
ADRENOCEPTOR AGONISTS
CLENBUTEROL
First draft prepared by
Dr K.N. Woodward
Veterinary Medicines Directorate
Ministry of Agriculture, Fisheries and Food
Addlestone, Surrey, United Kingdom
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.2 Biotransformation
2.1.3 Kinetics in humans
2.2 Toxicological studies
2.2.1 Acute toxicity studies
2.2.2 Short-term toxicity studies
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.4 Reproductive toxicity studies
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.6 Special studies on genotoxicity
2.2.7 Special studies on irritancy
2.2.8 Special studies on immunotoxicity
2.2.9 Special studies on pharmacodynamic effects
2.3 Observations in humans
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Clenbuterol is a ß-adrenoceptor agonist that exerts a potent
bronchiolytic effect by preferential action on ß2-adrenoceptors in
smooth muscle, resulting in the relaxation of bronchial smooth muscle
and a decrease in airway resistance. Similarly, through selective
binding to ß2-adrenoceptors on uterine smooth muscle cell membranes,
relaxation of the uterus (tocolysis) occurs. Clenbuterol hydrochloride
is used for the treatment of respiratory disease in horses and cattle
(0.8 µg/kg bw, twice daily) and as a tocolytic agent in cattle (a
single parenteral injection of 0.8 µg/kg bw). Although unapproved for
use as a repartitioning agent, it is used for this purpose in farm
animals at doses several orders of magnitude higher than the
recommended therapeutic dose.
Clenbuterol is manufactured as a 50 : 50 racemic mixture, most of
its pharmacological activity being associated with the laevo form. It
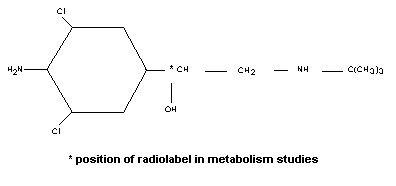
had not previously been evaluated by the Committee. The molecular
structure of clenbuterol is shown below.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Mice
Following a single intravenous dose of 5 mg/kg bw 14C-clenbu-
terol to pregnant mice, autoradiography demonstrated high levels of
radioactivity in the placenta with lower levels in the fetuses within
30 minutes after administration. Radioactivity was also found in the
fetal liver, thymus and spinal column. Total radioactivity in the
fetuses was significantly less than in maternal animals (Kopitar,
1969).
2.1.1.2 Rats
After oral administration of 2 mg/kg bw 14C-clenbuterol to
rats, approximately 60% of the radioactivity was found in the 48-hour
urine sample suggesting good absorption from the gastrointestinal
system. A further 20% was recovered from faeces over this time. Around
5-10% was recovered in urine and faeces in the 48-72 hour period.
Approximately 7% appeared in the bile. Levels in tissues were
relatively low, except for the liver, kidneys and lungs (Kopitar,
1970).
In a separate study using autoradiography, maximum distribution
after oral doses of 5 or 10 mg/kg bw occurred approximately 3 hours
after administration. Highest concentrations of radioactivity were
found in the lungs, liver, kidneys, pancreas and bone marrow, but some
radioactivity was also found in the brain, adrenal glands, and
skeletal and cardiac muscles (Kopitar, 1969).
When previously untreated rats were given a single oral dose of
5 mg/kg bw clenbuterol by gavage, plasma levels of the drug were
found to be 3-5 times lower than in rats pre-treated with the drug at
5 mg/kg bw per day for 6 months. The single dose was found to abolish
gastrointestinal peristalsis in the rat but after 6 months of compound
administration, peristalsis returned. This may explain the lower, but
prolonged plasma levels after the single administration, which
probably led to a depot effect in the gut (Kopitar & Zimmer, 1973).
Clenbuterol readily enters the placenta in the pregnant rat. At 3
hours after an intravenous or oral dose of 14C clenbuterol, there
was more radioactivity in the placenta than in the blood and tissues
of maternal animals or fetuses (Richter, 1982).
2.1.1.3 Dogs
When beagle dogs were given capsules containing 14C-clen-
buterol at a dose of 2.5 mg/kg bw, maximum blood and plasma levels
occurred 8 hours after dosing. Around 85% of the dose was recovered in
the urine after 96 hours, with 4-9% in the corresponding faecal
samples. Pretreating dogs for 5 weeks with 2.5 mg/kg bw per day
clenbuterol led to a slight increase in the rate of absorption in a
manner similar to that noted in rats (Zimmer, 1974a).
After single oral doses of 2.5 mg/kg bw, blood concentrations in
the dog were approximately twice those of rabbits given identical
doses (Zimmer, 1974b).
Clenbuterol was found to cross the placenta and enter the fetuses
in the dog when a single pregnant female was given an oral dose of
2.5 mg/kg bw 14C-clenbuterol. At 4 hours after administration the
concentration of radioactivity in fetal plasma was around 16% of that
in maternal plasma. Approximately 0.4% of the total dose administered
to the maternal animal was recovered in the fetuses at 4 hours after
administration (Rominger & Schrank, 1982).
2.1.1.4 Monkeys
After intravenous administration of 100 µg 14C-clenbuterol per
animal (approximately 30 µg/kg bw) to cynomolgus monkeys, around 60%
of the dose was recovered in the urine and 4% in the faeces within 72
hours of administration. There was biphasic elimination. It was not
possible to calculate the half-life for the first and rapid phase, but
the second phase half-life was 20-30 hours (Chasseaud et al.,
1978).
2.1.1.5 Baboons
Following a single intravenous dose of 2.5 mg/kg bw 14C-clen
buterol to male baboons, around 82% of the administered dose was
recovered in the urine and faeces within 5 days, with the majority of
this (72%) within 48 hours. Of the 82%, approximately 68% was recovered
in the urine and 14% in the faeces. A similar pattern was noted in
baboons given the drug orally. There was a rapid distribution phase and
the highest levels of radioactivity were found in the lungs, liver and
kidneys (Schmid, 1982).
Placental transfer of 14C-clenbuterol was investigated in a
single pregnant baboon given 3.3 mg/kg bw intravenously. The animal
was killed 3.5 hours after dosing, and around 1.5% of the total
administered close was found in the fetus (Schmid, 1980).
2.1.1.6 Cattle
In cattle given 0.8 µg/kg bw 14C-clenbuterol using a single
intramuscular injection, the liver was found to have the highest
concentrations of radioactivity. In muscle, soon after administration,
the radioactivity was due almost entirely to parent compound (Schmid,
1990a).
In a pilot study with a single cow given 0.8 µg/kg bw 14C-
clenbuterol orally, the first phase plasma elimination half-life
was 0.1 hours and the second phase elimination half-life 17 hours. The
maximum plasma concentration was 0.4 µg/ml with tmax at 8.5 hours.
Approximately 81% of the dose was excreted in the urine with 13% in
the faeces over the 96-hour period after dosing; around 1% was
excreted in the milk (Schmid & Zimmer, 1977a). Similar results were
noted after intramuscular (Schmid & Zimmer, 1977b) and intravenous
(Schmid, 1977) administration, but with the latter route plasma levels
rose much more rapidly. In both cases, radioactivity was found in the
faeces suggesting biliary excretion.
When calves were given 0.8 µg/kg bw 14C-clenbuterol intramus-
cularly, twice a day for 2 days and then orally twice a day for 3 days
in a consecutive manner, using a combination product also containing
trimethoprim and sulfadiazine, around 64% of the dose was excreted
during 15 days in urine and 5% in the faeces. The highest level of
radioactivity was found in injection site muscle, liver and kidney
(Hawkins et al., 1985a). Similar results were seen in another study
where cattle were given intramuscular injections of 14C-clenbuterol
(Cameron & Phillips, 1987a).
In cattle given 21 intramuscular doses of 14C-clenbuterol at a
dose level of 0.8 µg/kg bw per day, twice daily, the majority of the
dose was recovered in the urine. The plasma half-life following the
last dose was of the order of 4 days. Highest levels of radioactivity
were found in liver and kidney, with much lower levels in muscle and
fat (Hawkins et al., 1993a).
After administration of 7 µg/kg bw 14C-clenbuterol by the
intramuscular route to a pregnant cow, radioactivity crossed the
placenta and was found in fetal tissues, including skeletal tissues,
kidneys and liver, when the animal was killed 3 hours after the
dosing. Radioactivity was also found in the maternal and fetal eyes
(Baillie et al., 1980).
When lactating cattle were given oral or intramuscular doses of
14C-clenbuterol at a dose of 0.8 µg/kg bw, radioactivity was found
in the urine for 48-70 hours after administration. In the first 48
hours, the majority of the radioactivity in milk was attributable to
parent compound (Schmid, 1990b).
Lactating dairy cows treated with 0.8 µg/kg bw 14C-clen-
buterol, twice daily by the intramuscular route for 3 consecutive days,
and twice daily by the oral route for a further 2 days followed by a
single oral dose on day 6, were found to have milk concentrations in
the range of 0.76-1.03 µg/litre 12 hours after the first dose rising
to 2.14-2.6 µg/litre after 48 hours. They remained in the range 1.46
to 3.93 µg/litre throughout the dosing period. Highest tissue concen-
trations were at the injection site, liver and kidney (Hawkins et al.,
1985b). Similar results were obtained in another study where dairy
cattle were given oral, intravenous or intramuscular doses of 0.8 µg/kg
14C-clenbuterol. In this study approximately 2% of administered
radioactivity was found in milk (Cameron & Phillips, 1987b).
2.1.1.7 Horses
After administration of a single oral dose of 0.8 µg/kg bw
14C-clenbuterol to a horse, the maximum plasma concentration of
0.4 µg/ml was reached at approximately 2 hours. The elimination
half-life was 21 hours. After 144-168 hours, 81% of the administered
dose was recovered in urine. Following intravenous administration, 82%
of the administered dose was found in urine, with around 9% in faeces,
suggesting biliary excretion (Zimmer, 1977).
In a study with 10 horses, animals were given a single oral dose
of 0.8 µg/kg bw clenbuterol followed by a single intravenous dose 3
weeks later. A second group of 10 horses was treated in a similar
manner, but initially using the intravenous route with the oral dose
after 3 weeks. Approximately 20-50% of the oral dose (mean 36%) as
parent drug, was excreted over 108 hours in the urine, while,
following intravenous administration, some 17-70% of the dose
(mean 43%) was excreted in the 108-hour urine (Zupan, 1985).
In a study with a clenbuterol, trimethoprim and sulfadiazine
combination product, horses were given gelatin capsules containing
14C-clenbuterol at a dose of 0.8 µg/kg bw, twice daily for 10 days,
with a single oral dose on day 11. The majority of the dose (70-77%)
was excreted in the urine and 10-15% in the faeces. The maximum plasma
concentration was achieved 3-4 hours after the first administration
and the plasma half-life was 9-10 hours. The mean plasma concentration
was 0.6-1.2 µg/ml. Highest levels of radioactivity were found in the
liver and kidneys with very little in fat (Hawkins et al., 1984).
Similarly, when 12 horses were given 21 twice daily oral doses of
0.8 µg/kg bw 14C-clenbuterol, steady-state plasma levels were
rapidly achieved. Peak levels of 1-1.9 ng equivalents/ml occurred 1-3
hours after dosing. The terminal half-life was 22 hours. Around 75-91%
of the dose was excreted in the urine and 6-15% in the faeces. When
the horses were killed 3 or 12 hours and 9, 12 or 28 days after
dosing, the highest levels of radioactivity were found in the liver
and kidney. Levels in fat were extremely low (Johnston & Dunsire,
1993).
2.1.2 Biotransformation
The biotransformation of clenbuterol in rats, rabbits and dogs is
complex. Five urinary metabolites have been identified in dogs, and
eight in the rat and rabbit. Unchanged clenbuterol is the major
compound found in the urine of these species, and a range of oxidative
and conjugated metabolites are formed. Of these, 4-amino-3,5-dichloro-
mandelic acid, 3-amino-3,5-dichlorobenzoic acid and 4-amino-3,5-dichlo-
rohippuric acid are the major components. In the baboon, unchanged
parent drug is the major urinary component (18%), again with a range
of metabolites (Zimmer, 1971, 1974b; Johnston & Jenner, 1976; Schmid,
1982; Schmid & Prox, 1986).
In cattle, the major compound found in urine (28-52%) and in the
liver (50-80%) after administration of clenbuterol was the parent
compound. Small amounts of 4-amino-3,5-dichlorobenzoic acid were found
in urine and liver, and 4-amino-3,5-dichlorohippuric acid was detected
in urine (Baillie et al., 1980; Hawkins et al., 1993a). In
muscle, the main component was parent compound (Schmid, 1990a).
In horses also, parent clenbuterol was the major compound found
in urine (30-50%). It was also the major component in liver and kidney
(Zimmer, 1977; Hawkins et al., 1984; Johnston & Dunsire, 1993). A
study to examine specifically the nature of the metabolites in horse
liver confirmed clenbuterol as the major component, but with a smaller
quantity of a metabolite identified as 1-(4-amino-3,5-dichlorophenyl)-
1,2-ethanediol (Hawkins et al., 1993b).
None of the studies with any species revealed evidence of
significant covalent binding of clenbuterol or its metabolites.
Overall, the data suggest that the metabolism of clenbuterol is
similar in all the species studied and that the major differences are
quantitative rather than qualitative. The proposed metabolic pathways
are shown in figure 1.
2.1.3 Kinetics in humans
Following a single oral dose of 20 µg 14C-clenbuterol hydro-
chloride to volunteers, 67% of the administered dose was excreted in
the urine; faecal excretion was very low. The major compound present
in the urine was unchanged parent drug. The metabolic profile was
similar to that noted in animals (Zimmer, 1974c).
When a single dose of 20 µg 14C-clenbuterol hydrochloride was
administered orally to volunteers, the highest plasma level was 0.11
µg/litre, with a tmax, of 2-3 hours. Around 87% of the administered
radioactivity was detected in the urine; correspondingly lower levels
of radioactivity were found in the faeces.
In a repeat-dose phase of the same study, volunteers were given
40 µg 14C-clenbuterol for 2 days and then 20 µg for a further 2
days. Around 75% of the dose was detected in the urine. The metabolite
profile was similar to that noted in animals, and the major urinary
component was unchanged parent drug; the terminal half-life was around
34 hours (Zimmer, 1976).
Oral doses of 80 µg clenbuterol hydrochloride were given to 12
pregnant women in premature labour. These were followed 12 hours later
by 2 oral doses of 40 µg, and then by maintenance doses of 20 µg,
generally at 12-hour intervals. Blood samples were taken on days 2, 4,
6 and 8 of study, 3 hours after the morning dose. A steady-state
average concentration of 0.298 µg/litre was achieved, with a minimum
of 0.28 and a maximum of 0.344 µg/litre. Bioavailability was > 80%
(Rominger et al., 1987).
There are no data on in vivo percutaneous absorption of
clenbuterol in humans. However, data using a model which utilizes
excised human skin (Rohr et al., undated) suggests that some
absorption can occur (Boehringer, 1991b).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies after administration of
clenbuterol are summarized in Table 1.
Signs of toxicity in mice, rats and rabbits included lethargy,
increased heart rates, and tonic-clonic convulsions. After intravenous
dosing, death, when it occurred, did so either during injection or
immediately after it. In dogs, all the oral doses employed
(400-800 mg/kg bw) caused increases in heart rate. Salivation,
lethargy and tonic-clonic convulsions also occurred.
In cattle, single intravenous doses of up to 3.6 µg/kg bw
resulted in increases in heart and respiratory rates (Johnson, 1987).
Single oral doses of 2.4-12.0 µg/kg bw in horses resulted in sweating
and elevated heart rates and body temperatures at all dose levels
(Hamm & Erichsen, 1989).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Mice
Following a single intravenous dose of 5 mg/kg bw 14C-clenbu-
terol to pregnant mice, autoradiography demonstrated high levels of
radioactivity in the placenta with lower levels in the fetuses within
30 minutes after administration. Radioactivity was also found in the
fetal liver, thymus and spinal column. Total radioactivity in the
fetuses was significantly less than in maternal animals (Kopitar,
1969).
2.1.1.2 Rats
After oral administration of 2 mg/kg bw 14C-clenbuterol to
rats, approximately 60% of the radioactivity was found in the 48-hour
urine sample suggesting good absorption from the gastrointestinal
system. A further 20% was recovered from faeces over this time. Around
5-10% was recovered in urine and faeces in the 48-72 hour period.
Approximately 7% appeared in the bile. Levels in tissues were
relatively low, except for the liver, kidneys and lungs (Kopitar,
1970).
In a separate study using autoradiography, maximum distribution
after oral doses of 5 or 10 mg/kg bw occurred approximately 3 hours
after administration. Highest concentrations of radioactivity were
found in the lungs, liver, kidneys, pancreas and bone marrow, but some
radioactivity was also found in the brain, adrenal glands, and
skeletal and cardiac muscles (Kopitar, 1969).
When previously untreated rats were given a single oral dose of
5 mg/kg bw clenbuterol by gavage, plasma levels of the drug were
found to be 3-5 times lower than in rats pre-treated with the drug at
5 mg/kg bw per day for 6 months. The single dose was found to abolish
gastrointestinal peristalsis in the rat but after 6 months of compound
administration, peristalsis returned. This may explain the lower, but
prolonged plasma levels after the single administration, which
probably led to a depot effect in the gut (Kopitar & Zimmer, 1973).
Clenbuterol readily enters the placenta in the pregnant rat. At 3
hours after an intravenous or oral dose of 14C clenbuterol, there
was more radioactivity in the placenta than in the blood and tissues
of maternal animals or fetuses (Richter, 1982).
2.1.1.3 Dogs
When beagle dogs were given capsules containing 14C-clen-
buterol at a dose of 2.5 mg/kg bw, maximum blood and plasma levels
occurred 8 hours after dosing. Around 85% of the dose was recovered in
the urine after 96 hours, with 4-9% in the corresponding faecal
samples. Pretreating dogs for 5 weeks with 2.5 mg/kg bw per day
clenbuterol led to a slight increase in the rate of absorption in a
manner similar to that noted in rats (Zimmer, 1974a).
After single oral doses of 2.5 mg/kg bw, blood concentrations in
the dog were approximately twice those of rabbits given identical
doses (Zimmer, 1974b).
Clenbuterol was found to cross the placenta and enter the fetuses
in the dog when a single pregnant female was given an oral dose of
2.5 mg/kg bw 14C-clenbuterol. At 4 hours after administration the
concentration of radioactivity in fetal plasma was around 16% of that
in maternal plasma. Approximately 0.4% of the total dose administered
to the maternal animal was recovered in the fetuses at 4 hours after
administration (Rominger & Schrank, 1982).
2.1.1.4 Monkeys
After intravenous administration of 100 µg 14C-clenbuterol per
animal (approximately 30 µg/kg bw) to cynomolgus monkeys, around 60%
of the dose was recovered in the urine and 4% in the faeces within 72
hours of administration. There was biphasic elimination. It was not
possible to calculate the half-life for the first and rapid phase, but
the second phase half-life was 20-30 hours (Chasseaud et al.,
1978).
2.1.1.5 Baboons
Following a single intravenous dose of 2.5 mg/kg bw 14C-clen
buterol to male baboons, around 82% of the administered dose was
recovered in the urine and faeces within 5 days, with the majority of
this (72%) within 48 hours. Of the 82%, approximately 68% was recovered
in the urine and 14% in the faeces. A similar pattern was noted in
baboons given the drug orally. There was a rapid distribution phase and
the highest levels of radioactivity were found in the lungs, liver and
kidneys (Schmid, 1982).
Placental transfer of 14C-clenbuterol was investigated in a
single pregnant baboon given 3.3 mg/kg bw intravenously. The animal
was killed 3.5 hours after dosing, and around 1.5% of the total
administered close was found in the fetus (Schmid, 1980).
2.1.1.6 Cattle
In cattle given 0.8 µg/kg bw 14C-clenbuterol using a single
intramuscular injection, the liver was found to have the highest
concentrations of radioactivity. In muscle, soon after administration,
the radioactivity was due almost entirely to parent compound (Schmid,
1990a).
In a pilot study with a single cow given 0.8 µg/kg bw 14C-
clenbuterol orally, the first phase plasma elimination half-life
was 0.1 hours and the second phase elimination half-life 17 hours. The
maximum plasma concentration was 0.4 µg/ml with tmax at 8.5 hours.
Approximately 81% of the dose was excreted in the urine with 13% in
the faeces over the 96-hour period after dosing; around 1% was
excreted in the milk (Schmid & Zimmer, 1977a). Similar results were
noted after intramuscular (Schmid & Zimmer, 1977b) and intravenous
(Schmid, 1977) administration, but with the latter route plasma levels
rose much more rapidly. In both cases, radioactivity was found in the
faeces suggesting biliary excretion.
When calves were given 0.8 µg/kg bw 14C-clenbuterol intramus-
cularly, twice a day for 2 days and then orally twice a day for 3 days
in a consecutive manner, using a combination product also containing
trimethoprim and sulfadiazine, around 64% of the dose was excreted
during 15 days in urine and 5% in the faeces. The highest level of
radioactivity was found in injection site muscle, liver and kidney
(Hawkins et al., 1985a). Similar results were seen in another study
where cattle were given intramuscular injections of 14C-clenbuterol
(Cameron & Phillips, 1987a).
In cattle given 21 intramuscular doses of 14C-clenbuterol at a
dose level of 0.8 µg/kg bw per day, twice daily, the majority of the
dose was recovered in the urine. The plasma half-life following the
last dose was of the order of 4 days. Highest levels of radioactivity
were found in liver and kidney, with much lower levels in muscle and
fat (Hawkins et al., 1993a).
After administration of 7 µg/kg bw 14C-clenbuterol by the
intramuscular route to a pregnant cow, radioactivity crossed the
placenta and was found in fetal tissues, including skeletal tissues,
kidneys and liver, when the animal was killed 3 hours after the
dosing. Radioactivity was also found in the maternal and fetal eyes
(Baillie et al., 1980).
When lactating cattle were given oral or intramuscular doses of
14C-clenbuterol at a dose of 0.8 µg/kg bw, radioactivity was found
in the urine for 48-70 hours after administration. In the first 48
hours, the majority of the radioactivity in milk was attributable to
parent compound (Schmid, 1990b).
Lactating dairy cows treated with 0.8 µg/kg bw 14C-clen-
buterol, twice daily by the intramuscular route for 3 consecutive days,
and twice daily by the oral route for a further 2 days followed by a
single oral dose on day 6, were found to have milk concentrations in
the range of 0.76-1.03 µg/litre 12 hours after the first dose rising
to 2.14-2.6 µg/litre after 48 hours. They remained in the range 1.46
to 3.93 µg/litre throughout the dosing period. Highest tissue concen-
trations were at the injection site, liver and kidney (Hawkins et al.,
1985b). Similar results were obtained in another study where dairy
cattle were given oral, intravenous or intramuscular doses of 0.8 µg/kg
14C-clenbuterol. In this study approximately 2% of administered
radioactivity was found in milk (Cameron & Phillips, 1987b).
2.1.1.7 Horses
After administration of a single oral dose of 0.8 µg/kg bw
14C-clenbuterol to a horse, the maximum plasma concentration of
0.4 µg/ml was reached at approximately 2 hours. The elimination
half-life was 21 hours. After 144-168 hours, 81% of the administered
dose was recovered in urine. Following intravenous administration, 82%
of the administered dose was found in urine, with around 9% in faeces,
suggesting biliary excretion (Zimmer, 1977).
In a study with 10 horses, animals were given a single oral dose
of 0.8 µg/kg bw clenbuterol followed by a single intravenous dose 3
weeks later. A second group of 10 horses was treated in a similar
manner, but initially using the intravenous route with the oral dose
after 3 weeks. Approximately 20-50% of the oral dose (mean 36%) as
parent drug, was excreted over 108 hours in the urine, while,
following intravenous administration, some 17-70% of the dose
(mean 43%) was excreted in the 108-hour urine (Zupan, 1985).
In a study with a clenbuterol, trimethoprim and sulfadiazine
combination product, horses were given gelatin capsules containing
14C-clenbuterol at a dose of 0.8 µg/kg bw, twice daily for 10 days,
with a single oral dose on day 11. The majority of the dose (70-77%)
was excreted in the urine and 10-15% in the faeces. The maximum plasma
concentration was achieved 3-4 hours after the first administration
and the plasma half-life was 9-10 hours. The mean plasma concentration
was 0.6-1.2 µg/ml. Highest levels of radioactivity were found in the
liver and kidneys with very little in fat (Hawkins et al., 1984).
Similarly, when 12 horses were given 21 twice daily oral doses of
0.8 µg/kg bw 14C-clenbuterol, steady-state plasma levels were
rapidly achieved. Peak levels of 1-1.9 ng equivalents/ml occurred 1-3
hours after dosing. The terminal half-life was 22 hours. Around 75-91%
of the dose was excreted in the urine and 6-15% in the faeces. When
the horses were killed 3 or 12 hours and 9, 12 or 28 days after
dosing, the highest levels of radioactivity were found in the liver
and kidney. Levels in fat were extremely low (Johnston & Dunsire,
1993).
2.1.2 Biotransformation
The biotransformation of clenbuterol in rats, rabbits and dogs is
complex. Five urinary metabolites have been identified in dogs, and
eight in the rat and rabbit. Unchanged clenbuterol is the major
compound found in the urine of these species, and a range of oxidative
and conjugated metabolites are formed. Of these, 4-amino-3,5-dichloro-
mandelic acid, 3-amino-3,5-dichlorobenzoic acid and 4-amino-3,5-dichlo-
rohippuric acid are the major components. In the baboon, unchanged
parent drug is the major urinary component (18%), again with a range
of metabolites (Zimmer, 1971, 1974b; Johnston & Jenner, 1976; Schmid,
1982; Schmid & Prox, 1986).
In cattle, the major compound found in urine (28-52%) and in the
liver (50-80%) after administration of clenbuterol was the parent
compound. Small amounts of 4-amino-3,5-dichlorobenzoic acid were found
in urine and liver, and 4-amino-3,5-dichlorohippuric acid was detected
in urine (Baillie et al., 1980; Hawkins et al., 1993a). In
muscle, the main component was parent compound (Schmid, 1990a).
In horses also, parent clenbuterol was the major compound found
in urine (30-50%). It was also the major component in liver and kidney
(Zimmer, 1977; Hawkins et al., 1984; Johnston & Dunsire, 1993). A
study to examine specifically the nature of the metabolites in horse
liver confirmed clenbuterol as the major component, but with a smaller
quantity of a metabolite identified as 1-(4-amino-3,5-dichlorophenyl)-
1,2-ethanediol (Hawkins et al., 1993b).
None of the studies with any species revealed evidence of
significant covalent binding of clenbuterol or its metabolites.
Overall, the data suggest that the metabolism of clenbuterol is
similar in all the species studied and that the major differences are
quantitative rather than qualitative. The proposed metabolic pathways
are shown in figure 1.
2.1.3 Kinetics in humans
Following a single oral dose of 20 µg 14C-clenbuterol hydro-
chloride to volunteers, 67% of the administered dose was excreted in
the urine; faecal excretion was very low. The major compound present
in the urine was unchanged parent drug. The metabolic profile was
similar to that noted in animals (Zimmer, 1974c).
When a single dose of 20 µg 14C-clenbuterol hydrochloride was
administered orally to volunteers, the highest plasma level was 0.11
µg/litre, with a tmax, of 2-3 hours. Around 87% of the administered
radioactivity was detected in the urine; correspondingly lower levels
of radioactivity were found in the faeces.
In a repeat-dose phase of the same study, volunteers were given
40 µg 14C-clenbuterol for 2 days and then 20 µg for a further 2
days. Around 75% of the dose was detected in the urine. The metabolite
profile was similar to that noted in animals, and the major urinary
component was unchanged parent drug; the terminal half-life was around
34 hours (Zimmer, 1976).
Oral doses of 80 µg clenbuterol hydrochloride were given to 12
pregnant women in premature labour. These were followed 12 hours later
by 2 oral doses of 40 µg, and then by maintenance doses of 20 µg,
generally at 12-hour intervals. Blood samples were taken on days 2, 4,
6 and 8 of study, 3 hours after the morning dose. A steady-state
average concentration of 0.298 µg/litre was achieved, with a minimum
of 0.28 and a maximum of 0.344 µg/litre. Bioavailability was > 80%
(Rominger et al., 1987).
There are no data on in vivo percutaneous absorption of
clenbuterol in humans. However, data using a model which utilizes
excised human skin (Rohr et al., undated) suggests that some
absorption can occur (Boehringer, 1991b).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies after administration of
clenbuterol are summarized in Table 1.
Signs of toxicity in mice, rats and rabbits included lethargy,
increased heart rates, and tonic-clonic convulsions. After intravenous
dosing, death, when it occurred, did so either during injection or
immediately after it. In dogs, all the oral doses employed
(400-800 mg/kg bw) caused increases in heart rate. Salivation,
lethargy and tonic-clonic convulsions also occurred.
In cattle, single intravenous doses of up to 3.6 µg/kg bw
resulted in increases in heart and respiratory rates (Johnson, 1987).
Single oral doses of 2.4-12.0 µg/kg bw in horses resulted in sweating
and elevated heart rates and body temperatures at all dose levels
(Hamm & Erichsen, 1989).
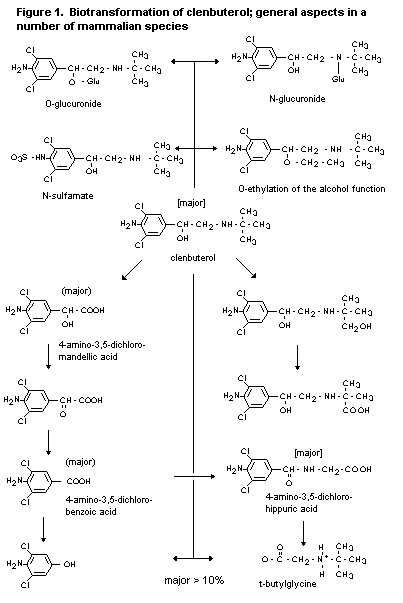 Table 1. Acute toxicity of clenbuterol
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M oral 80 Nishikawa et al., 1972
F oral 133 Nishikawa et al., 1972
M & F s.c 72 Nishikawa et al., 1972
M & F i.v 42 Nishikawa et al., 1972
M i.p 46 Nishikawa el al., 1972
M i.p 72 Nishikawa et al., 1972
Rat M & F oral 175 Nishikawa et al., 1972
M oral 82 Nishikawa et al., 1979
F oral 170 Nishikawa et al., 1979
M & F i.v 30 Nishikawa et al., 1972
M i.v 35 Nishikawa et al., 1979
F i.v 39 Nishikawa et al., 1979
M & F s.c 170 Nishikawa et al., 1972
M & F i.p 70 Nishikawa et al., 1972
M & F oral1 > 0.125 James, 1990a
Rabbit M & F i.v 84 Nishikawa et al., 1972
M & F dermal1 > 0.05 James, 1990b
Dog M & F oral 400-800 Pappritz, 1968
M & F i.v 45-52 Pappritz, 1972
1 Administered as a proprietary veterinary medicinal product
containing 25 µg clenbuterol per ml
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 15 male and 15 female ICR-JCL mice were given daily
oral doses of 0, 2.5, 12.5 or 62.5 mg/kg bw clenbuterol hydrochloride
as an aqueous solution, by stomach tube, for 30 days. Body weight gain
and food consumption were increased in all treated groups in a dose-
related manner. There were no adverse effects on haematology. The
animals from the highest dose group appeared weak and 6 animals died
during the course of the study.
At necropsy, there were significant increases in liver weights in
animals given 12.5 or 62.5 mg/kg bw per day. Ischemic heart damage was
noted in one animal given 12.5 mg/kg bw per day, and in two animals
from the highest dose group. The NOEL in this study was 2.5 mg/kg bw
per day (Kast, 1973a).
2.2.2.2 Rats
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily oral doses of 0, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by stomach tube, for 30 days. Signs
of toxicity were seen at the highest dose and these included
salivation, encrusted eyes and noses, and reduced body weight gain and
food consumption; 50% of these animal died during the course of the
study. Serum aspartate aminotransferase and glucose concentrations
were significantly reduced in animals of both sexes given 10 or
100 mg/kg bw per day.
Liver weights were significantly reduced in males given the
highest dose, while ischaemic lesions were seen in cardiac muscle in
one male and one female given this dose. The NOEL in this study was
1 mg/kg bw per day based on blood chemistry findings (Kast &
Tsunenari, 1973a).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given doses of 0, 1, 5, 25 or 75 mg/kg bw per day clenbuterol
hydrochloride in the feed for 6 months. At the highest dose level,
body weight gain and food consumption were reduced. During the first
week, a reduction in water consumption occurred in the animals given
this dose, and nine male and five female animals died during the
course of the study.
At necropsy, ischaemic lesions of the myocardium were noted
in all treated animals and there was a dose-related incidence and
severity. These lesions were also found in another group given
75 mg/kg bw per day clenbuterol hydrochloride and then allowed a
4-week recovery period without drug treatment. A NOEL was not
identified in this study (Kast & Tsunenari, 1973b).
Groups of 20 male and 20 female FW49/Biberach rats were fed diets
containing clenbuterol hydrochloride for 18 months. Further groups of
three male and three female rats were fed treated diets for 14 days,
followed by a recovery period; these animals were the subject of ECG
investigations. Another group of 10 female and 10 male rats was fed
the highest dietary level and then maintained for a 6-week recovery
period. The overall design of the study, including doses, is set out
below.
Dose (mg/kg bw 18-month phase 14-day ECG study2
per day) number of animals numbers of animals
males females males females
0 20 20 3 3
0.1 24 20 3 3
0.5 20 20 3 3
2.5 20 20 3 3
2.5 101 101 - -
1 subject to 6 week recovery period
2 subject to 21 day recovery period
Body weight gain in treated groups was slightly increased when
compared with control values. However, there were no overt signs of
toxicity, and no deaths occurred. There were no significant haemato-
logical effects. However, there were reductions in myocardial glycogen,
skeletal muscle glycogen and liver glycogen values in all treated
groups, but there was no clear dose response. Histochemical examination
of the myocardium showed a reduction in enzyme activity, particularly
in the lowest dose group.
The ECG examination revealed a bradycardia rather than the
expected tachycardia, but no explanation was provided for this. Heart
rates returned to normal 2 days into the recovery period. As the
results of this study appear to conflict with those of other studies
(reduction in heart rate rather than the expected increase), and as
the hearts were not adequately examined macroscopically or micro-
scopically, an NOEL cannot be identified from this study (Serbedija &
Bauer, 1973).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily intravenous doses of 0, 1, 4 or 16 mg/kg bw clenbuterol
hydrochloride for 30 days. Overt signs of toxicity included weakness
at the two highest dose levels and increased respiratory and heart
rates at the highest dose level. One female and one male in the 1 and
4 mg/kg bw per day dose groups respectively, died, while five male and
five female animals died at the highest dose. There was evidence of
hepatotoxicity (focal necrosis) in rats given the highest dose. A NOEL
was not identified in this study (Kast & Tsunenari, 1973c).
Groups of 30 male and 30 female Chbb: THOM (SPF) rats were
exposed by the inhalation route to 0 or 0.667 mg/m3 clenbuterol
hydrochloride in aqueous solution. A further two groups of 15 male and
15 female rats were exposed to 5.013 or 40.0 mg/m3. The exposures
were conducted for 0.5-2 hours per day, 7 days per week, for 13 weeks.
These exposures gave corresponding doses of 0, 0.01, 0.16 and
2.58 mg/kg bw per day. Groups of 10 male and 10 female control
animals, and 10 male and 10 female rats exposed to the highest
concentration were subjected to a 6-week recovery period without
further exposure to clenbuterol hydrochloride.
There was no drug-related mortality during the study. Increased
heart rates were noted in all treated rats. Animals exposed to the
highest concentration of clenbuterol hydrochloride showed myocardial
necrosis. Myocardial changes were noted in animals subject to the
6-week recovery period. As increases in heart rate were noted at all
concentrations, a NOEL was not identified in this study (Koellmer
et al., 1977).
Groups of 20 male and 20 female Charles River CD rats were
exposed to aerosols of clenbuterol hydrochloride in ethanol with
dichlorofluoromethane and 1,2-dichlorotetrafluoroethane as
propellants, using head-only exposure. Other groups served as controls
or ethanol/propellant controls. The doses were equivalent to up to
118 µg/kg bw per day.
The only treatment-related effects attributable to clenbuterol
hydrochloride were liver weight reduction and small aggregations of
alveolar macrophages, which were clearly dose-related (Clark et al.,
1979).
However, there were insufficient data on dosimetry to identify a
NOEL and in addition, there were no measurements of heart-rate or
other cardiac function parameters.
2.2.2.3 Dogs
Groups of three male and three female beagle dogs were given
starch capsules containing clenbuterol hydrochloride at doses of
0, 0.4, 4 and 20 mg/kg bw per day, daily for 13 weeks. The highest
dose was increased to 30 mg/kg bw per day from week 5 and to 40 mg/kg
bw per day from week 9. Water consumption was normal during the study,
but a slight decrease in appetite was noted in high-dose animals when
the dose was increased to 40 mg/kg bw per day. The rate of body weight
gain significantly fell from this point. One male and two females
given the highest dose appeared sedated after treatment. One of these
became increasingly agitated and breathless and died 2-3 minutes after
receiving the 23rd dose of 20 mg/kg bw per day. Tachycardia occurred
after the first dose in all treated animals and this persisted until
the following day. There was a decrease in its severity during
subsequent weeks, but it continued to be noted in all treated animals.
A shortening of the P-Q, Q-T and T-P intervals in the ECG,
corresponding to the increased heart rates, was seen in all treated
dogs. The dog that died was found to have a severe acute pulmonary
infection. Myocardial haemorrhage and necrosis, damage to the hepatic
parenchyma and severe haemorrhagic bronchopneumonia were noted. No
abnormalities occurred in any of the other animals. As dose-related
tachycardia occurred at all doses, a NOEL was not identified in this
study (Leuschner et al., 1969).
