
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
NORDIHYDROGUAIARETIC ACID
CHEMICAL NAMES Nordihydroguaiaretic acid;
ß,gamma-dimethyl-alpha,delta-bis-(3,4-
dihydroxyphenyl) butane; 4,4'-(2,3-
dimethyltetramethylene) dipyrocatechol
SYNONYM NDGA
EMPIRICAL FORMULA C18H22O4
STRUCTURAL FORMULA
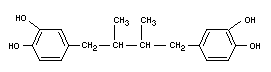 MOLECULAR WEIGHT 302.37
DESCRIPTION Nordihydroguaiaretic acid is a white to
greyish-white crystalline solid, which may be
prepared from an evergreen desert shrub,
Larrea divaricata. 1 g is soluble in 4 ml of
ethanol.
USE As an antioxidant in oils and foods.
Biological Data
Biochemical aspects
There does not appear to be any information on the metabolism of
NDGA.1 The effect of NDGA on enzyme systems has been studied.
Specific inhibition of peroxidase, catalase, and ethyl alcohol
dehydrogenase occurs with a concentration of 2x10-4 M of the
antioxidant. Non-specific inhibition of ascorbic acid oxidase,
D-amino-acid oxidase, the cyclophorase system and urease at a
concentration of 2x10-3 M has been described.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2000-5000 3
Mouse oral 2000-4000 3
Mouse intraperitoneal 550 3
Guinea-pig oral 830 3
Short-term studies
Guinea-pig. NDGA was found to be among the more strongly reacting
compounds among several antioxidants tested for their capacity to
induce skin sensitivity in the guinea-pig.4
Dog. NDGA was fed to young mongrel dogs at dietary levels of 0.1%
(3 dogs), 0.5% (4 dogs) and 1.0% (5 dogs) for 1 year. Adult dogs were
fed the same range of dietary concentrations, the numbers in the
groups being 2, 3 and 2 respectively, with 2 controls. The growth
curves of the young dogs showed some impairment in weight gain at the
0.5% levels but not at the 0.1% and 1.0% levels. No significant
pathological changes were found which were attributable to the
treatment and all the dogs were in good physical condition at the time
of sacrifice.5
Long-term studies
Mouse. Long-term studies were carried out using 0.25% and 0.5%
levels in mice. No deleterious effects on weight gain were observed,
nor any important histological changes.6
Rat. Chronic toxicity experiments were conducted over a period of 2
years, in which NDGA was compared with phenol, catechol and gum guaiac
in concentrations of 0.5% in rats. NDGA had little or no effect on
growth or food intake, except in the highest concentration, where
there was a temporary decrease in growth associated with a decreased
food intake. Histological study of the liver, spleen and kidneys
showed no significant effect. Necrosis of the liver was noted
occasionally in all groups, including controls.6
Concentrations of 0.1%, 0.5% and 1.0% of NDGA were used in another
series of rats. Haemorrhage into the caecum was observed in 50% of
the animals. In a larger series of tests on rats at the same
concentrations of NDGA, this haemorrhage did not occur. Cysts in the
mesentery were found in several rats on the higher concentrations of
NDGA.
Two-year toxicity tests on groups of 10 male rats at 0%, 0.1%. 0.25%,
0.5% and 1% NDGA in the diet showed that 0.5% was the lowest level
causing inflammatory caecal lesions and slight cystic enlargement of
lymph nodes near the caecum. Growth inhibition occurred after the
first 6 months at levels of 0.5% and 1%.3
In another experiment lasting 2 years, 0.5% of NDGA in the diet caused
massive caecal haemorrhages, with single and multiple cysts in the
mesentery in the angle of the junction between the small and large
intestines. During the first 6 months, inhibition of the growth rate
occurred only at the 1% level.3
(Work in progress) Groups of 20 rats (10 male and 10 female) were fed
the following diets: 0.5% NDGA, 0.5% NDGA plus aspirin, 1% NDGA, 1%
NDGA plus aspirin, and aspirin for 65 weeks. Growth has been retarded
in all test groups compared with the controls.a Haemoglobin and
haematocrit determinations were done at 16 and 23 weeks; all groups
where NDGA was given had lower readings than those of the controls.a
Two rats, one fed 0.5% NDGA plus aspirin, the other aspirin alone,
showed some ulceration of the stomach after 42 and 50 weeks on test.
