
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
o-PHENYLPHENOL
CHEMICAL NAMES Orthoxenol; o-hydroxydiphenyl;
o-hydroxydiphenyl
EMPIRICAL FORMULA C12H10O
STRUCTURAL FORMULA
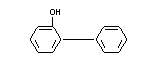 MOLECULAR WEIGHT 170.21
DEFINITION Contains not less then 98% of C12H10O and
conforms to the following specifications.
DESCRIPTION White, slightly yellow or pink flaky crystals
or solid, having a mild characteristic odour.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: Practically insoluble
Ethanol: Very soluble
B. Melting point: About 57°
C. An ethanolic solution (1 g in 10 ml) produces a green colour upon
addition of 10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Total ash: not more than 0.05%.
ASSAY
Weigh 2.000 g of o-phenylphenol, dissolve in 10 ml of 10% sodium
hydroxide solution by warming and dilute to 500.0 ml. Pipette 25.0 ml
into a 250-ml iodine flask and add 30.0 ml of 0.1 N bromide-bromate
TS and 50 ml of anhydrous methanol. Place the stopper in the flask
and add 10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise
the stopper slightly to allow the acid to flow down the sides inside
the flask, but retain a small amount of the acid in the well to act as
a seal. Mix the contents by swirling and allow it to react for
exactly 30 seconds at 25° ± 5°. Immediately add 10 ml of 20%
potassium iodide solution to the well and allow it to drain into the
assay flask as for the acid. Mix well, allow the solution to stand
for 5 minutes, shaking occasionally. Wash the stopper and the sides
of the flask with water. Titrate the liberated iodine with 0.1 N
sodium thiosulfate adding starch TS as the endpoint is approached.
Each ml of 0.1 N bromide-bromate TS consumed is equivalent to 4.255
mg of C12H10O.
SODIUM o-PHENYLPHENOL
CHEMICAL NAME Sodium o-phenylphenate
EMPIRICAL FORMULA C12H9ONa.4H2O
STRUCTURAL FORMULA
MOLECULAR WEIGHT 170.21
DEFINITION Contains not less then 98% of C12H10O and
conforms to the following specifications.
DESCRIPTION White, slightly yellow or pink flaky crystals
or solid, having a mild characteristic odour.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: Practically insoluble
Ethanol: Very soluble
B. Melting point: About 57°
C. An ethanolic solution (1 g in 10 ml) produces a green colour upon
addition of 10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Total ash: not more than 0.05%.
ASSAY
Weigh 2.000 g of o-phenylphenol, dissolve in 10 ml of 10% sodium
hydroxide solution by warming and dilute to 500.0 ml. Pipette 25.0 ml
into a 250-ml iodine flask and add 30.0 ml of 0.1 N bromide-bromate
TS and 50 ml of anhydrous methanol. Place the stopper in the flask
and add 10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise
the stopper slightly to allow the acid to flow down the sides inside
the flask, but retain a small amount of the acid in the well to act as
a seal. Mix the contents by swirling and allow it to react for
exactly 30 seconds at 25° ± 5°. Immediately add 10 ml of 20%
potassium iodide solution to the well and allow it to drain into the
assay flask as for the acid. Mix well, allow the solution to stand
for 5 minutes, shaking occasionally. Wash the stopper and the sides
of the flask with water. Titrate the liberated iodine with 0.1 N
sodium thiosulfate adding starch TS as the endpoint is approached.
Each ml of 0.1 N bromide-bromate TS consumed is equivalent to 4.255
mg of C12H10O.
SODIUM o-PHENYLPHENOL
CHEMICAL NAME Sodium o-phenylphenate
EMPIRICAL FORMULA C12H9ONa.4H2O
STRUCTURAL FORMULA
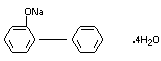 MOLECULAR WEIGHT 264.26
DEFINITION Contains not less then 97.0% of C12H9ONa.4H2O
and conforms to the following specifications.
DESCRIPTION White powder or flakes. When exposed to air it
absorbs carbon dioxide and releases free
o-phenylphenol which slowly sublimes.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: 122 g dissolve in 100 ml water
Ethanol: Very soluble
Methanol: 138 g dissolve in 100 ml methanol
B. An aqueous solution has a pH of about 12.7. When neutralized, a
precipitate of o-phenylphenol forms and, when filtered and
dried, this material melts at about 57° and its ethanolic
solution (1 g in 10 ml) produces a green colour upon addition of
10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Excess alkalinity (as NaOH): Not more than 1.0%.