Groups of three male and three female beagle dogs were given oral
doses of 0, 2.5 and 40 mg/kg bw per day clenbuterol hydrochloride in
gelatin capsules for 13 weeks. No animals died during the study, but
in the first week, all dogs given clenbuterol hydrochloride were
apathetic, had redness of the skin and eyes, mydriasis and fixed
pupils. Tachycardia was seen in all dogs. At 2.5 and 40 mg/kg bw per
day, significant increases in the activities of several serum enzymes,
including LDH and creatinine kinase, were found. The glycogen levels
of the left heart muscle were decreased. Myocardial necrosis was seen
in dogs given the 2.5 and 40 mg/kg bw per day doses. A NOEL was not
identified in this study (Pappritz & Bauer, 1973a).
Groups of three male and three female beagle dogs were given
gelatin capsules containing daily doses of 0, 0.1 or 0.5 mg/kg bw per
day clenbuterol hydrochloride for 1 year. A group of six male and six
female dogs were given 2.5 mg/kg bw per day in the same manner, and
three animals of each sex were maintained on a control diet for 6
weeks at the end of the study, as part of a recovery phase. No animals
died during the study. In the first week all drug-treated animals were
apathetic and had diffuse reddening of the skin. Tachycardia was noted
in all treated animals, as was mydriasis, episcleral vascular
injection and conjunctivitis.
There were no significant effects on food consumption or body
weights, although dogs given the highest dose gained slightly more
weight than did those in other groups. No haematological effects were
seen, but there were increases in some serum enzymes in treated
groups. Liver glycogen was decreased in dogs given the highest dose
and this remained decreased at the end of the 6-week recovery period.
Heart weights were increased in all the treated animals when
compared with controls, and myocardial necrosis was noted in the
papillary muscle of the left ventricle of all these dogs, including
those subject to the 6-week recovery period. There was no dose
relationship in the cardiotoxicity seen, but histochemical methods
revealed smaller decreases in the activities of several enzymes in
cardiac muscle of the 0.1 mg/kg bw per day group than in the other
treatment groups. A NOEL was not identified in this study
(Pappritz & Bauer, 1973b).
Groups of three male and three female beagle dogs were given
daily intravenous injections of 0, 1, 10 or 1000 µg/kg bw per day
clenbuterol hydrochloride for 30 days. Dogs given the highest dose of
clenbuterol hydrochloride were lethargic and all treated animals
developed tachycardia with associated decreases in the P-Q and Q-T
intervals in the ECG. At necropsy, one male given the highest dose was
found to have myocardial necrosis. A NOEL was not identified in this
study (Pappritz et al., 1973).
2.2.2.4 Monkeys
Groups of three male and three female cynomolgus monkeys were
exposed to atmospheric levels of clenbuterol hydrochloride as an
aerosol in ethanol, with dichlorofluoromethane and 1,2-dichlorotetra-
fluoroethane as propellants, using an oropharyngeal tube, giving doses
of 25, 50 and 150 µg/kg bw per day for 26 weeks. Half of the daily
metered dose was given each morning and the remainder in the afternoon.
Another group of three male and three female monkeys served as sham
controls with no exposure, while a further group of three male and
three female monkeys served as placebo controls and were exposed to
the same aerosol as treated animals, but without the clenbuterol
hydrochloride.
No signs of toxicity were seen in this study and food consumption
and body weights were normal except in high-dose females where a large
body weight gain occurred. Ophthalmoscopy, haematology, lung mechanics
and ventilation, blood chemistry and urinalyses were normal. A low
oxygen tension was noted in high-dose animals in week 10 of the study.
There were no drug-related changes in heart rate or ECGs. At macro-
scopic and histo-pathological examination, there were no drug-related
changes (Collins et al., 1979).
Thus, the NOEL in this study was of the order of 25 µg/kg bw per
day by the inhalation route, but this could not be identified as a
definitive study because of the problems in dosimetry in an inhalation
study of this type. For example, there were no data on how much drug
reached the lower respiratory tract and no information regarding blood
levels. Hence, the degree of systemic exposure was unknown.
2.2.2.5 Cattle
In calves given the therapeutic dose of 0.8 µg/kg bw per day
clenbuterol hydrochloride for 10 days by the oral or intravenous
routes, only transient tachycardia occurred. When given at 5 times the
therapeutic dose (4 µg/kg bw per day) for 10 days, tachycardia and
audibly increased heart force were the only effects noted. When given
to adult cattle at the therapeutic dose or twice the therapeutic dose
for periods of up to 9 days, by the oral route, the main effects were
tachycardia and falls in diastolic blood pressure (Fenner, 1982;
Cameron et al., 1992).
2.2.2.6 Horses
No clinically significant adverse effects were noted in horses
given 0.8-17.5 µg/kg bw per day clenbuterol hydrochloride by the oral
route for periods of up to 64 days. Transitory effects included
tachycardia, sweating, muscle tremors, hyperglycaemia and hypophos-
phataemia (Erichsen, 1988, 1989; Hamm & Erichsen, 1989; Owen et
al., 1990).
Clenbuterol, like other ß-agonists, leads to tachycardia and
hypotension. This probably results in reduced myocardial perfusion at
a time when oxygen demand is high because of increased cardiac rate.
The end result is hypoxia which probably leads to the necrotic lesions
seen in the left ventricular papillary muscles (Rosenblum et al.,
1965; De Busk & Harrison, 1969; Poynter & Spurling, 1971; Roberts &
Cohen, 1972; Magnusson & Hansson, 1973; Balazs & Bloom, 1982). This
explains the cardiac effects noted in the repeated dose (and other)
studies with clenbuterol.
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female (C57B/6 × DBA/2) mice were given
clenbuterol in the drinking-water at doses of 0.1, 1.0 and 25.0 mg/kg
bw per day for 2 years. Groups of 100 males and 100 females were given
drinking-water only over this period and served as controls. Treated
animals consumed slightly more food and water than controls, but showed
no overt signs of toxicity, except for a dose-related increase in body
weight in the first year and a decrease during the second year.
Survival after 2 years was good in males (78-86%) and acceptable in
females (54-60%).
At termination, there were significant changes in the weights of
a number of organs when compared with control values. These included
significant increases in the absolute heart weights of males given
1 mg/kg bw per day and in females given 25 mg/kg bw per day. There
were significant increases in relative heart weights in all treated
male and female mice. The incidences of non-neoplastic lesions,
including myocardial lesions, were not treatment related. There was no
increased incidence of any rumour type in treated mice when compared
with control values (Umemoto, 1984).
2.2.3.2 Rats
Groups of 48 male and 48 female Chbb:THOM rats were fed diets
containing clenbuterol hydrochloride, resulting in daily doses of
6.25, 12.5, or 25.0 mg/kg bw per day for 2 years. A further group of
72 rats of each sex was fed a diet containing no clenbuterol hydro-
chloride. As ß-sympathomiroetic agents are known to induce mesovarian
leiomyomas in some strains of rat, groups of 72 male and 72 female
Charles River Sprague-Dawley rats were also fed diets containing
clenbuterol hydrochloride at doses of 0 or 25 mg/kg bw per day for 2
years. After one control rat began to show signs of clenbuterol-related
effects, contamination of the feed preparation personnel and equipment
was confirmed, and the substance was then administered via the
drinking-water to ensure the same daily doses.
Treated rats appeared nervous, tense and aggressive, but no
effects on mortality were seen. Survival was in the range of 70-87% in
males and 50-71% in females. In both sexes and strains body weight was
reduced in a dose-related manner. Consumption of drinking-water was
significantly reduced in SD rats given the highest dose. At
termination, fibrosis of the subendocardial connective tissue was
frequently observed in all groups including controls, but the
incidence was higher in the Chbb:THOM rats, suggesting a drug-
related effect.
There was an increased incidence of mesovarian leiomyomas in the
female Sprague-Dawley rats, but not in the Chbb:THOM rats. The
incidence is shown in Table 2.
Table 2. Incidence of mesovarian leiomyomas in female rats treated with clenbuterol
hydrochloride for 2 years
Chbb:THOM Sprague-Dawley
Rats rats
Dose 0 6.25 12.5 25.0 0 25.0
(mg/kg bw per day)
Numbers with 0/72 0/48 0/48 0/72 0/72 11/72
Mesovarian
leiomyomas
per group
There was no increased incidence of any other tumour type
(Serbedija et al., 1982).
Leiomyomas of the smooth muscle of the uterus in mice and
mesovaria of rats have been reported following long-term treatment
with ß-agonists (Nelson & Kelly, 1971; Jack et al., 1983;
Amemiya et al., 1984; Gibson et al., 1987; Gopinath & Gibson,
1987; Sells & Gibson, 1987). They are not associated with genotoxicity
with these agents and are considered to be associated with adrenergic
stimulation. The ß-agonist salbutamol produced mesovarian leiomyomas
in rats, but these could be prevented by administration of the
ß-blocking agent propranolol; a similar phenomenon has been reported
in mice where the induction of leiomyomas of the uterus by medroxalol
was prevented by propranolol (Jack et al., 1983; Gibson et al.,
1987). There have been no reports of increased incidences of leiomyomas
in women following the use of ß-adrenergic agents (Poynter et al.,
1978).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
Groups of 17-20 pregnant female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage, from day 15 of gestation to 21 days
post-partum when dams and pups were sacrificed. The pups, including
those that died before day 21, were necropsied and X-rayed. Maternal
body weights were reduced at the end of the study in animals given
7 mg/kg bw per day clenbuterol hydrochloride. Those given the highest
dose were similar to the controls, but these animals were heavier at
the beginning of the study. Maternal food consumption was reduced in
all treated groups.
The number of pups born dead increased in a dose-related manner,
as did the number of pups dying after birth. At the highest dose all
the pups died, as shown below.
Body weights of pups were also reduced in a dose-related manner.
A NOEL was not identified in this study (Lehman, 1974).
Groups of 30 male and 30 female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water by gavage. Treatment of the male rats commenced 10
weeks prior to mating while that of females began 2 weeks prior to
mating. On day 14 of gestation 50% of the females were killed and the
uterine contents examined. The remaining female rats were allowed to
deliver normally and rear their pups until weaning. Dosing of all
females continued until they were killed on day 14 of gestation or
after weaning.
To investigate the cause of the high pup mortality in the study
of Lehman (1974), the litters of six control dams were exchanged with
litters from dams given 50 mg/kg bw per day. Surviving dams and pups
were necropsied at the end of the lactation period. Food consumption
was increased in treated animals when compared with control values.
However, body weight was significantly reduced in animals of both
sexes given the highest daily doses.
Pup weights at birth were reduced in all treated groups. There
was a dose-related decrease in the numbers of viable pups which was
statistically significant at 7 and 50 mg/kg bw per day. All the pups
in the 50 mg/kg bw per day group died on the first day of lactation
regardless of whether or not they were suckled by treated or control
dams. The majority of pups from control dams suckled by dams that were
given the 50 mg/kg bw per day dose survived.
There were no substance-related effects on fertility, corpora
lutea, implantation or resorption rates, and no malformations were
noted in pups from treated rats. The hearts of three pups from
high-dose females were examined histologically, but no abnormalities
were found (Lehman, 1975).
Groups of 34 male and 34 female Chbb:THOM rats were given daily
oral doses of 0, 1.5, 7.5 or 15 µg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage. The male rats were
treated for 70 days prior to mating and the females from 14 days prior
to mating up until the end of gestation. The pups were not treated.
Dosing of the females continued until the interim sacrifice or after
weaning.
On days 13-15 of gestation, 50 pregnant rats were killed and the
uterine contents examined. The remainder were allowed to give birth
naturally and the pups reared until 3 weeks old, except for two males
and two females in each litter which were reared until 10 weeks after
mating when certain behavioural tests were conducted (pupillary
reflex, photo-phobia response, hearing, behaviour on a rotating rod,
behaviour in a Y maze). The animals were then mated and allowed to
litter naturally.
There were no effects on fertility attributable to clenbuterol
treatment, and gestation length, numbers of corpora lutea,
implantation rates, incidences of resorptions, parental body
weights and food consumption were unaffected.
No compound-related effects were seen in the littering phase and
the pups gained weight normally. Performance in the behavioural tests
were similar in treated and control groups and the offspring of these
animals were normal in all respects. The NOEL was 15 µg/kg bw per day
(Lehman, 1980).
Similar results were obtained in an almost identical study on
rats using the same dose levels. Again, the NOEL was 15 µg/kg bw per
day (Lehman, 1981).
No teratogenic effects were seen in any of these reproductive
toxicity studies.
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 mated female SPF-FW 49 Biberach rats were given oral
doses of 0, 0.04, 0.2 or 1 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage on days 6-15 of gestation. No evidence
of maternal toxicity was seen and there were no compound-related
effects on the incidences of resorptions, or on litter and fetal
weights. There were no increased incidences of any type of
malformation. The NOEL was 1 mg/kg bw per day (Lehman, 1969a).
Groups of 25 mated female Sprague-Dawley rats were given oral
doses of 0, 0.01, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage, from days 9 to 14 of
gestation. Approximately 24 hours before the expected delivery date,
20 dams per dose level were killed and the uterine contents examined
while the remaining five pregnant animals in each group were allowed
to deliver naturally.
Overt signs of toxicity, including weakness, hypersensitivity and
bloody vaginal discharge were seen in rats given the highest daily
dose. Maternal body weights were reduced in dams given the 10 or
100 mg/kg bw per day doses. Animals given these doses had signifi-
cantly increased incidences of resorptions with corresponding
reductions in the numbers of viable fetuses. Mean litter weights were
reduced at both these doses, while mean fetal weight was significantly
reduced at the highest dose level.
The incidences of malformations were significantly increased at
the two highest dose levels used. Anomalies included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of the vertebrae (Kast, 1973b).
A study was conducted to verify the results of Kast (1973b).
Groups of 25 mated Sprague-Dawley rats were given 0, 0.01, 1, 10 or
100 mg/kg bw per day clenbuterol hydrochloride in distilled water, by
gavage, from days 8 to 17 of gestation. Approximately 24 hours before
the expected delivery date, 20 dams per group were killed and the
uterine contents examined. The remainder were allowed to deliver
naturally.
Overt signs of toxicity were similar to those in the previous
study and five dams given the highest dose died. The incidences of
resorptions were increased in animals given the highest dose; mean
litter weights and fetal weights were reduced. The incidences of
malformations were significantly increased at the two highest doses.
The types of malformation were similar to those seen in the study of
Kast (1973b).
In pups derived from animals allowed to deliver naturally, body
weights were increased at the highest dose. Swimming function was
retarded in this group. At 10 mg/kg bw per day, two pups were found to
have hydrocephalus while at the highest dose level, four pups were
found to have anophthalmia. No malformations were noted in pups from
the 0.01, 0.1 or 1 mg/kg bw per day groups. The NOEL in both these
studies was 1 mg/kg bw per day (Kast, 1975).
Groups of 20 Sprague-Dawley rats were exposed nose only to an
atmosphere containing clenbuterol hydrochloride during days 6-15 of
gestation. The estimated doses were 0, 19, 39 and 78 µg/kg bw per day.
There was no evidence of maternal toxicity or teratogenicity, but
there were dose-related increases in the incidences of skeletal
variations, indicative of fetotoxicity, at all dose levels (Palmer
et al., 1978).
2.2.5.2 Rabbits
Groups of 15 mated Russian/Biberach rabbits were given daily oral
doses of 0, 30, 100 or 300 µg/kg bw per day clenbuterol in distilled
water, by gavage, from days 6 to 18 of gestation. Three dams given the
300 µg/kg bw per day dose and one given the highest dose died during
the study. These deaths were due to bronchopneumonia. Several rabbits
in each group were found not to be pregnant. Despite these problems,
at least 10 litters from each dose group were available for
examination.
Dams in each dose group showed better weight gains than controls,
but there was no clear dose relationship. There were no adverse
effects on the incidences of resorptions, numbers of live fetuses,
fetal weights or the incidences of malformations. However, there were
increased incidences of delayed ossification, suggestive of
fetotoxicity, in the 100 and 300 µg/kg bw per day groups. The NOEL
was 30 µg/kg bw per day (Lehman, 1969b).
Groups of 10 female Russian/Biberach rabbits were given daily
oral doses of 0, 0.01, 1 or 50 mg/kg bw per day clenbuterol
hydrochloride, in distilled water by gavage, on days 8 to 16 of
gestation. There were no compound-related deaths, but body weight gain
and food intake was reduced at the highest dose level.
At the highest dose level, the incidence of resorptions was
significantly increased, and there was a corresponding decrease in
the numbers of viable fetuses. Mean litter and fetal weights were
significantly reduced at this level. There was an increased incidence
of malformations, notably cleft palate and synostosis, in pups from
dams given 50 mg/kg bw per day clenbuterol hydrochloride. The NOEL was
1 mg/kg bw per day (Kast, 1973c).
Groups of 13-16 mated New Zealand White rabbits were exposed to
doses of clenbuterol hydrochloride calculated to be 0, 48, 146 or
300 µg/kg bw per day by the inhalation route using a fine bore tube
sited over the tracheal opening. The heads of the animals were held in
an air extraction chamber to minimize rebreathing of aerosol. Dosing
took place on days 6-18 of gestation.
Signs of stress were observed in all groups as a result of the
exposure procedures. Signs included anorexia, lethargy and respiratory
distress, and some animals died in all groups, including the controls.
There was a high incidence of malformations in the control groups
which was probably related to the initial stress. There was no
increased incidence in animals given 146 or 300 µg/kg bw per day.
As a result of the high numbers of malformations seen in control
groups, no firm conclusions could be drawn from this study. However,
the data suggested that doses of up to 300 µg/kg bw per day clen-
buterol hydrochloride, when given by inhalation to pregnant rabbits on
days 6-18 of gestation, were not teratogenic (Palmer et al., 1980).
Taken together, the animal data suggested that clenbuterol
hydrochloride is teratogenic in rats and rabbits at relatively high
doses. There was evidence of fetotoxicity. The NOEL for maternal
toxicity and teratogenicity in the rat and rabbit following oral
administration was 1 mg/kg bw per day. However, the NOEL for
fetotoxicity in one study in the rabbit was 0.03 mg/kg bw per day.
2.2.6 Special studies on genotoxicity
Clenbuterol hydrochloride gave negative results in the Ames test
with Salmonella typhimurium strains, in a reversion test with
Escherichia coli strain WP2(P), in a gene mutation assay with
Chinese hamster V79 cells, in an in vivo mouse micronucleus test and
in an in vivo cytogenetic assay with Chinese hamster bone marrow.
In the mouse lymphoma test with L5178Y cells, no increased
incidences in mutation frequency were observed in the absence of
metabolic activation. However, with metabolic activation, one of two
experiments gave positive results at the two highest concentrations
used (Clements, 1992).
Viability counts were reduced at these concentrations and
sampling error may have led to these spurious results (Kirkland, 1992).
In an in vitro assay with human lymphocytes, at concentrations of
> 500 µg/ml, an increased incidence of chromosome aberrations was
noted in the absence (but not in the presence) of metabolic
activation. This was not dose-dependent.
The results are summarized in Table 3.
These data suggest that clenbuterol hydrochloride is not
genotoxic.
2.2.7 Special studies on irritancy
2.2.7.1 Skin irritation
Clenbuterol hydrochloride was applied to the shaved skin of six
Russian/Biberach rabbits, using an occlusive dressing, for a period of
28 days. No evidence of skin irritation was seen (Hewett & Notman,
1984). Similar results were noted in a further study of skin
irritation (James, 1990d).
2.2.7.2 Intramuscular tolerance
Intramuscular injection of 0.5 ml of a formulation containing
clenbuterol hydrochloride into the dorsal area of rabbits produced
only minimal haemorrhage, oedema and necrosis (Kreuzer, 1988).
2.2.7.3 Eye irritation
A formulated commercial product containing clenbuterol
hydrochloride (0.1 ml) was instilled into the left eye of six New
Zealand White rabbits. The right eye served as a control. Mild
irritation was noted and this resolved 48 hours after treatment
(Bailey, 1990).
2.2.8 Special studies on immunotoxicity
Clenbuterol hydrochloride, as a formulated commercial product,
was tested on the guinea-pig using the Buehler technique. The content
of active ingredient was 70.4 µg/ml in the formulation. It was not a
sensitizer in this study (James, 1990c).
Another study employed a 0.2% solution of clenbuterol hydro-
chloride in 30% aqueous ethanol utilizing guinea-pigs and the
Magnusson and Kligman maximization test involving the use of Freund's
adjuvant. No evidence of a sensitizing effect was found (Schuster,
1984).
Table 3. Results of genotoxicity studies on clenbuterol hydrochloride
Test system Test subject Concentration Results Reference
Bacterial Salmonella typhimurium 40-2500 µg/plate - Baumeister,
reversion TA98, TA100, TA 1535, 1978
TA1537, TA1538
Escherichia coli 40-1500 µg/plate
WP2(P)
Bacterial S. typhimurium 10-500 µg/plate - Ellenberger,
reversion TA98, TA 100, TA 1535, 1985
TA1537, TA1538
Forward Chinese hamster V79 10-100 µg/ml - Baumeister,
mutation fibroblasts, HGPRT 1985
assay locus
Forward Mouse lymphoma 300-800 µg/ml - Clements,
mutation L5178Y 1992
assay
In vitro human lymphocytes 177-2352 µg/ml +/- McEnaney,
cytogenetics 1992
In vivo mouse 0.006, 0.5, 5.0 - Friedmann,
micronucleus mg/kg bw per 1982
test day
In vivo Chinese hamster 19, 60, 186 - Holmstrom &
cytogenetics (bone marrow) mg/kg bw per MacGregor,
test day 1986
2.2.9 Special studies on pharmacodynamic effects
2.2.9.1 Studies in animals
Clenbuterol is a ß2-sympathomimetic agent with a wide range of
pharmacodynamic effects. It produces a potent, dose-dependent
bronchiolytic effect, which was demonstrated in guinea-pigs following
acetylcholine, histamine or bradykinin-induced bronchospasm. Similar
effects have been reported in cats and dogs. Clenbuterol exerts
positive chronotropic and inotropic effects on isolated atria. It
induces tachycardia in rats, dogs, cats and a variety of farm animals,
accompanied by reductions in systolic and diastolic blood pressure. In
the isolated uterine horn of the estrus rat, it exerts a pronounced
relaxing effect on serotonin, oxytocin and bradykinin-induced spasm.
It has also been shown to exert a relaxing effect on smooth muscle in
the guinea-pig ileum and to reduce intestinal mobility in the rat and
mouse.
Clenbuterol results in a glycogenolytic effect in the rat
myocardium associated with ß-stimulation. It has no effect on hepatic
glycogen. It causes a potent dose-dependent reduction of the gastric
secretion.
In mice, it causes decreases in spontaneous activity and also
leads to increases in barbiturate-induced sleeping time. The drug
results in dose-dependent striated muscle relaxation. However, it has
no antipyretic or analgesic effects in mice (Engelhardt, A., 1971;
Engelhardt, G., 1971, 1976).
Clenbuterol exerted major relaxing effects on spasms induced by
carbachol in guinea-pig trachea and lung. The effects were antagonised
by propranolol (Landry, 1983). It also led to decreases in perfusion
pressure of hind limb blood vessels, inhibitions of contractions of
the uterus, increases in atrial rate and inhibition of electrically
stimulated contractions of the ileum (O'Donnell, 1976). It improved
the rates of mucociliary clearance in the guinea-pig (Streller, 1981).
In beagle dogs, oral doses of clenbuterol hydrochloride at
1.5, 3.0, 7.5 and 15.0 µg/kg bw resulted in a dose-dependent
tachycardia at > 3.0 µg/kg bw. There was a slight tachyphylactic
effect demonstrated by a diminution of the intensity and duration of
the response. There was a dose-related reduction in blood pressure and
the changes in diastolic and systolic pressures ran in parallel. These
changes occurred at the lowest dose used (Ueberberg, 1976).
In cattle, intravenous doses of 0.6 µg/kg bw and 1.5 µg/kg bw
produced no cardiac effects, as shown by ECG examination, whereas
2.85 µg/kg bw produced pronounced tachycardia. A dose of 0.3 mg/kg bw
clenbuterol resulted in tocolysis lasting approximately 5 hours
(Ballarini, 1978). Different lag times in parturition may be induced
depending on the dose and route of treatment; no adverse effects on
the dam or calf were noted (Arbeiter & Thurner, 1977).
Four metabolites of clenbuterol that had been shown to be present
in the kidneys and liver of treated target animals were tested for
pharmacodynamic activity. Of these, only one, N-A 1141 (Figure 1) was
shown to have activity in the guinea-pig. Its bronchiolytic effect was
less than 20% that of clenbuterol, and, moreover, it accounted for
only 12% of residues in the liver and kidney 6 hours after treatment
(Engelhardt, 1971).
2.2.9.2 Studies in humans
Patients given 10 µg (0.167 µg/kg bw) clenbuterol by the
inhalation route showed no signs of tachycardia as determined by ECG.
There were slight decreases in blood pressure. When patients with
cardiac arrhythmia were given this dose of clenbuterol, none of the
patients showed any changes attributable to the drug (Hufnagel, 1974).
After inhalation exposure of patients with airways disease, onset
of broncholytic action occurred within 5 minutes and lasted for up to
6 hours. Pulmonary function effects were greatest with doses of >
5 µg (0.083 µg/kg bw); only a minimal effect was seen with 2.5 µg
(0.042 µg/kg bw) (Schuster et al., 1989).
2.3 Observations in humans
A single blinded cross-over study was carried out to examine the
acute bronchospasmolytic effect and possible side-effects following
oral administration of placebo and three doses of clenbuterol (1, 2.5
and 5 µg/day) in patients (three male and three female) with chronic
obstructive airway disease over a 3 day period. The drug was given
orally, diluted with water. Observations were carried out over a
2-hour period following dosing. The average age of the patients was
55.7 years and they had an average body weight of 73.16 kg.
None of the 3 doses produced any clear, consistent effects on
bronchial resistance, thoracic gas volume, radial pulse frequency or
blood pressure, and no side effects were seen.
The pharmacological NOEL in this study was 5 µg/day, equivalent
to 0.08 µg/kg bw per day (Kaik, 1978).
The bronchospasmolytic effect was examined in two groups of
patients:
Group A: ten patients aged 46-75 years with chronic obstructive
respiratory disease resulting from pulmonary tuberculosis.
Group B: five patients aged 56-67 years with chronic obstructive
respiratory disease not related to tuberculosis, plus five
patients aged 34-57 with bronchial asthma.
The bronchospasmolytic effect was examined after single oral doses of
1, 2.5, 5, 10, 20, 25 or 30 µg/person, and after a placebo dose.
In Group A patients, intrathoracic gas volume was significantly
reduced and vital capacity and pneumometer values significantly
increased at all dose levels. In Group B patients, airway resistance
was significantly reduced, but no dose relationship could be
demonstrated. No significant placebo effect was seen in either group.
When compared with placebo values, a significantly greater increase
for both vital capacity and pneumometer values was observed in Group
A, even at the lowest dose used in this group (5 µg). However, at the
two lowest doses used in Group B (1 and 2.5 µg), there were no
significant differences from placebo values. The pharmacological NOEL
in this study was 2.5 µg, equivalent to 0.042 µg/kg bw (Nolte &
Laumen, 1972; Nolte, 1980).
Children who consumed between 0.05-0.075 mg of clenbuterol showed
only mild tachycardia. A 30-year-old woman who consumed 30 tablets
equivalent to 0.6 mg clenbuterol (10 µg/kg approximately) developed
tachycardia and slight hypertension approximately 1 hour after
consumption. No tablet remains were found on gastric lavage, and
medicinal charcoal and a saline laxative were given. The following
day, the patient's pulse rate and blood pressure had returned to
normal (Boehringer, 1991a).
Patients (100+) administered doses of 20-60 µg/day (0.3-1.0 µg/kg
bw per day) for up to 1 year or 20 µg/day (0.3 µg/kg bw per day) for
up to 6 months showed no adverse effects except for slight tremor and
occasional, mild tachycardia (Laumen, 1978; Tullgren & Lins, 1987).
3. COMMENTS
The Committee considered toxicological data on clenbuterol,
including the results of acute, short-term and reproductive toxicity
studies, as well as studies on teratogenicity, genotoxicity and
carcinogenicity. Results of pharmacokinetic and pharmacodynamic
studies in animals and humans were also considered.
Clenbuterol is well absorbed after oral administration in a
number of animal species and in humans. An oral dose is largely and
rapidly excreted in the urine, and the majority of the remainder is
excreted in the faeces. The biotransformation of clenbuterol is
complex and a number of metabolites are formed. The major compound
found in a number of species was unchanged clenbuterol. After oral
administration of therapeutic doses to lactating cattle, clenbuterol
was found in the milk.
When radiolabelled clenbuterol was given orally to pregnant rats,
dogs, baboons and cattle, radioactivity was detected in the fetuses.
Clenbuterol was moderately toxic in mice and rats after oral
administration, LD50 values being in the range of 80-175 mg/kg bw.
It was less toxic in the dog (LD50 = 400-800 mg/kg bw). It was more
toxic after parenteral administration, with LD50 values in the range
of 30-85 mg/kg bw after intravenous administration. The main signs of
toxicity included lethargy, tachycardia and tonic-clonic convulsions
after oral administration.
The main effects noted in the repeat-dose studies were
tachycardia and, at higher doses, myocardial necrosis. These effects
are common with ß-agonist drugs. The myocardial necrosis was
considered to be secondary to hypoxia, due to reduced myocardial
perfusion at a time of high oxygen demand resulting from increased
cardiac rate.
In 30-day repeat-dose studies in mice and rats, NOELs of 2.5 and
1 mg/kg bw per day, respectively, were identified, largely based on
cardiac lesions. However, in a range of repeat-dose studies in rats
using doses of 0.01 to 100 mg/kg bw per day for durations of up to
18 months, administered through the oral, intravenous and inhalation
routes, no NOELs were identified. Effects were usually related to
cardiac function and were seen even at the lowest doses used.
Similarly, no NOELS could be identified in a range of repeat-dose oral
studies in dogs. These studies used doses ranging from 0.1 to 40 mg/kg
bw per day. In a 26-week inhalation study in cynomolgus monkeys, the
NOEL was 25 µg/kg bw per day, based on a number of observations
including cardiac effects.
No evidence of carcinogenicity was noted in a two-year oral study
in mice with doses of up to 25 mg/kg bw per day. In a two-year study
with doses of up to 25 mg/kg bw per day in the Chbb:THOM rat, no
evidence of carcinogenicity was seen. However, in Sprague-Dawley rats
given 25 mg clenbuterol/kg bw per day orally for 2 years, an increased
incidence of mesovarian leiomyomas occurred. With the related
compounds salbutamol in rats and medroxalol in mice, the effects could
be abolished by administration of the ß-blocking agent propranolol.
Mesovarian leiomyomas in rats and uterine leiomyomas in mice are known
to occur following long-term treatment with ß-adrenoceptor agonists
and the Committee concluded that these were due to adrenergic
stimulation and not to any genotoxic mechanism. Clenbuterol was not
genotoxic in a range of in vitro and in vivo genotoxicity studies.
Epidemiological studies indicate that there have been no
increased incidences of uterine leiomyomas in women following the use
of ß-adrenoceptor agonists.
Clenbuterol had no effects on fertility in a reproductive
toxicity study in rats using oral doses of 1-50 mg/kg bw per day from
10 weeks prior to mating in males and two weeks prior to mating in
females. However, doses of 50 mg/kg bw per day resulted in the deaths
of pups soon after birth. To investigate the cause of the high pup
mortality at this dose level, the litters of control dams were
exchanged with those from dams given 50 mg/kg bw per day. Pups from
rats given 50 mg/kg bw per day died on the first day of lactation
regardless of whether they suckled on treated or control dams. The
mechanism involved in this lethal effect is unknown. A NOEL was not
identified in this study, because pup weights at birth were reduced in
all treated animals.
In a reproductive toxicity study in which male rats were treated
with 1.5-15 µg clenbuterol/kg bw per day orally for 70 days prior to
mating and females with the same dose range for 14 days prior to
mating, no adverse effects on reproduction were noted. The NOEL was
15 µg/kg bw per day.
In teratogenicity studies in rats, oral doses of 10 and 100 mg/kg
bw per day produced teratogenic effects that included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of vertebrae. These were accompanied by signs of maternal
toxicity. The NOEL was 1 mg/kg bw per day. In three studies in rabbits
using doses of 30 µg to 50 mg per kg bw per day, signs of feto-
toxicity, including delayed ossification and cleft palate, occurred.
The NOEL was 30 µg/kg bw per day.
Clenbuterol produced a range of pharmacodynamic effects in a
number of animal species including tachycardia, hypertension and
muscle relaxing effects. These were seen at single doses as low as
0.8 µg/kg bw.
Four metabolites of clenbuterol that had been shown to be present
in the kidneys of treated target animals were tested for pharmaco-
logical activity. Of these, only one (N-A 1141) was shown to have
activity. Its broncholytic effect in the guinea-pig was less than
20% that of clenbuterol. In addition, it accounted for only 1-2% of
residues in the liver and kidney of target animals 6 hours after
treatment.
In humans, clenbuterol produced a bronchiolytic effect when a
single dose of 10 µg (0.167 µg/kg bw) was given by the inhalation
route, but no evidence of tachycardia was seen at this dose. With oral
doses of clenbuterol of up to 5 µg/day (0.08 µg/kg bw per day) over a
3-day period, there were no effects on bronchial resistance, thoracic
gas volume, cardiac rate or blood pressure. The NOEL in this study was
5 µg/day (0.08 µg/kg bw per day). In a study to investigate the
bronchospasmolytic effect in humans, patients with obstructive lung
disease were given oral doses of up to 30 µg per person. Patients
administered doses of 5 µg or more exhibited bronchospasmolytic
effects, and the pharmacological NOEL in this study was 2.5 µg per
person, equivalent to 0.04 µg/kg bw.
4. EVALUATION
The Committee considered the most relevant study for determining
the ADI to be that concerning the bronchospasmolytic effect in humans.
The patients had chronic obstructive airway disease and thus were
likely to be a very sensitive population for this effect. The NOEL
identified in this study (2.5 µg per person, equivalent to 0.04 µg/kg
bw) is approximately 25% of the dose in another study in which the
inhalation route was used, but in which cardiac effects were not
observed. This NOEL is approximately 50% of the oral dose used in
another study where, again, cardiac effects did not occur. Hence, this
NOEL for the bronchospasmolytic effect offers an additional safety
margin for cardiac effects. The Committee therefore established an ADI
of 0-0.004 µg/kg bw, based on the NOEL of 0.04 µg/kg bw per day for
pharmacodynamic effects in humans and a safety factor of 10.
5. REFERENCES
Amemiya, K., Kudoh, M., Suzuki, H., Saga, K., & Hosaka, K. (1984).
Toxicology of mabuterol. Arzneim.forsch, 34(11a), 1680-1684.
Arbeiter, K. & Thurner, M. (1977). Effect of the sympathomimetic
Planipart (NAB 365) on parturition in cattle. Tierärztl. Umsch.,
32, 423-427.
Bailey, D.E. (1990). Primary eye irritation study in rabbits with
Ventipulmin. Unpublished report No. TX-9005/Ventipulmin from Hazleton
Laboratories America, Inc., Vienna, VA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Baillie, H.W., Cameron, B.D., Draffan, G.H., & Schmid, J. (1980).
Investigations of the placental transfer of 14C-N-AB 365 CL in the
cow. Unpublished report No. 111674 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Balazs, T. & Bloom, S. (1982). Cardiotoxicity of adrenergic and
vasodilating antihypertensive drugs. In: van Stee, E.W. (ed.),
Cardiovascular Toxicology, Raven Press, New York, pp. 199-220.
Ballarini, G. (1978). Initial studies of the clinical pharmacology of
the ß2 sympathomimetic agent NAB 365 (Planipart) and its use for
pharmacological tocolysis in cows. Tierärztl. Umsch., 33, 421-427.
Baumeister, M. (1978). Mutagenicity studies with the substance
N-AB 365 CL in the plate incorporation assay (Ames test). Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Baumeister, M. (1985). Mutagenicity study with the substance
N-AB 365 CL in the V79 (HGPRT)-test. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Boehringer (1991a). Summary of case reports of overdosage with
clenbuterol. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Boehringer (1991b). Estimated transdermal absorption of clenbuterol
hydrochloride following skin contact with granules and injection
solutions for veterinary use. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987a). The residue kinetics of
[14C]-NAB 365 CL in the calf. Unpublished report No. 4371 from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987b). The residue kinetics of
(14C)-NAB 365 CL in the lactating cow. Unpublished report No. 4445
from Inveresk Research International. Submitted to Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, D.M., Crook, D., & Brown, G. (1992). Ventipulmin injection
solution and Ventipulmin granules. Target species tolerance study in
calves. Unpublished report No. BOI 139/921030 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Chasseaud, L.F., Cresswell D.G., & Savage J.A. (1978).
Pharmacokinetics of 14C-N-AB 365 during chronic inhalational
toxicity in cynomolgus monkeys. Unpublished report No. U79-0212 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Clark, G.C., Collins, C.J., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D.E., Edmonson, N., Lewis, D.J, & Mahjeed, S.K. (1979).
Investigation of the effects on rats of inhalation of NAB 365 CL for
26 weeks. Unpublished report No. BOI 88/78768 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Clements, J. (1992). Study to determine the ability of clenbuterol
hydrochloride to induce mutations at the thymidine kinase (tk) locus
in mouse lymphoma L5178Y cells using a fluctuation assay. Unpublished
report No. 2TKREBSG.002 from Hazleton Microtest. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Collins, C.J., Clark, G.C., Heywood, R., Street, A.E., Hardy, C.J.,
Cherry, C.P., Gibson, W.A., Gopinath, C., Harrington S.M., Edmonson,
N.A., & Howard, E. (1979). Investigation of the effects on monkeys of
inhalation of NAB 365 CL aerosol for a period of 26 weeks. Unpublished
report No. BOI 90/78712 from Huntingdon Research Centre. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
De Busk, R.F. & Harrison, D.C. (1969). The clinical spectrum of
papillary-muscle disease. N. Engl. J. Med., 281, 1458-1467.
