, hexamethylenetetramine produces sarcomas in rats after
subcutaneous injection and has a mutagenic action in insects.
It is concluded that, pending the evaluation of the results of work
now in progress, hexamethylenetetramine should not be used as a food
additive in human foodstuffs.
References
1. Malorny, G. (1964) FAO Symposium on the Significance of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/11
2. Amano, K. & Yamada, K. (1964) FAO Symposium on the Significanae of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/9
3. Malorny, G. & Rietbrock. N. (1962) Naturwissenschaften 49, 520
4. Malorny, G., Rietbrock, N. & Schassan, H. H. (1963) Arch. exp.
Path. Pharmak., 246, 62
5. Schassan, H. H. (1963) Inauguraldissertation, Hamburg
6. Hutschenreuter, H. (1956) Z. Lebensmitt.-Untersuch., 104, 161
7. Malorny. G. & Rietbrock. N. (1963) Vortrag auf der X. Tagung des
Ernährungswissenschaftlichen Beirats der deutschen Fischwirtschaft,
Bremen.
8. Malorny. G., Rietbrock. N. & Schneider, M. (1964) Arch. exp. Path.
Pharmak., 247, 381
9. Buss, J., Kuschinsky, K., Kewitz, H. & Koransky, W. (1964) Arch.
exp. Path. Pharmak., 247, 380
10. Malorny, G., Rietbrock, N. & Schneider, M. (1964) Der
Stoffwechsel des Formaldehyds. 1. Die Oxydation des Formaldehyds im
Blut (In preparation)
11. Malorny, G. & Reitbrock, N. (1964) Pharmakologisch-toxikologische
Wirkungen des Hexamethlentetramins und seines Spaltproduktes
Formaldehyd (In preparation)
12. Malorny, G., Netter, K. J. & Seidel, G. (1964) Untersuchungen
über die Hemmbarkeit enzymatischer Reaktionen durch Formaldehyd und
einige seiner Homologen (In Preparation)
13. Auerbach. C. (1951) Cold Spr. Harb. Symp. quant. Biol., 16, 199
14. Rapoport. I. A. (1946) C.R. Acad. Sci. USSR, 54, 65
15. Langecker. H. (1954) Arch. exp. Path. Pharmak., 221, 166
16. Brendel. R. (1964) Arzneimittel-Forsch., 14, 51
17. Kewitz, H. (1964) Work in progress, Institute for Veterinary
Pharmacology, Free University of Berlin
18. Malorny, G. (1964) Work in progresss, Pharmacological Institute,
University of Hamburg
19. Berglund, F. (1964) Work in progress, National Institute of
Public Health, Stockholm
20. Watangabe, G. & Sugimoto, S. (1955) Gann, 46, 365
 MOLECULAR WEIGHT 140.2
DESCRIPTION White odourless crystals, which sublime at
about 263°C. 1 g yields 1.2 of formaldehyde on
hydrolysis. One part is soluble in 1.5 ml of
water or 12.5 ml of alcohol.
USE As a preservative for fish, meat and pickles.
Biological Data
Biochemical aspects
Formaldehyde and formic acid occur in fresh unpreserved cod-fish
muscle.1,2
Formaldehyde and formic acid are excreted in the urine of dogs and
cats fed a meal of fish not treated with preservatives, the source of
these substances being trimethylamineoxide occurring naturally in fish
muscle.3,4,5 The concentration of free formaldehyde in the urine of
these dogs and cats given unpreserved fish was 4 to 5 times higher
than that found in human subjects who had consumed fish preserved with
hexamethylenetetramine. Humans fed almost exclusively on unpreserved
fish did not show an increase in the excretion of formaldehyde or
formic acid.3
Under acid conditions, or in the presence of proteins,
hexamethylenetetramine decomposes gradually yielding ammonia and
formaldehyde.6
Hexamethylenetetramine liberates free formaldehyde in the stomach.7
Intravenous infusion in dog of formaldehyde, 35 mg/kg as a 0.6%
solution during 8 to 10 minutes, was carried out. Two minutes after
completion of the infusion them was no increase in formaldehyde but a
high increase in the formic acid level in the blood. The formic acid
level decreased by 50% in 1 hour, by 75% after 2 hours, and was back
to normal after 4 hours. In vitro human blood oxidizes 30%
formaldehyde (0.7 mg to 4 ml blood) to formic acid within 4 hours.