Weigh 5.0 g into a 250-ml beaker, dissolve in 50 ml of water and
titrate with 1 N hydrochloric acid to a pH of 11.0 using a suitable
pH meter. Each ml of 1 N hydrochloric acid is equivalent to 40 mg
of sodium hydroxide.
ASSAY
Weigh 3.100 g of sodium o-phenylphenol, dissolve in water, adding a
few drops of 10% sodium hydroxide solution if necessary to clear any
turbidity, and dilute to 500.0 ml with water. Pipette 25.0 ml into a
250-ml iodine flask, and add 30.0 ml of 0.1 N bromide-bromate TS and
50 ml of anhydrous methanol. Place the stopper in the flask and add
10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise the
stopper slightly to allow the acid to flow down the sides of the
flask, but retain a small amount of the acid in the well to act as a
seal. Mix the contents by swirling and allow it to react for exactly
3O seconds at 25° ± 5°. Immediately add 10 ml of 20% potassium iodide
solution to the well and allow it to drain into the flask. Mix well
and allow the solution to stand for 5 minutes, shaking occasionally.
Wash the stopper and the sides of the flask with water and titrate the
liberated iodine with 0.1 N sodium thiosulfate adding starch TS as
the endpoint is approached. Each ml of 0.1 N bromide-bromate TS
consumed is equivalent to 6.608 mg of C12H9ONa.4H2O.
Biological Data
Biochemical aspects
Storage of o-phenylphenol has not been observed in rats. In feeding
experiments lasting 2 years, average values of 22 mg/100 g tissue were
found in the kidneys of rats on a 2% diet, and approximately 1 mg/100
g tissue in the kidneys of rats on a 0.2% diet.1
It is known that the o- and p-hydroxydiphenyls are highly
conjugated with glucuronic acids in the rabbit, but whether they form
ethereal sulfates is not known.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2700-3000 (approx.) 1,3
Cat oral 500 (approx.) 3
Short-term studies
Rat. Over a period of 32 days, groups of 15 male rats were fed
o-phenylphenol in daily doses of 2, 20 and 200 mg/kg body-weight.
No harmful effect was demonstrable in any of the groups.3
Five male and 5 female rats in each group were given by stomach tube
doses of 50, 100, 200 and 500 mg/kg body-weight for 5 days a week over
a period of 6 months. The only abnormality observed was a slight
increase in average liver and kidney weights in the animals at the 500
mg/kg dosage.1
When diets containing 0.1%, 0.3%, 1.0% and 2% of o-phenylphenol were
fed for 3 months to groups comprising 12 males and 12 females, slight
retardation of growth was observed in the 2% group. There was no
significant difference between the mortality of control and test
animals. There were doubtful increases in weight of liver, kidney and
spleen of certain rats of the 1% and 2% groups. No tissue changes
ware observed.
Dog. Daily doses of 1000 mg/kg of o-phenylphenol killed 2 dogs
within a month. Groups of 2 dogs each were fed o-phenylphenol for a
period of 1 year in daily amounts of 20, 200 and 500 mg/kg
body-weight; no effect related to the administration of
o-phenylphenol was observed. Haematological values, urinary sugar
and protein values, organ weights and histopathological examination of
the various tissues did not differ from the normal range.1
Man. A 5.0% solution of o-phenylphenol in sesame oil and a 0.1%
aqueous solution of the sodium salt tested on 200 subjects caused
neither primary skin irritation nor skin sensitization.1 The sodium
salt is slightly irritating in 0.5% aqueous solution and decidedly
irritating in 1.0% and 5% solutions.
Long-term studies
Rat. Male and female rate (25 of each sex per group) maintained for
2 years on diets containing 0.02% and 0.2% of o-phenylphenol showed
no adverse effects when compared with a control group, as judged by
growth, mortality, gross appearance, haematology, urinary sugar and
protein values, organ weights, tissue content of o-phenylphenol, and
histopathological examination of various tissues. A similar group of
rats maintained for 2 years on a diet containing 2% of
o-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilatation), and the presence of small amounts of o-phenylphenol in
the kidney tissues.1
Comment on experimental studies reported
From experiments on rats and dogs, o-phenylphenol does not seem to
be a very toxic substance. The noted difference in acute toxicity
between these animals and cats might be explained by the known high
sensitivity of cats to phenolic compounds. The long-term study in
rats is taken as a basis for the evaluation.1
Evaluation
Level causing no significant toxicological effect in the rat
0.2% (=2000 ppm) in the diet, equivalent to 100 mg/kg body-weight per
day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-1.0
Further Work Considered Desirable
Metabolic studies in experimental animals and man.