Examination of faeces for occult blood was done after 49 weeks; the
rats fed NDGA with no aspirin were all negative, but 5 rats fed
aspirin alone or aspirin plus NDGA showed traces of occult blood.7
Hamster. (Work in progress) Hamsters (15 male and 15 female) were
fed diets containing 0.1%, 0.5% and 1% NDGA for 65 weeks. Some deaths
which occurred at the 1% level may be attributed to the effect of NDGA
in the diet. Ulceration of the stomach was found in 3 hamsters at the
1% level after 48 weeks. At 22 weeks a marked decrease in haematocrit
values was found for females, but not in males, on 1% NDGA.
Examination of faeces for occult blood was negative in all groups.
The investigator points out that 1% NDGA in the diet of hamsters
produced a definite toxic effect, particularly in the female. Whether
the lower level will prove to be the no-effect level will depend on
the findings upon completion of the test, which is to he carried out
over the lifetime of the animals.7
Comment on experimental studies reported
The acute toxicity studies indicate that the guinea-pig is more
sensitive than the rat but no long-term studies with the former
species have been reported. The short-term studies in the dog suggest
that a 1% dietary level is well tolerated in this species although
there was some impairment of growth at the 0.5% level. The published
long-term studies in the rat and mouse provide little detailed
information and seem to have left some of the investigators in doubt
a No statistical evaluation has yet been done as these experiments
are not terminated; they are being carried out over the life span of
the animals.
about the acceptability of NDGA. The more recent long-term studies in
rat and hamster are still in progress and cannot be evaluated until
their completion.
Evaluation
Level causing no significant toxicological effect in the rat
From the limited information available, it is not possible to evaluate
the toxicological status of NDGA. No guidance can therefore be given
at present on the use of NDGA as an antioxidant in food.
Further Work Required
1. Further long-term studies in the rat and other species, including
dog.
2. Metabolic studies in animals and man.
References
1. Dacre. J. C. (1960) J. N.Z. Inst. Chem., 24, 161
2. Tappel, A. L. & Marr. A. G. (1954) J. Agr. Food Chem., 2, 554
3. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodard, G. (1951)
Advanc. Food Res., 3, 197
4. Griepentrog, F. (1961) Arzneimittel-Forsch., 11, 920
5. Anderson, W. D., Bell, B. J. & Bieter, R. N. (1955) Unpublished
report
6. Cranston, E. M., Jensen, M. J., Moren, A., Brey, T., Bell, E. T. &
Bieter, R. N. (1947) Fed. Proc., 6, 318
7. Mannell, W. A., Canadian Food and Drug Directorate, Ottawa (Letter
dated 23 September 1964)
MOLECULAR WEIGHT 302.37
DESCRIPTION Nordihydroguaiaretic acid is a white to
greyish-white crystalline solid, which may be
prepared from an evergreen desert shrub,
Larrea divaricata. 1 g is soluble in 4 ml of
ethanol.
USE As an antioxidant in oils and foods.
Biological Data
Biochemical aspects
There does not appear to be any information on the metabolism of
NDGA.1 The effect of NDGA on enzyme systems has been studied.
Specific inhibition of peroxidase, catalase, and ethyl alcohol
dehydrogenase occurs with a concentration of 2x10-4 M of the
antioxidant. Non-specific inhibition of ascorbic acid oxidase,
D-amino-acid oxidase, the cyclophorase system and urease at a
concentration of 2x10-3 M has been described.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2000-5000 3
Mouse oral 2000-4000 3
Mouse intraperitoneal 550 3
Guinea-pig oral 830 3
Short-term studies
Guinea-pig. NDGA was found to be among the more strongly reacting
compounds among several antioxidants tested for their capacity to
induce skin sensitivity in the guinea-pig.4
Dog. NDGA was fed to young mongrel dogs at dietary levels of 0.1%
(3 dogs), 0.5% (4 dogs) and 1.0% (5 dogs) for 1 year. Adult dogs were
fed the same range of dietary concentrations, the numbers in the
groups being 2, 3 and 2 respectively, with 2 controls. The growth
curves of the young dogs showed some impairment in weight gain at the
0.5% levels but not at the 0.1% and 1.0% levels. No significant
pathological changes were found which were attributable to the
treatment and all the dogs were in good physical condition at the time
of sacrifice.5
Long-term studies
Mouse. Long-term studies were carried out using 0.25% and 0.5%
levels in mice. No deleterious effects on weight gain were observed,
nor any important histological changes.6
Rat. Chronic toxicity experiments were conducted over a period of 2
years, in which NDGA was compared with phenol, catechol and gum guaiac
in concentrations of 0.5% in rats. NDGA had little or no effect on
growth or food intake, except in the highest concentration, where
there was a temporary decrease in growth associated with a decreased
food intake. Histological study of the liver, spleen and kidneys
showed no significant effect. Necrosis of the liver was noted
occasionally in all groups, including controls.6
Concentrations of 0.1%, 0.5% and 1.0% of NDGA were used in another
series of rats. Haemorrhage into the caecum was observed in 50% of
the animals. In a larger series of tests on rats at the same
concentrations of NDGA, this haemorrhage did not occur. Cysts in the
mesentery were found in several rats on the higher concentrations of
NDGA.