Addition of methylene blue (0.05% to 0.2%) results in a complete
oxidation of the formaldehyde.8
Experiments with 14C-labelled formaldehyde given by stomach tube to
rats and mice showed that 5 minutes after application the
radioactivity was distributed over the total body. After 12 hours,
approximately 40% had been expired as 14CO2, 10% had been excreted in
the urine, and 1% in the faeces. The homogenized whole animals
contained 20% of the radioactivity after 24 hours and 10% after 96
hours.9
Further work on the metabolism of hexamethylenetetramine and
formaldehyde is in progress.10,11,12
Special studies
Both hexamethylenetetramine13 and formaldehyde14 have been shown to
act as mutagens in Drosophila.
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat intravenous 9200 15
Short-term studies
Rat. Two groups of 5 male and 5 female rats received respectively
0.4 and 0.2 hexamethylenetetramine per day parenterally or by stomach
tube for 90 days. Groups of 15 male and 15 female rats were also
given 0.4 g hexamethylenetetramine by stomach tube for 333 days.
During the latter experiment 9 males and 3 females died compared with
1 male and 2 females in the control group. In both experiments no
macroscopic changes in organs were observed in the animals receiving
hexamethylenetetramine, nor did the body-weight of the test animals
vary from that of the controls. However, a citrus yellow colour of
the coat was noted.16
Cat. (Work In progress) Since October 1963, 0.124%
hexamethylenetetramine and 0.0375% formaldehyde have been fed in the
diet to groups of 5 and 4 cats respectively.17
Dog. (Work in progress) Since January 1963, 0.125%
hexamethylenetetramine and 0.375% formaldehyde have each been fed to
groups of 6 dogs. In November 1963, 5 litters born after the
commencement of the feeding trials were also included in the
experiment.17
Long-term studies
Mouse. (Work in progress) Since January 1963, 2 groups of 30 males
and 30 females of a special strain of mice with a high incidence of
spontaneous tumours (about 80% after 1 year) have been fed 1%
hexamethylenetetramine and 0.15% formaldehyde respectively.17
Rat. (work in progress) Since April 1963, rats have been fed 0.5%
and 1% hexamethylenetetramine and 0.05% and 0.15% formaldehyde.17
(Work in progress) A long-term, seven-generation feeding study on
rats was commenced in May 1963. 5 mg/kg and 50 mg/kg
hexamethylenetetramine are given in the drinking-water.18
(Work in progress) In March 1964, a three-generation chronic toxicity
test was begun, feeding rats 0.04%, 0.08% and 0.16%
hexamethylenetetramine in the diet.19
Repeated subcutaneous injections of 35-40% solutions of
hexamethylenetetramine produced local sarcomas in 8 out of 14 rats.20
(Work In progress) Two groups of rats (15 male and 15 female) are
being given twice weekly subcutaneous injections with 1 ml of a 40%
solution of hexamethylenetetramine and 0.1% formaldehyde respectively.
Two control groups, each having 7 male and 7 female rats, are being
given respectively NaCl and saccharose solution of equal osmotic
pressure to that of the 40% hexamethylenetetramine solution.
Evaluation
The toxicological data are considered to be insufficient for
evaluation of an acceptable level for use as a food additive in man.
In addition, hexamethylenetetramine produces sarcomas in rats after
subcutaneous injection and has a mutagenic action in insects.
It is concluded that, pending the evaluation of the results of work
now in progress, hexamethylenetetramine should not be used as a food
additive in human foodstuffs.
References
1. Malorny, G. (1964) FAO Symposium on the Significance of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/11
2. Amano, K. & Yamada, K. (1964) FAO Symposium on the Significanae of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/9
3. Malorny, G. & Rietbrock. N. (1962) Naturwissenschaften 49, 520
4. Malorny, G., Rietbrock, N. & Schassan, H. H. (1963) Arch. exp.
Path. Pharmak., 246, 62
5. Schassan, H. H. (1963) Inauguraldissertation, Hamburg
6. Hutschenreuter, H. (1956) Z. Lebensmitt.-Untersuch., 104, 161
7. Malorny. G. & Rietbrock. N. (1963) Vortrag auf der X. Tagung des
Ernährungswissenschaftlichen Beirats der deutschen Fischwirtschaft,
Bremen.