References
1. Hodge, H. C., Maynard, E. A., Blanchet, H. J. jr, Spencer, H. C. &
Rowe, V. K. (1952) J. Pharmcol. exp. Ther., 104, 202
2. Williams, R. T. (1959) Detoxication mechanisms, Chapmn & Hall,
London
3. MacIntosh, F. C. (1945) Analyst, 70, 334
MOLECULAR WEIGHT 264.26
DEFINITION Contains not less then 97.0% of C12H9ONa.4H2O
and conforms to the following specifications.
DESCRIPTION White powder or flakes. When exposed to air it
absorbs carbon dioxide and releases free
o-phenylphenol which slowly sublimes.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: 122 g dissolve in 100 ml water
Ethanol: Very soluble
Methanol: 138 g dissolve in 100 ml methanol
B. An aqueous solution has a pH of about 12.7. When neutralized, a
precipitate of o-phenylphenol forms and, when filtered and
dried, this material melts at about 57° and its ethanolic
solution (1 g in 10 ml) produces a green colour upon addition of
10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Excess alkalinity (as NaOH): Not more than 1.0%.
Weigh 5.0 g into a 250-ml beaker, dissolve in 50 ml of water and
titrate with 1 N hydrochloric acid to a pH of 11.0 using a suitable
pH meter. Each ml of 1 N hydrochloric acid is equivalent to 40 mg
of sodium hydroxide.
ASSAY
Weigh 3.100 g of sodium o-phenylphenol, dissolve in water, adding a
few drops of 10% sodium hydroxide solution if necessary to clear any
turbidity, and dilute to 500.0 ml with water. Pipette 25.0 ml into a
250-ml iodine flask, and add 30.0 ml of 0.1 N bromide-bromate TS and
50 ml of anhydrous methanol. Place the stopper in the flask and add
10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise the
stopper slightly to allow the acid to flow down the sides of the
flask, but retain a small amount of the acid in the well to act as a
seal. Mix the contents by swirling and allow it to react for exactly
3O seconds at 25° ± 5°. Immediately add 10 ml of 20% potassium iodide
solution to the well and allow it to drain into the flask. Mix well
and allow the solution to stand for 5 minutes, shaking occasionally.
Wash the stopper and the sides of the flask with water and titrate the
liberated iodine with 0.1 N sodium thiosulfate adding starch TS as
the endpoint is approached. Each ml of 0.1 N bromide-bromate TS
consumed is equivalent to 6.608 mg of C12H9ONa.4H2O.
Biological Data
Biochemical aspects
Storage of o-phenylphenol has not been observed in rats. In feeding
experiments lasting 2 years, average values of 22 mg/100 g tissue were
found in the kidneys of rats on a 2% diet, and approximately 1 mg/100
g tissue in the kidneys of rats on a 0.2% diet.1
It is known that the o- and p-hydroxydiphenyls are highly
conjugated with glucuronic acids in the rabbit, but whether they form
ethereal sulfates is not known.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2700-3000 (approx.) 1,3
Cat oral 500 (approx.) 3
Short-term studies
Rat. Over a period of 32 days, groups of 15 male rats were fed
o-phenylphenol in daily doses of 2, 20 and 200 mg/kg body-weight.
No harmful effect was demonstrable in any of the groups.3
Five male and 5 female rats in each group were given by stomach tube
doses of 50, 100, 200 and 500 mg/kg body-weight for 5 days a week over
a period of 6 months. The only abnormality observed was a slight
increase in average liver and kidney weights in the animals at the 500
mg/kg dosage.1
When diets containing 0.1%, 0.3%, 1.0% and 2% of o-phenylphenol were
fed for 3 months to groups comprising 12 males and 12 females, slight
retardation of growth was observed in the 2% group. There was no
significant difference between the mortality of control and test
animals. There were doubtful increases in weight of liver, kidney and
spleen of certain rats of the 1% and 2% groups. No tissue changes
ware observed.
