
THIAMPHENICOL
First draft prepared by
Dr R. Fuchs,
Department of Experimental Toxicology and Ecotoxicology,
Institute for Medical Research and Occupational Health,
Zagreb, Croatia
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.2 Toxicological studies
2.2.1 Acute toxicity studies
2.2.2 Short-term toxicity studies
2.2.3 Long-term toxicity/carcinogenicity study
2.2.4 Reproductive toxicity studies
2.2.5 Special studies on embryotoxicity and
teratogenicity
2.2.6 Special studies on genotoxicity
2.2.7 Special studies on immune responses
2.2.8 Special studies on microbiological effects
2.3 Observations in humans
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Thiamphenicol is a broad-spectrum antimicrobial agent,
structurally similar to chloramphenicol, used orally to control
infections in humans, pigs, poultry and non-ruminating cattle. It is
bacteriostatic for both Gram-positive and Gram-negative aerobes and
for some anaerobes. It has not been previously evaluated by the
Committee.
The molecular structure of thiamphenicol is shown below.
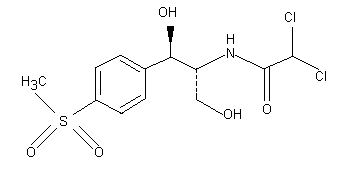 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
After i.v. administration in rats the half-life of thiamphenicol
was 46.3 minutes, compared to 21.5 minutes for chloramphenicol
(Ferrari & Della Bella, 1974).
Pretreatment of rats with phenobarbital increased thiamphenicol
half-life slightly from 46.3 to 55.2 minutes, whereas the
chloramphenicol half-life was reduced from 21.5 minutes to 9.3 minutes
(Della Bella et al., 1968a).
Following administration of 30 mg/kg to rats, thiamphenicol was
eliminated in the urine almost entirely in the unchanged form: 62%
following oral administration and 47% after i.m. administration (both
at 48 hours). No significant glucuro-conjugation products were found.
In the bile, 3.4% of the administered dose appeared unchanged and
10-12% appeared as conjugated products after 4 hours. Of the
administered dose, 36% was eliminated in faeces after 75 hours, almost
entirely as unchanged thiamphenicol. Distribution studies showed that
levels in the kidney and liver were higher than in plasma, while brain
concentrations were negligible (Gazzaniga, 1974).
2.1.1.2 Rabbits
The amount of metabolites recovered in urine and bile within 7
hours after i.v. administration was about 73% for thiamphenicol and
60% for chloramphenicol, and the percentage of glucuronide to total
amount recovered was 8% for thiamphenicol and 66% for chloramphenicol.
The recovery of thiamphenicol into the bile was only 1%, mostly in
unchanged form. About 3% of administered chloramphenicol was excreted
into the bile and two-thirds of this consisted of metabolites
(Uesugi et al., 1974).
2.1.1.3 Dogs
Following intraduodenal administration of 70 mg/kg thiamphenicol
or chloramphenicol, 30% of the thiamphenicol dose was found unchanged
in urine within 8 hours, whereas only 8.3% of chloramphenicol was
eliminated in the active form. After i.m. injection, the urinary
elimination of active antibiotics within 8 hours was 24.2% for
thiamphenicol but only 1.76% for chloramphenicol (Laplassote, 1962).
2.1.1.4 Pigs
To determine plasma and tissue concentrations of thiamphenicol in
pigs following dietary treatment, 16 male pigs, approximately 7 weeks
old, were fed thiamphenicol in the diet at a concentration of
900 mg/kg (equivalent to 30 mg/kg bw), twice daily for a period of 5
days. Three pigs were maintained as controls and fed basal diet only.
Venous blood samples were taken prior to treatment and at various
time-points during the study. The animals were killed at 4, 6, 8 and
10 days after treatment and samples of kidney, liver, muscle, fat and
lung taken. Concentrations of thiamphenicol in plasma were measured
following solvent extraction, using HPLC. On the second day of the
dosing period, scouring and swelling or redness of the anus/perineal
area were noted in all treated animals. The signs resolved within 4-10
days. The maximum mean plasma level of thiamphenicol (1.28 mg/litre)
was demonstrated 8 hours after the first dose. Mean levels were in the
range of 0.22-0.80 mg/litre during the dosing period and declined over
the withdrawal period to give concentrations below or close to the
limit of detection (0.01-0.08 mg/litre) from 4 hours to 5 days after
the end of treatment. The results of the analysis of tissue samples
were not reported (Redgrave et al., 1991).
2.1.1.5 Humans
After a 500 mg oral dose of each of thiamphenicol and
chloramphenicol, the plasma levels appear to be similar. A high level
of active thiamphenicol and a low level of chloramphenicol were found
in the urine (53.1% and 9.2%, respectively, after 24 hours). The
half-life of thiamphenicol is significantly increased in renal
insufficiency, but is almost unaffected by liver cirrhosis
(Azzolini et al., 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of thiamphenicol and thiamphenicol-glycinate
is given in Table 1.
The predominant clinical signs in the animal species tested were
sedation and piloerection after oral dosing, and dyspnoea, cyanosis,
motility disorders and respiratory arrest after parenteral
administration.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
The oral toxicity of thiamphenicol, when administered as an
aqueous suspension in 0.5% methocel for 13 week, has been investigated
in four groups of rats (Sprague-Dawley 30/sex/group). Thiamphenicol
was administered by oral gavage at dose levels of 30, 45, 65 or
100 mg/kg bw per day, while a fifth control group received 0.5%
methocel only. The first 15 animals of each group were killed on
completion of the treatment period, and the remaining 15 after a
recovery period of 8 weeks. Mortality during the treatment period was
elevated among animals of both sexes receiving 100 mg/kg bw per day,
and during the recovery period mortality was similar in all groups. At
levels of 65 and 100 mg/kg bw per day, pallor, hair loss, prostration,
hunched posture and flaccid musculature were seen. After the recovery
phase the incidence and the severity of symptoms were similar in all
groups including controls. Body weight stasis or loss was seen in
animals receiving 65 and 100 mg/kg bw per day, and after 8 weeks
recovery body weight was similar in all groups. Food intake was
reduced at dose levels of 45, 65 and 100 mg/kg bw per day, with
immediate improvement after cessation of treatment.
Table 1. Acute toxicity of thiamphenicol (TAP) and thiamphenicol-glycinate (TAP-G)
Species Sex Route LD50 (mg/kg bw) Reference
TAP TAP-G
Mouse M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1550 Bonanomi, 1978
M & F i.v. 450 Bonanomi, 1978
Rat M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1750 Bonanomi, 1978
M & F i.v. 470 Bonanomi, 1978
In all treated animals there were changes in erythrocyte
parameters, differential and total leukocyte counts and clotting
parameters, all of which were dose-related. After the recovery period
erythrocyte and leukocyte counts were still low in males treated with
65 and 100 mg/kg bw per day. Plasma levels of urea, triglycerides and
total protein were altered from week 7 in animals treated with
45 mg/kg bw per day and after the end of treatment also in males
receiving 30 mg/kg bw per day. Parameters associated with liver and
kidney function were affected at the two higher doses. After 8 weeks
full recovery was considered to have occurred. The weights of all the
main organs were reduced at dose levels of 65 and 100 mg/kg bw per
day, but after the recovery period only the testis weight was still
reduced. Postmortem examination revealed effects on the gastro-
intestinal tract and spleen in both sexes and in the liver, thymus and
testis of males at the highest dose level only. After 8 weeks only the
testis weights were still reduced. The erythroid/myeloid cell ratio
was increased in both sexes at doses of 65 or 100 mg/kg bw per day,
and after 8 weeks was still slightly higher than usual.
Treatment-related changes were seen in tissues with high cell
turnover rates; most of them recovered after a period of respite from
treatment. Animals in the groups treated with 30 and 45 mg/kg per day
showed no histopathological findings except hepatocytic reduced
basophilia (male, 45 mg/kg bw per day) and increased splenic extramed-
ullary haematopoiesis (female 45 mg/kg bw per day). Testicular
germinal epithelial deficit was present at doses above 45 mg/kg, and
caecal oedema and adnexal atrophy were present after the recovery
period at the highest dose level. The NOEL was 30 mg/kg bw per day
(Marubini et al., 1991).
In a 6-month toxicity study in the rat, thiamphenicol was
administered as an aqueous suspension in 2% gum arabic by stomach tube
6 days/week to 180 Wistar rats (30/sex/group) at dose levels of 0, 40
or 120 mg/kg bw per day. Body weight and food intake were recorded
twice weekly in the first 4 weeks of treatment, and thereafter only
once a week. At the end of weeks 4, 8, 16 and 24, ten animals from
each group were killed following collection of 2-hour urine sample.
Haematology, clinical chemistry assays and urinanalysis were performed
on all tested animals. Histological examinations were carried out on
the lungs, spermatozoa, blood and bone marrow smears and samples of
main organs. There was a decrease in food intake at a dose level of
120 mg/kg bw per day and a dose- and time-related decrease in body
weight gain in females. No effect was observed on erythropoiesis or on
hepatic or renal function. Urinanalysis showed the presence of albumin
and haemoglobin in the high-dose rats. No gross pathological
variations in organ weights were observed, but irritative changes in
the gastrointestinal mucosa and high incidence of monolateral
spontaneous hydronephrosis were seen both in treated and control
animals. Histopathological examination performed on control and
high-dose rats revealed a slight effect on the morphology of
spermatozoa in the high-dose group at the 8th, 16th and 24th week of
treatment (Della Bella et al., 1968b).
2.2.2.2 Rabbits
Thiamphenicol-glycinate was administered subcutaneously to groups
of rabbits ("Fauve de Bourgogne" seven/sex/group) at dose levels of
0, 25, 50 or 100 mg/kg bw per day, 6 days/week for 12 weeks. Animals
were weighed weekly and submitted to haematological (erythrocyte and
leukocyte counts with differentials) and biochemical (urea, reducing
sugars, chlorides) examinations before treatment, after 6 weeks and at
the end of the treatment period. After macroscopic examination of the
viscera, the various organs were examined histologically. During the
treatment two control animals and three in the high-dose group died
(no autopsies were carried out). A reduction in polymorphonuclear
neutrophils in male rabbits in all treated groups and a slight fall in
erythrocyte levels in high-dose females were observed. Anatomical and
pathological examination provided no evidence of damage attributable
to thiamphenicol-glycinate administration (Brunaud, 1965).
2.2.2.3 Dogs
In a 7-week study in beagle dogs (four/sex/group), thiamphenicol
was administered orally in gelatin capsules at dose levels of 0, 40 or
80 mg/kg bw per day. Two males and two female dogs were killed at the
end of the 7-week treatment, and the remaining animals were kept for
further 12 weeks without treatment before being killed. Behaviour and
body weights were recorded, haematology and clinical chemistry
examinations and urinanalysis were conducted on all animals pretest
and at various intervals during the study. Complete gross postmortem
examination, organ weighing and histopathological evaluation were
conducted on all animals. The animals in the two treated groups
developed diarrhoea soon after the beginning of treatment, which
spontaneously regressed in the low-dose group but persisted in the
high-dose group. At 80 mg/kg bw per day reduced food consumption with
loss of weight and muscular asthenia occurred, and vomiting was
observed in some cases. Four of these dogs were killed at the end of
the 4th week. Slight loss of weight was observed in two dogs in the
low-dose group.
Decreases in haematocrit, haemoglobin concentration and
erythrocyte count were seen in both treated groups, but did not appear
to be dose-related, and returned to normal on withdrawal of treatment.
Slight increase in proteinuria was observed in the last few weeks of
treatment. At 40 mg/kg bw per day superficial erosion of the gall
bladder mucosa and at 80 mg/kg bw per day haemorrhagic ulcers in the
gall bladder, diffuse muco-membranous enteritis and thymic involution
were seen in dogs killed at the end of treatment period. In dogs
killed after the 12-week recovery period no differences were observed
between treated and control animals. Histological examination after 7
weeks revealed in the high-dose group severe cholecystitis, chronic
sclerosing pancreatitis, enteritis, severe depletion of haematopoietic
marrow and lymphoid thymus depletion. Two dogs in the low-dose group
showed depletion of germinal epithelium in the testes and multinuc-
leated cells in seminiferous tubules, which were not seen in the high-
dose group. None of the changes observed at 7 weeks were detectable
in the animals kept for 12 weeks after cessation of treatment
(Bonanomi et al., 1978).
Thiamphenicol was administered orally in gelatin capsules to 24
beagle dogs (four/sex/group) at dose levels of 30, 60 or 120 mg/kg bw
per day for a period of 4 weeks. Physical observations, ophthalmo-
scopic examinations, and body weight and food consumption measurements
were performed before treatment and over selected intervals during the
treatment. Haematology, clinical chemistry and urinanalysis were
conducted on all animals pretest and at study termination. Complete
gross postmortem examinations, organ weight and histopathological
evaluation were conducted on all animals. The body weights of the
high-dose animals were slightly lower than those of controls at week 3
in both sexes, and at week 4 in males only. At 60 and 120 mg/kg bw per
day absolute and relative liver weights in male dogs were greater than
in controls, and relative liver weights were also increased in
females. Microscopically, hepatocellular hypertrophy was present in
the liver of mid- and high-dose animals, which correlated with the
increase in liver weights in these groups. No other parameter
evaluated showed evidence of adverse treatment-related effects. The
NOEL was 30 mg/kg bw per day (Kelly & Daly, 1990).
Thiamphenicol was administered orally in gelatin capsules to 56
beagle dogs (seven/sex/group) at dose levels of 15, 30 or 60 mg/kg bw
per day. After 6 months of treatment four animals/sex/group were
sacrificed, and the remaining three animals/sex/group were kept for a
2-month recovery period. Physical observations, ophthalmoscopic
examinations, body weight, food consumption, haematology and clinical
chemistry examinations and urinanalysis were conducted on all animals
pretest and on all surviving animals at selected intervals during the
treatment and recovery period. Complete gross postmortem examinations,
organ weight and histopathological evaluation were conducted on all
animals. One control male was found dead during the study. One male
and one female dog in the highest dose group were moribund and had to
be killed. Clinical signs prior to death included lethargy, poor food
consumption, emaciation, tremors and dehydration. Physical findings
related to thiamphenicol administration were tremors, lethargy,
irregular gait and excessive licking or chewing in the high-dose
group. Tremors were also present in the mid-dose animals. These signs
were seen during the last two months of the study and were not present
at the end of the recovery period. Body weights of the high-dose males
during the study were 4 to 18% lower than those of controls. Decreased
erythrocyte counts and mean haematocrit values were seen in high-dose
males at weeks 6 and 13 and at termination of the study, and in high-
and mid-dose females at week 13 and at termination of the study. After
the recovery period no differences were observed in the haematological
parameters between control and treated animals. No treatment-related
effects were seen in the bone marrow smear examinations. Mean serum
cholesterol and phospholipid levels of the mid-and high-dose males at
week 6 and 13 and at termination of the study were greater than
control values. The same parameters were elevated in high-dose females
at the end of the study. Mean serum glucose levels of males at
60 mg/kg bw per day and females at 30 and 60 mg/kg bw per day were
significantly increased. Mean fibrinogen values of high-dose females
were elevated at the end of the study. Relative liver weights were
increased at mid- and high-dose levels. Pathological lesions related
to treatment were seen in sections of the thymus (exacerbation of
involution), bone marrow (decreased cellularity), liver (centrilobular
necrosis and pigment deposition), testes (focal and diffuse tubular
atrophy) and oesophagus (ulceration) from high-dose animals of both
sexes, mostly occurring in animals killed in a moribund condition. No
alterations were noted in any of the tissues examined microscopically
from animals that were allowed to recover after treatment. The NOEL
was 15 mg/kg bw per day (Kelly & Daly, 1991).
2.2.2.4 Pigs
A study designed to determine tolerance in pigs to treatment with
thiamphenicol at three times the recommended dose for 5 days and at
the normal recommended dose for 15 days was conducted with 16 weaned
large white hybrid pigs (two pigs/sex/group). Thiamphenicol was
administered in the diet at dose levels of 30 or 90 mg/kg bw per day
for 5 days or 30 mg/kg bw per day for 15 days. The control group of
animals was fed basal diet only. Clinical signs, body weight and food
consumption were recorded. Blood, urine and faecal samples were
obtained before dosing and at various time-points during the study. No
significant treatment-related clinical abnormalities were noted. Body
weight changes and food consumption were within normal limits. No
consistent treatment-related differences in haematological and
biochemical parameters or in urinanalysis values were observed. The
authors concluded that treatment with thiamphenicol had no significant
adverse effects on general health, body weight, food consumption or
standard clinical pathology parameters (Roberts et al., 1989).
In a 4-week toxicity study, groups of pigs (Large White hybrid,
four/sex/group) were fed 25, 50 or 100 mg thiamphenicol/kg bw per day.
The control group of animals was fed basal diet only. Clinical signs,
body weight and food consumption were recorded. Blood, urine and
faecal samples were obtained before dosing and during week 4 for
clinical pathological investigations. At the end of the 4-week dosing
period, pigs were killed, and selected tissues were processed for
histological examination. In all groups treated with thiamphenicol,
swelling and erythema of the anus, vulva/testes and perineal area,
tail and hocks were observed on the second day of the dosing period.
These effects were consistent with scouring and irritancy, e.g., as a
result of disruption of normal gastrointestinal flora activity, and
disappeared within 1 to 13 days. All pigs remained in good health
thereafter. Slight reductions in body weight gain and food consumption
were noted at 50 and 100 mg/kg bw per day. At week 4 there was a
slight reduction in mean PCV, haemoglobin concentration and
erythrocyte counts in animals receiving 100 mg/kg bw per day and a
treatment-related reduction of urinary pH in all groups dosed with
thiamphenicol. At the end of the study, increases in liver and kidney
weights in pigs fed 50 and 100 mg/kg bw per day were observed. On
histological examination, treatment-related changes were found in the
highest dose group only: an increase in vacuolation and fat in renal
tubular epithelium and, in some animals, minimal diffuse hepatocyte
vacuolation and hepatocyte fat (Cameron et al., 1990).
2.2.3 Long-term toxicity/carcinogenicity study
2.2.3.1 Rats
As a range-finding study for the dose selection in a 2-year
carcinogenicity study, four groups of F-344 rats (10/sex/group)
were given drinking-water containing 0, 125, 250 or 500 mg/litre
thiamphenicol (equal to 9, 17 or 36 mg/kg bw per day for males and 12,
29 or 39 mg/kg bw per day for females) for 13 weeks. The examinations
at the end of the study covered clinical observations, water
consumption, body weight changes, haematological parameters, serum
biochemistry, organ weights and gross and microscopic appearance. In
haematological examinations anaemic changes were observed in males
treated with 250 mg/litre or more and high-dose females. Similar
changes, such as increased MCV and increased related counts, were
observed in males of the 125 mg/litre group and females of the 250 and
125 mg/litre groups. At autopsy, enlargement of the caecum was seen in
treated groups of both sexes. Histologically, the highest dose animals
showed decreased haematopoiesis of the bone marrow, decreased spermato-
genesis of the testis and sperm granulomas of the epididymis. Sperm
granulomas in the epididymis were also seen in some of the animals in
the 250 mg/litre group. Based on the results of this pilot study, a
2-year carcinogenicity study of thiamphenicol was performed in rats.
Three groups of F-344 rats (50/sex/group) were given drinking-water
containing 0, 125 or 250 mg thiamphenicol/litre (equal to 8 or 16
mg/kg bw per day for males and 9.7 or 19 mg/kg bw per day for females)
for 104 weeks. All surviving animals subjected to 4-week withdrawal of
the test chemical after the end of the treatment were killed for full
histopathological examinations. The high-dose animals showed decreased
body weight gain, but the incidence of rumours in treated groups was
not significantly higher than that of controls (Maekawa, 1996; summary
report only was available).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
In a fertility study on male Wistar rats, oral treatment with
thiamphenicol for 2 or 3 months (30 animals per dose level, 10 per
treatment time), at dose levels of 120, 180 or 240 mg/kg bw per day,
resulted in reduction in the number of tubular germinal cells, which
was more marked at the highest dose level. Ten animals of each group
were treated for 4 weeks, ten for 8 weeks and the last ten for 12
weeks. At the end of each test period 5 animals of each dose group
were killed and necropsied, while the remaining 5 rats were mated with
normal females. At 240 mg/kg bw per day extensive testicular
hypotrophy, together with severe depletion of the germinal epithelium
21 days after withdrawal of treatment, was seen. Histological changes
coincided with a reduction of the fertility index, which gradually
recovered within 50 days. Litters from matings between treated males
and normal females were normal in number and weight, and no morpho-
logical abnormalities were observed. The concentration ratio of
thiamphenicol between testes and plasma after administration of
240 mg/kg bw per day thiamphenicol was 1, indicating the absence of
accumulation in testes (Della Bella et al., 1967).
Groups of 21 Sprague-Dawley rats were given thiamphenicol orally
(30, 60 or 120 mg/kg bw per day daily) from day 15 of gestation to day
21 postpartum. In groups receiving 60 and 120 mg/kg bw per day a
higher post-implantation loss, slight weight reduction at birth and
increased rate of perinatal mortality were observed. No malformations
were observed. Development of pups was inhibited during the lactation
period with a consistent dose-dependent relationship. From day 30
postpartum a good recovery was observed in all groups. Sexual
behaviour and fertility of F1 animals were normal, and the F2
generation showed no signs of abnormal development (Bonanomi et
al., 1980).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Teratogenicity studies were carried out on 195 mature female
Wistar rats (15/group) given thiamphenicol orally at dose levels of
40, 80 or 160 mg/kg bw per day from days 1 to 21 of pregnancy and 80
or 960 mg/kg bw per day from days 1 to 7, 7 to 14 or 14 to 21 of
gestation. Thiamphenicol did not induce any teratogenic effects in any
of the four studies carried out. When the treatment period was 1-21
days, a dose-related increase in resorptions was noted, and newborns
had a high mortality rate in the second and third week of life,
particularly in the 40 mg/kg bw per day group. In rats treated on days
1-7 of gestation, a non-dose-related increase in resorptions and an
increased mortality in newborns in the third week after birth were
observed. When the treatment period was from 7 to 14 days, complete
resorption of fetuses occurred at 160 mg/kg bw per day. The mean
number of newborns per litter was reduced at 80 mg/kg bw per day and
there was a high mortality of newborns in the first week. An increased
mortality among newborns of the group treated with 80 mg/kg bw per day
during days 14-21 of gestation was observed (Bonanomi & De Paoli,
1969).
When the inhibition of mitochondrial functions induced by
thiamphenicol was compared with the inhibition of overall embryonic
development, it appeared that mitochondrial respiration was the
rate-limiting step for the embryotoxic effects of thiamphenicol.
Because of the lack of specificity of these effects, prenatal
mortality rather than teratogenic effects was seen (Bass et al.,
1978).
2.2.5.2 Rabbits
A teratogenicity study was performed with 50 New Zealand white
rabbits administered thiamphenicol orally at dose levels of 5, 30, 60
or 80 mg/kg bw per day from the 8th to the 16th day of pregnancy. The
highest dose resulted in a complete resorption of implantation due to
the toxic effects on the mothers. Data obtained in all treated groups
showed moderate fetal toxicity with a dose-related increase in
abortion rate and resorption. No skeletal malformations were found in
fetuses (Bonanomi et al., 1974).
Thiamphenicol in 0.5% Methocel K15M was administered daily by
oral gavage to female rabbits (16/group) from day 6 to day 18 of
gestation at doses of 1.25, 2.5 or 5.0 mg/kg bw per day. Control
animals received the vehicle alone. The females were killed on
gestation day 29 and subjected to postmortem examination. All fetuses
were examined for external and internal abnormalities and skeletal
changes. No clinical signs attributable to treatment were observed.
Mild signs of maternal toxicity were observed in mid- and high-dose
animals, indicated by reduction in body weight changes during the
treatment period.
Necropsy findings in females on gestation day 29 were incidental,
with no relation to treatment. Litter parameters and sex ratios did
not show any significant difference between groups. Mean fetal weight
was decreased in the high-dose group. Two fetuses in the mid-dose
group and one in the control group were malformed, with cleft palate,
abnormally shaped head, extra digits and incomplete flexure of the
hind limbs. The number of small fetuses in the high-dose group was
higher than in the control group. The few anomalies seen during the
internal examination were not considered to be treatment-related.
Skeletal examination revealed no differences between high-dose fetuses
and controls. The authors concluded that thiamphenicol administered by
oral gavage at concentrations of 1.25, 2.5 and 5 mg/kg per day had no
effects on pregnancy or embryo-fetal development. However, the
Committee concluded that the NOEL for embryotoxicity under the
conditions of this experiment was 1.25 mg/kg per day (Sisti, 1994).