Ellenberger, J. (1985). Clenbuterol (NAB-365-CL) testing for
point-mutagenic activity with Salmonella typhimurium. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, A. (1971). Pharmacological exposé of the substance NAB365.
Supplement to the exposé on the compound NAB 365. Supplementary
investigations (May 1976) on the pharmacology of the substance NAB 365
and its metabolites. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Engelhardt, G. (1971). Results of a comparative pharmacological
investigation of NAB 365 CL, NAB 365 CL-D and NAB365 CL. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, G. (1976). Pharmacological profile of the action of
NAB 365 (clenbuterol), a new bronchodilator with a selective effect on
the adrenergic ß2 receptors. Arzneim. Forsch., 26(7a), 1404-1420.
Erichsen, D.F. (1988). Dose titration of Ventipulmin syrup in horses
with chronic obstructive pulmonary disease (COPD). Trial
No: 37-295-86-3. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Erichsen, D.F. (1989). A determination of incremental dose tolerance
of Ventipulmin syrup in the horse. Trial No.: Range finder 84-VI.
Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica, Ingelheim am Rhein, Germany.
Fenner, A. (1982). Respiratory rate, minute volume and tidal volume in
clinically healthy fattening cattle and those with bronchopneumonia -
taking into account the effects of clenbuterol. Doctoral Thesis,
Veterinary Faculty, Ludwig-Maximilians University of Munich. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Friedmann, J.Ch. (1982). Study of the possible mutagenic activity of
the substance "NAB365Cl" evaluated by the micronucleus test (oral
route). Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Gibson, J.P., Sells, D.M., Cheng, H.C., & Yuh, L. (1987). Induction of
uterine leiomyomas in mice by medroxalol and prevention by propanolol.
Toxicol. Pathol., 15, 468-473.
Gopinath, C. & Gibson, W.A. (1987). Mesovarian leiomyomas in the rat.
Environ. Health Perspect., 73, 107-113.
Hamm, D. & Erichsen, D.F. (1989). Acute and subacute toxicity of
Ventipulmin. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Roberts, N.L., & Cameron,
D.M. (1984). The disposition of the combination product14C-NAB
365 CL/trimethoprim/sulphadiazine after oral administration to horses.
Unpublished report No. HRC/BOI 112/84912 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R., Roberts,
N.L., Cameron, D.M., Offer, J., & Fish, C. (1985a). The disposition of
the combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
calves. Unpublished report No. HRC/BOI 111/8468OB from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R.,
Roberts, N.L., & Cameron, D.M. (1985b). The disposition of the
combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
dairy cows. Unpublished report No. HRC/BOI 111/84680A from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A., & Cameron, D.M.
(1993a). The pharmacokinetics, metabolism and residues of
14C-clenbuterol (14C-N-AB 365 CL) following intramuscular
administration to calves. Unpublished report No. HRC/BOI 140/921418
from Huntingdon Research Centre. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., & Dighton, M.H. (1993b). Investigation of
the metabolic profiles in the livers of the horses following multiple
oral administration. Unpublished report No. BOI 149/931490 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hewett, Chr. & Notman J. (1984). Dermal tolerance test with repeated
topical application in the rabbit. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Holmstrom, M. & MacGregor, D.B. (1986). NAB 365 CL. Cytogenetic study
in Chinese hamsters. Unpublished report No 4085 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hufnagel, E. (1974). Results with a new broncholytic agent. Effect on
heart rate, blood pressure and ECG. Ärztl. Praxis, 28, 1350-1352.
Jack, D., Poynter, D., & Spurling, N.W. (1983). Beta-adrenoceptor
stimulants and mesovarian leiomyomas in the rat. Toxicology,
27, 315-320.
James, C.N. (1990a). Acute oral toxicity in rats with Ventipulmin
syrup. Unpublished report No. TX-9003/VENTIPULMIN. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990b). Acute dermal toxicity study in rabbits with
Ventipulmin syrup. Unpublished report No. TX-9002/VENTIPULMIN.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
James, C.N. (1990c). Guinea pig sensitisation study - Buehler method
using Ventipulmin syrup. Unpublished report No. Case #: 64551-05 from
Arthur D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990d). Primary dermal irritation study in rabbits with
Ventipulmin syrup. Unpublished report TX-9001/Ventipulmin from Arthur
D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Dunsire, J.P. (1993). Plasma kinetics, metabolism,
excretion and residue kinetics of [14C]-N-AB 365 CL following oral
administration to the horse. Unpublished report No. 8346 from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Jenner, W.N. (1976). Study of the metabolism of
14C-labelled N-AB 365 in the male baboon. Unpublished report from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, W. (1987). Ventipulmin solution Bovine safety trial.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kaik, G. (1978). Clinico-pharmacological tests using the ß2-sympa-
thomimetic NAB 365 (clenbuterol) in patients with obstructive
respiratory disease. Unpublished report from the Medical University
Clinic of Vienna. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973a). Subacute toxicity with compound NAB 365 CL on mice,
oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973b). Teratological testing with the compound N-AB 365-CL
on rats, oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973c). Teratological testing with the compound NAB-365 CL
on rabbits, oral administration. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1975). Teratological testing with the compound N-AB 365 CL
on rats, oral administration during the period of organogenesis.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973a). Subacute toxicity with compound
NAB 365 CL on rats, oral administration. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973b). Chronic toxicity of compound
NAB 365 CL on rats, oral administration per food. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973c). Subacute toxicity with compound
NAB 365 CL on rats, intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kirkland, D.J. (1992). Review of clenbuterol hydrochloride mutagen-
icity data in relation to the proposed acceptable daily intake.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Koellmer, H., Stotzer, H., & Paulini, K. (1977). Subacute toxicity
study of the substance N-AB 365-CL in rats using inhalation over a
period of 13 weeks. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1969). Autoradiographic investigations on the
distribution of [14C]-N-AB 365 CL in rats and pregnant mice
(ADME 1 B). Unpublished report No. U69-0108. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1970). Pharmacokinetic investigations with [14C]-N-AB
365 CL in rats (ADME 1 B). Unpublished report No. U69-0109. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein,
Germany.
Kopitar, Z & Zimmer, A. (1973). Effect of repeated doses of NAB 365 CL
on the pharmacokinetic profile in rats (ADME 1 D). Unpublished report
No. U73-0158. Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim
am Rhein, Germany.
Kreuzer, H. (1988). Intramuscular local tolerance in tests with
Ventipulmin injectable solution ad us. vet. in rabbits. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Landry, Y. (1983). Review of the chief pharmacological properties,
clinical structures and physiochemical properties of clenbuterol.
Unpublished report from Laboratoire d'Allergopharmacologie,
Strasbourg. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Laumen, F. (1978). Investigation on long-term treatment and cumulation
with clenbuterol. Med. Mon.schr., 29, 455-459.
Lehman, H. (1969a). Teratology investigations in pregnant rats with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1969b). Teratology investigations in pregnant rabbits with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1974). Testing of the compound NAB 365 CL for perinatal
toxicity in rats. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1975). Study with substance N-AB 365 CL for fertility
inhibiting, teratogenic and perinatal toxic effects in rats.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1980). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 78 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1981). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 73 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Otto, H., &
Dontenwill, W. (1969). Report on subacute toxicity studies of NAB 365
p. o.- batch Z 6191 in beagle dogs. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
McEnaney, S. (1992). Damaging potential of clenbuterol by its effects
on cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished report No. 2HLREBSG.002 from Hazleton Microtest. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Magnusson, G. & Hansson, E. (1973). Myocardial necrosis in the rat: a
comparison between isoprenaline, orciprenaline, salbutamol and
terbutaline. Cardiology, 58, 174-180.
Nelson, L.W. & Kelly, W.A. (1971). Mesovarian leiomyomas in rats in a
chronic toxicity study of soterenol hydrochloride. Vet. Pathol.,
8, 452-457.
Nishikawa, J., Tsunenari, Y., & Kast, A. (1972). Acute toxicity of NAB
365 CL in rats, mice and rabbits. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Nishikawa, J., Kast, A., & Klupp, H. (1979). Acute toxicity study with
clenbuterol (NAB 365 CL) in rats dosed orally or intravenously.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Nolte, D. (1980). Comments on the publication "Lung function tests
with the bronchospasmolytic agent NAB 365". Unpublished report.
Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim am
Rhein, Germany.
Nolte, D. & Laumen, F. (1972). Pulmonary function tests following the
bronchospasmolytic agent NAB 365. Unpublished report from Medical
Clinics and Polyclinics, Justus-Liebig University and Seltersberg
Sanatorium of Hessen State Insurance Association, Giessen, Germany.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
O'Donnell, S.R. (1976). Selectivity of clenbuterol (NAB 365) in
guinea-pig isolated tissues containing ß-adrenoceptors. Arch. Int.
Pharmacodyn., 224, 190-198.
Owen, R., Fischer, R., & Erichsen, D.F. (1990). Safety of Ventipulmin
syrup in horses: 64-day study trial No. 37-295-83-4. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Palmer, A.K., Edwards, J.A., & Collins, C.J. (1978). Effect of
NAB 365 CL metered aerosol on pregnancy of the rat. Unpublished report
No. BOI 85/78689 from Huntingdon Research Centre. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Palmer, A.K., Bottomley, A.M., & Collins, C.J. (1980). Effect of
NAB 365 CL metered aerosol on pregnancy of the New Zealand White
rabbit. Unpublished report No. BOI 86/79656 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Pappritz, G. (1968). Determination of ALD50 of the substance in the
dog after oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. (1972). Determination of the ALD50 Of NA-B 365 in the
dog following intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Pappritz, G. & Bauer, M. (1973a). Supplementary studies on the
subacute toxicity of substance N-AB 365 CL in dogs after oral
administration. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. & Bauer, M. (1973b). Chronic toxicity study of substance
NAB 365 in dogs following oral administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica, Ingelheim am
Rhein, Germany.
Pappritz, G., Bauer, M., & Eckenfels, A. (1973). Subacute toxicity of
NAB 265 CL in dogs following intravenous administration. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica,
Ingelheim am Rhein, Germany.
Poynter, D & Spurling, N.W. (1971). Some cardiac effects of
beta-adrenergic stimulants in animals. Postgrad. Med. J., 47, 21-25.
Poynter, D., Harris, D.M., & Jack, D. (1978). Salbutamol: lack of
evidence of tumour induction in man. Br. Med. J., 7 January.
Richter, I. (1982). Biochemical investigations of the placental
passage of 14C-clenbuterol in pregnant rats. Unpublished report
No. U82-0292. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Roberts, W.C., & Cohen, L.S. (1972). Left ventricular papillary
muscles. Description of the normal and a survey of conditions causing
them to be abnormal. Circulation, XLVI, 138-154.
Rohr, U.D., Haczkiewicz, K., Mohr, A., & Orton, B. (undated).
Validation of an excised human skin model for testing drug candidates
suited for transdermal drug delivery. Unpublished report from
Boehringer Ingelheim KG. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rominger, K.L. & Schrank, H. (1982). Placental transfer of
14C-clenbuterol in the dog. Unpublished report No. U82-0291.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Rominger, K.L., Förster, H., Hermer, M., Peil, H., & Wolf, M. (1987).
Clenbuterol plasma levels in patients under tocolytic treatment.
Unpublished report from Boehringer Ingelheim KG. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rosenblum, I., Wohl, A., & Stein, A.A. (1965). Studies in cardiac
necrosis. III. Metabolic effects of sympathomimetic amines producing
cardiac lesions. Toxicol. Appl. Pharmacol., 7, 344-351.
Schmid, J. (1977). Pilot pharmacokinetic investigations after
intravenous administration of N-AB 365 CL in a cow. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Schmid, J. (1980). Investigations of the placental transfer of
14C-N-AB 365 CL in the baboon. Unpublished report from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1982). Pharmacokinetics, metabolism and tissue
distribution of 14C-N-AB 365 CL in the baboon. Unpublished report
No. 2220 from Inveresk Research International. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990a). Plasma levels and residue analysis in cows and
calves. Unpublished report No. U90-0092. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990b). N-AB 365 CL in the cow's milk after oral and
intramuscular administration. Unpublished report No. U90-0099.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Prox, A. (1986). Isolation and structural elucidation of
NAB 365 CL metabolites from dog urine. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Zimmer, A. (1977a). Pilot pharmacokinetic studies
following oral administration (single and multiple dosing) of
14C N-AB 365 CL to a cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. & Zimmer, A. (1977b). Pilot pharmacokinetic investigations
after intramuscular administration (single and multiple doses) of
N-AB 365 CL in the cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schuster, A. (1984). Skin sensitisation study in guinea pigs.
Unpublished report No. U84-0673 from Boehringer Ingelheim KG.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schuster, D., Deichsel, G., & Feuerer, W. (1989). Dose effect study
with NAB 365 CL metered dose inhaler in patients with chronic
obstructive lung disease. Unpublished report from Dr. K. Thomae GmbH,
Medical Department. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Sells, D.M. & Gibson, J.P. (1987). Carcinogenicity studies with
medroxalol hydrochloride in rats and mice. Toxicol. Pathol.,
15, 457-467.
Serbedija, R. & Bauer, M. (1973). Toxicity study of the substance
NAB 365 CL in rats over a period of 18 months. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Serbedija, R., Lutzen, L., Puschner, H., & Hohbach, Ch. (1982).
Carcinogenicity study with the substance N-AB 365 CL in rats with oral
administration for a period of 2 years. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Streller, I. (1981). Effect of the ß-adrenergic agents fenoterol,
orciprenaline, clenbuterol, SOM 987 and SOM 1122 on the mucocilliary
clearance in the trachea of the anaesthetised guinea-pig. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Tullgren, A & Lins L-E. (1987). An open long-term tolerability study
on NAB 365 (clenbuterol). Unpublished report from Boehringer Ingelheim
AB, Stockholm, Sweden. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Ueberberg, H. (1976). Comparative study of the effects of NAB 365 CL
and terbutaline sulfate on the dog heart. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Umemoto, K. (1984). Carcinogenicity studies with the beta-adreno-
ceptor stimulant clenbuterol (NAB-365 CL) in mice by drinking water.
Unpublished report from Nippon Boehringer Ingelheim. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1971). Metabolism and species comparison in the rat,
rabbit and dog (ADME IV). Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974a). Comparison of the pharmacokinetic profile in the
dog with single and repeated dosage (ADME 1 D). Unpublished report
No. U73-0161. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Zimmer, A. (1974b). Pharmacokinetics and metabolism pattern in the
rabbit and dog (ADME I). Unpublished report No. U74-0116. Submitted to
WHO by Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974c). Metabolism in man; comparison with dog and rabbit,
using radioactive substance. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1976). Administration of clenbuterol in man. Single doses,
multiple doses, and metabolite samples. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Zimmer, A. (1977). Preliminary investigations of pharmacokinetics in a
horse. Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zupan, J. A. (1985). A comparative bioavailability study between oral
and injectable dosage forms of clenbuterol in horses. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Table 1. Acute toxicity of clenbuterol
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M oral 80 Nishikawa et al., 1972
F oral 133 Nishikawa et al., 1972
M & F s.c 72 Nishikawa et al., 1972
M & F i.v 42 Nishikawa et al., 1972
M i.p 46 Nishikawa el al., 1972
M i.p 72 Nishikawa et al., 1972
Rat M & F oral 175 Nishikawa et al., 1972
M oral 82 Nishikawa et al., 1979
F oral 170 Nishikawa et al., 1979
M & F i.v 30 Nishikawa et al., 1972
M i.v 35 Nishikawa et al., 1979
F i.v 39 Nishikawa et al., 1979
M & F s.c 170 Nishikawa et al., 1972
M & F i.p 70 Nishikawa et al., 1972
M & F oral1 > 0.125 James, 1990a
Rabbit M & F i.v 84 Nishikawa et al., 1972
M & F dermal1 > 0.05 James, 1990b
Dog M & F oral 400-800 Pappritz, 1968
M & F i.v 45-52 Pappritz, 1972
1 Administered as a proprietary veterinary medicinal product
containing 25 µg clenbuterol per ml
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 15 male and 15 female ICR-JCL mice were given daily
oral doses of 0, 2.5, 12.5 or 62.5 mg/kg bw clenbuterol hydrochloride
as an aqueous solution, by stomach tube, for 30 days. Body weight gain
and food consumption were increased in all treated groups in a dose-
related manner. There were no adverse effects on haematology. The
animals from the highest dose group appeared weak and 6 animals died
during the course of the study.
At necropsy, there were significant increases in liver weights in
animals given 12.5 or 62.5 mg/kg bw per day. Ischemic heart damage was
noted in one animal given 12.5 mg/kg bw per day, and in two animals
from the highest dose group. The NOEL in this study was 2.5 mg/kg bw
per day (Kast, 1973a).
2.2.2.2 Rats
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily oral doses of 0, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by stomach tube, for 30 days. Signs
of toxicity were seen at the highest dose and these included
salivation, encrusted eyes and noses, and reduced body weight gain and
food consumption; 50% of these animal died during the course of the
study. Serum aspartate aminotransferase and glucose concentrations
were significantly reduced in animals of both sexes given 10 or
100 mg/kg bw per day.
Liver weights were significantly reduced in males given the
highest dose, while ischaemic lesions were seen in cardiac muscle in
one male and one female given this dose. The NOEL in this study was
1 mg/kg bw per day based on blood chemistry findings (Kast &
Tsunenari, 1973a).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given doses of 0, 1, 5, 25 or 75 mg/kg bw per day clenbuterol
hydrochloride in the feed for 6 months. At the highest dose level,
body weight gain and food consumption were reduced. During the first
week, a reduction in water consumption occurred in the animals given
this dose, and nine male and five female animals died during the
course of the study.
At necropsy, ischaemic lesions of the myocardium were noted
in all treated animals and there was a dose-related incidence and
severity. These lesions were also found in another group given
75 mg/kg bw per day clenbuterol hydrochloride and then allowed a
4-week recovery period without drug treatment. A NOEL was not
identified in this study (Kast & Tsunenari, 1973b).
Groups of 20 male and 20 female FW49/Biberach rats were fed diets
containing clenbuterol hydrochloride for 18 months. Further groups of
three male and three female rats were fed treated diets for 14 days,
followed by a recovery period; these animals were the subject of ECG
investigations. Another group of 10 female and 10 male rats was fed
the highest dietary level and then maintained for a 6-week recovery
period. The overall design of the study, including doses, is set out
below.
Dose (mg/kg bw 18-month phase 14-day ECG study2
per day) number of animals numbers of animals
males females males females
0 20 20 3 3
0.1 24 20 3 3
0.5 20 20 3 3
2.5 20 20 3 3
2.5 101 101 - -
1 subject to 6 week recovery period
2 subject to 21 day recovery period
Body weight gain in treated groups was slightly increased when
compared with control values. However, there were no overt signs of
toxicity, and no deaths occurred. There were no significant haemato-
logical effects. However, there were reductions in myocardial glycogen,
skeletal muscle glycogen and liver glycogen values in all treated
groups, but there was no clear dose response. Histochemical examination
of the myocardium showed a reduction in enzyme activity, particularly
in the lowest dose group.
The ECG examination revealed a bradycardia rather than the
expected tachycardia, but no explanation was provided for this. Heart
rates returned to normal 2 days into the recovery period. As the
results of this study appear to conflict with those of other studies
(reduction in heart rate rather than the expected increase), and as
the hearts were not adequately examined macroscopically or micro-
scopically, an NOEL cannot be identified from this study (Serbedija &
Bauer, 1973).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily intravenous doses of 0, 1, 4 or 16 mg/kg bw clenbuterol
hydrochloride for 30 days. Overt signs of toxicity included weakness
at the two highest dose levels and increased respiratory and heart
rates at the highest dose level. One female and one male in the 1 and
4 mg/kg bw per day dose groups respectively, died, while five male and
five female animals died at the highest dose. There was evidence of
hepatotoxicity (focal necrosis) in rats given the highest dose. A NOEL
was not identified in this study (Kast & Tsunenari, 1973c).
Groups of 30 male and 30 female Chbb: THOM (SPF) rats were
exposed by the inhalation route to 0 or 0.667 mg/m3 clenbuterol
hydrochloride in aqueous solution. A further two groups of 15 male and
15 female rats were exposed to 5.013 or 40.0 mg/m3. The exposures
were conducted for 0.5-2 hours per day, 7 days per week, for 13 weeks.
These exposures gave corresponding doses of 0, 0.01, 0.16 and
2.58 mg/kg bw per day. Groups of 10 male and 10 female control
animals, and 10 male and 10 female rats exposed to the highest
concentration were subjected to a 6-week recovery period without
further exposure to clenbuterol hydrochloride.
There was no drug-related mortality during the study. Increased
heart rates were noted in all treated rats. Animals exposed to the
highest concentration of clenbuterol hydrochloride showed myocardial
necrosis. Myocardial changes were noted in animals subject to the
6-week recovery period. As increases in heart rate were noted at all
concentrations, a NOEL was not identified in this study (Koellmer
et al., 1977).
Groups of 20 male and 20 female Charles River CD rats were
exposed to aerosols of clenbuterol hydrochloride in ethanol with
dichlorofluoromethane and 1,2-dichlorotetrafluoroethane as
propellants, using head-only exposure. Other groups served as controls
or ethanol/propellant controls. The doses were equivalent to up to
118 µg/kg bw per day.
The only treatment-related effects attributable to clenbuterol
hydrochloride were liver weight reduction and small aggregations of
alveolar macrophages, which were clearly dose-related (Clark et al.,
1979).
However, there were insufficient data on dosimetry to identify a
NOEL and in addition, there were no measurements of heart-rate or
other cardiac function parameters.
2.2.2.3 Dogs
Groups of three male and three female beagle dogs were given
starch capsules containing clenbuterol hydrochloride at doses of
0, 0.4, 4 and 20 mg/kg bw per day, daily for 13 weeks. The highest
dose was increased to 30 mg/kg bw per day from week 5 and to 40 mg/kg
bw per day from week 9. Water consumption was normal during the study,
but a slight decrease in appetite was noted in high-dose animals when
the dose was increased to 40 mg/kg bw per day. The rate of body weight
gain significantly fell from this point. One male and two females
given the highest dose appeared sedated after treatment. One of these
became increasingly agitated and breathless and died 2-3 minutes after
receiving the 23rd dose of 20 mg/kg bw per day. Tachycardia occurred
after the first dose in all treated animals and this persisted until
the following day. There was a decrease in its severity during
subsequent weeks, but it continued to be noted in all treated animals.
A shortening of the P-Q, Q-T and T-P intervals in the ECG,
corresponding to the increased heart rates, was seen in all treated
dogs. The dog that died was found to have a severe acute pulmonary
infection. Myocardial haemorrhage and necrosis, damage to the hepatic
parenchyma and severe haemorrhagic bronchopneumonia were noted. No
abnormalities occurred in any of the other animals. As dose-related
tachycardia occurred at all doses, a NOEL was not identified in this
study (Leuschner et al., 1969).
Groups of three male and three female beagle dogs were given oral
doses of 0, 2.5 and 40 mg/kg bw per day clenbuterol hydrochloride in
gelatin capsules for 13 weeks. No animals died during the study, but
in the first week, all dogs given clenbuterol hydrochloride were
apathetic, had redness of the skin and eyes, mydriasis and fixed
pupils. Tachycardia was seen in all dogs. At 2.5 and 40 mg/kg bw per
day, significant increases in the activities of several serum enzymes,
including LDH and creatinine kinase, were found. The glycogen levels
of the left heart muscle were decreased. Myocardial necrosis was seen
in dogs given the 2.5 and 40 mg/kg bw per day doses. A NOEL was not
identified in this study (Pappritz & Bauer, 1973a).
Groups of three male and three female beagle dogs were given
gelatin capsules containing daily doses of 0, 0.1 or 0.5 mg/kg bw per
day clenbuterol hydrochloride for 1 year. A group of six male and six
female dogs were given 2.5 mg/kg bw per day in the same manner, and
three animals of each sex were maintained on a control diet for 6
weeks at the end of the study, as part of a recovery phase. No animals
died during the study. In the first week all drug-treated animals were
apathetic and had diffuse reddening of the skin. Tachycardia was noted
in all treated animals, as was mydriasis, episcleral vascular
injection and conjunctivitis.
There were no significant effects on food consumption or body
weights, although dogs given the highest dose gained slightly more
weight than did those in other groups. No haematological effects were
seen, but there were increases in some serum enzymes in treated
groups. Liver glycogen was decreased in dogs given the highest dose
and this remained decreased at the end of the 6-week recovery period.
Heart weights were increased in all the treated animals when
compared with controls, and myocardial necrosis was noted in the
papillary muscle of the left ventricle of all these dogs, including
those subject to the 6-week recovery period. There was no dose
relationship in the cardiotoxicity seen, but histochemical methods
revealed smaller decreases in the activities of several enzymes in
cardiac muscle of the 0.1 mg/kg bw per day group than in the other
treatment groups. A NOEL was not identified in this study
(Pappritz & Bauer, 1973b).
Groups of three male and three female beagle dogs were given
daily intravenous injections of 0, 1, 10 or 1000 µg/kg bw per day
clenbuterol hydrochloride for 30 days. Dogs given the highest dose of
clenbuterol hydrochloride were lethargic and all treated animals
developed tachycardia with associated decreases in the P-Q and Q-T
intervals in the ECG. At necropsy, one male given the highest dose was
found to have myocardial necrosis. A NOEL was not identified in this
study (Pappritz et al., 1973).
2.2.2.4 Monkeys
Groups of three male and three female cynomolgus monkeys were
exposed to atmospheric levels of clenbuterol hydrochloride as an
aerosol in ethanol, with dichlorofluoromethane and 1,2-dichlorotetra-
fluoroethane as propellants, using an oropharyngeal tube, giving doses
of 25, 50 and 150 µg/kg bw per day for 26 weeks. Half of the daily
metered dose was given each morning and the remainder in the afternoon.
Another group of three male and three female monkeys served as sham
controls with no exposure, while a further group of three male and
three female monkeys served as placebo controls and were exposed to
the same aerosol as treated animals, but without the clenbuterol
hydrochloride.
No signs of toxicity were seen in this study and food consumption
and body weights were normal except in high-dose females where a large
body weight gain occurred. Ophthalmoscopy, haematology, lung mechanics
and ventilation, blood chemistry and urinalyses were normal. A low
oxygen tension was noted in high-dose animals in week 10 of the study.
There were no drug-related changes in heart rate or ECGs. At macro-
scopic and histo-pathological examination, there were no drug-related
changes (Collins et al., 1979).
Thus, the NOEL in this study was of the order of 25 µg/kg bw per
day by the inhalation route, but this could not be identified as a
definitive study because of the problems in dosimetry in an inhalation
study of this type. For example, there were no data on how much drug
reached the lower respiratory tract and no information regarding blood
levels. Hence, the degree of systemic exposure was unknown.
2.2.2.5 Cattle
In calves given the therapeutic dose of 0.8 µg/kg bw per day
clenbuterol hydrochloride for 10 days by the oral or intravenous
routes, only transient tachycardia occurred. When given at 5 times the
therapeutic dose (4 µg/kg bw per day) for 10 days, tachycardia and
audibly increased heart force were the only effects noted. When given
to adult cattle at the therapeutic dose or twice the therapeutic dose
for periods of up to 9 days, by the oral route, the main effects were
tachycardia and falls in diastolic blood pressure (Fenner, 1982;
Cameron et al., 1992).
2.2.2.6 Horses
No clinically significant adverse effects were noted in horses
given 0.8-17.5 µg/kg bw per day clenbuterol hydrochloride by the oral
route for periods of up to 64 days. Transitory effects included
tachycardia, sweating, muscle tremors, hyperglycaemia and hypophos-
phataemia (Erichsen, 1988, 1989; Hamm & Erichsen, 1989; Owen et
al., 1990).
Clenbuterol, like other ß-agonists, leads to tachycardia and
hypotension. This probably results in reduced myocardial perfusion at
a time when oxygen demand is high because of increased cardiac rate.
The end result is hypoxia which probably leads to the necrotic lesions
seen in the left ventricular papillary muscles (Rosenblum et al.,
1965; De Busk & Harrison, 1969; Poynter & Spurling, 1971; Roberts &
Cohen, 1972; Magnusson & Hansson, 1973; Balazs & Bloom, 1982). This
explains the cardiac effects noted in the repeated dose (and other)
studies with clenbuterol.
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female (C57B/6 × DBA/2) mice were given
clenbuterol in the drinking-water at doses of 0.1, 1.0 and 25.0 mg/kg
bw per day for 2 years. Groups of 100 males and 100 females were given
drinking-water only over this period and served as controls. Treated
animals consumed slightly more food and water than controls, but showed
no overt signs of toxicity, except for a dose-related increase in body
weight in the first year and a decrease during the second year.
Survival after 2 years was good in males (78-86%) and acceptable in
females (54-60%).
At termination, there were significant changes in the weights of
a number of organs when compared with control values. These included
significant increases in the absolute heart weights of males given
1 mg/kg bw per day and in females given 25 mg/kg bw per day. There
were significant increases in relative heart weights in all treated
male and female mice. The incidences of non-neoplastic lesions,
including myocardial lesions, were not treatment related. There was no
increased incidence of any rumour type in treated mice when compared
with control values (Umemoto, 1984).
2.2.3.2 Rats
Groups of 48 male and 48 female Chbb:THOM rats were fed diets
containing clenbuterol hydrochloride, resulting in daily doses of
6.25, 12.5, or 25.0 mg/kg bw per day for 2 years. A further group of
72 rats of each sex was fed a diet containing no clenbuterol hydro-
chloride. As ß-sympathomiroetic agents are known to induce mesovarian
leiomyomas in some strains of rat, groups of 72 male and 72 female
Charles River Sprague-Dawley rats were also fed diets containing
clenbuterol hydrochloride at doses of 0 or 25 mg/kg bw per day for 2
years. After one control rat began to show signs of clenbuterol-related
effects, contamination of the feed preparation personnel and equipment
was confirmed, and the substance was then administered via the
drinking-water to ensure the same daily doses.
Treated rats appeared nervous, tense and aggressive, but no
effects on mortality were seen. Survival was in the range of 70-87% in
males and 50-71% in females. In both sexes and strains body weight was
reduced in a dose-related manner. Consumption of drinking-water was
significantly reduced in SD rats given the highest dose. At
termination, fibrosis of the subendocardial connective tissue was
frequently observed in all groups including controls, but the
incidence was higher in the Chbb:THOM rats, suggesting a drug-
related effect.
There was an increased incidence of mesovarian leiomyomas in the
female Sprague-Dawley rats, but not in the Chbb:THOM rats. The
incidence is shown in Table 2.
Table 2. Incidence of mesovarian leiomyomas in female rats treated with clenbuterol
hydrochloride for 2 years
Chbb:THOM Sprague-Dawley
Rats rats
Dose 0 6.25 12.5 25.0 0 25.0
(mg/kg bw per day)
Numbers with 0/72 0/48 0/48 0/72 0/72 11/72
Mesovarian
leiomyomas
per group
There was no increased incidence of any other tumour type
(Serbedija et al., 1982).
Leiomyomas of the smooth muscle of the uterus in mice and
mesovaria of rats have been reported following long-term treatment
with ß-agonists (Nelson & Kelly, 1971; Jack et al., 1983;
Amemiya et al., 1984; Gibson et al., 1987; Gopinath & Gibson,
1987; Sells & Gibson, 1987). They are not associated with genotoxicity
with these agents and are considered to be associated with adrenergic
stimulation. The ß-agonist salbutamol produced mesovarian leiomyomas
in rats, but these could be prevented by administration of the
ß-blocking agent propranolol; a similar phenomenon has been reported
in mice where the induction of leiomyomas of the uterus by medroxalol
was prevented by propranolol (Jack et al., 1983; Gibson et al.,
1987). There have been no reports of increased incidences of leiomyomas
in women following the use of ß-adrenergic agents (Poynter et al.,
1978).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
Groups of 17-20 pregnant female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage, from day 15 of gestation to 21 days
post-partum when dams and pups were sacrificed. The pups, including
those that died before day 21, were necropsied and X-rayed. Maternal
body weights were reduced at the end of the study in animals given
7 mg/kg bw per day clenbuterol hydrochloride. Those given the highest
dose were similar to the controls, but these animals were heavier at
the beginning of the study. Maternal food consumption was reduced in
all treated groups.
The number of pups born dead increased in a dose-related manner,
as did the number of pups dying after birth. At the highest dose all
the pups died, as shown below.
Body weights of pups were also reduced in a dose-related manner.
A NOEL was not identified in this study (Lehman, 1974).
Groups of 30 male and 30 female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water by gavage. Treatment of the male rats commenced 10
weeks prior to mating while that of females began 2 weeks prior to
mating. On day 14 of gestation 50% of the females were killed and the
uterine contents examined. The remaining female rats were allowed to
deliver normally and rear their pups until weaning. Dosing of all
females continued until they were killed on day 14 of gestation or
after weaning.
To investigate the cause of the high pup mortality in the study
of Lehman (1974), the litters of six control dams were exchanged with
litters from dams given 50 mg/kg bw per day. Surviving dams and pups
were necropsied at the end of the lactation period. Food consumption
was increased in treated animals when compared with control values.
However, body weight was significantly reduced in animals of both
sexes given the highest daily doses.
Pup weights at birth were reduced in all treated groups. There
was a dose-related decrease in the numbers of viable pups which was
statistically significant at 7 and 50 mg/kg bw per day. All the pups
in the 50 mg/kg bw per day group died on the first day of lactation
regardless of whether or not they were suckled by treated or control
dams. The majority of pups from control dams suckled by dams that were
given the 50 mg/kg bw per day dose survived.
There were no substance-related effects on fertility, corpora
lutea, implantation or resorption rates, and no malformations were
noted in pups from treated rats. The hearts of three pups from
high-dose females were examined histologically, but no abnormalities
were found (Lehman, 1975).
Groups of 34 male and 34 female Chbb:THOM rats were given daily
oral doses of 0, 1.5, 7.5 or 15 µg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage. The male rats were
treated for 70 days prior to mating and the females from 14 days prior
to mating up until the end of gestation. The pups were not treated.
Dosing of the females continued until the interim sacrifice or after
weaning.
On days 13-15 of gestation, 50 pregnant rats were killed and the
uterine contents examined. The remainder were allowed to give birth
naturally and the pups reared until 3 weeks old, except for two males
and two females in each litter which were reared until 10 weeks after
mating when certain behavioural tests were conducted (pupillary
reflex, photo-phobia response, hearing, behaviour on a rotating rod,
behaviour in a Y maze). The animals were then mated and allowed to
litter naturally.
There were no effects on fertility attributable to clenbuterol
treatment, and gestation length, numbers of corpora lutea,
implantation rates, incidences of resorptions, parental body
weights and food consumption were unaffected.
No compound-related effects were seen in the littering phase and
the pups gained weight normally. Performance in the behavioural tests
were similar in treated and control groups and the offspring of these
animals were normal in all respects. The NOEL was 15 µg/kg bw per day
(Lehman, 1980).
Similar results were obtained in an almost identical study on
rats using the same dose levels. Again, the NOEL was 15 µg/kg bw per
day (Lehman, 1981).
No teratogenic effects were seen in any of these reproductive
toxicity studies.
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 mated female SPF-FW 49 Biberach rats were given oral
doses of 0, 0.04, 0.2 or 1 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage on days 6-15 of gestation. No evidence
of maternal toxicity was seen and there were no compound-related
effects on the incidences of resorptions, or on litter and fetal
weights. There were no increased incidences of any type of
malformation. The NOEL was 1 mg/kg bw per day (Lehman, 1969a).
Groups of 25 mated female Sprague-Dawley rats were given oral
doses of 0, 0.01, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage, from days 9 to 14 of
gestation. Approximately 24 hours before the expected delivery date,
20 dams per dose level were killed and the uterine contents examined
while the remaining five pregnant animals in each group were allowed
to deliver naturally.
Overt signs of toxicity, including weakness, hypersensitivity and
bloody vaginal discharge were seen in rats given the highest daily
dose. Maternal body weights were reduced in dams given the 10 or
100 mg/kg bw per day doses. Animals given these doses had signifi-
cantly increased incidences of resorptions with corresponding
reductions in the numbers of viable fetuses. Mean litter weights were
reduced at both these doses, while mean fetal weight was significantly
reduced at the highest dose level.
The incidences of malformations were significantly increased at
the two highest dose levels used. Anomalies included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of the vertebrae (Kast, 1973b).
A study was conducted to verify the results of Kast (1973b).
Groups of 25 mated Sprague-Dawley rats were given 0, 0.01, 1, 10 or
100 mg/kg bw per day clenbuterol hydrochloride in distilled water, by
gavage, from days 8 to 17 of gestation. Approximately 24 hours before
the expected delivery date, 20 dams per group were killed and the
uterine contents examined. The remainder were allowed to deliver
naturally.
Overt signs of toxicity were similar to those in the previous
study and five dams given the highest dose died. The incidences of
resorptions were increased in animals given the highest dose; mean
litter weights and fetal weights were reduced. The incidences of
malformations were significantly increased at the two highest doses.
The types of malformation were similar to those seen in the study of
Kast (1973b).
In pups derived from animals allowed to deliver naturally, body
weights were increased at the highest dose. Swimming function was
retarded in this group. At 10 mg/kg bw per day, two pups were found to
have hydrocephalus while at the highest dose level, four pups were
found to have anophthalmia. No malformations were noted in pups from
the 0.01, 0.1 or 1 mg/kg bw per day groups. The NOEL in both these
studies was 1 mg/kg bw per day (Kast, 1975).
Groups of 20 Sprague-Dawley rats were exposed nose only to an
atmosphere containing clenbuterol hydrochloride during days 6-15 of
gestation. The estimated doses were 0, 19, 39 and 78 µg/kg bw per day.