Dog. Daily doses of 1000 mg/kg of o-phenylphenol killed 2 dogs
within a month. Groups of 2 dogs each were fed o-phenylphenol for a
period of 1 year in daily amounts of 20, 200 and 500 mg/kg
body-weight; no effect related to the administration of
o-phenylphenol was observed. Haematological values, urinary sugar
and protein values, organ weights and histopathological examination of
the various tissues did not differ from the normal range.1
Man. A 5.0% solution of o-phenylphenol in sesame oil and a 0.1%
aqueous solution of the sodium salt tested on 200 subjects caused
neither primary skin irritation nor skin sensitization.1 The sodium
salt is slightly irritating in 0.5% aqueous solution and decidedly
irritating in 1.0% and 5% solutions.
Long-term studies
Rat. Male and female rate (25 of each sex per group) maintained for
2 years on diets containing 0.02% and 0.2% of o-phenylphenol showed
no adverse effects when compared with a control group, as judged by
growth, mortality, gross appearance, haematology, urinary sugar and
protein values, organ weights, tissue content of o-phenylphenol, and
histopathological examination of various tissues. A similar group of
rats maintained for 2 years on a diet containing 2% of
o-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilatation), and the presence of small amounts of o-phenylphenol in
the kidney tissues.1
Comment on experimental studies reported
From experiments on rats and dogs, o-phenylphenol does not seem to
be a very toxic substance. The noted difference in acute toxicity
between these animals and cats might be explained by the known high
sensitivity of cats to phenolic compounds. The long-term study in
rats is taken as a basis for the evaluation.1
Evaluation
Level causing no significant toxicological effect in the rat
0.2% (=2000 ppm) in the diet, equivalent to 100 mg/kg body-weight per
day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-1.0
Further Work Considered Desirable
Metabolic studies in experimental animals and man.
References
1. Hodge, H. C., Maynard, E. A., Blanchet, H. J. jr, Spencer, H. C. &
Rowe, V. K. (1952) J. Pharmcol. exp. Ther., 104, 202
2. Williams, R. T. (1959) Detoxication mechanisms, Chapmn & Hall,
London
3. MacIntosh, F. C. (1945) Analyst, 70, 334
 MOLECULAR WEIGHT 170.21
DEFINITION Contains not less then 98% of C12H10O and
conforms to the following specifications.
DESCRIPTION White, slightly yellow or pink flaky crystals
or solid, having a mild characteristic odour.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: Practically insoluble
Ethanol: Very soluble
B. Melting point: About 57°
C. An ethanolic solution (1 g in 10 ml) produces a green colour upon
addition of 10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Total ash: not more than 0.05%.
ASSAY
Weigh 2.000 g of o-phenylphenol, dissolve in 10 ml of 10% sodium
hydroxide solution by warming and dilute to 500.0 ml. Pipette 25.0 ml
into a 250-ml iodine flask and add 30.0 ml of 0.1 N bromide-bromate
TS and 50 ml of anhydrous methanol. Place the stopper in the flask
and add 10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise
the stopper slightly to allow the acid to flow down the sides inside
the flask, but retain a small amount of the acid in the well to act as
a seal. Mix the contents by swirling and allow it to react for
exactly 30 seconds at 25° ± 5°. Immediately add 10 ml of 20%
potassium iodide solution to the well and allow it to drain into the
assay flask as for the acid. Mix well, allow the solution to stand
for 5 minutes, shaking occasionally. Wash the stopper and the sides
of the flask with water. Titrate the liberated iodine with 0.1 N
sodium thiosulfate adding starch TS as the endpoint is approached.
Each ml of 0.1 N bromide-bromate TS consumed is equivalent to 4.255
mg of C12H10O.
SODIUM o-PHENYLPHENOL
CHEMICAL NAME Sodium o-phenylphenate
EMPIRICAL FORMULA C12H9ONa.4H2O
STRUCTURAL FORMULA
MOLECULAR WEIGHT 170.21
DEFINITION Contains not less then 98% of C12H10O and
conforms to the following specifications.
DESCRIPTION White, slightly yellow or pink flaky crystals
or solid, having a mild characteristic odour.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: Practically insoluble
Ethanol: Very soluble
B. Melting point: About 57°
C. An ethanolic solution (1 g in 10 ml) produces a green colour upon
addition of 10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Total ash: not more than 0.05%.