2.2.6 Special studies on genotoxicity
The results of genotoxicity assays on thiamphenicol are given in
Table 2.
Table 2. Genotoxicity assays on thiamphenicol
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0.5-50 Negative Pinasi
TA98, TA100, µg/plate2 et al., 1990a
TA1535, TA1537,
TA1538
Gene conversion Saccharomyces 2.8-140.3 mM3 Negative Marca &
and mitotic cerevisiae Bonanomi,
crossing over1 1979
Gene mutation Chinese hamster 50-5000 Negative Pinasi
assay1 V79 cells µg/ml4 et al., 1990b
Chromosomal Cultured human 700-3250 Negative Mosesso &
aberrations1 lymphocytes µg/ml5 Driedger, 1989
DNA repair Primary rat 500 and 1000 Negative Bichet, 1985
test hepatocytes µg/kg6
In vivo Mouse bone 2500 and 5000 Negative Pinasi
micronucleus marrow mg/kg7 et al., 1990c
assay
1 Both with and without rat liver S9 fraction
2 Methyl methanesulfonate and cyclophosphamide were used as positive controls
3 2-Nitrofluorene, 9-aminoacridine, sodium azide and 2-aminoanthracene were used
as positive controls
4 Ethyl ethanesulfonate and N-dimethylnitrosoamine were used as positive controls
5 Mitomycin-C and cyclophosphamide were used as positive controls
6 2-Aminofluorene was used as positive control
7 Cyclophosphamide was used as positive control
2.2.7 Special studies on immune responses
The effects on spontaneous nephritis in NZB × OUW hybrid mice
(32-39 males/group) were investigated in a study involving lifetime
administration of thiamphenicol in feed at dose levels of 25, 50 and
250 mg/kg bw per day. Body weight, urinary protein, limited
haematology, organ weights and histopathology investigations were
reported. The prolonged treatment with thiamphenicol at dose rates of
> 50 mg/kg reduced the severity of the spontaneous renal disease and
significantly extended lifespan, compared to untreated controls. The
immunosuppressive effect of thiamphenicol was demonstrated
histologically by a reduction in immune-complex deposition in the
glomeruli. No evidence of malignancy or premalignant signs was seen
(Simpson et al., 1979).
2.2.8 Special studies on microbiological effects
2.2.8.1 In vivo
In a study of thiamphenicol-induced changes in mouse intestinal
microflora, 100 female albino mice were divided into 10 subgroups, and
five of these groups were treated with thiamphenicol at concentrations
of 40 µg/kg diet for 35 days. Samples of intestinal content were taken
from the caecum for bacteriological analysis before the treatment and
after 7, 14, 28 and 35 days. Microorganisms were isolated by preparing
serial dilutions of intestinal content, and the most representative
bacteria of the mouse intestinal microflora were cultured on specific
media. Their sensitivity to thiamphenicol was assessed by calculating
the minimal inhibitory concentration (MIC) of the drug. The results
show that the mean counts of various microorganisms did not differ
significantly between control mice and those fed with thiamphenicol.
The numbers of the bacterial populations did vary at the different
sampling times, and in some cases the difference from pre-treatment
results was significant, but confidence limits were the same in
treated and control mice killed at the same time. The investigation
shows that there were no appreciable differences in the type or amount
of bacterial flora related to thiamphenicol administration.
Differences in the distribution of genera such as Diplococcus sp.
and Escherichia sp. were similar to those in controls. The
thiamphenicol MIC values for the numerous strains tested indicate that
addition to feed at concentrations corresponding to the proposed MRL
of 40 µg/kg feed does not select for drug-resistant strains and has no
effect on the qualitative or quantitative composition of the
intestinal microflora. The MIC remained unchanged throughout the 35
days of the study (Poli, 1994).
2.2.8.2 In vitro
In vitro antibacterial activity of thiamphenicol against 489
bacterial isolates from infected animals was determined by the agar
dilution method. In the case of mycoplasmas, however, MICs were
determined by the broth dilution method. Depending on bacterial
strains tested estimations were made under aerobic or anaerobic
conditions. The results are presented in Table 3 (Albini, 1989).
Data on the sensitivity of normal components of human intestinal
microflora are presented in Table 4 (Sutter & Finegold, 1976;
Schioppacassi, 1992).
Table 3. Antibacterial activity of thiamphenicol against 489 animal pathogens
Organisms Number of MIC (µg/ml) Range
isolates
MIC50 MIC90
Bacteroides spp. 11 2 16 1 - 128
Bordetella spp. 9 32 32 16 - 32
Campylobacter spp. 17 8 16 4 - 16
Clostridium spp. 37 2 4 0.25 - 16
Corynebacterium spp. 10 2 16 2 - 16
Escherichia coli 61 128 >128 16 - > 128
Haemophilus
pleuropneumoniae 7 0.5 1 0.5 - 1
Micrococcus spp. 6 0.5 0.5 0.5 - 8
Mycoplasma spp. 9 1 2 0.125 - 4
Pasteurella spp. 71 1 2 0.25 - 128
Salmonella spp. 34 32 32 8 - > 128
Staphylococcus spp. 94 8 32 4 - > 128
Streptococcus spp. 123 2 4 0.5 - > 128
2.3 Observations in humans
Reversible dose-related bone marrow suppression is seen after
thiamphenicol treatment and is attributed to its inhibitory effect on
mitochondrial protein synthesis (Nijhof & Kroon, 1974). Reversible
inhibition of myeloid and erythroid colony growth by thiamphenicol,
resulting from an inhibition of mitochondrial protein synthesis, is
consistent with the reversible bone marrow suppression induced by this
drug (Yunis & Gross, 1975).
A study of clinical reports on the use of thiamphenicol in 16 631
cases from 1968 to 1977 in Japan revealed that blood disorders
occurred in 41 (0.46%) out of 8848 patients receiving thiamphenicol
glycinate therapy and 28 (0.36%) out of 7783 patients receiving
thiamphenicol. The disorders were dose-dependent, occurring mainly in
erythrocytes, and disappeared spontaneously on discontinuation of the
drug (Tomoeda & Yamamoto, 1981).
The para-nitro group of chloramphenicol has been shown to have
a central role in the pathogenesis of aplastic anaemia, probably as a
result of its reduction to the highly toxic nitroso metabolite, which
is a potent inhibitor of DNA synthesis. Thiamphenicol does not show
this activity and the absence of the para-nitro group is therefore
advanced as evidence that thiamphenicol cannot induce aplastic anaemia
(Murray et al., 1983).
Thiamphenicol has been used extensively in human medicine
for over 25 years. Total human exposure to thiamphenicol up to
1987 has been estimated at 130-650 million exposures, assuming an
average course of therapy of 7.5-15 g (Personal communication from
Dr S. Biressi, Zambon Group SpA, Italy to Dr R.D. Agostino,
Farmaquest Co.; submitted to WHO by Zambon Group SpA, Italy).
Epidemiological studies have not established any causal
association between thiamphenicol treatment and irreversible aplastic
anaemia (TAP Pharmaceuticals Inc., 1987). Statistical analysis of
data obtained in these studies support the argument that the risk of
aplastic anaemia from exposure to thiamphenicol is similar to the
background risk of idiopathic aplastic anaemia (from 1 in 200 000 to 1
in 800 000) (Kelly & Kaufman, 1989).
Table 4. Antibacterial activity of thiamphenicol against 261 strains of anaerobic bacteria isolated from humans
(From: Sutter & Finegold, 1976)
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Bacteroides fragilis 42 5 21 71 100
Bacteroides 59 9 24 51 90 100
melaninogenicus
Other Bacteroides 21 19 24 57 76 86 91 95 100
and Selenomonas
Fusobacterium 8 100
nucleatum
Other Fusobacterium 12 42 92 100
Peptococcus and 17 35 71 100
Gaffkya
Peptostreptococcus 15 33 47 93 100
Table 4. (cont'd).
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Anaerobic and 6 50 83 100
microaerophilic
streptococci
Gram-negative cocci 7 43 86 100
Eubacterium 7 43 57 100
Arachnia propionica 2 50 100
Propionibacterium 4 50 75 100
Actinomyces 16 25 56 94 100
Lactobacillus 10 10 30 50 90 100
Clostridium 8 100
perfringens
Other Clostridium 27 4 22 63 78 96 100
3. COMMENTS
Studies on thiamphenicol available for evaluation included data
on pharmacokinetics, acute toxicity, short-term toxicity, reproductive
toxicity, developmental toxicology, genotoxicity, and limited
information on long-term toxicity. Studies on microbiological effects
of thiamphenicol and epidemiological data on humans were also
considered by the Committee. The Committee noted that many of the
studies were conducted utilizing protocols that would not meet
contemporary standards, and therefore the substance was evaluated
under the procedures developed for drugs with a long history of use
(Annex 1, reference 104).
The pharmacokinetic data showed that the drug is rapidly absorbed
when administered by oral or parenteral routes. After intravenous
administration in rats, the half-life was estimated to be 46 minutes.
The main route of excretion in humans and animals is in the urine;
approximately 60% of an oral dose of 30 mg/kg bw was excreted
unchanged in the urine over a 24-hour period.
Single oral doses of thiamphenicol were of low toxicity to mice
and rats (LD50 > 3000 mg/kg bw).
Short-term oral toxicity studies with thiamphenicol were
performed in rats, dogs and pigs, the results are described in the
following paragraphs.
In a 13-week study in rats at dose levels of 30, 45, 65 or
100 mg/kg bw per day, increased mortality was observed among animals
given 100 mg/kg bw per day. In a 6-month study in rats, where the
highest dose used was 120 mg/kg bw per day, increased mortality was
not reported. In both studies, decrease in body weight gain during
treatment occurred at doses of > 65 mg/kg bw per day. Dose-related
decreases in red blood cell parameters, differential and total white
blood cell counts, and clotting parameters were observed in the
13-week study, but the same effects were not reported in the 6-month
study. Testicular germinal epithelial cell depletion was seen at doses
above 45 mg/kg bw per day in the 13-week study, and a dose of 30 mg/kg
bw per day was considered to be the NOEL.
Dogs were given 40 or 80 mg thiamphenicol/kg bw per day for 7
weeks. At both dose levels decreases in body weight were observed, as
well as reversible decreases in haematocrit, haemoglobin concentration
and erythrocyte count. At 40 mg/kg bw per day, superficial erosion of
the gall bladder mucosa was observed. The higher dose level resulted
in haemorrhagic ulcers in the gall bladder, diffuse mucomembranous
enteritis and early thymic involution. Two dogs in the low-dose group
had testicular germinal epithelial cell depletion and multinucleated
cells in the seminiferous tubules.
When thiamphenicol was given to dogs at doses of 30, 60 or
120 mg/kg bw per day for 4 weeks, the body weights of the high-dose
animals were slightly lower than those of controls. In the mid- and
high-dose groups, increases in absolute and relative liver weights
were observed. Hepatocellular hypertrophy was present in the liver of
dogs given 60 or 120 mg/kg bw per day.
In a 6-month study dogs were given thiamphenicol at doses of 15,
30 or 60 mg/kg bw per day. The body weights of high-dose males during
the study were up to 18% lower than those of controls. The main
haematological findings were decreases in red blood cell parameters at
the highest dose level. Increases were noted in mean serum cholesterol
level and phospholipid concentrations in males (30 and 60 mg/kg bw per
day groups) and females (60 mg/kg bw per day group), and in the mean
serum glucose concentration of males (60 mg/kg bw per day group) and
females (30 and 60 mg/kg bw per day groups). The relative liver
weights at the mid- and high-dose levels were increased. Histopatho-
logical lesions related to treatment were seen in the thymus (early
involution), bone marrow (decreased cellularity), testes (focal and
diffuse tubular atrophy) and oesophagus (ulceration) of high-dose
animals. The NOEL was 15 mg/kg bw per day.
In a 4-week study, pigs were treated with 25, 50 or 100 mg
thiamphenicol/kg bw per day. In the highest-dose group, slight
reduction in body weight gain, as well as reductions in mean packed
cell volume, haemoglobin concentration and erythrocyte counts, were
observed, and histological examination showed vacuolation in renal
tubular epithelial cells and mild diffuse hepatocyte vacuolation. In
all treated groups treatment-related reduction in urine pH was
observed.
A summary report of a two-year carcinogenicity study in rats,
including a range-finding study, was available to the Committee. Rats
were given 125 or 250 mg/kg thiamphenicol in drinking-water (equal to
8 or 16 mg/kg bw per day for males and 10 or 19 mg/kg bw per day for
females) for 104 weeks. The highest-dose animals showed a decrease in
body weight gain, but there was no significant increase in the
incidence of tumours in treated groups compared to control animals.
In a long-term study in mice (32-39 males/group), which was
designed primarily to investigate the effects of thiamphenicol on
immune responses, thiamphenicol was administered orally at doses of
25, 50 or 250 mg/kg bw per day. No evidence of neoplastic or
preneoplastic changes was observed.
In a study to determine the effect of thiamphenicol on fertility
in rats, the drug was administered orally at doses of 120, 180 or
240 mg/kg bw per day for 2 or 3 months. Thirty male rats were used per
dose level (10 per treatment period). From each treatment-period
group, half of the animals were killed for histopathological
examination at the given time intervals, while the remaining males
were mated with untreated females. Reductions in the number of
germinal epithelial cells in testes of all treated animals were
observed. These changes were present up to 21 days after termination
of treatment, and full recovery was observed by 50 days. Histological
changes correlated with the fertility index. Litters from matings
between treated males and non-treated females were normal in number
and no physical abnormalities were reported.
Thiamphenicol was given orally to rats from day 15 of gestation
to day 21 post-partum at doses of 30, 60 or 120 mg/kg bw per day. In
the mid-and high-dose groups, there were higher post-implantation
losses and increased rates of perinatal mortality. Physical develop-
ment of pups was inhibited during the lactation period in a dose-
dependent manner. Sexual behaviour and fertility of F1 animals
were normal, and animals in the F2 generation showed no
abnormalities.
In four teratogenicity studies in rats, thiamphenicol was
administered orally at dosages of 40, 80 or 160 mg/kg bw per day from
days 1 to 21 of pregnancy or of 80 or 960 mg/kg bw per day over
critical days of gestation (either 1-7, 1-21, 7-14 or 14-21). No
teratogenic effects were observed. In all animals treated from days 1
to 21, a dose-related increase in resorption was noted and newborn
pups had an elevated mortality rate.
A teratogenicity study was performed in rabbits using oral doses
of 5, 30, 60 or 80 mg/kg bw per day from days 8 to 16 of gestation.
Complete resorption of embryos occurred at 80 mg/kg bw per day. In
other treated groups, moderate fetal toxicity and dose-related
increases in abortion rate and resorption were reported. No
malformations were found in fetuses.
In another teratogenicity study, rabbits received oral doses of
thiamphenicol at doses of 1.25, 2.5 or 5 mg/kg bw per day from days 6
to 18 of gestation. Mild maternal toxicity was observed in mid- and
high-dose animals in the form of depressed body weights during the
treatment. No effects were observed on embryo-fetal development. The
NOEL was 1.25 mg/kg bw per day.
Thiamphenicol gave negative results in five in vitro
genotoxicity tests and in an in vivo micronucleus assay using mouse
bone marrow.
The Committee considered data from human epidemiological studies
and concluded that there was no evidence that thiamphenicol can induce
aplastic anaemia, in contrast to the structurally related compound,
chloramphenicol.
The Committee considered data from several in vitro studies on
the minimum inhibitory concentration (MIC) of thiamphenicol for a wide
range of animal and human pathogens as well as genera representative
of the human gut flora. The modal MIC50 value (minimum inhibitory
concentration of thiamphenicol giving complete inhibition of growth of
50% of cultures) was 1.68 µg/ml for 261 bacterial strains isolated
from humans. The following species were found to be the most
sensitive: Bacteroides, Fusobacteria, Propionibacteria and
Actinomyces. The Committee also noted that 40 µg thiamphenicol/kg
food given to mice over 35 days did not alter the intestinal
microflora in this species.
The Committee calculated a microbiological ADI for thiamphenicol
using the following formula:
Upper limit of MIC50 (µg/g) × mass of colonic content (g)
microbiological =
ADI fraction of oral × safety × human body
dose available factor weight (kg)
1.68 × 220
=
0.4 × 1 × 60
= 15 µg/kg bw
In calculating a microbiological ADI the Committee took the
following factors into consideration:
* Concentration: 1.68 µg/ml was the modal MIC50 for micro-
biological effects on human intestinal microflora (the density
was assumed to be 1 g/ml).
* Availability: the Committee calculated the available portion of
thiamphenicol as follows:
100% ingested - 60% excreted via = 40% bioavailable
urine within in the intestinal
24 hours tract
1 - 0.6 = 0.4
* Safety factor: the Committee concluded that the data deriving
from the microbiological studies (substantial amount of MIC data
covering a variety of microorganisms and in vivo data from
animal studies) provided sufficient information on microbio-
logical effects of thiamphenicol. It therefore adopted a safety
factor of 1 in the calculation.
4. EVALUATION
Taking into account the available toxicological and antimicrobial
data and the ADI based on antimicrobial activity, the Committee
concluded that the toxicological data provided the most appropriate
end-point for the evaluation of thiamphenicol. The Committee
established a temporary ADI of 0-6 µg/kg bw for thiamphenicol, based
on the NOEL of 1.25 mg/kg bw per day for maternal toxicity in the
teratogenicity study in rabbits and a safety factor of 200. The ADI
was designated "temporary" because only a summary report of the
carcinogenicity study in rats was available. Detailed reports of the
carcinogenicity study and the range-finding study used to establish
dose levels in that study are required for evaluation in 1999
(see Annex 4).
5. REFERENCES
Albini, E. (1989). In vitro antibacterial activity of thiamphenicol
against bacterial strains recently isolated from animal infectious
diseases. Unpublished report from Microbiology Laboratory, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Azzolini, F., Gazzaniga, A., Lodola, E., & Natangelo, R. (1972).
Elimination of chloramphenicol and thiamphenicol in subjects with
cirrhosis of the liver. Int. J. Clin. Pharmacol., 6, 130-134.
Bass, R., Oerter, D., Krowke, K., & Spielman H. (1978). Embryonic
development and mitochondrial function. III. Inhibition of respiration
and ATP generation in rat embryos by thiamphenicol. Teratology,
18, 93-102.
Bichet, N. (1985). Etude de génotoxicité éventuelle sur les
hépatocytes de rat par mesure de réparation du DNA. Unpublished report
No. 01147F from Sanofi Recherche, Montpellier, France. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. (1978). Acute toxicity study in rats and mice with
thiamphenicol and thiamphenicol glycinate HCl. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. & De Paoli, A.M. (1969). Teratogenicity study of oral
thiamphenicol in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, Pacei, E., & De Paoli, A.M. (1974). Teratogenicity study
of oral thiamphenicol in the rabbit. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, De Paoli, A.M., & Gazzaniga, A. (1978). Toxicity study
after repeated oral administration of thiamphenicol in the dog.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Bonanomi, L., Aliverti, V., Ornaghi, F., Losa, M., & Motta, F. (1980).
Thiamphenicol perinatal and postnatal toxicity study in the rat.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Brunaud, M. (1965). Thiamphenicol glycinate hydrochloride. Long-term
toxicity in the rabbit by subcutaneous route. Unpublished report from
Clin-Byla Laboratories, Paris, France. Submitted to WHO by Zambon
Group SpA, Bresso, Milan, Italy.
Cameron, D.M., Crook, D., Brown G., Gopinath, C., Farmer, H., & Offer,
J.M. (1990). Z 2041: 4-Week toxicity study in pigs. Unpublished report
No. ZBN 12/891518 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A. (1967).
Fertility study in male rat treated with thiamphenicol by the oral
route. Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Della Bella, D., Ferrari, V., Marca, G., & Bonanomi, L. (1968a).
Chloramphenicol metabolism in the phenobarbital induced rat.
Comparison with thiamphenicol. Biochem. Pharmacol., 17, 2381-2390.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A.
(1968b). Toxicity study of thiamphenicol after repeated oral
administration in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Ferrari, V. & Della Bella, D. (1974). Comparison of chloramphenicol
and thiamphenicol metabolism. Postgrad. Med. J., 50, 17-22.
Gazzaniga, A. (1974). Thiamphenicol - pharmacokinetics and metabolism
in animals. Unpublished report from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, C.M. & Daly I.W. (1990). A four week oral toxicity study in the
dog via capsule administration with Z 2041. Unpublished report
No. 88-3391 from Bio/dynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Kelly, C.M. & Daly, I.W. (1991). A six month oral toxicity study in
the dog via capsule administration with Z 2041 with a two month
recovery period. Unpublished report No. 88-3392 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, J.P. & Kaufman, D.W. (1989). Ant-infective drug use in relation
to the risk of agranulocytosis and aplastic anemia: A report from the
international agranulocytosis and aplastic anemia study.
Arch. Intern. Med., 149, 1036-1040.
Laplassote, J. (1962). Recherches expérimentales sur le thiopénicol:
activité antibactérienne, concentrations humorales, élimination.
Comparaison avec le chloramphénicol. Therapie, 16, 104-108.
Marca, G. & Bonanomi, L. (1979). Gene conversion and mitotic
crossing-over in Saccharomyces cerevisiae 6117 with thiamphenicol
with and without metabolic activation. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Maekawa, A. (1996). Summary report on two-year rat carcinogenicity
study of thiamphenicol. Submitted by Sasaki Institute, Tokyo, Japan to
the Japanese Ministry of Health and Welfare.
Marubini, M., Motta, F., Cerioli, A., & Finn, J.P. (1991). Z 2041.
Thirteen week oral toxicity study in rats followed by a recovery
period of 8 weeks. Unpublished report No. 1219 from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Mosesso, P. & Driedger, A. (1989). Chromosome abberations in human
lymphocytes cultured in vivo. Unpublished report No. LSC-RTC
116028-M-02989 from Life Science Research Roma Toxicology Centre SpA,
Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Murray, T.R., Downey, K.M., & Yunis, A.A. (1983).
Chloramphenicol-mediated DNA damage and its possible role in the
inhibitory effects of chloramphenicol on DNA synthesis.
J. Lab. Clin. Med., 102, 926-932.
Nijhof, W. & Kroon, A.M. (1974). The interference of chloramphenicol
and thiamphenicol with the biogenesis of mitochondria in animal
tissues. A possible clue to the toxic action. Postgr. Med. J.,
50 (Suppl. 5), 53-59.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990a). Mutagenicity
evaluation of Z 2041 in the Ames Salmonella-microsome plate test.
Unpublished report No. 1231 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990b). Mutagenicity
evaluation of Z 2041 in Chinese hamster V79 test. Unpublished report
No. 1243 from Toxicology Department, Zambon Research, Bresso, Milan,
Italy. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990c). Genotoxicity
evaluation of Z 2041 in the "in vivo" mouse micronucleus assay.
Unpublished report No. 1233 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Poli, G. (1994). Qualitative and quantitative changes in the mouse
intestinal microflora induced by chronic thiamphenicol at maximum
residual level (MRL) dose. Unpublished report from Facolta di Medicina
Vetarinaria, Universita di Milano, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Redgrave, V.A., Cameron, D.M., Anderson, A., & Maxwell, J.G. (1991).
Blood concentrations of thiamphenicol in pigs following dietary
administration of Z 2041. Unpublished report No. ZBN 11/901351 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Roberts, N.L., Cameron, D.M., Redgrave, V.A., Crook, D., & Brown G.
(1989). Z 2041. Tolerance in pigs. Unpublished report No. ZBN 10/89188
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Zambon Group SpA, Bresso, Milan, Italy.
Schioppacassi, G. (1992). Activity of thiamphenicol on indigenous
microorganisms of gastrointestinal tract. Unpublished report
No. DBI 1/92 from Zambon Research, Bresso, Milan, Italy. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Simpson, L.O., Aarons, I., & Howie, J.B. (1979). Thiamphenicol and
lupus nephritis. The effects of long-term therapy on kidney function
and pathology: a pilot study. Br. J. Exp. Pathol., 60, 45-57.
Sisti, R. (1994). Z 2041. Oral embryotoxicity study in rabbits.
Unpublished report No. RTC 4195/T/128/94 from Research Toxicology
Centre SpA, Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Sutter, V.L & Finegold, S.M. (1976). Susceptibility of anaerobic
bacteria to 23 antimicrobial agents. Antimicrob. Agents Chemoter.,
10, 736-752.
TAP Pharmaceuticals Inc. (1987). Issues relating to the safety of
thiamphenicol with special attention to the matter of aplastic anemia.
Unpublished report from TAP Pharmaceuticals Inc., Corte Mader, Canada.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Tomoeda, M. & Yamamoto, K. (1981). The hematologic adverse reaction
experience with thiamphenicol in Japan. In: Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy, Raven Press, New York,
pp. 103-110.