Two-year toxicity tests on groups of 10 male rats at 0%, 0.1%. 0.25%,
0.5% and 1% NDGA in the diet showed that 0.5% was the lowest level
causing inflammatory caecal lesions and slight cystic enlargement of
lymph nodes near the caecum. Growth inhibition occurred after the
first 6 months at levels of 0.5% and 1%.3
In another experiment lasting 2 years, 0.5% of NDGA in the diet caused
massive caecal haemorrhages, with single and multiple cysts in the
mesentery in the angle of the junction between the small and large
intestines. During the first 6 months, inhibition of the growth rate
occurred only at the 1% level.3
(Work in progress) Groups of 20 rats (10 male and 10 female) were fed
the following diets: 0.5% NDGA, 0.5% NDGA plus aspirin, 1% NDGA, 1%
NDGA plus aspirin, and aspirin for 65 weeks. Growth has been retarded
in all test groups compared with the controls.a Haemoglobin and
haematocrit determinations were done at 16 and 23 weeks; all groups
where NDGA was given had lower readings than those of the controls.a
Two rats, one fed 0.5% NDGA plus aspirin, the other aspirin alone,
showed some ulceration of the stomach after 42 and 50 weeks on test.
Examination of faeces for occult blood was done after 49 weeks; the
rats fed NDGA with no aspirin were all negative, but 5 rats fed
aspirin alone or aspirin plus NDGA showed traces of occult blood.7
Hamster. (Work in progress) Hamsters (15 male and 15 female) were
fed diets containing 0.1%, 0.5% and 1% NDGA for 65 weeks. Some deaths
which occurred at the 1% level may be attributed to the effect of NDGA
in the diet. Ulceration of the stomach was found in 3 hamsters at the
1% level after 48 weeks. At 22 weeks a marked decrease in haematocrit
values was found for females, but not in males, on 1% NDGA.
Examination of faeces for occult blood was negative in all groups.
The investigator points out that 1% NDGA in the diet of hamsters
produced a definite toxic effect, particularly in the female. Whether
the lower level will prove to be the no-effect level will depend on
the findings upon completion of the test, which is to he carried out
over the lifetime of the animals.7
Comment on experimental studies reported
The acute toxicity studies indicate that the guinea-pig is more
sensitive than the rat but no long-term studies with the former
species have been reported. The short-term studies in the dog suggest
that a 1% dietary level is well tolerated in this species although
there was some impairment of growth at the 0.5% level. The published
long-term studies in the rat and mouse provide little detailed
information and seem to have left some of the investigators in doubt
a No statistical evaluation has yet been done as these experiments
are not terminated; they are being carried out over the life span of
the animals.
about the acceptability of NDGA. The more recent long-term studies in
rat and hamster are still in progress and cannot be evaluated until
their completion.
Evaluation
Level causing no significant toxicological effect in the rat
From the limited information available, it is not possible to evaluate
the toxicological status of NDGA. No guidance can therefore be given
at present on the use of NDGA as an antioxidant in food.
Further Work Required
1. Further long-term studies in the rat and other species, including
dog.
2. Metabolic studies in animals and man.
References
1. Dacre. J. C. (1960) J. N.Z. Inst. Chem., 24, 161
2. Tappel, A. L. & Marr. A. G. (1954) J. Agr. Food Chem., 2, 554
3. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodard, G. (1951)
Advanc. Food Res., 3, 197
4. Griepentrog, F. (1961) Arzneimittel-Forsch., 11, 920
5. Anderson, W. D., Bell, B. J. & Bieter, R. N. (1955) Unpublished
report
6. Cranston, E. M., Jensen, M. J., Moren, A., Brey, T., Bell, E. T. &
Bieter, R. N. (1947) Fed. Proc., 6, 318
7. Mannell, W. A., Canadian Food and Drug Directorate, Ottawa (Letter
dated 23 September 1964)
 MOLECULAR WEIGHT 302.37
DESCRIPTION Nordihydroguaiaretic acid is a white to
greyish-white crystalline solid, which may be
prepared from an evergreen desert shrub,
Larrea divaricata. 1 g is soluble in 4 ml of
ethanol.