ASSAY
Weigh 2.000 g of o-phenylphenol, dissolve in 10 ml of 10% sodium
hydroxide solution by warming and dilute to 500.0 ml. Pipette 25.0 ml
into a 250-ml iodine flask and add 30.0 ml of 0.1 N bromide-bromate
TS and 50 ml of anhydrous methanol. Place the stopper in the flask
and add 10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise
the stopper slightly to allow the acid to flow down the sides inside
the flask, but retain a small amount of the acid in the well to act as
a seal. Mix the contents by swirling and allow it to react for
exactly 30 seconds at 25° ± 5°. Immediately add 10 ml of 20%
potassium iodide solution to the well and allow it to drain into the
assay flask as for the acid. Mix well, allow the solution to stand
for 5 minutes, shaking occasionally. Wash the stopper and the sides
of the flask with water. Titrate the liberated iodine with 0.1 N
sodium thiosulfate adding starch TS as the endpoint is approached.
Each ml of 0.1 N bromide-bromate TS consumed is equivalent to 4.255
mg of C12H10O.
SODIUM o-PHENYLPHENOL
CHEMICAL NAME Sodium o-phenylphenate
EMPIRICAL FORMULA C12H9ONa.4H2O
STRUCTURAL FORMULA
 MOLECULAR WEIGHT 264.26
DEFINITION Contains not less then 97.0% of C12H9ONa.4H2O
and conforms to the following specifications.
DESCRIPTION White powder or flakes. When exposed to air it
absorbs carbon dioxide and releases free
o-phenylphenol which slowly sublimes.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: 122 g dissolve in 100 ml water
Ethanol: Very soluble
Methanol: 138 g dissolve in 100 ml methanol
B. An aqueous solution has a pH of about 12.7. When neutralized, a
precipitate of o-phenylphenol forms and, when filtered and
dried, this material melts at about 57° and its ethanolic
solution (1 g in 10 ml) produces a green colour upon addition of
10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Excess alkalinity (as NaOH): Not more than 1.0%.
Weigh 5.0 g into a 250-ml beaker, dissolve in 50 ml of water and
titrate with 1 N hydrochloric acid to a pH of 11.0 using a suitable
pH meter. Each ml of 1 N hydrochloric acid is equivalent to 40 mg
of sodium hydroxide.
ASSAY
Weigh 3.100 g of sodium o-phenylphenol, dissolve in water, adding a
few drops of 10% sodium hydroxide solution if necessary to clear any
turbidity, and dilute to 500.0 ml with water. Pipette 25.0 ml into a
250-ml iodine flask, and add 30.0 ml of 0.1 N bromide-bromate TS and
50 ml of anhydrous methanol. Place the stopper in the flask and add
10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise the
stopper slightly to allow the acid to flow down the sides of the
flask, but retain a small amount of the acid in the well to act as a
seal. Mix the contents by swirling and allow it to react for exactly
3O seconds at 25° ± 5°. Immediately add 10 ml of 20% potassium iodide
solution to the well and allow it to drain into the flask. Mix well
and allow the solution to stand for 5 minutes, shaking occasionally.
Wash the stopper and the sides of the flask with water and titrate the
liberated iodine with 0.1 N sodium thiosulfate adding starch TS as
the endpoint is approached. Each ml of 0.1 N bromide-bromate TS
consumed is equivalent to 6.608 mg of C12H9ONa.4H2O.
Biological Data
Biochemical aspects
Storage of o-phenylphenol has not been observed in rats. In feeding
experiments lasting 2 years, average values of 22 mg/100 g tissue were
found in the kidneys of rats on a 2% diet, and approximately 1 mg/100
g tissue in the kidneys of rats on a 0.2% diet.1
It is known that the o- and p-hydroxydiphenyls are highly
conjugated with glucuronic acids in the rabbit, but whether they form
ethereal sulfates is not known.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2700-3000 (approx.) 1,3
Cat oral 500 (approx.) 3
Short-term studies
Rat. Over a period of 32 days, groups of 15 male rats were fed
o-phenylphenol in daily doses of 2, 20 and 200 mg/kg body-weight.