Uesugi, T., Ikeda, M., Hori, R., Katayama, K., & Arita, T. (1974).
Metabolism of thiamphenicol and comparative studies of its urinary and
biliary excretion with chloramphenicol in various species.
Chem. Pharm. Bull., 22, 2714-2722.
Yunis, A.A. & Gross, M.A. (1975). Drug-induced inhibition of myeloid
colony growth: protective effect of colony stimulating factor.
J. Lab. Clin. Med., 86, 499-504.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
After i.v. administration in rats the half-life of thiamphenicol
was 46.3 minutes, compared to 21.5 minutes for chloramphenicol
(Ferrari & Della Bella, 1974).
Pretreatment of rats with phenobarbital increased thiamphenicol
half-life slightly from 46.3 to 55.2 minutes, whereas the
chloramphenicol half-life was reduced from 21.5 minutes to 9.3 minutes
(Della Bella et al., 1968a).
Following administration of 30 mg/kg to rats, thiamphenicol was
eliminated in the urine almost entirely in the unchanged form: 62%
following oral administration and 47% after i.m. administration (both
at 48 hours). No significant glucuro-conjugation products were found.
In the bile, 3.4% of the administered dose appeared unchanged and
10-12% appeared as conjugated products after 4 hours. Of the
administered dose, 36% was eliminated in faeces after 75 hours, almost
entirely as unchanged thiamphenicol. Distribution studies showed that
levels in the kidney and liver were higher than in plasma, while brain
concentrations were negligible (Gazzaniga, 1974).
2.1.1.2 Rabbits
The amount of metabolites recovered in urine and bile within 7
hours after i.v. administration was about 73% for thiamphenicol and
60% for chloramphenicol, and the percentage of glucuronide to total
amount recovered was 8% for thiamphenicol and 66% for chloramphenicol.
The recovery of thiamphenicol into the bile was only 1%, mostly in
unchanged form. About 3% of administered chloramphenicol was excreted
into the bile and two-thirds of this consisted of metabolites
(Uesugi et al., 1974).
2.1.1.3 Dogs
Following intraduodenal administration of 70 mg/kg thiamphenicol
or chloramphenicol, 30% of the thiamphenicol dose was found unchanged
in urine within 8 hours, whereas only 8.3% of chloramphenicol was
eliminated in the active form. After i.m. injection, the urinary
elimination of active antibiotics within 8 hours was 24.2% for
thiamphenicol but only 1.76% for chloramphenicol (Laplassote, 1962).
2.1.1.4 Pigs
To determine plasma and tissue concentrations of thiamphenicol in
pigs following dietary treatment, 16 male pigs, approximately 7 weeks
old, were fed thiamphenicol in the diet at a concentration of
900 mg/kg (equivalent to 30 mg/kg bw), twice daily for a period of 5
days. Three pigs were maintained as controls and fed basal diet only.
Venous blood samples were taken prior to treatment and at various
time-points during the study. The animals were killed at 4, 6, 8 and
10 days after treatment and samples of kidney, liver, muscle, fat and
lung taken. Concentrations of thiamphenicol in plasma were measured
following solvent extraction, using HPLC. On the second day of the
dosing period, scouring and swelling or redness of the anus/perineal
area were noted in all treated animals. The signs resolved within 4-10
days. The maximum mean plasma level of thiamphenicol (1.28 mg/litre)
was demonstrated 8 hours after the first dose. Mean levels were in the
range of 0.22-0.80 mg/litre during the dosing period and declined over
the withdrawal period to give concentrations below or close to the
limit of detection (0.01-0.08 mg/litre) from 4 hours to 5 days after
the end of treatment. The results of the analysis of tissue samples
were not reported (Redgrave et al., 1991).
2.1.1.5 Humans
After a 500 mg oral dose of each of thiamphenicol and
chloramphenicol, the plasma levels appear to be similar. A high level
of active thiamphenicol and a low level of chloramphenicol were found
in the urine (53.1% and 9.2%, respectively, after 24 hours). The
half-life of thiamphenicol is significantly increased in renal
insufficiency, but is almost unaffected by liver cirrhosis
(Azzolini et al., 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of thiamphenicol and thiamphenicol-glycinate
is given in Table 1.
The predominant clinical signs in the animal species tested were
sedation and piloerection after oral dosing, and dyspnoea, cyanosis,
motility disorders and respiratory arrest after parenteral
administration.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
The oral toxicity of thiamphenicol, when administered as an
aqueous suspension in 0.5% methocel for 13 week, has been investigated
in four groups of rats (Sprague-Dawley 30/sex/group). Thiamphenicol
was administered by oral gavage at dose levels of 30, 45, 65 or
100 mg/kg bw per day, while a fifth control group received 0.5%
methocel only. The first 15 animals of each group were killed on
completion of the treatment period, and the remaining 15 after a
recovery period of 8 weeks. Mortality during the treatment period was
elevated among animals of both sexes receiving 100 mg/kg bw per day,
and during the recovery period mortality was similar in all groups. At
levels of 65 and 100 mg/kg bw per day, pallor, hair loss, prostration,
hunched posture and flaccid musculature were seen. After the recovery
phase the incidence and the severity of symptoms were similar in all
groups including controls. Body weight stasis or loss was seen in
animals receiving 65 and 100 mg/kg bw per day, and after 8 weeks
recovery body weight was similar in all groups. Food intake was
reduced at dose levels of 45, 65 and 100 mg/kg bw per day, with
immediate improvement after cessation of treatment.
Table 1. Acute toxicity of thiamphenicol (TAP) and thiamphenicol-glycinate (TAP-G)
Species Sex Route LD50 (mg/kg bw) Reference
TAP TAP-G
Mouse M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1550 Bonanomi, 1978
M & F i.v. 450 Bonanomi, 1978
Rat M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1750 Bonanomi, 1978
M & F i.v. 470 Bonanomi, 1978
In all treated animals there were changes in erythrocyte
parameters, differential and total leukocyte counts and clotting
parameters, all of which were dose-related. After the recovery period
erythrocyte and leukocyte counts were still low in males treated with
65 and 100 mg/kg bw per day. Plasma levels of urea, triglycerides and
total protein were altered from week 7 in animals treated with
45 mg/kg bw per day and after the end of treatment also in males
receiving 30 mg/kg bw per day. Parameters associated with liver and
kidney function were affected at the two higher doses. After 8 weeks
full recovery was considered to have occurred. The weights of all the
main organs were reduced at dose levels of 65 and 100 mg/kg bw per
day, but after the recovery period only the testis weight was still
reduced. Postmortem examination revealed effects on the gastro-
intestinal tract and spleen in both sexes and in the liver, thymus and
testis of males at the highest dose level only. After 8 weeks only the
testis weights were still reduced. The erythroid/myeloid cell ratio
was increased in both sexes at doses of 65 or 100 mg/kg bw per day,
and after 8 weeks was still slightly higher than usual.
Treatment-related changes were seen in tissues with high cell
turnover rates; most of them recovered after a period of respite from
treatment. Animals in the groups treated with 30 and 45 mg/kg per day
showed no histopathological findings except hepatocytic reduced
basophilia (male, 45 mg/kg bw per day) and increased splenic extramed-
ullary haematopoiesis (female 45 mg/kg bw per day). Testicular
germinal epithelial deficit was present at doses above 45 mg/kg, and
caecal oedema and adnexal atrophy were present after the recovery
period at the highest dose level. The NOEL was 30 mg/kg bw per day
(Marubini et al., 1991).
In a 6-month toxicity study in the rat, thiamphenicol was
administered as an aqueous suspension in 2% gum arabic by stomach tube
6 days/week to 180 Wistar rats (30/sex/group) at dose levels of 0, 40
or 120 mg/kg bw per day. Body weight and food intake were recorded
twice weekly in the first 4 weeks of treatment, and thereafter only
once a week. At the end of weeks 4, 8, 16 and 24, ten animals from
each group were killed following collection of 2-hour urine sample.
Haematology, clinical chemistry assays and urinanalysis were performed
on all tested animals. Histological examinations were carried out on
the lungs, spermatozoa, blood and bone marrow smears and samples of
main organs. There was a decrease in food intake at a dose level of
120 mg/kg bw per day and a dose- and time-related decrease in body
weight gain in females. No effect was observed on erythropoiesis or on
hepatic or renal function. Urinanalysis showed the presence of albumin
and haemoglobin in the high-dose rats. No gross pathological
variations in organ weights were observed, but irritative changes in
the gastrointestinal mucosa and high incidence of monolateral
spontaneous hydronephrosis were seen both in treated and control
animals. Histopathological examination performed on control and
high-dose rats revealed a slight effect on the morphology of
spermatozoa in the high-dose group at the 8th, 16th and 24th week of
treatment (Della Bella et al., 1968b).
2.2.2.2 Rabbits
Thiamphenicol-glycinate was administered subcutaneously to groups
of rabbits ("Fauve de Bourgogne" seven/sex/group) at dose levels of
0, 25, 50 or 100 mg/kg bw per day, 6 days/week for 12 weeks. Animals
were weighed weekly and submitted to haematological (erythrocyte and
leukocyte counts with differentials) and biochemical (urea, reducing
sugars, chlorides) examinations before treatment, after 6 weeks and at
the end of the treatment period. After macroscopic examination of the
viscera, the various organs were examined histologically. During the
treatment two control animals and three in the high-dose group died
(no autopsies were carried out). A reduction in polymorphonuclear
neutrophils in male rabbits in all treated groups and a slight fall in
erythrocyte levels in high-dose females were observed. Anatomical and
pathological examination provided no evidence of damage attributable
to thiamphenicol-glycinate administration (Brunaud, 1965).
2.2.2.3 Dogs
In a 7-week study in beagle dogs (four/sex/group), thiamphenicol
was administered orally in gelatin capsules at dose levels of 0, 40 or
80 mg/kg bw per day. Two males and two female dogs were killed at the
end of the 7-week treatment, and the remaining animals were kept for
further 12 weeks without treatment before being killed. Behaviour and
body weights were recorded, haematology and clinical chemistry
examinations and urinanalysis were conducted on all animals pretest
and at various intervals during the study. Complete gross postmortem
examination, organ weighing and histopathological evaluation were
conducted on all animals. The animals in the two treated groups
developed diarrhoea soon after the beginning of treatment, which
spontaneously regressed in the low-dose group but persisted in the
high-dose group. At 80 mg/kg bw per day reduced food consumption with
loss of weight and muscular asthenia occurred, and vomiting was
observed in some cases. Four of these dogs were killed at the end of
the 4th week. Slight loss of weight was observed in two dogs in the
low-dose group.
Decreases in haematocrit, haemoglobin concentration and
erythrocyte count were seen in both treated groups, but did not appear
to be dose-related, and returned to normal on withdrawal of treatment.
Slight increase in proteinuria was observed in the last few weeks of
treatment. At 40 mg/kg bw per day superficial erosion of the gall
bladder mucosa and at 80 mg/kg bw per day haemorrhagic ulcers in the
gall bladder, diffuse muco-membranous enteritis and thymic involution
were seen in dogs killed at the end of treatment period. In dogs
killed after the 12-week recovery period no differences were observed
between treated and control animals. Histological examination after 7
weeks revealed in the high-dose group severe cholecystitis, chronic
sclerosing pancreatitis, enteritis, severe depletion of haematopoietic
marrow and lymphoid thymus depletion. Two dogs in the low-dose group
showed depletion of germinal epithelium in the testes and multinuc-
leated cells in seminiferous tubules, which were not seen in the high-
dose group. None of the changes observed at 7 weeks were detectable
in the animals kept for 12 weeks after cessation of treatment
(Bonanomi et al., 1978).
Thiamphenicol was administered orally in gelatin capsules to 24
beagle dogs (four/sex/group) at dose levels of 30, 60 or 120 mg/kg bw
per day for a period of 4 weeks. Physical observations, ophthalmo-
scopic examinations, and body weight and food consumption measurements
were performed before treatment and over selected intervals during the
treatment. Haematology, clinical chemistry and urinanalysis were
conducted on all animals pretest and at study termination. Complete
gross postmortem examinations, organ weight and histopathological
evaluation were conducted on all animals. The body weights of the
high-dose animals were slightly lower than those of controls at week 3
in both sexes, and at week 4 in males only. At 60 and 120 mg/kg bw per
day absolute and relative liver weights in male dogs were greater than
in controls, and relative liver weights were also increased in
females. Microscopically, hepatocellular hypertrophy was present in
the liver of mid- and high-dose animals, which correlated with the
increase in liver weights in these groups. No other parameter
evaluated showed evidence of adverse treatment-related effects. The
NOEL was 30 mg/kg bw per day (Kelly & Daly, 1990).
Thiamphenicol was administered orally in gelatin capsules to 56
beagle dogs (seven/sex/group) at dose levels of 15, 30 or 60 mg/kg bw
per day. After 6 months of treatment four animals/sex/group were
sacrificed, and the remaining three animals/sex/group were kept for a
2-month recovery period. Physical observations, ophthalmoscopic
examinations, body weight, food consumption, haematology and clinical
chemistry examinations and urinanalysis were conducted on all animals
pretest and on all surviving animals at selected intervals during the
treatment and recovery period. Complete gross postmortem examinations,
organ weight and histopathological evaluation were conducted on all
animals. One control male was found dead during the study. One male
and one female dog in the highest dose group were moribund and had to
be killed. Clinical signs prior to death included lethargy, poor food
consumption, emaciation, tremors and dehydration. Physical findings
related to thiamphenicol administration were tremors, lethargy,
irregular gait and excessive licking or chewing in the high-dose
group. Tremors were also present in the mid-dose animals. These signs
were seen during the last two months of the study and were not present
at the end of the recovery period. Body weights of the high-dose males
during the study were 4 to 18% lower than those of controls. Decreased
erythrocyte counts and mean haematocrit values were seen in high-dose
males at weeks 6 and 13 and at termination of the study, and in high-
and mid-dose females at week 13 and at termination of the study. After
the recovery period no differences were observed in the haematological
parameters between control and treated animals. No treatment-related
effects were seen in the bone marrow smear examinations. Mean serum
cholesterol and phospholipid levels of the mid-and high-dose males at
week 6 and 13 and at termination of the study were greater than
control values. The same parameters were elevated in high-dose females
at the end of the study. Mean serum glucose levels of males at
60 mg/kg bw per day and females at 30 and 60 mg/kg bw per day were
significantly increased. Mean fibrinogen values of high-dose females
were elevated at the end of the study. Relative liver weights were
increased at mid- and high-dose levels. Pathological lesions related
to treatment were seen in sections of the thymus (exacerbation of
involution), bone marrow (decreased cellularity), liver (centrilobular
necrosis and pigment deposition), testes (focal and diffuse tubular
atrophy) and oesophagus (ulceration) from high-dose animals of both
sexes, mostly occurring in animals killed in a moribund condition. No
alterations were noted in any of the tissues examined microscopically
from animals that were allowed to recover after treatment. The NOEL
was 15 mg/kg bw per day (Kelly & Daly, 1991).
2.2.2.4 Pigs
A study designed to determine tolerance in pigs to treatment with
thiamphenicol at three times the recommended dose for 5 days and at
the normal recommended dose for 15 days was conducted with 16 weaned
large white hybrid pigs (two pigs/sex/group). Thiamphenicol was
administered in the diet at dose levels of 30 or 90 mg/kg bw per day
for 5 days or 30 mg/kg bw per day for 15 days. The control group of
animals was fed basal diet only. Clinical signs, body weight and food
consumption were recorded. Blood, urine and faecal samples were
obtained before dosing and at various time-points during the study. No
significant treatment-related clinical abnormalities were noted. Body
weight changes and food consumption were within normal limits. No
consistent treatment-related differences in haematological and
biochemical parameters or in urinanalysis values were observed. The
authors concluded that treatment with thiamphenicol had no significant
adverse effects on general health, body weight, food consumption or
standard clinical pathology parameters (Roberts et al., 1989).
In a 4-week toxicity study, groups of pigs (Large White hybrid,
four/sex/group) were fed 25, 50 or 100 mg thiamphenicol/kg bw per day.
The control group of animals was fed basal diet only. Clinical signs,
body weight and food consumption were recorded. Blood, urine and
faecal samples were obtained before dosing and during week 4 for
clinical pathological investigations. At the end of the 4-week dosing
period, pigs were killed, and selected tissues were processed for
histological examination. In all groups treated with thiamphenicol,
swelling and erythema of the anus, vulva/testes and perineal area,
tail and hocks were observed on the second day of the dosing period.
These effects were consistent with scouring and irritancy, e.g., as a
result of disruption of normal gastrointestinal flora activity, and
disappeared within 1 to 13 days. All pigs remained in good health
thereafter. Slight reductions in body weight gain and food consumption
were noted at 50 and 100 mg/kg bw per day. At week 4 there was a
slight reduction in mean PCV, haemoglobin concentration and
erythrocyte counts in animals receiving 100 mg/kg bw per day and a
treatment-related reduction of urinary pH in all groups dosed with
thiamphenicol. At the end of the study, increases in liver and kidney
weights in pigs fed 50 and 100 mg/kg bw per day were observed. On
histological examination, treatment-related changes were found in the
highest dose group only: an increase in vacuolation and fat in renal
tubular epithelium and, in some animals, minimal diffuse hepatocyte
vacuolation and hepatocyte fat (Cameron et al., 1990).
2.2.3 Long-term toxicity/carcinogenicity study
2.2.3.1 Rats
As a range-finding study for the dose selection in a 2-year
carcinogenicity study, four groups of F-344 rats (10/sex/group)
were given drinking-water containing 0, 125, 250 or 500 mg/litre
thiamphenicol (equal to 9, 17 or 36 mg/kg bw per day for males and 12,
29 or 39 mg/kg bw per day for females) for 13 weeks. The examinations
at the end of the study covered clinical observations, water
consumption, body weight changes, haematological parameters, serum
biochemistry, organ weights and gross and microscopic appearance. In
haematological examinations anaemic changes were observed in males
treated with 250 mg/litre or more and high-dose females. Similar
changes, such as increased MCV and increased related counts, were
observed in males of the 125 mg/litre group and females of the 250 and
125 mg/litre groups. At autopsy, enlargement of the caecum was seen in
treated groups of both sexes. Histologically, the highest dose animals
showed decreased haematopoiesis of the bone marrow, decreased spermato-
genesis of the testis and sperm granulomas of the epididymis. Sperm
granulomas in the epididymis were also seen in some of the animals in
the 250 mg/litre group. Based on the results of this pilot study, a
2-year carcinogenicity study of thiamphenicol was performed in rats.
Three groups of F-344 rats (50/sex/group) were given drinking-water
containing 0, 125 or 250 mg thiamphenicol/litre (equal to 8 or 16
mg/kg bw per day for males and 9.7 or 19 mg/kg bw per day for females)
for 104 weeks. All surviving animals subjected to 4-week withdrawal of
the test chemical after the end of the treatment were killed for full
histopathological examinations. The high-dose animals showed decreased
body weight gain, but the incidence of rumours in treated groups was
not significantly higher than that of controls (Maekawa, 1996; summary
report only was available).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
In a fertility study on male Wistar rats, oral treatment with
thiamphenicol for 2 or 3 months (30 animals per dose level, 10 per
treatment time), at dose levels of 120, 180 or 240 mg/kg bw per day,
resulted in reduction in the number of tubular germinal cells, which
was more marked at the highest dose level. Ten animals of each group
were treated for 4 weeks, ten for 8 weeks and the last ten for 12
weeks. At the end of each test period 5 animals of each dose group
were killed and necropsied, while the remaining 5 rats were mated with
normal females. At 240 mg/kg bw per day extensive testicular
hypotrophy, together with severe depletion of the germinal epithelium
21 days after withdrawal of treatment, was seen. Histological changes
coincided with a reduction of the fertility index, which gradually
recovered within 50 days. Litters from matings between treated males
and normal females were normal in number and weight, and no morpho-
logical abnormalities were observed. The concentration ratio of
thiamphenicol between testes and plasma after administration of
240 mg/kg bw per day thiamphenicol was 1, indicating the absence of
accumulation in testes (Della Bella et al., 1967).
Groups of 21 Sprague-Dawley rats were given thiamphenicol orally
(30, 60 or 120 mg/kg bw per day daily) from day 15 of gestation to day
21 postpartum. In groups receiving 60 and 120 mg/kg bw per day a
higher post-implantation loss, slight weight reduction at birth and
increased rate of perinatal mortality were observed. No malformations
were observed. Development of pups was inhibited during the lactation
period with a consistent dose-dependent relationship. From day 30
postpartum a good recovery was observed in all groups. Sexual
behaviour and fertility of F1 animals were normal, and the F2
generation showed no signs of abnormal development (Bonanomi et
al., 1980).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Teratogenicity studies were carried out on 195 mature female
Wistar rats (15/group) given thiamphenicol orally at dose levels of
40, 80 or 160 mg/kg bw per day from days 1 to 21 of pregnancy and 80
or 960 mg/kg bw per day from days 1 to 7, 7 to 14 or 14 to 21 of
gestation. Thiamphenicol did not induce any teratogenic effects in any
of the four studies carried out. When the treatment period was 1-21
days, a dose-related increase in resorptions was noted, and newborns
had a high mortality rate in the second and third week of life,
particularly in the 40 mg/kg bw per day group. In rats treated on days
1-7 of gestation, a non-dose-related increase in resorptions and an
increased mortality in newborns in the third week after birth were
observed. When the treatment period was from 7 to 14 days, complete
resorption of fetuses occurred at 160 mg/kg bw per day. The mean
number of newborns per litter was reduced at 80 mg/kg bw per day and
there was a high mortality of newborns in the first week. An increased
mortality among newborns of the group treated with 80 mg/kg bw per day
during days 14-21 of gestation was observed (Bonanomi & De Paoli,
1969).
When the inhibition of mitochondrial functions induced by
thiamphenicol was compared with the inhibition of overall embryonic
development, it appeared that mitochondrial respiration was the
rate-limiting step for the embryotoxic effects of thiamphenicol.
Because of the lack of specificity of these effects, prenatal
mortality rather than teratogenic effects was seen (Bass et al.,
1978).
2.2.5.2 Rabbits
A teratogenicity study was performed with 50 New Zealand white
rabbits administered thiamphenicol orally at dose levels of 5, 30, 60
or 80 mg/kg bw per day from the 8th to the 16th day of pregnancy. The
highest dose resulted in a complete resorption of implantation due to
the toxic effects on the mothers. Data obtained in all treated groups
showed moderate fetal toxicity with a dose-related increase in
abortion rate and resorption. No skeletal malformations were found in
fetuses (Bonanomi et al., 1974).
Thiamphenicol in 0.5% Methocel K15M was administered daily by
oral gavage to female rabbits (16/group) from day 6 to day 18 of
gestation at doses of 1.25, 2.5 or 5.0 mg/kg bw per day. Control
animals received the vehicle alone. The females were killed on
gestation day 29 and subjected to postmortem examination. All fetuses
were examined for external and internal abnormalities and skeletal
changes. No clinical signs attributable to treatment were observed.
Mild signs of maternal toxicity were observed in mid- and high-dose
animals, indicated by reduction in body weight changes during the
treatment period.
Necropsy findings in females on gestation day 29 were incidental,
with no relation to treatment. Litter parameters and sex ratios did
not show any significant difference between groups. Mean fetal weight
was decreased in the high-dose group. Two fetuses in the mid-dose
group and one in the control group were malformed, with cleft palate,
abnormally shaped head, extra digits and incomplete flexure of the
hind limbs. The number of small fetuses in the high-dose group was
higher than in the control group. The few anomalies seen during the
internal examination were not considered to be treatment-related.
Skeletal examination revealed no differences between high-dose fetuses
and controls. The authors concluded that thiamphenicol administered by
oral gavage at concentrations of 1.25, 2.5 and 5 mg/kg per day had no
effects on pregnancy or embryo-fetal development. However, the
Committee concluded that the NOEL for embryotoxicity under the
conditions of this experiment was 1.25 mg/kg per day (Sisti, 1994).
2.2.6 Special studies on genotoxicity
The results of genotoxicity assays on thiamphenicol are given in
Table 2.