8. Malorny. G., Rietbrock. N. & Schneider, M. (1964) Arch. exp. Path.
Pharmak., 247, 381
9. Buss, J., Kuschinsky, K., Kewitz, H. & Koransky, W. (1964) Arch.
exp. Path. Pharmak., 247, 380
10. Malorny, G., Rietbrock, N. & Schneider, M. (1964) Der
Stoffwechsel des Formaldehyds. 1. Die Oxydation des Formaldehyds im
Blut (In preparation)
11. Malorny, G. & Reitbrock, N. (1964) Pharmakologisch-toxikologische
Wirkungen des Hexamethlentetramins und seines Spaltproduktes
Formaldehyd (In preparation)
12. Malorny, G., Netter, K. J. & Seidel, G. (1964) Untersuchungen
über die Hemmbarkeit enzymatischer Reaktionen durch Formaldehyd und
einige seiner Homologen (In Preparation)
13. Auerbach. C. (1951) Cold Spr. Harb. Symp. quant. Biol., 16, 199
14. Rapoport. I. A. (1946) C.R. Acad. Sci. USSR, 54, 65
15. Langecker. H. (1954) Arch. exp. Path. Pharmak., 221, 166
16. Brendel. R. (1964) Arzneimittel-Forsch., 14, 51
17. Kewitz, H. (1964) Work in progress, Institute for Veterinary
Pharmacology, Free University of Berlin
18. Malorny, G. (1964) Work in progresss, Pharmacological Institute,
University of Hamburg
19. Berglund, F. (1964) Work in progress, National Institute of
Public Health, Stockholm
20. Watangabe, G. & Sugimoto, S. (1955) Gann, 46, 365
MOLECULAR WEIGHT 140.2
DESCRIPTION White odourless crystals, which sublime at
about 263°C. 1 g yields 1.2 of formaldehyde on
hydrolysis. One part is soluble in 1.5 ml of
water or 12.5 ml of alcohol.
USE As a preservative for fish, meat and pickles.
Biological Data
Biochemical aspects
Formaldehyde and formic acid occur in fresh unpreserved cod-fish
muscle.1,2
Formaldehyde and formic acid are excreted in the urine of dogs and
cats fed a meal of fish not treated with preservatives, the source of
these substances being trimethylamineoxide occurring naturally in fish
muscle.3,4,5 The concentration of free formaldehyde in the urine of
these dogs and cats given unpreserved fish was 4 to 5 times higher
than that found in human subjects who had consumed fish preserved with
hexamethylenetetramine. Humans fed almost exclusively on unpreserved
fish did not show an increase in the excretion of formaldehyde or
formic acid.3
Under acid conditions, or in the presence of proteins,
hexamethylenetetramine decomposes gradually yielding ammonia and
formaldehyde.6
Hexamethylenetetramine liberates free formaldehyde in the stomach.7
Intravenous infusion in dog of formaldehyde, 35 mg/kg as a 0.6%
solution during 8 to 10 minutes, was carried out. Two minutes after
completion of the infusion them was no increase in formaldehyde but a
high increase in the formic acid level in the blood. The formic acid
level decreased by 50% in 1 hour, by 75% after 2 hours, and was back
to normal after 4 hours. In vitro human blood oxidizes 30%
formaldehyde (0.7 mg to 4 ml blood) to formic acid within 4 hours.
Addition of methylene blue (0.05% to 0.2%) results in a complete
oxidation of the formaldehyde.8
Experiments with 14C-labelled formaldehyde given by stomach tube to
rats and mice showed that 5 minutes after application the
radioactivity was distributed over the total body. After 12 hours,
approximately 40% had been expired as 14CO2, 10% had been excreted in
the urine, and 1% in the faeces. The homogenized whole animals
contained 20% of the radioactivity after 24 hours and 10% after 96
hours.9
Further work on the metabolism of hexamethylenetetramine and
formaldehyde is in progress.10,11,12
Special studies
Both hexamethylenetetramine13 and formaldehyde14 have been shown to
act as mutagens in Drosophila.
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat intravenous 9200 15
Short-term studies
Rat. Two groups of 5 male and 5 female rats received respectively
0.4 and 0.2 hexamethylenetetramine per day parenterally or by stomach
tube for 90 days. Groups of 15 male and 15 female rats were also
given 0.4 g hexamethylenetetramine by stomach tube for 333 days.