No harmful effect was demonstrable in any of the groups.3
Five male and 5 female rats in each group were given by stomach tube
doses of 50, 100, 200 and 500 mg/kg body-weight for 5 days a week over
a period of 6 months. The only abnormality observed was a slight
increase in average liver and kidney weights in the animals at the 500
mg/kg dosage.1
When diets containing 0.1%, 0.3%, 1.0% and 2% of o-phenylphenol were
fed for 3 months to groups comprising 12 males and 12 females, slight
retardation of growth was observed in the 2% group. There was no
significant difference between the mortality of control and test
animals. There were doubtful increases in weight of liver, kidney and
spleen of certain rats of the 1% and 2% groups. No tissue changes
ware observed.
Dog. Daily doses of 1000 mg/kg of o-phenylphenol killed 2 dogs
within a month. Groups of 2 dogs each were fed o-phenylphenol for a
period of 1 year in daily amounts of 20, 200 and 500 mg/kg
body-weight; no effect related to the administration of
o-phenylphenol was observed. Haematological values, urinary sugar
and protein values, organ weights and histopathological examination of
the various tissues did not differ from the normal range.1
Man. A 5.0% solution of o-phenylphenol in sesame oil and a 0.1%
aqueous solution of the sodium salt tested on 200 subjects caused
neither primary skin irritation nor skin sensitization.1 The sodium
salt is slightly irritating in 0.5% aqueous solution and decidedly
irritating in 1.0% and 5% solutions.
Long-term studies
Rat. Male and female rate (25 of each sex per group) maintained for
2 years on diets containing 0.02% and 0.2% of o-phenylphenol showed
no adverse effects when compared with a control group, as judged by
growth, mortality, gross appearance, haematology, urinary sugar and
protein values, organ weights, tissue content of o-phenylphenol, and
histopathological examination of various tissues. A similar group of
rats maintained for 2 years on a diet containing 2% of
o-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilatation), and the presence of small amounts of o-phenylphenol in
the kidney tissues.1
Comment on experimental studies reported
From experiments on rats and dogs, o-phenylphenol does not seem to
be a very toxic substance. The noted difference in acute toxicity
between these animals and cats might be explained by the known high
sensitivity of cats to phenolic compounds. The long-term study in
rats is taken as a basis for the evaluation.1
Evaluation
Level causing no significant toxicological effect in the rat
0.2% (=2000 ppm) in the diet, equivalent to 100 mg/kg body-weight per
day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-1.0
Further Work Considered Desirable
Metabolic studies in experimental animals and man.
References
1. Hodge, H. C., Maynard, E. A., Blanchet, H. J. jr, Spencer, H. C. &
Rowe, V. K. (1952) J. Pharmcol. exp. Ther., 104, 202
2. Williams, R. T. (1959) Detoxication mechanisms, Chapmn & Hall,
London
3. MacIntosh, F. C. (1945) Analyst, 70, 334
MOLECULAR WEIGHT 264.26
DEFINITION Contains not less then 97.0% of C12H9ONa.4H2O
and conforms to the following specifications.
DESCRIPTION White powder or flakes. When exposed to air it
absorbs carbon dioxide and releases free
o-phenylphenol which slowly sublimes.
USE For the post-harvest treatment of fruits and
vegetables to protect against microbial damage.
IDENTIFICATION TESTS
A. Solubility: Water: 122 g dissolve in 100 ml water
Ethanol: Very soluble
Methanol: 138 g dissolve in 100 ml methanol
B. An aqueous solution has a pH of about 12.7. When neutralized, a
precipitate of o-phenylphenol forms and, when filtered and
dried, this material melts at about 57° and its ethanolic
solution (1 g in 10 ml) produces a green colour upon addition of
10% ferric chloride solution.
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Excess alkalinity (as NaOH): Not more than 1.0%.
Weigh 5.0 g into a 250-ml beaker, dissolve in 50 ml of water and
titrate with 1 N hydrochloric acid to a pH of 11.0 using a suitable
pH meter. Each ml of 1 N hydrochloric acid is equivalent to 40 mg
of sodium hydroxide.
ASSAY
Weigh 3.100 g of sodium o-phenylphenol, dissolve in water, adding a
few drops of 10% sodium hydroxide solution if necessary to clear any
turbidity, and dilute to 500.0 ml with water. Pipette 25.0 ml into a
250-ml iodine flask, and add 30.0 ml of 0.1 N bromide-bromate TS and
50 ml of anhydrous methanol. Place the stopper in the flask and add
10 ml of dilute (1 to 1) hydrochloric acid to the well. Raise the
stopper slightly to allow the acid to flow down the sides of the
flask, but retain a small amount of the acid in the well to act as a
seal. Mix the contents by swirling and allow it to react for exactly
3O seconds at 25° ± 5°. Immediately add 10 ml of 20% potassium iodide
solution to the well and allow it to drain into the flask. Mix well
and allow the solution to stand for 5 minutes, shaking occasionally.