Table 2. Genotoxicity assays on thiamphenicol
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0.5-50 Negative Pinasi
TA98, TA100, µg/plate2 et al., 1990a
TA1535, TA1537,
TA1538
Gene conversion Saccharomyces 2.8-140.3 mM3 Negative Marca &
and mitotic cerevisiae Bonanomi,
crossing over1 1979
Gene mutation Chinese hamster 50-5000 Negative Pinasi
assay1 V79 cells µg/ml4 et al., 1990b
Chromosomal Cultured human 700-3250 Negative Mosesso &
aberrations1 lymphocytes µg/ml5 Driedger, 1989
DNA repair Primary rat 500 and 1000 Negative Bichet, 1985
test hepatocytes µg/kg6
In vivo Mouse bone 2500 and 5000 Negative Pinasi
micronucleus marrow mg/kg7 et al., 1990c
assay
1 Both with and without rat liver S9 fraction
2 Methyl methanesulfonate and cyclophosphamide were used as positive controls
3 2-Nitrofluorene, 9-aminoacridine, sodium azide and 2-aminoanthracene were used
as positive controls
4 Ethyl ethanesulfonate and N-dimethylnitrosoamine were used as positive controls
5 Mitomycin-C and cyclophosphamide were used as positive controls
6 2-Aminofluorene was used as positive control
7 Cyclophosphamide was used as positive control
2.2.7 Special studies on immune responses
The effects on spontaneous nephritis in NZB × OUW hybrid mice
(32-39 males/group) were investigated in a study involving lifetime
administration of thiamphenicol in feed at dose levels of 25, 50 and
250 mg/kg bw per day. Body weight, urinary protein, limited
haematology, organ weights and histopathology investigations were
reported. The prolonged treatment with thiamphenicol at dose rates of
> 50 mg/kg reduced the severity of the spontaneous renal disease and
significantly extended lifespan, compared to untreated controls. The
immunosuppressive effect of thiamphenicol was demonstrated
histologically by a reduction in immune-complex deposition in the
glomeruli. No evidence of malignancy or premalignant signs was seen
(Simpson et al., 1979).
2.2.8 Special studies on microbiological effects
2.2.8.1 In vivo
In a study of thiamphenicol-induced changes in mouse intestinal
microflora, 100 female albino mice were divided into 10 subgroups, and
five of these groups were treated with thiamphenicol at concentrations
of 40 µg/kg diet for 35 days. Samples of intestinal content were taken
from the caecum for bacteriological analysis before the treatment and
after 7, 14, 28 and 35 days. Microorganisms were isolated by preparing
serial dilutions of intestinal content, and the most representative
bacteria of the mouse intestinal microflora were cultured on specific
media. Their sensitivity to thiamphenicol was assessed by calculating
the minimal inhibitory concentration (MIC) of the drug. The results
show that the mean counts of various microorganisms did not differ
significantly between control mice and those fed with thiamphenicol.
The numbers of the bacterial populations did vary at the different
sampling times, and in some cases the difference from pre-treatment
results was significant, but confidence limits were the same in
treated and control mice killed at the same time. The investigation
shows that there were no appreciable differences in the type or amount
of bacterial flora related to thiamphenicol administration.
Differences in the distribution of genera such as Diplococcus sp.
and Escherichia sp. were similar to those in controls. The
thiamphenicol MIC values for the numerous strains tested indicate that
addition to feed at concentrations corresponding to the proposed MRL
of 40 µg/kg feed does not select for drug-resistant strains and has no
effect on the qualitative or quantitative composition of the
intestinal microflora. The MIC remained unchanged throughout the 35
days of the study (Poli, 1994).
2.2.8.2 In vitro
In vitro antibacterial activity of thiamphenicol against 489
bacterial isolates from infected animals was determined by the agar
dilution method. In the case of mycoplasmas, however, MICs were
determined by the broth dilution method. Depending on bacterial
strains tested estimations were made under aerobic or anaerobic
conditions. The results are presented in Table 3 (Albini, 1989).
Data on the sensitivity of normal components of human intestinal
microflora are presented in Table 4 (Sutter & Finegold, 1976;
Schioppacassi, 1992).
Table 3. Antibacterial activity of thiamphenicol against 489 animal pathogens
Organisms Number of MIC (µg/ml) Range
isolates
MIC50 MIC90
Bacteroides spp. 11 2 16 1 - 128
Bordetella spp. 9 32 32 16 - 32
Campylobacter spp. 17 8 16 4 - 16
Clostridium spp. 37 2 4 0.25 - 16
Corynebacterium spp. 10 2 16 2 - 16
Escherichia coli 61 128 >128 16 - > 128
Haemophilus
pleuropneumoniae 7 0.5 1 0.5 - 1
Micrococcus spp. 6 0.5 0.5 0.5 - 8
Mycoplasma spp. 9 1 2 0.125 - 4
Pasteurella spp. 71 1 2 0.25 - 128
Salmonella spp. 34 32 32 8 - > 128
Staphylococcus spp. 94 8 32 4 - > 128
Streptococcus spp. 123 2 4 0.5 - > 128
2.3 Observations in humans
Reversible dose-related bone marrow suppression is seen after
thiamphenicol treatment and is attributed to its inhibitory effect on
mitochondrial protein synthesis (Nijhof & Kroon, 1974). Reversible
inhibition of myeloid and erythroid colony growth by thiamphenicol,
resulting from an inhibition of mitochondrial protein synthesis, is
consistent with the reversible bone marrow suppression induced by this
drug (Yunis & Gross, 1975).
A study of clinical reports on the use of thiamphenicol in 16 631
cases from 1968 to 1977 in Japan revealed that blood disorders
occurred in 41 (0.46%) out of 8848 patients receiving thiamphenicol
glycinate therapy and 28 (0.36%) out of 7783 patients receiving
thiamphenicol. The disorders were dose-dependent, occurring mainly in
erythrocytes, and disappeared spontaneously on discontinuation of the
drug (Tomoeda & Yamamoto, 1981).
The para-nitro group of chloramphenicol has been shown to have
a central role in the pathogenesis of aplastic anaemia, probably as a
result of its reduction to the highly toxic nitroso metabolite, which
is a potent inhibitor of DNA synthesis. Thiamphenicol does not show
this activity and the absence of the para-nitro group is therefore
advanced as evidence that thiamphenicol cannot induce aplastic anaemia
(Murray et al., 1983).
Thiamphenicol has been used extensively in human medicine
for over 25 years. Total human exposure to thiamphenicol up to
1987 has been estimated at 130-650 million exposures, assuming an
average course of therapy of 7.5-15 g (Personal communication from
Dr S. Biressi, Zambon Group SpA, Italy to Dr R.D. Agostino,
Farmaquest Co.; submitted to WHO by Zambon Group SpA, Italy).
Epidemiological studies have not established any causal
association between thiamphenicol treatment and irreversible aplastic
anaemia (TAP Pharmaceuticals Inc., 1987). Statistical analysis of
data obtained in these studies support the argument that the risk of
aplastic anaemia from exposure to thiamphenicol is similar to the
background risk of idiopathic aplastic anaemia (from 1 in 200 000 to 1
in 800 000) (Kelly & Kaufman, 1989).
Table 4. Antibacterial activity of thiamphenicol against 261 strains of anaerobic bacteria isolated from humans
(From: Sutter & Finegold, 1976)
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Bacteroides fragilis 42 5 21 71 100
Bacteroides 59 9 24 51 90 100
melaninogenicus
Other Bacteroides 21 19 24 57 76 86 91 95 100
and Selenomonas
Fusobacterium 8 100
nucleatum
Other Fusobacterium 12 42 92 100
Peptococcus and 17 35 71 100
Gaffkya
Peptostreptococcus 15 33 47 93 100
Table 4. (cont'd).
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Anaerobic and 6 50 83 100
microaerophilic
streptococci
Gram-negative cocci 7 43 86 100
Eubacterium 7 43 57 100
Arachnia propionica 2 50 100
Propionibacterium 4 50 75 100
Actinomyces 16 25 56 94 100
Lactobacillus 10 10 30 50 90 100
Clostridium 8 100
perfringens
Other Clostridium 27 4 22 63 78 96 100
3. COMMENTS
Studies on thiamphenicol available for evaluation included data
on pharmacokinetics, acute toxicity, short-term toxicity, reproductive
toxicity, developmental toxicology, genotoxicity, and limited
information on long-term toxicity. Studies on microbiological effects
of thiamphenicol and epidemiological data on humans were also
considered by the Committee. The Committee noted that many of the
studies were conducted utilizing protocols that would not meet
contemporary standards, and therefore the substance was evaluated
under the procedures developed for drugs with a long history of use
(Annex 1, reference 104).
The pharmacokinetic data showed that the drug is rapidly absorbed
when administered by oral or parenteral routes. After intravenous
administration in rats, the half-life was estimated to be 46 minutes.
The main route of excretion in humans and animals is in the urine;
approximately 60% of an oral dose of 30 mg/kg bw was excreted
unchanged in the urine over a 24-hour period.
Single oral doses of thiamphenicol were of low toxicity to mice
and rats (LD50 > 3000 mg/kg bw).
Short-term oral toxicity studies with thiamphenicol were
performed in rats, dogs and pigs, the results are described in the
following paragraphs.
In a 13-week study in rats at dose levels of 30, 45, 65 or
100 mg/kg bw per day, increased mortality was observed among animals
given 100 mg/kg bw per day. In a 6-month study in rats, where the
highest dose used was 120 mg/kg bw per day, increased mortality was
not reported. In both studies, decrease in body weight gain during
treatment occurred at doses of > 65 mg/kg bw per day. Dose-related
decreases in red blood cell parameters, differential and total white
blood cell counts, and clotting parameters were observed in the
13-week study, but the same effects were not reported in the 6-month
study. Testicular germinal epithelial cell depletion was seen at doses
above 45 mg/kg bw per day in the 13-week study, and a dose of 30 mg/kg
bw per day was considered to be the NOEL.
Dogs were given 40 or 80 mg thiamphenicol/kg bw per day for 7
weeks. At both dose levels decreases in body weight were observed, as
well as reversible decreases in haematocrit, haemoglobin concentration
and erythrocyte count. At 40 mg/kg bw per day, superficial erosion of
the gall bladder mucosa was observed. The higher dose level resulted
in haemorrhagic ulcers in the gall bladder, diffuse mucomembranous
enteritis and early thymic involution. Two dogs in the low-dose group
had testicular germinal epithelial cell depletion and multinucleated
cells in the seminiferous tubules.
When thiamphenicol was given to dogs at doses of 30, 60 or
120 mg/kg bw per day for 4 weeks, the body weights of the high-dose
animals were slightly lower than those of controls. In the mid- and
high-dose groups, increases in absolute and relative liver weights
were observed. Hepatocellular hypertrophy was present in the liver of
dogs given 60 or 120 mg/kg bw per day.
In a 6-month study dogs were given thiamphenicol at doses of 15,
30 or 60 mg/kg bw per day. The body weights of high-dose males during
the study were up to 18% lower than those of controls. The main
haematological findings were decreases in red blood cell parameters at
the highest dose level. Increases were noted in mean serum cholesterol
level and phospholipid concentrations in males (30 and 60 mg/kg bw per
day groups) and females (60 mg/kg bw per day group), and in the mean
serum glucose concentration of males (60 mg/kg bw per day group) and
females (30 and 60 mg/kg bw per day groups). The relative liver
weights at the mid- and high-dose levels were increased. Histopatho-
logical lesions related to treatment were seen in the thymus (early
involution), bone marrow (decreased cellularity), testes (focal and
diffuse tubular atrophy) and oesophagus (ulceration) of high-dose
animals. The NOEL was 15 mg/kg bw per day.
In a 4-week study, pigs were treated with 25, 50 or 100 mg
thiamphenicol/kg bw per day. In the highest-dose group, slight
reduction in body weight gain, as well as reductions in mean packed
cell volume, haemoglobin concentration and erythrocyte counts, were
observed, and histological examination showed vacuolation in renal
tubular epithelial cells and mild diffuse hepatocyte vacuolation. In
all treated groups treatment-related reduction in urine pH was
observed.
A summary report of a two-year carcinogenicity study in rats,
including a range-finding study, was available to the Committee. Rats
were given 125 or 250 mg/kg thiamphenicol in drinking-water (equal to
8 or 16 mg/kg bw per day for males and 10 or 19 mg/kg bw per day for
females) for 104 weeks. The highest-dose animals showed a decrease in
body weight gain, but there was no significant increase in the
incidence of tumours in treated groups compared to control animals.
In a long-term study in mice (32-39 males/group), which was
designed primarily to investigate the effects of thiamphenicol on
immune responses, thiamphenicol was administered orally at doses of
25, 50 or 250 mg/kg bw per day. No evidence of neoplastic or
preneoplastic changes was observed.
In a study to determine the effect of thiamphenicol on fertility
in rats, the drug was administered orally at doses of 120, 180 or
240 mg/kg bw per day for 2 or 3 months. Thirty male rats were used per
dose level (10 per treatment period). From each treatment-period
group, half of the animals were killed for histopathological
examination at the given time intervals, while the remaining males
were mated with untreated females. Reductions in the number of
germinal epithelial cells in testes of all treated animals were
observed. These changes were present up to 21 days after termination
of treatment, and full recovery was observed by 50 days. Histological
changes correlated with the fertility index. Litters from matings
between treated males and non-treated females were normal in number
and no physical abnormalities were reported.
Thiamphenicol was given orally to rats from day 15 of gestation
to day 21 post-partum at doses of 30, 60 or 120 mg/kg bw per day. In
the mid-and high-dose groups, there were higher post-implantation
losses and increased rates of perinatal mortality. Physical develop-
ment of pups was inhibited during the lactation period in a dose-
dependent manner. Sexual behaviour and fertility of F1 animals
were normal, and animals in the F2 generation showed no
abnormalities.
In four teratogenicity studies in rats, thiamphenicol was
administered orally at dosages of 40, 80 or 160 mg/kg bw per day from
days 1 to 21 of pregnancy or of 80 or 960 mg/kg bw per day over
critical days of gestation (either 1-7, 1-21, 7-14 or 14-21). No
teratogenic effects were observed. In all animals treated from days 1
to 21, a dose-related increase in resorption was noted and newborn
pups had an elevated mortality rate.
A teratogenicity study was performed in rabbits using oral doses
of 5, 30, 60 or 80 mg/kg bw per day from days 8 to 16 of gestation.
Complete resorption of embryos occurred at 80 mg/kg bw per day. In
other treated groups, moderate fetal toxicity and dose-related
increases in abortion rate and resorption were reported. No
malformations were found in fetuses.
In another teratogenicity study, rabbits received oral doses of
thiamphenicol at doses of 1.25, 2.5 or 5 mg/kg bw per day from days 6
to 18 of gestation. Mild maternal toxicity was observed in mid- and
high-dose animals in the form of depressed body weights during the
treatment. No effects were observed on embryo-fetal development. The
NOEL was 1.25 mg/kg bw per day.
Thiamphenicol gave negative results in five in vitro
genotoxicity tests and in an in vivo micronucleus assay using mouse
bone marrow.
The Committee considered data from human epidemiological studies
and concluded that there was no evidence that thiamphenicol can induce
aplastic anaemia, in contrast to the structurally related compound,
chloramphenicol.
The Committee considered data from several in vitro studies on
the minimum inhibitory concentration (MIC) of thiamphenicol for a wide
range of animal and human pathogens as well as genera representative
of the human gut flora. The modal MIC50 value (minimum inhibitory
concentration of thiamphenicol giving complete inhibition of growth of
50% of cultures) was 1.68 µg/ml for 261 bacterial strains isolated
from humans. The following species were found to be the most
sensitive: Bacteroides, Fusobacteria, Propionibacteria and
Actinomyces. The Committee also noted that 40 µg thiamphenicol/kg
food given to mice over 35 days did not alter the intestinal
microflora in this species.
The Committee calculated a microbiological ADI for thiamphenicol
using the following formula:
Upper limit of MIC50 (µg/g) × mass of colonic content (g)
microbiological =
ADI fraction of oral × safety × human body
dose available factor weight (kg)
1.68 × 220
=
0.4 × 1 × 60
= 15 µg/kg bw
In calculating a microbiological ADI the Committee took the
following factors into consideration:
* Concentration: 1.68 µg/ml was the modal MIC50 for micro-
biological effects on human intestinal microflora (the density
was assumed to be 1 g/ml).
* Availability: the Committee calculated the available portion of
thiamphenicol as follows:
100% ingested - 60% excreted via = 40% bioavailable
urine within in the intestinal
24 hours tract
1 - 0.6 = 0.4
* Safety factor: the Committee concluded that the data deriving
from the microbiological studies (substantial amount of MIC data
covering a variety of microorganisms and in vivo data from
animal studies) provided sufficient information on microbio-
logical effects of thiamphenicol. It therefore adopted a safety
factor of 1 in the calculation.
4. EVALUATION
Taking into account the available toxicological and antimicrobial
data and the ADI based on antimicrobial activity, the Committee
concluded that the toxicological data provided the most appropriate
end-point for the evaluation of thiamphenicol. The Committee
established a temporary ADI of 0-6 µg/kg bw for thiamphenicol, based
on the NOEL of 1.25 mg/kg bw per day for maternal toxicity in the
teratogenicity study in rabbits and a safety factor of 200. The ADI
was designated "temporary" because only a summary report of the
carcinogenicity study in rats was available. Detailed reports of the
carcinogenicity study and the range-finding study used to establish
dose levels in that study are required for evaluation in 1999
(see Annex 4).
5. REFERENCES
Albini, E. (1989). In vitro antibacterial activity of thiamphenicol
against bacterial strains recently isolated from animal infectious
diseases. Unpublished report from Microbiology Laboratory, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Azzolini, F., Gazzaniga, A., Lodola, E., & Natangelo, R. (1972).
Elimination of chloramphenicol and thiamphenicol in subjects with
cirrhosis of the liver. Int. J. Clin. Pharmacol., 6, 130-134.
Bass, R., Oerter, D., Krowke, K., & Spielman H. (1978). Embryonic
development and mitochondrial function. III. Inhibition of respiration
and ATP generation in rat embryos by thiamphenicol. Teratology,
18, 93-102.
Bichet, N. (1985). Etude de génotoxicité éventuelle sur les
hépatocytes de rat par mesure de réparation du DNA. Unpublished report
No. 01147F from Sanofi Recherche, Montpellier, France. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. (1978). Acute toxicity study in rats and mice with
thiamphenicol and thiamphenicol glycinate HCl. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. & De Paoli, A.M. (1969). Teratogenicity study of oral
thiamphenicol in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, Pacei, E., & De Paoli, A.M. (1974). Teratogenicity study
of oral thiamphenicol in the rabbit. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, De Paoli, A.M., & Gazzaniga, A. (1978). Toxicity study
after repeated oral administration of thiamphenicol in the dog.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Bonanomi, L., Aliverti, V., Ornaghi, F., Losa, M., & Motta, F. (1980).
Thiamphenicol perinatal and postnatal toxicity study in the rat.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Brunaud, M. (1965). Thiamphenicol glycinate hydrochloride. Long-term
toxicity in the rabbit by subcutaneous route. Unpublished report from
Clin-Byla Laboratories, Paris, France. Submitted to WHO by Zambon
Group SpA, Bresso, Milan, Italy.
Cameron, D.M., Crook, D., Brown G., Gopinath, C., Farmer, H., & Offer,
J.M. (1990). Z 2041: 4-Week toxicity study in pigs. Unpublished report
No. ZBN 12/891518 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A. (1967).
Fertility study in male rat treated with thiamphenicol by the oral
route. Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Della Bella, D., Ferrari, V., Marca, G., & Bonanomi, L. (1968a).
Chloramphenicol metabolism in the phenobarbital induced rat.
Comparison with thiamphenicol. Biochem. Pharmacol., 17, 2381-2390.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A.
(1968b). Toxicity study of thiamphenicol after repeated oral
administration in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Ferrari, V. & Della Bella, D. (1974). Comparison of chloramphenicol
and thiamphenicol metabolism. Postgrad. Med. J., 50, 17-22.
Gazzaniga, A. (1974). Thiamphenicol - pharmacokinetics and metabolism
in animals. Unpublished report from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, C.M. & Daly I.W. (1990). A four week oral toxicity study in the
dog via capsule administration with Z 2041. Unpublished report
No. 88-3391 from Bio/dynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Kelly, C.M. & Daly, I.W. (1991). A six month oral toxicity study in
the dog via capsule administration with Z 2041 with a two month
recovery period. Unpublished report No. 88-3392 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, J.P. & Kaufman, D.W. (1989). Ant-infective drug use in relation
to the risk of agranulocytosis and aplastic anemia: A report from the
international agranulocytosis and aplastic anemia study.
Arch. Intern. Med., 149, 1036-1040.
Laplassote, J. (1962). Recherches expérimentales sur le thiopénicol:
activité antibactérienne, concentrations humorales, élimination.
Comparaison avec le chloramphénicol. Therapie, 16, 104-108.
Marca, G. & Bonanomi, L. (1979). Gene conversion and mitotic
crossing-over in Saccharomyces cerevisiae 6117 with thiamphenicol
with and without metabolic activation. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Maekawa, A. (1996). Summary report on two-year rat carcinogenicity
study of thiamphenicol. Submitted by Sasaki Institute, Tokyo, Japan to
the Japanese Ministry of Health and Welfare.
Marubini, M., Motta, F., Cerioli, A., & Finn, J.P. (1991). Z 2041.
Thirteen week oral toxicity study in rats followed by a recovery
period of 8 weeks. Unpublished report No. 1219 from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Mosesso, P. & Driedger, A. (1989). Chromosome abberations in human
lymphocytes cultured in vivo. Unpublished report No. LSC-RTC
116028-M-02989 from Life Science Research Roma Toxicology Centre SpA,
Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Murray, T.R., Downey, K.M., & Yunis, A.A. (1983).
Chloramphenicol-mediated DNA damage and its possible role in the
inhibitory effects of chloramphenicol on DNA synthesis.
J. Lab. Clin. Med., 102, 926-932.
Nijhof, W. & Kroon, A.M. (1974). The interference of chloramphenicol
and thiamphenicol with the biogenesis of mitochondria in animal
tissues. A possible clue to the toxic action. Postgr. Med. J.,
50 (Suppl. 5), 53-59.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990a). Mutagenicity
evaluation of Z 2041 in the Ames Salmonella-microsome plate test.
Unpublished report No. 1231 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990b). Mutagenicity
evaluation of Z 2041 in Chinese hamster V79 test. Unpublished report
No. 1243 from Toxicology Department, Zambon Research, Bresso, Milan,
Italy. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990c). Genotoxicity
evaluation of Z 2041 in the "in vivo" mouse micronucleus assay.
Unpublished report No. 1233 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Poli, G. (1994). Qualitative and quantitative changes in the mouse
intestinal microflora induced by chronic thiamphenicol at maximum
residual level (MRL) dose. Unpublished report from Facolta di Medicina
Vetarinaria, Universita di Milano, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Redgrave, V.A., Cameron, D.M., Anderson, A., & Maxwell, J.G. (1991).
Blood concentrations of thiamphenicol in pigs following dietary
administration of Z 2041. Unpublished report No. ZBN 11/901351 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Roberts, N.L., Cameron, D.M., Redgrave, V.A., Crook, D., & Brown G.
(1989). Z 2041. Tolerance in pigs. Unpublished report No. ZBN 10/89188
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Zambon Group SpA, Bresso, Milan, Italy.
Schioppacassi, G. (1992). Activity of thiamphenicol on indigenous
microorganisms of gastrointestinal tract. Unpublished report
No. DBI 1/92 from Zambon Research, Bresso, Milan, Italy. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Simpson, L.O., Aarons, I., & Howie, J.B. (1979). Thiamphenicol and
lupus nephritis. The effects of long-term therapy on kidney function
and pathology: a pilot study. Br. J. Exp. Pathol., 60, 45-57.
Sisti, R. (1994). Z 2041. Oral embryotoxicity study in rabbits.
Unpublished report No. RTC 4195/T/128/94 from Research Toxicology
Centre SpA, Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Sutter, V.L & Finegold, S.M. (1976). Susceptibility of anaerobic
bacteria to 23 antimicrobial agents. Antimicrob. Agents Chemoter.,
10, 736-752.
TAP Pharmaceuticals Inc. (1987). Issues relating to the safety of
thiamphenicol with special attention to the matter of aplastic anemia.
Unpublished report from TAP Pharmaceuticals Inc., Corte Mader, Canada.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Tomoeda, M. & Yamamoto, K. (1981). The hematologic adverse reaction
experience with thiamphenicol in Japan. In: Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy, Raven Press, New York,
pp. 103-110.
Uesugi, T., Ikeda, M., Hori, R., Katayama, K., & Arita, T. (1974).
Metabolism of thiamphenicol and comparative studies of its urinary and
biliary excretion with chloramphenicol in various species.
Chem. Pharm. Bull., 22, 2714-2722.
Yunis, A.A. & Gross, M.A. (1975). Drug-induced inhibition of myeloid
colony growth: protective effect of colony stimulating factor.
J. Lab. Clin. Med., 86, 499-504.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
After i.v. administration in rats the half-life of thiamphenicol
was 46.3 minutes, compared to 21.5 minutes for chloramphenicol
(Ferrari & Della Bella, 1974).