There was no evidence of maternal toxicity or teratogenicity, but
there were dose-related increases in the incidences of skeletal
variations, indicative of fetotoxicity, at all dose levels (Palmer
et al., 1978).
2.2.5.2 Rabbits
Groups of 15 mated Russian/Biberach rabbits were given daily oral
doses of 0, 30, 100 or 300 µg/kg bw per day clenbuterol in distilled
water, by gavage, from days 6 to 18 of gestation. Three dams given the
300 µg/kg bw per day dose and one given the highest dose died during
the study. These deaths were due to bronchopneumonia. Several rabbits
in each group were found not to be pregnant. Despite these problems,
at least 10 litters from each dose group were available for
examination.
Dams in each dose group showed better weight gains than controls,
but there was no clear dose relationship. There were no adverse
effects on the incidences of resorptions, numbers of live fetuses,
fetal weights or the incidences of malformations. However, there were
increased incidences of delayed ossification, suggestive of
fetotoxicity, in the 100 and 300 µg/kg bw per day groups. The NOEL
was 30 µg/kg bw per day (Lehman, 1969b).
Groups of 10 female Russian/Biberach rabbits were given daily
oral doses of 0, 0.01, 1 or 50 mg/kg bw per day clenbuterol
hydrochloride, in distilled water by gavage, on days 8 to 16 of
gestation. There were no compound-related deaths, but body weight gain
and food intake was reduced at the highest dose level.
At the highest dose level, the incidence of resorptions was
significantly increased, and there was a corresponding decrease in
the numbers of viable fetuses. Mean litter and fetal weights were
significantly reduced at this level. There was an increased incidence
of malformations, notably cleft palate and synostosis, in pups from
dams given 50 mg/kg bw per day clenbuterol hydrochloride. The NOEL was
1 mg/kg bw per day (Kast, 1973c).
Groups of 13-16 mated New Zealand White rabbits were exposed to
doses of clenbuterol hydrochloride calculated to be 0, 48, 146 or
300 µg/kg bw per day by the inhalation route using a fine bore tube
sited over the tracheal opening. The heads of the animals were held in
an air extraction chamber to minimize rebreathing of aerosol. Dosing
took place on days 6-18 of gestation.
Signs of stress were observed in all groups as a result of the
exposure procedures. Signs included anorexia, lethargy and respiratory
distress, and some animals died in all groups, including the controls.
There was a high incidence of malformations in the control groups
which was probably related to the initial stress. There was no
increased incidence in animals given 146 or 300 µg/kg bw per day.
As a result of the high numbers of malformations seen in control
groups, no firm conclusions could be drawn from this study. However,
the data suggested that doses of up to 300 µg/kg bw per day clen-
buterol hydrochloride, when given by inhalation to pregnant rabbits on
days 6-18 of gestation, were not teratogenic (Palmer et al., 1980).
Taken together, the animal data suggested that clenbuterol
hydrochloride is teratogenic in rats and rabbits at relatively high
doses. There was evidence of fetotoxicity. The NOEL for maternal
toxicity and teratogenicity in the rat and rabbit following oral
administration was 1 mg/kg bw per day. However, the NOEL for
fetotoxicity in one study in the rabbit was 0.03 mg/kg bw per day.
2.2.6 Special studies on genotoxicity
Clenbuterol hydrochloride gave negative results in the Ames test
with Salmonella typhimurium strains, in a reversion test with
Escherichia coli strain WP2(P), in a gene mutation assay with
Chinese hamster V79 cells, in an in vivo mouse micronucleus test and
in an in vivo cytogenetic assay with Chinese hamster bone marrow.
In the mouse lymphoma test with L5178Y cells, no increased
incidences in mutation frequency were observed in the absence of
metabolic activation. However, with metabolic activation, one of two
experiments gave positive results at the two highest concentrations
used (Clements, 1992).
Viability counts were reduced at these concentrations and
sampling error may have led to these spurious results (Kirkland, 1992).
In an in vitro assay with human lymphocytes, at concentrations of
> 500 µg/ml, an increased incidence of chromosome aberrations was
noted in the absence (but not in the presence) of metabolic
activation. This was not dose-dependent.
The results are summarized in Table 3.
These data suggest that clenbuterol hydrochloride is not
genotoxic.
2.2.7 Special studies on irritancy
2.2.7.1 Skin irritation
Clenbuterol hydrochloride was applied to the shaved skin of six
Russian/Biberach rabbits, using an occlusive dressing, for a period of
28 days. No evidence of skin irritation was seen (Hewett & Notman,
1984). Similar results were noted in a further study of skin
irritation (James, 1990d).
2.2.7.2 Intramuscular tolerance
Intramuscular injection of 0.5 ml of a formulation containing
clenbuterol hydrochloride into the dorsal area of rabbits produced
only minimal haemorrhage, oedema and necrosis (Kreuzer, 1988).
2.2.7.3 Eye irritation
A formulated commercial product containing clenbuterol
hydrochloride (0.1 ml) was instilled into the left eye of six New
Zealand White rabbits. The right eye served as a control. Mild
irritation was noted and this resolved 48 hours after treatment
(Bailey, 1990).
2.2.8 Special studies on immunotoxicity
Clenbuterol hydrochloride, as a formulated commercial product,
was tested on the guinea-pig using the Buehler technique. The content
of active ingredient was 70.4 µg/ml in the formulation. It was not a
sensitizer in this study (James, 1990c).
Another study employed a 0.2% solution of clenbuterol hydro-
chloride in 30% aqueous ethanol utilizing guinea-pigs and the
Magnusson and Kligman maximization test involving the use of Freund's
adjuvant. No evidence of a sensitizing effect was found (Schuster,
1984).
Table 3. Results of genotoxicity studies on clenbuterol hydrochloride
Test system Test subject Concentration Results Reference
Bacterial Salmonella typhimurium 40-2500 µg/plate - Baumeister,
reversion TA98, TA100, TA 1535, 1978
TA1537, TA1538
Escherichia coli 40-1500 µg/plate
WP2(P)
Bacterial S. typhimurium 10-500 µg/plate - Ellenberger,
reversion TA98, TA 100, TA 1535, 1985
TA1537, TA1538
Forward Chinese hamster V79 10-100 µg/ml - Baumeister,
mutation fibroblasts, HGPRT 1985
assay locus
Forward Mouse lymphoma 300-800 µg/ml - Clements,
mutation L5178Y 1992
assay
In vitro human lymphocytes 177-2352 µg/ml +/- McEnaney,
cytogenetics 1992
In vivo mouse 0.006, 0.5, 5.0 - Friedmann,
micronucleus mg/kg bw per 1982
test day
In vivo Chinese hamster 19, 60, 186 - Holmstrom &
cytogenetics (bone marrow) mg/kg bw per MacGregor,
test day 1986
2.2.9 Special studies on pharmacodynamic effects
2.2.9.1 Studies in animals
Clenbuterol is a ß2-sympathomimetic agent with a wide range of
pharmacodynamic effects. It produces a potent, dose-dependent
bronchiolytic effect, which was demonstrated in guinea-pigs following
acetylcholine, histamine or bradykinin-induced bronchospasm. Similar
effects have been reported in cats and dogs. Clenbuterol exerts
positive chronotropic and inotropic effects on isolated atria. It
induces tachycardia in rats, dogs, cats and a variety of farm animals,
accompanied by reductions in systolic and diastolic blood pressure. In
the isolated uterine horn of the estrus rat, it exerts a pronounced
relaxing effect on serotonin, oxytocin and bradykinin-induced spasm.
It has also been shown to exert a relaxing effect on smooth muscle in
the guinea-pig ileum and to reduce intestinal mobility in the rat and
mouse.
Clenbuterol results in a glycogenolytic effect in the rat
myocardium associated with ß-stimulation. It has no effect on hepatic
glycogen. It causes a potent dose-dependent reduction of the gastric
secretion.
In mice, it causes decreases in spontaneous activity and also
leads to increases in barbiturate-induced sleeping time. The drug
results in dose-dependent striated muscle relaxation. However, it has
no antipyretic or analgesic effects in mice (Engelhardt, A., 1971;
Engelhardt, G., 1971, 1976).
Clenbuterol exerted major relaxing effects on spasms induced by
carbachol in guinea-pig trachea and lung. The effects were antagonised
by propranolol (Landry, 1983). It also led to decreases in perfusion
pressure of hind limb blood vessels, inhibitions of contractions of
the uterus, increases in atrial rate and inhibition of electrically
stimulated contractions of the ileum (O'Donnell, 1976). It improved
the rates of mucociliary clearance in the guinea-pig (Streller, 1981).
In beagle dogs, oral doses of clenbuterol hydrochloride at
1.5, 3.0, 7.5 and 15.0 µg/kg bw resulted in a dose-dependent
tachycardia at > 3.0 µg/kg bw. There was a slight tachyphylactic
effect demonstrated by a diminution of the intensity and duration of
the response. There was a dose-related reduction in blood pressure and
the changes in diastolic and systolic pressures ran in parallel. These
changes occurred at the lowest dose used (Ueberberg, 1976).
In cattle, intravenous doses of 0.6 µg/kg bw and 1.5 µg/kg bw
produced no cardiac effects, as shown by ECG examination, whereas
2.85 µg/kg bw produced pronounced tachycardia. A dose of 0.3 mg/kg bw
clenbuterol resulted in tocolysis lasting approximately 5 hours
(Ballarini, 1978). Different lag times in parturition may be induced
depending on the dose and route of treatment; no adverse effects on
the dam or calf were noted (Arbeiter & Thurner, 1977).
Four metabolites of clenbuterol that had been shown to be present
in the kidneys and liver of treated target animals were tested for
pharmacodynamic activity. Of these, only one, N-A 1141 (Figure 1) was
shown to have activity in the guinea-pig. Its bronchiolytic effect was
less than 20% that of clenbuterol, and, moreover, it accounted for
only 12% of residues in the liver and kidney 6 hours after treatment
(Engelhardt, 1971).
2.2.9.2 Studies in humans
Patients given 10 µg (0.167 µg/kg bw) clenbuterol by the
inhalation route showed no signs of tachycardia as determined by ECG.
There were slight decreases in blood pressure. When patients with
cardiac arrhythmia were given this dose of clenbuterol, none of the
patients showed any changes attributable to the drug (Hufnagel, 1974).
After inhalation exposure of patients with airways disease, onset
of broncholytic action occurred within 5 minutes and lasted for up to
6 hours. Pulmonary function effects were greatest with doses of >
5 µg (0.083 µg/kg bw); only a minimal effect was seen with 2.5 µg
(0.042 µg/kg bw) (Schuster et al., 1989).
2.3 Observations in humans
A single blinded cross-over study was carried out to examine the
acute bronchospasmolytic effect and possible side-effects following
oral administration of placebo and three doses of clenbuterol (1, 2.5
and 5 µg/day) in patients (three male and three female) with chronic
obstructive airway disease over a 3 day period. The drug was given
orally, diluted with water. Observations were carried out over a
2-hour period following dosing. The average age of the patients was
55.7 years and they had an average body weight of 73.16 kg.
None of the 3 doses produced any clear, consistent effects on
bronchial resistance, thoracic gas volume, radial pulse frequency or
blood pressure, and no side effects were seen.
The pharmacological NOEL in this study was 5 µg/day, equivalent
to 0.08 µg/kg bw per day (Kaik, 1978).
The bronchospasmolytic effect was examined in two groups of
patients:
Group A: ten patients aged 46-75 years with chronic obstructive
respiratory disease resulting from pulmonary tuberculosis.
Group B: five patients aged 56-67 years with chronic obstructive
respiratory disease not related to tuberculosis, plus five
patients aged 34-57 with bronchial asthma.
The bronchospasmolytic effect was examined after single oral doses of
1, 2.5, 5, 10, 20, 25 or 30 µg/person, and after a placebo dose.
In Group A patients, intrathoracic gas volume was significantly
reduced and vital capacity and pneumometer values significantly
increased at all dose levels. In Group B patients, airway resistance
was significantly reduced, but no dose relationship could be
demonstrated. No significant placebo effect was seen in either group.
When compared with placebo values, a significantly greater increase
for both vital capacity and pneumometer values was observed in Group
A, even at the lowest dose used in this group (5 µg). However, at the
two lowest doses used in Group B (1 and 2.5 µg), there were no
significant differences from placebo values. The pharmacological NOEL
in this study was 2.5 µg, equivalent to 0.042 µg/kg bw (Nolte &
Laumen, 1972; Nolte, 1980).
Children who consumed between 0.05-0.075 mg of clenbuterol showed
only mild tachycardia. A 30-year-old woman who consumed 30 tablets
equivalent to 0.6 mg clenbuterol (10 µg/kg approximately) developed
tachycardia and slight hypertension approximately 1 hour after
consumption. No tablet remains were found on gastric lavage, and
medicinal charcoal and a saline laxative were given. The following
day, the patient's pulse rate and blood pressure had returned to
normal (Boehringer, 1991a).
Patients (100+) administered doses of 20-60 µg/day (0.3-1.0 µg/kg
bw per day) for up to 1 year or 20 µg/day (0.3 µg/kg bw per day) for
up to 6 months showed no adverse effects except for slight tremor and
occasional, mild tachycardia (Laumen, 1978; Tullgren & Lins, 1987).
3. COMMENTS
The Committee considered toxicological data on clenbuterol,
including the results of acute, short-term and reproductive toxicity
studies, as well as studies on teratogenicity, genotoxicity and
carcinogenicity. Results of pharmacokinetic and pharmacodynamic
studies in animals and humans were also considered.
Clenbuterol is well absorbed after oral administration in a
number of animal species and in humans. An oral dose is largely and
rapidly excreted in the urine, and the majority of the remainder is
excreted in the faeces. The biotransformation of clenbuterol is
complex and a number of metabolites are formed. The major compound
found in a number of species was unchanged clenbuterol. After oral
administration of therapeutic doses to lactating cattle, clenbuterol
was found in the milk.
When radiolabelled clenbuterol was given orally to pregnant rats,
dogs, baboons and cattle, radioactivity was detected in the fetuses.
Clenbuterol was moderately toxic in mice and rats after oral
administration, LD50 values being in the range of 80-175 mg/kg bw.
It was less toxic in the dog (LD50 = 400-800 mg/kg bw). It was more
toxic after parenteral administration, with LD50 values in the range
of 30-85 mg/kg bw after intravenous administration. The main signs of
toxicity included lethargy, tachycardia and tonic-clonic convulsions
after oral administration.
The main effects noted in the repeat-dose studies were
tachycardia and, at higher doses, myocardial necrosis. These effects
are common with ß-agonist drugs. The myocardial necrosis was
considered to be secondary to hypoxia, due to reduced myocardial
perfusion at a time of high oxygen demand resulting from increased
cardiac rate.
In 30-day repeat-dose studies in mice and rats, NOELs of 2.5 and
1 mg/kg bw per day, respectively, were identified, largely based on
cardiac lesions. However, in a range of repeat-dose studies in rats
using doses of 0.01 to 100 mg/kg bw per day for durations of up to
18 months, administered through the oral, intravenous and inhalation
routes, no NOELs were identified. Effects were usually related to
cardiac function and were seen even at the lowest doses used.
Similarly, no NOELS could be identified in a range of repeat-dose oral
studies in dogs. These studies used doses ranging from 0.1 to 40 mg/kg
bw per day. In a 26-week inhalation study in cynomolgus monkeys, the
NOEL was 25 µg/kg bw per day, based on a number of observations
including cardiac effects.
No evidence of carcinogenicity was noted in a two-year oral study
in mice with doses of up to 25 mg/kg bw per day. In a two-year study
with doses of up to 25 mg/kg bw per day in the Chbb:THOM rat, no
evidence of carcinogenicity was seen. However, in Sprague-Dawley rats
given 25 mg clenbuterol/kg bw per day orally for 2 years, an increased
incidence of mesovarian leiomyomas occurred. With the related
compounds salbutamol in rats and medroxalol in mice, the effects could
be abolished by administration of the ß-blocking agent propranolol.
Mesovarian leiomyomas in rats and uterine leiomyomas in mice are known
to occur following long-term treatment with ß-adrenoceptor agonists
and the Committee concluded that these were due to adrenergic
stimulation and not to any genotoxic mechanism. Clenbuterol was not
genotoxic in a range of in vitro and in vivo genotoxicity studies.
Epidemiological studies indicate that there have been no
increased incidences of uterine leiomyomas in women following the use
of ß-adrenoceptor agonists.
Clenbuterol had no effects on fertility in a reproductive
toxicity study in rats using oral doses of 1-50 mg/kg bw per day from
10 weeks prior to mating in males and two weeks prior to mating in
females. However, doses of 50 mg/kg bw per day resulted in the deaths
of pups soon after birth. To investigate the cause of the high pup
mortality at this dose level, the litters of control dams were
exchanged with those from dams given 50 mg/kg bw per day. Pups from
rats given 50 mg/kg bw per day died on the first day of lactation
regardless of whether they suckled on treated or control dams. The
mechanism involved in this lethal effect is unknown. A NOEL was not
identified in this study, because pup weights at birth were reduced in
all treated animals.
In a reproductive toxicity study in which male rats were treated
with 1.5-15 µg clenbuterol/kg bw per day orally for 70 days prior to
mating and females with the same dose range for 14 days prior to
mating, no adverse effects on reproduction were noted. The NOEL was
15 µg/kg bw per day.
In teratogenicity studies in rats, oral doses of 10 and 100 mg/kg
bw per day produced teratogenic effects that included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of vertebrae. These were accompanied by signs of maternal
toxicity. The NOEL was 1 mg/kg bw per day. In three studies in rabbits
using doses of 30 µg to 50 mg per kg bw per day, signs of feto-
toxicity, including delayed ossification and cleft palate, occurred.
The NOEL was 30 µg/kg bw per day.
Clenbuterol produced a range of pharmacodynamic effects in a
number of animal species including tachycardia, hypertension and
muscle relaxing effects. These were seen at single doses as low as
0.8 µg/kg bw.
Four metabolites of clenbuterol that had been shown to be present
in the kidneys of treated target animals were tested for pharmaco-
logical activity. Of these, only one (N-A 1141) was shown to have
activity. Its broncholytic effect in the guinea-pig was less than
20% that of clenbuterol. In addition, it accounted for only 1-2% of
residues in the liver and kidney of target animals 6 hours after
treatment.
In humans, clenbuterol produced a bronchiolytic effect when a
single dose of 10 µg (0.167 µg/kg bw) was given by the inhalation
route, but no evidence of tachycardia was seen at this dose. With oral
doses of clenbuterol of up to 5 µg/day (0.08 µg/kg bw per day) over a
3-day period, there were no effects on bronchial resistance, thoracic
gas volume, cardiac rate or blood pressure. The NOEL in this study was
5 µg/day (0.08 µg/kg bw per day). In a study to investigate the
bronchospasmolytic effect in humans, patients with obstructive lung
disease were given oral doses of up to 30 µg per person. Patients
administered doses of 5 µg or more exhibited bronchospasmolytic
effects, and the pharmacological NOEL in this study was 2.5 µg per
person, equivalent to 0.04 µg/kg bw.
4. EVALUATION
The Committee considered the most relevant study for determining
the ADI to be that concerning the bronchospasmolytic effect in humans.
The patients had chronic obstructive airway disease and thus were
likely to be a very sensitive population for this effect. The NOEL
identified in this study (2.5 µg per person, equivalent to 0.04 µg/kg
bw) is approximately 25% of the dose in another study in which the
inhalation route was used, but in which cardiac effects were not
observed. This NOEL is approximately 50% of the oral dose used in
another study where, again, cardiac effects did not occur. Hence, this
NOEL for the bronchospasmolytic effect offers an additional safety
margin for cardiac effects. The Committee therefore established an ADI
of 0-0.004 µg/kg bw, based on the NOEL of 0.04 µg/kg bw per day for
pharmacodynamic effects in humans and a safety factor of 10.
5. REFERENCES
Amemiya, K., Kudoh, M., Suzuki, H., Saga, K., & Hosaka, K. (1984).
Toxicology of mabuterol. Arzneim.forsch, 34(11a), 1680-1684.
Arbeiter, K. & Thurner, M. (1977). Effect of the sympathomimetic
Planipart (NAB 365) on parturition in cattle. Tierärztl. Umsch.,
32, 423-427.
Bailey, D.E. (1990). Primary eye irritation study in rabbits with
Ventipulmin. Unpublished report No. TX-9005/Ventipulmin from Hazleton
Laboratories America, Inc., Vienna, VA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Baillie, H.W., Cameron, B.D., Draffan, G.H., & Schmid, J. (1980).
Investigations of the placental transfer of 14C-N-AB 365 CL in the
cow. Unpublished report No. 111674 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Balazs, T. & Bloom, S. (1982). Cardiotoxicity of adrenergic and
vasodilating antihypertensive drugs. In: van Stee, E.W. (ed.),
Cardiovascular Toxicology, Raven Press, New York, pp. 199-220.
Ballarini, G. (1978). Initial studies of the clinical pharmacology of
the ß2 sympathomimetic agent NAB 365 (Planipart) and its use for
pharmacological tocolysis in cows. Tierärztl. Umsch., 33, 421-427.
Baumeister, M. (1978). Mutagenicity studies with the substance
N-AB 365 CL in the plate incorporation assay (Ames test). Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Baumeister, M. (1985). Mutagenicity study with the substance
N-AB 365 CL in the V79 (HGPRT)-test. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Boehringer (1991a). Summary of case reports of overdosage with
clenbuterol. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Boehringer (1991b). Estimated transdermal absorption of clenbuterol
hydrochloride following skin contact with granules and injection
solutions for veterinary use. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987a). The residue kinetics of
[14C]-NAB 365 CL in the calf. Unpublished report No. 4371 from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987b). The residue kinetics of
(14C)-NAB 365 CL in the lactating cow. Unpublished report No. 4445
from Inveresk Research International. Submitted to Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, D.M., Crook, D., & Brown, G. (1992). Ventipulmin injection
solution and Ventipulmin granules. Target species tolerance study in
calves. Unpublished report No. BOI 139/921030 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Chasseaud, L.F., Cresswell D.G., & Savage J.A. (1978).
Pharmacokinetics of 14C-N-AB 365 during chronic inhalational
toxicity in cynomolgus monkeys. Unpublished report No. U79-0212 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Clark, G.C., Collins, C.J., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D.E., Edmonson, N., Lewis, D.J, & Mahjeed, S.K. (1979).
Investigation of the effects on rats of inhalation of NAB 365 CL for
26 weeks. Unpublished report No. BOI 88/78768 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Clements, J. (1992). Study to determine the ability of clenbuterol
hydrochloride to induce mutations at the thymidine kinase (tk) locus
in mouse lymphoma L5178Y cells using a fluctuation assay. Unpublished
report No. 2TKREBSG.002 from Hazleton Microtest. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Collins, C.J., Clark, G.C., Heywood, R., Street, A.E., Hardy, C.J.,
Cherry, C.P., Gibson, W.A., Gopinath, C., Harrington S.M., Edmonson,
N.A., & Howard, E. (1979). Investigation of the effects on monkeys of
inhalation of NAB 365 CL aerosol for a period of 26 weeks. Unpublished
report No. BOI 90/78712 from Huntingdon Research Centre. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
De Busk, R.F. & Harrison, D.C. (1969). The clinical spectrum of
papillary-muscle disease. N. Engl. J. Med., 281, 1458-1467.
Ellenberger, J. (1985). Clenbuterol (NAB-365-CL) testing for
point-mutagenic activity with Salmonella typhimurium. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, A. (1971). Pharmacological exposé of the substance NAB365.
Supplement to the exposé on the compound NAB 365. Supplementary
investigations (May 1976) on the pharmacology of the substance NAB 365
and its metabolites. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Engelhardt, G. (1971). Results of a comparative pharmacological
investigation of NAB 365 CL, NAB 365 CL-D and NAB365 CL. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, G. (1976). Pharmacological profile of the action of
NAB 365 (clenbuterol), a new bronchodilator with a selective effect on
the adrenergic ß2 receptors. Arzneim. Forsch., 26(7a), 1404-1420.
Erichsen, D.F. (1988). Dose titration of Ventipulmin syrup in horses
with chronic obstructive pulmonary disease (COPD). Trial
No: 37-295-86-3. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Erichsen, D.F. (1989). A determination of incremental dose tolerance
of Ventipulmin syrup in the horse. Trial No.: Range finder 84-VI.
Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica, Ingelheim am Rhein, Germany.
Fenner, A. (1982). Respiratory rate, minute volume and tidal volume in
clinically healthy fattening cattle and those with bronchopneumonia -
taking into account the effects of clenbuterol. Doctoral Thesis,
Veterinary Faculty, Ludwig-Maximilians University of Munich. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Friedmann, J.Ch. (1982). Study of the possible mutagenic activity of
the substance "NAB365Cl" evaluated by the micronucleus test (oral
route). Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Gibson, J.P., Sells, D.M., Cheng, H.C., & Yuh, L. (1987). Induction of
uterine leiomyomas in mice by medroxalol and prevention by propanolol.
Toxicol. Pathol., 15, 468-473.
Gopinath, C. & Gibson, W.A. (1987). Mesovarian leiomyomas in the rat.
Environ. Health Perspect., 73, 107-113.
Hamm, D. & Erichsen, D.F. (1989). Acute and subacute toxicity of
Ventipulmin. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Roberts, N.L., & Cameron,
D.M. (1984). The disposition of the combination product14C-NAB
365 CL/trimethoprim/sulphadiazine after oral administration to horses.
Unpublished report No. HRC/BOI 112/84912 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R., Roberts,
N.L., Cameron, D.M., Offer, J., & Fish, C. (1985a). The disposition of
the combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
calves. Unpublished report No. HRC/BOI 111/8468OB from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R.,
Roberts, N.L., & Cameron, D.M. (1985b). The disposition of the
combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
dairy cows. Unpublished report No. HRC/BOI 111/84680A from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A., & Cameron, D.M.
(1993a). The pharmacokinetics, metabolism and residues of
14C-clenbuterol (14C-N-AB 365 CL) following intramuscular
administration to calves. Unpublished report No. HRC/BOI 140/921418
from Huntingdon Research Centre. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., & Dighton, M.H. (1993b). Investigation of
the metabolic profiles in the livers of the horses following multiple
oral administration. Unpublished report No. BOI 149/931490 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hewett, Chr. & Notman J. (1984). Dermal tolerance test with repeated
topical application in the rabbit. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Holmstrom, M. & MacGregor, D.B. (1986). NAB 365 CL. Cytogenetic study
in Chinese hamsters. Unpublished report No 4085 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hufnagel, E. (1974). Results with a new broncholytic agent. Effect on
heart rate, blood pressure and ECG. Ärztl. Praxis, 28, 1350-1352.
Jack, D., Poynter, D., & Spurling, N.W. (1983). Beta-adrenoceptor
stimulants and mesovarian leiomyomas in the rat. Toxicology,
27, 315-320.
James, C.N. (1990a). Acute oral toxicity in rats with Ventipulmin
syrup. Unpublished report No. TX-9003/VENTIPULMIN. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990b). Acute dermal toxicity study in rabbits with
Ventipulmin syrup. Unpublished report No. TX-9002/VENTIPULMIN.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
James, C.N. (1990c). Guinea pig sensitisation study - Buehler method
using Ventipulmin syrup. Unpublished report No. Case #: 64551-05 from
Arthur D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990d). Primary dermal irritation study in rabbits with
Ventipulmin syrup. Unpublished report TX-9001/Ventipulmin from Arthur
D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Dunsire, J.P. (1993). Plasma kinetics, metabolism,
excretion and residue kinetics of [14C]-N-AB 365 CL following oral
administration to the horse. Unpublished report No. 8346 from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Jenner, W.N. (1976). Study of the metabolism of
14C-labelled N-AB 365 in the male baboon. Unpublished report from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, W. (1987). Ventipulmin solution Bovine safety trial.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kaik, G. (1978). Clinico-pharmacological tests using the ß2-sympa-
thomimetic NAB 365 (clenbuterol) in patients with obstructive
respiratory disease. Unpublished report from the Medical University
Clinic of Vienna. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973a). Subacute toxicity with compound NAB 365 CL on mice,
oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973b). Teratological testing with the compound N-AB 365-CL
on rats, oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973c). Teratological testing with the compound NAB-365 CL
on rabbits, oral administration. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1975). Teratological testing with the compound N-AB 365 CL
on rats, oral administration during the period of organogenesis.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973a). Subacute toxicity with compound
NAB 365 CL on rats, oral administration. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973b). Chronic toxicity of compound
NAB 365 CL on rats, oral administration per food. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973c). Subacute toxicity with compound
NAB 365 CL on rats, intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kirkland, D.J. (1992). Review of clenbuterol hydrochloride mutagen-
icity data in relation to the proposed acceptable daily intake.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Koellmer, H., Stotzer, H., & Paulini, K. (1977). Subacute toxicity
study of the substance N-AB 365-CL in rats using inhalation over a
period of 13 weeks. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1969). Autoradiographic investigations on the
distribution of [14C]-N-AB 365 CL in rats and pregnant mice
(ADME 1 B). Unpublished report No. U69-0108. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1970). Pharmacokinetic investigations with [14C]-N-AB
365 CL in rats (ADME 1 B). Unpublished report No. U69-0109. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein,
Germany.
Kopitar, Z & Zimmer, A. (1973). Effect of repeated doses of NAB 365 CL
on the pharmacokinetic profile in rats (ADME 1 D). Unpublished report
No. U73-0158. Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim
am Rhein, Germany.
Kreuzer, H. (1988). Intramuscular local tolerance in tests with
Ventipulmin injectable solution ad us. vet. in rabbits. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Landry, Y. (1983). Review of the chief pharmacological properties,
clinical structures and physiochemical properties of clenbuterol.
Unpublished report from Laboratoire d'Allergopharmacologie,
Strasbourg. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Laumen, F. (1978). Investigation on long-term treatment and cumulation
with clenbuterol. Med. Mon.schr., 29, 455-459.
Lehman, H. (1969a). Teratology investigations in pregnant rats with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1969b). Teratology investigations in pregnant rabbits with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1974). Testing of the compound NAB 365 CL for perinatal
toxicity in rats. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1975). Study with substance N-AB 365 CL for fertility
inhibiting, teratogenic and perinatal toxic effects in rats.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1980). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 78 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1981). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 73 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Otto, H., &
Dontenwill, W. (1969). Report on subacute toxicity studies of NAB 365
p. o.- batch Z 6191 in beagle dogs. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
McEnaney, S. (1992). Damaging potential of clenbuterol by its effects
on cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished report No. 2HLREBSG.002 from Hazleton Microtest. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Magnusson, G. & Hansson, E. (1973). Myocardial necrosis in the rat: a
comparison between isoprenaline, orciprenaline, salbutamol and
terbutaline. Cardiology, 58, 174-180.
Nelson, L.W. & Kelly, W.A. (1971). Mesovarian leiomyomas in rats in a
chronic toxicity study of soterenol hydrochloride. Vet. Pathol.,
8, 452-457.
Nishikawa, J., Tsunenari, Y., & Kast, A. (1972). Acute toxicity of NAB
365 CL in rats, mice and rabbits. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Nishikawa, J., Kast, A., & Klupp, H. (1979). Acute toxicity study with
clenbuterol (NAB 365 CL) in rats dosed orally or intravenously.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Nolte, D. (1980). Comments on the publication "Lung function tests
with the bronchospasmolytic agent NAB 365". Unpublished report.
Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim am
Rhein, Germany.
Nolte, D. & Laumen, F. (1972). Pulmonary function tests following the
bronchospasmolytic agent NAB 365. Unpublished report from Medical
Clinics and Polyclinics, Justus-Liebig University and Seltersberg
Sanatorium of Hessen State Insurance Association, Giessen, Germany.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
O'Donnell, S.R. (1976). Selectivity of clenbuterol (NAB 365) in
guinea-pig isolated tissues containing ß-adrenoceptors. Arch. Int.
Pharmacodyn., 224, 190-198.
Owen, R., Fischer, R., & Erichsen, D.F. (1990). Safety of Ventipulmin
syrup in horses: 64-day study trial No. 37-295-83-4. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Palmer, A.K., Edwards, J.A., & Collins, C.J. (1978). Effect of
NAB 365 CL metered aerosol on pregnancy of the rat. Unpublished report
No. BOI 85/78689 from Huntingdon Research Centre. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Palmer, A.K., Bottomley, A.M., & Collins, C.J. (1980). Effect of
NAB 365 CL metered aerosol on pregnancy of the New Zealand White
rabbit. Unpublished report No. BOI 86/79656 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Pappritz, G. (1968). Determination of ALD50 of the substance in the
dog after oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. (1972). Determination of the ALD50 Of NA-B 365 in the
dog following intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Pappritz, G. & Bauer, M. (1973a). Supplementary studies on the
subacute toxicity of substance N-AB 365 CL in dogs after oral
administration. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. & Bauer, M. (1973b). Chronic toxicity study of substance
NAB 365 in dogs following oral administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica, Ingelheim am
Rhein, Germany.
Pappritz, G., Bauer, M., & Eckenfels, A. (1973). Subacute toxicity of
NAB 265 CL in dogs following intravenous administration. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica,
Ingelheim am Rhein, Germany.
Poynter, D & Spurling, N.W. (1971). Some cardiac effects of
beta-adrenergic stimulants in animals. Postgrad. Med. J., 47, 21-25.
Poynter, D., Harris, D.M., & Jack, D. (1978). Salbutamol: lack of
evidence of tumour induction in man. Br. Med. J., 7 January.
Richter, I. (1982). Biochemical investigations of the placental
passage of 14C-clenbuterol in pregnant rats. Unpublished report
No. U82-0292. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Roberts, W.C., & Cohen, L.S. (1972). Left ventricular papillary
muscles. Description of the normal and a survey of conditions causing
them to be abnormal. Circulation, XLVI, 138-154.
Rohr, U.D., Haczkiewicz, K., Mohr, A., & Orton, B. (undated).
Validation of an excised human skin model for testing drug candidates
suited for transdermal drug delivery. Unpublished report from
Boehringer Ingelheim KG. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rominger, K.L. & Schrank, H. (1982). Placental transfer of
14C-clenbuterol in the dog. Unpublished report No. U82-0291.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Rominger, K.L., Förster, H., Hermer, M., Peil, H., & Wolf, M. (1987).
Clenbuterol plasma levels in patients under tocolytic treatment.
Unpublished report from Boehringer Ingelheim KG. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rosenblum, I., Wohl, A., & Stein, A.A. (1965). Studies in cardiac
necrosis. III. Metabolic effects of sympathomimetic amines producing
cardiac lesions. Toxicol. Appl. Pharmacol., 7, 344-351.
Schmid, J. (1977). Pilot pharmacokinetic investigations after
intravenous administration of N-AB 365 CL in a cow. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Schmid, J. (1980). Investigations of the placental transfer of
14C-N-AB 365 CL in the baboon. Unpublished report from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1982). Pharmacokinetics, metabolism and tissue
distribution of 14C-N-AB 365 CL in the baboon. Unpublished report
No. 2220 from Inveresk Research International. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990a). Plasma levels and residue analysis in cows and
calves. Unpublished report No. U90-0092. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990b). N-AB 365 CL in the cow's milk after oral and
intramuscular administration. Unpublished report No. U90-0099.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Prox, A. (1986). Isolation and structural elucidation of
NAB 365 CL metabolites from dog urine. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Zimmer, A. (1977a). Pilot pharmacokinetic studies
following oral administration (single and multiple dosing) of
14C N-AB 365 CL to a cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. & Zimmer, A. (1977b). Pilot pharmacokinetic investigations
after intramuscular administration (single and multiple doses) of
N-AB 365 CL in the cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schuster, A. (1984). Skin sensitisation study in guinea pigs.
Unpublished report No. U84-0673 from Boehringer Ingelheim KG.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schuster, D., Deichsel, G., & Feuerer, W. (1989). Dose effect study
with NAB 365 CL metered dose inhaler in patients with chronic
obstructive lung disease. Unpublished report from Dr. K. Thomae GmbH,
Medical Department. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Sells, D.M. & Gibson, J.P. (1987). Carcinogenicity studies with
medroxalol hydrochloride in rats and mice. Toxicol. Pathol.,
15, 457-467.
Serbedija, R. & Bauer, M. (1973). Toxicity study of the substance
NAB 365 CL in rats over a period of 18 months. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Serbedija, R., Lutzen, L., Puschner, H., & Hohbach, Ch. (1982).
Carcinogenicity study with the substance N-AB 365 CL in rats with oral
administration for a period of 2 years. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Streller, I. (1981). Effect of the ß-adrenergic agents fenoterol,
orciprenaline, clenbuterol, SOM 987 and SOM 1122 on the mucocilliary
clearance in the trachea of the anaesthetised guinea-pig. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Tullgren, A & Lins L-E. (1987). An open long-term tolerability study
on NAB 365 (clenbuterol). Unpublished report from Boehringer Ingelheim
AB, Stockholm, Sweden. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Ueberberg, H. (1976). Comparative study of the effects of NAB 365 CL
and terbutaline sulfate on the dog heart. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Umemoto, K. (1984). Carcinogenicity studies with the beta-adreno-
ceptor stimulant clenbuterol (NAB-365 CL) in mice by drinking water.
Unpublished report from Nippon Boehringer Ingelheim. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1971). Metabolism and species comparison in the rat,
rabbit and dog (ADME IV). Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974a). Comparison of the pharmacokinetic profile in the
dog with single and repeated dosage (ADME 1 D). Unpublished report
No. U73-0161. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Zimmer, A. (1974b). Pharmacokinetics and metabolism pattern in the
rabbit and dog (ADME I). Unpublished report No. U74-0116. Submitted to
WHO by Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974c). Metabolism in man; comparison with dog and rabbit,
using radioactive substance. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1976). Administration of clenbuterol in man. Single doses,
multiple doses, and metabolite samples. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Zimmer, A. (1977). Preliminary investigations of pharmacokinetics in a
horse. Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zupan, J. A. (1985). A comparative bioavailability study between oral
and injectable dosage forms of clenbuterol in horses. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Mice
Following a single intravenous dose of 5 mg/kg bw 14C-clenbu-
terol to pregnant mice, autoradiography demonstrated high levels of
radioactivity in the placenta with lower levels in the fetuses within
30 minutes after administration. Radioactivity was also found in the
fetal liver, thymus and spinal column. Total radioactivity in the
fetuses was significantly less than in maternal animals (Kopitar,
1969).