USE As an antioxidant in oils and foods.
Biological Data
Biochemical aspects
There does not appear to be any information on the metabolism of
NDGA.1 The effect of NDGA on enzyme systems has been studied.
Specific inhibition of peroxidase, catalase, and ethyl alcohol
dehydrogenase occurs with a concentration of 2x10-4 M of the
antioxidant. Non-specific inhibition of ascorbic acid oxidase,
D-amino-acid oxidase, the cyclophorase system and urease at a
concentration of 2x10-3 M has been described.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2000-5000 3
Mouse oral 2000-4000 3
Mouse intraperitoneal 550 3
Guinea-pig oral 830 3
Short-term studies
Guinea-pig. NDGA was found to be among the more strongly reacting
compounds among several antioxidants tested for their capacity to
induce skin sensitivity in the guinea-pig.4
Dog. NDGA was fed to young mongrel dogs at dietary levels of 0.1%
(3 dogs), 0.5% (4 dogs) and 1.0% (5 dogs) for 1 year. Adult dogs were
fed the same range of dietary concentrations, the numbers in the
groups being 2, 3 and 2 respectively, with 2 controls. The growth
curves of the young dogs showed some impairment in weight gain at the
0.5% levels but not at the 0.1% and 1.0% levels. No significant
pathological changes were found which were attributable to the
treatment and all the dogs were in good physical condition at the time
of sacrifice.5
Long-term studies
Mouse. Long-term studies were carried out using 0.25% and 0.5%
levels in mice. No deleterious effects on weight gain were observed,
nor any important histological changes.6
Rat. Chronic toxicity experiments were conducted over a period of 2
years, in which NDGA was compared with phenol, catechol and gum guaiac
in concentrations of 0.5% in rats. NDGA had little or no effect on
growth or food intake, except in the highest concentration, where
there was a temporary decrease in growth associated with a decreased
food intake. Histological study of the liver, spleen and kidneys
showed no significant effect. Necrosis of the liver was noted
occasionally in all groups, including controls.6
Concentrations of 0.1%, 0.5% and 1.0% of NDGA were used in another
series of rats. Haemorrhage into the caecum was observed in 50% of
the animals. In a larger series of tests on rats at the same
concentrations of NDGA, this haemorrhage did not occur. Cysts in the
mesentery were found in several rats on the higher concentrations of
NDGA.
Two-year toxicity tests on groups of 10 male rats at 0%, 0.1%. 0.25%,
0.5% and 1% NDGA in the diet showed that 0.5% was the lowest level
causing inflammatory caecal lesions and slight cystic enlargement of
lymph nodes near the caecum. Growth inhibition occurred after the
first 6 months at levels of 0.5% and 1%.3
In another experiment lasting 2 years, 0.5% of NDGA in the diet caused
massive caecal haemorrhages, with single and multiple cysts in the
mesentery in the angle of the junction between the small and large
intestines. During the first 6 months, inhibition of the growth rate
occurred only at the 1% level.3
(Work in progress) Groups of 20 rats (10 male and 10 female) were fed
the following diets: 0.5% NDGA, 0.5% NDGA plus aspirin, 1% NDGA, 1%
NDGA plus aspirin, and aspirin for 65 weeks. Growth has been retarded
in all test groups compared with the controls.a Haemoglobin and
haematocrit determinations were done at 16 and 23 weeks; all groups
where NDGA was given had lower readings than those of the controls.a
Two rats, one fed 0.5% NDGA plus aspirin, the other aspirin alone,
showed some ulceration of the stomach after 42 and 50 weeks on test.
Examination of faeces for occult blood was done after 49 weeks; the
rats fed NDGA with no aspirin were all negative, but 5 rats fed
aspirin alone or aspirin plus NDGA showed traces of occult blood.7
Hamster. (Work in progress) Hamsters (15 male and 15 female) were
fed diets containing 0.1%, 0.5% and 1% NDGA for 65 weeks. Some deaths
which occurred at the 1% level may be attributed to the effect of NDGA
in the diet. Ulceration of the stomach was found in 3 hamsters at the
1% level after 48 weeks. At 22 weeks a marked decrease in haematocrit
values was found for females, but not in males, on 1% NDGA.