During the latter experiment 9 males and 3 females died compared with
1 male and 2 females in the control group. In both experiments no
macroscopic changes in organs were observed in the animals receiving
hexamethylenetetramine, nor did the body-weight of the test animals
vary from that of the controls. However, a citrus yellow colour of
the coat was noted.16
Cat. (Work In progress) Since October 1963, 0.124%
hexamethylenetetramine and 0.0375% formaldehyde have been fed in the
diet to groups of 5 and 4 cats respectively.17
Dog. (Work in progress) Since January 1963, 0.125%
hexamethylenetetramine and 0.375% formaldehyde have each been fed to
groups of 6 dogs. In November 1963, 5 litters born after the
commencement of the feeding trials were also included in the
experiment.17
Long-term studies
Mouse. (Work in progress) Since January 1963, 2 groups of 30 males
and 30 females of a special strain of mice with a high incidence of
spontaneous tumours (about 80% after 1 year) have been fed 1%
hexamethylenetetramine and 0.15% formaldehyde respectively.17
Rat. (work in progress) Since April 1963, rats have been fed 0.5%
and 1% hexamethylenetetramine and 0.05% and 0.15% formaldehyde.17
(Work in progress) A long-term, seven-generation feeding study on
rats was commenced in May 1963. 5 mg/kg and 50 mg/kg
hexamethylenetetramine are given in the drinking-water.18
(Work in progress) In March 1964, a three-generation chronic toxicity
test was begun, feeding rats 0.04%, 0.08% and 0.16%
hexamethylenetetramine in the diet.19
Repeated subcutaneous injections of 35-40% solutions of
hexamethylenetetramine produced local sarcomas in 8 out of 14 rats.20
(Work In progress) Two groups of rats (15 male and 15 female) are
being given twice weekly subcutaneous injections with 1 ml of a 40%
solution of hexamethylenetetramine and 0.1% formaldehyde respectively.
Two control groups, each having 7 male and 7 female rats, are being
given respectively NaCl and saccharose solution of equal osmotic
pressure to that of the 40% hexamethylenetetramine solution.
Evaluation
The toxicological data are considered to be insufficient for
evaluation of an acceptable level for use as a food additive in man.
In addition, hexamethylenetetramine produces sarcomas in rats after
subcutaneous injection and has a mutagenic action in insects.
It is concluded that, pending the evaluation of the results of work
now in progress, hexamethylenetetramine should not be used as a food
additive in human foodstuffs.
References
1. Malorny, G. (1964) FAO Symposium on the Significance of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/11
2. Amano, K. & Yamada, K. (1964) FAO Symposium on the Significanae of
Fundamental Research in the Utilisation of Fish, Paper No. WP/II/9
3. Malorny, G. & Rietbrock. N. (1962) Naturwissenschaften 49, 520
4. Malorny, G., Rietbrock, N. & Schassan, H. H. (1963) Arch. exp.
Path. Pharmak., 246, 62
5. Schassan, H. H. (1963) Inauguraldissertation, Hamburg
6. Hutschenreuter, H. (1956) Z. Lebensmitt.-Untersuch., 104, 161
7. Malorny. G. & Rietbrock. N. (1963) Vortrag auf der X. Tagung des
Ernährungswissenschaftlichen Beirats der deutschen Fischwirtschaft,
Bremen.
8. Malorny. G., Rietbrock. N. & Schneider, M. (1964) Arch. exp. Path.
Pharmak., 247, 381
9. Buss, J., Kuschinsky, K., Kewitz, H. & Koransky, W. (1964) Arch.
exp. Path. Pharmak., 247, 380
10. Malorny, G., Rietbrock, N. & Schneider, M. (1964) Der
Stoffwechsel des Formaldehyds. 1. Die Oxydation des Formaldehyds im
Blut (In preparation)
11. Malorny, G. & Reitbrock, N. (1964) Pharmakologisch-toxikologische
Wirkungen des Hexamethlentetramins und seines Spaltproduktes
Formaldehyd (In preparation)
12. Malorny, G., Netter, K. J. & Seidel, G. (1964) Untersuchungen
über die Hemmbarkeit enzymatischer Reaktionen durch Formaldehyd und
einige seiner Homologen (In Preparation)
13. Auerbach. C. (1951) Cold Spr. Harb. Symp. quant. Biol., 16, 199
14. Rapoport. I. A. (1946) C.R. Acad. Sci. USSR, 54, 65
15. Langecker. H. (1954) Arch. exp. Path. Pharmak., 221, 166
16. Brendel. R. (1964) Arzneimittel-Forsch., 14, 51
17. Kewitz, H. (1964) Work in progress, Institute for Veterinary
Pharmacology, Free University of Berlin
18. Malorny, G. (1964) Work in progresss, Pharmacological Institute,
University of Hamburg
19. Berglund, F. (1964) Work in progress, National Institute of
Public Health, Stockholm
20. Watangabe, G. & Sugimoto, S. (1955) Gann, 46, 365