2.1.1.2 Rats
After oral administration of 2 mg/kg bw 14C-clenbuterol to
rats, approximately 60% of the radioactivity was found in the 48-hour
urine sample suggesting good absorption from the gastrointestinal
system. A further 20% was recovered from faeces over this time. Around
5-10% was recovered in urine and faeces in the 48-72 hour period.
Approximately 7% appeared in the bile. Levels in tissues were
relatively low, except for the liver, kidneys and lungs (Kopitar,
1970).
In a separate study using autoradiography, maximum distribution
after oral doses of 5 or 10 mg/kg bw occurred approximately 3 hours
after administration. Highest concentrations of radioactivity were
found in the lungs, liver, kidneys, pancreas and bone marrow, but some
radioactivity was also found in the brain, adrenal glands, and
skeletal and cardiac muscles (Kopitar, 1969).
When previously untreated rats were given a single oral dose of
5 mg/kg bw clenbuterol by gavage, plasma levels of the drug were
found to be 3-5 times lower than in rats pre-treated with the drug at
5 mg/kg bw per day for 6 months. The single dose was found to abolish
gastrointestinal peristalsis in the rat but after 6 months of compound
administration, peristalsis returned. This may explain the lower, but
prolonged plasma levels after the single administration, which
probably led to a depot effect in the gut (Kopitar & Zimmer, 1973).
Clenbuterol readily enters the placenta in the pregnant rat. At 3
hours after an intravenous or oral dose of 14C clenbuterol, there
was more radioactivity in the placenta than in the blood and tissues
of maternal animals or fetuses (Richter, 1982).
2.1.1.3 Dogs
When beagle dogs were given capsules containing 14C-clen-
buterol at a dose of 2.5 mg/kg bw, maximum blood and plasma levels
occurred 8 hours after dosing. Around 85% of the dose was recovered in
the urine after 96 hours, with 4-9% in the corresponding faecal
samples. Pretreating dogs for 5 weeks with 2.5 mg/kg bw per day
clenbuterol led to a slight increase in the rate of absorption in a
manner similar to that noted in rats (Zimmer, 1974a).
After single oral doses of 2.5 mg/kg bw, blood concentrations in
the dog were approximately twice those of rabbits given identical
doses (Zimmer, 1974b).
Clenbuterol was found to cross the placenta and enter the fetuses
in the dog when a single pregnant female was given an oral dose of
2.5 mg/kg bw 14C-clenbuterol. At 4 hours after administration the
concentration of radioactivity in fetal plasma was around 16% of that
in maternal plasma. Approximately 0.4% of the total dose administered
to the maternal animal was recovered in the fetuses at 4 hours after
administration (Rominger & Schrank, 1982).
2.1.1.4 Monkeys
After intravenous administration of 100 µg 14C-clenbuterol per
animal (approximately 30 µg/kg bw) to cynomolgus monkeys, around 60%
of the dose was recovered in the urine and 4% in the faeces within 72
hours of administration. There was biphasic elimination. It was not
possible to calculate the half-life for the first and rapid phase, but
the second phase half-life was 20-30 hours (Chasseaud et al.,
1978).
2.1.1.5 Baboons
Following a single intravenous dose of 2.5 mg/kg bw 14C-clen
buterol to male baboons, around 82% of the administered dose was
recovered in the urine and faeces within 5 days, with the majority of
this (72%) within 48 hours. Of the 82%, approximately 68% was recovered
in the urine and 14% in the faeces. A similar pattern was noted in
baboons given the drug orally. There was a rapid distribution phase and
the highest levels of radioactivity were found in the lungs, liver and
kidneys (Schmid, 1982).
Placental transfer of 14C-clenbuterol was investigated in a
single pregnant baboon given 3.3 mg/kg bw intravenously. The animal
was killed 3.5 hours after dosing, and around 1.5% of the total
administered close was found in the fetus (Schmid, 1980).
2.1.1.6 Cattle
In cattle given 0.8 µg/kg bw 14C-clenbuterol using a single
intramuscular injection, the liver was found to have the highest
concentrations of radioactivity. In muscle, soon after administration,
the radioactivity was due almost entirely to parent compound (Schmid,
1990a).
In a pilot study with a single cow given 0.8 µg/kg bw 14C-
clenbuterol orally, the first phase plasma elimination half-life
was 0.1 hours and the second phase elimination half-life 17 hours. The
maximum plasma concentration was 0.4 µg/ml with tmax at 8.5 hours.
Approximately 81% of the dose was excreted in the urine with 13% in
the faeces over the 96-hour period after dosing; around 1% was
excreted in the milk (Schmid & Zimmer, 1977a). Similar results were
noted after intramuscular (Schmid & Zimmer, 1977b) and intravenous
(Schmid, 1977) administration, but with the latter route plasma levels
rose much more rapidly. In both cases, radioactivity was found in the
faeces suggesting biliary excretion.
When calves were given 0.8 µg/kg bw 14C-clenbuterol intramus-
cularly, twice a day for 2 days and then orally twice a day for 3 days
in a consecutive manner, using a combination product also containing
trimethoprim and sulfadiazine, around 64% of the dose was excreted
during 15 days in urine and 5% in the faeces. The highest level of
radioactivity was found in injection site muscle, liver and kidney
(Hawkins et al., 1985a). Similar results were seen in another study
where cattle were given intramuscular injections of 14C-clenbuterol
(Cameron & Phillips, 1987a).
In cattle given 21 intramuscular doses of 14C-clenbuterol at a
dose level of 0.8 µg/kg bw per day, twice daily, the majority of the
dose was recovered in the urine. The plasma half-life following the
last dose was of the order of 4 days. Highest levels of radioactivity
were found in liver and kidney, with much lower levels in muscle and
fat (Hawkins et al., 1993a).
After administration of 7 µg/kg bw 14C-clenbuterol by the
intramuscular route to a pregnant cow, radioactivity crossed the
placenta and was found in fetal tissues, including skeletal tissues,
kidneys and liver, when the animal was killed 3 hours after the
dosing. Radioactivity was also found in the maternal and fetal eyes
(Baillie et al., 1980).
When lactating cattle were given oral or intramuscular doses of
14C-clenbuterol at a dose of 0.8 µg/kg bw, radioactivity was found
in the urine for 48-70 hours after administration. In the first 48
hours, the majority of the radioactivity in milk was attributable to
parent compound (Schmid, 1990b).
Lactating dairy cows treated with 0.8 µg/kg bw 14C-clen-
buterol, twice daily by the intramuscular route for 3 consecutive days,
and twice daily by the oral route for a further 2 days followed by a
single oral dose on day 6, were found to have milk concentrations in
the range of 0.76-1.03 µg/litre 12 hours after the first dose rising
to 2.14-2.6 µg/litre after 48 hours. They remained in the range 1.46
to 3.93 µg/litre throughout the dosing period. Highest tissue concen-
trations were at the injection site, liver and kidney (Hawkins et al.,
1985b). Similar results were obtained in another study where dairy
cattle were given oral, intravenous or intramuscular doses of 0.8 µg/kg
14C-clenbuterol. In this study approximately 2% of administered
radioactivity was found in milk (Cameron & Phillips, 1987b).
2.1.1.7 Horses
After administration of a single oral dose of 0.8 µg/kg bw
14C-clenbuterol to a horse, the maximum plasma concentration of
0.4 µg/ml was reached at approximately 2 hours. The elimination
half-life was 21 hours. After 144-168 hours, 81% of the administered
dose was recovered in urine. Following intravenous administration, 82%
of the administered dose was found in urine, with around 9% in faeces,
suggesting biliary excretion (Zimmer, 1977).
In a study with 10 horses, animals were given a single oral dose
of 0.8 µg/kg bw clenbuterol followed by a single intravenous dose 3
weeks later. A second group of 10 horses was treated in a similar
manner, but initially using the intravenous route with the oral dose
after 3 weeks. Approximately 20-50% of the oral dose (mean 36%) as
parent drug, was excreted over 108 hours in the urine, while,
following intravenous administration, some 17-70% of the dose
(mean 43%) was excreted in the 108-hour urine (Zupan, 1985).
In a study with a clenbuterol, trimethoprim and sulfadiazine
combination product, horses were given gelatin capsules containing
14C-clenbuterol at a dose of 0.8 µg/kg bw, twice daily for 10 days,
with a single oral dose on day 11. The majority of the dose (70-77%)
was excreted in the urine and 10-15% in the faeces. The maximum plasma
concentration was achieved 3-4 hours after the first administration
and the plasma half-life was 9-10 hours. The mean plasma concentration
was 0.6-1.2 µg/ml. Highest levels of radioactivity were found in the
liver and kidneys with very little in fat (Hawkins et al., 1984).
Similarly, when 12 horses were given 21 twice daily oral doses of
0.8 µg/kg bw 14C-clenbuterol, steady-state plasma levels were
rapidly achieved. Peak levels of 1-1.9 ng equivalents/ml occurred 1-3
hours after dosing. The terminal half-life was 22 hours. Around 75-91%
of the dose was excreted in the urine and 6-15% in the faeces. When
the horses were killed 3 or 12 hours and 9, 12 or 28 days after
dosing, the highest levels of radioactivity were found in the liver
and kidney. Levels in fat were extremely low (Johnston & Dunsire,
1993).
2.1.2 Biotransformation
The biotransformation of clenbuterol in rats, rabbits and dogs is
complex. Five urinary metabolites have been identified in dogs, and
eight in the rat and rabbit. Unchanged clenbuterol is the major
compound found in the urine of these species, and a range of oxidative
and conjugated metabolites are formed. Of these, 4-amino-3,5-dichloro-
mandelic acid, 3-amino-3,5-dichlorobenzoic acid and 4-amino-3,5-dichlo-
rohippuric acid are the major components. In the baboon, unchanged
parent drug is the major urinary component (18%), again with a range
of metabolites (Zimmer, 1971, 1974b; Johnston & Jenner, 1976; Schmid,
1982; Schmid & Prox, 1986).
In cattle, the major compound found in urine (28-52%) and in the
liver (50-80%) after administration of clenbuterol was the parent
compound. Small amounts of 4-amino-3,5-dichlorobenzoic acid were found
in urine and liver, and 4-amino-3,5-dichlorohippuric acid was detected
in urine (Baillie et al., 1980; Hawkins et al., 1993a). In
muscle, the main component was parent compound (Schmid, 1990a).
In horses also, parent clenbuterol was the major compound found
in urine (30-50%). It was also the major component in liver and kidney
(Zimmer, 1977; Hawkins et al., 1984; Johnston & Dunsire, 1993). A
study to examine specifically the nature of the metabolites in horse
liver confirmed clenbuterol as the major component, but with a smaller
quantity of a metabolite identified as 1-(4-amino-3,5-dichlorophenyl)-
1,2-ethanediol (Hawkins et al., 1993b).
None of the studies with any species revealed evidence of
significant covalent binding of clenbuterol or its metabolites.
Overall, the data suggest that the metabolism of clenbuterol is
similar in all the species studied and that the major differences are
quantitative rather than qualitative. The proposed metabolic pathways
are shown in figure 1.
2.1.3 Kinetics in humans
Following a single oral dose of 20 µg 14C-clenbuterol hydro-
chloride to volunteers, 67% of the administered dose was excreted in
the urine; faecal excretion was very low. The major compound present
in the urine was unchanged parent drug. The metabolic profile was
similar to that noted in animals (Zimmer, 1974c).
When a single dose of 20 µg 14C-clenbuterol hydrochloride was
administered orally to volunteers, the highest plasma level was 0.11
µg/litre, with a tmax, of 2-3 hours. Around 87% of the administered
radioactivity was detected in the urine; correspondingly lower levels
of radioactivity were found in the faeces.
In a repeat-dose phase of the same study, volunteers were given
40 µg 14C-clenbuterol for 2 days and then 20 µg for a further 2
days. Around 75% of the dose was detected in the urine. The metabolite
profile was similar to that noted in animals, and the major urinary
component was unchanged parent drug; the terminal half-life was around
34 hours (Zimmer, 1976).
Oral doses of 80 µg clenbuterol hydrochloride were given to 12
pregnant women in premature labour. These were followed 12 hours later
by 2 oral doses of 40 µg, and then by maintenance doses of 20 µg,
generally at 12-hour intervals. Blood samples were taken on days 2, 4,
6 and 8 of study, 3 hours after the morning dose. A steady-state
average concentration of 0.298 µg/litre was achieved, with a minimum
of 0.28 and a maximum of 0.344 µg/litre. Bioavailability was > 80%
(Rominger et al., 1987).
There are no data on in vivo percutaneous absorption of
clenbuterol in humans. However, data using a model which utilizes
excised human skin (Rohr et al., undated) suggests that some
absorption can occur (Boehringer, 1991b).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies after administration of
clenbuterol are summarized in Table 1.
Signs of toxicity in mice, rats and rabbits included lethargy,
increased heart rates, and tonic-clonic convulsions. After intravenous
dosing, death, when it occurred, did so either during injection or
immediately after it. In dogs, all the oral doses employed
(400-800 mg/kg bw) caused increases in heart rate. Salivation,
lethargy and tonic-clonic convulsions also occurred.
In cattle, single intravenous doses of up to 3.6 µg/kg bw
resulted in increases in heart and respiratory rates (Johnson, 1987).
Single oral doses of 2.4-12.0 µg/kg bw in horses resulted in sweating
and elevated heart rates and body temperatures at all dose levels
(Hamm & Erichsen, 1989).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Mice
Following a single intravenous dose of 5 mg/kg bw 14C-clenbu-
terol to pregnant mice, autoradiography demonstrated high levels of
radioactivity in the placenta with lower levels in the fetuses within
30 minutes after administration. Radioactivity was also found in the
fetal liver, thymus and spinal column. Total radioactivity in the
fetuses was significantly less than in maternal animals (Kopitar,
1969).
2.1.1.2 Rats
After oral administration of 2 mg/kg bw 14C-clenbuterol to
rats, approximately 60% of the radioactivity was found in the 48-hour
urine sample suggesting good absorption from the gastrointestinal
system. A further 20% was recovered from faeces over this time. Around
5-10% was recovered in urine and faeces in the 48-72 hour period.
Approximately 7% appeared in the bile. Levels in tissues were
relatively low, except for the liver, kidneys and lungs (Kopitar,
1970).
In a separate study using autoradiography, maximum distribution
after oral doses of 5 or 10 mg/kg bw occurred approximately 3 hours
after administration. Highest concentrations of radioactivity were
found in the lungs, liver, kidneys, pancreas and bone marrow, but some
radioactivity was also found in the brain, adrenal glands, and
skeletal and cardiac muscles (Kopitar, 1969).
When previously untreated rats were given a single oral dose of
5 mg/kg bw clenbuterol by gavage, plasma levels of the drug were
found to be 3-5 times lower than in rats pre-treated with the drug at
5 mg/kg bw per day for 6 months. The single dose was found to abolish
gastrointestinal peristalsis in the rat but after 6 months of compound
administration, peristalsis returned. This may explain the lower, but
prolonged plasma levels after the single administration, which
probably led to a depot effect in the gut (Kopitar & Zimmer, 1973).
Clenbuterol readily enters the placenta in the pregnant rat. At 3
hours after an intravenous or oral dose of 14C clenbuterol, there
was more radioactivity in the placenta than in the blood and tissues
of maternal animals or fetuses (Richter, 1982).
2.1.1.3 Dogs
When beagle dogs were given capsules containing 14C-clen-
buterol at a dose of 2.5 mg/kg bw, maximum blood and plasma levels
occurred 8 hours after dosing. Around 85% of the dose was recovered in
the urine after 96 hours, with 4-9% in the corresponding faecal
samples. Pretreating dogs for 5 weeks with 2.5 mg/kg bw per day
clenbuterol led to a slight increase in the rate of absorption in a
manner similar to that noted in rats (Zimmer, 1974a).
After single oral doses of 2.5 mg/kg bw, blood concentrations in
the dog were approximately twice those of rabbits given identical
doses (Zimmer, 1974b).
Clenbuterol was found to cross the placenta and enter the fetuses
in the dog when a single pregnant female was given an oral dose of
2.5 mg/kg bw 14C-clenbuterol. At 4 hours after administration the
concentration of radioactivity in fetal plasma was around 16% of that
in maternal plasma. Approximately 0.4% of the total dose administered
to the maternal animal was recovered in the fetuses at 4 hours after
administration (Rominger & Schrank, 1982).
2.1.1.4 Monkeys
After intravenous administration of 100 µg 14C-clenbuterol per
animal (approximately 30 µg/kg bw) to cynomolgus monkeys, around 60%
of the dose was recovered in the urine and 4% in the faeces within 72
hours of administration. There was biphasic elimination. It was not
possible to calculate the half-life for the first and rapid phase, but
the second phase half-life was 20-30 hours (Chasseaud et al.,
1978).
2.1.1.5 Baboons
Following a single intravenous dose of 2.5 mg/kg bw 14C-clen
buterol to male baboons, around 82% of the administered dose was
recovered in the urine and faeces within 5 days, with the majority of
this (72%) within 48 hours. Of the 82%, approximately 68% was recovered
in the urine and 14% in the faeces. A similar pattern was noted in
baboons given the drug orally. There was a rapid distribution phase and
the highest levels of radioactivity were found in the lungs, liver and
kidneys (Schmid, 1982).
Placental transfer of 14C-clenbuterol was investigated in a
single pregnant baboon given 3.3 mg/kg bw intravenously. The animal
was killed 3.5 hours after dosing, and around 1.5% of the total
administered close was found in the fetus (Schmid, 1980).
2.1.1.6 Cattle
In cattle given 0.8 µg/kg bw 14C-clenbuterol using a single
intramuscular injection, the liver was found to have the highest
concentrations of radioactivity. In muscle, soon after administration,
the radioactivity was due almost entirely to parent compound (Schmid,
1990a).
In a pilot study with a single cow given 0.8 µg/kg bw 14C-
clenbuterol orally, the first phase plasma elimination half-life
was 0.1 hours and the second phase elimination half-life 17 hours. The
maximum plasma concentration was 0.4 µg/ml with tmax at 8.5 hours.
Approximately 81% of the dose was excreted in the urine with 13% in
the faeces over the 96-hour period after dosing; around 1% was
excreted in the milk (Schmid & Zimmer, 1977a). Similar results were
noted after intramuscular (Schmid & Zimmer, 1977b) and intravenous
(Schmid, 1977) administration, but with the latter route plasma levels
rose much more rapidly. In both cases, radioactivity was found in the
faeces suggesting biliary excretion.
When calves were given 0.8 µg/kg bw 14C-clenbuterol intramus-
cularly, twice a day for 2 days and then orally twice a day for 3 days
in a consecutive manner, using a combination product also containing
trimethoprim and sulfadiazine, around 64% of the dose was excreted
during 15 days in urine and 5% in the faeces. The highest level of
radioactivity was found in injection site muscle, liver and kidney
(Hawkins et al., 1985a). Similar results were seen in another study
where cattle were given intramuscular injections of 14C-clenbuterol
(Cameron & Phillips, 1987a).
In cattle given 21 intramuscular doses of 14C-clenbuterol at a
dose level of 0.8 µg/kg bw per day, twice daily, the majority of the
dose was recovered in the urine. The plasma half-life following the
last dose was of the order of 4 days. Highest levels of radioactivity
were found in liver and kidney, with much lower levels in muscle and
fat (Hawkins et al., 1993a).
After administration of 7 µg/kg bw 14C-clenbuterol by the
intramuscular route to a pregnant cow, radioactivity crossed the
placenta and was found in fetal tissues, including skeletal tissues,
kidneys and liver, when the animal was killed 3 hours after the
dosing. Radioactivity was also found in the maternal and fetal eyes
(Baillie et al., 1980).
When lactating cattle were given oral or intramuscular doses of
14C-clenbuterol at a dose of 0.8 µg/kg bw, radioactivity was found
in the urine for 48-70 hours after administration. In the first 48
hours, the majority of the radioactivity in milk was attributable to
parent compound (Schmid, 1990b).
Lactating dairy cows treated with 0.8 µg/kg bw 14C-clen-
buterol, twice daily by the intramuscular route for 3 consecutive days,
and twice daily by the oral route for a further 2 days followed by a
single oral dose on day 6, were found to have milk concentrations in
the range of 0.76-1.03 µg/litre 12 hours after the first dose rising
to 2.14-2.6 µg/litre after 48 hours. They remained in the range 1.46
to 3.93 µg/litre throughout the dosing period. Highest tissue concen-
trations were at the injection site, liver and kidney (Hawkins et al.,
1985b). Similar results were obtained in another study where dairy
cattle were given oral, intravenous or intramuscular doses of 0.8 µg/kg
14C-clenbuterol. In this study approximately 2% of administered
radioactivity was found in milk (Cameron & Phillips, 1987b).
2.1.1.7 Horses
After administration of a single oral dose of 0.8 µg/kg bw
14C-clenbuterol to a horse, the maximum plasma concentration of
0.4 µg/ml was reached at approximately 2 hours. The elimination
half-life was 21 hours. After 144-168 hours, 81% of the administered
dose was recovered in urine. Following intravenous administration, 82%
of the administered dose was found in urine, with around 9% in faeces,
suggesting biliary excretion (Zimmer, 1977).
In a study with 10 horses, animals were given a single oral dose
of 0.8 µg/kg bw clenbuterol followed by a single intravenous dose 3
weeks later. A second group of 10 horses was treated in a similar
manner, but initially using the intravenous route with the oral dose
after 3 weeks. Approximately 20-50% of the oral dose (mean 36%) as
parent drug, was excreted over 108 hours in the urine, while,
following intravenous administration, some 17-70% of the dose
(mean 43%) was excreted in the 108-hour urine (Zupan, 1985).
In a study with a clenbuterol, trimethoprim and sulfadiazine
combination product, horses were given gelatin capsules containing
14C-clenbuterol at a dose of 0.8 µg/kg bw, twice daily for 10 days,
with a single oral dose on day 11. The majority of the dose (70-77%)
was excreted in the urine and 10-15% in the faeces. The maximum plasma
concentration was achieved 3-4 hours after the first administration
and the plasma half-life was 9-10 hours. The mean plasma concentration
was 0.6-1.2 µg/ml. Highest levels of radioactivity were found in the
liver and kidneys with very little in fat (Hawkins et al., 1984).
Similarly, when 12 horses were given 21 twice daily oral doses of
0.8 µg/kg bw 14C-clenbuterol, steady-state plasma levels were
rapidly achieved. Peak levels of 1-1.9 ng equivalents/ml occurred 1-3
hours after dosing. The terminal half-life was 22 hours. Around 75-91%
of the dose was excreted in the urine and 6-15% in the faeces. When
the horses were killed 3 or 12 hours and 9, 12 or 28 days after
dosing, the highest levels of radioactivity were found in the liver
and kidney. Levels in fat were extremely low (Johnston & Dunsire,
1993).
2.1.2 Biotransformation
The biotransformation of clenbuterol in rats, rabbits and dogs is
complex. Five urinary metabolites have been identified in dogs, and
eight in the rat and rabbit. Unchanged clenbuterol is the major
compound found in the urine of these species, and a range of oxidative
and conjugated metabolites are formed. Of these, 4-amino-3,5-dichloro-
mandelic acid, 3-amino-3,5-dichlorobenzoic acid and 4-amino-3,5-dichlo-
rohippuric acid are the major components. In the baboon, unchanged
parent drug is the major urinary component (18%), again with a range
of metabolites (Zimmer, 1971, 1974b; Johnston & Jenner, 1976; Schmid,
1982; Schmid & Prox, 1986).
In cattle, the major compound found in urine (28-52%) and in the
liver (50-80%) after administration of clenbuterol was the parent
compound. Small amounts of 4-amino-3,5-dichlorobenzoic acid were found
in urine and liver, and 4-amino-3,5-dichlorohippuric acid was detected
in urine (Baillie et al., 1980; Hawkins et al., 1993a). In
muscle, the main component was parent compound (Schmid, 1990a).
In horses also, parent clenbuterol was the major compound found
in urine (30-50%). It was also the major component in liver and kidney
(Zimmer, 1977; Hawkins et al., 1984; Johnston & Dunsire, 1993). A
study to examine specifically the nature of the metabolites in horse
liver confirmed clenbuterol as the major component, but with a smaller
quantity of a metabolite identified as 1-(4-amino-3,5-dichlorophenyl)-
1,2-ethanediol (Hawkins et al., 1993b).
None of the studies with any species revealed evidence of
significant covalent binding of clenbuterol or its metabolites.
Overall, the data suggest that the metabolism of clenbuterol is
similar in all the species studied and that the major differences are
quantitative rather than qualitative. The proposed metabolic pathways
are shown in figure 1.
2.1.3 Kinetics in humans
Following a single oral dose of 20 µg 14C-clenbuterol hydro-
chloride to volunteers, 67% of the administered dose was excreted in
the urine; faecal excretion was very low. The major compound present
in the urine was unchanged parent drug. The metabolic profile was
similar to that noted in animals (Zimmer, 1974c).
When a single dose of 20 µg 14C-clenbuterol hydrochloride was
administered orally to volunteers, the highest plasma level was 0.11
µg/litre, with a tmax, of 2-3 hours. Around 87% of the administered
radioactivity was detected in the urine; correspondingly lower levels
of radioactivity were found in the faeces.
In a repeat-dose phase of the same study, volunteers were given
40 µg 14C-clenbuterol for 2 days and then 20 µg for a further 2
days. Around 75% of the dose was detected in the urine. The metabolite
profile was similar to that noted in animals, and the major urinary
component was unchanged parent drug; the terminal half-life was around
34 hours (Zimmer, 1976).
Oral doses of 80 µg clenbuterol hydrochloride were given to 12
pregnant women in premature labour. These were followed 12 hours later
by 2 oral doses of 40 µg, and then by maintenance doses of 20 µg,
generally at 12-hour intervals. Blood samples were taken on days 2, 4,
6 and 8 of study, 3 hours after the morning dose. A steady-state
average concentration of 0.298 µg/litre was achieved, with a minimum
of 0.28 and a maximum of 0.344 µg/litre. Bioavailability was > 80%
(Rominger et al., 1987).
There are no data on in vivo percutaneous absorption of
clenbuterol in humans. However, data using a model which utilizes
excised human skin (Rohr et al., undated) suggests that some
absorption can occur (Boehringer, 1991b).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies after administration of
clenbuterol are summarized in Table 1.
Signs of toxicity in mice, rats and rabbits included lethargy,
increased heart rates, and tonic-clonic convulsions. After intravenous
dosing, death, when it occurred, did so either during injection or
immediately after it. In dogs, all the oral doses employed
(400-800 mg/kg bw) caused increases in heart rate. Salivation,
lethargy and tonic-clonic convulsions also occurred.
In cattle, single intravenous doses of up to 3.6 µg/kg bw
resulted in increases in heart and respiratory rates (Johnson, 1987).
Single oral doses of 2.4-12.0 µg/kg bw in horses resulted in sweating
and elevated heart rates and body temperatures at all dose levels
(Hamm & Erichsen, 1989).
 Table 1. Acute toxicity of clenbuterol
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M oral 80 Nishikawa et al., 1972
F oral 133 Nishikawa et al., 1972
M & F s.c 72 Nishikawa et al., 1972
M & F i.v 42 Nishikawa et al., 1972
M i.p 46 Nishikawa el al., 1972
M i.p 72 Nishikawa et al., 1972
Rat M & F oral 175 Nishikawa et al., 1972
M oral 82 Nishikawa et al., 1979
F oral 170 Nishikawa et al., 1979
M & F i.v 30 Nishikawa et al., 1972
M i.v 35 Nishikawa et al., 1979
F i.v 39 Nishikawa et al., 1979
M & F s.c 170 Nishikawa et al., 1972
M & F i.p 70 Nishikawa et al., 1972
M & F oral1 > 0.125 James, 1990a
Rabbit M & F i.v 84 Nishikawa et al., 1972
M & F dermal1 > 0.05 James, 1990b
Dog M & F oral 400-800 Pappritz, 1968
M & F i.v 45-52 Pappritz, 1972
1 Administered as a proprietary veterinary medicinal product
containing 25 µg clenbuterol per ml
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 15 male and 15 female ICR-JCL mice were given daily
oral doses of 0, 2.5, 12.5 or 62.5 mg/kg bw clenbuterol hydrochloride
as an aqueous solution, by stomach tube, for 30 days. Body weight gain
and food consumption were increased in all treated groups in a dose-
related manner. There were no adverse effects on haematology. The
animals from the highest dose group appeared weak and 6 animals died
during the course of the study.
At necropsy, there were significant increases in liver weights in
animals given 12.5 or 62.5 mg/kg bw per day. Ischemic heart damage was
noted in one animal given 12.5 mg/kg bw per day, and in two animals
from the highest dose group. The NOEL in this study was 2.5 mg/kg bw
per day (Kast, 1973a).
2.2.2.2 Rats
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily oral doses of 0, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by stomach tube, for 30 days. Signs
of toxicity were seen at the highest dose and these included
salivation, encrusted eyes and noses, and reduced body weight gain and
food consumption; 50% of these animal died during the course of the
study. Serum aspartate aminotransferase and glucose concentrations
were significantly reduced in animals of both sexes given 10 or
100 mg/kg bw per day.
Liver weights were significantly reduced in males given the
highest dose, while ischaemic lesions were seen in cardiac muscle in
one male and one female given this dose. The NOEL in this study was
1 mg/kg bw per day based on blood chemistry findings (Kast &
Tsunenari, 1973a).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given doses of 0, 1, 5, 25 or 75 mg/kg bw per day clenbuterol
hydrochloride in the feed for 6 months. At the highest dose level,
body weight gain and food consumption were reduced. During the first
week, a reduction in water consumption occurred in the animals given
this dose, and nine male and five female animals died during the
course of the study.
At necropsy, ischaemic lesions of the myocardium were noted
in all treated animals and there was a dose-related incidence and
severity. These lesions were also found in another group given
75 mg/kg bw per day clenbuterol hydrochloride and then allowed a
4-week recovery period without drug treatment. A NOEL was not
identified in this study (Kast & Tsunenari, 1973b).
Groups of 20 male and 20 female FW49/Biberach rats were fed diets
containing clenbuterol hydrochloride for 18 months. Further groups of
three male and three female rats were fed treated diets for 14 days,
followed by a recovery period; these animals were the subject of ECG
investigations. Another group of 10 female and 10 male rats was fed
the highest dietary level and then maintained for a 6-week recovery
period. The overall design of the study, including doses, is set out
below.
Dose (mg/kg bw 18-month phase 14-day ECG study2
per day) number of animals numbers of animals
males females males females
0 20 20 3 3
0.1 24 20 3 3
0.5 20 20 3 3
2.5 20 20 3 3
2.5 101 101 - -
1 subject to 6 week recovery period
2 subject to 21 day recovery period
Body weight gain in treated groups was slightly increased when
compared with control values. However, there were no overt signs of
toxicity, and no deaths occurred. There were no significant haemato-
logical effects. However, there were reductions in myocardial glycogen,
skeletal muscle glycogen and liver glycogen values in all treated
groups, but there was no clear dose response. Histochemical examination
of the myocardium showed a reduction in enzyme activity, particularly
in the lowest dose group.
The ECG examination revealed a bradycardia rather than the
expected tachycardia, but no explanation was provided for this. Heart
rates returned to normal 2 days into the recovery period. As the
results of this study appear to conflict with those of other studies
(reduction in heart rate rather than the expected increase), and as
the hearts were not adequately examined macroscopically or micro-
scopically, an NOEL cannot be identified from this study (Serbedija &
Bauer, 1973).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily intravenous doses of 0, 1, 4 or 16 mg/kg bw clenbuterol
hydrochloride for 30 days. Overt signs of toxicity included weakness
at the two highest dose levels and increased respiratory and heart
rates at the highest dose level. One female and one male in the 1 and
4 mg/kg bw per day dose groups respectively, died, while five male and
five female animals died at the highest dose. There was evidence of
hepatotoxicity (focal necrosis) in rats given the highest dose. A NOEL
was not identified in this study (Kast & Tsunenari, 1973c).
Groups of 30 male and 30 female Chbb: THOM (SPF) rats were
exposed by the inhalation route to 0 or 0.667 mg/m3 clenbuterol
hydrochloride in aqueous solution. A further two groups of 15 male and
15 female rats were exposed to 5.013 or 40.0 mg/m3. The exposures
were conducted for 0.5-2 hours per day, 7 days per week, for 13 weeks.
These exposures gave corresponding doses of 0, 0.01, 0.16 and
2.58 mg/kg bw per day. Groups of 10 male and 10 female control
animals, and 10 male and 10 female rats exposed to the highest
concentration were subjected to a 6-week recovery period without
further exposure to clenbuterol hydrochloride.
There was no drug-related mortality during the study. Increased
heart rates were noted in all treated rats. Animals exposed to the
highest concentration of clenbuterol hydrochloride showed myocardial
necrosis. Myocardial changes were noted in animals subject to the
6-week recovery period. As increases in heart rate were noted at all
concentrations, a NOEL was not identified in this study (Koellmer
et al., 1977).
Groups of 20 male and 20 female Charles River CD rats were
exposed to aerosols of clenbuterol hydrochloride in ethanol with
dichlorofluoromethane and 1,2-dichlorotetrafluoroethane as
propellants, using head-only exposure. Other groups served as controls
or ethanol/propellant controls. The doses were equivalent to up to
118 µg/kg bw per day.
The only treatment-related effects attributable to clenbuterol
hydrochloride were liver weight reduction and small aggregations of
alveolar macrophages, which were clearly dose-related (Clark et al.,
1979).
However, there were insufficient data on dosimetry to identify a
NOEL and in addition, there were no measurements of heart-rate or
other cardiac function parameters.
2.2.2.3 Dogs
Groups of three male and three female beagle dogs were given
starch capsules containing clenbuterol hydrochloride at doses of
0, 0.4, 4 and 20 mg/kg bw per day, daily for 13 weeks. The highest
dose was increased to 30 mg/kg bw per day from week 5 and to 40 mg/kg
bw per day from week 9. Water consumption was normal during the study,
but a slight decrease in appetite was noted in high-dose animals when
the dose was increased to 40 mg/kg bw per day. The rate of body weight
gain significantly fell from this point. One male and two females
given the highest dose appeared sedated after treatment. One of these
became increasingly agitated and breathless and died 2-3 minutes after
receiving the 23rd dose of 20 mg/kg bw per day. Tachycardia occurred
after the first dose in all treated animals and this persisted until
the following day. There was a decrease in its severity during
subsequent weeks, but it continued to be noted in all treated animals.
A shortening of the P-Q, Q-T and T-P intervals in the ECG,
corresponding to the increased heart rates, was seen in all treated
dogs. The dog that died was found to have a severe acute pulmonary
infection. Myocardial haemorrhage and necrosis, damage to the hepatic
parenchyma and severe haemorrhagic bronchopneumonia were noted. No
abnormalities occurred in any of the other animals. As dose-related
tachycardia occurred at all doses, a NOEL was not identified in this
study (Leuschner et al., 1969).
Groups of three male and three female beagle dogs were given oral
doses of 0, 2.5 and 40 mg/kg bw per day clenbuterol hydrochloride in
gelatin capsules for 13 weeks. No animals died during the study, but
in the first week, all dogs given clenbuterol hydrochloride were
apathetic, had redness of the skin and eyes, mydriasis and fixed
pupils. Tachycardia was seen in all dogs. At 2.5 and 40 mg/kg bw per
day, significant increases in the activities of several serum enzymes,
including LDH and creatinine kinase, were found. The glycogen levels
of the left heart muscle were decreased. Myocardial necrosis was seen
in dogs given the 2.5 and 40 mg/kg bw per day doses. A NOEL was not
identified in this study (Pappritz & Bauer, 1973a).
Groups of three male and three female beagle dogs were given
gelatin capsules containing daily doses of 0, 0.1 or 0.5 mg/kg bw per
day clenbuterol hydrochloride for 1 year. A group of six male and six
female dogs were given 2.5 mg/kg bw per day in the same manner, and
three animals of each sex were maintained on a control diet for 6
weeks at the end of the study, as part of a recovery phase. No animals
died during the study. In the first week all drug-treated animals were
apathetic and had diffuse reddening of the skin. Tachycardia was noted
in all treated animals, as was mydriasis, episcleral vascular
injection and conjunctivitis.
There were no significant effects on food consumption or body
weights, although dogs given the highest dose gained slightly more
weight than did those in other groups. No haematological effects were
seen, but there were increases in some serum enzymes in treated
groups. Liver glycogen was decreased in dogs given the highest dose
and this remained decreased at the end of the 6-week recovery period.
Heart weights were increased in all the treated animals when
compared with controls, and myocardial necrosis was noted in the
papillary muscle of the left ventricle of all these dogs, including
those subject to the 6-week recovery period. There was no dose
relationship in the cardiotoxicity seen, but histochemical methods
revealed smaller decreases in the activities of several enzymes in
cardiac muscle of the 0.1 mg/kg bw per day group than in the other
treatment groups. A NOEL was not identified in this study
(Pappritz & Bauer, 1973b).
Groups of three male and three female beagle dogs were given
daily intravenous injections of 0, 1, 10 or 1000 µg/kg bw per day
clenbuterol hydrochloride for 30 days. Dogs given the highest dose of
clenbuterol hydrochloride were lethargic and all treated animals
developed tachycardia with associated decreases in the P-Q and Q-T
intervals in the ECG. At necropsy, one male given the highest dose was
found to have myocardial necrosis. A NOEL was not identified in this
study (Pappritz et al., 1973).