Pretreatment of rats with phenobarbital increased thiamphenicol
half-life slightly from 46.3 to 55.2 minutes, whereas the
chloramphenicol half-life was reduced from 21.5 minutes to 9.3 minutes
(Della Bella et al., 1968a).
Following administration of 30 mg/kg to rats, thiamphenicol was
eliminated in the urine almost entirely in the unchanged form: 62%
following oral administration and 47% after i.m. administration (both
at 48 hours). No significant glucuro-conjugation products were found.
In the bile, 3.4% of the administered dose appeared unchanged and
10-12% appeared as conjugated products after 4 hours. Of the
administered dose, 36% was eliminated in faeces after 75 hours, almost
entirely as unchanged thiamphenicol. Distribution studies showed that
levels in the kidney and liver were higher than in plasma, while brain
concentrations were negligible (Gazzaniga, 1974).
2.1.1.2 Rabbits
The amount of metabolites recovered in urine and bile within 7
hours after i.v. administration was about 73% for thiamphenicol and
60% for chloramphenicol, and the percentage of glucuronide to total
amount recovered was 8% for thiamphenicol and 66% for chloramphenicol.
The recovery of thiamphenicol into the bile was only 1%, mostly in
unchanged form. About 3% of administered chloramphenicol was excreted
into the bile and two-thirds of this consisted of metabolites
(Uesugi et al., 1974).
2.1.1.3 Dogs
Following intraduodenal administration of 70 mg/kg thiamphenicol
or chloramphenicol, 30% of the thiamphenicol dose was found unchanged
in urine within 8 hours, whereas only 8.3% of chloramphenicol was
eliminated in the active form. After i.m. injection, the urinary
elimination of active antibiotics within 8 hours was 24.2% for
thiamphenicol but only 1.76% for chloramphenicol (Laplassote, 1962).
2.1.1.4 Pigs
To determine plasma and tissue concentrations of thiamphenicol in
pigs following dietary treatment, 16 male pigs, approximately 7 weeks
old, were fed thiamphenicol in the diet at a concentration of
900 mg/kg (equivalent to 30 mg/kg bw), twice daily for a period of 5
days. Three pigs were maintained as controls and fed basal diet only.
Venous blood samples were taken prior to treatment and at various
time-points during the study. The animals were killed at 4, 6, 8 and
10 days after treatment and samples of kidney, liver, muscle, fat and
lung taken. Concentrations of thiamphenicol in plasma were measured
following solvent extraction, using HPLC. On the second day of the
dosing period, scouring and swelling or redness of the anus/perineal
area were noted in all treated animals. The signs resolved within 4-10
days. The maximum mean plasma level of thiamphenicol (1.28 mg/litre)
was demonstrated 8 hours after the first dose. Mean levels were in the
range of 0.22-0.80 mg/litre during the dosing period and declined over
the withdrawal period to give concentrations below or close to the
limit of detection (0.01-0.08 mg/litre) from 4 hours to 5 days after
the end of treatment. The results of the analysis of tissue samples
were not reported (Redgrave et al., 1991).
2.1.1.5 Humans
After a 500 mg oral dose of each of thiamphenicol and
chloramphenicol, the plasma levels appear to be similar. A high level
of active thiamphenicol and a low level of chloramphenicol were found
in the urine (53.1% and 9.2%, respectively, after 24 hours). The
half-life of thiamphenicol is significantly increased in renal
insufficiency, but is almost unaffected by liver cirrhosis
(Azzolini et al., 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of thiamphenicol and thiamphenicol-glycinate
is given in Table 1.
The predominant clinical signs in the animal species tested were
sedation and piloerection after oral dosing, and dyspnoea, cyanosis,
motility disorders and respiratory arrest after parenteral
administration.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
The oral toxicity of thiamphenicol, when administered as an
aqueous suspension in 0.5% methocel for 13 week, has been investigated
in four groups of rats (Sprague-Dawley 30/sex/group). Thiamphenicol
was administered by oral gavage at dose levels of 30, 45, 65 or
100 mg/kg bw per day, while a fifth control group received 0.5%
methocel only. The first 15 animals of each group were killed on
completion of the treatment period, and the remaining 15 after a
recovery period of 8 weeks. Mortality during the treatment period was
elevated among animals of both sexes receiving 100 mg/kg bw per day,
and during the recovery period mortality was similar in all groups. At
levels of 65 and 100 mg/kg bw per day, pallor, hair loss, prostration,
hunched posture and flaccid musculature were seen. After the recovery
phase the incidence and the severity of symptoms were similar in all
groups including controls. Body weight stasis or loss was seen in
animals receiving 65 and 100 mg/kg bw per day, and after 8 weeks
recovery body weight was similar in all groups. Food intake was
reduced at dose levels of 45, 65 and 100 mg/kg bw per day, with
immediate improvement after cessation of treatment.
Table 1. Acute toxicity of thiamphenicol (TAP) and thiamphenicol-glycinate (TAP-G)
Species Sex Route LD50 (mg/kg bw) Reference
TAP TAP-G
Mouse M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1550 Bonanomi, 1978
M & F i.v. 450 Bonanomi, 1978
Rat M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1750 Bonanomi, 1978
M & F i.v. 470 Bonanomi, 1978
In all treated animals there were changes in erythrocyte
parameters, differential and total leukocyte counts and clotting
parameters, all of which were dose-related. After the recovery period
erythrocyte and leukocyte counts were still low in males treated with
65 and 100 mg/kg bw per day. Plasma levels of urea, triglycerides and
total protein were altered from week 7 in animals treated with
45 mg/kg bw per day and after the end of treatment also in males
receiving 30 mg/kg bw per day. Parameters associated with liver and
kidney function were affected at the two higher doses. After 8 weeks
full recovery was considered to have occurred. The weights of all the
main organs were reduced at dose levels of 65 and 100 mg/kg bw per
day, but after the recovery period only the testis weight was still
reduced. Postmortem examination revealed effects on the gastro-
intestinal tract and spleen in both sexes and in the liver, thymus and
testis of males at the highest dose level only. After 8 weeks only the
testis weights were still reduced. The erythroid/myeloid cell ratio
was increased in both sexes at doses of 65 or 100 mg/kg bw per day,
and after 8 weeks was still slightly higher than usual.
Treatment-related changes were seen in tissues with high cell
turnover rates; most of them recovered after a period of respite from
treatment. Animals in the groups treated with 30 and 45 mg/kg per day
showed no histopathological findings except hepatocytic reduced
basophilia (male, 45 mg/kg bw per day) and increased splenic extramed-
ullary haematopoiesis (female 45 mg/kg bw per day). Testicular
germinal epithelial deficit was present at doses above 45 mg/kg, and
caecal oedema and adnexal atrophy were present after the recovery
period at the highest dose level. The NOEL was 30 mg/kg bw per day
(Marubini et al., 1991).
In a 6-month toxicity study in the rat, thiamphenicol was
administered as an aqueous suspension in 2% gum arabic by stomach tube
6 days/week to 180 Wistar rats (30/sex/group) at dose levels of 0, 40
or 120 mg/kg bw per day. Body weight and food intake were recorded
twice weekly in the first 4 weeks of treatment, and thereafter only
once a week. At the end of weeks 4, 8, 16 and 24, ten animals from
each group were killed following collection of 2-hour urine sample.
Haematology, clinical chemistry assays and urinanalysis were performed
on all tested animals. Histological examinations were carried out on
the lungs, spermatozoa, blood and bone marrow smears and samples of
main organs. There was a decrease in food intake at a dose level of
120 mg/kg bw per day and a dose- and time-related decrease in body
weight gain in females. No effect was observed on erythropoiesis or on
hepatic or renal function. Urinanalysis showed the presence of albumin
and haemoglobin in the high-dose rats. No gross pathological
variations in organ weights were observed, but irritative changes in
the gastrointestinal mucosa and high incidence of monolateral
spontaneous hydronephrosis were seen both in treated and control
animals. Histopathological examination performed on control and
high-dose rats revealed a slight effect on the morphology of
spermatozoa in the high-dose group at the 8th, 16th and 24th week of
treatment (Della Bella et al., 1968b).
2.2.2.2 Rabbits
Thiamphenicol-glycinate was administered subcutaneously to groups
of rabbits ("Fauve de Bourgogne" seven/sex/group) at dose levels of
0, 25, 50 or 100 mg/kg bw per day, 6 days/week for 12 weeks. Animals
were weighed weekly and submitted to haematological (erythrocyte and
leukocyte counts with differentials) and biochemical (urea, reducing
sugars, chlorides) examinations before treatment, after 6 weeks and at
the end of the treatment period. After macroscopic examination of the
viscera, the various organs were examined histologically. During the
treatment two control animals and three in the high-dose group died
(no autopsies were carried out). A reduction in polymorphonuclear
neutrophils in male rabbits in all treated groups and a slight fall in
erythrocyte levels in high-dose females were observed. Anatomical and
pathological examination provided no evidence of damage attributable
to thiamphenicol-glycinate administration (Brunaud, 1965).
2.2.2.3 Dogs
In a 7-week study in beagle dogs (four/sex/group), thiamphenicol
was administered orally in gelatin capsules at dose levels of 0, 40 or
80 mg/kg bw per day. Two males and two female dogs were killed at the
end of the 7-week treatment, and the remaining animals were kept for
further 12 weeks without treatment before being killed. Behaviour and
body weights were recorded, haematology and clinical chemistry
examinations and urinanalysis were conducted on all animals pretest
and at various intervals during the study. Complete gross postmortem
examination, organ weighing and histopathological evaluation were
conducted on all animals. The animals in the two treated groups
developed diarrhoea soon after the beginning of treatment, which
spontaneously regressed in the low-dose group but persisted in the
high-dose group. At 80 mg/kg bw per day reduced food consumption with
loss of weight and muscular asthenia occurred, and vomiting was
observed in some cases. Four of these dogs were killed at the end of
the 4th week. Slight loss of weight was observed in two dogs in the
low-dose group.
Decreases in haematocrit, haemoglobin concentration and
erythrocyte count were seen in both treated groups, but did not appear
to be dose-related, and returned to normal on withdrawal of treatment.
Slight increase in proteinuria was observed in the last few weeks of
treatment. At 40 mg/kg bw per day superficial erosion of the gall
bladder mucosa and at 80 mg/kg bw per day haemorrhagic ulcers in the
gall bladder, diffuse muco-membranous enteritis and thymic involution
were seen in dogs killed at the end of treatment period. In dogs
killed after the 12-week recovery period no differences were observed
between treated and control animals. Histological examination after 7
weeks revealed in the high-dose group severe cholecystitis, chronic
sclerosing pancreatitis, enteritis, severe depletion of haematopoietic
marrow and lymphoid thymus depletion. Two dogs in the low-dose group
showed depletion of germinal epithelium in the testes and multinuc-
leated cells in seminiferous tubules, which were not seen in the high-
dose group. None of the changes observed at 7 weeks were detectable
in the animals kept for 12 weeks after cessation of treatment
(Bonanomi et al., 1978).
Thiamphenicol was administered orally in gelatin capsules to 24
beagle dogs (four/sex/group) at dose levels of 30, 60 or 120 mg/kg bw
per day for a period of 4 weeks. Physical observations, ophthalmo-
scopic examinations, and body weight and food consumption measurements
were performed before treatment and over selected intervals during the
treatment. Haematology, clinical chemistry and urinanalysis were
conducted on all animals pretest and at study termination. Complete
gross postmortem examinations, organ weight and histopathological
evaluation were conducted on all animals. The body weights of the
high-dose animals were slightly lower than those of controls at week 3
in both sexes, and at week 4 in males only. At 60 and 120 mg/kg bw per
day absolute and relative liver weights in male dogs were greater than
in controls, and relative liver weights were also increased in
females. Microscopically, hepatocellular hypertrophy was present in
the liver of mid- and high-dose animals, which correlated with the
increase in liver weights in these groups. No other parameter
evaluated showed evidence of adverse treatment-related effects. The
NOEL was 30 mg/kg bw per day (Kelly & Daly, 1990).
Thiamphenicol was administered orally in gelatin capsules to 56
beagle dogs (seven/sex/group) at dose levels of 15, 30 or 60 mg/kg bw
per day. After 6 months of treatment four animals/sex/group were
sacrificed, and the remaining three animals/sex/group were kept for a
2-month recovery period. Physical observations, ophthalmoscopic
examinations, body weight, food consumption, haematology and clinical
chemistry examinations and urinanalysis were conducted on all animals
pretest and on all surviving animals at selected intervals during the
treatment and recovery period. Complete gross postmortem examinations,
organ weight and histopathological evaluation were conducted on all
animals. One control male was found dead during the study. One male
and one female dog in the highest dose group were moribund and had to
be killed. Clinical signs prior to death included lethargy, poor food
consumption, emaciation, tremors and dehydration. Physical findings
related to thiamphenicol administration were tremors, lethargy,
irregular gait and excessive licking or chewing in the high-dose
group. Tremors were also present in the mid-dose animals. These signs
were seen during the last two months of the study and were not present
at the end of the recovery period. Body weights of the high-dose males
during the study were 4 to 18% lower than those of controls. Decreased
erythrocyte counts and mean haematocrit values were seen in high-dose
males at weeks 6 and 13 and at termination of the study, and in high-
and mid-dose females at week 13 and at termination of the study. After
the recovery period no differences were observed in the haematological
parameters between control and treated animals. No treatment-related
effects were seen in the bone marrow smear examinations. Mean serum
cholesterol and phospholipid levels of the mid-and high-dose males at
week 6 and 13 and at termination of the study were greater than
control values. The same parameters were elevated in high-dose females
at the end of the study. Mean serum glucose levels of males at
60 mg/kg bw per day and females at 30 and 60 mg/kg bw per day were
significantly increased. Mean fibrinogen values of high-dose females
were elevated at the end of the study. Relative liver weights were
increased at mid- and high-dose levels. Pathological lesions related
to treatment were seen in sections of the thymus (exacerbation of
involution), bone marrow (decreased cellularity), liver (centrilobular
necrosis and pigment deposition), testes (focal and diffuse tubular
atrophy) and oesophagus (ulceration) from high-dose animals of both
sexes, mostly occurring in animals killed in a moribund condition. No
alterations were noted in any of the tissues examined microscopically
from animals that were allowed to recover after treatment. The NOEL
was 15 mg/kg bw per day (Kelly & Daly, 1991).
2.2.2.4 Pigs
A study designed to determine tolerance in pigs to treatment with
thiamphenicol at three times the recommended dose for 5 days and at
the normal recommended dose for 15 days was conducted with 16 weaned
large white hybrid pigs (two pigs/sex/group). Thiamphenicol was
administered in the diet at dose levels of 30 or 90 mg/kg bw per day
for 5 days or 30 mg/kg bw per day for 15 days. The control group of
animals was fed basal diet only. Clinical signs, body weight and food
consumption were recorded. Blood, urine and faecal samples were
obtained before dosing and at various time-points during the study. No
significant treatment-related clinical abnormalities were noted. Body
weight changes and food consumption were within normal limits. No
consistent treatment-related differences in haematological and
biochemical parameters or in urinanalysis values were observed. The
authors concluded that treatment with thiamphenicol had no significant
adverse effects on general health, body weight, food consumption or
standard clinical pathology parameters (Roberts et al., 1989).
In a 4-week toxicity study, groups of pigs (Large White hybrid,
four/sex/group) were fed 25, 50 or 100 mg thiamphenicol/kg bw per day.
The control group of animals was fed basal diet only. Clinical signs,
body weight and food consumption were recorded. Blood, urine and
faecal samples were obtained before dosing and during week 4 for
clinical pathological investigations. At the end of the 4-week dosing
period, pigs were killed, and selected tissues were processed for
histological examination. In all groups treated with thiamphenicol,
swelling and erythema of the anus, vulva/testes and perineal area,
tail and hocks were observed on the second day of the dosing period.
These effects were consistent with scouring and irritancy, e.g., as a
result of disruption of normal gastrointestinal flora activity, and
disappeared within 1 to 13 days. All pigs remained in good health
thereafter. Slight reductions in body weight gain and food consumption
were noted at 50 and 100 mg/kg bw per day. At week 4 there was a
slight reduction in mean PCV, haemoglobin concentration and
erythrocyte counts in animals receiving 100 mg/kg bw per day and a
treatment-related reduction of urinary pH in all groups dosed with
thiamphenicol. At the end of the study, increases in liver and kidney
weights in pigs fed 50 and 100 mg/kg bw per day were observed. On
histological examination, treatment-related changes were found in the
highest dose group only: an increase in vacuolation and fat in renal
tubular epithelium and, in some animals, minimal diffuse hepatocyte
vacuolation and hepatocyte fat (Cameron et al., 1990).
2.2.3 Long-term toxicity/carcinogenicity study
2.2.3.1 Rats
As a range-finding study for the dose selection in a 2-year
carcinogenicity study, four groups of F-344 rats (10/sex/group)
were given drinking-water containing 0, 125, 250 or 500 mg/litre
thiamphenicol (equal to 9, 17 or 36 mg/kg bw per day for males and 12,
29 or 39 mg/kg bw per day for females) for 13 weeks. The examinations
at the end of the study covered clinical observations, water
consumption, body weight changes, haematological parameters, serum
biochemistry, organ weights and gross and microscopic appearance. In
haematological examinations anaemic changes were observed in males
treated with 250 mg/litre or more and high-dose females. Similar
changes, such as increased MCV and increased related counts, were
observed in males of the 125 mg/litre group and females of the 250 and
125 mg/litre groups. At autopsy, enlargement of the caecum was seen in
treated groups of both sexes. Histologically, the highest dose animals
showed decreased haematopoiesis of the bone marrow, decreased spermato-
genesis of the testis and sperm granulomas of the epididymis. Sperm
granulomas in the epididymis were also seen in some of the animals in
the 250 mg/litre group. Based on the results of this pilot study, a
2-year carcinogenicity study of thiamphenicol was performed in rats.
Three groups of F-344 rats (50/sex/group) were given drinking-water
containing 0, 125 or 250 mg thiamphenicol/litre (equal to 8 or 16
mg/kg bw per day for males and 9.7 or 19 mg/kg bw per day for females)
for 104 weeks. All surviving animals subjected to 4-week withdrawal of
the test chemical after the end of the treatment were killed for full
histopathological examinations. The high-dose animals showed decreased
body weight gain, but the incidence of rumours in treated groups was
not significantly higher than that of controls (Maekawa, 1996; summary
report only was available).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
In a fertility study on male Wistar rats, oral treatment with
thiamphenicol for 2 or 3 months (30 animals per dose level, 10 per
treatment time), at dose levels of 120, 180 or 240 mg/kg bw per day,
resulted in reduction in the number of tubular germinal cells, which
was more marked at the highest dose level. Ten animals of each group
were treated for 4 weeks, ten for 8 weeks and the last ten for 12
weeks. At the end of each test period 5 animals of each dose group
were killed and necropsied, while the remaining 5 rats were mated with
normal females. At 240 mg/kg bw per day extensive testicular
hypotrophy, together with severe depletion of the germinal epithelium
21 days after withdrawal of treatment, was seen. Histological changes
coincided with a reduction of the fertility index, which gradually
recovered within 50 days. Litters from matings between treated males
and normal females were normal in number and weight, and no morpho-
logical abnormalities were observed. The concentration ratio of
thiamphenicol between testes and plasma after administration of
240 mg/kg bw per day thiamphenicol was 1, indicating the absence of
accumulation in testes (Della Bella et al., 1967).
Groups of 21 Sprague-Dawley rats were given thiamphenicol orally
(30, 60 or 120 mg/kg bw per day daily) from day 15 of gestation to day
21 postpartum. In groups receiving 60 and 120 mg/kg bw per day a
higher post-implantation loss, slight weight reduction at birth and
increased rate of perinatal mortality were observed. No malformations
were observed. Development of pups was inhibited during the lactation
period with a consistent dose-dependent relationship. From day 30
postpartum a good recovery was observed in all groups. Sexual
behaviour and fertility of F1 animals were normal, and the F2
generation showed no signs of abnormal development (Bonanomi et
al., 1980).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Teratogenicity studies were carried out on 195 mature female
Wistar rats (15/group) given thiamphenicol orally at dose levels of
40, 80 or 160 mg/kg bw per day from days 1 to 21 of pregnancy and 80
or 960 mg/kg bw per day from days 1 to 7, 7 to 14 or 14 to 21 of
gestation. Thiamphenicol did not induce any teratogenic effects in any
of the four studies carried out. When the treatment period was 1-21
days, a dose-related increase in resorptions was noted, and newborns
had a high mortality rate in the second and third week of life,
particularly in the 40 mg/kg bw per day group. In rats treated on days
1-7 of gestation, a non-dose-related increase in resorptions and an
increased mortality in newborns in the third week after birth were
observed. When the treatment period was from 7 to 14 days, complete
resorption of fetuses occurred at 160 mg/kg bw per day. The mean
number of newborns per litter was reduced at 80 mg/kg bw per day and
there was a high mortality of newborns in the first week. An increased
mortality among newborns of the group treated with 80 mg/kg bw per day
during days 14-21 of gestation was observed (Bonanomi & De Paoli,
1969).
When the inhibition of mitochondrial functions induced by
thiamphenicol was compared with the inhibition of overall embryonic
development, it appeared that mitochondrial respiration was the
rate-limiting step for the embryotoxic effects of thiamphenicol.
Because of the lack of specificity of these effects, prenatal
mortality rather than teratogenic effects was seen (Bass et al.,
1978).
2.2.5.2 Rabbits
A teratogenicity study was performed with 50 New Zealand white
rabbits administered thiamphenicol orally at dose levels of 5, 30, 60
or 80 mg/kg bw per day from the 8th to the 16th day of pregnancy. The
highest dose resulted in a complete resorption of implantation due to
the toxic effects on the mothers. Data obtained in all treated groups
showed moderate fetal toxicity with a dose-related increase in
abortion rate and resorption. No skeletal malformations were found in
fetuses (Bonanomi et al., 1974).
Thiamphenicol in 0.5% Methocel K15M was administered daily by
oral gavage to female rabbits (16/group) from day 6 to day 18 of
gestation at doses of 1.25, 2.5 or 5.0 mg/kg bw per day. Control
animals received the vehicle alone. The females were killed on
gestation day 29 and subjected to postmortem examination. All fetuses
were examined for external and internal abnormalities and skeletal
changes. No clinical signs attributable to treatment were observed.
Mild signs of maternal toxicity were observed in mid- and high-dose
animals, indicated by reduction in body weight changes during the
treatment period.
Necropsy findings in females on gestation day 29 were incidental,
with no relation to treatment. Litter parameters and sex ratios did
not show any significant difference between groups. Mean fetal weight
was decreased in the high-dose group. Two fetuses in the mid-dose
group and one in the control group were malformed, with cleft palate,
abnormally shaped head, extra digits and incomplete flexure of the
hind limbs. The number of small fetuses in the high-dose group was
higher than in the control group. The few anomalies seen during the
internal examination were not considered to be treatment-related.
Skeletal examination revealed no differences between high-dose fetuses
and controls. The authors concluded that thiamphenicol administered by
oral gavage at concentrations of 1.25, 2.5 and 5 mg/kg per day had no
effects on pregnancy or embryo-fetal development. However, the
Committee concluded that the NOEL for embryotoxicity under the
conditions of this experiment was 1.25 mg/kg per day (Sisti, 1994).
2.2.6 Special studies on genotoxicity
The results of genotoxicity assays on thiamphenicol are given in
Table 2.
Table 2. Genotoxicity assays on thiamphenicol
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0.5-50 Negative Pinasi
TA98, TA100, µg/plate2 et al., 1990a
TA1535, TA1537,
TA1538
Gene conversion Saccharomyces 2.8-140.3 mM3 Negative Marca &
and mitotic cerevisiae Bonanomi,
crossing over1 1979
Gene mutation Chinese hamster 50-5000 Negative Pinasi
assay1 V79 cells µg/ml4 et al., 1990b
Chromosomal Cultured human 700-3250 Negative Mosesso &
aberrations1 lymphocytes µg/ml5 Driedger, 1989
DNA repair Primary rat 500 and 1000 Negative Bichet, 1985
test hepatocytes µg/kg6
In vivo Mouse bone 2500 and 5000 Negative Pinasi
micronucleus marrow mg/kg7 et al., 1990c
assay
1 Both with and without rat liver S9 fraction
2 Methyl methanesulfonate and cyclophosphamide were used as positive controls
3 2-Nitrofluorene, 9-aminoacridine, sodium azide and 2-aminoanthracene were used
as positive controls
4 Ethyl ethanesulfonate and N-dimethylnitrosoamine were used as positive controls
5 Mitomycin-C and cyclophosphamide were used as positive controls
6 2-Aminofluorene was used as positive control
7 Cyclophosphamide was used as positive control
2.2.7 Special studies on immune responses
The effects on spontaneous nephritis in NZB × OUW hybrid mice
(32-39 males/group) were investigated in a study involving lifetime
administration of thiamphenicol in feed at dose levels of 25, 50 and
250 mg/kg bw per day. Body weight, urinary protein, limited
haematology, organ weights and histopathology investigations were
reported. The prolonged treatment with thiamphenicol at dose rates of
> 50 mg/kg reduced the severity of the spontaneous renal disease and
significantly extended lifespan, compared to untreated controls. The
immunosuppressive effect of thiamphenicol was demonstrated
histologically by a reduction in immune-complex deposition in the
glomeruli. No evidence of malignancy or premalignant signs was seen
(Simpson et al., 1979).