Wash the stopper and the sides of the flask with water and titrate the
liberated iodine with 0.1 N sodium thiosulfate adding starch TS as
the endpoint is approached. Each ml of 0.1 N bromide-bromate TS
consumed is equivalent to 6.608 mg of C12H9ONa.4H2O.
Biological Data
Biochemical aspects
Storage of o-phenylphenol has not been observed in rats. In feeding
experiments lasting 2 years, average values of 22 mg/100 g tissue were
found in the kidneys of rats on a 2% diet, and approximately 1 mg/100
g tissue in the kidneys of rats on a 0.2% diet.1
It is known that the o- and p-hydroxydiphenyls are highly
conjugated with glucuronic acids in the rabbit, but whether they form
ethereal sulfates is not known.2
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
Rat oral 2700-3000 (approx.) 1,3
Cat oral 500 (approx.) 3
Short-term studies
Rat. Over a period of 32 days, groups of 15 male rats were fed
o-phenylphenol in daily doses of 2, 20 and 200 mg/kg body-weight.
No harmful effect was demonstrable in any of the groups.3
Five male and 5 female rats in each group were given by stomach tube
doses of 50, 100, 200 and 500 mg/kg body-weight for 5 days a week over
a period of 6 months. The only abnormality observed was a slight
increase in average liver and kidney weights in the animals at the 500
mg/kg dosage.1
When diets containing 0.1%, 0.3%, 1.0% and 2% of o-phenylphenol were
fed for 3 months to groups comprising 12 males and 12 females, slight
retardation of growth was observed in the 2% group. There was no
significant difference between the mortality of control and test
animals. There were doubtful increases in weight of liver, kidney and
spleen of certain rats of the 1% and 2% groups. No tissue changes
ware observed.
Dog. Daily doses of 1000 mg/kg of o-phenylphenol killed 2 dogs
within a month. Groups of 2 dogs each were fed o-phenylphenol for a
period of 1 year in daily amounts of 20, 200 and 500 mg/kg
body-weight; no effect related to the administration of
o-phenylphenol was observed. Haematological values, urinary sugar
and protein values, organ weights and histopathological examination of
the various tissues did not differ from the normal range.1
Man. A 5.0% solution of o-phenylphenol in sesame oil and a 0.1%
aqueous solution of the sodium salt tested on 200 subjects caused
neither primary skin irritation nor skin sensitization.1 The sodium
salt is slightly irritating in 0.5% aqueous solution and decidedly
irritating in 1.0% and 5% solutions.
Long-term studies
Rat. Male and female rate (25 of each sex per group) maintained for
2 years on diets containing 0.02% and 0.2% of o-phenylphenol showed
no adverse effects when compared with a control group, as judged by
growth, mortality, gross appearance, haematology, urinary sugar and
protein values, organ weights, tissue content of o-phenylphenol, and
histopathological examination of various tissues. A similar group of
rats maintained for 2 years on a diet containing 2% of
o-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilatation), and the presence of small amounts of o-phenylphenol in
the kidney tissues.1
Comment on experimental studies reported
From experiments on rats and dogs, o-phenylphenol does not seem to
be a very toxic substance. The noted difference in acute toxicity
between these animals and cats might be explained by the known high
sensitivity of cats to phenolic compounds. The long-term study in
rats is taken as a basis for the evaluation.1
Evaluation
Level causing no significant toxicological effect in the rat
0.2% (=2000 ppm) in the diet, equivalent to 100 mg/kg body-weight per
day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-1.0
Further Work Considered Desirable
Metabolic studies in experimental animals and man.
References
1. Hodge, H. C., Maynard, E. A., Blanchet, H. J. jr, Spencer, H. C. &
Rowe, V. K. (1952) J. Pharmcol. exp. Ther., 104, 202
2. Williams, R. T. (1959) Detoxication mechanisms, Chapmn & Hall,
London
3. MacIntosh, F. C. (1945) Analyst, 70, 334