2.2.2.4 Monkeys
Groups of three male and three female cynomolgus monkeys were
exposed to atmospheric levels of clenbuterol hydrochloride as an
aerosol in ethanol, with dichlorofluoromethane and 1,2-dichlorotetra-
fluoroethane as propellants, using an oropharyngeal tube, giving doses
of 25, 50 and 150 µg/kg bw per day for 26 weeks. Half of the daily
metered dose was given each morning and the remainder in the afternoon.
Another group of three male and three female monkeys served as sham
controls with no exposure, while a further group of three male and
three female monkeys served as placebo controls and were exposed to
the same aerosol as treated animals, but without the clenbuterol
hydrochloride.
No signs of toxicity were seen in this study and food consumption
and body weights were normal except in high-dose females where a large
body weight gain occurred. Ophthalmoscopy, haematology, lung mechanics
and ventilation, blood chemistry and urinalyses were normal. A low
oxygen tension was noted in high-dose animals in week 10 of the study.
There were no drug-related changes in heart rate or ECGs. At macro-
scopic and histo-pathological examination, there were no drug-related
changes (Collins et al., 1979).
Thus, the NOEL in this study was of the order of 25 µg/kg bw per
day by the inhalation route, but this could not be identified as a
definitive study because of the problems in dosimetry in an inhalation
study of this type. For example, there were no data on how much drug
reached the lower respiratory tract and no information regarding blood
levels. Hence, the degree of systemic exposure was unknown.
2.2.2.5 Cattle
In calves given the therapeutic dose of 0.8 µg/kg bw per day
clenbuterol hydrochloride for 10 days by the oral or intravenous
routes, only transient tachycardia occurred. When given at 5 times the
therapeutic dose (4 µg/kg bw per day) for 10 days, tachycardia and
audibly increased heart force were the only effects noted. When given
to adult cattle at the therapeutic dose or twice the therapeutic dose
for periods of up to 9 days, by the oral route, the main effects were
tachycardia and falls in diastolic blood pressure (Fenner, 1982;
Cameron et al., 1992).
2.2.2.6 Horses
No clinically significant adverse effects were noted in horses
given 0.8-17.5 µg/kg bw per day clenbuterol hydrochloride by the oral
route for periods of up to 64 days. Transitory effects included
tachycardia, sweating, muscle tremors, hyperglycaemia and hypophos-
phataemia (Erichsen, 1988, 1989; Hamm & Erichsen, 1989; Owen et
al., 1990).
Clenbuterol, like other ß-agonists, leads to tachycardia and
hypotension. This probably results in reduced myocardial perfusion at
a time when oxygen demand is high because of increased cardiac rate.
The end result is hypoxia which probably leads to the necrotic lesions
seen in the left ventricular papillary muscles (Rosenblum et al.,
1965; De Busk & Harrison, 1969; Poynter & Spurling, 1971; Roberts &
Cohen, 1972; Magnusson & Hansson, 1973; Balazs & Bloom, 1982). This
explains the cardiac effects noted in the repeated dose (and other)
studies with clenbuterol.
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female (C57B/6 × DBA/2) mice were given
clenbuterol in the drinking-water at doses of 0.1, 1.0 and 25.0 mg/kg
bw per day for 2 years. Groups of 100 males and 100 females were given
drinking-water only over this period and served as controls. Treated
animals consumed slightly more food and water than controls, but showed
no overt signs of toxicity, except for a dose-related increase in body
weight in the first year and a decrease during the second year.
Survival after 2 years was good in males (78-86%) and acceptable in
females (54-60%).
At termination, there were significant changes in the weights of
a number of organs when compared with control values. These included
significant increases in the absolute heart weights of males given
1 mg/kg bw per day and in females given 25 mg/kg bw per day. There
were significant increases in relative heart weights in all treated
male and female mice. The incidences of non-neoplastic lesions,
including myocardial lesions, were not treatment related. There was no
increased incidence of any rumour type in treated mice when compared
with control values (Umemoto, 1984).
2.2.3.2 Rats
Groups of 48 male and 48 female Chbb:THOM rats were fed diets
containing clenbuterol hydrochloride, resulting in daily doses of
6.25, 12.5, or 25.0 mg/kg bw per day for 2 years. A further group of
72 rats of each sex was fed a diet containing no clenbuterol hydro-
chloride. As ß-sympathomiroetic agents are known to induce mesovarian
leiomyomas in some strains of rat, groups of 72 male and 72 female
Charles River Sprague-Dawley rats were also fed diets containing
clenbuterol hydrochloride at doses of 0 or 25 mg/kg bw per day for 2
years. After one control rat began to show signs of clenbuterol-related
effects, contamination of the feed preparation personnel and equipment
was confirmed, and the substance was then administered via the
drinking-water to ensure the same daily doses.
Treated rats appeared nervous, tense and aggressive, but no
effects on mortality were seen. Survival was in the range of 70-87% in
males and 50-71% in females. In both sexes and strains body weight was
reduced in a dose-related manner. Consumption of drinking-water was
significantly reduced in SD rats given the highest dose. At
termination, fibrosis of the subendocardial connective tissue was
frequently observed in all groups including controls, but the
incidence was higher in the Chbb:THOM rats, suggesting a drug-
related effect.
There was an increased incidence of mesovarian leiomyomas in the
female Sprague-Dawley rats, but not in the Chbb:THOM rats. The
incidence is shown in Table 2.
Table 2. Incidence of mesovarian leiomyomas in female rats treated with clenbuterol
hydrochloride for 2 years
Chbb:THOM Sprague-Dawley
Rats rats
Dose 0 6.25 12.5 25.0 0 25.0
(mg/kg bw per day)
Numbers with 0/72 0/48 0/48 0/72 0/72 11/72
Mesovarian
leiomyomas
per group
There was no increased incidence of any other tumour type
(Serbedija et al., 1982).
Leiomyomas of the smooth muscle of the uterus in mice and
mesovaria of rats have been reported following long-term treatment
with ß-agonists (Nelson & Kelly, 1971; Jack et al., 1983;
Amemiya et al., 1984; Gibson et al., 1987; Gopinath & Gibson,
1987; Sells & Gibson, 1987). They are not associated with genotoxicity
with these agents and are considered to be associated with adrenergic
stimulation. The ß-agonist salbutamol produced mesovarian leiomyomas
in rats, but these could be prevented by administration of the
ß-blocking agent propranolol; a similar phenomenon has been reported
in mice where the induction of leiomyomas of the uterus by medroxalol
was prevented by propranolol (Jack et al., 1983; Gibson et al.,
1987). There have been no reports of increased incidences of leiomyomas
in women following the use of ß-adrenergic agents (Poynter et al.,
1978).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
Groups of 17-20 pregnant female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage, from day 15 of gestation to 21 days
post-partum when dams and pups were sacrificed. The pups, including
those that died before day 21, were necropsied and X-rayed. Maternal
body weights were reduced at the end of the study in animals given
7 mg/kg bw per day clenbuterol hydrochloride. Those given the highest
dose were similar to the controls, but these animals were heavier at
the beginning of the study. Maternal food consumption was reduced in
all treated groups.
The number of pups born dead increased in a dose-related manner,
as did the number of pups dying after birth. At the highest dose all
the pups died, as shown below.
Body weights of pups were also reduced in a dose-related manner.
A NOEL was not identified in this study (Lehman, 1974).
Groups of 30 male and 30 female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water by gavage. Treatment of the male rats commenced 10
weeks prior to mating while that of females began 2 weeks prior to
mating. On day 14 of gestation 50% of the females were killed and the
uterine contents examined. The remaining female rats were allowed to
deliver normally and rear their pups until weaning. Dosing of all
females continued until they were killed on day 14 of gestation or
after weaning.
To investigate the cause of the high pup mortality in the study
of Lehman (1974), the litters of six control dams were exchanged with
litters from dams given 50 mg/kg bw per day. Surviving dams and pups
were necropsied at the end of the lactation period. Food consumption
was increased in treated animals when compared with control values.
However, body weight was significantly reduced in animals of both
sexes given the highest daily doses.
Pup weights at birth were reduced in all treated groups. There
was a dose-related decrease in the numbers of viable pups which was
statistically significant at 7 and 50 mg/kg bw per day. All the pups
in the 50 mg/kg bw per day group died on the first day of lactation
regardless of whether or not they were suckled by treated or control
dams. The majority of pups from control dams suckled by dams that were
given the 50 mg/kg bw per day dose survived.
There were no substance-related effects on fertility, corpora
lutea, implantation or resorption rates, and no malformations were
noted in pups from treated rats. The hearts of three pups from
high-dose females were examined histologically, but no abnormalities
were found (Lehman, 1975).
Groups of 34 male and 34 female Chbb:THOM rats were given daily
oral doses of 0, 1.5, 7.5 or 15 µg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage. The male rats were
treated for 70 days prior to mating and the females from 14 days prior
to mating up until the end of gestation. The pups were not treated.
Dosing of the females continued until the interim sacrifice or after
weaning.
On days 13-15 of gestation, 50 pregnant rats were killed and the
uterine contents examined. The remainder were allowed to give birth
naturally and the pups reared until 3 weeks old, except for two males
and two females in each litter which were reared until 10 weeks after
mating when certain behavioural tests were conducted (pupillary
reflex, photo-phobia response, hearing, behaviour on a rotating rod,
behaviour in a Y maze). The animals were then mated and allowed to
litter naturally.
There were no effects on fertility attributable to clenbuterol
treatment, and gestation length, numbers of corpora lutea,
implantation rates, incidences of resorptions, parental body
weights and food consumption were unaffected.
No compound-related effects were seen in the littering phase and
the pups gained weight normally. Performance in the behavioural tests
were similar in treated and control groups and the offspring of these
animals were normal in all respects. The NOEL was 15 µg/kg bw per day
(Lehman, 1980).
Similar results were obtained in an almost identical study on
rats using the same dose levels. Again, the NOEL was 15 µg/kg bw per
day (Lehman, 1981).
No teratogenic effects were seen in any of these reproductive
toxicity studies.
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 mated female SPF-FW 49 Biberach rats were given oral
doses of 0, 0.04, 0.2 or 1 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage on days 6-15 of gestation. No evidence
of maternal toxicity was seen and there were no compound-related
effects on the incidences of resorptions, or on litter and fetal
weights. There were no increased incidences of any type of
malformation. The NOEL was 1 mg/kg bw per day (Lehman, 1969a).
Groups of 25 mated female Sprague-Dawley rats were given oral
doses of 0, 0.01, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage, from days 9 to 14 of
gestation. Approximately 24 hours before the expected delivery date,
20 dams per dose level were killed and the uterine contents examined
while the remaining five pregnant animals in each group were allowed
to deliver naturally.
Overt signs of toxicity, including weakness, hypersensitivity and
bloody vaginal discharge were seen in rats given the highest daily
dose. Maternal body weights were reduced in dams given the 10 or
100 mg/kg bw per day doses. Animals given these doses had signifi-
cantly increased incidences of resorptions with corresponding
reductions in the numbers of viable fetuses. Mean litter weights were
reduced at both these doses, while mean fetal weight was significantly
reduced at the highest dose level.
The incidences of malformations were significantly increased at
the two highest dose levels used. Anomalies included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of the vertebrae (Kast, 1973b).
A study was conducted to verify the results of Kast (1973b).
Groups of 25 mated Sprague-Dawley rats were given 0, 0.01, 1, 10 or
100 mg/kg bw per day clenbuterol hydrochloride in distilled water, by
gavage, from days 8 to 17 of gestation. Approximately 24 hours before
the expected delivery date, 20 dams per group were killed and the
uterine contents examined. The remainder were allowed to deliver
naturally.
Overt signs of toxicity were similar to those in the previous
study and five dams given the highest dose died. The incidences of
resorptions were increased in animals given the highest dose; mean
litter weights and fetal weights were reduced. The incidences of
malformations were significantly increased at the two highest doses.
The types of malformation were similar to those seen in the study of
Kast (1973b).
In pups derived from animals allowed to deliver naturally, body
weights were increased at the highest dose. Swimming function was
retarded in this group. At 10 mg/kg bw per day, two pups were found to
have hydrocephalus while at the highest dose level, four pups were
found to have anophthalmia. No malformations were noted in pups from
the 0.01, 0.1 or 1 mg/kg bw per day groups. The NOEL in both these
studies was 1 mg/kg bw per day (Kast, 1975).
Groups of 20 Sprague-Dawley rats were exposed nose only to an
atmosphere containing clenbuterol hydrochloride during days 6-15 of
gestation. The estimated doses were 0, 19, 39 and 78 µg/kg bw per day.
There was no evidence of maternal toxicity or teratogenicity, but
there were dose-related increases in the incidences of skeletal
variations, indicative of fetotoxicity, at all dose levels (Palmer
et al., 1978).
2.2.5.2 Rabbits
Groups of 15 mated Russian/Biberach rabbits were given daily oral
doses of 0, 30, 100 or 300 µg/kg bw per day clenbuterol in distilled
water, by gavage, from days 6 to 18 of gestation. Three dams given the
300 µg/kg bw per day dose and one given the highest dose died during
the study. These deaths were due to bronchopneumonia. Several rabbits
in each group were found not to be pregnant. Despite these problems,
at least 10 litters from each dose group were available for
examination.
Dams in each dose group showed better weight gains than controls,
but there was no clear dose relationship. There were no adverse
effects on the incidences of resorptions, numbers of live fetuses,
fetal weights or the incidences of malformations. However, there were
increased incidences of delayed ossification, suggestive of
fetotoxicity, in the 100 and 300 µg/kg bw per day groups. The NOEL
was 30 µg/kg bw per day (Lehman, 1969b).
Groups of 10 female Russian/Biberach rabbits were given daily
oral doses of 0, 0.01, 1 or 50 mg/kg bw per day clenbuterol
hydrochloride, in distilled water by gavage, on days 8 to 16 of
gestation. There were no compound-related deaths, but body weight gain
and food intake was reduced at the highest dose level.
At the highest dose level, the incidence of resorptions was
significantly increased, and there was a corresponding decrease in
the numbers of viable fetuses. Mean litter and fetal weights were
significantly reduced at this level. There was an increased incidence
of malformations, notably cleft palate and synostosis, in pups from
dams given 50 mg/kg bw per day clenbuterol hydrochloride. The NOEL was
1 mg/kg bw per day (Kast, 1973c).
Groups of 13-16 mated New Zealand White rabbits were exposed to
doses of clenbuterol hydrochloride calculated to be 0, 48, 146 or
300 µg/kg bw per day by the inhalation route using a fine bore tube
sited over the tracheal opening. The heads of the animals were held in
an air extraction chamber to minimize rebreathing of aerosol. Dosing
took place on days 6-18 of gestation.
Signs of stress were observed in all groups as a result of the
exposure procedures. Signs included anorexia, lethargy and respiratory
distress, and some animals died in all groups, including the controls.
There was a high incidence of malformations in the control groups
which was probably related to the initial stress. There was no
increased incidence in animals given 146 or 300 µg/kg bw per day.
As a result of the high numbers of malformations seen in control
groups, no firm conclusions could be drawn from this study. However,
the data suggested that doses of up to 300 µg/kg bw per day clen-
buterol hydrochloride, when given by inhalation to pregnant rabbits on
days 6-18 of gestation, were not teratogenic (Palmer et al., 1980).
Taken together, the animal data suggested that clenbuterol
hydrochloride is teratogenic in rats and rabbits at relatively high
doses. There was evidence of fetotoxicity. The NOEL for maternal
toxicity and teratogenicity in the rat and rabbit following oral
administration was 1 mg/kg bw per day. However, the NOEL for
fetotoxicity in one study in the rabbit was 0.03 mg/kg bw per day.
2.2.6 Special studies on genotoxicity
Clenbuterol hydrochloride gave negative results in the Ames test
with Salmonella typhimurium strains, in a reversion test with
Escherichia coli strain WP2(P), in a gene mutation assay with
Chinese hamster V79 cells, in an in vivo mouse micronucleus test and
in an in vivo cytogenetic assay with Chinese hamster bone marrow.
In the mouse lymphoma test with L5178Y cells, no increased
incidences in mutation frequency were observed in the absence of
metabolic activation. However, with metabolic activation, one of two
experiments gave positive results at the two highest concentrations
used (Clements, 1992).
Viability counts were reduced at these concentrations and
sampling error may have led to these spurious results (Kirkland, 1992).
In an in vitro assay with human lymphocytes, at concentrations of
> 500 µg/ml, an increased incidence of chromosome aberrations was
noted in the absence (but not in the presence) of metabolic
activation. This was not dose-dependent.
The results are summarized in Table 3.
These data suggest that clenbuterol hydrochloride is not
genotoxic.
2.2.7 Special studies on irritancy
2.2.7.1 Skin irritation
Clenbuterol hydrochloride was applied to the shaved skin of six
Russian/Biberach rabbits, using an occlusive dressing, for a period of
28 days. No evidence of skin irritation was seen (Hewett & Notman,
1984). Similar results were noted in a further study of skin
irritation (James, 1990d).
2.2.7.2 Intramuscular tolerance
Intramuscular injection of 0.5 ml of a formulation containing
clenbuterol hydrochloride into the dorsal area of rabbits produced
only minimal haemorrhage, oedema and necrosis (Kreuzer, 1988).
2.2.7.3 Eye irritation
A formulated commercial product containing clenbuterol
hydrochloride (0.1 ml) was instilled into the left eye of six New
Zealand White rabbits. The right eye served as a control. Mild
irritation was noted and this resolved 48 hours after treatment
(Bailey, 1990).
2.2.8 Special studies on immunotoxicity
Clenbuterol hydrochloride, as a formulated commercial product,
was tested on the guinea-pig using the Buehler technique. The content
of active ingredient was 70.4 µg/ml in the formulation. It was not a
sensitizer in this study (James, 1990c).
Another study employed a 0.2% solution of clenbuterol hydro-
chloride in 30% aqueous ethanol utilizing guinea-pigs and the
Magnusson and Kligman maximization test involving the use of Freund's
adjuvant. No evidence of a sensitizing effect was found (Schuster,
1984).
Table 3. Results of genotoxicity studies on clenbuterol hydrochloride
Test system Test subject Concentration Results Reference
Bacterial Salmonella typhimurium 40-2500 µg/plate - Baumeister,
reversion TA98, TA100, TA 1535, 1978
TA1537, TA1538
Escherichia coli 40-1500 µg/plate
WP2(P)
Bacterial S. typhimurium 10-500 µg/plate - Ellenberger,
reversion TA98, TA 100, TA 1535, 1985
TA1537, TA1538
Forward Chinese hamster V79 10-100 µg/ml - Baumeister,
mutation fibroblasts, HGPRT 1985
assay locus
Forward Mouse lymphoma 300-800 µg/ml - Clements,
mutation L5178Y 1992
assay
In vitro human lymphocytes 177-2352 µg/ml +/- McEnaney,
cytogenetics 1992
In vivo mouse 0.006, 0.5, 5.0 - Friedmann,
micronucleus mg/kg bw per 1982
test day
In vivo Chinese hamster 19, 60, 186 - Holmstrom &
cytogenetics (bone marrow) mg/kg bw per MacGregor,
test day 1986
2.2.9 Special studies on pharmacodynamic effects
2.2.9.1 Studies in animals
Clenbuterol is a ß2-sympathomimetic agent with a wide range of
pharmacodynamic effects. It produces a potent, dose-dependent
bronchiolytic effect, which was demonstrated in guinea-pigs following
acetylcholine, histamine or bradykinin-induced bronchospasm. Similar
effects have been reported in cats and dogs. Clenbuterol exerts
positive chronotropic and inotropic effects on isolated atria. It
induces tachycardia in rats, dogs, cats and a variety of farm animals,
accompanied by reductions in systolic and diastolic blood pressure. In
the isolated uterine horn of the estrus rat, it exerts a pronounced
relaxing effect on serotonin, oxytocin and bradykinin-induced spasm.
It has also been shown to exert a relaxing effect on smooth muscle in
the guinea-pig ileum and to reduce intestinal mobility in the rat and
mouse.
Clenbuterol results in a glycogenolytic effect in the rat
myocardium associated with ß-stimulation. It has no effect on hepatic
glycogen. It causes a potent dose-dependent reduction of the gastric
secretion.
In mice, it causes decreases in spontaneous activity and also
leads to increases in barbiturate-induced sleeping time. The drug
results in dose-dependent striated muscle relaxation. However, it has
no antipyretic or analgesic effects in mice (Engelhardt, A., 1971;
Engelhardt, G., 1971, 1976).
Clenbuterol exerted major relaxing effects on spasms induced by
carbachol in guinea-pig trachea and lung. The effects were antagonised
by propranolol (Landry, 1983). It also led to decreases in perfusion
pressure of hind limb blood vessels, inhibitions of contractions of
the uterus, increases in atrial rate and inhibition of electrically
stimulated contractions of the ileum (O'Donnell, 1976). It improved
the rates of mucociliary clearance in the guinea-pig (Streller, 1981).
In beagle dogs, oral doses of clenbuterol hydrochloride at
1.5, 3.0, 7.5 and 15.0 µg/kg bw resulted in a dose-dependent
tachycardia at > 3.0 µg/kg bw. There was a slight tachyphylactic
effect demonstrated by a diminution of the intensity and duration of
the response. There was a dose-related reduction in blood pressure and
the changes in diastolic and systolic pressures ran in parallel. These
changes occurred at the lowest dose used (Ueberberg, 1976).
In cattle, intravenous doses of 0.6 µg/kg bw and 1.5 µg/kg bw
produced no cardiac effects, as shown by ECG examination, whereas
2.85 µg/kg bw produced pronounced tachycardia. A dose of 0.3 mg/kg bw
clenbuterol resulted in tocolysis lasting approximately 5 hours
(Ballarini, 1978). Different lag times in parturition may be induced
depending on the dose and route of treatment; no adverse effects on
the dam or calf were noted (Arbeiter & Thurner, 1977).
Four metabolites of clenbuterol that had been shown to be present
in the kidneys and liver of treated target animals were tested for
pharmacodynamic activity. Of these, only one, N-A 1141 (Figure 1) was
shown to have activity in the guinea-pig. Its bronchiolytic effect was
less than 20% that of clenbuterol, and, moreover, it accounted for
only 12% of residues in the liver and kidney 6 hours after treatment
(Engelhardt, 1971).
2.2.9.2 Studies in humans
Patients given 10 µg (0.167 µg/kg bw) clenbuterol by the
inhalation route showed no signs of tachycardia as determined by ECG.
There were slight decreases in blood pressure. When patients with
cardiac arrhythmia were given this dose of clenbuterol, none of the
patients showed any changes attributable to the drug (Hufnagel, 1974).
After inhalation exposure of patients with airways disease, onset
of broncholytic action occurred within 5 minutes and lasted for up to
6 hours. Pulmonary function effects were greatest with doses of >
5 µg (0.083 µg/kg bw); only a minimal effect was seen with 2.5 µg
(0.042 µg/kg bw) (Schuster et al., 1989).
2.3 Observations in humans
A single blinded cross-over study was carried out to examine the
acute bronchospasmolytic effect and possible side-effects following
oral administration of placebo and three doses of clenbuterol (1, 2.5
and 5 µg/day) in patients (three male and three female) with chronic
obstructive airway disease over a 3 day period. The drug was given
orally, diluted with water. Observations were carried out over a
2-hour period following dosing. The average age of the patients was
55.7 years and they had an average body weight of 73.16 kg.
None of the 3 doses produced any clear, consistent effects on
bronchial resistance, thoracic gas volume, radial pulse frequency or
blood pressure, and no side effects were seen.
The pharmacological NOEL in this study was 5 µg/day, equivalent
to 0.08 µg/kg bw per day (Kaik, 1978).
The bronchospasmolytic effect was examined in two groups of
patients:
Group A: ten patients aged 46-75 years with chronic obstructive
respiratory disease resulting from pulmonary tuberculosis.
Group B: five patients aged 56-67 years with chronic obstructive
respiratory disease not related to tuberculosis, plus five
patients aged 34-57 with bronchial asthma.
The bronchospasmolytic effect was examined after single oral doses of
1, 2.5, 5, 10, 20, 25 or 30 µg/person, and after a placebo dose.
In Group A patients, intrathoracic gas volume was significantly
reduced and vital capacity and pneumometer values significantly
increased at all dose levels. In Group B patients, airway resistance
was significantly reduced, but no dose relationship could be
demonstrated. No significant placebo effect was seen in either group.
When compared with placebo values, a significantly greater increase
for both vital capacity and pneumometer values was observed in Group
A, even at the lowest dose used in this group (5 µg). However, at the
two lowest doses used in Group B (1 and 2.5 µg), there were no
significant differences from placebo values. The pharmacological NOEL
in this study was 2.5 µg, equivalent to 0.042 µg/kg bw (Nolte &
Laumen, 1972; Nolte, 1980).
Children who consumed between 0.05-0.075 mg of clenbuterol showed
only mild tachycardia. A 30-year-old woman who consumed 30 tablets
equivalent to 0.6 mg clenbuterol (10 µg/kg approximately) developed
tachycardia and slight hypertension approximately 1 hour after
consumption. No tablet remains were found on gastric lavage, and
medicinal charcoal and a saline laxative were given. The following
day, the patient's pulse rate and blood pressure had returned to
normal (Boehringer, 1991a).
Patients (100+) administered doses of 20-60 µg/day (0.3-1.0 µg/kg
bw per day) for up to 1 year or 20 µg/day (0.3 µg/kg bw per day) for
up to 6 months showed no adverse effects except for slight tremor and
occasional, mild tachycardia (Laumen, 1978; Tullgren & Lins, 1987).
3. COMMENTS
The Committee considered toxicological data on clenbuterol,
including the results of acute, short-term and reproductive toxicity
studies, as well as studies on teratogenicity, genotoxicity and
carcinogenicity. Results of pharmacokinetic and pharmacodynamic
studies in animals and humans were also considered.
Clenbuterol is well absorbed after oral administration in a
number of animal species and in humans. An oral dose is largely and
rapidly excreted in the urine, and the majority of the remainder is
excreted in the faeces. The biotransformation of clenbuterol is
complex and a number of metabolites are formed. The major compound
found in a number of species was unchanged clenbuterol. After oral
administration of therapeutic doses to lactating cattle, clenbuterol
was found in the milk.
When radiolabelled clenbuterol was given orally to pregnant rats,
dogs, baboons and cattle, radioactivity was detected in the fetuses.
Clenbuterol was moderately toxic in mice and rats after oral
administration, LD50 values being in the range of 80-175 mg/kg bw.
It was less toxic in the dog (LD50 = 400-800 mg/kg bw). It was more
toxic after parenteral administration, with LD50 values in the range
of 30-85 mg/kg bw after intravenous administration. The main signs of
toxicity included lethargy, tachycardia and tonic-clonic convulsions
after oral administration.
The main effects noted in the repeat-dose studies were
tachycardia and, at higher doses, myocardial necrosis. These effects
are common with ß-agonist drugs. The myocardial necrosis was
considered to be secondary to hypoxia, due to reduced myocardial
perfusion at a time of high oxygen demand resulting from increased
cardiac rate.
In 30-day repeat-dose studies in mice and rats, NOELs of 2.5 and
1 mg/kg bw per day, respectively, were identified, largely based on
cardiac lesions. However, in a range of repeat-dose studies in rats
using doses of 0.01 to 100 mg/kg bw per day for durations of up to
18 months, administered through the oral, intravenous and inhalation
routes, no NOELs were identified. Effects were usually related to
cardiac function and were seen even at the lowest doses used.
Similarly, no NOELS could be identified in a range of repeat-dose oral
studies in dogs. These studies used doses ranging from 0.1 to 40 mg/kg
bw per day. In a 26-week inhalation study in cynomolgus monkeys, the
NOEL was 25 µg/kg bw per day, based on a number of observations
including cardiac effects.
No evidence of carcinogenicity was noted in a two-year oral study
in mice with doses of up to 25 mg/kg bw per day. In a two-year study
with doses of up to 25 mg/kg bw per day in the Chbb:THOM rat, no
evidence of carcinogenicity was seen. However, in Sprague-Dawley rats
given 25 mg clenbuterol/kg bw per day orally for 2 years, an increased
incidence of mesovarian leiomyomas occurred. With the related
compounds salbutamol in rats and medroxalol in mice, the effects could
be abolished by administration of the ß-blocking agent propranolol.
Mesovarian leiomyomas in rats and uterine leiomyomas in mice are known
to occur following long-term treatment with ß-adrenoceptor agonists
and the Committee concluded that these were due to adrenergic
stimulation and not to any genotoxic mechanism. Clenbuterol was not
genotoxic in a range of in vitro and in vivo genotoxicity studies.
Epidemiological studies indicate that there have been no
increased incidences of uterine leiomyomas in women following the use
of ß-adrenoceptor agonists.
Clenbuterol had no effects on fertility in a reproductive
toxicity study in rats using oral doses of 1-50 mg/kg bw per day from
10 weeks prior to mating in males and two weeks prior to mating in
females. However, doses of 50 mg/kg bw per day resulted in the deaths
of pups soon after birth. To investigate the cause of the high pup
mortality at this dose level, the litters of control dams were
exchanged with those from dams given 50 mg/kg bw per day. Pups from
rats given 50 mg/kg bw per day died on the first day of lactation
regardless of whether they suckled on treated or control dams. The
mechanism involved in this lethal effect is unknown. A NOEL was not
identified in this study, because pup weights at birth were reduced in
all treated animals.
In a reproductive toxicity study in which male rats were treated
with 1.5-15 µg clenbuterol/kg bw per day orally for 70 days prior to
mating and females with the same dose range for 14 days prior to
mating, no adverse effects on reproduction were noted. The NOEL was
15 µg/kg bw per day.
In teratogenicity studies in rats, oral doses of 10 and 100 mg/kg
bw per day produced teratogenic effects that included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of vertebrae. These were accompanied by signs of maternal
toxicity. The NOEL was 1 mg/kg bw per day. In three studies in rabbits
using doses of 30 µg to 50 mg per kg bw per day, signs of feto-
toxicity, including delayed ossification and cleft palate, occurred.
The NOEL was 30 µg/kg bw per day.
Clenbuterol produced a range of pharmacodynamic effects in a
number of animal species including tachycardia, hypertension and
muscle relaxing effects. These were seen at single doses as low as
0.8 µg/kg bw.
Four metabolites of clenbuterol that had been shown to be present
in the kidneys of treated target animals were tested for pharmaco-
logical activity. Of these, only one (N-A 1141) was shown to have
activity. Its broncholytic effect in the guinea-pig was less than
20% that of clenbuterol. In addition, it accounted for only 1-2% of
residues in the liver and kidney of target animals 6 hours after
treatment.
In humans, clenbuterol produced a bronchiolytic effect when a
single dose of 10 µg (0.167 µg/kg bw) was given by the inhalation
route, but no evidence of tachycardia was seen at this dose. With oral
doses of clenbuterol of up to 5 µg/day (0.08 µg/kg bw per day) over a
3-day period, there were no effects on bronchial resistance, thoracic
gas volume, cardiac rate or blood pressure. The NOEL in this study was
5 µg/day (0.08 µg/kg bw per day). In a study to investigate the
bronchospasmolytic effect in humans, patients with obstructive lung
disease were given oral doses of up to 30 µg per person. Patients
administered doses of 5 µg or more exhibited bronchospasmolytic
effects, and the pharmacological NOEL in this study was 2.5 µg per
person, equivalent to 0.04 µg/kg bw.
4. EVALUATION
The Committee considered the most relevant study for determining
the ADI to be that concerning the bronchospasmolytic effect in humans.
The patients had chronic obstructive airway disease and thus were
likely to be a very sensitive population for this effect. The NOEL
identified in this study (2.5 µg per person, equivalent to 0.04 µg/kg
bw) is approximately 25% of the dose in another study in which the
inhalation route was used, but in which cardiac effects were not
observed. This NOEL is approximately 50% of the oral dose used in
another study where, again, cardiac effects did not occur. Hence, this
NOEL for the bronchospasmolytic effect offers an additional safety
margin for cardiac effects. The Committee therefore established an ADI
of 0-0.004 µg/kg bw, based on the NOEL of 0.04 µg/kg bw per day for
pharmacodynamic effects in humans and a safety factor of 10.
5. REFERENCES
Amemiya, K., Kudoh, M., Suzuki, H., Saga, K., & Hosaka, K. (1984).
Toxicology of mabuterol. Arzneim.forsch, 34(11a), 1680-1684.
Arbeiter, K. & Thurner, M. (1977). Effect of the sympathomimetic
Planipart (NAB 365) on parturition in cattle. Tierärztl. Umsch.,
32, 423-427.
Bailey, D.E. (1990). Primary eye irritation study in rabbits with
Ventipulmin. Unpublished report No. TX-9005/Ventipulmin from Hazleton
Laboratories America, Inc., Vienna, VA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Baillie, H.W., Cameron, B.D., Draffan, G.H., & Schmid, J. (1980).
Investigations of the placental transfer of 14C-N-AB 365 CL in the
cow. Unpublished report No. 111674 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Balazs, T. & Bloom, S. (1982). Cardiotoxicity of adrenergic and
vasodilating antihypertensive drugs. In: van Stee, E.W. (ed.),
Cardiovascular Toxicology, Raven Press, New York, pp. 199-220.
Ballarini, G. (1978). Initial studies of the clinical pharmacology of
the ß2 sympathomimetic agent NAB 365 (Planipart) and its use for
pharmacological tocolysis in cows. Tierärztl. Umsch., 33, 421-427.
Baumeister, M. (1978). Mutagenicity studies with the substance
N-AB 365 CL in the plate incorporation assay (Ames test). Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Baumeister, M. (1985). Mutagenicity study with the substance
N-AB 365 CL in the V79 (HGPRT)-test. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Boehringer (1991a). Summary of case reports of overdosage with
clenbuterol. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Boehringer (1991b). Estimated transdermal absorption of clenbuterol
hydrochloride following skin contact with granules and injection
solutions for veterinary use. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987a). The residue kinetics of
[14C]-NAB 365 CL in the calf. Unpublished report No. 4371 from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987b). The residue kinetics of
(14C)-NAB 365 CL in the lactating cow. Unpublished report No. 4445
from Inveresk Research International. Submitted to Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, D.M., Crook, D., & Brown, G. (1992). Ventipulmin injection
solution and Ventipulmin granules. Target species tolerance study in
calves. Unpublished report No. BOI 139/921030 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Chasseaud, L.F., Cresswell D.G., & Savage J.A. (1978).
Pharmacokinetics of 14C-N-AB 365 during chronic inhalational
toxicity in cynomolgus monkeys. Unpublished report No. U79-0212 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Clark, G.C., Collins, C.J., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D.E., Edmonson, N., Lewis, D.J, & Mahjeed, S.K. (1979).
Investigation of the effects on rats of inhalation of NAB 365 CL for
26 weeks. Unpublished report No. BOI 88/78768 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Clements, J. (1992). Study to determine the ability of clenbuterol
hydrochloride to induce mutations at the thymidine kinase (tk) locus
in mouse lymphoma L5178Y cells using a fluctuation assay. Unpublished
report No. 2TKREBSG.002 from Hazleton Microtest. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Collins, C.J., Clark, G.C., Heywood, R., Street, A.E., Hardy, C.J.,
Cherry, C.P., Gibson, W.A., Gopinath, C., Harrington S.M., Edmonson,
N.A., & Howard, E. (1979). Investigation of the effects on monkeys of
inhalation of NAB 365 CL aerosol for a period of 26 weeks. Unpublished
report No. BOI 90/78712 from Huntingdon Research Centre. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
De Busk, R.F. & Harrison, D.C. (1969). The clinical spectrum of
papillary-muscle disease. N. Engl. J. Med., 281, 1458-1467.
Ellenberger, J. (1985). Clenbuterol (NAB-365-CL) testing for
point-mutagenic activity with Salmonella typhimurium. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, A. (1971). Pharmacological exposé of the substance NAB365.
Supplement to the exposé on the compound NAB 365. Supplementary
investigations (May 1976) on the pharmacology of the substance NAB 365
and its metabolites. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Engelhardt, G. (1971). Results of a comparative pharmacological
investigation of NAB 365 CL, NAB 365 CL-D and NAB365 CL. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, G. (1976). Pharmacological profile of the action of
NAB 365 (clenbuterol), a new bronchodilator with a selective effect on
the adrenergic ß2 receptors. Arzneim. Forsch., 26(7a), 1404-1420.
Erichsen, D.F. (1988). Dose titration of Ventipulmin syrup in horses
with chronic obstructive pulmonary disease (COPD). Trial
No: 37-295-86-3. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Erichsen, D.F. (1989). A determination of incremental dose tolerance
of Ventipulmin syrup in the horse. Trial No.: Range finder 84-VI.
Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica, Ingelheim am Rhein, Germany.
Fenner, A. (1982). Respiratory rate, minute volume and tidal volume in
clinically healthy fattening cattle and those with bronchopneumonia -
taking into account the effects of clenbuterol. Doctoral Thesis,
Veterinary Faculty, Ludwig-Maximilians University of Munich. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Friedmann, J.Ch. (1982). Study of the possible mutagenic activity of
the substance "NAB365Cl" evaluated by the micronucleus test (oral
route). Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Gibson, J.P., Sells, D.M., Cheng, H.C., & Yuh, L. (1987). Induction of
uterine leiomyomas in mice by medroxalol and prevention by propanolol.
Toxicol. Pathol., 15, 468-473.
Gopinath, C. & Gibson, W.A. (1987). Mesovarian leiomyomas in the rat.
Environ. Health Perspect., 73, 107-113.