Examination of faeces for occult blood was negative in all groups.
The investigator points out that 1% NDGA in the diet of hamsters
produced a definite toxic effect, particularly in the female. Whether
the lower level will prove to be the no-effect level will depend on
the findings upon completion of the test, which is to he carried out
over the lifetime of the animals.7
Comment on experimental studies reported
The acute toxicity studies indicate that the guinea-pig is more
sensitive than the rat but no long-term studies with the former
species have been reported. The short-term studies in the dog suggest
that a 1% dietary level is well tolerated in this species although
there was some impairment of growth at the 0.5% level. The published
long-term studies in the rat and mouse provide little detailed
information and seem to have left some of the investigators in doubt
a No statistical evaluation has yet been done as these experiments
are not terminated; they are being carried out over the life span of
the animals.
about the acceptability of NDGA. The more recent long-term studies in
rat and hamster are still in progress and cannot be evaluated until
their completion.
Evaluation
Level causing no significant toxicological effect in the rat
From the limited information available, it is not possible to evaluate
the toxicological status of NDGA. No guidance can therefore be given
at present on the use of NDGA as an antioxidant in food.
Further Work Required
1. Further long-term studies in the rat and other species, including
dog.
2. Metabolic studies in animals and man.
References
1. Dacre. J. C. (1960) J. N.Z. Inst. Chem., 24, 161
2. Tappel, A. L. & Marr. A. G. (1954) J. Agr. Food Chem., 2, 554
3. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodard, G. (1951)
Advanc. Food Res., 3, 197
4. Griepentrog, F. (1961) Arzneimittel-Forsch., 11, 920
5. Anderson, W. D., Bell, B. J. & Bieter, R. N. (1955) Unpublished
report
6. Cranston, E. M., Jensen, M. J., Moren, A., Brey, T., Bell, E. T. &
Bieter, R. N. (1947) Fed. Proc., 6, 318
7. Mannell, W. A., Canadian Food and Drug Directorate, Ottawa (Letter
dated 23 September 1964)
MOLECULAR WEIGHT 302.37
DESCRIPTION Nordihydroguaiaretic acid is a white to
greyish-white crystalline solid, which may be
prepared from an evergreen desert shrub,
Larrea divaricata. 1 g is soluble in 4 ml of
ethanol.
USE As an antioxidant in oils and foods.
Biological Data
Biochemical aspects
There does not appear to be any information on the metabolism of
NDGA.1 The effect of NDGA on enzyme systems has been studied.
Specific inhibition of peroxidase, catalase, and ethyl alcohol
dehydrogenase occurs with a concentration of 2x10-4 M of the
antioxidant. Non-specific inhibition of ascorbic acid oxidase,
D-amino-acid oxidase, the cyclophorase system and urease at a
concentration of 2x10-3 M has been described.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2000-5000 3
Mouse oral 2000-4000 3
Mouse intraperitoneal 550 3
Guinea-pig oral 830 3
Short-term studies
Guinea-pig. NDGA was found to be among the more strongly reacting
compounds among several antioxidants tested for their capacity to
induce skin sensitivity in the guinea-pig.4
Dog. NDGA was fed to young mongrel dogs at dietary levels of 0.1%
(3 dogs), 0.5% (4 dogs) and 1.0% (5 dogs) for 1 year. Adult dogs were
fed the same range of dietary concentrations, the numbers in the
groups being 2, 3 and 2 respectively, with 2 controls. The growth
curves of the young dogs showed some impairment in weight gain at the
0.5% levels but not at the 0.1% and 1.0% levels. No significant
pathological changes were found which were attributable to the
treatment and all the dogs were in good physical condition at the time
of sacrifice.5
Long-term studies
Mouse. Long-term studies were carried out using 0.25% and 0.5%
levels in mice. No deleterious effects on weight gain were observed,
nor any important histological changes.6
Rat. Chronic toxicity experiments were conducted over a period of 2
years, in which NDGA was compared with phenol, catechol and gum guaiac
in concentrations of 0.5% in rats. NDGA had little or no effect on
growth or food intake, except in the highest concentration, where
there was a temporary decrease in growth associated with a decreased
food intake. Histological study of the liver, spleen and kidneys
showed no significant effect. Necrosis of the liver was noted
occasionally in all groups, including controls.6
Concentrations of 0.1%, 0.5% and 1.0% of NDGA were used in another
series of rats. Haemorrhage into the caecum was observed in 50% of
the animals. In a larger series of tests on rats at the same
concentrations of NDGA, this haemorrhage did not occur. Cysts in the
mesentery were found in several rats on the higher concentrations of
NDGA.