2.2.8 Special studies on microbiological effects
2.2.8.1 In vivo
In a study of thiamphenicol-induced changes in mouse intestinal
microflora, 100 female albino mice were divided into 10 subgroups, and
five of these groups were treated with thiamphenicol at concentrations
of 40 µg/kg diet for 35 days. Samples of intestinal content were taken
from the caecum for bacteriological analysis before the treatment and
after 7, 14, 28 and 35 days. Microorganisms were isolated by preparing
serial dilutions of intestinal content, and the most representative
bacteria of the mouse intestinal microflora were cultured on specific
media. Their sensitivity to thiamphenicol was assessed by calculating
the minimal inhibitory concentration (MIC) of the drug. The results
show that the mean counts of various microorganisms did not differ
significantly between control mice and those fed with thiamphenicol.
The numbers of the bacterial populations did vary at the different
sampling times, and in some cases the difference from pre-treatment
results was significant, but confidence limits were the same in
treated and control mice killed at the same time. The investigation
shows that there were no appreciable differences in the type or amount
of bacterial flora related to thiamphenicol administration.
Differences in the distribution of genera such as Diplococcus sp.
and Escherichia sp. were similar to those in controls. The
thiamphenicol MIC values for the numerous strains tested indicate that
addition to feed at concentrations corresponding to the proposed MRL
of 40 µg/kg feed does not select for drug-resistant strains and has no
effect on the qualitative or quantitative composition of the
intestinal microflora. The MIC remained unchanged throughout the 35
days of the study (Poli, 1994).
2.2.8.2 In vitro
In vitro antibacterial activity of thiamphenicol against 489
bacterial isolates from infected animals was determined by the agar
dilution method. In the case of mycoplasmas, however, MICs were
determined by the broth dilution method. Depending on bacterial
strains tested estimations were made under aerobic or anaerobic
conditions. The results are presented in Table 3 (Albini, 1989).
Data on the sensitivity of normal components of human intestinal
microflora are presented in Table 4 (Sutter & Finegold, 1976;
Schioppacassi, 1992).
Table 3. Antibacterial activity of thiamphenicol against 489 animal pathogens
Organisms Number of MIC (µg/ml) Range
isolates
MIC50 MIC90
Bacteroides spp. 11 2 16 1 - 128
Bordetella spp. 9 32 32 16 - 32
Campylobacter spp. 17 8 16 4 - 16
Clostridium spp. 37 2 4 0.25 - 16
Corynebacterium spp. 10 2 16 2 - 16
Escherichia coli 61 128 >128 16 - > 128
Haemophilus
pleuropneumoniae 7 0.5 1 0.5 - 1
Micrococcus spp. 6 0.5 0.5 0.5 - 8
Mycoplasma spp. 9 1 2 0.125 - 4
Pasteurella spp. 71 1 2 0.25 - 128
Salmonella spp. 34 32 32 8 - > 128
Staphylococcus spp. 94 8 32 4 - > 128
Streptococcus spp. 123 2 4 0.5 - > 128
2.3 Observations in humans
Reversible dose-related bone marrow suppression is seen after
thiamphenicol treatment and is attributed to its inhibitory effect on
mitochondrial protein synthesis (Nijhof & Kroon, 1974). Reversible
inhibition of myeloid and erythroid colony growth by thiamphenicol,
resulting from an inhibition of mitochondrial protein synthesis, is
consistent with the reversible bone marrow suppression induced by this
drug (Yunis & Gross, 1975).
A study of clinical reports on the use of thiamphenicol in 16 631
cases from 1968 to 1977 in Japan revealed that blood disorders
occurred in 41 (0.46%) out of 8848 patients receiving thiamphenicol
glycinate therapy and 28 (0.36%) out of 7783 patients receiving
thiamphenicol. The disorders were dose-dependent, occurring mainly in
erythrocytes, and disappeared spontaneously on discontinuation of the
drug (Tomoeda & Yamamoto, 1981).
The para-nitro group of chloramphenicol has been shown to have
a central role in the pathogenesis of aplastic anaemia, probably as a
result of its reduction to the highly toxic nitroso metabolite, which
is a potent inhibitor of DNA synthesis. Thiamphenicol does not show
this activity and the absence of the para-nitro group is therefore
advanced as evidence that thiamphenicol cannot induce aplastic anaemia
(Murray et al., 1983).
Thiamphenicol has been used extensively in human medicine
for over 25 years. Total human exposure to thiamphenicol up to
1987 has been estimated at 130-650 million exposures, assuming an
average course of therapy of 7.5-15 g (Personal communication from
Dr S. Biressi, Zambon Group SpA, Italy to Dr R.D. Agostino,
Farmaquest Co.; submitted to WHO by Zambon Group SpA, Italy).
Epidemiological studies have not established any causal
association between thiamphenicol treatment and irreversible aplastic
anaemia (TAP Pharmaceuticals Inc., 1987). Statistical analysis of
data obtained in these studies support the argument that the risk of
aplastic anaemia from exposure to thiamphenicol is similar to the
background risk of idiopathic aplastic anaemia (from 1 in 200 000 to 1
in 800 000) (Kelly & Kaufman, 1989).
Table 4. Antibacterial activity of thiamphenicol against 261 strains of anaerobic bacteria isolated from humans
(From: Sutter & Finegold, 1976)
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Bacteroides fragilis 42 5 21 71 100
Bacteroides 59 9 24 51 90 100
melaninogenicus
Other Bacteroides 21 19 24 57 76 86 91 95 100
and Selenomonas
Fusobacterium 8 100
nucleatum
Other Fusobacterium 12 42 92 100
Peptococcus and 17 35 71 100
Gaffkya
Peptostreptococcus 15 33 47 93 100
Table 4. (cont'd).
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Anaerobic and 6 50 83 100
microaerophilic
streptococci
Gram-negative cocci 7 43 86 100
Eubacterium 7 43 57 100
Arachnia propionica 2 50 100
Propionibacterium 4 50 75 100
Actinomyces 16 25 56 94 100
Lactobacillus 10 10 30 50 90 100
Clostridium 8 100
perfringens
Other Clostridium 27 4 22 63 78 96 100
3. COMMENTS
Studies on thiamphenicol available for evaluation included data
on pharmacokinetics, acute toxicity, short-term toxicity, reproductive
toxicity, developmental toxicology, genotoxicity, and limited
information on long-term toxicity. Studies on microbiological effects
of thiamphenicol and epidemiological data on humans were also
considered by the Committee. The Committee noted that many of the
studies were conducted utilizing protocols that would not meet
contemporary standards, and therefore the substance was evaluated
under the procedures developed for drugs with a long history of use
(Annex 1, reference 104).
The pharmacokinetic data showed that the drug is rapidly absorbed
when administered by oral or parenteral routes. After intravenous
administration in rats, the half-life was estimated to be 46 minutes.
The main route of excretion in humans and animals is in the urine;
approximately 60% of an oral dose of 30 mg/kg bw was excreted
unchanged in the urine over a 24-hour period.
Single oral doses of thiamphenicol were of low toxicity to mice
and rats (LD50 > 3000 mg/kg bw).
Short-term oral toxicity studies with thiamphenicol were
performed in rats, dogs and pigs, the results are described in the
following paragraphs.
In a 13-week study in rats at dose levels of 30, 45, 65 or
100 mg/kg bw per day, increased mortality was observed among animals
given 100 mg/kg bw per day. In a 6-month study in rats, where the
highest dose used was 120 mg/kg bw per day, increased mortality was
not reported. In both studies, decrease in body weight gain during
treatment occurred at doses of > 65 mg/kg bw per day. Dose-related
decreases in red blood cell parameters, differential and total white
blood cell counts, and clotting parameters were observed in the
13-week study, but the same effects were not reported in the 6-month
study. Testicular germinal epithelial cell depletion was seen at doses
above 45 mg/kg bw per day in the 13-week study, and a dose of 30 mg/kg
bw per day was considered to be the NOEL.
Dogs were given 40 or 80 mg thiamphenicol/kg bw per day for 7
weeks. At both dose levels decreases in body weight were observed, as
well as reversible decreases in haematocrit, haemoglobin concentration
and erythrocyte count. At 40 mg/kg bw per day, superficial erosion of
the gall bladder mucosa was observed. The higher dose level resulted
in haemorrhagic ulcers in the gall bladder, diffuse mucomembranous
enteritis and early thymic involution. Two dogs in the low-dose group
had testicular germinal epithelial cell depletion and multinucleated
cells in the seminiferous tubules.
When thiamphenicol was given to dogs at doses of 30, 60 or
120 mg/kg bw per day for 4 weeks, the body weights of the high-dose
animals were slightly lower than those of controls. In the mid- and
high-dose groups, increases in absolute and relative liver weights
were observed. Hepatocellular hypertrophy was present in the liver of
dogs given 60 or 120 mg/kg bw per day.
In a 6-month study dogs were given thiamphenicol at doses of 15,
30 or 60 mg/kg bw per day. The body weights of high-dose males during
the study were up to 18% lower than those of controls. The main
haematological findings were decreases in red blood cell parameters at
the highest dose level. Increases were noted in mean serum cholesterol
level and phospholipid concentrations in males (30 and 60 mg/kg bw per
day groups) and females (60 mg/kg bw per day group), and in the mean
serum glucose concentration of males (60 mg/kg bw per day group) and
females (30 and 60 mg/kg bw per day groups). The relative liver
weights at the mid- and high-dose levels were increased. Histopatho-
logical lesions related to treatment were seen in the thymus (early
involution), bone marrow (decreased cellularity), testes (focal and
diffuse tubular atrophy) and oesophagus (ulceration) of high-dose
animals. The NOEL was 15 mg/kg bw per day.
In a 4-week study, pigs were treated with 25, 50 or 100 mg
thiamphenicol/kg bw per day. In the highest-dose group, slight
reduction in body weight gain, as well as reductions in mean packed
cell volume, haemoglobin concentration and erythrocyte counts, were
observed, and histological examination showed vacuolation in renal
tubular epithelial cells and mild diffuse hepatocyte vacuolation. In
all treated groups treatment-related reduction in urine pH was
observed.
A summary report of a two-year carcinogenicity study in rats,
including a range-finding study, was available to the Committee. Rats
were given 125 or 250 mg/kg thiamphenicol in drinking-water (equal to
8 or 16 mg/kg bw per day for males and 10 or 19 mg/kg bw per day for
females) for 104 weeks. The highest-dose animals showed a decrease in
body weight gain, but there was no significant increase in the
incidence of tumours in treated groups compared to control animals.
In a long-term study in mice (32-39 males/group), which was
designed primarily to investigate the effects of thiamphenicol on
immune responses, thiamphenicol was administered orally at doses of
25, 50 or 250 mg/kg bw per day. No evidence of neoplastic or
preneoplastic changes was observed.
In a study to determine the effect of thiamphenicol on fertility
in rats, the drug was administered orally at doses of 120, 180 or
240 mg/kg bw per day for 2 or 3 months. Thirty male rats were used per
dose level (10 per treatment period). From each treatment-period
group, half of the animals were killed for histopathological
examination at the given time intervals, while the remaining males
were mated with untreated females. Reductions in the number of
germinal epithelial cells in testes of all treated animals were
observed. These changes were present up to 21 days after termination
of treatment, and full recovery was observed by 50 days. Histological
changes correlated with the fertility index. Litters from matings
between treated males and non-treated females were normal in number
and no physical abnormalities were reported.
Thiamphenicol was given orally to rats from day 15 of gestation
to day 21 post-partum at doses of 30, 60 or 120 mg/kg bw per day. In
the mid-and high-dose groups, there were higher post-implantation
losses and increased rates of perinatal mortality. Physical develop-
ment of pups was inhibited during the lactation period in a dose-
dependent manner. Sexual behaviour and fertility of F1 animals
were normal, and animals in the F2 generation showed no
abnormalities.
In four teratogenicity studies in rats, thiamphenicol was
administered orally at dosages of 40, 80 or 160 mg/kg bw per day from
days 1 to 21 of pregnancy or of 80 or 960 mg/kg bw per day over
critical days of gestation (either 1-7, 1-21, 7-14 or 14-21). No
teratogenic effects were observed. In all animals treated from days 1
to 21, a dose-related increase in resorption was noted and newborn
pups had an elevated mortality rate.
A teratogenicity study was performed in rabbits using oral doses
of 5, 30, 60 or 80 mg/kg bw per day from days 8 to 16 of gestation.
Complete resorption of embryos occurred at 80 mg/kg bw per day. In
other treated groups, moderate fetal toxicity and dose-related
increases in abortion rate and resorption were reported. No
malformations were found in fetuses.
In another teratogenicity study, rabbits received oral doses of
thiamphenicol at doses of 1.25, 2.5 or 5 mg/kg bw per day from days 6
to 18 of gestation. Mild maternal toxicity was observed in mid- and
high-dose animals in the form of depressed body weights during the
treatment. No effects were observed on embryo-fetal development. The
NOEL was 1.25 mg/kg bw per day.
Thiamphenicol gave negative results in five in vitro
genotoxicity tests and in an in vivo micronucleus assay using mouse
bone marrow.
The Committee considered data from human epidemiological studies
and concluded that there was no evidence that thiamphenicol can induce
aplastic anaemia, in contrast to the structurally related compound,
chloramphenicol.
The Committee considered data from several in vitro studies on
the minimum inhibitory concentration (MIC) of thiamphenicol for a wide
range of animal and human pathogens as well as genera representative
of the human gut flora. The modal MIC50 value (minimum inhibitory
concentration of thiamphenicol giving complete inhibition of growth of
50% of cultures) was 1.68 µg/ml for 261 bacterial strains isolated
from humans. The following species were found to be the most
sensitive: Bacteroides, Fusobacteria, Propionibacteria and
Actinomyces. The Committee also noted that 40 µg thiamphenicol/kg
food given to mice over 35 days did not alter the intestinal
microflora in this species.
The Committee calculated a microbiological ADI for thiamphenicol
using the following formula:
Upper limit of MIC50 (µg/g) × mass of colonic content (g)
microbiological =
ADI fraction of oral × safety × human body
dose available factor weight (kg)
1.68 × 220
=
0.4 × 1 × 60
= 15 µg/kg bw
In calculating a microbiological ADI the Committee took the
following factors into consideration:
* Concentration: 1.68 µg/ml was the modal MIC50 for micro-
biological effects on human intestinal microflora (the density
was assumed to be 1 g/ml).
* Availability: the Committee calculated the available portion of
thiamphenicol as follows:
100% ingested - 60% excreted via = 40% bioavailable
urine within in the intestinal
24 hours tract
1 - 0.6 = 0.4
* Safety factor: the Committee concluded that the data deriving
from the microbiological studies (substantial amount of MIC data
covering a variety of microorganisms and in vivo data from
animal studies) provided sufficient information on microbio-
logical effects of thiamphenicol. It therefore adopted a safety
factor of 1 in the calculation.
4. EVALUATION
Taking into account the available toxicological and antimicrobial
data and the ADI based on antimicrobial activity, the Committee
concluded that the toxicological data provided the most appropriate
end-point for the evaluation of thiamphenicol. The Committee
established a temporary ADI of 0-6 µg/kg bw for thiamphenicol, based
on the NOEL of 1.25 mg/kg bw per day for maternal toxicity in the
teratogenicity study in rabbits and a safety factor of 200. The ADI
was designated "temporary" because only a summary report of the
carcinogenicity study in rats was available. Detailed reports of the
carcinogenicity study and the range-finding study used to establish
dose levels in that study are required for evaluation in 1999
(see Annex 4).
5. REFERENCES
Albini, E. (1989). In vitro antibacterial activity of thiamphenicol
against bacterial strains recently isolated from animal infectious
diseases. Unpublished report from Microbiology Laboratory, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Azzolini, F., Gazzaniga, A., Lodola, E., & Natangelo, R. (1972).
Elimination of chloramphenicol and thiamphenicol in subjects with
cirrhosis of the liver. Int. J. Clin. Pharmacol., 6, 130-134.
Bass, R., Oerter, D., Krowke, K., & Spielman H. (1978). Embryonic
development and mitochondrial function. III. Inhibition of respiration
and ATP generation in rat embryos by thiamphenicol. Teratology,
18, 93-102.
Bichet, N. (1985). Etude de génotoxicité éventuelle sur les
hépatocytes de rat par mesure de réparation du DNA. Unpublished report
No. 01147F from Sanofi Recherche, Montpellier, France. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. (1978). Acute toxicity study in rats and mice with
thiamphenicol and thiamphenicol glycinate HCl. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. & De Paoli, A.M. (1969). Teratogenicity study of oral
thiamphenicol in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, Pacei, E., & De Paoli, A.M. (1974). Teratogenicity study
of oral thiamphenicol in the rabbit. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, De Paoli, A.M., & Gazzaniga, A. (1978). Toxicity study
after repeated oral administration of thiamphenicol in the dog.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Bonanomi, L., Aliverti, V., Ornaghi, F., Losa, M., & Motta, F. (1980).
Thiamphenicol perinatal and postnatal toxicity study in the rat.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Brunaud, M. (1965). Thiamphenicol glycinate hydrochloride. Long-term
toxicity in the rabbit by subcutaneous route. Unpublished report from
Clin-Byla Laboratories, Paris, France. Submitted to WHO by Zambon
Group SpA, Bresso, Milan, Italy.
Cameron, D.M., Crook, D., Brown G., Gopinath, C., Farmer, H., & Offer,
J.M. (1990). Z 2041: 4-Week toxicity study in pigs. Unpublished report
No. ZBN 12/891518 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A. (1967).
Fertility study in male rat treated with thiamphenicol by the oral
route. Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Della Bella, D., Ferrari, V., Marca, G., & Bonanomi, L. (1968a).
Chloramphenicol metabolism in the phenobarbital induced rat.
Comparison with thiamphenicol. Biochem. Pharmacol., 17, 2381-2390.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A.
(1968b). Toxicity study of thiamphenicol after repeated oral
administration in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Ferrari, V. & Della Bella, D. (1974). Comparison of chloramphenicol
and thiamphenicol metabolism. Postgrad. Med. J., 50, 17-22.
Gazzaniga, A. (1974). Thiamphenicol - pharmacokinetics and metabolism
in animals. Unpublished report from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, C.M. & Daly I.W. (1990). A four week oral toxicity study in the
dog via capsule administration with Z 2041. Unpublished report
No. 88-3391 from Bio/dynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Kelly, C.M. & Daly, I.W. (1991). A six month oral toxicity study in
the dog via capsule administration with Z 2041 with a two month
recovery period. Unpublished report No. 88-3392 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, J.P. & Kaufman, D.W. (1989). Ant-infective drug use in relation
to the risk of agranulocytosis and aplastic anemia: A report from the
international agranulocytosis and aplastic anemia study.
Arch. Intern. Med., 149, 1036-1040.
Laplassote, J. (1962). Recherches expérimentales sur le thiopénicol:
activité antibactérienne, concentrations humorales, élimination.
Comparaison avec le chloramphénicol. Therapie, 16, 104-108.
Marca, G. & Bonanomi, L. (1979). Gene conversion and mitotic
crossing-over in Saccharomyces cerevisiae 6117 with thiamphenicol
with and without metabolic activation. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Maekawa, A. (1996). Summary report on two-year rat carcinogenicity
study of thiamphenicol. Submitted by Sasaki Institute, Tokyo, Japan to
the Japanese Ministry of Health and Welfare.
Marubini, M., Motta, F., Cerioli, A., & Finn, J.P. (1991). Z 2041.
Thirteen week oral toxicity study in rats followed by a recovery
period of 8 weeks. Unpublished report No. 1219 from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Mosesso, P. & Driedger, A. (1989). Chromosome abberations in human
lymphocytes cultured in vivo. Unpublished report No. LSC-RTC
116028-M-02989 from Life Science Research Roma Toxicology Centre SpA,
Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Murray, T.R., Downey, K.M., & Yunis, A.A. (1983).
Chloramphenicol-mediated DNA damage and its possible role in the
inhibitory effects of chloramphenicol on DNA synthesis.
J. Lab. Clin. Med., 102, 926-932.
Nijhof, W. & Kroon, A.M. (1974). The interference of chloramphenicol
and thiamphenicol with the biogenesis of mitochondria in animal
tissues. A possible clue to the toxic action. Postgr. Med. J.,
50 (Suppl. 5), 53-59.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990a). Mutagenicity
evaluation of Z 2041 in the Ames Salmonella-microsome plate test.
Unpublished report No. 1231 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990b). Mutagenicity
evaluation of Z 2041 in Chinese hamster V79 test. Unpublished report
No. 1243 from Toxicology Department, Zambon Research, Bresso, Milan,
Italy. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990c). Genotoxicity
evaluation of Z 2041 in the "in vivo" mouse micronucleus assay.
Unpublished report No. 1233 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Poli, G. (1994). Qualitative and quantitative changes in the mouse
intestinal microflora induced by chronic thiamphenicol at maximum
residual level (MRL) dose. Unpublished report from Facolta di Medicina
Vetarinaria, Universita di Milano, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Redgrave, V.A., Cameron, D.M., Anderson, A., & Maxwell, J.G. (1991).
Blood concentrations of thiamphenicol in pigs following dietary
administration of Z 2041. Unpublished report No. ZBN 11/901351 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Roberts, N.L., Cameron, D.M., Redgrave, V.A., Crook, D., & Brown G.
(1989). Z 2041. Tolerance in pigs. Unpublished report No. ZBN 10/89188
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Zambon Group SpA, Bresso, Milan, Italy.
Schioppacassi, G. (1992). Activity of thiamphenicol on indigenous
microorganisms of gastrointestinal tract. Unpublished report
No. DBI 1/92 from Zambon Research, Bresso, Milan, Italy. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Simpson, L.O., Aarons, I., & Howie, J.B. (1979). Thiamphenicol and
lupus nephritis. The effects of long-term therapy on kidney function
and pathology: a pilot study. Br. J. Exp. Pathol., 60, 45-57.
Sisti, R. (1994). Z 2041. Oral embryotoxicity study in rabbits.
Unpublished report No. RTC 4195/T/128/94 from Research Toxicology
Centre SpA, Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Sutter, V.L & Finegold, S.M. (1976). Susceptibility of anaerobic
bacteria to 23 antimicrobial agents. Antimicrob. Agents Chemoter.,
10, 736-752.
TAP Pharmaceuticals Inc. (1987). Issues relating to the safety of
thiamphenicol with special attention to the matter of aplastic anemia.
Unpublished report from TAP Pharmaceuticals Inc., Corte Mader, Canada.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Tomoeda, M. & Yamamoto, K. (1981). The hematologic adverse reaction
experience with thiamphenicol in Japan. In: Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy, Raven Press, New York,
pp. 103-110.
Uesugi, T., Ikeda, M., Hori, R., Katayama, K., & Arita, T. (1974).
Metabolism of thiamphenicol and comparative studies of its urinary and
biliary excretion with chloramphenicol in various species.
Chem. Pharm. Bull., 22, 2714-2722.
Yunis, A.A. & Gross, M.A. (1975). Drug-induced inhibition of myeloid
colony growth: protective effect of colony stimulating factor.
J. Lab. Clin. Med., 86, 499-504.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
2.1.1.1 Rats
After i.v. administration in rats the half-life of thiamphenicol
was 46.3 minutes, compared to 21.5 minutes for chloramphenicol
(Ferrari & Della Bella, 1974).
Pretreatment of rats with phenobarbital increased thiamphenicol
half-life slightly from 46.3 to 55.2 minutes, whereas the
chloramphenicol half-life was reduced from 21.5 minutes to 9.3 minutes
(Della Bella et al., 1968a).
Following administration of 30 mg/kg to rats, thiamphenicol was
eliminated in the urine almost entirely in the unchanged form: 62%
following oral administration and 47% after i.m. administration (both
at 48 hours). No significant glucuro-conjugation products were found.