Hamm, D. & Erichsen, D.F. (1989). Acute and subacute toxicity of
Ventipulmin. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Roberts, N.L., & Cameron,
D.M. (1984). The disposition of the combination product14C-NAB
365 CL/trimethoprim/sulphadiazine after oral administration to horses.
Unpublished report No. HRC/BOI 112/84912 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R., Roberts,
N.L., Cameron, D.M., Offer, J., & Fish, C. (1985a). The disposition of
the combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
calves. Unpublished report No. HRC/BOI 111/8468OB from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R.,
Roberts, N.L., & Cameron, D.M. (1985b). The disposition of the
combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
dairy cows. Unpublished report No. HRC/BOI 111/84680A from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A., & Cameron, D.M.
(1993a). The pharmacokinetics, metabolism and residues of
14C-clenbuterol (14C-N-AB 365 CL) following intramuscular
administration to calves. Unpublished report No. HRC/BOI 140/921418
from Huntingdon Research Centre. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., & Dighton, M.H. (1993b). Investigation of
the metabolic profiles in the livers of the horses following multiple
oral administration. Unpublished report No. BOI 149/931490 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hewett, Chr. & Notman J. (1984). Dermal tolerance test with repeated
topical application in the rabbit. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Holmstrom, M. & MacGregor, D.B. (1986). NAB 365 CL. Cytogenetic study
in Chinese hamsters. Unpublished report No 4085 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hufnagel, E. (1974). Results with a new broncholytic agent. Effect on
heart rate, blood pressure and ECG. Ärztl. Praxis, 28, 1350-1352.
Jack, D., Poynter, D., & Spurling, N.W. (1983). Beta-adrenoceptor
stimulants and mesovarian leiomyomas in the rat. Toxicology,
27, 315-320.
James, C.N. (1990a). Acute oral toxicity in rats with Ventipulmin
syrup. Unpublished report No. TX-9003/VENTIPULMIN. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990b). Acute dermal toxicity study in rabbits with
Ventipulmin syrup. Unpublished report No. TX-9002/VENTIPULMIN.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
James, C.N. (1990c). Guinea pig sensitisation study - Buehler method
using Ventipulmin syrup. Unpublished report No. Case #: 64551-05 from
Arthur D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990d). Primary dermal irritation study in rabbits with
Ventipulmin syrup. Unpublished report TX-9001/Ventipulmin from Arthur
D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Dunsire, J.P. (1993). Plasma kinetics, metabolism,
excretion and residue kinetics of [14C]-N-AB 365 CL following oral
administration to the horse. Unpublished report No. 8346 from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Jenner, W.N. (1976). Study of the metabolism of
14C-labelled N-AB 365 in the male baboon. Unpublished report from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, W. (1987). Ventipulmin solution Bovine safety trial.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kaik, G. (1978). Clinico-pharmacological tests using the ß2-sympa-
thomimetic NAB 365 (clenbuterol) in patients with obstructive
respiratory disease. Unpublished report from the Medical University
Clinic of Vienna. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973a). Subacute toxicity with compound NAB 365 CL on mice,
oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973b). Teratological testing with the compound N-AB 365-CL
on rats, oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973c). Teratological testing with the compound NAB-365 CL
on rabbits, oral administration. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1975). Teratological testing with the compound N-AB 365 CL
on rats, oral administration during the period of organogenesis.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973a). Subacute toxicity with compound
NAB 365 CL on rats, oral administration. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973b). Chronic toxicity of compound
NAB 365 CL on rats, oral administration per food. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973c). Subacute toxicity with compound
NAB 365 CL on rats, intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kirkland, D.J. (1992). Review of clenbuterol hydrochloride mutagen-
icity data in relation to the proposed acceptable daily intake.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Koellmer, H., Stotzer, H., & Paulini, K. (1977). Subacute toxicity
study of the substance N-AB 365-CL in rats using inhalation over a
period of 13 weeks. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1969). Autoradiographic investigations on the
distribution of [14C]-N-AB 365 CL in rats and pregnant mice
(ADME 1 B). Unpublished report No. U69-0108. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1970). Pharmacokinetic investigations with [14C]-N-AB
365 CL in rats (ADME 1 B). Unpublished report No. U69-0109. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein,
Germany.
Kopitar, Z & Zimmer, A. (1973). Effect of repeated doses of NAB 365 CL
on the pharmacokinetic profile in rats (ADME 1 D). Unpublished report
No. U73-0158. Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim
am Rhein, Germany.
Kreuzer, H. (1988). Intramuscular local tolerance in tests with
Ventipulmin injectable solution ad us. vet. in rabbits. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Landry, Y. (1983). Review of the chief pharmacological properties,
clinical structures and physiochemical properties of clenbuterol.
Unpublished report from Laboratoire d'Allergopharmacologie,
Strasbourg. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Laumen, F. (1978). Investigation on long-term treatment and cumulation
with clenbuterol. Med. Mon.schr., 29, 455-459.
Lehman, H. (1969a). Teratology investigations in pregnant rats with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1969b). Teratology investigations in pregnant rabbits with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1974). Testing of the compound NAB 365 CL for perinatal
toxicity in rats. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1975). Study with substance N-AB 365 CL for fertility
inhibiting, teratogenic and perinatal toxic effects in rats.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1980). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 78 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1981). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 73 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Otto, H., &
Dontenwill, W. (1969). Report on subacute toxicity studies of NAB 365
p. o.- batch Z 6191 in beagle dogs. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
McEnaney, S. (1992). Damaging potential of clenbuterol by its effects
on cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished report No. 2HLREBSG.002 from Hazleton Microtest. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Magnusson, G. & Hansson, E. (1973). Myocardial necrosis in the rat: a
comparison between isoprenaline, orciprenaline, salbutamol and
terbutaline. Cardiology, 58, 174-180.
Nelson, L.W. & Kelly, W.A. (1971). Mesovarian leiomyomas in rats in a
chronic toxicity study of soterenol hydrochloride. Vet. Pathol.,
8, 452-457.
Nishikawa, J., Tsunenari, Y., & Kast, A. (1972). Acute toxicity of NAB
365 CL in rats, mice and rabbits. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Nishikawa, J., Kast, A., & Klupp, H. (1979). Acute toxicity study with
clenbuterol (NAB 365 CL) in rats dosed orally or intravenously.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Nolte, D. (1980). Comments on the publication "Lung function tests
with the bronchospasmolytic agent NAB 365". Unpublished report.
Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim am
Rhein, Germany.
Nolte, D. & Laumen, F. (1972). Pulmonary function tests following the
bronchospasmolytic agent NAB 365. Unpublished report from Medical
Clinics and Polyclinics, Justus-Liebig University and Seltersberg
Sanatorium of Hessen State Insurance Association, Giessen, Germany.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
O'Donnell, S.R. (1976). Selectivity of clenbuterol (NAB 365) in
guinea-pig isolated tissues containing ß-adrenoceptors. Arch. Int.
Pharmacodyn., 224, 190-198.
Owen, R., Fischer, R., & Erichsen, D.F. (1990). Safety of Ventipulmin
syrup in horses: 64-day study trial No. 37-295-83-4. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Palmer, A.K., Edwards, J.A., & Collins, C.J. (1978). Effect of
NAB 365 CL metered aerosol on pregnancy of the rat. Unpublished report
No. BOI 85/78689 from Huntingdon Research Centre. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Palmer, A.K., Bottomley, A.M., & Collins, C.J. (1980). Effect of
NAB 365 CL metered aerosol on pregnancy of the New Zealand White
rabbit. Unpublished report No. BOI 86/79656 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Pappritz, G. (1968). Determination of ALD50 of the substance in the
dog after oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. (1972). Determination of the ALD50 Of NA-B 365 in the
dog following intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Pappritz, G. & Bauer, M. (1973a). Supplementary studies on the
subacute toxicity of substance N-AB 365 CL in dogs after oral
administration. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. & Bauer, M. (1973b). Chronic toxicity study of substance
NAB 365 in dogs following oral administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica, Ingelheim am
Rhein, Germany.
Pappritz, G., Bauer, M., & Eckenfels, A. (1973). Subacute toxicity of
NAB 265 CL in dogs following intravenous administration. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica,
Ingelheim am Rhein, Germany.
Poynter, D & Spurling, N.W. (1971). Some cardiac effects of
beta-adrenergic stimulants in animals. Postgrad. Med. J., 47, 21-25.
Poynter, D., Harris, D.M., & Jack, D. (1978). Salbutamol: lack of
evidence of tumour induction in man. Br. Med. J., 7 January.
Richter, I. (1982). Biochemical investigations of the placental
passage of 14C-clenbuterol in pregnant rats. Unpublished report
No. U82-0292. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Roberts, W.C., & Cohen, L.S. (1972). Left ventricular papillary
muscles. Description of the normal and a survey of conditions causing
them to be abnormal. Circulation, XLVI, 138-154.
Rohr, U.D., Haczkiewicz, K., Mohr, A., & Orton, B. (undated).
Validation of an excised human skin model for testing drug candidates
suited for transdermal drug delivery. Unpublished report from
Boehringer Ingelheim KG. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rominger, K.L. & Schrank, H. (1982). Placental transfer of
14C-clenbuterol in the dog. Unpublished report No. U82-0291.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Rominger, K.L., Förster, H., Hermer, M., Peil, H., & Wolf, M. (1987).
Clenbuterol plasma levels in patients under tocolytic treatment.
Unpublished report from Boehringer Ingelheim KG. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rosenblum, I., Wohl, A., & Stein, A.A. (1965). Studies in cardiac
necrosis. III. Metabolic effects of sympathomimetic amines producing
cardiac lesions. Toxicol. Appl. Pharmacol., 7, 344-351.
Schmid, J. (1977). Pilot pharmacokinetic investigations after
intravenous administration of N-AB 365 CL in a cow. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Schmid, J. (1980). Investigations of the placental transfer of
14C-N-AB 365 CL in the baboon. Unpublished report from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1982). Pharmacokinetics, metabolism and tissue
distribution of 14C-N-AB 365 CL in the baboon. Unpublished report
No. 2220 from Inveresk Research International. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990a). Plasma levels and residue analysis in cows and
calves. Unpublished report No. U90-0092. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990b). N-AB 365 CL in the cow's milk after oral and
intramuscular administration. Unpublished report No. U90-0099.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Prox, A. (1986). Isolation and structural elucidation of
NAB 365 CL metabolites from dog urine. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Zimmer, A. (1977a). Pilot pharmacokinetic studies
following oral administration (single and multiple dosing) of
14C N-AB 365 CL to a cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. & Zimmer, A. (1977b). Pilot pharmacokinetic investigations
after intramuscular administration (single and multiple doses) of
N-AB 365 CL in the cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schuster, A. (1984). Skin sensitisation study in guinea pigs.
Unpublished report No. U84-0673 from Boehringer Ingelheim KG.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schuster, D., Deichsel, G., & Feuerer, W. (1989). Dose effect study
with NAB 365 CL metered dose inhaler in patients with chronic
obstructive lung disease. Unpublished report from Dr. K. Thomae GmbH,
Medical Department. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Sells, D.M. & Gibson, J.P. (1987). Carcinogenicity studies with
medroxalol hydrochloride in rats and mice. Toxicol. Pathol.,
15, 457-467.
Serbedija, R. & Bauer, M. (1973). Toxicity study of the substance
NAB 365 CL in rats over a period of 18 months. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Serbedija, R., Lutzen, L., Puschner, H., & Hohbach, Ch. (1982).
Carcinogenicity study with the substance N-AB 365 CL in rats with oral
administration for a period of 2 years. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Streller, I. (1981). Effect of the ß-adrenergic agents fenoterol,
orciprenaline, clenbuterol, SOM 987 and SOM 1122 on the mucocilliary
clearance in the trachea of the anaesthetised guinea-pig. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Tullgren, A & Lins L-E. (1987). An open long-term tolerability study
on NAB 365 (clenbuterol). Unpublished report from Boehringer Ingelheim
AB, Stockholm, Sweden. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Ueberberg, H. (1976). Comparative study of the effects of NAB 365 CL
and terbutaline sulfate on the dog heart. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Umemoto, K. (1984). Carcinogenicity studies with the beta-adreno-
ceptor stimulant clenbuterol (NAB-365 CL) in mice by drinking water.
Unpublished report from Nippon Boehringer Ingelheim. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1971). Metabolism and species comparison in the rat,
rabbit and dog (ADME IV). Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974a). Comparison of the pharmacokinetic profile in the
dog with single and repeated dosage (ADME 1 D). Unpublished report
No. U73-0161. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Zimmer, A. (1974b). Pharmacokinetics and metabolism pattern in the
rabbit and dog (ADME I). Unpublished report No. U74-0116. Submitted to
WHO by Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974c). Metabolism in man; comparison with dog and rabbit,
using radioactive substance. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1976). Administration of clenbuterol in man. Single doses,
multiple doses, and metabolite samples. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Zimmer, A. (1977). Preliminary investigations of pharmacokinetics in a
horse. Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zupan, J. A. (1985). A comparative bioavailability study between oral
and injectable dosage forms of clenbuterol in horses. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Table 1. Acute toxicity of clenbuterol
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M oral 80 Nishikawa et al., 1972
F oral 133 Nishikawa et al., 1972
M & F s.c 72 Nishikawa et al., 1972
M & F i.v 42 Nishikawa et al., 1972
M i.p 46 Nishikawa el al., 1972
M i.p 72 Nishikawa et al., 1972
Rat M & F oral 175 Nishikawa et al., 1972
M oral 82 Nishikawa et al., 1979
F oral 170 Nishikawa et al., 1979
M & F i.v 30 Nishikawa et al., 1972
M i.v 35 Nishikawa et al., 1979
F i.v 39 Nishikawa et al., 1979
M & F s.c 170 Nishikawa et al., 1972
M & F i.p 70 Nishikawa et al., 1972
M & F oral1 > 0.125 James, 1990a
Rabbit M & F i.v 84 Nishikawa et al., 1972
M & F dermal1 > 0.05 James, 1990b
Dog M & F oral 400-800 Pappritz, 1968
M & F i.v 45-52 Pappritz, 1972
1 Administered as a proprietary veterinary medicinal product
containing 25 µg clenbuterol per ml
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of 15 male and 15 female ICR-JCL mice were given daily
oral doses of 0, 2.5, 12.5 or 62.5 mg/kg bw clenbuterol hydrochloride
as an aqueous solution, by stomach tube, for 30 days. Body weight gain
and food consumption were increased in all treated groups in a dose-
related manner. There were no adverse effects on haematology. The
animals from the highest dose group appeared weak and 6 animals died
during the course of the study.
At necropsy, there were significant increases in liver weights in
animals given 12.5 or 62.5 mg/kg bw per day. Ischemic heart damage was
noted in one animal given 12.5 mg/kg bw per day, and in two animals
from the highest dose group. The NOEL in this study was 2.5 mg/kg bw
per day (Kast, 1973a).
2.2.2.2 Rats
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily oral doses of 0, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by stomach tube, for 30 days. Signs
of toxicity were seen at the highest dose and these included
salivation, encrusted eyes and noses, and reduced body weight gain and
food consumption; 50% of these animal died during the course of the
study. Serum aspartate aminotransferase and glucose concentrations
were significantly reduced in animals of both sexes given 10 or
100 mg/kg bw per day.
Liver weights were significantly reduced in males given the
highest dose, while ischaemic lesions were seen in cardiac muscle in
one male and one female given this dose. The NOEL in this study was
1 mg/kg bw per day based on blood chemistry findings (Kast &
Tsunenari, 1973a).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given doses of 0, 1, 5, 25 or 75 mg/kg bw per day clenbuterol
hydrochloride in the feed for 6 months. At the highest dose level,
body weight gain and food consumption were reduced. During the first
week, a reduction in water consumption occurred in the animals given
this dose, and nine male and five female animals died during the
course of the study.
At necropsy, ischaemic lesions of the myocardium were noted
in all treated animals and there was a dose-related incidence and
severity. These lesions were also found in another group given
75 mg/kg bw per day clenbuterol hydrochloride and then allowed a
4-week recovery period without drug treatment. A NOEL was not
identified in this study (Kast & Tsunenari, 1973b).
Groups of 20 male and 20 female FW49/Biberach rats were fed diets
containing clenbuterol hydrochloride for 18 months. Further groups of
three male and three female rats were fed treated diets for 14 days,
followed by a recovery period; these animals were the subject of ECG
investigations. Another group of 10 female and 10 male rats was fed
the highest dietary level and then maintained for a 6-week recovery
period. The overall design of the study, including doses, is set out
below.
Dose (mg/kg bw 18-month phase 14-day ECG study2
per day) number of animals numbers of animals
males females males females
0 20 20 3 3
0.1 24 20 3 3
0.5 20 20 3 3
2.5 20 20 3 3
2.5 101 101 - -
1 subject to 6 week recovery period
2 subject to 21 day recovery period
Body weight gain in treated groups was slightly increased when
compared with control values. However, there were no overt signs of
toxicity, and no deaths occurred. There were no significant haemato-
logical effects. However, there were reductions in myocardial glycogen,
skeletal muscle glycogen and liver glycogen values in all treated
groups, but there was no clear dose response. Histochemical examination
of the myocardium showed a reduction in enzyme activity, particularly
in the lowest dose group.
The ECG examination revealed a bradycardia rather than the
expected tachycardia, but no explanation was provided for this. Heart
rates returned to normal 2 days into the recovery period. As the
results of this study appear to conflict with those of other studies
(reduction in heart rate rather than the expected increase), and as
the hearts were not adequately examined macroscopically or micro-
scopically, an NOEL cannot be identified from this study (Serbedija &
Bauer, 1973).
Groups of 15 male and 15 female Sprague-Dawley JCL rats were
given daily intravenous doses of 0, 1, 4 or 16 mg/kg bw clenbuterol
hydrochloride for 30 days. Overt signs of toxicity included weakness
at the two highest dose levels and increased respiratory and heart
rates at the highest dose level. One female and one male in the 1 and
4 mg/kg bw per day dose groups respectively, died, while five male and
five female animals died at the highest dose. There was evidence of
hepatotoxicity (focal necrosis) in rats given the highest dose. A NOEL
was not identified in this study (Kast & Tsunenari, 1973c).
Groups of 30 male and 30 female Chbb: THOM (SPF) rats were
exposed by the inhalation route to 0 or 0.667 mg/m3 clenbuterol
hydrochloride in aqueous solution. A further two groups of 15 male and
15 female rats were exposed to 5.013 or 40.0 mg/m3. The exposures
were conducted for 0.5-2 hours per day, 7 days per week, for 13 weeks.
These exposures gave corresponding doses of 0, 0.01, 0.16 and
2.58 mg/kg bw per day. Groups of 10 male and 10 female control
animals, and 10 male and 10 female rats exposed to the highest
concentration were subjected to a 6-week recovery period without
further exposure to clenbuterol hydrochloride.
There was no drug-related mortality during the study. Increased
heart rates were noted in all treated rats. Animals exposed to the
highest concentration of clenbuterol hydrochloride showed myocardial
necrosis. Myocardial changes were noted in animals subject to the
6-week recovery period. As increases in heart rate were noted at all
concentrations, a NOEL was not identified in this study (Koellmer
et al., 1977).
Groups of 20 male and 20 female Charles River CD rats were
exposed to aerosols of clenbuterol hydrochloride in ethanol with
dichlorofluoromethane and 1,2-dichlorotetrafluoroethane as
propellants, using head-only exposure. Other groups served as controls
or ethanol/propellant controls. The doses were equivalent to up to
118 µg/kg bw per day.
The only treatment-related effects attributable to clenbuterol
hydrochloride were liver weight reduction and small aggregations of
alveolar macrophages, which were clearly dose-related (Clark et al.,
1979).
However, there were insufficient data on dosimetry to identify a
NOEL and in addition, there were no measurements of heart-rate or
other cardiac function parameters.
2.2.2.3 Dogs
Groups of three male and three female beagle dogs were given
starch capsules containing clenbuterol hydrochloride at doses of
0, 0.4, 4 and 20 mg/kg bw per day, daily for 13 weeks. The highest
dose was increased to 30 mg/kg bw per day from week 5 and to 40 mg/kg
bw per day from week 9. Water consumption was normal during the study,
but a slight decrease in appetite was noted in high-dose animals when
the dose was increased to 40 mg/kg bw per day. The rate of body weight
gain significantly fell from this point. One male and two females
given the highest dose appeared sedated after treatment. One of these
became increasingly agitated and breathless and died 2-3 minutes after
receiving the 23rd dose of 20 mg/kg bw per day. Tachycardia occurred
after the first dose in all treated animals and this persisted until
the following day. There was a decrease in its severity during
subsequent weeks, but it continued to be noted in all treated animals.
A shortening of the P-Q, Q-T and T-P intervals in the ECG,
corresponding to the increased heart rates, was seen in all treated
dogs. The dog that died was found to have a severe acute pulmonary
infection. Myocardial haemorrhage and necrosis, damage to the hepatic
parenchyma and severe haemorrhagic bronchopneumonia were noted. No
abnormalities occurred in any of the other animals. As dose-related
tachycardia occurred at all doses, a NOEL was not identified in this
study (Leuschner et al., 1969).
Groups of three male and three female beagle dogs were given oral
doses of 0, 2.5 and 40 mg/kg bw per day clenbuterol hydrochloride in
gelatin capsules for 13 weeks. No animals died during the study, but
in the first week, all dogs given clenbuterol hydrochloride were
apathetic, had redness of the skin and eyes, mydriasis and fixed
pupils. Tachycardia was seen in all dogs. At 2.5 and 40 mg/kg bw per
day, significant increases in the activities of several serum enzymes,
including LDH and creatinine kinase, were found. The glycogen levels
of the left heart muscle were decreased. Myocardial necrosis was seen
in dogs given the 2.5 and 40 mg/kg bw per day doses. A NOEL was not
identified in this study (Pappritz & Bauer, 1973a).
Groups of three male and three female beagle dogs were given
gelatin capsules containing daily doses of 0, 0.1 or 0.5 mg/kg bw per
day clenbuterol hydrochloride for 1 year. A group of six male and six
female dogs were given 2.5 mg/kg bw per day in the same manner, and
three animals of each sex were maintained on a control diet for 6
weeks at the end of the study, as part of a recovery phase. No animals
died during the study. In the first week all drug-treated animals were
apathetic and had diffuse reddening of the skin. Tachycardia was noted
in all treated animals, as was mydriasis, episcleral vascular
injection and conjunctivitis.
There were no significant effects on food consumption or body
weights, although dogs given the highest dose gained slightly more
weight than did those in other groups. No haematological effects were
seen, but there were increases in some serum enzymes in treated
groups. Liver glycogen was decreased in dogs given the highest dose
and this remained decreased at the end of the 6-week recovery period.
Heart weights were increased in all the treated animals when
compared with controls, and myocardial necrosis was noted in the
papillary muscle of the left ventricle of all these dogs, including
those subject to the 6-week recovery period. There was no dose
relationship in the cardiotoxicity seen, but histochemical methods
revealed smaller decreases in the activities of several enzymes in
cardiac muscle of the 0.1 mg/kg bw per day group than in the other
treatment groups. A NOEL was not identified in this study
(Pappritz & Bauer, 1973b).
Groups of three male and three female beagle dogs were given
daily intravenous injections of 0, 1, 10 or 1000 µg/kg bw per day
clenbuterol hydrochloride for 30 days. Dogs given the highest dose of
clenbuterol hydrochloride were lethargic and all treated animals
developed tachycardia with associated decreases in the P-Q and Q-T
intervals in the ECG. At necropsy, one male given the highest dose was
found to have myocardial necrosis. A NOEL was not identified in this
study (Pappritz et al., 1973).
2.2.2.4 Monkeys
Groups of three male and three female cynomolgus monkeys were
exposed to atmospheric levels of clenbuterol hydrochloride as an
aerosol in ethanol, with dichlorofluoromethane and 1,2-dichlorotetra-
fluoroethane as propellants, using an oropharyngeal tube, giving doses
of 25, 50 and 150 µg/kg bw per day for 26 weeks. Half of the daily
metered dose was given each morning and the remainder in the afternoon.
Another group of three male and three female monkeys served as sham
controls with no exposure, while a further group of three male and
three female monkeys served as placebo controls and were exposed to
the same aerosol as treated animals, but without the clenbuterol
hydrochloride.
No signs of toxicity were seen in this study and food consumption
and body weights were normal except in high-dose females where a large
body weight gain occurred. Ophthalmoscopy, haematology, lung mechanics
and ventilation, blood chemistry and urinalyses were normal. A low
oxygen tension was noted in high-dose animals in week 10 of the study.
There were no drug-related changes in heart rate or ECGs. At macro-
scopic and histo-pathological examination, there were no drug-related
changes (Collins et al., 1979).
Thus, the NOEL in this study was of the order of 25 µg/kg bw per
day by the inhalation route, but this could not be identified as a
definitive study because of the problems in dosimetry in an inhalation
study of this type. For example, there were no data on how much drug
reached the lower respiratory tract and no information regarding blood
levels. Hence, the degree of systemic exposure was unknown.
2.2.2.5 Cattle
In calves given the therapeutic dose of 0.8 µg/kg bw per day
clenbuterol hydrochloride for 10 days by the oral or intravenous
routes, only transient tachycardia occurred. When given at 5 times the
therapeutic dose (4 µg/kg bw per day) for 10 days, tachycardia and
audibly increased heart force were the only effects noted. When given
to adult cattle at the therapeutic dose or twice the therapeutic dose
for periods of up to 9 days, by the oral route, the main effects were
tachycardia and falls in diastolic blood pressure (Fenner, 1982;
Cameron et al., 1992).
2.2.2.6 Horses
No clinically significant adverse effects were noted in horses
given 0.8-17.5 µg/kg bw per day clenbuterol hydrochloride by the oral
route for periods of up to 64 days. Transitory effects included
tachycardia, sweating, muscle tremors, hyperglycaemia and hypophos-
phataemia (Erichsen, 1988, 1989; Hamm & Erichsen, 1989; Owen et
al., 1990).
Clenbuterol, like other ß-agonists, leads to tachycardia and
hypotension. This probably results in reduced myocardial perfusion at
a time when oxygen demand is high because of increased cardiac rate.
The end result is hypoxia which probably leads to the necrotic lesions
seen in the left ventricular papillary muscles (Rosenblum et al.,
1965; De Busk & Harrison, 1969; Poynter & Spurling, 1971; Roberts &
Cohen, 1972; Magnusson & Hansson, 1973; Balazs & Bloom, 1982). This
explains the cardiac effects noted in the repeated dose (and other)
studies with clenbuterol.
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
Groups of 50 male and 50 female (C57B/6 × DBA/2) mice were given
clenbuterol in the drinking-water at doses of 0.1, 1.0 and 25.0 mg/kg
bw per day for 2 years. Groups of 100 males and 100 females were given
drinking-water only over this period and served as controls. Treated
animals consumed slightly more food and water than controls, but showed
no overt signs of toxicity, except for a dose-related increase in body
weight in the first year and a decrease during the second year.
Survival after 2 years was good in males (78-86%) and acceptable in
females (54-60%).
At termination, there were significant changes in the weights of
a number of organs when compared with control values. These included
significant increases in the absolute heart weights of males given
1 mg/kg bw per day and in females given 25 mg/kg bw per day. There
were significant increases in relative heart weights in all treated
male and female mice. The incidences of non-neoplastic lesions,
including myocardial lesions, were not treatment related. There was no
increased incidence of any rumour type in treated mice when compared
with control values (Umemoto, 1984).
2.2.3.2 Rats
Groups of 48 male and 48 female Chbb:THOM rats were fed diets
containing clenbuterol hydrochloride, resulting in daily doses of
6.25, 12.5, or 25.0 mg/kg bw per day for 2 years. A further group of
72 rats of each sex was fed a diet containing no clenbuterol hydro-
chloride. As ß-sympathomiroetic agents are known to induce mesovarian
leiomyomas in some strains of rat, groups of 72 male and 72 female
Charles River Sprague-Dawley rats were also fed diets containing
clenbuterol hydrochloride at doses of 0 or 25 mg/kg bw per day for 2
years. After one control rat began to show signs of clenbuterol-related
effects, contamination of the feed preparation personnel and equipment
was confirmed, and the substance was then administered via the
drinking-water to ensure the same daily doses.
Treated rats appeared nervous, tense and aggressive, but no
effects on mortality were seen. Survival was in the range of 70-87% in
males and 50-71% in females. In both sexes and strains body weight was
reduced in a dose-related manner. Consumption of drinking-water was
significantly reduced in SD rats given the highest dose. At
termination, fibrosis of the subendocardial connective tissue was
frequently observed in all groups including controls, but the
incidence was higher in the Chbb:THOM rats, suggesting a drug-
related effect.
There was an increased incidence of mesovarian leiomyomas in the
female Sprague-Dawley rats, but not in the Chbb:THOM rats. The
incidence is shown in Table 2.
Table 2. Incidence of mesovarian leiomyomas in female rats treated with clenbuterol
hydrochloride for 2 years
Chbb:THOM Sprague-Dawley
Rats rats
Dose 0 6.25 12.5 25.0 0 25.0
(mg/kg bw per day)
Numbers with 0/72 0/48 0/48 0/72 0/72 11/72
Mesovarian
leiomyomas
per group
There was no increased incidence of any other tumour type
(Serbedija et al., 1982).
Leiomyomas of the smooth muscle of the uterus in mice and
mesovaria of rats have been reported following long-term treatment
with ß-agonists (Nelson & Kelly, 1971; Jack et al., 1983;
Amemiya et al., 1984; Gibson et al., 1987; Gopinath & Gibson,
1987; Sells & Gibson, 1987). They are not associated with genotoxicity
with these agents and are considered to be associated with adrenergic
stimulation. The ß-agonist salbutamol produced mesovarian leiomyomas
in rats, but these could be prevented by administration of the
ß-blocking agent propranolol; a similar phenomenon has been reported
in mice where the induction of leiomyomas of the uterus by medroxalol
was prevented by propranolol (Jack et al., 1983; Gibson et al.,
1987). There have been no reports of increased incidences of leiomyomas
in women following the use of ß-adrenergic agents (Poynter et al.,
1978).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
Groups of 17-20 pregnant female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage, from day 15 of gestation to 21 days
post-partum when dams and pups were sacrificed. The pups, including
those that died before day 21, were necropsied and X-rayed. Maternal
body weights were reduced at the end of the study in animals given
7 mg/kg bw per day clenbuterol hydrochloride. Those given the highest
dose were similar to the controls, but these animals were heavier at
the beginning of the study. Maternal food consumption was reduced in
all treated groups.
The number of pups born dead increased in a dose-related manner,
as did the number of pups dying after birth. At the highest dose all
the pups died, as shown below.
Body weights of pups were also reduced in a dose-related manner.
A NOEL was not identified in this study (Lehman, 1974).
Groups of 30 male and 30 female Chbb:THOM rats were given daily
oral doses of 0, 1, 7 or 50 mg/kg bw per day clenbuterol hydrochloride
in distilled water by gavage. Treatment of the male rats commenced 10
weeks prior to mating while that of females began 2 weeks prior to
mating. On day 14 of gestation 50% of the females were killed and the
uterine contents examined. The remaining female rats were allowed to
deliver normally and rear their pups until weaning. Dosing of all
females continued until they were killed on day 14 of gestation or
after weaning.
To investigate the cause of the high pup mortality in the study
of Lehman (1974), the litters of six control dams were exchanged with
litters from dams given 50 mg/kg bw per day. Surviving dams and pups
were necropsied at the end of the lactation period. Food consumption
was increased in treated animals when compared with control values.
However, body weight was significantly reduced in animals of both
sexes given the highest daily doses.
Pup weights at birth were reduced in all treated groups. There
was a dose-related decrease in the numbers of viable pups which was
statistically significant at 7 and 50 mg/kg bw per day. All the pups
in the 50 mg/kg bw per day group died on the first day of lactation
regardless of whether or not they were suckled by treated or control
dams. The majority of pups from control dams suckled by dams that were
given the 50 mg/kg bw per day dose survived.
There were no substance-related effects on fertility, corpora
lutea, implantation or resorption rates, and no malformations were
noted in pups from treated rats. The hearts of three pups from
high-dose females were examined histologically, but no abnormalities
were found (Lehman, 1975).
Groups of 34 male and 34 female Chbb:THOM rats were given daily
oral doses of 0, 1.5, 7.5 or 15 µg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage. The male rats were
treated for 70 days prior to mating and the females from 14 days prior
to mating up until the end of gestation. The pups were not treated.
Dosing of the females continued until the interim sacrifice or after
weaning.
On days 13-15 of gestation, 50 pregnant rats were killed and the
uterine contents examined. The remainder were allowed to give birth
naturally and the pups reared until 3 weeks old, except for two males
and two females in each litter which were reared until 10 weeks after
mating when certain behavioural tests were conducted (pupillary
reflex, photo-phobia response, hearing, behaviour on a rotating rod,
behaviour in a Y maze). The animals were then mated and allowed to
litter naturally.
There were no effects on fertility attributable to clenbuterol
treatment, and gestation length, numbers of corpora lutea,
implantation rates, incidences of resorptions, parental body
weights and food consumption were unaffected.
No compound-related effects were seen in the littering phase and
the pups gained weight normally. Performance in the behavioural tests
were similar in treated and control groups and the offspring of these
animals were normal in all respects. The NOEL was 15 µg/kg bw per day
(Lehman, 1980).
Similar results were obtained in an almost identical study on
rats using the same dose levels. Again, the NOEL was 15 µg/kg bw per
day (Lehman, 1981).
No teratogenic effects were seen in any of these reproductive
toxicity studies.
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Groups of 20 mated female SPF-FW 49 Biberach rats were given oral
doses of 0, 0.04, 0.2 or 1 mg/kg bw per day clenbuterol hydrochloride
in distilled water, by gavage on days 6-15 of gestation. No evidence
of maternal toxicity was seen and there were no compound-related
effects on the incidences of resorptions, or on litter and fetal
weights. There were no increased incidences of any type of
malformation. The NOEL was 1 mg/kg bw per day (Lehman, 1969a).
Groups of 25 mated female Sprague-Dawley rats were given oral
doses of 0, 0.01, 1, 10 or 100 mg/kg bw per day clenbuterol
hydrochloride in distilled water, by gavage, from days 9 to 14 of
gestation. Approximately 24 hours before the expected delivery date,
20 dams per dose level were killed and the uterine contents examined
while the remaining five pregnant animals in each group were allowed
to deliver naturally.
Overt signs of toxicity, including weakness, hypersensitivity and
bloody vaginal discharge were seen in rats given the highest daily
dose. Maternal body weights were reduced in dams given the 10 or
100 mg/kg bw per day doses. Animals given these doses had signifi-
cantly increased incidences of resorptions with corresponding
reductions in the numbers of viable fetuses. Mean litter weights were
reduced at both these doses, while mean fetal weight was significantly
reduced at the highest dose level.
The incidences of malformations were significantly increased at
the two highest dose levels used. Anomalies included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of the vertebrae (Kast, 1973b).
A study was conducted to verify the results of Kast (1973b).
Groups of 25 mated Sprague-Dawley rats were given 0, 0.01, 1, 10 or
100 mg/kg bw per day clenbuterol hydrochloride in distilled water, by
gavage, from days 8 to 17 of gestation. Approximately 24 hours before
the expected delivery date, 20 dams per group were killed and the
uterine contents examined. The remainder were allowed to deliver
naturally.
Overt signs of toxicity were similar to those in the previous
study and five dams given the highest dose died. The incidences of
resorptions were increased in animals given the highest dose; mean
litter weights and fetal weights were reduced. The incidences of
malformations were significantly increased at the two highest doses.
The types of malformation were similar to those seen in the study of
Kast (1973b).
In pups derived from animals allowed to deliver naturally, body
weights were increased at the highest dose. Swimming function was
retarded in this group. At 10 mg/kg bw per day, two pups were found to
have hydrocephalus while at the highest dose level, four pups were
found to have anophthalmia. No malformations were noted in pups from
the 0.01, 0.1 or 1 mg/kg bw per day groups. The NOEL in both these
studies was 1 mg/kg bw per day (Kast, 1975).
Groups of 20 Sprague-Dawley rats were exposed nose only to an
atmosphere containing clenbuterol hydrochloride during days 6-15 of
gestation. The estimated doses were 0, 19, 39 and 78 µg/kg bw per day.
There was no evidence of maternal toxicity or teratogenicity, but
there were dose-related increases in the incidences of skeletal
variations, indicative of fetotoxicity, at all dose levels (Palmer
et al., 1978).
2.2.5.2 Rabbits
Groups of 15 mated Russian/Biberach rabbits were given daily oral
doses of 0, 30, 100 or 300 µg/kg bw per day clenbuterol in distilled
water, by gavage, from days 6 to 18 of gestation. Three dams given the
300 µg/kg bw per day dose and one given the highest dose died during
the study. These deaths were due to bronchopneumonia. Several rabbits
in each group were found not to be pregnant. Despite these problems,
at least 10 litters from each dose group were available for
examination.
Dams in each dose group showed better weight gains than controls,
but there was no clear dose relationship. There were no adverse
effects on the incidences of resorptions, numbers of live fetuses,
fetal weights or the incidences of malformations. However, there were
increased incidences of delayed ossification, suggestive of
fetotoxicity, in the 100 and 300 µg/kg bw per day groups. The NOEL
was 30 µg/kg bw per day (Lehman, 1969b).
Groups of 10 female Russian/Biberach rabbits were given daily
oral doses of 0, 0.01, 1 or 50 mg/kg bw per day clenbuterol
hydrochloride, in distilled water by gavage, on days 8 to 16 of
gestation. There were no compound-related deaths, but body weight gain
and food intake was reduced at the highest dose level.
At the highest dose level, the incidence of resorptions was
significantly increased, and there was a corresponding decrease in
the numbers of viable fetuses. Mean litter and fetal weights were
significantly reduced at this level. There was an increased incidence
of malformations, notably cleft palate and synostosis, in pups from
dams given 50 mg/kg bw per day clenbuterol hydrochloride. The NOEL was
1 mg/kg bw per day (Kast, 1973c).
Groups of 13-16 mated New Zealand White rabbits were exposed to
doses of clenbuterol hydrochloride calculated to be 0, 48, 146 or
300 µg/kg bw per day by the inhalation route using a fine bore tube
sited over the tracheal opening. The heads of the animals were held in
an air extraction chamber to minimize rebreathing of aerosol. Dosing
took place on days 6-18 of gestation.