Two-year toxicity tests on groups of 10 male rats at 0%, 0.1%. 0.25%,
0.5% and 1% NDGA in the diet showed that 0.5% was the lowest level
causing inflammatory caecal lesions and slight cystic enlargement of
lymph nodes near the caecum. Growth inhibition occurred after the
first 6 months at levels of 0.5% and 1%.3
In another experiment lasting 2 years, 0.5% of NDGA in the diet caused
massive caecal haemorrhages, with single and multiple cysts in the
mesentery in the angle of the junction between the small and large
intestines. During the first 6 months, inhibition of the growth rate
occurred only at the 1% level.3
(Work in progress) Groups of 20 rats (10 male and 10 female) were fed
the following diets: 0.5% NDGA, 0.5% NDGA plus aspirin, 1% NDGA, 1%
NDGA plus aspirin, and aspirin for 65 weeks. Growth has been retarded
in all test groups compared with the controls.a Haemoglobin and
haematocrit determinations were done at 16 and 23 weeks; all groups
where NDGA was given had lower readings than those of the controls.a
Two rats, one fed 0.5% NDGA plus aspirin, the other aspirin alone,
showed some ulceration of the stomach after 42 and 50 weeks on test.
Examination of faeces for occult blood was done after 49 weeks; the
rats fed NDGA with no aspirin were all negative, but 5 rats fed
aspirin alone or aspirin plus NDGA showed traces of occult blood.7
Hamster. (Work in progress) Hamsters (15 male and 15 female) were
fed diets containing 0.1%, 0.5% and 1% NDGA for 65 weeks. Some deaths
which occurred at the 1% level may be attributed to the effect of NDGA
in the diet. Ulceration of the stomach was found in 3 hamsters at the
1% level after 48 weeks. At 22 weeks a marked decrease in haematocrit
values was found for females, but not in males, on 1% NDGA.
Examination of faeces for occult blood was negative in all groups.
The investigator points out that 1% NDGA in the diet of hamsters
produced a definite toxic effect, particularly in the female. Whether
the lower level will prove to be the no-effect level will depend on
the findings upon completion of the test, which is to he carried out
over the lifetime of the animals.7
Comment on experimental studies reported
The acute toxicity studies indicate that the guinea-pig is more
sensitive than the rat but no long-term studies with the former
species have been reported. The short-term studies in the dog suggest
that a 1% dietary level is well tolerated in this species although
there was some impairment of growth at the 0.5% level. The published
long-term studies in the rat and mouse provide little detailed
information and seem to have left some of the investigators in doubt
a No statistical evaluation has yet been done as these experiments
are not terminated; they are being carried out over the life span of
the animals.
about the acceptability of NDGA. The more recent long-term studies in
rat and hamster are still in progress and cannot be evaluated until
their completion.
Evaluation
Level causing no significant toxicological effect in the rat
From the limited information available, it is not possible to evaluate
the toxicological status of NDGA. No guidance can therefore be given
at present on the use of NDGA as an antioxidant in food.
Further Work Required
1. Further long-term studies in the rat and other species, including
dog.
2. Metabolic studies in animals and man.
References
1. Dacre. J. C. (1960) J. N.Z. Inst. Chem., 24, 161
2. Tappel, A. L. & Marr. A. G. (1954) J. Agr. Food Chem., 2, 554
3. Lehman, A. J., Fitzhugh, O. G., Nelson, A. A. & Woodard, G. (1951)
Advanc. Food Res., 3, 197
4. Griepentrog, F. (1961) Arzneimittel-Forsch., 11, 920
5. Anderson, W. D., Bell, B. J. & Bieter, R. N. (1955) Unpublished
report
6. Cranston, E. M., Jensen, M. J., Moren, A., Brey, T., Bell, E. T. &
Bieter, R. N. (1947) Fed. Proc., 6, 318
7. Mannell, W. A., Canadian Food and Drug Directorate, Ottawa (Letter
dated 23 September 1964)