In the bile, 3.4% of the administered dose appeared unchanged and
10-12% appeared as conjugated products after 4 hours. Of the
administered dose, 36% was eliminated in faeces after 75 hours, almost
entirely as unchanged thiamphenicol. Distribution studies showed that
levels in the kidney and liver were higher than in plasma, while brain
concentrations were negligible (Gazzaniga, 1974).
2.1.1.2 Rabbits
The amount of metabolites recovered in urine and bile within 7
hours after i.v. administration was about 73% for thiamphenicol and
60% for chloramphenicol, and the percentage of glucuronide to total
amount recovered was 8% for thiamphenicol and 66% for chloramphenicol.
The recovery of thiamphenicol into the bile was only 1%, mostly in
unchanged form. About 3% of administered chloramphenicol was excreted
into the bile and two-thirds of this consisted of metabolites
(Uesugi et al., 1974).
2.1.1.3 Dogs
Following intraduodenal administration of 70 mg/kg thiamphenicol
or chloramphenicol, 30% of the thiamphenicol dose was found unchanged
in urine within 8 hours, whereas only 8.3% of chloramphenicol was
eliminated in the active form. After i.m. injection, the urinary
elimination of active antibiotics within 8 hours was 24.2% for
thiamphenicol but only 1.76% for chloramphenicol (Laplassote, 1962).
2.1.1.4 Pigs
To determine plasma and tissue concentrations of thiamphenicol in
pigs following dietary treatment, 16 male pigs, approximately 7 weeks
old, were fed thiamphenicol in the diet at a concentration of
900 mg/kg (equivalent to 30 mg/kg bw), twice daily for a period of 5
days. Three pigs were maintained as controls and fed basal diet only.
Venous blood samples were taken prior to treatment and at various
time-points during the study. The animals were killed at 4, 6, 8 and
10 days after treatment and samples of kidney, liver, muscle, fat and
lung taken. Concentrations of thiamphenicol in plasma were measured
following solvent extraction, using HPLC. On the second day of the
dosing period, scouring and swelling or redness of the anus/perineal
area were noted in all treated animals. The signs resolved within 4-10
days. The maximum mean plasma level of thiamphenicol (1.28 mg/litre)
was demonstrated 8 hours after the first dose. Mean levels were in the
range of 0.22-0.80 mg/litre during the dosing period and declined over
the withdrawal period to give concentrations below or close to the
limit of detection (0.01-0.08 mg/litre) from 4 hours to 5 days after
the end of treatment. The results of the analysis of tissue samples
were not reported (Redgrave et al., 1991).
2.1.1.5 Humans
After a 500 mg oral dose of each of thiamphenicol and
chloramphenicol, the plasma levels appear to be similar. A high level
of active thiamphenicol and a low level of chloramphenicol were found
in the urine (53.1% and 9.2%, respectively, after 24 hours). The
half-life of thiamphenicol is significantly increased in renal
insufficiency, but is almost unaffected by liver cirrhosis
(Azzolini et al., 1972).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The acute toxicity of thiamphenicol and thiamphenicol-glycinate
is given in Table 1.
The predominant clinical signs in the animal species tested were
sedation and piloerection after oral dosing, and dyspnoea, cyanosis,
motility disorders and respiratory arrest after parenteral
administration.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
The oral toxicity of thiamphenicol, when administered as an
aqueous suspension in 0.5% methocel for 13 week, has been investigated
in four groups of rats (Sprague-Dawley 30/sex/group). Thiamphenicol
was administered by oral gavage at dose levels of 30, 45, 65 or
100 mg/kg bw per day, while a fifth control group received 0.5%
methocel only. The first 15 animals of each group were killed on
completion of the treatment period, and the remaining 15 after a
recovery period of 8 weeks. Mortality during the treatment period was
elevated among animals of both sexes receiving 100 mg/kg bw per day,
and during the recovery period mortality was similar in all groups. At
levels of 65 and 100 mg/kg bw per day, pallor, hair loss, prostration,
hunched posture and flaccid musculature were seen. After the recovery
phase the incidence and the severity of symptoms were similar in all
groups including controls. Body weight stasis or loss was seen in
animals receiving 65 and 100 mg/kg bw per day, and after 8 weeks
recovery body weight was similar in all groups. Food intake was
reduced at dose levels of 45, 65 and 100 mg/kg bw per day, with
immediate improvement after cessation of treatment.
Table 1. Acute toxicity of thiamphenicol (TAP) and thiamphenicol-glycinate (TAP-G)
Species Sex Route LD50 (mg/kg bw) Reference
TAP TAP-G
Mouse M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1550 Bonanomi, 1978
M & F i.v. 450 Bonanomi, 1978
Rat M & F oral > 5000 Bonanomi, 1978
M & F i.p. > 3000 1750 Bonanomi, 1978
M & F i.v. 470 Bonanomi, 1978
In all treated animals there were changes in erythrocyte
parameters, differential and total leukocyte counts and clotting
parameters, all of which were dose-related. After the recovery period
erythrocyte and leukocyte counts were still low in males treated with
65 and 100 mg/kg bw per day. Plasma levels of urea, triglycerides and
total protein were altered from week 7 in animals treated with
45 mg/kg bw per day and after the end of treatment also in males
receiving 30 mg/kg bw per day. Parameters associated with liver and
kidney function were affected at the two higher doses. After 8 weeks
full recovery was considered to have occurred. The weights of all the
main organs were reduced at dose levels of 65 and 100 mg/kg bw per
day, but after the recovery period only the testis weight was still
reduced. Postmortem examination revealed effects on the gastro-
intestinal tract and spleen in both sexes and in the liver, thymus and
testis of males at the highest dose level only. After 8 weeks only the
testis weights were still reduced. The erythroid/myeloid cell ratio
was increased in both sexes at doses of 65 or 100 mg/kg bw per day,
and after 8 weeks was still slightly higher than usual.
Treatment-related changes were seen in tissues with high cell
turnover rates; most of them recovered after a period of respite from
treatment. Animals in the groups treated with 30 and 45 mg/kg per day
showed no histopathological findings except hepatocytic reduced
basophilia (male, 45 mg/kg bw per day) and increased splenic extramed-
ullary haematopoiesis (female 45 mg/kg bw per day). Testicular
germinal epithelial deficit was present at doses above 45 mg/kg, and
caecal oedema and adnexal atrophy were present after the recovery
period at the highest dose level. The NOEL was 30 mg/kg bw per day
(Marubini et al., 1991).
In a 6-month toxicity study in the rat, thiamphenicol was
administered as an aqueous suspension in 2% gum arabic by stomach tube
6 days/week to 180 Wistar rats (30/sex/group) at dose levels of 0, 40
or 120 mg/kg bw per day. Body weight and food intake were recorded
twice weekly in the first 4 weeks of treatment, and thereafter only
once a week. At the end of weeks 4, 8, 16 and 24, ten animals from
each group were killed following collection of 2-hour urine sample.
Haematology, clinical chemistry assays and urinanalysis were performed
on all tested animals. Histological examinations were carried out on
the lungs, spermatozoa, blood and bone marrow smears and samples of
main organs. There was a decrease in food intake at a dose level of
120 mg/kg bw per day and a dose- and time-related decrease in body
weight gain in females. No effect was observed on erythropoiesis or on
hepatic or renal function. Urinanalysis showed the presence of albumin
and haemoglobin in the high-dose rats. No gross pathological
variations in organ weights were observed, but irritative changes in
the gastrointestinal mucosa and high incidence of monolateral
spontaneous hydronephrosis were seen both in treated and control
animals. Histopathological examination performed on control and
high-dose rats revealed a slight effect on the morphology of
spermatozoa in the high-dose group at the 8th, 16th and 24th week of
treatment (Della Bella et al., 1968b).
2.2.2.2 Rabbits
Thiamphenicol-glycinate was administered subcutaneously to groups
of rabbits ("Fauve de Bourgogne" seven/sex/group) at dose levels of
0, 25, 50 or 100 mg/kg bw per day, 6 days/week for 12 weeks. Animals
were weighed weekly and submitted to haematological (erythrocyte and
leukocyte counts with differentials) and biochemical (urea, reducing
sugars, chlorides) examinations before treatment, after 6 weeks and at
the end of the treatment period. After macroscopic examination of the
viscera, the various organs were examined histologically. During the
treatment two control animals and three in the high-dose group died
(no autopsies were carried out). A reduction in polymorphonuclear
neutrophils in male rabbits in all treated groups and a slight fall in
erythrocyte levels in high-dose females were observed. Anatomical and
pathological examination provided no evidence of damage attributable
to thiamphenicol-glycinate administration (Brunaud, 1965).
2.2.2.3 Dogs
In a 7-week study in beagle dogs (four/sex/group), thiamphenicol
was administered orally in gelatin capsules at dose levels of 0, 40 or
80 mg/kg bw per day. Two males and two female dogs were killed at the
end of the 7-week treatment, and the remaining animals were kept for
further 12 weeks without treatment before being killed. Behaviour and
body weights were recorded, haematology and clinical chemistry
examinations and urinanalysis were conducted on all animals pretest
and at various intervals during the study. Complete gross postmortem
examination, organ weighing and histopathological evaluation were
conducted on all animals. The animals in the two treated groups
developed diarrhoea soon after the beginning of treatment, which
spontaneously regressed in the low-dose group but persisted in the
high-dose group. At 80 mg/kg bw per day reduced food consumption with
loss of weight and muscular asthenia occurred, and vomiting was
observed in some cases. Four of these dogs were killed at the end of
the 4th week. Slight loss of weight was observed in two dogs in the
low-dose group.
Decreases in haematocrit, haemoglobin concentration and
erythrocyte count were seen in both treated groups, but did not appear
to be dose-related, and returned to normal on withdrawal of treatment.
Slight increase in proteinuria was observed in the last few weeks of
treatment. At 40 mg/kg bw per day superficial erosion of the gall
bladder mucosa and at 80 mg/kg bw per day haemorrhagic ulcers in the
gall bladder, diffuse muco-membranous enteritis and thymic involution
were seen in dogs killed at the end of treatment period. In dogs
killed after the 12-week recovery period no differences were observed
between treated and control animals. Histological examination after 7
weeks revealed in the high-dose group severe cholecystitis, chronic
sclerosing pancreatitis, enteritis, severe depletion of haematopoietic
marrow and lymphoid thymus depletion. Two dogs in the low-dose group
showed depletion of germinal epithelium in the testes and multinuc-
leated cells in seminiferous tubules, which were not seen in the high-
dose group. None of the changes observed at 7 weeks were detectable
in the animals kept for 12 weeks after cessation of treatment
(Bonanomi et al., 1978).
Thiamphenicol was administered orally in gelatin capsules to 24
beagle dogs (four/sex/group) at dose levels of 30, 60 or 120 mg/kg bw
per day for a period of 4 weeks. Physical observations, ophthalmo-
scopic examinations, and body weight and food consumption measurements
were performed before treatment and over selected intervals during the
treatment. Haematology, clinical chemistry and urinanalysis were
conducted on all animals pretest and at study termination. Complete
gross postmortem examinations, organ weight and histopathological
evaluation were conducted on all animals. The body weights of the
high-dose animals were slightly lower than those of controls at week 3
in both sexes, and at week 4 in males only. At 60 and 120 mg/kg bw per
day absolute and relative liver weights in male dogs were greater than
in controls, and relative liver weights were also increased in
females. Microscopically, hepatocellular hypertrophy was present in
the liver of mid- and high-dose animals, which correlated with the
increase in liver weights in these groups. No other parameter
evaluated showed evidence of adverse treatment-related effects. The
NOEL was 30 mg/kg bw per day (Kelly & Daly, 1990).
Thiamphenicol was administered orally in gelatin capsules to 56
beagle dogs (seven/sex/group) at dose levels of 15, 30 or 60 mg/kg bw
per day. After 6 months of treatment four animals/sex/group were
sacrificed, and the remaining three animals/sex/group were kept for a
2-month recovery period. Physical observations, ophthalmoscopic
examinations, body weight, food consumption, haematology and clinical
chemistry examinations and urinanalysis were conducted on all animals
pretest and on all surviving animals at selected intervals during the
treatment and recovery period. Complete gross postmortem examinations,
organ weight and histopathological evaluation were conducted on all
animals. One control male was found dead during the study. One male
and one female dog in the highest dose group were moribund and had to
be killed. Clinical signs prior to death included lethargy, poor food
consumption, emaciation, tremors and dehydration. Physical findings
related to thiamphenicol administration were tremors, lethargy,
irregular gait and excessive licking or chewing in the high-dose
group. Tremors were also present in the mid-dose animals. These signs
were seen during the last two months of the study and were not present
at the end of the recovery period. Body weights of the high-dose males
during the study were 4 to 18% lower than those of controls. Decreased
erythrocyte counts and mean haematocrit values were seen in high-dose
males at weeks 6 and 13 and at termination of the study, and in high-
and mid-dose females at week 13 and at termination of the study. After
the recovery period no differences were observed in the haematological
parameters between control and treated animals. No treatment-related
effects were seen in the bone marrow smear examinations. Mean serum
cholesterol and phospholipid levels of the mid-and high-dose males at
week 6 and 13 and at termination of the study were greater than
control values. The same parameters were elevated in high-dose females
at the end of the study. Mean serum glucose levels of males at
60 mg/kg bw per day and females at 30 and 60 mg/kg bw per day were
significantly increased. Mean fibrinogen values of high-dose females
were elevated at the end of the study. Relative liver weights were
increased at mid- and high-dose levels. Pathological lesions related
to treatment were seen in sections of the thymus (exacerbation of
involution), bone marrow (decreased cellularity), liver (centrilobular
necrosis and pigment deposition), testes (focal and diffuse tubular
atrophy) and oesophagus (ulceration) from high-dose animals of both
sexes, mostly occurring in animals killed in a moribund condition. No
alterations were noted in any of the tissues examined microscopically
from animals that were allowed to recover after treatment. The NOEL
was 15 mg/kg bw per day (Kelly & Daly, 1991).
2.2.2.4 Pigs
A study designed to determine tolerance in pigs to treatment with
thiamphenicol at three times the recommended dose for 5 days and at
the normal recommended dose for 15 days was conducted with 16 weaned
large white hybrid pigs (two pigs/sex/group). Thiamphenicol was
administered in the diet at dose levels of 30 or 90 mg/kg bw per day
for 5 days or 30 mg/kg bw per day for 15 days. The control group of
animals was fed basal diet only. Clinical signs, body weight and food
consumption were recorded. Blood, urine and faecal samples were
obtained before dosing and at various time-points during the study. No
significant treatment-related clinical abnormalities were noted. Body
weight changes and food consumption were within normal limits. No
consistent treatment-related differences in haematological and
biochemical parameters or in urinanalysis values were observed. The
authors concluded that treatment with thiamphenicol had no significant
adverse effects on general health, body weight, food consumption or
standard clinical pathology parameters (Roberts et al., 1989).
In a 4-week toxicity study, groups of pigs (Large White hybrid,
four/sex/group) were fed 25, 50 or 100 mg thiamphenicol/kg bw per day.
The control group of animals was fed basal diet only. Clinical signs,
body weight and food consumption were recorded. Blood, urine and
faecal samples were obtained before dosing and during week 4 for
clinical pathological investigations. At the end of the 4-week dosing
period, pigs were killed, and selected tissues were processed for
histological examination. In all groups treated with thiamphenicol,
swelling and erythema of the anus, vulva/testes and perineal area,
tail and hocks were observed on the second day of the dosing period.
These effects were consistent with scouring and irritancy, e.g., as a
result of disruption of normal gastrointestinal flora activity, and
disappeared within 1 to 13 days. All pigs remained in good health
thereafter. Slight reductions in body weight gain and food consumption
were noted at 50 and 100 mg/kg bw per day. At week 4 there was a
slight reduction in mean PCV, haemoglobin concentration and
erythrocyte counts in animals receiving 100 mg/kg bw per day and a
treatment-related reduction of urinary pH in all groups dosed with
thiamphenicol. At the end of the study, increases in liver and kidney
weights in pigs fed 50 and 100 mg/kg bw per day were observed. On
histological examination, treatment-related changes were found in the
highest dose group only: an increase in vacuolation and fat in renal
tubular epithelium and, in some animals, minimal diffuse hepatocyte
vacuolation and hepatocyte fat (Cameron et al., 1990).
2.2.3 Long-term toxicity/carcinogenicity study
2.2.3.1 Rats
As a range-finding study for the dose selection in a 2-year
carcinogenicity study, four groups of F-344 rats (10/sex/group)
were given drinking-water containing 0, 125, 250 or 500 mg/litre
thiamphenicol (equal to 9, 17 or 36 mg/kg bw per day for males and 12,
29 or 39 mg/kg bw per day for females) for 13 weeks. The examinations
at the end of the study covered clinical observations, water
consumption, body weight changes, haematological parameters, serum
biochemistry, organ weights and gross and microscopic appearance. In
haematological examinations anaemic changes were observed in males
treated with 250 mg/litre or more and high-dose females. Similar
changes, such as increased MCV and increased related counts, were
observed in males of the 125 mg/litre group and females of the 250 and
125 mg/litre groups. At autopsy, enlargement of the caecum was seen in
treated groups of both sexes. Histologically, the highest dose animals
showed decreased haematopoiesis of the bone marrow, decreased spermato-
genesis of the testis and sperm granulomas of the epididymis. Sperm
granulomas in the epididymis were also seen in some of the animals in
the 250 mg/litre group. Based on the results of this pilot study, a
2-year carcinogenicity study of thiamphenicol was performed in rats.
Three groups of F-344 rats (50/sex/group) were given drinking-water
containing 0, 125 or 250 mg thiamphenicol/litre (equal to 8 or 16
mg/kg bw per day for males and 9.7 or 19 mg/kg bw per day for females)
for 104 weeks. All surviving animals subjected to 4-week withdrawal of
the test chemical after the end of the treatment were killed for full
histopathological examinations. The high-dose animals showed decreased
body weight gain, but the incidence of rumours in treated groups was
not significantly higher than that of controls (Maekawa, 1996; summary
report only was available).
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
In a fertility study on male Wistar rats, oral treatment with
thiamphenicol for 2 or 3 months (30 animals per dose level, 10 per
treatment time), at dose levels of 120, 180 or 240 mg/kg bw per day,
resulted in reduction in the number of tubular germinal cells, which
was more marked at the highest dose level. Ten animals of each group
were treated for 4 weeks, ten for 8 weeks and the last ten for 12
weeks. At the end of each test period 5 animals of each dose group
were killed and necropsied, while the remaining 5 rats were mated with
normal females. At 240 mg/kg bw per day extensive testicular
hypotrophy, together with severe depletion of the germinal epithelium
21 days after withdrawal of treatment, was seen. Histological changes
coincided with a reduction of the fertility index, which gradually
recovered within 50 days. Litters from matings between treated males
and normal females were normal in number and weight, and no morpho-
logical abnormalities were observed. The concentration ratio of
thiamphenicol between testes and plasma after administration of
240 mg/kg bw per day thiamphenicol was 1, indicating the absence of
accumulation in testes (Della Bella et al., 1967).
Groups of 21 Sprague-Dawley rats were given thiamphenicol orally
(30, 60 or 120 mg/kg bw per day daily) from day 15 of gestation to day
21 postpartum. In groups receiving 60 and 120 mg/kg bw per day a
higher post-implantation loss, slight weight reduction at birth and
increased rate of perinatal mortality were observed. No malformations
were observed. Development of pups was inhibited during the lactation
period with a consistent dose-dependent relationship. From day 30
postpartum a good recovery was observed in all groups. Sexual
behaviour and fertility of F1 animals were normal, and the F2
generation showed no signs of abnormal development (Bonanomi et
al., 1980).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Teratogenicity studies were carried out on 195 mature female
Wistar rats (15/group) given thiamphenicol orally at dose levels of
40, 80 or 160 mg/kg bw per day from days 1 to 21 of pregnancy and 80
or 960 mg/kg bw per day from days 1 to 7, 7 to 14 or 14 to 21 of
gestation. Thiamphenicol did not induce any teratogenic effects in any
of the four studies carried out. When the treatment period was 1-21
days, a dose-related increase in resorptions was noted, and newborns
had a high mortality rate in the second and third week of life,
particularly in the 40 mg/kg bw per day group. In rats treated on days
1-7 of gestation, a non-dose-related increase in resorptions and an
increased mortality in newborns in the third week after birth were
observed. When the treatment period was from 7 to 14 days, complete
resorption of fetuses occurred at 160 mg/kg bw per day. The mean
number of newborns per litter was reduced at 80 mg/kg bw per day and
there was a high mortality of newborns in the first week. An increased
mortality among newborns of the group treated with 80 mg/kg bw per day
during days 14-21 of gestation was observed (Bonanomi & De Paoli,
1969).
When the inhibition of mitochondrial functions induced by
thiamphenicol was compared with the inhibition of overall embryonic
development, it appeared that mitochondrial respiration was the
rate-limiting step for the embryotoxic effects of thiamphenicol.
Because of the lack of specificity of these effects, prenatal
mortality rather than teratogenic effects was seen (Bass et al.,
1978).
2.2.5.2 Rabbits
A teratogenicity study was performed with 50 New Zealand white
rabbits administered thiamphenicol orally at dose levels of 5, 30, 60
or 80 mg/kg bw per day from the 8th to the 16th day of pregnancy. The
highest dose resulted in a complete resorption of implantation due to
the toxic effects on the mothers. Data obtained in all treated groups
showed moderate fetal toxicity with a dose-related increase in
abortion rate and resorption. No skeletal malformations were found in
fetuses (Bonanomi et al., 1974).
Thiamphenicol in 0.5% Methocel K15M was administered daily by
oral gavage to female rabbits (16/group) from day 6 to day 18 of
gestation at doses of 1.25, 2.5 or 5.0 mg/kg bw per day. Control
animals received the vehicle alone. The females were killed on
gestation day 29 and subjected to postmortem examination. All fetuses
were examined for external and internal abnormalities and skeletal
changes. No clinical signs attributable to treatment were observed.
Mild signs of maternal toxicity were observed in mid- and high-dose
animals, indicated by reduction in body weight changes during the
treatment period.
Necropsy findings in females on gestation day 29 were incidental,
with no relation to treatment. Litter parameters and sex ratios did
not show any significant difference between groups. Mean fetal weight
was decreased in the high-dose group. Two fetuses in the mid-dose
group and one in the control group were malformed, with cleft palate,
abnormally shaped head, extra digits and incomplete flexure of the
hind limbs. The number of small fetuses in the high-dose group was
higher than in the control group. The few anomalies seen during the
internal examination were not considered to be treatment-related.
Skeletal examination revealed no differences between high-dose fetuses
and controls. The authors concluded that thiamphenicol administered by
oral gavage at concentrations of 1.25, 2.5 and 5 mg/kg per day had no
effects on pregnancy or embryo-fetal development. However, the
Committee concluded that the NOEL for embryotoxicity under the
conditions of this experiment was 1.25 mg/kg per day (Sisti, 1994).
2.2.6 Special studies on genotoxicity
The results of genotoxicity assays on thiamphenicol are given in
Table 2.
Table 2. Genotoxicity assays on thiamphenicol
Test system Test object Concentration Results Reference
Ames test1 S. typhimurium 0.5-50 Negative Pinasi
TA98, TA100, µg/plate2 et al., 1990a
TA1535, TA1537,
TA1538
Gene conversion Saccharomyces 2.8-140.3 mM3 Negative Marca &
and mitotic cerevisiae Bonanomi,
crossing over1 1979
Gene mutation Chinese hamster 50-5000 Negative Pinasi
assay1 V79 cells µg/ml4 et al., 1990b
Chromosomal Cultured human 700-3250 Negative Mosesso &
aberrations1 lymphocytes µg/ml5 Driedger, 1989
DNA repair Primary rat 500 and 1000 Negative Bichet, 1985
test hepatocytes µg/kg6
In vivo Mouse bone 2500 and 5000 Negative Pinasi
micronucleus marrow mg/kg7 et al., 1990c
assay
1 Both with and without rat liver S9 fraction
2 Methyl methanesulfonate and cyclophosphamide were used as positive controls
3 2-Nitrofluorene, 9-aminoacridine, sodium azide and 2-aminoanthracene were used
as positive controls
4 Ethyl ethanesulfonate and N-dimethylnitrosoamine were used as positive controls
5 Mitomycin-C and cyclophosphamide were used as positive controls
6 2-Aminofluorene was used as positive control
7 Cyclophosphamide was used as positive control
2.2.7 Special studies on immune responses
The effects on spontaneous nephritis in NZB × OUW hybrid mice
(32-39 males/group) were investigated in a study involving lifetime
administration of thiamphenicol in feed at dose levels of 25, 50 and
250 mg/kg bw per day. Body weight, urinary protein, limited
haematology, organ weights and histopathology investigations were
reported. The prolonged treatment with thiamphenicol at dose rates of
> 50 mg/kg reduced the severity of the spontaneous renal disease and
significantly extended lifespan, compared to untreated controls. The
immunosuppressive effect of thiamphenicol was demonstrated
histologically by a reduction in immune-complex deposition in the
glomeruli. No evidence of malignancy or premalignant signs was seen
(Simpson et al., 1979).