Signs of stress were observed in all groups as a result of the
exposure procedures. Signs included anorexia, lethargy and respiratory
distress, and some animals died in all groups, including the controls.
There was a high incidence of malformations in the control groups
which was probably related to the initial stress. There was no
increased incidence in animals given 146 or 300 µg/kg bw per day.
As a result of the high numbers of malformations seen in control
groups, no firm conclusions could be drawn from this study. However,
the data suggested that doses of up to 300 µg/kg bw per day clen-
buterol hydrochloride, when given by inhalation to pregnant rabbits on
days 6-18 of gestation, were not teratogenic (Palmer et al., 1980).
Taken together, the animal data suggested that clenbuterol
hydrochloride is teratogenic in rats and rabbits at relatively high
doses. There was evidence of fetotoxicity. The NOEL for maternal
toxicity and teratogenicity in the rat and rabbit following oral
administration was 1 mg/kg bw per day. However, the NOEL for
fetotoxicity in one study in the rabbit was 0.03 mg/kg bw per day.
2.2.6 Special studies on genotoxicity
Clenbuterol hydrochloride gave negative results in the Ames test
with Salmonella typhimurium strains, in a reversion test with
Escherichia coli strain WP2(P), in a gene mutation assay with
Chinese hamster V79 cells, in an in vivo mouse micronucleus test and
in an in vivo cytogenetic assay with Chinese hamster bone marrow.
In the mouse lymphoma test with L5178Y cells, no increased
incidences in mutation frequency were observed in the absence of
metabolic activation. However, with metabolic activation, one of two
experiments gave positive results at the two highest concentrations
used (Clements, 1992).
Viability counts were reduced at these concentrations and
sampling error may have led to these spurious results (Kirkland, 1992).
In an in vitro assay with human lymphocytes, at concentrations of
> 500 µg/ml, an increased incidence of chromosome aberrations was
noted in the absence (but not in the presence) of metabolic
activation. This was not dose-dependent.
The results are summarized in Table 3.
These data suggest that clenbuterol hydrochloride is not
genotoxic.
2.2.7 Special studies on irritancy
2.2.7.1 Skin irritation
Clenbuterol hydrochloride was applied to the shaved skin of six
Russian/Biberach rabbits, using an occlusive dressing, for a period of
28 days. No evidence of skin irritation was seen (Hewett & Notman,
1984). Similar results were noted in a further study of skin
irritation (James, 1990d).
2.2.7.2 Intramuscular tolerance
Intramuscular injection of 0.5 ml of a formulation containing
clenbuterol hydrochloride into the dorsal area of rabbits produced
only minimal haemorrhage, oedema and necrosis (Kreuzer, 1988).
2.2.7.3 Eye irritation
A formulated commercial product containing clenbuterol
hydrochloride (0.1 ml) was instilled into the left eye of six New
Zealand White rabbits. The right eye served as a control. Mild
irritation was noted and this resolved 48 hours after treatment
(Bailey, 1990).
2.2.8 Special studies on immunotoxicity
Clenbuterol hydrochloride, as a formulated commercial product,
was tested on the guinea-pig using the Buehler technique. The content
of active ingredient was 70.4 µg/ml in the formulation. It was not a
sensitizer in this study (James, 1990c).
Another study employed a 0.2% solution of clenbuterol hydro-
chloride in 30% aqueous ethanol utilizing guinea-pigs and the
Magnusson and Kligman maximization test involving the use of Freund's
adjuvant. No evidence of a sensitizing effect was found (Schuster,
1984).
Table 3. Results of genotoxicity studies on clenbuterol hydrochloride
Test system Test subject Concentration Results Reference
Bacterial Salmonella typhimurium 40-2500 µg/plate - Baumeister,
reversion TA98, TA100, TA 1535, 1978
TA1537, TA1538
Escherichia coli 40-1500 µg/plate
WP2(P)
Bacterial S. typhimurium 10-500 µg/plate - Ellenberger,
reversion TA98, TA 100, TA 1535, 1985
TA1537, TA1538
Forward Chinese hamster V79 10-100 µg/ml - Baumeister,
mutation fibroblasts, HGPRT 1985
assay locus
Forward Mouse lymphoma 300-800 µg/ml - Clements,
mutation L5178Y 1992
assay
In vitro human lymphocytes 177-2352 µg/ml +/- McEnaney,
cytogenetics 1992
In vivo mouse 0.006, 0.5, 5.0 - Friedmann,
micronucleus mg/kg bw per 1982
test day
In vivo Chinese hamster 19, 60, 186 - Holmstrom &
cytogenetics (bone marrow) mg/kg bw per MacGregor,
test day 1986
2.2.9 Special studies on pharmacodynamic effects
2.2.9.1 Studies in animals
Clenbuterol is a ß2-sympathomimetic agent with a wide range of
pharmacodynamic effects. It produces a potent, dose-dependent
bronchiolytic effect, which was demonstrated in guinea-pigs following
acetylcholine, histamine or bradykinin-induced bronchospasm. Similar
effects have been reported in cats and dogs. Clenbuterol exerts
positive chronotropic and inotropic effects on isolated atria. It
induces tachycardia in rats, dogs, cats and a variety of farm animals,
accompanied by reductions in systolic and diastolic blood pressure. In
the isolated uterine horn of the estrus rat, it exerts a pronounced
relaxing effect on serotonin, oxytocin and bradykinin-induced spasm.
It has also been shown to exert a relaxing effect on smooth muscle in
the guinea-pig ileum and to reduce intestinal mobility in the rat and
mouse.
Clenbuterol results in a glycogenolytic effect in the rat
myocardium associated with ß-stimulation. It has no effect on hepatic
glycogen. It causes a potent dose-dependent reduction of the gastric
secretion.
In mice, it causes decreases in spontaneous activity and also
leads to increases in barbiturate-induced sleeping time. The drug
results in dose-dependent striated muscle relaxation. However, it has
no antipyretic or analgesic effects in mice (Engelhardt, A., 1971;
Engelhardt, G., 1971, 1976).
Clenbuterol exerted major relaxing effects on spasms induced by
carbachol in guinea-pig trachea and lung. The effects were antagonised
by propranolol (Landry, 1983). It also led to decreases in perfusion
pressure of hind limb blood vessels, inhibitions of contractions of
the uterus, increases in atrial rate and inhibition of electrically
stimulated contractions of the ileum (O'Donnell, 1976). It improved
the rates of mucociliary clearance in the guinea-pig (Streller, 1981).
In beagle dogs, oral doses of clenbuterol hydrochloride at
1.5, 3.0, 7.5 and 15.0 µg/kg bw resulted in a dose-dependent
tachycardia at > 3.0 µg/kg bw. There was a slight tachyphylactic
effect demonstrated by a diminution of the intensity and duration of
the response. There was a dose-related reduction in blood pressure and
the changes in diastolic and systolic pressures ran in parallel. These
changes occurred at the lowest dose used (Ueberberg, 1976).
In cattle, intravenous doses of 0.6 µg/kg bw and 1.5 µg/kg bw
produced no cardiac effects, as shown by ECG examination, whereas
2.85 µg/kg bw produced pronounced tachycardia. A dose of 0.3 mg/kg bw
clenbuterol resulted in tocolysis lasting approximately 5 hours
(Ballarini, 1978). Different lag times in parturition may be induced
depending on the dose and route of treatment; no adverse effects on
the dam or calf were noted (Arbeiter & Thurner, 1977).
Four metabolites of clenbuterol that had been shown to be present
in the kidneys and liver of treated target animals were tested for
pharmacodynamic activity. Of these, only one, N-A 1141 (Figure 1) was
shown to have activity in the guinea-pig. Its bronchiolytic effect was
less than 20% that of clenbuterol, and, moreover, it accounted for
only 12% of residues in the liver and kidney 6 hours after treatment
(Engelhardt, 1971).
2.2.9.2 Studies in humans
Patients given 10 µg (0.167 µg/kg bw) clenbuterol by the
inhalation route showed no signs of tachycardia as determined by ECG.
There were slight decreases in blood pressure. When patients with
cardiac arrhythmia were given this dose of clenbuterol, none of the
patients showed any changes attributable to the drug (Hufnagel, 1974).
After inhalation exposure of patients with airways disease, onset
of broncholytic action occurred within 5 minutes and lasted for up to
6 hours. Pulmonary function effects were greatest with doses of >
5 µg (0.083 µg/kg bw); only a minimal effect was seen with 2.5 µg
(0.042 µg/kg bw) (Schuster et al., 1989).
2.3 Observations in humans
A single blinded cross-over study was carried out to examine the
acute bronchospasmolytic effect and possible side-effects following
oral administration of placebo and three doses of clenbuterol (1, 2.5
and 5 µg/day) in patients (three male and three female) with chronic
obstructive airway disease over a 3 day period. The drug was given
orally, diluted with water. Observations were carried out over a
2-hour period following dosing. The average age of the patients was
55.7 years and they had an average body weight of 73.16 kg.
None of the 3 doses produced any clear, consistent effects on
bronchial resistance, thoracic gas volume, radial pulse frequency or
blood pressure, and no side effects were seen.
The pharmacological NOEL in this study was 5 µg/day, equivalent
to 0.08 µg/kg bw per day (Kaik, 1978).
The bronchospasmolytic effect was examined in two groups of
patients:
Group A: ten patients aged 46-75 years with chronic obstructive
respiratory disease resulting from pulmonary tuberculosis.
Group B: five patients aged 56-67 years with chronic obstructive
respiratory disease not related to tuberculosis, plus five
patients aged 34-57 with bronchial asthma.
The bronchospasmolytic effect was examined after single oral doses of
1, 2.5, 5, 10, 20, 25 or 30 µg/person, and after a placebo dose.
In Group A patients, intrathoracic gas volume was significantly
reduced and vital capacity and pneumometer values significantly
increased at all dose levels. In Group B patients, airway resistance
was significantly reduced, but no dose relationship could be
demonstrated. No significant placebo effect was seen in either group.
When compared with placebo values, a significantly greater increase
for both vital capacity and pneumometer values was observed in Group
A, even at the lowest dose used in this group (5 µg). However, at the
two lowest doses used in Group B (1 and 2.5 µg), there were no
significant differences from placebo values. The pharmacological NOEL
in this study was 2.5 µg, equivalent to 0.042 µg/kg bw (Nolte &
Laumen, 1972; Nolte, 1980).
Children who consumed between 0.05-0.075 mg of clenbuterol showed
only mild tachycardia. A 30-year-old woman who consumed 30 tablets
equivalent to 0.6 mg clenbuterol (10 µg/kg approximately) developed
tachycardia and slight hypertension approximately 1 hour after
consumption. No tablet remains were found on gastric lavage, and
medicinal charcoal and a saline laxative were given. The following
day, the patient's pulse rate and blood pressure had returned to
normal (Boehringer, 1991a).
Patients (100+) administered doses of 20-60 µg/day (0.3-1.0 µg/kg
bw per day) for up to 1 year or 20 µg/day (0.3 µg/kg bw per day) for
up to 6 months showed no adverse effects except for slight tremor and
occasional, mild tachycardia (Laumen, 1978; Tullgren & Lins, 1987).
3. COMMENTS
The Committee considered toxicological data on clenbuterol,
including the results of acute, short-term and reproductive toxicity
studies, as well as studies on teratogenicity, genotoxicity and
carcinogenicity. Results of pharmacokinetic and pharmacodynamic
studies in animals and humans were also considered.
Clenbuterol is well absorbed after oral administration in a
number of animal species and in humans. An oral dose is largely and
rapidly excreted in the urine, and the majority of the remainder is
excreted in the faeces. The biotransformation of clenbuterol is
complex and a number of metabolites are formed. The major compound
found in a number of species was unchanged clenbuterol. After oral
administration of therapeutic doses to lactating cattle, clenbuterol
was found in the milk.
When radiolabelled clenbuterol was given orally to pregnant rats,
dogs, baboons and cattle, radioactivity was detected in the fetuses.
Clenbuterol was moderately toxic in mice and rats after oral
administration, LD50 values being in the range of 80-175 mg/kg bw.
It was less toxic in the dog (LD50 = 400-800 mg/kg bw). It was more
toxic after parenteral administration, with LD50 values in the range
of 30-85 mg/kg bw after intravenous administration. The main signs of
toxicity included lethargy, tachycardia and tonic-clonic convulsions
after oral administration.
The main effects noted in the repeat-dose studies were
tachycardia and, at higher doses, myocardial necrosis. These effects
are common with ß-agonist drugs. The myocardial necrosis was
considered to be secondary to hypoxia, due to reduced myocardial
perfusion at a time of high oxygen demand resulting from increased
cardiac rate.
In 30-day repeat-dose studies in mice and rats, NOELs of 2.5 and
1 mg/kg bw per day, respectively, were identified, largely based on
cardiac lesions. However, in a range of repeat-dose studies in rats
using doses of 0.01 to 100 mg/kg bw per day for durations of up to
18 months, administered through the oral, intravenous and inhalation
routes, no NOELs were identified. Effects were usually related to
cardiac function and were seen even at the lowest doses used.
Similarly, no NOELS could be identified in a range of repeat-dose oral
studies in dogs. These studies used doses ranging from 0.1 to 40 mg/kg
bw per day. In a 26-week inhalation study in cynomolgus monkeys, the
NOEL was 25 µg/kg bw per day, based on a number of observations
including cardiac effects.
No evidence of carcinogenicity was noted in a two-year oral study
in mice with doses of up to 25 mg/kg bw per day. In a two-year study
with doses of up to 25 mg/kg bw per day in the Chbb:THOM rat, no
evidence of carcinogenicity was seen. However, in Sprague-Dawley rats
given 25 mg clenbuterol/kg bw per day orally for 2 years, an increased
incidence of mesovarian leiomyomas occurred. With the related
compounds salbutamol in rats and medroxalol in mice, the effects could
be abolished by administration of the ß-blocking agent propranolol.
Mesovarian leiomyomas in rats and uterine leiomyomas in mice are known
to occur following long-term treatment with ß-adrenoceptor agonists
and the Committee concluded that these were due to adrenergic
stimulation and not to any genotoxic mechanism. Clenbuterol was not
genotoxic in a range of in vitro and in vivo genotoxicity studies.
Epidemiological studies indicate that there have been no
increased incidences of uterine leiomyomas in women following the use
of ß-adrenoceptor agonists.
Clenbuterol had no effects on fertility in a reproductive
toxicity study in rats using oral doses of 1-50 mg/kg bw per day from
10 weeks prior to mating in males and two weeks prior to mating in
females. However, doses of 50 mg/kg bw per day resulted in the deaths
of pups soon after birth. To investigate the cause of the high pup
mortality at this dose level, the litters of control dams were
exchanged with those from dams given 50 mg/kg bw per day. Pups from
rats given 50 mg/kg bw per day died on the first day of lactation
regardless of whether they suckled on treated or control dams. The
mechanism involved in this lethal effect is unknown. A NOEL was not
identified in this study, because pup weights at birth were reduced in
all treated animals.
In a reproductive toxicity study in which male rats were treated
with 1.5-15 µg clenbuterol/kg bw per day orally for 70 days prior to
mating and females with the same dose range for 14 days prior to
mating, no adverse effects on reproduction were noted. The NOEL was
15 µg/kg bw per day.
In teratogenicity studies in rats, oral doses of 10 and 100 mg/kg
bw per day produced teratogenic effects that included hydrocephalus,
anasarca, umbilical hernia, anophthalmia, rib variations and
splintering of vertebrae. These were accompanied by signs of maternal
toxicity. The NOEL was 1 mg/kg bw per day. In three studies in rabbits
using doses of 30 µg to 50 mg per kg bw per day, signs of feto-
toxicity, including delayed ossification and cleft palate, occurred.
The NOEL was 30 µg/kg bw per day.
Clenbuterol produced a range of pharmacodynamic effects in a
number of animal species including tachycardia, hypertension and
muscle relaxing effects. These were seen at single doses as low as
0.8 µg/kg bw.
Four metabolites of clenbuterol that had been shown to be present
in the kidneys of treated target animals were tested for pharmaco-
logical activity. Of these, only one (N-A 1141) was shown to have
activity. Its broncholytic effect in the guinea-pig was less than
20% that of clenbuterol. In addition, it accounted for only 1-2% of
residues in the liver and kidney of target animals 6 hours after
treatment.
In humans, clenbuterol produced a bronchiolytic effect when a
single dose of 10 µg (0.167 µg/kg bw) was given by the inhalation
route, but no evidence of tachycardia was seen at this dose. With oral
doses of clenbuterol of up to 5 µg/day (0.08 µg/kg bw per day) over a
3-day period, there were no effects on bronchial resistance, thoracic
gas volume, cardiac rate or blood pressure. The NOEL in this study was
5 µg/day (0.08 µg/kg bw per day). In a study to investigate the
bronchospasmolytic effect in humans, patients with obstructive lung
disease were given oral doses of up to 30 µg per person. Patients
administered doses of 5 µg or more exhibited bronchospasmolytic
effects, and the pharmacological NOEL in this study was 2.5 µg per
person, equivalent to 0.04 µg/kg bw.
4. EVALUATION
The Committee considered the most relevant study for determining
the ADI to be that concerning the bronchospasmolytic effect in humans.
The patients had chronic obstructive airway disease and thus were
likely to be a very sensitive population for this effect. The NOEL
identified in this study (2.5 µg per person, equivalent to 0.04 µg/kg
bw) is approximately 25% of the dose in another study in which the
inhalation route was used, but in which cardiac effects were not
observed. This NOEL is approximately 50% of the oral dose used in
another study where, again, cardiac effects did not occur. Hence, this
NOEL for the bronchospasmolytic effect offers an additional safety
margin for cardiac effects. The Committee therefore established an ADI
of 0-0.004 µg/kg bw, based on the NOEL of 0.04 µg/kg bw per day for
pharmacodynamic effects in humans and a safety factor of 10.
5. REFERENCES
Amemiya, K., Kudoh, M., Suzuki, H., Saga, K., & Hosaka, K. (1984).
Toxicology of mabuterol. Arzneim.forsch, 34(11a), 1680-1684.
Arbeiter, K. & Thurner, M. (1977). Effect of the sympathomimetic
Planipart (NAB 365) on parturition in cattle. Tierärztl. Umsch.,
32, 423-427.
Bailey, D.E. (1990). Primary eye irritation study in rabbits with
Ventipulmin. Unpublished report No. TX-9005/Ventipulmin from Hazleton
Laboratories America, Inc., Vienna, VA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Baillie, H.W., Cameron, B.D., Draffan, G.H., & Schmid, J. (1980).
Investigations of the placental transfer of 14C-N-AB 365 CL in the
cow. Unpublished report No. 111674 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Balazs, T. & Bloom, S. (1982). Cardiotoxicity of adrenergic and
vasodilating antihypertensive drugs. In: van Stee, E.W. (ed.),
Cardiovascular Toxicology, Raven Press, New York, pp. 199-220.
Ballarini, G. (1978). Initial studies of the clinical pharmacology of
the ß2 sympathomimetic agent NAB 365 (Planipart) and its use for
pharmacological tocolysis in cows. Tierärztl. Umsch., 33, 421-427.
Baumeister, M. (1978). Mutagenicity studies with the substance
N-AB 365 CL in the plate incorporation assay (Ames test). Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Baumeister, M. (1985). Mutagenicity study with the substance
N-AB 365 CL in the V79 (HGPRT)-test. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Boehringer (1991a). Summary of case reports of overdosage with
clenbuterol. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Boehringer (1991b). Estimated transdermal absorption of clenbuterol
hydrochloride following skin contact with granules and injection
solutions for veterinary use. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987a). The residue kinetics of
[14C]-NAB 365 CL in the calf. Unpublished report No. 4371 from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, B.D. & Phillips, M.W.A. (1987b). The residue kinetics of
(14C)-NAB 365 CL in the lactating cow. Unpublished report No. 4445
from Inveresk Research International. Submitted to Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Cameron, D.M., Crook, D., & Brown, G. (1992). Ventipulmin injection
solution and Ventipulmin granules. Target species tolerance study in
calves. Unpublished report No. BOI 139/921030 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Chasseaud, L.F., Cresswell D.G., & Savage J.A. (1978).
Pharmacokinetics of 14C-N-AB 365 during chronic inhalational
toxicity in cynomolgus monkeys. Unpublished report No. U79-0212 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Clark, G.C., Collins, C.J., Heywood, R., Street, A.E., Gibson, W.A.,
Prentice, D.E., Edmonson, N., Lewis, D.J, & Mahjeed, S.K. (1979).
Investigation of the effects on rats of inhalation of NAB 365 CL for
26 weeks. Unpublished report No. BOI 88/78768 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Clements, J. (1992). Study to determine the ability of clenbuterol
hydrochloride to induce mutations at the thymidine kinase (tk) locus
in mouse lymphoma L5178Y cells using a fluctuation assay. Unpublished
report No. 2TKREBSG.002 from Hazleton Microtest. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Collins, C.J., Clark, G.C., Heywood, R., Street, A.E., Hardy, C.J.,
Cherry, C.P., Gibson, W.A., Gopinath, C., Harrington S.M., Edmonson,
N.A., & Howard, E. (1979). Investigation of the effects on monkeys of
inhalation of NAB 365 CL aerosol for a period of 26 weeks. Unpublished
report No. BOI 90/78712 from Huntingdon Research Centre. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
De Busk, R.F. & Harrison, D.C. (1969). The clinical spectrum of
papillary-muscle disease. N. Engl. J. Med., 281, 1458-1467.
Ellenberger, J. (1985). Clenbuterol (NAB-365-CL) testing for
point-mutagenic activity with Salmonella typhimurium. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, A. (1971). Pharmacological exposé of the substance NAB365.
Supplement to the exposé on the compound NAB 365. Supplementary
investigations (May 1976) on the pharmacology of the substance NAB 365
and its metabolites. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Engelhardt, G. (1971). Results of a comparative pharmacological
investigation of NAB 365 CL, NAB 365 CL-D and NAB365 CL. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Engelhardt, G. (1976). Pharmacological profile of the action of
NAB 365 (clenbuterol), a new bronchodilator with a selective effect on
the adrenergic ß2 receptors. Arzneim. Forsch., 26(7a), 1404-1420.
Erichsen, D.F. (1988). Dose titration of Ventipulmin syrup in horses
with chronic obstructive pulmonary disease (COPD). Trial
No: 37-295-86-3. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Erichsen, D.F. (1989). A determination of incremental dose tolerance
of Ventipulmin syrup in the horse. Trial No.: Range finder 84-VI.
Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica, Ingelheim am Rhein, Germany.
Fenner, A. (1982). Respiratory rate, minute volume and tidal volume in
clinically healthy fattening cattle and those with bronchopneumonia -
taking into account the effects of clenbuterol. Doctoral Thesis,
Veterinary Faculty, Ludwig-Maximilians University of Munich. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Friedmann, J.Ch. (1982). Study of the possible mutagenic activity of
the substance "NAB365Cl" evaluated by the micronucleus test (oral
route). Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Gibson, J.P., Sells, D.M., Cheng, H.C., & Yuh, L. (1987). Induction of
uterine leiomyomas in mice by medroxalol and prevention by propanolol.
Toxicol. Pathol., 15, 468-473.
Gopinath, C. & Gibson, W.A. (1987). Mesovarian leiomyomas in the rat.
Environ. Health Perspect., 73, 107-113.
Hamm, D. & Erichsen, D.F. (1989). Acute and subacute toxicity of
Ventipulmin. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Roberts, N.L., & Cameron,
D.M. (1984). The disposition of the combination product14C-NAB
365 CL/trimethoprim/sulphadiazine after oral administration to horses.
Unpublished report No. HRC/BOI 112/84912 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R., Roberts,
N.L., Cameron, D.M., Offer, J., & Fish, C. (1985a). The disposition of
the combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
calves. Unpublished report No. HRC/BOI 111/8468OB from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R.,
Roberts, N.L., & Cameron, D.M. (1985b). The disposition of the
combination product 14C-NAB 365 CL/trimethoprim/sulphadiazine in
dairy cows. Unpublished report No. HRC/BOI 111/84680A from Huntingdon
Research Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A., & Cameron, D.M.
(1993a). The pharmacokinetics, metabolism and residues of
14C-clenbuterol (14C-N-AB 365 CL) following intramuscular
administration to calves. Unpublished report No. HRC/BOI 140/921418
from Huntingdon Research Centre. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hawkins, D.R., Elsom, L.F., & Dighton, M.H. (1993b). Investigation of
the metabolic profiles in the livers of the horses following multiple
oral administration. Unpublished report No. BOI 149/931490 from
Huntingdon Research Centre. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Hewett, Chr. & Notman J. (1984). Dermal tolerance test with repeated
topical application in the rabbit. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Holmstrom, M. & MacGregor, D.B. (1986). NAB 365 CL. Cytogenetic study
in Chinese hamsters. Unpublished report No 4085 from Inveresk Research
International. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Hufnagel, E. (1974). Results with a new broncholytic agent. Effect on
heart rate, blood pressure and ECG. Ärztl. Praxis, 28, 1350-1352.
Jack, D., Poynter, D., & Spurling, N.W. (1983). Beta-adrenoceptor
stimulants and mesovarian leiomyomas in the rat. Toxicology,
27, 315-320.
James, C.N. (1990a). Acute oral toxicity in rats with Ventipulmin
syrup. Unpublished report No. TX-9003/VENTIPULMIN. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990b). Acute dermal toxicity study in rabbits with
Ventipulmin syrup. Unpublished report No. TX-9002/VENTIPULMIN.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
James, C.N. (1990c). Guinea pig sensitisation study - Buehler method
using Ventipulmin syrup. Unpublished report No. Case #: 64551-05 from
Arthur D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
James, C.N. (1990d). Primary dermal irritation study in rabbits with
Ventipulmin syrup. Unpublished report TX-9001/Ventipulmin from Arthur
D. Little, Inc., Cambridge, MA, USA. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Dunsire, J.P. (1993). Plasma kinetics, metabolism,
excretion and residue kinetics of [14C]-N-AB 365 CL following oral
administration to the horse. Unpublished report No. 8346 from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, A.M. & Jenner, W.N. (1976). Study of the metabolism of
14C-labelled N-AB 365 in the male baboon. Unpublished report from
Inveresk Research International. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Johnston, W. (1987). Ventipulmin solution Bovine safety trial.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kaik, G. (1978). Clinico-pharmacological tests using the ß2-sympa-
thomimetic NAB 365 (clenbuterol) in patients with obstructive
respiratory disease. Unpublished report from the Medical University
Clinic of Vienna. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973a). Subacute toxicity with compound NAB 365 CL on mice,
oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973b). Teratological testing with the compound N-AB 365-CL
on rats, oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1973c). Teratological testing with the compound NAB-365 CL
on rabbits, oral administration. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kast, A. (1975). Teratological testing with the compound N-AB 365 CL
on rats, oral administration during the period of organogenesis.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973a). Subacute toxicity with compound
NAB 365 CL on rats, oral administration. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973b). Chronic toxicity of compound
NAB 365 CL on rats, oral administration per food. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kast, A. & Tsunenari, Y. (1973c). Subacute toxicity with compound
NAB 365 CL on rats, intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Kirkland, D.J. (1992). Review of clenbuterol hydrochloride mutagen-
icity data in relation to the proposed acceptable daily intake.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Koellmer, H., Stotzer, H., & Paulini, K. (1977). Subacute toxicity
study of the substance N-AB 365-CL in rats using inhalation over a
period of 13 weeks. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1969). Autoradiographic investigations on the
distribution of [14C]-N-AB 365 CL in rats and pregnant mice
(ADME 1 B). Unpublished report No. U69-0108. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Kopitar, Z. (1970). Pharmacokinetic investigations with [14C]-N-AB
365 CL in rats (ADME 1 B). Unpublished report No. U69-0109. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein,
Germany.
Kopitar, Z & Zimmer, A. (1973). Effect of repeated doses of NAB 365 CL
on the pharmacokinetic profile in rats (ADME 1 D). Unpublished report
No. U73-0158. Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim
am Rhein, Germany.
Kreuzer, H. (1988). Intramuscular local tolerance in tests with
Ventipulmin injectable solution ad us. vet. in rabbits. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Landry, Y. (1983). Review of the chief pharmacological properties,
clinical structures and physiochemical properties of clenbuterol.
Unpublished report from Laboratoire d'Allergopharmacologie,
Strasbourg. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Laumen, F. (1978). Investigation on long-term treatment and cumulation
with clenbuterol. Med. Mon.schr., 29, 455-459.
Lehman, H. (1969a). Teratology investigations in pregnant rats with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1969b). Teratology investigations in pregnant rabbits with
the substance N-AB 365 CL. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1974). Testing of the compound NAB 365 CL for perinatal
toxicity in rats. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1975). Study with substance N-AB 365 CL for fertility
inhibiting, teratogenic and perinatal toxic effects in rats.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1980). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 78 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Lehman, H. (1981). Test of the substance N-AB 365 CL for fertility-
inhibiting, embryotoxic and foetotoxic effects in rats. Unpublished
report of study No. 73 D. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., Otto, H., &
Dontenwill, W. (1969). Report on subacute toxicity studies of NAB 365
p. o.- batch Z 6191 in beagle dogs. Unpublished report. Submitted to
WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
McEnaney, S. (1992). Damaging potential of clenbuterol by its effects
on cultured human lymphocytes using an in vitro cytogenetics assay.
Unpublished report No. 2HLREBSG.002 from Hazleton Microtest. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Magnusson, G. & Hansson, E. (1973). Myocardial necrosis in the rat: a
comparison between isoprenaline, orciprenaline, salbutamol and
terbutaline. Cardiology, 58, 174-180.
Nelson, L.W. & Kelly, W.A. (1971). Mesovarian leiomyomas in rats in a
chronic toxicity study of soterenol hydrochloride. Vet. Pathol.,
8, 452-457.
Nishikawa, J., Tsunenari, Y., & Kast, A. (1972). Acute toxicity of NAB
365 CL in rats, mice and rabbits. Unpublished report. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Nishikawa, J., Kast, A., & Klupp, H. (1979). Acute toxicity study with
clenbuterol (NAB 365 CL) in rats dosed orally or intravenously.
Unpublished report. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Nolte, D. (1980). Comments on the publication "Lung function tests
with the bronchospasmolytic agent NAB 365". Unpublished report.
Submitted to WHO by Boehringer Ingelheim GmbH, Ingelheim am
Rhein, Germany.
Nolte, D. & Laumen, F. (1972). Pulmonary function tests following the
bronchospasmolytic agent NAB 365. Unpublished report from Medical
Clinics and Polyclinics, Justus-Liebig University and Seltersberg
Sanatorium of Hessen State Insurance Association, Giessen, Germany.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
O'Donnell, S.R. (1976). Selectivity of clenbuterol (NAB 365) in
guinea-pig isolated tissues containing ß-adrenoceptors. Arch. Int.
Pharmacodyn., 224, 190-198.
Owen, R., Fischer, R., & Erichsen, D.F. (1990). Safety of Ventipulmin
syrup in horses: 64-day study trial No. 37-295-83-4. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Palmer, A.K., Edwards, J.A., & Collins, C.J. (1978). Effect of
NAB 365 CL metered aerosol on pregnancy of the rat. Unpublished report
No. BOI 85/78689 from Huntingdon Research Centre. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Palmer, A.K., Bottomley, A.M., & Collins, C.J. (1980). Effect of
NAB 365 CL metered aerosol on pregnancy of the New Zealand White
rabbit. Unpublished report No. BOI 86/79656 from Huntingdon Research
Centre. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Pappritz, G. (1968). Determination of ALD50 of the substance in the
dog after oral administration. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. (1972). Determination of the ALD50 Of NA-B 365 in the
dog following intravenous administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Pappritz, G. & Bauer, M. (1973a). Supplementary studies on the
subacute toxicity of substance N-AB 365 CL in dogs after oral
administration. Unpublished report. Submitted to WHO by Boehringer
Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Pappritz, G. & Bauer, M. (1973b). Chronic toxicity study of substance
NAB 365 in dogs following oral administration. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica, Ingelheim am
Rhein, Germany.
Pappritz, G., Bauer, M., & Eckenfels, A. (1973). Subacute toxicity of
NAB 265 CL in dogs following intravenous administration. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica,
Ingelheim am Rhein, Germany.
Poynter, D & Spurling, N.W. (1971). Some cardiac effects of
beta-adrenergic stimulants in animals. Postgrad. Med. J., 47, 21-25.
Poynter, D., Harris, D.M., & Jack, D. (1978). Salbutamol: lack of
evidence of tumour induction in man. Br. Med. J., 7 January.
Richter, I. (1982). Biochemical investigations of the placental
passage of 14C-clenbuterol in pregnant rats. Unpublished report
No. U82-0292. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Roberts, W.C., & Cohen, L.S. (1972). Left ventricular papillary
muscles. Description of the normal and a survey of conditions causing
them to be abnormal. Circulation, XLVI, 138-154.
Rohr, U.D., Haczkiewicz, K., Mohr, A., & Orton, B. (undated).
Validation of an excised human skin model for testing drug candidates
suited for transdermal drug delivery. Unpublished report from
Boehringer Ingelheim KG. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rominger, K.L. & Schrank, H. (1982). Placental transfer of
14C-clenbuterol in the dog. Unpublished report No. U82-0291.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Rominger, K.L., Förster, H., Hermer, M., Peil, H., & Wolf, M. (1987).
Clenbuterol plasma levels in patients under tocolytic treatment.
Unpublished report from Boehringer Ingelheim KG. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Rosenblum, I., Wohl, A., & Stein, A.A. (1965). Studies in cardiac
necrosis. III. Metabolic effects of sympathomimetic amines producing
cardiac lesions. Toxicol. Appl. Pharmacol., 7, 344-351.
Schmid, J. (1977). Pilot pharmacokinetic investigations after
intravenous administration of N-AB 365 CL in a cow. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Schmid, J. (1980). Investigations of the placental transfer of
14C-N-AB 365 CL in the baboon. Unpublished report from Inveresk
Research International. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1982). Pharmacokinetics, metabolism and tissue
distribution of 14C-N-AB 365 CL in the baboon. Unpublished report
No. 2220 from Inveresk Research International. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990a). Plasma levels and residue analysis in cows and
calves. Unpublished report No. U90-0092. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. (1990b). N-AB 365 CL in the cow's milk after oral and
intramuscular administration. Unpublished report No. U90-0099.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Prox, A. (1986). Isolation and structural elucidation of
NAB 365 CL metabolites from dog urine. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schmid, J. & Zimmer, A. (1977a). Pilot pharmacokinetic studies
following oral administration (single and multiple dosing) of
14C N-AB 365 CL to a cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schmid, J. & Zimmer, A. (1977b). Pilot pharmacokinetic investigations
after intramuscular administration (single and multiple doses) of
N-AB 365 CL in the cow. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Schuster, A. (1984). Skin sensitisation study in guinea pigs.
Unpublished report No. U84-0673 from Boehringer Ingelheim KG.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Schuster, D., Deichsel, G., & Feuerer, W. (1989). Dose effect study
with NAB 365 CL metered dose inhaler in patients with chronic
obstructive lung disease. Unpublished report from Dr. K. Thomae GmbH,
Medical Department. Submitted to WHO by Boehringer Ingelheim Vetmedica
GmbH, Ingelheim am Rhein, Germany.
Sells, D.M. & Gibson, J.P. (1987). Carcinogenicity studies with
medroxalol hydrochloride in rats and mice. Toxicol. Pathol.,
15, 457-467.
Serbedija, R. & Bauer, M. (1973). Toxicity study of the substance
NAB 365 CL in rats over a period of 18 months. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Serbedija, R., Lutzen, L., Puschner, H., & Hohbach, Ch. (1982).
Carcinogenicity study with the substance N-AB 365 CL in rats with oral
administration for a period of 2 years. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Streller, I. (1981). Effect of the ß-adrenergic agents fenoterol,
orciprenaline, clenbuterol, SOM 987 and SOM 1122 on the mucocilliary
clearance in the trachea of the anaesthetised guinea-pig. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
Tullgren, A & Lins L-E. (1987). An open long-term tolerability study
on NAB 365 (clenbuterol). Unpublished report from Boehringer Ingelheim
AB, Stockholm, Sweden. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Ueberberg, H. (1976). Comparative study of the effects of NAB 365 CL
and terbutaline sulfate on the dog heart. Unpublished report.
Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Umemoto, K. (1984). Carcinogenicity studies with the beta-adreno-
ceptor stimulant clenbuterol (NAB-365 CL) in mice by drinking water.
Unpublished report from Nippon Boehringer Ingelheim. Submitted to WHO
by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1971). Metabolism and species comparison in the rat,
rabbit and dog (ADME IV). Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974a). Comparison of the pharmacokinetic profile in the
dog with single and repeated dosage (ADME 1 D). Unpublished report
No. U73-0161. Submitted to WHO by Boehringer Ingelheim GmbH,
Ingelheim am Rhein, Germany.
Zimmer, A. (1974b). Pharmacokinetics and metabolism pattern in the
rabbit and dog (ADME I). Unpublished report No. U74-0116. Submitted to
WHO by Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1974c). Metabolism in man; comparison with dog and rabbit,
using radioactive substance. Unpublished report. Submitted to WHO by
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zimmer, A. (1976). Administration of clenbuterol in man. Single doses,
multiple doses, and metabolite samples. Unpublished report. Submitted
to WHO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim am
Rhein, Germany.
Zimmer, A. (1977). Preliminary investigations of pharmacokinetics in a
horse. Unpublished report. Submitted to WHO by Boehringer Ingelheim
Vetmedica GmbH, Ingelheim am Rhein, Germany.
Zupan, J. A. (1985). A comparative bioavailability study between oral
and injectable dosage forms of clenbuterol in horses. Unpublished
report. Submitted to WHO by Boehringer Ingelheim Vetmedica GmbH,
Ingelheim am Rhein, Germany.