2.2.8 Special studies on microbiological effects
2.2.8.1 In vivo
In a study of thiamphenicol-induced changes in mouse intestinal
microflora, 100 female albino mice were divided into 10 subgroups, and
five of these groups were treated with thiamphenicol at concentrations
of 40 µg/kg diet for 35 days. Samples of intestinal content were taken
from the caecum for bacteriological analysis before the treatment and
after 7, 14, 28 and 35 days. Microorganisms were isolated by preparing
serial dilutions of intestinal content, and the most representative
bacteria of the mouse intestinal microflora were cultured on specific
media. Their sensitivity to thiamphenicol was assessed by calculating
the minimal inhibitory concentration (MIC) of the drug. The results
show that the mean counts of various microorganisms did not differ
significantly between control mice and those fed with thiamphenicol.
The numbers of the bacterial populations did vary at the different
sampling times, and in some cases the difference from pre-treatment
results was significant, but confidence limits were the same in
treated and control mice killed at the same time. The investigation
shows that there were no appreciable differences in the type or amount
of bacterial flora related to thiamphenicol administration.
Differences in the distribution of genera such as Diplococcus sp.
and Escherichia sp. were similar to those in controls. The
thiamphenicol MIC values for the numerous strains tested indicate that
addition to feed at concentrations corresponding to the proposed MRL
of 40 µg/kg feed does not select for drug-resistant strains and has no
effect on the qualitative or quantitative composition of the
intestinal microflora. The MIC remained unchanged throughout the 35
days of the study (Poli, 1994).
2.2.8.2 In vitro
In vitro antibacterial activity of thiamphenicol against 489
bacterial isolates from infected animals was determined by the agar
dilution method. In the case of mycoplasmas, however, MICs were
determined by the broth dilution method. Depending on bacterial
strains tested estimations were made under aerobic or anaerobic
conditions. The results are presented in Table 3 (Albini, 1989).
Data on the sensitivity of normal components of human intestinal
microflora are presented in Table 4 (Sutter & Finegold, 1976;
Schioppacassi, 1992).
Table 3. Antibacterial activity of thiamphenicol against 489 animal pathogens
Organisms Number of MIC (µg/ml) Range
isolates
MIC50 MIC90
Bacteroides spp. 11 2 16 1 - 128
Bordetella spp. 9 32 32 16 - 32
Campylobacter spp. 17 8 16 4 - 16
Clostridium spp. 37 2 4 0.25 - 16
Corynebacterium spp. 10 2 16 2 - 16
Escherichia coli 61 128 >128 16 - > 128
Haemophilus
pleuropneumoniae 7 0.5 1 0.5 - 1
Micrococcus spp. 6 0.5 0.5 0.5 - 8
Mycoplasma spp. 9 1 2 0.125 - 4
Pasteurella spp. 71 1 2 0.25 - 128
Salmonella spp. 34 32 32 8 - > 128
Staphylococcus spp. 94 8 32 4 - > 128
Streptococcus spp. 123 2 4 0.5 - > 128
2.3 Observations in humans
Reversible dose-related bone marrow suppression is seen after
thiamphenicol treatment and is attributed to its inhibitory effect on
mitochondrial protein synthesis (Nijhof & Kroon, 1974). Reversible
inhibition of myeloid and erythroid colony growth by thiamphenicol,
resulting from an inhibition of mitochondrial protein synthesis, is
consistent with the reversible bone marrow suppression induced by this
drug (Yunis & Gross, 1975).
A study of clinical reports on the use of thiamphenicol in 16 631
cases from 1968 to 1977 in Japan revealed that blood disorders
occurred in 41 (0.46%) out of 8848 patients receiving thiamphenicol
glycinate therapy and 28 (0.36%) out of 7783 patients receiving
thiamphenicol. The disorders were dose-dependent, occurring mainly in
erythrocytes, and disappeared spontaneously on discontinuation of the
drug (Tomoeda & Yamamoto, 1981).
The para-nitro group of chloramphenicol has been shown to have
a central role in the pathogenesis of aplastic anaemia, probably as a
result of its reduction to the highly toxic nitroso metabolite, which
is a potent inhibitor of DNA synthesis. Thiamphenicol does not show
this activity and the absence of the para-nitro group is therefore
advanced as evidence that thiamphenicol cannot induce aplastic anaemia
(Murray et al., 1983).
Thiamphenicol has been used extensively in human medicine
for over 25 years. Total human exposure to thiamphenicol up to
1987 has been estimated at 130-650 million exposures, assuming an
average course of therapy of 7.5-15 g (Personal communication from
Dr S. Biressi, Zambon Group SpA, Italy to Dr R.D. Agostino,
Farmaquest Co.; submitted to WHO by Zambon Group SpA, Italy).
Epidemiological studies have not established any causal
association between thiamphenicol treatment and irreversible aplastic
anaemia (TAP Pharmaceuticals Inc., 1987). Statistical analysis of
data obtained in these studies support the argument that the risk of
aplastic anaemia from exposure to thiamphenicol is similar to the
background risk of idiopathic aplastic anaemia (from 1 in 200 000 to 1
in 800 000) (Kelly & Kaufman, 1989).
Table 4. Antibacterial activity of thiamphenicol against 261 strains of anaerobic bacteria isolated from humans
(From: Sutter & Finegold, 1976)
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Bacteroides fragilis 42 5 21 71 100
Bacteroides 59 9 24 51 90 100
melaninogenicus
Other Bacteroides 21 19 24 57 76 86 91 95 100
and Selenomonas
Fusobacterium 8 100
nucleatum
Other Fusobacterium 12 42 92 100
Peptococcus and 17 35 71 100
Gaffkya
Peptostreptococcus 15 33 47 93 100
Table 4. (cont'd).
Bacteria No. of Cumulative % susceptible to indicated concentration (µg/ml)
strains
tested
<0.1 0.5 1.0 2.0 4.0 8.0 16.0 32.0 64.0 128.0
Anaerobic and 6 50 83 100
microaerophilic
streptococci
Gram-negative cocci 7 43 86 100
Eubacterium 7 43 57 100
Arachnia propionica 2 50 100
Propionibacterium 4 50 75 100
Actinomyces 16 25 56 94 100
Lactobacillus 10 10 30 50 90 100
Clostridium 8 100
perfringens
Other Clostridium 27 4 22 63 78 96 100
3. COMMENTS
Studies on thiamphenicol available for evaluation included data
on pharmacokinetics, acute toxicity, short-term toxicity, reproductive
toxicity, developmental toxicology, genotoxicity, and limited
information on long-term toxicity. Studies on microbiological effects
of thiamphenicol and epidemiological data on humans were also
considered by the Committee. The Committee noted that many of the
studies were conducted utilizing protocols that would not meet
contemporary standards, and therefore the substance was evaluated
under the procedures developed for drugs with a long history of use
(Annex 1, reference 104).
The pharmacokinetic data showed that the drug is rapidly absorbed
when administered by oral or parenteral routes. After intravenous
administration in rats, the half-life was estimated to be 46 minutes.
The main route of excretion in humans and animals is in the urine;
approximately 60% of an oral dose of 30 mg/kg bw was excreted
unchanged in the urine over a 24-hour period.
Single oral doses of thiamphenicol were of low toxicity to mice
and rats (LD50 > 3000 mg/kg bw).
Short-term oral toxicity studies with thiamphenicol were
performed in rats, dogs and pigs, the results are described in the
following paragraphs.
In a 13-week study in rats at dose levels of 30, 45, 65 or
100 mg/kg bw per day, increased mortality was observed among animals
given 100 mg/kg bw per day. In a 6-month study in rats, where the
highest dose used was 120 mg/kg bw per day, increased mortality was
not reported. In both studies, decrease in body weight gain during
treatment occurred at doses of > 65 mg/kg bw per day. Dose-related
decreases in red blood cell parameters, differential and total white
blood cell counts, and clotting parameters were observed in the
13-week study, but the same effects were not reported in the 6-month
study. Testicular germinal epithelial cell depletion was seen at doses
above 45 mg/kg bw per day in the 13-week study, and a dose of 30 mg/kg
bw per day was considered to be the NOEL.
Dogs were given 40 or 80 mg thiamphenicol/kg bw per day for 7
weeks. At both dose levels decreases in body weight were observed, as
well as reversible decreases in haematocrit, haemoglobin concentration
and erythrocyte count. At 40 mg/kg bw per day, superficial erosion of
the gall bladder mucosa was observed. The higher dose level resulted
in haemorrhagic ulcers in the gall bladder, diffuse mucomembranous
enteritis and early thymic involution. Two dogs in the low-dose group
had testicular germinal epithelial cell depletion and multinucleated
cells in the seminiferous tubules.
When thiamphenicol was given to dogs at doses of 30, 60 or
120 mg/kg bw per day for 4 weeks, the body weights of the high-dose
animals were slightly lower than those of controls. In the mid- and
high-dose groups, increases in absolute and relative liver weights
were observed. Hepatocellular hypertrophy was present in the liver of
dogs given 60 or 120 mg/kg bw per day.
In a 6-month study dogs were given thiamphenicol at doses of 15,
30 or 60 mg/kg bw per day. The body weights of high-dose males during
the study were up to 18% lower than those of controls. The main
haematological findings were decreases in red blood cell parameters at
the highest dose level. Increases were noted in mean serum cholesterol
level and phospholipid concentrations in males (30 and 60 mg/kg bw per
day groups) and females (60 mg/kg bw per day group), and in the mean
serum glucose concentration of males (60 mg/kg bw per day group) and
females (30 and 60 mg/kg bw per day groups). The relative liver
weights at the mid- and high-dose levels were increased. Histopatho-
logical lesions related to treatment were seen in the thymus (early
involution), bone marrow (decreased cellularity), testes (focal and
diffuse tubular atrophy) and oesophagus (ulceration) of high-dose
animals. The NOEL was 15 mg/kg bw per day.
In a 4-week study, pigs were treated with 25, 50 or 100 mg
thiamphenicol/kg bw per day. In the highest-dose group, slight
reduction in body weight gain, as well as reductions in mean packed
cell volume, haemoglobin concentration and erythrocyte counts, were
observed, and histological examination showed vacuolation in renal
tubular epithelial cells and mild diffuse hepatocyte vacuolation. In
all treated groups treatment-related reduction in urine pH was
observed.
A summary report of a two-year carcinogenicity study in rats,
including a range-finding study, was available to the Committee. Rats
were given 125 or 250 mg/kg thiamphenicol in drinking-water (equal to
8 or 16 mg/kg bw per day for males and 10 or 19 mg/kg bw per day for
females) for 104 weeks. The highest-dose animals showed a decrease in
body weight gain, but there was no significant increase in the
incidence of tumours in treated groups compared to control animals.
In a long-term study in mice (32-39 males/group), which was
designed primarily to investigate the effects of thiamphenicol on
immune responses, thiamphenicol was administered orally at doses of
25, 50 or 250 mg/kg bw per day. No evidence of neoplastic or
preneoplastic changes was observed.
In a study to determine the effect of thiamphenicol on fertility
in rats, the drug was administered orally at doses of 120, 180 or
240 mg/kg bw per day for 2 or 3 months. Thirty male rats were used per
dose level (10 per treatment period). From each treatment-period
group, half of the animals were killed for histopathological
examination at the given time intervals, while the remaining males
were mated with untreated females. Reductions in the number of
germinal epithelial cells in testes of all treated animals were
observed. These changes were present up to 21 days after termination
of treatment, and full recovery was observed by 50 days. Histological
changes correlated with the fertility index. Litters from matings
between treated males and non-treated females were normal in number
and no physical abnormalities were reported.
Thiamphenicol was given orally to rats from day 15 of gestation
to day 21 post-partum at doses of 30, 60 or 120 mg/kg bw per day. In
the mid-and high-dose groups, there were higher post-implantation
losses and increased rates of perinatal mortality. Physical develop-
ment of pups was inhibited during the lactation period in a dose-
dependent manner. Sexual behaviour and fertility of F1 animals
were normal, and animals in the F2 generation showed no
abnormalities.
In four teratogenicity studies in rats, thiamphenicol was
administered orally at dosages of 40, 80 or 160 mg/kg bw per day from
days 1 to 21 of pregnancy or of 80 or 960 mg/kg bw per day over
critical days of gestation (either 1-7, 1-21, 7-14 or 14-21). No
teratogenic effects were observed. In all animals treated from days 1
to 21, a dose-related increase in resorption was noted and newborn
pups had an elevated mortality rate.
A teratogenicity study was performed in rabbits using oral doses
of 5, 30, 60 or 80 mg/kg bw per day from days 8 to 16 of gestation.
Complete resorption of embryos occurred at 80 mg/kg bw per day. In
other treated groups, moderate fetal toxicity and dose-related
increases in abortion rate and resorption were reported. No
malformations were found in fetuses.
In another teratogenicity study, rabbits received oral doses of
thiamphenicol at doses of 1.25, 2.5 or 5 mg/kg bw per day from days 6
to 18 of gestation. Mild maternal toxicity was observed in mid- and
high-dose animals in the form of depressed body weights during the
treatment. No effects were observed on embryo-fetal development. The
NOEL was 1.25 mg/kg bw per day.
Thiamphenicol gave negative results in five in vitro
genotoxicity tests and in an in vivo micronucleus assay using mouse
bone marrow.
The Committee considered data from human epidemiological studies
and concluded that there was no evidence that thiamphenicol can induce
aplastic anaemia, in contrast to the structurally related compound,
chloramphenicol.
The Committee considered data from several in vitro studies on
the minimum inhibitory concentration (MIC) of thiamphenicol for a wide
range of animal and human pathogens as well as genera representative
of the human gut flora. The modal MIC50 value (minimum inhibitory
concentration of thiamphenicol giving complete inhibition of growth of
50% of cultures) was 1.68 µg/ml for 261 bacterial strains isolated
from humans. The following species were found to be the most
sensitive: Bacteroides, Fusobacteria, Propionibacteria and
Actinomyces. The Committee also noted that 40 µg thiamphenicol/kg
food given to mice over 35 days did not alter the intestinal
microflora in this species.
The Committee calculated a microbiological ADI for thiamphenicol
using the following formula:
Upper limit of MIC50 (µg/g) × mass of colonic content (g)
microbiological =
ADI fraction of oral × safety × human body
dose available factor weight (kg)
1.68 × 220
=
0.4 × 1 × 60
= 15 µg/kg bw
In calculating a microbiological ADI the Committee took the
following factors into consideration:
* Concentration: 1.68 µg/ml was the modal MIC50 for micro-
biological effects on human intestinal microflora (the density
was assumed to be 1 g/ml).
* Availability: the Committee calculated the available portion of
thiamphenicol as follows:
100% ingested - 60% excreted via = 40% bioavailable
urine within in the intestinal
24 hours tract
1 - 0.6 = 0.4
* Safety factor: the Committee concluded that the data deriving
from the microbiological studies (substantial amount of MIC data
covering a variety of microorganisms and in vivo data from
animal studies) provided sufficient information on microbio-
logical effects of thiamphenicol. It therefore adopted a safety
factor of 1 in the calculation.
4. EVALUATION
Taking into account the available toxicological and antimicrobial
data and the ADI based on antimicrobial activity, the Committee
concluded that the toxicological data provided the most appropriate
end-point for the evaluation of thiamphenicol. The Committee
established a temporary ADI of 0-6 µg/kg bw for thiamphenicol, based
on the NOEL of 1.25 mg/kg bw per day for maternal toxicity in the
teratogenicity study in rabbits and a safety factor of 200. The ADI
was designated "temporary" because only a summary report of the
carcinogenicity study in rats was available. Detailed reports of the
carcinogenicity study and the range-finding study used to establish
dose levels in that study are required for evaluation in 1999
(see Annex 4).
5. REFERENCES
Albini, E. (1989). In vitro antibacterial activity of thiamphenicol
against bacterial strains recently isolated from animal infectious
diseases. Unpublished report from Microbiology Laboratory, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Azzolini, F., Gazzaniga, A., Lodola, E., & Natangelo, R. (1972).
Elimination of chloramphenicol and thiamphenicol in subjects with
cirrhosis of the liver. Int. J. Clin. Pharmacol., 6, 130-134.
Bass, R., Oerter, D., Krowke, K., & Spielman H. (1978). Embryonic
development and mitochondrial function. III. Inhibition of respiration
and ATP generation in rat embryos by thiamphenicol. Teratology,
18, 93-102.
Bichet, N. (1985). Etude de génotoxicité éventuelle sur les
hépatocytes de rat par mesure de réparation du DNA. Unpublished report
No. 01147F from Sanofi Recherche, Montpellier, France. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. (1978). Acute toxicity study in rats and mice with
thiamphenicol and thiamphenicol glycinate HCl. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L. & De Paoli, A.M. (1969). Teratogenicity study of oral
thiamphenicol in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, Pacei, E., & De Paoli, A.M. (1974). Teratogenicity study
of oral thiamphenicol in the rabbit. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Bonanomi, L, De Paoli, A.M., & Gazzaniga, A. (1978). Toxicity study
after repeated oral administration of thiamphenicol in the dog.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Bonanomi, L., Aliverti, V., Ornaghi, F., Losa, M., & Motta, F. (1980).
Thiamphenicol perinatal and postnatal toxicity study in the rat.
Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Brunaud, M. (1965). Thiamphenicol glycinate hydrochloride. Long-term
toxicity in the rabbit by subcutaneous route. Unpublished report from
Clin-Byla Laboratories, Paris, France. Submitted to WHO by Zambon
Group SpA, Bresso, Milan, Italy.
Cameron, D.M., Crook, D., Brown G., Gopinath, C., Farmer, H., & Offer,
J.M. (1990). Z 2041: 4-Week toxicity study in pigs. Unpublished report
No. ZBN 12/891518 from Huntingdon Research Centre, Huntingdon,
England. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A. (1967).
Fertility study in male rat treated with thiamphenicol by the oral
route. Unpublished report from Toxicology Department, Zambon Research,
Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Della Bella, D., Ferrari, V., Marca, G., & Bonanomi, L. (1968a).
Chloramphenicol metabolism in the phenobarbital induced rat.
Comparison with thiamphenicol. Biochem. Pharmacol., 17, 2381-2390.
Della Bella, D., Bonanomi, L., De Paoli, A.M., & Gazzaniga, A.
(1968b). Toxicity study of thiamphenicol after repeated oral
administration in the rat. Unpublished report from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Ferrari, V. & Della Bella, D. (1974). Comparison of chloramphenicol
and thiamphenicol metabolism. Postgrad. Med. J., 50, 17-22.
Gazzaniga, A. (1974). Thiamphenicol - pharmacokinetics and metabolism
in animals. Unpublished report from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, C.M. & Daly I.W. (1990). A four week oral toxicity study in the
dog via capsule administration with Z 2041. Unpublished report
No. 88-3391 from Bio/dynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Kelly, C.M. & Daly, I.W. (1991). A six month oral toxicity study in
the dog via capsule administration with Z 2041 with a two month
recovery period. Unpublished report No. 88-3392 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Kelly, J.P. & Kaufman, D.W. (1989). Ant-infective drug use in relation
to the risk of agranulocytosis and aplastic anemia: A report from the
international agranulocytosis and aplastic anemia study.
Arch. Intern. Med., 149, 1036-1040.
Laplassote, J. (1962). Recherches expérimentales sur le thiopénicol:
activité antibactérienne, concentrations humorales, élimination.
Comparaison avec le chloramphénicol. Therapie, 16, 104-108.
Marca, G. & Bonanomi, L. (1979). Gene conversion and mitotic
crossing-over in Saccharomyces cerevisiae 6117 with thiamphenicol
with and without metabolic activation. Unpublished report from
Toxicology Department, Zambon Research, Bresso, Milan, Italy.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Maekawa, A. (1996). Summary report on two-year rat carcinogenicity
study of thiamphenicol. Submitted by Sasaki Institute, Tokyo, Japan to
the Japanese Ministry of Health and Welfare.
Marubini, M., Motta, F., Cerioli, A., & Finn, J.P. (1991). Z 2041.
Thirteen week oral toxicity study in rats followed by a recovery
period of 8 weeks. Unpublished report No. 1219 from Toxicology
Department, Zambon Research, Bresso, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Mosesso, P. & Driedger, A. (1989). Chromosome abberations in human
lymphocytes cultured in vivo. Unpublished report No. LSC-RTC
116028-M-02989 from Life Science Research Roma Toxicology Centre SpA,
Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Murray, T.R., Downey, K.M., & Yunis, A.A. (1983).
Chloramphenicol-mediated DNA damage and its possible role in the
inhibitory effects of chloramphenicol on DNA synthesis.
J. Lab. Clin. Med., 102, 926-932.
Nijhof, W. & Kroon, A.M. (1974). The interference of chloramphenicol
and thiamphenicol with the biogenesis of mitochondria in animal
tissues. A possible clue to the toxic action. Postgr. Med. J.,
50 (Suppl. 5), 53-59.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990a). Mutagenicity
evaluation of Z 2041 in the Ames Salmonella-microsome plate test.
Unpublished report No. 1231 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990b). Mutagenicity
evaluation of Z 2041 in Chinese hamster V79 test. Unpublished report
No. 1243 from Toxicology Department, Zambon Research, Bresso, Milan,
Italy. Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Pinasi, C., Ferrini, S., & Ceriani, D. (1990c). Genotoxicity
evaluation of Z 2041 in the "in vivo" mouse micronucleus assay.
Unpublished report No. 1233 from Toxicology Department, Zambon
Research, Bresso, Milan, Italy. Submitted to WHO by Zambon Group SpA,
Bresso, Milan, Italy.
Poli, G. (1994). Qualitative and quantitative changes in the mouse
intestinal microflora induced by chronic thiamphenicol at maximum
residual level (MRL) dose. Unpublished report from Facolta di Medicina
Vetarinaria, Universita di Milano, Milan, Italy. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Redgrave, V.A., Cameron, D.M., Anderson, A., & Maxwell, J.G. (1991).
Blood concentrations of thiamphenicol in pigs following dietary
administration of Z 2041. Unpublished report No. ZBN 11/901351 from
Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by
Zambon Group SpA, Bresso, Milan, Italy.
Roberts, N.L., Cameron, D.M., Redgrave, V.A., Crook, D., & Brown G.
(1989). Z 2041. Tolerance in pigs. Unpublished report No. ZBN 10/89188
from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO
by Zambon Group SpA, Bresso, Milan, Italy.
Schioppacassi, G. (1992). Activity of thiamphenicol on indigenous
microorganisms of gastrointestinal tract. Unpublished report
No. DBI 1/92 from Zambon Research, Bresso, Milan, Italy. Submitted to
WHO by Zambon Group SpA, Bresso, Milan, Italy.
Simpson, L.O., Aarons, I., & Howie, J.B. (1979). Thiamphenicol and
lupus nephritis. The effects of long-term therapy on kidney function
and pathology: a pilot study. Br. J. Exp. Pathol., 60, 45-57.
Sisti, R. (1994). Z 2041. Oral embryotoxicity study in rabbits.
Unpublished report No. RTC 4195/T/128/94 from Research Toxicology
Centre SpA, Rome, Italy. Submitted to WHO by Zambon Group SpA, Bresso,
Milan, Italy.
Sutter, V.L & Finegold, S.M. (1976). Susceptibility of anaerobic
bacteria to 23 antimicrobial agents. Antimicrob. Agents Chemoter.,
10, 736-752.
TAP Pharmaceuticals Inc. (1987). Issues relating to the safety of
thiamphenicol with special attention to the matter of aplastic anemia.
Unpublished report from TAP Pharmaceuticals Inc., Corte Mader, Canada.
Submitted to WHO by Zambon Group SpA, Bresso, Milan, Italy.
Tomoeda, M. & Yamamoto, K. (1981). The hematologic adverse reaction
experience with thiamphenicol in Japan. In: Safety Problems Related to
Chloramphenicol and Thiamphenicol Therapy, Raven Press, New York,
pp. 103-110.
Uesugi, T., Ikeda, M., Hori, R., Katayama, K., & Arita, T. (1974).
Metabolism of thiamphenicol and comparative studies of its urinary and
biliary excretion with chloramphenicol in various species.
Chem. Pharm. Bull., 22, 2714-2722.
Yunis, A.A. & Gross, M.A. (1975). Drug-induced inhibition of myeloid
colony growth: protective effect of colony stimulating factor.
J. Lab. Clin. Med., 86, 499-504.
